Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
The clinical features, evaluation, and diagnosis of neonatal seizures will be reviewed here. The etiology and treatment of neonatal seizures and associated epilepsy syndromes are discussed separately. (See "Etiology and prognosis of neonatal seizures" and "Overview of neonatal epilepsy syndromes" and "Treatment of neonatal seizures" .)
EPIDEMIOLOGY
● Neonatal encephalopathy and hypoxic-ischemic encephalopathy
Neonatal Seizure Disorders
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prognosis |
- Key Points |
Neonatal seizures are abnormal electrical discharges in the central nervous system of neonates and usually manifest as stereotyped muscular activity or autonomic changes. Diagnosis is confirmed by electroencephalography; testing for causes is indicated. Treatment depends on the cause.
(See also Seizure Disorders in adults.)
Seizures occur in 1 to 5/1000 live births, and incidence increase in preterm infants and in low-birth-weight infants ( 1 ).
Seizures may be related to a serious neonatal problem and require immediate evaluation. Most neonatal seizures are focal, probably because generalization of electrical activity is impeded in neonates by lack of myelination and incomplete formation of dendrites and synapses in the brain.
Some neonates undergoing electroencephalography (EEG) to assess seizures or other symptoms of encephalopathy (eg, hypoactivity, decreased responsiveness) are found to have clinically silent seizures (≥ 20 seconds of rhythmic epileptiform electrical activity during an EEG but without any clinically visible seizure activity). Occasionally, clinically silent electrical activity is continuous and persists for > 20 minutes; at that point, it is defined as electrical status epilepticus.
1. Vasudevan C, Levene M : Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med 18(4):185–191, 2013. doi: 10.1016/j.siny.2013.05.008
Etiology of Neonatal Seizure Disorders
The abnormal central nervous system (CNS) electrical discharge may be caused by a
Primary intracranial process (eg, meningitis, ischemic stroke, encephalitis, intracranial hemorrhage, tumor, malformation)
Systemic problem (eg, hypoxia-ischemia, hypoglycemia, hypocalcemia, hyponatremia, other disorders of metabolism)
Seizures resulting from an intracranial process usually cannot be differentiated from seizures resulting from a systemic problem by their clinical features (eg, focal vs generalized).
Hypoxia-ischemia, the most common cause of neonatal seizures, may occur before, during, or after delivery (see Overview of Perinatal Respiratory Disorders ). Such seizures may be severe and difficult to treat, but they tend to abate after about 3 to 4 days. When neonatal hypoxia is treated with therapeutic hypothermia (usually whole-body cooling), seizures may be less severe but may recur during rewarming.
Ischemic stroke is more likely to occur in neonates with polycythemia , thrombophilia due to a genetic disorder, or severe hypotension but may occur in neonates without any risk factors. Stroke occurs typically in the middle cerebral artery distribution or, if associated with hypotension, in watershed zones. Seizures resulting from stroke tend to be focal and may cause apnea.
Neonatal infections such as meningitis and sepsis may cause seizures; in such cases, seizures are usually accompanied by other symptoms and signs. Group B streptococci and gram-negative bacteria are common causes of such infections in neonates. Encephalitis due to cytomegalovirus , herpes simplex virus , rubella virus , Treponema pallidum , Toxoplasma gondii , or Zika virus can also cause seizures.
Hypoglycemia is common among neonates whose mothers have diabetes , who are small for gestational age , or who have hypoxia-ischemia or other stresses. Seizures due to hypoglycemia tend to be focal and variable. Prolonged or recurrent hypoglycemia may permanently affect the CNS.
Intracranial hemorrhage , including subarachnoid , intracerebral, and intraventricular hemorrhage , may cause seizures. Intraventricular hemorrhage, which occurs more commonly in preterm infants , results from bleeding in the germinal matrix (an area that is adjacent to the ventricles and that gives rise to neurons and glial cells during development).
Hypernatremia
Hyponatremia can result from dilution (when too much water is given orally or IV particularly in the setting of hypovolemia, which, when severe enough, leads to increased antidiuretic hormone [ADH] levels despite the low serum osmolarity [nonosmotic ADH release]) or may follow sodium loss in stool or urine.
Hypocalcemia (serum calcium level < 7.5 mg/dL [ < 1.87 mmol/L]) is usually accompanied by a serum phosphorus level of > 3 mg/dL ( > 0.95 mmol/L) and can be otherwise asymptomatic. Risk factors for hypocalcemia include prematurity and a difficult birth. Hypocalcemia can also be a manifestation of DiGeorge syndrome (22q11.2 deletion syndrome).
Hypomagnesemia is a rare cause of seizures, which may occur when the serum magnesium level is < 1.4 mEq/L ( < 0.7 mmol/L). Hypomagnesemia often occurs with hypocalcemia and should be considered in neonates with hypocalcemia if seizures continue after adequate calcium therapy.
Inborn errors of metabolism (eg, amino or organic aciduria ) can cause neonatal seizures. Rarely,
CNS malformations can also cause seizures.
Maternal recreational substance use
Neonatal seizures may be familial; some have genetic causes. Benign familial neonatal convulsion is a potassium channelopathy inherited in an autosomal dominant pattern. Early infantile epileptic encephalopathy (Ohtahara syndrome) is a rare disorder associated with a variety of mutations.
Symptoms and Signs of Neonatal Seizure Disorders
Neonatal seizures are usually focal and may be difficult to distinguish from normal neonatal activity because they may manifest as chewing or bicycling movements. Common manifestations include migratory clonic jerks of extremities, alternating hemiseizures, and primitive subcortical seizures (which cause respiratory arrest, chewing movements, persistent eye deviations or nystagmoid movements, and episodic changes in muscle tone). Generalized tonic-clonic seizures are uncommon.
Clinically silent electrical seizure activity is often present after a hypoxic-ischemic insult (including perinatal asphyxia or stroke) and in neonates with CNS infections, especially after initial antiseizure medication treatment, which is more likely to stop clinical manifestations than electrical seizure activity.
Diagnosis of Neonatal Seizure Disorders
Electroencephalography (EEG)
Laboratory testing (eg, serum glucose, electrolytes, cerebrospinal fluid [CSF] analysis, urine and blood cultures; sometimes genetic testing)
Usually cranial imaging
Evaluation begins with a detailed family history and a physical examination.
Jitteriness (alternating contraction and relaxation of opposing muscles in the extremities) must be distinguished from true seizure activity. Jitteriness is usually stimulus-induced and can be stopped by holding the extremity still; in contrast, seizures occur spontaneously, and motor activity is felt even when the extremity is held still.
EEG is essential, and at times recording may need to be prolonged, especially when it is difficult to determine whether the neonate is having seizures. EEG is also helpful for monitoring response to treatment.
EEG should capture periods of active and quiet sleep and thus may require ≥ 2 hours of recording. A normal EEG with expected variation during sleep stages is a good prognostic sign; an EEG with diffuse severe abnormalities (eg, suppressed voltage or burst suppression pattern) is a poor one.
Bedside EEG with video monitoring for ≥ 24 hours may detect ongoing clinically silent electrical seizures, particularly in the first few days after a CNS insult.
Laboratory tests
Laboratory tests to look for underlying treatable disorders should be done immediately; tests include pulse oximetry; measurement of serum glucose, sodium, potassium, chloride, bicarbonate, calcium, and magnesium; and lumbar puncture for CSF analysis (cell count and differential, glucose, protein) and culture. Urine and blood cultures are also obtained.
The need for other metabolic tests (eg, arterial pH, blood gases, serum bilirubin, urine amino or organic acids) or tests for common recreationally used substances (passed to the neonate transplacentally or by breastfeeding) depends on the clinical situation.
Genetic testing must be considered for children with recurrent or refractory seizures of undetermined cause.
Imaging tests
Imaging tests are typically done unless the cause is immediately obvious (eg, glucose or electrolyte abnormality). MRI is preferred but may not be readily available; in such cases, head CT is done.
For very sick infants who cannot be moved to radiology, bedside cranial ultrasonography can be done; it may detect intraventricular but not subarachnoid hemorrhage. MRI or CT is done when infants are stable.
Head CT can detect intracranial bleeding and some brain malformations. MRI shows malformations more clearly and can detect ischemic tissue within a few hours of onset.
Magnetic resonance spectroscopy may help determine the extent of an ischemic injury or identify buildup of certain neurotransmitters associated with an underlying metabolic disorder.
Treatment of Neonatal Seizure Disorders
Treatment of cause
Antiseizure medications
Treatment of neonatal seizures is focused primarily on the underlying disorder and secondarily on seizures.
Treatment of the cause
For low serum glucose,
For hypocalcemia,
For hypomagnesemia, 0.2 mL/kg (100 mg/kg) of a 50% magnesium sulfate solution is given IM.
Bacterial infections are treated with antibiotics.
Herpes encephalitis
Antiseizure medications are used unless seizures stop quickly after correction of reversible disorders such as hypoglycemia, hypocalcemia, hypomagnesemia, hyponatremia, or hypernatremia.
Phenobarbital is continued IV, especially if seizures are frequent or prolonged. When the infant is stable, phenobarbital can be given orally at 3 to 4 mg/kg once/day. Therapeutic serum levels of phenobarbital are 20 to 40 mcg/mL (85 to 170 micromol/L), but higher levels are sometimes needed at least temporarily.
phenobarbital . It is given IV as a 20- to 60-mg/kg IV loading dose given at 2 to 5 mg/kg/minute, and therapy may be continued as 10 to 30 mg/kg IV every 12 hours. Therapeutic levels are not well-established in the neonate.
phenobarbital and levetiracetam
Neonates given IV antiseizure medications are closely observed; large doses and combinations of medications, particularly lorazepam plus phenobarbital , may result in respiratory depression.
The optimal duration of maintenance therapy is not known for any of the antiseizure medications and depends on the underlying etiology of seizures and on the presence of risk factors for seizure recurrence.
Prognosis for Neonatal Seizure Disorders
Prognosis depends on the etiology:
About 50% of neonates with seizures due to hypoxia-ischemia develop normally.
Most neonates with seizures due to a transient electrolyte disturbance (eg, hypocalcemia, hyponatremia) do well when seizures resolve after the disturbance is reversed and long-term antiseizure medications are not required.
Those with severe intraventricular hemorrhage have a high morbidity rate.
For idiopathic seizures or seizures due to malformations, earlier onset is associated with worse neurodevelopmental outcomes.
It is suspected, but not proved, that prolonged or frequent neonatal seizures may cause damage beyond that caused by the underlying disorder. There is concern that the metabolic stress of prolonged nerve cell firing during lengthy seizures may cause additional brain damage. When caused by acute injuries to the brain such as hypoxia-ischemia, stroke, or infection, neonates may have a series of seizures, but seizures typically abate after about 3 to 4 days; they may recur months to years later if brain damage has occurred. Seizures due to other conditions may be more persistent during the neonatal period.
Neonatal seizures usually occur in reaction to a systemic or central nervous system event (eg, hypoxia/ischemia, stroke, hemorrhage, infection, metabolic disorder, structural brain abnormality).
Neonatal seizures are usually focal and may be difficult to recognize; common manifestations include migratory clonic jerks of extremities, chewing movements, persistent eye deviations or nystagmoid movements, and episodic changes in muscle tone.
Electroencephalography is essential for diagnosis; laboratory testing and usually neuroimaging are done to identify the cause.
Treatment is directed at the cause.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Neonatal seizures—diagnostic options and treatment recommendations
Neugeborenenanfälle: diagnostische Optionen und Therapieempfehlungen
- Open access
- Published: 25 October 2022
- Volume 35 , pages 310–316, ( 2022 )
Cite this article
You have full access to this open access article

- Georgia Ramantani MD, Ph.D. 1 , 2 , 3 &
- Francesco Pisani 4
2539 Accesses
Explore all metrics
Seizures in neonates should prompt rapid evaluation to verify the diagnosis, determine etiology, and initiate appropriate treatment. Neonatal seizure diagnosis requires EEG confirmation and clinical observation alone is insufficient. Although most neonatal seizures are related to acute brain injury, some neonates present early-onset structural or metabolic/genetic epilepsy. Video-EEG monitoring, the gold standard for neonatal seizure detection and quantification, is resource-intensive and often unavailable, with amplitude-integrated EEG offering a reasonable alternative in guiding treatment. Whereas new-generation antiseizure medication (ASM), such as levetiracetam, appear promising, particularly in terms of tolerability, older-generation ASM, such as phenobarbital and phenytoin, are yet to be replaced. Acute treatment should aim at stopping both electroclinical and electrographic-only seizures. In neonates with acute provoked seizures, ASM should be discontinued without tapering after 72 h of seizure freedom and before hospital discharge.
Zusammenfassung
Epileptische Anfälle bei Neugeborenen sollten rasch evaluiert werden, um die Diagnose zu bestätigen, die Ursache festzustellen und eine angemessene Behandlung einzuleiten. Zur sicheren Diagnosestellung der Neugeborenenanfälle ist ein Elektroenzephalogramm (EEG) unerlässlich, da die klinische Beobachtung allein unzureichend ist. Obwohl die meisten Neugeborenenanfälle auf akute Erkrankungen des zentralen Nervensystems zurückzuführen sind, liegt bei manchen Neugeborenen eine früh beginnende strukturelle oder metabolische/genetische Epilepsie vor. Das Video-EEG-Monitoring, der Goldstandard für die Erkennung und Quantifizierung von Neugeborenenanfällen, ist ressourcenintensiv und oft nicht verfügbar, wobei das amplitudenintegrierte EEG (aEEG) eine gute Alternative in der Therapieüberwachung der Neugeborenenanfälle darstellt. Obwohl anfallsunterdrückende Medikamente der neueren Generation, wie Levetiracetam, aufgrund der besseren Verträglichkeit immer häufiger eingesetzt werden, bleiben die Medikamente der älteren Generation, wie Phenobarbital und Phenytoin, Mittel der ersten Wahl. Die Akutbehandlung sollte darauf abzielen, sowohl elektroklinische als auch rein elektrographische Anfälle anzuhalten. Bei Neugeborenen mit akut provozierten Anfällen sollte die Medikation nach 72 h Anfallsfreiheit und vor Entlassung beendet werden.
Similar content being viewed by others

Recent Advances in Neonatal Seizures
EEG Monitoring of the Epileptic Newborn
Avoid common mistakes on your manuscript.
Neonatal seizures are often the first and most common neurological sign in neonates. Seizures occur more frequently in the first week of life than at any other time, with a reported incidence of 1–4 in 1000 neonates, and higher rates reported in preterm neonates and in those with lower gestational age and birth weight [ 13 ]. Seizures may suggest the presence of a potentially treatable disorder and should therefore prompt a timely workup and treatment initiation [ 14 , 18 ]. Neonatal seizures, notably recurrent or prolonged, can cause further brain injury, thus increasing the risk for poor neurological outcomes, including cognitive and motor impairment and post-neonatal epilepsy [ 14 , 16 ]. Recent advances in diagnostics, including full EEG, amplitude-integrated EEG (aEEG), magnetic resonance imaging, metabolic and genetic testing, have drastically improved seizure detection and facilitated etiologic classification, paving the way for targeted treatment and potentially optimal outcomes. However, despite the integration of ground-breaking preclinical research and the implementation of cutting-edge technology in clinical research, treating neonatal seizures still poses a challenge for clinicians.
Neonatal seizures are unique since most relate to acute brain injuries such as hypoxic–ischemic encephalopathy (HIE), structural brain injury—including ischemic and hemorrhagic stroke-and metabolic derangements, usually of glucose and electrolytes—and central nervous system or systemic infections. Intraventricular hemorrhage in premature neonates and HIE in term neonates are the predominant etiology of acute provoked seizures. Only ~15% of neonatal seizures are related to neonatal-onset epilepsy [ 14 , 18 ] that may arise from structural brain abnormalities (structural epilepsies), such as malformations of cortical development, or genetic conditions (genetic epilepsies), such as ion channel and vitamin-dependent disorders [ 28 ]. Recurrent seizures in otherwise healthy neonates with overall negative findings may correspond to self-limited neonatal epilepsy with an excellent prognosis, whereas recurrent seizures in severely affected neonates with neurological deficit may indicate an early infantile developmental and epileptic encephalopathy with particularly poor prognosis.
Diagnostic options
Electroencephalography.
Although neonatal seizures have been commonly diagnosed based on clinical semiology alone, recent studies have shown that EEG confirmation is essential to avoid misdiagnosis and guide treatment. Not all clinically suspicious events in preterm or term neonates correspond to seizures and most neonatal seizures are electrographic-only. The high rate of uncoupling, meaning that electroclinical seizures become electrographic-only following the administration of anti-seizure medication (ASM)—notably phenobarbital and phenytoin—in affected neonates, adds to this challenge [ 3 , 20 , 27 ].
The American Clinical Neurophysiology Society (ACNS) has defined electrographic neonatal seizures as sudden, abnormal EEG events defined by a repetitive and evolving pattern with a voltage of > 2 μV and a duration of > 10 s. “Evolving” is defined as an unequivocal evolution in frequency, voltage, morphology, or location, i.e., increasing amplitude and decreasing frequency of discharges over time [ 26 ]. When these EEG changes are related to simultaneously coupled definite clinical signs, the seizure is defined as electro-clinical; otherwise, the seizure is defined as electrographic-only. However, the definition of neonatal seizures has been reconsidered, and their duration is currently not strictly defined but has to be sufficient to (1) demonstrate an evolution in frequency and morphology of the discharges and (2) allow for the recognition of onset, evolution, and resolution of an abnormal discharge [ 15 ], with the exception of myoclonic seizures and spasms. Interestingly, EEG seizure patterns in neonates often show a focal onset and evolution, although not necessarily reflecting a focal pathology [ 18 ]. Although no consensus definition of neonatal status epilepticus is yet available [ 15 ], this may be diagnosed when the summed duration of seizures comprises ≥ 50% of an arbitrarily defined 1‑h epoch.
Continuous video EEG (v-EEG) is the gold standard for reliable neonatal seizure detection, prompt treatment initiation, and monitoring. For neonates at high risk of seizures, the ACNS recommends v‑EEG for 24 h [ 23 ]. In a prospective cohort study of neonates with clinically suspicious events or electrographic seizures who underwent v‑EEG, the median time from the beginning of the recording to electrographic seizure detection was 7 h [ 9 ]. If the EEG background activity is stable and no seizures have occurred in 24 h, the monitoring may be discontinued, except for neonates with HIE, who have a particularly high risk of seizures and thus require v‑EEG throughout cooling and rewarming. An integrative approach to neuromonitoring in high-risk neonates with v‑EEG and, ideally, a multicamera setup instead of the standard single-camera can significantly increase diagnostic accuracy. However, v‑EEG is expensive, time-consuming, and moderately invasive, while its rapid installation and real-time interpretation are prohibitive since it would require the 24/7 availability of skilled technical and medical personnel [ 16 , 18 ]. To address this issue, a flexible frame of recommendations for neonatal units, encompassing different levels of complexity, local resources, and patient features, has been recently proposed [ 5 ]. Novel automated seizure detection algorithms and seizure-burden analyses developed for use in clinical settings may enhance seizure detection and provide decision support. Although no automated algorithm is reliable enough to replace experienced EEG specialists, future clinical trials will undoubtedly profit from these technological developments.
Amplitude-integrated EEG
Amplitude-integrated EEG (aEEG), with its time-compressed, one-or two-channel EEG trend presented on a semi-logarithmic scale, is increasingly applied as an adjuvant tool for continuous monitoring of affected neonates. Particularly in HIE, aEEG is an adjunctive criterion for the introduction of therapeutic hypothermia. Reducing electrode placement and interpretation time compared to conventional EEG, aEEG enables the assessment of the background activity and facilitates the earlier recognition of state changes. However, aEEG is considerably less accurate than conventional EEG for seizure detection, especially for seizures (1) arising outside the centro-temporal regions, (2) featuring a short duration, and (3) consisting of low-voltage slow discharges. However, seizures are generally more common in the central regions, and these seizure patterns can be correctly identified in 70–80% of cases since they are sampled by aEEG [ 24 ]. Although seizure detection by aEEG has been shown to miss 20% of neonatal seizures compared to full EEG, even when used by aEEG experts [ 25 ], aEEG-based seizure detection is still more reliable than clinical diagnosis alone [ 8 ]. Seizures can be detected in the aEEG as “saw-tooth-like” augmentations of the baseline amplitude but should be confirmed by examining the simultaneous EEG trace to rule out artifacts that pollute these unattended EEG recordings without simultaneous video.
Seizure semiology can reveal specific etiologies (Table 1 ). Myoclonic seizures should raise suspicions of a metabolic disorder such as non-ketotic hyperglycinemia, propionic acidemia, and vitamin B 6 -dependent epilepsy. Focal motor clonic seizures are more frequently acute provoked, corresponding to a focal cortical lesion such as stroke, intracranial hemorrhage, and, more rarely, cortical dysplasia [ 19 ]. Motor tonic seizures often arise in genetically determined developmental epileptic encephalopathy ( KCNQ2, CDKL5, STXBP1 -related epilepsy, etc.) if a cortical malformation or an acute provoked etiology is unlikely based on clinical history. Sequences of a tonic followed by a myoclonic or clonic phase (sequential seizures) are a hallmark of KCNQ2 -related epilepsy, the most common neonatal-onset genetic epilepsy. Epileptic spasms in neonates are rare, primarily found in metabolic disorders, such as vitamin B 6 -dependent epilepsy but also in cortical malformations or early-onset epileptic encephalopathy.
Classification
The Task Force on Neonatal Seizures established by the International League Against Epilepsy (ILAE) has recently introduced a diagnostic framework consisting of four domains: clinical presentation (high risk or suspicious clinical events), diagnosis (with EEG), manifestation (with or without clinical signs), and seizure types determined by the predominant clinical signs ( motor : automatisms, clonic, epileptic spasms, myoclonic, sequential, and tonic; non-motor : autonomic and behavioral arrest; and unclassified ) or without clinical signs [ 15 ]. In contrast to older classifications that derived from clinical semiology alone, this new classification emphasizes the critical role of EEG in the diagnosis of neonatal seizures. Paroxysmal clinical events without EEG confirmation are considered non-epileptic, and EEG-confirmed seizures are divided into electroclinical or electrographic-only seizures. Notably, neonatal seizures are generally considered focal at onset, although seizures such as spasms or myoclonic seizures may rapidly engage bilaterally distributed networks.
Differential diagnosis
The clinical differentiation of neonatal seizures from paroxysmal non-epileptic motor phenomena is particularly challenging if v‑EEG is unavailable. There is a wide range of abnormal and paroxysmal motor phenomena in neonates: tremor and jitteriness, benign neonatal sleep myoclonus, startle reflex, ocular movement disorders, paroxysmal dystonia, bilateral tonic stiffening, and hyperekplexia. Some clinical features may facilitate the differential diagnosis, including stimulus sensitivity (e.g., different stimuli, mainly auditory, for startle reflex, crying and stress for tremor and jitteriness, sudden visual stimuli or movement for paroxysmal tonic up/downward gaze), habituation (present in startle reflex but absent in hyperekplexia), and association with behavioral states (benign neonatal sleep myoclonus stops with arousal). Holding the affected limbs or repositioning the neonate can differentiate some paroxysmal non-epileptic from epileptic phenomena: Gentle restraint stops physiological tremors but does not influence epileptic events. The use of polygraphic v‑EEG is fundamental in uncovering these clinical conditions. Finally, non-epileptic paroxysmal events in the neonate are often symptomatic of an underlying pathology and should be evaluated just as systematically and thoroughly as epileptic seizures. Most abrupt changes in vital signs, such as blood pressure, heart rate, and respirations, recorded in neonates do not correspond to epileptic seizures; when these changes correspond to seizures, they are usually associated with motor phenomena or other clinical signs. In a retrospective v‑EEG study evaluating abrupt changes in vital signs, these were more likely to correspond to epileptic seizures when oxygen desaturation or apnea occurred, particularly in the presence of abnormal eye movements or abrupt tone changes [ 4 ].
Symptomatic treatment
Starting anti-seizure medication.
Particularly in HIE, both preclinical and clinical studies have provided evidence that prolonged or recurrent seizures themselves may augment injury to the developing brain beyond that of the underlying etiology [ 16 ]. For electrographic seizures, the most common seizure type in neonates, a recent study showed that those treated within 1 h of seizure onset had a significantly lower seizure burden and fewer seizures over the subsequent 24 h. This effect was absent if ASM was started > 1 h from seizure onset, suggesting that the impact of ASM on seizure burden is time-critical, with an optimal efficacy within 1 h of seizure onset. Despite this recent evidence, the optimal timepoint for treatment initiation in neonatal seizures remains ill-defined, whereas a cumulative electrographic seizure burden of > 30 s/h has been proposed as the entry criterion for randomization in therapeutic trials for neonatal seizures.
Medication selection
The optimal ASM management of neonatal seizures is still under debate. In a 2004 Cochrane review [ 2 ], the authors concluded that “there is little evidence from randomized controlled trials to support the use of any of the anticonvulsants currently used in the neonatal period.” Although neonatal seizure management pathways differ between institutions and settings, the most commonly used ASMs are phenobarbital (PB), phenytoin (PHT), and levetiracetam (LEV), with alternatives including lacosamide (LCM), and escalation to continuous midazolam (MDZ) infusion [ 11 ]. However, overall response rates to ASM are low, whereas the self-limited nature of acute provoked seizures and the potential neurotoxicity of ASM to the immature brain dictate caution. Standardized treatment protocols assessing ASM response in neonatal seizures may be crucial for improving outcomes and reducing adverse effects, morbidity, and mortality. Reassessing intravenous ASM efficacy 60 min from initiation has been linked to lower status epilepticus rates, lower PB concentrations, and shorter hospital stays.
The oldest and most popular first-line ASM, PB displays efficacy in only half of the cases and produces the phenomenon of electroclinical uncoupling in treated neonates. Moreover, PB has been associated with widespread neuronal apoptosis [ 1 ] and impaired synaptic maturation [ 7 ] in the animal model. It should be noted that these studies have been performed on heathy rodents and their results have neither been confirmed in humans nor has the benefit–risk ratio been evaluated in immature rodents with seizures. The main adverse effects of PB, including hypotension, respiratory suppression, and sedation, are particularly relevant for neonates with severe encephalopathy. Third-generation ASMs with a good efficacy and safety profile, particularly LEV, have emerged as novel treatment options for neonatal seizures [ 17 ]. One of the key arguments for LEV use in neonatal seizures is the more favorable pharmacokinetics, characterized by a linear clearance, few drug interactions, and a broad therapeutic index. By contrast, PB is linked to auto-inducible clearance with use and numerous drug interactions. However, PB was considerably more effective than LEV for treating neonatal seizures in a recent randomized controlled study, although higher rates of adverse effects were seen with PB [ 22 ].
Among second-line ASM, it should be noted that lidocaine reached a response rate of 68% in full-term neonates, higher than MDZ. Concerns regarding lidocaine toxicity, mainly of cardiac arrhythmias, have been eased following the introduction of new reduced-dose regimens. Nevertheless, given these potential cardiac side effects, lidocaine should not be combined with other cardiotoxic agents, e.g., phenytoin/fosphenytoin (PHT/FPHT). Little is known about the use of newer ASM, such as brivaracetam, in the neonatal period, while other, older ASMs have limited use in the acute phase because of the unavailability of an intravenous formulation. No specific ASM is indicated for preterm neonates, despite the vast differences in pharmacokinetics and brain maturation.
Finally, therapeutic hypothermia in HIE may alter the metabolism of ASM, particularly in neonates with multiorgan dysfunction. Hypothermia prolongs clearance of PHT with increased risk of bradycardia and of lidocaine with increased risk of cardiotoxicity. By contrast, the rewarming phase of hypothermia may accelerate ASM metabolism.
Etiologic treatment
The choice of treatment should be guided by the etiology of neonatal seizures since, in some cases, specific and efficacious treatment choices are available for affected neonates [ 14 , 18 ]. Inborn errors of metabolism, diagnosed based on clinical presentation as well as biochemical investigations and verified by genetics, represent a significant challenge that needs to be rapidly addressed to avoid metabolic decompensation and facilitate prognostic counseling. Since early diagnosis enables precision medicine, i.e., etiologic treatment in selected metabolic disorders and specific ASM in selected neonatal-onset genetic epilepsies [ 12 , 21 ], a diagnostic algorithm designed to detect these disease entities is required in all neonatal units. This algorithm should include a standardized and well-documented vitamin B 6 trial to identify defects in the ALDH7A1, PNPO, PLPBP gene or cases of severe congenital hypophosphatasia [ 18 ]. Finally, some early-onset genetic epilepsies may respond to sodium channel blockers such as carbamazepine, especially loss-of-function- KCNQ2 or gain-of-function SCN2A / SCN8A . Clinical data regarding the use of quinidine in gain-of-function KCNT1 are, however, inconclusive [ 6 ].
Treatment duration
Since neonatal seizures are acute provoked in their majority, it has been suggested to wean medication to a single ASM before discharge or even withdraw ASM altogether when (1) only single or rare seizures have occurred, (2) the neonate has been seizure-free for at least 48–72 h, and (3) the risk of recurrence is not considered particularly high. Discontinuation of ASM prior to hospital discharge has not been related to a higher risk of post-neonatal epilepsy or less favorable neurodevelopmental outcomes at the 2‑year follow-up. Therefore, while the World Health Organization recommends withdrawing ASM after 72 h of seizure freedom in those with normal EEG and neurological examination [ 29 ], others suggest expanding this recommendation to neonates with abnormal EEG and neurological examination [ 10 ]. However, if seizures are uncontrolled or neonatal-onset epilepsy has been diagnosed [ 14 ], ASM should be maintained and neonates should be referred to specialized neuropediatric clinics for further management.
Practical conclusion
Neonatal seizures are a medical emergency and should prompt rapid evaluation to determine the etiology and introduce symptomatic and/or etiologic therapy.
Treatment of the underlying cause (hypoxic–ischemic encephalopathy, infection, metabolic derangements, etc.) is critical for preventing further damage to the developing brain. Although video EEG is the gold standard for neonatal seizure detection and treatment surveillance, it is resource-intensive and often unavailable, with amplitude-integrated EEG offering a reasonable alternative to guide therapeutic decisions.
Whereas new-generation antiseizure medications (ASMs) appear promising, particularly in terms of tolerability, these have not yet replaced older-generation ASM. Acute treatment should aim at stopping both electroclinical and electrographic-only seizures, and ASM should be given in a predefined order and in sufficient dosages to achieve plasma levels in the higher therapeutic range, provided that the ASM is well tolerated.
In addition to efficacy, ASM choice should be guided by the potential adverse effects of the ASM and the state of the patient since renal, cardiovascular, and hepatic dysfunction are common.
In neonates with acute provoked seizures, ASM should be discontinued without tapering after 72 h of seizure freedom and before hospital discharge. Neonates with neonatal-onset epilepsy should be maintained on ASM and followed up closely in specialized neuropediatric clinics.
While diagnosing and treating neonatal seizures remains challenging, the development of novel, disease-modifying, or anti-epileptogenic therapies and new neuroprotective agents may ultimately improve neonatal seizure outcomes.
Bittigau P, Sifringer M, Genz K et al (2002) Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A 99:15089–15094. https://doi.org/10.1073/pnas.222550499
Article CAS PubMed PubMed Central Google Scholar
Booth D, Evans DJ (2004) Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004218.pub2
Article PubMed Google Scholar
Boylan GB, Stevenson NJ, Vanhatalo S (2013) Monitoring neonatal seizures. Semin Fetal Neonatal Med 18:202–208. https://doi.org/10.1016/j.siny.2013.04.004
Dang LT, Shellhaas RA (2016) Diagnostic yield of continuous video electroencephalography for paroxysmal vital sign changes in pediatric patients. Epilepsia 57:272–278. https://doi.org/10.1111/epi.13276
Article CAS PubMed Google Scholar
Dilena R, Raviglione F, Cantalupo G et al (2021) Consensus protocol for EEG and amplitude-integrated EEG assessment and monitoring in neonates. Clin Neurophysiol 132:886–903. https://doi.org/10.1016/j.clinph.2021.01.012
Fitzgerald MP, Fiannacca M, Smith DM et al (2019) Treatment responsiveness in KCNT1-related epilepsy. Neurother J Am Soc Exp Neurother 16:848–857. https://doi.org/10.1007/s13311-019-00739-y
Article CAS Google Scholar
Forcelli PA, Janssen MJ, Vicini S, Gale K (2012) Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol 72:363–372. https://doi.org/10.1002/ana.23600
Frenkel N, Friger M, Meledin I et al (2011) Neonatal seizure recognition—comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin Neurophysiol 122:1091–1097. https://doi.org/10.1016/j.clinph.2010.09.028
Glass HC, Shellhaas RA, Wusthoff CJ et al (2016) Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr 174:98–103.e1. https://doi.org/10.1016/j.jpeds.2016.03.035
Article PubMed PubMed Central Google Scholar
Glass HC, Soul JS, Chang T et al (2021) Safety of early discontinuation of Antiseizure medication after acute symptomatic neonatal seizures. JAMA Neurol 78:817–825. https://doi.org/10.1001/jamaneurol.2021.1437
Keene JC, Morgan LA, Abend NS et al (2021) Treatment of neonatal seizures: comparison of treatment pathways from 11 neonatal intensive care units. Pediatr Neurol. https://doi.org/10.1016/j.pediatrneurol.2021.10.004
Numis AL, Angriman M, Sullivan JE et al (2014) KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response. Neurology 82:368–370. https://doi.org/10.1212/WNL.0000000000000060
Pisani F, Facini C, Bianchi E et al (2018) Incidence of neonatal seizures, perinatal risk factors for epilepsy and mortality after neonatal seizures in the province of Parma, Italy. Epilepsia 59:1764–1773. https://doi.org/10.1111/epi.14537
Pisani F, Spagnoli C, Falsaperla R et al (2021) Seizures in the neonate: a review of etiologies and outcomes. Seizure 85:48–56. https://doi.org/10.1016/j.seizure.2020.12.023
Pressler RM, Cilio MR, Mizrahi EM et al (2021) The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE task force on neonatal seizures. Epilepsia 62:615–628. https://doi.org/10.1111/epi.16815
Ramantani G (2013) Neonatal epilepsy and underlying aetiology: to what extent do seizures and EEG abnormalities influence outcome? Epileptic Disord 15:365–375. https://doi.org/10.1684/epd.2013.0619
Ramantani G, Ikonomidou C, Walter B et al (2011) Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol 15:1–7. https://doi.org/10.1016/j.ejpn.2010.10.003
Ramantani G, Schmitt B, Plecko B et al (2019) Neonatal seizures-are we there yet? Neuropediatrics 50:280–293. https://doi.org/10.1055/s-0039-1693149
Santarone ME, Pietrafusa N, Fusco L (2020) Neonatal seizures: when semiology points to etiology. Seizure 80:161–165. https://doi.org/10.1016/j.seizure.2020.06.025
Scher MS, Alvin J, Gaus L et al (2003) Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol 28:277–280
Schubert-Bast S, Hofstetter P, Fischer D et al (2017) Sodium channel blockers in KCNQ2-encephalopathy: lacosamide as a new treatment option. Seizure 51:171–173. https://doi.org/10.1016/j.seizure.2017.08.005
Sharpe C, Reiner GE, Davis SL et al (2020) Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 145:e20193182. https://doi.org/10.1542/peds.2019-3182
Shellhaas RA, Chang T, Tsuchida T et al (2011) The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 28:611–617
Shellhaas RA, Soaita AI, Clancy RR (2007) Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics 120:770–777. https://doi.org/10.1542/peds.2007-0514
Toet MC, van der Meij W, de Vries LS et al (2002) Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 109:772–779
Tsuchida TN, Wusthoff CJ, Shellhaas RA et al (2013) American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol 30:161–173. https://doi.org/10.1097/WNP.0b013e3182872b24
Weiner SP, Painter MJ, Geva D et al (1991) Neonatal seizures: electroclinical dissociation. Pediatr Neurol 7:363–368. https://doi.org/10.1016/0887-8994(91)90067-u
Zuberi SM, Wirrell E, Yozawitz E et al (2022) ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 63:1349–1397. https://doi.org/10.1111/epi.17239
WHO (2011) Guidelines on Neonatal Seizures. World Health Organization, Geneva
Google Scholar
Download references
Acknowledgements
We thank the Vontobel Foundation (to G.R.) for funding.
INFORMATION: Vontobel-Stiftung, Prof. Dr. Georgia RAMANTANI
Open access funding provided by University of Zurich
Author information
Authors and affiliations.
Department of Neuropediatrics, University Children’s Hospital Zurich, Steinwiesstrasse 75, 8032, Zurich, Switzerland
Georgia Ramantani MD, Ph.D.
Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland
University of Zurich, Zurich, Switzerland
Child Neuropsychiatry Unit, Medicine and Surgery Department, University of Parma, Parma, Italy
Francesco Pisani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Georgia Ramantani MD, Ph.D. .
Ethics declarations
Conflict of interest.
G. Ramantani and F. Pisani declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Supplementary Information
Comprehensive list of references on the topic., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ramantani, G., Pisani, F. Neonatal seizures—diagnostic options and treatment recommendations. Z. Epileptol. 35 , 310–316 (2022). https://doi.org/10.1007/s10309-022-00534-4
Download citation
Accepted : 23 September 2022
Published : 25 October 2022
Issue Date : November 2022
DOI : https://doi.org/10.1007/s10309-022-00534-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neonatal seizures
- Term neonates
- Preterm neonates
- Anti-seizure medication
Schlüsselwörter
- Neugeborenenanfälle
- Termingeborene
- Frühgeborene
- Anfallsunterdrückende Medikation
- Find a journal
- Publish with us
- Track your research
Search In: All Awards Chapter Spotlights Chapters Congresses Documents Epigraph Articles Farewells News Regional Commissions Topical Commissions Translated Content Web Pages
Found {{ totalResults }} result s
{{ item.typename }}
- Guidelines & Reports
- Archive of Draft Guideline Comments
Treatment of Seizures in the Neonate: Guidelines and Consensus-based Recommendations
The ILAE clinical practice guideline development group for neonatal seizures has developed guidelines and expert-based consensus recommendations for the treatment of neonatal seizures. These recommendations were informed by an extensive systematic review and, where no sufficient evidence was available, on expert-based consensus via Delphi. We are seeking your comments prior to submitting to a journal for peer review.
The systematic review identified 218 studies. The proposal includes guidelines and consensus-based recommendations on first and second line treatment, when to stop antiseizure medication, the impact of therapeutic hypothermia on seizure burden, and use of pyridoxine and pyridoxal 5´-phosphate. An example of a suggested treatment pathway including doses and adverse events based on current evidence and expert recommendations is given.
Draft : Treatment of Seizures in the Neonate: Guidelines and Consensus-based Recommendations – Special Report from the ILAE Task Force on Neonatal Seizures
Appendix A | Appendix B | Appendix C
Public comments are now closed and available for review below.
16 January 2023
Thank you very much for the work done, the evidence presented indicates that phenobarbital continues with the first option, leaving different options in the second line. What is not clear is whether using electroencephalographic monitoring when treating seizures what is referred to in some bibliography as seizure burden.
Rosa Y Alvarado M
13 January 2023
Thank you for compiling this important guidance. We would like to suggest some comments for your consideration.
- There is no caveat included for the caution regarding the use phenytoin or carbamazepine as a second-line agent if a channelopathy is suspected (page 11) and further, for the incorporation of genetic testing in the treatment decision. While genetic variants in SCN1A and the associated Dravet Syndrome are unlikely to present in the neonatal period, we found that nearly 1% of the 800 neonates with seizures evaluated by a tested by a commercial genetic testing laboratory had a positive molecular diagnosis in SCN1A, for which the use of sodium-channel agents may not be appropriate (poster presented at the 2022 AES meeting by Grayson et al.)
- For Ref 32: Painter et al 1997; Phenobarbital and Phenytoin met criteria for success in 13/30 (43%) and 13/29 (45%) as the initial agent, and when the other agent was added to the regimen for neonates whose seizures persistent, seizures were controlled with phenobarbital in 5/13 (39%) and phenytoin in 4/15 (27%) neonates.
- For Ref 33: Sharpe et al 2020; Phenobarbital and Levetiracetam met criteria for success with dose escalation in 24/30 (80%) and 15/53 (28%) patients as the initial agent, and when the other agent was added to the regimen for neonates whose seizures persistent, seizures were controlled with phenobarbital in 20/37 (54%) and levetiracetam in 1/6 (17%) neonates, again with dose escalation in this second-line treated group.
- Further, the information presented argues for phenobarbital to be considered in the list of second-line agents as well. Phenobarbital was the only ASM evaluated in the Delphi process that was not recommended by the experts as second-line treatment. Given that it is the choice of first line agent, we feel there should be a comment on why it wasn’t recommended, particularly as there may be patients started on a sodium channel agent (as recommended in the guidelines) and did not respond. These would be highly appropriate patients for phenobarbital use.
- The recommendation (#3) to discharge babies whose seizures resolved off ASMs regardless of EEG and MRI findings is supported by the paper cited (ref 58). However, we think the response should be modulated somewhat, in that if seizures recur, obviously ASMs should be resumed. There is a chance that this recommendation could be taken too literally and could potentially obviate good clinical judgement.
- Finally, the guidance states that the research priorities for the treatment of neonatal seizures includes dose finding, pharmacokinetic and safety studies in neonates, however, there are no recommendations regarding the preferred formulation of these ASMs. If an intravenous formulation is recommended, this should be specifically mentioned in addition to the acceptability of other routes of administration including intranasal and rectal use of ASMs.
Celene Grayson, PhD and Cynthia Harden, MD
11 January 2023
Dear ILAE Task Force:
Thank you for drafting this important document - these guidelines will be helpful to clinicians managing seizures in neonates. We would like to offer several points for your consideration.
- The most recent search was done on 28 June 2020, and these guidelines are being drafted in 2023. We would suggest consideration of an updated search to ensure the most recent evidence is considered for these guidelines, and we have cited some of these publications. 1,2
- The search strategy focusing on studies that include only electrographic seizures could potentially exclude studies that evaluated seizure response based on other important outcomes related to their safety. We understand this is important when evaluating the efficacy of seizure treatment on seizure burden and to avoid including seizure mimics. However, it excludes studies that provide insight into other relevant factors related to ASM treatment, such as adverse effects that may impact important outcomes such as mortality and neurodevelopment. Safety studies typically require large numbers of patients, and neonatal studies that only include patients with electrographic seizures are not sufficient powered to detect safety related issues. Phenobarbital and phenytoin have been shown to be neurotoxic in animal models. 3-5 Additionally, phenobarbital has been associated with decreased cognitive and motor scores in some studies of infants and young children. 6-8 As the ultimate goal of ASM treatment is not simply seizure cessation but long term outcome, a search strategy that excludes studies evaluating adverse effects ASM does not provide a complete picture of long-term outcome. We would suggest that future search strategies consider real-world evidence, where not all infants will undergo continuous EEG monitoring, into consideration of evidence. Access to continuous EEG monitoring that is interpreted by trained pediatric neurologists may be limited, even in high-resource settings. This is especially important if these guidelines are to be utilized in low and middle income countries.
- The second-line treatment recommendations should consider risks such as mortality and neurodevelopmental outcome in recommendations between agents such as a phenytoin/fosphenytoin and levetiracetam. The current guideline draft states the level of evidence was very low for this recommendation and expert opinion was sought (with mixed results). As stated above, the authors’ search strategy limited to electrographic seizures excluded studies that could provide helpful information on adverse effects of ASMs. Adverse effects of phenytoin include arrhythmias, respiratory depression, and hypotension, which are serious and potentially lethal side-effects. 9 For example, a recent large US cohort study examined phenytoin compared to levetiracetam as second-line treatment following phenobarbital failure. 2 The comparative safety of these two medications in this study favors levetiracetam as second-line treatment.
Thank you for the opportunity to provide points for your consideration.
Dr. Elizabeth Sewell, Dr. Ravi Patel and Dr. Kaashif Ahmad
References :
- Glass HC, Shellhaas RA. Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures-Reply. JAMA Neurol. 2022;79(1):91-92.
- Sewell EK, Hamrick SEG, Patel RM, Bennett M, Tolia VN, Ahmad KA. Association between anti-seizure medication and outcomes in infants. J Perinatol. 2021.
- Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089-15094.
- Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug–induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52(12):e207-e211.
- Kaushal S, Tamer Z, Opoku F, Forcelli PA. Anticonvulsant drug–induced cell death in the developing white matter of the rodent brain. Epilepsia. 2016;57(5):727-734.
- Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. Journal of Perinatology. 2013;33(11):841-846.
- Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures—effects on intelligence and on seizure recurrence. New England Journal of Medicine. 1990;322(6):364-369.
- Sulzbacher S, Farwell JR, Temkin N, Lu AS, Hirtz DG. Late cognitive effects of early treatment with phenobarbital. Clinical pediatrics. 1999;38(7):387-394.
- El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin Fetal Neonatal Med. 2017;22(5):321-327.
10 January 2023
Thanks to all for the wonderful work.
I think is accessible and affordable even in LMIC. Anyway only levet we’re preferred in cardiac condition so if there’s no levet in the case of seizure due to channel channelopathy what could be the anti seizure choice.
Dear ILAE Task force,
We congratulate for the efforts made in providing advances in neonatal seizures management; in particular, we appreciate with enthusiasm your efforts in underlining the importance of the EEG/aEEG as indispensable tools for seizure detection and believe that such inputs from the scientific community are needed to strengthen the possibility to have 24-7 skilled personnel available in all settings; furthermore, we believe it is fundamental, as you did, to underline the need to withdraw ASMs as soon as possible, ideally before discharge home (recommendation 3).
Unfortunately, we are concerned that more than one point needs to be further assessed.
With regard to recommendation 6, the authors state that a trial of pyridoxine should be provided as add-on to ASM in neonates with seizures unresponsive to second-line ASM without an identified etiology. Both our experience and various reports from the literature, though, support the possibility that patients with vitamin B6-dependent epilepsy might show an initial response to common ASMs to, later, relapse with status epilepticus. 1,2 We believe updated guidelines should look deeper into this matter and suggest considering a trial of pyridoxine in all patients with seizures of unknown etiology as first-line treatment, even before ASMs’ administration, especially in neonates presenting with clinical features or EEG characteristics suggestive of vitamin B6- dependent epilepsy.
Furthermore, the authors’ enthusiasm in providing 'high' consensus-based evidence that Phenobarbital should always be administered as first-line ASM seems a bit incautious (recommendation 1). Historically, Phenobarbital has been largely used and this unavoidably justifies the high amount of literature available on its efficacy rates and side-effects. On the other hand, though, the scientific community has pronounced for decades on Phenobarbital’s long-term detrimental side effects on neurodevelopment. We believe the fact that to date there’s no 'universally' more effective alternative does not justify Phenobarbital’s administration in all patients, as suggested in your paper, even in those with low seizure burden, electrical only seizures or short, self-limiting seizures. It would probably be more prudent to at least address this issue in the paper and to underline the need to consider Phenobarbital’s side effects and inform parents when the decision to prescribe it is made. Furthermore, in your paper Phenobarbital is suggested as first choice in all cases but its’ overall efficacy remains quite unsatisfactory; this, unavoidably raises doubts and questions in readers. What the scientific community and clinicians worldwide expect is not anymore the 'holy grail' of neonatal seizures treatment, a medication that works for all patients, regardless of the underlying etiology and seizures features but the possibility to point towards a targeted, precision medicine; in 2021 our group published a systematic review on ASMs’ efficacy in treating neonatal seizures from different etiologies and found, among 66 included studies, that the same ASM provided different efficacy rates in different population of newborns, that were grouped altogether not by the ASM used, as in your paper, but by the underlying etiology. 3 In particular, some data pointed towards an overall satisfactory efficacy of Lidocaine for stroke, SCB for genetic channelopathies – as you well underlined – Lev for HIE and found that Lev was safe both in term and preterm newborns and in terms of both short- and long-term outcomes, not only in patients with cardiac comorbidities as you underlined based mainly on expert opinions. Indeed, the small number of patients enrolled and the not-so rigid methodology of most studies included both in our and in your work probably does not allow to replace current protocols but we feel the need for updated guidelines, such as yours, to support the efforts made by clinicians worldwide to point towards a precision therapy and feel concerned that the paper you provided does not completely follow the trend that clinical practice has conquered in the last years and risks perpetuating management strategies that have been shown to be unsatisfactory and in some cases even harmful.
Raffaele Falsaperla & Bruna Scalia
- Kluger G, Blank R, Paul K, Paschke E, Jansen E, Jakobs C, Wörle H, Plecko B. Pyridoxine-dependent epilepsy: normal outcome in a patient with late diagnosis after prolonged status epilepticus causing cortical blindness. Neuropediatrics. 2008 Oct;39(5):276-9. doi: 10.1055/s-0029-1202833. Epub 2009 Mar 17. PMID: 19294602.
- Lin J, Lin K, Masruha MR, Vilanova LC. Pyridoxine-dependent epilepsy initially responsive to phenobarbital. Arq Neuropsiquiatr. 2007 Dec;65(4A):1026-9. doi: 10.1590/s0004-282x2007000600023. PMID: 18094870.
- Falsaperla R, Scalia B, Giugno A, Pavone P, Motta M, Caccamo M, Ruggieri M. Treating the symptom or treating the disease in neonatal seizures: a systematic review of the literature. Ital J Pediatr. 2021 Apr 7;47(1):85. doi: 10.1186/s13052-021-01027-2. PMID: 33827647; PMCID: PMC8028713.
4 January 2023
The authors are to be congratulated for going forward on the path of preventing overtreatment by advocating discontinuation of antiseizure medication (ASM) before discharge home in infants who showed cessation of acute symptomatic seizures ( recommendation 3 ). However, their enthusiasm for phenobarbital as a first-line ASM ( recommendation 1 ) and treatment of all neonatal seizures including electrographic-only seizures ( recommendation 5 ) cause concern.
Phenobarbital has been shown to display considerable neurotoxicity in numerous animal models both in rodents and in monkeys. In newborn mice, a dosage of phenobarbital that reduces seizure burden in hypoxia-induced seizures (25 mg/kg) lacks any positive effect on histopathological injury, behavioral abnormality, or impaired memory function. 1 In neonatal rats with status epilepticus, phenobarbital and midazolam even worsen neuronal injury. 2 In neonatal rhesus monkeys, extensive degeneration of neurons and oligodendrocytes has been observed in various brain regions following the administration of phenobarbital and midazolam 3 that is not ameliorated by hypothermia. 4 A retrospective study of 280 human infants who were given ASM for neonatal seizures, a cumulative exposure of 100 mg phenobarbital/kg was associated with 8-9 points decrease of the Bayley Scales of Infant Development scores at 2 years of age, while the probability of cerebral palsy increased more than 2-fold. 5 Furthermore, low-dose phenobarbital (5 mg/kg/d) given prophylactically to infants and young children was associated with a decline of the intelligence quotient by 5-8 points in a large placebo-controlled randomized controlled trial. 6 Thus, phenobarbital may be the most efficacious ASM but its administration exerts considerable damage to the developing brain. Possibly, this is the price to pay for infants with heavy seizure burden, but for infants with short, self-limiting seizures, the remedy may be more dangerous than the disease.
Seizures by themselves have not been found to be linked to impaired neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. 7,8 As overall seizure burden is a marker of the extent of brain damage, the association between high seizure burden and neurodevelopmental impairment is trivial. Prolonged seizures appear to herald rather than cause poor outcome, at least in the setting of hypoxic-ischemic encephalopathy. Data from two prospective randomized controlled trials involving 33 infants 9 and 172 infants, 10 respectively, failed to suggest that treatment of subclinical seizures improves outcome in newborns with acute symptomatic seizures, contravening recommendation 5. Efforts aimed at stopping all seizures as rapidly and effectively as possible may do more harm than good.
Christoph Bührer
- Quinlan SMM, Rodriguez-Alvarez N, Molloy EJ, Madden SF, Boylan GB, Henshall DC, Jimenez-Mateos EM. Complex spectrum of phenobarbital effects in a mouse model of neonatal hypoxia-induced seizures. Sci Rep 2018;8(1):9986. doi: 10.1038/s415980‑18‑28044-2.
- Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Phenobarbital and midazolam increase neonatal seizure-associated neuronal injury. Ann Neurol 2017;82(1):115-120. doi: 10.1002/ana.24967.
- Noguchi KK, Fuhler NA, Wang SH, Capuano S 3rd, Brunner KR, Larson S, Crosno K, Simmons HA, Mejia AF, Martin LD, Dissen GA, Brambrink A, Ikonomidou C. Brain pathology caused in the neonatal macaque by short and prolonged exposures to anticonvulsant drugs. Neurobiol Dis 2021;149:105245. doi:10.1016/j.nbd.2020.105245.
- Ikonomidou C, Wang SH, Fuhler NA, Larson S, Capuano S 3rd, Brunner KR, Crosno K, Simmons HA, Mejia AF, Noguchi KK. Mild hypothermia fails to protect infant macaques from brain injury caused by prolonged exposure to antiseizure drugs. Neurobiol Dis 2022;171:105814. doi: 10.1016/j.nbd.2022.105814.
- Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol 2013;33(11):841-6. doi: 10.1038/jp.2013.116.
- Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med 1990;322(6):364‑9. doi: 10.1056/NEJM199002083220604.
- Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, Boylan GB. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol 2016;58(12):1242‑1248. doi: 10.1111/dmcn.13215.
- Basti C, Maranella E, Cimini N, Catalucci A, Ciccarelli S, Del Torto M, Di Luca L, Di Natale C, Mareri A, Nardi V, Pannone V, Di Fabio S. Seizure burden and neurodevelopmental outcome in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: A single center observational study. Seizure 2020;83:154-159. doi: 10.1016/j.seizure.2020.10.021.
- van Rooij LG, Toet MC, van Huffelen AC, Groenendaal F, Laan W, Zecic A, de Haan T, van Straaten IL, Vrancken S, van Wezel G, van der Sluijs J, Ter Horst H, Gavilanes D, Laroche S, Naulaers G, de Vries LS. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125(2):e358-66. doi: 10.1542/peds.2009-0136.
- Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, Francis-Pester S, de Waal K, Cheong JYL, Olischar M, Badawi N, Wong FY, Osborn DA, Rajadurai VS, Dargaville PA, Headley B, Wright I, Colditz PB; Newborn Electrographic Seizure Trial Investigators. Effect of treatment of clinical seizures vs electrographic seizures in full-term and near-term neonates: a randomized clinical trial. JAMA Netw Open 2021;4(12):e2139604. doi: 10.1001/jamanetworkopen.2021.39604.
25 December 2022
Nice work from the ILAE task Force.
Dr Sameer Zuberi visited Aga Khan University Hospital a month ago. Since then, we have started collecting data regarding recurrence of seizures in HIE noenatal age groups.
Although the majority of RCTs mentioned in this report are based on clinical seizures, electroclinical and specifically electrographic seizures are still topics of concern in a major part of world.
I question that in developing countries the majority of HIE neonates are Grade III, and suddenly withdrawing ASMs may result in recurrence.
Data from developing countries are still lacking. Early discontinuation of ASMs should be individualized in certain conditions.
Dr. Farhan Ali
20 December 2022
Thank you for this excellent work.
Dear Madam or Sir,
The draft mentions sodium channel blockers as a treatment options, but lists only phenytoin and carbamazepine as possible choices. Are there any reasons not to mention oxcarbazepine (which is, at least in Germany, nowadays much more widely used than carbamazepine) and lacosamide?
Sincerely yours,
Moritz Tacke
This is a valuable effort. The sentence on page 10 of the introduction [Electroencephalography (EEG or aEEG) is required for seizure diagnosis since most seizures in neonates have no clinical manifestations (electrographic-only)...] is very important.
EEG is not yet provided in many NICUs at point of diagnosis. I suggest more emphasis on need for EEG for certain diagnosis and to determine AED treatment duration in neonatal seizures.
David Rowitch
19 December 2022
I have great worries about consensus based guidelines as they risk perpetuating treatments or management protocols that may ultimately be shown to be harmful or ineffective. A non-evidenced based guideline risks imposing a harmful or dangerous treatment on ALL infants; this was the case for example with routine use of 100% oxygen for newborn resuscitation, a practice that was enshrined in consensus/expert opinion based guidelines which harmed countless babies. However, I recognise that all too often, evidence to support clinical practice is insufficient and also that consistency of practice within a centre has some benefits. Therefore when evidence is insufficient for an evidence-based guideline I strongly recommend that the authors include the following statements in their document: 1) the treatment uncertainties should be explained to parents; 2) it should be made explicit to neonatal centres that the existence of a guideline does not mean they cannot take part in a randomised controlled trial to address the treatment uncertainty; 3) all neonatal centres should be strongly advised to join high quality randomised studies to resolve such uncertainties; and 4) it should be explained to parents that in the face of uncertainty, randomisation is the most fair and ethical approach as it is the only way to provide every baby with an equal chance of receiving the (unknown) best treatment.
I appreciate the great effort from the authors to provide us with this recommendations. I would like to ask, if it is possible to address the following concerns:
- Treatment as a rescue-option in newborns with CNS insult and seizure-like activity that are being transported or waiting for transport to a specialized NICU center (an agent that will not interfere with the exam/EEG or with a short half-life).
- In acute metabolic disturbances and seizures (glycemia or electrolytes) ASM should be started while the metabolic issue is being corrected?
- Lidocaine should not be used if phenytoin was previously used.
- Treatment options/recommendations in refractory-seizures or neonatal status epilepticus after using 1st and 2nd line agents.
Oscar DeLaGarza
17 December 2022
Thank you. The methodology and scope for the recommendations is excellent.
I find some of the recommendations confusing, and I think need to be clearer particularly if aimed at non-neurology neonatal professionals in an acute/emergency setting.
Recommendation 1 implies all should have phenobarbital, but then outlines an exception. This is confusing.
Where citing sodium channel ASMs in a recommendation would be good to always list the examples as many will not think of ASMs in this way. In fact I would simply list the suitable drugs to consider rather than refer to them by this category.
For the first line ASM it implies solely family history of channelopathy is factor, whereas for second line, solely electro-clinical features of channelopathy in index case are important. Is this the intention?
I would like to be guided within the guidance as to the definition of channelopathy and what should make me consider a channelopathy in practice, given is a key determinant of different ASMs. Many will not be familiar with this channelopathy term.
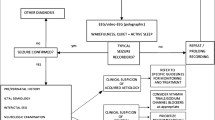
The flow diagram arrows are confusing and is hard to follow the logic. The vertical arrows imply next step or decision. The horizontal arrows imply caveats rather than a next step. I think a decision tree approach might make this much clearer so that you can follow through ASM choices based on presence or absence of the key caveats.
Colin Dunkley
15 December 2022
Dear ILAE Task Force,
I have gone through the consensus based recommendations for treatment of seizures in the neonates. Phenobarbitone is known to have negative neurodevelopmental effect especially on the developing brain, recommending it as first line treatment for seizure control needs further clarification in terms of duration and dosage and what should be our end point, clinical seizure control or electrographic improvement. Also stopping anti-seizure medications before discharge irrespective of neurological and electrographic changes needs further research and recommendations. The role neuroimaging can play an important role. The loading and maintenance dosage of levetiracetam also needs clarification.
Dr Rahul Sinha Pediatric Neurologist Command Hospital Chandimandir Panchkula Haryana India
The manuscript looks excellent and the recommendations are reasonable.
Dr Shripada Rao
13 December 2022
Role of EEG and aEEG especially in LMIC settings for management of Neonatal seizures should be clarified.
Rohit Anand
Indication for midazolam infusion, dose and escalation and tapering protocol.
Mayank Priyadarshi
Refractory seizures can be the first choice of treatment with oxcarbamazepine in channelopathy.
Rafid Abdulkadhim Atshan Alneghimsh
I am concerned that the first recommendation - that in neonates with seizures requiring antiseizure medication (ASM), phenobarbital should be the first-line ASM, does not highlight the fact that this is based only on data regarding seizure cessation efficacy, and we do not know if it will ultimately improve neurodevelopmental outcome- the ultimate endpoint we care about.
Although phenobarbital has proven highly effective at achieving seizure cessation, its net effect on neurodevelopment could still be negative, due to its neurotoxic effects.
Rat and primate neonatal studies have shown markedly accelerated neuronal apoptosis following a single standard dose of phenobarbital, (and most other antiseizure medications, phenytoin, midazolam, valproate). Other adverse effects caused by phenobarbital in the animal model include interference with cell proliferation and migration, axonal arborization, synaptogenesis and synaptic plasticity. 1-6
Fetal exposure has been shown to reduce cognitive outcomes in 2 case control studies. The magnitude of the effect was about 0.5 standard deviations. 7
Young children randomized to PHB for febrile seizure had persistently lower IQ test results after medications tapered. 8-11
This month at Neonatal Hot Topics conference, 7 December 2022, Washington DC Professor Rod Hunt, presented additional, yet unpublished, data from his recent study on the impact of aEEG monitoring on neurodevelopmental outcome. 12 This showed a dose dependent adverse impact of phenobarbital on IQ, a 3pt IQ drop for every 10 mg/kg phenobarbital received. On average in his cohort, neonates received 38 mg/kg of PHB. This association held after correction for the underlying severity of injury and for seizure burden.
Additionally, the recommendation does not not define when seizures require antiseizure medication.
A summary of the evidence that seizures are harmful per se and the remaining gaps in that evidence should be included in this guideline.
It should be highlighted that our evidence that seizures are harmful is most convincing for prolonged seizures in both animal and human studies. 13-16 In patients with low seizure burden, neurodevelopmental outcome is frequently normal. Boylan et al 17 demonstrate that a cut off maximal seizure burden of 13 minutes/ hour or total seizure burden of 40 minutes separate patients with abnormal outcomes from those with normal outcomes. In NEOLEV2 more than half the cohort had seizure burdens less than this.
It remains unclear in patients with low seizure burden, whether the risk of neurologic injury from the seizures is greater than the risks of neurotoxic injury from phenobarbital.
Unedited, the draft recommendation will lead to widespread increased use of phenobarbital for all neonatal seizures, potentially doing more harm than good.
Yours sincerely,
Dr Cynthia Sharpe NEOLEV2 Investigator
- Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353(2-3):191-206.
- Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007;323(1):165-73.
- Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012;340(3):558-66.
- Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug-induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52(12):e207-11.
- Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72(3):363-72.
- Kaindl AM, Asimiadou S, Manthey D, Hagen MV, Turski L, Ikonomidou C. Antiepileptic drugs and the developing brain. Cell Mol Life Sci. 2006;63(4):399-413.
- Reinisch JM, Sanders SA, Mortensen EL, Rubin DB. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA. 1995;274(19):1518-25.
- Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322(6):364-9.
- Sulzbacher S, Farwell JR, Temkin N, Lu AS, Hirtz DG. Late cognitive effects of early treatment with phenobarbital. Clin Pediatr (Phila). 1999;38(7):387-94.
- Calandre EP, Dominguez-Granados R, Gomez-Rubio M, Molina-Font JA. Cognitive effects of long-term treatment with phenobarbital and valproic acid in school children. Acta Neurol Scand. 1990;81(6):504-6.
- Camfield CS, Chaplin S, Doyle AB, Shapiro SH, Cummings C, Camfield PR. Side effects of phenobarbital in toddlers; behavioral and cognitive aspects. J Pediatr. 1979;95(3):361-5.
- Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, et al. Effect of Treatment of Clinical Seizures vs Electrographic Seizures in Full-Term and Near-Term Neonates: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(12):e2139604.
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155(3):318-23.
- Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Dev Neurosci. 2002;24(5):367-81.
- Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, et al. Treating EEG Seizures in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. Pediatrics. 2015;136(5):e1302-9.
- Zhou KQ, McDouall A, Drury PP, Lear CA, Cho KHT, Bennet L, et al. Treating Seizures after c-Ischemic Encephalopathy-Current Controversies and Future Directions. Int J Mol Sci. 2021;22(13).
- Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58(12):1242-8.
Thank you for the interesting study and is very needed in practice.
My comments:
- Page 10 needs to be clarified: 'Only one study with phenobarbital and levetiracetam used standardized adverse events tables and reported that there was a trend towards hypotension being more common with phenobarbital (n=17%) compared to levetiracetam (n=5%)'.
- Why is the Neonatal hypoglycemia not mentioned or included in the draft discussion?
Murad Al-Nusaif
12 December 2022
Clear guidelines need for the management of convulsive non convulsive status epileptics in neonates. Use of anesthetic agents, intranasal and rectal use of ASM can be mentioned.
Prof.Dr. M.A.Aleem
Subscribe to the ILAE Newsletter
To subscribe, please click on the button below.
Please send me information about ILAE activities and other information of interest to the epilepsy community
Clinical manifestations of neonatal seizures
Affiliations.
- 1 Department of Neurology, Stanford University School of Medicine, Palo Alto, CA, USA.
- 2 Departments of Neurology and Pediatrics, Stanford University School of Medicine, Palo Alto, CA, USA.
- PMID: 33599034
- DOI: 10.1111/ped.14654
Neonatal seizures present a unique diagnostic challenge with clinical manifestations often subtle or absent to the bedside observer. Seizures can be overdiagnosed in newborns with unusual paroxysmal movements and underdiagnosed in newborns without clinical signs of seizures. Electroclinical "uncoupling" also adds to the diagnostic challenge. Reliable diagnosis requires additional tools; continuous electroencephalogram (EEG) monitoring is the gold standard for diagnosis of neonatal seizures. Certain high-risk neonatal populations with known brain injury, such as stroke or hypoxic-ischemic encephalopathy, are most likely to benefit from continuous EEG. Studies have shown that risk stratification for continuous EEG has positive impact on care, including rapid and accurate diagnosis and treatment of neonatal seizures, which leads to reduced use of antiseizure medicines and length of hospital stay. This review describes common clinical manifestations of neonatal seizures, and clinical situations in which EEG monitoring to screen for seizures should be considered.
Keywords: electroencephalogram; epilepsy; neonatal; seizure; subclinical.
© 2021 Japan Pediatric Society.
Publication types
- Electroencephalography
- Hypoxia-Ischemia, Brain* / diagnosis
- Hypoxia-Ischemia, Brain* / therapy
- Infant, Newborn
- Infant, Newborn, Diseases*
- Seizures / diagnosis
- Seizures / etiology
- Case Report
- Open access
- Published: 16 May 2024
New and old lessons from a devastating case of neonatal E coli meningitis
- Tawny Saleh 1 ,
- Edwin Kamau 2 , 3 &
- Jennifer A. Rathe 1
BMC Pediatrics volume 24 , Article number: 339 ( 2024 ) Cite this article
67 Accesses
1 Altmetric
Metrics details
Neonatal Escherichia coli ( E coli ) meningitis results in significant morbidity and mortality. We present a case of a premature infant with extensive central nervous system (CNS) injury from recurrent E coli infection and the non-traditional methods necessary to identify and clear the infection.
Case Presentation
The infant was transferred to our institution’s pediatric intensive care unit (PICU) after recurrence of E coli CNS infection requiring neurosurgical intervention. He had been treated for early onset sepsis (EOS) with ampicillin and gentamicin for 10 days followed by rapid development of ampicillin-resistant E coli septic shock and meningitis after discontinuation of antibiotics. Sterility of the CNS was not confirmed at the end of 21 days of cefepime therapy and was subsequently followed by recurrent ampicillin-resistant E coli septic shock and CNS infection. Despite 6 weeks of appropriate therapy with sterility of CSF by traditional methods, he suffered from intractable seizures with worsening hydrocephalus. Transferred to our institution, he underwent endoscopic 3rd ventriculostomy with cyst fenestration revealing purulent fluid and significant pleocytosis. An additional 3 weeks of systemic and intraventricular antibiotics with cefepime and tobramycin were given but a significant CNS neutrophil-predominant pleocytosis persisted (average of \(\sim\) 21,000 cells/mm 3 ). Repeated gram stains, cultures, polymerase chain reaction (PCR) testing, and metagenomic next generation sequencing (NGS) testing of CSF were negative for pathogens but acridine orange stain (AO) revealed numerous intact rod-shaped bacteria. After the addition of ciprofloxacin, sterility and resolution of CSF pleocytosis was finally achieved.
Neonatal E coli meningitis is a well-known entity but unlike other bacterial infections, it has not proven amenable to shorter, more narrow-spectrum antibiotic courses or limiting invasive procedures such as lumbar punctures. Further, microbiologic techniques to determine CSF sterility suffer from poorly understood limitations leading to premature discontinuation of antibiotics risking further neurologic damage in vulnerable hosts.
Peer Review reports
Introduction
Neonatal E coli sepsis results in high morbidity and mortality due to complex host and environmental factors, but early identification and appropriate treatment limits progression to meningitis and/or significant parenchymal damage (encephalitis). Recurrence or relapse of CNS infection is a well-recognized entity of neonatal E coli meningitis with increased risk associated with the K1 virulence factor and signs of complicated infection (abnormalities on brain imaging: ventriculitis, abscess or clinical signs of CNS infection: seizures or altered mental status). Therefore, repeat LP to document sterility prior to the end of antibiotics has been a longstanding recommendation [ 1 , 2 ].
Neonatal EOS presents within the first 72 h of life [ 3 ]. Late onset sepsis (LOS) presents in the first 4 to 28 days of life. EOS pathogens colonize the infant as they pass through the vaginal canal. Risk of EOS increases with prolonged rupture of membranes and maternal fever or infection. Conversely, LOS pathogens are obtained from the neonates’ environment with significantly increased risk of severe infection for very low birthweight premature infants. Group B Streptococcal (GBS) and E coli are the most common pathogens responsible for EOS, with E coli rates rising, while a variety of gram positive and negative species are responsible for LOS without a particular pathogen predominance [ 3 , 4 ]. Empiric therapy for EOS is generally ampicillin and gentamicin [ 3 ]. If meningitis is a concern, cefotaxime or cefepime are recommended with ampicillin; gentamicin has limited CNS penetration due to its large, hydrophilic nature. Consequently, aminoglycosides can be used as an adjunct but are not reliable as the primary treatment for meningitis [ 3 , 5 ]. Recommendations for empiric antibiotic therapy for LOS now include a broad-spectrum 3rd or 4th generation cephalosporin +/- vancomycin due to the wide range of causative bacterial organisms and concerns for highly pathogenic gram-negative organisms [ 3 , 6 ]. However, recommendations for empiric therapy for EOS continue to be ampicillin and gentamicin.
Case presentation
A 3-month-old male born prematurely at 27 weeks gestation was transferred to our institution with recurrent E coli CNS infection. He was born via vaginal delivery after premature and prolonged rupture of membranes, with unremarkable prenatal labs. Within 48 h, the neonate became unstable with concern for EOS but blood cultures were negative; he was treated for culture negative sepsis with 10 days of ampicillin and gentamicin.
Within a week after completion of antibiotics, he went into septic shock with ampicillin-resistant E coli bacteremia (Fig. 1 A) complicated by intractable seizures, grade 3–4 intraventricular hemorrhage (IVH), and respiratory failure requiring intubation. Lumbar puncture (LP) was not performed secondary to patient instability. Cefepime was administered for 21 days for presumed E coli meningitis with some clinical improvement but seizures continued. Brain magnetic resonance imaging (MRI) and serial head ultrasounds were significant for global encephalomalacia and worsening hemorrhage with ventriculomegaly. No LP was performed at the end of therapy.

Cerebral Spinal Fluid (CSF), Serum, and Clinical Markers of Inflammation. A : Culture and susceptibility testing results from blood and CSF samples from the outside hospitals from the first and recurrent episode of E coli infection. Culture and testing results from UCLA after transfer. B : This graph demonstrates the pleocytosis measured in the CSF over the course of antibiotic treatment during the patient’s admission. Black bars demonstrate the levels of WBCs detected on sampling and the grey bars represent the proportion of those WBCs consisting of neutrophils. The start of ciprofloxacin is shown with a text box and arrow at the top of the graph. Neutrophil predominant CSF pleocytosis resolved after the introduction of ciprofloxacin. C : Serum and core temperature measurements of the patient at the time frame concerning for continued infection despite negative CSF gram stain, cultures, bacterial 16 S PCR testing, and unbiased pathogen NGS testing. All testing was negative for signs of systemic inflammation
Two weeks after completion of antibiotics, the patient again went into septic shock with ampicillin-resistant E coli bacteremia, meningitis, and ventriculitis. He was treated with cefepime and gentamicin. He was diagnosed via a positive blood culture and positive CSF cultures (same sensitivities, Fig. 1 A). Two subsequent CSF samples were negative by culture and stain, but were positive via a meningitis nucleic acid PCR panel for E coli after 5 and 6 weeks of therapy. Inflammatory markers and clinical signs of sepsis normalized by the 6th week of therapy with the exception of seizures. An MRI revealed multiple heterogenous cystic structures concerning for hydrocephalus as well as loculated areas consistent with infection and/or hemorrhage. After 8 weeks of antibiotics, he was experiencing increasing hydrocephalus and seizures; he was transferred to our institution for neurosurgical intervention.
Neurosurgery performed an endoscopic 3rd ventriculostomy with cyst fenestration and reservoir placement. The surgeons encountered diffuse, thick, purulent material in the accessible intra-parenchymal and -ventricular cysts. CSF studies demonstrated significant pleocytosis with a high percentage of neutrophils, but gram stain and cultures were negative. Cefepime was continued with the addition of systemic and intraventricular (IT) tobramycin. Despite an aggressive antibiotic regimen for 3 weeks (+ 8 weeks prior to transfer), serial CSF sampling continued to demonstrate significant neutrophil-predominant pleocytosis (Fig. 1 B). However, gram stains and cultures of the CSF remained negative. Further, metagenomic NGS tests performed at University of California San Francisco and 16 S bacterial long-range PCR performed at University of Washington did not detect any pathogenic genomic material. Systemic inflammatory markers were normal and clinical symptoms of infection were absent (Fig. 1 C).
It was unclear if the pleocytosis was due to inflammation from significant brain damage or continued E coli infection. Brain MRI demonstrated the presence of multiple, non-communicating cysts (Fig. 2 A). We hypothesized that cultures were negative not because the CNS was sterile but rather that there were sequestered areas of infection in the non-communicating brain cysts and the instillation of tobramycin may be affecting culture results. The clinical microbiology lab recommended an alternative stain with AO to help visualize any organisms that might be present. Assessment of several CSF samples with AO found multiple, rod-shaped, intact bacterial organisms (Fig. 2 B). None of the associated gram stains, cultures, PCR, nor NGS testing of these samples detected microorganisms. With concern for possible development of antibiotic resistance, a new secondary infection, and improved CNS penetration, we discontinued tobramycin and started ciprofloxacin with continuation of cefepime. Previous cultures demonstrated E coli sensitivity to ciprofloxacin (Fig. 1 A). Four weeks after the addition of ciprofloxacin, the CSF pleocytosis resolved and AO stains were negative. A ventricular peritoneal shunt was placed, and he has remained infection free for > 2 years.

MRI Brain Imaging and Acridine Orange (AO) Stains of CSF Samples Prior to Ciprofloxacin. A : MRI brain imaging of the patient axial and coronal views. Multiple discontinuous fluid filled cysts are noted throughout the brain with minimal remaining brain parenchyma. B : Sections of representative microscopic slides of patient’s CSF AO stains of two separate CSF collections obtained 1–2 days apart. Black arrows locate the rod-shaped intact organisms. The connected rods are consistent with images of actively dividing organisms such as E. coli
Given his recurrent infection and significant CNS damage, he was evaluated for a primary immune deficiency by Allergy and Immunology; the work up did not identify abnormalities on lymphocyte panel, quantitative immunoglobulins, CH50 testing, TLR function, etc. He is now > 2 years old. He has severe global developmental delays: he is blind, he speaks no words, he has mild hearing loss, he cannot support his head, roll over, crawl, nor use his limbs in purposeful movements, and lastly, he startles to sounds but does not respond to his name nor commands.
Discussion and conclusions
Bacterial meningitis in neonates and infants is a rare occurrence; incidence is estimated at \(\sim\) 1–2% for full term infants and 4–6% for preterm/very low birth-weight neonates [ 3 , 4 ]. However, EOS and LOS are far more common clinical presentations, especially in preterm infants, with the risk of progression to meningitis. As appreciated in this case, meningitis in these populations have a high likelihood of resulting in some amount of neurodevelopmental injuries with a range of severity [ 7 , 8 ]. Our patient experienced EOS and LOS in rapid succession suggesting that both instances resulted from infection with the same ampicillin-resistant E coli. In other words, his EOS was treated with gentamicin alone and may have predisposed to his LOS either because of partially treated E coli CNS infection or as the result of a new infection from translocation of colonizing E coli in his leaky, premature gastrointestinal tract. The high rates of E coli resistance increasing globally raises concerns about the safety of continued empiric use of ampicillin and gentamicin in EOS [ 9 ]. However, given the large number needed to treat secondary to the rare incidence of EOS progressing to bacterial meningitis, it is not clear that the subsequent trade off of increased antibiotic resistance is worth the small number of patients that would benefit from decreased morbidity and mortality. At this time, many argue the change is not warranted and that individual risk assessment and local antibiotic resistance rates are key in the decision to broaden antibiotics in EOS [ 3 , 9 ].
Neonatal E coli meningitis comes with a risk of recurrence, therefore, a repeat LP to confirm sterilization is recommended. Therapy should be extended if sterilization is not yet achieved at 21 days [ 10 , 11 ]. In the case presented here, sterilization was not assessed at the initial diagnosis of E coli meningitis nor at the end of therapy when the patient had stabilized. There are times when the decision is made to forgo proof of sterilization but this should only be considered in patients with uncomplicated infection and rapid clinical response to therapy; however, even that scenario is not a guarantee of bacterial clearance when it comes to neonatal E coli meningitis [ 10 ]. Unfortunately, our patient had intractable seizures and severe IVH concerning for ongoing infection. A repeat LP may have demonstrated evidence of continued infection resulting in prolongation of antibiotic therapy. As noted by Vissing et al. , neonatal E coli meningitis may be one of the clinical scenarios where pushing for shorter antibiotic courses and limiting invasive procedures is not providing benefit but increasing the chance for significant morbidity and mortality [ 10 ].
The recurrence of this patient’s E coli CNS infection resulted in global parenchymal infection with the formation of non-communicating cystic structures. Serially CSF sampling demonstrated notable pleocytosis and purulent fluid but bacterial gram stain and culture failed to detect organisms. PCR and NGS also failed to identify bacterial pathogens despite visual evidence on acridine orange stain of numerous, intact, rod-shaped bacteria. Due to extensive systemic and IV antibiotics, it is not surprising that the cultures were no growth. However, it was unexpected that serial CSF Gram stains, NGS, and PCR did not detect bacteria. No test is 100% sensitive and specific and all are subject to specific limitations. AO actually has a higher sensitivity compared to Gram stain but is not able to differentiate between gram-positive and -negative organisms, and is not used in the regular clinical microbiology workflow for CSF samples [ 12 , 13 ]. However, unknown to many physicians, the newer NGS and PCR diagnostic techniques are not reliable in detecting pathogens when using samples with high numbers of inhibitory materials and cells present, i.e. pleocytosis [ 14 , 15 ].
The addition of ciprofloxacin resulted in resolution of the CNS pleocytosis and bacterial rods on AO stain. Adjunct ciprofloxacin for neonatal E coli meningitis has been proposed for many years by some experts in France. However, there is not clear data demonstrating ciprofloxacin superiority to aminoglycosides or cephalosporins [ 16 ]. But, in this particular patient it may have provided improved bactericidal killing secondary to higher sensitivity or better CNS penetration compared to our initial regimen [ 17 ]. As the bacteria detected on AO did not grow in culture, we could not assess for the development of resistance after prolonged treatment with cefepime and aminoglycosides.
The infant presented here suffered severe consequences from recurrent, neonatal E coli CNS infection resulting in destruction of most of his brain parenchyma. Uncontrollable risk factors contributing to his poor outcome include his prematurity and infection with an E coli K1 strain. Premature infants even without CNS infection have a greater risk of long-term consequences [ 18 ]. K1 is a virulence factor that increases the ability of the bacteria to cross the blood brain barrier. However, initial treatment with cefepime rather than ampicillin and a repeat LP prior to stopping antibiotics may have altered the ultimate outcome of his infection. This case also illustrates the importance of understanding the limitations of classic sterilization testing as well as newer technologies. Identification of bacteria on AO stain stopped us from discontinuation of antibiotics prior to attaining true sterilization. The addition of ciprofloxacin allowed us to achieve sterilization in this patient though it is not entirely clear as to why it was successful. Key lessons from this patient include: (1) the use of AO stain when there are signs of continued infection despite negative GS and culture (2), always plan to repeat LPs to document sterilization in neonatal E coli meningitis unless the patient is clinically unstable, and (3) if patients are not clearing their CSF or have continued signs of clinical infection despite cefepime +/- an aminoglycoside, adjunct therapy with ciprofloxacin should be considered.
Data availability
The data presented in this report are available upon request from the corresponding author.
Abbreviations
Escherichia coli
Central nervous system
Polymerase chain reaction
Pediatric intensive care unit
Early onset sepsis
Late onset sepsis
Cerebral spinal fluid
Intraventricular hemorrhage
Lumbar puncture
Intraventricular
Magnetic resonance imaging
Next-Generation sequencing
Acridine orange
Group B Streptococcus
Vissing NH, Mønster MB, Nordly S, Dayani GK, Heedegaard SS, Knudsen JD et al. Relapse of neonatal Escherichia coli Meningitis: did we Miss something at First? Child (Basel). 2021;8(2).
Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. Feigin and Cherry’s textbook of pediatric infectious diseases. Philadelphia, PA: Elsevier; 2019.
Google Scholar
Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-Onset neonatal Sepsis 2015 to 2017, the rise of Escherichia coli, and the need for Novel Prevention Strategies. JAMA Pediatr. 2020;174(7):e200593.
Article PubMed PubMed Central Google Scholar
Gowda H, Norton R, White A, Kandasamy Y. Late-onset neonatal Sepsis-A 10-year review from North Queensland, Australia. Pediatr Infect Dis J. 2017;36(9):883–8.
Article PubMed Google Scholar
Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013;15(2):93–117.
Ellis JM, Rivera L, Reyes G, Castillo F, Marte P, Tejada M, et al. Cefepime cerebrospinal fluid concentrations in neonatal bacterial meningitis. Ann Pharmacother. 2007;41(5):900–1.
Flannery DD, Edwards EM, Coggins SA, Horbar JD, Puopolo KM. Late-Onset Sepsis among very Preterm infants. Pediatrics. 2022;150(6).
Thomas R, Bijlsma MW, Gonçalves BP, Nakwa FL, Velaphi S, Heath PT. Long-term impact of serious neonatal bacterial infections on neurodevelopment. Clin Microbiol Infect. 2023.
Liu Y, Zhu M, Fu X, Cai J, Chen S, Lin Y, et al. Causing neonatal Meningitis during 2001–2020: a study in Eastern China. Int J Gen Med. 2021;14:3007–16.
Vissing NH, Mønster MB, Nordly S, Dayani GK, Heedegaard SS, Knudsen JD et al. Relapse Neonatal Child (Basel). 2021;8(2).
Mulder CJ, van Alphen L, Zanen HC. Neonatal meningitis caused by Escherichia coli in the Netherlands. J Infect Dis. 1984;150(6):935–40.
Article CAS PubMed Google Scholar
Sharma S, Acharya J, Banjara MR, Ghimire P, Singh A. Comparison of acridine orange fluorescent microscopy and gram stain light microscopy for the rapid detection of bacteria in cerebrospinal fluid. BMC Res Notes. 2020;13(1):29.
Article CAS PubMed PubMed Central Google Scholar
Lauer BA, Reller LB, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J Clin Microbiol. 1981;14(2):201–5.
Oechslin CP, Lenz N, Liechti N, Ryter S, Agyeman P, Bruggmann R, et al. Limited Correlation of Shotgun Metagenomics Following Host Depletion and Routine Diagnostics for Viruses and Bacteria in low concentrated surrogate and clinical samples. Front Cell Infect Microbiol. 2018;8:375.
Hasan MR, Rawat A, Tang P, Jithesh PV, Thomas E, Tan R, et al. Depletion of human DNA in spiked clinical specimens for improvement of sensitivity of Pathogen Detection by Next-Generation sequencing. J Clin Microbiol. 2016;54(4):919–27.
Sáez-Llorens X, Castaño E, García R, Báez C, Pérez M, Tejeira F, et al. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob Agents Chemother. 1995;39(4):937–40.
Lipman J, Allworth A, Wallis SC. Cerebrospinal fluid penetration of high doses of intravenous ciprofloxacin in meningitis. Clin Infect Dis. 2000;31(5):1131–3.
Morniroli D, Tiraferri V, Maiocco G, De Rose DU, Cresi F, Coscia A, et al. Beyond survival: the lasting effects of premature birth. Front Pediatr. 2023;11:1213243.
Download references
Acknowledgements
Not applicable.
No grants were used to fund the preparation of this manuscript.
Author information
Authors and affiliations.
Department of Pediatrics, Division of Infectious Diseases, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Tawny Saleh & Jennifer A. Rathe
Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Edwin Kamau
Present address: Department of Pathology and Area Laboratory Services, Tripler Army Medical Center, Honolulu, HI, USA
You can also search for this author in PubMed Google Scholar
Contributions
TS, EK, and JR conceptualized the manuscript. TS reviewed literature and wrote the original draft. EK, TS, and JR edited the manuscript. TS and JR provided pediatrics infectious disease care and clinical decision making. EK created and provided images of the acridine orange stains in the microbiology lab. JR supervised the entire process of from development to submission of the manuscript.
Corresponding author
Correspondence to Jennifer A. Rathe .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Verbal and written informed consent were obtained from the parents of the patient for publication of the text and images of this case report. A copy of the written consent is available upon request.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Saleh, T., Kamau, E. & Rathe, J.A. New and old lessons from a devastating case of neonatal E coli meningitis. BMC Pediatr 24 , 339 (2024). https://doi.org/10.1186/s12887-024-04787-y
Download citation
Received : 30 June 2023
Accepted : 23 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12887-024-04787-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neonatal E coli meningitis
- Acridine orange stain
- Neonatal sepsis
- CSF sterility
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier Sponsored Documents

Neonatal seizures: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data
Serena pellegrin.
a Clinical Neuroscience, UCL-Institute of Child Health, London, UK
b Department of Child Neuropsychiatry, University of Verona, Verona, Italy
Flor M. Munoz
c Baylor College of Medicine, Department of Pediatrics, Houston, TX, USA

Michael Padula
d The Children’s Hospital of Philadelphia, PA, USA
Paul T. Heath
e Vaccine Institute, St Georges University of London, London, UK
f Syneos Health, Safety & Pharmacovigilance, Raleigh, NC, USA
g Department of Pediatrics, Dalhousie University, Halifax, NS, Canada
Jo Wilmshurst
h Department of Paediatric Neurology, Red Cross War Memorial Children’s Hospital, Neuroscience Institute, University of Cape Town, South Africa
Max Wiznitzer
i Rainbow Babies & Children’s Hospital, Cleveland, OH, USA
Manoja Kumar Das
j The INCLEN Trust International, New Delhi, India
Cecil D. Hahn
k Division of Neurology, The Hospital for Sick Children and Department of Paediatrics, University of Toronto, Toronto, Canada
Merita Kucuku
l National Agency for Medicines and Medical Devices, Tirana, Albania
James Oleske
m Department of Pediatrics, Rutgers – New Jersey Medical School, Newark, NJ, USA
Kollencheri Puthenveettil Vinayan
n Division of Pediatric Neurology, Department of Neurology, Amrita Institute of Medical Sciences, Cochin, Kerala, India
Elissa Yozawitz
o Saul R. Korey Department of Neurology, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, USA
Satinder Aneja
p Department of Pediatrics, School of Medical Sciences & Research, Sharda University, Gr Noida, India
Niranjan Bhat
q Center for Vaccine Innovation and Access PATH, Seattle, WA, USA
Geraldine Boylan
r INFANT Research Centre, University College Cork, Ireland
Sanie Sesay
s Clinical Sciences, Sanofi Pasteur, Marcy L’Etoile, France
Anju Shrestha
t Sanofi Pasteur, Global Pharmacovigilance, PA, USA
Janet S. Soul
u Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Beckie Tagbo
v Institute of Child Health, University of Nigeria Teaching Hospital, Nigeria
Jyoti Joshi
w Center for Disease Dynamics, Economics & Policy, New Delhi, India
x Medway NHS Foundation Trust, Kent, UK
Helena C. Maltezou
y Department for Interventions in Healthcare Facilities, Hellenic Center for Disease Control and Prevention, Athens, Greece
Jane Gidudu
z Centers for Disease Control and Prevention, Global Immunization Division, Atlanta, USA
Sonali Kochhar
aa Global Healthcare Consulting, New Delhi, India
ab Department of Public Health, Erasmus MC, University Medical Center, Rotterdam, the Netherlands
ac Department of Global Health, University of Washington, Seattle, USA
Ronit M. Pressler
ad Great Ormond Street Hospital for Children NHS Foundation Trust, London, UK
Associated Data
1. preamble, 1.1. need for developing case definitions and guidelines for data collection, analysis, and presentation for neonatal seizures as an adverse event following immunization.
Seizures are the most common neurological emergency in newborns and can be associated with significant mortality and neuro-developmental disability. Neonatal seizures are a major challenge for clinicians because of inconspicuous clinical presentation, variable electro-clinical correlation, and poor response to antiseizure drugs. It is well recognized that fever and infection can trigger seizures in young children and that this risk is enhanced in children with epilepsy. As immunization may cause a fever, vaccination can be a non-specific trigger for seizures in children [1] . On the other hand, children with epilepsy do not appear to be at increased risk of seizures following immunization [2] . It is unclear whether vaccination in newborns or maternal vaccination, is associated with a higher risk of neonatal seizures. However, as maternal immunization with established vaccines becomes more prevalent across multiple geographies, and new maternal vaccine candidates enter late-stage development, it is becoming increasingly important to create easily adopted standard definitions for adverse events potentially associated with these interventions. The Brighton Collaboration has previously published a case definition for seizures in children [3] but not for seizures in neonates.
1.1.1. Epidemiology of neonatal seizures
The reported prevalence and incidence of neonatal seizures vary considerably due to differences in study methodology, especially in the identification of neonatal seizures, and geographic setting [4] , [5] . The majority of seizures in neonates present without clinical signs [6] , [7] and can be recognized only with cEEG (conventional electroencephalography) monitoring, which has not been used in all studies. Therefore, the exact incidence of electrographic, clinically silent neonatal seizures in term and preterm babies is not known ( Table 1 , Table 2 ).
Incidence of neonatal seizures.
xLegend: NICU (neonatal intensive care unit), VLBW (<1500 g), LBW (≥1500–2499 g), NBW (≥2500–3999 g), HBW (≥4000 g), cEEG (conventional EEG), aEEG (amplitude-integrated EEG).
Etiology of neonatal seizures and reported relative frequency in high-, middle- and low- income countries.
Incidence. The reported incidence of neonatal seizures worldwide varies from 1.0–4.4 per 1000 livebirths in high-income countries (USA) [8] , [9] , [16] , to 5 per 1000 live births in upper middle-income countries (Iran) [13] . Reports from low- and middle-income countries are limited, but one study from Kenya reported an incidence of 39.5 per 1000 live births [15] . Among the preterm population, incidences vary considerably according to different methods of diagnosis. Based only on clinical observation the incidence of seizure in preterms has been reported to be 3.9–57.5 per 1000 live births [8] , [10] , [17] , whereas studies using amplitude-integrated electroencephalography (aEEG), reveal a seizure burden up to 48% [11] , [18] , [19] . However, it is well recognized that aEEG can be falsely positive particularly in preterm infants [20] . Studies using cEEG in preterms indicate an incidence of 4–9% in high-income countries (75% of which are electrographic-only seizures) [21] , [22] .
1.1.2. Etiology of neonatal seizures
The etiology of neonatal seizures is heterogeneous, and sometimes unknown, although the majority are due to hypoxia-ischemia, stroke or infections in term infants. In preterm infants, intraventricular hemorrhage is the commonest cause of seizure [29] , [30] .
The heterogeneity in the etiologic profile of neonatal seizures across geographies and economic strata is due to two main factors: differences in obstetric/perinatal care and access to electrodiagnostic techniques leading to differing rates of detection and diagnosis ( Table 2 ).
1.1.3. Timing of onset
The onset of neonatal seizures depends on etiology and is most common within the first week of life, with 25–55% occurring in the first 24 h [15] , [24] , [31] . Onset is generally later in preterm compared to term infants [29] .
1.1.4. Risk factors
Maternal risk factors for neonatal seizures include maternal age >40 years, nulliparous, diabetes mellitus, chorioamnionitis, traumatic delivery, prolonged second stage of labor, fetal distress, placental abruption, cord prolapse, and uterine rupture [23] .
Neonatal risk factors for seizures include the etiologies for seizure listed in Table 2 .
1.1.5. Outcomes
While a normal neurological outcome after neonatal seizures is reported in 25–40% of infants [21] , [32] , 15–30% develop cerebral palsy [32] , [33] , [34] ; 30–50% developmental delay [21] , [32] ; and 20–35% epilepsy [32] , [33] . The prognosis of neonatal seizures depends on the underlying etiology. However, there is evidence that seizures are independently associated with worse outcome [35] , [36] . Risk factors identified for poor outcome following neonatal seizures include prematurity/low birth weight, severity of HIE, high-grade intraventricular hemorrhage, persistently abnormal EEG background activity, seizure burden (electrographic seizure burden of >13 min/h), presence of neonatal status epilepticus (but not recurrent seizures), central nervous system infection and cerebral dysgenesis [4] , [26] , [35] , [37] , [38] . Death is reported among 7–25% of neonates with seizures in low-, middle-, and high-income countries [15] , [25] , [32] , [36] , mostly due to the underlying etiology. Mortality is higher among preterm and low-birthweight neonates (30–33%) [22] , [39] .
1.1.6. Pathophysiology of neonatal seizures
Developmental age-specific mechanisms influence the generation and phenotype of seizures. While there are some limitations in the use of animal models to study neonatal seizures, conclusions can be reached with consideration of the species-specific maturation rates in the system of interest [40] .
The neonatal period is a time of intense brain development. While cortical lamination is fully developed in the term infant, neurite outgrowth and synaptogenesis are continuing and are in their elementary stages. Brain myelination is immature. These factors limit the rapid propagation of neonatal seizures and their clinical presentation (with generalized, from onset, tonic-clonic seizures rarely occurring) [41] .
In the neonatal brain, the balance between excitatory versus inhibitory synapses is tipped in favor of excitation to permit robust activity-dependent synaptic formation, plasticity, and remodeling. Glutamate is the major excitatory neurotransmitter in the CNS with the involvement of AMPA and NMDA receptors and more expression and function than in the adult brain. For example, while, in the adult brain, γ-amino-butyric acid (GABA) usually induces membrane hyperpolarization, early in the developing brain it induces membrane depolarization by causing Cl¯ efflux rather than influx. The HCN channels, which are members of the K + channel super-family and important for maintenance of resting membrane potential and dendritic excitability, are also developmentally regulated. The immature brain has relatively low expression of the HCN1 isoform, which serves to reduce dendritic excitability in the adult brain [40] .
Genetic epilepsies with onset in the neonatal period reflect the structural and physiologic factors that can lead to neonatal seizures. These include ion channel function (e.g. KCNQ2), excitation-inhibition balance (e.g. pyridoxine-dependent epilepsy), brain development (e.g. ARX) and synaptic function (e.g. STXBP1) [42] . Some of the epilepsy syndromes with neonatal seizures have a favorable or “benign” prognosis (self-limiting familial neonatal seizures), however there exist severe epileptic encephalopathies with a poor outcome (neonatal myoclonic encephalopathy and early infantile epileptic encephalopathy or Ohtahara syndrome).
1.1.7. Diagnosis of neonatal seizures
The clinical diagnosis of neonatal seizures is challenging because many neonatal seizures either manifest with subtle clinical signs or remain entirely subclinical despite the presence of clear electrographic seizure activity on EEG.
Clinical manifestations of neonatal seizures may include focal motor movements or non-motor signs [79] , but manifestations are usually discreet and are often difficult to distinguish from other physiologic non-seizure movements such as eye deviation, automatisms, apnea and limb posturing [43] . Furthermore, numerous studies applying conventional EEG (cEEG) monitoring in neonatal cohorts have consistently demonstrated that the majority of neonatal seizures are subclinical [7] , [44] , especially in preterm infants [45] .
The diagnosis of neonatal seizures may be made by cEEG, amplitude-integrated EEG (aEEG) or by clinical signs alone. Gold-standard is capturing a seizure on cEEG (ictal EEG) because it provides the most direct and comprehensive assessment of neuronal activity. In comparison, aEEG is less accurate because it employs fewer electrodes over a smaller spatial area and the aEEG display is filtered and time-compressed making it harder to identify brief seizures. When aEEG is used together with a real-time EEG channel, the median sensitivity for seizure identification is 76% (range: 71–85%), and the median specificity is 85% (range: 39–96%). When aEEG was used without a real-time EEG channel, the median sensitivity is 39% (range: 25–80), and specificity is 95% (range 50–100) [46] . On the other hand, when the goal is identifying only the presence or absence of seizures in a neonate rather than individual seizures, the median sensitivity of aEEG with a real-time EEG channel rises to 85% (range: 70–90%).
Among neonates who present with clinically apparent seizures, antiseizure drugs commonly suppress clinical activity, but ongoing electrographic seizures persist, a phenomenon termed uncoupling [47] , [48] , [49] , [50] . Because of this uncoupling, which can also occur spontaneously, aEEG or cEEG monitoring is even more essential for the accurate assessment of response to therapy and seizure burden [51] . Practitioners should be aware of the limitations of the clinical assessment in over and under-diagnosing seizures, and aEEG or cEEG confirmation of clinically-diagnosed seizures should be sought whenever possible.
1.1.8. Differential diagnosis
Early recognition and accurate diagnosis of seizures in the neonatal period is essential for optimal management. However, the clinical diagnosis of seizures in neonates is also challenging because infants may present with abnormal movements that are non-epileptic but are mistaken for seizures leading to inappropriate treatment and unwarranted prognostic concern [52] . While the most common non-epileptic movements are generally benign and associated with a good prognosis, some may be associated with pathologic conditions. The video-EEG recording of the event can be very helpful to differentiate seizure from non-epileptic events. Seizures can coexist with non-epileptic manifestation in some patients. Table 3 summarizes the characteristics of the most common non-epileptic manifestation in newborns.
Differential diagnosis of neonatal seizures.
1.1.9. Neonatal seizures following maternal or neonatal vaccination
Maternal vaccination. A literature search conducted by the authors did not identify any reports of seizures among newborns born to women who received tetanus-diphtheria-acellular pertussis (Tdap), tetanus toxoid, tetanus-diphtheria (Td), seasonal or pandemic influenza vaccines, or in randomized controlled trials of investigational Group B Streptococcus or respiratory syncytial virus vaccines. A retrospective cohort study of pertussis among infants <63 days of age reported no seizures among 34 infants (median age 45 days) whose mothers received Tdap during pregnancy, while 14/336 (4%) infants of unvaccinated mothers developed seizures with pertussis infection (relative risk 0.96; 95% CI 0.94–0.98) [63] . There is currently no evidence of an association between vaccination during pregnancy and neonatal seizures.
Neonatal vaccination. In a study of claims in the United States National Vaccine Injury Compensation Program of seizures and/or encephalopathy allegedly caused by an immunization among children younger than two years during 1995–2005, a total of 90 claims (60%) concerned babies between 0 and 6 months of age but the number of neonates was not reported [64] . In 12 cases (7.2%) the final diagnostic impression by a pediatric neurologist was “infantile seizures”. This article provides no certainty about a causal effect because it is a summary of individual cases in a litigation setting. Another study found no increase in seizures or other neurologic events among healthy, full-term neonates who received hepatitis B vaccination versus controls [65] . In addition, there were no reports of neonatal seizures after polio or bacille Calmette-Guérin (BCG) vaccination, the vaccinations most commonly used in the neonatal period [66] .
1.1.10. Existing definitions for neonatal seizures
Several definitions of neonatal seizures exist ( Table 4 ). Neonatal seizures are traditionally defined as paroxysmal alterations in neurologic function (including motor, behavior and/or autonomic function) occurring in the first 28 days after birth of a term neonate or before 44 weeks of gestational age in a preterm infant [67] . It should be noted that this purely clinical definition of neonatal seizures is entirely arbitrary, resulting in both over and underestimation of the number of seizures in the newborn [7] . Several studies have shown the existence of considerable inter-observer variability among physicians and allied health professionals in the clinical diagnosis of seizures in the NICU [68] . According to the International League Against Epilepsy (ILAE), an epileptic seizure is defined as an electro-clinical phenomenon characterized by the transient occurrence of signs and symptoms due to an abnormal, excessive or synchronous neuronal activity in the brain [69] . Therefore, the identification of ictal discharges on the EEG (electrographic seizure) should be considered the gold standard for the accurate diagnosis of neonatal seizures (see Section 1.1.7 ). A recent World Health Organization’s (WHO) guideline on neonatal seizures also recommended the use of EEG for the confirmation of suspected neonatal seizures at all levels of care [27] .
Existing definitions of neonatal seizures.
Legend: ILAE (International League Against Epilepsy); ACNS (American Clinical Neurophysiology Society).
1.1.11. Classification of neonatal seizures
Neonatal seizures are focal, often subclinical [6] or have discreet clinical manifestations that are difficult to differentiate from movements of severely ill newborns [71] , [74] . Historically, seizure semiology in the neonatal period was considered to differ to those of other ages and therefore specific classification systems for neonates were developed. Some classification systems are based on direct observation only [71] , [75] , [76] , [77] , whereas others are based on clinical observation and video EEG [74] ( Table 5 ). However, there is no universally accepted classification in the neonatal period and therefore no common language to describe neonatal seizures. The 2017 ILAE Position Papers on Classification [77] , [78] are important updates on the terminology and etiology of seizures but specifically do not include neonatal seizures. A Neonatal Seizure Task Force of the ILAE has proposed a new framework that uses EEG and clinical seizure semiology to classify seizures in the neonatal period according to the predominant seizure type (electrographic only, motor, or non-motor) [79] . Motor seizures may be automatisms, clonic, epileptic spasms, myoclonic, sequential or tonic and non-motor seizures may be autonomic or behavior arrest seizures.
Classifications used for neonatal seizures.
Legend: ILAE (International League Against Epilepsy).
1.1.12. Need for a harmonized definition of neonatal seizures in the neonate
There is no uniformly accepted definition of neonatal seizures. This provides the opportunity to offer a definition that is practical and useful in the context of neonatal seizures following maternal and neonatal immunization, as data comparability across trials or surveillance systems will facilitate data interpretation and the assessment of vaccine safety, as well as promote the scientific understanding of neonatal seizures.
1.2. Methods for the development of the case definition and guidelines for data collection, analysis, and presentation for neonatal seizures as an adverse events following immunization
Following the process described in the overview papers [81] , [82] as well as on the Brighton Collaboration Website http://www.brightoncollaboration.org/internet/en/index/process.html , the Brighton Collaboration Neonatal Seizures Working Group was formed in 2018 and included members with clinical, academic, public health, industry backgrounds.
To guide the decision-making for the case definition and guidelines, we conducted a literature search using Medline, Embase and the Cochrane Central Register for English language articles reporting on seizures among neonates born to women vaccinated during pregnancy. In addition, we searched for clinical trials, passive and active surveillance reports, cohort and case-control studies of specific vaccines evaluated in pregnancy to capture additional reports of neonatal seizures and confirm the findings of our primary literature review. Only English language articles and articles referring to humans were selected for review. The primary search identified 82 articles excluding duplications of which 80 were excluded based on review of the title of abstract. The remaining two articles were excluded after review of the full text as they did not provide information regarding neonatal seizures and vaccines. A search for adverse events after maternal Tdap vaccination identified one relevant article that mentioned neonatal seizures.
We extended the search to include reports of neonates with seizure after immunization at birth, following the same methods described above. A total of 194 articles excluding duplications were identified. Based on abstract content we selected 12 articles for complete reading. Articles were excluded mainly because they presented no detailed information about the age of the vaccinated infants (e.g. “infants 0–6 months”) or the specific vaccination schedule. Finally, only one original article was selected for inclusion in our systematic review [65] .
1.3. Rationale for selected decisions about the case definition of neonatal seizures as an adverse event following immunization
The working group agreed that electrographically documented seizures with or without clinical manifestations represent the most accurate concept of neonatal seizures. There are several operational definitions for electrographic seizures in the newborn. According to the American Clinical Neurophysiology Society (ACNS), an electrographic seizure in a newborn is defined as a sudden, abnormal EEG event characterized by a rhythmic and evolving pattern with a minimum 2 µV peak-to-peak voltage and duration of at least 10 s. “Evolving” is defined as an unequivocal evolution in frequency, voltage, morphology, or location [73] . However, the working group considered at length the operational difficulties of a purely electrographic definition. The cut-off of 10 s of duration is arbitrary and does not include shorter clinical seizures e.g. myoclonic jerks or spasms. Prolonged EEG monitoring in the NICU on critically ill term/preterm newborns with multiple hemodynamic supports may be technically very demanding and may not be easily available in many centers, even in high-income countries. Another limiting factor will be the non-availability of adequate and appropriately trained personnel with special expertise in the recording and interpretation of EEG in the neonatal ICU setting.
Amplitude-integrated EEG (aEEG) can be a useful instrument but less accurate (see Section 1.1.7 for further details).
Clinical diagnosis of neonatal seizures is the least accurate parameter, although some clinical manifestations, such as focal clonic seizures or focal tonic seizures, particularly when seizures are stereotyped and recurrent, are highly indicative of epileptic seizures [68] . In contrast, events with generalized tonic posturing seen in infants with diffuse severe brain injury are usually of non-epiletic origin [28] .
1.3.1. Related terms of neonatal seizures
Neonatal period: begins at birth and ends at 28 completed days of life [83] .
Gestational age (GA): is a clinical term that applies to the estimated age of the fetus during pregnancy, generally given in weeks and days from the first day of the last menstrual period. According to the International Statistical Classification of Diseases and Related Health Problems (ICD-10) [84] , GA is used to classify three different periods in relation to delivery: preterm births (less than 37 weeks), term births (37–41 weeks) and post-term births (42 weeks or more). For additional information refer to the premature birth Case Definition of the Brighton Collaboration Preterm Birth Working Group [85] .
Neonatal seizures: relate to epileptic seizures in the neonatal period. It includes terms such as neonatal convulsions, neonatal fits, neonatal epilepsy and neonatal convulsive disorder (the latter two refer to a disorder with repeated unprovoked epileptic seizures, see below). The preferred term is neonatal seizure.
Epilepsy refers to a disorder with at least two unprovoked (or reflex) seizures occurring greater than 24 h apart or one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years [86] .
1.3.2. Focus of Brighton Collaboration case definition
The focus of the working group was to agree on a harmonized definition of neonatal seizures and the criteria to identify them, with different levels of diagnostic certainty. This will be useful also for the identification of neonatal seizures in the context of vaccination of mothers during pregnancy or neonatal vaccination.
1.3.3. Formulating a case definition that reflects diagnostic certainty: weighing specificity versus sensitivity
It needs to be emphasized that the grading of definition levels is entirely about diagnostic certainty, not the clinical severity of an event. Thus, a very severe clinical event may appropriately be classified as possible (level 3) or probable (level 2), rather than definite (level 1), if it could reasonably be of a non-epileptic etiology. Detailed information about the severity of the event should additionally always be recorded, as specified by the data collection guidelines.
The number of symptoms and/or signs that will be documented for each case may vary considerably. The case definition has been formulated such that the level 1 definition is highly specific for the condition. As maximum specificity normally implies a loss of sensitivity, two additional diagnostic levels have been included in the definition, offering a stepwise increase of sensitivity from level 1 down to level 3, while retaining an acceptable level of specificity at all levels. In this way, it is hoped that all possible cases of neonatal seizures can be captured.
1.3.4. Rationale for individual criteria or decision made related to the case definition
The working group agreed to a definition of neonatal seizures (see below) and to give different levels of certainty in the diagnosis (depending on the use of instrumental tools such as cEEG and aEEG or the sole clinical observation) in order to be effective and applicable in high-, middle- and low-income countries.
Pathology, radiology and laboratory findings are not included in the case definition, although they can provide important information regarding the causes of neonatal seizure.
1.3.5. Influence of treatment on the fulfilment of the case definition
The working group decided against using “treatment” or “treatment response” towards the fulfillment of the case definition of neonatal seizures.
A treatment response or failure is not in itself diagnostic, as less than 50% of neonatal seizures respond to the first line treatment (phenobarbital) [27] , [87] , [88] . At the same time, many antiseizure drugs have sedative or central nervous system depressant effects and may reduce the intensity or frequency of non-epileptic movements. It is only in certain circumstances, such as acute symptomatic seizures due to hypoglycemia or pyridoxine-dependent seizures, that specific treatments have diagnostic implications.
1.3.6. Timing post maternal immunization
Specific time-frames for the onset of symptoms of neonatal seizures following maternal immunization are not included. No information is available regarding the potential relevance of the timing of maternal immunization and the occurrence of neonatal seizures.
We postulate that a definition designed to be a suitable tool for testing causal relationships requires ascertainment of the outcome (e.g. neonatal seizures) independent from the exposure (e.g. maternal immunization). Therefore, to avoid selection bias, a restrictive time interval from maternal immunization to onset of neonatal seizures should not be an integral part of such a definition. Instead, where feasible, details of this interval should be assessed and reported as described in the data collection guidelines.
Furthermore, neonatal seizures often occur outside the controlled setting of a clinical trial or hospital. In some settings, it may be impossible to obtain a clear timeline of the event, particularly in low resource and rural settings. To avoid exclusion of such cases, this Brighton Collaboration case definition avoids setting arbitrary time-frames between maternal immunization and occurrence of the defined event.
1.4. Guidelines for data collection, analysis and presentation
As mentioned in the overview, the case definition is accompanied by guidelines which are structured according to the steps of conducting a clinical trial, i.e. data collection, analysis and presentation. Neither case definition nor guidelines are intended to guide or establish criteria for management of ill infants, children, or adults. Both were developed to improve data comparability.
1.5. Periodic review
Similar to all Brighton Collaboration case definitions and guidelines, review of the definition with its guidelines is planned on a regular basis (i.e. every three to five years) or more often if needed.
2. Case definition of neonatal seizures 2
Case definition
A neonatal seizure is defined as a transient electrographic change in the brain due to an abnormal, excessive or synchronous neuronal activity either with the occurrence of clinical signs (electro-clinical) or without them (electrographic-only), in the first 28 days of life in full-term infants. In the preterm infants (born <37 weeks of gestation), this definition applies up to 44 weeks of post menstrual age (PMA), considering the pattern of brain maturation.
Seizures confirmed by conventional EEG (cEEG) with or without clinical manifestations represent the most accurate concept of neonatal seizures; cEEG is considered the gold standard for neonatal seizure diagnosis (Level 1 – “definite” diagnosis). Ictal EEG refers to the epileptiform activity seen during a seizure in contrast to interictal discharges seen between seizures which are not diagnostic in neonates. Concomitant video recording is helpful although not a necessity and may be replaced by clinical observation during the EEG to determine a clinical-electrographic correlation.
Amplitude-integrated EEG (aEEG) or cerebral function monitoring can be a useful instrument but is less accurate than cEEG (see Section 1.1.7 ). The identification of seizures on the aEEG is considered a “probable” diagnosis of neonatal seizure (Level 2a).
As mentioned above, the clinical diagnosis of neonatal seizures is challenging and without EEG it is difficult to differentiate seizure from physiological or abnormal, but non-epileptic, movements (see Section 1.1.8 ). However, two seizure types are highly indicative of epileptic seizures, specifically focal tonic seizures (focal sustained stiffening/sustained increase in muscle contraction lasting a few seconds to minutes) or focal clonic (regularly rhythmic jerking, that involves the same muscle groups), which are not influenced by manual restraint [77] . Therefore, these seizure types also can be considered “probable seizures” (Level 2b) in the absence of a confirmation EEG, if observed by experienced medical personnel (a history of such events is not considered sufficient). The term “experienced medical personnel” refers to who routinely care for neonates and are familiar with the clinical presentation of neonatal seizures through training or clinical practice. Ideally this is a physician (not restricted to neonatology or neurology specialists), but in different settings also other professionals (such as advanced care provider, nurse, or individual such as midwife, health care worker) could diagnose “probable or possible seizures”, depending of their specific training in neonatal care.
As discussed in Section 1.1.11 , neonatal seizure types also include other motor or non-motor manifestations such as myoclonic jerks, epileptic spasms, automatisms, autonomic changes and behavioral arrest. Based only on clinical observation (without EEG confirmation) it is not possible to label these manifestations as definite neonatal seizures, however, they can be considered “possible” seizure (Level 3), if observed by experienced medical personnel (a history of such events is not considered sufficient). Generalized tonic events and bilateral hypermotor events are usually non-epileptic.
For further information on clinical manifestations and definitions of seizure types and epilepsy syndromes see https://www.epilepsydiagnosis.org/index.html .
LEVELS OF CERTAINTY
For All Levels of Diagnostic Certainty
Age 0–28 days in a full-term infant
Postmenstrual age of <44 weeks in a preterm infant (born <37 weeks of gestation)
Level 1 of diagnostic certainty

Level 2 of diagnostic certainty

Level 3 of diagnostic certainty

Notes for Levels of Certainty
2 sudden, abnormal EEG event characterized by repetitive and evolving pattern (in frequency, voltage, morphology, or location)
3 seizure confirmed with EEG and with clear clinical manifestation
4 seizure confirmed with EEG without clear clinical manifestation
5 regularly rhythmic jerking, that involves the same muscle groups and not influenced by manual restraint
6 focal sustained stiffening/sustained increase in muscle contraction lasting a few seconds to minutes and not influenced by manual restraint
7 someone who routinely cares for neonates and is familiar with the clinical presentation of neonatal seizures through training or clinical practice. Ideally this is a physician (not restricted to neonatology or neurology specialists), but in different settings also other professionals (such as advanced care provider, nurse, or individual such as midwife, health care worker) could diagnose “probable or possible seizures”, depending of their specific training in neonatal care
8 such as myoclonic, epileptic spasm, automatism, autonomic changes, behavioral arrest, but non-seizure events cannot be excluded without EEG [79]
3. Guidelines for data collection, analysis and presentation of neonatal seizures
It was the consensus of the Brighton Collaboration Neonatal Seizures Working Group to recommend the following guidelines to enable meaningful and standardized collection, analysis, and presentation of information about neonatal seizures. However, the implementation of all guidelines might not be possible in all settings. The availability of information may vary depending upon resources, geographical region, and whether the source of information is a prospective clinical trial, a post-marketing surveillance or epidemiological study, or an individual sporadic report of neonatal seizures. Also, these guidelines have been developed by this working group for guidance only and are not to be considered a mandatory requirement for data collection, analysis, or presentation.
3.1. Data collection
These guidelines represent a desirable standard for the collection of data on neonatal seizures following maternal immunization to allow for comparability of data and are recommended as an addition to data collected for the specific study question and setting. The guidelines are not specifically intended to guide the primary reporting of neonatal seizures to a surveillance system or study monitor, but they could potentially be adapted for these purposes. Investigators developing a data collection tool based on these data collection guidelines also need to refer to the criteria in the case definition, which are not repeated in these guidelines.
Guidelines numbered below have been developed to address data elements for the collection of adverse event information as specified in general drug safety guidelines by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, and the form for reporting of drug adverse events by the Council for International Organizations of Medical Sciences. These data elements include an identifiable reporter and patient, one or more prior maternal immunization, and a detailed description of the adverse event, in this case, of neonatal seizures following maternal immunization. The additional guidelines have been developed as guidance for the collection of additional information to allow for a more comprehensive understanding of neonatal seizures following maternal immunization.
3.1.1. Source of information/reporter
For all cases and/or all study participants (including mothers and infants, as appropriate), the following information should be recorded:
- (1) Date of report.
- (2) Name and contact information of person reporting 10 and/or diagnosing the neonatal seizures as specified by country-specific data protection law.
- (3) Name and contact information of the investigator responsible for the subject, as applicable.
- (4) Relation to the patient (e.g., clinician, nurse, family member [indicate relationship], other).
3.1.2. Vaccinee/Control
3.1.2.1. demographics.
For all cases and/or all study participants (including mothers and infants as appropriate), the following information should be recorded:
- (5) Case/study participant identifiers (e.g. first name initial followed by last name initial) or code (or in accordance with country-specific data protection laws).
- (6) Date of birth, age, and sex.
- (7) For neonates: gestational age and birth weight, twin status.
3.1.2.2. Clinical and immunization history
- (8) Past and current gynecological/obstetric history, medical history, including hospitalizations, underlying diseases/disorders, pre- immunization signs and symptoms including identification of indicators for, or the absence of, a history of allergy or other reactions to vaccines, vaccine components or medications; food allergy; allergic rhinitis; eczema; asthma. Any family history of seizure, neonatal/infant death (sibling), or congenital/genetic conditions should be recorded.
- (9) Any medication history (other than treatment for the event described) prior to, during, and after maternal immunization during pregnancy including prescription and non-prescription medication as well as medication or treatment with long half-life or long-term effect. (e.g. immunoglobulins, blood transfusion and immunosuppressant).
- (10) Maternal and infant immunization history (i.e. previous immunizations and any adverse event following immunization (AEFI), in particular occurrence of neonatal seizures after a previous immunization).
3.1.3. Details of maternal and infant immunizations
- (11) Date and time of maternal and infant immunization(s).
- (12) Description of vaccine(s) (name of vaccine, manufacturer, lot number, dose (e.g. 0.25 mL, 0.5 mL, etc.) and number of dose if part of a series of immunization s against the same disease).
- (13) The anatomical sites (including left or right side) of all immunizations (e.g. vaccine A in proximal left lateral thigh, vaccine B in left deltoid).
- (14) Route and method of administration (e.g. oral, intramuscular, intradermal, subcutaneous, and needle-free [including type and size], and vaccine vial [used/open vial or new vial] other injection devices).
- (15) Needle length and gauge.
3.1.4. The adverse event
- (16) For all cases at any level of diagnostic certainty and for reported events with insufficient evidence, the criteria fulfilled to meet the case definition should be recorded.
Specifically document:
- (17) Clinical description of signs and symptoms of neonatal seizures, seizure type [79] and if there was medical confirmation of the event (i.e. patient seen by appropriate health care provider 7 , and/or testing performed).
- (18) Date/time of onset 11 , first observation 12 and diagnosis 13 , duration and frequency of seizures (seizures/hour or seizures/day), last seizure 14 and final outcome 15 .
- • Measurement/testing [89] .
- • Minimum EEG standards for cEEG are described in the American Clinical Neurophysiology Society (ACNS) guidelines [73] , [89] .
- • Minimum aEEG standards are described by de Vries and Hellström-Westas ( https://doi.org/10.1136/adc.2004.062745 ) [90] and also in the American Clinical Neurophysiology Society (ACNS) guidelines ( https://www.acns.org/UserFiles/file/Guideline5-MinimumTechnicalStandardsforPediatricEEG_v1.pdf ) [73] .
- • Details of EEG (Date, type, duration, quality)
- • Results of electrolytes, blood gas, and serum glucose, calcium, magnesium, bilirubin as well as complete blood count and blood culture.
- • Other investigations depend on clinical presentation, history and availability and may include lumbar puncture, urine culture and toxicology (maternal toxicology screen), screen for relevant congenital infections, metabolic screen, and genetic testing.
- • Ultrasound and neuroimaging (MRI or CT scan) if available.
- (20) Treatment given for neonatal seizures, especially specify drug(s) and dosing.
- (21) Outcome 15 at last observation. Persistence beyond the neonatal period should be noted, ideally as late as 12–18 months.
- (22) Objective clinical evidence supporting classification of the event as “serious” according to regulatory standards 16 .
- (23) Maternal and infant exposures other than the maternal immunization, including those 24 h before and after immunization, and until delivery (e.g. food, medications, environmental, etc.) considered potentially relevant to the reported event.
3.1.5. Miscellaneous/general
The duration of surveillance for neonatal seizures should be predefined based on the neonatal period (see case definition – up to 28 days in term and up to 44 PMA in preterm infants). Events with onset of seizures after this time are not considered neonatal seizures although it is recognized that seizures may persist (onset of epilepsy).
Biologic characteristics of the vaccine (e.g. live attenuated versus inactivated component vaccines), biologic characteristics of the vaccine-targeted disease, biologic characteristics of the vaccinee (e.g. nutrition, underlying disease like immune-depressing illness) are not considered relevant for the choice of the duration of the surveillance for neonatal seizures.
- (24) The duration of follow-up reported during the surveillance period should be predefined likewise. It should aim to continue to resolution of the event.
- (25) Methods of data collection should be consistent within and between study groups, if applicable.
- (26) Follow-up of cases should attempt to verify and complete the information collected as outlined in data collection guidelines 1–23.
- (27) Investigators of patients with neonatal seizures should provide guidance to reporters to optimize the quality and completeness of the information provided.
- (28) Reports of neonatal seizures should be collected throughout the study period regardless of the time elapsed between maternal or infant immunization and the adverse event. If this is not feasible due to the study design, the study periods during which safety data are being collected should be clearly defined.
3.2. Data analysis
The following guidelines represent a desirable standard for analysis of data on neonatal seizures to allow for comparability of data and are recommended as an addition to data analyzed for the specific study question and setting.
- (29) Reported events should be classified in one of the following five categories including the three levels of diagnostic certainty. Events that meet the case definition should be classified according to the levels of diagnostic certainty as specified in the case definition. Events that do not meet the case definition should be classified in the additional categories for analysis.
- Level 1: Criteria as specified in the neonatal seizures case definition
- Level 2: Criteria as specified in the neonatal seizures case definition
- Level 3: Criteria as specified in the neonatal seizures case definition
- Event does not meet case definition
- Level 4: Reported neonatal seizures with insufficient evidence to meet the case definition 18
- Level 5: Not a case of neonatal seizures 19
Reporting of time intervals. (a) Subjects with neonatal seizures in relation to trimester of maternal immunization. (b) Subjects with neonatal seizures in relation to date of birth (maternal vaccination received any time during pregnancy).
Furthermore, it is useful to analyze time of onset of seizure because some etiologies have a definite time of onset. For preterm infants the age of onset is recorded as the corrected age and chronological age ( Table 6 b).
- (31) The period of occurrence is defined as the interval between the date of onset of the first seizure consistent with the definition and the last seizure 14 and/or final outcome 15 . If seizures persist beyond the neonatal period, this has to be noted. Whatever start and end are used, they should be used consistently within and across study groups.
- (32) If more than one measurement of a particular criterion is taken and recorded, the value corresponding to the greatest magnitude of the adverse experience could be used as the basis for analysis. Analysis may also include other characteristics like qualitative patterns of criteria defining the event.
- (33) The distribution of data (as numerator and denominator data) could be analyzed in predefined increments (e.g. measured values, times), where applicable. Increments specified above should be used. When only a small number of cases are presented, the respective values or time course can be presented individually.
- (34) Data on neonatal seizures obtained from subjects born to mothers receiving a vaccine should be compared with those obtained from an appropriately selected and documented control group(s) to assess background rates of neonatal seizures in non-exposed populations and should be analyzed by study arm and dose where possible, e.g. in prospective clinical trials.
3.3. Data presentation
These guidelines represent a desirable standard for the presentation and publication of data on neonatal seizures following maternal immunization to allow for comparability of data and are recommended as an addition to data presented for the specific study question and setting. Additionally, it is recommended to refer to existing general guidelines for the presentation and publication of randomized controlled trials, systematic reviews, and meta-analyses of observational studies in epidemiology (e.g. statements of Consolidated Standards of Reporting Trials (CONSORT) [91] , of Improving the quality of reports of meta-analyses of randomized controlled trials (QUORUM) [92] , and of Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [93] , respectively).
- (35) All reported events of neonatal seizures should be presented according to the categories listed in guideline 29 or other classification that is considered appropriate.
- (36) Data on possible neonatal seizures events should be presented in accordance with data collection guidelines 1–23 and data analysis guidelines 29–34.
- (37) Terms to describe neonatal seizures such as “low-grade”, “mild”, “moderate”, “high”, “severe” or “significant” are highly subjective, prone to wide interpretation, and should be avoided, unless clearly defined.
- (38) Data should be presented with numerator and denominator (n/N) (and not only in percentages), if available.
- (39) Although denominator data are usually not readily available for immunization safety surveillance, attempts should be made to identify approximate denominators. The source of the denominator data should be reported, and calculations of estimates be described (e.g. manufacturer data such as total doses distributed, reporting through Ministry of Health, coverage/population-based data, etc.). The incidence of cases in the study population should be presented and clearly identified as such in the text.
- (40) If the distribution of data is skewed, median and range are usually the more appropriate statistical descriptors than a mean. However, the mean and standard deviation should also be provided.
- • The study design;
- • The method, frequency and duration of monitoring for neonatal seizures;
- • The trial profile, indicating participant flow during a study including drop-outs and withdrawals to indicate the size and nature of the respective groups under investigation;
- • The type of surveillance (e.g. passive or active surveillance);
- • The characteristics of the surveillance system (e.g. population served, mode of report solicitation);
- • The search strategy in surveillance databases;
- • Comparison group(s), if used for analysis;
- • The instrument of data collection (e.g. standardized questionnaire, diary card, report form);
- • Whether the day of maternal immunization was considered “day one” or “day zero” in the analysis;
- • Whether the date of onset 2 and/or the date of first observation 3 and/or the date of diagnosis 4 was used for analysis; and
- • Use of this case definition for neonatal seizures, in the abstract or methods section of a publication 20 .
Notes for guidelines
10 If the reporting center is different from the vaccinating center, appropriate and timely communication of the adverse event should occur.
11 The date and/or time of onset is defined as the time within the neonatal period when the first sign or symptom indicative of neonatal seizures occurred. This may only be possible to determine in retrospect.
12 The date and/or time of first observation of the first sign or symptom indicative for neonatal seizures can be used if date/time of onset is not known.
13 The date of diagnosis of an episode is the day within the neonatal period when the event met the case definition at any level.
14 The end of the occurrence of neonatal seizures is defined as the time the subject no longer meets the case definition at the lowest level of the definition.
15 E.g. recovery to pre-event immunization health status, spontaneous resolution, therapeutic intervention, persistence of the event, sequelae, death.
16 An adverse event after immunization (AEFI) is defined as serious by international standards [94] if it meets one or more of the following criteria: (1) it results in death, (2) is life-threatening, (3) requires inpatient hospitalization or results in prolongation of existing hospitalization, (4) results in persistent or significant disability/incapacity, (5) is a congenital anomaly/birth defect, (6) is a medically important event or reaction.
17 To determine the appropriate category, the user should first establish, whether a reported event meets the criteria for the lowest applicable level of diagnostic certainty, e.g. Level three. If the lowest applicable level of diagnostic certainty of the definition is met, and there is evidence that the criteria of the next higher level of diagnostic certainty are met, the event should be classified in the next category. This approach should be continued until the highest level of diagnostic certainty for a given event could be determined. If the lowest level of the case definition is not met, it should be ruled out that any of the higher levels of diagnostic certainty are met and the event should be classified in categories four or five. The highest possible level of classification should be recorded for each event.
18 If the evidence available for an event is insufficient because information is missing, such an event should be categorized as “Reported neonatal seizures with insufficient evidence to meet the case definition”.
19 An event does not meet the case definition if investigation reveals a negative finding of a necessary criterion (necessary condition) for diagnosis. Such an event should be rejected and classified as “Not a case of neonatal seizures”.
20 Use of this document should preferably be referenced by referring to the respective link on the Brighton Collaboration website ( http://www.brightoncollaboration.org ).
4. Disclaimer
The findings, opinions and assertions contained in this consensus document are those of the individual scientific professional members of the working group. They do not necessarily represent the official positions of each participant’s organization (e.g., government, university, or corporation). Specifically, the findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their respective institutions.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
The authors are grateful for the support and helpful comments provided by the Brighton Collaboration Reference Group: Jorgen Bauwens, Julie Bettinger, Jan Bonhoeffer, Linda Eckert, Kathryn Edwards, Furaha Kyesi, Alex Mphuru, Victor Pakstan, Wan-Ting-Huang as well as by independent reviewers J. Helen Cross and Solomon L. Moshé.
2 The case definition should be applied when there is no clear alternative diagnosis for the reported event to account for the combination of symptoms.
Appendix A Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.05.031 .
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Appendix B.


IMAGES
VIDEO
COMMENTS
The occurrence of neonatal seizures may be the first, and perhaps the only, clinical sign of a central nervous system (CNS) disorder in the newborn infant. As such, seizures may indicate the presence of a potentially treatable etiology and should prompt an immediate evaluation to determine cause and to institute etiology-specific therapy.
Neonatal seizures are a commonly encountered neurologic condition in neonates.[1][2][3] They are defined as the occurrence of sudden, paroxysmal, abnormal alteration of electrographic activity at any point from birth to the end of the neonatal period.[3] During this period, the neonatal brain is developmentally immature.[4][5] Thus, neonatal seizures have unique pathophysiology and ...
Published May 3, 2023. N Engl J Med 2023;388: 1692 - 1700. DOI: 10.1056/NEJMra2300188. VOL. 388 NO. 18. Neonatal seizures are defined as seizures occurring within 4 weeks after birth in full-term ...
Clinical note. Neonatal seizures or neonatal convulsions are epileptic fits occurring from birth to the end of the neonatal period. 1-18 The neonatal period is the most vulnerable of all periods of life for developing seizures, particularly in the first 1-2 days to the first week from birth. They may be short-lived events lasting for a few ...
Although acute symptomatic seizures are more common in the neonatal period, they may be the first presentation of an epilepsy syndrome, with approximately 13% of neonatal seizures [2,23]. It is important to identify epilepsy syndrome and its etiology to determine its treatment and prognosis. ... · Clinical signs of neonatal seizures are ...
The purpose of this clinical practice guideline is to establish standard practices for the care of infants being evaluated, monitored, and/or treated for neonatal seizures. These guidelines have been developed to ensure that infants receive consistent and optimal care for neonatal seizures. II.
Clinical Presentation Neonatal seizures start focally but can spread to involve the entire body.2 Seizures that begin in a generalized fashion are rare. Clinical seizures in neonates can be
The designations employed and the presentation of the material in this publication do not imply the ... Neonatal seizures represent one of the most frequent neurological events in newborn ... 8. Where available, all clinical seizures in the neonatal period should be confirmed by electroencephalography. Strong, context-specific Not graded
Seizures resulting from an intracranial process usually cannot be differentiated from seizures resulting from a systemic problem by their clinical features (eg, focal vs generalized). Hypoxia-ischemia, the most common cause of neonatal seizures, may occur before, during, or after delivery (see Overview of Perinatal Respiratory Disorders). Such ...
Classification. The Task Force on Neonatal Seizures established by the International League Against Epilepsy (ILAE) has recently introduced a diagnostic framework consisting of four domains: clinical presentation (high risk or suspicious clinical events), diagnosis (with EEG), manifestation (with or without clinical signs), and seizure types determined by the predominant clinical signs (motor ...
Neonatal seizures are a frequent clinical manifestation in the neonatal period and they can present within the framework of four clinical scenarios, ... It should be noted that, although these four scenarios for neonatal seizure presentation are conceptually useful, in practice the categorization of patients into the scenarios A, B, C or D may ...
There is considerable variation in clinical practice regarding neonatal seizure management. 18-21 The most recent international guideline regarding neonatal seizure management was published in 2011 by the World Health Organization (WHO), ILAE, and International Bureau of Epilepsy, which included articles until 2008. 13 It was intended for ...
Sixth, a trial of pyridoxine may be attempted in neonates presenting with clinical features of vitamin B6-dependent epilepsy and seizures unresponsive to second-line ASM (expert agreement). Additional considerations include a standardized pathway for the management of neonatal seizures in each neonatal unit and informing parents/guardians about ...
The ILAE clinical practice guideline development group for neonatal seizures has developed guidelines and expert-based consensus recommendations for the treatment of neonatal seizures. These recommendations were informed by an extensive systematic review and, where no sufficient evidence was available, on expert-based consensus via Delphi.
How the timing of presentation can help determine the cause of the seizure Key components of the physical examination that should be conducted for neonates with seizure activity ... The clinical conundrum of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2002;86(2):F75-F77.
Diagnosis of neonatal seizures. Variability in the clinical presentation of neonatal seizures is the major factor of its misdiagnosis since it depends on clinical symptoms only, which may lead to overdiagnosis (excessive abnormal movement such as jitteriness, startle) or underdiagnosis (presence of electrical activity in areas away from the motor cortex) [].
Abstract. Neonatal seizures present a unique diagnostic challenge with clinical manifestations often subtle or absent to the bedside observer. Seizures can be overdiagnosed in newborns with unusual paroxysmal movements and underdiagnosed in newborns without clinical signs of seizures. Electroclinical "uncoupling" also adds to the diagnostic ...
Seizures are a common clinical indication of central nervous system damage or abnormality in neonates. We aimed to identify the etiologies, clinical characteristics, and radiological features of neonatal seizures. This is a cross-sectional, retrospective, descriptive study using data obtained from the neonatal intensive care unit in King ...
The postnatal history is also significant. Neonatal seizures in infants with an uneventful antenatal history and delivery may result from a postnatal cause. A history of tremulousness may suggest drug withdrawal or neonatal hypocalcemia. Temperature and/or blood pressure instability may suggest an infection; a sepsis workup may be required.
Seizures are a common occurrence in both term and preterm (PT) neonates. Despite their frequency and clinical significance, currently there are no clearly defined evidence-based guidelines to address major questions about their management. While diagnosis and management of seizures are often considered within the context of tertiary care health centres, seizures are a common manifestation in ...
Seizures in neonates are relatively common, with variable clinical manifestations. Their presence is often the first sign of neurologic dysfunction, and they are powerful predictors of long-term cognitive and developmental impairment. (See Prognosis.) Most seizures in the neonate are focal, although generalized seizures have been described in ...
Background Neonatal Escherichia coli (E coli) meningitis results in significant morbidity and mortality. We present a case of a premature infant with extensive central nervous system (CNS) injury from recurrent E coli infection and the non-traditional methods necessary to identify and clear the infection. Case Presentation The infant was transferred to our institution's pediatric intensive ...
Susan Quelly, PhD, RN, CNE University of Central Florida College of Nursing. 12201 Research Parkway Suite 300 Orlando, FL 32826 (407) 823-0645—Office.
Neonatal seizures are a major challenge for clinicians because of inconspicuous clinical presentation, variable electro-clinical correlation, and poor response to antiseizure drugs. It is well recognized that fever and infection can trigger seizures in young children and that this risk is enhanced in children with epilepsy.