Office: 919.785.1304 | Fax: 919.785.1306

- Donate to SUBR
- Copyright and Privacy Statements
- Why Are Animals Needed in Research?
- What About Using Fewer Animals or No Animals?
- Product and Environmental Safety Research
- What is Biomedical Research?
- Biomedical Research Definitions
- Research Options
- How Animals Help
- Member Organizations

WHAT IS BIOMEDICAL RESEARCH?
Biomedical research is the broad area of science that looks for ways to prevent and treat diseases that cause illness and death in people and in animals. This general field of research includes many areas of both the life and physical sciences.
Utilizing biotechnology techniques, biomedical researchers study biological processes and diseases with the ultimate goal of developing effective treatments and cures. Biomedical research is an evolutionary process requiring careful experimentation by many scientists, including biologists and chemists. Discovery of new medicines and therapies requires careful scientific experimentation, development, and evaluation.
Why are Animals Used in Biomedical Research?
The use of animals in some types of research is essential to the development of new and more effective methods for diagnosing and treating diseases that affect both humans and animals. Scientists use animals to learn more about health problems, and to assure the safety of new medical treatments. Medical researchers need to understand health problems before they can develop ways to treat them. Some diseases and health problems involve processes that can only be studied in living organisms. Animals are necessary to medical research because it is impractical or unethical to use humans.
Animals make good research subjects for a variety of reasons. Animals are biologically similar to humans. They are susceptible to many of the same health problems, and they have short life-cycles so they can easily be studied throughout their whole life-span or across several generations. In addition, scientists can easily control the environment around animals (diet, temperature, lighting), which would be difficult to do with people. Finally, a primary reason why animals are used is that most people feel it would be wrong to deliberately expose human beings to health risks in order to observe the course of a disease.
Animals are used in research to develop drugs and medical procedures to treat diseases. Scientists may discover such drugs and procedures using alternative research methods that do not involve animals. If the new therapy seems promising, it is tested in animals to see whether it seems to be safe and effective. If the results of the animal studies are good, then human volunteers are asked to participate in a clinical trial. The animal studies are conducted first to give medical researchers a better idea of what benefits and complications they are likely to see in humans.
A variety of animals provide very useful models for the study of diseases afflicting both animals and humans. However, approximately 95 percent of research animals in the United States are rats, mice, and other rodents bred specifically for laboratory research. Dogs, cats, and primates account for less than one percent of all the animals used in research.
Those working in the field of biomedical research have a duty to conduct research in a manner that is humane, appropriate, and judicious. CBRA supports adherence to standards of care developed by scientific and professional organizations, and compliance with governmental regulations for the use of animals in research.
Scientists continue to look for ways to reduce the numbers of animals needed to obtain valid results, refine experimental techniques, and replace animals with other research methods whenever feasible.
© California Biomedical Research Association
- Skip to primary navigation
- Skip to main content
- Skip to footer
Human Research Protection Program

Biomedical Research
What is biomedical research.
Biomedical scientists study human physiology and the treatment or understanding of disease. Biomedical research applies the principles of the physical sciences to medicine. Most biomedical research is conducted by physicians or biomedical scientists, but many studies are conducted by biologists, chemists, physicists, and other medical and scientific professionals.
Most biomedical research involves clinical trials, which are phased studies using human volunteers, designed to answer safety and efficacy questions about biologics, devices, pharmaceuticals, new therapies or new ways of using known treatments. Trials are often conducted in small group initially but expanding in later stages once safety and efficacy are demonstrated. Most clinical trials are FDA regulated, but there are some exceptions.
Types and Methods
- research on therapies ( e.g. , drugs, exercise, surgical interventions, or medical devices)
- diagnostic procedures ( e.g. , CAT scans, prenatal diagnosis through amniocentesis)
- preventive measures ( e.g. , vaccines, diet, or fluoridated toothpaste)
- studies of the human body while exercising, fasting, feeding, sleeping, or learning
- responding to such things as stress or sensory stimulation
- Studies comparing the functioning of a particular physiological system at different stages of development ( e.g. , infancy, childhood, adolescence, adulthood, or old age)
- Studies defining normal childhood development so that deviations from normal can be identified
- Records research – often used to develop and refine hypotheses
- research on the biochemical changes associated with AIDS
- research on the neurological changes associated with senile dementia
- Research on the human genome and genetic markers – for the purpose of creating new avenues for understanding disease processes and their eventual control
- research with animals
- research on preexisting samples of materials (tissue, blood, or urine) collected for other purposes, where the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects
- research based on records, when the data are recorded in such a manner that the individuals to whom the records pertain cannot be identified, either directly or through identifiers linked to them
Risk is the probability of harm or injury (physical, psychological, social, or economic) occurring as the result of participation in a research study. Biomedical researchers must consider the following risks when conducting their study:
- Social, psychological, or economic harm (See Social Behavioral Research for details)
- exercise-induced or repetition-exacerbated physical harm, such as carpal tunnel syndrome, stress fractures, asthma attacks, or heart attacks
- exposure to minor pain, discomfort (e.g. dizziness), or injury from invasive medical procedures
- possible side effects of drugs
Although most of the adverse effects that result from medical procedures or drugs are temporary, investigators must be aware of the potential for harm. The IRB will want to know how such outcomes will be minimized or addressed and is responsible for conducting a risk/benefit assessment.
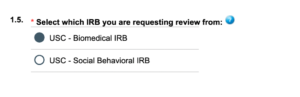
When submitting an application in iStar you will see the following in section 1.5.
Genomic Data Sharing (GDS)/Stem Cell Research
- USC Stem Cell Regenerative Medicine Initiative
- USC Stem Cell Research Oversight Committee (SCRO)
- NIH Genomic Data Sharing Policy (formerly ‘GWAS’)
- California Institute for Regenerative Medicine (CIRM)
- Genetics and Public Policy Center
- NIH: Stem Cell Research
- U.K. Human Fertilisation and Embryological Authority
- The Hinxton Group: An International Consortium on Stem Cells, Ethics and Law
- The International Society for Stem Cell Research (ISSCR)
- Human Genome Project: Educational Kit
3720 S. Flower Street, Suite 325 Los Angeles, CA 90089

- Announcements
- Getting Started
- Internal Staff Webpage
- Education & Certification
- HRPP Performance & Metrics
- Policies and Procedures
- Post Approval Monitoring (PAM)
- Youtube Channel
- Biospecimen & Data Repositories
- Investigational Drugs and Devices
- Investigator-Initiated Trials
- Emergency Research
- SBIRB Social Behavioral Research
- Student Researchers
- Requesting USC to Rely on an External IRB
- Requesting USC IRB to Act as the sIRB
- Starting a Research Trial: the Basics
- Forms and Templates
- FWA and IRB Registration Numbers
- IRB Member Toolbox
- IRB Review: How to
- IRB Submission Guidelines
- Levels of IRB Review
- Not Human Subjects Research (NHSR)
- Post IRB Review and Approval
- Privacy, Confidentiality, and Anonymity in Human Subjects Research
- Urgent Review

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

History of Medicine
About | Collections | Exhibitions | Research Tools | Copyright | Get Involved | Visit | Contact

- Introduction
- Health Care for Seamen
- Fighting the Spread of Epidemic Diseases
- The Beginnings of Organized Biomedical Research
- Notable Contributions to Biomedical Research
- Current Research
- Pure Food and Drugs
- Mental Health and Drug Abuse
- Health Care Delivery
- International Health
- Index of Images
Biomedical Research
Current research.
Following World War II, federal support for biomedical research was greatly expanded and so was the role of the National Institute of Health, which was renamed the National Institutes of Health to reflect the growth of research functions. Today NIH encompasses seventeen research institutes, two research divisions, the world's largest research hospital, the National Library of Medicine, the National Center for Human Genome Research, the National Center for Research Resources, and the Fogarty International Center. Its operating budget in 1990 was 7.6 billion dollars.
NIH conducts research on its Bethesda, Maryland campus and supports research throughout the United States by means of a competitive grant system. This research deals with every aspect of human biology and almost every disease and disability.
Some of the major topics of research today include: oncogenes and the question of how a normal cell becomes a cancer; the workings of immune cells and how they help to defend the body against cancer; the isolation of genes, including those for blood clotting factors; brain changes in Alzheimer's disease; the application of genetic engineering techniques to vaccine production; the discovery and isolation of bone growth factors; the study of neurotransmitters and modulation in the brain; cancer risk from passive smoking; new uses of the laser for the treatment of eye diseases; the construction of artificial chromosomes; the development and refinement of new body imaging technologies, such as magnetic resonance imaging (MRI) and, of course, the study of AIDS.
In 1968, Dr. Marshall W. Nirenberg of the National Heart, Lung, and Blood Institute became the first of four NIH Nobel laureates to date. He won the Nobel Prize in Physiology or Medicine for his work in translating the genetic code and its function in protein synthesis; he is shown here with his molecular models.
The National Heart, Lung, and Blood Institute has been a leader in conducting and supporting clinical studies of heart disease, including open heart surgery and artificial hearts, as well as educating the public about the prevention of heart attacks through control of high blood pressure, control of high levels of blood cholesterol, and cessation of smoking. Research advances and lifestyle changes have helped lower the death rate from heart disease by 43 percent since 1972.
Researcher at the National Heart, Lung, and Blood Institute's Laboratory of Kidney and Electrolyte Metabolism uses fluorescence microscopy to study kidney physiology, specifically kidney tubule transport. Other important research work on kidney function, such as improving the prevention and management of end-stage renal disease and improving dialysis techniques, is done at the National Institute of Diabetes and Digestive and Kidney Diseases.
Researcher at the National Heart, Lung, and Blood Institute engaged in the study of sickle cell anemia, a genetic blood disease which, in the United States, affects primarily Afro-Americans and is caused by an abnormal hemoglobin molecule. A new test, known as chorionic villus biopsy, promises to advance the prenatal diagnosis of genetic blood diseases, including sickle cell anemia, from the second trimester of pregnancy to the first. Tissue from the chorionic villi, hairlike projections of the membrane that surrounds the early embryo, can be removed before the 10th week of pregnancy and analyzed immediately for chromosomal or biochemical defects.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) has pioneered in the study and treatment of diabetes, the fifth leading cause of death in the United States. The NIDDK supported research which led to the development of the insulin pump. A physician instructs a patient in its use.
Dr. John Klippel of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) examines and measures the extension of the fingers of a patient with lupus, a disease characterized by eruption of scarred lesions and inflamed joints. Researchers at the NIAMS elaborated the underlying immunologic mechanisms in systemic lupus erythematosus (SLE), developed more effective treatments, and dramatically improved survival.
The development and testing of new and promising experimental drugs or vaccines for a variety of ailments is one of the key functions of the National Institutes of Health researchers.
Increasingly sophisticated instrumentation such as this fast atomic bombardment mass spectrometer, which separates chemical components, is being used at the FDA's Center for Biologics Evaluation and Research to determine the purity and potency of biologics and other products.
Laser technology is being applied to biomedical research by scientists at the FDA's Center for Biologics Evaluation and Research. Here a laser cell separation machine is separating a line of cells, such as T-cells, for immunology studies.
A computed tomography (CT) scan, a noninvasive method of getting good cross-sectional images of the body, is being done on a patient at the NIH's Warren U. Magnuson Clinical Center. The Clinical Center is the world's largest hospital devoted solely to biomedical research where physicians from all the different NIH institutes, together with the Center's staff, pursue clinical and laboratory studies related to patient care.
Legionnaires' disease was first recognized in July 1976, when a sudden outbreak of pneumonia, resulting in several deaths, occurred mostly in persons who had attended an American Legion convention in Philadelphia. Researchers Charles C. Shepard (1914-85) and Joseph E. McDade of the Centers for Disease Control and Prevention were the first in 1977 to identify the disease-causing bacterium -- Legionella pneumophilis, which is pictured here. Since then more than 20 species in the Legionella genus have been identified and the mystery surrounding many illnesses associated with them solved, in the grand tradition of the microbe hunters of the late 19th and early 20th centuries. Many of these modern day microbe hunters or epidemiologists were trained in the Centers for Disease Control and Prevention's Epidemic Intelligence Service, which was established by Dr. Alexander Laugmuir in 1951.
Researchers, such as this man in the Centers for Disease Control and Prevention's new maximum containment virology laboratory, use the most advanced technology available to study dangerous organisms like the Lassa, Machupo, Ebola, and AIDS viruses that cause deadly diseases for which no cure or vaccine exists. Statistics about these and other diseases are published in the Morbidity and Mortality Weekly Report (MMWR), a widely read publication around the world. Weekly reporting of morbidity and mortality statistics to the Public Health Service began in 1893. Various bureaus of the Service have published these reports. Since 1961, the Center for Disease Control and Prevention's Epidemiology Program Office has been responsible for publishing the MMWR.
Researcher at the National Eye Institute (NEI) tests the peripheral and central visual fields of a patient to find areas of visual loss. The NEI has helped pioneer the use of lasers in preventing visual loss from diabetes and other eye diseases, and is testing new medicines that may he able to prevent diabetes-induced damage to the retina and other tissues.
Researcher at the National Institute of Dental Research (NIDR) uses computers to measure very small changes in teeth and their surrounding tissues that are not detectable with conventional dental X-rays. Since its creation in 1948, the NIDR has been the primary sponsor of dental research in the United States. Much of its work was focused on preventing tooth decay, through such programs as community water fluoridation.
Dr. Robert C. Gallo, since 1972 chief of the National Cancer Institute's Laboratory of Tumor Cell Biology, is an internationally prominent investigator of human viruses and tumor cells. He played a leading role in isolating and characterizing the family of human viruses to which the AIDS causing virus, HIV (human immunodeficiency virus), belongs. Gallo and his colleagues are also responsible for the development of a blood test to detect HIV antibodies in blood collected for transfusions.
Vice-President George Bush addresses the AIDS Executive Committee during the centennial year of the National Institutes of Health. As the NIH enters its second century it faces one of its greatest research challenges -- a cure and vaccine against the deadly viral disease AIDS (acquired immunodeficiency syndrome), which has become the major scourge of the late twentieth century. To the left of the Vice-President is Dr. James B. Wyngaarden, director of the NIH, and to the right of the Vice-President is Dr. Anthony Fauci, director of the NIH's National Institute of Allergy and Infectious Diseases and coordinator of NIH research on AIDS.
The National Library of Medicine was established in 1836 as the Library of the Army Surgeon General's Office. The Armed Forces Institute of Pathology and its Medical Museum were founded in 1862 as the Army Medical Museum. Throughout their history the Army Medical Library and the Army Medical Museum often shared quarters. This red-brick building was built on the Mall in Washington, D.C., in 1887 for these two institutions. It was torn down in the late 1960s to make room for the Hirshorn Museum of Art. By an act of Congress in 1936 the Library collection was transferred from the Department of Defense to the Public Health Service of the Department of Health, Education and Welfare and renamed the National Library of Medicine. The Library moved to its current quarters in Bethesda, Maryland, on the campus of the National Institutes of Health in 1962.
Under the very able leadership of Dr. John Shaw Billings (1838-1913), a Civil War surgeon who served as director from 1865 to 1895, the Library increased in size from about 2,000 volumes to over 100,000 volumes of books and bound serials and expanded from serving primarily military medical officers to serving all physicians. It soon became the leading medical library in the United States and then the world. The first issue of Index Medicus, a comprehensive monthly medical bibliography with a subject and author index to articles published in medical journals around the world, was published in 1879.
The National Library of Medicine is now the world's largest medical research library. Its holdings include more than 3.5 million books, journals, technical reports, theses, pamphlets, photographs, manuscripts and audiovisual materials covering more than 40 biomedical areas and related subjects. The Library also houses one of the world's finest historical collections of rare medical texts and manuscripts. The 10-story Lister Hill Center was built in 1980 to house the Library's expanding computer facilities, audiovisual studios, and research and development laboratories. With materials in 70 languages and international exchange capabilities the Library can serve health professionals worldwide.
In order to keep up with the ever-increasing volume of biomedical literature that had to be included in the Index Medicus bibliography, the Library turned to computers in the early 1960s. An extensive computerized literature retrieval system, known as MEDLARS, became totally operational in 1964. It performed thousands of searches before on-line searching capabilities and data bases, such as MEDLINE, became available in 1971. Through MEDLINE, health professionals and other interested individuals have immediate access to more than 3 million journal article references accumulated since 1965 and growing at a rate of over 300,000 a year.
| Table of Contents | Next >
- Skip to main content
- Skip to "About this site"
- Departments
Language selection
- Search and menus
Infographic: What is biomedical research?
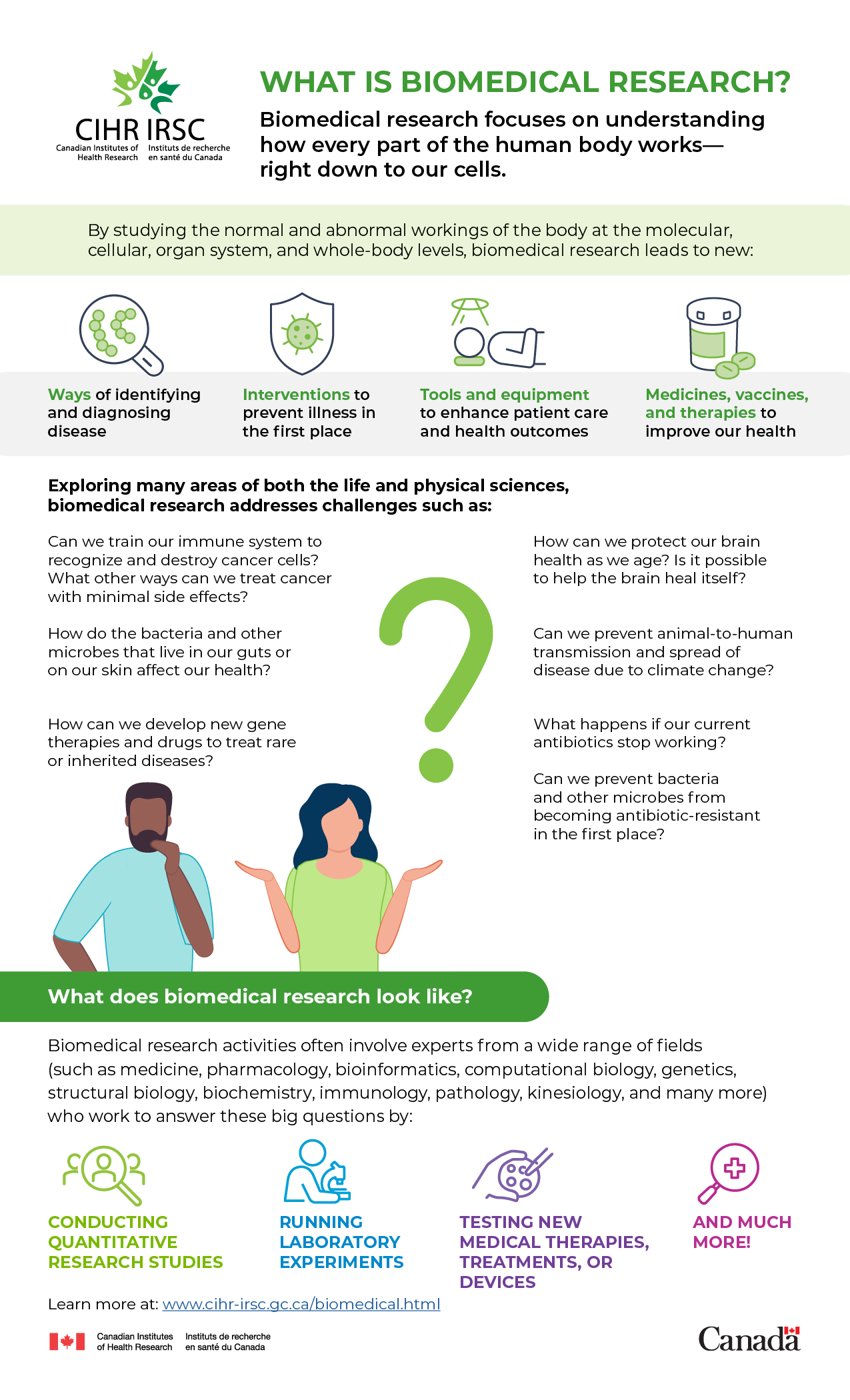
Biomedical research focuses on understanding how every part of the human body works—right down to our cells.
By studying the normal and abnormal workings of the body at the molecular, cellular, organ system, and whole-body levels, biomedical research leads to new:
- Ways of identifying and diagnosing disease
- Interventions to prevent illness in the first place
- Tools and equipment to enhance patient care and health outcomes
- Medicines, vaccines, and therapies to improve our health
Exploring many areas of both the life and physical sciences, biomedical research addresses challenges such as:
- Can we train our immune system to recognize and destroy cancer cells? What other ways can we treat cancer with minimal side effects?
- How do the bacteria and other microbes that live in our guts or on our skin affect our health?
- How can we develop new gene therapies and drugs to treat rare or inherited diseases?
- How can we protect our brain health as we age? Is it possible to help the brain heal itself?
- Can we prevent animal-to-human transmission and spread of disease due to climate change?
- What happens if our current antibiotics stop working? Can we prevent bacteria and other microbes from becoming antibiotic-resistant in the first place?
What does biomedical research look like?
Biomedical research activities often involve experts from a wide range of fields (such as medicine, pharmacology, bioinformatics, computational biology, genetics, structural biology, biochemistry, immunology, pathology, kinesiology, and many more) who work to answer these big questions by:
- Conducting quantitative research studies
- Running laboratory experiments
- Testing new medical therapies, treatments, or devices
- And much more!
Learn more by visiting the Biomedical research webpage.
Privacy notice
This site makes use of cookies . If you continue we'll assume you are happy to receive them.
- Read our cookie policy

Dedicated to excellence in biomedical science
- Specialist Advisory Panels
- Annual Report
- Chief Executive's biography
- Equity, Diversity and Inclusion
- Discussion groups
- IBMS library
- Media and press
- President's welcome
- Member benefits
- Applicants with overseas qualifications
- Help & FAQs
- Code of Conduct
- Professional indemnity cover
- IBMS Additions discount scheme
- Legal helpline
- Membership number login
- Request promotional items
- Update your details
- Laboratory Transformation and Improvement Program
- What is CPD?
- Science reading
- Choose IBMS Accredited
- Affiliated university societies
- President's Prize
- Certificate of Achievement
- Certificate of Competence by Equivalence
- Certificate of Attainment - Clinical Scientist
- Registration Training Portfolio
- Laboratory Training Approval
- Non-accredited degree assessment
- Return to practice
- Standards of Proficiency Updates 2023
- IBMS Mentoring Scheme
- Professional Guidance
- Sample Journey Videos
- Superlab Comics
- Do you know what happens to your sample?
What is biomedical science?
- Sample journey videos
- Cherie - Microbiologist
- Danny - Biochemist
- Jo - Histologist

Biomedical science is one of the broadest areas of modern science and underpins much of modern medicine - from determining the blood requirements of critically ill patients to identifying outbreaks of infectious diseases to monitoring biomarkers in cancer
Biomedical science staff mostly work in healthcare laboratories diagnosing diseases and evaluating the effectiveness of treatment by analysing fluids and tissue samples from patients. They provide the 'engine room' of modern medicine - 70% of diagnoses in the NHS are based on pathology results provided by laboratory services.
Handling over 150 million samples in the UK each year, every person at some point in their lives will benefit from the services of a biomedical scientist. If you have ever had a urine, blood, tissue or other sample taken by a doctor or nurse, chances are it will have been diagnosed by a biomedical scientist.
At the heart of healthcare
Biomedical scientists are at the heart of multi-disciplinary teams in healthcare. They provide other professionals with vital scientific information, allowing them to make informed clinical decisions, ensure blood stocks are adequate at critical times, matching blood to patients, measuring chemicals to monitor patient condition, investigating disease by looking at tumour samples and identifying micro-organisms in the fight against infection.
Protecting the public through registration
To protect the public, the term 'Biomedical Scientist' is a legally protected title. Anyone using the title must meet the Health & Care Professions Council (HCPC) standards and be HCPC registered.
Advancing knowledge and setting standards in biomedical science
With over 20,000 members in 30 different countries, the Institute of Biomedical Science is the leading professional body for scientists, support staff and students in the field of biomedical science. For over 100 years we have been dedicated to the promotion, development and delivery of excellence in biomedical science within all aspects of healthcare, and providing the highest standards of service to patients and the public.
By supporting members in their practice of biomedical science we set quality standards for the profession through: training, education, assessments, examinations and continuous professional development. Our publications and training events ensure members are kept up-to-date on the latest scientific developments and news. Through public relation and marketing campaigns we raise awareness of the vital role of biomedical science in healthcare and promote career opportunities in the profession.
Share this page:

- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents
Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Philosophy of Biomedicine
Despite the simple name, biomedicine is not simply the area of overlap between biology and medicine. It is a framework, a set of philosophical commitments, a global institution woven into Western culture and its power dynamics, and more. Biomedicine is the umbrella theoretical framework for most health science and health technology work done in academic and government settings. Western medical practices and the surrounding healthcare infrastructure are principally biomedical. Health-related corporations are predominantly biomedical in orientation (with the exception of a few homeopathic producers and other scattered outliers). International medical aid mainly offers biomedical resources. Biomedicine, in other words, is the name for how most powerful global institutions envision the relations between biological sciences and medicine.
The biomedical model is in fact so commonplace that it is easy to overlook how philosophically weighty (and contentious) its core commitments are: that health phenomena must be understood in terms of physical/biochemical entities and processes, that experimental techniques are the preferred means of acquiring and assessing health-related knowledge, and that human bodies are best understood as composed of a collection of subsidiary parts and processes (Krieger 2011: 130). In addition to critiquing those core commitments, philosophers have also disputed connected issues regarding the meanings of health and disease, the nature of biomedical knowledge and expertise, the value of reductionist thinking, the value of biomedicine’s global institutions, etc. Some philosophers have also proposed alternative frameworks for understanding the relations between biology and medicine. Though, relatively little literature is directed at biomedicine per se .
The fact that the philosophical literature is so critical is in part because
- biomedicine is so expansive that there is no shortage of flaws to identify, and
- biomedicine is deeply embedded in the current global order, and hence is tied directly or indirectly to all of the goods and ills of that order.
This entry will, as much as possible, avoid duplicating the work done in the thorough Philosophy of Medicine entry. That entry focuses on elucidating the work done by that branch of philosophy, which according to entry, has
dedicated journals and professional organizations, a relatively well-established canon of scholarly literature, and distinctive questions and problems. (Reiss & Ankeny 2016)
Most of the “medicine” examined in “philosophy of biomedicine” is medicine pursued within a biomedicine framework, so there is much overlap. Yet, this entry will accomplish something other than what that entry does. This entry will review the philosophical literature (and some literature in allied fields, such as history of medicine) that scrutinizes the biomedical framework itself, in whole or in part.
1.1 What is biomedicine?
1.2 the history of biomedicine, 2.1 ontology of the body and life in biomedicine, 2.2 biomedical conceptions of health and disease, 3.1 epistemology of expertise and experimental clinical data in biomedicine, 3.2 epistemology of basic and applied science in biomedicine, 3.3 epistemology of measurement in biomedicine, 3.4 reductionism in epistemology of biomedicine, 4. biomedicine as an institution, 5.1 traditional healing practices, 5.2 narrative and phenomenological approaches, 5.3 gentle medicine and population health framework approaches, other internet resources, related entries, 1. biomedicine as a framework for medicine + biology.
Consult with a primary care physician when feeling ill. Perhaps they send you to a medical lab that will take a blood sample to run a biochemical analysis. After the results arrive, consult with a physician specialist whose expertise is the particular bodily organ or system where the ailment is localized. The specialist prescribes a drug that has been mass-produced in a factory, after a biology laboratory extracted the active ingredient from a natural source, or perhaps synthesized it from scratch. The drug’s efficacy has been proven by testing the drug experimentally on two groups with the relevant disease, randomly sorted into an experimental group and a control group so that the drug’s measurable bodily effects could be scrutinized and counted. This is biomedicine. It has quickly grown to be so pervasive around the globe that it is difficult to characterize biomedicine.
As explored in this entry, and outlined in how the sections are divided in the entry, biomedicine has many facets. It is a very large and complex thing. It is a morphing historical product of the post-World War Two West. It is a contemporary global social institution. It is an epistemology of medical research and practice (albeit with factions). It is a set of ontological and metaphysical commitments. And more.
The US National Cancer Institute defines biomedicine as synonymous with “allopathic medicine, conventional medicine, mainstream medicine, orthodox medicine, and Western medicine” (NCI Other Internet Resources , accessed 12 March 2020). By contrast, A. E. Clarke, Mamo, Fishman, Shim, and Fosket (2003) takes an expansive view of the nature of biomedicine, contending that it is an evolving entity, a cohesive and developing whole that consists of elements ranging from the assertion that good health is a personal moral obligation to the increasing reliance on “computerization and data banking” (A. E. Clarke et al. 2003: 173).
Krieger offers a detailed examination of the history and philosophy of the “biomedicine approach,” and extracts three key tenets of the view.
Among the many features of a biomedical perspective, three stand out as fundamental regarding its approach to investigating disease (Lock & Gordon 1988; Fee & Krieger 1994; Krieger 1994; Lawrence & Weisz 1998; Cambrosio & Keating 2001; Bynum 2008). They are: First, specific to biomedicine: the domain of disease and its causes is restricted to solely biological, chemical, and physical phenomena; Second, shared with many natural sciences: an emphasis on laboratory research and technology and, as translated to health research, a discounting of research questions that cannot be studied by randomized clinical trials (or their analogs, e.g., “natural experiments”); and Third: an embrace of “reductionism,” a philosophical and methodological stance (discussed more fully below) that holds that phenomena are best explained by the properties of their parts. (Krieger 2011: 130)
Krieger’s account of biomedicine offers a compelling distillation of the philosophical commitments of biomedical science. Though since this is an entry on philosophy of biomedicine, the entry will be organized around philosophical dimensions and debates, rather than being organized directly around these core theoretical tenets (e.g., the upcoming section is on ontological and metaphysical commitments). Additionally, this entry will supplement the tenets identified by Krieger with discussion of connected issues, such as philosophical critiques of biomedicine as a social institution ( Section 4 ).
The terms “framework” and “approach” are used here to describe biomedicine, in an attempt to avoid narrowly overcommitting to any particular philosophical system. Biomedicine may or may not qualify as one of Thomas Kuhn’s “paradigms”, in the way that the heliocentric model of the solar system is a paradigm—a complex worldview in which the viewpoints cannot be straightforwardly translated back-and-forth with an alternative paradigm (i.e., if we try to translate the concepts of biomedicine to the pre-biomedical concepts of humoral theory, discussed in Section 1.2 (Kuhn 1962). Alternatively, biomedicine seems to qualify as one of Imre Lakatos’ Research Programmes, a different way of conceiving of the way that research communities intellectually cohere (Lakatos 1968). It’s a question of how biomedicine serves as a means of organizing and guiding research.
Without getting too lost in the technical criteria, biomedicine shares the Kuhnian paradigm quality of being incommensurable (or at least more or less so) with other conceptions of biology and health. Scholars of complementary medicine have spent enormous effort searching for ways that traditional medical practices—e.g., herbal treatments handed down over generations—can get a foothold in a medical world dominated by biomedicine. It is telling that there is no question that ‘proof’ of a treatment’s efficacy requires starting from scratch, by examining the herb’s chemical makeup, isolating and analyzing which chemical components are the active ingredients, and then testing safety and efficacy, all entirely within biomedicine’s rules. Whatever explanations might have been offered by herbalists (e.g., ‘it calms turmoil in the stomach’) are irrelevant untranslatable knowledge. Biomedicine also shares the Lakatosian Research Programme quality of (largely implicitly) dictating what sorts of internal dispute about biomedicine is permissible. Within the biomedical Research Programme, one is invited to question whether a particular experimental design is suitable; one is not permitted to reject experimentation entirely.
Biomedicine as a global institution coevolved with its characteristic philosophical positions. While key elements, such as laboratory biology, were present in the nineteenth century and even before, “World War II is usually presented as a turning point in the ‘biomedicalization’ process” (Löwy 2011: 117). The term “biomedicine” was first used between the two world wars as a shorthand for some of the medical and scientific work being done on radioactive materials (Keating & Cambrosio 2003; Löwy 2011: 49–55). But biomedicine only came into its own during the period of economic and social transformation in industrialized Western countries that followed World War Two:
In industrialized countries, the post-World War II era was also characterized by important increases in public funding for medical research, the extension of health insurance to large parts of the population (a process that, in nearly all the Western countries, the United States excepted, was also supported by the state), and the rapid growth of the pharmaceutical industry. (Löwy 2011: 117)
If biomedicine is a recent historical development, then what exactly came before it? Many different frameworks for health existed before the rise of biomedicine, though many such frameworks exist now despite the dominance of biomedicine. Some have been distinctly influenced by or merged with biomedicine. For instance, osteopathic medicine has gradually gone from a full alternative system of medicine (focusing on manipulation of body via pressure, stretching and other means of readjustment of bodily structure in order to rebalance internal processes), to an alternative track of medical education/practice that has in large part converged with the biomedical/allopathic medicine track (McClain 2017; Stark 2013).
Insofar as biomedicine formed in the West, it is helpful to see it in contrast to the medical and philosophical traditions that preceded it. The most influential Western tradition prior to biomedicine is the Hippocratic tradition—the views attributed to Hippocrates and over two millennia of re-interpretations, riffs, amendments, and other alterations. At the core of Hippocratic medicine were two views. First, it made a commitment to methodological naturalism—a refusal to attribute disease processes to the work of gods and other supernatural beings (Conrad, Neve, Nutton, Porter, & Wear 1995). Though, it is worth noting that for much of that history, elements of astrology, talismans, and some forms of magic were understood as essentially natural phenomena (e.g., the sun obviously affects health via sunburns and such, so it is not absurd to have inferred that planets could have their own subtle effects on bodies too). Second, it understood health as a matter of balance—and disease as a matter of imbalance—of the humors. Humoral theory was interpreted in many different ways, but centered on the idea that heath and disease are attributable to the interactions of anatomical humors (blood, phlegm, yellow bile, and black bile), which were in turn directly tied to a much larger cosmology of the elements (e.g., blood is linked to air, the springtime, a combination of heat and moisture, the heart, and the astrological influence of the planet Jupiter) (see additional internet resources below) (Conrad et al., 1995).
There is long-standing debate in health policy over exactly how successful the biomedical model has been over its relatively short historical trajectory. The growth of the biomedical model in Western countries occurred at the same time as other social and economic transformations (industrialization, urbanization, globalization, etc.) and at the same time as improvements in life expectancy and a large number of disease outcomes. The ‘received view’ of these historical relations is that social and economic changes allowed the growth of biomedicine, which in turn created new institutions, professional health care practices, and technologies that, in turn, caused vast improvements in health outcomes for the populations served by biomedical institutions. Penicillin is the go-to example for illustrating the positive health impacts of biomedicine (Löwy 2011). It is a medicine extracted from nature (mold), made into a widely available ‘magic bullet’ thanks to biomedical techniques of identifying its active components, and manufacturing controlled doses of it at reasonable cost at massive scale. Suffering and death from bacterial infections was rapidly alleviated; once hopeless diseases were suddenly curable with a few (usually) benign pills.
Thomas McKeown was an influential critic of the biomedical institution (including the work done during its early twentieth century prehistory), having authored the blockbuster volume The Role of Medicine: Dream, Mirage, or Nemesis (1976). McKeown reevaluated the historical record and available evidence to instead hypothesize that the social and economic changes in the West were more responsible for the health improvements than biomedicine’s growth. Put bluntly, he argued that twentieth century modernity (economic development, improved nutrition, etc.) caused populations in the West to get healthier over time, then modernity went on to also cause the growth of contemporary medicine/biomedicine, but the medical advances themselves played a generally secondary role in supporting the health improvements. His works has inspired decades worth of debates over the validity of his bold claims, which have been largely overturned or largely vindicated, depending on whom one asks (Link & Phelan 2002).
Health theorist Nancy Krieger shows how the activity of finding ratios of how much biomedical healthcare vs. socioeconomic environment contributed to health improvements since the twentieth century is the sort of debate that easily falls victim to the “fallacy of treating causes of population health as if they sum to 100%” (Krieger 2017). Causes overlap and interact in complex ways; e.g., individual diet, exercise, medications, and changing exposures to smoking, etc. have all been contributing to changes in heart disease rates. How much of the credit goes to biomedicine for studying the effects of exercise on heart attack risk, for helping make the case for the heart risks of smoking, and so on? Extracting and assessing the contributions of biomedicine, an enterprise fully embedded in Western countries’ social systems and economies, is not possible; biomedicine is firmly embedded. That is not to deny it is impossible to attribute health effects in particular populations to particular causes in general—there are methods allowing such calculations—just that causes’ interactions need to be taken into consideration (Krieger 2017).
2. Ontological and Metaphysical Commitments in Biomedicine
Biomedicine is built around a conception of disease as a disfunction of particular physical parts (organs, tissues, cells) of the body. Despite being hegemonic in the global research community, biomedicine’s ontological and metaphysical commitments are not self-evident, historically long-lived, nor universally embraced. In contrasting Classical Chinese Medicine with biomedicine, Lee explains that biomedicine is set apart by
the metaphysical thesis that only what is ascertainable by means of the five senses and by extension the use of instrumentation is real and exists
and a connected epistemic claim that legitimate health knowledge must come to us via those means (Lee 2017: 2).
What makes patients and their bodies special? Some philosophers of biomedicine have attempted to make headway in this question by provocatively asking what it means for biomedical sciences to be ‘chauvinist’ and whether this is proper. Biomedical discussions are in large part defined by what they exclude from discussion. Curiously, there are two sides to the coin of biological chauvinism: one that directly supports the biomedical framework and one that challenges it. Both help shed light on the relationship between biology and medicine.
Broadbent (2009) uses the helpful term, “biological chauvinism” to describe the way that biomedicine actively excludes consideration of entities and processes that don’t fit into its worldview: “a refusal to countenance causes of ill health that are not biological” (Broadbent 2009: 305). Biological chauvinists might appeal to ontological or epistemic commitments, e.g., the assumption that knowledge of the body is nothing but knowledge of organic chemical processes, and any processes not clearly reducible to such terms are not yet worthy of being given full consideration. But the strongest case for this chauvinism rests more on pragmatic grounds than on such philosophical assumptions. Biomedicine has built itself into a massive global institution and research enterprise while operating under that assumption (to repeat Krieger’s phrasing from Section 1.1 , the assumption is that “the domain of disease and its causes is restricted to solely biological, chemical, and physical phenomena” (Krieger 2011: 130)). This is a powerful argument, though it cuts both ways; Section 5 discusses critiques attempting to undercut the value of what biomedicine has indeed built while operating under that approach.
On the other side of the coin, “biochauvinism” is the term applied to the view that there is something philosophically unique about biological organisms—due to their basic nature and/or the limitations of human knowledge’s access to them—that makes them the biological world fundamentally unlike the rest of the world (Wolfe 2015). Vitalism—the view that life has some animating entity (along the lines of spirit) that animates matter into a living being—is one form of such biochauvinism (Wolfe 2015). Another form of biochauvinism is the use of phenomenology to assert that human lived experience partly operates within a space-time context that is different from that of a rock (Wolfe 2015). Wolfe finds that sort of view inside the influential phenomenological work of Maurice Merleau-Ponty (Wolfe 2015) (See Section 5 on phenomenology). Thus there are two rather opposite senses in which we can chauvinistically hold that the biological world is special:
- a specialness through what life excludes (medicine is nothing but applied biology) or
- a specialness through what life includes (an intangible vital spirit; a unique frame of time and space).
Version (1) of biomedicine is consistent with biomedicine and version (2) generally is not. The fact that the valorizing of biology can cut both ways helps to show, though, that biomedicine has staked out a sort of middle ground by insisting that understanding health and medicine is accomplished through understanding the innumerable subtleties of biological processes and substances, but that those biological processes and substances must not be so subtle or mysterious as to become untethered from properties and processes condoned by physics and chemistry.
Put another way, the biomedical framework of the body can be understood by considering what it excludes: spirit, vitality, and any other entity or property unknown to mainstream physicists or chemists. The concept Physicalism is closely related, but the term is perhaps not a perfect fit since it has taken on conceptual connotations and baggage through its use by analytic philosophers of metaphysics. The connection is that in the psychological biosciences, the “hard problem of consciousness” looms over everything:
It is widely agreed that experience arises from a physical basis, but we have no good explanation of why and how it so arises. Why should physical processing give rise to a rich inner life at all? It seems objectively unreasonable that it should, and yet it does. (Chalmers 1995)
While the human mind and consciousness attract a special sense of awe, the hard problem of consciousness is not all that different from the problems facing attempts to make sense of the patient as a whole. Patients live ; their bodies function every second of the day, via an astounding series of interconnected processes. It stretches the imagination to think that a vital spirit or such is giving unity to each life (human or non-); it stretches the imagination in another way to think that we humans are simply skin bags of chemical reactions.
The preceding section on purpose in biomedicine leads directly to the related issue of how health and disease are conceptualized in biomedicine. This is something that will only be dealt with very briefly here, in part to avoid duplicating the content in the entry on Concepts of Health and Disease . Debates over the meaning of health and disease—including how the two are connected—are central to philosophy of medicine. This entry will not attempt to summarize that complex literature, and rather focus on how conceptions of health and disease relate to biomedicine, including related disputes over how disease relates to disability justice matters, and ways in which purportedly objective biomedical conceptions of disease can be co-opted for dubious purposes.
Of the different philosophical accounts of health and disease, Christopher Boorse’s naturalistic Biostatistical Theory (BST) of disease gives the account that is perhaps most tightly linked with the philosophical commitments of biomedicine. Boorse’s revised account of the BST states:
The reference class is a natural class of organisms of uniform functional design; specifically, an age group of a sex of a species. A normal function of a part or process within members of the reference class is a statistically typical contribution by it to their individual survival [or] reproduction. Health in a member of the reference class is normal functional ability : the readiness of each internal part to perform all its normal functions on typical occasions with at least typical efficiency. A disease [later, pathological condition ] is a type of internal state which impairs health, i.e. , reduces one or more functional abilities below typical efficiency (Boorse 2014: 684, amending Boorse 1977: 562).
The idea is that we can wield insights of biochemistry, pathology, and evolutionary biology to together yield an objective means of ‘reading’ nature to tell us which states are pathological or diseased states, without the interference of messy and culturally rooted/biased evaluations. One line of rebuttal is that there is no principled way of objectively choosing a reference class against which to judge that a given part or process is falling short (my blood sugar levels are objectively too high compared to…what exactly?) (Kingma 2014). Even if that problem is resolvable, Krueger argues that an objective set of criteria for defining states as pathological or not would be a very limited accomplishment. Knowing that my blood sugar levels are objectively pathological does not really tell me much about what, if anything, I or my physician ought to do about that (Krueger 2015).
Fraught as it is to approach health via the functioning of components in the body, it is also quite conceptually difficult to measure health in a broader sense. Biomedical science practitioners tend to get twisted into conceptual and epistemic knots when attempting to measure general/overall health or well-being in a population (Hausman 2015). One key philosophical dispute among health scientists engaged in patient health measurement activities is over what sorts/amounts of knowledge patients have about their own health states, and how this knowledge ought to be accessed (McClimans forthcoming). How much can we glean about a patient’s physical comfort by asking them to rate their level of pain on a scale of 1–10? How do we solicit such information without repeating unethical patterns of trust or mistrust, rooted in racist/sexist/etc. stereotypes about patients (Buchman, Ho, & Goldberg 2017)?
The notion of disease is biological malfunction is also tied to a pair of critiques of the “medical model” of disability and of “medical model” of mental health. Both models are criticized for reducing the complexities of disability or psychological welfare to only the individual-level signs, symptoms, and variables recognized by biomedicine (see: Disability: Definitions, Models, Experience ). As traced in Hogan’s history of the concept, the medical model is either critiqued as insufficiently attentive to other dimensions (especially the social dynamics that drastically shape the relevant health experiences) or oppressive (since the model empowers biomedical experts to unilaterally dictate the disability/psychiatric categories, relevant evidence, diagnoses, treatments, etc.) (Hogan 2019). This is in part a dispute over functioning since debates over normality, difference, and pathology depend on whether/how we commit to the notion that bodies or parts thereof have functions at all. If one’s legs do not perform the function of walking, are the legs pathological? Is the person with those legs inherently diseased? Disabled? Worse off than if they had legs that could walk? One solution is to simply throw out the notion of normal functioning (Amundson 2000). Another related option is to embrace pluralism in the sense of accepting that the relationship between disability and well-being or health is complex, variable, and dependent on individual and social contingencies (Campbell & Stramondo 2017).
One philosophical complication is that a naturalistic concept of disease, like Boorse’s, does not prevent social processes from altering the standards and practices of how the boundaries of these natural categories are drawn in practice. Chronic diseases such as cardiovascular diseases and type 2 diabetes have been targeted by drug companies to not only create new treatments but to redraw the boundaries between healthy vs. pathological, including boundary zones such as “prediabetes” (Greene 2006). These efforts are in part accomplished via the design of clinical trials, which have the dangerous distinction of having very high epistemic value in the biomedical community, while remaining highly susceptible to manipulations to the experimental setup that push the evidence one direction or another (González-Moreno, Saborido, & Teira 2015).
While a naturalistic concept of disease is the most consistent with the ontological assumptions and methodological practices of biomedicine, that has not prevented biomedicine from accommodating a certain type of dissent from the biomedical tenet that biomedicine ought to focus on diseases and (only) the biological, chemical, and physical aspects thereof (Krieger 2011: 130). The World Health Organization (WHO)—founded in the same post-World War Two period as biomedicine—adopted a holistic positive concept of health at its founding. i.e., it said that health is the presence of complete well-being (including mental and social well-being) rather than just the absence of disease. This created a direct tension with the core tenets of biomedicine, especially the reduction of disease to a set of concrete observable and measurable physical bases (Valles 2018). Historically, the tension was resolved in the case of the WHO by the organization, shortly after its founding, effectively self-suppressing the use of its own definition of health in favor of pursuing a narrower set of initiatives to combat particular diseases (e.g., the celebrated Smallpox Eradication Program) (Irwin & Scali 2007). This seems to have been in part because cold war politics made it pragmatic to not antagonize the United States with pushing a health concept concerned with “social” well-being, sounding a bit reminiscent of social ism (Irwin & Scali 2007). While a WHO-type understanding of health is in genuine tension with the philosophical tenets of biomedicine, the tension is manageable in this case by simply pursuing areas of overlap between the different conceptions of disease (e.g., investing in infectious disease-control strategies for a population) while ignoring any purported aspects of health beyond the absence of pathologies (e.g., declining to invest in assessing how human-environment relations contribute to a population’s happiness or misery).
3. Epistemology of Biomedicine
There are philosophical disputes happening at the heart of the biomedical enterprise and those disputes offer a means of understanding the epistemological dimensions of biomedicine: the nature of evidence and knowledge in the framework. The most contentious of those internal biomedicine disputes center on what the epistemology of biomedicine ought to be, with the rise of “evidence-based medicine” (EBM) being the event that provoked the largest segment of these disputes. By examining the disputes surrounding EBM, we can gain a better understanding of the epistemology of biomedicine, as illustrated in work such as Solomon’s Making Medical Knowledge (2015).
Evidence-based medicine is a tricky concept, since its name misleadingly raises the question of who is practicing medicine that is not based on evidence (Goldenberg 2006). Instead, the dispute is over which evidence is best and how it ought to be used. For instance, physicians will often advise patients on which home treatments to use for minor lower back pain, with individual physicians varying in which treatments they recommend, even aside from differences in the advice based on differing patient characteristics. Ibuprofen? Acetaminophen? Heat and/or cold compresses? Exercise? The standard twentieth-century medical response would be to let physician groups (including consensus groups convened for this purpose) lay out the options and perhaps use their collective expertise to make a recommendation, but giving great leeway to individual physicians to use their individual accumulated expertise to choose another of the available options seen as viable options by their peers. EBM instead treats this as a matter to be largely decided by empirical research. I.e., we ought to run randomized control trial experiments that compare the efficacy of alternative options by randomly assigning patients with lower back pain into Treatment A or Treatment B —controlling for the single variable—and measure the effects on patients using predetermined metrics (change in reported pain severity, incidence of major side effects, etc.). After doing multiple experiments we can then do a “systematic review and meta-analysis” that compiles the data trends across parallel research studies and helps us build an evidence base for creating guidelines for which treatment clinicians ought to use. Those guidelines dictate the proper default practices, which individual clinicians can contravene if specific individual patient needs are in conflict (e.g., a patient history of not responding well to a certain medication).
EBM began as a self-described Kuhnian scientific revolution of biomedicine—a fundamental change of worldview that is incommensurable with the previous one; a paradigm shift (Evidence-Based Medicine Working Group 1992). The most fundamental dispute between advocates of the EBM movement and skeptics within the biomedical community is, in one sense, over the epistemic and power relationships between the two halves of biomedicine: biology and medicine. In biology, experimental methodologies and attempts to find generalizable population trends are valued highly; in clinical medicine, the single patient and the clinician’s accumulated (and ineffable) expert evaluation of them has long been valued very highly—much attention is paid to that individual patient’s contingencies (the particulars of their body and symptoms), with the clinician’s accumulated knowledge and know how being relied on when determining how to proceed. Evidence-based medicine is not a simplistic adoption of biology principles and their application to medicine; it is not the triumph of biology over medicine in biomedicine. Instead, EBM has helped to draw out deeper disagreements about what it means to do and use biomedical science well.
There is a large philosophical literature on evidence-based medicine, with the first monograph appearing in 2002 (Goodman 2002). Most of the literature takes at least a partially skeptical stance on EBM practitioners’ various hardline stances on evidentiary matters: the suitability of clinicians relying primarily on brief synopses that attempt to synthesize massive bodies of evidence (Borgerson 2009); the room for hidden biases in the process of evaluating medical data (Stegenga 2011), and more. EBM has also inspired passionate disputes among clinicians over how to apply its principles (Berwick 2005; Greenhalgh, Snow, Ryan, Rees, & Salisbury 2015). One line of criticism is that its aims are noble, but have been hijacked by bad actors, namely corporations that learned to that by getting involved in the production of randomized control trial evidence they could sway the evidence for the apparent safety and efficacy of their own products (Ioannidis 2016). Philosophy of EBM is given extensive discussion in the entry on Philosophy of Medicine , and as with the rest of this entry, this entry will avoid needless repetition and keep the focus on biomedicine per se .
Biomedicine is in a state of tension, between
- a history of resolving evidentiary disputes using mechanistic reasoning, consensus, and authority (see, e.g., Solomon [2015] on the roles played by ‘consensus conferences’) vs.
- a recent practice of creating predetermined evidentiary ranking/evaluating procedures designed to minimize the ability of biased or erratic human judgments to unduly influence the resolution of evidentiary disputes.
Clinical guidelines were once routinely created by what is pejoratively called GOBSAT: Good Old Boys Sat Around A Table (and reached a consensus by talking amongst themselves as leading experts in the subject matter). As Greenhalgh puts it, “it is a major achievement of the EBM movement that almost no guideline these days is produced by GOBSAT!” (Greenhalgh 2014: 7). It remains a point of philosophical contention just how epistemically different it is to generate clinical practice guidelines using a consensus-based model vs. an evidence-based model, in large part since, no matter what evidence is prioritized, a group of experts will ultimately need to interpret the data and make judgment calls on how to generate practical guidelines based on the compiled evidence (Djulbegovic & Guyatt 2019). Though even the meaning of clinical expertise is itself not clearly defined and agreed upon, a matter further complicated by the way that the meaning of expertise is tangled up with views about what roles expertise ought to be playing in clinical practice (Wieten 2018).
Evidence-based medicine prizes certain types of biomedical evidence—highly controlled and systematic evidence—more highly than others. But that preference is a matter of much contention. As noted above, experimental evidence from a randomized experiment testing a treatment (a “randomized control trial”) is held as the best sort of evidence: the gold standard. The one exception is that “meta-analyses” of multiple experimental studies are perhaps a platinum standard better than gold (Stegenga 2011), i.e., the thinking goes that the only evidence that might be better than such experimental data is a rigorous quantitative study rigorously looking for patterns and lessons by analyzing the results many rigorous experimental studies. While the rigidity of EBM thinking gets a great deal of criticism, there are elements of the EBM’s philosophy that implement the sort of pragmatism that many critics desire, such as an epistemic openness in the community to engage in “open-ended critical inquiry” (Goldenberg 2009).
The status of laboratory evidence is a major point of contention in philosophy of biomedicine disputes. “Bench science” is often used as a catchall term in EBM for a wide variety of laboratory evidence (biochemistry, pathology, digital models of drug metabolism, animal studies of a treatment, etc.), and that evidence is pushed to the very bottom of the evidence hierarchy (B. Clarke, Gillies, Illari, Russo, & Williamson 2014). For example, green tea contains epigallocatechin gallate (EGCG), which seems to be effective at killing cancer cells in petri dishes and in mice (Eisenstein 2019). EBM supporters would be inclined to look down on inferences that green tea is therefore a wise anti-cancer home health behavior, until there is at least epidemiological evidence that green tea drinkers genuinely do have better cancer outcomes. Even then, EBM supporters would want to know if the anti-cancer effects are due to EGCG, some combination of multiple components of green tea, or perhaps just the relaxing social act of having a hot beverage. Biomedicine has always valued the sort of research that investigates EGCG in petri dishes, in mice, or other models used on the laboratory bench, but the dispute is over how reliable one takes that research to be for guiding real medical decisions (ought physicians tell patients to drink green tea?).
The problem of how highly to value bench science is distinct from the general objection to hierarchies of evidence (Bluhm 2005). Even if evidence isn’t treated as inherently rankable in value, one key philosophical objection is that much of the biomedical research world is treated by EBM as less important than clinical patient experiments, or meta-analyses of such experimental data. Solomon explains that this position is in tension with a simultaneous trend in biomedicine, an increased concern with translational medicine (an effort to improve the process of moving candidate treatments from (the laboratory) bench to (the patient’s) bedside (Solomon 2011). This effort to better manage the biomedical research enterprise has brought with it,
a restoration of the recognition that clinical research requires an engagement with basic theory (e.g., physiological, genetic, biochemical) and a range of empirical techniques such as bedside observation, laboratory and animal studies. (Solomon 2011: 451)
In an openly conciliatory move, the “EBM+” group of philosophers and clinicians (see Other Internet Sources ), offers a defense of the view that mainstream EBM has erred by essentially relegating mechanistic evidence, such as EGCG having anti-cancer chemical properties, to a subsidiary role (B. Clarke et al. 2014).
While epidemiology-focused EBM is partly in tension with most of the rest of laboratory-focused biomedicine—needing it but seeing it as in service to randomized control trials on patients—both EBM and other branches of biomedicine are united by a valuing of precise measurement. While precise measurement is achievable in the biochemistry lab, the practical and philosophical challenges are thornier when doing measurement at the level of the whole person or the population. What is well-being (Alexandrova 2017)? By what standards can we call a population healthy (Hausman 2015)? What sorts of social structures, policies, and interventions are effective for promoting health (Valles 2018)? How do we identify which subgroups are left out of the benefits and prevent injustices being done to them (Maglo 2010, 2012)? What sorts of values are at stake when we debate biomedical evidence surrounding culturally issues such as birthing practices (McClimans 2017)? A common theme shared by these critiques is a concern about the fickleness and contingency of measurement leaving much room for practitioners’ values and motivations to shape the results of the measurement process, for good or for ill.
The biomedical quest for precise and objective measurements leaves some unsatisfied with the little room left for patient input in clinical care decision-making. What roles are there for patient input in the process of deciding the best course of treatment (Chin-Yee & Upshur, 2018)? According to critiques, by trying to sideline subjective factors in medical care and replace them with increasingly objective factors, EBM especially (even among than other biomedical perspectives), risks losing the humanistic aspects of medicine, an ethical loss and an epistemic loss. By treating patients’ desires, goals, and values as largely irrelevant or a source of interference with an objective process, EBM loses sight of medicine as a means of helping real humans’ real problems. Relatedly, by shutting the patient out of the process, the notion of the best or right treatment becomes hollowed out (McClimans forthcoming).
In the background is the important—and meta-epistemic—problem that physicians are not educated to be comfortable with uncertainty, and what to do in an objective measurement-focused field when one finds oneself lacking the desired evidence: inconclusive tests, treatments designed for patients unlike the one being treated, etc. The very topic of uncertainty is conspicuously absent from medical education (Tonelli & Upshur 2019).
In biomedicine, it appears much of medicine is reduced to applied biology—patients are just biochemical substances processes and medical knowledge is just a complicated form of biological knowledge. Keating and Cambrosio see the relationship between this reductionism in biomedicine (the reducing of medicine to biology) as more a matter of the two being aligned for complex historical and philosophical reasons, instead of that reductionism being philosophically central to biomedicine (Keating & Cambrosio 2003). According to them, after World War Two, the fields of biology and pathology negotiated the creation of “the institutional and epistemic hybrid we call biomedicine,” and neither component of this hybrid rules the other (Keating & Cambrosio 2003, p. 368). They see this as undermining claims of biomedicine being reductionist because such assertions implicitly or explicitly mean that (micro)biology gets epistemic priority because it is the true and stable foundation.
Part of the challenge is that there are multiple varieties of Reductionism in Biology . And there are a number of different reductionism disputes in biology, and the overlaps with biomedicine vary—e.g., Rosenberg’s defense of reductionism in genetics overlaps with biomedicine in the area of medical genetics (Rosenberg 2006). As discussed in Section 1 , it is very difficult to tell the difference between the core components of biomedicine and the bits of philosophy/technology/sociology that aren’t inherently part of biomedicine, but have gotten tangled up in it. The rationale for reductionism in biomedicine is explained with great care by Andersen:
Being able to successfully take a disease as complex as sickle cell anemia or Parkinson’s and reduce it to a single genetic error that cascades through various systems, even if that reduction holds for only a proper subset of cases, illustrates that reduction can be a powerful tool for research and explanation in medicine. It cannot be the only tool in the toolkit, since some diseases or dysfunctions may be only partially reducible, or for which only some cases are reducible. But it is a good working assumption, as Oppenheim and Putnam put it, in tackling a problem with an unknown etiology, to look for ways to reduce it to a few or even a single causal driver at a molecular level (Andersen 2016: 86).
In other words, the potential for success and record of prior successes is an argument for at least operating under the assumption that biomedical scholars ought to continue treating biomedical mysteries as biochemical puzzles for which the relevant pieces have not yet been identified or assembled.
Reductionism in biomedicine has been targeted by some committed critics. For instance, Marcum explains that the process of reducing the body to such component parts yields a vision of the body as machine, a radically dehumanizing move.
From the biomechanical point of view, the patient’s body is often perceived as a material object that can be reduced to a system of physical parts. That body is viewed as a machine composed of individual body parts, which can be fixed or exchanged with new parts, when broken. By reducing the patient’s body to an assemblage of body parts, the patient qua person vanishes (Marcum 2005: 318).
While the critique stands on its own, it remains an open question what other view ought to be adopted if not a reductionist biomechanical one. Marcum contrasts the view most directly to phenomenological views—which will be discussed in Section 5 —arguing that a phenomenological approach is better suited to advancing medicine, which he presents as being in a state of crisis.
EBM’s reliance on randomized control trials as a cornerstone of health data collection doubles down on the general reductionism of biomedicine. Randomized control trials assume that health variables can be observed, manipulated and controlled as largely independent units. Yet, is a well-established problem that we simply don’t know all of the variables that might confound a clinical experiment (Worrall 2007), so randomization can at most hope that the ‘confounders’ (e.g., unknown dietary factors that affect metabolization of a drug) are randomly distributed between the different treatment populations in an experiment. Taking the reductionism a step further, the goal of exactly measuring the differences between Treatment A and Treatment B requires EBM to only look for effects that are precisely measurable in the first place. This limitation is a deeply-rooted problem since even the official list of clinical signs and symptoms of a given disease can diverge from accumulated patient observations, and in cases such as some psychiatric conditions, the symptoms—or lack thereof—are overtly difficult to measure or factor into an assessment of treatment efficacy (e.g., one’s sense of self and/or emotional life) (Kendler 2016).
While reductionist and antireductionist approaches are in direct opposition, it is possible to take a pluralistic stance toward them: both can be welcomed into health science. For instance, this is the position taken by Campaner (2010). Though, as discussed in the next section, the power of the biomedical institution makes it such that biomechanical reductionist approaches can easily overwhelm alternative views.
The disputes over biomedicine’s virtues and flaws are tied to the other aspects of Western cultural-political-economic influence that coevolved with it, and travelled with it around the globe. The dynamics between the institution of biomedicine—rooted in wealthy Western countries—and low/middle-income countries is in part a manifestation of the philosophical complexities of the international political scene. See, for instance, the literature on International Distributive Justice .
As discussed in Section 1.2 , biomedicine co-evolved with the West’s social and economic institutions in the aftermath of World War Two. Of particular importance, the individualism of Western capitalism has meshed with biomedicine’s ontological view of the body as effectively separable from its social context. Briggs and Hallin describe the how contemporary media and biomedicine work to coproduce public knowledge about health and disease, a process they dub biomediatization (Briggs & Hallin 2016).
Metzl and Kirkland’s influential edited volume Against Health: How Health Became the New Morality lays out a related case for how biomedicine exercised undue influence over culture, including casting health ills as personal failings deserving of public shaming and stigmatization (Metzl & Kirkland 2010). As an illustration of the sort of stigmatizing strategies that they condemn as unacceptable, bioethicist Daniel Callahan ethically endorsed the social practice of ‘fat shaming’ as a means of promoting public health (Callahan 2016). Empirical arguments on the harms of stigmatization (Hatzenbuehler, Phelan, & Link 2013), and contrary ethical arguments (Dean 2018), both rebut stigma as an ethically viable health-promoting intervention.
Health/body stigma, public rhetoric over health, and moralism about health behaviors are all subjected to scrutiny in critiques of ‘self-care’ strategies, e.g., telling ‘overweight’/overtired/overworked employees feeling stressed by their jobs that they should take better care of themselves by doing yoga, more closely monitoring their eating at work, etc. The critique is that the biomedical model can recast social problems (including outright abuses—usually capitalist ones) as individual responsibilities. Self-care can go well when it empowers, such as the Women’s Health Movement of the 1960s and 1970s that fought against sexism by calling upon women to know their own bodies and value that knowledge (Bueter 2017). Or self-care can become regressive when it becomes
inner-directed, authoritarian, victim-blaming, manipulated by dominant forces, or diversionary from struggles for radical change in both medicine and the broader society. (Sidel & Sidel 1981: 656)
In recent discourse, in which the problem of stress has garnered increased attention,
controlling the health problems associated with living in stressful situations therefore becomes the responsibility of the individuals—all too often, the responsibility of individuals who are already disadvantaged by their economic status, their race, or their social position more generally. (Kaplan 2019: 116)
Biomedicine has been used as one prong of colonial power dynamics between Western institutions and non-Western peoples (including indigenous peoples) (see Millum & Emanuel 2012). Colonial governments and cultural imperialism have long undermined or directly attacked local/indigenous medical frameworks and institutions. And since biomedical education and practice are based on technologies and education from the West, low- and middle-income countries are left dependent on wealthy Western countries (Nunn 2009). As a result, local would-be biomedical practitioners often travel to the West for biomedical education and then have little incentive to return to their home countries to practice techniques for which they will have relatively few biomedical tools, lower pay, etc. This raises further ethical questions related to which entities have which individual/collective responsibilities to address this so-called ‘brain drain’ trend (Yuksekdag forthcoming). Meanwhile, non-governmental organizations such as the Red Cross offer medical aid, but generally in the form of sending temporary trained personnel from the West, rather than building local capacity for medical training and practice. And well-meaning Western biomedical students travel to the same low- and middle-income countries, with limited biomedical skills and little or no knowledge of the populations or health needs, seeking to help populations assumed to be incapable of helping themselves (Pinto & Upshur 2009).
5. Alternative Frameworks
As noted at the beginning of this entry, critiques of biomedicine tend to focus on elements of biomedicine rather than the whole. There are exceptions to this. This section will review some of the frameworks that have been offered in place of biomedicine, though they vary in how similar they remain to the biomedical framework. Before delving into the alternative frameworks, it is important to discuss two prominent critiques of biomedicine that are influential primarily as holistic criticisms and not so much as sources of alternative visions of what ought to be offered in place of biomedicine.
Michel Foucault ’s work, and use of the concepts biopower and biopolitics, remain touchstones for much of the critical discourse surrounding biomedicine. Foucault’s critique of modern medicine is part of a career critiquing other aspects of modernity, including the related topic of psychiatry. His famous work The Birth of the Clinic is written as a history, though in the process it highlighted aspects of biomedicine that other scholars went on to critique as well, either based on his critique or in parallel to it: dehumanization, reductionism, measurement and observation methods of dubious value, and the problem of the biomedicine’s institutional power (Foucault 1963 [2002]).
Ivan Illich’s Medical Nemesis is perhaps the most influential critique of biomedicine (Illich 1976). It combines critiques of (bio)medicine for failing at its own goals (iatrogenic disease—harms caused by medical treatment—are a large component) and for having the wrong goals in the first place (he valorizes death, disease, and pain as proper components of the human experience rather than enemies to be automatically and constantly opposed).
“Complementary, alternative, or integrative medicine” is biomedicine’s ‘big tent’ category for non-biomedical systems of health care (see NCCIH in Other Internet Resources ). Philosophers have pointed out that biomedicine, specifically evidence-based medicine, puts advocates of such non-biomedical systems into a no-win position. One such philosophical challenge is that some of the claims made by non-biomedical systems are claimed to be non-measurable experiences, e.g., the experience of Qi life force in traditional Chinese Medicine (Tonelli & Callahan 2001). This means that advocates have to either submit to the epistemological framework of biomedicine (which may be incapable of assessing some of the effects claimed to exist), or refusing to play by medicine’s rules and hence being dismissed by as quackery by biomedicine. Epistemically, it seems like the latter option is the better option, but it would require advocates to “work to develop new research designs and new standards of evidence that reflect their approach to medical care” (Borgerson 2005: 502).
Classical Chinese Medicine (CCM) remains practiced in communities around the world, and Lee draws out two core philosophical differences between CCM and biomedicine. First, CCM has
a process ontology—it considers causal relationships between events and processes to be foundational, rather than things. Furthermore, it implies complex causal relationships between events and processes which may be said to be multi-factorial and non-linear. (Lee 2017: 2)
Second, it is holistic
the universe and everything in it, including human beings, constitute wholes which are different from the sum of their parts, and which in turn are related and as well as inter-related with other wholes. (Lee 2017: 2)
It is within this philosophical system that treatments such as acupuncture and herbal/dietary remedies are used. While the gulf between biomedicine and CCM is wide, it is not entirely dissimilar from some Western scientific practices: CCM’s approach to health and the human body bear some resemblances to the ways that epidemiologists approach populations’ health and how ecologists approach ecosystems. Among other similarities, in both fields there is great respect for balance and dynamic interconnected processes (Lee forthcoming).
Ayurvedic medicine takes a somewhat similarly holistic and balance-oriented approach to the person and their health (Rastogi, 2014) (note also that holistic bodily balance is central to the Hippocractic medicine described in Section 1.2 ). One way that manifests in Ayurvedic practice—in contrast with biomedicine—is that the biological characteristics of two individuals might be similar but their recommended treatments might be quite different. For instance, while biomedicine routinely hands out standardized advice on what sorts of foods are best for someone of a given age and sex, Ayurvedic medicine rejects the assumption that such standardization within a single age-sex grouping is even a good default. Other factors play larger roles than in biomedicine, including bodily changes over the course of the day and one’s (internal) body type (affecting how one metabolizes specific foods, etc.). Complicating matters is that Ayurvedic medicine grew as a part of Hinduism in South Asia, and Hinduism itself is a substantially heterogeneous family of beliefs/practices that vary between communities (Desai 1988).
Among the numerous humanistic critiques of biomedicine, coming from the broad field of medical humanities (for an overview, see Marcum 2008), philosophers have paid particular attention to narrative and phenomenology (Ferry-Danini 2018).
Narrative medicine offers a revision to the biomedical model alternative based around the centrality of the story or narrative in human life (Charon 2006). This notion that narrative is central to human experience offers a variety of potential operationalizations in clinical biomedical practice. For instance, it places additional value on the patient consultation and asks for improved active listening skills among clinicians, who must learn to elicit, receive, and understand patients’ stories about their health conditions. Such listening has many potential benefits, including the potential to understand the meaning(s) of what human dignity means for a given patient, an important benefit given that biomedical settings are known for being “dehumanizing” (Parsons & Hooker 2010). Though, the proposition that humans are indeed narrative beings has itself been disputed (Woods 2011).
As shown by Carel, phenomenology’s value to medicine comes from its insistence on taking illness seriously (Carel 2016).
…we must enlist philosophical analysis in order to fully appreciate the existential transformation illness brings about. This transformation cannot be accounted for as merely physical or mental (in the case of psychiatric disorder) dysfunction. Rather, there is a need for a view of personhood as embodied, situated, and enactive, in order to explain how local changes to the ill person’s body and capacities modify her existence globally (Carel 2016: 14).
Biomedicine takes illness seriously, but the philosophical framework of biomedicine leaves no space for the notions of existential transformation as part of illness—disease is reduced to the state of a system in which there are malfunctioning parts (pathologies in body parts). See also the discussion of phenomenology in Section 3.4 .
Stegenga has argued in favor of “gentle medicine,” a conservative treatment mindset that stands in contrast to the biomedical pursuit of ever-more interventions (Stegenga 2018)—a pill for each ailment. This is in one sense a challenge to biomedicine more in quantity than in quality; he does not advocate for switching from pills to naturopathic diet-based treatments. He recommends this (purportedly gentler) conservatism as a response to an epistemic devaluing of biomedicine knowledge which he calls: “medical nihilism…the view that we should have little confidence in the effectiveness of medical interventions” (Stegenga 2018: 1). This follows in the loose tradition of other writings, such as the text by Illich discussed at the start of Section 5 (Illich 1976). But Stegenga’s critique and replacement remains a radical challenge to biomedicine in the sense that it undercuts the epistemic practices of biomedicine and pushes back on the institutional practices (and the related infrastructure, including massive pharmaceutical companies) by arguing that (bio)medical treatments ought to be applied sparingly.
Valles offers an alternative—the population health framework—that partly meshes with Stegenga’s concluding suggestion that “gentle medicine” ought to be accompanied by interventions that refocus health promotion efforts on social determinants of health, including “clean drinking water, better nutrition, and greater socio-economic equality” (Stegenga 2018: 198). Valles expounds and defends the merits of the population health framework, a view developed in response to frustrations with biomedicine that coalesced in the 1990s in Canada as an alternative theoretical framework, before expanding internationally (Evans, Barer, & Marmor 1994; Valles 2018). The population health framework is not nihilistic about medical care, but rather seeks to decenter medicine and healthcare in the overall pursuit of health; most of the problems and most promising solutions to ill health lay outside the scope of biomedicine (safer workplaces, an end to racist housing discrimination, neighborhoods where people can safely walk, socialize and play, etc.) (Valles 2018).
- Alexandrova, Anna, 2017, A Philosophy for the Science of Well-Being , Oxford: Oxford University Press. doi:10.1093/oso/9780199300518.001.0001
- Amundson, Ron, 2000, “Against Normal Function”, Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences , 31: 33–53. doi:10.1016/S1369-8486(99)00033-3
- Andersen, Holly K., 2016, “Reductionism in the Biomedical Sciences”, in The Routledge Companion to Philosophy of Medicine , Miriam Solomon , Jeremy R. Simon , and Harold Kincaid (eds), New York: Routledge, chapter 8.
- Berwick, D. M., 2005, “Broadening the View of Evidence-Based Medicine”, Quality and Safety in Health Care , 14(5): 315–316. doi:10.1136/qshc.2005.015669
- Bluhm, Robyn, 2005, “From Hierarchy to Network: A Richer View of Evidence for Evidence-Based Medicine”, Perspectives in Biology and Medicine , 48(4): 535–547. doi:10.1353/pbm.2005.0082
- Boorse, Christopher, 1977, “Health as a Theoretical Concept”, Philosophy of Science , 44(4): 542–573.
- –––, 2014, “A Second Rebuttal On Health”, Journal of Medicine and Philosophy , 39(6): 683–724. doi:10.1093/jmp/jhu035
- Borgerson, Kirstin, 2005, “Evidence-Based Alternative Medicine?”, Perspectives in Biology and Medicine , 48(4): 502–515. doi:10.1353/pbm.2005.0084
- –––, 2009, “Why Reading the Title Isn’t Good Enough: An Evaluation of the 4S Approach to Evidence-Based Medicine”, IJFAB: International Journal of Feminist Approaches to Bioethics , 2(2): 152–175. doi:10.3138/ijfab.2.2.152
- Briggs, Charles L. and Daniel C. Hallin, 2016, Making Health Public: How News Coverage Is Remaking Media, Medicine, and Contemporary Life , Abingdon, Oxon: Routledge. doi:10.4324/9781315658049
- Broadbent, Alex, 2009, “Causation and Models of Disease in Epidemiology”, Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences , 40(4): 302–311. doi:10.1016/j.shpsc.2009.09.006
- Bynum, William, 2008, The History of Medicine: A Very Short Introduction , Oxford: Oxford University Press.
- Buchman, Daniel Z, Anita Ho, and Daniel S Goldberg, 2017, “Investigating Trust, Expertise, and Epistemic Injustice in Chronic Pain”, Journal of Bioethical Inquiry , 14(1): 31–42. doi:10.1007/s11673-016-9761-x
- Bueter, Anke, 2017, “Androcentrism, Feminism, and Pluralism in Medicine”, Topoi , 36(3): 521–530. doi:10.1007/s11245-015-9339-y
- Bueter, Anke, 2017, “Androcentrism, Feminism, and Pluralism in Medicine”, International Encyclopedia of the Social & Behavioral Sciences , 36(3): 521–530. doi:10.1016/B0-08-043076-7/03143-0
- Callahan, Daniel, 2016, The Five Horsemen of the Modern World: Climate, Food, Water, Disease, and Obesity , New York: Columbia University Press.
- Cambosio, Alberto and Peter Keating, 2001: “Biomedical Sciences and Technology: History and Sociology” in Neil J. Smelser and Paul B. Baltes (eds.), International Encyclopedia of the Social & Behavioral Sciences , Amsterdam: Elsevier Ltd., 1222–1226.
- Campaner, Raffaella, 2010, “Reductionist and Antireductionist Stances in the Health Sciences”, in The Present Situation in the Philosophy of Science , Friedrich Stadler (ed.), Dordrecht: Springer Netherlands, 205–218. doi:10.1007/978-90-481-9115-4_17
- Campbell, Stephen M. and Joseph A. Stramondo, 2017, “The Complicated Relationship of Disability and Well-Being”, Kennedy Institute of Ethics Journal , 27(2): 151–184. doi:10.1353/ken.2017.0014
- Carel, Havi, 2016, Phenomenology of Illness , New York: Oxford University Press. doi:10.1093/acprof:oso/9780199669653.001.0001
- Chalmers, David J., 1995, “Facing Up to the Problem of Consciousness”, Journal of Consciousness Studies , 2(3): 200–219.
- Charon, Rita, 2006, Narrative Medicine: Honoring the Stories of Illness , New York: Oxford University Press.
- Chin-Yee, Benjamin and Ross Upshur, 2018, “Clinical Judgement in the Era of Big Data and Predictive Analytics”, Journal of Evaluation in Clinical Practice , 24(3): 638–645. doi:10.1111/jep.12852
- Clarke, Adele E., Laura Mamo, Jennifer R. Fishman, Janet K. Shim, and Jennifer Ruth Fosket, 2003, “Biomedicalization: Technoscientific Transformations of Health, Illness, and U.S. Biomedicine”, American Sociological Review , 68(2): 161–194. doi:10.2307/1519765
- Clarke, Brendan, Donald Gillies, Phyllis Illari, Federica Russo, and Jon Williamson, 2014, “Mechanisms and the Evidence Hierarchy”, Topoi , 33(2): 339–360. doi:10.1007/s11245-013-9220-9
- Conrad, Lawrence I., Michael Neve, Vivian Nutton, Roy Porter, and Andrew Wear, 1995, The Western Medical Tradition: 800 BC to AD 1800 , Cambridge: Cambridge University Press.
- Dean, Megan, 2018, “Eating Identities, ‘Unhealthy’ Eaters, and Damaged Agency”, Feminist Philosophy Quarterly , 4(3): article 3. doi:10.5206/fpq/2018.3.5778
- Desai, Prakash N., 1988, “Medical Ethics in India”, Journal of Medicine and Philosophy , 13(3): 231–255. doi:10.1093/jmp/13.3.231
- Djulbegovic, Benjamin and Gordon Guyatt, 2019, “Evidence vs Consensus in Clinical Practice Guidelines”, JAMA , 322(8): 725–726. doi:10.1001/jama.2019.9751
- Eisenstein, Michael, 2019, “Tea’s Value as a Cancer Therapy Is Steeped in Uncertainty”, Nature , 566(7742): S6–S7. doi:10.1038/d41586-019-00397-2
- Evans, Robert G., Morris L. Barer, and Theodore R. Marmor (eds.), 1994, Why Are Some People Healthy and Others Not? The Determinants of Health of Populations , New York: Aldine De Gruyter.
- Evidence-Based Medicine Working Group, 1992, “Evidence-Based Medicine: A New Approach to Teaching the Practice of Medicine”, JAMA , 268(17): 2420–2425. doi:10.1001/jama.1992.03490170092032
- Fee, Elizabeth and Nancy Krieger (eds.), 1994, Women’s Health, Politics, and Power: Essays on Sex/Gender, Medicine, and Public Policy , Amityville, NY: Baywood Publishers.
- Ferry–Danini, Juliette, 2010, “A New Path for Humanistic Medicine”, Theoretical Medicine and Bioethics , 39(1): 55–57. doi:10.1007/s11017-018-9433-4
- Foucault, Michel, 1963 [2002], Naissance de la Clinique , Paris: Presses Universitaires de France. Translated as The Birth of the Clinic: An Archaeology of Medical Perception , A. M. Sheridan (trans.), London: Routledge.
- Goldenberg, Maya J., 2006, “On Evidence and Evidence-Based Medicine: Lessons from the Philosophy of Science”, Social Science & Medicine , 62(11): 2621–2632. doi:10.1016/j.socscimed.2005.11.031
- –––, 2009, “Iconoclast or Creed?: Objectivism, Pragmatism, and the Hierarchy of Evidence”, Perspectives in Biology and Medicine , 52(2): 168–187. doi:10.1353/pbm.0.0080
- González-Moreno, María, Cristian Saborido, and David Teira, 2015, “Disease-Mongering through Clinical Trials”, Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences , 51: 11–18. doi:10.1016/j.shpsc.2015.02.007
- Goodman, Kenneth W., 2002, Ethics and Evidence-Based Medicine: Fallibility and Responsibility in Clinical Science , Cambridge: Cambridge University Press. doi:10.1017/CBO9780511545511
- Greene, Jeremy A., 2006, Prescribing by Numbers: Drugs and the Definition of Disease , Baltimore, MD: Johns Hopkins University Press.
- Greenhalgh, Trisha, 2014, How to Read a Paper: The Basics of Evidence-Based Medicine , fifth edition, Oxford: John Wiley & Sons. First edition 1997.
- Greenhalgh, Trisha, Rosamund Snow, Sara Ryan, Sian Rees, and Helen Salisbury, 2015, “Six ‘Biases’ against Patients and Carers in Evidence-Based Medicine”, BMC Medicine , 13: art. 200. doi:10.1186/s12916-015-0437-x
- Hatzenbuehler, Mark L., Jo C. Phelan, and Bruce G. Link, 2013, “Stigma as a Fundamental Cause of Population Health Inequalities”, American Journal of Public Health , 103(5): 813–821. doi:10.2105/AJPH.2012.301069
- Hausman, Daniel M., 2015, Valuing Health: Well-Being, Freedom, and Suffering , New York: Oxford University Press. doi:10.1093/acprof:oso/9780190233181.001.0001
- Hogan, Andrew J., 2019, “Moving Away from the ‘Medical Model’: The Development and Revision of the World Health Organization’s Classification of Disability”, Bulletin of the History of Medicine , 93(2): 241–269. doi:10.1353/bhm.2019.0028
- Illich, Ivan, 1976, Medical Nemesis: The Expropriation of Health , New York: Random House.
- Ioannidis, John P.A., 2016, “Evidence-Based Medicine Has Been Hijacked: A Report to David Sackett”, Journal of Clinical Epidemiology , 73: 82–86. doi:10.1016/j.jclinepi.2016.02.012
- Irwin, A. and E. Scali, 2007, “Action on the Social Determinants of Health: A Historical Perspective”, Global Public Health , 2(3): 235–256. doi:10.1080/17441690601106304
- Kaplan, Jonathan, 2019, “Self-Care as Self-Blame Redux: Stress as Personal and Political”, Kennedy Institute of Ethics Journal , 29(2): 97–123. doi:10.1353/ken.2019.0017
- Keating, Peter and Alberto Cambrosio, 2003, Biomedical Platforms: Realigning the Normal and the Pathological in Late-Twentieth-Century Medicine , Cambridge, MA: MIT Press.
- Kendler, Kenneth S., 2016, “Phenomenology of Schizophrenia and the Representativeness of Modern Diagnostic Criteria”, JAMA Psychiatry , 73(10): 1082–1092. doi:10.1001/jamapsychiatry.2016.1976
- Kingma, Elselijn, 2014, “Naturalism about Health and Disease: Adding Nuance for Progress”, Journal of Medicine and Philosophy , 39(6): 590–608. doi:10.1093/jmp/jhu037
- Krieger, Nancy, 1994, “Epidemiology and the Web of Causation: Has Anyone Seen the Spider?”, Social Science & Medicine , 39(6): 590–608. doi:10.1093/jmp/jhu037
- –––, 2011, Epidemiology and the People’s Health: Theory and Context , New York: Oxford University Press. doi:10.1093/acprof:oso/9780195383874.001.0001
- –––, 2017, “Health Equity and the Fallacy of Treating Causes of Population Health as If They Sum to 100%”, American Journal of Public Health , 107(4): 541–549. doi:10.2105/AJPH.2017.303655
- Krueger, James, 2015, “Theoretical Health and Medical Practice”, Philosophy of Science , 82(3): 491–508. doi:10.1086/681628
- Kuhn, Thomas S., 1962, The Structure of Scientific Revolutions , Chicago: University of Chicago Press.
- Lakatos, Imre, 1968, “Criticism and the Methodology of Scientific Research Programmes”, Proceedings of the Aristotelian Society , 69: 149–186. doi:10.1093/aristotelian/69.1.149
- Lawrence, Christopher and George Weisz (eds.), 1998, Greater than the Parts: Holism in Biomedicine, 1920–1950 , New York: Oxford University Press.
- Lee, Keekok, 2017, The Philosophical Foundations of Classical Chinese Medicine: Philosophy, Methodology, Science , Lanham, MD: Lexington Books.
- –––, forthcoming, “Epidemiology Is Ecosystem Science”, Synthese , first online: 26 February 2019. doi:10.1007/s11229-019-02129-5
- Link, Bruce G. and Jo C. Phelan, 2002, “McKeown and the Idea That Social Conditions Are Fundamental Causes of Disease”, American Journal of Public Health , 92(5): 730–732. doi:10.2105/AJPH.92.5.730
- Lock, Elizabeth and Deborah Gordon (eds.), 1988, Biomedicine Examined , Dordrecht: Kluwer Academic Publishers.
- Löwy, Ilana, 2011, “Historiography of Biomedicine: ‘Bio,’ ‘Medicine,’ and In Between”, Isis , 102(1): 116–122. doi:10.1086/658661
- Maglo, Koffi N., 2010, “Genomics and the Conundrum of Race: Some Epistemic and Ethical Considerations”, Perspectives in Biology and Medicine , 53(3): 357–372. doi:10.1353/pbm.0.0171
- –––, 2012, “Group-Based and Personalized Care in an Age of Genomic and Evidence-Based Medicine: A Reappraisal”, Perspectives in Biology and Medicine , 55(1): 137–154. doi:10.1353/pbm.2012.0006
- Marcum, James A., 2005, “Biomechanical and Phenomenological Models of the Body, the Meaning of Illness and Quality of Care”, Medicine, Health Care and Philosophy , 7(3): 311–320. doi:10.1007/s11019-004-9033-0
- –––, 2008, “Introduction: A Philosophy of Medicine?”, in his Humanizing Modern Medicine: An Introductory Philosophy of Medicine , (Philosophy and Medicine, 99), New York: Springer, 1–14.
- McClain, Elizabeth K., 2017, “Changes in Osteopathic Medical Education: The Journey Continues”, The Journal of the American Osteopathic Association , 117(4): 208–210. doi:10.7556/jaoa.2017.037
- McClimans, Leah, 2017, “Place of Birth: Ethics and Evidence”, Topoi , 36(3): 531–538. doi:10.1007/s11245-015-9353-0
- –––, forthcoming, “First Person Epidemiological Measures: Vehicles for Patient Centered Care”, Synthese , first online: 28 January 2019. doi:10.1007/s11229-019-02094-z
- McKeown, Thomas, 1976, The Role of Medicine: Dream, Mirage, or Nemesis , London: Nuffield Provincial Hospitals Trust.
- Metzl, Jonathan M. and Anna Kirkland (eds.), 2010, Against Health: How Health Became the New Morality , New York: New York University Press.
- Millum, Joseph and Ezekiel J. Emanuel (eds.), 2012, Global Justice and Bioethics , Oxford: Oxford University Press. doi:10.1093/acprof:osobl/9780195379907.001.0001
- Nunn, Amy, 2009, The Politics and History of AIDS Treatment in Brazil , New York: Springer New York. doi:10.1007/978-0-387-09618-6
- Parsons, Annie and Claire Hooker, 2010, “Dignity and Narrative Medicine”, Journal of Bioethical Inquiry , 7(4): 345–351. doi:10.1007/s11673-010-9254-2
- Pinto, Andrew D. and Ross E.G. Upshur, 2009, “Global Health Ethics for Students”, Developing World Bioethics , 9(1): 1–10. doi:10.1111/j.1471-8847.2007.00209.x
- Rastogi, Sanjeev, 2014, “Ayurvedic Principles of Food and Nutrition: Translating Theory into Evidence-Based Practice”, in Ayurvedic Science of Food and Nutrition , Sanjeev Rastogi (ed.), New York: Springer New York, 3–14. doi:10.1007/978-1-4614-9628-1_1
- Reiss, Julian and Rachel A. Ankeny, 2016, “Philosophy of Medicine”, in The Stanford Encyclopedia of Philosophy (Summer 2016), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/sum2016/entries/medicine/ >
- Rosenberg, Alexander, 2006, Darwinian Reductionism, or, How to Stop Worrying and Love Molecular Biology , Chicago: University of Chicago Press.
- Sidel, Victor W. and Ruth Sidel, 1981, “All Self-Care Is Not Solipsistic, but Selective Citation Surely Is: A Reply to Katz and Levin”, International Journal of Health Services , 11(4): 653–657. doi:10.2190/99DC-W7PD-2VLC-UUNB
- Solomon, Miriam, 2011, “Just a Paradigm: Evidence-Based Medicine in Epistemological Context”, European Journal for Philosophy of Science , 1(3): 451–466. doi:10.1007/s13194-011-0034-6
- –––, 2015, Making Medical Knowledge , New York: Oxford University Press. doi:10.1093/acprof:oso/9780198732617.001.0001
- Stark, Jane Eliza, 2013, “An Historical Perspective on Principles of Osteopathy”, International Journal of Osteopathic Medicine , 16(1): 3–10. doi:10.1016/j.ijosm.2012.10.001
- Stegenga, Jacob, 2011, “Is Meta-Analysis the Platinum Standard of Evidence?”, Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences , 42(4): 497–507. doi:10.1016/j.shpsc.2011.07.003
- –––, 2018, Medical Nihilism , Oxford: Oxford University Press. doi:10.1093/oso/9780198747048.001.0001
- Tonelli, Mark R. and Timothy C. Callahan, 2001, “Why Alternative Medicine Cannot Be Evidence-Based”, Academic Medicine , 76(12): 1213–1220. doi:10.1097/00001888-200112000-00011
- Tonelli, Mark R. and Ross E.G. Upshur, 2019, “A Philosophical Approach to Addressing Uncertainty in Medical Education”, Academic Medicine , 94(4): 507–511. doi:10.1097/ACM.0000000000002512
- Valles, Sean A., 2018, Philosophy of Population Health: Philosophy for a New Public Health Era , Abingdon, Oxon: Routledge.
- Wieten, Sarah, 2018, “Expertise in Evidence-Based Medicine: A Tale of Three Models”, Philosophy, Ethics, and Humanities in Medicine , 13(1): 2. doi:10.1186/s13010-018-0055-2
- Wolfe, Charles T., 2015, “Was Canguilhem a Biochauvinist? Goldstein, Canguilhem and the Project of Biophilosophy”, in Medicine and Society, New Perspectives in Continental Philosophy , Darian Meacham (ed.), (Philosophy and Medicine 120), Dordrecht: Springer Netherlands, 197–212. doi:10.1007/978-94-017-9870-9_12
- Woods, Angela, 2011, “The Limits of Narrative: Provocations for the Medical Humanities”, Medical Humanities , 37(2): 73–78. doi:10.1136/medhum-2011-010045
- Worrall, John, 2007, “Why There’s No Cause to Randomize”, The British Journal for the Philosophy of Science , 58(3): 451–488. doi:10.1093/bjps/axm024
- Yuksekdag, Yusuf, forthcoming, “Individual Responsibilities in Partial Compliance: Skilled Health Worker Emigration from Under-Served Regions”, Public Health Ethics , first online: 31 October 2019. doi:10.1093/phe/phz016
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
- NCI (National Cancer Institute), “ Biomedicine ”, in NCI Dictionary of Cancer Terms , accessed 12 March 2020.
- Institute for Philosophy in Biology and Medicine
- The World of Shakespeare’s Humors
- National Center for Complementary and Integrative Health (NCCIH)
disability: definitions and models | Foucault, Michel | health | justice: international distributive | medicine, philosophy of | Merleau-Ponty, Maurice | phenomenology | physicalism | reduction, scientific: in biology
Acknowledgments
I am indebted to Robyn Bluhm and Maya Goldenberg for their helpful comments on a draft of this work.
Copyright © 2020 by Sean Valles < valles @ msu . edu >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Medical research articles from across Nature Portfolio
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving the application of those already available. It can be viewed as encompassing preclinical research (for example, in cellular systems and animal models) and clinical research (for example, clinical trials).
The efficacy of dapagliflozin in a hierarchical kidney outcome in heart failure
Dapagliflozin improved a hierarchical composite outcome, including death, a worsening kidney disease event, and estimated glomerular filtration rate slope, compared with placebo, in patients with heart failure. This hierarchical outcome — analyzed with win statistics — might provide the statistical power to evaluate the effect of treatments on kidney function in heart failure trials.
Refining neoadjuvant immunotherapy for resectable lung cancer
In an era of expanding perioperative approaches for resectable non–small-cell lung cancer, new data demonstrate that dual neoadjuvant immunotherapy targeting PD-1 and LAG-3 is feasible; future analyses may enhance patient selection by identifying immune signatures predictive of response.
- Misty D. Shields
- Christine M. Lovly
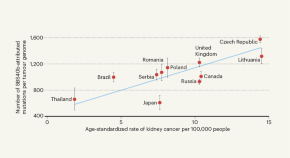
Genomics reveal unknown mutation-promoting agents at global sites
Genetic sequencing of human kidney cancers worldwide has revealed associations between geographical locations and specific mutation patterns, indicating exposure to known and unknown mutation-promoting agents.
- Irene Franco
Related Subjects
- Drug development
- Epidemiology
- Experimental models of disease
- Genetics research
- Outcomes research
- Paediatric research
- Preclinical research
- Stem-cell research
- Clinical trial design
- Translational research
Latest Research and Reviews
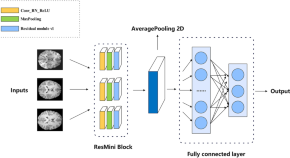
A ResNet mini architecture for brain age prediction
- Si-Yuan Duan
- Xiao-Lei Zhang
Maternal stress and breastfeeding outcomes in the NICU couplet care experience: a prospective cohort study
- Kimberly N. Doughty
- Caitlin Nichols
- Sarah N. Taylor
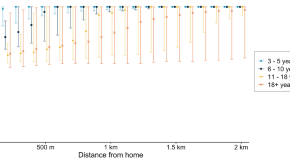
Reanalysis of cluster randomised trial data to account for exposure misclassification using a per-protocol and complier-restricted approach
- Suzanne M. Dufault
- Stephanie K. Tanamas
- Katherine L. Anders
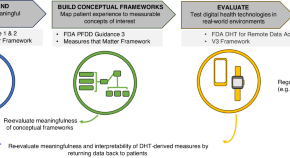
Patient-centricity in digital measure development: co-evolution of best practice and regulatory guidance
- Suvekshya Aryal
- Jennifer M. Blankenship
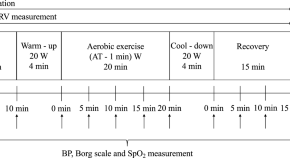
Effect of olfactory stimulation from aromatherapy on the autonomic nervous activity during aerobic exercises
- Katsuki Okada
- Koji Shimatani
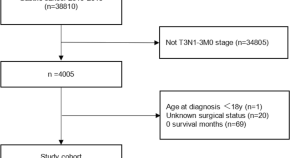
Comparison of different treatment strategies for T3N1-3 stage gastric cancer based on the SEER database
- Shuanghua Liu
News and Comment
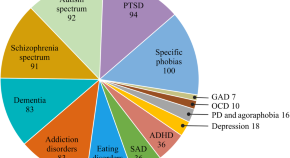
Clinical adoption of virtual reality in mental health is challenged by lack of high-quality research
Virtual reality has been found effective for some mental disorders, while for many others weak methodology prevents conclusive evidence. Similar to other digital technologies, the field has particular demands for conducting clinical research which currently remain poorly addressed. In this commentary, we discuss the unique issues associated with the incorporation of virtual reality in clinical research. In addition, we elaborate on the possibility that these challenges may also be consequences of current funding and publication schemes, and speculate on specific improvement approaches that might be more compatible with the characteristics of clinical virtual reality research.
- Benjamin Selaskowski
- Annika Wiebe
- Niclas Braun

Neglecting sex and gender in research is a public-health risk
The data are clear: taking sex and gender into account in research and using that knowledge to change health care could benefit billions of people.
- Cheryl Carcel
- Robyn Norton
Gut bacteria switch A and B blood types to ‘universal’
Enzymes produced by Akkermansia muciniphila generated group O blood by degrading A and B antigens, which could help solve blood donor shortages in the future.
- Karen O’Leary
Defining responsible use of AI chatbots in social care for older adults
Artificial intelligence (AI) chatbots offer the potential to enhance many aspects of social care for older adults, but also pose ethical risks. This Comment explores the responsible use of AI chatbots, which recognizes the distinct features of social care provision.
- Caroline Emmer De Albuquerque Green

Andrew S. Brierley (1967–2024)
Quantitative field ecologist who contributed to the fundamentals of polar science and pelagic ecology.
- Tom B. Letessier
- Martin J. Cox
- Alex D. Rogers
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
What does a biomedical scientist do?
Would you make a good biomedical scientist? Take our career test and find your match with over 800 careers.
What is a Biomedical Scientist?
Biomedical scientists uses scientific methods to investigate biological processes and diseases that affect humans and animals. They conduct experiments, analyze data, and interpret findings to improve our understanding of diseases and develop new treatments and cures. They also ensure the safety and efficacy of drugs and medical devices through clinical trials and regulatory processes.
The work of biomedical scientists covers a wide range of areas, including genetics, microbiology, immunology, and biochemistry. Various tools and techniques are used to study living organisms at the molecular and cellular levels, such as microscopy, DNA sequencing, and protein analysis. Biomedical scientists often collaborate with other healthcare professionals, such as physicians and nurses, to develop new diagnostics and treatments for diseases.
What does a Biomedical Scientist do?

The work of biomedical scientists has a profound impact on human health and has contributed to the development of numerous life-saving medical advances.
Duties and Responsibilities The duties and responsibilities of a biomedical scientist vary depending on their area of specialization and the specific role they play within their organization. However, some common responsibilities of biomedical scientists include:
- Conducting Research: Biomedical scientists design and conduct experiments to investigate biological processes and diseases. They use various laboratory techniques, including microscopy, DNA sequencing, and protein analysis, to study living organisms at the molecular and cellular levels. They collect and analyze data, interpret findings, and communicate results to other scientists and healthcare professionals.
- Developing New Treatments: Biomedical scientists work to develop new drugs, therapies, and medical devices to treat diseases. They conduct preclinical studies to test the safety and efficacy of new treatments, and they work with clinicians to design and conduct clinical trials to evaluate the effectiveness of new treatments in humans.
- Analyzing Samples: Biomedical scientists analyze biological samples, such as blood, tissue, and urine, to diagnose diseases and monitor treatment. They use laboratory techniques to detect and quantify biomarkers, such as proteins and DNA, that are associated with specific diseases.
- Ensuring Quality Control: Biomedical scientists are responsible for ensuring the quality and accuracy of laboratory tests and procedures. They follow established protocols and standard operating procedures, maintain laboratory equipment, and monitor laboratory safety to ensure compliance with regulatory requirements.
- Managing Laboratory Operations: Biomedical scientists may be responsible for managing laboratory operations, including supervising staff, developing and implementing laboratory policies and procedures, and ensuring that laboratory equipment is properly maintained and calibrated.
- Collaborating with Other Healthcare Professionals: Biomedical scientists collaborate with other healthcare professionals, including physicians, nurses, and pharmacists, to develop and implement treatment plans for patients. They communicate laboratory results and provide expert advice on the interpretation of test results.
- Teaching and Mentoring: Biomedical scientists may be responsible for teaching and mentoring students and junior researchers. They may develop and deliver lectures, supervise laboratory activities, and provide guidance and mentorship to students and trainees.
Types of Biomedical Scientists There are several different types of biomedical scientists, each with their own area of specialization and focus. Here are some examples of different types of biomedical scientists and what they do:
- Microbiologists : Microbiologists study microorganisms, including bacteria, viruses, and fungi. They investigate how these organisms cause disease, develop new treatments to combat infections, and develop new diagnostic tests to identify infectious agents.
- Immunologists : Immunologists study the immune system and its role in fighting disease. They investigate how the immune system responds to infectious agents, cancer cells, and other foreign substances, and they develop new treatments that harness the immune system to fight disease.
- Geneticists : Geneticists study genes and their role in disease. They investigate the genetic basis of diseases, such as cancer, and develop new diagnostic tests and treatments that target specific genetic mutations.
- Biochemists : Biochemists study the chemical processes that occur in living organisms. They investigate how cells and tissues produce and use energy, and they develop new drugs and therapies that target specific metabolic pathways.
- Toxicologists : Toxicologists study the effects of toxic substances on the body. They investigate how chemicals, pollutants, and other environmental factors can cause disease, and they develop strategies to prevent and mitigate the harmful effects of toxic exposures.
- Pharmacologists: Pharmacologists study the effects of drugs on the body. They investigate how drugs interact with cells and tissues, and they develop new drugs and therapies to treat disease.
- Medical Laboratory Scientists: Medical laboratory scientists, also known as clinical laboratory scientists, perform laboratory tests on patient samples to diagnose diseases and monitor treatment. They analyze blood, urine, tissue, and other samples using various laboratory techniques and instruments.
What is the workplace of a Biomedical Scientist like?
Biomedical scientists work in diverse settings, contributing to advancements in medical research, healthcare, and the understanding of diseases. The workplace of a biomedical scientist can vary based on their specific role, specialization, and the nature of their work.
Academic and Research Institutions: Many biomedical scientists are employed in universities, medical schools, and research institutions. In these settings, they conduct cutting-edge research, lead laboratory teams, and contribute to scientific discoveries. Academic biomedical scientists often split their time between conducting research, teaching students, and publishing their findings in scientific journals.
Hospitals and Healthcare Settings: Biomedical scientists play a crucial role in healthcare, especially in clinical laboratories and diagnostic facilities. They may be involved in analyzing patient samples, conducting medical tests, and interpreting results to assist in the diagnosis and treatment of diseases. Biomedical scientists working in hospitals collaborate with clinicians and healthcare professionals to ensure accurate and timely diagnostic information.
Biotechnology and Pharmaceutical Companies: The biotechnology and pharmaceutical industries employ biomedical scientists to drive innovation in drug discovery, development, and testing. In these settings, scientists work on designing experiments, conducting preclinical and clinical trials, and developing new therapeutic interventions. Biomedical scientists may also be involved in quality control, ensuring the safety and efficacy of pharmaceutical products.
Government Agencies and Public Health Organizations: Biomedical scientists can work for government agencies such as the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), or the Food and Drug Administration (FDA). In these roles, they contribute to public health research, policy development, and the regulation of healthcare products.
Nonprofit Research Organizations: Nonprofit organizations dedicated to medical research and public health also employ biomedical scientists. These organizations focus on specific diseases or health issues and work towards finding solutions, advancing knowledge, and advocating for improved healthcare practices.
Private Research Foundations: Biomedical scientists may work for private research foundations that fund and conduct medical research. These foundations often collaborate with academic institutions and industry partners to support innovative research projects with the potential to impact human health.
Collaborative and Interdisciplinary Teams: Biomedical scientists frequently collaborate with professionals from various disciplines, including bioinformaticians, clinicians, engineers, and statisticians. Interdisciplinary collaboration is common, especially in research projects that require a multifaceted approach to address complex health challenges.
Frequently Asked Questions
Biology related careers and degrees.
- Animal Scientist
- Bioinformatics Scientist
- Biomedical Scientist
- Biophysicist
- Biostatistician
- Cellular Biologist
- Comparative Anatomist
- Conservation Biologist
- Developmental Biologist
- Ecology Biologist
- Ecotoxicologist
- Entomologist
- Evolutionary Biologist
- Herpetologist
- Ichthyologist
- Immunologist
- Mammalogist
- Marine Biogeochemist
- Marine Biologist
- Marine Conservationist
- Marine Ecologist
- Marine Fisheries Biologist
- Marine Mammalogist
- Marine Microbiologist
- Microbiologist
- Molecular Biologist
- Neurobiologist
- Ornithologist
- Paleontologist
- Physiologist
- Systems Biologist
- Wildlife Biologist
- Wildlife Ecologist
- Zoo Endocrinologist
Related Degrees
- Animal Sciences
- Biostatistics
- Bioinformatics
- Cellular Biology
- Computational Biology
- Conservation Biology
- Evolutionary Biology
- Marine Biology
- Microbiology
- Molecular Biology
- Neurobiology
Continue reading
Science Related Careers and Degrees
- Anthropologist
- Archaeologist
- Astrophysicist
- Atmospheric Scientist
- Behavioral Scientist
- Biotechnician
- Biotechnologist
- Chemical Technician
- Climate Change Analyst
- Conservation Scientist
- Criminologist
- Cytogenetic Technologist
- Cytotechnologist
- Dairy Scientist
- Engineering Physicist
- Epidemiologist
- Food Science Technologist
- Food Scientist
- Forensic Pathologist
- Forensic Science Technician
- Forensic Scientist
- Geospatial Information Scientist
- Horticulturist
- Hydrologist
- Industrial Ecologist
- Materials Scientist
- Meteorologist
- Natural Sciences Manager
- Neuropsychologist
- Neuroscientist
- Oceanographer
- Particle Physicist
- Pathologist
- Pharmaceutical Scientist
- Political Scientist
- Poultry Scientist
- Social Scientist
- Sociologist
- Soil and Plant Scientist
- Soil and Water Conservationist
- Toxicologist
- Veterinary Pathologist
- Volcanologist
- Biochemistry
- Biomedical Sciences
- Criminology
- Dairy Science
- Environmental Science
- Food Science
- Horticulture
- Political Science
- Poultry Science
- Social Science
- Soil Science
Why We Need Biomedical Research
What is Biomedical Research
Research refers to a category of activities designed to contribute to or develop generalizable knowledge. Generalizable knowledge comprises theories, relationships or principles, or the collection of data on which they are based, that can be verified by accepted scientific techniques of inference and observation. In our current context, “research” includes both behavioral and medical studies relating to human health. When the term “research” is modified by the adjective “biomedical” it refers to health-related research. Advancement in disease prevention and medical care depends on comprehension of epidemiological findings or pathological and physiological processes, and at some stage requires a study involving human beings. The gathering, evaluation, and interpretation of data obtained from studies involving human beings significantly contribute to the betterment of human health.
Generally speaking, Biomedical research is the branch of science that focuses on the prevention and treatment of diseases that cause death and sickness in animals and human beings. This general branch of science includes various aspects of both the physical and life sciences. Biomedical researchers or scientists use biotechnology methods to study diseases and biological processes with the objective of developing effective cures and treatments. The biomedical research process is evolutionary and requires careful experimentation by several researchers including chemists and biologists. The Discovery of new therapies and medicines requires keen scientific experimentation, evaluation, and development.
Everybody in America has enjoyed the benefits of biomedical research. From the creation of new vaccines, drugs, or procedures to treat or prevent illnesses, to the safety testing of items we use in our day-to-day lives, biomedical researchers strive to better comprehend the causes and cure of illnesses. The similarities between laboratory animals and human beings have been crucial in biomedical research since they explain a lot about how human bodies function. The knowledge acquired from experimentation with laboratory animals has been successfully applied not only to humans but also to wildlife, pets, as well as other animals.
According to the WHO , biomedical research has dramatically increased life expectancy in America since its inception. For instance, the life expectancy in America in 1900 averaged 49 years, while this figure had grown to about 69 years by 2004. It is estimated that by 2030, 20% of the United States population will be sixty-five years or older, and the population segment comprising individuals aged 85 years and above could be more than ten million. This increase in the older population is to a great extent due to medical advances resulting from biomedical research.
Biomedical research is at the core of modern healthcare. Biomedical researchers diagnose illnesses and test the effectiveness of a wide range of potential cures by studying fluids and tissue samples from patients. Since its inception, biomedical research has led to several breath-taking biomedical discoveries. Every biomedical discovery is based on knowledge resulting from endless experiments conducted by different generations of biomedical researchers. Biomedical research offers essential information to medical practitioners to allow them to make optimal decisions.
Disease diagnosis involves looking for abnormalities in the structure of tissues. Biomedical researchers use specialized methods in screening cervical smears to look for components like sputum . They also conduct a variety of other types of fluid analysis for the diagnosis of illnesses such as diabetes. The recent advancement in biomedical research has mainly been due to the use of specialized cutting-edge technology used in various biomedical institutions and clinics.
About April Scott
View all posts by April Scott | Website
Related Posts
Other steps for biomedical research improvement, improving the biomedical research.

- Notices of Funding Opportunities
- NIH RePORTER
- Co-Funding Activities
- BSSR Clinical Trials
- Reports and Publications
- OBSSR-Supported Training
- Online Training Resources
- OBSSR Connector Monthly Newsletter
- The Director’s Voice Blog
- Research Spotlights
- BSSR News and Announcements
- All Upcoming Events and Meetings
- NIH Behavioral and Social Sciences Research Festival
- NIH Matilda White Riley Behavioral and Social Sciences Honors
- OBSSR Director’s Webinar Series
- Mission and History
- BSSR Definition
- BSSR Accomplishments
- Strategic Plan
- NIH Behavioral and Social Sciences Research Coordinating Committee (BSSR-CC)
- Coordination of NIH-wide Initiatives
- Staff Directory

- News and Events
Advancing Women’s Health Research and Innovation: A Conversation with Janine Clayton, Director of the NIH Office of Research on Women’s Health (ORWH)
In March 2024, President Joe Biden signed an Executive Order directing the most comprehensive set of executive actions ever to expand and improve women’s health. Its actions prioritize the integration of women’s health throughout the federal research portfolio and budget, galvanizing new research initiatives on various topics, including menopause and women’s midlife health.
The order will create a Fund for Women’s Health Research at the National Institutes of Health (NIH) to drive a cutting-edge, interdisciplinary research agenda and establish a nationwide network of research centers of excellence and innovation.
The Office of Behavioral and Social Sciences Research (OBSSR) has a history of supporting research initiatives addressing issues such as health disparities, opportunities for women in scientific fields, improvements in research inclusivity, and policy matters affecting women. We look forward to building on our collaborations with the Office of Research on Women’s Health (ORWH) to accelerate research to prevent, diagnose, and treat conditions that uniquely or disproportionately impact women.
I recently had the opportunity to connect with the Director of ORWH, Janine Clayton, M.D., FARVO about the importance of behavioral and social sciences research to women’s health and how the Executive Order can help address how we will work together to close gaps and accelerate the advancement of women's health research.
What are some of the major challenges in women’s health research that the Executive Order aims to address?
The Executive Order focuses on five key areas to support women’s health. These include prioritizing and enhancing investments; fostering innovation and discovery; expanding and leveraging data collection and analysis; strengthening coordination, infrastructure, and training; and improving women’s health across the lifespan.
By increasing funding and focusing on women’s health across the lifespan, there is an opportunity to better understand the diseases and conditions associated with women’s midlife and later years.
What role does behavioral and social science research play in improving health outcomes for women?
Much of the research at the NIH focuses on the biological perspectives that influence women’s health at the genetic, molecular, and physiological levels. However, it is imperative to understand how these internal factors interact with the external ones, such as social constructions of gender and sexuality and social determinants of health. These interactions have broad implications for women’s health outcomes.
Research has shown that lifestyle behaviors, such as healthy eating and regular physical activity can prevent conditions like heart attack and stroke. Simply recommending that women eat whole foods and exercise regularly does not consider how the social world influences individual behaviors. For example, some women may not have access to healthy foods because they live in an area without a grocery store. They may have physical disabilities that limit their ability to exercise or live in a neighborhood where it is not safe to be outside due to violence or air pollution.
Continued and expanded support for behavioral and social science research can enhance our understanding of the factors that influence women’s health. This support can help develop effective interventions that address health disparities and advance equity for women.
How is women’s health research linked to health equity?
To conduct equity-focused health research for women, we need to take an intersectional approach. This means looking at the differences among groups and understanding how various social factors influence women’s health outcomes.
Recognizing that women's experiences vary, it's important to tailor strategies for improving women’s health to account for these differences. For example, cultural factors significantly influence women's perceptions of menopause and affect their acceptance of biomedical approaches during this transition. Additionally, racial and ethnic minoritized groups may face obstacles in accessing proper care and can have challenges in finding menopause-related information and support.
To support equity-focused research, ORWH recently coordinated the release of a Notice of Special Interest on women’s health that highlights the NIH’s interest in receiving research applications focused on diseases and health conditions that predominantly affect women.
The Executive Order aims to improve the recruitment, enrollment, and retention of women in clinical trials. How do behavioral or social science play a role in achieving this goal?
As the focal point for women's health research, ORWH strives to ensure that women from understudied, underrepresented, and underreported (U3) populations are included in biomedical research to reduce health disparities. Behavioral and social science can shed light on the various obstacles that affect women’s willingness and ability to participate in clinical trials, such as caregiving responsibilities, work obligations, or concerns about discomfort.
For instance, historically there has been insufficient representation of African American women in clinical research. The Executive Order aims to reduce these gaps in representation by prioritizing the inclusion of women from all racial, ethnic, and socioeconomic backgrounds in clinical research.
OBSSR and ORWH promote implementation science to enhance the impact of research findings. Are there specific areas within women's health where implementation science is particularly important?
Implementation science is critical for ensuring the retention of women in clinical trials. The Executive Order recommends using technological and data science advances to reduce women’s barriers to access. Implementation science can investigate the multiple contextual factors that could impact women’s uptake of these advances.
Integrating user-centered design and community-based participatory research approaches in the development of innovative technological and data science strategies can increase the successful implementation of these tools and potentially increase women’s participation in clinical trials.
31 Center Drive, Building 31, Room B1C19 Bethesda, MD 20892
Email: [email protected]
Phone: 301-402-1146
NIH Virtual Tour
- Open access
- Published: 13 May 2024
SCIPAC: quantitative estimation of cell-phenotype associations
- Dailin Gan 1 ,
- Yini Zhu 2 ,
- Xin Lu 2 , 3 &
- Jun Li ORCID: orcid.org/0000-0003-4353-5761 1
Genome Biology volume 25 , Article number: 119 ( 2024 ) Cite this article
175 Accesses
2 Altmetric
Metrics details
Numerous algorithms have been proposed to identify cell types in single-cell RNA sequencing data, yet a fundamental problem remains: determining associations between cells and phenotypes such as cancer. We develop SCIPAC, the first algorithm that quantitatively estimates the association between each cell in single-cell data and a phenotype. SCIPAC also provides a p -value for each association and applies to data with virtually any type of phenotype. We demonstrate SCIPAC’s accuracy in simulated data. On four real cancerous or noncancerous datasets, insights from SCIPAC help interpret the data and generate new hypotheses. SCIPAC requires minimum tuning and is computationally very fast.
Single-cell RNA sequencing (scRNA-seq) technologies are revolutionizing biomedical research by providing comprehensive characterizations of diverse cell populations in heterogeneous tissues [ 1 , 2 ]. Unlike bulk RNA sequencing (RNA-seq), which measures the average expression profile of the whole tissue, scRNA-seq gives the expression profiles of thousands of individual cells in the tissue [ 3 , 4 , 5 , 6 , 7 ]. Based on this rich data, cell types may be discovered/determined in an unsupervised (e.g., [ 8 , 9 ]), semi-supervised (e.g., [ 10 , 11 , 12 , 13 ]), or supervised manner (e.g., [ 14 , 15 , 16 ]). Despite the fast development, there are still limitations with scRNA-seq technologies. Notably, the cost for each scRNA-seq experiment is still high; as a result, most scRNA-seq data are from a single or a few biological samples/tissues. Very few datasets consist of large numbers of samples with different phenotypes, e.g., cancer vs. normal. This places great difficulties in determining how a cell type contributes to a phenotype based on single-cell studies (especially if the cell type is discovered in a completely unsupervised manner or if people have limited knowledge of this cell type). For example, without having single-cell data from multiple cancer patients and multiple normal controls, it could be hard to computationally infer whether a cell type may promote or inhibit cancer development. However, such association can be critical for cancer research [ 17 ], disease diagnosis [ 18 ], cell-type targeted therapy development [ 19 ], etc.
Fortunately, this difficulty may be overcome by borrowing information from bulk RNA-seq data. Over the past decade, a considerable amount of bulk RNA-seq data from a large number of samples with different phenotypes have been accumulated and made available through databases like The Cancer Genome Atlas (TCGA) [ 20 ] and cBioPortal [ 21 , 22 ]. Data in these databases often contain comprehensive patient phenotype information, such as cancer status, cancer stages, survival status and time, and tumor metastasis. Combining single-cell data from a single or a few individuals and bulk data from a relatively large number of individuals regarding a particular phenotype can be a cost-effective way to determine how a cell type contributes to the phenotype. A recent method Scissor [ 23 ] took an essential step in this direction. It uses single-cell and bulk RNA-seq data with phenotype information to classify the cells into three discrete categories: Scissor+, Scissor−, and null cells, corresponding to cells that are positively associated, negatively associated, and not associated with the phenotype.
Here, we present a method that takes another big step in this direction, which is called Single-Cell and bulk data-based Identifier for Phenotype Associated Cells or SCIPAC for short. SCIPAC enables quantitative estimation of the strength of association between each cell in a scRNA-seq data and a phenotype, with the help of bulk RNA-seq data with phenotype information. Moreover, SCIPAC also enables the estimation of the statistical significance of the association. That is, it gives a p -value for the association between each cell and the phenotype. Furthermore, SCIPAC enables the estimation of association between cells and an ordinal phenotype (e.g., different stages of cancer), which could be informative as people may not only be interested in the emergence/existence of cancer (cancer vs. healthy, a binary problem) but also in the progression of cancer (different stages of cancer, an ordinal problem).
To study the performance of SCIPAC, we first apply SCIPAC to simulated data under three schemes. SCIPAC shows high accuracy with low false positive rates. We further show the broad applicability of SCIPAC on real datasets across various diseases, including prostate cancer, breast cancer, lung cancer, and muscular dystrophy. The association inferred by SCIPAC is highly informative. In real datasets, some cell types have definite and well-studied functions, while others are less well-understood: their functions may be disease-dependent or tissue-dependent, and they may contain different sub-types with distinct functions. In the former case, SCIPAC’s results agree with current biological knowledge. In the latter case, SCIPAC’s discoveries inspire the generation of new hypotheses regarding the roles and functions of cells under different conditions.
An overview of the SCIPAC algorithm
SCIPAC is a computational method that identifies cells in single-cell data that are associated with a given phenotype. This phenotype can be binary (e.g., cancer vs. normal), ordinal (e.g., cancer stage), continuous (e.g., quantitative traits), or survival (i.e., survival time and status). SCIPAC uses input data consisting of three parts: single-cell RNA-seq data that measures the expression of p genes in m cells, bulk RNA-seq data that measures the expression of the same set of p genes in n samples/tissues, and the statuses/values of the phenotype of the n bulk samples/tissues. The output of SCIPAC is the strength and the p -value of the association between each cell and the phenotype.
SCIPAC proposes the following definition of “association” between a cell and a phenotype: A group of cells that are likely to play a similar role in the phenotype (such as cells of a specific cell type or sub-type, cells in a particular state, cells in a cluster, cells with similar expression profiles, or cells with similar functions) is considered to be positively/negatively associated with a phenotype if an increase in their proportion within the tissue likely indicates an increased/decreased probability of the phenotype’s presence. SCIPAC assigns the same association to all cells within such a group. Taking cancer as the phenotype as an example, if increasing the proportion of a cell type indicates a higher chance of having cancer (binary), having a higher cancer stage (ordinal), or a higher hazard rate (survival), all cells in this cell type is positively associated with cancer.
The algorithm of SCIPAC follows the following four steps. First, the cells in the single-cell data are grouped into clusters according to their expression profiles. The Louvain algorithm from the Seurat package [ 24 , 25 ] is used as the default clustering algorithm, but the user may choose any clustering algorithm they prefer. Or if information of the cell types or other groupings of cells is available a priori, it may be supplied to SCIPAC as the cell clusters, and this clustering step can be skipped. In the second step, a regression model is learned from bulk gene expression data with the phenotype. Depending on the type of the phenotype, this model can be logistic regression, ordinary linear regression, proportional odds model, or Cox proportional hazards model. To achieve a higher prediction power with less variance, by default, the elastic net (a blender of Lasso and ridge regression [ 26 ]) is used to fit the model. In the third step, SCIPAC computes the association strength \(\Lambda\) between each cell cluster and the phenotype based on a mathematical formula that we derive. Finally, the p -values are computed. The association strength and its p -value between a cell cluster and the phenotype are given to all cells in the cluster.
SCIPAC requires minimum tuning. When the cell-type information is given in step 1, SCIPAC does not have any (hyper)parameter. Otherwise, the Louvain algorithm used in step 1 has a “resolution” parameter that controls the number of cell clusters: a larger resolution results in more clusters. SCIPAC inherits this parameter as its only parameter. Since SCIPAC gives the same association strength and p -value to cells from the same cluster, this parameter also determines the resolution of results provided by SCIPAC. Thus, we still call it “resolution” in SCIPAC. Because of its meaning, we recommend setting it so that the number of cell clusters given by the clustering algorithm is comparable to, or reasonably larger than, the number of cell types (or sub-types) in the data. We will see that the performance of SCIPAC is insensitive to this resolution parameter, and the default value 2.0 typically works well.
The details of the SCIPAC algorithm are given in the “ Methods ” section.
Performance in simulated data
We assess the performance of SCIPAC in simulated data under three different schemes. The first scheme is simple and consists of only three cell types. The second scheme is more complicated and consists of seven cell types, which better imitates actual scRNA-seq data. In the third scheme, we simulate cells under different cell development stages to test the performance of SCIPAC under an ordinal phenotype. Details of the simulation are given in Additional file 1.
Simulation scheme I
Under this scheme, the single-cell data consists of three cell types: one is positively associated with the phenotype, one is negatively associated, and the third is not associated (we call it “null”). Figure 1 a gives the UMAP [ 27 ] plot of the three cell types, and Fig. 1 b gives the true associations of these three cell types with the phenotype, with red, blue, and light gray denoting positive, negative, and null associations.
UMAP visualization and numeric measures of the simulated data under scheme I. All the plots in a–e are scatterplots of the two dimensional single-cell data given by UMAP. The x and y axes represent the two dimensions, and their scales are not shown as their specific values are not directly relevant. Points in the plots represents single cells, and they are colored differently in each subplot to reflect different information/results. a Cell types. b True associations. The association between cell types 1, 2, and 3 and the phenotype are positive, negative, and null, respectively. c Association strengths \(\Lambda\) given by SCIPAC under different resolutions. Red/blue represents the sign of \(\Lambda\) , and the shade gives the absolute value of \(\Lambda\) . Every cell is colored red or blue since no \(\Lambda\) is exactly zero. Below each subplot, Res stands for resolution, and K stands for the number of cell clusters given by this resolution. d p -values given by SCIPAC. Only cells with p -value \(< 0.05\) are colored red (positive association) or blue (negative association); others are colored white. e Results given by Scissor under different \(\alpha\) values. Red, blue, and light gray stands for Scissor+, Scissor−, and background (i.e., null) cells. f F1 scores and g FSC for SCIPAC and Scissor under different parameter values. For SCIPAC, each bar is the value under a resolution/number of clusters. For Scissor, each bar is the value under an \(\alpha\)
We apply SCIPAC to the simulated data. For the resolution parameter (see the “ Methods ” section), values 0.5, 1.0, and 1.5 give 3, 4, and 4 clusters, respectively, close to the actual number of cell types. They are good choices based on the guidance for choosing this parameter. To show how SCIPAC behaves under parameter misspecification, we also set the resolution up to 4.0, which gives a whopping 61 clusters. Figure 1 c and d give the association strengths \(\Lambda\) and the p -values given by four different resolutions (results under other resolutions are provided in Additional file 1: Fig. S1 and S2). In Fig. 1 c, red and blue denote positive and negative associations, respectively, and the shade of the color represents the strength of the association, i.e., the absolute value of \(\Lambda\) . Every cell is colored blue or red, as none of \(\Lambda\) is exactly zero. In Fig. 1 d, red and blue denote positive and negative associations that are statistically significant ( p -value \(< 0.05\) ). Cells whose associations are not statistically significant ( p -value \(\ge 0.05\) ) are shown in white. To avoid confusion, it is worth repeating that cells that are colored in red/blue in Fig. 1 c are shown in red/blue in Fig. 1 d only if they are statistically significant; otherwise, they are colored white in Fig. 1 d.
From Fig. 1 c, d (as well as Additional file 1: Fig. S1 and S2), it is clear that the results of SCIPAC are highly consistent under different resolution values, including both the estimated association strengths and the p -values. It is also clear that SCIPAC is highly accurate: most truly associated cells are identified as significant, and most, if not all, truly null cells are identified as null.
As the first algorithm that quantitatively estimates the association strength and the first algorithm that gives the p -value of the association, SCIPAC does not have a real competitor. A previous algorithm, Scissor, is able to classify cells into three discrete categories according to their associations with the phenotype. So, we compare SCIPAC with Scissor in respect of the ability to differentiate positively associated, negatively associated, and null cells.
Running Scissor requires tuning a parameter called \(\alpha\) , which is a number between 0 and 1 that balances the amount of regularization for the smoothness and for the sparsity of the associations. The Scissor R package does not provide a default value for this \(\alpha\) or a function to help select this value. The Scissor paper suggests the following criterion: “the number of Scissor-selected cells should not exceed a certain percentage of total cells (default 20%) in the single-cell data. In each experiment, a search on the above searching list is performed from the smallest to the largest until a value of \(\alpha\) meets the above criteria.” In practice, we have found that this criterion does not often work properly, as the truly associated cells may not compose 20% of all cells in actual data. Therefore, instead of setting \(\alpha\) to any particular value, we set \(\alpha\) values that span the whole range of \(\alpha\) to see the best possible performance of Scissor.
The performance of Scissor in our simulation data under four different \(\alpha\) values are shown in Fig. 1 e, and results under more \(\alpha\) values are shown in Additional file 1: Fig. S3. In the figures, red, blue, and light gray denote Scissor+, Scissor−, and null (called “background” in Scissor) cells, respectively. The results of Scissor have several characteristics different from SCIPAC. First, Scissor does not give the strength or statistical significance of the association, and thus the colors of the cells in the figures do not have different shades. Second, different \(\alpha\) values give very different results. Greater \(\alpha\) values generally give fewer Scissor+ and Scissor− cells, but there are additional complexities. One complexity is that the Scissor+ (or Scissor−) cells under a greater \(\alpha\) value are not a strict subset of Scissor+ (or Scissor−) cells under a smaller \(\alpha\) value. For example, the number of truly negatively associated cells detected as Scissor− increases when \(\alpha\) increases from 0.01 to 0.30. Another complexity is that the direction of the association may flip as \(\alpha\) increases. For example, most cells of cell type 2 are identified as Scissor+ under \(\alpha =0.01\) , but many of them are identified as Scissor− under larger \(\alpha\) values. Third, Scissor does not achieve high power and low false-positive rate at the same time under any \(\alpha\) . No matter what the \(\alpha\) value is, there is only a small proportion of cells from cell type 2 that are correctly identified as negatively associated, and there is always a non-negligible proportion of null cells (i.e., cells from cell type 3) that are incorrectly identified as positively or negatively associated. Fourth, Scissor+ and Scissor− cells can be close to each other in the figure, even under a large \(\alpha\) value. This means that cells with nearly identical expression profiles are detected to be associated with the phenotype in opposite directions, which can place difficulties in interpreting the results.
SCIPAC overcomes the difficulties of Scissor and gives results that are more informative (quantitative strengths with p -values), more accurate (both high power and low false-positive rate), less sensitive to the tuning parameter, and easier to interpret (cells with similar expression typically have similar associations to the phenotype).
SCIPAC’s higher accuracy in differentiating positively associated, negatively associated, and null cells than Scissors can also be measured numerically using the F1 score and the fraction of sign correctness (FSC). F1, which is the harmonic mean of precision and recall, is a commonly used measure of calling accuracy. Note that precision and recall are only defined for two-class problems, which try to classify desired signals/discoveries (so-called “positives”) against noises/trivial results (so-called “negatives”). Our case, on the other hand, is a three-class problem: positive association, negative association, and null. To compute F1, we combine the positive and negative associations and treat them as “positives,” and treat null as “negatives.” This F1 score ignores the direction of the association; thus, it alone is not enough to describe the performance of an association-detection algorithm. For example, an algorithm may have a perfect F1 score even if it incorrectly calls all negative associations positive. To measure an algorithm’s ability to determine the direction of the association, we propose a statistic called FSC, defined as the fraction of true discoveries that also have the correct direction of the association. The F1 score and FSC are numbers between 0 and 1, and higher values are preferred. A mathematical definition of these two measures is given in Additional file 1.
Figure 1 f, g show the F1 score and FSC of SCIPAC and Scissor under different values of tuning parameters. The F1 score of Scissor is between 0.2 and 0.7 under different \(\alpha\) ’s. The FSC of Scissor increases from around 0.5 to nearly 1 as \(\alpha\) increases, but Scissor does not achieve high F1 and FSC scores at the same time under any \(\alpha\) . On the other hand, the F1 score of SCIPAC is close to perfection when the resolution parameter is properly set, and it is still above 0.90 even if the resolution parameter is set too large. The FSC of SCIPAC is always above 0.96 under different resolutions. That is, SCIPAC achieves high F1 and FSC scores simultaneously under a wide range of resolutions, representing a much higher accuracy than Scissor.
Simulation scheme II
This more complicated simulation scheme has seven cell types, which are shown in Fig. 2 a. As shown in Fig. 2 b, cell types 1 and 3 are negatively associated (colored blue), 2 and 4 are positively associated (colored red), and 5, 6, and 7 are not associated (colored light gray).
UMAP visualization of the simulated data under a–g scheme II and h–k scheme III. a Cell types. b True associations. c , d Association strengths \(\Lambda\) and p -values given by SCIPAC under the default resolution. e Results given by Scissor under different \(\alpha\) values. f F1 scores and g FSC for SCIPAC and Scissor under different parameter values. h Cell differentiation paths. The four paths have the same starting location, which is in the center, but different ending locations. This can be considered as a progenitor cell type differentiating into four specialized cell types. i Cell differentiation steps. These steps are used to create four stages, each containing 500 steps. Thus, this plot of differentiation steps can also be viewed as the plot of true association strengths. j , k Association strengths \(\Lambda\) and p -values given by SCIPAC under the default resolution
The association strengths and p -values given by SCIPAC under the default resolution are illustrated in Fig. 2 c, d, respectively. Results under several other resolutions are given in Additional file 1: Fig. S4 and S5. Again, we find that SCIPAC gives highly consistent results under different resolutions. SCIPAC successfully identifies three out of the four truly associated cell types. For the other truly associated cell type, cell type 1, SCIPAC correctly recognizes its association with the phenotype as negative, although the p -values are not significant enough. The F1 score is 0.85, and the FSC is greater than 0.99, as shown in Fig. 2 f, g.
The results of Scissor under four different \(\alpha\) values are given in Fig. 2 e. (More shown in Additional file 1: Fig. S6.) Under this highly challenging simulation scheme, Scissor can only identify one out of four truly associated cell types. Its F1 score is below 0.4.
Simulation scheme III
This simulation scheme is to assess the performance of SCIPAC for ordinal phenotypes. We simulate cells along four cell-differentiation paths with the same starting location but different ending locations, as shown in Fig. 2 h. These cells can be considered as a progenitor cell population differentiating into four specialized cell types. In Fig. 2 i, the “step” reflects their position in the differentiation path, with step 0 meaning the start and step 2000 meaning the end of the differentiation. Then, the “stage” is generated according to the step: cells in steps 0 \(\sim\) 500, 501 \(\sim\) 1000, 1001 \(\sim\) 1500, and 1501 \(\sim\) 2000 are assigned to stages I, II, III, and IV, respectively. This stage is treated as the ordinal phenotype. Under this simulation scheme, Fig. 2 i also gives the actual associations, and all cells are associated with the phenotype.
The results of SCIPAC under the default resolution are shown in Fig. 2 j, k. Clearly, the associations SCIPAC identifies are highly consistent with the truth. Particularly, it successfully identifies the cells in the center as early-stage cells and most cells at the end of branches as last-stage cells. The results of SCIPAC under other resolutions are given in Additional file 1: Fig. S7 and S8, which are highly consistent. Scissor does not work with ordinal phenotypes; thus, no results are reported here.
Performance in real data
We consider four real datasets: a prostate cancer dataset, a breast cancer dataset, a lung cancer dataset, and a muscular dystrophy dataset. The bulk RNA-seq data of the three cancer datasets are obtained from the TCGA database, and that of the muscular dystrophy dataset is obtained from a published paper [ 28 ]. A detailed description of these datasets is given in Additional file 1. We will use these datasets to assess the performance of SCIPAC on different types of phenotypes. The cell type information (i.e., which cell belongs to which cell type) is available for the first three datasets, but we ignore this information so that we can make a fair comparison with Scissor, which cannot utilize this information.
Prostate cancer data with a binary phenotype
We use the single-cell expression of 8,700 cells from prostate-cancer tumors sequenced by [ 29 ]. The cell types of these cells are known and given in Fig. 3 a. The bulk data comprises 550 TCGA-PRAD (prostate adenocarcinoma) samples with phenotype (cancer vs. normal) information. Here the phenotype is cancer, and it is binary: present or absent.
UMAP visualization of the prostate cancer data, with a zoom-in view for the red-circled region (cell type MNP). a True cell types. BE, HE, and CE stand for basal, hillock, club epithelial cells, LE-KLK3 and LE-KLK4 stand for luminal epithelial cells with high levels of kallikrein related peptidase 3 and 4, and MNP stands for mononuclear phagocytes. In the zoom-in view, the sub-types of MNP cells are given. b Association strengths \(\Lambda\) given by SCIPAC under the default resolution. The cyan-circled cells are B cells, which are estimated by SCIPAC as negatively associated with cancer but estimated by Scissor as Scissor+ or null. c p -values given by SCIPAC. The MNP cell type, which is red-circled in the plot, is estimated by SCIPAC to be strongly negatively associated with cancer but estimated by Scissor to be positively associated with cancer. d Results given by Scissor under different \(\alpha\) values
Results from SCIPAC with the default resolution are shown in Fig. 3 b, c (results with other resolutions, given in Additional file 1: Fig. S9 and S10, are highly consistent with results here.) Compared with results from Scissor, shown in Fig. 3 d, results from SCIPAC again show three advantages. First, results from SCIPAC are richer and more comprehensive. SCIPAC gives estimated associations and the corresponding p -values, and the estimated associations are quantitative (shown in Fig. 3 b as different shades to the red or blue color) instead of discrete (shown in Fig. 3 d as a uniform shade to the red, blue, or light gray color). Second, SCIPAC’s results can be easier to interpret as the red and blue colors are more block-wise instead of scattered. Third, unlike Scissor, which produces multiple sets of results varying based on the parameter \(\alpha\) —a parameter without a default value or tuning guidance—typically, a single set of results from SCIPAC under its default settings suffices.
Comparing the results from our SCIPAC method with those from Scissor is non-trivial, as the latter’s outcomes are scattered and include multiple sets. We propose the following solutions to summarize the inferred association of a known cell type with the phenotype using a specific method (Scissor under a specific \(\alpha\) value, or SCIPAC with the default setting). We first calculate the proportion of cells in this cell type identified as Scissor+ (by Scissor at a specific \(\alpha\) value) or as significantly positively associated (by SCIPAC), denoted by \(p_{+}\) . We also calculate the proportion of all cells, encompassing any cell type, which are identified as Scissor+ or significantly positively associated, serving as the average background strength, denoted by \(p_{a}\) . Then, we compute the log odds ratio for this cell type to be positively associated with the phenotype compared to the background, represented as:
Similarly, the log odds ratio for the cell type to be negatively associated with the phenotype, \(\rho _-\) , is computed in a parallel manner.
For SCIPAC, a cell type is summarized as positively associated with the phenotype if \(\rho _+ \ge 1\) and \(\rho _- < 1\) and negatively associated if \(\rho _- \ge 1\) and \(\rho _+ < 1\) . If neither condition is met, the association is inconclusive. For Scissor, we apply it under six different \(\alpha\) values: 0.01, 0.05, 0.10, 0.15, 0.20, and 0.25. A cell type is summarized as positively associated with the phenotype if \(\rho _+ \ge 1\) and \(\rho _- < 1\) in at least four of these \(\alpha\) values and negatively associated if \(\rho _- \ge 1\) and \(\rho _+ < 1\) in at least four \(\alpha\) values. If these criteria are not met, the association is deemed inconclusive. The above computation of log odds ratios and the determination of associations are performed only on cell types that each compose at least 1% of the cell population, to ensure adequate power.
For the prostate cancer data, the log odds ratios for each cell type using each method are presented in Tables S1 and S2. The final associations determined for each cell type are summarized in Table S3. In the last column of this table, we also indicate whether the conclusions drawn from SCIPAC and Scissor are consistent or not.
We find that SCIPAC’s results agree with Scissor on most cell types. However, there are three exceptions: mononuclear phagocytes (MNPs), B cells, and LE-KLK4.
MNPs are red-circled and zoomed in in each sub-figure of Fig. 3 . Most cells in this cell type are colored red in Fig. 3 d but colored dark blue in Fig. 3 b. In other words, while Scissor determines that this cell type is Scissor+, SCIPAC makes the opposite inference. Moreover, SCIPAC is confident about its judgment by giving small p -values, as shown in Fig. 3 c. To see which inference is closer to the biological fact is not easy, as biologically MNPs contain a number of sub-types that each have different functions [ 30 , 31 ]. Fortunately, this cell population has been studied in detail in the original paper that generated this dataset [ 29 ], and the sub-type information of each cell is provided there: this MNP population contains six sub-types, which are dendritic cells (DC), M1 macrophages (Mac1), metallothionein-expressing macrophages (Mac-MT), M2 macrophages (Mac2), proliferating macrophages (Mac-cycling), and monocytes (Mono), as shown in the zoom-in view of Fig. 3 a. Among these six sub-types, DC, Mac1, and Mac-MT are believed to inhibit cancer development and can serve as targets in cancer immunotherapy [ 29 ]; they compose more than 60% of all MNP cells in this dataset. SCIPAC makes the correct inference on this majority of MNP cells. Another cell type, Mac2, is reported to promote tumor development [ 32 ], but it only composes less than \(15\%\) of the MNPs. How the other two cell types, Mac-cycling and Mono, are associated with cancer is less studied. Overall, the results given by SCIPAC are more consistent with the current biological knowledge.
B cells are cyan-circled in Fig. 3 b. B cells are generally believed to have anti-tumor activity by producing tumor-reactive antibodies and forming tertiary lymphoid structures [ 29 , 33 ]. This means that B cells are likely to be negatively associated with cancer. SCIPAC successfully identifies this negative association, while Scissor fails.
LE-KLK4, a subtype of cancer cells, is thought to be positively associated with the tumor phenotype [ 29 ]. SCIPAC successfully identified this positive association, in contrast to Scissor, which failed to do so (in the figure, a proportion of LE-KLK4 cells are identified as Scissor+, especially under the smallest \(\alpha\) value; however, this proportion is not significantly higher than the background Scissor+ level under the majority of \(\alpha\) values).
In summary, across all three cell types, the results from SCIPAC appear to be more consistent with current biological knowledge. For more discussions regarding this dataset, refer to Additional file 1.
Breast cancer data with an ordinal phenotype
The scRNA-seq data for breast cancer are from [ 34 ], and we use the 19,311 cells from the five HER2+ tumor tissues. The true cell types are shown in Fig. 4 a. The bulk data include 1215 TCGA-BRCA samples with information on the cancer stage (I, II, III, or IV), which is treated as an ordinal phenotype.
UMAP visualization of the breast cancer data. a True cell types. CAFs stand for cancer-associated fibroblasts, PB stands for plasmablasts and PVL stands for perivascular-like cells. b , c Association strengths \(\Lambda\) and p -values given by SCIPAC under the default resolution. Cyan-circled are a group of T cells that are estimated by SCIPAC to be most significantly associated with the cancer stage in the negative direction, and orange-circled are a group of T cells that are estimated by SCIPAC to be significantly positively associated with the cancer stage. d DE analysis of the cyan-circled T cells vs. all the other T cells. e DE analysis of the cyan-circled T cells vs. all the other cells. f Expression of CD8+ T cell marker genes in the cyan-circled cells and all the other cells. g DE analysis of the orange-circled T cells vs. all the other cells. h Expression of regulatory T cell marker genes in the orange-circled cells and all the other cells
Association strengths and p -values given by SCIPAC under the default resolution are shown in Fig. 4 b, c. Results under other resolutions are given in Additional file 1: Fig. S11 and S12, and again they are highly consistent with results under the default resolution. We do not present the results from Scissor, as Scissor does not take ordinal phenotypes.
In the SCIPAC results, cells that are most strongly and statistically significantly associated with the phenotype in the positive direction are the cancer-associated fibroblasts (CAFs). This finding agrees with the literature: CAFs contribute to therapy resistance and metastasis of cancer cells via the production of secreted factors and direct interaction with cancer cells [ 35 ], and they are also active players in breast cancer initiation and progression [ 36 , 37 , 38 , 39 ]. Another large group of cells identified as positively associated with the phenotype is the cancer epithelial cells. They are malignant cells in breast cancer tissues and are thus expected to be associated with severe cancer stages.
Of the cells identified as negatively associated with severe cancer stages, a large portion of T cells is the most noticeable. Biologically, T cells contain many sub-types, including CD4+, CD8+, regulatory T cells, and more, and their functions are diverse in the tumor microenvironment [ 40 ]. To explore SCIPAC’s discoveries, we compare T cells that are identified as most statistically significant, with p -values \(< 10^{-6}\) and circled in Fig. 4 d, with the other T cells. Differential expression (DE) analysis (details about DE analysis and other analyses are given in Additional file 1) identifies seven genes upregulated in these most significant T cells. Of these seven genes, at least five are supported by the literature: CCL4, XCL1, IFNG, and GZMB are associated with CD8+ T cell infiltration; they have been shown to have anti-tumor functions and are involved in cancer immunotherapy [ 41 , 42 , 43 ]. Also, IL2 has been shown to serve an important role in combination therapies for autoimmunity and cancer [ 44 ]. We also perform an enrichment analysis [ 45 ], in which a pathway called Myc stands out with a \(\textit{p}\text{-value}<10^{-7}\) , much smaller than all other pathways. Myc is downregulated in the T cells that are identified as most negatively associated with cancer stage progress. This agrees with current biological knowledge about this pathway: Myc is known to contribute to malignant cell transformation and tumor metastasis [ 46 , 47 , 48 ].
On the above, we have compared T cells that are most significantly associated with cancer stages in the negative direction with the other T cells using DE and pathway analysis, and the results could suggest that these cells are tumor-infiltrated CD8+ T cells with tumor-inhibition functions. To check this hypothesis, we perform DE analysis of these cells against all other cells (i.e., the other T cells and all the other cell types). The DE genes are shown in Fig. 4 e. It can be noted that CD8+ T cell marker genes such as CD8A, CD8B, and GZMK are upregulated. We further obtain CD8+ T cell marker genes from CellMarker [ 49 ] and check their expression, as illustrated in Fig. 4 f. Marker genes CD8A, CD8B, CD3D, GZMK, and CD7 show significantly higher expression in these T cells. This again supports our hypothesis that these cells are tumor-infiltrated CD8+ T cells that have anti-tumor functions.
Interestingly, not all T cells are identified as negatively associated with severe cancer stages; a group of T cells is identified as positively associated, as circled in Fig. 4 c. To explore the function of this group of T cells, we perform DE analysis of these T cells against the other T cells. The DE genes are shown in Fig. 4 g. Based on the literature, six out of eight over-expressed genes are associated with cancer development. The high expression of NUSAP1 gene is associated with poor patient overall survival, and this gene also serves as a prognostic factor in breast cancer [ 50 , 51 , 52 ]. Gene MKI67 has been treated as a candidate prognostic prediction for cancer proliferation [ 53 , 54 ]. The over-expression of RRM2 has been linked to higher proliferation and invasiveness of malignant cells [ 55 , 56 ], and the upregulation of RRM2 in breast cancer suggests it to be a possible prognostic indicator [ 57 , 58 , 59 , 60 , 61 , 62 ]. The high expression of UBE2C gene always occurs in cancers with a high degree of malignancy, low differentiation, and high metastatic tendency [ 63 ]. For gene TOP2A, it has been proposed that the HER2 amplification in HER2 breast cancers may be a direct result of the frequent co-amplification of TOP2A [ 64 , 65 , 66 ], and there is a high correlation between the high expressions of TOP2A and the oncogene HER2 [ 67 , 68 ]. Gene CENPF is a cell cycle-associated gene, and it has been identified as a marker of cell proliferation in breast cancers [ 69 ]. The over-expression of these genes strongly supports the correctness of the association identified by SCIPAC. To further validate this positive association, we perform DE analysis of these cells against all the other cells. We find that the top marker genes obtained from CellMarker [ 49 ] for the regulatory T cells, which are known to be immunosuppressive and promote cancer progression [ 70 ], are over-expressed with statistical significance, as shown in Fig. 4 h. This finding again provides strong evidence that the positive association identified by SCIPAC for this group of T cells is correct.
Lung cancer data with survival information
The scRNA-seq data for lung cancer are from [ 71 ], and we use two lung adenocarcinoma (LUAD) patients’ data with 29,888 cells. The true cell types are shown in Fig. 5 a. The bulk data consist of 576 TCGA-LUAD samples with survival status and time.
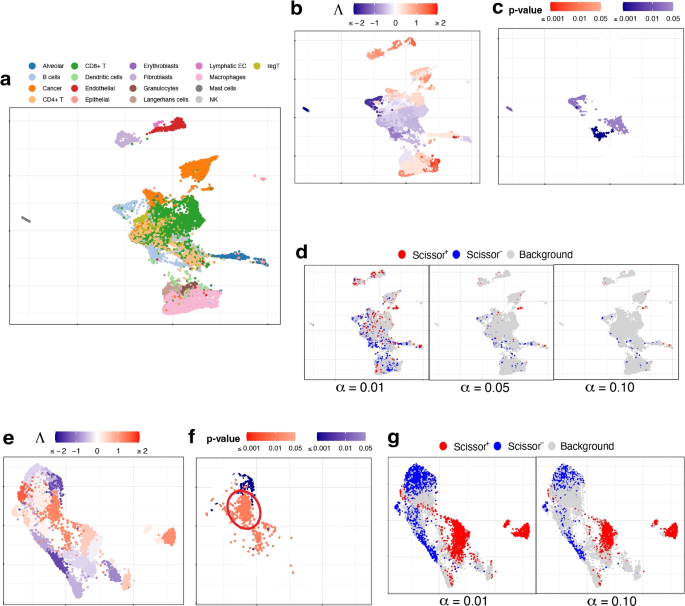
UMAP visualization of a–d the lung cancer data and e–g the muscular dystrophy data. a True cell types. b , c Association strengths \(\Lambda\) and p -values given by SCIPAC under the default resolution. d Results given by Scissor under different \(\alpha\) values. e , f Association strengths \(\Lambda\) and p -values given by SCIPAC under the default resolution. Circled are a group of cells that are identified by SCIPAC as significantly positively associated with the disease but identified by Scissor as null. g Results given by Scissor under different \(\alpha\) values
Association strengths and p -values given by SCIPAC are given in Fig. 5 b, c (results under other resolutions are given in Additional file 1: Fig. S13 and S14). In Fig. 5 c, most cells with statistically significant associations are CD4+ T cells or B cells. These associations are negative, meaning that the abundance of these cells is associated with a reduced death rate, i.e., longer survival time. This agrees with the literature: CD4+ T cells primarily mediate anti-tumor immunity and are associated with favorable prognosis in lung cancer patients [ 72 , 73 , 74 ]; B cells also show anti-tumor functions in all stages of human lung cancer development and play an essential role in anti-tumor responses [ 75 , 76 ].
The results by Scissor under different \(\alpha\) values are shown in Fig. 5 d. The highly scattered Scissor+ and Scissor− cells make identifying and interpreting meaningful phenotype-associated cell groups difficult.
Muscular dystrophy data with a binary phenotype
This dataset contains cells from four facioscapulohumeral muscular dystrophy (FSHD) samples and two control samples [ 77 ]. We pool all the 7047 cells from these six samples together. The true cell types of these cells are unknown. The bulk data consists of 27 FSHD patients and eight controls from [ 28 ]. Here the phenotype is FSHD, and it is binary: present or absent.
The results of SCIPAC with the default resolution are given in Fig. 5 e, f. Results under other resolutions are highly similar (shown in Additional file 1: Fig. S15 and S16). For comparison, results given by Scissor under different \(\alpha\) values are presented in Fig. 5 g. The agreements between the results of SCIPAC and Scissor are clear. For example, both methods identify cells located at the top and lower left part of UMAP plots to be negatively associated with FSHD, and cells located at the center and right parts of UMAP plots to be positively associated. However, the discrepancies in their results are also evident. The most pronounced one is a large group of cells (circled in Fig. 5 f) that are identified by SCIPAC as significantly positively associated but are completely ignored by Scissor. Checking into this group of cells, we find that over 90% (424 out of 469) come from the FSHD patients, and less than 10% come from the control samples. However, cells from FSHD patients only compose 73% (5133) of all the 7047 cells. This statistically significant ( p -value \(<10^{-15}\) , Fisher’s exact test) over-representation (odds ratio = 3.51) suggests that the positive association identified SCIPAC is likely to be correct.
SCIPAC is computationally highly efficient. On an 8-core machine with 2.50 GHz CPU and 16 GB RAM, SCIPAC takes 7, 24, and 2 s to finish all the computation and give the estimated association strengths and p -values on the prostate cancer, lung cancer, and muscular dystrophy datasets, respectively. As a reference, Scissor takes 314, 539, and 171 seconds, respectively.
SCIPAC works with various phenotype types, including binary, continuous, survival, and ordinal. It can easily accommodate other types by using a proper regression model with a systematic component in the form of Eq. 3 (see the “ Methods ” section). For example, a Poisson or negative binomial log-linear model can be used if the phenotype is a count (i.e., non-negative integer).
In SCIPAC’s definition of association, a cell type is associated with the phenotype if increasing the proportion of this cell type leads to a change of probability of the phenotype occurring. The strength of association represents the extent of the increase or decrease in this probability. In the case of binary-response, this change is measured by the log odds ratio. For example, if the association strength of cell type A is twice that of cell type B, increasing cell type A by a certain proportion leads to twice the amount of change in the log odds ratio of having the phenotype compared to increasing cell type B by the same proportion. The association strength under other types of phenotypes can be interpreted similarly, with the major difference lying in the measure of change in probability. For quantitative, ordinal, and survival outcomes, the difference in the quantitative outcome, log odds ratio of the right-tail probability, and log hazard ratio respectively are used. Despite the differences in the exact form of the association strength under different types of phenotypes, the underlying concept remains the same: a larger (absolute value of) association strength indicates that the same increase/decrease in a cell type leads to a larger change in the occurrence of the phenotype.
As SCIPAC utilizes both bulk RNA-seq data with phenotype and single-cell RNA-seq data, the estimated associations for the cells are influenced by the choice of the bulk data. Although different bulk data can yield varying estimations of the association for the same single cells, the estimated associations appear to be reasonably robust even when minor changes are made to the bulk data. See Additional file 1 for further discussions.
When using the Louvain algorithm in the Seurat package to cluster cells, SCIPAC’s default resolution is 2.0, larger than the default setting of Seurat. This allows for the identification of potential subtypes within the major cell type and enables the estimation of individual association strengths. Consequently, a more detailed and comprehensive description of the association between single cells and the phenotype can be obtained by SCIPAC.
When applying SCIPAC to real datasets, we made a deliberate choice to disregard the cell annotation provided by the original publications and instead relied on the inferred cell clusters produced by the Louvain algorithm. We made this decision for several reasons. Firstly, we aimed to ensure a fair comparison with Scissor, as it does not utilize cell-type annotations. Secondly, the original annotation might not be sufficiently comprehensive or detailed. Presumed cell types could potentially encompass multiple subtypes, each of which may exhibit distinct associations with the phenotype under investigation. In such cases, employing the Louvain algorithm with a relatively high resolution, which is the default setting in SCIPAC, enables us to differentiate between these subtypes and allows SCIPAC to assign varying association strengths to each subtype.
SCIPAC fits the regression model using the elastic net, a machine-learning algorithm that maximizes a penalized version of the likelihood. The elastic net can be replaced by other penalized estimates of regression models, such as SCAD [ 78 ], without altering the rest of the SCIPAC algorithm. The combination of a regression model and a penalized estimation algorithm such as the elastic net has shown comparable or higher prediction power than other sophisticated methods such as random forests, boosting, or neural networks in numerous applications, especially for gene expression data [ 79 ]. However, there can still be datasets where other models have higher prediction power. It will be future work to incorporate these models into SCIPAC.
The use of metacells is becoming an efficient way to handle large single-cell datasets [ 80 , 81 , 82 , 83 ]. Conceptually, SCIPAC can incorporate metacells and their representatives as an alternative to its default setting of using cell clusters/types and their centroids. We have explored this aspect using metacells provided by SEACells [ 81 ]. Details are given in Additional file 1. Our comparative analysis reveals that combining SCIPAC with SEACells results in significantly reduced performance compared to using SCIPAC directly on original single-cell data. The primary reason for this appears to be the subpar performance of SEACells in cell grouping, especially when contrasted with the Louvain algorithm. Given these findings, we do not suggest using metacells provided by SEACells for SCIPAC applications in the current stage.
Conclusions
SCIPAC is a novel algorithm for studying the associations between cells and phenotypes. Compared to the previous algorithm, SCIPAC gives a much more detailed and comprehensive description of the associations by enabling a quantitative estimation of the association strength and by providing a quality control—the p -value. Underlying SCIPAC are a general statistical model that accommodates virtually all types of phenotypes, including ordinal (and potentially count) phenotypes that have never been considered before, and a concise and closed-form mathematical formula that quantifies the association, which minimizes the computational load. The mathematical conciseness also largely frees SCIPAC from parameter tuning. The only parameter (i.e., the resolution) barely changes the results given by SCIPAC. Overall, compared with its predecessor, SCIPAC represents a substantially more capable software by being much more informative, versatile, robust, and user-friendly.
The improvement in accuracy is also remarkable. In simulated data, SCIPAC achieves high power and low false positives, which is evident from the UMAP plot, F1 score, and FSC score. In real data, SCIPAC gives results that are consistent with current biological knowledge for cell types whose functions are well understood. For cell types whose functions are less studied or more multifaceted, SCIPAC gives support to certain biological hypotheses or helps identify/discover cell sub-types.
SCIPAC’s identification of cell-phenotype associations closely follows its definition of association: when increasing the fraction of a cell type increases (or decreases) the probability for a phenotype to be present, this cell type is positively (or negatively) associated with the phenotype.
The increase of the fraction of a cell type
For a bulk sample, let vector \(\varvec{G} \in \mathbb {R}^p\) be its expression profile, that is, its expression on the p genes. Suppose there are K cell types in the tissue, and let \(\varvec{g}_{k}\) be the representative expression of the k ’th cell type. Usually, people assume that \(\varvec{G}\) can be decomposed by
where \(\gamma _{k}\) is the proportion of cell type k in the bulk tissue, with \(\sum _{k = 1}^{K}\gamma _{k} = 1\) . This equation links the bulk and single-cell expression data.
Now consider increasing cells from cell type k by \(\Delta \gamma\) proportion of the original number of cells. Then, the new proportion of cell type k becomes \(\frac{\gamma _{k} + \Delta \gamma }{1 + \Delta \gamma }\) , and the new proportion of cell type \(j \ne k\) becomes \(\frac{\gamma _{j}}{1 + \Delta \gamma }\) (note that the new proportions of all cell types should still add up to 1). Thus, the bulk expression profile with the increase of cell type k becomes
Plugging Eq. 1 , we get
Interestingly, this expression of \(\varvec{G}^*\) does not include \(\gamma _{1}, \ldots , \gamma _{K}\) . This means that there is no need actually to compute \(\gamma _{1}, \ldots , \gamma _{K}\) in Eq. 1 , which could otherwise be done using a cell-type-decomposition software, but an accurate and robust decomposition is non-trivial [ 84 , 85 , 86 ]. See Additional file 1 for a more in-depth discussion on the connections of SCIPAC with decomposition/deconvolution.
The change in chance of a phenotype
In this section, we consider how the increase in the fraction of a cell type will change the chance for a binary phenotype such as cancer to occur. Other types of phenotypes will be considered in the next section.
Let \(\pi (\varvec{G})\) be the chance of an individual with gene expression profile \(\varvec{G}\) for this phenotype to occur. We assume a logistic regression model to describe the relationship between \(\pi (\varvec{G})\) and \(\varvec{G}\) :
here the left-hand side is the log odds of \(\pi (\varvec{G})\) , \(\beta _{0}\) is the intercept, and \(\varvec{\beta }\) is a length- p vector of coefficients. In the section after the next, we will describe how we obtain \(\beta _{0}\) and \(\varvec{\beta }\) from the data.
When increasing cells from cell type k by \(\Delta \gamma\) , \(\varvec{G}\) becomes \(\varvec{G}^*\) in Eq. 3 . Plugging Eq. 2 , we get
We further take the difference between Eqs. 4 and 3 and get
The left-hand side of this equation is the log odds ratio (i.e., the change of log odds). On the right-hand side, \(\frac{\Delta \gamma }{1 + \Delta \gamma }\) is an increasing function with respect to \(\Delta \gamma\) , and \(\varvec{\beta }^T(\varvec{g}_{k} - \varvec{G})\) is independent of \(\Delta \gamma\) . This indicates that given any specific \(\Delta \gamma\) , the log odds ratio under over-representation of cell type k is proportional to
\(\lambda _k\) describes the strength of the effect of increasing cell type k to a bulk sample with expression profile \(\varvec{G}\) . Given the presence of numerous bulk samples, employing multiple \(\lambda _k\) ’s could be cumbersome and obscure the overall effect of a particular cell type. To concisely summarize the association of cell type k , we propose averaging their effects. The average effect on all bulk samples can be obtained by
where \(\bar{\varvec{G}}\) is the average expression profile of all bulk samples.
\(\Lambda _k\) gives an overall impression of how strong the effect is when cell type k over-represents to the probability for the phenotype to be present. Its sign represents the direction of the change: a positive value means an increase in probability, and a negative value means a decrease in probability. Its absolute value represents the strength of the effect. In SCIPAC, we call \(\Lambda _k\) the association strength of cell type k and the phenotype.
Note that this derivation does not involve likelihood, although the computation of \(\varvec{\beta }\) does. Here, it serves more as a definitional approach.
Definition of the association strength for other types of phenotype
Our definition of \(\Lambda _k\) relies on vector \(\varvec{\beta }\) . In the case of a binary phenotype, \(\varvec{\beta }\) are the coefficients of a logistic regression that describes a linear relationship between the expression profile and the log odds of having the phenotype, as shown in Eq. 3 . For other types of phenotype, \(\varvec{\beta }\) can be defined/computed similarly.
For a quantitative (i.e., continuous) phenotype, an ordinary linear regression can be used, and the left-hand side of Eq. 3 is changed to the quantitative value of the phenotype.
For a survival phenotype, a Cox proportional hazards model can be used, and the left-hand side of Eq. 3 is changed to the log hazard ratio.
For an ordinal phenotype, we use a proportional odds model
where \(j \in \{1, 2, ..., (J - 1)\}\) and J is the number of ordinal levels. It should be noted that here we use the right-tail probability \(\Pr (Y_{i} \ge j + 1 | X)\) instead of the commonly used cumulative probability (left-tail probability) \(\Pr (Y_{i} \le j | X)\) . Such a change makes the interpretation consistent with other types of phenotypes: in our model, a larger value on the right-hand side indicates a larger chance for \(Y_{i}\) to have a higher level, which in turn guarantees that the sign of the association strength defined according to this \(\varvec{\beta }\) has the usual meaning: a positive \(\Lambda _k\) value means a positive association with the phenotype-using the cancer stage as an example. A positive \(\Lambda _k\) means the over-representation of cell type k increases the chance of a higher cancer stage. In contrast, using the commonly used cumulative probability leads to a counter-intuitive, reversed interpretation.
Computation of the association strength in practice
In practice, \(\varvec{\beta }\) in Eq. 3 needs to be learned from the bulk data. By default, SCIPAC uses the elastic net, a popular and powerful penalized regression method:
In this model, \(l(\beta _{0}, \varvec{\beta })\) is a log-likelihood of the linear model (i.e., logistic regression for a binary phenotype, ordinary linear regression for a quantitative phenotype, Cox proportional odds model for a survival phenotype, and proportional odds model for an ordinal phenotype). \(\alpha\) is a number between 0 and 1, denoting a combination of \(\ell _1\) and \(\ell _2\) penalties, and \(\lambda\) is the penalty strength. SCIPAC fixes \(\alpha\) to be 0.4 (see Additional file 1 for discussions on this choice) and uses 10-fold cross-validation to decide \(\lambda\) automatically. This way, they do not become hyperparameters.
In SCIPAC, the fitting and cross-validation of the elastic net are done by calling the ordinalNet [ 87 ] R package for the ordinal phenotype and by calling the glmnet R package [ 88 , 89 , 90 , 91 ] for other types of phenotypes.
The computation of the association strength, as defined by Eq. 7 , does not only require \(\varvec{\beta }\) , but also \(\varvec{g}_k\) and \(\bar{\varvec{G}}\) . \(\bar{\varvec{G}}\) is simply the average expression profile of all bulk samples. On the other hand, \(\varvec{g}_k\) requires knowing the cell type of each cell. By default, SCIPAC does not assume this information to be given, and it uses the Louvain clustering implemented in the Seurat [ 24 , 25 ] R package to infer it. This clustering algorithm has one tuning parameter called “resolution.” SCIPAC sets its default value as 2.0, and the user can use other values. With the inferred or given cell types, \(\varvec{g}_k\) is computed as the centroid (i.e., the mean expression profile) of cells in cluster k .
Given \(\varvec{\beta }\) , \(\bar{\varvec{G}}\) , and \(\varvec{g}_k\) , the association strength can be computed using Eq. 7 . Knowing the association strength for each cell type and the cell-type label for each cell, we also know the association strength for every single cell. In practice, we standardize the association strengths for all cells. That is, we compute the mean and standard deviation of the association strengths of all cells and use them to centralize and scale the association strength, respectively. We have found such standardization makes SCIPAC more robust to the possible unbalance in sample size of bulk data in different phenotype groups.
Computation of the p -value
SCIPAC uses non-parametric bootstrap [ 92 ] to compute the standard deviation and hence the p -value of the association. Fifty bootstrap samples, which are believed to be enough to compute the standard error of most statistics [ 93 ], are generated for the bulk expression data, and each is used to compute (standardized) \(\Lambda\) values for all the cells. For cell i , let its original \(\Lambda\) values be \(\Lambda _i\) , and the bootstrapped values be \(\Lambda _i^{(1)}, \ldots , \Lambda _i^{(50)}\) . A z -score is then computed using
and then the p -value is computed according to the cumulative distribution function of the standard Gaussian distribution. See Additional file 1 for more discussions on the calculation of p -value.
Availability of data and materials
The simulated datasets [ 94 ] under three schemes are available at Zenodo with DOI 10.5281/zenodo.11013320 [ 95 ]. The SCIPAC package is available at GitHub website https://github.com/RavenGan/SCIPAC under the MIT license [ 96 ]. The source code of SCIPAC is also deposited at Zenodo with DOI 10.5281/zenodo.11013696 [ 97 ]. A vignette of the R package is available on the GitHub page and in the Additional file 2. The prostate cancer scRNA-seq data is obtained from the Prostate Cell Atlas https://www.prostatecellatlas.org [ 29 ]; the scRNA-seq data for the breast cancer are from the Gene Expression Omnibus (GEO) under accession number GSE176078 [ 34 , 98 ]; the scRNA-seq data for the lung cancer are from E-MTAB-6149 [ 99 ] and E-MTAB-6653 [ 71 , 100 ]; the scRNA-seq data for facioscapulohumeral muscular dystrophy data are from the GEO under accession number GSE122873 [ 101 ]. The bulk RNA-seq data are obtained from the TCGA database via TCGAbiolinks (ver. 2.25.2) R package [ 102 ]. More details about the simulated and real scRNA-seq and bulk RNA-seq data can be found in the Additional file 1.
Yofe I, Dahan R, Amit I. Single-cell genomic approaches for developing the next generation of immunotherapies. Nat Med. 2020;26(2):171–7.
Article CAS PubMed Google Scholar
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179(4):829–45.
Fan J, Slowikowski K, Zhang F. Single-cell transcriptomics in cancer: computational challenges and opportunities. Exp Mol Med. 2020;52(9):1452–65.
Article CAS PubMed PubMed Central Google Scholar
Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161(5):1187–201.
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202–14.
Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360(6385):176–82.
Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8(1):1–12.
Article Google Scholar
Abdelaal T, Michielsen L, Cats D, Hoogduin D, Mei H, Reinders MJ, et al. A comparison of automatic cell identification methods for single-cell RNA sequencing data. Genome Biol. 2019;20(1):1–19.
Article CAS Google Scholar
Luecken MD, Theis FJ. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol. 2019;15(6):e8746.
Article PubMed PubMed Central Google Scholar
Guo H, Li J. scSorter: assigning cells to known cell types according to marker genes. Genome Biol. 2021;22(1):1–18.
Pliner HA, Shendure J, Trapnell C. Supervised classification enables rapid annotation of cell atlases. Nat Methods. 2019;16(10):983–6.
Zhang AW, O’Flanagan C, Chavez EA, Lim JL, Ceglia N, McPherson A, et al. Probabilistic cell-type assignment of single-cell RNA-seq for tumor microenvironment profiling. Nat Methods. 2019;16(10):1007–15.
Zhang Z, Luo D, Zhong X, Choi JH, Ma Y, Wang S, et al. SCINA: a semi-supervised subtyping algorithm of single cells and bulk samples. Genes. 2019;10(7):531.
Johnson TS, Wang T, Huang Z, Yu CY, Wu Y, Han Y, et al. LAmbDA: label ambiguous domain adaptation dataset integration reduces batch effects and improves subtype detection. Bioinformatics. 2019;35(22):4696–706.
Ma F, Pellegrini M. ACTINN: automated identification of cell types in single cell RNA sequencing. Bioinformatics. 2020;36(2):533–8.
Tan Y, Cahan P. SingleCellNet: a computational tool to classify single cell RNA-Seq data across platforms and across species. Cell Syst. 2019;9(2):207–13.
Salcher S, Sturm G, Horvath L, Untergasser G, Kuempers C, Fotakis G, et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022;40(12):1503–20.
Good Z, Sarno J, Jager A, Samusik N, Aghaeepour N, Simonds EF, et al. Single-cell developmental classification of B cell precursor acute lymphoblastic leukemia at diagnosis reveals predictors of relapse. Nat Med. 2018;24(4):474–83.
Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177(5):1330–45.
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–20.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Disc. 2012;2(5):401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):1.
Sun D, Guan X, Moran AE, Wu LY, Qian DZ, Schedin P, et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat Biotechnol. 2022;40(4):527–38.
Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech Theory Exp. 2008;2008(10):P10008.
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM III, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–902.
Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Stat Methodol. 2005;67(2):301–20.
McInnes L, Healy J, Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 2018. arXiv preprint arXiv:1802.03426 .
Wong CJ, Wang LH, Friedman SD, Shaw D, Campbell AE, Budech CB, et al. Longitudinal measures of RNA expression and disease activity in FSHD muscle biopsies. Hum Mol Genet. 2020;29(6):1030–43.
Tuong ZK, Loudon KW, Berry B, Richoz N, Jones J, Tan X, et al. Resolving the immune landscape of human prostate at a single-cell level in health and cancer. Cell Rep. 2021;37(12):110132.
Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18(1):49–53.
Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72(4):621–7.
Raggi F, Bosco MC. Targeting mononuclear phagocyte receptors in cancer immunotherapy: new perspectives of the triggering receptor expressed on myeloid cells (TREM-1). Cancers. 2020;12(5):1337.
Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J. The B-side of cancer immunity: the underrated tune. Cells. 2019;8(5):449.
Wu SZ, Al-Eryani G, Roden DL, Junankar S, Harvey K, Andersson A, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021;53(9):1334–47.
Fernández-Nogueira P, Fuster G, Gutierrez-Uzquiza Á, Gascón P, Carbó N, Bragado P. Cancer-associated fibroblasts in breast cancer treatment response and metastasis. Cancers. 2021;13(13):3146.
Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Identification of cCAFs from metastatic cancer patients. Cancer Res. 2015;75(22):4681–7.
Arcucci A, Ruocco MR, Granato G, Sacco AM, Montagnani S. Cancer: an oxidative crosstalk between solid tumor cells and cancer associated fibroblasts. BioMed Res Int. 2016;2016. https://pubmed.ncbi.nlm.nih.gov/27595103/ .
Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers. 2016;8(2):19.
Ruocco MR, Avagliano A, Granato G, Imparato V, Masone S, Masullo M, et al. Involvement of breast cancer-associated fibroblasts in tumor development, therapy resistance and evaluation of potential therapeutic strategies. Curr Med Chem. 2018;25(29):3414–34.
Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24(7):986–93.
Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med. 2021;27(5):820–32.
Romero JM, Grünwald B, Jang GH, Bavi PP, Jhaveri A, Masoomian M, et al. A four-chemokine signature is associated with a T-cell-inflamed phenotype in primary and metastatic pancreatic cancer. Chemokines in Pancreatic Cancer. Clin Cancer Res. 2020;26(8):1997–2010.
Tamura R, Yoshihara K, Nakaoka H, Yachida N, Yamaguchi M, Suda K, et al. XCL1 expression correlates with CD8-positive T cells infiltration and PD-L1 expression in squamous cell carcinoma arising from mature cystic teratoma of the ovary. Oncogene. 2020;39(17):3541–54.
Hernandez R, Põder J, LaPorte KM, Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022:22:1–15. https://pubmed.ncbi.nlm.nih.gov/35217787/ .
Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, Sergushichev A. Fast gene set enrichment analysis. BioRxiv. 2016:060012. https://www.biorxiv.org/content/10.1101/060012v3.abstract .
Dang CV. MYC on the path to cancer. Cell. 2012;149(1):22–35.
Gnanaprakasam JR, Wang R. MYC in regulating immunity: metabolism and beyond. Genes. 2017;8(3):88.
Oshi M, Takahashi H, Tokumaru Y, Yan L, Rashid OM, Matsuyama R, et al. G2M cell cycle pathway score as a prognostic biomarker of metastasis in estrogen receptor (ER)-positive breast cancer. Int J Mol Sci. 2020;21(8):2921.
Zhang X, Lan Y, Xu J, Quan F, Zhao E, Deng C, et al. Cell Marker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 2019;47(D1):D721–8.
Chen L, Yang L, Qiao F, Hu X, Li S, Yao L, et al. High levels of nucleolar spindle-associated protein and reduced levels of BRCA1 expression predict poor prognosis in triple-negative breast cancer. PLoS ONE. 2015;10(10):e0140572.
Li M, Yang B. Prognostic value of NUSAP1 and its correlation with immune infiltrates in human breast cancer. Crit Rev TM Eukaryot Gene Expr. 2022;32(3). https://pubmed.ncbi.nlm.nih.gov/35695609/ .
Zhang X, Pan Y, Fu H, Zhang J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression. Med Sci Monit: Int Med J Exp Clin Res. 2018;24:8553.
Geyer FC, Rodrigues DN, Weigelt B, Reis-Filho JS. Molecular classification of estrogen receptor-positive/luminal breast cancers. Adv Anat Pathol. 2012;19(1):39–53.
Karamitopoulou E, Perentes E, Tolnay M, Probst A. Prognostic significance of MIB-1, p53, and bcl-2 immunoreactivity in meningiomas. Hum Pathol. 1998;29(2):140–5.
Duxbury MS, Whang EE. RRM2 induces NF- \(\kappa\) B-dependent MMP-9 activation and enhances cellular invasiveness. Biochem Biophys Res Commun. 2007;354(1):190–6.
Zhou BS, Tsai P, Ker R, Tsai J, Ho R, Yu J, et al. Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clin Exp Metastasis. 1998;16(1):43–9.
Zhang H, Liu X, Warden CD, Huang Y, Loera S, Xue L, et al. Prognostic and therapeutic significance of ribonucleotide reductase small subunit M2 in estrogen-negative breast cancers. BMC Cancer. 2014;14(1):1–16.
Putluri N, Maity S, Kommagani R, Creighton CJ, Putluri V, Chen F, et al. Pathway-centric integrative analysis identifies RRM2 as a prognostic marker in breast cancer associated with poor survival and tamoxifen resistance. Neoplasia. 2014;16(5):390–402.
Koleck TA, Conley YP. Identification and prioritization of candidate genes for symptom variability in breast cancer survivors based on disease characteristics at the cellular level. Breast Cancer Targets Ther. 2016;8:29.
Li Jp, Zhang Xm, Zhang Z, Zheng Lh, Jindal S, Liu Yj. Association of p53 expression with poor prognosis in patients with triple-negative breast invasive ductal carcinoma. Medicine. 2019;98(18). https://pubmed.ncbi.nlm.nih.gov/31045815/ .
Gong MT, Ye SD, Lv WW, He K, Li WX. Comprehensive integrated analysis of gene expression datasets identifies key anti-cancer targets in different stages of breast cancer. Exp Ther Med. 2018;16(2):802–10.
PubMed PubMed Central Google Scholar
Chen Wx, Yang Lg, Xu Ly, Cheng L, Qian Q, Sun L, et al. Bioinformatics analysis revealing prognostic significance of RRM2 gene in breast cancer. Biosci Rep. 2019;39(4). https://pubmed.ncbi.nlm.nih.gov/30898978/ .
Hao Z, Zhang H, Cowell J. Ubiquitin-conjugating enzyme UBE2C: molecular biology, role in tumorigenesis, and potential as a biomarker. Tumor Biol. 2012;33(3):723–30.
Arriola E, Rodriguez-Pinilla SM, Lambros MB, Jones RL, James M, Savage K, et al. Topoisomerase II alpha amplification may predict benefit from adjuvant anthracyclines in HER2 positive early breast cancer. Breast Cancer Res Treat. 2007;106(2):181–9.
Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23(30):7483–90.
Tanner M, Isola J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, Malmstrom P, et al. Topoisomerase II \(\alpha\) gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol. 2006;24(16):2428–36.
Arriola E, Moreno A, Varela M, Serra JM, Falo C, Benito E, et al. Predictive value of HER-2 and topoisomerase II \(\alpha\) in response to primary doxorubicin in breast cancer. Eur J Cancer. 2006;42(17):2954–60.
Järvinen TA, Tanner M, Bärlund M, Borg Å, Isola J. Characterization of topoisomerase II \(\alpha\) gene amplification and deletion in breast cancer. Gene Chromosome Cancer. 1999;26(2):142–50.
Landberg G, Erlanson M, Roos G, Tan EM, Casiano CA. Nuclear autoantigen p330d/CENP-F: a marker for cell proliferation in human malignancies. Cytom J Int Soc Anal Cytol. 1996;25(1):90–8.
CAS Google Scholar
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277–89.
Bremnes RM, Busund LT, Kilvær TL, Andersen S, Richardsen E, Paulsen EE, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11(6):789–800.
Article PubMed Google Scholar
Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, et al. Objective measurement and clinical significance of TILs in non–small cell lung cancer. J Natl Cancer Inst. 2015;107(3):dju435.
Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 2021;28(1):5–17.
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–80.
Wang Ss, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019;16(1):6–18.
van den Heuvel A, Mahfouz A, Kloet SL, Balog J, van Engelen BG, Tawil R, et al. Single-cell RNA sequencing in facioscapulohumeral muscular dystrophy disease etiology and development. Hum Mol Genet. 2019;28(7):1064–75.
Fan J, Li R. Variable selection via nonconcave penalized likelihood and its oracle properties. J Am Stat Assoc. 2001;96(456):1348–60.
Hastie T, Tibshirani R, Friedman JH, Friedman JH. The elements of statistical learning: data mining, inference, and prediction, vol. 2. New York: Springer; 2009.
Book Google Scholar
Baran Y, Bercovich A, Sebe-Pedros A, Lubling Y, Giladi A, Chomsky E, et al. MetaCell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 2019;20(1):1–19.
Persad S, Choo ZN, Dien C, Sohail N, Masilionis I, Chaligné R, et al. SEACells infers transcriptional and epigenomic cellular states from single-cell genomics data. Nat Biotechnol. 2023;41:1–12. https://pubmed.ncbi.nlm.nih.gov/36973557/ .
Ben-Kiki O, Bercovich A, Lifshitz A, Tanay A. Metacell-2: a divide-and-conquer metacell algorithm for scalable scRNA-seq analysis. Genome Biol. 2022;23(1):100.
Bilous M, Tran L, Cianciaruso C, Gabriel A, Michel H, Carmona SJ, et al. Metacells untangle large and complex single-cell transcriptome networks. BMC Bioinformatics. 2022;23(1):336.
Avila Cobos F, Alquicira-Hernandez J, Powell JE, Mestdagh P, De Preter K. Benchmarking of cell type deconvolution pipelines for transcriptomics data. Nat Commun. 2020;11(1):1–14.
Jin H, Liu Z. A benchmark for RNA-seq deconvolution analysis under dynamic testing environments. Genome Biol. 2021;22(1):1–23.
Wang X, Park J, Susztak K, Zhang NR, Li M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat Commun. 2019;10(1):380.
Wurm MJ, Rathouz PJ, Hanlon BM. Regularized ordinal regression and the ordinalNet R package. 2017. arXiv preprint arXiv:1706.05003 .
Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1.
Simon N, Friedman J, Hastie T. A blockwise descent algorithm for group-penalized multiresponse and multinomial regression. 2013. arXiv preprint arXiv:1311.6529 .
Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1.
Tibshirani R, Bien J, Friedman J, Hastie T, Simon N, Taylor J, et al. Strong rules for discarding predictors in lasso-type problems. J R Stat Soc Ser B Stat Methodol. 2012;74(2):245–66.
Efron B. Bootstrap methods: another look at the jackknife. In: Breakthroughs in statistics. New York: Springer; 1992. pp. 569–593.
Efron B, Tibshirani RJ. An introduction to the bootstrap. London: CRC Press; 1994.
Zappia L, Phipson B, Oshlack A. Splatter: simulation of single-cell RNA sequencing data. Genome Biol. 2017;18(1):174.
Gan D, Zhu Y, Lu X, Li J. Simulated datasets used in SCIPAC analysis. Zenodo. 2024. https://doi.org/10.5281/zenodo.11013320 .
Gan D, Zhu Y, Lu X, Li J. SCIPAC R package. GitHub. 2024. https://github.com/RavenGan/SCIPAC . Accessed 24 Apr 2024.
Gan D, Zhu Y, Lu X, Li J. SCIPAC source code. Zenodo. 2024. https://doi.org/10.5281/zenodo.11013696 .
Wu SZ, Al-Eryani G, Roden DL, Junankar S, Harvey K, Andersson A, et al. A single-cell and spatially resolved atlas of human breast cancers. Datasets. 2021. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE176078 . Gene Expression Omnibus. Accessed 1 Oct 2022.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Datasets. 2018. https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-6149 . ArrayExpress. Accessed 24 July 2022.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Datasets. 2018. https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-6653 . ArrayExpress. Accessed 24 July 2022.
van den Heuvel A, Mahfouz A, Kloet SL, Balog J, van Engelen BG, Tawil R, et al. Single-cell RNA sequencing in facioscapulohumeral muscular dystrophy disease etiology and development. Datasets. 2019. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE122873 . Gene Expression Omnibus. Accessed 13 Aug 2022.
Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71.
Download references
Review history
The review history is available as Additional file 3.
Peer review information
Veronique van den Berghe was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
This work is supported by the National Institutes of Health (R01CA280097 to X.L. and J.L, R01CA252878 to J.L.) and the DOD BCRP Breakthrough Award, Level 2 (W81XWH2110432 to J.L.).
Author information
Authors and affiliations.
Department of Applied and Computational Mathematics and Statistics, University of Notre Dame, Notre Dame, 46556, IN, USA
Dailin Gan & Jun Li
Department of Biological Sciences, Boler-Parseghian Center for Rare and Neglected Diseases, Harper Cancer Research Institute, Integrated Biomedical Sciences Graduate Program, University of Notre Dame, Notre Dame, 46556, IN, USA
Yini Zhu & Xin Lu
Tumor Microenvironment and Metastasis Program, Indiana University Melvin and Bren Simon Comprehensive Cancer Center, Indianapolis, 46202, IN, USA
You can also search for this author in PubMed Google Scholar
Contributions
J.L. conceived and supervised the study. J.L. and D.G. proposed the methods. D.G. implemented the methods and analyzed the data. D.G. and J.L. drafted the paper. D.G., Y.Z., X.L., and J.L. interpreted the results and revised the paper.
Corresponding author
Correspondence to Jun Li .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1. supplementary materials that include additional results and plots., additional file 2. a vignette of the scipac package., additional file 3. review history., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions

About this article
Cite this article.
Gan, D., Zhu, Y., Lu, X. et al. SCIPAC: quantitative estimation of cell-phenotype associations. Genome Biol 25 , 119 (2024). https://doi.org/10.1186/s13059-024-03263-1
Download citation
Received : 30 January 2023
Accepted : 30 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s13059-024-03263-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phenotype association
- Single cell
- RNA sequencing
- Cancer research
Genome Biology
ISSN: 1474-760X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 10, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Research shows impact of caregiving on parents' employment, health
by Tayler Shaw, CU Anschutz Medical Campus

When it comes to improving the lives of children with genetic conditions, medical research often focuses solely on the children. But there is an equally important population in need of research that a faculty member at the University of Colorado Department of Medicine is highlighting—the caregivers.
"We need to think very broadly about how to support not just the kids—yes, that matters—but also the people caring for those kids," said Liza Creel, Ph.D., a health services researcher and associate professor in the Division of Health Care Policy and Research. "I want to do work that informs policy to support caregivers and to help families."
Recently, at the CU Department of Medicine's Research Day , Creel presented her research on parents as medical caregivers, specifically looking at how caregiving is associated with their own health and employment.
"The health of these caregivers matters," said Creel, who is also involved in the Adult and Child Center for Outcomes Research and Delivery Science (ACCORDS). "These things are all connected."
Caregiving: A valuable but time-consuming role
Creel said the most recent survey data from 2021 estimates that 3.9% of children in the United States—which is nearly 3 million children—have a genetic condition. This includes inherited metabolic conditions , Down syndrome, blood disorders, and cystic fibrosis. However, capturing a true estimate of the prevalence of genetic conditions is difficult.
"There are many genetic conditions that are not captured in this definition, such as hearing loss," she said. "This likely underestimates the true prevalence, but right now, it's the best that we have."
These conditions are lifelong and require ongoing management and therapeutic interventions, she said. Research has found that children with genetic conditions have increased health care utilization and are more likely to have unmet health needs than other children.
"That's attributable to both the complexity of their needs and a very limited specialized workforce in the area of genetic medicine," Creel said.
Caregivers for children with genetic conditions provide a variety of support, such as care coordination of the health care system and insurance navigation, physical support, psychosocial support, navigation of the education system, and other services like transportation.
"Caregiving can be time consuming, and many things that you do as a caregiver have to happen in regular business hours," Creel said. "That suggests that there are potential implications on their participation in the labor market and the amount of time they have to take care of their own health needs."
Impact of caregiving on employment, health
To examine the associations between caregiving for a child with a genetic condition and the caregivers' health status and employment, Creel and her research team conducted a retrospective analysis using combined data from the 2016-2021 National Survey of Children's Health.
Since they had several years of data to analyze, Creel's team decided to also look at the prevalence of children with genetic conditions. They estimated that 4.4% of children in the U.S. have a genetic condition—roughly 0.5% higher than what the 2021 study showed.
Creel said her team measured caregivers' employment by using one specific variable that asked the caregiver, who was the person filling out the survey, if they had left the workforce in the past 12 months due to caring for a child.
They ultimately found that caregivers for children with genetic conditions had increased odds of leaving the workforce compared to other caregivers. Specifically, 12.6% of caregivers for children with genetic conditions stopped working, as compared to 8.5% of caregivers for children with other special health care needs.
"Overall, among caregivers of all children, 3.7% had left the workforce in the prior year due to caring for a child," Creel said.
The research also found increased odds of leaving the workforce for caregivers of Black children, American Indian children, children who receive care from others at least 10 hours a week, and children who needed care but did not receive it. On the flip side, caregivers with college degrees and those who are married had decreased odds of leaving the workforce.
Caregivers' health status was assessed based on self-reported responses to survey questions about physical and mental health . The data showed caregivers of children with genetic conditions, both mothers and fathers, reported fair or poor physical health and mental health at a higher rate than other caregivers.
For instance, 14% of mothers of a child with a genetic condition reported fair or poor physical health, as compared to 5.9% of mothers of a child who does not have a genetic condition .
"When a caregiver has poor or fair physical or mental health, the likelihood of leaving the workforce doubles," Creel said.
Why this research matters
Understanding the impact of caregiving on employment and caregivers' health matters for a multitude of reasons, Creel said.
In terms of health policy, the Administration for Community Living released a national strategy to support family caregivers in 2022, saying there are financial, physical, and emotional costs to being a caregiver.
"One study estimated that family caregivers lose over $500 billion in wages every year due to caregiving, and employers experience a financial loss as well," she said.
Understanding the impacts of caregiving is also relevant to clinical practice in a lot of ways, Creel said. For instance, the health care system focuses on offering clinical interventions and prevention efforts to improve a patient's well-being. However, to achieve this goal, there are a lot of factors and steps.
"There are these enabling resources, like whether a person has health insurance or the financial resources to access the care that they need," she said.
Other factors include caregivers' predisposing characteristics, and their real and perceived need for health care services.
"These things all influence the health behaviors that we perceive in the health system, including their use of services and their application of self-care. Both of which also really matter in terms of health outcomes," she said.
Ultimately, family caregiving is an area of policy, research, and clinical importance, Creel said. Both children with genetic conditions and their caregivers face substantial medical and care coordination needs. They also potentially face limited access to subspecialty care and other supports that facilitate access to needed care.
"Policies to support caregivers through respite, specialized childcare, coordination tools, and other resources may allow caregivers to focus their attention where it is most needed, allowing for a focus on their own health and without needing to leave the workforce," Creel said.
Explore further
Feedback to editors
Study investigates cardiac cell regeneration in search of novel therapeutics
5 minutes ago

Researchers profile clinical, gene and protein changes in 'brain fog' from long COVID
15 minutes ago

Researchers develop theory on traveling waves of activity in the human brain
21 minutes ago

Study indicates the rapid identification of stroke type is key to improving outcomes
36 minutes ago

Simple learning test may be used to diagnose autism at just six months of age
38 minutes ago
Researchers identify immunosuppressive pathway that helps newborn hearts regenerate in mouse models
40 minutes ago

'Trojan Horse' weight loss drug found to be more effective than available therapies
52 minutes ago
Research explains new method to engineer immune cells that could treat multiple cancer patients
58 minutes ago

Link between COVID-19 vaccine complication and rare 'common cold' blood disease
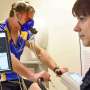
Consistent exercise changes how saturated fat is used by the body, study finds
Related stories.

Most caregivers are ill-prepared for their own hospitalization
May 2, 2024

Loneliness common among older informal caregivers, says report
May 7, 2024

Why women caregivers need more support to manage their responsibilities and well-being
Apr 29, 2024
Caregiving can be stressful, but it could also lower risk of depression: Study
Dec 12, 2023

Family caregivers who receive a high level of care from a family doctor found to have lower stress levels
Jun 26, 2023
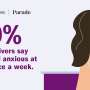
Survey shows importance of supporting family caregivers
Nov 30, 2022
Recommended for you

Researchers find microplastics in canine and human testicular tissue
21 hours ago

Scientists want to know how the smells of nature benefit our health
22 hours ago

The doctor is in… but what's behind them? Study reveals impacts of telehealth background settings
May 15, 2024

Two decades of studies suggest health benefits associated with plant-based diets, but caution urged

Racial disparities in childhood obesity on the rise in study of NYC public schools
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Global main menu
- School of Law
Will the US adopt IHRA’s anti-Semitism definition? What’s the controversy?
Professor Neve Gordon speaks to Al Jazeera about the problems of the US adopting the International Holocaust Remembrance Alliance (IHRA)’s definition of anti-Semitism.

Image by Wenhan Cheng from Pixabay
On 1 May, the United States House of Representatives passed a bill that would codify the IHRA’s definition of anti-Semitism, and the Senate – the upper house of Congress – is now expected to debate and vote on the bill. This has drawn some debate and controversy, as the IHRA’s definition has been accused of conflating criticism of the state of Israel and Zionism with anti-Semitism.
Israeli academic Neve Gordon , Professor of International Law and Human Rights at Queen Mary University of London and Vice President of BRISMES, argues that adopting this definition could brand critical Jewish voices as anti-Semitic. He said: “If I were to teach in a class the Human Rights Watch report stating that Israel is an apartheid state, I could be accused of anti-Semitism.”
Read the full article on Al Jazeera .
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Research Council (US) Committee to Study the National Needs for Biomedical, Behavioral, and Clinical Research Personnel. Research Training in the Biomedical, Behavioral, and Clinical Research Sciences. Washington (DC): National Academies Press (US); 2011.

Research Training in the Biomedical, Behavioral, and Clinical Research Sciences.
- Hardcopy Version at National Academies Press
5 Clinical Sciences Research
Reseach in the clinical sciences helps put into practice the discoveries that arise from the research in the fields described in the three previous chapters. Because the term “clinical research” is used to cover such a broad and diverse array of activities, its definition has proved to be controversial, primarily over the issue of whether the research does or does not require direct interaction with living patients or other human research subjects. The most expansive definition of clinical research is that agreed upon in 1998 at the Graylyn Clinical Research Consensus Development Conference, organized by the Association of American Medical Colleges (AAMC), the American Medical Association (AMA), and the Wake Forest University Medical Center. The Graylyn conferees defined
clinical research as a component of medical and health research intended to produce knowledge valuable for the understanding of human disease, preventing and treating illness, and promoting health. Clinical research involves interactions with patients, diagnostic clinical materials or data, or populations in any of the following areas: (1) disease mechanisms (etiopathogenesis); (2) bi-directional integrative (translational) research; (3) clinical knowledge, detection, diagnosis and natural history of disease; (4) therapeutic interventions including clinical trials of drugs, biologics, devices and instruments; (5) prevention (primary and secondary) and health promotion; (6) behavioral research; (7) health services research, including outcomes, and cost-effectiveness; (8) epidemiology; and (9) community-based trials. 1
This definition was adopted by the U.S. Congress in the Clinical Research Enhancement Act (P.L. 110-148) of November 2000.
In response to this definition, those determined to carve out and distinguish research requiring direct interaction with living patients coined the term patient-oriented research . Another distinction is commonly made for translational research , which describes research that explores the applicability of the results of basic research to clinical care (for example, in clinical trials, especially early Phase 1 or 2 trials). In addition, translational research may also include studies of how to facilitate the introduction of newly established clinical knowledge into broad clinical practice and the obstacles thereto, or it may describe studies of the clinical effectiveness or cost effectiveness of new knowledge applied in clinical practice across very large and diverse populations. A publication authored by members of the Institute of Medicine’s Clinical Research Roundtable 2 proposed that the first two of these different kinds of translational research, with their different strategies, technologies, time scales, training, and resource requirements, be distinguished as T1 and T2 and that the obstacles encountered be referred to as T1 blocks and T2 blocks, respectively. Subsequently, others have carried this terminology further by designating T3 blocks and even T4 blocks. This terminology has become widely accepted.
Despite the critical role that clinical research in all its forms plays in achieving the nation’s health goals, the clinical research enterprise has for years been underdeveloped. Recent scientific advances have begun to set the stage for a dramatic transformation of our capacity to diagnose, prevent, and treat disease and disability. But accomplishing this transformation will not only require the translation and wide-scale application of these increasingly remarkable basic research advances into health care practice, but will also demand profound changes in individual and group behaviors. The latter will not be achievable without substantially enhancing our understanding of individual and population behaviors, which in turn will require significantly greater investment in the social and behavioral sciences to accomplish the transformation of our health-care system from its primary focus on individual health care to a concentration on individual and population health maintenance .
Health services research, which involves the study of the efficiency, effectiveness, and costs of health care practices and systems, has become indispensable to understanding and informing the future of health care. Despite the promises of a more rational and equitable health care marketplace envisioned in the Health Care Reform Act, health care costs have been rising steadily for decades and consuming an increasing fraction of the nation’s gross domestic product. Expenditures in the United States on health care surpassed $2.3 trillion in 2008, more than three times the $714 billion spent in 1990, and over eight times the $253 billion spent in 1980. This relentless growth in costs, coupled with the aging of the American population, the severe economic recession, and the sharply rising federal deficit, is placing great strains on the private-sector, state, and federal systems used to finance health care, including private employer-sponsored health insurance coverage and public insurance programs such as Medicare and Medicaid.
The quality of the nation’s health care system has been an issue for many years. In 2001, the IOM, launched an effort to examine and recommend improvements in the nation’s quality of care. Successive IOM reports have highlighted the unacceptably poor status of our health care system as a whole, the high frequency and costs of medical errors resulting as much from systemic as individual failures, the almost unique failure of the health care industry in comparison with other sectors of the U.S. economy to adopt and exploit powerful new information technologies, and the shameful and adverse consequences of the continuing problem of the uninsured. Another effort to highlight the quality of health care began in 2003 with the publication of a series of reports by the Agency for Healthcare Research and Quality (AHRQ) that address the state of health care from the perspective of quality and disparity. These reports describe in great detail the impact of the organizational, administrative, financing, safety, access and other deficits of our cobbled-together health-care “system” on individuals, communities, businesses, and the entire nation. The need to address these major problems makes it imperative that “clinical research” be broadly conceived to encompass the assessment of health outcomes, cost-effectiveness, finance, access, information strategies, and other research related to the organization, deployment, utilization and quality of the nation’s health-care systems and services. At this time it is difficult to estimate the impact of the 2010 Patient Protection and Affordable Care Health Care Reform Act on the opportunities and challenges in clinical research.
There are many factors contributing to the continued underdevelopment of the clinical research enterprise. These include: (a) the extra time and expense required for clinical research training along with the inherent complexity, difficulty, and costs of patient-oriented clinical research, and the challenges these pose in competing successfully for sponsored research support, especially from National Institutes of Health, 3 (b) the sharply declining ability to cross-subsidize clinical research from hospital and faculty clinical practice income as a result of the major changes wrought in health care financing over the past 20 years, (c) the debt burden that inclines many physicians in training to forgo clinical research careers for the more likely rewards of clinical practice, and finally, (d) the still uncertain status of the full spectrum of clinical research within the culture of the academic health center, where traditionally, basic science and clinical prowess have often been valued more highly than clinical research. Notwithstanding this formidable array of deterrents, abundant anecdotal evidence indicates that physician-scientists who leave research careers often do so because of insufficient institutional support, a perceived lack of available mentors, licensure regulations, and role models and the attendant discouragement. 4
The need for increased investment in clinical research has been increasingly recognized in diverse funding programs—public, private, and philanthropic—as well as in academic medical and health centers. 5 These issues were addressed by Task Force II, a group assembled by the AAMC to analyze the problems posed by the need to develop the full potential of clinical research. A number of the recommendations of Task Force II have been realized, including the requirement by the accrediting bodies of medical schools (ACME) and residency programs Accreditation Council for Graduate Medical Education (ACGME), respectively, that all medical students and all residents be exposed to the principles of clinical research; having medical schools assume central oversight of clinical research training programs in order to ensure the “protected time” of trainee; and that academic medical centers invest in shared core facilities to support translational and clinical research.
Nevertheless, that this underinvestment continues is indicated by the remarkably small fraction of the total annual expenditures directed to health care that is invested in clinical research. The NIH is the single largest public-sector source of funding for clinical research, and its commitment to clinical research has increased substantially since the late 1990s, driven in part by the recommendations of the highly influential report of the NIH Director’s Panel on Clinical Research, chaired by David G. Nathan and released in December 1997. Although NIH support of clinical research awards during the proceeding two decades had remained largely constant at about 34 percent of total extramural research dollars, the NIH has now launched several well-received training awards for junior and mid-career physician scientists. There are also other support mechanisms, most notably the Clinical and Translational Science Awards (CTSAs), directed by the National Center for Research Resources (NCRR) and launched in 2006, all of which are transforming the quality and quantity of support of physician-scientists in universities and academic health centers. Much of the NIH funding for the CTSA has been recovered from closing down the General Clinical Research Centers (GCRC) program, begun in the 1960s to create a national network of such centers, situated primarily in academic health centers, and targeted initially to support what was then cutting-edge studies of metabolic diseases in human research subjects.
BOX 5-1 Recommendations from the Association of American Medical Colleges Task Force II Report, Promoting Translational and Clinical Science: The Critical Role of Medical Schools and Teaching Hospitals
Recommendation 1: Every future physician should receive a thorough education in the basic principles of translational and clinical research, both in medical school and during residency training.
Recommendation 2: The Liaison Committee on Medical Education (LCME) should add education in translational and clinical research to the requirements for medical school accreditation, and the Accreditation Council for Graduate Medical Education (ACGME) should embed understanding of translational and clinical research within its required core competencies.
Recommendation 3: Training for translational and clinical investigators should comprise completion of an advanced degree with a thesis project (or an equivalent educational experience), tutelage by an appropriate mentor, and a substantive postdoctoral training experience.
Recommendation 4: Sufficient support should be given to new junior faculty who are translational and clinical investigators to maximize their probability of success.
Recommendation 5: Training in translational and clinical research should be accelerated through comprehensive re-structuring so that these scientists can become independent clinicians and investigators at the earliest possible time.
Recommendation 6: Institutions, journals, the NIH, and other research sponsors should take steps to facilitate appropriate academic recognition of translational and clinical scientists for their contributions to collaborative research.
Recommendation 7: The NIH should modify the K23 and K24 awards to enhance their value in supporting clinical and translational research training and mentoring.
As of July 2010, 55 CTSAs had been funded in universities and academic health centers across the country, creating local, regional, and national systems to increase the efficiency and productivity of clinical and translational research and to develop ways to reduce the time it takes for clinical research to become available for use in treatments for patients. The NIH intends that there will be 60 centers when this program becomes fully implemented in 2012, although that number may increase. The CTSA—which require partnerships not only among academic medical institutions and health centers with other components of universities, but also with community hospitals, clinics, and health care practices—are truly creating increasing interest and excitement in clinical research across universities and their community partners, as well as attracting non-biomedical investigators from across universities into multidisciplinary clinical research programs. However, it is too early to predict the ultimate success of this program or whether it will achieve its ambitious goals.
Notwithstanding these positive steps to enhance training and support for physician-researchers in the clinical sciences, the past two decades have been particularly challenging for the funding of all academic health professionals and especially for the support of research activities in the clinical environment that are not clearly tied to specified funding streams. Clinical research, broadly defined, has yet to achieve the breadth and depth of currency it deserves.
To develop the nation’s clinical research capacity will require a sufficient workforce of highly trained clinician investigators in the several health research professions as well as Ph.D.s in the diverse areas of knowledge that are encompassed in the expansive definition of “clinical research.” Building this workforce will require enhanced support across the clinical research disciplines and will especially require supporting clinician-scientists, who must be accomplished in both their clinical and their scientific disciplines.
- DEFINING THE CLINICAL RESEARCH WORKFORCE
The clinical research workforce is as varied as the definition of the field. It consists of individuals with doctorates in the basic sciences, graduates of professional degree programs (mostly M.D.s), graduates of health sciences and public health programs, and dual- or multiple-degree holders. These scientists play an important role in improving the capabilities and the delivery of the nation’s health care, because their research spans the spectrum from discovery to delivery to critical assessment of delivery and the functioning of the health care enterprise. Some areas of research, however, are purely clinical, such as health services, oral health, and nursing, and they will be addressed in later chapters of this report. We also address individuals who fit the expansive Graylyn definition, which embraces research in health services and in the social and behavioral sciences; these topics likewise will similarly be addressed in later chapters of this report.
With this definition in place, it has proved difficult to analyze the specific number of individuals in the clinical research workforce because current workforce databases focus on their current research areas. Therefore, the basic workforce analysis for this report will include Ph.D.s with degrees in the health fields listed in Appendix C , as well as that fraction of the M.D. population in medical school clinical departments that conduct NIH-supported clinical research, along with doctorates with a degree from a foreign institution that are in some way identified as clinical researchers. A major shortcoming of this approach is that does not capture the complete workforce, especially M.D.s who are involved in the design and oversight of clinical trials and as well as those conducting research in non-medical areas of an academic institution or in industrial laboratories.
- EDUCATIONAL BACKGROUND OF THE CLINICAL RESEARCH WORKFORCE
The problems discussed in identifying those currently engaged in clinical research make it difficult to assess the educational background of clinical researchers in the same detail as is done for researchers in the biomedical and behavioral and social sciences, because such studies can only be done for those individuals who are currently participating in or have completed graduate programs that offer a Ph.D. in the clinical fields. The difficulty of such an approach to computing the overall workforce is underscored by the increasing numbers of Ph.D.s (both postdoctoral workers and faculty) from the basic biomedical sciences who are pursuing careers in clinical departments of medical schools and at major teaching hospitals. There are presently more of these Ph.D.s employed in the clinical departments than in basic sciences departments.
Many of these Ph.D.s, however, are likely to be involved in basic biomedical research, which happens to be performed in the labs of M.D. or M.D./Ph.D. scientists involved in biomedical research, albeit in a clinical department environment. At present there is no way of distinguishing between Ph.D.s conducting basic biomedical research from those involved in clinical research (see Figure 5-1 ).
Tenured and tenure-track faculty by type of medical school department, 1990–2009. SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
- GRADUATE STUDENTS
The following discussion draws on data from the National Science Foundation Survey of Graduate Students and Postdoctorates in Science and Engineering and records individuals who are studying in clinical departments (as defined in Appendix C ). The graduate student population in these clinical departments at doctoral-granting institutions grew by 67 percent from 2000 to 2008 (see Figure 5-2 ). The growth in the number of graduate students is greater than that in the other broad fields in this study where the size of the graduate population has increased more slowly. It should also be noted that the robust growth is primarily reflects an increase in the number of female graduate students (see Figure 5-2 ). (Nursing graduate students were excluded from the data, because many of these students will not receive a doctorate, and the pool of students pursuing a doctorate is discussed in the nursing chapter.)
Full-time graduate enrollment in the clinical sciences. SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
However, one has to be very cautious in interpreting the data of Figure 5-2 . Given the fact reported below that only about 2,000 students graduated with a Ph.D. and that the best available evidence suggests that the time to degree was not much more than six years, we have to assume that 40 percent of the students listed in Figure 5-2 either quit or graduated with an M.S. degree. This is supported by the observation (see Figure 5-3 ) that typically 30 to 40 percent of these students were self-supporting, a circumstance more characteristic of master’s students than of those pursuing the Ph.D. The type of financial support the students in the clinical sciences receive is quite different from that in the other fields ( Figure 5-3 ).
Mechanisms of support for full-time graduate students in the clinical sciences. SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
- GRADUATE SUPPORT AND THE ROLE OF THE NRSA IN TRAINING
Figure 5-3 shows the mechanisms of support for full-time graduate students in the clinical sciences. The number of traineeships and fellowships for graduate support in the clinical sciences has held relatively constant, at about 4,000 students each year over the past decade. Support for the increased number of students has largely come from increased teaching assistantships, research assistantships, and, especially, from self funding. The sources of external support have also changed over time with NIH support growing from 10 percent in 1979 to 25 percent in 2008, and non-federal support (excluding self-support) growing from 25 percent to 60 percent over the same time period (see Figure 5-4 ).
Sources of internal and external support of full-time graduate students in the clinical sciences. SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
NIH data for traineeships and fellowships shows a smaller number of National Research Service Awards (NRSAs) slots ranging from 823 in 2005 to 1,035 in 2008 (see Table 5-1 ). Like the other two broad fields in this study, support was rather constant in the 1990s. The decline in 2000 might be the result of higher stipend levels and the fixed NRSA budgets for the training programs. The difference among the numbers shown in Tables 5-1 , 5-2 , and Figure 5-4 is the result of NRSA support through other HHS agencies, primarily AHRQ.
NRSA Predoctoral Trainee and Fellowship Support in the Clinical Sciences (Excluding Health Services).
NRSA Postdoctoral Trainee and Fellowship Support in the Clinical Sciences (Excluding Health Services).
The growth in the graduate population is naturally reflected in the number of doctoral degrees in the clinical sciences fields, with more than a six-fold increase from the early 1970s, and much of the increase involves an increased participation by women. The modest increase in male Ph.D.s is similar to what has happened in the graduate population more generally ( Figure 5-5 ). The citizenship of doctorates in the clinical sciences differ from those in the biomedical sciences with about 16 percent awarded to temporary residents and 6 percent to permanent residents. However, minority participation accounted for about 12 percent of the degrees in 2008.
Doctoral degrees awarded in the clinical sciences. SOURCE: NSF. 2008. Survey of Earned Doctorates. Washington, DC: NSF.
- POSTDOCTORAL FELLOWS
Among Ph.D.s in the three fields, reviewed in this report, those in the clinical sciences are the least likely to have postdoctoral training, because less than 20 percent have traditionally planned such study versus the 30 percent and nearly 70 percent in the behavioral and biomedical sciences, respectively. It is likely that this small number of individuals specifically educated in research in the clinical sciences represents a minimum estimate of those involved in this type of research. One might add two additional categories to this postdoctoral pool, namely (a) individuals educated in basic biomedical research who have shifted to clinical research (and who may be expected to reside in clinical departments) and (b) international postdoctoral researchers trained in clinical research. One might be tempted to compute these numbers from the number of postdoctoral fellows in clinical departments. However it is clear that over the past two decades many Ph.D. postdoctorates and faculty in clinical departments have in fact conducted basic biomedical research, although the exact fraction of the total pool involved in clinical research is impossible to determine from the available data sources. Reflecting this point is the fact that the fraction of all postdoctoral fellows with medical degrees (not resident fellows) in clinical departments decreased from 61 percent in 1983 to 22 percent in 2008, while the number of foreign-educated postdoctoral fellows increased from 25 percent in 1983 to 45 percent in 2008.
Detailed data are not collected on the source of clinical research training support at the postdoctoral level by individual federal agency, but the type of training support, at least in academic institutions is available ( Figure 5-6 ). The traineeships and fellowships portion has been increasing at a slow rate, while in contrast the number of individuals on research grants has increased five-fold since the late 1970s. The NRSA contribution to postdoctoral training support mirrors the general trend for fellows and trainees, but at a lower level, because support is available from sources other than NRSA (see Table 5-2 ).
Academic postdoctoral support in the clinical sciences, 1979–2008. SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
- THE CLINICIAL RESEARCH WORKFORCE
It is extremely difficulty to determine the number of individuals contributing to the clinical research workforce from the available data. The primary sources of data are the NSF Survey of Doctorate Recipients and the AAMC Faculty Roster. In the former dataset Ph.D.s are classified by the area in which they receive their degree as defined according to the fields listed in Appendix C . Since these are considered to be clinical fields, we surmise that they are likely to be conducting clinical research. The AAMC dataset is comprehensive with regard to Ph.D.s in clinical departments in medical schools, but as mentioned earlier conducting research in a clinical department does not imply that the research is clinical. Indeed, it is quite likely that individuals with Ph.D.s in either basic sciences or clinical departments are conducting biomedical research. With this in mind, because individuals with different degrees conduct clinical research and no data source comprehensively captures their activities, it is best to look at the workforce from the perspective of the different degrees that lead to a clinical researcher. The basic clinical workforce, as described by the NSF data, is composed of those 23,282 individuals in 2006 with a Ph.D. in those clinical fields characterized in Appendix C . This number is the potential workforce of individuals employed or seeking employment. Those employed in S&E number 22,229. More current data on the Ph.D.s in the workforce are not available, but the AAMC roster of medical school faculty has data through 2009. The number of M.D.s conducting clinical research in medical schools can be estimated from the number with R01 support in 2006 at about 2,950 and 2,850 in 2009. In addition there were about 1,450 M.D.s in 2006 and 1,550 in 2009 with other non-R01 forms of grant support from the NIH. A longitudinal examination of NIH data 6 over a 40-year time span shows that the number of M.D.s applying for a first R01 grant has remained remarkably flat over most of that interval, and that in 2004 (the last year for which data were available) the number of M.D./Ph.D.s and M.D.s applying for a first R01 had become almost identical. Of course, neither of these counts captures the clinical researchers in the M.D. population that have support from non-NIH sources. As has been stressed repeatedly, even if we can ascertain the total number of M.D.s with R01 support it is still difficult to determine how many of these grants are for basic science alone.
Thus the overall workforce is composed of approximately 12,000 graduate students, 5,000 postdoctoral fellows, some 23,000 Ph.D.s beyond the postdoctoral stage, a number of M.D.s that is poorly defined but probably not more than 1,000, and an unknown number of foreign-born scientists working in this area. The total number then is at least 41,000, of which 24,000 completed their graduate and postdoctoral education (see Figure 5-7 ). The overall clinical workforce, including postdoctoral fellows, has grown significantly from about 2,850 in the early 1970s to the current level. Much of this growth has been in the academic sector, but the industrial sector has also shown a significant increase as is shown in Figure 5-6 . As was the case with the educational characteristics of clinical Ph.D.s, data on their career progression and employment characteristics are only well known for Ph.D.s from U.S. institutions. The steady growth in the academic sector in the past decade has been due in part to the employment of non-tenure-track faculty and other academics (usually research associates) who jointly made up about 40 percent of the faculty in 2006 (see Figure 5-8 ).
Employment sectors of the clinical workforce 1973–2006. SOURCE: NSF. Survey of Doctorate Recipients, 1973–2006. Washington, DC: NSF.
Academic appointments in the clinical sciences, 1973–2006. SOURCE: NSF. Survey of Doctorate Recipients, 1973–2006. Washington, DC: NSF.
Tenured and tenure-track faculty hold the majority of the positions, but their percentage has fallen from around 80 percent in the mid-1980s to 60 percent in 2006. This decline is not surprising, because there has been a movement by institutions toward temporary or soft money positions by institutions in many fields in recent years. This change in the composition of the faculty is confirmed in the AAMC data for medical schools, which show that from 1980 to 2009 the percentage of Ph.D. faculty in non-tenure-track positions in clinical departments increased from about 35 percent to near 60 percent (see Figure 5-9 ).
Tenure status of Ph.D.s in clinical departments in medical schools, 1980–2009. SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
A concern for the clinical research workforce is the increase in the age at which individuals receive their doctorate. From 1986 to 2006 the median age of the workforce has increased from being in the 41 to 43 age cohort in 1986 to the 51 to 52 cohort in 2006 ( Figure 5-10 ). The aging of the clinical workforce is also seen in the data from the AAMC Faculty Roster where the median age of the medical school faculty has increased from about 46 years to 52 years from 1989 to 2009 (see Figure 5-11 ).
FIGURE 5-10
Cumulative age distribution for the clinical workforce. SOURCE: NSF. Survey of Doctorate Recipients, 1973–2006. Washington, DC: NSF.
FIGURE 5-11
Age distribution of Ph.D.s on medical school faculty in clinical departments in 1989, 1999, and 2009. SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
The lower level of interest among postdoctoral training among those Ph.D. holders in fields listed in Appendix C is shown in Appendix Table F-5 and is reflected in the portion of the workforce that is working in postdoctoral positions. Only about 2 percent of the clinical U.S. Ph.D.s have held postdoctoral positions in recent years and almost all are in academic institutions. If the faculty in clinical departments is examined, the picture is somewhat different. There are about 8,000 U.S. citizens or permanent residents in these positions. The difference between in Appendix Table F-6 and Figure 5-12 is probably the result of Ph.D.s with biomedical degrees getting their training in clinical departments.
FIGURE 5-12
Clinical postdoctoral fellows by degree type. SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
Table F-5 also shows that minorities only represented 8.6 percent of the clinical research population in 2006, even though their numbers grew from about 100 in 1973 to a little more than 1,100 in 2006. This is better than in the biomedical sciences and about the same as in the behavioral and social sciences. The data show, as they did for the other fields, a small number of temporary residents in the research population, but since the data reflect only those individuals who were trained in U.S. institutions, there may be a larger percentage of temporary residents in the workforce with foreign doctorates.
Although the number of M.D. clinical researchers is not known exactly it appears that in recent years individuals with a Ph.D. have dominated the field. In the 1970s only 2,600 Ph.D.s made up the workforce, and only a few hundred degrees were awarded each year. There did not appear to be a change in the number of M.D.s in clinical research since the 1970s, even though the Ph.D. workforce grew by a factor of seven during that time. There may be several reasons for this change, but a primary one is probably the increased educational debt of medical school graduates. Except for graduates of dual-degree (e.g., M.D./Ph.D. or D.D.S.//Ph.D.) programs, most physicians and dentists today begin their professional careers with sizable educational debts.
In 2009, the AAMC reported that the average educational debt of current graduates was $156,456, with 79 percent of the graduates having a debt of at least $100,000 and 58 percent having a debt of at least $150,000. The level of educational debt for dental students is comparable to that of medical students. In 2006, the average it was more than $130,571, and 72 percent had an educational debt of more than $100,000. The increased debt results from the practice in dental schools that requires students to purchase their dental instruments during their clinical training. Although health care professionals are permitted to postpone payments on their student loans during NRSA or other authorized research training programs, this option may not be widely used, and even if it were used, additional training places financial and other burdens on a young physician.
Congress has authorized several educational loan repayment programs for M.D.s who enter clinical research training programs, for minority M.D.s who pursue clinical training, and for others pursuing designated career paths. There are perhaps a half-dozen different programs authorized, and the NIH has been vigorous in making these programs known to successful candidates. The explicit purpose of these programs is to mitigate educational debt burdens for M.D.s pursuing clinical research training. M.D. graduates from clinical research training programs (e.g., those receiving one of the several K awards) must have protected time to develop their independent research careers, an increasingly difficult situation in today’s increasingly competitive health care markets. Another obstacle is the limitation on salaries for NIH-funded physician-investigators. The cap is set annually set by Congress to be no more than that of an executive grade; this grade has varied in recent years between level II and level I. It is now set at $199,700, and although that is not an insignificant amount, it is below what many practicing clinicians or medical faculty can earn.
Dual-Degree Training
In addition to predoctoral and postdoctoral program support in the clinical sciences through the NRSA mechanism, dual-degree programs are another attractive option for health care professionals seeking clinical research training. The NIH currently, has three dual-degree training programs: (1) the Medical Scientist Training Program (MSTP), (2) individual M.D./Ph.D. fellowships, and (3) the Dental Scientist Training Program (DSTP).
These dual-degree programs are very attractive, because they provide students with several career options, and the level of educational debt that students are left with is much lower than that for regular M.D. students. The MSTP in the National Institute of General Medical Sciences (NIGMS) is the largest and oldest programs, dating back to 1964, and today it funds 880 students training at 35 medical schools and universities. An additional 31 MSTP trainees are supported by other institutes. Offering fellowships for M.D./Ph.D. training is more recent; they were instituted in 1989 by the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse to encourage dual-degree training in the areas of mental health, behavior, and neuroscience. The fellowship program is much smaller in scale, supporting about 140 new students each year. The latest type of dual-degree training to be introduced is the DSTP, which was created following the recommendations from the 1994 study of the NRSA program. The National Institute of Dental and Craniofacial Research supports about 90 dual-degree dental students through the T32 and F30 DSTP—in 16 different dental schools (only 2 of these schools do not have T32 DSTP trainees).
A student in a typical M.D./Ph.D. program begins intensive research training after the second year of medical school. At this point in their training, the students have had little exposure to clinical medicine and the challenges and research opportunities that are inherent therein. After three-plus years completing work required for the Ph.D. degree, the students return to the medical curriculum for the third and fourth years. For dual-degree graduates who elect to pursue full clinical specialty training, an additional three to five, or more, years typically ensue before the individuals can turn their attention fully to research. At that point, to begin an additional formal program of clinical research training is unappealing.
The M.D./Ph.D. programs were envisioned as a way to bring more M.D.s into clinical research, but in practice relatively few participants receive research training in clinical research methods, and only about 20 percent of the M.D./Ph.D.s actually go on to pursue clinical research careers. Educational debt does not appear to be the reason, because their debt averaged about $15,000 in 2006. Many have argued that these programs are not effective in training clinical researchers because of their structure. An analysis in 1996 of the fields of study chosen by MSTP participants found that nearly 60 percent of graduates from the late 1980s and early 1990s had their Ph.D.s in five basic science fields: biochemistry, neuroscience, molecular biology, cell biology, and pharmacology. As a consequence the work they were exposed to in their Ph.D. program was focused on basic research, and this attracted them to a research career in the biomedical sciences. As a result in their subsequent research careers, MSTP graduates focused almost entirely on laboratory-oriented research, albeit typically in clinical departments and in areas of relevance to that clinical discipline, and they sought NIH funding for such research projects at the same rate as Ph.D.s.
Recognizing this problem NIGMS has recommended that institutions provide broader opportunities within the M.D./ Ph.D. training mechanism. The institute issued new guidelines for the MSTP that urged medical schools with such training grants to extend their programs in order to give students “a breadth of doctoral research training opportunities” in fields including computer science, the social and behavioral sciences, economics, epidemiology, public health, bioengineering, biostatistics, and bioethics. However, most M.D./Ph.D. programs have been slow to respond, and there has been little change in the descriptions of the programs. And in most cases, the basic structure of two years/three years/two years persists.
In addition to formal dual M.D./Ph.D. programs, other approaches are being tried to attract M.D.s to clinical research. Examples include master’s level programs in specific clinical areas, which are becoming popular in some research-oriented medical schools and which may be designed to provide academic formal training in such areas as quantitative and methodological principles of medical genomics, epidemiology, biostatistics, clinical trial design and analysis, etc. These programs appear to be very attractive to medical students and may encourage them to pursue careers as physicians in clinical research.
Clearly, identifying optimal training mechanisms for attracting medical students to clinical research, and then structuring effective training programs to prepare the students and graduates for successful clinical research careers, remains a large challenge for the biomedical community and the funding agencies. Toward this end, the recent adoption by the ACME and the ACGME of recommendations from the AAMC’s Task Force II on Translational and Clinical Research, viz., that medical students and residents should be exposed to the basic principles of translational and clinical research and to the research challenges and opportunities therein, may over time increase the population of medical graduates with a keen interest in pursuing clinical research careers. Finding mechanisms that will encourage students in these dual-degree programs to conduct clinical research continues to be a challenge.
- RECOMMENDATIONS
Recommendation 5–1: The total number of NRSA positions awarded should remain at least at the 2008 level. Furthermore, training levels after 2008 should be commensurate with the rise in the total extramural research funding in the biomedical, clinical, and behavioral and social sciences. A decline in extramural research would also call for a decline in training.
Recommendation 5–2: The NIH, in consultation with academic medical leadership, should exercise leadership in identifying better training mechanisms for attracting medical students into translational and clinical research, and the NIH should fund pilot programs designed to implement promising new approaches to accomplishing that objective.
Summary of Report of the Graylyn Development Consensus Conference, November 1998, from Report 13 of the Council on Scientific Affairs (I-99), Update on Clinical Research. Available online at: http://www .ama-assn.org /ama/pub/article/2036-2392.html .
Sung, N.S., W.F. Crowley, Jr., M. Genel, P. Salber, L. Sandy, L.M. Sherwood, S.B. Johnson, V. Catanese, H. Tilson, K. Getz, E.L. Larson, D. Scheinberg, E.A. Reece, H. Slavkin, A. Dobs, J. Grebb, R.A. Martinez, A. Korn, and D. Rimoin. 2003. Central challenges facing the national clinical research enterprise. JAMA 289:1278–1287.
Kotchen, T.A., T. Lindquist, A. Miller Sostek, R. Hoffmann, K. Malik, and B. Stanfield. 2006. Outcomes of National Institutes of Health peer review of clinical grant applications. Journal of Investigative Medicine, 54:13–19.
Dickler et al. 2007. “New Physician-Investigators Receiving National Institutes of Health Research Project Grants.” JAMA 297(22):2496–2501.
AAMC. 2006. “Promoting Translational and Clinical Science: The Critical Role of Medical Schools and Teaching Hospitals.” Washington, DC: AAMC; and Dickler, H, Korn, D, and Gabbe, SG, PLoS Med. 2006;3. e378.
- Cite this Page National Research Council (US) Committee to Study the National Needs for Biomedical, Behavioral, and Clinical Research Personnel. Research Training in the Biomedical, Behavioral, and Clinical Research Sciences. Washington (DC): National Academies Press (US); 2011. 5, Clinical Sciences Research.
- PDF version of this title (3.5M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Clinical Sciences Research - Research Training in the Biomedical, Behavioral, an... Clinical Sciences Research - Research Training in the Biomedical, Behavioral, and Clinical Research Sciences
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
In Idaho, don’t say ‘abortion’? A state law limits teachers at public universities, they say
Idaho's public university professors say a law barring state employees from ‘promoting’ or ‘counseling in favor of’ abortion limits their ability to teach..

This story was published in partnership with the Center for Public Integrity , a newsroom that investigates inequality.
University of Idaho student Bergen Kludt-Painter started school in August 2022, a few months after a U.S. Supreme Court decision struck down Roe v. Wade. Soon after, abortion was banned in Idaho in almost all instances.
The political science major was eager to discuss the precedent-shattering case in class, but, she said, “we talked about everything except for abortion.”
During a political science course on how to write a research paper, her professor said he could not give her feedback on her chosen topic — abortion. The issue didn’t come up in her other political science classes either, even as state after state changed their abortion laws. Nor did abortion get mentioned in her Introduction to Women’s Gender and Sexuality Studies course.
“It wasn’t discussed,” she said, “which I found odd, personally, because it feels like something that would be relevant to talk about in a class like that.”
But few, if any, public university professors in Idaho are talking about or assigning readings on abortion these days. That’s due to a 2021 law that makes it illegal for state employees to “promote abortion” or “counsel in favor of abortion.” Professors have said those two phrases put them at risk of violating the law, known as the No Public Funds for Abortion Act , just for discussing abortion in class. The possible penalties include significant fines and even prison time.
Six named University of Idaho professors and two faculty unions filed a lawsuit against the state in August for violating their First Amendment right to free speech and academic freedom and their 14 th Amendment right to a clearly worded law. Lawyers from the American Civil Liberties Union are representing the professors.
“The more I heard about it, the more worried I was that I really can't teach my class in a responsible way without putting myself at risk,” said Aleta Quinn, an associate professor of philosophy for the University of Idaho and a plaintiff in the case.
Quinn teaches a course in biomedical ethics that typically features readings and class discussions about abortion. When she saw that the highest penalty for breaking the law was 14 years in prison, “I decided I would not — I couldn't — teach the subject of abortion.”
The bulk of the arguments in the case center on the due process clause of the 14th Amendment, which the Supreme Court has interpreted to mean that a statute “so vague that men of common intelligence must necessarily guess at its meaning” violates a person’s right to fair treatment under the law.
The case also raises an important First Amendment question about protections for academic freedom in America: Are public university professors exempt from laws that could otherwise govern the speech of state employees?
Supreme Court precedent suggests the government has significant leeway to regulate the speech of the people it employs while they are performing their professional duties.
Still, the most recent court opinion on the issue left open the question of how much that speech could be regulated for one key group: public university professors.
“We need not, and for that reason do not, decide whether the analysis we conduct today would apply in the same manner to a case involving speech related to scholarship or teaching,” then Justice Anthony Kennedy wrote in the 2006 majority opinion in Garcetti v. Ceballos .
The Supreme Court has not yet returned to that decision.
“So establishing that legal principle, in and of itself, is an important endeavor for those [Idaho] professors,” said Helen Norton, a professor of constitutional law at the University of Colorado who is not involved in the case.
Interestingly, none of the professors suing in the Idaho case are nursing instructors or even biology professors. They aren’t teaching anyone about the physical nature of abortion. Their concerns, as scholars of subjects like philosophy, political science, gender studies and English, are focused on whether they can speak about abortion as an ethical, political and historical issue.
For example, a sworn statement by an English professor named in the case explained that he used to assign Sallie Tisdale’s 1987 Harper’s Magazine essay, “We Do Abortions Here,” in one of his classes. The essay about her work as a nurse in an abortion clinic explores the complicated morality of helping women end their pregnancies. It’s also considered to be an example of powerful writing. He has now removed it from his syllabus.
Lawyers for the state of Idaho agree that professors fall under a different regulatory framework than other public employees when it comes to what they are permitted to say in the course of their duties. In their motion to dismiss the lawsuit, the state’s attorneys concede that settled law establishes protections for academics’ speech.
A month after the case was filed, Idaho’s attorney general, a defendant in the case, issued a non-binding opinion that the law does not apply to the “teaching or scholarship” of public university professors. If it did, Raul Labrador wrote, “the prohibition would likely be unconstitutional.”
A spokesperson for the attorney general’s office declined to respond to repeated requests for an interview.
Republican state Rep. Bruce Skaug, the sponsor of the No Public Funds for Abortion Act, later introduced legislation to create a specific protection for classroom discussion of abortion, but it failed to pass. Skaug did not respond to requests for an interview.
Rather than arguing about the First Amendment claim, lawyers for the state focused on the professors’ assertion that the law is unconstitutionally vague under the 14th Amendment.
“Plaintiffs have alleged that there is a law that prohibits them from teaching college courses concerning abortion, producing scholarship in favor of abortion, and grading papers concerning abortion,” the state’s lawyers write in the November motion to dismiss. “There is no such law in the state of Idaho.”
The state’s attorneys argue that any reasonable reader of the law would see that the statute refers only to the act of advising a specific person to have an abortion. As written, they argue the law could not be interpreted as a prohibition on, say, giving a strong grade on a writing assignment where the student had chosen to make an ethical argument in favor of abortion.
Because of the attorney general’s opinion and the “plain language” in the law, the state’s lawyers say the professors are imagining themselves to be at risk of prosecution when, in reality, no such risk exists.
Lawyers for the plaintiffs disagree. Federal courts have issued rulings with varied interpretations of the word “promote.” And the lawsuit offers numerous hypothetical situations in which a professor could be prosecuted for promoting abortion even if that were not their intent.
Norton, the University of Colorado law professor, said it was reasonable for the professors to question the law’s language.
“That’s shown so far to be the focus of the dispute — what does ‘promoting’ or ‘counseling’ mean?” she said. “And it seems like that’s an important thing to nail down.”
Because there’s no definition of the terms in the law, she said, “there’s absolutely room for folks to argue about whether or not we should be quick or slow to interpret broadly or narrowly.”
The current case challenging Idaho’s No Public Funds for Abortion Act does not directly include the state’s many other public employees, like social workers and school counselors, who are unlikely to qualify for any special First Amendment protections.
Public school teachers in the K-12 system do not have the same level of academic freedom protections as professors, either. But a high school history teacher could face the same concerns that speaking about abortion in class could be construed as either promoting or counseling in favor of it.
However, those employees would no longer have their speech curtailed if the professors prevail and a court strikes the law down.
That matters because Idaho’s restrictions surrounding abortion are so tight at this point that nearly every other action connected to encouraging abortion has been outlawed some other way. At this point, regulating how public employees speak about abortion is arguably the only thing the No Public Funds law still does. Opponents of the law have questioned why the state is fighting to uphold it, if not to limit speech about abortion.
Wendy Heipt, a reproductive rights attorney with Legal Voice who is working on a challenge to Idaho’s ban on helping minors travel to receive abortions without parental consent, calls the state Legislature “extremist.” She worries that the state has become a “testing ground” for the far right.
“You would notice [these laws] in Texas,” where more than 30 million people live, she said, “not Idaho,” home to less than 2 million.
Indeed, copycat travel ban bills restricting the movement of minors seeking an abortion were introduced in Alabama, Tennessee, Mississippi and Oklahoma this session, according to the Guttmacher Institute, a research and policy organization that works to advance sexual and reproductive health and rights.
No one interviewed for this story had heard about a copycat law that raised the same combination of First and 14th Amendment concerns as Idaho’s No Public Funds measure.
A judge heard the professors’ case in Idaho District Court in April. His decision on whether the preliminary injunction they’ve asked for will be granted is expected soon. The judge could also decide to dismiss the case, as the attorney general’s office has proposed. If the judge doesn’t dismiss the case, he will likely ask both parties to reconvene for another hearing before a final resolution.
In the meantime, professors are continuing to stay quiet about abortion in class.
For someone dedicated to the free exchange of ideas like Quinn, that silence feels wrong. When she started teaching, her goal was to make the world a slightly better place by helping young people learn how to think, not what to think. She feels like she’s not fulfilling her duty to her students by ignoring an ethical debate as relevant to daily life as abortion.
“Philosophy is thinking critically about ideas and concepts and arguments, and considering which arguments are stronger and which are weaker and how they apply and all their implications,” Quinn said. “My goal is to enable people to have the skills to evaluate positions on their own.”
Kludt-Painter, the University of Idaho student, is the president of the Young Democrats. But her issues with the No Public Funds law weren’t about the politics of abortion. It’s an education she wants and feels she is being at least partially denied.
“It's a form of censorship,” she said. “College students should be able to handle hearing about these difficult topics. And educators should be able to discuss them and have a free exchange of ideas without being worried about getting fired or having criminal charges be brought against them.”
Hayden Cassinelli, the vice president of the College Republicans at the University of Idaho, said the topic of abortion came up in one of his classes recently but was "quickly avoided" when a teaching assistant told students he couldn’t discuss it.
Despite Cassinelli’s opposition to abortion, the sophomore education major believes the topic should be discussed in class. He doesn’t think the No Public Funds law prevents such discussions. But he supported his university’s decision to issue guidance to professors in fall 2022, urging them to be cautious when talking about abortion.
"Given many professors' thoughts on abortion — including the fact that some of them may advocate for it and [encourage] a student to commit a crime — a temporary hold on any abortion-related discussion until legal clarity is established is a sound decision," Cassinelli wrote in an email.
Kludt-Painter thinks professors are just trying to protect their jobs when they avoid discussing abortion in class, but she wishes they didn’t feel that way.
“It takes away from the whole academic freedom thing that post-secondary education is supposed to be about,” she said.
share this!
May 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
What fire ants can teach us about making better self-healing materials
by Binghamton University

Fire ants form rafts to survive flooding, but how do those bonds work? And what can we learn from them? A Binghamton University, State University of New York professor is researching those questions to expand our knowledge of materials science.
When flooding hits a region where fire ants live, their survival response is to latch together to form a buoyant " raft " that floats and keeps the colony united. Think of it like a condensed, adaptive material where the building blocks—individual ants—are actually alive.
Binghamton University Assistant Professor Rob Wagner led a study as part of the Vernerey Soft Matter Mechanics Lab at University of Colorado Boulder in which researchers investigated the adaptive response of these living rafts. The goals are to understand how they autonomously morph and change their mechanical properties, and then incorporate the simplest and most useful discoveries into artificial materials.
"Living systems have always fascinated me, because they achieve things that our current engineered materials cannot—not even close," he said. "We manufacture bulk polymeric systems, metals and ceramics, but they're passive. The constituents don't store energy and then convert it to mechanical work the way every single living system does."
Wagner sees this storage and conversion of energy as essential to mimicking the smart and adaptive behaviors of living systems.
In their most recent publication in the Proceedings of the National Academy of Sciences , Wagner and his co-authors at University of Colorado investigated how fire ant rafts responded to mechanical load when stretched, and they compared the response of these rafts to dynamic, self-healing polymers.
"Many polymers are held together by dynamic bonds that break, but can reform," Wagner said. "When pulled slowly enough, these bonds have time to restructure the material so that—instead of fracturing—it flows like the slime our kids play with, or soft-serve ice cream. When pulled very fast, though, it breaks more like chalk. Since the rafts are held together by ants clinging onto one another, their bonds can break and reform. So, my colleagues and I thought they'd do the same thing."
But Wagner and his collaborators discovered that no matter what speed they pulled the ant rafts, their mechanical response was nearly the same, and they never flowed. Wagner speculates that the ants reflexively tighten and prolong their holds when they feel force because they want to stay together. They either turn down or turn off their dynamic behavior.
This phenomenon of bonds that grow stronger when force is applied to them is called catch bond behavior, and it likely enhances cohesion for the colony, which makes sense for survival.
"As you pull on typical bonds with some amount of force, they're going to let go sooner, and their lifetime goes down—you're weakening the bond by pulling on it. That is what you see in almost any passive system," Wagner said.
"But in living systems, because of their complexity, you can sometimes have catch bonds that hold on for longer durations under some range of applied force. Some proteins do this mechanistically and automatically, but it's not like the proteins are making a decision. They're just arranged in such a way that when a force is applied, it reveals these binding sites that latch or 'catch.'"
Wagner believes that mimicking these catch bonds in engineered systems could lead to artificial materials that exhibit autonomous, localized self-strengthening in regions of higher mechanical stress. This could enhance the lifetimes of biomedical implants, adhesives, fiber composites, soft robotics components and many other systems.
Collective insect aggregations like fire ant rafts already are inspiring researchers to develop materials with stimuli-responsive mechanical properties and behaviors. A paper in Nature Materials earlier this year—led by the Ware Responsive Biomaterials Lab at Texas A&M and including contributions from Wagner and his former thesis advisor, Professor Franck J. Vernerey—demonstrates how ribbons made of special gels or materials called liquid crystal elastomers can coil due to heating, and then entangle with each other to form condensed, solid-like structures that were inspired by these ants
"A natural progression of this work is to answer how we can get the interactions between these ribbons or other soft building blocks to 'catch' under load like the fire ants and some biomolecular interactions do," Wagner said.
Journal information: Proceedings of the National Academy of Sciences , Nature Materials
Provided by Binghamton University
Explore further
Feedback to editors

Bioengineered enzyme creates natural vanillin from plants in one step
5 minutes ago
Nanocarriers loaded with DNA relieve back pain, repairs damaged disk in mice

'Forever chemicals' found to rain down on all five Great Lakes
6 minutes ago
Study highlights pathoblockers as a future alternative to antibiotics
12 minutes ago

Floating robots reveal just how much airborne dust fertilizes the Southern Ocean—a key climate 'shock absorber'
16 minutes ago
Research team achieves rapid and reliable room-temperature phosphorescence chiral recognition
30 minutes ago
Low-temperature pulse irradiation technique enables flexible optoelectronic devices
41 minutes ago
A second chance for a new antibiotic agent
Nanobubble research to improve green hydrogen production
43 minutes ago
Quantum experts review major techniques for isolating Majoranas
52 minutes ago
Relevant PhysicsForums posts
Most dangerous chemicals.
16 hours ago
Potassium Iodide as a catalyst for Hydrogen Peroxide
19 hours ago
Very confused about Naunyn definition of acid and base
Ideas for a project in computational chemistry, why don't hydrogen ions have osmotic activity in living organisms.
May 6, 2024
Can you eat the Periodic Table?
Apr 23, 2024
More from Chemistry
Related Stories

Ant behavior inspires autonomous material assembly research
Jan 5, 2024

The physics of fire ant rafts could help engineers design swarming robots
Mar 2, 2022

Fire ants' raft building skills react as fluid forces change
Nov 26, 2019

Fire ant colonies could inspire molecular machines, swarming robots
Nov 5, 2018

Fire ants found to create 'appendages' on self-made rafts when put in water
Jul 1, 2021

Bio-inspired materials showcase potential for protective equipment and textiles
Mar 2, 2024
Recommended for you

Data-driven model rapidly predicts dehydrogenation barriers in solid-state materials
Temperature, time and blueberry wine: Researchers examine fermentation's effects on health-promoting compounds
A novel multifunctional catalyst turns methane into valuable hydrocarbons
21 hours ago

First direct imaging of radioactive cesium atoms in environmental samples
22 hours ago

Researchers identify drug compounds that can reduce prion protein levels in infected cells
23 hours ago

Bio-based resins could offer recyclable future for 3D printing
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

COMMENTS
Biomedical research is the science that seeks to prevent and treat diseases in people and animals. It involves various fields of biology and chemistry, and uses biotechnology techniques and animal models.
The University of Florida Cancer and Genetics Research Complex is an integrated medical research facility. Medical research (or biomedical research ), also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion ...
Basic biomedical research, which addresses mechanisms that underlie the formation and function of living organisms, ranging from the study of single molecules to complex integrated functions of humans, contributes profoundly to our knowledge of how disease, trauma, or genetic defects alter normal physiological and behavioral processes. Recent advances in molecular biology techniques and ...
Biomedical sciences. A biochemist engaged in bench research. Biomedical sciences are a set of sciences applying portions of natural science or formal science, or both, to develop knowledge, interventions, or technology that are of use in healthcare or public health. [1] Such disciplines as medical microbiology, clinical virology, clinical ...
Biomedical scientists bridge the gap between the basic sciences and medicine. The Ph.D. degree is the gateway to a career in biomedical research. Biomedical scientists: Think outside the box and are innovators. Are critical and analytical thinkers. Get excited by discovering new things. Look at biology and see previously unrecognized patterns.
The goal of basic biomedical research is to provide comprehensive and detailed understanding of the mechanisms that underlie the development and normal function of humans and other living organisms and thereby gain insights into the pathological and pathophysiological mechanisms that cause disease. A detailed understanding of these mechanisms and pathways is essential for identifying potential ...
Biomedical Research. Few topics in biomedical research, other than research funding and the JIF, the latter of which is a direct output of the peer review process, have galvanized opinion in biomedical researchers to the same degree as peer review with numerous erudite and often emotive statements that reflect on the innate bias of reviewers and editors, inconsistencies in reviewing standards ...
Biomedical research is the study of human physiology and disease using the principles of the physical sciences. It involves clinical trials, records research, and other methods that may have social, psychological, or physical risks for participants.
Cohort studies in a population group in which there has been exposure (e.g. industrial workers) Study of multiple exposures, such as the combined effect of oral contraceptives and smoking on myocardial infarction. Case control studies. Study of multiple end points, such as mortality from different causes.
Biomedical Research Current research. Following World War II, federal support for biomedical research was greatly expanded and so was the role of the National Institute of Health, which was renamed the National Institutes of Health to reflect the growth of research functions. Today NIH encompasses seventeen research institutes, two research ...
Biomedical research focuses on understanding how every part of the human body works—right down to our cells. By studying the normal and abnormal workings of the body at the molecular, cellular, organ system, and whole-body levels, biomedical research leads to new: Ways of identifying and diagnosing disease
A biomedical scientist is a scientist trained in biology, particularly in the context of medical laboratory sciences or laboratory medicine.These scientists work to gain knowledge on the main principles of how the human body works and to find new ways to cure or treat disease by developing advanced diagnostic tools or new therapeutic strategies.The research of biomedical scientists is referred ...
A simple definition for biomedical research is: postgraduate or doctoral research in any field related to medicine and biology that has the potential to heal and improve lives. What is a biomedical scientist? According to the Bureau of Labor Statistics, a biomedical scientist conducts research to improve human health. Biochemists focus on the ...
Biomedical science is one of the broadest areas of modern science and underpins much of modern medicine - from determining the blood requirements of critically ill patients to identifying outbreaks of infectious diseases to monitoring biomarkers in cancer. Biomedical science staff mostly work in healthcare laboratories diagnosing diseases and ...
Biomedical research depends largely on images. The huge advances in microscopy, imaging, visualization, and other tools have launched a plethora of interesting research not possible to glimpse 10 or 20 years ago. To visualize and manipulate images in the biomedical sciences, several software packages have been developed.
Philosophy of Biomedicine. First published Thu Apr 9, 2020. Despite the simple name, biomedicine is not simply the area of overlap between biology and medicine. It is a framework, a set of philosophical commitments, a global institution woven into Western culture and its power dynamics, and more. Biomedicine is the umbrella theoretical ...
Definition. Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or ...
Ethical lapses and emerging quandaries in biomedical research and practice have provided continual reminders of the need to emphasize scientific and medical ethics in the training of students and the oversight of researchers and health care providers. ... The White House Office of Science and Technology Policy finalized a federal definition of ...
Biomedical scientists uses scientific methods to investigate biological processes and diseases that affect humans and animals. They conduct experiments, analyze data, and interpret findings to improve our understanding of diseases and develop new treatments and cures. They also ensure the safety and efficacy of drugs and medical devices through clinical trials and regulatory processes.
Biomedical research is the branch of science that focuses on the prevention and treatment of diseases in animals and human beings. It involves various aspects of both the physical and life sciences, and uses biotechnology methods to study diseases and biological processes.
In March 2024, President Joe Biden signed an Executive Order directing the most comprehensive set of executive actions ever to expand and improve women's health. Its actions prioritize the integration of women's health throughout the federal research portfolio and budget, galvanizing new research initiatives on various topics, including menopause and women's midlife health.
Single-cell RNA sequencing (scRNA-seq) technologies are revolutionizing biomedical research by providing comprehensive characterizations of diverse cell populations in heterogeneous tissues [1, 2].Unlike bulk RNA sequencing (RNA-seq), which measures the average expression profile of the whole tissue, scRNA-seq gives the expression profiles of thousands of individual cells in the tissue [3,4,5 ...
They estimated that 4.4% of children in the U.S. have a genetic condition—roughly 0.5% higher than what the 2021 study showed. Creel said her team measured caregivers' employment by using one ...
Such is the case for the term "translational research," which is defined by the European Society of Translational Medicine as an interdisciplinary branch of biomedical science supported by 3 main pillars: benchside, bedside, and community ( 1 ). Defined in this way, translational research involves the application of scientific observations ...
Image by Wenhan Cheng from Pixabay. On 1 May, the United States House of Representatives passed a bill that would codify the IHRA's definition of anti-Semitism, and the Senate - the upper house of Congress - is now expected to debate and vote on the bill. This has drawn some debate and controversy, as the IHRA's definition has been ...
No meat, poultry or seafood, but dairy and eggs are OK. The new review analyzed 48 metanalyses that had investigated the impact of eating a vegetarian or vegan diet on the development of cancer ...
Reseach in the clinical sciences helps put into practice the discoveries that arise from the research in the fields described in the three previous chapters. Because the term "clinical research" is used to cover such a broad and diverse array of activities, its definition has proved to be controversial, primarily over the issue of whether the research does or does not require direct ...
Idaho's public university professors say a law barring state employees from 'promoting' or 'counseling in favor of' abortion limits their ability to teach. This story was published in ...
Collective insect aggregations like fire ant rafts already are inspiring researchers to develop materials with stimuli-responsive mechanical properties and behaviors. A paper in Nature Materials ...