- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Association between...
Association between antihypertensive treatment and adverse events: systematic review and meta-analysis
- Related content
- Peer review
- Ali Albasri , research fellow 1 ,
- Miriam Hattle , research associate 2 ,
- Constantinos Koshiaris , statistician 1 ,
- Anna Dunnigan , foundation year doctor 3 ,
- Ben Paxton , medical student 4 ,
- Sarah Emma Fox , medical student 4 ,
- Margaret Smith , senior statistician 1 5 ,
- Lucinda Archer , research associate 2 ,
- Brooke Levis , postdoctoral research fellow 2 ,
- Rupert A Payne , senior lecturer 6 ,
- Richard D Riley , professor of biostatistics 2 ,
- Nia Roberts , librarian 7 ,
- Kym I E Snell , lecturer 2 ,
- Sarah Lay-Flurrie , senior statistician 1 ,
- Juliet Usher-Smith , university lecturer 4 ,
- Richard Stevens , associate professor 1 ,
- F D Richard Hobbs , Nuffield professor of primary care 1 ,
- Richard J McManus , professor of primary care research 1 ,
- James P Sheppard , university research lecturer 1
- on behalf of the STRATIFY investigators
- 1 Nuffield Department of Primary Care Health Sciences, Radcliffe Primary Care Building, University of Oxford, Oxford, OX2 6GG, UK
- 2 School of Medicine, Keele University, Keele, UK
- 3 Oxford University Hospitals NHS Foundation Trust, Oxford, UK
- 4 Primary Care Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
- 5 NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
- 6 Centre for Academic Primary Care, Population Health Sciences, University of Bristol, Bristol, UK
- 7 Bodleian Health Care Libraries, University of Oxford, Oxford, UK
- Correspondence to: J P Sheppard james.sheppard{at}phc.ox.ac.uk (or @jamessheppard48 on Twitter)
- Accepted 14 January 2021
Objective To examine the association between antihypertensive treatment and specific adverse events.
Design Systematic review and meta-analysis.
Eligibility criteria Randomised controlled trials of adults receiving antihypertensives compared with placebo or no treatment, more antihypertensive drugs compared with fewer antihypertensive drugs, or higher blood pressure targets compared with lower targets. To avoid small early phase trials, studies were required to have at least 650 patient years of follow-up.
Information sources Searches were conducted in Embase, Medline, CENTRAL, and the Science Citation Index databases from inception until 14 April 2020.
Main outcome measures The primary outcome was falls during trial follow-up. Secondary outcomes were acute kidney injury, fractures, gout, hyperkalaemia, hypokalaemia, hypotension, and syncope. Additional outcomes related to death and major cardiovascular events were extracted. Risk of bias was assessed using the Cochrane risk of bias tool, and random effects meta-analysis was used to pool rate ratios, odds ratios, and hazard ratios across studies, allowing for between study heterogeneity (τ 2 ).
Results Of 15 023 articles screened for inclusion, 58 randomised controlled trials were identified, including 280 638 participants followed up for a median of 3 (interquartile range 2-4) years. Most of the trials (n=40, 69%) had a low risk of bias. Among seven trials reporting data for falls, no evidence was found of an association with antihypertensive treatment (summary risk ratio 1.05, 95% confidence interval 0.89 to 1.24, τ 2 =0.009). Antihypertensives were associated with an increased risk of acute kidney injury (1.18, 95% confidence interval 1.01 to 1.39, τ 2 =0.037, n=15), hyperkalaemia (1.89, 1.56 to 2.30, τ 2 =0.122, n=26), hypotension (1.97, 1.67 to 2.32, τ 2 =0.132, n=35), and syncope (1.28, 1.03 to 1.59, τ 2 =0.050, n=16). The heterogeneity between studies assessing acute kidney injury and hyperkalaemia events was reduced when focusing on drugs that affect the renin angiotensin-aldosterone system. Results were robust to sensitivity analyses focusing on adverse events leading to withdrawal from each trial. Antihypertensive treatment was associated with a reduced risk of all cause mortality, cardiovascular death, and stroke, but not of myocardial infarction.
Conclusions This meta-analysis found no evidence to suggest that antihypertensive treatment is associated with falls but found evidence of an association with mild (hyperkalaemia, hypotension) and severe adverse events (acute kidney injury, syncope). These data could be used to inform shared decision making between doctors and patients about initiation and continuation of antihypertensive treatment, especially in patients at high risk of harm because of previous adverse events or poor renal function.
Registration PROSPERO CRD42018116860.
Introduction
High blood pressure (hypertension) is one of the leading modifiable risk factors for cardiovascular disease worldwide, 1 and much healthcare resource is given to reducing blood pressure. In recent years, guidelines for hypertension management have recommended lower treatment targets 2 3 on the basis of trials that found benefit for cardiovascular risk reduction. 4 In patients with frailty and multimorbidity, however, these guidelines recommend clinical judgment because of potential risks from adverse effects of treatment. 3 5
In the UK, guidelines for managing patients with multimorbidity suggest doctors weigh the risk of diseases with the benefits and risks of treatments and make personalised treatment recommendations. 6 Such an approach is straightforward for the benefits of treatment when data exist from numerous meta-analyses of randomised controlled trials. 7 8 9 When attempting to judge the potential harms of treatment, however, few data are available to support decision making. Existing meta-analyses focus on the overall risk of adverse events, 10 11 making it difficult to distinguish between those events that might not be considered particularly serious, such as transient electrolyte abnormalities, and those resulting in severe complications and hospital admission, such as falls or acute kidney injury.
Currently few definitive data are available from meta-analyses of randomised controlled trials on the risks of specific harm outcomes that could be used to facilitate personalised decision making in patients with hypertension. We systematically reviewed evidence from trials and large observational studies to determine the association between antihypertensive treatment and specific adverse events such as falls, acute kidney injury, and electrolyte abnormalities.
We performed a systematic review and meta-analysis of randomised controlled trials and large observational studies examining the association between antihypertensive treatment and adverse events. The study is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. 12 The study protocol was registered on PROSPERO (international prospective register of systematic reviews) and is available online ( www.crd.york.ac.uk/prospero , CRD42018116860).
Search strategy
To capture all randomised controlled trials reporting the association between antihypertensive treatment and adverse events we searched Embase(OvidSP), Medline(OvidSP), Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library), and the Science Citation Index (Web of Science Core Collection). Searches were undertaken from inception of the databases until 14 April 2020, and no language restrictions were applied. In this review we focused on randomised controlled trials, which are less prone to bias from confounding by indication. 13 14 We also searched for large observational studies by interrogating the bibliographies of databases of electronic health records, but as few relevant data were identified and given the limitations of observational study designs we decided not to include them in the present study. Further studies were identified through searching the references of eligible full text articles and previous meta-analyses. Supplementary table 1 shows the full search strategy.
Selection of studies and inclusion and exclusion criteria
Eligible studies included participants aged 18 years or older, compared individuals receiving antihypertensive treatment (single agents) with those receiving placebo or no treatment, more antihypertensive drugs compared with fewer antihypertensive drugs, or one blood pressure target compared with another. Although these study designs examine different types of intervention, all compared more antihypertensive treatment with less antihypertensive treatment, enabling the potential association with adverse events to be determined. Trials were also required to present data describing the association between antihypertensive treatment and at least one adverse event. Randomised controlled trials were included if they reported 50 or more adverse events in each specific category or had at least 650 patient years of follow-up.
To ensure study selection and data analysis remained manageable by avoiding small, early phase mechanistic studies, we specified a priori the limit on patient years of follow-up and number of outcome events. We chose the specific criteria to ensure each included study was large enough to accrue outcome events and provide reliable effect estimates. These criteria assumed an incidence of the primary outcome (falls) of 7.8 events per 100 patient years of follow-up, which would accrue at least 50 outcome events in each study. 15
We excluded studies in specialist populations (children, pregnant women), and case reports, case series, or before and after studies. At least two members of the review team (AA, MS, BP, SF, CK, AD, JPS) independently reviewed study titles, abstracts, and full text articles. At each stage, the entire review team screened a proportion of articles to ensure consistency of decision making. Disagreements were resolved by a third reviewer (JPS).
Outcome measures
Outcomes of interest were prespecified based on those reported in recent large scale trials of blood pressure lowering treatment. 4 16 17 The primary outcome was falls, at any time point and by any definition given in the original study. Secondary outcomes were acute kidney injury, fractures, gout, electrolyte abnormalities (changes in potassium), hypotension, and syncope (eg, fainting) at any time point during trial follow-up. Acute kidney injury was defined as any outcome reported according to the KDIGO (kidney disease: improving global outcomes) definition. 18 All other outcomes were defined according to definitions given in the original study. Additional treatment efficacy outcomes of interest included cardiovascular death, myocardial infarction, stroke, and all cause mortality.
Data extraction and quality assessment
AA, MH, LA, AD, and BL extracted data from eligible studies. Two reviewers independently entered outcome data into a Microsoft Excel spreadsheet (2016 version, Redmond, WA). A second reviewer then manually cross checked these, referring to the original source data when discrepancies were identified. After an initial consistency check involving extraction of data from 10 articles, one reviewer extracted study descriptive data.
Data were extracted on populations studied, interventions tested, length of follow-up, effect measures (estimates and confidence intervals for rate ratios, odds ratios, and hazard ratios), and numbers of patients experiencing adverse events and cardiovascular or mortality outcomes.
The methodological quality and risk of bias of individual studies was assessed using the Cochrane risk of bias tool (for randomised controlled trials). 19
Data synthesis
Summary effect estimates describing the association between all antihypertensive drug classes (combined) and adverse events were derived using a random effects meta-analysis. For uncommon adverse events (approximately less than 10% of the population experience an event), rate ratios (for rate outcomes), odds ratios (for binary outcomes), and hazard ratios (for time-to-event outcomes) were considered reasonably similar and combined provided they had the same directional interpretation. 20 For uncommon outcomes, we label summary effect estimates as risk ratios. For more common cardiovascular disease outcomes, we synthesised rate ratios, odds ratios, and hazard ratios separately. We used restricted maximum likelihood estimation to fit the random effects model, with 95% confidence intervals derived using the Hartung-Knapp approach to account for uncertainty in heterogeneity estimates. 21 For studies with three treatment arms, we split binary and rate outcomes for the control arm into two equal groups. 22 This approach is not possible for the time-to-event outcomes, and therefore we made an approximate adjustment to the standard errors.
Heterogeneity was summarised using the estimate of between study variance (τ 2 ) and 95% prediction intervals for the treatment effect in a new study. The proportion of variability in effect estimates due to between study heterogeneity was summarised using I 2 .
Sensitivity analyses were undertaken focusing on adverse events reported as a reason for study withdrawal. Meta-regression was used to examine the association between observed treatment effects and study quality. Small study effects (potential publication bias) were explored using contour enhanced funnel plots for outcomes reported in 10 or more studies. 23 Prespecified subgroup analyses were conducted to examine the association between treatment and adverse events by antihypertensive drug class.
No other subgroup analyses were undertaken by patient level characteristics (eg, age), owing to the risk of ecological bias. 24 Aggregate data only allow relationships across studies to be examined, but these often do not reflect within study (participant level) relationships, because of aggregation bias and study level confounding. 25 26 For example, those studies with a higher mean age might also have a longer mean follow-up or a higher dose of the drug; hence it is difficult to disentangle these different associations, and interpreting across study associations as if they were interactions at the individual level is potentially misleading.
All analyses were undertaken using Stata version 16 (StataCorp, College Station, TX).
Patient and public involvement
This study was developed with the help of our patient and public advisor. As a member of our study advisory group, they commented on the study protocol. We also held a focus group with seven older adults during the study to discuss broader issues related to drugs for cardiovascular disease prevention and adverse events, which informed the interpretation of this work.
Study selection and characteristics
A total of 15 023 unique articles were identified from the literature searches, of which 119 records were screened from reference lists of included articles and previous meta-analyses. After screening of the title, abstract, and full text, 63 articles originating from 58 randomised controlled trials 4 16 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 were eligible for inclusion ( fig 1 ). The most common reason for exclusion at full text screening was lack of adverse event reporting (n=108) or inclusion of too few patient years of follow-up (n=104).

Selection of studies for inclusion in review. *Hand searches of reference lists of included studies and recent meta-analyses of blood pressure lowering trials 7 8 9
- Download figure
- Open in new tab
- Download powerpoint
A total of 280 638 participants were included in the primary analyses from 58 unique randomised controlled trials. Forty eight studies compared a single drug treatment with placebo and 10 studies compared a high blood pressure target with a lower blood pressure target in the intervention and control groups ( table 1 ). The remaining five studies either compared treatment with no treatment or compared multiple drugs with a single drug. The median duration of follow-up in the trials was 3 (interquartile range 2-4) years. Most studies were conducted in patients with at least one risk factor for cardiovascular disease in addition to hypertension.
Summary of included randomised controlled trials
- View inline
Quality assessment
Supplementary table 2 presents the risk of bias assessment for individual trials. Most of the trials (n=40, 69%) had a low risk of bias ( fig 2 ). Eight trials (14%) did not adequately blind outcome assessment of adverse events (or did not describe this adequately) and 12 (21%) did not adequately describe the randomisation process. Outcome reporting was complete in 52 trials (90%) trials.

Summary of risk of bias assessment across all included randomised controlled trials
Primary outcome
Seven randomised controlled trials reported data for the primary outcome of falls ( fig 3 ). Data were available from 29 481 patients experiencing 1790 events. Overall, no evidence was found of an association between antihypertensive treatment and falls (summary risk ratio 1.05, 95% confidence interval 0.89 to 1.24). Little evidence was found of between study heterogeneity in this association (τ 2 =0.009; I 2 =31.5%; P=0.372). Subgroup analyses by drug type did not reveal any evidence of associations between falls and specific antihypertensive drug classes, except for thiazide diuretics, although this was based on data from just one trial (supplementary figure 1). 71 More intensive treatment (ie, to lower blood pressure targets) was not associated with falls across four trials (supplementary figure 1).

Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and falls
Secondary outcomes
In analyses examining adverse events across all drug classes, antihypertensive treatment was associated with an increased risk of acute kidney injury (summary risk ratio 1.18, 95% confidence interval 1.01 to 1.39, n=15 studies; fig 4 ), hyperkalaemia (1.89, 1.56 to 2.30, n=26 studies), hypotension (1.97, 1.67 to 2.32, n=35 studies), and syncope (1.28, 1.03 to 1.59, n=16 studies) ( table 2 ; supplementary figures 2-4), although statistical heterogeneity was significant for most outcomes (τ 2 =0.037 to 1.374; I 2 =42.9% to 85.1%). Evidence was unclear of an association between antihypertensive treatment and fractures (0.93, 0.58 to 1.48, τ 2 =0.062, I 2 =53.8%, n=5 studies; supplementary figure 5) and gout (1.54, 0.63 to 3.75, τ 2 =1.612, I 2 =94.3%, n=12 studies; supplementary figure 7), although confidence intervals were wide, partly reflecting large between study heterogeneity.

Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and acute kidney injury
Main analyses showing meta-analysis results from trials reporting the association between antihypertensive treatment and adverse events and cardiovascular and mortality outcomes
Analyses of outcomes by specific drug class showed that drugs affecting the renin angiotensin-aldosterone system were associated with acute kidney injury (1.26, 1.03 to 1.56, τ 2 =0.030, I 2 =39.0%; n=9 studies; table 3 , supplementary figure 8) and hyperkalaemia (2.03, 1.67 to 2.48, τ 2 =0.063, I 2 =51.0%; n=20 studies; table 3 , supplementary figure 9). These effects were larger and had less between study heterogeneity than in analyses examining the association between all antihypertensive treatments and the same outcomes ( table 2 and table 3 ). Only a small number of studies assessed the association between diuretics and hypokalaemia (three studies) or gout (five studies), and the results of these were inconclusive ( table 3 ; supplementary figures 10 and 11). No other drug class specific associations with adverse events were observed in the stratified analyses (supplementary figures 12-14).
Summary of sensitivity analyses showing important drug class specific associations between antihypertensive treatment and specific adverse events
Cardiovascular and mortality outcomes
On average across studies examining outcomes using time-to-event analyses, antihypertensive treatment was associated with a reduction in cardiovascular death (hazard ratio 0.92, 95% confidence interval 0.86 to 0.99, τ 2 =0.011, I 2 =54.6%, n=21 studies; fig 5 ), all cause mortality (0.93, 0.88 to 0.98, τ 2 =0.008, I 2 =50.4%, n=32 studies; supplementary figure 15), and stroke (0.84, 0.76 to 0.93, τ 2 =0.013, I 2 =44.8%, n=17; supplementary figure 16) ( table 2 ). No clear evidence was found of an association between antihypertensive treatment and myocardial infarction (supplementary figure 17).

Random effects meta-analysis of randomised controlled trials examining the association between antihypertensive treatment and cardiovascular death
Sensitivity analyses
Meta-regression examining the relation between the observed treatment effects for each adverse event outcome and study quality found no clear evidence of an association (supplementary table 3). Funnel plots showed asymmetry (potential publication bias) for hyperkalaemia and hypotension events, with smaller studies missing for smaller effect estimates, but this was not evident for other adverse events examined (supplementary figures 18-22).
Supplementary figures 23-27 show the results of sensitivity analyses focusing on studies reporting adverse events that led to participant withdrawal from each trial (summarised in table 4 ). These analyses were limited to studies reporting acute kidney injury, gout, hyperkalaemia, hypotension, and syncope owing to availability of data. In these analyses, summary risk ratios for hyperkalaemia, hypotension, and syncope were increased compared with the primary analysis including all studies. However, there was no longer evidence that acute kidney injury was associated with antihypertensive treatment ( table 4 ).
Sensitivity analyses showing meta-analysis results focusing on trials reporting the association between antihypertensive treatment and adverse events which led to permanent withdrawal from a trial
Data from random effects meta-analyses of 58 randomised controlled trials and more than 280 000 patients with hypertension confirm the known benefit of antihypertensive treatment in reducing the risk of cardiovascular disease. 7 8 9 These data also confirm the association between antihypertensive treatment and adverse events 10 11 and show how this association varies across some drug classes and for mild (eg, hypotension without falls) and more severe (eg, acute kidney injury, syncope) adverse events. Despite a widely held belief, 95 96 no association was found between treatment and falls, but an association with syncope was observed, which is important as this can have a major impact on quality of life and health service use and could even result in death. 97 98 99 100
These data will inform shared decision making around initiation and continuation of antihypertensive treatment, especially in patients with a high absolute risk of certain adverse outcomes as a result of previous events or poor renal function. Such discussions will become increasingly important as patients age and develop frailty and multimorbidity that could put them at increased risk of adverse events. 101 102 103
Strengths and limitations of this study
More than 15 000 articles were screened for inclusion in this review and 58 randomised controlled trials including a large number of participants and adverse events were identified. Although power was likely to be sufficient to detect associations between antihypertensive treatment and adverse events, we observed statistically significant heterogeneity across studies, and the resulting prediction intervals were wide. Such heterogeneity might preclude pooling of some treatment effects, so caution should be exercised when interpreting the results. For acute kidney injury and hyperkalaemia events, the observed heterogeneity was partly explained by pooling of different drug classes, and heterogeneity was reduced when we focused on drugs that affect the renin angiotensin-aldosterone system. For other outcomes, the observed heterogeneity could not be explained by study quality or differences in the drug class examined in individual trials; however, populations of interest, interventions, comparators, and study designs varied widely across studies, which could have contributed to the observed variation.
As this review focused on adverse events, selective outcome reporting might also have been a problem. Evidence was found of publication bias for certain outcomes (hyperkalaemia and hypotension), confirming the findings of previous studies that showed adverse events are more likely to be reported in randomised controlled trials when they are statistically significant. 104 This is understandable in the context of single trial reporting, but it would be better for the evidence base if all adverse events were reported in clinical trials to enable more complete meta-analyses in the future. It is a limitation of this review that original study authors were not contacted for these additional data.
This review focused on large randomised controlled trials with the aim of including those with at least 50 adverse events (and therefore 650 patient years of follow-up). This restriction on study size was chosen to make the review more manageable in terms of screening and analysis and avoid inclusion of numerous small early phase mechanistic studies of varying methodological quality. The cut-off for this inclusion was chosen to ensure studies provided adequately powered estimates of association between treatment and outcomes. 15 It is possible that some useful trials could have been excluded, although many relevant trials were still available for inclusion.
Across all included trials, adverse events were poorly defined and probably varied across studies. For instance, many studies referred to syncope as an outcome, but did not say what type of syncopal event this might have included. A conservative approach to inclusion of outcomes was taken when possible, and only those explicitly stating the outcome of interest were included. For example, trials reporting hypotension or acute kidney injury were included, but those reporting hypotension or dizziness or renal impairment were excluded. Despite this approach, some studies were included that did not specify the thresholds used to define hypotension or acute kidney injury. This could have resulted in some relevant data for certain outcomes being missed, but this meant those that were included were likely to be sufficiently similar to enable pooling in a meta-analysis. Although the quality of adverse event ascertainment is likely to have varied between trials, it would not be expected to vary between treatment arms within trials. Thus it is unlikely that differences in the quality of adverse event ascertainment would have affected the relative treatment effects presented in this review.
We prespecified adverse events of interest based on those reported in recent large scale trials of blood pressure lowering treatment. 4 16 17 Other patient focused harm outcomes, such as weight gain, sexual dysfunction, fatigue, and exercise intolerance might exist that were reported in the original trials but not captured as part of this review. However, the reporting of these events is likely to vary because many have no standardised definitions. 105 106 107 Some might be captured but not reported. 108 It is also important to note that randomised controlled trials often select populations with less frailty and multimorbidity who are more likely to tolerate treatment. 109 Therefore, fewer adverse events might have been reported in the included trials than would be expected in the general population.
For outcomes included in meta-analyses, the time points at which they occurred varied across studies, and so the risk ratios and odds ratios provided relate to a summary across different times. We did synthesise hazard ratios when available, but these were rarely reported.
Comparison with other studies
Few previous meta-analyses have quantified the association between antihypertensive treatment and adverse events. Thomopoulos and colleagues examined the association between antihypertensive treatment and permanent discontinuation of treatment because of adverse events and found that antihypertensives were associated with a near doubling of risk (standardised relative risk 1.89, 95% confidence interval 1.51 to 2.39). 10 110 This was similar to findings from our sensitivity analyses focusing on permanent withdrawal as a result of hyperkalaemia, hypotension, and syncope events. These associations were stronger than those observed in the primary analysis focusing on all adverse event reporting. It is possible that these events were more likely to be reported in the intervention group when they were considered serious enough to lead to withdrawal. 104 Although the focus of this review was on adverse events, we found evidence for the beneficial effects of treatment on all cause mortality, cardiovascular mortality, and stroke, but not on myocardial infarction, as has been reported previously. 4 8
Frey and colleagues 11 focused on data from seven original studies investigating the harms of intensive blood pressure lowering targets (≤130 mm Hg) versus usual care (<140 mm Hg). Although this number of studies was insufficient to conduct a meta-analysis, the descriptive summary suggested that intensive blood pressure lowering might be associated with higher rates of serious adverse events. The present analysis included all trials of blood pressure lowering treatment enabling meta-analyses of the association between antihypertensive treatment and adverse events and how this association varies across mild and more severe adverse events. We identified an increased risk of acute kidney injury, hyperkalaemia, hypotension, and syncope with antihypertensive treatment.
Stratified analyses by drug class suggested that associations with acute kidney injury and hyperkalaemia were mostly driven by the use of drugs that affect the renin angiotensin-aldosterone system (eg, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and direct renin inhibitors). However, no evidence was found of an association with this class of drug and falls, fractures, gout, or hypokalaemia. In analyses that focused on patients prescribed diuretics, a 10-fold increase in the risk of hypokalaemia was observed, but this association was derived from only three trials, with high between study heterogeneity. The pooled effect had large confidence intervals and was not statistically significant. This null finding contrasted with previous studies that recommend routine monitoring of potassium to detect hypokalaemia in patients prescribed diuretics. 111 This could be explained by the small number of included trials examining this drug class.
Much debate exists in the literature on the association between antihypertensive treatment and falls. 95 96 112 113 Most data showing an association originate from observational studies, 112 113 which are prone to bias from confounding by indication. 14 Despite conflicting evidence, a wide held belief remains that antihypertensive treatment increases the risk of falls. 95 96 This study found no evidence for an association between treatment or lower blood pressure targets and falls, but an association was found with syncope. Although syncope is a common cause of falls, not all falls are caused by syncope and therefore not all falls will be related to blood pressure lowering treatment. 114 In addition, reporting of falls might vary among participants (ie, not all participants will be admitted to hospital or see their primary care doctor after a fall) and participants might be more likely to be withdrawn from a trial when experiencing events that could be considered precursors to falls and fractures (eg, hypotension). If this were the case and hypotension events are not dealt with by treating doctors, the incidence of serious falls and fractures associated with antihypertensive treatment could be greater in routine clinical practice.
Policy implications
The present data clearly show the benefits and harms of antihypertensive treatment for specific cardiovascular outcomes and adverse events. The data also highlight that certain adverse events might be specific to certain drug classes (eg, renin angiotensin-aldosterone system drugs and acute kidney injury or hyperkalaemia). This detail is important because some adverse events reported in randomised controlled trials might be considered relatively mild and worth the risk when weighed against the substantial benefits of treatment. These new data will allow patients and clinicians to take into consideration these benefits and risks, as has been recommended in clinical guidelines. 6 This is particularly important now that guidelines for the management of hypertension across the world increasingly recommend more intensive treatment, 2 3 5 115 but with conflicting blood pressure targets, meaning a personalised approach is required for each patient.
The present data should ideally be combined with information about an individual’s absolute risk of each harm outcome to make informed, personalised treatment decisions. This process is complex and requires real time data, which suggests that tools embedded in electronic health records will be the way forward. Further work is needed to understand better the results of this meta-analysis (which summarises average risk ratios across all participants and studies) in the context of individualised absolute risks so that treatment initiation and discontinuation can be targeted at those with the most to gain. 116 In the absence of such information, doctors should focus on patients who have experienced previous adverse events or have poor renal function. 17 110 117
Conclusions
This review found no evidence of an association between antihypertensive treatment and falls (primary outcome) or fractures but did show a variation in the association between antihypertensive treatment and mild (eg, hypotension without falls) and more severe (eg, acute kidney injury, syncope) adverse events. Some effects were found to be specific to the drug class used. In patients at high risk of drug harms because of previous adverse events or poor renal function, these data should be used to inform shared decision making between doctors and patients around initiation and continuation of antihypertensive treatment.
What is already known on this topic
Many meta-analyses exist of randomised controlled trials that examine the efficacy of antihypertensive treatment, but few have studied potential harms
Existing meta-analyses have focused on the association between antihypertensive treatment and all adverse events, grouping mild and more serious outcomes
The association between antihypertensive treatment and specific adverse events is unclear
What this study adds
In a meta-analysis of 58 randomised controlled trials, including 280 638 participants, no evidence was found of an association between antihypertensive treatment and falls (primary outcome) or fractures
Evidence was, however, found of an association between antihypertensive treatment and potentially both mild (hypotension) and more severe (acute kidney injury, syncope) adverse events
These data might be used to inform shared decision making between doctors and patients about the benefits and harms of initiation and continuation of antihypertensives, especially in those at high risk of harm because of previous adverse events or poor renal function
Acknowledgments
We thank Margaret Ogden for her advice as a patient and public contributor to this project, and Lucy Curtin for administrative support throughout the project.
The STRAtifying Treatments In the multi-morbid Frail elderlY (STRATIFY) investigators include the authors and: Amitava Banerjee, associate professor in clinical data science and honorary consultant cardiologist, Institute of Health Informatics, University College London; Andrew Clegg, professor of geriatric medicine, University of Leeds and Bradford Teaching Hospitals NHS Foundation Trust; John Gladman, professor of medicine of older people, School of Medicine, University of Nottingham; Simon Griffin, professor of primary care, Department of Public Health and Primary Care, Primary Care Unit, University of Cambridge; and Margaret Ogden, patient and public involvement advisor.
Contributors: JPS conceived the study and wrote the protocol with FDRH, RJM, RS, and RR. NR did the literature searches. AA, MS, BP, SF, CK, AD, and JPS screened articles for inclusion. MH, AA, LA, AD, and BL extracted data for analysis. MH undertook the meta-analysis and produced forest plots and summary results, under supervision of RR. AA and JPS wrote the first draft of the manuscript. All authors revised the manuscript and approved the final version. JPS is the guarantor for this work and accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author (JPS) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the Wellcome Trust and Royal Society through a Sir Henry Dale fellowship held by JPS (ref 211182/Z/18/Z) and the National Institute for Health Research (NIHR) School for Primary Care (project 430). JPS also receives funding through an NIHR Oxford Biomedical Research Centre (BRC) senior fellowship. RJMcM is supported by an NIHR senior investigator award. FDRH acknowledges part support from the NIHR SPCR, the NIHR CLAHRC Oxford, and the NIHR Oxford BRC. BL is supported by a Fonds de recherche du Québec – Santé Postdoctoral Training Fellowship. KIES is funded by an NIHR School for Primary Care Research launching fellowship. MS is supported by the NIHR Oxford BRC. SLF is part funded by the NIHR Oxford BRC and NIHR Applied Research Collaborations Oxford and Thames Valley. JUS was funded by a Cancer Research UK Prevention fellowship (C55650/A21464). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The sponsor and funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: authors had financial support from the Wellcome Trust, Royal Society, Cancer Research UK, Fonds de recherche du Québec–Santé and National Institute for Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
The manuscript’s guarantor (JPS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Data sharing: Requests for data sharing should be sent to the corresponding author at [email protected].
Dissemination to participants and related patient and public communities: No participants were included in this work. The findings of this work, including a lay summary of the results, will be made available on the study website ( www.phc.ox.ac.uk/research/stratified-treatments/studies/stratifying-treatments-in-the-multi-morbid-frail-elderly-stratify-antihypertensives ).
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- Lewington S ,
- Qizilbash N ,
- Collins R ,
- Prospective Studies Collaboration
- Whelton PK ,
- Aronow WS ,
- Williams B ,
- Spiering W ,
- ESC Scientific Document Group
- Wright JT Jr . ,
- Williamson JD ,
- SPRINT Research Group
- ↵ National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. NICE guideline [NG136]. https://www.nice.org.uk/guidance/ng136 . Published 2019.
- ↵ NICE. Overview | Multimorbidity: clinical assessment and management | Guidance | NICE. 2016. https://www.nice.org.uk/guidance/ng56 . Accessed May 29, 2020.
- Freeman M ,
- Brunström M ,
- Ettehad D ,
- Thomopoulos C ,
- Zanchetti A
- Gravestock I ,
- Pichierri G ,
- Steurer J ,
- Burgstaller JM
- Liberati A ,
- Tetzlaff J ,
- Altman DG ,
- PRISMA Group
- Caparrotta TM ,
- Colhoun HM ,
- Freemantle N ,
- Marston L ,
- Walters K ,
- Reynolds MR ,
- Stalenhoef PA ,
- Diederiks JP ,
- de Witte LP ,
- Schiricke KH ,
- Crebolder HFJ
- Cushman WC ,
- Byington RP ,
- ACCORD Study Group
- López-Jaramillo P ,
- HOPE-3 Investigators
- ↵ Section 2: AKI Definition . Kidney Int Suppl (2011) 2012 ; 2 : 19 - 36 . doi: 10.1038/kisup.2011.32 . pmid: 25018918 OpenUrl CrossRef PubMed
- Higgins JPT ,
- Gøtzsche PC ,
- Cochrane Bias Methods Group ,
- Cochrane Statistical Methods Group
- Perneger TV
- Hartung J ,
- Schwarzer G
- Sterne JA ,
- Sutton AJ ,
- Ioannidis JP ,
- Fisher DJ ,
- Carpenter JR ,
- Morris TP ,
- Freeman SC ,
- Debray TPA ,
- Friedman LM
- Verdecchia P ,
- Staessen JA ,
- Cardio-Sis investigators
- Chinese Cardiac Study (CCS-1) Collaborative Group
- Pfeffer MA ,
- Swedberg K ,
- CHARM Investigators and Committees
- McMurray JJV ,
- Östergren J ,
- Granger CB ,
- Hunsicker LG ,
- Clarke WR ,
- Collaborative Study Group
- Kjekshus J ,
- Rasmussen K ,
- Morimoto T ,
- DIME Investigators
- van Gijn J ,
- Jaap Kappelle L ,
- van Latum CJ ,
- Koudstaal PJ ,
- The Dutch TIA Trial Study Group
- African American Study of Kidney Disease and Hypertension Study Group
- EMPHASIS-HF Study Group
- Bertrand M ,
- Ferrari R ,
- EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators
- ↵ GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico . Lancet 1994 ; 343 : 1115 - 22 . https://www.ncbi.nlm.nih.gov/pubmed/7910229 . pmid: 7910229 OpenUrl PubMed Web of Science
- Disertori M ,
- Barlera S ,
- GISSI-AF Investigators
- Stratton I ,
- Gerstein HC ,
- Heart Outcomes Prevention Evaluation Study Investigators
- Beckett N ,
- Margolis KL ,
- Palermo L ,
- Vittinghoff E ,
- Wakefield DB ,
- Moscufo N ,
- Massie BM ,
- Carson PE ,
- McMurray JJ ,
- I-PRESERVE Investigators
- The MACB Study Group
- Hjalmarson A ,
- Goldstein S ,
- Fagerberg B ,
- MERIT-HF Study Group
- Greenberg G ,
- Medical Research Council Working Party
- Bigger JT ,
- Multicenter Diltiazem Postinfarction Trial Research Group
- Holman RR ,
- Haffner SM ,
- NAVIGATOR Study Group
- Desmet WJ ,
- Coussement P ,
- Maschio G ,
- Alberti D ,
- The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group
- Segurado R ,
- Kennelly S ,
- NILVAD Study Group
- ONTARGET Investigators
- ORIENT study investigators
- Braunwald E ,
- Domanski MJ ,
- Fowler SE ,
- PEACE Trial Investigators
- Diener HC ,
- PRoFESS Study Group
- MacMahon S ,
- Tzourio C ,
- PROGRESS Collaborative Group
- Izzo JL Jr . ,
- ROADMAP Trial Investigators
- ROADMAP investigators
- Silverman A ,
- Flather MD ,
- Shibata MC ,
- Coats AJS ,
- SENIORS Investigators
- Chalmers J ,
- Woodward M ,
- SHEP Cooperative Research Group
- Franse LV ,
- Di Bari M ,
- Applegate WB
- Hood WB Jr . ,
- SOLVD Investigators
- Benavente OR ,
- Coffey CS ,
- SPS3 Study Group
- Norwegian Multicenter Study Group
- Anderson C ,
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators
- Nesbitt SD ,
- Trial of Preventing Hypertension (TROPHY) Study Investigators
- Emanuele N ,
- VA NEPHRON-D Investigators
- Tognoni G ,
- Valsartan Heart Failure Trial Investigators
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators
- Velazquez EJ ,
- Valsartan in Acute Myocardial Infarction Trial Investigators
- Neurath HM ,
- Goldman AI ,
- Beckett NS ,
- Fletcher AE ,
- HYVET Study Group
- Parving HH ,
- Brenner BM ,
- ALTITUDE Investigators
- Solomon SD ,
- Aliskiren Study in Post-MI Patients to Reduce Remodeling (ASPIRE) Investigators
- Eichhorn EJ ,
- Krause-Steinrauf H ,
- Bristow MRLP ,
- Lavori PW ,
- Beta-Blocker Evaluation of Survival Trial Investigators
- Contreras G ,
- Birkenhäger W ,
- Torp-Pedersen C ,
- Carlsen JE ,
- Trandolapril Cardiac Evaluation (TRACE) Study Group
- Sleight P ,
- Dagenais G ,
- Benetos A ,
- Petrovic M ,
- Strandberg T
- O’Brien H ,
- Anne Kenny R
- Marrison VK ,
- Fletcher A ,
- da Silva RM
- Tinetti ME ,
- Rossignol P ,
- Mansfield KE ,
- Bhaskaran K ,
- Tomlinson LA
- Hróbjartsson A ,
- Phillips R ,
- Cornelius V
- Boutron I ,
- Finsterer J ,
- Sheppard JP ,
- Clayton JA ,
- Rodgers S ,
- Kahlaee HR ,
- Schneider CR
- Lipsitz LA ,
- Habtemariam D ,
- Humphrey LL ,
- Forciea MA ,
- Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians
- OPTIMISE Investigators
- Souverein PC ,
- Van Staa TP ,
- Egberts AC ,
- De la Rosette JJ ,
- Leufkens HG
New drug targets for hypertension: A literature review
Affiliations.
- 1 State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, People's Republic of China.
- 2 Heart Center and Beijing Key Laboratory of Hypertension, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, People's Republic of China. Electronic address: [email protected].
- 3 State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, People's Republic of China; Hypertension Center, Fuwai Hospital, State Key Laboratory of Cardiovascular Disease of China, National Center for Cardiovascular Diseases of China, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, People's Republic of China. Electronic address: [email protected].
- PMID: 33309796
- DOI: 10.1016/j.bbadis.2020.166037
Hypertension is one of the most prevalent cardiovascular diseases worldwide. However, in the population of resistant hypertension, blood pressure is difficult to control effectively. Moreover, antihypertensive drugs may have adverse effect currently. Hence, new therapeutic targets and treatments are needed to uncovered and exploited to control hypertension and its comorbidities. In the past, classical drug targets, such as the aldosterone receptor, aldosterone synthase, and ACE2/angiotensin 1-7/Mas receptor axis, have been investigated. Recently, vaccines and drugs targeting the gastrointestinal microbiome, which represent drug classes, have also been investigated for the management of blood pressure. In this review, we summarized current knowledge on classical and new drug targets and discussed the potential utility of new drugs in the treatment of hypertension.
Keywords: Aldosterone receptor antagonist; Angiotensin II; Blood pressure; Drug target; Hypertension.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Antihypertensive Agents / pharmacology*
- Antihypertensive Agents / therapeutic use
- Drug Development
- Drug Discovery*
- Gastrointestinal Microbiome / drug effects
- Hypertension / drug therapy*
- Hypertension / metabolism
- Hypertension / microbiology
- Hypertension / physiopathology
- Molecular Targeted Therapy*
- Renin-Angiotensin System / drug effects
- Signal Transduction / drug effects
- Antihypertensive Agents
- Open access
- Published: 27 March 2016
A review on prescribing patterns of antihypertensive drugs
- Noah Jarari 1 ,
- Narasinga Rao 2 ,
- Jagannadha Rao Peela ORCID: orcid.org/0000-0002-3782-4658 3 ,
- Khaled A. Ellafi 4 ,
- Srikumar Shakila 3 ,
- Abdul R. Said 3 ,
- Nagaraja Kumari Nelapalli 5 ,
- Yupa Min 6 ,
- Kin Darli Tun 7 ,
- Syed Ibrahim Jamallulail 8 ,
- Avinash Kousik Rawal 9 ,
- Ranjani Ramanujam 10 ,
- Ramesh Naidu Yedla 11 ,
- Dhilip Kumar Kandregula 12 ,
- Anuradha Argi 13 &
- Laxmi Teja Peela 14
Clinical Hypertension volume 22 , Article number: 7 ( 2015 ) Cite this article
24k Accesses
76 Citations
13 Altmetric
Metrics details
Hypertension continues to be an important public health concern because of its associated morbidity, mortality and economic impact on the society. It is a significant risk factor for cardiovascular, cerebrovascular and renal complications. It has been estimated that by 2025, 1.56 billion individuals will have hypertension. The increasing prevalence of hypertension and the continually increasing expense of its treatment influence the prescribing patterns among physicians and compliance to the treatment by the patients. A number of national and international guidelines for the management of hypertension have been published. Since many years ago, diuretics were considered as the first-line drugs for treatment of hypertension therapy; however, the recent guidelines by the Joint National Commission (JNC8 guidelines) recommend both calcium channel blockers as well as angiotensin-converting enzyme inhibitors as first-line drugs, in addition to diuretics. Antihypertensive drug combinations are generally used for effective long-term management and to treat comorbid conditions. This review focuses on the antihypertensive medication utilization, their cost factors, adherence to treatment by patients, and physicians’ adherence to guidelines in prescribing medications in different settings including Indian scenario. The antihypertensive medication prescribing pattern studies help in monitoring, evaluation and necessary modifications to the prescribing habits to achieve rational and cost-effective treatment. Additionally, periodic updating of recommended guidelines and innovative drug formulations, and prescription monitoring studies help in rational use of antihypertensive drugs, which can be tailored to suit the patients' requirements, including those in the developing countries.
Hypertension is the most common modifiable risk factor for cardiovascular diseases (CVD), stroke and renal failure [ 1 ]. It is the second leading cause of chronic kidney disease (CKD). It is estimated that more than one billion adults are hypertensive worldwide and this figure is projected to increase to 1.56 billion by the year 2025, which is an increase of 60 % from 2000. Cardiovascular diseases and Hypertension are accounting for loss of 4 % gross domestic product for low and middle income countries annually which is amounting 500 billion USD [ 2 ]. Clinical evidence suggests that lowering blood pressure (BP) with antihypertensive drugs reduces the risk of myocardial infarction, stroke, heart failure, revascularization procedures and end-stage renal diseases in hypertensive patients [ 3 ].
The increasing prevalence of hypertension has been attributed to population growth, ageing and behavioral risk factors, such as unhealthy diet, excess use of alcohol, sedentary lifestyle, obesity, and exposure to persistent stress. A whopping 9.4 million deaths occur worldwide every year because of hypertension [ 4 ], with it being responsible for about 50 % of mortality due to heart disease and stroke [ 5 ]. Epidemiological studies demonstrated that prevalence of hypertension is increasing rapidly in India, varying from 4 to 15 % in urban and 2-8 % in rural population [ 6 , 7 ].
Several guidelines have been developed worldwide for the management of hypertension, and these serve as reference standards for clinical practitioners. However, many clinicians practice their own prescribing pattern in treating hypertensive patients according to their clinical experience. Primary care physicians need to be empowered in appropriate and evidence-based management of hypertension. A review of these prescribing patterns and guideline-based use of antihypertensive medications can give better insights into the concept of personalised, yet cost-effective pharmacological management of hypertension.
Hypertension pharmacotherapy and guidelines
Antihypertensive drugs are prescribed mainly to reduce the morbidity and mortality caused by hypertension and its complications. Many a time, patients require more than one drug for effective control of hypertension. Various classes of antihypertensive drugs like diuretics, inhibitors of the renin-angiotensin system, calcium channel blockers (CCB) and beta blockers (BB) have been shown to reduce complications of hypertension and may be used for initial drugtherapy [ 8 ].
Since the need to improve the control of hypertension is well acknowledged, several guidelines on its classification and management have been developed. Some of the bodies which have developed guidelines are American Society of Hypertension/ International Society of hypertension (ASH/ISH), Joint National Committee (JNC) on Detection, Evaluation, and Treatment of High Blood Pressure, European Society of Hypertension (ESH)/European Society of Cardiology (ESC), National Institute for Health and Care Excellence (NICE) and Japanese Society of Hypertension. The JNC 8 guidelines published in 2014 are the most recent guidelines for the management of hypertension in different clinical settings. These guidelines were developed based on a systematic review of literature to help clinicians, especially the primary care physicians [ 3 ]. Despite these guidelines, and also evidence showing that hypertension is a major public health concern, many clinicians fail to assess BP routinely, and in those with a diagnosis of hypertension, do not start treatment or titrate the dosage of the drugs effectively [ 9 ]. The available guidelines recommend different goal BP levels and drug treatment options according to patients’ individual clinical need (see Table 1 ).
Studies have shown that the application of guidelines to clinical practice improve the treatment outcomes. According to a retrospective study by Jackson et al. on 19,258 patients, applying JNC-7 guidelines to practice helped in achieving better BP control. Blood pressure control in the before-JNC 7 cohort was 40.8 % vs. 49.3 % in the after-JNC 7 cohort ( p < 0.0001) [ 10 ].
In another older study conducted to assess whether the publication of JNC 6 (1997) and WHO/ISH (1999) guidelines, and the development of new drugs improved BP control, follow-up of 150 patients from 1991 to 2001 showed that BP control increased from 31 % initially, to 43 % in 1996 and finally to 57 % in 2001. Both younger and older patients showed similar improvement during these 10 years. The authors concluded that improved BP control was because of increased use of ACEIs and CCBs, lifestyle modifications and improved awareness about the disease condition and the need for effective management [ 11 ]. Jeschke et al. demonstrated that antihypertensive therapy prescribed by physicians specialized in complementary and alternative medicine (CAM) in Germany complied with the German Hypertension Society guidelines. Most patients were treated with conventional antihypertensives like BBs and ACEIs. A thiazide diuretic with ACEI was the most frequent combination prescribed [ 12 ].
Evaluating prescribing pattern of antihypertensive drugs
There have been several studies evaluating the prescribing pattern of antihypertensive drugs worldwide. Over the past 20 years, there has been a consistent increase in the use of ACEIs, ARBs and CCBs and many robustly conducted clinical studies have showed no consistent differences in antihypertensive efficacy, side effects and quality of life within these drug classes [ 13 ]. This has been supported by a retrospective time series data from 2007 to 2012 noted that the consumption of antihypertensive drugs in China nearly doubled [ 14 ]. The most frequently prescribed antihypertensive drug classes were CCBs and ARBs, with prescriptions of the latter increasing most rapidly [ 14 ].
Liu and Wang demonstrated that in 6,536 newly-diagnosed cases of uncomplicated hypertension, CCBs and BBs were the most prescribed antihypertensive medications. Surprisingly, the prescription rate of thiazide diuretics which are the least expensive, and well-known first-line antihypertensive therapy was low (8.3 % monotherapy and 19.9 % overall) [ 15 ].
Joseph et al. used Phadke’s criterion for assessment of appropriateness of prescribing. They observed that most patients were being treated with two or more drugs and CCBs were most frequently prescribed antihypertensive medicines. Similar to other studies, 67.92 % of the patients were prescribed more than one drug, with the most commonly used combination being CCB + BB + alpha-blocker (7.55 %). Based on Phadke’s evaluation criteria, 87.27 % of prescriptions were found rational [ 16 ].. In another drug utilization study, 645 prescriptions were analyzed. A total of 697 antihypertensive drugs prescribed, of which 33.57 % were ARBs, 16.79 % ACEIs, 13.63 % were BBs and 11.91 % CCBs. About 32 % of the antihypertensives prescribed were from the essential medicine list [ 17 ].
In a National Health and Nutrition Examination Survey conducted on subjects aged ≥18 years, it was observed that combination therapy regimens helped to achieve BP goals, with single-pill fixed dose combination (FDC) and multiple-pill combinations being associated with a 55 % and 26 % increased likelihood of BP control, respectively when compared to monotherapy. A significant increase in the use of multiple antihypertensive agents from 36.8 % to 47.7 % ( p < 0.01), with an increased use of thiazide diuretics, BBs, ACEIs, and ARBs by 23 %, 57 %, 31 %, and 100 %, respectively was observed [ 18 ].
Al-Drabah et al. observed that majority of subjects in their study were prescribed monotherapy, followed by two drugs. A few others required three of more drugs. While ACEIs were the most commonly prescribed monotherapy, diuretics were the most commonly prescribed drugs in combination therapy. The researchers further observed that target BP control was not achieved in most patients which imply that monotherapy may not be sufficient for achieving adequate BP control in majority of the patients [ 19 ]. The notable findings of various studies have been presented in Table 2 . As per our knowledge, there is no recent data on international variation in prescribing antihypertensive drugs, which can help clinicians to keep them updated with the recent trends.
Antihypertensive drug utilization and adherence
Antihypertensive medication utilization, adherence to treatment by patients, and physicians’ adherence to guidelines in prescribing medications have been studied in different settings. Many of them have noted full, partial or no-adherence in some studies. Studies suggest that formulators of guidelines should evolve treatment protocols which needs less frequent monitoring by physician, so as to suit developing countries patients. Globally, all guidelines address that guidelines are just to guide but physicians need to follow a patient-centric approach. Treatment strategies for developing countries, where access to health care system is less compared to developed countries, need to be simple, economic and forced time bound titration by the primary care physician and not by the specialist or the tertiary care physician, in order to reach maximum number of patients.
A study conducted in India pointed to a common trend that the study patients were on multiple therapies with at least two antihypertensives. This pattern is recommended by guidelines, which state that small doses of different classes of antihypertensive drug are more beneficial than a high dose of one [ 20 ]. In a recent study, it has been noted that in India, the antihypertensive utilization pattern is in accordance with the international guidelines for treatment of hypertension. There is considerable use of different antihypertensive drug combinations and such practice has a positive impact on the overall BP control [ 21 ].
In a meta-analysis, Murphy et al. noted that no consistent differences were observed in the rates of utilization or adherence to drugs for CVDs or diabetes in subjects living in urban and rural settings [ 22 ]. Odili et al. studied the role of physicians in the overall management of hypertension and their adherence to JNC 7, WHO/ISH and ESH guidelines. They concluded that physicians in this study fairly complied with hypertension management guidelines. However, they did not appear to recommend lifestyle modification to their patients [ 23 ]. On the contrary, a study conducted in Malaysia, observed that doctors poorly adhered to Malaysian Clinical Practice Guideline (CPG) in hypertensive patients with diabetes and left ventricular hypertrophy. A better hypertension control was seen with ACEIs and guidelines-adherent therapy [ 24 ].
In another study by Abdulameer et al., 85.30 % of the prescriptions were in accordance to guidelines [ 25 ]. It was observed that the treatment approach for cardiac complicated hypertension followed JNC 7 guidelines, except the lack of add-on therapy practice (ARBs, aldosterone antagonist). The prescribing practice was found in compliance with the Eritrean National treatment guideline 2003 [ 26 ]. In a multicenter study, it was noted that even though physicians self-reported that they were aware of and implement hypertension guidelines in daily practice, a significantly lower agreement rate between physicians’ practice and European guidelines was detected. It was also found that more than one-fourth of high risk hypertensive patients remained untreated, half of them remained uncontrolled, and almost 40 % of low-risk patients received medications unreasonably [ 27 ].
Interestingly, in another study, multifaceted comprehensive implementation of a hypertension guideline did not exert an impact on general practitioners’ prescribing of antihypertensive drugs for drug-treated patients with hypertension, even though the participating general practitioners rated themselves as highly motivated to treat according to the guidelines [ 28 ].
Table 3 summarizes the observations of above quoted studies. It can be noted that physicians seemed to be well-aware of clinical trials on compliance with hypertension treatment, which showed the compliance rates were good with monotherapy, average with two separate drugs (pills), poor when more than two pills were used and hence switched over from monotherapy to single pill FDC. This strategy will offset the side-effects of maximum dose of one class of drug, simultaneously attracting the synergistic effects of different classes of drugs at low doses. The other advantages of single pill FDC being low cost compared to multiple pills of different classes of drugs apart from better compliance.
Cost implications in antihypertensive drugs Use
The cost of medications has always been a barrier to an effective treatment. The increasing prevalence of hypertension and the continually increasing expense of its treatment influence the prescribing patterns among physicians and compliance to the treatment by the patients. In developing countries like India, unlike developed countries, patients are not covered by insurance schemes and are paying out of their pockets for their healthcare. Therefore, they would benefit if physicians provide better services based on rational and cost-effective drug prescription [ 29 ].
According to a cost analysis study by Rachana et al., alpha-blockers were the highest ranked in terms of cost utilized per year followed by ACEIs, ARBs, CCBs, BBs and diuretics in the same order. Thus they found diuretics to be the most cost-effective antihypertensive to be prescribed [ 30 ]. Similarly, Amira et al. observed that diuretics were the most cost-effective drugs for hypertension [ 31 ]. Additionally, the cost of drugs varied based on the type of hospitals, whether government or private, according to a study by Rimoy et al., the costs of nifedipine, bendrofluazide and frusemide were about five to six times higher in private hospitals than at the government-owned pharmacies [ 8 ].
Noteworthy is that adherence to guidelines while prescribing antihypertensive drugs results in substantial savings in prescription costs [ 32 ].
The presence of comorbidities further adds to the problem of increased economic burden. Osibogun and Okwor demonstrated a statistically significant association between co-morbid conditions and higher prescription costs with 73.7 % and 63.2 % of those with diabetes and renal disease respectively having prescription costs in the high cost group ( p < 0.05) [ 33 ]. The cost implication findings from the above studies are summarized in Table 4 .
Use of antihypertensives in special population
The management of hypertension needs special attention in patient population such as, elderly, pediatrics, pregnant women, and hypertension associated with co-morbidities. Often it qualifies for combination therapy to achieve target BP levels. There are several studies, which evaluated the prescription pattern of antihypertensive drugs in such patient population. In a prospective, observational study conducted on geriatric antihypertensive patients, it was noted that the most common drug classes prescribed were CCBs (37 %) and ACEIs (21 %), and amlodipine was the most commonly prescribed drug (37 %). The most common anti-hypertensive FDC prescribed was telmisartan + hydrochlorothiazide (15 %) and most common two drug combination therapy was amlodipine + atenolol (7 %) [ 34 ]. In another study by Fadare et al. , antihypertensive drugs accounted for 30.6 % of the total prescriptions of 220 elderly patients. The authors opined that physicians should be specifically trained regarding prescribing to the geriatric population [ 35 ].
An observational and cross-sectional prospective prescription audit study was carried-out to evaluate antihypertensive drug prescription patterns, rationality and adherence to JNC 7 guidelines in postmenopausal women. It was noted that ARBs were frequently prescribed as monotherapy and 31.6 % of patients were on a two-drug combination. Majority of the prescriptions showed non-adherence as per recommendations for pre-hypertension. The study concluded that except polypharmacy, antihypertensive prescription trends largely adhere to existing guidelines and are rational [ 36 ].
Though there is a scarcity of sufficient data in Indian context, some authors have evaluated antihypertensive medication use in hypertensive diabetes mellitus patients. In a cross-sectional study, Dhanaraj et al. observed that ACEIs were most commonly prescribed antihypertensives (59 %) and most of the patients (55 %) were on multiple drug therapy. In this study, although prescribing pattern of antihypertensives was in accordance with guidelines, there still remained a significant number of patients with uncontrolled hypertension [ 37 ]. In a similar patient population, Janagan et al. observed that most of the patients received more than one antihypertensive (75.2 %), with a combination of ACEIs and thiazide diuretics being the most common. This pattern was compliant with JNC7 guidelines [ 38 ] Hussain et al. conducted a retrospective, randomized, non-interventional study in 117 subjects to evaluate patterns of drug therapy among diabetic hypertensive patients with other complications. It was found that the most common drug administered for diabetes was metformin, whereas for hypertension, it was telmisartan. There was a positive relationship between fasting blood glucose and systolic blood pressure. The notable gap in the present prescribing pattern was found to be underutilization of diuretics [ 39 ].
Adolescent hypertensives seem to be undertreated, with only 23 % of them receiving antihypertensive prescription, according to a study by Yoon et al. Further, ACEIs were the most frequently prescribed monotherapy [ 40 ].
In a study by St. Peter et al. on hypertensive subjects on dialysis, the prescription patterns varied by dialysis modality in the initial six months. Further, it was observed that majority of patients who were on BBs, drugs inhibiting the renin angiotensin system, and dihydropyridine CCBs at 6 months of dialysis did not take prescriptions for these drugs by month 24. Additionally, the specific drugs prescribed varied based on factors like race/ethnicity, age and presence of comorbidities [ 41 ] The key observations of the studies discussed are summarized in Table 5 .
The continued challenges in the management of hypertension still need special attention. A number of national and international guidelines for the management of hypertension have been published highlighting mono- or combination therapy according to the BP levels and associated comorbidity. Worldwide, hypertension treatment strategies have varied widely over time in terms of initial drug of choice from diuretic to ACEI/ ARB/ CCB, from monotherapy to low dose combination single pill therapy. National health policy makers should consider evaluation and treatment of hypertension as a right in public health system for better outcomes in terms of morbidity and mortality from hypertension. The evaluation pattern, patient adherence to the treatment, physician adherence to hypertension management guidelines, cost implications and other data concerning comorbid conditions have been explored in many clinical studies. Inspite of these data and published guidelines, inconsistencies exist towards treatment approach, because of which physicians sometimes have to individualize the therapy, based on specific patient characteristics and response to treatment. In developing countries like India, more systematic studies are required on the evaluation of prescribing patterns and guideline-based antihypertensive medications’ use, which can be tailored to suit the patients' requirements.
Go AS, Mozaffarian D, Roger VL, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics – 2014 update: A report from the American Heart Association. Circulation. 2013;129:e28–292.
Article PubMed Google Scholar
World Health Organization (WHO). A global brief on hypertension. Available at: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ . Accessed on: 02 Jan 2015.
James PA, Oparil S, Carter BL, Eighth Joint National Committee (JNC 8) Members, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8), Supplemental Content. JAMA. 2014;311:507–20.
Article CAS PubMed Google Scholar
Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60.
Article PubMed Central PubMed Google Scholar
Causes of Death 2008 [online database]. Geneva, World Health Organization (http: // www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf .)
Gupta R, Gupta VP. Hypertension epidemiology in India: lessons from Jaipur Heart Watch. Current science. 2009;97(3):349–55.
Google Scholar
Sandozi T, Emani VK. Survey of prescription pattern of anti-hypertensive drugs in hypertensives and hypertension associated diabetics. Int J Pharm Bio Sci. 2010;1(4):23–6.
Rimoy GH, Justin-Temu M, Nilay C. Prescribing Patterns and Cost of Antihypertensive Drugs in Private Hospitals in Dar es Salaam, Tanzania. East Cent Afr J Pharm Sci. 2008;11:69–73.
Kotchen TA. The Search for Strategies to Control Hypertension. Circulation. 2010;122:1141–3.
Jackson JH, Sobolski J, Krienke R, Wong KS, Frech-Tamas F, Nightengale B. Blood pressure control and pharmacotherapy patterns in the United States before and after the release of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) Guidelines. J Am Board Fam Med. 2008;21:512–21.
Ohta Y, Tsuchihashi T, Fujii K, Matsumura K, Ohya Y, Uezono K, et al. Improvement of blood pressure control in a hypertension clinic: A 10-year follow-up study. J Hum Hypertens. 2004;18:273–8.
Jeschke E, Thomas O, Horst CV, Matthias K, Angelina B, Claudia MW, et al. Evaluation of prescribing patterns in a German network of CAM physicians for the treatment of patients with hypertension: A prospective observational study. BMC FamPract. 2009;10:78.
Caceres MC, Moyano P, Farinas H, Cobaleda J, Pijierro A, Darado P, et al. Trends in Antihypertensive Drug Use in Spanish Primary Health Care 1990–2012. AdvPharmacoepidemiol Drug Saf. 2015;3:172.
Xu H, He Y, Xu L, Yan X, Dai H. Trends and patterns of five antihypertensive drug classes between 2007 and 2012 in China using hospital prescription data. Int J Clin PharmacolTher. 2015;53:430–7.
Article Google Scholar
Liu PH, Wang JD. Antihypertensive medication prescription patterns and time trends for newly-diagnosed uncomplicated hypertension patients in Taiwan. BMC Health Serv Res. 2008;8:133.
Joseph S, Verghese N, Thomas L. A study on prescribing pattern of antihypertensive medications in a tertiary care hospital in Malabar region. Der Pharmacia Lettre. 2014;6(4):132–7.
Beg MA, Dutta S, Varma A, Kant R, Bawa S, Anjoom M, et al. Study on drug prescribing pattern in hypertensive patients in a tertiary care teaching hospital at Dehradun, Uttarakhand. Int J Med Sci Public Health. 2014;3(8):922–6. 26.
Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: The National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–14. 114.
Al-Drabah E, Irshaid Y, Yasein N, Zmeili S. Prescription pattern of antihypertensive drugs in Family Practice Clinics at Jordan University Hospital. Med Sci. 2013;2(1):469–88.
Xavier D, Noby M, Pradeep J, Prem P. Letter to the editor. Pattern of drug use in hypertension in a tertiary hospital; A cross sectional study in the inpatients ward. Indian J Pharmacol. 2001;33:456–7.
Shipra J, Prerna U, Jaswant G, Kumar A, Pushpawati J, Vikas S, et al. A systematic review of prescription pattern monitoring studies and their effectiveness in promoting rational use of medicines. Perspect Clin Res. 2015;6:86–90.
Murphy GK, McAlister FA, Weir DL, Tjosvold L, Eurich DT. Cardiovascular medication utilization and adherence among adults living in rural and urban areas: A systematic review and meta-analysis. BMC Public Health. 2014;14:544.
Odili VU, Oghagbon EK, Ugwa NA, Ochei UM, Aghomo OE. Adherence to international guidelines in the management of hypertension in a tertiary hospital in Nigeria. Trop J Pharm Res. 2008;7(2):945–52.
Ahmad N, Hassan Y, Tangiisuran B, Meng OL, Abd Aziz N, Khan AH. Guidelines adherence and hypertension control in an outpatient cardiology clinic in Malaysia. Trop J Pharm Res. 2012;11(4):665–72.
Abdulameer SA, Sahib MN, Aziz NA, Hassan Y, Abdul HA, Razzaq A, et al. Physician adherence to hypertension treatment guidelines and drug acquisition costs of antihypertensive drugs at the cardiac clinic: A pilot study. Patient Preference and Adherence. 2012;6:101–8.
Shobana J, Semere M, Sied M, Eyob T, Russom M. Prescribing pattern of anti - hypertensive drugs among hypertension patients with cardiac complications in Eritrea. Lat Am J Pharm. 2013;32(5):745–8.
Theodorou M, Stafylas P, Kourlaba G, Kaitelidou D, Maniadakis N and Papademetriou V. Physicians’ perceptions and adherence to guidelines for the management of hypertension: a national, multicentre, prospective study. Int J Hypertens. 2012:Article ID 503821. doi: 10.1155/2012/503821. Epub 2012 Nov 28.
Sipila R, Helin-Salmivaara A, Korhonen MJ, Ketola E. Change in antihypertensive drug prescribing after guideline implementation: A controlled before and after study. BMC Family Practice. 2011;12:87.
Karve AV, Chattar KB. Cost analysis study of oral antihypertensive agents available in Indian market. Int J Basic Clin Pharmacol. 2014;3:479–83.
Rachana PR, Anuradha HV, Shivamurthy MC. Antihypertensive prescribing patterns and cost analysis for primary hypertension: A retrospective study. J Clin Diagn Res. 2014;8(9):HC19–22.
Amira CO, Okubadejo NU. Antihypertensive pharmacotherapy in a developing economy: pattern, acquisition costs and conformity to international guidelines in a tertiary-care settingJsetting. J Human Hypertens. 2006;20:894–7.
Article CAS Google Scholar
Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA. 2004;291(15):1850–6.
Osibogun A, Okwor T. Anti-Hypertensive prescription and cost patterns in an outpatient department of a teaching hospital in Lagos State Nigeria. Op J PrevMedPrev Med. 2014;4:156–63.
Altaf M, Rasheed A, Mujtaba A, Mohammed S. Drug utilization evaluation of antihypertensives in geriatric patients in a tertiary care hospital. Int J Pharm Pharm Sci. 2014;6(9):261–4.
CAS Google Scholar
Fadare JO, Agboola SM, Opeke OA, Alabi RA. Prescription pattern and prevalence of potentially inappropriate medications among elderly patients in a Nigerian rural tertiary hospital. Ther Clin Risk Manag. 2013;9:115–20.
Tandon VR, Sharma S, Mahajan S, Mahajan A, Khajuria V, Mahajan V, et al. Antihypertensive drug prescription patterns, rationality, and adherence to Joint National Committee-7 hypertension treatment guidelines among Indian postmenopausal women. J Mid-life Health. 2014;5:78–83.
Dhanaraj E, Raval A, Yadav R, Bhansali A and Tiwari P. Prescription pattern of antihypertensive agents in T2DM patients visiting tertiary care centre in North India. Int J Hypertens. 2012; Article ID 520915. doi:10.1155/2012/520915. Epub 2012 Dec 18.
Janagan T, Kavitha R, Sridevi SA, Veerendra V. Prescription pattern of antihypertensive drugs used in hypertensive patients with associated type2 diabetes mellitus in a tertiary care hospital. Int J Pharm Res Rev. 2014;3(1):1–5.
Hussain Z, Sana A, Mohammed S, Razzaq MA. Patterns of drug therapy among diabetic hypertensive patients with other complications. Int J Pharm Pharm Sci. 2014;6(6):270–7.
Yoon EY, Cohn L, Rocchini A, Kershaw D, Freed G, Ascione F, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatr. 2012;129(1):e1–8.
St. Peter WL, Sozio SM, Shafi T, Ephraim PL, Luly J, McDermott A, et al. Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months. BMC Nephrol. 2013;14:249.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219.
Canadian Hypertension Education Program (CHEP) 2014 Recommendations. Hypertension treatment. Available at: http://www.hypertension.ca/en/chep. Accessed on: 02 Jan 2015.
American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36 suppl 1:S11–66.
Article PubMed Central Google Scholar
Kidney Disease; Improving Global Outcomes (KDIGO) Blood PressureWork Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337–14.
National Institute for Health and Clinical Excellence. Hypertension (CG127). Available at: http://www.nice.org.uk/guidance/cg127 . Accessed on: 02 Jan 2015.
Flack JM, Sica DA, Bakris G, et al. International Society on Hypertension in Blacks. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56(5):780–00.
Shin J, Park JB, Kim K, Kim JH, Yang DH, Pyun WB, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension. Part I - epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1.
Download references
Acknowledgement
I acknowledge my sincere thanks to Quest International University for giving full access to the online journals and providing reference books and journals in Library. My sincere thanks to the staff of department of Pharmacology to give resources including journals and study materials. I also extend my gratitude to the department of Medicine, Andhra Medical College, Visakhapatnam to facilitate my coauthors by providing relevant materials and case histories.
Author information
Authors and affiliations.
Department of Pharmacology, University of Benghazi, Benghazi, Libya
Noah Jarari
Department of Medicine, Andhra Medical College, Visakhapatnam, India
Narasinga Rao
Department of Biochemistry, Quest International University Perak, 227 The Teng Seng Plaza, Level 2, Jalan Raja Permaisuri Bainun, Ipoh, Perak, Malaysia
Jagannadha Rao Peela, Srikumar Shakila & Abdul R. Said
Libyan Cardiac Society, Department of Cardiology, Benghazi Medical Center, Benghazi University, Benghazi, Libya
Khaled A. Ellafi
Department of Physiology, Quest International University Perak, Ipoh, Malaysia
Nagaraja Kumari Nelapalli
Department of Pathology, Quest International University Perak, Ipoh, Malaysia
Department of Pathology, Management and Science University, Selangor, Malaysia
Kin Darli Tun
Department of Clinical Medicine, Hospital Raja Permaisuri Bainun, Ipoh, Malaysia
Syed Ibrahim Jamallulail
Department of Biochemistry, St. Mathews Medical University, Grand Cayman, Cayman Islands
Avinash Kousik Rawal
Department of Pharmacology, Dr Ambethkar Medical College, Bengaluru, India
Ranjani Ramanujam
Department of Medicine, Rangaraya Medical College, Kakinada, India
Ramesh Naidu Yedla
Department of Endocrinology, Andhra Medical College, Visakhapatnam, India
Dhilip Kumar Kandregula
Department of Human Genetics, Andhra University, Visakhapatnam, India
Anuradha Argi
Great Eastern Medical School, Srikakualm, India
Laxmi Teja Peela
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jagannadha Rao Peela .
Additional information
Competing interest.
The authors declare that they have no competing interests.
Authors’ contributions
NJ initiated the theme and provided references, NR given intellectual contribution being physician, JR is main coordinated the manuscript and corresponding author, KE provided intellectual cardiology content, SS provided open access to journals and helped in drafting, AR helped in getting references from library and added some matter, NK given physiological aspects and provided references, YM and KD given related information and pathophysiology of hypertension, SI provided intellectual contribution, AK participation in getting references and formatting work, RR has done major contribution in writing manuscript, RN given physician advices to manuscript, DK has given major guidelines in metabolic aspect, AA helped in typing and online access to various journals and LT helped in typing and language corrections. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Jarari, N., Rao, N., Peela, J.R. et al. A review on prescribing patterns of antihypertensive drugs. Clin Hypertens 22 , 7 (2015). https://doi.org/10.1186/s40885-016-0042-0
Download citation
Received : 26 June 2015
Accepted : 08 January 2016
Published : 27 March 2016
DOI : https://doi.org/10.1186/s40885-016-0042-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antihypertensive Drug
- Blood Pressure Control
- Telmisartan
- Joint National Committee
- Improve Blood Pressure Control
Clinical Hypertension
ISSN: 2056-5909
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Introduction
- Limitations
- Conclusions
- Article Information
DBP indicates diastolic blood pressure; GP, general practitioner; and SBP, systolic blood pressure.
SBP indicates systolic blood pressure.
a Other comorbidities include cerebrovascular disease, peripheral artery disease, chronic artery disease, chronic kidney disease, angina, and acute myocardial infarction.
Study Protocol and Statistical Analysis Plan
eAppendix 1. Exclusion Criteria
eAppendix 2. HBPM and Self-Titration Instructions Sheets
eAppendix 3. Individualized Adjustment Plan Sheet
eTable 1. Baseline Characteristics of the ADAMPA Trial Patients Completing the 24-Month Visit and Patients Not Completing the 24-Month Visit
eTable 2. Baseline Characteristics of the ADAMPA Trial Patients Completing the 24-Month Visit and Patients Not Completing the 24-Month Visit by Study Group
eTable 3. Home Blood Pressure Monitoring and Medication Changes at 24 Months
eTable 4. Self-Titration of Antihypertensive Medication in the Intervention Group (n=111) During the Whole Study Period and the Extension Phase
eTable 5. Differences in Systolic and Diastolic Blood Pressure Among Study Groups at 24 Months, Crude And Adjusted: Sensitivity Analysis Including All Patients Attending the 12-Month Visit (n=312) Using Multiple Imputation
eTable 6. Differences in Systolic and Diastolic Blood Pressure Among Study Groups at 24 Months, Crude and Adjusted: Sensitivity Analysis Including All Patients Attending the 12-Month Visit (n=312) Using the Last Observation Carried Forward
eTable 7. Number and Percentage of Patients Achieving Their Target BP at the Final Follow-Up Visit in the Intervention and Control Groups
eTable 8. Adverse Events During the Extension Follow-Up Period
eTable 9. Behavioural Risks and Health-Related Quality of Life at 12 Months and at the Final Follow-Up Visit
eTable 10. Health Care Services Utilization During the Extension Follow-Up Period
eFigure. Mean SBP and DBP in Follow-Up Visits at Baseline, 6, 12 Months and the Final Follow-Up Visit for the Intervention (n=111) and Control Groups (n=108)
Nonauthor Collaborators
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Martínez-Ibáñez P , Marco-Moreno I , García-Sempere A, et al. Long-Term Effect of Home Blood Pressure Self-Monitoring Plus Medication Self-Titration for Patients With Hypertension : A Secondary Analysis of the ADAMPA Randomized Clinical Trial . JAMA Netw Open. 2024;7(5):e2410063. doi:10.1001/jamanetworkopen.2024.10063
Manage citations:
© 2024
- Permissions
Long-Term Effect of Home Blood Pressure Self-Monitoring Plus Medication Self-Titration for Patients With Hypertension : A Secondary Analysis of the ADAMPA Randomized Clinical Trial
- 1 Health Services Research & Pharmacoepidemiology Unit, Fundació per al Foment de la Investigació Sanitària i Biomèdica de la Comunitat Valenciana (Fisabio), Valencia, Spain
- 2 Network for Research on Chronicity, Primary Care, and Health Promotion (RICAPPS), Spain
- 3 INCLIVA Health Research Institute, Valencia, Spain
Question Does a self-management intervention based on home blood pressure monitoring and self-titration of antihypertensive medication allow for better control of blood pressure compared with usual care at 24 months?
Findings In this prespecified secondary analysis of a randomized clinical trial of 219 patients with uncontrolled hypertension, a self-management intervention including home blood pressure monitoring and self-titration of antihypertensive medication resulted in a statistically significant reduction in systolic blood pressure (adjusted mean difference, −3.4 mm Hg) and diastolic blood pressure (adjusted mean difference, −2.5 mm Hg) at 24 months, with no increase in the use of health care resources or adverse events compared with usual care.
Meaning Hypertension self-management strategies for patients in primary care may be effective to control blood pressure in the longer term.
Importance Patient empowerment through pharmacologic self-management is a common strategy for some chronic diseases such as diabetes, but it is rarely used for controlling blood pressure (BP). Several trials have shown its potential for reducing BP in the short term, but evidence in the longer term is scarce.
Objective To evaluate the longer-term effectiveness of BP self-monitoring plus self-titration of antihypertensive medication vs usual care for patients with poorly controlled hypertension, with passive follow-up and primary-care nursing involvement.
Design, Setting, and Participants The ADAMPA (Impact of Self-Monitoring of Blood Pressure and Self-Titration of Medication in the Control of Hypertension) study was a randomized, unblinded clinical trial with 2 parallel arms conducted in Valencia, Spain. Included participants were patients 40 years or older, with systolic BP (SBP) over 145 mm Hg and/or diastolic BP (DBP) over 90 mm Hg, recruited from July 21, 2017, to June 30, 2018 (study completion, August 25, 2020). Statistical analysis was conducted on an intention-to-treat basis from August 2022 to February 2024.
Interventions Participants were randomized 1:1 to usual care vs an individualized, prearranged plan based on BP self-monitoring plus medication self-titration.
Main Outcomes and Measures The main outome was the adjusted mean difference (AMD) in SBP between groups at 24 months of follow-up. Secondary outcomes were the AMD in DBP between groups at 24 months of follow-up, proportion of patients reaching the BP target (SBP <140 mm Hg and DBP <90 mm Hg), change in behaviors, quality of life, health service use, and adverse events.
Results Among 312 patients included in main trial, data on BP measurements at 24 months were available for 219 patients (111 in the intervention group and 108 in the control group). The mean (SD) age was 64.3 (10.1) years, and 120 patients (54.8%) were female; the mean (SD) SBP was 155.6 (13.1) mm Hg, and the mean (SD) diastolic BP was 90.8 (7.7) mm Hg. The median follow-up was 23.8 months (IQR, 19.8-24.5 months). The AMD in SBP at the end of follow-up was −3.4 mm Hg (95% CI, −4.7 to −2.1 mm Hg; P < .001), and the AMD in DBP was −2.5 mm Hg (95% CI, −3.5 to −1.6 mm Hg; P < .001). Subgroup analysis for the main outcome showed consistent results. Sensitivity analyses confirmed the robustness of the main findings. No differences were observed between groups in behaviors, quality of life, use of health services, or adverse events.
Conclusions and Relevance In this secondary analysis of a randomized clinical trial, BP self-monitoring plus self-titration of antihypertensive medication based on an individualized prearranged plan used in primary care reduced BP in the longer term with passive follow-up compared with usual care, without increasing health care use or adverse events. These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.
Trial Registration ClinicalTrials.gov Identifier: NCT03242785
Hypertension, or elevated blood pressure (BP), is the number one risk factor for ischemic heart disease and stroke, the 2 leading causes of death globally. 1 , 2 Worldwide, management of high BP is suboptimal, 3 including in Europe, where more than half of patients with hypertension are unable to achieve adequate BP control, 4 despite the widespread availability of hypertension guidelines and the array of tools used to improve the delivery of long-term care, such as performance indicators, pay-for-performance schemes, and new information technologies.
The Chronic Care Model, an integrated framework widely adopted to guide the redesign of care based on the principles of patient-centeredness and evidence-based care, has proved to lead to better control of chronic conditions and to improve patient and economic outcomes. 5 In the case of hypertension, evidence from randomized clinical trials and systematic reviews has shown that home BP monitoring (HBPM), when combined with other interventions (such as lifestyle changes, multidisciplinary care, telemonitoring, or medication self-titration) may reduce BP levels, 6 - 13 although its effect appears to be strongly influenced by the intensity of cointerventions, 9 - 17 with little to no effect on BP control when used alone. 9 , 17 - 20 Overall, significant heterogeneity due to different inclusion criteria, organizational settings, self-monitoring regimens, the nature and intensity of cointerventions, target BPs in the included studies, or length of follow-up (with only 4 studies providing results at 12 months) calls for a cautious interpretation of the available evidence on hypertension self-management. Among these studies, 3 trials have addressed HBPM with medication self-titration, 1 from the US showing no effect at 6 months (in which the rate of medication self-titration was very low [approximately 20%]) and 2 from the UK showing a reduction in BP at 12 months. 13 - 15 Moreover, routine clinical practice conditions may differ notably from randomized experiments with stricter follow-up routines; therefore, naturalistic evidence of the effect of HBPM and medication self-titration under routine clinical conditions and during extended periods is still missing.
We conducted the ADAMPA (Impact of Self-Monitoring of Blood Pressure and Self-Titration of Medication in the Control of Hypertension) randomized clinical trial to evaluate the effectiveness of an intervention including self-monitoring of BP plus self-titration of antihypertensive medication (based on an individualized prearranged plan) and educational components vs usual care (also with educational components) for reducing BP in patients with poor BP control. At 12 months, we did not find statistical differences in systolic BP (SBP; primary outcome measure) or in diastolic BP (DBP) between the intervention and control groups, although a higher percentage of participants in the intervention group achieved their recommended BP threshold compared with the control group. 21 To gain additional knowledge on the effect of the intervention in the long term and under close to real-life conditions, we preplanned an additional 12-month extension of the study with passive follow-up. We present here the main findings of the ADAMPA trial at 24 months.
The ADAMPA study is a pragmatic (ie, tries to mimic routine clinical practices as much as possible), randomized, unblinded clinical trial with 2 parallel groups assigned at a ratio of 1:1 to self-management (which includes educational components, HBPM, and self-titration of antihypertensive medication based on a patient’s family physician’s preestablished adjustment plan) or to usual care (also with educational components). The clinical research ethics committee of Hospital Clínico Universitario de Valencia approved the study protocol, as did the Spanish Agency for Medicines and Health Products. All participants provided written informed consent prior to enrollment in the study. None of the health professionals involved in the ADAMPA study received any payment for the recruitment and follow-up of patients or their participation in the study. The study protocol has been previously published 22 and is available in Supplement 1 . This study followed the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline.
The ADAMPA study was conducted in a Health District of the Valencia Health System (VHS), an extensive network of public hospitals and primary health care centers in the region of Valencia in Spain, covering about 97% of the region’s population (5 million inhabitants). The VHS is funded and provided by the Valencia regional government as a part of the Spanish national health system, a public, universal, and mostly free at the point-of-care, decentralized health care system. 23 The VHS is geographically structured into 24 Health Districts, where each of the Health Districts includes a reference hospital and is subdivided into Primary Care Areas served by primary care centers.
Patients aged 40 years or older, with a diagnosis of hypertension in their electronic medical record, with uncontrolled hypertension (with a mean SBP reading on the reference arm of >145 mm Hg or a DBP of >90 mm Hg on the baseline examination), and voluntarily agreeing to join the study were eligible for inclusion (see eAppendix 1 in Supplement 2 for details on exclusion criteria). Recruitment took place from July 21, 2017, to June 30, 2018; main outcomes were captured at 12 months, and an extension of the study was preplanned, collecting a reduced set of outcome variables with passive follow-up up to 24 months (study completion, August 25, 2020).
Potentially eligible patients were recruited by family physicians who performed a preliminary examination and obtained written informed consent. Patients were randomized at a 1:1 allocation ratio, with a centralized online randomization system assigning participants to usual care vs self-management. We used a minimization strategy 24 considering age, sex, SBP higher than 160 mm Hg, and comorbidities (diabetes, cardiovascular disease, stroke, and chronic kidney disease). After randomization, a complete examination was scheduled by the research team (baseline visit); some patients either dropped out before that visit or were excluded because the examination revealed that they were not eligible and were mistakenly randomized. Losses to follow-up throughout the study and their reasons are detailed in Figure 1 .
The intervention has been described thoroughly elsewhere. 21 , 22 In short, family physicians defined individualized care plans and BP targets in conjunction with the patients, who received the recommendations for hypertension management from the clinical practice guidelines in force during the study period. 25 , 26 Patients were provided with a booklet containing user-friendly, educational materials on how to improve BP control, written instructions for self-monitoring at home, sheets for recording BP readings every morning and afternoon during the first week (7 days) of every month, and instructions for self-adjustment of medication (dose adjustment or add-on medication) when needed based on BP readings using a color traffic light system (eAppendix 2 and eAppendix 3 in Supplement 2 ). Patients were trained to use and were provided with a validated home BP monitor (Omron M3 model HEM-7131-E; Omron Healthcare Inc). During the first 12 months of follow-up, patients were instructed to routinely register their BP readings, BP-related health encounters, and medication self-titration schemes in the allotted sheets of their booklets, and they were contacted by telephone to clarify possible doubts. Patients had to self-adjust their medication without any additional contact with their family physician, other health care workers, or coaches when their SBP or DBP was above the predefined target for 4 or more days in the measurement week, and they continued taking the modified therapeutic regimen for 3 weeks when an appointment with the physician was scheduled. At this visit, the therapeutic plan was updated. In the extension phase, in which nurses were involved, patients in the intervention group continued HBPM and medication self-titration. Nurses received information about the trial and were aware of which patients were in the intervention and control groups of the ADAMPA study, but they did not have any specific role apart from their usual practice. They treated patients (face to face or by telephone) in the context of routine care without preplanned follow-up visits and provided support to enhance patients’ empowerment based on patient-specific needs.
Participants in the control group received routine hypertension care (health education included) during the whole study. All relevant concomitant care within the routine clinical practice was at the discretion of the attending family physician.
The main outcome in the extension phase was the adjusted mean difference (AMD) in SBP between the intervention and control groups at 24 months. Readings were taken by the family physician at baseline and at 6-, 12-, and 24-month follow-up visits, following measurement recommendations by the European Society of Hypertension and European Society of Cardiology (ESH–ESC) guidelines 25 , 26 using a validated home BP monitor (Omron M3 model HEM-7131-E). However, the final 24-month visit had to occur early for some patients due to the relocation of their physicians to a different Health District before the end of the 24-month follow-up period, resulting in a median follow-up of 23.8 months (IQR, 19.8-24.5 months). Secondary outcomes assessed at the final visit were the AMD in DBP between groups, the difference in the percentage of patients achieving the BP target (SBP <140 mm Hg and DBP <90 mm Hg), health-related quality of life (measured using the EuroQoL-5D 27 ), behavioral change (smoking, exercise, and body weight), use of health care resources, and occurrence of adverse events.
A sample size of 382 patients was estimated to have 90% power to detect a mean (SD) difference of 5 (15) mm Hg in SBP between groups (primary outcome) with a 2-tailed contrast and an α error of .05, which represents a clinically relevant difference based on previous trials. 9 , 14 , 15 For reasons unrelated to the study and detailed elsewhere, 21 we were able to randomize 364 patients, of whom 312 completed follow-up at 12 months; 219 patients attended the final visit of the extension phase.
Statistical analysis was conducted from August 2022 to February 2024. First, we described the characteristics of the patients who remained in the study during the extension period, using the χ 2 test for categorical variables and the t test for continuous variables. Second, in intention-to-treat analyses, we estimated the crude differences in SBP and DBP readings, with their corresponding 95% CIs, between baseline and 12 months and between baseline and the final visit, as well as the crude mean differences and the AMDs with their corresponding 95% CIs between groups in SBP and DBP at the final visit. Linear mixed-effects models were used to compare SBP and DBP between groups adjusting for sex, age, baseline SBP, obesity, and diabetes as fixed effects and for family physician as a random effect. Visual inspection of the residual plots did not show any major deviations from homoscedasticity or normality. Third, we performed several preplanned stratified analyses of between-group AMD in SBP at the final visit, with their corresponding 95% CIs, by sex, age group (40-64 years, 65-79 years, and ≥80 years), baseline SBP (<160 mm Hg vs ≥160 mm Hg), presence of diabetes and other comorbidities (cerebrovascular disease, peripheral artery disease, chronic kidney disease, angina, or acute myocardial infarction), diabetes plus elevated baseline SBP, obesity, overweight and obesity, and obesity plus elevated baseline SBP. Fourth, we estimated the proportion of patients achieving the BP target in the final visit using the recommendations established in the 2018 ESH-ESC guidelines, 26 as well as the difference in proportions between groups. Fifth, we estimated differences between groups in the final visit for behavioral outcomes (smoking, obesity, and sedentary lifestyle), health-related quality of life, and use of health care resources. In addition, the occurrence of adverse events during the extension follow-up period was also assessed.
Sensitivity analyses were performed to assess the robustness of the primary end point analyses. First, multiple-imputation analysis was performed, using a model-based approach for missing data based on a Markov chain Monte Carlo simulation. Second, we reran the main analysis including patients with missing BP measurements at 24 months using the last observation carried forward for those whose last BP measurements were at 12 months. A 2-sided P < .05 was considered significant. Analyses were performed using Stata, version 14 (StataCorp LLC), and R, version 3.6.0 (R Project for Statistical Computing).
From the 312 patients attending the 12-month visit, 219 (70.2%; 111 in the intervention group and 108 in the control group) remained in the study during the extension period and provided BP readings at the final follow-up visit. Of the 93 patients who did not attend the final visit, 75 experienced an early interruption of the trial extension follow-up due to restrictions for attending health care centers during the COVID-19 pandemic, 4 abandoned the study, 5 were lost to follow-up, and 6 did not attend the final visit for other causes ( Figure 1 ). Losses among study groups were similar.
Overall, 120 patients (54.8%) were women, 99 patients (45.2%) were men, the mean (SD) age was 64.3 (10.1) years, the mean (SD) baseline SBP was 155.6 (13.1) mm Hg, the mean (SD) DBP was 90.8 (7.7) mm Hg, 52 (23.7%) had diabetes, and 67 (30.6%) previously used HBPM ( Table 1 ). No differences between groups were observed in the baseline characteristics of the patients reaching the final follow-up visit. Compared with the 93 patients who did not attend the final visit, the 219 patients who attended the 24-month visit had a higher mean (SD) baseline DBP (90.8 [7.7] mm Hg in the intervention group vs 88.6 [8.6] mm Hg in the control group; P = .03) and were more likely to be housewives at baseline (14.6% [32 of 219] in the intervention group vs 4.3% [4 of 93] in the control group), although most had permanent work or were retired in both groups (78.5% [172 of 219] in the intervention group vs 86.0% [80 of 93] in the control group) (eTable 1 in Supplement 2 ). Baseline characteristics by study group, for patients who attended the 24-month visit and for patients who did not, are shown in eTable 2 in Supplement 2 .
There was an increase in prescriptions of antihypertensive drugs in both groups at 24 months; the mean number of antihypertensive medications at 24 months was higher in the intervention group than in the control group (2.4 [95% CI, 2.2-2.5] vs 2.1 [95% CI, 1.9-2.3]; P = .04) (eTable 3 in Supplement 2 ). Of the 111 patients in the intervention group who reached the final follow-up visit, 76 (68.5%) made at least 1 medication adjustment, 43 (38.7%) increased the medication dose, and 61 (55.0%) added on a new drug at least once according to their self-titration plan, with a mean number of adjustments for these patients of 2.7 (95% CI, 2.2-3.3) (eTable 4 in Supplement 2 ). During the extension phase, 27.9% of patients in the intervention group (31 of 111) self-adjusted their medication.
At the final visit, BP decreased in both groups from baseline (SBP: intervention group, −21.3 mm Hg; 95% CI, −24.5 to –18.2 mm Hg; control group, −18.6 mm Hg; 95% CI, −21.8 to −15.5 mm Hg) and DBP (intervention group, −9.4 mm Hg; 95% CI, −11.2 to −7.7 mm Hg; control group, −8.6 mm Hg; 95% CI, −10.1 to −7.0 mm Hg). The AMD between groups at 24 months were −3.4 mm Hg (95% CI, –4.7 to −2.1 mm Hg; P < .001) for SBP and −2.5 mm Hg (95% CI, −3.5 to −1.6 mm Hg; P < .001) for DBP ( Table 2 and eFigure in Supplement 2 ). The sensitivity analyses (using multiple imputation and carrying forward the last observation for those with missing BP measurements at 24 months) yielded similar results (eTable 5 and eTable 6 in Supplement 2 ).
Stratified unadjusted analyses showed no differences in SBP among subgroups at 24 months. Greater differences were observed for patients with diabetes (−9.8 mm Hg [95% CI, −18.2 to −1.4 mm Hg]) and patients with diabetes and SBP at baseline of 160 mm Hg or higher (−15.3 mm Hg [95% CI, −28.5 to −2.1 mm Hg]), although interaction terms were not statistically significant (diabetes, P = .08 for interaction; diabetes and SBP ≥160 mm Hg at baseline, P = .28 for interaction) ( Figure 2 ).
No differences were found with respect to the percentage of patients achieving the BP target at the final visit (64.0% [71 of 111] in the intervention group vs 53.7% [58 of 108] in the control group; difference, 10.3 [95% CI, −2.7 to 23.2]; P = .06) (eTable 7 in Supplement 2 ), the occurrence of adverse events, the change in behavioral risk factors, health-related quality of life, or use of health care resources (eTables 7-10 in Supplement 2 ), except for the mean (SD) number of visits to a primary care center without appointment, which was higher in the control group (0.2 [0.5] in the control group vs 0.06 [0.3] in the intervention group; P = .03) (eTable 10 in Supplement 2 ).
In this randomized clinical trial with a preplanned extension period of 12 months, with passive follow-up mimicking routine clinical conditions and a total follow-up close to 24 months, HBPM plus medication self-titration based on individualized care plans for patients with hypertension managed in primary care was associated with a reduction in SBP compared with usual care. An AMD of −3.4 mm Hg in SBP between groups at the final extension visit was observed, which, together with an AMD in DBP of −2.5 mm Hg, could result in clinical benefits if sustained over time; available evidence with up to 5 years of follow-up shows that similar BP reductions were associated with a 14% reduction in total cardiovascular events, a 28% reduction in strokes, and a 25% reduction in cardiovascular deaths. 28 Our results at 24 months suggest a maintenance effect of the intervention to reduce BP (eFigure in Supplement 2 ), with similar results to those shown at 12 months of follow-up. 21 Subgroup analyses for the primary outcome measure at the extension visit showed consistent results among subgroups. Although interaction terms were not significant, greater differences were observed among patients at higher risk, as seen in the TASMIN-SR (Targets and Self-Management for the Control of Blood Pressure in Stroke and at Risk Groups) study 15 (as compared with the TASMINH 2 [Telemonitoring and Self-Management in Hypertension 2] study 14 ); these differences could have clinically important implications, suggesting the potential of the intervention to reduce adverse outcomes among patients at higher risk. No other differences were found for the rest of the outcomes measured up to 24 months (percentage of patients achieving their BP targets, adverse events, health-related quality of life, behaviors, and use of health care resources), except for a slight reduction in the mean number of unplanned primary care visits in the intervention group.
Patient acceptance of the new treatment scheme, as well as an improvement in their capacity for self-management, may partly underlie the maintenance effect of the intervention for up to 24 months. Losses to follow-up in the extension phase were explained mainly by an early interruption of the trial extension follow-up due to restrictions for attending health care centers during the COVID-19 pandemic, rather than by lack of interest or patient commitment. Treatment intensity may be another relevant mediator. Overall, the mean number of antihypertensive medications increased in both groups from baseline, although it was slightly higher in the intervention group than in the control group at the end of the follow-up (2.4 vs 2.1). In addition to the treatment modifications made by their family physicians as part of routine care, 68.5% of patients in the intervention group attending the final visit self-adjusted their medication (eTable 4 in Supplement 2 ), a figure close to that of other studies. 14 During the extension phase, 27.9% of patients in the intervention group self-adjusted their medication. Finally, professional engagement and other aspects that patient empowerment entails (such as more knowledge, higher skills, and confidence to actively participate in their own care) may have played an essential role in the achievement of consistent long-term results. We conducted qualitative research with family physicians and nurses involved in the study and found that, overall, both groups valued the intervention and were highly supportive of its implementation at a system level, reporting a shared perception of better BP control, the key role of patient empowerment, and reduced therapeutic inertia in the intervention group compared with usual care. 29
Overall, HBPM as a single intervention has shown little or no effect in improving BP control. However, when combined with other interventions, such as interprofessional collaboration, education and training, active counseling, or medication titration strategies, relevant BP reductions have been observed, at least in the short term. 16 , 18 , 20 , 30 - 32 To our knowledge, few randomized studies have addressed BP self-management, 10 , 33 with only 1 trial to date assessing the effect of these interventions after 1 year. 10 , 34 The ADAMPA trial showed results consistent with the aforementioned studies at 12 months 10 , 21 , 33 and showed a maintenance effect beyond 1 year, consistent with the trial with long-term assessment. 34 No increase in adverse events, decrease in quality of life, or intensification of health services use (beyond the increase in antihypertensive medication prescriptions) was observed at 2 years. Nonetheless, the ADAMPA trial at 24 months differs from the latter study 34 in that it entailed a high degree of patient empowerment (mainly through self-adjustment of medication, without any kind of coaching) and telemonitoring was absent. The ADAMPA trial showed that a relatively simple and inexpensive intervention based on patient empowerment through HBPM and medication self-titration, with no telehealth components, was associated with a reduction in BP without an increase in adverse events or direct health care costs for up to 24 months.
Our study is subject to some limitations. First, 93 of the original 312 study participants (29.8%) attending the 12-month visit (main analysis) did not attend the 24-month visit; most of them (75 of 93 [80.6%]) experienced an early interruption of the trial extension follow-up due to restrictions for attending health care centers during the COVID-19 pandemic. Losses to follow-up were similar in both groups, and study participants attending the 24-month visit were also similar to the ones lost to follow-up. In addition, the sensitivity analysis including patients with missing BP measurements at 24 months using the last observation carried forward for those whose last BP measurements were at 12 months yielded similar results, suggesting a reduced potential bias. Second, the trial was unblinded to patients and professionals. This unblinding could lead to biases such as performance bias or the Hawthorne effect (where physicians and patients modify their behavior in response to being observed) or social desirability bias (patients overreporting positive behaviors or underreporting undesirable ones). Third, participating physicians managed patients in the intervention and control groups, which could result in a contamination bias, where some components of the intervention may have been extended to patients outside the intervention group of the study; if present, this effect would produce a result toward the null bias. Fourth, we did not evaluate the effect of HBPM on clinical outcomes. However, BP reduction has proved to be a reliable surrogate end point, highly correlated with reduced morbidity and cardiovascular mortality. 35 - 37 Fifth, our study was designed for a single primary end point at 12 months; thus, all results from the 24-month extension presented here should be considered nonconfirmatory. Sixth, our results should be extrapolated with caution because we applied strict inclusion and exclusion criteria, resulting in some relevant subgroups omitted in our study (for instance, patients with adequately controlled hypertension, who account for approximately half of all patients with hypertension; patients with very high BP; or pregnant women).
In this secondary analysis of a randomized clinical trial of self-management of BP including HBPM and self-titration of antihypertensive medication for patients with uncontrolled hypertension, a reduction in BP at 24 months was observed, with no increase in the use of health care resources or adverse events compared with usual care. These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.
Accepted for Publication: February 29, 2024.
Published: May 10, 2024. doi:10.1001/jamanetworkopen.2024.10063
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Martínez-Ibáñez P et al. JAMA Network Open .
Corresponding Author: Gabriel Sanfélix-Gimeno, PhD, Health Services Research & Pharmacoepidemiology Unit, Fisabio, Catalunya Av. 21; 46020 Valencia, Spain ( [email protected] ).
Author Contributions: Drs Sanfélix-Gimeno and Sanfélix-Genovés had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: P. Martínez-Ibáñez, Marco-Moreno, García-Sempere, Peiró, Barreira-Franch, Sanfélix-Gimeno, Sanfélix-Genovés.
Acquisition, analysis, or interpretation of data: Marco-Moreno, Peiró, L. Martínez-Ibáñez, Bellot-Pujalte, Avelino-Hidalgo, Escrig-Veses, Bóveda-García, Calleja-del-Ser, Robles-Cabaniñas, Hurtado, Rodríguez-Bernal, Giménez-Loreiro, Sanfélix-Gimeno, Sanfélix-Genovés.
Drafting of the manuscript: P. Martínez-Ibáñez, Marco-Moreno, García-Sempere, Barreira-Franch, Avelino-Hidalgo, Escrig-Veses, Sanfélix-Gimeno.
Critical review of the manuscript for important intellectual content: Marco-Moreno, Peiró, L. Martínez-Ibáñez, Bellot-Pujalte, Bóveda-García, Calleja-del-Ser, Robles-Cabaniñas, Hurtado, Rodríguez-Bernal, Giménez-Loreiro, Sanfélix-Gimeno, Sanfélix-Genovés.
Statistical analysis: Robles-Cabaniñas, Hurtado, Sanfélix-Gimeno.
Obtained funding: Marco-Moreno, García-Sempere, L. Martínez-Ibáñez, Bellot-Pujalte, Calleja-del-Ser, Sanfélix-Gimeno, Sanfélix-Genovés.
Administrative, technical, or material support: Marco-Moreno, Barreira-Franch, Avelino-Hidalgo, Bóveda-García, Calleja-del-Ser.
Supervision: P. Martínez-Ibáñez, Marco-Moreno, Peiró, Bóveda-García, Calleja-del-Ser, Sanfélix-Gimeno, Sanfélix-Genovés.
Conflict of Interest Disclosures: Drs García-Sempere, Peiró, Hurtado, Rodríguez-Bernal, and Sanfélix-Gimeno and Mrs Robles-Cabaniñas reported receiving grants from RTI Health Solutions (contract between Fisabio and RTI Health Solutions) outside the submitted work. Dr Peiró reported receiving personal fees from GSK and MSD outside the submitted work. Dr Sanfélix-Gimeno reported receiving personal fees from MSD outside the submitted work. No other disclosures were reported.
Funding/Support: The ADAMPA (Impact of Self-Monitoring of Blood Pressure and Self-Titration of Medication in the Control of Hypertension) study was funded by the Instituto de Salud Carlos III from the Spanish Ministry of Research, Innovation and Universities (grant Pl16/02130, cofinanced by the European Regional Development Fund) and had the collaboration of the SCReN Platform (Spanish Clinical Research Network from the Instituto de Salud Carlos III; grants PT13/0002/0031 and PT17/0017/0003).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The ADAMPA Research Group members are listed in Supplement 3 .
Meeting Presentations: The results of the ADAMPA extension phase were presented at the 38th International Conference on Pharmacoepidemiology & Therapeutic Risk Management of the International Society for Pharmaceutical Engineering; August 26, 2022; Copenhagen, Denmark; and at the 44th National Conference of the Sociedad Española de Médicos de Atención Primaria; October 7, 2022; Sevilla, Spain.
Data Sharing Statement: See Supplement 4 .
Additional Contributions: We acknowledge the trial sponsor INCLIVA Health Research Institute; its former scientific deputy director, Marta Peiró Signes, PharmD, PhD; the rest of the staff for their support; and all the participants for their invaluable contribution to this study. We also acknowledge Maria Teresa García, BSTE, Spanish Clinical Research Network, for her support with the electronic data capturing and quality checks. Signes and García were not compensated for their contributions.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Adverse Cardiovascular Outcomes and Antihypertensive Treatment: A Genome-Wide Interaction Meta-Analysis in the International Consortium for Antihypertensive Pharmacogenomics Studies
Add to collection, downloadable content.
- Other Affiliation: Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics and Precision Medicine, College of Pharmacy, University of Florida, Gainesville, FL, United States
- Other Affiliation: Clinical Pharmacology Department, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom
- Other Affiliation: Bioinformatics Research Center, Department of Statistics, North Carolina State University, Raleigh, NC, United States
- Other Affiliation: Biostatistics and Computational Biology Branch, National Institute of Environmental Health Sciences, Durham, NC, United States
- Other Affiliation: Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL, United States
- Other Affiliation: Cardiovascular Health Research Unit, Department of Medicine, University of Washington, Seattle, WA, United States
- Other Affiliation: Center for Public Health Genomics, University of Virginia, Charlottesville, VA, United States
- Other Affiliation: Department of Neurology, University of British Columbia, Vancouver, BC, Canada
- Other Affiliation: National Institute for Health Research, Barts Cardiovascular Biomedical Research Center, Queen Mary University of London, London, United Kingdom
- Other Affiliation: Research Division, Joslin Diabetes Center
- Other Affiliation: Division of Cardiovascular Medicine, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, United States
- Other Affiliation: Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milano, Italy
- Other Affiliation: Department of Clinical, Surgical and Experimental Science, University of Sassari, Medical School, Sassari, Italy
- Other Affiliation: Department of Medicine and Research Program for Clinical and Molecular Metabolism, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
- Other Affiliation: College of Public Health, Dean’s Office, University of Kentucky, Lexington, KY, United States
- Affiliation: School of Medicine, Department of Medicine
- We sought to identify genome-wide variants influencing antihypertensive drug response and adverse cardiovascular outcomes, utilizing data from four randomized controlled trials in the International Consortium for Antihypertensive Pharmacogenomics Studies (ICAPS). Genome-wide antihypertensive drug-single nucleotide polymorphism (SNP) interaction tests for four drug classes (β-blockers, n = 9,195; calcium channel blockers (CCBs), n = 10,511; thiazide/thiazide-like diuretics, n = 3,516; ACE-inhibitors/ARBs, n = 2,559) and cardiovascular outcomes (incident myocardial infarction, stroke, or death) were analyzed among patients with hypertension of European ancestry. Top SNPs from the meta-analyses were tested for replication of cardiovascular outcomes in an independent Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) study (n = 21,267), blood pressure (BP) response in independent ICAPS studies (n = 1,552), and ethnic validation in African Americans from the Genetics of Hypertension Associated Treatment study (GenHAT; n = 5,115). One signal reached genome-wide significance in the β-blocker-SNP interaction analysis (rs139945292, Interaction P = 1.56 × 10−8). rs139945292 was validated through BP response to β-blockers, with the T-allele associated with less BP reduction (systolic BP response P = 6 × 10−4, Beta = 3.09, diastolic BP response P = 5 × 10−3, Beta = 1.53). The T-allele was also associated with increased adverse cardiovascular risk within the β-blocker treated patients’ subgroup (P = 2.35 × 10−4, odds ratio = 1.57, 95% confidence interval = 1.23–1.99). The locus showed nominal replication in CHARGE, and consistent directional trends in β-blocker treated African Americans. rs139945292 is an expression quantitative trait locus for the 50 kb upstream gene NTM (neurotrimin). No SNPs attained genome-wide significance for any other drugs classes. Top SNPs were located near CALB1 (CCB), FLJ367777 (ACE-inhibitor), and CES5AP1 (thiazide). The NTM region is associated with increased risk for adverse cardiovascular outcomes and less BP reduction in β-blocker treated patients. Further investigation into this region is warranted.
- blood pressure
- antihypertensive agent
- Hypertension
- Pharmacogenomic Testing
- controlled study
- genome-wide association study
- antihypertensive therapy
- clinical outcome
- systolic blood pressure
- heart infarction
- Cardiovascular Diseases
- randomized controlled trial
- cardiovascular system
- Drug-Related Side Effects and Adverse Reactions
- single nucleotide polymorphism
- calcium channel blocking agent
- pharmacogenomics
- Polymorphism, Single Nucleotide
- major clinical study
- African Americans
- treatment response
- middle aged
- pharmacogenetic testing
- adverse drug reaction
- protein expression
- angiotensin 2 receptor antagonist
- hypertension
- African American
- cerebrovascular accident
- Blood Pressure
- cardiovascular disease
- cardiovascular risk
- Cardiovascular System
- diastolic blood pressure
- Middle Aged
- expression quantitative trait locus
- dipeptidyl carboxypeptidase inhibitor
- Genome-Wide Association Study
- Antihypertensive Agents
- meta analysis
- drug effect
- thiazide diuretic agent
- beta adrenergic receptor blocking agent
- https://doi.org/10.17615/47sy-yn42
- https://doi.org/10.1002/cpt.2355
- Clinical Pharmacology and Therapeutics
- National Eye Institute, NEI
- Carolinas HealthCare System, CHS, (75N92021D00006, UL1 TR000064, UL1 TR000135, UL1 TR000454)
- NIHR Imperial Biomedical Research Centre, BRC
- Mayo Foundation for Medical Education and Research, MFMER
- Helsingin ja Uudenmaan Sairaanhoitopiiri, HUS, (FP7‐HEALTHF4‐2007‐201550, R01 HL063082, R01 HL123782, T32 HL007457)
- National Institute of Diabetes and Digestive and Kidney Diseases, NIDDK
- University of Florida Opportunity Fund
- National Institute of General Medical Sciences, NIGMS, (U01GM074492)
- International Centre for Circulatory Health Charity
- Medical Research Council, MRC, (G952010)
- Abbott Pharmaceuticals, (R01 HL085251, R01 HL103612, R01 HL105756, R01 NS073346, U01 GM074492‐05S109, U01 NS038529)
- Sydäntutkimussäätiö
- National Institute of Environmental Health Sciences, NIEHS, (1‐HC‐1010, 1‐HC‐9035, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, N01‐HC‐95178, N01‐HC‐95179, ZIAES103338)
- Mayo Clinic
- National Center for Research Resources, NCRR, (UL1RR029890)
- RIKEN, (U01 GM074492-05S109)
- National Heart, Lung, and Blood Institute, NHLBI, (K01HL141690, R01HL063082, R01HL074730, R01HL085251, R01HL103612, R01HL105756, R01HL110380, R01HL110400, R01HL123782, R43HL095181, T32HL007457)
- National Institute for Health and Care Research, NIHR
- National Center for Advancing Translational Sciences, NCATS, (UL1TR000064, UL1TR000135, UL1TR000454)
- Centers for Disease Control and Prevention, CDC, (R01 HL074730, U01‐GM074492, UL1 RR092890)
- National Institute on Aging, NIA
- National Institute of Neurological Disorders and Stroke, NINDS, (R01NS073346, U01NS038529)
- National Institutes of Health, NIH
- Sigrid Juséliuksen Säätiö
- John Wiley and Sons Inc
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
- Open access
- Published: 10 May 2024
Association of RASGRP1 polymorphism with vascular complications in Chinese diabetic patients with glycemic control and antihypertensive treatment
- Jiecan Zhou ORCID: orcid.org/0000-0002-3037-3997 1 , 2 , 3 ,
- Bo Xu ORCID: orcid.org/0000-0003-0594-9103 1 , 2 ,
- Fazhong He 5 , 3 , 4 ,
- Yan Shu 6 ,
- Xiaoping Chen 1 , 3 , 4 ,
- Zhaoqian Liu 3 , 4 , 1 ,
- Bao Sun 7 , 3 , 4 &
- Wei Zhang 3 , 4 , 1
Cardiovascular Diabetology volume 23 , Article number: 166 ( 2024 ) Cite this article
184 Accesses
Metrics details
Studies have shown that RASGRP1 was potently associated with the onset of type 2 diabetes mellitus (T2DM), and RASGRP1 rs7403531 was significantly correlated with islet function in T2DM patients. However, the effect of RASGRP1 polymorphism on blood glucose and blood pressure in T2DM patients after continuous treatment has yet to be fully elucidated.
This study aimed to explore the association between RASGRP1 genetic polymorphism and cardiovascular complications in T2DM patients, so as to provide more evidence for the individualized treatment of T2DM patients.
We retrospectively analyzed a large-scale multicenter drug clinical study cohort that based on a 2 × 2 factorial (glucose control axis and blood pressure lowering axis) randomized controlled design, with follow-up for 5 years. The major vascular endpoint events included cardiovascular death, non-fatal stroke, coronary heart disease, new-onset or worsening renal disease, and diabetic retinopathy. RASGRP1 rs12593201, rs56254815 and rs7403531 were finally selected as candidate single nucleotide polymorphisms. Mixed linear model and Cox hazard ratio (HR) model were used for data analysis with IBM SPSS (version 20.0 for windows; Chicago, IL).
Our study enrolled 1357 patients with high-risk diabetes, with a mean follow-up duration of 4.8 years. RASGRP1 rs7403531 was associated with vascular events in hypoglycemic and antihypertensive therapy. Specifically, compared with CC carriers, patients with CT/TT genotype had fewer major microvascular events (HR = 0.41, 95% confidence interval (CI) 0.21–0.80, P = 0.009), and reduced the risk of major eye disease events (HR = 0.44, 95% CI 0.20–0.94, P = 0.03). For glucose lowering axis, CT/TT carriers had a lower risk of secondary nephropathy (HR = 0.48, 95% CI 0.25–0.92, P = 0.03) in patients with standard glycemic control. For blood pressure lowering axis, all cerebrovascular events (HR = 2.24, 95% CI 1.11–4.51, P = 0.025) and stroke events (HR = 2.07, 95% CI 1.03–4.15, P = 0.04) were increased in patients with CC genotype compared to those with CT/TT genotype in the placebo group, respectively. Furthermore, patients with CC genotype showed a reduced risk of major cerebrovascular events in antihypertensive group (HR = 0.36, 95% CI 0.15–0.86, P = 0.021). For RASGRP1 rs56254815, compared with the AA genotype carriers, the systolic blood pressure of AG/GG carriers in the antihypertensive group decreased by 1.5mmhg on average ( P = 0.04). In the placebo group, the blood pressure of AG/GG carriers was 1.7mmHg higher than that of AA carriers ( P = 0.02).
We found that patients with G allele of RASGRP1 (rs56254815) showed a better antihypertensive therapy efficacy in T2DM patients. The rs7403531 T allele could reduce the risk of major microvascular events and major eye diseases in T2DM patients receiving either hypoglycemic or antihypertensive therapy. Our findings suggest that RASGRP1 genetic polymorphism might predict the cardiovascular complications in T2DM patients.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease with rapidly increasing prevalence worldwide. Characteristics of T2DM include long-term elevation of blood glucose levels due to reduced insulin secretion by pancreatic islet cells and insulin resistance in peripheral tissue [ 1 , 2 ]. Persistent hyperglycemia can trigger a pathological cascade that leads to more serious diseases such as cardiovascular disease, renal failure, metabolic syndrome, or hormonal dysfunction [ 3 ].
In addition to well-known risk factors such as overweight, unhealthy lifestyle, metabolic changes, previous diagnosis of gestational diabetes, or family history of cardiovascular disease, genetic variations also play an important role in T2DM, with estimates ranging from 20 to 80%. So far, genome-wide association studies (GWAS) have identified at least 75 loci associated with T2DM or its traits, but there was no breakthrough in the understanding of the pathological mechanism of the disease or drug therapy [ 4 , 5 ]. Moreover, these genetic variations explain only 10–15% of the genetic probability of this diseases [ 6 ], and the study of genetic mechanisms is still an area of concern.
At present, there is increasing evidence that T2DM can also be defined as an auto-inflammatory disease, in which immune dysregulation disrupts insulin production in pancreatic islet beta cells and signal transduction to target tissues [ 7 , 8 ]. This overturns the traditional understanding of diabetes, suggesting that genes regulating immunity may influence the process of T2DM. RASGRP1 is a guanine nucleotide exchange factor (GEF) that activates RAS protein through the conversion of GDP to GTP initiate a cascade reaction of RAF/MEK/ERK signaling pathway downstream [ 9 ]. In its protein structure, RASGRP1 has an atypical pair of “EF” arms that bind to calcium ions and a diacylglycerol (DAG) binding domain (Fig. 1 A). Studies have shown that these two special domains are crucial for the function of RASGRP1 protein [ 10 ]. RASGRP1 is well known for regulating the immune response of T cells, B cells and other lymphocytes through the signaling cascade of RAS/RAF/MEK/ERK, influencing the progression of a large number of immune diseases such as acute leukemia (AML), solid tumors, lupus erythematosus (SLE) and their drug therapy [ 11 ]. Recent studies have shown that multiple single nucleotide polymorphisms (SNPs) in RASGRP1 gene were related to type 2 diabetes mellitus (T2DM) [ 12 , 13 ], suggesting that RASGRP1 may drive the process of T1DM through immunity, but whether it is also through a similar possible mechanism for T2DM is unclear.
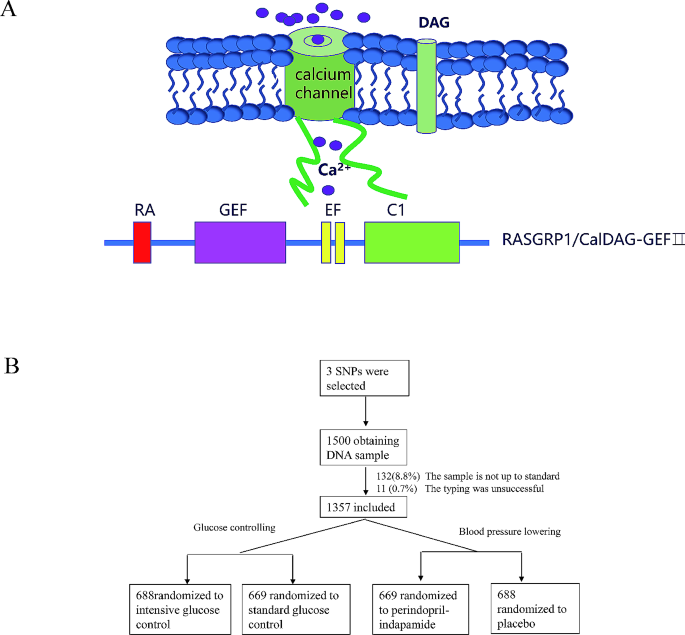
A The atypical “EF” pair of RASGRP1 binds to calcium ions with a diacylglycerol (DAG) binding domain B Overview of DNA sample collection and random grouping of participants
In recent years, many studies have reported that RASGRP1 was strongly associated with cardiovascular diseases and metabolic diseases [ 14 ]. Studies on European populations have found that RASGRP1 is negatively correlated with HbA1c [ 15 ]. A number of studies have identified rs7403531, a site located in the RASGRP1 gene intron 2, as a new susceptibility site for T2DM in Chinese populations [ 16 , 17 ], which has been verified in European populations derived from the DIAbetes Genetics Replication And meta-analysis (DIAGRAM) consortium data [ 18 ]. However, there is no linkage disequilibrium between this site and known susceptible sites of T1DM, suggesting that the pathogenesis of rs7403531-associated signal driving T2DM may be different from that of T1DM. RASGRP1 expressed by cardiomyocytes mediates the phosphorylation of p38 MAPK and the expression of periostin induced by Angiotensin II (AngII) [ 19 ]. Inhibiting the expression of RASGRP1 may contribute to the improvement in heart failure. RASGRP1 expressed in the kidney mediates the functional inhibition and expression of sodium-chloride co-transporter (NCC) in the distal convoluted tubule (DCT) cells induced by PE through the MAPK signaling pathway [ 20 , 21 ]. NCC is an important protein regulating blood pressure and a major target of thiazine antihypertensive drugs. This suggests that RASGRP1 is not only associated with the onset of T2DM, but also with the cardiovascular prognosis of T2DM treatment.
Many genetic variants that affect the susceptibility to T2DM are associated with the risk of vascular events [ 22 ], prompting us to explore the association between these reported pathogenic gene polymorphisms and vascular complications of T2DM. Previous studies in our research group have also found that TRIB3 rs2295490, POLR2A rs71541942, ATF6 rs12086247, SMARCD3 rs58125572 and other gene polymorphisms were significantly correlated with vascular complications under different hypoglycemic or hypertensive treatment modes [ 23 , 24 ]. Based on previous research strategies and a large T2DM clinical trial cohort, we conducted an association study between common genetic variations of RASGRP1 and vascular complications under hypoglycemic or hypertensive therapy, aiming to understand the genetic effects of T2DM on drug therapy from a more comprehensive perspective.
Study design and patients
We conducted a retrospective study on the cohort from Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) clinical trial at 61 centers in China, with a follow-up period of 5 years. Approval to conduct the trial was obtained from the ethics committee of each study center, and all participants provided written informed consent (registration number NCT00145925). Detailed rationale, design, follow-up schedule and clinical endpoints of ADVANCE trial have been described in previous studies [ 25 ].
In brief, it was a 2 × 2 factorial randomized controlled trial. Patients with T2DM were randomly (1:1) assigned to receive perindopril-indapamide or matching placebo for blood pressure control, and modified-release gliclazide based intensive or local standard therapy for glycaemic control. For blood pressure control, participants were treated for 6 weeks as run-in period with combination of perindopril and indapamide, then randomly grouped into fixed combination regimen (perindopril/indapamide, initially 2.0/0.625 mg daily, increasing to 4.0/1.25 mg daily after 3 months) or matching placebo. For hypoglycemic control, an open label, randomized protocol was implemented to an intensive glucose control or to local standard therapy based on local guidelines. The intensive glucose control was defined as the use of gliclazide modified release-based regimen (30–120 mg daily) and other oral agents, then insulin aiming for a HbA1c value of 6.5% or lower. The local standard treatment was defined as the patients who continue with their usual glucose control regimens, which may include any therapy except the use of gliclazide.
The major vascular endpoints include death from cardio-cerebral vascular diseases, nonfatal stroke or nonfatal myocardial infraction, and new or worsening renal or diabetic eye disease. Other vascular events such as cerebrovascular events (include death due to cerebrovascular disease, stroke, transient ischemic attack, and subarachnoid hemorrhage), coronary events (include myocardial infarction, angina pectoris, myocardial ischemia, and sudden death), heart disease (coronary heart disease, heart failure, atrial fibrillation), new or worsening microalbuminuria, and visual deterioration were also evaluated both jointly and separately. In our study, there was no other pre-specified criterion for the levels of blood pressure, HbA1c or other baseline clinical characteristics at entry.
SNP selection and genotyping
Candidate tag SNPs of RASGRP1 were initially selected based on literature, the database from NCBI ( https://www.ncbi.nlm.nih.gov/snp/ ), the 1000 Genomes Project ( https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/ ), and the Haploview 4.2 (Cambridge, MA, USA). Moreover, all of the selected SNPs had a minimum allele frequency > 5% in the Southern Han Chinese population. The SNPs rs12593201, rs56254815 and rs7403531 were genotyped by SNPscan™ (Genesky Biotechnologies, Shanghai, China). To evaluate the accuracy of the genotyping results, 5% of samples were genotyped in duplicate and showed 100% concordance. At last, a total of 1357 patients from 61 clinical centers were successfully genotyped.
Primer information for sequencing: rs12593201, 5F1-Seq: CATGGTAACAGGCACTACTGGTGTCTATATCC; 5F2-Seq: CATGGTAACAGGCACTACTGGTGTCTATACCT; 3 F-Seq: AAGCTTTTGTTGAGTATGTTTTCATAAGTTTG. rs56254815 5F1-Seq: GGTTTCCTTCCCACTTGAGCTAGAGTGC; 5F2-Seq: GGTTTCCTTCCCACTTGAGCTAGAGTGT; 3 F-Seq: AAAATGAAGGAAAATAAAGGAATGTCACC, rs7403531 5F1-Seq: CCATCTCAACTTTCCTTTATGGCTTGC; 5F2-Seq: CCATCTCAACTTTCCTTTATGGCTCGT; 3 F-Seq: TAGCATCTTCCCATAGATACTCATATGTGA.
Statistical analysis
Continuous data were presented as mean values ± standard deviation (SD) or mean (inter-quartile range, IQR), and frequencies or percentages for categorical variables. The Hardy–Weinberg equilibrium (HWE) for the study participants was assessed with the χ2-test. Comparisons of difference in baseline characteristics among the phenotypes were performed by independent-samples t-test or Wilcoxon rank sum test, appropriately. Mixed linear model was performed in plasma glucose, lipid levels and blood pressure between RASGRP1 genotype with adjustment for sex, age, duration of the disease, body mass index (BMI), combined medication and drug dosage. Cox proportional hazards models were used to investigate the relationship between genetic variation and the risk of vascular events, adjusting for history of vascular disease, baseline sex, age, duration of the disease, combined medication, drug dosage and clinical biochemical factors (i.e., potassium concentration and low-density lipoprotein). The cumulative risk of vascular related events was calculated as (1−hazard ratio [HR]) × 100%. Three genetic models (additive, dominant, and recessive genetic models) were used to test any differences of categorical variables and quantitative variables among the four treatment groups. A two-tailed P -value < 0.05 was considered significant. All the analyses were performed with IBM SPSS (version 20.0 for windows; Chicago, IL).
Baseline characteristics, genotyping results and outline of Major findings
We obtained 1500 peripheral venous blood samples in patients with T2DM from ADVANCE (China center), of which 132 (8.8%) samples were rejected due to sample quality and 11 (0.7%) samples were not genotyped successfully. Since typing errors can significantly reduce the degree of linkage disequilibrium between this site and other linkage sites, we intuitively estimated the overall quality of experimental data by constructing LD map of RASGRP1 gene (Supplement Fig. 1). The overall distribution scheme of the experiment was shown in Fig. 1 B. A total of 1357 DNA samples were successfully genotyped for RASGRP1 rs12593201, rs56254815, rs7403531. All of the sites were in Hardy-Weinberg equilibrium among random groups (Supplementary Table 1 ). In hypoglycemic therapy, there was no significant difference in the clinical baseline characteristics between the standard hypoglycemic group and the intensified hypoglycemic treatment group, and there was no significant difference between the antihypertensive treatment group and the placebo group (Supplementary Table 2 ).
Effects of RASGRP1 polymorphism on blood glucose and vascular complications in hypoglycemic therapy .
During the follow-up period, the mean HbA1c of patients in the intensive hypoglycemic treatment group was 6.77%, while that of the standard hypoglycemic group was 7.37%. We evaluated three gene models (dominant, recessive, additive) and used the results of the dominant model finally. For RASGRP1 12,593,201, the mean HbA1c level was 6.78% vs. 6.77% in the enhanced hypoglycemic group, and 7.29% vs. 7.42% in the standard glycemic control group (GG vs. GA/AA). For RASGRP1 rs56254815, the mean HbA1c level was 6.74% vs. 6.82% in the enhanced hypoglycemic group, and 7.36% vs. 7.37% in the standard glycemic control group (AA vs. AG/GG). For RASGRP1 rs7403531, the mean HbA1c level was 6.76% vs. 6.78% in the enhanced hypoglycemic group, and 7.31% vs. 7.40% in the standard glycemic control group (CC vs. CT/TT). After the analysis of the mixed linear model, we found that the genetic variation of RASGRP1 had no significant effect on the hypoglycemic effect in either the intensive hypoglycemic group or the standard hypoglycemic group (Fig. 2 ). In addition, fasting blood glucose (FPG) had similar trend with HbA1c during the follow-up period between different genotypes in each point, but also no statistical differences (data not shown).
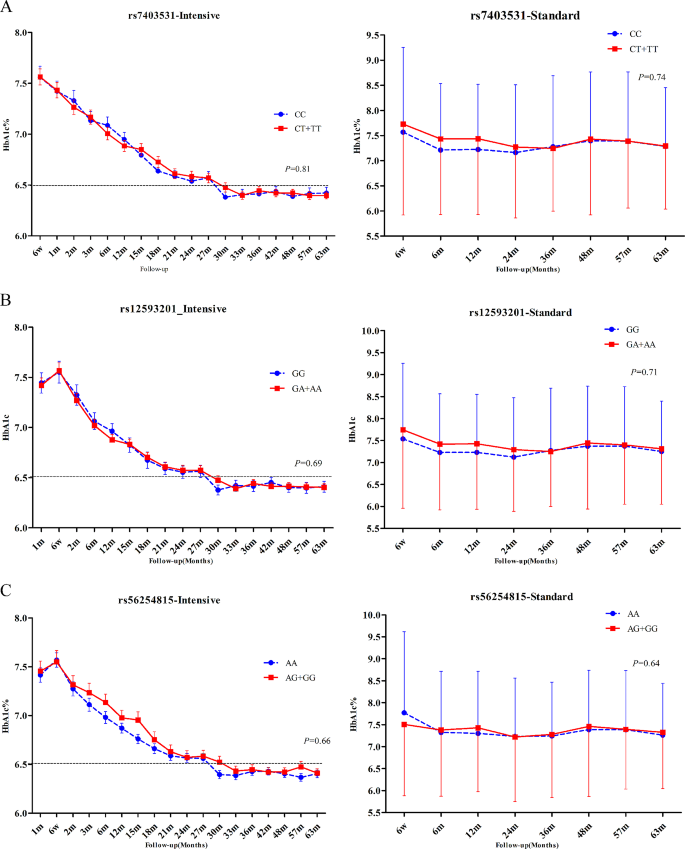
Effect of RASGRP1 gene variation on HbA1c (%) level. A – C were the responses of patients with T2DM to rs7403531, rs12593201 and rs56254815 under the enhanced hypoglycemic (left) and standard hypoglycemic (right) modes, respectively. Data were expressed as mean ± SEM, P values were estimated by the mixed linear model, and were adjusted according to baseline gender, age, course of disease, body mass index (BMI), different genotypes at the time of visit and drug dose
During follow-up period, 225 patients with T2DM developed major macrovascular and/or microvascular events. Among the three sites rs56254815 (Supplementary Table 3), rs7403531 (Supplementary Table 4), rs12593201 (Supplementary Table 5), rs7403531 was mainly closely associated with vascular events. In all patients, there were a total of 126 microvascular events, and the number of major microvascular events was 49 (7.1%) in patients who received intensive hypoglycemic therapy. The number of major microvascular events was 77 (11.5%) in patients who received standard oral glucose-lowering therapy. Compared with the standard hypoglycemic, intensive glucose control reduced major microvascular events (HR = 0.61, 95% confidence interval (CI) 0.42–0.89, P = 0.011) (Fig. 3 A). The number of major microvascular events in rs7403531 CT/TT carriers was 71 (8.5%), and the number of such vascular events in CC carriers was 55 (10.6%). Major microvascular events were reduced in CT/TT genotype carriers compared with CC carriers (HR = 0.41, 95% CI 0.21–0.80, P = 0.009) (Fig. 3 A). This suggests that intensive glucose-lowering in type 2 diabetic patients can reduce the risk of major microvascular events, while RASGRP1 rs7403531 CT/TT carriers are less likely to cause major microvascular events.
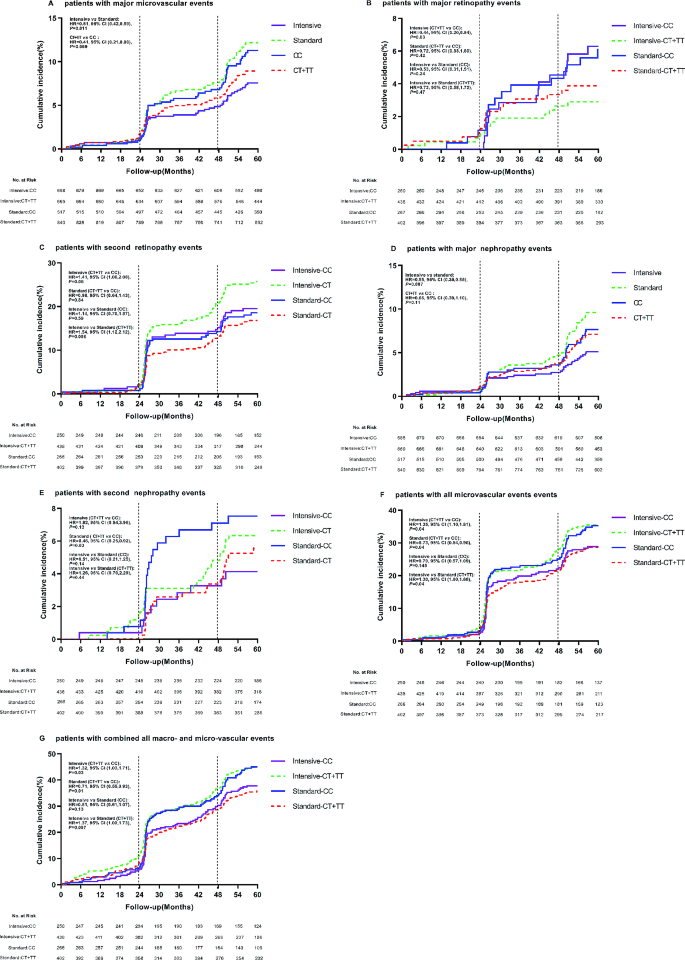
Effect of RASGRP1 (rs7403531) gene variation on cumulative risk at clinical endpoints according to glycemic control strategies. A – E , G display major microvascular events, major retinopathy events, minor retinopathy events, major nephropathy events, minor nephropathy events, all microvascular events, and all combined macroscopic and microscopic vascular events.The vertical line represents additional information about microvascular events (diabetic nephropathy and retinopathy) at 24 and 48 months of follow-up.The time of the event was recorded as the follow-up date.The therapeutic effects of RASGRP1 (rs7403531) CC and CT/TT genotypes (hazard ratio and p value) were treated with a backward LR survival-cox regression model, and the baseline data were corrected
As can be seen from Supplementary Table 6, RASGRP1 rs7403531 was closely related to eye disease events. We observed that CT/TT carriers who received intensive hypoglycemic therapy had a reduced risk of major eye disease events (HR = 0.44, 95% CI 0.20–0.94, P = 0.03), but an increased risk of minor eye disease (HR = 1.41, 95% CI 1.00–2.00, P = 0.05) compared with rs7403531 CC carriers. At the same time, in these gene carriers, the number of secondary eye disease events increased with intensive hypoglycemic therapy compared with standard therapy (HR = 1.54, 95% CI 1.12–2.12, P = 0.008) (Fig. 3 B and C).
Nephropathy-related vascular events were another complication associated with rs7403531 during hypoglycemic therapy. Among all T2DM patients, 33 (4.8%) patients in the intensive hypoglycemic group had major nephropathy events, while 60 (9.0%) patients in the standard group had major nephropathy events. Compared with standard hypoglycemic, intensive hypoglycemic reduced major kidney disease events (HR = 0.55, 95% CI 0.35–0.85, P = 0.007). and in all the T2DM patients, the number of major renal events in CC carriers was 37 (7.2%), while the number was 56 (6.7%) in CT/TT genotype patients, there were no significant differences between the two genotypes carriers (HR = 0.66, 95% CI 0.39–1.10, P = 0.11). CT/TT carriers had a lower risk of secondary nephropathy in the standard glucose-lowering group (HR = 0.48, 95% CI 0.25–0.92, P = 0.03) (Fig. 3 D and E).
Combined with all microvascular events, we found that rs7403531 CT/TT genotype carriers in the intensive hypoglycemic group increased the risk of all microvascular events (HR = 1.35, 95% CI 1.01–1.81, P = 0.04). And for CT/TT genotype carriers, intensive hypoglycemic control increased the risk of all microvascular events (HR = 1.30, 95% CI 1.00-1.68, P = 0.04). In the standard glucose-lowering group, the CT/TT genotype carriers had a reduced risk of all microvascular events (HR = 0.73, 95% CI 0.54–0.98, P = 0.04). When we combined all the microvascular events and major vascular events, we found that in the intensive hypoglycemic group, CT/TT genotype carriers would also increase the risk of all macrovascular events (HR = 1.32, 95% CI 1.03–1.71, P = 0.03), and in the standard hypoglycemic group, CT/TT genotype carriers had a reduced risk of all macrovascular event (HR = 0.71, 95% CI 0.55–0.93, P = 0.01). Consistent with this, intensive hypoglycemic increased the risk of such events in CT/TT patients (HR = 1.37, 95% CI 1.09–1.73, P = 0.007) (Fig. 3 F and G). It was suggested that intensive hypoglycemic therapy would increase the risk of vascular events in RASGRP1 rs7403531 CT/TT patients, while on the contrary, standard hypoglycemic therapy would benefit patients in terms of vascular complications.
Effects of RASGRP1 polymorphism on blood pressure and vascular complications in antihypertensive therapy .
During follow-up period, the systolic blood pressure (SBP) and diastolic blood pressure (DBP) values were 133.6mmHg and 75.2mmHg, respectively, in the T2DM patients treated with perdoperine-indapamide tablets. The mean SBP of the placebo matched group was 137.3mmHg and the mean DBP was 76.5mmHg. For RASGRP1 rs7403531, SBP increased by an average of 2.2 mmHg in CT + TT genotype patients compared with CC genotype carriers in the antihypertensive group, and by an average of 0.4 mmHg in the placebo group (without significant difference) (Fig. 4 A). For RASGRP1 rs12593201, the SBP of GA + AA carriers increased by an average of 1.3mmHg compared with the GG carriers of RASGRP1 (rs12593201) in the antihypertensive group (Fig. 4 B). Also in the placebo group, there was no difference in SBP between the two genotypes. For RASGRP1 rs56254815, compared with AA genotype carriers of RASGRP1 rs56254815, SBP of AG/GG carriers decreased by 1.5 mmHg on average ( P = 0.04), and DBP decreased by 1.1 mmHg on average in the antihypertensive group. In the placebo group, the blood pressure of AG/GG carriers increased by 1.7mmHg ( P = 0.02) compared with that of AA carriers (Fig. 4 C). There was no significant difference in DBP between genotypes for any of the three sites, either in the active antihypertensive group or the placebo group (data not shown). The above results suggested that patients with RASGRP1 rs56254815 AG/GG genotype had better response to antihypertensive therapy.
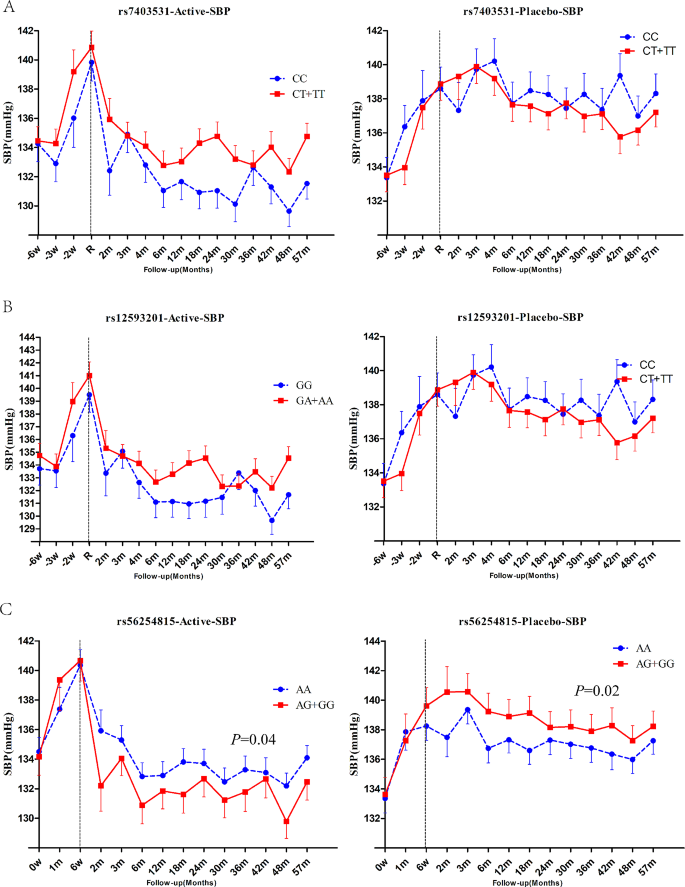
Effect of RASGRP1 genetic variation on systolic blood pressure response during follow-up between perindopril/indapamide treatment group and placebo group. A – C respectively represent the different responses of rs7403531, rs12593201 and rs56254815.Data were expressed as mean ± SEM, P values were estimated using a mixed linear model, and baseline gender, age, course of disease, body mass index (BMI), combined drugs and drug dose were corrected. The first 6 weeks were the elution period
In order to explore the effect of antihypertensive therapy on the prognosis of T2DM patients, we performed the same analysis on vascular complications in the targeted subgroup of antihypertensive therapy. It was found that rs7403531, rs56254815 and rs12593201 were significantly correlated with vascular complications after antihypertensive therapy (Supplementary Tables 6–9). According to the results of Supplement Fig. 1 , there was a linkage disequilibrium between rs7403531 and rs12593201 (D ‘=0.913, r 2 = 0.763), and the correlation between rs12593201 and vascular events may be caused by the linkage disequilibrium between rs7403531 and rs12593201. In our detailed analysis of rs7403531, we found that in the placebo group, compared with patients with CT/TT genotype, all cerebrovascular events increased in CC genotype patients (CC vs. CT/CC: HR = 2.24, 95% CI 1.11–4.51, P = 0.025). For blood pressure lowering axis, there was no significant difference in the occurrence of cerebrovascular events risk between CC and CT/TT genotypes (HR = 0.98, 95% CI 0.44–2.16, P = 0.96). Active antihypertensive therapy in CC patients resulted in approximately reduced risk of all cerebrovascular events. (antihypertensive therapy vs. placebo: HR = 0.47, 95% CI 0.21–1.03, P = 0.06) (Fig. 5 A). We also found that antihypertensive therapy for CC patients reduced the risk of major cerebrovascular events (HR = 0.36, 95% CI 0.15–0.86, P = 0.021) (Fig. 5 B). This suggested that patients with RASGRP1 rs7403531 CC genotype were more likely to trigger cerebrovascular events, and active antihypertensive therapy for this part of the population would reduce the risk of cerebrovascular events.
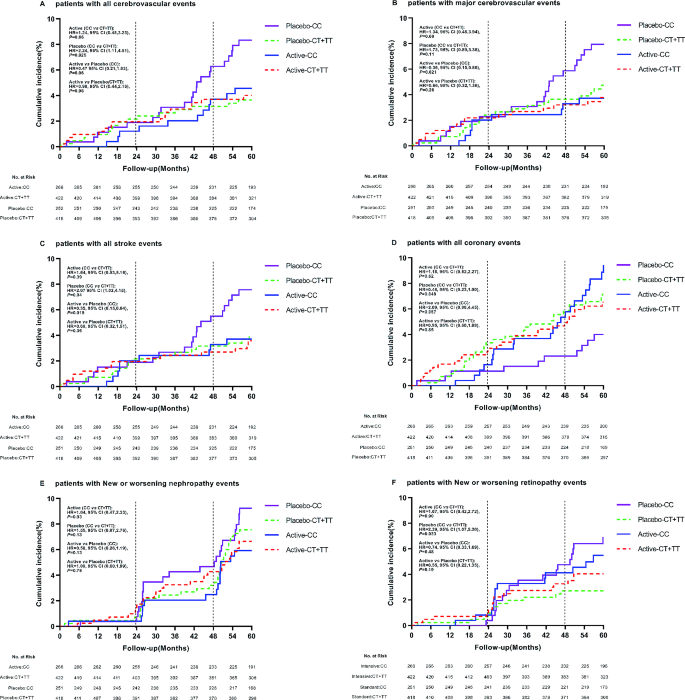
Effect of RASGRP1(rs7403531) genetic variation on clinical outcomes of coronary heart disease during follow-up between perindopril/indapamide treatment group and placebo group. The P values were estimated by the backward LR survival-cox regression model and the baseline data were corrected. A − F represent the cumulative incidence of all cerebrovascular events, major cerebrovascular events, all stroke events, all coronary heart disease events, new or worsening renal events, and new or worsening eye events, respectively
In the placebo group, patients with rs7403531 CC genotype have an increased number of stroke events compared with CT/TT genotype (HR = 2.07, 95% CI 1.03–4.15, P = 0.04), and antihypertensive therapy in these patients resulted in a reduced risk of all strokes (HR = 0.35, 95% CI 0.15–0.84, P = 0.019) (Fig. 5 C). However, the results of coronary heart disease events were opposite. In the placebo group, compared with CT/TT genotype patients, CC genotype patients have lower risk of coronary heart disease events (HR = 0.48, 95% CI 0.23-1.00, P = 0.049) (Fig. 5 D). This suggested that the rs7403531 CC genotype was inconsistent in the prognosis of cardiovascular events and cerebrovascular events, which might be related to the different mechanisms of its function in different tissues.
We also observed no significant effect of various treatments on major renal events (Fig. 5 E). In the placebo group, CC genotype carriers have a higher risk of developing major eye diseases compared to CT + TT genotype carriers (HR = 2.39, 95% CI 1.07–5.30, P = 0.033) (Fig. 5 F). For both CC genotype (HR = 0.74, 95% CI 0.33–1.69, P = 0.48) and CT/TT genotype (HR = 0.55, 95% CI 0.22–1.35, P = 0.19) patients, antihypertensive therapy did not alter the risk of major eye disease events.
For patients with T2DM complicated with hypertension, comprehensive control of blood lipid, blood pressure and blood glucose level are extremely important to reduce the risk of vascular disease in diabetics [ 26 ]. In this study, a 2 × 2 factorial design was used to explore the effectiveness of hypoglycemic treatment and antihypertensive treatment in all enrolled patients. In our cohort, T2DM patients receiving intensive glucose therapy were treated with metformin, insulin, glucosidase inhibitors and other glucose-lowering drugs to achieve HbA1c levels below 6.5%, in addition to gliclazide sustained release tablets. However, for T2DM patients receiving standard blood glucose regimen, the final overall HbA1c level remain above 7.0% according to the Chinese guidelines for diabetes prevention and treatment and the clinical practice by using metformin, sulfonylureas (except glycolide), insulin and other conventional hypoglycemic drugs. As depicted in Fig. 2 , in the study model of hypoglycemic therapy, the genetic variation of the three candidate sites of RASGRP1 did not significantly impact the HbA1c level of patients in all subgroups undergoing hypoglycemic therapy. Previous studies have shown that compared with non-diabetic or normal blood glucose group, diabetic or hyperglycemia group had lower RASGRP1 expression level, while RASGRP1 expression level was negatively correlated with HbA1c [ 15 ]. Nonetheless, the absence of association between RASGRP1 rs7403531 and HbA1c levels suggests that this locus is unlikely to influence the gene expression regulation.
In our study, we examined three candidate SNPs within the RASGRP1 gene, all of which exhibited MAF greater than 5% and were located in intron regions of RASGRP1 . Our analysis revealed no significant association between these SNPs and HbA1c levels in each treatment mode. Notably, two of these SNPs, excluding rs56254815, demonstrated a significant association with vascular complications in T2DM patients. Furthermore, a linkage disequilibrium analysis indicated that rs12593201 and rs7403531 were in strong linkage disequilibrium, suggesting a similar impact on the risk of vascular concurrent events among carriers. rs7403531 has been proved to be correlated with T2DM in a number of correlation studies in Chinese population [ 16 , 17 , 18 ]. Our findings indicated that rs7403531 played an important role in the pathogenesis of T2DM or in drug treatment model. We found that RASGRP1 rs56254815 AG/GG carriers exhibited a higher SBP. Such genotype carriers can receive better curative effect after receiving antihypertensive treatment.
In the hypoglycemic analysis model, our data showed that compared with the standard hypoglycemic, intensive glucose control reduced 5.6% main nephropathy and 4.4% main microvascular events, which was far from achieving UKPD research 25% [ 27 ], indicating that intensive glucose control of T2DM patients benefits less remarkable than most people think. In the whole enrolled population, the major microvascular events in rs7403531 CT/TT genotype carriers were decreased compared with CC genotype carriers, indicating that CT/TT genotype carriers were less likely to cause major microvascular events. Intensive hypoglycemic therapy resulted in a reduced risk of major eye events in CT/TT genotype carriers, and the T allele was a beneficial gene. In the standard glucose-lowering group, the T allele also benefited patients with secondary renal disease risk. In addition,, we also observed that enhanced glucose lowering increased the risk of secondary eye diseases such as vision loss, and that enhanced glucose lowering was more likely to occur in T allele carriers. In the combined analysis of all microvascular and macrovascular events, we found that carriers of the T allele increased the risk of all major microvascular events in the intensive hypoglycemic group, while in the standard hypoglycemic group, the risk of all major microvascular events was decreased. These findings suggest that in clinical practice, appropriate hypoglycemic treatment methods should be selected according to patients’ maximum benefit orientation based on comprehensive measurement. Intensive hypoglycemic treatment can benefit patients to some extent but increase the risk of alternative events. RASGRP1 rs740353 T allele increased the risk of all vascular events overall, yet it provided advantages in terms of major microvascular event.
In the antihypertensive analysis model, genetic variation of rs7403531 did not affect the efficacy of antihypertensive therapy. In the placebo group, compared with CT/TT genotype patients, CC genotype patients increased incidence of major eye events, stroke, and all cerebrovascular events, and aggressive antihypertensive treatment for CC genotype patients nearly reduced the risk of all cerebrovascular events. This suggests that CC genotype carriers were at high risk for T2DM with cerebrovascular events and should be actively treated with antihypertensive therapy. Nonetheless, these CC genotype patients presented a lower risk for concomitant coronary heart disease. Functionally, in myocardial tissue, RASGRP1 mediates angiotensin П-induced phosphorylation of p38 MAPK and expression of periostin protein [ 19 ], inhibiting the expression of RASGRP1 in the heart may help improve heart failure. Conversely, in brain tissue, phospholipase-c gamma activates Ras on golgi through RASGRP1 to induce the differentiation of nerve cells, the transformation of fibroblasts and radiation resistance and other physiological processes [ 28 ], and RASGRP1 is beneficial for brain function. This indicates the tissue-specific role of RASGRP1 , emphasizing that reasonable intervention in patients with different genotypes of rs7403531 contributes to the long-term prognosis of the disease.
In conclusion, our primary findings included: (1) RASGRP1 rs56254815 polymorphism was associated with antihypertensive efficacy. Compared with AA genotype carriers, AG/GG genotype T2DM patients had a higher blood pressure, and these patients showed better efficacy in antihypertensive therapy. (2) rs7403531 CC genotype patients were more likely to cause major eye diseases, cerebrovascular events, and stroke events, while coronary heart disease events are less likely to occur. For CC genotype patients, moderate antihypertensive treatment can be conducted. (3) Intensive hypoglycemic therapy could reduce major microvascular events, including major nephropathy. (4) rs7403531 T allele could reduce the risk of major microvascular events and major eye diseases, but intensive hypoglycemic lowering can increase the risk of overall vascular events in such carriers. Our findings may provide novel insight for personalized treatment for T2DM patients in clinic. However, the specific mechanism of RASGRP1 genetic variation that affecting vascular complications in T2DM patients remains to be studied.
Data availability
No datasets were generated or analysed during the current study.
Meier JJ, Bonadonna RC. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S113–119.
Article CAS PubMed PubMed Central Google Scholar
Walaszczyk E, Luijten M, Spijkerman AMW, et al. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: a systematic review and replication in a case-control sample of the lifelines study. Diabetologia. 2018;61:354–68.
Article CAS PubMed Google Scholar
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Article PubMed PubMed Central Google Scholar
Hivert MF, Vassy JL, Meigs JB. Susceptibility to type 2 diabetes mellitus–from genes to prevention. Nat Rev Endocrinol. 2014;10:198–205.
Fadista J, Vikman P, Laakso EO, et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A. 2014;111:13924–9.
Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med. 2016;48:e220.
Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–7.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107.
Zhu J, Tseng YH, Kantor JD, et al. Interaction of the ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci U S A. 1999;96:14911–8.
Ebinu JO, Bottorff DA, Chan EY, et al. RasGRP, a ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–6.
Kortum RL, Rouquette-Jazdanian AK, Samelson LE. Ras and extracellular signal-regulated kinase signaling in thymocytes and T cells. Trends Immunol. 2013;34:259–68.
Qu HQ, Grant SF, Bradfield JP, et al. Association of RASGRP1 with type 1 diabetes is revealed by combined follow-up of two genome-wide studies. J Med Genet. 2009;46:553–4.
Plagnol V, Howson JM, Smyth DJ, et al. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. 2011;7:e1002216.
Yang HC, Li HW. Analysis of homozygosity disequilibrium using whole-genome sequencing data. BMC Proc. 2014, 8:S15.
Taneera J, Lang S, Sharma A, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16:122–34.
Song C, Wang M, Fang H et al. Effects of variants of 50 genes on diabetes risk among the Chinese population born in the early 1960s. J Diabetes. 2019.
Li JY, Tao F, Wu XX, et al. Common RASGRP1 gene variants that Confer risk of type 2 diabetes. Genet Test Mol Biomarkers. 2015;19:439–43.
Article PubMed Google Scholar
Li H, Gan W, Lu L, et al. A genome-wide association study identifies GRK5 and RASGRP1 as type 2 diabetes loci in Chinese Hans. Diabetes. 2013;62:291–8.
Li L, Fan D, Wang C, et al. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–9.
Ko B, Joshi LM, Cooke LL, et al. Phorbol Ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci U S A. 2007;104:20120–5.
Ko B, Kamsteeg EJ, Cooke LL, et al. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Ren Physiol. 2010;299:F300–309.
Article CAS Google Scholar
Scott RA, Freitag DF, Li L, et al. A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med. 2016;8:341ra376.
Article Google Scholar
He F, Shu Y, Wang X, et al. Intensive glucose control reduces the risk effect of TRIB3, SMARCD3, and ATF6 Genetic Variation on Diabetic Vascular complications. Front Pharmacol. 2018;9:1422.
He F, Liu M, Chen Z, et al. Assessment of Human tribbles Homolog 3 genetic variation (rs2295490) effects on type 2 diabetes patients with glucose control and blood pressure lowering treatment. EBioMedicine. 2016;13:181–9.
Study rationale and design of ADVANCE. Action in diabetes and vascular disease–preterax and diamicron MR controlled evaluation. Diabetologia. 2001;44:1118–20.
Li P, Chen K, Nie Y, et al. Association of obesity with glucose, blood pressure, and lipid goals attainment in patients with concomitant diabetes and hypertension. Curr Med Res Opin. 2015;31:1623–31.
Turner RC, Cull CA, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK prospective diabetes study (UKPDS) Group. JAMA. 1999;281:2005–12.
Bivona TG, Perez De Castro I, Ahearn IM et al. Phospholipase cgamma activates Ras on the golgi apparatus by means of RasGRP1. Nature. 2003, 424:694–8.
Download references
This study was supported by the NSFC (82003872, 82104307), Natural Science Foundation of Hunan Province (2022JJ50163, 2023JJ20034, 2024JJ4080), Scientific Research Fund Project of Hunan Provincial Health Commission (20201973, B202313016776), Central government funds for guiding local scientific and Technological Development (2021QZY016), Hunan Province Clinical Medical Technology Innovation Guidance Project (2020SK51823, 2021SK51828, 2021SK51823), Hunan Provincial Clinical Medical Research Center for Drug Evaluation of major chronic diseases (2023SK4040). The Fund Project of University of South China (2020-25 and 2020-26), The Fund Project of Hengyang city (2020hcjz6713, 2020hcjz6716). The science and technology innovation Program of Hunan Province (2023RC3173).
Author information
Authors and affiliations.
The First Affiliated Hospital, Hunan Provincial Clinical Medical Research Center for Drug Evaluation of Major Chronic Diseases, Hengyang Medical School, University of South China, 421001, Hengyang, Hunan, China
Jiecan Zhou, Bo Xu, Xiaoping Chen, Zhaoqian Liu & Wei Zhang
The First Affiliated Hospital, Hengyang Clinical Pharmacology Research Center, Hengyang Medical School, University of South China, 421001, Hengyang, Hunan, China
Jiecan Zhou & Bo Xu
Department of Clinical Pharmacology, Xiangya Hospital, Central South University, 110 Xiangya Rode, Kaifu district, 410008, Changsha, Hunan, P.R. China
Jiecan Zhou, Fazhong He, Xiaoping Chen, Zhaoqian Liu, Bao Sun & Wei Zhang
Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Hunan Key Laboratory of Pharmacogenetics, Central South University, 410078, Changsha, Hunan, China
Fazhong He, Xiaoping Chen, Zhaoqian Liu, Bao Sun & Wei Zhang
Department of Pharmacy-Quality control section of medical department, Zhuhai People’s Hospital, Zhuhai Hospital Affiliated with Jinan University, Zhuhai, Guangdong, China
Department of Pharmaceutical Sciences, School of Pharmacy, University of Maryland, Baltimore, MD, USA
Department of Pharmacy, The Second Xiangya Hospital, Central South University, People’s Middle Street, Changsha, 410011, Hunan , P. R. China
You can also search for this author in PubMed Google Scholar
Contributions
Jiecan Zhou, Fazhong He, Wei Zhang were responsible for the study design. Jiecan Zhou, Fazhong He, Bao Sun were responsible for the experiments. Jiecan Zhou, Fazhong He, Bao Sun did the data processing and statistical analysis. Jiecan Zhou, Bo Xu, Fazhong He, Yan Shu wrote and interpreted the paper. Zhaoqian Liu, Xiaoping Chen, Wei Zhang were responsible for data management and contributed reagents and materials.
Corresponding authors
Correspondence to Jiecan Zhou , Bao Sun or Wei Zhang .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Competing interests
All authors declare no conflict of interest relevant to the subject matter or materials discussed in the article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhou, J., Xu, B., He, F. et al. Association of RASGRP1 polymorphism with vascular complications in Chinese diabetic patients with glycemic control and antihypertensive treatment. Cardiovasc Diabetol 23 , 166 (2024). https://doi.org/10.1186/s12933-024-02267-2
Download citation
Received : 22 January 2024
Accepted : 06 May 2024
Published : 10 May 2024
DOI : https://doi.org/10.1186/s12933-024-02267-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Polymorphism
- Vascular complications
- Type 2 diabetes
- Individual drug treatment
Cardiovascular Diabetology
ISSN: 1475-2840
- Submission enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 108, Issue 16
- Antihypertensive drug effects on long-term blood pressure: an individual-level data meta-analysis of randomised clinical trials
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-4493-9901 Dexter Canoy 1 , 2 ,
- http://orcid.org/0000-0001-9280-5241 Emma Copland 1 ,
- http://orcid.org/0000-0002-0576-8874 Milad Nazarzadeh 1 ,
- http://orcid.org/0000-0002-6784-8319 Rema Ramakrishnan 3 ,
- Ana-Catarina Pinho-Gomes 4 , 5 ,
- Abdul Salam 6 , 7 ,
- Jamie P Dwyer 8 ,
- Farshad Farzadfar 9 ,
- http://orcid.org/0000-0003-2247-8454 Johan Sundström 10 ,
- http://orcid.org/0000-0001-9800-5296 Mark Woodward 6 , 11 ,
- Barry R Davis 12 ,
- http://orcid.org/0000-0002-4807-4610 Kazem Rahimi 1 , 2
- Blood Pressure Lowering Treatment Trialists' Collaboration
- 1 Deep Medicine, Nuffield Department of Women's and Reproductive Health , University of Oxford , Oxford , UK
- 2 NIHR Oxford Biomedical Research Centre , Oxford University Hospitals NHS Foundation Trust , Oxford , Oxfordshire , UK
- 3 National Perinatal Epidemiology Unit, Nuffield Department of Population Health , University of Oxford , Oxford , UK
- 4 School of Population Health and Environmental Sciences, Faculty of Life Sciences and Medicine , King's College London , London , UK
- 5 Department of Community Medicine, Centre for Health Technology and Services Research , University of Porto , Porto , Portugal
- 6 The George Institute for Global Health , University of New South Wales , Sydney , New South Wales , Australia
- 7 The George Institute for Global Health India , Hyderabad , India
- 8 Vanderbilt University Medical Center , Nashville , Tennessee , USA
- 9 Endocrinology and Metabolism Institute , Tehran University of Medical Sciences , Tehran , Iran (the Islamic Republic of)
- 10 Department of Medical Sciences , Uppsala Universitet , Uppsala , Sweden
- 11 The George Institute for Global Health UK , Imperial College London , London , UK
- 12 Department of Biostatistics , University of Texas School of Public Health , Houston , Texas , USA
- Correspondence to Professor Kazem Rahimi, Deep Medicine, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; kazem.rahimi{at}wrh.ox.ac.uk
Objective Evidence from randomised trials of pharmacological treatments on long-term blood pressure (BP) reduction is limited. We investigated the antihypertensive drug effects on BP over time and across different participant characteristics.
Methods We conducted an individual patient-level data meta-analysis of 52 large-scale randomised clinical trials in the Blood Pressure Lowering Treatment Trialists’ Collaboration using mixed models to examine treatment effects on BP over 4 years of mean follow-up.
Results There were 363 684 participants (42% women), with baseline mean age=65 years and mean systolic/diastolic BP=152/87 mm Hg, and among whom 19% were current smokers, 49% had cardiovascular disease, 28% had diabetes and 69% were taking antihypertensive treatment at baseline. Drugs were effective in lowering BP showing maximal effect after 12 months and gradually attenuating towards later years. Based on measures taken ≥12 months postrandomisation, mean systolic/diastolic BP difference (95% CI) between more and less intense BP-lowering treatment was −11.1 (−11.3 to −10.8)/−5.6 (−5.7 to −5.4) mm Hg; between active treatment and placebo was −5.1 (−5.3 to −5.0)/−2.3 (−2.4 to −2.2) mm Hg; and between active and control arms for drug comparison trials was −1.4 (−1.5 to −1.3)/−0.6 (−0.7 to −0.6) mm Hg. BP reductions were observed across different baseline BP values and ages, and by sex, history of cardiovascular disease and diabetes and prior antihypertensive treatment use.
Conclusion These findings suggest that BP-lowering pharmacotherapy is effective in lowering BP, up to 4 years on average, in people with different characteristics. Appropriate treatment strategies are needed to sustain substantive long-term BP reductions.
- hypertension
- meta-analysis
- pharmacology
Data availability statement
Data may be obtained from a third party and are not publicly available. The governance of the BPLTTC have been reported previously. The BPLTTC is governed by the University of Oxford’s policies on research integrity and codes of practice and follows the university’s policy on the management of research data and records. Scientific activities using the BPLTTC data resource are overseen by the Steering Committee of the collaboration. All data shared with the BPLTTC are considered confidential and will not be provided to any third party. Requests for data should be made directly to the data custodians of individual trials.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/heartjnl-2021-320171
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Clinical guidelines for managing hypertension have invariably lowered the recommended blood pressure (BP) targets for patients at high risk of cardiovascular disease, 1–7 informed by evidence from large-scale randomised clinical trials (RCTs) and their meta-analyses showing substantial reductions in cardiovascular risk with more intensive BP-lowering treatment and independently of baseline BP values. 8–14 For most hypertensive patients, the lowered BP targets inevitably lead to a larger gap between their usual BP and the recommended target value, 15 16 requiring more intensive pharmacological treatment.
Attributing changes to treatment based on repeated measures of BP of an individual patient can be unreliable since measurements are subject to random fluctuations, regression to the mean, non-pharmacological effects and other sources of variability that can exceed true variability in treatment response. 17–19 However, it would be useful to have reliable information about the expected effects of drug treatment on BP levels over time from randomised comparisons to help interpret BP readings such as those obtained during clinical encounters. To date, randomised evidence on the effect of antihypertensive drugs on BP has come from efficacy trials with small numbers of highly selected participants and short follow-up durations. 20 Pooled evidence from RCTs using information from individual participants’ repeated BP measurements currently does not exist, which might explain why there is no guidance on the expected magnitudes of BP reduction with the various proposed treatment strategies and whether these reductions are expected to vary among people with different characteristics.
We addressed this evidence gap by using information from 52 trials involving 363 684 participants with individual-level data on repeated BP measurements over several years 21 to conduct a meta-analysis to quantify the unconfounded effects of BP-lowering drugs on BP over time and examine these effects across different subgroups.
The design of the current phase of the Blood Pressure Lowering Treatment Trialists’ Collaboration (BPLTTC) ( www.bplttc.org ), including the identification of eligible trials as well as data collection and harmonisation, has been described previously, 21 with the protocol registered with PROSPERO (CRD42018099283). Briefly, RCTs were eligible for inclusion if there was randomisation of patients between a BP-lowering agent and a placebo arm or inactive control, between various BP-lowering intensities or between various BP-lowering drugs. RCTs should have a follow-up of ≥1000 person-years in each randomly allocated arm to minimise the risk of small-study effects. 22 Trials without a drug comparison arm or without description of randomisation process were not eligible, nor were those conducted exclusively in patients with heart failure or investigating short-term interventions (eg, in acute care settings). The protocol for the current analyses was reviewed and approved by the BPLTTC Steering Committee prior to data analysis.
To analyse the data, we assigned each participant according to their random allocation in the individual trials, either to the active (or treatment) arm or to the control group separately for each trial design, as described in online supplemental methods and table S1 , and compared BP levels between these comparison groups. Our study outcomes were mean systolic and diastolic BP differences between comparison arms.
Supplemental material
Statistical analysis.
We used a one-stage approach to conduct the meta-analysis of repeated BP measurements over time and applied linear mixed models to estimate the effect of treatment on BP between comparison arms. We developed and compared models that accounted for clustering by trial and potential variability due to baseline BP and other trial-level and participant-level sources of heterogeneity, and determined the best fitting model for our data ( online supplemental methods 1 ). Our primary model was based on fixed treatment effect and fixed time effect but allowing for random intercepts at trial and participant levels and a random slope for follow-up duration at participant level.
We estimated BP values and their difference between comparison groups during the course of follow-up, separately by trial design. As the early phase of the treatment may involve adjustments to optimise treatment regimens such as dosage titration, 23 BP difference between treatment arms may not be maximally achieved until after this period. We therefore also analysed results with and without inclusion of BP measurements taken <12 months after randomisation. We used published aggregate information on achieved BP for each comparison arm to estimate individual-level follow-up values where follow-up BP measurements were not accessible ( online supplemental methods 1 ). We then investigated treatment effects stratified by participants’ baseline BP, age, sex, body mass index, history of cardiovascular disease and diabetes and prior use of antihypertensive medication, and assessed any heterogeneity by comparing models with and without an interaction term for the characteristic of interest and treatment allocation. Models were adjusted for baseline BP, age at recruitment and sex (except when used as stratification factors). We also ran sensitivity analyses that excluded data from each trial and examined results by study period (based on the year the trial has ended).
We used likelihood ratio test (for nested models) and the Akaike information criterion (for non-nested models) to compare models and reported estimates with their 95% CI and p values that were tested at 5% significance level (two tailed). We used R (V.3.4.4) 24 to analyse the data.

Patient and public involvement
There was no patient or public engagement in the design or conduct of this study.
Characteristics of trials and participants in the BPLTTC
The 52 included trials comprised of nine BP-lowering intensity trials, 21 placebo-controlled trials and 29 drug class comparison trials ( table 1 ), mostly conducted between 1990 and 2009 (eight trials conducted after 2009). Seven trials included both comparisons between drug classes as well as either intensity of BP-lowering or between active treatment and placebo. On average, the trials had 4 years of follow-up and eight BP measurements collected after baseline.
- View inline
Characteristics of trials and participants
The trials included 363 684 randomised participants (42% women) with a mean age of 65 years at baseline, including 6% aged ≥80 years. The mean baseline systolic/diastolic BP was 152/87 mm Hg (73% with ≥140 mm Hg systolic and 46% with ≥90 mm Hg diastolic BP) across all trials, with higher values for drug class comparison trials than the other designs ( table 1 ). At baseline, 49% of all participants had had a history of cardiovascular disease and a third a history of diabetes. Baseline BP was higher for older persons, in women and among those with lower body mass index, without cardiovascular disease or diabetes and no prior use of antihypertensive medications, as compared with their counterparts ( online supplemental table S2 ). Further details about study methods, design and risk of bias assessment for each trial are shown in online supplemental table S3 to S7 ).
Temporal BP patterns by treatment allocation
The temporal patterns of BP are shown in figure 1 (additional information in online supplemental table S8 ). Across all trial designs, BP fell during the first few months of follow-up in both study arms. For BP-lowering intensity and placebo-controlled trials, there was divergence in BP in the early follow-up period that increased over time—BP levels in the active arm were lowest at around 2 years after baseline. For drug class comparison trials, BP levels in both comparison arms remained similar during follow-up. The mean BP achieved in the active arm of BP-lowering intensity trials was substantially lower than those achieved in the active arms of the other trial designs. Results for all BP difference trials are shown in online supplemental figure S2 .
- Download figure
- Open in new tab
- Download powerpoint
Blood pressure (BP) trajectories according to different trial designs. Results are in red for active group and black for control group, from 3 months to 5 years of follow-up. Estimates were based on separate models for treatment and control groups, with random intercepts at individual and trial levels, a random slope for time at the individual level (see Method for details) and adjusted for baseline BP, age and sex. Baseline systolic/diastolic BP for active and control groups were: BP-lowering trials=151/88 mm Hg; placebo-controlled trials=146/83 mm Hg and drug class comparison trials=156/90 mm Hg. Estimated BP at specific time points are shown in online supplemental table S8 ). Results for all BP difference trials are shown in online supplemental figure S2 .
Effects of blood pressure (BP)-lowering treatment on mean BP at fixed follow-up time points and across all follow-up period. (A) Systolic BP; (B) Diastolic BP. For mean difference at fixed follow-up time periods, estimates were based on separate models for each time period with a fixed treatment effect and random intercept for individuals. For mean difference achieved across all time period (showing results based on all follow-up BP measures and measures obtained from 12 months until end of follow-up), estimates were based on fixed treatment effect and random intercepts at individual and trial levels, a random slope for time at the individual level. All mean difference values were adjusted for baseline BP, age and sex. The area of the square is inversely proportional to the variance of the estimated difference. Negative values indicate lower BP in the active than in the control group. Additional information provided in online supplemental table S9 and S10 ), and results for all BP difference trials are in online supplemental figure S3 .
Achieved net BP reduction by follow-up period
Figure 2 (additional details in online supplemental table S9 ) illustrates the varying estimates of the difference in BP between comparison groups at specific follow-up times. Consistent with the patterns of absolute BP levels, the estimated difference in BP achieved between the active and control groups tended to be lower in earlier than in later follow-up periods. For BP-lowering intensity trials, the difference in mean reductions in systolic and diastolic BP within 6 months from baseline were −4.2 (95% CI −4.4 to −4.0) mm Hg and −2.0 (95% CI −2.2 to −1.9) mm Hg, respectively, and over −10 mm Hg and −5 mm Hg reductions, respectively, based on measures taken at later follow-up periods. Similar patterns were seen for placebo-controlled trials (and BP difference trials, details shown in online supplemental figure S3 ), although this group achieved smaller magnitudes in mean BP reduction across all follow-up periods. Mean reductions were least for drug class comparison trials.
Effects of blood pressure (BP)-lowering treatment on mean BP, by baseline characteristics. (A) mean systolic BP difference; (B) mean diastolic BP difference. Estimates based on fixed treatment effect and random intercepts at individual and trial levels, a random slope for time at the individual level (see Method for details) and adjusted for baseline BP), age and sex except when these variables are used as stratification factors. The area of the square is inversely proportional to the variance of the estimated difference. Negative values indicate lower BP in the active than in the control group. Results for all BP difference trials are in online supplemental figure S4 . To provide context of background BP levels, baseline BP by these subgroups are shown in online supplemental table S2 .
Estimating overall achieved BP reduction between comparison groups
The time-related BP differences between comparison groups affected the overall achieved reduction in BP. Estimates based on BP measures obtained across all follow-up period were relatively smaller in magnitude than when the treatment phase of <12 months was excluded ( figure 2 , further details in online supplemental figure S3 and table S10 ). For example, for BP-lowering intensity and placebo-controlled trials, the overall mean systolic/diastolic BP reductions across the whole follow-up time were −7.6 (95% CI −7.7 to −7.4)/−3.7 (95% CI −3.8 to −3.6) mm Hg and −4.0 (95% CI −4.1 to −3.9)/−1.9 (95% CI −2.0 to −1.8) mm Hg, respectively; when using measurements taken ≥12 months from baseline, the achieved reductions were −11.1 (95% CI −11.3 to −10.8)/−5.6 (95% CI −5.7 to −5.4) mm Hg and −5.1 (95% CI −5.3 to −5.0)/−2.3 (95% CI −2.4 to −2.2) mm Hg, respectively.
Effects of treatment on BP reduction across different subgroups
Focusing on BP differences from ≥12 months from baseline, figure 3 and online supplemental figure S4 show treatment effects by different baseline characteristics. There were some variations in the magnitudes of BP reductions, notably by body mass index categories in BP-lowering intensity trials. Some trials disproportionately contributed more data in some subgroups so the results reflected features of these trial characteristics and design. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) and the Action to Control Cardovascular Risk in Diabetes (ACCORD) trial, which achieved substantive BP reductions, included participants with higher mean baseline body mass index whereas Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP) and Valsartan in Elderly Isolated Systolic Hypertensioon Study (VALISH) trials, which achieved modest BP reductions, included those with lower mean baseline body mass index ( online supplemental table S6 ). While there were variations in treatment effects across different subgroups, BP reductions were evident across these subgroups, even among those with baseline systolic BP <120 mm Hg and diastolic BP <70 mm Hg. For drug class comparison trials, BP differences overall and across subgroups were consistently small ( figure 3 ).
Sensitivity analyses
BP differences achieved by each trial are reported in online supplemental figure S5 . Results after excluding one trial at a time largely showed similar results as the overall estimates within each trial type ( online supplemental figure S6 ). There were little differences in the achieved BP reductions by study period except in placebo-controlled trials that achieved greater reductions in trials that ended before 2000 than in newer trials ( online supplemental table S11 ), due to some older trials that had far higher starting mean baseline BP values than the newer trials but with comparable treatment goals ( online supplemental table S6 and figure S5 ). Finally, online supplemental table S12 shows how the models we used fitted the data better and gave more conservative estimates than models that did not take into account time-related variations and other individual-level factors in treatment effects.
The analysis of individual-level data of 363 684 randomised participants of 52 large-scale RCTs, the largest of such meta-analysis to date, provides evidence to the overall and stratified effects of antihypertensive treatment on relatively long-term BP reduction. The magnitude of BP reduction varied by time after randomisation and the intended trial intervention. The predicted maximum effect of intervention became apparent about a year from randomisation, with some gradual attenuation several years later during follow-up. The net achieved BP reduction varied by trial design, with BP-lowering intensity trials achieving the largest mean reduction of over 11 mm Hg systolic BP after the first year of treatment. The effects were evident across patient subgroups, as defined by their baseline BP, age, sex, body size, history of cardiovascular disease or diabetes and prior use of antihypertensive treatment.
Randomised evidence on the expected effect of antihypertensive drugs on BP has been largely based on published information from efficacy trials. In a meta-analysis of 354 trials (N≈56 000), 20 half-standard dosages of one, two or three antihypertensive drugs led to systolic BP reductions of 6.7 mm Hg, 13.3 mm Hg and 19.9 mm Hg, respectively, from a pretreatment systolic/diastolic BP of 150/90 mm Hg. Our study is not directly comparable with this work, but it is notable that, in our study, the mean BP reductions were less pronounced than their estimates and that the full effects became evident only after a several months after initiating therapy. This discrepancy could be due to a number of reasons. Their meta-analysis included trials with relatively short follow-up duration (around 2–16 weeks), with some trials having potentially restricted their analysis to fully adherent participants. In contrast, we included large-scale trials with 4-year mean follow-up and performed analysis as per intention to treat. By design, many trials included in our study focused on achieving a target BP level or reduction, so the maximal physiologically feasible effect on BP reduction may not have been achieved. A substantial proportion of participants were on antihypertensive drugs at baseline, which could have further underestimated the magnitude of achieved BP reduction, although it should not have an impact on the net between-group BP reductions.
Current clinical practice guidelines typically recommend a gradual intensification of antihypertensive treatment over several weeks and monitoring of its response for the treated individual. 1–6 However, there is no clear guidance as to the expected change in BP on initiating treatment. To gauge treatment response without a counterfactual or ‘standard’ to compare against is difficult because of the multitude of other causes of BP change. 17 18 Estimates of longitudinal BP changes in our study may help mitigate exaggerated attributions of change in BP to treatment, while providing reassurance about achievable reductions in various groups of ‘at-risk’ individuals. Clinical guidelines also typically define specific BP targets that should be achieved for hypertension to be considered as ‘controlled’, although target levels set by different national guidelines vary. 1–6 While setting a target has practical advantages, it assumes that it is achievable on full implementation of the guidelines. However, population BP follows a distribution, with mean systolic BP≈130 mm Hg in Western populations and over 60% by age ≥60 years have values >140 mm Hg. 25 26 Among the most intensive treatment strategies in the clinical outcome trials were able to achieve an average of 10–15 mm Hg systolic BP reductions within a few months to several years (eg, SPRINT achieved 15 mm Hg systolic BP reduction ( online supplemental figure S5 ). With current evidence-based treatment recommendations, achieving a controlled BP for people with very high BP (eg, >150 mm Hg systolic), would be difficult to attain with the trialled regimens of pharmacologic treatment. 27 We do not imply that physiologically larger BP reductions are unachievable but rather intend to flag the limited evidence on pharmacological BP reductions of over 20 mm Hg in the long term. The achieved BP reduction estimated in our pooled analysis has implications not just for patients but also for healthcare providers whose performance will be assessed based on their patients achieving ‘controlled’ BP. Alternative monitoring strategies, such as the number of prescribed antihypertensives 28 for an individual as opposed to using a single BP target for all, are needed. Some translational implications of this study are described further in the online supplemental file 1 – clinical perspectives.
Recommendations for BP management in specific patient groups also remains controversial. The US guidelines suggest similar recommendations for people with and without pre-existing cardiovascular disease, 1 but the UK guidelines use a higher BP threshold for people without cardiovascular disease due to lack of any direct evidence of efficacy in this patient group. 29 Although there were some variations in the treatment effects in our stratified analyses, which were likely an artefact of trial design, BP reductions were evident across a wide range of baseline BP and other personal characteristics. Unsurprisingly, there was little difference in magnitude of BP reduction between comparison arms of drug comparison trials (overall and across subgroups). The BP values substantially fell from baseline in both arms, which is likely due to regression to the mean given the high baseline BP of patients in these trials. 17 The extent to which the estimated BP reductions will have an impact on existing evidence base, which have either been based on published information on average BP differences for each trial 8 or have not adjusted for achieved BP differences between trials, 30 requires further investigation.
A number of limitations need to be considered when interpreting our findings. Investigators or data custodians of some eligible trials could not be contacted (particularly for older trials) or were unwilling to take part in the collaboration. Nevertheless, the trials included in our collaboration generally have low risk of bias. Short-term effects of BP-lowering agents are well established, 20 and our findings extend these effects over a relatively longer period of follow-up of 4 years on average (few trials had over 5 years of follow-up). We could not compare drug classes based on standardised dosages, as most treatment interventions allowed titration or addition of other drug classes to achieve specific treatment goals ( online supplemental table S3 ). Investigators were allowed to add non-study antihypertensive treatment in some trials, which could have led to the dilution of treatment effects between trial arms or subgroups. Adherence to treatment had fallen towards the end of follow-up in most trials ( online supplemental table S5 ), which could partly explain why treatment effects were lower in these latter follow-up periods. We did not have full access to individual-level information about use of non-study drugs nor on adherence to treatment to be able to quantify their effects. Yet an important strength of our study is that it permitted comparison across subgroups while maintaining the advantage of the random allocation to treatment groups.
Our study highlights the role of pharmacological agents in effectively reducing BP over several years across individuals with a wide range of characteristics, although the achieved between-group reductions, even with the intensive BP-lowering regimens, were relatively modest. Given that large-scale trials have shown the effects of pharmacological BP reduction on improving clinical outcomes, the modest BP reductions estimated in our study should still be clinically meaningful. 14 Indeed, the estimates of long-term BP reduction in this study could inform treatment strategies and help in setting realistic treatment goals in the pharmacologic management of raised BP.
Key messages
What is already known on this subject.
Randomised evidence of the effects of antihypertensive drugs on achievable blood pressure reduction has been based on trials with small sample sizes and short treatment periods of several weeks; pooled analysis of randomised evidence to provide reliable estimates of achievable long-term blood pressure reduction from pharmacological treatment is lacking.
What might this study add?
This large-scale individual participant-level data meta-analysis has shown that the patterns of blood pressure reduction differed over time, with the maximum effect seen in intensive treatment strategies that achieved 11 mm Hg systolic blood pressure reduction on average after the first year of treatment. Beneficial effects were demonstrable over wide ranges of baseline blood pressure, ages and body sizes, in women and men, by history of cardiovascular disease or diabetes and by prior use of antihypertensive treatment.
How might this impact on clinical practice?
The efficacy of antihypertensive drugs was demonstrable across different population subgroups, although the achieved blood pressure reductions, even with trialled intensive regimens, were relatively modest. These findings could guide setting realistic treatment goals in the pharmacological management of raised blood pressure.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
The Blood Pressure Lowering Treatment Trialists’ Collaboration (BPLTTC) obtained approval to conduct this collaborative research from the Oxford Tropical Research Ethics Committee (OXTREX Reference: 545–14).
- Whelton PK ,
- Aronow WS , et al
- Williams B ,
- Spiering W , et al
- Joint Committee for Guideline Revision
- Umemura S ,
- Arima S , et al
- Kim G-H , et al
- Charchar F , et al
- Tsioufis K , et al
- Ettehad D ,
- Kiran A , et al
- MacMahon S ,
- Morris JK ,
- Blood Pressure Lowering Treatment Trialists' Collaboration ,
- Turnbull F ,
- Neal B , et al
- Zanchetti A ,
- Thomopoulos C ,
- Mahdavi M ,
- Parsaeian M ,
- Mohajer B , et al
- Sundström J , et al
- Stergiou GS ,
- Dolan E , et al
- Macaskill P , et al
- Morris JK , et al
- Nazarzadeh M , et al
- Kjaergard LL ,
- Villumsen J ,
- Rothwell PM ,
- Howard SC ,
- R Foundation for Statistical Computing
- British Heart Foundation
- World Health Organization
- Nazarzadeh M ,
- Pinho-Gomes A-C ,
- Atkins ER ,
- Hsu B , et al
- National Institute for Health and Care Excellence
- Brunström M ,
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Twitter @drdcanoy, @kazemr
Collaborators The Blood Pressure Lowering Treatment Trialists’ Collaboration Working Group: Dexter Canoy, Emma Copland, Milad Nazarzadeh, Rema Ramakrishnan, Ana-Catarina Pinho-Gomes, Abdul Salam, Jamie P Dwyer, Farshad Farzadfar, Johan Sundström, Mark Woodward, Barry R Davis and Kazem Rahimi. Steering committee: Kazem Rahimi (Chair) (Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK), Koon Teo (Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada), Barry R Davis (The University of Texas School of Public Health, Houston, Texas, USA), John Chalmers (The George Institute for Global Health, University of New South Wales, Sydney, Australia), Carl J Pepine (Department of Medicine, University of Florida, Gainesville, Florida, USA). Collaborating triallists: A Adler (UK Prospective Diabetes Study (UKPDS)), L Agodoa (African-American Study of Kidney Disease and Hypertension), A Algra (Dutch Transient Ischemic Attack Study), F W Asselbergs (Prevention of Renal and Vascular End-stage Disease Intervention Trial (PREVEND-IT)), N S Beckett (Hypertension in the Very Elderly Trial (HYVET)), E Berge (deceased) (Valsartan Antihypertensive Long-term Use Evaluation trial (VALUE trial)), H Black (Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE)), F P J Brouwers (PREVEND-IT), M Brown (International Nifedipine GITS Study: Intervention as a Goal in Hypertension (INSIGHT)), C J Bulpitt (European Working Party on High Blood Pressure in the Elderly, HYVET), R P Byington (Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT)), J Chalmers (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), Perindopril Protection Against Recurrent Stroke (PROGRESS)), W C Cushman (Action to Control Cardiovascular Risk in Diabetes), Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), Systolic Blood Pressure Intervention Trial (SPRINT)), J Cutler (ALLHAT), B R Davis (ALLHAT), R B Devereaux (Losartan Intervention For Endpoint Reduction in Hypertension (LIFE)), J P Dwyer (Irbesartan Diabetic Nephropathy Trial (IDNT)), R Estacio (Appropriate Blood Pressure Control in Diabetes (ABCD)), R Fagard (SYSTolic Hypertension in EURope (SYST-EUR)), K Fox (European Trial on Reduction of Cardiac Events with Perindopril among Patients with Stable Coronary Artery Disease), T Fukui (Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J)), A K Gupta (Anglo-Scandinavian Cardiac Outcomes Trial – Blood Pressure Lowering Arm (ASCOT-BPLA), R R Holman (UKPDS), Y Imai (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP)), M Ishii (Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B)), S Julius (VALUE), Y Kanno (Efficacy of Candesartan on Outcome in Saitama Trial (E-COST)), S E Kjeldsen (VALUE and LIFE), J Kostis (Systolic Hypertension in the Elderly Program (SHEP)), K Kuramoto (National Intervention Cooperative Study in Elderly Hypertensives), J Lanke (Swedish Trial in Old Patients with Hypertension-2 (STOP Hypertension-2), Nordic Diltiazem (NORDIL)), E Lewis (IDNT), J B Lewis (IDNT), M Lievre (Non-insulin-dependent diabetes, hypertension, microalbuminuria or proteinuria, cardiovascular events, and ramipril study), L H Lindholm (Captopril Prevention Project), STOP Hypertension-2 and NORDIL), S Lueders (The Morbidity and Mortality After Stroke, Eprosartan Compared With Nitrendipine for Secondary Prevention (MOSES)), S MacMahon (ADVANCE), G Mancia (INSIGHT), M Matsuzaki (The Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE)), M H Mehlum (VALUE), S Nissen (Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis), H Ogawa (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Heart Disease), T Ogihara (CASE-J, Combinations of OLMesartan and COPE), T Ohkubo (HOMED-BP), C R Palmer (INSIGHT), A Patel (ADVANCE), C J Pepine (International Verapamil SR-Trandolapril Study), M A Pfeffer (Prevention of Events with Angiotensin-Converting Enzyme Inhibition), B Pitt (PREVENT), N R Poulter (Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)), H Rakugi (CASE-J and Valsartan in Elderly Isolated Systolic Hypertension Study (VALISH)), G Reboldi (CARDIOvascolari del Controllo della Pressione Arteriosa SIStolica (Cardio-Sis)), C Reid (The Second Australian National Blood Pressure Study (ANBP2)), G Remuzzi (BErgamo NEphrologic DIabetes Complications Trial (BENEDICT)), P Ruggenenti (BENEDICT), T Saruta (CASE-J), J Schrader (MOSES), R Schrier (deceased) (ABCD), P Sever (ASCOT-BPLA), P Sleight (deceased) (CONVINCE, Heart Outcomes Prevention Evaluation (HOPE), Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND)), J A Staessen (SYST-EUR), H Suzuki (E-COST), L Thijs (SYST-EUR), K Ueshima (CASE-J and VALISH), S Umemoto (COPE), W H van Gilst (PREVEND-IT), P Verdecchia (Cardio-Sis), K Wachtell (LIFE), P Whelton (SPRINT), L Wing (ANBP2), M Woodward (ADVANCE and PROGRESS), Y Yui (JMIC-B), S Yusuf (HOPE, ONTARGET, PRoFESS and TRANSCEND), A Zanchetti (deceased) (European Lacidipine Study on Atherosclerosis, Verapamil in Hypertension and Atherosclerosis Study), Z Y Zhang (SYST-EUR). Other members: C Anderson, C Baigent, BM Brenner, R Collins, D de Zeeuw, J Lubsen, E Malacco, B Neal, V Perkovic, A Rodgers, P Rothwell, G Salimi-Khorshidi, J Sundström, F Turnbull, G Viberti and J Wang.
Contributors DC, EC and KR have full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors gave final approval of the version to be published. Concept and design: DC and KR. Data acquisition: KR, EC and DC. Data analysis or interpretation of data: DC, EC, RR, MN, A-C P-G, AS, JPD, FF, JS, MW, BRD and KR. Drafting of the manuscript: DC, EC, MW and KR. Critical revision of the manuscript for important intellectual content: MN, RR, A-C P-G, AS, JD, FF, JS and BRD. Statistical analysis: EC, RR, DC and MW. Obtained funding: KR and DC. Guarantor: KR and DC.
Funding This research was funded by the British Heart Foundation (PG/18/65/33872 and FS/19/36/34346), the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford Martin School. This manuscript was prepared using ACCORD, ALLHAT, HDFP, PEACE and SHEP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Centre and does not necessarily reflect the opinions or views of the ACCORD, ALLHAT, HDFP, PEACE and SHEP or the NHLBI. We acknowledge original depositors of the ANBP data and the Australian Data Archive and declare that those who carried out the original analysis and collection of the data bear no responsibility for the further analysis or interpretation of the data.
Disclaimer The funders had no role in the design or conduct of the study, data analysis, manuscript preparation or approval to submit for publication The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
Competing interests DC reports grants from the British Heart Foundation, during the conduct of the study. MN and AP reports grants from the British Heart Foundation outside the submitted work. JS reports ownership in companies providing services to Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer, Bayer, Pfizer and AstraZeneca outside the submitted work. MW reports personal fees from Amgen, Kyowa Kirin and Freeline outside the submitted work. KR reports grants from the British Heart Foundation, UK Research and Innovation Global Challenges Research Fund, Oxford Martin School, and National Institute for Health Research Oxford Biomedical Research Centre, during the conduct of the study, and personal fees from BMJ Heart and PLOS Medicine , outside the submitted work. EC, RR, AS, JD, FF and BRD declare no competing interests.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Google Releases A.I. That Can Predict How the Human Body’s Molecules Behave, Boosting Drug Discovery Research
Called AlphaFold 3, the latest update of the software models the interactions of proteins with DNA, RNA and other molecules for the first time
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/ChristianThorsberg_Headshot.png)
Christian Thorsberg
Daily Correspondent
:focal(960x544:961x545)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/f1/c4/f1c4ab29-c6b6-4088-a04a-b804c783b0fb/deepmind.webp)
Last week, Google released a much-anticipated upgrade for its AlphaFold software, which harnesses artificial intelligence to predict the shape and structure of molecules within the human body.
Any given molecule’s shape is indicative of its function and behavior, so biologists have long researched how chains of amino acids, the building blocks of proteins, fold into various shapes. The A.I. tool can accelerate and streamline this process, opening new avenues for breakthroughs—notably in vaccine and drug development.
AlphaFold 3, the newest update from Google DeepMind described last week in the journal Nature , builds on its previous two iterations. The software’s initial tease in 2018 offered potential for accurately predicting the three-dimensional structure of proteins, while its 2020 update, AlphaFold 2, came with significant improvements. In 2021, Google released an open-source version of AlphaFold, along with the predicted 3D structures of nearly all known proteins in the human body. The next year, two million predicted protein structures were shared.
But despite these leaps forward—which helped researchers map the human heart and better understand extinct birds’ eggs —AlphaFold 2 was limited in scope to modeling proteins.
“The AlphaFold 2 system only knew about amino acids, so it was of very limited utility for biopharma,” Mohammed AlQuraishi , a systems biologist at Columbia University who is not affiliated with Google DeepMind, tells MIT Technology Review ’s James O’Donnell.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/8e/cf/8ecf8233-17b3-47fe-a71c-38637ee9d744/deepmind2.png)
The newest version of the software can predict not only the shape of proteins, but also the structures of DNA, RNA and other molecules, such as ligands. Crucially, this update will allow researchers to better predict and study how different molecules in the human body geometrically interact with each other—and anticipate where a drug might bind to a protein.
This ability can “save months of experimental work and enable research that was previously impossible,” Deniz Kavi , a co-founder and chief executive of Tamarind Bio, a drug discovery start-up, tells the New York Times ’ Cade Metz. “This represents tremendous promise.”
Researchers could use the software update to probe some initial questions, including how proteins respond to DNA damage within the human body.
“It tells us a lot more about how the machines of the cell interact,” John Jumper , the director of Google DeepMind, tells the New York Times . “It tells us how this should work and what happens when we get sick.”

AlphaFold 3 offers researchers a level of confidence, often ranging from 40 percent to 80 percent, with each prediction it models, according to MIT Technology Review . Parts of a structure modeled with high confidence appear in blue, while the more uncertain regions appear in red. In some areas, its inaccuracy is a limitation—for modeling RNA-protein interactions, for example, the system isn’t yet highly exact.
Another potential drawback is the model’s ability to “hallucinate,” or produce false information. The team behind AlphaFold 3, in order to build its molecular library and function, borrowed methods from other A.I. models, such as OpenAI’s DALL-E 2 and Sora, that generate images and video. This improved AlphaFold 3’s capacity to produce large 3D images of molecular shapes, but leaves it liable to hallucinate. The team hopes to alleviate this issue with more training data, and in the paper, they note that hallucinated structures would typically be marked as low confidence.
Unlike its predecessor, the code for AlphaFold 3 will not be made open-source, and only a limited version, the AlphaFold Server , will be released publicly.
“This is a big advance for us,” Demis Hassabis , the CEO of Google DeepMind, tells WIRED ’s Will Knight. “This is exactly what you need for drug discovery: You need to see how a small molecule is going to bind to a drug, how strongly and also what else it might bind to.”
Get the latest stories in your inbox every weekday.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/ChristianThorsberg_Headshot.png)
Christian Thorsberg | READ MORE
Christian Thorsberg is an environmental writer and photographer from Chicago. His work, which often centers on freshwater issues, climate change and subsistence, has appeared in Circle of Blue , Sierra magazine, Discover magazine and Alaska Sporting Journal .
- Open access
- Published: 15 May 2024
Genetic variations in anti-diabetic drug targets and COPD risk: evidence from mendelian randomization
- Yue Su 1 na1 ,
- Youqian Zhang 2 na1 &
- Jinfu Xu 1
BMC Pulmonary Medicine volume 24 , Article number: 240 ( 2024 ) Cite this article
Metrics details
Previous research has emphasized the potential benefits of anti-diabetic medications in inhibiting the exacerbation of Chronic Obstructive Pulmonary Disease (COPD), yet the role of anti-diabetic drugs on COPD risk remains uncertain.
This study employed a Mendelian randomization (MR) approach to evaluate the causal association of genetic variations related to six classes of anti-diabetic drug targets with COPD. The primary outcome for COPD was obtained from the Global Biobank Meta-analysis Initiative (GBMI) consortium, encompassing a meta-analysis of 12 cohorts with 81,568 cases and 1,310,798 controls. Summary-level data for HbA1c was derived from the UK Biobank, involving 344,182 individuals. Positive control analysis was conducted for Type 2 Diabetes Mellitus (T2DM) to validate the choice of instrumental variables. The study applied Summary-data-based MR (SMR) and two-sample MR for effect estimation and further adopted colocalization analysis to verify evidence of genetic variations.
SMR analysis revealed that elevated KCNJ11 gene expression levels in blood correlated with reduced COPD risk (OR = 0.87, 95% CI = 0.79–0.95; p = 0.002), whereas an increase in DPP4 expression corresponded with an increased COPD incidence (OR = 1.18, 95% CI = 1.03–1.35; p = 0.022). Additionally, the primary method within MR analysis demonstrated a positive correlation between PPARG-mediated HbA1c and both FEV1 (OR = 1.07, 95% CI = 1.02–1.13; P = 0.013) and FEV1/FVC (OR = 1.08, 95% CI = 1.01–1.14; P = 0.007), and a negative association between SLC5A2-mediated HbA1c and FEV1/FVC (OR = 0.86, 95% CI = 0.74–1.00; P = 0.045). No colocalization evidence with outcome phenotypes was detected (all PP.H4 < 0.7).
This study provides suggestive evidence for anti-diabetic medications' role in improving COPD and lung function. Further updated MR analyses are warranted in the future, following the acquisition of more extensive and comprehensive data, to validate our results.
Peer Review reports
Introduction
Chronic obstructive pulmonary disease (COPD), is a progressive lung disease characterized by poorly reversible airflow blockage and persistent lung inflammation [ 1 ], contributing to increased resistance to airflow in the small conducting airways, reduced compliance of the lungs, and progressive airflow obstruction and air trapping [ 2 ]. Owing to the lung is a complex and vulnerable organ that is exposed to smoking, environmental degradation, and occupational hazards, the prevalence rate of COPD is increasing [ 3 ]. Based on the systematic analysis for the Global Burden Disease Study 2019, lower respiratory infection was the 3rd and COPD was the 6th cause of death, and the incidence rates vary by country and region, with higher rates in low- and middle-income countries and among people who smoke or work in industries with exposure to lung irritants [ 4 ]. The treatment for COPD includes medications, oxygen therapy, pulmonary rehabilitation, surgery, and lifestyle changes.
Diabetes mellitus is a chronic and metabolic disease, involving inappropriately increased blood glucose levels and systematic inflammatory responses accompanied by decreased insulin synthesis, insulin resistance (IR) or reduced metabolic response to insulin in many tissues [ 5 ]. To date, the major classes of oral antihyperglycemic medications include biguanides (e.g., metformin), thiazolidinedione (TZD), sulfonylureas, glucagon-like peptide 1 (GLP-1) receptor agonists, Insulin/insulin analogues (i.e., INSR), sodium-glucose cotransporter (SGLT2) inhibitors, dipeptidyl peptidase-IV (DPP-IV) inhibitors, and α-glucosidase inhibitors. Several studies have demonstrated the beneficial effect of antidiabetic drugs on COPD and its exacerbations. Pradhan et al. have demonstrated that GLP-1 receptor agonists and SGLT-2 inhibitors were capable of reducing severe exacerbations compared to sulfonylureas in patients diagnosed with COPD. However, the use of DPP-4 inhibitors did not clearly show a reduced risk of exacerbations in COPD [ 6 ]. Similarly, Au et al have suggested that SGLT2 inhibitor was associated with a decreased risk of incidence and exacerbations of obstructive airway diseases (OAD) compared with DPP4 inhibitor [ 7 ]. However, Hitchings et al. have found that metformin did not effectively decrease blood glucose levels in non-diabetic patients admitted to the hospital due to exacerbations of COPD, and no beneficial effect on CRP downregulation or clinical outcomes was identified [ 8 ]. However, to date, the role of antidiabetic drugs on COPD risk is inconclusive, and the confounding bias and conflicting results contribute to uncertain causation between antihyperglycemic medications and COPD.
Mendelian randomization is a statistical technique used in epidemiology and genetics to investigate causal relationships between an exposure (or risk factor) and an outcome, utilizing genetic variants or alleles as instrumental variables to estimate the causal effect of an exposure on an outcome. Importantly, MR is able to provide evidence for causality by leveraging genetic variants as instrumental variables, which helps overcome issues such as reverse causality and confounding over traditional observational studies and randomized controlled trials over traditional observational studies and randomized controlled trials. It is also less prone to biases introduced by self-reporting or recall bias. Hence, we performed a two-sample MR analysis in this study to explore the association of antidiabetic drugs with COPD.
Materials and methods
Study design.
The foundational data for this study were procured from publicly accessible, summary-level datasets originating from Genome-Wide Association Studies (GWAS) and Expression Quantitative Trait Loci (eQTL) studies. A description of summary statistics data sources is available in Supplementary file 1 : Table S1 and Supplementary file 2 . Figure 1 displays the details of the Mendelian Randomization (MR) design. All included GWAS studies have received approval from the relevant institutional review boards. As this research involves secondary analysis of publicly available data, no additional ethical approval was required.
Flowchart of the study analysis
Selection of genetic instrumental variables
In this study, the GeneCard database ( https://www.genecards.org/ ) and drug targets used in this MR study [ 9 ], we identified the final drug targets analysed, namely TZD (i.e. PPARG), SU (i.e. KCNJ11 and ABCC8), GLP-1 analogues (i.e. GLP1R), Insulin/insulin analogues (i.e. INSR), SGLT2 inhibitor (i.e. SLC5A2), DPP-IV inhibitor (i.e. DPP4), metformin (i.e. PRKAB1, ETFDH, etc .). Owing to the controversy surrounding metformin's protein target and the largely unknown molecular basis of its physiological effects [ 10 ], we excluded metformin from further analytical consideration.
Summary-level data for eQTLs were sourced from the eQTLGen Consortium ( https://www.eqtlgen.org/ ). We pinpointed common eQTLs single-nucleotide polymorphisms (SNPs) with a minor allele frequency (MAF) over 1% [ 11 ] that demonstrated a statistically significant correlation ( p < 5.0 × 10 −8 ) with PPARG, KCNJ11, GLP1R, INSR, SLC5A2 and DPP4 expression in blood. ABCC8 lacked significant eQTL levels and was therefore excluded. To generate genetic tools in this study, only cis eQTLs were included. These were defined as eQTLs situated within a 1 Mb range on either side of the encoding gene.
SNPs associated with HbA1c levels at genome-wide significance levels ( p < 5.0 × 10 −8 ) were selected from the target genes (± 100 kb of gene location) for each drug, based on the methodology used in the previous study [ 12 ]. HbA1c was obtained from UKB. To maximize the strength of the instrument for each drug, SNPs used as instruments were allowed to be in low weak linkage disequilibrium (r2 < 0.30) with each other. Finally, four drug targets were included in the study (PPARG, KCNJ11, GLP1R and SLC5A2). Additionally, we employed F-statistics (F = beta²/se²; beta for the SNP-exposure association; variance (se)) to examine the presence of weak instrumental variables [ 13 ]. The higher the F-statistic, the stronger the instrumental strength indicated. It was a requirement that all included SNPs have an F-statistic greater than 10. In MR analyses, MR-Steiger filtering was utilized to enhance the robustness of our findings by excluding variants manifesting stronger associations with the outcomes than with the exposure [ 13 ].
The primary outcome was COPD, derived from the database with the largest sample size of GWAS data available, the Global Biobank Meta-analysis Initiative (GBMI).
The GBMI [ 14 ], covering GWAS meta-analyses of 12 biobanks (BioMe, BioVU, Colorado Center for Personalised Medicine, Estonian Biobank, FinnGen, Generation Scotland, HUNT, Lifelines, Massachusetts General Brigham Biobank FinnGen, Generation Scotland, HUNT, Lifelines, Massachusetts General Brigham Biobank, Michigan Genomics Initiative, UCLA Precision Health Biobank, UK Biobank), ultimately including a European population of 81568 cases and 1310798 controls.
For secondary outcomes, lung function traits were examined, specifically Forced Expiratory Volume in 1-second (FEV1), Forced Vital Capacity (FVC), and the FEV1/FVC ratio. We utilized data from the most extensive presently available lung function GWAS by Shrine et al., which reported 279 genome-wide significant SNPs ( p < 5×10 −9 ) from a population of 321,407 of European ancestry [ 15 ]. The study adjusted for age, age 2 , height, and smoking status.
Statistical analyses
Primary mr analysis.
We employed the Summary-data-based Mendelian Randomization (SMR) approach to generate effect estimations using eQTLs as instrumental variables. This method enables a comprehensive exploration of the association between a specific gene's expression level and the desired outcome, utilizing summary-level data from both GWAS and eQTL studies. We used SMR software, version 1.03 ( https://cnsgenomics.com/software/smr/#Overview ), for allele harmonization and subsequent analytical procedures. The Wald ratio test was applied to individual instrumental variables (IVs), followed by the multiplicative random-effects inverse-variance-weighted (IVW) method for estimating causal associations of multiple IVs (≥ 2), supplemented by the MR-Egger and weighted median methods. The IVW weighting is directly proportional to the Wald ratio estimate for each SNP and inversely proportional to the variance estimate of the Wald ratio for each SNP. IVW provides reliable and efficient estimates when all genetic variations are considered valid. The weighted median method performs better when at least half of the genetic variations are considered invalid, whereas the MR-Egger method is applied when all genetic variations are considered invalid.
Genetic correlation analysis
Linkage Disequilibrium Score (LDSC) regression, applicable to summary-level GWAS data, serves as an effective approach to genetic correlation analysis of complex diseases or traits. It distinguishes true polygenic signals from confounding biases such as cryptic relatedness and population stratification. If the genetic correlation demonstrates both statistical and quantitative significance, the overall phenotype correlation cannot be entirely attributed to environmental confounding factors [ 16 ]. The LDSC tool ( https://github.com/bulik/ldsc ) assists us in evaluating the genetic correlations among HbA1c, COPD, FEV1, FVC, and the FEV1/FVC ratio.
Colocalization analysis
To confirm the shared causal genetic variation of eQTL implicated in the outcome phenotype as identified by MR, we conducted a colocalization analysis using the R package Coloc (version 3.2-1) [ 17 ]. The genetic variant showing the strongest association with the exposure in the MR assessment, indicated by the lowest P -value, was selected as the reference variant. Genetic variants within a range of ± 100 kb of this reference variant was included in the study. The LD reference panel was based on the 1000 Genomes v3 European ancestry dataset. The criterion for colocalization was a posterior probability exceeding 0.7 for a shared causal variant (posterior probability of hypothesis 4 > 0.7).
Sensitivity analysis
We initially conducted a positive control analysis to verify the validity of the two selected genetic instruments. As HbA1c reflects the average blood sugar level over the past 2–3 months due to long-term exposure of haemoglobin to blood sugar, we investigated the association of related exposures with HbA1c levels as a positive control study for eQTL tools. For the HbA1c GWAS tool, given that diabetes is the primary indication for hypoglycemic drugs, we conducted a positive control study by investigating the association of related exposures with T2DM.
Within the SMR analysis, we acknowledged that observed associations between gene expression and the outcome could result from a linkage scenario. To validate this, we utilized the heterogeneity in dependent instruments (HEIDI) test. A P -value less than 0.01 would indicate the possibility of an association due to linkage [ 18 ]. Considering potential horizontal pleiotropy, we initially identified other genes significantly associated with genetic IVs (5×10 −8 ) in close proximity to the top eQTL (within a 1 Mb window). We then performed SMR analysis to determine whether a single SNP was associated with the expression of multiple genes.
During the MR analysis, numerous tests were employed to ensure rigour and validity. Cochran's Q test was used to assess heterogeneity amongst the selected genetic variants, with a P -value of less than 0.05 indicating significant dissimilarity among the SNPs under investigation [ 16 ]. We further scrutinized any potential directional pleiotropy within our MR study by using MR-Egger regression [ 19 ]. Significant directional pleiotropy is indicated by a P -value less than 0.05 for the MR-Egger's intercept, despite this method's relative lack of precision [ 20 ]. We also employed the MR-PRESSO method to identify potential outliers and explore horizontal pleiotropy, inferred if the global P -value is under 0.05 [ 21 ]. This process allowed for the removal of outliers, thereby improving the accuracy of our correction. Following the Bonferroni adjustment for multiple testing, we regarded a P -value smaller than 0.0125 (derived from 0.05/4) as evidence of a statistically significant causal association. A P -value lower than 0.05 is considered to offer suggestive evidence of a possible causal relationship.
Genetic instruments selection and genetic correlation between phenotypes
We identified 1127, 330, 134, and 13 cis-eQTLs for the drug target genes PPARG, KCNJ11, GLP1R, and SLC5A2, respectively, from the eQTLGen Consortium. The most significant cis-eQTL SNP for each drug target gene was selected as a genetic tool. Furthermore, from the GWAS summary data for HbA1c levels, we identified 23, 3, 3, and 4 SNPs within or near the genes PPARG, KCNJ11, GLP1R, and SLC5A2, respectively (Supplementary file 1 : Table S2). The average F-statistic values of all instrument variations are 63.73, 81.69, 51.69, and 32.44, respectively, indicating robustness against weak instrument bias (Supplementary file 1 : Table S3). In the positive control study, significant associations were observed between exposure to each drug and HbA1c when using the IVs represented by eQTLs (Supplementary file 1 : Table S4). Similarly, significant associations were found between exposure to each medication and T2DM when using the IVs proposed by HbA1c GWAS, further confirming the effectiveness of the selected genetic instruments (Supplementary file 1 : Table S5).
LDSC analysis unveiled robust genetic correlations between HbA1c and COPD (genetic correlation = 0.2048, P = 3.52×10 −13 ), FEV1 (genetic correlation = -0.1179, P = 1.27×10 −7 ), and FVC (genetic correlation = -0.1342, P = 2.88×10 −9 ). A less pronounced genetic correlation was observed between HbA1c and FEV1/FVC (genetic correlation = 0.0221, P = 0.32). SNP-based liability-scale heritability h² for HbA1c, COPD, FEV1, FVC, and FEV1/FVC were 0.173, 0.011, 0.196, 0.191, and 0.189, respectively (Supplementary file 1 : Table S6).
Primary analysis
In Fig. 2 , the SMR analysis provided suggestive evidence that a one standard deviation (SD) increases in the blood expression of the KCNJ11 gene reduced the incidence of COPD by 13% (OR = 0.87, 95% CI = 0.79–0.95; P = 0.002). Additionally, a one SD increase in the expression of the DPP4 gene in the blood increased the incidence of COPD by 18% (OR = 1.18, 95% CI = 1.03–1.35; P = 0.022), implying that HMGCR agonists and DPP4 inhibitors may reduce the risk of COPD. No significant association was found between the expression of PPARG, GLP1R, INSR, and SLC5A2 and outcome phenotypes (Supplementary file 1 : Table S7).
Summary-data-based Mendelian randomization (SMR) association between expression of gene PPARG, KCNJ11, GLP lR, INSR, SLC5A2, or DPP4 and outcome phenotypes. SMR method was used to assess the association. COPD, chronic obstructive pulmonary disease; FEV 1, forced expiratory volume in 1-second;FVC, forced vital capacity; HEIDI, heterogeneity in dependent instruments.
In Fig. 3 , The MR analysis found strong evidence for an association between PPARG-mediated HbA1c and higher FEV1 (OR = 1.07, 95% CI = 1.02–1.13; P = 0.013) as well as FEV1/FVC (OR = 1.08, 95% CI = 1.01–1.14; P = 0.007), supporting a protective role of PPARG agonists on lung function (Fig. 2 , Table S 4 ). Suggestive evidence was observed for an association between SLC5A2-mediated HbA1c and lower FEV1/FVC (OR = 0.86, 95% CI = 0.74-1.00; P = 0.045), supporting a protective role of SLC5A2 inhibitors on lung function. No evidence was found of an association between HbA1c mediated by KCNJ11 or GLP1R and outcome phenotypes (Supplementary file 1 : Table S8). All genes demonstrated no evidence of colocalization with the risk of outcome phenotypes (posterior probability of hypothesis 4 < 0.7) (Supplementary file 1 : Table S9 and Supplementary file 3 : Figure S1-24).
Summary results of the primary MR analyses between HbA1c mediated by gene PPARG, KCNJ11, GLP lR, or SLC5A2 and outcome phenotypes. IVW, Inverse-variance-weight; MR, Mendelian randomization; COPD, chronic obstructive pulmonary disease; FEV 1, forced expiratory volume in 1-second; FVC, forced vital capacity.
The SMR analysis revealed that all observed associations were not due to linkage disequilibrium (HEIDI test P > 0.01), with the exception of associations between PPARG expression and FVC ( P = 0.009), and DPP4 expression and FEV1 ( P = 0.009) and FVC ( P = 0.004) (Supplementary file 1 : Table S10). We further explored potential horizontal pleiotropy between KCNJ11 and DPP4 expression and COPD and lung function, by examining associations between the expression of nearby genes significantly associated with the top eQTL SNPs of KCNJ11 and DPP4 and the outcomes. We identified 4 genes (including DPP4) and 8 genes (including KCNJ11) whose expressions were associated with instrumental variants (Table S). After screening, only two and eight genes, respectively, for DPP4 and KCNJ11, had available eQTLs at the genome-wide level ( P < 5.0 × 10 −8 ). The final results indicated that only KCNJ11 and DPP4 expression were significantly associated with COPD, ruling out the distortion of the results by horizontal pleiotropy (Supplementary file 1 : Table S11).
In MR analysis, the Cochran Q test found no heterogeneity in the main reported results (all P > 0.05; Table S 4 ), but unremovable heterogeneity was found in the PPARG-mediated HbA1c with FEV1 and FVC, and SLC5A2-mediated HbA1c with FVC. However, the intercepts in the MR-Egger regression and MR-PRESSO analyses indicated that the overall horizontal pleiotropy was not significant. Additionally, all instrumental variables passed MR-Steiger filtering, proving that the results remain robust (all P > 0.05; Supplementary file 1 : Table S8).
The present study demonstrated the association between antidiabetic drugs and respiratory diseases through the SMR method. We found robust genetic correlations between HbA1c and COPD and lung function, including FEV1, and FVC. Moreover, with every SD increase in the expression of the KCNJ11 gene in the blood, the incidence of COPD is reduced by 13%, implying that KCNJ11 agonists may reduce the risk of COPD. Likewise, every SD increase in the expression of the DPP4 gene in the blood, the incidence of COPD increases by 18%. No significant association was found between the expression of PPARG, GLP1R, INSR, and SLC5A2 and outcome phenotypes.
PPARG, KCNJ11, GLP1R, and SLC5A2 are common therapeutic targets of antihyperglycemic drugs which are capable of inducing cellular differentiation, reducing cellular proliferation and inducing apoptosis [ 22 ]. PPARG is a member of the nuclear receptor superfamily of ligand-dependent transcription factors that is predominantly expressed in adipose tissue, modulating insulin sensitization and glucose metabolism [ 23 ]. Moreover, PPARG also reduces the morbidity in the experimental models of asthma, COPD and acute lung injury [ 24 , 25 ]. Specifically, Solleti and colleagues have suggested that PPARG in the airway epithelial cell is able to modulate cigarette smoke-induced chemokine expression and emphysema susceptibility in mice [ 26 ]. Similarly, Karagiannis et al have found that PPARG is capable of controlling lipid uptake and transient storage in lipid droplets by influencing glucose levels [ 27 ]. GLP-1 as an incretin hormone possesses anti-inflammatory and immune-modulatory functions, and its receptor agonists contribute to decreased blood glucose levels [ 28 ]. Available studies have suggested that GLP-1 and its based agents have therapeutic potential in lung injury and COPD [ 29 , 30 ]. Wei et al have shown that GLP-1-based pharmaceuticals presented reduced occurrence in most chronic lung diseases, except for interstitial lung disease [ 31 ]. Dogan et al have demonstrated that liraglutide use leads to increased FVC but no benefits in FEV1/FVC and 6-min walking distance [ 32 ]. Moreover, Rogliani and colleagues have shown that FEV1, FVC and maximal expiratory flow are significantly elevated after the GLP-1R treatment but not in the control group and insulin cohorts [ 33 ]. SLC5A2, namely the SGLT2 gene, was reported to be the drug target for SGLT2 inhibitors, hence the spatial distribution of SLC5A2 would provide insights into the target tissues of SGLT2 inhibitors [ 34 ]. Takashima and colleagues have demonstrated that low-dose luseogliflozin ameliorates ischemic brain injury in mice without glucose-lowering effects [ 35 ]. Similarly, Joki et al have shown that tofogliflozin modulates pulmonary vascular remodelling in mice with left heart disease [ 36 ]. Importantly, Jeong and co-workers have suggested that empagliflozin is able to improve respiratory function, reduce ischemia/reperfusion (I/R)-induced pulmonary edema and inflammatory cytokine production and protein concentration in the bronchoalveolar lavage (BAL) fluid through ERK1/2-mediated signalling pathway [ 37 ]. However, no significant association was found between the expression of PPARG, GLP-1R, and SLC5A2 and outcome phenotypes through SMR analysis, while KCNJ11 and DPP4 inhibitors may reduce the risk of COPD.
KCNJ11 encodes pancreatic β cell KATP channel pore-forming subunit (Kir6.2) with a key role in insulin secretion and glucose homeostasis, and mutation in KCNJ11 contributes to impaired blood glucose control [ 38 ]. A national cohort study showed that SU application was related to lower risks of cardiovascular events, ventilation use, pulmonary pneumonia, and mortality in the patients with COPD and diabetes [ 39 ]. Consistently, Wang et al have suggested that a duration-dependent beneficial impact of SU on severe COPD exacerbation was proved in patients with diabetes [ 40 ]. DPP4 encodes dipeptidyl peptidase 4 expressed on most cell types to decrease the expression of GLP-1 in the intestinal tract [ 41 ]. Kotnala and colleagues have shown that alveolar macrophages isolated from COPD patients presented higher DPP4 expression than that of healthy individuals, and DPP4 inhibition may alleviate the severity of haemophilus influenzae-induced COPD [ 42 ]. Similarly, the level of serum DPP4 was elevated in the patients with acute COPD exacerbation (AECOPD) [ 43 ]. However, a clinical study by Au et al found that DPP4 inhibitor use did not reduce the OAD exacerbations [ 7 ].
MR analysis is a genetic epidemiological approach that takes advantage of minimizing bias due to confounding and reverse causality and thus improves the causal inference [ 44 ]. In the present study, we found that no significant association was found between the expression of PPARG, GLP1R, INSR, and SLC5A2 and outcome phenotypes. Two-sample MR analyses have suggested that PPARG or SLC5A2-mediated HbA1c play an important role in lung function, suggesting a protective role of SLC5A2 inhibitors on lung function. Additionally, there was no evidence of an association between HbA1c mediated by KCNJ11 or GLP1R and outcome phenotypes. In the SMR analysis, only KCNJ11 and DPP4 expression were significantly associated with COPD. However, the intercepts in the MR-Egger regression and MR-PRESSO analyses indicated that the overall horizontal pleiotropy was not significant.
The primary advantage of this study lies in employing genetic tools to represent drug exposure, a strategy that reduces potential bias from external factors and prevents the issue of reverse causation. Furthermore, we utilized two distinct genetic instruments to mimic the drug under investigation. This approach adds credibility to the obtained effect estimates as they support each other. Additionally, we conducted several sensitivity analyses to assess the reliability of the genetic instruments and the assumptions made in this study. However, there are several limitations in this study. First, while we have conducted a range of sensitivity analyses to evaluate the assumptions of the MR study, it is important to acknowledge that the possibility of confounding bias and/or horizontal pleiotropy cannot be entirely ruled out. Second, it is important to note that the anticipated effects of drugs based on genetic predictions may not align with actual therapeutic outcomes. The genetic variants that serve as instruments for drug exposure are present from birth and lasts throughout one's lifetime. Consequently, our analyses cannot capture the impact of exposure to antidiabetic medications during specific periods of life. Third, it is worth noting that the eQTLs and GWAS data utilized in our research primarily stem from individuals with European ancestry, hence, extending these findings to other population groups is essential. Lastly, due to the use of summary-level data, we were unable to conduct subgroup analyses. Therefore, it is imperative to conduct further MR studies using individual-level data to obtain more comprehensive and detailed information to decide the individuals who are likely to be taking these drugs.
To sum up, the MR analysis indicates a potential cause-and-effect connection between KCNJ11 and DPP4 inhibitors and COPD risk, recommending that clinical trials should be conducted to investigate whether antidiabetic drugs offer protective benefits against COPD. Additionally, more research is necessary to delve into the underlying mechanisms.
Availability of data and materials
The authors confirm that the data supporting the findings of this study within the article and its Supplementary material. Raw data analyzed for the present study are available from the corresponding author upon reasonable request.
Riley Craig, Frank S. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA. 2019;321(8):786–797.
MacNee W. ABC of chronic obstructive pulmonary disease: Pathology, pathogenesis, and pathophysiology. BMJ. 2006;332(7551):1202–4.
Article PubMed Central Google Scholar
Labaki WW, Han MLK. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–3. https://doi.org/10.1016/S2213-2600(20)30157-0 .
Article PubMed PubMed Central Google Scholar
Global Burden Disease (GBD). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019. Lancet. 2019;1204–222.
Limin Wang, Wen Peng, Zhenping Zhao, Mei Zhang, Zumin Shi, Ziwei Song, Xiao Zhang, Chun Li, Zhengjing Huang, Xiaomin Sun, Linhong Wang, Maigeng Zhou, Jing Wu, Youfa Wang. Prevalence and Treatment of Diabetes in China, 2013–2018.pdf. JAMA. 2021;326(24):2498–2506.
Pradhan R, et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ. 2022;379:e071380. https://doi.org/10.1136/bmj-2022-071380 .
Au PCM, et al. Association of Sodium-Glucose Cotransporter 2 Inhibitor vs Dipeptidyl Peptidase-4 Inhibitor Use With Risk of Incident Obstructive Airway Disease and Exacerbation Events Among Patients With Type 2 Diabetes in Hong Kong. JAMA Netw Open. 2023;6(1):e2251177. https://doi.org/10.1001/jamanetworkopen.2022.51177 .
Hitchings AW, Lai D, Jones PW, Baker EH. Metformin in severe exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2016;71(7):587–93. https://doi.org/10.1136/thoraxjnl-2015-208035 .
Tang B et al. Genetic Variation in Targets of Antidiabetic Drugs and Alzheimer Disease Risk. Neurology. 2022;(7):e650. LP-e659. https://doi.org/10.1212/WNL.0000000000200771 .
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85. https://doi.org/10.1007/s00125-017-4342-z .
Willer CJ, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83. https://doi.org/10.1038/ng.2797 .
Article CAS PubMed PubMed Central Google Scholar
Huang W, Xiao J, Ji J, Chen L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. Elife. 2021;10. https://doi.org/10.7554/eLife.73873 .
Bowden J, Del Greco F, Minelli MC, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6);1961–1974. https://doi.org/10.1093/ije/dyw220 .
Zhou W, et al. Global Biobank Meta-analysis Initiative: Powering genetic discovery across human disease. Cell Genomics. 2022;2(10):100192. https://doi.org/10.1016/j.xgen.2022.100192 .
Shrine N, et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51(3):481–93. https://doi.org/10.1038/s41588-018-0321-7 .
Tobin MD, Minelli C, Burton PR, Thompson JR. Commentary: development of Mendelian randomization: from hypothesis test to ‘Mendelian deconfounding’. Int J Epidemiol. 2004;33(1):26–9. https://doi.org/10.1093/ije/dyh016 .
Giambartolomei C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383. https://doi.org/10.1371/journal.pgen.1004383 .
Chauquet S, Zhu Z, O’Donovan MC, Walters JTR, Wray NR, Shah S. Association of Antihypertensive Drug Target Genes With Psychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2021;78(6):623–631. https://doi.org/10.1001/jamapsychiatry.2021.0005 .
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. https://doi.org/10.1007/s10654-017-0255-x .
Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18(1):312. https://doi.org/10.1186/s12916-020-01778-5 .
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7 .
Sarnobat D, Moffett RC, Flatt PR, Tarasov AI. Effects of first-line diabetes therapy with biguanides, sulphonylurea and thiazolidinediones on the differentiation, proliferation and apoptosis of islet cell populations. J Endocrinol Invest. 2022;45(1):95–103. https://doi.org/10.1007/s40618-021-01620-6 .
Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236–240. https://doi.org/10.4103/2231-4040.90879 .
Spears M, McSharry C, Thomson NC. Peroxisome proliferator-activated receptor-gamma agonists as potential anti-inflammatory agents in asthma and chronic obstructive pulmonary disease. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2006;36(12):1494–1504. https://doi.org/10.1111/j.1365-2222.2006.02604.x .
Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARgamma ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm Pharmacol Ther. 2006;19(1):39–46. https://doi.org/10.1016/j.pupt.2005.02.005 .
Article CAS PubMed Google Scholar
Solleti SK, et al. Airway epithelial cell PPARγ modulates cigarette smoke-induced chemokine expression and emphysema susceptibility in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309(3):L293–304. https://doi.org/10.1152/ajplung.00287.2014 .
Karagiannis F, et al. Lipid-Droplet Formation Drives Pathogenic Group 2 Innate Lymphoid Cells in Airway Inflammation. Immunity. 2020;52(4):620–34. https://doi.org/10.1016/j.immuni.2020.03.003 . e6.
Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–56. https://doi.org/10.1016/j.cmet.2018.03.001 .
Pang J, Feng JN, Ling W, Jin T. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs-Therapeutic potential exploration in lung injury. Acta Pharm Sin B. 2022;12(11):4040–4055. https://doi.org/10.1016/j.apsb.2022.06.003 .
Wang W, et al. The Role of Glucagon-Like Peptide-1 Receptor Agonists in Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2023;18:129–37. https://doi.org/10.2147/COPD.S393323 .
Wei J-P, Yang C-L, Leng W-H, Ding L-L, Zhao G-H. Use of GLP1RAs and occurrence of respiratory disorders: A meta-analysis of large randomized trials of GLP1RAs. Clin Respir J. 2021;15(7):847–850. https://doi.org/10.1111/crj.13372 .
Altintas Dogan AD, Hilberg O, Hess S, Jensen TT, Bladbjerg E-M, Juhl CB. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2022;17:405–14. https://doi.org/10.2147/COPD.S350133 .
Rogliani P, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med. 2019;154:86–92. https://doi.org/10.1016/j.rmed.2019.06.015 .
Article PubMed Google Scholar
Liu XZ, Zhang H. The Effect of Sodium Glucose Cotransporter 2 Inhibitors From a Human Genetic Perspective. Front Genet. 2021;12:2019–2022. https://doi.org/10.3389/fgene.2021.658012 .
Takashima M, et al. Low-dose sodium-glucose cotransporter 2 inhibitor ameliorates ischemic brain injury in mice through pericyte protection without glucose-lowering effects. Commun Biol. 2022;5(1):653. https://doi.org/10.1038/s42003-022-03605-4 .
Joki Y, Konishi H, Takasu K, Minamino T. Tofogliflozin, a sodium-glucose cotransporter 2 inhibitor, improves pulmonary vascular remodeling due to left heart disease in mice. J Cardiol. 2023;81(4)347–355. https://doi.org/10.1016/j.jjcc.2022.10.003 .
Huang D, et al. Empagliflozin Protects against Pulmonary Ischemia/Reperfusion Injury via an Extracellular Signal-Regulated Kinases 1 and 2-Dependent Mechanism. J Pharmacol Exp Ther. 2022;380(3):230. LP – 241. https://doi.org/10.1124/jpet.121.000956 .
Song J, Yang Y, Mauvais-Jarvis F, Wang Y-P, Niu T. KCNJ11, ABCC8 and TCF7L2 polymorphisms and the response to sulfonylurea treatment in patients with type 2 diabetes: a bioinformatics assessment. BMC Med Genet. 2017;18(1):64. https://doi.org/10.1186/s12881-017-0422-7 .
Yen F-S, Wei JC-C, Yu T-S, Hsu CY, Hsu C-C, Hwu C-M. Sulfonylurea Use in Patients with Type 2 Diabetes and COPD: A Nationwide Population-Based Cohort Study. Int J Environ Res. Public Health. 2022;19(22). https://doi.org/10.3390/ijerph192215013 .
Wang M-T, et al. Use of antidiabetic medications and risk of chronic obstructive pulmonary disease exacerbation requiring hospitalization: a disease risk score-matched nested case–control study. Respir Res. 2020;21(1):319. https://doi.org/10.1186/s12931-020-01547-1 .
Giugliano D, et al. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol. 2022;21(1):42. https://doi.org/10.1186/s12933-022-01474-z .
Kotnala S et al. Sep., Contribution of dipeptidyl peptidase 4 to non-typeable Haemophilus influenzae-induced lung inflammation in COPD. Clin. Sci. (Lond). 2021;135(17);2067–2083. https://doi.org/10.1042/CS20210099 .
Chang X-Y, et al. Expression and Clinical Significance of Serum Dipeptidyl Peptidase IV Chronic Obstructive Pulmonary Disease. Am J Med Sci. 2016;351(3):244–52. https://doi.org/10.1016/j.amjms.2015.12.011 .
Chen J, et al. Therapeutic targets for inflammatory bowel disease: proteome-wide Mendelian randomization and colocalization analyses. EBioMedicine. 2023;89:104494. https://doi.org/10.1016/j.ebiom.2023.104494 .
Download references
Acknowledgements
We thank all GWAS participants and investigators for making the summary statistics data publicly available.
This work was sponsored by the National Natural Science Foundation of China (81925001 to Jin-Fu Xu), the Innovation Program of Shanghai Municipal Education Commission (202101070007-E00097 to Jin-Fu Xu); the Program of Shanghai Municipal Science and Technology Commission (21DZ2201800 to Jin-Fu Xu). Shanghai Pujiang Program (22PJD065 to Yue Su).
Author information
Yue Su and Youqian Zhang contributed equally to this work.
Authors and Affiliations
Department of Respiratory and Critical Care Medicine, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, No. 507 Zhengmin Road, Shanghai, 200433, China
Yue Su & Jinfu Xu
Yangtze University, Jingzhou, Hubei Province, 434000, China
Youqian Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Material preparation, data collection and analysis were performed by YS and YQZ. Paper retouching by JFX. Format modification by YS and YQZ. The first draft of the manuscript was written by YS, YQZ and all authors commented on previous versions of the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Jinfu Xu .
Ethics declarations
Ethics approval and consent to participate.
The GWAS summary data included in this study were obtained from publicly available databases and therefore did not require additional ethics approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Su, Y., Zhang, Y. & Xu, J. Genetic variations in anti-diabetic drug targets and COPD risk: evidence from mendelian randomization. BMC Pulm Med 24 , 240 (2024). https://doi.org/10.1186/s12890-024-02959-1
Download citation
Received : 23 August 2023
Accepted : 09 March 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s12890-024-02959-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mendelian randomization
- Antidiabetic drugs
- Chronic obstructive lung disease
- Lung function
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Mini Review
- Special issue: Renal denervation: Evidence and challenges in clinical practice
- Published: 17 May 2024
Effects of renal denervation on the kidney: albuminuria, proteinuria, and renal function
- Daisuke Yamazaki 1 , 2 ,
- Yoshio Konishi 2 &
- Kento Kitada 1
Hypertension Research ( 2024 ) Cite this article
Metrics details
Renal denervation has attracted attention as a novel antihypertensive treatment for hypertensive patients who are poorly controlled by medicine. Clinical studies have shown the antihypertensive effects of renal denervation in patients with treatment-resistant hypertension. However, renal denervation potentially has other beneficial effects, such as improving glucose metabolism and cardioprotection beyond its antihypertensive effects. In this mini-review article, we summarize and discuss the effects of renal denervation on proteinuria, albuminuria, and renal function based on the recent findings of clinical studies, and review the renoprotective effects of renal denervation.
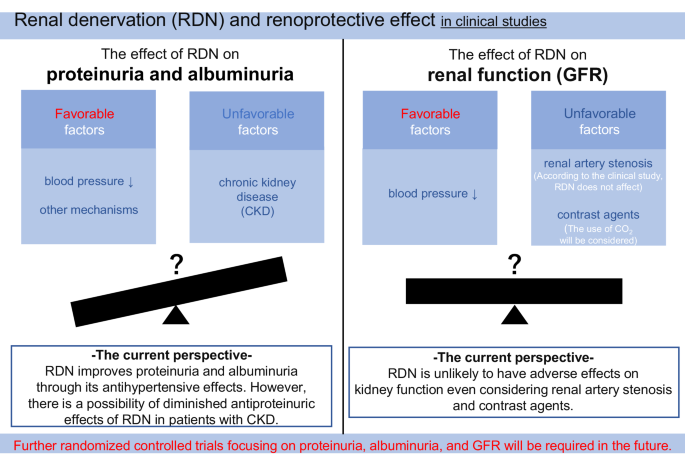
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
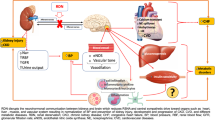
Possible organ-protective effects of renal denervation: insights from basic studies
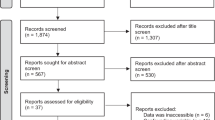
Effects of renal denervation on kidney function in patients with chronic kidney disease: a systematic review and meta-analysis
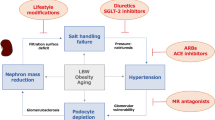
Nephrons, podocytes and chronic kidney disease: Strategic antihypertensive therapy for renoprotection
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
Article PubMed Google Scholar
Collaboration NCDRF. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–80.
Article Google Scholar
Faber JE, Brody MJ. Neural contribution to renal hypertension following acute renal artery stenosis in conscious rats. Hypertension. 1983;5:I155–64.
Article CAS PubMed Google Scholar
Katholi RE, McCann WP, Woods WT. Intrarenal adenosine produces hypertension via renal nerves in the one-kidney, one clip rat. Hypertension. 1985;7:I88–93.
Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the sympathetic nervous system and its modulation in renal hypertension. Front Med. 2018;5:82.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Bohm, Kario M, Kandzari K, Mahfoud DE, Weber MA F, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51.
Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86.
Kandzari, Bohm DE, Mahfoud M, Townsend F, Weber MA RR, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55.
Rafiq K, Fujisawa Y, Sherajee SJ, Rahman A, Sufiun A, Kobori H, et al. Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats. Diabetologia. 2015;58:2885–98.
Article CAS PubMed PubMed Central Google Scholar
Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–6.
Xia Z, Han L, Pellegrino PR, Schiller AM, Harrold LD, Lobato RL, et al. Safety and efficacy of renal denervation in patients with heart failure with reduced ejection fraction (HFrEF): a systematic review and meta-analysis. Heliyon. 2022;8:e08847.
Morisawa N, Kitada K, Fujisawa Y, Nakano D, Yamazaki D, Kobuchi S, et al. Renal sympathetic nerve activity regulates cardiovascular energy expenditure in rats fed high salt. Hypertens Res. 2020;43:482–91.
Eriguchi M, Tsuruya K, Haruyama N, Yamada S, Tanaka S, Suehiro T, et al. Renal denervation has blood pressure-independent protective effects on kidney and heart in a rat model of chronic kidney disease. Kidney Int. 2015;87:116–27.
Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–13.
Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, et al. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311–5.
Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–24.
Zhang ZH, Yang K, Jiang FL, Zeng LX, Jiang WH, Wang XY. The effects of catheter-based radiofrequency renal denervation on renal function and renal artery structure in patients with resistant hypertension. J Clin Hypertens. 2014;16:599–605.
Kiuchi MG, Graciano ML, Carreira MA, Kiuchi T, Chen S, Lugon JR. Long-term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens. 2016;18:190–6.
Verloop WL, Vink EE, Spiering W, Blankestijn PJ, Doevendans PA, Bots ML, et al. Effects of renal denervation on end organ damage in hypertensive patients. Eur J Prev Cardiol. 2015;22:558–67.
Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–7.
Hameed MA, Freedman JS, Watkin R, Ganeshan A, Dasgupta I. Renal denervation using carbon dioxide renal angiography in patients with uncontrolled hypertension and moderate to severe chronic kidney disease. Clin Kidney J. 2017;10:778–82.
Prasad B, Berry W, Goyal K, Dehghani P, Townsend RR. Central blood pressure and pulse wave velocity changes post renal denervation in patients with stages 3 and 4 chronic kidney disease: The Regina RDN Study. Can J Kidney Health Dis. 2019;6:2054358119828388.
Article PubMed PubMed Central Google Scholar
Xia M, Liu T, Chen D, Huang Y. Efficacy and safety of renal denervation for hypertension in patients with chronic kidney disease: a meta-analysis. Int J Hyperth. 2021;38:732–42.
Article CAS Google Scholar
Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–27.
Oliveras A, Armario P, Sans L, Clara A, Vazquez S, Molina L, et al. Organ damage changes in patients with resistant hypertension randomized to renal denervation or spironolactone: The DENERVHTA (Denervacion en Hipertension Arterial) study. J Clin Hypertens. 2018;20:69–75.
Sanders MF, Reitsma JB, Morpey M, Gremmels H, Bots ML, Pisano A, et al. Renal safety of catheter-based renal denervation: systematic review and meta-analysis. Nephrol Dial Transpl. 2017;32:1440–7.
Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–10.
Ott C, Mahfoud F, Mancia G, Narkiewicz K, Ruilope LM, Fahy M, et al. Renal denervation in patients with versus without chronic kidney disease: results from the Global SYMPLICITY Registry with follow-up data of 3 years. Nephrol Dial Transpl. 2022;37:304–10.
Mohammad AA, Nawar K, Binks O, Abdulla MH. Effects of renal denervation on kidney function in patients with chronic kidney disease: a systematic review and meta-analysis. J Hum Hypertens. 2024;38:29–44.
Sharafuddin MJ, Marjan AE. Current status of carbon dioxide angiography. J Vasc Surg. 2017;66:618–37.
Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261–6.
Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–19.
Solbu MD, Miroslawska A, Norvik JV, Eriksen BO, Steigen TK. Kidney function and markers of renal damage after renal denervation. Does method of measurement matter? The Reshape CV-Risk Study. J Clin Hypertens. 2021;23:954–62.
Lang D, Nahler A, Lambert T, Grund M, Kammler J, Kellermair J, et al. Anti-inflammatory effects and prediction of blood pressure response by baseline inflammatory state in catheter-based renal denervation. J Clin Hypertens. 2016;18:1173–9.
Lee H. Cystatin C in pregnant women is not a simple kidney filtration marker. Kidney Res Clin Pr. 2018;37:313–4.
Xin C, Xie J, Fan H, Sun X, Shi B. Association between serum cystatin C and thyroid diseases: a systematic review and meta-analysis. Front Endocrinol. 2021;12:766516.
Okura T, Jotoku M, Irita J, Enomoto D, Nagao T, Desilva VR, et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol. 2010;14:584–8.
Download references
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz ( https://jp.edanz.com/ac ) for editing a draft of this manuscript.
This work was partially supported by a Basic Research Grant of the Japanese Society of Hypertension (KK).
Author information
Authors and affiliations.
Department of Pharmacology, Faculty of Medicine, Kagawa University, Kagawa, 7610793, Japan
Daisuke Yamazaki & Kento Kitada
Division on Nephrology & Hypertension, Osaka City General Hospital, Osaka, 5340021, Japan
Daisuke Yamazaki & Yoshio Konishi
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, KK; investigation, DY, YK, and KK; writing—original draft preparation, DY and KK; writing—review & editing, DY, YK, and KK; supervision, YK, and KK; funding acquisition, KK.
Corresponding author
Correspondence to Kento Kitada .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Yamazaki, D., Konishi, Y. & Kitada, K. Effects of renal denervation on the kidney: albuminuria, proteinuria, and renal function. Hypertens Res (2024). https://doi.org/10.1038/s41440-024-01709-4
Download citation
Received : 28 February 2024
Revised : 02 April 2024
Accepted : 07 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1038/s41440-024-01709-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Renal denervation
- Blood pressure
- Renal function
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Methodist Debakey Cardiovasc J
- v.18(5); 2022

Pharmacotherapy for Essential Hypertension: A Brief Review
Behnam heidari.
1 Houston Methodist DeBakey Heart & Vascular Center, Houston Methodist, Houston, Texas, US
Eleonora Avenatti
Khurram nasir.
Hypertension is one of the leading causes of disability-adjusted life years and mortality, with approximately 15% prevalence worldwide. Most patients with hypertension from low- to high-income countries do not receive treatment. Among those who receive treatment, the majority remain undertreated and do not achieve their blood pressure goals. Therefore, new hypertension guidelines introduce more conscientious treatment strategies to maximize the probability of achieving the new strict blood pressure goals compared with the previous guidelines. Who should receive treatment for hypertension? Which antihypertensive medications have the strongest supporting data? Are generic and more affordable medications as effective as expensive brand medications? What are the different treatment strategies to maximize success in controlling blood pressure? Here, we briefly review pharmacotherapy for hypertension and provide answers to these questions as well as some other common questions regarding treatment of hypertension.
Introduction
In 2019, approximately 1.2 billion people globally were estimated to have hypertension—twice as many as the year 1990. 1 Hypertension is one of the leading causes of mortality and disability-adjusted life years worldwide, 2 , 3 and it remains one of the most important modifiable contributing factors to the burden of coronary artery disease, stroke, and chronic kidney disease. 2 , 3 , 4 Studies have shown that the risk of fatal cardiovascular events doubles for each 20-mm Hg increase in systolic or 10-mm Hg increase in diastolic blood pressure (BP). 5 Hypertension can be easily diagnosed, and a wide variety of inexpensive therapies are available to effectively control it. 1 , 6
Controlling hypertension is associated with a reduction in mortality and adverse cardiovascular outcomes, 7 and both non-pharmacological and pharmacological interventions are essential to treatment. 8 Non-pharmacological interventions include reducing dietary sodium, increasing consumption of fruits and vegetables, 9 , 10 a high-protein low-carbohydrate diet, 11 and losing weight, 12 all of which should be included throughout the treatment period ( Figure 1 ).

Non-pharmacological approaches to hypertension treatment. Attention to diet, salt and alcohol reduction, and physical activity need to be part of the therapeutic approach for all patients.
Pharmacological interventions were first established in the late 1950s with thiazide diuretics, the first class of medications to be studied in a clinical trial and the first to be used for treating hypertension. 13 , 14 Following the favorable outcome with thiazide diuretics, several other studies were conducted and showed the efficacy of other BP-lowering medications in controlling hypertension and preventing related complications. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 The landmark Treatment of Mild Hypertension Study—the first to compare the efficacy of different classes of BP-lowering medications—showed that various antihypertensive medications have considerable effect in reducing BP with minimal differences among different classes. 25 We still use most of these established BP medications to treat hypertension in our daily medical practice based on the findings of these landmark studies. 8 In this article, we discuss pharmacologic management of essential hypertension and briefly review the indications and use of different medication classes.
Defining Hypertension and When to Start Pharmacotherapy
Although major hypertension guidelines use different BP thresholds to define hypertension and its stages, they all use a combination of BP level and patient risk factors including atherosclerotic cardiovascular disease (ASCVD) risk for their recommendations on BP management ( Figure 2 ). The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guideline for treatment of BP defines normal BP as < 120/80 mm Hg. It recommends non-pharmacological therapy for elevated BP (120-129/< 80 mm Hg) and for stage 1 hypertension (130-139/80-90 mm Hg) without clinical ASCVD or 10-year ASCVD risk < 10%. Non-pharmacological plus pharmacological therapy is recommended for those with stage 1 hypertension with clinical ASCVD or 10-year ASCVD risk ≥ 10% and in all patients with stage 2 hypertension (BP ≥ 140/90 mm Hg). 8

Overview of recommendations for hypertension treatment by current international guidelines. ACC/AHA: American College of Cardiology/American Heart Association; ESC/ESH: European Society of Cardiology/European Society of Hypertension; ISH: International Society of Hypertension
The 2020 International Society of Hypertension (ISH) Global Hypertension Practice Guidelines, 26 and the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) guidelines 27 have a slightly higher threshold for pharmacotherapy of hypertension. The 2020 ISH Global Hypertension Practice Guidelines recommend immediate pharmacotherapy for patients who have grade 1 hypertension (BP ≥ 140-159/90-99 mm Hg) and clinical ASCVD, high risk of ASCVD, chronic kidney disease, diabetes mellitus, or hypertension-mediated organ damage. Pharmacotherapy is indicated for all patients who have grade 2 hypertension defined as BP ≥ 160/100 mm Hg regardless of their risk or comorbidities. 26 The 2018 ESC/ESH guidelines recommend immediate pharmacotherapy in grade-1 hypertension (BP 140-159/90-99 mm Hg) with renal disease, high risk of ASCVD, or hypertension-mediated organ damage. Grade 2 (BP 160-179/100-109 mm Hg) and grade 3 (BP ≥ 180/110 mm Hg) hypertension need pharmacotherapy regardless of comorbidities or ASCVD risk. 27
Medication Class Selection
There is a general recommendation for using one of three classes of medications—thiazide diuretics, angiotensin-converting enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARBs), or calcium channel blockers (CCBs)—as the initial therapy for patients with essential hypertension. 8 Several studies have shown that different BP-lowering medications have similar efficacy in treating hypertension and preventing its complications, with minimal differences between drug classes. 25 , 28 , 29 , 30 , 31 A meta-analysis studying 31 randomized trials demonstrated decreased major cardiovascular events following reduction of BP. This effect remained significant when patients were divided into two age groups (< 65 years or ≥ 65 years), showing both older and younger adults will benefit from treatment of hypertension. However, after analyzing the data for patients who were treated with diuretics, ACEi, ARBs, CCBs, and beta blockers, the study did not show any significant benefit for selecting one class of antihypertensive medications over another. 29 Another meta-analysis published a year later included 147 studies that were published between 1966 and 2007, and it analyzed the data for 464,164 patients. It showed that although beta blockers were associated with decreased coronary heart disease events if used shortly after a myocardial infarction, and CCBs were associated with a slightly lower risk of stroke, all antihypertensive medication classes were associated with a comparable reduction in coronary artery disease or stroke. Again, the result of this study confirmed the importance of controlling BP in different age groups regardless of the medication used. 30
A recent systematic review and meta-analysis that included 123 studies and 613,815 patients showed a significant reduction in major cardiovascular events and mortality for each 10 mm Hg reduction of systolic BP. This study showed that beta blockers were inferior to the other classes in preventing such adverse events, CCBs were superior in preventing stroke and inferior in preventing heart failure, and diuretics were superior in preventing heart failure. 31 A recent Cochrane systematic review article suggested that the use of low-dose thiazide diuretics, ACEi, or CCBs as first-line therapy have similar effects in reducing mortality and morbidity in patients with moderate-to-severe essential hypertension while beta blockers or high-dose thiazide diuretics might be less effective in reducing such outcomes. 32 Overall, in the absence of a specific indication for an antihypertensive medication, major guidelines recommend focusing on the treatment of hypertension and not the medication class selection to achieve the goal of reducing major cardiovascular events. 8 , 26 , 27 Table 1 shows some of the common uses and precautions for the most used antihypertensive medications.
Indications and contraindications for commonly used antihypertensive medications. ACE: angiotensin-converting enzyme inhibitor; Afib/flutter: atrial fibrillation/flutter; ARB: angiotensin II receptor blockers; CCB: calcium channel blocker; CKD: chronic kidney disease; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; MDD: major depressive disorder
Monotherapy or Multidrug Strategy
Although previous guidelines recommended starting treatment with monotherapy with gradual dose increase—switching to another class of medication, or adding a second medication if needed—recent guidelines recommend initiating two BP-lowering drugs simultaneously in most patients. 8 , 26 , 27 However, research shows that approximately 60% of patients with hypertension are not treated. This finding remains consistent worldwide across low- to high-income countries. Importantly, of the 40% of patients who receive treatment, approximately 65% do not achieve the target of 140/90 mm Hg. 33 In addition, the BP goal has been decreasing in the past several years, which makes it hard for monotherapy to achieve the new stricter goals. Increasing the dose of a single medication has little incremental BP-lowering effect and can potentially increase the risk of side effects. Importantly, most patients who participated in the published clinical trials were either started on multidrug therapy or needed to be treated with more than one BP-lowering medication throughout the trial. 29 , 30 , 31 , 32 Therefore, recent guidelines recommend initiating treatment with more than one BP-lowering medication in most patients with hypertension. 8 , 26 , 27
The 2018 ESC/ESH guideline recommends that monotherapy may be sufficient in patients with high normal BP (130-139/85-89 mm Hg) if their BP is close to the threshold of 140/90 mm Hg and non-pharmacological therapy has failed to control hypertension. Otherwise, it recommends the initiation of treatment with two different classes of medications for most patients who meet criteria for treatment as discussed above. 27 The 2020 ISH Global Hypertension Practice Guideline recommends initiating pharmacotherapy with low doses of two different classes of medications in patients with hypertension who require pharmacotherapy. 26 The 2017 ACC/AHA guideline recommends initiation of therapy with two first-line medications from different classes in patients with stage 2 hypertension (BP ≥ 140/90 mm Hg). Monotherapy can be considered in patients with stage 1 hypertension (BP = 130-138/80-90 mm Hg) with increasing the medication dose and adding other agents if needed to achieve BP goal < 130/80 mm Hg. 8
Morning, Evening, or Multiple Daily Dosing
The average nighttime BP is about 15% lower than daytime BP. Non-dipping phenomenon refers to the failure of BP to decrease by at least 10% of its daytime value while sleeping at night and is associated with adverse cardiovascular events. Some studies have recommended that moving BP medication doses to the evening can be associated with decreased non-dipping phenomenon. 34 , 35 , 36 , 37 , 38 Many other clinical trials have shown no significant difference in BP readings among patients who take BP-lowering medications in the morning or in the evening. 39 , 40 , 41 A Cochrane systematic review included 21 randomized controlled trials and analyzed the data for 1,993 patients with hypertension. It showed that evening dosing of BP medications can be associated with better BP control compared to morning administration. However, given that none of the studies reported clinically relevant outcome measures including mortality or cardiovascular events, the significance of this better BP control remains unclear. 42 Importantly, in the recently published TIME (Treatment In Morning versus Evening) study, 21,104 patients were randomly assigned to morning versus evening dosing of BP medications. This randomized clinical trial showed no significant difference in major cardiovascular events between the study groups and concluded that patients can take their BP-lowering medications at their convenience throughout the day. 43 Although splitting BP-lowering medication doses to more than once a day might seem to cause less fluctuations in BP levels, this increase in the number of daily doses of medications can be associated with decreased long-term medication adherence and increased treatment failure. 44 , 45 All three of the 2017 ACC/AHA, 2018 ESC/ESH, and the 2020 ISH Global Hypertension Practice Guidelines recommend once-daily dosing over multiple daily dosing to improve medication adherence and decrease the risk of treatment failure. They also recommend a single pill strategy (one pill that combines two different medications) for initial treatment of hypertension as an additional step for increasing the chance of medication adherence. However, they do not give any specific recommendations for morning or evening dosing of the medications. 8 , 26 , 27
Generic Versus Branded Drugs
Given the global burden of hypertension and the significant costs associated with treatment, the use of generic drugs offers potential benefits in terms of healthcare cost savings and hence availability of treatment for a wider population. Although guidelines based on the principle of bioequivalence and drug approval processes do not make any distinction in this regard, 46 adoption of generic drugs is sometimes hindered by doubts among providers and more often patients regarding efficacy and safety. 47 , 48 At the same time, studies directly comparing clinical efficacy are sparse and are not required by current regulations, which focus on biologic equivalence to then infer comparable clinical efficacy.
Meta-analyses have attempted to provide some guidance, with the caveat that often the included studies focused on determination of bioequivalence and are thus characterized by small sample size, short follow-up, and inclusion of mostly young healthy individuals. An initial large systematic review and meta-analysis of generic and brand-name drugs used to treat CV diseases (including beta blockers, diuretics, CCBs, ACEi) included 47 publications, 38 of which were randomized clinical trials, and did not find any evidence of superiority of innovator to generic drugs. 49 A more recent meta-analysis published by Manzoli et al. in 2016 substantially confirmed the same findings and further strengthened the safety of generic medications. 50
Large recent population datasets are in line with this conclusion. In an observational retrospective study in a dataset of 9,413,620 insured people, Ti et al. evaluated 17 branded versus generic pharmaceutical substances for the treatment of hypertension/heart failure, hyperlipidemia, and diabetes mellitus and compared the hazard ratios for all-cause death and major adverse cardiac and cardiovascular events. 51 The study concluded that generic versions were at least similar, if not superior, to branded medications. Two recent large community-based randomized controlled trials in a Chinese population followed a total of 29,000 hypertensive patients propensity score matched for use of brand versus generic medications. The results either showed no difference in the mean reduction in systolic BP, hypertension control rate, or CV outcomes 52 or demonstrated higher hospitalization rates for CVD in patients started with some of the branded drugs analyzed, possibly pointing to a difference in medication adherence in the brand prescription groups. 53 Indeed, in both studies the use of generic drugs unsurprisingly translated into lower medication costs.
Finally, a small open crossover randomized controlled trial in France allocated hypertensive patients to their usual antihypertensive treatment either exclusively with brand-name drugs for 6 weeks and then switching to generics for another 6 weeks or following the reverse order. 54 Twenty-four hour ambulatory BP monitoring demonstrated no significant impact of branded versus generic drug use (mean 24-h average BP of 129/77 vs 128/77 mm Hg for generic vs brand drugs, respectively). Taken together, these findings support the safety and efficacy of generic medications for BP treatment, with possible advantages in effectiveness given the lower economic burden on patients.
Less Commonly Used Medications
Other drug classes can be used to lower BP, namely beta blockers, alpha 1 blockers, central acting alpha 2 blockers, and direct vasodilators. These are all considered secondary agents because long-term use was not associated with survival benefit. 8 Their use should be considered only after exhausting other proven interventions including lifestyle changes and first-line drugs and having excluded forms of secondary hypertension. As such, these medications should be cautiously evaluated in each patient based on the presence of comorbidities and concomitant indications independent of BP control.
There are currently no data to support the use of beta blockers for the treatment of hypertension, barring the presence of specific comorbidities that represent a strong indication for beta blockade (ie, ischemic heart disease or heart failure). Data showed worse clinical outcomes with beta blockers compared to ARBs despite a similar drug-mediated reduction in BP values. 55
The ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack) trial was initially designed as a four-drug comparison with the alpha blocker doxazosin being tested against chlorthalidone amlodipine and lisinopril. The finding of increased risk of heart failure and stroke led to the premature cessation of the doxazosin arm and a re-evaluation of the role of alpha blockers in hypertension treatment. Current guidelines thus recommend the use of alpha 1 blockers (doxazosin, prazosin, and terazosin) as possible adjunct treatment in patients with benign prostatic hypertrophy. Of note, their use is associated with significant orthostatic hypotension, especially after the first administration, which is particularly concerning in older adults; therefore, a bedtime administration is thus usually recommended. 56
Clonidine is a centrally acting alpha 2 agonist that reduces BP levels through negative feedback on norepinephrine release. Given its mechanism of action, it is burdened by significant side effects (from peripherally mediated constipation and dry mouth to more substantial central nervous system side effects of drowsiness and sedation). Therefore, it is usually reserved as a last-line choice. Moreover, abrupt discontinuation is associated with significant rebound hypertension and tachycardia, thus discontinuation requires very gradual taper. Current guidelines 8 , 27 mention its use in a very limited setting of resistant hypertension and hypertensive emergency, although safer alternatives are often available as well.
Hydralazine and minoxidil exert their antihypertensive effect through direct vascular dilatation. The resulting compensatory reflex tachycardia and water retention require these drugs to be used in combination with a beta-blocker and diuretic 8 Moreover, specific side effects should also be considered, since hydralazine is associated with risk of drug-induced lupus and minoxidil is associated with hirsutism and risk of pericardial effusion. As such, the use of these medication has been declining, and current guidelines recommend their use in limited settings, including resistant hypertension and hypertension in pregnancy for hydralazine, although safer options are also available. 8
Approach to Resistant Hypertension
Resistant hypertension is defined as uncontrolled clinic BP (> 140/90 mm Hg) despite treatment with three antihypertensive medications with complementary mechanisms of action including a diuretic. Guidelines further specify that these three antihypertensive medications should include optimal doses of an ACEi or ARB, a CCB, and a diuretic. 57 When taking care of patients with difficult-to-control hypertension, it is critical to avoid misdiagnosis and accurately exclude the presence of secondary hypertension as well as pseudo-resistance. The latter can be attributed to incorrect techniques in BP measurements, white coat hypertension, medication nonadherence or patient intolerance to certain medications, and concomitant use of drugs or substances with hypertensive effects, including a high amount of dietary sodium.
Pseudo-resistance is a significant and underdiagnosed problem; hence, it needs to be considered and excluded to allow proper identification of patients with true resistant hypertension and focused treatment efforts. Salt consumption remains high in the general population, is associated with increased BP values, and is a significant barrier in hypertension management. A small Italian study using 24-hour urinary sodium excretion in patients with suspected resistant hypertension found that only 27% of the patients were following recommendations on salt consumption. 58 Reduction in salt consumption is associated with reduced BP, with an effect that is more evident in hypertensive patients than in normotensive controls. 10 Likewise, adherence to prescribed medications is a major factor in obtaining and maintaining BP control and thus preventing CVD. Estimation of adherence is difficult since methods to objectively confirm it are cumbersome and costly, and many different factors play into a patient’s willingness and ability to initiate and maintain a chronic treatment regimen. 45 Awareness of those factors and the risk of nonadherence is critical knowledge for the managing physician. These aspects of care should be emphasized in any patient-physician discussion as part of an effective hypertension management strategy. 59
Secondary causes of hypertension (renal arterial stenosis, hyperaldosteronism, obstructive sleep apnea, pheochromocytoma, etc) should be identified because they require different treatment approaches focused at addressing the underlying disease. 8 Of note, the relative frequency of these diseases varies significantly, as does the likelihood of complete resolution of hypertension once the underlying disease has been addressed. For example, it is reasonable to expect normalization of BP values once a rare pheochromocytoma has been surgically removed; however, in the more common scenario of a patient with obstructive sleep apnea, use of continuous positive air pressure might address the respiratory issues but the underlying comorbidities associated with such a phenotype will make continuation of hypertension treatment likely necessary. 60
The physiopathology of true resistant hypertension is still poorly understood. However, one of the most accepted hypothesis calls into play altered sodium homeostasis and inappropriate kidney-mediated sodium retention. The PATHWAY-2 (Optimum Treatment for Drug-Resistant Hypertension) study was a double-blind placebo-controlled crossover trial that evaluated the efficacy of medications targeting this system in improving BP control in resistant hypertension, ultimately sanctioning the superiority of spironolactone over alpha and beta blockers. 61 A total of 230 patients completed all treatment steps (12 weeks of once-daily treatment with each of spironolactone, bisoprolol, doxazosin and placebo on top of baseline BP treatment). Patients treated with spironolactone had the most BP reduction (mean reduction of 8.7 mm Hg compared to 4.03 mm Hg for doxazosin and 4.26 for bisoprolol).
Spironolactone has thus emerged as the drug of choice in this context and is currently recommended as the fourth drug to be added to the standard treatment of hypertension. 57 This holds true specifically for patients with normal potassium levels (K < 4.5 mEq/L) based on the limited but present risk of hyperkalemia in the PATHWAY 2 trial; for patients with higher K levels, a doubling of the thiazide diuretic dose is recommended. In case of intolerance to spironolactone, other potassium-sparing diuretics might be considered, including amiloride and eplerenone. The ACC guidelines are somewhat less direct, suggesting treatment with spironolactone while maximizing diuretic dosage and adding “other agents with different mechanism of action” to obtain BP control in resistant hypertension. Regarding the choices for the latter, the PATHWAY 2 trial supports the efficacy of bisoprolol and doxazosin in improving BP control in the setting of resistant hypertension, although the reduction was to a lesser degree compared with spironolactone. 61
Clonidine has been studied in this setting as well. In the ReHOT (Resistant Hypertension Optimal Treatment) trial, 187 patients were randomized to clonidine versus spironolactone as the fourth drug for BP control. Although inferior to spironolactone in the amount of 24-hour BP reduction in both systolic and diastolic values, clonidine achieved similar rates of BP control. 62 As mentioned above, significant side effects still hamper the use of clonidine, and safer options are available. Some data are also available to support the use of both hydralazine and minoxidil in this clinical setting. 63 However, they are used infrequently because of significant side effects of fluid retention and tachycardia. 27
Device-based treatment approaches have been evaluated given the substantial body of evidence linking the autonomic nervous system activity in the physiopathology of hypertension. 64 Renal denervation and chronic baroreceptor stimulation have been studied in randomized clinical trials with negative or conflicting results and safety concerns, and such approaches are thus not currently recommended. 65 , 66
Recent Developments
The selective sodium-glucose cotransporter-2 (SGLT2) receptor inhibitors (empagliflozin, canagliflozin, dapagliflozin) demonstrated BP-lowering effects through various mechanisms that may include natriuresis, osmotic diuresis, and reduction of the sympathetic tone. 67 The degree of actual BP reduction was quantified in the range of 2 mm Hg to 3 mm Hg in meta-analysis evaluation of available randomized clinical trials, a modest but nevertheless sizable impact. 68 Cardiovascular benefits of these medications are well proven, especially in the treatment and prevention of heart failure, and the additional effect on BP should be taken into consideration in devising a patient-specific strategy. 69
Glucagon-like peptide 1 receptor agonists (GLP1-RA), revolutionary drugs in term of medical management of obesity, positively impact BP in multiple ways above and beyond the expected positive effects of weight loss on hypertension. Indeed, the reduction in BP shown in clinical trials with GLP1-RA was observed early in the treatment, before significant weight loss occurred, suggesting independent action of these medications on hypertension. 70 The effect is sizable, with liraglutide and semaglutide demonstrating a reduction in systolic BP in the range of 3.5 to 5.6 mm Hg and 3.9 to 6.2 mm Hg, respectively, in their pivotal randomized clinical trials. 71 The proposed mechanisms for these antihypertensive effects might include natriuresis and increased urinary output, direct vasodilation via dedicated receptors in blood vessels, decreased sympathetic activity, or improved endothelial function through resolution of negative effects of hyperglycemia. 72
Both of these classes might represent an important ancillary medical strategy to optimize BP control in high-risk populations in which multiple comorbidities such as obesity, type 2 diabetes mellitus, and metabolic syndrome might be adequately addressed at the same time. 73
Hypertension is one of the leading causes of mortality and morbidity worldwide. Approximately 15% of the world population has hypertension, and most of these patients either do not receive any treatment or do not achieve BP target even if they are receiving treatment. Multiple studies have shown, and all major guidelines consistently recommend, that overall, the magnitude of BP reduction and not the use of a specific group of antihypertensive medication class is the major determinant of reducing adverse cardiovascular events. The use of multiple drug therapy as the initial approach and strategies that can improve patient compliance—including one-time dosing, the use of generic and more affordable medications, or using the medication that is well tolerated—can be associated with more consistent BP control and therefore more significant reduction in future events.
- Non-pharmacological intervention (salt reduction, weight management, appropriate diet, physical exercise, limitation of alcohol intake) are key strategies for hypertension treatment and should be implemented throughout the course of treatment for this patient population.
- First-line treatment agents include thiazide diuretics, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and calcium channel blockers.
- Combination treatment with two drugs from different classes is favored as initial treatment when pharmacotherapy is considered.
- There are no data to support the use of branded versus generic drugs or indicating a preferential timing for drug administration. Once-daily medication and combination pills might improve adherence.
- In difficult-to-control hypertension, pseudoresistance should be evaluated and addressed and secondary causes of hypertension should be considered; when a fourth drug is needed, spironolactone is the drug of choice.
CME Credit Opportunity
Houston Methodist is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Houston Methodist designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ . Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Click to earn CME credit: learn.houstonmethodist.org/MDCVJ-18.5 .
Competing Interests
The authors have no competing interests to declare.
The independent source for health policy research, polling, and news.
KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs
Alex Montero , Grace Sparks , Marley Presiado , and Liz Hamel Published: May 10, 2024
- Methodology
Key Findings
- The latest KFF Health Tracking Poll finds that about one in eight adults (12%) say they have ever taken a GLP-1 agonist – an increasingly popular class of prescription drugs used for weight loss and to treat diabetes or prevent heart attacks or strokes for adults with heart disease – including 6% who say they are currently taking such a drug. The share who report ever taking these drugs rises to four in ten (43%) among adults who have been told by a doctor that they have diabetes, a quarter who have been told they have heart disease, and one in five (22%) who have been told by a doctor that they are overweight or obese in the past five years 1 . Public awareness of GLP-1 drugs has increased in the past year, with about one-third (32%) of adults now saying they have heard “a lot” about these drugs, up from 19% in July 2023.
- Most adults who have taken GLP-1 drugs say they took them to treat a chronic condition including diabetes or heart disease (62%), while about four in ten say they took them primarily to lose weight.
- About half (54%) of all adults who have taken GLP-1 drugs say it was difficult to afford the cost, including one in five (22%) who say it was “very difficult.” While most insured adults who have taken these drugs say their insurance covered at least part of the cost, even among insured adults about half (53%) say the cost was difficult to afford 2 .
- While 8% of adults ages 65 and older say they have taken a GLP-1 medication for a chronic condition, just 1% say they have ever taken a GLP-1 drug to lose weight, which may reflect Medicare’s lack of coverage for prescription drugs used for weight loss. Nearly four in ten (37%) adults ages 65 and older report being told by a doctor they are overweight or obese in the past five years.
- With Medicare currently prohibited by law from covering prescription drugs used for weight loss, six in ten adults say they think Medicare should cover the cost of these drugs when prescribed for weight loss for people who are overweight, including more than half of Democrats, independents and Republicans. Similar shares of the public continue to support Medicare coverage of these drugs for weight loss even after hearing arguments for and against this proposal.
Use, Access and Affordability of GLP-1 Drugs
KFF’s latest Health Tracking Poll examines the public’s views and use of an increasingly popular group of drugs that include Ozempic, Wegovy, Mounjaro and others that belong to a class of prescription medications known as GLP-1 agonists 3 . GLP-1 drugs have garnered an increasing amount of media attention and some notable celebrity endorsements in the U.S., with much of the focus on their use for weight loss, though many of these drugs are also prescribed to treat diabetes or reduce risk of heart attack or stroke.
A large and increasing share of the public say they have heard about GLP-1 drugs, with about eight in ten (82%) adults saying they have heard at least “a little” and about three in ten (32%) saying they have heard “a lot” about these drugs. The share of the public who report having heard about these drugs has increased since July 2023 when seven in ten adults reported having heard at least “a little” about these drugs and one in five (19%) said they had heard “a lot.”
The share who say they have heard “a lot” about these drugs rises to at least four in ten among those who have ever been told by a doctor that they have diabetes (45%) or heart disease (41%) or have been told by a doctor in the past five years that they are overweight or obese (42%) – the primary conditions these drugs are prescribed for.
Across age groups, awareness of these drugs is highest among older adults. About four in ten adults ages 50 to 64 and 65 and older say they have heard “a lot” about GLP-1 drugs, compared to about one-third of adults ages 30-49 (32%) and one in six adults ages 18-29 (17%). Notably, older adults are more likely than their younger peers to have been told by a doctor that they have diabetes or heart disease.
Adults with annual household incomes of $90,000 or greater are more likely than those with lower household incomes to say they have heard “a lot” about these drugs.
Overall, 12% of adults say they have ever used GLP-1 drugs, including 6% who say they are currently using them. The share who report ever taking these drugs rises to about four in ten (43%) among adults who have been told by a doctor that they have diabetes, a quarter (26%) of adults who have been told they have heart disease, and one in five (22%) adults who have been told by a doctor that they are overweight or obese in the past five years (some of whom also have diabetes or heart disease).
Black adults are somewhat more likely than White adults to report ever taking these drugs (18% v. 10%), while 13% of Hispanic adults say they have taken these drugs. KFF’s analysis of Centers for Disease Control (CDC) data shows that Black and Hispanic adults in the U.S. have a higher rate of obesity than White adults. For additional information on obesity rates and racial disparities, see KFF’s policy watch: What are the Implications of New Anti-Obesity Drugs for Racial Disparities?
Similar shares of adults regardless of gender, income, or health insurance coverage report taking these drugs.
Among the 12% of adults who have ever taken GLP-1 drugs, most report taking them, at least in part, to treat a chronic condition like diabetes or heart disease, with fewer saying they took them only to lose weight. Among those who have taken these drugs, six in ten (62%) say they took them to treat a chronic condition like diabetes or heart disease, including about four in ten (39%) who took them only to treat a chronic condition and one in four (23%) who say they took them to both treat a chronic condition and to lose weight. About four in ten (38%) adults who have taken these drugs report using them only to lose weight.
Among all adults, 7% say they have taken or are taking these drugs to treat a chronic condition such as diabetes or heart disease – either alone (5%) or in combination with intent of losing weight (3%) – while 5% of adults report ever taking these drugs to lose weight but not to treat a chronic condition.
About one in five (19%) adults ages 50-64 say they have ever taken GLP-1 drugs, higher than the shares reported by other age groups. Among adults ages 50-64, 15% say they have taken GLP-1 drugs to treat a chronic condition and 5% say they’ve taken them for weight loss only. Few adults under age 50 report having taken these drugs to treat chronic conditions, but similar shares of 18–29-year-olds (7%) and 30–49-year-olds (6%) report having taken them for weight loss. Among adults ages 65 and over, 8% say they have taken a GLP-1 medication for a chronic condition, while just 1% say they have taken these drugs only to lose weight, which may be a reflection of Medicare’s lack of coverage for prescription drugs used for weight loss. Nearly four in ten (37%) adults ages 65 and older report being told by a doctor they are overweight or obese in the past five years.
Alongside the relatively high cost of GLP-1 drugs in the U.S., there have been recent reports of shortages or limited availability of these drugs occurring as demand increases. Recent news reports have emphasized that some adults are seeking generic or compounded versions of these drugs through sources such as medical spas or compounding pharmacies, which may sell products claiming to be name-brand GLP-1s that have not been vetted by the F.D.A.
About eight in ten (79%) adults who have taken GLP-1 drugs report getting these drugs or a prescription for them from their primary care doctor or a specialist, while fewer report getting them from an online provider or website (11%), a medical spa or aesthetic medical center (10%), or from somewhere else (2%).
In the U.S., list prices for GLP-1 drugs can range from $936 to $1,349 before insurance coverage, rebates or coupons. Most insured adults who have taken GLP-1 drugs say their insurance covered at least part of the cost. Among adults with health insurance who report ever taking these drugs, over half (57%) say their health insurance covered part of the cost of these drugs and they paid the rest, while one in four (24%) say their health insurance covered the full cost. One in five (19%) insured adults who have taken GLP-1s say they paid for the full cost themselves.
Despite the fact that few insured adults say they paid the full cost of these drugs themselves, many report difficulty affording them. About half of adults who have taken GLP-1s say it was difficult to afford the cost of these drugs. Among those who have taken these drugs, about half (54%) – including 53% of those with health insurance – say it was either “somewhat” or “very difficult” to afford to pay for these drugs, including one in five (22%) who say it was “very difficult,” including a similar share of adults with health insurance (23%).
Public Opinion on Medicare Coverage of GLP-1s for Weight Loss
While some Medicare drug plans cover the cost of some GLP-1s such as Ozempic or Wegovy when prescribed to treat diabetes or prevent heart attacks or strokes for adults with heart disease, Medicare is currently prohibited by law from covering drugs when prescribed for weight loss – for more information, see KFF’s issue brief on Medicare coverage of GLP-1s . KFF’s latest Health Tracking Poll finds that most adults think Medicare should cover the cost of these drugs when prescribed for weight loss for people who are overweight, with support remaining largely unchanged after hearing arguments for and against this proposal.
Overall, six in ten adults (61%), including similar shares across age groups, say they think Medicare should cover the cost of these drugs when prescribed for weight loss for people who are overweight, a share that rises to about seven in ten (71%) among those who have ever taken these drugs.
While more than half of adults across partisans say Medicare should cover the cost of these drugs for weight loss, Democrats (66%) are somewhat more likely than Republicans (55%) to say this.
Attitudes on some policy proposals may change when the public hears different arguments in favor or against certain proposals. After asking whether Medicare should cover the cost of GLP-1s when prescribed for weight loss for people who are overweight, the poll presented two different arguments for and against this proposal:
Argument against: Some people say that if Medicare covers the cost of these drugs, it could increase premiums paid by people with Medicare and place financial pressure on the Medicare program and the federal budget.
Argument in favor: Others say that if Medicare covers the cost of these drugs, it could help more people afford these medications and improve health and quality of life for people who are overweight.
After being presented with these arguments, the public’s attitudes remain largely unchanged, with six in ten adults still saying they think Medicare should cover the cost of these drugs when prescribed for weight loss for people who are overweight. Attitudes also remained largely unchanged among adults 65 and older, among those who have taken GLP-1s and those who have not, and among independents and Republicans. Among Democrats, there is a slight increase in the share who say Medicare should cover the cost after hearing these arguments (71% after v. 66% before).
- Health Costs
- Tracking Poll
- Prescription Drugs
- Heart Disease
- Medicare Part D
- TOPLINE & METHODOLOGY
news release
- Poll: 1 in 8 Adults Say They’ve Taken a GLP-1 Drug, Including 4 in 10 of Those with Diabetes and 1 in 4 of Those with Heart Disease
Also of Interest
- Medicare Spending on Ozempic and Other GLP-1s Is Skyrocketing
- A New Use for Wegovy Opens the Door to Medicare Coverage for Millions of People with Obesity
- What are the Implications of New Anti-Obesity Drugs for Racial Disparities?
- How Do Prices of Drugs for Weight Loss in the U.S. Compare to Peer Nations’ Prices?

IMAGES
VIDEO
COMMENTS
1. INTRODUCTION. Cardiovascular diseases (CVDs) are the leading cause of death globally, 1 and hypertension is the leading risk factor for CVD. 2 , 3 Antihypertensive drugs (AHTDs) are among the most commonly used prescription drugs worldwide. Drug regulatory agencies have approved many AHTDs primarily based on evidence of efficacy and safety from randomized controlled trials (RCTs).
Restrepo Guerrero A, Martinez V, Velez Rueda J, Portiansky E, De Giusti V, Ferrer E and Williams P (2023) Complexation of the Antihypertensive Drug Olmesartan with Zn: In Vivo Antihypertensive and Cardiac Effects, Biological Trace Element Research, 10.1007/s12011-023-03670-8, 202:1, (246-257), Online publication date: 1-Jan-2024.
Hypertension (HTN) is considered one of the leading causes of increased cardiovascular disease. Lowering blood pressure does reduce cardiovascular risks; maintaining systolic blood pressure of less than 130 mm Hg demonstrably prevents complications in patients with heart failure, diabetes, coronary artery disease, stroke, and other cardiovascular diseases. This activity discusses the ...
Conversely, some emerging antihypertensive drugs have antioxidant activity, which is currently attracting the attention of researchers. Among them are celiprolol, propranolol, nebivolol, and ...
Unfortunately, research on new antihypertensive drugs dramatically slowed over the past few years. We agree with Bhudia that the future in the management of hypertensive patients remains uncertain . However, significant progress is likely to come over the next few years from a combination of education and technology worldwide.
Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. ... Stricker BH, Wiholm B; EDIP Study Group of the European Pharmacovigilance Research Group. Antihypertensive medication and the risk of acute pancreatitis: the European case-control study on drug ...
Recent drug monitoring studies have revealed nonadherence to BP lowering therapy in 25% to 65% of patients with apparent treatment resistant hypertension (TRH). 5 - 9 In 24% to 34.5% of these individuals, who were prescribed 3-5 + antihypertensive medications, no antihypertensive medication was detected in blood or urine samples. The unmet ...
This review presents publication trends, characteristics, and quality of systematic reviews (SRs) of randomized controlled trials (RCTs) of antihypertensive drugs (AHTDs). Between 1985 and 2017, 1,173 SRs were published, and in the last 20 years, 10, 35, and 116 were published in the year 1996, 2006 …
Blood-pressure-lowering therapy with antihypertensive drugs can reduce the risk of cardiovascular morbidity and mortality in patients with hypertension. ... Research Institute for Radiation ...
Objective To examine the association between antihypertensive treatment and specific adverse events. Design Systematic review and meta-analysis. Eligibility criteria Randomised controlled trials of adults receiving antihypertensives compared with placebo or no treatment, more antihypertensive drugs compared with fewer antihypertensive drugs, or higher blood pressure targets compared with lower ...
Figure 5-2. Procedures of hypertension treatment in the absence of compelling indications. * 1 In elderly patients, administration should be started at 1/2 of the standard dose, and the dose ...
Hence, new therapeutic targets and treatments are needed to uncovered and exploited to control hypertension and its comorbidities. In the past, classical drug targets, such as the aldosterone receptor, aldosterone synthase, and ACE2/angiotensin 1-7/Mas receptor axis, have been investigated. Recently, vaccines and drugs targeting the ...
antihypertensive medications 10 3.4 Drug classes to be used as first-line agents 11 3.5 Combination therapy 13 3.6arget blood pressure T 16 3.7equency of re-assessment Fr 17 ... 5 Publication, implementation, evaluation and research gaps 24 5.1 Publication 24 5.2 Implementation and dissemination 24 5.3 Evaluation 24 5.4e updating of the ...
The translation of pharmacological research to the treatment of hypertension has been a continuous process, starting with drugs discovered 60 years ago, such as thiazide diuretics (1958) and currently finishing with the newest antihypertensive agent available on the market, the orally active direct renin-inhibitor aliskiren, discovered more ...
In another drug utilization study, 645 prescriptions were analyzed. A total of 697 antihypertensive drugs prescribed, of which 33.57 % were ARBs, 16.79 % ACEIs, 13.63 % were BBs and 11.91 % CCBs. About 32 % of the antihypertensives prescribed were from the essential medicine list [ 17 ].
Conclusions: Despite the modest sample, it appeared that even advanced hypertension can be controlled with lifestyle measures and 2 or 3 antihypertensive drugs, at modest or low dose. Lower drug dose has the advantages of less adverse events and potentially improved tolerability and compliance. References. 1. Mills KT, Stefanescu A, He J.
This systematic review and meta-analysis of 72 RCTs evaluates the effect of antihypertensive drugs morning versus evening dosing, which showed that evening dosing of antihypertensive drugs significantly reduced ambulatory BP parameters and lowered cardiovascular events but the effect was mainly driven by trials by Hermida et al. Unless the ...
Original research Antihypertensive drug effects on long-term blood pressure: an individual-level data meta-analysis of randomised clinical trials ... randomised evidence on the effect of antihypertensive drugs on BP has come from efficacy trials with small numbers of highly selected participants and short follow-up durations.20 Pooled evidence ...
There was an increase in prescriptions of antihypertensive drugs in both groups at 24 months; the mean number of antihypertensive medications at 24 months was higher in the intervention group than in the control group (2.4 [95% CI, 2.2-2.5] vs 2.1 [95% CI, 1.9-2.3]; P = .04) (eTable 3 in Supplement 2).
Other Affiliation: Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics and Precision Medicine, College of Pharmacy, University of Florida, Gainesville, FL, United States; ... We sought to identify genome-wide variants influencing antihypertensive drug response and adverse cardiovascular outcomes, utilizing ...
NCC is an important protein regulating blood pressure and a major target of thiazine antihypertensive drugs. ... (2020SK51823, 2021SK51828, 2021SK51823), Hunan Provincial Clinical Medical Research Center for Drug Evaluation of major chronic diseases (2023SK4040). The Fund Project of University of South China (2020-25 and 2020-26), The Fund ...
Objective Evidence from randomised trials of pharmacological treatments on long-term blood pressure (BP) reduction is limited. We investigated the antihypertensive drug effects on BP over time and across different participant characteristics. Methods We conducted an individual patient-level data meta-analysis of 52 large-scale randomised clinical trials in the Blood Pressure Lowering Treatment ...
That Can Predict How the Human Body's Molecules Behave, Boosting Drug Discovery Research. Called AlphaFold 3, the latest update of the software models the interactions of proteins with DNA, RNA ...
Previous research has emphasized the potential benefits of anti-diabetic medications in inhibiting the exacerbation of Chronic Obstructive Pulmonary Disease (COPD), yet the role of anti-diabetic drugs on COPD risk remains uncertain. This study employed a Mendelian randomization (MR) approach to evaluate the causal association of genetic variations related to six classes of anti-diabetic drug ...
Poor drug regulation in India is not a recent problem. The Indian drug market is full of look-alike, sound-alike (LASA) drugs which have not yet caught the attention of the media or the medical community. This viewpoint highlights the problem of LASA drugs and poor prescription practices and proposes solutions for involving all stakeholders in this unaddressed issue which is a huge public ...
Kandzari, Bohm DE, Mahfoud M, Townsend F, Weber MA RR, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results ...
Pharmacotherapy is indicated for all patients who have grade 2 hypertension defined as BP ≥ 160/100 mm Hg regardless of their risk or comorbidities. 26 The 2018 ESC/ESH guidelines recommend immediate pharmacotherapy in grade-1 hypertension (BP 140-159/90-99 mm Hg) with renal disease, high risk of ASCVD, or hypertension-mediated organ damage ...
Overall, 12% of adults say they have ever used GLP-1 drugs, including 6% who say they are currently using them. The share who report ever taking these drugs rises to about four in ten (43%) among ...
St. Jude Children's Research Hospital. St. Jude Children's Research Hospital is leading the way the world understands, treats and cures childhood cancer, sickle cell disease, and other life-threatening disorders. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood ...