An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.11(2); 2019 Feb


Planning and Conducting Clinical Research: The Whole Process
Boon-how chew.
1 Family Medicine, Universiti Putra Malaysia, Serdang, MYS
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question. Engagement with clinical experts, experienced researchers, relevant stakeholders of the research topic, and even patients can enhance the research question’s relevance, feasibility, and efficiency. Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental or non-experimental, sampling or census, and time features of the variables to be studied. The ultimate aims of research reporting are to present findings succinctly and timely. Concise, explicit, and complete reporting are the guiding principles in clinical studies reporting.
Introduction and background
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices. There are many ways a clinical research's findings can become invalid or less impactful including ignorance of previous similar studies, a paucity of similar studies, poor study design and implementation, low test agent efficacy, no predetermined statistical analysis, insufficient reporting, bias, and conflicts of interest [ 1 - 4 ]. Scientific, ethical, and moral decadence among researchers can be due to incognizant criteria in academic promotion and remuneration and too many forced studies by amateurs and students for the sake of research without adequate training or guidance [ 2 , 5 - 6 ]. This article will review the proper methods to conduct medical research from the planning stage to submission for publication (Table (Table1 1 ).
a Feasibility and efficiency are considered during the refinement of the research question and adhered to during data collection.
Epidemiologic studies in clinical and medical fields focus on the effect of a determinant on an outcome [ 7 ]. Measurement errors that happen systematically give rise to biases leading to invalid study results, whereas random measurement errors will cause imprecise reporting of effects. Precision can usually be increased with an increased sample size provided biases are avoided or trivialized. Otherwise, the increased precision will aggravate the biases. Because epidemiologic, clinical research focuses on measurement, measurement errors are addressed throughout the research process. Obtaining the most accurate estimate of a treatment effect constitutes the whole business of epidemiologic research in clinical practice. This is greatly facilitated by clinical expertise and current scientific knowledge of the research topic. Current scientific knowledge is acquired through literature reviews or in collaboration with an expert clinician. Collaboration and consultation with an expert clinician should also include input from the target population to confirm the relevance of the research question. The novelty of a research topic is less important than the clinical applicability of the topic. Researchers need to acquire appropriate writing and reporting skills from the beginning of their careers, and these skills should improve with persistent use and regular reviewing of published journal articles. A published clinical research study stands on solid scientific ground to inform clinical practice given the article has passed through proper peer-reviews, revision, and content improvement.
Systematic literature reviews
Systematic literature reviews of published papers will inform authors of the existing clinical evidence on a research topic. This is an important step to reduce wasted efforts and evaluate the planned study [ 8 ]. Conducting a systematic literature review is a well-known important step before embarking on a new study [ 9 ]. A rigorously performed and cautiously interpreted systematic review that includes in-process trials can inform researchers of several factors [ 10 ]. Reviewing the literature will inform the choice of recruitment methods, outcome measures, questionnaires, intervention details, and statistical strategies – useful information to increase the study’s relevance, value, and power. A good review of previous studies will also provide evidence of the effects of an intervention that may or may not be worthwhile; this would suggest either no further studies are warranted or that further study of the intervention is needed. A review can also inform whether a larger and better study is preferable to an additional small study. Reviews of previously published work may yield few studies or low-quality evidence from small or poorly designed studies on certain intervention or observation; this may encourage or discourage further research or prompt consideration of a first clinical trial.
Conceptual framework
The result of a literature review should include identifying a working conceptual framework to clarify the nature of the research problem, questions, and designs, and even guide the latter discussion of the findings and development of possible solutions. Conceptual frameworks represent ways of thinking about a problem or how complex things work the way they do [ 11 ]. Different frameworks will emphasize different variables and outcomes, and their inter-relatedness. Each framework highlights or emphasizes different aspects of a problem or research question. Often, any single conceptual framework presents only a partial view of reality [ 11 ]. Furthermore, each framework magnifies certain elements of the problem. Therefore, a thorough literature search is warranted for authors to avoid repeating the same research endeavors or mistakes. It may also help them find relevant conceptual frameworks including those that are outside one’s specialty or system.
Conceptual frameworks can come from theories with well-organized principles and propositions that have been confirmed by observations or experiments. Conceptual frameworks can also come from models derived from theories, observations or sets of concepts or even evidence-based best practices derived from past studies [ 11 ].
Researchers convey their assumptions of the associations of the variables explicitly in the conceptual framework to connect the research to the literature. After selecting a single conceptual framework or a combination of a few frameworks, a clinical study can be completed in two fundamental steps: study design and study report. Three study designs should be planned in sequence and iterated until satisfaction: the theoretical design, data collection design, and statistical analysis design [ 7 ].
Study designs
Theoretical Design
Theoretical design is the next important step in the research process after a literature review and conceptual framework identification. While the theoretical design is a crucial step in research planning, it is often dealt with lightly because of the more alluring second step (data collection design). In the theoretical design phase, a research question is designed to address a clinical problem, which involves an informed understanding based on the literature review and effective collaboration with the right experts and clinicians. A well-developed research question will have an initial hypothesis of the possible relationship between the explanatory variable/exposure and the outcome. This will inform the nature of the study design, be it qualitative or quantitative, primary or secondary, and non-causal or causal (Figure (Figure1 1 ).

A study is qualitative if the research question aims to explore, understand, describe, discover or generate reasons underlying certain phenomena. Qualitative studies usually focus on a process to determine how and why things happen [ 12 ]. Quantitative studies use deductive reasoning, and numerical statistical quantification of the association between groups on data often gathered during experiments [ 13 ]. A primary clinical study is an original study gathering a new set of patient-level data. Secondary research draws on the existing available data and pooling them into a larger database to generate a wider perspective or a more powerful conclusion. Non-causal or descriptive research aims to identify the determinants or associated factors for the outcome or health condition, without regard for causal relationships. Causal research is an exploration of the determinants of an outcome while mitigating confounding variables. Table Table2 2 shows examples of non-causal (e.g., diagnostic and prognostic) and causal (e.g., intervention and etiologic) clinical studies. Concordance between the research question, its aim, and the choice of theoretical design will provide a strong foundation and the right direction for the research process and path.
A problem in clinical epidemiology is phrased in a mathematical relationship below, where the outcome is a function of the determinant (D) conditional on the extraneous determinants (ED) or more commonly known as the confounding factors [ 7 ]:
For non-causal research, Outcome = f (D1, D2…Dn) For causal research, Outcome = f (D | ED)
A fine research question is composed of at least three components: 1) an outcome or a health condition, 2) determinant/s or associated factors to the outcome, and 3) the domain. The outcome and the determinants have to be clearly conceptualized and operationalized as measurable variables (Table (Table3; 3 ; PICOT [ 14 ] and FINER [ 15 ]). The study domain is the theoretical source population from which the study population will be sampled, similar to the wording on a drug package insert that reads, “use this medication (study results) in people with this disease” [ 7 ].
The interpretation of study results as they apply to wider populations is known as generalization, and generalization can either be statistical or made using scientific inferences [ 16 ]. Generalization supported by statistical inferences is seen in studies on disease prevalence where the sample population is representative of the source population. By contrast, generalizations made using scientific inferences are not bound by the representativeness of the sample in the study; rather, the generalization should be plausible from the underlying scientific mechanisms as long as the study design is valid and nonbiased. Scientific inferences and generalizations are usually the aims of causal studies.
Confounding: Confounding is a situation where true effects are obscured or confused [ 7 , 16 ]. Confounding variables or confounders affect the validity of a study’s outcomes and should be prevented or mitigated in the planning stages and further managed in the analytical stages. Confounders are also known as extraneous determinants in epidemiology due to their inherent and simultaneous relationships to both the determinant and outcome (Figure (Figure2), 2 ), which are usually one-determinant-to-one outcome in causal clinical studies. The known confounders are also called observed confounders. These can be minimized using randomization, restriction, or a matching strategy. Residual confounding has occurred in a causal relationship when identified confounders were not measured accurately. Unobserved confounding occurs when the confounding effect is present as a variable or factor not observed or yet defined and, thus, not measured in the study. Age and gender are almost universal confounders followed by ethnicity and socio-economic status.

Confounders have three main characteristics. They are a potential risk factor for the disease, associated with the determinant of interest, and should not be an intermediate variable between the determinant and the outcome or a precursor to the determinant. For example, a sedentary lifestyle is a cause for acute coronary syndrome (ACS), and smoking could be a confounder but not cardiorespiratory unfitness (which is an intermediate factor between a sedentary lifestyle and ACS). For patients with ACS, not having a pair of sports shoes is not a confounder – it is a correlate for the sedentary lifestyle. Similarly, depression would be a precursor, not a confounder.
Sample size consideration: Sample size calculation provides the required number of participants to be recruited in a new study to detect true differences in the target population if they exist. Sample size calculation is based on three facets: an estimated difference in group sizes, the probability of α (Type I) and β (Type II) errors chosen based on the nature of the treatment or intervention, and the estimated variability (interval data) or proportion of the outcome (nominal data) [ 17 - 18 ]. The clinically important effect sizes are determined based on expert consensus or patients’ perception of benefit. Value and economic consideration have increasingly been included in sample size estimations. Sample size and the degree to which the sample represents the target population affect the accuracy and generalization of a study’s reported effects.
Pilot study: Pilot studies assess the feasibility of the proposed research procedures on small sample size. Pilot studies test the efficiency of participant recruitment with minimal practice or service interruptions. Pilot studies should not be conducted to obtain a projected effect size for a larger study population because, in a typical pilot study, the sample size is small, leading to a large standard error of that effect size. This leads to bias when projected for a large population. In the case of underestimation, this could lead to inappropriately terminating the full-scale study. As the small pilot study is equally prone to bias of overestimation of the effect size, this would lead to an underpowered study and a failed full-scale study [ 19 ].
The Design of Data Collection
The “perfect” study design in the theoretical phase now faces the practical and realistic challenges of feasibility. This is the step where different methods for data collection are considered, with one selected as the most appropriate based on the theoretical design along with feasibility and efficiency. The goal of this stage is to achieve the highest possible validity with the lowest risk of biases given available resources and existing constraints.
In causal research, data on the outcome and determinants are collected with utmost accuracy via a strict protocol to maximize validity and precision. The validity of an instrument is defined as the degree of fidelity of the instrument, measuring what it is intended to measure, that is, the results of the measurement correlate with the true state of an occurrence. Another widely used word for validity is accuracy. Internal validity refers to the degree of accuracy of a study’s results to its own study sample. Internal validity is influenced by the study designs, whereas the external validity refers to the applicability of a study’s result in other populations. External validity is also known as generalizability and expresses the validity of assuming the similarity and comparability between the study population and the other populations. Reliability of an instrument denotes the extent of agreeableness of the results of repeated measurements of an occurrence by that instrument at a different time, by different investigators or in a different setting. Other terms that are used for reliability include reproducibility and precision. Preventing confounders by identifying and including them in data collection will allow statistical adjustment in the later analyses. In descriptive research, outcomes must be confirmed with a referent standard, and the determinants should be as valid as those found in real clinical practice.
Common designs for data collection include cross-sectional, case-control, cohort, and randomized controlled trials (RCTs). Many other modern epidemiology study designs are based on these classical study designs such as nested case-control, case-crossover, case-control without control, and stepwise wedge clustered RCTs. A cross-sectional study is typically a snapshot of the study population, and an RCT is almost always a prospective study. Case-control and cohort studies can be retrospective or prospective in data collection. The nested case-control design differs from the traditional case-control design in that it is “nested” in a well-defined cohort from which information on the cohorts can be obtained. This design also satisfies the assumption that cases and controls represent random samples of the same study base. Table Table4 4 provides examples of these data collection designs.
Additional aspects in data collection: No single design of data collection for any research question as stated in the theoretical design will be perfect in actual conduct. This is because of myriad issues facing the investigators such as the dynamic clinical practices, constraints of time and budget, the urgency for an answer to the research question, and the ethical integrity of the proposed experiment. Therefore, feasibility and efficiency without sacrificing validity and precision are important considerations in data collection design. Therefore, data collection design requires additional consideration in the following three aspects: experimental/non-experimental, sampling, and timing [ 7 ]:
Experimental or non-experimental: Non-experimental research (i.e., “observational”), in contrast to experimental, involves data collection of the study participants in their natural or real-world environments. Non-experimental researches are usually the diagnostic and prognostic studies with cross-sectional in data collection. The pinnacle of non-experimental research is the comparative effectiveness study, which is grouped with other non-experimental study designs such as cross-sectional, case-control, and cohort studies [ 20 ]. It is also known as the benchmarking-controlled trials because of the element of peer comparison (using comparable groups) in interpreting the outcome effects [ 20 ]. Experimental study designs are characterized by an intervention on a selected group of the study population in a controlled environment, and often in the presence of a similar group of the study population to act as a comparison group who receive no intervention (i.e., the control group). Thus, the widely known RCT is classified as an experimental design in data collection. An experimental study design without randomization is referred to as a quasi-experimental study. Experimental studies try to determine the efficacy of a new intervention on a specified population. Table Table5 5 presents the advantages and disadvantages of experimental and non-experimental studies [ 21 ].
a May be an issue in cross-sectional studies that require a long recall to the past such as dietary patterns, antenatal events, and life experiences during childhood.
Once an intervention yields a proven effect in an experimental study, non-experimental and quasi-experimental studies can be used to determine the intervention’s effect in a wider population and within real-world settings and clinical practices. Pragmatic or comparative effectiveness are the usual designs used for data collection in these situations [ 22 ].
Sampling/census: Census is a data collection on the whole source population (i.e., the study population is the source population). This is possible when the defined population is restricted to a given geographical area. A cohort study uses the census method in data collection. An ecologic study is a cohort study that collects summary measures of the study population instead of individual patient data. However, many studies sample from the source population and infer the results of the study to the source population for feasibility and efficiency because adequate sampling provides similar results to the census of the whole population. Important aspects of sampling in research planning are sample size and representation of the population. Sample size calculation accounts for the number of participants needed to be in the study to discover the actual association between the determinant and outcome. Sample size calculation relies on the primary objective or outcome of interest and is informed by the estimated possible differences or effect size from previous similar studies. Therefore, the sample size is a scientific estimation for the design of the planned study.
A sampling of participants or cases in a study can represent the study population and the larger population of patients in that disease space, but only in prevalence, diagnostic, and prognostic studies. Etiologic and interventional studies do not share this same level of representation. A cross-sectional study design is common for determining disease prevalence in the population. Cross-sectional studies can also determine the referent ranges of variables in the population and measure change over time (e.g., repeated cross-sectional studies). Besides being cost- and time-efficient, cross-sectional studies have no loss to follow-up; recall bias; learning effect on the participant; or variability over time in equipment, measurement, and technician. A cross-sectional design for an etiologic study is possible when the determinants do not change with time (e.g., gender, ethnicity, genetic traits, and blood groups).
In etiologic research, comparability between the exposed and the non-exposed groups is more important than sample representation. Comparability between these two groups will provide an accurate estimate of the effect of the exposure (risk factor) on the outcome (disease) and enable valid inference of the causal relation to the domain (the theoretical population). In a case-control study, a sampling of the control group should be taken from the same study population (study base), have similar profiles to the cases (matching) but do not have the outcome seen in the cases. Matching important factors minimizes the confounding of the factors and increases statistical efficiency by ensuring similar numbers of cases and controls in confounders’ strata [ 23 - 24 ]. Nonetheless, perfect matching is neither necessary nor achievable in a case-control study because a partial match could achieve most of the benefits of the perfect match regarding a more precise estimate of odds ratio than statistical control of confounding in unmatched designs [ 25 - 26 ]. Moreover, perfect or full matching can lead to an underestimation of the point estimates [ 27 - 28 ].
Time feature: The timing of data collection for the determinant and outcome characterizes the types of studies. A cross-sectional study has the axis of time zero (T = 0) for both the determinant and the outcome, which separates it from all other types of research that have time for the outcome T > 0. Retrospective or prospective studies refer to the direction of data collection. In retrospective studies, information on the determinant and outcome have been collected or recorded before. In prospective studies, this information will be collected in the future. These terms should not be used to describe the relationship between the determinant and the outcome in etiologic studies. Time of exposure to the determinant, the time of induction, and the time at risk for the outcome are important aspects to understand. Time at risk is the period of time exposed to the determinant risk factors. Time of induction is the time from the sufficient exposure to the risk or causal factors to the occurrence of a disease. The latent period is when the occurrence of a disease without manifestation of the disease such as in “silence” diseases for example cancers, hypertension and type 2 diabetes mellitus which is detected from screening practices. Figure Figure3 3 illustrates the time features of a variable. Variable timing is important for accurate data capture.

The Design of Statistical Analysis
Statistical analysis of epidemiologic data provides the estimate of effects after correcting for biases (e.g., confounding factors) measures the variability in the data from random errors or chance [ 7 , 16 , 29 ]. An effect estimate gives the size of an association between the studied variables or the level of effectiveness of an intervention. This quantitative result allows for comparison and assessment of the usefulness and significance of the association or the intervention between studies. This significance must be interpreted with a statistical model and an appropriate study design. Random errors could arise in the study resulting from unexplained personal choices by the participants. Random error is, therefore, when values or units of measurement between variables change in non-concerted or non-directional manner. Conversely, when these values or units of measurement between variables change in a concerted or directional manner, we note a significant relationship as shown by statistical significance.
Variability: Researchers almost always collect the needed data through a sampling of subjects/participants from a population instead of a census. The process of sampling or multiple sampling in different geographical regions or over different periods contributes to varied information due to the random inclusion of different participants and chance occurrence. This sampling variation becomes the focus of statistics when communicating the degree and intensity of variation in the sampled data and the level of inference in the population. Sampling variation can be influenced profoundly by the total number of participants and the width of differences of the measured variable (standard deviation). Hence, the characteristics of the participants, measurements and sample size are all important factors in planning a study.
Statistical strategy: Statistical strategy is usually determined based on the theoretical and data collection designs. Use of a prespecified statistical strategy (including the decision to dichotomize any continuous data at certain cut-points, sub-group analysis or sensitive analyses) is recommended in the study proposal (i.e., protocol) to prevent data dredging and data-driven reports that predispose to bias. The nature of the study hypothesis also dictates whether directional (one-tailed) or non-directional (two-tailed) significance tests are conducted. In most studies, two-sided tests are used except in specific instances when unidirectional hypotheses may be appropriate (e.g., in superiority or non-inferiority trials). While data exploration is discouraged, epidemiological research is, by nature of its objectives, statistical research. Hence, it is acceptable to report the presence of persistent associations between any variables with plausible underlying mechanisms during the exploration of the data. The statistical methods used to produce the results should be explicitly explained. Many different statistical tests are used to handle various kinds of data appropriately (e.g., interval vs discrete), and/or the various distribution of the data (e.g., normally distributed or skewed). For additional details on statistical explanations and underlying concepts of statistical tests, readers are recommended the references as cited in this sentence [ 30 - 31 ].
Steps in statistical analyses: Statistical analysis begins with checking for data entry errors. Duplicates are eliminated, and proper units should be confirmed. Extremely low, high or suspicious values are confirmed from the source data again. If this is not possible, this is better classified as a missing value. However, if the unverified suspicious data are not obviously wrong, they should be further examined as an outlier in the analysis. The data checking and cleaning enables the analyst to establish a connection with the raw data and to anticipate possible results from further analyses. This initial step involves descriptive statistics that analyze central tendency (i.e., mode, median, and mean) and dispersion (i.e., (minimum, maximum, range, quartiles, absolute deviation, variance, and standard deviation) of the data. Certain graphical plotting such as scatter plot, a box-whiskers plot, histogram or normal Q-Q plot are helpful at this stage to verify data normality in distribution. See Figure Figure4 4 for the statistical tests available for analyses of different types of data.

Once data characteristics are ascertained, further statistical tests are selected. The analytical strategy sometimes involves the transformation of the data distribution for the selected tests (e.g., log, natural log, exponential, quadratic) or for checking the robustness of the association between the determinants and their outcomes. This step is also referred to as inferential statistics whereby the results are about hypothesis testing and generalization to the wider population that the study’s sampled participants represent. The last statistical step is checking whether the statistical analyses fulfill the assumptions of that particular statistical test and model to avoid violation and misleading results. These assumptions include evaluating normality, variance homogeneity, and residuals included in the final statistical model. Other statistical values such as Akaike information criterion, variance inflation factor/tolerance, and R2 are also considered when choosing the best-fitted models. Transforming raw data could be done, or a higher level of statistical analyses can be used (e.g., generalized linear models and mixed-effect modeling). Successful statistical analysis allows conclusions of the study to fit the data.
Bayesian and Frequentist statistical frameworks: Most of the current clinical research reporting is based on the frequentist approach and hypotheses testing p values and confidence intervals. The frequentist approach assumes the acquired data are random, attained by random sampling, through randomized experiments or influences, and with random errors. The distribution of the data (its point estimate and confident interval) infers a true parameter in the real population. The major conceptual difference between Bayesian statistics and frequentist statistics is that in Bayesian statistics, the parameter (i.e., the studied variable in the population) is random and the data acquired is real (true or fix). Therefore, the Bayesian approach provides a probability interval for the parameter. The studied parameter is random because it could vary and be affected by prior beliefs, experience or evidence of plausibility. In the Bayesian statistical approach, this prior belief or available knowledge is quantified into a probability distribution and incorporated into the acquired data to get the results (i.e., the posterior distribution). This uses mathematical theory of Bayes’ Theorem to “turn around” conditional probabilities.
The goal of research reporting is to present findings succinctly and timely via conference proceedings or journal publication. Concise and explicit language use, with all the necessary details to enable replication and judgment of the study applicability, are the guiding principles in clinical studies reporting.
Writing for Reporting
Medical writing is very much a technical chore that accommodates little artistic expression. Research reporting in medicine and health sciences emphasize clear and standardized reporting, eschewing adjectives and adverbs extensively used in popular literature. Regularly reviewing published journal articles can familiarize authors with proper reporting styles and help enhance writing skills. Authors should familiarize themselves with standard, concise, and appropriate rhetoric for the intended audience, which includes consideration for journal reviewers, editors, and referees. However, proper language can be somewhat subjective. While each publication may have varying requirements for submission, the technical requirements for formatting an article are usually available via author or submission guidelines provided by the target journal.
Research reports for publication often contain a title, abstract, introduction, methods, results, discussion, and conclusions section, and authors may want to write each section in sequence. However, best practices indicate the abstract and title should be written last. Authors may find that when writing one section of the report, ideas come to mind that pertains to other sections, so careful note taking is encouraged. One effective approach is to organize and write the result section first, followed by the discussion and conclusions sections. Once these are drafted, write the introduction, abstract, and the title of the report. Regardless of the sequence of writing, the author should begin with a clear and relevant research question to guide the statistical analyses, result interpretation, and discussion. The study findings can be a motivator to propel the author through the writing process, and the conclusions can help the author draft a focused introduction.
Writing for Publication
Specific recommendations on effective medical writing and table generation are available [ 32 ]. One such resource is Effective Medical Writing: The Write Way to Get Published, which is an updated collection of medical writing articles previously published in the Singapore Medical Journal [ 33 ]. The British Medical Journal’s Statistics Notes series also elucidates common and important statistical concepts and usages in clinical studies. Writing guides are also available from individual professional societies, journals, or publishers such as Chest (American College of Physicians) medical writing tips, PLoS Reporting guidelines collection, Springer’s Journal Author Academy, and SAGE’s Research methods [ 34 - 37 ]. Standardized research reporting guidelines often come in the form of checklists and flow diagrams. Table Table6 6 presents a list of reporting guidelines. A full compilation of these guidelines is available at the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network website [ 38 ] which aims to improve the reliability and value of medical literature by promoting transparent and accurate reporting of research studies. Publication of the trial protocol in a publicly available database is almost compulsory for publication of the full report in many potential journals.
Graphics and Tables
Graphics and tables should emphasize salient features of the underlying data and should coherently summarize large quantities of information. Although graphics provide a break from dense prose, authors must not forget that these illustrations should be scientifically informative, not decorative. The titles for graphics and tables should be clear, informative, provide the sample size, and use minimal font weight and formatting only to distinguish headings, data entry or to highlight certain results. Provide a consistent number of decimal points for the numerical results, and with no more than four for the P value. Most journals prefer cell-delineated tables created using the table function in word processing or spreadsheet programs. Some journals require specific table formatting such as the absence or presence of intermediate horizontal lines between cells.
Decisions of authorship are both sensitive and important and should be made at an early stage by the study’s stakeholders. Guidelines and journals’ instructions to authors abound with authorship qualifications. The guideline on authorship by the International Committee of Medical Journal Editors is widely known and provides a standard used by many medical and clinical journals [ 39 ]. Generally, authors are those who have made major contributions to the design, conduct, and analysis of the study, and who provided critical readings of the manuscript (if not involved directly in manuscript writing).
Picking a target journal for submission
Once a report has been written and revised, the authors should select a relevant target journal for submission. Authors should avoid predatory journals—publications that do not aim to advance science and disseminate quality research. These journals focus on commercial gain in medical and clinical publishing. Two good resources for authors during journal selection are Think-Check-Submit and the defunct Beall's List of Predatory Publishers and Journals (now archived and maintained by an anonymous third-party) [ 40 , 41 ]. Alternatively, reputable journal indexes such as Thomson Reuters Journal Citation Reports, SCOPUS, MedLine, PubMed, EMBASE, EBSCO Publishing's Electronic Databases are available areas to start the search for an appropriate target journal. Authors should review the journals’ names, aims/scope, and recently published articles to determine the kind of research each journal accepts for publication. Open-access journals almost always charge article publication fees, while subscription-based journals tend to publish without author fees and instead rely on subscription or access fees for the full text of published articles.
Conclusions
Conducting a valid clinical research requires consideration of theoretical study design, data collection design, and statistical analysis design. Proper study design implementation and quality control during data collection ensures high-quality data analysis and can mitigate bias and confounders during statistical analysis and data interpretation. Clear, effective study reporting facilitates dissemination, appreciation, and adoption, and allows the researchers to affect real-world change in clinical practices and care models. Neutral or absence of findings in a clinical study are as important as positive or negative findings. Valid studies, even when they report an absence of expected results, still inform scientific communities of the nature of a certain treatment or intervention, and this contributes to future research, systematic reviews, and meta-analyses. Reporting a study adequately and comprehensively is important for accuracy, transparency, and reproducibility of the scientific work as well as informing readers.
Acknowledgments
The author would like to thank Universiti Putra Malaysia and the Ministry of Higher Education, Malaysia for their support in sponsoring the Ph.D. study and living allowances for Boon-How Chew.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The materials presented in this paper is being organized by the author into a book.
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Planning and Conducting Clinical Research: The Whole Process
Affiliation.
- 1 Family Medicine, Universiti Putra Malaysia, Serdang, MYS.
- PMID: 31058006
- PMCID: PMC6476607
- DOI: 10.7759/cureus.4112
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question. Engagement with clinical experts, experienced researchers, relevant stakeholders of the research topic, and even patients can enhance the research question's relevance, feasibility, and efficiency. Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental or non-experimental, sampling or census, and time features of the variables to be studied. The ultimate aims of research reporting are to present findings succinctly and timely. Concise, explicit, and complete reporting are the guiding principles in clinical studies reporting.
Keywords: clinical epidemiology; conceptual framework; literature review; research question; study designs; study reporting.
Publication types
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
Basics About Clinical Trials
What are clinical trials.
Clinical trials are research studies in which people volunteer to help find answers to specific health questions. When carefully conducted, they are the safest and fastest way to find new treatments and ways to improve health.
Clinical trials are conducted according to a plan, called a protocol, which describes:
- the types of patients who may enter the study
- the schedules of tests and procedures
- the drugs involved
- the dosages, or amount of the drug
- the length of the study
- what the researchers hope to learn from the study.
Volunteers who participate in the study must agree to the rules and terms outlined in the protocol. Similarly, researchers, doctors, and other health professionals who manage the clinical trials must follow strict rules set by the FDA. These rules make sure that those who agree to participate are treated as safely as possible.
Learn more about the basics of clinical trial participation, read first hand experiences from actual clinical trial volunteers, and see explanations from researchers at the NIH Clinical Research Trials and You Web site.
Why are clinical trials done?
Clinical trials are conducted for many reasons:
- to determine whether a new drug or device is safe and effective for people to use.
- to study different ways to use standard treatments or current, approved treatments so that they will be more effective, easier to use, or decrease certain side effects.
- to learn how to safely use a treatment in a population for which the treatment was not previously tested, such as children.
Who should consider clinical trials and why?
Some people participate in clinical trials because none of the standard (approved) treatment options have worked, or they are unable to tolerate certain side effects. Clinical trials provide another option when standard therapy has failed. Others participate in trials because they want to contribute to the advancement of medical knowledge.
Ensuring people from diverse backgrounds join clinical trials is key to advancing health equity. Participants in clinical trials should represent the patients that will use the medical products. This is often not the case—people from racial and ethnic minority and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products. Learn more about the clinical trial diversity initiative from the Office of Minority Health and Health Equity.
All clinical trials have guidelines, called eligibility criteria, about who can participate. The criteria are based on such factors as age, sex, type and stage of disease, previous treatment history, and other medical conditions. This helps to reduce the variation within the study and to ensure that the researchers will be able to answer the questions they plan to study. Therefore, not everyone who applies for a clinical trial will be accepted.
It is important to test drugs and medical products in the people they are meant to help. It is also important to conduct research in a variety of people, because different people may respond differently to treatments. FDA seeks to ensure that people of different ages, races, ethnic groups, and genders are included in clinical trials. Learn more about FDA’s efforts to increase diversity in clinical trials .
Where are clinical trials conducted?
Clinical trials can be sponsored by organizations (such as a pharmaceutical company), Federal offices and agencies (such as the National Institutes of Health or the U.S. Department of Veterans Affairs), or individuals (such as doctors or health care providers). The sponsor determines the location(s) of the trials, which are usually conducted at universities, medical centers, clinics, hospitals, and other Federally or industry-funded research sites.
Are clinical trials safe?
FDA works to protect participants in clinical trials and to ensure that people have reliable information before deciding whether to join a clinical trial. The Federal government has regulations and guidelines for clinical research to protect participants from unreasonable risks. Although efforts are made to control the risks to participants, some may be unavoidable because we are still learning more about the medical treatments in the study.
The government requires researchers to give prospective participants complete and accurate information about what will happen during the trial. Before joining a particular study, you will be given an informed consent document that describes your rights as a participant, as well as details about the study, including potential risks. Signing it indicates that you understand that the trial is research and that you may leave at any time. The informed consent is part of the process that makes sure you understand the known risks associated with the study.
What should I think about before joining a clinical trial?
Before joining a clinical trial, it is important to learn as much as possible. Discuss your questions and concerns with members of the health care team conducting the trial. Also, discuss the trial with your health care provider to determine whether or not the trial is a good option based on your current treatment. Be sure you understand:
- what happens during the trial
- the type of health care you will receive
- any related costs once you are enrolled in the trial
- the benefits and risks associated with participating.
What is FDA’s role in approving new drugs and medical treatments?
FDA makes sure medical treatments are safe and effective for people to use. We do not develop new therapies or conduct clinical trials. Rather, we oversee the people who do. FDA staff meet with researchers and perform inspections of clinical trial study sites to protect the rights of patients and to verify the quality and integrity of the data.
Learn more about the Drug Development Process .
Where can I find clinical trials?
One good way to find out if there are any clinical trials that might help you is to ask your doctor. Other sources of information include:
- FDA Clinical Trials Search. Search a database of Federally and privately supported studies available through clinicaltrials.gov. Learn about each trial’s purpose, who can participate, locations, and who to contact for more information.
- Clinicaltrials.gov. Conduct more advanced searches
- National Cancer Institute or call 1–800–4–CANCER (1–800–422–6237). Learn about clinical trials for people with cancer.
- AIDS Clinical Trials and Information Services (ACTIS) or call 1–800–TRIALS–A (1–800–874–2572). Locate clinical trials for people with HIV.
- AIDSinfo. Search a database of HIV/AIDS trials, sponsored by the National Institutes of Health’s National Library of Medicine.
What is a placebo and how is it related to clinical trials?
A placebo is a pill, liquid, or powder that has no treatment value. It is often called a sugar pill. In clinical trials, experimental drugs are often compared with placebos to evaluate the treatment’s effectiveness.
Is there a chance I might get a placebo?
In clinical trials that include placebos, quite often neither patients nor their doctors know who is receiving the placebo and how is being treated with the experimental drug. Many cancer clinical trials, as well as trials for other serious and life-threatening conditions, do not include placebo control groups. In these cases, all participants receive the experimental drug. Ask the trial coordinator whether there is a chance you may get a placebo rather than the experimental drug. Then, talk with your doctor about what is best for you.
How do I find out what Phase a drug is in as part of the clinical trial?
Talk to the clinical trial coordinator to find out which phase the clinical trial is in. Learn more about the different clinical trial phases and whether they are right for you.

What happens to drugs that don't make it out of clinical trials?
Most drugs that undergo preclinical (animal) research never even make it to human testing and review by the FDA. The drug developers go back to begin the development process using what they learned during with their preclinical research. Learn more about drug development .
Not a member?
Find out what The Global Health Network can do for you. Register now.
Member Sites A network of members around the world. Join now.
- 1000 Challenge
- ODIN Wastewater Surveillance Project
- Global Health Research Management
- UK Overseas Territories Public Health Network
- Global Malaria Research
- Global Outbreaks Research
- Sub-Saharan Congenital Anomalies Network
- Global Pathogen Variants
- Global Health Data Science
- AI for Global Health Research
- MRC Clinical Trials Unit at UCL
- Virtual Biorepository
- Epidemic Preparedness Innovations
- Rapid Support Team
- The Global Health Network Africa
- The Global Health Network Asia
- The Global Health Network LAC
- Global Health Bioethics
- Global Pandemic Planning
- EPIDEMIC ETHICS
- Global Vector Hub
- Global Health Economics
- LactaHub – Breastfeeding Knowledge
- Global Birth Defects
- Antimicrobial Resistance (AMR)
- Human Infection Studies
- EDCTP Knowledge Hub
- CHAIN Network
- Brain Infections Global
- Research Capacity Network
- Global Research Nurses
- ZIKAlliance
- TDR Fellows
- Global Health Coordinators
- Global Health Laboratories
- Global Health Methodology Research
- Global Health Social Science
- Global Health Trials
- Zika Infection
- Global Musculoskeletal
- Global Pharmacovigilance
- Global Pregnancy CoLab
- INTERGROWTH-21ˢᵗ
- East African Consortium for Clinical Research
- Women in Global Health Research
- Coronavirus
Research Tools Resources designed to help you.
- Site Finder
- Process Map
- Global Health Training Centre
- Resources Gateway
- Global Health Research Process Map
- About This Site
Downloadable Templates and Tools for Clinical Research
Welcome to global health trials' tools and templates library. please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. please click on the orange text to download each template., the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use them. to share your own templates and sops, or comment on these, please email [email protected]. we look forward to hearing from you.
These templates and tools are ordered by category, so please scroll down to find what you need.
To share your own templates and SOPs, or comment on these, please email [email protected]. We look forward to hearing from you!
- Webinar on community engagement in clinical research involving pregnant women
- Free Webinar: Science, technology and innovation for upskilling knowledge-based economies in Africa
- Open Public Consultation on “Strengthened cooperation against vaccine preventable diseases”
Trial Operations Trial Management Ethics and Informed Consent Resources Trial Design Data Management and Statistics
training
This is Degena Bahrey Tadesse from Tigray, Ethiopia. I am new for this web I am assistant professor in Adult Health Nursing Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award
I have learned lot..Thanks..
i was wondering why there is no SOP on laboratory procedures ?
Hi, Can you provide me the SOP for electronic signatures in Clinical trial
Do you have an "SOP for Telephonic site selection visit". Kindly Share on my registered mail ID
Thank you for sharing the resources. It is very kind of you.
Hi These tolls are very useful! Thank you
Do you have a task and responsability matrix template for clinical trial managment ? Best
I am very much happy to find myself here as a clinician
Dear Getrude
We have a free 14-module course on research ethics on our training centre; you'll receive a certificate if you complete all the modules and quizzes. You can take it in your own time. Just visit 'Training centre' in the tabs above, then 'short courses'.
Kind regards The Editorial Team
need modules on free online gcp course on research ethics
Estimados: me parece excelente el aporte que han hecho dado que aporta. por un lado a mejorar la transparencia del trabajo como a facilitar el seguimiento y supervisión de los mismos. Muchas gracias por ello
We also have an up to date list of global health events available here: https://globalhealthtrials.tghn.org/community/training-events/
Dear Nazish
Thank you, I am glad you found the seminars and the training courses useful. We list many training events (all relevant to Global Health, and as many of them as possible are either free or subsidised) on the 'community' web pages above. Keep an eye on those for events and activities which you can get involved with. Also, if you post an 'introduction' on the introduction group stating where you are from and your research interests, we can keep you updated of relevant local events.
Thanks so much. These are very helpful seminars. Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help.
Hi Nazish, and welcome to the Network. The items here are downloadable templates for you to use; it sounds like you may be seeking lectures and eLearning courses? If so - no problem! You can find free seminars with sound and slides here: https://globalhealthtrainingcentre.tghn.org/webinars/ , and you can find free, certified eLearning courses here: https://globalhealthtrials.tghn.org/elearning . Certificates are awarded for the eLearning courses for those scoring over 80% in the quiz at the end of each course. If you need anything else, do ask! Kind regards The Editorial Team
Hi, I am new to this website and also to the Clinical Research Industry for that matter I only am able to see the PDF of these courses, just wanted to know are these audio lectures and also happen to have audio clips that go with the pdf?
This site is impeccable and very useful for my job!!!!
Thank you for your kind comments.
Fantastic resources
I am delighted you found this website. I earlier introduced it to you because of your prolific interest in health care information and resource sharing....
Please Sign in (or Register ) to view further.
Useful Resources
Related articles.
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts BY Jai K Das
- 5 ways statistics can fool you—Tips for practicing clinicians BY Jai K Das
- How to prepare for a job interview and predict the questions you’ll be asked BY The Editorial Team
- Preparing for and Executing a Randomised Controlled Trial of Podoconiosis Treatment in Northern Ethiopia BY Henok Negussie, Thomas Addissie, Adamu Addissie, Gail Davey
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control BY WHO/ TDR
Most popular tags
- Archive (303)
- archive (104)
- data sharing (70)
- sharing (63)
- training (49)
- malaria (30)
- ACT consortium (25)
- informed consent (7)
- data management (6)
- trial management (6)
- careers (5)
- guidelines (5)
- monitoring (5)
- workshop (5)
- administration (4)
- clinical research (4)

- CREd Library , Planning, Managing, and Publishing Research
Developing a Five-Year Research Plan
Cathy binger and lizbeth finestack, doi: 10.1044/cred-pvd-path006.
The following is a transcript of the presentation videos, edited for clarity.
What Is a Research Plan, and Why Do You Need One?
Presented by Cathy Binger

First we’re going to talk about what a research plan is, why it’s important to write one, and why five years—why not one year, why not ten years. So we’ll do some of those basic things, then Liza is going to get down and dirty into the nitty-gritty of “now what” how do I go about writing that research plan.
First of all, what is a research plan? I’m sure some of you have taken a stab at these already. In case you haven’t, this is a real personalized map that relates your projects to goals. It’s exactly what it sounds like, it’s a plan of how you’re going to go about doing your research. It doesn’t necessarily just include research.
It’s something that you need to put a little time and effort into in the beginning. And then, if you don’t revisit it, it’s really a useless document. It’s something that you need to come back to repeatedly, at least annually, and you need to make it visible. So it’s not a document that sits around and once a year you pull it out and look at it.
It can and should be designed, especially initially, with the help of a mentor or colleague. And it does serve multiple purposes, with different lengths and different amounts of detail.
I forgot to say, too, getting started, the slides for this talk were started using as a jumping off point Ray Kent’s talk from last year. So some of the slides we’ve borrowed from him, so many thanks to him for that.

But why do we want to do a research plan? Well, to me the big thing is the vision. Dr. Barlow talked this morning about your line of research and really knowing where you want to go, and this is where that shows up with all the nuts and bolts in place.
What do you want to accomplish? What do you want to contribute? Most of you are at the stage in your career where maybe you have started out with that you want to change the world scenario and realized that whatever you wanted your first research project to be, really, is your entire career. You need to get that down to the point where it is manageable projects that you can do—this is where you map out what those projects are and set reasonable timelines for that.
You want to really demonstrate your independent thinking and your own creativity, whatever that is that you then establish as a PhD student, postdoc, and beyond—this is where you come back to, okay, here’s how I’m going to go about achieving all of that.
This next point, learning to realistically gauge how long it takes to achieve each goal, this for most of us is a phenomenally challenging thing to do. Most of us really overestimate what we can do in a certain amount of time, and we learn the hard way that you can’t, and that’s another reason why you keep coming back to these plans repeatedly and learning over time what’s really manageable, what’s really doable, so we can still reach our goals and be very strategic about how we do that.
When you’re not strategic, you just don’t meet the goals. Your time gets sucked into so many different things. We need to be really practical and strategic.
Everything we do is going to take longer than we think.
I think this last one is something that maybe we don’t talk about enough. Really being honest with ourselves about the role of research in our lives. Not all of you are at very high-level research universities. Some of you have chosen to go elsewhere, where research maybe isn’t going to be playing the same role as it is for other people. The research plan for someone at an R One research intensive university is going to look quite different from someone who is at a primary teaching university. We need to be open and practical about that.

Getting sidetracked. I love this picture, I just found this picture the other day. This feels like my life. You can get pulled in so many different directions once you are a professor. You will get asked to do a thousand different things. There are lots of great opportunities that are out there. Especially initially, it’s tempting to say yes to all of them. But if you’re going to be productive, you have to be very strategic. I’m going to be a little bit sexist against my own sex here for a minute, but my observation has been that women tend to fall into this a little bit more than men do in wanting to say yes and be people pleasers for everything that comes down the pike.
It is a professional skill to learn how to say no. And to do that in such a way that you are not burning bridges as you go down the path. That is a critical skill if you are going to be a successful researcher. I can’t tell you how many countless people I’ve seen who are very bright, very dedicated, have the skills that it takes in terms of doing the work—but then they are not successful because they’ve gotten sidetracked and they try to be too much of a good citizen, give too much service to the department, too much “sure I’ll take on that extra class” or whatever else comes down the line.
I just spoke with a professor recently who had something like five hours a week of office hours scheduled every single week for one class. Margaret is shaking her head like “are you kidding?” That’s crazy stuff. But he wanted to really support his students. His students loved him, but he was not going to get tenure. That’s the story.
So we have to be very thoughtful and strategic, and what can help you with this, and ASHA very firmly recognizes which is why we’re here—is that your mentors in your life should be there to help you learn these skills and learn what to say yes to, and learn what to say no to. I’ve learned to say things like, “Let me check with my mentor before I agree to that.” And it gives you a way out of that. The line that I use a lot is, “Let me check with my department head” or, I just said this to somebody last week, “I just promised my department head two weeks ago that I would only do X number of external workshops this year, so I’m going to have to turn this one down.” Those are really important skills to develop.
And having that research plan in place that you can go back to and say, know what, it’s not on my plan I can’t do it. If I do it—I have to go back to my research plan and figure out what I’m going to kick off in order to review this extra paper, in order to take on this extra task. The plan also helps me to know exactly what to say no to. And to be very direct and have a very strong visual.
I actually have my research plan up on a giant whiteboard in my office, so I can always go back to that and see where I am, and I can say, “Okay, what am I going to kick off of here? Nothing. Okay, I have to say no to whatever comes up.” Just be strategic. This is where I see most beginning professors really end up taking that wrong fork in the road—taking that right instead of that left, and ending up not being the successful researcher that they wanted to be.

What evidence supports research planning? This was something Ray Kent had found. That a recent analysis had found that postdoc scholars who developed a written plan with their postdoc advisers were much more productive than those who didn’t. And your performance during a postdoc—and I know many of you have either finished your postdoc or decided not to—so more simply, just during those first six years, the decisions you make really do establish the foundation for the rest of your professional life. It’s very important to get started and get off on the right foot.

I love this quote, I just found it the other day: “Productivity is never an accident. It is always the result of a commitment to excellence, intelligent planning, and focused effort.”
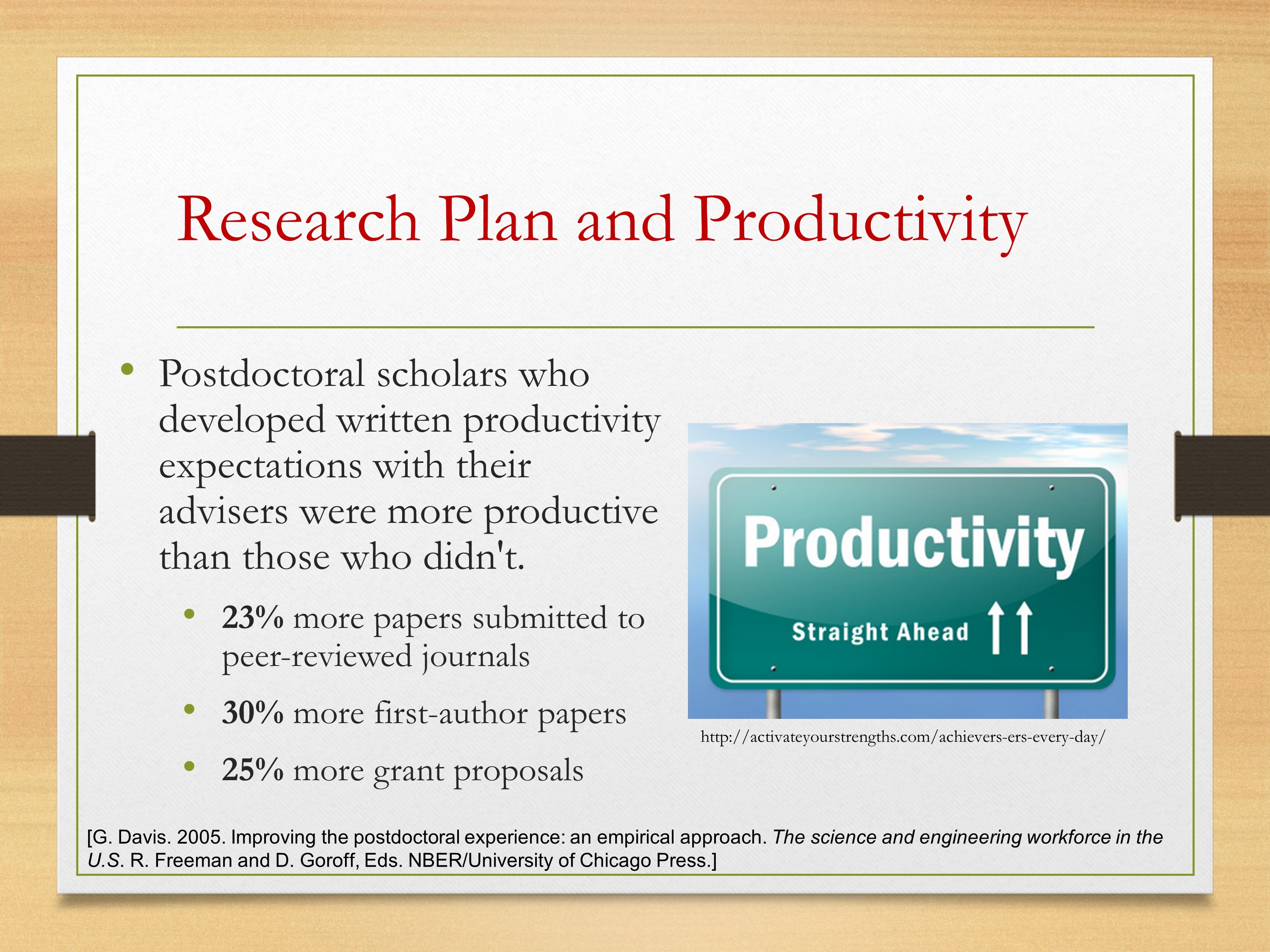
What we see with productivity is that postdoc scholars who developed written productivity expectations with their advisers were more productive than those who didn’t. You see 23% more papers submitted, 30% more first-author papers, and more grant proposals as well.

So why five years? I’m going to start with number 5. It’s long enough to build a program of research, but short enough to deal with changing circumstances. That’s really the long and the short of the matter. As well as these other things as well that I won’t take the time to go through point by point.
What Should a Five-Year Plan Include?
Presented by Lizbeth Finestack

So, thinking about a five-year research plan, I like to think about it like your major “To Do List.” It’s what you’re going to accomplish in five years. Start thinking: What is going to be on my to do list?

You can also think about it like: Okay, I have research. I’ve got to do research. Maybe think about this as one big bucket, or maybe one humongous silo. I have some farm themes going on. Cathy was just on a farm, so I thought I’d tie that in.
So here’s your big silo. You can call that your research silo.

But more realistically, you need to think about it like separate buckets, separate silos, where research is just one of those. Just like Cathy indicated, there’s going to be lots of other things coming up that you’re going to have to manage. They are going to have to be on your to do list, you need to figure out how to fit everything in.
What all those other buckets or silos are, are really going to depend on your job. And maybe the size of the silos, and the size of the buckets are going to vary depending on where you are, what the expectations are at your institution.
That’s important to keep in mind, and Cathy said this too, it’s not going to be the same for everyone. The five-year plan has to be your plan, your to do list.

Here are some buckets or some silos that I have on my list and the way that I break it up, this is just one example, take it or leave it.
The first three are all very closely related, right? Thinking about grants, thinking about research, thinking about publications. I’m going to define grants as actual writing, getting the grant, getting the money.
Research is what you’re going to do once you get that money. Steps you need to take before you are getting the money. Any sorts of projects, the lab work, that’s why I have the lab picture there. Of course, publications are part of the product—what’s coming out of the research—but it also cycles in because you need publications to support that you are a researcher to apply for funding and show you have this line of research that you’ve established and you’ll be able to continue. So, those first three are really closely related. And that’s where I’ll go next. And then have teaching and service you see here at the bottom.

So thinking about research, in that broad sense. As you’re writing your five-year plan you’re going to want to think of, “What’s my long-term goal?” There’s lots of ways to think of long-term goals. You could think, before I die, this is what I want to accomplish. For me I kind of have that. My long-term goal is that I’m going to find the most effective and efficient interventions for kids with language impairment. Huge broad goal. But within that I can start narrowing it down.
Where am I within that? Within the next five years or maybe the next ten years, what is it I want to accomplish towards that goal. Then start thinking about: In order to accomplish that goal, what are the steps I need to take? Starting to break it down a little bit. Then it’s also going to be really important to think: where are you going to start? Where are you now? What do you need to have happen? And is it reasonable to accomplish this goal within five years? Is it going to take longer? Maybe you could do it in a couple years? Start thinking about the timeline that’s going to work for you.
Then thinking about your goals—and everyone’s program is going to be different, like I said, there’s going to be a lot of individual needs, preferences. So it might be the case that you have this one long-term goal that you’re aiming for. Long-term goal in the sense of, maybe, what you want to study in your R01, perhaps something like that. But in order to get to that point, you’re going to have several short-term goals that need to be accomplished.
Or maybe it’s the case that you have two long-term goals. And with each of those you’re going to have multiple short-term goals that you’re working on. Maybe the scope of each of these long-term goals is a little bit less than in that first scenario.
Start thinking about my research, what I want to do, and how it might fit into these different circumstances.

Also thinking about your goals, this is a slide from Ray Kent from last year, was thinking about the different types of projects you might want to pursue, and thinking about ones that are definitely well on your way. They are safe bets. You have some funding. They are going to lead directly into your longer-term plan.
Those are going to be your front burner—things you can easily focus on. That said, don’t put everything there.
You can also have things on the back burner. Things that really excite you, might have huge benefits, big pay. But you don’t want to spend all of your time there because they could be pretty risky.
Start thinking about where you’re putting your time. Are you putting it all on this high-risk thing that if it doesn’t pan out you’re going to be in big trouble? Or balancing that somewhat with your front burner. Making that steady progress that will lead directly to help fund an R01 or whatever the mechanism that you’re looking for.
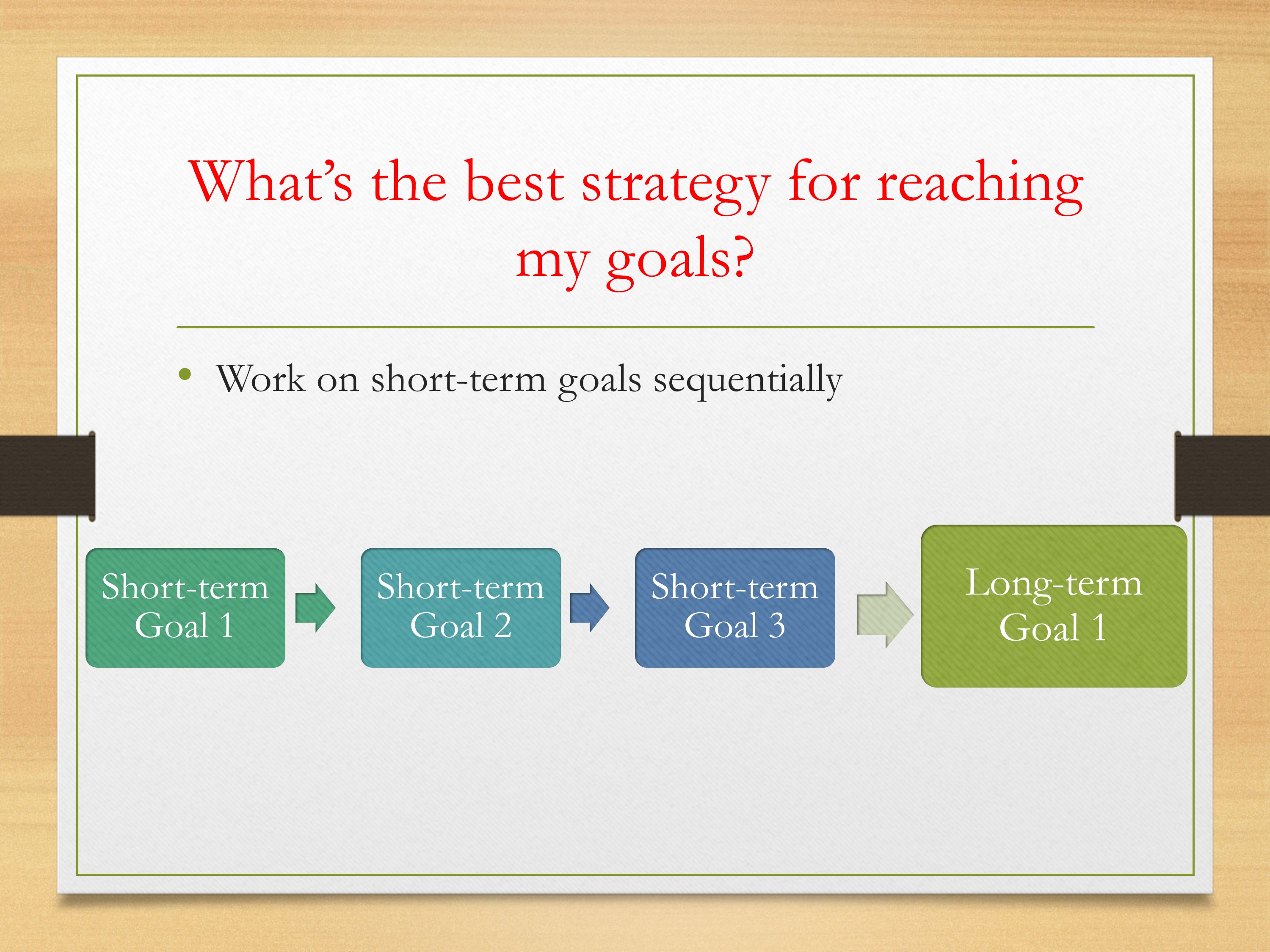
Then, thinking about your goals—if you have multiple long-term goals, or thinking about your short-term goals, you could think about your process. Is it something where you need to do study 1 then study 2, then study 3—each of those building on each other, that’s leading to that long-term goal. In many cases, that is the case, where you have to get information from the first study which is going to lead directly to the second study and so forth.
Or is it the case that you can be working on these three short-term goals simultaneously? Spreading your resources at the same time. Maybe it will take longer for any one study, but across a longer period of time you’ll get the information that you need to reach that long-term goal.
Lots and lots of different ways to go about it. The important thing is to think about what your needs are and what makes the most sense for you.
Here’s my own little personal example. Starting over here, I have my dissertation study. My dissertation study was this early efficacy study looking at one treatment approach using novel forms that really can’t generalize to anything too useful, but it was important.
Then I did a follow up study, where I was taking that same paradigm, looking to see where kids with typical development perform on the task. So I have these two studies, and they served as my preliminary studies for an R03. So I just finished an R03 where I was looking at different treatment approaching for kids with primary language impairment. At the same time, while conducting my R03, I’m also looking at some different approaches that might help with language development. Also conducting surveys to see what current practices are.
I have these three projects going on simultaneously, that are going to lead to a bigger pilot study that are going to feed directly into my R01. All of this will serve as preliminary data to go into an R01.
Start thinking about your projects, what you have. Maybe starting with your dissertation project or work that you’re doing as a postdoc as seeing how that can feed into your long-term goal. And really utilizing it, building on it, to your benefit.

That’s all fine and dandy. You can draw these great pictures. But you still have to break it down some more. It’s not like, “Oh, I’m just going to do this project.” There are other steps involved, and lots of the time these steps are going to be just as time consuming.
Starting to think about: well, if you have the funding. Saying, “I want to do this study, but I have no money to do it.” What are the steps in order to get the money to do it? Do you have a pilot study? What do you need?
Start thinking about the resources? Do you need to develop stimuli, protocols, procedures? Start working on that. All of these can be very time consuming, and if you don’t jump on that immediately, it’s going to delay when you can start that project.
Thinking about IRB. Relationships for recruitment, if you’re working with special populations especially? Do you have necessary personnel, grad students, people to help you with the project? Do you need to train them? What’s the timeline of the study?
Start thinking about all these pieces, and how they are going to fit in that timeline.
This is one way that might help you start thinking about the resources that you need. This is online—Ray Kent had it in his talk, and when I was doing my searches I came across it too and I have the website at the end. Just different ways to think about the resources you might need.
Let’s talk about mapping it out. You have your long-term goal. You have your short-term goals. You’re breaking it down thinking about all those little steps that you need to accomplish. We gotta put it on a calendar. When is it going to happen?
This is an example—you might have your five years. Each month plugging in what are you going to accomplish by that time. Maybe it’s when are grant applications due? It’s going to be important to put those on there to go what do I need to do to make that deadline. Maybe it’s putting when you’re going to get publications out. Things like that.
Honestly, looking at this drives me a little bit crazy, it seems a bit overwhelming. But it’s important to get to these details.
This is an example from, I did Lessons for Success a few years ago and they had their format for doing your plan. I wrote out all my projects, started thinking about all the different aspects. So if something like this works for you, by all means you could use that type of procedure.
Here’s a grid that Ray Kent showed last year. We’re breaking it down by semester. Thinking about each of your semesters, what manuscripts you’re going to be working on, what data collection, your grant applications. Starting to get into some of those other buckets: course preparation, conference submissions.
We also need to include teaching and service.
You probably can’t see this very well. This is similar to that last slide Ray Kent had used last year.
I have my five year plan: what studies I want to accomplish, start thinking about breaking it down.
Then at the beginning of each semester, I fill in a grid like this. Where at the top, I have each of my buckets. I have my grant bucket, my writing bucket which is going to include publications. I also include doing article reviews in my writing bucket, because that’s my writing time. My teaching bucket, my research bucket. Then at the end, my service bucket.
At the beginning of the semester, I think about the big things I want to accomplish. I list those at the top. Then at the beginning of each month, I say, okay what are the things I’m going to accomplish this month, write those in. Then at the beginning of each week, I start looking at whether I’m dedicating any time to the things I said I was going to do that month. I start listing those out saying, this is the amount of time I’m going to spend on that. Of course, I have to take data on what I actually do, so I plug in how much time I’m spending on each of the tasks. Then I graph it, because that’s rewarding to see how much time you’re spending on things, and I get a little side-tracked sometimes.
Think about a system that will help you keep on track, to make sure you’re meeting the goals that you want to meet in terms of your research. But also getting the other things done that you need to get done in terms of teaching and service.
Discussion and Questions
Compiled from comments made during the Pathways 2014 and 2015 conferences. (Video unavailable.)
Building Flexibility into Your Five-Year Plan Comments by Ray Kent, University of Wisconsin-Madison
The five-year plan is not a contract. It’s a map or a compass. A general set of directions to help you plan ahead. It’s not even a contract with yourself, because it will inevitably be revised in some ways.
Sometimes cool things land in your lap. Very often it turns out that through serendipity or whatever else, you find opportunities that are very enticing. Some of those can be path to an entirely new line of research. Some of them can be a huge distraction and a waste of time. It’s a really cool part of science that new things come along. If we put on blinders and say, “I’m committed to my research plan,” and we don’t look to the left or the right, we’re really robbing ourselves of much of the richness of the scientific life. Science is full of surprises, and sometimes those surprises are going to appear as research projects. The problem is you don’t want to redirect all your time and resources to those until you’re really sure they are going to pay off. I personally believe, some of those high risk but really appealing projects are things you can nurse along. You can devote some time and build some collaborations – far enough to determine how realistic and viable they are. That’s important because those things can be the core of your next research program.
It’s very easy to get overcommitted. We all know people who always say “yes”—and we know those people, and they are often disappointing because they can’t get things done. It’s important to have new directions, but limit them. Don’t say, “I’m going to have 12 new directions this year.” Maybe one or two. Weigh them carefully. Talk about them with other people to get a judgment about how difficult it might be to implement them. It enriches science: not only our knowledge, but the way we acquire new knowledge. A psychologist, George Miller—this is the guy with the magic number 7 +- 2—when we interviewed him years ago at Boystown, he said, “My conviction is that everybody should be able to learn a new area of study within three months.” That’s what he thought for a scientist was a goal.
The idea is that you can learn new things. And that’s very important because when you think of it in terms of a 30-year career, how likely is it that the project that you’re undertaking at age 28 is the same project you’ll be working on at age 68? Not very likely. You’re going to be reinventing yourself as a scientist. And reinventing yourself is one of the most important things you can do, because otherwise you’re going to be dead wood. Some projects aren’t worth carrying beyond five or ten years. They have an expiration date.
Building Risk into Your Five-Year Plan Comments by Ray Kent, University of Wisconsin-Madison
Your doctoral study should generally be low-risk research. As you move into a postdoctoral fellowship, think about having two studies—one low-risk, one high-risk with a potential for high impact. At this time you can begin to play the risk factor a little bit differently.
When you are tenure-track you can have a mix of significance with low-risk and high-risk studies. And when you are tenured, then you can go for high risk, clinical trials, and collaborations. Because you have established your independence, so you do not need to worry about losing your visibility. You can be recognized as a legitimate member of the team.
As you plan your career, you should take risk into account. Just as you manage your money taking risk into account, we should manage our careers taking risk into account. I have met people who did not really think about that, and they embarked on some very risky procedures and wasted a lot of time and resources with very little to show for it. For example, don’t put everything into an untested technology basket. You want to be using state of the art technology, but you want to be sure it is going to give you what you need.
Other Formats and Uses of Your Research Plan Audience Comments
- If you do your job right with your job talk, there’s a lot of cross-pollination between your job talk and your research plan. Ideally your job talk tells your colleagues that this is the long-term plan that you have. And they shouldn’t be surprised when you submit a more detailed research plan. They should say, “okay this is very consistent with the job talk.” In my view, the job talk should be a crystal summary of the major aspects of that research program. Of course, much of the talk will be about a specific project or two—but it should always be embedded within the larger program. That helps the audience keep sight of the fact that you are looking at the program. You can say that this is one project that I’ve done, and I plan to do more of these, and this is how they are conceptually related. That’s a good example of why the research plan has multiple purposes – it can be a research statement, it can be the core of your job talk, it can be the nature of your elevator message, and it can be a version of your research plan for a K award application or R01 application or anything else of that nature.
- I think what’s useful is to actually draft your NIH biosketch. The new biosketch has a section called “contributions to science.” It’s really helpful to think about all your projects. It’s hard to start with a blank sheet of paper. But to have it in the format of a biosketch can be really helpful.
Avoiding Overcommitment Audience Comments
- One of the things that is amazing about planning is that if you put an estimate on the level of effort for each part of your plan, you’ll quickly find that you are living three or four lives. Some 300% of your time is spent. It’s helpful for those of us who might share my lack of ability to see constraints or limitations to reel it back and say, “I have a lot on my plate.” Which allows you to say no—which is not something we all do very well when it comes to those nice colleagues and those people you want to impress nationally and connect with. But it allows you to look at what’s planned and go, “I don’t know where I’d find the time to do that.” Which will hopefully help you stay on track.
- I keep a to do list, but I also keep a “to not do” list. One of the things I will keep on my plan is the maximum number of papers I will review in a year. If I hit that number in March, that’s it. I say no to every other paper that comes down the pike. That’s something to work out with your mentor as far as what’s realistic and what’s okay for you. Every time I get a request, I think, “That’s my reading and writing time, so what am I willing to give up. If it means I won’t be able to write on my own paper this week, am I willing to do this?”
Staying on Schedule with Reading, Writing, and Reviewing Audience Comments
- You have to do what works for you. Some people do wait for big blocks of time for writing—which are hard to come by. But the most important thing is to block off your time. Put it on your schedule, or it is the first thing that will get pushed aside.
- Another thing I’ve done with some of my colleagues is writing retreats. So maybe once a year, twice a year, we’ll get together. Usually we’ll go to a hotel or somewhere, and we’re just writing. It’s a great way to get a jumpstart on a project. Like, I need to sit down and start this manuscript, and you can keep going once you’ve got that momentum.
- My input would be that you really have to write all the time, every day. It’s a skill. I’ve found that if I take time off, my writing deteriorates. It’s something you need to keep up with.
- I would look at it like a savings account that you put money into on a daily, weekly, monthly basis. The flip side of writing is reading. I would read constantly, widely, and not just in the discipline. That will give you not only a breadth in terms of your understanding of your field and the world around you, but it will also give you an incentive to make your own contributions. I think we don’t talk enough about the comprehensive side to this, and being receptive to the reading. I have a book, or something, by my bedside every night. And I read that until I fall asleep every night. And it’s done me in good stead over the years.
- Reviewing articles can help advance your career, but it is something you need to weigh carefully as a draw on your time. You get a lot from it. You get to see what’s out there. You get to see what’s coming down the pipe before publication. To me that’s a huge benefit. You get to learn from other people’s writing, and that’s part of your reading you get to do. But it is time consuming. And it depends on the kinds of papers you get. Sometimes you’re lucky and sometimes you’re not.
- If someone else is reviewing your grants and your articles, at some point you owe it back. You should at least be in break-even mode. Now, pre-tenure or postdoc your mentor should be doing that or senior faculty in the department. But there are so many articles to review. I review so many articles, but I am also at the tail end of my career. The bottom line is, if you don’t put on your schedule that if you don’t put time on your schedule for reading, reviewing articles forces you to look at and think about the literature, so you can be accomplishing what you owe back to the field—and at the same time, staying one step ahead knowledge wise. It forces you to do what you should be doing all along, which is keeping up with the literature.
Further Reading: Web Resources
Golash-Boza, T. (2014). In Response to Popular Demand, More on the 5-Year Plan. The Professor Is In . Available at http://theprofessorisin.com/2014/05/09/in-response-to-popular-demand-more-on-the-5-year-plan
Kelsky, K. (2010). The Five-Year Plan for Tenure-Track Professors. Get a life, PhD . Available at http://getalifephd.blogspot.com/2010/07/five-year-plan-for-tenure-track.html
National Association of Geoscience Teachers (NAGT). (2012). Planning Worksheets . Planning your Research Program (Available from the Science Education Resource Center at Carelton College Website at http://serc.carleton.edu/).
Pfirman, S., Bell, R., Culligan, P., Balsam, P. & Laird, J. (2008) . Maximizing Productivity and Recognition , Part 3: Developing a Research Plan. Science Careers. Available at http://sciencecareers.sciencemag.org/career_magazine/previous_issues/articles/2008_10_10/caredit.a0800148
Cathy Binger University of New Mexico
Lizbeth Finestack University of Minnesota
Based on a presentation and slides originally developed by Ray Kent, University of Wisconsin-Madison.
Presented at Pathways (2015). Hosted by the American Speech-Language-Hearing Association Research Mentoring Network.
Pathways is sponsored by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) through a U24 grant awarded to ASHA.
Copyrighted Material. Reproduced by the American Speech-Language-Hearing Association in the Clinical Research Education Library with permission from the author or presenter.

Clinical Research Education
More from the cred library, innovative treatments for persons with dementia, implementation science resources for crisp, when the ears interact with the brain, follow asha journals on twitter.

© 1997-2024 American Speech-Language-Hearing Association Privacy Notice Terms of Use
Forms, Tools, & Templates
Filter by Category(ies) Expand/Collapse Category Filter
- Budget/ Finance
- Communication
- Data Management
- OCR Applications
- Project Management
- Protocol Templates
- Recruitment
- Regulatory File/ Investigator Site File
- Source Documentation
- Study Start-Up
- Third Party/ Vendors
- Trial Documents
- Trial Master File
- Regulatory File
0">Matching of items
0 && show_pagination">Page of
There are no matching items
- Forms, Tools, & Templates Description Category(ies) Keyword(s)
- 02.04.02 Investigator's Brochure Addendum Log Track versions of the Investigator’s Brochure Trial Documents TrialDocuments
- 01.01.01 Work Instructions TMF - PennBox Instructions explaining training requirements, user roles, access, and use of the TMF in the Veeva Vault system Trial Master File TrialMasterFile Penn Box
- 01.01.01 Work Instructions TMF - Veeva Instructions explaining training requirements, user roles, access, and use of the TMF in the PennBox system. Trial Master File TrialMasterFile Veeva
- 01.02.03 Notice of Disclosure of FCOI Template for written disclosure of FCOI IND; IDE; Sponsor INDIDESponsor
- 01.02.03 Statement of Financial Interest - Sponsor Team Member Form to be used by Penn Faculty or staff of the Sponsor team to state presence/absence of financial interests for a particular study. Sponsor Sponsor
- 01.03.01 DSMB Charter Used to clearly describe how the DSMB will function, including frequency of meetings, data to be reviewed, how safety items will be communicated, etc. DSMB DSMB
- 01.03.02 DSMB Contact List Used to compile contact information for the DSMB members and their administrative support staff. May be useful as a reference during trial progress. DSMB DSMB
- 01.03.03 DSMB Meeting Minutes Used to document discussions during DSMB meetings. Includes documentation of attendance and disclosure of financial conflicts. DSMB DSMB
- 01.03.03 DSMB Outcome Email Letter Used to document the outcome of the DSMB meeting to provide to the PI(s). The PI may need this confirmation to provide to their IRB or other local regulatory review committee. DSMB DSMB
- 01.03.03_DSMB_Report Used to provide a summary of trial data to the DSMB. Specific section will differ by study, may be revised based on DSMB request. DSMB DSMB
- 01.03.05 DSMB Qualification Form Used to document potential DSMB member's agreement to serve as part of the DSMB. Includes description of confidentiality and disclosure of financial conflict of interest provisions. DSMB DSMB
- 01.05.01 Correspondence Log Template Log for documenting correspondence including the date, time, participants, and summary of discussion Project Management ProjectManagement
- 02.01.01 Investigator Brochure Template Guide for developing an Investigator's Brochure Trial Documents TrialDocuments Trial Document; Investigator; brochure
- 03.01.01 Expanded Access eIND Instructions for submitting an emergency IND request IND IND
- 03.01.01 Expanded Access sIND Instructions for submitting a (compassionate use) single patient IND request IND IND
- 03.01.01 IND Application Template Template for an application to the FDA for an Investigational New Drug (IND) IND; IDE; Sponsor INDIDESponsor IND, Sponsor, FDA
- 03.01.01 Submitting to the FDA This document describes the different way a submission can be sent to the FDA. It applies to clinical research (IND/IDE) and also to Expanded Access of drugs and devices. Sponsor Sponsor
- 03.03.02 IDE Progress Report Template Template for completing an IDE Progress Report IDE IDE
- 03.03.02 IND Annual Report Template Template for completing an IND Annual Report IND IND Investigator Annual Report
- 03.03.03 Closing an IND Guide to Sponsor How to discontinue an IND with the FDA. Definitions of the various IND statuses are included below for reference purposes. IND IND IND, Sponsor, FDA
- 03.04.01 Regulatory History Log Template for tracking all regulatory submissions of an IND/IDE IND; IDE; Sponsor INDIDESponsor
- 05.02.02 PI Signature Page The document to be signed by the Principal Investigator of a study at each revision of the study's protocol. Protocol Templates ProtocolTemplates
- 05.02.07 PI Qualification Form Template for documenting the qualifications of a Principal Investigator Sponsor Sponsor
- 05.02.10 Site FCOI This work instruction assists the Regulatory Specialists with understanding what documentation is required to ensure the qualification of the sponsor team members and individuals acting as vendors and to determine how qualifications should be filed in the Trial Master File (TMF).The sponsor must ensure qualification of its team members and vendors. IND; IDE INDIDE
- 05.02.19 IDE Investigator Agreements The investigator agreement of compliance to all requirements of the investigational plan, IDE regulations, and other applicable regulations of the FDA for investigational devices. IDE IDE Statement,Investigator
- 07.01.01 Work Instructions - Pharmacovigilance Work instructions on sponsor pharmacovigilance (safety) management. This includes a discussion of the sponsor responsibilities related to pharmacovigilance, as well as instructions on conducting the activities, and additional reference resources. Sponsor Sponsor
- 07.02.02 SAE Form Form used to document an SAE that occurs in a trial and to report the relevant information to the sponsor. Trial Documents TrialDocuments
- 09.01.01 GLP Qualification Guide Guide to ensuring vendors providing investigational product are qualified and follow Good Laboratory Practices Third Party/ Vendors ThirdPartyVendors
- 09.01.01 Manufacturing Evaluation The form to be used by Sponsors to conduct an initial manufacturing evaluation of quality for manufacturers. Third Party/ Vendors ThirdPartyVendors
- 09.02.03 Transfer of Obligations Guide to transferring IND/IDE sponsor obligations to another party IND; IDE INDIDE
- 21 CFR Part 11 for EMR Memo- Penn Medicine Electronic Health Records in Support of Clinical Research Communication; Study Start-Up CommunicationStudyStartUp part 11, 21 cfr part 11, EMR, compliance, pennchart
- CLIA, CAP, Lab References and Lab Director’s CV_HUP CLIA, CAP, Lab References and Lab Director’s CV. Documents obtained annually and posted with permission from Dept. of Pathology and Laboratory Medicine, Penn Medicine-HUP Regulatory File/ Investigator Site File; Source Documentation RegulatoryFileInvestigatorSiteFileSourceDocumentation CLIA, CAP, Lab References
- CLIA, CAP, Lab References and Lab Director’s CV_PRESBY CLIA, CAP, Lab References and Lab Director’s CV. Documents obtained annually and posted with permission from Dept. of Pathology and Laboratory Medicine, Penn Medicine-Presbyterian Regulatory File/ Investigator Site File; Source Documentation RegulatoryFileInvestigatorSiteFileSourceDocumentation CLIA, CAP, Lab References, Presby
- CRF Testing Script - Blank Template CRF Testing Script - Blank Template Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF Testing Tracker CRF Testing Tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF build and Schedule of Events tracker CRF build and Schedule of Events tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRSPR Email Listserve Clinical Research Staff Portal and Registry (CRSPR) is an email listserve to communicate important, timely information to Penn Medicine's Clinical Research Professionals. Needs PennKey login. Sign up by creating a profile, to receive emails from OCR, IRB, CHPS, etc. OCR Applications OCRApplications CRSPR, listserve, application, email, portal, registry
- Case Report Form Amendments Log Track Case Report Form (CRF) versions and changes Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile CRF
- Clinical Research Resource Feasibility Assessment Budget/ Finance BudgetFinance
- Clinical Trial Protocol Template This protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic, vaccine or device). Protocol Templates ProtocolTemplates
- Close Out Visit Checklist - Investigator Checklist for closing out a protocol Monitoring Monitoring
- Close Out Visit Checklist- Sponsor Investigator Checklist for closing out a protocol by a Sponsor or Sponsor Investigator Monitoring Monitoring
- Closing IDE Guide to Sponsor How to discontinue an IDE with the FDA. Definitions of the various IDE statuses are included below for reference purposes. IDE IDE
- Cost Finder Tool with research rates for both Hospital Billing (HB) and Professional Billing (PB), components of an item/service/procedure. Select at least one entity (HUP, PPMC, PAH OR CCH) prior to searching.Pricing may vary by entity. Ability to search by CPT code (preferred) or procedure name. OCR Applications; Study Start-Up OCRApplicationsStudyStartUp Cost Finder, finance, start up
- Data Safety Monitoring Plan (DSMP) With Guidance This template is intended to be used to develop a data safety monitoring plan. Monitoring Monitoring DSMP, monitoring, Data Safety Monitoring Plan
- Database Activation Authorization Database Activation Authorization Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Database Lockdown Checklist and CRMS Examples Database Lockdown Checklist and CRMS Examples Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crms, source
- Delegation of Responsibility and Signature Log Document study personnel and their role on the study, as delegated by the Principal Investigator. Also serves as a signature log to identify signatures on research forms. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Device Investigational Plan Template Guide to developing an Investigational Plan for medical device research IDE IDE
- Device Product Information This guidance document assists US IDE, IND, and foreign clinical research device applicant holders in determining what product information is needed and where to place device product information. IDE IDE
- Electronic Data Management Tool This tool is used to assist the determination of what data management tool would be most beneficial for your research study. Budget/ Finance BudgetFinance
- Electronic Database Build and Activation Summary Plan Electronic Database Build and Activation Summary Plan Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation data management , sponsor, source documentation
- GLP Vendor Qualification Form Template to qualify non-clinical vendors Third Party/ Vendors ThirdPartyVendors
- Good Laboratory Practice (GLP) Qualification Guide Guide to ensuring vendors providing investigational product are qualified and follow Good Laboratory Practices IND; IDE; Sponsor INDIDESponsor Third Party/Vendors; Sponsor; Investigational Product
- ICH GCP Essential Documents Table of regulatory files that should be maintained by the PI and Sponsor throughout a clinical research study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- IND Enabling Nonclinical Safety Study Work Instructions These work instructions are intended as guidance for personnel who (i) conduct or assist in the conduct of Nonclinical Safety Studies (NSS) to be submitted in support of an IND Application or (ii) have oversight of such activities. Although such studies usually occur prior to the submission of an IND, these work instructions also apply to NSS performed after IND approval and in support of clinical development. If an individual is subject to a FINANCIAL CONFLICT OF INTEREST (FCOI) management plan issued by the Vice Provost for Research, the conditions of the management plan will apply to the conduct of NSS. IND IND
- IND IDE Exemption Amendment Risk Assessment This form provides instructions, and may be used to document, the Sponsor Investigator’s risk assessment for minor changes to a protocol that has previously received an IND or IDE exemption determination. Protocol Templates; Sponsor ProtocolTemplatesSponsor
- IND-IDE Sponsor Responsibilities Guide Responsibilities of an IND/IDE Sponsor IND; IDE INDIDE
- IND-IDE Submision Planner Checklist to assist in preparing the final IND/IDE application package to be submitted to FDA. IND; IDE INDIDE
- Informed Consent Form (ICF) Version Log Tracking log of approvals for Informed Consent Form Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Investigational Device Exemption (IDE) Application Instructions Instructions for completing the application for an Investigational Device Exemption (IDE) IND; IDE; Sponsor INDIDESponsor
- Investigational Device Exemption (IDE) Application Template Template for an application to the FDA for an Investigational Device Exemption IND; IDE; Sponsor INDIDESponsor
- Investigational Device Exemption (IDE) Cover Letter Template Template cover letter to accompany the Investigational Device Exemption (IDE) application IND; IDE; Sponsor INDIDESponsor
- Investigational Product Accountability Study Log Study-level investigational product accountability log (all subjects on one log) Sponsor Sponsor
- Investigational Product Accountability Subject Log Subject specific investigational product accountability log Sponsor Sponsor
- Memo from Penn IRB Memo from Penn IRB regarding the IRB not disclosing names of its members. This is a necessary component of the IDE application. IDE IDE
- Monitoring Analysis Template This tool is intended to assist in the planning for monitoring a clinical trial. Monitoring Monitoring
- Monitoring Assessment Guidance Document This document is designed to assist study teams with completing an appropriate monitoring summary for the Penn IRB at the time of continuing review. Monitoring Monitoring
- Monitoring Close Out Visit Template Monitoring visit report template for the conduct of a close out monitoring visit at the end of a clinical trial. Monitoring; Sponsor; Trial Master File MonitoringSponsorTrialMasterFile
- Monitoring Findings Template Monitoring Findings Template Monitoring Monitoring findings, monitoring, site
- Monitoring Visit Report - Sponsor Summary Template for reporting monitoring findings to Sponsor Monitoring Monitoring
- Monitoring Visit Tracking Log Log used to document monitoring visits over the course of a trial Monitoring Monitoring
- Note to File Template To be used to create a Note to File which are written to identify a discrepancy or problem in the conduct of the clinical research study. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- On-Site Query Report Form Worksheet for documenting the identification and resolution of queries Monitoring Monitoring
- Participant Visit Schedule Tool for tracking the proposed and actual dates for subject study visits Study Start-Up StudyStartUp
- PennCRMS CRF Guidelines and Tips PennCRMS CRF Guidelines and Tips Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- PennChart - Patient Registration Form EMPI If you need your patient/subject setup in PennChart within 24 hours Budget/ Finance BudgetFinance
- Pre-Screen Phone Script (Incoming Call) Pre-screen phone script template for incoming calls from potential research participants IND; Recruitment; Regulatory File/ Investigator Site File INDRecruitmentRegulatoryFileInvestigatorSiteFile
- Pre-Screen Phone Script (Outgoing Call) Pre-screen phone script template for outgoing calls to potential research participants Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Principal Investigator (PI) Qualification Form Template for documenting the qualifications of a Principal Investigator Third Party/ Vendors ThirdPartyVendors PI; Qualification, principal investigator
- Principal Investigator Compliance Assessment (PICA) The Principal Investigator Compliance Assessment (PICA) is a tool which can be used to monitor or assess the overall conduct of a study. This document is required for all active, high risk studies conducted at Penn. Monitoring Monitoring
- Prospective Reimbursement Analysis/ Medicare Coverage Analysis - Budget Template Template for completing the prospective reimbursement analysis/ medicare coverage analysis and industry sponsored clinical research budget. Budget/ Finance; Regulatory File/ Investigator Site File BudgetFinanceRegulatoryFileInvestigatorSiteFile
- Prospective Study Design with no Investigational Product (IP) Template Use this template to develop a clinical research protocol that does not involve an investigational product. E.g. Comparative effectiveness study, a cohort design, case control study, etc. Protocol Templates ProtocolTemplates
- Protocol Adverse Event Log A Log for recording adverse events Regulatory File RegulatoryFile Adverse Event, Adverse, Event, Log
- Protocol Amendments Log Track versions of the IRB approved Protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Deviation Log To track all protocol deviations. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Training Log Log for documenting the training of research personnel on the research protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- RBA Business Admin Approval Tipsheet Tipsheet for the RBA. Shows how to route request for RBN to your BA and OCR Finance for RBN creation Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, RBN, Research Billing application, Research Billing number, finance, BA
- RBA New Request Tipsheet Tipsheet with steps on how to submit a new request for a Research Billing Number in the Research Billing Application Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, Research Billing Application, Finance, RBN, billing number
- Recording, Assessment, and Reporting of Deviations This document aims to help teams with the recording of deviations and exceptions of an approved protocol, and the reporting requirements to the Penn IRB and Sponsor IND; IDE; Regulatory File/ Investigator Site File; Sponsor INDIDERegulatoryFileInvestigatorSiteFileSponsor
- Recruitment Letter - Physician to Established Patient Template for recruitment letter from an external, referring physician to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter - Physician to Physician Letter Template Template for recruitment letter from one physician to another about a research study Recruitment Recruitment
- Recruitment Letter – PI to Patient Template Template for recruitment letter from the Principal Investigator to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter- Physician to Unknown Potential Subject Template for recruitment letter from a physician to a potential research subject about a research study Recruitment Recruitment
- Reportable Event Form (Electronic) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Reportable Event Form (Paper) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Research Billing Application (RBA) All studies utilizing UPHS services/procedures and/or Imaging Core/Service Center(s) will need to be registered in the Research Billing Application (RBA), regardless of the payor (insurance or research grant). A Research Billing Number (RBN) will be generated for those studies where all or a portion of visits/tests/procedures associated with the research protocol are being billed to the research grant. OCR Applications OCRApplications RBA, billing, research billing application, hospital, services, grant, payor, insurance
- Residual Balance Transfer Request Form Form used to request the residual balance to be transferred from appropriate contracts Budget/ Finance BudgetFinance
- Retrospective Study Protocol Template This protocol template is designed to facilitate the creation of a retrospective clinical research protocol. Protocol Templates ProtocolTemplates
- Risk Assessment Template for performing a clinical trial risk assessment for monitoring Monitoring Monitoring
- Screening and Enrollment Log Document subjects who have been screened and/or enrolled Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Single Patient Emergency Use of an Investigational Product Guide to requesting and managing a single patient emergency use of an investigational product. IND; IDE; Sponsor INDIDESponsor
- Site Guidance for Source Documentation This document contains information about Source Documentation for clinical trials. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Site Initiation Visit Checklist Checklist for conducting a Site Initiation Visit IND; IDE; Sponsor INDIDESponsor
- Site Qualification Report Tool for documenting the review and qualification assessment of a site and principal investigator Monitoring; Regulatory File/ Investigator Site File MonitoringRegulatoryFileInvestigatorSiteFile
- Site Visit Log Document the dates of Monitoring Visits Monitoring Monitoring
- Specimen Preparation Checklist Instructions for collecting, labeling and processing specimens for shipment Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Specimen Shipping Log Log the collection and shipment of specimens Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Sponsor - IDE Investigator List Template for the submission to the FDA-CDHR of the semi-annual list of investigators required by 21CFR 812.150 Monitoring; Regulatory File/ Investigator Site File MonitoringRegulatoryFileInvestigatorSiteFile
- Sponsor Monitoring Plan Guide Guide for developing an IND/IDE sponsor's monitoring plan IND; IDE; Sponsor INDIDESponsor
- Sponsor Welcome Letter Template for use in negotiating with an industry sponsor. Budget/ Finance BudgetFinance
- Study Admin File_Device Template for developing a study administration file or regulatory binder for a device trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Drug Template for developing a study administration file or regulatory binder for a drug trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Social Behavioral Template for developing a study administration file or regulatory binder for a social behavioral study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Feasibility Assessment Tool for assessing the resources, recruitment potential, and logistical considerations of a particular study Study Start-Up StudyStartUp
- Subject Contact Information Sheet Collect contact information for research subjects Source Documentation SourceDocumentation
- Subject Eligibility Checklist Template for documenting review of Inclusion and Exclusion criteria Source Documentation SourceDocumentation
- Testing CRFs Overview in PennCRMS Testing CRFs Overview in PennCRMS Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Trial Master File Template for a Trial Master File Trial Master File TrialMasterFile
- Understanding Using an EDC - PennCRMS W Understanding Using an EDC - PennCRMS Workflows Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, crms, sponsor, source, crf
- Vendor Qualification Guide Guide to qualifying a vendor IND; IDE; Sponsor INDIDESponsor
- W-9 IRS Form (No Form) Federal form used to report income paid to an individual Budget/ Finance BudgetFinance
- Zone 2 Core Document Change Control Sponsor checklist to document steps taken for an operationally compliant revision of core documents Sponsor Sponsor
Research Strategic Plan

In 2019, the Department of Medicine invested considerable effort and resources to devising a strategic plan that will provide a roadmap for our research mission today and into the future.
This work was guided by a Research Planning Committee that convened throughout the first half of 2019, reviewing the current state of research in the Department, generating recommendations for strengthening our research efforts, and developing the following plan. Many of our faculty and research administrators participated and contributed ideas as part of this process—through interviews, a survey, and robust discussions at the 2019 Research Retreat.
The result of this combined effort is the clear, direct, ambitious, and ultimately achievable research strategic plan that follows.
We identified five strategies for achieving our vision.
We will foster the success of our current faculty by enhancing our faculty development, mentoring, and funding programs while also strengthening the pipeline of the next generation of outstanding investigators in Medicine.
Lead: Andrew Alspaugh, MD
Initiatives:
- Strengthen faculty career development programs (Xunrong Luo, Matthew Crowley)
- Build a diverse and inclusive Department of Medicine (Laura Svetkey, Julius Wilder)
- Foster a culture of outstanding mentorship in the Department (Alspaugh, Cathleen Colon-Emeric)
- Expand physician-scientist recruitment and programmatic support (Rodger Liddle, Matt Hirschey)
- Launch a Department partnership hires program (Xunrong Luo, Chris Holley)
- Expand cadre of independent PhD investigators (Scott Palmer, Amy Porter-Tacoronte)
We will enhance our partnerships with other departments, centers, institutes, schools, and programs across Duke University.
Lead: David Simel, MD, vice chair for veterans affairs
- Duke Clinical Research Institute
- Duke Cancer Institute
- Durham VA Medical Center
- Duke Molecular Physiology Institute
- Pratt School of Engineering and MEDx
- Duke Human Vaccine Institute
- Duke Global Health Institute
- Center for Applied Genomics and Precision Medicine
We will solidify a leadership position in data science by leveraging the clinical disease expertise of our faculty; building our data assets; and improving our data collection, storage and analytics resources.
Lead: Chetan Patel, MD, vice chair for clinical affairs
- Cultivate DOM data assets into open science platform
- Augment biostatistics & bioinformatics resources
- Create new leadership role for data science
- Implement learning health units
- Continue implementation of Science Culture and Accountability Plan
We will foster a community and culture of rich scientific investigation by making research easier while achieving the highest levels of research integrity.
Lead: Erica Malkasian
- Provide outstanding grants and administrative support to investigators
- Position Duke as a leader in site-based research
- Develop next-generation biorepository capabilities
- Catalyze innovation and entrepreneurship
- Expand international research efforts
We will invest in emerging research content and method areas that leverage our strengths and address important unmet patient-centered medical needs.
Lead: Heather Whitson, MD
Cross-cutting themes:
- Immunology, inflammation & fibrosis
- Aging, resilience & pain
- Energy, obesity & metabolic disease
- Precision medicine
- Population health & disparities research
To learn more about our research strategies and initiatives, contact
- Scott Palmer, MD, MHS, Vice Chair for Research
- Saini Pillai, MBA, Senior Program Coordinator, Research
Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)

Thomas Boothby, MS, CCRP CRA II, Boston Scientific
Abstract : Research coordinators may transition to clinical research associates/monitors during their careers. This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process. A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one.
Disclosure: The author has a relevant financial relationship with respect to this article with Boston Scientific, where he is employed as a monitoring CRA.
Introduction
A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard monitoring visits. Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change.
When to Transition from Research Coordinator to CRA
While people naturally want to progress their careers as fast as possible, it is important to only make thetransition from research coordinator to CRA when the time is right. The grass is not always greener on the other side of the work fence.
The author knew that he was ready to make the transition from research coordinator to CRA because he felt that he had mastered all the tasks of a research coordinator. His job became stagnant, and he was looking for something better. Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators have the most difficult job. In the author’s opinion, they are often overworked and underpaid, and their contributions to the overall study are sometimes overlooked. Other reasons to make the transition from research coordinator to CRA include potential career progression and the opportunity to try something new. Some individuals may find that the travel component that goes along with being a monitor is a positive as well.
There are five stages of change according to a behavioral change model: pre-contemplation, contemplation, determination, action, and maintenance. In the pre-contemplation phase, people are not thinking about transitioning yet or may have obstacles in their daily lives that are preventing them from exploring new opportunities. When people are becoming serious about change, they are in the determination or action phases. During these phases, research coordinators who want to transition to CRAs might apply for new positions or become certified clinical research professionals (SOCRA CCRP®) as they try to gain new skills for the job market. When considering a transition from research coordinator to CRA, it is important to identify one’s place in the behavioral change model.
Qualifications and Background of CRAs
When the author was applying for CRA positions in 2015, he always saw a requirement for at least two years of experience as a monitor. This requirement is often a barrier to those looking to make this career transition. In 2010, ClinicalTrials.gov listed more than 100,000 clinical studies. By 2019, that number has increased to more than 300,000 clinical studies. The clinical research market has exploded over the last decade. More people are needed to monitor and to run clinical studies now than ever before. While some companies are less likely to require two years of monitoring experience now due to a depleted pool of candidates, these same companies may be more open to supplemental forms of experience such as certifications, course work, and on-the-job experience.
Thus, this is a great time to act on the decision to transition from research coordinator to CRA. From 2014 to 2024, the United States Bureau of Labor Statistics estimates that CRA positions will increase 14% annually. This increase in the job market, coupled with the high level of CRA turnover, could lead to a very strong job market in the future. At Boston Scientific, turnover among CRAs is fairly low due to the strong structure and principles. Many CRAs within Boston Scientific have been with the company for 10 to 20 years or longer.
Table 1 highlights the typical background of CRAs. Most CRAs are current or former nurses who have experience as a research coordinator or a research assistant. Many universities now offer bachelor’s, master’s, and certificate programs in clinical research as another form of training for these research related roles. In Michigan, where the author is from, Eastern Michigan University has a two-year master’s degree program in clinical research. Like the author, CRAs can often be a former research coordinator.
When the author was transitioning from research coordinator to CRA, he got his foot in the door by working closely with a monitor who still works for Boston Scientific. Relationships between research coordinators and CRAs can be contentious due to the nature of monitoring. Research coordinators should treat monitors and sponsor staff well and with respect, and they should treat monitoring visits as a learning opportunity and not a criticism of the coordinator’s work. These relationships do not need to be contentious. A good working relationship with a clinical research site’s CRAs can serve as a potential audition for a monitoring position.
CRAs typically have a clinical research certification, either SOCRA’s certified clinical research professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). Some companies provide tuition reimbursement for programs and certifications such as these as a way of employee enhancement. Research coordinators can participate in enrichment programs such as these and obtain certifications to help boost their resume and become more marketable to CROs and sponsors. When researching these programs, individuals must do their due diligence to ensure that the program or certification is offered by a legitimate organization and is accredited. Hiring managers know where to find the gold standards in clinical research programs and certifications, and those that do not fit this standard can even be viewed as a negative on ones resume.
The author is a SOCRA CCRP®, Certified Clinical Research Professional, which is an excellent indicator of knowledge for a monitoring position. The test includes knowledge of the regulations and the role of the monitor. There are also some CRO-development programs such as SOCRA’s Clinical Research Monitoring Conference and one-year certificate programs such as the Harvard Medical School global clinical scholar’s research training program.
Networking through the clinical research site’s CRAs and professional forums and groups such as SOCRA is a great way to find CRA positions and interact with other research professionals. At conferences, CROs often have booths in the exhibit hall where research coordinators can meet CRO staff, learn more about opportunities, and leave their resume with CRO staff.
A Typical Day in the Life of a CRA
The life of a CRA has its positives and negatives (Table 2). There are many things that the author wishes he knew before he became a monitor. The author works from home a great deal of the time. If he is not on the road visiting a clinical research site, he is working at home either preparing for a visit, writing follow-up visit letters, or performing other administrative work. Visit preparation and follow up is a crucial part of the home office work. CRAs have very strict compliance guidelines for completing monitoring visits and monitoring reports in a timely manner. Since recently becoming a lead CRA, the author has also been doing a great deal of administrative and compliance work with more of a global view of a clinical trial.
Some clinical research organizations (CROs) and sponsors have onsite monitors who can do remote visits and activities. Whether visits are onsite or remote, monitors are constantly in contact with clinical research sites to follow up on action items from monitoring visits or to answer protocol specific questions the site coordinators may have.
At most companies, about 60-80% of the monitor’s time is spent traveling to sites. The author currently covers all of Michigan, and he has covered other areas, including Wisconsin, New York, Pennsylvania, and Ohio. CRAs are often away for several days at a time depending on the current workload. This can be difficult on families and personal relationships. While the author travels extensively, there are some times when he travels more than others. Sometimes he does back-to-back visits and may be gone for several days at a time. After that, he may be home for several days. The extensive travel required of CRAs is a key consideration when exploring this career transition.
Being a CRA takes a great deal of self-discipline. Monitoring offers a flexible work arrangement, so monitors can work later in the day or take time off during the normal workday. However, if the CRA does not accomplish what he/she should accomplish, this will be glaringly obvious. Management and co-workers will immediately know if the CRA does not show up to meetings or has difficulty answering questions about his/her monitoring activities or their monitor role in general.
Starting a Monitoring Job
Boston Scientific has a rigorous onboarding process comprised of four to six months of training. After the author was hired as a CRA, he spent months learning the work instructions and going out on preceptor visits. In the beginning, the new CRA observes a senior CRA. Over time, the new CRA does more of the monitoring. By the end of the training, the new CRA is doing the monitoring visit, and the senior CRA is observing and making suggestions to the new hire on how the new hire can improve.
There are various levels of monitors at Boston Scientific: CRA I (for new hires), CRA II, and senior CRA. More experienced CRAs often mentor new CRAs. It is extremely helpful to find CRAs who can serve as mentors and answer questions.
CROs and sponsors have many systems that CRAs must learn. At Boston Scientific, these systems include electronic data capture, clinical trial management, auxiliary programs to help remote employees, and cloud-based filing systems. Being a CRA might be very difficult for people who are resistant to change or have difficulty with technology.
There are several types of monitoring (Table 3). The author would be considered a traditional CRA or monitor. By this, he does traditional onsite monitoring via annual or semi-annual visits to clinical research sites based upon the study’s monitoring plan. At smaller organizations, monitors may travel more often or may have an expanded territory to cover. It is important to ask how much travel is involved and how many monitors are on the team during the interview process. If a company has fewer monitors, more travel will be involved.
Many Boston Scientific protocols require annual monitoring visits. The author visits his clinical research sites at minimum once a year but generally 2-3 times per year. Some of the more difficult sites, high enrollers, and those that are more likely to be inspected by the U.S. Food and Drug Administration are monitored more often. Many sites are participating in more than one Boston Scientific study. For example, the author monitors a site in New York that is conducting several studies. He will monitor two studies during one visit. This saves him time and travel and saves the company money by reducing travel costs. Boston Scientific also uses a risk-based monitoring strategy.
In-house regulatory CRAs at Boston Scientific, called trial management CRAs, interact with the sites on regulatory matters, study startup, and study closure. They work primarily by email and lean on traditional CRAs such as the monitor to be the face of the company with the research coordinators and help ensure that tasks are completed on time. Many hospitals also run their own clinical studies and may have in-house monitors.
Boston Scientific does use remote monitoring in certain studies and circumstances. Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources.
Sponsor CRAs generally deal with one indication, while CRO CRAs can work on studies for different indications or therapies. In one month, for example, CRO CRAs may be doing four indications at four sites for four sponsors. This requires understanding a great deal of information and being able to use different systems. Good organization is key when working as a CRA, whether for a sponsor or a CRO.
Recently, the author progressed from a CRA II to a senior CRA. As a senior CRA, the author has a larger leadership role and is expected to participate more in training and mentoring other CRAs. Boston Scientific has some centralized monitoring that will look at certain metrics and internal documents to guide monitors in their daily monitoring activities. Monitors are closely linked to the trial managers who actually run the studies. They also deal with safety and data managers as well as their CRA manager and the director of operations. Boston Scientific recently created an associate clinical trial manager position as a way to slowly transition some staff members into clinical trial managers, and the author is also transitioning into this role.
One common drawback about this transition process from research coordinator to CRA is that a CRA is one step removed from patient care. Working directly with patients as a research coordinator is something that the author misses. It is important to remember that CRAs help protect patients who are participating in clinical studies at more of an indirect level. This ideology helps prevent burnout, especially when monitors are swamped with the many reports that are necessary as part of the monitoring process.
Checklist for Transitioning from Research Coordinator to CRA
Table 4 has a checklist for determining whether one is ready to make the transition from research coordinator to CRA. Prior to applying for positions, the research coordinator must consider his/her stage in the behavior change model. Unless the research coordinator is ready to transition to a CRA position, he/she should not do it. Becoming a CRA can be difficult without two to five years of research experience in medical devices, pharma, or academia in some capacity. A research coordinator who wants to transition to a CRA should work closely with current CRAs who can provide mentoring and networking opportunities as well as exploring other networking avenues such as SOCRA and ACRP forums, LinkedIn, and also attending the annual events or local events put on by these organizations.
It is important for research coordinators to bolster their resumes by completing supplemental training or certifications. Resumes should be up-to-date and attractive to potential employers. This means including details about accomplishments along with basic information such as job titles and education.
The research coordinator must also consider the travel demands of a CRA position, the types of monitoring to pursue, and his/her stage in the behavior change model. Travel is a major part of a CRA position and should be a focal point of your conversation with a hiring representative. Finally, the types of monitoring including central monitoring, remote monitoring, and regional monitoring should be considered.
Monitoring is a great job. It allows a lot of freedom. However, CRAs also have a great deal of responsibility. CRAs must be driven, willing to put in the time, and have the necessary work ethic while maintaining vigilance and holding others accountable for good clinical practices.
Typical Background of a CRA
- Nursing degree with a clinical research background
- Bachelor’s or master’s degree in clinical research
- Former/current research coordinator
- Clinical experience (medical assistant, registered nurse, or nurse practitioner)
- Clinical research certified ( SOCRA CCRP ® or ACRP-CP)
- Research experience/background
- Science/academic research background
The Life of a CRA
- This requires being self-motivated and driven
- Sometimes performing a combination of onsite and remote monitoring
- Email, etc.
- At times, CRAs are gone for several days at a time depending on current workload
- Visit preparation and follow-up is a crucial part of work at home
Types of Monitoring
- Annual or semi-annual visits based upon the monitoring plan
- Risk-based monitoring/central monitoring
- Remote monitoring
- In-house CRAs and regulatory CRAs
- Sponsor CRA/monitor
- CRO CRA/monitor
Checklist for Transitioning from a Clinical Research Coordinator
to a Monitoring CRA (Clinical Research Associate)
- 2-5 years of research experience as a research coordinator or research assistant
- Able to work with current CRAs as part of a mentorship or network with CRAs
- Completion of supplemental training or certifications to support career goals and bolster resume
- Explore networking avenues
- Up-to-date resume that is attractive to potential employers
- Able to meet travel demands of a CRA position
- Consideration of types of monitoring to pursue
- Stage in the behavior change model
7 thoughts on “Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)”
Your articles are always helpful and I always get something new to learn from them.
Research Update Organization
you are always giving something new. thank you for that.
Very clear and helpful article! The tables listing the different types of monitoring roles and the items to consider whether this is right transition are a great summary, too.
- Pingback: An overview of a career in clinical research: What jobs are available in this field? - Rebiz Zield
This is a very clear and collective article. The tables are the best helpful tips and resources for anyone interested for career advancement in clinical research. I really appreciate the author’s time and effort to sharing this article.
- Pingback: Career Growth Opportunities in Clinical Research
- Pingback: Introduction to Clinical Research: What Every Student Should Know - Drug Safety and Pharmacovigilance Course
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
Register to vote Register by 18 June to vote in the General Election on 4 July.
- Health and social care
- Research and innovation in health and social care
- The Future of UK Clinical Research Delivery: 2022 to 2025 implementation plan
- Department of Health & Social Care
The Future of Clinical Research Delivery: 2022 to 2025 implementation plan
Published 30 June 2022

© Crown copyright 2022
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/the-future-of-uk-clinical-research-delivery-2022-to-2025-implementation-plan/the-future-of-clinical-research-delivery-2022-to-2025-implementation-plan
Ministerial foreword
In 2021 we, the UK and devolved governments, set out our vision for the future of clinical research delivery. Saving and Improving Lives: The Future of UK Clinical Research Delivery lays out our ambition to create a world-leading UK clinical research environment that is more efficient, more effective and more resilient, with research delivery embedded across the NHS. We also set out our plans for 2021 to 2022 , as the first steps in delivering on the vision.
A digitally enabled, pro-innovation and people-centred clinical research environment is key to realising our ambitions to make the UK a world-leading hub for life sciences that delivers improved health outcomes for our citizens and attracts investment from all over the world. We will harness the explosion in innovative technologies to benefit patient outcomes and make a tangible difference to people’s lives across the UK. Clinical research is crucial to these efforts, as the lynchpin to driving improvements in healthcare.
As we emerge from the shadow of the pandemic and look to the future, we will work together to ensure that the UK is seen to be one of the best places in the world to deliver cutting-edge clinical research. We are working hard both to recover research delivery in the NHS and to use this moment as a catalyst for transformation, building increased resilience and embedding innovative practice as we go. The cross-sector partnerships built through the UK Clinical Research Recovery, Resilience and Growth ( RRG ) programme provide the strong foundations we need to succeed, drawing on expertise and support from industry, academia, charities, patients and the public, regulators, funders and the NHS.
Our vision was clear on the importance of unleashing the true potential of clinical research across the UK, addressing long-standing health inequalities and improving the lives of us all. We, the UK and devolved governments, are excited to set out the next stages as we look to turn our vision into a reality and build a clinical research system of the future.
Lord Kamall of Edmonton in the London Borough of Enfield Parliamentary Under Secretary of State for Technology, Innovation and Life Sciences Department of Health and Social Care
Robin Swann Minister for Health Northern Ireland Executive
Eluned Morgan Minister for Health and Social Services Welsh Government
Humza Yousaf Cabinet Secretary for Health and Social Care Scottish Government
Executive summary
In March 2021, we published our bold and ambitious 10 year vision: Saving and Improving Lives: The Future of UK Clinical Research Delivery . This was followed in June 2021 by The Future of UK Clinical Research Delivery: 2021 to 2022 implementation plan setting out the steps we would take to progress the vision in 2021 to 2022.
This phase 2 plan summarises the progress that we have made so far and the actions that we will take over the next 3 years, from 2022 to 2025, ensuring we make the progress necessary to achieve our vision in full by 2031.
This plan has been developed by the cross-sector UK Clinical Research RRG Programme in consultation with stakeholders from across the clinical research ecosystem. Our plan is centred around the 5 overarching themes identified in the vision:
- a sustainable and supported research workforce to ensure that healthcare staff of all backgrounds and roles are given the right support to deliver clinical research as an essential part of care
- clinical research embedded in the NHS so that research is increasingly seen as an essential part of healthcare to generate evidence about effective diagnosis, treatment and prevention
- people-centred research to make it easier for patients, service users and members of the public across the UK to access research and be involved in the design of research, and to have the opportunity to participate
- streamlined, efficient and innovative research so that the UK is seen as one of the best places in the world to conduct cutting-edge clinical research, driving innovation in healthcare
- research enabled by data and digital tools to ensure the best use of resources, leveraging the strength of UK health data assets to allow for more high-quality research to be delivered
We have made significant progress over the past year – a new combined review process has led to halving of approval times for new Clinical Trials of Investigational Medicinal Products (CTIMPs) since January 2022 compared to previous separate applications, streamlining the route through the regulatory journey for researchers, the world-leading £200 million data for research and development programme has been announced to invest in health data infrastructure in England with devolved administrations aligning and strengthening their infrastructure; and a new UK-wide professional accreditation scheme for Clinical Research Practitioners ( CRP ) has been launched to help double the size of this important workforce in the future.
However, the recovery of research delivery following the pandemic remains challenging. The Department for Health and Social Care (DHSC) and NHS England are taking firm action to address this, with the support of the devolved administrations, through the ‘Research Reset’ programme. We are committed not only to returning to pre-pandemic levels of performance, but to using this as an opportunity to reform and catalyse the transformation needed to create the flourishing, responsive and resilient system set out in our vision.
The phase 2 plan is aligned with funding confirmed through the government spending review for April 2022 to March 2025 and includes up to £150 million of additional funding from the National Institute for Health and Care Research ( NIHR ) and £25 million additional funding from RRG partners across the UK, complementing up to £200 million in England for the data for research and development programme announced in March 2022 and demonstrating the government’s ongoing commitment to delivering on the UK’s potential as a global life sciences superpower. This funding will enable RRG partners to:
- recover the UK’s capacity to deliver research through DHSC and NHS England’s Research Reset programme, and aligned work in the devolved administrations, aiming for 80% of all open studies on the NIHR Clinical Research Network (CRN) portfolio to be delivering to time and target by June 2023
- ensure we can recognise and support our expert workforce, and develop robust workforce plans, providing the basis for strategic investment in capacity development to support achievement of our vision in full
- broaden responsibility and accountability for research across the NHS, and improve measurement, visibility and recognition of those supporting the delivery of clinical research studies
- achieve a sector-wide, sustained shift in how studies are designed and delivered so that inclusive, practicable and accessible research is delivered with and for the people with the greatest need and in ways that enable us to tackle the greatest challenges facing the NHS
- streamline processes, strengthen our regulatory environment and ensure faster approval, set-up and delivery of studies with more predictability and less variation, as well as make it easier to understand and access the UK’s clinical research offer, thereby utilising the unique opportunity to develop a more flexible and improved regulatory model for clinical research outside the EU and improving our attractiveness as a leading destination to conduct cutting edge and global multi-centre clinical studies
- invest in the infrastructure and tools needed to implement people-centred, innovative data and digitally-enabled methods and increase partnership working across the health data ecosystem to ensure people across the whole of the UK can benefit from these approaches
The RRG programme will oversee the delivery of this plan, continuing to work in partnership with stakeholders across the sector and regularly revisiting the original vision to consider any further actions that will be needed to deliver it in full. In doing so, we will ensure that the NHS is able to tackle the healthcare challenges of the future and people across the UK and around the world will benefit from better health outcomes.
Further information about the RRG programme, including our delivery partners and governance , are available on the dedicated Recovery Resilience and Growth website . Detailed summaries of our progress to date and our future plans will be published on the site on an ongoing basis, providing a central point of information and updates about the programme and our progress towards achievement of the vision. You can also sign up to receive regular email updates on our progress.
UK-wide approach
Health policy is a devolved responsibility, where each of the UK administrations has distinct ownership over implementation. However, we are committed to delivering on a vision with a UK-wide reach and in pursuit of a common goal: to create a seamless and interoperable service across the UK to support clinical research delivery, shaping the future of healthcare and improving people’s lives.
We are therefore further strengthening a joined-up system, where sponsors of both commercial and non-commercial research can easily deliver studies across the UK and people can more easily participate. To ensure compatible and consistent ways of working across England, Scotland, Wales and Northern Ireland many commitments in this plan are focused on UK-wide implementation. Organisations such as MHRA ( Medicines and Healthcare products Regulatory Agency ) and systems such as IRAS ( Integrated Research Application System ) have a UK-wide reach and their actions will have impacts across the country. In some instances, actions are being led by a specific organisation on behalf of the UK, while others will be delivered through UK partnerships – recognising the different legislative and delivery contexts across the UK government and devolved administrations.
The needs of UK citizens and our health research system are broad and diverse. We are committed to maintaining a rich and balanced portfolio of studies in rare and common diseases, ranging from complex, intensive studies in small, highly targeted populations to pragmatic population health research in large cohorts, using a range of methodologies and methods as appropriate to the research questions.
Our vision focuses specifically on the future of UK clinical research delivery. Other types of research, including social care and public health research, are vitally important to provide the evidence necessary to support policy making and service delivery in these areas. Many partners involved in the RRG programme support this broader programme of research activity and other work programmes are underway to enable their development. We expect that many of the improvements we make in the clinical research environment will have benefits for other kinds of research and will work across our organisations and with wider groups of stakeholders to ensure the lessons are shared.
Research reset
As we recover from the pandemic, clinical research delivery is facing unprecedented challenges and there is an urgent need to reset the UK’s research portfolio so we can build for a stronger future.
The number of studies in the NHS is now higher than ever before. This is accounted for by the additional COVID-19 studies, other research that has remained on the portfolio from before the pandemic and has been paused or delayed, together with new studies being funded and coming into the system. In addition, the number of studies in set up is now much higher than pre-pandemic, further increasing the workload for NHS R&D offices and research delivery teams. This is taking place in the context of the recovery of wider NHS services and resourcing the high number of studies is challenging. Throughout this, the resilience of the workforce has been remarkable.
Recovery of the UK’s capacity to deliver clinical research is essential if we are to deliver the ambitions set out in this phase 2 plan. Indeed, many of the challenges the vision seeks to address have been exacerbated by the pandemic, so Research Reset and reform go hand in hand.
Since summer 2020, all delivery partners across the sector have been working to restore the diverse and balanced portfolio of studies which were impacted due to the COVID-19 pandemic. While this has had some positive impact, it has not resulted in the restoration of activity across all studies that were underway before the pandemic. We are taking further action through the Research Reset programme to build back a thriving, sustainable and diverse research and development portfolio within the NHS.
Our objective in implementing Research Reset is to give as many studies as possible the chance of completing and yielding results, generating the evidence needed to improve care and sustain our health system. However, this will require closure of studies that are not viable in the current context to free delivery resources in the system for those studies that can deliver. Lessons must also be learned to reform and increase the resilience of our research system. As part of this we have asked funders and research sponsors to review their active studies to assess the viability of delivering these within the capacity available.
Our aim is for 80% of all open studies on the NIHR CRN portfolio to be delivering to time and target by June 2023. We will take an agile approach to the Research Reset programme, continuously assessing whether further action is required with the input of stakeholders across the sector including patients and the public.
The devolved governments support this approach and we are working together across the UK to ensure synergistic arrangements are in place to promote the smooth delivery of cross-border studies. Each devolved administration will also review possible new eligibility criteria for national delivery support to ensure deliverability within available resources is feasible.
A sustainable and supported research workforce
The UK clinical research workforce has been fundamental to our collective success to date and will be critical to the achievement of our vision in the future. Healthcare and research staff of all backgrounds must be offered rewarding, challenging and exciting careers within clinical research, so that the most talented people can be brought into clinical research, including research delivery and R&D management, as a life-long career. This will help to bolster the capacity of the clinical research system and support a motivated and sustainable workforce. Collectively, we can realise the potential of UK clinical research to improve outcomes for people across the country, sustain our NHS and improve the economy.
Progress over the past 12 months:
- in England, to support the drive to recover the portfolio, DHSC provided over £30 million of additional funding via the NIHR Clinical Research Network (CRN) in the 2021 to 2022 financial year to increase research delivery capacity, especially in community settings and with a key focus on achieving flexibility and agility in the workforce. The Welsh Government provided £1.7 million to support additional capacity in order to achieve the recovery of non-COVID-19 research, including development of research capacity outside of hospital settings. £3 million of funding from the Department of Health in Northern Ireland has been provided to support the work of a Taskforce established to address clinical research recovery in Northern Ireland
- the NIHR , working with the devolved administrations, launched a UK census for nurses and midwives working in clinical research in order to understand the true size of this workforce. Data was also sought on location, speciality and banding or grade. It was able to identify that there are at least 7,469 research nurses and midwives across the UK and Ireland working at every level and within all areas of healthcare. This census demonstrates the breadth and depth of nurse and midwife involvement in research across the healthcare sector
- in June 2021, NIHR on behalf of the UK launched a new UK-wide professional accreditation scheme for Clinical Research Practitioners ( CRP ) as part of efforts to double the number of this important workforce over the next few years. Over 1,000 members have already signed up to the CRP directory
- NIHR also launched the UK wide Associate Principal Investigator Scheme, which aims to make research a routine part of clinical training so doctors, nurses and allied health professions can become the principle investigators of the future. Over 1,000 health and care professionals had registered for the scheme by April 2022
- in February 2022, Wales published a vision for research career pathways that outlines recommendations to improve support and encourage more health and social care professionals to embark on research careers
Phase 2 commitments
To continue this progress and build towards a sustainable and supported research workforce, we will ensure we can retain and recognise our expert staff and develop robust workforce plans to provide the basis for strategic investment in capacity development:
- the RRG Programme will lead the development of a cross-sector research workforce plan to support implementation of our vision in full. Developed over 2022 to 2023, this plan will guide additional investment in our workforce from 2024
- RRG partners will ensure workforce plans developed by key healthcare organisations include research requirements, particularly noting the knowledge and skills needed across the wider workforce to deliver research as an essential part of high-quality care. This will include the NHS People Plan , coordinating with Health Education England and DHSC, and equivalent plans in the devolved administrations
- NHS England, working with its partners is developing a comprehensive, long-term NHS workforce plan. This will include consideration of research requirements to support the delivery of high-quality care
- Health Education and Improvement Wales, working closely with Welsh Government and the NHS, will develop plans to support and facilitate the nursing, midwifery, allied healthcare professionals and health sciences professions in embracing research as part of their roles and career pathways. Through the development of competency and skills frameworks, Health and Care Research Wales is working to support the inclusion of research delivery roles
- NIHR will provide investment to support NHS R&D transformation, increase research capacity including nurses, midwives and allied healthcare professionals, and provide more opportunities for rewarding careers in research
- the RRG partners will expand the package of training programmes for the research workforce including through the RCP- NIHR Credentialing Scheme, the NIHR Associate PI scheme, the NIHR Nurse and Midwife Leaders Programme, an NHS England programme for executive nurses in Trusts and Integrated Care Systems (ICSs), and a research matron’s toolkit
- NIHR and the devolved administrations will invest in learning and support for researchers, so that they are equipped with the expertise and cultural competency to design and deliver people-centred studies to meet the needs of patients, service users and the public, including those from underserved communities and groups not traditionally served by research
- in support for NHS R&D transformation, Wales will invest in a new Health and Care Research Wales Faculty, which will include increased investment in the NHS Research Time scheme to help develop the next generation of principal and chief investigators in the NHS alongside enhanced mentorship schemes
Clinical research delivery embedded in the NHS
Our aim is to create a step change in the delivery of clinical research in the NHS, so that research is increasingly seen as an essential part of healthcare. Making research an intrinsic part of clinical care means that patients and service users can expect to have access to the most cutting-edge treatments and technologies. We want the NHS to actively participate in generating evidence about effective diagnosis, prevention and treatment through research. By acknowledging the important role of the whole of the healthcare workforce in clinical research delivery, we can ensure everyone is empowered to get involved in research and further boost overall capacity for research in the NHS and wider health system. Measuring clinical research will also support NHS leaders to drive behaviour change and incentivise more engagement in research activity. Finally, ensuring clinical research is embedded within the NHS will be essential in giving the UK the capacity to grow in an increasingly competitive global market.
Progress in Phase 1:
the UK Research and Development (UKRD) and NHS R&D Forum, with NIHR , developed the ‘Best Patient Care, Clinical Research and You’ online guide that aims to help busy non-research staff become more aware of the impact of research in their trust
the General Medical Council (GMC) published its position statement Normalising Research - Promoting Research for all Doctors
the Allied Health Professions’ Research and Innovation Strategy was published, addressing the key areas which impact research and innovation across all health professions in England
the NHS Chief Nursing Officer (CNO) for England published the strategic plan for research for nurses. The plan aims to create a people-centred research environment that empowers nurses to lead, participate in and deliver clinical research that is fully embedded in practice and professional decision making
together with existing strategies in the devolved administrations, we are continuing the development of UK-wide support for the key professional groups
In order to more deeply embed clinical research in the NHS, we will take action to broaden responsibility and accountability for research across the NHS, and improve measurement, visibility and recognition of those supporting the delivery of clinical research studies. The role of healthcare leaders and professionals will be vital in this:
NHS England and the devolved administrations will each develop clear and tangible plans to work towards embedding responsibility and accountability for research in healthcare delivery
- NHS England and the devolved administrations will use existing legal duties and planning frameworks to promote and facilitate research. Each administration will develop assurance frameworks and use existing channels such as annual reports and joint forward plans to help cement the importance of research as a core duty. In England this will include the implementation of the Health and Care Act . Integrated Care Boards (ICBs), NHS England and the Secretary of State for Health and Social Care will all have enhanced duties to report on how they are promoting and facilitating research. NHS England will also lead development of a research framework for ICBs to help them understand and fulfil the minimum expectations around research that the Health and Care Act sets. This will herald a significant shift in how research is considered within the NHS and drive a greater responsibility for more research activity across all sites. In Wales, we will explore opportunities provided through the development of the NHS Executive in Wales to strengthen the national oversight of NHS research
- we will work across the UK administrations to introduce new metrics and measures to increase the visibility and recognition for undertaking and supporting clinical research across NHS organisations
- NIHR , working in partnership with NHS England and the devolved governments, like the Scottish Health Research Register (SHARE), will continue to enhance the UK Be Part of Research platform through collaboration with other existing registries. National digital channels (for example the NHS App or NHS website) will feed into the Be Part of Research platform
The RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further in the next 3 years.
People-centred research
The vision set out our ambition for more people-centred research, designed to make it easier for patients, service users and members of the public to access research of relevance to them and be involved in its design. To achieve this, delivery of research in community, primary care and virtual settings needs to increase, with delivery designed around the needs of the people participating in it. Alongside this, we will ensure we maintain our world-leading specialist research infrastructure, which provides opportunities for people to access early-phase studies, complex therapies and devices.
- delivering studies such as PANORAMIC and IBS-RELIEVE has demonstrated the UK’s growing ability to harness technology and conduct studies virtually and in the community
- HRA and MHRA, in collaboration with NHS Research Scotland, Health and Social Care Northern Ireland (the equivalent to the NHS in Northern Ireland), and Health and Social Care Research Wales, have published UK-wide guidance on the set up of interventional research to enable research to be delivered across organisational boundaries and to help take research to where people might find it easier to take part, for example using hub and spoke models
- the NIHR led UK Working Group on Remote Trial Delivery published a report in June, which discussed the challenges and opportunities in remote trial delivery and provided guidance for researchers
- the NIHR Race Equality Framework was piloted by industry. This self-assessment tool helps organisations to improve racial equality in health and care research
- partners across the UK are working together to ensure patient and public involvement in research in a variety of ways including through regulation, ethics, payment for public contributors and development of new public engagement strategies. This includes the publication of a shared commitment to public involvement in research to ensure involvement is built into study design, delivery, and dissemination
- in Northern Ireland the Clinical Research Recovery Resilience and Growth Taskforce implementation plan includes a patient and public engagement and involvement sub-group, which is focused on the development of patient and public centred priorities, and an innovation sub-group planning approaches to innovative and people-centred trial design
- in Wales, the ‘Discover your Role’ programme is underway, with a co-created action plan to ensure that people are at the heart of new developments in research
- the NHS Research Scotland patient and public involvement workshop series completed and reported in September 2021. Findings from the workshops and the Scottish Patient Public Involvement Survey are informing work to support greater visibility and connectivity, increased diversity and representation, and a review of the current mechanisms for pre-award funding
- RRG partners have partnered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry to make it easy for researchers to fulfil their transparency responsibilities. Trial registration is the first step to ensuring research transparency from the outset, and from 2022 the HRA began automatic registration of clinical research with ISRCTN , taking the burden away from research sponsors and researchers
Our aim will be to achieve a sector-wide, sustained shift in how studies are designed and delivered so that inclusive, practicable and accessible research is delivered with and for the people with the greatest need and in ways that enable us to tackle the greatest challenges facing the NHS. The UK’s ability to deliver diverse trials and studies will also give us a competitive advantage on the global stage, attracting researchers from around the world to base their studies here:
- the HRA is leading a cross-sector project , co-produced with public contributors, to collect evidence about how high quality, people-centred clinical research is done well: finding out what matters most, what ‘good’ looks like and what might be making it difficult. It will make recommendations to help improve the way clinical research happens in the UK and disseminate information about actions and resources developed by partners
- NIHR will invest in the development of skills and tools for innovative trial delivery, increasing the confidence and ability of our researchers to design and deliver studies in people-centred ways
- NHS England will launch a toolkit that could be used by researchers across the UK to help them engage more effectively with selected underserved communities. NIHR will also promote increased use of the resources developed by the NIHR INCLUDE project project which enable researchers to increase inclusion of underserved communities in their research
- NIHR and NHS Digital will develop mechanisms to monitor the diversity of people participating in NIHR Clinical Research Network portfolio studies in England in order that we can understand where improvement is needed and what action will be most effective.
- in England, the NHS Accelerated Access Collaborative will invest in demand signalling (the process of identifying, prioritising and articulating the most important research questions) and horizon scanning (the process of identifying and better understanding emerging transformational technologies of potential benefit to the NHS and our communities) to improve identification of the most needed treatments and technologies and rapidly bring these into clinical use
- in Scotland, SHIP is leading the new Scottish Health and Industry Partnership Demand Signalling Plan. This new framework will support identification and decision making around key strategic challenges and operational pressures to accelerate NHS Scotland Re-mobilisation, Recovery and Re-design, aligning with delivery of the NHS Recovery Plan 2021 to 2026, and the Life Science Vision healthcare missions
- medical research charities play an important role in supporting people-centred research, utilising their contacts with patients and communities, and prioritising their needs when setting a research agenda. The Association of Medical Research Charities (AMRC) will be working with NIHR and NHS England to formalise this work – and will share findings once developed across the UK
The RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further within the next 3 years.
Work is also underway to improve access to research through digitised recruitment as detailed in the section on research delivery enabled by data and digital tools.
Streamlined, efficient and innovative research
Facilitating research to happen quickly and predictably will not only bolster our economy and status as a life sciences superpower, but will also drive innovation, which translates into improved care. We have the opportunity to develop a more flexible and improved regulatory model for clinical research outside the EU in the best interests of patients and the public, and since the publication of the vision we have been building towards our aims of supporting a more streamlined, efficient, and effective clinical research environment.
Progress in phase 1:
- in a new approach to licensing and regulation implemented by the MHRA, NICE, the All Wales Therapeutics and Toxicology Centre (AWTTC) and the Scottish Medicines Consortium (SMC), over 100 innovation passports have been issued through the Innovative Licensing and Access Pathway (ILAP), to robustly and safely support the path to market of the most innovative, transformative treatments
- the combined review from the MHRA and the UK Research Ethics Service, in collaboration with the HRA facilitates speedier set up for clinical research trials by requiring applicants to only make a single application for both Clinical Trial Authorisation (CTA) and Research Ethics Committee (REC) approval. Since January 2022, all new Clinical Trials of Investigational Medicinal Products (CTIMPS) in the UK have been benefiting from the combined review, halving the approval time compared with separate applications over the period 2018 to 2021
- the range of model UK contracts agreed with industry and the NHS has been expanded including the first UK-wide model Clinical Investigations Agreement (UK mCIA) for research in medical devices, and the first Model Confidentiality Disclosure Agreement (mCDA) for use by companies with potential NHS sites has also been launched
- the MHRA ran a public consultation on proposals for legislative changes for clinical research. The proposals aim to promote patient and public involvement in clinical research, increase the diversity of participants, streamline clinical research approvals, enable innovation, and enhance clinical research transparency. The consultation sought the views of the wider public, clinical research participants, researchers, developers, manufacturers, sponsors, investigators, and healthcare professionals to help shape this important future legislation and over 2,000 responses were received
- NHS England published refreshed guidance on Excess Treatment Costs (ETCs), expanding the framework to include studies where Clinical Commissioning Groups are the commissioner for the service where the study takes place and setting out the provider types which can utilise the national payment system in England. From April 2022 the provider thresholds for ETCs has been reduced, meaning that the number of providers who receive ETCs will increase
In our next phase of work, we will streamline processes, further strengthen our regulatory environment and ensure faster approval, set-up and delivery of studies with more predictability and less variation. Significant emphasis will be placed on reducing unwarranted variation in ways of working across sites and other research infrastructure, so that conducting clinical research in the UK is high quality, predictable and reliable. This will be particularly important for commercial contract research as speed and predictability is key to the UK’s competitiveness and our ability to attract global multi-centre research studies into the NHS.
The UK is globally recognised for its scientific expertise and dedicated research infrastructure. However, the devolved healthcare systems and competition between organisations has created a complex landscape which is difficult to navigate and creates barriers for researchers and companies. We will work across the UK clinical research system to ensure it is easier to understand and is attractive as a leading destination to conduct cutting edge clinical studies.
To improve research approvals and strengthen our regulatory frameworks:
- a single UK approval service will replace HRA and HCRW Approval and equivalent process in Northern Ireland and Scotland, and site permission and confirmation processes across the UK
- MHRA will work with HRA in continuing the development of IRAS to streamline health technology and medicines research, and HRA will explore whether it is viable to embed a fast-track ethics review as part of combined review
- HRA will lead UK-wide work to further expand the suite of model agreements, including decentralised and other innovative delivery models as well as particular fields of innovative products such as Advanced Therapy Medicinal Products
- following public consultation on proposals for legislative changes for clinical research, the MHRA is now carefully analysing the responses received, preparing a Government response and developing secondary legislation to improve and strengthen our clinical research legislation
- MHRA will support risk-proportionate trial conduct and monitoring, including through Good Clinical Practice (GCP) guidance and pragmatic investigator guidance, and will work with HRA to develop guidance on use of in vitro diagnostics (IVDs) in clinical research
- MHRA and HRA will also establish a comprehensive stakeholder reference group to assist with guidance generation on new legislation and ensure there is a common understanding of regulatory requirements that will enhance the UK’s international attractiveness as a place to conduct multinational trials
To improve study set-up:
- learning lessons from delivering COVID-19 research, we will enhance our early feedback service offer via the NIHR CRN to support study design that is optimised for delivery and explore how we can further match research delivery demand to capacity across the UK
- we will implement the UK-wide National Contract Value Review (NCVR), with the aim of expediting the costing elements of the contracting process across NHS Trusts to ensure costing does not delay study set-up. From 1 April 2022, the NCVR will begin to replace the current time-consuming process whereby each NHS organisation negotiates with each commercial sponsor for every study in order to agree bespoke contract value. The programme will be monitored throughout implementation to ensure lessons can be learnt and the process improved to ensure it achieves its aims. The existing single cost and contract review model in Scotland and across the NIHR Patient Recruitment Centres in England will integrate with NCVR as it develops, supporting more effective UK alignment and efficiency
- the Experimental Cancer Medicine Centre (ECMC) Network, with support from MHRA and HRA, will complete their pilot to set up Phase I oncology trials within 80 days of IRAS submission. Learning from this programme will be shared to enable improved set-up performance in other specialities
- RRG programme partners will identify and establish mechanisms to achieve efficient costing and contracting across other parts of the health system, supporting and enabling an increase in decentralised study designs and research taking place in primary care and community settings.
- DHSC and NHS England will lead a review of their current Excess Treatment Costs (ETC) process in England to review experiences of the policy and t explore how best we can support non-commercial research in the NHS
To make the UK offer easier to navigate:
- understand UK capabilities to deliver their study at all stages of the protocol development and delivery pathway
- connect with the right part of the system to help them at the right time
- access the network of expertise and resources available to create a package of support to deliver studies efficiently
- MHRA, NICE, AWTTC and SMC will work with partners across the UK to develop ILAP as an effective route into the UK research system, particularly through the development of a support toolkit
- the further development of IRAS will also provide navigation and signposting through the research journey, directing applicants to relevant guidance and advice. Through interfaces with other systems it will reduce burden and duplication
Research delivery enabled by data and digital tools
The UK’s health data offering is one of our global strengths due to our national health systems and cradle-to-grave healthcare records. Investing in data and digital tools, and making ethical use of them to support clinical research, for example by making it easier to recruit and follow-up participants, increases the efficiency and effectiveness of the clinical research process. These tools also increase the resilience and sustainability of the healthcare system and reduce the burden on the NHS workforce.
- the data strategy for health and social care in England was published in June 2022
- up to £200 million committed to support NHS-led health research (subject to business case) was announced on 2 March 2022 to invest in health data infrastructure to support research and development in England, with parallel activity in the devolved governments
the NHS-Galleri trial demonstrated the potential for the use of healthcare data to support rapid, large scale recruitment to and delivery of clinical studies in the NHS. The Accelerated Access Collaborative (AAC), led by NHS England, coordinated the design and set up of a 2 part, real-world demonstration project involving clinical data capture from NHS Digital and NIHR , and was a demonstrator for the ‘Find, Recruit and Follow-up’ service and NHS DigiTrials. The trial has already passed the halfway point in their recruitment of participants, with over 100,000 enrolled following the launch in autumn 2021
- each delivery partner funded as part of year one of the ‘Find Recruit and Follow-up’ service launched Minimum Viable Products (MVPs) of their services including: NHS DigiTrials, which has successfully facilitated 28 active trials through its service with a further 8 in application and 12 in pre-application; NIHR CRN launched its early stage ‘concierge’ service, with 2 companies and 4 data service providers as early users; and HRA, which agreed an approach to review by the Confidentiality Advisory Group which will enable more efficient study set up in future. In addition, the MHRA Clinical Practice Research Datalink (CPRD) has launched SPRINT (Speedy Recruitment into Trials ), a data-enabled research service that facilitates rapid feasibility and patient recruitment into industry sponsored phase 2 to 4 trials across the UK
- making use of real-world data (RWD) in and for clinical research is now a reality, supported by MHRA’s published guidance . This is the start of a series of guidelines to provide general points to consider for sponsors planning to conduct clinical research using RWD to support regulatory decision making
The next 3 years will see a revolution in how we use data across the health system. We will go further in utilising innovative data-driven methods and digital tools to transform the way we design, manage and deliver people-centred clinical research studies across the whole of the UK. We will achieve this by increasing the use of data and digital tools in recruitment and follow up, and by improving access to data via Trusted Research Environments (TREs: a type of Secure Data Environment, secure spaces where approved researchers can access rich, linked datasets) and through increased partnership working across the UK health data ecosystem.
We are very clear that the opportunity to use health data must be done in a way which is secure and trusted by members of the public, so governance and oversight processes must be both as efficient as possible and transparent, robust and trustworthy. Public trust and understanding of how data is being used to support research continues to be critical in developing appropriate activities. We will be working together to consider how to implement recommendations from the Goldacre Review , and ensuring that all work is supported by comprehensive public involvement and engagement activity.
To improve study planning, recruitment and follow-up:
- the Find, Recruit and Follow-up service will work across the 4 administrations to consider how activity can be expanded to include SAIL, Scottish Health Research Register, data infrastructure in Northern Ireland, NIHR BioResource and other key national data infrastructure, increasing opportunities for people to quickly and easily access research of relevance to them
- NHS DigiTrials and CPRD (via MHRA) will enable a significant increase in the scale of identification of people who match the eligibility criteria for specific studies in order that they can be given the opportunity to participate in research. They will also support increased use of routine healthcare data to streamline reporting of follow-up data, increasing predictability and releasing delivery capacity in the NHS
- in England, the Data for R&D Programme will invest in health data infrastructure for research and development, supported by comprehensive PPI and engagement throughout the programme, including embedded within its governance
- NIHR will invest in data and digital platforms such as Be Part of Research and NIHR BioResource, and provide the tools and support necessary to deliver virtual and decentralised studies. Increased interoperability between regulatory, NHS and NIHR platforms will enable further streamlining of processes for researchers
- in Wales, a digital recruitment programme will be developed through partnership between Health and Care Research Wales, SAIL Databank and the NHS Wales National Data Resource programme, to develop services that utilise data resources to drive research delivery. An Expert Working Group has been established to guide on the development of this ‘data for research’ programme. A pilot service has been funded to use SAIL data to provide rapid intelligence to aid placement of research trials in Wales to support most effective recruitment
- in Scotland, scoping work and stakeholder engagement is informing plans for developments to support increased use of NHS data and digital technology to accelerate clinical trial delivery, and for further development of the Scottish Health Research Register (SHARE) to support recruitment to health research studies. We will continue to support the already established regional NHS Scotland controlled data safe havens (Trusted Research Environments) and their collaboration with the newly established Research Data Scotland to support use of data in research. We will also look for opportunities to support research and innovation as part of the forthcoming Scottish Government Data Strategy for Health and Social Care
- in Northern Ireland, the RRG Taskforce data and digital sub-group will lead work to prepare the NI data infrastructure to support digitally-enabled trials and participate in UK-wide initiatives such as the ‘Find, Recruit and Follow-up’ service.
To improve access to data and TREs:
- over the next 3 years NHS England will build upon foundational investments made in 2021 and 2022 in an interoperable network of TREs. At a national level, we will expand the scale, scope and capacity of the NHS Digital TRE to enable more users to have timely and secure access to a range of national datasets. At a regional level, we will develop a small network of regional ‘Sub National TREs’ in England, each covering a population of more than 5 million citizens and enabling access to near real time, multimodal data particularly amenable to the development of AI algorithms
- the Data for R&D Programme within NHS England will expand the ability for researchers to access a range of rich linked genomic datasets, creating linkages across the various health data systems so that genomic data can be used to support innovation and patients and service users can benefit from the provision of innovative genomic healthcare. The Genome UK Implementation Coordination Group Data Working Group will lead work looking to link genomic datasets from across the UK, and federate these where appropriate, as set out in the Genome UK: shared commitments for UK-wide implementation 2022 to 2025
- in Scotland, we will continue to support the already established regional NHS controlled TREs and their collaboration with the newly established Research Data Scotland to support use of data in research
- in Wales, we will continue to invest and grow the internationally recognised expertise and TRE available via the SAIL Databank, offering national population coverage and secure access to billions of person-based records
- in Northern Ireland, the Honest Broker Service and the more recently established Northern Ireland TRE will be supported to further develop secure access to data for research. This will sit alongside a sustained public dialogue and progression of the enactment of secondary uses legislation to facilitate data access for research in Northern Ireland.
Connecting these developments into a coherent UK offer will bring added benefit, therefore to unite plans:
- the RRG programme will ensure strategic co-ordination of this work across the UK clinical research ecosystem, supporting progress and ensuring alignment of initiatives, as well as identifying key areas where we can go further within the next 3 years to take steps towards fully realising our overarching vision
- an RRG data and digital subgroup will be established to enhance collaboration across the sector and ensure people across the whole of the UK benefit from research delivered using data and/or digitally-enabled approaches
Governance, detailed plans and ongoing updates
The UK Clinical Research RRG programme will oversee the delivery of this plan, continuing to work in partnership with stakeholders across the sector and regularly revisit the original vision to consider any further actions needed to deliver on the 10 year vision. In doing so, we will ensure that the NHS is able to tackle the healthcare challenges of the future enabling people across the UK and around the world to benefit from better health outcomes.
Given the scope of the work and the fast pace of change in clinical research, we will keep the specifics of this plan under review via the RRG programme and adapt delivery as needed. This flexibility will allow us to meet emerging challenges and ensure that the outcomes are aligned to the most pressing issues to realise our shared ambitions.
Progress will be measured by the RRG Programme Board and the Ministerially-chaired Oversight Group, ensuring we are delivering on the commitments set out in this plan and that they are having the intended impact on the UK clinical research system. Specific measures for success will be published on the RRG website later in 2022.
We will publish a Phase 3 plan in 2025 to 2026 to align with the next government spending review period. The Phase 3 plan will showcase our progress and lay out the next steps needed to ensure the vision is delivered.
Achievement of our plan will require action across the whole sector, but by building on the foundations of collaboration and partnership that we have created through RRG programme we can collectively work through current challenges and see this vision become a reality.
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
NIH Releases Strategic Plan for Research on the Health of Women
The page you recommended will be added to the "what others are reading" feed on "My ACR".
The page you bookmarked will be added to the "my reading list" feed on "My ACR".
The National Institutes of Health (NIH) Office of Research on Women’s Health (ORWH) recently issued the NIH-Wide Strategic Plan for Research on the Health of Women 2024-2028 . The release of the strategic plan aligns with the President’s Executive Order to Advance Women’s Health Research and Innovation. This strategic plan is driven by three guiding principles:
• Consider the complex intersection among multiple factors that affect the health of women. • Include diverse populations of women in clinical research. • Integrate perspectives from a diverse workforce of scientists with differing skills, knowledge and experience. The plan focuses on five strategic research goals intended to advance research that examine multiple factors that influence the health of women; improve data science and data management practices to prevent and treat conditions affecting women; foster women scientists’ career development and promote scientific workforce training and education that advances the health of women; support basic and translational research to advance women’s health across the life course; and advance community-engaged science across the research and practice continuum. In addition to the release of the report, NIH Director Monica Bertagnolli, MD, released a video sharing her vision for the future of research on women’s health. The plan encourages the use of innovative tools, approaches and resources for research on the health of women, which may include novel imaging techniques and data science. Specifically mentioned is The RADx® Tech for Maternal Health Challenge, which accelerates the development of home-based and point-of-care maternal health diagnostic devices, wearables, or other remote sensing technologies to enable extension of care and improve health outcomes in maternity care deserts. For more information, contact Katie Grady , American College of Radiology® Government Affairs Director.
The clinical practice and dosimetric outcome of the manual adaptive planning during definitive radiotherapy for cervical cancer
- Open access
- Published: 27 May 2024
- Volume 150 , article number 280 , ( 2024 )
Cite this article
You have full access to this open access article

- Yi-Wei Wang 1 ,
- Min Chen 1 ,
- Wen-Tong Shen 1 &
- Hao-Ping Xu 1
76 Accesses
Explore all metrics
To evaluate the advantage of the manual adaptive plans comparing to the scheduled plans, and explored clinical factors predicting patients suitable for adaptive strategy.
Methods and materials
Eighty two patients with weekly online cone-beam computed tomography (CBCT) were enrolled. The re-CT simulation was performed after 15 fractions and a manual adaptive plan was developed if a significant deviation of the planning target volume (PTV) was found. To evaluate the dosimetric benefit, D98, homogeneity index (HI) and conformity index (CI) for the planning target volume (PTV), as well as D2cc of the bowel, bladder, sigmoid and rectum were compared between manual adaptive plans and scheduled ones. The clinical factors influencing target motion during radiotherapy were analyzed by chi-square test and logistic regression analysis.
The CI and HI of the manual adaptive plans were significantly superior to the scheduled ones ( P = 0.0002, 0.003, respectively), demonstrating a better dose coverage of the target volume. Compared to the scheduled plans, D98 of the manual adaptive plans increased by 3.3% ( P = 0.0002), the average of D2cc to the rectum, bladder decreased 0.358 Gy ( P = 0.000034) and 0.240 Gy ( P = 0.03), respectively. In addition, the chi-square test demonstrated that age, primary tumor volume, and parametrial infiltration were the clinical factors influencing target motion during radiotherapy. Multivariate analysis further identified the large tumor volume (≥ 50cm 3 , OR = 3.254, P = 0.039) and parametrial infiltration (OR = 3.376, P = 0.018) as the independent risk factors.
We found the most significant organ motion happened after 15 fractions during treatment. The manual adaptive plans improved the dose coverage and decreased the OAR doses. Patients with bulky mass or with parametrial infiltration were highly suggested to adaptive strategy during definitive radiotherapy due to the significant organ motion.
Similar content being viewed by others

Evaluation of PTV margins with daily iterative online adaptive radiotherapy for postoperative treatment of endometrial and cervical cancer: a prospective single-arm phase 2 study
Fan beam CT-guided online adaptive external radiotherapy of uterine cervical cancer: a dosimetric evaluation

Dosimetric benefit of an adaptive treatment by means of plan selection for rectal cancer patients in both short and long course radiation therapy
Avoid common mistakes on your manuscript.
Introduction
Locally advanced cervical cancer (LACC), which is defined as stage IB3-IVA according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system, accounts for more than 80% of all cases in patients with cervical cancer (Sung et al. 2021 ). Concurrent chemoradiotherapy is the standard of care for locally advanced cervical cancer (Cohen et al. 2019 ; Monk et al. 2007 ). However the tumor regression and deformation combined with organ motion during the course of fractionated RT remain challenges in clinical practice of IMRT use.
Often a significant reduction of cervix tumor is observed during the 5 weeks of radiotherapy (RT) (Mayr et al. 2002 , 1996 ). Besides the tumor regression, previous studies also demonstrated the intrafraction movements of pelvic organ, especially the movements of uterus, the filling status of bladder and rectum might result in variations in position and shape of the clinical target volume (CTV), and eventually cause geographical miss of the target and unnecessary volumes of organs-at-risk (OARs) included into high dose regions (Beadle et al. 2009 ; Chan et al. 2008 ; Lee et al. 2007 ). The most effective way to date is online adaptive radiotherapy (ART), which uses an imaging feedback loop to correct the deviation caused by tumor shrinkage and organ motion and modify the treatment plan accordingly (Shelley et al. 2021 ; Hall et al. 2022 ; Yan et al. 1997 ).
Despite the real-time online ART has provided improved clinical benefits (Tan et al. 2019 ), this technique needs intensive clinical effort, facing big challenges with clinical implementation, which make its utilization relatively limited (Qin et al. 2015 ), especially in low- and middle-income countries where cervical cancer has a greater prevalence. The time spent throughout the online ART workflow has been always an important consideration especially in a busy radiation oncology clinic. Radiation oncologists spend more time (usually more than 30 min per fraction) on verification of the recontouring and approving the replanning at each treatment fraction, while the patient was lying on the LINAC treatment couch during the whole process which would cause further changes of the bladder and rectum filling. In addition, all adaptive plans are automatically created and cannot be adjusted manually to better meet the whole team needs.
It would be beneficial to find an alternative workflow to avoid recontouring for every adaptive plan. A number of studies showed the largest uterus motion happened in the 2nd or 3rd week of treatment (Lee et al. 2007 ), and the most of tumor regression occurs about 21 days, or after 30.8 Gy of treatment (Lee et al. 2004 , and Bunt et al. 2006 ). Thus, we proposed an alternative offline ART, the manual adaptive radiotherapy, which only required one more CT simulation after 15 fractions during treatment, and the manual adaptive plan was created by conventional treatment planning systems. The offline ART course could be more easily implemented in clinics.
The goal of our study was to verify the appropriate time for manual adaptive planning during the fractionated treatment, compare the dosimetric outcomes of manual adaptive plans to the scheduled plans, and explore the clinical factors to guide patient selection for ART.
Materials and methods
Patients and treatment.
Consecutive patients with intact cervical cancer undergoing definitive treatment at our department who agreed to undergo twice CT simulation scans during treatment participated in this study. Between August 2019 and November 2022, 82 patients with histologically confirmed cervical cancer were included. Staging was performed according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) classification. The characteristic of patients, including age, BMI, FIGO stage, tumor size, histological type, and courses of concurrent chemotherapy, were recorded.
Patients underwent CT simulation for treatment planning (initial CT) in supine position with custom immobilization and 3-mm slice thickness. All patients were treated on a linear accelerator equipped with kilo-voltage CBCT (kV-CBCT) with IMRT using seven to nine coplanar fields and 6-MV photons. Treatment was planned using the Eclipse Treatment Planning System version 13.0 (Varian Medical Systems, Palo Alto, CA). In accordance with our treatment protocol all the patients received a combination of external beam radiation therapy (EBRT) with concurrent cisplatin of 40 mg/m 2 weekly, followed by high-dose-rate brachytherapy 6 Gy per fraction for 5 fractions.
The delineation of the clinical target volume and organs-at-risk is in accordance with RTOG consensus guidelines. The dose given to PTV was 45–50.4 Gy (25 or 28 fractions of 1.8 Gy), five fractions a week. Simultaneous integrated boost (SIB) of the parametrium and positive lymph nodes were applied if needed. And all patients received the high-dose-rate brachytherapy 6 Gy per fraction for 5 fractions. The total dose achieved 85–90 Gy (EQD2, biologically equivalent dose of 2 Gy per fraction).
All patients received at least a weekly cone-beam computed tomography (CBCT) scan throughout the whole EBRT course. The image datasets including one simulation CT for treatment planning (initial CT) and weekly CBCT images at the time of treatment per patient were collected. For each patient, the weekly CBCT images were projected rigidly to the initial CT image using matching of the bony structures to evaluate the uterine motion during radiation treatment. To minimize internal organ motion caused by bladder, patients were asked to empty bladder first and then to drink 500 ml of water 1 h before CT simulation as well as daily radiation treatments. To maintain an empty rectum, patients were asked to have a bowel movement or to use glycerin enema within 4 h before simulation and each of the radiation treatments.
Manual adaptive radiotherapy
Figure 1 showed the workflow procedure of the manual adaptive planning during radiotherapy. All patients underwent a second CT simulation scan (re-CT) after 15 fractions during treatment. The re-CT images were fused to the initial CT image set using bone matching, and the original PTV were copied to the re-CT images. The thresholds for re-plan action after 15 fractions of treatment were 1) if uterus was outside the original PTV or 2) if cervix/upper vagina was outside the original PTV. In this case, a new treatment plan (the manual adaptive plan) with the re-contoured target volume and OARs based on re-CT scan of the remaining fractions was calculated. Similarly, the delineation of the clinical target volume and organs-at-risk is in accordance with RTOG consensus guidelines.

Overview of workflow procedure of the manual adaptive planning during radiotherapy
The new plan utilizes the same standards and prescribed dose as the original plan, even being created by the same physicist, the primary target coverage objectives were as follows, more than 99% of the PTV volume to be covered by 95% of the prescription dose.
Monitoring uterine motion during external beam radiotherapy
In the median plane (sagittal plane through the midline of the body), we measured “line A” (Fig. 2 ) from the superior border of the fundus uteri to the superior border of the pubic symphysis, and measured “line B” (Fig. 2 ) from the anterior border of the uterus to the anterior border of the pubic symphysis in weekly CBCT images as well as the initial CT image per patient. The initial position of uterus was normalized to 0. We calculated ΔA and ΔB to evaluate the movement of uterus, and eventually got value “C” for comparison.

The definition of the distance to the boundary of the uterus in the median plane (sagittal plane through the midline of the body) on magnetic resonance images (an example of 1 patient, on a T2-weighted magnetic resonance image). A means the distance from the superior border of the fundus uteri to the superior border of the pubic symphysis. B means the distance from the anterior border of the uterus to the anterior border of the pubic symphysis
The ΔA, ΔB and C was calculated as follows,
“A0” means the distance from the superior border of the fundus uteri to the superior border of the pubic symphysis in initial CT image.
“B0” means the distance from the anterior border of the uterus to the anterior border of the pubic symphysis in initial CT image.
“n” represents the number of CBCT sessions.
The volumes of cervix gross tumor were computed by eclipse software.
Dose data acquisition
The original CT and the re-CT (15 fractions after) are fused and registered in the Varian Eclipse planning system according to the bone structure, and then the original plan is loaded onto the re-CT to generate the plan, named scheduled plan. To compare the planning target volume coverage between the manual adaptive plan and scheduled plan, the conformity index (CI) and homogeneity index (HI) were calculated. The values of dosimetric objectives obtained from the dose-volume histograms (DVHs) were compared. For the PTV, the dosimetric parameters included the mean dose (Dmean), the dose received by 98% of the target volume (D98), the dose received by 2% of the target volume (D2), homogeneity index (HI) and conformity index (CI). For the OARs, such as bowel, bladder, sigmoid colon and rectum, the dosimetric parameters referred to the maximum dose to 2 cc’s (D2cc). All these dosimetric parameters were compared between the manual adaptive plan and scheduled plan in the remaining radiation fractions after 3 weeks of treatment.
The HI was calculated as follows (Wu et al. 2003 ),
where D2, D98 and Dmean were defined as mentioned above.
The CI was calculated as follows (Paddick. 2000 ),
Vt, ref is the target volume covered by the reference isodose line, Vt is the volume of the PTV, and Vref is the total volume covered by the reference isodose line.
Statistical analysis
The SPSS software (version 19, SPSS Inc., Chicago, IL, USA) was conducted for the statistical analysis. Kruskal–Wallis with Dunn’s multiple comparisons test were used to analyze uterine motion during EBRT, and t-tests were used to compare the accumulated doses to the targets and OARs from the two planning scenarios. In addition, the chi-square test (or Fisher’s exact test when appropriate), univariate and multivariate logistic regression analysis were performed to evaluate the clinical factors influencing target motion. We defined p < 0.05 as statistically significant. This study was approved by the Medical Ethics Committee of the Ruijin Hospital (approval number:2024153). Written informed consent was not required because of the retrospective nature of the study.
Baseline characteristics of patients
Table S1 showed the clinical characteristics of these 82 LACC patients. The median age was 57 years (range 34–85 years) at time of diagnosis. And disease was restaged according to the 2018 FIGO staging system. The mean clinical tumor volume was 66.8cm3 (range 12–279.9cm 3 ). All patients underwent a second CT simulation scan (re-CT) after 15 fractions during treatment, and 41 patients completed the remaining fractions using a new treatment plan (the manual adaptive plan) due to significant deviation of the PTV. All patients completed EBRT followed by brachytherapy, while 66 patients received concurrent platinum-based chemotherapy.
Analysis of inter-fraction motion during EBRT
Table S2 summarized the motion of the uterus during the EBRT treatment period. The initial position of uterus was normalized to 0. As shown in the Table S2 , the maximum movement of the uterus happened three weeks after treatment started, the median value “C” and the maximum value “C”were 1.811 and 4.517, respectively. Kruskal–Wallis with Dunn’s multiple comparisons test showed that the median uterus movement after three weeks (3rd CBCT) was greater compared with the movement after one week (1st CBCT, P < 0.0001) and two weeks (2nd CBCT, P = 0.0045) after the treatment started. Additionally, the median uterus movement after three weeks and four weeks has no significant differences. Figure 3 showed the inter-fraction motion of uterus (Value C) of all the patients.
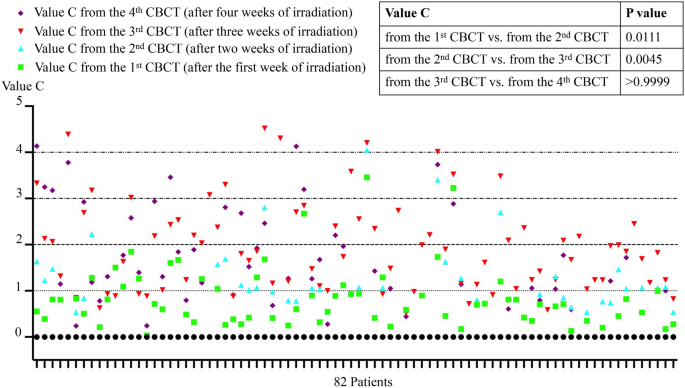
The inter-fraction motion of uterus (Value C) of all the patients. The difference of uterus positions during radiotherapy were compared by Kruskal–Wallis with Dunn’s multiple comparisons test
Target volume and OARs dose data analyses
Figure 4 a showed the mean CI of scheduled plan was 0.7711 ± 0.1382, which was lower than that of the manual adaptive plan 0.8586 ± 0.0354 (P = 0.0002). Similarly, the mean HI of the scheduled plan was 0.1764 ± 0.0779, which was higher than that of the manual adaptive plan 0.1469 ± 0.0776 (P = 0.003). Additionally, as shown in the Fig. 4 b, PTV D98 of manual adaptive plan was 2287.6 ± 59.8 cGy, which was higher than that of the scheduled plan (2212.9 ± 117.67 cGy, P = 0.0002). That is to say, the dose conformity, dose homogeneity and target coverage of PTV in manual adaptive plan is better than the scheduled plan (see Fig. 4 c). There is no difference in D2 and Dmean between the two groups (Table S3 ).

Comparison of the planning target volume coverage (PTV CI, HI, and D98) between the scheduled plan and manual adaptive plan. a Point plots of CI and HI for scheduled plan and manual adaptive plan, student’s t test, n = 41. b Point plots of accumulated dose to 98% volume for scheduled plan and manual adaptive plan, student’s t test, n = 41. c Comparison of the isodose distribution images of transverse for scheduled plan and manual adaptive plan of one patient. ***p < 0.001
Table 1 depict the differences in the D2cc of the organs-at-risk between the manual adaptive plan and scheduled plan. The D2cc of the rectum, bladder for the remaining fractions of manual adaptive plan was 2426.53 ± 67.66 cGy and 2502.75 ± 13.18 cGy, which was lower than that of scheduled plan, 2462.37 ± 74.37 cGy (P = 0.000034) and 2526.7 ± 14.32 cGy (P = 0.03), respectively. There is no difference in D2cc to the sigmoid and bowel between the two groups. The DVH lines for the two plans were displayed in Fig. S1 (an example of 1 patient).
Relationship between clinicopathological characteristics and replanning in LACC patients
Table 2 showed the clinicopathological characteristics of patients treated with scheduled plan or manual adaptive plan. Chi-square test showed that compared with the scheduled plan group, patients in the manual adaptive plan group were younger (age ≤ 60, 73.2% vs. 48.8%, P = 0.024) and with larger primary tumor volume (≥ 50cm 3 , 82.9% vs. 58.5%, P = 0.015). In addition, parametrial infiltration was found more in manual adaptive plan than in scheduled plan one, 75.6% (31/82) vs. 51.2% (21/82), P = 0.022.
In univariate analysis, the age (HR 2.864; 95%CI 1.138–7.209; P = 0.026), primary tumor volume (HR 3.440; 95%CI 1.236–9.576; P = 0.018), and parametrial infiltration (HR 2.952; 95%CI 1.154–7.556; P = 0.024) were the clinical factors significantly influencing target motion during EBRT. The multivariate analysis found that the tumor volume ≥ 50cm3 (HR 3.254; 95%CI 1.062–9.969; P = 0.039) and parametrial infiltration (HR 3.376; 95%CI 1.229–9.270; P = 0.018) were independent risk factors for replanning in patients with LACC (Table 3 ).
Substantial uterus motion and tumor shrinkage during radiotherapy significantly influence target coverage and OAR doses in LACC patients (Oh et al. 2014 ). There are previous studies demonstrating that oART can improve the target coverage and also reduce the dose of RT delivered to organs at risk in a variety of tumor sites (Hall et al. 2022 ). Online Adaptive Radiation Therapy (oART) is a radiation therapy technique that adjusts the treatment plan during the radiation therapy process based on changes in the patient's anatomical structure. This technology relies on advanced image-guided systems, such as cone-beam computed tomography (CBCT), to capture real-time images of the patient before each treatment fraction. If significant changes in the patient's anatomical structure are detected, such as tumor shrinkage, displacement, or shape alteration, the doctors can adjust the patients’ target volume and treatment plan by oART technology to ensure the accuracy and effectiveness of the treatment. During the online ART treatment, additional consideration regarding staffing and workflow are needed during each treatment, for example, it requires a radiation oncologist, medical physicist and radiation therapists to spend additional time at the machine daily (Shelley et al. 2021 ; Hall et al. 2022 ).
Thus, in our study, we introduced an alternative offline ART, the manual adaptive radiotherapy, which only required one more CT simulation during the definitive EBRT for cervical cancer. Our study analyzed the uterus mobility based on the weekly CBCT images, and found that the greatest variation in uterus position happened after 15 fractions of treatment. Thus, we recommend the patients underwent re-CT after 15 fractions during the manual adaptive radiotherapy, and ran the manual adaptive plan for the remaining fractions if significant deviation of the original PTV (1. if uterus was outside the PTV or 2. if cervix/upper vagina was outside the PTV). The new plan utilizes the same standards and prescribed dose as the original plan, even being created by the same physicist. We furthermore evaluated the potential dosimetric benefits compared with the scheduled plans, and explored the clinicopathological factors guiding patient selection for oART as well (for those have great variation in their uterus were suggested to underwent oART which will create a new plan according to their anatomical changes daily).
The key of offline ART is to determine the appropriate time for the replanning. A number of studies showed that the location of the uterus changes significantly in LACC patients during the EBRT course (Bunt et al. 2008 , Taylor et al. 2008 , Jadon et al. 2014 ), with the largest motion happened in the 2nd or 3rd week of treatment (Lee et al. 2007 ). In addition, it is reported that most of the tumor regression occurs during the first 3–4 weeks of treatment, with a mean tumor volume reduction of 50% after about 21 days, or after 30.8 Gy (Lee et al. 2004 , and Bunt et al. 2006 ). Consistent with these findings, our study analyzed the uterus mobility based on the weekly CBCT images, and found that the largest range of uterus movement happened after 15 fractions of treatment. All patients in our study underwent re-CT simulation after 15 fractions, and half of them completed the remaining fractions using a new treatment plan (the manual adaptive plan), due to the significant changes in target volume. Several studies have analyzed the dosimetric results on target coverage and dose to OARs when various adaptive procedures adopted. Creating an adaptive plan based on weekly CT/MRI images improved the dosimetric parameters by increasing the target coverage and decreasing the dose to OARs, such as the D2cc and V45Gy (Yock et al. 2021 ; Stewart et al. 2010 ). And the dosimetric parameters of adaptive plans based on re-CT simulation images after 15 fractions in our study were not inferior to those based on weekly CT/MRI images. We also found PTV D98 of the manual adaptive plans increased by 3.3% (2287.6 cGy vs. 2212.9 cGy, P = 0.0002), which was higher than that of 2.5% in the study with automated weekly replanning (Stewart et al. 2010 ).
For the OARs, Yock et al. described the decrease in the D2cc of the OAR resulting from the adaptive replan for cervical cancer patients. In their study, the D2cc to the bladder, bowel, rectum, and sigmoid colon for each fraction changed an average of − 0.02 Gy (SD = 0.09, p = 0.017), − 0.08 Gy (SD = 0.06, p < 0.001), − 0.07 Gy (SD = 0.07, p < 0.001), and − 0.04 Gy (SD = 0.05, p < 0.001) respectively (Yock et al. 2021 ). Consistent with the previous studies, ours showed that compared to the scheduled plans, the average of D2cc to the rectum and bladder in the manual adaptive plans decreased 0.358 Gy ( P < 0.001) and 0.240 Gy ( P = 0.03), respectively. However, there is no difference in D2cc to the sigmoid or bowel between the two plans, indicating the manual adaptive plan, which depends on numerous interrelated considerations regarding plan reoptimization, may not universally bring dosimetric benefits for all organs in all situations. On the contrary, Oh et al. analyzed the dosimetric effects of various adaptation strategies for cervix cancer. They observed dose reduction to the OAR was not at the level of clinically meaningful in most cases, though target shrinkage was observed (Oh et al. 2014 ). Therefore, the dosimetric advantages of manual adaptive plans in patients with LACC needed further validation in large-scale studies.
Unfortunately, financial and human resources remain barriers for the introduction of online adaptive radiotherapy in most low- and middle-income countries where cervical cancer has a greater prevalence, although the online ART improved CTV D98 and reduced normal tissue dose. In our study, we meant to find out who would benefit the most from the adaptive radiotherapy with daily replanning to save the time as well as human resources spending on the ART for all the patients. Thus, it’s necessary to identify patients who experience greater organ mobility during EBRT. In our study, the manual adaptive replanning workflow started when the uterus position observed beyond the PTV margin by re-CT after 15 fractions. We tried to explore the clinicopathological factors that may influence organ mobility during EBRT to establish a prediction model selecting patients the most suitable for the ART, which has not been reported in previous studies. The patients in our study with bulky mass (≥ 50cm 3 , OR = 3.254, P = 0.039) or with parametrial infiltration (OR = 3.376, P = 0.018) were highly suggested to receive adaptive replanning during definitive radiotherapy. Data from our study revealed that 82.9% of patients with tumor volume ≥ 50 cm 3 and 75.6% of patients with parametrial infiltration received raplanning. However, to date, there has been no study on the relationship between clinicopathological factors and organ mobility during EBRT. Therefore, further studies are needed to verify the value of these factors for predicting ART in patients with LACC.
The limitations of this study are the CBCT-guided ART and the limited sample size. However, kV CBCT is currently the most commonly available for online volumetric imaging, and in most cases differentiating target from surrounding tissues was not difficult (Khan et al. 2012 ). The MRI-guided online ART system has the potential advantages such as excellent soft-tissue definition and more accurate target delineation compared to CBCT-guided ART (Fields et al. 2016 ). And the results from our study need to be confirmed in a large sample of prospective studies. Despite these limitations, our study determined the appropriate time of manual adaptive replanning and further revealed that the tumor volume and parametrial infiltration may be two factors that predict the need for online ART during the EBRT for LACC patients, which has great significance.
In summary, we found the most significant organ motion happened after 15 fractions during treatment. The manual adaptive plan ran only once during the whole treatment time improved the dose coverage and decreased the volume of organs at risk in patients with LACC. Patients with bulky mass or with parametrial infiltration were highly suggested to receiving adaptive radiotherapy.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Beadle BM, Jhingran A, Salehpour M et al (2009) Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 73:235–241
Article PubMed Google Scholar
Chan P, Dinniwell R, Haider MA et al (2008) Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys 70:1507–1515
Cohen PA, Jhingran A, Oaknin A (2019) Cervical cancer. Lancet 393:169–182
Fields EC, Weiss E (2016) A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat Oncol 2(11):15
Article Google Scholar
Hall WA, Paulson E, Li XA et al (2022) Magnetic resonance linear accelerator technology and adaptive radiation therapy: an overview for clinicians. CA Cancer J Clin 72:34–56
Jadon R, Pembroke CA, Hanna CL et al (2014) A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 26:185–196
Article CAS PubMed Google Scholar
Khan A, Jensen LG, Sun S et al (2012) Optimized planning target volume for intact cervical cancer. Int J Radiat Oncol Biol Phys 83:1500–1505
Lee CM, Shrieve DC, Gaffney DK (2004) Rapid involution and mobility of carcinoma of the cervix. Int J Radiat Oncol Biol Phys 58:625–630
Lee JE, Han Y, Huh SJ et al (2007) Interfractional variation of uterine position during radical RT: weekly CT evaluation. Gynecol Oncol 104:145–151
Mayr NA, Magnotta VA, Ehrhardt JC et al (1996) Usefulness of tumor volumetry by magnetic resonance imaging in assessing response to radiation therapy in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 35:915–924
Mayr NA, Taoka T, Yuh WT et al (2002) Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys 52:14–22
Monk BJ, Tewari KS, Koh WJ (2007) Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol 25:2952–2965
Oh S, Stewart J, Moseley J, Kelly V et al (2014) Hybrid adaptive radiotherapy with on-line MRI in cervix cancer IMRT. Radiother Oncol 110:323–328
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical Note J Neurosurg 93(Suppl 3):219–222
PubMed Google Scholar
Qin A, Sun Y, Liang J, Yan D (2015) Evaluation of online/offline image guidance/adaptation approaches for prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys 91:1026–1033
Shelley CE, Barraclough LH, Nelder CL et al (2021) Adaptive radiotherapy in the management of cervical cancer: review of strategies and clinical implementation. Clin Oncol (R Coll Radiol) 33:579–590
Stewart J, Lim K, Kelly V et al (2010) Automated weekly replanning for intensity-modulated radiotherapy of cervix cancer. Int J Radiat Oncol Biol Phys 78:350–358
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
Tan LT, Tanderup DK, Kirisits DC et al (2019) Image-guided adaptive radiotherapy in cervical cancer. Semin Radiat Oncol. 29:284–298
Taylor A, Powell ME (2008) An assessment of interfractional uterine and cervical motion: implications for radiotherapy target volume definition in gynaecological cancer. Radiother Oncol 88:250–257
van de Bunt L, van der Heide UA, Ketelaars M et al (2006) Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression. Int J Radiat Oncol Biol Phys 64:189–196
van de Bunt L, Jurgenliemk-Schulz IM, de Kort GA et al (2008) Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need? Radiother Oncol 88:233–240
Wu Q, Mohan R, Morris M et al (2003) Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys 56:573–585
Yan D, Vicini F, Wong J et al (1997) Adaptive radiation therapy. Phys Med Biol 42:123–132
Yock AD, Ahmed M, Ayala-Peacock D et al (2021) Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum. J Appl Clin Med Phys 22:210–221
Article PubMed PubMed Central Google Scholar
Download references
This study was supported in part by the Scientific and Technological Innovation Action Plan of Shanghai Science and Technology Committee (No. 22Y31900103), Shanghai Pujiang Program (No. 2019PJD029), and Shanghai Sailing Program (No. 21YF1437500).
Author information
Authors and affiliations.
Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, No.197 Rui Jin Er Rd, Shanghai, 200025, China
Yi-Wei Wang, Min Chen, Wen-Tong Shen & Hao-Ping Xu
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Collection and assembly of data: Yi-Wei Wang, Min Chen and Wen-Tong Shen. Data analysis and interpretation: All authors. Manuscript writing: Yi-Wei Wang, and Hao-Ping Xu. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Wen-Tong Shen or Hao-Ping Xu .
Ethics declarations
Conflict of interest.
All authors declare no conflicts of interest associated with this manuscript.
Ethical approval
This retrospective study was performed using data from anonymized patients who received radiotherapy treatment between August 2019 and November 2022. Because of the nature of retrospective design and patient anonymization, the Institutional Ethics Committee of the affiliated Ruijin hospital of Shanghai Jiaotong University School of Medicine approved the retrospective study.
Consent to participate
The need for written informed consent was waived due to the retrospective nature of the study.
Consent for publication
All authors have consented to publication of the results presented in this manuscript.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (JPG 184 KB)
Supplementary file2 (docx 15 kb), supplementary file3 (docx 20 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Wang, YW., Chen, M., Shen, WT. et al. The clinical practice and dosimetric outcome of the manual adaptive planning during definitive radiotherapy for cervical cancer. J Cancer Res Clin Oncol 150 , 280 (2024). https://doi.org/10.1007/s00432-024-05809-z
Download citation
Received : 14 April 2024
Accepted : 17 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s00432-024-05809-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Locally advanced cervical cancer
- Radiotherapy
- Manual adaptive plan
- Clinical predictors
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Abstract. The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental ...
Watch this video to learn about the three phases of clinical trials. Clinical Research Phase Studies. Phase 1. Study Participants: 20 to 100 healthy volunteers or people with the disease/condition ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
the General Clinical Research Center (GCRC) statistician and consultant at the Harbor- UCLA Medical Center, she worked with both junior and senior inves-tigators developing protocols for clinical studies. Doing this work she identi ed the need for a book that would provide details of clinical design in an accessible format for all investigators.
The research protocol fulfils scientific, ethical, and organizational requirements so that the study may be conducted efficiently and according to the plan, thus standardizing the procedures for research personnel to follow.
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
5.5.4 Clinical Data Management Plan. The design of the research data lifecycle should be strategized in the clinical data management plan (CDMP). The exact content of the CDMP will vary on the type of trial, the number of sites involved, and the sponsor's specifications. Among the recommended items to include are:
Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award. Joshy Thomas 31 Oct 2017. I have learned lot..Thanks.. ... Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help. The Editorial Team 28 ...
The Clinical Research Education Library is supported in part by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health under award number U24-DC012078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Objectives. Identify responsibility for oversight of research. Specify the purpose and goals of Quality Management in research. Implement internal auditing system for Quality Management. Discuss components of a QM Plan for research teams. Understand how to utilize tools, report findings and develop corrective plans.
CRSPR Email Listserve Clinical Research Staff Portal and Registry (CRSPR) is an email listserve to communicate important, timely information to Penn Medicine's Clinical Research Professionals. Needs PennKey login. ... (FCOI) management plan issued by the Vice Provost for Research, the conditions of the management plan will apply to the conduct ...
Research Strategic Plan. In 2019, the Department of Medicine invested considerable effort and resources to devising a strategic plan that will provide a roadmap for our research mission today and into the future. This work was guided by a Research Planning Committee that convened throughout the first half of 2019, reviewing the current state of ...
Abstract: Research coordinators may transition to clinical research associates/monitors during their careers.This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process.
Clinical research is the lifeblood of the biopharmaceutical industry. In January 2024, there were more than 480,000 registered studies on ClinicalTrials.gov, a significant increase from three years ago, when there were 365,000. This uptick is driving demand for researchers and clinical trial participants—and the staggering amount of money and ...
to anticipate and respond to the special communications challenges posed by the conduct of clinical research. Designed to be accessible and relevant to a wide audience, Communications Handbook for Clinical Trials ... Implementing our plan: ongoing communication at multiple levels is key 51 Box 4.4. Internal communications within the Male ...
Our survey says … Their findings, published earlier this month in JNCI, the Journal of the National Cancer Institute, provide a snapshot in time of NCI cancer centers' initial progress in diversifying their research and cancer care workforce. The 87-item questionnaire, designed by Li, Law and others, assessed the domains, infrastructure, support and future plans of the centers.
This was followed in June 2021 by The Future of UK Clinical Research Delivery: 2021 to 2022 implementation plan setting out the steps we would take to progress the vision in 2021 to 2022. This ...
e13670 Background: The last decade has seen a paradigm shift in clinical research with the accessibility of trial data, facilitating secondary analyses and the development of novel predictive models. Vivli a non-profit entity established in 2018, epitomizes this movement with its global platform (vivli.org) dedicated to the facilitating access to and re-use of individual participant data from ...
The National Institutes of Health (NIH) Office of Research on Women's Health (ORWH) recently issued the NIH-Wide Strategic Plan for Research on the Health of Women 2024-2028. The release of the strategic plan aligns with the President's Executive Order to Advance Women's Health Research and Innovation. This strategic plan is driven by three guiding principles: • Consider the complex ...
FIVE YEAR PLAN: 2022-2027 STRATEGIES ENABLING ELEMENTS. 1.Accelerate Hiring & Retaining Research Intensive Faculty 2.Grow Research Strengths: Centers of Excellence, Growth Areas 3.Establish Research Growth as a SLU-wide Priority 4.Solidify SLU's Role as a Leading St. Louis Research University A.Increase Faculty Research Support B.Enhance ...
Propose To evaluate the advantage of the manual adaptive plans comparing to the scheduled plans, and explored clinical factors predicting patients suitable for adaptive strategy. Methods and materials Eighty two patients with weekly online cone-beam computed tomography (CBCT) were enrolled. The re-CT simulation was performed after 15 fractions and a manual adaptive plan was developed if a ...