
Chemistry Steps


General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
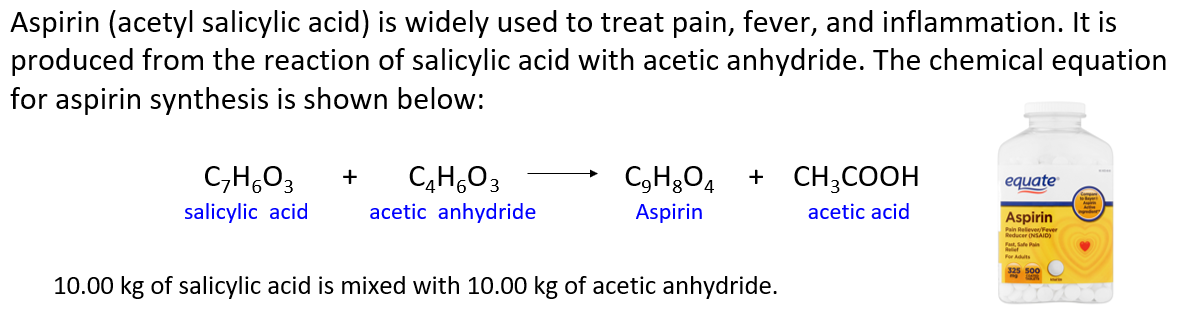
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

What is Stoichiometry? Examples and Practice
- The Albert Team
- Last Updated On: March 17, 2024

Have you ever wondered how chemists know exactly how much of each substance to use in a reaction? The answer lies in a fundamental concept called stoichiometry. This crucial aspect of chemistry helps scientists and students alike understand the quantitative relationships in chemical reactions, ensuring that no atom is wasted. In this post, we’ll delve into what stoichiometry is, explore some engaging stoichiometry examples, and provide you with practical stoichiometry practice problems. Whether you’re just getting started or looking to sharpen your skills, this guide will equip you with the knowledge and tools to tackle stoichiometry problems with confidence.
What We Review
What is Stoichiometry?
Stoichiometry is like the recipe for a cake, but instead of baking, we’re dealing with chemical reactions. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. When chemists conduct experiments, they need to know how much of each reactant to use and what amount of product to expect. Stoichiometry provides these answers, ensuring that chemical reactions are efficient and effective.
Understanding the Basics
To grasp stoichiometry, you first need to be familiar with a few key concepts:
- Moles: Just like a dozen represents 12 items, a mole is a specific number of molecules (approximately 6.022\times10^{23} molecules, known as Avogadro’s number).
- Molar Mass: This is the weight of one mole of a substance, typically expressed in grams per mole (g/mol). It tells you how much one mole of a substance weighs.
- Mole Ratios: These ratios come from the coefficients of a balanced chemical equation. They tell you the proportion of reactants and products in a reaction.
Real-World Applications

Stoichiometry isn’t just a topic in your chemistry textbook; it has real-world applications. Pharmacists use stoichiometry to mix medications, environmental scientists use it to track pollutant levels, and engineers apply it to design reactors. Understanding stoichiometry means you’re learning a skill that scientists and professionals use daily to make the world work.
Stoichiometry Examples
Understanding stoichiometry becomes clearer with practical examples. Let’s explore a couple of scenarios to illustrate how stoichiometry helps us solve chemical equations.
Example 1: Water Formation
Consider the chemical reaction where hydrogen and oxygen combine to form water:
If we start with 36.0 grams of H_2 and have an excess of O_2 , how many grams of H_2O can we produce? The molar mass of H_2 is 2.02 g/mol, and for H_2O , it’s 18.02 g/mol.
First, convert the mass of H_2 to moles:
Applying the mole ratio from the balanced equation, calculate the moles of H_2O produced:
Then, convert moles of H_2O to grams:
Thus, 36.0 grams of H_2 can produce 321.15 grams of H_2O .
Example 2: Ammonia Synthesis
Now, let’s examine another one of our stoichiometry examples. This problem examines the synthesis of ammonia ( NH_3 ) from nitrogen ( N_2 ) and hydrogen ( H_2 ) in a reaction conducted at STP (standard temperature and pressure):
If we begin with 22.4 liters of N_2 (1 mole at STP) and have an excess of H_2 , how many grams of NH_3 can be produced? The molar mass of NH_3 is 17.03 g/mol.
Using the mole ratio from the chemical equation:
Then, convert moles of NH_3 to grams:
This result shows that 22.4 liters of N_2 at STP can produce 34.06 grams of NH_3 .
Guided Stoichiometry Practice
To master stoichiometry, practice is key. In this section, we’ll explore strategies to approach stoichiometry problems and provide some guided practice examples. These strategies will help you develop a systematic approach to solving stoichiometry questions, enhancing your problem-solving skills.
Understanding the Approach
Identify What’s Given and What’s Unknown: Start by determining what information is provided in the problem and what you need to find. This could be the amount of reactants, products, or even the coefficients in a balanced chemical equation.
Write the Balanced Chemical Equation: Ensure you have the correct balanced chemical equation for the reaction you’re analyzing. This step is crucial as it sets the foundation for the stoichiometric calculations.
Use Mole Ratios: Utilize the coefficients from the balanced equation to establish mole ratios. These ratios are the heart of stoichiometry, allowing you to convert between amounts of reactants and products.
Perform the Calculations: With the mole ratios, you can perform calculations to find the unknown quantities. Remember to keep track of your units, ensuring they are consistent throughout the calculation.
Guided Example
Let’s practice with a guided example. Consider the combustion of propane ( C_3H_8 ) in oxygen to produce carbon dioxide and water:
Suppose you start with 44.1 grams of propane ( C_3H_8 ) and have an excess of oxygen ( O_2 ). How many grams of carbon dioxide ( CO_2 ) are produced?
Given: 44.1 grams of C_3H_8 The molar mass of C_3H_8 is 44.1 g/mol, and for CO_2 , it’s 44.0 g/mol.
Unknown: Grams of CO_2 produced
First, convert the mass of C_3H_8 to moles:
Mole Ratio: From the balanced equation, the mole ratio of C_3H_8 to CO_2 is 1:3.
Calculation:
Now, convert the moles of CO_2 to grams:
So, 44.1 grams of C_3H_8 produces 132.0 grams of CO_2 .
Stoichiometry Practice Problems
Now that you’ve seen stoichiometry in action through examples, it’s time to test your knowledge with some practice problems. Try to solve these problems on your own before checking the solutions provided. This will help you understand stoichiometry better and prepare you for similar problems in the future.
When magnesium burns in the air, it reacts with oxygen to form magnesium oxide. The balanced chemical equation for this reaction is:
If you start with 24.0 grams of magnesium ( Mg ) and have an excess of oxygen ( O_2 ), how many grams of magnesium oxide ( MgO ) will be produced? The molar mass of Mg is 24.3 g/mol, and MgO is 40.3 g/mol.
Hydrochloric acid reacts with sodium hydroxide to produce sodium chloride and water in the following reaction:
If 36.5 grams of hydrochloric acid ( HCl ) react with sufficient sodium hydroxide ( NaOH ), how many grams of sodium chloride ( NaCl ) are produced? Assume the molar mass of HCl is 36.5 g/mol and NaCl is 58.4 g/mol.
Nitrogen gas can react with hydrogen gas to form ammonia through the following reaction:
Calculate the amount of ammonia ( NH_3 ) produced (in grams) when 28.0 liters of nitrogen gas ( N_2 ) react with an excess of hydrogen gas ( H_2 ) at STP. The molar mass of NH_3 is [/latex]17.03[/latex] g/mol.
Stoichiometry Practice Problems Solutions
Here, we’ll go through the solutions to the stoichiometry practice problems we presented earlier. These solutions will help you understand the step-by-step process involved in solving stoichiometry problems.
Solution to Problem 1:
For the reaction 2Mg + O_2 \rightarrow 2MgO , we’re starting with 24.0 grams of Mg . First, convert the mass of Mg to moles:
Using the stoichiometry of the reaction, convert moles of Mg to moles of MgO (the ratio is 1:1):
Now, convert moles of MgO to grams:
So, 24.0 grams of Mg will produce 39.81 grams of MgO .
Solution to Problem 2:
For the reaction HCl + NaOH \rightarrow NaCl + H_2O , we start with 36.5 grams of HCl . Convert the mass of HCl to moles:
The mole ratio of HCl to NaCl is 1:1, so:
Now, convert moles of NaCl to grams:
Thus, 36.5 grams of HCl will produce 58.44 grams of NaCl .
Solution to Problem 3:
For the reaction N_2 + 3H_2 \rightarrow 2NH_3 , starting with 28.0 liters of N_2 at STP (which corresponds to 1.00 mole of N_2 ):
Using the stoichiometry of the reaction (1:2 ratio of N_2 to NH_3 ):
Convert moles of NH_3 to grams:
So, 28.0 liters of N_2 will produce 42.575 grams of NH_3 .
Stoichiometry is a cornerstone concept in chemistry that enables us to predict and quantify the outcomes of chemical reactions. By understanding the relationships between reactants and products, chemists can conduct reactions efficiently and effectively. Throughout this post, we’ve explored what stoichiometry is, provided clear examples, and offered practice problems to help you build your skills.
Remember, the key to mastering stoichiometry lies in practice. The more you work through problems, the more intuitive these concepts will become. Whether you’re balancing equations, calculating molar masses, or determining the amounts of reactants and products, each step you take strengthens your understanding of chemistry.
We encourage you to revisit these examples and practice problems regularly, and don’t hesitate to explore more complex scenarios as you become more comfortable with the basics. Stoichiometry is not just a topic for the classroom; it’s a tool that scientists use to make real-world decisions in industries, research, and environmental science.
So, keep practicing, stay curious, and remember that every chemical equation tells a story of transformation and interaction. With stoichiometry, you’re equipped to understand and narrate these fascinating stories of science.
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score

Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Physical Chemistry (Essentials) - Class 11
Course: physical chemistry (essentials) - class 11 > unit 3.
- Stoichiometry article
- Worked example: Calculating amounts of reactants and products
- Ideal stoichiometry
- Limiting reactant and reaction yields
- Worked example: Calculating the amount of product formed from a limiting reactant
- Limiting reagent stoichiometry
Stoichiometry and empirical formulae
Calculating mole fraction
Calculating the number of particles, solving across the equation using stoichiometric coefficients, empirical formulae, transaminase reaction, calculating mass percent, calculating limiting reactant, theoretical yield, handling complex math in answer choices, attribution:, want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page
Resource Topic: Stoichiometry
The mole, molarity, and density, autograded virtual labs, metals density problem, autograded virtual lab.
In this activity, students use the virtual lab to identify 3 unknown metals by measuring their density and comparing their measurements to the densities of known metals. In this randomized version, each student…
Creating a Stock Solution
In this activity, students use the virtual lab to create dilute solutions from a concentrated stock solution of acids or bases. They must first calculate the correct volumes of concentrated acid solution and water…
Scenario-Based Activities
Mixed reception, scenario-based activity.
An in-class activity in which students use molar mass calculations, the scientific method and basic knowledge of chemical reactions to solve a murder mystery. The activity begins with a 5 minute introductory video,…
[ChemVlab+] Powderade: Using Sports Drinks to Explore Concentration and Dilution
In this interactive activity, students first use color to determine which drinks are the most concentrated. Next students use the Virtual Lab to create solutions whose concentration matches that of two characters…
[ChemVlab+] The Factory: Using a City Water System to Explore Dilution
In this interactive activity, students must determine whether the factories in a fictional town are adhering to the guidelines the city has established for their emissions. The challenge is that, for some factories,…
[ChemVlab+] Gravimetric analysis and a closer look at drinking water
In this interactive activity, students first learn how gravimetric analysis can be used to determine the concentration of various species in water, through a combination of particulate-level representations and…
Simulations
Periodic table.
This simulation provides an interactive periodic table that also includes illustration of electron configurations in periodic trends. The schematic diagram to the right of the table plots the electron configurations…
This tutorial introduces the concept of the mole and how it is used in chemistry to connect macroscopic and molecular level scales. Practice is provided on the applied definition of the mole.
Solution Stoichiometry (Molarity)
This tutorial provides a quantitative overview of substances in solution and practice quantifying the amount of a substance in a solution. Guided practice in solution concentration calculations is provided.
Measuring Density
This tutorial explains the definition of density and explains how to perform density measurements. Guided practice in density calculations is also provided.
Calculating Molecular Weight
This tutorial provides instruction and practice on how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
Composition Stoichiometry
This tutorial provides instruction and practice converting between moles of a molecule and the moles of atoms that the molecule is composed of.

Significant Figures
This tutorial provides a brief review of the guides for determining how many significant figures to include when reporting your answer in a chemistry calculation. Guided practice in performing significant figures…
Dimensional Analysis/Stoichiometric Conversions
This tutorial provides a brief overview of dimensional analysis, including conversion between the amount of a substance expressed in "number of molecules" and the amount of a substance expressed in "moles of molecules".
Using Molecular Weight
This tutorial explains use the molecular weight to convert between the macroscopic scale (grams of a substance) and the microscopic scale (number of molecules of that substance). Guided practice performing molecular…
Making a Standard Solution from Another Solution: Dilution
This tutorial begins with the concept of concentration and expands this to the concept of dilution. It then provides and overview of the calculations and procedure for performing a dilution in the laboratory. Guided…
Virtual Labs
Glucose dilution problem, virtual lab.
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together…
Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to…
Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they…
Making Stock Solutions from Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution…
Identifying the Unknown Metal (Metals Density Problem)
In this activity, students use the virtual lab to identify an unknown metal by measuring its density and comparing their measurements to the densities of known metals.
Identifying an Unknown Liquid from its Density
In this activity students use the virtual lab to design an experiment to determine the identity of mislabeled bottles using the densities of the solutions inside.
Alcohol Density Problem
Determine the concentration of an alcohol solution from its density.
Reaction Stoichiometry and Limiting Reagents
Chemical remediation of arsenic.
In this problem, students determine the limiting reagent in a reaction involving the remediation of arsenic from drinking water.
Determining Reactants and Products in a Solution of DNA
In this limiting reagents problem, students are given random volumes and concentrations of DNA solutions and are asked to predict what will remain after a reaction has occurred. Students can check their prediction…
Determine the Concentration of Unknown Silver Nitrate Solution
In this limiting reagents problem, students are asked to determine the amount of silver nitrate dissolved in a solution by performing a reaction with solid NaCl. In this randomized activity, each student is given…
Determining Stoichiometric Coefficients
Students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients. In this randomized activity, each student receives a different reaction and…
Arsenic in Drinking Water
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. (Part of a larger online…
[ChemVlab+] Bioremediation of Oil Spills
Getting bacteria to eat oil is a powerful approach to cleaning up oil spills, and the first step is adding a bioremediation accelerator to form clumps that the bacteria will eat. In this activity, students perform…
Stoichiometry Applet
One of the first numerical problems encountered in introductory chemistry is that of "limiting reagents". This applet serves as a supplement to such calculations, providing imagery that helps students see beyond…
Reaction Stoichiometry
This tutorial introduces the concept of reaction stoichiometry, determining the amount of substance that is consumed or produced by a reaction. The tutorial then explains how to calculate how much of a reactant…
The Stoichiometry of Product Formation and Percent Yield
This tutorial provides on overview in determining the amount of product formed by a reaction. It explains how to perform calculations involving how much product was formed in a chemical reaction and explains theoretical…
Limiting Reagents
This tutorial describes how to determine the amount of each reactant that is consumed and each product that is produced in a given chemical reaction. Guided problems as well as a randomized calculation activity…
Gravimetric Determination of Arsenic
In this activity, students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients.
Stoichiometry and Solution Preparation Problem
In this limiting reagents problem, students mix together solutions in different ratios in an attempt to produce a final solution that contains only 1 product.
Textbook Style Limiting Reagents Problems
Textbook-style practice limiting reagent exercises with that can be used as a way to "predict and check" your answers using the virtual lab.
Textbook Style Limiting Reagents Problem II
In this activity, students practice with experiments involving limiting reagents and the test their knowledge to determine the concentration of an unknown solution.
Predicting DNA Concentration
In this limiting reagents problem, students are given specific concentrations of DNA solutions and are asked to predict what products and reactants will remain after a specific volumes are mixed and reaction has…
Unknown Concentration of DNA Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
Empirical Formula and Mixtures
Mineral composition.
In this randomized calculation activity, students calculate the empirical formula of a compound given its elemental analysis. Step-by-step support and feedback is provided for students who need additional help.
Composition Determination of a Mixture
In this activity, students calculate the percent composition of a mixture of two arsenic-containing minerals. Step-by-step support and feedback is provided for students who need additional help.
Empirical Formula Introduction
This tutorial defines empirical formula and discusses various ways in which it is used.
Determining the Empirical Formula of a Compund from Its Molecular Formula
This tutorial explains how to calculate an empirical formula when given a molecular formula. Guided practice in performing empirical formula calculations from molecular weight is provided.
Determining the Empirical Formula from an Elemental Analysis
This tutorial explains how to obtain a substance’s the empirical formula from an elemental analysis. It discusses how to compare the empirical formula obtained from an elemental analysis with that from a molecular…
Composition of Mixtures
This tutorial explains the advanced topic of using a chemical reaction, such as burning in oxygen, to determine the relative composition of a mixture. Guided practice in performing calculations that involve a mixture…
Gravimetric Analysis
The ChemCollective site and its contents are licensed under a Creative Commons Attribution 3.0 NonCommercial-NoDerivs License.

- Military Families
The official provider of online tutoring and homework help to the Department of Defense.
Check Eligibility

Higher Education
Improve persistence and course completion with 24/7 student support online.
How it Works

Public Libraries
Engage your community with learning and career services for patrons of all ages.

Corporate Partners
Support your workforce and their families with a unique employee benefit.
Get Started
Tutor.com is now part of The Princeton Review ! Learn more
- Testimonials
- Become a Tutor
Science - Chemistry
- Acids and Bases
- Atmospheric Chemistry
- Atom Characterization Experiments
- Atomic Models
- Atomic Orbitals
- Atomic Spectra
- Atomic Structure
- Balancing Chemical Equations
- Biochemistry
- Bond Polarity
- Changes of State
- Chemical Energy
- Chemical Equilibrium
- Chemical Laws
- Classification of Matter
- Colligative Properties
- Complex Ion Equilibria
- Concentration of Solutions
- Dalton's Atomic Theory
- Dimensional Analysis
- Effects on Solubility
- Effusion and Diffusion
- Electrochemistry
- Electrolytes
- Electromagnetic Radiation
- Electron Configuration
- Fission and Fusion
- Free Energy and Work
- Hybridization
- Intermolecular Forces
- Lewis Structures
- Limiting Reactant
- Modeling Kinetics
- Molecular Orbitals
- Molecular Structure
- Naming Covalent Compounds
- Naming Ionic Compounds
- Nature of Energy
- Nature of Matter
- Net Ionic Equations
- Organic Chemistry
- Oxidation-Reduction Reactions
- Percent Abundance
- Percent Composition
- Periodic Table
- Periodic Trends
- Physical vs. Chemical Changes
- Polymerization
- Pressure in Gases
- Quantum Numbers
- Radioactivity
- Scientific Method
- SI Unit Prefixes
- Solubility Equilibria
- Sources of Energy
- Spontaneous Processes
- Standard Enthalpy of Formation
Stoichiometry
- Temperature
- Tools for Measurement
- Types of Chemical Reactions
- Uncertainty in Measurement
- Units of Measurement
- Our Company
- Homework Resources
- Social Studies
- SAT/Test Prep
Proudly Serving
- Colleges & Universities
For more information call us at:
800-411-1970


Chemistry Unit 9: Stoichiometry Homework Pages
These high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of stoichiometry! This unit is meant to cover the basics of stoichiometry, the mole concept, empirical and molecular formulas, percent composition, limiting reactant problems, and percent yield problems.
This unit contains these pages:
1. Introduction to the Mole
2. Calculating Molar Mass from a Chemical Formula
3. Mass to Moles
4. More Mole Calculations
5. Writing Empirical Formulas from Chemical Formulas
6. Percent Composition from Chemical Formula
7. Decipher the Empirical Formula: Two Ways
8. Molecular Formula from the Empirical Formula and Molar Mass
9. Percent Composition, Empirical Formula, and Molecular Formula
10. Stoichiometry: Moles to Moles
11. Stoichiometry Word Problems: Given Moles of Reactants
12. Stoichiometry Word Problems: Given Mass
13. Stoichiometry Word Problems: Given Volumes and Densities
14. Limiting Reactant Problems: Step by Step
15. Limiting Reactant: A Big Word Problem
16. Percent Yield from Actual and Expected Yields
Each page will be unique. Each is designed to roughly cover the material that I would teach in an hour long class period. These are terrific for daily homework assignments because they don’t take too long to complete.
These pages have been carefully designed in Illustrator. I have created a unique set of questions to help students to review material taught in class and think deeper about the material. Many of the pages ask students to highlight or color something, to identify items in a diagram, to match related concepts, or interact with a topic in a new way. Many of the pages ask students to connect more than one concept; they are intended to help students see the bigger picture in each unit. A few pages ask students to use the internet to do a little research.
If you own any of my other resources, don’t worry about repeat pages. These homework pages are truly unique and separate from my activities. These homework pages will truly complement any activities or resources you already have or use in your class.
Homework Page Implementation Suggestions:
* First of all, I don’t grade it. I learned in my early teaching years that when I grade homework, I am rewarding students who copied off of their one studious friend the period before my class, and I am penalizing students who have limited educational time outside of school. I often give time at the end of the period to work on “homework” pages. Often, I start off the next day’s class with the answer key projected onto some sort of screen (ELMO or projector) so that students can check their answers as they walk in. My students know that they will do better in my class if they do the homework and I care about effort more than being correct.
* Answer keys are included (for almost all of the pages, where it makes sense to have an answer key). I designed these pages to be pretty simple to grade, if you want to do that.
* In my time as a teacher, I have noticed that for some reason, homework assignments that have more than one side of a page are just neglected by students. If I hand out a one sided homework page and tell them, here’s your homework, they say, yay, it’s just 1 page! They will often at least start it if not finish it before the end of the day. I really think there is a psychological barrier to starting an assignment with two sides. Call me crazy, but test it out! Try giving my homework assignments and watch your class actually do their homework!
* A way to save paper would be to print all of the homework assignments and copy them as a packet. This is great to give students all at once in the beginning of the unit, so they have every page in advance, which works great if they’re absent!
All files are non-editable PDFs. They are non-editable to protect the images that are copyrighted and purchased through licenses. Thanks for understanding!
(C) Bethany Lau
All Rights Reserved.
- Track Orders
- Shopping Bag
© 2018 Science with Mrs. Lau

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Stoichiometry (Worksheet)
- Last updated
- Save as PDF
- Page ID 90921
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Given the following reaction:
\[H_2SO_4 + NaOH \rightarrow Na_2SO_4 + H_2O\]
If it takes 27.4 mL of 0.768 M \(NaOH\) to titrate 16.7 mL of \(H_2SO_4\), what is the concentration of the \(H_2SO_4\) solution? (hint: balance the equation first)
\[NaOH + HCl \rightarrow H_2O + NaCl\]
If 24.5 mL of \(HCl\) solution is needed to titrate 33.0 mL of a 0.112 M \(NaOH\), what is the concentration of the \(HCl\) solution?
\[Ba(OH)_2 + HClO_4 \rightarrow Ba(ClO_4)_2 + H_2O\]
How many mL of 1.2 M \(HClO_4\) is needed to neutralize 5.8 mL of a 0.44 M \(Ba(OH)_2\) solution?
\[H_2SO_4 + Na_2CO_3 \rightarrow Na_2SO_4 + H_2O + CO_2\]
Calculate the molarity of the \(H_2SO_4\) solution if it takes 40.0 mL of \(H_2SO_4\) to neutralize 46.7 mL of a 0.364 M \(Na_2CO_3\) solution.
Contributors
- Mark Draganjac ( Arkansas State University )

COMMENTS
The stoichiometry of a balanced chemical equation identifies the maximum amount of product that can be obtained. The stoichiometry of a reaction describes the relative amounts of reactants and products in a balanced chemical equation. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant (s).
How many moles of water are produced when 57 moles of nitrogen are made? 3. Calculate the mass of aluminum oxide produced when 3.75 moles of aluminum burn in oxygen. Answers: 1A. 30 mol Ag 1B. 30 mol AgNO3. 1C. 20 mol H2O 1D. 10 mol NO. 2A. 38 mol N2H4 2B. 19 mol N2O4. 2C. 76 mol H2O.
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
Types of chemical reactions. Oxidation-reduction (redox) reactions. Worked example: Using oxidation numbers to identify oxidation and reduction. Balancing redox equations. Dissolution and precipitation. Precipitation reactions. Double replacement reactions. Single replacement reactions. Molecular, complete ionic, and net ionic equations.
At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. When chemists conduct experiments, they need to know how much of each reactant to use and what amount of product to expect. Stoichiometry provides these answers, ensuring that chemical reactions are efficient and ...
Stoichiometry Calculation Practice Worksheet 1. Calculate the number of moles of NaOH that are needed to react with 500.0 g of H 2 SO 4 according to the following equation: H 2 SO 4 + 2 NaOH Na 2 SO 4 + 2 H 2 O ANS: 10.19 mol 2. Calculate the mass of NH 3 that can be produced from the reaction of 125 g of NCl 3 according to the following equation:
Get help with your Stoichiometry homework. Access the answers to hundreds of Stoichiometry questions that are explained in a way that's easy for you to understand. Can't find the question you're looking for? Go ahead and submit it to our experts to be answered.
The goal is always to convert into moles, which is the language of stoichiometry. First, we should figure out the molar mass of ammonium phosphate trihydrate: H 1 8 N 3 O 7 P: ( 18 × 1) + ( 14 × 3) + ( 16 × 7) + 31 = 18 + 42 + 112 + 31 = 203 g / m o l. Next, we should figure out the moles of ammonium phosphate trihydrate we have on hand:
Chemistry: Stoichiometry - Problem Sheet 2. Directions: Solve each of the following problems. Show your work, including proper units, to earn full credit. ___ CaCl2 + ___ AgNO3. ___ Ca(NO3)2 + ___ AgCl. How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate? g AgCl 45 g CaCl 2.
Stoichiometry Homework ProblemSet. Stoichiometry Homework Problem Set. This problem set was developed by S.E. Van Bramer for Chemistry 145 at Widener University. Balance the following equations. Give the names of the reactants and the products. Given 10.0 grams of each reactant.
Flowchart of steps in stoichiometric calculations. Step 1: grams of A is converted to moles by multiplying by the inverse of the molar mass. Step 2: moles of A is converted to moles of B by multiplying by the molar ratio. Step 3: moles of B is converted to grams of B by the molar mass.
In this video we will learn how to calculate the amounts of products that will be created or the amount of reactants that will be required in a chemical reac...
Resource Topic: Stoichiometry The Mole, Molarity, and Density. Autograded Virtual Labs; Metals Density Problem Autograded Virtual Lab. In this activity, students use the virtual lab to identify 3 unknown metals by measuring their density and comparing their measurements to the densities of known metals.
Stoichiometry Problems You may work these problems individually, or with one partner. If you work with a partner, both people will get the same score on the assignment. 1. The reaction of calcium hydride with water can be used to prepare small quantities of hydrogen gas, as is done to fill weather-observation balloons.
www.njctl.org Chemistry Stoichiometry Homework Set 4: 1. No one likes cockroaches. Since the 1970's exterminators have used a chemical called diazinon, among others, to kill the insects. It is toxic to humans also and has been banned in the US since 2004 unless it is being used in agriculture. It is made from PCl 3 which is in turn ...
Q4. Given the following reaction: H2SO4 + Na2CO3 → Na2SO4 +H2O + CO2 H 2 S O 4 + N a 2 C O 3 → N a 2 S O 4 + H 2 O + C O 2. Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40.0 mL of H2SO4 H 2 S O 4 to neutralize 46.7 mL of a 0.364 M Na2CO3 N a 2 C O 3 solution.
Stoichiometry Homework. 1) How many grams of lithium sulfate will be formed when 25 grams of sulfuric acid react with an excess of lithium hydroxide in the following reaction? ____ H2SO4+ ____ LiOH ðà ____ Li2SO4+ ____ H2O 2) How many grams of sodium acetate are required to react completely with 30 grams of iron (III) n itrate in the ...
Stoichiometry. Stoichiometry is hard to spell and even harder to do. But with our free stoichiometry videos, worksheets, and reference materials, it's easy to review what you've learned in class and lock in the skills you'll need for more advanced chemistry topics.
PROBLEM 5.2.1.1. Write the balanced equation and determine the information requested. Don't worry about state symbols in these reactions. The number of moles and the mass (in grams) of chlorine, Cl 2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl. The number of moles and the mass (in milligrams) of diatomic ...
These high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of stoichiometry! This unit is meant to cover the basics of stoichiometry, the mole concept, empirical and molecular formulas, percent composition, limiting reactant problems, and percent yield problems. This unit contains these pages:
Download Full Book (PDF) Resources expand_more. Periodic Table. Physics Constants. Scientific Calculator. Reference expand_more. Reference & Cite. Tools expand_more. Help expand_more.