An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Exploring the relationship between the Mediterranean diet and weight loss maintenance: the MedWeight study
Dimitrios poulimeneas, costas a anastasiou, inês santos, james o hill, demosthenes b panagiotakos, mary yannakoulia.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Mary Yannakoulia, [email protected]
Issue date 2020 Oct 28.
This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Weight loss maintenance is crucial for obesity management, yet optimal dietary patterns for this period are not established. We aimed to explore the relationship between adherence to the Mediterranean diet and weight loss maintenance. Sample includes 565 adults (62 % women) of the MedWeight study. Eligible volunteers were those reporting intentional weight loss of ≥10 %, starting from a BMI ≥ 25 kg/m 2 , over 12 months prior to enrolment. Based on current weight, participants were characterised as maintainers (≤90 % maximum weight) or regainers (>95 % maximum weight). Socio-demographics and weight history were recorded. Dietary intake was assessed by two non-consecutive 24-h recalls within 10 d and analysed in energy, macronutrient and food group intakes. Adherence to the Mediterranean diet was assessed with the Mediterranean Diet Score (MedDietScore) (range 0–55, greater scores showing higher adherence). Protein intake was higher in maintainers than in regainers ( P < 0·001). When MedDietScore quartiles were considered, a linear trend for weight loss maintenance was revealed ( P < 0·05). After adjustment for basic demographic characteristics, being in the third or fourth quartile of the MedDietScore ( v. first) was associated with 2·30 (95 % CI 1·29, 4·09) and 1·88 (95% CI 1·10, 3·22) increased odds of maintenance. Regarding individual MedDietScore components, only fruit intake is associated with increased odds for maintenance (1·03 (95% CI 1·01, 1·06)). The leave-one-out approach revealed that at least six MedDietScore components were essential for the observed relationship. Higher adherence to the Mediterranean diet was associated with 2-fold increased likelihood of weight loss maintenance. Future studies should replicate these findings in non-Mediterranean populations as well.
Keywords: Dietary patterns, Mediterranean diet, Obesity, Weight control, Weight regain
The term Mediterranean diet is used to describe the traditional dietary pattern of the people residing in the Mediterranean basin, characterised by abundance of plant foods such as fruits, vegetables, either as main or side dish, cereals, including bread, legumes, nuts and seeds. Olive oil is the principal source of fat. The Mediterranean diet also includes moderate amounts of dairy products, low to moderate amounts of fish and poultry, red meat in low amounts and wine, consumed modestly, mostly with meals ( 1 , 2 ) . Strong evidence supports that higher adherence to this traditional dietary pattern is associated with greater longevity ( 3 ) and acts both as a preventive and therapeutic target for many prevailing non-communicable diseases ( 4 - 6 ) .
The Mediterranean diet has also been utilised as a whole-diet approach model within the framework of obesity prevention and management. Higher adherence to the Mediterranean diet has been found to be associated with lower body weight ( 7 ) , to prevent long-term weight gain ( 8 - 10 ) and to produce significant weight loss, with or without energy restriction ( 11 ) . Nevertheless, much less is known regarding its relationship with long-term weight loss maintenance.
Although weight loss maintenance is considered an integral part of obesity management, optimal dietary patterns for successful weight loss maintenance have not been established ( 12 ) . Few studies have evaluated different diets during the maintenance of weight loss. We have previously observed that an a posteriori -defined healthy dietary pattern was significantly associated with weight loss maintenance ( 13 ) . Similar results have been found for high-protein diets, with or without alterations in glycaemic index ( 14 , 15 ) . Two interventional weight loss studies with 6 months of follow-up suggest that adhering to the Mediterranean diet after weight loss may favour maintenance outcomes ( 16 , 17 ) . However, to the best of our knowledge, no study to date has examined the effects of the Mediterranean diet on maintaining weight loss for prolonged periods of time (i.e. more than 12 months). Our hypothesis was that higher adherence to the Mediterranean dietary pattern in the post-dieting period is associated with favourable outcomes for the maintenance of reduced body weight. To address this hypothesis, we explored the relationship between adherence to the Mediterranean diet and long-term weight loss maintenance in the MedWeight study ( 18 ) , a large cohort of weight loss maintainers and regainers, reporting lifestyle data for at least 12 months after initial weight loss.
Study design and population
The MedWeight study is an ongoing Greek registry of individuals with a history of overweight or obesity, residing in Greece. The detailed study protocol has been published elsewhere ( 18 ) . In short, inclusion criteria were (i) age 18–65 years, (ii) a lifetime maximum BMI ≥ 25 kg/m 2 and (iii) intentional weight loss of at least 10 % of maximum weight, in the period >12 months prior to enrolment. Currently, or within the previous year, pregnant women were excluded from sampling. Participation in the study was conducted through the study’s website ( medweight.hua.gr ). According to their current weight, the study’s algorithm automatically classified participants as maintainers, for attaining current weight ≤90 % of their maximum weight, or regainers, for attaining current weight >95 % of their maximum weight. To avoid overlap between groups, individuals with a weight of 90–95 % of their maximum weight were excluded from the study. This decision was based on (i) the weight loss maintenance definition by Wing & Hill ( 19 ) , suggesting that successful maintainers are those who maintain a loss of at least 10 % of their maximum weight for over 12 months and (ii) ascertaining that regainers were attaining a current body weight below the clinically meaningful weight loss range of 5–10 ( 20 ) . Then, eligible participants were asked to report on their current characteristics and habits (those at the time of recruitment). For the present analysis, 565 volunteers were analysed (69·7 % maintainers and 62·1 % women). The Ethics Committee of Harokopio University, Greece, approved the study protocol, and all participants provided electronic informed consent.
Demographics characteristics
Age (in years), sex (man/woman), education level that was assessed by years of formal education and family status categorised as (a) single, (b) married or cohabitating, (c) divorced or (d) widowed and afterwards coded as married or else (due to the small number of participants in categories c and d) were recorded.
Assessment of weight history and physical activity
Specific weight history questions were asked to the participants, regarding current anthropometric characteristics (weight (in kg) and height (in m), from which BMI was computed as weight/height 2 (kg/m 2 )), maximum weight ever reached and initial weight loss achieved (as percentage of maximum weight). For maintainers, additional questions regarding time maintaining weight loss and the amount of weight loss they are currently maintaining (as percentage of maximum weight) were computed.
Participants’ activity levels were assessed through the validated Greek short version of the International Physical Activity Questionnaire ( 21 ) . Volunteers reported how much time they spent during the previous week for vigorous and moderate activities and walking. This allowed for calculating total metabolic equivalent of task minutes (MET-minutes) of activity during the previous week, which were then converted to energy expenditure (kJ/d from physical activity), using the MET-minutes × weight/60 equation ( 22 )
Dietary intake assessment
Trained researchers conducted two non-consecutive telephone 24-h recall dietary recalls, based on the multiple-pass method ( 13 , 23 ) . All participants were asked for all food and beverages they consumed during the previous day. The same researcher conducted both dietary recalls within 10 d, with weekdays and weekends proportionally represented among participants. Researchers were blind to the maintenance status (maintainer or regainer) of the volunteers.
Recall data were analysed in terms of energy intake and macronutrients, using relevant software (Nutritionist Pro 2007; Axxya Systems). For Greek foods not available in the software databases, recipes were broken down to their original ingredients, and/ or the food label of the actual product was crosschecked with the nutrient analysis of the selected food in the database. Recall data were also analysed in terms of food group consumption ( 13 ) , and intake of all major food groups was estimated, including the core foods of the Mediterranean diet. Food group consumption was expressed as servings/week ( 24 ) , with the exception of olive oil which was expressed as frequency of using olive oil in meals.
Adherence to the Mediterranean diet
Adherence to the Mediterranean diet was assessed by the Mediterranean Diet Score (MedDietScore) proposed by Panagiotakos et al. ( 25 ) . The scoring is based on the weekly consumption of eleven food groups. For non-refined cereals, fruits, vegetables, legumes, potatoes, fish and olive oil, individuals who reported no consumption were assigned a score of 0, and scores of 1–5 are assigned for rare to daily consumption. For meat and meat products, poultry and full-fat dairy products, scores were assigned on a reverse scale. For alcohol intake, it is assumed that small amounts of consumption are beneficial, while high or zero consumption is detrimental. Thus, a score of 5 was assigned for consumption of <300 ml of alcohol/d and more than zero, a score of 0 was assigned for no consumption or for consumption of 700 ml/d or more and scores of 4–1 were assigned for consumption of 600–700, 500–600, 400–500 and 300–400 ml/d (100 ml has 12 g of ethanol concentration), respectively. The total score ranges from 0 to 55, with higher values indicating greater adherence to the Mediterranean dietary pattern.
Data distribution was graphically explored with Q–Q plots in order to assess normality. Normally distributed continuous variables were presented as means and standard deviations, and non-normally distributed variables as medians and quartiles (Ql, Q3). Independent-sample t tests and Mann–Whitney U tests for (normally and non-normally distributed, respectively) continuous variables and χ 2 tests for categorical variables were used to examine differences between maintainers and regainers. To further explore the relationship between weight loss maintenance and adherence to the Mediterranean diet, quartiles of the MedDietScore were calculated (≤21, 22–25, 26–30, ≥31); the association between adherence to the MedDietScore quartiles and maintenance status (i.e. maintainers or regainers) was examined applying multi-adjusted logistic regression (results are presented as OR and 95 % CI). Three models were employed, model 1: crude/unadjusted; model 2: adjusted for sex, age, marital status and years of education; model 3: adjusted for sex, age, marital status, years of education and energy intake and physical activity. We also performed additional adjustment for the variables described in model 3, for smoking habits (current or non-smoker) and sleep habits (mean duration of nocturnal sleep during the last month). Addition of variables in the nested models was based on their theoretically hypothesised association with the outcome. The Hosmer–Lemeshow test was used to evaluate models’ goodness of fit. Similarly, multi-adjusted logistic regression models were used to explore the relationship between the core food groups of the Mediterranean diet and weight loss maintenance. Additionally, in order to cross-validate the role of each component of the MedDietScore on weight loss maintenance, analyses based on the leave-one-out approach were conducted. In this regard, the MedDietScore was recalculated with ten instead of eleven items, and eleven new multi-adjusted models were estimated. Statistical analyses were performed using STATA version 15 software (M. Psarros & Associates).
Participants’ general characteristics, according to maintenance status, are presented in Table 1 . Maintainers exhibited maintenance of a 22·3 ( sd 9·4)% weight loss for a median period of 2·8 (Q1 1·7, Q3 5·7) years. Compared with regainers, maintainers were younger and less frequently married, had a lower current BMI and a higher initial weight loss (as percentage of maximum weight) ( P < 0·001 for all comparisons). Moreover, maintainers were found to be more active than regainers, reporting greater daily activity energy expenditure from physical activity (1552 (Q1 699, Q3 3017) v. 1109 (Q1 515, Q3 2272) kJ/d, P = 0·006). Current smoking was not different between groups (maintainers 36·6%; regainers 27·0%, P = 0·096). Maintainers reported greater nocturnal sleep duration than regainers (7·0 ( sd 1·2) v. 6·7 ( sd 1·3)h, P = 0·022).
Participants’ general characteristics, by maintenance status ( n 565) (Mean values and standard deviations; medians and quartiles; relative frequencies in percentages)
Comparisons of dietary intake by maintenance status are presented in Table 2 . On average, maintainers consumed more protein compared with regainers (0·98 (Q1 0·74, Q3 1·28) v. 0·82 (Q1 0·60, Q3 1·05) g/kg body weight, P < 0·001). Maintainers had marginally significantly higher consumption of fruit compared with regainers (8·8 ( sd 9·1) v. 7·3 ( sd 8·1) servings/week, P = 0·06). No other significant differences in the consumption of the core food groups of the Mediterranean diet, or in the total score of adherence to the Mediterranean diet, were found between maintainers and regainers.
Dietary intake and consumption of the core food groups of the Mediterranean diet, by maintenance status ( n 565). (Mean values and standard deviations; medians and quartiles)
EI, energy intake; BW, body weight; MedDietScore, Mediterranean Diet Score.
However, residual confounding may exist, and the aforementioned results from the crude analyses may be prone to bias due to sampling alterations. The estimated multi-adjusted logistic regression models showed a linear trend between quartiles of the MedDietScore and weight loss maintenance ( P < 0·05, see Table 3 ). After adjustment for basic demographic characteristics (sex, age, years of education and marital status), being in the third or the fourth quartile of the MedDietScore ( v. in the 1st) was associated with 2·30 (95 % CI 1·29, 4·09) and 1·88 (95 % CI 1·10, 3·22) increased odds for being a maintainer than a regainer, respectively. These findings remained significant after additional adjustment for energy intake and physical activity. Further adjustment for smoking habits and sleep habits did not affect statistically significant findings.
Logistic regression models describing the relationship between adherence to the Mediterranean diet and weight loss maintenance (Odds ratios and 95 % confidence intervals)
MedDietScore, Mediterranean Diet Score.
Adjusted for sex, age, years of education and marital status (married or not).
Adjusted for sex, age, years of education, marital status (married or not), energy intake (kJ/d) and physical activity energy expenditure (kJ/d).
No statistically significant results emerged from the multi-adjusted logistic regression models examining the relationship between food group consumption and weight loss maintenance, apart from the fruits group ( Table 4 ). Specifically, in the adjusted and fully adjusted models (for the confounders mentioned above), a significant trend for being a maintainer as compared with regainer towards increased consumption of weekly servings of fruit was revealed (OR/one fruit serving per week 1·03 (95 % CI 1·01, 1·06)); that is, every weekly serving of fruit increase was associated with 3% greater odds of being a maintainer.
Logistic regression models describing the relationship between the core food groups of the Mediterranean diet and weight loss maintenance (Odds ratios and 95 % confidence intervals)
The leave-one-out approach showed that the MedDietScore was not associated with weight loss maintenance in six of the eleven altered MedDietScore models. These were the scores that were calculated without the respective loadings from potatoes (OR 1·62 (95 % CI 0·91, 2·90)) without the respective loadings from potatoes, fruit (OR 1·70 (95 % CI 0·94, 3·05)), legumes (OR 1·38 (95 % CI 0·77, 2·47)), olive oil (OR 1·66 (95 % CI 0·94, 2·94)), red meat (OR 1·74 (95 % CI 0·99, 3·06)) and alcohol (OR 1·52 (95 % CI 0·88, 2·62)) weekly consumption.
This is one of the first studies exploring the relationship between adherence to the Mediterranean diet and long-term weight loss maintenance. Our results suggest that high adherence to the Mediterranean diet is associated with a 2-fold increased likelihood of weight loss maintenance in this large cohort of maintainers and regainers. This favourable association was mainly attributed to the Mediterranean diet as a whole dietary pattern. However, some components of this traditional pattern may largely contribute to the observed association.
Although mean adherence to the Mediterranean diet did not differ between maintainers and regainers, a linear trend for weight loss maintenance was revealed in the unadjusted model. After adjustment for potential confounding factors, our results highlighted that higher adherence to the Mediterranean diet confers benefits for long-term weight loss maintenance. Indeed, a large body of evidence supports our finding, given that scoring high (against low) in Mediterranean diet indexes is associated with favourable health and weight outcomes (i.e. better fasting glucose homeostasis indices ( 26 ) and lipid profile ( 27 ) , less longitudinal weight gain ( 28 ) and lower likelihood of obesity ( 29 ) ).
In the PREDIMED-Plus trial, a 6-month weight loss intervention based on energy-restricted Mediterranean diet, the intervention group continued to lose weight and enhance their adherence to the pattern from intervention end to the 12-month follow-up phase ( 16 ) . In addition, combination of a biphasic very low-energy diet (40 d) with two periods of maintenance (4 and 6 months) based on the Mediterranean diet has been associated with a reduction of about 15 kg and null weight regain in participants with obesity, over a 12-month period ( 17 ) . Thus, findings of these interventions, along with our results, support the beneficial role of the high adherence to the Mediterranean diet during the post-dieting period. There are several physiological mechanisms by which the Mediterranean diet may exert significant benefits for weight loss maintenance ( 30 ) . Specifically, this dietary pattern provides a large quantity of dietary fibre, which increases satiety and satiation through prolonged mastication, increased gastric detention and enhanced release of cholecystokinin; has a low energy density and a low glycaemic load, potentially leading to better appetite control; and has high water content, which taken together with the previously mentioned characteristics contribute to increased satiation and consequently to lower energy intake, thus promoting the maintenance of weight loss ( 30 ) .
Our results also support the beneficial role of protein intake in weight loss maintenance, as previously reported in other weight control registries ( 31 ) . While results from interventional studies remain controversial ( 32 ) , some studies suggest that modest increments in protein intake in the post-dieting period are associated with better maintenance of the lost weight, possibly through the effects of higher protein effect in satiety and increased energy expenditure ( 15 , 33 ) .
Furthermore, our results showed that the consumption of fruits, which are rich in dietary fibre and water and thus have low energy density, was positively associated with weight loss maintenance. Previous evidence demonstrated that increased intake of fruit was inversely associated with longitudinal weight gain ( 34 , 35 ) . Additionally, in the Weight Loss Maintenance Trial, a multi-centre, randomised controlled trial on the 6-month weight loss intervention followed by a 30-month maintenance phase, every increase in the serving of fruit in the maintenance phase is associated with 0–04 kg of weight loss ( 36 ) . The potential underlying mechanisms are largely unknown. A meta-analysis concluded that increased consumption of fruit and vegetables is unlikely to result in weight gain and may even produce modest long-term weight reduction ( 37 ) . We postulate that the observed relationship may reflect a possible substitution of high-energy snacks with fruit among maintainers, as a weight control behaviour. On the other hand, even though specific food groups may withhold promising aspects for weight loss maintenance, people do not consume foods in isolation. Decomposing a dietary pattern to its components may undermine the interactive or synergistic effects of foods that are consumed in combinations in real life ( 38 ) .
Consequently, analyses on the relationship between various combinations of food groups and weight loss maintenance highlighted the possibility that other food groups, in addition to fruit, may be of importance. In specific, the leave-one-out approach highlighted that higher consumption of not only fruit but also potatoes, legumes and olive oil, modest consumption of alcohol and lower consumption of red meat and products may be important for weight loss maintenance. Taken together, these results are supportive of the synergistic effect of the Mediterranean diet’s components ( 39 , 40 ) on weight loss maintenance, rather of the observed relationship being dependent on individual effects of single dietary constituents ( 29 ) . Similar results were also observed in the PREDIMED-Plus trial, where the intervention group reported greater adherence to the guidance regarding the Mediterranean diet’s components of fruits, vegetables, legumes, commercial bakery, sauces, added sugars, red meat and poultry than controls during the maintenance phase ( 16 ) .
The present study has several strengths. Findings reported here are from the largest European weight control registry. The inclusion of regainers in the sample allowed for direct comparisons of the measures acquired across long-term weight loss outcome groups. Dietary intake data were collected through thorough dietary assessment, by means that have been proven adequate for assessing nutrient intake on a population basis ( 41 ) . The cross-sectional nature of the present study reveals association, but not causality. On the other hand, we believe that our findings provide the basis for future experimental studies, given that performing trials for all possible prudent diets may not be entirely pragmatic or feasible. Although we performed various adjustments, other confounders, not taken under consideration by the present study, may have had an impact on the observed relationship (e.g. presence of co-morbidities or stress). We examined the adoption of the Mediterranean diet in a Mediterranean cohort, which may limit the generalisation of our results to other populations. Nevertheless, this dietary pattern can be transferable ( 42 , 43 ) and several studies showed that it has produced significant health outcomes to Mediterranean and non-Mediterranean populations alike ( 44 , 45 ) .
In conclusion, higher adherence to the Mediterranean diet was associated with a 2-fold increased likelihood of weight loss maintenance. Our results highlight the potential beneficial effect of this plant-based dietary pattern in long-term obesity management, as well as provide novel targets for diet planning during weight loss maintenance. Future research should explore the effects of the adoption of a Mediterranean dietary pattern in the post-dieting period through adequately designed interventions with long-term follow-up periods, as well as test the level of transferability of these findings to non-Mediterranean populations.
Acknowledgements
The authors express their gratitude to the volunteers of the Med Weight study.
The MedWeight study was initially funded by the Coca-Cola Foundation (2012–2015, KA 221; Principal Investigator: Mary Yannakoulia). D. P. received financial support by the Greek State Scholarship Foundation (MIS 5000432). Both funding bodies had no role in the design, analysis or writing of the present work.
M. Y. designed the research, revised the manuscript and had final responsibility for all aspects of the present work; D. P. contributed to data collection, drafted the manuscript and performed the analyses; C. A. A, D. B. P, I. S. and J. O. H. contributed to the interpretation of the data and revised the manuscript. All authors read and approved the final version prior to submission.
Abbreviation:
Mediterranean Diet Score
There are no conflicts of interest.
- 1. Willett WC, Sacks F, Trichopoulou A, et al. (1995) Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 61, 1402S–1406S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Kafatos A, Verhagen H, Moschandreas J, et al. (2000) Mediterranean diet of Crete: foods and nutrient content. J Am Diet Assoc 100, 1487–1493. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Eleftheriou D, Benetou V, Trichopoulou A, et al. (2018) Mediterranean diet and its components in relation to all-cause mortality: meta-analysis. Br J Nutr 120, 1081–1097. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Dinu M, Pagliai G, Casini A, et al. (2018) Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr 72, 30–43. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Esposito K, Maiorino MI, Bellastella G, et al. (2015) A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BM J Open 5, e008222. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Sofi F, Macchi C, Abbate R, et al. (2013) Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 17, 2769–2782. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. (2006) Association between the prevalence of obesity and adherence to the Mediterranean diet: the ATTICA study. Nutrition 22, 449–456. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Carlos S, De La Fuente-Arrillaga C, Bes-Rastrollo M, et al. (2018) Mediterranean diet and health outcomes in the SUN cohort. Nutrients 10, 439. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Agnoli C, Sieri S, Ricceri F, et al. (2018) Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr Diabetes 8, 22–22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Lassale C, Fezeu L, Andreeva VA, et al. (2012) Association between dietary scores and 13-year weight change and obesity risk in a French prospective cohort. Int J Obes 36,1455–1462. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Esposito K, Kastorini CM, Panagiotakos DB, et al. (2011) Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord 9, 1–12. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Yannakoulia M, Poulimeneas D, Mamalaki E, et al. (2019) Dietary modifications for weight loss and weight loss maintenance. Metabolism 92, 153–162. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Karfopoulou E, Brikou D, Mamalaki E, et al. (2017) Dietary patterns in weight loss maintenance: results from the MedWeight study. Eur J Nutr 56, 991–1002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Aller EE, Larsen TM, Claus H, et al. (2014) Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes (Lond) 38, 1511–1517. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Larsen TM, Dalskov SM, van Baak M, et al. (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363, 2102–2113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Salas-Salvado J, Diaz-Lopez A, Ruiz-Canela M, et al. (2019) Effect of a lifestyle intervention program with energy-restricted Mediterranean Diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus trial. Diabetes Care 42, 777–788. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Paoli A, Bianco A, Grimaldi KA, et al. (2013) Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients 5, 5205–5217. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Karfopoulou E, Anastasiou CA, Hill JO, et al. (2014) The MedWeight study: design and preliminary results. Med J Nutrition Metab 7, 201–210. [ Google Scholar ]
- 19. Wing RR & Hill JO (2001) Successful weight loss maintenance. Annu Rev Nutr 21, 323–341. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Jensen MD, Ryan DH, Apovian CM, et al. (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 63, 2985–3023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Papathanasiou G, Georgoudis G, Papandreou M, et al. (2009) Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hellenic J Cardiol 50, 283–294. [ PubMed ] [ Google Scholar ]
- 22. International Physical Activity Questionnaire (IPAQ) Group (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) – short and long forms. http://www.ipaq.ki.se (accessed October 2018).
- 23. Brikou D, Zannidi D, Karfopoulou E, et al. (2016) Breakfast consumption and weight-loss maintenance: results from the MedWeight study. Br J Nutr 115, 2246–2251. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Hellenic Ministry of Health and Welfare (1999) Dietary guidelines for adults in Greece. Arch Hell Med 5, 516–524. [ Google Scholar ]
- 25. Panagiotakos DB, Pitsavos C, Arvaniti F, et al. (2007) Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 44, 335–340. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Panagiotakos DB, Tzima N, Pitsavos C, et al. (2007) The association between adherence to the Mediterranean Diet and fasting indices of glucose homoeostasis: the ATTICA Study. J Am Coll Nutr 26, 32–38. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Magriplis E, Panagiotakos D, Mitsopoulou A-V, et al. (2019) Prevalence of hyperlipidaemia in adults and its relation to the Mediterranean diet: the Hellenic National Nutrition and Health Survey (HNNHS). Eur J Prev Cardiol 26, 1957–1967. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Beunza J-J, Toledo E, Hu FB, et al. (2010) Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr 92, 1484–1493. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Zappalà G, Buscemi S, Mulè S, et al. (2018) High adherence to Mediterranean diet, but not individual foods or nutrients, is associated with lower likelihood of being obese in a Mediterranean cohort. Eat Disord Stud Anorex Bulim Obes 23, 605–614. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Buckland G, Bach A & Serra-Majem L (2008) Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev 9, 582–593. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Paixão C, Dias CM, Jorge R, et al. (2020) Successful weight loss maintenance: a systematic review of weight control registries. Obes Rev 21, e13003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Kjolbaek L, Sorensen LB, Sondertoft NB, et al. (2017) Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr 106, 684–697. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Westerterp-Plantenga MS, Lejeune MP, Nijs I, et al. (2004) High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 28, 57–64. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Bertoia ML, Mukamal KJ, Cahill LE, et al. (2015) Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three Prospective Cohort Studies. PLoS Med 12, e1001878. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Vioque J, Weinbrenner T, Castelló A, et al. (2008) Intake of fruits and vegetables in relation to 10-year weight gain among Spanish adults. Obesity 16, 664–670. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Champagne CM, Broyles ST, Moran LD, et al. (2011) Dietary intakes associated with successful weight loss and maintenance during the Weight Loss Maintenance Trial. J Am Diet Assoc 111, 1826–1835. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Mytton OT, Nnoaham K, Eyles H, et al. (2014) Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health 14, 886. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13, 3–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Widmer RJ, Flammer AJ, Lerman LO, et al. (2015) The Mediterranean diet, its components, and cardiovascular disease. Am J Med 128, 229–238. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Yannakoulia M, Kontogianni M & Scarmeas N (2015) Cognitive health and Mediterranean diet: just diet or lifestyle pattern? Ageing Res Rev 20, 74–78. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Ma Y, Olendzki BC, Pagoto SL, et al. (2009) Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol 19, 553–559. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Martinez-Gonzalez MA, Hershey MS, Zazpe I, et al. (2017) Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 9, 1226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Murphy KJ & Parletta N (2018) Implementing a Mediterranean-style diet outside the Mediterranean region. Curr Atheroscler Rep 20, 28. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Cubillos L, Estrada Del Campo Y, Harbi K, et al. (2017) Feasibility and acceptability of a clinic-based Mediterranean-style diet intervention to reduce cardiovascular risk for Hispanic Americans with type 2 diabetes. Diabetes Educ 43, 286–296. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Sofi F, Macchi C, Abbate R, et al. (2014) Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 17, 2769–2782. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (345.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
REVIEW article
Adherence to a mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials.

- 1 Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, VIC, Australia
- 2 Centre for Physical Activity and Nutrition Research, Deakin University, Melbourne, VIC, Australia
The Mediterranean-style diet (MedDiet) involves substantial intake of fruits, vegetables, and fish, and a lower consumption of dairy, red meat, and sugars. Over the past 15 years, much empirical evidence supports the suggestion that a MedDiet may be beneficial with respect to reducing the incidence of cardiovascular disease, cancer, metabolic syndrome, and dementia. A number of cross-sectional studies that have examined the impact of MedDiet on cognition have yielded largely positive results. The objective of this review is to evaluate longitudinal and prospective trials to gain an understanding of how a MedDiet may impact cognitive processes over time. The included studies were aimed at improving cognition or minimizing of cognitive decline. Studies reviewed included assessments of dietary status using either a food frequency questionnaire or a food diary assessment. Eighteen articles meeting our inclusion criteria were subjected to systematic review. These revealed that higher adherence to a MedDiet is associated with slower rates of cognitive decline, reduced conversion to Alzheimer’s disease, and improvements in cognitive function. The specific cognitive domains that were found to benefit with improved Mediterranean Diet Score were memory (delayed recognition, long-term, and working memory), executive function, and visual constructs. The current review has also considered a number of methodological issues in making recommendations for future research. The utilization of a dietary pattern, such as the MedDiet, will be essential as part of the armamentarium to maintain quality of life and reduce the potential social and economic burden of dementia.
Neurocognitive Aging and the Potential Associated Risk Factors
An aging population raises the potential for increased incidence of cognitive impairment ( 1 ). Preservation of brain function and the reduction of risk of neurological disorders have become key issues for society. In addition to the need to find treatments for disorders, such as Alzheimer’s disease (AD), it is becoming increasingly apparent that early intervention is critical for the maintenance of brain health across the life span and reducing the risk of accelerated neurocognitive decline ( 2 ). There is mounting evidence that risk factors for cardiovascular disease, stroke, diabetes, and other later-life chronic health conditions also exacerbate age-associated cognitive decline, which can lead to AD ( 3 ). Research is increasingly focusing on understanding how interventions, such as improving nutritional status and modifying risk factors that may impinge directly and/or indirectly on brain functioning, can reduce the risk of neurocognitive impairment ( 4 ). Closer adherence to a traditional Mediterranean-style diet (MedDiet) may be beneficial and protective with respect to addressing such risk factors, ultimately reducing the rate of cognitive decline ( 5 ).
Neurocognitive decline across the life span is well documented. In particular, cognitive faculties that involve speed of processing and memory steadily decline from the third decade of life ( 6 ). For example, the speed of retrieval of previously learned spatial locations from short-term memory is reduced by around 50% from the third to the eighth decade of life ( 7 ). While this slowing of memory retrieval is observed in all individuals, there is considerable intersubject variability in cognitive trajectories across the life span, particularly in later life ( 8 ). Individuals who are still cognitively intact, but who have shown accelerated cognitive decline, are potentially at risk of dementia and AD ( 9 ). It has been suggested that our individual cognitive trajectories relate to our overall health, and, as such, are potentially modifiable ( 10 ).
Aging affects multiple biological systems and organs, including the brain ( 11 ). Much research has focused on the investigation of gross neuroanatomical changes with age. In recent years, this area has advanced through the adoption of high-resolution volumetric techniques, such as magnetic resonance imaging (MRI). Such methods reveal brain volume reduction at a rate of 5% per decade after the age of 40 years, with the rate of reduction possibly increasing after the age of 70 years ( 12 ). Other areas of investigation include structural and functional changes at the cellular level. Neurocognitive aging is related to a lifelong incidence of molecular damage, with progressive destruction of both biomolecular and cellular components ( 6 ). There are also neurochemical changes. For example, levels of brain-derived neurotrophic factor (BDNF) are known to reduce, which can impact synaptic plasticity and neurogenesis in the aging adult brain ( 13 ). Amyloid plaques and neurofibrillary tangles, the hallmarks of AD, have been extensively researched and occur commonly with age in cognitively intact individuals ( 12 ). Given the relevance of these neuropathological changes to AD, the origin of these changes and the potential risk for accelerated neurocognitive decline is being widely researched ( 14 , 15 ).
The cardiovascular system offers prime examples of the relationship between lifestyle, health, and aging. As we age, our arteries stiffen, and there is increased risk of cardiovascular disease, which has been associated with poor diet and lack of exercise ( 16 ). Stiffening of the arteries and increased blood pressure also increases the risk of stroke and neurodegeneration. Elevated systolic blood pressure has been shown to increase gray matter volume loss, which may eventually result in cognitive dysfunction ( 17 ). In addition to the consideration of increasing cardiovascular risks, it is important to also consider the important relationship between blood pressure, high cholesterol, and obesity ( 3 ). These three factors are associated with what is referred to as the metabolic syndrome, a cluster of conditions typically including increased blood pressure and elevated blood sugar levels that can be associated with excess body fat around the waist and abnormal cholesterol levels. When combined, these conditions may increase the risk of heart disease, stroke, and diabetes ( 18 ).
Investigation of physiological changes at the molecular level has helped to elucidate possible mechanisms of action for the aforementioned changes in neural and cardiovascular functioning. Inflammatory processes often increase with age and contribute to chronic disorders, such as arthritis, diabetes, and cardiovascular disease, as well as to neurodegenerative disorders, such as AD ( 19 ). It has also been proposed that age is associated with increased oxidative stress and free radical damage ( 20 ). Inflammation and oxidative stress may therefore be critical targets for the amelioration of declining health and brain function across the life span, which can potentially be addressed through improved nutrition and increased physical activity ( 21 ). However, as discussed below, there are very few controlled clinical trials that have utilized these simple interventions to investigate their effects on cognition in older individuals.

Nutrition and Cognitive Status
The variability between individuals’ cognitive abilities in later life has been well documented ( 11 , 12 , 22 , 23 ). The source of this variability has been related to both non-modifiable factors, such as age, gender, and genetics, as well as modifiable risk factors, such as stress, education, psychological well-being, and exercise ( 24 ).
Another particularly important modifiable risk factor for cognitive decline is dietary status. There is a wealth of literature, from both animal studies and human health research, indicating that diet can exert profound effects on biological aging ( 25 – 35 ). Diet can also affect other risk factors, such as inflammation and oxidation. Diets that are low in energy and that act to reduce oxidative stress may be protective against cognitive decline ( 25 , 36 ). Conversely, a diet that is high in energy and acts to increase oxidative stress may be considered a risk factor for impaired cognitive functioning ( 37 ).
Additionally, there are neurotrophic and neuroendocrine factors that may play a role in the cognitive responses that relate to food intake ( 38 ). Increasing consumption of fish and seafood by as little as one portion per month may be protective against stroke ( 39 ). Along with a healthy diet, there is evidence that low or moderate intake of alcohol may reduce cardiovascular and neurocognitive risk, with heavy drinkers tending to suffer more coronary infarcts and dementia ( 40 , 41 ).
The dietary B-complex vitamins, which include B1 (thiamine), B2 (riboflavin), B3 (niacin), B5 (pantothenate), B6 (biotin, folate), and B12 (cobalamin), are important regulators of neurotransmitter function ( 42 ). Vitamin B6, in particular, is an important cofactor for the enzymes that synthesize the neurotransmitters serotonin, epinephrine, and norepinephrine, and gamma-aminobutyric acid ( 43 ). B vitamins may therefore affect central metabolism, brain function, and the modulation of mood, given their role in neurotransmitter production ( 44 – 47 ). A lack of basic B vitamins (folic acid, B6, and B12) in the diet is also proposed to impact on the rate of brain atrophy associated with mild cognitive impairment (MCI) and with healthy aging ( 15 ).
It has also been proposed that a high intake of seafood and other sources of long-chain omega-3 polyunsaturated fats (LC-n3-FA) may have long-term beneficial effects on cognitive function ( 43 , 48 , 49 ). For example, energy balance and LC-n3-FA act via BDNF, and insulin-like growth factor-1 (IGF-1) can alter the expression of a number of protein pathways that are involved in neuronal function, plasticity, and neurogenesis ( 50 ). The addition of regular fish consumption in one’s diet has also been shown to maintain gray matter volumes in the hippocampus, precuneus, posterior cingulate, and orbital cortex ( 51 ). As shown in Figure 1 , these are examples of the many factors that can potentially modulate the action of nutrition on brain function.
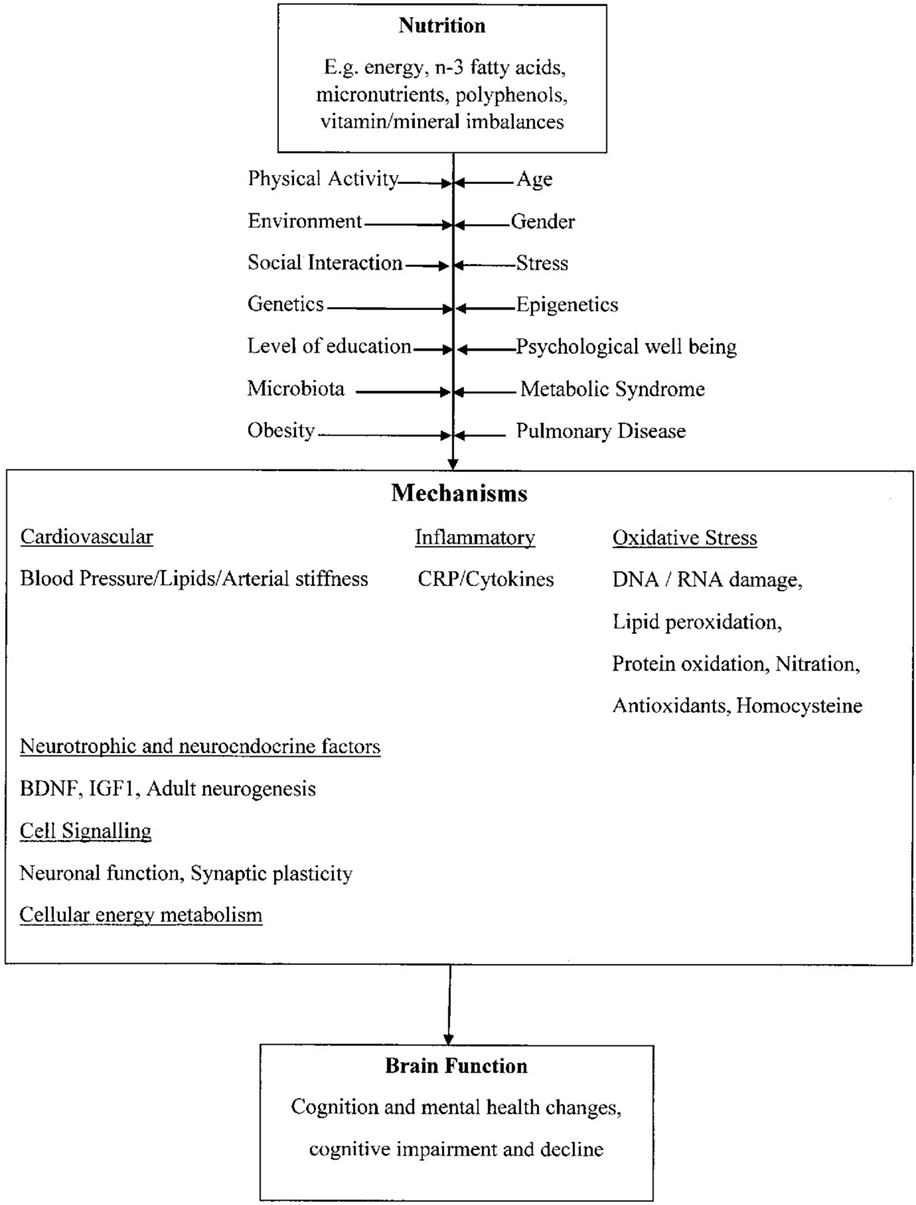
Figure 1. Proposed mechanisms that link nutrition to changes in brain function . Adapted from Dauncey ( 23 ), with permission to reprint from Cambridge Press and authority to alter.
The Mediterranean Diet
The MedDiet was initially investigated in studies that described the food consumption in countries in Southern Europe. In particular, the dietary intake of the Mediterranean countries of Italy, Yugoslavia, and Greece were investigated, as these populations demonstrated a higher than average life expectancy, lower rates of cardiovascular disease, cancer, and other chronic disorders ( 52 – 54 ). The key components of a MedDiet are abundant consumption of plant foods, such as leafy greens, fresh fruit and vegetables, cereals, beans, seeds, nuts, and legumes. The MedDiet is also low in dairy, has minimal red meats, and uses olive oil as its major source of fat ( 14 ).
Leafy vegetables are an important source of folate and other B vitamins ( 55 , 56 ). The MedDiet has been reported to be protective against diseases associated with chronic inflammation, cancer, diabetes, obesity, pulmonary disease, cardiovascular disease, and cognitive disorders ( 57 ). A diet with the nutritional qualities of the MedDiet has been shown to reduce homocysteine levels, considered a risk factor for age-associated cognitive decline ( 58 , 59 ). The MedDiet pattern is largely void of refined sugar, cholesterol, and trans fats, aspects of diet that are considered to be associated with poor cognitive outcomes in older age ( 60 , 61 ); with saturated fats impacting negatively on learning and memory and the potential for increasing metabolic distress ( 62 ). The effects of poor diet may be overcome by changing nutritional consumption, suggesting that a diet high in fruit and vegetables may assist in improving brain functioning over time ( 4 , 43 , 63 – 66 ).
There have been a substantial number of population/epidemiological studies that have investigated adherence to a MedDiet and health outcomes, such as the population of 22,043 adults in Greece who completed extensive food frequency questionnaires (FFQs) ( 26 ). Although this study did not review the effect of the MedDiet on cognition, it did indicate that adherence resulted in reduced mortality after 44 months of follow-up ( 26 ). Adherence to the MedDiet was also found to reduce the risk of myocardial infarction ( 27 ). The Italian Longitudinal Study on Aging, which was designed to assess the prevalence and incidence of certain chronic conditions in an older population, had a sample size of 5,632 subjects aged 65–68 years and found that an intake of 46 g per day or more of monounsaturated fatty acids, primarily from olive oil, as a part of the basic MedDiet, appeared to be protective against age-related changes in cognitive function ( 67 ). In the Chicago Health and Aging Project (CHAPS) longitudinal study that assessed the incidence of AD and the habitual diet of 6,158 participants, it was found that greater adherence to all three healthy dietary styles that were assessed, including the MedDiet, was associated with a reduced incidence of AD. In a cross-sectional analysis of an Australian study, it was found that MedDiet adherence was not related to cognitive function. However, the consumption of plant foods associated with the MedDiet had a positive relationship with improving physical function and general health, while being negatively associated with anxiety, depression, and perceived stress ( 68 ).
These studies have provided evidence that the MedDiet may be protective against cognitive decline. However, there are potential confounding issues associated with both cross-sectional and longitudinal population studies that may not take into account minor reactive and biological changes that cannot be identified by observation.
The Mediterranean Diet and Cognition – Evidence from Longitudinal and Prospective Trials?
Over the past 15 years, there has been substantial research into the impact of adherence to the MedDiet pattern with respect to overall cardiovascular health, cognitive health, and the potential to reduce the onset of dementia and AD. The understanding of what a MedDiet is and the potential biological markers and nutritional requirements of this diet have also been investigated. It is also important to segment the studies with clinical interventions from those that are considered observational studies.
This review focuses on studies that evaluate longitudinal and prospective trials in accordance with the specified criteria. The review explores our understanding of how a MedDiet may impact cognitive processes to gain an understanding of the cognitive endpoints following adherence to a MedDiet. Moreover, this review examines the various methodologies by which the MedDiet has been assessed and the assessment tools used to evaluate cognitive outcomes as well as identifying the key cognitive domains that that have been shown to be altered by adherence to the MedDiet.
Study Selection
The following key words were searched: cognition, cognitive measures, control group, non-interventional group, Mediterranean diet patterns, and Mediterranean diet. In addition to these key words, for inclusion, the following conditions had to be met: the study must be a randomized or non-randomized trial, longitudinal, or a prospective cohort study.
Articles were excluded if they were focused on dementia only, depression only, child or adolescent depression only, AD only, Parkinson’s disease only, stroke or cardiovascular disease only, diabetes only, or a review of published work.
A literature search was carried out with the focus on articles published between the years from 2000 to 2015. The following search engines were used: Directory of Open Access Journals, EBSCO Host Academic Search Complete Gale Cengage Academic One File, Google Scholar, Health Reference Center Academic, Health Reference Center (Gale), Highway Free Press, Journals @ Ovid, Journal reports, Medline, PubMed, Science Direct Freedom Collection, Scopus, Springer Link, Web of Science, Wiley Online Library, and Taylor & Frances Journal Complete.
The data extraction was carried out by one researcher, who completed the assessment in cooperation with the other authors to ensure that the capture of the information was relevant and consistent with the selection and exclusion criteria.
The data extracted from each article included (1) characteristics of the trial participants (number of participants, gender, and age); (2) study title, with respect to relevance and terminology used; (3) year of publication; (4) study design and country of origin; (5) sample size; (6) FFQ; (7) MedDiet assessment; (8) cognitive assessments used; (9) cognitive sub-measures; and (10) study outcomes.
A study was included in the review if it had a measure of a MedDiet, a Mediterranean Diet Score (MedDietS) based on a numeric point scale as a result of any form of food frequency assessment, or total food intake. In addition, studies were also included if they used a MedDiet intervention with other key dietary components.
Included Studies
Independent searches utilizing the selection criteria identified 173 trials, and after adjustment for duplicates, 129 articles remained for assessment. Of these, 79 were discarded after reviewing the abstracts and article content as they did not meet the criteria. This left the full text of 50 articles for general review. After allowing for the inclusion criteria and more extensive examination, 18 studies met the inclusion criteria. The selection procedure and the total number of eligible trials reviewed are shown in Figure 2 .
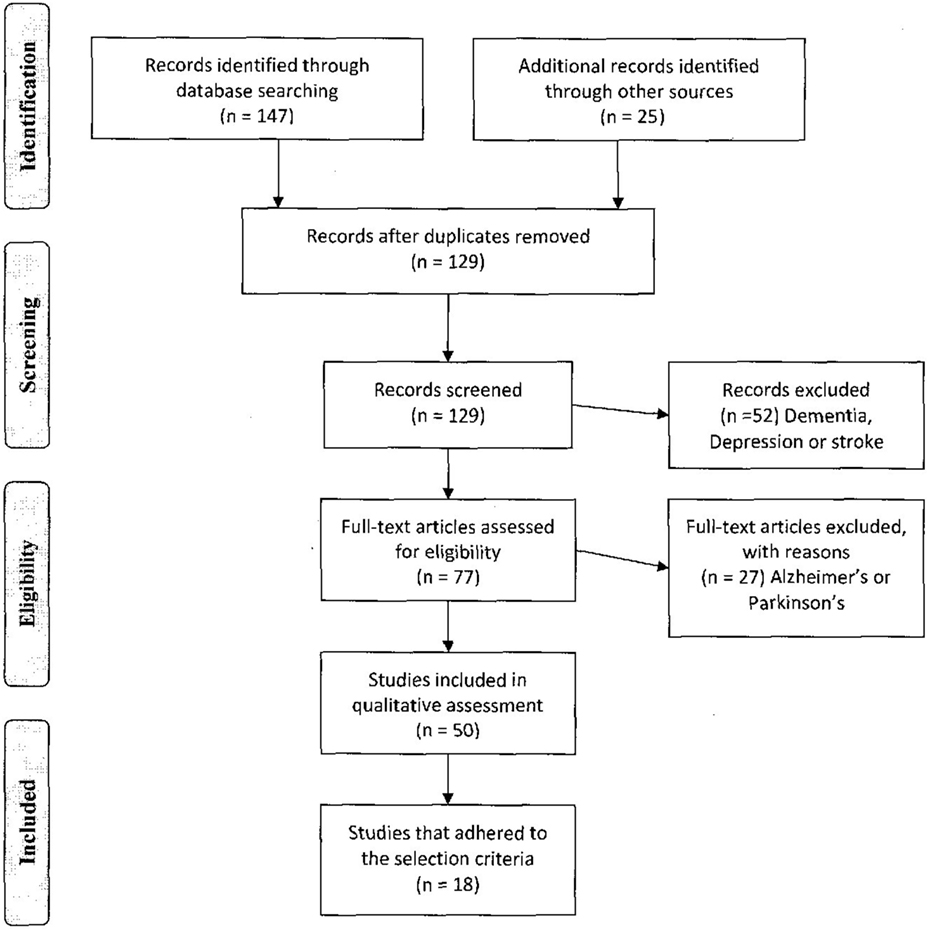
Figure 2. PRISMA diagram of the study selection process .
Table 1 shows the data extracted from each of the trials. In summary, the age range of the participants that were included in this selection varied considerably, with two studies including younger adults (19–40 years), three studies looking at middle age (45–65 years), nine in the range (65–75 years), and four in the range (75+ years).
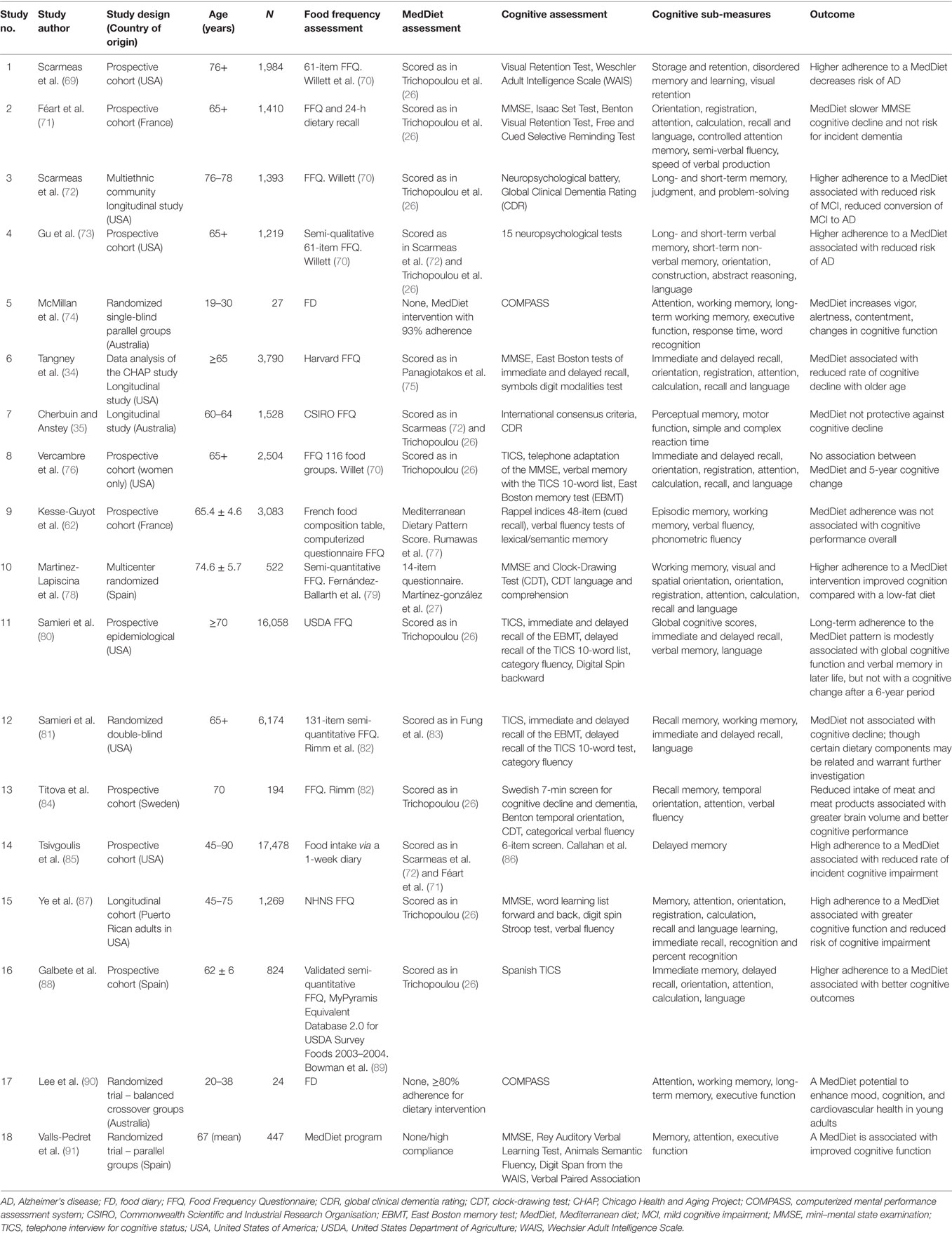
Table 1. Summary of the included studies .
These trials including a number of different study designs are presented in Table 1 .
Nutritional Intake Assessments
One of the key elements required to assess the adherence to the MedDiet is to gain an understanding of the volume of food intake and the particular food groups used for assessment. The common approach to achieve this outcome is to have participants complete a food diary (FD) or an assessment of their food consumption through the use of a FFQ at time points as part of the trial protocol. In all of the original research studies that met the selection criteria for this analysis, the participants had completed either an FFQ or an FD. Of the 18 studies identified, 14 utilized an FFQ, 3 utilized an FD, and 1 had a food program. The total number of categories of foods varied from a limited 20 groups through to 250 different food groups, with data being collected over a range of time frames.
Mediterranean Diet Scores
The assessment of the MedDietS has been discussed in the literature since 2003. This initial research identified 150 food groups and placed these into 14 inclusive food groups ( 26 ). A sex-specific median calculation was used to determine the baseline value to assess the MedDietS with the 14 inclusive food groups, or nutrients were considered, where some groups were combined to eventually calculate a score ranging from 0 to 9. The sex-specific median allows for a comparative cut off to be made between genders on food consumption ( 26 ). Beneficial foods, such as vegetables, legumes, fruits, nuts, cereals, and fish, were assigned a value of 0, if a person’s consumption was below the median, and a score of 1, if it was above the median. With food components considered to be detrimental to health, such as meat, poultry, and dairy, consumption above the median was scored as 0, and intake below the median was scored as 1. For alcohol, a score of 1 was given, provided consumption was within a specified range. When considering fat intake, the ratio of monounsaturated lipids to polyunsaturated lipids was evaluated with a higher ratio being more acceptable and a score of allocated accordingly.
Of the research included in this review, 10 papers referenced the Trichopoulou et al. ( 26 ) study to calculate a MedDietS, while 5 created a MedDietS utilizing a mean score. There are three papers that did not produce a MedDietS. When making an assessment of the MedDietS, 6 studies used mean scores, 11 used median scores, and 1 used a regression residual method. Three studies used a direct or total food amount. Although many studies utilized the original method to analyze the data, only four followed the sex-specific median approach. These were Articles 1, 3, 4, and 9 in Table 1 .
Cognitive Assessments
Generally, cognitive assessments utilize the mini–mental state examination (MMSE) ( 92 ). Of the 18 original research papers shown in Table 1 , 6 utilized this tool in combination with other tests. Another seven studies used cognitive assessments administered as part of neuropsychological test batteries and other scales. These included the East Boston tests of immediate and delayed recall, the clinical dementia rating scale, 6-item screeners, telephone interviews, category fluency, Stroop tests, clock-drawing, and others. All of which were used as paper-based assessments. Two studies used a computer-based assessment for cognition – the computerized mental performance assessment (COMPASS) ( 74 , 90 ). The cognitive domains that were assessed included measures of attention, working memory, long-term memory, and executive function.
Cognitive Results
Due to the heterogeneity of the selected studies that included a variety of experimental designs, cohorts, sampling times, as well as very diverse dietary and cognitive measures, it was not possible to pool data sets ( 102 , 103 ). Instead, we used the dietary assessment approach as a framework to report the cognitive outcomes from each study and discuss consistencies/inconsistencies more qualitatively.
Of the research that completed their assessment using a mean score to determine their MedDietS (Studies 6, 10, and 11), two found that higher adherence to the MedDiet resulted in slow rate of cognitive decline or improvements in cognition, including memory and immediate and delayed recall.
For those studies that used a median score for their MedDietS (Studies 2, 7, 8, 12, 13, 14, and 16), three showed that adherence to the MedDiet improved cognitive scores utilizing assessments of recognition memory, delayed recognition memory, or that this diet reduced the rate of cognitive decline utilizing assessments of working memory. In one study (Study 12), no relationship was found between the MedDiet intervention and cognitive decline; however, the authors did suggest that a specific food category, whole grains, merits further investigation with regard to cognition ( 81 ).
Four of the studies (Studies 1, 3, 4, and 9) also used a median score (sex-derived) to calculate the MedDietS. These studies analyzed the median score separately for men and women, as was done in the original study by Trichopoulou et al. ( 26 ). In three of these studies (Studies 1, 3, and 4), it was demonstrated that a higher adherence to a MedDiet was associated with a trend to reducing the risk of developing MCI ( 72 ), as measured using a global assessment and memory cognitive domains.
One study that did not use a mean or median calculation used a regression residual analysis method (Study 15). In this study, it was concluded that greater adherence to a MedDiet was associated with better cognitive function and a strong correlation with improvement in cognitive reserves when using MMSE, relating these outcomes to the cognitive domains of memory and attention ( 87 ).
In three studies, the absolute amount of food consumed in each category was used to assess adherence to a MedDiet. All reported high adherence to a MedDiet (Studies 5, 17, and 18). These studies demonstrated that a MedDiet supplemented with olive oil or nuts was associated with improved cognitive function and also that the MedDiet had the potential to enhance aspects of mood and cardiovascular function ( 74 , 90 , 91 ).
Finally, these trials were conducted in countries around the world with positive outcomes demonstrated both within and outside the Mediterranean region. There were six studies reporting positive cognitive outcomes in the USA, one study in France, three in Spain, one study in Sweden, and two in Australia.
This review has explored the contention that the MedDiet has a long-term positive effect on cognitive function. The results of the dietary pattern, cognitive decline, and dementia reviews also support the proposition that adherence to a MedDiet pattern is associated with less cognitive decline, dementia, or AD [69]. The primary aim of this review was to determine if adherence to a MedDiet produces favorable outcomes in relation to improved cognition or minimization of cognitive decline. Of the 18 research articles examined, 13 demonstrated that higher adherence to a MedDiet was related to either slowing the rate of cognitive decline, minimizing the conversion to AD or improving the cognitive function. Five of the 18 studies did not demonstrate that MedDiet adherence had a protective effect against cognitive decline or did not improve cognition. Irrespective of the study design, the studies that demonstrated improved cognitive outcomes examined the broad cognitive domains of attention, memory, and language. The studies that focused on the cognitive domains of motor or action did not report favorable outcomes when related to MedDiet adherence. The more specific cognitive domains that improved with MedDietS were memory, delayed recognition, executive function, long-term working memory, and visual constructs. These are mainly tests of fluid intelligence that decline with age ( 7 ). Interestingly, benefits to cognition afforded by the MedDiet were not exclusively in older individuals. The two studies included that were in younger adults both found improvements in cognition using computerized assessments.
It is noted that although there are several different designs within the studies examined, including randomized or non-randomized, that were longitudinal or prospective, and that may or may not have been interventional, it was difficult to assess if one or more particular designs reported more favorable outcomes with respect to adherence to a MedDiet and cognitive outcomes. It was also difficult to assess whether or not the calculation of the MedDietS, based on the original analysis of Trichopoulou et al. ( 26 ), yielded more favorable cognitive outcomes. More specific approaches utilizing a median, mean, or sex-derived determination of MedDietS were similarly difficult to conclude if one approach was better than the other with respect to cognitive outcomes, given the small number of studies and the mixed approaches for assessing cognition.
Six studies used the MMSE to examine a global measure of cognition. This measure was used mainly in trials examining cognitive decline and conversion to AD over a longer period of time. This seems reasonable given that the MMSE is a course tool for measuring cognition and is recommended mainly to identify accelerated aging and neurocognitive disorders, such as MCI and AD ( 93 ). Of the trials that used the MMSE to assess cognitive decline, three of the six trials showed either reduced cognitive decline or reduced conversion to MCI or AD. MMSE was also used to assess cognitive improvement – three out of six trials showed improvement in relation to adherence to a MedDietS. A higher rate of positive cognitive outcomes may have been realized if a more sensitive cognitive assessment tool was used. The studies that used the MMSE as their primary source of cognitive assessment measured cognitive decline as compared with the computer-based assessments that focused on cognitive improvement. The majority of the studies that used the MMSE also included cognitive assessments that measured memory, attention, language, and mood, such as the COMPASS, delayed recall from the Boston memory tests, the telephone interview of cognitive status (TICS) 10-word list, Stroop tests, verbal fluency measures, lexical semantic memory assessments, and clock-drawing tests. Only two of the trials used a computer-based assessment for cognition, the COMPASS, both showing evidence of improved cognitive functioning in a short-term study with a 10-day intervention. The non-computer-based neuropsychological assessments in general showed improved cognition or a reduced cognitive decline in relation to a MedDietS. There were 10 studies using these other neuropsychological assessments with 6 showing cognitive change with regard to a MedDietS.
The studies examined used a range of methods to derive a MedDietS. Some studies used an FD, while others used various FFQ assessments. An FFQ is a way to estimate the food consumed over an extended period 6–12 months. An FD is a more specific and detailed assessment conducted over a number of days to quantify what one actually eats. There is no standardized pattern or agreed types of foods included in these forms of dietary assessments. The foods consumed can be considered specific to a region or country of origin, and the foods vary between studies, as they have been conducted in different countries and geographic areas. Generally, the food intake is recorded as it is relevant to the specific cohort under investigation. The FFQ has been found to be reproducible and have good comparative validity. The FFQ has demonstrated dietary intervention effects to be relatively accurate ( 94 – 96 ). When converted to different languages, the validity of the FFQ was also reproducible, such as with the Brazilian, Chinese, and Italian populations ( 97 – 100 ).
Across the 18 studies, there was no standardization of the most appropriate FFQ. There was a large variation in the FFQs adopted, and the selection of food groups ranged from 70 to 250 different groups. It is also important to consider portion sizes, as some studies used food frequency alone. It is more appropriate for comparison to include portion size by food groups with respect to gender and ethnicity ( 101 ). Portion size needs to be determined based on the norms for the country under investigation and reported in grams per day for equivalence. The adherence to a standardized pattern of a MedDiet as demonstrated in the initial research conducted in 2003, Trichopoulou et al. ( 26 ) also needs to be assessed. This original research, which forms the focus of many of the studies conducted to date, used 14 all-inclusive food groups that were evaluated in grams per day, and consequently prospective studies need to adhere to this specific pattern of evaluation to ensure valid comparisons can be made.
To ensure that direct correlations can be made between studies, it would be of value to have a standardized computer-based FFQ that is easy to administer and simple to review. The use of a standardized FFQ that utilizes 14 all-inclusive food groups all evaluated to grams per day, and this would enable more accurate portions of food taken (grams per day) to be evaluated to obtain a MedDietS.
Interestingly, positive cognitive effects were found in countries around the world, including those outside of the Mediterranean region. Many of these positive studies used an approach where a mean or median is determined for each food category, and adherence for the individual is scored relative to this mean or median. In this way, habitual dietary habits are normalized with respect to the local region. Change over time is assessed relative to baseline scores to assess whether or not greater adherence supports better cognitive health. While this makes it difficult to compare absolute measures of dietary adherence in relation to a more traditional Mediterranean diet ( 26 ), it is encouraging that measurable shifts in adherence within a local population are effective in improving cognition or retarding cognitive decline. An important challenge will be to demonstrate a sustainable long-term shift in diet within a population that also confers cognitive benefits.
Conclusion and Future Directions
This review has analyzed longitudinal and prospective trials in accordance with the specified criteria to gain an understanding of how a MedDiet may impact cognitive processes. The level of adherence to the MedDiet has subsequently been evaluated against various cognitive domains to ascertain the potential protective nature of this diet pattern with respect to minimizing cognitive decline, improving cognitive decline, or reducing the incidence of conversion of MCI to AD.
The overall outcome from this review indicates that there is encouraging evidence that a higher adherence to a MedDiet is associated with improving cognition, slowing cognitive decline, or reducing the conversion to AD.
With few studies available to assess benefits of a MedDiet intervention in a healthy older population, it is recommended that further randomized controlled trials need to be conducted. These trials need to include a standard food consumption assessment that can be validated to determine the impact of cognitive changes over time. It is also recommended that future RCTs utilize validated computer-based cognitive batteries that are sensitive to cognitive faculties, which are compromised with age and potentially amenable to interventions, such as a MedDiet. Future studies should also consider the use of blood, cardio, and fecal biomarkers that will allow for mechanisms of action to be evaluated. Biomarkers will provide more direct measures of dietary status and will potentially elucidate important biological changes that relate to brain function and modifiable risk factors.
Further focused research in this area is important due to the expected extensive population aging over the next 20–30 years. The utilization of such a dietary pattern will be essential as part of the armamentarium to maintain quality of life and reduce the potential social and economic burden of dementia.
Author Contributions
There have been substantial contributions to the conception and design of this review by all authors, and all authors have been involved in revising the work critically for important intellectual content. The final approval of the version to be published has been agreed by all authors, and an agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work has been appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The resources of Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, Australia and the Centre for Physical Activity and Nutrition Research, Deakin University, Melbourne, Australia have been utilized to produce this review, and their contribution is extremely appreciated.
There have been no funding sources provided to any author in the development and final submission for this review.
1. Katsiardanis K, Diamantaras AA, Dessypris N, Michelakos T, Anastasiou A, Katsiardani KP, et al. Cognitive impairment and dietary habits among elders: the Velestino Study. J Med Food (2013) 16(4):343–50. doi:10.1089/jmf.2012.0225
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab (2007) 32(5):954–66. doi:10.1139/h07-085
3. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction – final report of the Lyon Diet Heart Study. Circulation (1999) 99(6):779–85. doi:10.1161/01.CIR.99.6.779
CrossRef Full Text | Google Scholar
4. Parrott MD, Greenwood CE. In: Weller NJ, Rattan SIS, editors. Dietary Influences on Cognitive Function with Aging: From High-Fat Diets to Healthful Eating . Oxen England: Blackwell publishing (2007).
Google Scholar
5. Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr (2015) 54(8):1311–21. doi:10.1007/s00394-014-0811-z
6. Peters R. Ageing and the brain. Postgrad Med J (2006) 82:84–8. doi:10.1136/pgmj.2005.036665
7. Pipingas A, Harris E, Tournier E, King R, Kras M, Stough CK. Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr Top Nutraceutical Res (2010) 8(2–3):79–87.
8. Christensen H, Kumar R. Cognitive changes and the ageing brain. In: Sachdev PS, editor. The Ageing Brain; The Neurobiological and Neuropsychiatry of Ageing . Netherlands: Swets & Zeiylinger (2003). p. 75–97.
9. Otsuka M, Yamaguchi K, Ueki A. Similarities and differences between Alzheimer’s disease and vascular dementia from the viewpoint of nutrition. Ann N Y Acad Sci (2002) 977:155–61. doi:10.1111/j.1749-6632.2002.tb04811.x
10. Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging (2003) 24:S123–7. doi:10.1016/s0197-4580(03)00051-4
11. Anderson MF, Åberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res (2002) 134(1–2):115–22. doi:10.1016/s0165-3806(02)00277-8
12. Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol (2003) 60(7):989–94. doi:10.1001/archneur.60.7.989
13. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci (2004) 27(10):589–94. doi:10.1016/j.tins.2004.08.001
14. Feart C, Samieri C, Alles B, Barberger-Gateau P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc Nutr Soc (2013) 72(1):140–52. doi:10.1017/s0029665112002959
15. Mathers JC. Nutrition and ageing: knowledge, gaps and research priorities. Proc Nutr Soc (2013) 72(2):246–50. doi:10.1017/s0029665112003023
16. Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol (2004) 14(2):186–91. doi:10.1016/j.conb.2004.03.001
17. Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology (2002) 59(5):713–9. doi:10.1212/WNL.59.5.713
18. Babio N, Toledo E, Estruch R, Ros E, Martinez-Gonzalez MA, Castaner O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. Can Med Assoc J (2014) 186(17):E649–57. doi:10.1503/cmaj.140764
19. Garcia-Arellano A, Ramallal R, Ruiz-Canela M, Salas-Salvado J, Corella D, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED Study. Nutrients (2015) 7(6):4124–38. doi:10.3390/nu7064124
20. Salthouse TA. Does the level at which cognitive change occurs change with age? Psychol Sci (2012) 23(1):18–23. doi:10.1177/0956797611421615
21. Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition, inflammation, and cognitive function. In: Savino W, Silva PO, Besedovsky H, editors. Neuroimmunomodulation: From Fundamental Biology to Therapy . Malden, MA: Wiley-Blackwell (2009). p. 164–75.
22. Kirkwood TBL. Understanding the odd science of aging. Cell (2005) 120(4):437–47. doi:10.1016/j.cell.2005.01.027
23. Dauncey MJ. New insights into nutrition and cognitive neuroscience. Proc Nutr Soc (2009) 68(4):408–15. doi:10.1017/s0029665109990188
24. Dauncey MJ. Nutrition, the brain and cognitive decline: insights from epigenetics. Eur J Clin Nutr (2014) 68(11):1179–85. doi:10.1038/ejcn.2014.173
25. Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev (2002) 82(3):637–72. doi:10.1152/physrev.00004.2002
26. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med (2003) 348(26):2599–608. doi:10.1056/NEJMoa025039
27. Martínez-González MA, Fernández-Jarne E, Serrano-Martínez M, Wright M, Gomez-Gracia E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur J Clin Nutr (2004) 58(11):1550–2. doi:10.1038/sj.ejcn.1602004
28. Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev (2004) 3(4):445–64. doi:10.1016/j.arr.2004.08.001
29. Panza F, Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Torres F, et al. Mediterranean diet and cognitive decline. Public Health Nutr (2004) 7(7):959–63. doi:10.1079/phn2004561
30. Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res (2006) 84(4):699–715. doi:10.1002/jnr.20979
31. Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. J Nutr (2009) 139(10):1944–9. doi:10.3945/jn.109.106427
32. Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive function in older adults. Curr Opin Clin Nutr Metab Care (2010) 13(1):14–8. doi:10.1097/MCO.0b013e3283331fe4
33. Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol (2010) 219(1–2):25–32. doi:10.1016/j.jneuroim.2009.11.010
34. Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr (2011) 93(3):601–7. doi:10.3945/ajcn.110.007369
35. Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH through life study. Am J Geriatr Psychiatry (2012) 20(7):635–9. doi:10.1097/JGP.0b013e31823032a9
36. Budge M, Johnston C, Hogervorst E, De Jager C, Milwain E, Iversen SD, et al. Plasma total homocysteine and cognitive performance in a volunteer elderly population. In: Kalaria RN, Ince P, editors. Vascular Factors in Alzheimer’s Disease . New York: New York Acad Science (2000). p. 407–10.
37. Butterfield DA, Castegna A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem (2002) 13(8):444–61. doi:10.1016/s0955-2863(02)00205-x
38. Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience (2004) 123(2):429–40. doi:10.1016/j.neuroscience.2003.09.020
39. He K, Song YQ, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Fish consumption and incidence of stroke – a meta-analysis of cohort studies. Stroke (2004) 35(7):1538–42. doi:10.1161/01.str.0000130856.31468.47
40. Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Mittleman MA, Siscovick DS. Prospective-study of alcohol consumption and risk of dementia in older adults. JAMA (2003) 289(11):1405–13. doi:10.1001/jama.289.11.1405
41. den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, van Duijn CM, et al. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. Am J Clin Nutr (2004) 80(4):992–7.
PubMed Abstract | Google Scholar
42. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy – a review. Nutrients (2016) 8(2):68. doi:10.3390/nu8020068
43. Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci (2008) 9(7):568–78. doi:10.1038/nrn2421
44. Gesch CB, Hammond SM, Hampson SE, Eves A, Crowder MJ. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. Randomised, placebo-controlled trial. Br J Psychiatry (2002) 181(JULY):22–8. doi:10.1192/bjp.181.1.22
45. Morris MC. Symposium 1: vitamins and cognitive development and performance nutritional determinants of cognitive aging and dementia. Proc Nutr Soc (2012) 71(1):1–13. doi:10.1017/s0029665111003296
46. Morley JE. Cognition and nutrition. Curr Opin Clin Nutr Metab Care (2014) 17(1):1–4. doi:10.1097/MCO.0000000000000005
47. Rathod R, Kale A, Joshi S. Novel insights into the effect of vitamin B-12 and omega-3 fatty acids on brain function. J Biomed Sci (2016) 23:17. doi:10.1186/s12929-016-0241-8
48. Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol (2009) 5(3):140–52. doi:10.1038/ncpneuro1044
49. Witte VA, Kerti L, Hermannstaedter HM, Fiebach JB, Schuchardt JP, Hahn A, et al. Effects of Omega-3 supplementation on brain structure and function in healthy elderly subjects. J Psychophysiol (2013) 27:45–45.
50. Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res Rev (2008) 7(1):49–62. doi:10.1016/j.arr.2007.04.003
51. Raji CA, Erickson KI, Lopez OL, Kuller LH, Gach HM, Thompson PM, et al. Regular fish consumption and age-related brain gray matter loss. Am J Prev Med (2014) 47(4):444–51. doi:10.1016/j.amepre.2014.05.037
52. Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, et al. The diet and 15-year death rate in the 7 countries study. Am J Epidemiol (1986) 124(6):903–15.
53. Feart C, Lorrain S, Coupez VG, Samieri C, Letenneur L, Paineau D, et al. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int (2013) 24(12):3031–41. doi:10.1007/s00198-013-2421-7
54. Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology (2013) 24(4):479–89. doi:10.1097/EDE.0b013e3182944410
55. Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B-12 and folate in relation to the development of Alzheimer’s disease. Neurology (2001) 56(9):1188–94. doi:10.1212/WNL.56.9.1188
56. Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on aging. Am J Clin Nutr (2005) 82(6):1346–52.
57. Gotsis E, Anagnostis P, Mariolis A, Vlachou A, Katsiki N, Karagiannis A. Health benefits of the Mediterranean diet: an update of research over the last 5 years. Angiology (2015) 66(4):304–18. doi:10.1177/0003319714532169
58. Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med (2002) 346(7):476–83. doi:10.1056/NEJMoa011613
59. Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Jacques PF, Selhub J, et al. Homocysteine and cognitive performance in the Framingham Offspring Study: age is important. Am J Epidemiol (2005) 162(7):644–53. doi:10.1093/aje/kwi259
60. Van Dyk K, Sano M. The impact of nutrition on cognition in the elderly. Neurochem Res (2007) 32(4–5):893–904. doi:10.1007/s11064-006-9241-5
61. Ordovas JM. Nutrition and cognitive health. In: Science T.G.O.f, editor. Foresight Mental Capital and Wellbeing Project . London: The Government Office of science (2008). p. 314.
62. Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, et al. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr (2013) 97(2):369–76. doi:10.3945/ajcn.112.047993
63. von Arnim CAF, Gola U, Biesalski HK. More than the sum of its parts? Nutrition in Alzheimer’s disease. Nutrition (2010) 26(7–8):694–700. doi:10.1016/j.nut.2009.11.009
64. Ferry M, Roussel AM. Micronutrient status and cognitive decline in ageing. Eur Geriatr Med (2011) 2(1):15–21. doi:10.1016/j.eurger.2010.11.014
65. Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm A-C. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci (2014) 17(6):241–51. doi:10.1179/1476830513y.0000000092
66. Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martinez-Lage P. Diet, cognition, and Alzheimer’s disease: food for thought. Eur J Nutr (2014) 53(1):1–23. doi:10.1007/s00394-013-0561-3
67. Maggi S, Zucchetto M, Grigoletto F, Baldereschi M, Candelise L, Scarpini E, et al. The Italian longitudinal-study on aging (ILSA): design and methods. Aging (Milano) (1994) 6(6):464–73. doi:10.1007/BF03324279
68. Crichton GE, Bryan J, Hodgson JM, Murphy KJ. Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite (2013) 70:53–9. doi:10.1016/j.appet.2013.06.088
69. Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol (2006) 63(12):1709–17. doi:10.1001/archneur.63.12.noc60109
70. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr (1995) 61(6 Suppl):1402S–6S.
71. Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA (2009) 302(6):638–48. doi:10.1001/jama.2009.1146
72. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol (2009) 66(2):216–25. doi:10.1001/archneurol.2008.536
73. Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis (2010) 22(2):483–92. doi:10.3233/jad-2010-100897
74. McMillan L, Owen L, Kras M, Scholey A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite (2011) 56(1):143–7. doi:10.1016/j.appet.2010.11.149
75. Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med (2007) 44(4):335–40. doi:10.1016/j.ypmed.2006.12.009
76. Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet (2012) 112(6):816–23. doi:10.1016/j.jand.2012.02.023
77. Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr (2009) 139(6):1150–6. doi:10.3945/jn.109.103424
78. Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry (2013) 84(12):1318–25. doi:10.1136/jnnp-2012-304792
79. Fernández-Ballarth JD, Lluis Pinol J, Zazpe I, Corella D, Carrasco P, Toledo E, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr (2010) 103(12):1808–16. doi:10.1017/s0007114509993837
80. Samieri C, Okereke OI, Devore EE, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr (2013) 143(4):493–9. doi:10.3945/jn.112.169896
81. Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology (2013) 24(4):490–9. doi:10.1097/EDE.0b013e318294a065
82. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semquantitative food frequency questionnaire among male health-professionals. Am J Epidemiol (1992) 135(10):1114–26.
83. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation (2009) 119(8):1093–100. doi:10.1161/circulationaha.108.816736
84. Titova OE, Ax E, Brooks SJ, Sjögren P, Cederholm T, Kilander L, et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol (2013) 48(12):1443–8. doi:10.1016/j.exger.2013.10.002
85. Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology (2013) 80(18):1684–92. doi:10.1212/WNL.0b013e3182904f69
86. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care (2002) 40(9):771–81. doi:10.1097/01.mlr.0000024610.33213.c8
87. Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet (2013) 113(2):276–81. doi:10.1016/j.jand.2012.10.014
88. Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, et al. Mediterranean diet and cognitive function: the sun project. J Nutr Health Aging (2015) 19(3):305–12. doi:10.1007/s12603-015-0441-z
89. Bowman SA, Friday JE, Moshfegh A. MyPyramid Equivalents Data-base 2.0 for USDA Survey Foods 2003-2004 [Online] . Beltsville: Food Surveys Research Group, Beltsville Human Nutrition Research Centre (2010).
90. Lee J, Pase M, Pipingas A, Raubenheimer J, Thurgood M, Villalon L, et al. Switching to a 10-day Mediterranean-style diet improves mood and cardiovascular function in a controlled crossover study. Nutrition (2015) 31(5):647–52. doi:10.1016/j.nut.2014.10.008
91. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martinez-Gonzalez MA, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med (2015) 175(7):1094–103. doi:10.1001/jamainternmed.2015.1668
92. Folstein MF, Folstein SE, McHugh PR. Mini-mental state-practical methods for grading cognitive state of patients for clinician. J Psychiatr Res (1975) 12(3):189–98. doi:10.1016/0022-3956(75)90026-6
93. Chapman KR, Bing-Canar H, Alosco ML, Steinberg EG, Martin B, Chaisson C, et al. Mini mental state examination and logical memory scores for entry into Alzheimer’s disease trials. Alzheimers Res Ther (2016) 8:9. doi:10.1186/s13195-016-0176-z
94. Watson P. Nutrition, the brain and prolonged exercise. Eur J Sport Sci (2008) 8(2):87–96. doi:10.1080/17461390801919086
95. Biltoft-Jensen A, Damsgaard CT, Andersen R, Ygil KH, Andersen EW, Ege M, et al. Accuracy of self-reported intake of signature foods in a school meal intervention study: comparison between control and intervention period. Br J Nutr (2015) 114(4):635–44. doi:10.1017/s0007114515002020
96. Shatenstein B, Payette H. Evaluation of the relative validity of the short diet questionnaire for assessing usual consumption frequencies of selected nutrients and foods. Nutrients (2015) 7(8):6362–74. doi:10.3390/nu7085282
97. Buscemi S, Rosafio G, Vasto S, Massenti FM, Grosso G, Galvano F, et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int J Food Sci Nutr (2015) 66(4):426–38. doi:10.3109/09637486.2015.1025718
98. Liu XD, Wang XR, Lin SH, Song QK, Lao XQ, Yu ITS. Reproducibility and validity of a food frequency questionnaire for assessing dietary consumption via the dietary pattern method in a Chinese rural population. PLoS One (2015) 10(7):e0134627. doi:10.1371/journal.pone.0134627
99. Pedraza DF, de Menezes TN. Food Frequency Questionnaire developed and validated for the Brazilian population: a review of the literature. Cien Saude Colet (2015) 20(9):2697–720. doi:10.1590/1413-81232015209.12602014
100. Tabacchi G, Filippi AR, Breda J, Censi L, Amodio E, Napoli G, et al. Comparative validity of the ASSO-Food Frequency Questionnaire for the web-based assessment of food and nutrients intake in adolescents. Food Nutr Res (2015) 59:26216. doi:10.3402/fnr.v59.26216
101. Fatihah F, Ng BK, Hazwanie H, Norimah AK, Shanita SN, Ruzita AT, et al. Development and validation of a food frequency questionnaire for dietary intake assessment among multi-ethnic primary school-aged children. Singapore Med J (2015) 56(12):687–94. doi:10.11622/smedj.2015190
102. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi:10.1002/sim.1186
103. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J (2003) 327(7414):557–60. doi:10.1136/bmj.327.7414.557
Keywords: nutrition, cognition, Mediterranean diet, clinical trials
Citation: Hardman RJ, Kennedy G, Macpherson H, Scholey AB and Pipingas A (2016) Adherence to a Mediterranean-Style Diet and Effects on Cognition in Adults: A Qualitative Evaluation and Systematic Review of Longitudinal and Prospective Trials. Front. Nutr. 3:22. doi: 10.3389/fnut.2016.00022
Received: 31 May 2016; Accepted: 05 July 2016; Published: 22 July 2016
Reviewed by:
Copyright: © 2016 Hardman, Kennedy, Macpherson, Scholey and Pipingas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roy J. Hardman, cmhhcmRtYW4mI3gwMDA0MDtzd2luLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
To assess the prevalence of the Mediterranean Diet amongst 12 Connecticut facilities, 3 methodology tools were used: a pre-validated quantitative questionnaire, qualitative interviews, and both qualitative and quantitative content analyses were conducted on dining menus.
Download sample Academic Papers along with scoring guidelines and scoring distributions. If you are using assistive technology and need help accessing these PDFs in another format, contact Services for Students with Disabilities at 212-713-8333 or by email at [email protected].
The Mediterranean Diet (MedDiet) is a term used to identify a dietary pattern originating from the unique multi-millennial interplay between natural food resources and the eating practices of people living in the Mediterranean basin. Scientific ...
Weight loss maintenance is crucial for obesity management, yet optimal dietary patterns for this period are not established. We aimed to explore the relationship between adherence to the Mediterranean diet and weight loss maintenance. Sample includes 565 adults (62 % women) of the MedWeight study.
Key Findings: In this prospective analysis on 2,023 Italian adults from the Moli-sani Study, increased adherence to a Mediterranean diet was associated with reduced low-grade inflammation after a median 12.7-year period. This was not accompanied by relevant changes in modifiable cardiovascular risk factors.
Closer adherence to a traditional Mediterranean-style diet (MedDiet) may be beneficial and protective with respect to addressing such risk factors, ultimately reducing the rate of cognitive decline (5). Neurocognitive decline across the life span is well documented.
AP® RESEARCH 2017 SCORING COMMENTARY Academic Paper Overview This performance task was intended to assess students’ ability to conduct scholarly and responsible research and articulate an evidence-based argument that clearly communicates the conclusion, solution, or answer to their stated research question.
• Generate a focused research question that is situated within or connected to a larger scholarly context or community; • Explore relationships between and among multiple works representing multiple perspectives within
We therefore conducted a systematic review of randomized controlled trials (RCTs) to determine the effect of the Mediterranean diet on weight loss and cardiovascular risk factor levels after ≥12 months.
Abstract: In biomedical literature, The Mediterranean Diet describes a healthy eating model, based on epidemiological findings on the predominant eating practices in Crete and Southern Italy in the 1960s.