An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed, diabetes in america, affiliations.
- 1 Senior Advisor and Director for Diabetes Epidemiology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
- 2 Senior Research Analyst, Social & Scientific Systems, Inc., Silver Spring, MD
- 3 Science Writer/Editor, Chicago, IL
- 4 Epidemiologist, Division of Health and Nutrition Examination Surveys, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD
- 5 Professor of Medicine, Harvard Medical School, and Physician, Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Boston, MA
- 6 Chief, Epidemiology and Statistics Branch, Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA
- 7 Chief, Diabetes Epidemiology and Clinical Research Section, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Phoenix, AZ
- 8 Distinguished Professor, Division of Epidemiology, Department of Family Medicine and Public Health, University of California, San Diego, La Jolla, CA
- 9 Professor of Pediatrics, Division of Endocrinology and Diabetes, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA
- 10 Distinguished Service Professor of Medicine and Epidemiology, Johns Hopkins University, Baltimore, MD
- 11 Professor, University of Washington, and Staff Physician, Veterans Affairs Puget Sound, Seattle, WA
- 12 Professor, Departments of Internal Medicine and Epidemiology, University of Michigan, Ann Arbor, MI
- 13 Professor of Medicine, Department of Medicine, Division of Endocrinology and Metabolism, Georgetown University Hospital, and Senior Scientist, MedStar Health Research Institute, Hyattsville, MD
- 14 Director, Emory Global Diabetes Research Center, Ruth and O.C. Hubert Professor of Global Health and Epidemiology, Rollins School of Public Health, and Professor of Medicine, School of Medicine, Emory University, Atlanta, GA
- 15 Professor of Pediatrics and Medicine, Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO
- 16 Director, Division of Diabetes, Endocrinology, and Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
- PMID: 33651524
- Bookshelf ID: NBK567985
Diabetes in America, 3rd Edition, is a compilation and assessment of epidemiologic, public health, clinical, and clinical trial data on diabetes and its complications in the United States. It was published by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, during 2016–2018. The intended audience is the wide range of individuals in the research community, clinicians, health policy makers, and individuals with diabetes, as well as their caregivers and family members.
Section I: Spectrum of Diabetes: Descriptive epidemiology of diabetes in the United States based on national surveys and community-based studies, including prevalence and incidence; sociodemographic, metabolic, and lifestyle characteristics; genetics and risk factors for developing diabetes; and unique aspects of diabetes in younger and older populations, and in pregnant women (chapters 1–16)
Section II: Complications of Diabetes and Related Conditions: The myriad complications that affect patients with diabetes, including mortality (chapters 17–36)
Section III: Prevention and Medical Care for Diabetes: Clinical trials and studies to prevent diabetes; medication use and self-care practices, health care utilization, and quality of care; and economic aspects including health insurance and health care costs (chapters 37–42)
PubMed Disclaimer
- Steering Committee
- Editorial Board
- External Reviewers
- CHAPTER 1. Classification and Diagnosis of Diabetes
- CHAPTER 2. Prevalence and Incidence of Type 1 Diabetes Among Children and Adults in the United States and Comparison With Non-U.S. Countries
- CHAPTER 3. Prevalence and Incidence of Type 2 Diabetes and Prediabetes
- CHAPTER 4. Gestational Diabetes
- CHAPTER 5. Preexisting Diabetes and Pregnancy
- CHAPTER 6. Other Specific Types of Diabetes
- CHAPTER 7. Monogenic Forms of Diabetes
- CHAPTER 8. Sociodemographic Characteristics of Persons With Diabetes
- CHAPTER 9. Physical and Metabolic Characteristics of Persons With Diabetes and Prediabetes
- CHAPTER 10. Lifestyle Characteristics Among People With Diabetes and Prediabetes
- CHAPTER 11. Risk Factors for Type 1 Diabetes
- CHAPTER 12. Genetics of Type 1 Diabetes
- CHAPTER 13. Risk Factors for Type 2 Diabetes
- CHAPTER 14. Genetics of Type 2 Diabetes
- CHAPTER 15. Diabetes in Youth
- CHAPTER 16. Diabetes in Older Adults
- CHAPTER 17. Acute Metabolic Complications in Diabetes
- CHAPTER 18. Heart Disease and Diabetes
- CHAPTER 19. Stroke and Diabetes
- CHAPTER 20. Peripheral Arterial Disease, Foot Ulcers, Lower Extremity Amputations, and Diabetes
- CHAPTER 21. Epidemiology of Ocular Functions and Diseases in Persons With Diabetes
- CHAPTER 22. Kidney Disease in Diabetes
- CHAPTER 23. Peripheral and Autonomic Neuropathy in Diabetes
- CHAPTER 24. Diabetes and Cognitive Impairment
- CHAPTER 25. Impact of Sleep and Circadian Disturbances on Glucose Metabolism and Type 2 Diabetes
- CHAPTER 26. Liver and Gallbladder Disease in Diabetes
- CHAPTER 27. Gastrointestinal Manifestations of Diabetes
- CHAPTER 28. Urologic Diseases and Sexual Dysfunction in Diabetes
- CHAPTER 29. Cancer and Diabetes
- CHAPTER 30. Infections Associated With Diabetes
- CHAPTER 31. Oral Health and Diabetes
- CHAPTER 32. Bone and Joint Complications in Diabetes
- CHAPTER 33. Psychiatric and Psychosocial Issues Among Individuals Living With Diabetes
- CHAPTER 34. Diabetes and Disability
- CHAPTER 35. Mortality in Type 1 Diabetes
- CHAPTER 36. Mortality Trends in Type 2 Diabetes
- CHAPTER 37. Prevention of Type 1 Diabetes
- CHAPTER 38. Prevention Of Type 2 Diabetes
- CHAPTER 39. Medication Use and Self-Care Practices in Persons With Diabetes
- CHAPTER 40. Health Care Utilization and Costs of Diabetes
- CHAPTER 41. Quality of Care in People With Diabetes
- CHAPTER 42. Health Insurance and Diabetes
- APPENDIX 1. Conversions
Similar articles
- Diabetes in America [Internet]. Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, editors. Lawrence JM, editor, et al. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023–. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023–. PMID: 38117921 Free Books & Documents. Review.
- FreeStyle Libre Flash Glucose Self-Monitoring System: A Single-Technology Assessment [Internet]. Bidonde J, Fagerlund BC, Frønsdal KB, Lund UH, Robberstad B. Bidonde J, et al. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2017 Aug 21. Report from the Norwegian Institute of Public Health No. 2017-07. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2017 Aug 21. Report from the Norwegian Institute of Public Health No. 2017-07. PMID: 29553668 Free Books & Documents. Review.
- Preexisting Diabetes and Pregnancy. Kitzmiller JL, Ferrara A, Peng T, Cissell MA, Kim C. Kitzmiller JL, et al. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug. CHAPTER 5. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug. CHAPTER 5. PMID: 33651557 Free Books & Documents. Review.
- Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet]. LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. LeBlanc EL, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
- Cesarean section. [No authors listed] [No authors listed] Netw Res Triangle Park N C. 1989;10(4):10-1. Netw Res Triangle Park N C. 1989. PMID: 12342592
Publication types
- Search in PubMed
- Search in MeSH
- Add to Search
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- NCBI Bookshelf

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 26 May 2024
An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory
- Tongyue Yang 1 ,
- Feng Qi 2 , 3 ,
- Feng Guo 1 ,
- Mingwei Shao 1 ,
- Yi Song 1 ,
- Gaofei Ren 1 ,
- Zhao Linlin 1 ,
- Guijun Qin 1 &
- Yanyan Zhao ORCID: orcid.org/0000-0001-6294-9447 1
Molecular Medicine volume 30 , Article number: 71 ( 2024 ) Cite this article
423 Accesses
Metrics details
Diabetes mellitus, a chronic metabolic disease, often leads to numerous chronic complications, significantly contributing to global morbidity and mortality rates. High glucose levels trigger epigenetic modifications linked to pathophysiological processes like inflammation, immunity, oxidative stress, mitochondrial dysfunction, senescence and various kinds of cell death. Despite glycemic control, transient hyperglycemia can persistently harm organs, tissues, and cells, a latent effect termed "metabolic memory" that contributes to chronic diabetic complications. Understanding metabolic memory's mechanisms could offer a new approach to mitigating these complications. However, key molecules and networks underlying metabolic memory remain incompletely understood. This review traces the history of metabolic memory research, highlights its key features, discusses recent molecules involved in its mechanisms, and summarizes confirmed and potential therapeutic compounds. Additionally, we outline in vitro and in vivo models of metabolic memory. We hope this work will inform future research on metabolic memory's regulatory mechanisms and facilitate the development of effective therapeutic compounds to prevent diabetic complications.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated blood glucose caused by deficiency or resistance to insulin (Joslin 1946 ). Chronic hyperglycemia can lead to multiple organ injury, thereby causing various complications, such as diabetic retinopathy (DR), diabetic kidney disease (DKD), and diabetic cardiovascular disorders (Zheng et al. 2018 ). Epidemiological studies have revealed that DM has emerged as a significant threat to human mortality. At present, the International Diabetes Federation (IDF) estimates that DM affects approximately 536.6 million adults worldwide, and that number is expected to increase to 783.2 million by 2045 (Sun et al. 2022 ).
In addition to its high incidence, the pathogenesis of diabetic complications is also very complex. In the early stages of DM, hyperglycemia induces oxidative stress and excessive advanced glycation end product (AGE) formation (Domingueti et al. 2016 ). As the disease progresses, protein glycation and mitochondrial DNA (mtDNA) damage to respiratory chain components can in turn exacerbate oxidative stress injury (Bhatti et al. 2022 ). Metabolic imbalance then promotes inflammation through binding receptors for glycation products to cause senescence or cell death (Takahashi et al. 2022 ; Phoenix et al. 2022 ; Teodoro et al. 2018 ). These structural changes can lead to various diabetes-related vascular complications (Teodoro et al. 2018 ). To improve the mechanisms described above, multiple novel hypoglycemic agents, such as sodium glucose co-transporter 2 inhibitor (SGLT2i), dipeptidyl peptidase 4 inhibitors (DPP4i) and glucagon-like peptide 1 receptor agonists (GLP-1RAs), have been applied in clinical practice (Mouhayyar et al. 2020 ; Nathan et al. 2013 ; Zhang and Wu 2014 ) (Mostafa et al. 2016 ; Mostafa et al. 2015 ). However, early hyperglycemia can still lead to a variety of diabetic complications. Fortunately, the novel concept of “metabolic memory” may explain this phenomenon. Metabolic memory, also known as hyperglycemic memory, arises from the enduring presence of an underlying driver. The persistence of cellular changes and characteristics represents the organism's recovery of a prior metabolic state, potentially playing a pivotal role in the etiology of DM and its chronic complications (Reddy et al. 2015 ).
In this comprehensive review, we aim to delve into the research chronology and distinct characteristics of metabolic memory. Additionally, we present a summary of the diverse molecular mechanisms that govern its regulation. By emphasizing its prevalence and profound implications, we highlight the significance of metabolic memory in various chronic diabetes complications. Furthermore, we delve into potential mechanisms and pharmacological advancements related to metabolic memory. Additionally, we consolidate information on various in vitro and in vivo models of metabolic memory. We hope that this review can offer valuable insights into the intricacies of metabolic memory, thereby paving the way for novel therapeutic strategies for the treatment of DM and its complications.
Overview of metabolic memory
The metabolic memory of diabetes refers to the observation that patients are vulnerable to developing diabetic complications due to early hyperglycemia, even if effective hypoglycemic agents are taken to maintain blood glucose within normal levels in the later stage of DM. As shown in Fig. 1 A, in 1987, Engerman et al. (Engerman and Kern 1987 ) first described the phenomenon of metabolic memory that decreased hyperglycemia to normal levels after 2.5 years of exposure in diabetic dogs, and the incidence of DR was still high (Engerman and Kern 1987 ). In addition, high glucose caused an increase in fibronectin and collagen IV expression that could not be reversed even after restoration to normal levels in diabetic rats in 1990 (Roy et al. 1990 ). In 1993, Hammes et al. (Hammes et al. 1993 ) further described the exposure time more accurately. Their research indicated that islet transplantation to diabetic rats could prevent the occurrence of DR within 6 weeks after onset. However, at 12 weeks after onset, DR still occurred. Later, in 2003, the Diabetes Control and Complications Trial (DCCT) with further follow-up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (DCCT/EDIC), where the concept of "metabolic memory" was first proposed, demonstrated that initial hyperglycemia still increased the risk of long-term diabetic complications, although the HbA1c of the intensive treatment group and conventional treatment group was maintained at similar levels (Writing Team 2003 ). In 2008, the United Kingdom Prospective Diabetes Study (UKPDS) again demonstrated the term “legacy effect”, in which early intensive glucose lowering can lead to long-term benefits in patients with newly diagnosed type 2 diabetes (Holman et al. 2008 ; Ranjit Unnikrishnan et al. 2011 ). Both "metabolic memory" and the “legacy effect” refer to the long-term effects of blood glucose on macrovascular and microvascular complications of diabetes. However, the concept of metabolic memory may focus on the negative effect of hyperglycemia impairment, while the legacy effect mainly focuses on the positive influence of effective treatments.
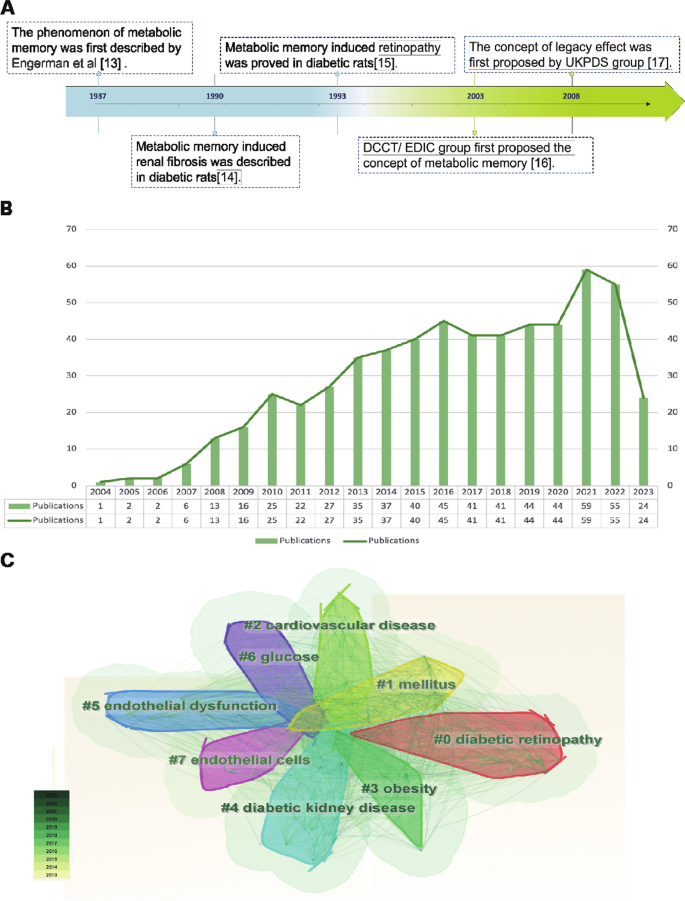
Overview of metabolic memory. A Chronological depiction of key events in the development of metabolic memory. B , C Bibliometric analysis exploring the intersection of metabolic memory and diabetic complications. Search criteria were set as follows: TS = ((“metabolic memory” OR “hyperglycemic memory”) AND (“diabetes” OR “diabetic”)) with a date range of DOP = (2013–08-01/2023–08-01). B Illustration of the annual trend in the number of published articles. C Clustered view of the key terms and concepts emerging from the literature
The bibliometric analysis of the research published on metabolic memory in the decade following its formal designation in 2004. Based on the information provided by the Web of Science (webofscience.com), we analyzed the scientific output related to metabolic memory and diabetes from 2000 to 2022. In total, 579 articles were identified. The trend of research related to metabolic memory and diabetes is displayed in Fig. 1 B, which shows a steady upward trend since its official naming in 2004, particularly in 2021–2022. Cluster analysis of high-frequency keywords related to metabolic memory and diabetes was performed using CiteSpace (Fig. 1 C). The clustering outcomes revealed a preponderance of research centering on the interplay between metabolic memory and diabetes, with a focus on DR, cardiovascular disease, endothelial dysfunction, DKD and obesity. Notably, obesity is intricately intertwined with glycemic and metabolic homeostasis, as evident in previous studies (El-Mesallamy et al. 2013 ; Aboouf et al. 2015 ; Khella et al. 2017 ). However, Zapata et al. ( 2022 ) also observed that obesity elicits a persistent metabolic imprint that persists despite weight loss, phenotypically resembling metabolic memory. Despite this, the existing literature consistently associates metabolic memory with glycemic fluctuations. This preponderance of findings can be partially attributed to the inherent constraints of bibliometric analysis, including the challenges associated with the precision and breadth of bibliographic databases, the absence of contextual understanding, and potential biases towards high-impact journals or specific research domains. Consequently, there is a pressing need for further exploration in this realm to clarify the intricate relationships among obesity, metabolic memory, and glycemic fluctuations. Our review primarily centered on metabolic memory and the potential long-term health implications of transient abnormalities in glucose metabolism.
The main molecular mechanisms of metabolic memory
The underlying mechanisms of metabolic memory and diabetic complications include inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. In fact, these mechanisms involve crosstalk with each other (Galicia-Garcia, et al. 2020 ; Berezin 2016 ). Epigenetic modifications can lead to inflammation, oxidative stress, and senescence, which in turn can be regulated by these mechanisms (Fig. 2 ).
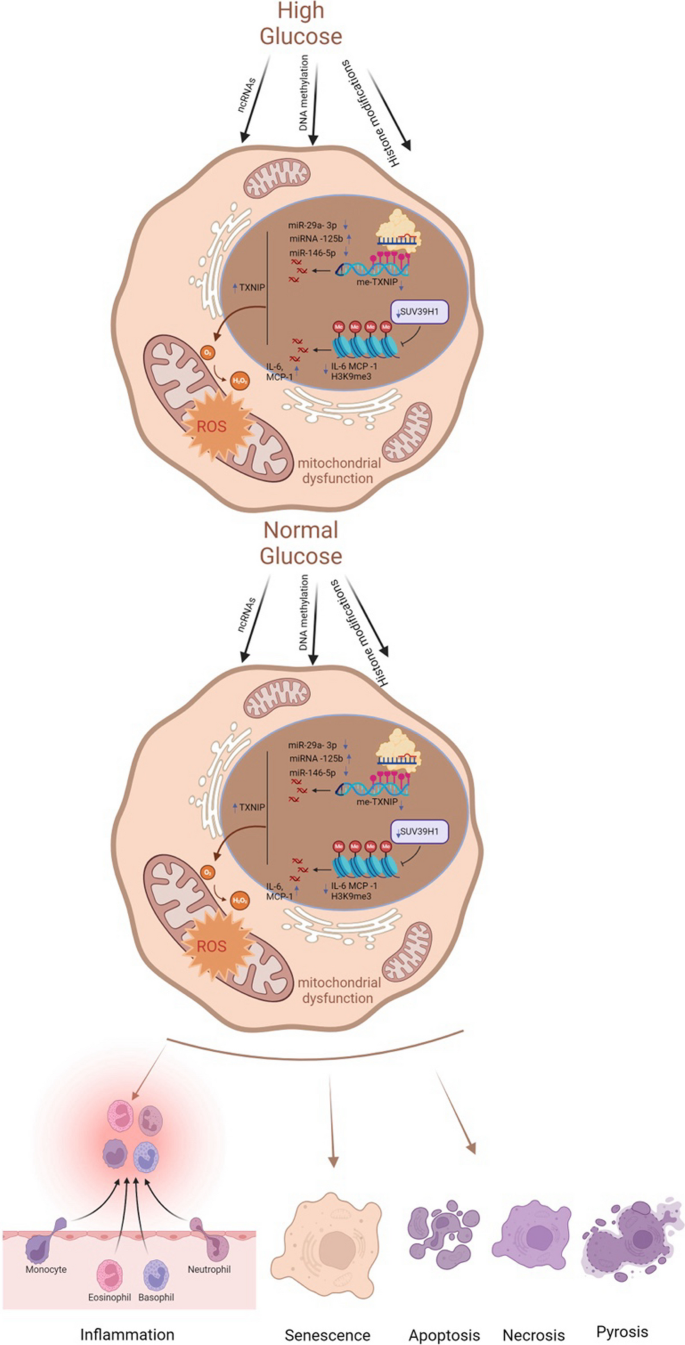
Key molecular mechanisms of metabolic memory. Despite the normalization of glucose levels, epigenetic modifications, inflammatory and immune responses, oxidative stress, mitochondrial dysfunction, cellular senescence, and apoptosis persist. These processes constitute the core molecular mechanisms underlying metabolic memory. ncRNAs noncoding RNAs, TXNIP thioredoxin-interacting protein, me-TXNIP thioredoxin-interacting protein, IL-6 interleukin-6, MCP-1 monocyte chemotactic protein 1, H3K9me3 trimethylated histone H3 at lysine 9, ROS reactive oxygen species
Epigenetic mechanisms involved in metabolic memory
Epigenetic mechanisms, including DNA methylation, histone modifications and noncoding RNAs (ncRNAs), can influence transcription activity and the generation of a heritable phenotype without changing DNA sequences (Goldberg et al. 2007 ). Emerging studies have indicated a key role for epigenetic modifications in the regulation of physiological and pathological processes associated with diabetic complications and metabolic memory (Chen and Natarajan 2022 ). Thus, this section mainly focuses on various modifications involved in hyperglycemic memory.
DNA methylation
DNA methylation, the most stable and widely reported epigenetic mechanism, is considered the primary transcriptional regulator. To investigate the relationship between hyperglycemic memory and DNA methylation, Chen et al. ( 2016 ) selected patients with type 1 diabetes mellitus (T1DM) from DCCT and EDIC studies. They discovered twelve distinctively annotated differentially methylated loci that exhibited a strong association with hyperglycemia and were intricately linked to diabetic complications. Notably, among these loci, thioredoxin-interacting protein ( TXNIP ) is a pivotal gene in the pathogenesis of diabetic complications. Transient hyperglycemic episodes were found to trigger hypomethylation at the 3’ untranslated region (3′ UTR) of TXNIP, leading to persistently elevated expression of this protein in peripheral blood cells (Thielen and Shalev 2018 ). This, in turn, triggered oxidative stress and triggered apoptotic and pyroptotic processes (Choi and Park 2023 ). Moreover, Park et al. ( 2014 ) derived foot fibroblasts from patients with diabetes with or without ulcers and from nondiabetic subjects without foot ulcers. Then, foot fibroblasts from patients with DM were cultured for four passages under normoglycemic conditions, and global and genome-wide DNA methylation profiles were used to identify alterations in DNA methylation. Their results illustrated that DNA methylation and metabolic memory were associated with poor wound healing outcomes in patients with diabetic foot ulceration. Similarly, proximal tubular epithelial cells (PTECs) derived from patients with or without diabetes were cultured via normoglycemic culture for four passages. After integrative omics analysis, multiple changes in DNA methylation sites were detected; among these changes, HNF4A may regulate epigenetic and hyperglycemic memory in DKD (Bansal et al. 2020 ).
In summary, these studies suggest that DNA methylation plays a vital role in metabolic memory and diabetic complications. In addition, as DNA methylation is involved in hyperglycemic memory, a review speculated that emerging m6A RNA methylation may also be a potential mechanism (Kumari et al. 2021 ). However, this theory remains to be confirmed in the future.
Histone modifications
Histones, including the corehistones H2A, H2B, H3, and H4 and the linker histone H1, can bind tightly to DNA to form nucleosome structures. Histone posttranslational modifications (HPTMs) refer to covalent modifications in which different modifications are added to one or several amino acid residues on the tails of histones. The modified histones change the loose or tight binding state between histones and DNA to effectively regulate gene transcription. The most common HPTMs are acetylation (Kac) and methylation (Kme) (Jin and Jeong 2023 ; Sun et al. 2023 ). Filgueiras et al. ( 2017 ) demonstrated that STAT1/MyD88 mRNA and protein levels remained elevated for a minimum of six days in macrophages from diabetic mice. This upregulation could be attenuated by the histone acetyltransferase (HAT) inhibitor anacardic acid. Furthermore, in the skeletal muscle tissue of diabetic mice, persistent enhanced Ped/Pea-15 expression was related to histone H3 lysine 4 monomethylation (H3K4me1) but not histone H3 Lys27 acetylation (H3K27Ac). The high expression of H3K4me1 remained stable even after re-exposure to 5 mM glucose-containing medium. However, there was a prompt loss of acetylation at K27 on histone H3 and a reduction in p300 recruitment at Ped/Pea-15 (Vastolo et al. 2018 ). In addition to H3K4me1, H3K9me3, a crucial repressive and relatively stable epigenetic chromatin mark, also contributes to metabolic memory in vascular smooth muscle cells (VSMCs) derived from db/db mice. The persistent downregulation of H3K9me3 and the inflammatory phenotype could be reversed by overexpressing suppressor of variegation 3–9 homolog 1 (Suv39h1), which is a histone methyltransferase (Sun et al. 2023 ). H3K4me1 and H3K9me3 also regulate metabolic memory in CMs and vascular endothelial cells, respectively (Yu et al. 2012 ; Okabe et al. 2012 ; Mao et al. 2019 ). Regrettably, relatively few studies on HPTMs and metabolic memory, especially some emerging HPTMs, such as lactylation, ubiquitination and glycosylation.
ncRNAs, which mainly include microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), play a vital role in diabetes and its complications as well as multidrug resistance (Li et al. 2022a ; Mahmoud et al. 2021 ). As another major mechanism of epigenetic regulation, ncRNAs can regulate gene expression by modulating protein synthesis at the posttranscriptional and translational levels (Taft et al. 2010 ). miRNAs, a class of endogenous single-stranded RNAs composed of 20–22 nucleotides, can participate in regulating posttranscriptional gene expression by binding to target mRNAs (Krol et al. 2010 ). Currently, various miRNAs have been reported to participate in metabolic memory and diabetic complications. To identify hyperglycemic memory-related miRNAs in human aortic endothelial cells, Zhong et al. ( 2015 ) used a miRCURY LNA array to screen for transcriptional changes in the normal glucose, high glucose and metabolic memory groups. After validation in vitro and in vivo, miR-125b, miR-29a-3p, and miR-146a-5p were shown to potentially be important for metabolic memory. Notably, miR-125b was the only miRNA confirmed to be related to metabolic memory, specifically targeting Suv39h1 to promote inflammation in VSMCs from diabetic mice (Villeneuve et al. 2010 ). Subsequently, Costantino et al. ( 2016 ) screened 268 miRNAs that remained significantly altered after 3 weeks of intensive glycemic control with insulin from heart samples. The majority of miRNAs related to metabolic memory effects, according to an ingenuity pathway analysis, regulate the myocardial pathways of apoptosis, autophagy, oxidative stress, fibrosis, hypertrophy and heart failure. Regrettably, they verified miRNA expression in left ventricular samples from controls, diabetic mice, and diabetic mice treated with insulin without further exploring the underlying mechanisms involved. In addition, miR-23b-3p has been proven to regulate high glucose-induced metabolic memory via the SIRT1-dependent signaling pathway in DR (Zhao et al. 2016 ). However, in-depth studies of the links between key lncRNAs and the crosstalk between lncRNAs and miRNAs in metabolic memory and diabetic complications still need further exploration.
Inflammation, immunity, oxidative stress and mitochondrial dysfunction
High blood glucose can induce chronic metabolic inflammation, which contributes to the development of various complications (Nedosugova et al. 2022 ). Monocytes and macrophages, crucial components of immunity, participate in inflammation in diabetic complications. The proinflammatory activation of macrophages within the liver and adipose tissue can initiate the recruitment and promotion of macrophage polarization, thereby inducing these cells to secrete inflammatory cytokines, including IL-1β, IL-6, and TNF-α. This, in turn, results in immune imbalance, highlighting the critical role of macrophage activation in the pathogenesis of inflammatory conditions (Bleriot et al. 2023 ; Ding et al. 2022 ). To further investigate the intricate relationships among inflammation, immunity, and metabolic memory, Mossel et al. ( 2020 ) investigated metabolic memory in primary human macrophages. Their findings revealed that even after normalizing glucose levels, the expression of S100A9 and S100A12 remained elevated, potentially due to transient hyperglycemia-induced histone methylation at the promoters of these genes. In addition, innate immune cells, which are integral to diabetes-related complications, can establish nonspecific immunological memory (trained immunity) through epigenetic regulation. Thiem et al. ( 2021 ) established both in vitro and in vivo trained immunity models using bone marrow cell transplantation and monocyte isolation. Their study demonstrated that glucose modulation of innate immune cell histone methylation levels can persist, leading to increased glycolysis and exacerbated inflammatory responses even after glucose normalization. Given these insights, diabetes and its complications related to oxidative stress and inflammation, as well as immunity, can significantly benefit from vitamin E intake (Hamdy et al. 2009 ).
In addition to the aforementioned factors, oxidative stress and mitochondrial dysfunction play essential roles in metabolic memory (Peng, et al. 2020 ). An imbalance between oxidative and antioxidative processes gives rise to oxidative stress, which can trigger lipid accumulation, inflammation, and fibrosis in diabetic complications (Zhang et al. 2020 ). Reactive oxygen species (ROS), a hallmark of oxidative stress, encompass a range of free radicals, including superoxide anions, hydroxyl and peroxyl radicals, and other compounds capable of generating free radicals (Halliwell 2006 ). Since mitochondria are key intracellular sources of ROS, mitochondrial dysfunction is intimately linked to oxidative stress (Cojocaru, et al. 2023 ). Multiple studies have established that oxidative stress and mitochondrial dysfunction are integral to the mechanism of metabolic memory in diabetic complications, particularly in the progression of DR (Wang et al. 2018 ; Zhong and Kowluru 2013 ; Voronova et al. 2017 ; Drzewoski et al. 2009 ). Sirtuin-1 (SIRT-1) functions as a modulator of antioxidant defense, energy metabolism, and organelle homeostasis, making it a key player in oxidative stress and mitochondrial dysfunction in various diseases (Kung, et al. 2021 ; Li et al. 2022b ). Lee et al. ( 2022 ) demonstrated that SIRT-1 was a link between hyperglycemic memory and oxidative stress and mitochondrial dysfunction in DR. Additionally, Kowluru et al. ( 2023 ) provided evidence that transient hyperglycemia results in a persistent imbalance in mitochondrial fission, mitophagy, and new mitochondrial formation, ultimately leading to oxidative stress in DR. Beyond mitochondrial dysfunction, oxidative stress intersects with other organelle dysfunctions, including endoplasmic reticulum (ER) stress, Golgi apparatus stress, and lysosomal homeostasis (Maamoun et al. 2019 ; Gong et al. 2022 ; Jiang et al. 2011 ). However, the intricate relationships between these processes remain largely unexplored and require further investigation.
Senescence and cell death
Cellular senescence, a type of permanent proliferative arrest without cell death, is divided into epigenetically induced senescence, oxidative stress-induced senescence and DNA damage-induced senescence (Hernandez-Segura et al. 2018 ). The process of senescence is closely related to programmed cell death (PCD) (Galluzzi and Myint 2023 ). When cellular damage cannot be efficiently repaired, irreversible dysfunction of cells can lead to PCD, including apoptosis, autophagy, pyroptosis and ferroptosis (Moujalled et al. 2021 ). Recently, a p21 -dependent pathway was identified that contributes to senescence and hyperglycemic memory in DKD (Al-Dabet et al. 2022 ). Furthermore, Mansour et al. ( 2023 ) demonstrated that overexpressed p21 can lead to senescence and increase the expression of BAX, a pro-apoptotic gene, to alleviate apoptosis. These results indirectly illustrate that p21, a key gene in metabolic memory, also participates in senescence and apoptosis and may be a promising target. Moreover, in DR, temporary high glucose could lead to consistent upregulation of miR-195 to decrease the expression of its target gene Bcl-2 , which is an antiapoptotic gene (Liu et al. 2019a ). This research suggested that epigenetic mechanisms, as representative ncRNAs, may interact with senescence and cell death in hyperglycemic memory. Nevertheless, how do other types of cell death regulate metabolic memory in diabetic complications? This question is still unanswered.
Metabolic memory and chronic complications of DM
Multiple large-scale clinical trials have verified that early intensive glycemic control can reduce the incidence and progression of macrovascular and microvascular complications of diabetes, including diabetic cardiovascular disorders, DKD, DR, and diabetic foot disease (DF) (C., I. 2003 ; Cuore et al. 2023 ; Brown et al. 2010 ; Nathan et al. 2014 ; Aiello et al. 2014 ), which is basically consistent with the results of our bibliometric analysis (Fig. 1 C). Numerous studies have also used experiments to elucidate the mechanisms underlying this clinical phenomenon in diabetic complications (Yamagishi et al. 2017 ; Zhong et al. 2023 ; Kato and Natarajan 2019 ). Thus, in this section, we will discuss the relationship between metabolic memory and chronic complications of DM (Fig. 3 ).
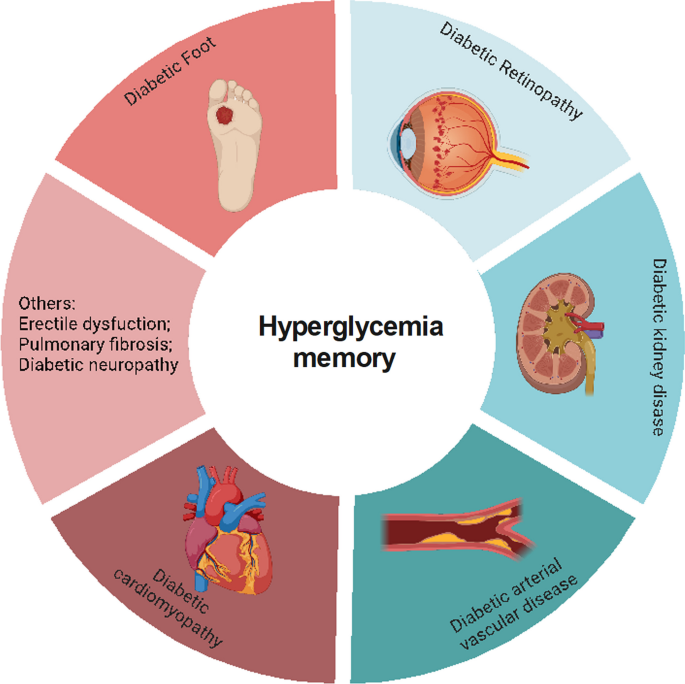
Metabolic memory and chronic complications of diabetes. Hyperglycemia can trigger a range of diabetic complications, including diabetic cardiomyopathy, diabetic arterial vascular disease, diabetic kidney disease, diabetic retinopathy, and diabetic foot. This figure illustrates the intricate relationship between metabolic memory and these chronic conditions
Diabetic cardiovascular disorders and metabolic memory
Diabetic cardiovascular disorders, including diabetic cardiomyopathy (DCM) and arterial vasculopathy, are the leading causes of death among patients with diabetes (Fang et al. 2004 ). Elevated blood glucose stimulates inflammation, regulates immune cells, and promotes the production of cytotoxic free radicals, thereby attacking myocardial cells and vascular endothelial cells (Johnson et al. 2022 ; Xie et al. 2022 ). Under the action of these mechanisms triggered by high glucose, damaged cells further secrete harmful irritants, which promote the transdifferentiation of other cell types into cardiac fibroblasts (Cheng et al. 2023 ). Subsequently, various adhesion molecules and adipokines, such as adiponectin, influence these fibroblasts, activating them to migrate and aggregate, thus exacerbating myocardial and vascular injury (El-Mesallamy et al. 2011 ). However, evidence from clinical trials has indicated that even with intensified blood glucose control, patients with diabetes are still at risk for diabetic cardiovascular diseases due to metabolic memory. This section discusses the relationship between diabetic cardiovascular diseases and metabolic memory.
DCM and metabolic memory
DCM is a cardiovascular complication that arises from DM and causes alterations in cardiac structure and function, independent of hypertension, coronary atherosclerotic heart disease, or any other known cardiac risk factors (Jia et al. 2018 ). Previous studies have established that metabolic dysfunction in cardiomyocytes, myocardial interstitial fibrosis, abnormal calcium transients in cardiomyocytes, and cardiac autonomic neuropathy play pivotal roles in the pathogenesis of DCM (Palomer et al. 2018 ; Marwick et al. 2018 ). Roy et al. ( 1990 ) demonstrated that fibronectin mRNA expression increased even after blood glucose returned to normal in streptozotocin (STZ)-induced diabetic rats. Given the mechanisms and manifestations of DCM, metabolic memory may play a key role in its development and progression (Zhan et al. 2022 ). Additionally, previous studies have shown that miR-320 mediates apoptosis in DCM (Su et al. 2020 ). Moreover, multiple studies have suggested that cluster of differentiation 36 (CD36) regulates free fatty acid uptake in DCM, and CD36-deficient patients and CD36 knockout mice exhibit a significant reduction in the myocardial uptake of long-chain fatty acids (LCFAs) (Zhang et al. 2021 ). A recent study revealed a connection between these factors, revealing that miR-320 serves as a central ncRNA in metabolic memory and positively interacts with CD36 to alleviate diastolic dysfunction caused by hyperglycemic memory in cardiomyocytes (Zhan et al. 2023 ). This finding offers novel insights into the pathogenesis of DCM and its molecular functions.
Diabetic arterial vasculopathy and metabolic memory
Elevated blood glucose can inflict substantial harm on both the microvascular and macrovascular systems, ultimately leading to endothelial dysfunction, atherosclerosis, and various vascular complications (Li et al. 2023 ). Observations from studies such as the EDIC and UKPDS revealed that individuals in the intensive treatment group developed fewer microvascular and macrovascular diseases (C., I. 2003 ; Retnakaran et al. 2006 ). Jax et al. ( 2010 ) argued that structural alterations, including perivascular fibrosis of microvessels, can exert a direct impact on upstream arteries, gradually leading to endothelial dysfunction and, subsequently, the development of atherosclerosis. The endothelium, the largest organ of the body, plays a pivotal role in regulating the functionality of blood vessels. Persistent hyperglycemia leads to oxidative stress, inflammation, and abnormal mitochondrial metabolism, all of which contribute to endothelial dysfunction (Wang et al. 2022 ). Remarkably, even when transient hyperglycemic conditions revert to normal glycemic levels, oxidative stress and inflammatory factors persist within aortic endothelial cells (El-Osta et al. 2008 ). Damaged endothelial cells lose their functionality and undergo a process known as endothelial-to-mesenchymal transition (EndMT), during which they transform into mesenchymal cells or myofibroblasts, thereby contributing to pathological fibrosis (Xu and Kovacic 2023 ; Bischoff 2019 ). Previous research has shown that hyperglycemic memory can also trigger EndMT and fibrosis (Al-Dabet et al. 2022 ). In this context, miR-27a, a ncRNA closely associated with EndMT and fibrosis, has been further implicated in the NF-κB/miR-27a-3p/NRF2/ROS/TGF-β/EndMT feedback loop, which regulates metabolic memory in endothelial cells (Liu et al. 2019b ; Yao et al. 2022 ). Reddy et al. ( 2016 ) demonstrated that the expression of miR-504 remains persistently high in diabetic VSMCs even after several passages of in vitro culture, enhancing ERK1/2 activation and VSMC dysfunction in atherosclerosis and restenosis.
In summary, metabolic memory is intricately linked to oxidative stress, inflammation, and fibrosis and plays a pivotal role in the pathogenesis of DCM and diabetic arterial vasculopathy. The involvement of ncRNAs, such as miR-320 and miR-27a, points to complex regulatory mechanisms underlying these processes. Nevertheless, other miRNAs, such as miR-423, miR-499, and miR-199a, have been implicated in metabolic memory and the diabetic heart, but further investigation is needed to fully elucidate their roles (Costantino et al. 2016 ).
DKD and metabolic memory
DKD is one of the most common and severe complications of DM and is also the leading cause of end-stage kidney disease (ESKD) in the general population (Novak et al. 2016 ; Collins, et al. 2011 ). The minimal functional unit of the kidney is the nephron, which consists of the glomerulus and renal tubule. Hyperglycemia can cause or exacerbate injuries in both the glomerulus and renal tubule to induce renal dysfunction.
Glomerular injury and metabolic memory
The glomeruli are composed of glomerular endothelial cells (GECs), mesangial cells, podocytes and parietal epithelial cells. As GECs serve as the primary barrier to exposure to high glucose conditions, they can initiate crosstalk between mesangial cells and podocytes. Hyperglycemia can increase the permeability of GECs, alter the glycocalyx and induce GEC apoptosis (Dou and Jourde-Chiche 2019 ). On the one hand, damaged GECs regulate the expression and secretion of endothelin-1 (ET-1), nitric oxide (NO), endothelial nitric oxide synthase (eNOS) and VEGF family members, thereby aggravating the dysfunction of other cell types, including mesangial cells and podocytes (Thomas and Ford Versypt 2022 ; Mahtal et al. 2021 ; Zou et al. 2019 ). Conversely, dysfunction in mesangial cells and podocytes can also deleteriously affect GECs through the regulation of VEGF expression (Fu, et al. 2022 ; Bartlett et al. 2016 ). This intricate crosstalk among glomerular cells plays a pivotal role in the pathogenesis and progression of glomerular injury. Notably, even after the restoration of normoglycemia, the damage to these cells persists. Li et al. ( 2022c ) demonstrated that Sirt7 cooperates with ELK1 to participate in metabolic memory and DKD through the modulation of DAPK3 expression and endothelial inflammation both in vitro and in vivo. Similarly, for podocytes, the expression of SHP-1 remains elevated despite the reduction in blood glucose levels achieved by insulin treatment for the last two months in diabetic mice (Lizotte et al. 2016 ). Additionally, free fatty acids, such as palmitate, contribute significantly to the development of insulin resistance. Thus, Novak et al. ( 2016 ) further demonstrated that a high-fat diet or palmitate can alter H3K36me2 and H3K27me3 on the promoter region of the FOXO1 gene, thereby regulating metabolic memory in podocytes. This comprehensive understanding of the interactions and responses among glomerular cells highlights the complexity and persistence of glomerular injury in patients with diabetes.
Tubular injury and metabolic memory
The injury of tubular epithelial cells (TECs), which account for the largest proportion of all cell types in the kidney, is an essential link in the pathogenesis of DKD (Vallon and Thomson 2020 ). On the one hand, hyperglycemia can cause structural alterations in renal tubules, including renal tubule atrophy, tubular cell hypertrophy, thickening of the tubular basement membrane and tubulointerstitial fibrosis (Slyne et al. 2015 ; Pourghasem et al. 2015 ). On the other hand, high glucose conditions can also lead to inflammation, programmed cell death, senescence and mitochondrial dysfunction in TECs (Zhou et al. 2023 ; Shen et al. 2022 ; Chang et al. 2021 ). Among them, cellular senescence in TECs is related to epigenetic modifications, which are the core mechanism of metabolic memory (Shen et al. 2022 ; Tonna et al. 2010 ). Recent research identified p21 as a key hyperglycemic memory-related gene that regulates TEC senescence in DKD, and activated protein C (aPC), an enzyme that epigenetically inhibits redox p66Shc, could inhibit p21 methylation to ameliorate metabolic memory and senescence (Al-Dabet et al. 2022 ).
In conclusion, metabolic memory is an emerging mechanism in glomerular and tubular injury. Regrettably, studies on the role of metabolic memory in DKD are rare, especially studies on mesangial cells and the crosstalk between different cell types in the kidney. A recent study on the multimodal integration of single nucleus RNA (snRNA-seq) and an assay for transposase-accessible chromatin sequencing (snATAC-seq) in DKD may provide more information on the epigenetic regulation of chromatin accessibility, which could contribute to the long-term expression of DKD and metabolic memory-related genes (Wilson et al. 2022 ). However, further studies are still needed.
DR and metabolic memory
DR, characterized as a neurodegenerative and microangiopathic disease, is the major cause of visual impairment in patients with diabetes, accounting for approximately 30 to 40% of cases (Ting et al. 2016 ; Altmann and Schmidt 2018 ). Hyperglycemia remains the major factor that contributes to the development and progression of DR (Cheung et al. 2010 ). The pathophysiological mechanisms underlying DR are complex and include oxidative stress, inflammation, autophagy, cellular dysfunction and cell death. The inflammatory cascades are primarily triggered by oxidative stress. Both inflammation and oxidative stress stimulate retinal autophagy, which leads to cellular dysfunction and cell death in nerve cells, endothelial cells and pericytes. All these factors may interact with each other, ultimately contributing to the development of DR (Wei et al. 2022 ; Madsen-Bouterse and Kowluru 2008 ).
Coincidentally, multiple studies have shown that the mechanisms mentioned above regulate hyperglycemic memory to affect DR pathogenesis (Liu et al. 2023 ). Metabolic memory-induced retinopathy was initially observed in diabetic dogs, which indicated that DR was not improved by good glycemic control (Engerman and Kern 1987 ). Tewari et al. ( 2012 ) reported that despite the restoration of normoglycemia in retinal endothelial cells, hypermethylation of POLG1 promoters did not change, which resulted in mtDNA replication dysfunction. Liu et al. ( 2019a ) demonstrated that miR-195 remained upregulated in human retinal pigment epithelial cells (RPEs) following three days of culture under high glucose conditions and subsequent normalization to normal glucose levels for another three days, leading to mitochondrial dysfunction-induced apoptosis. Furthermore, Astragalus polysaccharide (APS) attenuated the expression of miR-195 in a dose-dependent manner. Recent studies have demonstrated that the pathogenesis of metabolic memory-induced microvascular dysfunction in DR is regulated by mitochondrial dysfunction, which can be ameliorated by dopamine, mdivi-1 and leflunomide (Lee et al. 2022 ; Kowluru and Alka 2023 ; Mohammad and Kowluru 2022 ). Therefore, mitochondrial dysfunction may be the core mechanism of metabolic memory in DR. Both DKD and DR are microvascular complications of diabetes, and the kidneys and eyes are mitochondria-rich organs. However, studies on the relationship between mitochondrial dysfunction and hyperglycemia in DKD are limited and may be worthy of further research.
Epigenetic modifications also play a vital role in the progression of DR. The high glucose-induced histone 3 lysine 4 (H3K4) hypomethylation status of retinal Sod2 remains persistent even after reversing hyperglycemia (Zhong and Kowluru 2013 ). Mishra et al. ( 2014 ) also proved that as termination of hyperglycemia injury cannot change H3K4 methylation, the binding activity of the transcription factor Nrf2 remains compromised, which leads to oxidative stress. Furthermore, numerous miRNAs also participate in metabolic memory in DR. Apart from miR-195 mentioned above, miR-23b-3p regulates the miR-23b-3p/SIRT1/NF-κB feedback loop to maintain metabolic memory in DR (Zhao et al. 2016 ). Nevertheless, the mechanisms of DNA methylation, lncRNA or other epigenetic modifications are still relatively unknown.
DF and metabolic memory
DF, a common and severe complication of DM, is a major cause of extremity amputation, and the worldwide prevalence of DF is 6.3% (Zhang et al. 2017 ; Afonso, et al. 2021 ). The risk factors involved in the progression of DF are diabetic neuropathy, vascular insufficiency and immunological dysfunction (Noor et al. 2015 ). As there were no obvious improvements in wound healing even when glycemic control was achieved in patients with DM, metabolic memory may participate in DF (Zhao et al. 2021 ; Berlanga-Acosta et al. 2023 ). Del Cuore et al. ( 2023 ) used single nucleotide polymorphism (SNP) analysis in a population with diabetic foot disease. Their results indicated that patients with DF showed predominant expression of the VEGF C2578A CC polymorphism and reduced expression of the AC allele. They also found that miR-217-5p and miR-503-5p may be involved in regulating hyperglycemic memory in DF. Inflammation and DNA methylation are involved in metabolic memory, which is also a key mechanism in DR (Acosta et al. 2008 ; Deng et al. 2023 ). The genome-wide DNA methylation profiles of foot fibroblasts indicated that the change in DNA methylation was associated with metabolic memory, especially in patients with poor wound healing outcomes of diabetic foot ulceration (Park et al. 2014 ). Zhao et al. ( 2021 ) further demonstrated that transient hyperglycemia upregulated DNA methyltransferase 1 (DNMT1) expression, leading to the persistent hypermethylation of Ang-1 during subsequent normoglycemia, which induced inflammation and endothelial dysfunction in vitro and in vivo. These findings implied that epigenetic modifications are a hub contributor to metabolic memory in DR, although the present research is still limited.
Other diabetic complications and metabolic memory
As high blood glucose can injure multiple tissues and organs, hyperglycemic memory is also associated with other chronic complications of DM. Erectile dysfunction (ED) is a common complication of DM, with an approximate prevalence of 35–90% (Malavige and Levy 2009 ). A retrospective case‒control study showed that early hyperglycemia exposure could have long-term effects on erectile function in patients with DM, which could be sustained even after good glycemic control (Hui et al. 2021 ). A previous study showed that hyperglycemia could induce endothelial cell injury to cause microvascular leakage in the lung, which can further lead to pulmonary fibrosis (Lee et al. 2022 ). Jeon et al. ( 2023 ) further illustrated that high glucose-induced microvascular leakage and fibrosis in the lung could not be alleviated even after good blood glucose control. Furthermore, the pathophysiological mechanisms of diabetic neuropathy (DN) are epigenetic modifications, inflammation, oxidative stress and mitochondrial dysfunction, which are similar to the mechanisms of metabolic memory (Jankovic, et al. 2021 ). Thus, a review suggested that metabolic memory may also take part in the development of DN (Jankovic, et al. 2021 ). Regrettably, directly relevant research is rare, so more solid evidence is needed.
In summary, numerous studies have demonstrated that metabolic memory plays an important role in the progression of multiple chronic complications in patients with DM.
Potential therapeutic drugs for metabolic memory
Numerous molecular compounds have been proven to act on the key mechanisms of hyperglycemic memory, such as epigenetic modifications, inflammation, and senescence (Table 1 ). In addition, some molecular compounds may also regulate metabolism, but there is a lack of clear evidence supporting this possibility. Thus, in this section, we summarize the progress of current studies on metabolic memory-related potential drugs for treating diabetes and its complications.
SGI-1027, a highly lipophilic small-molecule inhibitor of DNMT1 based on its quinoline structure, potently inhibits DNA methylation, thereby suppressing senescence, apoptosis, and fibrosis (Sun et al. 2018 ; Gao et al. 2022 ; Wang et al. 2019 ). In DKD, DNMT1 regulates senescence and fibrosis by modulating the DNA methylation status of p21 (Al-Dabet et al. 2022 ). Given these findings, we hypothesize that SGI-1027 may hold promise for mitigating hyperglycemia memory and hyperglycemic memory-induced senescence, apoptosis, and fibrosis.
Chaetocin, a small-molecule natural product isolated from Chaetomium fungi, can regulate several mechanisms of metabolic memory, such as apoptosis, oxidative stress, autophagy and immune function (Jiang et al. 2021 ). SUV39H1 regulated sustained inflammation in vascular cells that were transiently cultured in high glucose through the modification of H3K9me3 (Villeneuve et al. 2008 ). Moreover, chaetocin can also decrease histone H3K9me3 levels at the promoter of the p21 WAF1 gene, which has also been proven to be a hyperglycemic memory-related gene in DKD (Al-Dabet et al. 2022 ; Lin et al. 2016 ). Interestingly, miR-125b , a key ncRNA that regulates hyperglycemic memory, plays an upstream role in the regulation of inflammatory genes in diabetic mice by downregulating SUV39H1 (Villeneuve et al. 2010 ; Wang and Chang 2011 ). These results further support the notion that chaetocin or a miR-125b inhibitor may be effective inhibitors of metabolic memory.
Research on these drugs is currently only at the experimental stage due to safety and other reasons, so their clinical use is still limited. Targeting the site of metabolic processes without interfering with regular metabolic processes is still a challenge. However, understanding the mechanisms of metabolic memory in diabetic complications is benefit in exploring new therapeutic approaches.
- Models of metabolic memory
The concept of metabolic memory was proposed in 2003, with studies involving insulin treatment groups and an average follow-up duration of 6.5 years (Pop-Busui et al. 2009 ). More recently, Al-Dabet et al. ( 2022 ) used SGLT2i for 7.2 ± 0.8 months to manage hyperglycemia and evaluated urinary P21 expression as a marker of persistent tubular damage in DKD. Li et al. ( 2022c ) tested DAPK3 in kidney tissue from DKD patients with poor HbA1c (10.2 ± 3.9) and those with good glycemic control (HbA1c 5.4 ± 0.5). However, crucial details such as the hypoglycemic medications used and the duration of glycemic control were omitted from their study. Hui et al. ( 2021 ) divided participants into three groups: a glycemic control group (regular treatment with normal glycemic levels in the past 5 years), a glycemic non-control group (non-regular treatment with poor glycemic control in the past 5 years), and a metabolic memory group (regular treatment and normal glycemic levels in the past year but non-regular treatment with poor glycemic control a year ago). Nevertheless, they also did not describe the hypoglycemic medications used in detail. Given the inherent challenges in controlling variables in clinical research, the majority of studies have resorted to animal and cell models to investigate the mechanisms underlying metabolic memory. Regrettably, there is a lack of consistency in the models of metabolic memory, both in vitro and in vivo. Thus, Table 2 lists different models used in different studies.
For in vitro models of metabolic memory, most researchers used high glucose conditions in cultured cells and then changed the glucose concentration to a normal level to simulate intensive treatment in DCCT/EDIC studies. In the majority of studies exploring diabetes and its chronic complications, a hyperglycemic exposure period of 48–72 h is typically considered representative of chronic hyperglycemia with long-term deleterious effects. This holds true for conditions such as DKD, DF, and diabetic cardiomyopathy (Hu et al. 2024 ; Wang et al. 2024 ; Li, et al. 2024 ; Song et al. 2023 ; Feng et al. 2023 ). However, it is worth noting that the duration of exposure to high glucose, as well as the periods of exposure to normal glucose, vary significantly across studies examining metabolic memory. In DKD, Al-Dabet et al. ( 2022 ) Mouse primary tubular cells were cultured under high-glucose conditions for 24 h and then at normal levels for another 24 h. However, Li et al. ( 2022c ) Human glomerular endothelial cells were exposed to high levels of glucose for 3 days, followed by 3 days under normal conditions. Although these studies cultured cells for different durations, they equally distributed the time to high and normal glucose levels. Zhong et al. ( 2021 ) compared different in vitro models of metabolic memory in DF. Interestingly, different models performed similarly, and they used cultured human aortic endothelial cells for 1 day under high glucose and 6 days under normal glucose conditions. Another uncommon method involves deriving human proximal tubular epithelial cells from people with type 2 diabetes and culturing them under normal glucose conditions for 4 passages to establish a metabolic memory model (Bansal et al. 2020 ). Regrettably, there are no accepted standards for metabolic memory models in vitro , and most studies have chosen these models directly. However, for different cell lines, comparing different culture times might be more accurate.
For in vivo models of metabolic memory, the majority of studies have utilized insulin to lower blood glucose levels in diabetic mice or rats. Notably, in 2022, Al-Dabet et al. ( 2022 ) reported a novel attempt to employ SGLT2i in the construction of a metabolic memory model. Although they presumably considered the renal benefits of SGLT2i, they did not compare its effectiveness with that of insulin in model establishment. The duration of glycemic control in these studies varied significantly, ranging from a minimum of 3 weeks in rats with DKD treated with insulin to a maximum of 4 months in rats with DR also treated with insulin (Li et al. 2022c ; Mohammad and Kowluru 2022 ). This wide range raises the following question: what is the optimal duration for the construction of in vivo models of metabolic memory? Furthermore, does this duration differ across various diabetic complications?
In this review, we explored the regulatory mechanism of metabolic memory, including inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. Then, we discuss the function of metabolic memory in diabetic complications. In addition, we analyzed confirmed and potential metabolic memory-related inhibitors. Finally, we also summarized the in vitro and in vivo models of metabolic memory. In conclusion, metabolic memory might be a vital mechanism in the occurrence and development of various diabetic complications and is a promising therapeutic target for preventing the progression of complications.
There have been extensive studies on the essential mechanisms and regulators of metabolic memory, oxidative stress, mitochondrial dysfunction and apoptosis, which are the most studied and are mainly focused on DR. On the one hand, these traditional mechanisms may be the core mechanisms of metabolic memory and need to be validated more widely for the treatment of different diabetic complications. However, emerging mechanisms, such as senescence, ferroptosis, and pyroptosis, also deserve further exploration. In addition, animal and cellular models of metabolic memory are still controversial. Thus, we speculated that for different cell lines and methods for generating diabetic models, preexperiments may be necessary. Furthermore, numerous key molecules, including miR-320 , p21 and Sod2 , have been identified. However, there are currently no well-recognized markers that can represent metabolic memory in vitro or in vivo, such as ColI and fibronectin for fibrosis, let alone in vitro diagnostic markers used in clinical practice. In addition, small molecule drugs, such as polysaccharide, dopamine and aPC, have also been found to alleviate hyperglycemic memory to mitigate diabetic complications. We also speculated that SGI-1027 and chaetocin may be potential molecular compounds against metabolic memory. Regrettably, these drugs have been limited to animal and cellular models.
In the future, further studies of metabolic memory may start from the following perspectives: (1) develop explicit animal and cellular models of metabolic memory; (2) elucidate the mechanisms of metabolic memory and further uncover more hub molecules that regulate metabolic memory to obtain representative markers; (3) transform small molecule compounds that can be used to regulate metabolic memory in clinical practice; and (4) extensively study the crosstalk between lncRNAs and miRNAs; however, regrettably, pertinent research on the intricate network of lncRNAs and other ncRNAs remains to be conducted.
Although there have been certain studies that have summarized the relationship between metabolic memory and DKD, DR or epigenetic modification, studies on the connection between metabolic memory and chronic complications of DM, along with potential therapeutic drugs, remain scarce. Notably, our review is the first to comprehensively summarize various models of metabolic memory, given that the exact duration and severity of high-glucose toxicity are still elusive. Despite our efforts to comprehensively review the pathogenesis of metabolic memory in diverse chronic complications of DM, we acknowledge certain limitations, including the constraints of our research perspectives. Nevertheless, with further in-depth exploration of metabolic memory in chronic DM complications and elucidation of its underlying mechanisms, we are confident that this study will pave the way for reliable and innovative therapeutic targets that can retard or even arrest the progression of DM and its associated complications. Additionally, we intend to conduct regular systematic summaries in this field of research.
Availability of data and materials
The data of this study are included within the paper.
Abbreviations
Diabetes mellitus
Diabetic retinopathy
Diabetic kidney disease
International Diabetes Federation
Advanced glycation end product
Mitochondrial DNA
Sodium glucose co-transporter 2 inhibitor
Dipeptidyl peptidase 4 inhibitors
Glucagon-like peptide 1 receptor agonists
Diabetes Control and Complications Trial
Epidemiology of Diabetes Interventions and Complications
United Kingdom Prospective Diabetes Study
Noncoding RNAs
Thioredoxin-interacting protein
3’ Untranslated region
Histone posttranslational modifications
Type 2 diabetes mellitus
Suppressor of variegation 3–9 homolog 1
Histone H3 lysine 4 monomethylation
Long noncoding RNAs
Diabetic foot
Diabetic cardiomyopathy
Streptozotocin
Cluster of differentiation 36
Endothelial-to-mesenchymal transition
Vascular smooth muscle cells
End-stage kidney disease
Glomerular endothelial cells
Endothelin-1
Tubular epithelial cells
Activated protein C
Single-nucleus RNA
Assay for transposase-accessible chromatin sequencing
Retinal pigment epithelial cells
Histone 3 lysine 4
DNA methyltransferase 1
Diabetic neuropathy
Endoplasmic reticulum
Reactive oxygen species
Transglutaminase
Human embryonic kidney cells
Boston University mouse proximal tubular cells
Nonobese diabetic
Tert-butylhydroquinone
Glutathione
Adeno-associated virus
5-Aza-deoxycytidine
Proximal tubular cells
Human retinal endothelial cells
Bovine retinal endothelial cells
Aboouf MA, et al. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance. Diabetes Res Clin Pract. 2015;109(1):40–7.
Article CAS PubMed Google Scholar
Acosta JB, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530–9.
Article PubMed PubMed Central Google Scholar
Afonso AC, et al. Biofilms in diabetic foot ulcers: impact, risk factors and control strategies. Int J Mol Sci 2021;22(15).
Aiello LP, D.E.R. Group (2014) Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 37(1): 17–23
Al-Dabet MM, et al. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat Commun. 2022;13(1):5062.
Article CAS PubMed PubMed Central Google Scholar
Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci 2018;19(1).
Bansal A, et al. Integrative omics analyses reveal epigenetic memory in diabetic renal cells regulating genes associated with kidney dysfunction. Diabetes. 2020;69(11):2490–502.
Bartlett CS, Jeansson M, Quaggin SE. Vascular growth factors and glomerular disease. Annu Rev Physiol. 2016;78:437–61.
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S176–83.
Article PubMed Google Scholar
Berlanga-Acosta J, et al. Endogenous biological drivers in diabetic lower limb wounds recurrence: hypothetical reflections. Int J Mol Sci 2023;24(12).
Bhatti JS, et al. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184:114–34.
Bischoff J. Endothelial-to-mesenchymal transition. Circ Res. 2019;124(8):1163–5.
Bleriot C, et al. Inflammatory and immune etiology of type 2 diabetes. Trends Immunol. 2023;44(2):101–9.
Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7(7):369–75.
Chang J, et al. Update on the mechanisms of tubular cell injury in diabetic kidney disease. Front Med (lausanne). 2021;8: 661076.
Chen Z, Natarajan R. Epigenetic modifications in metabolic memory: what are the memories, and can we erase them? Am J Physiol Cell Physiol. 2022;323(2):C570–82.
Chen Z, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113(21):E3002–11.
Cheng Y, et al. Central role of cardiac fibroblasts in myocardial fibrosis of diabetic cardiomyopathy. Front Endocrinol (lausanne). 2023;14:1162754.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Choi EH, Park SJ. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp Mol Med. 2023;55(7):1348–56.
Cojocaru KA, et al. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants (Basel), 2023;12(3).
Collins AJ, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl):A7, e1-420.
PubMed Google Scholar
Costantino S, et al. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–6.
Del Cuore A, et al. Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction. Cardiovasc Diabetol. 2023;22(1):148.
Deng JY, et al. Targeting DNA methylation and demethylation in diabetic foot ulcers. J Adv Res 2023.
Ding Q, et al. Inflammation-related epigenetic modification: the bridge between immune and metabolism in type 2 diabetes. Front Immunol. 2022;13: 883410.
Domingueti CP, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–45.
Dou L, Jourde-Chiche N. Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins (Basel), 2019;11(10).
Drzewoski J, Kasznicki J, Trojanowski Z. The role of “metabolic memory” in the natural history of diabetes mellitus. Pol Arch Med Wewn. 2009;119(7–8):493–500.
El Mouhayyar C, et al. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. 2020;2020:1762164.
El-Mesallamy HO, et al. Adiponectin and E-selectin concentrations in relation to inflammation in obese type 2 diabetic patients with coronary heart disease(s). Minerva Endocrinol. 2011;36(3):163–70.
CAS PubMed Google Scholar
El-Mesallamy HO, Hamdy NM, Sallam AA. Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. 2013;50(5):679–85.
El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–17.
Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–12.
Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67.
Feng Q, et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am J Chin Med. 2023;51(4):997–1018.
Filgueiras LR, et al. Imbalance between HDAC and HAT activities drives aberrant STAT1/MyD88 expression in macrophages from type 1 diabetic mice. J Diabetes Complications. 2017;31(2):334–9.
Fu J, et al. Regeneration of glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy. JCI Insight, 2022;7(5).
Galicia-Garcia U. et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci, 2020. 21(17).
Galluzzi L, Myint M. Cell death and senescence. J Transl Med. 2023;21(1):425.
Gao Q, et al. Inhibition of DNA methyltransferase aberrations reinstates antioxidant aging suppressors and ameliorates renal aging. Aging Cell. 2022;21(1): e13526.
Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8.
Gong ZG, et al. Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J Hazard Mater. 2022;423(Pt A): 127110.
Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–22.
Hamdy NM, Suwailem SM, El-Mesallamy HO. Influence of vitamin E supplementation on endothelial complications in type 2 diabetes mellitus patients who underwent coronary artery bypass graft. J Diabetes Complications. 2009;23(3):167–73.
Hammes HP, et al. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol vis Sci. 1993;34(6):2092–6.
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–53.
Holman RR, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Hu K, et al. MicroRNA-221-3p inhibits the inflammatory response of keratinocytes by regulating the DYRK1A/STAT3 signaling pathway to promote wound healing in diabetes. Commun Biol. 2024;7(1):300.
Hui J, et al. Effects of “metabolic memory” on erectile function in diabetic men: a retrospective case-control study. Andrology. 2021;9(1):288–96.
Jankovic M, et al, Genetic and epigenomic modifiers of diabetic neuropathy. Int J Mol Sci 2021;22(9).
Jax TW. Metabolic memory: a vascular perspective. Cardiovasc Diabetol. 2010;9:51.
Jeon HY, et al. Simultaneous attenuation of hyperglycemic memory-induced retinal, pulmonary, and glomerular dysfunctions by proinsulin C-peptide in diabetes. BMC Med. 2023;21(1):49.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Jiang Z, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50(8):907–17.
Jiang H, et al. Chaetocin: a review of its anticancer potentials and mechanisms. Eur J Pharmacol. 2021;910: 174459.
Jin ML, Jeong KW. Histone modifications in drug-resistant cancers: from a cancer stem cell and immune evasion perspective. Exp Mol Med. 2023;55(7):1333–47.
Johnson J, et al. Oxidative stress in neutrophils: implications for diabetic cardiovascular complications. Antioxid Redox Signal. 2022;36(10–12):652–66.
Joslin EP. Diabetes mellitus. N Engl J Med. 1946;234:476.
Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–45.
Khella MS, et al. The (FTO) gene polymorphism is associated with metabolic syndrome risk in Egyptian females: a case-control study. BMC Med Genet. 2017;18(1):101.
Kowluru RA, Alka K. Mitochondrial quality control and metabolic memory phenomenon associated with continued progression of diabetic retinopathy. Int J Mol Sci 2023;24(9).
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610.
Kumari N, et al. The potential role of m6A RNA methylation in diabetic retinopathy. Exp Eye Res. 2021;208: 108616.
Kung HC, et al. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson's disease. Biomedicines 2021;9(8).
Lee YJ, et al. Dopamine ameliorates hyperglycemic memory-induced microvascular dysfunction in diabetic retinopathy. FASEB J. 2022;36(12): e22643.
Li C, et al. Non-coding RNAs in diabetes mellitus and diabetic cardiovascular disease. Front Endocrinol (lausanne). 2022a;13: 961802.
Li Q, et al. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1alpha-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022b;13: 859723.
Li X, et al. Sirt7 associates with ELK1 to participate in hyperglycemia memory and diabetic nephropathy via modulation of DAPK3 expression and endothelial inflammation. Transl Res. 2022c;247:99–116.
Li Y, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8(1):152.
Li SS, et al. Diabetes promotes myocardial fibrosis via AMPK/EZH2/PPAR-gamma signaling pathway. Diabetes Metab J 2024.
Lin SH, et al. Histone methyltransferase Suv39h1 attenuates high glucose-induced fibronectin and p21(WAF1) in mesangial cells. Int J Biochem Cell Biol. 2016;78:96–105.
Liu P, et al. Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol Med. 2019a;25(1):21.
Liu T, et al. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019b;227:64–73.
Liu DD, et al. Epigenetic modifications and metabolic memory in diabetic retinopathy: beyond the surface. Neural Regen Res. 2023;18(7):1441–9.
Lizotte F, et al. Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes. 2016;65(12):3705–17.
Maamoun H, et al. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol. 2019;10:70.
Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–27.
Mahmoud MM, Sanad EF, Hamdy NM. MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration. Environ Sci Pollut Res Int. 2021;28(28):36984–7000.
Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Front Med (lausanne). 2021;8: 659013.
Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–47.
Mansour MA, et al. P21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers (Basel), 2023;15(4).
Mao R, et al. Enhancer RNAs: a missing regulatory layer in gene transcription. Sci China Life Sci. 2019;62(7):905–12.
Marwick TH, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51.
Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic Biol Med. 2014;75:129–39.
Mohammad G, Kowluru RA. Mitochondrial dynamics in the metabolic memory of diabetic retinopathy. J Diabetes Res. 2022;2022:3555889.
Mossel DM, et al. Epigenetic regulation of S100A9 and S100A12 expression in monocyte-macrophage system in hyperglycemic conditions. Front Immunol. 2020;11:1071.
Mostafa AM, et al. Glucagon-like peptide 1 (GLP-1)-based therapy upregulates LXR-ABCA1/ABCG1 cascade in adipocytes. Biochem Biophys Res Commun. 2015;468(4):900–5.
Mostafa AM, et al. Effect of vildagliptin and pravastatin combination on cholesterol efflux in adipocytes. IUBMB Life. 2016;68(7):535–43.
Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–44.
Nathan DM, DER Group, The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37(1):9-16.
Nathan DM, D.E.R. Group (2014) The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37(1): 9–16
Nedosugova LV, et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines 2022;10(5).
Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192–9.
Novak M, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care. 2016;39(11):1940–7.
Okabe J, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110(8):1067–76.
Palomer X, Pizarro-Delgado J, Vazquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci. 2018;39(5):452–67.
Park LK, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9(10):1339–49.
Peng QH, et al. Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci Rep 2020;40(1).
Phoenix A, Chandran R, Ergul A. Cerebral microvascular senescence and inflammation in diabetes. Front Physiol. 2022;13: 864758.
Pop-Busui R, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22):2886–93.
Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6(3):120–7.
PubMed PubMed Central Google Scholar
Ranjit Unnikrishnan I, Anjana RM, Mohan V (2011) Importance of controlling diabetes early—the concept of metabolic memory, legacy effect and the case for early insulinisation. J Assoc Physicians India. 59(Suppl): 8–12
Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55.
Reddy MA, et al. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36(5):864–73.
Retnakaran R et al (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55(6): 1832–9
Roy S, et al. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87(1):404–8.
Shen S, Ji C, Wei K. Cellular senescence and regulated cell death of tubular epithelial cells in diabetic kidney disease. Front Endocrinol (lausanne). 2022;13: 924299.
Slyne J, et al. New developments concerning the proximal tubule in diabetic nephropathy: in vitro models and mechanisms. Nephrol Dial Transplant. 2015;30(Suppl 4):60–7.
Article Google Scholar
Song Y, et al. Novel lncRNA-prader willi/angelman region RNA, SNRPN neighbour (PWARSN) aggravates tubular epithelial cell pyroptosis by regulating TXNIP via dual way in diabetic kidney disease. Cell Prolif. 2023;56(2): e13349.
Su D, et al. Tcf3-activated lncRNA Gas5 regulates newborn mouse cardiomyocyte apoptosis in diabetic cardiomyopathy. J Cell Biochem. 2020;121(11):4337–46.
Sun N, et al. DNMTs inhibitor SGI-1027 induces apoptosis in Huh7 human hepatocellular carcinoma cells. Oncol Lett. 2018;16(5):5799–806.
CAS PubMed PubMed Central Google Scholar
Sun H, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
Sun Y, et al. Epigenetic regulation of mesenchymal stem cell aging through histone modifications. Genes Dis. 2023;10(6):2443–56.
Taft RJ, et al. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–39.
Takahashi P, et al. Transcript expression profiles and microRNA regulation indicate an upregulation of processes linked to oxidative stress, DNA repair, cell death, and inflammation in type 1 diabetes mellitus patients. J Diabetes Res. 2022;2022:3511329.
Teodoro JS, et al. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2018;9:1857.
Tewari S, et al. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophthalmol vis Sci. 2012;53(8):4881–8.
Thielen L, Shalev A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr Opin Endocrinol Diabetes Obes. 2018;25(2):75–80.
Thiem K, et al. Hyperglycemic memory of innate immune cells promotes in vitro proinflammatory responses of human monocytes and murine macrophages. J Immunol. 2021;206(4):807–13.
Thomas HY, Ford Versypt AN. Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J Biol Eng. 2022;16(1):19.
Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–77.
Tonna S, et al. Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nat Rev Nephrol. 2010;6(6):332–41.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317–36.
Vastolo V, et al. High-fat diet unveils an enhancer element at the Ped/Pea-15 gene responsible for epigenetic memory in skeletal muscle. Metabolism. 2018;87:70–9.
Villeneuve LM, et al. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105(26):9047–52.
Villeneuve LM, et al. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59(11):2904–15.
Voronova V, et al. Interpretation of metabolic memory phenomenon using a physiological systems model: what drives oxidative stress following glucose normalization? PLoS ONE. 2017;12(2): e0171781.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14.
Wang Z, et al. Metabolic memory in mitochondrial oxidative damage triggers diabetic retinopathy. BMC Ophthalmol. 2018;18(1):258.
Wang JY, et al. Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett. 2019;24:10.
Wang M, et al. Endothelial dysfunction and diabetic cardiomyopathy. Front Endocrinol (lausanne). 2022;13: 851941.
Wang H, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating Nrf2/HO-1 signaling pathway in diabetic nephropathy. Adv Sci (weinh). 2024;11(10): e2305563.
Wei L, et al. The pathophysiological mechanisms underlying diabetic retinopathy. Front Cell Dev Biol. 2022;10: 963615.
Wilson PC, et al. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat Commun. 2022;13(1):5253.
Writing Team for the Diabetes C, I. Complications Trial/Epidemiology of Diabetes, and G. Complications Research (200) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290(16): 2159–67
Xian Y, et al. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(10):2009–17.
Xie L, et al. Emerging roles of sodium glucose cotransporter 2 (SGLT-2) inhibitors in diabetic cardiovascular diseases: focusing on immunity inflammation and metabolism. Front Pharmacol. 2022;13: 836849.
Xu Y, Kovacic JC. Endothelial to mesenchymal transition in health and disease. Annu Rev Physiol. 2023;85:245–67.
Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: a perspective on the concept of metabolic memory. J Diabetes. 2017;9(2):141–8.
Yao Y, et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc Res. 2022;118(1):196–211.
Yu XY, et al. High levels of glucose induce “metabolic memory” in cardiomyocyte via epigenetic histone H3 lysine 9 methylation. Mol Biol Rep. 2012;39(9):8891–8.
Zapata RC, et al. Adipocytes control food intake and weight regain via Vacuolar-type H(+) ATPase. Nat Commun. 2022;13(1):5092.
Zhan J, et al. Hyperglycemic memory in diabetic cardiomyopathy. Front Med. 2022;16(1):25–38.
Zhan J, et al. Positive feedback loop of miR-320 and CD36 regulates the hyperglycemic memory-induced diabetic diastolic cardiac dysfunction. Mol Ther Nucleic Acids. 2023;31:122–38.
Zhang E, Wu Y. Metabolic memory: mechanisms and implications for diabetic vasculopathies. Sci China Life Sci. 2014;57(8):845–51.
Zhang P, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. 2017;49(2):106–16.
Zhang P, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600.
Zhang X, et al. CD36 signaling in diabetic cardiomyopathy. Aging Dis. 2021;12(3):826–40.
Zhao S, et al. miR-23b-3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1-dependent signalling pathway. Diabetologia. 2016;59(3):644–54.
Zhao J, et al. Transient high glucose causes persistent vascular dysfunction and delayed wound healing by the DNMT1-mediated Ang-1/NF-kappaB pathway. J Invest Dermatol. 2021;141(6):1573–84.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Zhong Q, Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol vis Sci. 2013;54(1):244–50.
Zhong X, et al. The microRNAs in the pathogenesis of metabolic memory. Endocrinology. 2015;156(9):3157–68.
Zhong Y, et al. Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: a narrative review. Int J Biol Macromol. 2023;259(Pt 1): 128182.
Zhou X, et al. Role of renal tubular programed cell death in diabetic kidney disease. Diabetes Metab Res Rev. 2023;39(2): e3596.
Zou HH, et al. Endothelial cells secreted endothelin-1 augments diabetic nephropathy via inducing extracellular matrix accumulation of mesangial cells in ETBR(-/-) mice. Aging (albany NY). 2019;11(6):1804–20.
Download references
Acknowledgements
We thank the Translational Medicine Center, the First Affiliated Hospital of Zhengzhou University, for their support.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (U200410394), The Research and Innovation Team Project of the First Affiliated Hospital of Zhengzhou University (QNCXTD2023015) and 2021 Henan Province Health Young and Middle-aged Discipline Leader Training Project.
Author information
Authors and affiliations.
Division of Endocrinology, Department of Internal Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
Tongyue Yang, Feng Guo, Mingwei Shao, Yi Song, Gaofei Ren, Zhao Linlin, Guijun Qin & Yanyan Zhao
Traditional Chinese Medicine Integrated Department of Nephrology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
Research Institute of Nephrology, Zhengzhou University, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
TY, YZ and QF conceptualized and wrote the manuscript. TY, FG, MS, YS, GR, LZ and YZ contributed to manuscript writing. QF, GQ and YZ reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript being submitted.
Corresponding author
Correspondence to Yanyan Zhao .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Authors, hereby consent to the publication of my paper "An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory" in Molecular Medicine. I confirm that the content is accurate and that I am aware of any potential disclosures. I waive any rights to claim authorship and hold the publisher harmless from any liabilities arising from publication.
Competing interests
The authors declare no conflicts of interest that pertain to this work.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yang, T., Qi, F., Guo, F. et al. An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory. Mol Med 30 , 71 (2024). https://doi.org/10.1186/s10020-024-00824-9
Download citation
Received : 25 January 2024
Accepted : 29 April 2024
Published : 26 May 2024
DOI : https://doi.org/10.1186/s10020-024-00824-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metabolic memory
- Diabetic complications
- Molecular mechanisms
- Treatment progress
Molecular Medicine
ISSN: 1528-3658
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Reference Manager
- Simple TEXT file
People also looked at
Hypothesis and theory article, type 2 diabetes mellitus: a pathophysiologic perspective.

- Department of Medicine, Duke University, Durham, NC, United States
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5–10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years, and their effectiveness may simply be due to lowering the dietary contribution to glucose and insulin levels, which then leads to improvements in hyperglycemia and hyperinsulinemia. Treatments for T2DM that lead to improvements in glycemic control and reductions in blood insulin levels are sensible based on this pathophysiologic perspective. In this article, a pathophysiological argument for using carbohydrate restriction to treat T2DM will be made.
Introduction
Type 2 Diabetes Mellitus (T2DM) is characterized by a persistently elevated blood glucose, or an elevation of blood glucose after a meal containing carbohydrate ( 1 ) ( Table 1 ). Unlike Type 1 Diabetes which is characterized by a deficiency of insulin, most individuals affected by T2DM have elevated insulin levels (fasting and/or post glucose ingestion), unless there has been beta cell failure ( 2 , 3 ). The term “insulin resistance” (IR) has been used to explain why the glucose levels remain elevated even though there is no deficiency of insulin ( 3 , 4 ). Attempts to determine the etiology of IR have involved detailed examinations of molecular and intracellular pathways, with attribution of cause to fatty acid flux, but the root cause has been elusive to experts ( 5 – 7 ).
Table 1 . Definition of type 2 diabetes mellitus.
How Much Glucose Is in the Blood?
Keeping in mind that T2DM involves an elevation of blood glucose, it is important to understand how much glucose is in the blood stream to begin with, and then the factors that influence the blood glucose—both exogenous and endogenous factors. The amount of glucose in the bloodstream is carefully controlled—approximately 5–10 grams in the bloodstream at any given moment, depending upon the size of the person. To calculate this, multiply 100 milligrams/deciliter × 1 gram/1,000 milligrams × 10 deciliters/1 liter × 5 liters of blood. The “zeros cancel” and you are left with 5 grams of glucose if the individual has 5 liters of blood. Since red blood cells represent about 40% of the blood volume, and the glucose is in equilibrium, there may be an extra 40% glucose because of the red blood cell reserve ( 8 ). Adding the glucose from the serum and red blood cells totals about 5–10 grams of glucose in the entire bloodstream.
Major Exogenous Factors That Raise the Blood Glucose
Dietary carbohydrate is the major exogenous factor that raises the blood glucose. When one considers that it is common for an American in 2021 to consume 200–300 grams of carbohydrate daily, and most of this carbohydrate is digested and absorbed as glucose, the body absorbs and delivers this glucose via the bloodstream to the cells while attempting to maintain a normal blood glucose level. Thinking of it in this way, if 200–300 grams of carbohydrates is consumed in a day, the bloodstream that holds 5–10 grams of glucose and has a concentration of 100 milligrams/deciliter, is the conduit through which 200,000–300,000 milligrams (200 grams = 200,000 milligrams) passes over the course of a day.
Major Endogenous Factors That Raise the Blood Glucose
There are many endogenous contributors that raise the blood glucose. There are at least 3 different hormones that increase glucose levels: glucagon, epinephrine, and cortisol. These hormones increase glucose levels by increasing glycogenolysis and gluconeogenesis ( 9 ). Without any dietary carbohydrate, the normal human body can generate sufficient glucose though the mechanism of glucagon secretion, gluconeogenesis, glycogen storage and glycogenolysis ( 10 ).
Major Exogenous Factors That Lower the Blood Glucose
A reduction in dietary carbohydrate intake can lower the blood glucose. An increase in activity or exercise usually lowers the blood glucose ( 11 ). There are many different medications, employing many mechanisms to lower the blood glucose. Medications can delay sucrose and starch absorption (alpha-glucosidase inhibitors), slow gastric emptying (GLP-1 agonists, DPP-4 inhibitors) enhance insulin secretion (sulfonylureas, meglitinides, GLP-1 agonists, DPP-4 inhibitors), reduce gluconeogenesis (biguanides), reduce insulin resistance (biguanides, thiazolidinediones), and increase urinary glucose excretion (SGLT-2 inhibitors). The use of medications will also have possible side effects.
Major Endogenous Factors That Lower the Blood Glucose
The major endogenous mechanism to lower the blood glucose is to deliver glucose into the cells (all cells can use glucose). If the blood glucose exceeds about 180 milligrams/deciliter, then loss of glucose into the urine can occur. The blood glucose is reduced by cellular uptake using glut transporters ( 12 ). Some cells have transporters that are responsive to the presence of insulin to activate (glut4), others have transporters that do not require insulin for activation. Insulin-responsive glucose transporters in muscle cells and adipose cells lead to a reduction in glucose levels—especially after carbohydrate-containing meals ( 13 ). Exercise can increase the glucose utilization in muscle, which then increases glucose cellular uptake and reduce the blood glucose levels. During exercise, when the metabolic demands of skeletal muscle can increase more than 100-fold, and during the absorptive period (after a meal), the insulin-responsive glut4 transporters facilitate the rapid entry of glucose into muscle and adipose tissue, thereby preventing large fluctuations in blood glucose levels ( 13 ).
Which Cells Use Glucose?
Glucose can used by all cells. A limited number of cells can only use glucose, and are “glucose-dependent.” It is generally accepted that the glucose-dependent cells include red blood cells, white blood cells, and cells of the renal papilla. Red blood cells have no mitochondria for beta-oxidation, so they are dependent upon glucose and glycolysis. White blood cells require glucose for the respiratory burst when fighting infections. The cells of the inner renal medulla (papilla) are under very low oxygen tension, so therefore must predominantly use glucose and glycolysis. The low oxygen tension is a result of the countercurrent mechanism of urinary concentration ( 14 ). These glucose-dependent cells have glut transporters that do not require insulin for activation—i.e., they do not need insulin to get glucose into the cells. Some cells can use glucose and ketones, but not fatty acids. The central nervous system is believed to be able to use glucose and ketones for fuel ( 15 ). Other cells can use glucose, ketones, and fatty acids for fuel. Muscle, even cardiac muscle, functions well on fatty acids and ketones ( 16 ). Muscle cells have both non-insulin-responsive and insulin-responsive (glut4) transporters ( 12 ).

Possible Dual Role of an Insulin-Dependent Glucose-Transporter (glut4)
A common metaphor is to think of the insulin/glut transporter system as a key/lock mechanism. Common wisdom states that the purpose of insulin-responsive glut4 transporters is to facilitate glucose uptake when blood insulin levels are elevated. But, a lock serves two purposes: to let someone in and/or to keep someone out . So, one of the consequences of the insulin-responsive glut4 transporter is to keep glucose out of the muscle and adipose cells, too, when insulin levels are low. The cells that require glucose (“glucose-dependent”) do not need insulin to facilitate glucose entry into the cell (non-insulin-responsive transporters). In a teleological way, it would “make no sense” for cells that require glucose to have insulin-responsive glut4 transporters. Cells that require glucose have glut1, glut2, glut3, glut5 transporters—none of which are insulin-responsive (Back to the key/lock metaphor, it makes no sense to have a lock on a door that you want people to go through). At basal (low insulin) conditions, most glucose is used by the brain and transported by non-insulin-responsive glut1 and glut3. So, perhaps one of the functions of the insulin-responsive glucose uptake in muscle and adipose to keep glucose OUT of the these cells at basal (low insulin) conditions, so that the glucose supply can be reserved for the tissue that is glucose-dependent (blood cells, renal medulla).
What Causes IR and T2DM?
The current commonly espoused view is that “Type 2 diabetes develops when beta-cells fail to secrete sufficient insulin to keep up with demand, usually in the context of increased insulin resistance.” ( 17 ). Somehow, the beta cells have failed in the face of insulin resistance. But what causes insulin resistance? When including the possibility that the environment may be part of the problem, is it possible that IR is an adaptive (protective) response to excess glucose availability? From the perspective that carbohydrate is not an essential nutrient and the change in foods in recent years has increased the consumption of refined sugar and flour, maybe hyperinsulinemia is the cause of IR and T2DM, as cells protect themselves from excessive glucose and insulin levels.
Insulin Is Already Elevated in IR and T2DM
Clinical experience of most physicians using insulin to treat T2DM over time informs us that an escalation of insulin dose is commonly needed to achieve glycemic control (when carbohydrate is consumed). When more insulin is given to someone with IR, the IR seems to get worse and higher levels of insulin are needed. I have the clinical experience of treating many individuals affected by T2DM and de-prescribing insulin as it is no longer needed after consuming a diet without carbohydrate ( 18 ).
Diets Without Carbohydrate Reverse IR and T2DM
When dietary manipulation was the only therapy for T2DM, before medications were available, a carbohydrate-restricted diet was used to treat T2DM ( 19 – 21 ). Clinical experience of obesity medicine physicians and a growing number of recent studies have demonstrated that carbohydrate-restricted diets reverse IR and T2DM ( 18 , 22 , 23 ). Other methods to achieve caloric restriction also have these effects, like calorie-restricted diets and bariatric surgery ( 24 , 25 ). There may be many mechanisms by which these approaches may work: a reduction in glucose, a reduction in insulin, nutritional ketosis, a reduction in metabolic syndrome, or a reduction in inflammation ( 26 ). Though there may be many possible mechanisms, let's focus on an obvious one: a reduction in blood glucose. Let's assume for a moment that the excessive glucose and insulin leads to hyperinsulinemia and this is the cause of IR. On a carbohydrate-restricted diet, the reduction in blood glucose leads to a reduction in insulin. The reduction in insulin leads to a reduction in insulin resistance. The reduction in insulin leads to lipolysis. The resulting lowering of blood glucose, insulin and body weight reverses IR, T2DM, AND obesity. These clinical observations strongly suggest that hyperinsulinemia is a cause of IR and T2DM—not the other way around.
What Causes Atherosclerosis?
For many years, the metabolic syndrome has been described as a possible cause of atherosclerosis, but there are no RCTs directly targeting metabolic syndrome, and the current drug treatment focuses on LDL reduction, so its importance remains controversial. A recent paper compared the relative importance of many risk factors in the prediction of the first cardiac event in women, and the most powerful predictors were diabetes, metabolic syndrome, smoking, hypertension and BMI ( 27 ). The connection between dietary carbohydrate and fatty liver is well-described ( 28 ). The connection between fatty liver and atherosclerosis is well-described ( 29 ). It is very possible that the transport of excess glucose to the adipose tissue via lipoproteins creates the particles that cause the atherosclerotic damage (small LDL) ( Figure 1 ) ( 30 – 32 ). This entire process of dietary carbohydrate leading to fatty liver, leading to small LDL, is reversed by a diet without carbohydrate ( 26 , 33 , 34 ).
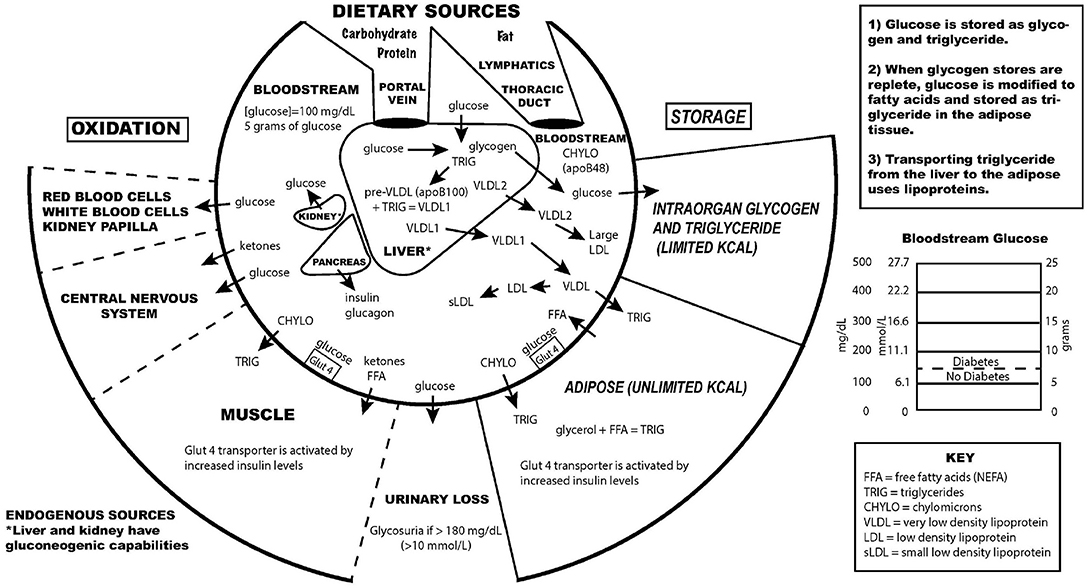
Figure 1 . Key aspects of the interconnection between glucose and lipoprotein metabolism.
Reducing dietary carbohydrate in the context of a low carbohydrate, ketogenic diet reduces hyperglycemia and hyperinsulinemia, IR and T2DM. In the evaluation of an individual for a glucose abnormality, measure the blood glucose and insulin levels. If the insulin level (fasting or after a glucose-containing meal) is high, do not give MORE insulin—instead, use an intervention to lower the insulin levels. Effective ways to reduce insulin resistance include lifestyle, medication, and surgical therapies ( 23 , 35 ).
The search for a single cause of a complex problem is fraught with difficulty and controversy. I am not hypothesizing that excessive dietary carbohydrate is the only cause of IR and T2DM, but that it is a cause, and quite possibly the major cause. How did such a simple explanation get overlooked? I believe it is very possible that the reductionistic search for intracellular molecular mechanisms of IR and T2DM, the emphasis on finding pharmaceutical (rather than lifestyle) treatments, the emphasis on the treatment of high total and LDL cholesterol, and the fear of eating saturated fat may have misguided a generation of researchers and clinicians from the simple answer that dietary carbohydrate, when consumed chronically in amounts that exceeds an individual's ability to metabolize them, is the most common cause of IR, T2DM and perhaps even atherosclerosis.
While there has historically been a concern about the role of saturated fat in the diet as a cause of heart disease, most nutritional experts now cite the lack of evidence implicating dietary saturated fat as the reason for lack of concern of it in the diet ( 36 ).
The concept of comparing medications that treat IR by insulin-sensitizers or by providing insulin itself was tested in the Bari-2D study ( 37 ). Presumably in the context of consuming a standard American diet, this study found no significant difference in death rates or major cardiovascular events between strategies of insulin sensitization or insulin provision.
While lifestyle modification may be ideal to prevent or cure IR and T2DM, for many people these changes are difficult to learn and/or maintain. Future research should be directed toward improving adherence to all effective lifestyle or medication treatments. Future research is also needed to assess the effect of carbohydrate restriction on primary or secondary prevention of outcomes of cardiovascular disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
EW receives royalties from popular diet books and is founder of a company based on low-carbohydrate diet principles (Adapt Your Life, Inc.).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care . (2016) 39 (Suppl. 1):S13–22. doi: 10.2337/dc16-S005
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. (1984) 74:1238–46. doi: 10.1172/JCI111533
3. Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. (2005) 34:49–62. doi: 10.1016/j.ecl.2004.12.001
4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7
6. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
7. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. (2000) 106:171–6. doi: 10.1172/JCI10583
8. Guizouarn H, Allegrini B. Erythroid glucose transport in health and disease. Pflugers Arch. (2020) 472:1371–83. doi: 10.1007/s00424-020-02406-0
9. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. (2017) 13:572–87. doi: 10.1038/nrendo.2017.80
10. Tondt J, Yancy WS, Westman EC. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr Res Rev. (2020) 33:260–70. doi: 10.1017/S0954422420000050
11. Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. (2013) 36:e177. doi: 10.2337/dc13-0965
12. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. (2013) 34:121–38. doi: 10.1016/j.mam.2012.07.001
13. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. (2002) 3:267–77. doi: 10.1038/nrm782
14. Epstein FH. Oxygen and renal metabolism. Kidney Int. (1997) 51:381–5. doi: 10.1038/ki.1997.50
15. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. (2006) 26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258
16. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
17. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
18. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
CrossRef Full Text | Google Scholar
19. Allen F. The treatment of diabetes. Boston Med Surg J. (1915) 172:241–7. doi: 10.1056/NEJM191502181720702
20. Osler W, McCrae T. The Principles and Practice of Medicine . 9th ed. New York and London: Appleton & Company (1923).
21. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. (2021) 131:e142246. doi: 10.1172/JCI142246
22. Steelman GM, Westman EC. Obesity: Evaluation and Treatment Essentials . 2nd ed. Boca Raton: CRC Press, Taylor & Francis Group (2016). 340 p.
23. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
24. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. (2011) 54:2506–14. doi: 10.1007/s00125-011-2204-7
25. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. (2010) 33:1438–42. doi: 10.2337/dc09-2107
26. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol. (2018) 17:56. doi: 10.1186/s12933-018-0698-8
27. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. (2021) 6:437–47. doi: 10.1001/jamacardio.2020.7073
28. Duwaerts CC, Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
29. Zhang L, She Z-G, Li H, Zhang X-J. Non-alcoholic fatty liver disease: a metabolic burden promoting atherosclerosis. Clin Sci Lond Engl. (1979) 134:1775–99. doi: 10.1042/CS20200446
30. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. (1995) 62:19–29. doi: 10.1093/ajcn/62.1.19
31. Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol. (2000) 74 (Suppl. 1):S17–22. doi: 10.1016/S0167-5273(99)00107-2
32. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
33. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769. doi: 10.7326/0003-4819-140-10-200405180-00006
34. Tendler D, Lin S, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. (2007) 52:589–93. doi: 10.1007/s10620-006-9433-5
35. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. (1995) 222:339–50. doi: 10.1097/00000658-199509000-00011
36. Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:844–57. doi: 10.1016/j.jacc.2020.05.077
37. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med . (2009) 360:2503–15. doi: 10.1056/NEJMoa0805796
Keywords: type 2 diabetes, insulin resistance, pre-diabetes, carbohydrate-restricted diets, hyperinsulinemia, hyperglycemia
Citation: Westman EC (2021) Type 2 Diabetes Mellitus: A Pathophysiologic Perspective. Front. Nutr. 8:707371. doi: 10.3389/fnut.2021.707371
Received: 09 May 2021; Accepted: 20 July 2021; Published: 10 August 2021.
Reviewed by:
Copyright © 2021 Westman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric C. Westman, ewestman@duke.edu
This article is part of the Research Topic
Carbohydrate-restricted Nutrition and Diabetes Mellitus
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 February 2021
To tackle diabetes, science and health systems must take into account social context
- Jacqueline A. Seiglie ORCID: orcid.org/0000-0001-9278-4516 1 , 2 ,
- Devaki Nambiar ORCID: orcid.org/0000-0001-5682-6109 3 , 4 , 5 , 6 ,
- David Beran 7 , 8 &
- J. Jaime Miranda ORCID: orcid.org/0000-0002-4738-5468 6 , 9 , 10 , 11 , 12
Nature Medicine volume 27 , pages 193–195 ( 2021 ) Cite this article
4769 Accesses
11 Citations
31 Altmetric
Metrics details
- Developing world
- Endocrinology
An increasing amount of publications are recognizing that a person’s risk of diabetes and diabetes outcomes are influenced largely by social determinants of health. This renewed understanding of disease should influence health provision and diabetes research, but will it?
In a year marked by the centenary of the discovery of insulin 1 and outstanding scientific progress in the therapeutic management of diabetes mellitus 2 , 3 , these advances have been contrasted by the sobering disparities that, although present before the COVID-19 pandemic, have been unmasked by this crisis for people living with diabetes. While diabetes is now well recognized as an important biological risk factor for poor COVID-19 outcomes, the disproportionate impact of this pandemic on socially vulnerable people with diabetes has laid bare the profound importance of the social determinants of health in the tackling of health inequalities 4 , 5 . These disparities are particularly alarming when one considers that among the nearly half-billion people with diabetes globally, three out of every four people with diabetes live in low- and middle-income countries (LMICs) 6 . The renewed attention on the social determinants of health in relation to diabetes in the wake of the COVID-19 pandemic has revealed the sheer complexity of tackling health inequalities and the inadequacy of existing metrics, frameworks and approaches 7 . The question is: amid the astounding scientific progress accomplished in the management of diabetes, what does a social-determinants framework add to the field, particularly in LMICs?
It has long been recognized that health is strongly influenced by social determinants—the conditions in which people are born, grow, live, work, and age, including the health system 8 . In fact, the concept of the social determinants of health was woven into the very foundations of modern public health, which at the turn of the 19th century inspired pioneers such as Rudolf Virchow to posit that social medicine was inextricably intertwined with the politics of social justice 9 , 10 . Further evidence on the strong linkage between the social determinants and their impact on disease emerged throughout the 20th century 11 and, in 2008, culminated in the report of the World Health Organization’s Commission on the Social Determinants of Health 8 , which called for a global movement to recognize their importance in the tackling of health inequalities, with the goal of closing the health gap within a generation. Yet more than a decade since that call for action was put forth, the COVID-19 crisis has abruptly highlighted the lack of progress achieved in tackling inequalities, making visible the linkages and the gross weaknesses of living conditions, health and well-being, and the impact of this on multiple facets of long-term chronic conditions 12 , 13 .
In their timely article ‘Social determinants of health and diabetes: a scientific review’, published in Diabetes Care 4 , the authors provide an overview of key definitions and social-determinant frameworks of diabetes and outline detailed recommendations for their implementation into community sectors, diabetes research and clinical practice in the USA. The review builds on pragmatic scientific and consensus statements published before the COVID-19 pandemic 14 , 15 and categorizes social determinants of diabetes into the following five domains: socioeconomic status; neighborhood and physical environment; food environment; healthcare; and social context 4 . Each of these domains encompass aspects of life circumstances that influence how a person’s risk of diabetes and diabetes outcomes are shaped—a phenomenon that occurs largely outside of the health system 16 . While there is robust evidence to support this framework, much of this evidence has been generated in high-income contexts. Less is known about the social determinants of diabetes in LMICs, which are highly heterogeneous and may be subject to additional societal pressures, such as socio-political and protracted conflicts 17 , environmental pollutants 18 , and corporate and commercial power 19 , 20 , as well as constrained health systems with limited capacity to manage chronic conditions that rely on continuity of care 21 .
Whereas much of the scientific progress in diabetes has gravitated toward the domain of pharmacological interventions, diabetes ‘exists’ within a much broader context, with factors that influence its development throughout the life course. For instance, an adverse intrauterine and postnatal environment may influence the development of insulin resistance and the onset of metabolic disease later in life 22 . When these early life disadvantages are coupled with social and commercial determinants that catalyze the development of overweight and obesity 19 , the cumulative burden of these life-course circumstances, which disproportionately affect vulnerable segments of the population, can be difficult to overcome even within equitable health systems. Notably, solutions for mitigating the risk of diabetes may very well lie outside the sphere of health systems, as demonstrated by a housing experiment showing that the opportunity to move to a neighborhood with a lower poverty rate was associated with a lower prevalence of severe obesity and poorly controlled diabetes 16 . As contended by others, “a diagnosis is rarely a solution to problems caused by poverty and inequality” 23 . Furthermore, there is a strong socioeconomic patterning of diabetes and other non-communicable diseases at the population level, whose rise in LMICs is occurring in the context of a complex interplay of epidemiological, demographic and nutritional transitions 24 , 25 . Within this framework, diabetes and other non-communicable diseases are posited to rise initially among affluent groups before shifting to groups of low socioeconomic status 26 , 27 . It is critical to recognize this socioeconomic patterning of diabetes, ideally before the burden of diabetes shifts among populations facing socioeconomic and other vulnerabilities, which requires that additional obstacles, manifold in quantity and complexity, be overcome.
While the social-determinants framework is fundamental for understanding elements largely outside of the health system that contribute to the risk of diabetes and its outcomes, insightful medical anthropologists have long proposed the broader framework of ‘syndemic’ (‘synergistic epidemic’) theory 28 , 29 . A syndemic refers to the clustering of two or more diseases within a population that contributes to, and results from, persistent social and economic inequalities 28 , 30 —a concept that is particularly relevant in the context of the intersection between COVID-19 and diabetes and that highlights social determinants as core components of the effort to eradicate disparities 30 . Indeed, it has been proposed that in tackling COVID-19 and its interaction with non-communicable diseases, “the syndemic nature of the threat we face means that a more nuanced approach is needed if we are to protect the health of our communities” 30 . A ‘syndemic lens’ can both lead to a deeper understanding of the reasons for the clustering of diabetes in certain populations and inform targeted policies to address broader structural and political forces that impact both the development of diabetes and diabetes-related outcomes 31 . This framework requires a nuanced understanding of context-dependent factors that influence disease development and progression, as well as investment in research that can tackle the biosocial complexities of diabetes 28 , 29 , 32 .
The strengthening of health systems for the provision of diabetes care in LMICs is critical. However, the development of health systems that solely prioritize short-term care delivery, relying on patient–provider interaction or health-system inputs, is likely to fall short in tackling both the rising burden of diabetes and its associated disparities. This core challenge, of recognizing the limitations of the current models of care, is poignantly captured by this reflection on patients’ real-world experience of living with type 2 diabetes in Peru: “a patient’s agency to manage their [type 2 diabetes] is affected by a multiplicity of factors acting together: the burden of treatment, the chronicity of poverty, the immediate social context, the deficiencies in the health system, as well as the financial burden of dietary change and medication adherence…the interaction among all these factors in an individual’s vulnerability to ill-health have been recognized by critical medical anthropologists who developed the syndemics framework to acknowledge the role of broader political, economic, and social structures in the individual lives of people, their health, and responses to health” 33 .
How can the COVID-19 crisis be leveraged to revisit the approach to diabetes care in LMICs through a syndemic framework? Diabetes has been long considered a suitable ‘tracer’ condition of international benchmarking of health systems, since it is well defined, fairly easy to diagnose and common 34 . Given the enormous and rapidly growing prevalence of diabetes in LMICs, as well as its social patterning, we argue that diabetes is also a suitable tracer of health disparities 35 . Through its recognition as such, the approach to understanding the growing burden of diabetes in LMICs can be broadened beyond its biology and within its tightly linked social, economic and structural context 29 , 32 .
The publication of scientific statements on the importance of social-determinant frameworks is a great step forward 4 , 14 , 15 . However, will this translate into research efforts and changes in this arena beyond the exploration of disparities in high-income contexts? The incorporation of a social-determinants framework will require both a conceptual shift in the collective approach to the prevention and management of diabetes, beyond pathophysiology and pharmacotherapy, and concomitant investment in upstream factors as an integral aspect of the diabetes field. Funders, governments, the private sector and researchers should consider the critical importance of the social determinants of diabetes as the world moves toward the ‘new normal’ after COVID-19, to ensure a more equitable society in which health is not vitiated by underlying social and economic factors. The centenary of the discovery of insulin is a reminder not only of the impact science and medicine can have on health but also that if governments and civil society, including academia, do not play a role in addressing disparities, advances will be sequestered among the privileged.
And we return to our question: what does a social-determinants ‘lens’ add to the field, particularly in the LMIC context? The multi-faceted challenges imposed by COVID-19 and the neglected rising burden of diabetes in LMICs present a key opportunity for remembering the caution and the bold call to action put forward by the World Health Organization’s Commission on the Social Determinants of Health in 2008: social injustice is killing people on a grand scale, and the health gap must be closed within a generation. It follows that ‘building back better and fairer’ 36 will require rather more than advances in pharmacotherapy and technologies. Life course, the whole of society and intergenerational approaches ought to guide responses, attempting to tackle multiple drivers at once. Indeed, contending with the global, national and local legacies of power (asymmetries) lies at the heart of addressing diabetes—and the ailing health systems and societies its burden reflects—in the coming decades.
Hegele, R. A. & Maltman, G. M. Lancet Diabetes Endocrinol. 8 , 971–977 (2020).
Article CAS PubMed Google Scholar
Sheahan, K. H., Wahlberg, E. A. & Gilbert, M. P. Postgrad. Med. J. 96 , 156–161 (2020).
Article PubMed Google Scholar
Zannad, F. et al. Lancet 396 , 819–829 (2020).
Hill-Briggs, F. et al. Diabetes Care https://doi.org/10.2337/dci20-0053 (2021).
Sosa-Rubí, S.G. et al. Diabetes Care https://doi.org/10.2337/dc20-2192 (2020).
International Diabetes Federation. https://www.diabetesatlas.org/en/resources/ (accessed 18 December 2019).
Gianella, C., Gideon, J. & Romero, M. J. Can. J. Dev. Stud . https://doi.org/10.1080/02255189.2020.1843009 (2020).
World Health Organization. https://www.who.int/publications-detail-redirect/WHO-IER-CSDH-08.1 (2008).
Nambiar, D. & Muralidharan, A. (eds.) The Social Determinants of Health in India: Concepts, Processes, and Indicators https://doi.org/10.1007/978-981-10-5999-5 (Springer Singapore, 2017).
Reese, D. M. West. J. Med. 169 , 105–108 (1998).
CAS PubMed PubMed Central Google Scholar
Marmot, M. G., Rose, G., Shipley, M. & Hamilton, P. J. J. Epidemiol. Community Health 32 , 244–249 (1978).
Article CAS PubMed PubMed Central Google Scholar
Nambiar, D., Bhaumik, S., Pal, A. & Ved, R. BMC Health Serv. Res. 20 , 1077 (2020).
Article PubMed PubMed Central Google Scholar
Pesantes, M. A. et al. Rev. Peru. Med. Exp. Salud Publica 37 , 541–546 (2020).
Hill, J. O. et al. Diabetes Care 36 , 2430–2439 (2013).
Thornton, P. L. et al. Ann. NY Acad. Sci. 1461 , 5–24 (2020).
Ludwig, J. et al. N. Engl. J. Med. 365 , 1509–1519 (2011).
Boulle, P., Kehlenbrink, S., Smith, J., Beran, D. & Jobanputra, K. Lancet Diabetes Endocrinol. 7 , 648–656 (2019).
Liu, F. et al. Environ. Pollut. 252 , 1235–1245 (2019).
McKee, M. & Stuckler, D. Am. J. Public Health 108 , 1167–1170 (2018).
de Lacy-Vawdon, C. & Livingstone, C. BMC Public Health 20 , 1022 (2020).
Beran, D. Curr. Diab. Rep. 15 , 20 (2015).
Lawlor, D. A., Davey Smith, G. & Ebrahim, S. Diabetes Care 26 , 97–103 (2003).
Burgess, R. Nature https://doi.org/10.1038/d41586-020-01313-9 (2020).
Popkin, B. M. Public Health Nutr. 1 , 5–21 (1998).
Omran, A. R. Milbank Mem. Fund Q. 49 , 509–538 (1971).
Jiwani, S. S. et al. Lancet Glob. Health 7 , e1644–e1654 (2019).
Jaacks, L. M. et al. Lancet Diabetes Endocrinol. 7 , 231–240 (2019).
Singer, M., Bulled, N., Ostrach, B. & Mendenhall, E. Lancet 389 , 941–950 (2017).
Mendenhall, E., Kohrt, B. A., Norris, S. A., Ndetei, D. & Prabhakaran, D. Lancet 389 , 951–963 (2017).
Horton, R. Lancet 396 , 874 (2020).
Mendenhall, E. Lancet 396 , 1731 (2020).
Saxena, A. & Mendenhall, E. Soc. Sci. Med . https://doi.org/10.1016/j.socscimed.2020.113503 (2020).
Pesantes, M. A., Tetens, A., Valle, A. D. & Miranda, J. J. Hum. Organ. 78 , 85–96 (2019).
Article Google Scholar
Nolte, E., Bain, C. & McKee, M. Diabetes Care 29 , 1007–1011 (2006).
Taype-Rondan, A., Lazo-Porras, M., Moscoso-Porras, M., Moreano-Sáenz, M. & Miranda, J. J. Br. J. Gen. Pract. 66 , 197–197 (2016).
Marmot, M. The BMJ Opinion https://blogs.bmj.com/bmj/2020/12/15/michael-marmot-post-covid-19-we-must-build-back-fairer/ (15 December 2020).
Download references
Author information
Authors and affiliations.
Diabetes Unit, Massachusetts General Hospital, Boston, MA, USA
Jacqueline A. Seiglie
Department of Medicine, Harvard Medical School, Boston, MA, USA
The George Institute for Global Health, New Delhi, Delhi, India
Devaki Nambiar
Faculty of Medicine, University of New South Wales, Sydney, Australia
Prasanna School of Public Health, Manipal Academy of Higher Education, Karnataka, India
The Bernard Lown Scholars in Cardiovascular Health Program, Harvard TH Chan School of Public Health, Boston, MA, USA
Devaki Nambiar & J. Jaime Miranda
Division of Tropical and Humanitarian Medicine, Hôpitaux Universitaires de Genève, Geneva, Switzerland
David Beran
Faculty of Medicine, University of Geneva, Geneva, Switzerland
Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia, Lima, Peru
J. Jaime Miranda
CRONICAS Center of Excellence in Chronic Diseases, Universidad Peruana Cayetano Heredia, Lima, Peru
The George Institute for Global Health, Sydney, Australia
Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, London, UK
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Jacqueline A. Seiglie or J. Jaime Miranda .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Seiglie, J.A., Nambiar, D., Beran, D. et al. To tackle diabetes, science and health systems must take into account social context. Nat Med 27 , 193–195 (2021). https://doi.org/10.1038/s41591-021-01231-x
Download citation
Published : 01 February 2021
Issue Date : February 2021
DOI : https://doi.org/10.1038/s41591-021-01231-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
How could taxes on sugary drinks and foods help reduce the burden of type 2 diabetes.
- Alan Reyes-García
- Isabel Junquera-Badilla
- Ana Basto-Abreu
Current Diabetes Reports (2023)
Multimorbidity
- Søren T. Skou
- Frances S. Mair
- Susan M. Smith
Nature Reviews Disease Primers (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
Health Information
- About NIDDK
- Español
The health information here is informed by NIDDK research, reviewed by doctors, and provided to help you understand the diseases and conditions you or your loved ones may face.
- Diabetes Basics
- Nerve Damage (Diabetic Neuropathies)
- Prediabetes & Insulin Resistance
Digestive Diseases
- Irritable Bowel Syndrome (IBS)
- Crohn's Disease
- Colon Polyps
Kidney Disease
- Chronic Kidney Disease (CKD)
- Simple Kidney Cysts
- Kidney Stones
Weight Management
- Prescription Medications to Treat Overweight & Obesity
- Helping Your Child Who Is Overweight
- Weight-loss (Bariatric) Surgery
Liver Disease
- Hepatitis C
- Nonalcoholic Fatty Liver Disease & NASH
Urologic Diseases
- Bladder Control Problems in Women (Urinary Incontinence)
- Erectile Dysfunction
Endocrine Diseases
- Adrenal Insufficiency & Addison's Disease
- Pregnancy & Thyroid Disease
- Hypothyroidism (Underactive Thyroid)
Diet & Nutrition
- About Food Portions
- Diabetes Diet & Eating
- Nutrition for Advanced Chronic Kidney Disease (CKD) in Adults
Blood Diseases
- Anemia of Inflammation & Chronic Disease
- Sickle Cell Trait & Other Hemoglobinopathies & Diabetes
Diagnostic Tests
- Upper GI Endoscopy
- Colonoscopy
- The A1C Test & Diabetes
Health Statistics

Epidemiologic, public health, and clinical data on diabetes, digestive, kidney, liver, and urologic diseases, as well as overweight and obesity
Healthy Moments Radio
Listen to health tips from Dr. Rodgers in his weekly 1-minute episodes.
- Caregiving & Alzheimer’s Disease
- Meal Timing in Weight Management
- Understanding Weight Loss Procedure Options
- How Anti-Obesity Medicines Work
- Emerging Trends in Weight Management
- Bowel Control Problems: Treatments Are Available
For Health Professionals

Community Health & Outreach

View program guides and toolkits on NIDDK-related conditions and diseases with your community.
Is Participating in Clinical Trials Right for You?
Clinical trials offer hope for many people and an opportunity to help researchers find better treatments for others in the future.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Reversing Type 2 Diabetes: A Narrative Review of the Evidence
Sarah j hallberg.
1 Virta Health, 535 Mission Street, San Francisco, CA 94105, USA; moc.htlaehatriv@einimahs
2 Indiana University Health Arnett, Lafayette, IN 47904, USA; gro.htlaehui@nubzaht
3 Indiana University School of Medicine, Indianapolis, IN 46202, USA
Victoria M Gershuni
4 Department of Surgery, Perelman School of Medicine University of Pennsylvania, Philadelphia, PA 19104, USA; moc.liamg@dminuhsregairotciv
Tamara L Hazbun
Shaminie j athinarayanan.
Background: Type 2 diabetes (T2D) has long been identified as an incurable chronic disease based on traditional means of treatment. Research now exists that suggests reversal is possible through other means that have only recently been embraced in the guidelines. This narrative review examines the evidence for T2D reversal using each of the three methods, including advantages and limitations for each. Methods: A literature search was performed, and a total of 99 original articles containing information pertaining to diabetes reversal or remission were included. Results: Evidence exists that T2D reversal is achievable using bariatric surgery, low-calorie diets (LCD), or carbohydrate restriction (LC). Bariatric surgery has been recommended for the treatment of T2D since 2016 by an international diabetes consensus group. Both the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) now recommend a LC eating pattern and support the short-term use of LCD for weight loss. However, only T2D treatment, not reversal, is discussed in their guidelines. Conclusion: Given the state of evidence for T2D reversal, healthcare providers need to be educated on reversal options so they can actively engage in counseling patients who may desire this approach to their disease.
1. Introduction
According to 2017 International Diabetes Federation (IDF) statistics, there are approximately 425 million people with diabetes worldwide [ 1 ]. In the United States, there are an estimated 30.3 million adults living with diabetes, and its prevalence has been rising rapidly, with at least 1.5 million new diabetes cases diagnosed each year [ 2 ]. Diabetes is a major public health epidemic despite recent advances in both pharmaceutical and technologic treatment options.
Type 2 diabetes (T2D) has long been identified as an incurable chronic disease. The best outcome that has been expected is amelioration of diabetes symptoms or slowing its inevitable progression. Approximately 50% of T2D patients will need insulin therapy within ten years of diagnosis [ 3 ] Although in the past diabetes has been called chronic and irreversible, the paradigm is changing [ 4 , 5 ].
The recent 2016 World Health Organization (WHO) global report on diabetes added a section on diabetes reversal and acknowledged that it can be achieved through weight loss and calorie restriction [ 4 ]. “Diabetes reversal” is a term that has found its way into scientific articles and the lay press alike; “remission” has also been used. While the exact criteria are still debated, most agree that a hemoglobin A1c (HbA1c) under the diabetes threshold of 6.5% for an extended period of time without the use of glycemic control medications would qualify [ 6 ]. Excluding metformin from the glycemic control medications list, as it has indications beyond diabetes, may also be a consideration [ 7 , 8 ]. Likewise, terms such as “partial” (HbA1c <6.5 without glycemic control medications for 1 year) or “complete” (HbA1c <5.7 without glycemic control medications for 1 year) remission have been defined by an expert panel as more evidence accumulates that points to the possibility of avoiding the presumably progressive nature of T2D [ 9 ]. It is important to note that the term “cure” has not been applied to T2D, as there does exist the potential for re-occurrence, which has been well documented in the literature.
Despite the growing evidence that reversal is possible, achieving reversal is not commonly encouraged by our healthcare system. In fact, reversal is not a goal in diabetes guidelines. Specific interventions aimed at reversal all have one thing in common: they are not first-line standard of care. This is important, because there is evidence suggesting that standard of care does not lead to diabetes reversal. This raises the question of whether standard of care is really the best practice. A large study by Kaiser Permanente found a diabetes remission rate of 0.23% with standard of care [ 10 ]. The status quo approach will not reverse the health crisis of diabetes.
A significant number of studies indicate that diabetes reversal is achievable using bariatric surgery, while other approaches, such as low-calorie diets (LCD) or carbohydrate restriction (LC), have also shown effectiveness in an increasing number of studies. This review will examine each of these approaches, identifying their beneficial effects, supporting evidence, drawbacks, and degree of sustainability.
2. Materials and Methods
A literature search was performed as appropriate for narrative reviews, including electronic databases of PubMed, EMBASE, and Google Scholar from 1970 through December 2018. We reviewed English-language original and review articles found under the subject headings diabetes, bariatric surgery, metabolic surgery, very low-calorie diet, calorie restriction, low carbohydrate diet, ketogenic diet, diabetes remission, and diabetes reversal. References of the identified publications were searched for more research articles to include in this review. Selected studies were reviewed and evaluated for eligibility for inclusion in this review based on their relevance for diabetes reversal and remission. Either remission or reversal needed to be discussed in the paper or the results were consistent with these terms for inclusion. Randomized clinical trials and intervention-based studies were given emphasis for inclusion.
A total of 99 original articles containing information pertaining to diabetes reversal or remission were included in this narrative review.
3. Results and Discussion
3.1. bariatric surgery.
Bariatric surgery has long been recognized as a potential treatment for both morbid obesity and the metabolic processes that accompany it, specifically T2D. While the efficacy of T2D reversal depends on the choice of procedure, there is unilateral improvement in glycemia following operation [ 11 ], and bariatric surgery has been found to be superior to intensive T2D medical management. Accordingly, in 2016, the second Diabetes Surgery Summit (DSS-II) released recommendations, endorsed by 45 medical and scientific societies worldwide, to use bariatric surgery as a treatment for T2D (bariatric surgery is currently approved by the 2016 recommendations for adults with a body mass index (BMI) >40, or >35 kg/m 2 with obesity-related comorbidities) [ 12 ]. Of interest is the consistent finding that glycemic improvements occur rapidly, often within hours to days, and precede weight loss, which likely represents the enteroendocrine responses to altered flow of intestinal contents (i.e., bile acid signaling and changes in microbiota and their metabolome) [ 13 , 14 , 15 , 16 , 17 , 18 , 19 ].
The most commonly performed bariatric surgeries in the United States include laparoscopic and robotic Roux-en-Y Gastric Bypass (RYGB) or Sleeve Gastrectomy (SG). While surgical treatment is based on the principles of restriction and intestinal malabsorption, evidence suggests that there are more complex mechanisms at play. Bariatric surgery has consistently been shown to dramatically and rapidly improve blood glucose [ 20 ] while allowing decreased oral hypoglycemic medications and insulin use, effectively reversing diabetes in up to 80% of patients [ 21 ] in the short term. In addition to early post-operative improvement in blood glucose and insulin sensitivity, bariatric surgery has also been shown to cause alterations in GI hormone release, including ghrelin, leptin, cholecystokinin (CCK), peptide-tyrosine-tyrosine (PYY), and glucagon-like peptide 1 (GLP-1), that may impact feeding behavior via the gut–brain axis in addition to modulating euglycemia [ 22 ]. Furthermore, microbial changes in the human gut have been linked to obesity, and surgical alterations to gastrointestinal anatomy have been associated with dramatic changes in gut microbiota populations with reversion from an “obesogenic” to a lean bacterial population [ 13 , 14 , 16 , 19 , 23 , 24 ].
Long-term outcomes from bariatric surgery depend on multiple factors, including type of surgery performed, patient comorbidities, patient readiness for lifelong dietary change, and ongoing surveillance. While bariatric surgery has been demonstrated to be safe and effective overall, it is important to recognize that it is not without risks. Each patient must weigh the risks and benefits associated with untreated morbid obesity versus those associated with surgery or effective dietary management and choose accordingly. Surgery of any type can be associated with complications leading to morbidity or mortality; the complication rates have been stated to be as high as 13% and 21% for SG and RYGB, respectively. The postoperative mortality rate is 0.28–0.34% for SG and 0.35–0.79% for RYGB; in comparison, an elective laparoscopic cholecystectomy is associated with overall complication rates of 9.29% and with a 30-day mortality rate of 0.15–0.6%, depending on the series [ 25 , 26 ]. Significant complications include anastomotic leak or hemorrhage, post-operative readmission, need for reoperation, post-operative hypoglycemia, dumping syndrome, worsening acid reflux, marginal ulceration, and micronutrient deficiencies [ 25 , 26 , 27 , 28 , 29 ].
It is important to consider that while short-duration studies have shown early resolution of comorbidities following bariatric procedures, when followed for multiple decades, there may be decreased efficacy of disease resolution and increased incidence of hospital admission long-term. Long-term reversal of T2D and true glucose homeostasis remain uncertain. Weight loss after surgery is a significant predictor of a return to euglycemia post-operatively. Multiple studies have reported initial T2D remission rates as high as 80% [ 30 , 31 ], however, long-term remission is less durable. The five-year follow-up outcomes of the SLEEVEPASS RCT found complete or partial remission of T2D in 37% of SG and 45% of RYGB patients, which is similar to other studies showing long-term T2D remission in up to a third of patients [ 32 ]. In the large prospective cohort study Longitudinal Assessment of Bariatric Surgery 2 (LABS-2), the investigators found that long-term diabetes remission after RYGB was higher than predicted by weight loss alone, which suggests that the surgery itself impacts metabolic factors that contribute to disease management [ 31 ]. Similarly, the STAMPEDE trial—an RCT that followed 150 patients with T2D who were randomized to intensive medical intervention (IMT) versus IMT plus RYGB versus IMT plus SG for diabetes resolution (defined as HbA1c <6.0%) and followed for five years—revealed increased rates of T2D resolution with RYGB (29%) and SG (23%) compared to IMT alone (5%) ( Figure 1 ). The surgery cohort also demonstrated greater weight loss and improvements in triglycerides, HDL, need for insulin, and overall quality of life [ 33 , 34 , 35 ].

( A ) Mean changes of hemoglobin A1c (HbA1c) from baseline to last published date for each study retrieved to represent the three methods of reversal; ( B ) mean changes of weight from baseline to last published date for each studies retrieved to represent the three methods of reversal. Note: We chose these three studies to represent the three methods of reversal based on publication date and relevance to diabetes reversal. Note that baseline characteristics differ. Surgery trial examined by sleeve gastrectomy and Roux-en-Y gastric bypass separately and were represented as sleeve and bypass in the graph. Surgery: STAMPEDE [ 34 , 35 ]. Low-calorie diets (LCD): DIRECT [ 65 , 66 ]; carbohydrate restriction (LC): IUH [ 99 , 107 ].
Despite the likelihood of improved glycemic control, there are significant financial costs for the patient, health system, and insurance companies associated with bariatric surgery (U.S. average of $14,389) [ 36 ]. Despite the high initial cost of surgery, Pories and colleagues found that prior to surgery, patients spend over $10,000 per year on diabetes medications; after RYGB, the annual cost falls to less than $2000, which represents an $8000 cost savings at the individual level [ 30 ]. Furthermore, economic analyses show that surgery is likely to be cost-effective, especially in patients who are obese [ 37 , 38 ]. In a clinical effectiveness review of the literature that included 26 trials extracted from over 5000 references, Picot et al. found that bariatric surgery was a more effective intervention for weight loss than non-surgical options; however, there was extreme heterogeneity and questionable long-term adherence to the non-surgical interventions [ 39 ]. After surgery, metabolic syndrome improved, and there were higher rates of T2D remission compared to the non-surgical groups [ 39 ]. Further, while there were improvements in comorbidities after surgery independent of bariatric procedure, there was also an increased likelihood of adverse events. While the overall event rate remained low, major adverse events included medication intolerance, need for reoperation, infection, anastomotic leakage, and venous and thromboembolic events [ 39 ].
It is imperative to consider that one of the requirements of qualifying for bariatric surgery is demonstration of at least six months of unsuccessful attempts at weight loss using traditional dietary and exercise advice according to the 2016 Recommendations [ 12 ]. There are, however, no requirements as to what weight loss strategy is employed, which may represent a time point where dietary intervention, including low-calorie, ketogenic, or carbohydrate-restricted diets, should be utilized. At least two recent clinical trials have demonstrated safety and efficacy in pre-operative very low-carbohydrate ketogenic diets before bariatric surgery for increasing weight loss and decreasing liver volume [ 40 , 41 ].
Furthermore, despite technically adequate surgery, an alarming number of patients may still experience weight regain and/or recurrence of comorbid obesity-associated conditions. In these patients, effective strategies for dietary intervention are even more important. Approximately 10–15% of patients fail to lose adequate weight (failure defined as <50% of excess weight) or demonstrate significant weight regain after bariatric surgery without evidence of an anatomic or technical reason [ 42 ]. Additionally, in 25–35% of patients who undergo surgery, significant weight regain (defined as >15% of initial weight loss) occurs within two to five years post-operatively [ 43 ]. These patients often require further medical management with weight loss medications, further dietary and behavioral intervention, and, for some, reoperation. Reoperation can be for either revision for further weight loss (narrowing of the gastric sleeve, conversion of VSG to RYGB , and increasing the length of the roux limb) or reversal of RYGB due to health concerns, most commonly associated with malnutrition. A small cohort of patients (4%) may experience severe weight loss with significant malnutrition leading to hospitalization in over 50%, mortality rates of 18%, and need for reversal of RYGB anatomy. While the incidence of RYGB reversal is unknown, based upon a systematic review that included 100 patients spanning 1985–2015, the rate of reversal parallels the increasing rate of bariatric surgery [ 44 ].
In the short term, T2D reversal rates with surgery have been reported to be as high as 80%, with an additional 15% demonstrating partial improvement in T2D despite still requiring medication [ 17 ]. Within one week after RYGB, patients experience improved fasting hepatic insulin clearance, reduced basal de novo glucose production, and increased hepatic insulin sensitivity; by three months and one year after surgery, patients have improved beta-cell sensitivity to glucose, increased GLP-1 secretion from the gut, and improved insulin sensitivity in muscle and fat cells [ 45 ]. Over time, T2D remission rates remain high but do decline; Purnell and colleagues reported three-year remission rates of 68.7% after RYGB [ 29 ]. However, Pories published results from a 14-year prospective study with mean follow-up of 7.6 years, and found 10-year remission rates remained around 83% [ 46 ]. In a 10-year follow-up study of participants from the Swedish Obese Subjects (SOS) study that prospectively followed patients who underwent bariatric surgery, the authors reported a 72% ( n = 342) and 36% ( n = 118) recovery rate from T2D for RYGB at two years and 10 years, respectively [ 47 ].
The long-term metabolic impact and risk reduction from surgery remain high in a substantial number of patients and this route to reversal clearly has the most robust data to support its use. As evidenced by the dramatic improvements in metabolic state that precede weight loss, bariatric surgery is far more than merely a restrictive and/or malabsorptive procedure. Large shifts in bile acid signaling in the lumen of the small intestine, gut nutrient sensing, and changes in the microbiota community appear to greatly impact overall host health. Further research is ongoing using both basic and translational science models to identify the role of these various hormones and metabolites; perhaps there will be a way to one day harness the beneficial effects of bariatric surgery without the need for anatomic rearrangement.
3.2. Low-Calorie Diets (LCD)
As diabetes rates have risen to unprecedented levels [ 1 , 2 ], the number of studies examining diabetes reversal using non-surgical techniques has increased. A handful of studies have reported successful weight loss with decreased insulin resistance, plasma glucose, and medication use following a LCD. As early as 1976, Bistrian et al. [ 48 ] reported that a very low-calorie protein-sparing modified fast allowed for insulin elimination in all seven obese patients with T2D. The average time to insulin discontinuation was only 6.5 days, and the longest was 19 days. In a study by Bauman et al., a low-calorie diet of 900 kcal, including 115 g of protein, led to significant improvement in glycemic control that was mainly attributed to improvements in insulin sensitivity [ 49 ]. Furthermore, a study conducted in obese T2D patients found that a LCD and gastric bypass surgery were equally effective in achieving weight loss and improving glucose and HbA1c levels in the short term [ 50 ]. Weight loss, however, persisted in the diet-treated patients only for the first three months, indicating difficulty with long-term maintenance [ 47 ]. Similarly, other studies also reported similar pattern of early blood glucose normalization without medication use, but the improvements were not sustained long-term [ 51 , 52 , 53 ]. Likewise, the study by Wing et al., even though reported significant and greater improvements of HbA1c at 1 year in the intermittently delivered very low-calorie diet, the HbA1c improvement was not significantly different than what was reported in the patients receiving low-calorie diet (LCD) throughout the one year period [ 54 ]. Furthermore, the glycemic improvements observed at 1 year were not maintained through 2-years, even though the group with intermittent very low-calorie diet had less medication requirement than the group in the LCD arm at 2 years [ 54 ]. Lastly, micronutrient deficiencies with the use of calorie restricted diets has been shown and supplementation and monitoring for deficiencies is a consideration with their use [ 55 , 56 ].
While these previous studies were not assessing diabetes remission or reversal rate per se, they demonstrated the effectiveness of calorie restriction in achieving weight loss and improved glycemic control, which are the core goals of reversal. In 2003, the Look AHEAD trial randomized 5145 overweight or obese patients with T2D to an intervention group that received either an intensive lifestyle intervention (ILI) including calorie restriction and increased physical activity or to a control group that included diabetes support and education (DSE) [ 57 ]. Post hoc analysis of this study revealed that at one year, 11.5% of the participants in the ILI group achieved remission (partial or complete); however, remission rates subsequently decreased over time (9.2% at year two and 7.3% at year four). Nevertheless, the remission rates achieved through ILI were three to six times higher than those achieved in the DSE group. Lower baseline HbA1c, greater level of weight loss, shorter duration of T2D diagnosis, and lack of insulin use at baseline predicted higher remission rate in ILI participants [ 58 ].
Following the Look AHEAD study, other studies have evaluated a LCD for diabetes remission [ 59 , 60 , 61 ]. Most of these studies assessed remission over a short period of time in a small study sample. Bhatt et al. reported that six of the 12 individuals achieved partial remission at the end of the three-month intervention [ 61 ]. Ades et al. studied an intensive lifestyle program including calorie restriction and exercise, and reported that eight of the 10 individuals with recently diagnosed T2D achieved partial remission at six months, including one with complete remission [ 60 ]. The study ended at six months, therefore long term sustainability was not assessed. Another study assessing a one-year diabetes remission retrospectively among those undergoing 12 weeks of the intensive weight loss program “Why Wait” had a much lower remission rate of 4.5%, with 2.3% of them achieving partial remission, while another 2.3% had complete remission [ 59 ]. This study suggests that long-term maintenance of remission is a challenge. Moreover, diabetes remission was more likely reported in those who had a shorter diabetes duration, lower baseline HbA1c, and were taking fewer hypoglycemic medications [ 59 , 61 ].
An initial 2011 diabetes reversal study by Taylor and colleagues showed that a very low-calorie diet of 600 Kcal/day not only normalized glucose, HbA1c, and hepatic insulin sensitivity levels within a week, but also led to decreased hepatic and pancreatic triacylglycerol content and normalization of the insulin response within eight weeks [ 62 ]. At 12 weeks post-intervention, many of the improvements were maintained, but over a quarter of the patients had an early recurrence of diabetes. Further, average weight regain during the 12 weeks post-intervention was 20% [ 62 ]. As a follow-up to the 2011 study, the same group performed a larger and longer study with eight weeks of a very low-calorie meal replacement (624–700 kcal/day) followed by two weeks of solid food replacement and a weight maintenance program of up to six months [ 63 ]. In this study, those who achieved a fasting blood glucose of <7 mmol/L (<126 mg/dL) were categorized as responders, while others were categorized as non-responders. At six months, 40% of participants who initially responded to the intervention were still in T2D remission which was defined by achieving a fasting plasma glucose of <7mmol/L; the majority of those who remitted (60%) had a shorter diabetes duration (<4 years) [ 63 ].
These short-term studies were the foundation for a community-based cluster-randomized clinical trial called DiRECT (Diabetes Remission Clinical Trial). DiRECT enrolled a sample of 306 relatively healthy participants with T2D (people on insulin or with a diabetes duration longer than six years were excluded) [ 64 ] ( Figure 1 ). They were cluster randomized to either standard diabetes care or an intervention using low-calorie meal replacement diet (825–853 kcal/day) for three to five months, followed by stepwise food re-introduction and a long-term weight maintenance program. At one-year follow-up, 46% of patients met the study criteria of diabetes remission (HbA1c <6.5% without antiglycemic medications) [ 64 ] and at two years the remission rate was 36% [ 65 ]. The DiRECT study has extended their follow-up an additional three years to assess the long-term impact on remission.
Taken together, evidence suggests that a LCD is effective in reversing diabetes in the short term up to two years, and its effectiveness was predominantly demonstrated in those with shorter duration since diabetes diagnosis. It is important to note that a substantial level of calorie restriction is needed to generate a sufficient level of weight loss for reversing diabetes. Short-term intervention with moderate energy restriction and metformin for modest weight loss was not as effective in reversing diabetes as compared to standard diabetes care [ 66 ]. Lifestyle intervention with severe energy restriction may have some deleterious effect on the body composition and physiology, which poses a concern for long-term health [ 67 ]. Furthermore, long-term achievement of diabetes remission, adherence to the diet, and weight loss maintenance after the diet remain a challenge. Studies have also suggested that physiological and metabolic adaptation of the body in response to caloric restriction may shift energy balance and hormonal regulation of weight toward weight regain after weight loss [ 67 , 68 ]. Thus, it is crucial that future studies are directed towards assessing the long-term sustainability of diabetes remission led by LCD and feasibility of this diet on the physiological adaptation and body composition changes.
3.3. Carbohydrate-Restricted Diets (LC)
Before the discovery of insulin in 1921, low carbohydrate (LC) diets were the most frequently prescribed treatment for diabetes [ 69 , 70 ]. The paradigm shifted both with the development of exogenous insulin and later with the emergence of the low-fat diet paradigm. A diet low in fat, which by default is high in carbohydrate, became the standard recommendation in guidelines around the globe [ 71 ]. Rather than preventing elevations in glucose, the goal became maintenance of blood sugar control via the increased use of glycemic control medications, including insulin [ 72 ]. Over the last decade, clinical studies have begun to resurrect the pre-insulin LC dietary approach. In response to the new evidence on the efficacy of carbohydrate restriction, low-carbohydrate has recently been endorsed as an eating pattern by the ADA and the European Association for the Study of Diabetes (EASD) [ 5 , 73 ]. In addition, the Veterans Affairs/Department of Defense (VA/DOD) guidelines now recommend carbohydrate restriction as low as 14% of energy intake in its most recent guidelines for treatment of diabetes (VA) [ 74 ].
LC diets are based on macronutrient changes rather than a focus on calorie restriction [ 75 ]. Although the exact definition varies, a low-carbohydrate diet usually restricts total carbohydrates to less than 130 grams per day, while a very low-carbohydrate or ketogenic diet usually restricts total carbohydrates to as low as 20–30 grams per day. Protein consumption is generally unchanged from a standard ADA diet (around 20% of intake), with the remaining energy needs met by fat from either the diet or mobilized body fat stores. Carbohydrate sources are primarily non-starchy vegetables with some nuts, dairy, and limited fruit [ 75 ].
A total of 32 separate trials examining carbohydrate restriction as a treatment for T2D were found when our search was performed [ 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 ]. However, for reasons that may include varied levels of carbohydrate restriction and differing levels of support given, not all studies had results that would be consistent with diabetes reversal. A number of shorter-term trials have found a significant between-group advantage of a low-carbohydrate intervention for T2D [ 80 , 84 , 92 , 97 ]. Data from longer-term trials are limited, and in some follow-up studies, the between-group advantage seen initially was lost or reduced, although it often remains significantly improved from baseline. This raises the question of long-term sustainability using this approach. Due to heterogeneity in methodology and definition of carbohydrate restriction, the ability to fully examine T2D reversal based on the existing studies is limited. Based upon a recent systematic review of LC, it appears that the greatest improvements in glycemic control and greatest medication reductions have been associated with the lowest carbohydrate intake [ 109 ]. In consideration of these limitations, it appears important to assess the level of carbohydrate restriction, support or other methods given to encourage sustainability, and length of follow-up.
A study comparing an ad libitum very low-carbohydrate (<20 g total) diet to an energy-restricted low-glycemic diet in T2D found greater reduction in HbA1c, weight, and insulin levels in the low-carbohydrate arm [ 89 ]. Additionally, 95% of participants in the low-carbohydrate arm reduced or eliminated glycemic control medications, compared to 62% in the low glycemic index arm at 24 weeks. Instruction was given in a one-time session with a dietician and included take-home materials for reference. A slightly longer study (34 weeks) trial [ 85 ] found that a very low-carbohydrate ketogenic diet intervention (20–50 g net carbs per day) resulted in HbA1c below the threshold for diabetes in 55% of the patients, compared to 0% of patients in the low-fat arm. The education sessions were all online and included behavior modification strategies and mindful eating which was aimed to address binge eating. New lessons were emailed to the patients weekly for the first 16 weeks and then every two weeks for the remainder of the study.
A small (34 participants) one-year study of an ad libitum, very low-carbohydrate diet compared to a calorie-restricted moderate carbohydrate diet found a significant reduction in HbA1c between groups favoring the low-carbohydrate arm [ 86 ]. At one year, 78% of participants who began the trial with a HbA1c above 6.5% no longer met the cutoff for the diagnosis of diabetes, no longer required any non-metformin medication, and significantly reduced or eliminated metformin. Total kilocalorie intake was not significantly different between the two groups, even with moderate carbohydrate restriction. Despite equal energy intake, the low carbohydrate group lost significantly more weight and had improved glycemic control, which indicates a potential mechanistic role for carbohydrate restriction itself. The support given was 19 classes over the 12-month period, tapering in frequency over time.
Another one-year trial [ 76 ] found significant HbA1c reduction in the subset of patients with diabetes ( n = 54) assigned to an ad libitum low-carbohydrate diet (<30 total grams per day), compared to an energy-restricted low-fat diet. These results remained significant after adjusting the model for weight loss, indicating an effect of the carbohydrate reduction itself. The support given was four weekly sessions during the first month, followed by monthly sessions for the remaining 11 months.
A metabolic ward study on 10 patients with T2D [ 96 ] found that 24-h glucose curves normalized within two weeks on a very low-carbohydrate diet (<21 g total per day). This was in addition to medication reduction and elimination including insulin and sulfonylureas After accounting for body water changes, the average weight loss during the two-week period was 1.65 kg (average of <2% total body weight which is similar to the results of bariatric surgery, where normoglycemia is seen prior to significant weight loss. Interestingly, despite the diet being ad libitum other than the carbohydrate limit, the average energy intake decreased by 1000 kcal per day. Assuming no further change in glycemic control, HbA1c would be 5.6% after eight weeks, which would represent a reduction of 23% from baseline. The fact that HbA1c reductions were greater than in other, longer-term outpatient studies may indicate that support of dietary changes is the key to longer-term success.
In our published trial providing significant support through the use of a continuous care intervention (CCI), we examined using a low-carbohydrate diet aimed at inducing nutritional ketosis in patients with T2D ( n = 262), compared with usual care T2D patients ( n = 87) [ 98 ] ( Figure 1 ). At one year, the HbA1c decreased by 1.3% in the CCI, with 60% of completers achieving a HbA1c below 6.5% without hypoglycemic medication (not including metformin). Overall, medications were significantly reduced, including complete elimination of sulfonylureas and reduction or elimination of insulin therapy in 94% of users. Most cardiovascular risk factors showed significant improvement [ 110 ]. The one-year retention rate was 83%, which indicates that a non-calorie-restricted, low-carbohydrate intervention can be sustained. Improvements were not observed in the usual care patients. The newly released two-year results of this trial [ 106 ] show sustained improvements in normoglycemia, with 54% of completers maintaining HbA1c below 6.5% without medication or only on metformin. The retention rate at two years was 74%, further supporting the sustainability of this dietary intervention for diabetes reversal. Weight loss of 10% was seen at 2-years despite no intentional caloric restriction instruction. Additionally, this trial involved participants with a much longer duration of diabetes (8.4 years on average) than other nutrition trial interventions [ 58 , 64 , 65 ] and did not exclude anyone taking exogenous insulin. As duration of T2D and insulin use have both been identified to be negative factors in predicting remission after bariatric surgery [ 111 , 112 ], the 2-year results of this trial may be even more significant.
It is interesting to note that most studies utilize ad libitum intake in the carbohydrate-restricted arm. Despite this, in studies that have tracked energy intake, spontaneous calorie restriction has occurred [ 113 , 114 ]. In many trials where energy intake has been prescribed or weight loss has been equal, an advantage has been seen in glycemic control, weight, or both in the low-carbohydrate arm [ 86 , 91 , 107 ]. A better understanding of the role that caloric intake, whether prescribed or spontaneous, plays in the overall success is important. In cases of spontaneous energy intake reduction, elucidating the specific mechanism behind this reduction would help in the overall personalization of this approach.
Multiple studies have evaluated side effects or potential complications of carbohydrate restriction. The diet has been found to be safe and well tolerated although long term hard outcome data is lacking and should be a focus of future research. A transient rise in uric acid early in very low-carbohydrate restriction without an associated increase in gout or kidney stones has been documented [ 84 , 98 , 100 ]. Blood urea nitrogen (BUN) has been found to increase and decrease in different studies without an associated change in kidney function [ 87 , 98 , 100 , 115 , 116 ]. Recently, bone mineral density has been found to be unchanged despite significant weight loss after two years of a ketogenic diet intervention in patients with T2D [ 108 ]. While most studies show an improvement or no change in LDL-C levels in patients with T2D on a low-carbohydrate diet, there have been two studies that have found an increase in LDL-C in participants with T2D [ 99 , 111 ]. In one of the studies that found an increase in calculated LDL-C, a non-significant reduction in measured ApoB lipoproteins and unchanged non-HDL cholesterol were seen. Monitoring LDL-C or a measured value of potentially atherogenic lipoproteins such as ApoB should be considered. Lastly, micronutrient deficiency has been seen with a carbohydrate restricted diet, supplementation and monitoring should be given consideration with this intervention [ 56 ].
Although the use of very low-carbohydrate diets for diabetes reversal shows promising results, the lack of longer-term follow-up studies remains a limitation. Follow up is limited to two years, and therefore longer-term studies are needed to determine the sustainability of the metabolic improvements. Determining the appropriate method of support may be a key to the overall success with disease reversal.
Additional evidence has become available in recent years suggesting that diabetes reversal is a possible alternative to consider in place of traditional diabetes treatment and management. In this paper, we provide a review of three methods that have been shown to successfully reverse type 2 diabetes. The current body of evidence suggests that bariatric surgery is the most effective method for overall efficacy and prolonged remission, even though concerns associated with surgical complications, treatment cost and complete lifestyle modification after surgery remain challenges for wide adoption of this approach. While both the LCD and LC dietary approaches are convincing for reversing diabetes in the short term (up to two years), long term maintenance of diabetes remission is still unproven. There are limited available data supporting long term maintenance of weight loss and its associated glycemic improvements in response to LCD; similarly, long-term adherence to a low carbohydrate diet will likely remain an obstacle without the development of proper patient education and optimal support for long-term behavioral change. Moreover, research in understanding the mechanism of diabetes reversibility in all three approaches and its overlapping mechanistic pathways are lacking; this is an area for future research emphasis.
There are similar identified negative predictors of remission for all three approaches. These factors include longer diabetes duration and increased severity, lower BMI, advanced age, poor glycemic control, and low C-peptide levels (indicating decreased endogenous insulin production) [ 117 ]. Further exploration into the heterogeneity of these factors will help personalize the approach, determine realistic goals for each patient, and should be considered during treatment discussions. Ongoing research into algorithm development will be helpful in this regard.
5. Conclusions
Overall, as a society we can no longer afford or tolerate the continued rising rates of diabetes. Despite many barriers within the healthcare system as a whole, providers are responsible on a daily basis for the lives of patients caught up in this unprecedented epidemic. The current standard of care may be suitable for some, but others would surely choose reversal if they understood there was a choice. The choice can only be offered if providers are not only aware that reversal is possible but have the education needed to review these options in a patient-centric discussion.
Acknowledgments
We thank James McCarter and Stephen Phinney for their edits, which greatly improved the manuscript.
Abbreviations:
| C | carbohydrate restriction |
| DSS-II | Diabetes Surgery Summit |
| RYGB | Roux-en-Y Gastric Bypass |
| SG | Sleeve Gastrectomy |
| CCK | cholecystokinin |
| PYY | peptide-tyrosine-tyrosine |
| GLP-1 | glucagon-like peptide 1 |
| LABS-2 | Longitudinal Assessment of Bariatric Surgery 2 |
| LCD | low-calorie diet |
| IMT | intensive medical intervention |
| DiRECT | Diabetes Remission Clinical Trial |
| ASD | European Association for the Study of Diabetes |
| VA/DOD | Veterans Affairs/Department of Defense |
Author Contributions
Conceptualization, S.J.H. and S.J.A. Investigation, S.J.H., V.M.G., T.L.H., S.J.A. Writing—original draft, S.J.H., V.M.G., S.J.A. Writing—review and editing, S.J.H., V.M.G., T.L.H., S.J.A. All authors approved of the final manuscript.
Conflicts of Interest
S.J.H. is an employee and shareholder of Virta Health, a for-profit company that provides remote diabetes care using a low-carbohydrate nutrition intervention, and serves as an advisor for Atkins Corp. V.M.G. has no conflicts of interest to declare. T.L.H. is an employee of Virta Health. S.J.A. is an employee and shareholder of Virta Health.

COMMENTS
Diabetes remains a substantial public health issue. Type 2 diabetes, which makes up the bulk of diabetes cases, is largely preventable and, in some cases, potentially reversible if identified and managed early in the disease course. However, all evidence indicates that diabetes prevalence is increasing worldwide, primarily due to a rise in obesity caused by multiple factors. Preventing and ...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Type 2 diabetes affects more than 34 million U.S. adults and significantly increases the risk of cardiovascular events, microvascular disease, and premature death. 1 Tight glycemic, blood-pressure ...
Diabetes publishes original research about the physiology and pathophysiology of diabetes mellitus. Submitted manuscripts can report any aspect of laboratory, animal, or human research. ... Each month, the Editors of Diabetes select an original research article to designate Paper of the Month. View these exclusive articles in a freely ...
Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes). ... Research Highlights 20 May 2024 ...
New estimates published this week in The Lancet indicate that more than 1·31 billion people could be living with diabetes by 2050 worldwide. That's 1·31 billion people living with a disease that causes life-altering morbidity, high rates of mortality, and interacts with and exacerbates many other diseases. The increase in prevalence (up from 529 million in 2021) is expected to be driven by ...
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR ...
A paper published in 2014 provided the first demonstration of the use of a bihormonal closed-loop system under free-living conditions in adults and adolescents with type 1 diabetes. Read more.
Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number affected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). Early detection and proactive management are crucial for prevention and mitigation of microvascular and macrovascular complications and mortality burden. Access to novel therapies ...
Introduction. Insulin resistance and β-cell dysfunction are the 2 major hallmarks of type 2 diabetes mellitus (T2DM) that appear as the result of disturbed homeostasis [].Failure of β-cells (∼80% of their β-cell function) and insulin resistance in muscles and the liver is a vicious triumvirate responsible for the core physiological defects.
1. Introduction. Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic disorders worldwide and its development is primarily caused by a combination of two main factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin [].Insulin release and action have to precisely meet the metabolic demand; hence, the ...
16 May 2024. 14 May 2024. 11 May 2024. 10 May 2024. Journal of Diabetes Research publishes articles related to type 1 and type 2 diabetes. Topics include etiology, pathogenesis, management, and prevention of diabetes, as well as associated complications such as nephropathy.
We randomly assigned patients with type 2 diabetes and chronic kidney disease (defined by an estimated glomerular filtration rate [eGFR] of 50 to 75 ml per minute per 1.73 m 2 of body-surface area ...
Journal of Diabetes is an international, imminently open access platform for diabetes research, therapeutics, and education. Uniting East and West, researchers and practitioners are invited to submit papers spanning all aspects of epidemiology, etiology, pathogenesis, management, complications, and prevention of diabetes including the molecular ...
Diabetes in America, 3rd Edition, is a compilation and assessment of epidemiologic, public health, clinical, and clinical trial data on diabetes and its complications in the United States. ... 14 Director, Emory Global Diabetes Research Center, Ruth and O.C. Hubert Professor of Global Health and Epidemiology, Rollins School of Public Health, ...
Prevention of Cardiovascular Disease in Type 1 Diabetes. C. Manrique-Acevedo, I.B. Hirsch, and R.H. EckelN Engl J Med 2024;390:1207-1217. More than half of newly diagnosed cases of type 1 diabetes ...
Pre-diabetes. Current guidelines for the delay and prevention of type 2 diabetes mellitus recommend for people with prediabetes to lose at least 7% of their body weight. Here, we advocate to use ...
The bibliometric analysis of the research published on metabolic memory in the decade following its formal designation in 2004. Based on the information provided by the Web of Science (webofscience.com), we analyzed the scientific output related to metabolic memory and diabetes from 2000 to 2022.
The molecular genetics of diabetes received extensive attention in recent years by many prominent investigators and research groups in the biomedical field. A large array of mutations and single nucleotide polymorphisms in genes that play a role in the various steps and pathways involved in glucose metabolism and the development, control and ...
PDF | The systematic review widely focused on diabetes and the developments in nutrition and diets required to prevent or control all types of diabetes.... | Find, read and cite all the research ...
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5-10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years ...
MLA. APA. Chicago. University of Barcelona. "New therapeutic targets to fight type 2 diabetes." ScienceDaily. ScienceDaily, 7 June 2024. <www.sciencedaily.com / releases / 2024 / 06 / 240607121434 ...
The research could lead to a better availability of beta cells for future research purposes. Type 1 and type 2 diabetes results when beta cells in the pancreas fail to produce enough insulin, the ...
Diabetes mellitus (DM), o r simply diabetes, is a group of metabolic diseases in which a person has. high blood sugar, either because the body does not produce enough insulin, or because cells do ...
In a year marked by the centenary of the discovery of insulin 1 and outstanding scientific progress in the therapeutic management of diabetes mellitus 2,3, these advances have been contrasted by ...
Background. A − β + ketosis-prone diabetes (KPD) in adults is characterized by presentation with diabetic ketoacidosis (DKA), negative islet autoantibodies, and preserved β-cell function in persons with a phenotype of obesity-associated type 2 diabetes (T2D).The prevalence of KPD has not been evaluated in children. We investigated children with DKA at "T2D" onset and determined the ...
Online Ahead of Print. Erratum: 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals. Clin Diabetes 2024;42:222 (doi: 10.2337/cd24-a016)
Two review papers address the complications that are non-traditionally linked to type 2 diabetes, although currently under exhaustive research: bone health and non-alcoholic fatty liver disease [9,10]. The multifaceted nature of type 2 diabetes is clearly visualized owing to the holistic angle used by these approaches.
Clinical trials offer hope for many people and an opportunity to help. researchers find better treatments for others in the future. Learn more. Discover NIDDK-supported information for improving public health, including topics about diabetes, kidney disease, digestive diseases, and more.
In this paper, we provide a review of three methods that have been shown to successfully reverse type 2 diabetes. ... Diabetes Prevention Program Research Group Long-term effects of metformin on diabetes prevention: Identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention outcomes study ...