Content types
- Infographics
- Case studies
- Press releases
- Career Advice
- Contracting/Freelancing
- Digital health
- Employee Engagement & Retention
- Employer Advice
- Employer Brand
- Life Science news
- Proclinical News
- Working in Recruitment
- Workplace Diversity

Latest jobs
US$180000 - US$210000 per annum + Highly Competitive Salary
Cambridge, USA
Proclinical is seeking a dynamic leader to drive the overall digital strategy and implementation roadmap in alignment with business objectives and priorities.
Boston, USA
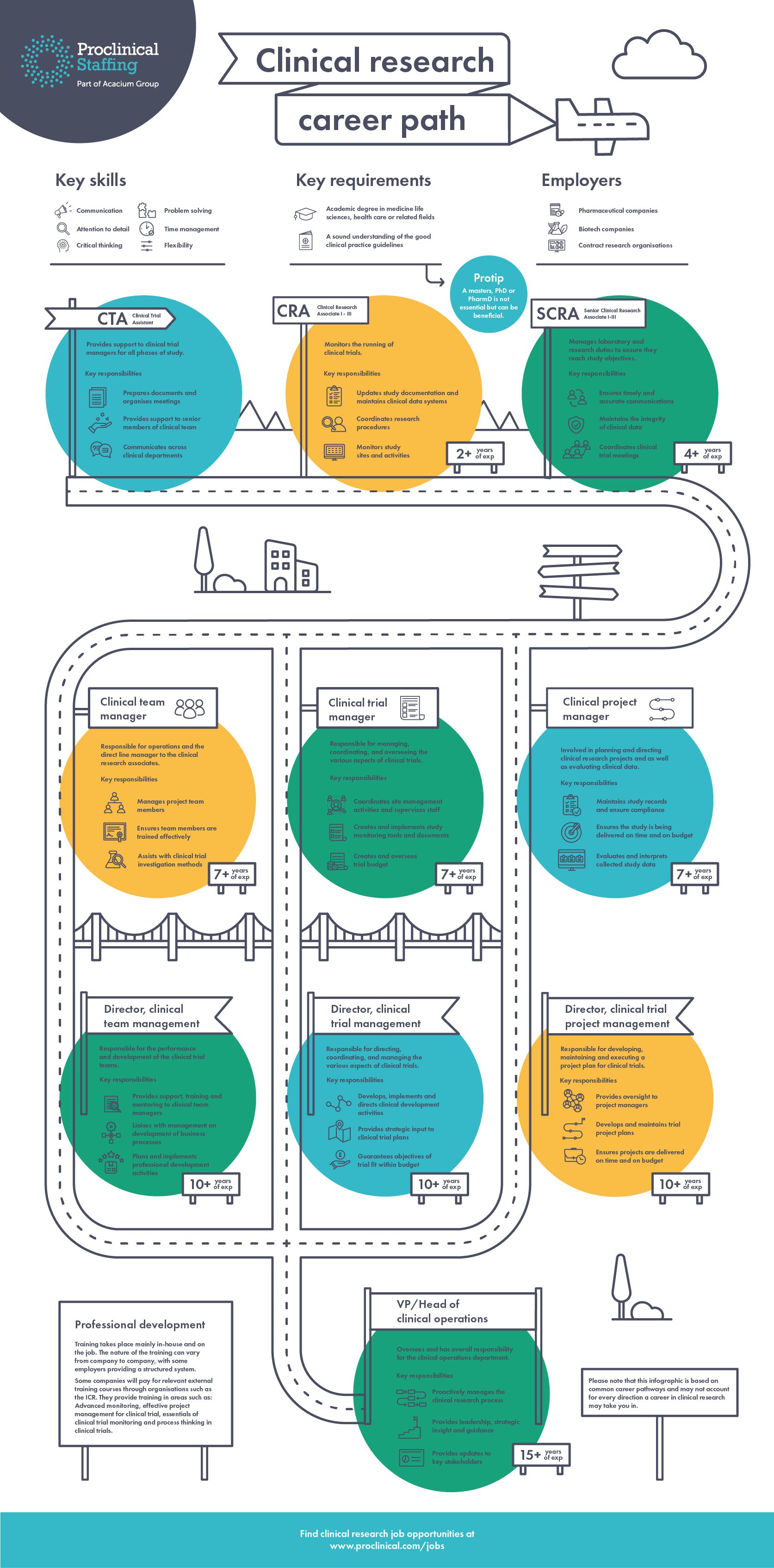
Infographic: Clinical research career paths
How to grow your career in clinical research
Clinical research is a competitive but growing field and provides rewarding career opportunities if you have qualifications or experience within life sciences . A career in clinical research involves playing a role in helping your employer conduct studies to ensure new treatments are safe and effective for patients. There are a variety of way to progress a career in the clinical research field with pharma, biotech, medical device companies and CROs all offering opportunities for professionals, such as the clinical research jobs listed on our website.
With the right experience, clinical professionals will have the potential to progress all the way to clinical director or even VP level. Once you have senior CRA level, there are typically three main routes a career in clinical research can take. You will either be a manager of the trial process, a manager of people or a project manager:
Clinical trial management:
Usually responsible for the managing, coordinating and overseeing various aspects of the trial. Typical duties will involve supervising staff, implementing study tools and documents, overseeing trial budgets and providing strategic input into trials.
Clinical team management:
Responsible for acting as the direct line manager to associates. Clinical team managers will manage and support team members, ensure staff are trained effectively, review the effectiveness of business processes and plan professional development activities.
Clinical project management:
Involved with the planning and directing of clinical trials and is also responsible for evaluating clinical data. Day-to-day tasks include, maintaining study records, ensuring activities are being delivered on time and on budget, interpreting study data and developing trial plans.
Here we discover what it takes to lead a fulfilling career in clinical research and the most common career pathways:
Looking to progress your career in clinical research?
We are hiring now for positions in clinical operations and clinical development within pharma and medical device companies and CROs. See our latest clinical research vacancies and apply today, or simply send us your CV to be considered for new positions as they become available.
Latest Posts

5 recent FDA oncology approvals that could change the future of healthcare

by Awonke Paul
.png)
(VIDEO) Bridging the Gap: Candidates & Employers in Clinical Research

by Theodora Savlovschi - Wicks

Mid-sized biotech, consultant clinical data management solution

by Proclinical Staffing

Top 10 women in life sciences today

by Hannah Burke
.png)
Top 10 drugs with patents due to expire in the next five years

Leading remotely: How to retain remote workers

Proclinical broadens its network of offices by opening in Munich

Who are the top 10 medical device companies in the world in 2023?

by Dove Jociute

Proclinical honoured with International Recruitment Company of the Year award

Who are the top 10 pharmaceutical companies in the world? (2023)

£600 - £650 per day
London, England
Proclinical is seeking a dedicated Manager of Global Trial Optimisation.

Highly Competitive Salary
Proclinical is seeking a dynamic leader to spearhead the North America's digital strategy and implementation roadmap. This is a permanent position located in Boston, MA.

£93 - £115 per hour
Uxbridge, England
Proclinical is seeking a dedicated and strategic Health Economics and Reimbursement Lead.

Highly Competitive
Dreux, France
Proclinical is seeking a dedicated Clinical Supply Chain Project Manager.

Philadelphia, USA
Proclinical is seeking a dedicated and dynamic Research Project Manager to support the operation of the Ovarian Cancer Research Center Tumor BioTrust Collection.

Hamburg, Germany
Proclinical is seeking a Senior Financial Analyst/Controller to join our finance team

Zürich, Switzerland
Proclinical is a currently supporting a Medical Technology client based in Zurich, Switzerland as they recruit a Team Lead to join and lead their Master Data team.

Plainsboro, USA
Proclinical is seeking a dedicated and dynamic individual for the role of Associate - SES Operations (Buyer). This is a contract position located in Plainsboro, NJ.

Career advice
- Proclinical Staffing
- Proclinical Consulting
- Proclinical Executive
- Proclinical Engage
- Job Opportunities
- Our Services
- Submit vacancy
- Insights and Advice
- Meet the Team
Register a new account

- Modern slavery agreement
- Gender pay gap
- Privacy policy
- Carbon Reduction Plan PPN 06/21
- Skip to main menu
- Skip to user menu
10 Clinical Research Career Paths
- Industry Features
- General Careers Advice
In 2020, the global Clinical Trials market was estimated at $44.3 billion, and this is expected to grow at an annual rate of 5.7% between 2021 and 2028. The National Institute for Health Research (NIHR) also recorded that between April 2020 and March 2021, 1,390,483 participants took part in Clinical Research across England, which is almost double the numbers from the previous year.
In this article, we look at 10 different career paths within Clinical Research, with an outline of some of the most common responsibilities for each role…
Clinical Trials Manager / Administrator
Clinical Trials Managers / Administrators are responsible for the administrative aspects of clinical trials. Their duties often include:
- Preparing essential documents and ensuring documentation is kept private and confidential.
- Attending safety and study start-up meetings and coordinating investigator meetings.
- Managing clinical trial supplies.
- Reviewing trial protocols and identifying any protocol issues.
- Processing and tracking payments to investigator sites.
More information on the role of a Clinical Trials Manager can be found here.
Clinical Research Associate (CRA)
CRAs are responsible for organising and administering clinical trials and are typically involved in all stages of a trial, from identifying investigator sites to closing down the trial. The responsibilities of a CRA can include:
- Identifying suitable facilities to be used as trial sites and selecting an investigator to be responsible for the site.
- Briefing trial investigators and instructing clinicians on how the trial should be conducted.
- Writing up clinical trial methodologies and designing trial materials.
- Monitoring the progress of clinical trials and preparing final reports.
- Designing and authenticating data collection forms and managing regulatory applications/approvals.
More information on the role of a Clinical Research Associate can be found here.
Clinical Project Manager
Clinical Project Managers are responsible for managing the workers involved in clinical research projects, ensuring protocol compliance whilst coordinating projects to meet clinical objectives. The main responsibilities of a Clinical Project Manager may include:
- Overseeing the enrolment of subjects into clinical trials by assessing the eligibility of potential subjects and tracking the enrolment status of suitable participants.
- Ensuring compliance with protocols and informing investigators of any protocol issues.
- Monitoring study activities to ensure the study remains on schedule and is kept within allocated budgets.
- Maintaining records of study activity, including records of side effect data.
More information on the role of a Clinical Project Manager can be found here.
Pharmacovigilance / Drug Safety Officer
Pharmacovigilance Officers, also known as Drug Safety Officers, are responsible for ensuring that new and existing drugs on the market are safe for patients, and for identifying any issues with these drugs. They may be responsible for:
- Monitoring the effectiveness of new drugs and pharmaceutical products already on the market.
- Monitoring adverse effects to new or existing drugs and flag any early warning signs of these to minimise risk.
- Conducting interviews with patients and healthcare professionals.
- Completing safety update reports and conducting safety audits.
Study Start Up Associate
Study Start Up Associates are integral in making sure that clinical research sites are well prepared to begin a new trial. They can be involved in the following:
- Executing start-up activities before site activation including preparing consent forms, identifying new investigator sites, allocating study budgets, and supporting patient recruitment and retention.
- Ensuring physicians working at research sites are prepared to begin trials.
- Obtaining appropriate ethics and regulatory approvals and ensuring research operations comply with protocols.
- Analysing study start-up metrics to ensure efficiency and identifying areas for development, including in terms of start-up timelines.
More information on the role of a Study Start Up Associate can be found here.
Clinical Research Nurse
Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include:
- Helping to develop new treatments and care pathways for patients.
- Aiding data collection activities.
- Ensuring patients give full consent prior to being enrolled in clinical trials and making sure patients fully understand all aspects of the study before doing so.
- Assisting the principal investigator with pre-study preparation and study start-up activities, including preparing protocols for regulatory and ethical approval, and attending investigator meetings.
- Arranging appointments for potential and enrolled trial participants.
More information on the role of a Clinical Research Nurse can be found here.
Clinical Research Scientist
Clinical Research Scientists are responsible for undertaking medical research in research labs to find more effective ways of diagnosing and curing a variety of illnesses. They may also be responsible for:
- Interacting with patients taking experimental treatments to understand the effectiveness of these treatments and to investigate new ways of improving their wellbeing.
- Working with other medical staff to advise on how to use products and equipment already on or coming to the market.
- Analysing data to further develop treatments and test any new methods of diagnosis and treatment.
Clinical Investigator
Clinical Investigators ensure that the investigation is meeting research expectations and is conducted in line with the investigator statement, investigational plan, and all necessary regulations. By doing so, they protect the welfare of clinical trial participants as well as the integrity of the resulting data. Their responsibilities can include:
- Meeting specific guidelines and/or requirements set by applicable regulatory and ethical bodies.
- Conducting or supervising research to ensure the investigational plan and corresponding study protocols are being followed.
- Notifying relevant bodies of any changes in research activity, including any unanticipated obstacles that may introduce risk to study participants.
- Ensuring informed consent has been obtained from all participants.
- Maintaining records of the clinical studies and preparing reports to be sent to investigation sponsors and other relevant bodies.
Patient Recruitment Specialist
Patient Recruitment Specialists are responsible for recruitment-related activities. Their main responsibilities include:
- Recruiting participants in line with protocol-specific inclusion and exclusion criteria.
- Tracking recruitment progress and developing new and existing recruitment strategies.
- Contacting potential participants to assess eligibility and to schedule site visits.
- Ensure patient information is accurately collected and entered into the relevant database and is protected.
Biostatistician
Biostatisticians provide statistical support to clinical studies and work across all study phases. Typically, their work can include:
- Obtaining clinical data from the Clinical Data Manager to undertake necessary statistical analyses. Interpreting the meanings of statistical outputs resulting from different analyses.
- Assisting the Clinical Trial Manager in writing up the final technical paper for the study, sharing findings from statistical analyses.
- Analysing safety and efficacy data and applying statistical methods to develop the science of data analysis.
More information on the role of a Biostatistician can be found here.
Current Opportunities in Clinical Research…
Take a look at current opportunities in Clinical Research here and set up job alerts to be notified of the latest opportunities in the industry.
* Article updated March 2024
Related links
- Jobs in Clinical Research
- More Careers Advice
Share this article
Related articles

Nouscom Appoints Leading Cancer Drug Development Expert, Neil Gallagher, MD, PhD, as Independent Advisor to its Board and Chair of its R&D Committee

AbbVie and Gilgamesh Pharmaceuticals Announce Collaboration and Option-to-License Agreement to Develop Next-Generation Therapies for Psychiatric Disorders

Recce Pharmaceuticals Reports Positive Preclinical Data of RECCE® 327 in Lung Infection Pilot Study
Latest articles, about kancera.

News & information
Keep up with the latest from r&d partners, 10 clinical research career paths and progression opportunities, felicia rodriguez.
- October 30, 2023
Clinical research careers contribute to the development of safe and effective treatments and therapies for patients. The responsibilities may vary based on the organization, therapeutic area, and specific study requirements, but they all share the common goal of advancing medical science and improving healthcare outcomes.
Progressing in clinical research jobs involves a combination of experience, education, certifications, and networking. All of these are fairly essential for career growth, although the specific path and opportunities may vary depending on your interests, the organization you work for, and the area you specialize in.
In this guide, we explore some of the many careers in clinical research, from entering the profession to potential progression opportunities.
Clinical research career paths
Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities.
1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol adherence. They coordinate study visits, maintain documentation, and communicate with investigators.
2. Clinical research associate (CRA) Entry-level clinical research associate jobs involve monitoring clinical trial sites, verifying data, ensuring regulatory compliance and study protocols, and assessing the safety and wellbeing of study subjects.
3. Clinical trial manager Clinical trial managers oversee all operations of a clinical trial, from study initiation to close-out. They manage budgets, timelines, and teams of CRAs, ensuring that trials are executed successfully.
4. Clinical project manager Clinical project managers manage and oversee multiple trials within a program. They collaborate with cross-functional teams, manage resources, and ensure that each phase of a project aligns with organizational goals.
5. Regulatory affairs specialist Regulatory affairs specialists are largely responsible for the administrative side of compliance. They compile, submit, and maintain regulatory documents for approval. You’ll have to stay informed on changing regulations and liaise with regulatory agencies, ensuring that all policies are adhered to.
6. Data manager Data managers manage clinical trial data, overseeing data collection, cleaning, and database management. They ensure data quality and see to it that all standards are followed, working closely with biostatisticians.
7. Clinical research scientist Clinical research scientists design study protocols, collect and analyze data, and interpret results. They also write study reports and publish findings in scientific journals.
8. Medical monitor Medical monitors oversee patient and subject safety during the trial, review adverse events, and make recommendations for study adjustments or halts based on medical knowledge.
9. Clinical quality assurance auditor Auditors conduct regular inspections and audits to adhere to regulations and quality standards. They identify non-compliance issues and recommend corrective actions.
10. Clinical research consultant Consultants provide expert guidance on various aspects of clinical research, including study design, regulatory strategies, and data analysis. They work independently or with organizations to solve complex problems.
Clinical research progression opportunities
To advance in clinical research careers, you can further your education by pursuing advanced degrees. These might include a Master’s in Clinical Research or an MBA. You could also obtain certifications relevant to your role, like the Clinical Research Professional (CCRP) or Project Management Professional (PMP).
Networking can also help you to get ahead by building rewarding relationships, connecting with peers and mentors in the industry and learning from more senior professionals. This could involve attending conferences, joining professional organizations, and asking to shadow leaders.
Here’s more detail on clinical research progression, and areas the above ten roles can move into.
1. Senior CRC or Clinical Research Associate (CRA) Clinical research coordinators (CRCs) can progress to a senior CRC or clinical research associate (CRA). Responsibilities include more independent study management, training junior coordinators, and handling complex trials.
2. Senior CRA or clinical trial manager Clinical research associates (CRAs) can progress to a senior CRA or clinical trial manager position. The duties of senior clinical research associate jobs are focused on starting to mentor junior CRAs. Clinical trial managers take on even more responsibility by overseeing an entire team of CRAs.
3. Clinical project manager or senior clinical trial manager Clinical trial managers can progress to clinical project manager roles, which involve managing the entire project portfolio. Senior clinical trial managers then demonstrate their capabilities by handling significantly more complex trials.
4. Director of clinical operations Clinical project managers can look to become directors of clinical operations. Directors play a pivotal role in overseeing the management and execution of clinical research programs within an organization, including teams of project managers and CRAs.
5. Regulatory affairs manager or director Over time, regulatory affairs specialists might be able to take up a manager position, which would involve handling larger portfolios of products. Eventually, you could become a director, overseeing an entire regulatory department.
6. Senior data manager or clinical data scientist Data managers often move into senior roles, which means managing larger datasets. You could then set your sights on becoming a data scientist, specializing in data analysis.
7. Senior research scientist or director of clinical research With experience, clinical research scientists can aim to become senior scientists. You would take on more significant research projects, possibly with the goal of becoming a director. This would mean running an entire research department.
8. Chief medical officer (CMO) Medical monitors can progress to chief medical officers (CMOs), who are responsible for all medical aspects of clinical research within an organization.
9. Senior auditor or quality assurance manager Later in their careers, clinical quality assurance auditors might become senior auditors, overseeing an audit team. You could then take up a quality assurance manager position, managing the entire quality assurance program.
10. Clinical research consultant As a consultant, your options for progression are slightly different. Rather than looking to move into a different role, the goal is usually to build a larger client base and gain expertise in specific therapeutic areas. Your earnings and reputation can then grow as you become an expert in the field.
Progressing your career with R&D Partners clinical research staffing agency
R&D Partners are dedicated to helping you excel in the rewarding field of clinical research. As experienced clinical research job recruiters, we understand that a rewarding career in this industry can shape the future of healthcare, making a positive impact on people’s lives.
Whether you’re looking for entry-level clinical research associate jobs or senior leadership roles, our goal is to provide you with insights, strategies, and guidance to chart your path to success in this ever-evolving industry. As partners to many leading life science organizations on the east and west coast, we can bring you exclusive career opportunities not available anywhere else.
Contact our friendly team to discuss your career options, or browse our current opportunities in clinical research careers.
Our global FSP & strategic staffing firm specializes in Scientific, Clinical Research, and Engineering.
Explore our top job opportunities.
We offer life sciences niche functional service provider & strategic staffing solutions to help you reach your goals.

Who’s Who in Clinical Research – A Complete Guide (2022)
- by Kunal Sampat
- February 25, 2022
- in Clinical Operations , General

Almost everyone I’ve met in clinical research has accidentally discovered this hidden profession.
Your interest in science and medicine somehow got you involved in clinical research and clinical trial management.
But didn’t you wish you had a crystal clear understanding of “Who’s Who In Clinical Research?”
That’s when this post comes handy.
Clinical Research is sometimes also referred to as Medical Affairs or Clinical Affairs.
Aside from the naming nuances, the pillars of clinical research remain the same. This is true for pharmaceuticals, medical devices, and biologics.
In this post, you’ll learn the functional areas that form an ideal clinical research team at a sponsor or clinical research organization (CRO).
For each function, I’ve highlighted why the group exists and their general mindset.
We’ll wrap up our discussion with adjacent departments that interact with clinical research professionals.
So let’s get started:
Clinical Project Management
I’d like to start with my personal favorite clinical research role, Project Management.
The reason why the project management role is so interesting is because it provides a holistic view of clinical trial management and clinical research.
“General Contractor” for the study
The project management team is made up of Clinical Project Managers and Program Managers.
In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers.
Project managers are the “General Contractors” of clinical research. They are accountable of all aspects of a clinical trial.
Go-to person for clinical trial budget, timelines, resources
Developing and actively managing clinical trial finances, timelines, and resource allocation is necessary for a smooth trial execution. This responsibility falls under the project management team.
After mapping out trial assumptions such as number of sites, patients, enrollment period, monitoring, a project manager will develop the study budget and timeline with key milestones.
Depending on the size and reporting structure of the company, project resources are either managed by the project manager or by functional managers.
Clinical project manager is able to connect the dots
A great project manager is able to connect two seemingly unrelated issues and assess the impact on the project.
Let me give you an example.
Say you’re in the midst of securing FDA approval to start a new clinical trial. You find out that the FDA has follow-up questions. This is going to delay the First Patient In (FPI) date. Your investigational devices with limited shelf-life are ready to be shipped to the sites.
A great PM will recognize the downstream effects of study startup delays. For example, she’ll start planning for the additional devices. Not only that but the PM will also adjust the study budget.
Clinical project manager anticipates issues
In addition, to be able to connect the dots, a project manager also anticipates issues.
For instance, a clinical site may be enrolling at a rapid pace. During a periodic data review meeting, the project manager finds out that the site has a significant number of protocol deviations.
Rather than letting the clinical site continue enrollment, the PM decides to put the site enrollment on hold until the compliance issues are fully addressed and resolved.
The PM anticipates the negative impact of compliance issues on the overall trial. If the PM does not anticipate the issue, it could have negative consequences on the final results and major audit findings for both the sponsor and the clinical site.
Clinical project manager can visualize and paint the big picture
A clinical trial has many moving parts. The objective of most trials is secure product approval, indication approval or assess the long term safety of the product after it is approved.
Keeping this end goal in mind, a clinical project manager needs to have a thorough understanding of the key milestones. If challenges arise (which they will), the project manager needs to communicate the challenges effectively to the right stakeholders.
It is also not uncommon for a project manager to paint the big picture for the team. This is necessary to prevent the team from digressing on irrelevant topics or getting distracted.
Clinical project manager is not afraid to get his/her hands dirty
When I started my career, I felt that the project manager’s primary job was to tell other people what to do and by when it needs to be done.
That certainly did not turn out to be true.
Most of us desire a “perfect” project with no issues or challenges. This is however not reality.
There will be obstacles along the way that a project manager will need to overcome.
For example, let’s consider a clinical site that urgently needs investigational devices to enroll a patient the next morning. Unfortunately, the FedEx package drop-off time is 4:00 pm and the pick-up truck has left for the day. The only way to get the device to the site in time for the procedure is to drop off the device package to the airport. A great project manager would drive to the airport and drop off that package.
Operations may be further subdivided in Internal Operations and External Operations. Members of the operations team usually have titles such as clinical research associate or clinical research manager.
CRA champions site start-up
The in-house operations team is primarily responsible for clinical trial site start-up. This team ensures that each site has the most recent version of the site start-up packet. The start-up packet consists of the protocol, ICF template, and other study specific documents.
The in-house operations team also facilitates clinical contract negotiation with the site, which includes the study budget.
CRA understands devil is in the details
Once the study-specific ICF template is sent to the sites, the site’s personnel review and edit the ICF to meet their site-specific IRB/ Ethics Committee requirements. The redlined ICF is then sent to the in-house operations team for final review and approval.
Similar to the ICF redlines, the CRAs also receive redlines on the clinical contracts.
This means that in-house operation is constantly inundated with multiple documents and redlines. This requires the in-house team to be detailed oriented and organized. An error can have significant ramifications including potential audit findings or lawsuits.
CRA’s life revolves site management and support
The in-house operations team is available for site management and support. Aside from ICF and clinical contract questions, it is usual for sites to contact their in-house CRA with questions about clinical protocol, study specific requirements, investigational device restocking, and more.
Field CRAs are also known as monitors.
In-house operations and field operations roles can sometimes overlap. In some organizations, field and in-house operations teams are combined into one group, namely Clinical Operations.
CRA is the face of the study for clinical sites
A field team is the “face of a study” for a clinical site. Most field operation team members work remotely from their home office. They travel to clinical sites on a regular basis for monitoring and site support.
The field CRA is also responsible for conducting pre-study visits, site initiation visits and study close-out visits.
A field CRA is responsible for monitoring clinical trial data at the site.
A field CRA needs to have thorough understanding of the study protocol, Good Clinical Practice (GCP) and regulations applicable to the conduct of clinical trials.
A clinical trial database has numerous fields. Depending on the monitoring plan, a CRA needs to review and verify the accuracy of the source data at the site.
For example, the CRA ensures the patient informed consent was signed prior to the procedure, serious adverse events were reported on time or protocol deviations were addressed appropriately. This work requires attention to details and deep focus to be done right.
CRA helps site succeed on all fronts – start-up, enrollment, compliance
Site coordinators can get overwhelmed with multiple trials. Many clinical sites are also nonprofit organization with limited funding and resources to support clinical research.
A field CRA can help their clinical site be successful by promptly answering all questions during study start-up, enrollment and follow-up.
In addition, it’s not uncommon for a CRA to prep sites prior to any scheduled audits.
The job of the field CRA is not “police” the site but rather be the site’s champion and help a site be successful in their research efforts.
Products are brought to market based on clinical data. Government agencies, medical community, and patients believe in the power of data.
It is no easy task to collect and clean hundreds of clinical trial data points in a compliant manner. This is where the data management team comes to play.
Data manager decides how data will be collected and cleaned
One of the key responsibilities of a data manager is to develop case report forms (CRF) for the collection of clinical trial data. These days most data is collected via electronic data capture (EDC) forms.
A well-designed and thought out CRF can be of great value to the sponsor in the long run.
For example, data collected in a case report form can not only help secure product or indication approvals but also forms the basis of publications and presentations.
Data manager’s life revolves around database locks
Once all patients have completed their primary endpoint visit and the data has been cleaned, the final step is to lock the database.
Database lock is a very important milestone not only for data management but also for the entire organization.
Database locks can be a very stressful time for data managers, sites and CRAs. The CRAs are frantically working with the clinical sites to resolve open queries. A database is usually not locked till open queries are resolved.
Once the database lock occurs, the biostatistics is able to analyze the data and generate tables and graphs.
Data manager communicates study metrics
Without data metrics, you would have no way of knowing whether your sites are completing the CRFs in a timely manner.
Let’s consider a 1000 patient clinical study at 65 sites with 3-year patient follow-up. For each patient there are 20-30 case report forms with multiple fields in each form. It becomes increasingly complex to determine what data is missing, incorrectly entered or not available.
A data management team can create custom reports that can provide data metrics by site, by visit or by patient. This information makes it efficient for sites and CRAs to address data entry gaps and query resolution.
Biostatistics (or Biometrics)
Statisticians are the numerical brain behind a clinical study. Statistics is a very broad field with numerous data analysis methods.
Biostatistician is the key driver behind trial design
One of the key components of clinical trial design is the sample size. Simply speaking, you want to know how many patients are needed in order for the clinical study to be statistically sound.
For many studies, regulatory agencies review the statistical analysis plan (SAP) as part of the trial design review.
Life revolves around p-values, trial power and performance goals
P-values, power and performance goals are the geeky pieces of information that statisticians care about.
Statisticians want to understand if the trial results (good or bad) are replicable in the real world.
They attempt to understand the probability of the certain benefits or risks to re-occur in real world once the medical products are commercially available.
Shares clinical study results using tables and graphs
The Statistical Analysis Plan (SAP) specifies how the data will be analyzed.
Once the clinical data is analyzed, it is presented beautifully in the form of tables and graphs. This is a key task as it forms the foundation of how the trial results will be communicated to the outside world.
Tables are graphs utilized in clinical summary reports, annual updates, presentations and publications. Tables and graphs are also included on product Information For Use (IFU) documents and Patient Guides.
When the product is approved to be sold commercially, sales and marketing teams use government approved tables and graphs to promote the use of the drug or device.
Clinical Safety
This is a very interesting role for anyone who wants to be closest to the medical aspects of any clinical trial.
Safety touches most aspects of a clinical trial including the protocol, patient informed consent, patient safety outcomes in the final study report, Instructions for Use (IFU) document and more.
Understands regulatory requirements around patient safety
Regulatory agencies are most concerned about the safety of the clinical trial procedure and investigational device or drug.
The safety team has a thorough understanding of regulatory requirements pertaining to the safety of any clinical trial.
When patients are enrolled in a clinical trial, they may experience an adverse event. Clinical sites, sponsors and CROs are required to meet strict adverse event reporting requirements. Adverse event reporting requirements can vary by country.
A safety team member has a clear understanding of regulatory requirements and helps ensure safety compliance.
Life revolves around adverse events management
A safety monitor champions all safety aspects of a clinical trial such as adverse events, clinical trial procedure risks, and device/ drug risks.
When a patient participates in a clinical trial, he or she may experience adverse events, also known as AEs. These AEs are reviewed by the safety team.
In some cases, there may be unexpected serious adverse events (SAEs) that may impact patient safety. When such events occur, the safety team evaluates and communicates the adverse event information with stakeholders such as clinical trial sites, patients, and regulatory agencies.
Manages Clinical Events Committee (CEC) and Data Monitoring Committee (DMC)
When patients experience AEs, the clinical sites reports the AEs to the trial sponsor or CRO. The safety team reviews reported AEs and collects relevant medical records.
A subset of these AEs and the corresponding relevant medical records are sent to a physician committee, known as the Clinical Events Committee (CEC). The safety team manages the selection and operation of a CEC.
The basic premise of a clinical trial is that it’s a drug or medical device experiment on human beings. Participation in a clinical trial usually involves safety risks. Some safety risks are anticipated and others aren’t.
For unanticipated risks during the enrollment phase, a sponsor may recruit a data safety monitoring board (DMC). The DMC ensures there is no risk to patients in the trial.
Not all trials require DMC as the duration of enrollment might be too small to detect a safety signal. Similar to CEC, the safety team leads the selection and management of a DMC.
Clinical Quality
Throughout my career, I’ve worked with clinical quality experts that either “police” every step in the clinical process or serve as a partner and resource to other clinical research functions.
In both cases, the ultimate goal of any clinical quality personnel is to keep you, your clinical trial and your organization out of trouble.
Ensures compliance in all aspects of a clinical trial
As we’ve discussed earlier, clinical research is a highly regulated industry. With regulations, comes compliance. Clinical research professionals need to comply with government regulations, Good Clinical Practice (GCP) and operating procedures.
You may ask, “What’s the purpose of all this compliance”?
Well, the simple answer is that the outcome of a clinical trial sets a new medical standard or leads to an update of an existing medical standard.
Would you trust a drug or device that is brought to market based on a non-compliant clinical trial?
Probably not.
Clinical quality wants no audit Findings
Passing an audit is the ultimate test of any clinical research organization. This is especially true for audits by regulatory agencies.
Audit findings are painful not just for the quality team but for the entire clinical team. Significant audit findings also raise doubts about the robustness of a clinical trial.
Life revolves around Standard Operating Procedures (SOPs)
For instance, in the US, FDA regulations dictate how a clinical trial should be conducted. These regulations form the basis of standard operating procedures (SOPs).
A great quality associate helps a clinical research organization create and maintain a practical set of SOPs.
It is important to note that the burden of creating SOPs does not lie with clinical quality. Other clinical research functions are responsible for drafting and finalizing their own role-specific SOPs.
Understands Corrective and Preventive Action Plan (CAPA)
Since many aspects of clinical trials are managed by humans, the process is prone to errors. Errors can happen due to oversight, gap in a SOP, or mismanagement. A quality personnel initiates a CAPA when errors are discovered.
The goal of a CAPA is to document the error and take necessary steps prevent the error from happening in the future.
CAPAs are frowned up and are never a good thing for any organization. More CAPAs means there is greater risk, which in turn reduces the public’s confidence in the clinical trial outcomes, product or company.
Medical Writing
Clinical trial information is communicated with all stakeholders via clinical documents such as the protocol, clinical reports and manuscripts.
A medical writer leads the task for assimilating trial information and putting it together in a logical way.
Understands scientific content
A medical writer has an in-depth understanding of the therapeutic area and previous clinical and preclinical information on the drug or device.
Given the countless number of medical products and trials, it’s not unusual to learn trial or product specific information after joining the project.
Life revolves around writing protocols, reports or manuscripts
As a medical writer, you need to enjoy the process of writing. If you don’t enjoy writing, it will be painful to write several hundred pages of clinical trial documents on a regular basis.
A medical writer has a unique ability to communicate high-level clinical trial strategy vision in a document such a protocol or report.
Regulatory decisions are made based on these documents. Therefore it is extremely important for a writer to understand what regulators are looking for.
Detailed oriented with focus on punctuation, grammar and formatting
A medical writer is responsible for writing key clinical documents such as the protocol, clinical reports, safety charters, and more.
Punctuation, grammatical errors or typos can reduce the reviewers trust in the final document. Such errors make the company look sloppy in front of regulators, clinical sites or trial sponsor.
Additionally, a medical writer needs to master writing software such as Microsoft Word. Inability to effectively use such software will cause issues with document formatting, resulting in delays and frustration.
Medical Science
A scientist is responsible for the clinical trial strategy.
In some organizations, the role of the scientist may be combined with that of the medical writer or that of project management.
Brainchild behind the overall clinical strategy
Prior to the start of any clinical trial, a lot of work is put into designing a cost-effective, safe and effective study.
Depending on the trial design, there may be quite a bit of back-and-forth between the regulator and the sponsor or CRO.
A clinical trial can begin enrolling patients only after the regulatory alignment is obtained.
The scientist champions the development of clinical documents and communication necessary to secure this regulatory alignment.
Life revolves around designing a trial that meets the primary endpoint
If you remember your life as a student (or maybe you’re still a student), your final grade on the test depends on how well you do on the finals.
Along the same lines, the success of any clinical trial is determined on whether or not it meets a pre-specified primary endpoint.
The trial endpoints are clearly stated in the protocol. Once the primary endpoint is met, the medical product will most likely be approved for commercialization.
Medical science wants high-quality clinical trial data
The success of a clinical trial is hinged on high-quality clinical trial data. But what does “high quality” mean?
High-quality data is error-free, accurate, and complete. Missing or incomplete data due to missed visits or sloppy data entry can cause headaches for many clinical stakeholders, primarily the clinical scientist.
Also, there can be a tendency to collect more data than needed for “just in case” scenarios such as unanticipated requests from regulators or potential for interesting publications in future years.
Clinical Systems and Solutions
Not too long ago, clinical trial data was collected on paper case report forms. Then it became incredibly hard to manage data queries, keep track of complete forms and manual entry of data from paper into an electronic database.
With the help of technology, electronic data capture (EDC) solutions were developed. Now site staff can directly enter data in the case report forms.
The Systems and Solutions team is responsible for managing clinical technology solutions such as the EDC. They are also responsible for onboarding new technology solutions and retiring old solutions that no longer add value to the organization.
Technology backbone for clinical trials
More than ever before, technology is becoming an integral part of clinical research. The system and solutions group is the technology backbone for clinical trials.
Want to incorporate wearable technology in a clinical study?
Or build an iPhone app to organize study team contact?
Clinical systems and solutions group is your go-to team.
Depending on the size of the company, this role may be a separate function within clinical research or part of the information technology (IT) organization.
Focused on clinical research software and applications
Clinical research teams utilize a few core technology solutions such as the Trial Master File (TMF), Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Integrated Web/Voice Response System (IxRS), and electronic Informed Consent (eConsent).
The TMF allows you to store study and site level documents in electronic format.
CTMS is useful for managing study contact information, monitoring trip reports, site and vendor payments and more.
EDC allows sponsors to design electronic case report forms and allows sites to remotely enter data into the system.
IxRS serves to register patients to a specific treatment.
In addition, there may be other systems for safety management and reporting, protocol deviation and monitoring visit management.
Understands regulatory requirements for technology solutions
Regulators are interested in ensuring clinical trial technology is compliant with the law.
At the most basic level, technology solutions need to maintain an audit trail, protect patient personal health information (PHI) and encrypt sensitive clinical trial information.
Systems and solutions teams need to have an in-depth understanding of regulatory requirements pertaining to technology solutions.
Regulators such as the FDA frequently publish guidance documents related to a specific technology product.
Guidance documents can be easily accessed via the FDA website and can serve as a great resource for anyone wanting to dig deep in a specific clinical technology area.
Senior Management
Senior management is ultimately accountable for the success (or failure) of any clinical trial.
Key senior management functions in a clinical research organization include directors of each of the functional teams described above.
Depending on how the organization is structured, a clinical director may oversee more than one clinical trial or clinical function.
Accountable for all aspects of a clinical program
Providing adequate oversight is the key role of a senior manager.
Oversight is not the same thing as “micro managing” an employee or a task. For certain critical deliverables, a senior manager may be more hands-on.
Strategic focus – Budget, Timelines, Resource Allocation, Procedure Compliance, Scientific Robustness
Clinical trial costs can run into millions of dollars. By controlling project timelines and resources, a senior manager can control expenses.
In addition, it is crucial to ensure the trial is conducted in compliance with regulations and internal procedures.
Finally, if the study is not scientifically robust, the clinical trial may not get regulatory approval.
It is not possible for one individual to be competent in all these areas. For this reason, a senior management team is needed to collaboratively achieve strategic goals.
Interested in Key Performance Indicators (KPIs)
Do you have a retirement account?
Or go to the doctor for an annual check-up?
If so, you’re probably paying attention to the performance of your retirement fund or your health over a period of time.
Along the same lines, senior managers are interested in a set of KPIs that track the performance of a clinical trial against a standard or threshold.
For example, regulators want to ensure patients sign the correct version of the informed consent form (ICF). Signing an incorrect ICF version is a major compliance issue. Thus ICF deviation rate can be a KPI.
Since senior managers are not involved in the day-to-day operations of the trial, they assess the health of a project by reviewing KPI metrics. If KPI metrics trend towards non-compliance, senior managers will take steps to address the issues.
Other Clinical Trial Stakeholders
A clinical department frequently collaborates and seeks help from other teams. The section below covers the key teams that interact and support clinical teams on a regular basis.
Since the focus of this post is clinical research, the role of these adjacent functions has been described keeping in mind the clinical context.
Regulatory Affairs (RA)
Regulatory Affairs (RA) is responsible for regulatory strategy and submissions. For a clinical trial, a RA team member is generally the primary contact person for the regulator such as the US FDA, Japan PMDA, China FDA.
Deep understanding of regulatory process and requirements
Ready to start a clinical trial? Or address clinical trial deficiencies with the regulator?
You’ll want to start with your regulatory affairs team. Clinical trials are conducted in compliance with regulations. RA can educate you on the clinical documents needed to meet regulatory requirements in a given geography.
A regulatory affairs expert has an in-depth understanding of the regulatory process and requirements.
Adept at reviewing and editing clinical submission documents
Once the clinical team has created the necessary documents such as the protocol, statistical analysis plan or the clinical summary report, a regulatory affairs associate performs a detailed review of the documents.
In addition, regulators have specific formatting requirements such as cover letters and forms that need to be completed with every submission.
Focus is on regulatory agency alignment on all aspects of clinical research
There is a specific way of communicating clinical trial information with regulators. This communication style is very different from the day-to-day communication amongst clinical research team members. A seasoned regulatory affairs expert understands this nuance and is able to communicate effectively with regulators.
Seeks new medical product/indication approvals
Success of a regulatory affairs team is measured by product and indication approvals. It’s no easy task to get a product to market, especially for new therapies.
Health Economics and Outcomes Research (HEOR)
Getting medical products or indications approvals is always great news.
But here’s the deal.
You need someone to pay for the approved drug or device. If payors such as insurance companies or Medicare are not willing to provide the reimbursement, it can significantly hurt a company’s bottom line.
This is where HEOR comes into play. A HEOR team helps create a body of evidence that the new drug or device has health benefits such as improved quality of life.
Deep understanding of payor process and requirements
A HEOR expert can help you develop a robust protocol and case report, so you can proactively collect the clinical data needed for reimbursement.
HEOR has an in-depth understanding of country-specific requirements. Their work leads to maximum medical product reimbursement for the company.
Understands medical product impact on Quality of Life (QoL)
Let’s say your friend experienced a heart attack and got a stent. Now 10 years have passed. Your friend has not experienced any new heart problems. He feels great, goes for a morning run and is able to spend quality time with his family.
How would you rate his quality of life? Excellent, right?
HEOR pays much attention to a patient’s quality of life. In addition to being safe and effective, medical products need to have a positive impact on a patient’s quality of life.
There are many tools available to measure such quality of life. Clinical trials include quality of life questionnaires for patients to complete. This data is then analyzed and utilized to secure reimbursement approvals.
Seeks to maximize medical product reimbursement
One of the primary roles of the HEOR team is to ensure the medical product receives maximum reimbursement possible.
Reimbursement can vary significantly based on country laws or availability of new health data. For example, Japan offers higher reimbursement for newer medical products but then decreases reimbursement in future years.
Clinical Research Organizations (CROs) and Consultants
Some sponsors hire full-time staff to manage their trials. Some outsource all clinical research tasks to CROs and consultants. While others may use a combination of internal and external resources.
Supports sponsor by filling in resource and talent gaps
It is expensive to build a full clinical team, especially for an organization with limited financial resources. CROs can help fill the talent gap for each of the clinical research roles discussed in this post.
Work is billable
A major chunk of clinical trial costs is full-time salaries paid for each of the clinical roles explained in this post.
CROs and consultants charge by the hour or by deliverable. The “pay by the hour” or “pay by deliverable” model gives sponsors greater flexibility and control on expenses.
People that work for a CRO may be full time employees or contractors. At any given time, a CRO or a consultant may be working on multiple projects for different sponsors.
Can help reduce fixed overhead expenses
For smaller organizations, steady cash flow can be an issue. Therefore having a full clinical team means paying for employee salaries, office space, health insurance and other benefits. This can be cost prohibitive.
Hiring a CRO or consultant can reduce fixed expenses, giving companies greater flexibility with their limited resources.
Clinical research cannot be conducted without legal support.
You’d open yourself to all sorts of risks if you didn’t have a lawyer on your team.
Complete understanding of legal landscape
A lawyer with clinical research experience has an understanding of intellectual property law (patents, trademarks), legal entities i.e. how the hospitals or suppliers are structured from legal standpoint, supplier contracts, subject injury and liabilities, and more.
Seeks legal compliance in a clinical trial
A lawyer will be extremely happy if a company is successfully able to conduct a clinical trial in a legally compliant manner.
For instance, there is great risk to patients participating in clinical trials. If the legal language on the informed consent form or clinical trial contract between the site and sponsor is inadequate or unclear, it can lead to lawsuits.
Lawsuits impact the company’s finances, brand and reputation. A legal team ensures the company does not end up in legal troubles with any of the clinical trial stakeholders.
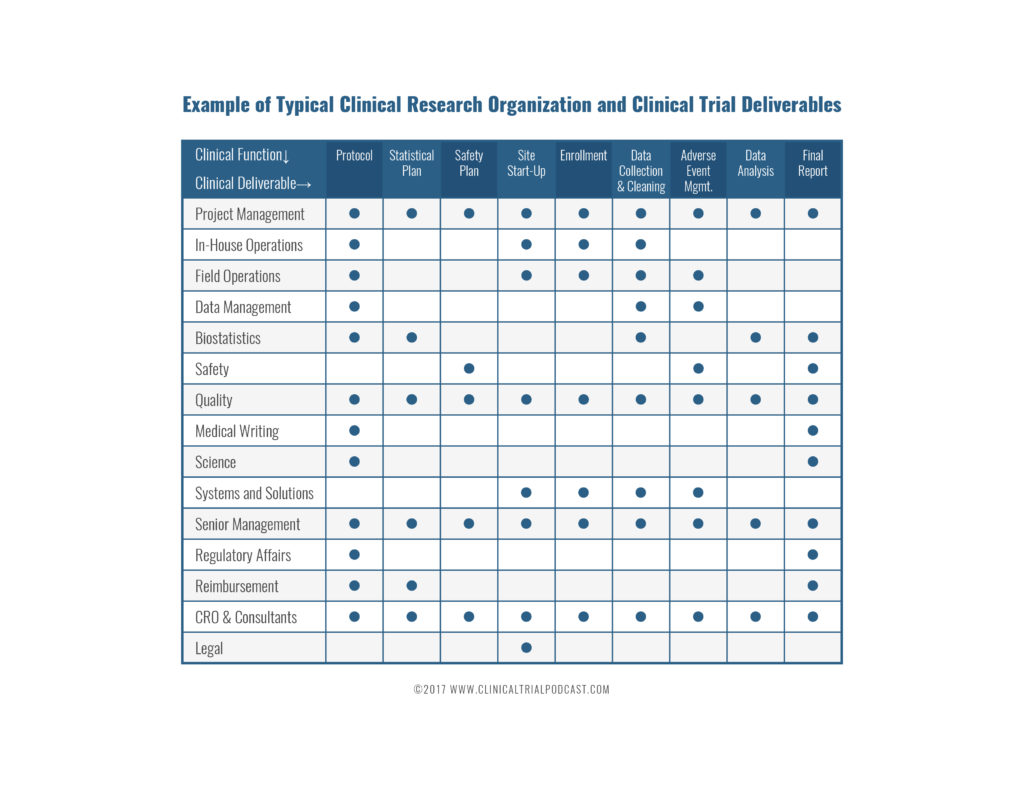
By now you have an in-depth understanding of all clinical research functions and teams that support clinical trials.
Clinical research and clinical trial management involve cross-functional teams. The chart below summarizes the roles of different clinical research team members.
Which clinical research function interests you the most? Leave your response in the comments section below.
Clinical Data Management with Mariam Mirgoli
Clinical research billing for small to medium sites with kristi etchberger, 25 thoughts on "who’s who in clinical research – a complete guide (2022)", ultimate guide to clinical trial costs - clinical trial podcast, 25 soft skills to boost your clinical research career – clinical trial podcast, 10 smart strategies to getting response from clinical site coordinators – clinical trial podcast, 5 ways to get clinical research associate experience – clinical trial podcast, 5 ways to engage clinical trial sites with technology | florence healthcare, conversation with clinical research expert | marshall cool, how to get a clinical research job | clinical trial podcast, how to get clinical research associate (cra) experience – clinical trial podcast, how to become a cra – career keg, 9 essential components of a clinical trial agreement - clinical trials arena.
Sonia Menezes
I have a masters in clinical research and work experience at an oncology site. I’m looking to get into to industry and medical writing is the most interesting to me. I wanted opinions on what people in the industry think about the field and whether it is too niche to start your with?
Kunal Sampat
Hi Sonia, I would suggest starting as a medical writer in clinical research. There is a huge demand for great medical writers. There are several medical writing groups on LinkedIn that you may want to consider joining. Reach to people in those groups and find out how you can get started. It’s not a small niche and the opportunities are endless. You can always move in different directions as long as you remain curious and interested in other roles. Goodluck!
mamtha srinivas
hi I have worked in clinicalresearch for last 4 years. i have an opportunity to work under investigator directly .I need to search for new projects.Guide me how to go about consulting for projects.
Hi Mamtha, I would recommend you follow the steps outlined in the blog post. https://clinicaltrialpodcast.com/get-a-clinical-research-job/ . Let me know if you have any additional questions. Goodluck!
Subuloye Olufunke
Hi, Please for someone thinking of going into the clinical research field with no experience, would you suggest getting an online training or getting a certification in one of the Universities? Which one has a better chance of getting a job considering the fact that there is no experience. Thanks.
An informative read. As an clinical research novice it imparts more knowledge about the field.
great, thanks for the feedback
Kunal, you’ve missed the Risk Manager, a critical role per ICH E6 R2, and expect even more important in upcoming R3. Love your work!!!
Thank you, Fran for your thoughtful comment. Safety monitors and quality associates can also serve as Risk Managers
This is such an informative post. I’m a Registered Dietitian and another RD informed me that RDs make good Project Managers in research; she works as one. I came across this post when looking for the “job ladder” and responsibilities involved in the field and its exactly what I needed. Thank you for compiling it!
raveena aher
Hey thanks for sharing this blog over here. It seems useful to start career in clinical research. We will look forward for more updates.
It is a pleasure to hear you share such useful information.
Moses Musitwa
Safety monitors and quality associates is some fascinating role,as a medical doctor, medical educator and manager which role would suite me because am spoiled for choice.
Safety monitors and quality associates are fantastic clinical research positions for doctors to pursue.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994.

Careers in Clinical Research: Obstacles and Opportunities.
- Hardcopy Version at National Academies Press
1 Introduction
Health care in the United States has improved markedly over the past five decades, in large measure because of the advances in health research that have been supported by a myriad of federal agencies, industry, the private nonprofit sector, and research institutions. Diverse teams of scientists composed of basic scientists, physicians, nurses, dentists, pharmacists, and other health professionals have been involved in research spanning a spectrum from fundamental biological discoveries about life processes, to behavioral and social research, to clinical and population-based studies, and to research on the organization, financing, and delivery of health care services. For example, imaging technology that allows investigators to peer into the human brain and observe the circuitry and biochemical reactions as humans construct thoughts and words is now available. Progress in genome mapping promises to provide monumental advances in understanding of genetic diseases and to aid investigators in finding biological therapies. Rational drug design is allowing researchers to custom design pharmacologic agents that can act on specific tissues, organs, or cell receptors and treat a broad spectrum of human maladies. Entirely new approaches to the treatment, cure, and prevention of human diseases are evolving with the availability of biological products and gene therapies. Research methods are being developed to permit investigators to evaluate the outcomes and effectiveness of health care practices. Research in these and other areas has formed a dynamic synergy that has positioned the United States at the forefront of innovation in medicine.
Despite all the advances, however, signs of stress are surfacing throughout the health care and health research systems. The soaring costs of health care and the escalating number of uninsured and underinsured people in the United States have thrown health issues into the policy arena at all levels of government. In medicine, highly subspecialized medical training, a declining interest by U.S. medical students in primary care training, and shortages of physicians willing to practice in rural or inner-city areas are all cited as symptoms of a worsening problem. The emergence of human immunodeficiency virus infection has demonstrated that new diseases can arise unexpectedly, and that a multifaceted approach spanning a variety of fields of research and a range of professional research scientists is needed to develop fundamental knowledge about a disease process, diagnosis, effective therapies, and prevention strategies and to assess the subsequent outcomes of health care practices. This can only be accomplished with highly talented and well-trained researchers in all areas of research, from basic to clinical research to outcomes and health services research.
Research is a highly social and political process of communication, interpersonal relationships, and scientific exchange that seeks to describe, explain, and modify biological and pathological processes. Researchers develop hypotheses and test them by collecting and analyzing data. The results add to existing knowledge. The unique feature of clinical research that distinguishes it from laboratory research is the direct involvement of human subjects. Although both laboratory and clinical research employ the same scientific principles for experimental procedures, the use of human subjects increases the complexities of scientific investigations. Whereas laboratory studies can more easily control for as many variables as possible to yield reproducible results, clinical research involves more heterogeneous populations, often is more expensive, takes longer to develop, requires long periods of time for data collection, and may be difficult to reproduce (Kimes et al., 1991). To advance medical care in patients, however, research must be performed in populations of patients with diseases.
Many research activities performed by a broad spectrum of professionals fall under the rubric of clinical research. Whereas many kinds of clinical research require similar skills and abilities, others may require different tools to achieve research objectives. Examples of how earlier investigations have influenced today's medical care are well known. Present research studies will improve tomorrow's medical practice, while future clinical research opportunities will affect care in the twenty-first century. What is the scope of clinical research, and what are the settings for conducting clinical research and the opportunities for future research?
- Scope Of Clinical Research
Clinical research is a relatively new discipline. Although the American Society for Clinical Investigation was formed in 1908, clinical advances prior to the 1950s were often based on imprecise observations by practicing clinicians (Cadman, 1994; Fox et al., 1992). In 1948 the British published the first randomized clinical trial, evaluating streptomycin in the treatment of malaria (Medical Research Council, 1948); the first clinical trial published in the United States was a study evaluating the effectiveness of penicillin for treating pneumoccocal pneumonia (Austrian et al., 1951). As methods for large-scale clinical studies became more refined, investigators gained an appreciation for new study designs, methodological advances, and the power of statistical analysis that permitted the validation of small differences between treatment regimens. Clinical research quickly became accepted as scholarly work and as an academic discipline (Fye, 1991; Ledley, 1991).
A major paradigm shift in clinical research was initiated in the 1970s when human cells were grown in vitro. As a result, some forms of clinical research could be performed on human cell lines grown in culture. This initiated a period some refer to as reductionism in which patients were no longer used as the primary focus of clinical research. This idea was extended by using the techniques of molecular biology, which permitted the study of human nucleic acid alterations in disease instead of requiring the study of the entire patient. Yet, in the final analysis, the application of these discoveries to improve medical care requires that these findings be used on the whole patient.
In parallel, during the last decade the discipline of clinical research has undergone a remarkable evolution in the scope, sophistication, and power of its methodologies. Changes have occurred in the approach to data collection, experimental design, and data analysis, and these changes provide a stronger basis for clinical research. In addition, understanding of the pathogenesis of diseases has provided more precise concepts of preclinical and subclinical disease states. The application of molecular epidemiology is a prime example of these changes in clinical research. Now the results of new biology are ripe for application to improve medical care, but many fear that a talented cohort of clinical investigators has not been prepared to translate these fundamental advances into improved medical care.
The revolution of fundamental research discovery is expected to accelerate in the future, driven by the explosion of science in biotechnology, molecular biology, computer technology, diagnostic systems, decision modeling, and clinical measurements technology. The sophisticated methods for clinical research require investigators with the requisite talents to design excellent clinical studies, recruit adequate numbers of research subjects, and analyze the large amounts of data collected. The need for cross-disciplinary teams to accomplish the objectives of multicenter, complex clinical trials is readily apparent. It is clear that training for a career in clinical research must be as rigorous as training for a career in the traditional basic sciences. Understanding of both the basic sciences and the evaluative sciences is essential to the success of clinical researchers. Moreover, novice clinical investigators require the same mentoring and nurturing in a supportive environment as those engaged in fundamental research disciplines if they are to develop into mature, independent scientists who remain competitive and productive over an extended period of time.
Numerous advances can be cited to describe opportunities in clinical research; the following allow one to comprehend their broad scope. One dramatic example of progress in fundamental research that has opened up immeasurable clinical research opportunities is the discovery in 1989 of the gene that is mutated in patients with cystic fibrosis. The gene was identified by using the advanced methodologies of positional gene mapping. Investigators delineated the nature of the mutation that leads to the production of a defective protein in the membranes of cells from patients with cystic fibrosis patients. Subsequent research demonstrated that this protein is associated with a membrane channel involved in the transport of chloride ions. This understanding has led to a number of chemical approaches to treat the disease. In addition, new efforts are under way to treat or cure the pulmonary manifestations of the disease by employing methods that are being developed in DNA transfer therapy. Research that is now being conducted in the laboratory will soon be carried over to use in patients with cystic fibrosis. Clinical investigators are crucial to the performance of this work and in bringing these novel therapies into common practice. Their participation will also be necessary to help determine how to deliver the technology efficiently, under what conditions and to which patients, and to assess the outcomes of these new therapies.
Hundreds, if not thousands, of other genetic disease are now being studied in the same fashion. As knowledge about the underlying genetic mechanisms for these diseases grows, new treatment approaches developed from basic laboratory techniques will be carried forward into clinical trials. In addition, genetic factors are being defined in diseases that have been regarded as multifactorial. For example, breast cancer scientists are on the threshold of discovering the genes that regulate its occurrence. Thus, approaches to modify the expression of these genes may be useful in the treatment of breast cancer. Genes that regulate the formation of atherogenic lesions in arteries, abnormalities that lead to coronary artery disease and heart disease have recently been identified. Blocking the activities of these genes using antisense gene therapy has been shown to block the progression of atherogenic lesions in arteries in animal models. On the basis of results of these promising studies, antisense gene therapies are being developed for use in humans. Novel therapies directed at blocking the genetic expression of the factors that determine at herogenesis as well as genetically directed products that can prevent or reverse these effects may be developed in the future and may lead to treatments or cures for ischemic heart disease and some forms of stroke. Clearly, clinical investigators will be critical for developing and testing these new therapies to determine their safety, efficacy, effectiveness, and cost-effectiveness in humans. Clinical investigators will also play a role in discussions regarding ethical considerations such as genetic testing and elucidating the behavioral or environmental factors influencing genetic diseases.
Although new therapies are being developed rapidly and require extensive clinical testing, old or current therapies should be rigorously evaluated as well. During the past few years several groups have initiated studies to examine the outcomes of current therapies for particular diseases or conditions (Eddy, 1984; Roper et al., 1984). For example, a broad-based research team has been investigating the treatment for benign prostate hypertrophy in a patient population in Maine (Wennberg et al., 1988). By taking into consideration the behavioral and social attributes of patients, the outcomes of the various treatments have been assessed. Not all treatment regimens are viewed favorably by patients, who have various needs and desired outcomes. Thus, the outcomes of particular therapies require sophisticated scientific methods to determine the effectiveness of therapy in patients with different expectations and needs. Other examples of opportunities for outcomes research can be cited by examining the topics under investigation by the Patient Outcomes Research Teams funded by the Agency for Health Care Policy and Research, such as low back pain, joint replacement, incontinence, and others. The research methods and tools used by those investigators are every bit as sophisticated as those needed to clone genes or isolate and characterize proteins. Similar studies in other fields of medical practice using these novel methodologies will be critical in the future.
The diversity of the preceding examples is a small sampling from a field rich in opportunity for improving medical care for millions of people in this country and around the world. An important interface in bringing these technologies to patients is the clinical investigator—the bridging scientist. The remarkable progress that has been evidenced in fundamental biology brings with it parallel opportunities for investigations in human populations. The realm of biomedical research can be viewed as a spectrum, with fundamental research occurring throughout the spectrum, some of which uses humans to answer crucial questions about human health and behavior. Thus, there is no discontinuity between fundamental biological science and clinical investigation. Indeed, it is progress throughout this research spectrum that frames the opportunities for progress in clinical research.
Increasing levels of sophistication and the assurance of an ample supply of excellent clinical investigators to carry technological advances to medical practice remain critical issues if the country is to continue to improve its health care system. There is growing evidence, however, of a discontinuity in the process of translating new research discoveries into improved health care; the process is further threatened by a potential lack of well-trained clinical investigators to provide the bridge to bring these discoveries into improved medical care (Kelley, 1988).
In the 20 years following World War II, bountiful resources were provided by the federal government to support research, primarily at the nation's research universities and medical schools (U.S. Department of Health, Education, and Welfare, Public Health Service, 1976). This paradigm of peer-reviewed, university-based research has been attributed to the wisdom and foresight of Vannevar Bush (Bush, 1945). Resources were not only plentiful for supporting research but numerous programs were also initiated to build the physical research infrastructure and train more highly talented scientists (Institute of Medicine, 1990). The biomedical research community responded, and the nation's health research capacity expanded significantly. During this period research that involved interactions with human subjects, possibly with the exception of psychological studies, was primarily the domain of physician-scientists. Many of these physician-scientists were motivated to pursue research careers because of the rapid advances in biomedicine and the potential to become critical players in medical discovery. Others may have pursued research to avoid military service in an unpopular war in Southeast Asia. Nonetheless, after completing their clinical training residencies, many physicians sought fellowships at the National Institutes of Health (NIH) and subsequently moved into academic and research positions around the country. Whatever their motivation, most of these scientists have contributed to the fount of knowledge that serves as the basis of modern health care.
In the late 1970s and early 1980s, many leaders in the medical research community expressed concern about a perceived decline in the participation rates of physicians engaged in all aspects of biomedical research (DiBona, 1979; Gill, 1984; Kelley, 1980 and 1985; Thier et al., 1980; Wyngaarden, 1979). This perception was supported by data demonstrating that the ratio of M.D.s to Ph.D.s successfully obtaining research grant awards from NIH was declining. More alarming was the notion that individuals who were highly trained in patient care and who were considered the technology transfer agents were not seeking rigorous scientific training, which widened the gap between basic research discoveries and application of these advances to improved health care (Glickman 1985; Healy, 1988). Furthermore, although some physicians were seeking training in the basic biological sciences, there was a perception that few were being trained to develop and test hypotheses in human subjects or populations (Forrest, 1980). Ironically, data show that the number of full-time faculty in medical schools has grown by more than 20,000 over the past decade, to nearly 65,000. (Data from the Association of American Medical Schools report that medical school faculty totaled 65,000 in 1990, whereas data collected for the Liaison Committee for Medical Education reports that faculty totals were nearly 80,000.) It has been hypothesized that this growth reflects a growing dependence on medical center profits to offset increasing constraints on research funds and shrinking subsidies for graduate medical education (Chin, 1985; Hughes et al., 1991). Although faculty members are required to perform scholarly activity, there appears to be an increasing demand on the clinical faculty to derive revenue through patient care. Furthermore, the growth in clinical faculty may have increased tensions between the faculty in basic science departments and those in the clinical departments. These tensions may arise because basic science faculty fear that their research funding base is being eroded by growing research activities in the clinical departments, and the growing number of clinical faculty bringing in patient care dollars positions the latter on a firmer financial footing. There is also a perception that some academic clinicians pursue research as a secondary interest and are not serious investigators. Many of these clinicians also feel that they cannot obtain tenure by performing human subject research, where the results may not be realized for many years and funding is believed to be extremely difficult to obtain. Moreover, those clinicians who focus on human research fear that they are perceived as second-rate scientists by their colleagues who perform fundamental research in both clinical and basic science departments.
A cause and effect has been difficult, if not impossible, to prove. Determining the size of the cohort of clinical investigators is fraught with error, because no database currently exists to track these investigators. Moreover, there has been no systematic way to collect and analyze data on the number of individuals who choose to perform clinical investigations, the availability of training pathways, or the outcomes of those few programs that do exist. Although many believe that quantitative factors such as debt and economic status directly influence decisions to pursue academic and research careers, there appear to be no measures for factoring in personal considerations such as the effects of mentors and role models, the desire to spend time with one's family, or having leisure time to pursue other personal interests. The growing base of fundamental science, the increasing complexity of medical care and understanding outcomes or effectiveness research, the difficulty (real or perceived) in obtaining research funding, and countless demands on an investigator's time all seem to weigh heavily against pursuing a career in research, particularly research that involves interactions with human subjects. The many employment sectors that require this expertise, such as federal agencies and industry, are also obstacles to conducting a thorough analysis.
Although most attention has been focused on the plight of physician-scientists, many other professional groups are experiencing similar difficulties in the area of human research. As in medicine, training for research careers in other professions is often fragmented, and the career pathways that young trainees should pursue are not clearly delineated. Although many of these other professions also provide outstanding training for delivering care, their programs may not be specifically structured for developing research capabilities. Thus, the Institute of Medicine ( IOM ) sought to undertake an analysis of the problems affecting the career paths leading to clinical research.
- Origins Of The Study
IOM has had an ongoing concern about the problems in the biomedical research arena, and particularly those problems confronting researchers who perform studies that require human subject participation. In 1988, IOM was commissioned by NIH to conduct a study to assess the availability of resources for performing research using patients. The committee was asked to consider a series of issues, including the effects of changes in the health care system on the environment for clinical research; how to improve the recruitment of medical students and residents into clinical research careers; identification of barriers to translating basic research advances into clinical practice; how to improve the relationships among clinical researchers, federal sponsors, and industry; the organization of clinical research; and how to stimulate interest in evaluative clinical sciences. Whereas that committee was asked to examine clinical research in the narrow sense of human subject research, the data from NIH that were available to the committee included all research on humans or human materials approved by institutional review boards, as indicated on Public Health Service grant application form number 398. This included research on all human material such as DNA, RNA, proteins, cells, or body fluids for in use in vitro studies, not necessarily material related to a patient's disease or involving the patient. Moreover, the committee was not able to glean any information from the private sector, either for-profit or nonprofit, to construct a complete picture of the resource base for patient-oriented clinical research.
Following the release of its report, Resources for Clinical Investigation (Institute of Medicine, 1988), the IOM Board on Health Sciences Policy convened a working group to reexamine issues related to clinical research. The working group recognized that the heterogeneous nature of the research training pathways for physician-scientists and the broad spectrum of research questions pursued by those investigators had complicated earlier analyses. The working group met twice to develop a strategy for exploring problems associated with the clinical research training pathways, particularly for physician-scientists. The working group sought to refine an approach that would isolate only the small portion of physician-scientist training that it felt was in a particularly vulnerable stage—patient-oriented clinical research—and did not attempt to address all the problems associated with physicians engaging in basic or health services research.
In December 1989, the National Research Council released the quadrennial report Biomedical and Behavioral Research Scientists: Their Training and Supply (National Research Council, 1989), which examined research training supported by the Public Health Service through National Research Service Awards (NRSAs). Although that report presented a detailed analysis of the doctoral biomedical and behavioral research workforce and recommended the numbers of NRSA trainees that should be supported, it paid scant attention to physician-scientists and largely ignored dentist- and nurse-scientists. The reasons for these omissions remain unclear, but they probably are the result of the inability to develop clearly defined populations of scientists in these professions. Whereas physicians, dentists, and nurses engage in a broad spectrum of fundamental research activities, they are critical players in clinical research. Although this group of scientists has often been referred to as clinical researchers because of their clinical degrees, they might be more appropriately referred to as clinician-researchers. Furthermore, the population of doctoral scientists engaged in human research also has remained undefined.
Following the release of the 1989 NRSA study, IOM 's Committee on Policies for Allocating Health Sciences Research Funds released a report in 1990, Funding Health Sciences Research: A Strategy to Restore Balance (Institute of Medicine, 1990). That committee also acknowledged that the limited understanding of the physician-scientist population and barriers to effective training of that population hampered the committee's attempts to recommend ways to overcome the barriers confronting those investigators. Thus, they recommended that a thorough analysis be performed on physician-scientists to clarify many of these issues.
- Charge To The Committee
The Committee on Addressing Career Paths for Clinical Research was formed in 1991 and was charged with identifying and evaluating issues in the education and training pathways for individuals pursuing careers in clinical investigation. In particular, the committee was asked to investigate ways to improve the quality of training for clinical investigators and to delineate pathways for individuals pursuing careers in clinical investigation in nursing, dentistry, medicine, and other related health professions engaged in human research. The committee was charged with the following: defining clinical research, how to stimulate individuals to pursue careers in clinical investigation, how to define appropriate curricula for training, how to identify mechanisms to bridge the gap between the basic and clinical sciences, how to address funding mechanisms for clinical investigation, how to establish measures of success in clinical research other than obtaining R01 grant support, how to encourage academic and industrial institutions to protect and reward these valuable investigators, and how to ensure adequate support mechanisms for retaining clinical researchers. For comparison, the committee also examined the pathways that lead physicians toward careers in basic research. The study focused on how existing structures and mechanisms in the federal government, universities, and industry might be used in new and innovative ways to foster careers for these groups of researchers.
The chair of the National Research Council appointed a 16-member committee to address the questions posed in the committee's charge. The committee was composed of active researchers and research administrators with expertise in nursing, dentistry, evaluative clinical sciences, surgery, epidemiology, and various medical subspecialties. The committee viewed several areas as deserving special attention, and these were addressed by task forces, including task forces in surgery, dentistry, nursing, and clinical psychology. The complete task force reports are included as appendixes to this report.
- Defining Clinical Research
The first item on the committee's agenda was to derive a working definition of clinical research. Various definitions have been used to describe or inventory research and development activities. Many lexicons classify research and development expenditures into the following three general categories: (1) basic research, (2) applied research, and (3) development. Although this classification scheme is useful for describing various research activities for budgetary purposes, it becomes less appropriate for describing cross-disciplinary clinical research, which may encompass portions of each of these categories.
Classification schemes often portray a linear progression of scientific knowledge from basic biological research, to applied research and development, and to improved diagnosis, treatment, and prevention of human disease. Many would argue, however, that a broad spectrum of research activities, from the most basic discoveries of nature to the application of knowledge in humans to understand and treat disease, would more accurately portray biomedical research ( Figure 1-1 ). Furthermore, research activity throughout the spectrum could be bidirectional or demonstrate circular feedback loops for generating new hypotheses. For example, many basic biomedical research questions arise from disease processes first observed in patients. Moreover, the boundaries between many of these subcategories are indistinct, with varying degrees of overlap and movement over time.
Diagram depicting the broad spectrum of clinical research, examples of training pathways, and possible sources of research funding. (Adapted from Kelley, 1988.)
Several clinical research classification methodologies have been attempted, each with its own limitations. Clinical research encompasses a vast range of research activities that are conducted by investigators in numerous disciplines. Ahrens has categorized the disparate activities encompassed under the rubric of clinical research into the following seven areas (Ahrens, 1992, pp. 40-48):
Studies on the mechanisms of human disease
- refinements in characterizations of disease processes
- explorations of unresolved questions in human biology
Studies on management of disease
- evaluations of new diagnostic and therapeutic techniques and devices
- drug trials (phases II, III, IV)
- studies of patient compliance and prevention measures
- searches for accurate prognostic markers
In vitro studies on materials of human origin
Animal models of human health or disease
Field surveys
Development of new technologies
Assessment of health care delivery.
All seven categories of research are essential to the progress of medical care and, ultimately, to the prevention of disease. Because the boundaries between these areas are indistinct, individuals can be working in more than one category at any given time.
The committee sought to derive a definition of clinical research that would cut across artificial boundaries to describe the universe of clinical research in terms of research activities or goals. Although there is a large amount of basic biological research that is not directly relevant to specific human diseases, such laboratory-based preclinical bench research may have direct links to understanding normal human function and disease. For example, control of human or retroviral gene expression as well as animal or cellular models of normal or diseased biological processes in humans is often clinically relevant, and under some classification schemes it is defined as clinical research.
At the other end of the spectrum is research on human subjects and populations that have direct application for understanding the prevention, diagnosis, and treatment of human disease by exploiting disciplines such as health services research, clinical epidemiology, and outcomes assessment. Undoubtedly, clinical research includes phase I-III human clinical trials to assess the effectiveness of new methods of intervention or patient management in defined populations. A body of research is also directed at understanding motivational factors for disease prevention and screening and the social and emotional impacts of disease and treatment by employing the disciplines of psychosocial, behavioral, and educational research, which can be considered clinical research. Thus, the committee agreed that there is a continuum of research spanning a wide range of activities that can be regarded as clinical research.
The committee emphasizes that clinical research is not simply that research performed by physicians or other professionals holding clinical degrees. Clearly, many scientists holding doctorates in the basic sciences are performing research that is very clinical in nature; many physicians are also outstanding basic scientists. Although it is very difficult to arrive at an unambiguous definition that will be agreeable to all parties, the committee believes that clinical research should be directed toward the elucidation of human biology and disease, and the control thereof.
The committee emphasized that a common definition should be as broad and inclusive as possible to accurately reflect the population of biomedical scientists generating knowledge about human ''disease." Furthermore, the committee acknowledges that clinical researchers may be performing research in more than one category; they may move back and forth along the spectrum as their line(s) of investigation matures or new research questions evolve. Thus, the committee proposes the following definition:
Clinical investigation , broadly defined, includes all studies intended to produce knowledge valuable to understanding the prevention, diagnosis, prognosis, treatment, or cure of human disease. This includes biomedical and health services research carried out in humans, usually by health care professionals, as well as research in organs, tissues, cells, subcellular elements, proteins, and genes derived from humans. It may also include the study of micro-organisms as well as studies of other members of the animal kingdom when this research is directed toward human disease.
Whereas this definition is suitably inclusive for all the researchers engaged in clinical research in the broadest sense, the committee identified specific areas that it believes need particular attention. The evolution of the new biology has begun to erode the perceived boundaries among the various medical disciplines as well as the boundaries between basic and clinical research. Moreover, the importance of basic research or other training experiences for teaching research methodology and study design to young clinical investigators cannot be overstated. Thus, the committee felt compelled to develop a broad definition for clinical research and then to focus on areas that it believes need immediate remediation to foster continued progress in clinical research. The special theme and focus of this study was patient-oriented clinical research, defined as that which requires "hands-on" participation with a human subject as opposed to the entire spectrum of clinical research. Interpreting its charge, the committee recognized that many professions are engaged in clinical research, including dentistry, nursing, pharmacy, osteopathic medicine, and the behavioral sciences, among others, and sought to include the perspectives of members of those professions as well. Nevertheless, the committee reinforced the common theme of the study and posed the following global questions about clinical research and the clinical research workforce:
- What can clinical research accomplish now and in the future to improve medical care?
- Is the current clinical research community poised and prepared to accomplish these goals?
- If the clinical research community is not prepared to accomplish these goals, what is the evidence that there is either inadequate clinical investigation or an inadequate number of well-trained clinical investigators to meet this need?
- What are the best approaches or best vehicles for change to improveclinical investigation and ensure a supply of highly competent clinicalinvestigators to meet these needs and accomplish the research goals?
- Limits On The Scope Of The Study
Although the committee developed a broad definition of clinical research, the major focus of the study was clinical research in which patients serve as the research subjects, often referred to as patient-oriented, patient-related , or preferably, human research . This category of clinical research includes research activities such as the characterization of healthy and diseased human function; evaluation of new diagnostic, therapeutic, and prognostic techniques, approaches, and devices; medical decision making; patient compliance and disease prevention research; health education research; drug trials; and the assessment of health care practices on patient populations. Thus, the committee's deliberations focused on the issues surrounding the preparation and training of clinical researchers who are engaged in research that requires the direct participation of human subjects. Lastly, although the committee frequently mentions areas of potential clinical research opportunities, it was not charged with developing a research agenda in clinical research and uses the examples only for reference.
- Conduct Of The Study
During the course of the study, the committee held four meetings to develop strategies and to analyze data. The committee used a variety of approaches to expand its expertise by involving as many avenues of input as possible to achieve its objectives, including four subcommittees, three task forces, a workshop, 11 commissioned papers, and information gained through solicitations of written input and interviews.
Subcommittees
First, the committee divided its members into the following four subcommittees to identify problems along the career pathways of clinical researchers: (1) undergraduate and precollege science education and research training, (2) research training during health professional school, (3) postdoctoral clinical research training, and (4) nurturing clinical research faculty. These subcommittees were convened separately to identify issues confronting their respective portions of the pathways and to develop approaches to collecting and analyzing data that could be used to draw conclusions.
Task Forces
Three task forces were convened in the spring of 1992 to address clinical research issues specific to (1) nursing and clinical psychology, (2) dentistry, and (3) surgery. Each of these task forces was chaired by a member of the committee and the membership was selected from those in the profession. They were charged with the following:
- Describe the clinical research performed by researchers in their respective professions and emphasize how it is different from that in other professions.
- Determine how many researchers in their profession are engaging in clinical research and estimate how many are needed.
- — Identify what needs to be enhanced or changed to encourage recruitment and the retention of clinical researchers in the profession.
- — Identify the funding sources for clinical research in their profession.
- — Explore the training backgrounds of the current cohort of clinical researchers in the profession.
- — Identify the education and training requirements for preparing clinical researchers in the profession.
- — Recommend changes necessary to address new clinical research questions for the profession in the future.
- — Describe how changes can be implemented or interwoven into existing organizational structures.
- — Identify the research training resources for individuals pursuing careers in clinical research for the profession.
- Recommend possible solutions to improving the career pathways leading to clinical research.
The complete task force reports can be found in Appendixes A , B , and C at the end of this report.
In June 1992 the committee sponsored a one-and-one-half-day workshop entitled "Clinical Research and Research Training: Spotlight on Funding." The overall goal of the workshop was to analyze training and research funding data and to explore innovative approaches to the training and support of clinical investigators. The first day of the workshop focused on the roles and responsibilities of research sponsors including the federal government, industry, the private nonprofit sector, third-party payers, and academic health centers and research institutions. The second day concentrated on the organizational barriers to clinical research training as well as the funding available for training. A transcript of the meeting was made for the use of the committee in preparing this report, but the committee chose not to publish a separate workshop proceedings.
Commissioned Papers
The committee commissioned 11 background papers to analyze topics of particular importance to the committee's deliberations. Although the findings of the papers are incorporated into this report, the committee felt that the papers were of such high-quality and made such significant contributions toward a better understanding of clinical research careers that they encouraged the authors to publish them separately. The following is a list of the paper titles and authors:
"Early Exposure to Research: Opportunities and Effects," by Marsha Lake Matyas of the American Association for the Advancement of Science.
"Advisers, Mentors, and Role Models in Graduate and Professional Education: Implications for the Recruitment, Training, and Retention of Physician-Investigators," by Judith P. Swazey of the Acadia Institute.
"The Effectiveness of Federally Supported Research Training in Preparing Clinical Investigators: Important Questions but Few Answers," by Georgine Pion of Vanderbilt University.
"Considerations of Educational Debt and the Selection of Clinical Research Careers," by Robert L. Beran of the Association of American Medical Colleges.
"Models of Postdoctoral Training for Clinical Research," by Thomas Lee and Lee Goldman of the Brigham and Women's Hospital.
"Models of Postdoctoral Training for Clinical Research," by David Atkins, Richard A. Deyo, Richard K. Albert, Donald J. Sherrard, and Thomas S. Inui of the University of Washington.
"Role of the GCRC in Establishing Career Paths in Clinical Research," by Charles Pak of the University of Texas Health Science Center.
"The Image of the Clinical Investigator," by Edwin Cadman of Yale University.
"University-Industry Relationships in Clinical Research: University Perspective," by David A. Blake of Johns Hopkins University.
"Roles and Responsibilities of Resident Review Committees and Certification Boards in Promoting Research Careers," by Linda Blank of the American Board of Internal Medicine.
"Clinical Research in Allied Health," by Leopold G. Selker of the University of Illinois.
Grants Analysis
The committee also undertook a detailed analysis of R01 grant awards that have been approved by institutional review boards to determine the fraction of awards that are truly patient-oriented, apart from those that use human materials or body fluids. Because the R01 pool represents about 55 percent of the total extramural funds awarded by NIH and because of the large time commitment required to read through grant files, the committee chose to limit the analysis to R01-type grant awards that were considered by initial review groups (study sections) in the Division of Research Grants ( DRG ). Of the more than 16,000 R01 grants active in fiscal year 1991, about 14,535 were reviewed by DRG study sections; of those, about 4,284 indicated the involvement of human subjects or materials. Of this 4,284, a random sample of 450 from 11 institutes was used for this analysis. The committee reviewed grants provided by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Deafness and Communicative Disorders, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institute of Child and Human Development, National Institute of Neurological Diseases and Stroke, National Institute of Allergy and Infectious Diseases, National Institute of Aging, National Institute of General Medical Sciences, National Institute of Diabetes, Digestive and Kidney Diseases, and National Eye Institute. Since the committee convened task forces on nursing and dentistry that evaluated grants in these disciplines, it did not include grants from the National Institute of Nursing Research (formerly the National Center for Nursing Research at the time the task force was convened) or the National Institute for Dental Research in the analysis. Furthermore, because National Institute of Mental Health, National Institute of Drug Abuse, and National Institute of Alcohol and Alcohol Abuse were not officially part of NIH at the start of this project and the transfer of these institutes was not assured in mid-June of 1992, grants from these institutes also were not included in the analysis. The results of this analysis are presented in Chapter 3 .
To supplement the information gleaned from each of the aforementioned mechanisms and to add breadth to the material available to the committee, IOM staff undertook several interviews of staff in various federal agencies, including NIH, the Food and Drug Administration, the Agency for Health Care Policy and Research, and Alcohol Drug Abuse and Mental Health Administration. Many of the data for the study came from NIH staff, to whom the committee is truly indebted. Because of the broad nature of the study, many sectors, public as well as private, contributed valuable information. Appendix E recognizes the many individuals who made important contributions to the report and are not cited elsewhere.
- Structure Of The Report
This report presents the findings from all the aforementioned methods of data collection and analysis. The following chapters elaborate on the issues the committee explored, presents its findings and conclusions, and offers its recommendations for improving clinical research career pathways. Chapter 2 examines the employment sectors and issues and obstacles confronting established clinical investigators, with an emphasis on academic clinical investigators. Chapter 3 discusses the available resources for funding clinical research. The obstacles and barriers to training pathways are presented in Chapter 4 . Chapter 5 explores the academic-industry relationships and the roles and responsibilities of investigators in these alliances.
- Cite this Page Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994. 1, Introduction.
- PDF version of this title (5.0M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Introduction - Careers in Clinical Research Introduction - Careers in Clinical Research
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers


Professional & Continuing Studies
Career opportunities in clinical research.
July 20, 2021

Field offers opportunities for professionals with health, science or technology background
Career opportunities in the clinical research field are many and varied, with employment settings ranging from pharmaceutical and biotechnology, to medical device companies, contract research organizations, hospitals, educational institutions, independent contractors and more.
Many professionals with a strong science or healthcare related background — such as nurses, pharmacists, medical technologists, physicians and more — are well-positioned to join the clinical research field. Here are 10 career paths in the field.
1. Clinical research coordinator
Also called research nurses, site managers and clinical study coordinators , clinical research coordinators (CRCs) are responsible for the daily operations of clinical research studies at sites like hospitals, independent medical practices, universities, medical schools and other research companies and institutions. CRCs may provide direct care or treatment to patients participating in a study, work with the study’s principal investigators, recruit study subjects and more. The background of most CRCs is in nursing and other healthcare professions.
2. Clinical research associate
The clinical research associate or CRA, also called a clinical monitor or trial monitor , works with clinical trial sites to monitor studies and ensure that standard regulatory, operations and ethical standards are being followed. CRAs may be employed by pharmaceutical companies, government research agencies or medical research institutes, and often travel between study sites for monitoring visits, as well as conducting virtual visits. CRAs often obtain their clinical research experience through working several years as CRCs.
3. Clinical research data specialist
The importance of data management and analysis in clinical research cannot be overstated. This includes data from research studies and electronic medical records, as well as other data from disparate systems and sources. Opportunities around clinical research data include data analyst, data manager, data coordinator, data associate and more.
4. Clinical investigator or clinical researcher 5. Research pharmacist
Life scientists, physicians, pharmacists and other scientists are at the core of clinical research. Their work drives the innovations and breakthroughs that result in improved outcomes and treatments for current and future patients. Roles may include research scientist or investigator as part of a research team or as principal investigator, lead scientist, etc.
6. Clinical project manager
Clinical research is a complex enterprise with multiple stakeholders, teams, tasks and challenges. Clinical trials project managers must have an advanced understanding of project management techniques and methodologies and apply them to coordinate all aspects of a clinical study.
7. Clinical laboratory technologist or technician 8. Medical laboratory scientist
Medical laboratory technologists and scientists are directly involved in preparing and analyzing research specimens in the lab. They may be employed in hospital-based research labs, pharmaceutical and biomedical companies, or other research institutions. Medical laboratory technicians usually have an associate degree and advance to medical laboratory scientist with a bachelor’s degree and experience in the field.
9. Medical/scientific writer 10. Marketing and communications professional
Writing, marketing and communication professionals with a medical or scientific background play a vital role in research. They write materials for scientific publications and other documents such as abstracts, posters, manuscripts, scientific presentations. They may be involved in grant-writing, publicity campaigns, scientific documentation, patient and stakeholder communications, and more.
Do you have a passion for making a difference?
“Clinical research is a field for individuals who have a passion for leaving their footprint in research as well as seeing the impact their efforts make to humankind,” said Michele Welch, program director and lead faculty for the University of Delaware’s Clinical Trials Management Online Certificate Program .
“The University of Delaware’s Clinical Trials Management Certificate program has been designed to help individuals understand the challenges that the industry faces today and provides an overview of opportunities for career change,” explained Welch, a clinical research professional with over 20 years’ experience in global pharmaceutical drug development.
UD certificate program
UD’s Clinical Trials Management Certificate program is aimed at healthcare professionals who want to learn more about the clinical research and new product development process in today’s environment, which includes device, biologicals and drug research. Curriculum aligns with Association of Clinical Research Professionals certification exam. Generally offered three times a year with start dates in February, May and September, this online 14-week program covers the logistics of site management, including start up, maintenance, and close out, with the corresponding collection and management of data, as well as regulatory, privacy and compliance issues.
UD students and UD alumni are eligible for a discount when enrolling in this program. Discounts are also available for military personnel and veterans, and groups of two or more individuals from one business or organization. For more information including a detailed curriculum, or to register, visit the Clinical Trials Management Certificate or write to [email protected] .
Learn more or register now!
Career resources in clinical research
- Society of Clinical Research Associates (SOCRA) — Clinical Research Job Opportunities
- Association of Clinical Research Professionals (ACRP) — Career Opportunities
- Careers in Clinical Research

501 South College Avenue, Newark, DE 19716
- Programs & Courses
- Student Resources
- Osher Lifelong Learning
- COVID-19, Health & Safety
- Weather Announcements
- PCS Admin (PCS Staff Only)
- Privacy Policy
- Refund & Registration Policies
Thursday, May 16@ 11am EDT: A Holistic Approach to Parkinson’s Disease Endpoint Data Collection

- Medical Devices
- Peer Circles
- Xtalks Clinical Edge
- Xtalks Spotlight
- Life Science Podcast
- Food Industry Podcast
- Quizzes & Polls
- Search Jobs
- Talent Acquisition & Branding Bundle
- Career Insights
- Contact Us to Host Your Webinars
- Webinar Production & Marketing Tips
- Frequently Asked Questions
- Join Xtalks
Clinical Research Jobs: How to Choose the Right Career Path
Share this:.
- Click to share on LinkedIn (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to email a link to a friend (Opens in new window)

To start your career or find your next clinical research job, explore Xtalks Job Search's current openings.
Clinical research is part of a growing industry that provides individuals with rewarding career options and a ladder to climb. Working in the clinical trials industry allows clinical researchers to work with patients and bring new drugs to market.
There are many professions under the clinical research umbrella. To become a professional in this field, one must begin by achieving a bachelor’s degree in a science-related field. After that, different paths need to be taken for different job titles.
Wondering which clinical research job is right for you? Read more to learn about job titles within clinical research, including job descriptions, average annual salaries and the level of experience required to land the role.
To start applying to clinical research jobs now, check out the Xtalks Job Search to find a job that best suits you.
Clinical Research Coordinator
Clinical research coordinator job description.
A clinical research coordinator works under the principal investigator’s direction, which is a person responsible for designing, conducting and managing clinical trials. The job involves supervising the trials and gathering patients by recruiting and screening them to make sure they fit into the inclusion criteria needed for the clinical trial. They are also in charge of ensuring that materials and supplies are safe and keep track of all the documents required during the study period.
Clinical research coordinators must ensure regulations are met and both safety and governmental rules are applied. Additionally, they may need to find and apply for funding and grants for the research, which entails putting together a budget plan for all the costs involved in conducting a trial.
Once that is completed and the trial begins, the clinical research coordinator will be responsible for ensuring that all team members are compliant with the rules and regulations. They will also ensure quality and organization is kept up to par to produce accurate reports.
Finally, the clinical research coordinator monitors the participants in the study to ensure they’re receiving quality medical care, but also to identify and record any adverse events that occurred during the clinical trial.
How To Become a Clinical Research Coordinator
To become a clinical research coordinator, one must earn a bachelor’s degree in a science-related field. This could then lead an applicant to receive an entry-level position in a clinical organization. A blend of education and experience within the clinical research field is required to be a professional clinical research coordinator, therefore, obtaining a clinical research certification allows employers to recognize that the knowledge required for the job has been acquired.
If an applicant has previous medical experience, then acquiring a certificate is all that is needed to receive a job in a clinical research coordinator position. However, if an applicant has no medical experience, some institutions offer degree programs to prepare them for entry-level employment, leading the applicant to be better equipped to pass a clinical research certification.
Clinical Research Coordinator Salary
According to Career Explorer , the average salary for a clinical research coordinator in the US is $112,044 annually. The wages can start at $57,324 per year and go up to $219,000 per year. The number of clinical research coordinator jobs is expected to grow by 10 percent by the year 2026.
Clinical Research Assistant
Clinical research assistant job description.
A clinical research assistant is in charge of finding subjects for the clinical trial but is also involved in collecting, analysing and evaluating data and results received from the study.
Clinical research assistants are also responsible for preparing the lab, volunteers and taking samples, or checking the vitals for volunteers involved in the clinical trial.
A clinical research assistant’s job is to help the principal investigator of the clinical trial ensure they stay on track and are complying with the ethics and scientific requirements needed to achieve a successful trial. Being a clinical research assistant is an excellent place to start in the clinical research career path; it helps make landing a clinical research associate job down the line more achievable.
How to Become a Clinical Research Assistant
Because this is an entry-level position, becoming a clinical research assistant has minimal requirements, including obtaining a Bachelor of Science and some experience in the field that is being researched in the clinical trial.
According to Salary , a clinical research assistant in the US makes around $64,000 annually. The average salary ranges between a low of $55,000 and a high of $73,900, depending on the person’s education, certifications, experience and additional skills that they could bring to the job.
Clinical Research Associate
Clinical research associate job description.
A clinical research associate organizes and administers clinical trials of new or current drugs to study the benefits and risks of using them. They help manage and monitor the clinical trials’ different phases while focusing more on data, accuracy and quality control. The main difference between a clinical research associate and a clinical research coordinator is that an associate does not interact with patients but has more responsibility for data quality.
Some of the more detailed responsibilities of a clinical research associate include preparing site reports; ensuring that only qualified individuals are working as site staff and are adequately trained; ensuring that data collected is accurate and verified; making sure that the drugs used in the trial are returned or destroyed once the study is complete; and making sure that all documentation and information needed for a final report is completed to be sent to the sponsor.
How to Become a Clinical Research Associate
Becoming a clinical research associate can be a tough process as companies that conduct clinical trials must follow strict regulations; therefore, recruitment is selective. Thus, competition is high and previous experience, such as being a clinical research assistant, is required. Two years of monitoring experience, including roles as a clinical trial administrator or a clinical project assistant, is required to be qualified for a clinical research associate job.
Clinical Research Associate Salary
A clinical research associate makes $97,368 per year as an average base salary. However, the wage can differ depending on years of experience, starting at $81,505 for one to two years of experience, and $124,022 for individuals with six to nine years of experience.
Clinical Research Specialist
Clinical research specialist job description.
Like other clinical research job roles, clinical research specialists conduct their work in labs and offices. They control teams of professionals that plan for projects that are aimed at advancing treatments through clinical trials.
This high-level position requires advanced communication skills, professional knowledge within the clinical research field, the ability to manage a team and expertise in analyzing clinical data.
Clinical research specialists are responsible for developing protocols for the study, including the study design, risk assessments and research tools needed to ensure that the study is conducted with ethics and accuracy. Additionally, they are responsible for coordinating and managing the team by providing them with materials and information needed to progress in the study. They are also in charge of monitoring and ensuring that the team is compliant with protocols. Finally, they could be asked to analyze, document and prepare reports that would later be presented to the larger medical community.
How to Become a Clinical Research Specialist
To become a clinical research specialist, candidates are expected to have earned a bachelor’s degree in a health-related field and have previous management skills. Many employers prefer applicants to have received a postgraduate degree such as a Master of Science. Employers will also seek out applicants with a nursing degree or any other healthcare-related degree.
Furthermore, gaining experience in a relevant clinical research field is of utmost importance in order to understand the larger picture of the job. Interpersonal skills and a good understanding of the regulations set out by the FDA around appropriate documentation for clinical research are critical parts of this career path.
Clinical Research Specialist Salary
A clinical research specialist’s salary is around $82,308 per year.
Clinical Research Scientist
Clinical research scientist job description.
Clinical research scientists are responsible for performing scientific research and clinical investigations to study diseases and other illnesses to advance diagnosis and treatments. They also assist physicians in diagnosing patients by identifying health conditions.
Clinical research scientists may interact with patients depending on the stage of research the study is in. Nevertheless, they do assist doctors in providing an effective treatment plan for patients.
Furthermore, clinical research scientists mainly work in labs where they could interact with infectious specimens, requiring them to be familiar with best practices around handling patient samples. Additionally, troubleshooting minor problems related to lab equipment is also required to succeed in the job.
Clinical research scientists will also be expected to be proficient in computer software that aids in producing necessary graphs, slides and documents to present their findings to others as required by their employer. They also need to stay up to date with scientific findings published in the literature to build on previous research.
How to Become a Clinical Research Scientist
To become a clinical research scientist, a bachelor’s degree in a science-related field is required. Majors such as biology, biochemistry, pharmacology and, if possible, clinical research, aid in achieving the first steps in becoming a clinical research scientist. To act as a principal investigator in research, obtaining a master’s degree and a PhD is highly recommended. Having a doctoral degree will allow an applicant to achieve a lead researcher job within clinical research.
Experience in clinical research has helped many people become a clinical research scientist, with previous positions as a clinical research associate or assistant being an asset. Earning certifications and gaining further experience will aid in achieving leadership roles.
Clinical Research Scientist Salary
According to Payscale , the average salary for a clinical research scientist is $103,927 annually, with a base salary of $75,295.
A clinical research job can be rewarding as clinical trials develop new therapies to improve patients’ quality of life. The outlook for a career in clinical research is promising.
According to the results of a survey from the Association of Clinical Research Professionals (ACRP) , monthly job posting for clinical trial positions increased by 9.3 percent over the last three years. This is because there has been a 12.2 percent growth in the number of clinical trials from 2016 to 2019.
Furthermore, there is a lot of investment in this sector because of the level of innovation taking place within the clinical research field. As clinical trials continue to deliver data supporting the approval of new drugs and devices, more investments are being made on an international scale, providing a lot of growth in this industry.
Grand View Research states that “the global clinical trial market size was estimated at $44.3 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 5.7 percent from 2021 to 2028.” Furthermore, the market size value in 2021 is estimated to be $47 billion with a forecasted revenue of $69.5 billion in 2028.
To start your career or find your next clinical research job, explore Xtalks Job Search’s current openings .
Related Vitals
Top 10 best healthcare companies to work for in 2024: a forbes list breakdown.

Top 15 Best Food Companies to Work For in 2024, According to Forbes

Healthcare Consulting Jobs: How to Succeed in This Competitive Field

Medical Science Liaison Jobs: Everything You Need to Know

Xtalks Talent Acquisition & Branding Bundle: Position Your Organization to Attract Elite Talent

Related Webinars
Advancing spatial biology to the clinic: the power of multiplex immunofluorescence-based biomarkers.
Unlocking precision medicine: An overview of Illumina’s TSO500
Comparability of Five Alternative AAV9 Downstream Processes, from Ultracentrifugation to Chromatography
Running Successful Neuropathology Studies: Sampling and Preparing Nervous System Tissues for Microscopic Evaluation

A Pathway to Better Health: How Supply Chains Deliver on the Promise of GLP-1 Drugs

Join or login to leave a comment
Subscribe to our newsletters.
Not a member yet? Sign up for our newsletters and receive webinars, vitals, and updates for your topic of choice
- Xtalks Vitals
- Vitals Life Science
- Vitals Food Industry
- Vitals Medical Devices
- Vitals Webinar Production Tips
- First Name *
- Last Name *
Sign up for our newsletters and receive webinars, vitals and updates for your topic of choice.

The Clinical Trials Team - Roles & Responsibilities

In a research study, a clinical trial tests a new medical treatment or a new way of using an existing treatment to see whether it will be a better way to avoid and screen for diagnosing or treating a disease. Purpose of clinical trial:
A research study that is performed on individuals for evaluation of a medical, surgical, or behavioral intervention.
Clinical Research Careers
Clinical Research Associate (CRA)
Clinical Research Coordinator (CRC)
Drug Safety Monitor (PV)
Clinical Trial Assistant (CTA)
Clinical Research Nurse (CRN)
Medical Monitor (MM)
Principal Investigator (PI)
All Research Professionals (ICH GCP)
Types of clinical trials:
Prevention trials
Screening trials
Case control studies
Cohort studies
Cross sectional studies
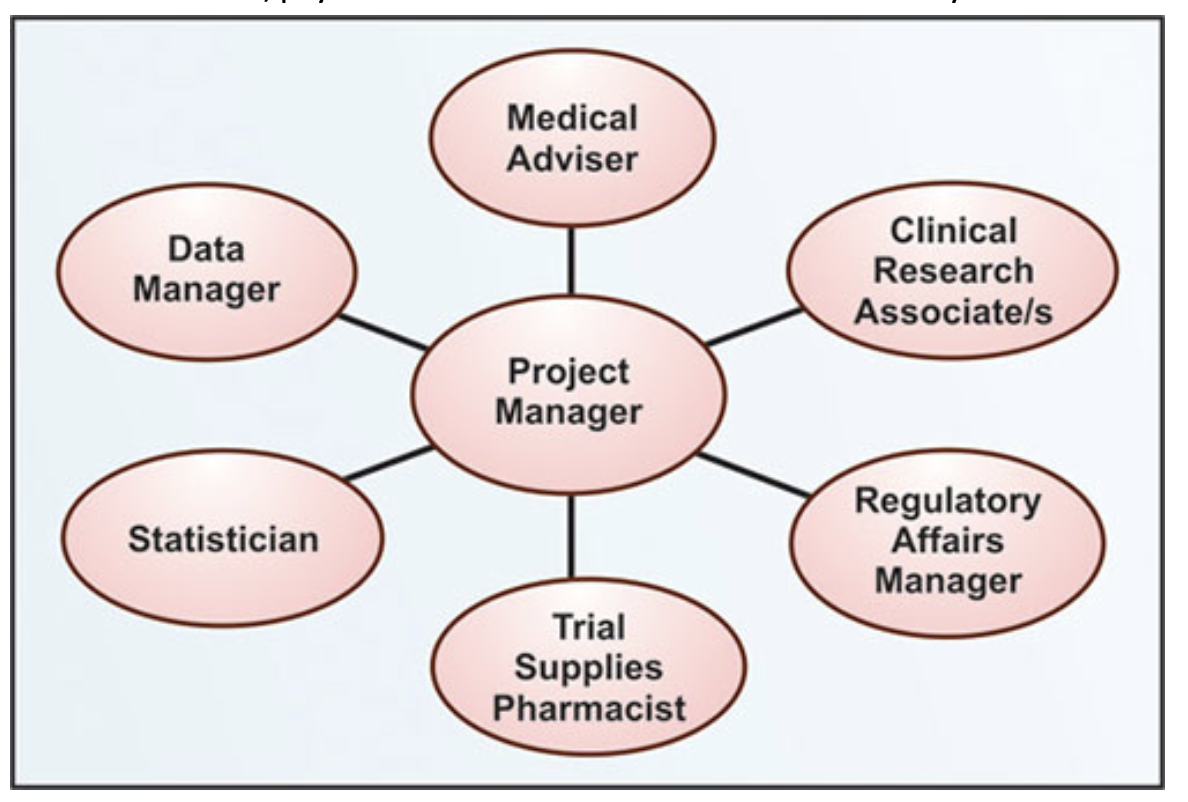
Figure no. 1: clinical trial team flowchart
Clinical research trial team:
The success of a quality clinical research program is essential for developing and maintaining an impeccable clinical research trial team. It is the main component of a research program because total time and effort for conducting a clinical trial; nurses and data managers each contribute more than 30%. On the other hand, physician’s contribution to clinical research is only 9%.
Roles and Responsibilities of clinical trial personnel
Clinical research team:
Participants are provided with information about the clinical trial.
The content of the informed consent is explained.
Reporting of adverse events or drug reactions.
report suspected misconduct.
Protect the integrity and confidentiality of records and data during the clinical study
Responsibilities:
Appropriate training
Following of GCP standard
Following required protocols
Investigator:
• Following ethical principles.
• Provide education programs.
• Design and conduct clinical trials for policies and procedures.
• Refer to GCP course for training.
• Determines the scientific, technical, and administrative aspects of the research project.
Responsibilities:
• Conduction of trial, statement, protocol, and applicable regulations.
• Protection of rights and welfare of participants.
• Obtaining informed consent.
• Maintenance of proper records.
• Management of all safety reports and financial disclosure reports.
Figure no. 2: Roles of clinical research controller.
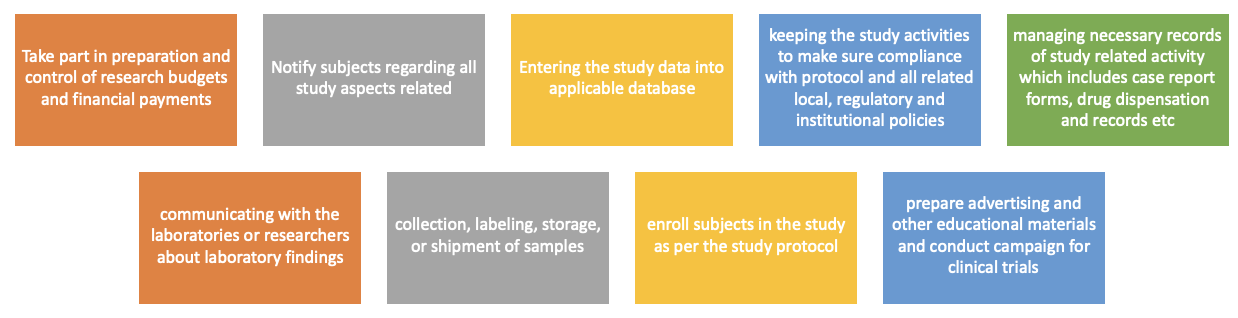
Figure no. 3: Responsibilities of clinical research controller.
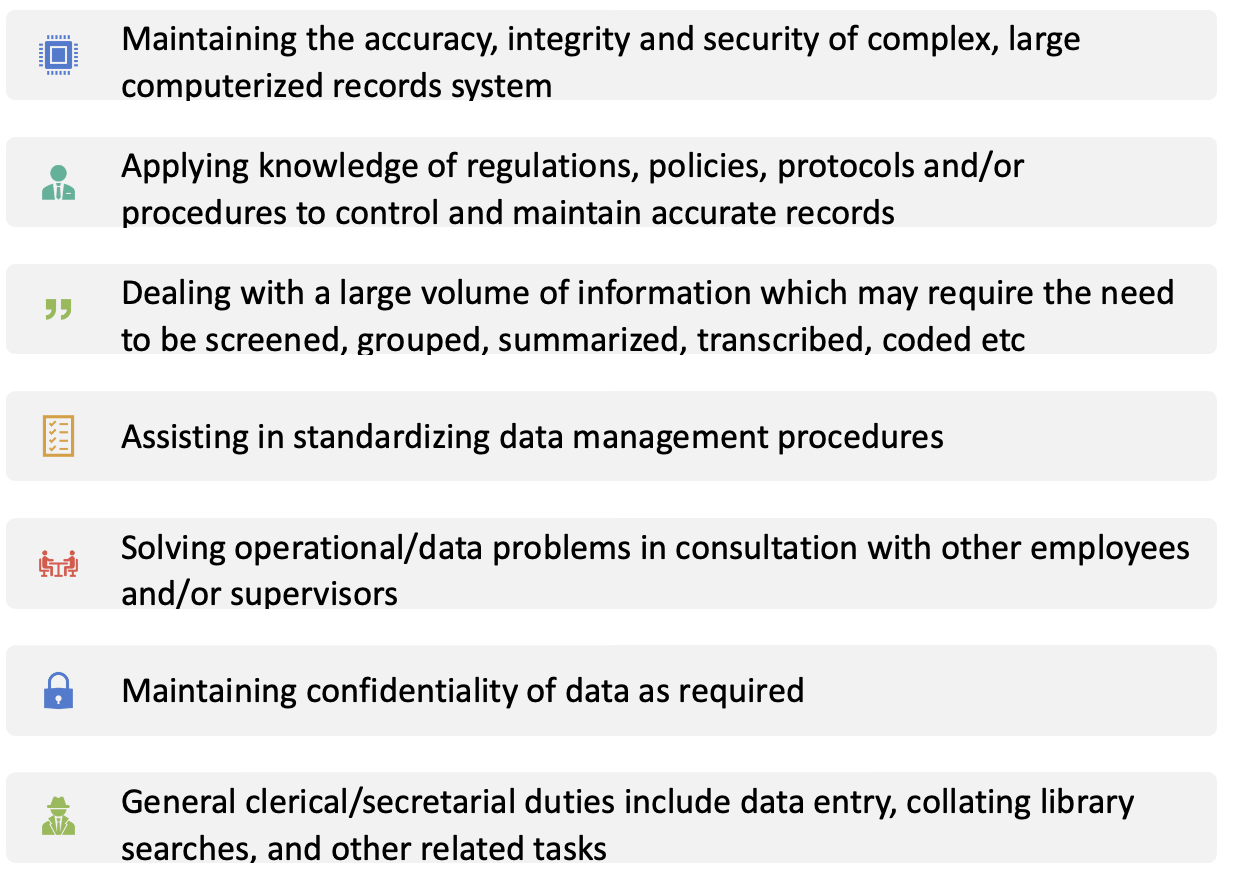
Figure no. 6: Responsibilities of data manager.
Sponsor:
Selection of qualified investigators.
Ensures proper monitoring of the clinical trial.
References: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092661/ - The Clinical Research Team https://clinicaltrialpodcast.com/clinical-research/ - 15 Clinical Research Job Roles & Responsibilities (2021) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3051859/ - Clinical Investigator Responsibilities https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6042393/ - How to engage stakeholders in research: design principles to support improvement
Efficacy Insight: Revealing its Meaning in Clinical Research
Good documentation in clinical trials.
- Grants & Funding Opportunities
- Study Design, Data & Analysis
- Conducting Clinical Research
- Community Engagement
- Education & Training
- Implementation Science
- Commercialization
- Recruitment & Retention
- Research Space
- News & Announcements
- Policies & Regulations
- Our Mission and Partners
- What is Translational Science?
- Leadership Team
- Use of services policy
- Cite the grant
- Contact / Directions
UNC Chapel-Hill:
- Forgot your password?
- Forgot your username?
- Create an account
The North Carolina CRO Collaborative was initiated by the NC Biotech Center in an effort to increase knowledge about career pathways in the clinical research industry and to help individuals prepare for careers in clinical research.
The Collaborative is comprised of representatives from the NC Biotech Center, academic institutions, clinical sites, and contract research organizations (CROs).
Career Pathways in Clinical Research
The first project undertaken by the group was to compile job profiles for entry-level clinical research positions in CROs and clinical sites. These job profiles provide a summary of the entry-level job role, essential duties, and salary range followed by example career pathways that illustrate possible next steps in that job category.
To assist individuals in preparing for these positions, each job profile includes a section on the required education/experience and knowledge, skills and abilities needed to be a competitive applicant.
View/download job profiles (pdf)
Biostatistician Clinical Research Technician Clinical Research Associate (CRA) In-House Clinical Research Associate Clinical Research Coordinator Data Research Coordinator Financial Analyst Nurse Consultant Project Support Safety Assistant
North Carolina CRO Collaborative

related services
- K12 Program
- TL1 Program
- Supplement Grants
- R Writing Groups
- Career Development Award Writing Groups
- Mock Review
- Career Pathways
- Master of Science in Clinical Research
- Education Team
- About Education
In partnership with:

Brinkhous-Bullitt, 2 nd floor 160 N. Medical Drive Chapel Hill, NC 27599

© 2008-2024 The North Carolina Translational and Clinical Sciences (NC TraCS) Institute at The University of North Carolina at Chapel Hill The content of this website is solely the responsibility of the University of North Carolina at Chapel Hill and does not necessarily represent the official views of the NIH accessibility | contact
- About Research & Innovation
- Advanced Cardiovascular Care
- Health Equity
- Inflammation
- Maternal and Fetal Medicine
- Musculoskeletal Care
- Neuroscience
- Opioid & Pain
- Research Centers
- Departments
- About the Office of Research
- Funding & Proposal Development
- Regulatory Review & Compliance
- Research Project Management
- Cores & Resources
- Education & Training
- Peter Arvan Lab
- Antiphospholipid Syndrome Research Labs
- J. Michelle Kahlenberg Lab
- John Varga Lab (ScleroLab)
- Mulholland Lab
- Pasca di Magliano Lab
- Raghavendran Lab
- ALS Center of Excellence
- Institute for Heart & Brain Health
- Center for Basic & Translational Science
- Center for Bioethics and Social Sciences in Medicine
- Biomedical Research Core Facilities
- IT Route Map
- Clinical Research Route Map
- Commercialization Route Map
- Great Minds, Greater Discoveries
- Research Scouts
- Meet the ROMS Team
- ROMS Fellowship Application Information
- Working with a ROMS Fellow
- Pandemic Research Recovery
- Research Climate Council
- Support for Outstanding Research
- Research News Trivia
- Frequently Asked Questions (FAQ)
- Billing Calendar & Study Applications
- CRAO Frequently Asked Questions (FAQ)
- Pre-Onboarding Considerations
- Access Biospecimen
- Biospecimen Processing
- Biospecimen Inventory
- Regulatory & Governance
- Equipment & Instrumentation
- Central Biorepository Pricelist
- CBR Frequently Asked Questions (FAQ)
- Clinical Trials Support Units
- Clinical Research Coordinator Career Ladder
- Clinical Trials Management Systems: Accounts & Support
- Research Pharmacy
- Off-Campus Clinical Research
- Statistical Support for Large-Scale Clinical Trials
- MCRU Services
- MCRU Lab Study Team Guidelines
- MCRU Frequently Asked Questions (FAQ)
- Michigan Medicine Site Profile
- CTSO Frequently Asked Questions (FAQ)
- Self-Serve Data Tools
- Custom Data Request
- Data & Biospecimen Sharing
- Student Access to Patient Data
- FFMI fastPACE
- Frankel Cardiovascular Center Innovation Program
- Rogel Cancer Center Innovation Program
- Global Health Commercialization Competition
- Biomedical Innovation 101
- Certificate in Biomedical Innovation & Entrepreneurship
- Fast Forward Medical Innovation Fellowship Program
- Biotech Career Development Program
- Drug Discovery & Development Course
- Innovation Studio
- Oncology Drug Discovery & Development (3D) Workshop
- FFMI fastPACE Train-the-Trainer
- Intellectual Property in the Academic Setting
- Academic Drug Discovery
- Medical Device Regulation
- Idea to Impact Webinar Series
- 6 Tips for Customer Discovery
- Interacting with Industry
- Ask the Experts: A Biomedical Innovation Forum
- Business Development
- Michigan Biomedical Venture Fund
- R01 Boot Camp
- Facility & Resource Profile Templates
- BMRC Bridging Support Program for Biomedical Research
- Medical School Limited Submissions
- Budgeting & Costs
- NIH Specifics, Tips & Tricks
- Grant Routing Deadlines
- Proposal Review Checklists
- Department Contacts
- Authorized Signers
- Research Data Analytics
- Grant Processing Advisory Committee (GPAC)
- Post-Award Advisory Committee (PAAC)
- Grants Frequently Asked Questions (FAQ)
- Board Nominations Sought
- Informed Consent & Assent Templates
- IRBMED Seminar Series
- Information for IRB Members
- Information for Study Subjects
- SignNow Electronic Signature Service
- IRBMED Frequently Asked Questions (FAQ)
- IRBMED Contacts & Roster

A path for career progression.
In 2022, Michigan Medicine implemented a career ladder for all staff currently performing clinical research coordinator work.
Research is one of the primary missions of Michigan Medicine. Recognizing the talent and expertise of these individuals to our mission as well as professionalizing the role was one of the top priorities identified by department chairs as part of their strategy to transform the clinical trials enterprise at Michigan Medicine. In recent years, the number of individuals performing important work to administer research has increased and the market titles assigned to these individuals have not been consistent across the organization. This series of job titles within clinical research will be a more consistent way to classify employees based on their responsibilities, competencies, and experience. This series also provides employees with a clear path for career progression within Michigan Medicine.
The following are market titles in this career ladder:
- Clinical Research Assistant ( 103921 )
- Clinical Research Technician ( 103922 )
- Clinical Research Coordinator Associate ( 103923 )
- Clinical Research Coordinator Intermediate ( 103924 )
- Clinical Research Coordinator Senior ( 103925 )
- Clinical Research Coordinator Lead ( 103926 )
- Clinical Research Project Manager ( 103927 )
View the Michigan Medicine policy outlining the required use of the Clinical Research Coordinator (CRC) career ladder.
A summary of the job descriptions is available in U-M Career Path Navigator, as linked above, and in Michigan Medicine Total Rewards. U-M supervisors and staff can review the detailed job descriptions (requires login) and summary job grid for the most appropriate title based on position responsibilities and an individual’s competencies. After determining the appropriate title, download the respective template (requires login) for job postings.
To ensure consistency of use across the organization, the Senior, Lead or Project Manager titles will require review by CRC Governance Board prior to job posting, candidate offer, and reclassifications.
As we professionalize the clinical research coordinator role at Michigan Medicine, professional certification is a key component of career progression in our competency- and training-based framework. ACRP CCRC , SoCRA CCRP , or equivalent certification is required for any positions at CRC – Associate or higher with new employees permitted six months from start date to acquire.
The expense of certification will be the responsibility of the hiring individual (e.g., PI) or central unit (e.g., Department, Division, Center, Institute, Program, etc.). The hiring individual or central unit will be responsible for the costs associated with clinical research coordinators maintaining their certification, such as continuing education and recertification fees. The source of funds for these expenses may include institutional funds, gift funds, or sponsored projects, as appropriate and proportional to the activity of the individual.
For more information on certification reimbursements, click here .
The CRC Career Ladder competency domains:
- Scientific Concepts
- Ethical and Participant Safety Concerns
- Investigational Products Development and Regulations
- Clinical Study Operations
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
These competencies were developed by the Joint Task Force for Clinical Trial Competency , an international team of investigators, educators, and clinical research professionals. This framework defines the knowledge, skills, and attitudes necessary for conducting safe, ethical, and high-quality clinical research.
CRC SharePoint Resource Library
Requirements to Use of the Clinical Research Coordinator Career Ladder Policy
Clinical Research Coordinator Certification Requirements Policy
Clinical Research Coordinator Reimbursements for Certification
The Clinical Trials Support Office is a unit of the Medical School Office of Research, where our mission is to foster an environment of innovation and efficiency that serves the Michigan Medicine research community and supports biomedical science from insight to impact.
We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- News & Stories
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School
Global Footer Secondary Navigation
Skip to job results
Skip to refine results
- Skip to main menu
- Skip to user menu
Clinical research Jobs - find worldwide career opportunities
- Refine results
Refine your search
- Clinical Remove selection
- Bioinformatician 8
- Director 5
- Faculty Member 16
- Head of Department 5
- Health Professional 5
- PhD Position 6
- Postdoctoral 10
- Principal Investigator 7
- Professor 17
- Researcher 17
- More…
- China 13
- United States 9
- Germany 6
- Asia Pacific 4
- Italy 3
- United Kingdom 3
- Singapore 2
- Denmark 2
- India 1
- Belgium 1
- $20,000 - $29,999 1
- $40,000 - $49,999 2
- $60,000 - $69,999 1
- $70,000 - $99,999 1
- $100,000 - $149,999 3
- $150,000 or more 2
- Full time 46
- Permanent 24
- Fixed term 22
- PhD 34
- Masters 6
- Professional certification 1
- MD 5
- Direct Employer 29
- Academia 32
- Industry 1
- Government 1
- Hospital 10
- Charity/NGO 2
Found 46 jobs
Nature Careers offers clinical research positions for every career stage, including researcher and Postdoc positions, as well as Faculty members and Head of Department, all over the world.
Clinician Researcher/Group Leader in Cancer Cell Therapies
- Herston, Brisbane (AU)
- A competitive remuneration package to be negotiated with the right candidate
- QIMR Berghofer
An excellent opportunity is available for a Group Leader with expertise in cellular therapies to join the Cancer Research program at QIMR Berghofer.
View details Clinician Researcher/Group Leader in Cancer Cell Therapies
- Save Clinician Researcher/Group Leader in Cancer Cell Therapies You need to sign in or create an account to save
High-Level Talents at the First Affiliated Hospital of Nanchang University
- Nanchang, Jiangxi, China
- Competitive salary
- The First Affiliated Hospital of Nanchang University
For clinical medicine and basic medicine; basic research of emerging inter-disciplines and medical big data.
View details High-Level Talents at the First Affiliated Hospital of Nanchang University
- Save High-Level Talents at the First Affiliated Hospital of Nanchang University You need to sign in or create an account to save
Two Junior Research Group Leaders (f/d/m) for Photonics
- 07743, Jena (DE)
- "-"
- Friedrich-Schiller-Universität Jena
Friedrich Schiller University is a traditional University with a strong research profile based in the heart of Germany. As a University covering al...
View details Two Junior Research Group Leaders (f/d/m) for Photonics
- 12 days ago
- Save Two Junior Research Group Leaders (f/d/m) for Photonics You need to sign in or create an account to save
W2 Professorship with tenure track to W3 in Animal Husbandry (f/m/d)
- Göttingen (Stadt), Niedersachsen (DE)
- Georg-August-Universität Göttingen
The Faculty of Agricultural Sciences at the University of Göttingen invites applications for a temporary professorship with civil servant status (g...
View details W2 Professorship with tenure track to W3 in Animal Husbandry (f/m/d)
- 16 days ago
- Save W2 Professorship with tenure track to W3 in Animal Husbandry (f/m/d) You need to sign in or create an account to save
W1 professorship for „Tissue Aspects of Immunity and Inflammation“
- University of Luebeck
Kiel University (CAU) and the University of Lübeck (UzL) are striving to increase the proportion of qualified female scientists in research and tea...
View details W1 professorship for „Tissue Aspects of Immunity and Inflammation“
- Save W1 professorship for „Tissue Aspects of Immunity and Inflammation“ You need to sign in or create an account to save
W1 professorship for "Congenital and adaptive lymphocyte regulation"
- Kiel, Schleswig-Holstein (DE)
- Universität Kiel - Medizinische Fakultät
View details W1 professorship for "Congenital and adaptive lymphocyte regulation"
- Save W1 professorship for "Congenital and adaptive lymphocyte regulation" You need to sign in or create an account to save
Position Recruitment of Guangzhou Medical University
- Guangzhou, Guangdong, China
- Very competitive salary with funds
- Guangzhou Medical University
Seeking talents around the world.
View details Position Recruitment of Guangzhou Medical University
- 17 days ago
- Save Position Recruitment of Guangzhou Medical University You need to sign in or create an account to save
Qiushi Chair Professor
- Hangzhou, Zhejiang, China
- Attractive salary packages are offered, negotiable based on individual qualifications and needs.
- Zhejiang University
Distinguished scholars with notable achievements and extensive international influence.
View details Qiushi Chair Professor
- 26 days ago
- Save Qiushi Chair Professor You need to sign in or create an account to save
ZJU 100 Young Professor
- Competitive salary and comprehensive support in housing, research funding, and team building
Promising young scholars who can independently establish and develop a research direction.
View details ZJU 100 Young Professor
- Save ZJU 100 Young Professor You need to sign in or create an account to save
Postdoctoral Research Associate position at University of Oklahoma Health Sciences Center
- Oklahoma City, Oklahoma
- University of Oklahoma Health Sciences Center
Postdoctoral Research Associate position at University of Oklahoma Health Sciences Center The Kamiya Mehla lab at the newly established Departmen...
View details Postdoctoral Research Associate position at University of Oklahoma Health Sciences Center
- 29 days ago
- Save Postdoctoral Research Associate position at University of Oklahoma Health Sciences Center You need to sign in or create an account to save
Call for Global Talents Recruitment Information of Nankai University
- Tianjin, China
- Competitive salary with research funds
- Nankai University
Nankai University welcomes global outstanding talents to join for common development.
View details Call for Global Talents Recruitment Information of Nankai University
- 34 days ago
- Save Call for Global Talents Recruitment Information of Nankai University You need to sign in or create an account to save
Director of Research
- Chennai, Tamil Nadu (IN)
- Open to negotiation
- Cancer Institute (W.I.A)
Applications are invited for the post of Director of Research at Cancer Institute (WIA), Chennai, India.
View details Director of Research
- 40 days ago
- Save Director of Research You need to sign in or create an account to save
Global Talent Recruitment (Scientist Positions)
- Beijing, China
- Competitive salaries, which can be further negotiated for particularly outstanding individuals.
- Changping Laboratory
Global Talent Gathering for Innovation, Changping Laboratory Recruiting Overseas High-Level Talents.
View details Global Talent Recruitment (Scientist Positions)
- 43 days ago
- Save Global Talent Recruitment (Scientist Positions) You need to sign in or create an account to save
Zhejiang Provincial Hospital of Chinese Medicine on Open Recruitment of Medical Talents and Postdocs
- The First Affiliated Hospital of Zhejiang Chinese Medical University
Director of Clinical Department, Professor, Researcher, Post-doctor
View details Zhejiang Provincial Hospital of Chinese Medicine on Open Recruitment of Medical Talents and Postdocs
- 47 days ago
- Save Zhejiang Provincial Hospital of Chinese Medicine on Open Recruitment of Medical Talents and Postdocs You need to sign in or create an account to save
Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Warmly Welcomes Talents Abroad
- No. 3, Qingchun East Road, Hangzhou, Zhejiang (CN)
- Competitive salary and fringe benefits.
- Sir Run Run Shaw Hospital Affiliated with Zhejiang University School of Medicine
“Qiushi” Distinguished Scholar, Zhejiang University, including Professor and Physician
View details Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Warmly Welcomes Talents Abroad
- Save Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Warmly Welcomes Talents Abroad You need to sign in or create an account to save
Postdoctoral Fellowships at West China Hospital/West China School of Medicine of Sichuan University
- Chengdu, Sichuan, China
- West China School of Medicine/West China Hospital
Open to PhD students, PhD, Post-Doc and residents.
View details Postdoctoral Fellowships at West China Hospital/West China School of Medicine of Sichuan University
- Save Postdoctoral Fellowships at West China Hospital/West China School of Medicine of Sichuan University You need to sign in or create an account to save
Welcome Global Talents to West China Hospital/West China School of Medicine of Sichuan University
Top Talents; Leading Talents; Excellent Overseas Young Talents on National level; Overseas Young Talents
View details Welcome Global Talents to West China Hospital/West China School of Medicine of Sichuan University
- Save Welcome Global Talents to West China Hospital/West China School of Medicine of Sichuan University You need to sign in or create an account to save
Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUST
- Wuhan, Hubei, China
- HUST will offer globally competitive salary packages
- School of Optical and Electronic Information, Huazhong University of Science and Technology
Job Opportunities: Leading talents, young talents, overseas outstanding young scholars, postdoctoral researchers.
View details Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUST
- Save Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUST You need to sign in or create an account to save
Global Talent Recruitment of Xinxiang Medical University in 2024
- Xinxiang, Henan, China
- It will be remunerated on a case-by-case basis and equipped with academic teams.
- Xinxiang Medical University
Top-notch talents, leading talents in science and technology, and young and middle-aged outstanding talents.
View details Global Talent Recruitment of Xinxiang Medical University in 2024
- 5 days left
- Save Global Talent Recruitment of Xinxiang Medical University in 2024 You need to sign in or create an account to save
Senior Research Scientist
- New York (US)
- $75,000 - $100,000
- Memorial Sloan Kettering Cancer Center (MSK)
MSK is seeking an experienced Scientist to join their NIH funded laboratory dedicated to gene target identification and drug discovery in soft tissue
View details Senior Research Scientist
- 4 days left
- Save Senior Research Scientist You need to sign in or create an account to save

- Clinical Research Job Opportunities
The road to your future starts here!
To post a job, please click here .
Careers & Services
- Clinical Research Services

Coordinator, Clinical Research Program - Gastroenterology Research
- Requisition #: 165663
- Department: Gastroenterology Research
- Location: Houston, TX
- Posted Date: 5/14/2024
- Requisition ID: 165663
- Employment Status: Full-Time
- Employee Status: Regular
- Work Week: Days
- Minimum Salary: US Dollar (USD) 55,500
- Midpoint Salary: US Dollar (USD) 69,500
- Maximum Salary : US Dollar (USD) 83,500
- FLSA: non-exempt and eligible for overtime pay
- Fund Type: Soft
- Work Location: Hybrid Onsite/Remote
- Pivotal Position: No
- Referral Bonus Available?: No
- Relocation Assistance Available?: No
- Science Jobs: No
Be more at MD Anderson
- Applicant Rights & Notices
- EEO / Accessibility
- mdanderson.org
© 2024 The University of Texas MD Anderson Cancer Center

Assistant Clinical Research Coordinator
🔍 stanford, california, united states.
Stanford University School of Medicine and the Heart Center Clinical and Translational Research Program (CTRP) is seeking an Assistant Clinical Research Coordinator (ACRC) to p erform administrative support duties related to the collection of clinical data and/or the coordination of clinical studies in pediatric cardiology. Work under supervision of the principal investigator and/or study coordinator/supervisor.
Duties include:
· Schedule and/or call subjects for appointments; contact participants with reminders or other requirements.
· Prepare, distribute, and process questionnaires.
· Perform clerical duties in the preparation of regulatory documents. Maintain all forms and documents, including consent forms and master subject logs. File all appropriate correspondence.
· Assist with the screening, recruiting, and obtaining consent of study participants. Review medical records and/or perform telephone or in-person interviews to gather data, as needed.
· Administer standard study questionnaires and tests, score test measurements and questionnaires, and code data for computer entry. Perform quantitative review of forms, tests, and other measurements for completeness and accuracy.
· Extract data from source documents for research studies as directed. Collect data and complete case report forms.
· Prepare, process, and ship specimens/samples accurately under well-defined requirements.
· Order and maintain equipment and supplies.
· Process study compensation payments and thank you letters to subjects upon completion of trial activities. Assist with post-study activities, as needed.
- - Other duties may also be assigned
~ All members of the Department of Pediatrics are engaged in continuous learning and improvement to foster a culture where diversity, equity, inclusion, and justice are central to all aspects of our work. The Department collectively and publicly commits to continuously promoting anti-racism and equity through its policies, programs, and practices at all levels. ~
DESIRED QUALIFICATIONS:
- Excellent oral and written communication skills
- Proficiency in using computers, software, and web-based applications
- Proficiency in English and Spanish (verbal and written) required.
EDUCATION & EXPERIENCE (REQUIRED):
Two-year college degree and one year of relevant experience or an equivalent combination of experience, education, and training.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
General knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
PHYSICAL REQUIREMENTS*:
· Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
· Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
· Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
* - Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
· Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
· May require extended or unusual work hours, including weeknights and weekends, based on research requirements and business needs.
Position may be required to support studies that are conducted at off-site clinics (within a 50-mile radius of the main campus). Incumbent will need to provide own transportation with the ability to get to/from these off-site clinics.
Stanford University provides pay ranges representing its good faith estimate of what the University reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location, and external market pay for comparable jobs. The pay range for this position working in the California Bay area is $25.48-$31.25.
- Schedule: Full-time
- Job Code: 1012
- Employee Status: Regular
- Department URL: http://pediatrics.stanford.edu/
- Requisition ID: 103246
- Work Arrangement : Hybrid Eligible
My Submissions
Track your opportunities.
Similar Listings
Stanford, California, United States
📁 Research
Post Date: 1 day ago
Post Date: Apr 03, 2024
Post Date: 6 days ago
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
- China Mainland
- Saudi Arabia
- Türkiye
- United Arab Emirates
- South Africa
- Australia and New Zealand
- Belgium and Luxembourg
- Czech Republic & Slovakia
- Netherlands
- Poland & The Baltics
- Switzerland
- United Kingdom
Careers at Gilead

Search Open Positions
Be part of Gilead’s journey as we strive to solve some of the world’s biggest healthcare challenges.
Working at Gilead
Gilead has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a better, healthier world for all people. We have commercialized more than 25 innovative medicines, helping to transform treatment for people living with HIV, viral hepatitis, cancer and other life-threatening diseases. Through our ongoing bold and transformative science, we’re driving innovations that have the potential to become the next generation of life-changing medicines.
Our employees include some of the most talented innovators in the biopharmaceutical industry. We are always looking for the next wave of passionate people interested in joining an intellectually stimulating, socially responsible company with a track record of addressing serious illnesses and public health issues around the world. Every member of our team plays a critical and visible role in helping to discover, develop and deliver innovative therapeutics for people with life-threatening diseases. Because the impossible is not impossible. It’s what’s next.
Employment Fraud Warning
It has come to our attention that there are cases of fraudulent job postings that claim to offer positions with Gilead Sciences. Some involve individuals posing as Gilead recruiters and representatives of Gilead’s Talent Acquisition team who may request interviews and even provide false job offers in an attempt to gain sensitive personal information.
We are committed to providing a safe and trustworthy environment. We only require applicants to apply directly through our official careers portal operated by Workday. This portal allows candidates to access their profiles and review any updates or formal offers. Candidates that are selected for a position will be contacted directly by an official Gilead recruiter
Gilead does not use third-party communication applications such as Signal to conduct interviews. Gilead does not attach offer letters via email, require emailed direct deposit information or require purchase of equipment through mailed checks as part of our onboarding process.
You may report any fraudulent behavior at [email protected] . If you have been defrauded or suspect identity theft as a result of an employment scam, please contact your local law enforcement.
Related Jobs
United States - California - Foster City
Canada - Alberta - Edmonton
Learn More About Who We Are

Get to know the talented people who are helping Gilead create a better, healthier world for all people.

We’re growing a diverse workforce to help drive innovation at Gilead.

We’re collaborating with our partners to improve access to care around the world.
Equal Opportunities
As an equal opportunity employer, Gilead Sciences, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non-job-related characteristics or other prohibited grounds specified in applicable federal, state and local laws. It is also Gilead’s policy to comply with all applicable federal, state and local laws respecting consideration of unemployment status in making hiring decisions. For more information about equal employment opportunity protections, please view the EEO is the Law poster.
Notice: Employee Polygraph Protection Act
Your Rights Under the Family and Medical Leave Act
Pay Transparency Nondiscrimination Provision
To ensure reasonable accommodations are provided to individuals protected by Section 503 of the Rehabilitation Act of 1973, the Vietnam Era Veterans' Readjustment Act of 1974, and Title I of the Americans with Disabilities Act of 1990, applicants who require accommodation in the job application process may contact [email protected] for assistance.
A Message from Gilead’s Talent Acquisition Team
We encourage all qualified individuals who are passionate about our mission and want to help make a difference through our work to apply online. Candidates who are selected for further consideration will be contacted by a member of the company’s internal recruiting team.

Native Health Navigator Program Connects Tribes to Cancer Care
Stories@Gilead - April 24, 2024 - 4 min read

Gilead Helps Put African Nation on Path to Eliminate Hepatitis C
Stories@Gilead - March 27, 2024 - 1 min read
Gilead’s FOCUS Program Helps Zero In on Early HIV Detection
Stories@Gilead - March 21, 2024 - 2 min read

Gilead Helps Launch Türkiye’s First HIV Testing Week
Stories@Gilead - March 07, 2024 - 4 min read
Winn Awards Help Physicians Solve Healthcare Disparities
Stories@Gilead - February 28, 2024 - 5 min read

From the Margins: Gilead's Book Uniquely Captures What it's Like to be Marginalized by Disease
Stories@Gilead - January 23, 2024 - 1 min read

Breaking Barriers to Breast Cancer Care in Rural Areas
Stories@Gilead - October 25, 2023 - 4 min read
Advancing Global Health Equity in Tropical Regions
Stories@Gilead - October 09, 2023 - 4 min read

Gilead’s Commitment to Advancing Health Equity for Black Women and Ending the HIV Epidemic
Stories@Gilead - September 13, 2023 - 4 min read
Intersititial body copy
Clinical Research Coordinator Associate/Technician
How to apply.
A cover letter is required for consideration for this position and should be attached as the first page of your resume. The cover letter should address your specific interest in the position and outline skills and experience that directly relate to this position.
The Program in Research and Innovation on Maternal and Neonatal Outcomes (PRIMO), housed within the Department of Obstetrics and Gynecology seeks a Clinical Research Coordinator to join our fast-growing obstetrics research team. The ideal candidate will assist with several maternal and obstetrical health research studies and have previous research experience in women?s health. This person will be key in providing clinical trial study support and day to day management of multiple research projects focused on improving women and infant health. This position requires a flexible schedule, with some evening and weekend coverage, and ability to work at various Michigan Medicine locations.
CRC STATEMENT:
This clinical research coordinator (CRC) position may provide study coordination for multiple clinical research studies depending on complexity that range from moderate to complex. Coordinator experience and mastery of all job duties from the CRC-Technician position on the Michigan Medicine CRC Career Ladder is required. This position should be able to perform tasks and make decisions independently, consistently, and accurately, and demonstrate that they have achieved a moderate level of expertise in all their skills and abilities resulting in high quality work. It is presumed that this position is able to apply their skills to a broad range of different types of clinical studies, navigate available resources appropriately, effectively use all tools and job aids at their disposal and operate e-clinical technologies with a reasonable degree of proficiency. This position can perform the majority of tasks independently and perform quality checks of their work. They also know where and how to identify appropriate resources and support and are able to discern when to escalate issues needing additional intervention. Key behavioral competency descriptors include demonstrate, implement, execute, and use.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Why Join Michigan Medicine?
Michigan Medicine is one of the largest health care complexes in the world and has been the site of many groundbreaking medical and technological advancements since the opening of the U-M Medical School in 1850. Michigan Medicine is comprised of over 30,000 employees and our vision is to attract, inspire, and develop outstanding people in medicine, sciences, and healthcare to become one of the worlds most distinguished academic health systems. In some way, great or small, every person here helps to advance this world-class institution. Work at Michigan Medicine and become a victor for the greater good.
What Benefits can you Look Forward to?
- Excellent medical, dental and vision coverage effective on your very first day
- 2:1 Match on retirement savings
Responsibilities*
Characteristic Duties and Responsibilities:
Expert level knowledge, skills, and abilities within all 8 competency domains is expected:
- Scientific Concepts and Research Design
- Ethical Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
Additional duties will include:
Study Interactions and Clinical Coordinator Responsibilities
- Assist multiple investigators and collaborators to monitor patient recruitment and develop plans to enhance recruitment
- Screen, recruit, approach and consent pregnant women, giving study overview while being sensitive to environment and patients involved.
- Attain in-depth understanding of study protocol and objectives to assist with successful implementation of all study procedures
- Explaining studies thoroughly, reviewing informed consent, answering questions and following GCP/IRB and obtaining consent
- Execute study visits and study related procedures
- Triage complex study concerns appropriately
- Collect of human biospecimens, as well as processing , preparing and shipping specimens to outside institutions according to study protocol requirements.
- Submit Human Subjects Incentive Program (HSIP) requests
- Coordinate with study teams at other institutions to stay up to date on study protocols and other relevant research tasks for multi-site studies
- Collaborates with medical staff to facilitate and optimize the care of research patients
- Develop and monitor protocols and infrastructure for clinical studies
- Track, document and report on study progress
- Review real time medical records to match potential research participants with inclusion/exclusion criteria for active studies
- Schedule subject visits and follow up interactions by facilitating communication between clinic/unit staff and investigators/study team
- Perform study-specific testing and oversee specialized research devices and equipment
- Investigate, modify, and integrate new procedures as needed
- Serve as primary liaison between study staff, subjects, investigators, other departments, and sponsors.
- Working with Research Pharmacy in ordering and obtaining study medication
- Travelling to various Michigan medicine site
- Various duties as needed
Data Related
- Create case report forms, questionnaires and study related documents
- Complete study documentation in various data systems
- Responsible for data entry, management, cleaning and database creation for several studies
- Triage complex data concerns appropriately
- Abstract data from the medical record
- Review collected data and perform data quality assurance of the collected data
- Create reports on the completeness and quality of the collected data
Regulatory & Study reporting
- Assist PI and study team in maintaining IRB (eResearch) applications including scheduled continuing reviews, adverse event reporting (events that either occurs in UM research subjects or subjects from other centers in multi-site studies) and other reportable information and occurrences.
- Assist PI with identifying and grading adverse events.
- Ensure compliance with study protocols, good clinical practice guidelines and FDA regulations
- Working with Research Pharmacy, study medication and chain of custody
- Assist with any regulatory and institutional and external monitoring visits
Other duties as assigned
- Various duties as needed
Supervision Received:
This position reports directly to a CRC-Lead, CRC-Project Manager, a unit Administrator, Director, or Faculty Principal Investigator.
Supervision Exercised:
Required Qualifications*
- Bachelor?s degree in Health Science or an equivalent combination of related education and experience is necessary.
- Certification is required through Association of Clinical Research Professionals ( ACRP ) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association ( SOCRA ) as a Certified Clinical Research Professionals (CCRP) or equivalent. Candidates must be eligible to register or take the exam at date of hire and the certification must be completed or passed etc . within six months of date of hire. (Please review eligibility criteria from SoCRA or ACRP prior to applying.)
- Minimum 2 years of directly related experience in clinical research and clinical trials is necessary. (Please review SoCRA?s Definition of a Clinical Research Professional for qualifying experience prior to applying.)
Technician:
- Associate degree in Health Science or an equivalent combination of related education and experience is necessary.
- Minimum 1 year of directly related experience in clinical research and clinical trials is necessary. (Please review SoCRA?s Definition of a Clinical Research Professional for qualifying experience prior to applying.) or An advanced degree in a health-related areas such as: Health Sciences, Behavioral Sciences, Public Health, Health Care Administration, Clinical Research Administration, Social Work, Psychology, Epidemiology, Foreign MD. or Minimum 3 years of human subject experience (clinical, lab or health regulations) such as related patient care, related community health and wellness, related clinical information, and research.
- Previous experience with chart abstraction and/or data entry
- Flexible work schedule
- Excellent verbal and written communication skills
- Excellent interpersonal and organizational skills, and the ability to work effectively with diverse groups
- Demonstrated ability to work well under time constraints and meet deadlines
- Demonstrated ability to prioritize and exercise good judgement
- High attention to detail and accuracy
- Demonstrated ability to work independently with minimal supervision as well as work as part of a team.
- Demonstrated problem solving, conflict resolution, analytical, and critical thinking skills
- Demonstrated coordination, time management and communication skills
- Demonstrated ability to perform the majority of tasks independently and perform quality checks of their work
- Personal transportation to support various work locations
Desired Qualifications*
Associate :
- 4+ years of direct related experience
Technician:
- Bachelor?s degree in Health Science or an equivalent combination of related education and experience is desirable.
- An understanding of medical terminology, experience in a large complex health care setting, ability to effectively communicate with staff and faculty of all levels, and knowledge of university policies and procedures is desirable.
- Previous experience in women?s health and/or a maternal population
- Experience with the OnCore clinical trial management system (CTMS)
- Previous experience with MiChart, RedCap, and Qualtrics
- Previous experience with sample processing and shipping
Work Schedule
General hours are Mon-Fri within the 7am-5pm range. Some evening (5pm-9pm) and weekend hours may be required depending on the study needs.
Work Locations
- Primary location is at the main medical campus in University Hospital South (UH South); with regular work activities at VonVoigtlander Womens Hospital.
- This position may include some travel to Michigan Medicine clinical sites such as West Ann Arbor.
Underfill Statement
This position may be underfilled at the CRC-Technician title based on selected candidates? qualifications.
Additional Information
Michigan Medicine is firmly committed to advancing inclusion, diversity, equity, accessibility, and belonging, which are core to the culture and values of the Medical School Office of Research. Our community supports recruiting and cultivating a diverse workforce as a reflection of our commitment to serve the diverse people of Michigan and the world. We strive to create a work culture where each team member feels respected, valued, and safe.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
Data Professional Reflects on Her ‘Journey Back into Clinical Research’ During AAPI Heritage Month
Blog May 15, 2024

Kiruthika Arumugam, MS, Clinical Data Operator, Natera, Inc.
In recognition of May being Asian American and Pacific Islander (AAPI) Heritage Month , ACRP reached out for some thoughts on the importance of the event to Kiruthika Arumugam, MS, a Clinical Data Operator with Natera, Inc., and Treasurer for the ACRP Northern California Chapter . Before moving to the San Francisco Bay area, she studied biotechnology in India and gained several years of clinical research industry experience there in research and development and clinical data management through work with Avesthagen and IQVIA.
ACRP: Can you share some details on where you grew up, what you studied, how you first got drawn into clinical research, and what kind of duties you have now?
Arumugam: Having grown up in Tamil Nadu, India, my fascination with science emerged early in my childhood, fueling a lifelong dream of becoming a scientist. Pursuing this passion, I attained a master’s degree in biotechnology and commenced my professional journey in oncology, specifically delving into the early stages of drug discovery for breast and prostate cancer.
Driven by a profound curiosity for clinical research, I transitioned into the clinical data realm, starting my career as a clinical data assistant. Upon relocating to the United States, I encountered a waiting period for my work visa, during which I prioritized my familial responsibilities. Despite focusing on family for several years, my ambition to become a clinical trial manager remained steadfast. To stay abreast of industry advancements, I pursued a certificate in Clinical Trial Design and Management at the University of California Santa Cruz, Silicon Valley extension.
My introduction to ACRP led to active involvement in the Northern California Chapter, initially as the Outreach Committee Chair and currently as the Treasurer since 2020. Engaging with this organization has refined my organizational and interpersonal skills, ensuring I stay updated on the latest developments in the field and showcasing my ability to adapt to new systems and tools to support educational initiatives.
Joining Natera as a clinical data operator marks my reentry into clinical research. My responsibilities include accessioning patient samples with high efficiency and accuracy, as well as creating new orders on the laboratory inventory management system while conducting necessary checks to ensure proper accessioning. This role signifies the beginning of my journey back into clinical research, with much more to accomplish in the future.
ACRP: What does AAPI Heritage Month mean in your personal and professional life?
Arumugam: AAPI Heritage Month holds distinct significance in both my personal and professional realms. On a personal level, it’s a time of introspection and celebration, where I reconnect with my cultural heritage and familial traditions. It’s a moment to honor the journeys of my ancestors, whose resilience and sacrifices have paved the way for my own opportunities and experiences. AAPI Heritage Month allows me to embrace and share the diverse tapestry of cultures, languages, and customs that make up my identity, fostering a sense of pride and belonging within the larger AAPI community.
Professionally, AAPI Heritage Month serves as a platform for recognition and advocacy within the workplace. It’s an opportunity to amplify the voices and contributions of AAPI colleagues, highlighting their talents, expertise, and leadership. By promoting diversity and inclusion, organizations can harness the unique perspectives and experiences of AAPI individuals, fostering innovation and driving positive change.
ACRP: From what you’ve seen, can you characterize how active persons of AAPI heritage are as professionals and participants in clinical research in the U.S. versus what would be an ideal situation? What can/should ACRP and similar organizations do to address any gaps in this area?
Arumugam: From what I’ve seen, individuals of AAPI heritage are actively engaged in the field of clinical research in the United States, contributing their expertise and insights across various roles and sectors. However, there are notable disparities in representation, particularly in leadership positions and decision-making roles, compared to their presence in the overall population. In an ideal scenario, the participation and influence of AAPI professionals in clinical research would be proportionate to their demographic representation. Achieving this ideal involves addressing systemic barriers and biases that hinder the advancement and recognition of AAPI professionals in the field.
To bridge these gaps, organizations can take proactive steps:
- Advocacy and Representation: Advocate for increased representation of AAPI professionals in leadership positions and decision-making bodies within the organization and industry at large. Actively support the nomination and promotion of AAPI candidates for leadership roles and ensure their voices are heard in shaping policies and initiatives.
- Community Engagement: Engage with AAPI communities through outreach programs, partnerships, and collaborations to build trust and facilitate participation in clinical research. Address cultural and language barriers to ensure equitable access to research opportunities and resources.
By implementing these strategies, ACRP and similar organizations can work toward creating a more inclusive and equitable clinical research environment that harnesses the full potential of AAPI professionals and promotes diversity and excellence in the field. I trust that ACRP is already addressing these concerns, and collectively, we are working to bridge these gaps.
ACRP: Are there often clinical trials sponsored in your area, or trials that could be conducted, to adequately serve the local AAPI population in terms of conditions that affect them more than other populations? For any that are conducted, is patient recruitment/outreach for persons of AAPI heritage a well-developed process or more of a challenge each time?
Arumugam: Clinical trials conducted in areas with substantial AAPI populations may indeed focus on health conditions that disproportionately affect this community, such as certain types of cancer (e.g., liver, stomach), hepatitis B, diabetes, and mental health issues. However, ensuring adequate representation of AAPI individuals in these trials can pose challenges.
Patient recruitment and outreach efforts targeting the AAPI community vary in effectiveness. In some cases, these efforts are well-developed and employ culturally sensitive strategies, collaborating with community organizations, ethnic media outlets, and healthcare providers serving AAPI communities. These initiatives aim to raise awareness about clinical trials, provide education on research participation, and address cultural and linguistic barriers.
However, in other instances, patient recruitment for AAPI individuals may be more challenging. Barriers such as language differences, cultural stigmas, and mistrust of the healthcare system can impede recruitment efforts. Overcoming these barriers requires additional resources and tailored approaches. To address these challenges, collaboration between clinical trial sponsors, research institutions, and community organizations is essential. Culturally competent recruitment strategies may involve leveraging trusted community leaders, providing language-specific materials, offering culturally appropriate incentives, and ensuring that trial staff are trained in cultural sensitivity.
By prioritizing inclusive recruitment practices and actively engaging with AAPI communities, clinical trials can better serve the needs of this population and ensure that research findings are applicable and beneficial to all segments of society.
Edited by Gary Cramer
Miami, Florida
Seattle, Washington

Health Equity Expert Values AAPI Heritage Month as Chance to Advocate for Diversity in Clinical Trials

Keeping Up with the Clinical Research Compliance Landscape
Jobs in the acrp career center.
- Research Fellow | Nemours
- Research Scientist | Nemours
- Research Manager | Catholic Health
- Skip to main menu
- Skip to user menu
Clinical Research Coordinator Supervisor, Department of Surgery

- Ability to prioritize, focus, work independently and be flexible when the unexpected occur
- Excellent, demonstrated oral and written communication skills
- Excellent customer service skills
- Prior experience supervising and leading a team
- Ability to work well in a team environment and to work with individuals from diverse backgrounds
- Ability to set priorities and to work comfortably in a fast-paced, deadline driven environment
- Exceptional interpersonal and communication skills and demonstrated ease problem solving in group settings
- Ability to readily accept routine interruptions in order to meet “real time” / “just in time” learning needs and support of co-workers
- Ability to demonstrate successful implementation of time management skills
- Ability to multi-task and prioritize autonomously
- Exceptional attention to detail and data accuracy
- Proficient with multiple computer applications, including but not limited to: Microsoft Word, Microsoft Excel, spreadsheet management, PDF file creation/use, online EDC database/maintenance, web-based research, network & online file maintenance & management, and custom report generation.
- Demonstrated experience fostering a diverse faculty, staff, and student environment or a commitment to do so at VCU
- Bachelor’s degree or equivalent combination of education and experience
- Research experience in an academic medical center strongly desired
Share this job
Get job alerts
Create a job alert and receive personalized job recommendations straight to your inbox.
Similar jobs
Academic testing coordinator.
- Toledo, Ohio, United States
Research Associate in Apiculture
- Frankfort, Kentucky, United States
Post-Doctoral Research Associate in Sustainable Agriculture

IMAGES
VIDEO
COMMENTS
Clinical research is a competitive but growing field and provides rewarding career opportunities if you have qualifications or experience within life sciences. A career in clinical research involves playing a role in helping your employer conduct studies to ensure new treatments are safe and effective for patients.
Clinical Research Nurse. Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include: Helping to develop new treatments and care pathways for patients. Aiding data collection activities.
Abstract: Research coordinators may transition to clinical research associates/monitors during their careers.This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process.
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job. Respondents stressed the importance of flexibility and persistence ...
Clinical research career paths. Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities. 1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol ...
The project management team is made up of Clinical Project Managers and Program Managers. In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers. Project managers are the "General Contractors" of clinical research. They are accountable of all aspects of a clinical trial.
There are several different career paths in clinical research, from technical positions to administrative positions. Clinical Research Associate (CRA), Field Monitor, Clinical Monitor or Trial Monitor. The clinical research associate supervisors and monitors are requested and paid by sponsors who hire them to help with the administrative parts ...
Task Forces. Three task forces were convened in the spring of 1992 to address clinical research issues specific to (1) nursing and clinical psychology, (2) dentistry, and (3) surgery. Each of these task forces was chaired by a member of the committee and the membership was selected from those in the profession.
To earn this certification, you must have one of the following: At least two years of clinical research experience or 3,500 hours of part-time experience in the past five years. A degree in clinical research and at least one year of full-time experience. A certificate in clinical research, a bachelor's or associate degree in health science ...
Field offers opportunities for professionals with health, science or technology background Career opportunities in the clinical research field are many and varied, with employment settings ranging from pharmaceutical and biotechnology, to medical device companies, contract research organizations, hospitals, educational institutions, independent contractors and more. Many professionals with a ...
Clinical Research Coordinator Salary. According to Career Explorer, the average salary for a clinical research coordinator in the US is $112,044 annually. The wages can start at $57,324 per year and go up to $219,000 per year. The number of clinical research coordinator jobs is expected to grow by 10 percent by the year 2026.
Clinical research trial team: The success of a quality clinical research program is essential for developing and maintaining an impeccable clinical research trial team. It is the main component of a research program because total time and effort for conducting a clinical trial; nurses and data managers each contribute more than 30%.
Here's a list of steps on how to find clinical research jobs: 1. Obtain qualifications. When looking for a clinical research job, you may have a better chance of receiving an employment offer if you possess sufficient education and certification requirements. Most clinical researchers have a bachelor's degree in life science or a health discipline.
The first project undertaken by the group was to compile job profiles for entry-level clinical research positions in CROs and clinical sites. These job profiles provide a summary of the entry-level job role, essential duties, and salary range followed by example career pathways that illustrate possible next steps in that job category.
This series also provides employees with a clear path for career progression within Michigan Medicine. The following are market titles in this career ladder: Clinical Research Assistant ( 103921) Clinical Research Technician ( 103922) Clinical Research Coordinator Associate ( 103923) Clinical Research Coordinator Intermediate ( 103924)
Found 46 jobs. Nature Careers offers clinical research positions for every career stage, including researcher and Postdoc positions, as well as Faculty members and Head of Department, all over the ...
Clinical Trial Assistant (CTA) Clinical Research Associate (CRA) or Study Monitor. Drug Safety Specialist. Biostatistician. Study Manager/Project Manager. Data Scientist, Clinical Data Coordinator, Analyst, or Manager. Quality Assurance Specialist, Auditor. Clinical Business Analyst. Medical Writer.
Clinical Research Coordinator. Alpha Clinical Research Centers, LLC 5.0. Clermont, FL. $45,000 - $60,000 a year. Full-time. Monday to Friday + 1. Easily apply. A minimum of two-year oncology or clinical research experience; Attends pertinent educational or study activities and reviews current literature relevant to….
Software Developer 3. The University of Washington, National Alzheimer's Coordinating Center (NACC) Seattle, WA. Details. Manager. Protocol and Correlative Development Manager. Hoosier Cancer Research Network. Indianapolis, IN. Details.
JOB SPECIFIC COMPETENCIES 1)Research Projects: Establishes a system for development of research programs including writing, processing, submission and maintenance of protocols. ... Attends all the different institutional meeting regarding clinical research and maintain the research updates log for the department.
Clinical Research Associate II. Precision for Medicine. Remote. $113,000 - $152,900 a year. Easily apply. Site management or equivalent experience in clinical research. Responsibilities will be dependent upon the type and timing of the program to which the CRA II is…. Posted 6 days ago ·.
Stanford University School of Medicine and the Heart Center Clinical and Translational Research Program (CTRP) is seeking an Assistant Clinical Research Coordinator (ACRC) to p erform administrative support duties related to the collection of clinical data and/or the coordination of clinical studies in pediatric cardiology. Work under supervision of the principal investigator and/or study ...
Equal Opportunities . As an equal opportunity employer, Gilead Sciences, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression ...
This clinical research coordinator (CRC) position may provide study coordination for multiple clinical research studies depending on complexity that range from moderate to complex. Coordinator experience and mastery of all job duties from the CRC-Technician position on the Michigan Medicine CRC Career Ladder is required. This position should be ...
Pros "Work life balance good" (in 29 reviews) "Work culture is good and working time is flexible" (in 54 reviews) "Good administration, benefits, salary, learning, and food" (in 24 reviews) "Good work environment for a fresher" (in 28 reviews) "Very good employee friendly environment" (in 14 reviews)
With more than 16,500 members, the Association of Clinical Research Professionals (ACRP) is the only non-profit organization solely dedicated to representing, supporting, and advocating for clinical research professionals. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing ...
Summer Undergraduate Research Fellowship Program (SURF) UT Dallas Green Fellows; Supporting a Culture of Clinical Trials. Our research teams help plan, conduct, fund, administer, and report on clinical trials across the broad spectrum of health conditions and diseases. More than 1,000 trials are currently underway, including these areas of ...
20% Clinical Skills - Complete VCUHS orientation and training modules as required for the department & clinical research projects. Conduct applicable clinical activities only as approved & required for each clinical research project. - Any clinical skill must be conducted in VCUHS approved clinical areas only.
University Title : Senior Clinical Research Coordinator (4-6), Research & Innovation ORP Eligibility : No Vaccination Requirements : Please note that if you are employed as a university employee working in any of the health system's facilities, you will need to follow VCU Health System policies, which will include but will not be limited to ...
Today's top 236 Research jobs in Moscow, Moscow City, Russia. Leverage your professional network, and get hired. New Research jobs added daily.