Stem Cell Research & Therapy


Call for Papers: Clinical and Preclinical Evidence in Regenerative Cardiology
Edited by: Armin Attar, MD, PhD , Shiraz University of Medical Sciences, Iran Submission Status: Open until 15 April 2025

Call for Papers: Mesenchymal Stem Cell (MSCs) and MSC-Derived Extracellular Vesicles: Roles in Regenerative Medicine and Beyond
Edited by: Hongcui Cao, PhD , Zhejiang University, China Submission Status: Open until 15 April 2025

Call for Papers: Clinical Trials in Regenerative Medicine and Their Challenges
Edited by: Akihiro Umezawa, PhD, National Research Institute for Child Health and Development, Japan Submission Status: Open until 31 March 2025
Featured articles
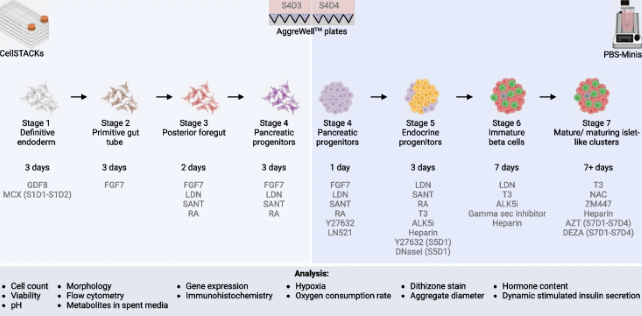
Metabolic switching, growth kinetics and cell yields in the scalable manufacture of stem cell-derived insulin-producing cells
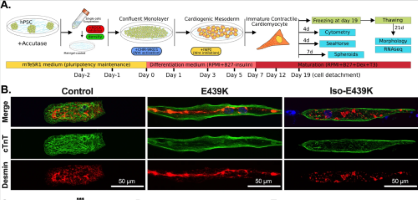
Critical contribution of mitochondria in the development of cardiomyopathy linked to desmin mutation
- Most accessed
- Collections
How to enhance MSCs therapeutic properties? An insight on potentiation methods
Authors: Cynthia Aylín García Guerrero, Paloma Fuentes, María Jesús Araya, Farida Djouad, Patricia Luz-Crawford, Ana María Vega-Letter and Claudia Altamirano
Cytokine priming enhances the antifibrotic effects of human adipose derived mesenchymal stromal cells conditioned medium
Authors: Marianela Brizio, Mathieu Mancini, Maximilien Lora, Sydney Joy, Shirley Zhu, Benoit Brilland, Dieter P. Reinhardt, Dominique Farge, David Langlais and Inés Colmegna
Adipose-derived stem cells apoptosis rejuvenate radiation-impaired skin in mice via remodeling and rearranging dermal collagens matrix
Authors: Yufan Zhu, Xu Liu, Xihang Chen and Yunjun Liao
Genetically modified mesenchymal stromal cells: a cell-based therapy offering more efficient repair after myocardial infarction
Authors: Congwang Xu, Yuanyuan Xie and Bin Wang
Endocytosis as a critical regulator of hematopoietic stem cell fate —implications for hematopoietic stem cell and gene therapy
Authors: Prathibha Babu Chandraprabha and Saravanabhavan Thangavel
Most recent articles RSS
View all articles
Stem cells: past, present, and future
Authors: Wojciech Zakrzewski, Maciej Dobrzyński, Maria Szymonowicz and Zbigniew Rybak
Ethical issues in stem cell research and therapy
Authors: Nancy MP King and Jacob Perrin
Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors
Authors: Paola Romina Amable, Rosana Bizon Vieira Carias, Marcus Vinicius Telles Teixeira, Ítalo da Cruz Pacheco, Ronaldo José Farias Corrêa do Amaral, José Mauro Granjeiro and Radovan Borojevic
CAR T cells in solid tumors: challenges and opportunities
Authors: Faroogh Marofi, Roza Motavalli, Vladimir A. Safonov, Lakshmi Thangavelu, Alexei Valerievich Yumashev, Markov Alexander, Navid Shomali, Max Stanley Chartrand, Yashwant Pathak, Mostafa Jarahian, Sepideh Izadi, Ali Hassanzadeh, Naghmeh Shirafkan, Safa Tahmasebi and Farhad Motavalli Khiavi
Stem cells: a potential treatment option for kidney diseases
Authors: Dongwei Liu, Fei Cheng, Shaokang Pan and Zhangsuo Liu
Most accessed articles RSS
Topical collection Clinical and Preclinical Evidence in Regenerative Cardiology Edited by Armin Attar
Topical collection Mesenchymal Stem Cell (MSCs) and MSC-Derived Extracellular Vesicles: Roles in Regenerative Medicine and Beyond Edited by Hongcui Cao
Topical collection Clinical Trials in Regenerative Medicine and Their Challenges Edited by Akihiro Umezawa
Topical collection Stromal cells and progenitor cells for osteoarticular regeneration Edited by Christian Jorgensen Topical collection Organoids and Tissue/Organ Chips Edited by Albert J Banes and Rajashekhar Gangaraju
Thematic series Adult stem cells in retinal diseases: where do we go from here? Edited by Rajashekhar Gangaraju
Thematic series Stem cell therapy of COVID-19 and other respiratory diseases Edited by Hong-Long James Ji, Michael A. Matthay and Yuanlin Song
Thematic series NK cells as the next option for cancer treatment Edited by Michael O'Dwyer
Cross-journal collection Coronavirus research highlights
Thematic series Stem cells and gene editing Edited by Stephen H. Tsang
Cross-journal collection Pluripotent Stem Cells
Thematic series Regenerative neurology Edited by Simon Koblar and Anne Hamilton-Bruce
Thematic series Extracellular vesicles and regenerative medicine Edited by Jeffrey Karp, Kelvin Ng and Armand Keating
Cross-journal collection Mesenchymal stem/stromal cells – an update Edited by Richard Schäfer and Selim Kuci
Cross-journal collection Biology and clinical applications of stem cells for autoimmune and musculoskeletal disorders Edited by Christian Jorgensen and Anthony Hollander
Thematic series Functional imaging in regenerative medicine Edited by Timothy O'Brien and Rocky Tuan
Thematic series Emerging investigators Edited by Timothy O'Brien and Rocky Tuan
Thematic series Stem cells and genitourinary regeneration Edited by John D Jackson
Thematic series Cardiovascular regeneration Edited by Ronald Li
Thematic series Physical influences on stem cells Edited by Gordana Vunjak-Novakovic
Thematic series Stem cell research in the Asia-Pacific Edited by Oscar Lee, Songtao Shi, Yufang Shi and Ying Jin
Thematic series Clinical applications of stem cells Edited by Mahendra Rao
Review series Immunology and stem cells Edited by Christian Jorgensen
Review series Epigenetics and regulation
Review series Stem cell niche
Review series Induced pluripotent stem cells

Trending articles in Stem Cell Research & Therapy
Click here to view which Articles have been shared the most in the last quarter!
Stem Cell Research & Therapy: 10th Anniversary
To mark the 10th year anniversary, we have reviewed the milestone achievements and highlighted some of the best content selected by our Editors-in-Chief and Associate Editors. Read more here .
Editors-in-Chief
Rocky S Tuan, The Chinese University of Hong Kong, Hong Kong SAR, China Timothy O'Brien, University of Galway, Ireland: Prof Timothy O’Brien is the Established Professor of Medicine in the College of Medicine, Nursing and Health Sciences at University of Galway and Consultant Endocrinologist in University Hospital Galway. He established the Regenerative Medicine Institute (REMEDI) with Prof Frank Barry in 2004 and is currently co-PI of the SFI-funded Centre for Research and Medical Devices (CÚRAM). Prof O’Brien’s research explores the synergies between gene therapy, stem/stromal cell therapy and biomaterials science with a key interest in the development of therapies for diabetic complications. The objective of his research is to translate research findings in the laboratory to clinical trials.
Journal submission information
Before you submit your manuscript to Stem Cell Research & Therapy , please ensure you have read and noted the below:
- Authors are requested to provide institutional email addresses. At the minimum, at least the corresponding author and submitting author should provide an institutional email address.
- Image integrity and standards: Cropped gels and blots can be included in the main text if it improves the clarity and conciseness of the presentation. In such cases, the cropping of the blot must be clearly evident and must be mentioned in the figure legend. Corresponding uncropped full-length gels and blot must be included in the supplementary files. These uncropped images should indicate where they were cropped, be labelled as in the main text and placed in a single supplementary figure. The manuscript's figure legends should state that 'Full-length blots/gels are presented in Supplementary Figure X'. Further information can be found under 'Digital image integrity' which are detailed on our Standards of Reporting page.
- Authors should clearly declare in the Acknowledgement Section if there is any work that was outsourced or data not collected by authors.
- Authors might be asked to provide original source data (raw data / original data / individual data points) if the reviewers or editors have any question.
- Authors should provide these information in the ethics declaration if the study involve human participants, human material, human data, or animals: (1) Title of the approved project; (2) Name of the institutional approval committee or unit; (3) Approval number; (4) Date of approval. Authors might be asked to provide ethics approval documents if the reviewers or editors have any question.
Aims and scope
Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials. The journal also provides reviews, commentaries, reports and methods.
Topics covered include, but are not limited to:
- Adult stem cells
- Animal models
- Biophysics and mechanobiology
- Cancer stem cells
- Cell culture and manufacture
- Clinical studies
- Disease modeling and drug screening
- Embryonic stem cells
- Ethical, legal and social aspects
- Genetics and epigenetics
- Genomics/proteomics/metabolomics
- Induced pluripotent stem cells
- Regenerative medicine
- Stem cell differentiation, proliferation and migration
- Stem cell therapy/transplantation
- Technologies
- Tissue engineering and biomaterials
Announcing the launch of In Review
Stem Cell Research & Therapy , in partnership with Research Square, is now offering In Review. Authors choosing this free optional service will be able to:
- Share their work with fellow researchers to read, comment on, and cite even before publication
- Showcase their work to funders and others with a citable DOI while it is still under review
- Track their manuscript - including seeing when reviewers are invited, and when reports are received
Editors' quotes

- Lucy Smith ORCID: orcid.org/0000-0002-7142-4471 1 ,
- Rebecca Quelch-Cliffe 1 ,
- Felicity Liu 1 ,
- Alejandro Hidalgo Aguilar 1 &
- Stefan Przyborski ORCID: orcid.org/0000-0001-7613-525X 1 , 2
Pluripotent stem cells have the ability to differentiate into all cells and tissues within the human body, and as a result they are attractive resources for use in basic research, drug discovery and regenerative medicine. In order to successfully achieve this application, starting cell sources ideally require in-depth characterisation to confirm their pluripotent status and their ability to differentiate into tissues representative of the three developmental germ layers. Many different methods to assess potency are employed, each having its own distinct advantages and limitations. Some aspects of this characterisation process are not always well standardised, particularly techniques used to assess pluripotency as a function. In this article, we consider the methods used to establish cellular pluripotency and subsequently analyse characterisation data for over 1590 human pluripotent cell lines from publicly available repositories in the UK and USA. In particular, we focus on the teratoma xenograft assay, its use and protocols, demonstrating the level of variation and the frequency with which it is used. Finally, we reflect on the implications of the findings, and suggest in vitro alternatives using modern innovative technology as a way forward.
Graphical Abstract
Avoid common mistakes on your manuscript.
Introduction
Pluripotency is defined as the ability of a cell population to self-renew and produce differentiated progeny derived from all three developmental germ layers; ectoderm, mesoderm and endoderm. Thorough confirmation of this property in stem cell lineages is crucial for their successful use in downstream applications, particularly in regenerative medicine and methods of tissue differentiation. This is especially pertinent when the variability in differentiation capacity of pluripotent stem cell (PSC) lines is considered [ 1 ], and is of importance when selecting a lineage for experimentation. Ideally the lineage should consist of a pure PSC population with the ability to generate a high yield of differentiated progeny that exhibit appropriate physiological function. Moreover, PSCs must be safe for use in clinical applications without risk of dedifferentiation or development of a malignant phenotype. Standardisation of the processes involved in PSC culture, including derivation, banking [ 2 , 3 , 4 ], characterisation [ 5 ], storage and maintenance [ 6 ] continues and will be imperative to the successful routine use of PSCs. However, consistently low yields, impurities and immature phenotypes from differentiation protocols can hinder the adoption of PSCs for routine use in the laboratory and prevent transition of potential therapies to the clinic. Such heterogeneity of PSC differentiation capacity is likely to be due to numerous diverse influences including genetic variation and microenvironmental effects within the culture dish [ 1 , 7 , 8 ].
Various methods have been developed to assess pluripotency, ranging from simple morphological analysis to complex animal experiments (see Tables 1 and 2 ). Each has its own advantages and limitations, according to the ease with which these assays can be performed and the data that can be generated. More specifically, these techniques can be further subdivided into those which assess pluripotency as a state, and those which assess pluripotency as a function. The importance of this distinction is clear when considering the fact that the identification of molecular pluripotency signatures which are commonly observed in pluripotent populations, i.e. identifying pluripotency as a state, does not necessarily provide any indication regarding the differentiation capacity, i.e. pluripotent function, of a given population or highlight subtle heterogeneities between different PSCs [ 1 , 5 , 9 , 10 ]. This in itself can be an issue when selecting the appropriate PSC lineage. Accordingly, assays which assess pluripotency as a function (also termed developmental potency, differentiation potential or developmental/differentiation capacity) are imperative to the characterisation process and involve a wide range of in vitro and in vivo techniques. For many years, the classic teratoma xenograft assay has been considered the ‘gold standard’ method [ 11 , 12 ]. The formation of highly complex, mature, morphologically identifiable tissues derived from the three germ layers is considered empirical proof of PSC differentiation capacity (see Fig. 1 ). Teratoma data has been regarded essential in the characterisation of new PSC lines, and has previously been endorsed by the International Stem Cell Banking Initiative [ 5 ].

Representative differentiated tissue structures observed in xenograft teratomas formed from engraftment of human embryonic stem cells into an immune deficient mouse host: The identification of structures from the three germ layers within a xenograft teratoma via histological analysis is sufficient to confirm pluripotency in putative human stem cell populations. Images are Haematoxylin and Eosin (H&E) stained unless stated otherwise: A ) Low magnification image of a typical teratoma, showing diversity of complex yet disorganised tissue structures from the three primary germ layers; B ) Neuroepithelial tissues organised in a neural rosette structure; C ) Intestinal epithelium structures with villus-like projections; D ) Weighert’s staining highlighting cartilage tissue (blue) surrounded by rudimentary bone tissue (red); E ) Masson’s Trichrome staining highlighting extracellular matrix rich areas (blue) surrounding striated muscle tissues (red); F ) Pseudostratified epithelial lumen structure surrounded by varied connective tissues. Scale bars: 100 µm ( E ) and 50 µm ( B , C , D , F )
The teratoma xenograft assay is also considered the most rigorous method of confirming the pluripotency of human PSCs [ 12 , 13 ], and generally, involves the implantation of an undifferentiated putative PSC population into a subcutaneous or internal location in an immunocompromised murine host. The subsequent formation of a tumour with evidence of tissues derived from each the three primary germ layers is indicative of pluripotency and assay success (See Fig. 1 ). Tetraploid complementation assays [ 14 ] and germ line transmission [ 15 ] are used to confirm developmental potential in non-human PSCs; these techniques can assess pluripotency even more comprehensively by demonstrating ability to direct differentiation for the formation of a fully competent organism capable of reproduction. Given that these methods involve the formation of chimeras, they cannot be used to characterise human stem cell lineages due to obvious ethical and legal restrictions.
The teratoma xenograft assay also has applications in developmental biology/organogenesis [ 16 , 17 , 18 , 19 , 20 ], cancer research [ 21 ], the study of pluripotency [ 22 ] and is considered to provide a wealth of data beyond the mere ability of PSCs to form the three germ layers. But while the teratoma assay is highly useful, it does have limitations. The need for a mouse host to conduct the assay puts it at odds with the now widespread practice of reducing animal use in research [ 23 ], and experimental success is not guaranteed. Variability in protocols and reporting is well recognised, however the scope and effects of this may be under appreciated, as these severely compromise data comparability and transparency, bringing into question the capability of the PSCs under analysis. In addition, many of these variable parameters influence differentiation trajectory [ 24 , 25 , 26 , 27 ]. The need for assay standardisation has been acknowledged by many [ 11 , 12 , 28 ], and while some attempts have been made [ 29 , 30 ], these have not yet been widely adopted by the field.
There has been some drive to develop novel methodologies to assess the pluripotency, differentiation capacity and malignancy of PSCs. PluriTest is perhaps the most well-known, using an open access gene expression database of known PSCs to which microarray data from putative PSCs can be compared to assess pluripotency and any technical or biological variation between lineages [ 31 , 32 ]. While these bioinformatics methods may provide strength of accuracy through use of large datasets, there are concerns regarding the use of a limited set of markers and the ability to detect subtle differences in lineage biases [ 33 ]. There have also been attempts to develop novel in vitro techniques to assess pluripotency, driven by advances in cell culture technology, the need to reduce animal usage, and to improve experimental accuracy, consistency and reproducibility. The ability to form complex tissue-like structures in vitro has been revolutionised through the development of technology that enhances the cellular microenvironment, including using three-dimensional culture systems such as physical scaffolds and hydrogels, perfused cultures and co-culture with other cell populations [ 34 , 35 , 36 , 37 ]. These methods can better recapitulate aspects and cues of the in vivo microenvironment, improving the ability to form tissue structures in vitro . This pursuit of complexity and consistency may resolve some of the issues associated with the teratoma xenograft assay and provide standardised and reproducible in vitro methods to investigate and confirm the differentiation capacity of PSCs.
In this article, we assess original characterisation data for human PSC lines registered for use at major UK and USA repositories in order to investigate trends in the methods employed to characterise the differentiation potential of PSCs. In particular, we focus on the combination of methods used to determine pluripotency and the frequency and reporting of the teratoma xenograft assay. While the lack of standardisation in assay reporting and performance continues to be appreciated [ 12 , 28 ], it is of utmost importance to continue to challenge the currently accepted process. By assessing whether the methods used to characterise novel PSCs are suitable and sufficient, the scientific community as a whole can determine whether recommended practices are being adopted, if the teratoma xenograft assay can continue to be regarded as a ‘gold standard’ method and ultimately, innovate the most suitable methods to conclude whether a lineage in question is truly pluripotent.
Materials and Methods
Literature search and analysis, research strategy and aims.
An overall research strategy was determined and aims identified prior to commencing the literature search. Our assessment focus on the availability and characterisation processes used on human PSCs deposited at major cell banks in the United Kingdom (UK) and the United States of America (USA). This represents a significant body of data and much is freely available and readily accessible as a consequence of cell lineages being available for public use.
The aims of this literature review were to determine:
Whether all cell lines had characterisation data available for inspection.
What specific methods were used to assess pluripotency within the cell lines upon derivation.
Whether a teratoma xenograft assay had been performed.
If the teratoma assay had been performed, what parameters had been used and how had the assay been reported.
Study Selection
Major UK cell banks holding human PSCs were identified as: the National Institute for Biological Standards and Control (NIBSC) Research Grade Stem Cell Catalogue and the European Collection of Authenticated Cell Cultures (ECACC). Major US cell banks holding human PSCs were identified as: National Institutes for Health (NIH) Embryonic Stem Cell Registry and the American Type Culture Collection (ATCC).
For all repositories, complete lists of all human embryonic and induced PSCs were obtained from the online catalogues and collated, resulting in a total number of 1790 cell lines at the time of data collection. Lists were checked and duplicated lines removed, giving a final number of 1590 cell lines for analysis.
Data Acquisition and Analysis
Extensive literature searches were carried out to acquire the characterisation data for the human PSC lines. For each cell line, the aim was to find the original derivation and characterisation information in a journal article or failing that, to use the data from characterisation performed by the cell bank. Research included specific searches for each named cell line on hpscreg.eu and Cellosaurus to identify depositors and whether direct links to original articles could be found. Following this, individual searches were undertaken for each cell line in PubMed, Science Direct and Google Search to locate relevant information. For cell lines where no information could be found, original depositors were contacted to request the necessary data or papers.
Characterisation Analysis
Characterisation methods were split into two groups, pluripotent state and pluripotent function, depending on the aspect of pluripotency the technique assessed. Pluripotent state analyses were defined as any technique which assessed the presence of specific proteins or genes associated with pluripotency and included immunocytochemistry, flow cytometry, qPCR, alkaline phosphatase assay, karyotypic analysis and bioinformatic assessments such as PluriTest. Pluripotent function analyses were defined as any technique which involved in assessing the differentiation potential of pluripotent cells and included spontaneous differentiation (2D), embryoid body formation, directed differentiation (2D and 3D methods) and the teratoma xenograft assay.
Teratoma Parameter Analysis
Key assay parameters, many of which have been previously shown to impact on teratoma formation, were chosen and compared between cell lines. These were: number of cells transplanted, anatomical location and details concerning the murine host. Assay endpoint and success rate were also determined to be key parameters that could influence the interpretation of tumour growth.
Data Presentation
Microsoft Excel 2019 was used to collate and analyse the data, GraphPad Prism 5 was used for data visualisation.
Generation of In Vivo and In Vitro Samples
Examples of data shared in this review have been generated from analysis of tissue blocks from previously published work [ 35 ]. Briefly, the following methods were used to generate these materials:
PSC cell culture: Human H9 embryonic stem cells were used for this work. Cells maintained in feeder free conditions in 6 well plates (Greiner Bio One, Stonehouse, UK) coated with Matrigel hESC qualified matrix (Corning, Flintshire, UK) in mTESR plus medium (Stem Cell Technologies, Cambridge, UK) prepared according to the manufacturer’s instructions, as previously described in [ 35 ].
Teratoma xenograft assay: H9 cells were maintained in feeder free conditions as described above, until required for the assay. Cells were detached from culture conditions using 0.25% trypsin/ 2mM EDTA (Fisher Scientific, Loughborough, UK) and counted using the Trypan Blue Exclusion Assay to obtain viable cell numbers. The teratoma assay was then performed as described in [ 35 ]. All procedures were completed under licence and permission according to the guidelines of the Home Office, United Kingdom.
Use of advanced 3D cell culture technology to assess differentiation potential of PSCs: H9 cells were routinely maintained in feeder free conditions before cells were detached from plates using ReLeSR (Stem Cell Technologies), using ROCK inhibitor to enable the survival of cells in a single cell suspension. 3D tissue models were generated as described in [ 35 ].
Characterisation Data was Available for more than 80% of PSC Lines at Public Repositories, yet those Lacking were Exclusively Embryonic Stem Cells
During the data collection phase, human PSC lines listed on the major UK and US repositories and cell banks were collated. Following the exclusion of duplicated cell lines, a total of 1590 original cell lines were identified as eligible for assessment. Each cell line was then extensively researched using a number of online resources in order to obtain the initial characterisation data. Original depositors were also contacted for cell lines where characterisation data was more difficult to source. In the event that initial characterisation data was unavailable, characterisation performed by the repository or cell bank was used for the analysis. This mainly applied to induced pluripotent stem cells (iPSCs), which had been derived on a larger scale as part of two projects by the cell banks themselves (EBiSC and HiPSC) (1,129 total cell lines). Of the 1590 lineages identified, 89% (1418) had basic characterisation data pertaining to pluripotency assessment available for analysis, whereas 11% did not have any characterisation data available from the primary literature or from initial analyses conducted by the cell bank (Fig. 2 A). This proportion of lineages lacking data were exclusively embryonic stem (ES) cell lines. When this is considered as a fraction of the embryonic stem cell lines assessed in the study, the percentage without characterisation data is much higher, at 41% (Fig. 2 A).
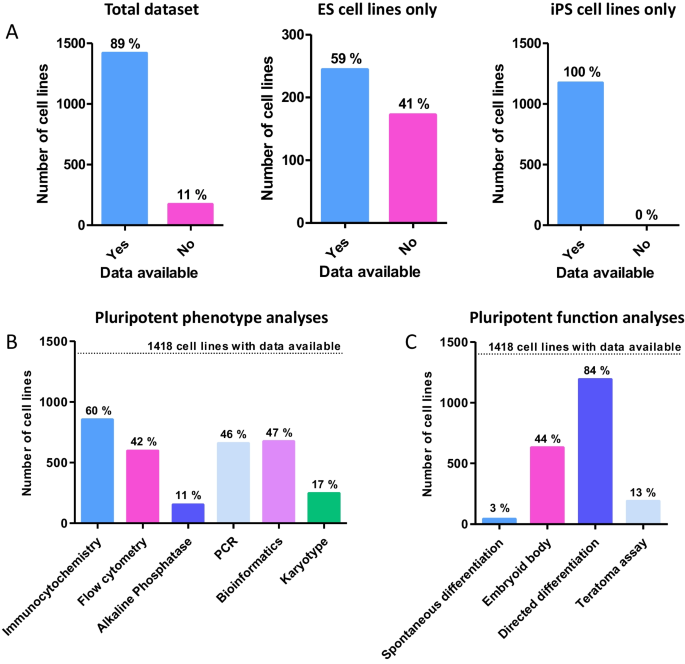
Analysis of characterisation data availability for hPSC cell lines and breakdown of the techniques used to determine pluripotency: Initial analyses focused on the availability of data in relation to cell line type as well as the techniques used to characterise hPSCs. A ) Data availability for cell lines as compared to the entire dataset. Almost 90% of cell lines had some accessible characterisation data. A significant proportion of ES cells did not have any characterisation data available, 172 out of a total of 412 ES cell lines in the study. Some form of characterisation data was found for all 1174 iPSC lines surveyed. B ) Breakdown of phenotypic analyses used to characterise hPSC lines. Phenotypic analyses had a smaller range of percentages, indicating diversity of methods available. Only immunocytochemistry was used on more than half of the cell lines surveyed, likely due to its simplicity and accessibility. C ) Breakdown of functional approaches used to characterise hPSC lines. The use of functional analyses varied considerably depending on the technique. Directed differentiation (2D or 3D) was overwhelmingly the most popular in either category, used for 84% of cell lines (1191 total). This fits with the hypothesis that many PSC lines are derived for a specific purpose or study, but may be skewed by iPSCs derived in large scale studies and the high throughput nature of the technique
Nearly all Cell Lines with Characterisation Data had at Least One Phenotypic and One Functional Assessment Performed
Of the PSCs for which data could be obtained, 98% of cell lines had at least one phenotypic and one functional assessment performed. Of those methods considered to assess the pluripotent phenotype (immunocytochemistry, flow cytometry and AP assay), immunocytochemistry was overwhelmingly the most popular method with 60% of lineages having had the analysis completed (Fig. 2 B). This was followed relatively closely by flow cytometry, at 42%. Performance of the AP assay appeared low, and this was mainly skewed by its use with iPSC lines (although only 55% of ES cells lines had the AP assay performed). Of the functional analyses assessing developmental potential, specific directed differentiation was the most popular method, with around 84% of the cell lines having had this completed (Fig. 2 C). These methods were varied and included differentiation into cardiomyocytes, neurones and trophoblast lineages, as well as more general germ layer differentiation. In contrast, only 13% of the cell lines assessed had a teratoma xenograft assay performed (Figs. 2 C, 3 A). Of those that had the teratoma assay performed, 94% were ES cell lines, whereas only 6% were iPS cell lines (Fig. 3 B). Of the ESC lines analysed just under half (41%, Fig. 3 C) of ESCs had a teratoma assay performed, whilst of the 1174 iPSC lines analysed in this study an overwhelmingly high 99% had not been characterised using the method.

Evaluation of frequency of teratoma assay performance and analysis of PSC types on which the assay is performed: Analysis of teratoma assay use was performed in relation to data accessibility and each hPSC type. A ) Percentage of registered human PSCs which had a teratoma assay performed, as a proportion all cell lines with characterisation data available. Only 13% of PSC lineages with accessible data had pluripotency confirmed using the teratoma assay, equating to 184 out of a total of 1418 cell lines. B ) Of those cell lines that had the assay performed, 94% were ESC lines, whereas only 6% were iPSC lines. C ) Percentage teratoma assays performed according to cell line type. For both ESCs and iPSCs, more cell lines did not have the teratoma assay performed that those that did. The difference is perhaps more surprising for ESCs which are generally derived in much smaller studies than iPSCs are (and with the associated skewing from the larger datasets)
Teratoma Assay Protocols Continue to Lack Standardisation, with the Most Common Response Being that Parameters and Procedures are Unreported
Deeper analysis into the protocols used to conduct the teratoma xenograft assay revealed a lack of congruency in this data subset. A number of key protocol parameters were assessed in depth (Fig. 4 ). Initial cell seeding number varied (Fig. 4 A); some papers cited the use of a single cell suspension without a specific cell number, many papers used a specific initial cell seeding number but these ranged from less than 1 million to over 5 million cells per injection. A number of protocols used a range of values in the same assay set up, whereas some used highly arbitrary measures such as cell clumps or wells of a plate. Most commonly however, the number of cells used in the protocol was not specified (45% of 188 cell lines which had the assay performed).
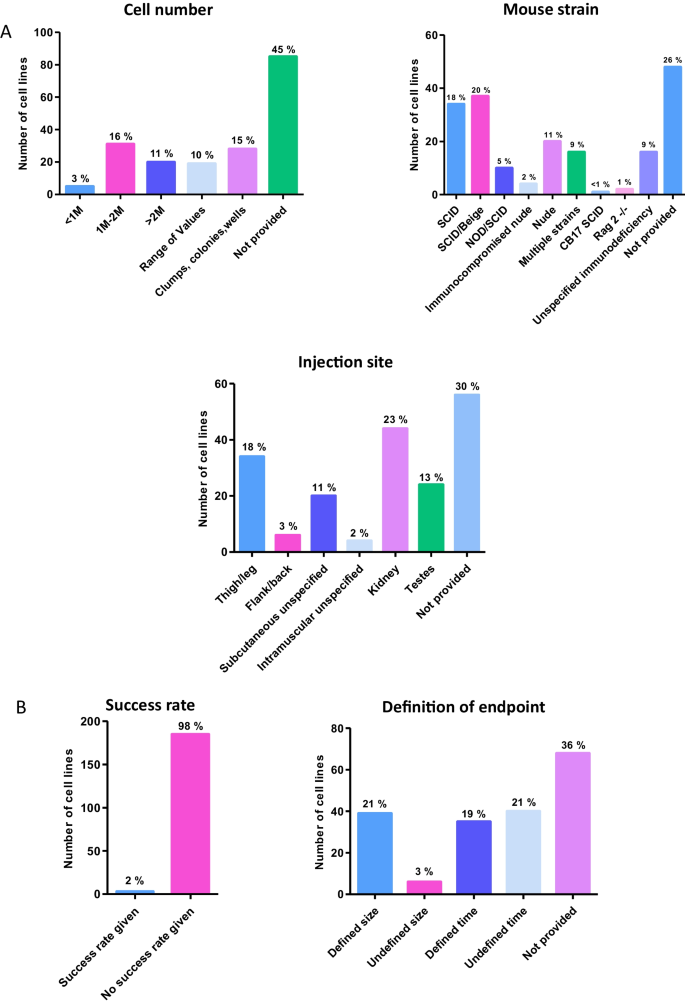
Analysis of the key experimental parameters and definitive aspects of the teratoma xenograft assay: In depth analysis of key teratoma assay protocol parameters provides additional useful data. A ) Assay protocol parameters: Around 30% of assays provided specific data on the number of cells injected. Multiple studies used vague references such as clumps/wells/colonies, or a range of values. While SCID and SCID/Beige mouse strains were the most popular, a wide range of immuodeficient mouse hosts were used in this selection of teratoma assays, yet most frequently this information was not provided. Analysis showed some preference for thigh/leg or kidney injection sites, with a small number of studies not specifying the exact location but providing some information (subcutaneous or intramuscular). Most frequently the information concerning injection site was not provided. B ) Assay completion parameters: Success rates can provide data on the effectiveness of protocols, although fear of judgement may prevent some from publishing this data. Of the 188 teratoma assays performed, only 3 provided information on teratoma formation success rates. Xenograft experiments may be brought to an end based on certain criteria. Such criteria could impact on assay results, and variability in this may prevent comparison. More assays used a defined endpoint than did not, but the modal response was that again the information was not provided
The mouse strain used and anatomical site for injection for the teratoma xenograft were also assessed (Fig. 4 A). Seven different immunodeficient mouse strains were used in the protocols analysed. In addition, some studies used multiple different immunodeficient mouse models as tumour hosts. While SCID/Beige was the most frequently used (20%), very closely followed by SCID (18%), around one third of the studies either did not detail the specific immunodeficiency of the mouse or did not provide any data about the mouse population used. As for the anatomical site where the PSC population was implanted, the use of renal (23%) and thigh/leg (18%) sites for injection was relatively common, but once again the modal response was that the anatomical site was not specified within the protocol (30% of 188 cell lines).
Information concerning tumour formation success rate and definition of the assay endpoint are also found to be generally lacking. Success rate was not provided for all but three of the cell lines assessed (Fig. 4 B). Definition of the assay endpoint was broadly split into two categories, those based on the size of the tumour, and those based on time the tumour was left to grow but again, the manner in which the endpoint of the assay was determined was not provided for many of these studies (36%) (Fig. 4 B).
Alternative In Vitro Methods can Achieve Similar Levels of Tissue Complexity and Diversity to that Observed in the In Vivo Teratoma Assay
The ‘gold standard’ status of the teratoma assay is largely due to the fact that the complex, mature nature and diverse range of tissues found in successful teratomas has not previously been replicated in vitro . Previous work in our group [ 35 , 37 ] has shown that tissue complexity similar to that observed in a teratoma can be achieved by combining EB formation with a porous scaffold (Fig. 5 ). Multiple small teratoma-like structures can be formed on the surface of the scaffold, with H&E staining demonstrating the varied nature of the in vitro structures in comparison to in vivo teratomas. Evidence of tissues derived from the three developmental germ layers, the key criterion for determining teratoma success, can be clearly observed in the in vitro teratoma model (Fig. 6 ). Highly similar structures can be produced using both experimental methods, as demonstrated by histological analysis.
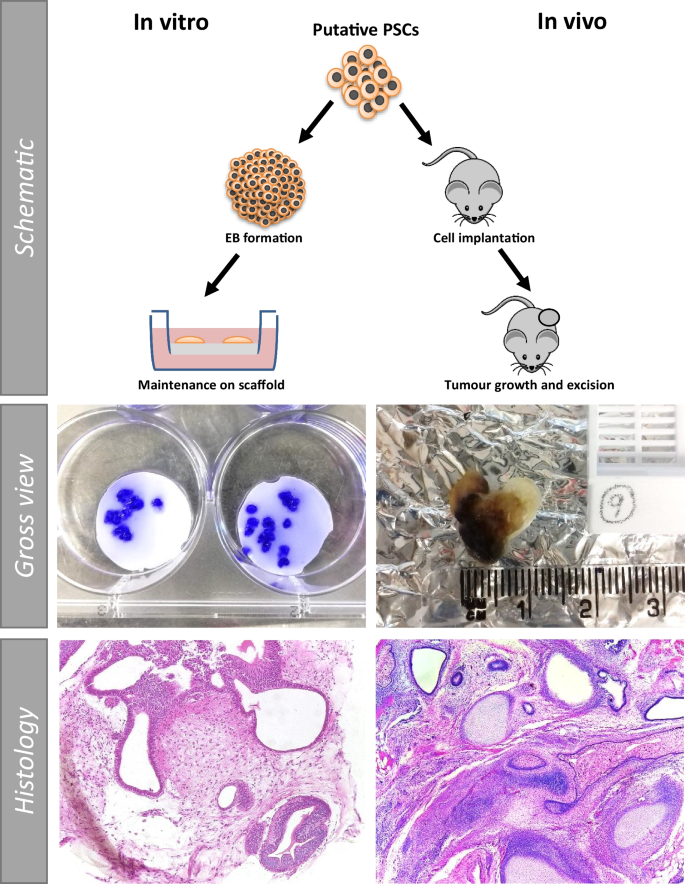
Schematic of technique comparison of in vitro vs in vivo structures derived from human pluripotent stem cells using either method: The in vitro method has a number of advantages, including the ability to generate multiple 3D teratoma structures from the same cell population in a single experiment, allowing for the assessment of a wider sample set. The gross view images of in vitro preparations (left), show individual crystal violet stained mini teratoma models. Low magnification histological staining highlights the diverse tissue structures which form using both techniques, demonstrating that teratoma structures exhibiting tissue complexity and diversity can be achieved utilising either in vitro or in vivo techniques
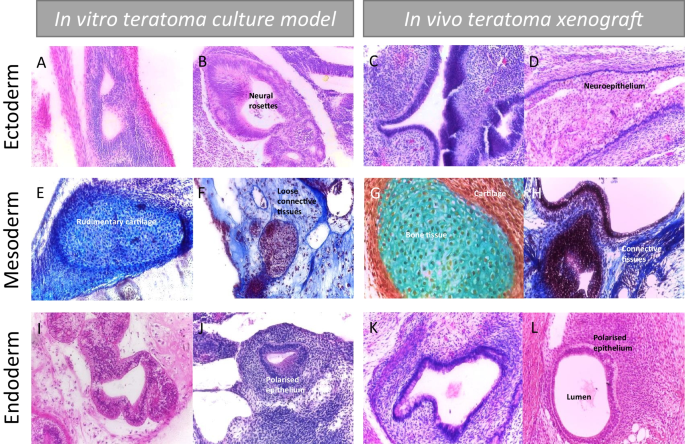
Assessment of functional pluripotency by teratoma tissue structures representative of different germ layers derived from hPSCs using either in vitro or in vivo techniques: Side by side histological comparison of samples from the in vitro and in vivo techniques shows the similarity in the tissue structures which form. Importantly, all three developmental germ layers can be identified in the in vitro sample, validating it against the simple criteria of the in vivo assay as a method for assessing functional pluripotency. A - D ) H&E staining highlights neural rosettes and neuroepithelial structures in both sample types, confirming the presence of ectodermal derivatives. E – H ) Masson’s Trichrome (MT) staining ( E , F , H ) and Weighert’s (WG) staining ( G ) indicate the structure and identity of the mesodermal structures within each sample type. Complex fibroblast derived extracellular matrix is demonstrated by blue MT staining of connective tissues, with evidence of diverse matrices noted in images E and F . Blue WG stain in image G highlights bone formation, surrounded by cartilage in red – rudimentary structures of this can be seen in the in vitro images. I - L ) H&E staining clearly highlights the epithelial structures within the two samples. Polarised, organised cells surrounding a central luminal space can be observed across the samples, demonstrating the complexity of the tissue structures present
Two previous studies have been performed to assess the characterisation of PSCs and the use of the teratoma xenograft assay within the stem cell field. In 2010, Müller and colleagues focused very specifically on whether the teratoma assay was used in articles which described the establishment of novel PSC lines [ 12 ]. While the study clearly argued that standardising the way the assay was performed and reported was essential to its successful use, and commented on protocols being ‘poorly reported’, much of the evidence was anecdotal, therefore not revealing the true extent of the variability issue. More recently, Montilla-Rojo published a review examining the teratoma xenograft assay as a tool to investigate both pluripotency and malignancy, providing an assessment of parameters used and considering the transparency of reporting in light of the ARRIVE guidelines for the use of animals in research [ 28 , 86 ]. While this study does demonstrate the issues surrounding protocol variability, ultimately it focuses on articles which described use of a teratoma assay, therefore providing little information as to the frequency of assay use within the field. In this study, we have reported on the use of all techniques named in the characterisation of publicly available cell lines at UK and US repositories (NIH, ECACC, MRC, ATCC), whether these are sufficient to definitively determine pluripotency and the frequency with which the teratoma assay was used as part of this characterisation. As a consequence of our analysis, questions are raised about whether the teratoma xenograft assay still features as a key method in the characterisation human PSCs or if it has been superseded by other techniques. If so, do our observations guide us to the selection of a new ‘gold standard’ approach?
Characterisation Data is not Routinely Published for ES Cell Populations
Initial assessment revealed that only 11% of cell lines did not have any characterisation data available, which seems relatively reasonable. However, the majority of these were embryonic (ES) cell lines, and it is concerning that almost half (41%) of the ES cell lines surveyed had no reported characterisation data. This may be due to the fact that such data were simply not published, which is possible if the lines had been derived intended for in-house use. However, this finding in itself raises separate issues not covered in this review on the requirement/responsibility of publishing cell line characterisation data, particularly if the cell line in question is eventually made publicly available.
The Consistent, Concurrent use of Phenotypic and Functional Methods is Encouraging, with a Tendency Towards Simple, Rapid Techniques
The characterisation of putative human PSCs is crucial to ascertaining their identity as a true stem cell population. Many analyses focus on the pluripotent phenotype, providing a ‘snap shot’ assessment of cellular status through the detection of surface markers, expression of transcription factors and enzymes known to be present in PSCs. This is unsurprising, and likely due to the inexpensive nature of these techniques and the ease with which these analyses can be performed in almost any laboratory, without the need for highly specialist equipment. However, as has already been noted, pluripotent phenotype does not necessarily correlate with developmental potential, due to the fact that the mechanisms and signalling pathways which maintain pluripotency and ability to differentiate are distinct from those which execute lineage determination and differentiation [ 1 ]. Even powerful technologies such as single cell RNA sequencing, which can be used to quantitatively assess heterogeneity in pluripotent states and subpopulations within PSC lineages [ 55 , 56 ], may not be able to detect these differences. Thus the use of additional methods is required to ascertain more thoroughly whether PSCs can successfully differentiate into mature, complex tissue structures representative of each germ layer.
It was encouraging to note that almost all cell lines examined in this study had at least one phenotypic and one functional assay performed. There is a trend towards simple, rapid methods such as immunocytochemistry and flow cytometry, which use a set of well-defined pluripotency markers as a basis for confirming pluripotency (see Table 1 ) [ 5 ]. As for functional assays, specific directed in vitro differentiation is highly favoured, which is a relatively straightforward method; it can be conducted within standard cell culture facilities and confirmed by differentiation marker expression. While the use of simple and rapid methods is less of an issue for general phenotypic assessments, such low demand techniques may neglect some of the finer aspects of functional characterisation, particularly the ability of cells to self-organise and achieve the functional maturity as seen in highly differentiated, organised tissue structures from mature teratoma xenografts.
The Teratoma Assay is not Routinely Performed on iPSC Lines, Which may Result in an Incomplete Characterisation and Understanding of Cellular Behaviour
The teratoma xenograft assay has long been held as the definitive method to assess the differentiation capacity of human PSCs, and yet it was performed in only 13% of cell lines in this study, which seems at odds with its previous ‘gold standard’ status. Of the teratoma assays that were conducted, most were on ES cell lineages. In this study, we found that 72% of ES cell lines and only 0.9% of iPSC lines within the identified repositories had the assay performed. It is likely that this is due to the high-throughput nature by which new iPSC lineages are derived, rendering the assessment of developmental potential by a teratoma xenograft an unfeasible task if performed for each new line. There is no doubt that directed differentiation can provide a wealth of information regarding differentiation capacity, with some studies suggesting that in vitro differentiation could replace the teratoma assay for differentiation assessment [ 33 , 87 ]. However, there is currently no standardised replacement for the teratoma assay which can definitively assess PSC differentiation and simultaneously provide information on malignancy. This is particularly relevant when we recall the functional heterogeneity between PSC populations expressing the same established pluripotency markers [ 1 , 9 ], and that current reprogramming technologies are another source of potential variation for iPSCs. Incomplete reprogramming can lead to epigenetic anomalies, such as variability in X chromosome inactivation, insufficient silencing of source lineage DNA modifications and aberrant DNA methylation, all of which have the potential to impact on cellular behaviour, including differentiation capacity and phenotype stability in disease lines [ 88 , 89 ]. While there seems to be consensus on the ability of the teratoma assay to provide malignancy data, recent studies have provided conflicting evidence regarding the ability of the assay to successfully identify incompletely reprogrammed iPSCs [ 33 ]. One study cites that these cells may form tumour masses similar to teratomas which do not contain the three developmental germ layers, and are open to misinterpretation [ 90 ]. On the one hand, the characterisation of these iPSCs could be considered incomplete due to a lack of teratoma assay to confirm differentiation capacity alongside more subtle aspects of cellular behaviour, such as malignancy. Yet conversely, the conflicting evidence on incompletely reprogrammed cells brings into question the suitability of the teratoma assay for assessing iPSC potency at all.
High Variability in all Parameters and a Lack of Protocol Reporting Prevents Standardisation of the Teratoma Assay
Protocols used in the teratoma xenograft assay continue to demonstrate a high level of variability [ 12 , 28 ]. Major parameters such as cell number, injection site and mouse strain differ considerably between studies, all of which are well known to impact on differentiation trajectory of PSCs, therefore affecting the tissues which form in the tumour [ 24 , 25 , 26 , 72 ]. This is in addition to the natural biological variation inherent in such an assay through the use of an animal host. Such variation can be an issue when the xenograft assay is used to directly compare the differentiation capabilities of novel PSC lines, as if the microenvironmental cues provided to the cells on implantation are directly influencing their behaviour, the true differentiation capabilities and any subtle lineage biases or changes in potency may not be noted. This hampers reproducibility and leads to an inability to determine the suitability of a specific PSC line for an intended application, as well as difficulties in monitoring PSC performance over time for quality assurance. Overall, protocol analysis shows a lack of any commonalities that could be used to standardise the assay, with the modal response being that protocol data was not reported. The lack of transparency surrounding teratoma protocol reporting is not consistent with the expectations of rigour associated with robust scientific research.
The period of tumour growth and assay endpoint are other parameters which ultimately impact on the differentiation and maturity of tissues derived from the implanted PSCs. In part, this is dependent on whether the assay is stopped according to length of study, tumour dimensions, or factors concerning the welfare of the host. This is also variable and it is evident that teratomas which have been left to grow for longer will have a greater likelihood of forming larger, more complex and mature tissue structures. Choosing to end a teratoma assay based on a specific time frame at least allows for comparison within a study, but the same cannot be said if biological dimensions are employed, as biological variability in tumour growth could lead to significantly different xenograft growth periods. Regardless of when the endpoint is determined, the need for a full and detailed analysis of the resultant tumour is paramount. Assessment by a pathologist or other suitably trained personnel is recommended due to the presence of partially differentiated and immature structures [ 91 ]. Histological analysis should also be performed at separate sites within the tumour to account for variation across the tumour mass and avoid missing specific tissue structures. The way in which histological analysis was performed is not often provided in detail and the assessment of protocols examined herein. In this study, it was noted that a total of 22 cell lines included details that histological analysis had been performed by a trained pathologist (whether clinical, veterinarian or commercial).
The success rate of a teratoma xenograft study may also provide information regarding the health of the PSCs tested, protocol success and assay utility, as well as insights into the developmental potential of PSC lineages which have been genetically manipulated [ 22 , 33 , 92 , 93 ]). Yet, only three cell lines out of all those surveyed provided success rate data; two ES cell lines (Nott-1 and Nott-2) which were derived in the same paper [ 94 ], and one iPS cell line (DXR0109B) [ 38 ]. The teratoma formation success rates were 60%, 100% and 25% respectively. This is a wide range and includes high failure rates, which may indicate the hesitancy of some to publish these data, given the inherent unreliability of the assay. The scarcity of success rate data also highlights a lack of clarity in reporting the number of animals used and therefore the number of biological or technical repeats performed. The regular omission of such key data seems at odds with the tight reporting regulations surrounding the use of animals.
The Teratoma Xenograft Assay is not Often Performed, with Limitations and Technical Requirements Outweighing a Small Number of Strengths
The data presented herein and the recent study by Montilla-Roja et al. (2023), correlate strongly with that obtained around a decade ago [ 12 ]. While the proportion of ES cell lines with teratoma xenograft data appears to be higher (contrast 72% with Müller’s finding of 44%), differences in the datasets and relative numbers of each PSC type in the studies make it difficult to determine whether this is actually as a result of increased use of the assay. Similarly, and more recently, the difference in the number of iPSC lines which have had the assay performed is likely reflective of improved efficiency in derivation techniques, resulting in more lines and the inability to perform such an extensive characterisation.
As noted, only 13% of PSC lines in this study used the teratoma xenograft approach as a strategy to assess developmental potential. This may be due to a number of reasons including high experimental costs, need for appropriate licensing, labour intensive set up and inability to scale up, requirement for specialist technical skill and extended experimental time. The use of an animal host in a time when there is a sustained effort by the scientific community to find satisfactory alternatives to animal studies may well be the most influential factor in the lack of uptake. Ultimately, the lack of protocol information prevents replication and comparison, and while implementing measures such as minimum reporting standards could help remedy these issues (as has previously been seen with microarray studies and animal research [ 12 , 86 , 95 ]), the lack of standardisation remains evident. The International Society for Stem Cell Research (ISSCR) have recently produced a series of standards for the characterisation of human PSCs, which includes minimum analysis criteria, suggested analyses and minimum reporting standards for the various analyses, including the teratoma xenograft assay.
PluriTest has been suggested as an alternative method to assess pluripotency, given its ability to rapidly screen and compare to a large, evolving dataset comprised of well-characterised PSC lines. Concerns have been raised that bioinformatics methods such as this may not be able to provide sufficient information on malignant potential, a crucial aspect of pre-clinical safety assessment, whereas others reason that as data on differentiation defective or malignant cell lines is added to the database ‘PluriTest gains power to discriminate subtler characteristics of pluripotent cells’ [ 87 ]. This 2018 study by the International Stem Cell Initiative also noted that, while combining methods such as EB formation and PluriTest could provide a good overview of functional pluripotency, the final recommendation was that different analytical methods were required depending on the intended application [ 87 ]. As an ultimate application of many PSCs could be regenerative medicine, stringent methods are required to assess both malignant potential and differentiation potential in order to maintain patient safety. The teratoma xenograft assay is deemed to be the only assay currently able to assess the malignant potential of PSCs [ 28 , 87 ], yet it has also been described as not having an acceptable level of reproducibility for iPSCs intended for the clinic [ 27 ] and there are concerns over the ability of the assay to identify incompletely reprogrammed cells. Accordingly, new approaches able to accurately and reproducibly assess both parameters are required.
Innovative Three-dimensional Cell Culture Technologies Offer a Feasible Alternative to the Teratoma Assay
Although limited like all methods and models, in vitro studies by their nature are more reproducible and controllable, allowing for greater standardisation and comparability, and their greater throughput is also advantageous, enabling parallel studies to achieve large datasets. There have been recent developments where advancements in cell culture technologies have been applied to assess the developmental potential of PSCs in vitro and, in some cases, enabling the study of tissue formation and equalling the capability of the teratoma xenograft assay. The ability to form specific cell populations and complex single tissues from PSCs has been demonstrated by many, using varied techniques to create constructs similar to those in vivo [ 96 , 97 , 98 ]. Highly detailed culture protocols and expensive reagents/equipment can be prohibitive, and experiments such as these are perhaps too complex to use routinely in order to demonstrate the formation of tissue derivatives representative of all three germ layers. However, simpler methods which enhance the culture microenvironment to permit sufficient time for complex tissue structures to spontaneously develop have been used by various researchers to achieve the equivalency of the teratoma assay in vitro . Through using bioreactors to monitor culture conditions over long term studies, PSCs have been able to spontaneously produce teratoma like masses which are significantly more complex than EBs in a series of promising studies [ 81 , 83 , 99 ]. In our own laboratory, we combined EB formation and culture on a porous polystyrene scaffold (Fig. 5 ) to extend EB viability to the point where highly complex tissue structures can be clearly identified which show a very strong resemblance to those observed in teratoma xenografts (Fig. 6 ) [ 35 , 37 ]. By allowing PSCs sufficient time to differentiate, similar to the bioreactor studies, PSC derived EBs were able to form diverse structures from the three germ layers, meeting the key criterion which underlines teratoma assay success. The controllability of this method, compatibility with varied analytical methods and ability to perform high throughput studies by culturing multiple EBs on the same scaffold are strong advantages, indicating the promising nature of this novel method for assessing pluripotent capacity. Some basic work regarding cellular malignancy and impaired differentiation capacity has been performed through the use of embryonal carcinoma cell populations in this system; these cells formed lineage restricted structures as expected and showed positive staining for pluripotency marker Oct4, indicating the presence of embryonal carcinoma elements in the tissue structure [ 37 ]. However, further optimisation is needed to fully explore these properties, using a wider range of differentiation defective and potentially malignant PSC populations, before this culture method can be considered a direct replacement for the teratoma assay.
The development and application of research tools such as these will allow researchers to achieve high levels of consistency and standardisation in the assessment of functional pluripotency. As such techniques become adopted over time, it is expected that the field will agree on a suite of new ‘gold standard’ methods and determine new criteria for an in vitro pluripotency assay, particularly one which enables the formation of recognisable tissue rudiments representative of all three germ layers and provides information on potential malignancy.
Conclusions and Future Perspectives
In this article, we have discussed the methods used to characterise human PSCs, focusing in particular on the use of functional pluripotency testing to confirm differentiation potential. Through analysing the characterisation data of around 1500 PSC lines held at public cell banks/repositories, we have shown that while most had data available and had at least one functional assay performed, the classical approach using the ‘gold standard’ teratoma xenograft assay was only undertaken on 13% of cell lines in the study. The assay varies significantly in terms of protocols used and level of detail provided. This analysis significantly advances the conclusions of similar studies and concurs with the guidelines of major PSC institutions, such that standardising characterisation processes is essential to the progression and authenticity of PSC lineages used in the field, particularly in light of increased derivation of novel iPSCs, and PSCs use in clinical applications. We also note that standardisation of the xenograft approach has failed to materialise over time, with there being no clear replacement technique which can encompass the ability of the assay to assess differentiation capacity and malignant potential. As such, a new method must be sought to replace the previous ‘gold standard’ assay.
With the advent of modern culture technology and the need to reduce animal usage, we show how alternative in vitro approaches are now available that have the added benefit of achieving much of the same outcome as the original teratoma xenograft assay, focusing in particular on a method established within our laboratory using EBs and a porous scaffold. This method shows promise, forming structures which resemble those seen in teratoma tumours and fitting the success criteria, and could be optimised further to be considered a total direct replacement for the teratoma xenograft assay. As well as simply expanding the number of PSC lines used to gather valuable data on how different PSC populations behave within the in vitro system, perhaps leaning towards iPSCs given their rapidly expanding use, additional studies need to be conducted to assess differentiation defective PSCs, incompletely reprogrammed PSCs and potentially malignant PSCs. Direct comparison studies between PSCs would also be of benefit, as would quality monitoring studies on PSC populations over time, to ascertain whether the assay is powerful enough to discriminate subtle differences as a result of derivation/reprogramming method, disease status or genetic drift, and offer benefits above and beyond the teratoma assay, which has ultimately been hampered by a basic lack of standardisation.
Employing modern technology to enhance the growth environment of differentiating PSCs in vitro provides the opportunity to create improved strategies for assessing PSC function and their ability to form mature, organised tissue structures which may in time become a new ‘gold standard’ approach to assay potency. These methods have the potential to more readily provide essential information concerning the full characterisation of newly derived PSC cell lines, which in turn, will contribute to greater understanding of the developmental potential across different PSC lineages within the field, information that will be of significant benefit to the scientific community.
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Code Availability
Not applicable.
Cahan, P., & Daley, G. Q. (2013). Origins and implications of pluripotent stem cell variability and heterogeneity. Nature Reviews Molecular Cell Biology, 14 (6), 357–368.
Article CAS PubMed PubMed Central Google Scholar
Crook, J. M., Hei, D., & Stacey, G. (2010). The International Stem Cell Banking Initiative (ISCBI): Raising standards to bank on. In Vitro Cellular and Developmental Biology-Animal, 46 (3–4), 169–172.
Article PubMed Google Scholar
Stacey, G. N., Crook, J. M., Hei, D., & Ludwig, T. (2013). Banking human induced pluripotent stem cells: Lessons learned from embryonic stem cells? Cell Stem Cell, 13 (4), 385–388.
Article CAS PubMed Google Scholar
The International Stem Cell Banking Initiative. (2009). Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Reviews and Reports, 5 (4), 301–314.
Article Google Scholar
International Stem Cell Initiative, Adewumi, O., Aflatoonian, B., Ahrlund-Richter, L., Amit, M., Andrews, P. W., et al. (2007). Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nature Biotechnology, 25 (7), 803–16.
Akopian, V., Andrews, P. W., Beil, S., Benvenisty, N., Brehm, J., Christie, M., et al. (2010). Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cellular and Developmental Biology-Animal, 46 (3), 247–258.
Engel, M., Do-Ha, D., Muñoz, S. S., & Ooi, L. (2016). Common pitfalls of stem cell differentiation: A guide to improving protocols for neurodegenerative disease models and research. Cellular and Molecular Life Sciences, 73 (19), 3693–3709.
Stewart, M. H., Bendall, S. C., Levadoux-Martin, M., & Bhatia, M. (2010). Clonal tracking of hESCs reveals differential contribution to functional assays. Nature Methods, 7 (11), 917–922.
Ortmann, D., & Vallier, L. (2017). Variability of human pluripotent stem cell lines. Current Opinion in Genetics & Development, 46 , 179–185.
Article CAS Google Scholar
Palmqvist, L., Glover, C. H., Hsu, L., Lu, M., Bossen, B., Piret, J. M., et al. (2005). Correlation of murine embryonic stem cell gene expression profiles with functional measures of pluripotency. Stem Cells, 23 (5), 663–680.
Buta, C., David, R., Dressel, R., Emgård, M., Fuchs, C., Gross, U., et al. (2013). Reconsidering pluripotency tests: Do we still need teratoma assays? Stem Cell Research, 11 (1), 552–562.
Article PubMed PubMed Central Google Scholar
Müller, F. J., Goldmann, J., Löser, P., & Loring, J. F. (2010). A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell, 6 (5), 412–414.
Brivanlou, A. H., Gage, F. H., Jaenisch, R., Jessell, T., Melton, D., & Rossant, J. (2003). Setting standards for human embryonic stem cells. Science, 300 (5621), 913–916.
Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W., & Roder, J. C. (1993). Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 90 (18), 8424–8428.
Peng, X., Liu, T., Shi, C., Zhang, L., Wang, Y., Zhao, W., et al. (2014). Germline transmission of an embryonic stem cell line derived from BALB/c cataract mice. PLoS ONE, 9 (3), e90707.
Aleckovic, M., & Simón, C. (2008). Is teratoma formation in stem cell research a characterization tool or a window to developmental biology? Reproductive Biomedicine Online, 17 (2), 270–280.
Al-Jomard, R., & Amarin, Z. (2017). The use of human mature cystic ovarian teratoma as a model to study the development of human lymphatics. Anatomy & Cell Biology, 50 (2), 104–106.
Hultman, I., Björk, L., Blomberg, E., Sandstedt, B., & Ährlund-Richter, L. (2014). Experimental teratoma: At the crossroad of fetal- and onco-development. Seminars in Cancer Biology, 1 (29), 75–79.
Li, H., Gao, L., Du, J., Ma, T., Ye, Z., & Li, Z. (2021). To better generate organoids, what can we learn from teratomas? Frontiers in Cell and Developmental Biology, 9 . [Internet]. [cited 2024 Apr 17]. Available from: https://www.frontiersin.org/articles/ https://doi.org/10.3389/fcell.2021.700482
Przyborski, S. A. (2005). Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells, 23 (9), 1242–1250.
Jamil, S., Cedervall, J., Hultman, I., Ali, R., Margaryan, N. V., Rasmuson, A., et al. (2013). Neuroblastoma cells injected into experimental mature teratoma reveal a tropism for embryonic loose mesenchyme. International Journal of Oncology, 43 (3), 831–838.
Gutierrez-Aranda, I., Ramos-Mejia, V., Bueno, C., Munoz-Lopez, M., Real, P. J., Mácia, A., et al. (2010). Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells (Dayton, Ohio), 28 (9), 1568–1570.
Smith, R. (2001). Animal research: The need for a middle ground. BMJ, 322 (7281), 248–249.
Dressel, R. (2011). Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Seminars in Immunopathology, 33 (6), 573–591.
Lee, A. S., Tang, C., Cao, F., Xie, X., van der Bogt, K., Hwang, A., et al. (2009). Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle (Georgetown, Tex.), 8 (16), 2608–2612.
Prokhorova, T. A., Harkness, L. M., Frandsen, U., Ditzel, N., Schrøder, H. D., Burns, J. S., et al. (2009). Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem cells and Development, 18 (1), 47–54.
Sullivan, S., Stacey, G. N., Akazawa, C., Aoyama, N., Baptista, R., Bedford, P., et al. (2018). Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Medicine, 13 (7), 859–866.
Montilla-Rojo, J., Bialecka, M., Wever, K. E., Mummery, C. L., Looijenga, L. H. J., Roelen, B. A. J., et al. (2023). Teratoma assay for testing pluripotency and malignancy of stem cells: Insufficient reporting and uptake of animal-free methods—a systematic review. International Journal of Molecular Sciences, 24 (4), 3879.
Gropp, M., Shilo, V., Vainer, G., Gov, M., Gil, Y., Khaner, H., et al. (2012). Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS ONE, 7 (9), e45532.
Masuda, S., Yokoo, T., Sugimoto, N., Doi, M., Fujishiro, S. H., Takeuchi, K., et al. (2012). A simplified in vitro teratoma assay for pluripotent stem cells injected into rodent fetal organs. Cell Medicine, 3 (1–3), 103–12.
Müller, F. J., Schuldt, B. M., Williams, R., Mason, D., Altun, G., Papapetrou, E. P., et al. (2011). A bioinformatic assay for pluripotency in human cells. Nature Methods, 8 (4), 315–317.
Müller, F. J., Brändl, B., & Loring, J. F. (2008). Assessment of human pluripotent stem cells with PluriTest. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute. Available from: http://www.ncbi.nlm.nih.gov/books/NBK133282/ . Accessed May 2024.
Bouma, M. J., van Iterson, M., Janssen, B., Mummery, C. L., Salvatori, D. C. F., & Freund, C. (2017). Differentiation-defective human induced pluripotent stem cells reveal strengths and limitations of the teratoma assay and in vitro pluripotency assays. Stem Cell Reports, 8 (5), 1340–1353.
Giandomenico, S. L., Sutcliffe, M., & Lancaster, M. A. (2021). Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nature Protocols, 16 (2), 579–602.
Hidalgo Aguilar, A., Smith, L., Owens, D., Quelch, R., & Przyborski, S. (2022). Recreating tissue structures representative of teratomas in vitro using a combination of 3D cell culture technology and human embryonic stem cells. Bioengineering, 9 (5), 185.
Nestor, M. W., Paull, D., Jacob, S., Sproul, A. A., Alsaffar, A., Campos, B. A., et al. (2013). Differentiation of serum-free embryoid bodies from human induced pluripotent stem cells into networks. Stem Cell Research, 10 (3), 454–463.
Smith, L. A., Hidalgo Aguilar, A., Owens, D. D. G., Quelch, R. H., Knight, E., Przyborski, S. A. (2021). Using advanced cell culture techniques to differentiate pluripotent stem cells and recreate tissue structures representative of teratoma xenografts. Frontiers in Cell and Developmental Biology, 9 . [Internet]. [cited 2021 May 14]. Available from: https://www.frontiersin.org/articles/ https://doi.org/10.3389/fcell.2021.667246/full
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131 (5), 861–872.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282 (5391), 1145–1147.
O’Connor, M. D., Kardel, M. D., & Eaves, C. J. (2011). Functional assays for human embryonic stem cell pluripotency. Methods in Molecular Biology (Clifton, NJ), 690 , 67–80.
Štefková, K., Procházková, J., & Pacherník, J. (2015). Stem Cells International . Alkaline Phosphatase in Stem Cells. [cited 2018 Jul 29]. Available from: https://www.hindawi.com/journals/sci/2015/628368/
O’Connor, M. D., Kardel, M. D., Iosfina, I., Youssef, D., Lu, M., Li, M. M., et al. (2008). Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells (Dayton, Ohio), 26 (5), 1109–1116.
Nethercott, H. E., Brick, D. J., & Schwartz, P. H. (2011). Immunocytochemical analysis of human pluripotent stem cells. Methods in Molecular Biology (Clifton, NJ), 767 , 201–220.
Brimble, S. N., Sherrer, E. S., Uhl, E. W., Wang, E., Kelly, S., Merrill, A. H., et al. (2007). The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells (Dayton, Ohio), 25 (1), 54–62.
Natunen, S., Satomaa, T., Pitkänen, V., Salo, H., Mikkola, M., Natunen, J., et al. (2011). The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency-associated type 1 lactosamine epitope. Glycobiology, 21 (9), 1125–1130.
Torres-Padilla, M. E., & Chambers, I. (2014). Transcription factor heterogeneity in pluripotent stem cells: A stochastic advantage. Development, 141 (11), 2173–2181.
Matic, I., Antunovic, M., Brkic, S., Josipovic, P., Mihalic, K. C., Karlak, I., et al. (2016). Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Macedonian Journal of Medical Sciences, 4 (1), 9–16.
Zangrossi, S., Marabese, M., Broggini, M., Giordano, R., D’Erasmo, M., Montelatici, E., et al. (2007). Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells, 25 (7), 1675–1680.
Ho, M. S, H., Fryga, A., & Laslett, A. L. (2011). Flow cytometric analysis of human pluripotent stem cells. In: Human pluripotent stem cells (p. 221–30). [Internet]. Humana Press. [cited 2018 Jul 29]. (Methods in Molecular Biology). Available from: https://link.springer.com/protocol/ https://doi.org/10.1007/978-1-61779-201-4_16
Aghaeepour, N., Finak, G., Hoos, H., Mosmann, T. R., Gottardo, R., Brinkman, R., et al. (2013). Critical assessment of automated flow cytometry data analysis techniques. Nature Methods, 10 (3), 228–238.
Lugli, E., Roederer, M., & Cossarizza, A. (2010). Data analysis in flow cytometry: The future just started. Cytometry Part A: Journal of Quantitative Cell Science, 77 (7), 705–713.
Lund, R. J., Nikula, T., Rahkonen, N., Närvä, E., Baker, D., Harrison, N., et al. (2012). High-throughput karyotyping of human pluripotent stem cells. Stem Cell Research, 9 (3), 192–195.
Yilmaz, A., Peretz, M., Aharony, A., Sagi, I., & Benvenisty, N. (2018). Defining essential genes for human pluripotent stem cells by CRISPR–Cas9 screening in haploid cells. Nature Cell Biology, 20 (5), 610.
Sperger, J. M., Chen, X., Draper, J. S., Antosiewicz, J. E., Chon, C. H., Jones, S. B., et al. (2003). Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proceedings of the National Academy of Sciences, 100 (23), 13350–13355.
Kolodziejczyk, A. A., Kim, J. K., Tsang, J. C. H., Ilicic, T., Henriksson, J., Natarajan, K. N., et al. (2015). Single cell RNA-sequencing of pluripotent states unlocks modular transcriptional variation. Cell Stem Cell, 17 (4), 471–485.
Nguyen, Q. H., Lukowski, S. W., Chiu, H. S., Senabouth, A., Bruxner, T. J. C., Christ, A. N., et al. (2018). Single-cell RNA-seq of human induced pluripotent stem cells reveals cellular heterogeneity and cell state transitions between subpopulations. Genome Research, 28 (7), 1053–1066.
Tsankov, A. M., Akopian, V., Pop, R., Chetty, S., Gifford, C. A., Daheron, L., et al. (2015). An improved ScoreCard to assess the differentiation potential of human pluripotent stem cells. Nature Biotechnology, 33 (11), 1182–1192.
Schmidt, M., Zeevaert, K., Elsafi Mabrouk, M. H., Goetzke, R., & Wagner, W. (2023). Epigenetic biomarkers to track differentiation of pluripotent stem cells. Stem Cell Reports, 18 (1), 145–158.
Beattie, G. M., Lopez, A. D., Bucay, N., Hinton, A., Firpo, M. T., King, C. C., et al. (2005). Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells (Dayton, Ohio), 23 (4), 489–495.
Eiselleova, L., Matulka, K., Kriz, V., Kunova, M., Schmidtova, Z., Neradil, J., et al. (2009). A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells (Dayton, Ohio), 27 (8), 1847–1857.
Schuldiner, M., Yanuka, O., Itskovitz-Eldor, J., Melton, D. A., & Benvenisty, N. (2000). Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 97 (21), 11307–11312.
Sheridan, S. D., Surampudi, V., & Rao, R. R. (2012). Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells International, 1 (2012), e738910.
Google Scholar
Itskovitz-Eldor, J., Schuldiner, M., Karsenti, D., Eden, A., Yanuka, O., Amit, M., et al. (2000). Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Molecular Medicine, 6 (2), 88–95.
Gothard, D., Roberts, S. J., Shakesheff, K. M., & Buttery, L. D. (2009). Controlled embryoid body formation via surface modification and avidin–biotin cross-linking. Cytotechnology, 61 (3), 135–144.
Van Winkle, A. P., Gates, I. D., & Kallos, M. S. (2012). Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells, Tissues, Organs, 196 (1), 34–47.
Wu, J., Rostami, M. R., Olaya, D. P. C., & Tzanakakis, E. S. (2014). Oxygen transport and stem cell aggregation in stirred-suspension bioreactor cultures. PLoS ONE, 9 (7), e102486.
Wesselschmidt, R. L. (2011). The teratoma assay: An in vivo assessment of pluripotency. Methods in Molecular Biology (Clifton, NJ), 767 , 231–241.
Stevens, L. C. (1970). The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Developmental Biology, 21 (3), 364–382.
Stevens, L. C. (1959). Embryology of testicular teratomas in strain 129 mice. JNCI Journal of the National Cancer Institute, 23 (6), 1249–1295.
CAS PubMed Google Scholar
Stevens, L. C., & Little, C. C. (1954). Spontaneous testicular teratomas in an inbred strain of mice. Proceedings of the National Academy of Sciences of the United States of America, 40 (11), 1080–1087.
Stevens, L. C., & Varnum, D. S. (1974). The development of teratomas from parthenogenetically activated ovarian mouse eggs. Developmental Biology, 37 (2), 369–380.
Cooke, M. J., Stojkovic, M., & Przyborski, S. A. (2006). Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells and Development, 15 (2), 254–259.
Hentze, H., Soong, P. L., Wang, S. T., Phillips, B. W., Putti, T. C., & Dunn, N. R. (2009). Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Research, 2 (3), 198–210.
Kothapalli, C. R., & Kamm, R. D. (2013). 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials, 34 (25), 5995–6007.
Shao, Y., Taniguchi, K., Townshend, R. F., Miki, T., Gumucio, D. L., & Fu, J. (2017). A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nature Communications, 8 (1), 208.
Olmer, R., Engels, L., Usman, A., Menke, S., Malik, M. N. H., Pessler, F., et al. (2018). Differentiation of human pluripotent stem cells into functional endothelial cells in scalable suspension culture. Stem Cell Reports, 10 (5), 1657–1672.
Wolfe, R. P., & Ahsan, T. (2013). Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnology and Bioengineering, 110 (4), 1231–1242.
Ramaiahgari, S. C., den Braver, M. W., Herpers, B., Terpstra, V., Commandeur, J. N. M., van de Water, B., et al. (2014). A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Archives of Toxicology, 88 (5), 1083-95.
Pazzano, D., Mercier, K. A., Moran, J. M., Fong, S. S., DiBiasio, D. D., Rulfs, J. X., et al. (2000). Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnology Progress, 16 (5), 893–896.
Strüver, K., Friess, W., & Hedtrich, S. (2017). Development of a perfusion platform for dynamic cultivation of in vitro skin models. Skin Pharmacology and Physiology, 30 (4), 180–189.
Gerlach, J. C., Hout, M., Edsbagge, J., Björquist, P., Lübberstedt, M., Miki, T., et al. (2010). Dynamic 3D culture promotes spontaneous embryonic stem cell differentiation in vitro. Tissue Engineering Part C: Methods, 16 (1), 115–121.
King, J. A., & Miller, W. M. (2007). Bioreactor development for stem cell expansion and controlled differentiation. Current Opinion in Chemical Biology, 11 (4), 394–398.
Stachelscheid, H., Wulf-Goldenberg, A., Eckert, K., Jensen, J., Edsbagge, J., Björquist, P., et al. (2013). Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. Journal of Tissue Engineering and Regenerative Medicine, 7 (9), 729–741.
Rivron, N. C., Frias-Aldeguer, J., Vrij, E. J., Boisset, J. C., Korving, J., Vivié, J., et al. (2018). Blastocyst-like structures generated solely from stem cells. Nature, 557 (7703), 106.
Mackinlay, K. M., Weatherbee, B. A., Souza Rosa, V., Handford, C. E., Hudson, G., Coorens, T., et al. (2021). An in vitro stem cell model of human epiblast and yolk sac interaction. eLife, 10 , e63930.
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biology, 18 (7), e3000410.
International Stem Cell Initiative. (2018). Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nature Communications, 9 (1), 1925.
Wang, Z., Zheng, J., Pan, R., & Chen, Y. (2021). Current status and future prospects of patient-derived induced pluripotent stem cells. Human Cell, 34 (6), 1601–1616.
Liang, G., & Zhang, Y. (2013). Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell, 13 (2), 149–159.
De Los, A. A., Ferrari, F., Xi, R., Fujiwara, Y., Benvenisty, N., Deng, H., et al. (2015). Hallmarks of pluripotency. Nature, 525 (7570), 469–478.
Andrews, P. W., Baker, D., Benvinisty, N., Miranda, B., Bruce, K., Brüstle, O., et al. (2015). Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regenerative Medicine, 10 (2 Suppl), 1–44.
Lee, M. O., Moon, S. H., Jeong, H. C., Yi, J. Y., Lee, T. H., Shim, S. H., et al. (2013). Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proceedings of the National Academy of Sciences, 110 (35), E3281–E3290.
Tao, H., Chen, X., Wei, A., Song, X., Wang, W., Liang, L., et al. (2018). Stem Cells International . Comparison of teratoma formation between embryonic stem cells and parthenogenetic embryonic stem cells by molecular imaging. [cited 2018 May 18]. Available from: https://www.hindawi.com/journals/sci/2018/7906531/
Priddle, H., Allegrucci, C., Burridge, P., Munoz, M., Smith, N. M., Devlin, L., et al. (2010). Derivation and characterisation of the human embryonic stem cell lines, NOTT1 and NOTT2. In Vitro Cellular and Developmental Biology-Animal, 46 (3–4), 367–375.
Brazma, A., Hingamp, P., Quackenbush, J., Sherlock, G., Spellman, P., Stoeckert, C., et al. (2001). Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nature Genetics, 29 (4), 365–371.
Beauchamp, P., Moritz, W., Kelm, J. M., Ullrich, N. D., Agarkova, I., Anson, B. D., et al. (2015). Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Engineering Part C: Methods, 21 (8), 852–861.
Forster, R., Chiba, K., Schaeffer, L., Regalado, S. G., Lai, C. S., Gao, Q., et al. (2014). Human intestinal tissue with adult stem cell properties derived from pluripotent stem cells. Stem Cell Reports, 2 (6), 838–852.
Gledhill, K., Guo, Z., Umegaki-Arao, N., Higgins, C. A., Itoh, M., & Christiano, A. M. (2015). Melanin transfer in human 3D skin equivalents generated exclusively from induced pluripotent stem cells. PLoS ONE, 10 (8), e0136713.
Gerlach, J. C., Lübberstedt, M., Edsbagge, J., Ring, A., Hout, M., Baun, M., et al. (2010). Interwoven four-compartment capillary membrane technology for three-dimensional perfusion with decentralized mass exchange to scale up embryonic stem cell culture. Cells, Tissues, Organs, 192 (1), 39–49.
Download references
This project was completed thanks to funding from The Anatomical Society (LS) and the Biotechnology and Biosciences Research Council (BB/K011405/1) (RQ) and the Consejo Nacional de Ciencia y Tecnología (AHA).
Author information
Authors and affiliations.
Department of Biosciences, Durham University, Durham, England
Lucy Smith, Rebecca Quelch-Cliffe, Felicity Liu, Alejandro Hidalgo Aguilar & Stefan Przyborski
Reprocell Europe Ltd, NETPark, Sedgefield, England
Stefan Przyborski
You can also search for this author in PubMed Google Scholar
Contributions
LS, RQ and FVPSL conducted the literature searches and compiled the data. LS completed the data analysis for the literature search. LS, RQ and AH performed the experimental work. LS, RQ, AH and SP composed and revised the manuscript. SP had oversight of the project.
Corresponding author
Correspondence to Stefan Przyborski .
Ethics declarations
Ethics approval, consent to participate, consent for publication, conflicts of interest.
Author SP collaborates and acts as a technical consultant for Reprocell Europe Ltd. The other authors declare no other conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Smith, L., Quelch-Cliffe, R., Liu, F. et al. Evaluating Strategies to Assess the Differentiation Potential of Human Pluripotent Stem Cells: A Review, Analysis and Call for Innovation. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10793-5
Download citation
Accepted : 23 September 2024
Published : 28 September 2024
DOI : https://doi.org/10.1007/s12015-024-10793-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pluripotent stem cells
- Pluripotency
- Differentiation
- Teratoma xenograft
- Cell culture technology
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Stem-cell research articles from across Nature Portfolio
Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells are the source of all tissues, understanding their properties helps in our understanding of the healthy and diseased body's development and homeostasis.
Latest Research and Reviews
p21 Regulates Wnt-Notch balance via DREAM/MMB/Rb-E2F1 and maintains intestinal stem cell homeostasis
- Liangxia Jiang
Heterogeneous expression of the atypical chemokine receptor ACKR3 in glioblastoma patient-derived tissue samples and cell cultures
- Amandine Kuppens
- Virginie Neirinckx
Outcomes of patients undergoing allogeneic haematopoietic stem cell transplantation for congenital amegakaryocytic thrombocytopenia; a study on behalf of the PDWP of the EBMT
- Clémence Aldebert
- Selim Corbacioglu

Post-thaw CD34+ cell recovery likely degraded under extreme graft platelet concentrations
- Gustavo C. Duarte
- Leandro Ladvanszky
- Wen-Hua Wei
Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges
- Yunlong Shan
- Mengying Zhang
- Guangji Wang
Oxidative stress and regulation of adipogenic differentiation capacity by sirtuins in adipose stem cells derived from female patients of advancing age
- Anne Bernhardt
- Maria Wartenberg
News and Comment
Impact of jacie accreditation on safety of patient care: healthcare providers perspective from a tertiary care stem cell transplant center in saudi arabia.
- Mohamed Bayoumy
- Ahlam Almasari
- Wasil Jastaniah

Wine without alcohol: medicine without doctors!
- Shaun R. McCann
Durable remission of refractory and advanced stage mycosis fungoides/sezary syndrome utilizing an “outpatient” alemtuzumab, fludarabine-based reduced intensity allogeneic hematopoietic cell transplantation
- Ramaprasad Srinivasan
- Richard Childs
Real-world outcomes of tandem ASCT in newly diagnosed multiple myeloma patients with standard risk features: a single-center analysis
- Andrea Poveda-García
- Estela Ruiz
- Valentín Cabañas
Unleashing the power of biomaterials to enhance organoid differentiation and function
Biomaterials are revolutionizing organoid development by offering tunable platforms that provide instructive cues, which enhance cell fate transitions, tissue-level functions and reproducibility. These advances are crucial for harnessing the translational potential of organoids.
- Samira Musah
- Hamidreza Arzaghi
The formation and maintenance of peri-implant fibrosis requires skeletal cells expressing the leptin receptor
The cellular composition of fibrous tissue around orthopaedic implants and the genetic pathways involved in its formation are unclear. We find that leptin-receptor-expressing cells activate the adhesion G-protein-coupled receptor F5 (ADGRF5) pathway to form peri-implant fibrous tissue. Inhibition of ADGRF5 in leptin-receptor-expressing cells can prevent and reverse peri-implant fibrosis.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Stem cells: past, present, and future
Affiliations.
- 1 Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland. [email protected].
- 2 Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland.
- 3 Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland.
- PMID: 30808416
- PMCID: PMC6390367
- DOI: 10.1186/s13287-019-1165-5
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Keywords: Differentiation; Growth media; Induced pluripotent stem cell (iPSC); Pluripotency; Stem cell derivation; Stem cells; Teratoma formation assay; Tissue banks; Tissue transplantation.
PubMed Disclaimer
Conflict of interest statement
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oocyte development and formation of…
Oocyte development and formation of stem cells: the blastocoel, which is formed from…
Changes in the potency of…
Changes in the potency of stem cells in human body development. Potency ranges…
Spontaneous differentiation of hESCs causes…
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There…
Culturing of pluripotent stem cells…
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells…
Retroviral-mediated transduction induces pluripotency in…
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their…
Stem cell experiments on animals.…
Stem cell experiments on animals. These experiments are one of the many procedures…
Localization of stem cells in…
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and…
Use of inner cell mass…
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate…
Similar articles
- Derivation of Human Induced Pluripotent Stem Cell (iPSC) Lines and Mechanism of Pluripotency: Historical Perspective and Recent Advances. Chhabra A. Chhabra A. Stem Cell Rev Rep. 2017 Dec;13(6):757-773. doi: 10.1007/s12015-017-9766-9. Stem Cell Rev Rep. 2017. PMID: 28918520 Review.
- Future prospects for regenerated heart using induced pluripotent stem cells. Fujita J, Fukuda K. Fujita J, et al. J Pharmacol Sci. 2014;125(1):1-5. doi: 10.1254/jphs.14r01cp. Epub 2014 Apr 16. J Pharmacol Sci. 2014. PMID: 24739283 Review.
- Induced pluripotent stem cells for the treatment of stroke: the potential and the pitfalls. Yu F, Li Y, Morshead CM. Yu F, et al. Curr Stem Cell Res Ther. 2013 Sep;8(5):407-14. doi: 10.2174/1574888x113089990052. Curr Stem Cell Res Ther. 2013. PMID: 23895059 Review.
- Integration of induced pluripotent stem cell-derived endothelial cells with polycaprolactone/gelatin-based electrospun scaffolds for enhanced therapeutic angiogenesis. Tan RP, Chan AHP, Lennartsson K, Miravet MM, Lee BSL, Rnjak-Kovacina J, Clayton ZE, Cooke JP, Ng MKC, Patel S, Wise SG. Tan RP, et al. Stem Cell Res Ther. 2018 Mar 21;9(1):70. doi: 10.1186/s13287-018-0824-2. Stem Cell Res Ther. 2018. PMID: 29562916 Free PMC article.
- 3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation. Gasparotto M, Bellet P, Scapin G, Busetto R, Rampazzo C, Vitiello L, Shah DI, Filippini F. Gasparotto M, et al. Int J Mol Sci. 2022 Feb 3;23(3):1736. doi: 10.3390/ijms23031736. Int J Mol Sci. 2022. PMID: 35163657 Free PMC article.
- Chondrogenic Potential of Umbilical Cord-Derived Mesenchymal Stromal Cells: Insights and Innovations. Jeyaraman N, Jeyaraman M, Muthu S, Balaji S, Ramasubramanian S, Patro BP. Jeyaraman N, et al. Indian J Orthop. 2024 Aug 24;58(10):1349-1361. doi: 10.1007/s43465-024-01239-8. eCollection 2024 Oct. Indian J Orthop. 2024. PMID: 39324097 Review.
- A Comprehensive Review of the Role of Stem Cells in Neuroregeneration: Potential Therapies for Neurological Disorders. Deokate N, Acharya S, Patil R, Shaikh SM, Karwa V. Deokate N, et al. Cureus. 2024 Aug 22;16(8):e67506. doi: 10.7759/cureus.67506. eCollection 2024 Aug. Cureus. 2024. PMID: 39310492 Free PMC article. Review.
- New method to induce neurotrophin gene expression in human adipose-derived stem cells in vitro . Altememy D, Kashani MHG, Fateme A, Khosravian P. Altememy D, et al. J Adv Pharm Technol Res. 2024 Jul-Sep;15(3):214-219. doi: 10.4103/JAPTR.JAPTR_390_23. Epub 2024 Jul 22. J Adv Pharm Technol Res. 2024. PMID: 39290551 Free PMC article.
- 3-D bioprinted human-derived skin organoids accelerate full-thickness skin defects repair. Zhang T, Sheng S, Cai W, Yang H, Li J, Niu L, Chen W, Zhang X, Zhou Q, Gao C, Li Z, Zhang Y, Wang G, Shen H, Zhang H, Hu Y, Yin Z, Chen X, Liu Y, Cui J, Su J. Zhang T, et al. Bioact Mater. 2024 Sep 4;42:257-269. doi: 10.1016/j.bioactmat.2024.08.036. eCollection 2024 Dec. Bioact Mater. 2024. PMID: 39285913 Free PMC article.
- Effect of placental mesenchymal stem cells on promoting the healing of chronic burn wounds. Xiao J, Zhang Q, Wu B, Wang M, Zhu Y, Zhao D, Zhao F, Xie Y. Xiao J, et al. Heliyon. 2024 Aug 22;10(17):e36584. doi: 10.1016/j.heliyon.2024.e36584. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281490 Free PMC article.
- Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58 - PubMed
- Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 . - PubMed
- Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; 10.2217/rme-2018-0095. - PubMed
- Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011;29(12):1121–1144. - PMC - PubMed
- Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Call for papers - Stem cell research
Guest editors.
Yun Chang, PhD, The Hong Kong Polytechnic University, China Xiaojun Lian, PhD, Pennsylvania State University, USA
Submission Status: Open | Submission Deadline: 25 June 2025

Articles will undergo the journal’s standard peer-review process and are subject to all of the journal’s standard policies . Articles will be added to the Collection as they are published.
The Editors have no competing interests with the submissions which they handle through the peer review process. The peer review of any submissions for which the Editors have competing interests is handled by another Editorial Board Member who has no competing interests.
NIH Stem Cell Information
Nih guidelines for human stem cell research.
SUMMARY: The National Institutes of Health (NIH) is hereby publishing final "National Institutes of Health Guidelines for Human Stem Cell Research" (Guidelines).
On March 9, 2009, President Barack H. Obama issued Executive Order 13505: Removing Barriers to Responsible Scientific Research Involving Human Stem Cells. The Executive Order states that the Secretary of Health and Human Services, through the Director of NIH, may support and conduct responsible, scientifically worthy human stem cell research, including human embryonic stem cell (hESC) research, to the extent permitted by law.
These Guidelines implement Executive Order 13505, as it pertains to extramural NIH-funded stem cell research, establish policy and procedures under which the NIH will fund such research, and helps ensure that NIH-funded research in this area is ethically responsible, scientifically worthy, and conducted in accordance with applicable law. Internal NIH policies and procedures, consistent with Executive Order 13505 and these Guidelines, will govern the conduct of intramural NIH stem cell research.
EFFECTIVE DATE: These Guidelines are effective on July 7, 2009.
SUMMARY OF PUBLIC COMMENTS ON DRAFT GUIDELINES: On April 23, 2009 the NIH published draft Guidelines for research involving hESCs in the Federal Register for public comment, 74 Fed. Reg. 18578 (April 23, 2009). The comment period ended on May 26, 2009.
The NIH received approximately 49,000 comments from patient advocacy groups, scientists and scientific societies, academic institutions, medical organizations, religious organizations, and private citizens. The NIH also received comments from members of Congress. This Notice presents the final Guidelines together with the NIH response to public comments that addressed provisions of the Guidelines.
Title of the Guidelines, Terminology, and Background :
Respondents felt the title of the NIH draft guidelines was misleading, in that it is entitled "National Institutes of Health Guidelines for Human Stem Cell Research," yet addresses only one type of human stem cell. The NIH notes that although the Guidelines pertain primarily to the donation of embryos for the derivation of hESCs, one Section also applies to certain uses of both hESCs and human induced pluripotent stem cells. Also, the Guidelines discuss applicable regulatory standards when research involving human adult stem cells or induced pluripotent stem cells constitutes human subject research. Therefore, the title of the Guidelines was not changed.
Respondents also disagreed with the definition of human embryonic stem cells in the draft Guidelines, and asked that the NIH define them as originating from the inner cell mass of the blastocyst. The NIH modified the definition to say that human embryonic stem cells "are cells that are derived from the inner cell mass of blastocyst stage human embryos, are capable of dividing without differentiating for a prolonged period in culture, and are known to develop into cells and tissues of the three primary germ layers."
Financial Gain
Respondents expressed concern that derivers of stem cells might profit from the development of hESCs. Others noted that because the stem cells eligible for use in research using NIH funding under the draft Guidelines are those cells that are subject to existing patents, there will be insufficient competition in the licensing of such rights. These respondents suggested that this could inhibit research, as well as increase the cost of any future clinical benefits. The Guidelines do not address the distribution of stem cell research material. It is, however, the NIH's expectation that stem cell research materials developed with NIH funds, as well as associated intellectual property and data, will be distributed in accordance with the NIH’s existing policies and guidance, including "Sharing Biomedical Research Resources, Principles and Guidelines for Recipients of NIH Grants and Contracts" and "Best Practices for the Licensing of Genomic Inventions." http://www.ott.nih.gov/policy/policies_and_guidelines.aspx Even where such policies are not directly applicable, the NIH encourages others to refrain from imposing on the transfer of research tools, such as stem cells, any conditions that hinder further biomedical research. In addition, the Guidelines were revised to state that there should be documentation that "no payments, cash or in kind, were offered for the donated embryos."
Respondents were concerned that donor(s) be clearly "apprised up front by any researchers that financial gain may come from the donation and that the donor(s) should know up front if he/she will share in the financial gain." The Guidelines address this concern by asking that donor(s) was/were informed during the consent process that the donation was made without any restriction or direction regarding the individual(s) who may receive medical benefit from the use of the stem cells, such as who may be the recipients of cell transplants. The Guidelines also require that the donor(s) receive(s) information that the research was not intended to provide direct medical benefit to the donor(s); that the results of research using the hESCs may have commercial potential, and that the donor(s) would not receive financial or any other benefits from any such commercial development.
IRB Review under the Common Rule
Respondents suggested that the current regulatory structure of IRB review under the Common Rule (45 C.F.R. Part 46, Subpart A) addresses the core ethical principles needed for appropriate oversight of hESC derivation. They noted that IRB review includes a full review of the informed consent process, as well as a determination of whether individuals were coerced to participate in the research and whether any undue inducements were offered to secure their participation. These respondents urged the NIH to replace the specific standards to assure voluntary and informed consent in the draft Guidelines with a requirement that hESC research be reviewed and approved by an IRB, in conformance with 45 C.F.R. Part 46, Subpart A, as a prerequisite to NIH funding. Respondents also requested that the NIH create a registry of eligible hESC lines to avoid burdensome and repetitive assurances from multiple funding applicants. The NIH agrees that the IRB system of review under the Common Rule provides a comprehensive framework for the review of the donation of identifiable human biological materials for research. However, in the last several years, guidelines on hESC research have been issued by a number of different organizations and governments, and different practices have arisen around the country and worldwide, resulting in a patchwork of standards. The NIH concluded that employing the IRB review system for the donation of embryos would not ameliorate stated concerns about variations in standards for hESC research and would preclude the establishment of an NIH registry of hESCs eligible for NIH funding, because there would be no NIH approval of particular hESCs. To this end and response to comments, these Guidelines articulate policies and procedures that will allow the NIH to create a Registry. These Guidelines also provide scientists who apply for NIH funding with a specific set of standards reflecting currently recognized ethical principles and practices specific to embryo donation that took place on or after the issuance of the Guidelines, while also establishing procedures for the review of donations that took place before the effective date of the Guidelines.
Federal Funding Eligibility of Human Pluripotent Cells from Other Sources
Respondents suggested that the allowable sources of hESCs potentially available for federal funding be expanded to include hESC lines from embryos created expressly for research purposes, and lines created, or pluripotent cells derived, following parthenogenesis or somatic cell nuclear transfer (SCNT). The Guidelines allow for funding of research using hESCs derived from embryos created using in vitro fertilization (IVF) for reproductive purposes and no longer needed for these purposes, assuming the research has scientific merit and the embryos were donated after proper informed consent was obtained from the donor(s). The Guidelines reflect the broad public support for federal funding of research using hESCs created from such embryos based on wide and diverse debate on the topic in Congress and elsewhere. The use of additional sources of human pluripotent stem cells proposed by the respondents involve complex ethical and scientific issues on which a similar consensus has not emerged. For example, the embryo-like entities created by parthenogenesis and SCNT require women to donate oocytes, a procedure that has health and ethical implications, including the health risk to the donor from the course of hormonal treatments needed to induce oocyte production.
Respondents noted that many embryos undergo Pre-implantation Genetic Diagnosis (PGD). This may result in the identification of chromosomal abnormalities that would make the embryos medically unsuitable for clinical use. In addition, the IVF process may also produce embryos that are not transferred into the uterus of a woman because they are determined to be not appropriate for clinical use. Respondents suggested that hESCs derived from such embryos may be extremely valuable for scientific study, and should be considered embryos that were created for reproductive purposes and were no longer needed for this purpose . The NIH agrees with these comments. As in the draft, the final Guidelines allow for the donation of embryos that have undergone PGD.
Donation and Informed Consent
Respondents commented in numerous ways that the draft Guidelines are too procedurally proscriptive in articulating the elements of appropriate informed consent documentation. This over-reliance on the specific details and format of the informed consent document, respondents argued, coupled with the retroactive application of the Guidelines to embryos already donated for research, would result in a framework that fails to appreciate the full range of factors contributing to the complexity of the informed consent process. For example, respondents pointed to several factors that were precluded from consideration by the proposed Guidelines, such as contextual evidence of the consent process, other established governmental frameworks (representing local and community influences), and the changing standards for informed consent in this area of research over time. Respondents argued that the Guidelines should be revised to allow for a fuller array of factors to be considered in determining whether the underlying ethical principle of voluntary informed consent had been met. In addition to these general issues, many respondents made the specific recommendation that all hESCs derived before the final Guidelines were issued be automatically eligible for Federal funding without further review, especially those eligible under prior Presidential policy, i.e., "grandfathered." The final Guidelines seek to implement the Executive Order by issuing clear guidance to assist this field of science to advance and reach its full potential while ensuring adherence to strict ethical standards. To this end, the NIH is establishing a set of conditions that will maximize ethical oversight, while ensuring that the greatest number of ethically derived hESCs are eligible for federal funding. Specifically, for embryos donated in the U.S. on or after the effective date of the Guidelines, the only way to establish eligibility will be to either use hESCs listed on the NIH Registry, or demonstrate compliance with the specific procedural requirements of the Guidelines by submitting an assurance with supporting information for administrative review by the NIH. Thus, for future embryo donations in the United States, the Guidelines articulate one set of procedural requirements. This responds to concerns regarding the patchwork of requirements and guidelines that currently exist.
However, the NIH is also cognizant that in the more than a decade between the discovery of hESCs and today, many lines were derived consistent with ethical standards and/or guidelines developed by various states, countries, and other entities such as the International Society for Stem Cell Research (ISSCR) and the National Academy of Sciences (NAS). These various policies have many common features, rely on a consistent ethical base, and require an informed consent process, but they differ in details of implementation. For example, some require specific wording in a written informed consent document, while others do not. It is important to recognize that the principles of ethical research, e.g., voluntary informed consent to participation, have not varied in this time period, but the requirements for implementation and procedural safeguards employed to demonstrate compliance have evolved. In response to these concerns, the Guidelines state that applicant institutions wishing to use hESCs derived from embryos donated prior to the effective date of the Guidelines may either comply with Section II (A) of the Guidelines or undergo review by a Working Group of the Advisory Committee to the Director (ACD). The ACD, which is a chartered Federal Advisory Committee Act (FACA) committee, will advise NIH on whether the core ethical principles and procedures used in the process for obtaining informed consent for the donation of the embryo were such that the cell line should be eligible for NIH funding. This Working Group will not undertake a de novo evaluation of ethical standards, but will consider the materials submitted in light of the principles and points to consider in the Guidelines, as well as 45 C.F.R. Part 46 Subpart A. Rather than “grandfathering,” ACD Working Group review will enable pre-existing hESCs derived in a responsible manner to be eligible for use in NIH funded research.
In addition, for embryos donated outside the United States prior to the effective date of these Guidelines, applicants may comply with either Section II (A) or (B). For embryos donated outside of the United States on or after the effective date of the Guidelines, applicants seeking to determine eligibility for NIH research funding may submit an assurance that the hESCs fully comply with Section II (A) or submit an assurance along with supporting information, that the alternative procedural standards of the foreign country where the embryo was donated provide protections at least equivalent to those provided by Section II (A) of these Guidelines. These materials will be reviewed by the NIH ACD Working Group, which will recommend to the ACD whether such equivalence exists. Final decisions will be made by the NIH Director. This special consideration for embryos donated outside the United States is needed because donation of embryos in foreign countries is governed by the laws and policies of the respective governments of those nations. Although such donations may be responsibly conducted, such governments may not or cannot change their national donation requirements to precisely comply with the NIH Guidelines. The NIH believes it is reasonable to provide a means for reviewing such hESCs because ethically derived foreign hESCs constitute an important scientific asset for the U.S.
Respondents expressed concern that it might be difficult in some cases to provide assurance that there was a "clear separation" between the prospective donor(s)’ decision to create embryos for reproductive purposes and the donor(s)’ decision to donate the embryos for research purposes. These respondents noted that policies vary at IVF clinics, especially with respect to the degree to which connections with researchers exist. Respondents noted that a particular clinic’s role may be limited to the provision of contact information for researchers. A clinic that does not have any particular connection with research would not necessarily have in place a written policy articulating the separation contemplated by the Guidelines. Other respondents noted that embryos that are determined not to be suitable for medical purposes, either because of genetic defects or other concerns, may be donated prior to being frozen. In these cases, it is possible that the informed consent process for the donation might be concurrent with the consent process for IVF treatment. Respondents also noted that the initial consent for IVF may contain a general authorization for donating embryos in excess of clinical need, even though a more detailed consent is provided at the actual time of donation. The NIH notes that the Guidelines specifically state that consent should have been obtained at the time of donation, even if the potential donor(s) had given prior indication of a general intent to donate embryos in excess of clinical need for the purposes of research. Accordingly, a general authorization for research donation when consenting for reproductive treatment would comply with the Guidelines, so long as specific consent for the donation is obtained at the time of donation. In response to comments regarding documentation necessary to establish a separation between clinical and research decisions, the NIH has changed the language of the Guidelines to permit applicant institutions to submit consent forms, written policies or other documentation to demonstrate compliance with the provisions of the Guidelines. This change should provide the flexibility to accommodate a range of practices, while adhering to the ethical principles intended.
Some respondents want to require that the IVF physician and the hESC researcher should be different individuals, to prevent conflict of interest. Others say they should be the same person, because people in both roles need to have detailed knowledge of both areas (IVF treatment and hESC research). There is also a concern that the IVF doctor will create extra embryos if he/she is also the researcher. As a general matter, the NIH believes that the doctor and the researcher seeking donation should be different individuals. However, this is not always possible, nor is it required, in the NIH's view, for ethical donation.
Some respondents want explicit language (in the Guidelines and/or in the consent) stating that the embryo will be destroyed when the inner cell mass is removed. In the process of developing guidelines, the NIH reviewed a variety of consent forms that have been used in responsible derivations. Several had extensive descriptions of the process and the research to be done, going well beyond the minimum expected, yet they did not use these exact words. Given the wide variety and diversity of forms, as well as the various policy, statutory and regulatory obligations individual institutions face, the NIH declines to provide exact wording for consent forms, and instead endorses a robust informed consent process where all necessary details are explained and understood in an ongoing, trusting relationship between the clinic and the donor(s).
Respondents asked for clarification regarding the people who must give informed consent for the donation of embryos for research. Some commenters suggested that NIH should require consent from the gamete donors, in cases where those individuals may be different than the individuals seeking reproductive treatment. The NIH requests consent from “the individual(s) who sought reproductive treatment” because this/these individual(s) is/are responsible for the creation of the embryo(s) and, therefore, its/their disposition. With regard to gamete donation, the risks are associated with privacy and, as such, are governed by requirements of the Common Rule, where applicable.
Respondents also requested clarification on the statement in the draft Guidelines noting that "although human embryonic stem cells are derived from embryos, such stem cells are not themselves human embryos." For the purpose of NIH funding, an embryo is defined by Section 509, Omnibus Appropriations Act, 2009, Pub. L. 111-8, 3/11/09, otherwise known as the Dickey Amendment, as any organism not protected as a human subject under 45 C.F.R. Part 46 that is derived by fertilization, parthenogenesis, cloning or any other means from one or more human gametes or human diploid cells. Since 1999, the Department of Health and Human Services (HHS) has consistently interpreted this provision as not applicable to research using hESCs, because hESCs are not embryos as defined by Section 509. This long-standing interpretation has been left unchanged by Congress, which has annually reenacted the Dickey Amendment with full knowledge that HHS has been funding hESC research since 2001. These guidelines therefore recognize the distinction, accepted by Congress, between the derivation of stem cells from an embryo that results in the embryo’s destruction, for which federal funding is prohibited, and research involving hESCs that does not involve an embryo nor result in an embryo’s destruction, for which federal funding is permitted.
Some respondents wanted to ensure that potential donor(s) are either required to put their "extra" embryos up for adoption before donating them for research, or are at least offered this option. The Guidelines require that all the options available in the health care facility where treatment was sought pertaining to the use of embryos no longer needed for reproductive purposes were explained to the potential donor(s). Since not all IVF clinics offer the same services, the healthcare facility is only required to explain the options available to the donor(s) at that particular facility.
Commenters asked that donor(s) be made aware of the point at which their donation decision becomes irrevocable. This is necessary because if the embryo is de-identified, it may be impossible to stop its use beyond a certain point. The NIH agrees with these comments and revised the Guidelines to require that donor(s) should have been informed that they retained the right to withdraw consent for the donation of the embryo until the embryos were actually used to derive embryonic stem cells or until information which could link the identity of the donor(s) with the embryo was no longer retained, if applicable.
Medical Benefits of Donation
Regarding medical benefit, respondents were concerned that the language of the Guidelines should not somehow eliminate a donor's chances of benefitting from results of stem cell research. Respondents noted that although hESCs are not currently being used clinically, it is possible that in the future such cells might be used for the medical benefit of the person donating them. The Guidelines are meant to preclude individuals from donating embryos strictly for use in treating themselves only or from donating but identifying individuals or groups they do or do not want to potentially benefit from medical intervention using their donated cells. While treatment with hESCs is one of the goals of this research, in practice, years of experimental work must still be done before such treatment might become routinely available. The Guidelines are designed to make it clear that immediate medical benefit from a donation is highly unlikely at this time. Importantly, it is critical to note that the Guidelines in no way disqualify a donor from benefitting from the medical outcomes of stem cell research and treatments that may be developed in the future.
Monitoring and Enforcement Actions
Respondents have expressed concern about the monitoring of funded research and the invocation of possible penalties for researchers who do not follow the Guidelines. A grantee's failure to comply with the terms and conditions of award, including confirmed instances of research misconduct, may cause the NIH to take one or more enforcement actions, depending on the severity and duration of the non-compliance. For example, the following actions may be taken by the NIH when there is a failure to comply with the terms and conditions of any award: (1) Under 45 CFR 74.14, the NIH can impose special conditions on an award, including but not limited to increased oversight/monitoring/reporting requirements for an institution, project, or investigator; and (2) under 45 CFR 74.62 the NIH may impose enforcement actions, including but not limited to withholding funds pending correction of the problem, disallowing all or part of the costs of the activity that was not in compliance, withholding further awards for the project, or suspending or terminating all or part of the funding for the project. Individuals and institutions may be debarred from eligibility for all Federal financial assistance and contracts under 2 CFR Part 376 and 48 CFR Subpart 9.4, respectively. The NIH will undertake all enforcement actions in accordance with applicable statutes, regulations, and policies.
National Institutes of Health Guidelines for Research Using Human Stem Cells
These Guidelines apply to the expenditure of National Institutes of Health (NIH) funds for research using human embryonic stem cells (hESCs) and certain uses of induced pluripotent stem cells (See Section IV). The Guidelines implement Executive Order 13505.
Long-standing HHS regulations for Protection of Human Subjects, 45 C.F.R. 46, Subpart A establish safeguards for individuals who are the sources of many human tissues used in research, including non-embryonic human adult stem cells and human induced pluripotent stem cells. When research involving human adult stem cells or induced pluripotent stem cells constitutes human subject research, Institutional Review Board review may be required and informed consent may need to be obtained per the requirements detailed in 45 C.F.R. 46, Subpart A. Applicants should consult http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html .
It is also important to note that the HHS regulation, Protection of Human Subjects , 45 C.F.R. Part 46, Subpart A, may apply to certain research using hESCs. This regulation applies, among other things, to research involving individually identifiable private information about a living individual, 45 C.F.R. § 46.102(f). The HHS Office for Human Research Protections (OHRP) considers biological material, such as cells derived from human embryos, to be individually identifiable when they can be linked to specific living individuals by the investigators either directly or indirectly through coding systems. Thus, in certain circumstances, IRB review may be required, in addition to compliance with these Guidelines. Applicant institutions are urged to consult OHRP guidances at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html
To ensure that the greatest number of responsibly derived hESCs are eligible for research using NIH funding, these Guidelines are divided into several sections, which apply specifically to embryos donated in the U.S. and foreign countries, both before and on or after the effective date of these Guidelines. Section II (A) and (B) describe the conditions and review processes for determining hESC eligibility for NIH funds. Further information on these review processes may be found at www.NIH.gov . Sections IV and V describe research that is not eligible for NIH funding.
These guidelines are based on the following principles:
- Responsible research with hESCs has the potential to improve our understanding of human health and illness and discover new ways to prevent and/or treat illness.
- Individuals donating embryos for research purposes should do so freely, with voluntary and informed consent.
As directed by Executive Order 13505, the NIH shall review and update these Guidelines periodically, as appropriate.
For the purpose of these Guidelines, "human embryonic stem cells (hESCs)" are cells that are derived from the inner cell mass of blastocyst stage human embryos, are capable of dividing without differentiating for a prolonged period in culture, and are known to develop into cells and tissues of the three primary germ layers. Although hESCs are derived from embryos, such stem cells are not themselves human embryos. All of the processes and procedures for review of the eligibility of hESCs will be centralized at the NIH as follows:
- that were created using in vitro fertilization for reproductive purposes and were no longer needed for this purpose;
- that were donated by individuals who sought reproductive treatment (hereafter referred to as "donor(s)") and who gave voluntary written consent for the human embryos to be used for research purposes; and
- All options available in the health care facility where treatment was sought pertaining to the embryos no longer needed for reproductive purposes were explained to the individual(s) who sought reproductive treatment.
- No payments, cash or in kind, were offered for the donated embryos.
- Policies and/or procedures were in place at the health care facility where the embryos were donated that neither consenting nor refusing to donate embryos for research would affect the quality of care provided to potential donor(s).
- Decisions related to the creation of human embryos for reproductive purposes should have been made free from the influence of researchers proposing to derive or utilize hESCs in research. The attending physician responsible for reproductive clinical care and the researcher deriving and/or proposing to utilize hESCs should not have been the same person unless separation was not practicable.
- At the time of donation, consent for that donation should have been obtained from the individual(s) who had sought reproductive treatment. That is, even if potential donor(s) had given prior indication of their intent to donate to research any embryos that remained after reproductive treatment, consent for the donation for research purposes should have been given at the time of the donation.
- Donor(s) should have been informed that they retained the right to withdraw consent for the donation of the embryo until the embryos were actually used to derive embryonic stem cells or until information which could link the identity of the donor(s) with the embryo was no longer retained, if applicable.
- that the embryos would be used to derive hESCs for research;
- what would happen to the embryos in the derivation of hESCs for research;
- that hESCs derived from the embryos might be kept for many years;
- that the donation was made without any restriction or direction regarding the individual(s) who may receive medical benefit from the use of the hESCs, such as who may be the recipients of cell transplants.;
- that the research was not intended to provide direct medical benefit to the donor(s);
- that the results of research using the hESCs may have commercial potential, and that the donor(s) would not receive financial or any other benefits from any such commercial development;
- whether information that could identify the donor(s) would be available to researchers.
- By complying with Section II (A) of the Guidelines; or
The materials submitted must demonstrate that the hESCs were derived from human embryos: 1) that were created using in vitro fertilization for reproductive purposes and were no longer needed for this purpose; and 2) that were donated by donor(s) who gave voluntary written consent for the human embryos to be used for research purposes.
The Working Group will review submitted materials, e.g., consent forms, written policies or other documentation, taking into account the principles articulated in Section II (A), 45 C.F.R. Part 46, Subpart A, and the following additional points to consider. That is, during the informed consent process, including written or oral communications, whether the donor(s) were: (1) informed of other available options pertaining to the use of the embryos; (2) offered any inducements for the donation of the embryos; and (3) informed about what would happen to the embryos after the donation for research.
- For embryos donated outside the United States before the effective date of these Guidelines, applicants may comply with either Section II (A) or (B). For embryos donated outside of the United States on or after the effective date of the Guidelines, applicants seeking to determine eligibility for NIH research funding may submit an assurance that the hESCs fully comply with Section II (A) or submit an assurance along with supporting information, that the alternative procedural standards of the foreign country where the embryo was donated provide protections at least equivalent to those provided by Section II (A) of these Guidelines. These materials will be reviewed by the NIH ACD Working Group , which will recommend to the ACD whether such equivalence exists. Final decisions will be made by the NIH Director.
- NIH will establish a new Registry listing hESCs eligible for use in NIH funded research. All hESCs that have been reviewed and deemed eligible by the NIH in accordance with these Guidelines will be posted on the new NIH Registry.
Prior to the use of NIH funds, funding recipients should provide assurances, when endorsing applications and progress reports submitted to NIH for projects using hESCs, that the hESCs are listed on the NIH registry.
This section governs research using hESCs and human induced pluripotent stem cells, i.e., human cells that are capable of dividing without differentiating for a prolonged period in culture, and are known to develop into cells and tissues of the three primary germ layers. Although the cells may come from eligible sources, the following uses of these cells are nevertheless ineligible for NIH funding, as follows:
- Research in which hESCs (even if derived from embryos donated in accordance with these Guidelines) or human induced pluripotent stem cells are introduced into non-human primate blastocysts.
- Research involving the breeding of animals where the introduction of hESCs (even if derived from embryos donated in accordance with these Guidelines) or human induced pluripotent stem cells may contribute to the germ line.
- NIH funding of the derivation of stem cells from human embryos is prohibited by the annual appropriations ban on funding of human embryo research (Section 509, Omnibus Appropriations Act, 2009, Pub. L. 111-8, 3/11/09), otherwise known as the Dickey Amendment.
- Research using hESCs derived from other sources, including somatic cell nuclear transfer, parthenogenesis, and/or IVF embryos created for research purposes, is not eligible for NIH funding.
Raynard S Kington, M.D., Ph.D. Acting Director, NIH

Stem Cell Research
Lab resource: stem cell line, last update 5 february 2020.
This Special Issue collates Lab Resource articles published in Stem Cell Research. Lab Resource articles detail the establishment of new pluripotent stem cell lines derived from embryos, generated by SCNT and reprogramming or the establishment of genetically modified stem cell sub-lines. For more information on requirements and submission of this article type please go to https://www.journals.elsevier.com/stem-cell-research/lab-resources
Actions for selected articles
Select all / Deselect all
Derivation of the human embryonic stem cell line RCe006-A (RC-2)
Generation of spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cell line sca3.a11, derivation of the human embryonic stem cell line rce007-a (rc-3), generation of spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cell line sca3.b11, derivation of hybrid es cell lines from two different strains of mice, genomic imprinting defect in zfp57 mutant ips cell lines, generation of induced pluripotent stem cells (ipscs) from an alzheimer's disease patient carrying a m146i mutation in psen1, generation of induced pluripotent stem cells (ipscs) from an alzheimer's disease patient carrying an a79v mutation in psen1, induced pluripotent stem cells derived from bernard-soulier syndrome patient's peripheral blood cells with a p.phe55ser mutation in the gpix gene, a feeder- and xeno-free human induced pluripotent stem cell line obtained from primary human dermal fibroblasts with epigenetic repression of reprogramming factors expression: gpcci001-a, generation of sibling-matched induced pluripotent stem cell lines from spinal and bulbar muscular atrophy patients, generation of an induced pluripotent stem cell line that mimics the disease phenotypes from a patient with fanconi anemia by conditional complementation, generation and characterization of two ipsc lines from human epicardium-derived cells, reporting on methods to generate and purify rodent and human oligodendrocytes from different sources, generation of human induced pluripotent stem cell lines from human dermal fibroblasts using a modified rna system, generation of an ipsc line from a patient with gtp cyclohydrolase 1 ( gch1 ) deficiency: hdmc0061i-gch1, generation of ipsc line epihuvec from human umbilical vein endothelial cells, generation of poikiloderma with neutropenia (pn) induced pluripotent stem cells (ipscs), reprogramming of mouse amniotic fluid cells using a piggybac transposon system, generation and characterization of the human ipsc line pbmc1-ips4f1 from adult peripheral blood mononuclear cells, derivation of genea015 human embryonic stem cell line, derivation of genea016 human embryonic stem cell line, derivation of genea043 human embryonic stem cell line, derivation of genea042 human embryonic stem cell line, derivation of genea047 human embryonic stem cell line, derivation of genea057 human embryonic stem cell line, derivation of genea052 human embryonic stem cell line, episomal plasmid-based generation of induced pluripotent stem cells from fetal femur-derived human mesenchymal stromal cells, generation and characterization of human ipsc lines derived from a primary hyperoxaluria type i patient with p.i244t mutation, generation of induced pluripotent stem cells (ipscs) from an alzheimer's disease patient carrying a l150p mutation in psen-1, generation of a human ipsc line from a patient with a defect of intergenomic communication, lymphoblast-derived integration-free ips cell line from a 65-year-old alzheimer's disease patient expressing the trem2 p.r47h variant, generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using an integration free vector, generation of a human ipsc line from a patient with a mitochondrial encephalopathy due to mutations in the gfm1 gene, derivation of the human embryonic stem cell line rcm1, withdrawn: generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using integration free sendai technology, generation of human embryonic stem cell line expressing green fluorescent protein, generation of kcl037 clinical grade human embryonic stem cell line, generation of kcl038 clinical grade human embryonic stem cell line, generation of human ipsc line grx-mcips4f-a2 from adult peripheral blood mononuclear cells (pbmcs) with spanish genetic background, induced pluripotent stem cells (ipscs) derived from frontotemporal dementia patient's peripheral blood mononuclear cells, human amniotic epithelial cells as feeder layer to derive and maintain human embryonic stem cells from poor-quality embryos, derivation of lif-independent mouse ips cells with modified oct4, derivation of genea002 human embryonic stem cell line, generation of an ips cell line from bone marrow derived mesenchymal stromal cells from an elderly patient, development of buffalo ( bubalus bubalis ) embryonic stem cell lines from somatic cell nuclear transferred blastocysts, generation of ipsc lines from primary human chorionic villi cells, human embryonic stem cells derived from abnormal blastocyst donated by marfan syndrome patient, generation of ipsc lines from a nijmegen breakage syndrome patient, generation of ipsc lines from primary human amniotic fluid cells, generation of human control ips cell line chopwt9 from healthy adult peripheral blood mononuclear cells, transgene-free human induced pluripotent stem cell line (hs5-sv.hips) generated from cesarean scar-derived fibroblasts, generation of kcl032 clinical grade human embryonic stem cell line, lymphoblast-derived integration-free ips cell line from a 69-year-old male, generation of integration free induced pluripotent stem cells from fibrodysplasia ossificans progressiva (fop) patients from urine samples, human embryonic stem cells derived from abnormal blastocyst donated by glucose-6-phosphate dehydrogenase deficiency patient, generation of a human ipsc line from a patient with leigh syndrome, induced pluripotent stem cells (ipscs) derived from a patient with frontotemporal dementia caused by a r406w mutation in microtubule-associated protein tau (mapt), induced pluripotent stem cells (ipscs) derived from a pre-symptomatic carrier of a r406w mutation in microtubule-associated protein tau (mapt) causing frontotemporal dementia, generation of ipsc line ipsc-fh2.1 in hypoxic conditions from human foreskin fibroblasts, generation of spinocerebellar ataxia type 2 patient-derived ipsc line h271, generation of an isogenic, gene-corrected control cell line of the spinocerebellar ataxia type 2 patient-derived ipsc line h271, generation of spinocerebellar ataxia type 2 patient-derived ipsc line h266, derivation of human embryonic stem cell from spinal muscular atrophy patient, generation of an isogenic, gene-corrected control cell line of the spinocerebellar ataxia type 2 patient-derived ipsc line h196, generation of transgenic human embryonic stem cell line bjnhem20–ociad1-ov, generation of kcl031 clinical grade human embryonic stem cell line, generation of kcl034 clinical grade human embryonic stem cell line, generation of kcl040 clinical grade human embryonic stem cell line, generation of kcl039 clinical grade human embryonic stem cell line, generation of a heterozygous knockout human embryonic stem cell line for the ociad1 locus using crispr/cas9 mediated targeting: bjnhem20-ociad1-crispr-39, induced pluripotent stem cells (ipscs) derived from a patient with frontotemporal dementia caused by a p301l mutation in microtubule-associated protein tau (mapt), generation of ipsc line hel24.3 from human neonatal foreskin fibroblasts, generation of ipsc line hel47.2 from healthy human adult fibroblasts, derivation of human induced pluripotent stem cells through continued exposure of oct4-induced plastic human fibroblasts to reprogramming media, generation and characterization of human ipsc line generated from mesenchymal stem cells derived from adipose tissue, generation of kcl016 research grade human embryonic stem cell line carrying a mutation in vhl gene, episomal-based generation of an ips cell line from human fetal foreskin fibroblasts, generation of a human control ipsc line with a european mitochondrial haplogroup u background, generation of human ips cell line ihfib3.2 from dermal fibroblasts, generation of ipsc line mu011.a-hips from homozygous α-thalassemia fetal skin fibroblasts, generation of integration-free induced pluripotent stem cells from a patient with familial mediterranean fever (fmf), derivation of huntington disease affected genea090 human embryonic stem cell line, generation of kcl036 research grade human embryonic stem cell line carrying a mutation in the htt gene, generation of an induced pluripotent stem cell line from a patient with hereditary multiple endocrine neoplasia 2a (men2a) syndrome with ret mutation, murine transgenic ips cell line for monitoring and selection of cardiomyocytes, generation of an induced pluripotent stem cell line from a patient with chronic myeloid leukemia (cml) resistant to targeted therapies, generation of a gene-corrected isogenic control cell line from an alzheimer's disease patient ipsc line carrying a a79v mutation in psen1, integration-free erythroblast-derived human induced pluripotent stem cells (ipscs) from an individual with ataxia-telangiectasia (a-t), generation of a human induced pluripotent stem cell (ipsc) line from a patient carrying a p33t mutation in the pdx1 gene, generation of a human induced pluripotent stem cell (ipsc) line from a patient with family history of diabetes carrying a c18r mutation in the pdx1 gene, induced pluripotent stem cells (ipsc) created from skin fibroblasts of patients with prader-willi syndrome (pws) retain the molecular signature of pws, generation of hexa -deficient hipscs from fibroblasts of a tay-sachs disease patient, generation of induced pluripotent stem cells (ipscs) from a retinoblastoma patient carrying a c.2663g > a mutation in rb1 gene, generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using integration free sendai virus technology, a human ve-cadherin-tdtomato and cd43-green fluorescent protein dual reporter cell line for study endothelial to hematopoietic transition, generation, genome edition and characterization of ipsc lines from a patient with coenzyme q 10 deficiency harboring a heterozygous mutation in coq4 gene, generation of a tle1 homozygous knockout human embryonic stem cell line using crispr-cas9, generation of a tle3 heterozygous knockout human embryonic stem cell line using crispr-cas9, generation of induced pluripotent stem cells (ipscs) from a hereditary spastic paraplegia patient carrying a homozygous y275x mutation in cyp7b1 (spg5).
ISSN: 1873-5061
Copyright © 2024 Elsevier B.V. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Mice and stem cell research

What are stem cells and why are they important?

Where are they collected from?
Stem cells are found at all stage in life, but for practical purposes are regarded as being from three stages of development: early embryos, fetuses, and adults. Scientists collect embryonic stem cells from the inner layer of early embryos, usually of rodents, when they are about five days old and consist of a double-layered ball of cells. These embryos can be created using test tube (in-vitro) fertilisation. The cells can be implanted into other animals, even of a different species, without being rejected by the body's immune system. Fetal stem cells are collected from blood taken from the placental end of the umbilical cord. There is thus a plentiful supply of cells that are normally discarded. Although each sample is relatively small, it is possible to make the stem cells contained therein multiply. Adult stem cells are harder to find, with the notable exception of bone marrow cells that give rise to the different kinds of blood cells. Bone marrow stem cells can be induced to proliferate by injecting a growth factor. This makes them spill over into the bloodstream, from where they are easily collected. Skin stem cells have been isolated in mice, and have been used to grow new skin in recipient mice. Embryonic stem cells give rise to any of the 216 kinds of cell in the body, but adult stem cells are more specialised—for example, bone marrow stem cells normally only form blood cells. However, they seem to 'change direction' (transdifferentiate) when injected into other tissues such as heart and brain, and some research is aimed at growing stem cells in the laboratory and 'taking back' (dedifferentiating) adult stem cells to become more versatile and less able to provoke an immune reaction. We know that stem cells from adult mice, when grown in mouse or chick embryos, revert to an unspecialised state and become identical to the surrounding tissue, depending on which cell layer they are injected into. The extent to which stem cells transdifferentiate is uncertain; some of them also seem to fuse with host cells, forming pluripotent cells, and recent (2004) debate and experiment has aimed at finding out the extent of these two factors. In October 2004 a third quality of stem cells was discovered. Scientists at the University of California in San Diego found, amazingly, that when embryonic stem cells — just 15 cells, taken from normal mice — are injected into the abdomen of mice pregnant with fetuses carrying a genetic heart defect, the pups are born with normal hearts. The stem cells do not cross the placenta into the fetuses, but secrete hormone-like chemicals that play an essential role of normal growth and development. to their chemical action. Two of these chemicals have been identified — they are called IGF1 and WNT5a.
Stem cell transplants today
Following successful animal research, stem cell transplants are now routine in the treatment of several types of cancer. They enable patients to undergo high-dose chemotherapy despite its toxic effect on bone marrow, as the patient can afterwards be given their own or a donor’s bone marrow stem cells, which engraft back into the bone. A further development, pioneered in animals and now entering clinical practice, is to use donor stem cells to treat certain serious and often fatal inherited blood disorders such as Fanconi anaemia, severe combined immune deficiency, and, potentially, sickle cell disease and thalassaemia. The patient’s own bone marrow, which produces diseased blood cells, is destroyed using high-dose chemotherapy and replaced by donor stem cells.
Bone marrow stem cells for tissues other than blood
Research has aimed to replace various missing tissues using bone marrow stem cells, and there seems to be evidence that this happens at least some of the time. The problem researchers have faced is knowing whether, some months later, any of the cells in the tissue being studied (usually heart or brain) is derived from the injected stem cells. The presence of apparently newly-generated tissue could also be due to the body’s existing capacity to repair itself or to environmental influences, which include oxygen lack and convulsions. Even learning and exercise can stimulate new nerve cell production. One way round this problem is to inject male cells into female bodies, wait a few months, and see if the brain or other organ contains Y-chromosomes, which are only normally found in males. It is also essential to check whether the Y-chromosomes are in new nerve cells and are not —as sometimes happens—merely the product of fusion of the injected stem cells with existing cells. Scientists have developed techniques to do this. A further problem is that a wait of six months between giving the transplant and killing a rodent for study represents a quarter of its lifespan.
The heart, blood vessels, and blood
During heart attacks — myocardial infarction — there is cell death in the area deprived of oxygen. Early (2001) research in rats and mice suggested that when bone marrow stem cells were injected into the heart, new heart muscle cells grew. It was then tried in humans, and clinicians thought that heart muscle was regenerated: the patients' heart seemed stronger and it was presumed that this was due to new muscle formation. In April 2004, two papers in the prestigious science journal Nature challenged these observations and the science underpinning them. Using state-of-the-art genetic tools, they discovered that bone marrow stem cells showed little or no capacity to turn into heart muscle. Instead, they turned into blood cells. Any functional improvement, they said, seems to have been caused by growth of new blood vessels, which has proved an unexpected side effect of treatment. In view of the potential hazards of mistaken cellular identity, reports of bone marrow stem cell versatility are now undergoing rigorous scrutiny. Better, but mixed, results came from research using muscle stem cells. When patients’ hearts were injects with their own muscle stem cells, there was an over-riding improvement in heart function in most patients, but some developed an irregular heartbeat, probably because the transplanted cells failed to become electrically linked to the rest of the heart. The best animal results yet (May 2004) came when embryonic stem cells were transplanted within a man-made 3D structure into damaged hearts of rodents which had suffered heart attacks. In the same month there were reports of successful human grafts, where it was shown that new heart cells were derived from injected umbilical cord stem cells. In animals, fetal heart cells graft easily into the heart, adopt the identity of adult heart cells, and become electrically coupled. However, their use in humans is ethically problematic. The search is now on for heart precursor cells and to discover the signals that guide the way they migrate, renew themselves, and become adult heart cells.
Making new muscle
New muscle cells have been formed in mouse muscle in the test-tube, and in living mice. In both cases the cells were reprogrammed by signals from surrounding cells, suggesting that adult tissues may be able to instruct transplanted cells to adopt the fates appropriate to their new location. Muscle regeneration has also resulted from injecting several types of stem cells — from bone marrow, from human knee joints, and from newborn mouse muscles — repairing damage similar to muscular dystrophy. Injured muscle seems to send distress signals around the body that summon the cells. But there are still doubts about whether adult stem cells can be used to provide effective treatments for diseases such as muscular dystrophy.
The brain and nervous system
The brain is immune privileged, which means that it does not reject cells from other animals or even other species, and this makes it a prime target for stem cell therapy. Fetal brain stem cells are relatively easy to collect and grow in the laboratory, and can divide indefinitely with no tendency to tumour growth. Research to date shows similar problems to that of heart research. Stem cells transplants have improved (but not cured) most (but not all) mice with Parkinson's, stroke, EAE (an induced form of multiple sclerosis) spinal cord injury, motor neurone disease and other conditions. Some of the injected stem cells create an environment that protects and aids the survival of the host nerve cells. In the spinal cord, only a tiny proportion of injected cells become new motor nerves that penetrate muscle. In normal brains after injury nerve stem cells cluster round the injury site and generate new stem cells, though it is self-evident that they do not produce sufficient to fully repair the damage done. In the eye, injected stem cells attach to a type of retinal cell called astrocytes. However, unless they are genetically modified, they form new blood vessels, which can obstruct clear vision. In May 2004 it was reported that three cancer patients, all female, who had received stem cell transplants from their brothers formed new brain support cells. The new cells contained Y-chromosomes, which proves they were of male origin, but the findings do not rule out fusion between bone marrow and other stem cells. Purkinje nerve cells in the cerebellum can fuse with bone marrow cells in both mice and humans.
Digestive organ diseases; diabetes
Research in rats and mice shows that pancreatic, liver, and bone marrow stem cells can transform into insulin-producing cells. Stem cell research may also cure Hirschprung disease, a genetic condition wherein a defect stops nerve stem cells forming nerves that control the small intestine. With diabetes, some researchers have found that bone marrow stem cells transdifferentiated into pancreatic beta cells, whereas other research teams have failed to see this. In July 2004 The Lancet commented: "As in every emerging field in biology, early reports seem conflicting and confusing. Embryonic and adult stem cells are potential sources for [pancreatic] beta-cell replacement and merit further scientific investigation. Discrepancies between different results need to be reconciled. Fundamental processes in determining the differentiation pathways of stem cells need to be elucidated, so that rigorous and reliable differentiation protocols can be established. Encouraging studies in rodent models may ultimately set the stage of large-animal studies and translational investigation."
Further information
The Royal Society policy pages on cloning and stem cells , includes official statements, comprehensive information and links. Edinburgh University Institute for Stem Cell Research answers questions such as What are stem cells? and Why are scientists so interested in them? The Cambridge Stem Cell Research Institute opened in June 2004. The Medical Research Council's booklet titled Stem cells focuses on MRC-funded research into stem cells, including the exciting potential these have for repairing damaged body tissues and replacing them with healthy new cells. The National Institute for Medical Research has a 2001 "Mill Hill Essay" on stem cell therapy and research. Use of human stem cells in research raises ethical, legal, religious, and policy questions which were addressed in a 1999 report of the American Association for the Advancement of Science and the Institute for Civil Society . Mice in the world of stem cell biology , Geraldine Guasch & Elaine Fuchs, 2005, Nature Genetics supplement, 37, 1201. Stem Cells. Stem Cell Science in the UK (2008) Human Tissue Authority. Hope and hype, an analysis of stem cells in the media , November 2011, NHS Choices. IMAGE©ISTOCKPHOTO.COM/DRA_SCHWARTZ
Featured news

Inviting protestors to talk

The animal research behind a new device to treat epilepsy

Ten organisations account for half of all animal research in Great Britain in 2023
Subscribe to our newsletter.
Get the latest articles and news from Understanding Animal Research in your email inbox every month. For more information, please see our privacy policy .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Stem Cell Therapy: From Idea to Clinical Practice
Regenerative medicine is a new and promising mode of therapy for patients who have limited or no other options for the treatment of their illness. Due to their pleotropic therapeutic potential through the inhibition of inflammation or apoptosis, cell recruitment, stimulation of angiogenesis, and differentiation, stem cells present a novel and effective approach to several challenging human diseases. In recent years, encouraging findings in preclinical studies have paved the way for many clinical trials using stem cells for the treatment of various diseases. The translation of these new therapeutic products from the laboratory to the market is conducted under highly defined regulations and directives provided by competent regulatory authorities. This review seeks to familiarize the reader with the process of translation from an idea to clinical practice, in the context of stem cell products. We address some required guidelines for clinical trial approval, including regulations and directives presented by the Food and Drug Administration (FDA) of the United States, as well as those of the European Medicine Agency (EMA). Moreover, we review, summarize, and discuss regenerative medicine clinical trial studies registered on the Clinicaltrials.gov website.
1. Introduction
Despite the progress in medical science, there still exist various diseases in the world for which there is no suitable treatment. People affected by incurable disorders typically use treatment methods intended to decrease the somatic and psychological symptoms and, in these situations, the physician offers treatment methods only to manage the disease, not treat it. Therefore, researchers are attempting to develop new treatment methods to not only control the symptoms of, but also to treat those diseases for which no cure is available at present.
Regenerative medicine is considered a promising new source of treatment for untreatable diseases in modern science [ 1 ]. Regenerative medicine is a multidisciplinary field including cell biology, genetic, biomechanics, material science, and computer science [ 2 , 3 ], the ultimate target of which is returning normal function to defective cells and tissues [ 4 ]. Since the discovery of stem cells and the spread of awareness regarding their unique properties, they have been defined as therapeutic agents for organ and tissue repair, and so are widely considered good candidates for regenerative medicine, due to their many potential applications [ 5 ]. Regenerative medicine is now regarded as an alternative to traditional drug-based treatments by researchers who study its potential applications in various diseases, including degenerative diseases, among others [ 6 , 7 , 8 , 9 , 10 ]. The main concept of regenerative medicine is implied tissue/organ regeneration using cells and, to reach this target, different kinds of cells have been used. However, various studies have indicated that cell therapy is restricted by a few limitations. In recent years, different alternatives have been introduced for cell therapy in order to resolve these limitations, including the improved application of stem cells for the restoration of tissue, such as the combination of cells with scaffolds, cell cultures with suitable biochemical properties, gene editing, and the immunomodulation of stem cells, as well as the use of stem cell derivatives [ 11 , 12 , 13 , 14 , 15 ]; however, the use of these alternatives clinically may be postponed, as more preclinical studies are required due to their status as newer technologies [ 16 ].
Stem cells are a group of immature cells that have the potential to build and recover every tissue/organ in the body due to their unique proliferative, differentiation, and self-renewal abilities [ 17 ]. Stem cells provide therapeutic effects which improve physical development by regenerating damaged cells to assist in organ recovery. Relying on the natural abilities of stem cells, researchers have used their biological mechanisms for stem-cell-based therapy. The mechanisms of action through which stem cells can promote the regeneration of tissue are diverse, including (1) inhibition of inflammation cascades [ 18 , 19 ], (2) reduction of apoptosis [ 20 , 21 ], (3) cell recruitment [ 22 , 23 ], (4) stimulation of angiogenesis [ 24 , 25 ], and (5) differentiation [ 26 ]. The cause of a disease is a vital consideration in selecting the proper stem cell mechanism and in the regeneration of tissue/organs using stem cells. Many examinations must be carried out to determine the main mechanisms involved in treatment when these cells are to be used in clinical practice, and the convergence of stem cell therapeutic mechanisms and disease mechanisms is expected to increase the chance of developing cures through stem cell applications.
From 1971 to 2021, 40,183 research papers were published regarding stem-cell-based therapies. All of these studies were conducted around discoveries and for the goal of “Stem Cell Therapy” based on the therapeutic efficacy of stem cells [ 27 ]. As basic stem cell research has soared over the past few years, “translation research”, a relatively new field of research, has recently greatly developed, making use of basic research results to develop new treatments. Although many articles on stem-cell-based therapies are published annually and their number increases every year, the number of clinical trial studies has not increased rapidly. Furthermore, among these studies, only a small portion of them can receive full regulatory approval for verification as treatment methods. Although one reason for this difference is due to the need for various prerequisite preclinical studies before carrying out a clinical trial study, the main reason is due to the sharply defined guidelines which prevent the translation of many preclinical studies to clinical trials.
In this review, we provide a general overview regarding the translation of stem cell therapies from idea to clinical service. Understanding the step-by-step knowledge underlying the translation of ideas to medical services is the first step in introducing a new treatment method. In this review, we divide this pathway into four levels, including idea evaluation, preclinical studies, clinical trial studies, and clinical practice. We focus not only on understanding each level’s requirements, but also discuss how an idea is assessed during the transition from one level to the next and, finally, move on to marketing.
2. From Idea to Preclinical Study
If a researcher has an idea regarding regenerative medicine using stem cells that inspires their use in a study, it must first be evaluated. During the evaluation step, it is important to select the target disease and make sure that the mechanism causing the disease is understood. Disease-related mechanisms refer to the cellular and molecular processes by which a particular disorder is caused [ 28 , 29 ], and stem-cell-based therapies are considered a treatment method intended to compensate for the disruption caused by such mechanisms in order to finally restore the defective tissue. Multiple mechanisms cause diseases [ 30 , 31 , 32 ]; however, stem cells, with their tremendous differentiation, self-renewal, angiogenesis, anti-inflammation, anti-apoptotic, and immunomodulatory potentials, as well as their capacity for induction of growth factor secretion and cell signaling, can affect these mechanisms [ 33 , 34 , 35 , 36 , 37 ].
After subject evaluation, preclinical studies should be carried out to determine whether the idea has any potential to treat the disease, and the safety of the final product should be assessed in an animal model of the target disease [ 38 , 39 , 40 ]. Preclinical studies are composed of in vitro and in vivo studies. In vitro experiments are performed with biological molecules and cells based on various hypotheses and, during the in vitro evaluation, a new treatment method is assayed in this controlled environment [ 39 ]. In contrast, during in vivo studies, as controlling all biological entities is impossible, the new product may be affected by various factors and thus present different effects. The general purpose of a preclinical study is to present scientific evidence supporting the performance of a clinical study, and the following are required for a decision to move forward to clinical study: (i) the feasibility and establishment of the rationale (e.g., validation, separation of active ingredients in vitro, and determination of its mechanism in vivo), (ii) establishment of a pharmacologically effective capacity (e.g., secure initial dose verification), (iii) optimization of administration route and usage (e.g., safe administration method, repeated administration, and interval verification), (iv) identification and verification of the potential activity and toxicity (e.g., toxicity analysis according to single and repetitive testing), (v) identification of the potential for special toxicity (e.g., genetic, carcinogenic, immunological, and neurotoxic analyses), and (vi) determination of whether to continue or discontinue development of the treatment [ 41 , 42 ].
3. From Preclinical Study to Clinical Trial
In principle, any idea regarding stem cell therapy should be assessed using comprehensive studies (i.e., in vitro and in vivo) before a clinical trial is considered, and the results of these studies should be proved by competent authorities. It can be easy during an in vitro study to create manipulative biological environments such as through the use of genetic mutation, drug testing, and pharmaceuticals, and it is easy to observe changes through the application of manipulated variables through living cells [ 43 , 44 , 45 ]. However, given the many associated variables, such as molecular transport through circulating blood and organ interactions, it is hard to say whether such a study can completely mimic the in vivo environment [ 43 , 44 , 45 ]. Before application in patients, in vivo experiments are conducted after in vitro experiments in order to overcome these weaknesses.
Many researchers use rodents for in vivo studies, due to their anatomical, physiological, and genetic similarities to humans, as well as their other unique advantages including small size, ease of maintenance, short life cycle, and abundant genetic resources [ 46 ]. The strength of in vivo studies is that they can supplement the limitations of in vitro studies, and the outcomes of their applications can be inferred in humans through the use of human-like biological environments. To establish in vivo experiments for stem cell therapies, the most correlated animal model should be selected depending on the specific safety aspects to be evaluated. Where possible, cell-derived drugs made for humans should be used for proof-of-concept and safety studies [ 47 ]. Homogeneous animal models can also be utilized as the most correlated systems in proof-of-concept studies [ 48 ].
Furthermore, in vivo studies require ethical responsibilities and obligations to be upheld according to experimental animal ethics. In other words, unnecessary and unethical experiments must be avoided. Summing up the above, we can see that both in vitro and in vivo approaches are used in preclinical studies, which should be carried out before clinical trial applications based on various interests.
Several factors must be considered in different in vitro and in vivo studies, including cell type determination, cell dose specification, route of administration, and safety and efficiency.
3.1. Stem Cell Source Determination
As expectations rise for regenerative treatment through the application of stem cell therapies, the number of applications of various types and stem cell sources has increased, and stem cell therapies have diversified from autologous to allogenic to iPSCs. These stem cell treatments can vary in risk, depending on the cell manufacturing process [ 49 ], among other factors, and in clinical experience, such that all types of stem cell treatments must be evaluated on the same basis [ 50 ]. Therefore, the strengths and weaknesses of each type of stem cell should be identified in order to determine the maximum therapeutic effect of stem cells in various diseases. This will enable us to build disease-targeted stem cells by applying the appropriate stem cells to the appropriate diseases. Below, we briefly discuss the characteristics of various stem cells.
3.1.1. Mesenchymal Stem Cells (MSCs)
MSCs are lineage-committed cells that divide into mesenchymal systems, primarily fatty cells, chondrocytes, and osteocytes [ 51 ]. It is well known that MSCs can be differentiated into dry cells, nerve cells, glioma cells, and skeletal muscle cells under proper in vitro culture conditions [ 52 , 53 , 54 , 55 , 56 , 57 ]. MSCs are primarily derived from myeloid and adipose tissues [ 58 , 59 ]. At present, MSCs are also isolated from many other tissues, such as the retina, liver, gastric mucosa, tendon, cartilage, placenta, cord blood, and blood [ 60 , 61 , 62 , 63 ]. The biggest characteristics of MSCs are their immunosuppressive functions, which prevent the proliferation of activated T cells through immunosuppressive cytokine secretion and suppression of programmed cell death signaling [ 64 , 65 ]. Due to this role, they have been spotlighted as a potential treatment for immune-related inflammation and disease. The initial clinical application of MSCs was in a case of patients with severe graft versus host disease (GVHD), and these cells have since been well applied in clinical practice, as evidenced through various studies [ 66 , 67 , 68 ].
MSCs have a variety of characteristics according to their organ of origin [ 69 ]. BM-MSCs, which are isolated from bone marrow, are useable in both autologous and allogenic contexts, and can perform stromal functions. However, the process of cell isolation from bone marrow is not only accompanied by the risk of pain and infection, but also has a lower efficiency of collection than other MSC sources. Furthermore, these cells have a longer doubling time (DT) in comparison to MSCs derived from other sources (approximately 60 h) [ 70 ]. Compared to BM-MSCs, AD-MSCs are not only easy to collect, but are also 100 to 500 times more efficient to harvest and have a shorter DT (approximately 20 h) [ 71 ]. However, these are adipose-derived stem cells that have a strong characteristic of adipogenic differentiation, such that they can be suggested as a valid alternative to BM-MSCs, but their nature must be considered regarding proper culture and body environment. Furthermore, there are concerns that these factors may affect the efficacy of treatment, as the amount of cytokines secreted is significantly lower when compared to BM-MSCs [ 72 ]. MSCs extracted from the umbilical cord (UC-MSCs) have come into the spotlight to compensate for these issues: UC-MSCs not only have the advantage of being easily collected compared to other stem cells, but also avoid ethical or donor age issues. They have superior proliferation and differentiation capabilities compared to BM-MSCs and AD-MSCs, and their DT has been reported as 24 h [ 69 , 73 ]. UC-MSCs are currently a subject of concern, as although they are easy to store frozen for a long time (e.g., in a cord blood bank), the cell survival rate and success rate during extraction are not high, due to exposure to cryogenic protectors during cryogenic storage [ 73 ]. Furthermore, as the cells are isolated from other organs, they have limited self-renewal capacity, and their senescence is faster than in other stem cells in long-term cultivation [ 66 , 74 ].
3.1.2. Hematopoietic Stem Cells (HSCs)
HSCs can be differentiated into cells from all hematopoietic systems present in the bone marrow and chest glands, namely myeloid cells and lymphocytes. HSCs can be obtained at good levels from adult bone marrow, the placenta, and cord blood. They can cause immunological problems such as transplant rejection. Nevertheless, they have been shown to be an effective treatment method in various diseases, including leukemia, malignant lymphoma, and regenerative anemia, as well as congenital metabolism, congenital immunodeficiency, nonresponsive autoimmune disease, and solid cancer to date. Furthermore, HSCs are the only stem cell type approved for stem cell treatment by the Food and Drug Administration (FDA) [ 75 , 76 ].
3.1.3. Embryonic Stem Cells (ESCs)
ESCs have established cell lines that can be maintained through in vitro culture. They are pluripotent cells that can be differentiated into almost any type of cell present in the body, and can be differentiated in vitro by adding external factors to the culture medium or by genetic modification. However, they may form teratomas, which are composed of various forms of cells derived from the endoderm, mesoderm, and exoderm, when transplanted into an acceptable host [ 77 ].
3.1.4. Induced Pluripotent Stem Cells (iPSCs)
iPSCs are artificially created stem cells. These cells are made by reprogramming adult somatic cells such as fibroblast cells. They share many of the characteristics of ESCs, including self-renewability, pluripotent differentiation, and malformed species performance. Unfortunately, these cells have little scientific evidence regarding changes in cell-specific regulatory pathways, gene expression, and epigenetic regulation. These characteristics pose a risk of tissue chimerism or cell dysfunction [ 78 ].
In summary, although the FDA-approved stem cell type is HSCs from healthy donors, a variety of issues have been raised, including a lack of donors and immune rejection. Therefore, we need to understand the characteristics of stem cells in order to handle them accordingly and overcome their disadvantages while maximizing their advantages. As stem cells derived from various sources have different characteristics, capabilities, potential, and efficiency, selecting the right source of stem cells that is appropriate for the target can be effective in assuring treatment efficiency.
3.2. Cell Dose Specification
The effective range of administration (i.e., dosage) of stem cells or stem-cell-derived products used in treatment should be determined through in vivo and in vitro studies. The safe and effective treatment capacity must be identified and, where possible, the minimum effective capacity must also be determined. When administered to vulnerable areas such as the central nervous system and myocardium, it has been reported that conducting normal dosage determination tests is unlikely. Thus, if the results of nonclinical studies can safety demonstrate treatment validity, it may be appropriate to conduct early human clinical trials with doses that may indicate therapeutic effects [ 79 ].
Will a high cell dose have better effects, considering only the effectiveness of stem cells? We answer this question below. An increasing dose of CD34 + cells (0.5 × 10 5 per mouse) has been shown to have positive effects, stimulating multilineage hematopoiesis at early stages and increasing the magnitude of reconstitution at post-transplant stages. Furthermore, improved T-cell reconstitution was correlated with higher cell doses of stem cells, compared to lower cell doses [ 80 ]. However, a few studies related to acute myeloblastic leukemia (AML) have reported that high doses of HSCs were correlated with restored function and rapid hematological and immunological recovery, but these results were not unconditional. In this study, a higher dose of HSCs (≥7 × 10 6 /kg) resulted in poorer outcomes and a higher relapse rate than the lower dose of HSCs (<1 × 10 6 /kg) [ 81 ]. In preclinical studies on heart disease, Golpanian et al. have demonstrated, through comparison of some preclinical studies for optimized cell dose, the therapeutic effects of stem cell types (i.e., allogenic and autologous MSCs), as well as the proper cell dose of stem cells and route of administration (direct epicardial and intravenous) in heart disease. Their results showed that the total number of cells used was different, but were inconsistent with the hypothesis that a higher number of cells would have higher therapeutic efficacy [ 82 ]. Therefore, these conclusions suggest that the currently reported data do not provide a decisive answer, such that sufficient and detailed early-stage studies may be needed before proceeding with clinical trials.
3.3. Route of Administration
Stem cells have been extensively studied under various disease conditions, depending on their type and characteristics. At this time, the route of administration should not be overlooked in favor of the number of stem cells transplanted. Several reports have shown that engraftment ability typically has a lower rate of reaching target organs relative to the number of transplanted cells, and does not have a temporary longer duration [ 83 , 84 ].
The methods of stem cell administration can largely be divided into local and systemic transmission. Local transmission involves specific injections through various manipulations and direct intra-organ injections, such as intraperitoneal (IP), intramuscular, and intracardiac injections. Systemic transmission uses vascular pathways, such as intravenous (IV) and intra-arterial (IA) methods. According to the publications in the literature, IV is the most common method, followed by intrasplenic and IP [ 85 , 86 , 87 ]. In a liver disease model, IV was shown to be not only suitable for targeting the liver, but also showed better liver regeneration effects than other routes of administration [ 85 , 88 ]. Intracardial injection showed better cell retention in heart disease, while intradermal injection showed better treatment in skin diseases [ 89 , 90 ]. Hence, we can determine that, in the context of these various diseases, the routes of administration should be different depending on the target organ. Many researchers have suggested that intravascular injection is a minimally invasive procedure, but it also poses a risk of clogged blood vessels, such that direct intravascular injection increases the risk of requiring open-air operations [ 91 ]. Clinical trials have reported that the number of cells and treatment efficacy under the same conditions, as in preclinical studies, are not significant, but also differ in significance depending on the route of administration [ 92 , 93 ]. Therefore, researchers should continue to study which cells are appropriate for a given route of administration—even within the same disease—based on many precedents [ 82 ]. In addition, researchers should explore the appropriate routes of administration for safer and more effective therapeutic effects.
3.4. Manipulation of Cell Transplantation for Safety and Efficiency Improvement of Administration
All medical treatments have benefits and risks. It is not particularly safe to apply these unproven stem cell treatments to patients. As expectations for regenerative treatment through stem cell therapies increase, the application of various administration pathways, including through the spinal cord, subcutaneous, and intramuscular, as well as the stem cell therapies themselves, have been diversifying, from autologous to homogenous to iPS. These stem cell treatments can vary in risk, depending on the cell type manufacturing process among other factors, and they differ in clinical experience, such that all types of stem cell treatments must be evaluate on the same basis. Furthermore, it should only be in limited and justified contexts that stem cells which can proliferate and have all-purpose differentiation remain in a final product.
Unfortunately, the only safe stem cells that have been employed in regenerative medicine so far are omnipotent stem cells, such as HSCs and MSCs, which are isolated from their self-origin [ 94 ]. Unfortunately, potential clinical applications using iPSCs and ESCs face many hurdles, as they present higher risk, including the possibility of rejection, teratoma formation, and genomic instability [ 95 ]. Hence, many researchers have attempted to overcome stem cell tracking for safety assessment. To check the engraftment and the remaining amount of stem cells, they have been labeled using BrdU, CM-Dil, and iron oxide nanoparticles, and visualized using Magnetic resonance imaging (MRI) [ 84 , 96 , 97 ].
A close analysis of the distribution patterns of administrative sites and target organs is required, as well as whether a distribution across the body is expected, and the organ that the cells are predicted to be distributed through should undergo a full-term analysis, including evaluation at administrative sites. To date, studies have reported assessments in the brain, lungs, heart, spleen, testicles, ovaries, kidneys, pancreas, bone marrow, blood, and lymph nodes, including areas of administration [ 98 ].
Some researchers have carried out the detection of transplanted UC-MSCs delivered by IV injection in the lung, heart, spleen, kidney, and liver. According to their results, the transplanted cells were not detected in other organs, except the lung and liver, for 7 days. In the lung and liver, the detected cells persisted at least 7 days after the transplant [ 99 ]. Furthermore, in a study comparing BM-MSCs and UC-MSCs in terms of cell tracking, they reported on the persistence of stem cells according to the route of administration used. In the results of the comparison of intracardiac and intravenous routes, the transplanted stem cells were detected in the lung for 10 days, but the signal disappeared after 21 days [ 100 ]. In other research, the stem cells were transplanted with using a biomaterial scaffold. The AD-MSCs were transplanted with hyaluronic acid/alginate hydrogel through intradermal injection, and could be detected by CM-Dil staining for 30 days [ 101 ]. These studies may show that the transplanted cells localized to the damaged organs through their homing ability, but the results of these previous studies seem to indicate that the residual volume and the residual date vary significantly depending on the target disease, organs, and type of stem cells. The cell residual means the survival of the cell, which represents the risk of formation of tumors. To overcome the problem of teratoma formation, the following results have been reported: According to one study, ESCs showed the following rates of teratoma formation: 100% under the kidney capsule, 60% intratesticular, 25–100% subcutaneous, and 12.5% intramuscular. To overcome this problem, the investigators performed a co-injection with Matrigel into an animal model. According to their results, subcutaneous implantation of ESCs in the presence of Matrigel appeared to be the most efficient, reproducible, and easiest approach for preventing teratoma formation, other than only using ESCs [ 102 ]. Moreover, cellular products derived from iPSCs have higher potential as potential cell sources in personalized medicine [ 103 ]. Their applicability is currently limited due to concerns regarding the potential risk of serious transplant-related side effects, such as tumor formation due to residual pluripotent cells [ 104 ]. Hence, a recent study reported the establishment of an optimized tool for therapeutic intervention that allows for controlled specific and selective ablation of iPSCs through the use of LVCAGs–transgenic iPSCs [ 104 ].
Unlike MSCs, which are generally considered immune-tolerant as an immunomodulator, transplantation of ESCs and HSCs requires close examination of the matching of histocompatibility antigen (HLA) between the donor and beneficiary [ 105 , 106 ]. Although homogeneous mesenchymal stem cells are known to have immunogenicity in immune-active rodent models and are quickly removed from the peripheral blood, studies have shown that a few MSCs remain for weeks to months. Therefore, it is recommended to conduct a study to assess the persistence of MSCs in the cell preparations administered, in order to assess the risk of stem cell removal. Therefore, for stem cell therapies that have undergone extensive in vitro manipulation such as long-term cell culture—including those derived from ESCs and iPSCs—both oncogenicity and genetic stability must be evaluated before clinical research begins. Furthermore, we must constantly review and study the latest research on safety, as well as the effects of regeneration using stem cells, and discuss and study the potential of regenerative medicine [ 107 , 108 , 109 , 110 , 111 ].
As discussed earlier, in vitro and in vivo preclinical studies are the direction of current research, and encompass the tasks that need to be completed. If we reinforce the current strengths and weaknesses based on the preceding content, we are already a step closer to developing stem cell treatments.
4. From Clinical Trial to Clinical Practice
Before a treatment is applied in humans (i.e., patients), preclinical study must involve checking whether the effect of treatment will be positive or negative and, if there are any negative effects, the researcher must check the safety possibilities at every step. Due to concerns relating to treatment using stem-cell-based products, deciding whether preclinical studies are sufficient for translating to clinical trials raises several issues that must be assessed by competent authorities. An application for a clinical trial should be submitted to the Food and Drug Administration (FDA), the European Medicine Agency (EMA), or another organization, based on the country [ 112 ].
The FDA is responsible for certifying clinical trial studies for stem-cell-based products in the United States [ 113 ]. If a new drug is introduced to a clinical investigator which has not been approved by the FDA, an Investigational New Drug (IND) application may need to be submitted [ 114 ]. The IND application includes data from animal pharmacology and toxicology studies, clinical protocols, and investigator information [ 115 ]. A lack of preclinical support (e.g., in vitro and in vivo studies) can lead to required modification or disapproval. If the FDA has announced that an IND requires modifications (meaning that the application is intended to secure approval but has not yet been approved), the results of the preclinical studies were deemed insufficient or inadequate for translation to clinical trial study, such that further study must be completed, after which an amended IND should be submitted.
The FDA has published guidelines for the submission of an IND in the Code of Federal Regulations (CFR). These regulations are presented in 21 CFR part 210, 211 (Current Good Manufacturing Practice (cGMP)), 21 CFR part 312 (Investigational New Drug Application), 21 CFR 610 (General Biological Product Standards), and 21 CFR 1271 (Human Cells, Tissues, and Cellular and Tissue-Based Products) [ 116 , 117 , 118 ]. These guidelines have been issued for the development of stem cell products with the highest standards of safety and potential effective translation to clinical trial studies.
The FDA issued 21 CFR parts 210 and 211to ensure the quality of the final products [ 119 ]. The 21 CFR part 210 contains the minimum current good manufacturing practice (cGMP) considered at the stages of manufacturing, processing, packing, or holding of a drug, while the 21 CFR part 211 contains the cGMP for producing final products. The 21 CFR 211 includes FDA guidelines for personnel, buildings and facilities, equipment, and control of components, process, packaging, labeling, holding, and so on, all of which are critical for pharmaceutical production [ 116 , 117 , 118 , 119 , 120 , 121 ]. The requirements for IND submission and conducting clinical trial studies, reviewed by the FDA in the 21 CFR part 312 (Investigational New Drug Applications), includes exemptions that are described in detail in 312.2 (general provisions). Such exemptions do not require an IND to be submitted, but other studies must present an IND based on 21 CFR part 312. The section, 21 CFR part 312, provides different information, including the requirements for an IND, its content and format, protocols, general principles of IND submission, and so on. In addition, the FDA describes the administrative actions of IND submission, the responsibilities of sponsors and investigators, and so on, in this section [ 116 , 117 , 122 ]. The 21 CFR part 610 contains general biological product standards for final product characterization. The master cell bank (MCB) or working cell bank (WCB) used as a source for stem-cell-based final products must be tested before the release or use of the product in humans. The MCB and WCB should be tested for sterility, mycoplasma, purity, identity, and potency, among other tests based on the final products (e.g., viability, stability, phenotypes), before use at the clinical level. The FDA provides all required information regarding general biological product standards in this section, including release requirements, testing requirements, labeling standards, and so on [ 116 , 117 , 123 , 124 ]. The 21 CFR part 1271 focuses on introducing the regulations for human cells, tissues, and cellular and tissue-based products (HCT/P’s), in order to ensure adequate control for preventing the transmission of communicable disease from cell/tissue products. Current Good Tissue Practice (GTP) is a part of 21 CFR part 1271, where the purpose of GTP is to present regulations for the establishment and maintenance of quality control for prevention of introduction, transmission, or spread of communicable diseases, including regulations for personnel, procedures, facilities, environmental control, equipment, and so on [ 125 , 126 , 127 , 128 ].
The EMA is an agency in the European Union (EU) which is responsible for evaluating any investigational medical products (IMPs) in order to make sure that the final product is safe and efficient for public use. When planning to introduce a new drug for a clinical trial in Europe, one may be required to submit clinical trial applications to the EMA for IMPs. Clinical trial applications for IMPs include summaries of chemical, pharmacological, and biological preclinical data (e.g., from in vivo and in vitro studies) [ 129 ]. The EMA has presented different regulations to support the development of safe and efficient products for public usage, including Regulation (EC) No. 1394/2007, Directive 2004/23/EC, Directive 2006/17/EC, Directive 2006/86/EC, Directive 2001/83/EC, Directive 2001/20/EC, and Directive 2003/94/EC.
Regulation (EC) No. 1394/2007 defines the criteria for regulation regarding ATMPs. Advanced therapy products (ATMPs) are focused on gene therapy medicinal products (GTMP), somatic cell therapy medicinal products (sCTMP), tissue-engineered products (TEP), and combined ATMPs, which refers to a combination of two different medical technologies. Regulation (EC) No. 1394/2007 includes the requirements to be used in development, manufacturing, or administration of ATMPS [ 130 , 131 , 132 ]. Directive 2004/23/EC, Directive 2006/17/EC, and Directive 2006/86/EC define standards for safety and quality, as well as technical requirements for donation, procurement, testing, preservation, storage, and distribution of tissue and cells intended for human applications [ 133 , 134 , 135 ]. Directive 2001/83/EC applies to medicinal products for human use [ 136 ]. Directive 2001/20/EC presents the regulations for the implantation of products in clinical trials in the EU [ 137 ]; however, this directive will be replaced by regulation (EU) No. 536/2014. Regulation (EU) No. 536/2014 was adapted by the European Parliament in 2014, and provides regulation for clinical trials on medical products intended for human use. The new EU regulation comes into effect on 31 January 2022 and aims to coordinate all clinical trials performed throughout the EU, using clinical trials submitted into CTIS (Clinical Trials Information System). The definition of regulation (EU) No. 536/2014 as a homogeneous regulation serves an important role in the EU, as all member states of the EU can be involved in multicenter clinical trials using international coordination, thus allowing larger patient populations [ 138 ]. Directive 2003/94/EC provides Good Manufacturing Practice (GMP) Guidelines in relation to medicinal products or IMPs intended for human use [ 139 ]. All process and application requirements for the IMP application are present in the regulations and directives of the EMA. After presenting an IND/IMP to the regulatory authority responsible for clinical trial oversight (FDA or EMA), the application will be reviewed in accordance with the FDA/EMA criteria and, if assured of the protection of humans enrolled in the clinical study, the application will be approved by the investigational review boards (IRBs) in the United States or Ethics Committees (ECs) in the European Union. Clinical trial studies are composed of different steps where, at each step, products are assessed using different quality and quantity measurements by the responsible agency. An efficient clinical trial study should address the safety and efficiency of new stem cell products in each of the different steps, and it is important to complete each step based on defined instructions and regulations, as the results of previous steps are needed to move forward.
Almost all clinical trial studies that have been approved for testing in humans have been registered online ( https://www.clinicaltrials.gov/ accessed 12 December 2021). Our search on this website revealed more than 6500 records for interventional studies registered using “Stem Cells” up to December 2021. The recorded clinical trials can be analyzed from different aspects.
Recruiting status: The recruiting status of these studies indicated that 18% of these studies were ongoing (recruitment) and 42% were completed ( Figure 1 ). Although completed, suspended, terminated, and withdrawn studies are all terms used for studies that have ended, each is used to describe a different status. Completed studies are those that have ended normally and the participants were completely enrolled in the study. Suspended, terminated, and withdrawn studies are studies that stopped early; however, the participant enrolment status differs between them. A suspended study may start again, but nobody can continue to participate in terminated or withdrawn studies [ 140 , 141 ].

Status of clinical trials using stem cells.
Type of disease: Stem-cell-based therapy is a new approach for the treatment of various diseases in different clinical trial studies. Blood and lymph diseases are the most common diseases that have benefited from this new approach ( Figure 2 ). Blood and lymph diseases refer to any type of disorder related to blood and lymph deficiency or abnormality, such as anemia, blood protein disorder, bone marrow disease, leukemia, hemophilia, thalassemia, thrombophilia, lymphatic disease, lymphoproliferative disease, thymoma, and so on. In addition, various clinical trial studies have been performed using stem cells to treat immune system disease; neoplasm, heart, and blood disease; and gland- and hormone-related disease ( Figure 2 ). However, this does not mean that all of these studies had great results, nor does it mean that all of these studies introduced a new treatment method; some of these clinical trial studies were only intended to increase treatment efficiency, compare different types of treatment methods, or analyze various parameters after the administration of stem cells into the body.

Diseases considered in clinical trials using stem cells.
Autologous vs. Allogenic: Stem-cell-based products for use in clinical trial studies can be divided into two categories: autologous and allogeneic stem cells. In autologous stem cell therapy, the stem cells are collected from the patient’s own body. Culture-expanded autologous stem cells are autologous stem cells that are expanded before transplantation, and can be divided into two groups: modified and unmodified expanded autologous stem cells. If autologous stem cells were transplanted to the donor immediately after collection, this is a nonexpanded autologous stem cell treatment. The use of these cells usually has fewer restrictions for receiving clinical trial authorization. The classification of allogenic stem cells is similar to that of autologous stem cells, except that allogeneic stem cells are collected from a healthy donor. The use of these cells requires more prerequisite tests, in order to check the donor’s health. Allogenic stem cells have been used more than autologous stem cells in the clinical trial studies (46.34% vs. 44.51%), as shown in Figure 3 .

Applied stem cell types in clinical trials using stem cells.
Phase: Clinical trial studies are conducted in different phases. In each phase, the purpose of study, the number of participants, and the follow-up duration may differ. A new phase of clinical trials should not be started unless the results of the completed phase(s) have been reviewed by competent authorities, in order to that certify the results of the completed phase(s) are valid for authorization of the start a new phase of the clinical trial. For this purpose, at the end of each phase of a clinical trial study, competent authorities evaluate whether the new drug is safe, efficient, and effective for the treatment of the target disease ( Figure 4 ).

Status of clinical phase within clinical trials using stem cells.
Early Phase I emphasizes the effects of the drug on the human body and how the drug is processed in the body.
Phase I of a clinical trial is carried out to ensure that a new treatment is safe and to determine how the new medicine works in humans. The FDA has estimated that about 70% of the studies pass this phase.
In Phase II, the accurate dose is determined and initial data on the efficiency and possible side effects are collected. The FDA has estimated that roughly 33% of the studies move to the next phase.
Phase III evaluates the safety and effectiveness of products. The result of this phase is submitted to the FDA/EMA for new product approval, which allows manufacturing and marketing of the drug. The FDA has estimated that 25%–30% of the drugs pass at this phase.
Phase IV take place after the approval of new products and is carried out to determine the public safety of the new product [ 142 , 143 , 144 ].
The number of participants and the duration: A new stem cell product is eligible for marketing after completing successful clinical trial phases. As the new product has been used on volunteers and the effects/side effects of the drug have also been followed for a long time throughout the different phases, it is now possible to make a decision regarding its introduction to the market for public use. The number of participants and the duration of long-term follow-up in each study and each phase differ ( Figure 5 and Figure 6 ). The number of volunteers that participate in each phase of a clinical trial study varies, as each phase has a different target. The FDA has recommended 20–80, 100–300, and several hundred to thousands of volunteers for Phase I, Phase II, and Phase III, respectively [ 144 , 145 ]. Although the FDA has defined a range for enrolments per phase, the number of participants can vary depending on the type of disease. The number of participants for clinical studies in rare diseases will be lower than when studying common diseases. Searching for stem cells in clinicaltrial.gov, studies can be found with only one participant (e.g., NCT02235844, NCT02383654, NCT03979898, and NCT01142856). The sponsor/investigator must provide the FDA with strong documentation regarding the selection of such a number of volunteers. The volunteers for each clinical trial study, before attending, should be informed about the enrolment criteria of each study, possible side effects, and the advantages of the study.

Enrolment of clinical trials using stem cells.

The duration of each clinical trial study using stem cells.
Age of participants: Roughly 190,000 people participated in all the completed clinical trial studies using stem cells that had been registered. Each clinical study was performed in different age groups, which differed among the various studies depending on the type of drug, type of disease, and sponsor decision, as shown in Figure 7 .

The age of patients participating in clinical trials using stem cells.
Number of clinical trial studies: The number of clinical trial studies increased gradually from 2000 to 2014, although it fluctuated after 2014 but did not change significantly ( Figure 8 ). The reason for this increase in 2014 is not clear, but it may have been related to the introduction of the first advanced medicinal therapy product containing stem cells (Holoclar) by the EMA in 2014–2015 [ 146 ].

The proportion of clinical trials using stem cells by year: ( A ) the proportion of new clinical trial studies using stem cells by year (green bar) and the proportion of registration results accordingly (orange color line); ( B ) the proportion of completed registered clinical trial studies using stem cells by year (blue bar) and the updated results of completed clinical trial studies using stem cells by year (orange line).
Place of study: According to economic website reports, the cell therapy market has grown significantly in recent years, and it is expected to grow more in the coming years; therefore, many countries have begun research in this field. Our data from clinicaltrial.gov showed that the United States has conducted the most clinical trials using stem cells ( Figure 9 ). Government agencies, industry, individuals, universities, and private organizations have all invested in stem-cell-based therapy. The number of stem-cell-based companies has rapidly increased in recent years, and a brief overview of the submitted clinical trial studies indicated that the studies were mostly aimed at introducing therapeutic products for clinical applications. Therefore, we can expect the introduction of stem-cell-based products to the market.

The registered and completed clinical trial studies using stem cells according to participating countries: ( A ) top 10 participating countries with registered clinical trials using stem cells; and ( B ) top 10 countries based on the completion of registered clinical trials using stem cells.
As indicated above, translational research from the laboratory to clinical services has many layers which must be passed through, each with its own requirements and measurements. Therefore, the only way to introduce a new stem-cell-based product onto the market is for competent authorities to make sure that the discovery is safe and effective for its intended human use, and that the product has successfully passed all of the clinical trial stages.
5. Challenges and Future Directions
One of the most important issues regarding the introduction of a new product for use in humans through a clinical trial is evaluation of its safety. Although many clinical trials have been performed using stem cells for the treatment of various diseases, as stem-cell-based therapies are one of the newest groups of therapeutic products in medicine, it is very hard to introduce new products based on stem cells onto the market, as many different parameters must be evaluated. There are several concerns regarding stem-cell-based therapies, including genetic instability after long-term expansion, stem cell migration to inappropriate regions of the body, immunological reaction, and so on. However, all challenges depend on the type of stem cell (e.g., embryonic stem cell, adult stem cell, iPS), type of disease, route of administration, and many other factors. Almost all researchers in the field of stem cell therapy believe that despite stem cells having great potential to treat disease through their intrinsic potential, unproven stem-cell-based therapies that have not been shown to be safe or effective may be accompanied by very serious health risks. In order to receive clinical trial approval from a competent regulatory authority, different tests must be performed for each study phase, and the results of one study should not be generalized to another study. The FDA and EMA have defined different regulations to ensure that stem-cell-based products are consistently controlled through the use of different preclinical studies (in vitro and in vivo). Based on these preclinical data, the FDA and EMA have the authority to approve a clinical trial study, as discussed in this review.
Another challenge that researchers and companies face is the duration of a clinical trial study before a stem-cell-based product can be introduced onto the market. At present, hematopoietic progenitor cells are the only FDA-approved product for use in patients with defects in blood production, while other stem-cell-based products used in clinical trials have not yet been introduced to the market.
In the past few years, several clinical trials have been conducted using stem cells, most of which have indicated the safety and high efficiency of stem-cell-based therapies. An attractive future option for regenerative medicine is the use of cell derivatives, including exosomes, amniotic fluid, Wharton’s jelly, and so on, for the treatment of diseases. Recently, the safety and efficiency of these products have been evaluated and optimized in preclinical studies. In addition, regenerative medicine using modified stem cells and combinations of stem cells with scaffolds and chemicals to overcome stem cell therapy challenges and increase the associated efficiency are two important future directions of research. However, establishing a safe method for stem cell modification and moving this technology toward clinical trial studies requires many preclinical studies.
The regenerative medicine market is developing and, due to encouraging findings in preclinical studies and predictable economic benefits, competition has increased between companies focused on the development of cell products. Therefore, government agencies, industries, individuals, universities, and private organizations have invested heavily into the development of the regenerative medicine market in recent years, such that we can be more hopeful about the future of stem-cell-based therapies.
6. Conclusions
In recent years, regenerative medicine has become a promising treatment option for various diseases. Due to their therapeutic potential, including the inhibition of inflammation or apoptosis, cell recruitment, stimulation of angiogenesis, and differentiation, stem cells can been seen as good candidates for regenerative medicine. In the last 50 years, more than 40,000 research papers have focused on stem-cell-based therapies. In this review study, we present a general overview of the translation of stem cell therapy from scientific ideas to clinical applications. Multiple mechanisms causing disease could be reversed by stem cells, due to their tremendous therapeutic potential. However, preclinical studies including in vitro and in vivo experiments are necessary to evaluate the potential of stem-cell-based treatments. Through preclinical research, it is possible to present scientific evidence and optimal treatment options for subsequent clinical studies. Before starting a clinical trial based on preclinical data, the application must be approved by a relevant regulatory administration, such as the FDA, EMA, or another organization. If the application is for the use of a new drug (including stem cells) which has never been tested before, the submission of an IND is required for FDA approval. Approximately 50% of clinical trials using stem cells take 2 to 5 years to complete. To minimize possible side effects, every new stem cell product should be approved for clinical marketing only after completing Phase I–IV clinical trials successfully. Interestingly, the number of stem-cell-based companies aimed at introducing clinical applications has rapidly increased in recent years. Therefore, it may be possible to find stem-cell-based products on the clinical market in the near future. As described in this paper, there are several steps that should be carried out on the path from the laboratory to the clinical setting. To develop new stem-cell-based medicine for the clinical market, researchers should follow the guidelines suggested by the relevant authorities. Through these well-controlled development processes, researchers can achieve safe and effective stem-cell-based therapies, thus brings their research ideas into the clinical field.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
This review funded by National Institutes of Health grant: R01HD087417-01A1, R01HD094378-01, R01HD094380-01, R01HD100367-01, R01HD100563, R01HD100563.
Conflicts of Interest
The author has no conflicts of interest to declare.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Lab Resource - Editor's Choice Article Collection. Lab Resource articles are short, structured articles detailing the establishment and characterization of new pluripotent stem cell lines, generated by SCNT and reprogramming or the establishment of genetically modified stem cell sub-lines.. Collected below is a compilation of high quality Lab Resource articles, recently published in Stem Cell ...
About the journal. Stem Cell Research is dedicated to publishing high-quality manuscripts focusing on the biology and applications of stem cell research. Submissions to Stem Cell Research, may cover all aspects of stem cells, including embryonic stem cells, tissue-specific stem cells, cancer stem cells, developmental …. View full aims & scope.
Embryonic stem cells have been utilized in the past for research, but ethical concerns have led to them being replaced largely by stem cells derived from other origins. 12 Common tissues from which adult oligopotent and unipotent stem cells are isolated include bone marrow, adipose tissue, and trabecular bone. 13 Bone marrow has traditionally ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
Communicating Impactful Stem Cell Research. Stem Cell Reports, the ISSCR's open-access, online journal, has continued to publish impactful stem cell research while serving the stem cell community.In the last 12 months, the journal has published primary research Articles, Reviews, and Perspectives across a breadth of stem cell science—from fundamental research to translational discoveries ...
Since stem cells were first discovered, researchers have identified distinct stem cell populations in different organs and with various functions, converging on the unique abilities of self ...
Stem Cell Research & Therapy (2023) Despite many reports of putative stem-cell-based treatments in genetic and degenerative disorders or severe injuries, the number of proven stem cell therapies ...
Cell Stem Cell is a broad-spectrum journal that covers the entire spectrum of stem cell biology. Topics covered include embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, stem cell differentiation, epigenetics, stem cell genomics and systems biology, genome reprogramming, cancer stem cells, stem cell niches, stem-cell-based disease models, nuclear transfer ...
Generation of the induced pluripotent stem cell line (ZSPHARi001-A) from a patient with recessive dystrophic epidermolysis bullosa carrying compound heterozygous mutation in the COL7A1 gene. Yeye Zhang, Jun Fan, Guangzhao Lu, Genying Xu, Qianzhou Lv. Article 102672.
Stem cells articles from across Nature Portfolio. Atom. RSS Feed. Stem cells are cells that have the capacity to self-renew by dividing and to develop into more mature, specialised cells. Stem ...
Stem cell-based therapies. Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies ().Stem cells offer the perfect solution when there is a need for tissue and organ ...
An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials. The journal also provides reviews, commentaries, reports and methods.
Pluripotent stem cells have the ability to differentiate into all cells and tissues within the human body, and as a result they are attractive resources for use in basic research, drug discovery and regenerative medicine. In order to successfully achieve this application, starting cell sources ideally require in-depth characterisation to confirm their pluripotent status and their ability to ...
Abstract. The purpose of this editorial is to highlight recent developments in molecular biology tools and techniques in stem cell research and their applications to human diseases. Recent advancements in stem cell research and regenerative medicine are offering immense hope to cure human diseases and injuries, such as cancer, diabetes ...
Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells are the source of all tissues, understanding their ...
The ethical implications of stem cell research are often described in terms of risks, side effects, safety, and therapeutic value, which are examples of so-called hard impacts. Hard impacts are typically measurable and quantifiable. To understand the broader spectrum of ethical implications of stem cell research on science and society, it is ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory ...
Adult stem cells are the only stem cell type that has shown evidence of success when it comes to patients, and treating patients is supposedly the ultimate goal for stem cell research, certainly the justification for the huge sums of money poured into the field. By the end of 2012, over 1 million people around the globe had already received ...
Stem cell research has emerged as a promising field with the potential to revolutionize regenerative medicine and disease treatment. Recent advancements include the development of more efficient methods for generating induced pluripotent stem cells (iPSCs), which can be derived from adult cells and reprogrammed to an embryonic-like state.
Stem Cell Research is dedicated to publishing high-quality manuscripts focusing on the biology and applications of stem cell research. ... Lab resources. Graphical review. Research papers: Research papers should not exceed 55,000 characters (including spaces, references, methods, figure legends, and an abstract of 200 words or less). We accept ...
The Executive Order states that the Secretary of Health and Human Services, through the Director of NIH, may support and conduct responsible, scientifically worthy human stem cell research, including human embryonic stem cell (hESC) research, to the extent permitted by law. These Guidelines implement Executive Order 13505, as it pertains to ...
Stem cell therapy is a novel therapeutic approach that utilizes the unique properties of stem cells, including self-renewal and differentiation, to regenerate damaged cells and tissues in the human body or replace these cells with new, healthy and fully functional cells by delivering exogenous cells into a patient. 7 Stem cells for cell-based ...
To populate the knowledge resource, it manually curates literature and patent reports, integrates information provided by cell line collections and companies developing/distributing cell lines, and increasingly from direct submissions by research groups and consortia. 20% of the entries in Cellosaurus are pluripotent stem cell lines, in total ...
Lab Resource: Stem Cell Line. Last update 5 February 2020. This Special Issue collates Lab Resource articles published in Stem Cell Research. Lab Resource articles detail the establishment of new pluripotent stem cell lines derived from embryos, generated by SCNT and reprogramming or the establishment of genetically modified stem cell sub-lines.
The National Institute for Medical Research has a 2001 "Mill Hill Essay" on stem cell therapy and research. Use of human stem cells in research raises ethical, legal, religious, and policy questions which were addressed in a 1999 report of the American Association for the Advancement of Science and the Institute for Civil Society.
From 1971 to 2021, 40,183 research papers were published regarding stem-cell-based therapies. All of these studies were conducted around discoveries and for the goal of "Stem Cell Therapy" based on the therapeutic efficacy of stem cells . As basic stem cell research has soared over the past few years, "translation research", a ...