- français
- español
- português

Related Links
A practical guide for health researchers.

View Statistics
Description, other identifiers, collections.
- EMRO Publications
Show Statistical Information
- 4. Regional Office for the Eastern Mediterranean
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Trending Articles
- Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. GBD 2021 Risk Factors Collaborators. Lancet. 2024. PMID: 38762324
- A modern way to teach and practice manual therapy. Kerry R, et al. Chiropr Man Therap. 2024. PMID: 38773515 Free PMC article. Review.
- Burden of disease scenarios for 204 countries and territories, 2022-2050: a forecasting analysis for the Global Burden of Disease Study 2021. GBD 2021 Forecasting Collaborators. Lancet. 2024. PMID: 38762325
- Oncogenic fatty acid oxidation senses circadian disruption in sleep-deficiency-enhanced tumorigenesis. Peng F, et al. Cell Metab. 2024. PMID: 38772364
- Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. Bhatt SP, et al. N Engl J Med. 2024. PMID: 38767614
Latest Literature
- Am J Clin Nutr (1)
- Cochrane Database Syst Rev (2)
- J Biol Chem (6)
- Nature (52)
- PLoS One (110)
- Proc Natl Acad Sci U S A (15)
- Science (45)
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Search by keyword
- Search by citation
Page 1 of 69
Fidelity, pragmatism and the “grey line” in between—exploring the delivery of a pragmatic physical activity randomised controlled trial—a secondary analysis
Intervention fidelity in health services research has been poor with a reported lack of understanding about what constitutes pragmatic adaptation of interventions and what constitutes failure to maintain inter...
- View Full Text
The quality of reporting in case reports of permanent neonatal diabetes mellitus: a cross-sectional study
Although randomized trials and systematic reviews provide the best evidence to guide medical practice, many permanent neonatal diabetes mellitus (PNDM) studies have been published as case reports. However, the...
Stacked probability plots of the extended illness-death model using constant transition hazards – an easy to use shiny app
Extended illness-death models (a specific class of multistate models) are a useful tool to analyse situations like hospital-acquired infections, ventilation-associated pneumonia, and transfers between hospital...
Weighted metrics are required when evaluating the performance of prediction models in nested case–control studies
Nested case–control (NCC) designs are efficient for developing and validating prediction models that use expensive or difficult-to-obtain predictors, especially when the outcome is rare. Previous research has ...
Identification of patients’ smoking status using an explainable AI approach: a Danish electronic health records case study
Smoking is a critical risk factor responsible for over eight million annual deaths worldwide. It is essential to obtain information on smoking habits to advance research and implement preventive measures such ...
Multimorbidity prevalence and health outcome prediction: assessing the impact of lookback periods, disease count, and definition criteria in health administrative data at the population-based level
Health administrative databases play a crucial role in population-level multimorbidity surveillance. Determining the appropriate retrospective or lookback period (LP) for observing prevalent and newly diagnose...
Leveraging machine learning for predicting acute graft-versus-host disease grades in allogeneic hematopoietic cell transplantation for T-cell prolymphocytic leukaemia
Orphan diseases, exemplified by T-cell prolymphocytic leukemia, present inherent challenges due to limited data availability and complexities in effective care. This study delves into harnessing the potential ...
An evaluation of computational methods for aggregate data meta-analyses of diagnostic test accuracy studies
A Generalized Linear Mixed Model (GLMM) is recommended to meta-analyze diagnostic test accuracy studies (DTAs) based on aggregate or individual participant data. Since a GLMM does not have a closed-form likeli...
Machine learning models for abstract screening task - A systematic literature review application for health economics and outcome research
Systematic literature reviews (SLRs) are critical for life-science research. However, the manual selection and retrieval of relevant publications can be a time-consuming process. This study aims to (1) develop...
Weibull parametric model for survival analysis in women with endometrial cancer using clinical and T2-weighted MRI radiomic features
Semiparametric survival analysis such as the Cox proportional hazards (CPH) regression model is commonly employed in endometrial cancer (EC) study. Although this method does not need to know the baseline hazar...
Using Bayesian statistics in confirmatory clinical trials in the regulatory setting: a tutorial review
Bayesian statistics plays a pivotal role in advancing medical science by enabling healthcare companies, regulators, and stakeholders to assess the safety and efficacy of new treatments, interventions, and medi...
Protocol implementation during the COVID-19 pandemic: experiences from a randomized trial of stress ulcer prophylaxis
During the COVID-19 pandemic, many intensive care units (ICUs) halted research to focus on COVID-19-specific studies.
Propensity score analysis for health care disparities: a deweighting approach
Propensity score weighting is a useful tool to make causal or unconfounded comparisons between groups. According to the definition by the Institute of Medicine (IOM), estimates of health care disparities shoul...
A novel non-negative Bayesian stacking modeling method for Cancer survival prediction using high-dimensional omics data
Survival prediction using high-dimensional molecular data is a hot topic in the field of genomics and precision medicine, especially for cancer studies. Considering that carcinogenesis has a pathway-based path...
Missingness mechanisms and generalizability of patient reported outcome measures in colorectal cancer survivors – assessing the reasonableness of the “missing completely at random” assumption
Patient-Reported Outcome Measures (PROM) provide important information, however, missing PROM data threaten the interpretability and generalizability of findings by introducing potential bias. This study aims ...
Using brief reflections to capture and evaluate end-user engagement: a case example using the COMPASS study
Use of participatory research methods is increasing in research trials. Once partnerships are established with end-users, there is less guidance about processes research teams can use to successfully incorpora...
The establishment of a multiple myeloma clinical registry in the Asia–Pacific region: The Asia–Pacific Myeloma and Related Diseases Registry (APAC MRDR)
Multiple myeloma (MM) is the second most common haematological cancer worldwide. Along with related diseases including monoclonal gammopathy of undetermined significance (MGUS), plasma cell leukaemia (PCL) and...
Elucidating vaccine efficacy using a correlate of protection, demographics, and logistic regression
Vaccine efficacy (VE) assessed in a randomized controlled clinical trial can be affected by demographic, clinical, and other subject-specific characteristics evaluated as baseline covariates. Understanding the...
Correction: Impact of sampling and data collection methods on maternity survey response: a randomised controlled trial of paper and push‑to‑web surveys and a concurrent social media survey
The original article was published in BMC Medical Research Methodology 2023 23 :10
Problematic meta-analyses: Bayesian and frequentist perspectives on combining randomized controlled trials and non-randomized studies
In the literature, the propriety of the meta-analytic treatment-effect produced by combining randomized controlled trials (RCT) and non-randomized studies (NRS) is questioned, given the inherent confounding in...
Ascertaining the Francophone population in Ontario: validating the language variable in health data
Language barriers can impact health care and outcomes. Valid and reliable language data is central to studying health inequalities in linguistic minorities. In Canada, language variables are available in admin...
Stability analysis and numerical evaluations of a COVID-19 model with vaccination
A novel (nonlinear) mathematical model for the transmission of Coronavirus 19 (COVID-19) with eight compartments and considering the impact of vaccination is examined in this manuscript. The qualitative behavi...
Arrhythmia detection by the graph convolution network and a proposed structure for communication between cardiac leads
One of the most common causes of death worldwide is heart disease, including arrhythmia. Today, sciences such as artificial intelligence and medical statistics are looking for methods and models for correct an...
Multimorbidity in middle-aged women and COVID-19: binary data clustering for unsupervised binning of rare multimorbidity features and predictive modeling
Multimorbidity is typically associated with deficient health-related quality of life in mid-life, and the likelihood of developing multimorbidity in women is elevated. We address the issue of data sparsity in ...
Evaluation of respondent-driven sampling in seven studies of people who use drugs from rural populations: findings from the Rural Opioid Initiative
Accurate prevalence estimates of drug use and its harms are important to characterize burden and develop interventions to reduce negative health outcomes and disparities. Lack of a sampling frame for marginali...
Reporting of interventional clinical trial results in an academic center: a survey of completed studies
The dissemination of clinical trial results is an important scientific and ethical endeavour. This survey of completed interventional studies in a French academic center describes their reporting status.
Interpretable machine learning in predicting drug-induced liver injury among tuberculosis patients: model development and validation study
The objective of this research was to create and validate an interpretable prediction model for drug-induced liver injury (DILI) during tuberculosis (TB) treatment.
Application of causal inference methods in individual-participant data meta-analyses in medicine: addressing data handling and reporting gaps with new proposed reporting guidelines
Observational data provide invaluable real-world information in medicine, but certain methodological considerations are required to derive causal estimates. In this systematic review, we evaluated the methodol...
Log odds of positive lymph nodes (LODDS)-based novel nomogram for survival estimation in patients with invasive micropapillary carcinoma of the breast
Invasive micropapillary carcinoma (IMPC) of the breast is known for its high propensity for lymph node (LN) invasion. Inadequate LN dissection may compromise the precision of prognostic assessments. This study...
Outlier detection in spatial error models using modified thresholding-based iterative procedure for outlier detection approach
Outliers, data points that significantly deviate from the norm, can have a substantial impact on statistical inference and provide valuable insights in data analysis. Multiple methods have been developed for o...
Bayesian modeling of spatially differentiated multivariate enamel defects of the children’s primary maxillary central incisor teeth
The analysis of dental caries has been a major focus of recent work on modeling dental defect data. While a dental caries focus is of major importance in dental research, the examination of developmental defec...
The potential impact fraction of population weight reduction scenarios on non-communicable diseases in Belgium: application of the g-computation approach
Overweight is a major risk factor for non-communicable diseases (NCDs) in Europe, affecting almost 60% of all adults. Tackling obesity is therefore a key long-term health challenge and is vital to reduce prema...
Spatial-temporal Bayesian accelerated failure time models for survival endpoints with applications to prostate cancer registry data
Prostate cancer is the most common cancer after non-melanoma skin cancer and the second leading cause of cancer deaths in US men. Its incidence and mortality rates vary substantially across geographical region...
Describing the content of trial recruitment interventions using the TIDieR reporting checklist: a systematic methodology review
Recruiting participants to clinical trials is an ongoing challenge, and relatively little is known about what recruitment strategies lead to better recruitment. Recruitment interventions can be considered comp...
Methods of determining optimal cut-point of diagnostic biomarkers with application of clinical data in ROC analysis: an update review
An important application of ROC analysis is the determination of the optimal cut-point for biomarkers in diagnostic studies. This comprehensive review provides a framework of cut-point election for biomarkers ...
Segmentation of patients with small cell lung cancer into responders and non-responders using the optimal cross-validation technique
The timing of treating cancer patients is an essential factor in the efficacy of treatment. So, patients who will not respond to current therapy should receive a different treatment as early as possible. Machi...
Bioequivalence trials for the approval of generic drugs in Saudi Arabia: a descriptive analysis of design aspects
This retrospective analysis aimed to comprehensively review the design and regulatory aspects of bioequivalence trials submitted to the Saudi Food and Drug Authority (SFDA) since 2017.
Overcoming denominator problems in refugee settings with fragmented electronic records for health and immigration data: a prediction-based approach
Epidemiological studies in refugee settings are often challenged by the denominator problem, i.e. lack of population at risk data. We develop an empirical approach to address this problem by assessing relation...
Optimal futility stopping boundaries for binary endpoints
Group sequential designs incorporating the option to stop for futility at the time point of an interim analysis can save time and resources. Thereby, the choice of the futility boundary importantly impacts the...
Assessment of the E-value in the presence of bias amplification: a simulation study
The E-value, a measure that has received recent attention in the comparative effectiveness literature, reports the minimum strength of association between an unmeasured confounder and the treatment and outcome...
Methodological insights into ChatGPT’s screening performance in systematic reviews
The screening process for systematic reviews and meta-analyses in medical research is a labor-intensive and time-consuming task. While machine learning and deep learning have been applied to facilitate this pr...
Evaluating methods for risk prediction of Covid-19 mortality in nursing home residents before and after vaccine availability: a retrospective cohort study
SARS-CoV-2 vaccines are effective in reducing hospitalization, COVID-19 symptoms, and COVID-19 mortality for nursing home (NH) residents. We sought to compare the accuracy of various machine learning models, e...
A database for oncological research and quality assurance: implementation and first experiences with the University Clinical Cancer Registry Regensburg
Legal requirements, certification specifications, as well as the demand for real world data on cancer research and treatment led to the decision to establish the University Clinical Cancer Registry Regensburg....
Socio-environmental predictors of diabetes incidence disparities in Tanzania mainland: a comparison of regression models for count data
Diabetes is one of the top four non-communicable diseases that cause death and illness to many people around the world. This study aims to use an efficient count data model to estimate socio-environmental fact...
Estimating individualized treatment effects using an individual participant data meta-analysis
One key aspect of personalized medicine is to identify individuals who benefit from an intervention. Some approaches have been developed to estimate individualized treatment effects (ITE) with a single randomi...
Development of the multivariate administrative data cystectomy model and its impact on misclassification bias
Misclassification bias (MB) is the deviation of measured from true values due to incorrect case assignment. This study compared MB when cystectomy status was determined using administrative database codes vs. ...
An empirical evaluation of approximate and exact regression-based causal mediation approaches for a binary outcome and a continuous or a binary mediator for case-control study designs
In the causal mediation analysis framework, several parametric regression-based approaches have been introduced in past years for decomposing the total effect of an exposure on a binary outcome into a direct e...
Implementation and external validation of the Cambridge Multimorbidity Score in the UK Biobank cohort
Patients with multiple conditions present a growing challenge for healthcare provision. Measures of multimorbidity may support clinical management, healthcare resource allocation and accounting for the health ...
Utilization of EHRs for clinical trials: a systematic review
Clinical trials are of high importance for medical progress. This study conducted a systematic review to identify the applications of EHRs in supporting and enhancing clinical trials.
An analysis of published study designs in PubMed prisoner health abstracts from 1963 to 2023: a text mining study
The challenging nature of studies with incarcerated populations and other offender groups can impede the conduct of research, particularly that involving complex study designs such as randomised control trials...
Important information
Editorial board
For authors
For editorial board members
For reviewers
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 4.0 - 2-year Impact Factor 7.0 - 5-year Impact Factor 2.055 - SNIP (Source Normalized Impact per Paper) 1.778 - SJR (SCImago Journal Rank)
2023 Speed 40 days submission to first editorial decision for all manuscripts (Median) 210 days submission to accept (Median)
2023 Usage 4,638,094 downloads 3,126 Altmetric mentions
- More about our metrics
Peer-review Terminology
The following summary describes the peer review process for this journal:
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published: Review reports. Reviewer Identities reviewer opt in. Author/reviewer communication
More information is available here
- Follow us on Twitter
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Medical research articles from across Nature Portfolio
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving the application of those already available. It can be viewed as encompassing preclinical research (for example, in cellular systems and animal models) and clinical research (for example, clinical trials).

mRNA therapy is safe for treating the inherited metabolic condition propionic acidaemia
Propionic acidaemia is an inheirited metabolic condition caused by a lack of a liver enzyme, which leads to accumulation of toxic compounds. In a first-in-human trial, a therapeutic messenger RNA drug (mRNA-3927) led to restored enzyme activity, was well tolerated and showed a promising dose-dependent reduction of potentially life-threatening clinical events.
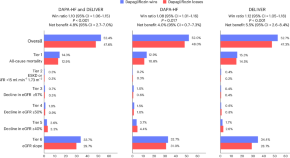
The efficacy of dapagliflozin in a hierarchical kidney outcome in heart failure
Dapagliflozin improved a hierarchical composite outcome, including death, a worsening kidney disease event, and estimated glomerular filtration rate slope, compared with placebo, in patients with heart failure. This hierarchical outcome — analyzed with win statistics — might provide the statistical power to evaluate the effect of treatments on kidney function in heart failure trials.
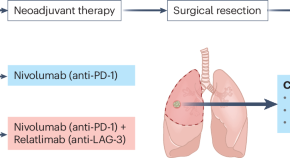
Refining neoadjuvant immunotherapy for resectable lung cancer
In an era of expanding perioperative approaches for resectable non–small-cell lung cancer, new data demonstrate that dual neoadjuvant immunotherapy targeting PD-1 and LAG-3 is feasible; future analyses may enhance patient selection by identifying immune signatures predictive of response.
- Misty D. Shields
- Christine M. Lovly
Related Subjects
- Drug development
- Epidemiology
- Experimental models of disease
- Genetics research
- Outcomes research
- Paediatric research
- Preclinical research
- Stem-cell research
- Clinical trial design
- Translational research
Latest Research and Reviews
Multimodal preoperative imaging for transcatheter mitral valve replacement in the domestic sheep model
- John P. Carney
- Richard W. Bianco
Balanced opioid-free anesthesia with lidocaine and esketamine versus balanced anesthesia with sufentanil for gynecological endoscopic surgery: a randomized controlled trial
- Qing-yun Zhang
Surgical treatment for recurrent thoracic ventral intradural arachnoid cyst secondary to tuberculous meningitis: a case report
- Yushi Sakamoto
- Takayoshi Shimizu
- Shuichi Matsuda
Seroprevalence of Treponema pallidum infection in Brazilian indigenous people: a cross-sectional study
- Marcelo S. Barbosa
- Júlio Henrique F. S. Queiroz
- Simone Simionatto
Improved differentiation of cavernous malformation and acute intraparenchymal hemorrhage on CT using an AI algorithm
- Jung Youn Kim
- Hye Jeong Choi
- Hwangseon Ju
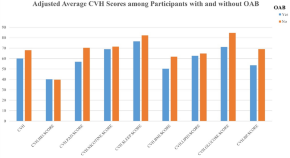
Association between life’s essential 8 and overactive bladder
- Guoliang Feng
- Shaoqun Huang
- Hongyang Gong
News and Comment
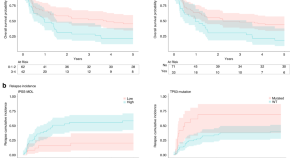
Molecular alterations monitoring in myelodysplastic patients receiving an allogeneic hematopoietic stem cell transplantation after a reduced-intensity conditioning regimen
- Marie Robin
- Olivier Nibourel
- Emmanuel Curis
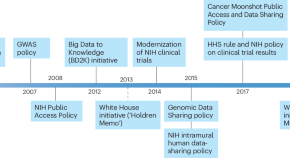
Shooting the moon: the National Cancer Institute’s implementation framework for data sharing policies
The National Cancer Institute’s Office of Data Sharing is implementing a framework for data sharing and public access policies, which will empower innovation and maximize therapeutic benefits for the cancer community.
- Emily S. Boja
- Jaime M. Guidry Auvil
Placebo response in RCT for antidepressant may not always be the ‘villain’ to fight: are KOR antagonists possibly affecting the intrinsic placebo response?
- Emilio Merlo Pich
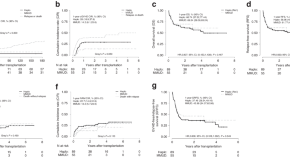
Haploidentical vs. mismatched unrelated donor transplants with posttransplant cyclophosphamide-based GVHD prophylaxis
- Dipenkumar Modi
- Seongho Kim
- Joseph P. Uberti
Trials that infected people with common colds can inform today’s COVID-19 challenge trials
- Jonathan Ewbank
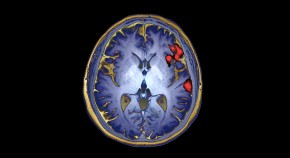
First ‘bilingual’ brain-reading device decodes Spanish and English words
Artificial-intelligence system allows a man who cannot speak coherently to have a conversation in the language of his choice.
- Amanda Heidt
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Investig
- v.3(4); 2019 Dec

Clinical research study designs: The essentials
Ambika g. chidambaram.
1 Children's Hospital of Philadelphia, Philadelphia Pennsylvania, USA
Maureen Josephson
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research.
Introduction
In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the “real world” setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of the population being studied. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2
From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental. 3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome. Experimental studies, on the other hand, are hypothesis testing studies. It involves an intervention that tests the association between the exposure and outcome. Each study design is different, and so it would be important to choose a design that would most appropriately answer the question in mind and provide the most valuable information. We will be reviewing each study design in detail (Figure 1 ).

Overview of clinical research study designs
Observational study designs
Observational studies ask the following questions: what, who, where and when. There are many study designs that fall under the umbrella of descriptive study designs, and they include, case reports, case series, ecologic study, cross‐sectional study, cohort study and case‐control study (Figure 2 ).

Classification of observational study designs
Case reports and case series
Every now and then during clinical practice, we come across a case that is atypical or ‘out of the norm’ type of clinical presentation. This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case. 4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity.
Ecologic study
Ecological studies are observational studies that provide a description of population group characteristics. That is, it describes characteristics to all individuals within a group. For example, Prentice et al 5 measured incidence of breast cancer and per capita intake of dietary fat, and found a correlation that higher per capita intake of dietary fat was associated with an increased incidence of breast cancer. But the study does not conclude specifically which subjects with breast cancer had a higher dietary intake of fat. Thus, one of the limitations with ecologic study designs is that the characteristics are attributed to the whole group and so the individual characteristics are unknown.
Cross‐sectional study
Cross‐sectional studies are study designs used to evaluate an association between an exposure and outcome at the same time. It can be classified under either descriptive or analytic, and therefore depends on the question being answered by the investigator. Since, cross‐sectional studies are designed to collect information at the same point of time, this provides an opportunity to measure prevalence of the exposure or the outcome. For example, a cross‐sectional study design was adopted to estimate the global need for palliative care for children based on representative sample of countries from all regions of the world and all World Bank income groups. 6 The limitation of cross‐sectional study design is that temporal association cannot be established as the information is collected at the same point of time. If a study involves a questionnaire, then the investigator can ask questions to onset of symptoms or risk factors in relation to onset of disease. This would help in obtaining a temporal sequence between the exposure and outcome. 7
Case‐control study
Case‐control studies are study designs that compare two groups, such as the subjects with disease (cases) to the subjects without disease (controls), and to look for differences in risk factors. 8 This study is used to study risk factors or etiologies for a disease, especially if the disease is rare. Thus, case‐control studies can also be hypothesis testing studies and therefore can suggest a causal relationship but cannot prove. It is less expensive and less time‐consuming than cohort studies (described in section “Cohort study”). An example of a case‐control study was performed in Pakistan evaluating the risk factors for neonatal tetanus. They retrospectively reviewed a defined cohort for cases with and without neonatal tetanus. 9 They found a strong association of the application of ghee (clarified butter) as a risk factor for neonatal tetanus. Although this suggests a causal relationship, cause cannot be proven by this methodology (Figure 3 ).

Case‐control study design
One of the limitations of case‐control studies is that they cannot estimate prevalence of a disease accurately as a proportion of cases and controls are studied at a time. Case‐control studies are also prone to biases such as recall bias, as the subjects are providing information based on their memory. Hence, the subjects with disease are likely to remember the presence of risk factors compared to the subjects without disease.
One of the aspects that is often overlooked is the selection of cases and controls. It is important to select the cases and controls appropriately to obtain a meaningful and scientifically sound conclusion and this can be achieved by implementing matching. Matching is defined by Gordis et al as ‘the process of selecting the controls so that they are similar to the cases in certain characteristics such as age, race, sex, socioeconomic status and occupation’ 7 This would help identify risk factors or probable etiologies that are not due to differences between the cases and controls.
Cohort study
Cohort studies are study designs that compare two groups, such as the subjects with exposure/risk factor to the subjects without exposure/risk factor, for differences in incidence of outcome/disease. Most often, cohort study designs are used to study outcome(s) from a single exposure/risk factor. Thus, cohort studies can also be hypothesis testing studies and can infer and interpret a causal relationship between an exposure and a proposed outcome, but cannot establish it (Figure 4 ).

Cohort study design
Cohort studies can be classified as prospective and retrospective. 7 Prospective cohort studies follow subjects from presence of risk factors/exposure to development of disease/outcome. This could take up to years before development of disease/outcome, and therefore is time consuming and expensive. On the other hand, retrospective cohort studies identify a population with and without the risk factor/exposure based on past records and then assess if they had developed the disease/outcome at the time of study. Thus, the study design for prospective and retrospective cohort studies are similar as we are comparing populations with and without exposure/risk factor to development of outcome/disease.
Cohort studies are typically chosen as a study design when the suspected exposure is known and rare, and the incidence of disease/outcome in the exposure group is suspected to be high. The choice between prospective and retrospective cohort study design would depend on the accuracy and reliability of the past records regarding the exposure/risk factor.
Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret.
Case‐control studies based within a defined cohort
Case‐control studies based within a defined cohort is a form of study design that combines some of the features of a cohort study design and a case‐control study design. When a defined cohort is embedded in a case‐control study design, all the baseline information collected before the onset of disease like interviews, surveys, blood or urine specimens, then the cohort is followed onset of disease. One of the advantages of following the above design is that it eliminates recall bias as the information regarding risk factors is collected before onset of disease. Case‐control studies based within a defined cohort can be further classified into two types: Nested case‐control study and Case‐cohort study.
Nested case‐control study
A nested case‐control study consists of defining a cohort with suspected risk factors and assigning a control within a cohort to the subject who develops the disease. 10 Over a period, cases and controls are identified and followed as per the investigator's protocol. Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for the control that was selected early in the study to develop the disease and become a case in the latter part of the study.
Case‐cohort Study
A case‐cohort study is similar to a nested case‐control study except that there is a defined sub‐cohort which forms the groups of individuals without the disease (control), and the cases are not matched on calendar time or length of follow‐up with the control. 11 With these modifications, it is possible to compare different disease groups with the same sub‐cohort group of controls and eliminates matching between the case and control. However, these differences will need to be accounted during analysis of results.
Experimental study design
The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality. As a result, the design of the study requires meticulous planning and resources to provide an accurate result.
The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison). 1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
Experimental study designs can be divided into 3 broad categories: clinical trial, community trial, field trial. The specifics of each study design are explained below (Figure 5 ).

Experimental study designs
Clinical trial
Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. It is considered a gold standard approach for epidemiological research. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy. 12 Lind divided twelve scorbutic sailors into six groups of two. Each group received the same diet, in addition to a quart of cider (group 1), twenty‐five drops of elixir of vitriol which is sulfuric acid (group 2), two spoonfuls of vinegar (group 3), half a pint of seawater (group 4), two oranges and one lemon (group 5), and a spicy paste plus a drink of barley water (group 6). The group who ate two oranges and one lemon had shown the most sudden and visible clinical effects and were taken back at the end of 6 days as being fit for duty. During Lind's time, this was not accepted but was shown to have similar results when repeated 47 years later in an entire fleet of ships. Based on the above results, in 1795 lemon juice was made a required part of the diet of sailors. Thus, clinical trials can be used to evaluate new therapies, such as new drug or new indication, new drug combination, new surgical procedure or device, new dosing schedule or mode of administration, or a new prevention therapy.
While designing a clinical trial, it is important to select the population that is best representative of the general population. Therefore, the results obtained from the study can be generalized to the population from which the sample population was selected. It is also as important to select appropriate endpoints while designing a trial. Endpoints need to be well‐defined, reproducible, clinically relevant and achievable. The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that are biologically related to the ideal endpoint. Surrogate endpoints need to be reproducible, easily measured, related to the clinical outcome, affected by treatment and occurring earlier than clinical outcome. 2
Clinical trials are further divided into randomized clinical trial, non‐randomized clinical trial, cross‐over clinical trial and factorial clinical trial.
Randomized clinical trial
A randomized clinical trial is also known as parallel group randomized trials or randomized controlled trials. Randomized clinical trials involve randomizing subjects with similar characteristics to two groups (or multiple groups): the group that receives the intervention/experimental therapy and the other group that received the placebo (or standard of care). 13 This is typically performed by using a computer software, manually or by other methods. Hence, we can measure the outcomes and efficacy of the intervention/experimental therapy being studied without bias as subjects have been randomized to their respective groups with similar baseline characteristics. This type of study design is considered gold standard for epidemiological research. However, this study design is generally not applicable to rare and serious disease process as it would unethical to treat that group with a placebo. Please see section “Randomization” for detailed explanation regarding randomization and placebo.
Non‐randomized clinical trial
A non‐randomized clinical trial involves an approach to selecting controls without randomization. With this type of study design a pattern is usually adopted, such as, selection of subjects and controls on certain days of the week. Depending on the approach adopted, the selection of subjects becomes predictable and therefore, there is bias with regards to selection of subjects and controls that would question the validity of the results obtained.
Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature. 1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Cross‐over clinical trial
In cross‐over clinical trial study design, there are two groups who undergoes the same intervention/experiment at different time periods of the study. That is, each group serves as a control while the other group is undergoing the intervention/experiment. 14 Depending on the intervention/experiment, a ‘washout’ period is recommended. This would help eliminate residuals effects of the intervention/experiment when the experiment group transitions to be the control group. Hence, the outcomes of the intervention/experiment will need to be reversible as this type of study design would not be possible if the subject is undergoing a surgical procedure.
Factorial trial
A factorial trial study design is adopted when the researcher wishes to test two different drugs with independent effects on the same population. Typically, the population is divided into 4 groups, the first with drug A, the second with drug B, the third with drug A and B, and the fourth with neither drug A nor drug B. The outcomes for drug A are compared to those on drug A, drug A and B and to those who were on drug B and neither drug A nor drug B. 15 The advantages of this study design that it saves time and helps to study two different drugs on the same study population at the same time. However, this study design would not be applicable if either of the drugs or interventions overlaps with each other on modes of action or effects, as the results obtained would not attribute to a particular drug or intervention.
Community trial
Community trials are also known as cluster‐randomized trials, involve groups of individuals with and without disease who are assigned to different intervention/experiment groups. Hence, groups of individuals from a certain area, such as a town or city, or a certain group such as school or college, will undergo the same intervention/experiment. 16 Hence, the results will be obtained at a larger scale; however, will not be able to account for inter‐individual and intra‐individual variability.
Field trial
Field trials are also known as preventive or prophylactic trials, and the subjects without the disease are placed in different preventive intervention groups. 16 One of the hypothetical examples for a field trial would be to randomly assign to groups of a healthy population and to provide an intervention to a group such as a vitamin and following through to measure certain outcomes. Hence, the subjects are monitored over a period of time for occurrence of a particular disease process.
Overview of methodologies used within a study design
Randomization.
Randomization is a well‐established methodology adopted in research to prevent bias due to subject selection, which may impact the result of the intervention/experiment being studied. It is one of the fundamental principles of an experimental study designs and ensures scientific validity. It provides a way to avoid predicting which subjects are assigned to a certain group and therefore, prevent bias on the final results due to subject selection. This also ensures comparability between groups as most baseline characteristics are similar prior to randomization and therefore helps to interpret the results regarding the intervention/experiment group without bias.
There are various ways to randomize and it can be as simple as a ‘flip of a coin’ to use computer software and statistical methods. To better describe randomization, there are three types of randomization: simple randomization, block randomization and stratified randomization.
Simple randomization
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers. 17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Hence, it is more challenging in studies with a small sample size.
Block randomization
In block randomization, the subjects of similar characteristics are classified into blocks. The aim of block randomization is to balance the number of subjects allocated to each experiment/intervention group. For example, let's assume that there are four subjects in each block, and two of the four subjects in each block will be randomly allotted to each group. Therefore, there will be two subjects in one group and two subjects in the other group. 17 The disadvantage with this methodology is that there is still a component of predictability in the selection of subjects and the randomization of prognostic factors is not performed. However, it helps to control the balance between the experiment/intervention groups.
Stratified randomization
In stratified randomization, the subjects are defined based on certain strata, which are covariates. 18 For example, prognostic factors like age can be considered as a covariate, and then the specified population can be randomized within each age group related to an experiment/intervention group. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient. But, with this methodology the covariates will need to be measured and determined before the randomization process. The sample size will help determine the number of strata that would need to be chosen for a study.
Blinding is a methodology adopted in a study design to intentionally not provide information related to the allocation of the groups to the subject participants, investigators and/or data analysts. 19 The purpose of blinding is to decrease influence associated with the knowledge of being in a particular group on the study result. There are 3 forms of blinding: single‐blinded, double‐blinded and triple‐blinded. 1 In single‐blinded studies, otherwise called as open‐label studies, the subject participants are not revealed which group that they have been allocated to. However, the investigator and data analyst will be aware of the allocation of the groups. In double‐blinded studies, both the study participants and the investigator will be unaware of the group to which they were allocated to. Double‐blinded studies are typically used in clinical trials to test the safety and efficacy of the drugs. In triple‐blinded studies, the subject participants, investigators and data analysts will not be aware of the group allocation. Thus, triple‐blinded studies are more difficult and expensive to design but the results obtained will exclude confounding effects from knowledge of group allocation.
Blinding is especially important in studies where subjective response are considered as outcomes. This is because certain responses can be modified based on the knowledge of the experiment group that they are in. For example, a group allocated in the non‐intervention group may not feel better as they are not getting the treatment, or an investigator may pay more attention to the group receiving treatment, and thereby potentially affecting the final results. However, certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention such as quitting smoking.
Placebo is defined in the Merriam‐Webster dictionary as ‘an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as drug)’. 20 A placebo is typically used in a clinical research study to evaluate the safety and efficacy of a drug/intervention. This is especially useful if the outcome measured is subjective. In clinical drug trials, a placebo is typically a drug that resembles the drug to be tested in certain characteristics such as color, size, shape and taste, but without the active substance. This helps to measure effects of just taking the drug, such as pain relief, compared to the drug with the active substance. If the effect is positive, for example, improvement in mood/pain, then it is called placebo effect. If the effect is negative, for example, worsening of mood/pain, then it is called nocebo effect. 21
The ethics of placebo‐controlled studies is complex and remains a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances:
Where no proven intervention exists, the use of placebo, or no intervention, is acceptable; or
Where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the patients who receive any intervention less effective than the best proven one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.
Extreme care must be taken to avoid abuse of this option”. 22
Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Bias has been defined as “any systematic error in the design, conduct or analysis of a study that results in a mistaken estimate of an exposure's effect on the risk of disease”. 23 There are multiple types of biases and so, in this review we will focus on the following types: selection bias, information bias and observer bias. Selection bias is when a systematic error is committed while selecting subjects for the study. Selection bias will affect the external validity of the study if the study subjects are not representative of the population being studied and therefore, the results of the study will not be generalizable. Selection bias will affect the internal validity of the study if the selection of study subjects in each group is influenced by certain factors, such as, based on the treatment of the group assigned. One of the ways to decrease selection bias is to select the study population that would representative of the population being studied, or to randomize (discussed in section “Randomization”).
Information bias is when a systematic error is committed while obtaining data from the study subjects. This can be in the form of recall bias when subject is required to remember certain events from the past. Typically, subjects with the disease tend to remember certain events compared to subjects without the disease. Observer bias is a systematic error when the study investigator is influenced by the certain characteristics of the group, that is, an investigator may pay closer attention to the group receiving the treatment versus the group not receiving the treatment. This may influence the results of the study. One of the ways to decrease observer bias is to use blinding (discussed in section “Blinding”).
Thus, while designing a study it is important to take measure to limit bias as much as possible so that the scientific validity of the study results is preserved to its maximum.
Overview of drug development in the United States of America
Now that we have reviewed the various clinical designs, clinical trials form a major part in development of a drug. In the United States, the Food and Drug Administration (FDA) plays an important role in getting a drug approved for clinical use. It includes a robust process that involves four different phases before a drug can be made available to the public. Phase I is conducted to determine a safe dose. The study subjects consist of normal volunteers and/or subjects with disease of interest, and the sample size is typically small and not more than 30 subjects. The primary endpoint consists of toxicity and adverse events. Phase II is conducted to evaluate of safety of dose selected in Phase I, to collect preliminary information on efficacy and to determine factors to plan a randomized controlled trial. The study subjects consist of subjects with disease of interest and the sample size is also small but more that Phase I (40–100 subjects). The primary endpoint is the measure of response. Phase III is conducted as a definitive trial to prove efficacy and establish safety of a drug. Phase III studies are randomized controlled trials and depending on the drug being studied, it can be placebo‐controlled, equivalence, superiority or non‐inferiority trials. The study subjects consist of subjects with disease of interest, and the sample size is typically large but no larger than 300 to 3000. Phase IV is performed after a drug is approved by the FDA and it is also called the post‐marketing clinical trial. This phase is conducted to evaluate new indications, to determine safety and efficacy in long‐term follow‐up and new dosing regimens. This phase helps to detect rare adverse events that would not be picked up during phase III studies and decrease in the delay in the release of the drug in the market. Hence, this phase depends heavily on voluntary reporting of side effects and/or adverse events by physicians, non‐physicians or drug companies. 2
We have discussed various clinical research study designs in this comprehensive review. Though there are various designs available, one must consider various ethical aspects of the study. Hence, each study will require thorough review of the protocol by the institutional review board before approval and implementation.
CONFLICT OF INTEREST
Chidambaram AG, Josephson M. Clinical research study designs: The essentials . Pediatr Invest . 2019; 3 :245‐252. 10.1002/ped4.12166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Mortality in Patients Hospitalized for COVID-19 vs Influenza in Fall-Winter 2023-2024
- 1 Clinical Epidemiology Center, VA St Louis Health Care System, St Louis, Missouri
In the first year of the COVID-19 pandemic, risk of death in people hospitalized for COVID-19 was substantially higher than in people hospitalized for seasonal influenza. 1 , 2 The risk of death due to COVID-19 has since declined. In fall-winter 2022-2023, people hospitalized for COVID-19 had a 60% higher risk of death compared with those hospitalized for seasonal influenza. 3 New variants of SARS-CoV-2 have continued to appear, including the emergence of JN.1, the predominant variant in the US since December 24, 2023. 4 This study evaluated the risk of death in a cohort of people hospitalized for COVID-19 or seasonal influenza in fall-winter 2023-2024.
Read More About
Xie Y , Choi T , Al-Aly Z. Mortality in Patients Hospitalized for COVID-19 vs Influenza in Fall-Winter 2023-2024. JAMA. Published online May 15, 2024. doi:10.1001/jama.2024.7395
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Guidelines and Guidance Library
- Core Practices
- Isolation Precautions Guideline
- Disinfection and Sterilization Guideline
- Environmental Infection Control Guidelines
- Hand Hygiene Guidelines
- Multidrug-resistant Organisms (MDRO) Management Guidelines
- Catheter-Associated Urinary Tract Infections (CAUTI) Prevention Guideline
- Tools and resources
- Evaluating Environmental Cleaning
What to know
This guideline provides recommendations for isolation precautions in healthcare settings.
Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007)
Print Version of Guidelines
Updates, infection control.
CDC provides information on infection control and clinical safety to help reduce the risk of infections among healthcare workers, patients, and visitors.
For Everyone
Health care providers, public health.
- Member Login
WCRI Medical Price Index for Workers’ Compensation, 16th Edition (MPI-WC)
By rebecca (rui) yang, olesya fomenko.
May 16, 2024 Related Topics: Medical Costs , Medical Price Index
Since medical costs are a constant focus for policymakers and system stakeholders in state workers’ compensation systems, this annual study provides comparisons of prices paid for medical professional services across 36 states from 2008 to 2023. The study focuses on professional services (evaluation and management, physical medicine, surgery, major and minor radiology, neurological testing, pain management injections, and emergency care) billed by physicians, physical therapists, and chiropractors.
Unlike other indices measuring how prices for professional services change, this index is a more relevant benchmark of medical inflation in workers’ compensation as it focuses on those services commonly provided to workers with injuries. Since workers’ compensation price regulations are set at the state level, this study provides a state-level price index to help policymakers and stakeholders conduct meaningful comparisons of prices paid across states and to monitor price trends in relation to changes in fee schedules.
This edition covers 36 states that represent 88 percent of the workers’ compensation benefits paid in the United States. These states are Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Oklahoma, Oregon, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and Wisconsin.
WCRI Medical Price Index for Workers’ Compensation, 16th Edition (MPI-WC) . Rebecca Yang and Olesya Fomenko. May 2024. WC-24-20.
(This is a free report. If you have a member user name and password, please log into the website to access this report. All others can access the report for free by adding it to the shopping cart and going through the checkout process.)
Copyright: WCRI
Share this report

pdf, 399 KB
Reports are free for members.
If you are a member, please login here
*Temporary membership will allow you to download the study represented here
Research Questions:
- How do prices paid for medical professional services for treating workers with injuries in my state compare with other states?
- How are prices in my state changing?
- Is the price trend in my state part of a national phenomenon or are the reasons unique to my state and, therefore, subject to local policy issues?
Contact WCRI
To obtain your member login or to answer any questions or concern you may have, please contact us here.
- What is your question about? Select Membership Press Website Research Other
- Describe question
- Submit form Submit
FREE REPORT

The increasing costs of medical care for treating injured workers have been a focus of public policymakers and system stakeholders in many states. To help them conduct meaningful comparisons of prices paid across states, and to monitor the price trends in relation to changes in fee schedules and network participation, this annual study creates an index for the actual prices paid for professional services across based on a marketbasket of commonly used services for treating injured workers.

The increasing costs of medical care for treating injured workers have been a focus of public policymakers and system stakeholders in many states. Download our 31-state study to compare medical prices paid across states and to monitor trends in relation to policy choices and changes in fee schedules.

IMAGES
VIDEO
COMMENTS
Abstract. 1 Basics of Medical Research Research in any field is an enterprise that carries its own risks and benefits. One may make heavy investment in terms of time, money and expertise yet the ...
Health research methodology: A guide for training in research methods Chapter 1 Research and Scientific Methods 1.1 Definition Research is a quest for knowledge through diligent search or investigation or experimentation aimed at the discovery and interpretation of new knowledge. Scientific method is a systematic
Clinical research is necessary to establish the safety and effective-ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed to answer important scientifi c and health
In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research.
Guide 56: "The research compass": An introduction to research in medical education19 twice as high in the case-group compared to the control-group, indicating an association between the predictor and the criterion, i.e. disciplinary action by a Medical Board. FIguRE 4: Example of a cohort and a case-control study.
Biomedical scientists bridge the gap between the basic sciences and medicine. The Ph.D. degree is the gateway to a career in biomedical research. Biomedical scientists: Think outside the box and are innovators. Are critical and analytical thinkers. Get excited by discovering new things. Look at biology and see previously unrecognized patterns.
A comprehensive guide to health research that covers qualitative and quantitative methods and ethical principles. It is a useful resource for students, field researchers, and teachers of health research methodologies.
Health-related Research Involving Humans Prepared by the Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the ... The Council for International Organizations of Medical Sciences (CIOMS) acknowledges the contribution of the Working Group for the revision of the CIOMS Ethical Guidelines. In 2011, the ...
Study design is the procedure under which a study is carried out Study design is the procedure under which a study is. Two main categories. •Observation: •Identify subjects, then. •Observe and record characteristics. •Experiment. •Identify subjects, •Place in common context, •Intervene, then.
Research INTRODUCTION These guidelines outline principles that should be followed at Harvard Medical School when conducting research. They are a supplement to the Guidelines for Investigators in Scientific Research, first issued in February 1988. Clinical research may be defined as investigations involving
MEDICAL RESEARCH. Medical research needs to be conducted to increase knowledge about the human species, its social/natural environment and to combat disease/infirmity in humans. Research should be conducted in a manner conducive to and consistent with dignity and well-being of the participant; in a professional and transparent manner; and ...
PubMed is a comprehensive database of biomedical literature from various sources, including MEDLINE, life science journals, and online books. You can search for citations, access full text content, and explore topics related to health, medicine, and biology. PubMed also provides advanced search options and tools for researchers and clinicians.
The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical research and review articles, and editorial opinion on a wide variety of topics of ...
View Full Text ; View PDF ; Stacked probability plots of the extended illness-death model using constant transition hazards - an easy to use shiny app. Extended illness-death models (a specific class of multistate models) are a useful tool to analyse situations like hospital-acquired infections, ventilation-associated pneumonia, and transfers between hospital...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management.
and research methodologies and of medical educators as scholars. MedEd research clearly benefits from a multitude of perspectives and close collaboration among medical educators and behavioral scientists. 1-2 . 1 Norman G. Fifty years of medical education research: waves of migration. Med Educ 2011; 45: 785-91. 2 Kuper A, Whitehead C.
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving ...
e medical students who participated in this study reported poor knowledge about antimicrobial stew-ardship. When knowledge was compared between the groups, the medical students who were in their clini-cal training years had higher awareness and attitudes towards both antimicrobial resistance and antimicrobial stewardship.
Research team identifies a new way to treat prostate cancer May 22 2024, by Marilyn Perkins 1/4. Active Stat5 increases protein levels of full-length androgen receptor (AR-FL)
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
of $1,861 more in medical costs annually than people who are a healthy weight, and severe adult obesity was linked to $3,097 in excess annual costs per person.3 Additional research is needed to understand if GLP-1 interventions lead to lower medical costs in the longer term. The average monthly list price for semaglutide is over $1,000.
In the first year of the COVID-19 pandemic, risk of death in people hospitalized for COVID-19 was substantially higher than in people hospitalized for seasonal influenza. 1,2 The risk of death due to COVID-19 has since declined. In fall-winter 2022-2023, people hospitalized for COVID-19 had a 60% higher risk of death compared with those hospitalized for seasonal influenza. 3 New variants of ...
Appendix A: Type and Duration of Precautions Recommended for Selected Infections and Conditions. Appendix A: Table 1. History of Guidelines for Isolation Precautions in Hospitals. Appendix A: Table 2. Clinical Syndromes or Conditions Warranting Empiric Transmission-Based Precautions in Addition to Standard Precautions. Appendix A. Table 3.
May 16, 2024 Related Topics: Medical Costs, Medical Price Index Since medical costs are a constant focus for policymakers and system stakeholders in state workers' compensation systems, this annual study provides comparisons of prices paid for medical professional services across 36 states from 2008 to 2023.
Application limit: 250 applications. Center: The Biomedical Advanced Research and Development Authority (BARDA) Division of Research Innovation and Ventures (DRIVe) Location: Washington, DC. Pay: Commensurate with qualifications and experience. Travel Required: 25%. You are expected to travel for this position. Service: Excepted.