- Open access
- Published: 09 August 2023

The effectiveness of theory-based smoking cessation interventions in patients with chronic obstructive pulmonary disease: a meta-analysis
- Mengjing Han 1 na1 ,
- Yingping Fu 1 na1 ,
- Quanyue Ji 1 ,
- Xiaoli Deng 2 &
- Xuewen Fang 2
BMC Public Health volume 23 , Article number: 1510 ( 2023 ) Cite this article
3440 Accesses
1 Citations
15 Altmetric
Metrics details
Smoking cessation can effectively reduce the risk of death, alleviate respiratory symptoms, and decrease the frequency of acute exacerbations in patients with chronic obstructive pulmonary disease (COPD). Effective smoking cessation strategies are crucial for the prevention and treatment of COPD. Currently, clinical interventions based on theoretical frameworks are being increasingly used to help patients quit smoking and have shown promising results. However, theory-guided smoking cessation interventions have not been systematically evaluated or meta-analyzed for their effectiveness in COPD patients. To improve smoking cessation rates, this study sought to examine the effects of theory-based smoking cessation interventions on COPD patients.
We adhered to the PRISMA guidelines for our systematic review and meta-analysis. The Cochrane Library, Web of Science, PubMed, Embase, Wanfang, CNKI, VIP Information Services Platform, and China Biomedical Literature Service System were searched from the establishment of the database to April 20, 2023. The study quality was assessed using the Cochrane Collaboration's risk assessment tool for bias. The revman5.4 software was used for meta-analysis. The I 2 test was used for the heterogeneity test, the random effect model and fixed effect model were used for meta-analysis, and sensitivity analysis was performed by excluding individual studies.
A total of 11 RCTs involving 3,830 patients were included in the meta-analysis. Results showed that theory-based smoking cessation interventions improved smoking cessation rates, quality of life, and lung function in COPD patients compared to conventional nursing. However, these interventions did not significantly affect the level of nicotine dependence in patients.
Theory-based smoking cessation intervention as a non-pharmacologically assisted smoking cessation strategy has a positive impact on motivating COPD patients to quit smoking and improving their lung function and quality of life.
Trial registration
PROSPERO registration Number: CRD42023434357.
Peer Review reports
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung disease characterized by persistent respiratory symptoms and airflow limitation caused by airway and/or alveolar abnormalities, as defined by the 2023 Global Initiative for Chronic Obstructive Lung Disease (GOLD) [ 1 ]. In China, the overall prevalence of COPD is 8.6%, with a rate of 13.7% in the population over 40 years old [ 2 ]. Smoking is a major risk factor for COPD, with smokers having 10.92 times the risk of developing COPD compared to non-smokers [ 3 ]. Additionally, smoking COPD patients have more respiratory symptoms than non-smokers and higher mortality rates [ 4 ]. Smoking cessation is considered the most effective and cost-effective strategy for preventing and treating COPD [ 5 ]. For COPD smokers, it is important to adopt effective methods to control their smoking behavior [ 6 ]. However, smoking cessation is challenging, and conventional approaches may not be effective for all patients. Although conventional smoking cessation methods such as telephone hotlines [ 7 ], medication [ 8 ], and comprehensive interventions [ 9 ] have been shown to improve patients' smoking cessation rates and lung function to some extent, patients' smoking cessation behavior is highly influenced by their health knowledge and behavior change.
Therefore, some scholars have attempted to use theory-guided interventions to improve COPD patients' smoking cessation rates, achieving good results. Currently, the theories related to the management of smoking cessation in COPD include "timing theory" [ 10 ], "theory of planned behavior" [ 11 ], "the 5A nursing model" [ 12 ], and "cognitive-behavioral theory" [ 13 ]. The timing theory was proposed by Canadian scholars Cameron et al [ 10 ]. According to this theory, targeted intervention should be implemented according to the disease stage of patients, emphasizing the importance of understanding the different stages of the disease, focusing on the patients themselves, increasing their confidence in treating the disease, improving their current negative behaviors and emotions, and ultimately achieving a positive health outcome [ 14 , 15 ]. The planned behavior theory was proposed by Ajzen [ 11 ], who believed that individual behavior is mainly influenced by individual behavioral intentions, including attitudes, subjective norms, and perceived behavioral control. Attitude refers to the positive or negative evaluation and experience of behavior; subjective norms refer to the social pressure felt when adopting behavior; and perceived behavioral control refers to self-efficacy and control over behavior [ 16 , 17 ]. The 5A nursing model [ 12 ] includes five components: assess, advise, agree, assist, and arrange. The aim is to improve patients' self-efficacy and self-management skills [ 18 , 19 ]. Cognitive-behavioral theory is an integration of cognitive theory and behavioral theory that utilizes methods to change negative cognitions, beliefs, and behaviors [ 13 ]. Cognitive-behavioral interventions involve selecting theories related to cognition and/or behavior, considering individual, behavioral, and environmental factors, and designing intervention plans based on the individual's understanding of behavior change and available resources. This approach promotes the formation of healthy behaviors and corrects negative ones [ 20 ]. Theory-based smoking cessation interventions are designed to provide patients with the knowledge, skills, and support necessary to quit smoking successfully [ 21 ]. By understanding these theories, healthcare providers can design interventions that are tailored to the individual patient's needs and increase the likelihood of successful smoking cessation [ 22 ].
Currently, there has yet to be a systematic evaluation or meta-analysis of the effectiveness of theory-based smoking cessation interventions in COPD patients. Therefore, this study aims to synthesize randomized controlled trials of theory-based smoking cessation interventions in COPD patients and evaluate their effectiveness and impact on patients through meta-analysis, providing evidence-based medicine for their clinical application.
Our aim was to evaluate the effectiveness of theory-based smoking cessation interventions in patients with COPD.
We followed the Cochrane Collaboration's Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 23 ]. The review protocol is registered on the PROSPERO database (Registration No: CRD42023434357).
Literature search
Two researchers searched for RCT studies published in the Cochrane Library, Web of Science, PubMed, Embase, Wanfang Knowledge Service Platform, CNKI, VIP Resource Integration Service Platform, and China Biomedical Literature Database. The search terms included chronic obstructive pulmonary disease*/chronic obstructive lung disease*/COPD, smoking/smoking cessation/smoking intervention, theory/model/theoretical. We conducted the search by combining subject terms and free words, and expanded our search by tracing the references included in the study in a snowball manner. The retrieval deadline for this search is from the establishment of the database up until April 20, 2023.
Study selection
The inclusion and exclusion criteria were formulated according to the Population, Intervention, Comparison, Outcome, Study design (PICOS) framework. Inclusion criteria: (i) the study participants met the diagnostic criteria for COPD of the Chinese Medical Association Respiratory Disease Society (2021 revised edition) [ 24 ] and also met the relevant criteria for tobacco dependence in the Chinese Clinical Smoking Cessation Guidelines (2015 edition) [ 25 ]; (ii) the intervention was based on theoretical smoking cessation methods; (iii) the outcome indicators: at least one of smoking cessation rate, nicotine dependence level, lung function, quality of life, clinical composite symptom score, and number of clinical symptom exacerbations; (iv) the study type was a randomized controlled trial. Exclusion criteria: Exclusion criteria: (i) duplicate publications; (ii) there were no relevant outcome indicators; (iii) literature with incomplete data and outcome index data that cannot be transformed and used; (iv) literature of low quality (based on a Cochrane Collaboration risk of bias assessment quality grade of C).
Quality assessment
The Cochrane Collaboration's risk of bias assessment tool (RoB 2.0) [ 26 ] was used to evaluate the methodological quality of the included studies. Involving seven items: (i) random sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinding of outcome assessment, (v) incomplete outcome data (loss to follow-up or withdrawal), (vi) selective reporting, (vii) other biases. High-risk, low-risk, and unclear were used to evaluate the risk of bias for each item. If all of the above criteria are fully met, the study quality level is A, indicating a low possibility of various biases occurring. If some of the above criteria are met, the study quality level is B, indicating a moderate possibility of bias occurring. If none of the above criteria are met, the study quality level is C, indicating a high possibility of bias occurring. In the event of disagreement between the two researchers, a third-party researcher should be consulted to reach a consensus.
Data extraction
Two researchers independently screened articles, extracted data, and cross-checked them. The data were extracted according to the designed extraction strategy, which included: (i) basic information of the included studies, including title, first author, publication year, abstract, and source of the literature; (ii) study characteristics, including sample size, age of the experimental and control groups, and intervention measures; (iii) outcome indicators, including observation indicators, measurement tools or assessment criteria, measurement values, and research conclusions.
Data synthesis and analysis
RevMan5.4 software was used for meta-analysis. The heterogeneity test was performed using the I 2 test. If P>0.1 and I 2 <50%, heterogeneity was considered acceptable, and the fixed effect model was selected; if P ≤0.1 and I 2 ≥50%, indicated that there was heterogeneity among studies, and the random effect model was selected. A sensitivity analysis was conducted to identify sources of heterogeneity. The effect size of count data was expressed as odds ratio (OR) with a 95% confidence interval (CI), while continuous data were expressed as mean difference (MD) or standardized mean difference (SMD) with a 95% confidence interval (CI).
Literature search outcomes
We searched 431 relevant articles in the database and obtained one article by reading the references to related studies. The EndNote software was applied to remove 207 duplicate literatures. 156 articles were excluded based on reading the titles and abstracts, as they included non-randomized controlled trials, inconsistent research subjects, and poor correlation. Further reading of the full text was re-screened to exclude 58 papers with the same data, outcome indicators that did not match, data that could not be translated into application, and lower quality. Ultimately, we included 11 articles [ 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ] in our analysis, consisting of 9 Chinese-language articles [ 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ] and 2 English-language articles [ 36 , 37 ]. A total of 3830 patients were included, including 1989 in the experimental group and 1841 in the control group. The literature screening process and results are shown in Fig. 1 .
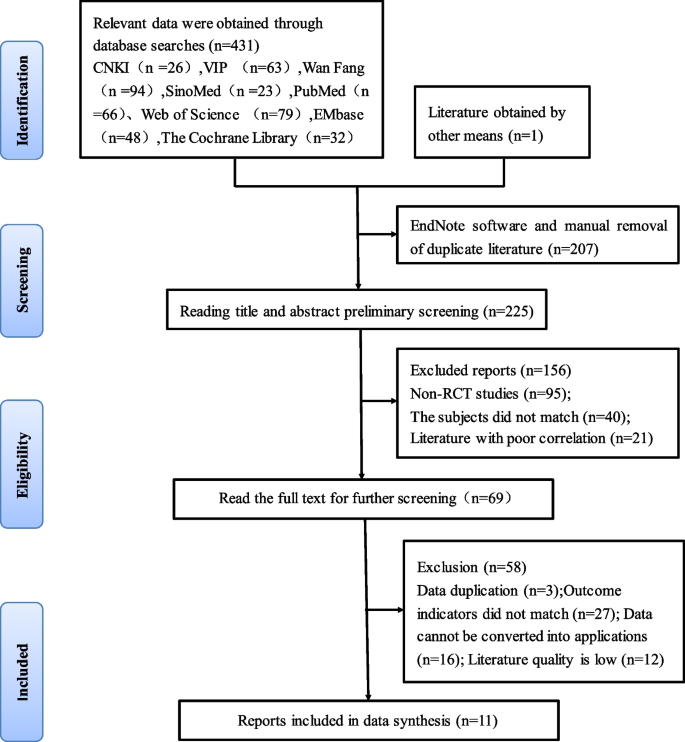
Flow chart of literature screening
The basic characteristics of studies
11 RCTs published between 2013 and 2023 were included in the meta-analysis. The studies were based on three different theories, including seven on the timing theory [ 27 , 28 , 29 , 30 , 31 , 32 , 33 ], two on the 5A nursing model [ 34 , 35 ], and two on the cognitive-behavioral theory [ 36 , 37 ]. One study on the theory of planned behavior [ 38 ] was not included in the meta-analysis because it was not an RCT. The basic characteristics of the literature are shown in Table 1 .
Two researchers evaluated and graded the 11 included studies according to the RTC bias risk assessment tool [ 26 ] provided by the Cochrane Collaboration. The results are shown in Table 2 and Fig. 2 . All studies were graded B in quality. Ten studies [ 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 ] described the generation of randomized sequences, with seven studies [ 27 , 28 , 29 , 30 , 32 , 35 , 37 ] using random number tables for grouping, one study [ 31 ] using odd-even numbering for grouping, one study [ 34 ] grouping according to patient preference, and one study [ 36 ] mentioning randomization but not specifying the method used. None of the 11 studies had any dropouts or missing data reports, and the experimental and control groups were comparable in terms of baseline levels before the intervention ( P > 0.05). This suggests that the methodological quality of the included literature is fair, the risk of bias is low, and the credibility of the evidence is high.
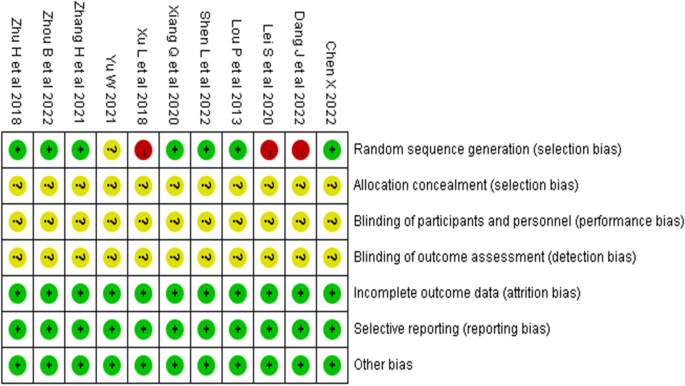
Risk of bias summary
Meta-analysis results and sensitivity analysis
Smoking cessation rates.
Ten studies [ 27 , 28 , 29 , 33 , 35 , 36 , 37 ] were evaluated for smoking cessation rates. Four studies [ 27 , 28 , 30 , 32 ] reported smoking cessation rates at one month after the intervention, and nine studies [ 27 , 29 , 30 , 31 , 32 , 33 , 35 , 36 , 37 ] reported smoking cessation rates at six months after the intervention. Fewer studies reported smoking cessation rates at three and twelve months after the intervention, so they were not included in the meta-analysis. The heterogeneity test was conducted, I 2 =48% and P =0.03, and the heterogeneity was acceptable. A fix-effects model was used for analysis, which showed that smoking cessation interventions at different intervention times were more effective in increasing smoking cessation rates than the control group [ OR =4.04, 95%CI (3.23, 5.06), P <0.001, Fig. 3 ].
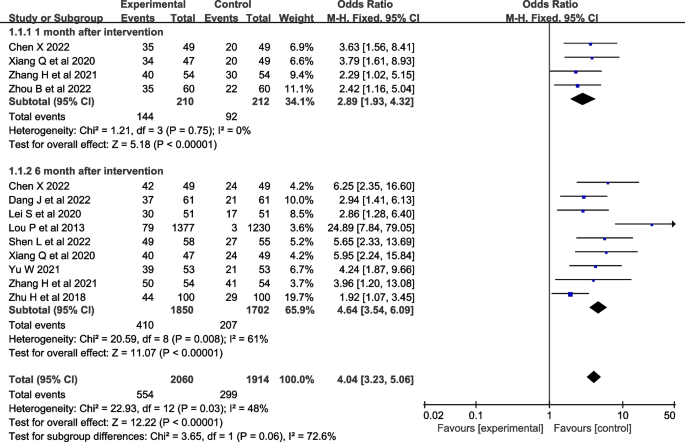
Forest plot of smoking cessation rate
Nicotine dependence
Seven studies [ 27 , 28 , 29 , 30 , 31 , 32 , 37 ] evaluated nicotine dependence. However, one study [ 27 ] used percentile and interquartile range to describe nicotine dependence, two studies [ 29 , 32 ] used percentile and interquartile range to describe nicotine dependence, and four studies [ 28 , 30 , 31 , 37 ] described nicotine dependence as mild, moderate, and severe, so four studies [ 28 , 30 , 31 , 37 ] were included in the meta-analysis. A heterogeneity test was conducted, resulting in an I 2 =71% and P <0.001. A random-effects model was used for analysis, which showed that the effect of theory-based smoking cessation interventions on nicotine dependence could not be determined [ OR =1.00, 95%CI (0.78, 1.29), P <0.001, Fig. 4 ]. Sensitivity analysis was performed by excluding individual studies, and the results still showed significant heterogeneity, indicating that the heterogeneity was stable.
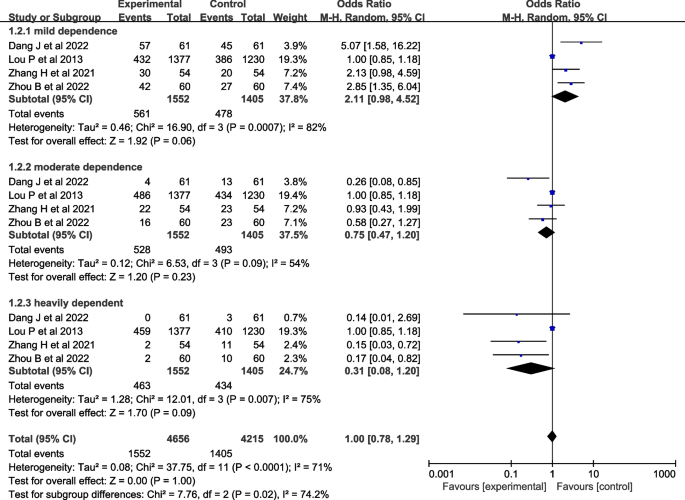
Forest plot of nicotine dependence level
Pulmonary function
Seven studies [ 28 , 29 , 30 , 32 , 33 , 35 , 36 ] evaluated lung function, including FEV1 (forced expiratory volume in the first second) [ 28 , 29 , 32 , 33 , 34 , 35 ], FEV1/Pre (ratio of forced expiratory volume in the first second to estimated vital capacity) [ 30 , 32 , 33 , 34 , 35 , 36 ], and FEV1/FVC (ratio of forced expiratory volume in the first second to forced vital capacity) [ 28 , 30 , 32 , 33 , 35 , 36 ]. The heterogeneity test showed that there was significant heterogeneity in FEV1 and FEV1/FVC among the studies ( I 2 >50%, P <0.001), and there was no heterogeneity in FEV1/Pre ( I 2 =0%, P =0.86). A random-effects model was used for analysis, which showed that the effect of theory-based smoking cessation interventions on lung function was better in the experimental group than in the control group [ MD =0.51, 95% CI (0.28, 0.74), P <0.001, Fig. 5 ]. Sensitivity analysis was performed by excluding individual studies, and the results still showed significant heterogeneity, indicating that the heterogeneity was stable.
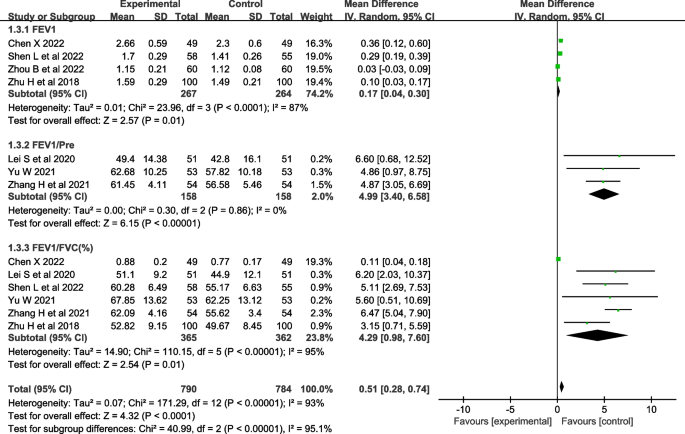
Forest plot of lung function
Quality of life
Four studies [ 29 , 30 , 31 , 35 ] evaluated quality of life. One study [ 29 ] used the Seattle COPD questionnaire [ 39 ] for evaluation, and three studies [ 30 , 31 , 35 ] used the St. George's Respiratory Questionnaire (SGRQ) [ 40 ] for evaluation, so three studies [ 30 , 31 , 35 ] were included in the meta-analysis. The heterogeneity test showed that there was significant heterogeneity ( I 2 =78%, P <0.001). A random-effects model was used for analysis, which showed that the effect of theory-based smoking cessation interventions on quality of life was better in the experimental group than in the control group [ MD =-4.87, 95% CI (-6.34, -3.40), P < 0.001, Fig. 6 ]. Sensitivity analysis was performed by excluding individual studies, and the results still showed significant heterogeneity, indicating that the heterogeneity was stable.
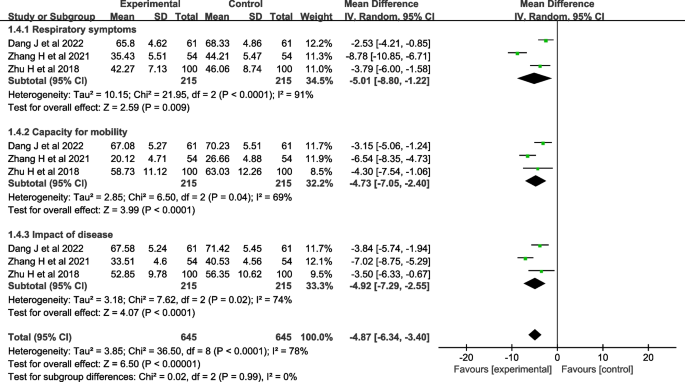
Forest plot of quality of life
Clinical symptom score
Two studies [ 28 , 34 ] reported clinical symptom scores, which are not suitable for meta-analysis because of the paucity of literature. Both studies [ 28 , 34 ] showed that the clinical composite symptom scores were significantly lower in the experimental group than in the control group ( P <0.05).
Frequency of clinical symptom exacerbation
Two studies [ 33 , 34 ] reported the frequency of clinical symptom exacerbation, which was not suitable for meta-analysis due to the small number of studies. The two studies [ 33 , 34 ] both showed that the frequency of clinical symptom aggravation in the experimental group was significantly lower than that in the control group ( P <0.05).
This study conducted a meta-analysis of data from 11 randomized controlled trials to assess the effectiveness of smoking cessation interventions in patients with COPD. This meta-analysis demonstrated that based on timing theory [ 10 ], 5A nursing model [ 12 ], and cognitive behavioral theory [ 13 ] smoking cessation interventions significantly improved smoking cessation rates, lung function, and quality of life in COPD patients. However, these interventions did not significantly affect nicotine dependence levels.
The timing theory proposes that smoking cessation strategies should be targeted based on the disease stage of COPD patients. This approach emphasizes understanding the different stages of the disease, improving negative behaviors, and increasing patients' confidence to quit smoking [ 27 , 28 , 29 , 30 , 31 , 32 , 33 ]. The 5A nursing model involves individualized assessment, setting goals, and providing help and regular follow-up to change COPD patients' cognition of the disease and the harm of smoking so that they can establish correct health beliefs [ 34 , 35 ]. Cognitive behavioral theory emphasizes the importance of addressing patients' smoking-related thoughts and behaviors for successful smoking cessation [ 36 , 37 ]. Healthcare providers can develop interventions by targeting the specific needs of patients at each stage of the disease, identifying the underlying causes of their smoking behavior, and selecting an appropriate rationale. The goal is to help COPD patients develop effective strategies to quit smoking and manage their disease symptoms. This study provides valuable insights into the effectiveness of theory-based smoking cessation interventions for COPD patients.
Theory-based smoking cessation interventions can improve the smoking cessation rate of COPD patients
The findings of this study suggest that theory-based smoking cessation interventions can improve smoking cessation rates in patients with COPD. Given the strong association between COPD and smoking, it is crucial to address smoking cessation as a key component of COPD management [ 41 ]. Previous studies mainly used smoking cessation drugs to relieve withdrawal symptoms or used auxiliary methods to improve the success rate of smokers who wanted to quit, but not all patients were willing to accept or needed to use smoking cessation drugs to quit successfully [ 42 , 41 , 42 , 43 , 44 ]. The positive impact of theory-based smoking cessation interventions on smoking cessation rates can be attributed to their emphasis on understanding patients' individual needs, motivations, and barriers to quitting smoking, as well as providing tailored support and strategies to overcome these challenges. By addressing the psychological aspects of smoking behavior and incorporating behavioral change theories, these interventions can help patients develop the necessary skills and confidence to successfully quit smoking. The use of theory-based interventions is particularly promising because it allows for a more systematic and evidence-based approach to smoking cessation. It is more conducive for patients to form a strong desire to quit smoking and take action to bring about more effective and sustainable smoking cessation effects for patients. The sensitivity analysis showed that the heterogeneity among the studies included in the meta-analysis was acceptable, indicating that the evidence results were relatively reliable.
The effect of theory-based smoking cessation interventions on nicotine dependence levels is uncertain
Nicotine dependence, also known as tobacco dependence, is a chronic disease [ 45 ]. A considerable number of COPD patients, know the harm of smoking and have the intention to quit, but because they are addicted to smoking, it is difficult to quit, which means that their degree of tobacco dependence has not improved and they still have a high risk of relapse after discharge [ 46 ]. The lack of significant effect on nicotine dependence levels may be due to several factors, including the relatively short duration of the interventions and follow-up periods in the included studies, as well as potential differences in the measurement and reporting of nicotine dependence levels across studies. For patients, in addition to providing professional and scientific help throughout the smoking cessation process, better results can be achieved by combining drug control and encouraging family members to provide adequate emotional support throughout the process. It is recommended that future studies be guided by theory and combined with pharmacological control to investigate the improvement effect.
Theory-based smoking cessation interventions improve lung function and quality of life in COPD patients
Lung function is the gold standard for diagnosing and evaluating the severity of COPD, which can objectively reflect the degree of airflow restriction or obstruction in patients [ 47 ]. Due to the intake of a large amount of nicotine, tar, and some radioactive substances, COPD smokers have a serious impact on their lung health, which not only causes inflammatory changes but also threatens the lung function of the body's respiratory system [ 48 ]. As the duration of smoking increases, the lung function of patients also decreases, which further triggers a series of lung diseases and reduces their quality of life [ 49 , 50 ], so it is urgent to control their smoking behavior.
The improvement in lung function observed in this meta-analysis is consistent with previous research showing that smoking cessation can lead to significant improvements in lung function and reduce the risk of COPD exacerbations. By helping patients quit smoking, theory-based interventions may contribute to slowing down the progression of COPD and improving patients' overall respiratory health. The observed improvement in quality of life is also an important finding, as COPD is known to have a significant impact on patients' physical, emotional, and social well-being. By addressing both the physical and psychological aspects of smoking behavior, theory-based interventions may help improve patient’s overall well-being and quality of life.
Limitations
Several limitations of this study remain: (i) Due to language limitations, only publicly available Chinese and English literature was searched, which may result in incomplete literature collection; (ii) The included studies did not mention allocation concealment and blinding methods, resulting in medium-quality research quality, which may affect the reliability of the results to some extent. It is hoped that subsequent relevant research will further improve the rigor of allocation concealment and blinding methods to achieve higher quality levels. (iii) Currently, most studies only report short-term effects of theory-based smoking cessation interventions on COPD patients.
The findings of this study demonstrated that implementing theory-based smoking cessation interventions in conventional healthcare can have a positive effect on the smoking cessation rate, lung function, and quality of life of COPD patients. It is recommended that these interventions be widely implemented in clinical practice. Further investigation is required to confirm these findings due to the limitations in the standardization and homogeneity of the included studies.
Availability of data and materials
The study is conducted using open-source data from published articles. Additional data can be made available upon request to Mengjing Han([email protected]).
Liang Z, Wang F, Chen Z, et al. Updated key points interpretation of global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Chinese General Practice. 2023;26(11):1287–98.
Google Scholar
Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17.
PubMed Google Scholar
Salvi SS, Brashier BB, Londhe J, et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res. 2020;21(1):50.
CAS PubMed PubMed Central Google Scholar
Li X, Wu Z, Xue M, et al. Smoking status affects clinical characteristics and disease course of acute exacerbation of chronic obstructive pulmonary disease: a prospectively observational study. Chron Respir Dis. 2020;17:1479973120916184.
PubMed PubMed Central Google Scholar
Hirai K, Tanaka A, Homma T, et al. Characteristics of and reasons for patients with chronic obstructive pulmonary disease to continue smoking, quit smoking, and switch to heated tobacco products. Tob Induc Dis. 2021;19(1):85.
Thomson NC. The role of smoking in asthma and chronic obstructive pulmonary disease overlap. Immunol Allergy Clin North Am. 2022;42(3):615–30.
McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5(5):CD011425.
Antoniu SA, Buculei I, Mihaltan F, et al. Pharmacological strategies for smoking cessation in patients with chronic obstructive pulmonary disease: a pragmatic review. Expert Opin Pharmacother. 2021;22(7):835–47.
CAS PubMed Google Scholar
Saeed MI, Sivapalan P, Eklöf J, et al. TOB-STOP-COP (TOBacco STOP in COPd trial): study protocol-a randomized open-label, superiority, multicenter, two-arm intervention study of the effect of “high-intensity” vs. “low-intensity” smoking cessation intervention in active smokers with chronic obstructive pulmonary disease. Trials. 2020;21(1):730.
Cameron JI, Gignac MA. “Timing It Right”: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns. 2008;70(3):305–14.
Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. Journal of Applied Social Psychology. 2002;32(4):665–83.
Martínez C, Castellano Y, Andrés A, et al. Factors associated with implementation of the 5A’s smoking cessation model. Tob Induc Dis. 2017;1(1):15:41.
Zeng G, Wang W, Qin X. Research progress on the theory and mechanism of health behavior intervention. Chinese Nursing Research. 2021;35(8):1428–34.
Jiang X, Gu Q, Jiang Z, et al. Effect of family-centered nursing based on timing it right framework in patients with acute cerebral infarction. Am J Transl Res. 2021;13(4):3147–55.
Xu Y, Song W, Wang Q, et al. The effect of a psychological nursing intervention program based on the “Timing it Right” (TIR) framework on elderly patients’ anxiety, psychology, and self-efficacy. Am J Transl Res. 2021;13(8):9600–6.
Bosnjak M, Ajzen I, Schmidt P. The Theory of Planned Behavior: Selected Recent Advances and Applications. Eur J Psychol. 2020;16(3):352–6.
Şanlıtürk D, Ayaz-Alkaya S. The Effect of a Theory of Planned Behavior Education Program on Asthma Control and Medication Adherence: A Randomized Controlled Trial. J Allergy Clin Immunol Pract. 2021;9(9):3371–9.
Zhang X, Lai M, Wu D, et al. The Effect of 5A nursing intervention on living quality and self-care efficacy of patients undergoing chemotherapy after hepatocellular carcinoma surgery. Am J Transl Res. 2021;13(6):6638–45.
Rokni S, Rezaei Z, Noghabi AD, et al. Evaluation of the effects of diabetes self-management education based on 5A model on the quality of life and blood glucose of women with gestational diabetes mellitus: an experimental study in eastern Iran. J Prev Med Hyg. 2022;63(3):E442–7.
Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis. 2020;15(1):903–19.
Bhatt G, Goel S, Soundappan K, et al. Theoretical constructs of smoking cessation among current tobacco smokers in India: a secondary analysis of Global Adult Tobacco Survey-2 (2016–2017). BMJ Open. 2022;12(1): e050916.
Campbell KA, Fergie L, Coleman-Haynes T, et al. Improving behavioral support for smoking cessation in pregnancy: What are the barriers to stopping and which behavior change techniques can influence these? Application of theoretical domains framework. Int J Environ Res Public Health. 2018;15(2):359.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Chronic obstructive pulmonary Disease Group, Respiratory Branch, Medical Association, et al. Guidelines for the management and Treatment of chronic obstructive pulmonary Disease (2021 revised). Chinese J Tubercul Resp Dis. 2021;44(3):170–205.
National health and family planning commission of the people’s republic of China. Chinese clinical smoking cessation guidelines (2015 edition). Chinese J Health Management. 2016;10(2):88–95.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Xiang Q, Zhang C, Xu S, et al. Effects of a smoking cessation intervention based on Timing It Right in patients with chronic obstructive pulmonary disease. Chinese Journal of Nursing. 2020;55(5):684–9.
Zhou B, Chui Y, Wang X. Application of smoking cessation intervention based on timing theory in elderly patients with COPD complicated with tobacco dependence. Chinese Journal of Modern Nursing. 2022;28(36):5084–9.
Shen L, Zhang Q, Wang J, et al. Application effect of timing theory based smoking cessation intervention in patients with chronic obstructive pulmonary disease. Modern Nurse. 2022;29(10):24–7.
Zhang H, Yan X. Clinical effect of smoking cessation intervention based on timing theory on patients with chronic obstructive pulmonary disease. Journal of Clinical and Pathological Research. 2021;41(9):2052–8.
Dang J. Effect of wooden ball training combined with timing theory of smoking cessation intervention on cardiopulmonary endurance and quality of life of patients with chronic obstructive pulmonary disease in stable stage. Medical Journal of Liaoning. 2022;36(2):49–52.
Chen X. Application of smoking cessation intervention based on timing theory in patients with chronic obstructive pulmonary disease. The Journal of Medical Theory and Practice. 2022;35(2):343–5.
Yu W. Effect of a staged smoking cessation intervention program based on timing theory on the prognosis of elderly COPD patients. Zhejiang Clinical Medicine Journal. 2021;23(11):1642–4.
Xu L, Hu X, Sun H, et al. Application of smoking cessation intervention based on 5A model in patients with chronic obstructive pulmonary disease. Nursing Practice and Research. 2018;15(10):33–5.
Zhu H, Zhang Y, Zeng Y. Effects of smoking cessation intervention on smoking cessation efficacy in patients with chronic obstructive pulmonary disease. Academic Journal of Guangzhou Medical University. 2018;46(02):86–9.
Lei S, Li M, Duan W, et al. The long-term outcomes of tobacco control strategies based on the cognitive intervention for smoking cessation in COPD patients. RESP MED. 2020;172:106155.
Lou P, Zhu Y, Chen P, et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Fam Pract. 2013;14:91.
Xiang Q, Zeng H, Yu F, et al. Application of smoking cessation intervention based on planned behavior theory in community patients with chronic obstructive pulmonary disease. Journal of Nursing Administration. 2022;22(12):853–7+867.
Tu SP, McDonell MB, Spertus JA, et al. A new self-administered questionnaire to monitor health-related quality of life in patients with COPD. Ambulatory Care Quality Improvement Project (ACQUIP) Investigators. Chest. 1997;112(2):614–22.
Hardin M, Rennard SI. What’s New with the St George’s Respiratory Questionnaire and Why Do We Care? Chronic Obstr Pulm Dis. 2017;4(2):83–6.
Liu Z, Xiao D, Wang C. Smoking cessation is the most effective measure for the prevention and treatment of chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases. 2017;40(12):894–7.
Wei X, Guo K, Shang X, et al. Effects of different interventions on smoking cessation in chronic obstructive pulmonary disease patients: a systematic review and network meta-analysis. Int J Nurs Stud. 2022;136(1): 104362.
van Eerd EA, van der Meer RM, van Schayck OC, et al. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;1(8):CD010744.
Feng L, Lv X, Wang Y, et al. Developments in smoking cessation interventions for patients with chronic obstructive pulmonary disease in the past 5 years: a scoping review. Expert Rev Respir Med. 2022;16(7):749–64.
Wills L, Kenny PJ. Addiction-related neuroadaptations following chronic nicotine exposure. J Neurochem. 2021;157(5):1652–73.
Hashimoto R, Tomioka H, Wada T, et al. Outcomes and predictive factors for successful smoking cessation therapy in COPD patients with nicotine dependence. Respir Investig. 2020;58(5):387–94.
Neder JA, Torres JP, Milne KM, et al. Lung Function Testing in Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2020;41(3):347–66.
Keogan S, Alonso T, Sunday S, et al. Lung function changes in patients with chronic obstructive pulmonary disease (COPD) and asthma exposed to secondhand smoke in outdoor areas. J Asthma. 2021;58(9):1169–75.
Ding Q, Li J, Xu S, et al. Different smoking statuses on survival and emphysema in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17(1):505–15.
Boulet LP, Boulay ME, Milot J, et al. Longitudinal comparison of outcomes in patients with smoking-related asthma-COPD overlap and in non-smoking asthmatics with incomplete reversibility of airway obstruction. Int J Chron Obstruct Pulmon Dis. 2019;14(1):493–8.
Download references
Acknowledgements
Not applicable.
This study was supported by the Open Program of the Clinical Medical Center of the First People's Hospital of Yunnan Province, PRC (Grant NO. 2021LCZXXF-HX05).
Author information
MengjingHan and YingpingFu contributed equally to this work.
Authors and Affiliations
Yunnan University of Chinese Medicine, Kunming, Yunnan, People’s Republic of China
Mengjing Han, Yingping Fu & Quanyue Ji
The First People’s Hospital of Yunnan Province, Kunming, Yunnan, People’s Republic of China
Xiaoli Deng & Xuewen Fang
You can also search for this author in PubMed Google Scholar
Contributions
Research plan and framework: Mengjing Han. Data acquisition and analysis: Mengjing Han, Yingping Fu. Methodological approach: Xiaoli Deng, Xuewen Fang. Validation: Quanyue Ji. Drafting of the manuscript: Mengjing Han, Yingping Fu. Critical revision: Mengjing Han, Xiaoli Deng, Xuewen Fang. The work was equally contributed by Mengjing Han and Yingping Fu.
Corresponding author
Correspondence to Xiaoli Deng .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Search terms and strategies.
Additional file 2.
PRISMA Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Han, M., Fu, Y., Ji, Q. et al. The effectiveness of theory-based smoking cessation interventions in patients with chronic obstructive pulmonary disease: a meta-analysis. BMC Public Health 23 , 1510 (2023). https://doi.org/10.1186/s12889-023-16441-w
Download citation
Received : 28 June 2023
Accepted : 02 August 2023
Published : 09 August 2023
DOI : https://doi.org/10.1186/s12889-023-16441-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic obstructive pulmonary disease
- Smoking cessation
- Clinical trial
- Meta-analysis
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 12, Issue 6
- Systematic review of changed smoking behaviour, smoking cessation and psychological states of smokers according to cigarette type during the COVID-19 pandemic
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-2567-2845 Hae-ryoung Chun 1 ,
- Eunsil Cheon 1 ,
- http://orcid.org/0000-0002-5094-6107 Ji-eun Hwang 2
- 1 Graduate School of Public Health , Seoul National University , Gwanak-gu , Seoul , Republic of Korea
- 2 College of health science , Dankook University , Chungnam , Republic of Korea
- Correspondence to Dr Ji-eun Hwang; loshjeve{at}gmail.com
Objectives Although the global COVID-19 pandemic has increased interest in research involving high-risk smokers, studies examining changed smoking behaviours, cessation intentions and associated psychological states among smokers are still scarce. This study aimed to systematically review the literature related to this subject.
Design A systematic review of published articles on cigarettes and COVID-19 -related topics
Data sources Our search was conducted in January 2021. We used the keywords COVID-19, cigarettes, electronic cigarettes (e-cigarettes) and psychological factors in PubMed and ScienceDirect and found papers published between January and December 2020.
Data selection We included articles in full text, written in English, and that surveyed adults. The topics included smoking behaviour, smoking cessation, psychological state of smokers and COVID-19-related topics.
Data extraction and synthesis Papers of low quality, based on quality assessment, were excluded. Thirteen papers were related to smoking behaviour, nine papers were related to smoking cessation and four papers were related to psychological states of smokers.
Results Owing to the COVID-19 lockdown, cigarette users were habituated to purchasing large quantities of cigarettes in advance. Additionally, cigarette-only users increased their attempts and willingness to quit smoking, compared with e-cigarette-only users.
Conclusions Owing to the COVID-19 outbreak, the intention to quit smoking was different among smokers, according to cigarette type (cigarette-only users, e-cigarette-only users and dual users). With the ongoing COVID-19 pandemic, policies and campaigns to increase smoking cessation intentions and attempts to quit smoking among smokers at high risk of COVID-19 should be implemented. Additionally, e-cigarette-only users with poor health-seeking behaviour require interventions to increase the intention to quit smoking.
- public health
- mental health
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bmjopen-2021-055179
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
This study is the first to systematically review the literature regarding changes in smoking behaviours, cessation intentions and psychological states of smokers, based on the study period, country and age of participants during the COVID-19 pandemic.
According to the Risk of Bias Assessment Tool for Non-randomised Studies, three researchers cross-checked and evaluated the articles’ quality and excluded papers with a high risk of bias.
Using the backward snowballing method, screening for article selection was strictly performed by checking for any additional papers.
Since the articles collected for this systematic review were only cross-sectional designs, it was difficult to determine causality; therefore, only associations were presented.
Introduction
On 30 January 2020, the WHO declared COVID-19, an infectious disease of the respiratory tract characterised by SARS, 1 a public health emergency of international concern. 2 In 2020, a total of 350 594 336 cases of COVID-19 were reported, including 7 893 839 deaths. 3 4 Due to the pandemic, different standards and steps to control the spread of the disease have been adopted worldwide, but almost all countries have implemented lockdown and social distancing measures to prevent COVID-19 infection.
Smoking is a high-risk factor for COVID-19 infection, and many systematic reviews and meta-analyses report the association between COVID-19 and smoking. 5 6 Since tobacco is associated with chronic lung disease, physicians could expect more cigarette users to develop a severe form of COVID-19, 5 given the effect of tobacco on respiratory disease and immune function. 7 Smoking is associated with negative progression and side effects of COVID-19. 6 Smoking, including current or previous smoking history, significantly increases the severity and risk of death from COVID-19. 8 Similar to cigarette use, e-cigarette use can cause oxidative stress and inflammatory response in the lungs, making e-cigarette users more susceptible to bacterial or viral infections. 9 Although no literature indicates that cigarettes are the direct cause of COVID-19 infection, smokers have a higher risk of severe COVID-19 symptoms than non-smokers. 10 Previous studies have suggested several public health messages to continue focusing on smoking cessation efforts; however, smoking rates have not decreased. 11
Few investigations have focused on whether smoking behavior 12 and cessation efforts 13 have changed during the COVID-19 pandemic. Previous studies have shown that smoking has decreased 12 and quitting attempts have increased 13 during the pandemic. Since lockdowns and various social distancing measures were implemented in the early stages of the COVID-19 pandemic, they have the potential to affect smoking behaviour and cessation intention. In some countries, it is expected that smoking behaviour will change given the difficulty in freely purchasing tobacco products as non-essential items; individuals are locked down under the pandemic and are required to stay at home for prolonged periods. 14 Changes in daily life due to social distancing and lockdowns are expected to help reduce smoking consumption. 15 Therefore, it is necessary to examine the factors influencing smoking behaviour and cessation intention in the early stages of the pandemic, which have been investigated in the literature.
There are insufficient studies on smokers’ negative psychological states during the COVID-19 pandemic. Further research is needed because the relationship between negative psychological states and health behaviour is unclear. 16 People experience higher stress levels than usual because of social isolation, employment insecurity, finances uncertainty, responsibilities and concerns about illness from the virus. 17 Therefore, many cigarette users smoke to relieve stress and negative emotions, 18 but it is often counterproductive. 19 Previous research has shown that negative psychological states, such as stress, anxiety and depression, can influence tobacco-use behaviour. 20
There are insufficient studies to compare the smoking behaviour, cessation intention and psychological state of cigarette and e-cigarette users during the COVID-19 pandemic. 14 The daily use of e-cigarettes among e-cigarette users slightly increased during the COVID-19 lockdown 21 ; therefore, it is necessary to investigate whether smoking behaviour changes according to the cigarette type. Cigarette users are aware that they engage in harmful health behaviours. However, it is unclear whether e-cigarettes help cigarette users quit smoking. 22 E-cigarette users believe that e-cigarettes are less harmful to their health as they contain relatively fewer harmful ingredients than cigarettes. 23 Therefore, they believe that smoking e-cigarettes is an effort to quit smoking. 23
In addition, after the COVID-19 pandemic, smoking behaviour may change by age depending on the cigarette type. The use of e-cigarettes increases among persons in their early 20s compared with old persons. 24 In addition, it is known that older persons are more vulnerable to COVID-19 infection and can develop severe infection symptoms. 25 Therefore, there may be an increase in the older persons’ intention to quit smoking cigarettes. However, young people who use e-cigarettes may not be less willing to quit smoking.
Many studies have investigated whether smoking is a high-risk factor for COVID-19. 6 8 However, relatively few studies have been published on whether smoking behaviour, cessation intention and psychological state have changed during the COVID-19 pandemic. In the future, it is expected that this study will establish a scientific basis and policy response to smoking behaviour, cessation intention and psychological state based on cigarette type, both during and post COVID-19. Therefore, we conducted a systematic review based on the hypothesis that there would be changes in smoking behaviour, cessation intention and psychological states of adult smokers, depending on the tobacco product used during the COVID-19 pandemic.
Literature search strategy
We systematically reviewed the data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, shown in the Supplementary file. We searched articles using MeSH terms ‘COVID-19, depression, stress, psychological, anxiety, tobacco, tobacco products, tobacco use and electronic nicotine delivery system’ in January 2021. Only papers published between January and December 2020 were included in the study. All search terms are listed in online supplemental table 1 .
Supplemental material
A literature search was conducted on PubMed using the following MeSH terms: ‘(depression OR stress OR anxiety OR psychology) AND ((tobacco OR tobacco products OR tobacco use OR electronic nicotine delivery system) AND COVID-19))’ in the title or the abstract in 2020. A literature search was conducted on ScienceDirect using the following search terms: ‘COVID-19 AND (e-cigarette OR “electronic cigarette” OR “electronic nicotine delivery” OR “vaping” OR “heat not burn” OR “heated tobacco product”)’, as well as COVID-19 AND (tobaccos OR cigarette OR kretek OR bidis OR “pipe tobacco” OR cigarillos).
Inclusion and exclusion criteria
Figure 1 shows a flowchart of the database searches, followed by the exclusion/inclusion strategy. The inclusion criteria were cases that did not meet the exclusion criteria. In the first phase ( figure 1 ), certain types of articles were excluded, including comments, letters, editorials, viewpoints, correspondence and articles without full text. In addition, duplicate works and those not written in English and studies that did not include humans or adults were excluded. In the next phase ( figure 1 ), studies unrelated to tobacco or COVID-19 were excluded. In addition, articles were excluded if the topics were not related to changes in tobacco use and mental health caused by COVID-19. Finally, articles with a high risk of bias were excluded using the Risk of Bias Assessment Tool for Non-randomised Studies (RoBANS) ( figures 2 and 3 ). 26
- Download figure
- Open in new tab
- Download powerpoint
Flowchart diagram. The flowchart shows the article selection process, the criteria for exclusion of articles and the number of articles excluded.
Assessment of the risk of bias in identified studies using the RoBANS questionnaire. Author judgement for each RoBANS domain for the included articles. Green indicates a low risk of bias, yellow indicates unclear risk and red indicates a high risk of bias. The six domains are ‘comparability of groups, participant selection, confounding variables, measurement of exposure, blinding of the outcome, incomplete outcome data, selective outcome reporting’. RoBANS, Risk of Bias Assessment Tool for Non-randomised Studies.
Assessment summary of the risk of bias in identified studies using the RoBANS questionnaire. Author judgement for each RoBANS domain for the included articles. Green circles indicate low risk of bias, yellow circles indicate unclear risk and red circles indicate high risk of bias. The study numbers indicate the order of the articles presented in table 1 . RoBANS, Risk of Bias Assessment Tool for Non-randomised Studies.
Data extraction
Two independent reviewers (H-rC and J-eH) screened the literature and assessed each paper by reading the titles, abstracts and full texts. Three authors extracted data from studies that fulfilled our inclusion criteria independently (H-rC, J-eH and EC). The primary data points included were the following: study details (author, journal, publication date, country, study design, study period and funding), the total number of participants, types of cigarettes, changes in smoking behaviour, cessation intention and psychological state.
Two authors (H-rC and EC) independently assessed the quality of included studies using the RoBANS. 26 If the opinions of the two authors were different, the quality evaluation was completed through further discussion with another researcher (J-eH). The RoBANS contains six domains: selection of participants, confounding variables, measurement of exposure, blinding of outcome assessments, incomplete outcome data and selective outcome reporting ( figures 2 and 3 ). The risk of bias was divided into three stages (high, unclear or low risk). When the risk of bias in a study was high, it was excluded.
Using the backward snowballing method, 27 28 we checked the references of the articles that met the inclusion criteria. We checked the titles, journals, abstracts and full texts of the references of the articles. We excluded articles that did not meet the inclusion criteria. In addition, articles not related to the subject of this study were excluded. As a result, no additional papers were added.
Literature search and literature selection results
A total of 1659 papers were found in PubMed and ScienceDirect, and 905 non-original papers were excluded. Eleven papers without full text and four papers not written in English were excluded. In addition, 413 papers were selected, excluding 102 papers that were not conducted in humans, 114 papers that were not conducted in adults and 110 duplicate papers after the first screening phase. Papers unrelated to tobacco (n=200) and COVID-19 (n=21) were excluded. In addition, a total of 18 papers were included, excluding 166 papers that were not related to tobacco behaviour and psychological states that changed during the COVID-19 pandemic as well as eight papers targeting COVID-19 patients or medical personnel in the first screening phase.
Assessment result of the risk of bias
Thirteen papers were selected after excluding five papers with a high risk of bias, as determined through quality evaluation of the 18 papers using the RoBANS ( figures 2 and 3 ).
Characteristics of included papers by three subjects
All 13 selected papers were published in 2020; three were conducted in the USA, three in the UK, two in Italy, two in Turkey and one in China, India and Australia. All were cross-sectional design studies that used surveys. Comments, letters, editorials, viewpoints and correspondence were not included. In the included papers, the number of study participants varied from 93 to 52 002, and all participants were adults. We also presented the average age of the participants in the papers or the ratio by age group. Eight papers targeted cigarette-only users, and five reviewed e-cigarette and cigarette users together ( table 1 ). Table 1 shows that smokers’ smoking behaviour, cessation intentions and psychological states changed due to the COVID-19 pandemic, presented in all 13 papers. Seven papers studied changes in smoking behaviour, eight papers examined changes in smoking cessation and five papers examined changes in smokers’ psychological state after the COVID-19 outbreak ( table 1 ).
- View inline
Integrating results of the 13 selected papers
Changes in frequency of smoking occurred in the early stages of the COVID-19 pandemic, regardless of cigarette type. However, smoking cessation intentions and attempts were higher among cigarette-only users than e-cigarette-only users. When the average age of participants was presented, and only papers surveyed according to the cigarette type were examined, the smoking behaviour of cigarette-only users was found to increase in the paper with an average age of 22. In a study targeting participants on average in their 50s or older, cigarette-only users showed a higher intention to quit smoking than e-cigarette-only users. Regardless of the average age of participants, in most studies, the amount of e-cigarette-only users’ usage did not decrease, and the intention to quit smoking did not increase. In addition, it was found that all smokers, regardless of the tobacco product used, had increased stress and anxiety during the COVID-19 pandemic, and the more they did not intend to quit, the more stressed they were. We present detailed information on smoking behaviour, smoking cessation and psychological state changes according to cigarette type during the COVID-19 pandemic in the following six subheadings.
Changes in smoking behaviour in e-cigarette users (or dual users) during the COVID-19 outbreak
According to Soule et al , 29 the higher the reliance on e-cigarettes, the higher the incidence of cigarette use. There have also been increasing efforts to reduce e-cigarette use among dual users. However, mastery of e-cigarette-specific behaviours during the additional time spent at home improved e-cigarette skills and increased e-cigarette use. 29 According to the article by Caponnetto et al , 14 daily cigarette consumption by dual users of cigarettes and e-cigarettes and cigarette users decreased slightly. Due to the COVID-19 outbreak, purchasing products, including tobacco products, has changed; individuals buy large numbers of products at once.
Changes in smoking behaviour in cigarette-only users during the COVID-19 outbreak
According to the results of articles by Cancello et al 11 and Ren et al , 30 only cigarette consumption increased by 38% and 30%, respectively. Compared with before the COVID-19 pandemic, Chopra et al showed a significant decrease in smoking consumption (0.02 (0.03), p<0.05). 12 However, in the study by Ozcelik and Yilmaz Kara, 31 67.5% of participants reported no change in smoking behaviour, which cannot be interpreted as a significant change in smoking behaviour during the COVID-19 pandemic.
Changes in intentions and attempts to quit smoking among e-cigarette users (or dual users) during the COVID-19 outbreak
Tattan-Birch et al 13 found that a few cigarette-only users and e-cigarette-only users attempted to quit smoking due to the COVID-19 outbreak. Approximately, 1 in 10 current e-cigarette-only users reported an attempt to quit vaping because of COVID-19. Soule et al reported that users with high reliance on e-cigarettes exerted less effort to reduce product usage than users with low reliance. 29 According to a study by Caponnetto et al , while most cigarette-only users have considered quitting smoking, most e-cigarette-only users have not considered stopping e-cigarette use. 14 According to a study by Chertok et al , 36.7% of all smokers attempted to quit smoking during the pandemic regardless of cigarette type; people who quit smoking due to COVID-19 perceived smoking as a high-risk factor COVID-19 infection. 32
Changes in intentions and attempts to quit smoking among cigarette users during the COVID-19 outbreak
According to Jackson et al , 33 COVID-19 shutdowns have increased attempts to quit smoking. According to the results of the article by Kayhan Tetik et al , 34 which compared the success rates of smoking cessation before and during the pandemic, the COVID-19 outbreak effectively promoted smoking cessation. Only 12.8% of people who quit smoking during the COVID-19 outbreak did not restart smoking. According to Ozcelik and Yilmaz Kara, 31 people with coronaphobia exhibited a significantly higher decrease or cessation in smoking than those with no change in smoking behaviour or increased smoking consumption. A study of cigarette-only users found that the number of attempts to quit smoking had increased in the USA. 32 35 In the UK, all studies conducted on cigarette-only users found that attempts to quit smoking had increased. 13 36 In the case of cigarette-only users, there are more successful quitting smoking cases than e-cigarette-only users. 13
The association between smoking and psychological changes in e-cigarette users (or dual users) during the COVID-19 outbreak
According to Soule et al , the greater the dependence on e-cigarettes, the higher the concern about COVID-19 infection than those with low dependence. 29 In the article by Kowitt et al , the higher the awareness regarding the risk of contracting COVID-19, regardless of cigarette type, the greater the willingness to quit (B=0.38, p<0.001) and the attempt to quit smoking (OR: 1.31, 95% CI 1.04 to 1.64). 35 In addition, as social distancing (B=0.11, p=0.01) was enforced more strongly, the willingness to quit smoking increased. 35
The association between smoking and psychological changes in cigarette users during the COVID-19 outbreak
Stanton et al found that people with increased tobacco consumption had a higher risk of depression, anxiety and stress. 16 Jackson et al reported that compared with non-smokers, current and former cigarette-only users had increased stress from COVID-19. 37 Ozcelik et al indicated that among people with coronaphobia, those who reduced or stopped smoking felt more anxious than those who increased their smoking frequency. 31
We examined the changes in smoking behaviour, cessation intentions and psychological states caused by the COVID-19 outbreak in smokers (cigarette-only users, e-cigarette-only users and dual users). Regardless of the cigarette type, the amount of smoking either increased or decreased in some cases. However, compared with e-cigarette-only users, most cigarette-only users’ intentions and attempts to cease smoking increased. In addition, regardless of the type of cigarette, smokers showed a negative psychological state due to COVID-19. It was found that attempts and intentions to cease smoking increased as dependence on cigarettes decreased. Additionally, social distancing strengthened and awareness about COVID-19 increased.
The amount of smoking done by e-cigarette-only users increased with increased dependency. For dual users, those who use cigarettes and e-cigarettes, efforts to reduce product usage increased; however, there was a tendency to buy cigarettes in bulk, increase e-cigarette usage skills and increase their use. This is thought to result from a tendency to recognise that using e-cigarettes is relatively less harmful to health than smoking cigarettes. 23 According to previous studies, many e-cigarette users recognise that e-cigarettes are relatively less harmful to health, their own and others, 23 because they contain far fewer harmful components 38 than cigarettes. However, it is not yet clear how e-cigarettes can help cigarette users quit smoking. 22 For this reason, e-cigarette users should consider the risks of using e-cigarettes as much as cigarettes.
Cigarette-only users’ efforts to reduce product usage and quit smoking during the pandemic increased, especially when the average age group was high. In other words, the higher the age group, the higher the risk of COVID-19 infection and symptom severity. Therefore, it was found that the use of cigarettes decreased. 25 Efforts to reduce product usage by e-cigarette users, regardless of age, decreased. In particular, the higher the dependence of e-cigarette users, the higher were the worries related to COVID-19; however, efforts to reduce the amount of smoking were lower among users with low e-cigarette dependence. This could be because e-cigarette users think that using e-cigarettes is an attempt to quit smoking. 23 Therefore, e-cigarette users’ intentions to quit smoking have not increased, even during the pandemic. A cohort analysis conducted in the USA found that e-cigarette users did not quit smoking, compared with cigarette-only users, and more than half of e-cigarette users still used e-cigarettes a year later. 39 In another study, it was unclear whether e-cigarette users were more likely to quit smoking. 40 Since many studies are still being conducted on the cessation effect of e-cigarettes, it should be recognised that e-cigarette use could be harmful to health.
Regardless of the cigarette type, the higher the awareness of the risks associated with contracting COVID-19, the higher the level of social distancing since the start of COVID-19 and intent to quit smoking. In the case of cigarette-only users, coronaphobia increased smoking cessation intentions. Among them, those who reduced smoking felt more anxious than those who increased their amount of smoking. Smokers were more likely to quit after understanding the health risks of smoking due to the ‘vulnerability hypothesis’, wherein smokers who care about their health would be inclined to quit smoking when they know it makes them vulnerable to certain diseases. 41 42 This presents the same context as the results of this systematic review.
In contrast, previous studies have shown that smokers have higher levels of depression, anxiety, stress and increased smoking amounts. According to a study by Klemperer et al , 43 28.3% of patients reduced their tobacco use due to fear of infectious diseases, while 30.3% showed increased tobacco use and anxiety levels in both groups. However, in the literature surveyed in this study, it was found that the higher the level of anxiety, the higher the attempt to quit smoking. Therefore, further research exploring the relationship between mental health and smoking behaviours during the COVID-19 pandemic using standardised tools to measure mental health conditions, such as anxiety, is needed.
Increased awareness of COVID-19 risk has led to increased smoking cessation attempts, which can be considered a desirable outcome for physical health in the long run. However, for mental health, including anxiety, depression and increased stress during the pandemic, governments’ response to address the increasing trend of smoking should be to conduct campaigns for risk awareness to protect their citizens’ mental health. Research on the association between cigarette smoking and COVID-19 increases; the WHO also suggests that smokers may be considered at high risk of contracting COVID-19. However, because of the lack of research on the relationship between the use of liquid e-cigarettes and COVID-19, the willingness or attempts to quit e-cigarette use has not increased compared with cigarette-only users or dual users. Therefore, this study was able to review only cross-sectional studies. As smokers have a high risk of contracting certain diseases, both during and after the COVID-19 pandemic, further studies on the risk of e-cigarettes and their impact on smoking cessation, especially regarding COVID-19 infection, should be conducted.
This study is the first to systematically review the literature regarding changes in smoking behaviours, cessation intentions and psychological states of smokers, based on the study period, country and age of participants during the COVID-19 pandemic. Since this study aimed to investigate people’s smoking behaviour, smoking cessation and psychological state in the early stages of the COVID-19 pandemic in 2020, all studies used questionnaire tools. There were no articles that had conducted qualitative or interventional studies on this topic. Therefore, only cross-sectional studies were included. This study only systematically reviewed articles published between January and December 2020. According to the results of this study, as social distancing and lockdown were strengthened in the early stages of the pandemic, smoking behaviour, smoking cessation intention and psychological changes appeared. However, in 2021, as vaccinations began to occur worldwide, social distancing decreased, as did people’s willingness to participate in social distancing and stay-at-home. 44 Accordingly, smoking behaviour, smoking cessation and psychological status in 2021 may differ from that in 2020. When reviewing articles published in 2021, it is necessary to examine the changes in smoking behaviour, smoking cessation and psychological state of smokers while considering vaccine variables.
However, it is meaningful that this study looked at smoking usage behaviour during the COVID-19 pandemic based on cigarette type. This study could serve as primary data to establish effective strategies that focus on specific tobacco-product users by suggesting different smoking behaviours, smoking cessation intentions and psychological changes based on cigarette type.
In this study, we examined changes in smoking behaviours, smoking cessation intentions and psychological states of smokers during COVID-19 according to cigarette type. We conducted a systematic review of 13 papers to suggest future research and policy directions that the government may implement for COVID-19 and smokers. During the COVID-19 pandemic, cigarette-only users’ consumption did not change significantly, but dual users and e-cigarette-only users with a high dependence on e-cigarettes showed increased consumption. Cigarette users’ quit intentions, attempts and cessation success rates increased, and even those with coronaphobia showed increased intent to quit smoking. On the other hand, users of e-cigarettes demonstrated fewer attempts to quit. As the COVID-19 pandemic continues, policies and campaigns should be implemented to increase the intent and attempt to stop smoking. Research and interventions on COVID-19, smoking and e-cigarette use should also be conducted in the medium to long term.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Le Faou A-L , et al
- Organization WH
- Our World in Data
- Vardavas C ,
- Umnuaypornlert A ,
- Kanchanasurakit S ,
- Lucero-Prisno DEI , et al
- Sladewski K , et al
- Lungarella G ,
- Cancello R ,
- Soranna D ,
- Zambra G , et al
- Singh V , et al
- Tattan-Birch H ,
- Jackson S , et al
- Caponnetto P ,
- Inguscio L ,
- Saitta C , et al
- Stanton R ,
- Khalesi S , et al
- Fancourt D ,
- ↵ Collision of the COVID-19 and addiction epidemics. American College of physicians . Ann Intern Med 2020 . doi:10.7326/M20-1212
- García-Álvarez L ,
- Fuente-Tomás LDla ,
- Adriaens K ,
- Van Gucht D ,
- Etter J-F ,
- Schroeder R ,
- Jensen JW , et al
- Garibaldi BT , et al
- Lee YJ , et al
- Greenhalgh T ,
- Snipes W , et al
- Li Z , et al
- Ozcelik N ,
- Yilmaz Kara B
- Chertok IRA
- Jackson SE ,
- Garnett C ,
- Kayhan Tetik B ,
- Tekinemre G ,
- Kowitt SD ,
- Cornacchione Ross J ,
- Jarman KL , et al
- Shahab L , et al
- Goniewicz ML ,
- Gawron M , et al
- Pierce JP ,
- Benmarhnia T ,
- Chen R , et al
- Pasquereau A ,
- Guignard R ,
- Andler R , et al
- Borrelli B ,
- Dunsiger S , et al
- Weinstein ND
- Klemperer EM ,
- Peasley-Miklus C , et al
- Andersson O ,
- Campos-Mercade P ,
- Meier AN , et al
- Tekinemre IG ,
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors H-rC designed the study, analysed and interpreted the data, and wrote the manuscript. J-eH and EC contributed to the study design and interpretation of the results. H-rC and J-eH took full responsibility for the study and access to all data and controlled the decision to publish.
Funding This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Ministry of Science and ICT (MSIT) (Number 2020R1C1C1012562).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
A comparative study on tobacco cessation methods: A quantitative systematic review
- Quantitative Health Sciences
Research output : Contribution to journal › Review article › peer-review
Background: During recent years, there have been many advances in different types of pharmacological and non-pharmacological tobacco control treatments. In this study, we aimed to identify the most effective smoking cessation methods used in quit based upon a review of the literature. Methods: We did a search of PubMed, limited to English publications from 2000 to 2012. Two trained reviewers independently assessed titles, abstracts and full texts of articles after a pilot inter-rater reliability assessment which was conducted by the author (GH). The total number of papers and their conclusions including recommendation of that method (positive) or not supporting (negative) was computed for each method. The number of negative papers was subtracted from the number of positive ones for each method. In cases of inconsistency between the two reviewers, these were adjudicated by author. Results: Of the 932 articles that were critically assessed, 780 studies supported quit smoking methods. In 90 studies, the methods were not supported or rejected and in 62 cases the methods were not supported. Nicotine replacement therapy (NRT), Champix and Zyban with 352, 117 and 71 studies respectively were the most supported methods and e-cigarettes and non-Nicotine medications with one case were the least supported methods. Finally, NRT with 39 and Champix and education with 36 scores were the most supported methods. Conclusions: Results of this review indicate that the scientific papers in the most recent decade recommend the use of NRT and Champix in combination with educational interventions. Additional research is needed to compare qualitative and quantitative studies for smoking cessation.
- Systematic review
- Tobacco cessation
- Tobacco control
ASJC Scopus subject areas
- Public Health, Environmental and Occupational Health
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- Tobacco Use Cessation Medicine & Life Sciences 100%
- nicotine Social Sciences 68%
- Systematic Reviews Medicine & Life Sciences 53%
- Varenicline Medicine & Life Sciences 28%
- Smoking Cessation Medicine & Life Sciences 19%
- smoking Social Sciences 18%
- Tobacco Medicine & Life Sciences 6%
- Tobacco Products Medicine & Life Sciences 6%
T1 - A comparative study on tobacco cessation methods
T2 - A quantitative systematic review
AU - Heydari, Gholamreza
AU - Masjedi, Mohammadreza
AU - Ahmady, Arezoo Ebn
AU - Leischow, Scott J.
AU - Lando, Harry A.
AU - Shadmehr, Mohammad Behgam
AU - Fadaizadeh, Lida
PY - 2014/6
Y1 - 2014/6
N2 - Background: During recent years, there have been many advances in different types of pharmacological and non-pharmacological tobacco control treatments. In this study, we aimed to identify the most effective smoking cessation methods used in quit based upon a review of the literature. Methods: We did a search of PubMed, limited to English publications from 2000 to 2012. Two trained reviewers independently assessed titles, abstracts and full texts of articles after a pilot inter-rater reliability assessment which was conducted by the author (GH). The total number of papers and their conclusions including recommendation of that method (positive) or not supporting (negative) was computed for each method. The number of negative papers was subtracted from the number of positive ones for each method. In cases of inconsistency between the two reviewers, these were adjudicated by author. Results: Of the 932 articles that were critically assessed, 780 studies supported quit smoking methods. In 90 studies, the methods were not supported or rejected and in 62 cases the methods were not supported. Nicotine replacement therapy (NRT), Champix and Zyban with 352, 117 and 71 studies respectively were the most supported methods and e-cigarettes and non-Nicotine medications with one case were the least supported methods. Finally, NRT with 39 and Champix and education with 36 scores were the most supported methods. Conclusions: Results of this review indicate that the scientific papers in the most recent decade recommend the use of NRT and Champix in combination with educational interventions. Additional research is needed to compare qualitative and quantitative studies for smoking cessation.
AB - Background: During recent years, there have been many advances in different types of pharmacological and non-pharmacological tobacco control treatments. In this study, we aimed to identify the most effective smoking cessation methods used in quit based upon a review of the literature. Methods: We did a search of PubMed, limited to English publications from 2000 to 2012. Two trained reviewers independently assessed titles, abstracts and full texts of articles after a pilot inter-rater reliability assessment which was conducted by the author (GH). The total number of papers and their conclusions including recommendation of that method (positive) or not supporting (negative) was computed for each method. The number of negative papers was subtracted from the number of positive ones for each method. In cases of inconsistency between the two reviewers, these were adjudicated by author. Results: Of the 932 articles that were critically assessed, 780 studies supported quit smoking methods. In 90 studies, the methods were not supported or rejected and in 62 cases the methods were not supported. Nicotine replacement therapy (NRT), Champix and Zyban with 352, 117 and 71 studies respectively were the most supported methods and e-cigarettes and non-Nicotine medications with one case were the least supported methods. Finally, NRT with 39 and Champix and education with 36 scores were the most supported methods. Conclusions: Results of this review indicate that the scientific papers in the most recent decade recommend the use of NRT and Champix in combination with educational interventions. Additional research is needed to compare qualitative and quantitative studies for smoking cessation.
KW - Methods
KW - Systematic review
KW - Tobacco cessation
KW - Tobacco control
UR - http://www.scopus.com/inward/record.url?scp=84902957792&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=84902957792&partnerID=8YFLogxK
M3 - Review article
AN - SCOPUS:84902957792
SN - 2008-7802
JO - International Journal of Preventive Medicine
JF - International Journal of Preventive Medicine
Does Quitting Smoking Affect Depressive Symptoms? A Longitudinal Study Based on Treatment-Seeking Smokers with a History of Depressive Episode
- Original Article
- Open access
- Published: 06 May 2024
Cite this article
You have full access to this open access article

- Elizabeth Moss-Alonso ORCID: orcid.org/0009-0008-0782-4893 1 ,
- Carmela Martínez-Vispo 1 ,
- Ana López-Durán 1 &
- Elisardo Becoña 1
136 Accesses
Explore all metrics
This study analyses changes in depressive symptomatology as a function of smoking status over time after a cognitive-behavioural intervention for smoking cessation among smokers with a history of depressive episode. The sample comprised 215 smokers with antecedents of depressive episode (M age =45.03; 64.7% female). Depressive symptoms were assessed using BDI-II at baseline, end of intervention and at 3-, 6- and 12-month follow-ups. Depression was examined according to smoking status at 12-month follow-up: abstainers, relapsers and smokers. The linear mixed model showed a significant effect for time ( F = 11.26, p < .001) and for the interaction between smoking status and time ( F = 9.11, p < .001) in the variations in depression. Abstinent participants at 12 months experienced a reduction in depressive symptomatology. This change was significant when comparing abstainers to smokers and relapsers. The present study suggests an association between abstinence and reductions in depressive symptomatology for smokers with a history of depressive episode after an intervention for smoking cessation.
Similar content being viewed by others
Depression and smoking cessation: evidence from a smoking cessation clinic with 1-year follow-up, cognitive-behavioral treatment with behavioral activation for smokers with depressive symptomatology: study protocol of a randomized controlled trial, does deterioration in mental health after smoking cessation predict relapse to smoking.
Avoid common mistakes on your manuscript.
Smoking is one of the leading modifiable causes of death and disability worldwide (US Department of Health and Human Services [USDHHS], 2020 ). Even though smoking prevalence has decreased over the past decades (World Health Organization [WHO], 2023 ), the prevalence of smokers with mental health disorders does not follow the same pattern. Weinberger et al. ( 2020 ) analysed the changes in smoking prevalence from 2005 to 2017, finding a significant decrease in smoking prevalence both among people with and without depression. However, these authors point out that the decrease in smoking prevalence was noticeably lower for smokers with depression.
The association between depression and smoking is well-established and has been extensively studied. People with depression are at higher risk of becoming smokers, and smoking seems to be associated with later depression (Fluharty et al., 2017 ; Sánchez-Villegas et al., 2021 ). Also, smokers with depression encounter more significant barriers to smoking cessation than those without depression (Ranjit et al., 2020 ) and show higher levels of nicotine dependence (Bainter et al., 2020 ), stronger urges to smoke when anticipating the relief from a negative mood state (Berlin & Singleton, 2008 ), higher relapse rates (Huffman et al., 2018 ; Zvolensky et al., 2015 ) and a more intense withdrawal syndrome when compared to smokers with lower levels of depressive symptomatology (Tucker et al., 2022 ).
Due to the difficulties that depressed smokers encounter in quitting and remaining abstinent, efforts are being made to improve smoking cessation interventions. For example, behavioural activation is a mood management component that has been gaining in popularity as an option to address this issue (Audrain-McGovern et al., 2023 ; Busch et al., 2017 ; Martínez-Vispo et al., 2019 ). Other efforts also include a combination of both behavioural activation and contingency management (Secades-Villa et al., 2019 ).
In contrast to all the factors that may hinder smoking cessation, smokers with mental health issues seem to be as motivated to quit as the general population (Siru et al., 2009 ). Some studies even suggest that higher levels of psychological distress are associated with higher motivation to quit smoking (Kastaun et al., 2022 ). The same is true for smokers with a past or current history of major depression; motivation to quit is high, and various attempts to quit are made among this population (Quinn et al., 2022 ). Considering that depressive disorders are classified as one of the leading causes of non-fatal health loss at a global level (WHO, 2017 ) and that tobacco smoking poses increased health risk, it is crucial to foster any strength among this population, such as high motivation, to promote and achieve successful smoking cessation. Therefore, continued research is warranted to improve smoking cessation interventions for smokers with depression.
Unfortunately, there is a common fear that smoking cessation among people with current or past major depression or other mental health disorders will worsen mental health conditions. This fear is reflected in the reluctance of mental health professionals to provide smoking cessation interventions (Lembke et al., 2007 ). Notwithstanding the reluctance, evidence suggests that smoking cessation improves depressive symptomatology among the general population (Hahad et al., 2022 ; Rodríguez-Cano et al., 2016 ), and other mental health variables such as depressive symptoms, anxiety and stress levels among clinical and general populations (Taylor et al., 2021 ). However, when studies target smokers with past or current depressive episode, results are mixed. For instance, a study conducted by Liu et al. ( 2021 ) concludes that abstinence does not result in lower depression in smokers with a history of major depressive disorder (MDD), but continued smoking does result in worse depressive symptomatology over time. Furthermore, Blalock et al. ( 2008 ) found that among a population of smokers with a history of major depression, 44% of abstainers reported a remission of their depressive disorder at the 3-month follow-up. On the other hand, other studies, like the one conducted by Glassman et al. ( 2001 ), conclude that smokers with a history of major depression who quit are at increased risk of depressive episode for at least the initial 6 months of abstinence. Therefore, further research is called for (Weinberger et al., 2013 ).
The present study aims to clarify further the relationship between smoking cessation and depression in people with a history of depressive episode. Changes in depressive symptomatology were examined based on smoking status over time after having received a cognitive-behavioural intervention to quit smoking. The analysis was conducted in a sample of smokers with antecedents of depressive episode.
Participants
The initial sample was composed of 409 smokers who participated in a cognitive-behavioural intervention for smoking cessation in the Smoking Cessation and Addictive Disorders Unit at the University of Santiago de Compostela. The participants were recruited between 2015 and 2021.
To be included in the study, participants had to be 18 years old or older, willing to participate in the psychological intervention, smoke a minimum of six cigarettes per day and meet the criteria for past or current major depressive episode as assessed by the Major Depressive Episode Screener (MDE; Muñoz, 1998 ).
Exclusion criteria included the following: co-occurring substance use disorder (cocaine, cannabis, alcohol and/or opioids); diagnosis of a severe mental disorder (psychotic disorder and/or bipolar disorder); use of any tobacco products other than cigarettes; participation in the previous year in an effective psychological or pharmacological intervention for smoking cessation; and failing to attend the first intervention session.
After assessment and considering the criteria, a total of 194 participants were excluded for the following reasons: 1 smoker had co-occurring substance use disorder; 11 presented a diagnosis of a severe mental disorder; 8 had received an effective intervention for smoking cessation in the past year; 13 did not attend the first intervention session; and 161 participants did not meet criteria for a past or current depressive episode. The final sample on which the analysis was conducted comprised 215 smokers.
For this study, participants were grouped according to their smoking status at the 12-month follow-up: abstainers ( n = 58), relapsers ( n = 76) and smokers ( n = 81). Abstinent participants were defined as those who self-reported not smoking during the 30 days before the follow-up conducted 12 months after the end of the intervention. Relapsers were defined as those who self-reported not smoking even one puff for the past 24 h at the last intervention session but reported smoking at any of the follow-ups conducted at 3, 6 or 12 months. Finally, the smoker category was defined as those participants who did not quit throughout the intervention sessions.
Abstinence was biochemically validated through measurements of carbon monoxide (CO) in exhaled breath using Bedfont’s Micro + Smokerlyzer (Bedfont Scientific Ltd., Maidstone, Kent, UK). The cut point for CO measurements was 5 particles per million (ppm) in exhaled breath (Benowitz et al., 2020 ). Any score over 5 ppm indicated that the participant was a smoker. Due to the situation generated by the coronavirus pandemic in 2020 (COVID-19) and the social distancing measures that were established, it was impossible to biochemically validate abstinence for 43.1% of our sample, leading to our having to rely exclusively on self-reported abstinence for this part of the sample.
In the context of this research, participants were assessed using a semi-structured interview and a set of questionnaires to collect socio-demographic variables and assess depression and smoking-related factors.
The following questionnaires were used for the pre-intervention assessment:
The Smoking Habit Questionnaire (SHQ; Becoña, 1994 ). The SHQ is a questionnaire used to gather general information on socio-demographic and smoking variables, such as age, sex, cigarettes per day (CPD) or educational level.
The Major Depressive Episode (MDE) Screener (Muñoz, 1998 ). The MDE Screener is a hetero-applied questionnaire that is used to identify past or current major depressive episodes.
The Beck Depression Inventory II (BDI-II; Beck et al., 2006 ). The BDI-II is a 21-item questionnaire that evaluates the presence and severity of depressive symptomatology. Participants completed this questionnaire at pre-intervention, post-intervention and at the 3-, 6- and 12-month follow-ups.
A cognitive-behavioural smoking cessation intervention was applied in this study. The intervention was administered by counselling psychologists or clinical psychologists and was divided into eight weekly group sessions. Some of the components of this intervention were the following: self-report of daily cigarette use, graphic representation of daily consumption, psychoeducation about tobacco, strategies and activities for the avoidance and attenuation of withdrawal symptoms and craving, nicotine fading, stimulus control, behavioural activation and relapse prevention (Becoña, 2007 ; Martínez-Vispo et al., 2019 ).
The present study followed all ethical principles and was approved by the Bioethics Committee of the University of Santiago de Compostela.
Analytical Strategy
Descriptive analyses based on smoking status at the 12-month follow-up (abstainers, smokers, and relapsers) were conducted for socio-demographic variables and pre-intervention depressive symptomatology and smoking-related factors. Additionally, differences in these data were examined using chi-square tests for qualitative variables, to which Bonferroni corrections for multiple comparisons were applied, and ANOVAs for continuous variables. Post hoc analyses were conducted to identify which groups differed from each other and in what direction.
In order to further assess the longitudinal changes in depressive symptomatology in the three groups, we applied a repeated measures analysis of variance (ANOVA), with time (pre-intervention, post-intervention, 3-, 6- and 12-month follow-ups) as the within-subject factor and smoking status at the 12-month follow-up (abstainers, smokers and relapsers) as the between subject factor. Greenhouse–Geisser correction was used to correct for the non-sphericity of the data.
Linear mixed modelling (LMM) was conducted to compare the effect of smoking status at the 12-month follow-up (abstainers, smokers and relapsers) on depression measures over time with Bonferroni correction for multiple comparisons. Restricted maximum likelihood (REML) estimation was used to account for missing data. Significant effects were followed up using pairwise comparisons.
All effects were reported with their corresponding confidence interval (CI), which was established at 95%. Any p -value lower than 0.05 was considered significant. All the analyses were conducted with SPSS version 28.
Participant’s descriptive data based on smoking status at the 12-month follow-up is presented in Table 1 . No significant differences were found as a function of smoking status at the 12-month follow-up for socio-demographic variables (sex, marital status, education level and employment) or for the pre-intervention BDI-II score. However, significant differences were found as a function of age and CPD.
Post hoc tests revealed that smokers at the 12-month follow-up were significantly older than relapsers ( F 2,212 = 3.91, SE = 1.73, p = .02) and that smokers consumed significantly more CPD at pre-intervention than abstainers ( F 2,212 = 4.34, SE = 1.43, p = .003) and relapsers ( F 2,212 = 3.13, SE = 1.33, p = .02).
After examining the differences in depressive symptomatology between abstainers, smokers and relapsers at each point in time through the ANOVA (see Table 2 ), no statistical differences were found at baseline or at the 3-month follow-up. Notwithstanding, the analysis showed statistical differences between groups at 6- and 12-month follow-ups. At the end of the intervention, smokers were significantly more depressed than abstainers and relapsers. At the 6- and 12-month follow-ups, relapsers’ depressive symptomatology seemed to return back to the levels displayed by smokers. Due to this tendency, at the 6- and 12-month follow-ups, both smokers and relapsers were significantly more depressed than abstainers. This tendency can be observed in Fig. 1 .
Evolution over time of depressive symptomatology as measured by the BDI-II, according to smoking status at 12-month follow-up
The repeated measures analysis showed differences in depressive symptoms according to smoking status at 12-month follow-up (F GG (6.406, 186.341) = 2.636, p = .015, η 2 = 0.059), adjusting for cigarettes per day at baseline (F GG, p > .05).
The mixed linear model showed a significant effect for time ( F = 11.26, p < .001) and for the smoking status × time interaction ( F = 9.11, p < 0001) in the change of the BDI-II scores. Concretely, abstinent participants experienced a significant reduction in depressive symptoms over time compared to relapsers and smokers (Table 3 ). After quitting smoking, participants who were abstinent at the 12-month follow-up had a mean reduction of 8.09 in their BDI-II scores from pre-intervention to the last follow-up, indicating an improvement of depressive symptomatology over time. BDI-II scores remained similar in the relapsed (from 13.55 at pre-intervention to 12.36 at the 12-month follow-up) and smoker group (from 14.11 to 14.28). The pairwise contrast revealed that mean change was statistically significant when comparing abstinent participants with smokers (mean difference − 5.22; SE = 1.37, p < .001, 95% CI [−7.82, −2.70]) and relapsed participants (mean difference − 3.20; SE = 1.3, p = .048, 95% CI [−6.39, −0.02]). No significant differences were found between smokers and relapsed participants (mean difference 2.02; SE = 1.30, p = .371, 95% CI [−1.13, 5.16]).
The aim of the present study was to investigate changes in depressive symptomatology over time after a cognitive-behavioural intervention for smoking cessation, among smokers with a history of depressive episode. The results revealed that at the end of the intervention, participants with a history of depressive episode who were abstinent or who had relapsed at the 12-month follow-up were significantly less depressed than smokers. At the 6- and 12-month follow-ups, relapsers and smokers displayed more depressive symptomatology than those who remained abstinent at 12 months.
According to the literature, smokers with depressive symptoms or with a history of depression are more likely to relapse in the first month after smoking cessation (Cooper et al., 2016 ). Our data align with the literature, with relapses occurring mainly between the end of the intervention and the 3-month follow-up. As the relapse occurs and the smoking habit is reestablished, our results point toward a gradual increase in depressive symptomatology in the group of relapsers. At the 6- and 12-month follow-ups, there are no significant differences in depressive symptomatology between smokers and relapsers. Depressive symptoms return to levels similar to pre-intervention scores for the group of relapsers, whereas abstainers’ depression levels continue to decrease over time. This might explain the absence of significant differences in depression at the 3-month follow-up.
The mixed linear model that was performed showed a significant effect of being abstinent at the 12-month follow-up on depressive symptomatology reduction over time. Thus, smoking cessation is associated with an improvement of depressive symptomatology from pre-intervention to the 12-month follow-up among smokers with a history of depressive episode who manage to quit and maintain abstinence over time. These results are in line with those obtained for treatment-seeking smokers regardless of depression history. In studies like the one conducted by Rodríguez-Cano et al. ( 2016 ), abstainers and relapsers at the 12-month follow-up were significantly less depressed than smokers at the end of a cognitive-behavioural intervention for smoking cessation. Moreover, participants who managed to maintain abstinence at the 12-month follow-up continued to experience a decrease in depressive symptomatology in contrast with smokers and also with relapsers, whose symptoms started to increase as relapses took place. Similar results are also obtained for smokers with mental health issues (Taylor et al., 2021 ; Wu et al., 2023 ). In the systematic review conducted by Taylor et al., it is concluded that smoking cessation is associated with improvements in anxiety, depression and mixed depressed and anxious symptomatology when maintaining abstinence within a range of a few weeks to years, in comparison to continuing to smoke. Wu et al. also found that after a pharmacological intervention for smoking cessation, managing to maintain abstinence for at least 15 weeks and up to 24 weeks was associated with less anxiety and less depression in contrast with continuous smoking.
Conversely, other studies have not found decreases in depressive symptomatology after smoking cessation for smokers with a history of depressive episode. For instance, Liu et al. ( 2021 ) analysed depression severity over a 1-year follow-up period after a smoking cessation intervention among treatment-seeking smokers with past, current or no major depressive episode history. The authors found that continuous smoking seemed to be significantly associated with an increase in depressive symptomatology after a smoking cessation intervention, rather than abstinence being related to significant decreases in depressive symptoms. The high baseline depression scores among the participants in the study might explain this. Glassman et al. ( 2001 ) examined smokers with a past history of major depression, finding that at the 3- and 6-month follow-ups, abstainers were at a significantly higher risk of developing another episode of major depression than smokers. The results of this study do not refer to depression symptomatology severity. Also, a significant amount of data was missing at the follow-up among those who did not quit smoking after the intervention.
The results of the present study suggest that maintaining abstinence over time, especially in the long term, would reduce depressive symptomatology. Therefore, it is essential to promote smoking cessation among smokers in general and specifically among those with a history of depression to improve both physical and mental health.
This study contributes to the growing body of research that supports the fact that smoking cessation is associated with improvements in psychological variables, not only among treatment-seeking smokers in general but also among those with mental health issues. Dissipating the worry in patients that depression may get worse or more difficult to manage after smoking cessation is a critical practical implication of the present study. It is crucial to highlight studies like the one conducted by Prochaska et al. ( 2019 ), which concludes that exposing smokers with mental health issues to a smoking cessation ad campaign that features success stories of smokers with depression (i.e. achieving abstinence, improving mood) increases motivation to quit and quit attempts. Motivation to quit is a well-established key factor for attaining abstinence (Piñeiro et al., 2016 ). Therefore, it is vital to continue conducting studies in this line that strengthen the idea that smoking cessation is not only possible among people with mental health issues, but it is also beneficial due to improvements in both physical and mental health. Moreover, the results obtained not only have implications for smokers with a history of depressive episode but also for the health professionals who treat them. Mental health professionals perceive many obstacles to smoking cessation among people with mental health issues, hold negative attitudes towards quitting smoking and are more permissive when it comes to smoking (Sheals et al., 2016 ). They also ask less about smoking status, offer advice for smoking cessation less frequently and fear that smoking cessation will worsen symptoms (Cerci, 2023 ). Additionally, mental health professionals believe it should be the patients who demand help with smoking cessation (Zeeman et al., 2023 ). The results of the present study can be used as a motivation and a means for dissipating fears and deconstructing negative attitudes for healthcare professionals to offer and provide smoking cessation interventions to patients with mental health issues.
Among the limitations of this study, it is important to highlight the absence of biochemical validation for 43.1% of the sample. To analyse the possible impact this may have had on the data, we ran tests to compare abstinence rates between participants who had biochemical validation and those whose abstinence was self-reported. The analysis revealed no significant group differences (χ 2 = 0.54, p = .461). Additionally, smoking cessation literature suggests that when in-person contact is not feasible, self-reported abstinence seems to be a reliable measure (Benowitz et al., 2020 ; West et al., 2005 ). Another limitation is that our results are not translatable to the general population of smokers or to smokers with a history of depressive episode who do not seek help to quit smoking. It is also relevant that the assessment questionnaires used for this study were self-reported, and such questionnaires can lead to a social desirability bias when reporting on depressive symptomatology.
The present study also has some strengths. Firstly, this study analyses the evolution of depressive symptoms after a smoking cessation intervention in a specific sample of smokers with a history of depressive episode, filling the gap in the literature that usually studies variations in psychological variables among non-specific populations (Taylor et al., 2021 ). Therefore, this study can be generalised to smokers who seek intervention for smoking cessation and who may be struggling to quit smoking due to psychological variables such as a history of depressive episode. Furthermore, the study offers an extensive time frame for follow-ups, allowing one to observe the variation of depressive symptomatology up to 1 year after the intervention, strengthening the conclusion that depression seems to decrease after smoking cessation. Finally, the present study includes an analysis of smokers, abstainers and relapsers in contrast with previous studies that generally analyse smokers versus abstainers (Wu et al., 2023 ).
Future research is warranted to analyse more specifically the changes that can be observed in depressive symptomatology for people with a history of depressive episode, and, consequently, to study in more detail its relationship with smoking relapse. Furthermore, due to the differences found in baseline smoking volume between abstainers, smokers and relapsers, it would be advisable for future studies to consider the impact of the number of cigarettes smoked per day on depressive symptoms and abstinence.
In conclusion, this study investigates the association between smoking cessation and depression among smokers with antecedents of depressive episode, confirming important reductions in depression for abstainers over time. The analysis presented can not only be used as support to encourage smokers with depression to achieve abstinence in smoking cessation interventions but also to encourage people struggling with depressive episodes to quit smoking as a means to improve mental health.
Audrain-McGovern, J., Wileyto, E. P., Ashare, R., Albelda, B., Manikandan, D., & Perkins, K. A. (2023). Behavioral activation for smoking cessation and the prevention of smoking cessation-related weight gain: A randomized trial. Drug and Alcohol Dependence , 244. https://doi.org/10.1016/j.drugalcdep.2023.109792 .
Bainter, T., Selya, A. S., & Oancea, S. C. (2020). A key indicator of nicotine dependence is associated with greater depression symptoms, after accounting for smoking behavior. PloS One , 15 (5). https://doi.org/10.1371/journal.pone.0233656 .
Beck, A. T., Steer, R. A., & Brown, G. K. (2006). BDI-II. Beck Depression Inventory-Second Edition. Manual . The Psychological Corporation.
Google Scholar
Becoña, E. (1994). Evaluación de la conducta de fumar [Assessment of smoking behavior]. In J. L. Graña, (Ed.), Conductas adictivas: Teoría, evaluación y tratamiento [Addictive behaviors: Theory, assessment, and treatment] (pp. 403–454). Debate.
Becoña, E. (2007). Programa para dejar de fumar [Program to quit smoking]. Nova Galicia Edicións.
Benowitz, N. L., Bernert, J. T., Foulds, J., Hecht, S. S., Jacob, P., Jarvis, M. J., Joseph, A., Oncken, C., & Piper, M. E. (2020). Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine & Tobacco Research , 22 (7), 1086–1097. https://doi.org/10.1093/ntr/ntz132 .
Article Google Scholar
Berlin, I., & Singleton, E. G. (2008). Nicotine dependence and urge to smoke predict negative health symptoms in smokers. Preventive Medicine , 47 (4), 447–451. https://doi.org/10.1016/j.ypmed.2008.06.008 .
Article CAS PubMed Google Scholar
Blalock, J. A., Robinson, J. D., Wetter, D. W., Schreindorfer, L. S., & Cinciripini, P. M. (2008). Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychology of Addictive Behaviors , 22 (1), 122–128. https://doi.org/10.1037/0893-164X.22.1.122 .
Article PubMed Google Scholar
Busch, A. M., Tooley, E. M., Dunsiger, S., Chattillion, E. A., Srour, J. F., Pagoto, S. L., Kahler, C. W., & Borrelli, B. (2017). Behavioral activation for smoking cessation and mood management following a cardiac event: Results of a pilot randomised controlled trial. Bmc Public Health , 17 (1). https://doi.org/10.1186/s12889-017-4250-7 .
Cerci, D. (2023). Staff perspectives on smoking cessation treatment in German psychiatric hospitals. Journal of Public Health . https://doi.org/10.1007/s10389-022-01811-2 .
Cooper, J., Borland, R., McKee, S. A., Yong, H. H., & Dugué, P. A. (2016). Depression motivates quit attempts but predicts relapse: Differential findings for gender from the International Tobacco Control Study. Addiction , 111 (8), 1438–1447. https://doi.org/10.1111/add.13290 .
Article PubMed PubMed Central Google Scholar
Fluharty, M., Taylor, A. E., Grabski, M., & Munafò, M. R. (2017). The association of cigarette smoking with depression and anxiety: A systematic review. Nicotine & Tobacco Research , 19 (1), 3–13. https://doi.org/10.1093/ntr/ntw140 .
Glassman, A. H., Covey, L. S., Stetner, F., & Rivelli, S. (2001). Smoking cessation and the course of major depression: A follow-up study. The Lancet , 357 , 1929–1932. https://doi.org/10.1016/S0140-6736(00)05064-9 .
Article CAS Google Scholar
Hahad, O., Beutel, M., Gilan, D. A., Michal, M., Schulz, A., Pfeiffer, N., König, J., Lackner, K., Wild, P., Daiber, A., & Münzel, T. (2022). The association of smoking and smoking cessation with prevalent and incident symptoms of depression, anxiety, and sleep disturbance in the general population. Journal of Affective Disorders , 313 , 100–109. https://doi.org/10.1016/j.jad.2022.06.083 .
Huffman, A. L., Bromberg, J. E., & Augustson, E. M. (2018). Lifetime depression, other mental illness, and smoking cessation. American Journal of Health Behavior , 42 (4), 90–101. https://doi.org/10.5993/AJHB.42.4.9 .
Kastaun, S., Brose, L. S., Scholz, E., Viechtbauer, W., & Kotz, D. (2022). Mental health symptoms and associations with tobacco smoking, dependence, motivation, and attempts to quit: Findings from a population survey in Germany (DEBRA Study). European Addiction Research , 28 (4), 287–296. https://doi.org/10.1159/000523973 .
Lembke, A., Johnson, K., & DeBattista, C. (2007). Depression and smoking cessation: Does the evidence support psychiatric practice? Neuropsychiatric Disease and Treatment , 3 (4), 487–493.
PubMed PubMed Central Google Scholar
Liu, N. H., Wu, C., Pérez-Stable, E. J., & Muñoz, R. F. (2021). Longitudinal association between smoking abstinence and depression severity in those with baseline current, past, and no history of major depressive episode in an international online tobacco cessation study. Nicotine & Tobacco Research , 23 (2), 267–275. https://doi.org/10.1093/ntr/ntaa036 .
Martínez-Vispo, C., Rodríguez-Cano, R., López-Durán, A., Senra, C., Fernández, D., Río, E., & Becoña, E. (2019). Cognitive-behavioral treatment with behavioral activation for smoking cessation: Randomized controlled trial. PloS One , 14 (4). https://doi.org/10.1371/journal.pone.0214252 .
Muñoz, R. (1998). Preventing major depression by promoting emotion regulation: A conceptual framework and some practical tools. International Journal of Mental Health Promotion , 1 , 23–40.
Piñeiro, B., López-Durán, A., Río, D., Martínez, E. F., Brandon, Ú., T. H., & Becoña, E. (2016). Motivation to quit as a predictor of smoking cessation and abstinence maintenance among treated Spanish smokers. Addictive Behaviors , 53 , 40–45. https://doi.org/10.1016/j.addbeh.2015.09.017 .
Prochaska, J. J., Gates, E. F., Davis, K. C., Gutierrez, K., Prutzman, Y., & Rodes, R. (2019). The 2016 Tips from former Smokers® campaign: Associations with quit intentions and quit attempts among smokers with and without mental health conditions. Nicotine & Tobacco Research , 21 (5), 576–583. https://doi.org/10.1093/ntr/nty241 .
Quinn, M. H., Olonoff, M., Bauer, A. M., Fox, E., Jao, N., Lubitz, S. F., Leone, F., Gollan, J. K., Schnoll, R., & Hitsman, B. (2022). History and correlates of smoking cessation behaviors among individuals with current or past major depressive disorder enrolled in a smoking cessation trial. Nicotine & Tobacco Research , 24 (1), 37–43. https://doi.org/10.1093/ntr/ntab147 .
Ranjit, A., Latvala, A., Kinnunen, T. H., Kaprio, J., & Korhonen, T. (2020). Depressive symptoms predict smoking cessation in a 20-year longitudinal study of adult twins. Addictive Behaviors , 108. https://doi.org/10.1016/j.addbeh.2020.106427 .
Rodríguez-Cano, R., López-Durán, A., del Río, E. F., Martínez-Vispo, C., Martínez, Ú., & Becoña, E. (2016). Smoking cessation and depressive symptoms at 1-, 3-, 6-, and 12-months follow-up. Journal of Affective Disorders , 191 , 94–99. https://doi.org/10.1016/j.jad.2015.11.042 .
Sánchez-Villegas, A., Gea, A., Lahortiga-Ramos, F., Martínez-González, J., Molero, P., & Martínez-González, M. (2021). Á. Bidirectional association between tobacco use and depression risk in the SUN cohort study. Adicciones . https://doi.org/10.20882/adicciones.1725 .
Secades-Villa, R., González-Roz, A., Vallejo-Seco, G., Weidberg, S., García-Pérez, Á., & Alonso-Pérez, F. (2019). Additive effectiveness of contingency management on cognitive behavioural treatment for smokers with depression: Six-month abstinence and depression outcomes. Drug and Alcohol Dependence , 204. https://doi.org/10.1016/j.drugalcdep.2019.06.003 .
Sheals, K., Tombor, I., McNeill, A., & Shahab, L. (2016). A mixed-method systematic review and meta-analysis of mental health professionals’ attitudes toward smoking and smoking cessation among people with mental illnesses. Addiction , 111 (9), 1536–1553. https://doi.org/10.1111/add.13387 .
Siru, R., Hulse, G. K., & Tait, R. J. (2009). Assessing motivation to quit smoking in people with mental illness: A review. Addiction , 104 (5), 719–733. https://doi.org/10.1111/j.1360-0443.2009.02545.x .
Taylor, G. M., Lindson, N., Farley, A., Leinberger-Jabari, A., Sawyer, K., Naudé, T. W., Theodoulou, R., King, A., Burke, N., C., & Aveyard, P. (2021). Smoking cessation for improving mental health. The Cochrane Database of Systematic Reviews , 3 (3). https://doi.org/10.1002/14651858.CD013522.pub2 .
Tucker, C. J., Bello, M. S., Weinberger, A. H., D’Orazio, L. M., Kirkpatrick, M. G., & Pang, R. D. (2022). Association of depression symptom level with smoking urges, cigarette withdrawal, and smoking reinstatement: A preliminary laboratory study. Drug and Alcohol Dependence , 232 . https://doi.org/10.1016/j.drugalcdep.2022.109267 .
U. S. Department of Health and Human Services (USDHHS). (2020). Smoking cessation: A report of the Surgeon General . US Department of Health and Human Services, Public Health Services, Office of the Surgeon General.
Weinberger, A. H., Mazure, C. M., Morlett, A., & McKee, S. A. (2013). Two decades of smoking cessation treatment research on smokers with depression: 1990–2010. Nicotine & Tobacco Research , 15 (6), 1014–1031. https://doi.org/10.1093/ntr/nts213 .
Weinberger, A. H., Chaiton, M. O., Zhu, J., Wall, M. M., Hasin, D. S., & Goodwin, R. D. (2020). Trends in the prevalence of current, daily, and nondaily cigarette smoking and quit ratios by depression status in the US: 2005–2017. American Journal of Preventive Medicine , 58 (5), 691–698. https://doi.org/10.1016/j.amepre.2019.12.023 .
West, R., Hajek, P., Stead, L., & Stapleton, J. (2005). Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction , 100 (3), 299–303. https://doi.org/10.1111/j.1360-0443.2004.00995.x .
World Health Organization (2017). Depression and other common mental disorders. Global health estimates . Geneva: World Health Organization. https://www.who.int/publications/i/item/depression-global-health-estimates .
World Health Organization (2023). WHO report on the global tobacco epidemic, 2023: Protect people from tobacco smoke. Geneva: World Health Organization. https://www.who.int/publications/i/item/9789240077164 .
Wu, A. D., Gao, M., Aveyard, P., & Taylor, G. (2023). Smoking cessation and changes in anxiety and depression in adults with and without psychiatric disorders. Journal of the American Medical Association , 6 (5). https://doi.org/10.1001/jamanetworkopen.2023.16111 .
Zeeman, A. D., Jonker, J., Groeneveld, L., de Krijger, E. M., & Meijer, E. (2023). Clients are the problem owners’: A qualitative study into professionals’ and clients’ perceptions of smoking cessation care for smokers with mental illness. Advances in Mental Health . https://doi.org/10.1080/18387357.2023.2262627 .
Zvolensky, M. J., Bakhshaie, J., Sheffer, C., Perez, A., & Goodwin, R. D. (2015). Major depressive disorder and smoking relapse among adults in the United States: A 10-year, prospective investigation. Psychiatry Research , 226 (1), 73–77. https://doi.org/10.1016/j.psychres.2014.11.064 .
Download references
Acknowledgements
We thank all the participants in this study, and María Ramos-Carro for all the support during the elaboration of this research paper.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by the Ministerio de Economía y Competitividad (Project PSI2015-66755-R), and Ministerio de Ciencia e Innovación (Project PID2019-109400RB-100) of Spain and co-financed by FEDER (European Regional Development Fund).
Author information
Authors and affiliations.
Smoking and Addictive Disorders Unit, Faculty of Psychology, Campus Vida, University of Santiago de Compostela, Rúa de Xosé María Súarez Núñez, Santiago de Compostela, 15782, Galicia, Spain
Elizabeth Moss-Alonso, Carmela Martínez-Vispo, Ana López-Durán & Elisardo Becoña
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation, E.M.A., C.M.V., A.L. and E.B.; methodology, E.M.A., C.M.V., A.L. and E.B.; validation, C.M.V., A.L. and E.B.; formal analysis, C.M.V. and E.M.A.; investigation, E.M.A., C.M.V., A.L. and E.B.; resources, E.B.; data curation, E.M.A., C.M.V. and A.L.; writing—original draft preparation, E.M.A.; writing—review and editing, C.M.V., A.L. and E.B; visualisation, E.M.A., C.M.V., A.L. and E.B.; supervision, A.L. and E.B; project administration, E.B. and A.L.; funding acquisition, E.B. and A.L.
Corresponding author
Correspondence to Elizabeth Moss-Alonso .
Ethics declarations
Ethics approval and consent to participate.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Moss-Alonso, E., Martínez-Vispo, C., López-Durán, A. et al. Does Quitting Smoking Affect Depressive Symptoms? A Longitudinal Study Based on Treatment-Seeking Smokers with a History of Depressive Episode. Int J Ment Health Addiction (2024). https://doi.org/10.1007/s11469-024-01317-w
Download citation
Accepted : 25 April 2024
Published : 06 May 2024
DOI : https://doi.org/10.1007/s11469-024-01317-w

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Depressive symptomatology
- Smoking cessation
- Cognitive-behavioural intervention
- 12-month follow-up
- History of major depressive episode
- Find a journal
- Publish with us
- Track your research
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Anniversary
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 32, Issue e2
- Effectiveness of e-cigarettes as aids for smoking cessation: evidence from the PATH Study cohort, 2017–2019
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Ruifeng Chen 1 ,
- http://orcid.org/0000-0002-0075-7471 John P Pierce 1 , 2 ,
- Eric C Leas 1 ,
- Tarik Benmarhnia 3 ,
- David R Strong 1 , 2 ,
- Martha M White 2 ,
- http://orcid.org/0000-0002-1152-0621 Matthew Stone 1 ,
- Dennis R Trinidad 1 ,
- Sara B McMenamin 1 ,
- Karen Messer 1 , 2
- 1 Herbert Wertheim School of Public Health and Human Longevity Science , University of California San Diego , La Jolla , California , USA
- 2 Moores Cancer Center , University of California San Diego , La Jolla , California , USA
- 3 Scripps Institution of Oceanography , University of California San Diego , La Jolla , California , USA
- Correspondence to Dr John P Pierce, Herbert Wertheim School of Public Health and Human Longevity Science, University of California San Diego, La Jolla, California, USA; jppierce{at}ucsd.edu
Objective To assess the effectiveness of e-cigarettes in smoking cessation in the USA from 2017 to 2019, given the 2017 increase in high nicotine e-cigarette sales.
Methods In 2017, the PATH Cohort Study included data on 3578 previous year smokers with a recent quit attempt and 1323 recent former smokers. Respondents reported e-cigarettes or other products used to quit cigarettes and many covariates associated with e-cigarette use. Study outcomes were 12+ months of cigarette abstinence and tobacco abstinence in 2019. We report weighted unadjusted estimates and use propensity score matched analyses with 1500 bootstrap samples to estimate adjusted risk differences (aRD).
Results In 2017, 12.6% (95% CI 11.3% to 13.9%) of recent quit attempters used e-cigarettes to help with their quit attempt, a decline from previous years. Cigarette abstinence for e-cigarette users (9.9%, 95% CI 6.6% to 13.2%) was lower than for no product use (18.6%, 95% CI 16.0% to 21.2%), and the aRD for e-cigarettes versus pharmaceutical aids was −7.3% (95% CI −14.4 to –0.4) and for e-cigarettes versus any other method was −7.7% (95% CI −12.2 to –3.2). Only 2.2% (95% CI 0.0% to 4.4%) of recent former smokers switched to a high nicotine e-cigarette. Subjects who switched to e-cigarettes appeared to have a higher relapse rate than those who did not switch to e-cigarettes or other tobacco, although the difference was not statistically significant.
Conclusions Sales increases in high nicotine e-cigarettes in 2017 did not translate to more smokers using these e-cigarettes to quit smoking. On average, using e-cigarettes for cessation in 2017 did not improve successful quitting or prevent relapse.
- electronic nicotine delivery devices
- Surveillance and monitoring
Data availability statement
The data are in a Restricted Use File that is available to approved researchers. National Addiction and HIV Data Archive Program. Population Assessment of Tobacco and Health (PATH) Study [United States] Restricted-Use Files (ICPSR 36231). NIH; National Institute on Drug Abuse.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/tobaccocontrol-2021-056901
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Electronic cigarettes (e-cigarettes), which were first sold in the USA in 2007, had become a popular cessation aid for US smokers by 2014–2016. 1 2 From 2013 to 2017 US sales of e-cigarettes almost doubled, 3 which was associated with rapid uptake among adolescents. 4 If there was a similar increase in e-cigarette usage attributed to smoking cessation (either as a cessation aid or an alternative nicotine source) and effectiveness was demonstrated, we would expect that successful cigarette cessation would increase in the population.
Randomised clinical trials (RCTs) are the optimal design to assess the efficacy of e-cigarettes as smoking cessation aids. To date, a number of RCTs have addressed the role of e-cigarettes as an aid to quitting cigarettes, and a recent systematic review concluded, with moderate certainty, that e-cigarettes improve cessation by an estimated four additional successful quitters per 100 quit attempters when compared with nicotine replacement therapy (NRT). 5 However, RCTs are usually conducted under optimal conditions, which means that they may not translate to the effectiveness of the product in community settings. 6 Analyses of the Population Assessment of Tobacco and Health (PATH) Study 7 have not found that e-cigarettes improve cessation. 8 9
To date, no trials have been reported that test the hypothesis that cigarette smokers are able to switch to e-cigarettes and maintain their nicotine habit without relapsing to cigarette smoking. A recent PATH Study analysis found that those who switched to e-cigarettes between 2014 and 2016 were more likely to relapse to cigarette smoking by 2017 than those who were free from all tobacco including e-cigarettes between 2014 and 2016. 10 However, the e-cigarette market has changed dramatically since 2016. JUUL Labs introduced nicotine salt technology in 2015 and high nicotine concentration pods (ie, 5% nicotine by weight). 11 On the back of an innovative marketing campaign, JUUL became the most popular US e-cigarette in 2017 12 13 when over 50% of all e-cigarette products sold had high (>4%) nicotine concentrations. 3 Increasing the nicotine concentration in e-cigarette liquid increases nicotine exposure for users, 14–16 and high nicotine JUUL users have blood nicotine concentrations similar to cigarette smokers, which some argue may be a prerequisite for successfully switching to e-cigarettes. 17 Thus, in 2017, recent former smokers had the opportunity to switch to e-cigarettes with a much higher nicotine concentration than was possible for those in earlier years, which could reduce relapse to cigarette smoking.
The PATH Study is a nationally representative longitudinal study that can address questions on the effectiveness of e-cigarettes in reducing cigarette smoking. However, for longitudinal studies to address whether a product may cause an outcome such as smoking cessation requires careful analysis. The critical point is that groups must be as comparable as possible across variables that might be related to the study outcome. 18 In RCTs, randomisation of product usage usually achieves this effect. In observational studies it is necessary to control for the variables associated with using e-cigarettes, particularly those that are also associated with longer term cigarette cessation (eg, motivation to quit). Some published analyses of PATH Study data 19–21 have not required that the control group has a recent quit attempt. Given that e-cigarettes are seen as a popular way to quit cigarettes, 1 such an analytical decision means that the control group will be very different from the e-cigarette user group as it will include many people who are not trying to quit, thus significantly biasing the conclusions in favour of an e-cigarette effect. 22
In this paper, our starting population are PATH Study respondents who were established smokers in 2016. To address the hypothesis that e-cigarettes are an effective cigarette cessation aid, we limit our consideration to those who reported a quit attempt in the year prior to the 2017 (W4) survey and compare how cessation aids used were associated with 12+ months of cigarette/tobacco abstinence at the 2019 (W5) survey (see study flowchart in online supplemental file 1 ). To address whether switching to e-cigarettes improves maintenance of cigarette abstinence, we focus on those who were recent former smokers in 2017 (W4) and compare relapse to cigarette smoking in 2019 (W5) among those who switched to e-cigarettes versus those who did not use any tobacco or e-cigarette product.
Supplemental material
Data sources.
The PATH Study is a US nationally representative cohort study. A screener survey of a stratified address-based sample of households oversampled tobacco users, young adults aged 18–24 and African Americans for the adult cohort. 7 The first four survey waves (W1–4) were at annual intervals starting in 2013–14 (W1), and W5 (2019) was conducted ~2 years after W4 (2017). The initial household screener had a 54% response rate and the adult survey response rates were 74.0%, 83.2%, 78.4% and 73.5% for W1–4, respectively. Among initial screened households, 27 757 adults were interviewed at W4 and an additional new replenishment sample of 6065 adults were added to the cohort to adjust for attrition and reset the cohort sample size, thus reducing the magnitude of weighting required to provide population estimates. 23 The weighted response rate for W4 replenishment household screener was 52.8% and the response rate of the adult survey was 68.0% at W4 and 88.0% at W5. The Westat Institutional Review Board approved the study and all respondents provided written informed consent. Data were obtained from available restricted use files. 23
Study sample
The W4 (2017) total sample included both a continuing cohort and an added refreshment sample (see online supplemental file 1 ). For longitudinal analyses requiring earlier data we are limited to the continuing cohort subset (those with W1–W3 data). For each PATH survey, lifetime 100+ cigarette smokers were asked if they “currently smoke every day, some days, or not at all”. 23 Thus, in this paper the continuing cohort are drawn from those who were current daily or some-day smokers at W3 (2016). For the added refreshment sample at W4 (2017), we assessed previous year smoking from: “Around this time 12 months ago, did you smoke cigarettes every day, some days or not at all?”.
To investigate whether e-cigarettes are an effective cigarette cessation aid, we identified recent quit attempters from the W4 question: “In the past 12 months, have you tried to quit cigarettes completely?” A positive response was made by 3578 previous year established smokers. To investigate whether switching to e-cigarettes helps prevent relapse to cigarettes, we identified recent former smokers at W4 from a “not at all” response to the current cigarette smoking question among previous year established smokers (n=1323).
Use of e-cigarette or other products
To identify products used to help quit attempts, W4 quit attempters were asked: “Thinking back to the last time you tried to quit cigarettes in the past 12 months”, followed by three separate types of questions: “did you use an e-cigarette/(other non-cigarette tobacco product) to help you quit?”; “did you use a nicotine patch, gum, inhaler, nasal spray, lozenge or pill?”; and “did you use Chantix, varenicline, Wellbutrin, Zyban or bupropion?”.
To identify recent former smokers who had switched to an alternative nicotine source, we used the current use question (responses of every day, some days or not at all) for each of the following products: e-cigarettes, cigars, cigarillo, filtered cigars, pipes, hookah, snus and smokeless products. E-cigarette users were asked: “What concentration of nicotine do you usually use?” with eight response categories ranging from 0% to 4+%, as well as don't know.
Study outcome
At W5 (2019) current cigarette and other tobacco use was assessed from responses to the current use question for each product. To assess duration of abstinence from cigarettes, recent former smokers were asked: “In the past 12 months, have you smoked a cigarette/(used product), even one or two puffs/times?” Cigarette abstinence includes those who were using e-cigarettes or other tobacco products. Tobacco abstinence requires abstinence from all tobacco and e-cigarettes. This question was asked for all tobacco products as well as e-cigarettes. Duration of abstinence came from the question: “About how long has it been since you last smoked a cigarette/puffed from an electronic nicotine product?”
Study covariates
PATH Study investigators identified and measured potential confounders for e-cigarette and cessation analyses and demonstrated that these were mismatched between e-cigarette users and control participants. 9 Most of these variables were best measured when participants were still smokers at W3 (2016) and are only available for the continuing cohort. They include sociodemographic variables (age, sex, education, race, ethnicity, income), cigarette smoking status (daily or non-daily), tobacco dependence index, 24 time since last quit attempt, cigarette consumption, e-cigarette use status (any use or no use), interest in quitting cigarettes, self-efficacy about quitting, smoke-free home, exposure to smoking, perceived harm of cigarettes and e-cigarettes, cigarette pack-years, age began regular smoking, insurance status and health-related covariates (external/internal mental health symptoms, existence of smoking-related disease). Questions for each covariate and univariate distributions by product used in the quit attempt are shown in online supplemental file 2,3 .
To test whether switching to e-cigarettes prevented relapse, we used the same set of covariates with the following exceptions: (1) we added duration of cigarette abstinence at W4 (2017); (2) we changed the source of the smoke-free home measure from W3 (2016) to W4 (2017). Details of these covariates with univariate distributions by product used are shown in online supplemental file 4,5 .
Statistical analyses
All analyses were conducted in R (version 3.6.1). For unadjusted analyses using total samples (continuing + refreshment), estimates were weighted using W4 single wave weights 23 and variance estimates for confidence intervals were calculated using replicate weights constructed using a balanced repeated replications procedure with Fay adjustment (ρ=0.3). 7 Sample characteristics were explored using weighted proportions with 95% confidence limits. The adjusted analyses were restricted to the continuing cohort only and used W1–W5 longitudinal survey weights. 23
For the adjusted propensity score matching analysis we created 1500 bootstrap samples for each hypothesis test. Within each bootstrap sample we used simple imputation (R package ‘Mice’) for missing data from all the covariates, and we identified the optimal set of covariates prior to estimating the propensity score as follows. To select variables we used the LASSO with the Akaike Information Criterion (AIC). 25 26 The optimal set of covariates was the one that returned the smallest AIC. Then, for each exposure separately, we calculated a propensity score for each participant by estimating the unweighted probability of membership in the e-cigarette use group using logistic regression adjusting for the optimised set of covariates. Using the estimated propensity score, we matched up to two controls for each case (nearest neighbour matching using R package ‘Matchit’) 27 within the a priori calliper distance of 0.1. Cases that did not have a match meeting these criteria were omitted from the sample (<10% for each matching). For each matched bootstrap sample we used logistic regression with survey weights (R package ‘survey’) to estimate the average risk difference between the two matched groups for each outcome. The model included an indicator of the matched pair (or triple) and an indicator of use of e-cigarettes or not. The risk difference was estimated by the bootstrap mean estimate and the confidence intervals were calculated using the 95% bootstrap quantiles. To assess e-cigarettes as a cigarette cessation aid we compared 12+ months of cigarette abstinence between (1) any e-cigarette for quit attempt versus anyone who did not use an e-cigarette; and (2) any e-cigarette versus NRT or pharmaceutical aid only for quit attempt. We also compared those who used e-cigarettes only versus NRT or pharmaceutical aid only in a sensitivity analysis. To assess if e-cigarettes prevent relapse to cigarettes, we estimated the risk difference in rates of relapse to cigarette smoking between any e-cigarette versus no e-cigarette at W4. Current use of NRT and pharmaceutical aids was only collected in relation to the last quit attempt.
Characteristics of tobacco use among recent quit attempters
There were no differences between the continuing cohort and the combined continuing cohort and refreshment sample (ie, total W4 sample) in any of the following key measures ( table 1 ). In 2017 (W4), 32.8% (95% CI 31.8% to 33.9%) of previous year established smokers reported a recent quit attempt in the year prior to W4 and 12.4% (95% CI 11.6% to 13.3%) were recent former smokers at W4. Among recent quit attempters, 12.6% (95% CI 11.3% to 13.9%) reported using e-cigarettes to help in their last quit attempt (8.7% e-cigarettes only, 3.2% e-cigarettes and NRT/pharmaceutical aid, 0.5% e-cigarettes and other tobacco products, 0.2% used 3+ products); 2.5% (95% CI 1.9% to 3.1%) used non-e-cigarette tobacco products (2.1% non-e-cigarette tobacco products only); 20.6% (95% CI 18.9% to 22.3%) used NRT or a pharmaceutical aid only and 64.3% (95% CI 62.4% to 66.1%) did not use any product.
- View inline
Characteristics of PATH Study Wave 4 tobacco use
Among recent former cigarette smokers in 2017 (W4), 15.3% had switched to e-cigarettes (daily: 9.1% (95% CI 7.1% to 11.0%); non-daily: 6.2% (95% CI 4.7% to 7.7%); 10.4% e-cigarettes only) and 15.9% (95% CI 13.6% to 18.2%) reported use of another tobacco product (11.5% cigar family, 2.9% smokeless, 3.6% other or multiple products) and 68.8% (95% CI 65.9% to 71.8%) reported not using any tobacco or e-cigarette. Among those who had switched to e-cigarettes, only 2.2% (95% CI 0.0% to 4.4%) reported using e-cigarettes with concentration >4% (see online supplemental file 6 ) and 1.9% (95% CI 0.4% to 3.4%) reported using JUUL e-cigarettes. This supplement also presents the 2019 (W5) data for recent former smokers who switched to e-cigarettes as this proportion increased to 22.0% (95% CI 19.6% to 24.5%) compared with the 15.3% observed at W4, with 19.9% of them using high nicotine content e-cigarettes.
Characteristics of recent quit attempters who used e-cigarettes
The use of e-cigarettes to aid a quit attempt was higher in 18–50-year-old subjects than in those aged 50+ years, higher in those who had attended college than in those who did not complete high school, higher in non-Hispanic white people than in other race ethnicities, higher in those with incomes >$35 000 than in those with lower incomes, higher in 2016 (W3) daily smokers than in non-daily smokers and higher in 2016 (W3) e-cigarette users ( table 2 ). Similar use patterns were observed for recent former smokers (see online supplemental file 3, 5 ), although the lower sample size of recent former smokers resulted in some wide confidence intervals.
Characteristics of recent quit attempters reported at PATH Wave 4 by use of non-cigarette tobacco products on last quit attempt prior to Wave 4
Successful quitting at W5 among quit attempters in year prior to W4
Unadjusted successful quitting in the total samples (continuing + refreshment).
Among those who used e-cigarettes in their last quit attempt prior to W4 (2017), 9.9% (95% CI 6.6% to 13.2%) were abstinent from cigarettes for 12+ months but not all tobacco at W5, which was lower than those who used NRT or pharmaceutical aid only (15.2%, 95% CI 12.3% to 18.1%) or those who did not use any product in the quit attempt (18.6%, 95% CI 16.0% to 21.2%), with similar patterns between the total sample and the continuing cohort ( table 3 ). Considering abstinence for 12+ months from all tobacco including e-cigarettes, the proportion who used e-cigarettes for the quit attempt (3.5%, 95% CI 1.5% to 5.5%) was considerably lower than those who used NRT or pharmaceutical aid only (12.5%, 95% CI 9.6% to 15.4%) or who did not use any product when attempting to quit (13.9%, 95% CI 11.4% to 16.5%). For both abstinence from cigarettes and abstinence from all tobacco (including e-cigarettes), our data suggest that those who used e-cigarettes to help them quit had a similar outcome to those who used another non-cigarette combustible (eg, cigar) or smokeless tobacco product (eg, snus) ( table 3 ).
Abstinence for 12+ months at Wave 5 among smokers who tried to quit prior to Wave 4 according to products used to assist during last quit attempt prior to Wave 4
Among recent former smokers who had switched to daily use of e-cigarettes in 2017 (W4), 43.2% (95% CI 32.5% to 54.0%) had successfully quit cigarette smoking by 2019 (W5), which was similar to those who used e-cigarettes on a non-daily basis or to those who switched to another tobacco product, whether daily or non-daily ( table 4 ). All estimates of successful quitting for those who switched to another nicotine source were below the lower confidence bound for those who reported no tobacco use in 2017 (W4) (52.9%, 95% CI 47.8% to 58.0%), although confidence intervals overlapped. Among those who had relapsed between 2017 (W4) and 2019 (W5), 15–20% had made another quit attempt (re-quit) and were abstinent at the time of the 2019 (W5) survey, although there were no differences across categories in the duration of these re-quit attempts.
Unadjusted cigarette smoking status at Wave 5 among recent former cigarette smokers* by use of non-cigarette tobacco products assessed at Wave 4
Adjusted successful quitting in the continuing cohort
Propensity score matching achieved comparable study groups for variables associated with e-cigarette use at W4 (2017) (see online supplemental file 7-9 ). However, the perception that e-cigarettes were less harmful than cigarettes fell from 23.8% (95% CI 23.1% to 24.5%) in 2016 (W3) to 16.4% (95% CI 15.9% to 17.0%) in 2019 (W5) (see online supplemental file 10 ). Among quit attempters, those who used an e-cigarette as an aid had a lower 12+ month cigarette abstinence rate than those who did not (adjusted risk difference (aRD) −7.7, 95% CI −12.2 to −3.2). Similarly, using an e-cigarette as an aid resulted in a lower 12+ month cigarette abstinence rate than using NRT or a pharmaceutical aid (aRD −7.3, 95% CI −14.4 to −0.4) ( figure 1A ). When the outcome was 12+ months abstinence from cigarettes, e-cigarettes or any other tobacco product, these results were essentially the same with the aRD showing that e-cigarette use had between 7.4% and 6.4% lower abstinence than either not using e-cigarettes or using a pharmaceutical aid ( figure 1B ). The sensitivity analysis estimating the aRD between e-cigarette only users and NRT or pharmaceutical aid only users produced similar results.
- Download figure
- Open in new tab
- Download powerpoint
The adjusted risk difference (RD) in the rate of 12+ months of cigarette/tobacco abstinence for quit attempters by comparing the use of e-cigarettes versus no product use and the use of e-cigarettes versus use of nicotine replacement therapy (NRT) or pharmaceutical aid only during the last quit attempt in the year prior to Wave 4. (A) 12+ months of cigarette abstinence; (B) 12+ months of tobacco abstinence. Analyses using propensity score matching followed by logistic regression adjustment. Bootstrap samples were created to make statistical inference (details given in the section on Statistical Analyses). Covariates used for propensity score matching include: age, sex, education, race, ethnicity, income, cigarette smoking status at W3, time since last quit attempt, tobacco dependence index, cigarette consumption at W3, duration of previous quit attempt reported at W4, interest in quitting cigarettes, self-efficacy about quitting, smoke-free home, exposure to smoking, perceived harm of cigarettes and e-cigarettes, cigarette pack-years, age began regular smoking, insurance status, external mental health symptoms, internal mental health symptoms and existence of smoking-related disease. Missing data were imputed using simple imputation for each bootstrap sample. Cigarette abstinence does not include abstinence from e-cigarettes or other tobacco products. Tobacco abstinence includes no use of e-cigarette, cigar, cigarillo, filtered cigar, pipe, hookah, snus and smokeless tobacco.
Propensity score matching achieved highly comparable groups among recent former smokers who had switched to e-cigarettes compared with those who had not ( online supplemental file 7 ). The e-cigarette group appeared to have a higher relapse rate by W5 (2019) than those who did not use any tobacco or e-cigarette product (aRD 9.4%, 95% CI −5.0% to 22.8%); however, this did not reach statistical significance.
In this analysis of the most recent PATH Study data, smokers who reported using e-cigarettes to help them in their most recent cigarette quit attempt were less rather than more likely than other quit attempters to achieve either successful cigarette cessation or to become tobacco and e-cigarette free. Rather than e-cigarettes adding four additional successful cigarette quitters per 100 quit attempters compared with pharmaceutical aid users as concluded by a systematic review of RCT data, 5 in this study e-cigarette use was associated with seven fewer successful quitters per 100 quit attempters. Furthermore, switching to e-cigarettes did not reduce the risk of relapse to cigarette smoking compared with other recent former smokers. Instead, nearly 60% of recent former smokers who were daily e-cigarette users had relapsed to cigarette smoking by 2019 (W5).
Between 2013 and 2018 there was a rapid increase in both the number of e-cigarette products available in the USA (now >800) and in the total unit sales, with over 40% sales growth between 2016 and 2017 alone. 3 This rapid growth has been attributed to the introduction and effective marketing of high nicotine e-cigarettes, initially by JUUL Labs. 28 The high nicotine JUUL e-cigarette has been noted as the closest match to cigarettes in both nicotine delivery and user satisfaction, 29 which should make it one of the best candidates as a product to which smokers could switch in order to maintain their nicotine habit. 30 Thus, it was surprising that, just as sales for JUUL were surging in the marketplace, the use of e-cigarettes as a cessation aid fell from 17.4% of recent quit attempters in PATH W3 8 to 12.4% at PATH W4. However, by 2019 this situation had changed, at least among recent former smokers, with 22% switching to e-cigarettes and ~4% using high nicotine concentration e-cigarettes. Our analysis suggests that the 2017 JUUL marketing campaigns were not effective in encouraging smokers to use JUUL products to help with quit attempts, unlike their effectiveness in encouraging young people to initiate nicotine use with their products. 4 31 32 However, when we looked ahead to 2019, recent former smokers had started using high nicotine e-cigarettes. The effectiveness of high nicotine e-cigarettes at preventing relapse will require another follow-up PATH survey.
This study has both advantages and limitations. The PATH Study is a large cohort of a representative sample of the US population with a rigorous methodology, including biological samples to validate self-reported cigarette smoking. 7 In previous reports, biomarker concentrations indicate that self-reporting is valid. 33 This study included a large group of potential confounders that were measured prior to the target quit attempt and propensity score matching was used to achieve highly comparable groups. Each PATH survey collects detailed current use of a comprehensive set of tobacco products and detailed duration of abstinence of recently used products, allowing a comparison of the effectiveness of a wide range of potential products to help smokers quit. However, this study is observational and the exposure variable was not under experimental control. While our analytical design adjusted for potential confounding variables, other variables that were unmeasured confounders limit causal inference.
In 2017, a time of rapid growth in e-cigarette sales in the USA and increasing nicotine content in e-cigarette liquids, no such growth was seen in the use of e-cigarettes for cessation. In this study, smokers trying to quit or interested in switching to another nicotine delivery system were not early adopters of the high nicotine e-cigarettes such as JUUL, which have been reported as the closest products to resembling the experience of cigarette smoking. This analysis did not show a cessation benefit from using e-cigarettes either to help a cessation attempt or as a substitute for cigarette smoking. However, there is evidence that cigarette smokers were starting to use high nicotine e-cigarettes by 2019 and further follow-up in PATH is needed to see whether these changes result in future cessation benefit.
What this paper adds
What is already known on this subject.
Randomised clinical trials indicate e-cigarettes have efficacy in helping smokers quit
US cohort studies have not demonstrated effectiveness in the real world
Starting in 2017, JUUL high nicotine e-cigarettes became the most popular e-cigarette brand and overall e-cigarette sales increased markedly
What important gaps in knowledge exist on this topic?
The influence of the increased nicotine content of e-cigarettes on US smokers’ ability to quit cigarette smoking is not known
What this study adds
Despite a large increase in e-cigarette sales, the proportion who used e-cigarettes to help quit cigarettes declined and in 2017 only 2.2% of recent former smokers were using high nicotine e-cigarettes
Those who used e-cigarettes to aid their cigarette quit attempt in the year prior to the 2017 survey were less likely to have successfully quit by 2019 compared with those who used a pharmaceutical aid or no product at all
E-cigarette use did not prevent recent former smokers from relapsing to cigarettes
However, the usage of high nicotine e-cigarettes for cessation increased in 2019, suggesting that this question needs to be addressed again in the 2021 PATH survey
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants but IRB for University of California San DiegoProject #181462 exempted this study Participants gave informed consent to participate in the study before taking part.
- Caraballo RS ,
- Shafer PR ,
- Patel D , et al
- Romberg AR ,
- Miller Lo EJ ,
- Cuccia AF , et al
- Johnston L ,
- O'Malley PM , et al
- Hartmann-Boyce J ,
- McRobbie H ,
- Lindson N , et al
- Motschman CA ,
- Wray JM , et al
- Ambrose BK ,
- Conway KP , et al
- Pierce JP ,
- Leas EC , et al
- Benmarhnia T ,
- Chen R , et al
- Kealey S , et al
- Prochaska JJ ,
- Gammon DG ,
- Marynak KL , et al
- Soule EK , et al
- Bansal-Travers M ,
- Grana R , et al
- Eissenberg T , et al
- Goldenson NI ,
- Fearon IM ,
- Buchhalter AR , et al
- Stratton KR ,
- Reynolds LM ,
- Collins JM , et al
- Glasser AM ,
- Vojjala M ,
- Cantrell J , et al
- Kalkhoran S ,
- Benmarhnia T , et al
- National Addiction & HIV Data Archive Program
- Strong DR ,
- Pearson J ,
- Ehlke S , et al
- Tibshirani R
- Wallisch C ,
- King G , et al
- Jackler RK ,
- Ramamurthi D
- Phillips-Waller A ,
- Smith KM , et al
- Patterson JG ,
- LaPolt DT ,
- Miranda AR , et al
- Johnston LD ,
- O'Malley PM
- Centers for Disease Control and Prevention
- Rostron BL ,
- Chang JT , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Press release
Twitter @MatthewDavStone
Contributors JPP is responsible for the overall content and is the guarantor of this paper. JPP and RC conceptualised and designed the study, drafted the initial manuscript and reviewed and revised the manuscript. JPP and TB acquired funding for the study. TB and KM had input into conceptualisation and supervised the methodology and all analyses undertaken. They also reviewed and revised the manuscript for important intellectual content. ECL, SBM, DRS, MDS, DT and MMW had input into the study conceptualisation and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding Supported by the National Institutes of Health (grant R01CA234539) and by the Tobacco-Related Disease Research ProgramProgramme of the University of California, Office of the President (grants 28IR-0066 and T31IR-1584).
Disclaimer Neither funding source had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Further, the funders of the PATH Study had no role in the analysis or interpretation of the data, its preparation, review or approval of this manuscript or decision to submit it for publication. All data used are available in a restricted public use file.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2022
Tobacco and nicotine use
- Bernard Le Foll 1 , 2 ,
- Megan E. Piper 3 , 4 ,
- Christie D. Fowler 5 ,
- Serena Tonstad 6 ,
- Laura Bierut 7 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 8 , 9 ,
- Prabhat Jha 10 &
- Wayne D. Hall 11 , 12
Nature Reviews Disease Primers volume 8 , Article number: 19 ( 2022 ) Cite this article
34k Accesses
70 Citations
96 Altmetric
Metrics details
- Disease genetics
- Experimental models of disease
- Preventive medicine
Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will die prematurely from tobacco-related complications. In addition, people who smoke have a significant reduction in their quality of life. Neurobiological findings have identified the mechanisms by which nicotine in tobacco affects the brain reward system and causes addiction. These brain changes contribute to the maintenance of nicotine or tobacco use despite knowledge of its negative consequences, a hallmark of addiction. Effective approaches to screen, prevent and treat tobacco use can be widely implemented to limit tobacco’s effect on individuals and society. The effectiveness of psychosocial and pharmacological interventions in helping people quit smoking has been demonstrated. As the majority of people who smoke ultimately relapse, it is important to enhance the reach of available interventions and to continue to develop novel interventions. These efforts associated with innovative policy regulations (aimed at reducing nicotine content or eliminating tobacco products) have the potential to reduce the prevalence of tobacco and nicotine use and their enormous adverse impact on population health.
Similar content being viewed by others

Associations between classic psychedelics and nicotine dependence in a nationally representative sample
Use of electronic cigarettes and heated tobacco products during the covid-19 pandemic.
Smoking cessation behaviors and reasons for use of electronic cigarettes and heated tobacco products among Romanian adults
Introduction.
Tobacco is the second most commonly used psychoactive substance worldwide, with more than one billion smokers globally 1 . Although smoking prevalence has reduced in many high-income countries (HICs), tobacco use is still very prevalent in low-income and middle-income countries (LMICs). The majority of smokers are addicted to nicotine delivered by cigarettes (defined as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10) or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)). As a result of the neuro-adaptations and psychological mechanisms caused by repeated exposure to nicotine delivered rapidly by cigarettes, cessation can also lead to a well-characterized withdrawal syndrome, typically manifesting as irritability, anxiety, low mood, difficulty concentrating, increased appetite, insomnia and restlessness, that contributes to the difficulty in quitting tobacco use 2 , 3 , 4 .
Historically, tobacco was used in some cultures as part of traditional ceremonies, but its use was infrequent and not widely disseminated in the population. However, since the early twentieth century, the use of commercial cigarettes has increased dramatically 5 because of automated manufacturing practices that enable large-scale production of inexpensive products that are heavily promoted by media and advertising. Tobacco use became highly prevalent in the past century and was followed by substantial increases in the prevalence of tobacco-induced diseases decades later 5 . It took decades to establish the relationship between tobacco use and associated health effects 6 , 7 and to discover the addictive role of nicotine in maintaining tobacco smoking 8 , 9 , and also to educate people about these effects. It should be noted that the tobacco industry disputed this evidence to allow continuing tobacco sales 10 . The expansion of public health campaigns to reduce smoking has gradually decreased the use of tobacco in HICs, with marked increases in adult cessation, but less progress has been achieved in LMICs 1 .
Nicotine is the addictive compound in tobacco and is responsible for continued use of tobacco despite harms and a desire to quit, but nicotine is not directly responsible for the harmful effects of using tobacco products (Box 1 ). Other components in tobacco may modulate the addictive potential of tobacco (for example, flavours and non-nicotine compounds) 11 . The major harms related to tobacco use, which are well covered elsewhere 5 , are linked to a multitude of compounds present in tobacco smoke (such as carcinogens, toxicants, particulate matter and carbon monoxide). In adults, adverse health outcomes of tobacco use include cancer in virtually all peripheral organs exposed to tobacco smoke and chronic diseases such as eye disease, periodontal disease, cardiovascular diseases, chronic obstructive pulmonary disease, stroke, diabetes mellitus, rheumatoid arthritis and disorders affecting immune function 5 . Moreover, smoking during pregnancy can increase the risk of adverse reproductive effects, such as ectopic pregnancy, low birthweight and preterm birth 5 . Exposure to secondhand cigarette smoke in children has been linked to sudden infant death syndrome, impaired lung function and respiratory illnesses, in addition to cognitive and behavioural impairments 5 . The long-term developmental effects of nicotine are probably due to structural and functional changes in the brain during this early developmental period 12 , 13 .
Nicotine administered alone in various nicotine replacement formulations (such as patches, gum and lozenges) is safe and effective as an evidence-based smoking cessation aid. Novel forms of nicotine delivery systems have also emerged (called electronic nicotine delivery systems (ENDS) or e-cigarettes), which can potentially reduce the harmful effects of tobacco smoking for those who switch completely from combustible to e-cigarettes 14 , 15 .
This Primer focuses on the determinants of nicotine and tobacco use, and reviews the neurobiology of nicotine effects on the brain reward circuitry and the functioning of brain networks in ways that contribute to the difficulty in stopping smoking. This Primer also discusses how to prevent tobacco use, screen for smoking, and offer people who smoke tobacco psychosocial and pharmacological interventions to assist in quitting. Moreover, this Primer presents emerging pharmacological and novel brain interventions that could improve rates of successful smoking cessation, in addition to public health approaches that could be beneficial.
Box 1 Tobacco products
Conventional tobacco products include combustible products that produce inhaled smoke (most commonly cigarettes, bidis (small domestically manufactured cigarettes used in South Asia) or cigars) and those that deliver nicotine without using combustion (chewing or dipping tobacco and snuff). Newer alternative products that do not involve combustion include nicotine-containing e-cigarettes and heat-not-burn tobacco devices. Although non-combustion and alternative products may constitute a lesser risk than burned ones 14 , 15 , 194 , no form of tobacco is entirely risk-free.
Epidemiology
Prevalence and burden of disease.
The Global Burden of Disease Project (GBDP) estimated that around 1.14 billion people smoked in 2019, worldwide, increasing from just under a billion in 1990 (ref. 1 ). Of note, the prevalence of smoking decreased significantly between 1990 and 2019, but increases in the adult population meant that the total number of global smokers increased. One smoking-associated death occurs for approximately every 0.8–1.1 million cigarettes smoked 16 , suggesting that the estimated worldwide consumption of about 7.4 trillion cigarettes in 2019 has led to around 7 million deaths 1 .
In most populations, smoking prevalence is much higher among groups with lower levels of education or income 17 and among those with mental health disorders and other co-addictions 18 , 19 . Smoking is also more frequent among men than women (Figs 1 – 3 ). Sexual and/or gender minority individuals have disproportionately high rates of smoking and other addictions 17 , 20 . In addition, the prevalence of smoking varies substantially between regions and ethnicities; smoking rates are high in some regions of Asia, such as China and India, but are lower in North America and Australia. Of note, the prevalence of mental health disorders and other co-addictions is higher in individuals who smoke compared with non-smokers 18 , 19 , 21 . For example, the odds of smoking in people with any substance use disorder is more than five times higher than the odds in people without a substance use disorder 19 . Similarly, the odds of smoking in people with any psychiatric disorder is more than three times higher than the odds of smoking in those without a psychiatric diagnosis 22 . In a study in the USA, compared with a population of smokers with no psychiatric diagnosis, subjects with anxiety, depression and phobia showed an approximately twofold higher prevalence of smoking, and subjects with agoraphobia, mania or hypomania, psychosis and antisocial personality or conduct disorders showed at least a threefold higher prevalence of smoking 22 . Comorbid disorders are also associated with higher rates of smoking 22 , 23 .
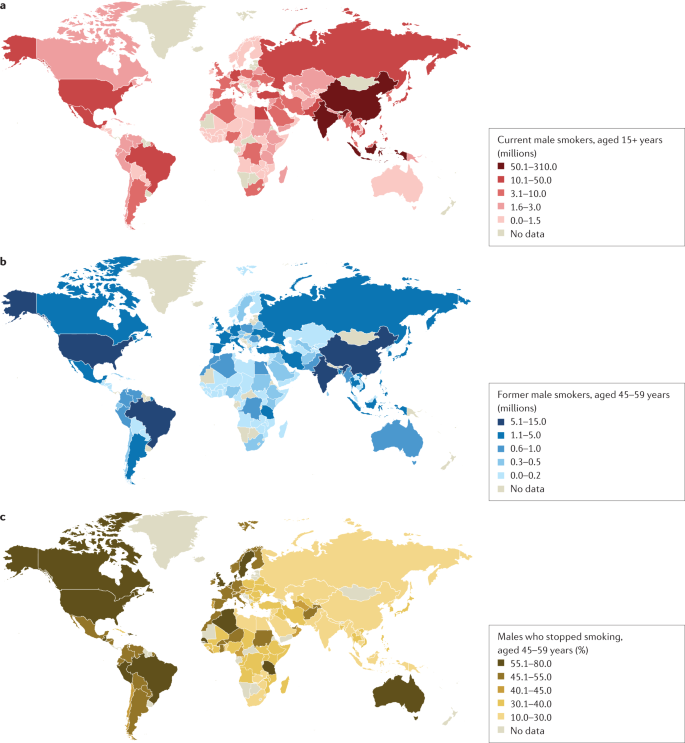
a | Number of current male smokers aged 15 years or older per country expressed in millions. b | Former male smokers aged 45–59 years per country expressed in millions. c | Former male smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for male smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among males is less variable than among females. Data from ref. 1 .
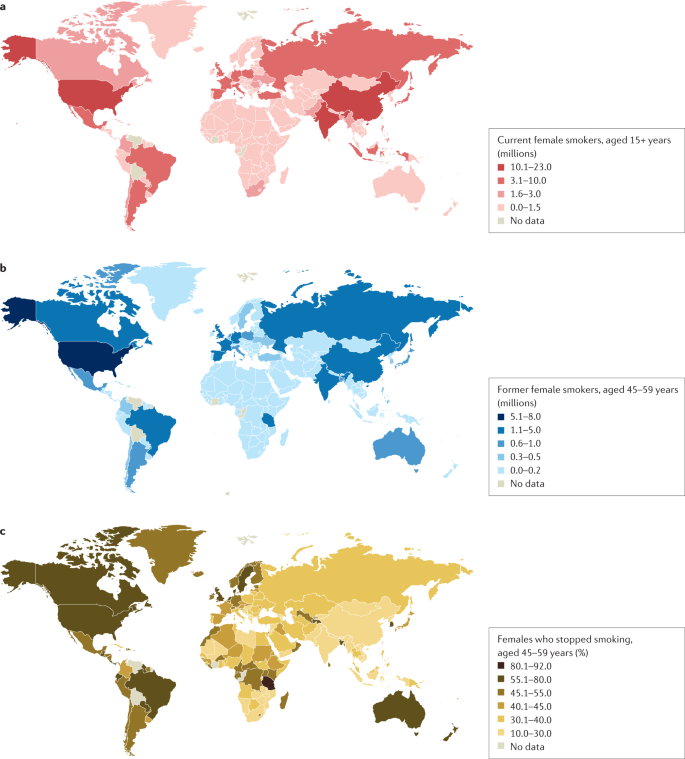
a | Number of current female smokers aged 15 years or older per country expressed in millions. b | Former female smokers aged 45–59 years per country expressed in millions. c | Former female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for female smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among females is much lower in East and South Asia than in Latin America or Eastern Europe. Data from ref. 1 .
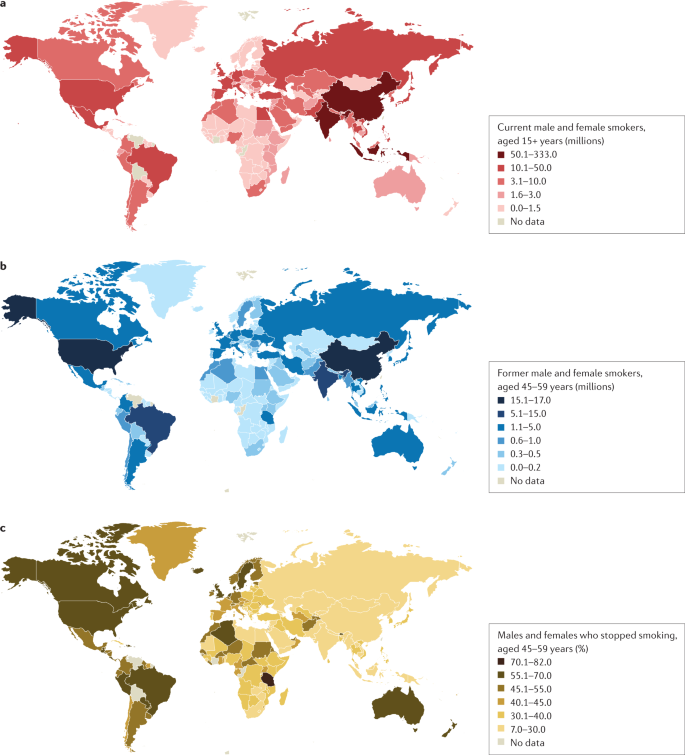
a | Number of current male and female smokers aged 15 years or older per country expressed in millions. b | Former male and female smokers aged 45–59 years per country expressed in millions. c | Former male and female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for the period 2015–2019 from countries with direct smoking surveys. Cessation rates are higher in high-income countries, but also notably high in Brazil. Cessation is far less common in South and East Asia and Russia and other Eastern European countries, and also low in South Africa. Data from ref. 1 .
Age at onset
Most smokers start smoking during adolescence, with almost 90% of smokers beginning between 15 and 25 years of age 24 . The prevalence of tobacco smoking among youths substantially declined in multiple HICs between 1990 and 2019 (ref. 25 ). More recently, the widespread uptake of ENDS in some regions such as Canada and the USA has raised concerns about the long-term effects of prolonged nicotine use among adolescents, including the possible notion that ENDS will increase the use of combustible smoking products 25 , 26 (although some studies have not found much aggregate effect at the population level) 27 .
Smoking that commences in early adolescence or young adulthood and persists throughout life has a more severe effect on health than smoking that starts later in life and/or that is not persistent 16 , 28 , 29 . Over 640 million adults under 30 years of age smoke in 22 jurisdictions alone (including 27 countries in the European Union where central efforts to reduce tobacco dependence might be possible) 30 . In those younger than 30 years of age, at least 320 million smoking-related deaths will occur unless they quit smoking 31 . The actual number of smoking-related deaths might be greater than one in two, and perhaps as high as two in three, long-term smokers 5 , 16 , 29 , 32 , 33 . At least half of these deaths are likely to occur in middle age (30–69 years) 16 , 29 , leading to a loss of two or more decades of life. People who smoke can expect to lose an average of at least a decade of life versus otherwise similar non-smokers 16 , 28 , 29 .
Direct epidemiological studies in several countries paired with model-based estimates have estimated that smoking tobacco accounted for 7.7 million deaths globally in 2020, of which 80% were in men and 87% were current smokers 1 . In HICs, the major causes of tobacco deaths are lung cancer, emphysema, heart attack, stroke, cancer of the upper aerodigestive areas and bladder cancer 28 , 29 . In some lower income countries, tuberculosis is an additional important cause of tobacco-related death 29 , 34 , which could be related to, for example, increased prevalence of infection, more severe tuberculosis/mortality and higher prevalence of treatment-resistant tuberculosis in smokers than in non-smokers in low-income countries 35 , 36 .
Despite substantial reductions in the prevalence of smoking, there were 34 million smokers in the USA, 7 million in the UK and 5 million in Canada in 2017 (ref. 16 ), and cigarette smoking remains the largest cause of premature death before 70 years of age in much of Europe and North America 1 , 16 , 28 , 29 . Smoking-associated diseases accounted for around 41 million deaths in the USA, UK and Canada from 1960 to 2020 (ref. 16 ). Moreover, as smoking-associated diseases are more prevalent among groups with lower levels of education and income, smoking accounts for at least half of the difference in overall mortality between these social groups 37 . Any reduction in smoking prevalence reduces the absolute mortality gap between these groups 38 .
Smoking cessation has become common in HICs with good tobacco control interventions. For example, in France, the number of ex-smokers is four times the number of current smokers among those aged 50 years or more 30 . By contrast, smoking cessation in LMICs remains uncommon before smokers develop tobacco-related diseases 39 . Smoking cessation greatly reduces the risks of smoking-related diseases. Indeed, smokers who quit smoking before 40 years of age avoid nearly all the increased mortality risks 31 , 33 . Moreover, individuals who quit smoking by 50 years of age reduce the risk of death from lung cancer by about two-thirds 40 . More modest hazards persist for deaths from lung cancer and emphysema 16 , 28 ; however, the risks among former smokers are an order of magnitude lower than among those who continue to smoke 33 .
Mechanisms/pathophysiology
Nicotine is the main psychoactive agent in tobacco and e-cigarettes. Nicotine acts as an agonist at nicotinic acetylcholine receptors (nAChRs), which are localized throughout the brain and peripheral nervous system 41 . nAChRs are pentameric ion channels that consist of varying combinations of α 2 –α 7 and β 2 –β 4 subunits, and for which acetylcholine (ACh) is the endogenous ligand 42 , 43 , 44 . When activated by nicotine binding, nAChR undergoes a conformational change that opens the internal pore, allowing an influx of sodium and calcium ions 45 . At postsynaptic membranes, nAChR activation can lead to action potential firing and downstream modulation of gene expression through calcium-mediated second messenger systems 46 . nAChRs are also localized to presynaptic membranes, where they modulate neurotransmitter release 47 . nAChRs become desensitized after activation, during which ligand binding will not open the channel 45 .
nAChRs with varying combinations of α-subunits and β-subunits have differences in nicotine binding affinity, efficacy and desensitization rate, and have differential expression depending on the brain region and cell type 48 , 49 , 50 . For instance, at nicotine concentrations found in human smokers, β 2 -containing nAChRs desensitize relatively quickly after activation, whereas α 7 -containing nAChRs have a slower desensitization profile 48 . Chronic nicotine exposure in experimental animal models or in humans induces an increase in cortical expression of α 4 β 2 -containing nAChRs 51 , 52 , 53 , 54 , 55 , but also increases the expression of β 3 and β 4 nAChR subunits in the medial habenula (MHb)–interpeduncular nucleus (IPN) pathway 56 , 57 . It is clear that both the brain localization and the type of nAChR are critical elements in mediating the various effects of nicotine, but other factors such as rate of nicotine delivery may also modulate addictive effects of nicotine 58 .
Neurocircuitry of nicotine addiction
Nicotine has both rewarding effects (such as a ‘buzz’ or ‘high’) and aversive effects (such as nausea and dizziness), with the net outcome dependent on dose and others factors such as interindividual sensitivity and presence of tolerance 59 . Thus, the addictive properties of nicotine involve integration of contrasting signals from multiple brain regions that process reward and aversion (Fig. 4 ).
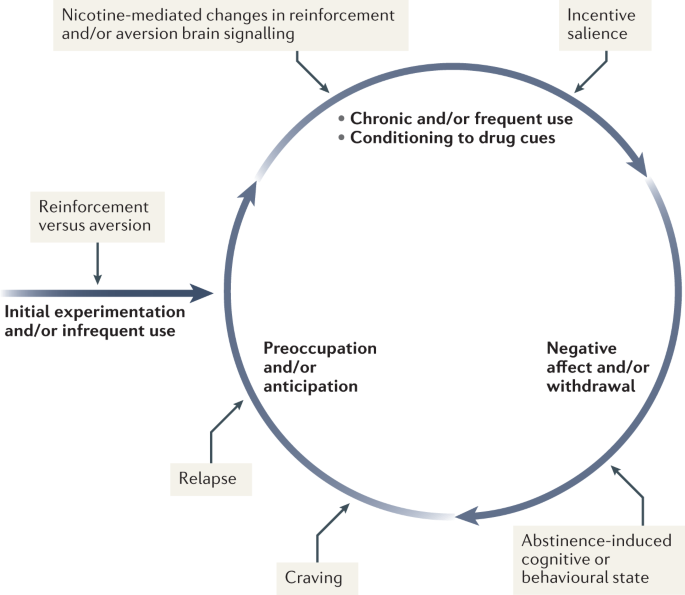
During initial use, nicotine exerts both reinforcing and aversive effects, which together determine the likelihood of continued use. As the individual transitions to more frequent patterns of chronic use, nicotine induces pharmacodynamic changes in brain circuits, which is thought to lead to a reduction in sensitivity to the aversive properties of the drug. Nicotine is also a powerful reinforcer that leads to the conditioning of secondary cues associated with the drug-taking experience (such as cigarette pack, sensory properties of cigarette smoke and feel of the cigarette in the hand or mouth), which serves to enhance the incentive salience of these environmental factors and drive further drug intake. When the individual enters into states of abstinence (such as daily during sleep at night or during quit attempts), withdrawal symptomology is experienced, which may include irritability, restlessness, learning or memory deficits, difficulty concentrating, anxiety and hunger. These negative affective and cognitive symptoms lead to an intensification of the individual’s preoccupation to obtain and use the tobacco/nicotine product, and subsequently such intense craving can lead to relapse.
The rewarding actions of nicotine have largely been attributed to the mesolimbic pathway, which consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens and prefrontal cortex 60 , 61 , 62 (Fig. 5 ). VTA integrating circuits and projection regions express several nAChR subtypes on dopaminergic, GABAergic, and glutamatergic neurons 63 , 64 . Ultimately, administration of nicotine increases dopamine levels through increased dopaminergic neuron firing in striatal and extrastriatal areas (such as the ventral pallidum) 65 (Fig. 6 ). This effect is involved in reward and is believed to be primarily mediated by the action of nicotine on α 4 -containing and β 2 -containing nAChRs in the VTA 66 , 67 .
Multiple lines of research have demonstrated that nicotine reinforcement is mainly controlled by two brain pathways, which relay predominantly reward-related or aversion-related signals. The rewarding properties of nicotine that promote drug intake involve the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). By contrast, the aversive properties of nicotine that limit drug intake and mitigate withdrawal symptoms involve the fasciculus retroflexus projection from the medial habenula (MHb) to the interpeduncular nucleus (IPN). Additional brain regions have also been implicated in various aspects of nicotine dependence, such as the prefrontal cortex (PFC), ventral pallidum (VP), nucleus tractus solitarius (NTS) and insula (not shown here for clarity). All of these brain regions are directly or indirectly interconnected as integrative circuits to drive drug-seeking and drug-taking behaviours.
Smokers received brain PET scans with [ 11 C]PHNO, a dopamine D 2/3 PET tracer that has high sensitivity in detecting fluctuations of dopamine. PET scans were performed during abstinence or after smoking a cigarette. Reduced binding potential (BP ND ) was observed after smoking, indicating increased dopamine levels in the ventral striatum and in the area that corresponds to the ventral pallidum. The images show clusters with statistically significant decreases of [ 11 C]PHNO BP ND after smoking a cigarette versus abstinence condition. Those clusters have been superimposed on structural T1 MRI images of the brain. Reprinted from ref. 65 , Springer Nature Limited.
The aversive properties of nicotine are mediated by neurons in the MHb, which project to the IPN. Studies in rodents using genetic knockdown and knockout strategies demonstrated that the α 5 -containing, α 3 -containing and β 4 -containing nAChRs in the MHb–IPN pathway mediate the aversive properties of nicotine that limit drug intake, especially when animals are given the opportunity to consume higher nicotine doses 68 , 69 , 70 , 71 , 72 . In addition to nAChRs, other signalling factors acting on the MHb terminals in the IPN also regulate the actions of nicotine. For instance, under conditions of chronic nicotine exposure or with optogenetic activation of IPN neurons, a subtype of IPN neurons co-expressing Chrna5 (encoding the α 5 nAChR subunit) and Amigo1 (encoding adhesion molecule with immunoglobulin-like domain 1) release nitric oxide from the cell body that retrogradely inhibits MHb axon terminals 70 . In addition, nicotine activates α 5 -containing nAChR-expressing neurons that project from the nucleus tractus solitarius to the IPN, leading to release of glucagon-like peptide-1 that binds to GLP receptors on habenular axon terminals, which subsequently increases IPN neuron activation and decreases nicotine self-administration 73 . Taken together, these findings suggest a dynamic signalling process at MHb axonal terminals in the IPN, which regulates the addictive properties of nicotine and determines the amount of nicotine that is self-administered.
Nicotine withdrawal in animal models can be assessed by examining somatic signs (such as shaking, scratching, head nods and chewing) and affective signs (such as increased anxiety-related behaviours and conditioned place aversion). Interestingly, few nicotine withdrawal somatic signs are found in mice with genetic knockout of the α 2 , α 5 or β 4 nAChR subunits 74 , 75 . By contrast, β 2 nAChR-knockout mice have fewer anxiety-related behaviours during nicotine withdrawal, with no differences in somatic symptoms compared with wild-type mice 74 , 76 .
In addition to the VTA (mediating reward) and the MHb–IPN pathway (mediating aversion), other brain areas are involved in nicotine addiction (Fig. 5 ). In animals, the insular cortex controls nicotine taking and nicotine seeking 77 . Moreover, humans with lesions of the insular cortex can quit smoking easily without relapse 78 . This finding led to the development of a novel therapeutic intervention modulating insula function (see Management, below) 79 , 80 . Various brain areas (shell of nucleus accumbens, basolateral amygdala and prelimbic cortex) expressing cannabinoid CB 1 receptors are also critical in controlling rewarding effects and relapse 81 , 82 . The α 1 -adrenergic receptor expressed in the cortex also control these effects, probably through glutamatergic afferents to the nucleus accumbens 83 .
Individual differences in nicotine addiction risk
Vulnerability to nicotine dependence varies between individuals, and the reasons for these differences are multidimensional. Many social factors (such as education level and income) play a role 84 . Broad psychological and social factors also modulate this risk. For example, peer smoking status, knowledge on effect of tobacco, expectation on social acceptance, exposure to passive smoking modulate the risk of initiating tobacco use 85 , 86 .
Genetic factors have a role in smoking initiation, the development of nicotine addiction and the likelihood of smoking cessation. Indeed, heritability has been estimated to contribute to approximatively half of the variability in nicotine dependence 87 , 88 , 89 , 90 . Important advances in our understanding of such genetic contributions have evolved with large-scale genome-wide association studies of smokers and non-smokers. One of the most striking findings has been that allelic variation in the CHRNA5 – CHRNA3 – CHRNB4 gene cluster, which encodes α 5 , α 3 and β 4 nAChR subunits, correlates with an increased vulnerability for nicotine addiction, indicated by a higher likelihood of becoming dependent on nicotine and smoking a greater number of cigarettes per day 91 , 92 , 93 , 94 , 95 . The most significant effect has been found for a single-nucleotide polymorphism in CHRNA5 (rs16969968), which results in an amino acid change and reduced function of α 5 -containing nAChRs 92 .
Allelic variation in CYP2A6 (encoding the CYP2A6 enzyme, which metabolizes nicotine) has also been associated with differential vulnerability to nicotine dependence 96 , 97 , 98 . CYP2A6 is highly polymorphic, resulting in variable enzymatic activity 96 , 99 , 100 . Individuals with allelic variation that results in slow nicotine metabolism consume less nicotine per day, experience less-severe withdrawal symptoms and are more successful at quitting smoking than individuals with normal or fast metabolism 101 , 102 , 103 , 104 . Moreover, individuals with slow nicotine metabolism have lower dopaminergic receptor expression in the dopamine D2 regions of the associative striatum and sensorimotor striatum in PET studies 105 and take fewer puffs of nicotine-containing cigarettes (compared with de-nicotinized cigarettes) in a forced choice task 106 . Slower nicotine metabolism is thought to increase the duration of action of nicotine, allowing nicotine levels to accumulate over time, therefore enabling lower levels of intake to sustain activation of nAChRs 107 .
Large-scale genetic studies have identified hundreds of other genetic loci that influence smoking initiation, age of smoking initiation, cigarettes smoked per day and successful smoking cessation 108 . The strongest genetic contributions to smoking through the nicotinic receptors and nicotine metabolism are among the strongest genetic contributors to lung cancer 109 . Other genetic variations (such as those related to cannabinoid, dopamine receptors or other neurotransmitters) may affect certain phenotypes related to smoking (such as nicotine preference and cue-reactivity) 110 , 111 , 112 , 113 , 114 , 115 .
Diagnosis, screening and prevention
Screening for cigarette smoking.
Screening for cigarette smoking should happen at every doctor’s visit 116 . In this regard, a simple and direct question about a person’s tobacco use can provide an opportunity to offer information about its potential risks and treatments to assist in quitting. All smokers should be offered assistance in quitting because even low levels of smoking present a significant health risk 33 , 117 , 118 . Smoking status can be assessed by self-categorization or self-reported assessment of smoking behaviour (Table 1 ). In people who smoke, smoking frequency can be assessed 119 and a combined quantity frequency measure such as pack-year history (that is, average number of cigarettes smoked per day multiplied by the number of years, divided by 20), can be used to estimate cumulative risk of adverse health outcomes. The Association for the Treatment of Tobacco Use and Dependence recommends that all electronic health records should document smoking status using the self-report categories listed in Table 1 .
Owing to the advent of e-cigarettes and heat-not-burn products, and the popularity of little cigars in the US that mimic combustible cigarettes, people who use tobacco may use multiple products concurrently 120 , 121 . Thus, screening for other nicotine and tobacco product use is important in clinical practice. The self-categorization approach can also be used to describe the use of these other products.
Traditionally tobacco use has been classified according to whether the smoker meets criteria for nicotine dependence in one of the two main diagnostic classifications: the DSM 122 (tobacco use disorder) and the ICD (tobacco dependence) 123 . The diagnosis of tobacco use disorder according to DSM-5 criteria requires the presence of at least 2 of 11 symptoms that have produced marked clinical impairment or distress within a 12-month period (Box 2 ). Of note, these symptoms are similar for all substance use disorder diagnoses and may not all be relevant to tobacco use disorder (such as failure to complete life roles). In the ICD-10, codes allow the identification of specific tobacco products used (cigarettes, chewing tobacco and other tobacco products).
Dependence can also be assessed as a continuous construct associated with higher levels of use, greater withdrawal and reduced likelihood of quitting. The level of dependence can be assessed with the Fagerström Test for Nicotine Dependence, a short questionnaire comprising six questions 124 (Box 2 ). A score of ≥4 indicates moderate to high dependence. As very limited time may be available in clinical consultations, the Heaviness of Smoking Index (HSI) was developed, which comprises two questions on the number of cigarettes smoked per day and how soon after waking the first cigarette is smoked 125 . The HSI can guide dosing for nicotine replacement therapy (NRT).
Other measures of cigarette dependence have been developed but are not used in the clinical setting, such as the Cigarette Dependence Scale 126 , Hooked on Nicotine Checklist 127 , Nicotine Dependence Syndrome Scale 128 , the Wisconsin Inventory of Smoking Dependence Motives (Brief) 129 and the Penn State Cigarette Dependence Index 130 . However, in practice, these are not often used, as the most important aspect is to screen for smoking and encourage all smokers to quit smoking regardless of their dependence status.
Box 2 DSM-5 criteria for tobacco use disorder and items of the Fagerström Test for nicotine dependence
DSM-5 (ref. 122 )
Taxonomic and diagnostic tool for tobacco use disorder published by the American Psychiatric Association.
A problematic pattern of tobacco use leading to clinically significant impairment or distress as manifested by at least two of the following, occurring within a 12-month period.
Tobacco often used in larger amounts or over a longer period of time than intended
A persistent desire or unsuccessful efforts to reduce or control tobacco use
A great deal of time spent in activities necessary to obtain or use tobacco
Craving, or a strong desire or urge to use tobacco
Recurrent tobacco use resulting in a failure to fulfil major role obligations at work, school or home
Continued tobacco use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of tobacco (for example, arguments with others about tobacco use)
Important social, occupational or recreational activities given up or reduced because of tobacco use
Recurrent tobacco use in hazardous situations (such as smoking in bed)
Tobacco use continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco use
Tolerance, defined by either of the following.
A need for markedly increased amounts of tobacco to achieve the desired effect
A markedly diminished effect with continued use of the same amount of tobacco
Withdrawal, manifesting as either of the following.
Withdrawal syndrome for tobacco
Tobacco (or a closely related substance, such as nicotine) taken to relieve or avoid withdrawal symptoms
Fagerström Test for Nicotine Dependence 124
A standard instrument for assessing the intensity of physical addiction to nicotine.
How soon after you wake up do you smoke your first cigarette?
Within 5 min (scores 3 points)
5 to 30 min (scores 2 points)
31 to 60 min (scores 1 point)
After 60 min (scores 0 points)
Do you find it difficult not to smoke in places where you should not, such as in church or school, in a movie, at the library, on a bus, in court or in a hospital?
Yes (scores 1 point)
No (scores 0 points)
Which cigarette would you most hate to give up; which cigarette do you treasure the most?
The first one in the morning (scores 1 point)
Any other one (scores 0 points)
How many cigarettes do you smoke each day?
10 or fewer (scores 0 points)
11 to 20 (scores 1 point)
21 to 30 (scores 2 points)
31 or more (scores 3 points)
Do you smoke more during the first few hours after waking up than during the rest of the day?
Do you still smoke if you are so sick that you are in bed most of the day or if you have a cold or the flu and have trouble breathing?
A score of 7–10 points is classified as highly dependent; 4–6 points is classified as moderately dependent; <4 points is classified as minimally dependent.
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Young people who do not start smoking cigarettes between 15 and 25 years of age have a very low risk of ever smoking 24 , 131 , 132 . This age group provides a critical opportunity to prevent cigarette smoking using effective, evidence-based strategies to prevent smoking initiation and reduce escalation from experimentation to regular use 131 , 132 , 133 , 134 , 135 .
Effective prevention of cigarette uptake requires a comprehensive package of cost-effective policies 134 , 136 , 137 to synergistically reduce the population prevalence of cigarette smoking 131 , 135 . These policies include high rates of tobacco taxation 30 , 134 , 137 , 138 , widespread and rigorously enforced smoke-free policies 139 , bans on tobacco advertising and promotions 140 , use of plain packaging and graphic warnings about the health risks of smoking 135 , 141 , mass media and peer-based education programmes to discourage smoking, and enforcement of laws against the sale of cigarettes to young people below the minimum legal purchase age 131 , 135 . These policies make cigarettes less available and affordable to young people. Moreover, these policies make it more difficult for young people to purchase cigarettes and make smoking a much less socially acceptable practice. Of note, these policies are typically mostly enacted in HICs, which may be related to the declining prevalence of smoking in these countries, compared with the prevalence in LMICs.
Pharmacotherapy
Three evidence-based classes of pharmacotherapy are available for smoking cessation: NRT (using nicotine-based patches, gum, lozenges, mini-lozenges, nasal sprays and inhalers), varenicline (a nAChR partial agonist), and bupropion (a noradrenaline/dopamine reuptake inhibitor that also inhibits nAChR function and is also used as an antidepressant). These FDA-approved and EMA-approved pharmacotherapies are cost-effective smoking cessation treatments that double or triple successful abstinence rates compared with no treatment or placebo controls 116 , 142 .
Combinations of pharmacotherapies are also effective for smoking cessation 116 , 142 . For example, combining NRTs (such as the steady-state nicotine patch and as-needed NRT such as gum or mini-lozenge) is more effective than a single form of NRT 116 , 142 , 143 . Combining NRT and varenicline is the most effective smoking cessation pharmacotherapy 116 , 142 , 143 . Combining FDA-approved pharmacotherapy with behavioural counselling further increases the likelihood of successful cessation 142 . Second-line pharmacotherapies (for example, nortriptyline) have some potential for smoking cessation, but their use is limited due to their tolerability profile.
All smokers should receive pharmacotherapy to help them quit smoking, except those in whom pharmacotherapy has insufficient evidence of effectiveness (among adolescents, smokeless tobacco users, pregnant women or light smokers) or those in whom pharmacotherapy is medically contraindicated 144 . Table 2 provides specific information regarding dosing and duration for each FDA-approved pharmacotherapy. Extended use of pharmacotherapy beyond the standard 12-week regimen after cessation is effective and should be considered 116 . Moreover, preloading pharmacotherapy (that is, initiating cessation medication in advance of a quit attempt), especially with the nicotine patch, is a promising treatment, although further studies are required to confirm efficacy.
Cytisine has been used for smoking cessation in Eastern Europe for a long time and is available in some countries (such as Canada) without prescription 145 . Cytisine is a partial agonist of nAChRs and its structure was the precursor for the development of varenicline 145 . Cytisine is at least as effective as some approved pharmacotherapies for smoking cessation, such as NRT 146 , 147 , 148 , and the role of cytisine in smoking cessation is likely to expand in the future, notably owing to its much lower cost than traditional pharmacotherapies. E-cigarettes also have the potential to be useful as smoking cessation devices 149 , 150 . The 2020 US Surgeon General’s Report concluded that there was insufficient evidence to promote cytisine or e-cigarettes as effective smoking cessation treatments, but in the UK its use is recommended for smoking cessation (see ref. 15 for regularly updated review).
Counselling and behavioural treatments
Psychosocial counselling significantly increases the likelihood of successful cessation, especially when combined with pharmacotherapy. Even a counselling session lasting only 3 minutes can help smokers quit 116 , although the 2008 US Public Health Service guidelines and the Preventive Services Task Force 151 each concluded that more intensive counselling (≥20 min per session) is more effective than less intensive counselling (<20 min per session). Higher smoking cessation rates are obtained by using behavioural change techniques that target associative and self-regulatory processes 152 . In addition, behavioural change techniques that will favour commitment, social reward and identity associated with changed behaviour seems associated with higher success rates 152 . Evidence-based counselling focuses on providing social support during treatment, building skills to cope with withdrawal and cessation, and problem-solving in challenging situations 116 , 153 . Effective counselling can be delivered by diverse providers (such as physicians, nurses, pharmacists, social workers, psychologists and certified tobacco treatment specialists) 116 .
Counselling can be delivered in a variety of modalities. In-person individual and group counselling are effective, as is telephone counselling (quit lines) 142 . Internet and text-based intervention seem to be effective in smoking cessation, especially when they are interactive and tailored to a smoker’s specific circumstances 142 . Over the past several years, the number of smoking cessation smartphone apps has increased, but there the evidence that the use of these apps significantly increases smoking cessation rates is not sufficient.
Contingency management (providing financial incentives for abstinence or engagement in treatment) has shown promising results 154 , 155 but its effects are not sustained once the contingencies are removed 155 , 156 . Other treatments such as hypnosis, acupuncture and laser treatment have not been shown to improve smoking cessation rates compared with placebo treatments 116 . Moreover, no solid evidence supports the use of conventional transcranial magnetic stimulation (TMS) for long-term smoking cessation 157 , 158 .
Although a variety of empirically supported smoking cessation interventions are available, more than two-thirds of adult smokers who made quit attempts in the USA during the past year did not use an evidence-based treatment and the rate is likely to be lower in many other countries 142 . This speaks to the need to increase awareness of, and access to, effective cessation aids among all smokers.
Brain stimulation
The insula (part of the frontal cortex) is a critical brain structure involved in cigarette craving and relapse 78 , 79 . The activity of the insula can be modulated using an innovative approach called deep insula/prefrontal cortex TMS (deep TMS), which is effective in helping people quit smoking 80 , 159 . This approach has now been approved by the FDA as an effective smoking cessation intervention 80 . However, although this intervention was developed and is effective for smoking cessation, the number of people with access to it is limited owing to the limited number of sites equipped and with trained personnel, and the cost of this intervention.
Quality of life
Generic instruments (such as the Short-Form (SF-36) Health Survey) can be used to evaluate quality of life (QOL) in smokers. People who smoke rate their QOL lower than people who do not smoke both before and after they become smokers 160 , 161 . QOL improves when smokers quit 162 . Mental health may also improve on quitting smoking 163 . Moreover, QOL is much poorer in smokers with tobacco-related diseases, such as chronic respiratory diseases and cancers, than in individuals without tobacco-related diseases 161 , 164 . The dimensions of QOL that show the largest decrements in people who smoke are those related to physical health, day-to-day activities and mental health such as depression 160 . Smoking also increases the risk of diabetes mellitus 165 , 166 , which is a major determinant of poor QOL for a wide range of conditions.
The high toll of premature death from cigarette smoking can obscure the fact that many of the diseases that cause these deaths also produce substantial disability in the years before death 1 . Indeed, death in smokers is typically preceded by several years of living with the serious disability and impairment of everyday activities caused by chronic respiratory disease, heart disease and cancer 2 . Smokers’ QOL in these years may also be adversely affected by the adverse effects of the medical treatments that they receive for these smoking-related diseases (such as major surgery and radiotherapy).
Expanding cessation worldwide
The major global challenge is to consider individual and population-based strategies that could increase the substantially low rates of adult cessation in most LMICs and indeed strategies to ensure that even in HICs, cessation continues to increase. In general, the most effective tools recommended by WHO to expand cessation are the same tools that can prevent smoking initiation, notably higher tobacco taxes, bans on advertising and promotion, prominent warning labels or plain packaging, bans on public smoking, and mass media and educational efforts 29 , 167 . The effective use of these policies, particularly taxation, lags behind in most LMICs compared with most HICs, with important exceptions such as Brazil 167 . Access to effective pharmacotherapies and counselling as well as support for co-existing mental health conditions would also be required to accelerate cessation in LMICs. This is particularly important as smokers living in LMICs often have no access to the full range of effective treatment options.
Regulating access to e-cigarettes
How e-cigarettes should be used is debated within the tobacco control field. In some countries (for example, the UK), the use of e-cigarettes as a cigarette smoking cessation aid and as a harm reduction strategy is supported, based on the idea that e-cigarette use will lead to much less exposure to toxic compounds than tobacco use, therefore reducing global harm. In other countries (for example, the USA), there is more concern with preventing the increased use of e-cigarettes by youths that may subsequently lead to smoking 25 , 26 . Regulating e-cigarettes in nuanced ways that enable smokers to access those products whilst preventing their uptake among youths is critical.
Regulating nicotine content in tobacco products
Reducing the nicotine content of cigarettes could potentially produce less addictive products that would allow a gradual reduction in the population prevalence of smoking. Some clinical studies have found no compensatory increase in smoking whilst providing access to low nicotine tobacco 168 . Future regulation may be implemented to gradually decrease the nicotine content of combustible tobacco and other nicotine products 169 , 170 , 171 .
Tobacco end games
Some individuals have proposed getting rid of commercial tobacco products this century or using the major economic disruption arising from the COVID-19 pandemic to accelerate the demise of the tobacco industry 172 , 173 . Some tobacco producers have even proposed this strategy as an internal goal, with the idea of switching to nicotine delivery systems that are less harmful ( Philip Morris International ). Some countries are moving towards such an objective; for example, in New Zealand, the goal that fewer than 5% of New Zealanders will be smokers in 2025 has been set (ref. 174 ). The tobacco end-game approach would overall be the best approach to reduce the burden of tobacco use on society, but it would require coordination of multiple countries and strong public and private consensus on the strategy to avoid a major expansion of the existing illicit market in tobacco products in some countries.
Innovative interventions
The COVID-19 pandemic has shown that large-scale investment in research can lead to rapid development of successful therapeutic interventions. By contrast, smoking cessation has been underfunded compared with the contribution that it makes to the global burden of disease. In addition, there is limited coordination between research teams and most studies are small-scale and often underpowered 79 . It is time to fund an ambitious, coordinated programme of research to test the most promising therapies based on an increased understanding of the neurobiological basis of smoking and nicotine addiction (Table 3 ). Many of those ideas have not yet been tested properly and this could be carried out by a coordinated programme of research at the international level.
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021). This study summarizes the burden of disease induced by tobacco worldwide .
Google Scholar
West, R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32 , 1018–1036 (2017).
PubMed PubMed Central Google Scholar
West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD 6 , 277–283 (2009).
PubMed Google Scholar
Fagerström, K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob. Res. 14 , 75–78 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 2 , 739–748 (1950).
CAS PubMed PubMed Central Google Scholar
Royal College of Physicians. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases (Pitman Medical Publishing, 1962).
Henningfield, J. E., Smith, T. T., Kleykamp, B. A., Fant, R. V. & Donny, E. C. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology 233 , 3829–3848 (2016).
Le Foll, B. & Goldberg, S. R. Effects of nicotine in experimental animals and humans: an update on addictive properties. Hand. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_12 (2009).
Article Google Scholar
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control. 21 , 87–91 (2012).
Hall, B. J. et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 120 , 103–108 (2014).
Musso, F. et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191 , 159–169 (2007).
CAS PubMed Google Scholar
Goriounova, N. A. & Mansvelder, H. D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2 , a012120 (2012).
Fagerström, K. O. & Bridgman, K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addictive Behav. 39 , 507–511 (2014).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9 , CD010216 (2021).
Jha, P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. eLife https://doi.org/10.7554/eLife.49979 (2020).
Article PubMed PubMed Central Google Scholar
Palipudi, K. M. et al. Social determinants of health and tobacco use in thirteen low and middle income countries: evidence from Global Adult Tobacco Survey. PLoS ONE 7 , e33466 (2012).
Goodwin, R. D., Pagura, J., Spiwak, R., Lemeshow, A. R. & Sareen, J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol. Depend. 118 , 127–133 (2011).
Weinberger, A. H. et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002-14: emerging disparities in vulnerable populations. Addiction 113 , 719–728 (2018).
Evans-Polce, R. J., Kcomt, L., Veliz, P. T., Boyd, C. J. & McCabe, S. E. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am. J. Psychiatry 177 , 1073–1081 (2020).
Hassan, A. N. & Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiatry https://doi.org/10.4088/JCP.20m13637 (2021).
Article PubMed Google Scholar
Smith, P. H., Mazure, C. M. & McKee, S. A. Smoking and mental illness in the U.S. population. Tob. Control. 23 , e147–e153 (2014).
Bourgault, Z., Rubin-Kahana, D. S., Hassan, A. N., Sanches, M. & Le Foll, B. Multiple substance use disorders and self-reported cognitive function in U.S. adults: associations and sex-differences in a nationally representative sample. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.797578 (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health 6 , e472–e481 (2021).
Warner, K. E. How to think–not feel–about tobacco harm reduction. Nicotine Tob. Res. 21 , 1299–1309 (2019).
Soneji, S. et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171 , 788–797 (2017).
Levy, D. T. et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control. 28 , 629–635 (2019).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014). This review covers the impact of tobacco, of quitting smoking and the importance of taxation to impact prevalence of smoking .
Jha, P. & Peto., R. in Tobacco Tax Reform: At the Crossroads of Health and Development . (eds Marquez, P. V. & Moreno-Dodson, B.) 55–72 (World Bank Group, 2017).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 , 341–350 (2013).
Banks, E. et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 13 , 38 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Jha, P. et al. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 , 1137–1147 (2008).
Chan, E. D. et al. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis 94 , 544–550 (2014).
Wang, M. G. et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 11 , 873–887 (2018).
Jha, P. et al. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet 368 , 367–370 (2006).
Jha, P., Gelband, H, Irving, H. & Mishra, S. in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (eds Vaccarella, S et al.) 161–166 (IARC, 2018).
Jha, P. Expanding smoking cessation world-wide. Addiction 113 , 1392–1393 (2018).
Jha, P. Avoidable global cancer deaths and total deaths from smoking. Nat. Rev. Cancer 9 , 655–664 (2009).
Wittenberg, R. E., Wolfman, S. L., De Biasi, M. & Dani, J. A. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177 , 108256 (2020).
Boulter, J. et al. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl Acad. Sci. USA 84 , 7763–7767 (1987).
Couturier, S. et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 , 847–856 (1990).
Picciotto, M. R., Addy, N. A., Mineur, Y. S. & Brunzell, D. H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84 , 329–342 (2008).
Changeux, J. P. Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41 , 67–70 (2018).
McKay, B. E., Placzek, A. N. & Dani, J. A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74 , 1120–1133 (2007).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20 , 92–98 (1997).
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S. & Dani, J. A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 , 3176–3185 (2003).
Gipson, C. D. & Fowler, C. D. Nicotinic receptors underlying nicotine dependence: evidence from transgenic mouse models. Curr. Top. Behav. Neurosci. 45 , 101–121 (2020).
Hamouda, A. K. et al. Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self-administration and withdrawal symptoms. Neuropharmacology 190 , 108568 (2021).
Lallai, V. et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro https://doi.org/10.1523/ENEURO.0051-19.2019 (2019).
Benwell, M. E., Balfour, D. J. & Anderson, J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 50 , 1243–1247 (1988).
Perry, D. C., Davila-Garcia, M. I., Stockmeier, C. A. & Kellar, K. J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289 , 1545–1552 (1999).
Marks, M. J. et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12 , 2765–2784 (1992).
Le Foll, B. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233 , 1829–1835 (2016).
Meyers, E. E., Loetz, E. C. & Marks, M. J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 130 , 1–8 (2015).
Zhao-Shea, R., Liu, L., Pang, X., Gardner, P. D. & Tapper, A. R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 23 , 2327–2335 (2013).
Jensen, K. P., Valentine, G., Gueorguieva, R. & Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237 , 1359–1369 (2020).
Villanueva, H. F., James, J. R. & Rosecrans, J. A. Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95 , 349–350 (1989).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653 , 278–284 (1994).
Nisell, M., Nomikos, G. G., Hertel, P., Panagis, G. & Svensson, T. H. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22 , 369–381 (1996).
Rice, M. E. & Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7 , 583–584 (2004).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50 , 911–921 (2006).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76 , 116–129 (2012).
Le Foll, B. et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39 , 415–424 (2014). This brain imaging study identified the brain areas in which smoking elevates dopamine levels .
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436 , 103–107 (2005). This article discusses the implication of the β 2 - containing nAChRs in the VTA in mammalian cognitive function .
Picciotto, M. R. et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 , 173–177 (1998). This article discusses the implication of the β 2 - containing nAChRs in addictive effects of nicotine .
Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 , 597–601 (2011). This article discusses the implication of the α5 nicotinic receptor located in the MHb in a mechanism mediating the aversive effects of nicotine .
Elayouby, K. S. et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0127-19.2020 (2020).
Ables, J. L. et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl Acad. Sci. USA 114 , 13012–13017 (2017).
Frahm, S. et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70 , 522–535 (2011).
Jackson, K. J. et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334 , 137–146 (2010).
Tuesta, L. M. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20 , 708–716 (2017).
Salas, R., Pieri, F. & De Biasi, M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci. 24 , 10035–10039 (2004).
Salas, R., Sturm, R., Boulter, J. & De Biasi, M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29 , 3014–3018 (2009).
Jackson, K. J., Martin, B. R., Changeux, J. P. & Damaj, M. I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325 , 302–312 (2008).
Le Foll, B. et al. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52 , 86–93 (2014).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315 , 531–534 (2007). This article discusses the implication of the insular cortex in tobacco addiction .
Ibrahim, C. et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10 , 720 (2019).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20 , 397–404 (2021). This study validated the utility of deep insula/prefrontal cortex rTMS for smoking cessation .
Le Foll, B., Forget, B., Aubin, H. J. & Goldberg, S. R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 13 , 239–252 (2008).
Kodas, E., Cohen, C., Louis, C. & Griebel, G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194 , 161–171 (2007).
Forget, B. et al. Noradrenergic α1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35 , 1751–1760 (2010).
Garrett, B. E., Dube, S. R., Babb, S. & McAfee, T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob. Res. 17 , 892–897 (2015).
Polanska, K., Znyk, M. & Kaleta, D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 22 , 72 (2022).
Volkow, N. D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry 177 , 113–116 (2020).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98 , 23–31 (2003).
Carmelli, D., Swan, G. E., Robinette, D. & Fabsitz, R. Genetic influence on smoking–a study of male twins. N. Engl. J. Med. 327 , 829–833 (1992).
Broms, U., Silventoinen, K., Madden, P. A. F., Heath, A. C. & Kaprio, J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res. Hum. Genet. 9 , 64–72 (2006).
Kendler, K. S., Thornton, L. M. & Pedersen, N. L. Tobacco consumption in Swedish twins reared apart and reared together. Arch. Gen. Psychiat 57 , 886–892 (2000).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69 , 6848–6856 (2009).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165 , 1163–1171 (2008). This study demonstrates that nAChR gene variants are important in nicotine addiction .
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16 , 24–35 (2007).
Berrettini, W. et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13 , 368–373 (2008).
Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103 , 1544–1552 (2008).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42 , 448–453 (2010).
Ray, R., Tyndale, R. F. & Lerman, C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 23 , 252–261 (2009).
Bergen, A. W. et al. Drug metabolizing enzyme and transporter gene variation, nicotine metabolism, prospective abstinence, and cigarette consumption. PLoS ONE 10 , e0126113 (2015).
Mwenifumbo, J. C. et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin. Pharmacol. Ther. 83 , 115–121 (2008).
Raunio, H. & Rahnasto-Rilla, M. CYP2A6: genetics, structure, regulation, and function. Drug Metab. Drug Interact. 27 , 73–88 (2012).
CAS Google Scholar
Patterson, F. et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 84 , 320–325 (2008).
Rodriguez, S. et al. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J. Psychopharmacol. 25 , 915–923 (2011).
Strasser, A. A., Malaiyandi, V., Hoffmann, E., Tyndale, R. F. & Lerman, C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob. Res. 9 , 511–518 (2007).
Liakoni, E. et al. Effects of nicotine metabolic rate on withdrawal symptoms and response to cigarette smoking after abstinence. Clin. Pharmacol. Ther. 105 , 641–651 (2019).
Di Ciano, P. et al. Influence of nicotine metabolism ratio on [11C]-(+)-PHNO PET binding in tobacco smokers. Int. J. Neuropsychopharmacol. 21 , 503–512 (2018).
Butler, K. et al. Impact of Cyp2a6 activity on nicotine reinforcement and cue-reactivity in daily smokers. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab064 (2021).
Benowitz, N. L., Swan, G. E., Jacob, P. 3rd, Lessov-Schlaggar, C. N. & Tyndale, R. F. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 , 457–467 (2006).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51 , 237–244 (2019).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49 , 1126–1132 (2017).
Chukwueke, C. C. et al. The CB1R rs2023239 receptor gene variant significantly affects the reinforcing effects of nicotine, but not cue reactivity, in human smokers. Brain Behav. 11 , e01982 (2021).
Ahrens, S. et al. Modulation of nicotine effects on selective attention by DRD2 and CHRNA4 gene polymorphisms. Psychopharmacology 232 , 2323–2331 (2015).
Harrell, P. T. et al. Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology 233 , 351–360 (2016).
Lerman, C. et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 31 , 231–242 (2006).
Le Foll, B., Gallo, A., Le Strat, Y., Lu, L. & Gorwood, P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 20 , 1–17 (2009).
Chukwueke, C. C. et al. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 10 , 4085 (2020).
The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am. J. Prev. Med. 35 , 158–176 (2008).
Hackshaw, A., Morris, J. K., Boniface, S., Tang, J. L. & Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360 , j5855 (2018).
Qin, W. et al. Light cigarette smoking increases risk of all-cause and cause-specific mortality: findings from the NHIS cohort study. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph17145122 (2020).
Rodu, B. & Plurphanswat, N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct. J. 16 , 50 (2019).
Kasza, K. A. et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 376 , 342–353 (2017).
Richardson, A., Xiao, H. & Vallone, D. M. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob. Res. 14 , 927–932 (2012).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders 5th edn (American Psychiatric Association, 2013).
World Health Organization. Tobacco fact sheet. WHO https://www.who.int/news-room/fact-sheets/detail/tobacco (2021).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 , 1119–1127 (1991).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., Rickert, W. & Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 84 , 791–799 (1989).
Etter, J. F., Le Houezec, J. & Perneger, T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28 , 359–370 (2003).
DiFranza, J. R. et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 156 , 397–403 (2002).
Shiffman, S., Waters, A. & Hickcox, M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6 , 327–348 (2004).
Smith, S. S. et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob. Res. 12 , 489–499 (2010).
Foulds, J. et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 17 , 186–192 (2015).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the Surgeon General (Centers for Disease Control and Prevention, 2012).
World Health Organization. Tobacco control to improve child health and development. Thematic brief (WHO, 2021).
Lantz, P. M. et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob. Control. 9 , 47–63 (2000).
Leão, T., Kunst, A. E. & Perelman, J. Cost-effectiveness of tobacco control policies and programmes targeting adolescents: a systematic review. Eur. J. Public Health 28 , 39–43 (2018).
Royal College of Physicians. Smoking and health 2021: a coming of age for tobacco control? (RCP, 2021).
Higashi, H. et al. Cost effectiveness of tobacco control policies in Vietnam: the case of population-level interventions. Appl. Health Econ. Health Policy 9 , 183–196 (2011).
Ranson, M. K., Jha, P., Chaloupka, F. J. & Nguyen, S. N. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 4 , 311–319 (2002).
International Agency for Research on Cancer. I ARC Handbooks of Cancer Prevention: Tobacco control Vol. 14 (IARC, 2011).
Frazer, K. et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2 , CD005992 (2016).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
McNeill, A. et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 4 , CD011244 (2017).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General (Department of Health and Human Services, 2020).
Lindson, N. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 4 , CD013308 (2019).
Krist, A. H. et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325 , 265–279 (2021).
Tutka, P. & Zatonski, W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 58 , 777–798 (2006).
Courtney, R. J. et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 326 , 56–64 (2021).
Walker, N. et al. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 371 , 2353–2362 (2014). This study validated the utility of cytisine for smoking cessation .
West, R. et al. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 365 , 1193–1200 (2011).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23 , 1–82 (2019).
Walker, N. et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir. Med. 8 , 54–64 (2020).
Siu, A. L., U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 163 , 622–634 (2015).
Black, N. et al. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: a systematic review and meta-regression. Addiction 115 , 2008–2020 (2020).
Center for Substance Abuse and Treatment. Detoxification and Substance Abuse Treatment (Center for Substance Abuse and Treatment, 2006).
Cahill, K., Hartmann-Boyce, J. & Perera, R. Incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub5 (2015).
Secades-Villa, R., Aonso-Diego, G., García-Pérez, Á. & González-Roz, A. Effectiveness of contingency management for smoking cessation in substance users: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 88 , 951–964 (2020).
Cahill, K. & Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub4 (2011).
Trojak, B. et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 , 1168–1174 (2015).
Wing, V. C. et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 , 221–230 (2013).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 , 742–749 (2014).
Goldenberg, M., Danovitch, I. & IsHak, W. W. Quality of life and smoking. Am. J. Addict. 23 , 540–562 (2014).
Heikkinen, H., Jallinoja, P., Saarni, S. I. & Patja, K. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob. Res. 10 , 1199–1207 (2008).
Moayeri, F., Hsueh, Y. A., Dunt, D. & Clarke, P. Smoking cessation and quality of life: insights from analysis of longitudinal Australian data, an application for economic evaluations. Value Health 24 , 724–732 (2021).
Taylor, G. M. et al. Smoking cessation for improving mental health. Cochrane Database Syst. Rev. 3 , CD013522 (2021).
López-Nicolás, Á., Trapero-Bertran, M. & Muñoz, C. Smoking, health-related quality of life and economic evaluation. Eur. J. Health Econ. 19 , 747–756 (2018).
Morris, A. Linking nicotine addiction and T2DM. Nat. Rev. Endocrinol. 16 , 6 (2020).
Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 298 , 2654–2664 (2007).
World Health Organization. WHO report on the global tobacco epidemic (WHO, 2019).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 , 1340–1349 (2015). This study tested the impact of reducing the quantity of nicotine present in cigarettes on smoking .
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 331 , 123–125 (1994).
Benowitz, N. L. & Henningfield, J. E. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 22 , i14–i17 (2013).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377 , 1111–1114 (2017).
Hall, W. & West, R. Thinking about the unthinkable: a de facto prohibition on smoked tobacco products. Addiction 103 , 873–874 (2008).
Ioannidis, J. P. A. & Jha, P. Does the COVID-19 pandemic provide an opportunity to eliminate the tobacco industry? Lancet Glob. Health 9 , e12–e13 (2021).
Smokefree. Smokefree 2025. Smokefree https://www.smokefree.org.nz/smokefree-in-action/smokefree-aotearoa-2025 (2021).
Morgan, C. J., Das, R. K., Joye, A., Curran, H. V. & Kamboj, S. K. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38 , 2433–2436 (2013).
Elsaid, S., Kloiber, S. & Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 167 , 25–75 (2019).
Butler, K. & Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin. Drug Discov. 15 , 1065–1080 (2020).
D’Souza, D. C. et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6 , 35–45 (2019).
Robinson, J. D. et al. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 23 , 291–303 (2018).
Gueye, A. B. et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw068 (2016).
Yammine, L. et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob. Res. 23 , 1682–1690 (2021).
Eren-Yazicioglu, C. Y., Yigit, A., Dogruoz, R. E. & Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14 , 614884 (2020).
Vanderkam, P. et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta-analysis. Addiction 116 , 1011–1020 (2021).
Sokoloff, P. & Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 , 2–19 (2017).
David, S. P., Lancaster, T., Stead, L. F., Evins, A. E. & Prochaska, J. J. Opioid antagonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003086.pub3 (2013).
Ray, L. A. et al. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry 178 , 818–828 (2021).
Mooney, M. E. et al. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100 , 344–352 (2016).
Justinova, Z., Le Foll, B., Redhi, G. H., Markou, A. & Goldberg, S. R. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology 233 , 1791–1800 (2016).
Le Foll, B., Wertheim, C. E. & Goldberg, S. R. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci. Lett. 443 , 236–240 (2008).
Franklin, T. R. et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol. Depend. 103 , 30–36 (2009).
Lotfy, N., Elsawah, H. & Hassan, M. Topiramate for smoking cessation: systematic review and meta-analysis. Tob. Prev. Cessat. 6 , 14 (2020).
Shanahan, W. R., Rose, J. E., Glicklich, A., Stubbe, S. & Sanchez-Kam, M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res. 19 , 944–951 (2017).
Higgins, G. A., Fletcher, P. J. & Shanahan, W. R. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205 , 107417 (2020).
Stead, L. F. & Lancaster, T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005231.pub2 (2007).
Download references
Acknowledgements
B.Le F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and the Addiction Psychiatry Chair from the University of Toronto. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank H. Fu (University of Toronto) for assistance with Figs 1–3.
Author information
Authors and affiliations.
Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
Bernard Le Foll
Departments of Family and Community Medicine, Psychiatry, Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada
Department of Medicine, University of Wisconsin, Madison, WI, USA
Megan E. Piper
University of Wisconsin Center for Tobacco Research and Intervention, Madison, WI, USA
Department of Neurobiology and Behaviour, University of California Irvine, Irvine, CA, USA
Christie D. Fowler
Section for Preventive Cardiology, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
Serena Tonstad
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Laura Bierut
Institute of Mental Health, Peking University Sixth Hospital, Peking University, Beijing, China
National Institute on Drug Dependence, Peking University Health Science Center, Beijing, China
Centre for Global Health Research, Unity Health Toronto, University of Toronto, Toronto, Ontario, Canada
- Prabhat Jha
National Centre for Youth Substance Use Research, The University of Queensland, St Lucia, Queensland, Australia
Wayne D. Hall
Queensland Alliance for Environmental Health Sciences, The University of Queensland, Woolloongabba, Queensland, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.Le F.); Epidemiology (P.J. and W.D.H.); Mechanisms/pathophysiology (C.D.F., L.B., L.L. and B.Le F.); Diagnosis, screening and prevention (P.J., M.E.P., S.T. and B.Le F.); Management (M.E.P., S.T., W.D.H., L.L. and B.Le F.); Quality of life (P.J. and W.D.H.); Outlook (all); Conclusions (all). All authors contributed substantially to the review and editing of the manuscript.
Corresponding author
Correspondence to Bernard Le Foll .
Ethics declarations
Competing interests.
B.Le F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.Le F. has received some in-kind donations of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.Le F. has obtained industry funding from Canopy (through research grants handled by CAMH or the University of Toronto), Bioprojet, ACS, Indivior and Alkermes. B.Le F. has received in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. B.Le F. has been an advisor to Shinoghi. S.T. has received honoraria from Pfizer the manufacturer of varenicline for lectures and advice. All other authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Disease Primers thanks Jamie Brown, Elisardo Iglesias and Ming Li for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CTRI website: https://d3futrf33lk36a.cloudfront.net/wp-content/uploads/sites/240/2019/04/Meds-Chart.pdf
Philip Morris International: https://www.pmi.com/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Le Foll, B., Piper, M.E., Fowler, C.D. et al. Tobacco and nicotine use. Nat Rev Dis Primers 8 , 19 (2022). https://doi.org/10.1038/s41572-022-00346-w
Download citation
Accepted : 07 February 2022
Published : 24 March 2022
DOI : https://doi.org/10.1038/s41572-022-00346-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated mrna- and mirna-sequencing analyses unveil the underlying mechanism of tobacco pollutant-induced developmental toxicity in zebrafish embryos.
- Jiasheng Chen
- Xiaoping Zhong
Journal of Translational Medicine (2024)
Influence of substance use on male reproductive health and offspring outcomes
- Jamie O. Lo
- Jason C. Hedges
- Charles A. Easley
Nature Reviews Urology (2024)
Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
- Guillermo Paraje
- Mauricio Flores Muñoz
Nature Medicine (2024)
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
- Qiulian Peng
- Jiayan Zhang
Plant and Soil (2024)
Nicotinic regulation of microglia: potential contributions to addiction
- Alexa R. Soares
- Marina R. Picciotto
Journal of Neural Transmission (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Smoking Cessation—The Role of Healthcare Professionals and Health Systems
Key findings from the 2020 surgeon general’s report.
- Smoking cessation reduces risk for many adverse health effects, including poor reproductive health outcomes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), and cancer. Quitting smoking is also beneficial to those who have been diagnosed with heart disease and COPD.
- More than three out of five U.S. adults who have ever smoked cigarettes have quit. Although a majority of cigarette smokers make a quit attempt each year, less than one-third use cessation medications approved by the U.S. Food and Drug Administration (FDA) or behavioral counseling to support quit attempts.
- Considerable disparities exist in the prevalence of smoking across the U.S. population, with higher prevalence in some subgroups. Similarly, the prevalence of key indicators of smoking cessation—quit attempts, receiving advice to quit from a health professional, and using cessation therapies—also varies across the population, with lower prevalence in some subgroups.
- Smoking cessation medications approved by the FDA and behavioral counseling are cost-effective cessation strategies. Cessation medications approved by the FDA and behavioral counseling increase the likelihood of successfully quitting smoking, particularly when used in combination. Using combinations of nicotine replacement therapies can further increase the likelihood of quitting.
- Insurance coverage for smoking cessation treatment that is comprehensive, barrier-free, and widely promoted increases the use of these treatment services, leads to higher rates of successful quitting, and is cost-effective.
- E-cigarettes, a continually changing and heterogeneous group of products, are used in a variety of ways. Consequently, it is difficult to make generalizations about efficacy for cessation based on clinical trials involving a particular e-cigarette, and there is presently inadequate evidence to conclude that e-cigarettes, in general, increase smoking cessation.
Smoking in the U.S.
Since the first Surgeon General’s report on smoking and health was released in 1964, cigarette smoking among U.S. adults has declined from nearly 43% to a low of nearly 14% in 2018. Despite this progress, smoking remains the leading preventable cause of death and disease in the United States. Additionally, smoking-related illnesses continue to cost the nation more than $300 billion every year.

Smoking Cessation Saves Lives and Money
Tobacco dependence is a chronic, relapsing condition driven by addiction to nicotine. But cessation treatment can help people quit. The 2020 Surgeon General’s Report highlights the latest evidence on the benefits of smoking cessation. The evidence is clear – one of the most important actions people can take to improve their health is to quit smoking, no matter how old they are or how long they’ve been smoking.
Smoking cessation:
- Reduces the risk of premature death, improves health, and enhances quality of life. Quitting can add as much as a decade to life expectancy.
- Reduces the risk for many adverse health effects, including poor reproductive health outcomes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), and 12 types of cancer.
- Benefits people already diagnosed with coronary heart disease or COPD.
- Benefits the health of pregnant women and that of their fetuses and babies.
- Reduces the financial burden that smoking places on people who smoke, healthcare systems, and society.
Most Adults Who Smoke Want to Quit
The report highlights the progress made in reducing smoking in the U.S.:
- Nearly 70% of adults who smoke say they want to quit.
- Over 50% of adults who smoke try to quit each year.
- Three in five adults who ever smoked cigarettes have quit.
The report also presents findings that underscore the challenges we face to further reduce smoking:
- Over 40% of adults who smoke do not receive advice to quit from a healthcare professional.
- Fewer than one in three adults who smoke use cessation counseling or FDA-approved medications when trying to quit.
- Fewer than one in 10 U.S. adults successfully quit smoking each year.
Evidence-Based Cessation Treatments Work
Tobacco use and dependence often requires repeated intervention and long-term support to help patients quit. The report outlines an array of effective treatments and resources, including:
- Counseling and medication —Each is effective when used alone, and using them together can more than double the chances of quitting.
- Combining medications —Compared to using a single form of nicotine replacement therapy (NRT), combining long-acting NRT (e.g., patch) with a short-acting NRT (e.g., lozenge) increases the chances of quitting.
- Tobacco quitlines —Proactive counseling from quitlines increases the chances of quitting when used alone or together with cessation medication. Text messaging and web-based cessation interventions can also help people successfully quit smoking.
You Can Help Your Patients Quit
- Advising them to quit
- Offering brief counseling
- Prescribing cessation medications
- Connecting them to additional resources, like a quitline
- Following up with continued support to help prevent relapse
- Every member of the care team can help. Delegating these tasks can improve efficiency and support a coordinated-care approach.
- Providing cessation treatment is reimbursable and can help meet quality measures.
E-Cigarettes and Adult Cessation
Many adults who smoke are interested in using e-cigarettes to quit cigarettes. Research is uncertain on whether e-cigarettes, in general, increase smoking cessation. Some research suggests that using e-cigarettes containing nicotine is associated with greater smoking cessation than using e-cigarettes that don’t contain nicotine, and some research suggests that more frequent use of e-cigarettes is associated with greater smoking cessation than less frequent use. However, e-cigarettes are not currently approved by the FDA as a quit smoking aid, and more research is needed on whether e-cigarettes are effective for smoking cessation and to better understand the health effects of e-cigarettes.
The use of any tobacco products, including e-cigarettes, is not safe for youth, young adults, and pregnant women, as well as adults who do not currently use tobacco products. In order for adult smokers to achieve any meaningful health benefits from e-cigarettes, they would need to fully switch to e-cigarettes and stop smoking cigarettes and other tobacco products completely. Among those who have switched completely, the ultimate goal should be to also stop using e-cigarettes completely to achieve the maximum health benefit.
Health System and Population-Level Interventions
The Surgeon General’s Report reviews effective health system and population-level interventions that can promote cessation and extend the reach and use of clinical treatments. Health systems can adopt policies and changes to integrate tobacco dependence treatment into routine care and make it easier for healthcare teams to deliver treatment (e.g., integrating cessation content into electronic health records). Additionally, comprehensive, barrier-free smoking cessation insurance coverage that is widely promoted can increase the use of evidence-based treatments and cessation. Population-level interventions, such as raising the price of cigarettes, adopting comprehensive smokefree policies, implementing mass media campaigns, requiring pictorial health warnings, and maintaining comprehensive statewide tobacco control programs also support and increase smoking cessation.
Smoking Cessation Resources for Healthcare Professionals
- Clinical Practice Guideline for Treating Tobacco Use and Dependence
- CDC’s Office on Smoking and Health
- Million Hearts
- Smokefree.gov
For information about:
- quitting smoking, visit www.CDC.gov/quit
- the Surgeon General’s report, visit www.CDC.gov/CessationSGR
Download (PDF–227 KB)
To receive email updates about Smoking & Tobacco Use, enter your email address:
- Tips From Former Smokers ®
- Division of Cancer Prevention and Control
- Lung Cancer
- National Comprehensive Cancer Control Program
- Division of Reproductive Health

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Time for a Focus on Cessation of E-Cigarettes
- 1 Department of Psychiatry, Yale School of Medicine, New Haven, Connecticut
- 2 Yale Cancer Center, New Haven, Connecticut
- Original Investigation Cytisinicline for Vaping Cessation in Adults Using Nicotine E-Cigarettes Nancy A. Rigotti, MD; Neal L. Benowitz, MD; Judith J. Prochaska, PhD, MPH; Daniel F. Cain, BSc; Julie Ball, MS; Anthony Clarke, PhD; Brent A. Blumenstein, PhD; Cindy Jacobs, PhD, MD JAMA Internal Medicine
E-cigarettes, which were introduced in the US market in 2007, have experienced tremendous growth in the US and globally and are the focus of an intense and often controversial public health debate concerning harms and benefits. The most important potential benefit of e-cigarettes is to assist with cigarette smoking cessation and reduce exposure to cigarette-related harm among those who completely switch to e-cigarettes. 1 However, e-cigarettes can also produce harm and serve to initiate and maintain nicotine addiction in populations for whom there is no potential public health benefit of use. 2 Indeed, e-cigarette use prevalence is highest and rising among youth and young adults, many of whom have never smoked cigarettes. Among cigarette smokers, dual use of e-cigarettes and cigarettes is common, and many who had successfully quit smoking in the past are reinitiating nicotine use with e-cigarettes. Many youth and adults who use e-cigarettes experience nicotine addiction and physical dependence and are keen to quit e-cigarettes. 3 However, in the national and worldwide discourse about the public health benefits vs harm of e-cigarettes there has been minimal focus on how to support individuals who want to stop using e-cigarettes, leading to a critical lack of evidence-based treatments.
Read More About
Krishnan-Sarin S , Fucito LM. Time for a Focus on Cessation of E-Cigarettes. JAMA Intern Med. Published online May 06, 2024. doi:10.1001/jamainternmed.2024.1310
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Video consultation and treatment in the community smoking cessation therapy success rates in patients with mental illness: a randomized controlled trial
Affiliations.
- 1 Psychiatric Department, Mental Health Services, Region of Southern Denmark, University Hospital of Southern Denmark, Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.
- 2 Psychiatric Department, Mental Health Services, Region of Southern Denmark, University Hospital of Southern, Odense, Denmark.
- 3 Health Promotion and Prevention, Vejle, Denmark.
- 4 Division of Interdisciplinary Research and Practice, School of Health and Social Care, University of Essex, Colchester, UK.
- 5 Department of Health Promotion, Africa Centre for Epidemiology, Accra, Accra North, Ghana.
- PMID: 38385357
- DOI: 10.1080/08039488.2024.2318305
Purpose: Smoking is the single factor with the highest impact on reducing life expectancy of patients with mental illness. Patients experience difficulty in participating in smoking cessation programs but are concerned about the impact of tobacco on their health and finances. Smoking cessation advice via videoconferencing might be an alternative to an ordinary in-person consultation.
Material and method: Randomized controlled trial with follow-up at 6 months. We included patients with diagnoses of schizophrenia and affective disorder from psychiatric outpatient clinics. Intervention 1 involved daily video consultations; intervention 2 was treatment as usual.
Results: Seventy patients were included. For both/all groups/interventions, rates of smoking cessation were 45% and predictors for a 50% reduction in smoking were antipsychotic medication load [odds ratio (OR) 0.54; p = 0.045] and number of nicotine patches (OR 1.02; p = 0.06). Predictors for a reduction in the number of cigarettes to < 10 were antipsychotic medication load (OR 0.52; p = 0.04), number of nicotine patches (OR 1.01; p = 0.02) and number of cigarettes at baseline [OR 0.95 ( p = 0.09); adjusted OR 0.94 ( p = 0.02)]. Patients prevented weight gain during the cessation period.
Conclusion: The smoking cessation rate was high. One of the reasons for the high cessation rate was that the intervention was carried out by highly experienced and professionally qualified staff. In addition, we used free nicotine patches to increase the patients' motivation to quit smoking. It is very important that we introduce these results into our clinical work with the patients.
Keywords: Smoking cessation; physical health; randomized controlled trial; severe mental illness; video conferencing.
Publication types
- Randomized Controlled Trial
- Research Support, Non-U.S. Gov't
- Antipsychotic Agents / therapeutic use
- Follow-Up Studies
- Middle Aged
- Mood Disorders / therapy
- Schizophrenia* / therapy
- Smoking Cessation* / methods
- Tobacco Use Cessation Devices
- Treatment Outcome
- Videoconferencing*
- Weight Gain
- Antipsychotic Agents

COMMENTS
Smoking cessation, defined as quitting or the discontinuation of tobacco smoking, reduces the risk of smoking-related diseases and premature death [3, 28, 29]. ... (ERSC) at the Ottawa Hospital Research Institute. A working group (WG) of Task Force members and external content experts was formed for development of the topic, refinement of the ...
Smoking cessation can effectively reduce the risk of death, alleviate respiratory symptoms, and decrease the frequency of acute exacerbations in patients with chronic obstructive pulmonary disease (COPD). Effective smoking cessation strategies are crucial for the prevention and treatment of COPD. Currently, clinical interventions based on theoretical frameworks are being increasingly used to ...
Objectives Although the global COVID-19 pandemic has increased interest in research involving high-risk smokers, studies examining changed smoking behaviours, cessation intentions and associated psychological states among smokers are still scarce. This study aimed to systematically review the literature related to this subject. Design A systematic review of published articles on cigarettes and ...
Although considerable progress has been made in reducing cigarette smoking since the first U.S. Surgeon General's report was released in 1964 (USDHHS 2014), in 2018, 13.7% of U.S. adults (34.2 million people) were still current cigarette smokers (Creamer et al. 2019).
Smoking Cessation: A Report of the Surgeon General. US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. ... , 120 there is a need for research on interventions to help dual users of conventional cigarettes ...
We read with great interest a research letter by Thomson B. and Islami F.(1) where the presented temporal context of smoking cessation effects on cause-specific mortality reduction calls for the urgency of smoking cessation and early intervention in smokers. ... Overall smoking cessation benefits compared to smoking are undisputed.(2) However ...
Conclusions: Results of this review indicate that the scientific papers in the most recent decade recommend the use of NRT and Champix in combination with educational interventions. Additional research is needed to compare qualitative and quantitative studies for smoking cessation. KW - Methods. KW - Systematic review. KW - Tobacco cessation
This study analyses changes in depressive symptomatology as a function of smoking status over time after a cognitive-behavioural intervention for smoking cessation among smokers with a history of depressive episode. The sample comprised 215 smokers with antecedents of depressive episode (Mage=45.03; 64.7% female). Depressive symptoms were assessed using BDI-II at baseline, end of intervention ...
A growing body of research points to the ... Source data are provided with this paper. ... A. et al. Impact of tobacco control policies on smoking prevalence and quit ratios in 27 European Union ...
Objective To assess the effectiveness of e-cigarettes in smoking cessation in the USA from 2017 to 2019, given the 2017 increase in high nicotine e-cigarette sales. Methods In 2017, the PATH Cohort Study included data on 3578 previous year smokers with a recent quit attempt and 1323 recent former smokers. Respondents reported e-cigarettes or other products used to quit cigarettes and many ...
Objectives: Although the global COVID-19 pandemic has increased interest in research involving high-risk smokers, studies examining changed smoking behaviours, cessation intentions and associated psychological states among smokers are still scarce. This study aimed to systematically review the literature related to this subject. Design: A systematic review of published articles on cigarettes ...
This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychologi- ... Definitions of smoking and smoking cessation Tobacco smoking consists of drawing into the mouth, and usually the lungs, smoke ... has been the subject of by far the largest volume of research and is the most harmful
Smoking cessation was measured after at least six months, using the most rigorous definition available, on an intention-to-treat basis. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for smoking cessation for each study, where possible. ... inconsistency and imprecision. Future research should aim to match any additional ...
Abstract. Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will ...
Smoking cessation: Reduces the risk of premature death, improves health, and enhances quality of life. Quitting can add as much as a decade to life expectancy. Reduces the risk for many adverse health effects, including poor reproductive health outcomes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), and 12 types of cancer.
This systematic review aimed to identify existing smoking cessation interventions for this cohort diagnosed with breast, head and neck, lung and cervical cancers (linked to risk). Systematic searches of Pubmed, Embase, Psych Info and CINAHL from 1 January 2015 to 15 December 2020 were conducted. Included studies examined the characteristics of ...
quit earlier in life • Reduces the financial burden that smoking places on people who smoke, healthcare systems, and society . Smoking cessation . improves health, saves lives, and reduces financial burden. ` Research is uncertain on whether e-cigarettes, in general, increase smoking cessation. ` Some research suggests that using e-cigarettes
Background and objectives: Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide.This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychological, pharmacological and social factors that contribute to uptake and maintenance of ...
The most important potential benefit of e-cigarettes is to assist with cigarette smoking cessation and reduce exposure to cigarette-related harm among those who completely switch to e-cigarettes. 1 However, e-cigarettes can also produce harm and serve to initiate and maintain nicotine addiction in populations for whom there is no potential ...
Search 218,364,397 papers from all fields of science. Search. ... with behavioral support, demonstrated smoking cessation efficacy and excellent tolerability, offering new nicotine dependence treatment options. ... the state of the emerging evidence about e-cigarettes and health and makes recommendations for the improvement of this research and ...
Patients experience difficulty in participating in smoking cessation programs but are concerned about the impact of tobacco on their health and finances. Smoking cessation advice via videoconferencing might be an alternative to an ordinary in-person consultation. Material and method: Randomized controlled trial with follow-up at 6 months. We ...
There is also emerging data suggesting that e-cigarettes may facilitate smoking cessation but further research is needed to compare the effectiveness and safety of e-cigarettes compared to other nicotine replacement therapies [13,14]. Consistent with the U.S. FDA regulatory oversight and the U.S. Surgeon General report, it may be prudent to ...
Smoking cessation produces immediate and substantial health ... We can only talk about this with a paper and pen but the govt will never stop manufacturing the cigarettes ... Previous research suggests that people in socioeconomically deprived neighbourhoods smoke more than those in affluent neighbourhoods, independently of individual-level ...