- Insights blog

How to publish your research
A step-by-step guide to getting published.
Publishing your research is an important step in your academic career. While there isn’t a one-size-fits-all approach, this guide is designed to take you through the typical steps in publishing a research paper.
Discover how to get your paper published, from choosing the right journal and understanding what a peer reviewed article is, to responding to reviewers and navigating the production process.
Step 1: Choosing a journal

Choosing which journal to publish your research paper in is one of the most significant decisions you have to make as a researcher. Where you decide to submit your work can make a big difference to the reach and impact your research has.
It’s important to take your time to consider your options carefully and analyze each aspect of journal submission – from shortlisting titles to your preferred method of publication, for example open access .
Don’t forget to think about publishing options beyond the traditional journals format – for example, open research platform F1000Research , which offers rapid, open publication for a wide range of outputs.
Why choose your target journal before you start writing?
The first step in publishing a research paper should always be selecting the journal you want to publish in. Choosing your target journal before you start writing means you can tailor your work to build on research that’s already been published in that journal. This can help editors to see how a paper adds to the ‘conversation’ in their journal.
In addition, many journals only accept specific manuscript formats of article. So, by choosing a journal before you start, you can write your article to their specifications and audience, and ultimately improve your chances of acceptance.
To save time and for peace of mind, you can consider using manuscript formatting experts while you focus on your research.

How to select the journal to publish your research in
Choosing which journal to publish your research in can seem like an overwhelming task. So, for all the details of how to navigate this important step in publishing your research paper, take a look at our choosing a journal guide . This will take you through the selection process, from understanding the aims and scope of the journals you’re interested in to making sure you choose a trustworthy journal.
Don’t forget to explore our Journal Suggester to see which Taylor & Francis journals could be right for your research.
Go to guidance on choosing a journal
Step 2: Writing your paper
Writing an effective, compelling research paper is vital to getting your research published. But if you’re new to putting together academic papers, it can feel daunting to start from scratch.
The good news is that if you’ve chosen the journal you want to publish in, you’ll have lots of examples already published in that journal to base your own paper on. We’ve gathered advice on every aspect of writing your paper, to make sure you get off to a great start.
How to write your paper
How you write your paper will depend on your chosen journal, your subject area, and the type of paper you’re writing. Everything from the style and structure you choose to the audience you should have in mind while writing will differ, so it’s important to think about these things before you get stuck in.
Our writing your paper guidance will take you through everything you need to know to put together your research article and prepare it for submission. This includes getting to know your target journal, understanding your audiences, and how to choose appropriate keywords.
You can also use this guide to take you through your research publication journey .

You should also make sure you’re aware of all the Editorial Policies for the journal you plan to submit to. Don’t forget that you can contact our editing services to help you refine your manuscript.
Discover advice and guidance for writing your paper
Step 3: Making your submission
Once you’ve chosen the right journal and written your manuscript, the next step in publishing your research paper is to make your submission .
Each journal will have specific submission requirements, so make sure you visit Taylor & Francis Online and carefully check through the instructions for authors for your chosen journal.
How to submit your manuscript
To submit your manuscript you’ll need to ensure that you’ve gone through all the steps in our making your submission guide. This includes thoroughly understanding your chosen journal’s instructions for authors, writing an effective cover letter, navigating the journal’s submission system, and making sure your research data is prepared as required.
You can also improve your submission experience with our guide to avoid obstacles and complete a seamless submission.

To make sure you’ve covered everything before you hit ‘submit’ you can also take a look at our ‘ready to submit’ checklist (don’t forget, you should only submit to one journal at a time).
Understand the process of making your submission
Step 4: Navigating the peer review process
Now you’ve submitted your manuscript, you need to get to grips with one of the most important parts of publishing your research paper – the peer review process .
What is peer review?
Peer review is the independent assessment of your research article by independent experts in your field. Reviewers, also sometimes called ‘referees’, are asked to judge the validity, significance, and originality of your work.
This process ensures that a peer-reviewed article has been through a rigorous process to make sure the methodology is sound, the work can be replicated, and it fits with the aims and scope of the journal that is considering it for publication. It acts as an important form of quality control for research papers.

Peer review is also a very useful source of feedback, helping you to improve your paper before it’s published. It is intended to be a collaborative process, where authors engage in a dialogue with their peers and receive constructive feedback and support to advance their work.
Almost all research articles go through peer review, although in some cases the journal may operate post-publication peer review, which means that reviews and reader comments are invited after the paper is published.
If you’ll like to feel more confident before getting your work peer reviewed by the journal, you may want to consider using an in-depth technical review service from experts.
Understanding peer review
Peer review can be a complex process to get your head around. That’s why we’ve put together a comprehensive guide to understanding peer review . This explains everything from the many different types of peer review to the step-by-step peer review process and how to revise your manuscript. It also has helpful advice on what to do if your manuscript is rejected.
Visit our peer review guide for authors
Step 5: The production process
If your paper is accepted for publication, it will then head into production . At this stage of the process, the paper will be prepared for publishing in your chosen journal.
A lot of the work to produce the final version of your paper will be done by the journal production team, but your input will be required at various stages of the process.
What do you need to do during production?
During production, you’ll have a variety of tasks to complete and decisions to make. For example, you’ll need to check and correct proofs of your article and consider whether or not you want to produce a video abstract to accompany it.
Take a look at our guide to the production process to find out what you’ll need to do in this final step to getting your research published.
Your research is published – now what?
You’ve successfully navigated publishing a research paper – congratulations! But the process doesn’t stop there. Now your research is published in a journal for the world to see, you’ll need to know how to access your article and make sure it has an impact .
Here’s a quick tip on how to boost your research impact by investing in making your accomplishments stand out.
Below you’ll find helpful tips and post-publication support. From how to communicate about your research to how to request corrections or translations.
How to access your published article
When you publish with Taylor & Francis, you’ll have access to a new section on Taylor & Francis Online called Authored Works . This will give you and all other named authors perpetual access to your article, regardless of whether or not you have a subscription to the journal you have published in.
You can also order print copies of your article .
How to make sure your research has an impact
Taking the time to make sure your research has an impact can help drive your career progression, build your networks, and secure funding for new research. So, it’s worth investing in.
Creating a real impact with your work can be a challenging and time-consuming task, which can feel difficult to fit into an already demanding academic career.
To help you understand what impact means for you and your work, take a look at our guide to research impact . It covers why impact is important, the different types of impact you can have, how to achieve impact – including tips on communicating with a variety of audiences – and how to measure your success.

Keeping track of your article’s progress
Through your Authored Works access , you’ll be able to get real-time insights about your article, such as views, downloads and citation numbers.
In addition, when you publish an article with us, you’ll be offered the option to sign up for email updates. These emails will be sent to you three, six and twelve months after your article is published to let you know how many views and citations the article has had.
Corrections and translations of published articles
Sometimes after an article has been published it may be necessary to make a change to the Version of Record . Take a look at our dedicated guide to corrections, expressions of concern, retractions and removals to find out more.
You may also be interested in translating your article into another language. If that’s the case, take a look at our information on article translations .
Go to your guide on moving through production
Explore related posts
Insights topic: Get published
Use a trusted editing service to help you get published
5 practical tips for writing an academic article

5 ways to avoid the wrong journal and find the right one

Publishing in a scholarly journal: Part one, the publishing process
As a psychology student or early career psychologist, you might be thinking about publishing your first paper in a scholarly journal. There are several important steps and points to consider as you embark on your publishing journey. Not sure where to start? We’ve got you covered!
Recognizing that not all young academics get all of their questions about publication answered in their respective training programs, we crowdsourced from trainees and early career psychologists using an anonymous Twitter poll and direct solicitation from various students and colleagues known to the authors, this three-part article series includes frequently asked questions about the publication process with answers from the Editor-in-Chief of Experimental and Clinical Psychopharmacology ( ECP ), William Stoops, the Associate Editor of ECP , Raina Pang, and a past ECP Editorial Fellow, Daniel Bradford. Part one focuses on crucial publishing insights for future authors; part two examines the role of the editorial board; and part three sheds light on peer review.
Choosing a journal
How does one choose a journal in which to publish and what factors (impact factor, journal content) should be considered?
In general, the most important factor to consider when choosing where to submit your article is the fit of the manuscript to the scope and profile of the journal; Aside from the quality of the science and writing, this is the largest factor that will determine whether a manuscript is accepted to a journal. To determine fit, one should examine the journal description, usually found on the journal website.
Additionally, it is helpful to browse the journal to see whether it has published articles on the same topic and with similar methods to the manuscript you are submitting.
In addition to the above, you may also consider online search engines, which can help generate a list of journals that may be appropriate for the manuscript being submitted:
- JournalFinder
- Springer Nature: Journal suggester
- Enago’s Open Access Journal Finder
- Journal/Author Name Estimator
Can you submit a paper to multiple journals at once?
No. Submitting a paper to multiple journals at once contravenes publishing guidelines and presents serious ethical concerns.
Is there a uniform format that I should submit my manuscript in?
Make sure to carefully read the manuscript submission instructions available on every journal’s webpage. Although there are certain rules that most journals follow (e.g. formatting in APA Style), each journal provides specific guidelines about certain aspects, for example the information that must be included within the manuscript.
What’s a predatory journal?
A predatory journal is a counterfeit publication that imitates that of a legitimate, respected publisher. Predatory publishers use various techniques to trick scholars into submitting their article for publication. A predatory publisher will usually solicit articles via email, emphasizing a publishing fee and touting a quick turnaround that often omits peer review.
Although the publishing fee is a red flag when it comes to identifying a predatory journal, not all journals that charge a publishing fee are predatory (see next question for more information). For tips on how to identify a predatory journal, see the following resources:
- Scholars beware
- How to avoid predatory publishers
Publishing fees
Does it usually cost money to publish?
It’s important to note that many journals do not charge the author(s) or their institution to publish an article. There are exceptions, however.
Some journals may charge a fee for publishing the article in a particular format. For example, some authors prefer or require their figures to be printed in color. Because printing in color costs more to the publisher, some journals may require a fee for each figure to be printed in color. Other journals may print one color figure for free, but charge for every additional color figure.
An increasing number of journals are also adding open access options which, when chosen, require fees paid by the author or their institution. Further, some reputable journals have recently gone entirely open access and thus require a fee to publish (the fee varies by journal). Open access journals are free to read for all and do not receive revenue from journal subscriptions—therefore, in many cases, an article publishing fee is charged to offset the cost of publishing (e.g., peer review management, production costs).
For example, APA’s open access journal Technology, Mind, and Behavior charges a $1,200 article processing charge (APC), however an author may apply for an APC waiver if they are unable to pay via grant, institutional funding, or by other means outlined on the journal website.
As such, it is important to recognize that journals charging a fee are not necessarily “predatory”—it’s crucial to consider other factors to figure out the legitimacy of the publication.
What is the difference between an open access journal and the open science movement?
Open access is a publishing model in which the author pays a fee to publish; the reader is able to access the article for free. Some journals are entirely open access, while others are “hybrid”—providing both a subscription as well as an open access publishing option.
Open science , on the other hand, is a movement towards increased transparency in publishing. It goes beyond open access, offering guidelines on the type of information that authors should include in their manuscript: for example, APA Style JARS provide guidelines for the details that authors should include in their methods section. Open science initiatives include data sharing, preregistration, preprints, registered reports, and more. The goals of open science initiatives are to increase openness and collaboration, and to improve reproducibility of science and research discovery.
Licensing and copyright
How does licensing and copyright work?
Authors usually own the copyright of their original work and are free to share, without limitation, any version of their articles prior to the final text (after the journal proofing / copy editing process). However, licensing of article versions and individual publisher stances on sharing of accepted articles vary and change frequently. Fortunately, there are many resources to help authors keep track of individual policies. For example, the Sherpa Romeo website includes a conveniently searchable tool of journals’ copyright and open access policies on a journal-by-journal basis.
Publishing tips
Expert insights on key topics and best practices in publishing.
Read insights
- APA Journals ™ Publishing Resource Center
- Author resource center
- Reviewer Resource Center
- Editor resource center
- APA and Affiliated Journals
APA Publishing Insider
APA Publishing Insider is a free monthly newsletter with tips on APA Style, open science initiatives, active calls for papers, research summaries, and more.
Contact Journals
Unfortunately we don't fully support your browser. If you have the option to, please upgrade to a newer version or use Mozilla Firefox , Microsoft Edge , Google Chrome , or Safari 14 or newer. If you are unable to, and need support, please send us your feedback .
We'd appreciate your feedback. Tell us what you think! opens in new tab/window
7 steps to publishing in a scientific journal
April 5, 2021 | 10 min read
By Aijaz Shaikh, PhD
Before you hit “submit,” here’s a checklist (and pitfalls to avoid)
As scholars, we strive to do high-quality research that will advance science. We come up with what we believe are unique hypotheses, base our work on robust data and use an appropriate research methodology. As we write up our findings, we aim to provide theoretical insight, and share theoretical and practical implications about our work. Then we submit our manuscript for publication in a peer-reviewed journal. For many, this is the hardest part of research. In my seven years of research and teaching, I have observed several shortcomings in the manuscript preparation and submission process that often lead to research being rejected for publication. Being aware of these shortcomings will increase your chances of having your manuscript published and also boost your research profile and career progression.

Dr Aijaz Shaikh gives a presentation.
In this article, intended for doctoral students and other young scholars, I identify common pitfalls and offer helpful solutions to prepare more impactful papers. While there are several types of research articles, such as short communications, review papers and so forth, these guidelines focus on preparing a full article (including a literature review), whether based on qualitative or quantitative methodology, from the perspective of the management, education, information sciences and social sciences disciplines.
Writing for academic journals is a highly competitive activity, and it’s important to understand that there could be several reasons behind a rejection. Furthermore, the journal peer-review process is an essential element of publication because no writer could identify and address all potential issues with a manuscript.
1. Do not rush submitting your article for publication.
In my first article for Elsevier Connect – “Five secrets to surviving (and thriving in) a PhD program” – I emphasized that scholars should start writing during the early stages of your research or doctoral study career. This secret does not entail submitting your manuscript for publication the moment you have crafted its conclusion. Authors sometimes rely on the fact that they will always have an opportunity to address their work’s shortcomings after the feedback received from the journal editor and reviewers has identified them.
A proactive approach and attitude will reduce the chance of rejection and disappointment. In my opinion, a logical flow of activities dominates every research activity and should be followed for preparing a manuscript as well. Such activities include carefully re-reading your manuscript at different times and perhaps at different places. Re-reading is essential in the research field and helps identify the most common problems and shortcomings in the manuscript, which might otherwise be overlooked. Second, I find it very helpful to share my manuscripts with my colleagues and other researchers in my network and to request their feedback. In doing so, I highlight any sections of the manuscript that I would like reviewers to be absolutely clear on.
2. Select an appropriate publication outlet.
I also ask colleagues about the most appropriate journal to submit my manuscript to; finding the right journal for your article can dramatically improve the chances of acceptance and ensure it reaches your target audience.
Elsevier provides an innovative Journal Finder opens in new tab/window search facility on its website. Authors enter the article title, a brief abstract and the field of research to get a list of the most appropriate journals for their article. For a full discussion of how to select an appropriate journal see Knight and Steinbach (2008).
Less experienced scholars sometimes choose to submit their research work to two or more journals at the same time. Research ethics and policies of all scholarly journals suggest that authors should submit a manuscript to only one journal at a time. Doing otherwise can cause embarrassment and lead to copyright problems for the author, the university employer and the journals involved.
3. Read the aims and scope and author guidelines of your target journal carefully.
Once you have read and re-read your manuscript carefully several times, received feedback from your colleagues, and identified a target journal, the next important step is to read the aims and scope of the journals in your target research area. Doing so will improve the chances of having your manuscript accepted for publishing. Another important step is to download and absorb the author guidelines and ensure your manuscript conforms to them. Some publishers report that one paper in five does not follow the style and format requirements of the target journal, which might specify requirements for figures, tables and references.
Rejection can come at different times and in different formats. For instance, if your research objective is not in line with the aims and scope of the target journal, or if your manuscript is not structured and formatted according to the target journal layout, or if your manuscript does not have a reasonable chance of being able to satisfy the target journal’s publishing expectations, the manuscript can receive a desk rejection from the editor without being sent out for peer review. Desk rejections can be disheartening for authors, making them feel they have wasted valuable time and might even cause them to lose enthusiasm for their research topic. Sun and Linton (2014), Hierons (2016) and Craig (2010) offer useful discussions on the subject of “desk rejections.”
4. Make a good first impression with your title and abstract.
The title and abstract are incredibly important components of a manuscript as they are the first elements a journal editor sees. I have been fortunate to receive advice from editors and reviewers on my submissions, and feedback from many colleagues at academic conferences, and this is what I’ve learned:
The title should summarize the main theme of the article and reflect your contribution to the theory.
The abstract should be crafted carefully and encompass the aim and scope of the study; the key problem to be addressed and theory; the method used; the data set; key findings; limitations; and implications for theory and practice.
Dr. Angel Borja goes into detail about these components in “ 11 steps to structuring a science paper editors will take seriously .”
Learn more in Elsevier's free Researcher Academy opens in new tab/window
5. Have a professional editing firm copy-edit (not just proofread) your manuscript, including the main text, list of references, tables and figures.
The key characteristic of scientific writing is clarity. Before submitting a manuscript for publication, it is highly advisable to have a professional editing firm copy-edit your manuscript. An article submitted to a peer-reviewed journal will be scrutinized critically by the editorial board before it is selected for peer review. According to a statistic shared by Elsevier, between 30 percent and 50 percent of articles submitted to Elsevier journals are rejected before they even reach the peer-review stage, and one of the top reasons for rejection is poor language. A properly written, edited and presented text will be error free and understandable and will project a professional image that will help ensure your work is taken seriously in the world of publishing. On occasion, the major revisions conducted at the request of a reviewer will necessitate another round of editing. Authors can facilitate the editing of their manuscripts by taking precautions at their end. These include proofreading their own manuscript for accuracy and wordiness (avoid unnecessary or normative descriptions like “it should be noted here” and “the authors believe) and sending it for editing only when it is complete in all respects and ready for publishing. Professional editing companies charge hefty fees, and it is simply not financially viable to have them conduct multiple rounds of editing on your article. Applications like the spelling and grammar checker in Microsoft Word or Grammarly are certainly worth applying to your article, but the benefits of proper editing are undeniable. For more on the difference between proofreading and editing, see the description in Elsevier’s WebShop.
6. Submit a cover letter with the manuscript.
Never underestimate the importance of a cover letter addressed to the editor or editor-in-chief of the target journal. Last year, I attended a conference in Boston. A “meet the editors” session revealed that many submissions do not include a covering letter, but the editors-in-chief present, who represented renewed and ISI-indexed Elsevier journals, argued that the cover letter gives authors an important opportunity to convince them that their research work is worth reviewing.
Accordingly, the content of the cover letter is also worth spending time on. Some inexperienced scholars paste the article’s abstract into their letter thinking it will be sufficient to make the case for publication; it is a practice best avoided. A good cover letter first outlines the main theme of the paper; second, argues the novelty of the paper; and third, justifies the relevance of the manuscript to the target journal. I would suggest limiting the cover letter to half a page. More importantly, peers and colleagues who read the article and provided feedback before the manuscript’s submission should be acknowledged in the cover letter.
7. Address reviewer comments very carefully.
Editors and editors-in-chief usually couch the acceptance of a manuscript as subject to a “revise and resubmit” based on the recommendations provided by the reviewer or reviewers. These revisions may necessitate either major or minor changes in the manuscript. Inexperienced scholars should understand a few key aspects of the revision process. First, it important to address the revisions diligently; second, is imperative to address all the comments received from the reviewers and avoid oversights; third, the resubmission of the revised manuscript must happen by the deadline provided by the journal; fourth, the revision process might comprise multiple rounds. The revision process requires two major documents. The first is the revised manuscript highlighting all the modifications made following the recommendations received from the reviewers. The second is a letter listing the authors’ responses illustrating they have addressed all the concerns of the reviewers and editors. These two documents should be drafted carefully. The authors of the manuscript can agree or disagree with the comments of the reviewers (typically agreement is encouraged) and are not always obliged to implement their recommendations, but they should in all cases provide a well-argued justification for their course of action.
Given the ever increasing number of manuscripts submitted for publication, the process of preparing a manuscript well enough to have it accepted by a journal can be daunting. High-impact journals accept less than 10 percent of the articles submitted to them, although the acceptance ratio for special issues or special topics sections is normally over 40 percent. Scholars might have to resign themselves to having their articles rejected and then reworking them to submit them to a different journal before the manuscript is accepted.
The advice offered here is not exhaustive but it’s also not difficult to implement. These recommendations require proper attention, planning and careful implementation; however, following this advice could help doctoral students and other scholars improve the likelihood of getting their work published, and that is key to having a productive, exciting and rewarding academic career.
Acknowledgements
I would like to thank Professor Heikki Karjaluoto, Jyväskylä University School of Business and Economics for providing valuable feedback on this article.
Sun, H., & Linton, J. D. (2014).
Structuring papers for success: Making your paper more like a high impact publication than a desk reject opens in new tab/window
Technovation.
Craig, J. B. (2010).
Desk rejection: How to avoid being hit by a returning boomerang opens in new tab/window
Family Business Review
Hierons, R. M. (2016).
The dreaded desk reject opens in new tab/window
, Software Testing, Verification and Reliability .
Borja, A (2014):
11 steps to structuring a science paper editors will take seriously
Elsevier Connect
Knight, L. V., & Steinbach, T. A. (2008).
Selecting an appropriate publication outlet: a comprehensive model of journal selection criteria for researchers in a broad range of academic disciplines opens in new tab/window
, International Journal of Doctoral Studies .
Tewin, K. (2015).
How to Better Proofread An Article in 6 Simple Steps opens in new tab/window ,
Day, R, & Gastel, B: How to write and publish a scientific paper. Cambridge University Press (2012)
Contributor
Aijaz shaikh, phd.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Hum Reprod Sci
- v.10(1); Jan-Mar 2017
Preparing and Publishing a Scientific Manuscript
Padma r. jirge.
Department of Reproductive Medicine, Sushrut Assisted Conception Clinic Shreyas Hospital, Kolhapur, Maharashtra, India
Publishing original research in a peer-reviewed and indexed journal is an important milestone for a scientist or a clinician. It is an important parameter to assess academic achievements. However, technical and language barriers may prevent many enthusiasts from ever publishing. This review highlights the important preparatory steps for creating a good manuscript and the most widely used IMRaD (Introduction, Materials and Methods, Results, and Discussion) method for writing a good manuscript. It also provides a brief overview of the submission and review process of a manuscript for publishing in a biomedical journal.
B ACKGROUND
T he publication of original research in a peer-reviewed and indexed journal is the ultimate and most important step toward the recognition of any scientific work. However, the process starts long before the write-up of a manuscript. The journal in which the author wishes to publish his/her work should be chosen at the time of conceptualization of the scientific work based on the expected readership.
The journals do provide information on the “scope of the journal,” which specifies the scientific areas relevant for publication in the journal, and “instructions to authors,” which need to be adhered to while preparing a manuscript.
The publication of scientific work has become mandatory for scientists or specialists holding academic affiliations, and it is now desirable even at an undergraduate level. Despite a plethora of forums for presenting the original research work, very little of it ever gets published in a scientific journal, and even if it does, the manuscripts are usually from the same few institutions.[ 1 , 2 ] It serves the purpose of academic recognition; and certain publications may even contribute to shaping various national policies. An academic appointment, suitable infrastructure, and access to peer-reviewed journals are considered as the facilitators for publishing.[ 3 ]
The lack of technical and writing skills, institutional hurdles, and time constraints are considered as the major hurdles for any scientific publication.[ 3 ] In addition, the majority of clinicians in India are involved in providing healthcare in the private sector in individually owned hospitals or those governed by small groups of doctors. This necessitates performing a multitude of tasks apart from providing core clinical care and, hence, poses an additional limiting factor because of the long and irregular working hours.
It is extremely challenging to dedicate some time for research and writing in such a scenario. However, it is a loss to science if this group of skilled clinicians does not contribute to medical literature.
Maintaining the ethics and science of research and understanding the norms of preparing a manuscript are very important in improving the quality and relevance of clinical research in our country. This article brings together various aspects to be borne in mind while creating a manuscript suitable for publication. The inputs provided are relevant to all those interested, irrespective of whether they have an academic or institutional affiliation. While the prospect of becoming an author of a published scientific work is exciting, it is important to be prepared for minor or major revisions in the original article and even rejection. However, persevering in this endeavor may help preserving one’s work and contribute to the promotion of science.[ 4 , 5 ]
Important considerations for writing a manuscript include the following:
- (1) Conceptualization of a clinically relevant scientific work.
- (2) Choosing an appropriate journal and an alternative one.
- (3) Familiarizing with instructions to authors.
- (4) Coordination and well-defined task delegation within the team and involvement of a biostatistician from the conception of the study.
- (5) Preparing a skeletal framework for writing the manuscript.
- (6) Delegating time for thinking and writing at regular intervals.
S TEPS I NVOLVED IN M ANUSCRIPT P REPARATION
A manuscript should both be informative and readable. Even though the concept is clear in the authors’ mind, it is important to remember that they are introducing some new work for the readers, and, hence, appropriate organization of the manuscript is necessary to make the purpose and importance of the work clear to the readers.
- (1) Choosing the appropriate journal for publication : The preferred choice of journal should be one of the first steps to be considered, as mentioned earlier. The guidelines for authors may change with time and, hence, should be referred to at regular intervals and conformed to. The choice of journal principally depends on the target readers, and it may be necessary to have one or more journals in mind in case of nonacceptance from the journal of first choice. A journal’s impact factor is to be considered while choosing an appropriate journal.
Majority of the biomedical journals with good impact factor have specific authorship criteria.[ 8 ] This prevents problems related to ghost authorship and honorary authorship. Ghost authorship refers to a scenario wherein an author’s name is omitted to hide financial relationships with private companies; honorary authorship is naming someone who has not made substantial contribution to the work, either due to pressure from colleagues or to improve the chances of publication.[ 9 ]
Most of the journals conform to the authorship criteria defined by the International Committee of Medical Journal Editors.[ 10 ] They are listed as the following:
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; ANDDrafting the work or revising it critically for important intellectual content; ANDFinal approval of the version to be published; ANDAgreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Some journals require authors to declare their contributions to the research work and manuscript preparation. This helps to prevent honorary and ghost authorship and encourages authors to be more honest and accountable.[ 11 ]
Keywords : are mentioned at the bottom of the Abstract section. These words denote the important aspects of the manuscript and help identify the manuscripts by electronic search engines. Most of the journals specify the number of keywords required, usually between 4 and 8. They need to be simple and specific to the manuscript; a good title contains majority of the keywords.
The general flow of the manuscript follows an IMRaD (Introduction, Materials and Methods, Results, and Discussion) structure. Even though this has been recommended since the early 20 th century, most of the authors started following it since the 1970s.[ 13 ]
Important components of the Introduction section

A common error while writing an introduction is an attempt to review the entire evidence available on the topic. This becomes confusing to the reader, and the purpose and importance of the study in question gets submerged in the plethora of information provided. Issues mentioned in the Introduction section will need to be addressed in the Discussion section, and it is important to avoid repetitions and overlapping. Some may prefer to write the Introduction section after preparing the draft of the Materials and Methods and Results sections.
The last paragraph in the Introduction section defines the aim of the study or the study question using active verbs. If there is more than one aim for the study, specify the primary aim and address the secondary aims in a separate sentence. It is recommended that the Introduction section should not occupy more than 10–15% of the entire text.[ 14 ]
This is followed by a detailed description of the study protocol. At times, some of the methods used may be very elaborate and not very relevant to majority of the readers, for example, if polymerase chain reaction (PCR) is used for diagnosis, the type of PCR performed should be mentioned in this section, but the entire procedure need not be elaborated in the “methods” section. Either a relevant reference can be provided or the procedural details can be given online as supplemental data.
It is important to mention both the generic and brand names of all the drugs used along with the name of the manufacturer and the place of manufacturing. Similarly, all the hematological, biochemical, hormonal assays, and radiological investigations performed should provide the specifications of the equipment used and its manufacturer’s details. For many biochemical and endocrine parameters, it is preferred that the intra- and interassay coefficients of variation are provided. In addition, the standard units of measurements and the internationally accepted abbreviations should be used.[ 18 ]
There are online guidelines available to maintain uniformity in reporting the different types of studies such as Consolidated Standards of Reporting Trials (CONSORT) for randomized controlled trials, Strengthening the Reporting of Observational studies in Epidemiology (STROBE) for observational studies, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for systematic reviews.[ 19 ] Adherence to these guidelines improves the clarity and completeness of reporting.
Statistical analysis : One of the most important deterrents for publishing clinical research is the inability to choose and perform appropriate statistical analysis. With the availability of various user-friendly software systems, an increasing number of the researchers are comfortable performing complex analyses without additional assistance. However, it is still a common practice to involve biostatisticians for this purpose. Coordination between the clinicians and biostatisticians is very important for sample size calculation, creation of a proper data set, and its subsequent analysis. It is important to use the appropriate statistical methodologies for a more complete representation of the data to improve the quality of a manuscript.[ 20 ] It may be helpful to refer to a recent review of the most widely used statistical analyses and their application in clinical research for a better data presentation.[ 20 ] There is some evidence that structured training involving data analysis, manuscript writing, and submission to indexed journals improves the quality of submitted manuscripts even in a low-resource setting.[ 21 ] Short, online certificate courses on biostatistics are available free of cost from many universities across the globe. The important aspects regarding the Materials and Methods section are summarized in Table 2 .
Important components of the Materials and Methods section

The results of the study are summarized in the form of tables and figures. Journals may have limitations on the number of figures and tables, as well as the rows and columns in tables. The text should only highlight the findings recorded in the tables and figures and should not repeat every detail.[ 16 ] Primary analysis should be presented in a separate paragraph. Any secondary analysis performed in view of the results seen in the primary analysis should be mentioned separately [ Table 3 ].
Important components of the Results section

When comparing two groups, it is a good practice to mention the data pertaining to the study group followed by that of the control group and to maintain the same order throughout the section. No adjectives should be used while comparing, except for the statistical significance of the findings. The Results section is written in the past tense, and the numerical values should be presented with a maximum of one decimal place.
Statistical significance as shown by P-value, if accompanied by odds ratio and 95% confidence interval gives important information of direction and size of treatment effect. The measures of central tendencies should be followed by the appropriate measures of variability (mean and standard deviation; median and interquartile range). Relative measures should be accompanied by absolute values (percentage and actual value).[ 22 ] The interpretation of results solely based on bar diagrams or line graphs could be misleading, and a more complete data may be presented in the form of box plots or scatter plots.[ 20 ]
The strengths and weaknesses of the study should be discussed in a separate paragraph. This makes way for implications for clinical practice and future research.[ 16 , 23 ]
The section ends with a conclusion of not more than one to two sentences. The Conclusion section summarizes the study findings in the context of evidence in the field. The important components of the Discussion section are summarized in Table 4 [ Figure 1 ].
Important components of the Discussion section

The hourglass structure of the Introduction and Discussion sections
A referencing tool such as EndNote™ may be used to store and organize the references. The references at the end of the manuscript need to be listed in a manner specified by the journal. The common styles used are Vancouver, Harvard, APA, etc.[ 24 ] Despite continued efforts, standardization to one global format has not yet become a reality.[ 25 ]
It is important to understand the evidence in the referenced articles to write meaningful Introduction and Discussion sections. Online search engines such as Pubmed, Medline, and Scopus are some of the sources that provide abstracts from indexed journals. However, a full-text article may not always be available unless one has subscription for the journals. Those with institutional attachments, authors, and even the research division of pharmaceutical companies may be unconventional but helpful sources for procuring full-text articles. Individual articles can be purchased from certain journals as well.
- (9) Acknowledgements : This section follows the Conclusion section. People who have helped in various aspects of the concerned research work, statistical analysis, or manuscript preparation, but do not qualify to be authors for the study, are acknowledged, preferably with their academic affiliations.[ 26 ]
The aforementioned section provides the general guidelines for preparing a good manuscript. However, an exhaustive list of available guidelines and other resources to facilitate good research reporting are provided by the Enhancing the Quality and Transparency of Health Research network ( http://www.equator-network.org ).
A DDITIONAL F ACTORS I NFLUENCING THE M ANUSCRIPT Q UALITY
- (1) Plagiarism : Plagiarism is a serious threat to scientific publications and is described by the office of Research Integrity as “theft or misappropriation of intellectual property and the substantial unattributed textual copying of another’s work and the representation of them as one’s own original work.” The primary responsibility of preventing plagiarism lies with the authors. It is important to develop the skill of writing any manuscript in one’s own words and when quoting available evidence, substantiate with appropriate references. However, the use of plagiarism detection tools and a critical analysis by the editorial team prior to submitting an article for peer review are also equally important to prevent this menace.[ 29 ] The consequences of plagiarism could range from disciplinary charges such as retraction of the article to criminal charges.[ 30 ]
- (2) Language : One of the important limitations to publication is the problem of writing in English. This can be minimized by seeking help from colleagues or using the language editing service provided by many of the journals.
- (3) Professional medical writing support : In recent years, it is acknowledged that the lack of time and linguistic constraints prevent some of the good work from being published. Hence, the role of professional medical writing support is being critically evaluated. Declared professional medical writing support is found to be associated with more complete reporting of clinical trial results and higher quality of written English. Medical writing support may play an important role in raising the quality of clinical trial reporting.[ 31 ] The role of professional medical writers should be acknowledged in the Acknowledgements section.[ 32 ]
S UBMISSION TO J OURNALS AND R EVIEWING P ROCESS
The submission of manuscripts is now exclusively an online exercise. The basic model of submission in any journal comprises the following: the title file or first page file, article file, image files, videos, charts, tables, figures, and copyright/consent forms. It is important to keep all the files ready in a folder before starting the submission process. When submitting images, it is important to have good quality, well-focused images with good resolution.[ 33 ] Some journals may offer the choice of selecting preferred reviewers to the authors and hence, one must be prepared for this. Once the manuscript is submitted, the status can be periodically checked. With minor variations, a submitted article goes through the following review process: The Editor allocates it to one of the editorial team members who checks for the suitability for publication in the journal. It is checked for plagiarism as well at this stage. The article then goes for peer review to two to three reviewers. The review process may take 4–6 weeks, at the end of which, the reviewers submit their remarks, and “article decision” is made, which could be an advice for minor/major revisions, rewriting the whole manuscript for specific reasons, acceptance without any changes (very rare), or rejection. It is important to take into consideration all the comments of the reviewers and incorporate the necessary changes in the manuscript before resubmitting. However, if the manuscript is rejected, revise to incorporate the valid suggestions given by the reviewers and consider submitting to another journal in the field. This should be effected without delay overcoming the disappointment so that the research still remains valid in the context of time.
P REDATORY J OURNALS
Some of the well-known journals provide an “open access” option to the authors, wherein if the manuscript is published, it is accessible to all the readers online free of cost. However, the authors need to pay a certain fee to make their manuscript an open access article. In addition, some of the well-known journals published by reputed publishers such as BioMed Central (BMC) and Public Library of Science (PLoS) have online “open access” journals, where the manuscripts are published for a fee but are subjected to the conventional scrutiny process, and the readers can access the full-text article.[ 34 ] The Directory of Open Access Journals, http://doaj.org , is an online directory that indexes and provides access to high-quality, open access, peer-reviewed journals. However, many online open access journals are mushrooming, which provide a legitimate face for an illegitimate publication process lacking basic industry standards, sound peer review practices, and solid basis in publication ethics. Such journals are known as “predatory journals.”[ 35 ] The pressure of needing to have scientific publications and the lack of knowledge regarding predatory journals may encourage authors to submit their articles to such journals. Currently, it is not easy to identify predatory journals, and authors should seek such information proactively from mentors, journal websites, and recent and relevant published literature. In addition, editorial oversights (editors and editorial board members), peer review practices, the quality of published articles, indexing, access, citations and ethical practices are important aspects to be considered while choosing an appropriate journal.[ 36 ]
A relevant research hypothesis and research conducted within the ethical framework are of utmost importance for clinical research. The natural progression from here is the manuscript preparation, a daunting process for most of the clinicians involved in clinical research. Choosing a journal that provides an appropriate platform for the manuscript, conforming to the instructions specific for the journal, and following certain simple guidelines can result in successful preparation and publishing of scientific work. Allocating certain time at regular intervals for writing and maintaining discipline and perseverance in this regard are very important prerequisites to achieve the goal of successful publication.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
R EFERENCES
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
How to publish your paper
On this page, journal specific instructions, nature journal pledge to authors, how to publish your research in a nature journal, editorial process, about advance online publication, journals' aop timetable, frequently asked questions.
For more information on how to publish papers in a specific Nature Portfolio title, please visit the author instructions page for the journal that is of interest to you.
Top of page ⤴
Editors of the Nature journals strive to provide authors with an outstandingly efficient, fair and thoughtful submission, peer-review and publishing experience. Authors can expect all manuscripts that are published to be scrutinized for peer-review with the utmost professional rigor and care by expert referees who are selected by the editors for their ability to provide incisive and useful analysis. Editors weigh many factors when choosing content for Nature journals, but they strive to minimize the time taken to make decisions about publication while maintaining the highest possible quality of that decision.
After review, editors work to increase a paper's readability, and thereby its audience, through advice and editing, so that all research is presented in a form that is both readable to those in the field and understandable to scientists outside the immediate discipline. Research is published online without delay through our Advance Online Publication system. Nature journals provide more than 3,000 registered journalists with weekly press releases that mention all research papers to be published. About 800,000 registered users receive e-mailed tables of contents, and many papers are highlighted for the nonspecialist reader on the journal's homepage, contents pages and in News and Views.
Throughout this process, the editors of Nature journals uphold editorial, ethical and scientific standards according to the policies outlined on the author and referee site as well as on our journal websites. We periodically review those policies to ensure that they continue to reflect the needs of the scientific community, and welcome comments and suggestions from scientists, either via the feedback links on the author and referees' website or via our author blog, Nautilus , or peer-review blog, Peer to Peer .
The Nature journals comprise the weekly, multidisciplinary Nature, which publishes research of the highest influence within a discipline that will be of interest to scientists in other fields, and fifteen monthly titles, publishing papers of the highest quality and of exceptional impact: Nature Biotechnology, Nature Cell Biology, Nature Chemical Biology, Nature Chemistry, Nature Climate Change, Nature Communications, Nature Genetics, Nature Geoscience, Nature Immunology, Nature Materials, Nature Medicine, Nature Methods, Nature Nanotechnology, Nature Neuroscience, Nature Photonics, Nature Physics, Nature Protocolsand Nature Structural and Molecular Biology. These journals are international, being published and printed in the United States, the United Kingdom and Japan. See here for more information about the relationship between these journals.
Nature and the Nature monthly journals have Impact Factors that are among the highest in the world. The high prestige of these journals brings many rewards to their authors, but also means that competition for publication is severe, so many submissions have to be declined without peer-review.
The Nature journals differ from most other journals in that they do not have editorial boards, but are instead run by professional editors who consult widely among the scientific community in making decisions about publication of papers. This article is to provide you with an overview of the general editorial processes of these unique journals. Although the journals are broadly similar and share editorial policies , all authors should consult the author information pages of the specific Nature journal before submitting, to obtain detailed information on criteria for publication and manuscript preparation for that journal, as some differences exist.
The following sections summarise the journals' editorial processes and describe how manuscripts are handled by editors between submission and publication. At all stages of the process, you can access the online submission system and find the status of your manuscript.
Presubmission enquiries
Many Nature journals allow researchers to obtain informal feedback from editors before submitting the whole manuscript. This service is intended to save you time — if the editors feel it would not be suitable, you can submit the manuscript to another journal without delay. If you wish to use the presubmission enquiry service, please use the online system of the journal of your choice to send a paragraph explaining the importance of your manuscript, as well as the abstract or summary paragraph with its associated citation list so the editors may judge the manuscript in relation to other related work. The editors will quickly either invite you to submit the whole manuscript (which does not mean any commitment to publication), or will say that it is not suitable for the journal. If you receive a negative response, please do not reply. If you are convinced of the importance of your manuscript despite editors' reservations, you may submit the whole manuscript using the journal's online submission system. The editors can then make a more complete assessment of your work. Note that not all Nature journals offer a presubmission enquiry service.
Initial submission
When you are ready to submit the manuscript, please use the online submission system for the journal concerned. When the journal receives your manuscript, it will be assigned a number and an editor, who reads the manuscript, seeks informal advice from scientific advisors and editorial colleagues, and compares your submission to other recently published papers in the field. If the manuscript seems novel and arresting, and the work described has both immediate and far-reaching implications, the editor will send it out for peer review, usually to two or three independent specialists. However, because the journals can publish only a few of the manuscripts in the field or subfield concerned, many manuscripts have to be declined without peer review even though they may describe solid scientific results.
Transfers between Nature journals
In some cases, an editor is unable to offer publication, but might suggest that the manuscript is more suitable for one of the other Nature journals. If you wish to resubmit your manuscript to the suggested journal, you can simply follow the link provided by the editor to transfer your manuscript and the reviewers' comments to the new journal. This process is entirely in your control: you can choose not to use this service and instead to submit your manuscript to any other Nature or nature research journal, with or without including the reviewers' comments if you wish, using the journal's usual online submission service. For more information, please see the manuscript transfers page .
Peer review
The corresponding author is notified by email when an editor decides to send a manuscript for review. The editors choose referees for their independence, ability to evaluate the technical aspects of the paper fully and fairly, whether they are currently or recently assessing related submissions, and whether they can review the manuscript within the short time requested.
You may suggest referees for your manuscript (including address details), so long as they are independent scientists. These suggestions are often helpful, although they are not always followed. Editors will honour your requests to exclude a limited number of named scientists as reviewers.
Decisions and revisions
If the editor invites you to revise your manuscript, you should include with your resubmitted version a new cover letter that includes a point-by-point response to the reviewers' and editors' comments, including an explanation of how you have altered your manuscript in response to these, and an estimation of the length of the revised version with figures/tables. The decision letter will specify a deadline, and revisions that are returned within this period will retain their original submission date.
Additional supplementary information is published with the online version of your article if the editors and referees have judged that it is essential for the conclusions of the article (for example, a large table of data or the derivation of a model) but of more specialist interest than the rest of the article. Editors encourage authors whose articles describe methods to provide a summary of the method for the print version and to include full details and protocols online. Authors are also encouraged to post the full protocol on Nature Protocols' Protocol Exchange , which as well as a protocols database provides an online forum for readers in the field to add comments, suggestions and refinements to the published protocols.
After acceptance
Your accepted manuscript is prepared for publication by copy editors (also called subeditors), who refine it so that the text and figures are readable and clear to those outside the immediate field; choose keywords to maximize visibility in online searches as well as suitable for indexing services; and ensure that the manuscripts conform to house style. The copy editors are happy to give advice to authors whose native language is not English, and will edit those papers with special care.
After publication
All articles are published in the print edition and, in PDF and HTML format, in the online edition of the journal, in full. Many linking and navigational services are provided with the online (HTML) version of all articles published by the Nature journals.
All articles and contact details of corresponding authors are included in our press release service, which means that your work is drawn to the attention of all the main media organizations in the world, who may choose to feature the work in newspaper and other media reports. Some articles are summarized and highlighted within Nature and Nature Portfolio publications and subject-specific websites.
Journals published by Nature Portfolio do not ask authors for copyright, but instead ask you to sign an exclusive publishing license . This allows you to archive the accepted version of your manuscript six months after publication on your own, your institution's, and your funder's websites.
Disagreements with decisions
If a journal's editors are unable to offer publication of a manuscript and have not invited resubmission, you are strongly advised to submit your manuscript for publication elsewhere. However, if you believe that the editors or reviewers have seriously misunderstood your manuscript, you may write to the editors, explaining the scientific reasons why you believe the decision was incorrect. Please bear in mind that editors prioritise newly submitted manuscripts and manuscripts where resubmission has been invited, so it can take several weeks before letters of disagreement can be answered. During this time, you must not submit your manuscript elsewhere. In the interests of publishing your results without unnecessary delay, we therefore advise you to submit your manuscript to another journal if it has been declined, rather than to spend time on corresponding further with the editors of the declining journal.
Nature journals offer Advance Online Publication (AOP).
We believe that AOP is the best and quickest way to publish high-quality, peer-reviewed research for the benefit of readers and authors. Papers published AOP are the definitive version: they do not change before appearing in print and can be referenced formally as soon as they appear on the journal's AOP website. In addition, Nature publishes some papers each week via an Accelerated Article Preview (AAP) workflow. For these papers, we upload the accepted manuscript to our website as an AAP PDF, without subediting of text, figures or tables, but with some preliminary formatting. AAP papers are clearly indicated by a watermark on each page of the online PDF.
Each journal's website includes an AOP table of contents, in which papers are listed in order of publication date (beginning with the most recent). Each paper carries a digital object identifier (DOI), which serves as a unique electronic identification tag for that paper. As soon as the issue containing the paper is printed, papers will be removed from the AOP table of contents, assigned a page number and transferred to that issue's table of contents on the website. The DOI remains attached to the paper to provide a persistent identifier.
Nature publishes many, but not all, papers AOP, on Mondays and Wednesdays.
For the monthly Nature journals publishing primary research, new articles are uploaded to the AOP section of their web sites once each week. Occasionally, an article may be uploaded on other days.
The monthly Nature Reviews journals also upload new articles to the AOP section of their web sites once each week.
Q. Which articles are published AOP?
A. Original research is published AOP — that is, Articles and Letters, and for the Nature journals that publish them, Brief Communications. Associated News and Views articles may be published with the AOP Article or Letter or when the papers are published in the print/online edition of the journal. Nature occasionally publishes other article types AOP, for example News and Commentaries.
Q. Is the AOP version of the article definitive?
A. Yes. Only the final version of the paper is published AOP, exactly as it will be published in the printed edition. The paper is thus complete in every respect except that instead of having a volume/issue/page number, it has a DOI (digital object identifier). This means that the paper can be referenced as soon as it appears on the AOP site by using the DOI. Nature also publishes some papers each week via an Accelerated Article Preview workflow, where the accepted version of the paper is uploaded as a PDF to our website without subediting of text, figures and tables, but with some preliminary formatting. These papers are clearly identified by a watermark on each page of the PDF.
Q. What is a Digital Object Identifier?
A. The DOI is an international, public, "persistent identifier of intellectual property entities" in the form of a combination of numbers and letters. For Nature Portfolio journals, the DOI is assigned to an item of editorial content, providing a unique and persistent identifier for that item. The DOI system is administered by the International DOI Foundation, a not-for-profit organization. CrossRef, another not-for-profit organization, uses the DOI as a reference linking standard, enables cross-publisher linking, and maintains the lookup system for DOIs. Nature Portfolio is a member of CrossRef.
Q. What do the numbers in the DOI signify?
A. The DOI has two components, a prefix (before the slash) and a suffix (after the slash). The prefix is a DOI resolver server identifer (10) and a unique identifier assigned to the publisher—for example, the identifier for Nature Portfolio is 1038 and the entire DOI prefix for an article published by Nature Portfolio is 10.1038. The suffix is an arbitrary number provided by the publisher. It can be composed of numbers and/or letters and does not necessarily have any systematic significance. Each DOI is registered in a central resolution database that associates it with one or more corresponding web locations (URLs). For example, the DOI 10.1038/ng571 connects to http://dx.doi.org/10.1038/ng571.
Q. Can I use the DOI in a reference citation?
A. Yes, instead of giving the volume and page number, you can give the paper's DOI at the end of the citation. For example, Nature papers should be cited in the form;
Author(s) Nature advance online publication, day month year (DOI 10.1038/natureXXX).
After print publication, you should give the DOI as well as the print citation, to enable readers to find the paper in print as well as online. For example;
Author(s) Nature volume, page (year); advance online publication, day month year (DOI 10.1038/natureXXX).
Q. How can I use a DOI to find a paper?
A. There are two ways:
- DOIs from other articles can be embedded into the linking coding of an article's reference section. In Nature journals these appear as "|Article|" in the reference sections. When |Article| is clicked, it opens another browser window leading to the entrance page (often the abstract) for another article. Depending on the source of the article, this page can be on the Nature Portfolio's site or a site of another publisher. This service is enabled by CrossRef.
- A DOI can be inserted directly into the browser. For example, for the DOI 10.1038/ng571, typing http://dx.doi.org/10.1038/ng571 brings up the entrance page of the article.
Q. What is the official publication date?
A. Many journals, and most abstracting and indexing services (including Medline and Thomson-Reuters) cite the print date as the publication date. Publishers usually state both the 'online publication date' and the 'print publication date'. Nature Portfolio publishes both dates for our own papers, in the hope that scientific communities, as well as abstracting and indexing services, will recognize these dates.
We endeavour to include both the online publication date and the usual print citation in reference lists of Nature Portfolio papers, where a paper has been published online before being published in print. Given the use of the DOI in locating an online publication in the future, we encourage authors to use DOIs in reference citations.
For legal purposes (for example, establishing intellectual property rights), we assume that online publication constitutes public disclosure. But this is for the courts to decide; Nature Portfolio's role as a publisher is to provide clear documentation of the publication history, online and in print.
Q. Must I be a subscriber to read AOP articles?
A. Yes. AOP papers are the same as those in the print/online issues: while abstracts are freely available on any Nature Portfolio journal's web site, access to the full-text article requires a paid subscription or a site license.
Q. Does Medline use DOIs?
A. Medline currently captures DOIs with online publication dates in its records, and is developing an enhanced level of support for the DOI system.
Q. Does Thomson-Reuters use DOIs?
A. Thomson Reuters captures DOIs in its records at the same time as the volume/issue/page number. Therefore, it is not using the DOI to capture information before print publication, but rather as an additional piece of metadata.
Q. How does AOP affect the Impact Factor?
A. Impact factors are calculated by Thomson-Reuters. At present, Thomson-Reuters bases its calculations on the date of print publication alone, so until or unless it changes its policy, AOP has no effect on impact factors.
Q. What are the page numbers in PDFs of AOP papers?
A. For convenience, the PDF version of every AOP article is given a temporary pagination, beginning with page 1. This is unrelated to the final pagination in the printed article.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
How to Write and Publish a Research Paper for a Peer-Reviewed Journal
- Open access
- Published: 30 April 2020
- Volume 36 , pages 909–913, ( 2021 )
Cite this article
You have full access to this open access article

- Clara Busse ORCID: orcid.org/0000-0002-0178-1000 1 &
- Ella August ORCID: orcid.org/0000-0001-5151-1036 1 , 2
286k Accesses
19 Citations
706 Altmetric
Explore all metrics
Communicating research findings is an essential step in the research process. Often, peer-reviewed journals are the forum for such communication, yet many researchers are never taught how to write a publishable scientific paper. In this article, we explain the basic structure of a scientific paper and describe the information that should be included in each section. We also identify common pitfalls for each section and recommend strategies to avoid them. Further, we give advice about target journal selection and authorship. In the online resource 1 , we provide an example of a high-quality scientific paper, with annotations identifying the elements we describe in this article.
Similar content being viewed by others

How to Choose the Right Journal

The Point Is…to Publish?

Writing and publishing a scientific paper
Explore related subjects.
- Artificial Intelligence
Avoid common mistakes on your manuscript.
Introduction
Writing a scientific paper is an important component of the research process, yet researchers often receive little formal training in scientific writing. This is especially true in low-resource settings. In this article, we explain why choosing a target journal is important, give advice about authorship, provide a basic structure for writing each section of a scientific paper, and describe common pitfalls and recommendations for each section. In the online resource 1 , we also include an annotated journal article that identifies the key elements and writing approaches that we detail here. Before you begin your research, make sure you have ethical clearance from all relevant ethical review boards.
Select a Target Journal Early in the Writing Process
We recommend that you select a “target journal” early in the writing process; a “target journal” is the journal to which you plan to submit your paper. Each journal has a set of core readers and you should tailor your writing to this readership. For example, if you plan to submit a manuscript about vaping during pregnancy to a pregnancy-focused journal, you will need to explain what vaping is because readers of this journal may not have a background in this topic. However, if you were to submit that same article to a tobacco journal, you would not need to provide as much background information about vaping.
Information about a journal’s core readership can be found on its website, usually in a section called “About this journal” or something similar. For example, the Journal of Cancer Education presents such information on the “Aims and Scope” page of its website, which can be found here: https://www.springer.com/journal/13187/aims-and-scope .
Peer reviewer guidelines from your target journal are an additional resource that can help you tailor your writing to the journal and provide additional advice about crafting an effective article [ 1 ]. These are not always available, but it is worth a quick web search to find out.
Identify Author Roles Early in the Process
Early in the writing process, identify authors, determine the order of authors, and discuss the responsibilities of each author. Standard author responsibilities have been identified by The International Committee of Medical Journal Editors (ICMJE) [ 2 ]. To set clear expectations about each team member’s responsibilities and prevent errors in communication, we also suggest outlining more detailed roles, such as who will draft each section of the manuscript, write the abstract, submit the paper electronically, serve as corresponding author, and write the cover letter. It is best to formalize this agreement in writing after discussing it, circulating the document to the author team for approval. We suggest creating a title page on which all authors are listed in the agreed-upon order. It may be necessary to adjust authorship roles and order during the development of the paper. If a new author order is agreed upon, be sure to update the title page in the manuscript draft.
In the case where multiple papers will result from a single study, authors should discuss who will author each paper. Additionally, authors should agree on a deadline for each paper and the lead author should take responsibility for producing an initial draft by this deadline.
Structure of the Introduction Section
The introduction section should be approximately three to five paragraphs in length. Look at examples from your target journal to decide the appropriate length. This section should include the elements shown in Fig. 1 . Begin with a general context, narrowing to the specific focus of the paper. Include five main elements: why your research is important, what is already known about the topic, the “gap” or what is not yet known about the topic, why it is important to learn the new information that your research adds, and the specific research aim(s) that your paper addresses. Your research aim should address the gap you identified. Be sure to add enough background information to enable readers to understand your study. Table 1 provides common introduction section pitfalls and recommendations for addressing them.

The main elements of the introduction section of an original research article. Often, the elements overlap
Methods Section
The purpose of the methods section is twofold: to explain how the study was done in enough detail to enable its replication and to provide enough contextual detail to enable readers to understand and interpret the results. In general, the essential elements of a methods section are the following: a description of the setting and participants, the study design and timing, the recruitment and sampling, the data collection process, the dataset, the dependent and independent variables, the covariates, the analytic approach for each research objective, and the ethical approval. The hallmark of an exemplary methods section is the justification of why each method was used. Table 2 provides common methods section pitfalls and recommendations for addressing them.
Results Section
The focus of the results section should be associations, or lack thereof, rather than statistical tests. Two considerations should guide your writing here. First, the results should present answers to each part of the research aim. Second, return to the methods section to ensure that the analysis and variables for each result have been explained.
Begin the results section by describing the number of participants in the final sample and details such as the number who were approached to participate, the proportion who were eligible and who enrolled, and the number of participants who dropped out. The next part of the results should describe the participant characteristics. After that, you may organize your results by the aim or by putting the most exciting results first. Do not forget to report your non-significant associations. These are still findings.
Tables and figures capture the reader’s attention and efficiently communicate your main findings [ 3 ]. Each table and figure should have a clear message and should complement, rather than repeat, the text. Tables and figures should communicate all salient details necessary for a reader to understand the findings without consulting the text. Include information on comparisons and tests, as well as information about the sample and timing of the study in the title, legend, or in a footnote. Note that figures are often more visually interesting than tables, so if it is feasible to make a figure, make a figure. To avoid confusing the reader, either avoid abbreviations in tables and figures, or define them in a footnote. Note that there should not be citations in the results section and you should not interpret results here. Table 3 provides common results section pitfalls and recommendations for addressing them.
Discussion Section
Opposite the introduction section, the discussion should take the form of a right-side-up triangle beginning with interpretation of your results and moving to general implications (Fig. 2 ). This section typically begins with a restatement of the main findings, which can usually be accomplished with a few carefully-crafted sentences.
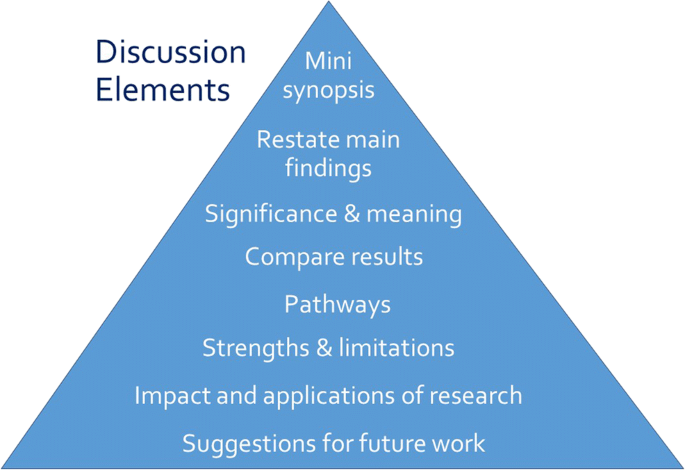
Major elements of the discussion section of an original research article. Often, the elements overlap
Next, interpret the meaning or explain the significance of your results, lifting the reader’s gaze from the study’s specific findings to more general applications. Then, compare these study findings with other research. Are these findings in agreement or disagreement with those from other studies? Does this study impart additional nuance to well-accepted theories? Situate your findings within the broader context of scientific literature, then explain the pathways or mechanisms that might give rise to, or explain, the results.
Journals vary in their approach to strengths and limitations sections: some are embedded paragraphs within the discussion section, while some mandate separate section headings. Keep in mind that every study has strengths and limitations. Candidly reporting yours helps readers to correctly interpret your research findings.
The next element of the discussion is a summary of the potential impacts and applications of the research. Should these results be used to optimally design an intervention? Does the work have implications for clinical protocols or public policy? These considerations will help the reader to further grasp the possible impacts of the presented work.
Finally, the discussion should conclude with specific suggestions for future work. Here, you have an opportunity to illuminate specific gaps in the literature that compel further study. Avoid the phrase “future research is necessary” because the recommendation is too general to be helpful to readers. Instead, provide substantive and specific recommendations for future studies. Table 4 provides common discussion section pitfalls and recommendations for addressing them.
Follow the Journal’s Author Guidelines
After you select a target journal, identify the journal’s author guidelines to guide the formatting of your manuscript and references. Author guidelines will often (but not always) include instructions for titles, cover letters, and other components of a manuscript submission. Read the guidelines carefully. If you do not follow the guidelines, your article will be sent back to you.
Finally, do not submit your paper to more than one journal at a time. Even if this is not explicitly stated in the author guidelines of your target journal, it is considered inappropriate and unprofessional.
Your title should invite readers to continue reading beyond the first page [ 4 , 5 ]. It should be informative and interesting. Consider describing the independent and dependent variables, the population and setting, the study design, the timing, and even the main result in your title. Because the focus of the paper can change as you write and revise, we recommend you wait until you have finished writing your paper before composing the title.
Be sure that the title is useful for potential readers searching for your topic. The keywords you select should complement those in your title to maximize the likelihood that a researcher will find your paper through a database search. Avoid using abbreviations in your title unless they are very well known, such as SNP, because it is more likely that someone will use a complete word rather than an abbreviation as a search term to help readers find your paper.
After you have written a complete draft, use the checklist (Fig. 3 ) below to guide your revisions and editing. Additional resources are available on writing the abstract and citing references [ 5 ]. When you feel that your work is ready, ask a trusted colleague or two to read the work and provide informal feedback. The box below provides a checklist that summarizes the key points offered in this article.

Checklist for manuscript quality
Data Availability
Michalek AM (2014) Down the rabbit hole…advice to reviewers. J Cancer Educ 29:4–5
Article Google Scholar
International Committee of Medical Journal Editors. Defining the role of authors and contributors: who is an author? http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authosrs-and-contributors.html . Accessed 15 January, 2020
Vetto JT (2014) Short and sweet: a short course on concise medical writing. J Cancer Educ 29(1):194–195
Brett M, Kording K (2017) Ten simple rules for structuring papers. PLoS ComputBiol. https://doi.org/10.1371/journal.pcbi.1005619
Lang TA (2017) Writing a better research article. J Public Health Emerg. https://doi.org/10.21037/jphe.2017.11.06
Download references
Acknowledgments
Ella August is grateful to the Sustainable Sciences Institute for mentoring her in training researchers on writing and publishing their research.
Code Availability
Not applicable.
Author information
Authors and affiliations.
Department of Maternal and Child Health, University of North Carolina Gillings School of Global Public Health, 135 Dauer Dr, 27599, Chapel Hill, NC, USA
Clara Busse & Ella August
Department of Epidemiology, University of Michigan School of Public Health, 1415 Washington Heights, Ann Arbor, MI, 48109-2029, USA
Ella August
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ella August .
Ethics declarations
Conflicts of interests.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
(PDF 362 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Busse, C., August, E. How to Write and Publish a Research Paper for a Peer-Reviewed Journal. J Canc Educ 36 , 909–913 (2021). https://doi.org/10.1007/s13187-020-01751-z
Download citation
Published : 30 April 2020
Issue Date : October 2021
DOI : https://doi.org/10.1007/s13187-020-01751-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Manuscripts
- Scientific writing
- Find a journal
- Publish with us
- Track your research
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Happiness Hub Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- College University and Postgraduate
- Academic Writing
- Research Papers
How to Write and Publish Your Research in a Journal
Last Updated: May 26, 2024 Fact Checked
Choosing a Journal
Writing the research paper, editing & revising your paper, submitting your paper, navigating the peer review process, research paper help.
This article was co-authored by Matthew Snipp, PhD and by wikiHow staff writer, Cheyenne Main . C. Matthew Snipp is the Burnet C. and Mildred Finley Wohlford Professor of Humanities and Sciences in the Department of Sociology at Stanford University. He is also the Director for the Institute for Research in the Social Science’s Secure Data Center. He has been a Research Fellow at the U.S. Bureau of the Census and a Fellow at the Center for Advanced Study in the Behavioral Sciences. He has published 3 books and over 70 articles and book chapters on demography, economic development, poverty and unemployment. He is also currently serving on the National Institute of Child Health and Development’s Population Science Subcommittee. He holds a Ph.D. in Sociology from the University of Wisconsin—Madison. There are 13 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 707,574 times.
Publishing a research paper in a peer-reviewed journal allows you to network with other scholars, get your name and work into circulation, and further refine your ideas and research. Before submitting your paper, make sure it reflects all the work you’ve done and have several people read over it and make comments. Keep reading to learn how you can choose a journal, prepare your work for publication, submit it, and revise it after you get a response back.
Things You Should Know
- Create a list of journals you’d like to publish your work in and choose one that best aligns with your topic and your desired audience.
- Prepare your manuscript using the journal’s requirements and ask at least 2 professors or supervisors to review your paper.
- Write a cover letter that “sells” your manuscript, says how your research adds to your field and explains why you chose the specific journal you’re submitting to.

- Ask your professors or supervisors for well-respected journals that they’ve had good experiences publishing with and that they read regularly.
- Many journals also only accept specific formats, so by choosing a journal before you start, you can write your article to their specifications and increase your chances of being accepted.
- If you’ve already written a paper you’d like to publish, consider whether your research directly relates to a hot topic or area of research in the journals you’re looking into.

- Review the journal’s peer review policies and submission process to see if you’re comfortable creating or adjusting your work according to their standards.
- Open-access journals can increase your readership because anyone can access them.

- Scientific research papers: Instead of a “thesis,” you might write a “research objective” instead. This is where you state the purpose of your research.
- “This paper explores how George Washington’s experiences as a young officer may have shaped his views during difficult circumstances as a commanding officer.”
- “This paper contends that George Washington’s experiences as a young officer on the 1750s Pennsylvania frontier directly impacted his relationship with his Continental Army troops during the harsh winter at Valley Forge.”

- Scientific research papers: Include a “materials and methods” section with the step-by-step process you followed and the materials you used. [5] X Research source
- Read other research papers in your field to see how they’re written. Their format, writing style, subject matter, and vocabulary can help guide your own paper. [6] X Research source

- If you’re writing about George Washington’s experiences as a young officer, you might emphasize how this research changes our perspective of the first president of the U.S.
- Link this section to your thesis or research objective.
- If you’re writing a paper about ADHD, you might discuss other applications for your research.

- Scientific research papers: You might include your research and/or analytical methods, your main findings or results, and the significance or implications of your research.
- Try to get as many people as you can to read over your abstract and provide feedback before you submit your paper to a journal.

- They might also provide templates to help you structure your manuscript according to their specific guidelines. [11] X Research source

- Not all journal reviewers will be experts on your specific topic, so a non-expert “outsider’s perspective” can be valuable.

- If you have a paper on the purification of wastewater with fungi, you might use both the words “fungi” and “mushrooms.”
- Use software like iThenticate, Turnitin, or PlagScan to check for similarities between the submitted article and published material available online. [15] X Research source

- Header: Address the editor who will be reviewing your manuscript by their name, include the date of submission, and the journal you are submitting to.
- First paragraph: Include the title of your manuscript, the type of paper it is (like review, research, or case study), and the research question you wanted to answer and why.
- Second paragraph: Explain what was done in your research, your main findings, and why they are significant to your field.
- Third paragraph: Explain why the journal’s readers would be interested in your work and why your results are important to your field.
- Conclusion: State the author(s) and any journal requirements that your work complies with (like ethical standards”).
- “We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.”
- “All authors have approved the manuscript and agree with its submission to [insert the name of the target journal].”

- Submit your article to only one journal at a time.
- When submitting online, use your university email account. This connects you with a scholarly institution, which can add credibility to your work.

- Accept: Only minor adjustments are needed, based on the provided feedback by the reviewers. A first submission will rarely be accepted without any changes needed.
- Revise and Resubmit: Changes are needed before publication can be considered, but the journal is still very interested in your work.
- Reject and Resubmit: Extensive revisions are needed. Your work may not be acceptable for this journal, but they might also accept it if significant changes are made.
- Reject: The paper isn’t and won’t be suitable for this publication, but that doesn’t mean it might not work for another journal.

- Try organizing the reviewer comments by how easy it is to address them. That way, you can break your revisions down into more manageable parts.
- If you disagree with a comment made by a reviewer, try to provide an evidence-based explanation when you resubmit your paper.

- If you’re resubmitting your paper to the same journal, include a point-by-point response paper that talks about how you addressed all of the reviewers’ comments in your revision. [22] X Research source
- If you’re not sure which journal to submit to next, you might be able to ask the journal editor which publications they recommend.

Expert Q&A
You might also like.

- If reviewers suspect that your submitted manuscript plagiarizes another work, they may refer to a Committee on Publication Ethics (COPE) flowchart to see how to move forward. [23] X Research source Thanks Helpful 0 Not Helpful 0

- ↑ https://www.wiley.com/en-us/network/publishing/research-publishing/choosing-a-journal/6-steps-to-choosing-the-right-journal-for-your-research-infographic
- ↑ https://link.springer.com/article/10.1007/s13187-020-01751-z
- ↑ https://libguides.unomaha.edu/c.php?g=100510&p=651627
- ↑ https://www.canberra.edu.au/library/start-your-research/research_help/publishing-research
- ↑ https://writingcenter.fas.harvard.edu/conclusions
- ↑ https://writing.wisc.edu/handbook/assignments/writing-an-abstract-for-your-research-paper/
- ↑ https://www.springer.com/gp/authors-editors/book-authors-editors/your-publication-journey/manuscript-preparation
- ↑ https://apus.libanswers.com/writing/faq/2391
- ↑ https://academicguides.waldenu.edu/library/keyword/search-strategy
- ↑ https://ifis.libguides.com/journal-publishing-guide/submitting-your-paper
- ↑ https://www.springer.com/kr/authors-editors/authorandreviewertutorials/submitting-to-a-journal-and-peer-review/cover-letters/10285574
- ↑ https://www.apa.org/monitor/sep02/publish.aspx
- ↑ Matthew Snipp, PhD. Research Fellow, U.S. Bureau of the Census. Expert Interview. 26 March 2020.
About This Article

To publish a research paper, ask a colleague or professor to review your paper and give you feedback. Once you've revised your work, familiarize yourself with different academic journals so that you can choose the publication that best suits your paper. Make sure to look at the "Author's Guide" so you can format your paper according to the guidelines for that publication. Then, submit your paper and don't get discouraged if it is not accepted right away. You may need to revise your paper and try again. To learn about the different responses you might get from journals, see our reviewer's explanation below. Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
RAMDEV GOHIL
Oct 16, 2017
Did this article help you?
David Okandeji
Oct 23, 2019
Revati Joshi
Feb 13, 2017
Shahzad Khan
Jul 1, 2017
Apr 7, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life
- How it works

How To Publish A Research Paper? | A Step-By-Step Guide
Published by Alvin Nicolas at September 23rd, 2024 , Revised On September 23, 2024
The process of publishing a research paper can be confusing to many students or first-time authors. It requires careful planning, attention to detail, using academic sources and submitting your manuscript through the submission system.
After writing a research paper comes the most significant step of all. Yes, of course, we are talking about getting it published in a journal. This blog provides you with a step-by-step guide on how to publish your research paper and share it with academic professionals all over the world.
What Is A Research Paper?
A research paper is a piece of academic writing that presents your interpretation, evaluation and findings on a specific topic. It involves extensive research data collection through qualitative and quantitative methods to validate your hypothesis .
A research paper is not easy to write, as it involves understanding the research paper format and guidelines of many journals. It typically consists of an abstract, introduction, literature review, methodologies, results and discussion. But it doesn’t end here, as you need to publish it in well-known journals to create an impact on your work worldwide.

Advantages Of Publishing A Research Paper
Before we dive into the process of publishing a research paper, let’s understand the advantages and benefits of publishing a research paper.
| Your research work can help you get academic recognition and appreciation and advance your career. | |
| Your research work can create an impact and advance knowledge in academic fields. | |
| Publishing a research paper as an undergraduate or as a first-time author can help you get academic funding and credit. It also improves your academic record. | |
| Your research work will provide you with a sense of satisfaction and accomplishment. | |
| When experts check your work for accuracy and transparency, you will get to learn more from your peers. |
Criteria For Publishing A Research Paper
A well-crafted research paper proves to be a valuable resource in academic fields. However, it should meet specific criteria to be eligible for publishing in journals. These criteria can vary from journal to journal, however, here are some common requirements to publish a research paper:
Originality
The research paper should be original and should not have been published anywhere else or previously in some other journal. Also, it should provide advanced knowledge on the chosen topic. The figures included in the manuscript should not be published anywhere else.
High-Technical Standards
Any research methodology or reagent used in the research should be mentioned in a comprehensive manner. The experiment must be carried out properly, with the sample size large enough for robust results. Moreover, the data presented must support the conclusions drawn.
Scientific Merit
The manuscript must be clear and concise for peers and other academic researchers to understand. The research must be ethical and of the highest standards with clear objectives. All ethical considerations should be taken into review such as transparency, accuracy, data privacy, participant’s consent and animal welfare in the case of experiments.
Relevance To Journal
The research paper must be relevant to the journal approached for publishing, and it must focus on the key areas of it. Every journal has its publication criteria, ethical considerations, figure assessment and more, which should all be taken into account.
Standard English
Authors should avoid using unambiguous words that are difficult to understand. Each journal urges that the research work be in standard English. In case of any problem, authors can seek scientific editing services or manuscript editing services online for ease.
Hire an Expert Writer
Orders completed by our expert writers are
- Formally drafted in an academic style
- Free Amendments and 100% Plagiarism Free – or your money back!
- 100% Confidential and Timely Delivery!
- Free anti-plagiarism report
- Appreciated by thousands of clients. Check client reviews
Steps In Publishing A Research Paper
Publishing a research paper involves a great deal of steps. Here is the process of publishing a research paper:
Step 1: Choose A Journal
The first step in getting your paper published is choosing the right journal. The best way to identify the best journal for your paper is by looking at the reference section of your manuscript. Journals only publish manuscripts of topics they have previously published.
Moreover, the tone, format and writing style must be similar to that of articles and papers published by the journal. Another way to find the right journal is by using a journal finder. This helps specify journals related to your work and also mentions journal rankings. For a better understanding, the following questions must be taken into view while selecting a journal for your paper:
- What is my target audience?
- Which journal fits my manuscript?
- Which journals are ranked better and have a higher impact?
- Which journals are open access and which are subscription-based?
- What is the publishing fee for journals?
Some well-known online journals in academic fields are:
- ResearchGate
- Chemical Reviews
- Nature Medicine
- World Psychiatry
Step 2: Prepare Your Paper
After selecting a journal, you should prepare your paper for submission. The tone, structure and format must be according to the journal you chose. Certain journals have a particular format for how tables, figures, and other materials are presented. You should always keep in mind your target journal, and your audience, and use keywords accordingly. Lastly, it is necessary to be aware of the editorial policies of the journal, to avoid any complications in future.
Step 3: Editing And Revising
Before submitting your paper, it is wise to revise it and correct any factual errors and knowledge gaps that might have occurred.
Step 4: Submit Your Paper
After completing the above steps, submit your paper to the journal. Each journal will have specific journal requirements that should be followed. There is no set time when you can expect to hear from the editor. However, a desk rejection may occur if the editor rejects your paper or sends it back for revision and resubmission.
Step 5: The Peer-Review Process
A peer review is an independent assessment of your work by experts in your field. A few research papers get immediate acceptance from peer-reviewed journals. However, rejection is not something to be scared of. It simply means that the journal requires changes before the publication can be considered. Often you will be asked to revise your paper and resubmit it for further feedback. You can also track your paper by the reference number given to you by the journal.
Step 6: The Production Process
When your paper is accepted for publication, it heads into production. Then it is prepared for publishing in the journal that has accepted your research work.
Step 7: Share And Promote
When your research paper is successfully published, access your article on its impact. Sharing research and making notable achievements can get you recognition in the field.
Frequently Asked Questions
How to publish a research paper in an international journal.
To publish a research paper in an international journal, it is necessary to research international journals that specialise in your field. Your paper should adhere to all international standards. Meeting international researchers and developing connections can also be a plus point. Hence, you can submit a cover letter that highlights your work and its need to be published internationally .
What is the average time to publish a research paper?
The process of publication can vary from journal to journal. It can take a few months to over a year.
How to publish a legal research paper?
To submit your legal research paper, you need to choose a solid legal topic. Write a well-structured paper on it, citing any sources and mentioning all references. Lastly, submit it to legal journals that align with your work.
You May Also Like
College life is one of the essential experiences that everyone should have in their life, especially when in their early 20’s.
Use LaTeX for dissertations due to its precise formatting control. However, familiarity and collaboration are needed before deciding.
This article contains information about the dissertation Gantt charts for research, features of Gantt charts, templates of Gantt charts, and their benefits.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works
- Search Search
- CN (Chinese)
- DE (German)
- ES (Spanish)
- FR (Français)
- JP (Japanese)
- Open science
- Booksellers
- Peer Reviewers
- Springer Nature Group ↗
Publish an article
- Roles and responsibilities
- Signing your contract
- Writing your manuscript
- Submitting your manuscript
- Producing your book
- Promoting your book
- Submit your book idea
- Manuscript guidelines
- Book author services
- Book author benefits
- Publish a book
- Publish conference proceedings
Join thousands of researchers worldwide that have published their work in one of our 3,000+ Springer Nature journals.
Step-by-step guide to article publishing
1. Prepare your article
- Make sure you follow the submission guidelines for that journal. Search for a journal .
- Get permission to use any images.
- Check that your data is easy to reproduce.
- State clearly if you're reusing any data that has been used elsewhere.
- Follow our policies on plagiarism and ethics .
- Use our services to get help with English translation, scientific assessment and formatting. Find out what support you can get .
2. Write a cover letter
- Introduce your work in a 1-page letter, explaining the research you did, and why it's relevant.
3. Submit your manuscript
- Go to the journal homepage to start the process
- You can only submit 1 article at a time to each journal. Duplicate submissions will be rejected.
4. Technical check
- We'll make sure that your article follows the journal guidelines for formatting, ethics, plagiarism, contributors, and permissions.
5. Editor and peer review
- The journal editor will read your article and decide if it's ready for peer review.
- Most articles will be reviewed by 2 or more experts in the field.
- They may contact you with questions at this point.
6. Final decision
- If your article is accepted, you'll need to sign a publishing agreement.
- If your article is rejected, you can get help finding another journal from our transfer desk team .
- If your article is open access, you'll need to pay a fee.
- Fees for OA publishing differ across journals. See relevant journal page for more information.
- You may be able to get help covering that cost. See information on funding .
- We'll send you proofs to approve, then we'll publish your article.
- Track your impact by logging in to your account
Get tips on preparing your manuscript using our submission checklist .
Each publication follows a slightly different process, so check the journal's guidelines for more details
Open access vs subscription publishing
Each of our journals has its own policies, options, and fees for publishing.
Over 600 of our journals are fully open access. Others use a hybrid model, with readers paying to access some articles.
Publishing your article open access has a number of benefits:
- Free to access and download
- Reaches a wider global audience
- 1.6x more citations
- 6x more downloads
- 4.9 average Altmetric attention (vs 2.1 subscription)
It's free to publish your article in a subscription journal, but there are fees for publishing open access articles. You'll need to check the open access fees for the journal you choose.
Learn more about open access
Get help with funding.
Many organisations require you to publish your research open access. It's worth checking with your supervisor and colleagues to understand your organisation's approach.
Many funders and institutions will cover your open access publishing fees. To find out if your fees are covered, take a look at our funding agreements .
We also offer discounts for researchers in some geographical regions. See regions with reduced fees
Learn more about funding
Choose a journal.
We have 3,000+ journals to choose from, covering a wide range of topics. The best way to find a relevant journal is to search by keyword.
Once you've chosen a journal, check the submission guidelines to see the open access fees.
Search all journals
Get support.
We offer editing, translation, data presentation and formatting services to help you at each step.
Author support for publishing
Knowledge resources for scientists, author tutorials.
If you have a question about a specific journal, check the submission guidelines. If you still need help, contact us .
- Tools & Services
- Account Development
- Sales and account contacts
- Professional
- Press office
- Locations & Contact
We are a world leading research, educational and professional publisher. Visit our main website for more information.
- © 2024 Springer Nature
- General terms and conditions
- Your US State Privacy Rights
- Your Privacy Choices / Manage Cookies
- Accessibility
- Legal notice
- Help us to improve this site, send feedback.

Guide to Getting Published in Journals
- Why publish in journals?
- Identifying potential journals
- Creating a journal comparison spreadsheet
- Aims & Scope
- Editorial Board
- How different journals approach peer review
- Different open access models
- Interpreting traditional metrics like the Impact Factor
- Alternative metrics
- Ethics and malpractice statements
- Recognising and avoiding predatory journals
- Instructions for authors
- Submitting your paper
Introduction
What is a journal? And why is important to publish your work in one? Finding the right journal for your work can make a big difference to the way it is received, so the process of selecting a journal can be an important one.
There are several key benefits to publishing research in journals:
DISCOVERABILITY
- Publishing in journals can give your work visibility among other researchers in your field, outside of your immediate circle of contacts and colleagues.
- Journals can makes your work more discoverable, as they are already being read by circles of interested readers.
- Journals often have sophisticated distribution networks, placing work into libraries, organisations and institutes, and through letterboxes of readers around the world.
CONTRIBUTING TO THE RECORDS OF RESEARCH IN THE FIELD
- Journal publication helps to preserve your work in the permanent records of research in the field.
- Adding your work to this record involves you in the active research community for a topic, helping to expand your professional network, increasing potential for collaboration and interaction with peers.
- Publishing your work through visible sources helps others to learn. By adding your experiences to the literature of the field, it helps to build the corpus of knowledge in your subject area.
THE BENEFITS OF PEER REVIEW
- The peer review process helps improve the presentation and communication of research. The feedback can help you to frame your arguments in the most effective ways, and may even present valuable new insights into your own work. In addition, the peer review process can also help you reach peers and senior members of the research community by having journal editors, editorial boards and reviewers read your work.
DISSEMINATION AND IMPACT
- Selecting the appropriate journals can help add information to the public discussion of contemporary topics, beyond academic circles.
- You may be required by funding agencies to publish your work in certain journals, as open access, or meeting other criteria stipulated in your grant award.
- As well as the publication itself, particular journals may help you to engage with audiences, and meet requirements to achieve or provide certain impact metrics, evidence of engagement and interaction with your work.
CAREER ADVANCEMENT
- Publishing in particular journals can be an essential component to advance your career, by meeting necessary assessment criteria and output performance targets.
PREVENTING DUPLICATION OF EFFORT
- And last but by no means least, publishing your work can prevent waste and increase efficiencies, by enabling others to build on your achievements or avoid unnecessary duplication of efforts.
As you can see, your choice of journal can make a significant difference to the impact your paper may have. With much to consider, choosing the right journal for your research is both important and difficult.
The different modules will dig further into the ideas presented here, helping you identify the journals that will maximise the potential in your paper, reach the most appropriate audiences, and enhance your career.
- << Previous: Getting started
- Next: Identifying potential journals >>
- Last Updated: Sep 18, 2023 1:28 PM
- URL: https://ifis.libguides.com/journal-publishing-guide
- Hirsh Health Sciences
- Webster Veterinary
Scholarly Publishing
- Choosing a journal to publish in
Evaluating journals
Predatory publishers, journal directories, article analyzers & journal suggesters, undergraduate research journals, tools to measure journal impact.
- Author's rights, copyright & permissions
- Funding & discounts for open access publishing
- Resources for publishing & sharing your work
- Your scholarly profile
Any questions?

How can you identify journals to publish your work in? To start, look at the journals you read, that your colleagues read and publish in, and at who you cite in your work. Is there a pattern to those journals?
There are also additional tools that you can use to identify & evaluate journals you're considering publishing in. Browse this section of the guide to learn more about evaluating a journal; tools to use for finding appropriate journals such as journal directories & article analyzers; tools to measure the impact of a journal; and finding an undergraduate research journal to publish in.
When considering a journal as a potential place to publish, here are some things you might ask yourself:
Is the journal the right place for my work?
- Does the subject matter covered in the journal match your scholarship?
- Do the types of articles published and article length guidelines match with what you want to submit?
- Who is the audience of the journal?
Is this a trusted journal?
Look for journals where you can answer yes to many of the following questions:
- Can you identify the publisher? Are they affiliated with an organization you're familiar with? Is there contact information present?
- Do the affiliations & backgrounds of the editorial board and authors publishing in the journal appear to be appropriate for the subject matter of the journal?
- Are articles peer-reviewed?
- Does the journal have an ISSN, and do articles have DOIs?
- Are the journal's copyright policies & any fees to publish clear? If you'd like to publish open access, are there options?
- Web of Science for journals spanning the humanities, social sciences, and STEM fields (select "Publication Name" from the drop down menu next to the search box)
- Scopus for journals in the social sciences and STEM fields
- SciFinder for journals in Chemistry and related fields (select "Journal" under the References bar)
- PubMed for life sciences, biomedical, clinical, and public/community health journals (choose "Journal" from the drop down menu next to the search box)
- JSTOR for journals spanning the arts, humanities, social sciences, and sciences (scroll down and search using the "Publication Title" search box)
You can also look at the Think Check Submit checklist, use a journal evaluation tool [pdf] , or talk to the library!
"Predatory journals and publishers are entities that prioritize self-interest at the expense of scholarship and are characterized by false or misleading information, deviation from best editorial and publication practices, a lack of transparency, and/or the use of aggressive and indiscriminate solicitation practices."
Grudniewicz, Agnes, et. al. (2019). Predatory journals: No definition, no defence. Nature (London) , 576 (7786), 210–212. https://doi.org/10.1038/d41586-019-03759-y .
Visit the website for the journal and consider the questions in the Evaluating journals section above. Some red flags include:
- The journal is not listed in the Directory of Open Access Journals (DOAJ)
- It's not listed in Ulrichs (Tufts login required), which is an authoritative source on publisher information, including Open Access titles
- It's not widely available within major databases
- You don't recognize previously published authors or members of the editorial board
- The journal isn't affiliated with a university or scholarly organization you are familiar with
- You can't easily identify if they have author processing fees and/or how much they cost.
- The journal doesn't appear professional - look for an impact factor, an ISSN, DOIs for individual articles, and easy to find contact information
- There isn't clear information about a peer-review process, or the journal promises extremely fast turn-around times to publishing that don't allow enough time for review
Use these resources to browse for an appropriate journal for your work, or to research a title that you're considering publishing in.
- Directory of Open Access Journals Use DOAJ to search or browse high-quality, peer-reviewed open access journal titles in all subjects and languages.
- MLA Directory of Periodicals Find out information for thousands of journals and book series that cover literature, literary theory, dramatic arts, folklore, language, linguistics, pedagogy, rhetoric and composition, and the history of printing and publishing.
- Ulrichsweb Ulrichsweb is the authoritative source of bibliographic and publisher information on more than 300,000 periodicals of all types: academic and scholarly journals, Open Access publications, peer-reviewed titles, popular magazines, newspapers, newsletters, and more from around the world.
If you've written an article but aren't sure where to submit it, these tools can help. They use your article's title, keywords, abstract, or full text to find journals that have published similar articles. The description for each resource below notes if it's limited to a specific publisher or discipline.
- B!SON Open Access Journal Finder Enter the title, abstract, and/or references of your paper to find an open access journal suitable to publish in.
- JSTOR Text Analyzer Drag and drop a copy of your article into the Text Analyzer, and the tool will find similar content in JSTOR. Consider the journals that those papers are published in.
- Jane (Journal Author/Name Estimator) Enter your article title and/or abstract of the paper in the box, and click on 'Find journals', 'Find authors' or 'Find Articles'. Jane will then compare your document to millions of documents indexed in Medline to find the best matching journals, authors or articles.
- Elsevier Journal Finder Elsevier Journal Finder uses smart search technology and field-of-research specific vocabularies to match your article to Elsevier journals.
- IEEE Publication Recommender Searches 170+ periodicals and 1500+ conferences from IEEE, provides factors such as Impact Factor and Submission-To-Publication Time.
- ChronosHub Journal Finder Browse, search, filter, sort, and compare more than 40,000 journals to find the right journal without worry about publishing in compliance with your funders’ Open Access policy.
Undergraduate research journals aren't indexed in many of the sources we typically use for finding journals, so lists of academic journals focused on publishing undergraduate research compiled by universities and organizations are good starting places for finding a place to publish your work:
- Undergraduate Research Journal Listing from the Council on Undergraduate Research
- Where to Publish Your Research (compiled by Sacred Heart University)
- Undergraduate Research Journals (compiled by University of Nebraska)
- Undergraduate Research Journals (compiled by CUNY)
- Student Journals hosted on the bepress platform
Some things to consider while looking for an undergraduate research journal to publish your scholarship in include:
- Is there a submission deadline?
- Does the journal appear to be currently publishing?
- Are the journal's copyright policies clear?
- Journal Citation Reports Provides Impact Factors, and Eigenfactors and Article Influence Scores for science and social science journals.
- Scopus Journal Analyzer Use the Journal Analyzer to compare up to 10 Scopus sources on a variety of parameters: CiteScore, SJR (Scimago Journal and Country Rank), and SNIP (source normalised impact per paper)
Read more about these tools & measures on Hirsh Library's Measuring Research Impact guide .
- << Previous: Home
- Next: Author's rights, copyright & permissions >>
- Last Updated: Aug 6, 2024 4:37 PM
- URL: https://researchguides.library.tufts.edu/publishing
We Trust in Human Precision
20,000+ Professional Language Experts Ready to Help. Expertise in a variety of Niches.
API Solutions
- API Pricing
- Cost estimate
- Customer loyalty program
- Educational Discount
- Non-Profit Discount
- Green Initiative Discount1
Value-Driven Pricing
Unmatched expertise at affordable rates tailored for your needs. Our services empower you to boost your productivity.
- Special Discounts
- Enterprise transcription solutions
- Enterprise translation solutions
- Transcription/Caption API
- AI Transcription Proofreading API
Trusted by Global Leaders
GoTranscript is the chosen service for top media organizations, universities, and Fortune 50 companies.
GoTranscript
One of the Largest Online Transcription and Translation Agencies in the World. Founded in 2005.
Speaker 1: Hi, I'm Aisha. You are watching Educational Hub. In this tutorial, we discuss about how to publish manuscripts in journal. First we need to understand that. What is manuscript? A manuscript was, traditionally, any document written by hand, or, once practical typewriters became available, typewritten, as opposed to mechanically printed or reproduced in some indirect or automated way. Why do we need to publish research paper? Your published paper can help in the public understanding of a research question. Publishing helps establish you as an expert in your field of knowledge. Peer-reviewed publication provides evidence that helps in the evaluation of merit of research funding requests. Now going to our main topic. How to publish manuscripts in journal? First open the journal you want to submit the manuscript. As you are the submitting the manuscript for the first time in this journal, so first register yourself with the HEM journal and give all the information such as name, a fealtion, and other required data and then complete the registration process by clicking finish. After completing the registration process go to main page of journal and click on submit the paper click. After clicking on submit the paper the option of username and password will appear. Give your username and password and click on login. Then after click on author desk board and start submitting your paper. While submitting the paper the first page will ask for t-tile of the manuscript and abstract of the manuscript. Step 2. In second step you will upload the manuscript files tables figures files. Step 3. Third step you will add your co-authors and their a fealtion. Step 4. Fourth step you will add details of the manuscript such as number of words figures and tables. Step 5. In last step you will read all the information you added in the online system to remove any mistake and also system will generate manuscript pdf file for proofread. Add keyword and attach cover letter for submission of manuscript. The authors will proofread the manuscript and remove any mistakes if found. If author founds it's ok then author click on submit and the manuscript will be submitted to the journal. The author will receive confirmation email with manuscript id that your paper has been received by the journal. After that the paper is submitted to the journal the editor will check the manuscript and if it is found suitable for publication then it will be sent for review if it is not found then it will be rejected. If the paper is sent for review then the whole process will take months depending upon journals. When the paper is finally accepted then you will receive a acceptance email. When you proofread the manuscript and done ok then it will be published in that final version in few days time. That's it you have a published paper. Wish you best of luck with your manuscript. Thank you for watching. Like share and subscribe my channel for more informative videos about research.

CoB Research Experience for Undergraduates (REU)
- Learn More About a Topic
- Find Scholarly Articles
- Use Scholarly Articles
- Organizing Your Research
- Cite Your Sources
- Libraries Events - Reading Purple
Question? Ask me!

What is a Scholarly Article?
- Defining scholarly articles
- Peer-review
- Other article types
Scholarly articles are articles written by experts and researchers in a field of study to educate or share new discoveries and research. You can use scholarly articles to find out about new innovations, research methodologies, and to dive more deeply into understanding the themes and subtopics of your field of research.
Other names: Scholarly articles are sometimes also called peer-reviewed articles or academic articles. However, not all scholarly articles are peer reviewed.
How to identify a scholarly article:
- Investigate the publisher. Most scholarly articles are published in peer-reviewed journals, e.g. Strategic Management Journal or Journal of Labor Economics
- Browse the structure of the article. Scholarly articles often contain multiples sections and have headers such as introduction, literature review, methodology, discussion, conclusion, etc.
- Check for in-text citations and a large bibliography at the end of the article
- Take note of the language an author uses to write their article. Scholarly articles often use jargon - or specific terms that are used and understood by other professionals in the field. This can make them tricky to understand by others without deeper expertise of the topic.
If you are using a Libraries' database, use a filter to narrow your search to just scholarly articles. This is helpful if you do not want news or magazine articles to show up in your search results.
What is Peer Review?
Some journals require articles to go through a peer review process before accepting it for publication. This means an article is reviewed by other experts in the field to check for accuracy and relevancy before a journal will publish it.
Why Does This Matter?
Many researchers consider peer review articles as a gold standard in research as they have already been evaluated by a panel of experts. Not all scholarly articles are peer reviewed and while both can be helpful to use in your research, you may need to evaluate scholarly articles for quality just like you would a news article before using it in your research project.
How Can I Tell a Scholarly Article from a Peer Reviewed Journal?
Try googling a journal's name. Many times the journal will mention if they require peer review in their about us, but you can also look at their submission criteria or article requirements to find out what process they use.
For example, this is on the about page for the Journal of Business :
"The Journal of Business (JoB) is a peer-reviewed journal with the focus on research articles and case studies in all academic fields of business discipline. The scope of the journal covers the broad range of areas related to business studies including interdisciplinary topics and newly developing areas of business. Submissions comprise research articles – both theoretical and empirical, case studies and reviews of the literature."
When you are searching in a database there may be other articles that you may come across that are not scholarly, but that can be helpful to you in other ways.
Grey Literature
As the name implies, these articles fall into a sort of grey area, they aren't quite scholarly, but they also don't fit anywhere else. The types of articles you might find that fit into this category are:
- Government documents
- Reports, policies, and white papers either produced by an organization, company, or government agency
- Thesis and dissertations, that graduate students produce as part of their degree requirements
- Conference proceedings that researchers produce to share their research with others in more informal ways than through publication
Trade Journals and Magazines
Trade journals are also not the same as a scholarly journal, though they might be easy to confuse. Trade journals are written for professionals in a trade or industry and cover practical topics that impact their career. These articles are written more like news or magazine articles and are meant for working professionals to learn more about innovative technology, relevant news, and current events that impact their industry.
For example, say you decide to pursue a career in managing a fitness center or gym. A relevant trade journal for you would be National Fitness.
How to Get the Most Out of a Scholarly Article
Because of the technical content and level of prior knowledge the author(s) expect their readers to have; being able to get the most out of a scholarly article is a skill that takes time and practice to get good at.
When you are looking for scholarly articles that fulfill your research needs it can be time consuming to read each one top to bottom to determine whether or not it is relevant to your project. Instead try reading the article out of order to determine whether or not a scholarly article is relevant to your project.
- First, read the abstract - if RELEVANT then read the...
- Discussion and/or Conclusion - if still RELEVANT read the...
- Methodology and/or Literature Review - if SOUND, examine the...
- Argument - if BIAS is limited, check the...
- References or bibliography - for other sources you can use in your research
Start by reading a scholarly article in this order. If what you read sounds relevant, then move on to reading the next section. At any time if they article no longer meets your needs, stop reading and move on. Once you complete reading an article out of order, and you determine that it is relevant to your project, it can be helpful to read the article again from top to bottom and annotate your thoughts as you go.
How to Evaluate Scholarly Articles for Quality
Since many scholarly articles go through a peer review process, it can be quicker and easier to evaluate. However, there are still a few things to investigate before using a scholarly article in your research.
Examine the Article
- How current is the article? Don't just look at when the article was published, but also scan the references to make sure the author(s) used current sources.
- Is the methodology used sound? This may require deeper expertise of the methods used in your field to evaluate. Talk with your faculty mentor about the articles you found to help determine what is appropriate for your field.
- Is this relevant to my research topic? Consider if and how the article you found is relevant to your research topic. Does it provide background on your topic or relate to your research in other ways? Even if an article is well cited, it won't be helpful to you if it doesn't connect to your topic.
Investigate Beyond the Article
- Does the journal require peer review?
- Is this journal well regarded by other experts?
- What expertise do the authors have on this topic?
- What other works have these authors published?
- What is it - https://www.nature.com/articles/d41586-023-03974-8
- Tools like Retraction Watch can be helpful
It can be helpful to google the author and publisher to see what you can find out about them.
Evaluating a source is to explore the source. You do not need to answer all the questions above each time you evaluate a source. Over time you will become familiar with well regarded journals and authors in your field. All research skills take practice, the more you use this skill the faster and better you will become at it.
- Retraction Watch A database that tracks retractions of scholarly articles
- Retraction - Example This article was retracted from the journal Environmental Science and Pollution research due to a number of concerns including including but not limited to a compromised peer review process, inappropriate or irrelevant references and citation behavior.
- << Previous: Find Scholarly Articles
- Next: Organizing Your Research >>
- Last Updated: Sep 27, 2024 11:54 AM
- URL: https://guides.lib.jmu.edu/reu
Business Journal Daily | The Youngstown Publishing Company

Cleveland Fed Names Pfajfar to Lead Center for Inflation Research
CLEVELAND – The Federal Reserve Bank of Cleveland has appointed Damjan Pfajfar to lead the bank’s Center for Inflation Research. Pfajfar will oversee a team of economists who study inflation. He also will lead efforts to augment the inflation-related resources the center provides for researchers, policymakers and the public.
Pfajfar joins the bank from the board of governors of the Federal Reserve System, where he served 10 years in the monetary affairs and research and statistics divisions, most recently as group manager in the monetary studies section. His research focuses on inflation, macroeconomics, and monetary economics. He is published widely in top economics journals and has extensive research experience focused on inflation expectations.
Pfajfar holds a BS in economics from the University of Ljubljana, an MS in economics from the University of Warwick, and a PhD in economics from the University of Cambridge.
The Center for Inflation Research publishes research as well as several popular inflation indicators and surveys, such as daily inflation “nowcasting” estimates and the Survey of Firms’ Inflation Expectations (SoFIE). The center also provides educational resources on its Inflation 101 page and organizes inflation-related events, such as the Inflation: Drivers and Dynamics Conference being held at the Cleveland Fed Oct. 24-25.
Copyright 2024 The Business Journal, Youngstown, Ohio.
Related News

Getting Ahead
First Commonwealth Bankers Receive PBA Recognition
September 27
INDIANA, Pa. – Two members of the First Commonwealth Bank team are recipients of the Pennsylvania Bankers Association’s 2024 Future Under 40 Awards. Larissa Murphy, advertising and digital content manager, and Eric Gavazzi, operations manager, were selected for the PBA’s…

Shaklee elected to Phi Kappa Phi Board of Directors
YOUNGSTOWN, Ohio – Ronald Shaklee, professor emeritus of geography at Youngstown University, was recently elected to serve on the board of directors for The Honor Society of Phi Kappa Phi, the nation’s oldest and most selective collegiate honor society for…

WCBA Associate Dean Inducted into Barberton Schools Hall of Fame
YOUNGSTOWN, Ohio – Christina Saenger, associate dean of the Williamson College of Business Administration at Youngstown State University and 1999 graduate of Barberton High School, has been inducted into the Academic Hall of Fame for Barberton City Schools. “Christina is…
Subscribe to The Business Journal
Just $99/year or $10/month will get you full access to all of our articles every month.
Already a subscriber? Log In
Subscribe For Full Access
Flipbooks are available to paid subscribers only. Subscribe now or log in for access.
- Faculty Issues
The Prestige Factor Propping Up Academic Publishers
A federal antitrust lawsuit against a group of megapublishers highlights how academia’s system of rewarding researchers for publishing in certain journals has undermined their leverage.
By Kathryn Palmer
You have / 5 articles left. Sign up for a free account or log in.

The lawsuit a neuroscientist filed earlier this month accuses the six largest academic publishers of colluding to create a business model that diverts money from scientific research “into their pockets.”
DrAfter123/Getty Images
A prolific neuroscientist is accusing some of the same companies that published her work in top-tier peer-reviewed journals of conspiring “to hold the careers of scholars hostage” in the name of maximizing profits, according to a federal antitrust lawsuit filed earlier this month.
It’s the latest instance of scholarly pushback against the $19 billion academic publishing industry , which often relies on the unpaid work of career-focused scholars to generate exclusive—and highly lucrative—content.
The lawsuit aims to upend that system, though experts are skeptical that the legal challenge can spur broader changes on its own. That’s because academics have for decades relied on publishing their research in prestigious journals to advance their careers—and that pressure has only intensified as the job market has become increasingly competitive.
Most Popular
- Universities of Wisconsin fires Joe Gow again
- Almost no education research is replicated, new article shows
- Tenured Jewish prof. says she's fired for pro-Palestine post
“Regardless of its legal validity, the lawsuit is likely to have an explosive societal effect,” Sven Fund, managing director of Reviewer Credits, the peer-review-expert network, wrote in a recent post for The Scholarly Kitchen . “Not just legal issues are at stake, but the legitimacy of business models in scholarly publishing.”
It could take years for the courts to decide if the case against the six largest for-profit publishers of peer-reviewed academic journals—Elsevier, Wolters Kluwer, John Wiley & Sons, Sage Publications, Taylor & Francis and Springer Nature—and their trade association, the International Association of Scientific, Technical and Medical Publishers (STM), has merit. If the publishers are in fact found guilty of colluding to control the publishing market, the lawyers who filed the case want them not only to dissolve their agreements but also to pay out an undetermined amount in damages to make up for scholars’ “artificially deflated wages.”
Regardless of the case’s outcome, the antitrust complaint illuminates broader discussions about who has power in a system that rewards academics with promotions, tenure and research grants for publishing their work in big-name journals with a high impact factor, such as Nature , The Lancet and Advanced Science , which are published by Springer, Elsevier and Wiley, respectively.
The Impact Factor
Some researchers’ career success still hinges on the prestige of the journal publishing their work because of the impact factor metric, which is based in part on how often researchers cite papers published in those journals. Thus, several experts say, the lawsuit also exposes how the scholarly community’s obsession with prestige has undermined its leverage in the market.
Academe’s reliance on such metrics to appraise the value of scholarly work has created a market niche publishers have filled, said Roger Schonfeld, vice president of Organizational Strategy and Libraries, Scholarly Communication, and Museums at Ithaka S+R.
And for better or worse, so long as there’s competition for grant funding and professorships, “someone’s going to have to help them evaluate who to hire and who to fund,” he said. “And whoever does that is going to be in a position of power.”
On paper this system has rewarded the lawsuit’s plaintiff, Lucina Uddin, a professor at the University of California, Los Angeles, who has published more than 175 academic articles and provided peer-review services to 150-plus journals, including those published by the companies she’s suing.
But like most academics competing for jobs in a saturated market, she’s done nearly all of that work for free. Meanwhile, for-profit publishers are using that work to turn massive profits by charging authors hundreds—or even thousands—of dollars in article processing fees and selling pricey journal subscriptions to academic libraries. Scholars who can’t pay don’t get their work published.
That imbalance of power is at the heart of Uddin’s suit, which accuses the six publishers and STM of colluding to create a “scheme” that’s not only hampered scientific advances but also “unlawfully divert[ed] billions of taxpayer dollars every year from science” into the companies’ coffers. Uddin filed the lawsuit on behalf of other scientists and scholars. If the court certifies its class action status, then the suit could include hundreds of thousands of additional plaintiffs.
Editors' Picks
- How Much Do Students Really Read?
- Re: Your Recent Email to Your Professor
- Facing Budget Cuts and Faculty Pushback, Brandeis President Resigns
Is It a ‘Scheme’?
The suit alleges that the six academic publishers—which own 53 percent of academic journals—have been able to carry out the so-called scheme by forming a “cartel” through STM and fixing the price of peer-review services at zero. Those journals received more than $10 billion in revenue in 2023.
In so doing, the suit says, publishers “agreed to coerce scholars into providing their labor for nothing by expressly linking their unpaid labor with their ability to get their manuscripts published in the publisher defendants’ journals,” which can boost a researcher’s curriculum vitae in what the complaint characterizes as the “‘publish or perish’ world of academia.”
The suit also accuses the publishers of agreeing not to compete with one another by requiring scholars to submit their manuscripts to a single journal at a time and prohibiting them from sharing findings while their manuscripts are under peer review. That enables publishers to “behave as though the scientific advancements set forth in the manuscripts are their property,” it reads.
The three elements of the alleged scheme work in concert “to create a set of rules that cement the [publishers’] market dominance and maximize the amount of money they can divert from scientific research into their pockets,” the suit argues. “The Scheme has been remarkably profitable for the Publisher Defendants, while doing tremendous damage to science and the public interest.”
All six of the publishers named in the suit declined to comment on the case to Inside Higher Ed , though a spokesperson for Wiley said in an email that the publisher believes the claims are “without merit.”
But Christopher Jon Sprigman, a law professor and co-director of the Engelberg Center on Innovation Law and Policy at New York University School of Law, who specializes in antitrust law, isn’t so sure.
“These rules are intensely anticompetitive,” he said. “As the complaint hints, to join the [STM] and stay in good standing with the industry, [publishers] have to essentially agree to impose the rules.”
‘Their Own Worst Enemies’
Although the judicial system will get the final say, Sprigman said the issues the suit raises are a reminder that by focusing on the profile of a particular journal to judge the value of a scholar’s research, academics have made themselves “their own worst enemies.”
“Science and knowledge are paying an enormous tax to commercial publishers for this prestige economy for which they depend on commercial publishers,” he said. “If academics generally had other prestige mechanisms they could refer to—such as actually reading the articles more carefully when making tenure decisions—they wouldn’t depend so much on the publishers.”
Dean Harvey, one of the lawyers representing Uddin, told Inside Higher Ed that at the very least he’s hopeful the case will “trigger more conversations among academics about alternative systems,” and that inciting competition between megapublishers “will drive solutions we can’t even anticipate right now.”
Academic-led backlash against for-profit publishers has been building for years.
In the early 2010s, a group of scientists launched the ongoing Cost of Knowledge protest movement and called on academics to boycott Elsevier’s business practices by refusing to publish, peer review or serve on the editorial boards of their journals; more than 20,000 people have signed the petition to date. In 2019, the University of California system canceled its contract with Elsevier after failed negotiations over the company’s open-access fees and paywalls, though they have since agreed to a new contract .
During the pandemic, a years-old idea to pay peer reviewers for their labor resurged among some scholars. And this summer, authors who had published with Wiley and Taylor & Francis sounded the alarm when the publishers made millions by selling their data to tech companies that are using it to help train proprietary AI models.
But Dave Hansen, executive director of the Authors Alliance and a lawyer with copyright expertise, said he’s not convinced that forcing the largest publishing companies to pay peer reviewers or compete for articles “is going to change much about what promotion and tenure committees think of those journals or those publishers.”
Instead, he said, “That change has to come from within the universities,” which need “to get serious about article-level metrics and evaluating the scholarship divorced from the idea that it’s in any particular journal.”
To that end, Hansen added, Uddin’s case, in illustrating the way some publishers have the alleged market power to “twist the way scholarship gets published,” is “a fantastic vehicle for promoting these kinds of conversations.”

Reimagining the Academic Calendar for Student Success
Starting in January, Montclair State University will operate on a three-term model, increasing lea
Share This Article
More from research.

‘Red Wedding’: Storied Stanford Creative Writing Program Laying Off Lecturers
The university says creative writing faculty recommended returning its Jones Lectureships to their “original intent”

When a U.S. Presidential Candidate Is Called a ‘DEI Hire’
The first Black woman to be a major party’s nominee for president is facing conservative attacks on her race.

Taylor & Francis AI Deal Sets ‘Worrying Precedent’ for Academic Publishing
The publisher didn’t give authors any notice before selling access to its data to Microsoft for $10 million.
- Become a Member
- Sign up for Newsletters
- Learning & Assessment
- Diversity & Equity
- Career Development
- Labor & Unionization
- Shared Governance
- Academic Freedom
- Books & Publishing
- Financial Aid
- Residential Life
- Free Speech
- Physical & Mental Health
- Race & Ethnicity
- Sex & Gender
- Socioeconomics
- Traditional-Age
- Adult & Post-Traditional
- Teaching & Learning
- Artificial Intelligence
- Digital Publishing
- Data Analytics
- Administrative Tech
- Alternative Credentials
- Financial Health
- Cost-Cutting
- Revenue Strategies
- Academic Programs
- Physical Campuses
- Mergers & Collaboration
- Fundraising
- Research Universities
- Regional Public Universities
- Community Colleges
- Private Nonprofit Colleges
- Minority-Serving Institutions
- Religious Colleges
- Women's Colleges
- Specialized Colleges
- For-Profit Colleges
- Executive Leadership
- Trustees & Regents
- State Oversight
- Accreditation
- Politics & Elections
- Supreme Court
- Student Aid Policy
- Science & Research Policy
- State Policy
- Colleges & Localities
- Employee Satisfaction
- Remote & Flexible Work
- Staff Issues
- Study Abroad
- International Students in U.S.
- U.S. Colleges in the World
- Intellectual Affairs
- Seeking a Faculty Job
- Advancing in the Faculty
- Seeking an Administrative Job
- Advancing as an Administrator
- Beyond Transfer
- Call to Action
- Confessions of a Community College Dean
- Higher Ed Gamma
- Higher Ed Policy
- Just Explain It to Me!
- Just Visiting
- Law, Policy—and IT?
- Leadership & StratEDgy
- Leadership in Higher Education
- Learning Innovation
- Online: Trending Now
- Resident Scholar
- University of Venus
- Student Voice
- Academic Life
- Health & Wellness
- The College Experience
- Life After College
- Academic Minute
- Weekly Wisdom
- Reports & Data
- Quick Takes
- Advertising & Marketing
- Consulting Services
- Data & Insights
- Hiring & Jobs
- Event Partnerships
4 /5 Articles remaining this month.
Sign up for a free account or log in.
- Sign Up, It’s FREE
Physical Review Research
- Collections
- Editorial Team
- Open Access
Demonstration of Maxwell demon-assisted Einstein-Podolsky-Rosen steering via superconducting quantum processor
Z. t. wang, ruixia wang, peng zhao, z. h. yang, yun-hao shi, kaixuan huang, kai xu, yong-sheng zhang, heng fan, s. p. zhao, meng-jun hu, and haifeng yu, phys. rev. research 6 , l032073 – published 26 september 2024.
- No Citing Articles
Supplemental Material
- ACKNOWLEDGMENTS
The concept of Maxwell demon plays an essential role in connecting thermodynamics and information theory, while entanglement and nonlocality are fundamental features of quantum theory. Given the rapid advancements in the field of quantum information science, there is a growing interest and significance in investigating the connection between Maxwell demon and quantum correlation. The majority of research endeavors thus far have been directed toward the extraction of work from quantum correlation through the utilization of Maxwell demon. Recently, a novel concept called Maxwell demon-assisted Einstein-Podolsky-Rosen (EPR) steering has been proposed, which suggests that it is possible to simulate quantum correlation by doing work. This seemingly counterintuitive conclusion is attributed to the fact that Alice and Bob need classical communication during EPR steering task, a requirement that does not apply in the Bell test. In this study, we demonstrate Maxwell demon-assisted EPR steering with superconducting quantum circuits. By compiling and optimizing a quantum circuit to be implemented on a 2D superconducting chip, we were able to achieve a steering parameter of S 2 = 0.770 ± 0.005 in the case of two measurement settings, which surpasses the classical bound of 1 / 2 by 12.6 standard deviations. In addition, experimental observations have revealed a linear correlation between the nonlocality demonstrated in EPR steering and the work done by the demon. Considering the errors in practical operation, the experimental results are highly consistent with theoretical predictions. Our findings not only suggest the presence of a Maxwell demon loophole in the EPR steering, but also contribute to a deeper comprehension of the interplay between quantum correlation, information theory, and thermodynamics.
- Received 26 February 2024
- Accepted 2 September 2024
DOI: https://doi.org/10.1103/PhysRevResearch.6.L032073
Published by the American Physical Society under the terms of the Creative Commons Attribution 4.0 International license. Further distribution of this work must maintain attribution to the author(s) and the published article's title, journal citation, and DOI.
Published by the American Physical Society
Physics Subject Headings (PhySH)
- Research Areas
Authors & Affiliations
- 1 Beijing Academy of Quantum Information Sciences , Beijing 100193, China
- 2 Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences , Beijing 100190, China
- 3 School of Physical Sciences, University of Chinese Academy of Sciences , Beijing 100190, China
- 4 CAS Center for Excellence in Topological Quantum Computation, UCAS, Beijing 100190, China
- 5 Laboratory of Quantum Information, University of Science and Technology of China , Hefei 230026, China
- 6 Synergetic Innovation Center of Quantum Information and Quantum Physics, University of Science and Technology of China , Hefei 230026, China
- 7 Hefei National Laboratory, University of Science and Technology of China , Hefei 230088, China
- 8 Songshan Lake Materials Laboratory , Dongguan 523808, China
- * These authors contributed equally to this work.
- † Contact author: [email protected]
- ‡ Contact author: [email protected]
- § Contact author: [email protected]
Article Text
Vol. 6, Iss. 3 — September - November 2024
Subject Areas
- Condensed Matter Physics
- Quantum Physics

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
The Maxwell Demon-assisted EPR steering task. (a) Bob receives qubits sent by Alice, meanwhile the demon sneaks into Bob's device along with the qubits. All qubits are prepared in the same pure state. (b) The demon gains access to the information of the measurement setting σ ̂ k by entangling itself with Bob's random basis generator. The demon then randomly rotates the qubits into one of the eigenstates of σ ̂ k before Bob performs measurements. (c) Bob announces his measurement setting σ ̂ k to Alice, meanwhile the demon secretly sends an additional 1 bit of information to Alice, which is mixed in the communication channel between Bob and Alice. The 1 bit information tells Alice which eigenstate of σ ̂ k it has chosen to rotate the qubits into. (d) Based on the information provided by Bob and the demon, Alice responds to Bob regarding her result A k . (a)–(d) are repeated multiple times, and Bob combines the results to calculate the steering parameter S m . If S m exceeds a certain classical bound, then Bob becomes convinced that Alice has the ability to remotely manipulate his state. It should be noted that the demon may not do operations for every run, and in this case, Alice answers Bob randomly.
(a) Quantum circuit representation of Maxwell demon-assisted EPR steering with two measurement settings { σ ̂ Z , σ ̂ X } . (b) Quantum circuit representation without the Maxwell demon. (c) Schematic diagram of a superconducting quantum processor in which the physical qubits q 0 − q 4 correspond to the logical qubits q 0 − q 4 in the quantum circuit. In the practical experiment, we execute circuit (a) or circuit (b) with a certain probability p to simulate the case that the demon performs operations probabilistically.
(a) Experimental results verify the linear relationship between the probability of demon operation p and the steering parameter S 2 , as predicted by theory. The largest value of S 2 is measured to be 0.770 ± 0.005 , which exceeds the classical bound of 1 / 2 by 12.6 standard deviations. (b) Simulation results show that the deviation between the experiment and the ideal case is primarily caused by depolarizing noise in the actual circuits.
Sign up to receive regular email alerts from Physical Review Research
Reuse & Permissions
It is not necessary to obtain permission to reuse this article or its components as it is available under the terms of the Creative Commons Attribution 4.0 International license. This license permits unrestricted use, distribution, and reproduction in any medium, provided attribution to the author(s) and the published article's title, journal citation, and DOI are maintained. Please note that some figures may have been included with permission from other third parties. It is your responsibility to obtain the proper permission from the rights holder directly for these figures.
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.
- Appointments
- Our Providers
- For Physicians
Clinical cancer research in the U.S. increasingly dominated by pharmaceutical industry sponsors
SEATTLE – September 27, 2024 – Researchers at Fred Hutch Cancer Center identified a substantial increase over the past decade in the proportion of patients with cancer in the U.S. who participate in pharmaceutical industry sponsored clinical trials compared to those conducted with federal government support. Published in The Journal of Clinical Oncology and presented at the ASCO Quality Care Symposium , these findings reveal trends of underinvestment in federally funded studies, flat enrollment counts in federally funded studies over more than a decade and a growing reliance on industry to conduct cancer research.
The study showed that between 2018 and 2022, industry sponsored trials enrolled over eight times more patients than federally sponsored trials. For adult trials, industry enrolled nearly 10 times more patients. These ratios have also grown substantially over time. Compared to a decade earlier (2008-2012), the proportion of enrollments attributable to industry vs. federal support increased from 4.8 to 9.6 in adults, and from 0.7 to 2.3 in children. The study was conducted in more than 26,000 cancer clinical studies in adults and children using data from clinicaltrials.gov.
The magnitude of difference revealed by the study, which was the first to comprehensively evaluate the comparative roles of industry and federal sponsors in supporting patient enrollments to cancer studies, took the researchers by surprise.
“We recognized that industry was playing an increasing role in cancer clinical research compared to decades ago,” said Joseph Unger, PhD, MS , a health services researcher and biostatistician at Fred Hutch and lead author of the study. “But we didn’t realize the difference was this dramatic.”
Federally funded cancer research studies play a critical role due to their demographic diversity. For example, three times more Black participants were enrolled in federally supported research than in industry funded research. In addition to investigating cancer treatments, federally funded research covers a broad set of clinical research questions, such as combining treatment modalities or examining whether approved drugs work in other cancers. Industry funded cancer research predominately aims to support new drug approvals.
“Underinvestment in federally funded cancer clinical research results in missed opportunities for scientific, clinical and populations advances,” said Unger. “Federally funded cancer clinical trials have contributed to more than 14 million life years gained over four decades and have helped improve clinical care guidelines for patients.”
Despite the need for more federally funded cancer clinical research trials, researchers are quick to point out the importance of both federally funded and industry funded cancer clinical trials.
“Industry investment in cancer clinical research has accelerated precision oncology and cancer immunotherapy tremendously,” commented Unger, noting the important role of industry in new drug discovery. “However, with increased federal investment in cancer research as well, we could see even greater strides in treatment options for patients with cancer.”
The study was funded by Fred Hutch. Unger reports the following disclosures: serves as a consultant for the American Cancer Society, AstraZeneca and Loxo Oncology/ Eli Lilly Company, which is unrelated to the current work.
Media Contact: Claire Hudson [email protected] 206-667-2210
Fred Hutchinson Cancer Center
Fred Hutchinson Cancer Center unites individualized care and advanced research to provide the latest cancer treatment options while accelerating discoveries that prevent, treat and cure cancer and infectious diseases worldwide.
Based in Seattle, Fred Hutch is an independent, nonprofit organization and the only National Cancer Institute-designated cancer center in Washington. We have earned a global reputation for our track record of discoveries in cancer, infectious disease and basic research, including important advances in bone marrow transplantation, immunotherapy, HIV/AIDS prevention and COVID-19 vaccines. Fred Hutch operates eight clinical care sites that provide medical oncology, infusion, radiation, proton therapy and related services. Fred Hutch also serves as UW Medicine’s cancer program.
- Public Health Sciences
- biostatistics
- health economics
- Joseph Unger
- Patients & Families
- Donors & Volunteers
- Science Professionals
- Medical Professionals
- Industry Partnerships
- Press Release
For the Media
- Contact Media Relations
- News Releases
- Media Coverage
- About Fred Hutch
- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Braunwald's Corner
- ESC Guidelines
- EHJ Dialogues
- Issue @ a Glance Podcasts
- CardioPulse
- Weekly Journal Scan
- European Heart Journal Supplements
- Year in Cardiovascular Medicine
- Asia in EHJ
- Most Cited Articles
- ESC Content Collections
- Author Guidelines
- Submission Site
- Why publish with EHJ?
- Open Access Options
- Submit from medRxiv or bioRxiv
- Author Resources
- Self-Archiving Policy
- Read & Publish
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Journals Career Network
- About European Heart Journal
- Editorial Board
- About the European Society of Cardiology
- ESC Publications
- War in Ukraine
- ESC Membership
- ESC Journals App
- Developing Countries Initiative
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, conclusions, acknowledgements, supplementary data, declarations, congenital heart defects in children born after assisted reproductive technology: a conartas study.
Christina Bergh and Ulla-Britt Wennerholm contributed equally to the study.
- Article contents
- Figures & tables
- Supplementary Data
Nona Sargisian, Max Petzold, Eva Furenäs, Mika Gissler, Anne Lærke Spangmose, Sara Malchau Lauesgaard, Signe Opdahl, Anja Pinborg, Anna-Karina A Henningsen, Kjersti Westvik-Johari, Kristiina Rönö, Christina Bergh, Ulla-Britt Wennerholm, Congenital heart defects in children born after assisted reproductive technology: a CoNARTaS study, European Heart Journal , 2024;, ehae572, https://doi.org/10.1093/eurheartj/ehae572
- Permissions Icon Permissions
Children born after assisted reproductive technology (ART) have worse perinatal outcomes compared with spontaneously conceived children. This study investigates whether children conceived after ART have a higher risk of congenital heart defects (CHDs) compared with children born after spontaneous conception (SC).
All 7 747 637 liveborn children in Denmark (1994–2014), Finland (1990–2014), Norway (1984–2015), and Sweden (1987–2015), where 171 735 children were conceived after ART, were included. National ART and medical birth registry data were cross-linked with data from other health and population registries. Outcomes were major CHDs, severe CHDs, 6 hierarchical CHD lesion groups, and 10 selected major CHDs, diagnosed prenatally or up to 1 year of age (Denmark, Finland, and Sweden) and prenatally or at birth (Norway). The association between ART and CHDs was assessed with multivariable logistic regression analysis, with adjustment for available confounders.
Major CHDs were detected in 3159 children born after ART (1.84%) and in 86 824 children born after SC [1.15%; adjusted odds ratio (AOR) 1.36; 95% confidence interval (CI) 1.31–1.41]. Risk was highest in multiples, regardless of conception method. Severe CHDs were detected in 594 children born after ART (0.35%) and in 19 375 children born after SC (0.26%; AOR 1.30; 95% CI 1.20–1.42). Risk was similar between ICSI and IVF and between frozen and fresh embryo transfer.
Assisted reproductive technology–conceived children have a higher prevalence of major CHDs, being rare, but severe conditions. The absolute risks are, however, modest and partly associated with multiple pregnancies, more prevalent in ART.

The main findings were that assisted reproductive technology (ART) was associated with an increased risk of major congenital heart defects (CHDs) as well as severe CHDs in liveborn children with follow-up to 1 year of age, compared with spontaneous conception (SC). Children born from a multifetal pregnancy had the highest risk of CHDs, but ART was also associated with an increased risk in singletons. No significant difference was found between singletons born after intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF) or between fresh and frozen embryo transfer. AOR, adjusted odds ratio; ASD, atrial septal defect; CI, confidence interval; FET, frozen embryo transfer; HLHS, hypoplastic left heart syndrome; VSD, ventricular septal defect.
See the editorial comment for this article ‘Assisted reproductive technology and heart defects: what’s real and what’s not?’, by N. Auger, https://doi.org/10.1093/eurheartj/ehae549 .
The field of reproductive medicine is growing due to advancements in assisted reproductive technology (ART). 1 More than 10 million children are so far conceived through ART worldwide, and currently, 3.0% of children in Europe and 2.3% in the USA are born after ART. 2–4
Health outcomes for children born after ART continue to be in focus due to the widespread use of ART, the increasing number of children born after ART, and the fast development of new ART procedures. Many systematic reviews, meta-analyses, and large observational studies show an association between ART and low birth weight (LBW) and preterm birth (PTB). Although multiple births are the most important cause of the increased risk of PTB and LBW in ART-conceived children compared with children born after spontaneous conception, risk of these outcomes is also higher in ART-conceived singletons. 5–8 Several meta-analyses and original studies have found that birth defects are more common in children born after ART compared with children born after spontaneous conception. Estimates of excess risk range between 30% and 70%. 7–10 A systematic review, including 29 studies, by Qin et al. 7 reported birth defects in 5.7% [95% confidence interval (CI) 4.7%–6.9%] of singletons born after in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) and in 3.9% (95% CI 3.1%–4.8%) of singletons born after spontaneous conception. Most studies show no difference in the frequency of birth defects in children born after IVF vs. ICSI, nor in children born after fresh vs. frozen-thawed embryo transfer (FET). 10 , 11 The increased rate of birth defects found among children born after ART could partly be explained by parental subfertility according to a recent study from Australia. 12
Congenital heart defects (CHDs) refer to structural anomalies of the heart and the intrathoracic vessels present during pregnancy or at birth. 13 Congenital heart defects are the most commonly occurring birth defects, accounting for ∼50% of all major birth defects, affecting ∼1%–2% of children in the general population. 14–17 Although many CHDs are recorded at birth and a few later in life, the true incidence of CHDs remains largely unknown due to lack of identification in pregnancies ending in miscarriages, terminations, or stillbirths. 13 , 18 , 19 Heart defects are a major paediatric health concern and remain the leading cause of mortality from birth defects. 20 Several systematic reviews and cohort studies have found an increased risk of CHDs in children born after ART. 21–23 A recent review including 41 cohort and case–control studies with 25 856 children born after ART showed an increased risk of CHDs in the ART-conceived group as compared with spontaneous conception [SC; pooled odds ratio (OR) 1.45; 95% CI 1.20–1.76]. 21 Congenital heart defects are more common in twins compared with singletons, 24 , 25 and a recent study found that most of the association between ART and CHD was mediated by twinning. 26 For specific CHDs, conflicting results have been reported. 21 , 27
Using nationwide data from four Nordic countries, we assessed the risk of major CHDs in ART-conceived liveborn children compared with children born after spontaneous conception. We further explored if risk of any specific CHD was increased in children born after ART and if specific assisted reproductive techniques were associated with CHDs.
Data sources
The Committee of Nordic ART and Safety (CoNARTaS) was established in 2008 to evaluate short- and long-term health consequences of ART in children and their mothers. 28 The unique personal identity number, assigned to all residents in the Nordic countries, enabled individual-level data linkage between children and their mothers and between different registries. 29 Data from national ART registries, medical birth registries (MBRs), national patient registries (NPRs), cause of death registries, and population registries were cross-linked. Data for this study were obtained from Denmark (1994–2014), Finland (1990–2014), Norway (1984–2015), and Sweden (1987–2015). Due to incompleteness of the Swedish ICD-8 codes for 1985 and 1986, we chose to exclude these 2 years for Sweden. Details on our cohort and the registries used are given in Supplementary data online , Table S1 .
Study population
Inclusion criteria were all liveborn singletons, twins, and higher-order multiples born after ART and SC (i.e. conception without ART) during the study period. Assisted reproductive technology is defined according to Zegers-Hochschild et al. , 30 i.e. ‘all interventions that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of reproduction’.
Stillbirths were excluded due to low data quality on birth defects in these pregnancies. Information on terminations of pregnancies due to birth defects was not available.
Outcome variables
We defined children with CHDs as having a CHD diagnosis at birth and up to 1 year of age, in the MBR, NPR, or cause of death registry. For Norway, follow-up stopped at birth. All diagnoses were coded according to the International Classification of Diseases (ICD), Eighth Revision (ICD-8); Ninth Revision (ICD-9); and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). 31
The primary outcome was major CHDs diagnosed up to 1 year of age. Major CHDs were defined according to the European Concerted Action on Congenital Anomalies and Twins (EUROCAT) as ICD-10 Q20-Q26, and with corresponding ICD-8 and ICD-9 codes 32 (see Supplementary data online , Table S2 ). In line with EUROCAT, we did not consider minor defects such as patent ductus arteriosus in preterm babies (<37 weeks) and isolated patent foramen ovale as major CHDs. 33
Secondary outcomes were (i) severe CHDs, (ii) CHDs according to the hierarchical classification of Botto et al. , 34 and (iii) 10 selected specific major CHDs. The severe CHD subgroup consists of 26 major CHDs classified as severe CHDs according to EUROCAT, which is based on Dolk et al. 35 (see Supplementary data online , Table S2 ). The Botto classification of CHDs has been designed for use in aetiological and observational studies. 34 , 36 , 37 Here, all CHDs are grouped in a hierarchical arrangement into six lesion groups, lesion group 1 being the most severe (see Supplementary data online , Table S3 ). Lesion group 1 includes conotruncal defects (such as tetralogy of Fallot, transposition of the great vessels, common arterial trunk, and aortopulmonary septal defects); lesion group 2 includes non-conotruncal defects [such as endocardial cushion defects, a common ventricle, and hypoplastic left heart syndrome (HLHS)]; lesion group 3 coarctation of the aorta; lesion group 4 ventricular septal defects (VSDs); and lesion group 5 atrial septal defects. Lesion group 6 includes all other CHD diagnoses and circulatory system anomalies not included in lesion groups 1–5. In individuals with several CHDs, only the most severe CHD was included. The 10 selected specific major CHDs were common arterial truncus, double outlet right ventricle, complete transposition of the great vessel, isomerism of atrial appendages with asplenia or polysplenia, atrioventricular septal defect, tetralogy of Fallot, pulmonary valve atresia, tricuspid atresia and stenosis, HLHS, and coarctation aortae (see Supplementary data online , Table S2 ). These specific CHDs were mainly selected based on medical knowledge and previous studies. 38 , 39
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. 40
Statistical methods
Logistic regression analysis was performed estimating crude and adjusted ORs for CHD, with 95% CIs. We estimated the risk of major CHDs, severe CHDs, the 6 CHD lesion groups, and the 10 selected major CHDs within 1 year of age for Danish, Finnish, and Swedish children, and by the time of birth for Norwegian children since data on CHDs in Norway were available solely from the birth records.
Children born after ART were compared with children conceived spontaneously. Multiples included twins, triplets, and higher-order multiple births. Moreover, ART-conceived singletons were compared with spontaneously conceived singletons, ART-conceived multiples with spontaneously conceived multiples, ART-conceived multiples with ART-conceived singletons, and spontaneously conceived multiples with spontaneously conceived singletons. Where data were available (Denmark, Norway, and Sweden), we further compared singletons conceived using ICSI with singletons conceived using conventional IVF, and singletons conceived using frozen embryos with singletons conceived using fresh embryos. Singletons born after ICSI, IVF, or frozen embryo transfer (FET) were also compared with spontaneously conceived singletons.
The choice of covariates was based on previous studies and a thorough consideration of the existing knowledge of risk factors. 38 , 41–44 Adjustments were made for child’s year of birth (continuous variable), country of birth, maternal age at delivery (continuous variable), parity (nulliparous/parous), maternal smoking, maternal pre-gestational diabetes types 1 and 2, and history of maternal non-chromosomal CHDs. There were few missing data for most of these covariates except for smoking ( Table 1 ). Missing data for smoking were set as no smoking in the main analysis. No imputation was conducted for other missing data. The significance level was set to 5%. All data analyses were calculated using STATA (version 18).
Characteristics of study population by mode of conception and country of birth (Denmark 1994–2014, Finland 1990–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | All countries . | Denmark . | Finland . | Norway . | Sweden . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | = 7 747 637 liveborn children . | = 1 355 267 liveborn children . | = 1 497 341 liveborn children . | = 1 865 484 liveborn children . | = 3 029 545 liveborn children . | |||||
| . | ART . | SC . | ART . | SC . | ART . | SC . | ART . | SC . | ART . | SC . |
| . | = 171 735 . | = 7 575 902 . | = 45 801 . | = 1 309 466 . | = 29 691 . | = 1 467 650 . | = 34 042 . | = 1 831 442 . | = 62 201 . | = 2 967 344 . |
| Year of birth, n (%) | ||||||||||
| 1984–90 | 1610 (0.9) | 898 290 (11.9) | 0 | 0 | 53 (0.2) | 65 393 (4.5) | 877 (2.6) | 383 757 (21.0) | 680 (1.1) | 449 140 (15.1) |
| 1991–95 | 11 681 (6.8) | 1 321 614 (1.4) | 1299 (2.8) | 138 138 (10.6) | 2864 (9.7) | 321 476 (21.9) | 2464 (7.2) | 297 356 (16.2) | 5054 (8.1) | 564 644 (19.0) |
| 1996–2000 | 28 709 (16.7) | 1 333 763 (17.6) | 8532 (18.6) | 327 305 (25.0) | 6941 (23.4) | 283 333 (19.3) | 4313 (12.7) | 292 015 (15.9) | 8923 (14.4) | 431 110 (14.5) |
| 2001–05 | 36 092 (21.0) | 1 332 414 (17.6) | 11 798 (25.8) | 313 094 (23.9) | 6359 (21.4) | 276 400 (18.8) | 6586 (19.4) | 276 926 (15.1) | 11 349 (18.3) | 465 994 (15.7) |
| 2006–10 | 45 519 (26.5) | 1 414 225 (18.7) | 13 156 (28.7) | 310 143 (23.7) | 6974 (23.5) | 291 949 (19.9) | 9411 (27.7) | 293 014 (16.0) | 15 978 (25.7) | 519 119 (17.5) |
| 2011–15 | 48 124 (28.0) | 1 275 596 (16.8) | 11 016 (24.1) | 220 786 (16.9) | 6500 (21.9) | 229 099 (15.6) | 10 391 (30.5) | 288 374 (15.8) | 20 217 (32.5) | 537 337 (18.1) |
| Birth weight, (%) | ||||||||||
| Very low, <1500 g | 5215 (3.1) | 54 992 (0.7) | 1566 (3.5) | 10 331 (0.8) | 845 (2.9) | 9504 (0.7) | 1233 (3.6) | 14 584 (0.8) | 1571 (2.5) | 20 573 (0.7) |
| Low, <2500, g | 27448 (16.0) | 319 685 (4.2) | 8463 (18.5) | 61 229 (4.7) | 4783 (16.1) | 56 334 (3.8) | 6103 (17.9) | 81 588 (4.5) | 8099 (13.0) | 120 534 (4.1) |
| Macrosomia, ≥4000g | 20 516 (12.0) | 1 425 820 (18.8) | 4725 (10.3) | 238 103 (18.2) | 3521 (11.9) | 272 464 (18.6) | 3933 (11.6) | 355 748 (19.4) | 8337 (13.4) | 559 505 (18.9) |
| Missing data on birth weight | 755 (0.4) | 36 271 (0.5) | 463 (1.0) | 24 570 (1.9) | 13 (0.04) | 3405 (0.2) | 35 (0.1) | 1556 (0.1) | 244 (0.4) | 6740 (0.2) |
| Gestational age, (%) | ||||||||||
| Extremely preterm, <28 + 0 weeks | 1984 (1.2) | 20 482 (0.3) | 626 (1.4) | 3640 (0.3) | 325 (1.1) | 3613 (0.3) | 455 (1.3) | 5352 (0.3) | 578 (0.9) | 7877 (0.3) |
| Very preterm, <32 + 0 weeks | 6120 (3.6) | 64 821 (0.9) | 1869 (4.1) | 12 178 (0.9) | 970 (3.3) | 10 834 (0.7) | 1400 (4.1) | 16 984 (0.9) | 1881 (3.0) | 24 825 (0.8) |
| Preterm, <37 + 0 weeks | 31 367 (18.3) | 436 471 (5.8) | 9264 (20.2) | 78 548 (6.0) | 5725 (19.3) | 77 520 (5.3) | 6867 (20.2) | 108 524 (5.9) | 9511 (15.3) | 171 879 (5.8) |
| Post-term, ≥42 + 0 weeks | 3296 (5.3) | 513 869 (6.8) | 1491 (3.3) | 82 959 (6.3) | 924 (3.1) | 67 415 (4.6) | 1557 (4.6) | 158 092 (8.6) | 3296 (5.3) | 205 403 (6.9) |
| Missing data on gestational age | 652 (0.4) | 128 984 (1.7) | 298 (0.7) | 28 949 (2.2) | 57 (0.2) | 6755 (0.5) | 246 (0.7) | 89 599 (4.9) | 51 (0.1) | 3681 (0.1) |
| Plurality, (%) | ||||||||||
| Singletons | 127 275 (74.1) | 7 380 916 (97.4) | 31 077 (67.9) | 1 269 638 (97.0) | 22 044 (74.2) | 1 431 723 (97.6) | 24 117 (70.8) | 1 783 926 (97.4) | 50 037 (80.4) | 2 895 629 (97.6) |
| Twins | 42 459 (24.7) | 190 426 (2.5) | 14 332 (31.3) | 38 810 (3.0) | 7198 (24.2) | 35 222 (2.4) | 9364 (27.5) | 46 356 (2.5) | 11 565 (18.6) | 70 038 (2.4) |
| Triplets and higher | 2001 (1.2) | 4504 (0.1) | 392 (0.9) | 1018 (0.1) | 449 (1.5) | 705 (0.1) | 561 (1.7) | 1160 (0.1) | 599 (1.0) | 1621 (0.1) |
| Missing data on plurality, | 0 | 56 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 |
| Male sex, (%) | 87 785 (51.1) | 3 887 791 (51.3) | 23 211 (50.7) | 672 079 (51.3) | 15 197 (51.2) | 749 952 (51.1) | 17 456 (51.3) | 940 571 (51.4) | 31 921 (51.3) | 1 525 189 (51.4) |
| Missing data on sex, | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Age at delivery years, mean (SD) | 33.4 (4.2) | 29.3 (5.2) | 33.1 (4.2) | 29.7 (4.9) | 33.4 (4.7) | 29.3 (5.3) | 33.2 (4.7) | 28.8 (5.2) | 33.7 (4.2) | 29.3 (5.2) |
| Age at delivery (years), (%) | ||||||||||
| <25 | 2773 (1.6) | 1 401 083 (18.5) | 715 (1.6) | 186 390 (14.2) | 695 (2.3) | 280 446 (19.1) | 506 (1.5) | 394 183 (21.5) | 857 (1.4) | 540 064 (18.2) |
| 25–29 | 28 888 (16.8) | 2 574 096 (34.0) | 8278 (18.1) | 454 202 (34.7) | 5424 (18.3) | 489 753 (33.4) | 6098 (17.9) | 637 419 (34.8) | 9088 (14.6) | 992 722 (33.5) |
| 30–34 | 70 849 (41.3) | 2 371 342 (31.3) | 19 848 (43.3) | 453 307 (34.6) | 11 776 (39.7) | 446 451 (30.4) | 14 402 (42.3) | 538 859 (29.4) | 24 823 (39.9) | 932 725 (31.4) |
| 35–39 | 56 593 (33.0) | 1 027 140 (13.6) | 13 813 (30.2) | 184 443 (14.1) | 8956 (30.2) | 204 704 (14.0) | 11 203 (32.9) | 221 121 (12.1) | 22 621 (36.4) | 416 872 (14.1) |
| >40 | 12 632 (7.4) | 202 241 (2.7) | 3147 (6.9) | 31 124 (2.4) | 2840 (9.6) | 46 296 (3.2) | 1833 (5.4) | 39 860 (2.2) | 4812 (7.7) | 84 961 (2.9) |
| Primiparous, (%) | 116 520 (67.9) | 3 163 682 (41.8) | 30 490 (66.6) | 554 283 (42.3) | 2138 (67.8) | 593 361 (40.4) | 21 733 (63.8) | 756 578 (41.3) | 44 159 (71.0) | 1 259 460 (42.4) |
| Missing data on parity, (%) | 569 (0.33) | 19 165 (0.3) | 560 (1.2) | 17 719 (1.4) | 9 (0.03) | 1374 (0.1) | 0 | 0 | 0 | 0 |
| BMI (kg/m ), (% of non-missing) | ||||||||||
| <18.5 | 2565 (2.4) | 132 058 (3.5) | 942 (3.4) | 25 980 (4.2) | 391 (2.6) | 22 448 (3.7) | 310 (0.2) | 10 619 (4.1) | 922 (1.7) | 73 011 (3.1) |
| 18.5–24.9 | 68 169 (63.4) | 2 415 098 (63.4) | 18 050 (64.6) | 385 134 (62.5) | 9840 (65.3) | 375 550 (62.6) | 5878 (61.0) | 158 114 (61.7) | 34 411 (62.8) | 1 496 300 (64.0) |
| 25.0–29.9 | 25 989 (24.2) | 851 663 (22.4) | 6196 (22.2) | 129 398 (21.0) | 3302 (21.9) | 130 144 (21.7) | 2298 (23.8) | 56 411 (22.0) | 14 193 (25.9) | 535 710 (22.9) |
| ≥30 | 10 746 (10.0) | 410 734 (10.8) | 2774 (9.9) | 75 920 (12.3) | 1543 (10.2) | 72 271 (12.0) | 1154 (12.0) | 31 139 (12.2) | 5275 (9.6) | 231 404 (9.9) |
| Missing data for BMI, (% of total) | 64 256 (37.4) | 3 766 349 (49.7) | 17 839 (39.0) | 693 037 (52.9) | 14 615 (49.2) | 867 237 (59.1) | 24 402 (71.7) | 1 575 159 (86.0) | 7400 (11.9) | 630 919 (21.3) |
| Smoking, (%) | 10 120 (5.9) | 934 433 (12.3) | 3956 (8.6) | 194 514 (14.9) | 1815 (6.1) | 224 950 (15.3) | 1735 (5.1) | 129 171 (7.1) | 2614 (4.2) | 385 798 (13.0) |
| Missing data on smoking, (%) | 12 938 (7.5) | 1 199 059 (15.8) | 3217 (7.0) | 132 470 (10.1) | 449 (1.5) | 37 317 (2.5) | 5703 (16.8) | 868 891 (47.4) | 3569 (5.7) | 160 381 (5.4) |
| Educational level , , (%) | ||||||||||
| Low | 59 160 (43.0) | 2 972 924 (51.7) | 24 096 (52.6) | 775 820 (59.3) | 8675 (29.2) | 604 510 (41.2) | NA | NA | 26 389 (42.4) | 1 592 594 (53.7) |
| Middle | 45 314 (32.9) | 1 604 375 (27.9) | 13 662 (29.8) | 342 853 (26.2) | 11 349 (38.2) | 466 541 (31.8) | NA | NA | 20 303 (32.6) | 794 981 (26.8) |
| High | 27 952 (20.3) | 765 000 (13.3) | 7 463 (16.3) | 154 271 (11.8) | 8078 (27.2) | 237 162 (16.2) | NA | NA | 12 411 (20.0) | 373 567 (12.6) |
| Missing data on education, (%) | 5267 (3.8) | 402 161 (7.0) | 580 (1.3) | 36 522 (2.8) | 1589 (5.4) | 159 437 (10.9) | NA | NA | 3098 (5.0) | 206 202 (6.7) |
| Pre-gestational diabetes (%) | 1515 (0.88) | 53 709 (0.71) | 517 (1.13) | 16 105 (1.23) | 372 (1.25) | 14 266 (0.97) | 268 (0.79) | 9352 (0.51) | 358 (0.58) | 13 986 (0.47) |
| Non-chromosomal CHD, (%) | 648 (0.38) | 26 017 (0.34) | 167 (0.36) | 4649 (0.36) | 159 (0.54) | 7868 (0.54) | 54 (0.16) | 1739 (0.09) | 268 (0.43) | 11 761 (0.40) |
| Chromosomal defects, (%) | 661 (0.38) | 5925 (0.08) | 232 (0.51) | 1368 (0.10) | 107 (0.36) | 2066 (0.14) | 28 (0.08) | 872 (0.05) | 294 (0.47) | 1619 (0.05) |
| Non-chromosomal CHD, (%) | NA | NA | NA | NA | NA | NA | NA | NA | 243 (0.14) | 10 448 (0.35) |
| Chromosomal defects, (%) | NA | NA | NA | NA | NA | NA | NA | NA | 217 (0.35) | 768 (0.03) |
| , (%) | ||||||||||
| IVF | 81 900 (57.7) | - | 25 024 (54.6) | - | NA | - | 20 093 (59.0) | - | 36 783 (59.1) | - |
| ICSI | 55 126 (38.8) | - | 18 118 (39.6) | - | NA | - | 11 590 (34.1) | - | 25 418 (40.9) | - |
| Missing data on IVF/ICSI | 5018 (3.5) | - | 2659 (5.8) | - | NA | - | 2359 (6.9) | - | 0 | - |
| Single embryo transfer | 52 130 (36.7) | - | 10 191 (22.3) | - | NA | - | 10 303 (30.3) | - | 31 636 (50.9) | |
| Fresh embryo transfer | 115 408 (81.2) | - | 41 022 (89.6) | - | NA | - | 25 630 (75.3) | - | 48 756 (78.4) | - |
| Frozen embryo transfer | 22 630 (15.9) | - | 4761 (10.4) | - | NA | - | 4424 (13.0) | - | 13 445 (21.6) | - |
| Missing data on fresh/frozen cycle | 4006 (2.8) | - | 18 (0.04) | - | NA | - | 3988 (11.7) | - | 0 | - |
| Cleavage stage embryo | 130 079 (91.6) | - | 43 621 (95.2) | - | NA | - | 30 219 (88.8) | - | 56 239 (90.4) | - |
| Blastocysts | 7663 (5.4) | - | 1287 (2.8) | - | NA | - | 414 (1.2) | - | 5962 (9.6) | - |
| Missing data on embryo stage | 4302 (3.0) | - | 893 (2.0) | - | NA | - | 3409 (10.0) | - | 0 | - |
| Donated oocytes | 1410 (1.0) | - | 710 (1.6) | - | NA | - | 0 | - | 700 (1.1) | - |
| Donated sperm | 2994 (2.8) | - | 1911 (4.2) | - | NA | - | NA | - | 1083 (1.7) | - |
| . | All countries . | Denmark . | Finland . | Norway . | Sweden . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | = 7 747 637 liveborn children . | = 1 355 267 liveborn children . | = 1 497 341 liveborn children . | = 1 865 484 liveborn children . | = 3 029 545 liveborn children . | |||||
| . | ART . | SC . | ART . | SC . | ART . | SC . | ART . | SC . | ART . | SC . |
| . | = 171 735 . | = 7 575 902 . | = 45 801 . | = 1 309 466 . | = 29 691 . | = 1 467 650 . | = 34 042 . | = 1 831 442 . | = 62 201 . | = 2 967 344 . |
| Year of birth, n (%) | ||||||||||
| 1984–90 | 1610 (0.9) | 898 290 (11.9) | 0 | 0 | 53 (0.2) | 65 393 (4.5) | 877 (2.6) | 383 757 (21.0) | 680 (1.1) | 449 140 (15.1) |
| 1991–95 | 11 681 (6.8) | 1 321 614 (1.4) | 1299 (2.8) | 138 138 (10.6) | 2864 (9.7) | 321 476 (21.9) | 2464 (7.2) | 297 356 (16.2) | 5054 (8.1) | 564 644 (19.0) |
| 1996–2000 | 28 709 (16.7) | 1 333 763 (17.6) | 8532 (18.6) | 327 305 (25.0) | 6941 (23.4) | 283 333 (19.3) | 4313 (12.7) | 292 015 (15.9) | 8923 (14.4) | 431 110 (14.5) |
| 2001–05 | 36 092 (21.0) | 1 332 414 (17.6) | 11 798 (25.8) | 313 094 (23.9) | 6359 (21.4) | 276 400 (18.8) | 6586 (19.4) | 276 926 (15.1) | 11 349 (18.3) | 465 994 (15.7) |
| 2006–10 | 45 519 (26.5) | 1 414 225 (18.7) | 13 156 (28.7) | 310 143 (23.7) | 6974 (23.5) | 291 949 (19.9) | 9411 (27.7) | 293 014 (16.0) | 15 978 (25.7) | 519 119 (17.5) |
| 2011–15 | 48 124 (28.0) | 1 275 596 (16.8) | 11 016 (24.1) | 220 786 (16.9) | 6500 (21.9) | 229 099 (15.6) | 10 391 (30.5) | 288 374 (15.8) | 20 217 (32.5) | 537 337 (18.1) |
| Birth weight, (%) | ||||||||||
| Very low, <1500 g | 5215 (3.1) | 54 992 (0.7) | 1566 (3.5) | 10 331 (0.8) | 845 (2.9) | 9504 (0.7) | 1233 (3.6) | 14 584 (0.8) | 1571 (2.5) | 20 573 (0.7) |
| Low, <2500, g | 27448 (16.0) | 319 685 (4.2) | 8463 (18.5) | 61 229 (4.7) | 4783 (16.1) | 56 334 (3.8) | 6103 (17.9) | 81 588 (4.5) | 8099 (13.0) | 120 534 (4.1) |
| Macrosomia, ≥4000g | 20 516 (12.0) | 1 425 820 (18.8) | 4725 (10.3) | 238 103 (18.2) | 3521 (11.9) | 272 464 (18.6) | 3933 (11.6) | 355 748 (19.4) | 8337 (13.4) | 559 505 (18.9) |
| Missing data on birth weight | 755 (0.4) | 36 271 (0.5) | 463 (1.0) | 24 570 (1.9) | 13 (0.04) | 3405 (0.2) | 35 (0.1) | 1556 (0.1) | 244 (0.4) | 6740 (0.2) |
| Gestational age, (%) | ||||||||||
| Extremely preterm, <28 + 0 weeks | 1984 (1.2) | 20 482 (0.3) | 626 (1.4) | 3640 (0.3) | 325 (1.1) | 3613 (0.3) | 455 (1.3) | 5352 (0.3) | 578 (0.9) | 7877 (0.3) |
| Very preterm, <32 + 0 weeks | 6120 (3.6) | 64 821 (0.9) | 1869 (4.1) | 12 178 (0.9) | 970 (3.3) | 10 834 (0.7) | 1400 (4.1) | 16 984 (0.9) | 1881 (3.0) | 24 825 (0.8) |
| Preterm, <37 + 0 weeks | 31 367 (18.3) | 436 471 (5.8) | 9264 (20.2) | 78 548 (6.0) | 5725 (19.3) | 77 520 (5.3) | 6867 (20.2) | 108 524 (5.9) | 9511 (15.3) | 171 879 (5.8) |
| Post-term, ≥42 + 0 weeks | 3296 (5.3) | 513 869 (6.8) | 1491 (3.3) | 82 959 (6.3) | 924 (3.1) | 67 415 (4.6) | 1557 (4.6) | 158 092 (8.6) | 3296 (5.3) | 205 403 (6.9) |
| Missing data on gestational age | 652 (0.4) | 128 984 (1.7) | 298 (0.7) | 28 949 (2.2) | 57 (0.2) | 6755 (0.5) | 246 (0.7) | 89 599 (4.9) | 51 (0.1) | 3681 (0.1) |
| Plurality, (%) | ||||||||||
| Singletons | 127 275 (74.1) | 7 380 916 (97.4) | 31 077 (67.9) | 1 269 638 (97.0) | 22 044 (74.2) | 1 431 723 (97.6) | 24 117 (70.8) | 1 783 926 (97.4) | 50 037 (80.4) | 2 895 629 (97.6) |
| Twins | 42 459 (24.7) | 190 426 (2.5) | 14 332 (31.3) | 38 810 (3.0) | 7198 (24.2) | 35 222 (2.4) | 9364 (27.5) | 46 356 (2.5) | 11 565 (18.6) | 70 038 (2.4) |
| Triplets and higher | 2001 (1.2) | 4504 (0.1) | 392 (0.9) | 1018 (0.1) | 449 (1.5) | 705 (0.1) | 561 (1.7) | 1160 (0.1) | 599 (1.0) | 1621 (0.1) |
| Missing data on plurality, | 0 | 56 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 |
| Male sex, (%) | 87 785 (51.1) | 3 887 791 (51.3) | 23 211 (50.7) | 672 079 (51.3) | 15 197 (51.2) | 749 952 (51.1) | 17 456 (51.3) | 940 571 (51.4) | 31 921 (51.3) | 1 525 189 (51.4) |
| Missing data on sex, | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Age at delivery years, mean (SD) | 33.4 (4.2) | 29.3 (5.2) | 33.1 (4.2) | 29.7 (4.9) | 33.4 (4.7) | 29.3 (5.3) | 33.2 (4.7) | 28.8 (5.2) | 33.7 (4.2) | 29.3 (5.2) |
| Age at delivery (years), (%) | ||||||||||
| <25 | 2773 (1.6) | 1 401 083 (18.5) | 715 (1.6) | 186 390 (14.2) | 695 (2.3) | 280 446 (19.1) | 506 (1.5) | 394 183 (21.5) | 857 (1.4) | 540 064 (18.2) |
| 25–29 | 28 888 (16.8) | 2 574 096 (34.0) | 8278 (18.1) | 454 202 (34.7) | 5424 (18.3) | 489 753 (33.4) | 6098 (17.9) | 637 419 (34.8) | 9088 (14.6) | 992 722 (33.5) |
| 30–34 | 70 849 (41.3) | 2 371 342 (31.3) | 19 848 (43.3) | 453 307 (34.6) | 11 776 (39.7) | 446 451 (30.4) | 14 402 (42.3) | 538 859 (29.4) | 24 823 (39.9) | 932 725 (31.4) |
| 35–39 | 56 593 (33.0) | 1 027 140 (13.6) | 13 813 (30.2) | 184 443 (14.1) | 8956 (30.2) | 204 704 (14.0) | 11 203 (32.9) | 221 121 (12.1) | 22 621 (36.4) | 416 872 (14.1) |
| >40 | 12 632 (7.4) | 202 241 (2.7) | 3147 (6.9) | 31 124 (2.4) | 2840 (9.6) | 46 296 (3.2) | 1833 (5.4) | 39 860 (2.2) | 4812 (7.7) | 84 961 (2.9) |
| Primiparous, (%) | 116 520 (67.9) | 3 163 682 (41.8) | 30 490 (66.6) | 554 283 (42.3) | 2138 (67.8) | 593 361 (40.4) | 21 733 (63.8) | 756 578 (41.3) | 44 159 (71.0) | 1 259 460 (42.4) |
| Missing data on parity, (%) | 569 (0.33) | 19 165 (0.3) | 560 (1.2) | 17 719 (1.4) | 9 (0.03) | 1374 (0.1) | 0 | 0 | 0 | 0 |
| BMI (kg/m ), (% of non-missing) | ||||||||||
| <18.5 | 2565 (2.4) | 132 058 (3.5) | 942 (3.4) | 25 980 (4.2) | 391 (2.6) | 22 448 (3.7) | 310 (0.2) | 10 619 (4.1) | 922 (1.7) | 73 011 (3.1) |
| 18.5–24.9 | 68 169 (63.4) | 2 415 098 (63.4) | 18 050 (64.6) | 385 134 (62.5) | 9840 (65.3) | 375 550 (62.6) | 5878 (61.0) | 158 114 (61.7) | 34 411 (62.8) | 1 496 300 (64.0) |
| 25.0–29.9 | 25 989 (24.2) | 851 663 (22.4) | 6196 (22.2) | 129 398 (21.0) | 3302 (21.9) | 130 144 (21.7) | 2298 (23.8) | 56 411 (22.0) | 14 193 (25.9) | 535 710 (22.9) |
| ≥30 | 10 746 (10.0) | 410 734 (10.8) | 2774 (9.9) | 75 920 (12.3) | 1543 (10.2) | 72 271 (12.0) | 1154 (12.0) | 31 139 (12.2) | 5275 (9.6) | 231 404 (9.9) |
| Missing data for BMI, (% of total) | 64 256 (37.4) | 3 766 349 (49.7) | 17 839 (39.0) | 693 037 (52.9) | 14 615 (49.2) | 867 237 (59.1) | 24 402 (71.7) | 1 575 159 (86.0) | 7400 (11.9) | 630 919 (21.3) |
| Smoking, (%) | 10 120 (5.9) | 934 433 (12.3) | 3956 (8.6) | 194 514 (14.9) | 1815 (6.1) | 224 950 (15.3) | 1735 (5.1) | 129 171 (7.1) | 2614 (4.2) | 385 798 (13.0) |
| Missing data on smoking, (%) | 12 938 (7.5) | 1 199 059 (15.8) | 3217 (7.0) | 132 470 (10.1) | 449 (1.5) | 37 317 (2.5) | 5703 (16.8) | 868 891 (47.4) | 3569 (5.7) | 160 381 (5.4) |
| Educational level , , (%) | ||||||||||
| Low | 59 160 (43.0) | 2 972 924 (51.7) | 24 096 (52.6) | 775 820 (59.3) | 8675 (29.2) | 604 510 (41.2) | NA | NA | 26 389 (42.4) | 1 592 594 (53.7) |
| Middle | 45 314 (32.9) | 1 604 375 (27.9) | 13 662 (29.8) | 342 853 (26.2) | 11 349 (38.2) | 466 541 (31.8) | NA | NA | 20 303 (32.6) | 794 981 (26.8) |
| High | 27 952 (20.3) | 765 000 (13.3) | 7 463 (16.3) | 154 271 (11.8) | 8078 (27.2) | 237 162 (16.2) | NA | NA | 12 411 (20.0) | 373 567 (12.6) |
| Missing data on education, (%) | 5267 (3.8) | 402 161 (7.0) | 580 (1.3) | 36 522 (2.8) | 1589 (5.4) | 159 437 (10.9) | NA | NA | 3098 (5.0) | 206 202 (6.7) |
| Pre-gestational diabetes (%) | 1515 (0.88) | 53 709 (0.71) | 517 (1.13) | 16 105 (1.23) | 372 (1.25) | 14 266 (0.97) | 268 (0.79) | 9352 (0.51) | 358 (0.58) | 13 986 (0.47) |
| Non-chromosomal CHD, (%) | 648 (0.38) | 26 017 (0.34) | 167 (0.36) | 4649 (0.36) | 159 (0.54) | 7868 (0.54) | 54 (0.16) | 1739 (0.09) | 268 (0.43) | 11 761 (0.40) |
| Chromosomal defects, (%) | 661 (0.38) | 5925 (0.08) | 232 (0.51) | 1368 (0.10) | 107 (0.36) | 2066 (0.14) | 28 (0.08) | 872 (0.05) | 294 (0.47) | 1619 (0.05) |
| Non-chromosomal CHD, (%) | NA | NA | NA | NA | NA | NA | NA | NA | 243 (0.14) | 10 448 (0.35) |
| Chromosomal defects, (%) | NA | NA | NA | NA | NA | NA | NA | NA | 217 (0.35) | 768 (0.03) |
| , (%) | ||||||||||
| IVF | 81 900 (57.7) | - | 25 024 (54.6) | - | NA | - | 20 093 (59.0) | - | 36 783 (59.1) | - |
| ICSI | 55 126 (38.8) | - | 18 118 (39.6) | - | NA | - | 11 590 (34.1) | - | 25 418 (40.9) | - |
| Missing data on IVF/ICSI | 5018 (3.5) | - | 2659 (5.8) | - | NA | - | 2359 (6.9) | - | 0 | - |
| Single embryo transfer | 52 130 (36.7) | - | 10 191 (22.3) | - | NA | - | 10 303 (30.3) | - | 31 636 (50.9) | |
| Fresh embryo transfer | 115 408 (81.2) | - | 41 022 (89.6) | - | NA | - | 25 630 (75.3) | - | 48 756 (78.4) | - |
| Frozen embryo transfer | 22 630 (15.9) | - | 4761 (10.4) | - | NA | - | 4424 (13.0) | - | 13 445 (21.6) | - |
| Missing data on fresh/frozen cycle | 4006 (2.8) | - | 18 (0.04) | - | NA | - | 3988 (11.7) | - | 0 | - |
| Cleavage stage embryo | 130 079 (91.6) | - | 43 621 (95.2) | - | NA | - | 30 219 (88.8) | - | 56 239 (90.4) | - |
| Blastocysts | 7663 (5.4) | - | 1287 (2.8) | - | NA | - | 414 (1.2) | - | 5962 (9.6) | - |
| Missing data on embryo stage | 4302 (3.0) | - | 893 (2.0) | - | NA | - | 3409 (10.0) | - | 0 | - |
| Donated oocytes | 1410 (1.0) | - | 710 (1.6) | - | NA | - | 0 | - | 700 (1.1) | - |
| Donated sperm | 2994 (2.8) | - | 1911 (4.2) | - | NA | - | NA | - | 1083 (1.7) | - |
ART, assisted reproductive technology; BMI, body mass index; CHD, congenital heart defect; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; NA, not available; SC, spontaneous conception.
a Data for Denmark, Finland, and Sweden (denominator ART, 137 693; SC, 5 744 460).
b Highest educational level according to the International Standard Classification of Education (ISCED2011): ISCED < 5, primary, secondary, or post-secondary non tertiary education; ISCED 5–6, first stage of tertiary education (bachelor or equivalent); and ISCED 7–8, second stage of tertiary education (master, doctorate, or more). 45 .
c Data for Denmark, Norway, and Sweden (denominator ART = 142 044).
d Data for Denmark and Sweden (denominator ART = 108 002).
e Oocyte donation not permitted in Norway during the study period.
Sensitivity analyses
We performed several sensitivity analyses on major CHDs. First, we performed a sensitivity analysis restricting the analysis to those with known data on smoking. Second, maternal highest educational level (low, medium, and high) 45 was included as a covariate when this information was available (Denmark, Finland, and Sweden). Third, a sensitivity analysis was performed including Finnish data with validated data on major CHDs and excluding those which after further evaluation by the Finnish malformation registry were considered as minor CHDs (see Supplementary data online , Table S1 ). 46 A fourth sensitivity analysis on major CHDs and severe CHDs was performed restricting the cohort to infants born 2006–15. Between 2004 and 2007, all countries had introduced the second trimester ultrasound including foetal anomaly screening (gestational week 18–21). Lastly, a sensitivity analysis was performed on Swedish data, adjusting also for paternal CHD.
Baseline characteristics
A total of 7 747 637 liveborn children were included, 171 735 (2.2%) were born after ART and 7 575 902 (97.8%) were born after SC ( Figure 1 ). The proportion of multiples was 25.9% (44 460 of 171 735) in the ART group and 2.6% (194 930 of 7 575 902) in the spontaneously conceived group.

Flow chart of the study population
Baseline characteristics are presented in Table 1 . Children conceived by ART were more often born preterm (<37 weeks; 18.3% vs. 5.8%) or with LBW (<2500 g; 16.0% vs. 4.2%) than children born after SC. Women who conceived by ART were more likely to be older at birth (≥35 years; 40.4% vs. 16.3%), to be primiparous (67.9% vs. 41.8%), to have a high educational level (16.3% vs. 10.1%), and to be non-smokers (86.6% vs. 71.9%). The prevalence of pre-gestational diabetes was 0.88% in women who conceived using ART vs. 0.71% in women who conceived spontaneously. Corresponding figures for maternal non-chromosomal CHDs were 0.38% vs. 0.34% and for obesity (body mass index ≥ 30 kg/m 2 ) 10.0% vs. 10.8%.
Major congenital heart defects
The rate of major CHDs varied somewhat between countries as indicated in Table 2 and Figure 2 .

Forest plot showing the ORs for independent covariates of risk of major congenital heart defects in children born after assisted reproductive technology vs. children conceived spontaneously. Footnote: Please observe that in this figure, child’s year of birth and maternal age are expressed in categories for better visualization. In all statistical models, however, both variables are analysed as continuous variables, resulting in slightly different AOR and 95% CI for comparison ART vs. SC
Risk of congenital heart defects in liveborn children conceived by assisted reproductive technology vs. spontaneous conception for all countries and by country (Denmark 1994–2014, Finland 1990–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No. of liveborn children . | Risk of CHD, ART vs. spontaneous conception . | ||
|---|---|---|---|---|
| . | ART . | Spontaneous conception . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| diagnosed within the first year of life | ||||
| All countries / (%) | 3159/171 735 (1.84) | 86 824/7 575 902 (1.15) | 1.62 (1.56–1.68) < .001 | 1.36 (1.31–1.41) < .001 |
| Denmark / (%) | 879/45 801 (1.92) | 14 467/1 309 466 (1.10) | 1.75 (1.64–1.88) < .001 | 1.70 (1.58–1.82) < .001 |
| Finland / (%) | 592/29 691 (1.99) | 19 341/1 467 650 (1.32) | 1.52 (1.40–1.65) < .001 | 1.33 (1.22–1.45) < .001 |
| Norway (at birth) / (%) | 380/34 042 (1.12) | 10 630/1 831 442 (0.58) | 1.93 (1.74–2.14) < .001 | 1.34 (1.21–1.49) < .001 |
| Sweden / (%) | 1308/62 201 (2.10) | 42 386/2 967 344 (1.43) | 1.48 (1.40–1.57) < .001 | 1.22 (1.16–1.29) < .001 |
| diagnosed within the first year of life | ||||
| All countries / (%) | 594/171 735 (0.35) | 19 375/7 575 902 (0.26) | 1.35 (1.25–1.47) < .001 | 1.30 (1.20–1.42) < .001 |
| Denmark / (%) | 137/45 801 (0.30) | 3042/1 309 466 (0.23) | 1.29 (1.09–1.53) .004 | 1.28 (1.07–1.53) .006 |
| Finland / (%) | 117/29 691 (0.39) | 4320/1 467 650 (0.29) | 1.34 (1.11–1.61) .002 | 1.27 (1.06–1.54) .011 |
| Norway (at birth) / (%) | 90/34 042 (0.26) | 3642/1 831 442 (0.20) | 1.33 (1.08–1.64) .008 | 1.23 (0.99–1.52) .060 |
| Sweden / (%) | 250/62 201 (0.40) | 8371/2 967 344 (0.28) | 1.43 (1.26–1.62) < .001 | 1.37 (1.20–1.56) < .001 |
| Lesion group 1 conotruncal | ||||
| All countries / (%) | 194/171 735 (0.11) | 6314/7 575 902 (0.08) | 1.36 (1.18–1.56) < .001 | 1.23 (1.06–1.42) .006 |
| Denmark / (%) | 49/45 801 (0.11) | 1255/1 309 466 (0.10) | 1.12 (0.84–1.49) .450 | 1.14 (0.86–1.52) .366 |
| Finland / (%) | 36/29 691 (0.12) | 1205/1 467 650 (0.08) | 1.48 (1.06–2.06) .021 | 1.46 (1.04–2.04) .027 |
| Norway (at birth) / (%) | 35/34 042 (0.10) | 965/1 831 442 (0.05) | 1.95 (1.39–2.74) < .001 | 1.45 (1.03–2.04) .033 |
| Sweden / (%) | 74/62 201 (0.12) | 2889/2 967 344 (0.10) | 1.22 (0.97–1.54) .088 | 1.20 (0.95–1.51) .134 |
| Lesion group 2 Non-conotruncal | ||||
| All countries / (%) | 158/171 735 (0.09) | 5700/7 575 902 (0.08) | 1.22 (1.04–1.43) .013 | 1.27 (1.08–1.50) .003 |
| Denmark / (%) | 42/45 801 (0.09) | 835/1 309 466 (0.06) | 1.44 (1.05–1.96) .022 | 1.40 (1.02–1.92) .035 |
| Finland / (%) | 34/29 691 (0.11) | 1038/1 467 650 (0.07) | 1.62 (1.15–2.28) .006 | 1.45 (1.02–2.04) .036 |
| Norway (at birth) / (%) | 32/34 042 (0.09) | 1787/1 831 442 (0.10) | 0.96 (0.68–1.37) .834 | 1.19 (0.84–1.70) .325 |
| Sweden / (%) | 50/62 201 (0.08) | 2040/2 967 344 (0.07) | 1.17 (0.88–1.55) .274 | 1.12 (0.84–1.48) .441 |
| Lesion group 3 coarctation aortae | ||||
| All countries / (%) | 105/171 735 (0.06) | 3397/7 575 902 (0.04) | 1.36 (1.12–1.66) .002 | 1.22 (0.999–1.49) .051 |
| Denmark / (%) | 20/45 801 (0.04) | 522/1 309 466 (0.04) | 1.10 (0.70–1.71) .689 | 1.06 (0.67–1.66) .815 |
| Finland / (%) | 19/29 691 (0.06) | 1081/1 467 650 (0.07) | 0.87 (0.55–1.37) .543 | 0.79 (0.50–1.24) .305 |
| Norway (at birth) / (%) | 10/34 042 (0.03) | 313/1 831 442 (0.02) | 1.72 (0.92–3.23) .092 | 1.14 (0.60–2.15) .689 |
| Sweden / (%) | 56/62 201 (0.09) | 1481/2 967 344 (0.05) | 1.80 (1.38–2.36) < .001 | 1.57 (1.20–2.06) .001 |
| Lesion group 4 VSD | ||||
| All countries / (%) | 1212/171 735 (0.71) | 36 219/7 575 902 (0.48) | 1.48 (1.40–1.57) < .001 | 1.21 (1.14–1.29) < .001 |
| Denmark / (%) | 185/45 801 (0.40) | 3812/1 309 466 (0.29) | 1.39 (1.20–1.61) < .001 | 1.39 (1.20–1.62) < .001 |
| Finland / (%) | 333/29 691 (1.12) | 10 677/1 467 650 (0.73) | 1.55 (1.39–1.73) < .001 | 1.39 (1.25–1.56) < .001 |
| Norway (at birth) | 153/34 042 (0.45) | 3661/1 831 442 (0.20) | 2.25 (1.92–2.65) < .001 | 1.41 (1.20–1.66) < .001 |
| Sweden / (%) | 541/62 201 (0.87) | 18 069/2 967 344 (0.61) | 1.43 (1.31–1.56) < .001 | 1.16 (1.06–1.26) .001 |
| Lesion group 5 ASD | ||||
| All countries / (%) | 720/171 735 (0.42) | 13 787/7 575 902 (0.18) | 2.31 (2.14–2.49) < .001 | 1.60 (1.48–1.73) < .001 |
| Denmark / (%) | 288/45 801 (0.63) | 3372/1 309 466 (0.26) | 2.45 (2.17–2.77) < .001 | 2.21 (1.96–2.50) < .001 |
| Finland / (%) | 23/29 691 (0.08) | 1038/1 467 650 (0.07) | 1.10 (0.72–1.66) .666 | 0.90 (0.59–1.37) .623 |
| Norway (at birth) / (%) | 82/34 042 (0.24) | 1559/1 831 442 (0.09) | 2.83 (2.27–3.54) < .001 | 1.75 (1.40–2.19) < .001 |
| Sweden / (%) | 327/62 201 (0.53) | 7818/2 967 344 (0.26) | 2.00 (1.79–2.24) < .001 | 1.38 (1.23–1.54) < .001 |
| Lesion group 6 other CHDs | ||||
| All countries / (%) | 770/171 735 (0.45) | 21 407/7 575 902 (0.28) | 1.59 (1.48–1.71) < .001 | 1.48 (1.37–1.59) < .001 |
| Denmark n/N (%) | 295/45 801 (0.64) | 4671/1 309 466 (0.36) | 1.81 (1.61–2.04) < .001 | 2.09 (1.85–2.35) < .001 |
| Finland / (%) | 147/29 691 (0.50) | 4302/1 467 650 (0.29) | 1.69 (1.44–2.00) < .001 | 1.50 (1.27–1.77) < .001 |
| Norway (at birth) / (%) | 68/34 042 (0.20) | 2345/1 831 442 (0.13) | 1.56 (1.23–1.99) < .001 | 1.13 (0.88–1.44) .332 |
| Sweden / (%) | 260/62 201 (0.42) | 10 089/2 967 344 (0.34) | 1.23 (1.09–1.39) .001 | 1.27 (1.12–1.44) < .001 |
| . | No. of liveborn children . | Risk of CHD, ART vs. spontaneous conception . | ||
|---|---|---|---|---|
| . | ART . | Spontaneous conception . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| diagnosed within the first year of life | ||||
| All countries / (%) | 3159/171 735 (1.84) | 86 824/7 575 902 (1.15) | 1.62 (1.56–1.68) < .001 | 1.36 (1.31–1.41) < .001 |
| Denmark / (%) | 879/45 801 (1.92) | 14 467/1 309 466 (1.10) | 1.75 (1.64–1.88) < .001 | 1.70 (1.58–1.82) < .001 |
| Finland / (%) | 592/29 691 (1.99) | 19 341/1 467 650 (1.32) | 1.52 (1.40–1.65) < .001 | 1.33 (1.22–1.45) < .001 |
| Norway (at birth) / (%) | 380/34 042 (1.12) | 10 630/1 831 442 (0.58) | 1.93 (1.74–2.14) < .001 | 1.34 (1.21–1.49) < .001 |
| Sweden / (%) | 1308/62 201 (2.10) | 42 386/2 967 344 (1.43) | 1.48 (1.40–1.57) < .001 | 1.22 (1.16–1.29) < .001 |
| diagnosed within the first year of life | ||||
| All countries / (%) | 594/171 735 (0.35) | 19 375/7 575 902 (0.26) | 1.35 (1.25–1.47) < .001 | 1.30 (1.20–1.42) < .001 |
| Denmark / (%) | 137/45 801 (0.30) | 3042/1 309 466 (0.23) | 1.29 (1.09–1.53) .004 | 1.28 (1.07–1.53) .006 |
| Finland / (%) | 117/29 691 (0.39) | 4320/1 467 650 (0.29) | 1.34 (1.11–1.61) .002 | 1.27 (1.06–1.54) .011 |
| Norway (at birth) / (%) | 90/34 042 (0.26) | 3642/1 831 442 (0.20) | 1.33 (1.08–1.64) .008 | 1.23 (0.99–1.52) .060 |
| Sweden / (%) | 250/62 201 (0.40) | 8371/2 967 344 (0.28) | 1.43 (1.26–1.62) < .001 | 1.37 (1.20–1.56) < .001 |
| Lesion group 1 conotruncal | ||||
| All countries / (%) | 194/171 735 (0.11) | 6314/7 575 902 (0.08) | 1.36 (1.18–1.56) < .001 | 1.23 (1.06–1.42) .006 |
| Denmark / (%) | 49/45 801 (0.11) | 1255/1 309 466 (0.10) | 1.12 (0.84–1.49) .450 | 1.14 (0.86–1.52) .366 |
| Finland / (%) | 36/29 691 (0.12) | 1205/1 467 650 (0.08) | 1.48 (1.06–2.06) .021 | 1.46 (1.04–2.04) .027 |
| Norway (at birth) / (%) | 35/34 042 (0.10) | 965/1 831 442 (0.05) | 1.95 (1.39–2.74) < .001 | 1.45 (1.03–2.04) .033 |
| Sweden / (%) | 74/62 201 (0.12) | 2889/2 967 344 (0.10) | 1.22 (0.97–1.54) .088 | 1.20 (0.95–1.51) .134 |
| Lesion group 2 Non-conotruncal | ||||
| All countries / (%) | 158/171 735 (0.09) | 5700/7 575 902 (0.08) | 1.22 (1.04–1.43) .013 | 1.27 (1.08–1.50) .003 |
| Denmark / (%) | 42/45 801 (0.09) | 835/1 309 466 (0.06) | 1.44 (1.05–1.96) .022 | 1.40 (1.02–1.92) .035 |
| Finland / (%) | 34/29 691 (0.11) | 1038/1 467 650 (0.07) | 1.62 (1.15–2.28) .006 | 1.45 (1.02–2.04) .036 |
| Norway (at birth) / (%) | 32/34 042 (0.09) | 1787/1 831 442 (0.10) | 0.96 (0.68–1.37) .834 | 1.19 (0.84–1.70) .325 |
| Sweden / (%) | 50/62 201 (0.08) | 2040/2 967 344 (0.07) | 1.17 (0.88–1.55) .274 | 1.12 (0.84–1.48) .441 |
| Lesion group 3 coarctation aortae | ||||
| All countries / (%) | 105/171 735 (0.06) | 3397/7 575 902 (0.04) | 1.36 (1.12–1.66) .002 | 1.22 (0.999–1.49) .051 |
| Denmark / (%) | 20/45 801 (0.04) | 522/1 309 466 (0.04) | 1.10 (0.70–1.71) .689 | 1.06 (0.67–1.66) .815 |
| Finland / (%) | 19/29 691 (0.06) | 1081/1 467 650 (0.07) | 0.87 (0.55–1.37) .543 | 0.79 (0.50–1.24) .305 |
| Norway (at birth) / (%) | 10/34 042 (0.03) | 313/1 831 442 (0.02) | 1.72 (0.92–3.23) .092 | 1.14 (0.60–2.15) .689 |
| Sweden / (%) | 56/62 201 (0.09) | 1481/2 967 344 (0.05) | 1.80 (1.38–2.36) < .001 | 1.57 (1.20–2.06) .001 |
| Lesion group 4 VSD | ||||
| All countries / (%) | 1212/171 735 (0.71) | 36 219/7 575 902 (0.48) | 1.48 (1.40–1.57) < .001 | 1.21 (1.14–1.29) < .001 |
| Denmark / (%) | 185/45 801 (0.40) | 3812/1 309 466 (0.29) | 1.39 (1.20–1.61) < .001 | 1.39 (1.20–1.62) < .001 |
| Finland / (%) | 333/29 691 (1.12) | 10 677/1 467 650 (0.73) | 1.55 (1.39–1.73) < .001 | 1.39 (1.25–1.56) < .001 |
| Norway (at birth) | 153/34 042 (0.45) | 3661/1 831 442 (0.20) | 2.25 (1.92–2.65) < .001 | 1.41 (1.20–1.66) < .001 |
| Sweden / (%) | 541/62 201 (0.87) | 18 069/2 967 344 (0.61) | 1.43 (1.31–1.56) < .001 | 1.16 (1.06–1.26) .001 |
| Lesion group 5 ASD | ||||
| All countries / (%) | 720/171 735 (0.42) | 13 787/7 575 902 (0.18) | 2.31 (2.14–2.49) < .001 | 1.60 (1.48–1.73) < .001 |
| Denmark / (%) | 288/45 801 (0.63) | 3372/1 309 466 (0.26) | 2.45 (2.17–2.77) < .001 | 2.21 (1.96–2.50) < .001 |
| Finland / (%) | 23/29 691 (0.08) | 1038/1 467 650 (0.07) | 1.10 (0.72–1.66) .666 | 0.90 (0.59–1.37) .623 |
| Norway (at birth) / (%) | 82/34 042 (0.24) | 1559/1 831 442 (0.09) | 2.83 (2.27–3.54) < .001 | 1.75 (1.40–2.19) < .001 |
| Sweden / (%) | 327/62 201 (0.53) | 7818/2 967 344 (0.26) | 2.00 (1.79–2.24) < .001 | 1.38 (1.23–1.54) < .001 |
| Lesion group 6 other CHDs | ||||
| All countries / (%) | 770/171 735 (0.45) | 21 407/7 575 902 (0.28) | 1.59 (1.48–1.71) < .001 | 1.48 (1.37–1.59) < .001 |
| Denmark n/N (%) | 295/45 801 (0.64) | 4671/1 309 466 (0.36) | 1.81 (1.61–2.04) < .001 | 2.09 (1.85–2.35) < .001 |
| Finland / (%) | 147/29 691 (0.50) | 4302/1 467 650 (0.29) | 1.69 (1.44–2.00) < .001 | 1.50 (1.27–1.77) < .001 |
| Norway (at birth) / (%) | 68/34 042 (0.20) | 2345/1 831 442 (0.13) | 1.56 (1.23–1.99) < .001 | 1.13 (0.88–1.44) .332 |
| Sweden / (%) | 260/62 201 (0.42) | 10 089/2 967 344 (0.34) | 1.23 (1.09–1.39) .001 | 1.27 (1.12–1.44) < .001 |
ART, assisted reproductive technology; ASD, atrial septal defect; CHD, congenital heart defects; CI, confidence interval; OR, odds ratio; VSD, ventricular septal defect.
a Major CHDs and severe CHDs: Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, and maternal CHD, in the analysis of all countries. Adjustment for child’s year of birth, maternal age, parity, maternal smoking, maternal diabetes, and maternal CHD, in the analysis of the specific countries. Botto lesion groups 1–6: Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, and maternal CHD in the analysis of all countries. Only adjustment for child’s year of birth and maternal age in the analysis of the specific countries.
b Major CHDs and severe CHDs according to the EUROCAT 1.5 definition. 32 , 33 .
c Lesion groups 1–6 according to Botto et al. 34
Major CHDs diagnosed up to 1 year of age were detected in 3159 children born after ART (1.84%) and in 86 824 children born after SC (1.15%; adjusted OR 1.36; 95% CI 1.31–1.41; P < .001; Table 2 ). Among children with major CHDs, 193 children (6.1%) in the ART group and 5472 (6.3%) in the spontaneously conceived group had a concomitant chromosomal aberration. Associations of each covariate with major CHDs are illustrated in Figure 2 . The strongest associations were seen for maternal pre-gestational diabetes (OR 2.72; 95% CI 2.59–2.86) and maternal CHDs (OR 3.80; 95% CI 3.59–4.02).
Major CHDs were detected among 1.62% ( n = 2059) of singletons born after ART and among 1.11% ( n = 82 119) of singletons born after SC (adjusted OR 1.19; 95% CI 1.14–1.24; P < .001; Table 3 ). No significant difference was seen for multiples conceived after ART vs. multiples conceived after SC ( Table 3 ).
Risk of congenital heart defects in singletons conceived by assisted reproductive technology vs. spontaneous conception and multiples conceived by assisted reproductive technology vs. spontaneous conception (Denmark 1994–2014, Finland 1990–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No. of singletons . | No. of multiples . | Risk of CHD in ART singletons vs. singletons born after spontaneous conception . | Risk of CHD in ART multiples vs. multiples born after spontaneous conception . | ||||
|---|---|---|---|---|---|---|---|---|
| . | ART = 127 275 . | Spontaneous conception = 7 380 916 . | ART = 44 460 . | Spontaneous conception = 194 930 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs diagnosed within the first year of life, (%) | 2059 (1.62) | 82 119 (1.11) | 1100 (2.47) | 4707 (2.41) | 1.46 (1.40–1.53) < .001 | 1.19 (1.14–1.24) < .001 | 1.03 (0.96–1.10) .455 | 0.94 (0.88–1.01) .085 |
| Severe CHDs diagnosed within the first year of life, (%) | 399 (0.31) | 18 539 (0.25) | 195 (0.44) | 836 (0.43) | 1.25 (1.13–1.38) < .001 | 1.20 (1.09–1.33) < .001 | 1.02 (0.87–1.20) .778 | 0.97 (0.82–1.14) .669 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | ||||||||
| Lesion group 1 conotruncal, (%) | 138 (0.11) | 6057 (0.08) | 56 (0.13) | 257 (0.13) | 1.32 (1.12–1.56) .001 | 1.20 (1.01–1.42) .042 | 0.96 (0.72–1.28) .757 | 0.84 (0.62–1.24) .273 |
| Lesion group 2 non-conotruncal, (%) | 99 (0.08) | 5499 (0.07) | 59 (0.13) | 201 (0.10) | 1.04 (0.86–1.27) .671 | 1.12 (0.92–1.37) .260 | 1.29 (0.96–1.72) .088 | 1.14 (0.83–1.55) .420 |
| Lesion group 3 coarctation aortae, (%) | 64 (0.05) | 3221 (0.04) | 41 (0.09) | 176 (0.09) | 1.15 (0.90–1.48) .261 | 1.00 (0.78–1.28) .988 | 1.02 (0.73–1.44) .903 | 1.03 (0.72–1.49) .855 |
| Lesion group 4 VSD, (%) | 882 (0.69) | 34 552 (0.47) | 330 (0.74) | 1667 (0.86) | 1.48 (1.39–1.59) < .001 | 1.15 (1.07–1.23) < .001 | 0.87 (0.77–0.98) .018 | 0.90 (0.79–1.02) .086 |
| Lesion group 5 ASD, (%) | 427 (0.34) | 12 569 (0.17) | 293 (0.66) | 1218 (0.62) | 1.97 (1.79–2.17) < .001 | 1.30 (1.17–1.43) < .001 | 1.06 (0.93–1.20) .412 | 0.95 (0.83–1.09) .400 |
| Lesion group 6 other CHDs, (%) | 449 (0.35) | 20 221 (0.27) | 321 (0.72) | 1186 (0.61) | 1.29 (1.17–1.42) < .001 | 1.20 (1.09–1.32) < .001 | 1.19 (1.05–1.34) .006 | 1.01 (0.88–1.15) .879 |
| . | No. of singletons . | No. of multiples . | Risk of CHD in ART singletons vs. singletons born after spontaneous conception . | Risk of CHD in ART multiples vs. multiples born after spontaneous conception . | ||||
|---|---|---|---|---|---|---|---|---|
| . | ART = 127 275 . | Spontaneous conception = 7 380 916 . | ART = 44 460 . | Spontaneous conception = 194 930 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs diagnosed within the first year of life, (%) | 2059 (1.62) | 82 119 (1.11) | 1100 (2.47) | 4707 (2.41) | 1.46 (1.40–1.53) < .001 | 1.19 (1.14–1.24) < .001 | 1.03 (0.96–1.10) .455 | 0.94 (0.88–1.01) .085 |
| Severe CHDs diagnosed within the first year of life, (%) | 399 (0.31) | 18 539 (0.25) | 195 (0.44) | 836 (0.43) | 1.25 (1.13–1.38) < .001 | 1.20 (1.09–1.33) < .001 | 1.02 (0.87–1.20) .778 | 0.97 (0.82–1.14) .669 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | ||||||||
| Lesion group 1 conotruncal, (%) | 138 (0.11) | 6057 (0.08) | 56 (0.13) | 257 (0.13) | 1.32 (1.12–1.56) .001 | 1.20 (1.01–1.42) .042 | 0.96 (0.72–1.28) .757 | 0.84 (0.62–1.24) .273 |
| Lesion group 2 non-conotruncal, (%) | 99 (0.08) | 5499 (0.07) | 59 (0.13) | 201 (0.10) | 1.04 (0.86–1.27) .671 | 1.12 (0.92–1.37) .260 | 1.29 (0.96–1.72) .088 | 1.14 (0.83–1.55) .420 |
| Lesion group 3 coarctation aortae, (%) | 64 (0.05) | 3221 (0.04) | 41 (0.09) | 176 (0.09) | 1.15 (0.90–1.48) .261 | 1.00 (0.78–1.28) .988 | 1.02 (0.73–1.44) .903 | 1.03 (0.72–1.49) .855 |
| Lesion group 4 VSD, (%) | 882 (0.69) | 34 552 (0.47) | 330 (0.74) | 1667 (0.86) | 1.48 (1.39–1.59) < .001 | 1.15 (1.07–1.23) < .001 | 0.87 (0.77–0.98) .018 | 0.90 (0.79–1.02) .086 |
| Lesion group 5 ASD, (%) | 427 (0.34) | 12 569 (0.17) | 293 (0.66) | 1218 (0.62) | 1.97 (1.79–2.17) < .001 | 1.30 (1.17–1.43) < .001 | 1.06 (0.93–1.20) .412 | 0.95 (0.83–1.09) .400 |
| Lesion group 6 other CHDs, (%) | 449 (0.35) | 20 221 (0.27) | 321 (0.72) | 1186 (0.61) | 1.29 (1.17–1.42) < .001 | 1.20 (1.09–1.32) < .001 | 1.19 (1.05–1.34) .006 | 1.01 (0.88–1.15) .879 |
ART, assisted reproductive technology; ASD, atrial septal defect; CHD, congenital heart defect; CI, confidence interval; OR, odds ratio; VSD, ventricular septal defect.
a Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, and maternal CHD.
Multiples born after ART had an absolute risk of major CHDs of 2.47% ( n = 1100; adjusted OR 1.70; 95% CI 1.58–1.84; P < .001 vs. singletons conceived after ART; Table 4 ). Multiples born after SC had an absolute risk of major CHDs of 2.41% ( n = 4705; adjusted OR 2.17; 95% CI 2.10–2.24; P < .001 vs. spontaneously conceived singletons; Table 4 ).
Risk of congenital heart defects in multiples born after ART vs. singletons born after assisted reproductive technology and multiples born after spontaneous conception vs. singletons born after spontaneous conception (Denmark 1994–2014, Finland 1990–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No of singletons . | No of multiples . | Risk of CHD in ART multiples vs. ART singletons . | Risk of CHD in SC multiples vs. SC singletons . | ||||
|---|---|---|---|---|---|---|---|---|
| . | ART = 127 275 . | SC = 7 380 916 . | ART = 44 460 . | SC =194 930 . | Crude OR (95% CI) value . | Adjusted OR* (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs diagnosed within the first year of life, (%) | 2059 (1.62) | 82 119 (1.11) | 1100 (2.47) | 4705 (2.41) | 1.54 (1.43–1.66) < .001 | 1.70 (1.58–1.84) < .001 | 2.20 (2.13–2.26) < .001 | 2.17 (2.10–2.24) < .001 |
| Severe CHD diagnosed within the first year of life, (%) | 399 (.31) | 18 539 (0.25) | 195 (0.44) | 836 (0.43) | 1.40 (1.18–1.66) < .001 | 1.46 (1.22–1.75) < .001 | 1.71 (1.60–1.83) < .001 | 1.70 (1.58–1.82) < .001 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | ||||||||
| Lesion group 1 conotruncal, (%) | 138 (0.11) | 6057 (0.08) | 56 (0.13) | 257 (0.13) | 1.16 (0.85–1.59) .344 | 1.19 (0.86–1.65) .281 | 1.61 (1.42–1.82) < .001 | 1.58 (1.39–1.79) < .001 |
| Lesion group 2 non-conotruncal, (%) | 99 (0.08) | 5499 (0.07) | 59 (0.13) | 201 (0.10) | 1.71 (1.24–2.36) .001 | 1.63 (1.17–2.28) .004 | 1.38 (1.20–1.59) < .001 | 1.37 (1.19–1.57) < .001 |
| Lesion group 3 coarctation aortae, (%) | 64 (0.05) | 3221 (0.04) | 41 (0.09) | 176 (0.09) | 1.83 (1.24–2.72) .002 | 2.05 (1.36–3.11) .001 | 2.07 (1.78–2.41) < .001 | 2.05 (1.76–2.39) < .001 |
| Lesion group 4 VSD, (%) | 882 (0.69) | 34 552 (0.47) | 330 (0.74) | 1667 (0.86) | 1.07 (0.94–1.22) .286 | 1.26 (1.10–1.44) .001 | 1.83 (1.75–1.93) < .001 | 1.83 (1.74–1.92) < .001 |
| Lesion group 5 ASD, (%) | 427 (0.34) | 12 569 (0.17) | 293 (0.66) | 1218 (0.62) | 1.97 (1.70–2.29) < .001 | 2.49 (2.13–2.92) < .001 | 3.69 (3.47–3.91) < .001 | 3.50 (3.30–3.71) < .001 |
| Lesion group 6 Other CHDs, (%) | 449 (0.35) | 20 221 (0.27) | 321 (0.72) | 1186 (0.61) | 2.05 (1.78–2.37) < .001 | 1.93 (1.66–2.34) < .001 | 2.23 (2.10–2.36) < .001 | 2.21 (2.08–2.34) < .001 |
| . | No of singletons . | No of multiples . | Risk of CHD in ART multiples vs. ART singletons . | Risk of CHD in SC multiples vs. SC singletons . | ||||
|---|---|---|---|---|---|---|---|---|
| . | ART = 127 275 . | SC = 7 380 916 . | ART = 44 460 . | SC =194 930 . | Crude OR (95% CI) value . | Adjusted OR* (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs diagnosed within the first year of life, (%) | 2059 (1.62) | 82 119 (1.11) | 1100 (2.47) | 4705 (2.41) | 1.54 (1.43–1.66) < .001 | 1.70 (1.58–1.84) < .001 | 2.20 (2.13–2.26) < .001 | 2.17 (2.10–2.24) < .001 |
| Severe CHD diagnosed within the first year of life, (%) | 399 (.31) | 18 539 (0.25) | 195 (0.44) | 836 (0.43) | 1.40 (1.18–1.66) < .001 | 1.46 (1.22–1.75) < .001 | 1.71 (1.60–1.83) < .001 | 1.70 (1.58–1.82) < .001 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | ||||||||
| Lesion group 1 conotruncal, (%) | 138 (0.11) | 6057 (0.08) | 56 (0.13) | 257 (0.13) | 1.16 (0.85–1.59) .344 | 1.19 (0.86–1.65) .281 | 1.61 (1.42–1.82) < .001 | 1.58 (1.39–1.79) < .001 |
| Lesion group 2 non-conotruncal, (%) | 99 (0.08) | 5499 (0.07) | 59 (0.13) | 201 (0.10) | 1.71 (1.24–2.36) .001 | 1.63 (1.17–2.28) .004 | 1.38 (1.20–1.59) < .001 | 1.37 (1.19–1.57) < .001 |
| Lesion group 3 coarctation aortae, (%) | 64 (0.05) | 3221 (0.04) | 41 (0.09) | 176 (0.09) | 1.83 (1.24–2.72) .002 | 2.05 (1.36–3.11) .001 | 2.07 (1.78–2.41) < .001 | 2.05 (1.76–2.39) < .001 |
| Lesion group 4 VSD, (%) | 882 (0.69) | 34 552 (0.47) | 330 (0.74) | 1667 (0.86) | 1.07 (0.94–1.22) .286 | 1.26 (1.10–1.44) .001 | 1.83 (1.75–1.93) < .001 | 1.83 (1.74–1.92) < .001 |
| Lesion group 5 ASD, (%) | 427 (0.34) | 12 569 (0.17) | 293 (0.66) | 1218 (0.62) | 1.97 (1.70–2.29) < .001 | 2.49 (2.13–2.92) < .001 | 3.69 (3.47–3.91) < .001 | 3.50 (3.30–3.71) < .001 |
| Lesion group 6 Other CHDs, (%) | 449 (0.35) | 20 221 (0.27) | 321 (0.72) | 1186 (0.61) | 2.05 (1.78–2.37) < .001 | 1.93 (1.66–2.34) < .001 | 2.23 (2.10–2.36) < .001 | 2.21 (2.08–2.34) < .001 |
ART, assisted reproductive technology; ASD, atrial septal defect; CHD, congenital heart defect; CI, confidence interval; OR, odds ratio; SC, spontaneous conception; VSD, ventricular septal defect.
c Lesion groups 1–6 according to Botto et al . 34
Table 5 shows the results for singletons born after ICSI ( n = 42 385), IVF ( n = 59 244), and SC ( n = 5 949 193). Major CHDs were detected among 1.67% ( n = 709) of singletons born after ICSI and among 1.51% ( n = 895) singletons born after IVF (adjusted OR 1.07; 95% CI 0.97–1.18; P = .200; Table 5 ).
Risk of congenital heart defects by type of in vitro fertilization treatment (intracytoplasmic sperm injection or in vitro fertilization) in singletons conceived by assisted reproductive technology and spontaneous conception (Denmark 1994–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No of singletons . | Risk of CHD, ICSI vs. IVF . | Risk of CHD, ICSI vs. SC . | Risk of CHD, IVF vs. SC . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | ICSI = 42 385 . | IVF = 59 244 . | SC = 5 949 193 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs (%) | 709 (1.67) | 895 (1.51) | 63 675 (1.07) | 1.11 (1.00–1.22) .041 | 1.07 (0.97–1.18) .200 | 1.57 (1.46–1.69) < .001 | 1.21 (1.12–1.31) < .001 | 1.42 (1.33–1.52) < .001 | 1.14 (1.07–1.22) < .001 |
| Severe CHDs (%) | 124 (0.29) | 180 (0.30) | 14 390 (0.24) | 0.96 (0.77–1.21) .746 | 0.93 (0.74–1.17) .536 | 1.21 (1.01–1.44) .035 | 1.17 (0.98–1.40) .085 | 1.26 (1.08–1.46) .002 | 1.19 (1.03–1.39) .019 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | |||||||||
| Lesion group 1 conotruncal, (%) | 37 (0.09) | 70 (0.12) | 4896 (0.08) | 0.74 (0.50–1.10) .136 | 0.73 (0.49–1.10) .130 | 1.06 (0.77–1.47) .721 | 0.94 (0.68–1.30) .688 | 1.44 (1.13–1.82) .003 | 1.28 (1.01–1.62) .046 |
| Lesion group 2 non-conotruncal, (%) | 31 (0.07) | 42 (0.07) | 4500 (0.08) | 1.03 (0.65–1.64) .895 | 1.03 (0.64–1.67) .889 | 0.97 (0.68–1.38) .852 | 1.14 (0.80–1.63) .459 | 0.94 (0.69–1.27) .676 | 1.00 (0.74–1.36) .995 |
| Lesion group 3 coarctation aortae, (%) | 21 (0.05) | 28 (0.05) | 2185 (0.04) | 1.05 (0.60–1.85) .870 | 0.99 (0.56–1.76) .977 | 1.35 (0.88–2.07) .172 | 1.06 (0.69–1.63) .795 | 1.29 (0.89–1.87) .185 | 1.07 (0.73–1.56) .733 |
| Lesion group 4 VSD, (%) | 270 (0.64) | 356 (0.60) | 24 349 (0.41) | 1.06 (0.90–1.24) .468 | 1.03 (0.87–1.21) .752 | 1.56 (1.38–1.76) < .001 | 1.08 (0.96–1.22) .198 | 1.47 (1.32–1.63) < .001 | 1.09 (0.98–1.21) .115 |
| Lesion group 5 ASD, (%) | 191 (0.45) | 209 (0.35) | 11 598 (0.19) | 1.28 (1.05–1.56) .014 | 1.18 (0.97–1.44) .101 | 2.32 (2.01–2.67) < .001 | 1.42 (1.23–1.64) < .001 | 1.81 (1.58–2.08) < .001 | 1.22 (1.06–1.41) .005 |
| Lesion group 6 other CHDs, (%) | 159 (0.38) | 190 (0.32) | 16 147 (0.27) | 1.17 (0.95–1.45) .144 | 1.17 (0.94–1.45) .163 | 1.38 (1.18–1.62) < .001 | 1.33 (1.14–1.56) < .001 | 1.18 (1.02–1.36) .022 | 1.14 (0.99–1.32) .079 |
| . | No of singletons . | Risk of CHD, ICSI vs. IVF . | Risk of CHD, ICSI vs. SC . | Risk of CHD, IVF vs. SC . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | ICSI = 42 385 . | IVF = 59 244 . | SC = 5 949 193 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs (%) | 709 (1.67) | 895 (1.51) | 63 675 (1.07) | 1.11 (1.00–1.22) .041 | 1.07 (0.97–1.18) .200 | 1.57 (1.46–1.69) < .001 | 1.21 (1.12–1.31) < .001 | 1.42 (1.33–1.52) < .001 | 1.14 (1.07–1.22) < .001 |
| Severe CHDs (%) | 124 (0.29) | 180 (0.30) | 14 390 (0.24) | 0.96 (0.77–1.21) .746 | 0.93 (0.74–1.17) .536 | 1.21 (1.01–1.44) .035 | 1.17 (0.98–1.40) .085 | 1.26 (1.08–1.46) .002 | 1.19 (1.03–1.39) .019 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | |||||||||
| Lesion group 1 conotruncal, (%) | 37 (0.09) | 70 (0.12) | 4896 (0.08) | 0.74 (0.50–1.10) .136 | 0.73 (0.49–1.10) .130 | 1.06 (0.77–1.47) .721 | 0.94 (0.68–1.30) .688 | 1.44 (1.13–1.82) .003 | 1.28 (1.01–1.62) .046 |
| Lesion group 2 non-conotruncal, (%) | 31 (0.07) | 42 (0.07) | 4500 (0.08) | 1.03 (0.65–1.64) .895 | 1.03 (0.64–1.67) .889 | 0.97 (0.68–1.38) .852 | 1.14 (0.80–1.63) .459 | 0.94 (0.69–1.27) .676 | 1.00 (0.74–1.36) .995 |
| Lesion group 3 coarctation aortae, (%) | 21 (0.05) | 28 (0.05) | 2185 (0.04) | 1.05 (0.60–1.85) .870 | 0.99 (0.56–1.76) .977 | 1.35 (0.88–2.07) .172 | 1.06 (0.69–1.63) .795 | 1.29 (0.89–1.87) .185 | 1.07 (0.73–1.56) .733 |
| Lesion group 4 VSD, (%) | 270 (0.64) | 356 (0.60) | 24 349 (0.41) | 1.06 (0.90–1.24) .468 | 1.03 (0.87–1.21) .752 | 1.56 (1.38–1.76) < .001 | 1.08 (0.96–1.22) .198 | 1.47 (1.32–1.63) < .001 | 1.09 (0.98–1.21) .115 |
| Lesion group 5 ASD, (%) | 191 (0.45) | 209 (0.35) | 11 598 (0.19) | 1.28 (1.05–1.56) .014 | 1.18 (0.97–1.44) .101 | 2.32 (2.01–2.67) < .001 | 1.42 (1.23–1.64) < .001 | 1.81 (1.58–2.08) < .001 | 1.22 (1.06–1.41) .005 |
| Lesion group 6 other CHDs, (%) | 159 (0.38) | 190 (0.32) | 16 147 (0.27) | 1.17 (0.95–1.45) .144 | 1.17 (0.94–1.45) .163 | 1.38 (1.18–1.62) < .001 | 1.33 (1.14–1.56) < .001 | 1.18 (1.02–1.36) .022 | 1.14 (0.99–1.32) .079 |
ART, assisted reproductive technology; ASD, atrial septal defect; CHD, congenital heart defect; CI, confidence interval; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation; OR, odds ratio; SC, spontaneous conception; VSD, ventricular septal defect.
a Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, maternal CHD, and fresh and frozen embryo transfer.
b Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, and maternal CHD.
c Major CHDs and severe CHDs according to the EUROCAT 1.5 definition. 32 , 33 .
d Lesion groups 1–6 according to Botto et al . 34
Table 6 presents the results for singletons born after frozen ( n = 18 875) and fresh ( n = 83 649) embryo transfer and SC ( n = 5 949 193). The occurrence of major CHDs among singletons born after FET was 1.82% ( n = 343) and among singletons born after fresh embryo transfer 1.54% ( n = 1286; adjusted OR 1.04; 95% CI 0.91–1.18; P = .603).
Risk of congenital heart defects by frozen and fresh embryo transfer in singletons conceived by assisted reproductive technology and spontaneous conception (Denmark 1994–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No of singletons . | Risk of CHD, frozen vs. fresh embryo transfer . | Risk of CHD, frozen embryo transfer vs. SC . | ||||
|---|---|---|---|---|---|---|---|
| . | Frozen embryo transfer = 18 875 . | Fresh embryo transfer = 83 649 . | Spontaneous conception = 5 949 193 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs , (%) | 343 (1.82) | 1286 (1.54) | 63 675 (1.07) | 1.19 (1.05–1.34) .006 | 1.04 (0.91–1.18) .603 | 1.71 (1.54–1.90) < .001 | 1.20 (1.08–1.34) .001 |
| Severe CHDs , (%) | 64 (0.34) | 244 (0.29) | 14 390 (0.24) | 1.16 (0.88–1.53) .283 | 1.04 (0.77–1.41) .784 | 1.40 (1.10–1.79) .007 | 1.30 (1.02–1.67) .037 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | |||||||
| Lesion group 1 conotruncal, (%) | 24 (0.13) | 85 (0.10) | 4896 (0.08) | 1.25 (0.80–1.97) .332 | 1.18 (0.72–1.95) .504 | 1.55 (1.03–2.31) .033 | 1.33 (0.89–1.98) .171 |
| Lesion group 2 non-conotruncal, (%) | 15 (0.08) | 58 (0.07) | 4500 (0.08) | 1.15 (0.65–2.02) .638 | 1.43 (0.77–2.66) .261 | 1.05 (0.63–1.74) .848 | 1.26 (0.76–2.09) .375 |
| Lesion group 3 coarctatio aortae, (%) | 12 (0.06) | 38 (0.05) | 2185 (0.04) | 1.40 (0.73–2.68) .310 | 1.13 (0.56–2.29) .726 | 1.73 (0.98–3.05) .058 | 1.22 (0.69–2.16) .488 |
| Lesion group 4 VSD, (%) | 137 (0.73) | 493 (0.59) | 24 349 (0.41) | 1.23 (1.02–1.49) .031 | 1.00 (0.82–1.23) .963 | 1.78 (1.50–2.11) < .001 | 1.06 (0.89–1.25) .516 |
| Lesion group 5 ASD, (%) | 91 (0.48) | 320 (0.38) | 11 598 (0.19) | 1.26 (1.00–1.59) .051 | 1.04 (0.80–1.34) .766 | 2.48 (2.02–3.05) < .001 | 1.33 (1.08–1.63) .008 |
| Lesion group 6 other CHDs, (%) | 64 (0.34) | 292 (0.35) | 16 147 (0.27) | 0.97 (0.74–1.27) .833 | 0.95 (0.70–1.29) .755 | 1.25 (0.98–1.60) .075 | 1.20 (0.93–1.53) .155 |
| . | No of singletons . | Risk of CHD, frozen vs. fresh embryo transfer . | Risk of CHD, frozen embryo transfer vs. SC . | ||||
|---|---|---|---|---|---|---|---|
| . | Frozen embryo transfer = 18 875 . | Fresh embryo transfer = 83 649 . | Spontaneous conception = 5 949 193 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Major CHDs , (%) | 343 (1.82) | 1286 (1.54) | 63 675 (1.07) | 1.19 (1.05–1.34) .006 | 1.04 (0.91–1.18) .603 | 1.71 (1.54–1.90) < .001 | 1.20 (1.08–1.34) .001 |
| Severe CHDs , (%) | 64 (0.34) | 244 (0.29) | 14 390 (0.24) | 1.16 (0.88–1.53) .283 | 1.04 (0.77–1.41) .784 | 1.40 (1.10–1.79) .007 | 1.30 (1.02–1.67) .037 |
| Major CHDs diagnosed within the first year of life according to the hierarchic classification of Botto | |||||||
| Lesion group 1 conotruncal, (%) | 24 (0.13) | 85 (0.10) | 4896 (0.08) | 1.25 (0.80–1.97) .332 | 1.18 (0.72–1.95) .504 | 1.55 (1.03–2.31) .033 | 1.33 (0.89–1.98) .171 |
| Lesion group 2 non-conotruncal, (%) | 15 (0.08) | 58 (0.07) | 4500 (0.08) | 1.15 (0.65–2.02) .638 | 1.43 (0.77–2.66) .261 | 1.05 (0.63–1.74) .848 | 1.26 (0.76–2.09) .375 |
| Lesion group 3 coarctatio aortae, (%) | 12 (0.06) | 38 (0.05) | 2185 (0.04) | 1.40 (0.73–2.68) .310 | 1.13 (0.56–2.29) .726 | 1.73 (0.98–3.05) .058 | 1.22 (0.69–2.16) .488 |
| Lesion group 4 VSD, (%) | 137 (0.73) | 493 (0.59) | 24 349 (0.41) | 1.23 (1.02–1.49) .031 | 1.00 (0.82–1.23) .963 | 1.78 (1.50–2.11) < .001 | 1.06 (0.89–1.25) .516 |
| Lesion group 5 ASD, (%) | 91 (0.48) | 320 (0.38) | 11 598 (0.19) | 1.26 (1.00–1.59) .051 | 1.04 (0.80–1.34) .766 | 2.48 (2.02–3.05) < .001 | 1.33 (1.08–1.63) .008 |
| Lesion group 6 other CHDs, (%) | 64 (0.34) | 292 (0.35) | 16 147 (0.27) | 0.97 (0.74–1.27) .833 | 0.95 (0.70–1.29) .755 | 1.25 (0.98–1.60) .075 | 1.20 (0.93–1.53) .155 |
a Adjustment for child’s year of birth, country of birth, maternal age, parity, maternal smoking, maternal diabetes, maternal CHD, and IVF/ICSI.
d Lesion groups 1–6 according to Botto et al. 34
Severe congenital heart defects
Severe CHDs were detected in 594 children born after ART (0.35%) and in 19 375 children born after SC (0.26%; adjusted OR 1.30; 95% CI 1.20–1.42; P < .001; Table 2 ). Severe CHDs occurred among 0.31% ( n = 399) singletons born after ART and among 0.25% ( n = 18 539) singletons born after SC (adjusted OR 1.20; 95% CI 1.09–1.33; P < .001; Table 3 ). In multiples born after ART, the prevalence of severe CHDs was 0.44% ( n = 195; adjusted OR 1.46; 95% CI 1.22–1.75; P < .001 vs. singletons conceived after ART; Table 4 ). In multiples born after SC, the prevalence of severe CHDs was 0.43% ( n = 836; adjusted OR 1.70; 95% CI 1.58–1.82; P < .001 vs. spontaneously conceived singletons; Table 4 ). No significant difference in risk of severe CHDs was seen for multiples born after ART vs. multiples born after SC ( Table 3 ).
Severe CHDs occurred among singletons born after ICSI in 0.29% ( n = 124) and among singletons born after IVF in 0.30% ( n = 180; adjusted OR 0.93; 95% CI 0.74–1.17; P = .536; Table 5 ). In singletons born after FET, the prevalence of severe CHDs was 0.34% ( n = 64), and among singletons born after fresh embryo, transfer the prevalence was 0.29% ( n = 244; adjusted OR 1.04; 95% CI 0.77–1.41; P = .784).
Information on smoking was missing in ∼15% of the study population. A sensitivity analysis with no imputation on smoking did not alter results (adjusted OR for major CHDs 1.35; 95% CI 1.30–1.40, P < .001 for all countries combined, singletons and multiples).
In a second sensitivity analysis, we excluded observations from Norway and added an adjustment for maternal highest educational level, data which were not available for Norway. Including singletons and multiples from Denmark, Finland, and Sweden, the adjusted OR for major CHDs was 1.36 (95% CI 1.30–1.41, P < .001).
The analysis including Finnish data with validated major CHDs showed similar results as the main analysis (adjusted OR for major CHDs 1.35; 95% CI 1.30–1.41, P < .001 for all countries combined, and adjusted OR 1.31; 95% CI 1.19–1.44, P < .001 for Finland).
Including 2 783 464 infants born between 2006 and 2015, small differences in rates of any major CHDs and severe CHDs were found, compared with the whole time period (see Supplementary data online , Table S4 ). For major CHDs, all countries combined, the absolute rates during this period were 1.88% for ART and 1.42% for SC and 0.34% and 0.25% for severe CHDs, respectively. For Denmark, the absolute rates for both major CHDs and severe CHDs decreased, while for the other Nordic countries, the rates during the more recent time period stayed almost unchanged or varied slightly up or down. The adjusted ORs remain, however, rather unchanged (major CHDs, adjusted OR 1.31, 95% CI 1.24–1.37, P < .001; severe CHDs, adjusted OR 1.35, 95% CI 1.20–1.52, P < .001). Lastly, including only Swedish data and adding paternal CHDs as a covariate did not change the results for major CHDs (adjusted OR 1.22; 95% CI 1.16–1.29, P < .001).
Congenital heart defects according to the classification of Botto
According to the hierarchical CHD classification, the risk of CHDs was higher in children born after ART than in spontaneously conceived children for five of the six lesion groups: conotruncal defects, non-conotruncal defects, VSD, ASD, and other CHDs ( Table 2 ).
Singletons born after ART had increased risk for four lesion groups compared with spontaneously conceived singletons: conotruncal defects, VSD, ASD, and other CHDs ( Table 3 ).
For multiples born after ART vs. singletons born after ART a higher risk was seen for five of the six lesion groups: non-conotruncal defects, coarctation aortae, VSD, ASD, and other CHDs, and for multiples born after SC vs. spontaneously conceived singletons, the risk was increased for all six lesion groups ( Table 4 ).
In singletons born after ART, no difference was seen between ICSI and IVF ( Table 5 ), or between frozen and fresh embryo transfer ( Table 6 ) for any of the six lesion groups.
Selected specific congenital heart defects
We analysed 10 selected major CHDs ( Table 7 ). In singletons, significantly increased risks in ART were seen for three CHDs: isomerism of atrial appendages, atrioventricular septal defects, and tetralogy of Fallot. Also including multiples, increased risk was seen also for pulmonary valve atresia (see Supplementary data online , Table S5 ).
Risk of selected major congenital heart defects in singletons conceived by assisted reproductive technology vs. spontaneous conception (Denmark 1994–2014, Finland 1990–2014, Norway 1984–2015, and Sweden 1987–2015)
| . | No. of children . | Risk of CHD, ART vs. spontaneous conception . | ||
|---|---|---|---|---|
| . | ART = 127 275 . | SC = 7 380 916 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Common arterial truncus (Q20.0 ), (%) | 15 (0.01) | 613 (0.01) | 1.42 (0.85–2.37) .180 | 1.50 (0.89–2.52) .126 |
| Double outlet right ventricle (Q20.1 ), (%) | 23 (0.02) | 831 (0.01) | 1.61 (1.06–2.43) .025 | 1.26 (0.83–1.91) .281 |
| Complete transposition of the great vessel (Q20.3 ), (%) | 46 (0.04) | 2676 (0.04) | 1.00 (0.74–1.33) .983 | 0.99 (0.74–1.33) .949 |
| Isomerism of atrial appendages with asplenia or polysplenia (Q20.6 ), (%) | 11 (0.01) | 196 (0.003) | 3.25 (1.77–5.97) < .001 | 2.79 (1.50–5.18) < .001 |
| Atrioventricular septal defect (Q21.2 ), (%) | 90 (0.07) | 3327 (0.05) | 1.57 (1.27–1.93) < .001 | 1.28 (1.03–1.58) .023 |
| Tetralogy of Fallot (Q21.3 ), (%) | 60 (0.05) | 2272 (0.03) | 1.53 (1.19–1.98) < .001 | 1.34 (1.03–1.73) .028 |
| Pulmonary valve atresia (Q22.0 ), (%) | 29 (0.02) | 1407 (0.02) | 1.20 (0.83–1.73) .342 | 1.45 (1.00–2.10) .052 |
| Tricuspid atresia and stenosis (Q22.4 ), (%) | 9 (0.01) | 447 (0.01) | 1.17 (0.60–2.26) .645 | 1.37 (0.70–2.67) .357 |
| Hypoplastic left heart syndrome (Q23.4 ), (%) | 26 (0.02) | 1607 (0.02) | 0.94 (.64–1.38) .747 | 1.05 (0.71–1.54) .825 |
| Coarctation aortae (Q25.1 ), (%) | 80 (0.06) | 4112 (0.06) | 1.13 (0.90–1.41) .285 | 0.97 (0.78–1.22) .808 |
| . | No. of children . | Risk of CHD, ART vs. spontaneous conception . | ||
|---|---|---|---|---|
| . | ART = 127 275 . | SC = 7 380 916 . | Crude OR (95% CI) value . | Adjusted OR (95% CI) value . |
| Common arterial truncus (Q20.0 ), (%) | 15 (0.01) | 613 (0.01) | 1.42 (0.85–2.37) .180 | 1.50 (0.89–2.52) .126 |
| Double outlet right ventricle (Q20.1 ), (%) | 23 (0.02) | 831 (0.01) | 1.61 (1.06–2.43) .025 | 1.26 (0.83–1.91) .281 |
| Complete transposition of the great vessel (Q20.3 ), (%) | 46 (0.04) | 2676 (0.04) | 1.00 (0.74–1.33) .983 | 0.99 (0.74–1.33) .949 |
| Isomerism of atrial appendages with asplenia or polysplenia (Q20.6 ), (%) | 11 (0.01) | 196 (0.003) | 3.25 (1.77–5.97) < .001 | 2.79 (1.50–5.18) < .001 |
| Atrioventricular septal defect (Q21.2 ), (%) | 90 (0.07) | 3327 (0.05) | 1.57 (1.27–1.93) < .001 | 1.28 (1.03–1.58) .023 |
| Tetralogy of Fallot (Q21.3 ), (%) | 60 (0.05) | 2272 (0.03) | 1.53 (1.19–1.98) < .001 | 1.34 (1.03–1.73) .028 |
| Pulmonary valve atresia (Q22.0 ), (%) | 29 (0.02) | 1407 (0.02) | 1.20 (0.83–1.73) .342 | 1.45 (1.00–2.10) .052 |
| Tricuspid atresia and stenosis (Q22.4 ), (%) | 9 (0.01) | 447 (0.01) | 1.17 (0.60–2.26) .645 | 1.37 (0.70–2.67) .357 |
| Hypoplastic left heart syndrome (Q23.4 ), (%) | 26 (0.02) | 1607 (0.02) | 0.94 (.64–1.38) .747 | 1.05 (0.71–1.54) .825 |
| Coarctation aortae (Q25.1 ), (%) | 80 (0.06) | 4112 (0.06) | 1.13 (0.90–1.41) .285 | 0.97 (0.78–1.22) .808 |
ART, assisted reproductive technology; CI, confidence interval; OR, odds ratio.
a Adjustment for child’s year of birth, country of birth, maternal age.
b ICD-10 codes. Corresponding included ICD-8 and ICD-9 codes are shown in Supplementary data online , Table S2 . A child can have more than one CHD diagnosis.
In this large cohort study of 7.7 million liveborn children, including more than 171 000 children born after ART, we found that ART was associated with an increased risk of major CHDs as well as severe CHDs in both the overall ART population and in the ART singleton population, compared with spontaneously conceived children. Multiples, regardless of conception method, were associated with the highest risk of CHDs. Similar risks were observed in multiples conceived by ART and spontaneous conception, but this comparison is limited by the fact that we were missing information about chorionicity. The lower rate of monochorionic multiples in ART may give a false low risk in ART. Children conceived with ICSI did not seem to have an increased risk for CHDs compared with children conceived with IVF, and no significant difference was found between fresh and FET ( Structured Graphical Abstract ). The estimates were robust without any major changes after adjustments for available confounders or in sensitivity analyses.
Consistent with previous studies, our data showed higher occurrence of CHDs in pregnancies conceived by ART compared with spontaneously conceived pregnancies. 21–23 For specific CHDs, conflicting results have been reported. A large US study, including more than 11 million live births (singletons and multiples), of which 71 050 were conceived by ART, found a nearly three-fold increased risk of cyanotic CHDs in children born after ART, compared with children born after spontaneous conception, in adjusted analysis. 47 In a meta-analysis from 2018, Giorgione et al. analysed some specific CHDs in ART and spontaneously conceived singletons and multiples. They found lower occurrence of tetralogy of Fallot and transposition of the great arteries in the ART group. However, results were based on few events in the ART group. 21 In contrast, a French case–control study of 1583 CHD cases and 4104 controls (singletons and multiples) assessing four different major structural CHDs found 2.4-fold odds of tetralogy of Fallot in children born after ART. 27
The overall risk of birth defects in our cohort has been explored in a previous study. 11 The study showed an increased risk of major birth defects in singletons conceived using ICSI with fresh embryo transfer compared with spontaneously conceived singletons. The risk was increased for most organ systems including the heart. Detailed data on type of CHD group or specific diagnoses were not reported. Further, multifetal pregnancies, an important mediator in risk of CHDs, were not included in our previous study.
Congenital heart defects are a heterogeneous group of diseases including both severe, life-threatening defects and minor abnormalities. 34 , 48 , 49 While most children with CHDs survive to adulthood, health issues persist for many children with CHDs when they grow up. 50 , 51 Children and adolescents with CHDs have an 11-fold increased risk of ischaemic stroke, compared with the general population, although absolute risk is low. 48 , 52 For adults with CHDs, the risks of pulmonary arterial hypertension and endocarditis are increased. 53 , 54 Further, for young adults with CHDs, 1 in 12 develop atrial fibrillation, and 1 in 10 of these develop congestive heart failure, before 42 years of age. 52 , 55
The aetiology of CHDs is mainly unknown, but chromosomal abnormalities and other genetic and environmental factors are considered to predispose to CHDs. 36 , 56 Congenital heart defects may be part of a malformation syndrome due to chromosomal aneuploidy, such as Down syndrome (trisomy 21), Edward syndrome (trisomy 18), Patau syndrome (trisomy 13), Turner syndrome (monosomy X), and Klinefelter syndrome (XXY), or Mendelian syndromes as Alagille–Holt–Oram syndrome and Noonan syndrome. Several environmental risk factors have been identified for CHDs, including both young and advanced maternal age, high parity, smoking, obesity, maternal diabetes, and use of drugs during pregnancy, e.g. antiepileptic and antidepressant drugs. 38 , 41–44 , 57–61 Furthermore, women with a history of CHDs are considered to be at increased risk of having offspring with CHDs. 38 , 39 , 62 , 63 Also, low socioeconomic status has been found to be associated with CHDs. 64 , 65
Prenatal screening with foetal echocardiography for CHDs has been proposed to be beneficial for ART-conceived pregnancies. 21 , 66 , 67 and screening by foetal echocardiography is recommended by the American Heart Association for ART pregnancies. 66 Improved detection rate prenatally may offer the possibility for foetal therapy and/or specialized planning of delivery. However, this screening is still controversial and may cause increased costs and anxiety for the parents. 68–70 Further research is required to determine whether screening with foetal echocardiography, in addition to routine prenatal screening, will reduce morbidity and mortality for ART-conceived children when a major CHD is detected prenatally. In addition, preimplantation genetic testing may identify some CHDs of genetic origin and thereby contribute to decrease the CHDs among liveborn children.
Recent research has hypothesized that the placenta has a role in the development of CHDs, since placental vascular resistance has a direct impact on foetal circulation and thereby the developing foetal heart. 71 Children born with CHDs have smaller placentas with increased vascular abnormalities. 72 Further, studies also show a strong association between preeclampsia and CHDs, especially in early-onset and severe preeclampsia. 73 , 74 Pregnancies conceived with ART, in particular after FET, are associated with increased risk of preeclampsia, both for singleton and multifetal pregnancies. 75–77 An association between preeclampsia and CHD would, however, be expected to translate into a higher risk of CHD after FET which was not observed in the present study.
Twin pregnancies, especially monochorionic twins are associated with a higher risk of CHDs. 78 In recent years, multi-foetal pregnancies in ART have been declining, due to the introduction of the single embryo transfer policy. 79 However, the incidence of twin pregnancies continues to be elevated in ART-conceived pregnancies. 3 , 80
The main strength of this study is the large population with pooled nationwide data cross-linked from several high-quality national registries. Moreover, we explored specific CHD groups and specific assisted reproductive techniques. Detailed information enabled sub-analysis and adjustment for several confounders and comparisons according to multiplicity.
Some limitations should be considered when interpreting the results. Despite similar demography and healthcare systems, the rate of CHDs varied somewhat between countries. The follow-up for Norway was limited to birth, explaining the lower rate of CHDs in Norway. The reason for discrepancies between the other Nordic countries is not known but may be due to differences in registration policies and screening for foetal anomalies. The detection rate of major CHDs prenatally has increased substantially over time, as shown in Denmark leading to an increased termination of pregnancies, with a subsequent decrease in live-birth incidence of major CHDs. 18 This change might have had an impact on the results in this study, particularly since a greater proportion of the ART cohort are born in later years in this study. There were some differences in prenatal screening routines in the four Nordic countries during the study period. All countries had introduced a second trimester ultrasound (gestational week 18–21) between 2004–07 including foetal organ screening and where the large majority of women participated. Norway had a second trimester prenatal screening ultrasound during the whole study period. A first-trimester ultrasound to assess the nuchal fold and determine the risk of aneuploidy was more variably introduced with a higher frequency in Denmark and Finland. Although sensitivity analyses showed some differences in rates of major and severe CHDs in live births in the later years, this seemed to occur in similar way for both ART and spontaneous conception, resulting in only minor changes in adjusted ORs. However, still these changes over time in combination with the much increasing ART population are considered a limitation.
Furthermore, a limitation of this study is the lack of information on CHDs in miscarriages, termination of pregnancies, and stillbirths. This may result in bias if ART-conceived pregnancies have a different probability of prenatal diagnosis with subsequent termination compared with spontaneously conceived pregnancies. A French study by Tararbit et al. 81 found however no difference between ART and SC when evaluating the probability of prenatal diagnosis or termination of pregnancy for CHDs. A previous study on singletons from our cohort indicated similar risk of stillbirth after fresh and frozen embryo transfer compared with singletons conceived without medical assistance. 82 One study limitation is that we relied only on registry data and ICD codes with the potential for miscoding. Some ICD codes, e.g. the codes for VSDs, do not differentiate between severe and less severe CHDs, and we should have needed more data on echocardiography and surgical and other procedures for correct classification. However, we have used different classifications of major CHDs to identify the most complex CHDs. Furthermore, a sensitivity analysis using data from the Finnish birth defects registry with validated major CHDs showed similar results as the main result.
Children conceived after ovulation induction and intrauterine insemination were included in the SC group. This misclassification will, if anything, dilute the association between ART and CHD. 12 Other limitations are that we did not have information about causes of infertility and data on specific techniques used in assisted reproduction was not available from Finland. Finally, as in all observational studies residual confounding by unknown or unmeasured factors may remain.
Congenital heart defects are serious, although rare conditions. This large study reports a higher occurrence of CHDs after ART conception, both severe and less severe. The highest rates of CHDs were observed in children born in multiple pregnancies. No difference in CHDs was found between ICSI and IVF and neither between children born after fresh or frozen transfer. The findings of the current study should be conveyed to patients undergoing counselling before ART. Although the risk for major CHDs is higher in children born after ART, the absolute increase in risks seems to be modest. This study also emphasizes the importance of single embryo transfer to avoid the increased risks in multifetal pregnancies.
We thank all national registries providing individual data for this study.
Supplementary data are available atInterest European Heart Journal online.
Disclosure of Interest
All authors declare no disclosure of interest for this contribution.
Data Availability
The data that support the findings of this study are not available due to regulations restricted by law. Storage of data is arranged and ensured by Statistics Denmark. Research was feasible after receiving approvals from the Ethics Committees and registry-keeping authorities in each country. Administration of microdata from the national registries is regulated by the General Data Protection Regulation (GDPR) ensured by Statistics Denmark. Contact information for Statistics Denmark: Division of Research Services Statistics Denmark Sejrøgade 11 DK-2100 Copenhagen Denmark E-mail: [email protected] Phone: + 45 39 17 31 30.
The CoNARTaS has been supported by the Nordic Trial Alliance: a pilot project jointly funded by the Nordic Council of Ministers and NordForsk ( https://www.nordforsk.org/sv/research-areas/nordic-trial-alliance ) (grant number 71450; A.P.), the Central Norway Regional Health Authorities (grant number 46045000), the Norwegian Cancer Society (grant number 182356-2016; S.O.], the Nordic Federation of Obstetrics and Gynaecology [grant numbers NF13041, NF15058, NF16026, NF17043, and NF399 (2022–23)], the Interreg Öresund-Kattegat-Skagerrak European Regional Development Fund (ReproUnion project; AP), and the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-70940; C.B.), and the Hjalmar Svensson Foundation (U.-B.W.). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethical Approval
Ethical approvals were obtained from Ethical Committee in Gothenburg, Sweden (Dnr 214-12, T422-12, T516-15, T233-16, T300-17, T1144-17, T121-18, T1071-18, 2019-02347, 2022-00903-02), and in Norway from the Regional Committee for Medical and Health Research Ethics (REK-Nord, 2010/1909). There are no requirements for ethical approval for registry-based studies in Denmark and Finland.
Pre-registered Clinical Trial Number
ISRCTN 11780826.
De Geyter C , Calhaz-Jorge C , Goossens V , Magli CM , Smeenk J , Vesela K , et al. EuMAR: a roadmap towards a prospective, cycle-by-cycle registry of medically assisted reproduction in Europe . Hum Reprod Open 2023 ; 2023 : hoad011 . 10.1093/hropen/hoad011
Google Scholar
ART fact sheet 2023. https://www.eshre.eu/Press-Room/Resources/Fact-sheets (21 December 2023, date last accessed).
European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) ; Smeenk J , Wyns C , De Geyter C , Kupka M , Bergh C , et al. ART in Europe, 2019: results generated from European registries by ESHREdagger . Hum Reprod 2023 ; 38 : 2321 – 38 . 10.1093/humrep/dead197
Centers for Disease Control and Prevention. 2021 Assisted reproductive technology fertility clinic and national summary report . US Dept of Health and Human Services; 2023. https://www.cdc.gov/art/reports/2021/index.html (28 December 2023, date last accessed)
McDonald SD , Han Z , Mulla S , Murphy KE , Beyene J , Ohlsson A . Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses . Eur J Obstet Gynecol Reprod Biol 2009 ; 146 : 138 – 48 . 10.1016/j.ejogrb.2009.05.035
Jackson RA , Gibson KA , Wu YW , Croughan MS . Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis . Obstet Gynecol 2004 ; 103 : 551 – 63 . 10.1097/01.AOG.0000114989.84822.51
Qin JB , Sheng XQ , Wu D , Gao SY , You YP , Yang TB , et al. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis . Arch Gynecol Obstet 2017 ; 295 : 285 – 301 . 10.1007/s00404-016-4250-3
Pandey S , Shetty A , Hamilton M , Bhattacharya S , Maheshwari A . Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis . Hum Reprod Update 2012 ; 18 : 485 – 503 . 10.1093/humupd/dms018
Hansen M , Kurinczuk JJ , Milne E , de Klerk N , Bower C . Assisted reproductive technology and birth defects: a systematic review and meta-analysis . Hum Reprod Update 2013 ; 19 : 330 – 53 . 10.1093/humupd/dmt006
Zhao J , Yan Y , Huang X , Li Y . Do the children born after assisted reproductive technology have an increased risk of birth defects? A systematic review and meta-analysis . J Matern Fetal Neonatal Med 2020 ; 33 : 322 – 33 . 10.1080/14767058.2018.1488168
Henningsen A-KA , Opdahl S , Wennerholm U-B , Tiitinen A , Rasmussen S , Romundstad LB , et al. Risk of congenital malformations in live-born singletons conceived after intracytoplasmic sperm injection: a nordic study from the CoNARTaS group . Fertil Steril 2023 ; 120 : 1033 – 41 . 10.1016/j.fertnstert.2023.07.003
Venetis C , Choi SKY , Jorm L , Zhang X , Ledger W , Lui K , et al. Risk for congenital anomalies in children conceived with medically assisted fertility treatment: a population-based cohort study . Ann Intern Med 2023 ; 176 : 1308 – 20 . 10.7326/M23-0872
Dolk H , Loane M , Garne E . The prevalence of congenital anomalies in Europe . Adv Exp Med Biol 2010 ; 686 : 349 – 64 . 10.1007/978-90-481-9485-8_20
Marelli AJ , Mackie AS , Ionescu-Ittu R , Rahme E , Pilote L . Congenital heart disease in the general population: changing prevalence and age distribution . Circulation 2007 ; 115 : 163 – 72 . 10.1161/CIRCULATIONAHA.106.627224
Leirgul E , Fomina T , Brodwall K , Greve G , Holmstrøm H , Vollset SE , et al. Birth prevalence of congenital heart defects in Norway 1994–2009–a nationwide study . Am Heart J 2014 ; 168 : 956 – 64 . 10.1016/j.ahj.2014.07.030
Socialstyrelsen.se . Övervakning av fosterskador. https://www.socialstyrelsen.se/statistik-och-data/register/medicinska-fodelseregistret/ (21 December 2023, date last accessed).
Giang KW , Mandalenakis Z , Fedchenko M , Eriksson P , Rosengren A , Norman M , et al. Congenital heart disease: changes in recorded birth prevalence and cardiac interventions over the past half-century in Sweden . Eur J Prev Cardiol 2023 ; 30 : 169 – 76 . 10.1093/eurjpc/zwac227
Lytzen R , Vejlstrup N , Bjerre J , Petersen OB , Leenskjold S , Dodd JK , et al. Live-born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013 . JAMA Cardiol 2018 ; 3 : 829 – 37 . 10.1001/jamacardio.2018.2009
Jepson BM , Metz TD , Miller TA , Son SL , Ou Z , Presson AP , et al. Pregnancy loss in major fetal congenital heart disease: incidence, risk factors and timing . Ultrasound Obstet Gynecol 2023 ; 62 : 75 – 87 . 10.1002/uog.26231
World Health Organization . MORTALITY DATABASE. https://platform.who.int/mortality/themes/theme-details/topics/indicator-groups/indicator-group-details/MDB/congenital-heart-anomalies (21 December 2023, date last accessed).
Giorgione V , Parazzini F , Fesslova V , Cipriani S , Candiani M , Inversetti A , et al. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis . Ultrasound Obstet Gynecol 2018 ; 51 : 33 – 42 . 10.1002/uog.18932
Talebi T , Mohsen-Pour N , Hesami M , Maleki M , Kalayinia S . The association between in vitro fertilization and intracytoplasmic sperm injection treatment and the risk of congenital heart defects . J Matern Fetal Neonatal Med 2022 ; 35 : 7471 – 85 . 10.1080/14767058.2021.1949705
Hoorsan H , Mirmiran P , Chaichian S , Moradi Y , Hoorsan R , Jesmi F . Congenital malformations in infants of mothers undergoing assisted reproductive technologies: a systematic review and meta-analysis study . J Prev Med Public Health 2017 ; 50 : 347 – 60 . 10.3961/jpmph.16.122
Mastroiacovo P , Castilla EE , Arpino C , Botting B , Cocchi G , Goujard J , et al. Congenital malformations in twins: an international study . Am J Med Genet 1999 ; 83 : 117 – 24 . 10.1002/(sici)1096-8628(19990312)83:2<117::aid-ajmg7>3.0.co;2-4
Best KE , Rankin J . Increased risk of congenital heart disease in twins in the north of England between 1998 and 2010 . Heart 2015 ; 101 : 1807 – 12 . 10.1136/heartjnl-2015-307826
Wen SW , Miao Q , Taljaard M , Lougheed J , Gaudet L , Davies M , et al. Associations of Assisted Reproductive Technology and Twin Pregnancy With Risk of Congenital Heart Defects . JAMA Pediatr 2020 ; 174 : 446 – 54 . 10.1001/jamapediatrics.2019.6096
Tararbit K , Lelong N , Thieulin AC , Houyel L , Bonnet D , Goffinet F , et al. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation . Hum Reprod 2013 ; 28 : 367 – 74 . 10.1093/humrep/des400
Opdahl S , Henningsen AA , Bergh C , Gissler M , Romundstad LB , Petzold M , et al. Data resource profile: Committee of Nordic Assisted Reproductive Technology and Safety (CoNARTaS) cohort . Int J Epidemiol 2020 ; 49 : 365 – 366f . 10.1093/ije/dyz228
Laugesen K , Ludvigsson JF , Schmidt M , Gissler M , Valdimarsdottir UA , Lunde A , et al. Nordic Health Registry-based research: a review of health care systems and key registries . Clin Epidemiol 2021 ; 13 : 533 – 54 . 10.2147/CLEP.S314959
Zegers-Hochschild F , Adamson GD , Dyer S , Racowsky C , de Mouzon J , Sokol R , et al. The International Glossary on Infertility and Fertility Care, 2017 . Hum Reprod 2017 ; 32 : 1786 – 801 . 10.1093/humrep/dex234
World Health Organization . International Statistical Classification of Diseases and Related Health Problems (ICD). https://www.who.int/standards/classifications/classification-of-diseases (23 December 2023, date last accessed).
EUROCAT . Subgroups of Congenital Anomalies. https://eu-rd-platform.jrc.ec.europa.eu/system/files/public/eurocat/Guide_1.5_Chapter_3.3.pdf (21 December 2023, date last accessed).
EUROCAT . Minor anomalies and other conditions for exclusion. https://eu-rd-platform.jrc.ec.europa.eu/system/files/public/eurocat/Guide_1.5_Chapter_3.2.pdf (21 January 2024, date last accessed).
Botto LD , Lin AE , Riehle-Colarusso T , Malik S , Correa A . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies . Birth Defects Res A Clin Mol Teratol 2007 ; 79 : 714 – 27 . 10.1002/bdra.20403
Dolk H , Loane M , Garne E ; European Surveillance of Congenital Anomalies (EUROCAT) Working Group . Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005 . Circulation 2011 ; 123 : 841 – 9 . 10.1161/CIRCULATIONAHA.110.958405
Liu S , Joseph KS , Luo W , León JA , Lisonkova S , Van den Hof M , et al. Effect of folic acid food fortification in Canada on congenital heart disease subtypes . Circulation 2016 ; 134 : 647 – 55 . 10.1161/CIRCULATIONAHA.116.022126
Mandalenakis Z , Giang KW , Eriksson P , Liden H , Synnergren M , Wåhlander H , et al. Survival in children with congenital heart disease: have we reached a peak at 97%? J Am Heart Assoc 2020 ; 9 : e017704 . 10.1161/JAHA.120.017704
Oyen N , Poulsen G , Boyd HA , Wohlfahrt J , Jensen PK , Melbye M . Recurrence of congenital heart defects in families . Circulation 2009 ; 120 : 295 – 301 . 10.1161/CIRCULATIONAHA.109.857987
Calcagni G , Digilio MC , Sarkozy A , Dallapiccola B , Marino B . Familial recurrence of congenital heart disease: an overview and review of the literature . Eur J Pediatr 2007 ; 166 : 111 – 6 . 10.1007/s00431-006-0295-9
Cuschieri S . The STROBE guidelines . Saudi J Anaesth 2019 ; 13 : S31 – 4 . 10.4103/sja.SJA_543_18
Miller A , Riehle-Colarusso T , Siffel C , Frias JL , Correa A . Maternal age and prevalence of isolated congenital heart defects in an urban area of the United States . Am J Med Genet A 2011 ; 155A : 2137 – 45 . 10.1002/ajmg.a.34130
Feng Y , Yu D , Chen T , Liu J , Tong X , Yang L , et al. Maternal parity and the risk of congenital heart defects in offspring: a dose-response meta-analysis of epidemiological observational studies . PLoS One 2014 ; 9 : e108944 . 10.1371/journal.pone.0108944
Lee LJ , Lupo PJ . Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: a systematic review and metaanalysis . Pediatr Cardiol 2013 ; 34 : 398 – 407 . 10.1007/s00246-012-0470-x
Zhang TN , Huang XM , Zhao XY , Wang W , Wen R , Gao SY . Risks of specific congenital anomalies in offspring of women with diabetes: a systematic review and meta-analysis of population-based studies including over 80 million births . PLoS Med 2022 ; 19 : e1003900 . 10.1371/journal.pmed.1003900
International Standard Classification of Education (ISCED). Statistics explained. https://ec.europa.eu/eurostat/statistics-explained/index.php? title=Main_Page (1 February 2024, date last accessed).
The Finnish Institute for Health and Wellfare . Quality description of the Finnish Register for Congenital Malformations. https://thl.fi/en/statistics-and-data/data-and-services/quality-and-statistical-principles/quality-descriptions/congenital-anomalies (21 January 2024, date last accessed).
Shechter-Maor G , Czuzoj-Shulman N , Spence AR , Abenhaim HA . The effect of assisted reproductive technology on the incidence of birth defects among livebirths . Arch Gynecol Obstet 2018 ; 297 : 1397 – 403 . 10.1007/s00404-018-4694-8
Mandalenakis Z , Rosengren A , Skoglund K , Lappas G , Eriksson P , Dellborg M . Survivorship in children and young adults with congenital heart disease in Sweden . JAMA Intern Med 2017 ; 177 : 224 – 30 . 10.1001/jamainternmed.2016.7765
Baumgartner H , Backer D , Babu-Narayan J , Budts SV , Chessa W , Diller M , et al. 2020 ESC guidelines for the management of adult congenital heart disease . Eur Heart J 2021 ; 42 : 563 – 645 . 10.1093/eurheartj/ehaa554
Bouma BJ , Sieswerda GT , Post MC , Ebels T , van Kimmenade R , de Winter RJ , et al. New developments in adult congenital heart disease . Neth Heart J 2020 ; 28 : 44 – 9 . 10.1007/s12471-020-01455-5
Fedchenko M , Mandalenakis Z , Giang KW , Rosengren A , Eriksson P , Dellborg M . Long-term outcomes after myocardial infarction in middle-aged and older patients with congenital heart disease-a nationwide study . Eur Heart J 2021 ; 42 : 2577 – 86 . 10.1093/eurheartj/ehaa874
Mandalenakis Z , Rosengren A , Lappas G , Eriksson P , Hansson PO , Dellborg M . Ischemic stroke in children and young adults with congenital heart disease . J Am Heart Assoc 2016 ; 5: e003071 . 10.1161/JAHA.115.003071
Bouma BJ , Mulder BJ . Changing landscape of congenital heart disease . Circ Res 2017 ; 120 : 908 – 22 . 10.1161/CIRCRESAHA.116.309302
Snygg-Martin U , Giang KW , Dellborg M , Robertson J , Mandalenakis Z . Cumulative incidence of infective endocarditis in patients with congenital heart disease: a nationwide, case-control study over nine decades . Clin Infect Dis 2021 ; 73 : 1469 – 75 . 10.1093/cid/ciab478
Mandalenakis Z , Rosengren A , Lappas G , Eriksson P , Gilljam T , Hansson PO , et al. Atrial fibrillation burden in young patients with congenital heart disease . Circulation 2018 ; 137 : 928 – 37 . 10.1161/CIRCULATIONAHA.117.029590
Blue GM , Kirk EP , Sholler GF , Harvey RP , Winlaw DS . Congenital heart disease: current knowledge about causes and inheritance . Med J Aust 2012 ; 197 : 155 – 9 . 10.5694/mja12.10811
Mamasoula C , Bigirumurame T , Chadwick T , Addor MC , Cavero-Carbonell C , Dias CM , et al. Maternal age and the prevalence of congenital heart defects in Europe, 1995–2015: a register-based study . Birth Defects Res 2023 ; 115 : 583 – 94 . 10.1002/bdr2.2152
Cai GJ , Sun XX , Zhang L , Hong Q . Association between maternal body mass index and congenital heart defects in offspring: a systematic review . Am J Obstet Gynecol 2014 ; 211 : 91 – 117 . 10.1016/j.ajog.2014.03.028
Weston J , Bromley R , Jackson CF , Adab N , Clayton-Smith J , Greenhalgh J , et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child . Cochrane Database Syst Rev 2016 ; 11 : CD010224 . 10.1002/14651858.CD010224.pub2
Pugnaloni F , Felici A , Corno AF , Marino B , Versacci P , Putotto C . Gender differences in congenital heart defects: a narrative review . Transl Pediatr 2023 ; 12 : 1753 – 64 . 10.21037/tp-23-260
Furu K , Kieler H , Haglund B , Engeland A , Selmer R , Stephansson O , et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design . BMJ 2015 ; 350 : h1798 . 10.1136/bmj.h1798
Burn J , Brennan P , Little J , Holloway S , Coffey R , Somerville J , et al. Recurrence risks in offspring of adults with major heart defects: results from first cohort of British collaborative study . Lancet 1998 ; 351 : 311 – 6 . 10.1016/S0140-6736(97)06486-6
Chou HH , Chiou MJ , Liang FW , Chen LH , Lu TH , Li CY . Association of maternal chronic disease with risk of congenital heart disease in offspring . CMAJ 2016 ; 188 : E438 – 46 . 10.1503/cmaj.160061
Miao Q , Dunn S , Wen SW , Lougheed J , Yang P , Davies M , et al. Association between maternal marginalization and infants born with congenital heart disease in Ontario Canada . BMC Public Health 2023 ; 23 : 790 . 10.1186/s12889-023-15660-5
Li X , Sundquist J , Hamano T , Zoller B , Sundquist K . Neighbourhood deprivation, individual-level and familial-level socio-demographic factors and risk of congenital heart disease: a nationwide study from Sweden . Int J Behav Med 2016 ; 23 : 112 – 20 . 10.1007/s12529-015-9488-9
Donofrio MT , Moon-Grady AJ , Hornberger LK , Copel JA , Sklansky MS , Abuhamad A , et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association . Circulation 2014 ; 129 : 2183 – 242 . 10.1161/01.cir.0000437597.44550.5d
The American Institute of Ultrasound in Medicine (AIUM) and Collaborators . AIUM practice parameter for the performance of fetal echocardiography . J Ultrasound Med 2020 ; 39 : E5 – 16 . 10.1002/jum.15188
Patil AS , Nguyen C , Groff K , Wu J , Elliott J , Gunatilake RP . Severity of congenital heart defects associated with assisted reproductive technologies: case series and review of the literature . Birth Defects Res 2018 ; 110 : 654 – 61 . 10.1002/bdr2.1228
Bjorkman KR , Bjorkman SH , Ferdman DJ , Sfakianaki AK , Copel JA , Bahtiyar MO . Utility of routine screening fetal echocardiogram in pregnancies conceived by in vitro fertilization . Fertil Steril 2021 ; 116 : 801 – 8 . 10.1016/j.fertnstert.2021.04.035
Spurr RR , Conwell JA , Young LT , Lewin MB , Edwards LA , Arya B . Utility of screening fetal echocardiogram following normal anatomy ultrasound for in vitro fertilization pregnancies . Pediatr Cardiol 2022 ; 43 : 1349 – 53 . 10.1007/s00246-022-02857-5
Camm EJ , Botting KJ , Sferruzzi-Perri AN . Near to one’s heart: the intimate relationship between the placenta and fetal heart . Front Physiol 2018 ; 9 : 629 . 10.3389/fphys.2018.00629
Snoep MC , Aliasi M , van der Meeren LE , Jongbloed MRM , DeRuiter MC , Haak MC . Placenta morphology and biomarkers in pregnancies with congenital heart disease—a systematic review . Placenta 2021 ; 112 : 189 – 96 . 10.1016/j.placenta.2021.07.297
Auger N , Fraser WD , Healy-Profitos J , Arbour L . Association between preeclampsia and congenital heart defects . JAMA 2015 ; 314 : 1588 – 98 . 10.1001/jama.2015.12505
Liu J , Zhao G , Xie J , Wu S , Li B , Yao J . There is a strong association between early preeclampsia and congenital heart defects: a large population-based, retrospective study . Gynecol Obstet Invest 2021 ; 86 : 40 – 7 . 10.1159/000506804
Opdahl S , Henningsen AA , Tiitinen A , Bergh C , Pinborg A , Romundstad PR , et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group . Hum Reprod 2015 ; 30 : 1724 – 31 . 10.1093/humrep/dev090
Chih HJ , Elias FTS , Gaudet L , Velez MP . Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses . BMC Pregnancy Childbirth 2021 ; 21 : 449 . 10.1186/s12884-021-03938-8
Hoff Petersen S , Westvik-Johari K , Spangmose AL , Pinborg A , Romundstad LB , Bergh C , et al. Risk of hypertensive disorders in pregnancy after fresh and frozen embryo transfer in assisted reproduction: a population-based cohort study with within-sibship analysis . Hypertension 2023 ; 80 : e6 – 16 . 10.1161/HYPERTENSIONAHA.122.19689
Gijtenbeek M , Shirzada MR , Ten Harkel ADJ , Oepkes D , C Haak M . Congenital heart defects in monochorionic twins: a systematic review and meta-analysis . J Clin Med 2019 ; 8 : 902 . 10.3390/jcm8060902
Thurin A , Hausken J , Hillensjo T , Jablonowska B , Pinborg A , Strandell A , et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization . N Engl J Med 2004 ; 351 : 2392 – 402 . 10.1056/NEJMoa041032
Sunderam S , Kissin DM , Zhang Y , Jewett A , Boulet SL , Warner L , et al. Assisted reproductive technology surveillance—United States, 2018 . MMWR Surveill Summ 2022 ; 71 : 1 – 19 . 10.15585/mmwr.ss7104a1
Tararbit K , Lelong N , Jouannic JM , Goffinet F , Khoshnood B . Is the probability of prenatal diagnosis or termination of pregnancy different for fetuses with congenital anomalies conceived following assisted reproductive techniques? A population-based evaluation of fetuses with congenital heart defects . BJOG 2015 ; 122 : 924 – 31 . 10.1111/1471-0528.13345
Westvik-Johari K , Lawlor DA , Romundstad LB , Bergh C , Wennerholm UB , Gissler M , et al. Risk of stillbirth and neonatal death in singletons born after fresh and frozen embryo transfer: cohort study from the committee of nordic assisted reproduction technology and safety . Fertil Steril 2023 ; 119 : 265 – 76 . 10.1016/j.fertnstert.2022.10.020
Author notes
- congenital heart defects
- fertilization in vitro
- embryo transfer
- reproductive techniques, assisted
- sperm injections, intracytoplasmic
Email alerts
Companion article.
- Assisted reproductive technology and heart defects: what’s real and what’s not?
Related articles in PubMed
Citing articles via, looking for your next opportunity, affiliations.
- Online ISSN 1522-9645
- Print ISSN 0195-668X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
How to Publish a Research Paper in Journal. Publishing a research paper in a journal is a crucial step in disseminating scientific knowledge and contributing to the field. Here are the general steps to follow: Choose a research topic: Select a topic of your interest and identify a research question or problem that you want to investigate ...
Every year, we accept and publish more than 470,000 journal articles so you are in safe hands. Publishing in an Elsevier journal starts with finding the right journal for your paper. We have tools, resources and services to help you at each stage of the publication journey to enable you to research, write, publish, promote and track your article.
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open. ... At this stage, the technical editor will also add requests to ensure the paper, if published ...
The first step in publishing a research paper should always be selecting the journal you want to publish in. Choosing your target journal before you start writing means you can tailor your work to build on research that's already been published in that journal.
How does one choose a journal in which to publish and what factors (impact factor, journal content) should be considered? In general, the most important factor to consider when choosing where to submit your article is the fit of the manuscript to the scope and profile of the journal; Aside from the quality of the science and writing, this is the largest factor that will determine whether a ...
3. Read the aims and scope and author guidelines of your target journal carefully. Once you have read and re-read your manuscript carefully several times, received feedback from your colleagues, and identified a target journal, the next important step is to read the aims and scope of the journals in your target research area.
Communicating research findings is an essential step in the research process. Often, peer-reviewed journals are the forum for such communication, yet many researchers are never taught how to write a publishable scientific paper. In this article, we explain ...
B ACKGROUND. The publication of original research in a peer-reviewed and indexed journal is the ultimate and most important step toward the recognition of any scientific work.However, the process starts long before the write-up of a manuscript. The journal in which the author wishes to publish his/her work should be chosen at the time of conceptualization of the scientific work based on the ...
A. Original research is published AOP — that is, Articles and Letters, and for the Nature journals that publish them, Brief Communications. Associated News and Views articles may be published ...
Communicating research findings is an essential step in the research process. Often, peer-reviewed journals are the forum for such communication, yet many researchers are never taught how to write a publishable scientific paper. In this article, we explain the basic structure of a scientific paper and describe the information that should be included in each section. We also identify common ...
Publishing research is only half the battle — promoting it to the right audience is equally important in achieving impact. This is essential when publishing research into alternative methods, as this can help to increase acceptance of such approaches. ... By implementing these strategies, published work from the journal can be effectively ...
Publishing a research paper in a peer-reviewed journal allows you to network with other scholars, get your name and work into circulation, and further refine your ideas and research. Before submitting your paper, make sure it reflects all the work you've done and have several people read over it and make comments.
Publishing a research paper as an undergraduate or as a first-time author can help you get academic funding and credit. It also improves your academic record. Self-Fulfilment: ... Each journal urges that the research work be in standard English. In case of any problem, authors can seek scientific editing services or manuscript editing services ...
If your article is accepted, you'll need to sign a publishing agreement. If your article is rejected, you can get help finding another journal from our transfer desk team. 7. Payment. If your article is open access, you'll need to pay a fee. Fees for OA publishing differ across journals. See relevant journal page for more information.
The most fundamental ingredient is excellent research. Work with the best scientists you can, in the best lab you can find. You will absorb the most about doing excellent science if you are surrounded by it during your training. ... Good news: The journal wants to publish your paper. Still, only on rare occasions will reviewers recommend that ...
There are several key benefits to publishing research in journals: DISCOVERABILITY. Publishing in journals can give your work visibility among other researchers in your field, outside of your immediate circle of contacts and colleagues. Journals can makes your work more discoverable, as they are already being read by circles of interested readers.
Undergraduate research journals aren't indexed in many of the sources we typically use for finding journals, so lists of academic journals focused on publishing undergraduate research compiled by universities and organizations are good starting places for finding a place to publish your work:
This article provides an overview of writing for publication in peer-reviewed journals. While the main focus is on writing a research article, it also provides guidance on factors influencing journal selection, including journal scope, intended audience for the findings, open access requirements, and journal citation metrics.
Successfully publishing a research paper in international journals is a key part of a researcher's work and can shape the trajectory of their careers. This not only helps advance the knowledge in a researcher's field of work but also helps them build networks and even secure funding for new research in the long run. However, irrespective of the discipline, a beginner with even a brilliant ...
Choosing a journal that's right for your research can be more complex than it seems. You want to publish in a journal that will help your study to reach its intended audience. Your research has a better chance of attracting readers, accumulating citations, and impacting the field when your colleagues can easily find it.
Why do we need to publish research paper? Your published paper can help in the public understanding of a research question. Publishing helps establish you as an expert in your field of knowledge. Peer-reviewed publication provides evidence that helps in the evaluation of merit of research funding requests. Now going to our main topic.
Explore the latest peer-reviewed articles and learn how to publish your work in Research in Materials Science. Browse; Search. Close search. ... Find a journal; Search calls for papers; Journal Suggester; Open access publishing ... Register to receive personalised research and resources by email. Sign me up.
Investigate the publisher. Most scholarly articles are published in peer-reviewed journals, e.g. Strategic Management Journal or Journal of Labor Economics; Browse the structure of the article. Scholarly articles often contain multiples sections and have headers such as introduction, literature review, methodology, discussion, conclusion, etc.
This article was published in Research on Social Work Practice. View All Journal Metrics. Article usage * Total views and downloads: 0 * Article usage tracking started in December 2016. ... If you have access to journal content via a personal subscription, university, library, employer or society, select from the options below:
His research focuses on inflation, macroeconomics, and monetary economics. He is published widely in top economics journals and has extensive research experience focused on inflation expectations. Pfajfar holds a BS in economics from the University of Ljubljana, an MS in economics from the University of Warwick, and a PhD in economics from the ...
A federal antitrust lawsuit against a group of megapublishers highlights how academia's system of rewarding researchers for publishing in certain journals has undermined their leverage. A prolific neuroscientist is accusing some of the same companies that published her work in top-tier peer-reviewed journals of conspiring "to hold the careers of scholars hostage" in the name of ...
Published by the American Physical Society under the terms of the Creative Commons Attribution 4.0 International license. Further distribution of this work must maintain attribution to the author(s) and the published article's title, journal citation, and DOI. Published by the American Physical Society
BACKGROUND: BMP9 (bone morphogenetic protein 9) is a member of the TGF-β (transforming growth factor β) family of cytokines with pleiotropic effects on glucose metabolism, fibrosis, and lymphatic development. However, the role of BMP9 in myocardial infarction (MI) remains elusive. METHODS: The expressional profiles of BMP9 in cardiac tissues and plasma samples of subjects with MI were ...
SEATTLE - September 27, 2024 - Researchers at Fred Hutch Cancer Center identified a substantial increase over the past decade in the proportion of patients with cancer in the U.S. who participate in pharmaceutical industry sponsored clinical trials compared to those conducted with federal government support. Published in The Journal of Clinical Oncology and presented at the ASCO Quality ...
The main findings were that assisted reproductive technology (ART) was associated with an increased risk of major congenital heart defects (CHDs) as well as severe CHDs in liveborn children with follow-up to 1 year of age, compared with spontaneous conception (SC).