- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
About The BMJ
- Resources for authors
- Forms, policies, and ethics
- Authorship & contributorship
The International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals ( ICMJE Recommendations 2018 ) recommend that authorship be based on the following four criteria:
• Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND • Drafting the work or revising it critically for important intellectual content; AND • Final approval of the version to be published; AND • Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
In addition to being accountable for the parts of the work he or she has done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors.
We include only one corresponding author per article. Any further contribution details (eg, equal contribution) must be included in the contributors or acknowledgement sections at the end of the article.
The BMJ requires that all those designated as authors should meet all four ICMJE criteria for authorship, and all who meet the four criteria should be identified as authors. We recognise only natural persons over 18 years of age as authors. These authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work. The criteria are not intended for use as a means to disqualify colleagues from authorship who otherwise meet authorship criteria by denying them the opportunity to meet criterion #s 2 or 3. Therefore, all individuals who meet the first criterion should have the opportunity to participate in the review, drafting, and final approval of the manuscript.
The individuals who conduct the work are responsible for identifying who meets these criteria and ideally should do so when planning the work, making modifications as appropriate as the work progresses. The corresponding author takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and typically ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements, are properly completed, although these duties may be delegated to one or more coauthors.
When a large multi-author group has conducted the work, the group ideally should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship, including approval of the final manuscript, and they should be able to take public responsibility for the work and should have full confidence in the accuracy and integrity of the work of other group authors. They will also be expected as individuals to complete conflict-of-interest disclosure forms.
The byline of the article identifies who is directly responsible for the manuscript, and Medline lists as authors whichever names appear on the byline. If the byline includes a group name, Medline will list the names of individual group members who are authors or who are collaborators, sometimes called non-author contributors, if there is a note associated with the byline clearly stating that the individual names are elsewhere in the paper and whether those names are authors or collaborators.
At The BMJ we want authors to assure us that all authors included on a paper fulfil the criteria of authorship. In addition we want assurance that there is no one else who fulfils the criteria but has not been included as an author.
When we encounter disagreements among authors we follow guidance from the Committee on Publication Ethics (COPE)—see here and here .
AI technologies will not be accepted as an author(s) of any content submitted to BMJ for publication. BMJ only recognises humans as being capable of authorship since they must be accountable for the work.
Contributorship
The BMJ lists contributors in two ways. Firstly, we publish a list of authors' names at the beginning of the paper and, secondly, we list contributors (some of whom may not be included as authors) at the end of the paper, giving details of who did what in planning, conducting, and reporting the work. This is a good place to include contributions by patients or members of the public who have assisted as research volunteers, giving their names and specific roles. We encourage authors to fully acknowledge the contribution of patients and the public to their research where appropriate.
One or more of these contributors are listed as guarantors of the paper. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. See Maintaining the integrity of the scientific record.
Contributorship and guarantorship are concepts that were applied first to original research papers, and are sometimes hard to define for other articles. Each contributorship statement should make clear who has contributed what to the planning, conduct, and reporting of the work described in the article, and should identify one, or occasionally more, contributor(s) as being responsible for the overall content as guarantor(s). For articles in The BMJ that do not report original research - such as editorials, clinical reviews, and education and debate - please state who had the idea for the article, who performed the literature search, who wrote the article, and who is the guarantor (the contributor who accepts full responsibility for the finished article, had access to any data, and controlled the decision to publish). For non-research articles that include case reports such as lessons of the week, drug points, and interactive case reports, please also state who identified and/or managed the case(s).
Researchers must determine among themselves the precise nature of each person's contribution, and we encourage open discussion among all participants. See Authorship is dying; long live contributorship.
Alteration to authorship or contributorship
Any change in authors and/or contributors after initial submission must be approved by all authors. This applies to additions, deletions, change of order to the authors, or contributions being attributed differently. Any alterations must be explained to the editor. The editor may contact any of the authors and/or contributors to ascertain whether they have agreed to any alteration.
Group authorship
If there is a very large number of authors we may ask for confirmation that everyone listed met the ICMJE criteria for authorship. If they did, we may then require that the authors form a group whose name will appear in the article byline.
We appreciate that authors may be concerned that their work will not be properly recognised if they form a group, but this is unfounded. Medline guidance can handle group authorship and still give each individual due credit:
"When a group name for a specific consortium, committee, study group, or the like appears in an article byline, the personal names of the members of that group may be published in the article text. Such names are entered as collaborator names for the Medline citation."
Key points:
• A Medline citation may contain an array of personal author names, group (or corporate) author names, and collaborator names. • Personal author names are included in Medline when the author names appear in the article byline, or are explicitly identified anywhere else in the text of the article as the authors or as the members of the writing group or writing committee for the article. • Group author names (also known as corporate, organization or collective names) are included in Medline when such names appear in the article byline. • When a group name for a specific consortium, committee, study group, or the like appears in an article byline, the personal names of the members of that group may be published in the article text. Such names are entered as collaborator names for the Medline citation. • Collaborator names are entered for a Medline citation only when a group (corporate) author name is present for the citation. • More than one group name may appear for a citation, and a group name may appear along with personal author names. • For articles that represent a formal guideline or practice guideline, the name of the guideline-issuing body is entered as a group name for the Medline citation, even if that name does not appear in the article byline.
What this means for The BMJ 's authors
a) if authors form a group for the article's main byline they will also be listed individually:
• As collaborators in the article's Medline/PubMed record; • As authors in a group authorship statement at the end of the article on thebmj.com; and • As contributors in the contributorship statement at the end of the article on thebmj.com.
b) however, for The BMJ 's research articles with many authors, where those authors do not opt to form a group, we will not be able to publish a BMJ pico in the print issue of The BMJ . Such research articles will be for online only (thebmj.com) publication only.
Here's a research article in The BMJ with group authorship as it appeared on Medline, with all collaborators clearly listed as individuals:
http://www.ncbi.nlm.nih.gov/pubmed/20123835
And here's how the individual authors for that article were listed on thebmj.com:
1. What appeared at the top of the article and was dowloadable to citation manager:
Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries. Wei-Hong Zhang, Catherine Deneux-Tharaux, Peter Brocklehurst, Edmund Juszczak, Matthew Joslin, Sophie Alexander, on behalf of the EUPHRATES Group. BMJ 2010;340:c293, doi: 10.1136/bmj.c293 (Published 1 February 2010)
2. What appeared at the end of the article in an authorship statement:
The following are members of EUPHRATES (EUropean Project on obstetric Haemorrhage, Reduction, Attitudes, Trial and Early warning System): Sophie Alexander (project leader, Belgium), Diogo Ayres-de-Campos (Portugal), Istvan Berbik (Hungary), Marie-Hélène Bouvier-Colle (France), Gérard Bréart (France), Peter Brocklehurst (UK), Vicenç Cararach (Spain), Anna Maria Marconi (Italy), Catherine Deneux-Tharaux (France), Risto Erkkola (Finland), Mathias Klein (Austria), Jens Langhoff-Roos (Denmark), Alison Macfarlane (UK), Walter Prendiville (Republic of Ireland), Jos van Roosmalen (Netherlands), Babill Stray-Pedersen (Norway), Carolyn Troeger (Switzerland), Clare Winter (UK), and Wei-Hong Zhang (Belgium). Also see web extra for a list of people who helped in each country.
3. What appeared at the end of the article in the contributorship statement:
Contributors: W-HZ designed data collection tools, monitored data collection for the whole trial, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. She is guarantor. CD-T implemented the trial in France, analysed the data, and drafted and revised the paper. PB analysed the data and drafted and revised the paper. EJ wrote the statistical analysis plan, monitored data collection for the whole trial, and revised the draft paper. MJ designed data collection tools,, monitored data collection for the whole trial, and revised the draft paper. SA initiated the collaborative project, designed data collection tools, implemented the trial for the all countries, monitored data collection for the whole trial, analysed the data, and drafted and revised the paper. All members of EUPHRATES designed the trial. Diogo Ayres-de-Campos, Istvan Berbik, Marie-Hélène Bouvier-Colle, Vicenç Cararach, Risto Erkkola, Mathias Klein, Walter Prendiville, Jos van Roosmalen, Babill Stray-Pedersen, and Carolyn Troeger implemented the trial in, respectively, Portugal, Hungary, France, Spain, Finland, Austria, Republic of Ireland, Netherlands, Norway, and Switzerland, and revised the draft paper. Gérard Bréart analysed the data and revised the draft paper. Alison Macfarlane and Clare Winter revised the draft paper.
- Publishing model
- Editorial staff
- Advisory panels
- Explore The BMJ
- BMJ Student
- How green is The BMJ?
- Sources of revenue
- Article types and preparation
- Article submission
- Competing interest policy
- Copyright, open access, and permission to reuse
- Patient consent and confidentiality
- Research Ethics
- BMJ papers audit
- Guidance for new authors
- BMJ Christmas issue
- Resources for advertisers and sponsors
- Resources for BMA members
- Resources for media
- Resources for subscribers
- Resources for readers
- Resources for reviewers
- About The BMJ app
- Poll archive
- International jobs
This week's poll
Read related article
See previous polls
Defining the Role of Authors and Contributors
Page Contents
- Why Authorship Matters
- Who Is an Author?
- Non-Author Contributors
- Artificial Intelligence (AI)-Assisted Technology
1. Why Authorship Matters
Authorship confers credit and has important academic, social, and financial implications. Authorship also implies responsibility and accountability for published work. The following recommendations are intended to ensure that contributors who have made substantive intellectual contributions to a paper are given credit as authors, but also that contributors credited as authors understand their role in taking responsibility and being accountable for what is published.
Editors should be aware of the practice of excluding local researchers from low-income and middle-income countries (LMICs) from authorship when data are from LMICs. Inclusion of local authors adds to fairness, context, and implications of the research. Lack of inclusion of local investigators as authors should prompt questioning and may lead to rejection.
Because authorship does not communicate what contributions qualified an individual to be an author, some journals now request and publish information about the contributions of each person named as having participated in a submitted study, at least for original research. Editors are strongly encouraged to develop and implement a contributorship policy. Such policies remove much of the ambiguity surrounding contributions, but leave unresolved the question of the quantity and quality of contribution that qualify an individual for authorship. The ICMJE has thus developed criteria for authorship that can be used by all journals, including those that distinguish authors from other contributors.
2. Who Is an Author?
The ICMJE recommends that authorship be based on the following 4 criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or reviewing it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
In addition to being accountable for the parts of the work done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors.
All those designated as authors should meet all four criteria for authorship, and all who meet the four criteria should be identified as authors. Those who do not meet all four criteria should be acknowledged—see Section II.A.3 below. These authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work. The criteria are not intended for use as a means to disqualify colleagues from authorship who otherwise meet authorship criteria by denying them the opportunity to meet criterion #s 2 or 3. Therefore, all individuals who meet the first criterion should have the opportunity to participate in the review, drafting, and final approval of the manuscript.
The individuals who conduct the work are responsible for identifying who meets these criteria and ideally should do so when planning the work, making modifications as appropriate as the work progresses. We encourage collaboration and co-authorship with colleagues in the locations where the research is conducted. It is the collective responsibility of the authors, not the journal to which the work is submitted, to determine that all people named as authors meet all four criteria; it is not the role of journal editors to determine who qualifies or does not qualify for authorship or to arbitrate authorship conflicts. If agreement cannot be reached about who qualifies for authorship, the institution(s) where the work was performed, not the journal editor, should be asked to investigate. The criteria used to determine the order in which authors are listed on the byline may vary, and are to be decided collectively by the author group and not by editors. If authors request removal or addition of an author after manuscript submission or publication, journal editors should seek an explanation and signed statement of agreement for the requested change from all listed authors and from the author to be removed or added.
The corresponding author is the one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer-review, and publication process. The corresponding author typically ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and disclosures of relationships and activities are properly completed and reported, although these duties may be delegated to one or more co-authors. The corresponding author should be available throughout the submission and peer-review process to respond to editorial queries in a timely way, and should be available after publication to respond to critiques of the work and cooperate with any requests from the journal for data or additional information should questions about the paper arise after publication. Although the corresponding author has primary responsibility for correspondence with the journal, the ICMJE recommends that editors send copies of all correspondence to all listed authors.
When a large multi-author group has conducted the work, the group ideally should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship, including approval of the final manuscript, and they should be able to take public responsibility for the work and should have full confidence in the accuracy and integrity of the work of other group authors. They will also be expected as individuals to complete disclosure forms.
Some large multi-author groups designate authorship by a group name, with or without the names of individuals. When submitting a manuscript authored by a group, the corresponding author should specify the group name if one exists, and clearly identify the group members who can take credit and responsibility for the work as authors. The byline of the article identifies who is directly responsible for the manuscript, and MEDLINE lists as authors whichever names appear on the byline. If the byline includes a group name, MEDLINE will list the names of individual group members who are authors or who are collaborators, sometimes called non-author contributors, if there is a note associated with the byline clearly stating that the individual names are elsewhere in the paper and whether those names are authors or collaborators.
3. Non-Author Contributors
Contributors who meet fewer than all 4 of the above criteria for authorship should not be listed as authors, but they should be acknowledged. Examples of activities that alone (without other contributions) do not qualify a contributor for authorship are acquisition of funding; general supervision of a research group or general administrative support; and writing assistance, technical editing, language editing, and proofreading. Those whose contributions do not justify authorship may be acknowledged individually or together as a group under a single heading (e.g. "Clinical Investigators" or "Participating Investigators"), and their contributions should be specified (e.g., "served as scientific advisors," "critically reviewed the study proposal," "collected data," "provided and cared for study patients," "participated in writing or technical editing of the manuscript").
Because acknowledgment may imply endorsement by acknowledged individuals of a study’s data and conclusions, editors are advised to require that the corresponding author obtain written permission to be acknowledged from all acknowledged individuals.
Use of AI for writing assistance should be reported in the acknowledgment section.
4. Artificial Intelligence (AI)-Assisted Technology
At submission, the journal should require authors to disclose whether they used artificial intelligence (AI)-assisted technologies (such as Large Language Models [LLMs], chatbots, or image creators) in the production of submitted work. Authors who use such technology should describe, in both the cover letter and the submitted work in the appropriate section if applicable, how they used it. For example, if AI was used for writing assistance, describe this in the acknowledgment section (see Section II.A.3). If AI was used for data collection, analysis, or figure generation, authors should describe this use in the methods (see Section IV.A.3.d). Chatbots (such as ChatGPT) should not be listed as authors because they cannot be responsible for the accuracy, integrity, and originality of the work, and these responsibilities are required for authorship (see Section II.A.1). Therefore, humans are responsible for any submitted material that included the use of AI-assisted technologies. Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased. Authors should not list AI and AI-assisted technologies as an author or co-author, nor cite AI as an author. Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI. Humans must ensure there is appropriate attribution of all quoted material, including full citations.
Next: Disclosure of Financial and Non-Financial Relationships and Activities, and Conflicts of Interest
Keep up-to-date Request to receive an E-mail when the Recommendations are updated.
Subscribe to Changes
- Research Process
- Manuscript Preparation
- Manuscript Review
- Publication Process
- Publication Recognition
- Language Editing Services
- Translation Services

What is a Corresponding Author?
- 3 minute read
- 425.2K views
Table of Contents
Are you familiar with the terms “corresponding author” and “first author,” but you don’t know what they really mean? This is a common doubt, especially at the beginning of a researcher’s career, but easy to explain: fundamentally, a corresponding author takes the lead in the manuscript submission for publication process, whereas the first author is actually the one who did the research and wrote the manuscript.
The order of the authors can be arranged in whatever order suits the research group best, but submissions must be made by the corresponding author. It can also be the case that you don’t belong in a research group, and you want to publish your own paper independently, so you will probably be the corresponding author and first author at the same time.
Corresponding author meaning:
The corresponding author is the one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process. Normally, he or she also ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements, are properly completed, although these duties may be delegated to one or more co-authors.
Generally, corresponding authors are senior researchers or group leaders with some – or a lot of experience – in the submission and publishing process of scientific research. They are someone who has not only contributed to the paper significantly but also has the ability to ensure that it goes through the publication process smoothly and successfully.
What is a corresponding author supposed to do?
A corresponding author is responsible for several critical aspects at each stage of a study’s dissemination – before and after publication.
If you are a corresponding author for the first time, take a look at these 6 simple tips that will help you succeed in this important task:
- Ensure that major deadlines are met
- Prepare a submission-ready manuscript
- Put together a submission package
- Get all author details correct
- Ensure ethical practices are followed
- Take the lead on open access
In short, the corresponding author is the one responsible for bringing research (and researchers) to the eyes of the public. To be successful, and because the researchers’ reputation is also at stake, corresponding authors always need to remember that a fine quality text is the first step to impress a team of peers or even a more refined audience. Elsevier’s team of language and translation professionals is always ready to perform text editing services that will provide the best possible material to go forward with a submission or/and a publication process confidently.
Who is the first author of a scientific paper?
The first author is usually the person who made the most significant intellectual contribution to the work. That includes designing the study, acquiring and analyzing data from experiments and writing the actual manuscript. As a first author, you will have to impress a vast group of players in the submission and publication processes. But, first of all, if you are in a research group, you will have to catch the corresponding author’s eye. The best way to give your work the attention it deserves, and the confidence you expect from your corresponding author, is to deliver a flawless manuscript, both in terms of scientific accuracy and grammar.
If you are not sure about the written quality of your manuscript, and you feel your career might depend on it, take full advantage of Elsevier’s professional text editing services. They can make a real difference in your work’s acceptance at each stage, before it comes out to the public.
Language Editing Services by Elsevier Author Services:
Through our Language Editing Services , we correct proofreading errors, and check for grammar and syntax to make sure your paper sounds natural and professional. We also make sure that editors and reviewers can understand the science behind your manuscript.
With more than a hundred years of experience in publishing, Elsevier is trusted by millions of authors around the world.
Check our video Elsevier Author Services – Language Editing to learn more about Author Services.
Find more about What is a corresponding author on Pinterest:

What is Journal Impact Factor?

What is a Good H-index?
You may also like.

How to Make a PowerPoint Presentation of Your Research Paper

How to Submit a Paper for Publication in a Journal
Input your search keywords and press Enter.

In this section:
- Advertising and sponsorship
- Authorship and contributorship
- Competing interests
- Copyright and authors’ rights
- Correction and retraction policies
- Data protection policy
- Data sharing
- Editor Roles & Responsibilities
- Gender identity and race & ethnicity data in ScholarOne
- Patient and public partnership
- Patient consent and confidentiality
- Peer Review Terms and Conditions
- Publication embargo
- Reproducing third party illustrative materials
- Research Ethics
- Rapid responses
- Scientific misconduct
- Tobacco Policy
- Trial registration
This policy ensures that contributors who have made substantive intellectual contributions to an article are given credit and that contributors understand their role in taking responsibility and being accountable for what is published. Contributors are either author contributors (meaning that they meet all four authorship criteria – see below) or non-author contributors.
BMJ credits and lists contributors in two ways:
- Authorship – we publish a list of authors’ names at the beginning of the paper in the byline
- Contributorship – we publish a contributorship statement at the end of the paper, giving details of who did what in planning, conducting, and reporting the work. This should include all author contributors and may include non-author contributors.
We also publish an acknowledgements statement at the end of the paper, detailing those who helped in carrying out the research but that have not been recognised as contributors, and for personal expressions of gratitude.
Submitting author
Corresponding author, joint first authorship, collaborators (group authorship), deceased authors, alteration to authorship, acknowledgements.
The International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals ( ICMJE Recommendations 2019 ) recommend that authorship be based on the following four criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
BMJ requires that all those designated as authors should meet all four ICMJE criteria for authorship, and all who meet the four criteria should be identified as author contributors. We recognise only natural persons (an individual human being, as opposed to a private or public organisation) as authors. These authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work. The criteria should not be used to disqualify colleagues from authorship who otherwise meet authorship criteria by denying them the opportunity to meet criterion number 2 or 3. Therefore, all individuals who meet the first criterion should have the opportunity to participate in the review, drafting and final approval of the manuscript.
Contributors who have contributed materially to the paper but whose contributions do not justify authorship should be described clearly in the contributorship statement .
In addition to being accountable for the parts of the work they have done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors.
Submitting authors should provide assurance that all authors included on a paper fulfil the criteria of authorship. We also ask for assurance that there is no one else who fulfils the criteria that has been excluded as an author.
When we encounter disagreements among authors we follow guidance from the Committee on Publication Ethics (COPE).
The submitting author takes primary responsibility for submitting the article to the journal using our manuscript submission system ScholarOne and for communicating with the journal during the article submission, peer review and revision process. They ensure that all of the journal’s administrative requirements are properly completed. These include, but are not limited to, providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements. These tasks may be delegated to one or more co-authors, but the submitting author remains responsible for them.
When you submit your article through our submission system you will be asked to provide a name, email address and institutional affiliation for all author contributors. In the final published article author names, institutions and addresses will be taken from these completed fields and not from the submitted Word document.
Affiliations listed should be those where the work was carried out at the time the research/article was written. If institution details appear incorrectly these can be directly amended under ‘Actions’ by selecting the ‘Edit’ drop down next to each author.
All author contributors receive a confirmation email when an article has been submitted and when a final decision is made.
The submitting author should assign the corresponding author when providing author details (see below for more information about the corresponding author role). The submitting author and corresponding author can be the same person.
The corresponding author, as listed on ScholarOne, takes primary responsibility for completing all necessary actions after acceptance of the manuscript and communicating with the journal and with readers after publication. All email communication from BMJ will be sent to the corresponding author including:
- The timeline for your article proof with a link to Publishing at Work where you can track your article’s status
- If your article will be published open access or in colour in the print edition of the journal, you will receive an email from Rightslink with payment options and instructions. If you are not making the payment yourself, you may forward the email to the person or organisation that will be paying on your behalf
- A link to review and approve the proof when available
- Confirmation that your article has been published online
- Notifications when a response has been posted to your article
Find out more about what to expect when your article has been accepted .
Although we include only one corresponding author on ScholarOne for email communication, multiple authors can be listed with correspondence information in the author byline of the final published article. This information can be included at the article proof stage, after acceptance.
Note, the policy for The BMJ differs and can be found here
Joint first authors can be indicated by the inclusion of the statement ‘X and X contributed equally to this paper’ in the contributorship statement.
Collaborators are a large group of multi-author contributors (e.g. a specific consortium, committee, study group or the like). Collaborators should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship as detailed above. They will also be expected as individuals to complete conflict-of-interest disclosure forms and provide a summary in the relevant section.
The collaborator group name(s) should be included in the main author list on ScholarOne. The collaborator group name(s) followed by the individual names should also be listed in the ‘Collaborator’ field on ScholarOne. BMJ will list the author group name(s) in the author byline, with the full list of individual names included in a collaborator statement at the end of the article. Details of the group’s contributions should also be listed in the ‘Contributorship statement’ field on ScholarOne.
If the journal is indexed in PubMed (MEDLINE and/or PubMed Central), the group name will be listed in the author byline and the names of individual group members entered as collaborators on the PubMed record to ensure individual due credit.
The BMJ Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries
PubMed record >>
BMJ Open Establishing a core outcome set for treatment of uncomplicated appendicitis in children: study protocol for an international Delphi survey
AI technologies will not be accepted as an author(s) of any content submitted to BMJ for publication. BMJ only recognises humans as being capable of authorship since they must be accountable for the work.
Deceased persons deemed appropriate as authors should be highlighted to the Editorial Assistant when submitting your article and should also be included in your contributorship statement.
If an author’s affiliation has changed during the course of the work, the author may either list the affiliation at the time that the research (or most significant portion of the research) was conducted, or their current affiliation, or both. The change of affiliation can be explained in an acknowledgements section.
Any change in authors after initial submission and before publication must be approved by all authors. This applies to additions, deletions, a change of order to the authors’ names or a change to the attribution of contributions. Any alterations must be explained to the Editor. The Editor may contact any of the authors and/or contributors to ascertain whether they have agreed to any alteration.
Contributorship statement
A contributorship statement is required for every article submitted and should outline who has contributed what to the planning, conduct and reporting of the work described in the article. A contributorship statement should include author contributors, non author contributors and group author contributors (collaborators). Contributors who have contributed materially to the paper but whose contributions do not justify authorship should be described clearly in the contributorship statement; for example, “served as scientific advisors”, “critically reviewed the study proposal”, “collected data” or “provided and cared for study patients”.
Researchers must determine among themselves the precise nature of each person’s contribution, and we encourage open discussion among all participants to reach a consensus.
This is also the appropriate place to include contributions by patients or members of the public who have assisted as research volunteers, giving their names and specific roles. We encourage authors to fully acknowledge the contribution of patients and the public to their research where appropriate.
All individuals named in the contributorship statement must give permission to be included, as readers may infer their endorsement of the data and conclusions of the paper. It is the responsibility of the submitting author to ensure that permission is obtained and to be able to provide evidence of this if required.
Each contributorship statement must make clear who is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
- To ensure transparent declaration of AI, authors should:
2. Transparent declaration includes a description of:
- What AI technology was used (the name of the technology)
- Why this AI technology was used (the reason for its use)
- How the AI technology was used (what the task of the technology was)
- Consider including a summary of the input, output, and the way in which the AI output was reviewed on the part of the authors as supplementary files or additional information for the editor to review. The editor may ask for more information and/or for information to be added to the content for internal use and/or for publication.
An acknowledgements statement may be included at the end of the paper, detailing those who helped in carrying out the research but who have not been recognised as contributors, as well as for personal expressions of gratitude.
Because acknowledgment may imply endorsement by acknowledged individuals of a study’s data and conclusions, authors are strongly advised to obtain permission to be acknowledged from all acknowledged individuals before submitting to any BMJ journal.
A uthor name change requests
As an inclusive publisher, BMJ wishes to ensure a smooth process and experience to facilitate author name changes after publication. For more information on how to request an author name change in an existing publication see our corrections policies.
Last updated: March 2023
Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.
Should student or supervisor be corresponding author for publications based on student research?
Papers published from an academic project (MSc or PhD) usually have two authors; the first author is the student who mainly conducted the research, and the second author is the professor who supervised the projects.
The corresponding author is the one who take the responsibility of a paper, and thus, some believe that students are not yet prepared to take this responsibility.
Ideally, who should be corresponding author for papers published by MSc or PhD projects?
I understand that it mainly depends on personal agreements and preferences, but I want to know which case is more reasonable from academic logic?
- publications
- professorship
- 2 If students are not yet prepared , how can they ever prepare unless they're going to be corresponding author? – gerrit Commented Sep 3, 2013 at 22:12
- 9 “Papers published from an academic project usually have two authors” — sometimes , but not usually – F'x Commented Sep 3, 2013 at 22:19
- 1 @F'x I wanted to define a simplified example. – Googlebot Commented Sep 3, 2013 at 22:24
- 2 @fedja: So then why are some answers talking about people still being around 5-10 years later? – JeffE Commented Sep 5, 2013 at 2:19
- 3 corresponding author is responsible to answer questions of both editors and readers — Weird. In my field, that's the responsibility of all authors. – JeffE Commented Sep 24, 2013 at 13:40
9 Answers 9
Since the ordering of authors differs between fields the meaning and usefulness of a corresponding author also varies. In fields I am familiar with, the corresponding author is usually the same as the "first author" (quotes because it may not be literally the first). Many journals therefore do not explicitly identify a first author unless different from the "first". There are then several cases where the corresponding author may need to be identified. One example is when a person lacking a permanent academic address is first author. Then the supervisor may take on the responsibility for the paper and be corresponding author. This can be important since it can be near impossible to track down someone who has left academia and so the supervisor stands for continuity in terms of contact. There are many variants on this and in some cases, a person heading a project or who by legal obligations carries responsibility for a project may be identified as corresponding author. This could be the case with some governmental organisations where communications are funnelled through hierarchies for bureaucratic reasons. I am sure there are lots of examples good and bad but the main purpose of identifying corresponding author, unless first, is so that anyone requiring more information can go directly to the main source for such.
So based on this background and the field you are in you may find a good way to determine corresponding author. In most cases, I would say it is the person who has done the most work, or the one who "owns" the project. It is not clear in some cases whether it is the student or the advisor who should be corresponding author. One also has to weigh in the intellectual work behind the project as a whole and from that perspective the person who has done the work, perhaps a detail in a much bigger perspective, may not be the appropriate person for details although that person has done most of the work for the paper in question. So in some cases the question is definitely harder to answer. Not being corresponding author, does not necessarily detract much from being first author since such details are not visible in literature searches and CVs.
"The corresponding author is the one who take the responsibility of a paper". I've never heard this before. For example: http://sciencecareers.sciencemag.org/career_magazine/previous_issues/articles/2010_04_16/caredit.a1000039 says "The corresponding author is the point of contact for editors, readers, and outside researchers who have questions about the contents of the paper. Often, the corresponding author is also the last author, but she or he may be listed first or even in the middle of the author list."
All authors take responsibility for the paper (or should). The point of the corresponding author is who to contact if you want to correspond about the paper. If this were someone who was likely to move institution (because they are finishing, or have finished their study), they are going to be hard to contact, so make it someone who's likely to hang around for a while. I've never seen anyone take any notice of who the corresponding author is.
- 6 They can give their permanent contact addresses not their current affiliation that they will change soon. That's why some people put their Gmail or yahoo mail addresses in their papers! – sajjadG Commented Sep 24, 2013 at 13:13
I was always the corresponding author; my advisor(s) thought it was good for me, and they had other things to do than to fiddle around with LaTeX...
So, to answer your question, I think it is good for phd students to be the corresponding author; besides, if there is any trouble, you have always your advisor/coauthor to ask.
- I think this conflates the author who is doing the actual submission process (almost always the trainee) with the person with email address listed on the paper (in my experience, often the supervisor even if the trainee went through the forms). – AJK Commented Jan 2, 2017 at 0:45
It varies widely, not only on your field's customs, but also on individual research groups. In the research groups I have worked in, and worked with, in chemical engineering , the corresponding author is usually the most “perennial” researcher, i.e. usually the PI/professor . The idea is to ensure that the corresponding author is a faculty member, meaning he is the person most likely to still be around in 5 to 10 years' time to answer questions about the work. (In that time, PhD students and post-docs may change field completely, exit academia, etc.)
Also, the PI is usually the one who gets to keep the archives (raw data, lab notebooks, etc.) in the long term, so it makes sense that way.
I think it mostly depends upon the mutual understanding between the supervisor(PI) and the student. I had a similar case with my PhD colleague. She wanted to be a corresponding author but the adviser of the study group (a large scale multidisciplinary study) denied which could be due to the factors mentioned above such as: the PI will be staying there at least for few years however the student might leave the institute or even academia.
In my opinion, it is very helpful for a PhD student be the corresponding author because being a corresponding author will improve some skills: experience in answering critiques from the reviewers, writing, giving reasonable explanations and so on. More importantly, it is the student who did most of the work for the publication and will be able to give answers to most of the queries from the reviewers.
This is something that ought to be agreed on in discussion between the student and supervising professor. Ideally, this decision should be made from the start of the research.
In my own situation, when I was completing my PhD, the 4 papers published had me as both the first author and corresponding author. My advisor told me that part of the research process would be to field any and all questions, concerns and queries that come from the paper.
Edited (in response to question edit): From an academic point of view, it can be argued that the student is the primary researcher, hence expert in that specific topic, hence would be the only one who can completely answer any questions.
I'm (as the postgrad and lead author) the corresponding author on a paper, rather than my supervisor, which is common here. I've had a few queries on the experiment and equipment, which realistically, as I did the work, and I'm not so busy as my supervisor I'm better placed to deal with.
When I was working as senior resident,the department head was the first author and also the corresponding author in all the scientific papers written by me. I thought that a senior person is better placed to answer any outside researcher's question easily and be available for years instead of a student who is likely to leave the institution once study period is completed. But once the internet facility has come, the point of being permanent or regular has become irrelevant and anybody can be contacted anywhere over E-mail.I feel that only those with maximum involvement in the scientific work should be the corresponding author as he only knows well about the work and can give reply on his own to any outside researcher's question regarding the contents of the paper.
Most of the time students never know how, what and where to write,they purely depends upon their supervisor,who is directly involved in this exercise.Paying regards is another factor that is also linked and making a segregation in teacher and taught is a good practice.Supervising any task is not easy,it requires complete involvement in form of legal, responsibilities & other issues as well.Therefore CA deserve proper place with dignity in research papers as it also carries a message.
You must log in to answer this question.
Not the answer you're looking for browse other questions tagged phd publications citations professorship thesis ..
- Featured on Meta
- Upcoming sign-up experiments related to tags
Hot Network Questions
- DSP Puzzle: Advanced Signal Forensics
- Was BCD a limiting factor on 6502 speed?
- Cloud masking ECOSTRESS LST data
- "All due respect to jazz." - Does this mean the speaker likes it or dislikes it?
- What could explain that small planes near an airport are perceived as harassing homeowners?
- Geometry question about a six-pack of beer
- Do known physical systems all have a unique time evolution?
- Is arxiv strictly for new stuff?
- Could space habitats have large transparent roofs?
- Determine the voltages Va and V1 for the circuit
- Is it this limit impossible or not exists?
- How can I take apart a bookshelf?
- Can I get a refund for ICE due to cancelled regional bus service?
- Where does someone go with Tzara'as if they are dwelling in a Ir Miklat?
- Viewport Shader Render different from 1 computer to another
- What was the first game to intentionally use letterboxing to indicate a cutscene?
- Can I route audio from a macOS Safari PWA to specific speakers, different from my system default?
- How will the ISS be decommissioned?
- Folk stories and notions in mathematics that are likely false, inaccurate, apocryphal, or poorly founded?
- Why depreciation is considered a cost to own a car?
- What is the meaning of '"It's nart'ral" in "Pollyanna" by Eleanor H. Porter?
- D&D 3.5 Shadow Magic / Shadow Conjuration: Can an illusionist create a quasi-real shadow of ANY monster? Or only those on the summon monster lists?
- Sets of algebraic integers whose differences are units
- Visit USA via land border for French citizen
What roles Co-author and Corresponding Author play in Research Papers

Introduction

In current academic research, nothing exists in isolation. Good research requires collaboration, thus giving rise to the guild of co-authors and corresponding authors. These terms often raise questions about their significance and differences. Let’s delve into the distinctions between co-authors and corresponding authors, their roles, and how to appropriately mention the corresponding author in a paper.
Co-author vs. Corresponding Author: Unveiling the Differences
Co-author Meaning
- A co-author is a researcher who has contributed significantly to a research paper, sharing responsibility for its content and findings.
- Co-authors collaborate to design experiments, analyze results, and contribute to the overall intellectual content of the paper.
Corresponding Author Meaning
- The corresponding author is the designated point of contact for the paper. They facilitate communication with the journal, handle revisions, and address queries.
- The corresponding author isn’t necessarily the primary contributor but takes on administrative responsibilities.
How to Mention the Corresponding Author in a Paper
- Typically, the corresponding author’s name and contact information are provided at the top of the first page of the paper.
- Including an asterisk (*) next to the corresponding author’s name and explaining their role in a footnote is common practice.
- Mention the corresponding author’s email address for efficient communication.
Co-author vs. Second Author: Clarifying the Distinction
- Co-author: Holds equal responsibility for the content contributed substantially.
- Second Author: Holds a significant role but might not have been as involved as co-authors.
Who Should Be the Corresponding Author?
- Usually, the corresponding author is a senior researcher who can effectively handle communication.
- The corresponding author need not be the primary author; any co-author familiar with the research can take on this role.
Differences Between Co-author and Corresponding Author
- Responsibility : Co-authors share content responsibility; the corresponding author manages communication.
- Involvement : Co-authors are deeply involved in research; the corresponding author handles administrative aspects.
- Listing : All co-authors are listed in the byline; only the corresponding author’s contact details are visible.
- Primary Contribution : Co-authors contribute intellectually; the corresponding author manages logistics.
Main Author vs. Corresponding Author: Unraveling the Contrast
- Main Author : Often referred to as the first author, contributes significantly to research and writing.
- Corresponding Author : Handles communication, edits, and revisions after accepting the paper.
Collaborative Writing: Can Two Authors Pen a Book Together?
- Multiple authors can co-write a book, combining their expertise and perspectives.
The Merits of Being a Co-author
- Learning Opportunity : Co-authoring exposes you to diverse ideas and research methods.
- Networking : Collaboration connects you with other researchers in your field.
- Shared Workload : Co-authors distribute the research and writing burden.
Conclusion: Navigating the Authorship Landscape
Understanding the roles of co-authors and corresponding authors is vital in the intricate realm of academic authorship. Collaborative efforts enrich research and foster academic growth. As you embark on research journeys, remember the unique contributions of co-authors and the crucial responsibilities shouldered by corresponding authors. So, cheer up if you are a co-author or corresponding author; your contributions to this evolving knowledge domain are unparalleled.
Explore, Collaborate, and Illuminate. Connect with us at Manuscriptedit.com .
Related Posts
Open access journals are vanishing from the web, internet archive stands ready to fill in the gaps.
The advent of Open access journals was expected to revolutionize the academic journal publication sector. Various factors were driving the rise of open access journals, especially in the field of scientific studies. Some of the most common factors, such as time required to get published in conventional peer-reviewed journals or the high rate of rejections, […]
Types of Peer Review
Following the submission of the manuscript, a preliminary review round is conducted by the editor to determine whether the manuscript is consistent with the journal’s aim and scope. To analyze the research’s quality, it is moved to the desk of peer reviewers before getting published. This collective process is termed as “Peer review.” It is […]
Is the world of academia facing the gender disparity issue?
Gender-biased selection is a prominent concern in academics. This gender disparity is not only restricted to scientists and researchers, but also in evidence during the selection of peer reviewers. Peer review is a vital process before acceptance of a paper in order to evaluate the research methods and validate the findings. It is conducted by subject […]
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
On this page
Authorship: inclusion & ethics in global research, consortia authorship, author contribution statements, author identification, author name change.
- Nature Portfolio journals' editorials
Authorship provides credit for a researcher's contributions to a study and carries accountability. Authors are expected to fulfil the criteria below (adapted from McNutt et al ., Proceedings of the National Academy of Sciences, Feb 2018, 201715374; DOI: 10.1073/pnas.1715374115; licensed under CC BY 4.0 ):
Each author is expected to have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; or have drafted the work or substantively revised it
AND to have approved the submitted version (and any substantially modified version that involves the author's contribution to the study);
AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Nature Portfolio journals encourage collaboration with colleagues in the locations where the research is conducted, and expect their inclusion as co-authors when they fulfil all authorship criteria described above. Contributors who do not meet all criteria for authorship should be listed in the Acknowledgements section.
Nature Portfolio journals reserve the right not to consider non-primary research manuscripts that have been authored by medical writers. Writing assistance should be acknowledged in all article types.
Nature Portfolio journals do not require all authors of a research paper to sign the letter of submission, nor do they impose an order on the list of authors. Submission to a Nature Portfolio journal is taken by the journal to mean that all the listed authors have agreed all of the contents, including the author list and author contribution statements. The corresponding author is responsible for having ensured that this agreement has been reached that all authors have agreed to be so listed, and have approved the manuscript submission to the journal, and for managing all communication between the journal and all co-authors, before and after publication. The corresponding author is also responsible for submitting a competing interests' statement on behalf of all authors of the paper; please refer to our competing interests' policy for more information.
It is expected that the corresponding author (and on multi-group collaborations, at least one member of each collaborating group, usually the most senior member of each submitting group or team, who accepts responsibility for the contributions to the manuscript from that team) will be responsible for the following with respect to data, code and materials: (adapted from McNutt et al., Proceedings of the National Academy of Sciences, Feb 2018, 201715374; DOI: 10.1073/pnas.1715374115; licensed under CC BY 4.0 ):
- ensuring that data, materials, and code comply with transparency and reproducibility standards of the field and journal;
- ensuring that original data/materials/code upon which the submission is based are preserved following best practices in the field so that they are retrievable for reanalysis;
- confirming that data/materials/code presentation accurately reflects the original;
- foreseeing and minimizing obstacles to the sharing of data/materials/code described in the work
- ensuring that all authors (or group leaders in multi-lab collaborations) have certified the author list and author contributions
At submission, the corresponding author must include written permission from the authors of the work concerned for mention of any unpublished material cited in the manuscript (for example others' data, in press manuscripts, personal communications or work in preparation). The corresponding author also must clearly identify at submission any material within the manuscript (such as figures) that has been published previously elsewhere and provide written permission from authors of the prior work and/or publishers, as appropriate, for the re-use of such material.
After acceptance, the corresponding author is responsible for the accuracy of all content in the proof, including the names of co-authors, addresses and affiliations.
After publication, the corresponding author is the point of contact for queries about the published paper. It is their responsibility to inform all co-authors of any matters arising in relation to the published paper and to ensure such matters are dealt with promptly. Authors of published material have a responsibility to inform the journal immediately if they become aware of any aspects that requires correction.
Any changes to the author list after submission, such as a change in the order of the authors or the deletion or addition of authors, must be approved by every author. Changes of authorship by adding or deleting authors, and/or changes in Corresponding Author, and/or changes in the sequence of authors are not permitted after acceptance of a manuscript. Nature Portfolio journal editors are not in a position to investigate or adjudicate authorship disputes before or after publication. Such disagreements, if they cannot be resolved amongst authors, should be directed to the relevant institutional authority.
The primary affiliation for each author should be the institution where the majority of their work was done. If an author has subsequently moved, the current address may also be stated. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Top of page ⤴
Nature Portfolio journals encourage collaboration with colleagues in the locations where the research is conducted, and expect their inclusion as co-authors when they fulfill all authorship criteria described above. Contributors who do not meet all criteria for authorship should be listed in the Acknowledgements section. We urge researchers to carefully consider researcher contributions and authorship criteria when involved in multi-region collaborations involving local researchers so as to promote greater equity in research collaborations.
We encourage researchers to follow the recommendations set out in the Global Code of Conduct for Research in Resource-Poor Settings when designing, executing and reporting their research and to provide a disclosure statement in their manuscript that covers the aspects listed below (drawn from the Global Code of Conduct) Editors may at their discretion ask authors to provide a disclosure statement taking these questions into account; the disclosure can be requested during peer review, shared with reviewers and published in the final paper as an “Ethics & Inclusion statement” in the Methods section. Our general policies on Research ethics and Reporting standards can be found here and here .
Has the research included local researchers throughout the research process – study design, study implementation, data ownership, intellectual property and authorship of publications?
Is the research locally relevant and has this been determined in collaboration with local partners?
Please describe whether roles and responsibilities were agreed amongst collaborators ahead of the research and whether any capacity-building plans for local researchers were discussed.
Would this research have been severely restricted or prohibited in the setting of the researchers? If yes, please provide details on specific exceptions granted for this research in agreement with local stakeholders.
Where appropriate, has the study been approved by a local ethics review committee? If not, please explain the reasons.
Where animal welfare regulations, environmental protection and biorisk-related regulations in the local research setting were insufficient compared to the setting of the researchers, please describe if research was undertaken to the higher standards.
Does the research result in stigmatization, incrimination, discrimination or otherwise personal risk to participants? If yes, describe provisions to ensure safety and well- being of participants.
If research involves health, safety, security or other risk to researchers, describe any risk management plans undertaken.
Have any benefit sharing measures been discussed in case biological materials, cultural artefacts or associated traditional knowledge has been transferred out of the country?
Please indicate if you have taken local and regional research relevant to your study into account in citations.
A collective of authors can be listed as a consortium. If necessary, individual authors can be listed in both the main author list and as a member of a consortium. All authors within a consortium must be listed at the end of the paper. If it is necessary to include a list of consortium members that did not directly contribute to the paper, this list can be placed in the Supplementary Information. To facilitate submission of manuscripts with large author lists, please consult the journal editor before submission.
Nature Portfolio journals encourage transparency by publishing author contribution statements. Authors are required to include a statement of responsibility in the manuscript, including review-type articles, that specifies the contribution of every author. The level of detail varies; some disciplines produce manuscripts that comprise discrete efforts readily articulated in detail, whereas other fields operate as group efforts at all stages. Author contribution statements are included in the published paper. This Nature Editorial describes the policy in more detail.
Nature Portfolio journals also allow one set of co-authors to be specified as having contributed equally to the work and one set of co-authors to be specified as having jointly supervised the work. Other equal contributions are best described in author contribution statements. Corresponding authors have specific responsibilities (described above).
As part of our efforts to improve transparency and unambiguous attribution of scholarly contributions, corresponding authors of published papers must provide their Open Researcher and Contributor Identifier (ORCID) iD; co-authors are encouraged to provide ORCiD iDs. More information about Springer Nature’s support for ORCiD iDs and journals participating in the ORCiD mandate can be found here .
An author who has changed their name for reasons such as gender transition or religious conversion may request for their name, pronouns and other relevant biographical information to be corrected on papers published prior to the change. The author can choose for this correction to happen silently, in which case there will be no note flagging the change on either the pdf or the html of the paper, or alternatively they may do so by a formal public Author Correction.
For authors who’ve changed their name and wish to correct it on their published works, please see SNCS Contact Form: Inclusive Name Change Policy : Springer Nature Support .
Nature Portfolio journals' editorials:
- New framework aims to improve inclusion and ethics in global research collaborations amid wider efforts to end exploitative practices. Nature. Nature addresses helicopter research and ethics dumping , June 2022.
- Corresponding authors should not neglect their responsibility to a journal or their co-authors. Nature Nanotechnology . A matter of duty , December 2012.
- Why do we need statements to define the contributions made by each author? Nature Photonics. Contributors, guests, and ghosts , June 2012.
- Nature Nanotechnology. The responsibilities of authors.
- Nature Cell Biology. Attribution and accountability .
- Nature Physics . What did you do?
- Nature Photonics. Combating plagiarism.
- Nature. Authorship policies.
- Individual contributions should be carefully evaluated when compiling the author list of a scientific paper. Nature Materials. Authorship matters, February 2008.
- How the responsibilities of co-authors for a scientific paper's integrity could be made more explicit. Nature . Who is accountable? 1 November 2007.
- The problems of unjustified authorship. Nature Materials . Authorship without authorization , November 2004.
Nature is encouraging authors of papers to say who did what. Nature . Author contributions , 3 June 1999.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
To read this content please select one of the options below:
Please note you do not have access to teaching notes, multiple co-first authors, co-corresponding authors and co-supervisors: a synthesis of shared authorship credit.
Online Information Review
ISSN : 1468-4527
Article publication date: 26 March 2021
Issue publication date: 14 October 2021
Authorship is the ultimate status of intellectual recognition in academic publishing. Although fairly robust guidelines have already been in place for a considerable amount of time regarding authorship criteria and credit, such as those by the International Committee of Medical Journal Editors or Contributor Roles Taxonomy, the lack of reliable verification techniques hamper their accuracy, thereby reducing the validity of authorship claims in such statements. This paper aims to focus on the authorship status and responsibilities of co-first authors and co-corresponding authors.
Design/methodology/approach
To appreciate authorship responsibilities in this subset of authors, the broader academic authorship literature, as well as position statements, rules and guidelines, were consulted.
Academic publishing that relies on metrics is a global multi-billion-dollar business, so strict measures to assess and confirm authorship, which can be intellectually or financially “profitable” among academics that game such metrics, are needed. The current assessment is that there are inconsistent rules for equally credited authors such as co-first authors, co-corresponding authors and co-supervisors. In shared and collaborative authorship, there are also shared authorship-related responsibilities, but these are infrequently discussed, or tend to only be dealt with broadly.
Originality/value
Within the wider, and important, discussion about authorship, which is one of the most central issues in academic publishing, there has been a limited focus on equally credited authors such as co-first authors, co-corresponding authors and co-supervisors. This paper expands and fortifies that discussion.
- Authors' rights
- Contribution
- Corresponding author
- Entitlement
- Equal contributor
- Equally credited author (ECA)
- Science, technology, engineering and mathematics or medicine (STEMM)
Acknowledgements
Author contributions: The author contributed fully to the intellectual discussion underlying this paper, literature exploration, writing, reviews and editing and accepts responsibility for the content, analyses and interpretation herein. Conflicts of interest: The author declares no conflicts of interest of relevance to this topic.
Teixeira da Silva, J.A. (2021), "Multiple co-first authors, co-corresponding authors and co-supervisors: a synthesis of shared authorship credit", Online Information Review , Vol. 45 No. 6, pp. 1116-1130. https://doi.org/10.1108/OIR-06-2020-0219
Emerald Publishing Limited
Copyright © 2021, Emerald Publishing Limited
Related articles
All feedback is valuable.
Please share your general feedback
Report an issue or find answers to frequently asked questions
Contact Customer Support
How to Decide the First Author and Corresponding Author in a Manuscript

When a scholarly/academic paper is produced, the researchers participating in the work must assign a first author and corresponding author. This is a challenging decision and sometimes there’s conflict because the positions can also indicate status (whether real or perceived).
The first author and corresponding author, ideally, are decided during the research and through a mutual agreement among the authors. It’s made based on an understanding of the role and significance of the positions. The author order generally indicates the amount of contribution.
The first author is considered to have contributed more than the second author, and so forth, until reaching the author in the last position. A shared first author (co-first author) or shared corresponding author (co-corresponding author), however, isn’t out of the question.
The last position may also be prestigious – considered the senior author or principal investigator. One of the authors in the list will also be the corresponding author. This means they coordinate the publication process (such as arranging editing and communicating with the journal and with other inquiries) and have their contact information shown upfront in the work.
- What you’ll learn in this post
- The differences between first author and corresponding author (and what’s a senior author?).
- How first author and corresponding author are defined.
- How to fairly determine which author(s) will fulfill which role(s).
- When and why the same person might fulfill both roles.
Determining the author order
What defines the first author, some of the main duties of a first author are:, what defines a corresponding author, some of the corresponding author’s main duties are:, and who is the senior author, a few words on guarantors, can the first and the corresponding authors be the same person, how do you decide who does what role what are the potential ethical issues.
The order of authors should reasonably correspond to how the authors contributed to the work. It also implies specific credit and responsibilities that go with being in these positions. Working it out over a cup of tea or coffee as soon as possible can help to avoid disputes, and even mediation , later on.
The issue of determining a senior author is also a bit challenging. You need to understand these roles and responsibilities. Don’t just think about who gets the most credit.
Publishing in a scientific journal or any peer-reviewed publication, including preprints and poster presentations , makes research visible to the greater public. It brings discoveries and insights into the eyes of the main experts around the world. This, in turn, builds the author’s reputation as a researcher.
Having a good list of published papers can also help achieve some career goals, such as getting a degree, a promotion or, as a scientist, getting funding to continue with research. The number of first-author papers may also be looked upon as a positive metric.
An “author” (having authorship) of a paper must meet certain criteria for their contributions. The International Committee of Medical Journal Editors (ICJME) guidelines state authorship must at least include:
- made substantial contributions to work
- approved the final version
- assumed responsibility and accountability for what is published
However, while the minimum requirements for authorship are generally agreed upon, the credit/responsibility given to a specific author list isn’t so clear. The position on the list can determine the author’s expected duties and show the readers how the author contributed to the work.
So let’s take a deeper look at the definitions of these positions. Hopefully, this will help in your decision-making process. It may in fact affect your future career and status.
Effective English Writing
Get insights from a real journal editor!
This quick handy PDF highlights what to do (and what NOT to do) when writing your research manuscript.
Our experts show you how to write effectively for better readability and faster publication!
Free PDF e-book

The first author is usually the person who makes the greatest practical/intellectual contributions to the work.
This person might have co-authors’ assistance with specific tasks, but they are the main responsible one for acquiring and analyzing the data, and for writing the final manuscript.
The first position in the authorship list of a paper is the most attractive one. First authors will have their (last) name mentioned in every future citation of the work, no matter how many other authors there are.
There can also be co-first authors. This is common in projects that require different areas of expertise. It’s also used where it’s hard to figure out who made a larger contribution. Two or even three authors can be listed as equal contributors.
The co-first authors are denoted by an asterisk or other symbol (for example, “ Author A*, Author B*, Author C, Author D.. ”) and a note on the first page.
But even then, the person listed first will continue to be the most visible. This is because of how citations are created. To give equal credit to both first authors, an alternative is to cite the paper as “ Author A & Author B et al .”., instead of “ Author A et al. ”
- Make intellectual contributions to the work. Participate in the conception and planning of the study; define aims and trace a methodological approach to achieve them.
- Generate the data. For instance, perform experiments, conduct literature reviews, write programming code, etc.
- Analyze the results. Generate graphs , tables , and illustrations to convey the data, and perform statistical analyses when needed.
- Write and edit the manuscript.
- Help the corresponding author with referees’ queries when the paper is under revision.

The corresponding author is responsible for bringing together the manuscript, and for the whole process of submitting it to a journal, up to (hopefully!) final acceptance. Of course, the corresponding author also must meet the academic authorship requirements.
The ICJME defines a corresponding author as someone who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process.
In that sense, the corresponding author is also responsible for ensuring that all the journal’s administrative requirements are fulfilled. This can include providing documents related to ethics committee approval, data and signatures from all authors, and conflict of interest (COI) statements.
In line with this, the Committee on Publication Ethics (COPE) says the corresponding author should be someone willing to fulfill all obligations the journal stipulates ( COPE Discussion Document , 2014).
The corresponding author’s contact details are included in the article. This makes them the representative for inquiries about the work. A good corresponding author must therefore be readily available. All communications with journals or readers should be done in a timely way.
English ability is also a big help if you’re the corresponding author and you want to publish in English. You’re usually the person whom I , as the editor, will be communicating with when you choose an editing service.
- Certify the manuscript contains all the necessary parts, it is appropriately organized, and it complies with the journal’s requirements. Upload the manuscript and other files.
- Make sure all authors have reviewed and approved the final version of the manuscript before submission. Get signed consent.
- Be in charge of all communications related to the paper. Distribute notifications to all authors (e.g., emails, peer review feedback , decision letters).
- Meet all deadlines, communicate with the authors and editors efficiently, and follow time schedules for publication.
- Ensure all editorial and submission policies are followed.
Note: Although there’s common sense on the main roles of a corresponding author, some of the responsibilities involved can change from one journal to another (e.g., see this list , created by the National Academy of Sciences, with different journals and their respective requirements).
The senior author is the person who provides the intellectual input and helps to design the study and the protocols to be followed. This is especially because they’re experts in that field of research.
They are also sometimes the financial driving force behind the study and generally supervise several projects. For these reasons, they are also known as the “ principal investigator .” They usually have broad experience in publishing processes, and their names appear in the last positions of the author list.
Honestly, the senior author is often the lab leader or simply the person in charge. For the work they’ve put into this to date, they earned this honor.
Some journals now ask for one author on a paper to be listed as a guarantor. The guarantor:
- accepts official responsibility for the overall integrity of the manuscript (including ethics, data handling, reporting of results, and study conduct)
- does not act as the primary correspondent for the manuscript
- ensures all statements in the manuscript are true to his knowledge
The guarantor can be the same as the corresponding author, or can be another of the authors.
It’s often recommended that the Principal Investigator or Senior Researcher on a manuscript act as the guarantor as they will be responsible for the study supervision already; however, this is not explicitly required.
Yes, first authors can also be corresponding authors in a manuscript. In fact, it’s quite common.
The main conflict here is when authors equate being a corresponding author with seniority. Senior authors are often viewed as the perfect corresponding authors because of all the qualities they have, as mentioned.
However, as discussed, a corresponding author is charged with communicating with editors and readers only. Journal editors usually see this as an administrative role. Therefore, the corresponding author doesn’t necessarily have to be the seniormost author.
While there’s a special responsibility involved in this role, it’s not supposed to be a mark of distinction. Also, most senior authors will probably have less available time to reply to queries during the submission review process. And they may not have time to respond to reader queries in the future.
So, first authors should be expected to serve as corresponding authors. This is the case as long as they’re consistently involved in the study and know-how to go through the submission/publication process.
This includes deciding on the need for scientific editing if the English needs improving. The role can also help them gain experience in corresponding with journals and general readers.

Even when the first and corresponding authors aren’t the same person some of their duties can be shared.
While a corresponding author can help the first author, or main author, with data analysis, for example, the first author can help the corresponding author prepare the documents for submission.
The same applies to other authors. Deciding who does what role should be clearly discussed and defined beforehand. Ideally, researchers involved in the study should have regular meetings to clarify responsibilities and update the status of the work. New co-authors may be included and other members may end their involvement along the way. Doing this planning may help prevent conflicts regarding academic authorship and help manage any disputes (Albert & Wager, 2009).
There are many possible reasons for conflict (you can find many examples of real cases on COPE’s website ). Disputes around first authorship are more common because this is the most prestigious position and an important measure of productivity. This happens, for example, when two authors both claim they contributed the most. This issue can be solved by proposing co-first authorship, or by using a system to quantify their contributions and then decide who should go first.
There can also be disagreements when the senior author wants to be the first author, or main author. For example, they may need more or higher-impact publications as the first author. Or they wrote the manuscript and believe this entitles them to be the first author.
Disputes on who will be the corresponding author are less likely. That’s because the role, as mentioned, doesn’t have any special distinction other than a visible name and contact. However, some senior authors may still want this role and occasionally there’s some conflict.
Ideally, the corresponding author should be decided among the others. It’s also possible to share the position, have shared corresponding authors; e.g. both the first and senior authors are co-corresponding authors. This may actually work out well if one is available and the other isn’t.
Every participant should feel free to seek clarity throughout the collaboration. Consider having a written document ( see this example PDF file on APA) in place as guidance ( COPE Discussion Document , 2014; Guidelines on Authorship and Acknowledgement , n.d.).
Before publication, authors should reunite to check the previous responsibilities list and create a final version of the documents. This includes detailed information on the type and extent of the contribution of each person involved. For categories of contributions, see the Contributor Roles Taxonomy [CRediT] website (McNutt et al., 2018). To quantify contributions, different proposed systems can be found in the literature (APA, for example, proposed a scorecard – see their Helpful Tools files).
As several journals now request and publish information about each author’s contributions, such documents can be essential. This can help in creating standards that will improve transparency in the system of scientific publishing. That, in turn, greatly reduces ethical concerns and authorship disputes.
Our Publication Support team is ready to hear from you if you want to accelerate your path to publication. And explore valuable research services that can help increase your impact and avoid ethical mishaps.
This is a guest post from Adam Goulston, PsyD, MBA, MS, MISD, ELS. Adam runs the Asia-based science marketing and PR company Scize . He has worked as an in-house Senior Language Editor, as well as a manuscript editor, with Edanz.
Albert, T., & Wager, E. (2009). How to handle authorship disputes: A guide for new researchers. Committee on Publication Ethics. https://doi.org/10.24318/cope.2018.1.1
COPE Discussion Document: Authorship. (2014). Committee on Publication Ethics. https://doi.org/10.24318/cope.2019.3.3
Guidelines on Authorship and Acknowledgement. (n.d.). Retrieved from https://research.fas.harvard.edu/links/guidelines-authorship-and-acknowledgement
McNutt, M. K., Bradford, M., Drazen, J. M., Hanson, B., Howard, B., Jamieson, K. H., Kiermer, V., Marcus, E., Pope, B. K., Schekman, R., Swaminathan, S., Stang, P. J., & Verma, I. M. (2018). Transparency in authors’ contributions and responsibilities to promote integrity in scientific publication. Proceedings of the National Academy of Sciences, 115(11), 2557-2560. https://doi.org/10.1073/pnas.1715374115

- Researcher Services
- English Editing
- EXCITED by the SCIENCE
- Smart Tools
- Journal Selector
- About Edanz
- Privacy Policy
- Terms & Conditions
- Services & Pricing
- 特定商取引法に基づく表記
Instructions for Authors
Contact Monica Mungle for help if edits are needed to the top section.
Original Investigation
Caring for the critically ill patient, brief report, research letter, systematic review (without meta-analysis), narrative review, special communication, clinical challenge, diagnostic test interpretation, a piece of my mind, letter to the editor, letter in reply.
- Randomized Clinical Trial
- Parallel-Design Double-blind Trial
- Crossover Trial
- Equivalence and Noninferiority Trial
- Cluster Trial
- Nonrandomized Controlled Trial
Meta-analysis
- Cohort Study
- Case-Control Study
- Cross-sectional Study
- Case Series
- Economic Evaluation
- Decision Analytical Model
- Comparative Effectiveness Research
- Genetic Association Study
- Diagnostic/Prognostic Study
- Quality Improvement Study
- Survey Study
- Qualitative Study
Manuscript Submission
Copies of previous editorial and reviewer comments, cover letter, manuscript style, manuscript components, recommended file sizes, manuscript file formats, abbreviations, units of measure, names of drugs, devices, and other products, gene names, symbols, and accession numbers, reproduced and re-created material, online-only supplements and multimedia.
What to Expect
Editorial and Peer Review
The jama network advantage.
- JAMA-Express
Authorship Form and Publishing Agreement
Publication.
- Postpublication Online Commenting
Reprints/e-Prints
Corrections, previous publication, related manuscripts and reports, and preprints, previous or planned meeting presentation or release of information, embargo policy, research article public access, depositing in repositories, and discoverability.
Editorial Policies for Authors
Authorship and Disclosures
Authorship criteria and contributions, role of the corresponding author, changes in authorship, name change policy, group authorship, conflicts of interest and financial disclosures, funding/support and role of funder/sponsor, data access, responsibility, and analysis, acknowledgment section, equator reporting guidelines, use of causal language, timeliness of data, statistical methods and data presentation, reporting demographic information for study participants, ethical approval of studies and informed consent, patient identification, use of ai in publication and research, personal communications and unpublished data, manuscripts that pose security risks.
Journal Policies, Forms, Resources
Decisions and Management of Editorial Conflicts of Interest
Publishing agreement, unauthorized use.
- Patient Permission Form
- AMA Manual of Style
- EQUATOR Network
- About This Journal
Contact Information
JAMA , Kirsten Bibbins-Domingo, PhD, MD, MAS, Editor in Chief, 330 N Wabash Ave, Chicago, IL 60611-5885; telephone: (312) 464-4444; fax: (312) 464-5824; email: [email protected] . Manuscripts should be submitted online at http://manuscripts.jama.com .
Determine My Article Type
Categories of articles.
Original Investigation full info
Clinical trial Meta-analysis Intervention study Cohort study Case-control study Epidemiologic assessment Survey with high response rate Cost-effectiveness analysis Decision analysis Study of screening and diagnostic tests Other observational study
- ≤5 tables and/or figures
- Structured abstract
Data Sharing Statement
Follow EQUATOR Reporting Guidelines
Caring for the Critically Ill Patient full info
Original research reports, preferably clinical trials or systematic reviews that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care.
- See also requirements for Clinical Trial , Meta-analysis , and Systematic Review
Brief Report full info
Short reports of original studies or evaluations or unique, first-time reports of clinical case series.
It is very rare for this journal to publish case reports.
- 15 references
- ≤3 tables and/or figures
Research Letter full info
Concise, focused reports of original research. Can include any of the study types listed under Original Investigation.
- ≤6 references
- ≤2 small tables and/or figures
- No Abstract or Key Points
Back to top
Clinical Review and Education
Systematic Review (without meta-analysis) full info
This article type requires a presubmission inquiry. See the "full info" below for requirements and contact information.
Critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ).
- 50-75 references
- A PRISMA-style flow diagram should be included as an online supplement
- Include a table with ratings of the quality of the studies/evidence
- Subtitle should be "A Systematic Review"
Narrative Review full info
Up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines.
The focus should be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment.
These reviews should address a specific question or issue that is relevant for clinical practice.
- 2000-3500 words
- 3-part structured abstract
- No Key Points
- Subtitle should be "A Review"
Special Communication full info
This journal publishes very few of these types of articles.
These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
- 50 references
- ≤4 tables and/or figures
- Requires a presubmission inquiry
Clinical Challenge full info
Presents an actual patient case with a specific disease or condition with an accompanying clinical image.
- "What Would You Do Next?" with 4 single-phrase plausible treatment options describing possible courses of action with 1 being preferred
- Case presentation: 250 words
- Discussion: 500-600 words
- ≤10 references
- 1-2 small figures
- Patient permission required
Diagnostic Test Interpretation full info
This article requires a presubmission inquiry.
Presentation of the results of a diagnostic test from a single patient with exploration of the clinical application of the test result; intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
- How Do You Interpret These Test Results? (or What Would You Do Next?) with 4 plausible responses
- Case presentation: 200 words
- Discussion: 650 words
Viewpoint full info
May address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article.
- 1200 words (or 1000 words with 1 small table or figure)
- ≤7 references at submission
- ≤3 authors, with no more than 2 affiliations per author
A Piece of My Mind full info
Personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession.
- ≤1600 words
- Patient permission may be needed
Poetry full info
Original poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer.
- No longer than 44 lines
Correspondence
Letter to the Editor full info
Letters discussing a recent article in this journal should be submitted within 4 weeks of the article's publication in print.
- ≤5 references (1 of which should be to the recent article)
Letter in Reply full info
Replies by authors of original articles to letters from readers.
Determine My Study Type
Randomized Clinical Trial full info
A trial that prospectively assigns participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like.
- ≤5 tables and/or figures, including CONSORT flow diagram
- Subtitle should be "A Randomized Clinical Trial"
- Trial registration and ID
- Trial protocol
- CONSORT checklist
- Follow CONSORT Reporting Guidelines
Parallel-Design Double-blind Trial full info
A randomized trial that prospectively assigns participants to 2 or more groups to receive different interventions. Participants and those administering the interventions are unaware of which intervention individual participants are receiving.
Crossover Trial full info
A trial in which participants receive more than 1 of the treatments under investigation, usually in a randomly determined sequence, and with a prespecified amount of time (washout period) between sequential treatments.
Equivalence and Noninferiority Trial full info
A trial designed to assess whether the treatment or intervention under study (eg, a new intervention) is no worse than an existing alternative (eg, an active control). In these trials, authors must prespecify a margin of noninferiority that is consistent with all relevant studies and within which the new intervention can be assumed to be no worse than the active control.
Cluster Trial full info
A trial that includes random assignment of groups rather than individuals to intervention and control groups.
Nonrandomized Controlled Trial full info
A trial that prospectively assigns groups or populations to study the efficacy or effectiveness of an intervention but in which the assignment to the intervention occurs through self-selection or administrator selection rather than through randomization. Control groups can be historic, concurrent, or both. This design is sometimes called a quasi-experimental design.
- ≤5 tables and/or figures, including a trial flow diagram
- Subtitle should be "A Nonrandomized Controlled Trial"
- TREND checklist
Meta-analysis full info
A systematic review that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate.
- Subtitle should include "A Meta-analysis"
- Follow PRISMA Reporting Guidelines or MOOSE Reporting Guidelines
Cohort Study full info
An observational study that follows a group (cohort) of individuals who are initially free of the outcome of interest. Individuals in the cohort may share some underlying characteristic, such as age, sex, diagnosis, exposure to a risk factor, or treatment.
- Follow STROBE Reporting Guidelines
Case-Control Study full info
An observational study designed to determine the association between an exposure and outcome in which study participants are selected by outcome. Those with the outcome (cases) are compared with those without the outcome (controls) with respect to an exposure or event. Cases and controls may be matched according to specific characteristics (eg, age, sex, or duration of disease).
Cross-sectional Study full info
An observational study of a defined population at a single point in time or during a specific interval, in which exposure and outcome are ascertained simultaneously.
Case Series full info
An observational study that describes a selected group of participants with similar exposure or treatment and without a control group. A case series may also involve observation of larger units such as groups of hospitals or municipalities, as well as smaller units such as laboratory samples.
- Follow Reporting Guidelines
Economic Evaluation full info
A study using formal, quantitative methods to compare 2 or more treatments, programs, or strategies with respect to their resource use and expected outcomes. This includes cost-effectiveness, cost-benefit, and cost-minimization analyses.
- Follow CHEERS Reporting Guidelines
Decision Analytical Model full info
A mathematical modeling study that compares consequences of decision options by synthesizing information from multiple sources and applying mathematical simulation techniques, usually with specific software. Reporting should address the relevant non-cost aspects of the CHEERS guideline.
Comparative Effectiveness Research full info
A study that compares different interventions or strategies to prevent, diagnose, treat, and monitor health conditions to determine which work best for which patients, under what circumstances, and are associated with the greatest benefits and harms.
- Follow ISPOR Reporting Guidelines
Genetic Association Study full info
A study that attempts to identify and characterize genomic variants that may be associated with susceptibility to multifactorial disease.
- Follow STREGA Reporting Guidelines
Diagnostic/Prognostic Study full info
A prospective study designed to develop, validate, or update the diagnostic or prognostic accuracy of a test or model.
- Follow STARD Reporting Guidelines or TRIPOD Reporting Guidelines
Quality Improvement Study full info
A study that uses data to define, measure, and evaluate a health care practice or service to maintain or improve the appropriateness, quality, safety, or value of that practice or service.
- Follow SQUIRE Reporting Guidelines
Survey Study full info
A survey study includes a representative sample of individuals who are asked to describe their opinions, attitudes, or behaviors. Survey studies should have sufficient response rates (generally ≥60%) and appropriate characterization of nonresponders to ensure that nonresponse bias does not threaten the validity of the findings.
- Follow AAPOR Best Practices for Survey Research
- Optional: Survey instrument as supplemental file
Qualitative Study full info
A study based on observation and interview with individuals that uses inductive reasoning and a theoretical sampling model and that focuses on social and interpreted, rather than quantifiable, phenomena and aims to discover, interpret, and describe rather than to test and evaluate. This includes mixed-methods studies that combine quantitative and qualitative designs in a sequential or concurrent manner.
- Follow SRQR Reporting Guidelines or COREQ Reporting Guidelines
These reports typically include randomized trials (see Clinical Trial ), intervention studies, cohort studies, case-control studies, epidemiologic assessments, other observational studies, surveys with high response rates (see Reports of Survey Research ), cost-effectiveness analyses and decision analyses (see Reports of Cost-effectiveness Analyses and Decision Analyses ), and studies of screening and diagnostic tests (see also Reports of Diagnostic Tests ). Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are original research reports, preferably clinical trials, or systematic reviews (see above classifications for manuscript submission requirements by category of article) that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care. Manuscripts that provide new insights into the diagnosis, prognosis, and treatment of critically ill patients, as well as those that explore pathophysiological, technological, ethical, or other related aspects of critical care medicine, are welcome. Follow EQUATOR Reporting Guidelines . For reports of original data and systematic reviews, a structured abstract is required; see instructions for preparing Abstracts for Reports of Original Data or Abstracts for Reviews . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are short reports of original studies or evaluations or unique, first-time reports of clinical case series. Follow EQUATOR Reporting Guidelines . A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Recommended length: 1200 words (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 3 tables and/or figures and no more than 15 references. Note: It is very rare for this journal to publish case reports.
Research Letters are concise, focused reports of original research. These should not exceed 600 words of text and 6 references and may include up to 2 tables or figures. Online supplementary material is only allowed for brief additional and absolutely necessary methods but not for any additional results or discussion. The text should include the full name, academic degrees, and institutional affiliation for each author and the email address for the corresponding author. Other persons who have contributed to the study may be indicated in an Acknowledgment, with their permission, including their academic degrees, affiliation, contribution to the study, and an indication if compensation was received for their role. Letters must not duplicate other material published or submitted for publication. In general, Research Letters should be divided into the following sections: Introduction, Methods, Results, and Discussion. They should not include an abstract or key points, but otherwise should follow all of the guidelines in Manuscript Preparation and Submission Requirements . Letters not meeting these specifications are generally not considered.
This article type requires a presubmission inquiry to [email protected] .
The journal will consider 2 types of review articles:
Systematic Reviews
These types of Review articles differ by the scope and level of analysis of the literature searches and the titles used. Systematic Reviews require a complete systematic search of the literature using multiple databases, covering many years, and grading of the quality of the cited evidence. Narrative Reviews do not require a rigorous literature search but should rely on evidence and should be written by established experts in the field. See below for more detail on each type of Review.
Titles for these Reviews should include a concise description of the main topic. Use specific and not overly broad wording for the title; the type of review should be indicated in the subtitle. For example:
Behavioral Treatment of Obesity: A Systematic Review
Behavioral Treatment of Obesity: A Review (note: the word "narrative" is not included in the subtitle)
Systematic Reviews are critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention. Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ). Systematic Reviews should address a specific question or issue that is relevant for clinical practice and provide an evidence-based, balanced, patient-oriented review on a focused topic. Follow EQUATOR Reporting Guidelines .
The basic structure of manuscripts reporting Systematic Reviews should include the following: Abstract (structured abstract of no more than 350 words); Introduction (150-250 words); Methods (150-250 words); Results (1000-1250 words, with the following subsections, if appropriate, depending on the specific question or issue addressed: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); Discussion (1000 words); and Conclusions (2-3 sentences).
Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. For an example of a published Systematic Review, see JAMA . 2014;312(6):631-640 and below for the general structure of a Systematic Review article.
Prospective authors interested in submitting a review manuscript should prepare a detailed outline of the proposed article. There should also be a brief summary of the extent and quality of the literature supporting the proposed review. Alternatively, if a draft of the manuscript has been completed, this can be sent. Prospective authors should also summarize their publication record in the field. Send this information to the editorial office via email to Mary McDermott, MD, at [email protected] .
Specific Components of a Systematic Review
Key Points (75-100 words)
This feature provides a quick structured synopsis of the Review, following 3 key points: Question, Findings, and Meaning. Limit to no more than 100 words. This is different from the Abstract.
Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Abstract (350 words)
A structured abstract is required; Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Introduction (150-250 words)
The first 2 to 3 sentences of the Introduction should draw in readers such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). The epidemiology of the disease or condition should be briefly summarized and generally should include disease prevalence and incidence. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments reported in trials with a minimum follow-up of 2 years including 80% of the original cohort).
Methods/Literature Search (150-250 words)
The literature search should be as current as possible, ideally with end dates within a month or two before manuscript submission. A search of the primary literature should be conducted, including multiple bibliographic databases (eg, PubMed/MEDLINE, Embase, CINAHL, PsycINFO). This can be facilitated by collaborating with a medical librarian to help with the search.
Briefly describe characteristics of the literature searched and included in the review, following the PRISMA reporting guidelines , including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, screening process, language limitations, and inclusion and exclusion criteria. The rating system used to evaluate the quality of the evidence should be specified (see table below) and the methods used to evaluate quality should be described, including number of quality raters, how agreement on quality ratings was assessed, and how disagreements on quality ratings were resolved.
The highest-quality evidence (eg, randomized clinical trials, meta-analyses, systematic reviews, and high-quality prospective cohort studies) should receive the greatest emphasis. Clinical practice guidelines ordinarily should not be used as a primary component of the evidence base for the systematic review, although relevant guidelines should be addressed in the Discussion section of the article.
The search methods should be described in sufficient detail so the search can be reproduced based on the information provided in the manuscript. A summary of the methods of the literature search including this information should be included in the main article; details can be included in an online-only supplement. A PRISMA-style flow diagram showing this information should also be included as an online-only supplement. In addition, a completed PRISMA checklist should be submitted for the items completed that apply to systematic reviews (the checklist items that apply to meta-analyses do not need to be completed for systematic reviews without meta-analysis). The checklist will be used during review but will not be published.
Results (1000-1250 words)
First, briefly report the results of the literature search, including the number of articles reviewed and included, numbers of various types of studies (eg, clinical trials, cohort studies) included, and the aggregate numbers of patients included in the reviewed studies. Also provide a brief summary of the quality of the evidence. Details of this information can be included in a PRISMA-style flow diagram and table(s).
Next, the subsections listed below should generally appear in the Results sections of most Reviews although all of these subsections may not be necessary for some topics, depending on the specific question or issue addressed. The word counts following each subsection are suggested to assist with keeping the overall Results section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a clinician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review of the literature, either performed by the author of the Review or published in the form of a high-quality review or guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included. Discussion (Approximately 1000 words)
Key findings should be summarized in the first paragraph of the Discussion section. All statements made should be supported by evidence. It is very important to not simply list findings from the studies reviewed. This information is best presented in tables. The Discussion should provide a critical synthesis of data and information based on the results of the review, an assessment of the quality of studies summarized, and a description of how studies can be interpreted and used to guide clinical practice. The limitations of the evidence and of the review should be discussed, and gaps in evidence should be addressed. A discussion of controversial or unresolved issues and topics in need of future research also should be included.
Clinical Practice Guidelines: In the Discussion section, describe current clinical practice guidelines, relevant to the topic of the review, if available, and whether the conclusions of this review agree with, or disagree with, the current clinical practice guidelines. If this is done and there is more than 1 guideline, a table should be prepared comparing the major features that differ between the guidelines. Guideline quality should be discussed using the standards outlined for the JAMA Clinical Guidelines Synopsis .
Conclusions
Include a 2- to 3-sentence summary of the major conclusions of the review.
Construct tables that summarize the search results. Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns should include the name of the treatment, strength of evidence supporting the treatment, the treatment's effect (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Ratings of the quality of the evidence. Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
| Quality Rating Scheme for Studies and Other Evidence | |
|---|---|
| 1 | Properly powered and conducted randomized clinical trial; systematic review with meta-analysis |
| 2 | Well-designed controlled trial without randomization; prospective comparative cohort trial |
| 3 | Case-control studies; retrospective cohort study |
| 4 | Case series with or without intervention; cross-sectional study |
| 5 | Opinion of respected authorities; case reports |
There are several other preferred systems for rating the quality of evidence in Review articles. For Reviews that synthesize findings from numerous studies into a single summary recommendation, use the rating scale shown above or the Oxford Centre for Evidence-based Medicine's Levels of Evidence and Grades of Recommendation or the recommendations in the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . For reviews that include diagnostic studies, use The Rational Clinical Examination Levels of Evidence table .
Follow additional instructions for preparation and submission of Tables .
A PRISMA-style flow diagram should be included as an online supplement that summarizes the results of the literature search and the numbers of articles/records/studies and patients/participants represented in the studies identified, screened, eligible, and included in the final review.
Additional figures that illustrate pathophysiology or clinical presentation may be considered. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Follow additional instructions for preparation and submission of Figures and Video .
Narrative Reviews on clinical topics provide an up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines. The focus of Narrative Reviews will be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment. These reviews should address a specific question or issue that is relevant for clinical practice. Narrative Reviews do not require (but may include) a systematic review of the literature search. Recommendations should be supported with evidence and should rely on recent systematic reviews and guidelines, if available, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
The basic structure of manuscripts reporting Narrative Reviews should include the following: Abstract (structured abstract of no more than 300 words); Introduction (150-250 words); Methods, if included (150-250 words); Discussion/Observations (1000-1250 words, with the following subsections, if appropriate: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); and Conclusions (2-3 sentences).
Typical length: 2000-3500 words (maximum), with no more than a total of 5 tables and/or figures, and no more than 50-75 references. For an example of this type of article, see JAMA . 2015;314(23):2544-2554 .
Specific Components of a Narrative Review
Abstract (300 words)
Narrative Review articles should include a 3-part structured abstract of no more than 300 words using the headings listed below:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
The first 2 to 3 sentences of the Introduction should draw in readers in such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). Briefly summarize the epidemiology of the disease. This information should include disease prevalence and incidence and perhaps discussion of the presence and frequency of any relevant subpopulations and any geographic or seasonal variations of the disease if these are relevant. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments).
Methods (150-250 words)
A Methods section is not required for Narrative Reviews, but may be included to summarize a literature search that was conducted for this Review. If included, briefly describe the characteristics of the literature searched and included in the review, including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, and any process used to evaluate the literature.
Discussion/Observations (1000-1250 words)
The principal observations of the Narrative Review generally should include the subsections listed below, although each section may not be necessary for some topics. The word counts following each subsection are suggested to assist with keeping the overall Observations section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a physician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review or a high-quality guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included.
For most Narrative Reviews, tables should be included that summarize the epidemiology, diagnostic tools, and therapies available for the disease. In some cases, these 3 topics may not all be relevant to the review topic and tables may be appropriately modified to fit the review. Include a fourth table that compares the findings of the review and current clinical practice recommendations or diagnostic and therapeutic uncertainty or controversies.
Table 1: Major epidemiologic and burden of disease facts Table 2: Major diagnostic tools available Table 3: Major therapies available Table 4: Current clinical practice recommendations and/or diagnostic and therapeutic uncertainty, and controversies
Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns may include the treatment, strength of evidence supporting the treatment, the effect of the treatment (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief explanatory comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Figures that illustrate pathophysiology or clinical presentation may be included. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Note: This journal publishes very few of these types of articles. These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
A structured abstract is required. Maximum length: 3000 words of text (not including tables, figures, or references) with no more than a total of 4 tables and/or figures and no more than 50 references. For a recently published example, see JAMA . 2019;322(20):1996-2016 .
Clinical Challenge presents an actual patient scenario about a specific disease or condition with an accompanying clinical image.
Authors should provide 4 single-phrase plausible treatment options describing possible courses of action with one of these being the most correct response for the question "What Would You Do Next?" Manuscripts should include a brief discussion of the relevant clinical issues and provide well-supported (evidence-based) explanations discussing the 4 potential courses of action. For a recently published example, see JAMA . 2022;327(24):2448-2449. doi:10.1001/jama.2022.8384 .
All diagnostic and treatment recommendations should be supported by referencing recent authoritative texts or journal articles. Preferably, these recommendations should be supported by governmental or multisociety guidelines, clinical trials, meta-analyses, or systematic reviews. The text should have a maximum length of 850 words, consisting of no more than 250 words for the case presentation, question, and 4 one-sentence answers, followed by no more than 600 words that include the diagnosis and a brief discussion. There should be no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. There should be no more than 10 references, and no more than 2 small figures totaling 3 image components (Figure 1, with no more than 2 components, for the case presentation; and Figure 2, with no more than 1 component, for the diagnosis and discussion).
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or the first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
In addition, the JAMA Network Patient Permission form must be completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. Please read Patient Identification before submitting your manuscript.
The image and case presentation should be from the same patient and must not have been published previously. In some cases, additional figures may be included to accompany the answer explanations (see description of additional figure(s) above). All images submitted should be high-quality .jpg or .tif files. Submit the original version of all image files at the highest resolution possible without labels. In general, the original image file should have a minimum resolution of 350 dpi at a width of about 5 inches. Do not increase the original resolution, resize, or crop the image; where applicable, we will crop to maintain patient confidentiality. If any labels, arrowheads, or A/B panel indicators are desired, provide a separate labeled version of the figure(s) for reference. All labels will be reformatted to journal style.
For more information on how to submit figures, see Figures.
We would like to receive common problems presenting uncommonly, rather than unusual or rare conditions (ie, "zebras"). These cases should be of interest to clinicians; they should be problems that clinicians are likely to encounter and have an outstanding image that illustrates the disorder and contributes to the diagnostic challenge.
Manuscripts not meeting these guidelines will not be considered.
Diagnostic Test Interpretation presents the results of a diagnostic test from a single patient and explores the clinical application of the test result. The Diagnostic Test Interpretation is intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
The diagnostic test result must be obtained from the care of an actual patient and must include that patient's written permission. The JAMA Network Patient Permission form should be read and completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. The results of laboratory, pathologic, or radiographic tests are appropriate but clinical images are not. Results of the diagnostic test of interest (and related tests) and the range of reference values should be included after the case. Authors of manuscripts based on clinical images should consult the instructions for Clinical Challenge .
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
Manuscripts for Diagnostic Test Interpretation should have the following sections:
Case presentation. The case presentation should be brief and focus on the diagnostic test in question. At the end of the case presentation the pertinent diagnostic test results and reference ranges should be provided (200 words). Include: JAMA Exclude: Specialty Journals, JNO Comments: How do you interpret these test results? How do you interpret these test results? (or What would you do next?) Four plausible responses should be provided. While most Diagnostic Test Interpretation articles will pose the question "How do you interpret these results?" a subset may more appropriately focus on the next best step regarding workup of the abnormal test result. In these cases, the question "How do you interpret these test results?" can be replaced with "What would you do next?" Either question should be presented in the format of a multiple choice question with a single correct (or best) answer. The answers may be brief phrases or short sentences, should be similar in length, and should be arranged alphabetically by first word in the answer. Response options should not describe treatments (about 50 words). Include: CAR,ONC Exclude: JAMA, DER, IMD, NEU, OPH, PED, OTO, PSY, SUR, JNO Comments: How do you interpret these test results? Test characteristics. A brief review of the diagnostic test should be provided (approximately 200 words). For biomarkers, this should include a brief description of the related physiology. Test accuracy should be reported using sensitivity and specificity or likelihood ratios, and predictive values should be provided for common clinical scenarios. Please use likelihood ratios whenever possible, since they do not depend on disease prevalence. The prevalence of the disease should be stated so that the pretest probability may be estimated. For example, "For patients with a typical disease prevalence of 10%, the predictive values of positive and negative test results are approximately 50% and 1%, respectively." Discussion of the application and utility of the diagnostic test should be based on a high-quality systematic review or authoritative practice guideline. If a more recent, original study supersedes or adds meaningfully to the prior synthesis of research, that article also should be cited. The approximate fee for the test should be provided. For example, some fees for laboratory tests can be obtained from the Medicare fee schedules . Radiology procedure fees can be found at the Medicare Physician Fee Schedule website . Application of test result to this patient. A brief discussion of how the diagnostic test result will facilitate the next steps in a patient's management should be presented. Please also address the correct answer to the question about test interpretation in this section (200 words). What Are Alternative Diagnostic Testing Approaches? If there are different testing strategies that can be used to evaluate patients to establish a diagnosis, please discuss them (100 words). Patient Outcome. Long-term follow-up (most recent as possible) regarding the patient's condition and outcome of treatment is necessary (100 words). Clinical Bottom Line. Please provide a bulleted list of 3-5 items that reflect the most important message readers should obtain from this article.
The overall text of the manuscript should have a maximum of 850 words, no more than 10 references, and no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. The case presentation must not have been previously published.
For an example of this article type, see JAMA . 2022;327(13):1284-1285. doi:10.1001/jama.2022.2037 .
If there are questions about patient identifiability, please contact the editorial office. Authors interested in submitting a manuscript for Diagnostic Test Interpretation should contact the editorial office prior to manuscript preparation and submission by sending an email to Kristin Walter at [email protected] .
Viewpoints may address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article. Viewpoints should be well focused, scholarly, and clearly presented but should not include the findings of new research or data that have not been previously published.
Viewpoints must have no more than 3 authors. Editors encourage diversity of gender, race, ethnicity, geographic location, and discipline for Viewpoint authors, and the first author should have sufficient expertise and experience with the topic to provide an authoritative opinion. The text should include the full name, academic degrees, and no more than 2 institutional affiliations for each author. Maximum length: up to 1200 words of text—or 1000 words of text with 1 small table or figure—and no more than 7 references, which should be as current as possible. Viewpoints not meeting these guidelines will not be considered.
Most essays published in A Piece of My Mind are personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession. If the patient(s) described in these manuscripts is identifiable, a Patient Permission form , which provides consent for publication, must be completed and signed by the patient(s) or family member(s) and submitted with the manuscript. Manuscripts that describe identifiable patients that do not have a signed form will not be reviewed. Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Fictional or composite accounts are not permitted.
Manuscripts are not published anonymously or pseudonymously and must have no more than 3 authors. All manuscripts must be submitted formally via the journal's manuscript submission system; we do not review drafts or unfinished manuscripts prior to submission. Length limit: 1600 words.
Poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer, will be considered. Poems should be original, not previously published or under consideration elsewhere, no longer than 44 lines, and with individual lines no longer than 55 characters (including spaces). Authors should submit each poem separately (ie, one poem per submission record, and only one author per poem). Submissions containing multiple poems will be returned with instructions to split into individual files. Do not submit artwork, music/audio, or other accompanying materials, which are not considered. All poems must be submitted online via the online manuscript submission and review system . Authors of poems that are accepted for publication are required to complete Authorship Forms and transfer copyright to the publisher as part of a publishing agreement. An email with links to the Authorship Form will be sent to authors for completion before final acceptance. Author requests to republish poems are generally granted by our permissions department following a formal request.
Questions about submitting poems (but not submissions) may be sent to [email protected] .
Letters discussing a recent article in this journal should be submitted within 4 weeks of publication of the article in print. 3 Letters received after 4 weeks will rarely be considered. Letters should not exceed 400 words of text and 5 references, 1 of which should be to the recent article. Letters may have no more than 3 authors. The text should include the full name, academic degrees, and a single institutional affiliation for each author and the email address for the corresponding author. Letters must not duplicate other material published or submitted for publication and should not include unpublished data. Letters not meeting these specifications are generally not considered. Letters being considered for publication ordinarily will be sent to the authors of the original article, who will be given the opportunity to reply. Letters will be published at the discretion of the editors and are subject to abridgement and editing for style and content. To read more about Letters, see the AMA Manual of Style .
Replies by authors should not exceed 500 words of text and 6 references. They should have no more than 3 authors.
Clinical Trial
These manuscripts include reports of Randomized Clinical Trials, Parallel-Design Double-blind Trials, Crossover Trials, Equivalence and Noninferiority Trials, Cluster Trials, and Nonrandomized Controlled Trials.
The ICMJE defines a clinical trial as any research project that prospectively assigns human participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. 4 Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like. All manuscripts reporting clinical trials, including those limited to secondary exploratory or post hoc analysis of trial outcomes, must include the following:
- Copy of the original trial protocol, including the complete statistical analysis plan and any amendments. The journal recommends using the SPIRIT reporting guidelines when preparing original protocols (see Protocols ).
- CONSORT flow diagram (see Figure ).
- Completed trial checklist (see Checklist ).
- Registry at an appropriate online public clinical trial registry (see Trial Registration requirements).
- A Data Sharing Statement to indicate if data will be shared or not. Specific questions regarding the sharing of data are included in the manuscript submission system.
For additional guidance on reporting Randomized Clinical Trial, Parallel-Design Double-blind Trial, Crossover Trial, Equivalence and Noninferiority Trial, Cluster Trial, and Nonrandomized Controlled Trial, see Study Types .
Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria, or data sources, and how these were selected for the study); the essential features of any interventions; the primary and secondary outcome measures (consistent with those reported in the trial protocol); the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions.
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Randomized Clinical Trial" or, for Nonrandomized Controlled Trials, "A Nonrandomized Controlled Trial." To read more about clinical trials, see the AMA Manual of Style .
Trial Registration:
In concert with the ICMJE, JAMA Network requires, as a condition of consideration for publication, registration of all trials in a public trials registry that is acceptable to the ICMJE (ie, the registry must be owned by a not-for-profit entity, be publicly accessible, and require the minimum registration data set as described by ICMJE). 4 , 8 , 9
Acceptable trial registries include the following and others listed at http://www.icmje.org :
- anzctr.org.au
- clinicaltrials.gov
- trialregister.nl
- umin.ac.jp/ctr
All clinical trials, regardless of when they were completed, and secondary analyses of original clinical trials must be registered before submission of a manuscript based on the trial. Secondary data analyses of primary (parent) clinical trials should not be registered as separate clinical trials, but instead should reference the trial registration number of the primary trial. Please note: for clinical trials starting patient enrollment after July 2005, trials must have been registered before onset of patient enrollment. For trials that began before July 2005 but that were not registered before September 13, 2005, trials must have been registered before journal submission. Trial registry name, registration identification number, and the URL for the registry should be included at the end of the abstract and also in the space provided on the online manuscript submission form.
Authors of manuscripts reporting clinical trials must submit trial protocols (including the complete statistical analysis plan) along with their manuscripts. Protocols in non-English languages should be translated into English. This should include the original approved protocol and statistical analysis plan, and all subsequent amendments to either document. Do not submit a summary version that was published as an article in another journal. If the manuscript is accepted, the protocol and statistical analysis plan will be published as a supplement.
CONSORT Flow Diagram and Checklist:
Manuscripts reporting the results of randomized trials must include the CONSORT flow diagram showing the progress of patients throughout the trial. The CONSORT checklist also should be completed and submitted with the manuscript. 10
Figure. Profile of a Randomized Clinical Trial

Trial Protocol
These manuscripts are documents that describe the organization and plan for a randomized clinical trial, including the trial's objective(s), design, methodology, all outcomes to be measured, and statistical analysis plan. All trial protocol manuscripts must include a copy of the trial protocol including the complete statistical analysis plan (see Protocols ). All clinical trials that have begun randomization must be registered at an appropriate online public registry (see Trial Registration requirements). Follow SPIRIT Reporting Guidelines .
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Trial Protocols . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Trial Protocol."
These manuscripts are systematic, critical assessments of literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention, and that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate. All articles or data sources should be searched for and selected systematically for inclusion and critically evaluated, and the search and selection process should be described in the manuscript. The specific type of study or analysis, population, intervention, exposure, and tests or outcomes should be described for each article or data source. The data sources should be as current as possible, ideally with the search having been conducted within several months of manuscript submission. Authors of reports of meta-analyses of clinical trials should submit the PRISMA flow diagram and checklist . Authors of meta-analyses of observational studies should submit the MOOSE checklist . Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Meta-analysis . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Meta-analysis." To read more about meta-analyses, see the AMA Manual of Style .
Other Observational Studies
These manuscripts include Cohort Study, Case-Control Study, Cross-sectional Study, Case Series, Economic Evaluation, Decision Analytical Model, Comparative Effectiveness Research, Genetic Association Study, Diagnostic/Prognostic Study, Quality Improvement Study, Survey Study, and Qualitative Study. Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions or exposures; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references.
Format My Manuscript
Manuscript preparation and submission requirements.
All manuscripts must be submitted online via the online manuscript submission and review system .
At the time of submission, complete contact information (affiliation, postal/mail address, email address, and telephone numbers) for the corresponding author is required. First and last names, email addresses, and institutional affiliations of all coauthors are also required. After the manuscript is submitted, the corresponding author will receive an acknowledgment confirming receipt and a manuscript number. Authors will be able to track the status of their manuscripts via the online system. After manuscript submission, all authors of papers under consideration for publication will be sent a link to the Authorship Form to complete and submit. See other details in these instructions for additional requirements. 2 , 4
As recommended by the ICMJE, "if the manuscript has been submitted previously to another journal, it is helpful to include the previous editors' and reviewers' comments with the submitted manuscript, along with the authors' responses to those comments." 4 It is not uncommon for manuscripts to have been submitted to and peer reviewed by other journals and sharing this information will not bias an editor's decision for this journal. Thus, authors are encouraged to submit these previous comments in their entirety and indicate how they have revised the manuscript in response to these comments, which may expedite the review process. In the submission system, there is a file type for Previous Peer Review and Editorial Comments.
Include a cover letter and complete contact information for the corresponding author (affiliation, postal/mail address, email address, and telephone number) and whether the authors have published, posted, or submitted any related papers from the same study (see Previous Publication, Related Manuscripts and Reports, and Preprints ).
Manuscripts should be prepared in accordance with the AMA Manual of Style , 11th edition, 2 and/or the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals . 4
Include in the manuscript file a title page, abstract, text, references, and as appropriate, figure legends and tables. Start each of these sections on a new page, numbered consecutively, beginning with the title page. Figures should be submitted as separate files (1 file per figure) and not included in the manuscript text.
We recommend individual file sizes of no more than 500 kB and not exceeding 1 MB, with the total size for all files not exceeding 5 MB (not including any video files).
For submission and review, please submit the manuscript as a Word document. Do not submit your manuscript in PDF format.
Use 10-, 11-, or 12-point font size, double-space text, and leave right margins unjustified (ragged).
The title page should be the first page of your manuscript file. It should include a manuscript title; the full names, highest academic degrees, and affiliations of all authors (if an author's affiliation has changed since the work was done, the new affiliation also should be listed); name and complete contact information for corresponding author; and manuscript word count (not including title, abstract, acknowledgment, references, tables, and figure legends).
Titles should be concise, specific, and informative. 2(p8) Please limit the length of titles to 100 characters (including spaces) for reports of research and other major articles and 60 characters for shorter article types such as opinion articles and Letters as well as for subtitles to major articles. For scientific manuscripts, do not use overly general titles, declarative titles, titles that include the direction of study results, or questions as titles. For reports of clinical trials, meta-analyses, and systematic reviews, include the type of study as a subtitle (eg, A Randomized Clinical Trial, A Meta-analysis, A Systematic Review). For reports of other types of research, do not include study type or design in the title or subtitle. Depending on the context, avoid inclusion of specific locations (eg, state, province, or country) and specific years. To read more about titles, see the AMA Manual of Style .
In the manuscript, include a separate section called "Key Points" before the Abstract.
This feature provides a quick structured synopsis of the findings of your manuscript (required only for research and review manuscripts), following 3 key points: Question, Findings, and Meaning. Limit this section to 75-100 words or less.
Question: Focused question based on the study hypothesis or goal/purpose. Limit to 1 sentence. Findings: Results of the study/review. Include the design (eg, clinical trial, cohort study, case-control study, meta-analysis). Focus on primary outcome(s) and finding(s). Do not emphasize secondary outcomes. Report basic numbers only but state if results are statistically significant or not significant; do not include results of statistical tests or measures of variance (see example below). Can include 1 to 2 sentences. Meaning: Key conclusion and implication based on the primary finding(s). Limit to 1 sentence. Example of Research Article Question: What is the immunogenicity of an inactivated influenza A vaccine with and without adjuvant? Findings: In this randomized clinical trial that included 980 adults, the proportion achieving an effective antibody response was 84% with adjuvant vs 2% without adjuvant, a significant difference. Meaning: In an influenza pandemic the use of an adjuvant with inactivated influenza A vaccine may be warranted. Include: All Journals except JNO and JHF Exclude: JNO and JHF Comments: Example of Review Article Example of Review Article Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Include a structured abstract for reports of original data, meta-analyses, and systematic reviews. Abstracts should be prepared in JAMA Network style—see instructions for preparing abstracts below. Abstracts are not required for Editorials, Viewpoints, and special features. No information should be reported in the abstract that does not appear in the text of the manuscript. To read more about abstracts, see the AMA Manual of Style .
Abstracts for Reports of Original Data:
Reports of original data should include an abstract of no more than 350 words using the headings listed below. For brevity, parts of the abstract may be written as phrases rather than complete sentences. Each section should include the following content:
Importance: The abstract should begin with a sentence or 2 explaining the clinical (or other) importance of the study question. Objective: State the precise objective or study question addressed in the report (eg, "To determine whether..."). If more than 1 objective is addressed, the main objective should be indicated and only key secondary objectives stated. If an a priori hypothesis was tested, it should be stated. Design: Describe the basic design of the study and include the specific study type (eg, randomized clinical trial, cohort, cross-sectional, case-control, case series, survey, meta-analysis, bibliometric analysis). State the years of the study and the duration of follow-up. For older studies (eg, those completed >3 years ago), add the date of the analysis being reported. If applicable, include the name of the study (eg, the Framingham Heart Study). As relevant, indicate whether observers were blinded to patient groupings, particularly for subjective measurements. Setting: Describe the study setting to assist readers to determine the applicability of the report to other circumstances, for example, multicenter, population-based, primary care or referral center(s), etc. Participants: State the clinical disorders, important eligibility criteria, and key sociodemographic features of patients (or other study participants). The numbers of eligible participants and how they were selected should be provided, including the number approached but who refused or were excluded. For selection procedures, these terms should be used, if appropriate: random sample (where random refers to a formal, randomized selection in which all eligible individuals have a fixed and usually equal chance of selection); population-based sample; referred sample; consecutive sample; volunteer sample; convenience sample. If matching is used for comparison groups, characteristics that are matched should be specified. In follow-up studies, the proportion of participants who completed the study must be indicated.
Note: The preceding 3 sections are usually combined for accepted papers during the editing process as "Design, Setting, and Participants," but for manuscript submission these sections should be kept separate.
Intervention(s) (for clinical trials) or Exposure(s) (for observational studies): The essential features of any interventions, or exposures, should be described, including their method and duration. The intervention, or exposure, should be named by its most common clinical name, and nonproprietary drug names should be used. Main Outcome(s) and Measure(s): Indicate the primary study outcome measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurements unfamiliar to a general medical readership. Results: Summary demographic information (eg, characteristics such as sex and age) and the number of study participants should be reported in the first sentence of the Results paragraph. The main outcomes of the study should be reported and quantified, including final included/analyzed sample. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P =.13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Measures of relative risk also may be reported (eg, relative risk, hazard ratios) and should include confidence intervals. Studies of screening and diagnostic tests should report sensitivity, specificity, and likelihood ratio. If predictive value or accuracy is reported, prevalence or pretest likelihood should be given as well. All randomized clinical trials should include the results of intention-to-treat analysis as well. In intervention studies, the number of patients withdrawn because of adverse effects should be given. Approaches such as number needed to treat to achieve a unit of benefit may be included when appropriate. All surveys should include response/participation rates. Conclusions and Relevance: Provide only conclusions of the study that are directly supported by the results. Give equal emphasis to positive and negative findings of equal scientific merit. Also, provide a statement of relevance indicating implications for clinical practice or health policy, avoiding speculation and overgeneralization. The relevance statement may also indicate whether additional study is required before the information should be used in clinical settings. Trial Registration: For clinical trials only (not nontrial observational studies), the name of the trial registry, registration number, and URL of the registry must be included. See Trial Registration .
Abstracts for Meta-analysis:
Manuscripts reporting the results of meta-analyses should include an abstract of no more than 350 words using the headings listed below. The text of the manuscript should also include a section describing the methods used for data sources, study selection, data extraction, and data synthesis. Each heading should be followed by a brief description:
Importance: A sentence or 2 explaining the importance of the systematic review question that is used to justify the meta-analysis. Objective: State the precise primary objective of the meta-analysis. Indicate whether the systematic review for the meta-analysis emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being analyzed. Data Sources: Succinctly summarize data sources, including years searched. The search should include the most current information possible, ideally with the search being conducted within several months before the date of manuscript submission. Potential sources include computerized databases and published indexes, registries, meeting abstracts, conference proceedings, references identified from bibliographies of pertinent articles and books, experts or research institutions active in the field, and companies or manufacturers of tests or agents being reviewed. If a bibliographic database is used, state the exact indexing terms used for article retrieval, including any constraints (for example, English language or human study participants). If abstract space does not permit this level of detail, summarize sources in the abstract including databases and years searched, and place the remainder of the information in the Methods section. Study Selection: Describe inclusion and exclusion criteria used to select studies for detailed review from among studies identified as relevant to the topic. Details of selection should include particular populations, interventions, outcomes, or methodological designs. The method used to apply these criteria should be specified (for example, blinded review, consensus, multiple reviewers). State the proportion of initially identified studies that met selection criteria. Data Extraction and Synthesis: Describe guidelines (eg, PRISMA , MOOSE ) used for abstracting data and assessing data quality and validity. The method by which the guidelines were applied should be stated (for example, independent extraction by multiple observers). Indicate whether data were pooled using a fixed-effect or random-effects model. Main Outcome(s) and Measure(s): Indicate the primary study outcome(s) and measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurement unfamiliar to a general medical readership. Results: Provide the number of studies and patients/participants in the analysis and state the main quantitative results of the review. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Meta-analyses should state the major outcomes that were pooled and include odds ratios or effect sizes and, if possible, sensitivity analyses. Evaluations of screening and diagnostic tests should include sensitivity, specificity, likelihood ratios, receiver operating characteristic curves, and predictive values. Assessments of prognosis should summarize survival characteristics and related variables. Major identified sources of variation between studies should be stated, including differences in treatment protocols, co-interventions, confounders, outcome measures, length of follow-up, and dropout rates. Conclusions and Relevance: The conclusions and their applications (clinical or otherwise) should be clearly stated, limiting interpretation to the domain of the review.
Abstracts for Systematic Reviews or Special Communications:
Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Abstracts for Narrative Reviews or Special Communications:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
Ratings of the quality of the evidence
Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
Do not use abbreviations in the title or abstract and limit their use in the text. Expand all abbreviations at first mention in the text. To read more about abbreviation use, see the AMA Manual of Style .
Laboratory values are expressed using conventional units of measure, with relevant Système International (SI) conversion factors expressed secondarily (in parentheses) only at first mention. Articles that contain numerous conversion factors may list them together in a paragraph at the end of the Methods section. In tables and figures, a conversion factor to SI should be presented in the footnote or legend. The metric system is preferred for the expression of length, area, mass, and volume. For more details, see the Units of Measure conversion table on the website for the AMA Manual of Style . 2
To read more about units of measure, click here .
Use nonproprietary names of drugs, devices, and other products and services, unless the specific trade name of a drug is essential to the discussion. 2(pp567-569) In such cases, use the trade name once and the generic or descriptive name thereafter. Do not include trademark symbols. To read more about names of drugs, see the AMA Manual of Style .
Authors describing genes or related structures in a manuscript should include the names and official symbols provided by the US National Center for Biotechnology Information (NCBI) or the HUGO Gene Nomenclature Committee . Before submission of a research manuscript reporting on large genomic data sets (eg, protein or DNA sequences), the data sets should be deposited in a publicly available database, such as NCBI's GenBank , and a complete accession number (and version number if appropriate) must be provided in the Methods section or Acknowledgment of the manuscript. To read more about gene nomenclature, see the AMA Manual of Style .
JAMA does not republish text, tables, figures, or other material from other publishers, except under rare circumstances. Please delete any such material and replace with originals.
The submission and publication of content created by artificial intelligence, language models, machine learning, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
Authors are responsible for the accuracy and completeness of their references and for correct text citation. Number references in the order they appear in the text; do not alphabetize. In text, tables, and legends, identify references with superscript arabic numerals. When listing references, follow AMA style and abbreviate names of journals according to the journals list in PubMed . List all authors and/or editors up to 6; if more than 6, list the first 3 followed by "et al." Note: Journal references should include the issue number in parentheses after the volume number.
Examples of reference style:
Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficileinfection. JAMA . 2014;312(17):1772-1778. Murray CJL. Maximizing antiretroviral therapy in developing countries: the dual challenge of efficiency and quality [published online December 1, 2014]. JAMA . doi:10.1001/jama.2014.16376 Centers for Medicare & Medicaid Services. CMS proposals to implement certain disclosure provisions of the Affordable Care Act. http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4221 . Accessed January 30, 2012. McPhee SJ, Winker MA, Rabow MW, Pantilat SZ, Markowitz AJ, eds. Care at the Close of Life: Evidence and Experience . New York, NY: McGraw Hill Medical; 2011.
For more examples of electronic references, click here .
Tables and Figures
Restrict tables and figures to those needed to explain and support the argument of the article and to report all outcomes identified in the Methods section. Number each table and figure and provide a descriptive title for each. Every table and figure should have an in-text citation. Verify that data are consistently reported across text, tables, figures, and supplementary material.
See also Tables and Figures .
Frequency data should be reported as "No. (%)," not as percentages alone (exception, sample sizes exceeding ~10,000). Whenever possible, proportions and percentages should be accompanied by the actual numerator and denominator from which they were derived. This is particularly important when the sample size is less than 100. Do not use decimal places (ie, xx%, not xx.xx%) if the sample size is less than 100. Tables that include results from multivariable regression models should focus on the primary results. Provide the unadjusted and adjusted results for the primary exposure(s) or comparison(s) of interest. If a more detailed description of the model is required, consider providing the additional unadjusted and adjusted results in supplementary tables.
Tables have a minimum of 2 columns. Comparisons must read across the table columns.
Do not duplicate data in figures and tables. For all primary outcomes noted in the Methods section, exact values with measures of uncertainty should be reported in the text or in a table and in the Abstract, and not only represented graphically in figures.
Pie charts and 3-D graphs should not be used and should be revised to alternative graph types.
Bar graphs should be used to present frequency data only (ie, numbers and rates). Avoid stacked bar charts and consider alternative formats (eg, tables or splitting bar segments into side-by-side bars) except for comparisons of distributions of ordinal data.
Summary data (eg, means, odds ratios) should be reported using data markers for point estimates, not bars, and should include error bars indicating measures of uncertainty (eg, SDs, 95% CIs). Actual values (not log-transformed values) of relative data (for example, odds ratios, hazard ratios) should be plotted on log scales.
For survival plots, include the number at risk for each group included in the analysis at intervals along the x-axis scale. For any figures in which color is used, be sure that colors are distinguishable.
All symbols, indicators, line styles, and colors in statistical graphs should be defined in a key or in the figure legend. Axes in statistical graphs must have labels. Units of measure must be provided for continuous data.
Note: All figures are re-created by journal graphics experts according to reporting standards using the JAMA Network style guide and color palette.
- Number all tables in the order of their citation in the text.
- Include a brief title for each table (a descriptive phrase, preferably no longer than 10 to 15 words).
- Include all tables at the end of the manuscript file.
- Refer to Categories of Articles for limits on the number of tables.
- NOTE: Do not embed tables as images in the manuscript file or upload tables in image formats, and do not upload tables as separate files.
Table Creation
Use the table menu in the software program used to prepare the text. Tables can be built de novo using Insert→Table or copied into the text file from another document (eg, Word, Excel, or a statistical spreadsheet).
Avoid using tabs, spaces, and hard returns to set up the table; such tables will have to be retyped, creating delays and opportunities for error.
Tables should be single-spaced and in a 10- or 12-point font (do not shrink the point size to fit the table onto the page). Do not draw extra lines or rules—the table grid will display the outlines of each cell.
Missing data and blank space in the table field (ie, an empty cell) may create ambiguity and should be avoided; use abbreviations such as NA for not applicable or not available. Each piece of data needs to be contained in its own cell. Do not try to align cells with hard returns or tabs; alignment will be imposed in the production system if the manuscript is accepted. To show an indent, add 2 spaces.
When presenting percentages, include numbers (numerator and denominator).
Include statistical variability where applicable (eg, mean [SD], median [IQR]). For additional detail on requirements for data presentation in tables, see Statistical Methods and Data Presentation .
Place each row of data in a separate row of cells, and note that No. (%) and measures of variability are presented in the same cell as in the example Table 1 below:
Table 1. Baseline Values in the Editors' Health Study

SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259.
Note that JAMA Network journals report laboratory values in conventional units. In a table, provide a footnote with the conversion factor to SI units. For a calculator of SI and conventional units, see the AMA Manual of Style . 2
To present data that span more than 1 row, merge the cells vertically. For example, in Table 2 the final column presents the P value for overall age comparisons.
Table 2. Blood Pressure Values Stratified by Age

The table should be constructed such that the primary comparison reads horizontally. For example, see Table 3 (incorrect) and Table 4 (correct).
Table 3. Patient Data by Study Group

Table 4. Patient Data by Study Group

If a table must be continued, repeat the title and column headings on the second page, followed by "(continued)."
Table Footnotes
Footnotes to tables may apply to the entire table, portions (eg, a column), or an individual entry.
The order of the footnotes is determined by the placement in the table of the item to which the footnote refers.
When both a footnote letter and reference number follow data in a table, set the superscript reference number first followed by a comma and the superscript letter.
Use superscript letters (a, b, c) to mark each footnote and be sure each footnote in the table has a corresponding note (and vice versa).
List abbreviations in the footnote section and explain any empty cells.
If relevant, add a footnote to explain why numbers may not sum to group totals or percentages do not add to 100%.
For more detail on the components and recommended structure of tables, see the AMA Manual of Style . 2
Number all figures (graphs, charts, photographs, and illustrations) in the order of their citation in the text. The number of figures should be limited. Avoid complex composite or multipart figures unless justified. See Categories of Articles for limits on the number of figures and/or tables according to article type.
For initial manuscript submissions, figures must be of sufficient quality and may be embedded at the end of the file for editorial assessment and peer review. If a revision is requested and before a manuscript is accepted, authors will be asked to provide figures that meet the requirements described in Figure File Requirements for Publication .
Graphs, charts, some illustrations, titles, legends, keys, and other elements related to figures in accepted manuscripts will be re-created and edited according to JAMA Network style and standards prior to publication. Online-only figures will not be edited or re-created (see Online-Only Supplements and Multimedia ).
Image Integrity
Preparation of scientific images (clinical images, radiographic images, micrographs, gels, etc) for publication must preserve the integrity of the image data. Digital adjustments of brightness, contrast, or color applied uniformly to an entire image are permissible as long as these adjustments do not selectively highlight, misrepresent, obscure, or eliminate specific elements in the original figure, including the background. Selective adjustments applied to individual elements in an image are not permissible. Individual elements may not be moved within an image field, deleted, or inserted from another image. Cropping may be used for efficient image display or to deidentify patients but must not misrepresent or alter interpretation of the image by selectively eliminating relevant visual information. Juxtaposition of elements from different parts of a single image or from different images, as in a composite, must be clearly indicated by the addition of dividing lines, borders, and/or panel labels.
The submission and publication of images created by artificial intelligence, machine learning tools, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
When inappropriate images or image adjustments are detected by the journal staff, authors will be asked for an explanation and will be requested to submit the image as originally captured prior to any adjustment, cropping, or labeling. Authors may be asked to resubmit the image prepared in accordance with the above standards.
Acceptable Figure Files for Initial Submission and Review
Each figure for the main article may be uploaded as a separate file or appended to the end of the manuscript with the figure titles and legends. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ). Note: If a revision is requested and before acceptance, authors must upload each figure for the main article as a separate file and follow the instructions in Figure File Requirements for Publication .
See the Table of Figure Requirements for additional guidance for specific types of figures for suggested resolution and file formats. In general each figure should be no larger than 1 MB.
Figure File Requirements for Publication
Each figure for the main article must be uploaded as a separate file. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ).
See the Table of Figure Requirements for additional guidance and file formats for specific types of figures.
Files created by vector programs are best for accurately plotting and maintaining data points. JAMA Network journals are unable to use file formats native to statistical software applications to prepare figures for publication; most statistical software programs allow users to save or export files in digital vector formats.
Images created digitally (by digital camera or electronically created illustrations) must meet the minimum resolution requirements at the time of creation. Electronically increasing the resolution of an image after creation causes a breakdown of detail and will result in an unacceptable poor-quality image. Each component of a composite image must be uploaded separately at submission and individually meet the minimum resolution requirement.
Color photographs should be submitted in RGB mode using profiles such as Adobe RGB or sRGB. Digital cameras capture images in RGB. Do not change any color settings once the file is on the computer. Black-and-white photographs (eg, radiographs, ultrasound images, CT and MRI scans, and electron micrographs) can be submitted in either RGB or grayscale modes.
Figure Titles and Legends (Captions)
At the end of the manuscript, include a title for each figure. The figure title should be a brief descriptive phrase, preferably no longer than 10 to 15 words. A figure legend (caption) can be used for a brief explanation of the figure or markers if needed and expansion of abbreviations. For photomicrographs, include the type of specimen, original magnification or a scale bar, and stain in the legend. For gross pathology specimens, label any rulers with unit of measure. Digitally enhanced images must be clearly identified in the figure legends as enhanced or manipulated, eg, computed tomographic scans, magnetic resonance images, photographs, photomicrographs, x-ray films.
Figures With Labels, Arrows, or Other Markers
Photographs, clinical images, photomicrographs, gel electrophoresis, and other types that include labels, arrows, or other markers must be submitted in 2 versions: one version with the markers and one without. Provide an explanation for all labels, arrows, or other markers in the figure legend. The Figure field in the File Description tab of the manuscript submission system allows for uploading of 2 versions of the same figure.
Number of Figures
Refer to Categories of Articles because there may be a limit on the number of figures by article type.
General Figure Guidelines
- Primary outcome data should not be presented in figures alone. Exact values with measure of variability should be reported in the text or table as well as in the abstract.
- All symbols, indicators (including error bars), line styles, colors, and abbreviations should be defined in a legend.
- Each axis on a statistical graph must have a label and units of measure should be labeled.
- Do not use pie charts, 3-D graphs, and stacked bar charts as these are not appropriate for accurate statistical presentation of data and should be revised to another figure type or converted to a table.
- Error bars should be included in both directions, unless only 1-sided variability was calculated.
- Values for ratio data—odds ratios, relative risks, hazard ratios—should be plotted on a log scale. Values for ratio data should not be log transformed.
- For footnotes, use letters (a, b, c, etc) not symbols.
- Do not submit figures with more than 4 panels unless otherwise justified.
- See the AMA Manual of Style for more guidance on figure types and components.
For images featuring patients or other identifiable persons, it is not acceptable to use black bars across the eyes in an attempt to deidentify. Cropping may be acceptable as long as the condition under discussion is clearly visible and necessary anatomic landmarks display. If the person in the image is possibly identifiable (not only by others but also by her/himself), permission for publication is required (see Patient Identification ).
Table of Figure Requirements

To present frequency data (numbers or percentages). Each bar represents a category.
Bar graphs are typically vertical but when categories have long titles or there are many of them, they may run horizontally.
The scale on the frequency axis should begin at 0, and the axis should not be broken.
If the data plotted are a percentage or rate, error bars may be used to show statistical variability.
Acceptable File Formats for Initial Submission: .ai, .bmp, .docx, .emf, .eps, .jpg, .pdf, .ppt, .psd, .tif, .wmf, .xls
Acceptable File Formats for Revision and Publication: .ai, .emf, .eps, .pdf, .wmf, .xls

To demonstrate the relationship between 2 or more quantitative variables, such as changes over time.
The dependent variable appears on the vertical axis (y) and the independent variable on the horizontal axis (x); the axes should be continuous, not broken.
Flow diagram

To show participant recruitment and follow-up or inclusions and exclusions (such as in a systematic review).
Acceptable File Formats for Initial Submission: .ai, .docx, .emf, .eps, .jpg, .pdf, .ppt
Acceptable File Formats for Revision and Publication: .ai, .docx, .emf, .eps, .pdf
Survival plot

To display the proportion or percentage of individuals (represented on the y-axis) remaining free of or experiencing a specific outcome over time (represented on the x-axis).
The curve should be drawn as a step function (not smoothed).
The number of individuals followed up for each time interval (number at risk) should be shown underneath the x-axis.
Box-and-whisker plot (box plot)

To show data distribution from 1 or more groups, particularly aggregate/summary data.
Each element should be described (the ends of the boxes, the middle line, and the whiskers). Data points that fall beyond the whiskers are typically shown as circles.
Forest plot

To illustrate summary data, particularly in meta-analyses and systematic reviews.
The data are presented both tabularly and graphically.
The sources (with years and citations, when relevant) should comprise the first column.
Provide indicators of both directions of results at the top of the plot on either side of the vertical line (eg, favors intervention).
Typically, proportionally sized boxes represent the weight of each study and a diamond shows the overall effect at the bottom of the plot.

To display quantitative data other than counts or frequencies on a single scaled axis according to categories on a baseline (horizontal or vertical). Point estimates are represented by discrete data markers, preferably with error bars (in both directions) to designate variability.
Scatterplot

To show individual data points plotted according to coordinate values with continuous, quantitative x- and y-axis scales.
A curve that is generated mathematically may be fitted to the data to summarize the relationship among the variables.
Illustration

To explain physiological mechanisms, describe clinical maneuvers and surgical techniques, or provide orientation to medical imaging.
Required minimum resolution for publication: ≥350 ppi
Acceptable File Formats for Initial Submission: .ai, .docx, .eps, .jpg, .pdf, .ppt, .psd., tif
Acceptable File Formats for Revision and Publication: .ai, .eps, .jpg, .pdf, .psd, .tif
Photographs and other clinical images

To display clinical findings, experimental results, or clinical procedures, including medical imaging, photomicrographs, clinical photographs, and photographs of biopsy specimens.
Legends for photomicrographs should include details about the type of stain used and magnification.
Acceptable File Formats for Initial Submission: .eps, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .eps, .jpg, .psd, .tif
Line drawings

To illustrate anatomy or procedures.
Line drawings are almost always black and white.
Required minimum resolution for publication: ≥600 ppi
Acceptable File Formats for Initial Submission: .docx, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .jpg, .psd, .tif
Authors may submit supporting material to accompany their article for online-only publication when there is insufficient space to include the material in the print article. This material should be important to the understanding and interpretation of the report and should not repeat material in the print article. The amount of online-only material should be limited and justified. Online-only material should be original and not previously published.
Online-only material will undergo editorial and peer review with the main manuscript. If the manuscript is accepted for publication and if the online-only material is deemed appropriate for publication by the editors, it will be posted online at the time of publication of the article as additional material provided by the authors. This material will not be edited or formatted; thus, authors are responsible for the accuracy and presentation of all such material.
Online-only material should be submitted in a single Word document with pages numbered consecutively. Each element included in the online-only material should be cited in the text of the main manuscript (eg, eTable in the Supplement) and numbered in order of citation in the text (eg, eTable 1, eTable 2, eFigure 1, eFigure 2, eMethods). The first page of the online-only document should list the number and title of each element included in the document.
Online-Only Text
Online-only text should be set in Times New Roman font, 10 point in size, and single-spaced. The main heading of the online-only text should be in 12 point and boldface; subheadings should be in 10 point and boldface.
Online-Only References
All references cited within the online-only document must be included in a separate reference section, including those that also were cited in the main manuscript. They should be formatted just as in the main manuscript and numbered and cited consecutively in the online-only material.
Online-Only Tables
Online-only tables should be inserted in the document and numbered consecutively according to the order of citation as eTable 1, eTable 2, etc. All online-only tables should be cited in the relevant text of the main manuscript. The text and data in online tables should be Arial font, 10 point in size, and single-spaced. The table title should be set in Arial font, 12 point, and bold. Headings within tables should be set in 10 point and bold. Table footnotes should be set in 8 point and single-spaced. See also instructions for Tables above. If a table runs on to subsequent pages, repeat the column headers at the top of each page. Wide tables may be presented using a landscape orientation.
If data are better displayed in a separate Excel file, this can be submitted, provided that the Excel file is cited as an eTable and is numbered in the order cited in the text. If multiple Excel files of data are submitted, these should be placed in a single Excel file, with multiple tabs (sheets) at the bottom of the file. The first tab (sheet) should include a table of contents with eTable numbers and titles, and the subsequent tabs (sheets) should be labeled as eTable 1, eTable 2, etc. Please note: the journal is not a data repository; large data sets should be deposited into publicly accessible data repositories, and a link should be provided in the Methods or Results section and the Data Sharing Statement .
Online-Only Figures
Online-only figures should be inserted in the document and numbered consecutively according to the order of citation as eFigure 1, eFigure 2, etc. All online-only figures should be cited in the relevant text of the main manuscript. Figure titles should be set in Arial font, 12 point, bold, and single-spaced. Text within figures should be set as Arial font, 10 point. Figure legends should be set in 8 point and single-spaced. Graphs and diagrams should be exported directly out of the software application used to create them in a vector file format, such as .wmf, and then inserted into the Word document. Image file formats such as .jpg, .tif, and .gif are generally not suitable for graphs. Photographs, including all radiological images, should be prepared as .jpg (highest option) or .tif (uncompressed) files at a resolution of 300 dpi and width of 3-5 inches, but the resolution of photographic files with an original resolution <300 dpi should not be increased digitally to achieve a 300-dpi resolution. Photographs should be inserted in the document with the "Link to File" button turned off. Wide figures may be presented using a landscape orientation.
For editorial and review of an initial submission, submit videos according to the following specifications:
- Acceptable file formats: .mov, .wmv, .mpg, .mpeg, .mp4, or .avi
- Maximum file size: ≤25 MB
- Preferred dimensions: 1920x1080 (HD) or greater (4k UHD footage is acceptable)
- Minimum dimensions: 640 pixels wide by 360 pixels deep
- Recommended frame rate: 24 fps (or 23.976 fps), 25 and 30 fps (or 29.97 fps)
- Maximum length: ≤5 minutes
- Desired aspect ratio: 4:3 (standard) or 16:9 (widescreen)
- If compression is required to reduce file size for uploading, please use a minimum bit rate of 10,000 kbit/s – 20,000 kbit/s
- When filming, please use a landscape orientation, not a portrait orientation. This is especially important when filming video or taking photographs with a smartphone or a mobile device.
Verify that the videos are viewable in QuickTime or Windows Media Player before uploading.
For each video, provide an in-text citation (eg, Video 1). At the end of the manuscript file, include a title (a brief phrase, preferably no longer than 10 to 15 words) and a caption that includes the file format and a brief explanation for each video. The same title and caption must be entered in the designated fields in the manuscript submission system when uploading each video. If multiple video files are submitted, number them in the order in which they should be viewed.
If patient(s) are identifiable in the video, authors must submit a Patient Permission form completed and signed by each patient. See also Patient Identification .
If the author does not hold copyright to the video, the author must obtain permission for the video to be published in the journal. This permission must be for unrestricted use in all print, online, and licensed versions of the journal.
NOTE: If your manuscript and accompanying videos are accepted for publication, the video files will be placed into a journal video frame and will be edited by JAMA Network video production staff according to journal style. In addition, a JAMA Network staff person may contact you to resubmit your videos to meet our production specifications. For example, a larger size may be needed, and if your videos were submitted with embedded text such as titles, annotations, labels, or captions, we will ask you to remove the text at this stage and resubmit the video without text, and JAMA Network video production will re-create all text using our house style.
Guidelines for Optimal Video Quality
- Use plenty of diffuse light; avoid shadows.
- Use the appropriate white-balance based on your lighting conditions. Different cameras have different settings, but most have presets for incandescent (yellow) light, fluorescent light, daylight, and tungsten light. Please make sure to select the correct one so that the color of your footage renders accurately.
- Do not overexpose the image; a bit underexposed is preferable.
- Use a tripod. This is especially important in close-ups.
- Avoid excessive zooming. Use the optical zoom only; do not use a digital zoom.
- Turn off all camera special effects.
- Avoid using autofocus. Manual focus is more accurate. Keep the camera at a fixed distance from the subject.
- Instruct people on camera to speak clearly and face the camera when speaking. Try to avoid large movements while speaking or immediately after speaking. Allow pauses before and after speaking for easier editing.
- If the situation permits, ensure that individuals being filmed are not wearing white clothing or clothing with busy patterns or stripes, especially shirts, jackets, and ties. Subdued medium blue, brown, tan, beige, and green colors all work well for shirt and clothing choices.
- Do not include an introduction by the physician as a "talking head" explaining a procedure. All footage should be of the procedure or relevant subject matter only.
- Record a few extra seconds before and after each cut or after changing the camera's position. This allows for easier editing.
Additional Considerations for Filming Surgical Procedures
- Coordinate with the surgical staff to establish a vantage point for the camera that has a clear view of the surgical field.
- Before the procedure, if the situation permits, identify the surgical staff's positions for access into and out of the surgical field to ensure there is no immediate obstruction of the camera.
- During the procedure, avoid typical obstructions of the camera's main view such as arms reaching across the field or soiled surgical sponges. Where possible, keep the heads, hands, and any instruments away from the immediate sightline of the camera. This will ensure that all moments of the procedure are captured in full view and focus.
- If the situation permits a choice of glove type, use brown or tan. White gloves reflect bright light; vividly colored surgical gloves can distract the viewer from the teaching point of the video.
- If the situation permits, avoid rapid movements for procedural steps that should be noticed and understood. To demonstrate a key moment or use of an instrument, movement that is deliberate and steady will allow a standard camera to focus properly.
For editorial and review of an initial submission, submit audio files according to the following minimum requirements:
- Acceptable file formats: .mp3, .wav, or .aiff
- Maximum file size: 25 MB
- To achieve the best quality, use a setting of 256 kbps or higher for stereo or 128 kbps or higher for mono.
- Sampling rate should be either 44.1 kHz or 48 kHz.
- Bit rate should be either 16 or 24 bit.
- To avoid audible clipping noise, please make sure that audio levels do not exceed 0 dBFS.
For each audio file, provide an in-text citation. At the end of the manuscript, include a title (a brief phrase, preferably no longer than 10-15 words) and a caption that includes the file format and a brief explanation for each audio.
NOTE: If your manuscript is accepted for publication, JAMA Network video production staff may contact you to request an original uncompressed audio file in .wav or .aiff format. There is no maximum file size requirement for publication at this stage.
After Submission
Authors will be sent notifications of the receipt of manuscripts and editorial decisions by email. During the review process, authors can check the status of their submitted manuscript via the online manuscript submission and review system . Authors should not disclose the fact that their manuscript has been submitted to anyone, except coauthors and contributors, without permission of the editor.
All submitted manuscripts are reviewed initially by one of the editors. Manuscripts are evaluated according to the following criteria: material is original and timely, writing is clear, study methods are appropriate, data are valid, conclusions are reasonable and supported by the data, information is important, and topic has general interest to readers of this journal. From these basic criteria, the editors assess a paper's eligibility for publication. Manuscripts with insufficient priority for publication are rejected promptly. Other manuscripts are sent to expert consultants for peer review. The journal uses a single-anonymized peer review process: peer reviewer identities are kept confidential (unless reviewers choose to reveal their names in their formal reviews); author identities are made known to reviewers. The existence of a manuscript under review is not revealed to anyone other than peer reviewers and editorial staff. Peer reviewers are required to maintain confidentiality about the manuscripts they review and must not divulge any information about a specific manuscript or its content to any third party without prior permission from the journal editors. Reviewers are instructed to not submit confidential manuscripts, abstracts, or other text into a chatbot, language model, or similar tool. At submission, authors may choose to have manuscripts that are not accepted by the journal referred to one of the JAMA Network specialty journals and/or JAMA Network Open along with reviewers' comments (if available). Information from submitted manuscripts may be systematically collected and analyzed as part of research to improve the quality of the editorial or peer review process. Identifying information remains confidential. Final decisions regarding manuscript publication are made by an editor who does not have any relevant conflicts of interest.
At the time of manuscript submission, authors may preselect the option to have their manuscript and reviewers' comments automatically referred to one of the JAMA Network specialty journals if the manuscript is not accepted by JAMA .
JAMA -EXPRESS
JAMA -EXPRESS provides rapid peer review and publication of major clinical trials and other original research studies that have immediate or public health importance. Authors who wish to have manuscripts considered for JAMA -EXPRESS should send the manuscript file and a request letter to [email protected] or call (312) 464-4444. Authors will be notified promptly whether the manuscript is approved for rapid peer review. Authors of those manuscripts determined not to qualify for rapid review may be invited to submit the manuscript for further consideration under the standard review process.
Authors may appeal decisions. All appeals are reviewed by the editor in chief, on a case-by-case basis, or a designated editor if the editor in chief is recused from the review.
After Revision/Acceptance
All authors are required to complete an Authorship Form and Publishing Agreement. See Authorship Criteria and Contributions .
Accepted manuscripts are edited in accordance with the AMA Manual of Style , 2 and returned to the corresponding author (or her/his designee) for approval. Authors are responsible for all statements made in their work, including changes made during editing and production that are authorized by the corresponding author.
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, until it is published without permission of the editor or as described in the guidance on Previous or Planned Meeting Presentaton or Release of Information and Embargo Policy .
If accepted for publication, all articles are published quickly in one of JAMA 's weekly print/online issues; selected articles are published Online First.
After Publication
Postpublication correspondence.
For accepted manuscripts, the corresponding author will be asked to respond to letters to the editor.
Reprints and e-prints may be ordered online when the edited manuscript is sent for approval to the corresponding author.
Requests to publish corrections should be sent to the editorial office. Errors and requests for corrections are reviewed by editors and authors, and, if warranted, a Correction notice summarizing the errors and corrections is published promptly and linked online to the original article, and the original article is corrected online with the date of correction. 15
First and last authors of peer-reviewed articles are eligible to receive CME credit. See CME From the JAMA Network .
About Previous Release of Information, Embargo, and Access
Manuscripts are considered with the understanding that they have not been published previously and are not under consideration by another publication.
Copies of all related or similar manuscripts and reports by the same authors (ie, those containing substantially similar content or using the same, similar, or a subset of data) that have been previously published or posted electronically or are under consideration elsewhere must be provided at the time of manuscript submission. All related previously published articles should be cited as references and described in the submitted manuscript along with explanation of how the submitted manuscript differs from the related previously published article(s).
Manuscripts that have been previously posted on a preprint server may be submitted for consideration for publication. When the manuscript is submitted, authors must provide information about the preprint, including a link to it and a description of whether the submitted manuscript has been revised or differs from the preprint.
See also Previous or Planned Meeting Presentation or Release of Information and Research Article Public Access, Depositing in Repositories, and Discoverability.
Meeting presentation: A complete manuscript submitted to the journal following or prior to presentation at a scientific meeting or publication of preliminary findings elsewhere (ie, as an abstract) is eligible for consideration for publication. Authors considering presenting or planning to present the work at an upcoming scientific meeting should indicate the name and date of the meeting on the manuscript submission form. For accepted papers, the editors may be able to coordinate publication with the meeting presentation. Authors of submitted papers, including those accepted but not yet published, should not disclose the status of such papers during such meeting presentations that occur before the work is published. Authors who present information contained in a manuscript that is under consideration by this journal during scientific or clinical meetings should not distribute complete reports (ie, copies of manuscripts) or full data presented as tables and figures to conference attendees or journalists. Publication of abstracts in print and online conference proceedings, as well as posting of slides or videos from the scientific presentation on the meeting website, is acceptable. However, for manuscripts under consideration by this journal, publication of full reports in meeting proceedings or online, issuing detailed news releases reporting the results of the study that go beyond the meeting abstract, or participation in formal news conferences will ordinarily jeopardize chances for publication of the submitted manuscript in this journal. 5 Media coverage of presentations at scientific meetings will not jeopardize consideration, but direct release of information through press releases or news media briefings may preclude consideration of the manuscript by this journal. 5 Rare instances of papers reporting public health emergencies should be discussed with the editor. Authors submitting manuscripts or letters to the editor regarding adverse drug or medical device reactions, reportable diseases, etc, should also report this information to the relevant government agency.
Authors should not release information about accepted manuscripts via social media until publication.
See also Previous Publication, Related Manuscripts and Reports, and Preprints . For more information, see the AMA Manual of Style .
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, without permission of the editor until it is published. All information regarding the content and publication date of accepted manuscripts is strictly confidential. Unauthorized prepublication release of accepted manuscripts and information about planned publication date may result in rescinding the acceptance and rejecting the paper. This policy applies to all categories of articles, including research, review, opinion, correspondence, etc. Information contained in or about accepted articles cannot appear in print, audio, video, or digital form or be released by the news media until the specified embargo release date. 2 , 5 See also Previous or Planned Meeting Presentation or Release of Information .
The journal makes all JAMA research articles free public access 6 months after publication on the journal website.
Authors of research articles may deposit the accepted version (ie, the peer-reviewed manuscript that you submitted on which this decision is based) of the manuscript in a repository of your choice on or after the date of publication provided that it links to the final published version on the journal website. You may not deposit the published article (version of record), which is the final copyedited, formatted, and proofed version published by the journal. The journal will deposit a copy of the published research article into PubMed Central (PMC) at the time of publication, where it will be publicly available 6 months after publication. A few weeks after publication, you may obtain your PMCID on the PMC site at: https://www.ncbi.nlm.nih.gov/pmc/pmctopmid/ . These options apply only to research articles. Non-research articles may not be deposited into repositories.
In addition, the journal will add metadata to all articles to ensure web-based search engine discoverability and will provide publicly discoverable information about your article to PubMed/Medline and numerous other bibliographic databases on the day of publication.
Author Responsibilities
Most of the JAMA Network journals' editorial policies for authors are summarized in these instructions. Citations and links to the AMA Manual of Style: A Guide for Authors and Editors 2 and other publications with additional information are also provided.
Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. 2 One or more authors should take responsibility for the integrity of the work as a whole, from inception to published article. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE), 4 authorship credit should be based on the following 4 criteria:
- substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; and
- drafting of the work or reviewing it critically for important intellectual content; and
- final approval of the version to be published; and
- agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Each author should be accountable for the parts of the work he or she has done. In addition, each author should be able to identify which coauthors are responsible for specific other parts of the work and should have confidence in the integrity of the contributions of any coauthors.
All those designated as authors should meet all 4 criteria for authorship, and all who meet the 4 criteria should be identified as authors. Those who do not meet all 4 criteria should be acknowledged (see Acknowledgment Section ).
All authors (ie, the corresponding author and each coauthor) must read, complete, and submit an electronic Authorship Form with required statements on Authorship Responsibility, Criteria, and Contributions; Confirmation of Reporting Conflicts of Interest and Funding; and Publishing Agreement. 2(pp128-133) In addition, authors are required to identify their specific contributions to the work described in the manuscript. Requests by authors to designate equal contributions or shared authorship positions (eg, co-first authorship) may be considered if justified and within reason. 6 An email with links to the Authorship Form will be sent to authors for completion after manuscripts have been submitted.
For reports of original data, authors' specific contributions will be published in the Acknowledgment section (see Manuscript Preparation and Submission Requirements , Acknowledgment section ). 2 All other persons who have made substantial contributions to the work reported in this manuscript (eg, data collection, analysis, or writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions and affiliations in an Acknowledgment in the manuscript. Written permission to include the names of individuals in the Acknowledgment section must be obtained.
Nonhuman artificial intelligence, language models, machine learning, or similar technologies do not qualify for authorship. If these models or tools are used to create content or assist with writing or manuscript preparation, authors must take responsibility for the integrity of the content generated by these tools. Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or Methods section if this is part of formal research design or methods. See also Use of AI in Publication and Research , Reproduced and Re-created Material , and Image Integrity .
The authors also must certify that the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere (see also About Previous Release of Information, Embargo, and Access ). 2 Authors of manuscripts reporting original data or systematic reviews must provide an access to data statement from 1 or 2 named authors, often the corresponding author (see also Data Access, Responsibility, and Analysis ). If requested, authors should be prepared to provide the data and must cooperate fully in obtaining and providing the data on which the manuscript is based for examination by the editors or their assignees.
A single corresponding author (or coauthor designee in the event that the corresponding author is unavailable) will serve on behalf of all coauthors as the primary correspondent with the editorial office during the submission and review process. If the manuscript is accepted, the corresponding author will review an edited manuscript and proof, make decisions regarding release of information in the manuscript to the news media or federal agencies, handle all postpublication communications and inquiries, and will be identified as the corresponding author in the published article.
The corresponding author also is responsible for ensuring that the Acknowledgment section of the manuscript is complete (see Acknowledgment Section ) and that the conflict of interest disclosures reported in the Acknowledgment section of the manuscript are accurate, up-to-date, and consistent with the information provided in each author's potential conflicts of interest section in the Authorship Form (see Conflicts of Interest and Financial Disclosures ).
The corresponding author also must complete the Acknowledgment statement part of the Authorship Form confirming that all persons who have contributed substantially but who are not authors are identified in the Acknowledgment section and that written permission from each person acknowledged has been obtained (see Acknowledgment Section ).
Requests for co-corresponding authors will be considered on a very limited basis if justified, but no more than 2 co-corresponding authors will be permitted. In such cases, a primary corresponding author must be designated as the point of contact responsible for all communication about the manuscript and article, manage the tasks described above, and will be listed first in the corresponding author section. 6 To read more about the role and responsibilities of corresponding authors, see the AMA Manual of Style .
Authors should determine the order of authorship among themselves and should settle any disagreements before submitting their manuscript. Changes in authorship (ie, order, addition, and deletion of authors) should be discussed and approved by all authors. Any requests for such changes in authorship after initial manuscript submission and before publication should be explained in writing to the editor in a letter or email from all authors. 2(pp128-133)
The JAMA Network recognizes that authors may change their names for personal reasons, and the editors respect authors' rights to autonomy and privacy in this regard. Authors who request confidential name changes after publication because of changes in identity, marital status, religion, or other reasons may have their names changed in articles without indication of the reason for the change and without a formal correction notice. If an author prefers this change to be public, a formal Correction notice can be issued, with or without the reason per author preference. The journal will not request the approval of coauthors, but the requesting author may wish to notify coauthors if this change will affect subsequent citations to the article. The requester may be asked to notify the corresponding author about this change to the published article; alternatively, the journal may inform the corresponding author of this change (without explaining the reason for the change). The journal will make this change to the online and PDF versions of the published article and will notify postpublication indexes and databases as a standard process but cannot guarantee when or if the change will be reflected in these indexes and databases.
If authorship is attributed to a group (either solely or in addition to 1 or more individual authors), all members of the group must meet the full criteria and requirements for authorship as described above, and all group member authors must complete Authorship Forms. 6 If all members of a group do not meet all authorship criteria, a group must designate 1 or more individuals as authors or members of a writing group who meet full authorship criteria and requirements and who will take responsibility for the group. 2 , 6 Group names should appear at the end of the byline and should not be interspersed within the list of individually named authors. Group authors may not be included for article types with limited numbers of authors (eg, opinion articles).
For articles with a large number of authors (eg, >50), a long list of authors will not fit in the byline of a print/PDF version of the article. In such cases, a group byline will be recommended with the individual names of each author listed at the end of the article. All author names would still be individually indexed, displayed, and easily searchable in bibliographic records such as PubMed. 6
Nonauthor Collaborators: Other group members who do not meet the criteria for authorship (eg, investigators, advisors, assistants) may be identified. For group author manuscripts, a Nonauthor Collaborator Template (with names, academic degrees, institution, location, role/contribution, and subgroup) must be completed during revision. The template will be available to authors with the request for revision. The collaborators will be published in an online Supplement based on this template and will be deposited to PubMed.
To read more about authorship, click here .
A conflict of interest may exist when an author (or the author's institution or employer) has financial or personal relationships or affiliations that could influence (or bias) the author's decisions, work, or manuscript. All authors are required to report potential conflicts of interest including specific financial interests relevant to the subject of their manuscript in the Acknowledgment section of the manuscript 2 and in the Disclosure of Potential Conflicts of Interest section of the Authorship Form. Note: These forms will be requested after a manuscript has been submitted, but authors should also include conflict of interest disclosures in the Acknowledgment section of the submitted manuscript.
Definitions and Terms of Conflicts of Interest Disclosures:
Authors are expected to provide detailed information about all relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript) including, but not limited to, employment, affiliation, funding and grants received or pending, consultancies, honoraria or payment, speakers' bureaus, stock ownership or options, expert testimony, royalties, donation of medical equipment, or patents planned, pending, or issued.
Following the guidelines of the ICMJE, 4 the definitions and terms of such disclosures include
Any potential conflicts of interest "involving the work under consideration for publication" (during the time involving the work, from initial conception and planning to present), Any "relevant financial activities outside the submitted work" (over the 3 years prior to submission), and Any "other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing" what is written in the submitted work (based on all relationships that were present during the 3 years prior to submission).
Authors without conflicts of interest, including relevant financial interests, activities, relationships, and affiliations, should indicate such in their disclosures and include a statement of no such interests in the Acknowledgment section of the manuscript. Failure to include this information in the manuscript may delay evaluation and review of the manuscript. Authors should err on the side of full disclosure and should contact the editorial office if they have questions or concerns.
Although many universities and other institutions and organizations have established policies and thresholds for reporting financial interests and other conflicts of interest, the JAMA Network requires complete disclosure of all relevant financial relationships and potential financial conflicts of interest, regardless of amount or value. For example, authors of a manuscript about hypertension should report all financial relationships they have with all manufacturers and owners of products, devices, tests, and services used in the management of hypertension, not only those relationships with entities whose specific products, devices, tests, and services are mentioned in the manuscript. If authors are uncertain about what constitutes a relevant financial interest or relationship, they should contact the editorial office.
For all accepted manuscripts, the corresponding author will have been asked to confirm that each coauthor's disclosures of conflicts of interest and relevant financial interests, activities, relationships, and affiliations and declarations of no such interests are accurate, up-to-date, and consistent with the disclosures reported in the Acknowledgment section of the manuscript because this information will be published in the Acknowledgment section of the article. Decisions about whether such information provided by authors should be published, and thereby disclosed to readers, are usually straightforward. Although editors are willing to discuss disclosure of specific conflicts of interest with authors, JAMA Network policy is one of complete disclosure of all potential conflicts of interest, including relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript). The policy requiring disclosure of conflicts of interest applies for all manuscript submissions, including letters to the editor. If an author's disclosure of potential conflicts of interest is determined to be inaccurate or incomplete after publication, a correction will be published to rectify the original published disclosure statement, and additional action may be taken as necessary.
All authors must also complete the Disclosure of Potential Conflicts of Interest section of the Authorship Form. 7
All financial and material support for the research and the work should be clearly and completely identified in an Acknowledgment section of the manuscript. At the time of submission, information on the funding source (including grant identification) must also be completed via the online manuscript submission and review system. The specific role of the funding organization or sponsor in each of the following should be specified: "design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication." 7 To read more about reporting funding and other support, see the AMA Manual of Style .
For all reports (regardless of funding source) containing original data, at least 1 named author (eg, the principal investigator), and no more than 2 authors, must indicate that she or he "had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis." 7 This exact statement should be included in the Acknowledgment section at the end of the manuscript. Modified statements or generic statements indicating that all authors had such access are not acceptable. In addition, for all reports containing original data, the names and affiliations of all authors (or other individuals) who conducted and are responsible for the data analysis must be indicated in the Acknowledgment section of the manuscript. If the individual who conducted the analysis is not named as an author, a detailed explanation of his/her contributions and reasons for his/her involvement with the data analysis should be included.
For all reports of research, authors are required to provide a Data Sharing Statement to indicate if data will or will not be shared. Specific questions regarding the sharing of data are included in the manuscript submission system. If authors choose to share or not share data, this information will be published in a Data Sharing Statement in an online supplement linked to the published article. Authors will be asked to identify the data, including individual patient data, a data dictionary that defines each field in the data set, and supporting documentation (eg, statistical/analytic code), that will be made available to others; when, where, and how the data will be available (eg, a link to a data repository); types of analyses that are permitted; and if there will be any restrictions on the use of the data. Authors also have the option to explain why data may not be shared. A list of generalist public repositories that authors may consider using is available from the National Library of Medicine .
The Acknowledgment section is the general term for the list of contributions, disclosures, credits, and other information included at the end of the text of a manuscript but before the references. The Acknowledgment section includes authors' contributions; information on author access to data; disclosure of potential conflicts of interest, including financial interests, activities, relationships, and affiliations; sources of funding and support; an explanation of the role of funder(s)/sponsor(s); names, degrees, and affiliations of participants in a large study or other group (ie, collaborators); any important disclaimers; information on previous presentation of the information reported in the manuscript; and the contributions, names, degrees, affiliations, and indication if compensation has been received for all persons who have made substantial contributions to the work but who are not authors. 2
All other persons who have made substantial contributions to the work reported in the manuscript (eg, data collection, analysis, and writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions in an Acknowledgment in the manuscript.
Authors must obtain written permission to include the names of all individuals included in the Acknowledgment section, and the corresponding author must confirm that such permission has been obtained in the Authorship Form.
Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or the Methods section if this is part of formal research design or methods. This should include a description of the content that was created or edited and the name of the language model or tool, version and extension numbers, manufacturer, date(s) of use, and confirmation that the authors take responsibility for the integrity of the content generated. (Note: this does not include basic tools for checking grammar, spelling, references, etc.) See also Use of AI in Publication and Research and Statistical Analysis Subsection .

Requirements for Reporting
Authors of research articles should follow the EQUATOR Reporting Guidelines . See specific Study Types for detailed guidance on reporting.
Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording. To read more about use of causal language, see the AMA Manual of Style .
Research reports should be timely and current and should be based on data collected as recently as possible. Manuscripts based on data from randomized clinical trials should be reported as soon as possible after the trial has ended, ideally within 1 year after follow-up has been completed.
For cohort studies, the date of final follow-up should be no more than 5 years before manuscript submission. Likewise, data used in case-control or cross-sectional studies should have been collected as recently as possible, but no more than 5 years before manuscript submission. Manuscripts in which the most recent data have been collected more than 5 years ago ordinarily will receive lower priority for publication; thus, authors of such manuscripts should provide a detailed explanation of the relevance of the information in light of current knowledge and medical practice as well as the most recent date(s) of analysis of the study.
General Considerations
Authors are encouraged to consult "Reporting Statistical Information in Medical Journal Articles." 1 In the Methods section, describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to reproduce the reported results. Such description should include appropriate references to the original literature, particularly for uncommon statistical methods. For more advanced or novel methods, provide a brief explanation of the methods and appropriate use in the text and consider providing a detailed description in an online supplement.
In the reporting of results, when possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty, such as confidence intervals (see Reporting Standards and Data Presentation ). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important quantitative information. For observational studies, provide the numbers of observations. For randomized trials, provide the numbers randomized. Report losses to observation or follow up (see Missing Data ). For multivariable models, report all variables included in models, and report model diagnostics and overall fit of the model when available (see Statistical Procedures ).
Define statistical terms, abbreviations, and symbols, if included. Avoid nontechnical uses of technical terms in statistics, such as correlation, normal, predictor, random, sample, significant, trend. Do not use inappropriate hedge terms such as marginal significance or trend toward significance for results that are not statistically significant. Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording.
Sample Size Calculations
For randomized trials, a statement of the power or sample size calculation is required (see the EQUATOR Network CONSORT Guidelines ). For observational studies that use an established population, a power calculation is not generally required when the sample size is fixed. However, if the sample size was determined by the researchers, through any type of sampling or matching, then there should be some justification for the number sampled. In any case, describe power and sample size calculations at the beginning of the Statistical Methods section, following the general description of the study population.
Descriptive Statistics
It is generally not necessary to provide a detailed description of the methods used to generate summary statistics, but the tests should be briefly noted in the Methods section (eg, ANOVA or Fisher exact test).
Statistical Procedures
Identify regression models with more than 1 independent variable as multivariable and regression models with more than 1 dependent variable as multivariate. Report all variables included in models, as well as any mathematical transformations of those variables. Provide the scientific rationale (clinical, statistical, or otherwise) for including variables in regression models.
For regression models fit to dependent data (eg, clustered or longitudinal data), the models should account for the correlations that arise from clustering and/or repeated measures. Failure to account for such correlation will result in incorrect estimates of uncertainty (eg, confidence intervals). Describe how the model accounted for correlation. For example, for an analysis based on generalized estimating equations, identify the assumed correlation structure and whether robust (or, sandwich) variance estimators were used. Or, for an analysis based on mixed-effects models, identify the assumed structure for the random effects, such as the level of random intercepts and whether any random slopes were included. Fixed-effects estimation should be described as conditional likelihood. Avoid the term fixed effects for describing covariates.
Missing Data
Report losses to observation, such as dropouts from a clinical trial or those lost to follow-up or unavailable in an observational study. If some participants are excluded from analyses because of missing or incomplete data, provide a supplementary table that compares the observed characteristics between participants with complete and incomplete data. Consider multiple imputation methods to impute missing data and include an assessment of whether data were missing at random. Approaches based on "last observation carried forward" should not be used.
Primary Outcomes, Multiple Comparisons, and Post Hoc Comparisons
Both randomized and observational studies should identify the primary outcome(s) before the study began, as well as any prespecified secondary, subgroup, and/or sensitivity analyses. Comparisons arrived at during the course of the analysis or after the study was completed should be identified as post hoc. For analyses of more than 1 primary outcome, corrections for multiple testing should generally be used. For secondary outcomes, address multiple comparisons or consider such analyses as exploratory and interpret them as hypothesis-generating. The reporting of all outcomes should match that included in study protocols. For randomized clinical trials, protocols with complete statistical analysis plans should be cited in the Methods section and submitted as online supplementary content. Randomized clinical trials should be primarily analyzed according to the intention-to-treat approach. Deviations from strict intention-to-treat analysis should be described as "modified intention-to-treat," with the modifications clearly described.
Statistical Analysis Subsection
At the end of the Methods section, briefly describe the statistical tests used for the analysis. State any a priori levels of significance and whether hypothesis tests were 1- or 2-sided. Also include the statistical software used to perform the analysis, including the version and manufacturer, along with any extension packages (eg, the svy suite of commands in Stata or the survival package in R). Do not describe software commands (eg, SAS proc mixed was used to fit a linear mixed-effects model). If analysis code is included, it should be placed in the online supplementary content.
Reporting Standards and Data Presentation
Analyses should follow EQUATOR Reporting Guidelines and be consistent with the protocol and statistical analysis plan, or described as post hoc.
When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. If P values are reported, follow standard conventions for decimal places: for P values less than .001, report as " P <.001"; for P values between .001 and .01, report the value to the nearest thousandth; for P values greater than or equal to .01, report the value to the nearest hundredth; and for P values greater than .99, report as " P >.99." For studies with exponentially small P values (eg, genetic association studies), P values may be reported with exponents (eg, P = 1×10 −5 ). In general, there is no need to present the values of test statistics (eg, F statistics or χ² results) and degrees of freedom when reporting results.
For secondary and subgroup analyses, there should be a description of how the potential for type I error due to multiple comparisons was handled, for example, by adjustment of the significance threshold. In the absence of some approach, these analyses should generally be described and interpreted as exploratory, as should all post hoc analyses.
For randomized trials using parallel-group design, there is no validity in conducting hypothesis tests regarding the distribution of baseline covariates between groups; by definition, these differences are due to chance. Because of this, tables of baseline participant characteristics should not include P values or statements of statistical comparisons among randomized groups. Instead, report clinically meaningful imbalances between groups, along with potential adjustments for those imbalances in multivariable models. To read more about statistical tests and data presentation, see the AMA Manual of Style .
Researchers are encouraged to report studies that include diverse and representative participants and to indicate participant inclusion and exclusion criteria and how the findings generalize to the population(s) that are the focus of or are compatible with the research question. Aggregate, deidentified demographic information (eg, age, sex, race and ethnicity, and socioeconomic indicators) should be reported for all research reports along all prespecified outcomes. Demographic variables collected for a specific study should be reported in the Methods section. Demographic information assessed should be reported in the Results section, either in the main article or in an online supplement or both. If any demographic characteristics that were collected are not reported, the reason should be stated. Summary demographic information (eg, baseline characteristics of study participants) should be reported in the first line of the Results section of Abstracts.
Reporting Age
Study inclusion or exclusion criteria by age or age group should be defined in the Methods section. Stratification by age groups should be based on relevance to disease, condition, or population (eg, <5 or >65 years). The ages for study participants should be reported in aggregate (ie, mean and SD or median and IQR or range) in the Results section.
Reporting Sex and Gender
The term sex should be used when reporting biological factors and gender should be used when reporting gender identity or psychosocial/cultural factors. The methods used to obtain information on sex, gender, or both (eg, self-reported, investigator observed or classified, or laboratory test) should be explained in the Methods section. 12 The distribution of study participants or samples should be reported in the Results section, including for studies of humans, tissues, cells, or animals. All participants should be reported, not just the category that represents the majority of the sample. Studies that address pregnancy should follow these recommendations, and if the gender identity of participants was not assessed, use the terms "pregnant participants," "pregnant individuals," "pregnant patients," etc, as appropriate.
In research articles, sex or gender should be reported and defined, and how sex or gender was assessed should be described. Whenever possible, all main outcomes should be reported by sex (or gender if appropriate). In nonresearch reports, choose sex-neutral terms that avoid bias, suit the material under discussion, and do not intrude on the reader's attention.
Reporting Race and Ethnicity
The Methods section should include an explanation of who identified participant race and ethnicity and the source of the classifications used (eg, self-report or selection, investigator observed, database, electronic health record, survey instrument).
If race and ethnicity categories were collected for a study, the reasons that these were assessed also should be described in the Methods section. If collection of data on race and ethnicity was required by the funding agency, that should be noted.
Specific racial and ethnic categories are preferred over collective terms, when possible. Authors should report the specific categories used in their studies and recognize that these categories will differ based on the databases or surveys used, the requirements of funders, and the geographic location of data collection or study participants. Categories included in groups labeled as "other" should be defined.
Categories should be listed in alphabetical order in text and tables.
Race and ethnicity of the study population should be reported in the Results section.
For additional information, see " Updated Guidance on Reporting Race and Ethnicity in Medical and Science Journals " and the Summary Guide for Preferred Terms When Reporting Race and Ethnicity .
For all manuscripts reporting data from studies involving human participants or animals, formal review and approval, or formal review and waiver, by an appropriate institutional review board or ethics committee is required and should be described in the Methods section. 2(p226) For those investigators who do not have formal ethics review committees, the principles outlined in the Declaration of Helsinki should be followed. 13 For investigations of humans, state in the Methods section the manner in which informed consent was obtained from the study participants (ie, oral or written) and whether participants received a stipend. Authors of research studies involving humans should not make independent determinations of exemption or exclusion of IRB or ethical review; they should cite the institutional or regulatory policy for that determination and indicate if the data are deidentified and publicly available or protected by prior consent or privacy safeguards. Editors may request that authors provide documentation of the formal review and recommendation from the institutional review board or ethics committee responsible for oversight of the study.
A signed statement of informed consent to publish patient descriptions, photographs, video, and pedigrees should be obtained from all persons (parents or legal guardians for minors) who can be identified (including by the patients themselves) i/n such written descriptions, photographs, or pedigrees and should be submitted with the manuscript and indicated in the Acknowledgment section of the manuscript. Such persons should be offered the opportunity to see the manuscript before its submission. 2(pp229-232)
Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Only those details essential for understanding and interpreting a specific case report or case series should be provided. Although the degree of specificity needed will depend on the context of what is being reported, specific ages, race/ethnicity, and other sociodemographic details should be presented only if clinically or scientifically relevant and important. 2 Cropping of photographs to remove identifiable personal features that are not essential to the clinical message may be permitted as long as the photographs are not otherwise altered. Please do not submit masked photographs of patients. Patients' initials or other personal identifiers must not appear in an image.
Patient Permission Form:
The Patient Permission form for publication of identifying material is available here . Translated versions in Arabic, Chinese, French, German, Hindi, Italian, Japanese, Portuguese, and Spanish are available on request.
AI Used in Manuscript Preparation
When traditional and generative AI technologies are used to create, review, revise, or edit any of the content in a manuscript, authors should report in the Acknowledgment section the following:
- Name of the AI software platform, program, or tool
- Version and extension numbers
- Manufacturer
- Date(s) of use
- A brief description of how the AI was used and on what portions of the manuscript or content
- Confirmation that the author(s) take responsibility for the integrity of the content generated
Note this guidance does not apply to basic tools for checking grammar, spelling, references, and similar.
AI Used in Research
When AI (eg, large language model [LLM] or natural language processing [NLP], supervised or unsupervised machine learning [ML] for predictive/prescriptive or clustering tasks, chatbots, or similar other technologies) is used as part of a scientific study, authors should:
- Follow relevant reporting guidelines for specific study designs when they exist and report each recommended guideline element with sufficient detail to enable reproducibility.
- Avoid inclusion of identifiable patient information in text, tables, and figures.
- Be aware of copyright and intellectual property concerns - if including content (text, images) generated by AI, and indicate rights or permissions to publish that content as determined by the AI service or owner.
Also address the following:
Methods Section
- Include the study design and, if a relevant reporting guideline exists, indicate how it was followed, with sufficient detail to enable reproducibility.
- Describe how AI was used for specific aspects of the study (eg, to generate or refine study hypotheses, assist in the generation of a list of adjustment variables, create graphs to show visual relationships).
- For studies using LLMs, provide the name of the platform or program, tool, version, and manufacturer; specify dates and prompt(s) used and their sequence and any revisions to prompts in response to initial outputs.
- For studies reporting ML and algorithm development, include details about data sets used for development, training, and validation. Clearly state if algorithms were trained and tested only on previously collected or existing data sets or if the study includes prospective deployment. Include the ML model and describe the variables and outcome(s) and selection of the fine-tuning parameters. Describe any assumptions involved (eg, log linearity, proportionality) and how these assumptions were tested.
- Indicate the metric used to evaluate the performance of the algorithms, including bias, discrimination, calibration, reclassification, and others as appropriate.
- Indicate the methods used to address missing data.
- Indicate institutional review board/ethics review, approval, waiver, or exemption.
- Describe methods or analyses included to address and manage AI-related methodologic bias and inaccuracy of AI-generated content.
- Indicate, when appropriate, if sensitivity analyses were performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
- Provide a data sharing statement, including if code will be shared.
Results Section
- When reporting comparisons, provide performance assessments (eg, against standard of care), include effect sizes and measures of uncertainty (eg, 95% CIs) and other measurements such as likelihood ratios, and include information about performance errors, inaccurate or missing data, and sufficient detail for others to reproduce the findings.
- Report the results of analyses to address methodologic bias and population representation.
- If examples of generated text or content are included in tables or figures, be sure to indicate the source and licensing information, as noted above.
Discussion Section
- Discuss the potential for AI-related bias and what was done to identify and mitigate such bias.
- Discuss the potential for inaccuracy of AI-generated content and what was done to identify and manage this.
- Discuss generalizability of findings across populations and results of analyses performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
A signed statement of permission should be included from each individual identified as a source of information in a personal communication or as a source for unpublished data, and the date of communication and whether the communication was written or oral should be specified. 2(p199) Personal communications should not be included in the list of references but added to the text parenthetically.
Authors and reviewers are expected to notify editors if a manuscript could be considered to report dual use research of concern (ie, research that could be misused by others to pose a threat to public health and safety, agriculture, plants, animals, the environment, or material). 14 The editor in chief will evaluate manuscripts that report potential dual use research of concern and, if necessary, consult additional reviewers.
Journal Policies
Final decisions regarding manuscript publication are made by the editor in chief or a designated editor who does not have any relevant conflicts of interest. The journal has a formal recusal process in place to help manage potential conflicts of interest of editors. In the event that an editor has a conflict of interest with a submitted manuscript or with the authors, the manuscript, review, and editorial decisions are managed by another designated editor without a conflict of interest related to the manuscript.
All authors are required to complete and submit a Publishing Agreement that is part of the journal's electronic Authorship Form. In this agreement, authors will transfer copyright or a publication license; or indicate that they are employed by a federal government; or indicate that they are an employee of an institution that considers the work in the manuscript a work for hire, in which case an authorized representative of that institution will assign copyright or a publication license on the author's behalf.
Published articles become the permanent property of the American Medical Association (AMA) and may not be published elsewhere without written permission. Unauthorized use of the journal's name, logo, or any content for commercial purposes or to promote commercial goods and services (in any format, including print, video, audio, and digital) is not permitted by the JAMA Network or the AMA.
1. Cummings P, Rivara FP. Reporting statistical information in medical journal articles. Arch Pediatr Adolesc Med . 2003;157(4):321-324. doi:10.1001/archpedi.157.4.321
2. Iverson C, Christiansen S, Flanagin A, et al. AMA Manual of Style: A Guide for Authors and Editors . 11th ed. Oxford University Press; 2020. http://www.amamanualofstyle.com
3. Golub RM. Correspondence course: tips for getting a letter published in JAMA . JAMA . 2008;300(1):98-99. doi:10.1001/jama.300.1.98
4. International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Updated May 2023. Accessed May 18, 2023. http://www.icmje.org/recommendations/
5. Fontanarosa PB, Flanagin A, DeAngelis CD. Update on JAMA 's policy on release of information to the public. JAMA . 2008;300(13):1585-1587. doi:10.1001/jama.300.13.1585
6. Fontanarosa P, Bauchner H, Flanagin A. Authorship and team science. JAMA . 2017;318(24):2433-2437. doi:10.1001/jama.2017.19341
7. Fontanarosa PB, Flanagin A, DeAngelis CD. Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies. JAMA . 2005;294(1):110-111. doi:10.1001/jama.294.1.110
8. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA . 2004;292(11):1363-1364. doi:10.1001/jamainternmed.2014.6933
9. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Is this clinical trial fully registered? a statement from the International Committee of Medical Journal Editors. JAMA . 2005;293(23):2927-2929. doi:10.1001/jama.293.23.jed50037
10. The CONSORT Group. The CONSORT statement. Updated 2014. Accessed September 23, 2016. http://www.consort-statement.org/consort-2010
11. American Association for Public Opinion Research. Best practices for survey research. Accessed March 23, 2023. https://aapor.org/standards-and-ethics/best-practices/
12. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA . 2016;316(18):1863-1864. doi:10.1001/jama.2016.16405
13. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA . 2013;310(20):2191-2194. doi:10.1001/jama.2013.281053
14. Journal Editors and Authors Group. Statement on scientific publication and security. Science . 2003;299(5610):1149. doi:10.1126/science.299.5610.1149 . Published correction appears in Science . 2003;299(5614):1845.
15. Christiansen S, Flanagin A. Correcting the medical literature: "to err is human, to correct divine." JAMA . 2017;318(9):804-805. doi:10.1001/jama.2017.11833
Last Updated: June 13, 2024
Quick Links
- Why Publish in JAMA
- Submit and Track Your Manuscript
- Author Reprints
- Permissions Requests
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 27 June 2024
Embolization treatment of right pulmonary artery agenisis with patent ductus arteriosus causing pulmonary hypertension and hemoptysis: a case report and literature review
- Fan Wei 1 ,
- Chang Yaowen 1 &
- Wang Wenhui 1
Journal of Cardiothoracic Surgery volume 19 , Article number: 391 ( 2024 ) Cite this article
38 Accesses
Metrics details
As the pediatric patient with right pulmonary artery agenesis (PAA) matured, she progressively presented symptoms of pulmonary hypertension and hemoptysis. There is limited clinical literature on this condition, and currently, there is no consensus regarding its diagnosis and treatment. This article presents a case study of a 16-year-old female patient with right pulmonary artery hypoplasia, providing a comprehensive summary and analysis of her developmental progression, pathology, diagnosis, and treatment.
Peer Review reports
Right PAA is a rare congenital developmental anomaly initially documented by Frantzel in 1868, with a post-mortem incidence of 0.0005% [ 1 ]. Over 70% of patients present with associated congenital heart disease, encompassing common malformations such as tetralogy of Fallot, atrial septal defect, coarctation of the aorta, persistent pulmonary trunk, aortic stenosis, transposition of the great arteries, pulmonary atresia, and pulmonary stenosis [ 2 , 3 ]. The prevalence of right PAA is double that of the left; however left PAA demonstrates stronger association with congenital heart disease [ 4 ]. Diagnosis typically occurs during childhood for most patients and common complications include recurrent pulmonary infections, hemoptysis, chest pain and Eisenmenger syndrome. Clinical indicators facilitating early diagnosis comprise features like pulmonary hypertension and congestive heart failure [ 5 ]. The mortality rate for individuals with right PAA experiencing pulmonary hemorrhage ranges from 27 to 48% [ 2 , 6 ], further increasing when accompanied by congenital heart disease.
The 16-year-old female patient presented with intermittent hemoptysis over a three-day period, estimated at approximately 150 ml. The episodes occurred seasonally during winter. She was diagnosed with right PAA thirteen years ago and has exhibited limited exercise tolerance (less than 150 m), along with symptoms of anemia, palpitations, and a preference for squatting. Blood tests revealed the following results: pH: 7.503, pCO₂: 21.80mmHg, sO₂: 90.00%, pO₂: 58.60mmHg, red blood cells: 2.90 × 10¹²/L, hemoglobin:46 g/L; prothrombin time:16.2s; international normalized ratio:1.48; brain natriuretic peptide:533pg/ml. Enhanced chest CT indicated abnormalities in the right pulmonary artery (Fig. 1 ) and multiple small vessels originating from the neck arteries in the mediastinum and right lung parenchyma. Echocardiography suggested congenital right PAA with patent ductus arteriosus (PDA)(measuring approximately 9 mm in width with shunt velocity of 223 cm/s and pressure gradient of 20mmHg), mild narrowing of the descending aorta leading to secondary pulmonary hypertension (estimated systolic pressure in the pulmonary artery at rest is 83mmHg = brachial systolic pressure—left-to-right shunt pressure difference), main pulmonary trunk diameter measures 31 mm; overall cardiac enlargement is noted particularly on the right side forming a D-shaped configuration; trivial tricuspid regurgitation is observed along with slight dilation of the right coronary artery; minimal pericardial effusion present; both lungs exhibit diffuse interstitial edema. The patient’s WHO functional class was III while her ESC/ERS risk stratification for pulmonary hypertension was intermediate risk level. To control hemoptysis and alleviate pulmonary arterial hypertension selective embolization therapy targeting systemic collateral arteries was performed via Seldinger puncture using a 5 F catheter (YASHIRO) through the right femoral artery. The angiogram showed abnormal communication between bronchial arteries-pulmonary arteries(Fig. 2 ); visualization also demonstrated anomalous connections between branches such as thyrocervical trunk, internal mammary, and intercostal arteries to spinal cord arteries(Figs. 3 and 4 ). Embolization using polyvinyl alcohol particles(350–560 μm) and gelatin sponge particles(350–560 μm、1000–1400 μm) was conducted to occlude these systemic collateral vessels, resulting in no residual abnormal systemic circulation after embolization. Tadalafil, a phosphodiesterase 5 inhibitor, and guanylate cyclase stimulator were administered post-embolization to assist in reducing pulmonary arterial pressures. During follow-up at six months, the patient reported no further episodes of hemoptysis, enjoyed normal physical activity(with continuous exertion exceeding 6 h including running without limitation).

The chest CT scan with three-dimensional reconstruction of a 13-year-old female revealed the absence of the right pulmonary artery (arrow) and patent ductus arteriosus

Left (right arrow) and right (left arrow) bronchial arteries open at the thoracic level of T5

Right costocervical trunk, subclavian artery, thyrocervical trunk, and internal thoracic artery were developed (arrows from left to right)

Right intercostal artery (left arrow) and spinal artery (right arrow) were developed
Development and pathology
During embryonic development, the proximal portion of the bilateral sixth aortic arch undergoes differentiation into the main trunk and left and right branches of the pulmonary artery, while the distal portion on the left transforms into the ductus arteriosus and the right portion regresses [ 1 , 6 ]. If there is a failure in primitive displacement and rotation of the sixth aortic arch, it results in discontinuity between its proximal portion and the pulmonary artery within the lung. However, originating from the pulmonary bud, the distal portion of the pulmonary artery can maintain a certain degree of branching structure, providing an anatomical basis for abnormal blood supply from bronchial and other systemic arteries. PAA are typically accompanied by varying degrees of underdevelopment in ipsilateral lung tissue, which impacts systemic circulation arteries’ origin and distribution [ 7 ]. Abnormal systemic circulation arteries may originate from bronchial, intercostal, intrathoracic, thyrocervical trunk, subclavian, or hypogastric arteries; fewer cases have been reported involving blood supply from coronary arteries. In children with unilateral PAA where all cardiac output flows to one lung side due to excessive blood flow increasing shear stress on endothelial cells leading to long-term decline in vascular bed elasticity as well as excessive sensitivity to vasoconstrictive substances [ 8 ]. Additionally reduced relative size of affected side’s pulmonary vascular bed along with continuous opening of systemic circulation arteries leads to increased risk for adult-onset pulmonary hypertension that tends to stabilize as open arterial numbers stabilize during adulthood [ 8 ].
Children with PAA may develop recurrent pulmonary infections (37%), reduced exercise tolerance (40%), pulmonary hypertension (44%), and hemoptysis (20%) as they age, or remain asymptomatic until adulthood [ 3 , 9 , 10 ]. Physical examination may reveal chest asymmetry, abnormal breath sounds on the affected side, and a generally normal electrocardiogram. When a chest X-ray shows increased lung transparency and hypoplasia of the lung (contralateral displacement of the trachea, fewer pulmonary vascular segments), along with fine linear opacities in the peripheral lung, suspicion of PAA is high; however, it needs to be differentiated from diseases causing diffuse changes in one lung field such as bronchiectasis, hypoplastic lung, scimitar syndrome, Swyer-James syndrome, mediastinal tumor, fibrotic mediastinitis, and pulmonary venous occlusive disease [ 11 , 12 ]. CT three-dimensional reconstruction is an important preoperative diagnostic method; angiography is reserved for individuals requiring surgical intervention due to its invasiveness [ 2 ]. Common methods for measuring pulmonary artery pressure include tricuspid regurgitation velocity method, pulmonary valve regurgitation method, and intracardiac shunt method. Right ventricular enlargement and thickening as well as widening of the right ventricular outflow tract are suggestive but lack diagnostic significance for patients with unilateral absence of a pathway in the pulmonary artery [ 13 ]. In this case study patient’s right subclavian artery systolic pressure was used along with catheter left-to-right shunt pressure difference for estimation.
Treatment of disease
Refined sentence: The literature on PAA leading to pulmonary hypertension is primarily based on case studies and adult clinical staging. Early diagnosis and intervention are essential principles. Treatment aims to reduce risk stratification (low-risk) and ensure unrestricted normal activities. Prognosis depends largely on the type of cardiovascular disease associated with pulmonary hypertension and the severity of the condition [ 14 ]. In children with right PAA hypoplasia, the ductus arteriosus maintains a balance between systemic and pulmonary circulation blood flow, ensuring cardiac function. While ligation is considered the optimal treatment for PDA patients, it may further restrict pulmonary circulation, disrupt equilibrium, and impede oxygen exchange [ 15 ]. This limitation has been a primary reason for not performing ductus arteriosus ligation thus far; currently, monitoring cardiovascular hemodynamics is deemed most beneficial [ 16 , 17 ]. A retrospective study suggests that early ductus arteriosus ligation is linked to severe bronchial artery hypoplasia; children with right pulmonary artery hypoplasia are likely to exhibit more severe symptoms [ 18 ].The insufficient blood flow in the right lung leads to a decreased delivery of phagocytes to the inflammatory site, reduced carbon dioxide exchange rate, and secondary bronchial constriction and mucus retention, resulting in recurrent pulmonary infections. While systemic circulation improvement enhances pulmonary perfusion to some extent, it also elevates the risk of pulmonary hypertension and hemoptysis [ 7 ]. Hemoptysis occurs spontaneously over several years and often follows a seasonal pattern, worsening with age [ 19 ]. The most optimal treatment for children with PAA involves utilizing autologous pericardium or artificial materials for pulmonary artery vascular reconstruction. This approach can promote lung development and alleviate vascular system damage as long as the relative integrity of the pulmonary arterial tree is maintained. However, challenges arise due to mismatch between implant size and the child’s developing body, necessitating staged procedures. Any lung surgery for a child with such anomalies will be complicated by PDA and open systemic arteries. In cases where surgical removal of infected sites fails to control recurrent pulmonary infections and hemoptysis, unilateral lung resection becomes the last resort option. Unfortunately, mortality rates are extremely high after unilateral lung resection among patients with accompanying anemia, pulmonary hypertension, and acute respiratory failure due to these anomalies. Selective systemic circulation arterial embolization proves effective in controlling coughing fits along with managing both pulmonary hypertension and infection [ 15 ], offering good hemostatic effects while minimizing trauma; this procedure can be repeated if necessary. It is crucial to ensure complete embolization of responsible vessels in order to avoid missed embolizations that could lead to higher recurrence rates and mortality [ 20 , 21 ].Following selective embolization of the systemic arteries, there was a reduction in blood flow in the right pulmonary circulation and a decrease in pulmonary arterial pressure, with no significant impact on oxygen exchange or lung lobe development (16 years old). For abnormal open systemic circulation arteries (arterial fistula), larger diameter permanent solid embolic agents are preferred due to their irregular surfaces that are more prone to clotting and can prevent occlusion of aberrant small vessels. In cases of suspected spinal or coronary arteries, non-permanent embolic agents or no embolization is recommended. Embolization should be followed by targeted drug therapy as per the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension and the 2021 Chinese Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension [ 22 ]. These guidelines recommend single drug therapy for patients with pulmonary hypertension; low-risk group patients should receive endothelin receptor antagonists (such as Bosentan), while high-risk group patients should be given prostacyclin analogues and prostacyclin receptor agonists (such as Epoprostenol). The patient in this case belongs to a moderate-risk group and was administered Phosphodiesterase 5 inhibitors and guanylate cyclase stimulators (such as Tadalafil). If clinical symptoms do not improve after single drug therapy, early combination targeted drugs can be considered, especially for WHO functional grade IV patients who may benefit from an initial combined treatment strategy. Supportive treatments such as diuretics and oxygen therapy can also be provided, along with long-term use of vasodilators which have been shown to improve survival rates [ 23 , 24 ].
In conclusion, for pediatric patients with PAA and PDA, selective embolization of systemic circulation arteries can alleviate pulmonary hypertension and represents a viable therapeutic option for those ineligible for revascularization. Continuous monitoring of cardiovascular hemodynamic changes is advised, while early ductus arteriosus ligation is not recommended.
Data availability
No datasets were generated or analysed during the current study.
Seedat F, Kalla IS, Feldman C. Unilateral absent pulmonary artery in an adult - A diagnostic and therapeutic challenge. Respir Med Case Rep. 2017;22:238–42. https://doi.org/10.1016/j.rmcr.2017.09.004 . PMID: 28951831; PMCID: PMC5604951.
Article PubMed PubMed Central Google Scholar
Li W, Ma L, Xia S, et al. Early single-stage surgical revascularization of pulmonary artery in unilateral absence of a pulmonary artery. J Cardiothorac Surg. 2021;16(1):80. https://doi.org/10.1186/s13019-021-01481-3 . PMID: 33849614; PMCID: PMC8045296.
Reading DW, Oza U. Unilateral absence of a pulmonary artery: a rare disorder with variable presentation. Proc (Bayl Univ Med Cent). 2012;25(2):115–8. https://doi.org/10.1080/08998280.2012.11928802 . PMID: 22481838; PMCID: PMC3310505.
Article PubMed Google Scholar
Kruzliak P, Syamasundar RP, Novak M, et al. Unilateral absence of pulmonary artery: pathophysiology, symptoms, diagnosis and current treatment Arch. Cardiovasc Dis. 2013;106:448–54.
Google Scholar
Mehta K, Mehta K, Bhattarai V, et al. Congenital unilateral absence of right pulmonary artery with VSD and wide aortopulmonary window in an adult patient: a case report. Ann Med Surg (Lond). 2023;85(2):291–4. The doi: 10.1097 / MS9.0000000000000213. PMID: 36845809; PMCID: PMC9949833.
Mistrot JJ, Bernhard WF, Rosenthal A et al. Tetralogy of Fallot with a single pulmonary artery: operative repair. Ann Thorac Surg. 1977;23(3):249 – 53. https://doi.org/10.1016/s0003-4975(10)64118-5 . PMID: 849032.
Kollu R, Uligada S, Kotamraju S, et al. Proximal interruption of pulmonary artery: spectrum of radiological findings with emphasis on chest radiograph and contrast-enhanced computed tomography (CECT). Cureus. 2022;14(12):e32916. 10.7759/. cureus.32916.PMID: 36699783; PMCID: PMC9873200.
PubMed PubMed Central Google Scholar
Weldetsadik AY, Kebede A, Godu BG, et al. Unilateral pulmonary vein atresia presenting with recurrent hemoptysis and bronchial varices in an Ethiopian adolescent: a case report. J Med Case Rep. 2023;17(1):246. https://doi.org/10.1186/s13256-023-03956-4 . PMID: 37269023; PMCID: PMC10239153.
Dunphy L, Talwar A, Patel N, et al. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations. BMJ Case Rep. 2021;14(1):e238385. : 10.1136/ bcr-2020-238385.PMID: 33419752; PMCID: PMC7799076.
Ten Harkel AD, Blom NA, Ottenkamp J. Isolated unilateral absence of a pulmonary artery: a case report and review of the literature. Chest. 2002;122(4):1471-7. https://doi.org/10.1378/chest.122.4.1471 . PMID: 12377882.
Bouros D, Pare P, Panagou P et al. The varied manifestation of pulmonary artery agenesis in adulthood. Chest. 1995;108(3):670-6. https://doi.org/10.1378/chest.108.3.670 . PMID: 7656614.
Raymond A, Pedretti E, Privitera G, et al. Neonatal diagnosis of isolated absence of the right pulmonary artery: a case report and review of the literature. Ital J Pediatr. 2018;44(1):27. https://doi.org/10.1186/s13052-018-0465-1 . PMID: 29463283; PMCID: PMC5819273.
Koga H, Hidaka T, Miyako K et al. Age-related clinical characteristics of isolated congenital unilateral absence of a pulmonary artery. Pediatr Cardiol. 2010; 31(8):1186-90. doi: 10.1007/ s00246-010-9787-5.Epub 2010 Sep 3.PMID: 20814784.
Hu Z, Fan S. Progress in the application of echocardiography in neonatal pulmonary hypertension. J Matern Fetal Neonatal Med. 2024;37(1):2320673. https://doi.org/10.1080/14767058.2024.2320673 . Epub 2024 Mar 12. PMID: 38475689.
Omachi N, Ishikawa H, Hara M, et al. The impact of bronchial artery embolisation on the quality of life of patients with haemoptysis: a prospective observational study. Eur Radiol. 2021;31(7):5351–60. doi: 10.1007/ s00330-020-07533-x.Epub 2021 Jan 6. PMID: 33409794; PMCID: PMC8213576.
Pugnaloni F, Doni D, Lucente M, et al. Ductus Arteriosus in fetal and perinatal life. J Cardiovasc Dev Dis. 2024;11(4):113. https://doi.org/10.3390/jcdd11040113 . PMID: 38667731; PMCID: PMC11050351.
Article CAS PubMed PubMed Central Google Scholar
Gopagondanahalli KR, Abdul Haium AA, Vora SJ, et al. Serial tissue doppler imaging in the evaluation of bronchopulmonary dysplasia-associated pulmonary hypertension among extremely preterm infants: a prospective observational study. Front Pediatr. 2024;12:1349175. https://doi.org/10.3389/fped.2024.1349175 . PMID: 38646509; PMCID: PMC11026596.
Shimada K, Hirose M, Hamabe L, et al. Evaluation of Alacepril Administration in Canine Patent Ductus Arteriosus according to plasma chymase activity. Anim (Basel). 2024;14(7):1078. https://doi.org/10.3390/ani14071078 . PMID: 38612317; PMCID: PMC11010901.
Article Google Scholar
Söderström F, Sindelar R, Olsson KW, et al. Active versus restrictive ligation strategy for patent ductus arteriosus - A retrospective two-center study of extremely preterm infants born between 22 + 0 and 25 + 6 weeks of gestational age. Early Hum Dev. 2024;191:105976. Epub 2024 Feb 29. PMID: 38452632.
Loo YJ, Thomas R, Yagnik L. Isolated unilateral absence of pulmonary artery (IUPAD) in adults: a case series and review of literature. Respirol Case Rep. 2022;11(1):e01073. 10.1002/ rcr2.1073. .PMID: 36523545; PMCID: PMC9745554.
Kanno K, Maehara T, Yamamoto T, et al. Isolated unilateral absence of the pulmonary artery. Clin Case Rep. 2019;7(12):2583–4. 10.1002/ ccr3.2515. .PMID: 31893107; PMCID: PMC6935673.
Humbert M, Kovacs G, Hoeper MM, ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879. https://doi.org/10.1183/13993003.00879-2022 . PMID: 36028254.
Frood R, Karthik S, Mirsadraee S, et al. Bronchial artery embolisation for massive haemoptysis: Immediate and Long-Term Outcomes-A Retrospective Study. Pulm Ther. 2020;6(1):107–17. doi: 10.1007/ s41030-020-00112-x.Epub 2020 Mar 17.PMID: 32185642; PMCID: PMC7229022.
Kruzliak P, Syamasundar RP, Novak M et al. Unilateral absence of pulmonary artery: pathophysiology, symptoms, diagnosis and current treatment. Arch Cardiovasc Dis. 2013 Aug-Sep; 106, (8–9): 448 – 54. Doi: 10.1016 / j.a CVD 2013.05.004. Epub 2013 Aug 9. PMID: 23938302.
Download references
This work is supported by Natural science foundation (22JR11RA036).
Author information
Authors and affiliations.
Department of Interventional medicine, The First Clinical Medical College of Lanzhou University, the First Hospital of Lanzhou University, Lanzhou, 730000, Gansu Province, China
Fan Wei, Chang Yaowen & Wang Wenhui
You can also search for this author in PubMed Google Scholar
Contributions
All authors have the same contribution.
Corresponding author
Correspondence to Wang Wenhui .
Ethics declarations
Ethical approval.
This study was approved by the Ethics Committee of the First Hospital of Lanzhou University, in line with the Helsinki-International Declaration.
Competing interests
The authors declare no competing interests.
No potential conflict of interest was reported by the author(s).
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wei, F., Yaowen, C. & Wenhui, W. Embolization treatment of right pulmonary artery agenisis with patent ductus arteriosus causing pulmonary hypertension and hemoptysis: a case report and literature review. J Cardiothorac Surg 19 , 391 (2024). https://doi.org/10.1186/s13019-024-02883-9
Download citation
Received : 22 January 2024
Accepted : 15 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s13019-024-02883-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pulmonary artery agenesis
- Pulmonary hypertension
Journal of Cardiothoracic Surgery
ISSN: 1749-8090
- General enquiries: [email protected]
Calculation of the minimum clinically important difference (MCID) using different methodologies: case study and practical guide
- Original Article
- Open access
- Published: 28 June 2024
Cite this article
You have full access to this open access article

- Anita M. Klukowska 1 , 2 ,
- W. Peter Vandertop 1 ,
- Marc L. Schröder 3 &
- Victor E. Staartjes ORCID: orcid.org/0000-0003-1039-2098 4
39 Accesses
Explore all metrics
Introduction
Establishing thresholds of change that are actually meaningful for the patient in an outcome measurement instrument is paramount. This concept is called the minimum clinically important difference (MCID). We summarize available MCID calculation methods relevant to spine surgery, and outline key considerations, followed by a step-by-step working example of how MCID can be calculated, using publicly available data, to enable the readers to follow the calculations themselves.
Thirteen MCID calculations methods were summarized, including anchor-based methods, distribution-based methods, Reliable Change Index, 30% Reduction from Baseline, Social Comparison Approach and the Delphi method. All methods, except the latter two, were used to calculate MCID for improvement of Zurich Claudication Questionnaire (ZCQ) Symptom Severity of patients with lumbar spinal stenosis. Numeric Rating Scale for Leg Pain and Japanese Orthopaedic Association Back Pain Evaluation Questionnaire Walking Ability domain were used as anchors.
The MCID for improvement of ZCQ Symptom Severity ranged from 0.8 to 5.1. On average, distribution-based methods yielded lower MCID values, than anchor-based methods. The percentage of patients who achieved the calculated MCID threshold ranged from 9.5% to 61.9%.
Conclusions
MCID calculations are encouraged in spinal research to evaluate treatment success. Anchor-based methods, relying on scales assessing patient preferences, continue to be the “gold-standard” with receiver operating characteristic curve approach being optimal. In their absence, the minimum detectable change approach is acceptable. The provided explanation and step-by-step example of MCID calculations with statistical code and publicly available data can act as guidance in planning future MCID calculation studies.
Similar content being viewed by others
Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions.

The anchor design of anchor-based method to determine the minimal clinically important difference: a systematic review
Values derived from patient reported outcomes in spine surgery: a systematic review of the minimal clinically important difference, substantial clinical benefit, and patient acceptable symptom state
Avoid common mistakes on your manuscript.
The notion of minimum clinically important difference (MCID) was introduced to establish thresholds of change in an outcome measurement instrument that are actually meaningful for the patient. Jaeschke et al . originally defined it “as the smallest difference in score in the domain of interest which the patient perceives as beneficial and which would mandate, in the absence of troublesome side-effects and excessive cost, a change in the patient’s management” [ 1 ].
In many clinical trials statistical analyses only focuses on intergroup comparisons of raw outcome scores using parametric/non-parametric tests and deriving conclusions based on the p -value. Using the classical threshold of p- value < 0.05 only suggests that the observed effect is unlikely to have occurred by chance, but it does not equate to a change that is clinically meaningful for the patient [ 2 ]. Calculating MCID scores, and using them as thresholds for “treatment success”, ensures that patients’ needs and preferences are considered and allows for comparison of proportion of patients experiencing a clinically relevant improvement among different groups [ 3 ]. Through MCID, clinicians can better understand the impact of an intervention on their patients’ lives, sample size calculations can become more robust and health policy makers may decide which treatments deserve reimbursement [ 4 , 5 , 6 ].
The MCID can be determined from the patient’s perspective, where it is the patient who decides whether a change in their health was meaningful [ 4 , 7 , 8 , 9 ]. This is the most common “gold-standard” approach and one that we will focus on. Occasionally, the clinician’s perspective can also be used to determine MCID. However, MCID for a clinician may not necessarily mean an increase in a patient’s functionality, but rather a change in disease survival or treatment planning [ 10 ]. MCID can also be defined at a societal level, as e.g. improvement in a patient’s functionality significant enough to aid their return to work [ 11 ].
MCID thresholds are intended to assess an individual’s clinical improvement and ought not to be applied to mean scores of entire groups post-intervention, as doing so may falsely over-estimate treatment effectiveness. It is also noteworthy to mention that obtained MCID values are not treatment-specific but broadly disease category-specific. They rely on a patient’s perception of clinical benefit, which is influenced by their diagnosis and subsequent symptoms, not just treatment modality.
In this study, we summarize available MCID calculation methods and outline key considerations when designing a MCID study, followed by a step-by-step working example of how MCID can be calculated.
Navigating the case study
To illustrate the MCID methods and to enable the reader to follow the practical calculation guide of different MCID values, based on the described methods along the way, a previously published data set of 84 patients, as described in Minetama et al ., was used based on CC0.10 license [ 12 ]. Data can be downloaded at https://data.mendeley.com/datasets/vm8rg6rvsw/1 . The statistical R code can be found in Supplementry content 1 including instructions on formatting the data set for MCID calculations The title of different MCID methods in the paper (listed below) and their number correspond to the same title and respective number in the R code. All analyses in this case study were carried out using R version 2023.12 + 402 (The R Foundation for Statistical Computing, Vienna Austria) [ 13 ].
The aim of Minetama et al . was to assess the effectiveness of supervised physical therapy (PT) with unsupervised at-home-exercises (HE) in patients with lumbar spinal stenosis (LSS). The main inclusion criteria were presence of neurogenic intermittent claudication and pain/or numbness in the lower extremities with or without back pain and > 50 years of age; diagnosis of LSS confirmed on MRI and a history of ineffective response to therapy for ≥ 3 months. Patients were then randomized into a 6-week PT or HE programme [ 12 ]. All data was pooled, as a clinically significant benefit for patients is independent of group allocation and because MCID is disease-specific. Therefore, the derived MCID will be applicable to most patients with lumbar spinal stenosis, irrespective of treatment modality. Change scores were calculated by subtracting baseline scores from follow-up scores.
MCID calculation methods
There are multiple approaches to calculate MCID, mainly divided into anchor-based and distribution-based methods (Fig. 1 ) [ 4 , 10 , 14 , 15 , 16 , 17 ]. Before deciding on the method, it needs to be defined whether the calculated MCID will be for improvement or deterioration [ 18 ]. Most commonly, MCID is used to measure improvement (as per Jaeschke et al . definition) [ 1 , 4 , 7 , 14 , 15 , 16 , 19 , 20 ]. The value of MCID for improvement should not be directly applied in reverse to determine whether a decrease in patients' scores signifies a clinically meaningful deterioration – those are two separate concepts [ 18 ]. In addition, the actual MCID value ought to be applied to post-intervention score of an individual patient (not the overall score for the whole group), to determine whether, at follow-up, he or she experienced a change equating to MCID or more, compared to their baseline score. Such patient is then classified as “responders”.

Flow diagram presenting range of Minimum clinically important difference calculation methods stratified into anchor, distribution-based and “other” described in the study. MCID, Minimum Clinically Important Difference; MIC, Minimal Important Change
According to the Consensus-based Standards for the selection of health measurement instruments (COSMIN) guidelines, the “anchor-based” approach is regarded as the “gold-standard” [ 21 , 22 , 23 ]. In this approach, we determine the MCID of a chosen outcome measurement, based on whether a pre-defined MCID (usually derived from another published study) was achieved by an external criterion, known as the anchor, usually another patient-reported outcome measure (PROM) or an objective test of functionality [ 4 , 7 , 8 , 15 , 16 , 17 , 18 , 20 ]. It is best to use scales which allow the patient to rate the specific aspect of their health related to the disease of interest post-intervention compared to baseline on a Likert-type scale. This scale may range, for example, from “much worse”, “somewhat worse”, “about the same”, “somewhat better”, to “much better”, such as the established Global Assessment Rating tool [ 7 , 8 , 24 , 25 ]. Depending on the scale, some studies determine MCID by calculating change scores for patients who only ranked themselves as “somewhat better”, and some only consider patients who ranked themselves as “much better” [ 7 , 25 , 26 , 27 , 28 , 29 ]. This discrepancy is likely an explanation for a range of MCID for a single outcome measure dependent on the methodology. There appears to be no singular “correct” approach. One of the alternatives to the Global assessment rating is the use of the health transition item (HTI) from the SF-36 questionnaire, where patients are asked about their overall health compared to one year ago [ 7 , 30 , 31 ]. Although quick and easy to conduct, the patient’s response may be influenced by comorbid health issues other than those targeted by intervention. Nevertheless, any anchor where the patient is the one to decide what change is clinically meaningful, captures the true essence of the MCID. One should however, be mindful of the not easily addressed recall bias with such anchors – patients at times do not reliably remember their baseline health status [ 32 ]. Moreover, what the above anchors do not consider is, whether the patient would still choose the intervention for the same condition despite experiencing side-effects or cost. That can be addressed through implementing anchors such as the Satisfaction with Results scale described in Copay et al ., who found that MCID values based on the Satisfaction with Results scale were slightly higher than those derived from HTI-SF-36 [ 7 , 33 ].
Other commonly used outcome scales, such as Oswestry Disability Index (ODI), Roland–Morris Disability Questionnaire (RMDQ), Visual Analogue Scale (VAS), or EQ5D-3L Health-Related Quality of Life, can also act as anchors [ 7 , 14 , 16 , 34 , 35 ]. In such instances, patients complete the “anchor” questionnaire at baseline and post-intervention and the MCID of that anchor is derived from a previous publication [ 12 , 16 , 35 ]. Before deciding on the MCID, full understanding of how it was derived in that previous publication is crucial. Ideally, this should be done for a population similar to our study cohort, with comparable follow-up periods [ 18 , 20 ]. Correlations between the anchor instrument and the investigated outcome measurement instrument must be recorded, and ought to be at least moderate (> 0.05), as that is the best indicator of construct validity (whether both the anchor instrument and outcome instrument represent a similar construct of patient health) [ 18 , 36 ]. If such correlation is not available, the anchor-based MCID credibility instrument is available to aid in assessing construct proximity between the two [ 36 , 37 ].
Once the process for selecting an anchor and classifying “responders” and “non-responders” is established, the MCID can be calculated. The outcome instrument of interest will be defined as an outcome for which we want to calculate the MCID. The first anchor-based method (within-patient change) focuses on the average improvement seen among clear responders in the anchor. The between-patient change anchor-based method additionally subtracts the average improvement seen among non-responders (unchanged and/or worsened) and consequently ends up with a smaller MCID value. Finally, an anchor-based method based on Receiver Operating Characteristic (ROC) curve analysis–that can be considered the current “gold standard”- also exists, which effectively looks at the MCID calculation as a sort of diagnostic instrument and aims to improve the discriminatory performance of our MCID threshold. In the following paragraphs, the three anchor-based methods are described in more detail. The R code (Supplementry Content 1 ) enables the reader to follow the text and to calculate MCID for the Zurich Claudication Questionnaire (ZCQ) Symptom Severity domain, based on a publicly available dataset [ 12 ].
Choice of outcome measurement instruments for MCID calculation case study
The chosen outcome measurement instrument in this case study for which MCID for improvement will be calculated is ZCQ Symptom Severity domain [ 12 ]. The ZCQ is composed of three subscales: symptom severity (7 questions, score per question ranging from 1 to 5 points); physical function (5 questions, score per question ranging from 1 to 4 points) and patient satisfaction with treatment scale (6 questions, score per question ranging from to 4 points). Higher scores indicate greater disability/worse satisfaction [ 38 ]. To visualize different MCID values, Numeric Rating Scale (NRS) for Leg Pain (score from 0 “no pain” to 10 “worse possible pain) and Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ) Walking Ability domain are chosen, as they showed high responsiveness in patients with LSS post-operatively [ 39 ].Through 25 questions, the JOABPEQ assesses five distinctive domains: pain-related symptoms, lumbar spine dysfunction, walking ability, impairment in social functioning and psychological disturbances. The score for each domain ranges from 0 to 100 points (higher score indicating better health status) [ 40 ]. The correlation of ZCQ symptom severity with NRS Leg Pain and JOABPEQ Walking Ability domain, is 0.56 and − 0.51, respectively [ 39 ]. For a patient to be classified as a “responder”, using the NRS for Leg pain or JOABPEQ walking ability, the score at 6-week follow-up must have improved by 1.6 points or 20 points, respectively [ 7 , 40 , 41 ].
This publicly available dataset does not report patient satisfaction or any kind of global assessment rating.
To enable calculation of global assessment rating-based MCID methods for educational purposes, despite very limited availability of studies providing MCID for deterioration of JOABPEQ, we decided to stratify patients in this dataset into the three following groups, based on the JOABPEQ Walking Ability as an anchor: likely improved (change score above 20 points according to Kasai et al . ), no significant change (− 20– + 20 points change score), and likely deteriorated (lower than − 20 points change score) [ 41 ]. As obtained MCID values were expected to be negative, all values, for clarity of presentation, were multiplied by − 1, except in Method (IX), where graphical data distribution was shown.
The different methods in detail
Method (i) calculating mcid using “within-patient” score change.
The first method focuses on calculating the change between baseline and post-intervention score of our outcome instrument, for each patient classified as a “responder”. A “responder” is a patient who, at follow-up, has achieved the pre-defined MCID of the anchor (or ranks themselves high enough on Global assessment rating type scale based on our methodology). The MCID is then defined as the mean change in the outcome instrument of interest of those classified as “responders” [ 4 , 7 , 16 , 31 ].
The corresponding R-Code formula is described in Step 5a of Supplementry Content 1 . Calculated within-patient MCID of ZCQ Symptom Severity based on NRS Leg Pain and JOABPEQ Walking Ability domain was 4.4 and 4.2, respectively.
Method (II) calculating MCID using “between-patient” score change
In this approach, the mean change in our outcome instrument is calculated for not only “responders” but also for “non-responders”. “Non-responders” are patients who did not achieve the pre-defined MCID of our anchor or who did not rank themselves high enough (unchanged, or sometimes: unchanged + worsened) on Global Assessment Rating type scale according to our methodology. The minimum clinically important difference of our outcome instrument is then defined as the difference between the mean change scores of “responders” and “non-responders” [ 4 , 7 , 16 , 19 ].
The corresponding R-Code formula is described in Step 5b of Supplementry content 1 . Calculated between-patient MCID of ZCQ Symptom Severity based on NRS Leg Pain and JOABPEQ Walking Ability domain was 3.5 and 2.8, respectively.
Method (III) calculating MCID using the ROC analysis
Here the MCID is derived through ROC analysis to identify the “threshold” score of our outcome instrument that best discriminates between “responders” and “non-responders” of the anchor [ 4 , 7 , 16 , 19 , 27 ]. To understand ROC, one must familiarize oneself with the concept of sensitivity and specificity. In ROC analysis, sensitivity is defined as the ability of the test to correctly detect “true positives”, which in this context refers to patients who have achieved a clinically meaningful change.
“False negative” would be a patient, who was classified as “non-responder” but is really a “responder”. Specificity is defined as the ability of a test to correctly detect a “true negative” result- a patient who did not achieve a clinically meaningful change – a “non-responder” [ 25 ].
A “false positive” would be a patient, who was classified as a “responder” but who was a “non-responder”. Values for sensitivity and specificity range from 0 to 1. Sensitivity of 1 means that the test can detect 100% of “true positives”’ (“responders”), while specificity of 1 reflects the ability to detect 100% of “true negatives” (“non-responders”). It is unclear what the minimum sensitivity and specificity should be for a “gold-standard” MCID, which is why the most established approach is to opt for a MCID threshold that maximizes both sensitivity and specificity at the same time, which can be done using ROC analysis [ 4 , 7 , 25 , 31 , 42 ]. During ROC analysis, the “closest-to-(0,1)-criterion” (the top left most point of the curve) or the Youden index are the two methods to automatically determine the optimal threshold point [ 43 ].
When conducting the ROC analysis, the Area under the curve (AUC) is also determined–a measure of how well the MCID threshold discriminates responders and non-responders in general. Values in AUC can range 0–1. An AUC of 0.5 signifies that the score discriminates no better than random chance, whereas a value of 1 means that the score perfectly discriminates between responders and non-responders. In the literature, an AUC of 0.7 and 0.8 is deemed fair (acceptable), while ≥ 0.8 to < 0.9 is considered good and values ≥ 0.9 are considered excellent [ 44 ]. Calculating the AUC provides a rough estimate of how well the chosen MCID threshold performs. The corresponding R-Code formula is described in Step 5c of Supplementry content 1 . Statistical package pROC was used. The calculated MCID of ZCQ symptom severity based on NRS Leg Pain and JOABPEQ Walking Ability domain was for both 1.5.
Calculation of MCID through distribution-based methods
Calculation of MCID using the distribution-based approach focuses on statistical properties of the dataset [ 7 , 14 , 16 , 27 , 45 ]. Those methods are objective, easy to calculate, and in some cases, yield values close to anchor-based MCID. The advantage of this approach is that it does not rely on any external criterion or require additional studies on previously established MCIDs or other validated “gold standard” questionnaires for the specific disease in each clinical setting. However, it fails to include the patient’s perspective of a clinically meaningful change, which will be discussed later in this study. In this sense, distribution-based methods focus on finding MCID thresholds that enable mathematical distinction of what is considered a changed vs. unchanged score, whereas anchor-based methods focus on finding MCID thresholds which represent a patient-centered, meaningful improvement.
Method (IV) calculating MCID through Standard Error of Measurement (SEM)
The standard error of measurement conceptualizes the reliability of the outcome measure, by determining how repeated measurements of an outcome may differ from the “true score”. Greater SEM equates to lower reliability, which is suggestive of meaningful inconsistencies in the values produced by the outcome instrument despite similar measuring conditions. Hence, it has been theorized that 1 SEM is equal to MCID, because a change score ≥ 1 SEM, is unlikely to be due to measurement error and therefore is also more likely to be clinically meaningful [ 46 , 47 ]. The following formula is used: [ 1 , 7 , 35 , 46 , 48 ].
The ICC, also called reliability coefficient, signifies level of agreement or consistency between measurements taken on different occasions or by different raters [ 49 ]. There are various ways of calculating the ICC depending on the used model with values < 0.5, 0.5– 0.75, 0.75–0.9 and > 0.90 indicating poor, moderate, good and excellent reliability, respectively [ 49 ]. While a value of 1 × SEM is probably the most established way to calculate MCID, in the literature, a range of multiplication factors for SEM-based MCID have been used, including 1.96 SEM or even 2.77 SEM to identify a more specific threshold for improvement [ 48 , 50 ]. The corresponding R-Code formula is described in Step 6a of Supplementry Content 1 . The chosen ZCQ Symptom Severity ICC was 0.81 [ 51 ]. The SEM-based MCID was 1.9.
Method (V) calculating MCID through Effect Size (ES)
Effect size (ES) is a standardized measure of the strength of the relationship or difference between two variables [ 52 ]. It is described by Cohen et al . as “degree to which the null hypothesis (there is no difference between the two groups) is false”. It allows for direct comparison of different instruments with different units between studies. There are multiple forms to calculate ES, but for the purpose of MCID calculations, the ES represents the number of SDs by which the post-intervention score has changed from baseline score. It is calculated based on the following formula incorporating the average change score divided by the SD of the baseline score: [ 52 ].
According to Cohen et al . 0.2 is considered small ES, 0.5 is moderate ES and 0.8 or more is large ES [ 53 ]. Most commonly, a change score with an ES of 0.2 is considered equivalent to MCID [ 7 , 16 , 31 , 54 , 55 , 56 ]. Using this method, we are basically identifying the mean change score (in this case reflecting the MCID) that equates to an ES of 0.2: [ 7 , 55 ].
Practically, if a patient experienced small improvement in an outcome measure post intervention, the ES will be smaller than for a patient who experienced a large improvement in outcomes measure. The corresponding R-Code formula is described in Step 6b of Supplementry Content 1 . The ES-based MCID was 0.9.
Method (VI) calculating MCID through Standardized Response Mean (SRM)
The Standardized Response Mean (SRM) aims to gauge the responsiveness of an outcome similarly to ES. Initially described by Cohen et al . as a derivative of ES assessing differences of paired observations in a single sample, later renamed as SRM, it is also considered an “index of responsiveness” [ 38 , 53 ]. However, the denominator is SD of the change scores–not the SD of the baseline scores–while the numerator remains the average change score from baseline to follow-up: [ 10 , 45 , 57 , 58 , 59 ].
Similarly, to Cohen’s rule of interpreting ES, it has been theorized that responsiveness can be considered low if SRM is 0.2–0.5, moderate if > 0.5–0.8 and large if > 0.8 [ 58 , 59 , 60 ]. Again, a change score equating to SRM of 0.2 (although SRM of 1/3 or 0.5 were also proposed) can be considered MCID, although studies have used the overall SRM as MCID as well [ 45 , 54 , 56 , 61 ]. However, since SRM is a standardized index, similarly to ES, the aim of the SRM-based method ought to be to identify a change score that indicates responsiveness of 0.2: [ 61 ].
Similar to the ES-based method, the SRM-based approach for calculating the MCID is not commonly used in in spine surgery studies [ 14 ]. It is a measure of responsiveness, which is the ability to detect change over time in a construct to be measured by the instrument, and ought to be therefore calculated for the study-specific change score rather than extrapolated as a “universal” MCID threshold to other studies. The corresponding R-Code formula is described in Step 6c of Supplementry Content 1 . The SRM-based MCID was 0.8.
The limitation of using Method (V) and (VI) in MCID calculations will be later described in Discussion.
Method (VII) calculating MCID through SD
Standard Deviation represents the average spread of individual data points around the mean value of the outcome measure. Norman et al . found in their review of studies using MCID in health-related quality of life instruments that most studies had an average ES of 0.5, which equated to clinically meaningful change score of 0.5 × SD of baseline score [ 7 , 16 , 30 ].
The corresponding R-Code formula is described in Step 6d of Supplementry content 1 . The SD-based MCID was 2.1.
Method (VIII) calculating MCID through 95% Minimum Detectable Change (MDC)
The MDC is defined as the minimal change below which there is a 95% chance that it is due to measurement error of the outcome measurement instrument: [ 7 , 61 ].
Usually, value corresponding to z is the desired level of confidence, which for 95% confidence level is 1.96. Although MDC–like all distribution-based methods–does not consider whether a change is clinically meaningful, the calculated MCID should be at least the same or greater than MDC to enable distinguishing true mathematical change from measurement noise. The 95% MDC calculation, is the most common distribution-based approach in spinal surgery, and it appears to most closely resemble anchor-derived MCID values, as demonstrated by Copay et al . [ 7 , 14 , 62 ]. The corresponding R-Code formula is described in Step 6e of Supplementry Content 1 . The 95% MDC was 5.1.
Method (IX) calculating MCID through Reliable Change Index
Another less frequently applied method through which “responders and “non-responders” can be classified but which does not rely on an external criterion is the Reliable Change Index (RCI), also called the Jacobson–Truax index [ 63 , 64 ]. It indicates whether an individual change score is statistically significantly greater than a change in score that could have occurred due to random measurement error alone [ 63 ].
In theory, a patient can be considered to experience a statistically reliably identifiable improvement ( p < 0.05), if the individual RCI is > 1.96. Again, it does not reflect whether the change is clinically meaningful for the patient but rather that the change should not be attributed to measurement error alone and likely has a component of true score change. Therefore, this method is discouraged in MCID calculations as it relies on statistical properties of the sample and not patient preferences–as all distribution-based methods do [ 65 ]. In the example of Bolton et al . who focused on the Bournemouth Questionnaire in patients with neck pain, RCI was subsequently used to discriminate between “responders” and “non-responders”. The ROC analysis approach was then used to determine the MCID [ 64 ]. The corresponding R-Code formula is described in Step 6f of Supplementry Content 1 . Again, pROC package was used. The ROC-derived MCID was 2.5.
Other methods
Method (x) calculating mcid through anchor-based minimal important change (mic) distribution model.
In theory, combining anchor- and distribution-based methods could yield superior results. Some suggestions include averaging the values of various methods, simply combining two different methods (i.e. both an anchor-based criterion such as ROC-based MCID from patient satisfaction and 95% MDC-based MCID have to both be met to consider a patient as having achieved MCID) [ 25 ]. In 2007, de Vet et al . introduced a new visual method of MCID calculations that does not only combine but also integrates both anchor- and distribution-based calculations [ 25 ]. In addition, their method allows the calculation of both MCID for improvement and for deterioration, as these can differ.
In short form, using an anchor, patients were divided into three “importantly improved”, “not importantly changed” and “importantly deteriorated” groups (Fig. 2 ) . Then distribution expressed in percentiles of patients who “importantly improved”, “importantly deteriorated” and “not importantly changed” were plotted on a graph. This is the anchor-based part of the approach, ensuring that MCID thresholds chosen have clinical value.
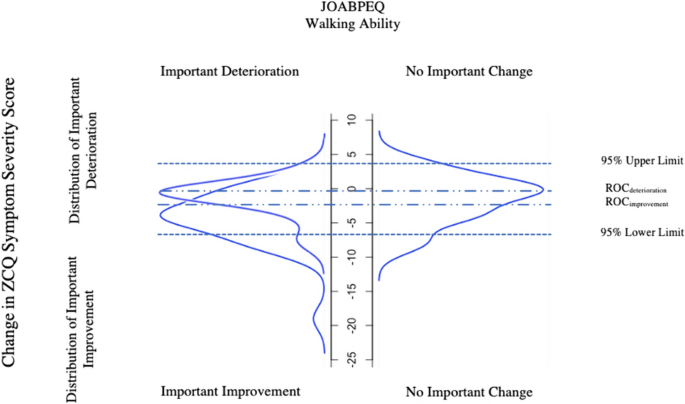
Distribution of the Zurich Claudication Questionnaire Symptom Severity change scores for patients categorized as experiencing “important improvement”, “no important change” or “important deterioration” in JOABPEQ walking ability as an anchor (Method (X)). For ZCQ Symptom Severity score to improve, the actual value must decrease explaining the negative values in the model. ROC , Receiver Operating Characteristic; ZCQ , Zurich Claudication Questionnaire; JOABPEQ , Japanese Orthopaedic Association Back Pain Evaluation Questionnaire
The second part of the approach is then entirely focused on the group of patients determined by the anchor to be “unchanged”, and can be either distribution- or anchor-based:
In the first and more anchor-based method, the ROC-based method described in Method (III) is applied to find the threshold for improvement (by finding the ROC-based threshold point that optimizes sensitivity and specificity of identifying improved vs unchanged patients) or for deterioration (by finding the ROC-based threshold point that optimizes sensitivity and specificity of identifying deteriorated vs unchanged patients). For example, the threshold for improvement is found by combining the improved and unchanged groups, and then testing out different thresholds for discriminating those two groups from each other. The optimal point on the resulting ROC curve based on the closest-to-(0,1)-criterion is then found.
In the second method, which is distribution-based, the upper 95% (for improvement) and lower 95% (for deterioration) limits are found based solely on the group of patients determined to be unchanged. The following formula is used (instead, subtracting instead of adding the 1.645 × SD for deterioration or improvement, respectively): [ 25 ]
The corresponding R-Code formula can be found under Step 7a in Supplementry Content 1 . The model is presented in Fig. 2 . The 95% upper limit and 95% lower limit was 4.1 and − 7.2 respectively. The ROC-derived MCID using RCI was − 2.5 (important improvement vs unchanged) and − 0.5 (important deterioration vs unchanged). For the purpose of the model, MCID values were not multiplied by − 1 but remained in original form.
Method (XI) calculating MCID as 30% Reduction from Baseline
In recent years, a simple 30% reduction from baseline values has been introduced as an alternative to MCID calculations [ 66 ]. It has been speculated that absolute-point changes are difficult to interpret and have limited value in context of “ceiling” and “floor” effects (i.e. values that are on the extreme spectra of the measurement scale) [ 4 ]. To overcome this, Khan et al . found that 30% reduction in PROMs has similar effectiveness as traditional anchored or distribution-based methods in detecting patients with clinically meaningful differences post lumbar spine surgery [ 15 ]. The corresponding R-Code formula can be found under Step 7b in Supplementry Content 1 .
Method (XII) Calculating MCID through Delphi method
The Delphi Method is a systemic approach using the collective opinion of experts to establish a consensus regarding a medical issue [ 67 ]. It has mostly been used to develop best practice guidelines [ 68 ]. However, it can also be used to aid MCID determination [ 69 ]. The method focuses on distributing questionnaires or surveys to panel of members. The anonymized answers are grouped together and shared again with the expert panel in subsequent rounds. This allows the experts to reflect on their opinions and consider strengths and weaknesses of the others response. The process is repeated until consensus is reached. Ensuring anonymity, this prevents any potential bias linked to a specific participant’s concern about their own opinion being viewed or influenced by other personal factors [ 67 ].
Method (XIII) calculating MCID through Social Comparison Approach
The final approach is asking patients to compare themselves to other patients, which requires time and resources [ 70 ]. In a study by Redelmeier et al . patients with chronic obstructive pulmonary disease in a rehabilitation program were organized into small groups and observed each other at multiple occasions [ 70 ]. Additionally, each patient was paired with another participant and had a one-to-one interview with them discussing different aspects of their health. Finally, each patient anonymously rated themselves against their partner on a scale “much better”, “somewhat better”, “a little bit better”, “about the same”, “a little bit worse” “somewhat worse” and “much worse”. MCID was then calculated based on the mean change score of patients who graded themselves as “a little bit better” (MCID for improvement) or a “little bit worse” (MCID for deterioration), like in the within-patient change and between-patient change method described in Method (I) and (II) [ 70 ].
Substantial Clinical Benefit
Over the years, it has been noted that MCID calculations based either purely on distribution-based method or only group of patients rating themselves as “somewhat better” or “slightly better” does not necessarily constitute a change that patients would consider beneficial enough “to mandate, in the absence of troublesome side effects and excessive cost, to undergo the treatment again” [ 3 , 24 ]. Therefore, the concept of substantial clinical benefit (SCB) has been introduced as a way of identifying a threshold of clinical success of intervention rather than a “floor” value for improvement- that is MCID [ 24 ]. For example, in Carreon et al ., ROC derived SCB “thresholds” were defined as a change score with equal sensitivity and specificity to distinguish “much better” from “somewhat better” patients post cervical spinal fusion [ 71 ]. Glassman et al . on the other hand used ROC derived SCB thresholds to discriminate between “much better” and “about the same” patients following lumbar spinal fusion. The authors stress that SCB and MCID are indeed separate entities, and one should not be used to derive the other [ 24 ]. Thus, while the methods to derive SCB and MCID thresholds can be carried out similarly based on anchors, the ultimate goal of applying SCB versus MCID is different.
Using the various methods explained above, overall, MCID for improvement for ZCQ Symptoms Severity domain ranged from 0.8 to 5.1 (Table 1 ). Here, the readers obtained results can be checked for correctness. On average distribution-based MCID values were lower than anchor-based MCID values. Within distribution-based approach, method (VIII) “Minimum detectable change” resulted in MCID of 5.1, which exceeded the MCID’s derived using the “gold-standard” anchor-based approaches. The average MCID based on anchor of NRS Leg pain and JOABPEQ walking ability was 3.1 and 2.8, respectively. Dependent on methods used, percentage of responders to HE and PT intervention fell within range of 9.5% for “30% Reduction from Baseline” method to 61.9% using ES- and SRM-based method (Table 2 ). Method (X) is graphically presented in Fig. 2 .
As demonstrated above, the MCID is dependent upon the methodology and the chosen anchor, highlighting the necessity for careful preparation in MCID calculations. The lowest MCID of 0.8 was calculated for Method (VI) being SRM. Logically, if a patient on average had a baseline ZCQ Symptom Severity score of 23.2, an improvement of 0.8 is unlikely to be clinically meaningful, even if rounded up. It rather informs on the measurement error property of our instrument as explained by COSMIN. Additionally, the distribution-based methods rely on statistical properties of the sample, which varies from cohort to cohort making it only generalizable to patient groups with similar SD but not applicable to others with a different spread of data [ 52 ]. Not surprisingly, anchor-based methods considering patient preferences yielded on average higher MCID values than distribution-based methods, which again varied from anchor to anchor. The mean MCID for improvement calculated for NPRS Leg Pain was 3.1, while for JOABPEQ Walking Ability it was 2.8—such similar values prove the importance of selecting responsive anchors with at least moderate correlations. Despite assessing different aspects of LSS disease, the MCID remained comparable in this specific case.
Interestingly, Method (VIII) MDC yielded the highest value of 5.1, exceeding the “gold-standard” ROC-derived MCID. This suggests that, in this example, using this ROC-derived MCID in clinical practice would be illogical, as the value falls within the measurement error determined by MDC. Here it would be appropriate to choose MDC approach as the MCID. Interestingly, ROC-derived MCID values based on Global Assessment Rating like stratification of patients based on their JOABPEQ Walking Ability (Method X) yielded higher MCID, than in Method (III). This may be attributed to a more a balanced distribution of “responders” and “non-responders” (only unchanged patients) in Method (X), unlike in the latter (Method III) where patients were strictly categorized into “responders” and “non-responders” (including both deteriorated and unchanged). This further highlights the importance of using global assessment rating type scales in determining the extent of clinical benefit.
Although ES-based (Method (V)) and SRM-based (Method (VI)) MCID calculations have been described in the literature, ES and SRM were originally created to quantify the strength of relationship between scores of two samples (in case of ES) and change score of paired observations in one sample (in case of SRM) [ 53 , 58 , 59 ]. They do offer an alternative to MCID calculations. However, verification with other MCID calculation methods, ideally anchor-based, is strongly recommended. As seen in this case study and other MCID’s derived similarly, they often result small estimates [ 7 , 55 ]. There is also no consensus regarding the choice of SD of Change Score vs. SD of Baseline Score as denominator. Additionally, whether the calculated MCID (mean change score) should represent value, such as the ES is 0.2 indicating small effect, or value should be 0.5 suggesting moderate effect is currently arbitrary and often relies on the researcher’s preference [ 53 , 55 , 59 ]. Both ES and SRM can be used to assess whether the overall change score observed in single study is suggestive of a clinically meaningful benefit in that specific cohort or in case of SRM, whether the outcome measure is responsive. However, it is our perspective that extending such value as “MCID” from one study to another is not recommended.
One can argue whether there is even a place for distribution-based methods in MCID calculations. They ultimately fail to provide an MCID value that meets the original definition of Jaeschke et al . “of smallest change in the outcome that the patient would identify as important”. At no point are patients asked about what constitutes a meaningful change for them, and the value is derived from statistical properties of the sample solely [ 1 ]. Nevertheless, conduction of studies on MCID implementing scales such as Global Assessment Rating is time-consuming and performing studies for each patient outcome and each disease is likely not feasible. Distribution-based methods still have some merit in that they–like the 95% MDC method—can help distinguish measurement noise and inaccuracy from true change. Even if anchor-based methods should probably be used to define MCID thresholds, they ought to be supported by a calculation of MDC so that it can be decided whether the chosen threshold makes sense mathematically (i.e., can reliably be distinguished from measurement inaccuracies) as seen in our case study.
Calculating MCID for different diagnoses
Previously, MCID thresholds for outcome measurement instruments were calculated for generic populations, such as patients suffering from low back pain. More recently, MCID values for commonly used PROMs in spine surgery, such as ODI, RMDQ or NRS have been calculated for more narrowly defined diagnoses, such as lumbar disc herniation (LDH) or LSS. The question arises as to whether a separate MCID is needed for all the different spinal conditions. In general, establishing an MCID specific to these patient groups is only recommended if these patient’s perception of meaningful change is different from that of low back pain in general. Importantly, again, the MCID should not be treatment-specific, but rather broadly disease specific. Therefore, it is advisable to use MCID based on patients who had the most similar disease characteristics to our cohort. For example, an MCID for NRS Back Pain based on study group composed of different types of lumbar degenerative disease, may in some cases, be applied to study cohort composed solely of patients with LDH. However, no such extrapolation should be performed for populations with back pain secondary to malignancy, due to a totally different pathogenesis and associated symptoms that may influence the ability to detect a clinically meaningful change in the above NRS Back Pain such as fatigue or anorexia.
Study cohort characteristics that influence MCID
Regardless of robust methodology, it can be expected that it is impossible to obtain the same MCID on different occasions even in the same population due to the inherent subjectivity of what is perceived as “clinically beneficial” and day-to-day symptom fluctuation. However, it was found that patients who have worse baseline scores, reflecting e.g., more advanced disease, require greater overall change at follow-up to report it as clinically meaningful [ 72 ]. One should also be mindful of “regression to the mean” where extremely high or low-scoring patients then subsequently score closer to baseline at second measurement [ 73 ]. Therefore, adequate cohort characteristics need to be presented, for the readers to judge how generalizable the MCID may be to their study cohort. If a patient pre-operatively experiences NRS Leg Pain of 1, and the MCID is 1.6, they cannot achieve MCID at all, as the maximum possible change score is smaller than the MCID threshold (“floor effect”). A similar situation can occur with patients closer to the higher end of the scale (“ceiling effect”). The general rule is, that if at least 15% of the study cohort has the highest or lowest possible score for a given outcome instrument, one can expect significant “ceiling/floor effects” [ 50 ]. One way to overcome this, is through transferring absolute MCID scores to percentage change scores [ 4 , 45 ]. However, percentage change scores only account for high baseline scores, if high baseline scores indicate larger disability (as seen with ODI) and have a possibility of larger change. If a high score in an instruments reflects better health status (as seen in in SF-36), than percentage change scores will increase the association with baseline score [ 4 ]. In general, it is important to consider which patient to exclude from certain analyses when applying MCID: For example, patients without relevant disease preoperatively (for example, those exhibiting so-called “patient-accepted symptom states”, PASS) should probably be excluded altogether when reporting the percentage of patients achieving MCID [ 74 ].
Establishing reliable thresholds for MCID is key in clinical research and forms the basis of patient-centered treatment evaluations when using patient-reported outcome measures or objective functional tests. Calculation of MCID thresholds can be achieved using a variety of different methods, each yielding completely different results, as is demonstrated in this practical guide. Generally, anchor-based methods relying on scales assessing patient preferences/satisfaction or global assessment ratings continue to be the “gold-standard” approach- the most common being ROC analysis. In the absence of appropriate anchors, the distribution-based MCID based on the 95% MDC approach is acceptable, as it appears to yield the most similar results compared to anchor-based approaches. Moreover, we recommend using it as a supplement to any anchor-based MCID thresholds to check if they can reliably distinguish true change from measurement inaccuracies. The explanation provided in this practical guide with step-by-step examples along with public data and statistical code can add as guidance for future studies calculating MCID thresholds.
Jaeschke R, Singer J, Guyatt GH (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 10:407–415. https://doi.org/10.1016/0197-2456(89)90005-6
Article CAS PubMed Google Scholar
Concato J, Hartigan JA (2016) P values: from suggestion to superstition. J Investig Med 64:1166. https://doi.org/10.1136/jim-2016-000206
Article PubMed PubMed Central Google Scholar
Zannikos S, Lee L, Smith HE (2014) Minimum clinically important difference and substantial clinical benefit: Does one size fit all diagnoses and patients? Semin Spine Surg 26:8–11. https://doi.org/10.1053/j.semss.2013.07.004
Article Google Scholar
Copay AG, Subach BR, Glassman SD et al (2007) Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 7:541–546. https://doi.org/10.1016/j.spinee.2007.01.008
Article PubMed Google Scholar
Lanario J, Hyland M, Menzies-Gow A et al (2020) Is the minimally clinically important difference (MCID) fit for purpose? a planned study using the SAQ. Euro Respirat J. https://doi.org/10.1183/13993003.congress-2020.2241
Neely JG, Karni RJ, Engel SH, Fraley PL, Nussenbaum B, Paniello RC (2007) Practical guides to understanding sample size and minimal clinically important difference (MCID). Otolaryngol Head Neck Surg 136(1):14–18. https://doi.org/10.1016/j.otohns.2006.11.001
Copay AG, Glassman SD, Subach BR et al (2008) Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry disability index, medical outcomes study questionnaire short form 36, and pain scales. Spine J 8:968–974. https://doi.org/10.1016/j.spinee.2007.11.006
Andersson EI, Lin CC, Smeets RJ (2010) Performance tests in people with chronic low back pain: responsiveness and minimal clinically important change. Spine 35(26):E1559-1563. https://doi.org/10.1097/BRS.0b013e3181cea12e
Mannion AF, Porchet F, Kleinstück FS, Lattig F, Jeszenszky D, Bartanusz V, Dvorak J, Grob D (2009) The quality of spine surgery from the patient’s perspective. Part 1: the core outcome measures index in clinical practice. Euro Spine J 18:367–373. https://doi.org/10.1007/s00586-009-0942-8
Crosby RD, Kolotkin RL, Williams GR (2003) Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 56:395–407. https://doi.org/10.1016/S0895-4356(03)00044-1
Gatchel RJ, Mayer TG (2010) Testing minimal clinically important difference: consensus or conundrum? Spine J 10:321–327. https://doi.org/10.1016/j.spinee.2009.10.015
Minetama M, Kawakami M, Teraguchi M et al (2019) Supervised physical therapy vs. home exercise for patients with lumbar spinal stenosis: a randomized controlled trial. Spine J 19:1310–1318. https://doi.org/10.1016/j.spinee.2019.04.009
R Core Team (2021) R A Language and Environment for Statistical Computing
Chung AS, Copay AG, Olmscheid N, Campbell D, Walker JB, Chutkan N (2017) Minimum clinically important difference: current trends in the spine literature. Spine 42(14):1096–1105. https://doi.org/10.1097/BRS.0000000000001990
Khan I, Pennings JS, Devin CJ, Asher AM, Oleisky ER, Bydon M, Asher AL, Archer KR (2021) Clinically meaningful improvement following cervical spine surgery: 30% reduction versus absolute point-change MCID values. Spine 46(11):717–725. https://doi.org/10.1097/BRS.0000000000003887
Gautschi OP, Stienen MN, Corniola MV et al (2016) Assessment of the minimum clinically important difference in the timed up and go test after surgery for lumbar degenerative disc disease. Neurosurgery. https://doi.org/10.1227/NEU.0000000000001320
Kulkarni AV (2006) Distribution-based and anchor-based approaches provided different interpretability estimates for the hydrocephalus outcome questionnaire. J Clin Epidemiol 59:176–184. https://doi.org/10.1016/j.jclinepi.2005.07.011
Wang Y, Devji T, Qasim A et al (2022) A systematic survey identified methodological issues in studies estimating anchor-based minimal important differences in patient-reported outcomes. J Clin Epidemiol 142:144–151. https://doi.org/10.1016/j.jclinepi.2021.10.028
Parker SL, Godil SS, Shau DN et al (2013) Assessment of the minimum clinically important difference in pain, disability, and quality of life after anterior cervical discectomy and fusion: clinical article. J Neurosurg Spine 18:154–160. https://doi.org/10.3171/2012.10.SPINE12312
Carrasco-Labra A, Devji T, Qasim A et al (2021) Minimal important difference estimates for patient-reported outcomes: a systematic survey. J Clin Epidemiol 133:61–71. https://doi.org/10.1016/j.jclinepi.2020.11.024
Prinsen CAC, Mokkink LB, Bouter LM et al (2018) COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 27:1147–1157. https://doi.org/10.1007/s11136-018-1798-3
Article CAS PubMed PubMed Central Google Scholar
Mokkink LB, de Vet HCW, Prinsen CAC et al (2018) COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 27:1171–1179. https://doi.org/10.1007/s11136-017-1765-4
Terwee CB, Prinsen CAC, Chiarotto A et al (2018) COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 27:1159–1170. https://doi.org/10.1007/s11136-018-1829-0
Glassman SD, Copay AG, Berven SH et al (2008) Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am 90:1839–1847. https://doi.org/10.2106/JBJS.G.01095
de Vet HCW, Ostelo RWJG, Terwee CB et al (2007) Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res 16:131–142. https://doi.org/10.1007/s11136-006-9109-9
Solberg T, Johnsen LG, Nygaard ØP, Grotle M (2013) Can we define success criteria for lumbar disc surgery? Acta Orthop 84:196–201. https://doi.org/10.3109/17453674.2013.786634
Power JD, Perruccio AV, Canizares M et al (2023) Determining minimal clinically important difference estimates following surgery for degenerative conditions of the lumbar spine: analysis of the Canadian spine outcomes and research network (CSORN) registry. The Spine Journal 23:1323–1333. https://doi.org/10.1016/j.spinee.2023.05.001
Asher AL, Kerezoudis P, Mummaneni PV et al (2018) Defining the minimum clinically important difference for grade I degenerative lumbar spondylolisthesis: insights from the quality outcomes database. Neurosurg Focus 44:E2. https://doi.org/10.3171/2017.10.FOCUS17554
Cleland JA, Whitman JM, Houser JL et al (2012) Psychometric properties of selected tests in patients with lumbar spinal stenosis. Spine J 12:921–931. https://doi.org/10.1016/j.spinee.2012.05.004
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41:582–592. https://doi.org/10.1097/01.MLR.0000062554.74615.4C
Parker SL, Mendenhall SK, Shau DN et al (2012) Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same-level recurrent lumbar stenosis: understanding clinical versus statistical significance. J Neurosurg Spine 16:471–478. https://doi.org/10.3171/2012.1.SPINE11842
Gatchel RJ, Mayer TG, Chou R (2012) What does/should the minimum clinically important difference measure?: a reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain 28:387. https://doi.org/10.1097/AJP.0b013e3182327f20
Lloyd H, Jenkinson C, Hadi M et al (2014) Patient reports of the outcomes of treatment: a structured review of approaches. Health Qual Life Outcomes 12:5. https://doi.org/10.1186/1477-7525-12-5
Beighley A, Zhang A, Huang B et al (2022) Patient-reported outcome measures in spine surgery: a systematic review. J Craniovertebr Junction Spine 13:378–389. https://doi.org/10.4103/jcvjs.jcvjs_101_22
Ogura Y, Ogura K, Kobayashi Y et al (2020) Minimum clinically important difference of major patient-reported outcome measures in patients undergoing decompression surgery for lumbar spinal stenosis. Clin Neurol Neurosurg 196:105966. https://doi.org/10.1016/j.clineuro.2020.105966
Wang Y, Devji T, Carrasco-Labra A et al (2023) An extension minimal important difference credibility item addressing construct proximity is a reliable alternative to the correlation item. J Clin Epidemiol 157:46–52. https://doi.org/10.1016/j.jclinepi.2023.03.001
Devji T, Carrasco-Labra A, Qasim A et al (2020) Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ 369:m1714. https://doi.org/10.1136/bmj.m1714
Stucki G, Daltroy L, Liang MH et al (1996) Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine 21:796
Fujimori T, Ikegami D, Sugiura T, Sakaura H (2022) Responsiveness of the Zurich claudication questionnaire, the Oswestry disability index, the Japanese orthopaedic association back pain evaluation questionnaire, the 8-item short form health survey, and the Euroqol 5 dimensions 5 level in the assessment of patients with lumbar spinal stenosis. Eur Spine J 31:1399–1412. https://doi.org/10.1007/s00586-022-07236-5
Fukui M, Chiba K, Kawakami M et al (2009) JOA back pain evaluation questionnaire (JOABPEQ)/ JOA cervical myelopathy evaluation questionnaire (JOACMEQ) the report on the development of revised versions April 16, 2007: the subcommittee of the clinical outcome committee of the Japanese orthopaedic association on low back pain and cervical myelopathy evaluation. J Orthop Sci 14:348–365. https://doi.org/10.1007/s00776-009-1337-8
Kasai Y, Fukui M, Takahashi K et al (2017) Verification of the sensitivity of functional scores for treatment results–substantial clinical benefit thresholds for the Japanese orthopaedic association back pain evaluation questionnaire (JOABPEQ). J Orthop Sci 22:665–669. https://doi.org/10.1016/j.jos.2017.02.012
Glassman SD, Carreon LY, Anderson PA, Resnick DK (2011) A diagnostic classification for lumbar spine registry development. Spine J 11:1108–1116. https://doi.org/10.1016/j.spinee.2011.11.016
Perkins NJ, Schisterman EF (2006) The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163:670–675. https://doi.org/10.1093/aje/kwj063
Nahm FS (2022) Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol 75:25–36. https://doi.org/10.4097/kja.21209
Angst F, Aeschlimann A, Angst J (2017) The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J Clin Epidemiol 82:128–136. https://doi.org/10.1016/j.jclinepi.2016.11.016
Wyrwich KW, Tierney WM, Wolinsky FD (1999) Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 52:861–873. https://doi.org/10.1016/s0895-4356(99)00071-2
Wolinsky FD, Wan GJ, Tierney WM (1998) Changes in the SF-36 in 12 months in a clinical sample of disadvantaged older adults. Med Care 36:1589–1598
Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD (1999) Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care 37:469–478. https://doi.org/10.1097/00005650-199905000-00006
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
McHorney CA, Tarlov AR (1995) Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 4:293–307
Hara N, Matsudaira K, Masuda K et al (2016) Psychometric assessment of the Japanese version of the Zurich claudication questionnaire (ZCQ): reliability and validity. PLoS ONE 11:e0160183. https://doi.org/10.1371/journal.pone.0160183
Kazis LE, Anderson JJ, Meenan RF (1989) Effect sizes for interpreting changes in health status. Med Care 27:S178–S189. https://doi.org/10.1097/00005650-198903001-00015
Cohen J (1988) Statistical power analysis for the behavioral sciences. L Erlbaum Associates, Hillsdale, NJ
Franceschini M, Boffa A, Pignotti E et al (2023) The minimal clinically important difference changes greatly based on the different calculation methods. Am J Sports Med 51:1067–1073. https://doi.org/10.1177/03635465231152484
Samsa G, Edelman D, Rothman ML et al (1999) Determining clinically important differences in health status measures: a general approach with illustration to the health utilities index mark II. Pharmacoeconomics 15:141–155. https://doi.org/10.2165/00019053-199915020-00003
Wright A, Hannon J, Hegedus EJ, Kavchak AE (2012) Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther 20:160–166. https://doi.org/10.1179/2042618612Y.0000000001
Stucki G, Liang MH, Fossel AH, Katz JN (1995) Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J Clin Epidemiol 48:1369–1378. https://doi.org/10.1016/0895-4356(95)00054-2
Liang MH, Fossel AH, Larson MGS (1990) Comparisons of five health status instruments for orthopedic evaluation. Med Care 28:632–642
Middel B, Van Sonderen E (2002) Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr care. https://doi.org/10.5334/ijic.65
Revicki D, Hays RD, Cella D, Sloan J (2008) Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61:102–109. https://doi.org/10.1016/j.jclinepi.2007.03.012
Woaye-Hune P, Hardouin J-B, Lehur P-A et al (2020) Practical issues encountered while determining minimal clinically important difference in patient-reported outcomes. Health Qual Life Outcomes 18:156. https://doi.org/10.1186/s12955-020-01398-w
Parker SL, Mendenhall SK, Shau D et al (2012) Determination of minimum clinically important difference in pain, disability, and quality of life after extension of fusion for adjacent-segment disease. J Neurosurg Spine 16:61–67. https://doi.org/10.3171/2011.8.SPINE1194
Jacobson NS, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 59:12–19
Bolton JE (2004) Sensitivity and specificity of outcome measures in patients with neck pain: detecting clinically significant improvement. Spine 29(21):2410–2417. https://doi.org/10.1097/01.brs.0000143080.74061.25
Blampied NM (2022) Reliable change and the reliable change index: Still useful after all these years? Cogn Behav Ther 15:e50. https://doi.org/10.1017/S1754470X22000484
Asher AM, Oleisky ER, Pennings JS et al (2020) Measuring clinically relevant improvement after lumbar spine surgery: Is it time for something new? Spine J 20:847–856. https://doi.org/10.1016/j.spinee.2020.01.010
Barrett D, Heale R (2020) What are Delphi studies? Evid Based Nurs 23:68–69. https://doi.org/10.1136/ebnurs-2020-103303
Droeghaag R, Schuermans VNE, Hermans SMM et al (2021) Evidence-based recommendations for economic evaluations in spine surgery: study protocol for a Delphi consensus. BMJ Open 11:e052988. https://doi.org/10.1136/bmjopen-2021-052988
Henderson EJ, Morgan GS, Amin J et al (2019) The minimum clinically important difference (MCID) for a falls intervention in Parkinson’s: a delphi study. Parkinsonism Relat Disord 61:106–110. https://doi.org/10.1016/j.parkreldis.2018.11.008
Redelmeier DA, Guyatt GH, Goldstein RS (1996) Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 49:1215–1219. https://doi.org/10.1016/s0895-4356(96)00206-5
Carreon LY, Glassman SD, Campbell MJ, Anderson PA (2010) Neck disability index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J 10:469–474. https://doi.org/10.1016/j.spinee.2010.02.007
Wang Y-C, Hart DL, Stratford PW, Mioduski JE (2011) Baseline dependency of minimal clinically important improvement. Phys Ther 91:675–688. https://doi.org/10.2522/ptj.20100229
Tenan MS, Simon JE, Robins RJ et al (2021) Anchored minimal clinically important difference metrics: considerations for bias and regression to the mean. J Athl Train 56:1042–1049. https://doi.org/10.4085/1062-6050-0368.20
Staartjes VE, Stumpo V, Ricciardi L et al (2022) FUSE-ML: development and external validation of a clinical prediction model for mid-term outcomes after lumbar spinal fusion for degenerative disease. Eur Spine J 31:2629–2638. https://doi.org/10.1007/s00586-022-07135-9
Download references
Open access funding provided by University of Zurich. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Neurosurgery, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam, The Netherlands
Anita M. Klukowska & W. Peter Vandertop
Department of Neurosurgery, University Clinical Hospital of Bialystok, Bialystok, Poland
Anita M. Klukowska
Department of Neurosurgery, Park Medical Center, Rotterdam, The Netherlands
Marc L. Schröder
Machine Intelligence in Clinical Neuroscience and Microsurgical Neuroanatomy (MICN) Laboratory, Department of Neurosurgery, Clinical Neuroscience Center, University Hospital Zurich, University of Zurich, Zurich, Switzerland
Victor E. Staartjes
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Victor E. Staartjes .
Ethics declarations
Conflict of interest.
The authors declare that the article and its content were composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TXT 6 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Klukowska, A.M., Vandertop, W.P., Schröder, M.L. et al. Calculation of the minimum clinically important difference (MCID) using different methodologies: case study and practical guide. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08369-5
Download citation
Received : 03 May 2024
Revised : 17 May 2024
Accepted : 10 June 2024
Published : 28 June 2024
DOI : https://doi.org/10.1007/s00586-024-08369-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Minimum clinically important difference
- Anchor-based methods
- Distribution-based methods
- Change scores
- Clinical outcomes
- Spine surgery
- Find a journal
- Publish with us
- Track your research
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
An evaluation on the effect of water-saving renovation on a large-scale irrigation district: a case study in the north china plain.

1. Introduction
2. materials and methods, 2.1. research methods, 2.2. data sources, 2.3. an overview of the research area, 2.3.1. natural conditions, 2.3.2. socioeconomic benefits, 2.3.3. irrigation engineering, 3.1. the outcomes of water-saving renovation in the irrigation district, 3.1.1. spatial pattern of water-saving renovation, 3.1.2. time pattern of water-saving renovation, 3.2. temporal and spatial evolution of irrigation water use, 3.2.1. spatial evolution of irrigation water use, 3.2.2. time evolution of irrigation water use, 3.3. temporal and spatial evolution of groundwater depth in irrigation district, 3.3.1. spatial evolution of groundwater depth, 3.3.2. time evolution of groundwater burial depth, 4. discussion, 4.1. analysis of water-saving renovation, 4.2. analysis of changes in irrigation water use, 4.3. groundwater impact analysis, 5. conclusions, author contributions, data availability statement, conflicts of interest.
- Fan, X.; Qin, J.; Xu, L.; Liu, S.; Gu, S.; Lyu, M. Construction and empirical analysis of the evaluation index system for the water-saving level of large-sized irrigation districts. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2021 , 37 , 99–107. [ Google Scholar ]
- Zan, Z.; Yue, W.; Zhao, H.; Cao, C.; Wu, F.; Lin, P.; Wu, J. Analysis of the Scale Effect and Temporal Stability of Groundwater in a Large Irrigation District in Northwest China. Agronomy 2023 , 13 , 2172. [ Google Scholar ] [ CrossRef ]
- Mi, L.; Tian, J.; Si, J.; Chen, Y. Eco-hydrological effects of agricultural water-saving in the Yinchuan Plain, Northwest China. Reg. Environ. Change 2022 , 23 , 13. [ Google Scholar ] [ CrossRef ]
- Xu, D.; Li, Y.; Gong, S.; Zhang, B. Impacts of climate change on agricultural water management and its coping strategies. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2019 , 35 , 79–89. [ Google Scholar ]
- Contor, B.A.; Taylor, R.G. Why Improving Irrigation Efficiency Increases Total Volume of Consumptive Use. Irrig. Drain. 2013 , 62 , 273–280. [ Google Scholar ] [ CrossRef ]
- Pfeiffer, L.; Lin, C.-Y.C. Does efficient irrigation technology lead to reduced groundwater extraction? Empirical Evidence. J. Environ. Econ. Manag. 2014 , 67 , 189–208. [ Google Scholar ] [ CrossRef ]
- Grafton, J.W.R.Q.; Perry, C.J.; Molle, F.; Ringler, C.; Steduto, P.; Udall, B.; Wheeler, S.A. The paradox of irrigation efficiency. Science 2018 , 361 , 748–758. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, L.; Ma, Q.; Zhao, Y.; Wu, X.; Yu, W. Determining the influence of irrigation efficiency improvement on water use and consumption by conceptually considering hydrological pathways. Agric. Water Manag. 2019 , 213 , 674–681. [ Google Scholar ] [ CrossRef ]
- Kang, S. Accelerate the modernization and renovation of irrigation areas to fill the gaps in national food security. China Water Resour. 2020 , 9 , 1–5. [ Google Scholar ]
- Wen, Y.; Wan, H.; Shang, S. A monthly distributed water and salt balance model in irrigated and non-irrigated lands of arid irrigation district with shallow groundwater table. J. Hydrol. 2023 , 616 , 128811. [ Google Scholar ] [ CrossRef ]
- Hao, N.; Sun, P.; He, W.; Yang, L.; Qiu, Y.; Chen, Y.; Zhao, W. Water Resources Allocation in the Tingjiang River Basin: Construction of an Interval-Fuzzy Two-Stage Chance-Constraints Model and Its Assessment through Pearson Correlation. Water 2022 , 14 , 2928. [ Google Scholar ] [ CrossRef ]
- Tsuchihara, T.; Yoshimoto, S.; Shirahata, K.; Nakazato, H.; Ishida, S. Analysis of groundwater-level fluctuation and linear regression modeling for prediction of initial groundwater level during irrigation of rice paddies in the Nasunogahara alluvial fan, central Japan. Environ. Earth Sci. 2023 , 82 , 473. [ Google Scholar ] [ CrossRef ]
- Chen, M.; Luo, Y.; Shen, Y.; Han, Z.; Cui, Y. Driving force analysis of irrigation water consumption using principal component regression analysis. Agric. Water Manag. 2020 , 234 , 106089. [ Google Scholar ] [ CrossRef ]
- Malamos, N.; Koutsoyiannis, D. Field survey and modelling of irrigation water quality indices in a Mediterranean island catchment: A comparison between spatial interpolation methods. Hydrol. Sci. J. 2018 , 63 , 1447–1467. [ Google Scholar ] [ CrossRef ]
- Zhang, D.; Qin, M.; Yuan, Q.; Fan, J.; Fan, G.; Ma, Z.; Chai, J. Kriging interpolation reconstruction of surrounding rock moisture content field. Environ. Earth Sci. 2023 , 82 , 575. [ Google Scholar ] [ CrossRef ]
- Liu, S.; Wu, F.; Zai, S.; Hao, S.; Leghari, S.J.; Li, L. An Optimized Cloud Model Algorithm Adapted to Comprehensive Benefit Evaluation of Water-Saving Irrigation. Pol. J. Environ. Stud. 2021 , 30 , 3713–3726. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, Q.; Bai, Q.J.; Nie, K.K.; Wang, H.; Zeng, X.M. Multi-objective optimization allocation of regional water resourcesbased on NSGA-III algorithm and TOPSIS decision. J. Drain. Irrigation Mach. Eng. 2022 , 40 , 1233–1240. [ Google Scholar ]
- Zhao, Q. Optimization of Water Resources Allocation in Yahekou Irrigation District of Henan Province Based on the Three Red Lines Management Mechanism ; Xi’an University of Technology: Xi’an, China, 2022. [ Google Scholar ]
- Jaiswal, R.K.; Ali, S.; Jain, S.; Galkate, R.V.; Krishan, G.; Lohani, A.K.; Kumar, S. Monitoring and modelling approaches for quantitative assessment of irrigation return flows in a command. Environ. Earth Sci. 2024 , 83 , 161. [ Google Scholar ] [ CrossRef ]
- Saha, S.; Chakma, N.; Sam, K. Responses of rural livelihood with limited access to water resources: A case from water-scarce region of West Bengal, India, Environment. Dev. Sustain. 2023 , 361 , 1–27. [ Google Scholar ] [ CrossRef ]
- Sun, Y.; Li, X.; Shi, H.; Ma, H.; Wang, W.; Jian, W. Evolution Mechanism of Soil Sainization in Hetao Irrigation District underCondition of Water-saving Reform Based on Remote Sensing Technology. J. Agric. Mach. 2022 , 12 , 366–379. [ Google Scholar ]
- Liu, X.; Wang, X.; Guo, H.; An, X. Benefit Allocation in Shared Water-Saving Management Contract Projects Based on Modified Expected Shapley Value. Water Resour. Manag. 2020 , 35 , 39–62. [ Google Scholar ] [ CrossRef ]
- Hur, A.Y.; Page, M.A.; Guest, J.S.; Ploschke, C.M. Financial analysis of decentralized water reuse systems in mission critical facilities at U.S. Army installations. Environ. Sci. Water Res. Technol. 2024 , 10 , 603–613. [ Google Scholar ] [ CrossRef ]
- Zhong, L. Analysis on investment performance and influencing factors of farmland water conservancy infrastructure in various regions of China based on SFA method. In Proceedings of the 2021 International Conference on Statistics, Applied Mathematics, and Computing Science, CSAMCS 2021, Nanjing, China, 26–28 November 2021. [ Google Scholar ]
- Zai, S.; Feng, X.; Wu, F.; Li, L. Effect of time scales on probability of irrigation water requirement of farmland. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2018 , 34 , 96–102. [ Google Scholar ]
- Niu, X.; Lu, C.; Zhang, Y.; Zhang, Y.; Wu, C.; Saidy, E.; Liu, B.; Shu, L. Hysteresis response of groundwater depth on the influencing factors using an explainable learning model framework with Shapley values. Sci. Total Environ. 2023 , 904 , 166662. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Qin, J.; Xiaoni, L.; Zhitian, L.; Huibin, G.; Wenjie, Z.; Zhaxi, N.; Wenlong, H. Response and prediction of groundwater depth changes to climate change in the Huaibei Plain of China. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2022 , 38 , 136–145. [ Google Scholar ]
- Perry, C. Efficient irrigation; inefficient communication; flawed recommendations. Irrig. Drain. 2007 , 56 , 367–378. [ Google Scholar ] [ CrossRef ]
- Manekar, A.; Jena, S.; Panda, R.K. Estimation of Groundwater Evapotranspiration and Extinction Depth Using Diurnal Water Table Fluctuation and Remote Sensing Observations: A Case Study in Agriculture-Dominated Watershed of Eastern India. Water Conserv. Sci. Eng. 2024 , 9 , 4. [ Google Scholar ] [ CrossRef ]
- do Nascimento, T.V.M.; de Oliveira, R.P.; de Melo, M.T.C. Impacts of large-scale irrigation and climate change on groundwater quality and the hydrological cycle: A case study of the Alqueva irrigation scheme and the Gabros de Beja aquifer system. Sci. Total Environ. 2024 , 907 , 168151. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Canal Name | Quantity | Length (km) |
|---|---|---|
| Main canal | 2 | 86.279 |
| Divided main canal | 8 | 197.968 |
| Branch canal | 118 | 680.657 |
| Divided canal | 28 | 134.125 |
| Year | Canal Lining (m) | Canal Lining (m ) | Anti-Seepage Reinforcement (m) | Landslide Control (m) | Canal Side Road (m) | Bridge | Canal System Buildings | Drainage Canal Lining (m) | Investment (USD Million) |
|---|---|---|---|---|---|---|---|---|---|
| 1998 | 4150 | 178,601 | / | 1747 | / | / | 1 | / | 2.07 |
| 1999 | 5204 | 71,834 | 6800 | / | / | / | 315 | / | 2.49 |
| 2000 | 9200 | 93,830 | 3750 | / | / | / | 24 | / | 1.66 |
| 2001 | 18,500 | 108,673 | / | / | / | / | 186 | / | 1.10 |
| 2002 | / | 203,328 | 5283 | / | / | / | 286 | / | 2.22 |
| 2003 | 41,542 | 1071,149 | 3970 | / | / | / | 46 | / | 2.21 |
| 2004 | 24,443 | 109,184 | 100 | / | / | / | 46 | / | 2.23 |
| 2005 | 11,798 | 184,685 | / | 500 | / | / | 27 | / | 2.76 |
| 2006 | 15,965 | 170,309 | / | 150 | 5000 | / | 6 | / | 2.49 |
| 2007 | 14,000 | 117,163 | 80 | 330 | 3500 | 7 | 9 | / | 1.10 |
| 2008 | 59,745 | 903,696 | / | / | 8000 | 31 | 17 | / | 7.73 |
| 2009 | 16,295 | 214,976 | 31,300 | / | / | 12 | 2 | / | 2.97 |
| 2010 | 66,931 | 736,242 | / | 2600 | 3000 | 44 | 249 | / | 10.02 |
| 2011 | 57,028 | 569,251 | / | 1408 | / | 65 | 119 | 464 | 6.68 |
| 2012 | 94,225 | 784,854 | 900 | 40 | 5000 | 59 | 350 | 1400 | 12.02 |
| 2013 | 82,212 | 513,547 | / | / | 9767 | 221 | 408 | / | 11.58 |
| 2014 | 35,902 | 197,017 | 6118 | / | 10,400 | 114 | 291 | 2917 | 11.14 |
| 2015 | 83,635 | 799,890 | / | 1777 | 6000 | 177 | 287 | 3400 | 22.68 |
| 2016 | 163,545 | 895,414 | / | 17,198 | 2200 | 481 | 854 | 2247 | 28.90 |
| 2017 | 9677 | 162,648 | 560 | / | 11,550 | 395 | 571 | / | 5.25 |
| 2018 | 15,132 | 225,318 | / | / | / | 71 | 127 | / | 7.27 |
| 2019 | 30,175 | 253,226 | / | / | / | 146 | 176 | / | 8.58 |
| 2020 | 1500 | 12,600 | / | / | / | / | 14 | / | 1.13 |
| 2021 | / | 0 | / | / | / | / | 4 | / | 0.26 |
| Variable Correlation Indicators (r) | Capital Investment (10,000 CNY/a) | |
|---|---|---|
| Changes in channel lining area (m /a) | Correlation coefficient | 0.617 ** |
| Significance (bilateral) | 0.004 | |
| Variable | Regression Coefficient (B) | Significance Level (Sig) | Coefficient of Determination (R ) |
|---|---|---|---|
| Changes in channel lining area (m /a) | 33.712 | 0.004 | 0.381 |
| Benefit-Related Indicators | Changes in Channel Lining Area | |
|---|---|---|
| Changes in total irrigation water use | Correlation coefficient (r) | −0.538 ** |
| Significance (bilateral) | 0.010 | |
| Changes in total irrigation water saved | Correlation coefficient (r) | 0.547 ** |
| Significance (bilateral) | 0.003 | |
| Variable | Regression Coefficient (B) | Significance Level (Sig) | R |
|---|---|---|---|
| Changes in total irrigation water use | −18.669 | 0.010 | 0.290 |
| Changes in total irrigation water saved | 11.939 | 0.003 | 0.300 |
| Benefit-Related Indicators | The Cumulative Lining Area of the County (m ) | Annual Average Precipitation (mm) | |
|---|---|---|---|
| The groundwater depth in FC (m) | Correlation coefficient | 0.906 ** | −0.768 * |
| Significance (bilateral) | 0.002 | 0.026 | |
| The groundwater depth in SQ (m) | Correlation coefficient | 0.791 * | −0.831 * |
| Significance (bilateral) | 0.019 | 0.011 | |
| The groundwater depth in WC (m) | Correlation coefficient | 0.824 * | −0.837 ** |
| Significance (bilateral) | 0.012 | 0.010 | |
| The groundwater depth in XY (m) | Correlation coefficient | 0.942 ** | −0.790 * |
| Significance (bilateral) | 0.000 | 0.020 | |
| The groundwater depth in TH (m) | Correlation coefficient | 0.533 | −0.832 * |
| Significance (bilateral) | 0.174 | 0.010 | |
| Regression Analysis Coefficient | The Groundwater Depth in FC (m) | The Groundwater Depth in SQ (m) | The Groundwater Depth in WC (m) | The Groundwater Depth in XY (m) | The Groundwater Depth in TH (m) |
|---|---|---|---|---|---|
| Regression coefficient of channel lining area (B) | 4.08 × 10 | 5.67 × 10 | 5.23 × 10 | 2.81 × 10 | 1.06 × 10 |
| Significance (bilateral) of channel lining area (Sig) | 0.002 | 0.019 | 0.012 | 0 | 0.174 |
| Canal lining area R | 0.82 | 0.625 | 0.678 | 0.888 | 0.284 |
| Annual average precipitation regression coefficient (B) | −1.5 × 10 | −1.5 × 10 | −0.8 × 10 | −1.4 × 10 | −0.8 × 10 |
| Annual average precipitation significance level (Sig) | 0.026 | 0.011 | 0.01 | 0.02 | 0.01 |
| Annual average precipitation (R ) | 0.589 | 0.690 | 0.700 | 0.624 | 0.692 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Liu, S.; Wu, F.; Li, P.; Wang, D.; Feng, X.; Wang, Z.; Yan, L.; Zhang, Z.; Li, Y.; Ji, M.; et al. An Evaluation on the Effect of Water-Saving Renovation on a Large-Scale Irrigation District: A Case Study in the North China Plain. Agronomy 2024 , 14 , 1434. https://doi.org/10.3390/agronomy14071434
Liu S, Wu F, Li P, Wang D, Feng X, Wang Z, Yan L, Zhang Z, Li Y, Ji M, et al. An Evaluation on the Effect of Water-Saving Renovation on a Large-Scale Irrigation District: A Case Study in the North China Plain. Agronomy . 2024; 14(7):1434. https://doi.org/10.3390/agronomy14071434
Liu, Shaobo, Feng Wu, Puyang Li, Dayang Wang, Xuefang Feng, Zonghua Wang, Lu Yan, Zhengan Zhang, Yuying Li, Mingfei Ji, and et al. 2024. "An Evaluation on the Effect of Water-Saving Renovation on a Large-Scale Irrigation District: A Case Study in the North China Plain" Agronomy 14, no. 7: 1434. https://doi.org/10.3390/agronomy14071434
Article Metrics
Further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Search Menu
- Sign in through your institution
- Computer Science
- Earth Sciences
- Information Science
- Life Sciences
- Materials Science
- Science Policy
- Advance Access
- Special Topics
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About National Science Review
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Powerful qtl mapping and favorable allele mining in an all-in-one population: a case study of heading date.
These authors equally contributed to this work
- Article contents
- Figures & tables
- Supplementary Data
Pengfei Wang, Ying Yang, Daoyang Li, Zhichao Yu, Bo zhang, Xiangchun Zhou, Lizhong Xiong, Jianwei Zhang, Yongzhong Xing, Powerful QTL mapping and favorable allele mining in an all-in-one population: a case study of heading date, National Science Review , 2024;, nwae222, https://doi.org/10.1093/nsr/nwae222
- Permissions Icon Permissions
The multiparent advanced generation intercross (MAGIC) population is characterized with great potentials in power and resolution of QTL mapping, but SNP-based GWAS does not fully play its potential. In this study, a MAGIC population of 1021 lines was developed from four Xian and four Geng varieties from 5 subgroups of rice. A total of 44,000 genes showed functional polymorphisms among eight parents, including frameshift variations or premature stop codon variations, which provides the potential to map almost all genes of the MAGIC population. Principal component analysis results showed that the MAGIC population had a weak population structure. A high-density bin map of 24,414 bins was constructed. Segregation distortion occurred in the regions possessing the genes underlying genetic incompatibility and gamete development. SNP-based association analysis and bin-based linkage analysis identified 25 significant loci and 47 QTLs for heading date, including 14 known heading date genes. The mapping resolution of genes is dependent on genetic effects with offset distances of less than 55 kb for major effect genes and less than 123 kb for moderate effect genes. Four causal variants and noncoding structure variants were identified to be associated with heading date. Three to four types of alleles with strong, intermediate, weak, and no genetic effects were identified from eight parents, providing flexibility for the improvement of rice heading date. In most cases, japonica rice carries weak alleles, and indica rice carries strong alleles and nonfunctional alleles. These results confirmed that the MAGIC population provides the exceptional opportunity to detect QTLs, and its use is encouraged for mapping genes and mining favorable alleles for breeding.
Author notes
| Month: | Total Views: |
|---|---|
| June 2024 | 115 |
Email alerts
Citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 2053-714X
- Print ISSN 2095-5138
- Copyright © 2024 China Science Publishing & Media Ltd. (Science Press)
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Case report
- Open access
- Published: 26 June 2024
Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC), new name and new problems: an illustration of one case with atypical morphology and biology
- Arnault Tauziède-Espariat 1 , 2 , 3 ,
- Lelio Guida 4 ,
- Volodia Dangouloff-Ros 5 , 6 ,
- Nathalie Boddaert 5 , 6 ,
- Gaëlle Pierron 7 , 8 ,
- Delphine Guillemot 7 ,
- Julien Masliah-Planchon 7 , 8 ,
- Lauren Hasty 1 ,
- Alice Métais 1 , 2 , 3 ,
- Fabrice Chrétien 1 &
- Pascale Varlet 1 , 2 , 3
Acta Neuropathologica Communications volume 12 , Article number: 104 ( 2024 ) Cite this article
111 Accesses
3 Altmetric
Metrics details
A novel histomolecular tumor of the central nervous system (CNS), the “diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC),” has recently been identified, based on a distinct DNA methylation profile and has been added to the 2021 World Health Organization Classification of CNS Tumors. This glioneuronal tumor mainly affects the supratentorial area in children and recurrently presents with a monosomy of chromosome 14. Herein, we report the case of a DNA-methylation based diagnosis of DGONC having atypical features, such as pseudo-rosettes and the absence of a chromosome 14 monosomy, thus rendering its diagnosis very challenging. Because of the wide variety of morphologies harbored by DGONC, a large range of differential diagnoses may be hypothesized from benign to malignant. Interestingly, the current case, like one previously reported, exhibited a co-expression of OLIG2, synaptophysin and SOX10, without GFAP immunopositivity. This particular immunophenotype seems to be a good indicator for a DGONC diagnosis. The classification of DGONC amongst glioneuronal or embryonal tumors is still debated. The clinical (a pediatric supratentorial tumor), morphological (from a benign oligodendroglioma-like tumor with microcalcifications and possible neuropil-like islands to a malignant embryonal tumor with a possible spongioblastic pattern), and immunohistochemical (co-expression of OLIG2 and synaptophsyin) profiles resemble CNS, neuroblastoma, FOXR2- activated and may potentially bring them together in a future classification. Further comprehensive studies are needed to conclude the cellular origin of DGONC and its prognosis.
Introduction
The “diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters” (DGONC) is a new tumor type recently isolated by a distinct DNA-methylation profile and introduced in the latest World Health Organization (WHO) Classification of Brain Tumors [ 1 , 2 ]. These tumors frequently harbor a chromosome 14 monosomy [ 1 , 2 ]. To date, 44 cases have been reported in the literature and very few data concerning their histopathological, radiological and clinical features are available [ 1 , 3 , 4 , 5 ]. The question concerning its classification within the chapter of glioneuronal or embryonal tumors is still debated [ 2 ]. Herein, we report an atypical case of DNA-methylation proven DGONC without a chromosome 14 monosomy and histopathological features resembling a Central Nervous System (CNS) neuroblastoma, FOXR2 -activated (CNS, NB-FOXR2).
Case presentation
A previously healthy 11-year-old boy presented with an episode of generalized seizure. A computerized tomodensitometry scan of the head showed a slightly hyperdense, heterogeneous cortical mass with calcifications in the left central sulcus. Bone scalloping was observed next to the tumor (Fig. 1 A). Cerebral magnetic resonance imaging showed the mass to have high signal intensity on T2-weighted images, heterogeneous contrast enhancement, high signal intensity on diffusion weighted images, with an intermediate apparent diffusion coefficient and low cerebral blood flow measured by arterial spin labeling perfusion (Fig. 1 B-F). The mass was surgically excised. Microscopically, we observed a densely cellular proliferation, infiltrating the brain parenchyma (Fig. 2 D). The tumor presented various histopathological patterns with pseudo-rosettes, spongioblastic, and oligodendroglioma-like features (Fig. 2 A-C). Some tumor cells were multinucleated (Fig. 2 B). Signs of malignancy were obvious (microvascular proliferation, a high mitotic index of 29 mitoses per 1.6 mm 2 , an elevated MIB-1 labeling index (30%) and necrosis (Fig. 2 D-E). Microcalcifications were observed (Fig. 2 F ) . There were no perivascuclar lymphocytic infiltrations, xanthomatous cells, eosinophilic granular bodies or Rosenthal fibres. Immunohistochemistry revealed INI1, BRG1, H3K27me3 and ATRX to be maintained. The tumor cells diffusely expressed OLIG2, synaptophysin, MAP2, NeuN (not shown) and SOX10 but did not express GFAP (Fig. 2 G-K). Synaptophysin revealed the presence of neuropil islands (Fig. 2 I). Neurofilament staining confirmed the tumor’s diffuse growth pattern (Fig. 2 L). No extravascular expression of CD34 was present. Lin28A, IDH1R132H and BRAFV600E were negative. DNA-methylation analysis classified the tumor as a DGONC (v12.8 with a calibrated score 0.99) (Fig. 3 ). The copy number variation analysis, generated from the DNA methylation profile, failed to reveal a chromosome 14 monosomy, but revealed a gain of chromosome 17q (Fig. 3 ). The DNA sequencing analysis found a PIK3R1 mutation. The RNA-sequencing analysis failed to reveal any fusion.
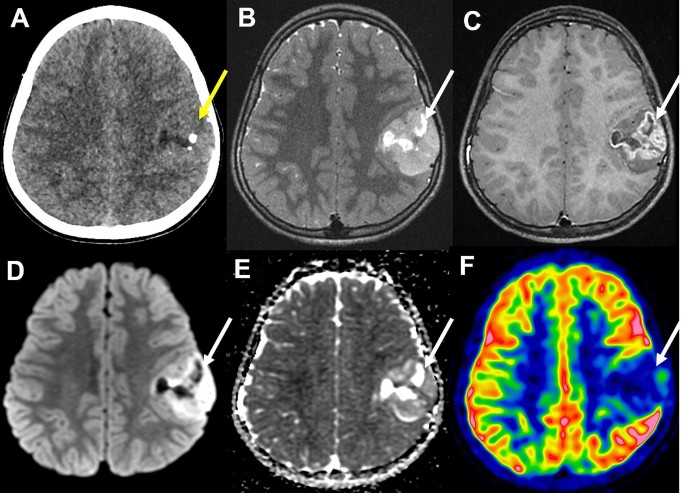
Radiological features of the tumor. ( A ) A computerized tomodensitometry scan showed a slightly hyperdense heterogeneous cortical mass with calcifications in the left central sulcus. Bone scalloping was observed next to the tumor. Magnetic resonance imaging showed the mass to have high signal intensity on T2-weighted images ( B ), heterogeneous contrast enhancement ( C ), high signal intensity on diffusion weighted images ( D ) with intermediate apparent diffusion coefficient ( E ) and low cerebral blood flow by arterial spin labeling perfusion ( F )

Histopathological features. ( A ) A highly cellular tumor showing varied histopathological patterns composed of pseudorosettes (HPS, magnification 400x), oligodendroglioma-like features and nuclear clusters (HPS, magnification 400x) ( B ) and spongioblastic arrangements (HPS, magnification 400x) ( C ). Microvascular proliferation (HPS, magnification 400x) ( D ) and microcalcifications ( E ) were present (HPS, magnification 400x). MIB1-labeling index was high (magnification 400x) ( F ). Diffuse expression of OLIG2 by tumor cells (magnification 400x) ( G ) and synaptophysin with perivascular strong staining (magnification 400x) ( H ) and neuropil-like islands (magnification 400x) ( I ). Diffuse expression of SOX10 by tumor cells (magnification 400x) ( J ), without immunopositivity for GFAP staining only residual astrocytes (magnification 400x) ( K ). Neurofilament staining confirming the diffuse growth pattern of the tumor (magnification 400x) ( L ). HPS: Hematoxylin phloxin saffron. Scale bars represent 50 μm

Results from DNA-methylation profiling analysis. Copy number variation analysis from DNA-methylation profiling analysis showed a partial gain of chromosome 7q and a gain of chromosome 17q and classified the tumor as a diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters
Discussion and conclusions
DGONC affect predominantly children (85% of reported cases) with a median age of 9 years-old (ranging from 2 to 75) without sex predominance (female to male ratio of 1.1) [ 1 , 3 , 4 , 5 ]. They are found in supratentorial locations outside the ventricles [ 1 , 3 , 4 , 5 ]. The tumors show variations in differentiation and cellularity, from moderately cellular oligodendroglioma-like features, microcalcifications and neuropil islands to highly cellular tumors, explaining why reported cases have previously been identified as primitive neuroectodermal tumors, anaplastic oligodendrogliomas, glioblastomas, extraventricular neurocytomas, or low-grade gliomas and dysembryoplastic neuroepithelial tumors [ 1 , 3 , 4 , 5 ]. DGONC is a provisional tumor type and its classification among glioneuronal vs. embryonal tumors is debated [ 2 ]. Herein, we present for the first time a case harboring pseudo-rosettes. The variety of histopathology and grading (neurocytic from embryonal morphology with neuropil islands and spongioblastic features) in association with the co-expression of OLIG2 and neuronal markers may morphologically resemble CNS NB-FOXR2 [ 2 , 6 ]. However, in contrast to the aforementioned tumors, DGONC expresses SOX10, which may potentially be important for a diagnosis [ 7 ]. A chromosome 1q gain, which is almost always observed in CNS NB-FOXR2, may also be encountered in DGONC (19/35 reported cases, 54%) [ 1 , 5 , 6 ]. The monosomy of chromosome 14, which is a desirable diagnostic criterion for the current WHO classification, may be exceptionally absent (1/44 reported cases, 2%) [ 1 , 2 , 3 , 4 , 5 ], as was the case in our observation. Because of its morphological heterogeneity and immunoprofile (co-expression of OLIG2 and synaptophysin), other differential diagnoses include glioneuronal tumors kinase-fused, glioneuronal tumors with an ATRX alteration, kinase fusion and anaplastic features (GTAKA), embryonal tumors with a BRD4::LEUTX fusion, CNS tumors with a BCOR alteration, pediatric diffuse high-grade gliomas, IDH- and H3-WT (with MYCN amplification), but also diffuse leptomeningeal glioneuronal tumors, polymorphous low-grade neuroepithelial tumors of the young, multinodular and vacuolating neuronal tumors, and extraventricular neurocytomas. All of these diagnoses can be ruled out by DNA-sequencing, RNA-sequencing, and DNA-methylation profiling analyses. Additional molecular features may be observed in DGONC, particularly GNAS mutations, a PIK3CA mutation [ 1 ], or PIK3R1 mutations, as in our case.
In a case similar to ours, Benesch et al. [ 3 ] reported homogeneous imaging characteristics of nine DGONC, including sharply delineated cortical-subcortical supra-tentorial masses, with small mass effect, bone remodeling, intermediate diffusion restriction, and variable contrast uptake. These imaging features may resemble embryonal tumors, except for diffusion, which is less consistently restricted in DGONC, arguing radiologically for a less aggressive behavior. Very few clinical data are available concerning the outcome of DGONC. Data from the literature show that 4/22 (18%) cases presented a progression [ 1 , 3 , 4 , 5 ] with a median progression-free survival of 18 months (ranging from 4 to 26) [ 1 , 3 ] and only two patients were deceased from their disease at the end of follow-up (2/25 cases, 8%) [ 1 , 3 , 4 , 5 ], 25 and 96 months after the initial diagnosis [ 1 ]. Interestingly, three cases were treated by total resection alone, and did not present any recurrence at the end of follow-up [ 3 , 5 ], one of them 108 months after the initial diagnosis [ 3 ]. Further series are needed to determine if the different morphologies (well and poorly differentiated) are correlated to different outcomes.
In conclusion, we expanded the characterization of DGONC with a novel case, which does not harbor a chromosome 14 monosomy and presents histopathological features similar to an embryonal tumor. Further studies are needed to characterize in detail this rare type of tumor and to conclude its cellular origin and prognosis.
Deng MY, Sill M, Sturm D, Stichel D, Witt H, Ecker J et al (2020) Diffuse glioneuronal tumour with oligodendroglioma-like features and nuclear clusters (DGONC) - a molecularly defined glioneuronal CNS tumour class displaying recurrent monosomy 14. Neuropathol Appl Neurobiol 46:422–430
Article CAS PubMed Google Scholar
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A (2016) WHO classification of tumours of the central nervous system. Intl. Agency for Research, City, pp 94–97
Google Scholar
Benesch M, Perwein T, Apfaltrer G, Langer T, Neumann A, Brecht IB et al (2022) MR Imaging and clinical characteristics of diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters. AJNR Am J Neuroradiol 43:1523–1529
Article CAS PubMed PubMed Central Google Scholar
Howie C, Ahmad T, McFadden K, Crooks B, McNeely PD, Walling S et al (2022) Diagnostics and prospective outcome of a diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters after surgical resection (DGONC): a case report. Neuro-Oncol Adv 4:vdac170
Article Google Scholar
Pickles JC, Mankad K, Aizpurua M, Paine SM, Bridges LR, Carceller F et al (2021) A case series of diffuse Glioneuronal Tumours with Oligodendroglioma-like features and nuclear clusters (DGONC). Neuropathol Appl Neurobiol 47:464–467
Article PubMed PubMed Central Google Scholar
Sturm D, Orr BA, Toprak UH, Hovestadt V, Jones DTW, Capper D et al (2016) New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164:1060–1072
Tauziède-Espariat A, Figarella-Branger D, Métais A, Uro-Coste E, Maurage C-A, Lhermitte B et al (2023) CNS neuroblastoma, FOXR2-activated and its mimics: a relevant panel approach for work-up and accurate diagnosis of this rare neoplasm. Acta Neuropathol Commun 11:43
Download references
Acknowledgements
We would like to thank the laboratory technicians at GHU Paris Neuro Sainte-Anne for their assistance with DNA-methylation analyses.
The authors declare that they have not received any funding.
Author information
Authors and affiliations.
Department of Neuropathology, GHU Paris - Psychiatry and Neuroscience, Sainte-Anne Hospital, 1, rue Cabanis, Paris, 75014, France
Arnault Tauziède-Espariat, Lauren Hasty, Alice Métais, Fabrice Chrétien & Pascale Varlet
Institut de Psychiatrie et Neurosciences de Paris (IPNP), UMR S1266, INSERM, IMA-BRAIN, Paris, France
Arnault Tauziède-Espariat, Alice Métais & Pascale Varlet
Laboratory of Somatic Genetics, Université de Paris, France3 Curie Institute Hospital, Paris, Paris, France
Department of pediatric Neurosurgery, APHP Hopital Universitaire Necker Enfants Malades, Université Paris-Cité, Paris, Paris, France
Lelio Guida
Department of pediatric Radiology, APHP, Hôpital Universitaire Necker Enfants Malades, Paris, France
Volodia Dangouloff-Ros & Nathalie Boddaert
Institut Imagine, INSERM U1299 and UMR 1163, Université Paris-Cité, Paris, France
Paris-Sciences-Lettres, Curie Institute Research Center, INSERMU830, Paris, France
Gaëlle Pierron, Delphine Guillemot & Julien Masliah-Planchon
Curie Institute Hospital, Laboratory of Somatic Genetics, Paris, France
Gaëlle Pierron & Julien Masliah-Planchon
You can also search for this author in PubMed Google Scholar
Contributions
ATE, LG, and VDR compiled the MRI and clinical records; ATE, AM, and PV conducted the neuropathological examinations; DG, GP, and JMP conducted the molecular studies; ATE, LH, and PV drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Arnault Tauziède-Espariat .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by GHU Paris Psychiatrie et Neurosciences, Sainte-Anne Hospital’s local ethic committee.
Consent for publication
The patient signed informed consent forms before treatment began.
Competing interests
The authors declare that they have no conflicts of interest directly related to the topic of this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tauziède-Espariat, A., Guida, L., Dangouloff-Ros, V. et al. Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC), new name and new problems: an illustration of one case with atypical morphology and biology. acta neuropathol commun 12 , 104 (2024). https://doi.org/10.1186/s40478-024-01822-y
Download citation
Received : 08 April 2024
Accepted : 14 June 2024
Published : 26 June 2024
DOI : https://doi.org/10.1186/s40478-024-01822-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chromosome 14
Acta Neuropathologica Communications
ISSN: 2051-5960
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Med (Lausanne)
- PMC11199683
Vibrio vulnificus infection from insect bites in Shanghai: a case report
1 Department of Traditional Chinese Surgery, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
2 Longhua Clinical Medical College of Shanghai University of Traditional Chinese Medicine, Shanghai, China
Gururaja Perumal Pazhani, SRM Institute of Science and Technology, India
Associated Data
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Infection with Vibrio vulnificus is associated with high rates of amputation and mortality. Alterations in the global climate have heightened the risk of atypical infections caused by this pathogen.
Case presentation
In the case report we describe, a 75-year-old man residing in a coastal city contracted Vibrio vulnificus secondary to an insect bite.
Discussion and conclusion
This case underscores the importance for clinicians of recognizing that early administration of appropriate antibiotics in patients with non-traditional routes of Vibrio vulnificus infection can significantly reduce rates of amputation and mortality.
Vibrio vulnificus , a Gram-negative bacterium, inhabits brackish waters in warm coastal regions. Infections typically arise from consuming raw or undercooked seafood or through exposure of open wounds to contaminated seawater or seafood ( 1 , 2 ). Vibrio vulnificus is a highly pathogenic bacterium associated with a mortality rate of up to 33% ( 1 ). The mortality rate for wound infections caused by Vibrio vulnificus can reach 18% ( 3 ). In addition to classical infection pathways, clinicians often overlook atypical routes of transmission in clinical practice. Increasing evidence suggests that under conditions of high emissions and global warming, the endemic range of Vibrio vulnificus is expanding annually. Moreover, the role of terrestrial animals, plants, and insects as vectors is likely to elevate the risk of infection ( 4 , 5 ). This case serves as a crucial reminder for clinicians to remain vigilant regarding atypical transmission routes of Vibrio vulnificus and provides evidence supporting insect-mediated transmission of the bacterium.
The patient is a 75-year-old male who resides in Shanghai, China, approximately 70 km from the nearest coastline. His medical history includes hypertension, which has been managed with long-term medication.
On the morning of September 17, 2022, while taking a walk in his community, the patient sustained an insect bite on the middle finger of his left hand. The bite, small with a diameter of 2–3 mm, caused only minor stinging pain and no other immediate discomfort. The patient disinfected the bite site but did not cover it with a waterproof bandage. The wound scabbed over within a few hours. In interviews, he and his family denied any exposure to raw fish, seawater, uncooked seafood or its juices, marine-related products, or fishing activities, as well as the use of water that might have been contaminated. No similar symptoms were reported by others in his vicinity. Nineteen hours post-injury, the patient experienced localized pain in his left middle finger, along with swelling and redness of the hand. He sought treatment at a local hospital, where he received an intramuscular injection of dexamethasone and was prescribed cefaclor capsules, olopatadine hydrochloride, and topical halomethasone cream. Despite these interventions, his symptoms persisted and the condition worsened. Twenty-four hours after the injury, the affected skin darkened, and purple blisters formed ( Figure 1 ). Twenty-eight hours post-injury, the patient presented at our hospital’s emergency department with symptoms consistent with severe inflammation. Notably, transparent blisters had formed between the second and third fingers of the left hand, and the left upper extremity exhibited pronounced erythema and pain. The patient’s body temperature was elevated at 38.5°C, and he reported dizziness. Upon admission, laboratory findings revealed a white blood cell count of 10.48 × 10^9/L and a B-type natriuretic peptide level of 1,427 pg/mL; however, hepatic and renal functions remained within normal ranges.

28 h after being bitten.
Based on the findings, the physician diagnosed gangrene accompanied by insect bites on the left upper extremity. Treatment included administration of fosfomycin for its antimicrobial properties, low molecular weight heparin calcium to prevent thrombosis, and agents to protect the gastric mucosa. Concurrently, fluid from the blister was aspirated and submitted for microbiological analysis. On the first day of hospitalization, the microbiology laboratory detected Vibrio vulnificus in the submitted sample using microbial protein analysis techniques, with the finding confirmed by a second test. Based on the antimicrobial susceptibility report, the medication regimen was adjusted to include targeted antibiotics. During the hospital stay, the patient underwent two decompression surgeries on the skin and subcutaneous tissue of the left hand, with daily dressing changes and concurrent antibiotic and symptomatic treatment. The patient experienced pain in the left hand during these dressing changes, but no other symptoms of discomfort were reported. Subsequently, at the patient’s request, treatment was continued at another hospital.
Given that the initial presentation of hemorrhagic blisters coincided with the site of an insect bite, we hypothesize that the insect bite facilitated the patient’s infection with Vibrio vulnificus .
Vibrio vulnificus , a naturally occurring Gram-negative bacterium, is commonly found in warm, brackish waters with low salinity across the globe ( 6 ). Vibrio vulnificus infection can manifest within 4 h, with untreated cases potentially resulting in a mortality rate of up to 100% within 3 days ( 7 ). Individuals over the age of 40, particularly men, are predominantly affected by Vibrio vulnificus infections ( 8 ). These infections typically manifest as acute fever, chills, hemorrhagic bullae, and septic shock.
Vibrio vulnificus , traditionally associated with seafood, is increasingly being reported in freshwater contexts. Exposure to freshwater fauna, flora, and insects has been identified as a contributing factor to the rising incidence of infection ( 9 , 10 ). Consequently, clinicians must maintain vigilance for non-traditional routes of infection. Early administration of appropriate antibiotics is crucial to reduce the rates of amputation and mortality.
Data availability statement
Ethics statement.
Ethical approval was not required for the studies involving humans because this article is a descriptive case report with no interventional treatment and no ethical issues involved. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XH: Writing – original draft. HQ: Visualization, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The construction project for the Shanghai Municipal Health Commission inheritance and innovation team of the Shanghai-style Traditional Chinese Medicine (2021LPTD-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.

COMMENTS
3. Affiliation: The names of organizations for each author: 4. Corresponding author: Write the full name of the corresponding author and all contact details including email and mobile number: Abstract: 5. Background: what does this case report add to the medical literature? 6. Case summary: chief complaint, diagnosis, intervention, and outcome: 7.
The corresponding author takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and typically ensures that all the journal's administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation ...
Because acknowledgment may imply endorsement by acknowledged individuals of a study's data and conclusions, editors are advised to require that the corresponding author obtain written permission to be acknowledged from all acknowledged individuals. Use of AI for writing assistance should be reported in the acknowledgment section. 4.
A corresponding author is someone who: Can assume responsibility for all communication with the co-authors and the journal and can ensure that this communication is transparent. Is willing to represent the team and take responsibility for the research output submitted on its behalf, especially in the event of a dispute.
Corresponding author meaning: The corresponding author is the one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process. Normally, he or she also ensures that all the journal's administrative requirements, such as providing details of authorship ...
The corresponding author is also important or the most senior author in ... In this review paper we show - through personal real case studies and testimonials of writing CPC accumulated over the ...
The corresponding author, i.e., the author with whom the Editor communicates, is responsible for the integrity of the manuscript contents such as the veracity of the results, the lack of plagiarism, the contribution of each author, and other issues related to production of the manuscript. ... it seems intuitive that the corresponding should for ...
Senior Editor, Editage Insights. Researcher coach since 2015. Not all journals allow more than one corresponding author for a single study, but sometimes the research team needs a co-corresponding author. Authors need to keep this in mind and send pre-submission inquiries if they wish to have two corresponding authors for a paper.
One of the relevant examples is Academic Medicine where corresponding author Twitter handles are increasingly listed in the ... Egilman DS, Krumholz HM. Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation. JAMA. 2008; 299:1800-1812. doi: 10.1001/jama.299.15.1800 ...
The submitting author and corresponding author can be the same person. Corresponding author. The corresponding author, as listed on ScholarOne, takes primary responsibility for completing all necessary actions after acceptance of the manuscript and communicating with the journal and with readers after publication. All email communication from ...
The corresponding author is the one who take the responsibility of a paper, and thus, some believe that students are not yet prepared to take this responsibility. ... a person heading a project or who by legal obligations carries responsibility for a project may be identified as corresponding author. This could be the case with some ...
Corresponding author: * [email protected] ABSTRACT It is generally understood that the corresponding author (CA) is responsible for all communications related to the submission of a ... tific publishers are summarized below as case studies. Case 1: Elsevier and the International Committee of Medical Journal Editors
Co-authors collaborate to design experiments, analyze results, and contribute to the overall intellectual content of the paper. Corresponding Author Meaning. The corresponding author is the designated point of contact for the paper. They facilitate communication with the journal, handle revisions, and address queries.
The corresponding author is often the group leader or a senior researcher whose contact address is not likely to change in the near future. In cases where the main contributor of the study is also the group leader, he or she can be both the first and corresponding author for the study. Related reading: What corresponding authors are expected to ...
Authorship. Authorship provides credit for a researcher's contributions to a study and carries accountability. Authors are expected to fulfil the criteria below (adapted from McNutt et al ...
A person who gave advice on case management without being directly involved. A person who critically appraised the manuscript before submission for publication. A person who carried out a search of the literature to contextualize the report. A person who provided medical writing services. A person who copy-edited the manuscript. rder of autho.
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
Conflicts of interest: The author declares no conflicts of interest of relevance to this topic. Citation Teixeira da Silva, J.A. (2021), "Multiple co-first authors, co-corresponding authors and co-supervisors: a synthesis of shared authorship credit", Online Information Review , Vol. 45 No. 6, pp. 1116-1130.
The corresponding author is usually the one who had the main idea and invited other collaborators or delegated tasks. While all authors are responsible for the work and review the final version of the manuscript with a fine-tooth comb, the corresponding author is ultimately in charge of submitting the paper and is responsible for the overall ...
The first author and corresponding author, ideally, are decided during the research and through a mutual agreement among the authors. ... first authors should be expected to serve as corresponding authors. This is the case as long as they're consistently involved in the study and know-how to go through the submission/publication process ...
Include a cover letter and complete contact information for the corresponding author (affiliation, postal/mail address, email address, and telephone number) and whether the authors have published, ... Include the design (eg, clinical trial, cohort study, case-control study, meta-analysis). Focus on primary outcome(s) and finding(s). Do not ...
This paper analyses the influence of a Brazilian institution delivering the corresponding author on its scientific citation impact, distinguishing between its collaborative papers with foreign institutions and ... "case studies" are used (Katz & Martin, 1997). Co-authorship reflects the formal result of the work of a group of researchers. The ...
iv Goal of Case Study: The goal of this case study is to consolidate the knowledge and improve practices of the participants to detect, response to and control/prevent threating pandemics. Learning Objectives: By the end of the teaching session, participants will be able to: 1. State the case definition of suspected, probable and confirmed cases of novel
The emergence of the case cost theory gives inevitable guidance to the study of how to measure the carbon sink in the jungle. The forest carbon sink project carried out by the international director is the application of the case cost theory, which shows people's recognition and face up to this new concept. 3.2.3. Ecological Capital Theory
As the pediatric patient with right pulmonary artery agenesis (PAA) matured, she progressively presented symptoms of pulmonary hypertension and hemoptysis. There is limited clinical literature on this condition, and currently, there is no consensus regarding its diagnosis and treatment. This article presents a case study of a 16-year-old female patient with right pulmonary artery hypoplasia ...
According to the Consensus-based Standards for the selection of health measurement instruments (COSMIN) guidelines, the "anchor-based" approach is regarded as the "gold-standard" [21,22,23].In this approach, we determine the MCID of a chosen outcome measurement, based on whether a pre-defined MCID (usually derived from another published study) was achieved by an external criterion ...
The construction of water-saving renovation projects can bring substantial benefits to the development of agriculture, but it may also be accompanied by negative impacts, especially in a large-scale irrigation district. Hence, there is always controversy, and it is vital and necessary to investigate the effectiveness of water-saving renovation. In this study, the Yahekou irrigation district ...
In this study, a MAGIC population of 1021 lines was developed from four Xian and four Geng varieties from 5 subgroups of rice. A total of 44,000 genes showed functional polymorphisms among eight parents, including frameshift variations or premature stop codon variations, which provides the potential to map almost all genes of the MAGIC population.
A novel histomolecular tumor of the central nervous system (CNS), the "diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC)," has recently been identified, based on a distinct DNA methylation profile and has been added to the 2021 World Health Organization Classification of CNS Tumors. This glioneuronal tumor mainly affects the supratentorial area in ...
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author. Ethics statement Ethical approval was not required for the studies involving humans because this article is a descriptive case report with no interventional treatment and no ethical ...