- Biology Article
- Photosynthesis And Chemiosmotic Hypothesis

Chemiosmotic Hypothesis - Overview
Ever wonder how Photosynthesis helps in the creation of energy in plants? This is everything you need to know about the Chemiosmotic Hypothesis
What is Photosynthesis?
Photosynthesis is a biological process where plants create food and oxygen using solar energy. The process requires the plant to produce glucose, which is then used by the plant in the production of chemicals required for growth. Or it could be stored as starch and reconstituted into glucose when the plant needs it. Lastly, it could be used in the process of respiration, thereby liberating stored energy in the molecules.
Explore more: Photosynthesis
Chemiosmotic Hypothesis
In 1961, British biochemist by the name of Peter Dennis Mitchell theorized the Chemiosmotic hypothesis, which explains how the energy molecules (ATP: Adenosine triphosphate) are created during photosynthesis. His work was awarded the Nobel Prize as it provided a deeper insight into the complete process of ATP production within the chloroplasts.
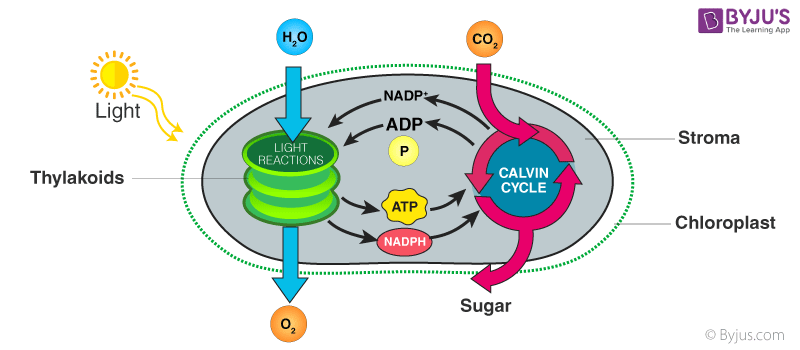
NADP (Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+) gets generated along with ATP during the light reaction or photochemical phase. These components are the key elements of photosynthesis. During the process, they are used in the dark reaction or Calvin Cycle to produce sugar molecules (final product).
The Process Of Chemiosmotic Hypothesis
In this process, ATP- Adenosine triphosphates are produced as the result of the proton gradient that exists across the thylakoid membrane. The fundamental components necessary for the chemiosmosis process are proton gradient, ATP synthase, and proton pump. The enzyme which is required for the synthesis of ATP molecules is called ATP synthase.
The enzyme ATP synthase consists of two subunits, namely: F0 and F1. The F0 subunit is involved in the proton transportation across the membrane, which causes changes in F1 configuration leading to the activation of enzymes . The enzyme phosphorylates ADP (meaning: to add a phosphate group) and converts ADP molecules to ATP molecules. The proton gradient that exists across the membrane is the primary driving force of ATP synthase.
In the light reaction phase of photosynthesis, chlorophyll absorbs light with the help of photosystems. This results in hydrolysis, where water molecules are split, releasing electrons and protons in the process. The released electrons get excited and move to a higher energy level and are carried by the electron transport system.
Meanwhile, the released protons from the stroma begin accumulating inside the membrane. This results in the creation of a proton gradient, a product of the electron transport chain . The small quantity of the resultant protons is used by photosystem I to reduce NADP+ to NADPH by electrons from the photolysis of water. Eventually, the proton gradient collapses, and it releases energy and protons that are carried out back to the stroma via F0 of ATP synthase. This resultant energy induces changes in F1 configuration and this, in turn, activates the ATP synthase, which converts ADP to ATP.
Also Read: Light Dependent Reactions
Learn more in detail about the chemiosmotic hypothesis, generation of ATP molecules and other related topics at BYJU’S Biology

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
| BIOLOGY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- COVID-19 Tracker
- Biochemistry
- Anatomy & Physiology
- Microbiology
- Neuroscience
- Animal Kingdom
- NGSS High School
- Latest News
- Editors’ Picks
- Weekly Digest
- Quotes about Biology
Chemiosmosis
Reviewed by: BD Editors
Chemiosmosis Definition
Chemiosmosis is when ions move by diffusion across a semi-permeable membrane, such as the membrane inside mitochondria. Ions are molecules with a net electric charge, such as Na + , Cl – , or specifically in chemiosmosis that generates energy, H + . During chemiosmosis, ions move down an electrochemical gradient, which is a gradient of electrochemical potential (a form of potential energy). Since chemiosmosis is a type of diffusion, ions will move across a membrane from areas of high concentration to areas of low concentration. Ions also move to balance out the electric charge across a membrane.
Function of Chemiosmosis
Chemiosmosis is involved in the production of adenosine triphosphate (ATP), which is the main molecule used for energy by the cell. In eukaryotes, ATP is produced through the process of cellular respiration in the mitochondria. First, the molecules NADH and FADH 2 , obtained from the citric acid cycle, pass electrons down an electron transport chain, which releases energy. This energy allows protons (H + ) to travel down a proton gradient via chemiosmosis. This in turn provides the energy for the enzyme ATP synthase to make ATP. The flow of these protons down the gradient turns the rotor and stalk of the ATP synthase, which makes it possible for a phosphate group to join with adenosine diphosphate (ADP), forming ATP. The production of ATP during respiration is called oxidative phosphorylation. Through oxygen and glucose, ATP is ultimately created through the phosphorylation of ADP. In aerobic respiration, 38 ATP molecules are formed per glucose molecule. Since chemiosmosis plays a role in the creation of ATP during this process, without chemiosmosis, organisms would not be able to produce the energy that they need to live.
The idea that ATP is synthesized through chemiosmosis was first proposed in 1961 by Dr. Peter D. Mitchell. At the time, this was controversial, because it was more widely accepted that there was some intermediate molecule that stored energy from the electron transport chain. However, an intermediate molecule was never found, and eventually research showed that the chemiosmosis theory was correct. Mitchell would later go on to win the Nobel Prize in Chemistry in 1976 for his contributions to science.
Examples of Chemiosmosis
Although chemiosmosis is often generally defined as the movement of ions across a membrane, it is really only used in the context of talking about the movement of H + ions during the production of ATP. The most common method involving chemiosmosis in the production of ATP is cellular respiration in the mitochondria, the process of which is discussed above. All eukaryotic organisms have mitochondria, so chemiosmosis is involved in ATP production through cellular respiration in the vast majority of different types of organisms, from animals to plants to fungi to protists. However, even though archaea and bacteria do not have mitochondria, they also use chemiosmosis to produce ATP through photophosphorylation. This process also involves an electron transport chain, proton gradient, and chemiosmosis of H + , but it takes place across the inner membrane of the bacterium or archaeon, since they have no mitochondria.
Plants produce ATP during photosynthesis in the chloroplast in addition to the ATP they generate through cellular respiration in mitochondria. The process is again similar: during photosynthesis, light energy excites electrons, which flow down an electron transport chain, which in turn allows H + ions to travel through a membrane in the chloroplast. Some bacteria, such as cyanobacteria, also use photosynthesis.
The similarities between these ATP production methods are more than just coincidence; both mitochondria and chloroplasts are thought to have evolved from free-living bacteria. This theory is called the endosymbiotic theory. This theory hypothesizes that that had symbiotic relationships with other cells, aiding them by producing energy in return for a place to live inside the cell. Over time, these bacteria became inextricable from the cells they resided in. The fact that mitochondria and chloroplasts have their own, separate, DNA supports this idea. This is why the chemiosmosis is used in generally the same way whether ATP is being produced in a mitochondrion, chloroplast, or bacterium.
Related Biology Terms
- Glucose – A simple sugar that has an important role in metabolism and energy production.
- Adenosine triphosphate (ATP) – The main molecule used for energy in cells.
- Ion – A molecule with a net electric charge due to gaining or losing an electron.
- Diffusion – Movement of molecules from an area of high concentration to an area of low concentration.
1. Which organism does not have mitochondria? A. Human B. Mushroom C. Bacteria D. Fern
2. Chemiosmosis involving what ion is part of the process of generating ATP? A. Na + B. H + C. Cl – D. H –
3. Chemiosmosis can occur in what cell organelle? A. Mitochondrion B. Chloroplast C. Nucleus D. Choices A and B
Cite This Article
Subscribe to our newsletter, privacy policy, terms of service, scholarship, latest posts, white blood cell, t cell immunity, satellite cells, embryonic stem cells, popular topics, pituitary gland, homeostasis, amino acids, adenosine triphosphate (atp), photosynthesis.
Chemiosmotic theory
Definition noun A theory postulated by the biochemist Peter Mitchell in 1961 to describe ATP synthesis by way of a proton electrochemical coupling. Accordingly, hydrogen ions ( proton s) are pumped from the mitochondrial matrix to the intermembrane space via the hydrogen carrier protein s while the electrons are transferred along the electron transport chain in the mitochondrial inner membrane. As the hydrogen ion s accumulate in the intermembrane space, an energy-rich proton gradient is established. As the proton gradient becomes sufficiently intense the hydrogen ions tend to diffuse back to the matrix (where hydrogen ions are less) via the ATP synthase (a transport protein). As the hydrogen ion s diffuse (through the ATP synthase ) energy is released which is then used to drive the conversion of ADP to ATP (by phosphorylation ). Supplement This theory was not previously well accepted until a great deal of evidence for proton pumping by the complexes of the electron transfer chain emerged. This began to favor the chemiosmotic hypothesis, and in 1978, Peter Mitchell was awarded the Nobel Prize in Chemistry. Also called: chemiosmotic (coupling) hypothesis See also: chemiosmosis , mitochondrion
Last updated on February 24th, 2022
You will also like...

Chromosomes X and Y and Sex Determination
This tutorial looks at sex determination via the sex chromosomes, X and Y. Read it to get more info on X and Y chromosom..
Circulation
The circulatory system is key to the transport of vital biomolecules and nutrients throughout the body. Learn about the ..
DNA Structure & DNA Replication
DNA is a double helix structure comprised of nucleotides. A nucleotide, in turn, is made up of phosphate molecule, deoxy..

Animal Water Regulation
Animals adapt to their environment in aspects of anatomy, physiology, and behavior. This tutorial will help you understa..
The Evolution of Cell Organelles
The nucleus containing the genetic material, DNA, and the mitochondria, well-identified as the "powerhouse of the cell",..

A Balanced Diet – Carbohydrates and Fat
Apart from vitamins, the human body also requires high energy sources such as carbohydrates and fats. If you want an ove..
Related Articles...

No related articles found

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Chemiosmosis
Chemiosmosis is the process by which hydrogen ions (protons) diffuse to the other side of the biological membrane from high to low concentration. It creates a difference in their concentration (electrochemical gradient) between the two sides of the semi-permeable membrane. The gradient energy synthesizes adenosine triphosphate ( ATP ) in the cell.
It is the final part of oxidative phosphorylation . The initial part is the electron transport chain.
Where Does Chemiosmosis Occur
In eukaryotes, chemiosmosis occurs in the mitochondria during aerobic cellular respiration and in the chloroplasts during photosynthesis . Since prokaryotes lack these organelles, chemiosmosis occurs in their plasma.
However, chemiosmosis requires a membrane to allow the electrochemical gradient to develop.
Function of Chemiosmosis
Chemiosmosis directly results in the synthesis of ATP. Thus, it is the primary energy source through cellular respiration within the cell. Any defect in the pathway would devoid the cell from producing ATP through oxidative phosphorylation .
Chemiosmotic Theory
Peter D. Mitchell first proposed the chemiosmotic theory in 1961. His theory suggests chemiosmosis is driven by an electrochemical proton gradient across the inner mitochondrial membrane that produces ATP in cells.
How does Chemiosmosis Produce ATP in Cellular Respiration
Before chemiosmosis can start, the electrochemical needs to be established with the help of high-energy molecules, NADH and FADH 2 . These compounds were formed during glucose oxidation through glycolysis in the cytoplasm to form pyruvate.
The pyruvate is further metabolized to acetyl CoA and then to citric acid or citrate through a series of steps in the citric acid cycle in the mitochondrial matrix. This cycle, with reducing intermediates like NAD and FAD, form NADH and FADH 2 .
These intermediates act as electron carriers in the electron transport chain . When the electrons move down the electron transport chain, protons are pumped across the mitochondria’s inner membrane, creating an electrochemical gradient, which eventually phosphorylates ADP to ATP. This final step is called chemiosmosis.
Steps of Chemiosmosis
The steps below describe how chemiosmosis occurs in the mitochondria of eukaryotes:
- The sources of electrons in the electron transport chain are high-energy electron carriers like NADH and FADH 2 . NADH provides electrons to Complex I while FADH 2 provides electrons to Complex II
- As electrons move along the electron transport chain cause from a higher to a lower energy level, energy is released. The flow of electrons in ETC is:
Complex I -> Complex II -> Coenzyme Q -> Complex III -> Cytochrome c -> complex IV -> Oxygen
- The hydrogen ions or protons are in lower concentration inside the mitochondrial matrix. The energy liberated by electrons helps to pump protons into the intermembranous space against their concentration gradient . The energy of electrons is stored as an electrochemical gradient
- With the increase in the concentration of protons in the intermembranous space, they move down their concentration gradient along the proton channel in the ATP synthase. This process is coupled to turn the proton ring and liberate energy
- The enzyme ATP synthase uses this energy to phosphorylate ADP, forming ATP on the stroma
How Many ATP Does Chemiosmosis Produce
At the end of aerobic respiration , around 32 ATPs are produced from one glucose molecule . Among these, 2 ATPs are from glycolysis, 4 from the Krebs cycle , and the remaining 28 from chemiosmosis.
Inhibition of Chemiosmosis
Chemiosmosis is inhibited by inhibitors of the electron transport chain or uncouplers. They are protein channels that provide an alternative path for entering the stroma without the involvement of ATP synthase. The energy of the electrochemical gradient gets wasted as heat.
2, 4-dinitrophenol, desaspidin, and dicoumarol are typical examples of uncouplers. The drug aspirin is also an uncoupling agent.
Chemiosmosis in Chloroplasts
In photosynthetic cells of eukaryotes, chemiosmosis occurs in the thylakoid membranes of the chloroplast . It takes place during light-dependent reactions of photosynthesis, where the energy of photons is used to make ATP for dark reactions.
In Non-cyclic Electron Flow
Here, the photoexcited electrons pass through the ETC but do not return to photosystem I.
As photoexcited electrons from the photosystem I move through the electron transport chain, energy is liberated that pumps protons from the stroma of chloroplasts into the lumen of thylakoids. The flow of electrons is:
Photosystem I -> Plastoquinone -> Cytochrome Complex -> Plastocyanin -> Photosystem II -> Ferredoxin -> NADP
The protons move down the concentration gradient back into the stroma, passing through the proton channel of ATP synthase, rotating the ring, and liberating energy, which is used to generate ATP from ADP
In Cyclic Electron Flow
At the end of each cycle, the photoexcited electrons pass through the ETC and return to photosystem I. The flow of electrons is:
Photosystem II -> Ferredoxin -> Cytochrome Complex -> Plastocyanin -> Photosystem II
The mechanism of ATP production is the same as the non-cyclic flow of electrons.
Chemiosmosis in Prokaryotes
Due to the absence of membrane-bound organelles, chemiosmosis in prokaryotes such as bacteria and archaea occurs in the cell membrane . The basic process is similar to mitochondria and chloroplast in eukaryotes.
- What is Chemiosmosis? – Definition & Process – Study.com
- Chemiosmosis and Oxidative Phosphorylation – Bio.libretexts.org
- Chemiosmosis – Alevelbiology.co.uk
- What’s Chemiosmosis? – Ats.doit.wisc.edu
- An Update of the Chemiosmotic Theory – Ncbi.nlm.nih.gov
Article was last reviewed on Friday, February 3, 2023
Related articles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
- Daily Lessons
- Get your widget
How to pronounce chemiosmotic hypothesis in American English ( 1 out of 1 ):
Enabled javascript is required to listen to the english pronunciation of 'chemiosmotic hypothesis'..
Definition:
Click on any word below to get its definition:, nearby words:, you may want to improve your pronunciation of 'chemiosmotic hypothesis' by saying one of the nearby words below:.
- hypothetical
- hypertension
- hypothalamus
- hypocritical
- hypothetically
- hypothesized
When you begin to speak English, it's essential to get used to the common sounds of the language, and the best way to do this is to check out the phonetics. Below is the UK transcription for 'hypothesis' :
- Modern IPA: hɑjpɔ́θəsɪs
- Traditional IPA: haɪˈpɒθəsɪs
- 4 syllables : "hy" + "POTH" + "uh" + "sis"
Test your pronunciation on words that have sound similarities with 'hypothesis' :
- hypothesize
- hopelessness
Tips to improve your English pronunciation:
Here are 4 tips that should help you perfect your pronunciation of 'chemiosmotic hypothesis' :.
- Record yourself saying 'chemiosmotic hypothesis' in full sentences , then watch yourself and listen. You'll be able to mark your mistakes quite easily.
- Look up tutorials on Youtube on how to pronounce 'chemiosmotic hypothesis'.
- Focus on one accent : mixing multiple accents can get really confusing especially for beginners, so pick one accent ( US or UK ) and stick to it.
To further improve your English pronunciation, we suggest you do the following:
- Work on word/sentence reduction : in some countries, reducing words and sentences can be seen as informal but in the United States, it's completely normal and part of everyday conversation (eg: what are you going to do this weekend → what you gonna do this weekend). Check out gonna and wanna for more examples.
- Work on your intonation: stress, rhythm and intonation patterns are not easy to master in English but they are crucial to make others understand what you say. It's what expresses the mood, attitude and emotion. Check out Youtube, it has countless videos related to this subject .
- Subscribe to 1 or more English teaching channels on Youtube: it's free and it covers the core topics of the English language. Check out Rachel and Mike channels to name just a few.
Dictionary not available Known issues Mother tongue required Content quota exceeded Subscription expired Subscription suspended Feature not available Login is required

pronouncekiwi - How To Pronounce Chemiosmotic hypothesis
Pronouncekiwi, listen to the pronunciation of chemiosmotic hypothesis and learn how to pronounce chemiosmotic hypothesis correctly..
| Catalan Pronunciation | |||
| Chinese (Mandarin) Pronunciation | |||
| Chinese (China) Pronunciation | |||
| Chinese (Hong Kong) Pronunciation | |||
| Chinese (Taiwan) Pronunciation | |||
| Danish Pronunciation | |||
| Danish Pronunciation | |||
| Danish Pronunciation | |||
| Danish Pronunciation | |||
| Dutch Pronunciation | |||
| Dutch Pronunciation | |||
| Dutch Pronunciation | |||
| Dutch Pronunciation | |||
| English (Australia) Pronunciation | |||
| English (Australia) Pronunciation | |||
| English (Australia) Pronunciation | |||
| English (Australia) Pronunciation | |||
| English (Canada) Pronunciation |
| English (UK) Pronunciation | |||
| English (UK) Pronunciation | |||
| English (UK) Pronunciation | |||
| English (UK) Pronunciation | |||
| English (UK) Pronunciation | |||
| English (UK) Pronunciation | |||
| English (India) Pronunciation | |||
| English (India) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (USA) Pronunciation | |||
| English (Welsh) Pronunciation | |||
| Finnish Pronunciation | |||
| French (France) Pronunciation | |||
| French (France) Pronunciation | |||
| French (France) Pronunciation | |||
| French (France) Pronunciation | |||
| French (France) Pronunciation | |||
| French (Canada) Pronunciation | |||
| French (Canada) Pronunciation | |||
| French (Canada) Pronunciation | |||
| German Pronunciation | |||
| German Pronunciation | |||
| German Pronunciation | |||
| German Pronunciation | |||
| German Pronunciation | |||
| German Pronunciation | |||
| Hindi Pronunciation | |||
| Icelandic Pronunciation | |||
| Icelandic Pronunciation | |||
| Italian Pronunciation | |||
| Italian Pronunciation | |||
| Italian Pronunciation | |||
| Italian Pronunciation | |||
| Italian Pronunciation | |||
| Japanese Pronunciation | |||
| Japanese Pronunciation | |||
| Japanese Pronunciation | |||
| Japanese Pronunciation | |||
| Korean Pronunciation | |||
| Korean Pronunciation | |||
| Norwegian Pronunciation | |||
| Norwegian Pronunciation | |||
| Norwegian Pronunciation | |||
| Polish Pronunciation | |||
| Polish Pronunciation | |||
| Polish Pronunciation | |||
| Polish Pronunciation | |||
| Polish Pronunciation | |||
| Polish Pronunciation | |||
| Portuguese (Brazil) Pronunciation | |||
| Portuguese (Brazil) Pronunciation | |||
| Portuguese (Brazil) Pronunciation | |||
| Portuguese (Brazil) Pronunciation | |||
| Portuguese (Brazil) Pronunciation | |||
| Portuguese (Portugal) Pronunciation | |||
| Portuguese (Portugal) Pronunciation | |||
| Portuguese (Portugal) Pronunciation | |||
| Portuguese (Portugal) Pronunciation | |||
| Russian Pronunciation | |||
| Russian Pronunciation | |||
| Russian Pronunciation | |||
| Russian Pronunciation | |||
| Romanian Pronunciation | |||
| Slovak Pronunciation | |||
| Spanish (USA) Pronunciation | |||
| Spanish (USA) Pronunciation | |||
| Spanish (Castilian) Pronunciation | |||
| Spanish (Castilian) Pronunciation | |||
| Spanish (Spain) Pronunciation | |||
| Spanish (Spain) Pronunciation | |||
| Spanish (Spain) Pronunciation | |||
| Spanish (Spain) Pronunciation | |||
| Spanish (Spain) Pronunciation | |||
| Spanish (Mexico) Pronunciation | |||
| Swedish Pronunciation | |||
| Swedish Pronunciation | |||
| Swedish Pronunciation | |||
| Turkish Pronunciation | |||
| Turkish Pronunciation | |||
| Welsh Pronunciation | |||
| Ukrainian Pronunciation |
Simply select a language and press on the speaker button to listen to the pronunciation of the word. Leave a vote for your preferred pronunciation.
- How To Pronounce chemi
- How To Pronounce Chemi Doron
- How To Pronounce Chemi Shalev
- How To Pronounce chemi-
- How To Pronounce Chemi-ionization
- How To Pronounce chemia
- How To Pronounce chemia biologiczna
- How To Pronounce chemia organiczna
- How To Pronounce chemiach
- How To Pronounce chemiae
- How To Pronounce chemiam
- How To Pronounce chemiami
- How To Pronounce chemiarum
- How To Pronounce chemias
- How To Pronounce Chemiatry
- How To Pronounce Chemiautomatics Design Bureau
- How To Pronounce Chemiazid
- How To Pronounce Chemic
- How To Pronounce Chemic (atom)
- How To Pronounce Chemic (disambiguation)
- How To Pronounce Chemic (school)
- How To Pronounce Chemic (scientist)
- How To Pronounce chemica
- How To Pronounce chemicae
- How To Pronounce chemicage
- How To Pronounce Chemical
- How To Pronounce Chemical & Engineering News
- How To Pronounce Chemical (Crashdiet song)
- How To Pronounce Chemical (Crashdïet song)
- How To Pronounce Chemical (disambiguation)
- How To Pronounce Chemical (Joseph Arthur song)
- How To Pronounce Chemical (medicine)
- How To Pronounce Chemical (science)
- How To Pronounce Chemical (song)
- How To Pronounce Chemical Abortion
- How To Pronounce Chemical Abstract Services
- How To Pronounce Chemical Abstracts
- How To Pronounce Chemical Abstracts Service
- How To Pronounce Chemical Abstracts Service Registry Number
- How To Pronounce Chemical Abstracts Service Source Index
- How To Pronounce Chemical abundance
- How To Pronounce Chemical accident
- How To Pronounce Chemical accident in Gorlivka (2013)
- How To Pronounce Chemical Accidents
- How To Pronounce Chemical activity
- How To Pronounce Chemical addiction
- How To Pronounce Chemical addition wastewater treatment
- How To Pronounce Chemical additives
- How To Pronounce Chemical affinities
- How To Pronounce Chemical Affinity
- How To Pronounce Chemical agent
- How To Pronounce Chemical Agent Identification Set
- How To Pronounce Chemical Agent Identification Sets
- How To Pronounce Chemical Agent Resistant Coating
- How To Pronounce Chemical Agents
- How To Pronounce Chemical Ali
- How To Pronounce Chemical amount
- How To Pronounce Chemical analog
- How To Pronounce Chemical analogue
- How To Pronounce Chemical analyses
- How To Pronounce Chemical Analysis
- How To Pronounce Chemical analyst
- How To Pronounce Chemical analysts
- How To Pronounce Chemical and Biological Arms Control Institute
- How To Pronounce Chemical and Biological Warfare
- How To Pronounce Chemical and Engineering News
- How To Pronounce Chemical and pharmaceutical patent
- How To Pronounce Chemical and pharmaceutical patents
- How To Pronounce Chemical Assault
- How To Pronounce Chemical Atom
- How To Pronounce Chemical attack
- How To Pronounce Chemical attack on Behbahan battalion
- How To Pronounce Chemical Automatics Design Bureau
- How To Pronounce Chemical balance
- How To Pronounce Chemical Bank
- How To Pronounce Chemical Bank (Michigan)
- How To Pronounce Chemical Bank of New York
- How To Pronounce Chemical Banking
- How To Pronounce Chemical Banking Corp.
- How To Pronounce Chemical Banking Corporation
- How To Pronounce Chemical barriers
- How To Pronounce Chemical base
- How To Pronounce Chemical basis for love
- How To Pronounce Chemical bath deposition
- How To Pronounce Chemical Beam Epitaxy
- How To Pronounce Chemical Biological Incident Response Force
- How To Pronounce Chemical Biology
- How To Pronounce Chemical Biology (journal)
- How To Pronounce Chemical Biology Laboratory
- How To Pronounce chemical bomb
- How To Pronounce Chemical Bond
- How To Pronounce Chemical bonding
- How To Pronounce Chemical bonding model
- How To Pronounce Chemical Bonds
- How To Pronounce Chemical brain preservation
- How To Pronounce Chemical breakdown
- How To Pronounce Chemical Breaks
- How To Pronounce Chemical Bros
- How To Pronounce Chemical Bros.
- How To Pronounce Chemical Brothers
- How To Pronounce Chemical Brothers discography
- How To Pronounce Chemical Brothers, The
- How To Pronounce Chemical buffer
- How To Pronounce Chemical buffers
- How To Pronounce Chemical Building, Field's Point Wastewater Treatment Facility
- How To Pronounce Chemical Building, Fields Point Sewage Treatment Plant
- How To Pronounce Chemical burn
- How To Pronounce Chemical burns
- How To Pronounce Chemical burns to the eye
- How To Pronounce Chemical capacitance
- How To Pronounce Chemical carcinogen
- How To Pronounce Chemical cardioversion
- How To Pronounce Chemical carrier
- How To Pronounce Chemical castration
- How To Pronounce chemical castrations
- How To Pronounce Chemical cell
- How To Pronounce Chemical chameleon
- How To Pronounce Chemical Change
- How To Pronounce Chemical Changes
- How To Pronounce Chemical chaperone
- How To Pronounce Chemical chaperones
- How To Pronounce Chemical chirality
- How To Pronounce Chemical chirality in popular fiction
- How To Pronounce Chemical Chords
- How To Pronounce Chemical City
- How To Pronounce Chemical class
- How To Pronounce Chemical classification
- How To Pronounce Chemical clock
- How To Pronounce Chemical Coast
- How To Pronounce Chemical Coast Line
- How To Pronounce Chemical colitis
- How To Pronounce Chemical coloring of metals
- How To Pronounce Chemical Communications
- How To Pronounce Chemical companies
- How To Pronounce Chemical company
- How To Pronounce Chemical compatibility
- How To Pronounce Chemical complex
- How To Pronounce Chemical components
- How To Pronounce Chemical composition
- How To Pronounce Chemical composition of living beings
- How To Pronounce chemical compositions
- How To Pronounce Chemical compound
- How To Pronounce Chemical compound CS
- How To Pronounce Chemical compound lime
- How To Pronounce Chemical compound magnesia
- How To Pronounce Chemical Compound Microarray
- How To Pronounce Chemical compound salt
- How To Pronounce Chemical compounds
- How To Pronounce Chemical computer
- How To Pronounce Chemical computing

Microbe Notes
Chemiosmosis: Definition, Components, Mechanisms, Uses
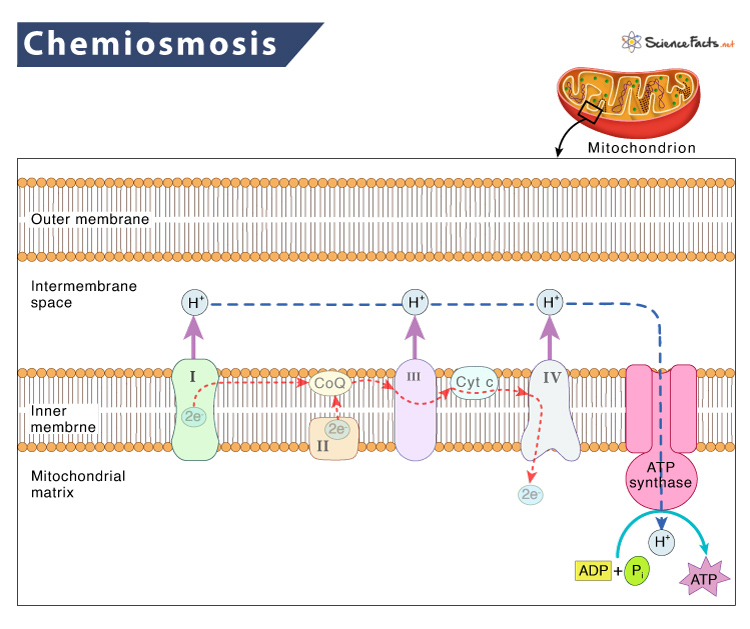
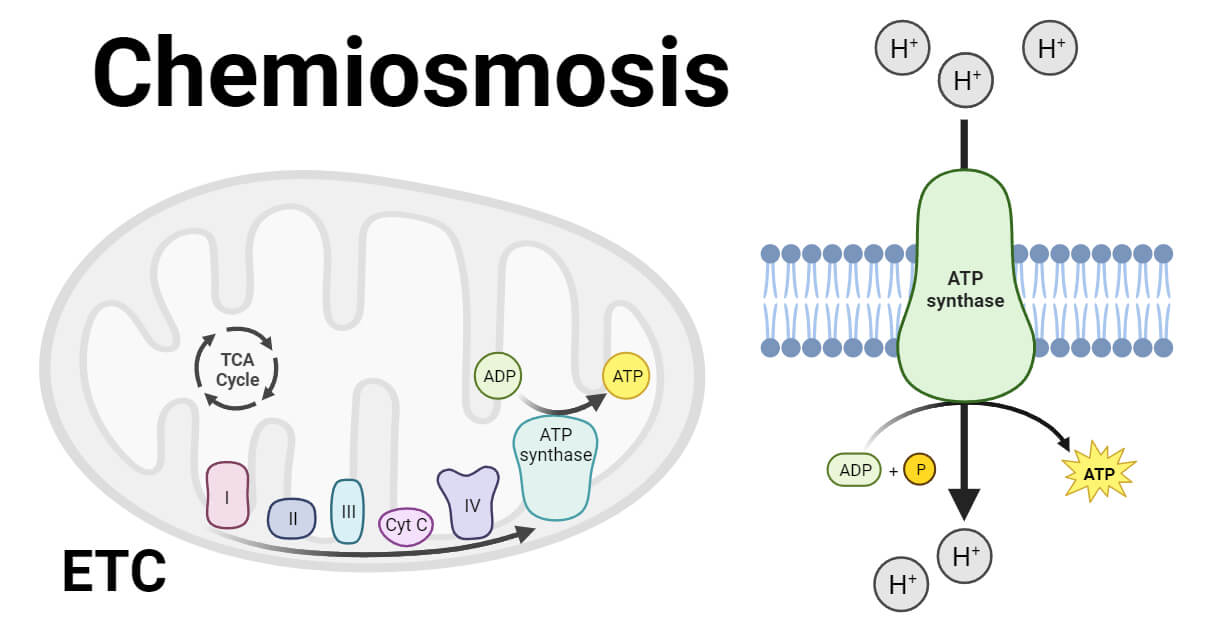
Chemiosmosis is a fundamental mechanism by which cells transfer and utilize energy, vital for various biological processes. Chemiosmosis involves the creation of a proton gradient through the electron transport chain, driving ATP synthesis via ATP synthase .
- The process tightly links electron transport with ATP synthesis, illustrating the interconnectedness of cellular respiration and photosynthesis.
- Chemiosmosis is not confined to a specific cell type or organism, playing a crucial role in both aerobic and anaerobic respiration and photosynthesis .
- Ongoing research continues to unveil new insights into chemiosmosis, expanding our understanding of cellular bioenergetics and presenting potential applications in drug discovery and bioenergetics research.
Energy is the currency of life, vital for cellular processes. Organisms utilize energy to perform various functions, such as growth, movement, and maintenance of biological structures. The flow of energy is crucial for survival and functioning at the cellular level.
Table of Contents
Interesting Science Videos
The Role of Chemiosmosis in Cellular Energy Production
Chemiosmosis is a fundamental process involved in cellular energy production, specifically in adenosine triphosphate (ATP) formation. ATP is the primary energy currency in cells, providing the necessary energy for cellular activities.
At the same time, chemiosmosis involves the movement of protons across a membrane, generating a proton gradient that powers ATP synthesis through ATP synthase.
It plays a critical role in both cellular respiration and photosynthesis, key processes that sustain life.
Components and Mechanisms of Chemiosmosis
Electron transport chain (etc).
The electron transport chain (ETC) is a series of protein complexes and molecules embedded in the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). During cellular respiration or photosynthesis, ETC facilitates the movement of electrons through these complexes, powering the creation of a proton gradient.
Proton Gradient Creation Across a Membrane
As electrons move through the ETC, protons (H⁺ ions) are pumped from the mitochondrial matrix (or the stroma in chloroplasts) to the intermembrane space (or thylakoid space in chloroplasts).
It creates a concentration gradient of protons, establishing a potential energy difference across the membrane.
Overview of ATP Synthase and its Function in Chemiosmosis
ATP synthase is an enzyme complex located in the inner mitochondrial membrane (or thylakoid membrane in chloroplasts). It serves as a molecular turbine powered by the proton gradient. Protons flow back into the matrix (or stroma) through ATP synthase.
Its energy is harnessed to convert ADP (adenosine diphosphate) and inorganic phosphate (Pi) into ATP (adenosine triphosphate), the primary energy currency of the cell.
Proton Gradient Formation
Electron transfer and proton pumping in the etc.
Electron transfer involves a series of redox reactions where electrons are passed from one complex to another in the ETC. This transfer is coupled with the pumping of protons across the membrane, contributing to the formation of the proton gradient.
Accumulation of Protons in a Compartment or Intermembrane Space
Protons, being positively charged, accumulate in the intermembrane space (or thylakoid space) due to the active pumping by protein complexes in the ETC. This accumulation creates a high proton concentration in this compartment.
Establishment of an Electrochemical Gradient
The accumulation of protons results in both a chemical gradient (difference in proton concentration) and an electrical gradient (due to the positive charge of protons). Together, these gradients constitute the electrochemical gradient essential for ATP synthesis.
ATP Synthesis by ATP Synthase
ATP synthase comprises two main components: the rotor (F0 subunit) embedded in the membrane and the catalytic knob (F1 subunit) facing the mitochondrial matrix (or stroma). The rotor spins as protons flow through it, activating the catalytic sites.
Role of ATP Synthase in ATP Production
The spinning rotor of ATP synthase causes conformational changes in the catalytic knob, facilitating the synthesis of ATP from ADP and inorganic phosphate (Pi) as protons move through ATP synthase.
Coupling of Proton Movement and ATP Synthesis
The flow of protons through ATP synthase provides the energy needed to drive the formation of ATP. Protons moving from high concentration (intermembrane space) to low concentration (matrix or stroma) force the rotor to spin, ultimately leading to ATP production in a process directly linked to the proton gradient.
Significance of Chemiosmosis
Importance in cellular respiration and photosynthesis.
Chemiosmosis is a fundamental process in both cellular respiration and photosynthesis. It enables the production of adenosine triphosphate (ATP), the energy currency of cells. During cellular respiration, it occurs in the inner mitochondrial membrane, while in photosynthesis, it happens in the thylakoid membrane of chloroplasts.
Linkage to Metabolic Processes and Energy Storage
Chemiosmosis is intrinsically linked to various metabolic processes within cells. It plays a crucial role in energy conversion and storage. The proton gradient generated through chemiosmosis drives the synthesis of ATP, facilitating energy storage for cellular activities and maintaining metabolic balance.
Contribution to Maintaining Cellular Homeostasis
Chemiosmosis contributes significantly to cellular homeostasis by regulating the balance of ions and pH levels across cellular membranes. This is vital for maintaining optimal conditions within cells, ensuring proper enzymatic activity, and supporting various cellular functions.
Examples and Applications of Chemiosmosis
Connection to aerobic and anaerobic respiration.
In aerobic respiration, chemiosmosis occurs during the electron transport chain in the mitochondria, facilitating the synthesis of ATP. In anaerobic respiration, while the process varies slightly, the underlying principle of chemiosmosis remains crucial for ATP production.
Relevance in Photosynthetic Electron Transport
In photosynthesis, chemiosmosis is a pivotal part of the light-dependent reactions that take place in the thylakoid membrane of chloroplasts. It allows for the conversion of light energy into chemical energy (ATP) through the movement of protons and ATP synthase.
Application in Drug Discovery and Bioenergetics Research
Understanding chemiosmosis is essential in drug discovery, particularly for targeting ATP synthesis in diseases related to mitochondrial dysfunction. Additionally, it is a vital area of study in bioenergetics research, providing insights into cellular energy production and potential avenues for therapeutic interventions.
Chemiosmosis serves as a linchpin in cellular respiration and photosynthesis, fueling the production of ATP crucial for energy storage and metabolism.
Its role in maintaining cellular homeostasis and its diverse applications, from drug discovery to bioenergetics research, underscore its profound significance in the intricate machinery of life processes.
Understanding chemiosmosis not only enriches our comprehension of fundamental biological mechanisms but also holds promise for innovative therapeutic and scientific advancements.
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/07%3A_Cellular_Respiration/7.12%3A_Oxidative_Phosphorylation_-_Chemiosmosis_and_Oxidative_Phosphorylation
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/chemiosmosis
- https://alevelbiology.co.uk/notes/chemiosmosis/
- https://bioprinciples.biosci.gatech.edu/05-respiration-chemiosmosis-and-oxidative-phosphorylation-2/
- https://www.wikidoc.org/index.php/Chemiosmosis
- https://www.jove.com/science-education/10743/atp-synthase-and-chemiosmosis
- https://www.mytutor.co.uk/answers/14800/A-Level/Biology/How-does-chemiosmosis-work/
About Author
Krisha Karki
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- To save this word, you'll need to log in. Log In
chemiosmotic
Definition of chemiosmotic
Word history.
chemi- + osmotic
1957, in the meaning defined above
Dictionary Entries Near chemiosmotic
chemiphotic
Cite this Entry
“Chemiosmotic.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/chemiosmotic. Accessed 8 Jun. 2024.
Medical Definition
Medical definition of chemiosmotic.
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
What's the difference between 'fascism' and 'socialism', more commonly misspelled words, commonly misspelled words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, popular in wordplay, the words of the week - june 7, 8 words for lesser-known musical instruments, 9 superb owl words, 10 words for lesser-known games and sports, etymologies for every day of the week, games & quizzes.

Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.
Is Chemiosmosis a hypothesis or a theory?
I was trying to find out whether chemiosmosis is a hypothesis or a theory. Naturally, I first searched on Wikipedia, but the article on chemiosmosis uses both the words, which is confusing.
The chemiosmotic theory Peter D. Mitchell proposed the chemiosmotic hypothesis in 1961. The theory suggests essentially...
...the weight of evidence began to favor the chemiosmotic hypothesis , and in 1978 Peter Mitchell was awarded...
In chemiosmotic theory transmembrane ATP synthases are...
Then I found several papers; some of them use the word hypothesis like this one , this one and several others, but few others use the word theory like this one , and this one .
To my knowledge, a hypothesis is an educated guess based on certain data that acts as a foundation for further investigation while a theory explains a natural phenomenon that is validated through observation and experimentation but I am not aware of any experiments conducted by scientists to test this?
In summary:
Is Chemiosmosis a hypothesis or a theory, and what experiment has been performed to test it?
- biochemistry
- mitochondria
- bioenergetics
- chloroplasts
- 1 $\begingroup$ Edited your question so that now it's not obvious that it's actually two ;-) $\endgroup$ – David Feb 10, 2023 at 17:44
- 2 $\begingroup$ I have now edited the Wikipedia page, changing theory to hypothesis. We'll see how long it lasts before someone tries to revert it, and what the eventual outcome may be. In part I did this to demonstrate that Wikipedia entries can be edited by anyone (unless they are politically controversial etc.) and that they are a communal effort with no single formal editor. You cannot assume that they are correct, only that they are correctable. $\endgroup$ – David Feb 11, 2023 at 11:05
Although there is a case for closing this question (it is two questions, the first of which is more linguistics, logic or philosophy of science, and the second well-covered in text books) I have chosen to answer it, mainly because neither of the terms hypothesis or theory describe the current status of the proposal (the neutral description I shall employ initially). I have restructured my original answer putting the science first, and providing more information on this.
Mitchell’s Proposal
As the title of his original 1961 Nature paper makes clear (“Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism”) Mitchell was addressing the following question: How is the free energy released from the electron transport chain — involved in the oxidation of NADH and FADH 2 by oxygen — used to phosphorylate ADP to ATP.
At that time it was assumed that this was by a mechanism in which the energy was used to generate a chemical compound, X~P, containing a phosphate bond with a high free energy of hydrolysis, which could be used to phosphorylate ADP to ATP. (This was influenced by the ‘substrate-level’ phosphorylation occurring in glycolysis.) In brief, Mitchell proposed that the phosphorylation did not involve any compound, X~P, but was catalysed by an ATP synthase enzyme powered by an electrical gradient, established across the membrane in which the electron transport chain resided.
Mitchell proposed a possible mechanism for oxidative phosphorylation in terms of conventional physics and chemistry. Just as the postulated X~P, had not been identified, neither had the components of his proposed chemi-osmotic mechanism. The paper focused on its quantitative feasibility (making it challenging reading for many biological scientists), although it did also argue that the idea was consistent with a series of experimental observations that were difficult to explain in terms of substrate-level phosphorylation.
It was a hypothesis
Regardless of the interpretation of dictionary definitions, considered below, it was a hypothesis because that was the word Mitchell used in the original paper, cited above, a paper in which the word theory does not occur.
As well as misquoting history, it would seem presumptuous to use different terms to refer to the proposal of the original author, a proposal which resulted in his award of the Nobel Prize.
It is a fact
The mechanism of oxidative phosphorylation has now been explored in great detail and the general features of Mitchell’s hypothesis have been generally validated. It can be considered as much a fact as any other in our general conception of cellular biochemistry.
The famous experiment that resulted in widespread acceptance of the hypothesis was the so-called acid-bath experiment of Uribe and Jagendorf . In this experiment ATP was generated in the dark and without electron transport by loading the inner space of the grana disk membranes of spinach chloroplasts with protons.
More recently the structures of all the proteins involved in the chemiosmotic mechanism have now been determined, including that of the ATP synthase , the molecular machine which uses the electrochemical gradient to phosphorylate ADP to ATP. and the components of the electron transport chain .
Dictionaries
Merriam–Webster, on-line , is quite dogmatic about the difference between the words hypothesis and theory :
A hypothesis is an assumption made before any research has been done. It is formed so that it can be tested to see if it might be true. A theory is a principle formed to explain the things already shown in data. Because of the rigors of experiment and control, it is much more likely that a theory will be true than a hypothesis.
But is this dogmatism justified, and how does it apply to the current problem? Mitchell’s proposal was made to be tested (as in a hypothesis) but it was intended to explain the observed phenomenon (as in a theory) of oxidative phosphorylation.
An extensive article in Wikipedia begins with a more modest statement:
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon.
And chemi-osmosis is a proposed explanation for the phenomenon of ATP synthesis in double-membrane systems; so by this token Mitchell’s choice of words seems justified.
Major dictionaries record how words are or were used in practice . In some contexts there are sharp distinctions in usage of particular words, where in others the same words are used interchangeably. In modern biological science, unlike philosophy, logic or the numerical sciences, laws, theories and hypotheses are generally of no great concern, and the writers quoted may merely feel that the word ‘theory’ (‘Einstein’s’ theory, ‘Darwin’s theory’) has more gravitas than ‘hypothesis’, a word they perhaps use to describe ideas about relatively humble scientific problems of their own.
…or they may just find it easier to spell.
- $\begingroup$ Understood, I don't think I possibly know how to merge my two questions now, but I'll sure keep that point in mind for my future questions. Again, thanks! $\endgroup$ – Aditya Kumar Panda Feb 9, 2023 at 14:35
- $\begingroup$ also reg the textbook i'll try to get my hands on one $\endgroup$ – Aditya Kumar Panda Feb 9, 2023 at 14:38
- $\begingroup$ Revised my answer. Principle the same, but more information on theory and evidence for it. $\endgroup$ – David Feb 10, 2023 at 17:41
You must log in to answer this question.
Not the answer you're looking for browse other questions tagged biochemistry mitochondria bioenergetics chloroplasts ., hot network questions.
- What should I get paid for if I can't work due to circumstances outside of my control?
- How is this function's assembly implementing the conditional?
- Can campaign promises be enforced by a contract, or has it ever happened they were?
- Integrating slower growing functions to find a faster growing function
- Is it legal to deposit a check that says pay to the order of cash
- Effects if a human was shot by a femtosecond laser
- What terminal did David connect to his IMSAI 8080?
- Find the limit of an integral using Darboux/Riemann sums
- Which ability checks are rolled for a shove attack?
- What legal reason, if any, does my bank have to know if I am a dual citizen of the US?
- How to make sub "array" to aligned with the outer "array" in equation in LaTeX?
- Do you have an expression saying "when you are very hungry, bread is also delicious for you" or similar one?
- How often does systemd journal collect/read logs from sources
- What’s the history behind Rogue’s ability to touch others directly without harmful effects in the comics?
- Fiber product of schemes and tensor calculations
- Can I paraphrase an conference paper I wrote in my dissertation?
- Who are the mathematicians interested in the history of mathematics?
- Quick release inside of thru axle?
- Why is the magnitude of the cross product equal to the parallelogram spanned by the two vectors?
- Does Japanese advertises selling something with full price?
- Grigio meaning, in Shiras's Children of the Atom
- Reducing the gap in the Lemma environment with enumerate
- Accumulated charge in conductors
- Which program is used in this shot of the movie "The Wrong Woman"
ResearchTweet
Chemiosmosis: definition, mechanism, and function.
- Post published: August 28, 2021
- Reading time: 11 mins read
Table of Contents
Chemiosmosis definition.
Chemiosmosis is a biological process that involves transporting ions (such as protons) to the other side of a membrane, resulting in an electrochemical gradient that may be utilised to drive ATP production.
What is Chemiosmosis?
Chemiosmosis is the process of diffusion of ions (usually H + ions, also known as protons) across a selectively permeable membrane and thus proton gradient developed. In many cells, proton gradient provides the energy for the synthesis of ATP.
With the aid of the proteins buried in the membrane, the gradient also encourages the ions to return passively. By passively, we mean that the ions will migrate from a high-concentration location to a low-concentration area. Water molecules flow passively in this mechanism, comparable to osmosis.
Chemiosmosis, on the other hand, includes the movement of ions across the membrane, whereas osmosis involves the movement of water molecules. Both processes, however, need a gradient. This is known as an osmotic gradient in osmosis. Osmosis is caused by pressure variations between the two sides of the membrane.
In chemiosmosis, an electrochemical gradient, such as a proton gradient, drives the flow of ions. Chemiosmosis is not just comparable to osmosis. It’s also comparable to assisted diffusion and other kinds of passive transfer. It works on the same basis. Ions travel downward.
Membrane proteins also assist in the transport of molecules to the other side of the membrane. Membrane proteins aid ion movement across the membrane, which is not easily permeable to ions due to its bilipid structure. These proteins in the membrane function as a temporary shuttle, a conduit, or a tunnel, allowing them to move around more easily.
Membrane proteins are used in chemiosmosis to transport particular ions. Furthermore, unlike an active transport system, it does not require chemical energy (e.g., ATP). The development of an ion gradient in chemiosmosis results in the generation of potential energy, which is sufficient to drive the process.
Chemiosmosis can happen anywhere. It happens in eukaryotes during cellular respiration and photosynthesis in the mitochondria and chloroplasts. Chemiosmosis will occur in the cell membrane of prokaryotes since they lack these organelles.
Chemiosmotic Theory
Chemiosmosis is driven by an electrochemical proton gradient that is required for the synthesis of ATP, according to the chemiosmotic theory. Peter D. Mitchell (1920–1992), a British scientist, presented this idea. Mitchell’s idea, on the other hand, was not immediately accepted until a solid foundation for proton pumping was built. With the discovery of ATP synthase and the pH difference across the thylakoid, the bioenergetics field began to question his hypothesis’ validity.
He was aware of the phenomenon of membrane potential in the 1960s, in which the inner side of the membrane was negatively charged in relation to its surroundings. At the time, ATP was also recognised as the cell’s primary energy currency. However, the actual mechanism through which living creatures create ATP is unknown. The organelles responsible for ATP production have long been recognised as mitochondria.
However, it was unclear how these organelles produced ATP. It was previously thought to be related to phosphorylation at the substrate level (as what happens in glycolysis). Mitchell suggested that chemiosmosis might potentially create ATP.
He demonstrated that ATP production is linked to a proton gradient electrochemically. This laid the groundwork for understanding how oxidative phosphorylation leads to ATP production.
Chemiosmosis Model
Chemiosmosis is an ATP-producing energy-coupling process used by living organisms. It is one of the most important stages in cellular respiration in respiring cells.
Because mitochondria produces the majority of ATPs, it is known as the cell’s powerhouse. It is dedicated to the production of ATP. The organelle is a double-membraned structure, so keep that in mind. An outer membrane and an inner membrane make up the mitochondrial membrane. Both layers are made up of lipid layers that prevent ions from passing through them easily.
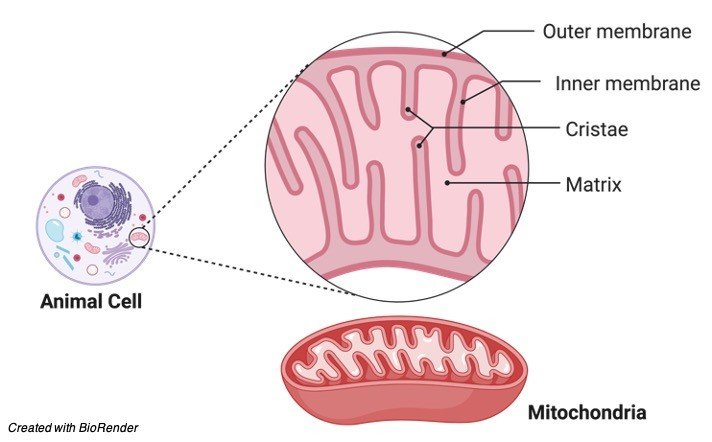
The intermembrane gap is the space between the two membranes. Cristae are many infoldings in the inner membrane. The mitochondrial matrix is the region within the inner membrane. The citric acid cycle, a cyclic metabolic mechanism in which food molecules are churned to create energy-rich phosphate compounds, takes place in the matrix.
The pyruvate produced by glycolysis is transformed into acetyl CoA, which is then transported to the mitochondrion where it is completely oxidised and degraded into carbon dioxide. The citric acid cycle generates one ATP for every pyruvate molecule via substrate phosphorylation.
The electron transport chain (ETC) and the enzyme ATP synthase are integrated into the mitochondrial membrane, so oxidative phosphorylation will provide the majority of the ATP.
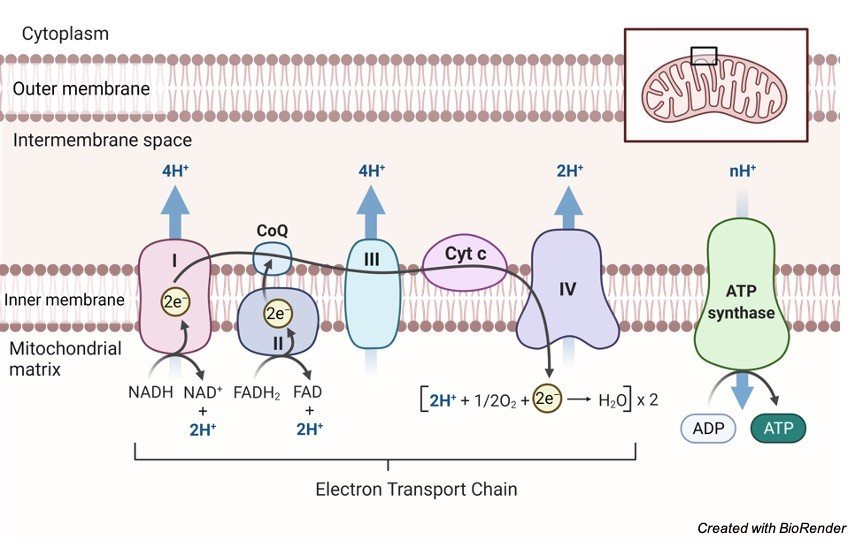
Most of the high-energy electrons are transported to NAD + and FAD through redox processes, resulting in NADH (and H + ) and FADH2, respectively. The electrons will be shuttled to the ETC for oxidative phosphorylation by these electron-carrying molecules. Every ETC member performs a redox reaction as the electrons move along the chain, receiving and giving electrons.
When electrons are transferred to the last electron acceptor, molecular oxygen, the electron flow will come to a halt. Water is formed as a result of the reaction: 2 H + + 12 O 2 H 2 O. ATP is not produced by ETC. Instead, as electrons pass through, ETC members push H+ (protons) into the intermembrane gap.
Protons accumulate on one side of the membrane as they are pushed across it. A proton (H+) gradient is created as a result of this. The proton-motive force was the name given to it by scientists. The energy created by the transport of protons (or electrons) through an energy-transducing membrane is defined by them.
Through the ATP synthase channel, protons will flow down to their gradient, i.e., from the intermembrane space to the matrix. When protons release energy as they traverse the ATP synthase, the hydrogen ion migration leads to ATP production. The energy causes the enzyme’s rotor and rod to revolve. The enzyme is then triggered to use this force to create an ATP molecule by forming a high-energy bond between the ADP molecule and the inorganic phosphate (Pi). ADP + Pi ATP is the reaction.

Chemiosmosis Fucntion
It’s all about energy coupling in chemiosmosis. The production of a proton motive force is the link between chemiosmosis and ATP synthesis. As previously stated, chemiosmosis is the process that drives ATP production via oxidative phosphorylation in cellular respiration.
The electrons from the citric acid cycle (which breaks down pyruvate-turned-acetyl coenzyme A into carbon dioxide) are transferred to electron carriers and transported to the ETC. The proton motive force generated by protons accumulating on one side of the membrane during energy transfer in the ETC via a series of redox reactions will be utilised to synthesise ATP from ADP and inorganic phosphate.
As a result, there will be no proton motive force for ATP synthase to employ during ATP synthesis if chemiosmosis is absent. As a result, fewer ATP end products will be produced without the need for chemiosmosis. In photosynthesis, where chemiosmosis is also a critical stage in ATP generation, a similar effect can be predicted.
Chemiosmosis in Chloroplast
Chemiosmosis occurs in the mitochondria of eukaryotes, as previously stated. Apart from the mitochondria, photosynthetic eukaryotes like plants have another organelle in which chemiosmosis occurs: the chloroplast.
The chloroplast is the organelle that plays the most important role in photosynthesis. It possesses a light-harvesting thylakoid system. As a result, it acts as the site of light responses (or light-dependent processes). The stroma is the stroma of the chloroplast matrix. The thick fluid comprises enzymes, chemicals, and other substrates that are engaged in dark reactions (or light-independent processes).
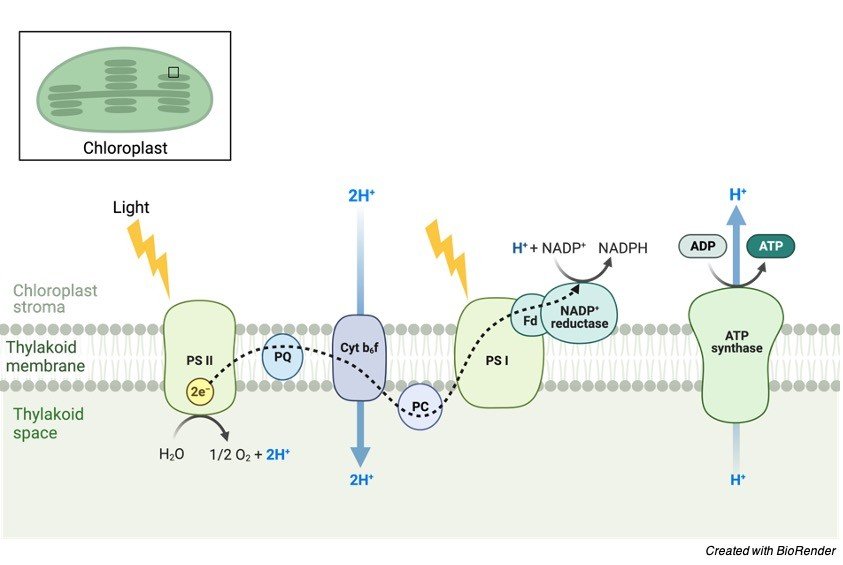
Chemiosmosis occurs in the thylakoid of chloroplasts. This membrane system contains its own ATP synthase and transport chain. The source of energy is one of the key distinctions between chemiosmosis in mitochondria and chloroplasts. The high-energy electrons in mitochondria are taken from the food molecules (through a redox reaction), whereas the source in the chloroplast is photons collected from the light source.
The proton (H+) gradient is formed by the accumulation of H+ ions in the thylakoid compartment (i.e., the space inside the thylakoid). The H+ ions can come from three sources:
(1) water splitting during light processes,
(2) protons translocated across the thylakoid membrane when electrons travel through the transport chain, and
(3) stromal H+ ions taken up by NADP+.
Because the quantity of H+ ions inside the thylakoid compartment (lumen) is higher, they will diffuse into the stroma via passing through the ATP synthases implanted in the thylakoid membrane.
Chemiosmosis in Prokaryotic Cells
Chemiosmosis occurs in the cell membrane of prokaryotes such as bacteria and archaea, which lack mitochondria and chloroplasts. When a proton gradient occurs on the other side of the membrane, hydrogen ions (protons) travel across the cellular membrane via ATP synthase (a transport protein).
During electron transport and redox processes, hydrogen ions accumulate as they are forcibly pushed to the other side, forming a proton gradient. As additional hydrogen ions accumulate on the other side of the membrane, they will return to the cell via passing through the ATP synthase. Energy is released when they pass through, and it is used to convert ADP to ATP via phosphorylation.
Chemiosmosis vs Oxidative Phosphorylation
Chemiosmosis is the method through which oxidative phosphorylation generates ATP directly. ATP synthase, on the other hand, will be unable to do so without the proton motive force generated by the ETC, which pushes protons (H+) to the other side of the membrane as electrons travel through the chain.
The metabolic process of oxidative phosphorylation creates ATP from the energy generated by a series of redox events in the ETC. As a result, electron transport-linked phosphorylation is another name for it. Because molecular oxygen is the ultimate electron acceptor, it is an aerobic process. This distinguishes it from the other type of phosphorylation, namely substrate-level phosphorylation, in which ATP is produced directly from an intermediary substrate. In contrast, oxidative phosphorylation is an indirect method of ATP synthesis. It is combined with chemiosmosis, which involves the movement of protons through the membrane.

Chordata: Definition, Characteristic, and Examples
Chemiosmosis citations.
- Quantum Mechanics predicts evolutionary biology. Prog Biophys Mol Biol . 2018 Jul;135:11-15.
- Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab . 2012 Sep;23(9):451-8.
- What is the Role of Lipid Membrane-embedded Quinones in Mitochondria and Chloroplasts? Chemiosmotic Q-cycle versus Murburn Reaction Perspective. Cell Biochem Biophys . 2021 Mar;79(1):3-10.
Similar Post:

Top 10 Free Resume Builders of 2024
Microscope, Microscope Parts, Labeled Diagram, and Functions

26 Postdoctoral Fellowships at New York University, New York, United States

22 Postdoctoral Fellowships at Princeton University, New Jersey, United States

34 Postdoctoral Fellowships at Imperial College London, England

31 Postdoctoral Fellowships at Stanford University, California, United States

28 Postdoctoral Fellowships at King’s College London, England

15 Postdoctoral Fellowships at Northwestern University, Illinois, United States

35 Postdoctoral Fellowships at University of Cambridge, England

23 Postdoctoral Fellowships at Moffitt Cancer Center, Florida, United States

31 Postdoctoral Fellowships at University of Edinburgh, Scotland

13 Postdoctoral Fellowships at Queen’s University Belfast, Northern Ireland
You might also like.
Homozygous : Definition, Examples, and Traits

Thymine: Structure, Definition, and Functions
Cell Lysis: an Overview, Definition, Types, and Importance

Hypotonic Solution : Definition and Examples

Fully Funded PhD in Neuroscience at University of North Carolina-Chapel Hill

Starch Hydrolysis Test: Result, Principle, Procedure, and Reagents
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

Get 3X More Success with Our Ready-to-Use Academic CV Templates!
Our CV Templates Land You in Harvard, MIT, Oxford, and Beyond!

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.5.2: Chemiosmosis and Oxidative Phosphorylation
- Last updated
- Save as PDF
- Page ID 75142

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Describe how the energy obtained from the electron transport chain powers chemiosmosis and discuss the role of hydrogen ions in the synthesis of ATP
During chemiosmosis, electron carriers like NADH and FADH donate electrons to the electron transport chain. The electrons cause conformation changes in the shapes of the proteins to pump H+ across a selectively permeable cell membrane. The uneven distribution of H + ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient) owing to the hydrogen ions’ positive charge and their aggregation on one side of the membrane.
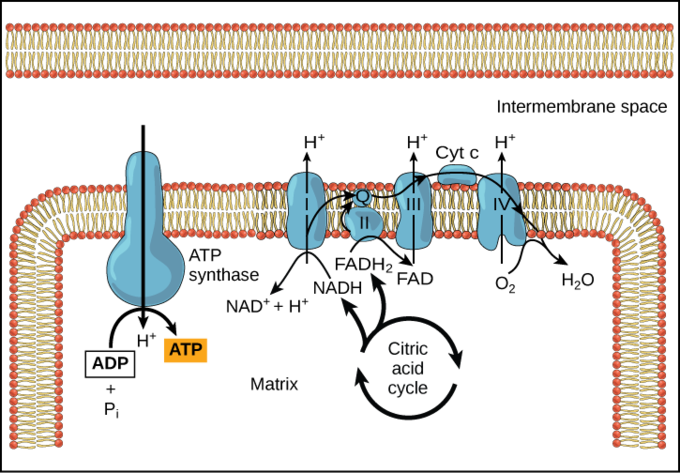
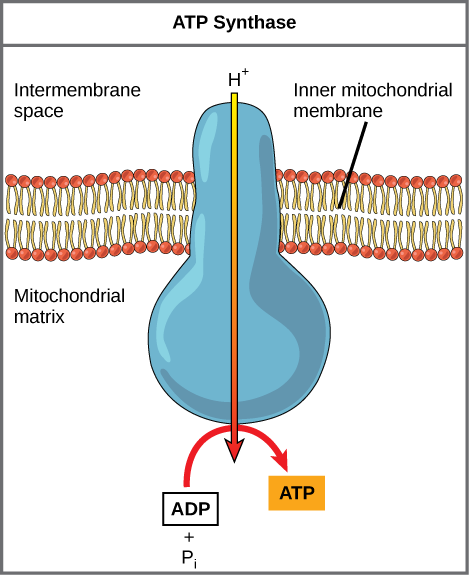
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to spontaneously diffuse back across into the matrix, driven by their electrochemical gradient. However, many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through a membrane protein called ATP synthase. This protein acts as a tiny generator turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of this molecular machine harnesses the potential energy stored in the hydrogen ion gradient to add a phosphate to ADP, forming ATP.
Chemiosmosis is used to generate 90 percent of the ATP made during aerobic glucose catabolism. The production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. It is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium and water is formed.
- During chemiosmosis, the free energy from the series of reactions that make up the electron transport chain is used to pump hydrogen ions across the membrane, establishing an electrochemical gradient.
- Hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through a membrane protein called ATP synthase.
- As protons move through ATP synthase, ADP is turned into ATP.
- The production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation.
- ATP synthase : An important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate (ATP).
- oxidative phosphorylation : A metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate (ATP).
- chemiosmosis : The movement of ions across a selectively permeable membrane, down their electrochemical gradient.

Chemiosmotic hypothesis
July 4, 2023 Dr. Asha Jyoti Biochemistry 0
| Know in one minute about the Chemiosmotic hypothesis and FADH ). O splits into proton electrons and oxygen. |
Introduction
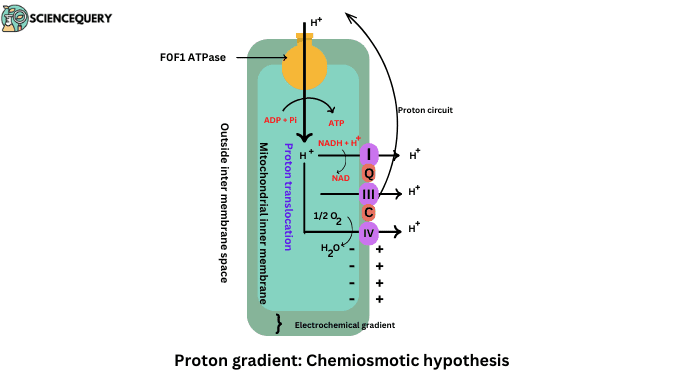
- The chemiosmotic hypothesis explains the mechanism of ATP Synthesis.
- It was given by Peter Mitchell in 1961.
- According to this hypothesis, the energy released from electrochemical potential is by the pumping of protons across the inner mitochondrial membrane.
- The energy in this electrochemical potential or proton motive force can be converted into ATP.
- Proton motive force is generated by the pumping of Protons.
- The energy released during electron transport is stored in the form of an electrochemical gradient of protons across the membrane.
- Proton means H + ions and gradient means difference. So on the two different sites of a membrane, there will be some differences in protons.
- At one site the proton concentration will be much higher. In other sites, the H + ion concentration will be much lower which will develop a gradient or difference.
- For maintaining equilibrium H + ions will be moved from one site to another site and they will generate ATP from ADP & Pi.
- Respiratory Complex I, Complex II, and Complex IV worked as a proton pump and generated electrochemical gradients.
- Protons are pumped out of the inner mitochondrial membrane to the matrix through the FoF1 complex. FoF1 complex is also known as ATP synthase enzyme.

ATP synthase is located in the inner mitochondrial membrane. It consists of two parts Fo and F1.
Fo particle:- it is embedded in the membrane. It involves the movement of the H + proton from intermembrane space to matrix.
F1 Particles:- It is a head-like part that is projected from the surface of the membrane to a matrix.
It involves in synthesis of ATP by the combination of ADP + Pi → ATP
Chemiosmotic Synthesis of ATP
- According to the chemiosmotic hypothesis, the proton gradient or proton motive force is responsible for ATP Synthesis.
How to proton motive force generate
In excess amounts, Protons accumulate in the lumen of thylakoid and the inner membrane of mitochondria.
When H + ion concentration Will be higher they move towards lower concentration through the ATP synthase enzyme.
Accumulation of Protons
In chloroplast.
During photosynthesis, the H + ion accumulates in the lumen.
In Mitochondria
During cellular respiration, the proton accumulates in the inner membrane of the mitochondria and moves toward the matrix.
The inner mitochondrial membrane is impermeable to protons so their pumping results in the generation of the proton gradient.
ATP Synthesis in Chloroplast
ATP Synthesis reaction occurs in the thylakoid membrane of Chloroplast.
Protons accumulate in the lumen of thylakoid that’s why the proton gradient is established. Protons moved from the lumen to the membrane.
Factors that are involved in Proton transportation
- Plastoquinone
- FNR (ferredoxin NADP reductase),
- Photolysis of water or splitting of water molecules
Plastoquinone (PQ) : it is an electron transporter. Remove proton from stroma while electron transport.
PQ → PQH 2
FNR: FNR brings protons from the stroma to the lumen of the thylakoid. This enzyme is responsible for the conversion of NAD to NADPH.
Photolysis of Water: During light reaction, H 2 O splits in oxygen, electron, and proton in Photosystem II. These hydrogen-positive ions transport to the lumen of the thylakoid
2 H 2 O → 4H+ 4e + O 2
When proton Concentration is higher in the lumen of thylakoid they move to the membrane of the thylakoid membrane through ATP synthase enzyme. Where they bind with ADP and Pi and produce ATP.
The movement of Protons from higher concentration to lower concentration generates the Proton motive force which is responsible for the rotation of F1 particles.
What does the chemiosmotic hypothesis claim
The chemiosmotic hypothesis claims that the proton gradient is responsible for ATP Synthesis.
Experimental Proof of the chemiosmotic hypothesis
- It was given by A. Jangendory and B. Uribe in 1966. The process of ATP Synthesis takes place in the inner mitochondrial membrane and thylakoid membrane of Chloroplast.
- Thylakoid vesicles were isolated by these two scientists. Thylakoid membrane-bound with FoF1 particles. ATP synthase consists of these two particles Fo and F1.
- Thylakoid vesicles have these two particles. Firstly put these vesicles in a buffer solution (pH-4) until the same pH inside of the Vesicle. The pH of the buffer solution is four.
- When vesicles have 4 pH, put the vesicles in another Solution that has 8 pH, and ADP and inorganic Phosphate (Pi) is also present. Then ATP Synthesis will start.
- pH difference means variation in H+ ion concentration across the membrane that variation is responsible for the ATP Synthesis.
- So this experiment proved that the difference in proton gradient is responsible For ATP Synthesis.
- The process of synthesizing ATP from ADP and Pi coupled with ETC is Known as oxidative phosphorylation
Chemiosmotic model
- Chemiosmotic models explain the ATP Synthesis in the thylakoid of Chloroplast.
- These are the following steps of the chemiosmotic model which explained ATP Synthesis.
- Site of Proton accumulation:- Proton accumulates in the lumen of the thylakoid.
Proton accumulation
Through photolysis of water:- H 2 O split into proton electrons and oxygen. These protons are stored in the lumen of thylakoids. Proton concentration will be higher in the lumen.
Movement of the proton:- Proton moved from high concentration to lower concentration. In the lumen, pH is lower, and on the other side high pH approx. 7. This pH variation established the proton gradient or proton motive force.
ATP Synthesis:- Proton actively moved from ATP synthase. Movement of protons responsible for the rotation of the FoF1 complex. Their ADP and Pi convert into ATP.
Chemiosmosis Vs Oxidative Phosphorylation
| Definition Synthesizing of ATP from ADP & Pi coupled with ETC is known as Oxidative Phosphorylation. | ATP molecules produced from proton gradients are known as chemiosmotic hypotheses. | |
| ATP Synthesis occurs in the inner mitochondrial membrane. | ATP Synthesis occurs in the matrix of mitochondria. | |
| Energy is derived from the Oxidation of hydrogen acceptors like NADH2 and FADH2. They are formed in the Krebs cycle from the Oxidation of glucose | Energy derived from proton gradient for ATP Synthesis. | |
| Electron transporters are involved in the binding of O to NADH or FADH . | Electron transporters are not involved. Only plastoquinone involved | |
|
| Proton motive force not applied. Osmosis reaction has not occurred. | Proton motive force generated in ATP synthase enzyme. Osmosis reaction is found. Protons moved from high concentrations to lower concentrations. |
Factors for the chemiosmotic generation of ATP
ATP synthase enzyme: ATP synthase enzyme activity is most important for ATP Synthesis.
Proton gradient: Proton gradient most important factor of ATP Synthesis.
Plastoquinone: Plastoquinone is an electron transporter. Plastoquinone transports protons into lumen.
Ferredoxin NADP reductase: It is responsible for the conversion of NAD to NADPH and for releasing protons into the lumen. It helps in proton accumulation.
Proton Concentration: Proton Concentration is the most important factor. High proton Concentration generates proton gradients and protons move from high concentration to lower concentration.
What does the chemiosmotic hypothesis explain?
Chemiosmotic explains the mechanism of ATP Synthesis through the proton gradient.
What is the chemiosmotic theory of ATP Synthesis?
For the synthesis of ATP, the energy is released from the proton gradient by the pumping of protons across the inner mitochondrial membrane or thylakoid membrane. This process is called the chemiosmotic theory of ATP Synthesis.
What are the 3 steps of chemiosmosis?
These are the three steps of chemiosmosis:-
1. Accumulation of proton
2. Movement of proton and proton gradient generates
3. ATP synthesized
Karp’s Cell and Molecular Biology: Concepts and Experiments, 8th Edition
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
Essentials of Biochemistry (for Medical Students). By Pankaja Naik, Shivananda Nayak B.
Biochemistry 4th edition, U. Satyanarayana & U. Chakrapani.
Written By: Richa Pachori
Copyright © 2024 | WordPress Theme by MH Themes
- School Guide
- Class 11 Syllabus
- Class 11 Revision Notes
- Maths Notes Class 11
- Physics Notes Class 11
- Chemistry Notes Class 11
- Biology Notes Class 11
- NCERT Solutions Class 11 Maths
- RD Sharma Solutions Class 11
- Math Formulas Class 11
- CBSE Class 11 Biology Notes
Chapter 1: The Living World
- Diversity In The Living World
- Binomial Nomenclature - Definition, Rules, Classification and Examples
- Taxonomic Hierarchy In Biological Classification
- Genus and Family
- Difference Between Phylum and Class
- Taxonomical Aids
- Botanical Gardens
- The Living World - Introduction, Classification, Characteristics, FAQs
Chapter 2: Biological Classification
- Biological Classification
- Kingdom Monera - Definition, Classification, Characteristics, Examples
- Archaebacteria
- Eubacteria - Structure, Characteristics, Classification, and Types
- Kingdom Protista
- Chrysophytes
- Dinoflagellates
- Slime Moulds
- Protozoans - Structure, Classification, Characteristics, Examples
- Kingdom Fungi
- Phycomycetes
- Ascomycetes - Introduction, Characteristics, Reproduction, Importance
- Basidiomycetes
- Deuteromycetes
- Kingdom Plantae - Class 11 Biology
- Kingdom Animalia - Definition, Classification, Characteristics
Chapter 3: Plant Kingdom
- What is Plant Kingdom?
- Algae - Definition, Characteristics, Types and Examples
- Chlorophyceae
- Phaeophyceae - Overview, Characteristics, Importance, Examples
- Rhodophyceae
- Bryophytes | Class 11 Biology
- Pteridophytes
- Gymnosperms - Definition, Characteristics, Uses and Examples
- Angiosperms
- Difference between Angiosperms and Gymnosperms
Chapter 4: Animal Kingdom
- Animal Kingdom
- Classification of Animal Kingdom
- Levels of Organization in Animals
- Symmetry in Animals - Definition, Types and Importance
- Diploblastic And Triploblastic Organization
- Classification of Animals
- Phylum Porifera
- Phylum Coelenterata | Class 11 Biology
- Phylum Ctenophora
- Platyhelminthes
- Phylum Aschelminthes
- Phylum Annelida
- Phylum Arthropoda
- Phylum Mollusca
- Phylum Echinodermata
- Phylum Hemichordata
- Phylum Chordata
Chapter 5: Morphology of Flowering Plants
- Morphology of Flowering Plants
- Root System in Plants - Types and Functions of Root
- Stem - Characteristics and Functions
- Inflorescence
- Morphology of Flower - Definition, Structure, Parts, Examples
- Parts of a Flower and their Functions
- Androecium - Definition, Components, Structure, Functions
- Gynoecium - Definition, Concept, Parts, Functions
- What is a Fruit?
- Structure Of A Dicotyledonous Seed
- Structure Of Monocotyledonous Seed
- Semi Technical Description of a Flowering Plant - Class 11 Biology
- Fabaceae - Overview, Characteristics, Classification, Importance
- Solanaceae - Characteristics, Importance, Examples
Chapter 6: Anatomy of Flowering Plants
- Meristematic Tissues | Class 11 Biology
- Permanent Tissues
- Why are Xylem and Phloem called Complex Tissues?
- Epidermal Tissue System: Its Functions and Tissue in Plant
- Difference between Dicot and Monocot Root
- Monocot and Dicot Stems - Definition, Structure, Characteristics, Examples
- Describe the Internal Structure of a Dorsiventral Leaf
- Isobilateral (Monocotyledonous) Leaf - Definition, Features, Structure, Examples
- Secondary Growth
- Cork Cambium
Chapter 7: Structural Organization In Animals
- NCERT Notes of Class 11 Biology Chapter 7 Structural Organisation in Animals
- Structural Organization in Animals
- Epithelial Tissue - Introduction, Characteristics, Types, Importance
- Connective Tissue - Definition, Functions, Types, Examples
- Organ System
- Morphology of Earthworm
- Earthworm Anatomy
- Morphology of Cockroach
- Anatomy of Cockroach
- Morphology and Anatomy of Frogs
Chapter 8: Cell-The Unit of Life
- Cell the Unit of Life Class 11 Notes CBSE Biology Chapter 8
- Prokaryotic Cells
- Cell Envelope - Definition, Classification, Types, Functions
- Ribosomes and Inclusion Bodies
- Eukaryotic Cells
- Cell Membrane
- Endomembrane System - Overview, Structure, and Functions
- Mitochondria
- Golgi Apparatus
- Plastids - Definition, Classification, Structure, Functions
- Cytoskeleton - Definition, Structure, Components, Functions
- Cilia And Flagella - Definition, Structure, Functions and FAQs
- What is Nucleus? | Class 11 Biology
Chapter 9: Biomolecules
- Biomolecules - Definition, Structure, Classification, Examples
- How To Analyze Chemical Composition?
- What are Metabolites - Primary and Secondary Metabolites
- Biomacromolecules - Definition, Types, Functions, Significance
- Proteins - Definition, Structure, Significance, Examples
- Polysaccharides
- Nucleic Acid - Definition, Function, Structure, and Types
- Protein Structure - Primary, Secondary, Tertiary, Quaternary
- Metabolic Basis For Living | CBSE Class 11 Biology Chapter 9
- Enzymes - Definition, Structure, Classification, Examples
- How Do Enzymes Bring About Such High Rates Of Chemical Conversions?
- Nature of Enzyme Action
- Mechanism of Enzymes Action
- Factors Affecting Enzyme Activity
Chapter 10: Cell Cycle and Cell Division
- Cell Division: Mitosis & Meiosis, Different Phases of Cell Cycle
- Mitosis - Overview, Phases, & Significance Class Notes
- Mitosis Cell Division: Definition, Stages and Diagram
- Meiosis - Definition, Stages, Function and Purpose
Chapter 11: Photosynthesis in Higher Plants
- Where Does Photosynthesis Take Place?
- Photosynthetic Pigments
- What Is Light Dependent Reaction?
- Electron Transport System (ETS) And Oxidative Phosphorylation
- Cyclic and Non-cyclic Photo-phosphorylation
Chemiosmotic Hypothesis
- Where are the Atp and Nadph Used?
- Calvin Cycle Notes Class 11
- C4 Cycle of Photosynthesis
- Photorespiration - Definition, Diagram, Process, Significance
- Factors Affecting Photosynthesis
Chapter 12: Respiration in Plants
- Respiration In Plants Class 11 Notes
- Do Plants Breathe?
- What is Glycolysis ?
- Fermentation: Meaning, Process, Types and Importance
- Aerobic Respiration
- Tricarboxylic Acid Cycle - Overview, Stages, Roles, Significance
- Amphibolic Pathway
- Respiratory Quotient
Chapter 13: Plant-Growth and Development
- Plant Growth - Definition, Types, Factors Affecting, Examples
- Phases of Growth In Plants - Growth Rates
- Differentiation, Dedifferentiation and Redifferentiation in Plant Growth
- Plant Growth and Development
- Physiological Effects Of Plant Growth Regulators
- Vernalization
- Seed Dormancy
Chapter 14: Breathing and Exchange of Gases
- NCERT Notes Class 11 Biology Chapter 14 - Breathing and Exchange of Gases
- Human Respiratory System
- Inspiration and Expiration
- Lung Volumes And Capacities
- Oxygen Transport in Blood
- Transport of Carbon Dioxide in the Blood
- Regulation Of Respiration
- Respiratory System Disorders - Definition, Causes, Types, Symptoms
Chapter 15: Body Fluids and Circulation
- Body Fluids and Circulation
- Plasma And Formed Elements
- Blood Groups - ABO Blood Group and Rh Group System
- Blood Coagulation
- Lymphatic System
- Circulatory Pathways
- Cardiac Cycle | Class 11 Biology
- Electrocardiogram (ECG)
- Double Circulation
- Regulation of Cardiac Activity
- Disorders of the Circulatory System
Chapter 16: Excretory Products and their Elimination
- Excretory Products and their Elimination
- Human Excretory System
- Mechanism of Urine Formation
- Functions of Renal Tubules
- Mechanism Of Concentration Of The Filtrate
- Micturition - The process of Urination
- Role of Other Organs In Excretion
- Disorders Of The Excretory System
Chapter 17: Locomotion and Movement
- NCERT Notes for Class 11 Biology Chapter 17: Locomotion and Movement
- Locomotion And Movement
- Muscular Tissue
- Muscular and Skeletal Disorders
Chapter 18: Neural Control and Coordination
- Class 11 Biology NCERT Notes Chapter 18 - Neural Control and Coordination
- Neural Tissue
- Generation And Conduction Of Nerve Impulse - NCERT Notes
- Transmission of Nerve Impulses
- Central Nervous System
- Reflex Action
- Anatomy and Physiology of Human Eye
- Anatomy and Physiology of Human Ear
Chapter 19: Chemical Coordination and Integration
- NCERT Notes for Class 11 Biology Chapter 19: Chemical Coordination and Integration
- Endocrine System
- Hypothalamus - Function, Hormones and Disorder
- Pituitary Gland
- Pineal Gland - Function, Structure, Location, and Disorders
- Thyroid Gland - Anatomy, Function and Clinical Aspects
- Parathyroid Gland - Functions and Disorders
- What is Thymus Gland?
- Adrenal Gland
- Testes - Anatomy and Functions
- Ovary - Female Reproductive System
- Hormones of Heart, Kidney And Gastrointestinal
NCERT Solution
- NCERT Solutions Class 11- Biology
- NCERT Solutions Class 11 Biology Chapter 1 Living World
- NCERT Solutions for Class 11 Biology Chapter 2 - Biological Classification
- NCERT Solutions for Class 11 Biology Chapter 3 - Plant Kingdom
- NCERT Solutions Class 11 Biology Chapter 4 Animal Kingdom
- NCERT Solutions for Class 11 Biology Chapter 5: Morphology of Flowering Plants
- NCERT Solutions for Class 11 Biology Chapter 7 Structural Organisation in Animals
- NCERT Solutions for Class 11 Biology Chapter 8 Cell The Unit of Life
- NCERT Solutions for class 11 Biology Chapter 9 - Biomolecules
- NCERT Solutions Class 11 Biology Chapter 10 Cell Cycle and Cell Division
- NCERT Solutions Chapter 11 of Class 11 Biology - Photosynthesis in Higher Plants
- NCERT Solutions for Class 11 Biology Chapter 12 Respiration in Plants
- NCERT Solutions for Class 11 Biology Chapter 13 - Plant Growth and Development
- NCERT Solutions of Class 11 Chapter 14 Breathing and Exchange of Gases
- NCERT Solutions Class 11 Biology Chapter 15 Body Fluids and Circulation
- NCERT Solutions for Class 11 Biology Chapter 16 Excretory Products and Their Elimination
- NCERT Solutions for Class 11 Biology Chapter 17 Locomotion and Movement
- NCERT Solutions for Class 11 Biology Chapter 18 Neural Control and Coordination
- NCERT Solutions for Class 11 Biology Chapter 19 - Chemical Coordination and Integration
The chemiosmotic hypothesis explains how ATP is synthesized in mitochondria through the movement of protons across the inner mitochondrial membrane, driving ATP synthase to produce ATP. The chemiosmotic hypothesis is given by Peter Mitchell. In this article, we will cover the chemiosmotic hypothesis, its process, and its functions.
Table of Content
What is Photosynthesis?
What is chemiosmosis.
- what is the function of chemiosmotic hypothesis
Chemiosmotic Hypothesis Process
Conclusion – chemiosmotic hypothesis.
- FAQs on Chemiosmosis Hypothesis
The process through which a plant transforms light energy into chemical energy to produce food is known as photosynthesis . In the presence of chlorophyll, plants use water, carbon dioxide, and sunlight to make food or energy in the form of sugar, and as a by-product they release oxygen. This suggests that light energy is used as a catalyst in chemical synthesis or reaction.
Certain bacteria and prokaryotes also use this to prepare their food; it is not just for green plants. The chloroplast is an important cell organelle in green plants and algae. It contains the pigment chlorophyll and facilitates synthesis. The leaves, flowers, stems, sepals , and plastids all contain chlorophyll.
Chemiosmosis refers to the process by which ions move over a semi-permeable membrane , such as the membrane within mitochondria . Molecules containing a net electric charge are called ions. Examples include the specialized usage of Na + , Cl – , and H + in chemiosmosis to generate energy.
During chemiosmosis, ions move along an electrochemical gradient or a gradient of electrochemical potential (a form of potential energy). Chemiosmosis is a type of diffusion that causes ions to move from areas of high concentration to areas of low concentration across a membrane. Ions also move to balance the electric charge across a membrane.

Chemiosmotic Hypothesis Diagram
The biological process by which ATP synthase produces ATP molecules is known chemiosmotic hypothesis . An explanation of how energy molecules (ATP: Adenosine triphosphate) are produced during photosynthesis is provided by the Chemiosmotic theory, which was put forth by a British biochemist, Peter Dennis Mitchell in 1961.
The chemiosmotic hypothesis is a theory that explains how cells produce energy. It suggests that energy is generated through the movement of ions across a membrane, creating an electrochemical gradient. This gradient powers the production of ATP, the cell’s energy currency. In simpler terms, it’s like a battery powering the cell’s activities by moving ions across a membrane. This process is important for cellular functions including metabolism and cell signaling.
Nicotinamide adenine dinucleotide phosphate, often known as NADP or NADP + , is created with ATP during the light reaction or photochemical phase. These constitute the essential components of photosynthesis. They are used to produce sugar molecules throughout the dark reaction or Calvin cycle .
The proton gradient that exists across the thylakoid membrane is what causes the ATP- Adenosine Triphosphates to be created in this process. The proton gradient, ATP synthase, and proton pump are important elements required for the chemiosmosis process. ATP synthase is the name of the enzyme that is necessary for the synthesis of ATP molecules. Two subunits designated F0 and F1, make up the enzyme ATP synthase. To move protons across the membrane, the F0 subunit is necessary. This alters the F1 subunit’s conformation, which activates enzymes . By adding a phosphate group to ADP, the enzyme phosphorylates it, turning it into ATP. Across the membrane, there is a proton gradient, which acts as ATP synthase’s main propulsion source.
Chlorophyll absorbs light with the aid of photosystems during the light response stage of photosynthesis. As a result, the water molecules split, releasing protons and electrons in the process. This is known as hydrolysis. The electron transport system carries the liberated electrons as they become energized and proceed to a higher energy level.
In the meantime, the stroma’s released protons start assembling inside the membrane. As a result, a proton gradient is produced, which is a by-product of the electron transport chain. Photosystem I use the few remaining protons to convert NADP+ to NADPH using electrons from the photolysis of water. The proton gradient eventually collapses, releasing energy and protons that are then transported back to the stroma by ATP synthase F0. ADP is converted to ATP by the ATP synthase when the F1 conformation is altered by the resulting energy.
What is the Function of Chemiosmotic Hypothesis?
Some of the functions of chemiosmotic hypothesis are:
- The chemiosmotic hypothesis explains how cells produce energy.
- It describes the process of ATP synthesis through the movement of ions across a membrane.
- This hypothesis is essential for understanding cellular respiration and photosynthesis.
- It provides a framework for studying the role of ion gradients in various cellular processes.
- Understanding the chemiosmotic hypothesis helps in developing treatments for diseases related to energy metabolism
In conclusion, photosynthesis is a fundamental process by which plants convert light energy into chemical energy, using chlorophyll and various molecules to produce sugars and release oxygen. Chemiosmosis, as part of cellular processes, elucidates how ions move across membranes to generate energy, crucial for ATP synthesis. The Chemiosmotic Hypothesis, proposed by Peter Dennis Mitchell, outlines this mechanism, highlighting its significance in cellular metabolism and signaling. Understanding this process not only advances our comprehension of photosynthesis and respiration but also offers insights into developing treatments for energy-related diseases. Through the proton gradient, ATP synthase, and electron transport chain, chemiosmosis efficiently synthesizes ATP, powering cellular activities essential for life.
Also Read: The Pressure Flow or Mass Flow Hypothesis Photosynthesis Cyclic and Non-cyclic Photo-phosphorylation Where are the ATP and NADPH Used? ATP Synthesis in Mitochondria
FAQs on Chemiosmotic Hypothesis
Why is it called chemiosmotic theory.
It’s called Chemiosmotic Theory because it explains energy production via ion movement across membranes.
What are the 4 Tenets of Chemiosmotic Theory?
The four tenets include ion movement generating a gradient, ATP synthesis driven by gradient dissipation, coupling of ATP synthesis to electron transport, and ATP synthase as the catalyst.
What is the Chemiosmotic Theory of ATP Synthesis?
The Chemiosmotic Theory of ATP Synthesis proposes that ATP is produced as ions move across a membrane, dissipating an electrochemical gradient.
What are the Steps of Chemiosmosis?
Steps of Chemiosmosis involve ion movement across membranes, creating a gradient, which powers ATP synthesis via ATP synthase, and the subsequent release of ATP for cellular use.
What Ion Undergoes Chemiosmosis as Part of the Production of ATP?
The process by which protons (H+) move down a proton gradient during cellular respiration is known as chemiosmosis. Adenosine diphosphate (ADP) and a phosphate group are joined by the enzyme ATP synthase as a result, creating ATP.
What Molecule is Produced by Chemiosmosis, and Who Proposed the Chemiosmotic Hypothesis?
ATP synthase’s role in the creation of ATP molecules naturally. The Chemiosmotic theory describes how energy molecules (ATP: adenosine triphosphate) are produced during photosynthesis and was put out by a British biochemist by the name of Peter Dennis Mitchell in 1961. He received the Nobel Prize for his contribution to the discovery of this idea.
What Cell Organelle is Capable of Chemiosmosis?
During cellular respiration in the mitochondria and photosynthesis in the chloroplasts, chemiosmosis takes place. These two procedures both produce ATP.
Please Login to comment...
Similar reads.
- Biology-Class-11
- Plant-Physiology
- School Biology
- School Learning
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

IMAGES
VIDEO
COMMENTS
The chemiosmotic hypothesis. Peter D. Mitchell proposed the chemiosmotic hypothesis in 1961. In brief, the hypothesis was that most adenosine triphosphate (ATP) synthesis in respiring cells comes from the electrochemical gradient across the inner membranes of mitochondria by using the energy of NADH and FADH 2 formed during the oxidative breakdown of energy-rich molecules such as glucose.
Chemiosmotic Hypothesis. It is the biological process of producing ATP molecules through the action of ATP synthase. In 1961, British biochemist by the name of Peter Dennis Mitchell theorized the Chemiosmotic hypothesis, which explains how the energy molecules (ATP: Adenosine triphosphate) are created during photosynthesis.
Chemiosmotic hypothesis. Definition. noun. A theory postulated by the biochemist Peter Mitchell in 1961 to describe ATP synthesis by way of a proton electrochemical coupling. Accordingly, hydrogen ion s ( proton s) are pumped from the mitochondrial matrix to the intermembrane space via the hydrogen carrier protein s while the electrons are ...
Oxidative phosphorylation is a vital cellular respiration process that generates ATP. It involves the oxidation of NADH and FADH2 and phosphorylation. The process creates a hydrogen gradient, enabling chemiosmosis and ATP synthesis. This energy conversion is essential for all life forms, from bacteria to sharks.
According to the chemiosmotic theory, chemiosmosis is driven by an electrochemical proton gradient essential during the production of ATP. This theory was proposed by Peter D. Mitchell (1920 - 1992), a British biochemist. (Ref. 1) Mitchell's hypothesis, however, was not accepted instantly until a substantive groundwork on proton pumping was ...
Chemiosmosis is when ions move by diffusion across a semi-permeable membrane, such as the membrane inside mitochondria. Ions are molecules with a net electric charge, such as Na +, Cl -, or specifically in chemiosmosis that generates energy, H +. During chemiosmosis, ions move down an electrochemical gradient, which is a gradient of ...
The chemiosmotic theory explains the functioning of electron transport chains. According to this theory, the tranfer of electrons down an electron transport system through a series of oxidation-reduction reactions releases energy. This energy allows certain carriers in the chain to transport hydrogen ions (H + or protons) across a membrane.
Figure 4.10.1 4.10. 1: Chemiosmosis demo. This is a direct evidence that a gradient of protons can be harnessed to the synthesis of ATP. Several kinds of evidence support the chemiosmotic theory of ATP synthesis in chloroplasts. When isolated chloroplasts are illuminated, the medium in which they are suspended becomes alkaline — ….
Chemiosmotic theory. Definition. noun. A theory postulated by the biochemist Peter Mitchell in 1961 to describe ATP synthesis by way of a proton electrochemical coupling. Accordingly, hydrogen ions ( proton s) are pumped from the mitochondrial matrix to the intermembrane space via the hydrogen carrier protein s while the electrons are ...
Chemiosmosis is the process by which hydrogen ions (protons) diffuse to the other side of the biological membrane from high to low concentration. It creates a difference in their concentration (electrochemical gradient) between the two sides of the semi-permeable membrane. The gradient energy synthesizes adenosine triphosphate ( ATP) in the cell.
Here are 4 tips that should help you perfect your pronunciation of 'chemiosmotic hypothesis':. Break 'chemiosmotic hypothesis' down into sounds: say it out loud and exaggerate the sounds until you can consistently produce them.; Record yourself saying 'chemiosmotic hypothesis' in full sentences, then watch yourself and listen.You'll be able to mark your mistakes quite easily.
Figure 7.12.1 7.12. 1: Chemiosmosis: In oxidative phosphorylation, the hydrogen ion gradient formed by the electron transport chain is used by ATP synthase to form ATP. If the membrane were open to diffusion by the hydrogen ions, the ions would tend to spontaneously diffuse back across into the matrix, driven by their electrochemical gradient.
Listen to the audio pronunciation of Chemiosmotic hypothesis on pronouncekiwi How To Pronounce Chemiosmotic hypothesis: Chemiosmotic hypothesis pronunciation Unlock premium audio pronunciations.
Chemiosmosis is a fundamental process involved in cellular energy production, specifically in adenosine triphosphate (ATP) formation. ATP is the primary energy currency in cells, providing the necessary energy for cellular activities. At the same time, chemiosmosis involves the movement of protons across a membrane, generating a proton gradient ...
The chemiosmotic hypothesis was first proposed by Peter D. Mitchell in 1961. 13 The basic assumption of the chemiosmotic theory is derived from the important observation that the IMM is generally impermeable to ions, but it keeps the permeability of H +. The composition of the OMM is similar to those of the sarcolemma (SL) and ER/SR in ...
chemiosmotic: [adjective] relating to or being a theory that seeks to explain the mechanism of ATP formation in oxidative phosphorylation by mitochondria and chloroplasts without recourse to the formation of high-energy intermediates by postulating the formation of an energy gradient of hydrogen ions across the organelle membranes that results ...
I was trying to find out whether chemiosmosis is a hypothesis or a theory. Naturally, I first searched on Wikipedia, but the article on chemiosmosis uses both the words, which is confusing.. The chemiosmotic theory. Peter D. Mitchell proposed the chemiosmotic hypothesis in 1961. The theory suggests essentially.....the weight of evidence began to favor the chemiosmotic hypothesis, and in 1978 ...
Chemiosmosis is the process of diffusion of ions (usually H + ions, also known as protons) across a selectively permeable membrane and thus proton gradient developed. In many cells, proton gradient provides the energy for the synthesis of ATP. With the aid of the proteins buried in the membrane, the gradient also encourages the ions to return ...
Figure 7.5.2.1 7.5.2. 1: Chemiosmosis: In oxidative phosphorylation, the hydrogen ion gradient formed by the electron transport chain is used by ATP synthase to form ATP. If the membrane were open to diffusion by the hydrogen ions, the ions would tend to spontaneously diffuse back across into the matrix, driven by their electrochemical gradient.
Chemiosmotic hypothesis: Definition: Definition Synthesizing of ATP from ADP & Pi coupled with ETC is known as Oxidative Phosphorylation. ATP molecules produced from proton gradients are known as chemiosmotic hypotheses. Location in cell: ATP Synthesis occurs in the inner mitochondrial membrane.
The chemiosmotic hypothesis is a theory that explains how cells produce energy. It suggests that energy is generated through the movement of ions across a membrane, creating an electrochemical gradient. This gradient powers the production of ATP, the cell's energy currency. In simpler terms, it's like a battery powering the cell's ...
The chemiosmotic hypothesis is a biological mechanism proposed in 1961 by a British biochemist named Peter Dennis Mitchell.. It is the mechanism by which ATP molecules are synthesised by the activity of ATP synthase.; It is a process that describes how ATP molecules or energy molecules are formed as a result of the process of photosynthesis.; The Nobel Prize in Chemistry was granted to the ...
Before the widespread acceptance of the chemiosmotic hypothesis, many biochemists searched for the 'high-energy intermediate' coupling electron transfer to ATP synthesis. The fascinating histories of Dr Mitchell, the Glynn Research Institute and the chemiosmotic hypothesis have been reported previously [18-20].