- Structure Of Glucose And Fructose

Structure of Glucose and Fructose
What is glucose.
Glucose is a group of carbohydrates which is a simple sugar with a chemical formula C 6 H 12 O 6 . It is made of six carbon atoms and an aldehyde group . Therefore, it is referred to as aldohexose. It exists in two forms viz open-chain (acyclic) form or ring (cyclic) form.
The primary source of energy required for living organisms is glucose. Plants and algae prepare glucose during the process of photosynthesis with the help of water, sunlight, and carbon dioxide. It is naturally found in fruits and honey. Glucose in animals is obtained by the process of glycogenolysis.
Table of Contents
Recommended videos.
- Steps to Draw Open Chain Structure of a Glucose Molecule
- Steps to Draw a Ring Structure of a Glucose Molecule
- Glucose Properties
Structure of Fructose
Open chain and ring structure of a fructose molecule, glucose and fructose are which isomers.
- Frequently Asked Questions – FAQs

1. Steps to Draw Open Chain Structure of a Glucose Molecule
Follow the steps given below to draw an acyclic form of glucose.
Step 1: Draw 6 carbon atoms
Step 2: Draw extended arms for all the carbon atoms excluding the first one.
Step 3: Now draw hydrogen to carbon bond such that four are on one side and one on the other side.
Step 4: The remaining spaces should be filled with an OH group. (Important – transpose
(OH) to —> (HO) for the left side to show that the oxygen is bonded to carbon)
Step 5: Complete the ends with two single-bonded hydrogen bond and one double-bonded carbon.
The open chain structure of glucose was given by Baeyer however these structure could not explain why glucose fails to react with Schiff base, sodium bisulphate or the process of mutarotation. Haworth introduced cyclic structure of glucose which confirms the existence of alpha and beta forms of glucose, mutarotation etc.

Open Chain Structure of a Glucose Molecule
2. Steps to Draw a Ring Structure of a Glucose Molecule
Follow the steps given below to draw a cyclic form of glucose.
Step 1: Construct a hexagon
Step 2: Draw carbon atoms at 5 consecutive edges.
Step 3: Attach an oxygen atom at the left out edge.
Step 4: Attach the 4 carbon atoms with H and OH groups.
Step 5: Complete the structure by attaching the left out carbon atom to two hydrogen atoms, OH group, and one carbon atom.

Ring Structure of a Glucose Molecule
Glucose Properties:
| Glucose Formula | C H O |
| Glucose Molar mass | 180.156 g/mol |
| Glucose Chemical Formula | C H O |
| IUPAC Name | -Glucose |
Also, Check ⇒ Amylose
Fructose is a monosaccharide which is a simple sugar with a chemical formula C 6 H 12 O 6 . It is also called as fruit sugar. It was discovered in the year 1847 by a French chemist Augustin-Pierre Dubrunfaut. It consists of a 6-carbon polyhydroxy ketone.
Crystalline fructose occurs as a cyclic six-membered structure delinquent to the stability of the hemiketal and internal hydrogen-bonding . This form is called D-fructopyranose. It mainly occurs in honey, vine fruits, most root vegetables, berries, and flowers. Commercially it can be obtained from sugar beets, maize, and sugar cane.
The cyclic structure of fructose is given below:
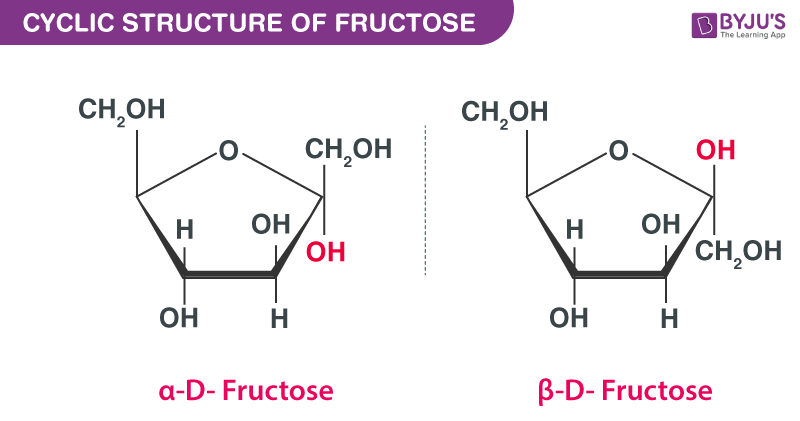
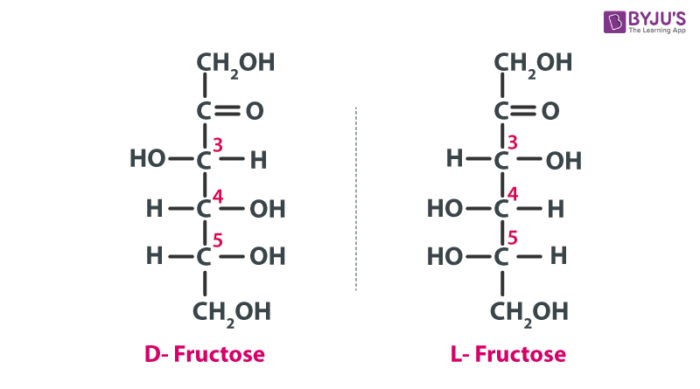
The Fischer projections of D- and L-fructose is given below:
Fructose Properties
Dry fructose appears as a white, crystalline solid which is sweet, and odourless. It is soluble in alcohol, water, and ether.
| Fructose Formula | C H O |
| Fructose Molar mass | 180.156 g/mol |
| Fructose Chemical Formula | C H O |
| Density | 1.694 g/cm |
| Melting point | 103°C |
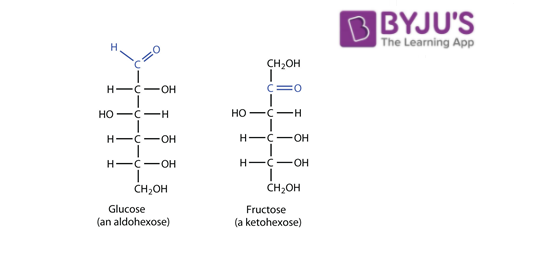
Glucose and fructose are functional isomers of each other because they have same molecular formula that is C 6 H 12 O 6 But different functional group in their chemical formula. Glucose has aldehyde group while fructose has ketone as functional group. They differ in the nature of the functional group. Glucose is an aldehyde and fructose is a ketone.
Frequently Asked Questions – FAQs
What are the different types of glucose.
The D-isomer, D-glucose, also known as dextrose, is commonly found in nature, but the L-isomer, L-glucose, is not. Hydrolysis of carbohydrates such as dairy sugar (lactose), plant sugar (sacrose), maltose, cellulose, glycogen, etc. can be used to obtain glucose.
What are fructose and glucose?
Both fructose and glucose are simple sugars of monosaccharides. When digested, both starch and sugar, whether sucrose or high-fructose corn syrup (HCFS), produce big quantities of glucose.
Why Glucose is a monosaccharide?
Monosaccharides (mono- = “one ;” sacchar- = “sugar”) are simple sugars, of which glucose is the most prevalent.
Where does glucose come from?
The food we consume comes from glucose or sugar. The prevalent sources of glucose are carbohydrates such as fruit, bread pasta, and cereals. In our stomachs, these foods are split into sugar and then absorbed into the bloodstream.
What is the function of fructose?
Like glucose, fructose provides the cells with energy. Cells process fructose to obtain energy through a method called aerobic breathing, which mainly involves burning fructose in the presence of oxygen to generate ATP, the molecule of cellular energy.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
The given cyclic structure of fructose is wrong, see it has 7 carbons.
Resolved, updated and corrected the image. Thank you.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

6.1: Structure and Function- Carbohydrates

Carbohydrates are commonly described as sugars, or saccharides, from the Greek word for sugar. The simplest carbohydrates are called monosaccharides. An example is glucose. Monosaccharides can be joined to make larger molecules. Disaccharides contain two monosaccharides. Sucrose is a disaccharide, containing both fructose and glucose. Mono and disaccharides are sometimes referred to as simple sugars. Polysaccharides are chains of many sugar subunits. Examples include glycogen and cellulose, both of which are polymers of glucose (configured differently).
Carbohydrates are literally “hydrates of carbon.” This name derives from the generalized formula of simple monosaccharides, which can be written in the form of C x (H 2 O) x , where x is a digit typically between 3 and 8. Not all sugars have this formula, however. Deoxyribose, the sugar found in every nucleotide in a DNA molecule lacks one oxygen and thus has the formula C 5 H 10 O 4 .
Carbohydrates are important in cells as energy sources (especially glucose, glycogen, and amylose), as markers of cellular identity (oligosaccharides on the surface of cells of multicellular organisms), as structural components (cellulose holding up plants), and as constituents of nucleotides (ribose in RNA, deoxyribose in DNA).
The building blocks of all carbohydrates are the monosaccharides.
Shown below are Fischer projection formulas for a group of common monosaccharides. Fischer projection formulas are similar but not identical to organic structural formulas. Carbons in the sugar are represented with the elemental symbol C at the end of the chain, but also are represented by vertices (such as carbon 1 in D-Ribose below) and by intersecting perpendicular lines (carbons 2, 3, and 4 in D-Ribose).
The tetrahedral arrangement around the carbons in the chain of a monosaccharide are represented as flat, with 90 degree bond angles, in the Fischer projection. This is not an accurate representation of the three-dimensional molecules. To interpret these structures as 3D models, each carbon within the chain can be considered in sequence. The bonds shown vertically in the Fischer projection are oriented back, away from the viewer, while the horizontal bonds (to H and OH) emerge forward, out of the plane of view.
Fischer projections make for easy drawing and comparison of carbohydrate structure but their interpretation is prone to error.
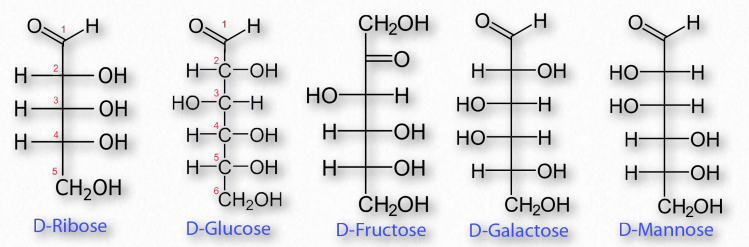
Figure 2.148 – Common sugar structures
Monosaccharides
The most common monosaccharides include glucose, fructose, galactose, ribose, and mannose. Of these sugars, all but one (fructose) exists as an aldehyde. Fructose and some other less well known sugars are ketones. Figure 2.148 shows the structure of these sugars.
By convention, the letters ‘ose’ at the end of a biochemical name flags a molecule as a sugar. Thus, there are glucose, galactose, sucrose, and many other ‘-oses’. Other descriptive nomenclature involves use of a prefix that tells how many carbons the sugar contains. For example, glucose, which contains six carbons, is described as a hexose. The following list shows the prefixes for numbers of carbons in a sugar:
Other prefixes identify whether the sugar contains an aldehyde group (aldo-) or a ketone (keto-) group. Prefixes may be combined. Glucose, which is a 6-carbon sugar with an aldehyde group, can be described as an aldohexose. The list that follows gives the common sugars and their descriptors. •Ribose = aldo-pentose
•Glucose = aldo-hexose
•Galactose = aldo-hexose
•Mannose = aldo-hexose
•Fructose = keto-hexose
Diastereomers
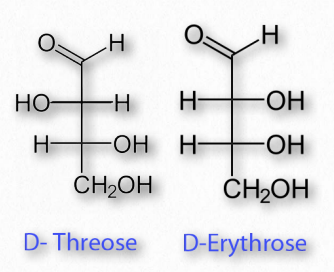
Figure 2.149 – Diastereomers
Sugars may have multiple chiral carbons and thus differ from each other in the configuration of groups around those asymmetric carbons. Two sugars having the same chemical form (aldoses, for example) and the same number of carbons, but that differ only in the stereochemical orientations of their carbons are referred to as diastereomers (Figure 2.149). For example, glucose, galactose, and mannose all have the formula of C6H12O6, but are chemically distinct from each other in the orientation of groups around the carbons within them.
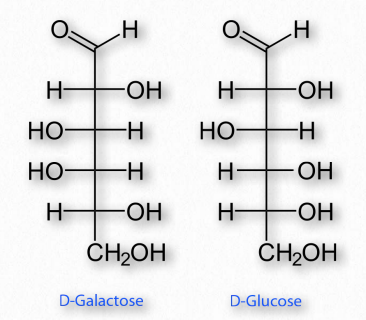
Figure 2.150 – Epimers – D-Galactose and D-Glucose differ only in the configuration of carbon #4
Enantiomers and epimers
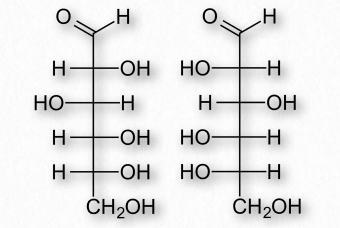
Figure 2.151 – Enantiomers – D-Glucose (left) and L-Glucose (right) are mirror
If two sugars are identical except for having one chiral carbon arranged differently (such as images glucose and galactose – Figure 2.150), they are considered epimers of one another. If two sugars are mirror images of each other, they are enantiomers (Figure 2.151). Biochemical notation uses the letters D and L to describe monosaccharide stereochemistry in a very particular way. As a result, one enantiomer will be given an L designation while the other is D. So L-glucose is the mirror image of D-glucose.
Movie 2.6 – Conversion of glucose from a straight chain form to a ring form
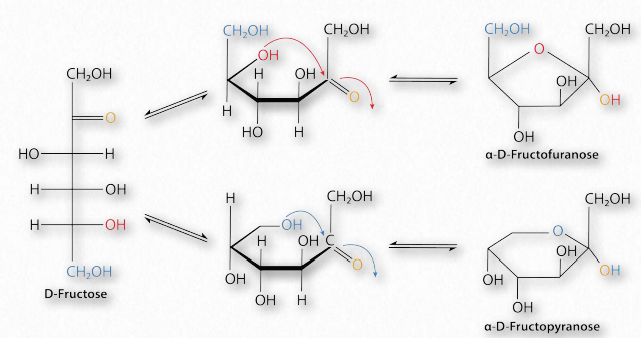
Figure 2.152 – Conversion of D-fructose between furanose (top right), linear (left), and pyranose (bottom right) forms Image by Pehr Jacobson
Sugars with five and six carbons can readily cyclize (Figure 2.152, Movie 2.6) in solution. When they do, a new asymmetric carbon is created that didn’t exist in the same sugars when they were in the straight chain form, as the carbon to oxygen double bond converts to an alcohol. This carbon has a special name – it is called the anomeric carbon and (like the other asymmetric carbons in sugars) it can have the hydroxyl in two different positions. These positions are referred to as α and β . Sugars, such as α-D-glucose and β-D-glucose that differ only in the configuration of the anomeric carbon are referred to as anomers (Figure 2.153).
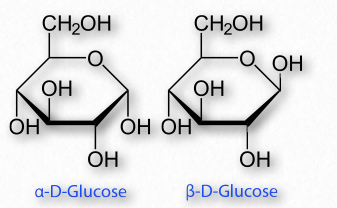
Figure 2.153 – Anomers – α-DGlucose and β-D-Glucose differ only in the configuration of the anomeric carbon #1
Sugars cyclizing to form rings with five atoms in them (see fructose in Figure 2.128) are referred to as furanoses (named for furan) and those forming rings with six atoms, such as glucose in the same figure, are called pyranoses (named for pyran). The carbonyl carbon becomes the anomeric carbon in the ring by binding to the oxygen of a hydroxyl elsewhere in the chain. α- and β- forms of a given sugar can readily “flip” between each form in solution, so long as the anomeric hydroxyl is free, because the bonding in cyclic forms is unstable, so molecules interconvert in solution. Most pentoses and hexoses can form both furanose and pyranose structures (Figure 2.152).
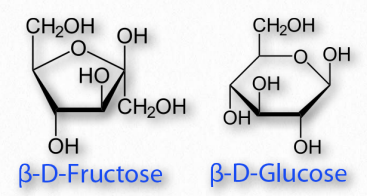
Figure 2.154 – A furanose (left) and a pyranose (right)
Linking the anomeric hydroxyl to another group will create a structure called a glycoside which will remain locked in whichever α- or β- configuration they were in when the anomeric hydroxyl was altered.
Boat/chair conformations
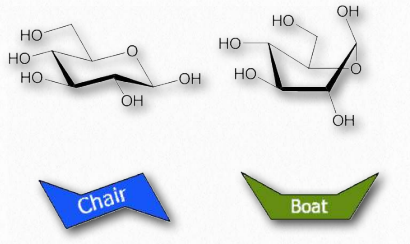
Figure 2.155 – Chair and boat forms of glucose
Orbitals of carbon prefer to be in tetrahedral conformations and this means that the bonds between carbons in a ring do not lie flat. Indeed, rings “pucker” to try to accommodate this tendency, giving rise to different 3D forms for any given sugar. Some of these forms resemble boat structures, which others resemble chairs or envelopes (Figure 2.155). The stablest (and thus most abundant) of these forms have all of the hydoxyls in the equatorial positions, resulting in less steric hindrance.
Modified monosaccharides
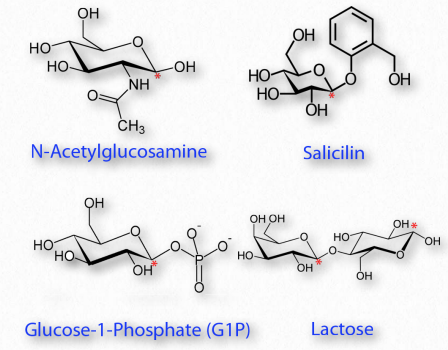
Figure 2.156 – Modified sugars. Locations of glycosidic carbon indicated with red asterisks. All are glycosides except N-acetylglucosamine
Many chemical modifications can occur on sugar residues (Figure 2.156). Common ones include oxidation, reduction, phosphorylation, and substitution of an amine or an acetylamine for a hydroxyl. The ones that affect the anomeric hydroxyl group make glycosides (Figure 2.157), whereas modifications that don’t affect the anomeric hydroxyl, (glucose-6-phosphate, for example), do not.
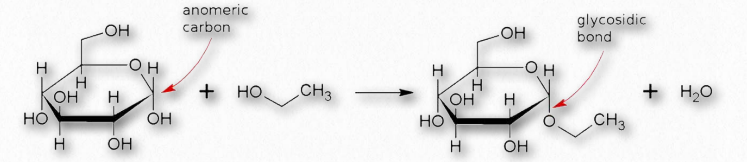
Figure 2.157 – Formation of a glycosidic bond
Oxidation/reduction

Figure 2.158 – A positive Benedict’s test starting at left and moving right Wikipedia
The last considerations for simple sugars relative to their structure are their chemical reactivity and modification. Sugars that are readily oxidized are called ‘reducing sugars’ because their oxidation causes other reacting molecules to be reduced. A test for reducing sugars is known as Benedict’s test. In it, sugars are mixed and heated with an alkaline solution containing Cu++. Reducing sugars will donate an electron to Cu++, converting it to Cu+, which will produce cuprous oxide Cu2O, as an orange precipitate (Figure 2.158). Since Cu++ solution is blue, the change of color provides an easy visual indication of a reducing sugar.
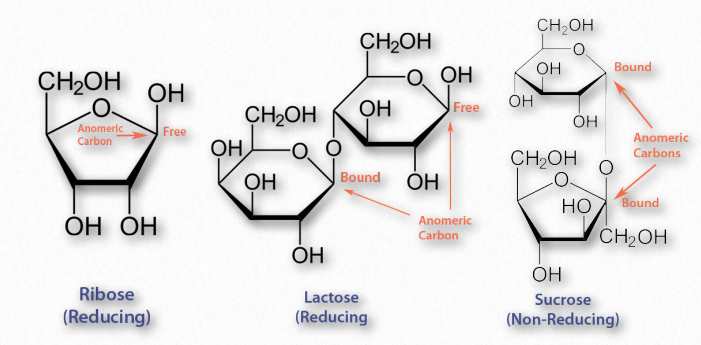
Figure 2.159 – Reducing and non-reducing sugars
The aldehyde group of aldoses is very susceptible to oxidation, whereas ketoses are less so, but can easily be oxidized if, like fructose, they contain an α-hydroxyl and can tautomerize to an aldose. Most monosaccharides are reducing sugars. This includes all of the common ones galactose, glucose, fructose, ribose, xylose, and mannose. Some disaccharides, such as lactose and maltose are reducing sugars since they have at least one anomeric carbon free, allowing that part of the sugar to linearize and yield an aldose. Sucrose, on the other hand has no anomeric carbons free – both are involved in a glycosidic linkage, so they cannot linearize and thus it is not a reducing sugar.
Oxidation and reduction of sugars can occur in cells. As we will see, phosphorylation of sugars occurs routinely during metabolism.
Glucuronic acid
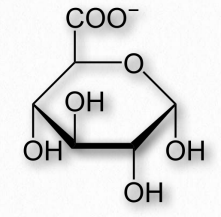
Figure 2.160 – Glucuronic acid
One oxidation product of glucose is glucuronic acid, a six carbon molecule where the CH 2 OH on carbon six is oxidized to a carboxylic acid (Figure 2.160). Related oxidized sugars include galacturonic acid and mannuronic acid. Glucuronic acid is commonly conjugated to other molecules in the liver/bile by UDP-glucuronyltransferase enzymes to make the molecules more water soluble for excretion, since the carboxyl group of glucoronic acid ionizes readily at physiological pH. The reactions are usually done starting with glucuronic acid linked to UDP (UDPGlucuronic Acid). In addition, glucuronic acid is made from a UDP-glucose precursor. Glucuronic acid is a common constituent of glycosaminoglycans, proteoglycans, and glycoglycerolipids. Glucuronic acid is found in heparin, dermatan sulfate, chondroitin sulfate, hyaluronic acid, and keratan sulfate. Glucuronic acid is also a precursor of ascorbic acid (Vitamin C) in organisms that synthesize this compound.
Sugar alcohols
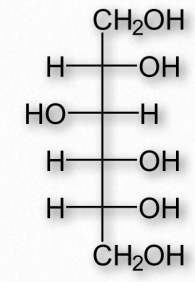
Figure 2.161 – Sorbitol (also called glucitol)
Reduction of aldoses or ketoses by hydrogenation produces the corresponding sugar alcohols. The compounds are widely used as thickeners of food or as artificial sweeteners, due to their ability to stimulate sweet receptors on the tongue. Common sugar alcohols (sugar progenitor in parentheses) include glycerol (glyceraldehyde), xylitol (xylose), sorbitol (Figure 2.161 – from glucose), galactitol (galactose), arabitol (arabinose), and ribitol (ribose). Most of these compounds have a sweetness of between 0.4 and 1.0 times as sweet as sucrose, but provide considerably fewer calories per weight. Xylitol is the sweetest of them with a sweetness equal to that of sucrose.
Figure 2.162 – Structure of sucralose
Sugar alcohols are used sometimes to mask the aftertaste of other artificial sweeteners. Many of them also produce a cooling sensation upon dissolving, due to that being an endothermic process for them, resulting in a pleasant mouth sensation. Last, they are poorly absorbed by intestines, and so have a low glycemic index.
Artificial sweeteners
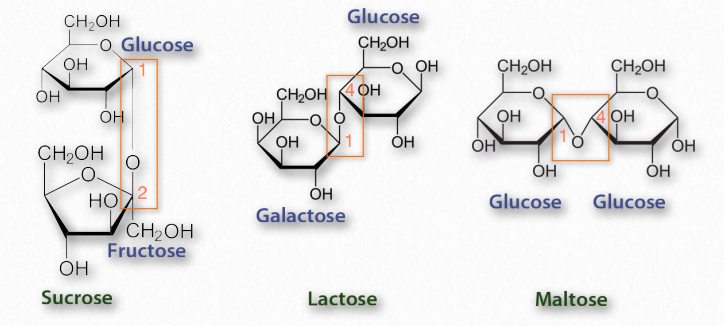
Figure 2.163 – Common disaccharides – glycosidic bonds in rectangles
Artificial sweeteners are compounds that stimulate taste receptors for sweetness, but are metabolized for energy inefficiently at best. Such compounds frequently are many times sweeter than table sugar (sucrose) on a weight/weight basis and are referred to as “intensely sweet.” Most of the artificial sweeteners are not carbohydrates, but rather are able to stimulate the same sweet receptors that sugar does. Seven such compounds are approved for use in the U.S. – stevia, aspartame, sucralose, neotame, acesulfame potassium, saccharin, 1 1 1 2 4 4 and advantame. The sugar alcohol known as sorbitol is also sometimes used as an artificial sweetener.
Disaccharides
Disaccharides (Figure 2.163) are made up of two monosaccharides. The most common ones include sucrose (glucose and fructose), lactose (galactose and glucose), and maltose (glucose and glucose). All of the common disaccharides contain at least one glycosidic bond. We name the disaccharides according to which carbons are linked to each other and the how the anomeric carbon of the glycosidic bond is configured. Lactose, for example, is described as β-Dgalactopyranosyl-(1→4)-D-glucose, or more succinctly as having an α-1,4 glycosidic bond.
Oligosaccharides
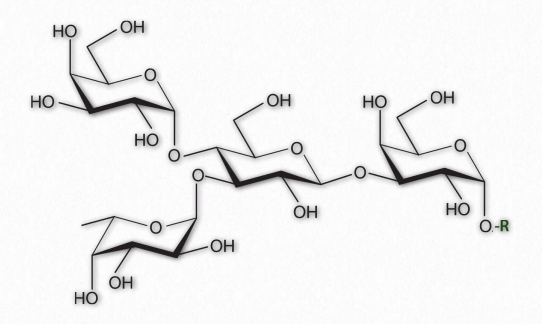
Figure 2.164 – A Branched oligosaccharide attached to an RGroup
As their name implies, oligosaccharides (Figure 2.164) are comprised of a few (typically 3 to 9) sugar residues. These often, but not always contain modified sugars. Unlike all of the other saccharides, oligosaccharides are not typically found unattached to other cellular structures. Instead, oligosaccharides are found bound, for example, to sphingolipids (making cerebrosides or gangliosides) or proteins (making glycoproteins).
Oligosaccharides in membrane glycoproteins play important roles in cellular identity/ recognition. The patterns of oligosaccharides displayed on the extracellular face of the plasma membrane acts as a sort of barcode that identifies specific cell types. The immune system recognizes these identity tags in the body. “Foreign” oligosaccharide structures trigger the immune system to attack them. While this provides a very good defense against invading cells of an organism, it also can pose significant problems when organs are transplanted from one individual into another, with rejection of donated organs, in some cases.
Organelle targeting
The oligosaccharides that are attached to proteins may also determine their cellular destinations. Improper glycosylation or errors in subsequent sugar modification patterns can result in the failure of proteins to reach the correct cellular compartment.
For example, inclusion cell disease (also called I-cell disease) arises from a defective phosphotransferase in the Golgi apparatus. This enzyme normally catalyzes the addition of a phosphate to a mannose sugar attached to a protein destined for the lysosome. In the absence of a functioning enzyme, the unphosphorylated glycoprotein never makes it to the lysosome and is instead exported out of the cell where it accumulates in the blood and is excreted in the urine. Individuals with Icell disease suffer developmental delays, abnormal skeletal development, and restricted joint movement.
Glycosylation
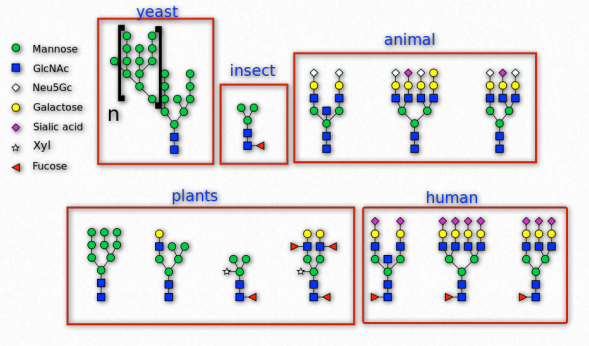
Figure 2.165 – N-linked glycosylation in various organisms Wikipedia
Sugars are commonly attached to proteins in a process called glycosylation. Typically the attachment is to a hydroxyl or other functional group. The majority of proteins synthesized in the endoplasmic reticulum are glycosylated.
N-glycans on cell surfaces play roles in the immune system. The immunoglobulin types (IgG, IgA, IgE, IgD, and IgM) have distinct glycosylation patterns that confer unique functions by affecting their affinities for immune receptors. Glycans also are important in self/non-self identity is tissue rejection and autoimmune diseases.
Glycoproteins
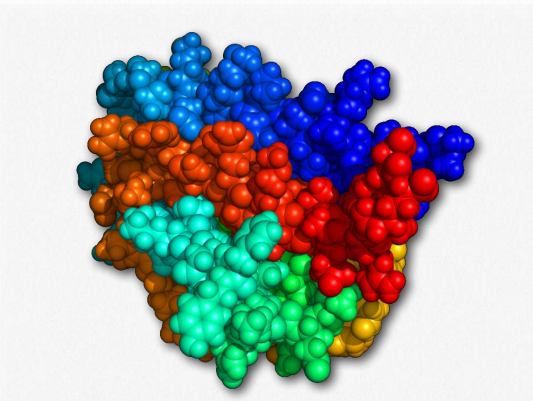
Figure 2.170 – Erythropoietin Wikipedia
Glycoproteins are a very diverse collection of saccharide-containing proteins with many functions. Attachment of the saccharide to the protein is known as glycosylation. Secreted extracellular proteins and membrane proteins with exposed extracellular regions are often glycosylated. Saccharides attached to these may be short (oligosaccharides) or very large (polysaccharides). Glycoproteins play important roles in the immune system in antibodies and as components of the major histocompatibility complex (MHC). They are important for interactions between sperms and eggs, in connective tissues and are abundant in egg whites and blood plasma. Two glycoproteins (gp41 and gp120) are part of the HIV viral coat and are important in the infection process. Some hormones, such as erythropoietin, human chorionic gonadotropin, follicle-stimulating hormone and luteinizing hormone are also glycoproteins.
Glycation is a chemical process (nonenzymatic) that occurs when a protein or lipid covalently binds to a sugar, such as glucose or fructose. Glycation differs from glycosylation in that the latter process is controlled by enzymes and results in specific attachment of specific sugars to biomolecules. Glycation, by contrast, is driven by two properties of monosaccharides 1) their chemistry and 2) their concentration. Glycations may be endogenous (occurring in an organism) or exogenous (occurring external to an organism).
Exogenous glycation arises most commonly as a result of cooking of food and this results in attachment of sugars to lipids and/or proteins to form advanced glycation endproducts (AGEs). At temperatures above 120°C, AGE production occurs readily and contributes to the taste and the appearance of the food we eat.
Browning of food, for example, is a product of glycation and is enhanced as the sugar content of a food increases. Browning of french fries is often enhanced, for example, by adding sugar to them. The formation of a crust of bread or the toasting of bread are other examples. These glycations are products of the Maillard reaction in which a reactive sugar carbonyl group combines with a nucleophilic amine of an amino acid. The process is favored in an alkaline environment, when amines are less protonated. The formation of the harder shell of a pretzel, for example, results from addition of lye to the exterior. At higher temperatures, though, a carcinogen known as acrylamide can be formed by reactions involving asparagine.
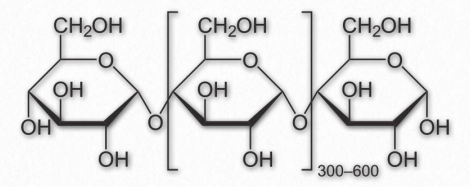
Figure 2.171 – Repeating unit of amylose
Endogenous glycation, on the other hand, arises with a frequency that is proportional to the concentration of free sugar in the body. These occur most frequently with fructose, galactose, and glucose in that decreasing order and are detected in the bloodstream. Both proteins and lipids can be glycated and the accumulation of endogenous advanced glycation endproducts (AGEs) is associated with Type 2 diabetes, as well as in increases in cardiovascular disease (damage to endothelium, cartilage, and fibrinogen), peripheral neuropathy (attack of myelin sheath), and deafness (loss of myelin sheath).
The formation of AGEs increases oxidative stress, but is also thought to be exacerbated by it. Increased oxidative stress, in turn causes additional harm. Damage to collagen in blood cells causes them to stiffen and weaken and is a factor in hardening of the arteries and formation of aneurysms, respectively. One indicator of diabetes is increased glycation of hemoglobin in red blood cells, since circulating sugar concentration are high in the blood of diabetics. Hemoglobin glycation is measured in testing for blood glucose control in diabetic patients.
| Homopolymer | Monomeric Unit |
| Glycogen | Glucose |
| Cellulose | Glucose |
| Amylose | Glucose |
| Callose | Glucose |
| Chitin | N-acetylglucosamine |
| Xylan | Xylose |
| Mannan | Mannose |
| Chrysolaminarin | Glucose |
Polysaccharides
Long polymers of sugar residues are called polysaccharides and can be up to many thousands of units long. Polysaccharides are found free (not attached to other molecules) or bound to other cellular structures such as proteins. Some polysaccharides are homopolymers (contain only one kind of sugar). Others are heteropolymers (glycosaminoglycans, hemicellulose). Polysaccharides function in energy storage (nutritional polysaccharides, such as glycogen, amylose, amylopectin, e.g.), structure enhancement (chitin, cellulose, e.g.), and lubrication (hyaluronic acid, e.g.). These individual categories of polysaccharides are discussed below.
Nutritional polysaccharides
This group of polysaccharides is used exclusively for storage of sugar residues. They are easily easily broken down by the organism making them, allowing for rapid release of sugar to meet rapidly changing energy needs.
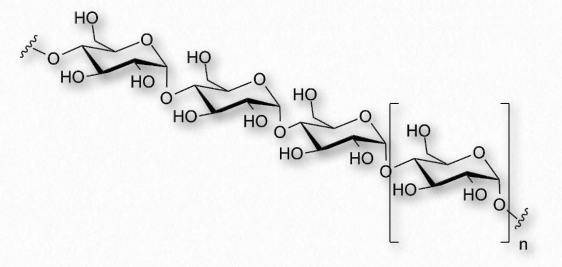
Figure 2.172 – Another view of amylose
Amylose has the simplest structure of any of the nutritional polysaccharides, being made up solely of glucose polymers linked only by α-1,4 bonds (Figure 2.171 & 2.172). (Note that the term ‘starch’ is actually a mixture of amylose and amylopectin). Amylose is insoluble in water and is harder to digest than amylopectin (see below). The complexing of amylopectin with amylose facilitates its water Figure 2.172 – Another view of amylose solubility and its digestion. Amylose is produced in plants for energy storage and since plants don’t have rapidly changing demands for glucose (no muscular contraction, for example), its compact structure and slow breakdown characteristics are consistent with plants’ needs.
Amylopectin and glycogen
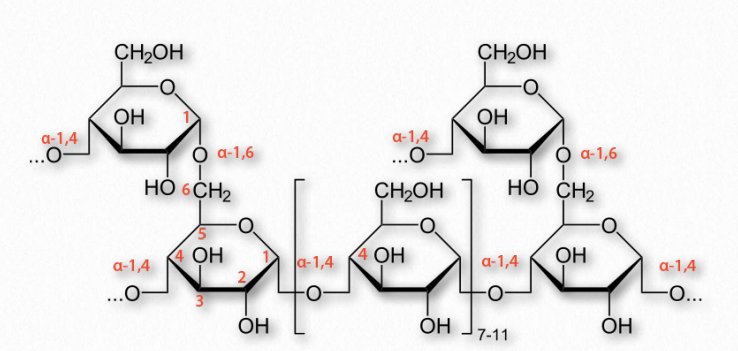
Figure 2.173 – Structure of glycogen
More complicated homopolymers of glucose are possessed by amylopectin in plants and glycogen (Figure 2.173) in animals. Both compounds contain long glucose chains with α-1,4 bonds like amylose, but unlike amylose, these long chains have branches of α-1,6 bonds. Amylopectin is the less-branched of the two, having such bonds about every 25-30 residues, whereas glycogen has branches about every 8-12 residues.
Branching plays important roles in increasing water solubility and in providing more “ends” to the polymer. In animals, glycogen is broken down starting at the ends, so more ends means more glucose can be released quickly. Again, plants, which have a lower need for quick release of glucose than animals get by with less branching and fewer ends.
Structural polysaccharides
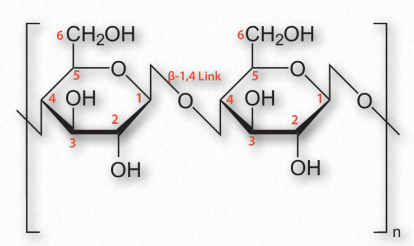
Figure 2.174 – Cellulose with β-1,4 links between glucose sugars
An additional function of polysaccharides in cells relates to structure. Cellulose, which is a polymer of glucose with exclusive β-1,4 linkages between the units (Figure 2.174) is an important structural component of plants and fungi cells. Notably, most non-ruminant animals are unable to digest this polymer, as they lack the enzyme known as cellulase.
Ruminants, such as cattle, however, contain in their rumen a bacterium that possesses this enzyme and allows them to obtain glucose energy from plants. Another group of polysaccharides found in plant cell walls is the hemicelluloses. This class of molecules encompasses several branched heteropolymers of (mostly) D-pentose sugars along with a few hexoses and L-sugars as well. Hemicelluloses are shorter than cellulose (500-3000 sugars versus 7000-15,000 sugars).
Monomer sugars of polysaccharides besides glucose include xylose, mannose, galactose, rhamnose, and arabinose. Xylose is usually present in the greatest amount (Figure 2.175).
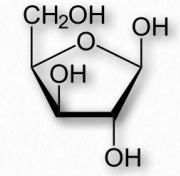
Figure 2.175 Xylose
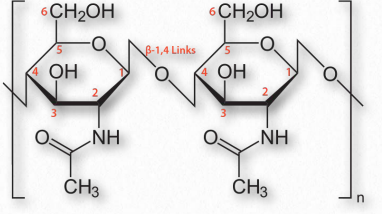
Figure 2.176 – Chitin with β-1,4 links between N-acetylglucosamine sugars
Chitin (Figure 2.176) is another structural polysaccharide, being comprised of N-acetylglucosamine units joined by β-1,4 linkages. It is a primary component of the cell walls of fungi and is also prominent in the exoskeletons of arthropods and insects, as well as the beaks and internal shells of cephalopods (Figure 2.177). Chitin’s structure was solved by Albert Hofmann in 1929. It is like cellulose except for the acetylamine group replacing the hydroxyl on position 2. This change allows hydrogen bonding to occur between adjacent polymers, thus providing greater strength.
Another group of structural polysaccharides is the pectins (Figure 2.178). These com pounds are present in most primary plant cell walls and are abundant in non-woody parts of terrestrial plants. They are rich in galacturonic acid (α-1,4 links with no branches – Figure 2.179) and are used commercially as a gelling agent in jams/jellies, as well as a stabilizer in fruit juices and milk drinks. Pectin consumption may result in reduced blood cholesterol levels due to its tendency to 1) bind cholesterol and 2) to increase viscosity in the intestinal tract, thus reducing absorption of cholesterol from food. Pectins also trap carbohydrates in the digestive system and reduce their rate of absorption.
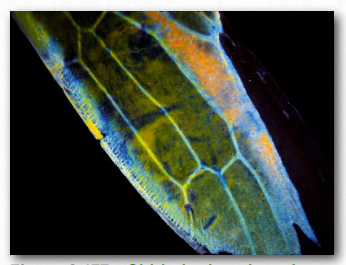
Figure 2.177 – Chitin in the wing of a sap beetle Wikipedia

Figure 2.178 – A powdered form of pectin
Lectins are not carbohydrates, but proteins that specifically bind to carbohydrate molecules found in animals and plants (where they are known as phytohemagglutinins) and are each highly specific for certain sugars. They function in cellular and molecular recognition, as well as cell adhesion. One lectin recognizes hydrolytic enzymes containing mannose-6-phosphate and targets them to be delivered to lysosomes. In the innate immune system, a mannose binding lectin helps defend against invading microbes. Other lectins have roles in inflammation and autoimmune disorders.
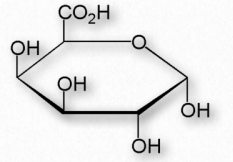
Figure 2.179 – α-DGalacturonic acid – An important component of pectin polymers
Some viruses and bacteria use lectins to recognize and bind specific carbohydrate residues on the surface of target cells. Flu virus, for example, carries a lectin known as hemagglutinin (Figure 2.180) that binds to sialic acid and is essential for entrance of the virus into the target cell. After binding, the viral particle enters by endocytosis after the hemagglutinin has been cleaved by a protease.
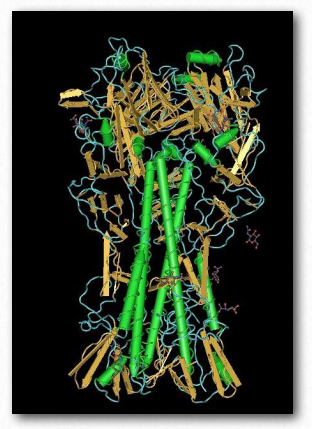
Figure 2.180 – Hemagglutinin
After replication of the virus inside of the cell, hemagglutinin and and a viral enzyme known as neuraminidase cluster in the cell membrane. Viral RNA and associated viral proteins cluster near this membrane site and new viruses bud off in a portion of the cell’s membrane after the hemagglutinin-sialic acid link to the infected cell is released by the neuraminidase cutting the bond between the sialic acid and the rest of the cell surface carbohydrate. Drugs, such as tamiflu, that interfere with neuraminidase work by preventing release of the viral particle. Unreleased particles will tend to aggregate and not function.
Some viral glycoproteins from hepatitis C virus may attach to lectins on the surface of liver cells in their infectious cycle. The bacterium Helicobacter pylori uses a cell surface lectin to bind oligosaccharides on epithelial cells lining the stomach. One lectin known as ricin is a very powerful toxin. It is produced in the endosperm of seeds of the castor oil plant and is of concern as a bioterrorism weapon as a result of its acute toxicity when inhaled or ingested.
Lectins were discovered originally in plants and have been most studied in legumes, but lectins are now known to be widely dispersed in nature. In the immune system, a mannan binding lectin (MBL) helps mediate the first defenses against microorganisms. Other immune system lectins are thought to modulate inflammatory processes and probably play a role in self/non-self recognition that is at the root of rejection of transplanted organs.
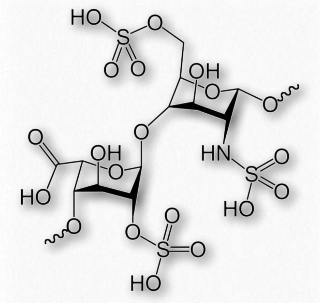
Figure 2.186 – Repeating sulfated disaccharide in heparin
Heparin (Figures 2.186 & 2.187) is a modified polysaccharide whose biological function is unclear, but whose ability to prevent clotting of blood is used for medical purposes. Heparin does not dissolve blood clots. Rather, it acts to prevent conversion of fibrinogen to fibrin.
Whether or not heparin is actually used by the body for its anticoagulation property is uncertain. It is stored in the secretory granules of mast cells and released at the point of injury and it has been proposed it is a protection against bacteria and other foreign materials.
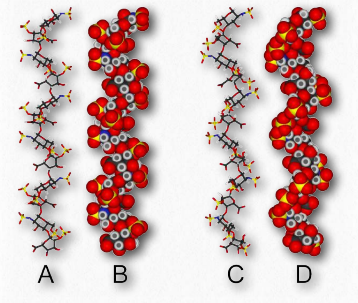
Figure 2.187 – Two structures for heparin
Heparin has abundant sulfates and is, in fact, the molecule with the highest negative charge density known. Its size varies from 3 kDa to 30 kDa, with an average of about 15 kDa. The repeating disaccharide of 2-Osulfated iduronic acid and 6-O-sulfated, N- sulfated glucosamine, occupies about 85% of the molecule. Copper salts of heparin help stimulate the synthesis of blood vessels (angiogenic).
Hyaluronic acid
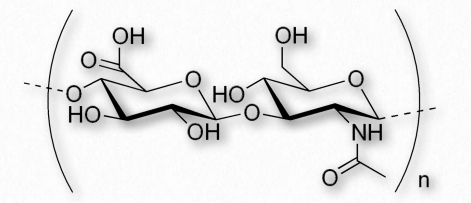
Figure 2.188 – Repeating disaccharide of hyaluronic acid
Hyaluronic acid (also known as hyaluronan or hyaluronate) is a glycosaminoglycan found in connective, epithelial, and nerve tissues. It is an unusual glycosaminoglycan (Figure 2.188), lacking sulfate, is made by hyaluronan synthases on the inner face of the plasma membrane and has a molecular weight in the millions. An average adult body contains about 15 grams of HA, one third of which is replaced every day. The repeating unit in hyaluronic acid is a disaccharide structure of D-glucuronic acid joined to D-N-acetylglucosamine. The compound, which can have upwards of 25,000 units of the disaccharide, is delivered directly into the extracellular matrix by enzymes from its plasma membrane site of synthesis.It is an important component of the extracellular matrix, where it assists in cell proliferation and migration. The polymer provides an open hydrated matrix to facilitate general cell migration whereas directed cell migration occurs via the interaction between hyaluronic acid and specific cell surface receptors. HA interaction with the receptor RHAMM (Receptor for Hyaluronan Mediated Motility) has been shown to be involved in wound repair as well as tumor progression.
Synovial Fluid
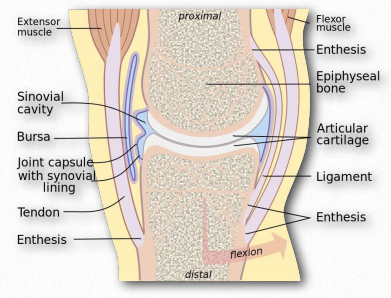
Figure 2.189 – Synovial fluid in joint lubrication Wikipedia
The function of hyaluronic acid has traditionally been described as providing lubrication in synovial fluid (the lubricating material in animal joints – Figure 2.189). Along with the proteoglycan called lubricin, hyaluronic acid turns water into lubricating material. Hyaluronic acid is present as a coat around each cell of articular cartilage and forms complexes with proteoglycans that absorb water, giving resilience (resistance to compression) to cartilage. Aging causes a decrease in size of hyaluronans, but an increase in concentration.
Function in skin
Hyaluronic acid is a major component of skin and has functions in tissue repair. With exposure to excess UVB radiation, cells in the dermis produce less hyaluronan and increase its degradation.
For some cancers the plasma level of hyaluronic acid correlates with malignancy. Hyaluronic acid levels have been used as a marker for prostate and breast cancer and to follow disease progression. The compound can to used to induce healing after cataract surgery. Hyaluronic acid is also abundant in the granulation tissue matrix that replaces a fibrin clot during the healing of wounds. In wound healing, it is thought that large polymers of hyaluronic acid appear early and they physically make room for white blood cells to mediate an immune response.
Breakdown of hyaluronic acid is catalyzed by enzymes known as hyaluronidases. Humans have seven types of such enzymes, some of which act as tumor suppressors. Smaller hyaluronan fragments can induce inflammatory response in macrophages and dendritic cells after tissue damage. They can also perform proangiogenic functions.
each of two isomers with different configurations of atoms around one of several asymmetric carbon atoms present
Introductory Biochemistry Copyright © by Carol Higginbotham is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
NOTIFICATIONS
Glucose structures.
- + Create new collection
The molecular formula of glucose is C 6 H 12 O 6 .
The structure of the glucose molecule can be either a linear or a ring arrangement.
In this animation, this symbol will represent a glucose molecule.

Food macromolecules
The purpose of this animation is to show visually how glucose molecules can be assembled to form simple sugars and large macromolecular carbohydrates such as starch and cellulose. To use this ...
See our newsletters here .
Would you like to take a short survey?
This survey will open in a new tab and you can fill it out after your visit to the site.
Talk to our experts
1800-120-456-456
- Structure of Glucose and Fructose

Glucose vs Fructose
Glucose is defined as a monosaccharide and is found in all the primary carbohydrates such as in the table sugar starch. Glucose is also known as grape sugar or blood sugar, and it is represented as a six-membered ring that forms a pyranose ring structure. Glucose is aldohexose and is the primary and the most preferred energy source of the body. It is found in starch.
Fructose is defined as a monosaccharide and is found in fruits and vegetables. In fructose, the glycemic index is lower as compared to glucose. Compared to Glucose, the binding fructose to cellular protein is seven times faster. It is also referred to as D- fructose or fruit sugar and its functional group are known as ketone. Glucose is known to be primarily metabolized in the liver and is not found in starch.
Structure of Glucose
Glucose is defined as a group of carbohydrates, a simple sugar having a chemical formula C 6 H 12 O 6 . It is composed of six carbon atoms, including an aldehyde group. Thus, we can refer to this as aldohexose. It exists in two forms, which are either in the open-chain (acyclic) form or ring (cyclic) form. The major source of energy needed for living organisms is given as glucose. Algae and plants prepare glucose during the photosynthesis process with the help of water , carbon dioxide, and sunlight. It is naturally found in honey and fruits. The glycogenolysis process obtains glucose present in animals.
How to draw an Open-Chain Structure of the Glucose Molecule
The required steps to draw an acyclic form of glucose are:
Step 1: Draw six carbon atoms.
Step 2: Draw extended arms for all the carbon atoms.
Step 3: Draw a hydrogen atom to carbon bond in such a way that four will be on one side, one on the other side
Step 4: Fill up the rest of the spaces with an OH group.
Step 5: Finally, complete both the ends with two single-bonded hydrogen bonds with one double-bonded carbon.
Dry fructose looks like a white coloured crystalline solid, which is odourless and sweet. Fructose is soluble in ether, in water, and also in alcohol.
Baeyer showed the open-chain structure of the glucose compound. However, these structures have faced difficulties in explaining why glucose fails to react with Schiff base, sodium bisulphate or the mutarotation process. Haworth introduced the cyclic structure of glucose that confirms the existence of alpha and beta forms of mutarotation, glucose, and more.
(Image will be uploaded soon)

Steps to draw the Ring Structure of a Glucose Molecule
Follow the below steps to draw a cyclic form of glucose.
Step 1: Firstly, construct a hexagon
Step 2: Then, draw carbon atoms at five consecutive edges.
Step 3: Thereafter, attach an oxygen atom at the left out edge.
Step 4: Now, attach the four carbon atoms with OH and H groups.
Step 5: Complete the entire structure by attaching the left out carbon atom to two hydrogen atoms, one carbon atom and OH group.
Glucose Properties
Glucose Chemical Formula | C6H12O6 |
Glucose Molar mass | 180.156 g/mol |
IUPAC Name | D-Glucose |
Fructose Ring Structure
Fructose is described as a monosaccharide which is given as a simple sugar having a chemical formula C 6 H 12 O 6 . It is also known as fruit sugar and was discovered in 1847 by a French chemist named Augustin-Pierre Dubrunfaut. It is composed of a 6-carbon polyhydroxy ketone.
Crystalline fructose takes place as a cyclic six-membered structure, which is delinquent to the stability of the internal hydrogen bonding and hemiketal. This form is known as D-fructopyranose. It primarily takes place in vine fruits, honey, most root vegetables, flowers, and berries. It can be obtained Commercially from maize, sugar beets, and sugar cane.
Open Chain Structure and Ring Structure of a Fructose Molecule
The cyclic structure of fructose is given below:
The Fischer projections of D-fructose and L-fructose can be given as follows:
Fructose Properties
Dry fructose appears as a white coloured crystalline solid, which is odourless and sweet. It is soluble in ether, water, and alcohol.
Properties of Fructose
Fructose Molar mass | 180.156 g/mol |
Fructose Formula | C6H12O6 |
Density | 1.694 g/cm3 |
Fructose Chemical Formula | C6H12O6 |
Melting point | 103°C |
The Difference between the Glucose and Fructose Compounds
Known as constitutional isomers, which means that there is a difference in the bond connectivity. Glucose is an aldehyde, and fructose is a ketone. When they become cyclized by the formation of hemiketal/hemiacetal, glucose becomes a 6-ring sugar and on the other side, fructose becomes a five-ring sugar. Glucose is the most preferred form of energy of the body and every cell in the body is capable of metabolizing the glucose compound. AS far as Fructose is concerned, only the Liver is capable of metabolizing fructose. This is the reason why Fructose intake should be restricted.
D-Glucose Chemistry
In the glucose molecule, a hydrogen and oxygen atom group is bonded to the carbon atom. On the other side of the glucose molecule, there exists a double-bonded oxygen atom. Looking at the D-glucose’s Fisher model with the double-bonded oxygen atom, which is pointed down, the hydrogen and oxygen group present at the top of the atom points towards the right.
L-Glucose Chemistry
D-glucose and L-glucose are composed of similar atoms. The only difference between these two structures can be displayed using the Fisher model. In the Fisher model, unlike D-glucose, the hydrogen and oxygen group of atoms present in L-glucose points to the left. If these two specific molecules faced each other, they would look like a reflection of one another.

FAQs on Structure of Glucose and Fructose
1. Explain the Structure of Glucose.
Glucose is a group of carbohydrates, a simple sugar that has the chemical formula C 6 H 12 O 6 .
Glucose is composed of six carbon atoms, including an aldehyde group. Glucose can be referred to as aldohexose. It exists in two forms, which are either in the open-chain (acyclic) form or the ring (cyclic) form. Glucose is considered a major source of energy that is needed for living organisms.
2. Explain the Fructose Ring Structure.
Fructose is a monosaccharide which is a simple sugar that has a chemical formula C 6 H 12 O 6 . Fructose is also called fruit sugar and it was discovered in 1847 by a French chemist, Augustin-Pierre Dubrunfaut. Fructose is composed of a 6-carbon polyhydroxy ketone. The crystalline fructose takes place as a cyclic six-membered structure, that is delinquent to the stability of the internal hydrogen-bonding and hemiketal. Fructose can be obtained commercially from maize, sugar beets, and sugar cane.
3. Explain Glucose and Fructose?
Both the compounds Glucose and Fructose are given as simple sugars or monosaccharides. Both Glucose and Fructose can be said to be similar as they both contain the same amount of calories and they occur naturally in certain fruits as well as in some foods. They are both different in their chemical structures and in the way the human body digests and processes them.
Glucose is less fat producing as compared to Fructose.
4. How to Draw the Ring Structure of a Glucose Molecule?
The required steps to draw the Ring Structure, or a cyclic form, of glucose, are:
Step 2: Draw carbon atoms at five consecutive edges.
Step 3: Attach an oxygen atom.
Step 4: Attach the four carbon atoms with OH and H groups.
Step 5: Finally, complete the entire structure by attaching the left out carbon atom to two hydrogen atoms, one carbon atom and the OH group.
5. Where are study materials available?
In Chemistry, the ‘Structure of Glucose and Fructose’ Chapter is as important a chapter as all the other chapters in Chemistry. It is necessary to be able to practice some of the important questions to be able to do well. The online portal, Vedantu.com offers important questions along with answers and other very helpful study material on the Structure of Glucose and Fructose, which have been formulated in a well structured, and in an easy to understand manner. These study materials and solutions are all important and are very easily accessible from Vedantu.com and can be downloaded for free.
6. Explain various types of glucose?
The d-isomer, d-glucose, which is also called dextrose, is commonly found in nature, but the l-glucose, l-isomer, is not found commonly in nature. Hydrolysis of carbohydrates like plant sugar (sucrose), dairy sugar (lactose), cellulose, maltose, glycogen is used to obtain glucose.
7. Where does glucose produce from?
The food we consume is produced either from sugar or glucose. The prevalent sources of glucose are given as carbohydrates, such as bread pasta, cereals. In our stomachs, these types of foods are split into sugar, and then they are absorbed into the bloodstream.
8. Why is Glucose a monosaccharide?
Monosaccharides (to explain clearly, mono- = “one;” sacchar- = “sugar”) are given as simple sugars, where glucose is the most prevalent compound.
9. What is the function of fructose?
Similar to glucose, fructose also provides energy cells. These cells process fructose to gain energy using a method known as aerobic breathing, which primarily involves burning fructose in the presence of oxygen to generate ATP, which is the molecule of cellular energy.
A Level Biology
Instant Access to A Level Biology Revision
Sign up now to get access to the entire library of a level biology resources for all exam boards.
If you're ready to pass your A-Level Biology exams, become a member now to get complete access to our entire library of revision materials.
Join over 22,000 learners who have passed their exams thanks to us!
Sign up below to get instant access!
Or try a sample...
Not ready to purchase the revision kit yet? No problem. If you want to see what we offer before purchasing, we have a free membership with sample revision materials.
Signup as a free member below and you'll be brought back to this page to try the sample materials before you buy.
Introduction
Linear chain, closed chain, d and l isomers, alpha and beta isomers, epimers of glucose, photosynthesis, gluconeogenesis, entry into the cell, krebs cycle, fermentation, hypoglycemia, hyperglycemia, is glucose a sugar, is glucose soluble in water, what are the functions of glucose, what are the normal blood glucose levels.
Glucose is the most important monosaccharide present in our body. It belongs to the hexose category of monosaccharides. Glucose provides energy to all the cells in our body except the cardiac myocytes. Excess glucose is stored in the body in the form of storage molecules.
Glucose is present in human blood within a specific range. Different medical conditions can arise if the blood glucose levels fall or increase above this range. In this article, we will discuss the structure of glucose in its various forms, properties, isomers, metabolism in the human body, and medical conditions associated with it. So, keep reading.
Glucose is a monosaccharide made up of six carbon atoms. It is the most important hexose present in our body. The molecular formula of glucose is represented as C 6 H 12 O 6 . The structural formula of glucose can be represented in two ways;
- Closed ring
The linear chain structure and the ring structure co-exist in equilibrium with each other in an aqueous solution. Both these forms are interconvertible.
When the glucose structure is represented in the form of an open chain, it can be seen to have a linear chain of six carbon atom. One hydroxyl group is attached to each carbon atom, except the first carbon. The first carbon of glucose is a part of the aldehyde group. Because of the presence of the aldehydic functional group, glucose is also known as an aldohexose.
Glucose makes a ring when it is dissolved in an aqueous solution. The ring formed by glucose is hexagonal in structure. Five carbon atoms and one oxygen atom, belonging to the aldehydic functional group, make the corners or angles of the hexagon. This ring structure of glucose is known as glucopyranose.
Isomers are the molecules that have the same molecular formula but differ with respect to the arrangement of atom in the three-dimensional space. The number of isomers of a molecule depends on the number of chiral carbons in it. A chiral carbon is the one that is attached to four different groups of atoms. The formula to find the number of isomers based on chiral carbons is as follows;
Number of isomers = 2 n (here, n=number of chiral carbons)
Except for the first and the last carbon atom, the other four carbon atoms in glucose are chiral. Thus, glucose has 2 4 =16 isomers.
When in ring form, each of these 16 isomers can have one of the two possible orientations; alpha or beta. Thus, glucose actually has 32 isomers.
The two different structural forms of glucose are as follows;
When dissolved in solution, each of them can have one of the following ring structure.
- Alpha-Glucose
- Beta-Glucose
These are the two isomeric forms of glucose that differ in the optical properties.
The D-glucose is dextrorotatory. When a ray of light is passed through a solution of D-glucose, it bends the light in the right direction. It is the most abundant form of glucose present in nature. When the structure of D-glucose is drawn on a paper, the -OH groups are written on the right side of carbon atoms, except the 3 rd carbon atom that has the -OH group on its left side.
The L-Glucose is levorotatory. It bends the light rays to the left when passed through its aqueous solution. It is normally present in living cells. Its structure is opposite to that of D-glucose i.e. the -OH groups are attached to the left side of carbon atoms except the third carbon.
When either D-glucose or L-glucose is dissolved in water, it forms a hexagonal ring structure known as glucopyranose. The ring formed by each of these molecules can have alpha or beta orientation.
In alpha-D-glucose or alpha-L-glucose, the hydroxyl group attached to the carbonyl carbon or the first carbon is on the side of the ring opposite to that of the sixth carbon.
On the other hand, in beta-D-glucose or beta-L-glucose, the hydroxyl group of the carbonyl carbon is on the same side of the ring as the sixth carbon.
Epimers are the molecules that differ in structure around one carbon atom only. Glucose has eight epimers.
All these eight epimers have D-form and L-form making a total of 16 isomers.
Each of the 16-isomers can have either an alpha ring or a beta ring when dissolved in aqueous solution.
The following are the important properties of glucose that should be kept in mind.
- It is sweet in taste
- It is a polar compound that readily dissolves in water
- It is a reducing sugar that gives positive Benedict’s test
- Like other monosaccharides, it cannot undergo hydrolysis.
- Upon reduction, it forms alcohol like sorbitol
- The oxidation of glucose yields carboxylic acids like pyruvic acid
- It can also undergo fermentation and ester formation
Glucose is abundantly present in nature.
Plants are the major source of glucose as they make it by the process of photosynthesis. It is present in free form in all fruits being abundantly present in dates, figs, grapes, etc. In the form of large storage molecule starch, it is present in the roots and tubers of plants.
In humans, glucose is present in free form in blood, cerebrospinal fluid, and other tissue fluids. It is also stored in cells in the form of large storage molecule glycogen.
Glucose is mainly synthesized in plants by the process of photosynthesis. However, animals are also capable of synthesizing glucose to some extent from non-carbohydrate sources by a process known as gluconeogenesis.
A brief detail of these processes is mentioned below.
It is a process of food production that takes place in plants in the presence of sunlight. Photosynthesis is a chain of chemical reactions that uses energy from sunlight and converts water and carbon dioxide into glucose and oxygen.
This process takes place in special organelles of plant cells called chloroplasts. Chloroplasts are the factories of glucose production. They are present in almost all plant cells, being abundantly present in leaves. They impart the green colors to plants.
The process of photosynthesis is divided into two steps, light reactions and dark reactions.
- Light reactions involve the capturing of energy present in photons of sunlight in the form of ATP and NADPH.
- Dark reactions involve the carbon fixing reactions that convert carbon dioxide molecules into glucose using the energy provided by ATP and NADPH. They are not dependent on light and are also called collectively known as the Calvin cycle.
This process involves the synthesis of glucose from non-carbohydrate sources in animals. Not all the animal cells are capable of carrying out gluconeogenesis. In humans it only takes place in two organs;
- Liver (majorly)
- Kidneys (to a small extent)
Cells present in other organs of our body lack enzymes for gluconeogenesis.
Amino acids provide the carbon skeleton for the synthesis of glucose. The process is a reverse of glycolysis and takes place in two steps.
- In the first step, the carbon skeleton of an amino acid is converted into pyruvic acid or pyruvate within the mitochondria. The pyruvate molecule is then transported outside the mitochondria into the cytoplasm.
- The second step is the reverse of glycolysis in which a glucose molecule is generated from two pyruvate molecules within the cytoplasm of a cell.
Both these steps use energy in the form of ATP and NADH. The glucose molecules thus formed are released into the blood so that they can be transported to the other cells of the body.
Metabolism
The first step of glucose metabolism in human body is the entry of glucose molecules into the cells. Once inside the cytoplasm, glucose molecules can undergo oxidation to yield energy or they can be stored in the form of glycogen.
Glucose molecules are large and thus, cannot cross the plasma membrane freely. They require special protein channels for entry into the cell, called glucose transporter or GLUT.
Different cells have different types of GLUT. The most common forms of GLUT along with the cells having them are listed below.
- GLUT-1 is present in blood-brain barrier and heart
- GLUT-2 is present in liver, pancreas and small intestine
- GLUT-3 is present in brain
- GLUT-4 is present in skeletal muscles and adipose tissues
These glucose transporters have different affinities for the glucose molecules and allow their entry into the cell at different concentrations. It should also be noted that GLUT-4 is dependent on glucose.
Once the glucose molecules have entered a cell, they can be oxidized to obtain energy. The energy thus obtained is used to carry out various functions performed by the cell.
Oxidation involves two processes; an oxygen-independent glycolysis, and oxygen-dependent Krebs cycle.
Glycolysis is the process of breaking down a molecule of glucose into two pyruvic acid molecules.
It takes place within the cytoplasm of cells. Glycolysis can occur in the presence or absence of oxygen.
The net product of glycolysis is two molecules of glucose, two ATP molecules, two NADH molecules, and two water molecules.
The pyruvate molecules are transported into the mitochondria for further processing. Here, the two pyruvate molecules are converted into two acetyl-CoA molecules.
The acetyl-CoA molecules are then passed through a cyclic process for complete oxidation into carbon dioxide. This cyclic process is called the Krebs cycle or citric acid cycle.
It is an oxygen-dependent process that takes place only in aerobic organisms.
This process yields two carbon dioxide molecules, three NADH molecules and one FADH 2 molecule from one acetyl-CoA.
The alternative to Krebs cycle in non-aerobic organisms as well as humans in the absence of oxygen is fermentation. In this process, the pyruvate molecule is converted into lactic acid molecule using NADH as a reducing agent.
Clinical conditions
Glucose is present in human blood in free as well as dissolved form. The normal range of blood glucose levels are 60-100 mg/dL in fasting condition, and 170-200mg/dL in non-fasting condition. Any increase or decrease in this level results in some serious clinical conditions mentioned below.
It is a condition in which blood glucose levels fall below 60mg/dL in fasting conditions.
It is characterized by pale, cold and clammy skin, very rapid pulse, tachycardia, with normal or shallow breathing. As the hypoglycemia progresses, the person becomes irritable, confused, aggressive and uncoordinated, finally resulting in unconsciousness. Other symptoms include headache, dizziness, tremors, and convulsions.
Hypoglycemia is a medical emergency and should be treated immediately.
It is usually associated with alcohol consumption, too much insulin administration, or unexpected exercise in diabetic patients.
It is characterized by blood glucose levels above 200mg/dL in fasting conditions.
The signs and symptoms of hyperglycemia include flushed, hot and dry skin, weak and rapid pulse, low blood pressure, and low breathing.
The three classical signs of hyperglycemia include;
- Polyphagia (excessive food intake)
- Polydipsia (excessive thirst)
- Polyuria (excessive urine output)
It is a gradual condition that develops over time. It is associated with excessive food intake, insufficient insulin, insulin resistance, or an infectious disease.
- Glucose is the most important monosaccharide that provides energy to cells present in our bodies.
- It is an aldohexose having an aldehydic group and multiple hydroxyl groups attached to six carbon atoms.
- Its structure can be represented by an open-chain structure or a closed ring.
- Glucose has 16 isomers. Each of these isomers can have either alpha ring or beta ring when dissolved in aqueous solution.
- D-glucose and L-glucose are the two optical isomers of glucose.
- The properties of glucose resemble other monosaccharides.
- It is majorly present in fruits.
- Plants make glucose from carbon dioxide and water by using sunlight as an energy source in the process of photosynthesis.
- Animals can also make glucose by the process of gluconeogenesis.
- Once the glucose has entered the cells, it can either be used as an energy source can be converted into glycogen for use in the future.
- Oxidation of glucose in aerobic organisms involves glycolysis and the Krebs cycle.
- The alternative of the Krebs cycle in non-aerobic organisms and bacteria is fermentation.
- Increase or decrease in blood glucose levels can result in either of the two clinical conditions, hypoglycemia and hyperglycemia.
Frequently Asked Questions
The term sugar is often used for glucose. Glucose is the simplest form of sugar that is made up of six carbon atoms. It is a monosaccharide belonging to the category of hexoses.
Being the simplest sugar, glucose is soluble in water. All monosaccharides are soluble in water. Glucose is also a monosaccharide that has six carbon atoms in its structure.
Glucose is the main source of energy for us. All carbohydrates that we consume in our diet are broken down into glucose molecules that are carried to the cell. Cells majorly use glucose to obtain energy and use it in various cellular processes.
The normal blood glucose level is less than 200 mg/dl two hrs after a meal. If it is more than that, the person may be diabetic.
- Matthews, C. E.; K. E. Van Holde; K. G. Ahern (1999) Biochemistry. 3rd edition. Benjamin Cummings. ISBN 0-8053-3066-6
- N.A.Campbell (1996) Biology (4th edition). Benjamin Cummings NY. p.23 ISBN 0-8053-1957-3
- Kwan, Lam Peng (2000). Biology- A course for O Level. p. 59. ISBN 9810190964 .
- “Carbohydrates” . Chemistry for Biologists. Royal Society of Chemistry. Retrieved 10 March 2017.
- https://commons.wikimedia.org/wiki/File:Gluconeogenesis-es.svg
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Physiology, glucose metabolism.
Mihir N. Nakrani ; Robert H. Wineland ; Fatima Anjum .
Affiliations
Last Update: July 17, 2023 .
- Introduction
Glucose is central to energy consumption. Carbohydrates and proteins ultimately break down into glucose, which then serves as the primary metabolic fuel of mammals and the universal fuel of the fetus. Fatty acids are metabolized to ketones. Ketones cannot be used in gluconeogenesis. Glucose serves as the major precursor for the synthesis of different carbohydrates like glycogen, ribose, deoxyribose, galactose, glycolipids, glycoproteins, and proteoglycans. On the contrary, in plants, glucose is synthesized from carbon dioxide and water (photosynthesis) and stored as starch. At the cellular level, glucose is usually the final substrate that enters the tissue cells and converts to ATP (adenosine triphosphate).
ATP is the energy currency of the body and is consumed in multiple ways, including the active transport of molecules across cell membranes, contraction of muscles and performance of mechanical work, synthetic reactions that help to create hormones, cell membranes, and other essential molecules, nerve impulse conduction, cell division and growth, and other physiologic functions. [1]
- Issues of Concern
The average fasting blood glucose concentration (no meal within the last 3 to 4 hours) is between 80 to 90 mg/dl. On average, postprandial blood glucose may rise to 120 to 140 mg/dl, but the body's feedback mechanism returns the glucose to normal within 2 hours. During starvation, the liver provides glucose to the body through gluconeogenesis, synthesizing glucose from lactate and amino acids.
We can summarize blood glucose regulation and its clinical significance in the following ways:
- The liver serves as a buffer for blood glucose concentration
After a meal, there is a rise in blood glucose levels, which raises insulin secretion from the pancreas simultaneously. Insulin causes glucose to be deposited in the liver as glycogen; then, during the next few hours, when blood glucose concentration falls, the liver releases glucose back into the blood, decreasing fluctuations.
Clinical significance: During severe liver disease, it is impossible to maintain blood glucose concentration.
- Insulin and glucagon work together to maintain normal glucose concentration.
High blood glucose causes insulin secretion, which concomitantly lowers blood glucose levels as glucose is driven from extracellular to intracellular. Conversely, a fall in blood glucose stimulates glucagon secretion, which in turn raises blood glucose levels.
Clinical significance: Impaired and insufficient insulin secretion leads to diabetes mellitus.
- CNS control of blood glucose levels
Low blood glucose level is sensed by the hypothalamus, leading to activation of the sympathetic nervous system to maintain glucose levels and avoid severe hypoglycemia.
- Effect of growth hormone and cortisol.
Prolonged hypoglycemia for hours and days leads to the secretion of growth hormone and cortisol that maintain blood glucose levels by increasing fat utilization and decreasing the rate of glucose utilization by cells. [2]
- Cellular Level
Following are the critical steps in the utilization of glucose at the cellular level-
- Transport of glucose through the cell membrane
For glucose to be utilizable in most tissue cells, it needs to be transported across the cell membrane into the cytoplasm. Glucose cannot readily diffuse through because of its high molecular weight. Transport is made possible through protein carrier molecules; this is known as facilitated diffusion, and it takes place down the gradient from high concentration to low concentration.
There is an exception for the gastrointestinal tract and renal tubules. Here, glucose is transported actively by sodium-glucose co-transport against the concentration gradient.
- Role of insulin in glucose metabolism
The rate of glucose/carbohydrate utilization is under the control of the rate of insulin secretion from the pancreas. Normally, the amount of glucose that can diffuse in the cells is limited except for liver and brain cells. This diffusion is significantly increased by insulin to 10 times or more.
- Phosphorylation of glucose
As soon as glucose enters the cell, it becomes phosphorylated to glucose-6-phosphate. This reaction is mediated by glucokinase in the liver and hexokinase in most other cells. This phosphorylating step serves to capture glucose inside the cell. It is irreversible mostly except in liver cells, intestinal epithelial cells, and renal tubular epithelial cells where glucose phosphatase is present in these locations, which is reversible.
This glucose can then either be utilized immediately for the release of energy through glycolysis, a multi-step procedure to release energy in the form of ATP, or it can be stored as glycogen(polysaccharide). Liver and muscle cells store large amounts of glycogen for later utilization to release glucose by glycogenolysis, ie, the breakdown of glucose. [3]
- Development
In a developing fetus, regulated glucose exposure is imperative to normal growth because glucose is the primary energy form used by the placenta. In late gestation, fetal glucose metabolism is essential to the development of skeletal muscles, fetal liver, fetal heart, and adipose tissue. Three components that are crucial to fetal glucose metabolism are maternal serum glucose concentration, maternal glucose transport to the placenta, which is impacted by the amount of glucose the fetus uses, and finally, fetal pancreas insulin production.
Fetal insulin secretion gradually increases during the gestational period. Pulsatile peaks in glucose levels are beneficial to insulin secretion; however, constant hyperglycemia down-regulates insulin sensitivity and glucose tolerance. [4] In mothers with elevated serum glucose levels, adverse fetal impacts include congenital abnormalities, fetal macrosomia, and stillbirth. [5]
- Organ Systems Involved
- Nervous system: The pancreas performs autonomic function through the sympathetic and parasympathetic innervation of the pancreas. The brain itself also houses insulin receptors in multiple regions, including the hypothalamus, cerebellum, hippocampus, among other areas.
- Pancreas: The pancreas is behind the stomach in the right upper quadrant of the abdomen. The endocrine functionality of the pancreas regulates glucose homeostasis.
- Liver: Glycogenesis and gluconeogenesis are the storing and releasing of glucose, respectively. These processes occur using insulin, glucagon, and hepatocyte derived factors.
- Gut: Hormones in the gut are released in response to the ingestion of nutrients. These hormones are involved in appetite, glucose production, gastric emptying, and glucose removal.
- Adipocytes: Adipose tissue secretes adipokines, which regulate insulin release through their involvement in glucose metabolism, control of food intake, and insulin gene expression. [6]
Glucose metabolism involves multiple processes, including glycolysis, gluconeogenesis, glycogenolysis, and glycogenesis. Glycolysis in the liver is a process that involves various enzymes that encourage glucose catabolism in cells. One enzyme, in particular, glucokinase, allows the liver to sense serum glucose levels and to utilize glucose when serum glucose levels rise, for example, after eating. During periods of fasting, when there is no glucose consumption, for example, overnight while asleep, gluconeogenesis takes place.
Gluconeogenesis happens when there is glucose synthesis from non-carbohydrate components in the mitochondria of liver cells. Additionally, during fasting periods, the pancreas secretes glucagon, which begins glycogenolysis. In glycogenolysis, glycogen, the stored form of glucose, is released as glucose. The process of synthesizing glycogen is termed glycogenesis and occurs when excess carbohydrates exist in the liver. [7]
Glucose tolerance is regulated with the circadian cycle. In the morning, humans typically have their peak glucose tolerance for metabolism. Afternoon and evenings are a trough for oral glucose tolerance. This trough likely occurs because pancreatic beta-cells are also most responsive in the morning—similarly, glycogen storage components peak in the evening. Adipose tissue is most sensitive to insulin in the afternoon. The varied timings of fuel utilization throughout the day compose the cycle of glucose metabolism. [8]
Glycolysis is the most crucial process in releasing energy from glucose, the end product of which is two molecules of pyruvic acid. It occurs in 10 successive chemical reactions, leading to a net gain of two ATP molecules from one molecule of glucose.
The overall efficiency for ATP formation is only approximately forty-three percent, with the remaining 57 percent lost in the form of heat. The next step is the conversion of pyruvic acid to acetyl coenzyme A. This reaction utilizes coenzyme A, releasing two carbon dioxide molecules and four hydrogen atoms. No ATP forms at this stage, but the four released hydrogen atoms participate in oxidative phosphorylation, later releasing six molecules of ATP. The next step is the breakdown of acetyl coenzyme A and the release of energy in the form of ATP in the Kreb cycle or the tricarboxylic acid cycle, taking place in the cytoplasm of the mitochondrion. [9]
- Related Testing
- HbA1c. The HbA1C value indicates a 2 to 3 month average of a patient’s glycemic control. Since the HbA1C value summarizes long-term glycemic control, it is frequently used to evaluate patients with long-standing hyperglycemia, as seen in patients with diabetes, and to forecast the risk of diabetic complications. [10]
- Fasting Plasma Glucose . Plasma blood glucose level is measured after a period of fasting, typically at least 8 hours. A value greater than 126 mg/dL is associated with diabetes. [11]
- Random Plasma Glucose. A random plasma glucose measurement is sampled sometime after dietary intake was last ingested. A value greater than 200 mg/dL is highly suggestive of diabetes. [12]
- Oral Glucose Tolerance Test. All pregnant women should receive gestational diabetes mellitus (GDM) screening through an orally consumed glucose challenge and subsequent plasma blood glucose measurement. [13]
- C-Peptide . C-peptide is a quantitative measurement of beta-cell function in an individual’s pancreas. Measured via urine or serum samples, a C-peptide value aids in the evaluation and management of diabetes. [14]
- Autoantibody . The presence of autoantibodies, including islet autoantibody, insulin autoantibody, insulinoma-associated antigen-2 autoantibodies, and anti-glutamic acid decarboxylase (GAD) autoantibodies, among others, are suggestive of auto-immune response as is seen in type 1 diabetes. [15] [16]
- Pathophysiology
Although not completely understood, Type 1 and Type 2 diabetes differ in their pathophysiology. Both are considered polygenic diseases, meaning multiple genes are involved, likely with multifactorial environmental influences, including gut microbiome composition and environmental pollutants, among others. Type 1 diabetes is the autoimmune destruction of the body’s pancreatic beta cells, which produce insulin hormone. Without the insulin hormone, the body is unable to regulate blood glucose control. Type 1 diabetes more commonly presents in childhood and persists through adulthood, equally affects males and females, and has the highest prevalence of diagnosis in European White race individuals. Life expectancy for an individual with Type 1 diabetes is reduced by an estimated 13 years.
Type 2 diabetes results when pancreatic beta cells cannot produce enough insulin to meet metabolic needs. Additionally, as adipose cells are deposited in the patient’s liver and muscle, insulin resistance becomes a prominent feature of Type 2 diabetes. Therefore, individuals with more adipose deposition, typically with higher body fat content and an obese BMI, more commonly have type 2 diabetes.
Type 2 diabetes is more common, as 95% of individuals with overall diabetes have Type 2 Diabetes. Type 2 diabetes is more common among adult and older adult populations; however, youth are demonstrating rising rates of type 2 diabetes. Type 2 diabetes is slightly more common in males (6.9%) than in females (5.9%). It is also more common in individuals of Native American, African American, Hispanic, Asian, and Pacific Islander race or ethnicity. [17]
- Clinical Significance
Poor glucose metabolism leads to diabetes mellitus. According to the American Diabetes Association, the prevalence of diabetes in the year 2015 was 9.4%, with people aged 65 and older forming the largest group. Every year, 1.5 million Americans receive a diagnosis of diabetes. As the seventh-highest cause of mortality in the United States, diabetes mellitus poses a concerning healthcare challenge with large amounts of yearly expenditures, morbidity, and death.
Diabetes is classified into two types-
Type 1 DM- due to deficient insulin secretion. Key features of this type are-
- Immune-mediated in over 90% of cases.
- It can occur in any age group but is most common in children and young adults.
- Circulating insulin is virtually absent, leading to a catabolic state with exogenous insulin required for treatment.
Type 2 DM- due to insulin resistance with a defect in compensatory insulin secretion. Key features of this type are-
- This condition occurs predominantly in adults but is now increasingly present in children and adolescents.
- Genetic and environmental factors combine, leading to insulin resistance and beta-cell loss.
- Treatment entails lifestyle changes and oral anti-diabetic drugs.
Uncontrolled diabetes poses a significantly increased risk of developing macrovascular disease, especially coronary, cerebrovascular, and peripheral vascular disease. It also increases the chances of microvascular disease, including retinopathy, nephropathy, and neuropathy. [18]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Diagram of the relationship between the processes of carbohydrate metabolism, including glycolysis, gluconeogenesis, glycogenesis, glycogenolysis, fructose metabolism, and galactose metabolism Contributed by Wikimedia User: Eschopp, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, (more...)
Disclosure: Mihir Nakrani declares no relevant financial relationships with ineligible companies.
Disclosure: Robert Wineland declares no relevant financial relationships with ineligible companies.
Disclosure: Fatima Anjum declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Nakrani MN, Wineland RH, Anjum F. Physiology, Glucose Metabolism. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Comments on metabolic needs for glucose and the role of gluconeogenesis. [Eur J Clin Nutr. 1999] Review Comments on metabolic needs for glucose and the role of gluconeogenesis. Brosnan JT. Eur J Clin Nutr. 1999 Apr; 53 Suppl 1:S107-11.
- Physiology, Glucose. [StatPearls. 2024] Physiology, Glucose. Hantzidiamantis PJ, Awosika AO, Lappin SL. StatPearls. 2024 Jan
- Physiology, Adenosine Triphosphate. [StatPearls. 2024] Physiology, Adenosine Triphosphate. Dunn J, Grider MH. StatPearls. 2024 Jan
- Review From the Photosynthesis to Hormone Biosynthesis in Plants. [Plant Pathol J. 2024] Review From the Photosynthesis to Hormone Biosynthesis in Plants. Choi HW. Plant Pathol J. 2024 Apr; 40(2):99-105. Epub 2024 Apr 1.
- Review [Methylglyoxal--test for biological dysfunctions of homeostasis and endoecology, low cytosolic glucose level, and gluconeogenesis from fatty acids]. [Ter Arkh. 2010] Review [Methylglyoxal--test for biological dysfunctions of homeostasis and endoecology, low cytosolic glucose level, and gluconeogenesis from fatty acids]. Titov VN, Dmitriev LF, Krylin VA. Ter Arkh. 2010; 82(10):71-7.
Recent Activity
- Physiology, Glucose Metabolism - StatPearls Physiology, Glucose Metabolism - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Carbohydrates: structure and Function
Jul 26, 2014
341 likes | 1.28k Views
Carbohydrates: structure and Function. By Dr. Amr S. Moustafa , MD, PhD. Objectives. To understand: The structure of carbohydrates of physiological significance The main role of carbohydrates in providing and storing of energy The structure and function of glycosaminoglycans. OVERVIEW.
Share Presentation
- complex carbohydrates
- functional sugar group
- structural formula
- carboxyl groups
- large complexes

Presentation Transcript
Carbohydrates: structure and Function By Dr. Amr S. Moustafa, MD, PhD
Objectives To understand: • The structure of carbohydrates of physiological significance • The main role of carbohydrates in providing and storing of energy • The structure and function of glycosaminoglycans
OVERVIEW Carbohydrates: The most abundant organic molecules in nature The empiric formula is (CH2O)n, “hydrates of carbon” Carbohydrates: provide important part of energy in diet Act as the storage form of energy in the body are structural component of cell membranes
OVERVIEW CONT’D • Many diseases associated with disorders of carbohydrate metabolism including: Diabetes mellitus Galactosemia Glycogen storage diseases Lactose intolerance
CLASSIFICATION • Monosaccharides: Simple sugar • Disaccharides: 2 monosaccharide units • Oligosaccharides: 3-10 monosaccharide units • Polysaccharides: more than 10 sugar units Homopolysaccharides & heteropolysaccharides
Monosaccharides Further classified based on: 1. No. of carbon atoms 2. Functional sugar group: Aldehyde group – aldoses Keto group – ketoses
Monosaccharides CONT’D
Disaccharides • Joining of 2 monosaccharides by O-glycosidic bond: Maltose (α-1, 4)= glucose + glucose Sucrose (α-1,2) = glucose + fructose Lactose (β-1,4) = glucose + galactose
Disaccharides CONT’D Lactose
Polysaccharides • Homopolysaccharides: Branched: Glycogen and starch (α-glycosidic polymer) Unbranched: Cellulose (β-glycosidic polymer) • Heteropolysaccharides: e.g., glycosaminoglycans (GAGs)
Isomerism • Isomers Compounds having samechemical formula but different structural formula The No. of isomers depends on the No. of asymmetric C
Aldo-Keto Isomers Example: Glucose (Aldose) and Fructose (Ketose)
Epimers • Epimers CHO dimers that differ in configuration around only one specific carbon atom -Glucose and galactose, C4 -Glucose and Mannose, C2 Galactose and mannose are not epimers
Enantiomers (D- and L-Forms) Structures that are mirror images of each other are designated as D- and L- sugars based on the position of –OH grp on the asymmetric carbon farthest from the carbonyl carbon Majority of sugars in humans are D-sugars
α- and β-Forms • Cyclization of Monosaccharides Monosaccharides with 5 or more carbon are predominantly found in the ring form • The aldehyde or ketone grp reacts with the –OH grp on the same sugar • Cyclization creates an anomericcarbon (former carbonyl carbon) generating the α and β configurations
Mutarotation In solution, the cyclic α and βanomers of a sugar are in equilibrium with each other, and can be interconverted spontaneously Fischer Projection Haworth Projection Fischer Projection
Sugar Isomers • Aldo-keto • Epimers • D- and L-Forms • α- and β-anomers
Reducing Sugars • If the O on the anomeric C of a sugar is not attached to any other structure (Free),that sugar can act as a reducing agent • Reducing sugars reduce chromogenic agents like Benedict’s reagent or Fehling’s solution to give a colored periceptate • Urine is tested for the presence of reducing sugars using these colorimetric tests
Reducing Sugars CONT’D • Examples: Monosaccharides Maltose and Lactose Sucrose is non-reducing, Why?
Complex Carbohydrates • Carbohydrates attached to non-carbohydrate structures by glycosidic bonds (O- or N-type) e.g., 1. Purine and pyrimidine bases in nucleic acids 2. Bilirubin 3. Proteins in glycoproteins and proteoglycans 4. Lipids found in glycolipids
Glycosidic Bonds • N-Glycosidic • O-Glycosidic
Glycosaminoglycans (GAGs) • Glycosaminoglycans (GAGs) are large complexes of negativelycharged heteropolysaccharide chains • are associated with a small amount of protein, forming proteoglycans, which consist of over 95 percent carbohydrate • bind with large amounts of water, producing the gel-like matrix that forms body's ground substance • The viscous, lubricating properties of mucous secretions also result from GAGs, which led to the original naming of these compounds as mucopolysaccharides
Glycosaminoglycans (GAGs) • GAGs are linear polymers of repeating disaccharide units [acidic sugar-amino sugar]n • The amino sugar (usually sulfated) is either D-glucosamine or D-galactosamine • The acidic sugar is either D-glucuronic acid or L-iduronic acid • GAGs are strongly negatively-charged: carboxyl groups of acidic sugars Sulfate groups
Resilience of GAGs Relationship between glycosaminoglycan structure and function • Because of negative charges, the GAG chains tend to be extended in solution and repel each other and when brought together, they "slip" past each other This produces the "slippery" consistency of mucous secretions and synovial fluid • When a solution of GAGs is compressed, the water is "squeezed out" and the GAGs are forced to occupy a smaller volume. When the compression is released, the GAGs spring back to their original, hydrated volume because of the repulsion of their negative charges This property contributes to the resilience of synovial fluid and the vitreous humor of the eye
Members of GAGs Examples of GAGs are: • Chondroitin sulfates: Most abundant GAG • Keratan sulfates: Most heterogeneous GAGs • Hyaluronic acid: Compared to other GAGs, it is unsulfated and not covalently attached to protein • Heparin: Unlike other GAGs that are extracellular, heparin is intracellular and serves as an anticoagulant
Take home Message Structure and function of carbohydrates • Mono-, Di-, and Poly-saccharides • Sugar Isomers: Aldo-keto, epimers, D- and L-, α- and β-anomers • Complex carbohydrates: e.g., Glycosaminoglycans and proteoglycans • Structure and function of GAGs • Examples of GAGs: chondroitin sulfate, keratin sulfate, hyaluronic acid and heparin
- More by User

Carbohydrates
Carbohydrates. By Henry Wormser, Ph.D. Professor of Medicinal Chemistry PSC 3110 Fall 2010. Reading in Garrett & Grisham textbook. Chapter 7 pages 205- 240 – (quite complete discourse on carbohydrate structure and function with some emphasis on cell surfaces)
3.33k views • 160 slides
Chapter 5a: The Structure and Function of Macromolecules (Carbohydrates)
Chapter 5a: The Structure and Function of Macromolecules (Carbohydrates). Important Point:. If you are having trouble understanding lecture material: Try reading your text before attending lectures. And take the time to read it well!. Many macromolecules consist of polymers
488 views • 26 slides
Ch. 7: Membrane Structure and Function
Ch. 7: Membrane Structure and Function. Introduction. The plasma membrane is selectively permeable . The macromolecules that make up the PM are lipids, proteins, and carbohydrates. Phospholipids make up most of the PM. Phospholipids are amphipathic molecules.
528 views • 38 slides

Biology EOC Highlight Review
Biology EOC Highlight Review. Courtesy of Mr. S. Russillo. Organic Compounds. All living things are made of organic compounds. Contain the element Carbon Carbohydrates, Proteins, Lipids, Nucleic Acids. Carbohydrates. Monomer- monosaccharide Function- energy source and structure
1.21k views • 102 slides
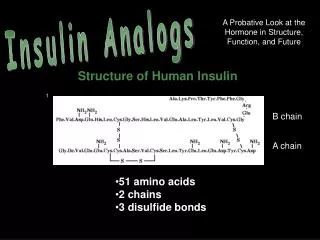
Insulin Analogs
Insulin Analogs. A Probative Look at the Hormone in Structure, Function, and Future. Structure of Human Insulin. 1. B chain. A chain. 51 amino acids 2 chains 3 disulfide bonds. Function of Human Insulin. Glucoregulation Cell Type Hormones Duration of Contact Metabolism Carbohydrates
351 views • 9 slides

Proteins: Structure and Function
Proteins: Structure and Function. Proteins. Cellular Overview Functions Key Properties Core Topics Amino Acids: properties, classifications, pI Primary Structure, Secondary Structure, and Motifs Tertiary Structure Fibrous vs. Globular Quaternary Structure. Amazing Proteins: Function.
1.09k views • 63 slides

UNDERLINED PURPLE WORDS MUST BE RECORDED IN YOUR DICTIONARY
UNDERLINED PURPLE WORDS MUST BE RECORDED IN YOUR DICTIONARY . OVERVIEW. WATER / DISSOLVING WHAT IS A BIOMACROMOLECULE CARBOHYDRATES STRUCTURE AND FUNCTION (Mono, di, polysaccharides) AMINO ACIDS PEPTIDE POLYPEPTIDE PROTEIN STRUCTURE AND FUNCTION NUCLEIC ACID RNA/DNA
596 views • 47 slides

Structure and Function
Structure and Function. Structure and Function in the Origin. S tructures developed through descent by modification modulated by natural selection. Connects structure with function through adaptation. Influence of Naturphilosophie. Archetype or Urplan Development of taxa within the plan.
616 views • 23 slides

1.12k views • 102 slides
Biochemistry
Biochemistry. Reviewing the 4 macromolecules of life!!! Objectives Describe the structure and functions of carbohydrates, lipids, proteins and nucleic acids Describe the structure and function of the cells membrane (phospholipid bilayer)
909 views • 26 slides

Carbohydrates. Annie Rotman, Allie Queler, Maddie Bovi, Max Coren and Nate Cole. Elements that are in Carbohydrates. There are 3 elements that make up carbohydrates: -Oxygen -Carbon -Hydrogen. Details about Structure.
489 views • 9 slides
Chapter 5- The Structure and Function of Macromolecules Carbohydrates
Chapter 5- The Structure and Function of Macromolecules Carbohydrates . AP BIOLOGY. Macromolecules . Carbohydrates Proteins Lipids Nucleic Acids . Carbohydrates. Polymer Vs. Monomer. Polymer : A large molecule made up of identical or similar building blocks Ex. Polysaccaride Starches
1.16k views • 42 slides

CARBOHYDRATES
CARBOHYDRATES. CARBOHYDRATES. It is the body’s most preferred energy source. CARBOHYDRATES. Provides energy to every living cells Also provides many vitamins and minerals that the body needs. CARBOHYDRATES. Building block—sugar molecules Single or chains. CARBOHYDRATES.
646 views • 28 slides

CARBOHYDRATES . Dr. Madushani Silva (MBBS) North Colombo Teaching Hospital – Ragama. Carbohydrates. Carbohydrates are called carbohydrates because they are essentially hydrates of carbon (i.e. they are composed of carbon and water and have a composition of (CH 2 O) n. .
1.01k views • 72 slides
Carbohydrates. Compare and contrast the four groups of organic molecules in terms of their structure and functions. Recognize the monomers of each group of organic molecules. Learning Targets:. Carbohydrates are compounds made up of carbon, hydrogen, and oxygen atoms.
1.1k views • 18 slides

Carbohydrates Review
Carbohydrates Review. 1 . What is a Carbohydrate ?. Carbohydrates. A carbohydrate is any of the group of organic compounds consisting carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1. Examples include sugar, starch, cellulose, and gums. . This structure represents a polymer.
304 views • 19 slides
Cell Structure and Function Notes
Cell Structure and Function Notes. Organelles Means “little organs” Specialized mini organs inside of a cell. Cell Membrane Structure Double layer sheet called a lipid bilayer Flexible and strong Proteins and carbohydrates are embedded in the lipid bilayer Function
672 views • 22 slides

Carbohydrates. AVS 305. Basic Structure. (CH 2 O) n Aldehyde or Ketone D form is biologically more abundant than L form CHOs make up most of the organic matter on earth. Roles. Energy DNA and RNA framework Structure Linked to proteins or lipids. Nomenclature. Monosaccharide
254 views • 13 slides

The IUPS Physiome Project Auckland Bioengineering Institute & Maurice Wilkins Centre
BMSW 2008, Bangalore, India. The IUPS Physiome Project Auckland Bioengineering Institute & Maurice Wilkins Centre University of Auckland, NZ. Genes. mRNA. Proteins Lipids Carbohydrates. Cell structure -function. Tissue structure -function. Organ structure -function. Clinical medicine.
538 views • 26 slides
2.6. Carbohydrates. 2.6. Carbohydrates. Carbohydrates are made from CARBON, HYDROGEN and OXYGEN. They STORE ENERGY in plants and animals. Plant cell walls depend on the structural role of some carbohydrates. CARBOHYDRATES. MONOSACCHARIDES. DISACCHARIDES. POLYSACCHARIDES.
631 views • 9 slides

Nutrient Information
Nutrient Information. Carbohydrates. What do carbohydrates do for us? 1 major function: Give you energy to run, jump and even blink your eyes. What are carbohydrates and where you get them:. Simple Carbohydrates: One to two glucose molecules linked together Found in :
432 views • 28 slides

1.13k views • 102 slides

COMMENTS
Glucose Structure - Diagrams, Examples, Physical ...
Structure Of Glucose and Fructose
6.1: Structure and Function- Carbohydrates
The molecular formula of glucose is C 6 H 12 O 6. The structure of the glucose molecule can be either a linear or a ring arrangement. In this animation, this symbol will represent a glucose molecule. Curious Minds is a Government initiative jointly led by the Ministry of Business, Innovation and Employment, the Ministry of Education and the ...
Chitin is made up of a modified b-D-glucose called N-acetylglucosamine with b(1→4) glycosidic bonds. Like cellulose, chitin is a strong material with many uses, one of which is a surgical thread that biodegrades as a wound heals. Chitin is present in many insects' exoskeletons and serves to protect them from water.
How to draw an Open-Chain Structure of the Glucose Molecule. The required steps to draw an acyclic form of glucose are: Step 1: Draw six carbon atoms. Step 2: Draw extended arms for all the carbon atoms. Step 3: Draw a hydrogen atom to carbon bond in such a way that four will be on one side, one on the other side.
Carbohydrates (article) | Chemistry of life
Pathophysiology of diabetes: An overview - PMC
Structure of Glucose, Preparation, Uses, Properties ...
Summary. Glucose is the most important monosaccharide that provides energy to cells present in our bodies. It is an aldohexose having an aldehydic group and multiple hydroxyl groups attached to six carbon atoms. Its structure can be represented by an open-chain structure or a closed ring. Glucose has 16 isomers.
Physiology, Glucose Metabolism - StatPearls
Presentation Transcript. Carbohydrates: structure and Function By Dr. Amr S. Moustafa, MD, PhD. Objectives To understand: • The structure of carbohydrates of physiological significance • The main role of carbohydrates in providing and storing of energy • The structure and function of glycosaminoglycans. OVERVIEW Carbohydrates: The most ...