ART User Guide
What is ART?
The Assisted Referral Tool (ART) was developed by the NIH Center for Scientific Review (CSR) to recommend potentially appropriate study sections, based on the scientific content of a user’s grant application. The information you provide ART is only used to recommend study sections and is not stored or persisted. The recommendations made by ART are solely for the benefit of the user.
How ART Works
ART uses natural language processing and large-scale machine learning technology to make recommendations. It uses indexed representations of both the application text that the user enters as well as all the grant application data used to train the models.
As of August, 2020, 175 study sections are represented in ART. Models have been trained and validated for every pair of study sections , for a total of nearly 15,000 unique pairwise models. The corpora of text for training the models are drawn from up to ten years of indexed text for each study section.
After the user’s application text has been entered, it is compacted into an indexed form and sent to each of the 15,000 pairwise classification models. Each pairwise model is a support vector machine (SVM) that determines which of the two study sections comprising that model is more appropriate for the application text in question. The indexed application text is thus sent to the bank of 15,000 SVM models, each of which votes on a study section.
Choosing the Mode of Operation
When you enter the ART landing page, you are asked a couple of questions to choose the operating mode:
There are essentially two modes of ART: recommending study sections and recommending SBIT/STTR Special Emphasis Panels. These two modes involve completely different models that are trained independently. That is, none of the SBIR/STTR models are used in the primary mode, nor are study section models used in SBIR/STTR mode. Whereas study sections are recommended at the SRG (study section) level, SBIR/STTR SEPs are recommended at the IRG (parent) level.
The Animal Usage checkbox is used to filter the list of potentially relevant study sections. If the checkbox is left open, all study section models are included in the search. If the checkbox is checked, however, study sections with less than 5% animal research are omitted from the search.
Extracting Scientific Concepts
When you enter your application text into the main textbox (and your application title into the title textbox), your application is indexed in two ways. First, the RCDC ERA UberIndexer resource extracts scientific concepts from the RCDC Thesaurus and weights them according to relative frequency in the text. (See https://report.nih.gov/rcdc/index.aspx for a description of RCDC). Concepts found in the title are given full weight. Second, a novel indexing scheme based on a self-learned dictionary of n-grams is used to provide an alternative index, intended to capture new concepts that may not be found in a curated thesaurus. The two indices are concatenated into a single vector representing the text you entered. All the grant applications used to train the models (essentially ten years’ worth of grant applications) are similarly indexed. ART does not store the documents used to train the models nor their indices, but rather only the model representations. Your text and the extracted indices are discarded when your job is complete.
Recommending Study Sections
The vector representing the concatenated index of your text is sent to the bank of 15,000 pairwise SVM models (or a smaller bank in the case of the SBIR/STTR SEPs), each of which votes on which of its two study sections is more appropriate for your application. Study sections are ranked according to those that received the most pairwise votes. A variety of criteria are used to select how many (generally three to six) study sections are chosen as having “Strong” relevance as well as how many (also three to six) additional study sections are indicated as having “Possible” relevance. Within these two groups, the study sections are listed alphabetically.
In the example below, four study sections are listed in the “Strong” group and five in the “Possible” group. Note that the listing of the study sections is alphabetic within a group by the SRG abbreviation. For each study section, links to the SRG as well as the parent IRG descriptions are provided, as well as a link to the SRG roster. Users are encouraged to browse these links to determine whether the recommended study sections are a match for their research.
Tips for Using ART
1. Entering the Title is optional but strongly recommended – ART can operate without text entered into the Title box but this is not recommended. Scientific concepts found in the Title are given full weight by the ERA RCDC Uberindexer, matching the indexing scheme of applications as they were used to train the models. The user could enter the text in the main text box but this does not allow the Uberindexer to apply full weight to title concepts.
2. Entering both Abstract and Specific Aims is recommended – In general, an abundance of text improves performance. ART ignores stop words such as “Abstract” and “Specific Aim” intended to delimit sections of text. Therefore, it is in your interest to include both the Abstract and the Specific Aims from your application. You can just copy and paste the text, section headers and all. However, we do not recommend including sections other than Abstract and Specific Aims. ART will not filter out other sections based on section header.
3. Minimum text requirement – ART requires that at least 10 scientific concepts from the RCDC Thesaurus be identified by the Uberindexer from your text, comprising both the Title and main text box combined. For ART to classify accurately, it needs a strong enough “signal” to overcome potential “noise.” Empirical testing revealed that a signal strength threshold of 10 concepts is necessary to ensure accurate classifications.
Keeping ART Updated
As CSR updates its study sections periodically, so too must ART be updated. This involves training new models for the new study sections and retiring models for study sections that are being phased out. Three or four new releases of ART are issued per year, to keep up with changes in study sections. For the new models to be accurate, there need to be enough assignments of applications to the new study sections. In some cases, if too few assignments are available for accurate classification, ART may resort to business rules redirecting recommendations from retiring models to the new study sections, until the new models can stand on their own in a future release. For every new release of ART, a comprehensive testing and validation suite is applied on all study sections.


Writing For Reviewers
Remember the review criteria .
For more information on the scoring criteria and how reviewers use them please see Scoring and Summary Statements For more insight into what reviewers expect to find in your application, please see NIH Review Guidelines for the given grant type and the Review Criteria.
Remember the review criteria when writing an application. Take special note of any opportunity-specific criteria or statements in Section V of the chosen notice of funding opportunity.
As you write your application, think of it as an integrated whole. Reviewers mostly focus on the Research Strategy, but other sections count too.
Your overall impact score will reflect reviewers' judgment of two broad concepts: importance and likelihood.
- Importance —the significance and innovation of the research problem—whether the scientific question is justified and able to move the frontier of knowledge forward.
- Likelihood —the ability that the Principal Investigator(s), can achieve the ends, as judged by the experimental design, the rigor of that design , the expertise of the team, and the resources available.
Together, importance and likelihood form impact .
Your Reviewers Are Your Audience
For more details about the review process please see Understand the Review Process . For more details about how reviewers assess rigor and transparency, see Reviewer Guidance on Rigor and Transparency
Study sections usually have about 20 peer reviewers, but not all reviewers have the same role. As a study section can have dozens or even hundreds of applications; not all reviewers read all applications. Instead, the Scientific Review Officer (SRO) assigns a small number of reviewers to assess the application and relies on the expertise of the assigned reviewers to focus the study section’s discussion of the application. Applications are assigned primary and secondary reviewers plus at least one additional reader, because their expertise is closest to the application field. Do not expect the other members of the study section to be experts in the field or familiar with the specific science in your application.
The assigned reviewers read the application thoroughly, write a critique before the meeting, and assign preliminary scores for each review criterion as well as an initial overall impact score. The primary reviewer presents an application's topic, strengths, and weaknesses to the group, and other assigned reviewers may comment. Write and organize the application so the primary reviewer can readily grasp what you are proposing and be well poised to explain it to the others. These initial remarks launch the group discussion, the basis for the overall impact score that each of the reviewers gives an application. More than anything else, the impact score sets up the percentile or ranking of an application, which has a significant impact on the application's funding fate. Note that all reviewers will score the application —even those who weren’t specifically assigned to the application. Read more at NIH Center for Scientific Review.
Writing for the Reviewers
In the various parts of the application, use different approaches to reach out to the audience to accommodate different levels of knowledge about your techniques and field. Assigned reviewers are often at the top of a given field, they will know where the opportunities are, and have strong views about what research should be conducted to move the field forward. Looking at their research and talking to other experts in the field can provide insight on how to meet reviewer expectations. However, it is critical to not neglect the other reviewers by writing at a level they will understand. Addressing all your reviewers is key if you're proposing highly innovative research.
By describing how the research gap is critical and the outcomes of the proposal are high-impact will demonstrate to reviewers why NINDS should prioritize the application. Describe the strong potential for the application to have a high impact on its field of science; that the approach is logical, rigorous, and innovative; and the required expertise, resources, and intuitional support are present. Citations and other references in the Approach should highlight expertise of the team. Emphasize the project's significance and (to a lesser extent innovation) in several places to meet the needs of the full reviewer audience.
Help the assigned reviewers advocate for the application by organizing and writing so the assigned reviewers can readily find and understand the goals, significance, robustness, and feasibility of the application. Emphasize critical points that the assigned reviewers can convey to the study section. Assigned reviewers will review your entire application to make this assessment. However, for non-assigned reviewers, the Abstract, Aims, and Significance section of the Research Strategy along with the figures contained within are often the most utilized source of information regarding your application. Be sure your application is cleanly written, free of typos, and that critical points are emphasized and understandable by reviewers who are experts in other fields. It may be beneficial to ask investigators working in a different field to read the application and provide feedback on readability well in advance of the submission date.
Study Sections and Members
For investigator-initiated R01, R21, and R03 applications, NIH encourages applicants to request a study section they feel would be most receptive to the application. Study section assignment requests are made using the PHS Assignment Request Form . You can also note what expertise are needed to review the application and identify any reviewers who should not review the application. Do not request reviewers by name, as this may lead them to be disqualified. Please note that assignment to a requested study section is not guaranteed, particularly:
- If using continuous submission to apply outside of standard due dates, NIH may refer the application to a relevant study section or to a special emphasis panel.
- If a standing study section member has a conflict of interest, NIH may refer the application to another study section with the expertise to review the application fairly. If no such study section exists, NIH may use a special emphasis panel for review.
After identifying a proposed study section, determine how its membership may affect the presentation of your proposed research. The reviewers need to share the perspective that the proposed work is vital to the field. It's critical to choose a study section that would embrace both the field and the direction of the proposed research. Review the study section roster, identify the reviewers who are most likely to be assigned to your application, and research their work to better understand how they may perceive your application.
However, if the study section has appropriate expertise but there is concern about a particular reviewer, inform the Scientific Review Officer when applying. NIH will ensure all applications receive a fair review.
comments Want to contact NINDS staff? Please visit our Find Your NINDS Program Officers page to learn more about contacting Program Officers, Grants Management Specialists, Scientific Review Officers, and Health Program Specialists.

- NIH Grants & Funding
- Blog Policies
NIH Extramural Nexus
Encouraging Use of the PHS Assignment Request Form in Applications
You have likely come across the Public Health Service (PHS) assignment request form when putting together your grant application. It’s optional, but we encourage applicants to fill it out.
This form is available in nearly all competing NIH application form packages and allows you to provide specific application assignment and review information to referral and review staff.
Applicants may suggest NIH Institute/Center/Office (ICO) assignments, particular study sections, names of people who may have a conflict with reviewing the application, and areas of expertise needed for the review. The information provided is not included in the assembled application and is only shared with appropriate NIH review staff, not with reviewers or others at NIH.
Several resources exist to help identify which NIH ICO or study section may be appropriate. For instance, you can search RePORTER for grants where related research was funded and reviewed (see also this blog ). The NIH Center for Scientific Review’s Assisted Referral Tool can also help you identify potentially appropriate study sections .
Please keep in mind that suggestions for peer reviewers are not allowed on the form (or the cover letter or anywhere else in the application). The PHS form clearly states “do not provide names of individuals” you would like to review your application.
The main reason we do not allow these suggestions is due to potential conflicts of interest between the applicant and reviewer. Any recommendations of reviewers that an applicant provides are ignored. We will likely even be more hesitant to recruit them to serve on the study section because of the possible unforeseen conflicts of interest.
Overall, the information provided on the PHS Assignment Request form is quite helpful for NIH review staff, who refer to it when considering the best course of action for your application’s timely review. Our staff will carefully consider your suggestions, which are also balanced with many other factors when making appropriate ICO and review group assignments.
Check out this NIH All About Grants podcast for more ( MP3 / Transcript ).
RELATED NEWS
I wonder how carefully CSR reads these forms. Recently I requested assignment to an institute as primary, who wanted to receive it, and instead it was assigned to the secondary institute that had already rejected the application. I eventually was able to get this switched but it took a great deal of time and effort.
I in my experience CSR does not use the information to assign proposal to study sections
It might encourage use of this form to know more about how the NIH assigns study sections. I assume that the NIH might use tools like the ones listed on this post; therefore, if I do the same and send the results on the PHS form it seems redundant. If NIH does not use these tools, why not? And what researcher perspectives on desirable assignments are useful?
I have has similar experience as previous comments. Two of my recent proposals were not assigned to the study section requested. This has happened in the past too.
I agree with the above investigators’ comments. In my experience, Public Health Service (PHS) assignment request form has not been helpful to me as an investigator. I encountered many instances that my applications were assigned by the CSR to a different institute or study section I did not requested. I wrote to argue many times without success. In a latest instance last year, I specifically requested to exclude a potential reviewer from reviewing my application in the Public Health Service (PHS) assignment request form who I believe is historically very biased and impartial to investigators of color. Yet CSR and my SRO still assigned my application(s) to this reviewer to review my application with nearly 2 pages’ critiques on my research strategy alone and a score 8 out of 9!
If the CSR encourages the investigators to use the Public Health Service (PHS) assignment request form, my suggestion is that the CSR and SRO should consider the investigators’ requests and concerns.
I’ve had similar experiences as the other commenters – multiple times I have submitted the form and then been assigned another study section with no strong rationale provided as to why, and it has been very difficult to get it changed (only successful about half of the time).
After working very hard on a grant for months and tailoring it to a specific audience (and having the salary of me and my team dependent on it), it is very disappointing to have a grant reviewed by a section that has different expectations and background knowledge, with little transparency provided throughout the process.
CSR appreciates information provided by investigators. The information is considered in making assignments to funding institutes, review groups, and in identifying conflicts of interest. However, requests cannot always be honored. There are many reasons for assignments made to groups other than those requested. For example, sometimes a request is made for assignment to a funding institute that does not participate in the funding opportunity announcement under which the application was submitted. In making assignments to review groups, investigator suggestions are considered but within the parameters of the published study section descriptions. The need to manage conflicts of interest also contribute to assignment decisions. Questions about funding institute assignment may be directed to the CSR Division of Receipt and Referral at [email protected] and questions about review group assignment should be directed to the assigned scientific review officer, identified in your eRA Commons account.
Before submitting your comment, please review our blog comment policies.
Your email address will not be published. Required fields are marked *

Subscribe Follow Watch
NIH Prevention-Related Study Sections
Research applications are reviewed either by the Center for Scientific Review (CSR) or one of the NIH Institutes or Centers (ICs) specified in the funding opportunity . You may request assignment to a particular study section; however, while NIH staff consider all requests, in some cases the reviewing IC is predetermined and requests cannot be honored.
- Alert: New simplified review framework for research applications with due dates on or after Jan. 25, 2025.
- How applications are assigned
- How to request a specific study section
To get the most accurate information about the review of your application—including if it will be reviewed by CSR or an IC—refer to “Section V. Application Review Information” of the specific funding opportunity to which you are submitting. If the peer review contact is at one of the ICs, the review will be held there; if the peer review contact is at CSR (or no contact is listed) the review is likely being held at CSR.
You are also strongly encouraged to:
- Talk with the scientific/research contact listed in “Section VII. Agency Contacts” to learn more about the funding opportunity itself or the type of research the IC is interested in supporting.
- Reach out to the peer review contact listed in “Section VII. Agency Contacts” with questions about the review or your application’s assignment.
Once an application is assigned to a study section (sometimes called a review committee or a Scientific Review Group—SRG), the review process is organized and overseen by a Scientific Review Officer (SRO) to ensure it is fair and unbiased.
Center for Scientific Review Study Sections
About 70% of research applications are reviewed by CSR study sections, which are organized by scientific discipline or research area. CSR reviews most investigator-initiated research project grant (many R and U), fellowship (F), and small business (SBIR/STTR) applications; however, always check Sections V. and VII. (“Application Review Information” and “Agency Contacts,” respectively) of the specific funding opportunity to which you are submitting.
- Keyword or topic search
- Assisted Referral Tool (ART)
NIH Institute and Center Study Sections
NIH ICs review applications specific to their missions and needs. Typically, these include program project (P), institutional training award (T), and career development award (K) applications, as well those submitted in response to IC-initiated Requests for Applications (RFAs). The review location of these applications is predetermined, so always check Sections V. and VII. (“Application Review Information” and “Agency Contacts,” respectively) of the specific funding opportunity to which you are submitting.
- IC standing study section rosters
- IC SEP rosters
- NIH Institutes, Centers, and Offices

Tools to Help select a Study Section
By bouvier grant group.
- April 13, 2023
We stay current on NIH happenings and would be delighted to keep you informed.
Did you know that there are several NIH-sponsored tools that applicants can use to determine which study section is most appropriate for their application? First, NIH RePORTER , where you can search for funded projects by a myriad of search criteria, including specific terms in a project title, project abstract, or keywords; PI name; organization; organization type; I&C; and more.
Second, NIH Center for Scientific Review’s (CSR) Assisted Referral Tool (ART) can help investigators determine what study section is the best fit for their application. The ART requests that users enter their application title (optional), abstract, and specific aims. The ART algorithm uses the text provided to find the optimal study section.
While the Assignment Request Form isn’t a 100% guarantee that the request will be honored,
it increases the odds that your application ends up in the optimal study section.

Author: Dr. Meg Bouvier
Margaret Bouvier received her PhD in 1995 in Biomedical Sciences from the Mount Sinai School of Medicine. After an NINDS post-doctoral fellowship, she worked as a staff writer for long-standing NIH Director Dr. Francis Collins in the Office of Press, Policy, and Communications for the Human Genome Project and NHGRI. Since 2007, Meg has specialized in editing and advising on NIH submissions, and began offering virtual courses in 2015. She's recently worked with more than 40% of the nation's highest-performing hospitals*, four of the top 10 cancer hospitals, three of the top five medical schools for research, and 14 NCI-designated cancer centers. Her experience at NIH as both a bench scientist and staff writer greatly informs her approach to NIH grantwriting. She has helped clients land over half a billion in federal funding. Bouvier Grant Group is a woman-owned small business.
*Our clients include 9 of the top 22 hospitals as recognized by the 2023/24 US News & World Report honor roll
Categories:
- Meg Bouvier Blog , NIH grantwriting
Related posts
You may also be interested in.

Intro to the NIH and NSF SBIR/STTR programs

Why Knowing IC Funding Priorities Is Important
How can we help.
Let’s have a conversation about how we can help your faculty improve their NIH grantsmanship skills.

- Course Library
Quick Links
- Privacy Policy
- Terms of Use
Contact Info
- 6 University Drive, Ste. 206, #237 Amherst, MA 01002
- 413-200-9155
- [email protected]
- Weekdays 9 AM - 5 PM ET
- We value your privacy and will never sell your information.
Wait! Subscribe to our monthly newsletter for the latest NIH news, grantwriting tips, and more.


- About Grants
PHS Assignment Information
Nih institutes/centers(ics).
NIH Institutes/Centers are the funding Components of NIH. More Information about the scientific missions/Centers can be found by browsing the links in the section "Funding Components of NIH". You may also use the NIHRePORTER System for keyword searches which can identify applications funded by the various NIH Institutes/Centers. A tool in NIHRePORTER, called "Matchmaker", allows you to paste your abstract/specific aims in a query box and get information about which NIH Institutes/Centers may have funded similar work in the past. NIH Institute/Center requests are not required. However, if you wish to make an assignment request, please use the correct short abbreviation for that institute.
| Short Abbreviation | NIH Institute/Center |
| NCI |
|
| NEI |
|
| NHLBI |
|
| NHGRI |
|
| NIA |
|
| NIAAA |
|
| NIAID |
|
| NIAMS |
|
| NIBIB |
|
| NICHD |
|
| NIDCD |
|
| NIDCR | |
| NIDDK |
|
| NIDA |
|
| NIEHS |
|
| NIGMS |
|
| NIMH |
|
| NIMHD |
|
| NINDS |
|
| NINR |
|
| NLM |
|
| FIC |
|
| NCCIH |
|
| NCATS |
|
| ORIP |
|
CDC Centers, Institutes and Offices (CIOs)
| Short Abbreviation | CDC Centers, Institutes and Offices |
| NCIRD | |
| NCEZID | |
| NCHHSTP | |
| NCBDDD | |
| NCCDPHP | |
| NCEH | |
| NCIPC | |
| NIOSH | |
| CGH (Global Health) | |
| COTPER (Terrorism) | |
| NCHS |
Other Department of Health and Human Services Funding Components
With the exception of SBIR/STTR programs, applications assigned to other operating divisions (see list, below) of the Department of Health and Human Services will be reviewed there. See Section VII (Agency Contacts) section of the Funding Opportunity for additional information about these reviews.
| Short Abbreviation | NIH Agency Partner |
| AHRQ |
|
| CDC |
|
| FDA |
|
| ACF |
|
| ACL |
|
IC vs CSR Review
Although the majority of NIH reviews are conducted by the Center for Scientific Review (CSR), some applications are reviewed in-house at one of the NIH Institutes/Centers. The Peer Review Contact listed in Section VII (Agency Contacts) section of the Funding Opportunity will be able to provide additional information about the review. If the Peer Review contact is listed as being at one of the NIH Institute/Centers, the review will be held there; if they are listed as being at CSR (or no contact is listed) the review is likely being held at CSR. If your application is getting reviewed at an IC, then you do not need to request a study section. See our Locus of Review FAQ for more information.
Center for Scientific Review (CSR) Study Sections and Special Emphasis Panels
Applications are reviewed in Study Sections (Scientific Review Groups, SRGs) or Special Emphasis Panels (SEPs). Integrated Review groups (IRGs) are clusters of SRGs and SEPs that are related based on scientific discipline. You may also use the NIHRePORTER system to learn more about which study sections reviewed funded applications. Click here to see the complete listing of CSR Study Sections and Special Emphasis Panels Click here to see the complete listing of CSR Integrated Review Groups
This page last updated on: May 8, 2018
- Bookmark & Share
- E-mail Updates
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

Application Assigned to a Review Group
The Division of Receipt and Referral (DRR) in NIH's Center for Scientific Review (CSR) checks your application for administrative and formatting requirements. DRR also assigns the application to a scientific review group or special emphasis panel (SEP) at CSR or an NIH institute or center (IC). For more information, check The Assignment Process .
For investigator-initiated R01, R21, and R03 applications, the institute and review group assignments are based on the science proposed in the application. If you included an assignment request with your application using the PHS Assignment Request Form, NIH staff take that into consideration as well.
For applications in response to an initiative such as an IC request for applications (RFA), applications would all be assigned to that IC, which may establish one or more funding opportunity-specific SEPs. These SEPs may be convened by the NIAID Scientific Review Program (SRP) or by CSR.
To determine your application’s likely locus of review, check your chosen notice of funding opportunity. See "Section V. Application Review Information" and scroll down to subsection "2. Review and Selection Process." The first sentence of this section will tell you whether your application will be reviewed at CSR or at NIAID SRP.
Here are general rules of thumb about who will be reviewing your application, based on application type:
CSR review —For applications in response to program announcements (PAs), including parent announcements and most notices of special interest, the most appropriate study section in CSR will conduct the initial peer review. Potentially, many integrated review groups (IRGs) could review applications responding to a single PA.
Note: for NIAID, there is an exception to the rule above for career development (K) and training (T) awards. For K and T applications, NIAID SRP oversees the initial peer review instead of CSR.
Institute review —Instead of CSR, institutes like NIAID oversee initial peer review of applications submitted for the following:
- Some investigator-initiated applications, such as program project applications, investigator-initiated clinical trials, and resource grant applications
*For RFAs in which multiple NIH institutes are participating, the lead sponsoring institute usually conducts the review.
If you apply to a program announcement with special receipt, referral, and/or review considerations (PAR), your application might go to CSR or an institute. In either case, a SEP is likely to review your application. That means instead of requesting assignment to an IRG in the PHS Assignment Request Form, we suggest that you focus on describing the scientific expertise necessary to review your application.
NIH Checks Your Application
After your application moves to DRR in CSR, staff there assign it a number and make sure it conforms with administrative and formatting requirements.
Your application gets a unique identification number that looks like this: 1 R01 AI183723 02 A1 S1. Learn how to interpret the parts of the application number using the NIH definition of application identification number . NIH staff will typically refer to your grant or application using that number.
Be aware that DRR will perform a manual check of your application and may return your application to you without a peer review for any of the reasons we described previously at Act Now to Avoid Post-Submission Rejection on Submit an Application .
A late application is not acceptable without proper justification; for more information, read Late Applications and Post-Submission Materials .
Applications Are Assigned to an Institute and Integrated Review Group
At that point, DRR assigns an investigator-initiated application to an IRG, an umbrella organization comprising several study sections, and then to the study section that will perform the initial peer review. It also assigns your application to an institute or center for potential funding.
You may have requested these assignments in your PHS Assignment Request Form, as we described at Use the PHS Assignment Request Form . While DRR often complies with these requests, it is not required to, and DRR staff may make different assignments based on NIH referral guidelines and workload.
Within 7 to 10 days after you apply, you should find your initial assignment information in the Commons.
Study Section. At first, the Commons may show your IRG instead of your study section. During the next few days, DRR will update this information with the study section assignment. If you don't see your study section assignment within 2 weeks, call DRR at 301-435-0715 .
Institute. After NIAID receives notice of your application, our Referral and Policy Analysis Branch assigns it to one of our program divisions using our internal referral guidelines. SRP will organize a SEP to conduct the review of your application with others received in response to the same funding opportunity.
Do You Have the Right Reviewers?
Approximately 30 days before the review meeting, CSR or SRP will post your study section's updated roster in the Commons. Although standing member rosters for CSR study sections are always available on the CSR website, study section rosters may change from one review cycle to the next, so you won't know exactly who is on the panel until CSR posts updated rosters.
After the roster appears in the Commons, make sure the expertise you need is represented on the committee. If it is not, or if you are concerned that there are problematic competitors on the roster, notify your scientific review officer (SRO).
If you used the PHS Assignment Request Form and did not get the study section you requested, check carefully that the one you are assigned to meets your needs. CSR may have more than one study section with the expertise you require and sometimes creates ad hoc groups to fill in gaps. Find out more from your SRO.

Requesting a Change in Study Section
If you submitted an investigator-initiated R01, R21, or R03 application and feel your application was not assigned to an appropriate study section, you can request a change:
- Check the CSR Integrated Review Groups to find an alternative. Find roster links at the top of the study section pages.
- Discuss your alternative with the chief of the IRG for your assigned study section. Find his or her contact information in the CSR Staff Directory or ask your SRO if you cannot find it online. (Don’t contact the listed reviewers.)
To make your request, email [email protected] requesting a new assignment and briefly stating the rationale for the change. Below is an example of an acceptable and an unacceptable request.
- Acceptable: "The focus of study section X seems to be more on the structural biology of molecules of immunologic importance. Since my application proposes to develop new antibodies for Phase I human studies, the clinical perspective of reviewers on study section Y is critical to appreciate the approaches I have taken."
- Not acceptable: "I don't want X reviewers but want Y instead."
Do You Have the Right Institute?
Did you request assignment to an institute in your application? We explained how at Use the PHS Assignment Request Form .
If you didn't get the right assignment or you think you requested the wrong institute, contact CSR's Division of Receipt and Referral at [email protected] and briefly state the rationale for the change as above.
Your application stands the best chance of getting funded if it goes to an institute that considers the research high priority.
Previous Step
Have questions.
Contact your assigned scientific review officer, found in your eRA Commons account or in your notice of funding opportunity. If you do not have a scientific review officer, go to Scientific Review Program Contacts .
Related Rules & Policies:
- Request for Primary IC Assignment SOP
- Late Applications SOP
- Dual Assignments SOP
| Revised May 13, 2024 |
| For additional assistance, please contact the . |
|
PHS Assignment Request Form
For assistance with the information required on this form, please refer to the appropriate application guide on the How to Apply page.
NOTE: The forms in these topics reflect FORMS-H, which must be used for applications with due dates on or after January 25, 2023 (see guide notice NOT-OD-22-195 . Also see the annotated form set and summary of changes for Forms-H.) For due dates on or before January 24, 2023, use FORMS-G.
Steps for Filling out the Form
The PHS Assignment Request form is used for capturing assignment requests.
This form replaces certain information from the application cover letter attachment, and should be used to:
- request an assignment to an institute/center for funding consideration
- request an assignment to a specific study section for initial peer review
- identify individuals who may not be appropriate to review their application
- identify scientific areas of expertise needed to review the application
This form is only visible to receipt and referral staff and scientific review officers, who may need to act on the information.
For guidance on completing the form in ASSIST, refer to the steps below.
Adding an Assignment Request Form
To add an Assignment Request form:
- Select the Add Optional Form button from the Actions navigation panel.
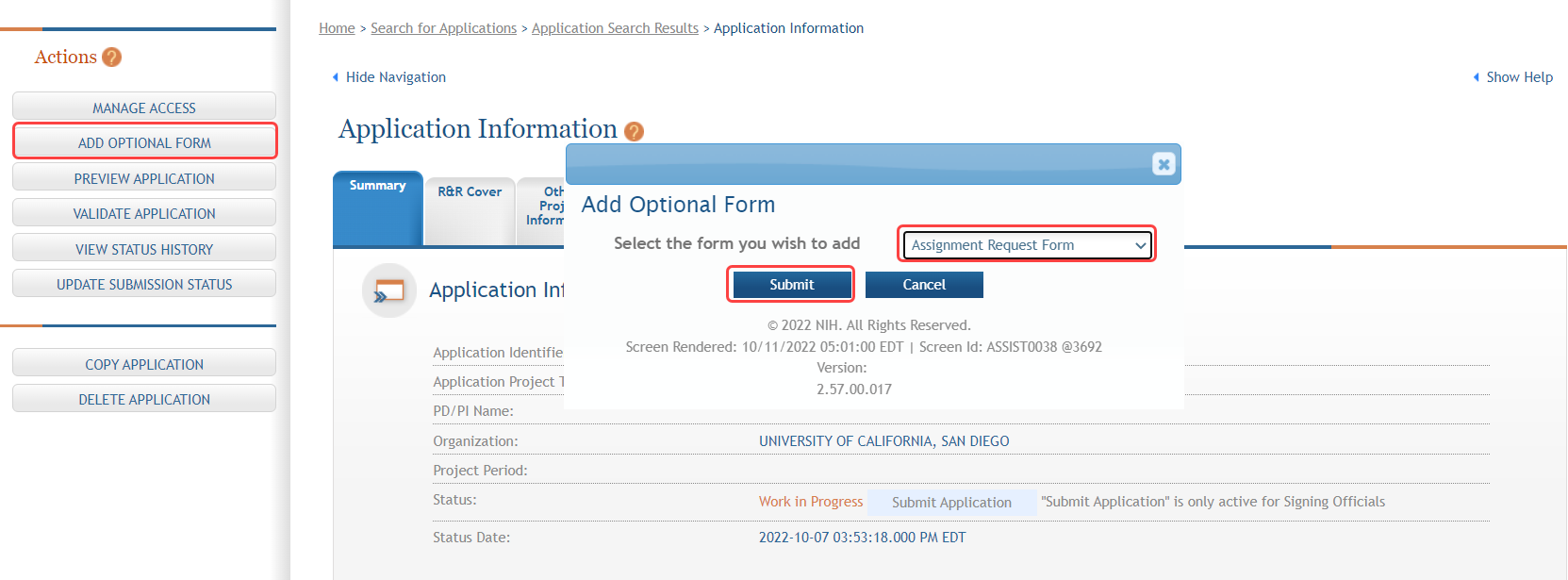
- Complete the required fields and any other appropriate information. Required fields are marked with an asterisk (*).
- To save the information and keep the form open for further editing, select the Save and Keep Lock options.
- To save the information and close the form, select the Save and Release Lock button.
- To delete the form and return to the original application, select the Remove Form button.
NOTE: Selecting the Cancel and Release Lock button - followed by the Continue button on the confirmation - returns the form to read-only and does not save any of the entered information onto the form.
Viewing and Editing the Assignment Request Form
To view and/or edit an assignment request form:
Once the Save and Release Lock button has been clicked, the details of the form are displayed as read-only.
To further modify the form, select the Edit button.
|
| For feedback on the online help, please email the ' + 'eRA Communications Office' + ' . |
- Work & Careers
- Life & Arts
Political deepfakes top list of malicious AI use, DeepMind finds
To read this article for free register for ft edit now.
Once registered, you can:
- Read this article and many more, free for 30 days with no card details required
- Enjoy 8 thought-provoking articles a day chosen for you by senior editors
- Download the award-winning FT Edit app to access audio, saved articles and more
- Global news & analysis
- Expert opinion
- Special features
- FirstFT newsletter
- Videos & Podcasts
- Android & iOS app
- FT Edit app
- 10 gift articles per month
Explore our full range of subscriptions.
Why the ft.
See why over a million readers pay to read the Financial Times.
International Edition
Environment | The West is warming and drying so fast that a…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Twitter (Opens in new window)
Digital Replica Edition
- Latest Headlines
Environment
- Transportation
- News Obituaries
Environment | The West is warming and drying so fast that a crucial drought-monitoring tool can’t keep up, study says
U.s. drought monitor’s weekly reports impact disaster relief, government decision-making.

Every week since 1999, the U.S. Drought Monitor has published a new map showing drought conditions across the country, with five categories of drought severity depicted in shades of yellows, oranges and reds. Policymakers and elected leaders in Colorado and other states use the map to make critical decisions about water use, campfire bans, declarations of emergency and more.
And multiple federal agencies use the map to determine how much financial aid is filtered to ranchers and farmers in times of drought.
But what was once considered an exceptional, rare drought is no longer so rare, the study found.
An “exceptional drought” — the most severe category of drought, depicted in dark red — should occur in a region only 2% of the time, according to the monitor’s guidelines. But some areas of the western U.S. have been in exceptional drought 18% of the time, according to a study published this spring in AGU Advances , a scientific journal. An exceptional drought is also more harmful than it was when the monitor was founded more than two decades ago, the study states.
“What is the value to a decision-maker of a map that is just red all of the time?” said Justin Mankin , a professor at Dartmouth College and the study’s lead author. “It doesn’t help you triage resources.”
In Colorado, the severity, length and breadth of droughts can have substantial impacts on the state’s $47 billion agriculture industry. Swaths of the state are so often in drought that brief reprieves from dryness merit news stories — as in 2023, when the Drought Monitor declared the state drought-free for the first time since 2019.

But dryness has returned and nearly half of Colorado is now in drought or has near-drought conditions, according to the monitor’s most recent report .
Mankin and the other study authors explored two ways to better incorporate climate change into the monitor reports, but both have drawbacks.
Those in charge of the Drought Monitor — the National Drought Mitigation Center at the University of Nebraska-Lincoln, the National Oceanic and Atmospheric Administration and the U.S. Department of Agriculture — could create a new category for drought that is more severe than “exceptional drought.” Scientists have similarly proposed creating a new Category 6 for measuring hurricanes as they intensify due to climate change.
Another solution could be to adjust the data used as the “normal” baseline for the Drought Monitor so that it includes more recent drier years.
The Department of Agriculture last year updated its plant hardiness map to incorporate more recent data indicative of climate change. The map helps farmers and gardeners decide what and when to plant based on their location.
Making such a shift with the Drought Monitor, however, would mute the existence of climate change and would minimize impacts on people affected by aridification, Mankin said.
The Drought Monitor is a crucial tool, he said, and there will be no silver bullet or simple solution to adapt it to climate change. Instead, he said, “a constellation of fixes and investments” is needed.
Get more Colorado news by signing up for our Mile High Roundup email newsletter.
- Report an Error
- Submit a News Tip
More in Environment

Environment | Here’s where Colorado’s wolves — and at least one new pup — traveled in June

Colorado News | Pueblo County wildfire grows to 275 acres, no containment

Outdoors | Why you might have to share the trail with cows while hiking on Colorado’s public lands

Outdoors | Colorado fourteener rules and restrictions; here’s what you need to know
- Latest CDC Covid-19 Info
- CSR Extranet
- Staff Directory
- Contact CSR
- For Applicants
CSR’s primary role is to handle the receipt and review of ~ 75% of the grant applications that NIH receives. NIH separates the review process from funding decisions.
For Reviewers
Reviewers are critical to our mission to see that NIH grant applications receive, fair, independent, expert, and timely scientific reviews. We appreciate the generosity with which reviewers give their time.
News & Policy
The latest news and policy updates from CSR. Read about our outreach programs and publications.
Study Sections
Applications are reviewed in study sections (Scientific Review Group, SRG). Review Branches (RBs) are clusters of study sections based on scientific discipline.
Review Panels & Dates
- Planning & Writing
Submission Process
Most competing grant applications to NIH require electronic submission using the SF424 (R&R) application forms. Electronic submission involves two separate systems working together – the federal portal Grants.gov and the NIH eRA Commons . The Office of Extramural Research provides information on electronic submission and submission options . (Paper applications may only be submitted if required by the funding opportunity announcement.) The Division of Receipt and Referral (DRR) in the Center for Scientific Review (CSR) receives and checks for compliance all applications submitted to NIH. Then the DRR assigns each application: 1) to one or more institutes or centers for funding consideration and 2) to a study section (scientific review group) to evaluate the scientific and technical merit of the application.
The applicant organization submits applications prepared by the principal investigator(s) to NIH through Grants.gov , a single access point for all 26 federal grant-making agencies. The eRA Commons is an online interface where a grant applicant can check the submitted grant application for errors and warnings and view the final image.
Investigators are encouraged to include the PHS Assignment Request Form in their application. The form is optional. Use it if you wish to:
- request assignment to a specific awarding institute or center (e.g. NIMH)
- request a specific study section or that it not be assigned to a specific study section
- list individuals who should not review your application
- identify scientific areas of expertise needed to evaluate your application (do not enter names of experts)
CSR offers a number of ways to identify an appropriate study section including searching for a study section or using the CSR Assisted Referral Tool , and you can use NIH RePorter to identify where similar funded proposals were reviewed. Applicants may wish to contact scientific review officers or the DRR (301-435-0715) with specific questions about a potential assignment.
A cover letter may be included (as a PDF attachment) with your application. Cover letters may not be used for assignment requests but instead should address important information, not covered by the PHS Assignment Request Form, such as eligibility for continuous submission or the reason for a late submission.
Permission is not given in advance for a late submission. For standard due dates, NIH has established a window of consideration in which applications must be received in order to have a possibility of acceptance. All late applications must include a cover letter that provides details on the specific reasons for the delay. Further information is found in the NIH Late Application Policy . A special opportunity for continuous submission of R01, R21, and R34 applications with standard due dates is available for appointed members of NIH study sections and advisory groups. NIH Guide Notice 20-060 details the application receipt period for assignment to a particular advisory council round.
Applicants should make sure that submissions are complete and correct. The NIH policy on post submission materials limits the materials that may be accepted after submission; missing pages or corrected pages may not be submitted.
Noncompeting continuation progress reports are not sent to CSR or submitted through Grants.gov but are submitted using the PHS 2590 to a separate central location and then distributed to the funding institute or center. Contract proposals are sent directly to the soliciting institute or center and administrative supplements are sent directly to the institute or center that is funding the parent grant.
Compliance Check
For electronic submissions, the Funding Opportunity Announcement (FOA) determines the validations that will be applied in processing receipt of the application by the NIH eRA Commons. If errors are identified in the validation process, the errors must be addressed and a changed/corrected application resubmitted to Grants.gov. Applicants are encouraged to start the submission process early enough to allow for error correction and still have an error-free, on-time submission by 5 p.m. local time on the due date.
The DRR checks for compliance with important NIH policies as follows:
- The application must be complete and must contain sufficient information for the review group to evaluate the scientific and technical merit.
- Simultaneous submissions of similar, essentially identical, or identical applications to one or more components of the PHS are not allowed.
- Resubmission applications cannot be submitted until the summary statement from review of the prior submission is posted in eRA Commons. Subsequent applications may be submitted as new (A0) or as an A1 resubmission. Resubmission applications must include an Introduction that discusses the previous review, and the text should be marked to show where changes have been made. A1 resubmission applications must be submitted within 37 months of the original application.
- Applications submitted in response to a Request for Applications (RFA) are normally new applications. If an RFA submission is not successful, a subsequent application should be submitted as a new application , not a resubmission.
- Applications that are changing activity code should also be submitted as new applications .
- Approval is needed for applications requesting $500,000 or more in direct costs: NIH policy requires that any competing application (new, renewal, resubmission, revision) requesting $500,000 or more in direct costs in any year must be accepted by an institute or center prior to assignment for review. Investigators need to contact the institute or center at least six weeks prior to the submission of the application. Note that any Facilities and Administrative costs of subcontracts are not included .
- Approval is also needed for conference grant (R13/U13) applications .
- Applications proposing research Human Embryonic Stem Cells must indicate the registration number of the cell lines to be used or include a statement that one from the NIH registry will be used.
- Format of applications: Applications are checked to make sure that they follow the font style, type size, page limits, margin size and other requirements specified in the application instructions. Noncompliant applications may be withdrawn from the review and funding consideration process.
- An eRA Commons User Name must be provided for all Principal Investigators for all applications.
- At least three reference letters are required for fellowship applications (predoctoral, postdoctoral, and senior fellowships) and mentored research career development award applications by the application due date.
Other important aspects of a grant application such as information on human subjects research, use of vertebrate animals, and plans for resource sharing are scrutinized at other stages of the grant process. Applications that do not address all the critical components may be delayed in the review process or for potential funding.
Last updated: 02/22/2023 10:34
- More Social Media from NIH

- You are here:
- American Chemical Society
- Discover Chemistry
Some landfill ‘burps’ contain airborne PFAS, study finds
FOR IMMEDIATE RELEASE
“Landfill Gas: A Major Pathway for Neutral Per- and Polyfluoroalkyl Substance (PFAS) Release” Environmental Science & Technology Letters
Many municipal landfills “burp” gas from decomposing organic matter rather than letting it build up. And burps from buried waste containing per- and polyfluoroalkyl substances (PFAS) can release these “forever chemicals” into the air, say researchers in ACS’ Environmental Science & Technology Letters. Their study reports unexpectedly high levels of airborne PFAS at three landfills and demonstrates that vented gases and liquid by-products called leachates could transport similar amounts of these contaminants to the environment.

Many municipal landfills “burp” gas from decomposing organic matter rather than letting it build up. And burps from buried waste containing per- and polyfluoroalkyl substances (PFAS) can release these “forever chemicals” into the air, say researchers in ACS’ Environmental Science & Technology Letters. Their study reports unexpectedly high levels of airborne PFAS at three landfills and demonstrates that vented gases and liquid by-products called leachates could transport similar amounts of these contaminants to the environment.
Some consumer products and commercial waste, such as children’s clothing , cosmetics and wastewater treatment sludge solids , contain PFAS — and they ultimately end up in landfills. Timothy Townsend and colleagues previously established that PFAS-containing waste can contaminate the water that seeps through landfills. This leachate is usually captured and treated before entering the environment. Landfills also produce gas that can be captured and controlled, but unlike leachate, it’s often released untreated. The burped gas is mostly made up of methane and carbon dioxide; however, two recent studies also discovered a subset of airborne PFAS called fluorotelomer alcohols, which have the potential to be toxic when inhaled and can be transported long distances. Since the prevalence of PFAS-contaminated landfill vapors isn’t yet widely known, Townsend, Ashley Lin and their team wanted to identify and measure them in vented gas at three sites in Florida.
The researchers pumped landfill gas from pipes through cartridges filled with resin that captured the airborne PFAS. They freed the compounds from the cartridges with organic solvents and analyzed the extracts for 27 neutrally charged PFAS, including fluorotelomer alcohols. Surprisingly, some of the fluorotelomer alcohol levels were up to two orders of magnitude higher than previous studies at other landfills. Three of these alcohols (abbreviated 6:2, 8:2 and 10:2) comprised most of the vaporized contaminants measured at each site. The researchers also collected leachate samples at the Florida sites and analyzed them for ionic PFAS commonly found in water samples. From this data, they estimated that the annual amount of fluorine (as a proxy for PFAS content) leaving the landfills through gas emissions could be similar to, or even greater than, the amount leaving through leachates.
Because landfills are repositories for PFAS, this work indicates that vented gas from these sites should be considered in future mitigation and management strategies to reduce potential inhalation exposure and release to the environment. Some landfills burn the vapors or trap them for energy production, and the team suggests that further research is needed to determine the degree of removal these treatments provide for airborne contaminants.
The authors acknowledge funding from the Florida Department of Environmental Protection and from the U.S. Environmental Protection Agency under the Science to Achieve Results grant program.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News . ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org .
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom newsroom@acs.org
Related Content

More From This Series

Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

COMMENTS
The Assisted Referral Tool (ART) was developed by the NIH Center for Scientific Review (CSR) to recommend potentially appropriate study section. The information you provide ART is only used to recommend study sections and is not stored or persisted. The recommendations made by ART are solely for the benefit of the user. Assisted Referral Tool.
You can use the Matchmaker function or a text search. 2. Use CSR's Assisted Referral Tool (ART) to match your abstract or specific aims to a study section/scientific review group. 3. Use the Assignment Request Form in your application to: Suggest assignment to an institute that might be interested in funding your application (e.g., NCI, NIAID).
Assisted Referral Tool (ART) Recommend study sections directly. You will be given a list of the best matching of the 190 active SRG panels. Recommend SBIR/STTR Special Emphasis Panels. If you are applying for a SBIR/STTR grant, select this option. Applications are assigned for review based on relevance of that application to the guidelines of ...
NIH encourages you to submit an Assignment Request Form with your application. The form allows you to: Express a preference for a particular scientific review group (or "study section") Express a preference for a specific awarding component (an NIH Institute or Center) Let us know of potential reviewers who you feel might have a conflict of ...
The Assisted Referral Tool (ART) was developed by the NIH Center for Scientific Review (CSR) to recommend potentially appropriate study sections, based on the scientific content of a user's grant application. The information you provide ART is only used to recommend study sections and is not stored or persisted.
The Assisted Referral Tool (ART) was developed by the NIH Center for Scientific Review (CSR) to recommend potentially appropriate study sections using text from your application. Using these recommendations, you then have the opportunity to suggest the study section for the first level of scientific review of your application in the PHS ...
Here's what you can expect, assignment-wise. Within 7 to 10 days after you submit your application: You should find your initial assignment information in the eRA Commons. If a CSR review branch is listed instead of a specific study section, don't worry. CSR will update this information with your study section assignment in the next few days.
Use the optional PHS Assignment Request Form to list expertise needed to review your application, exclude reviewers, and request an institute assignment. For investigator-initiated R01, R21, and R03 applications, we also advise you to request assignment to the most appropriate study section of reviewers. In any case, it's a good idea to ...
This limits flexibility for honoring assignment preference requests. You do not need to make an entry in all six boxes of the "Study Section Assignment Request" section. Assign to Study Section: You may enter up to three preferences for SRGs/SEPs in the boxes in the "Assign to Study Section" row. Use one box per individual SRG/SEP preference ...
For investigator-initiated R01, R21, and R03 applications, NIH encourages applicants to request a study section they feel would be most receptive to the application. Study section assignment requests are made using the PHS Assignment Request Form.You can also note what expertise are needed to review the application and identify any reviewers who should not review the application.
Review Branches. Review activities of the Center for Scientific Review (CSR) are organized into Review Branches (RBs). Each RB represents a cluster of study sections around a general scientific area. Applications generally are assigned first to an RB, and then to a specific study section within that RB for evaluation of scientific merit.
Rationale for assignment suggestions (optional) Enter the rationale (i.e., why you think the assignment is appropriate) for your Awarding Component and Study Section suggestions. Your answer can have a maximum of 1000 characters. List individuals who should not review your application and why (optional)
For example, sometimes a request is made for assignment to a funding institute that does not participate in the funding opportunity announcement under which the application was submitted. In making assignments to review groups, investigator suggestions are considered but within the parameters of the published study section descriptions.
NIH Prevention-Related Study Sections. Research applications are reviewed either by the Center for Scientific Review (CSR) or one of the NIH Institutes or Centers (ICs) specified in the funding opportunity. You may request assignment to a particular study section; however, while NIH staff consider all requests, in some cases the reviewing IC is ...
Assisted Referral Tool: The Assisted Referral Tool (ART) was developed by the NIH Center for Scientific Review (CSR) to recommend potentially appropriate study section. You enter the title and abstract of your submission, it will analyze the text and suggest a study section based on keywords. Assignment Request form:
The ART requests that users enter their application title (optional), abstract, and specific aims. The ART algorithm uses the text provided to find the optimal study section. While the Assignment Request Form isn't a 100% guarantee that the request will be honored, it increases the odds that your application ends up in the optimal study section.
A tool in NIHRePORTER, called "Matchmaker", allows you to paste your abstract/specific aims in a query box and get information about which NIH Institutes/Centers may have funded similar work in the past. NIH Institute/Center requests are not required. However, if you wish to make an assignment request, please use the correct short abbreviation ...
Within 7 to 10 days after you apply, you should find your initial assignment information in the eRA Commons. You can monitor the status of your application, including the study section to which it is assigned, on the Status Tab in eRA Commons. At first, the Status Tab may show your integrated review group instead of your specific study section.
If you don't see your study section assignment within 2 weeks, call DRR at 301-435-0715. Institute. After NIAID receives notice of your application, our Referral and Policy Analysis Branch assigns it to one of our program divisions using our internal referral guidelines. SRP will organize a SEP to conduct the review of your application with ...
PHS Assignment Request Form. For assistance with the information required on this form, please refer to the appropriate application guide on the How to Apply page.. NOTE: The forms in these topics reflect FORMS-H, which must be used for applications with due dates on or after January 25, 2023 (see guide notice NOT-OD-22-195.Also see the annotated form set and summary of changes for Forms-H.)
Any dual assignments are indicated by the additional two-letter code. The review assignment, including the name of the study section/special emphasis panel and the name, address, and telephone number of the scientific review officer (SRO). The SRO is now the primary point of contact for the investigator throughout the peer review process.
5.1 Assignment: The Book Thief - Introduction INSTRUCTIONS: Complete the sections below after reading the Lesson Overview, Introduction, and Vocabulary sections in Canvas. When you respond to the Quick Write prompt, write in complete sentences and include details from the text as needed. Quick Write Memory and punishment are two topics that come up often in The Book Thief.
EDSE 517 Computer Applications for Special Populations Major Assignment 2 - Technology Tools Overview Students will select a broad technology topic to research, describe, and analyze based on the needs of an actual student or developed case study. A list of technology topics (i.e. word prediction) will be provided by the instructor. Students will then select two specific technologies within ...
The study is the first of its kind by DeepMind, Google's AI unit led by Sir Demis Hassabis, and is an attempt to quantify the risks from the use of generative AI tools, which the world's ...
Helpful resources for finding a CSR study section are the study section guidelines, and the Assignment Request Tool. You can find both on our home page: https://public.csr.nih.gov. You also can use the Assignment Request form in your application to tell us the types of expertise needed to appropriately review your grant application.
The West is warming and drying so fast that a crucial drought-monitoring tool can't keep up, study says U.S. Drought Monitor's weekly reports impact disaster relief, government decision-making
CSR offers a number of ways to identify an appropriate study section including searching for a study section or using the CSR Assisted Referral Tool, and you can use NIH RePorter to identify where similar funded proposals were reviewed. Applicants may wish to contact scientific review officers or the DRR (301-435-0715) with specific questions ...
Many municipal landfills "burp" gas from decomposing organic matter rather than letting it build up. And burps from buried waste containing per- and polyfluoroalkyl substances (PFAS) can release these "forever chemicals" into the air, say researchers in ACS' Environmental Science & Technology Letters.Their study reports unexpectedly high levels of airborne PFAS at three landfills and ...