

NIH FAQs: Biosketch and Other Support
Download the pdf, biosketch faqs.
For additional information, see the NIH Biosketch and Biosketch FAQ pages.
What recent changes has NIH made to the Biosketch format?
NIH has updated the format and instructions for the Other Support and Biographical Sketch pages to facilitate full disclosure of existing requirements. NIH removed Section D from non-fellowship biosketches and “Research Support” from Section D for fellowship biosketches. Details on ongoing and completed research projects/support from the past three years can be included within the personal statement (Section A).
Section B, “Position and Honors” has been renamed “Positions, Scientific Appointments, and Honors” and the order changed from chronological to reverse chronological. See the Biosketch and FAQ webpages and March 2021 notice NOT-OD-21-073 for additional details. NIH has updated the SciENcv Biosketch template.
What needs to be included in the new Biosketch format?
NIH requires applicants to list all positions, honors, and scientific appointments both domestic and foreign held by senior/key personnel that are relevant to an application, including affiliations with foreign entities or governments. This includes titled academic, professional, or institutional appointments whether or not remuneration is received, and whether full-time, part-time, or voluntary (including adjunct, visiting, or honorary).
Does my appointment at a foreign institution need to be reported even if it is not related to my NIH Supported work?
Yes, all appointments need to be reported in the Biosketch and, if related to research endeavors, in Other Support as well.
The Biosketch instructions state that all positions and scientific appointments must be provided. Does this refer to active positions and appointments, or all positions a researcher has ever held?
The Biosketch must include all current positions and scientific appointments.
What is the effective date for the new Biographical Sketch formats?
The new formats are required for application due dates on or after January 25, 2022.
Other Support FAQs
General other support.
For additional information, see NIH Other Support and FAQ pages.
What recent changes has NIH made to the Other Support format?
NIH has updated the format and instructions for the Other Support and Biographical Sketch pages to facilitate full disclosure of existing requirements. Requirements were clarified in the 2019 notice NOT-OD-19-114 , which included disclosure of in-kind and foreign support, including support provided directly to the individual PI/key personnel (not to the institution). Details on the format changes can be found on NIH’s Other Support webpage and in the March 2021 notice NOT-OD-21-073 .
What is the effective date for the new Other Support format?
The new format is required for application due dates, JIT, and RPPR submissions on or after January 25, 2022.
Are there additional documentation requirements with the revised format and instructions for the Other Support page?
Yes. Beginning January 25, 2022, all principal investigators and other senior/key personnel are required to electronically sign the Other Support document, prior to its submission to NIH, certifying that the information is accurate and complete. NIH and SciENcv are currently developing an Other Support template, estimated to be rolled out sometime in 2022. When NIH transitions to the use of SciENcv to generate Other Support, the signature/certification will be integrated into that process.
The revised formats require documentation of Person Months (Calendar/Academic/Summer) per budget period for each source of support listed, as well as for in-kind support, as applicable. In-kind support is included as a separate section on the Other Support form.
If other support submissions include foreign activities and resources, recipients will be required to submit copies of contracts, grants, and any other agreement specific to senior/key personnel foreign appointments, affiliations, and/or employment with a foreign institution. The supporting copies must be included with the Other Support submission to NIH beginning January 25, 2022. Penn is operationalizing this as follows:
- Foreign contracts and agreements to the PD/PI or key personnel in their individual capacity that are included in Other Support should be submitted to Penn’s Office of Research Services (ORS) with the Other Support/RPPR document.
- Penn/ORS will assist with document translation as needed and will submit the forms to NIH as requested by the agency.
- When Penn is the subawardee, please submit Other Support documents to ORS for preview prior to sharing them with the prime sponsor.
What should be disclosed in Other Support?
NIH has indicated in NOT-OD-19-114 and other resources that Other Support includes “…all resources made available to a researcher in support of and/or related to all of their research endeavors, regardless of whether or not they have monetary value” and regardless of location, including but not limited to:
- Resources and financial support from all foreign and domestic entities available to the researcher irrespective of whether provided through the applicant organization, another domestic or foreign organization, or directly to an individual.
- Note: If intended for use on the project being proposed, include as part of the Facilities and Other Resources or Equipment section and not in Other Support.
- Includes consulting during summer months for 9-month appointments.
- Anticipated co-authorship or joint publication is a useful indicator that the activity should be disclosed as Other Support.
Should start-up support provided by the applicant organization be included in Other Support?
Start-up packages provided by Penn should not be included in Other Support. Start-up packages from outside organizations, including foreign entities, must be included in Other Support.
Should gifts be reported in Other Support?
No, gifts should not be reported in Other Support. Gifts are resources provided where there is no expectation of anything (e.g., time, services, specific research activities, money, etc.) in return. If a gift was provided to Penn for a specific research activity or there is an expectation of an associated time commitment, it must be reported in Other Support.
Should training grants or prizes be included in other support?
No, training grants and prizes are not required to be disclosed in Other Support.
Should core facilities or shared equipment be included in Other Support?
No. Other Support includes resources uniquely available to the researcher and does not include institutional resources that are made broadly available to faculty and staff.
What should I do if I am not sure if something needs to be included as Other Support?
In the interest of full transparency, recipients should err on the side of disclosure. Researchers should consult with their institutional officials for guidance to ensure compliance with institutional and NIH policies.
What should I do if I realize that a resource was not included/reported in Just-in-Time or the Research Performance Progress Report?
Notify the appropriate school/department and/or Proposal and Award Management team contact within Penn’s Office of Research Services immediately. They will work with you to submit updated Other Support information to NIH.
The new Other Support format page includes a chart that asks for the Year (YYYY). Is this calendar or fiscal year?
It is calendar year. If reporting person-months that span two calendar years, enter the latter year. For example, if the budget period runs from June 2019 through May 2020, enter “2020” for the year and include the corresponding person months.
Do PD/PI's need to report effort for the current year or for all years?
PD/PI's need to report effort for all years.
With the new Other Support format page, are pending grants now required on Other Support pages submitted for RPPRs?
If other support is being submitted due to changes in active support, pending support would also be included. Updated other support is only required at the time of RPPR submission if there has been a change in the active other support of senior/key personnel.
Am I required to report on consulting arrangements or other internal contracts, such as retention agreements, that are within Penn's contract/agreement?
No, activities that fall within the home institution's agreement do not need to be reported in other support. This is applicable for both domestic and foreign institutions.
Can Other Support be signed with a wet signature or a typed name?
No, wet and typed names will not be accepted as signatures. Electronic signatures are required. PIs and Senior/Key Personnel may use the electronic signature software of their choice, and in alignment with their institutional practices (Adobe at Penn).
Outside Appointments, Consulting, and Collaborations
What outside/consulting activities should i report in other support.
When research will be conducted as part of consulting activities that fall outside of an individual's institutional appointment, it must be reported as Other Support. Consulting roles that involve the design, conduct, or reporting of research may be considered to involve the “conduct of research.” Anticipated co-authorship or joint publication is a useful indicator that the activity should be disclosed as Other Support.
Foreign-sponsored talent recruitment program participation or paid research should be disclosed. National Security Presidential Memorandum-33, on U.S. Government-Supported Research and Development National Security Policy, defines “foreign government-sponsored talent recruitment programs” as “an effort directly or indirectly organized, managed, or funded by a foreign government or institution to recruit S&T professionals or students.”
Consulting or professional service arrangements that do not involve research or are purely advisory, do not need to be reported. Consulting engagements that are relevant to a researcher’s professional expertise should be included in the biosketch under Scientific Appointments.
If I have an unpaid (or paid) appointment at a foreign university with access to lab space, research materials, and staff, should it be reported as Other Support?
Yes. Even when not receiving monetary compensation, the lab space, materials, and staff are resources made available for and/or related to your research efforts. Other payments, such as travel or living expenses associated with this appointment must also be reported.
Joint or dual appointments under formal agreements with Penn that include lab or other research space and resources (e.g., CHOP) are institutional resources and do not need to be listed in Other Support. Office/lab space provided to a researcher in support of or related to their research that is not due to a formal joint or dual appointment with Penn should be listed as Other Support.
If I have a 9-month appointment and spend two months at a foreign university during the summer conducting research under a foreign award, should this be reported as Other Support?
Yes, as it involves the conduct of research and supports and/or relates to your research endeavors.
Should service on advisory committees or boards, such as data safety monitoring committees or scientific advisory boards, be included in Other Support?
Service on advisory committees and boards does not need to be disclosed as Other Support unless it involves the conduct of research (e.g., writing protocols or analyzing data). It should be included as a “Scientific Appointment” on the Biosketch.
If key personnel on an NIH grant in the U.S. are collaborating with another scientist in the U.S. whose experiments, conducted with funds awarded to their institution, have directly benefitted their research, should this be reported as Other Support? What about collaborations with a former graduate student/postdoc or other researcher with their own lab at a non-U.S. university?
Yes. Other Support includes domestic and foreign research collaborations that directly benefit an individual’s research endeavors. Anticipated co-authorship or joint publication is a useful indicator that the activity should be disclosed as Other Support.
If the collaborator is contributing a significant scientific element to an NIH-supported project from a non-U.S. location it could be considered a “foreign component.” In such cases, prior approval would be required. Departmental or ORS staff can assist with prior approval requests. A foreign component is the performance of a significant scientific element of the NIH-supported project outside of the United States. Examples of activities that may be considered a significant element of the project include:
- collaborations anticipated to result in co-authorship
- use of facilities or instrumentation at a foreign site
- receipt of financial support or resources from a foreign entity
I have a number of long-standing collaborations with foreign scientists and entities. Do I need to be concerned about these collaborative research efforts?
NIH and other U.S. research funding agencies have indicated their continued and strong support for collaborative research efforts, both domestic and foreign. Agencies are seeking disclosure of collaborations consistent with biosketch, other support, and FCOI requirements, as applicable, to allow for appropriate funding decisions and grants management (e.g., to identify any scientific, budgetary, or commitment overlap; ensure sufficient levels of effort are committed to the project; and identify potential conflicts of interest).
Do I need to include internal Penn awards in Other Support?
Yes, internal awards need to be included in Other Support. However, discretionary or research funds provided by Penn as part of an academic appointment (e.g., named professorship) do not need to be disclosed as other support.
Should consulting activities included in Other Support display calendar months effort, or is N/A appropriate for effort, when serving as a consultant?
Consulting should include estimates for the amount paid or to be paid, rather than time and effort reflected in calendar months. Therefore, it will not count towards the 12 calendar months of effort.
Is supporting documentation required for a research contract listed in Other Support in which the Sponsor is a U.S. subsidiary of a foreign organization?
Supporting documentation is required specifically for foreign appointments, agreements, and activities. Use professional judgment to determine whether the agreement is primarily (e.g., 51 percent or more) with the foreign or domestic entity within the organization. If the agreement is primarily with the foreign organization, then it would require supporting documentation.
If I have a partial appointment at a foreign institution and hold grants at that foreign institution, does NIH require copies of the foreign appointment contract AND copies of any grant agreements I have at the foreign institution?
Yes. Supporting documentation for both must be provided.
Do I need to provide copies of foreign grants, contracts, and agreements that are still pending and not yet finalized?
Copies of grants, contracts and other written agreements are only required for active support.
In-kind Support
Do in-kind contributions that will be used for the project being proposed need to be included in other support.
If the in-kind contribution is intended for use on the project being proposed, the information must be included as part of the Facilities and Other Resources or Equipment section of the application and does not need to be replicated in Other Support. If an in-kind contribution is not intended for use on the project being proposed, then the information must be included as part of Other Support.
If I have an exchange student, post-doc, or visiting scholar working on research activities in my lab who is paid a salary by their home university in a foreign country, does that need to be included in Other Support?
Yes, since they are performing research activities, their work in the lab is a resource available in support of the PD/PI or other senior/key personnel’s research endeavors and must be reported as an in-kind contribution in Other Support.
A student working for class credit, including those on a student visa, is not considered in-kind support. This would be considered institutional support and therefore not reportable as Other Support. Students supported by an NIH T32 and working on an R01 with, or under the direction of, the PI or senior/key personnel are not considered Other Support.
If I am mentoring a post-doc or graduate student who is individually funded through an outside institution (e.g., foundation, home university), does that need to be disclosed as an in-kind resource in Other Support?
If the relationship is solely a mentor/mentee arrangement, with no research activities, then it is not a resource and does not need to be reported. If the post-doc or graduate student is performing research activities in support of the PD/PI or other senior/key personnel’s research endeavors, then their support must be reported as an in-kind resource.
How should I list materials (e.g., data, samples, etc.) received from external collaborators in Other Support?
Information on materials received from collaborators that support an investigator’s research should include the source, a summary of the in-kind contribution, and the estimated value. Only resources uniquely available to the researcher (e.g., not widely or commercially available) must be reported.
How should I value tissue samples, cell lines, animal models, and other materials as an in-kind contribution toward my research endeavors?
Researchers should strive to estimate the value based on the cost of comparable materials. If there is no comparable information, indicate that there is “no market value available.”
For in-kind resources that do not have associated effort, can zero effort be entered?
Yes, for in-kind resources with no associated time commitment, researchers can list zero effort, but must provide the estimated dollar value of the in-kind resource. The effort and dollar value cannot both be zero.
Does completed in-kind support need to be included in Other Support?
No, only active and pending in-kind support must be included.
When disclosing materials received from external collaborators on Other Support how far back in time should I go? Should materials provided 20 years ago be disclosed, if they are still in use?
Materials provided within the past 3 years, that are still in use, must be included in Other Support.
Do I need to disclose unpaid volunteers working in my lab as Other Support?
Unpaid volunteers need to be disclosed if they serve as a resource available in support of an individual’s research endeavors.
Cost/Budget Reporting
Should the other support documents include annual total cost or only annual direct cost.
Other Support submissions should provide the total award amount, direct and indirect, for the entire project period, not just the annual budget period, as indicated in NOT-OD-19-114 .
When providing the total award amount for an NIH funded grant project, should the researcher provide what was requested in the initial application, or the amount of funding listed on the current Notice of Award?
Researchers should list the total award amount, direct and indirect, for the entire project period, based on the most recent Notice of Award.
When a researcher works on a subaward to an NIH grant that is awarded to another institution, how should that information be included in Other Support?
The researcher should provide the project number and PD/PI name for the prime award. All other information, including the total award amount and person months, should be specific to the subaward.
I have a non-U.S. subrecipient for a project. How should the non-U.S. investigators report Other Support? Do they need to disclose their employment agreements with the subaward recipient institution?
The non-U.S. investigators must follow the same rules as U.S. investigators. They do not need to disclose their employment agreements with the subaward recipient institution, but they must disclose any Other Support, including any appointments with additional entities that involve research or access to resources for research endeavors.

- Contact SPA
- Institutional Information
- Responsibility Matrix
- Responsible Offices
- PI Eligibility / Project Leadership
- Obtaining SPA Review and Endorsement
- Common Elements of Proposals
- Non-Salary Administrative Expenses
- Other Direct Costs
- Travel Costs
- Equipment Costs
- Supplies and Materials
- DHHS Salary Rate Cap
- Administrative Salaries
- Student Compensation Wages vs Stipends
- Budget Justifications
- Facilities and Administrative Cost Rates
- Employee Fringe Benefits
- Graduate Student Fees and Tuition Remission
- NIH/SAMHSA/AHRQ Salary Rates
- Requesting Cost Sharing from the Vice Chancellor for Research
- Fulfilling and Documenting Cost Sharing
- NSF Supported Events – Code of Conduct Policy
- NSF Safe and Inclusive Working Environments for Off-Campus or Off-Site Research
- A Plan to Promote Safe Environments at Conferences Supported by NIH Grants and Cooperative Agreements
- NIH Biosketch & Other Support
- State of California Guidance
- Review for Tobacco Industry Sponsors
- Frequently Asked Questions
- Procurement Services COI Guidance
- Doing Research at the VA
- Gift vs. Grant vs. Contract
- Applications & Forms
NIH Biosketch & Other Support
The National Institutes of Health (NIH) has issued new requirements for Other Support reporting and Biographical Sketches (Biosketches) submitted on or after January 25, 2022.
The information provided below summarizes these new requirements.
- Biosketch and Other Support Checklist
- Summary of Changes to Biosketch and Other Support
- NIH Disclosures Table Pre-Award and Post-Award Disclosures Relating to the Biosketch and Other Support
- Federal Sponsored Projects Requirements For more information on NIH and other federal agencies' requirements
- NIH Other Support sample template
How to Comply with NIH Biosketch Requirements
A biosketch is a streamlined version of your cv (curriculum vitae) requested by most funding agencies and institutions. nih and nsf both use standard biosketch formats that are periodically updated. foundation and industry sponsors may also have specific requirements when requesting your cv/biosketches., how to comply with nih other support reporting, nih requires disclosure of all resources made available to a researcher in support of and/or related to any of their research endeavors, regardless of whether they have monetary value and regardless of whether they are based at uci..
Research and Economic Development
Nih biographical sketch and other support.
Most grant applications require a Biographical Sketch (biosketch), an abbreviated record of your accomplishments. If you are engaged in research, even as a collaborator, you will need a biosketch. The National Institute of Health (NIH) Biosketch is the most common format. It is similar to a CV, but is limited to five (5) pages with information pertinent to the application.
NIH encourages researchers to create an NIH biosketch online using SciENcv (Science Experts Network Curriculum Vitae) to integrate information from your NIH eRA Commons profile and your bibliography in NCBI .
Changes to the NIH Biosketch Format
There have been changes to the NIH Biosketch and the Other Support page. The NIH issued notice NOT-OD-21-073 , Upcoming Changes to the NIH Biographical Sketch and Other Support, and subsequently issued notice NOT-OD-21-110 which delayed the required used of the new biosketch and other support page. NIH requires researchers to use the updated NIH Biosketches and Other Support format page for all Applications, Just-In-Time requests, and Research Performance Progress Reports (RPPRs) with due dates on and after January 25, 2022.
There are two NIH Biosketch types: (i) Non-Fellowship Biosketch and (ii) Fellowship Biosketch. Major changes are noted below:
- Section A - Personal Statement may include details on ongoing and completed research projects from the past three years that they want to draw attention
- Section B - Positions and Honors has been renamed Positions, Scientific Appointments, and Honors
- Section D - This section was removed for non-Fellowship Biosketches. For the Fellowship Biosketch, this section has been updated to remove Research Support
Suggestions for Researchers
1. Create a My NCBI account and link the account to your eRA Commons account.
2. Populate your My Bibliography and keep the publication and research products list updated.
3. Play around with SciENcv . See which parts of the new Biosketch are auto-populated.
4. Try creating a Biosketch with data from ORCID , eRA Commons and My Bibliography .
5. Assign delegates to help manage your My Bibliography and SciENcv .
6. Consider a hybrid approach of using the Word Template and SciENcv .
Option 1 - Using SciENcv to Create NIH Biosketches
Researchers are encouraged to use SciENcv to create an NIH (non-Fellowship) Biosketch or NIH Fellowship Biosketch, as applicable.
What Is SciENcv?
SciENcv stands for Science Expert Network Curriculum Vitae. It is a researcher profile system for all individuals associated with research investments from federal agencies.
- In SciENcv, researchers can document their education, employment, research activities, publications, honors, research grants, and other professional contributions.
- My NCBI users can create multiple SciENcv profiles in official biographical sketch formats for NIH and NSF, which can be used for grant submissions.
To create the new biosketch in SciENcv:
- Click on Manage SciENcv at the bottom of the SciENcv box in My NCBI Biosketch .
- At the following page (*see Illustration below) under Create a New Document , click on the applicable NIH biosketch format.
- Start with a blank document
- Existing Document (i.e, from an existing biosketch)
- External source (eRA Commons, ORCiD, NSF)
*Illustration
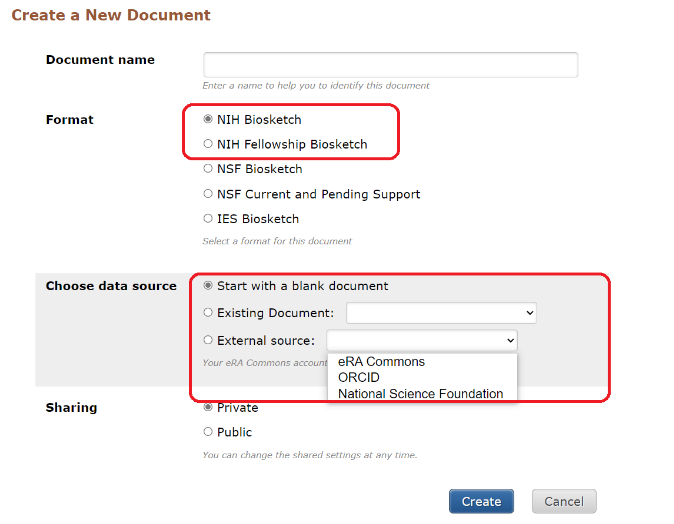
SciENcv is connected to My Bibliography, and can be connected with ORCiD too. Users can directly import up to four desired citations from either My Bibliography or ORCiD into their biosketches.
Notes: Biosketch Creation
SciENcv is not required for creating a NIH biosketch in the new format.
Users are recommended to have their My Bibliography accounts created and updated before using SciENcv to create a biosketch.
SciENcv users can also create biosketches using data stored in their ORCID records. By linking an ORCID account to an NCBI account, users will be able to auto-populate biosketches using the personal statement, education, employment, publications and research awards information stored in ORCID records. See My NCBI – ORCID Author Data Integration with SciENcv.
Links for Creating Biosketches Using SciENcv
SciENcv Website - http://www.ncbi.nlm.nih.gov/sciencv
SciENcv Help - http://www.ncbi.nlm.nih.gov/books/NBK154494/
SciENcv FAQs - http://www.ncbi.nlm.nih.gov/sciencv/faqs/
SciENcv YouTube Video Tutorial - https://www.youtube.com/watch?v=PRWy-3GXhtU&feature=youtu.be
My Bibliography Help - http://www.ncbi.nlm.nih.gov/books/NBK53595/
My NCBI Documentation - http://www.ncbi.nlm.nih.gov/books/NBK3843/
More information about SciENcv

Option 2 - Using a Word Template to Create NIH Biosketches
Researchers can opt to use a blank template in Word to create a Biographical Sketch Format Page. Links to the blank format page are provided below (along with instructions, samples, FAQs and other resources).
For Non-Fellowship Biosketch:
- Instructions for Biographical Sketch These instructions will be incorporated into the NIH Application Form Instructions with the next update by FY 2022.
- SAMPLE: Non-fellowship biosketch
- NIH Pre-award and Post-award Disclosures Relating to the Biographical Sketch and Other Support
For Fellowship Biosketch:
- Predoctoral Fellowship biosketch sample (Word)
- Postdoctoral Fellowship biosketch sample (Word)
Complete the education block. Begin with the baccalaureate or other initial professional education, such as nursing. Include postdoctoral, residency, and clinical fellowship training, as applicable, listing each separately.
For each entry provide:
- the name and location of the institution
- the degree received (if applicable)
- the month and year of end date (or expected end date). For fellowship applicants only, also include the month and year of start date.
- the field of study (for residency entries, the field of study should reflect the area of residency training)
Following the education block, complete Sections A-D of the biographical sketch
Section A: Personal Statement
Briefly describe why you are well-suited for your role(s) in this project. Relevant factors may include: aspects of your training; your previous experimental work on this specific topic or related topics; your technical expertise; your collaborators or scientific environment; and/or your past performance in this or related fields, including ongoing and completed research projects from the past three years that you want to draw attention to (previously captured under Section D. Research Support).
You may cite up to four publications or research products that highlight your experience and qualifications for this project. Research products can include, but are not limited to, audio or video products; conference proceedings such as meeting abstracts, posters, or other presentations; patents; data and research materials; databases; educational aids or curricula; instruments or equipment; models; protocols; and software or netware. Use of hyperlinks and URLs to cite these items is not allowed.
It is permissible to cite interim research products. Note: Interim research products have specific citation requirements. (See related Frequently Asked Questions for more information.)
Please note the following additional instructions for ALL applicants/candidates:
- If you wish to explain factors that affected your past productivity, such as family care responsibilities, illness, disability, or military service, you may address them in this "A. Personal Statement" section.
- Indicate whether you have published or created research products under another name.
- You may mention specific contributions to science that are not included in Section C. (Do not present or expand on materials that should be described in other sections of this Biosketch or application.)
- Figures, tables, or graphics are not allowed.
Note the following instructions for specific subsets of applicants/candidates:
- For institutional research training, institutional career development, or research education grant applications: Faculty who are not senior/key persons are encouraged, but are not required, to complete the "A. Personal Statement" section.
- For dissertation research awards (e.g., R36), Applicants should, in addition to addressing the points noted above, also include a description of their career goals, their intended career trajectory, and their interest in the specific areas of research designated in the FOA.
- Candidates for research supplements to promote diversity in health-related research should, in addition to addressing the points noted above, also include a description of their general scientific achievements and/or interests, specific research objectives, and career goals. Indicate any current source(s) of educational funding.
[ Source: NIH ]
List in reverse chronological order all current positions and scientific appointments both domestic and foreign, including affiliations with foreign entities or governments. This includes titled academic, professional, or institutional appointments, whether or not remuneration is received and whether full-time, part-time, or voluntary (including adjunct, visiting, or honorary). High school students and undergraduates may include any previous positions. For individuals who are not currently located at the applicant organization, include the expected position at the applicant organization and the expected start date.
List any relevant academic and professional achievements and honors. In particular:
- Students, postdoctorates, and junior faculty should include scholarships, traineeships, fellowships, and development awards, as applicable.
- Clinicians should include information on any clinical licensures and specialty board certifications that they have achieved.
Section C: Contributions to Science
All senior/key persons should complete the "Contributions to Science" section except candidates for research supplements to promote diversity in health-related research who are high school students, undergraduates, and post-baccalaureates.
Briefly describe up to five of your most significant contributions to science. The description of each contribution should be no longer than one half page, including citations.
While all applicants may describe up to five contributions, graduate students and postdoctorates may wish to consider highlighting two or three they consider most significant.
For each contribution, indicate the following:
- the historical background that frames the scientific problem;
- the central finding(s);
- the influence of the finding(s) on the progress of science or the application of those finding(s) to health or technology; and
- your specific role in the described work.
For each contribution, you may cite up to four publications or research products that are relevant to the contribution. If you are not the author of the product, indicate what your role or contribution was. Note that while you may mention manuscripts that have not yet been accepted for publication as part of your contribution, you may cite only published papers to support each contribution. Research products can include audio or video products (see the NIH Grants Policy Statement , Section 2.3.7.7: Post-Submission Grant Application Materials); conference proceedings such as meeting abstracts, posters, or other presentations; patents; data and research materials; databases; educational aids or curricula; instruments or equipment; models; protocols; and software or netware. Use of hyperlinks and URLs to cite these items is not allowed.
It is permissible to cite interim research products. Note: Interim research products have specific citation requirements. (See related Frequently Asked Questions for more information.)
You may provide a URL to a full list of your published work. This URL must be to a Federal Government website (.gov). Providing a URL to a list of published work is not required; however, if desired, then NIH recommends using My Bibliography . See the "URL to Published Works" tab for more information on providing a link to a list of published works.
Descriptions of contributions may include a mention of research products under development, such as manuscripts that have not yet been accepted for publication. These contributions do not have to be related to the project proposed in this application.
*Section D: Scholastic Performance
NOTE: For Non-Fellowship NIH Biosketch, Section D is removed. For Fellowship NIH Biosketch, Section D has been revised. It is now for Scholastic Performance only
*Note that only the following types of applicants must complete this section:
- applicants for predoctoral and postdoctoral fellowships
- applicants to dissertation research grants (e.g., R36)
- candidates for research supplements to promote diversity in health-related research from the undergraduate through postdoctoral levels
Scholastic Performance
Predoctoral applicants/candidates (including undergraduates and post-baccalaureates): List by institution and year all undergraduate and graduate courses, with grades. In addition, explain any grading system used if it differs from a 1-100 scale; an A, B, C, D, F system; or a 0-4.0 scale. Also indicate the levels required for a passing grade.
Postdoctoral applicants: List by institution and year all graduate scientific and/or professional courses with grades. In addition, explain any grading system used if it differs from a 1-100 scale; an A, B, C, D, F system; or a 0-4.0 scale. Also indicate the levels required for a passing grade.
URL to Published Works
The Contributions to Science Section offers investigators the option to include a URL to a full list of published work. NIH recommends that investigators use "My Bibliography" as NIH can assure reviewers that their anonymity will be protected if they review publications at that site. A URL for a publication list is optional and, if provided, must be to a government website (.gov) such as My Bibliography.
- Use the My Bibliography “Sharing” feature to obtain a URL.
- Click the “Make it Public” link.
- A URL will appear.
- Copy and paste the entire URL string to the template.
- Private/Public settings are flexible.
- Check the box below the Contributions to Science section.
Include link to complete list of published work in My Bibliography. (Selecting this option will make the list public.)
Spell the URL in full, beginning with "http://."
Do not embed the link as hyperlinked text. The link will not remain active after processing.
The URL to a full list of published work is not required .
Only one URL is allowed in the Biosketch. Any URLs other than the List of Published Works to a government website will not be allowed.
[Source: NIH]
My Bibliography
My Bibliography is a reference tool that allows researchers to:
- save references of their scholarly works directly from PubMed
- add references manually using the built-in template
If your NCBI account is linked to eRA Commons, you can use your My Bibliography to view whether your publications comply with the NIH Public Access Policy, start the compliance process for applicable journal articles if not in compliance, and associate your publications to awards when applicable.
You may add a delegate in your NCBI account to manage your My Bibliography.
ORCID (pronounced "orkid") stands for Open Researcher and Contributor ID. ORCID is an open, non-profit, and community-driven effort to create and maintain a registry of unique researcher identifiers. An ORCID iD acts as a unique identifier for a person, much like each publication in PubMed has a PubMed ID.
Why do I need an ORCID identifier (ORCID iD)?
While not mandatory, publishers and funding agencies are increasingly adopting ORCID as a tool to manage submissions and applications. At some point in the future, having an ORCID iD and using ORCID as a tool may be required. Note: ORCID is currently required for NIH fellowship and Career Development applications.
An ORCID iD is unique, and it distinguishes you from other researchers with similar or the same names. On average, a name in PubMed could be referencing 8 authors. Having an ORCID, you can quickly identify which publications are yours. ORCID is also transferrable to other institutions.
For new researchers, an ORCID iD offers a way to have an accurate record of your scholarly output from the very beginning. You can use it on your CV, departmental webpage, email signature, in professional directories and more.
How do I create an ORCID iD?
Once your ORCID ID has been created, click on the Create or Connect your ORCID ID link in your Commons Personal Profile and log into ORCID. You will then be prompted to authorize NIH to access your personal ORCID profile (as illustrated below).

Populating Scholarly Works in ORCID
Researchers have several ways to add their scholarly works in their ORCID profiles:
- Search and link
- Add PubMed ID (PMID)
- Add BibText
- Add manually
Adding a Delegate
Researchers are able to add one or more delegates or proxy in ORCID to manage their ORCID records and updating scholarly works. To add a delegate, go to Account Settings, scroll down to Trusted Individuals. Add the name or email address in the box below Trusted Individuals. Other ORCID users can grant permission for you to update their records. A trusted individual does not need to be another researcher but must have an ORCID iD.
Information on other active and pending support may be requested (often as part of Just-in-Time procedures for grant applications or in progress reports) to ensure there is no scientific, budgetary, or commitment overlap. “Other Support” is sometimes referred to as “current and pending support” or “active and pending support.”
Other Support includes all resources made available to a researcher in support of and/or related to all of their research endeavors, regardless of whether or not they have monetary value and regardless of whether they are based at the institution the researcher identifies for the current grant . This includes but is not limited to:
- Resources and/or financial support from all foreign and domestic entities, that are available to the researcher. This includes but is not limited to, financial support for laboratory personnel, and provision of high-value materials that are not freely available (e.g., biologics, chemical, model systems, technology, etc.). Institutional resources, such as core facilities or shared equipment that are made broadly available, should not be included in Other Support, but rather listed under Facilities and Other Resources.
- Consulting agreements, when the PD/PI or other senior/key personnel will be conducting research as part of the consulting activities. Non-research consulting activities are not Other Support.
- In-kind contributions, e.g. office/laboratory space, equipment, supplies, or employees or students supported by an outside source. If the time commitment or dollar value of the in-kind contribution is not readily ascertainable, the recipient must provide reasonable estimates.
Other support does not include training awards, prizes, or gifts. Gifts are resources provided where there is no expectation of anything (e.g. time, services, specific research activities, money, etc.) in return. An item or service given with the expectation of an associated time commitment is not a gift and is instead an in-kind contribution and must be reported as such.
Other Support information is requested for:
- Program Directors, training faculty, and other individuals involved in the oversight of training grants
- Individuals categorized as Other Significant Contributors
Updated Requirements Effective January 25, 2022 ( NOT-OD-21-073 )
Effective January 25, 2022 , NIH requires the following:
- Supporting documentation, which includes copies of contracts/agreements specific to senior/key-personnel foreign appointments and/or employment with a foreign institution for all foreign activities and resources that are reported in Other Support. If the contracts/agreements are not in English, recipients must provide translated copies.
- Immediate notification of undisclosed Other Support. When a recipient organization discovers that a PI or other Senior/Key personnel on an active NIH grant failed to disclose Other Support information outside of Just-in-Time or the RPPR, as applicable, the recipient must submit updated Other Support to the Grants Management Specialist named in the Notice of Award as soon as it becomes known.
Additional information on Other Support can be found in the Grants Policy Statement .
Using the NIH Word Template to Create Other Support
NIH is finalizing the SciENcv template for Other Support and anticipates that the template will be available in the spring of 2022. Until the SciENcv template for Other Support is available, applicants and recipients are required to use the Word Format page to prepare Other Support information.
The form must be converted to a PDF, and electronically signed prior to submission.* (NIH requires senior/key personnel to certify the accuracy of their Other Support disclosures by electronically signing their Other Support form.) This is an interim process while NIH moves to the use of SciENcv templates for Other Support. Researchers may use the electronic signature software of their choice, and in alignment with institutional practices. A typed name is not an electronic signature and is not acceptable. Two options are available for electronic signature: (i) converting the final Word file to Adobe PDF and adding your Adobe authenticated signature or (ii) uploading the final Word file into DocuSign for signature. Both options include supporting documentation to reasonably authenticate that the appropriate the individual signed the form, as is required by the NIH. Such documentation must be made available upon request in accordance with 45 CFR Part 75.364.
- Other Support sample
- Instructions on How to Flatten a PDF
Workshop: Changes to the NIH Biographical Sketch & Other Support
NIH requires researchers to use the new format for NIH Biosketches and Other Support for submissions of applications and progress reports for due dates on or after January 25, 2022. These two (identical) workshops will address such forthcoming changes/requirements.
Note: Each workshop will contain the same content, so please attend the one which is most convenient
Date/Time :
Friday, January 14, 2022, Noon - 1PM via ZOOM
Join Zoom Meeting - https://ucr.zoom.us/j/91455209470
Meeting ID: 914 5520 9470
Thursday, January 20, 2022, Noon - 1PM via ZOOM
Join Zoom Meeting - https://ucr.zoom.us/j/97550587030
Meeting ID: 975 5058 7030
Audience: UCR researchers and research administrators who ares and interested to learn about the new format and requirements.
Instructors:
Ursula Prins, PreAward Manager, Sponsored Programs Administration Robert Chan, Post-Award Manager, Sponsored Programs Administration
For those who were unable to attend one of the above workshops, or attended but would like a refresher, here is a pre-recorded powerpoint presentation with audio. Note: A UCR NetID is required to access this presentation.
QUICK LINKS
- SPA Departmental Assignments
- Frequently Asked Questions
- Roles and Responsibilities Matrix
- Proposal Preparation / Submission
- Preaward Administration
- Award Negotiation and Setup
- Post-Award Administration
- Outgoing Subawards
- Clinical Trials
- Data Management Resources
- DOE's Disclosure Requirements for Current & Pending Support
- Ethical and Responsible Conduct of Research
- Export Control
- Foreign Engagement
- Kuali Research
- Material Transfer Agreements
- NASA Restrictions on Funding Activity with China
- NIH Changes to Biosketch and Other Support
- NSF Broader Impacts
- PI and Award Transfers
- Reports (Annual, On Demand)
- Research Administrators INC
- Staff Directory
- UCR Contracting Guide
- Uniform Guidance
- UC Research Website
- UCOP AB20 Webpage
Creating an NIH Biosketch
- NIH Overview
- Overview of the NIH Biosketch
- Tools to assist in Biosketch creation
- Section Overview: Demographic Information
- Section Overview: Personal Statement
- Section Overview: Positions, Scientific Appointments, and Honors
- Section Overview: Contribution to Science
- Citation Tips
- My Bibliography
- Metric Statements in your NIH Biosketch
- Resources & Help
Who and why?
Completion of the NIH Biosketch is part of the “Apply for Grant Funding” Portion of the grants process overview. The biosketch allows applicants to:
- Describe the magnitude and significance of their scientific contributions (including publications)
- Provide detailed information about their research experience in the context of the proposed project
Biosketches are required from all senior/key personnel and other significant contributors that are applying for the grant
- NIH Grants & Funding: How to Apply
The NIH Biosketch Form
The NIH provides detailed instructions and samples on their website to assist you in the creation of your biosketch. The current template is approved for use through 9/30/2024 . The total biosketch cannot be longer than five pages in length. Margins may be no narrower than ½ inch. Font of 11 point or larger is required, as long as the font type has no more than 15 characters per linear inch (including characters and spaces) -- some 11 point fonts are more compressed than 15 characters per inch, so the recommended font is Arial 11 point font.
The form includes four sections:
- general demographic information
- a personal statement
- a section to list your positions, scientific appointment, and honors
- a section to note your contributions to science
Special Note: Important changes were made to the NIH Biosketch form, effective May 25, 2021.
- Section D will be completely deleted for non-fellowship sketches. Instead, investigators are encouraged to mention important funding support in the text of Section A: Personal Statement including ongoing and completed research projects from the past three years that you want to draw attention to (previously known as research support).
- Section D will omit "Research Support" for fellowship sketches . Section D will now be solely dedicated to scholastic performance.
- NIH Biosketch Format Pages, Instructions and Samples
- You might want to consider having more than one version of your biosketch if you work in a variety of disciplines or conduct your own research but also are a mentor to new faculty or students.
- While there is no requirement on writing in first or third person voice, biosketches generally sound better when written in first person. If you prefer to write in third person voice, just be consistent. Do not write the personal statement in third person and contributions in first person voice.
- << Previous: NIH Overview
- Next: Tools to assist in Biosketch creation >>
- Last Updated: Mar 8, 2024 9:01 AM
- URL: https://libguides.galter.northwestern.edu/NIH-biosketch

- NIH Grants & Funding
- Blog Policies
NIH Extramural Nexus
New Central Email Inbox for Biosketch and Other Support Inquiries
Have you checked out our other support or biosketch resources and still have questions? Send your inquiries to our new central email inbox [email protected] .
See NOT-OD-21-122 for details.
RELATED NEWS
Under the new guidelines for a biosketch, under section A where we listed ongoing/completed research, will we still have to include “goals” to each.
In section A, applicants can highlight ongoing/completed ongoing and completed research projects from the past three years that they want to draw attention to. The “goals” are not required, but can be included if the applicant wishes to highlight that information.
Why was the “Research Support” section made optional in the latest biosketch changes? As a reviewer, I find it highly informative to know what current/past support an applicant has received but many applicants are now simply choosing not to include any such information in section A. NIH funding can be found on REPORTER, but not if the applicant is not the PI and non-NIH funding is also not searchable. It’s not clear what the goal was with this change.
Will administrators have more time to prepare and submit JIT? We normally only have a business few days to submit JIT, but now that the other supports are so complicated and require signatures, will we be given more time?
Do funds with the below characteristics need to be included in Other Support? My university defines “gift” somewhat differently than the NIH, and I would like to confirm that gifts like the one described below meet the NIH definition of a “gift” and therefore do not need to be included as other support. – The fund description includes language that the gift is designed for research and educational purposes within the PI’s lab, however specific research objectives are not indicated – The fund defaults to the department if the PI departs the university – The funds do NOT have effort requirements or specific deliverables and there is no stated completion date – No peer-reviewed proposals were required to obtain funding
We recommend consulting with your Office of Sponsored Research. The determination that something is a gift would be made by the institution, in accordance with the institutional policies, and documented accordingly. NIH defines gifts as resources provided where there is no expectation of anything (e.g., time, services, specific research activities, money, etc.) in return.
NIH policy indicates that the researcher must use the NIH Other Support template in Word (effective for proposals due 1/25/2022). Could an institution to convert NIH’s Word template into a fillable-pdf (with additional fields added rior to convertion thereby enabling the disclosure of up to 5 or 10 Active awards)? This would be more convenient to the researcher to then use Adobe signature.
Before submitting your comment, please review our blog comment policies.
Your email address will not be published. Required fields are marked *

- Administration and Staff
- Organization Chart
- OUHSC Centers of Excellence
- Funding Opportunities
- Internal Deadlines
- Sponsor Review Criteria
- Post-Submission Requests
- NIH Review and Award Cycle
- Biosketch and Other Support Update
- Data Management and Sharing Plan
- Grants Team
- Clinical Trials
- Industry-Sponsored Research Budgets
- Common Topics in Research Agreement Negotiations
- Roles & Responsibilities
- Master & Template Agreements
- Industry Research & Clinical Trials Team
- Contracts Team
- Research Requirements
- Conflict of Interest Policies & Guidance
- Find Sponsored Program Administrators
- Federal Uniform Guidance
- OUHSC Faculty Salary Adjustment
- OUHSC/VA Appointments
- International Activities
- Institutional Information
- Institutional Officials
- F&A Rates
- Fringe Benefits Rates
- Tuition & Fees
- Quick Links
- Proposal Development Support
- SoonerTrack II Support
- Institutional Research Core Facility
- Other Shared Resources
- High Throughput Screening Facility
- Laboratory of Biomolecular Structure and Function
- Reseach Imaging Facility
- Office of Technology Commercialization (OTC)
- Grants & Contract Accounting (GCA)
- Research Clinical Informatics Resources
- Clinical Research Informatics Oversight Committee (CRIOC)
- Office of Export Controls
- Office of Compliance
- Division of Comparative Medicine
- Institutional Animal Care & Use Committee (IACUC)
- Office of Human Research Participant Protection (HRPP)
- Institutional Biosafety Committee (IBC)
- Equipment Inventory
- Research Integrity Training
- Research Integrity Training FAQs
- Third Party Requests for Information from Faculty or Staff FAQs
- Authorship Disputes
- Reporting Possible Research Misconduct
- Related Resources
- Career Opportunities
- Animal Resources
- Industry Contracts & Clinical Trials
- Core Services Team
- Grants & Contracts Team
- Office of Technology Commercialization
NIH Other Support and Biographical Sketches Requirements
On April 28, 2021, the NIH released "Implementation of Changes to the Biographical Sketch and Other Support Format Page" ( NOT-OD-21-110 ). In summary:
- Effective May 25, 2021, the NIH expects applicants and recipients to use the updated biosketch and other support formats for applications, Research Performance Progress Reports (RPPR), and Just-in-Time (JIT) Reports. Immediate use of the updated format is preferred but not required until January 25, 2022 .
- Effective January 25, 2022, the NIH will require the use of the updated formats for any submissions on or after January 25, 2022 (or anything with a due date on or after that date). Failure to follow the appropriate formats on or after January 25, 2022, may cause an application to be withdrawn from or delay consideration of funding by the NIH.
Additional information can be found below. We recommend that you bookmark these pages and begin to update your biosketch and other support.
- NIH Biosketch format pages and instructions and FAQs
- NIH Other Support format pages and FAQs
Note that NIH is now requiring PIs and senior/key personnel to electronically certify that their Other Support information is true, complete and accurate to the best of their knowledge. ORA’s preferred electronic signatures for Other Support forms are Adobe Sign or DocuSign. If other electronic signatures are used, they must be verified signatures with a digital ID.
Each senior/key personnel must electronically sign their respective NIH Other Support form. The version with the original electronic signature will need to be retained by the department, to be made available to NIH and/or ORA upon request. ORA will only keep the final flattened versions on file as part of the submission package and cannot serve as the central repository for the original signed copies of the Other Support.
Additional Information and Guidance
- ORA/VPR NIH Other Support and Biographical Sketch Webinars .
- The following FAQs related to the NIH Other Support format pages were collected from researchers and administrators at OUHSC. They are meant to supplement NIH's FAQs. Please refer to NIH’s websites for additional information and guidance on this topic.
NIH Other Support Frequently Asked Questions (FAQs) (PDF)
Updated: April 17, 2023
- National Institute of General Medical Sciences

- Biophysics, Biomedical Technology, and Computational Biosciences
- Genetics and Molecular, Cellular, and Developmental Biology
- Pharmacology, Physiology, and Biological Chemistry
- Research Capacity Building
- Training, Workforce Development, and Diversity
- Contacts by Research Area
- Funding Opportunities and Notices
- Post Award Information
- Submitting an Application
- NIH RePORTER
- Dashboard of TWD Funded Programs
- High School and Undergraduate
- Postbaccalaureate and Graduate Students
- Postdoctoral, Early Career, and Faculty
- Workforce Development
- Contact Information
- Division Structure and Programs
- Enhancing Diversity in Training Programs
- Evaluation Resources
- Laboratory Safety and Guidelines
- Training Resources
- Institutional Development Award (IDeA)
- Native American Research Centers for Health (NARCH)
- Science Education Partnership Awards (SEPA)
- Support for Research Excellence (SuRE)
- DRCB Staff Contacts
- Current NIGMS Funding Opportunities
- Parent Announcements for Investigator-Initiated Applications
- Research Project Grants (NIH Parent R01)
- Research With Activities Related to Diversity (ReWARD)
- Maximizing Investigators' Research Awards (MIRA)
- Instrumentation Grant Program for Resource-Limited Institutions (RLI-S10)
- Undergraduate-Focused Institutions
- Small Business Research
- Multidisciplinary Teams/Collaborative Research
- Technology Development
- Research Resources
- Clinical Studies and Trials
- Conferences and Scientific Meetings
- Administrative Supplements
- All Funding Opportunities
- How to Apply
- Grant Application and Review Process
- NIGMS Funding Policies
- Post-Award Information
- Talking to NIH Staff About Your Application and Grant
- Considerations for Multiple Principal Investigator (MPI) Applications
- Attribution of NIH/NIGMS Support
- Message to NIGMS Investigators
- Research Using Human Subjects or Specimens
- Data Management and Sharing Plan Expectations for NIGMS Grantees
- STEM Teaching Resources
- Coloring Pages
- Educator's Corner
- Image & Video Gallery
- NIGMS-Supported Resources
- Protein Alphabet
- Biomedical Beat Blog
- Featured Topics
- Past Campaigns
- Past Releases and Announcements
- Media Coverage
- Feedback Loop Blog
- NIGMS Events
- Webinars for the NIGMS Training Community
- Face to Face with Program Directors
- Grant Writing Webinar Series for Institutions Building Research and Research Training Capacity
- Image and Video Gallery
- Director's Corner
- Organization and Staff
- Staff Directory
- Budget, Financial Management, and Congressional Material
- Strategic Plans
- Data Integration, Modeling, and Analytics
- Advisory Council
- Communications and Public Liaison Branch
- Job Vacancies
- Visitor Information
Highlight Header
Highlight Body
Related Information
Answers to frequently asked questions about mira par-22-180.
Answers to Questions Cover the Following Topics:
MIRA Program Description
Eligibility information, application and submission information, budget information, review information, post review issues, award process information, post award concerns, prior approval issues, what is the nigms mira program and what is its purpose.
- A single NIGMS grant per PI to provide support for NIGMS-related research in an investigator’s lab. Distinct features of MIRA include increased award length, stability, flexibility, and reduced administrative burden
- PI relinquishes other NIGMS support with some exceptions, e.g., resource, training, small business grants
- Award levels determined based on the PI's current NIGMS support. MIRA budgets for New Investigators will generally be $250,000 in annual direct costs
- Renewals – lower application numbers, higher success rates, flexible funding levels with the possibility of budget increases
What distinguishes MIRA from traditional NIH programs?
The three key differences are:
- The scope of the research supported by the MIRA, which encompasses the broad program of the investigator's laboratory that is within the mission of NIGMS, in contrast to a narrowly focused project(s).
- The flexibility that program-level support provides to the investigators, allowing them to pursue new research directions as opportunities arise. This flexibility is reflected in the form of the MIRA application, which shifts emphasis away from specific aims and details of proposed experiments and toward the importance of the overall research questions. The review process and review criteria emphasize the potential impact of the work over details of the approach.
- The possibility of funding a renewal application that is deemed meritorious but not as strong as would have been expected, based on the previous budget or other factors, for the full 5 years but at a lower level, allowing the PI’s research program to continue, albeit at a reduced scale.
How much time/effort must recipients devote to MIRA? How many calendar months?
MIRA provides support for a major research effort in the investigator's laboratory, leading to a requirement that he or she must devote at least 51 percent of their total research effort to the MIRA. Research effort per this NOFO is calculated differently than professional effort in that research effort does not include effort expended toward teaching, administration not directly related to the PD's/PI's research, and/or clinical duties and needs to be expressed in person-months.
For example, if an investigator has two awards (including the MIRA) with 50 percent total effort between them, the investigator must devote a minimum of 25.5 percent effort to MIRA (50 percent research effort x 51 percent MIRA effort requirement = 25.5 percent must be devoted to the MIRA), regardless of the amount of salary support requested. This would be equal to 3.06 calendar months of effort (25.5 percent x 12 calendar months = 3.06 months). In another example, if an investigator spends 25 percent of the PI’s time on awards, the PI must devote at least 12.75 percent effort to MIRA (25 percent research effort x 51 percent MIRA effort requirement = 12.75 percent effort to the MIRA). This is equal to 1.53 calendar months (12.75 percent x 12 calendar months = 1.53 calendar months).
PIs must devote at least 51 percent of their research effort, but a higher level of PI research effort may be requested, if well justified. The total research effort should be calculated based on an investigator's expected level of research effort should the MIRA application be funded.
Investigators cannot simultaneously hold another award that requires 50 percent research effort (such as an R35 from another institute of the NIH or a DP1 Pioneer Award). NIGMS will not award a MIRA while such awards are active or applications for any such awards are pending. The pending applications must be withdrawn before the MIRA will be issued.
How will NIGMS staff members verify if a PD’s/PI’s effort requirement is met?
NIGMS staff will verify that the MIRA research effort requirement is met by using the Other Support documentation. A PD’s/PI’s effort on an award for which the purpose is education, training or enhancing workforce diversity, including R25s, will be excluded from the calculation of research effort. The sum of all research effort reported on the Other Support page inclusive of the effort to be committed to the MIRA will be used as the PD’s/PI’s total research effort level. A PD’s/PI’s effort on MIRA should be equal to or greater than 51% of the total research effort.
How much salary can be charged to MIRA?
Salary may be charged based on the institutional base salary level for up to an amount commensurate with the number of calendar months of effort committed to the MIRA. A lower level of salary support may be charged; NIGMS does not consider there to be an obligatory relationship between percent of annual effort and percent of annual salary recovered from the grant.
What scientific areas of research are appropriate for support by MIRA?
Any research area within the mission of NIGMS is eligible for support on a MIRA. Research areas supported by NIGMS are outlined on the NIGMS website. Potential applicants are strongly encouraged to confer with the program official managing their qualifying award(s) or relevant research portfolio(s) before submitting a MIRA application.
Can technology development be included?
Yes. Technology development can be included either as the sole focus of the application or integrated with addressing novel biological hypotheses.
Can a MIRA support clinical/translational research?
Yes. Clinical and translational research within the NIGMS mission may be supported through a MIRA. Pre-application discussion of clinical/translational research projects with an NIGMS program director managing the qualifying award(s) or relevant research portfolio(s) is encouraged. Such research can also be added after award of the MIRA, as a change in scope, provided NIH approval is obtained prior to initiating the studies.
Can I continue to work with my current collaborators?
Yes. NIGMS strongly encourages collaborative research. However, the MIRA concept is based on the idea that NIGMS will provide support to an individual investigator's research program. Collaborators will work together because of their mutual interest in a research topic or area. In rare cases where a collaborator's efforts are well-justified, essential to the research program of the MIRA, and cannot be supported by the collaborator, a consortium agreement can be included.
Can I collaborate with an NIH intramural scientist?
Yes, a MIRA investigator can collaborate with an intramural scientist, but no funds can be provided to the intramural laboratory from the MIRA. If involvement with an intramural lab is a substantial part of the investigator's research program, a MIRA may not be a suitable means of support.
Can I work with foreign collaborators?
Yes. NIGMS supports international collaborative research efforts, and investigators are encouraged to pursue scientifically productive collaborations. In rare cases where a foreign collaborator's efforts are well-justified, represent a unique scientific opportunity, are essential to the research program of the MIRA, and cannot be supported by the foreign collaborator, a consortium agreement with a foreign institution can be included.
Can you clarify how much flexibility is meant by "flexibility to pursue new research directions"?
This needs to be considered on a case-by-case basis and requires the application of reasonable judgment by both investigators and NIGMS staff. The extension of studies on a problem from one organism to another would be very reasonable. Extending a project into another within the mission of NIGMS would also be reasonable. When in doubt, you should discuss proposed changes with the appropriate NIGMS program director. The flexibility to pursue new research directions does NOT mean a MIRA PI could submit an overlapping or even duplicate application to other NIH Institute(s)/Center(s), other federal agencies, or private foundations. Receiving funding on overlapping applications could lead to a budget reduction or termination of MIRA. Please contact your program officer for further discussions about these issues.
What would be considered within or outside the scope of a MIRA?
Potential indicators of changes in scope include the addition, or a change in the approved use, of human subjects, vertebrate animals, select agents or human embryonic stem cells. These changes require appropriate documentation and prior approval by NIGMS before the work is initiated. Work that migrates into the mission of another NIH institute or center would be considered out of scope, and it would be appropriate for this work to transition out of MIRA to grants supported by the relevant NIH institute or center.
Can a PI apply for a MIRA and NIGMS R01, R15, or R21 grants concurrently?
On submission of a MIRA application, the PI cannot submit another research grant including R01, R15 or R21 to NIGMS until the MIRA application has been reviewed and a summary statement issued. Please refer to PAR 22-180 for applications that are allowed pending review at the same time as your MIRA application.
What other NIGMS funding opportunities can a PI apply for concurrently with a MIRA application?
These applications are allowed to be pending review* at the same time with a MIRA application:
- Grants supporting resources for the research community;
- Collaborative Program Grant for Multidisciplinary Teams (RM1) grants;**
- Biomedical Technology Development and Dissemination (RM1) grants;***
- Sepsis Human Biospecimen Collections (R21/R33) Phased Innovation Awards;****
- Grants supporting training, workforce development, or diversity building activities;
- Grants supporting multi-site clinical research , both clinical trials and observational studies, with costs for patient recruitment, protocol-related expenses, and/or community resource activities (e.g., specimen banking, dissemination);
- INBRE (P20), COBRE (P20/P30) or IDeA-CTRs (U54), SEPA, or NARCH;
- SBIR/STTR grants;
- Conference grants;
*Applications are defined as ‘pending review’ until a summary statement is issued.
** MIRA applicants and recipients can participate as a PD/PI on a Collaborative Program Grant for Multidisciplinary Teams (RM1) as part of the 51% effort required by the MIRA but may not receive additional funds from this RM1.
*** For the Biomedical Technology Development and Dissemination (BTDD) grants, the PD/PI must maintain 51% research effort toward their MIRA, and 3 person months' effort toward the BTDD. The BTDD award can only provide support in a MIRA PD/PI's laboratory for late-stage technology development and dissemination efforts.
**** For the Sepsis Human Biospecimen Collections (R21/R33) Phased Innovation Awards, if a New Investigator is issued the R33 portion of the phased award prior to the MIRA, they are no longer eligible as a New Investigator to receive the MIRA.
How can I decide if MIRA is right for my circumstances?
Decide whether a single NIGMS program, with a budgetary constraint on well-funded labs, is worth the advantages provided by the MIRA program. The advantages include 5 years of funding, more scientific flexibility, greater funding stability and reduced administrative burden.
Why does NIGMS think that this initiative will enhance the quality of science generated by its community of grantees?
MIRA-supported investigators will have the flexibility to pursue the science they want to do as it evolves, rather than being held to specific aims that they proposed before they received a grant. In this way, they will have greater flexibility to try ideas that might be considered high-risk. MIRA is expected to result in more stable funding of investigators, better continuity of effort and better ability to keep well-trained personnel in the laboratory. MIRA is also expected to broaden the distribution of funding among laboratories, enabling more of the nation's highly talented and promising investigators to participate.
Can the topic of a MIRA be multiple distinct research directions?
MIRA is intended to enable consolidation of NIGMS support for multiple projects that may be disparate, so there is no obligation to develop a single unifying theme. Applicants should directly address the rationale underlying the balance of effort and the resources dedicated to each activity, and how the activities are distinct or complementary.
What NIGMS grants are qualifying awards for a MIRA application?
A single-PI R01-equivalent award (defined here as R01, R35, R37, DP1, DP2, SC1, or NRNM U01) from NIGMS is a qualifying award for a MIRA application.
What if I don’t have an active NIGMS grant?
New Investigators (those who have not competed successfully for a substantial, independent NIH research award) working in the mission areas of NIGMS will be eligible to apply for a MIRA if they have not received substantial funding as a subproject leader on a multi-component NIH award, except for project leaders and pilot project leaders on NIGMS COBRE, NIGMS IDeA-CTR or NIGMS NARCH awards who are eligible to apply for a MIRA. Potential applicants are strongly encouraged to contact NIGMS program staff to discuss the relevance of their research program to the NIGMS mission before submitting a MIRA application. Applications that fall outside of the NIGMS mission will be withdrawn prior to review.
PD/PIs who are currently Early Stage Investigators (ESIs) should apply through the ESI MIRA NOFO and should not apply through PAR 22-180 .
What is the time window for submission of a new MIRA application?
A new MIRA application can be submitted at any time prior to the original project end date, but no later than the end of the fiscal year following the one in which the qualifying award was originally set to expire, regardless of the status and duration of a no-cost extension. For example, if the qualifying R01-equivalent award ends in fiscal year 2022 (October 1, 2021 to September 30, 2022), a MIRA application needs to be submitted before September 30, 2023.
Can I renew my MIRA?
Yes, NIGMS encourages PDs/PIs who are currently funded with an NIGMS MIRA to renew their MIRAs, including both established investigator MIRAs and ex-early-stage investigator (ESI) MIRAs. Information on MIRA renewal success rate and budget increases can be found in this NIGMS Feedback Loop post .
What is the time window for submission of a renewal MIRA application?
A renewal MIRA application should be submitted no later than the end of the second fiscal year following the one in which the funded MIRA was originally set to expire. For example, if the current MIRA expires in fiscal year 2022 (October 1, 2021 to September 30, 2022), a renewal MIRA application needs to be submitted before September 30, 2024. NIGMS will not accept applications from MIRA grantees for awards that would begin prior to the end date of the current MIRA, with the exceptions listed in the NOFO.
Can I submit a renewal application early and propose a start date that is after the project end date of my active MIRA?
MIRAs will be funded in the fiscal year (e.g. FY2024 starts on October 1, 2023 and ends on September 30, 2024) of Council review. A renewal application that is submitted too early may lead to a truncation of the current MIRA, even if the proposed start date is after the project end date of the current MIRA. Please consider the Key Dates, especially the Council Review Date and the Earliest Start Date in the NOFO before deciding when to submit the renewal application.
Why do MIRA renewal applications have a longer time window for eligibility?
The MIRA to be renewed is the only qualifying award for the PD/PI. In contrast, a PD/PI may have two R01s as qualifying awards and thus could have multiple opportunities to submit a new MIRA application.
Will early submissions be allowed?
NIGMS will not accept applications from MIRA grantees for awards that would begin prior to the end date of the current MIRA, with the exceptions listed in the NOFO.
Suppose my MIRA application does not score well enough to be funded. May I reapply for a MIRA next year?
A PD/PI may submit another MIRA application as long as the PD/PI is eligible. Note that each application will be considered as a new application, not as a resubmission.
Does the eligibility time window include grants in no-cost extension status?
The original project expiration date of the qualifying award will be the sole determinant. Please see the previous questions about the time windows for new and renewal applications. The qualifying award could be in no-cost extension when a new or renewal MIRA application is submitted.
Can I submit a R01 application before my MIRA is due for renewal?
Because MIRA supports the research program of a PD/PI, NIGMS will not accept a R01 application with a project start date before the end date of the MIRA. MIRA Renewals have a high success rate. Unless your research program has changed significantly and MIRA is no longer optimal for supporting your lab, renewing MIRA would be a better option in most cases.
Can I be a PI on a new MPI R01 application before my MIRA is due for renewal?
NIGMS expects MIRA PDs/PIs to collaborate with other investigators using funds from their MIRA awards. NIGMS will not accept MPI R01 applications with MIRA awardees listed as one of the PDs/PIs unless the requested start date is after the MIRA project end date and the MIRA PD/PI does not have a MIRA application pending review.

Can I be a PI or a project lead on a NIGMS Collaborative Program Grant for Multidisciplinary Teams or other multiple component project?
Yes, a MIRA PD/PI may participate in a RM1 project as part of the PD’s/PI’s 51% research effort. The MIRA PD/PI will not request or receive research funds from the RM1 while the PD’s/PI’s research program is supported by MIRA.
If an investigator has two or more R01-equivalent grants, do they all have to meet the eligibility requirements for applying for the MIRA?
No, an investigator needs only one eligible R01-equivalent award in a given fiscal year to be eligible to apply for the MIRA.
If I am the PI on one or two multi-PI R01s, am I eligible to apply?
No. Eligibility is restricted to PDs/PIs with at least one NIGMS single-PI R01-equivalent award.
Are individuals at all stages of their careers eligible?
Investigators with a qualifying NIGMS award are eligible to apply for a MIRA in the time window defined in the NOFO. New Investigators (those who have not competed successfully for a substantial, independent NIH research award) working in the mission areas of NIGMS are eligible as described in the eligibility section. Early state investigators working in the NIGMS mission areas should apply for a MIRA only through the MIRA NOFO for ESIs .
May two or more scientists apply as a team for an NIGMS MIRA?
No. This NOFO is intended to provide support for the NIGMS mission-related research program of a single independent investigator.
Are scientists in the NIH intramural research program eligible to apply?
No, NIH intramural research program scientists are not eligible to apply. A MIRA application can include a collaboration with an intramural scientist, but no funds can be provided to the intramural laboratory via MIRA. A MIRA will not be converted to a cooperative agreement. Therefore, if the intramural researcher would have primary responsibility for a significant component of a MIRA program, a MIRA may not be a suitable means of support.
Can I apply for a MIRA if I have grants from other NIH Institute(s)/Center(s) in addition to my qualifying awards from NIGMS?
Yes. Investigators may have support from other NIH components or from other sources. However, that support in itself does not confer eligibility to apply for a MIRA. Investigators are eligible if they meet the eligibility requirement as defined in the NOFO ( PAR 22-180 ) and are able to commit 51 percent of their total research effort to the MIRA.
Can I combine all my NIH grants into a MIRA?
Research supported by non-NIGMS sources may not be included in the base of the MIRA budget, and NIGMS funds will not be awarded to support the non-NIGMS research. Please note that, consistent with NIGMS' policy on Support of Research in Well-Funded Laboratories , if your laboratory receives a large amount of research support from non-NIGMS sources such that your total funding is in excess of $1,500,000 annual total costs, your application will receive additional scrutiny by the NIGMS Advisory Council and, if awarded, your MIRA budget may be reduced.
Can I submit an application for the continuation of my current NIGMS grant support and apply for MIRA at the same time?
No. NIGMS will not accept a new or renewal R01 application from a PD/PI until after the summary statement of a pending MIRA application is released. NIGMS will not accept a MIRA application from a PD/PI until after the summary statement of a pending R01 application is released.
Are Howard Hughes Medical Institute investigators eligible to apply for MIRA? What about individuals with other types of substantial, unrestricted laboratory grant support?
Yes. HHMI investigators are eligible to apply for MIRA. The relationship between the work supported by HHMI and the work to be supported by NIGMS must be carefully explained but need not be separated into different projects and different specific aims. However, study sections, the National Advisory General Medical Sciences Council, and NIGMS staff will take total support, including PI salary support by HHMI, into account when considering the appropriate budget level of a MIRA. Investigators with other types of substantial, unrestricted laboratory support will be similarly considered on a case-by-case basis.
If I have an appointment at more than one institution, can I apply for a separate MIRA through each institution?
No. An investigator can only have one MIRA, which should be submitted by the institution where the PI primarily conducts their research program. Under rare circumstances, a subcontract might be permitted to support a part of the research program that is based at a different institution.
Can I keep my MIRA if I obtain a second appointment at another domestic or foreign institution?
A PD/PI is expected to commit at least 51% of their total research effort to MIRA as originally reviewed and approved. Significant change of research environment will be evaluated by NIGMS staff on a case-by-case basis, and may lead to termination of the MIRA award.
Can I request an extension of my eligibility status?
You may request to extend your eligibility to apply for a MIRA for reasons including medical issues, disability, family care responsibilities, extended periods of clinical training, natural disasters, and active-duty military service. Please submit your request at [email protected] . Your request will be determined by NIGMS staff on a case-by-case basis.
Is there a limit on the number of applications that can be submitted by a PI? By an institution?
An investigator may be the PI on only one application for a MIRA in any one review cycle. There is no limit on the number of applications that can be submitted by an institution, provided they are for support of the research programs of different independent eligible investigators.
Can I get advice on my ideas for submission of a MIRA application?
You may discuss your ideas with the program officer who administers your current NIGMS applications and awards. New Investigators are strongly encouraged to contact the program officer who manages scientific portfolios relevant to what you propose to study in your MIRA application.
What became of the specific aims section of the grant application?
Specific aims are not allowed because a goal of MIRA is to move the scientific enterprise away from a focus on narrowly defined research projects with detailed specific aims and to refocus attention on the larger picture and potential overall impact of the research. If your institution's system for submitting a grant application gives you a warning and will not allow you to skip the specific aims page, enter the following text in the appropriate place: "Per the NOFO instructions, no specific aims are to be submitted."
How should I prepare the Current and Pending Support attachment?
Provide information on current and pending research support at the time of application as instructed in PAR 22-180 , which is different than the Other Support reported as part of your Just-in-Time information. Be sure to distinguish the direct costs per year that support research in the investigator's laboratory from support that goes to other investigators. See the MIRA webpage for an example.
Why is the research strategy section only six pages?
Because the goal of MIRA is to focus the investigator's and reviewers' attention on the higher level questions about significance and impact of the research program, details in the research plan can be reduced. Furthermore, changes in the biosketch mean that much of the needed information about the recent past research contributions of the investigator can be presented there, rather than in the research plan.
Do I need to include a Plan for Enhancing Diverse Perspective (PEDP)?
Yes. As stated in PAR 22-180, all MIRA applications must include a summary of strategies to advance the scientific and technical merit of the proposed project through expanded inclusivity. Please follow instructions in the NOFO when preparing your PEDP. For additional help, refer to the NIH BRAIN Initiative website for detailed information on the key elements and examples and FAQs of a PEDP.
Do I need to include a data management and sharing plan?
Yes, effective January 25, 2023, NIH implemented a new policy to promote the sharing of scientific data. A data management and sharing plan is required in MIRA applications. Include information on genomic data sharing (GDS) ( https://sharing.nih.gov/genomic-data-sharing-policy ) when applicable. Use "Other Plan(s)" attachment field on PHS 398 Research Plan Form to upload the data management and sharing plan.
Please refer to NIGMS Data Management and Sharing Plan for more information.
Can a MIRA support human subjects and clinical/translational research?
Yes. Clinical and translational research within the NIGMS mission may be supported through a MIRA. Clinical research must be completely within the context of the NIGMS clinical areas (anesthesiology, clinical pharmacology, sepsis, injury and critical illness). Mechanistic clinical trials are permitted in MIRA when the mechanistic study is an essential part of the research program. Clinical research that involves recruitment of human subjects at more than one site or the substantial financial support of multiple collaborators and subcontractors is not allowed, because these fixed commitments are not consistent with the highly flexible nature of the MIRA program. Clinical trials that are designed to test safety and efficacy of interventions (Phase I, Phase II, Phase III) for the purpose of future clinical treatment and/or regulatory approval are not permitted in MIRA. Pre-application discussion of clinical/translational research projects with an NIGMS program director is encouraged.
How do I prepare the Protection of Human Subjects section?
There is no change in the format/requirements for the Protection of Human Subjects section in the MIRA application. MIRA applicants should use the PHS Human Subjects and Clinical Trials Information form . Please note that study records should be entered for delayed start human subjects studies, i.e. a study that can be described at the time of application but will not begin at the time of award. The delayed onset human subjects study section only applies to studies for which definite plans for human subjects involvement cannot be described at the time of application.
Is delayed-onset human subjects research permitted in a MIRA?
Yes, both delayed start and delayed-onset human subjects research are permitted in MIRA awards. MIRA awardees should submit a prior approval request to their program director to add human subjects research to their MIRA. Prior approval requests should contain IRB approval of the human subjects study as well as a complete PHS Human Subjects and Clinical Trials Information form .
What approvals are required to add human subject studies to my MIRA project or to initiate "delayed onset" human subject studies?
MIRA awardees can submit a prior approval request via their AOR to the assigned NIGMS Grants Management Specialist to add human subjects research to their MIRA or to initiate "delayed onset" studies . Prior approval requests should contain IRB approval of the human subjects study as well as a complete PHS Human Subjects and Clinical Trials Information form .
What may I include in the appendix?
Please follow instructions in this Guide Notice when preparing your appendix materials. Do not use the appendix to circumvent the page limits.
When should the institutional letter of support be submitted?
The institutional letter of support should be submitted as part of the Just-in-Time information. This letter from the institution's authorized organizational representative must state that the institution is aware of and accepts the condition that other NIGMS research awards must be relinquished as a condition of receiving a MIRA and that if chosen to receive an award, the PI will commit a minimum of 51 percent of their total research effort to MIRA activities.
What is the policy on acceptance of late applications?
Applications that miss the due date and time are subjected to the NIH Policy on Late Application Submission .
Can I include a collaborator?
Financial support for a collaborator is not anticipated in most cases of MIRA, and such requests must be extremely well-justified. For example, one request that has been approved is for the MIRA PI to continue working with a longtime collaborator in order to gain access to the collaborator’s collection of cells from human subjects with mitochondrial disorders, which is not available anywhere else in the world, and the collaborator had no other funding to maintain this collaboration.
Should I submit letters of support?
A letter of support should be included from a collaborator who will receive financial support from the MIRA, which is not anticipated in most cases. Other letters of support are not encouraged. A MIRA PI will have the flexibility in future research directions and may or may not require any specific support.
Can you provide examples of "contributions to the research community"?
The MIRA program wants to provide recognition during the review to PIs who have made significant contributions to the research community. These contributions are not limited to, but are exemplified by, the following examples:
- Providing a research resource that is widely used, e.g., maintaining a research organism database.
- Running an effective program that gives high school or undergraduate students from diverse backgrounds experience and mentoring in biomedical research.
- Significant service to scientific professional societies, peer review panels, or journal editorial boards.
Who will be the scientific point of contact for my MIRA application?
The names and email addresses of the scientific contacts for distinct scientific areas. Once applications are received, they will be referred to the most relevant program official based on internal NIGMS referral procedures and guidelines. The program official assigned to your application should be visible to you in the eRA Commons by the time the application is reviewed, and the name and contact information for the program official should appear at the top of the summary statement.
Can I request a budget increase to expand my research program when I convert my R01(s) to a MIRA?
A strategic goal of NIGMS is to fund as broad a portfolio of meritorious investigators as possible, giving them the resources they need to carry out important research. For this reason, and to improve overall returns on investments, NIGMS pays close attention to budget levels in all funding decisions, and gives large budget increases only in highly compelling cases. As stated in the NOFO, most investigators should ask for budgets similar to what their overall NIGMS funding has averaged over the past three years. On average, investigators are likely to get higher budgets through MIRA than through NIGMS R01s.
Can I request a budget increase when I renew my MIRA?
Yes, you can. As reported in this FBL post , investigators have received budget increases when they renewed their MIRAs in the past. We anticipate similar budget increases for future MIRA renewals. As described on our funding policies page , multiple factors are considered when staff makes budget recommendations. Although there will be different budget scenarios and exceptions, as a guide, if your current MIRA budget is $250-275k or less, requesting a budget of $300k for your renewal would be reasonable.
How will the funding level for a new MIRA be established?
For well-funded investigators (generally with more than $400,000 direct costs from NIGMS), a new MIRA will likely be somewhat lower than the current/recent total budget in recognition of the stability, flexibility, decrease in administrative burden, and increased length of award that the MIRA provides. Investigators should keep in mind of NIGMS Policy that NIGMS is unlikely to award more than 2 R01s to a PI, so the MIRA is likely to be a better choice for most well-funded investigators.
In general, if an investigator's current total research support from NIGMS is between $250,000 and $400,000 in direct costs, the award may be about the same, reduced, or slightly increased.
If an investigator's current NIGMS research funding is more modest (less than $250,000), the MIRA will likely be funded at $250,000 unless the PI has significant other support.
MIRA budgets for New Investigators will generally be $250,000 in annual direct costs.
Because every situation is different, budget determinations are done with a great deal of consideration and on a case-by-case basis.
Can I request money for equipment in the budget?
Yes. You may request money for equipment in any year as long as there is appropriate justification. Note that this is not intended as a mechanism to acquire "big-ticket" items that may be covered under instrument-specific funding opportunities.
What is the maximum allowable budget?
Applications may request no more than $750,000 in direct costs including Equipment per year for a period of 5 years. However, investigators are expected to request what is actually well-justified for their research program. In general, the request should be commensurate with the investigator's stable base of NIGMS research funding. Cost efficiency is one of the goals of the MIRA program and will be one of the considerations in review and funding decisions.
MIRA budgets for New Investigators will generally be $250,000 in annual direct costs, not including equipment costs.
Should I include an itemized budget request in my MIRA application?
No, an itemized budget request is not required and will not be accepted. Instead, provide a total budget request for each budget period as instructed below.
Note: The budget instructions below have been updated to include instructions for the Data Management and Sharing Costs in accordance with NOT-GM-24-012 . Please see https://nigms.nih.gov/Research/Pages/data-management-and-sharing-plan.aspx for more information on the Data Management and Sharing policy.
R&R Budget
All instructions in the SF424 (R&R) Application Guide must be followed.
Itemized budget information is not required and will not be accepted. Instead, a total requested direct cost amount for each budget period, including requests for equipment purchase, is required. Other than years when equipment is requested, it is expected that the annual total direct costs in Section G. will remain constant in all years.
All instructions in the SF424 (R&R) Application Guide must be followed except as detailed below. While a 10-year R&R Detailed Budget form is provided in the application package, applicants may not request more than 5 years of support.
In order to allow submission of a budget request without filling out detailed yearly budgets, some basic information must be completed in order for NIH to successfully process the budget form. For each budget period:
- Select the appropriate Budget Type.
- Provide the Budget Period Start Date and End Date.
- Section A: Senior/Key Persons Provide an entry for the PD/PI, including the appropriate level of effort, and enter $0 for Requested Salary and $0 for Fringe Benefits. Entering $0 does not imply that the PD/PI will not receive any salary or fringe benefit support from the grant.
- In Section C: Enter budget request for equipment. Provide complete justification using the Budget Justification.
- In Section F: "Other Direct Costs" add a line item titled 'Requested Direct Costs' and provide the total request for that budget period including any costs requested for Data Management and Sharing Costs . Investigators with existing NIGMS research grants may request up to $750,000 Direct Costs including Equipment. New Investigators may request up to $250,000 Direct Costs excluding Equipment . NOTE: If equipment is requested in Section C, do not add the equipment budget in Section F. Other than when equipment is requested it is expected that the total annual direct costs requested will remain constant in future years.
- In Section H: Enter your institutional "indirect cost type," "indirect cost rate," "indirect cost base" and "funds requested." NOTE: Sections B, D, and E of the detailed budget page should be left blank.
Should I include a separate budget form if consortium/contractual costs are requested?
No, consortium costs are expected to be rare and must be extremely well justified. In the rare instance these costs are requested, the consortium/subaward direct costs (not including consortium F&A) should be included in the "Requested Direct Costs." in Section F. of the Budget Form. In the Budget Justification, provide an estimate of the total consortium/subaward costs (direct costs plus F&A costs) for each budget period, rounded to the nearest $1,000. Also, list the individuals and organizations with whom consortium/contractual arrangements have been made and indicate whether the collaborating institution is foreign or domestic.
What information should be included in the budget justification?
Note: This information has been updated to include instructions for the Data Management and Sharing Costs.
Justification for budget categories detailed below should be included in the budget justification. Additional information beyond these categories should not be provided. For MIRA renewal applications, justify any increase in funding requested over the previous project period. For applications for new MIRA grants, justify any requested increase in support relative to NIGMS research support over the previous three years.
Data Management and Sharing Costs : NIGMS' expectation is that most NIGMS grantees will not need to significantly change their data management and sharing practices and thus additional costs, if any, will be modest.
Justification for any requested Data Management and Sharing costs should be clearly labeled as "Data Management and Sharing Justification" and include any requested direct costs dollar amount. If no costs are requested for DMS, state "0" for the requested dollar amount . Follow instructions in the SF424 Application Guide for the Data Management and Sharing Justification .
Equipment : Justification for the need of equipment as well as equipment quote(s) may be included here.
Consortium/Contractual Arrangements are not anticipated in most cases and must be extremely well justified. List the individuals/organizations with whom consortium or contractual arrangements have been made and indicate whether the collaborating institution is foreign or domestic. Provide the total consortium/subaward costs for each budget period listing the total direct costs and indirect (F&A) costs separately.
F&A base : Explain any exclusions applied to the F&A base calculation.
Can I request Facilities and Administrative (F&A) Costs?
Yes, F&A costs should be applied as you normally would for an NIH research grant and requested in the application as per the instructions in the SF424 R&R application guide.
Should I include year-on-year inflationary increase for salaries or supplies?
No. Annual increases in any direct cost category are not allowed. Except when equipment is requested, NIGMS expects the budget to remain constant for all years for the entire project period. If increases are included in the submitted total budget, the increases will be removed from requested funds and may result in decreases in the total budgets of the years where they were included.
If I submit a renewal application for my MIRA, is it possible to receive an increased budget?
Yes, MIRAs with modest budgets that have been very productive and score very well could receive budget increases. The most recent data on MIRA renewal success rate and MIRA budget .
What is the timeline for submission, review, and award?
For applications submitted for the January due date, the review will be in July and the award will generally start between December 1 of the same year and March 1 of the following year. For applications submitted for the May due date, the review will be in November and the award will generally start between April 1 and June 1 of the following year. Some applications may be funded toward the end of the fiscal year.
What happens to my multiple component GM awards when I receive a MIRA?
If you receive funds as a project leader on an NIGMS multi-component grant (i.e., P01, P50, or RM1), your MIRA funding level will be adjusted to remove any overlap with the multi-component grant. You will be required to relinquish your funding from the multi-component grant at the end of its current competitive segment.
The project period of my NIGMS award was ended early to start a MIRA. Will I be able to use my existing unobligated balance?
Yes. Grants on which a project period is ended early may initiate the first no cost extension, thereby remaining active and retaining the unobligated balance. Funds should continue to be expended on the aims of the active grants that are or will be in no cost extensions.
How will MIRA applications be reviewed?
MIRA applications will be reviewed at the Center for Scientific Review. Below are the links to the current standing MIRA review panels.
https://public.csr.nih.gov/StudySections/DBIB/GGG/MRAA https://public.csr.nih.gov/StudySections/DBIB/BCMB/MRAB https://public.csr.nih.gov/StudySections/DBIB/CB/MRAC https://public.csr.nih.gov/StudySections/DBIB/CDB/MRAD https://public.csr.nih.gov/StudySections/DBIB/MBBC/MRAE https://public.csr.nih.gov/StudySections/DBIB/MGG/MRAF
In addition to the chartered MIRA study sections, Special Emphasis Panels can also be organized as needed.
Applications received for the January receipt dates will be reviewed in June/July for consideration by the National Advisory General Medical Sciences Council in September, with the earliest possible awards beginning in December. Applications received in May will be reviewed in October/November for consideration by the National Advisory General Medical Sciences Council in January of the following year, with the earliest possible awards beginning in April.
Will the reviewers have expertise in the subject area of my application?
Yes. The Center for Scientific Review will ensure that reviewers have the relevant expertise to review the application, bearing in mind that a MIRA is intended to support a broad program of research and the breadth of research areas that are encompassed by the NIGMS mission. Thus, reviewers will be expected to bring a broad perspective rather than detailed expertise.
How can I find the roster for the study section that will review my application?
Rosters will be available 15 days before the study section meeting.
How will the review process for MIRA differ from that for R01 research grant applications?
All applicants will receive a summary statement containing the reviewers' critiques, as well as a resume and summary of the discussion. Since MIRA applications have a relatively high success rates, the anticipation is that most of these MIRA applications will be discussed. Reviewers will be asked to provide a single overall impact score and will not provide individual criterion scores. This is intended to shift emphasis away from details of the application and the approach, and to emphasize the potential impact of the investigator's research program on the field. Reviewers will be asked for guidance on whether the budget should be increased, decreased, or stay essentially the same as the average level of NIGMS support over the previous several years. However, award levels will be based primarily on the parameters described in the Section IV, Budget Information.
How do the review criteria differ from those for an R01 research grant application?
The review criteria are the same, but the wording has been modified to emphasize the review of the investigator's overall NIGMS-relevant research program rather than a specific, narrowly focused project with highly tailored specific aims. Reviewers should emphasize MIRA-specific aspects of significance, investigator qualifications, innovation, approach, and environment.
Can I appeal the review of my MIRA application submitted to PAR-22-180?
Yes. Appeals are allowed for PARs.
If my application for MIRA is not funded, will I be able to prepare a resubmission?
No, but if you are eligible, you may submit a new application for a receipt date in the next fiscal year.
What will be Council's involvement in the second level peer review of MIRA applications?
The summary statements for MIRA applications will be made available to the National Advisory General Medical Sciences Council in the same way as for other applications.
How is scientific overlap between a MIRA and my other grants or applications evaluated?
MIRA grantees may apply for and receive research grants from other NIH institutes or centers or from other funding agencies. However, it is important to ensure the work supported by the MIRA is distinct from that supported by other sources or under review or funding consideration. A key test of scientific overlap is whether two grants would both be cited as having supported the same publication. If they would be, it is an indication that there may be scientific overlap between the two grants. NIGMS staff always evaluate the possibility of overlap with other active or pending grants prior to making an award. In addition, NIGMS staff look at the grants PIs cite on their papers at the time of each research performance progress report to check for possible scientific overlap between grants. They also evaluate the research the PI is currently conducting, and compare this to the work being supported by the PI’s other research support. If it is determined that scientific overlap exists, the MIRA grant may be adjusted or terminated.
When should I submit Just-in-Time information?
NIGMS will notify applicants when to submit Just-in-Time information. This information will be requested for all applications under consideration for funding, but the request is not meant to imply anything about the probability of an award. Be sure that the information provides a complete and accurate accounting of all awards that support research in the investigator's laboratory, as well as any pending applications.
As stated in the section III, a letter from the institution's authorized organizational official is required verifying that the institution is aware of and accepts the condition that other NIGMS research awards must be relinquished as a condition of receiving a MIRA and providing a statement that if chosen to receive an award, the PI will commit a minimum of 51 percent of their total research effort to MIRA activities.
How will MIRA funding decisions be made?
NIGMS staff will carefully consider the study section and advisory council recommendations, including the scores and language in the summary statement, Just-in-Time information and recent history of NIGMS, NIH and other grant support, in making recommendations about whether to fund a grant and at what level. As with all funding decisions, NIGMS also considers the breadth and diversity of the Institute's research portfolio .
How will investigators be informed about the process?
Investigators who contact NIGMS staff will be provided feedback in the usual way on the reviews and their prospects for receiving a MIRA. As always, NIGMS staff cannot make any definite statements until the plan to make an award is approved at an appropriate level. The Notice of Award is the only official notice of a government commitment to fund a MIRA.
What if I decline the MIRA after I am notified?
Once the PI is notified by program staff that NIGMS intends to fund a MIRA and the recommended funding level, the PI should reply by email with their intention to accept the MIRA within one week. If the PI declines the planned award, the institution should withdraw the application immediately. If the MIRA is declined, the PI has the option to pursue other programs to obtain NIGMS funding, but NIGMS will not assume any obligations as a result of this decision.
When will I find out if I will not receive a MIRA?
Applicants will not be informed that they will NOT receive an award, because an award may be made at a later date. MIRA applications in response to this PAR will remain under consideration until the end of the fiscal year following the year of submission of the MIRA application.
What is the principle for MIRA award level determination?
The initial budget determination is the level of annual funding for the MIRA. The MIRA funding level corresponds to the annual direct costs in the absence of other continued NIGMS funding. The MIRA award level reflects adjustments that are made to the MIRA funding level to account for overlap with the PI's current NIGMS grants along with the addition of the appropriate Facilities and Administrative Costs.
What is the anticipated success rate for this NOFO?
NIGMS anticipates that the success rate will be the same as or higher than that of established investigators' success rates on renewing NIGMS R01 grants. The success rates for new and renewal MIRA applications for fiscal year 2021 are published in this NIGMS FBL post .
Will carryover of an unobligated balance from one budget period of the MIRA to another be permitted?
Yes. Automatic carry over authority will apply to MIRA. Standard NIH and NIGMS policies apply.
I am a MIRA PI who is also a co-investigator on someone else's NIGMS grant. How will that grant be affected and how does that affect my MIRA funding?
If the MIRA application proposed to continue work on an NIGMS grant for which he or she is currently a co-investigator, the applicant's effort will be subsumed into the MIRA. When the MIRA is funded, NIGMS will inform the PI of the other grant that the MIRA PI can no longer receive funding as a co-investigator on the grant and that the PI may rebudget those funds in accordance with all relevant established policies. If after being awarded a MIRA, the MIRA PI wishes to be listed as an unpaid collaborator on another PI's NIGMS grant application, this is permitted, but the MIRA PI cannot request salary or receive funds in any form from another NIGMS award.
With acceptance of the MIRA, the special terms and conditions on the MIRA Notice of Award stipulate the existing NIGMS grants from which the MIRA PI can continue to receive funding. A MIRA PI who is a co-investigator cannot receive funds from NIGMS grants not specified on the MIRA Notice of Award.
How will existing administrative supplements to affected grants be handled?
Diversity supplements will be included in the MIRA funding level consideration and also in offsets taken to determine the MIRA award level in each year. Equipment supplements will be excluded from MIRA budget base calculations.
How will NIGMS multiple-PI R01s be affected?
NIGMS multiple-PI R01s where the MIRA PI is one of the PIs (whether contact PI or not) will continue through the end of the current project period. These funds will be considered in setting the MIRA funding level and an offset will be taken in the first year and each of the remaining years on a prorated basis. Depending on the funding recommendation, once the multi-PI R01 award ends, the MIRA award level may or may not increase.
The remaining multiple-PIs can submit a renewal application. The MIRA PI can continue to be listed as an unpaid collaborator in the renewal application, but the MIRA PI cannot request any salary or receive funds in any form from the renewal application. The MIRA PI’s support must be provided from the MIRA grant.
What kinds of grants will not be affected by the MIRA?
Non-affected grants include NIGMS grants that support research resources, training, workforce development or diversity building, clinical trials, SBIR/STTRs, conference grants, cooperative agreements, and the portion of a multiple component grant that is strictly a core. Funds currently supporting these activities will not be included in the MIRA funding level.
How should investigators attribute support from their MIRA on publications?
It is very important for PIs to accurately attribute grant support on their publications . The terms and conditions of all NIH awards , including MIRAs, state that all research publications supported in whole or in part by NIH must include a specific acknowledgment of NIH grant support, such as: "Research reported in this publication was supported by [name of the Institute(s), Center, or other NIH offices] of the National Institutes of Health under award number [specific NIH grant number(s) in this format: R01GM987654] ."
(If you have more than one grant, only cite the grant(s) that supported the research described in the article.) In addition, prior to award of any NIH competing grant application, PIs are required to provide a complete list of all their active and pending other support and, in the subsequent annual progress reports, describe any changes in other support, including new funding received: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-19-114.html .
Will MIRAs be eligible for administrative supplements?
MIRAs will be eligible for certain types of administrative supplements as described in the NOFO ( PAR 22-180 ).
Can I convert my MIRA back to an R01?
Once a MIRA has been issued, that grant cannot be converted to an R01 award. The terms and conditions of the MIRA will continue through the end of the project period unless the institution chooses to relinquish the grant early. At the end of the project period, the investigator will have the option to apply for continuation of the MIRA or to apply for a new R01 or other forms of support. At the end of the MIRA, an R01 submission must have a requested start date that is after the project end date of the MIRA.
What, if anything, will be different about the annual reporting required for MIRA?
Annual reports will be required using the Research Performance Progress Report (RPPR). The NOFO includes some additional instructions consistent with the broader goals of the research program supported by MIRA and the absence of detailed specific aims. Additional information is requested that describes any new program directions within the NIGMS research mission, discussion of how the work continues to be of high impact, and the relationship of any new other support to the activities supported by MIRA. These reporting requirements are more specific for MIRA than for an R01 research project grant.
How will NIGMS manage overlap with other grants that may be awarded after MIRA?
Changes in other support must be reported in the RPPR. The relationship between other support and work supported by MIRA should be explained. NIGMS will assess whether there is sufficient scientific and budgetary overlap to warrant adjustment of the MIRA.
Can I adjust my effort on MIRA?
The requirement for at least 51 percent of total research effort must be met for the entire project period of MIRA. A PD/PI will not be permitted to reduce their effort level on MIRA to less than 51 percent of their total research effort.
Will a change of PI be allowed?
Formally, the MIRA is awarded to the institution in support of a project, not to an individual person. The institution has the right to request prior approval by NIH for the replacement of the PI. However, given the very intimate association of the ideas, expertise and record of productivity of the specific investigator with the program of research described in the research plan of the MIRA application and the scientific merit of the application as determined during peer review, it is doubtful that NIGMS would approve a permanent change of PI. A temporary change may be allowed with prior approval under circumstances such as sabbatical leave, medical condition, disability or personal or family situations such as child or eldercare needs.
What happens if the PI becomes unable to carry out the duties as PI or will be absent for more than 3 months at a time for any reason?
Standard NIH and NIGMS policies will apply.
How will NIGMS handle changes in senior/key personnel on a MIRA?
Senior/key personnel named in the Notice of Award may be replaced or eliminated from the budget with NIH written prior approval. Standard NIH and NIGMS policies apply. See NIH Grants Policy Statement regarding key personnel for full details.
Will a change of grantee institution be allowed?
Who will be the program officer for my mira.
If you currently have grants administered by two or more different NIGMS program officials, the program director for your MIRA will be assigned by considering the scientific areas included in the MIRA application, NIGMS internal referral guidelines, and discussion among NIGMS staff.
Regarding allowable exceptions to the restriction on obtaining other funding from NIGMS after a MIRA award is made, what is meant by “funding for clinical trials”?
This generally refers to a clinical activity that is not part of the MIRA investigator’s own research program, for example, serving as a site PI for a multi-site clinical trial. That is often a structured activity staffed by physicians, nurses, and personnel who are working in a professional care-giving capacity and not in a research role.
Can an NIH Director’s Transformative Research Award (tR01) be assigned to and awarded by NIGMS while I am funded with a MIRA?
No. A tR01 is assigned to a specific institute at NIH based on the scientific area of the proposed research. If your tR01 proposes research that falls within the scientific mission of NIGMS, it would be considered to overlap with your MIRA, even if the proposed work in the tR01 application is different from what was described in the MIRA application. A MIRA PD/PI is encouraged to contact their PO before submitting a tR01 application to NIH.
What changes will require NIH written prior approval?
What approvals are required to add animal studies to my mira project, or when studies included as delayed onset in the application are to be initiated.
Prior approval is needed for the following:
- A change in scope, including, but not limited to, a change from the approved use of vertebrate animals or involvement of human subjects, select agents or human embryonic stem cells
- Additional no cost-extensions beyond a first no-cost extension or late notification of an initial no-cost extension
- Change in status of the PI or senior/key personnel named in the Notice of Award
- Change in grantee organization or organization status
- Addition of a foreign component if not included in the original application, including a significant new foreign collaboration, requires NIGMS' prior approval; however, MIRA funds may be used to support a subcontract at a foreign institution only if the collaboration is essential to the PI's research program, represents a unique scientific opportunity and cannot be supported by the collaborator.
You will need to notify your NIGMS program officer and grants management specialist of any significant changes to your animal research. Addition of animal studies and/or initiation of "delayed onset" studies requires NIGMS and NIH Office of Laboratory Welfare (NIH OLAW) administrative review prior to the start of these animal studies if you will be using funds from your MIRA. Approval from your IACUC for any new animal studies that will be carried out or for any significant changes to your approved animal studies is also necessary. You will need to provide an updated VAS section and NIH OLAW will need to provide their written approval that you may proceed with your animal studies using your NIGMS MIRA funds including for the purchase of any animals. For details, please see NIH Notice NOT-OD-14-126 .
If you conduct research involving animals on your MIRA without your IACUC approval, NIH may reduce the amount of your award or request return of funds, and you may not use any animal data obtained during this time for any activity related to the grant award.
MIRA awardees can submit a prior approval request in writing via their AOR to the assigned grants management specialist to add human subjects research to their MIRA or to initiate "delayed onset" studies. Prior approval requests should contain a complete Protection of Human Subjects section as described in the current MIRA notice of funding opportunity.
If my MIRA is in a No Cost Extension (NCE), do I still need to meet the minimum level of effort requirement on the MIRA of 51% total research effort?
The requirement of MIRA 51% total research effort applies for the duration of the MIRA, even during a no-cost extension. However, for exceptional circumstances, written prior approval for the reduction in PI/PD effort below the MIRA 51% total research effort can be requested. The recipient is reminded that active awards must have a measurable level of effort. Written prior approval requests should be submitted at least 30 days before the effective date of the change. If the request is emailed, it must provide evidence of the AOR's approval; a cc to the AOR is not acceptable.

Connect With Us: Facebook Instagram Linkedin Subscriptions YouTube
- Your Privacy
- Accessibility
- Disclaimers
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- National Institutes of Health: NIH...Turning Discovery Into Health®
- 45 Center Drive MSC 6200
- Bethesda, MD 20892-6200
- 301-496-7301

Internal Resources
Honors and awards, 2024 college of medicine – tucson faculty excellence awards.
Deadline for completed submissions extended to Friday – November 17, 2023.
All of these competitive awards are acknowledged by a plaque at a COM-T Awards Ceremony on February 28, 2024.
Self-nominations and nominations from colleagues will be accepted.
Please use the link provided after each award to submit the required materials.
Incomplete submissions will not be considered.
Clinical Excellence Award Nomination Information
Clinical Excellence Awards (2 awards available)
The Annual College of Medicine – Tucson Clinical Excellence Award recognizes physician faculty members at the Assistant or Associate Professor level for outstanding clinical care. Nominations of physician faculty are welcome from all members of the College of Medicine – Tucson community: faculty, staff, residents, administrators and students.
The selection of the person receiving this award will be based on the following criteria:
- Delivers consistently outstanding clinical care
- Has an approximate clinical effort of .50 or higher FTE
- Is readily available for consultation with other faculty, demonstrating an outstanding ability to assist and share expertise with colleagues
- Encourages medical students and residents to ask questions and express their ideas or opinions
- Uses innovative methods to assure a positive clinical outcome
- Is humanistic in his/her approach to patient care and interaction with colleagues and other healthcare professionals
- Demonstrates a sincere commitment to patient care and patient access to care
To be considered for the award, please submit the following information:
- Personal statement/philosophy of clinical care (no more than one single-spaced page)
- A biosketch (CV or NIH Biosketch are acceptable)
- Letters of support from patients/students/trainees/colleagues/division chief/department head (minimum of 2, no more than 5)
Please Submit All Materials Here
Submissions will be reviewed and awardees selected by a committee made up of COM-T and health system leadership.
Research Excellence Award Nomination Information
Research Excellence Awards
These awards recognize faculty investigators at the Assistant or Associate Professor level who have demonstrated outstanding research efforts and advancement of science.
1. Clinical Investigator Award:
This award recognizes an investigator who writes, designs, and runs clinical trials or clinical research studies at the College of Medicine – Tucson. The recipient is involved on a national stage for their disease/area of focus and demonstrates success in moving the field forward based on their work. The selection of the person receiving this award will be based on the following criteria:
- Actively engaged in clinical trials or clinical research, serving as site and/or national PI
- Consistently accrues open clinical trials to support COM-T’s clinical research efforts
- Is readily available to research personnel and research participants to answer questions, manage queries, and sign-off on required documentation
- Designs and runs investigator-initiated trials
- Publishes research results in high-impact journals
- Is recognized on the national or international level as a clinical research thought leader in his/her area of expertise
2. Basic and Translational Investigator Award:
This award recognizes an investigator who conducts research aimed at basic science and translational research discoveries or the development of patents, technology, or devices.
- Actively engaged in basic research supported by externally peer-reviewed funding OR serves as a liaison between basic researcher and the clinic to translate scientific discoveries into clinical research
- Consistently contributing to the basic or translational research mission of the COM-T
- Makes additional contributions to COM-T research mission through service or mentoring of trainees at any level
- Recognized on the national or international level as a basic/translational research thought leader
To be considered for these awards, please submit the following information:
- Personal statement and description of the impact of your work in your field (no more than one single-spaced page)
- A biosketch (please only submit a biosketch - do not submit CV)
- Letters of support from colleagues/division chief/department head (minimum of 1, no more than 3)
Submissions will be reviewed by a committee selected by the Office of Research. Reviewers will consider the nominee’s rank as Assistant or Associate Professor and evaluate according to expectations of that rank. Final awards approval will be made by the Vice Dean of Research and Graduate Studies and Associate Dean of Clinical and Translational Research.
DEI Excellence Award Nomination Information
DEI Excellence Awards
1. Individual (2 awards available)
The Diversity, Equity and Inclusion Award honors the cumulative contributions of a faculty member to advance diversity, equity and inclusion (DEI) at UACOMT. Contributions can include, but are not limited to, the mentoring of diverse students, residents/fellows, and faculty; advising students or student organizations; curriculum development, academic teaching, research, or service.
- Candidate demonstrates a sustained record of serving as an advocate for DEI.
- Candidate must have contributed in significant ways to strengthen the overall climate of inclusion at UACOMT.
- Candidate advances inclusion and equity with contributions that have resulted in a positive campus outcome.
2. Departmental (1 award available)
This award honors a department that has had a demonstrable impact in shaping and driving the DEI agenda at UACOMT; and demonstrates the sustained value it has added to the institution. Nominations should indicate how the nominee meets one or more of the following criteria:
Engaged in an initiative that is considered a best practice that impacts DEI within the department, institution, and/or community”. Implemented successful initiatives that have involved or led to real changes in “the way we do things around here.” Implemented steps toward fostering diversity in the workplace with programs that maximize the potential of all people.
Nomination Process: A nominator may choose to complete and submit a nomination for an individual or department. Final submission requires:
- Completed nomination form - Click Here to Download Form
- Two letters of support (no more than 500 words each) for the nominee (individual or department) on how they significantly promoted diversity, equity, and inclusion among UACOMT faculty, staff, students, and residents/fellows.
- CV or biosketch for Individual Award nominations
Submissions will be reviewed and awardees selected by the Faculty Diversity Advisory Committee.
GME Excellence Award Nomination Information
Excellence in Graduate Medical Education (GME) (2 awards available)
The Award for Excellence in Graduate Medical Education (GME) is open to any faculty member who, in the past year, has demonstrated outstanding effort in advancing the educational quality of Residents and/or Fellows. Innovative teaching methods, implementation of best practices, and outstanding curricular development are some examples.
Selection will be based on the impact the faculty member’s work has had with regard to:
- Optimizing the quality of education in their program]
- Enhancing GME at COM-T
- Contributing to GME in their specialty at the national level
- Having an impact on the advancement of GME beyond their program/specialty
In order to be considered for this award, please submit the following:
- Personal statement and description of your work and the impact it has made (no more than one single-spaced page).
- Letter of support from department head or Designee
- (optional) Letters of support from no more than 2 additional colleagues
Letters of support should illustrate how the nominee’s work has had a significant positive impact in GME and what characteristics have made them unusually effective.
Nominations will be reviewed and awardees will be selected by a group of GME education leaders.
WIMS GRACE Excellence Awards
2024 college of medicine – tucson mentoring awards.
Deadline for completed submissions is Thursday – November 9, 2023.
We invite all COM-T faculty to nominate a senior faculty or peer mentor who has contributed to their professional development and advancement. Nominations will be reviewed and awardees selected by the Dean’s Council on Faculty Affairs.
These faculty mentors will be recognized at a COM-T Awards Ceremony on February 28, 2024.
Please use the link provided to submit required materials.
These faculty mentors will be recognized at a COM-T Awards Ceremony on February 28, 2024 .
Mentoring Award Nomination Information
Is there a faculty member who has contributed to your success, fostered your development or opened doors for you?
If so, please give your mentor the opportunity to be recognized with a COM-T Faculty Mentoring Award!
We invite all COM-T faculty to nominate a senior faculty or peer mentor who has contributed to their professional development and advancement. Nominations will be reviewed and awardees selected by the Dean’s Council on Faculty Affairs. These faculty mentors will be recognized at a COM-T Awards Ceremony on February 28, 2024.
Nominations should be no more than one page and should address how your mentor has demonstrated excellence across the following areas:
- Sharing of time and accessibility to mentees
- Supporting the mentee’s development with concrete evidence of successful achievement of goals or project completion
- Fostering a positive and inclusive culture
- Expanding mentee’s network and professional connections
- Serving as a role model for colleagues by maintaining high standards for excellence in their own discipline and across the institution
Specific eligibility:
- Previous winners are eligible for nomination two years from their previous win.
- View previous winners here .
- Both nominator and nominee must be current faculty members of the College of Medicine – Tucson.
The deadline for receipt of nominations is November 9, 2023.

Faculty Mentoring Awards

Faculty Teaching Awards

Faculty Excellence Awards

Grants Awarded for Health Sciences Research

Health Sciences Awards and Honors
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

- Policy & Compliance
- Updates To NIH Institutional Training Grant Applications
Updates to NIH Institutional Training Grant Applications
This page provides details on the updates being made to NIH institutional training grant applications for due dates on or after January 25, 2025.
On this page:
- Application updates
- Peer review updates
- Notices, reports, and blogs
- Training, resources, and FAQs
- Contact information
NIH has made significant investments to develop, implement, assess and disseminate innovative, effective approaches to research training and mentoring and to prepare trainees for a variety of career paths in the biomedical research workforce. Additionally, NIH established the UNITE initiative to identify structural barriers and promote equity in the NIH-supported biomedical research ecosystem. Through these initiatives, the research community has identified the need for:
- Broader outreach activities to foster awareness of research training opportunities for potential trainees from all backgrounds, including individuals from underrepresented groups ,
- Targeted recruitment activities to diversify training program applicant pools, and
- Increased mentorship opportunities to facilitate trainee success (see Re-envisioning NIH Supported Postdoctoral Training ; UNITE Listening Sessions )
NIH will leverage the lessons of these initiatives to enhance institutional research training programs, including by enhancing opportunities to strengthen mentor training.
Applicability:
The updates will apply to applications that use the following activity codes:
- Institutional Training – T series, e.g., T15, T32, T34, T35, T37, T90/R90, TL1, TL4
- International Institutional Training – D43, D71, U2R
- Institutional Career Development – K12, KL2
Application Updates
The updates to NIH Institutional Training Grant Applications include three key changes:
- The Recruitment Plan to Enhance Diversity will be its own attachment in the PHS 398 Research Training Program Plan Form.
- Mentor training expectations will be more clearly defined in the parent T32 Notice of Funding Opportunity (NOFO).
- Institutional Training data tables will be updated to reduce burden and promote consistent information collection across training programs.
Updates to PHS 398 Research Training Program Plan Form
The “Recruitment Plan to Enhance Diversity” will be moved from being nested within the Program Plan attachment to being a separate attachment within the PHS 398 Research Training Plan Form . The Recruitment Plan to Enhance Diversity Attachment will:
- Continue to be required for all training grant activity codes except U2R, and all D-series activity codes.
- Have a three-page limit, consistent with the page limits for “Plan for Instruction in the Responsible Conduct of Research” and “Plan for Instruction in Methods for Enhancing Reproducibility.”
Defining Mentor Training Expectations in Training Programs
The Parent T32 NOFO will incorporate new language outlining expectations for mentor training and oversight into the program considerations, application instructions, and review criteria.
Programs should consider the following, in addition to other evidence-informed curricula, as potential mentor training components and are encouraged to adapt to program and trainee needs:
- Aligning expectations
- Maintaining effective communication
- Fostering independence
- Assessing scholars’ understanding of scientific research
- Enhancing professional development
- Addressing equity and inclusion
- Articulating your mentoring philosophy and plan
Reviewers will assess the mentor training expectations included in the application.
Updates to Data Tables
Institutional Training data tables will be updated to reduce burden, focus on trainee outcomes, and promote consistent information collection across training programs. For example:
- Tables 1 and 2: Applicants will be expected to provide data only for the training stage(s) reflected in the proposed program.
- Table 5 (Publications of Those in Training) will be reorganized so that the first column is the trainee (instead of the faculty member), and applicants will be allowed to include interim research products to which the trainee contributed.
- Table 6 (Applicants, Entrants, and their Characteristics for the Past Five Years) will no longer ask for trainee characteristics related to prior academic and research experience.
- Table 8 (Program Outcomes: Predoctoral and Postdoctoral) will no longer include Part II “Those Clearly Associated with the Training Grant.”
Peer Review Updates
Institutional training awards will retain the five scored review criteria. For example, for Training Grants (Ts), reviewers will continue to score Training Program and Environment, Training Program Director(s)/Principal Investigator(s), Preceptors/Mentors, Trainees, and Training Record when determining the overall impact score.
NIH will now include “Training in the Responsible Conduct of Research” and “Recruitment Plan to Enhance Diversity” as items that contribute to the overall impact score. These items will move from “Additional Review Considerations” and will be included as “Additional Review Criteria.” As such reviewers will evaluate the “Training in the Responsible Conduct of Research” and the “Recruitment Plan to Enhance Diversity” while determining scientific and technical merit, and in providing an overall impact score.
Notices, Reports, and Blogs
- Updates to NIH Institutional Training Grant Applications for Due Dates on or After January 25, 2025: NOT-OD-24-129 – May 31, 2024
- Updates to NIH Training Grant Applications - Registration Open for June 5, 2024 Webinar: NOT-OD-24-124 – May 8, 2024
- Open Mike Announcing updates to NIH Institutional Training Grant applications and required data tables – May 16, 2024
Training, Resources, and FAQs
Webinars and Videos
Learn more about the application and peer review updates for institutional training grants and have the opportunity to have your questions answered at our live events.
Slide Decks
Slide decks for use in public presentations.
Public FAQs
Answers to some of your most frequent questions
Contact Information
For those with questions, please direct all inquiries to: [email protected]
This page last updated on: May 28, 2024
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

COMMENTS
A biographical sketch (also referred to as biosketch) documents an individual's qualifications and experience for a specific role in a project. ... and resources necessary to carry out the proposed research. NIH biosketches must conform to a specific format. ... Direct specific biosketch and other support related questions to nihosbiosketch@nih ...
Details on ongoing and completed research projects/support from the past three years can be included within the personal statement (Section A). Section B, "Position and Honors" has been renamed "Positions, Scientific Appointments, and Honors" and the order changed from chronological to reverse chronological. See the Biosketch and FAQ ...
Guide to NIH Biosketch and Other Support Disclosures. Introduction . This document is intended to help reporting individuals (i.e. researchers and/or Key Personnel) identify and ... I. Section A:pplicants may A now include details on ongoing and completed research projects from the past three years that they want to drawattention to within the
NIH Biosketch & Other Support: Harvard FAQs . Revised November 30, 2021 . Please review the National Institutes of Health (NIH) Frequently Asked Questions (FAQs) on . Other Support and Biosketches. The Harvard responses below include requirements and instructions effective for due dates on or after . January 25, 2022. and are meant to ...
Perspective: The NIH incidents we have seen so far involve 87 institutions with confirmed violations of NIH rules by over 150 scientists. NIH funds scientists across the country and even across the globe. Each year, NIH awards more than 60,000 research and training grants. These support approximately 300,000 researchers.
17th Floor. 2716 South StreetPhiladelphia, PA19146United States. eSPA. Electronic Research Administration. eSPA Training Sessions. NIH Data Management and Sharing Plan Policy & Implementation. Proposal Preparation. NIH Biosketch and Other Support Guidance. Published on Aug 05, 2022 · Last Updated 9 months 3 weeks ago.
As announced in March, updated biosketch and other support format pages and instructions are available for use in applications, Just-in-Time (JIT) Reports, and Research Performance Progress Reports (RPPRs). Use of the new format pages is preferred immediately and required for due dates and submissions on or after January 25, 2022 (NOT-OD-21-110). This represents a change from the original May ...
In an effort to support strong collaboration between Federal research agencies, NIH has made every effort to align the Biographical Sketch (Biosketch), Other Support format page and Application Form Instructions with the guidance issued by the Office of Science and Technology Policy Joint Committee on the Research Environment.
*New* In-Kind Support Job Aid. NIH has also released a Disclosures Table to aid Investigators in determining what types of information must be disclosed. Please find this Table at the link below: NIH Pre-award and Post-award Disclosures Relating to the Biographical Sketch and Other Support. Sample Questions from Brown Staff & Faculty
This notice describes implementation of the updated Other Support and Biosketch format pages and associated instructions, as outlined in NOT-OD-21-073.. To align applicant and recipient systems with NIH's longstanding policy requirements and application requirement updates, NIH expects applicants and recipients to use the updated biosketch and other support format for applications, Just-in ...
Does NIH require disclosure of recently completed support in Other Support? The Biosketch instructions state that all positions and scientific appointments must be provided. Does this refer to active positions and appointments, or all positions a researcher has ever held? Look for the and stickers in our biosketch FAQs and other support FAQs ...
ongoing and completed research projects from the past three years. This was previously known as "Research Support" which was included in Section D of the biosketch. • Section B: Positions, Scientific Appointments, and Honors. Individuals completing a biosketch must list, in reverse chronological order, all positions and
NIH Biosketch & Other Support The National Institutes of Health (NIH) has issued new requirements for Other Support reporting and Biographical Sketches (Biosketches) submitted on or after January 25, 2022. The information provided below summarizes these new requirements. Resources Biosketch and Other Support Checklist Summary of Changes to Biosketch and Other Support NIH Disclosures Table…
NIH requires researchers to use the updated NIH Biosketches and Other Support format page for all Applications, Just-In-Time requests, and Research Performance Progress Reports (RPPRs) with due dates on and after January 25, 2022. There are two NIH Biosketch types: (i) Non-Fellowship Biosketch and (ii) Fellowship Biosketch.
Completion of the NIH Biosketch is part of the "Apply for Grant Funding" Portion of the grants process overview. ... Personal Statement including ongoing and completed research projects from the past three years that you want to draw attention to (previously known as research support). Section D will omit "Research Support" for fellowship ...
Send your inquiries to our new central email inbox [email protected]. See NOT-OD-21-122 for details. biosketch other support. Reminders About Financial Conflicts of Interest and Other Support. September 16, 2022. Tags: FCOI, other support, Research integrity. New and Updated Other Support FAQs Now Available. March 10, 2022.
Create Biosketches. Along with any principal investigators, include a biographical sketch with research support information for everyone you designate as senior/key personnel or other significant contributors (OSCs). This includes consultants and technical staff with senior/key personnel or OSC designations, even if they are not paid a salary ...
On March 12, 2021, NIH provided detailed information on the updated biosketch and other support formats in NOT-OD-21-073. It is critical for all NIH applicants and awardees, including PIs, senior/key personnel, and grants administrators, to read it in its entirety. Additional information can be found below. We recommend that you bookmark these ...
- Research Support (part of Biosketch): Highlights your/colleagues accomplishments as scientists. Used by the reviewers to assess your qualifications for a specific role in the proposed project, and to evaluate the overall qualifications of the research team. - Other Support (not part of Biosketch): NIH staff may request complete and up-to ...
NIH is adopting the Biographical Sketch Common Form and the Current and Pending (Other) Support Common Form in 2025 as per the White House Office of Science and Technology Policy (OSTP) memorandum on Policy Regarding Use of Common Disclosure Forms for applications and Research Performance Progress Reports (RPPRs) submitted on or after May 2025.. The Common Forms represent a collaborative ...
Effective January 25, 2022, the NIH will require changes to biosketches. Below is an abbreviated guide. New Biosketches are slightly different for Fellowship Grants (F-awards, R36 and research supplements for undergraduate researchers to promote diversity in health-related research) vs all others, such as RO1s, R21, R35.. Changes required for non-fellowship Biosketches by submission dates on ...
The terms and conditions of all NIH awards, including MIRAs, state that all research publications supported in whole or in part by NIH must include a specific acknowledgment of NIH grant support, such as: "Research reported in this publication was supported by [name of the Institute(s), Center, or other NIH offices] of the National Institutes ...
A biosketch (CV or NIH Biosketch are acceptable) Letter of support from department head or Designee (optional) Letters of support from no more than 2 additional colleagues; Letters of support should illustrate how the nominee's work has had a significant positive impact in GME and what characteristics have made them unusually effective.
Effective May 2025, NIH will be adopting the Common Forms for Biographical Sketch and Current and Pending (Other) Support as part of the directive from Guidance for Implementing National Security Presidential Memorandum (NSPM)-33. The Common Forms are part of a separate OMB collection, currently approved under 3145-0279.
Up-to-date NIH biosketch (NIH instructions) or curriculum vitae. ... CFAR Support: CFAR support (P30 AI027757) must be acknowledged in all publications and presentations derived ... please complete the Behavioral Innovations Research Consultation Form. • Biomarkers, Prevention and Interventions for HIV-associated Malignancies & NCDs Core ...
0925-0001/0002 (Rev. 08/12) Page Biographical Sketch Format Page BIOGRAPHICAL SKETCH ... resumed my research projects and collaborations and successfully competed for NIH support. In summary, I ... Completed Research Support . K02 AG442898 Hunt (PI) 02/01/02-01/31/05 . Drug Abuse in the Elderly ...
A personal statement describing research training and career goals that relate to the TL1 focus (1,500-word limit, approximately 3 pages); An NIH biosketch or curriculum vitae (CV); Letters of recommendation from at least two academic faculty familiar with the applicant's work (2-page limit per letter).
PHS 398 Fillable Forms - 3/2020 Revision. The links below allow for the downloading of individual and combined form files in MS Word and PDF formats. Some of the files are large and may take a few minutes to download. Fillable Individual PHS 398 Forms. (These forms are to be used only with paper submissions using the PHS 398.
May 29, 2024. Introduction: The UofL School of Public Health and Information Sciences (SPHIS) and School of Medicine (SOM) are soliciting applications for pilot projects funded with support from Deans Cardarelli and Bumpous. The Pilot Project Program was implemented to foster novel research forming the basis for new NIH R01 grant applications.
Application Updates. The updates to NIH Institutional Training Grant Applications include three key changes: The Recruitment Plan to Enhance Diversity will be its own attachment in the PHS 398 Research Training Program Plan Form. Mentor training expectations will be more clearly defined in the parent T32 Notice of Funding Opportunity (NOFO).