
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Case Study: Cystic Fibrosis - CER
- Last updated
- Save as PDF
- Page ID 26446
This page is a draft and is under active development.
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Part I: A Case of Cystic Fibrosis
Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they thought was normal for a child with a cold. Upon arriving at the emergency room, the attending pediatrician noted that salt crystals were present on Zoey's skin and called Dr. Weyland, a pediatric pulmonologist. Dr. Weyland suspects that baby Zoey may be suffering from cystic fibrosis.
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
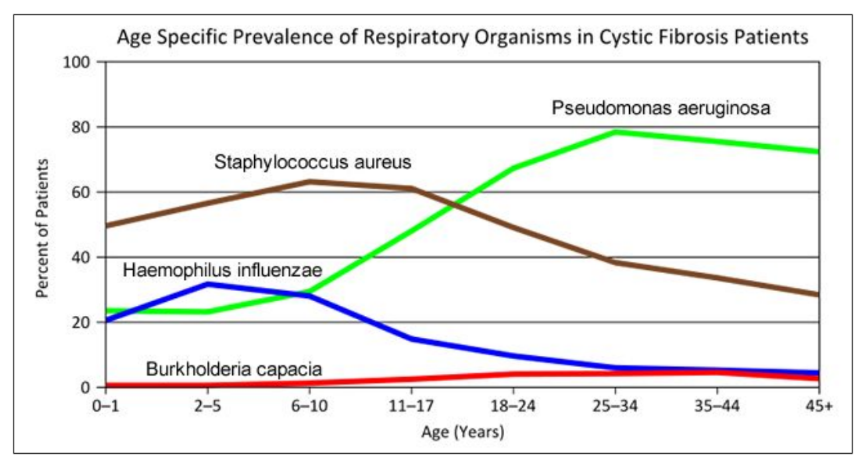
- "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die" This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
- What symptoms lead Dr. Weyland to his initial diagnosis?
- Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime. What organism is most likely affecting baby Zoey?
- What do you think is the most dangerous time period for a patient with CF? Justify your answer.
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
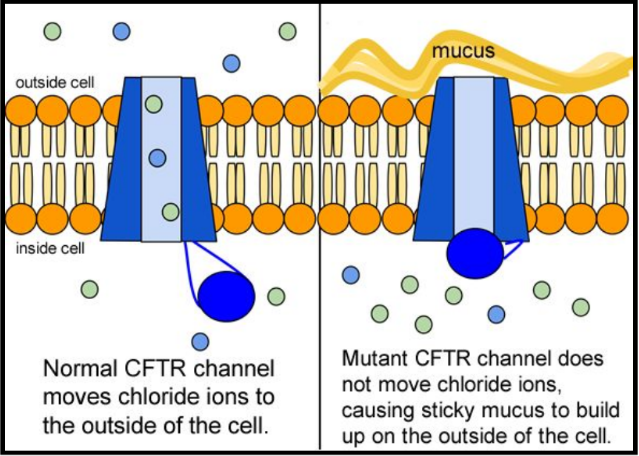
5. Suggest a molecular fix for a mutated CFTR channel. How would you correct it if you had the ability to tinker with it on a molecular level?
6. Why would treatment that targets the CFTR channel not be effective for 2⁄3 of those with cystic fibrosis?
7. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
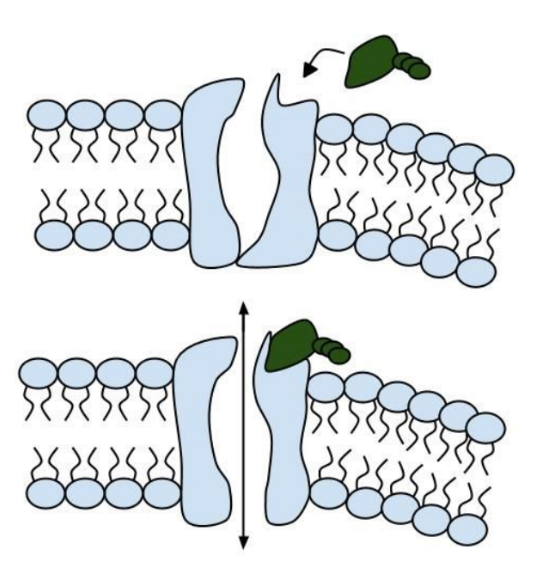
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
In the case of CFTR, chloride ions travel in and out of the cell through the channel’s guarded pore as a means to control the flow of water in and out of cells. In cystic fibrosis patients, this delicate salt/water balance is disturbed, most prominently in the lungs, resulting in thick coats of mucus that eventually spur life-threatening infections. Shown below are several mutations linked to CFTR:
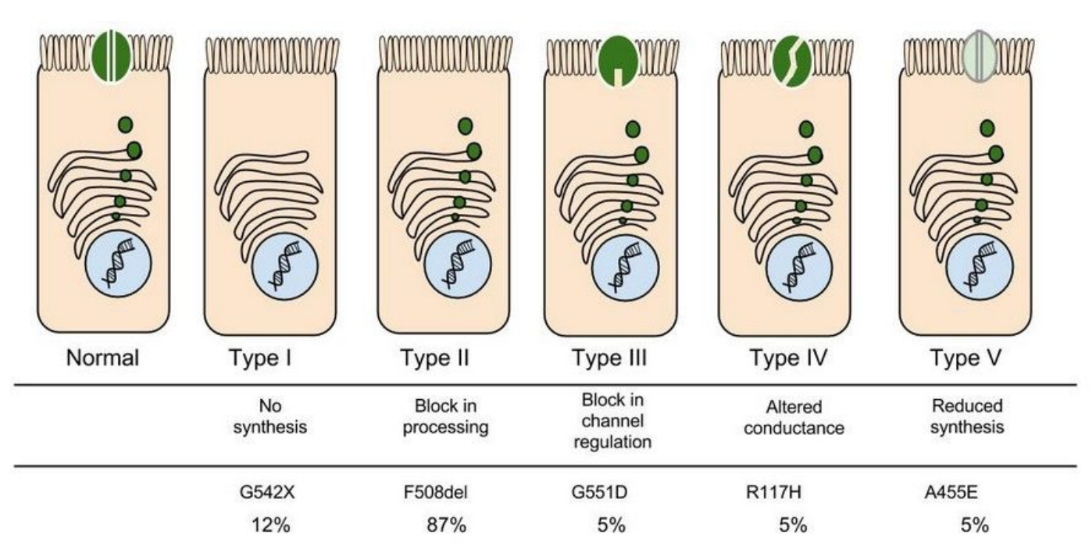
8. Which mutation do you think would be easiest to correct. Justify your answer. 9. Consider what you know about proteins, why does the “folding” of the protein matter?
Part IV: Open sesame
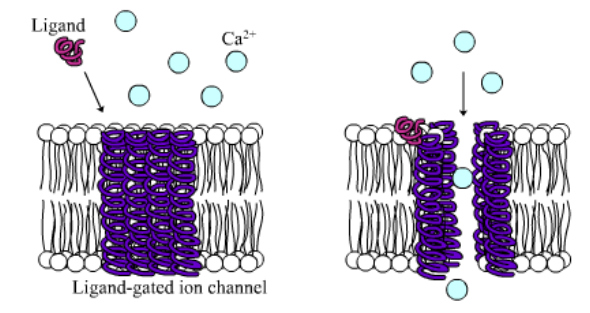
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remains obscure.
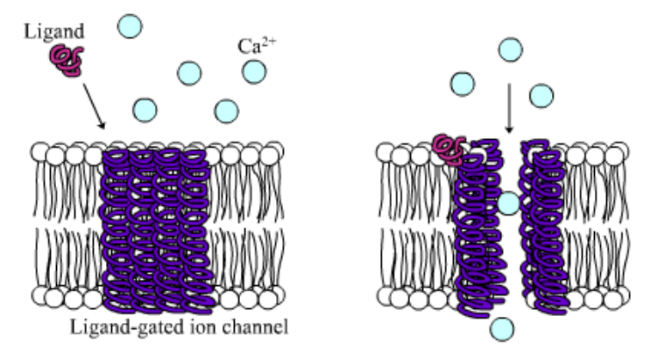
10. Compare the action of the ligand-gated channel to how an enzyme works.
11. Consider the model of the membrane channel, What could go wrong to prevent the channel from opening?
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
13. Label the image below to show how the ligand-gated channel for CFTR works. Include a summary.
Part V: Can a Drug Treat Zoey’s Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from his lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor TM is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were age over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
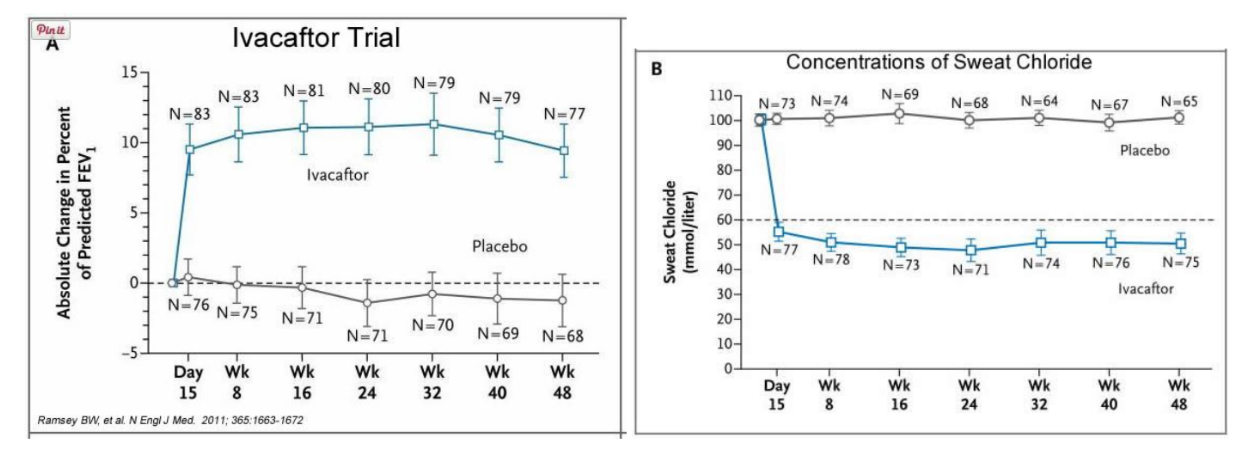
14. What is FEV? Describe a way that a doctor could take a measurement of FEV.
15. Why do you think it was important to have placebos in both of these studies?
16. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacafor? Defend your choice.
17. Take a look at the mutations that can occur in the cell membrane proteins from Part III. For which mutation do you think Ivacaftor will be most effective? Justify your answer.
18. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey’s Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
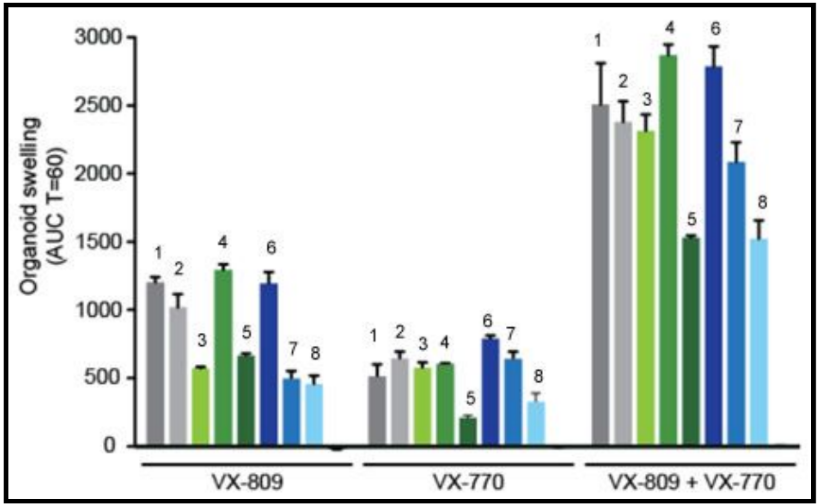
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids. The graph below shows how each drug works for 8 different patients (#1-#8)
19. Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart. If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim. Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 19: Case Study: Cystic Fibrosis
Julie M. Skrzat; Carole A. Tucker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction.
- Examination: Age 2 Months
- Evaluation, Diagnosis, and Prognosis
- Intervention
- Conclusion of Care
- Examination: Age 8 Years
- Examination: Age 16 Years
- Recommended Readings
- Full Chapter
- Supplementary Content
C ystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation ( Cystic Fibrosis Foundation, 2019a ), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease prevalence varies greatly by ethnicity, with the highest prevalence occurring in Western European descendants and within the Ashkenazi Jewish population.
The CF gene, located on chromosome 7, was first identified in 1989. The disease process is caused by a mutation to the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein. This mutation alters the production, structure, and function of cyclic adenosine monophosphate (cAMP), a dependent transmembrane chloride channel carrier protein found in the exocrine mucus glands throughout the body. The mutated carrier protein is unable to transport chloride across the cell membrane, resulting in an electrolyte and charge imbalance. Diffusion of water across the cell membrane is thus impaired, resulting in the development of a viscous layer of mucus. The thick mucus obstructs the cell membranes, traps nearby bacteria, and incites a local inflammatory response. Subsequent bacterial colonization occurs at an early age and ultimately this repetitive infectious process leads to progressive inflammatory damage to the organs involved in individuals with CF.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Publications
- Conferences & Events
- Professional Learning
- Science Standards
- Awards & Competitions
- Instructional Materials
- Free Resources
- American Rescue Plan
- For Preservice Teachers
- NCCSTS Case Collection
- Science and STEM Education Jobs
- Interactive eBooks+
- Digital Catalog
- Regional Product Representatives
- e-Newsletters
- Bestselling Books
- Latest Books
- Popular Book Series
- Prospective Authors
- Web Seminars
- Exhibits & Sponsorship
- Conference Reviewers
- National Conference • Denver 24
- Leaders Institute 2024
- National Conference • New Orleans 24
- Submit a Proposal
- Latest Resources
- Professional Learning Units & Courses
- For Districts
- Online Course Providers
- Schools & Districts
- College Professors & Students
- The Standards
- Teachers and Admin
- eCYBERMISSION
- Toshiba/NSTA ExploraVision
- Junior Science & Humanities Symposium
- Teaching Awards
- Climate Change
- Earth & Space Science
- New Science Teachers
- Early Childhood
- Middle School
- High School
- Postsecondary
- Informal Education
- Journal Articles
- Lesson Plans
- e-newsletters
- Science & Children
- Science Scope
- The Science Teacher
- Journal of College Sci. Teaching
- Connected Science Learning
- NSTA Reports
- Next-Gen Navigator
- Science Update
- Teacher Tip Tuesday
- Trans. Sci. Learning
MyNSTA Community
- My Collections
Maggie’s Illness
Protein Structure and Function in Cystic Fibrosis
By Michaela Gazdik Stofer
Share Start a Discussion

This directed case study examines the molecular basis of cystic fibrosis to emphasize the relationship between the genetic code stored in a DNA sequence and the encoded protein’s structure and function. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein that functions to help maintain salt and water balance along the surface of the lung and gastrointestinal tract. This case introduces students to “Maggie,” who has just been diagnosed with cystic fibrosis. The students must identify the mutation causing Maggie’s disease by transcribing and translating a portion of the wildtype and mutated CFTR gene. Students then compare the three-dimensional structures of the resulting proteins to better understand the effect a single amino acid mutation can have on the overall shape of a protein. Students also review the concepts of tonicity and osmosis to examine how the defective CFTR protein leads to an increase in the viscosity of mucus in cystic fibrosis patients. This case was developed for use in an introductory college-level biology course but could also be adapted for use in an upper-level cell or molecular biology course.
Download Case
Date Posted
- Generate a protein sequence through transcription and translation of a given DNA gene sequence.
- Explain the chemistry of amino acid side chains and their importance in protein folding.
- Describe how a mutation in a protein sequence leads to changes in the overall tertiary structure of the protein.
- Examine various levels of protein structure using Cn3D to view three-dimensional protein structures from NCBI’s Entrez Structure database.
- Relate the loss of function of the CFTR protein to the physiological causes of cystic fibrosis.
Protein structure; transcription; translation; DNA mutation; cystic fibrosis; genetic disease; protein function; protein folding; protein; CFTR; Cn3D
Subject Headings
EDUCATIONAL LEVEL
Undergraduate lower division, Undergraduate upper division
TOPICAL AREAS
TYPE/METHODS
Teaching Notes & Answer Key
Teaching notes.
Case teaching notes are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Teaching notes are intended to help teachers select and adopt a case. They typically include a summary of the case, teaching objectives, information about the intended audience, details about how the case may be taught, and a list of references and resources.
Download Notes
Answer Keys are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Download Answer Key
Materials & Media
Supplemental materials.
The following two files should be viewed with the Cn3D software to view a single domain of the CFTR and ∆F508 CFTR proteins.
You may also like
Web Seminar
Join us on Tuesday, June 4, 2024, from 7:00 PM to 8:30 PM ET, to learn about the free lesson plans and storyline units designed for high school studen...
Join us on Thursday, October 24, 2024, from 7:00 PM to 8:00 PM ET, to learn about all NSTA Teacher Awards available and how to apply.Did you come up w...
Cystic fibrosis occurs when the cystic fibrosis transmembrane conductance regulator (CFTR) protein is either not made correctly, or not made at all. By understanding how the protein is made, scientists have been able to develop treatments that target the protein and restore its function.
- The cystic fibrosis transmembrane conductance regulator (CFTR) protein helps to maintain the balance of salt and water on many surfaces in the body, such as the surface of the lung.
- The CFTR protein is a particular type of protein called an ion channel. In the lung, the CFTR ion channel moves chloride ions from inside the cell to outside the cell.
Researchers are still trying to learn more about the structure of the CFTR protein so that they can find new and better ways to help improve the function of the protein in people with CF.
The cystic fibrosis transmembrane conductance regulator (CFTR) protein helps to maintain the balance of salt and water on many surfaces in the body, such as the surface of the lung. When the protein is not working correctly, chloride — a component of salt — becomes trapped in cells. Without the proper movement of chloride, water cannot hydrate the cellular surface. This leads the mucus covering the cells to become thick and sticky, causing many of the symptoms associated with cystic fibrosis.
To understand how mutations in the CFTR gene cause the protein to become dysfunctional, it is important to understand how the protein is normally made, and how it helps to move water and chloride to the cell surface.
What Are Proteins?
Proteins are tiny machines that do specific jobs within a cell. The instructions for building each protein are encoded in DNA. Proteins are assembled from building blocks called amino acids. There are 20 different amino acids. All proteins are made up of chains of these amino acids connected together in different orders, like different words that are written using the same 26 letters of the alphabet. The DNA instructions tell the cell which amino acid to use at each position in the chain to make a specific protein.
The CFTR protein is made up of 1,480 amino acids. Once the CFTR protein chain is made, it is folded into a specific 3-D shape. The CFTR protein is shaped like a tube that goes through the membrane surrounding the cell, like a straw goes through the plastic top on a cup.

What Does the CFTR Protein Do?
The CFTR protein is a particular type of protein called an ion channel. An ion channel moves atoms or molecules that have an electrical charge from inside the cell to outside, or from outside the cell to inside. In the lung, the CFTR ion channel moves chloride ions from inside the cell to outside the cell. To get out of the cell, the chloride ions move through the center of the tube formed by the CFTR protein.
Once the chloride ions are outside the cell, they attract a layer of water. This water layer is important because it allows tiny hairs on the surface of the lung cells, called cilia, to sweep back and forth. This sweeping motion moves mucus up and out of the airways.
How Do Problems With the CFTR Protein Cause CF?
In people with CF, mutations in the CFTR gene can cause the following problems with the CFTR protein:
- It doesn't work well
- It isn't produced in sufficient quantities
- It is not produced at all
When any of these problems occur, the chloride ions are trapped inside the cell, and water is no longer attracted to the space outside the cell. When there is less water outside the cells, the mucus in the airways becomes dehydrated and thickens, causing it to flatten the cilia. The cilia can't sweep properly when thick, sticky mucus weighs them down.
Because the cilia can't move properly, mucus gets stuck in the airways, making it difficult to breathe. In addition, germs caught in the mucus are no longer expelled from the airway, allowing them to multiply and cause infections. Thick mucus in the lungs and frequent airway infections are some of the most common problems people with CF face.
Researchers Are Still Studying the Basic Structure
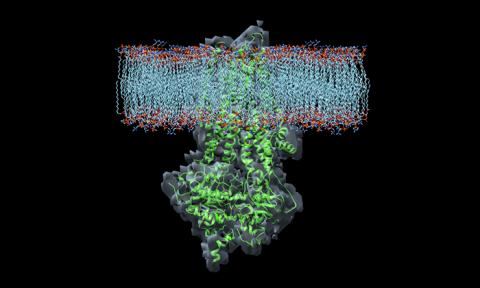
Because the 3-D shape of CFTR is so complex, it was not until early 2017 that the first high-resolution pictures were developed. These pictures have given researchers important clues about where drugs bind the protein, how they affect its function, and how to develop new CF therapies. In the future, pictures showing the protein in an “open” position, where salt can move through, will be even more helpful to researchers developing new CF therapies.
CF Genetics: The Basics Article | 6 min read
Types of CFTR Mutations Article | 9 min read
Restore CFTR: Exploring Treatments for Rare and Nonsense Mutations Article | 8 min read
Find Out More About Your Mutations Article | 3 min read

( 1-800-344-4823 ) Mon - Thu, 9 am - 7 pm ET Fri, 9 am - 3 pm ET
We can help you reset your password using the email address linked to your BioOne Complete account.

- BioOne Complete
- BioOne eBook Titles
- By Publisher
- About BioOne Digital Library
- How to Subscribe & Access
- Library Resources
- Publisher Resources
- Instructor Resources
- FIGURES & TABLES
- SUPPLEMENTAL CONTENT
- DOWNLOAD PAPER SAVE TO MY LIBRARY
Scientific modeling is a practice that we use frequently in our undergraduate biomedical applications course for nonscience majors. We use case studies in which students apply course concepts to create cause- and-effect models. In this article, we describe a case study assessment on protein synthesis that examines the use of CRISPR to bring back the mammoth (i.e., de-extinction). Students learn about protein synthesis throughout the course and work on various case study scenarios to apply those concepts. Their final assessment is a team project to illustrate how protein synthesis is influenced by gene editing, including gene expression and its regulation, transcription, translation, protein structure and function, and the ultimate impact on an organism's phenotype. Although we use this case study as an assessment, it is also appropriate as a class activity in which students practice modeling the CRISPR gene-editing system.

KEYWORDS/PHRASES
Publication title:, collection:, publication years.

The Biology Corner
Biology Teaching Resources

Case Study – Cystic Fibrosis

This case study explores the relationship between the cell membrane and breathing difficulties that occur as a result of the genetic disorder cystic fibrosis. Students look at specific channel proteins in the cell membrane that affect the movement of chloride ions.
Different mutations result in different problems with these protein channels. Treatment options depend on the type of mutation present.
This is intended for an AP Biology, and includes advanced data analysis and interpretation of graphics. Students works in small groups to solve problems and work through some of the difficult concepts presented.
HHMI has a great animation of the CFTR protein and how changes in its shape result in a non-functioning channel. I often supplement this lesson with several resources for discussion. I may pause the group work to share additional information.
2020 Remote Edition also available. This one was made slightly easier with fewer questions for students to do on their own. The remote edition is divided into Part 1 and Part 2 so students can receive feedback and have discussions before completing the exercise.
Grade Level: 11-12 | Time Required: 1-2 hours
HS-LS1-1 Construct an explanation based on evidence for how the structure of DNA determines the structure of proteins which carry out the essential functions of life through systems of specialized cells
Shannan Muskopf
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 29 July 2020
Cystic fibrosis drugs target the malformed proteins at the root of the disease
- Sarah DeWeerdt 0
Sarah DeWeerdt is a freelance writer in Seattle, Washington.
You can also search for this author in PubMed Google Scholar
Over the course of two decades spent developing treatments for the genetic lung disease cystic fibrosis, biologist Fredrick Van Goor has had hundreds of conversations with patients. But he remembers one in particular.

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 583 , S2-S4 (2020)
doi: https://doi.org/10.1038/d41586-020-02106-w
This article is part of Nature Outlook: Cystic fibrosis , an editorially independent supplement produced with the financial support of third parties. About this content .
Related Articles

- Drug discovery
- Therapeutics
- Medical research
Bitter taste TAS2R14 activation by intracellular tastants and cholesterol
Article 22 MAY 24

AlphaFold3 — why did Nature publish it without its code?
Editorial 22 MAY 24
How to kill the ‘zombie’ cells that make you age
News Feature 15 MAY 24
Trials that infected people with common colds can inform today’s COVID-19 challenge trials
Correspondence 21 MAY 24

Targeting RNA opens therapeutic avenues for Timothy syndrome
News & Views 24 APR 24
Anti-ageing antibodies revive the immune system
News & Views 27 MAR 24
Ozempic keeps wowing: trial data show benefits for kidney disease
News 24 MAY 24
Neural pathways for reward and relief promote fentanyl addiction
News & Views 22 MAY 24
Covalent targeted radioligands potentiate radionuclide therapy
Professor, Division Director, Translational and Clinical Pharmacology
Cincinnati Children’s seeks a director of the Division of Translational and Clinical Pharmacology.
Cincinnati, Ohio
Cincinnati Children's Hospital & Medical Center
Data Analyst for Gene Regulation as an Academic Functional Specialist
The Rheinische Friedrich-Wilhelms-Universität Bonn is an international research university with a broad spectrum of subjects. With 200 years of his...
53113, Bonn (DE)
Rheinische Friedrich-Wilhelms-Universität
Recruitment of Global Talent at the Institute of Zoology, Chinese Academy of Sciences (IOZ, CAS)
The Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS), is seeking global talents around the world.
Beijing, China
Institute of Zoology, Chinese Academy of Sciences (IOZ, CAS)
Full Professorship (W3) in “Organic Environmental Geochemistry (f/m/d)
The Institute of Earth Sciences within the Faculty of Chemistry and Earth Sciences at Heidelberg University invites applications for a FULL PROFE...
Heidelberg, Brandenburg (DE)
Universität Heidelberg
Postdoc: deep learning for super-resolution microscopy
The Ries lab is looking for a PostDoc with background in machine learning.
Vienna, Austria
University of Vienna
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Learn about CFTR, the chloride channel that defective in cystic fibrosis.
The cystic fibrosis transmembrane conductance regulator (CFTR) is defective in cystic fibrosis (CF). This protein is a channel that sits on the surface of cells and transports chloride and other molecules, such as bicarbonate. The gene that encodes the CFTR protein, which is also called CFTR , is located on chromosome 7. Mutations in this gene lead to CF. Since the discovery of the CFTR gene in 1989, more than 2,500 mutations have been identified.

Cystic fibrosis is caused by mutations in the CFTR gene, which encodes a chloride channel located on the surface of certain epithelial cells.

CFTR Channel
The CFTR protein is composed of 1,480 amino acids—the building blocks of all proteins—and is located on the surface of many cells in the body. The CFTR protein contains a single chain of amino acids that are grouped in five functional regions called domains. Two transmembrane domains (TMD1 and TMD2), two cytoplasmic nucleotide-binding domains (NBD1 and NBD2) and a regulatory (R) domain make up the CFTR protein. Each domain has a special function when it comes to transporting chloride through the cell surface. Therefore, mutations in different domains cause a range of CF symptoms depending on the extent that chloride transport is affected. Mutations in CFTR often affect the three-dimensional structure of the protein and prevent CFTR from reaching the membrane.
The location of the CFTR protein, which is found in several organs, determines where the symptoms of CF occur. The organs that are typically involved in CF are the skin, pancreas and lungs.
Sweat Gland
People with CF has very salty sweat. The sweat gland secretes salt and water some of which is typically reabsorbed in the sweat duct. This reabsorption process is markedly abnormal in people with CF. Chloride transport is virtually eliminated because CFTR located on the surface of the cells in the sweat duct is defective. The lack of CFTR function leads to excess chloride in the sweat of people with CF. The high chloride concentration in the sweat can be used to diagnose people with CF.
The airways are covered with a thin, layer of liquid called airway surface liquid (ASL) and a mucus gel layer. The mucus layer traps bacteria and foreign particles, while cilia on the surface of airway cells constantly move the particles out of the lungs and toward the mouth. This process, called mucociliary clearance is an important defense mechanism that protects the lungs from infection. The ASL also contains antiproteases, antioxidants, antibodies and other substances that work together to neutralize or destroy invading organisms without damaging the lungs. In CF airways, decreased chloride transport is coupled with excess sodium reabsorption out of the ASL. Since water follows the flow of sodium the ASL and the mucus gel layer become dehydrated.
The exocrine pancreas produces enzymes that digest food. Most people with CF do not make pancreatic enzymes leading to a problem called pancreatic insufficiency. The pancreatic duct cells also secrete bicarbonate into the intestine to neutralize stomach acid via the CFTR channel. The inability to neutralize stomach acid contributes to malabsorption in many people with CF.
Select the “Lung – Airway Cell” or “Sweat Gland Cell” above to compare the functionality of a normal cell to a cell with CF.
Cellular processing.

Coding, construction and placement of the CFTR protein.
Construction and placement of the CFTR protein in the cell membrane occurs in distinct phases. Located on the long (q) arm of chromosome 7 at position 31.2, the CFTR gene is comprised of 27 exons that encode its genetic sequence (1). An exon is a portion of a DNA that contains the code for a protein structure. The CFTR gene is transcribed into a single strand of RNA within the cell nucleus (2); regions that are not needed to make the protein are spliced out, producing the final messenger RNA (mRNA) (3).
The mRNA leaves the nucleus (4) and is translated into protein by ribosomes in the endoplasmic reticulum, or ER (5). A number of proteins called chaperones (6), facilitate folding of the new CFTR protein and its to the Golgi apparatus (7) where sugars are added. The CFTR protein then travels (8) to cell surface (9).
More than 2500 different mutations in the CFTR gene have been described. Most of these mutations either substitute one base – the building material of DNA – for another, or delete a small number of DNA bases. The most common CFTR mutation, present in approximately 70 percent of people with CF, is F508del. This mutation is caused by the deletion of three base pairs of the CFTR gene leading to the loss of an amino acid called phenylalanine, abbreviated F, in the CFTR protein.
Everyone receives one copy of the CFTR gene from each parent. To have CF, a mutation must be present on both copies of the CFTR gene, but the mutations do not have to be the same. If a person received one normal gene and one mutated gene, he or she will not have CF, but will be a CFTR mutation “carrier”. One in 31 Americans has one CFTR gene mutation.
Mutations in the CFTR gene can lead to different changes in the CFTR protein. These changes are grouped into 6 classes. People with CF who have some residual CFTR function (Classes 4, 5 & 6) tend to have milder or later onset of symptoms.
Six functional classes of CFTR gene mutations have been described

CFTR channel mutations
Class 1 mutations
No CFTR protein is produced. Class 1 mutations can be due to early termination of CFTR protein production or large regions of mutated CFTR DNA.
Class 2 mutations
Defective trafficking of CFTR, which does not reach the surface of the cell. F508del is a class 2 mutation.
Class 3 mutations
The CFTR protein reaches the cell surface but it does not function. G551D is a class 3 mutation.
Class 4 mutations
The CFTR protein reaches the cell surface but chloride transport through the channel is defective.
Class 5 mutations
The CFTR channel is normal but the amount of protein at the cell surface is decreased.
Class 6 mutations
The CFTR channel is not stable at the cell surface so the amount of protein at the cell surface is decreased.
Effects on Other Channels
When CFTR is defective other channels, including the outwardly rectifying chloride channel (ORCC), the epithelial sodium channel (ENaC), a potassium channel known as ROMK1 and a chloride/bicarbonate exchanger, do not work properly. In addition, other chloride channels present on the surface of epithelial cells may be affected in the CF airways. These “alternative” chloride channels have been proposed as a therapeutic target to enhance chloride transport.
The ORCC is found on the surface of many epithelial cells. Normal CFTR facilitates the transport of adenosine triphosphate (ATP), an energy-carrying molecule, to the outside of the cell, activating ORCC. It is unknown whether CFTR itself or an associated channel actually transports the ATP. However, the mutant CFTR is not able to perform the function of transporting ATP.
The ENaC, a sodium channel found on the surface of epithelial cells, is made up of four subunits: two alpha, one beta and one gamma. Each subunit consists of two transmembrane helices. CFTR also influences the function of ENaC in the lung by decreasing its activity, however, the mechanism by which this occurs is unclear.
As suggested by its name, the chloride/bicarbonate exchanger transports one bicarbonate molecule out the cell for every chloride that it transports into the cell. The chloride is derived from the efflux of chloride through CFTR. Therefore, if CFTR is not functional the activity of this channel will be greatly reduced.
Several other chloride channels are present on the cell surface. The one that may be most influenced by CFTR is the CaCC or calcium-activated chloride channel. The exact protein that creates this channel has yet to be defined. However, it is known that the channel is modulated by the P2Y2 receptor which is activated by ATP. Therefore, the activity of this channel could be influenced by decreased ATP associated with mutant CFTR..

CFTR regulates the function of other ion channels located within the cell membrane.
Related Resources
Adult guide to cystic fibrosis, cfind the words, cfunky crossword.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Future Sci OA
- v.1(2); 2015 Sep

Cystic fibrosis – a multiorgan protein misfolding disease
Douglas fraser-pitt.
1 NovaBiotics Ltd, Cuickshank Building, Craibstone, Aberdeen, AB21 9TR UK
Deborah O’Neil
Cystic fibrosis (CF) is a heterogeneous multiorgan disease caused by mutations in the CFTR gene leading to misfolding (and other defects) and consequent dysfunction of CFTR protein. The majority of mutations cause a severe CF phenotype, and people with this condition will require a wide variety of medical interventions and therapies throughout their lives to address the symptoms of their condition. CF affects many different organ systems, but the most serious consequence of the disease is degeneration of lung function due to chronic respiratory infection and colonization of the airways with opportunistic microbial pathogens. Improvements in therapeutics, particularly the effective use of antibiotics, have led to significant gradual increases in life expectancy. There remains, however, a continuing need for newer, safer and more effective antimicrobials and mucolytic agents to maintain and improve our ability to combat CF lung infections before other curative approaches which target the root cause of the disease become available.
Cystic fibrosis
Cystic fibrosis (CF) is an inherited, multiorgan, multifactorial protein misfolding disease with its major pathologic impact being on respiratory function. Digestive, reproductive and other co-morbidities are also common in CF patients; a life-shortening disease that affects around 1 in 2500 babies of Caucasian ethnicity. Symptoms of the condition vary between individuals, but historically this condition was associated with mortality in infancy. Today, with improvements in the management of the condition, and application of the range of treatments and protocols required for management of the CF patient, many of those affected live into their 40s or beyond. Other factors in improved life expectancies are the recent development of novel, personalized medicinal therapies and advances in transplant surgery such that a child born with CF in the UK today can expect to live into the 5th or 6th decade of their life [ 1 ]. Here we discuss the genetic and protein-level basis for cystic fibrosis, current treatments and latest developments in the treatment of this condition.
The vast majority of affected individuals will have a mutation in the CFTR gene, encoded on human chromosome 7. Cystic fibrosis is an autosomal recessive disease but the spectrum and nature of CF symptoms are largely dependent upon the type of mutation(s) and their interactions. It is often described as a heterogeneous genetic disorder and genotyping of CF patients is the basis of tailored genetic counseling, epidemiological studies and the development of personalized medicine (i.e., mutation-specific therapies combined with symptom specific supportive treatments/care). The ‘sweat test’ which determines secreted chloride levels in sweat (abnormal levels being a hallmark of the condition) and antenatal ultrasound are the nonspecific diagnostic approaches currently employed for CF [ 2 ].
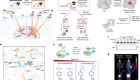
Over 1000 different mutations in the CFTR gene have been associated with cystic fibrosis, but some mutations are more common than others and there are ethnic and regional preponderances. Mutations can be classified by the way they alter the fate of the CFTR protein. There are currently six recognized classes of mutation, and three of the most common are illustrated in Figure 1 . The fully functioning wild-type protein is a transmembrane ion channel with two membrane-spanning domains (MSDs) which span the membrane six times. It has two nucleotide binding domains (NBDs) and a regulatory domain (R) and transports chloride (and other anions) along the electrochemical gradient. Mutations of the CFTR gene lead to either misfolding and consequent degradation or dysfunction/altered expression of the CFTR protein or can also prevent translation of the CFTR protein.

Class II mutations include the common F508del (or ΔF508) which lead to the misfolding of CFTR protein and subsequent polyubiquitination and destruction by the cell proteasome. Class III mutations (such as G551D) are also misfolded, but may be transported to the plasma membrane. They are either poorly regulated or nonfunctional and are subsequently degraded by the cell. Class IV mutations lead to a receptor with reduced chloride conductance, whilst class V mutations lead to reduced expression levels of CFTR. Class VI mutations lead to a higher turnover of CFTR at the plasma membrane. Class IV, V and VI mutations lead to a nonclassical or atypical CF phenotype and are not shown.
Protein misfolding of the CFTR results in to a buildup of intracellular chloride ions which is thought to draw in sodium ions and water down electrochemical and osmotic gradients. One theory is that this leads to dehydration of the surface airway fluid and creates the thick, sticky mucous seen in individuals with CF, and that this creates an environment susceptible to colonization with opportunistic pathogens. Although this was the prevailing theory for many years it is likely that defects in CFTR function also have effects on innate immunity at the airway epithelial cell surface. CFTR also transports thiocyanate ions (SCN - ), which are a key component of the innate immune defense. Thiocyanate and hypothiocyanite (OSCN - ) are able to react with potentially damaging innate immune mediators and are thereby thought to have a protective role in protecting the lung during inflammation which may be absent in people with CF [ 3 ].
CFTR misfolding also impact the digestive and reproductive systems. People with CF can have gastrointestinal symptoms associated with malabsorption of dietary nutrients, particularly fats, due to reduced enzyme secretion in the pancreas. Some of these symptoms can be addressed through diet management and the use of enzyme replacement therapies. In the reproductive system, some men with mutations in CFTR can have bilateral absence of the vas deferens. Although these affected individuals are usually fertile (i.e., they do produce viable sperm) they would require assisted reproductive technologies to reproduce as the vas deferens have usually degraded prior to birth due to clogging with thick mucus. Although these symptoms will have significant impacts on the quality of life of CF patients, it is the lung infections with opportunistic microbial pathogens such as the bacteria Pseudomonas aeruginosa and Burkholderia cepacia and the fungi Aspergillus fumigatus and consequent respiratory degeneration and failure which are the ultimate cause of death in most individuals with CF. Repeated infections over the duration of their lifetime has an impact upon lung capacity and architecture. Improved physiotherapy regimes, infection prevention strategies and antibiotic therapy have had some of the biggest impacts on improved life expectancy, but the ultimate aim as a means to a cure versus treatment would be to restore the functionality of the misfolded CFTR in CF patients.
Class I, II & III mutations & protein folding & function
A wide variety of mutations in CFTR have been characterized which have been linked with the cystic fibrosis phenotype. As described above ( Figure 1 ), these can be categorized based upon the impact of the mutation on the fate of the CFTR protein. People who are homozygous for class I, II and III mutations most often have a severe cystic fibrosis phenotype and some examples of these classes of CFTR mutation are explored here in more detail. Class IV, V and VI mutations often leave some residual CFTR function and therefore have a less severe phenotype.
F508del-CFTR, or delta-F508 (ΔF508), is the most common CFTR mutation leading to cystic fibrosis [ 4 ] F508del-CFTR is a class II mutation. A deletion of three nucleotides in the gene leads to the deletion of the phenylalanine residue at position 508 of the polypeptide chain. Although the protein is fully translated, the absence of this phenylalanine residue leads to misfolding of the CFTR protein in the endoplasmic reticulum and the protein is subsequently degraded. Perhaps surprisingly, wild-type CFTR is also subject to some degree of proteasomal degradation at this stage, but a proportion of functioning wild-type CFTR protein makes it to the plasma membrane allowing for normal chloride ion transport, and this is not the case in people homozygous for F508del-CFTR. The cell proteasomal machinery and the action of ubiquitinases were found to be essential for the recognition and rapid degradation of the misfolded F508del-CFTR protein [ 5 ]. A chain of ubiquitin molecules, covalently linked together at Lys48, are needed for the recognition of a labeled protein by the cell proteasome. This phenomenon is known as polyubiquitination [ 6 ]. One of the therapeutic goals in cystic fibrosis has been to develop drugs which can assist the correct folding of F508del-CFTR to bypass this cellular quality control step and to restore some function.
Class I mutations (such as G542X) are thought to be the genetic basis for as many as 10% of CF cases [ 7 ]. This class of mutation is defined as impairing the translation of the protein and is caused by the creation of premature stop codons leading to the production of truncated proteins and consequently no functioning CFTR at the plasma membrane. In this specific case the codon for glycine residue (G) at position 542 on the polypeptide chain has been mutated from GGA to the stop codon TGA [ 8 ].
Class III mutations, such as G551D, also results in some changes to the structure and function of the CFTR protein. This specific mutation is a missense mutation which leads to an aspartate (D) reside at position 551 instead of the amino acid glycine (G). This effectively swaps a small nonpolar amino acid for a negatively charged amino acid. It is the most common specific mutation in this rare class, and results in a severe CF phenotype. Although the CFTR protein is not removed by cellular quality control systems and is trafficked to the plasma membrane, the mutation leads to the abolition of ATP-dependent gating of the ion channel. This means it is much more likely that channel will be in a ‘closed’ conformation when compared with wild-type channels and it leads to impairment in anion conductance [ 9 ].
Unmet medical need – an orphan disease
Cystic fibrosis is defined as an orphan disease in that it affects fewer than 200,000 individuals in the USA and has a similar incidence elsewhere in the developed world. The US Orphan Drug Act of 1983 and equivalent legislation worldwide (Australia, Japan and Europe predominantly) provides regulatory and commercial incentives to develop treatments for rare/ultrarare diseases to counterbalance the low numbers of patients requiring medicinal treatments. Orphan drug schemes were introduced to address the very low levels of R&D activity in the rare disease space and allowed for the creation and success of a number of highly innovative biotechnology companies who have come to dominate the orphan drug discover space which ‘big pharma’ shunned until recently in favor of mass-market ‘blockbuster’ drug discovery and development strategies.
Despite the range of medical interventions CF patients depend on to maintain any quality of life, there are a very limited number of targeted, specific CF therapies and there had been very little progress as regards the discovery of new treatments until very recent times. Despite the market incentives orphan schemes provide and a recent resurgence of R&D efforts in this space, CF remains a highly complex, challenging clinical problem to solve and in the absence of a cure, there remains a need to develop more effective, long-term therapeutics to tackle various aspects of the condition; a priority being well-tolerated, resistance-free medicines for treating and preventing infection in the airways of CF patients and as a consequence improving lung function.
In addition to commercial efforts to develop new CF treatments, research into rare diseases (as well as patient support and disease awareness) in the UK is also supported through research councils UK (RCUK) and charitable trusts like the Cystic Fibrosis Trust, and Rare Disease UK (RDUK). Funding supports projects seeking to understand the fundamental mechanisms of CF and molecular epidemiology, from blue-skies research to applied science which target the development of new therapies. Seed funding, such as that provided by the National Organization for Rare Disorders (NORD) in the USA has been used to develop new research ideas into commercial possibilities, which have also been supported by large US charities such as the Cystic Fibrosis Foundation. CF research will continue in both the academic and commercial sectors, but in the commercial sectors many of the recent developments in CF therapeutics have come from smaller biotechnology firms, in line with trends in orphan drug discovery activities overall.
Current treatment options
There is no cure for CF, and treatments largely address multiple symptoms and not the underlying cause of the disease (i.e., protein misfolding). There are a wide range of medical interventions required for CF patients (summarized in Table 1 ) which have to be tailored to the individual patient and which depend on their specific symptoms, disease etiology, age and general health.
Table 1.
Patients with the most common severe phenotypes will have experienced many if not most of those listed above during the course of their lives. The Cystic Fibrosis Foundation and other bodies assess the evidence of treatment efficacies gathered through Cochrane reviews and other scientific literature to make informed recommendations for CF patients and their support teams [ 10 ].
† Daily treatments for those who require them.
CPT: Chest physiotherapy
The most successful therapeutic intervention in terms of reducing the morbidity and mortality in CF has been the use of antibiotic therapy for the treatment of CF associated lung disease. Inhaled antibiotics such as tobramycin [ 32 ] and colistin [ 33 ] have had a significant impact on mortality [ 34 ]. Inhalation minimises the systemic side effects of long term antibiotic use.
Many of the treatments described above target respiratory symptoms as infection leads to a gradual deterioration in lung function in CF patients. Bilateral lung transplant surgery is only suitable for a limited number CF patients when their lung function has been badly affected. There is a shortage of donor organs and there are inherent risks with major surgery. CF patients who are evaluated for this procedure would also need to be on anti-rejection medication for life. Antibiotic therapy is often necessary to optimize the surgical outcome, and colonization with certain microbes would preclude surgery.
Recent developments in treatments which target the underlying causes of CF
A few recent advances in CF therapies address the underlying causes of CF. Kalydeco™ (Ivacaftor/VX-770; Vertex, MA, USA) was approved by the FDA in 2012 as a treatment for people with the CF class III mutation G551D. This rare gating mutation, discussed above, has also been nicknamed the ‘Celtic’ mutation because of its prevalence in NW Europe, with Ireland having the highest prevalence of G511D CF at 5.7% [ 11 ]. Ivacaftor (VX-770), has been demonstrated to improve lung function in people with at least one G551D allele [ 12 ]. It is thought that VX-770 directly binds the CFTR protein and alters the regulation of the protein in a phosphorylation-dependent manner, which increases the likelihood that the channel will be in the ‘open’ conformation, therefore restoring some function [ 13 ]. Phase III clinical trials have demonstrated a 10.7% increase in forced expiratory volume (FEV 1 ) measurements of lung function in comparison to controls, and an improvement of 14.2% in FEV 1 measurements over the course of the trial.
Another Vertex (Boston, USA) compound, lumacaftor (also known as VX-809), is in development as a treatment for the much more abundant F508del-CFTR class II mutation. This compound partially corrects some of the protein misfolding so that some of the protein escapes endoplasmic reticulum-associated degradation and is translocated to the cell surface. In vitro experiments have demonstrated some restoration of chloride transport across the membrane is restored in cells treated with VX-809, and it is thought that this compound works by stabilizing the conformation of membrane-spanning domain 1 (MSD1) [ 14 ]. Further in vitro experimental data on lung cells showed that combination of VX-809 treatment with the potentiator VX-770 (Ivacaftor) enhanced chloride transport [ 15 ]. Phase IIa clinical trials using lumacaftor alone demonstrated safety and tolerability of the compound, but failed to improve clinical outcomes measured by FEV 1 [ 16 ], therefore, Phase III clinical trials in 2014 examined treatments with lumacaftor alone and in combination with ivacaftor. Results from this trial were published very recently and demonstrate improvements in treated versus predicted FEV 1 of 2.6–4.0% and reduced exacerbation rates [ 31 ]. The impact of the drugs in the target patients population seems to be greater on minimizing exacerbations than FEV1 so distinct from the monotherapy approach with ivacaftor alone. A US FDA approval decision for the combination therapy is expected in July 2015. Although it remains to be seen if early intervention with any of the potentiator/corrector therapies will rescue lung function and reduce morbidity in the long term, these treatments do provide hope along with other supportive therapies, treatments that tackle the folding and function of the CFTR protein might extend the lives and reduce morbidity for people living with CF.
Ataluren is another small molecule in development as a potential therapeutic for certain CF genotypes that targets the CFTR. It is being developed by PTC therapeutics and may also be a potential treatment for some people with Duchenne muscular dystrophy and some other rare conditions as it may overcome problems caused by mutations which lead to premature stop codons, (or nonsense mutations) [ 17 ]. It is thought to work by forcing the read-through of transcripts of genes containing a nonsense mutation and has been the subject of Phase II clinical trials in adults and children with rare class I mutations of CF such as G542X (described above) which have demonstrated some improvements in chloride transport in sinus membranes and improved some clinical parameters. It is orally bioavailable and has potential to treat a number of genetic conditions caused by nonsense mutations [ 18 ].
Other pharmacological therapies are being developed to address the symptoms and altered physiology in CF that results from CFTR misfolding. These include enhancing thiocyanate levels [ 19 ] and direct [ 20 , 21 ] or indirect [ 22 , 23 ] methods for supplementing nitric oxide in the lungs (which is reduced in CF patients [ 24 , 25 ]). Some groups are also investigating the possibility of promoting autophagy via pharmacological means, as this is also known to be dysregulated in people with CF [ 26 ].
Novel antimicrobials are badly needed in CF, as well as more widely in treating infectious disease, because of the well known problem of microbial resistance to current antibiotics recently addressed in a UK HM Government report chaired by Jim O'Neill [ 35 ]. The development of new antibiotics will hopefully increase the range of therapeutic options for CF patients, and those which can be used in combination with other drugs to reduce the development of antibiotic resistance. Combinatorial antibiotic therapy in CF is in development, such as recent clinical trials using inhaled fosfomycin together with tobramycin [ 36 ]. Individual or combined therapies which tackle more than one of the consequences of CF, such as co-delivery of DNAseI with Ciprofloxacin [ 37 ] could also prove to be successful. Lynovex ® , is a compound in clinical development by NovaBiotics Ltd (Aberdeen, UK) and has potential to be used as an adjunct therapy in CF as it has been demonstrated to have antibacterial, antibiofilm and mucoactive activities [ 38 ]. Also existing antibiotics, such as aztreonam [ 39 ] and levofloxacin [ 40 ] have been reformulated for inhalation and have been, or are being, re-purposed for use in treating CF lung disease. Inhaled macrolides [ 41 ], such as azithromycin, are also being used primarily because of potential anti-inflammatory [ 42 ] and anti-virulence [ 43 ] properties secondary to their well-known antimicrobial activity as this is thought to be less important when many common CF pathogens such as P. aeruginosa are regularly found to be resistant [ 44 ].
The therapy candidates currently in development and those recently approved represent important milestones in CF management, but these pharmacological interventions have not yet been demonstrated to be wholly curative. An alternative approach which has been also been a focus of research, is gene therapy. In theory, replacing a faulty gene in all the affected cells with a stably expressed healthy gene could be curative for CF lung disease. Obstacles to gene therapy in CF include the heterogeneous genetic nature of the disease, difficulties in transfecting lung tissue, finding suitable and tolerable genetic transfer agents or vectors, and obtaining stable expression of the wt CFTR protein. Although there have been a number of preclinical and a few clinical developments with regards to gene therapy for CF, Cochrane systemic review analysis of the literature do not yet support topical gene therapy as a treatment for CF [ 27 ]. Work continues, and one possible vector for use in future topical gene therapy for CF is the lentiviral vector [ 28 ]. The potential for genome editing in stem cells used for therapy in CF is also being investigated [ 29 ]. The most recent gene therapy clinical trial data examined nebulised liposomal delivery of plasmid DNA into the lungs of CF patients [ 45 ]. The results, although described as modest, suggested the stabilisation of lung function in CF patients receiving gene therapy compared with control with a 3.7% improvement in predicted FEV 1 .
Although limited in number, the new therapeutics under investigation for CF, including those which enable the correct folding of the CFTR protein demonstrate promise for the future. Efforts are being driven largely by the biotechnology and academic sectors from where the required innovation is being derived. As discussed elsewhere, for example by Fanen et al. , 2014 [ 30 ], customized and combinatorial therapeutics based upon patient genotype, and clinical need will be a major focus of future research and development.
Cystic fibrosis is a rare genetic, orphan disease. The many genetic causes of the condition largely converge by the impact on the expression, or function of, the CFTR protein. Mutations have been categorised into different classes on the basis of how they effect the function of CFTR and the consequences can be mild to severe disease phenotypes. There are ethnic and geographical patterns in the frequency of different mutations, but F508del-CFTR, which causes a severe disease phenotype, is the most common, and is caused by misfolding of the protein channel. CF is a multi-organ disease, but the most serious impact in people with the severe disease phenotype, is on the respiratory system and the increased susceptibility of individuals to colonisation and infection of the airways by bacteria and fungi. The most successful treatments to date have been the use of prophylactic and inhaled antibiotics, which have treated infectious exacerbations, and managed microbial colonisation of the lungs over long periods of time, and helped to delay the progressive loss of lung function over time. Other therapeutics which have been in use for a long time also tackle the symptoms of this condition, such as the use of mucolytics to break up CF sputum. Although there have been steady increases in median mortality for people with CF, over recent decades there were very few new therapies for this condition and none of the standard drug treatments tackled the underlying cause of the disease. Only surgical intervention through transplantation had the capacity to correct the cause of the disease by replacing diseased organs. In the last few years there have been a number of promising developments, including pharmacological therapies, termed correctors and potentiators, which can correct misfolding and improve the function of the CFTR and thus target the cause of the condition.
Future perspective
The clinical consequences of the vast majority of CF cases have been well described for decades (although there are still accounts of patients with rare mutations and atypical presentations). The physiological consequences are also now beginning to be understood: Dysregulated ion transportation across epithelial cell layers at various body sites, and the subsequent impact on body secretions is the major contributor to disease phenotype. This understanding has helped us to develop therapeutics which target the underlying cause of the disease (CFTR) and those which tackle the consequences of disease, including mucolytics, antibiotics to address respiratory infections associated with CF and digestive enzyme replacements to supplement the patient’s low secretion levels in the digestive tract.
The impact on CFTR malfunction on all aspects of innate immunity in the lung is still not fully understood or quantified. As this develops over the next few years, therapeutics supporting immunity, or replacing lost components of immunity, might make an impact in the CF field.
Advances in molecular biology have meant that the genetic component of CF is now well catalogued, and genotyping of CF patients is now well established. Our understanding of the impact of protein folding of CFTR, and potentially other proteins, as well as CF cell biology is still being fully elucidated however. Recent breakthroughs in pharmacological modification of CFTR were made through screening for active compounds, rather than rational design, but further understanding CFTR protein misfolding, cell quality control systems, and ways to modify these might lead to second and third generations of correctors and potentiators of the underlying cause of CF.
Pharmacological interventions which target the cause of CF are a breakthrough but are not curative. Earlier intervention studies in children with CF have yet to be conducted. Although even with new and improved CFTR modifiers, it is highly likely that therapies which tackle the consequences of CF will still be required. Multi-active or combinatorial therapies could tackle both the causes and consequences of CF. The struggle for new and effective antibiotics in the face of antibiotic resistance is also of increasing relevance to CF, where unavoidable chronic antibiotic use encourages resistance development and patients are susceptible to new and emerging pathogens.
Executive summary
- Cystic fibrosis (CF) is a disease caused by aberrant expression, or misfolding, of CFTR.
- Mutations affecting protein folding and function: Many genetic mutations have been associated with CF, but the impact of these mutations can be classified (class I-VI) by how they affect the expression, or folding of, CFTR.
- The clinical consequences of low abundance of functioning CFTR protein or of a malfunctioning CFTR can mild or very serious, and they are driven by dysregulation of ion transport across epithelial surfaces.
- Unmet clinical need – an orphan disease: CF is a rare genetic condition, and within this there are people with common and rare genotypes with varying clinical symptoms. Researching and developing new therapeutics to serve this complex condition which affects relatively small numbers with regards to whole populations has required different incentives and regulatory approaches.
- Current treatment options: antibiotics for CF-associated lung disease have contributed to the gradual improvement in median life-span for people with CF but new developments in this area are needed. Inhaled delivery of antibiotics is in common use and can be used in long term therapy. Other therapeutic options for reducing sputum viscocity in the lungs, such as DNAse l have also made a positive impact. Replacement enzyme therapy and dietetic input can improve nutritional status and morbidity, but surgical interventions in the GI tract are sometimes necessary. Up until recently, lung transplant was also the only way of tackling the underlying cause of CF and is still required for eligible patient's whose lung function has declined significantly.
- Recent developments in treatments which target the underlying causes of CF: the CF research landscape has shifted dramatically and positively of late. After decades absent of any breakthroughs, we have seen recent advances in new therapeutic interventions that target the underlying cause and also the debilitating symptoms of this life-limiting protein-misfolding disease (e.g., inhaled antibiotics, novel CFTR corrector and potentiator therapies) and an increase in late stage CF drug research (e.g. CFTR gene therapy/editing) and development. These advances are significant for the CF community and also the clinical and scientific field as translation of these breakthroughs to clinical candidates/products has only come about from a much better understanding of disease mechanism and pathology.
Financial & competing interests disclosure
D O'Neil is Chief Executive Officer of NovaBiotics Ltd. D Fraser-Pitt is an employee of NovaBiotics Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

IMAGES
VIDEO
COMMENTS
the CFTR protein is a growth factor receptor four don the surface of lung cells. False; the CFTR protein is a membrane protein responsible for controlling the movement of chloride in and out of cells. in which organelle (s) does transcription occur in eukaryotes. nucleus. in prokaryotes, translation happens after transcription and RNA ...
A Case of Cystic Fibrosis - ANSWER KEY The student version of this activity available in these formats: ... Answers vary, since the channel is blocked, suggest to unblock the channel or keep it open in some way ... Case Study - Cystic Fibrosis (Remote Edition) KEY 1. "Woe to that child which when kissed on the forehead tastes salty. ...
This case study is a follow-up to the Cystic Fibrosis Case Study where students explore how changes in transport proteins affects the movement of ions, resulting in a build-up of chloride ions and the symptoms of the disease.. Students were introduced to the idea that different mutations can cause differences in the transport proteins, but in the first version, the origin of these mutations ...
CFTR Gene. Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and chloride channel in vertebrates that is encoded by the CFTR gene. This gene is found on chromosome 7 and is 4400 nucleotides in length. The gene encodes the CFTR protein that acts as a channel across the membrane to transport chloride ions.
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system. When the CFTR protein is defective, epithelial cells can't regulate the way that ...
Case study 1: Cystic fibrosis. cystic fibrosis. Click the card to flip 👆. A genetic disorder that occurs in people with two copies of a certain recessive allele; characterized by an excessive secretion of mucus and consequent vulnerability to infection; fatal if untreated. Click the card to flip 👆.
Cystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide.According to the Cystic Fibrosis Foundation (Cystic Fibrosis Foundation, 2019a), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births.The disease prevalence varies greatly by ethnicity, with the ...
2.1. Characteristics of the human cystic fibrosis gene and encoded CFTR protein. Cystic fibrosis is caused by pathogenic mutations in a single large gene located on human chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein. 1, 2, 3 CFTR belongs to the ABC (ATP‐binding cassette) family of proteins, a large group of related proteins that share ...
Description. This is the answer key for the case study on cystic fibrosis where students explore how children are diagnosed with CF, how CF mutations affect transport across the cell membrane, and how two drugs can be used to treat the disease. The activity is used in AP Biology class and requires students to complete a CER (claim, evidence ...
This directed case study examines the molecular basis of cystic fibrosis to emphasize the relationship between the genetic code stored in a DNA sequence and the encoded protein's structure and function. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein that functions to help ...
The new modulators of CFTR protein synthesis could facilitate the additional exploration needed to better understand the unfolding clinical biology of CFTR in human disease, even as they ... Crosignani A. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcome. Hepatol Baltim Md. 2002; 36 (6):1374-1382 ...
The cystic fibrosis transmembrane conductance regulator (CFTR) protein helps to maintain the balance of salt and water on many surfaces in the body, such as the surface of the lung. The CFTR protein is a particular type of protein called an ion channel. In the lung, the CFTR ion channel moves chloride ions from inside the cell to outside the cell.
This case study asks students to examine a case of cystic fibrosis. As students read the symptoms and gather evidence about membrane proteins, they learn that CF is really a disorder of membrane permeability. ... The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis ...
Then students use this information to explain how the cell types are different. The gene therapy case study on cystic fibrosis has students model protein synthesis and challenges students to apply concepts of protein synthesis, gene expression regulation, and the effects of gene therapy on protein structure and function.
A progressive, genetic disease that causes persistent lung infections and limits the ability to breathe over time. where is CF usually? In the lungs, the mucus clogs the airways and traps germs, like bacteria, leading to infections, inflammation, respiratory failure, and other complications. what are the subjective findings that are consistent ...
Name: _____ Date: ___/___/___ Period: ____ Cystic Fibrosis and Protein Synthesis Introduction Cystic fibrosis is an inherited disease that is marked by the buildup of thick, sticky mucus that can damage the lungs and many other organs. Cystic fibrosis affects the viscosity of the mucus lining of the lungs. Mucus is a collection of many substances including enzymes, proteins and muc
This case study explores the relationship between the cell membrane and breathing difficulties that occur as a result of the genetic disorder cystic fibrosis. Students look at specific channel proteins in the cell membrane that affect the movement of chloride ions. Different mutations result in different problems with these protein channels.
In the case of F508del, the loss of a single amino acid about one-third of the way along the CFTR protein leads to two defects: the resulting protein has trouble making its way to the cell ...
Introduction: Cystic Fibrosis (CF) is a recessive autosomal, progressive and multisystemic genetic disease. Its pathophysiological basis is the mutation in a gene whose function is to code the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein, responsible for regulating the transport of chlorine in the apical
The cystic fibrosis transmembrane conductance regulator (CFTR) is defective in cystic fibrosis (CF). This protein is a channel that sits on the surface of cells and transports chloride and other molecules, such as bicarbonate. The gene that encodes the CFTR protein, which is also called CFTR, is located on chromosome 7. Mutations in this gene lead to CF. Since the discovery of the CFTR gene in ...
Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide . Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease.
Cystic fibrosis (CF) is a heterogeneous multiorgan disease caused by mutations in the CFTR gene leading to misfolding (and other defects) and consequent dysfunction of CFTR protein. The majority of mutations cause a severe CF phenotype, and people with this condition will require a wide variety of medical interventions and therapies throughout their lives to address the symptoms of their ...