Last updated 20/06/24: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .

Login Alert

- > Journals
- > Journal of Relationships Research
- > Volume 9
- > Intimacy Through Casual Sex: Relational Context of...

Article contents
Intimacy through casual sex: relational context of sexual activity and affectionate behaviours.
Published online by Cambridge University Press: 17 September 2018
Little is known about the role of affectionate behaviours — factors traditionally understood within the context of romantic relationships — in uncommitted ‘casual sex’ encounters. In a sample of U.S. undergraduate emerging adults aged 18–25 years ( N = 639) we conducted a preliminary internet-based questionnaire investigation into the role of affectionate behaviours — operationalised here as cuddling, spending the night and cuddling, foreplay, and eye gazing — across two sexual relationship contexts: (committed) traditional romantic relationships and (uncommitted) casual sex encounters. While affectionate behaviours were desired more often in romantic relationships than in casual sexual encounters, many respondents (both men and women) engaged in these affectionate behaviours during casual sexual encounters as well. This was especially pronounced in those who expressed a preference for casual sex encounters over romantic relationships: in a casual sex context these participants were about 1.5 times as likely to cuddle, 1.5 times as likely to spend the night and cuddle, and nearly 5 times as likely to engage in foreplay with a partner. The current study emphasises the importance of considering relationship context in sexuality and relationship research, and the need for further theoretical and empirical research on dimensions of intimacy, including affection, in people's diverse romantic and sexual lives.
Access options

This article has been cited by the following publications. This list is generated based on data provided by Crossref .
- Google Scholar
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Justin R. Garcia (a1) (a2) , Amanda N. Gesselman (a1) , Sean G. Massey (a3) , Susan M. Seibold-Simpson (a4) and Ann M. Merriwether (a5) (a6)
- DOI: https://doi.org/10.1017/jrr.2018.10
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Emotional Outcomes of Casual Sexual Relationships and Experiences: A Systematic Review
Affiliations.
- 1 Department of Human Development and Family Science, Virginia Polytechnic Institute and State University.
- 2 Department of Psychology, Morningside College.
- 3 Nebraska Center for Research on Children, Youth, Families and Schools, University of Nebraska- Lincoln.
- PMID: 32991206
- PMCID: PMC8579856
- DOI: 10.1080/00224499.2020.1821163
Casual sexual relationships and experiences (CSREs) are common and emotionally significant occurrences. Given the uncommitted, often emotionally complicated nature of CSREs, researchers have asked whether these experiences may have positive and/or negative emotional consequences. We reviewed 71 quantitative articles examining emotional outcomes of CSREs, including subjective emotional reactions (e.g., excitement, regret) and emotional health (e.g., depression, self-esteem). Overall, people evaluated their CSREs more positively than negatively. In contrast, CSREs were associated with short-term declines in emotional health in most studies examining changes in emotional health within a year of CSRE involvement. Emotional outcomes of CSREs differed across people and situations. Women and individuals with less permissive attitudes toward CSREs experienced worse emotional outcomes of CSREs. Alcohol use prior to CSREs, not being sexually satisfied, and not knowing a partner well were also associated with worse emotional outcomes. These findings suggest directions for prevention/intervention related to CSREs. For example, skill-building related to sexual decision-making may help individuals decide whether, and under what circumstances, CSREs are likely to result in positive or negative emotional outcomes. In addition, the limitations of extant research suggest directions for future inquiry (e.g., examining whether verbal and nonverbal consent practices predict emotional outcomes of CSREs).
PubMed Disclaimer
Conflict of interest statement
Conflict of Interest: The authors declare that they have no conflict of interest.
PRISMA Flow Diagram
Similar articles
- Sexual Behaviors, Satisfaction, and Intentions to Engage in Casual Sexual Relationships and Experiences in Emerging Adulthood. Hawkins SE, DeLuca HK, Claxton SE, Baker EA. Hawkins SE, et al. Arch Sex Behav. 2023 May;52(4):1575-1591. doi: 10.1007/s10508-022-02508-z. Epub 2022 Dec 21. Arch Sex Behav. 2023. PMID: 36542273
- Evaluations and Future Plans After Casual Sexual Experiences: Differences Across Partner Type. Wesche R, Claxton SE, Lefkowitz ES, van Dulmen MHM. Wesche R, et al. J Sex Res. 2018 Nov-Dec;55(9):1180-1191. doi: 10.1080/00224499.2017.1298714. Epub 2017 Mar 24. J Sex Res. 2018. PMID: 28339298 Free PMC article.
- Psychological Well-Being as a Predictor of Casual Sex Relationships and Experiences among Adolescents: A Short-Term Prospective Study. Dubé S, Lavoie F, Blais M, Hébert M. Dubé S, et al. Arch Sex Behav. 2017 Aug;46(6):1807-1818. doi: 10.1007/s10508-016-0914-0. Epub 2017 Feb 22. Arch Sex Behav. 2017. PMID: 28229246 Free PMC article.
- Consequences of Casual Sex Relationships and Experiences on Adolescents' Psychological Well-Being: A Prospective Study. Dubé S, Lavoie F, Blais M, Hébert M. Dubé S, et al. J Sex Res. 2017 Oct;54(8):1006-1017. doi: 10.1080/00224499.2016.1255874. Epub 2016 Dec 23. J Sex Res. 2017. PMID: 28010123 Free PMC article.
- Sexuality (and Lack Thereof) in Adolescence and Early Adulthood: A Review of the Literature. Boislard MA, van de Bongardt D, Blais M. Boislard MA, et al. Behav Sci (Basel). 2016 Mar 17;6(1):8. doi: 10.3390/bs6010008. Behav Sci (Basel). 2016. PMID: 26999225 Free PMC article. Review.
- Psychological and Psychosexual Adjustment in University Students as a Function of Sexual Activity and Relationship Type. Castro Á, Correa AB. Castro Á, et al. Int J Sex Health. 2023 Nov 29;35(4):543-554. doi: 10.1080/19317611.2023.2264285. eCollection 2023. Int J Sex Health. 2023. PMID: 38601808
- Sex, Gender and Class: An Analysis of Chilean Young People's Intimate Life. Tello-Navarro F, Gómez-Urrutia V, Hidalgo-Ortiz JP. Tello-Navarro F, et al. Int J Sex Health. 2024 Jan 24;36(1):46-58. doi: 10.1080/19317611.2024.2303516. eCollection 2024. Int J Sex Health. 2024. PMID: 38600899
- Navigating Love in a Post-Pandemic World: Understanding Young Adults' Views on Short- and Long-Term Romantic Relationships. Mengzhen L, Lim DHJ, Berezina E, Benjamin J. Mengzhen L, et al. Arch Sex Behav. 2024 Feb;53(2):497-510. doi: 10.1007/s10508-023-02738-9. Epub 2023 Nov 20. Arch Sex Behav. 2024. PMID: 37985563
- Positive, Negative, or Mixed Feelings? A Person-Centered Approach to Consequences of First Penile-Vaginal Intercourse in College Students. Vasilenko SA, Walters TL, Clark AN, Lefkowitz ES. Vasilenko SA, et al. Arch Sex Behav. 2022 Nov;51(8):3993-4006. doi: 10.1007/s10508-022-02379-4. Epub 2022 Aug 16. Arch Sex Behav. 2022. PMID: 35974120 Free PMC article.
- Allison R, & Risman BJ (2013). A double standard for “hooking up”: How far have we come toward gender equality? Social Science Research, 42, 1191–1206. 10.1016/j.ssresearch.2013.04.006 - DOI - PubMed
- *Bachtel MK (2013). Do hookups hurt? Exploring college students’ experiences and perceptions. Journal of Midwifery & Women’s Health, 58, 41–48. 10.1111/j.1542-2011.2012.00266.x - DOI - PubMed
- Backstrom L, Armstrong EA, & Puentes J (2012). Women’s negotiation of cunnilingus in college hookups and relationships. Journal of Sex Research, 49, 1–12. 10.1080/00224499.2011.585523 - DOI - PubMed
- *Bancroft J, Janssen E, Strong D, Carnes L, Vukadinovic Z, & Long JS (2003). Sexual risk-taking in gay men: The relevance of sexual arousability, mood, and sensation seeking. Archives of Sexual Behavior, 32, 555–572. 10.1023/A:1026041628364 - DOI - PubMed
- Barrios RJ, & Lundquist JH (2012). Boys just want to have fun? Masculinity, sexual behaviors, and romantic intentions of gay and straight males in college. Journal of LGBT Youth, 9, 271–296. 10.1080/19361653.2012.716749 - DOI
Publication types
- Search in MeSH
Related information
Grants and funding.
- P30 MH052776/MH/NIMH NIH HHS/United States
- T32 MH019985/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- Taylor & Francis
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Does Casual Sex Harm College Students’ Well-Being? A Longitudinal Investigation of the Role of Motivation
- February 2014
- Archives of Sexual Behavior 44(4)

- New York University
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Am J Sex Educ
- Michelle Burbage

- J AM COLL HEALTH
- Yushan Zhao
- Jessica M. Dennis

- J BUS ETHICS

- Maša Černilec
- PERS INDIV DIFFER
- Susan Sprecher

- Panyan Shen

- INT J CLIN HLTH PSYC

- Kathleen A. Bogle
- M. Rosenberg

- J.G. La Guardia
- ARCH SEX BEHAV

- Miranda Gaub

- L.R. Derogatis

- Melinda S. Harper
- J.W. Dickson
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

Casual Sex: The Hidden Truth
Those who engage in it fit no set psychological profile..
Posted May 2, 2022 | Reviewed by Devon Frye
- The Fundamentals of Sex
- Find a sex counsellor near me
- Historically frowned upon, casual sex has become integrated into the culture’s lore and sexual repertoire as an acceptable sexual choice.
- Those who partake in casual sex do not share the same psychological profile, defy crude stereotypes, and are not fated toward similar futures.
- Women express less interest in casual sex than men in part because the casual sex they get is often bad.
- In general, casual sex is not a strong predictor of life outcome.
American culture has a complex relationship with casual sex. Historically frowned upon, particularly for women, it has become increasingly integrated into the culture’s lore and sexual repertoire as an acceptable, widely practiced choice.
Predictably, the subject has been attracting increasing scientific attention . One rather consistent finding in the casual sex literature concerns an apparent gender gap: Women tend to report lower motivation for—and lower satisfaction with—casual sex encounters.
Evolutionary psychologists have argued that this gap is innate—the product of women’s evolved tendency toward sexual selectivity. In our evolutionary past, having sex meant having babies, and having a baby entails heavy investment and higher risk for women. Thus for them, sex is best considered seriously, not casually.
Yet evolution tends to select for strategic diversity, rather than uniformity, the better to assure a species' survival over changing conditions. A casual sex preference may therefore prove adaptive for some women in certain contexts, such as when looking to acquire needed resources or switch to a better mate.
In addition, we have also evolved to construct complex cultures, the dictates of which may override those of our biological program. To wit: Sex has evolved as a reproductive strategy, yet most of the sex happening right now around the world is not for the purpose of reproduction. In fact, most people having sex right now have taken intentional steps—through the use of cultural technology—to avoid reproduction. Thus, while biology may have a say, people’s attitudes about casual sex are bound to also be influenced by the social context in which they reside.
For example, most people find that pleasurable sex is preferable to non-pleasurable sex. People who find casual sex unpleasant are less likely to want or benefit from it. The prevalent cultural script for casual sex involves behaviors likely to produce orgasm in men (e.g., fellatio) but not those more likely to produce orgasm in women (e.g., cunnilingus).
Indeed, research suggests that this orgasm gap may account for the gender difference in people's attitudes about casual sex. Psychologist Jennifer Piemonte of the University of Michigan and colleagues, in a set of three studies involving more than 1,500 participants, found that "men are more likely to orgasm during casual sex, and people who orgasm during casual sex are more likely to experience positive reactions afterward. Therefore, while gender may be one way to describe the discrepancy in how positive people feel following casual sex, orgasm explains it.”
In a recent (2022) paper , the researchers Terri Conley and Verena Klein of the University of Michigan argue that a central reason for gender differences in this area is that "women and men are treated to different experiences of what is called ' sexuality ' and 'having sex.'” Women, more specifically, "experience substantially worse sex than men do."
The authors note that women face several unique barriers to sexual enjoyment. First, the structure of the clitoris (mostly hidden) and vagina (an internal organ) along with traditional social mores (“good girls don’t”) make girls less likely to become familiar and comfortable with their genitalia and its pleasure functions. Women are more likely to be judged negatively for their sexual appetites and face a higher threat of both social stigma and sexual violence . These myriad structural and social obstacles may increase women’s sexual apprehension and serve to reduce sexual trust and desire.
By way of analogy, the construct of "sex" may resonate differently for women and men in the same way that the construct of "police" resonates differently in black vs. white communities—a consequence of systematically divergent social experiences.
Another line of inquiry has sought to discover the consequences and implications of casual sex in the lives of participants. Contrary to early fears, research suggests that current engagement in casual sex is not associated with people’s expectations for involvement in future committed relationships and marriage . Casual sex activity does not patently denote a rejection of committed sex.

Research on the costs and benefits of casual sex has yielded mixed results. For example, Rose Wesche of Virginia Polytechnic Institute and State University and colleagues recently (2021) reviewed 71 studies examining emotional outcomes of casual sex relationships (CSREs), including emotional reactions (excitement, regret) and emotional health ( depression , self-esteem ). “Overall, people evaluated their CSREs more positively than negatively. In contrast, CSREs were associated with short-term declines in emotional health in most studies examining changes in emotional health within a year of CSRE involvement.”
Overall, research suggests that regardless of gender, casual sex does not reliably predict life outcomes. For example, a 2009 longitudinal study by Marla Eisenberg of the University of Minnesota and colleagues analyzed a diverse sample of 1,311 sexually active young adults, finding that “scores of psychological well-being were generally consistent across sex partner categories, and no significant associations between partner type and well-being were found in adjusted analyses.” The authors concluded: “Young adults who engage in casual sexual encounters do not appear to be at greater risk for harmful psychological outcomes than sexually active young adults in more committed relationships.”
Summarizing the research on the issue, Zhana Vrangalova, a casual sex researcher at New York University, wrote : “The most frequent finding for both sexes is one of no significant relationship” between casual sex and wellbeing.
This is not entirely surprising. Individual wellbeing is multiply determined, and the effects of any one factor may easily be drowned out over time by many others. Life is also contextual; the meaning and effects of any event depend heavily on the individual characteristics of the players and the environmental conditions in which they operate.
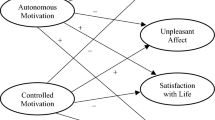
This general truth appears to hold here. When casual sex is found to predict future outcomes, it predicts different consequences for different people depending on several variables. One potent variable in this equation is a person’s motivation. For example, research suggests that having casual sex for “non-autonomous” reasons (i.e., due to self-imposed pressures, external contingencies and controls, or complete lack of intentionality) predicts lower self-esteem, higher depression and anxiety , and more physical symptoms. Those who report “autonomous motivation,” on the other hand (i.e., emanating from one’s self) do not tend to suffer adverse consequences.
Another factor at play is sociosexuality, a dimension of personality that describes people’s comfort with and preference for sexual activity in the absence of love or commitment. Research has shown that those with "unrestricted" orientation, regardless of gender, typically report higher well being after having casual sex while restricted individuals show no such differences
Despite its growing prevalence and acceptance, casual sex is still associated with certain negative cultural myths. One of these regards the notion that women pursuing casual sex have low self-esteem.
Recently (2020), the researcher Jaimie Krems of Oklahoma State University and colleagues set out to test the truth-value of this notion. Across six experiments with U.S. participants (N = 1,469), they found that “both men and women stereotype women (but not men) who have casual sex as having low self-esteem.” Alas, across experiments, the participants’ own sexual behavior was uncorrelated with their self-esteem. In other words, the participants' self-reports in effect refuted the validity of their own stereotyped beliefs.
In sum, it appears that casual sex is not here to replace emotionally committed sex in the lives of most people. Those who partake in casual sex do not share the same psychological profile, can not be fitted usefully into crude stereotypes, and are not fated toward similar futures. Casual sex is good when it’s good casual sex: freely chosen, in alignment with one’s authentic values, and involving a competent partner.
Facebook image: Sergio Sico/Shutterstock

Noam Shpancer, Ph.D., is a professor of psychology at Otterbein University and a practicing clinical psychologist in Columbus, Ohio.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Does Casual Sex Harm College Students’ Well-Being? A Longitudinal Investigation of the Role of Motivation
- Original Paper
- Published: 05 February 2014
- Volume 44 , pages 945–959, ( 2015 )
Cite this article

- Zhana Vrangalova 1
8992 Accesses
67 Citations
242 Altmetric
29 Mentions
Explore all metrics
Engagement in casual sex (or hooking up) is generally feared to have negative well-being consequences; however, empirical evidence is inconclusive, pointing toward potential moderators. Using self-determination theory (SDT), we hypothesized that well-being following hookups would depend on the type and level of motivation for hooking up. A university-wide sample of 528 undergraduates completed online surveys at the beginning (T1) and end (T3) of one academic year. After controlling for demographics, personality traits (i.e., neuroticism and extraversion), prior casual and romantic sex, and T1 well-being, having genital hookups between T1 and T3 for non-autonomous reasons (i.e., due to self-imposed pressures, external contingencies and controls, or complete lack of intentionality) was linked to lower self-esteem, higher depression and anxiety, and more physical symptoms. Autonomous hookup motivation (i.e., emanating from one’s self) was not linked to any outcomes. Compared to peers without hookups, those with high non-autonomy in their hookups typically had inferior well-being; this was not true of those with low non-autonomy hookups. Gender differences, implications for SDT and casual sex research, and implications for educational programs and clinical work are discussed.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
The Impact of Autonomous and Controlled Sexting Motivations on Subjective Well-being and Relationship Quality

Was it Good for You? Gender Differences in Motives and Emotional Outcomes Following Casual Sex

Associations Between Motives for Casual Sex, Depression, Self-Esteem, and Sexual Victimization
The results were virtually identical, albeit somewhat weaker, when the amotivation item was excluded from the non-autonomous motivation score or when controlled motivation and amotivation weree treated as separate variables (data available on request).
Initial analyses also controlled for sexual orientation (heterosexual vs. nonheterosexual) and race (White vs. Nonwhite). Neither was significant and both were excluded from final models.
Initial analyses also controlled for interactions between T1–T3 hookups and all control variables (as recommended by Yzerbyt, Muller, & Judd, 2004 ); most of these interactions were non-significant and, in all cases, had no impact on the main results, so we excluded them from the final analyses.
Allison, R., & Risman, B. J. (2013). A double standard for “hooking up”: How far have we come toward gender equality? Social Science Research, 42 , 1191–1206. doi: 10.1016/j.ssresearch.2013.04.006 .
Article PubMed Google Scholar
Apostolopoulos, Y., Sönmez, S., & Yu, C. H. (2002). HIV-risk behaviours of American spring break vacationers: A case of situational disinhibition? International Journal of STD and AIDS, 13 , 733–743. doi: 10.1258/095646202320753673 .
Armstrong, E. A., England, P., & Fogarty, A. C. K. (2012). Accounting for women’s orgasm and sexual enjoyment in college hookups and relationships. American Sociological Review, 77 , 435–462. doi: 10.1177/0003122412445802 .
Article Google Scholar
Bailey, J. M., Kirk, K. M., Zhu, G., Dunne, M. P., & Martin, N. G. (2000). Do individual differences in sociosexuality represent genetic or environmentally contingent strategies? Evidence from the Australian twin registry. Journal of Personality and Social Psychology, 78 , 537–545. doi: 10.1037//0022-3514.78.3.537 .
Bancroft, J., Janssen, E., Carnes, L., Goodrich, D., & Strong, D. (2004). Sexual activity and risk taking in young heterosexual men: The relevance of sexual arousability, mood and sensation seeking. Journal of Sex Research, 41 , 181–192. doi: 10.1080/00224490409552226 .
Baron, R. M., & Kenny, D. A. (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology, 51 , 1173–1182. doi: 10.1037/0022-3514.51.6.1173 .
Baumeister, R. F., & Leary, M. R. (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117 , 497–529. doi: 10.1037/0033-2909.117.3.497 .
Baumeister, R. F., & Twenge, J. M. (2002). Cultural suppression of female sexuality. Review of General Psychology, 6 , 166–203. doi: 10.1037//1089-2680.6.2.166 .
Bersamin, M. M., Zamboanga, B. L., Schwartz, S. J., Donnellan, M. B., Hudson, M., Weisskirch, R. S., … Caraway, S. J. (2013). Risky business: Is there an association between casual sex and mental health among emerging adults? Journal of Sex Research . doi: 10.1080/00224499.2013.772088 .
Bogle, K. A. (2008). Hooking up: Sex, dating, and relationships on campus . New York: New York University Press.
Google Scholar
Brunell, A. B., & Webster, G. D. (2013). Self-determination and sexual experience in dating relationships. Personality and Social Psychology Bulletin . doi: 10.1177/0146167213485442 .
Buss, D. M., & Schmitt, D. P. (1993). Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review, 100 , 204–232. doi: 10.1037//0033-295X.100.2.204 .
Campbell, A. (2008). The morning after and the night before. Human Nature, 19 , 157–173. doi: 10.1007/s12110-008-9036-2 .
Coleman, P. K., Rue, V. M., Spence, M., & Coyle, C. T. (2008). Abortion and the sexual lives of men and women: Is casual sexual behavior more appealing and more common after abortion? International Journal of Clinical and Health Psychology, 8 , 77–91.
Cook, C., Heath, F., & Thompson, R. L. (2002). A meta-analysis of response rates in Web- or Internet-based surveys. Educational and Psychological Measurement, 60 , 821–836. doi: 10.1177/00131640021970934 .
Cooper, M. L. (2002). Alcohol use and risky sexual behavior among college students and youth: Evaluating the evidence. Journal of Studies on Alcohol (Suppl. 14), 101–117.
Cooper, M. L., Shapiro, C. M., & Powers, A. M. (1998). Motivations for sex and risky sexual behavior among adolescents and young adults: A functional perspective. Journal of Personality and Social Psychology, 75 , 1528–1558. doi: 10.1037/0022-3514.75.6.1528 .
Costa, P. T., & McCrae, R. R. (1980). Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. Journal of Personality and Social Psychology, 38 , 668–678. doi: 10.1037/0022-3514.38.4.668 .
Crawford, M., & Popp, D. (2003). Sexual double standards: A review and methodological critique of two decades of research. Journal of Sex Research, 40 , 13–26. doi: 10.1080/00224490309552163 .
de Graaf, H., & Sandfort, T. G. M. (2004). Gender differences in affective responses to sexual rejection. Archives of Sexual Behavior, 33 , 395–403. doi: 10.1023/B:ASEB.0000028892.63150.be .
Deci, E. L., & Ryan, R. M. (1985). The General Causality Orientations Scale: Self-determination in personality. Journal of Research in Personality, 19 , 109–134. doi: 10.1016/0092-6566(85)90023-6 .
Deci, E. L., & Ryan, R. M. (2000). The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry, 11 , 227–268. doi: 10.1207/S15327965PLI1104_01 .
Derogatis, L. R. (1993). BSI: Administration, scoring and procedures manual (3rd ed.). Minneapolis, MN: National Computer Systems.
Donelan, M. B., Oswald, F. L., Baird, B. M., & Lucas, R. E. (2006). The Mini-IPIP Scales: Tiny-yet-effective measures of the Big Five factors of personality. Psychological Assessment, 18 , 192–203. doi: 10.1037/1040-3590.18.2.192 .
Eisenberg, M. E., Ackard, D. M., Resnick, M. D., & Neumark-Sztainer, D. (2009). Casual sex and psychological health among young adults: Is having ‘friends with benefits’ emotionally damaging? Perspectives on Sexual and Reproductive Health, 41 , 231–237. doi: 10.1363/4123109 .
Emmons, R. A. (1991). Personal strivings, daily life events, and psychological and physical well-being. Journal of Personality, 59 , 453–472. doi: 10.1111/j.1467-6494.1991.tb00256.x .
Eshbaugh, E. M., & Gute, G. (2008). Hookups and sexual regret among college women. Journal of Social Psychology, 148 , 77–89. doi: 10.3200/SOCP.148.1.77-90 .
Fielder, R. L., & Carey, M. P. (2010a). Predictors and consequences of sexual “hookups” among college students: A short-term prospective study. Archives of Sexual Behavior, 39 , 1105–1119. doi: 10.1007/s10508-008-9448-4 .
Article PubMed Central PubMed Google Scholar
Fielder, R. L., & Carey, M. P. (2010b). Prevalence and characteristics of sexual hookups among first-semester female college students. Journal of Sex and Marital Therapy, 36 , 346–359. doi: 10.1080/0092623X.2010.488118 .
Fielder, R. L., Carey, K. B., & Carey, M. P. (2013). Are hookups replacing romantic relationships? A longitudinal study of first-year female college students. Journal of Adolescent Health . doi: 10.1016/j.jadohealth.2012.09.001 .
Fiesta Frog. (2013). Top party schools and universities 2012 – 2013 school year . Retrieved from http://www.fiestafrog.com/blog/top-party-schools-universities-2012-party-schools-nightlife/ .
Gagné, M., & Deci, E. L. (2005). Self-determination theory and work motivation. Journal of Organizational Behavior, 26 , 331–362. doi: 10.1002/job.322 .
Gangestad, S. W., & Simpson, J. A. (2000). The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences, 23 , 573–644. doi: 10.1017/S0140525X0000337X .
Garcia, J. R., & Reiber, C. (2008). Hook-up behavior: A biopsychosocial perspective. Journal of Social, Evolutionary, and Cultural Psychology, 2 , 192–208.
Garcia, J. R., Reiber, C., Massey, S. G., & Merriwether, A. M. (2012). Sexual hookup culture: A review. Review of General Psychology, 16 , 161–176. doi: 10.1037/a0027911 .
Gentzler, A. L., & Kerns, K. A. (2004). Associations between insecure attachment and sexual experiences. Personal Relationships, 11 , 249–265. doi: 10.1111/j.1475-6811.2004.00081.x .
Gilmartin, S. K. (2006). Changes in college women’s attitudes toward sexual intimacy. Journal of Research on Adolescence, 16 , 429–454. doi: 10.1111/j.1532-7795.2006.00501.x .
Glenn, N., & Marquardt, E. (2001). Hooking up, hanging out, and hoping for Mr. Right: College women on dating and mating today . Research report of the Institute for American Values. Retrieved April 15, 2007 from http://www.americanvalues.org/Hooking_Up.pdf .
Greiling, H., & Buss, D. (2000). Women’s sexual strategies: The hidden dimension of extra-pair mating. Personality and Individual Differences, 28 , 929–963. doi: 10.1016/S0191-8869(99)00151-8 .
Grello, C. M., Welsh, D. P., & Harper, M. S. (2006). No strings attached: The nature of casual sex in college students. Journal of Sex Research, 43 , 255–267. doi: 10.1080/00224490609552324 .
Grello, C. M., Welsh, D. P., Harper, M. S., & Dickson, J. W. (2003). Dating and sexual relationship trajectories and adolescent functioning. Adolescent and Family Health, 3 , 103–111.
Guay, F., Ratelle, C. F., & Chanal, J. (2008). Optimal learning in optimal contexts: The role of self-determination in education. Canadian Psychology, 49 , 233–240. doi: 10.1037/a0012758 .
Gute, G., & Eshbaugh, E. M. (2008). Personality as a predictor of hooking up among college students. Journal of Community Health Nursing, 25 , 26–43. doi: 10.1080/07370010701836385 .
Haselton, M. G., & Buss, D. M. (2001). The affective shift hypothesis: The functions of emotional changes following sexual intercourse. Personal Relationships, 8 , 357–369. doi: 10.1111/j.1475-6811.2001.tb00045.x .
Hill, C. A., & Preston, L. K. (1996). Individual differences in the experience of sexual motivation: Theory and measurement of dispositional sexual motives. Journal of Sex Research, 33 , 27–45. doi: 10.1080/00224499609551812 .
Holman, A., & Sillars, A. (2012). Talk about “hooking up”: The influence of college student social networks on non-relationship sex. Health Communication, 27 , 205–216. doi: 10.1080/10410236.2011.575540 .
Jenkins, S. S. (2004). Gender and self-determination in sexual motivation. Dissertation Abstracts International: Section B: The Sciences and Engineering, 64 (12-B), 6330.
Jonason, P. K., Li, N. P., & Richardson, J. (2011). Positioning the booty-call relationship on the spectrum of relationships: Sexual but more emotional than one-night stands. Journal of Sex Research, 46 , 460–470. doi: 10.1080/00224499.2010.497984 .
Kenney, S. R., Thadani, V., Ghaidarov, T., & LaBrie, J. W. (2013). First-year college women’s motivations for hooking up: A mixed methods examination of normative peer perceptions and personal hookup participation. International Journal of Sexual Health . doi: 10.1080/19317611.2013.786010 .
Kraaykamp, G. (2002). Trends and countertrends in sexual permissiveness: Three decades of attitude change in the Netherlands 1965–1995. Journal of Marriage and the Family, 64 , 225–239. doi: 10.1111/j.1741-3737.2002.00225.x .
Kreager, D. A., & Staff, J. (2009). The sexual double standard and adolescent peer acceptance. Social Psychology Quarterly, 72 , 143–164. doi: 10.1177/019027250907200205 .
La Guardia, J. G., & Patrick, H. (2008). Self-determination theory as a fundamental theory of close relationships. Canadian Psychology, 49 , 201–209. doi: 10.1037/a0012760 .
Lambert, T. A., Kahn, A. S., & Apple, K. J. (2003). Pluralistic ignorance and hooking up. Journal of Sex Research, 40 , 129–133. doi: 10.1080/00224490309552174 .
Levin, R. J. (2007). Sexual activity, health and well-being: The beneficial roles of coitus and masturbation. Sexual and Relationship Therapy, 22 , 135–148. doi: 10.1080/14681990601149197 .
Lewis, M. A., Granato, H., Blayney, J. A., Lostutter, T. W., & Kilmer, J. R. (2012). Predictors of hooking up sexual behavior and emotional reactions among U.S. college students. Archives of Sexual Behavior, 41 , 1219–1229. doi: 10.1007/s10508-011-9817-2 .
Manning, W. D., Giordano, P. C., & Longmore, M. A. (2006). Hooking up: The relationship contexts of ‘non-relationship’ sex. Journal of Adolescent Research, 21 , 459–483. doi: 10.1177/0743558406291692 .
Manning, W. D., Longmore, M. A., & Giordano, P. C. (2005). Adolescents’ involvement in non-romantic sexual activity. Social Science Research, 34 , 384–407. doi: 10.1016/j.ssresearch.2004.03.001 .
Marks, M. J. (2008). Evaluations of sexually active men and women under divided attention: A social cognitive approach to the sexual double standard. Basic and Applied Social Psychology, 30 , 84–91. doi: 10.1080/01973530701866664 .
Marks, M. J., & Fraley, R. C. (2005). The sexual double standard: Fact or fiction? Sex Roles, 52 , 175–186. doi: 10.1007/s11199-005-1293-5 .
Marks, M. J., & Fraley, R. C. (2006). Confirmation bias and the sexual double standard. Sex Roles, 54 , 19–26. doi: 10.1007/s11199-006-8866-9 .
McIlhaney, J. S., & Bush, F. M. (2008). Hooked: New science on how casual sex is affecting our children . Chicago, IL: Northfield Publishing.
Meier, A. M. (2007). Adolescent first sex and subsequent mental health. American Journal of Sociology, 112 , 1811–1847. doi: 10.1086/512708 .
Mendle, J., Ferrero, J., Moore, S. R., & Harden, K. P. (2013). Depression and adolescent sexual activity in romantic and nonromantic relational contexts: A genetically-informative sibling comparison. Journal of Abnormal Psychology, 122 , 51–63. doi: 10.1037/a0029816 .
Meston, C. M., & Buss, M. P. (2007). Why humans have sex? Archives of Sexual Behavior, 36 , 477–507. doi: 10.1007/s10508-007-9175-2 .
Monahan, K. C., & Lee, J. M. (2008). Adolescent sexual activity: Links between relational context and depressive symptoms. Journal of Youth and Adolescence, 37 , 917–927. doi: 10.1007/s10964-007-9256-5 .
Oliver, M. B., & Hyde, J. S. (1993). Gender differences in sexuality: A meta-analysis. Psychological Bulletin, 114 , 29–51. doi: 10.1037/0033-2909.114.1.29 .
Olmstead, S. B., Pasley, K., & Fincham, F. D. (2013). Hooking up and penetrative hookups: Correlates that differentiate college men. Archives of Sexual Behavior, 42 , 573–583. doi: 10.1007/s10508-012-9907-9 .
Owen, J., & Fincham, F. D. (2011). Young adults’ emotional reactions after hooking up encounters. Archives of Sexual Behavior, 40 , 321–330. doi: 10.1007/s10508-010-9652-x .
Owen, J., Fincham, F. D., & Moore, J. (2011). Short-term prospective study of hooking up among college students. Archives of Sexual Behavior, 40 , 331–341. doi: 10.1007/s10508-010-9697-x .
Owen, J., Quirk, K., & Fincham, F. D. (2013). Toward a more complete understanding of reactions to hooking up among college women. Journal of Sex & Marital Therapy. doi: 10.1080/0092623X.2012.751074 .
Owen, J. J., Rhoades, G. K., Stanley, S. M., & Fincham, F. D. (2010). “Hooking up” among college students: Demographic and psychosocial correlates. Archives of Sexual Behavior, 39 , 653–663. doi: 10.1007/s10508-008-9414-1 .
Paul, E. L. (2006). Beer goggles, catching feelings, and the walk of shame: The myths and realities of the hookup experience. In D. C. Kirkpatrick, S. Duck, & M. K. Foley (Eds.), Relating difficulty: The process of constructing and managing difficult relationships (pp. 141–160). Mahwah, NJ: Lawrence Erlbaum Associates.
Paul, E. L., & Hayes, K. A. (2002). The casualties of ‘casual’ sex: A qualitative exploration of the phenomenology of college students’ hookups. Journal of Social and Personal Relationships, 19 , 639–661. doi: 10.1177/0265407502195006 .
Paul, E. L., McManus, B., & Hayes, A. (2000). “Hookups”: Characteristics and correlates of college students’ spontaneous and anonymous sexual experience. Journal of Sex Research, 37 , 76–88. doi: 10.1080/00224490009552023 .
Paul, E. L., Wenzel, A., & Harvey, J. (2009). Hookups: A facilitator or a barrier to relationship initiation and intimacy development? In S. Sprecher, A. Wenzel, & J. Harvey (Eds.), Handbook of relationship initiation (pp. 375–390). New York: Psychology Press.
Petersen, J. L., & Hyde, J. S. (2010). A meta-analytic review of research on gender differences in sexuality, 1993–2007. Psychological Bulletin, 136 , 21–38. doi: 10.1037/a0017504 .
Peterson, Z. D., & Muehlenhard, C. L. (2007). What is sex and why does it matter? A motivational approach to exploring individuals; definitions of sex. Journal of Sex Research, 44 , 256–268. doi: 10.1080/00224490701443932 .
Randolph, K. K. (2013). The list is out: Top 10 party schools of 2013 . Retrieved from http://college.monster.com/news/articles/2174-the-list-is-out-top-10-party-schools-of-2013?page=10 .
Regan, P. C., & Dreyer, C. S. (1999). Lust? Love? Status? Young adults’ motives for engaging in casual sex. Journal of Psychology and Human Sexuality, 11 , 1–24. doi: 10.1300/J056v11n01_01 .
Reiber, C., & Garcia, J. R. (2010). Hooking up: Gender differences, evolution, and pluralistic ignorance. Evolutionary Psychology, 8 , 390–404.
Romero-Daza, N., & Freidus, A. (2008). Female tourists, casual sex, and HIV risk in Costa Rica. Qualitative Sociology, 31 , 169–187. doi: 10.1007/s11133-008-9096-y .
Rosenberg, M. (1965). Society and the adolescent self-image . Princeton, NJ: Princeton University Press.
Ryan, R. M., & Connell, J. P. (1989). Perceived locus of causality and internalization: Examining reasons for acting in two domains. Journal of Personality and Social Psychology, 57 , 749–761. doi: 10.1037/0022-3514.57.5.749 .
Ryan, R. M., & Deci, E. L. (2008). A self-determination theory approach to psychotherapy: The motivational basis for effective change. Canadian Psychology, 49 , 186–193. doi: 10.1037/a0012753 .
Ryan, R. M., Deci, E. L., Grolnick, W. S., & LaGuardia, J. G. (2006). The significance of autonomy and autonomy support in psychological development and psychopathology. In D. Cicchetti & D. Cohen (Eds.), Developmental psychopathology: Vol. 1: Theory and methods (2nd ed., pp. 795–849). New York: Wiley
Sakaguchi, K., Sakai, Y., Ueda, K., & Hasegawa, T. (2007). Robust association between sociosexuality and self-monitoring in heterosexual and non-heterosexual Japanese. Personality and Individual Differences, 43 , 815–825. doi: 10.1016/j.paid.2007.02.006 .
Schmitt, D. P. (2005). Is short-term mating the maladaptive result of insecure attachment? A test of competing evolutionary perspectives. Personality and Social Psychology Bulletin, 31 , 747–768. doi: 10.1177/0146167204271843 .
Schmitt, D. P., Shackelford, T. K., & Buss, D. M. (2001a). Are men really more ‘oriented’ toward short-term mating than women? Psychology, Evolution and Gender, 3 , 211–239. doi: 10.1080/14616660110119331 .
Schmitt, D. P., Shackelford, T. K., Duntley, J., Tooke, W., & Buss, D. M. (2001b). The desire for sexual variety as a key to understanding basic human mating strategies. Personal Relationships, 8 , 425–455. doi: 10.1111/j.1475-6811.2001.tb00049.x .
Shulman, S., Walsh, S. D., Weisman, O., & Schelyer, M. (2009). Romantic contexts, sexual behavior, and depressive symptoms among adolescent males and females. Sex Roles, 61 , 850–863. doi: 10.1007/s11199-009-9691-8 .
Stepp, L. S. (2007). Unhooked: How young women pursue sex, delay love, and lose at both . New York: Riverhead Books.
Teixeira, P. J., Carraҫa, E. V., Markland, D., Silva, M. N., & Ryan, R. M. (2012). Exercise, physical activity, and self-determination theory: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 9 , 78–108. doi: 10.1186/1479-5868-9-78 .
Thornton, A., & Young-DeMarco, L. (2001). Four decades of trends in attitudes toward family issues in the United States: The 1960s through the 1990s. Journal of Marriage and the Family, 63 , 1009–1037. doi: 10.1111/j.1741-3737.2001.01009.x .
Townsend, J. M. (1995). Sex without emotional involvement: An evolutionary interpretation of sex differences. Archives of Sexual Behavior, 24 , 173–206. doi: 10.1007/BF01541580 .
Townsend, J. M., & Wasserman, T. H. (2011). Sexual hookups among college students: Sex differences in emotional reactions. Archives of Sexual Behavior, 40 , 1173–1181. doi: 10.1007/s10508-011-9841-2 .
Vrangalova, Z., Bukberg, R., & Gerulf, R. (2013). Birds of a feather? Not when it comes to sexual permissiveness. Journal of Social and Personal Relationships . doi: 10.1177/0265407513487638 .
Weaver, S. J., & Herold, E. S. (2000). Casual sex and women: Measurement and motivational issues. Journal of Psychology and Human Sexuality, 12 , 23–41. doi: 10.1300/J056v12n03_02 .
Weinstein, N., & Ryan, R. M. (2010). When helping helps: Autonomous motivation for prosocial behavior and its influence on well-being for the helper and recipient. Journal of Social and Personality Psychology, 98 , 222–244. doi: 10.1037/a0016984 .
Wentland, J. J., & Reissing, E. D. (2011). Taking casual sex not too casually: Exploring definitions of casual sexual relationships. The Canadian Journal of Human Sexuality, 20 , 75–91.
Whipple, B., Knowles, J., & Davis, J. (2003). The health benefits of sexual expression [White Paper]. New York: Planned Parenthood Federation of America, Inc., and the Society for the Scientific Study of Sexuality. [Updated 2007 by Gianotten, W. L., & Golub, D.]
Young, L., & Wang, Z. (2004). The neurobiology of human mating. Nature Neuroscience, 7 , 1048–1054. doi: 10.1038/nn1327 .
Yzerbyt, V. Y., Muller, D., & Judd, C. M. (2004). Adjusting researchers’ approach to adjustment: On the use of covariates when testing interactions. Journal of Experimental Social Psychology, 40 , 424–431. doi: 10.1016/j.jesp.2003.10.001 .
Download references
Acknowledgments
This research was partially supported by a grant-in-aid from the Foundation for Scientific Study of Sexuality, a grant-in-aid from the Society for the Psychological Study of Social Issues, and a grant from the Human Ecology Alumni Association, Cornell University, all awarded to Zhana Vrangalova for conducting her doctoral dissertation research. I would like to thank Rachel Mack, Melany Bradshaw, and Vickie Liang for their help with data collection and preparation.
Author information
Authors and affiliations.
Department of Human Development, Cornell University, B40 Martha Van Rensselaer Hall, Ithaca, NY, 14850, USA
Zhana Vrangalova
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zhana Vrangalova .
Rights and permissions
Reprints and permissions
About this article
Vrangalova, Z. Does Casual Sex Harm College Students’ Well-Being? A Longitudinal Investigation of the Role of Motivation. Arch Sex Behav 44 , 945–959 (2015). https://doi.org/10.1007/s10508-013-0255-1
Download citation
Received : 19 April 2013
Revised : 19 July 2013
Accepted : 26 August 2013
Published : 05 February 2014
Issue Date : May 2015
DOI : https://doi.org/10.1007/s10508-013-0255-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Autonomous motivation
- Psychological well-being
- Self-determination theory
- Find a journal
- Publish with us
- Track your research

Cover Story
Sexual hook-up culture
With more emerging adults having casual sex, researchers are exploring psychological consequences of such encounters.
By Justin R. Garcia, The Kinsey Institute for Research in Sex, Gender, and Reproduction, Indiana University, Bloomington; and Chris Reiber, Sean G. Massey, and Ann M. Merriwether, Binghamton University, State University of New York
February 2013, Vol 44, No. 2
Print version: page 60
20 min read

Welcome to ‘CE Corner'
"CE Corner" is a quarterly continuing education article offered by the APA Office of CE in Psychology. This feature will provide you with updates on critical developments in psychology, drawn from peer-reviewed literature and written by leading psychology experts. "CE Corner" appears in the February 2012 , April , July/August and November issues of the Monitor.
Upon successful completion of the test (a score of 75 percent or higher), you can print your CE certificate immediately. APA will immediately send you a "Documentation of CE" certificate. The test fee is $25 for members; $35 for nonmembers. The APA Office of CE in Psychology retains responsibility for the program. For more information, call (800) 374-2721, ext. 5991.
CE credits: 1
Exam items: 10
Learning objectives:
- Describe the concept and context of contemporary sexual hook-up culture and behavior.
- Review the current research on psychological and health consequences of emerging adults' uncommitted sexual activity.
- Discuss the role of uncommitted sexual behavior, and larger social-sexual scripts, on the lives and experiences of emerging adult college students.
It is an unprecedented time in the history of human sexuality. In the United States, the age when people first marry and reproduce has been pushed back dramatically, while at the same time the age of puberty has dropped, resulting in an era in which young adults are physiologically able to reproduce but not psychologically or socially ready to "settle down" and begin a family (Bogle, 2007; Garcia & Reiber, 2008).
These developmental shifts, research suggests, are some of the factors driving the increase in sexual "hookups," or uncommitted sexual encounters, part of a popular cultural change that has infiltrated the lives of emerging adults throughout the Western world.
Hookups are becoming more engrained in popular culture, reflecting both evolved sexual predilections and changing social and sexual scripts. Hook-up activities may include a wide range of sexual behaviors, such as kissing, oral sex and penetrative intercourse. However, these encounters often transpire without any promise of — or desire for — a more traditional romantic relationship.
In this article, we review the literature on sexual hookups and consider the research on the psychological consequences of casual sex. This is a transdisciplinary literature review that draws on the evidence and theoretical tensions between evolutionary theoretical models and sociocultural theory. It suggests that these encounters are becoming increasingly normative among adolescents and young adults in North America and can best be understood from a biopsychosocial perspective.
Today's hook-up culture represents a marked shift in openness and acceptance of uncommitted sex.
A cultural revolution
Hookups — defined in this article as brief uncommitted sexual encounters between individuals who are not romantic partners or dating each other — have emerged from more general social shifts taking place during the last century. Hookups began to become more frequent in the 1920s, with the upsurge of automobiles and novel entertainment, such as movie theaters. Instead of courting at home under a parent's watchful eye, young adults left the home and were able to explore their sexuality more freely.
By the 1960s, young adults became even more sexually liberated, with the rise of feminism, widespread availability of birth control and growth of sex-integrated college party events. Today, sexual behavior outside of traditional committed romantic pair-bonds has become increasingly typical and socially acceptable (Bogle, 2007, 2008).
Influencing this shift in sexuality is popular culture. The media have become a source of sex education, filled with often inaccurate portrayals of sexuality (Kunkel et al., 2005). The themes of books, plots of movies and television shows, and lyrics of numerous songs all demonstrate a permissive sexuality among consumers. The media suggest that uncommitted sex, or hookups, can be both physically and emotionally enjoyable and occur without "strings." The 2009 film "Hooking Up," for example, details the chaotic romantic and sexual lives of adolescent characters. Another film, "No Strings Attached," released in 2011, features two friends negotiating a sexual, yet nonromantic, component of their relationship. Popular pro-hookup same-sex representations have also emerged in television series like "Queer as Folk" and "The L-Word."
When it comes to real life, most of today's young adults report some casual sexual experience. The most recent data suggest that between 60 percent and 80 percent of North American college students have had some sort of hook-up experience. This is consistent with the view of emerging adulthood (typical college age) as a period of developmental transition (Arnett, 2000), exploring and internalizing sexuality and romantic intimacy, now including hookups (Stinson, 2010).
Although much of the current research has been done on college campuses, among younger adolescents, 70 percent of sexually active 12- to 21-year-olds reported having had uncommitted sex within the last year (Grello et al., 2003). Similarly, in a sample of seventh, ninth and 11th graders, 32 percent of participants had experienced sexual intercourse and 61 percent of sexually experienced teenagers reported a sexual encounter outside a dating relationship; this represents approximately one-fifth of the entire sample (Manning et al., 2006).
Affective responses to hooking up
On average, both men and women appear to have higher positive affect than negative affect after a hookup. In one study, among participants who were asked to characterize the morning after a hookup, 82 percent of men and 57 percent of women were generally glad they had done it (Garcia & Reiber, 2008). The gap between men and women is notable and demonstrates an average sex difference in affective reactions.
Similarly, in a study of 832 college students, 26 percent of women and 50 percent of men reported feeling positive after a hookup, and 49 percent of women and 26 percent of men reported a negative reaction (the remainders for each sex had a mix of both positive and negative reactions; Owen et al., 2010).
However, both sexes also experience some negative affect as well. In a qualitative study that asked 187 participants to report their feelings after a typical hookup, 35 percent reported feeling regretful or disappointed, 27 percent good or happy, 20 percent satisfied, 11 percent confused, 9 percent proud, 7 percent excited or nervous, 5 percent uncomfortable, and 2 percent desirable or wanted (Paul & Hayes, 2002). However, this same study found that feelings differed during hookups compared with after: During a typical hookup, 65 percent of participants reported feeling good, aroused, or excited, 17 percent desirable or wanted, 17 percent nothing in particular or were focused on the hookup, 8 percent embarrassed or regretful, 7 percent nervous or scared, 6 percent confused, and 5 percent proud (Paul & Hayes, 2002).
Hook-up regret
A number of studies have looked at regret with respect to hookups and have documented the negative feelings men and women may feel after casual sex. In a large Web-based study of 1,468 undergraduate students, participants reported a variety of consequences: 27.1 percent felt embarrassed, 24.7 percent reported emotional difficulties, 20.8 percent experienced loss of respect, and 10 percent reported difficulties with a steady partner (Lewis et al., 2011). In another recent study conducted on a sample of 200 undergraduate students in Canada, 78 percent of women and 72 percent of men who had uncommitted sex (including vaginal, anal, and/or oral sex) reported a history of experiencing regret following such an encounter (Fisher et al., 2012).
Fisher et al. (2012) also found few sex differences in reasons for regret, with better quality sex reducing the degree of regret reported. It appears the method of asking participants whether and when they had experienced regret (i.e., ever, last hookup, or typical hookup) produces a sex difference, but in terms of categorical presence, most emerging adults experienced a kaleidoscope of reactions. This is consistent with Stinson's (2010) message of sexual development requiring experimentation, including trial and error, good feelings and bad feelings.
In a study of 270 sexually active college-age students, 72 percent regretted at least one instance of previous sexual activity (Oswalt, Cameron, & Koob, 2005). In a report of 152 female undergraduate students, 74 percent had either a few or some regrets from uncommitted sex: 61 percent had a few regrets, 23 percent had no regrets, 13 percent had some regrets and 3 percent had many regrets (Eshbaugh & Gute, 2008).
Another study identified two types of sexual encounters that were particularly predictive of regret: engaging in penetrative intercourse with someone known less than 24 hours and engaging in penetrative intercourse with someone only once. Among a sample of 1,743 individuals who had experienced a one-night stand, Campbell (2008) showed that most men and women had combinations of both positive and negative affective reactions following this event. Campbell also found that men had stronger feelings of being "sorry because they felt they used another person," whereas women had stronger feelings of "regret because they felt used." Again, both men and women had experienced some sexual regret, but women were more negatively impacted by some hook-up experiences.
Hook-up culture and mental health
An individual history of hook-up behavior has been associated with a variety of mental health factors. In a study of 394 young adults followed across a university semester, those with more depressive symptoms and greater feelings of loneliness who engaged in penetrative sex hookups subsequently reported a reduction in both depressive symptoms and feelings of loneliness (Owen et al., 2011). At the same time, participants who reported fewer depressive symptoms and fewer feelings of loneliness who engaged in penetrative sex hookups subsequently reported an increase in both depressive symptoms and feelings of loneliness (Owen et al., 2011). In another study, among 291 sexually experienced individuals, people who had the most regret after uncommitted sex also had more symptoms of depression than those who had no regret (Welsh et al., 2006). However, in the same sample, women's but not men's degree of depressive symptoms increased with number of previous sex partners within the last year (Welsh et al., 2006).
In the first study to investigate the issue of self-esteem and hookups, both men and women who had ever engaged in an uncommitted sexual encounter had lower overall self-esteem scores compared with those without uncommitted sexual experiences (Paul et al., 2000). The potential causal direction of the relationship between self-esteem and uncommitted sex is yet unclear (Fielder & Carey, 2010; Paul et al., 2000).
Just as multiple motivations can be in conflict, a person's affective reactions during and after a hookup can be in conflict. Discrepancies between behaviors and desires, particularly with respect to social-sexual relationships, have dramatic implications for physical and mental health. Despite the allure of engaging in uncommitted sex, research shows that people engage in these behaviors even when they feel uncomfortable doing so (Lambert et al., 2003; Reiber & Garcia, 2010). In addition, people overestimate others' comfort with hookups and assign variable meanings to those behaviors (Lambert et al., 2003; Reiber & Garcia, 2010). Misperception of sexual norms is one potential driver for people to behave in ways they do not personally endorse. In a replication and extension of Lambert et al.'s (2003) study, Reiber and Garcia (2010) found that 78 percent of people overestimated others' comfort with many different sexual hook-up behaviors, with men particularly overestimating women's actual comfort with a variety of sexual behaviors in hookups.
Hook-up scenarios may include feelings of pressure and performance anxiety, contributing to feelings of discomfort. In Paul et al.'s (2000) study on hookups, 16 percent of participants felt pressured during their typical hookup. In this sample, 12 percent of participants felt out of control when intercourse was not involved, while 22 percent felt out of control when sexual intercourse took place. (Note that this study asked participants about typical hookups, and although this is informative for general patterns, it does not capture specific factors influencing specific individual scenarios. For instance, it is unclear how one might rate a "typical" hookup if one instance involved sexual coercion and regret while another, before or after, was consenting and more enjoyable.)
Hookups can result in guilt and negative feelings. In a study of 169 sexually experienced men and women surveyed in singles bars, when presented with the statement, "I feel guilty or would feel guilty about having sexual intercourse with someone I had just met," 32 percent of men and 72 percent of women agreed (Herold & Mewhinney, 1993). The percentage of women expressing guilt was more than twice that of men. This is consistent with a classic study by Clark and Hatfield (1989), which found that men are much more likely than women to accept casual sex offers from people they find attractive. Conley (2011) replicated and extended this finding, demonstrating that, under certain conditions of perceived comfort, the gender differences in acceptance of casual sex are diminished.
Qualitative descriptions of hookups reveal relative gender differences in terms of feelings afterward, with women displaying more negative reactions than men (Paul & Hayes, 2002). This is also consistent with earlier work demonstrating a sex difference, with women generally identifying more emotional involvement in seemingly "low investment" (i.e., uncommitted) sexual encounters than men (Townsend, 1995). Moreover, in a study of 140 (109 female, 31 male) first-semester undergraduates, women, but not men, who had engaged in intercourse during a hookup showed higher rates of mental distress (Fielder & Carey, 2010). Possibly contributing to findings on gender differences in thoughts of worry, in a sample of 507 undergraduate students, more women than men hoped that a relationship would develop following a hookup. Only 4.4 percent of men and 8.2 percent of women (6.45 percent of participants) expected a traditional romantic relationship as an outcome, while 29 percent of men and 42.9 percent of women (36.57 percent of participants) ideally wanted such an outcome (Garcia & Reiber, 2008). It is possible that regret and negative consequences result from individuals attempting to negotiate multiple desires. It is likely that a substantial portion of emerging adults today are compelled to publicly engage in hookups while desiring both immediate sexual gratification and more stable romantic attachments.
Hook-up culture and sexual risk
Despite the prevalence of positive feelings, hookups can include negative outcomes, such as emotional and psychological injury, sexual violence, sexually transmitted infections and unintended pregnancy. Despite those risks, a qualitative study of 71 college students (39 women and 32 men) found that nearly half of participants were not concerned about contracting sexually transmitted diseases from intercourse during a hookup, and most were unconcerned about contracting diseases from fellatio or cunnilingus in hookups (Downing-Matibag & Geisinger, 2009).
Compounding disease risks, people who hook up are more likely to have concurrent sexual partners (Paik, 2010b). Moreover, in a sample of 1,468 college students, among the 429 students who had engaged in oral sex, anal sex or vaginal intercourse in their most recent hookup, only 46.6 percent reported using a condom (Lewis et al., 2011).
In terms of condom use, another issue of concern involving hookups is the high comorbidity with substance use. As part of a larger study, in a sample of several thousand people ages 15 to 25, men and women who had used marijuana or cocaine in the previous 12 months were also more likely than nonusers to have had nonmonogamous sex in the past 12 months (van Gelder et al., 2011). More specifically, in one study of undergraduate students, 33 percent of those who reported they had uncommitted sex indicated their motivation was "unintentional," likely due to alcohol and other drugs (Garcia & Reiber, 2008). In Fielder and Carey's (2010) study among 118 first-semester female college students, participants reported that 64 percent of uncommitted sexual encounters followed alcohol use, with the average occurring after consuming three alcoholic drinks. Similarly, another study found that nearly 61 percent of undergraduate students used alcohol, with an average of 3.3 alcoholic drinks, during their most recent hookup (Lewis et al., 2011).
Not all hook-up encounters are necessarily wanted or consensual. People occasionally consent to a sexual act but do not necessarily want sex (Peterson & Muehlenhard, 2007). In a sample of 178 college students, participants noted that most of their unwanted sex occurred in the context of hookups: 77.8 percent during a hookup, 13.9 percent in an ongoing relationship and 8.3 percent on a date (Flack et al., 2007). Similarly, in a sample of 761 women students, approximately 50 percent of women reported at least one experience of unwanted sex (Hill, Garcia, & Geher, 2012). Of those women, 70 percent experienced unwanted sex in the context of a hookup and 57 percent in the context of a committed romantic relationship (Hill et al., 2012).
Even more worrisome, a proportion of hookups also involve nonconsensual sex. In a study by Lewis et al. (2011), 86.3 percent of participants portrayed their most recent hook-up experience as one they wanted to have, while 7.6 percent indicated that their most recent hookup was an experience they did not want to have or to which they were unable to give consent. Unwanted and nonconsensual sexual encounters are more likely occurring alongside alcohol and substance use.
Alcohol use has also been associated with a type of hookup: The greatest alcohol use was associated with penetrative sexual hookups, less alcohol use with nonpenetrative hookups, and the least amount of alcohol use occurred among those who did not hook-up (Owen, Fincham, & Moore, 2011). In one study of men and women who had engaged in an uncommitted sexual encounter that included vaginal, anal or oral sex, participants reported their intoxication levels: 35 percent were very intoxicated, 27 percent were mildly intoxicated, 27 percent were sober and 9 percent were extremely intoxicated (Fisher, Worth, Garcia, & Meredith, 2012). Alcohol may also serve as an excuse, purposely consumed as a strategy to protect the self from having to justify hook-up behavior later (Paul, 2006).
Although alcohol and drugs are likely a strong factor, it is still largely unclear what role individual differences play in shaping decisions to engage in hookups. In a sample of 394 young adults, the strongest predictor of hook-up behavior was having previously hooked up — those who engaged in penetrative sex hookups were 600 percent more likely than others to repeat this over the course of a university semester (Owen et al., 2011). Other factors may include media consumption, personality and biological predispositions. Garcia, MacKillop, et al. (2010) demonstrated an association between dopamine D4 receptor gene polymorphism (DRD4 VNTR) and uncommitted sexual activity among 181 young men and young women. Although genotypic groups in this study did not vary in terms of overall number of sexual partners, individuals with a particular "risk-taking" variant of the dopamine D4 receptor gene (DRD4 VNTR; also associated with substance abuse) were shown to have a higher likelihood of having uncommitted sexual encounters (including infidelity and one-night stands); however, no sex differences were observed. This suggests that biological factors that contribute to motivating the different contexts of sexual behavior for both men and women may be fairly sexually monomorphic (Garcia & Reiber, 2008; Garcia, Reiber, et al., 2010). This may, in some cases, point to fairly stable individual differences.
Sex differences in hook-up behaviors
Some research has considered the interactions of sex and individual differences in predicting hook-up behavior. The Mating Intelligence Scale, designed to measure an individual's cognitive abilities in the evolutionary domain of mating (see Geher & Kaufman, 2011), was used to assess hook-up behavior in a sample of 132 college students. Young men higher in mating intelligence were more likely than others to have hooked up with strangers, acquaintances and friends, while young women higher in mating intelligence were only more likely than others to have had more hook-up experiences with acquaintances (O'Brien, Geher, Gallup, Garcia, & Kaufman, 2009). The authors proposed that given the potential risks and costs of sex to females, sex with strangers would be disadvantageous; and because women do not generally report having sexual motives toward opposite-sex friends (Bleske-Rechek & Buss, 2001), women with high mating intelligence were likely striking the optimal balance, whereas men high in mating intelligence were obtaining maximum sexual encounters (O'Brien et al., 2009).
Still unclear are the degree to which hookups may result in positive reactions, and whether young men and young women are sexually satisfied in these encounters. Fine (1988) has argued that sex negativity is even more pronounced when directed at women and, further, that the possibility of desire seems to be missing from the sexual education of young women. This discrepancy in the socialization and education of men and women may be a significant influence on behavioral patterns and outcomes in sexual hookups.
Armstrong, England and Fogarty (2009) addressed sexual satisfaction in a large study of online survey responses from 12,295 undergraduates from 17 different colleges. Participants were asked about oral sex rates and orgasm in their most recent hookup and most recent relationship sexual event. In this study, men reported receiving oral sex both in hookups and in relationships much more than women. In first-time hookups that involved oral sex, 55 percent included only men receiving oral sex, 19 percent only women receiving oral sex, and 27 percent both mutually receiving; in last relationship sexual activity, 32 percent included only men receiving oral sex, 16 percent included only women receiving oral sex, and 52 percent included both mutually receiving.
In both contexts, men also reached orgasm more often than women. In first-time hookups, 31 percent of men and 10 percent of women reached orgasm; in last relationship sexual activity, 85 percent of men and 68 percent of women reached orgasm. Armstrong et al. (2009) concluded with an important message: "A challenge to the contemporary sexual double standard would mean defending the position that young women and men are equally entitled to sexual activity, sexual pleasure, and sexual respect in hookups as well as relationships. To achieve this, the attitudes and practices of both men and women need to be confronted. Men should be challenged to treat even first hookup partners as generously as the women they hook up with treat them."
Uncommitted sex, now being explored across a variety of disciplines and theoretical perspectives, is best understood as a biopsychosocial phenomenon. Evidence suggests that both pleasure and reproductive motives may influence these sexual patterns, as seen in participants' reactions following uncommitted sex. Further, the findings that a majority of both men and women are motivated to engage in hookups, but often desire a more romantic relationship, are consistent with a nuanced perspective that takes into account changing social scripts, new patterns of development, and the cross-cultural and biological centrality of the pair-bond (Fisher, 1992; Gray & Garcia, 2013).
By definition, sexual hookups provide the allure of sex without strings attached. Despite their increasing social acceptability, however, developing research suggests that sexual hookups may leave more strings attached than many participants might first assume.
Justin R. Garcia, MS, PhD, is CTRD Research Fellow at The Kinsey Institute for Research in Sex, Gender, and Reproduction and member of the Center for the Integrative Study of Animal Behavior and the Cognitive Science Program at Indiana University, Bloomington. He is co-author of "Evolution and Human Sexual Behavior" (Harvard University Press, 2013).
Chris Reiber, PhD, MPH, is interim associate dean for research for Harpur College of Arts and Sciences, director of the graduate program in biomedical anthropology, and associate professor of anthropology at Binghamton University, SUNY.
Sean G. Massey, PhD, is a research associate professor in the women, gender and sexuality studies program at Binghamton University, SUNY. He received his doctorate from the Graduate Center of the City University of New York. His research focuses on the psychology of prejudice and privilege, research and policy, sexual behaviors and the study of LGBT lives.
Ann M. Merriwether, PhD, is a lecturer in psychology and human development at Binghamton University, SUNY. She received her doctorate from Pennsylvania State University in the area of developmental psychology. Her research focuses on the development of reproductive health attitudes and sexual socialization.
Read the Journal Article
- This article is condensed from "Sexual Hookup Culture: A Review," in Review of General Psychology , 2012, Vol. 16, No. 2, 161–176. Read the full article, which includes all references, and a more detailed theoretical review (PDF, 125KB).
Letters to the Editor
- Send us a letter
- Library of Congress National Book Festival Announces Full Author Lineup
- National Ambassador for Young People’s Literature Unveils New Video Series
- New Exhibition “Collecting Memories” to Open in new David M. Rubenstein Treasures Gallery
- 25 Audio Treasures Selected for National Recording Registry
- Poet Mary Oliver's Papers, Fund for New Event Series Gifted to Library
- Library of Congress
Papers of Talk Show Host and Sex Therapist Ruth Westheimer Open for Research at Library of Congress

- Pinterest LinkedIn E-Mail
Papers of Talk Show Host and Sex Therapist Ruth Westheimer Open for Research at Library of Congress Papers and Correspondence of “Dr. Ruth” Acquired in 2022, Now Open for Research
The papers of talk show host and sex therapist Ruth Westheimer have been acquired by the Library of Congress and are now opening for research in the Library’s Manuscript Division.
Westheimer became a household name as “Dr. Ruth” in the 1980s, filling radio airwaves, television screens, and bookshelves with advice on sex and relationships. Westheimer was a pioneering voice in sex education, speaking openly about sex and the male and female anatomy at a time when such topics were publicly taboo.
The collection contains thousands of letters sent by listeners of her radio program, viewers of her television show, and readers of her books, providing insight into the sexual questions and concerns of her audience. There are also some of Westheimer’s written responses to those letters. The papers also document the dynamic rise in popularity of “Dr. Ruth.” There are publicity packets, production files and show notes related to Westheimer’s shows.
“I am delighted that the many letters I received requesting sexual advice will now be available to the research community,” Westheimer said. “I hope it helps us better understand the issues people struggled with then and also serves to promote better awareness today.”
Westheimer first went on the air in 1980 on WYNY-FM, broadcasting in New York City. Only radio listeners with antennae strong enough to pick up New York’s 97.1 FM station could hear her German-accented voice until 1984, when NBC Radio nationally syndicated her program, “Sexually Speaking.” Her audience grew with the premiere of “Good Sex! with Dr. Ruth Westheimer” on Lifetime Television in 1984. Both listeners and viewers sent in questions, comments, and requests for information or the occasional autograph.
Researchers may contact the Manuscript Reading Room for more information about this collection. The collection finding aid will be posted online next month. Researchers consulting the collection will be required to sign a form promising not to disclose personally identifiable information found in letters sent by the public to Westheimer.
The Library of Congress is the world’s largest library, offering access to the creative record of the United States — and extensive materials from around the world — both on-site and online. It is the main research arm of the U.S. Congress and the home of the U.S. Copyright Office. Explore collections, reference services and other programs and plan a visit at loc.gov ; access the official site for U.S. federal legislative information at congress.gov ; and register creative works of authorship at copyright.gov .
Media Contacts: Brett Zongker, [email protected] , Elaina Finkelstein, [email protected] PR 24-054 06/21/2024 ISSN 0731-3527
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 June 2024
A disease-associated gene desert directs macrophage inflammation through ETS2
- C. T. Stankey ORCID: orcid.org/0000-0001-5710-1716 1 , 2 , 3 na1 ,
- C. Bourges ORCID: orcid.org/0000-0001-8122-0475 1 na1 ,
- L. M. Haag ORCID: orcid.org/0000-0002-3754-5317 4 na1 ,
- T. Turner-Stokes 1 , 2 ,
- A. P. Piedade 1 ,
- C. Palmer-Jones 5 , 6 ,
- I. Papa ORCID: orcid.org/0000-0003-3167-7623 1 ,
- M. Silva dos Santos ORCID: orcid.org/0000-0003-2404-8490 7 ,
- Q. Zhang 8 ,
- A. J. Cameron ORCID: orcid.org/0000-0002-7065-9033 9 ,
- A. Legrini 9 ,
- T. Zhang 9 ,
- C. S. Wood 9 ,
- F. N. New ORCID: orcid.org/0000-0001-6213-4731 10 ,
- L. O. Randzavola 2 ,
- L. Speidel 11 , 12 ,
- A. C. Brown 13 ,
- A. Hall 14 , 15 ,
- F. Saffioti ORCID: orcid.org/0000-0001-7635-9931 6 , 14 ,
- E. C. Parkes 1 ,
- W. Edwards 16 ,
- H. Direskeneli 17 ,
- P. C. Grayson 18 ,
- L. Jiang 19 ,
- P. A. Merkel 20 , 21 ,
- G. Saruhan-Direskeneli ORCID: orcid.org/0000-0002-6903-7173 22 ,
- A. H. Sawalha ORCID: orcid.org/0000-0002-3884-962X 23 , 24 , 25 , 26 ,
- E. Tombetti 27 , 28 ,
- A. Quaglia 15 , 29 ,
- D. Thorburn 6 , 14 ,
- J. C. Knight ORCID: orcid.org/0000-0002-0377-5536 13 , 30 , 31 ,
- A. P. Rochford 5 , 6 ,
- C. D. Murray 5 , 6 ,
- P. Divakar 10 ,
- M. Green 32 ,
- E. Nye 32 ,
- J. I. MacRae ORCID: orcid.org/0000-0002-1464-8583 7 ,
- N. B. Jamieson ORCID: orcid.org/0000-0002-9552-4725 9 ,
- P. Skoglund 11 ,
- M. Z. Cader 16 , 33 ,
- C. Wallace ORCID: orcid.org/0000-0001-9755-1703 16 , 34 ,
- D. C. Thomas ORCID: orcid.org/0000-0002-9738-2329 16 , 33 &
- J. C. Lee ORCID: orcid.org/0000-0001-5711-9385 1 , 5 , 6
Nature volume 630 , pages 447–456 ( 2024 ) Cite this article
147k Accesses
1047 Altmetric
Metrics details
- Autoimmunity
- Functional genomics
- Immunogenetics
Increasing rates of autoimmune and inflammatory disease present a burgeoning threat to human health 1 . This is compounded by the limited efficacy of available treatments 1 and high failure rates during drug development 2 , highlighting an urgent need to better understand disease mechanisms. Here we show how functional genomics could address this challenge. By investigating an intergenic haplotype on chr21q22—which has been independently linked to inflammatory bowel disease, ankylosing spondylitis, primary sclerosing cholangitis and Takayasu’s arteritis 3 , 4 , 5 , 6 —we identify that the causal gene, ETS2 , is a central regulator of human inflammatory macrophages and delineate the shared disease mechanism that amplifies ETS2 expression. Genes regulated by ETS2 were prominently expressed in diseased tissues and more enriched for inflammatory bowel disease GWAS hits than most previously described pathways. Overexpressing ETS2 in resting macrophages reproduced the inflammatory state observed in chr21q22-associated diseases, with upregulation of multiple drug targets, including TNF and IL-23. Using a database of cellular signatures 7 , we identified drugs that might modulate this pathway and validated the potent anti-inflammatory activity of one class of small molecules in vitro and ex vivo. Together, this illustrates the power of functional genomics, applied directly in primary human cells, to identify immune-mediated disease mechanisms and potential therapeutic opportunities.
Similar content being viewed by others
The tidyomics ecosystem: enhancing omic data analyses
Genotype × environment interactions in gene regulation and complex traits

Genome-wide association studies
Nearly 5% of humans live with an autoimmune or inflammatory disease. These heterogeneous conditions, ranging from Crohn’s disease and ulcerative colitis (collectively inflammatory bowel disease (IBD)) to psoriasis and lupus, all require better therapies, but only 10% of drugs entering clinical development ever become approved treatments 2 . This high failure rate is mainly due to a lack of efficacy 8 and reflects our poor understanding of disease mechanisms. Genetics provides a unique opportunity to address this, with hundreds of loci now directly linked to the pathogenesis of immune-mediated diseases 9 . Indeed, drugs that target pathways implicated by genetics have a far higher chance of being effective 10 .
However, to fully realize the potential of genetics, knowledge of where risk variants lie must be translated into an understanding of how they drive disease 9 . Animal models can help with this, especially for coding variants in conserved genes 11 , 12 . Unfortunately, most risk variants do not lie in coding DNA, but in less-well-conserved, non-coding genomic regions. Resolving the biology at these loci is a formidable task, as the same DNA sequence can function differently depending on the cell type and/or external stimuli 9 . Most non-coding variants are thought to affect gene regulation 13 , but difficulties identifying causal genes, which may lie millions of bases away, and causal cell types, which may only express implicated genes under certain conditions, have hindered efforts to identify disease mechanisms. For example, although genome-wide association studies (GWASs) have identified over 240 IBD risk loci 3 , including several possible drug targets, fewer than 10 have been mechanistically resolved.
Molecular mechanisms at chr21q22
Some genetic variants predispose to multiple diseases, highlighting both their biological importance and an opportunity to study shared disease mechanisms. One notable example is an intergenic region on chromosome 21q22 (chr21q22), where the major allele haplotype predisposes to five inflammatory diseases 3 , 4 , 5 , 6 . Such regions, which were originally termed ‘gene deserts’ owing to their lack of coding genes, often contain GWAS hits but are poorly understood. To test for a shared disease mechanism, we performed co-localization analyses and confirmed that the genetic basis for every disease was the same, meaning that a common causal variant(s) and a shared molecular effect was responsible (Fig. 1a and Extended Data Fig. 1 ). As these heterogeneous diseases are all immune mediated, we reasoned that this locus must contain a distal enhancer that functioned in immune cells. By examining H3K27ac chromatin immunoprecipitation–sequencing (ChIP–seq) data, which marks active enhancers and promoters, we identified a monocyte/macrophage-specific enhancer within the locus (Fig. 1b ). Monocytes and macrophages have a key role in many immune-mediated diseases, producing cytokines that are often targeted therapeutically 14 .
a , Disease associations at chr21q22. The red points denote the IBD 99% credible set. Co-localization results for each disease versus IBD. PP.H3, posterior probability of independent causal variants; PP.H4, posterior probability of shared causal variant. b , Immune cell H3K27ac ChIP–seq at chr21q22. IBD GWAS results are shown. NK cells, natural killer cells. rpm, reads per million. c , The ETS2 eQTL in resting monocytes, with co-localization versus IBD association. Macrophage promoter-capture Hi-C (pcHi-C) data at the disease-associated locus. d , Experimental schematic for studying the chr21q22 locus in inflammatory (TPP) macrophages. e , ETS2 , BRWD1 and PSMG1 mRNA expression during TPP stimulation, measured using PrimeFlow RNA assays. Data are from one representative donor out of four. f , Relative ETS2 , BRWD1 and PSMG1 expression (mean fluorescence intensity (MFI)) in chr21q22-edited macrophages versus unedited cells. n = 4. Data are mean ± s.e.m. Statistical analysis was performed using two-way analysis of variance (ANOVA)). g , SuSiE fine-mapping posterior probabilities for IBD-associated SNPs at chr21q22 (99% credible set). h , Macrophage MPRA at chr21q22. Data are oligo coverage (top), enhancer activity (sliding-window analysis with significant enhancer activity highlighted; middle) and expression-modulating effects of SNPs within the enhancer (bottom). For the box plots, the centre line shows the median, the box limits show the interquartile range, and the whiskers represent the minimum and maximum values. n = 8. False-discovery rate (FDR)-adjusted P values were calculated using QuASAR-MPRA (two-sided). i , Inflammatory macrophage PU.1 ChIP–seq peaks at chr21q22. Bottom, magnification of the location of rs2836882 and the nearest predicted PU.1 motif. j , BaalChIP analysis of allele-specific PU.1 ChIP–seq binding at rs2836882 in two heterozygous macrophage datasets (data are mean ± 95% posterior distribution of allelic balance). Total counts shown as a pie chart. k , Allele-specific ATAC–seq reads at rs2836882 in monocytes from 16 heterozygous donors (including healthy controls and patients with ankylosing spondylitis). Statistical analysis was performed using two-sided Wilcoxon matched-pair tests. l , H3K27ac ChIP–seq data from risk (top) or non-risk (bottom) allele homozygotes at rs2836882. Data are shown from two out of four donors. FDR-corrected P values were calculated using MEDIPS (two-sided). The diagrams in d and e were created using BioRender.
Source Data
We next sought to identify the gene regulated by this enhancer. Although the associated locus lacks coding genes, there are several nearby candidates that have been highlighted in previous studies, including PSMG1 , BRWD1 and ETS2 (refs. 3 , 4 , 5 , 6 , 15 ) (Fig. 1a ). Using promoter-capture Hi-C and expression quantitative locus (eQTL) data from human monocytes ( Methods ), we found that the disease-associated locus physically interacts with the promoter of ETS2 —the most distant candidate gene (around 290 kb away)—and that the risk haplotype correlates with higher ETS2 expression (Fig. 1c ). Indeed, increased ETS2 expression in monocytes and macrophages, either at rest or after early exposure to bacteria, was found to have the same genetic basis as inflammatory disease risk (Extended Data Fig. 1c ). To directly confirm that ETS2 was causal, we used CRISPR–Cas9 to delete the 1.85 kb enhancer region in primary human monocytes before culturing these cells with inflammatory ligands, including TNF (a pro-inflammatory cytokine), prostaglandin E2 (a pro-inflammatory lipid) and Pam3CSK4 (a TLR1/2 agonist) (TPP model; Fig. 1d and Extended Data Fig. 2a–c ). This model was designed to mimic chronic inflammation 16 , and better resembles disease macrophages than classical IFNγ-driven or IL-4-driven models 17 (Extended Data Fig. 2 ). As flow cytometry antibodies were not available for the candidate genes, we used PrimeFlow to measure the dynamics of mRNA expression and detected increased levels of all three genes ( ETS2 , BRWD1 and PSMG1 ) after TPP stimulation of unedited monocytes (Fig. 1e ). Deletion of the chr21q22 enhancer did not affect BRWD1 or PSMG1 expression, but the upregulation of ETS2 was profoundly reduced (Fig. 1f ), confirming that this pleiotropic locus contains a distal ETS2 enhancer.
To identify the causal variant, we performed statistical fine-mapping in a large IBD GWAS 3 . Unfortunately, this did not resolve the association owing to high linkage disequilibrium between candidate single-nucleotide polymorphisms (SNPs) ( Methods and Fig. 1g ). We therefore used a functional approach to first delineate the active enhancers at the locus, and then assess whether any candidate SNPs might alter enhancer activity. This method, massively parallel reporter assay (MPRA), simultaneously tests enhancer activity in thousands of short DNA sequences by coupling each to a uniquely barcoded reporter gene 18 . Sequences that alter gene expression are identified by normalizing the barcode counts in mRNA, extracted from transfected cells, to their matching counts in the input DNA library. After adapting MPRA for primary macrophages ( Methods and Extended Data Fig. 3 ), we synthesized a pool of overlapping oligonucleotides to tile the 2 kb region containing all candidate SNPs, and included oligonucleotides with either risk or non-risk alleles for every variant. The resulting library was transfected into inflammatory macrophages from multiple donors, ensuring that a physiological repertoire of transcription factors could interact with the genomic sequences. Using a sliding-window analysis, we identified a single 442 bp focus of enhancer activity (chromosome 21: 40466236–40466677, hg19; Fig. 1h ) that contained three (out of seven) candidate SNPs. Two of these polymorphisms were transcriptionally inert, but the third (rs2836882) had the strongest expression-modulating effect of any candidate SNP, with the risk allele (G) increasing transcription, consistent with the ETS2 eQTL (Fig. 1h and Extended Data Fig. 1b ). This SNP was in the credible set of every co-localizing molecular trait, and lay within a macrophage PU.1 ChIP–seq peak (Fig. 1i ). PU.1 is a non-classical pioneer factor in myeloid cells 19 that can bind to DNA, initiate chromatin remodelling (thereby enabling other transcription factors to bind) and activate transcription 20 . To determine whether rs2836882 might affect PU.1 binding, we identified PU.1 ChIP–seq data from heterozygous macrophages and tested for allelic imbalances in binding. Despite not lying within a canonical PU.1 motif, strong allele-specific binding was detected, with over fourfold greater binding to the rs2836882 risk allele (Fig. 1i,j ). This was replicated by genotyping PU.1-bound DNA in macrophages from five heterozygous donors (Extended Data Fig. 4a–f ). Moreover, assay for transposase-accessible chromatin with sequencing (ATAC–seq) analysis of monocytes and macrophages from rs2836882 heterozygotes revealed allelic differences in chromatin accessibility that were consistent with differential binding of a pioneer factor (Fig. 1k and Extended Data Fig. 4g ).
To test for allele-specific enhancer activity at the endogenous locus, we performed H3K27ac ChIP–seq analysis of inflammatory macrophages from rs2836882 major and minor allele homozygotes. While most chr21q22 enhancer peaks were similar between these donors, the enhancer activity overlying rs2836882 was significantly stronger in major (risk) allele homozygotes (Fig. 1l and Extended Data Fig. 4h ), contributing to an approximate 2.5-fold increase in activity across the locus (Extended Data Fig. 4i ). Collectively, these data reveal a mechanism whereby the putative causal variant at chr21q22—identified by its functional effects in primary macrophages—promotes binding of a pioneer factor, enhances chromatin accessibility and increases activity of a distal ETS2 enhancer.
Macrophage inflammation requires ETS2
ETS2 is an ETS-family transcription factor and proto-oncogene 21 , but its exact role in human macrophages is unclear, with previous studies using either cell lines or complex mouse models and assessing a limited number of potential targets 22 , 23 , 24 , 25 , 26 . This has led to contradictory reports, with ETS2 being described as both necessary and redundant for macrophage development 27 , 28 , and both pro- and anti-inflammatory 22 , 23 , 24 , 25 , 26 . To clarify the role of ETS2 in human macrophages, and determine how dysregulated ETS2 expression might contribute to disease, we first used a CRISPR–Cas9-based loss-of-function approach (Fig. 2a ). To control for off-target effects, two gRNAs targeting different ETS2 exons were designed, validated and individually incorporated into Cas9 ribonucleoproteins for transfection into primary monocytes. These produced on-target editing in around 90% and 79% of cells, respectively, and effectively reduced ETS2 expression (Extended Data Fig. 2d–f ). Cell viability and macrophage marker expression were unaffected, suggesting that ETS2 was not required for macrophage survival or differentiation (Extended Data Fig. 2g,h ). By contrast, pro-inflammatory cytokine production, including IL-6, IL-8 and IL-1β, was markedly reduced after ETS2 disruption (Fig. 2b ), whereas IL-10—an anti-inflammatory cytokine—was less affected. TNF was not assessed as it had been added exogenously. We next investigated whether other macrophage functions were affected. Using fluorescently labelled particles that are detectable by flow cytometry, we found that phagocytosis was similarly impaired after ETS2 disruption (Fig. 2c ). We also tested extracellular reactive oxygen species (ROS) production—a major contributor to inflammatory tissue damage 29 . Disrupting ETS2 profoundly reduced the macrophage oxidative burst—most likely by decreasing expression of key NADPH oxidase components (Fig. 2d and Extended Data Fig. 5a ). Together, these data suggest that ETS2 is essential for multiple inflammatory functions in human macrophages.
a , Experimental schematic for studying ETS2 in inflammatory (TPP) macrophages. The diagram was created using BioRender. b , Cytokine secretion after ETS2 disruption. Heat map of relative cytokine levels from ETS2 -edited versus unedited macrophages. n = 8. c , Phagocytosis of fluorescently labelled zymosan particles by ETS2 -edited and unedited macrophages (non-targeting control (NTC)) (left). Data are from one representative donor out of seven. Right, the phagocytosis index (the product of the proportion and MFI of phagocytosing cells). n = 7. d , ROS production by ETS2 -edited and unedited macrophages. Data from one representative donor out of six (left). Right, NADPH oxidase component expression in ETS2 -edited and unedited macrophages (western blot densitometry). n = 7. Source gels are shown in Supplementary Fig. 1 . RLU, relative light units. e , RNA-seq analysis of differentially expressed genes in ETS2 -edited versus unedited TPP macrophages (limma with voom transformation, two-sided). n = 8. The horizontal line denotes the FDR-adjusted significance threshold. f , fGSEA of differentially expressed genes between ETS2 -edited and unedited TPP macrophages. The results of selected GO Biological Pathways are shown. The dot size denotes the unadjusted P value (two-sided), and the colour denotes normalized enrichment score (NES). g , The log 2 [fold change (FC)] of genes differentially expressed by chr21q22 enhancer deletion, plotted against their fold change after ETS2 editing. The percentages denote upregulated (red) and downregulated (blue) genes. The coloured points (blue or red) represent differentially expressed genes after ETS2 editing (FDR < 0.1, two-sided). For c and d , data are mean ± s.e.m. Statistical analysis was performed using two-sided Wilcoxon tests ( b – d ); * P < 0.05.
To understand the molecular basis for these effects, we performed RNA sequencing (RNA-seq) of ETS2 -edited and unedited inflammatory macrophages from multiple donors. Disrupting ETS2 led to widespread transcriptional changes, with reduced expression of many inflammatory genes (Fig. 2e ). These included cytokines (such as TNFSF10/TRAIL , TNFSF13 , IL1A and IL1B ), chemokines (such as CXCL3 , CXCL5 , CCL2 and CCL5 ), secreted effector molecules (such as S100A8 , S100A9 , MMP14 and MMP9 ), cell surface receptors (such as FCGR2A , FCGR2C and TREM1 ), pattern-recognition receptors (such as TLR2 , TLR6 and NOD2 ) and signalling molecules (such as MAP2K , GPR84 and NLRP3 ). To better characterize the pathways affected, we performed gene set enrichment analysis (fGSEA) using the Gene Ontology (GO) Biological Pathways dataset. This corroborated the functional deficits, with the most negatively enriched pathways (downregulated by ETS2 disruption) being related to macrophage activation, inflammatory cytokine production, phagocytosis and ROS production (Fig. 2f ). Genes involved in macrophage migration were also downregulated, but those relating to monocyte-to-macrophage differentiation were unaffected—consistent with ETS2 being required for inflammatory functions but not for monocyte-derived macrophage development. Fewer genes were upregulated after ETS2 disruption (Fig. 2e ), but positive enrichment was noted for aerobic respiration and oxidative phosphorylation (OXPHOS; Fig. 2f )—metabolic processes that are linked to anti-inflammatory phenotypes 30 . Notably, these transcriptional effects were not due to major changes in chromatin accessibility, although enhancer activity was generally reduced (Extended Data Fig. 2j,k ). As expected, deletion of the chr21q22 enhancer phenocopied both the transcriptional and functional effects of disrupting ETS2 (Fig. 2g and Extended Data Fig. 5a–e ). Collectively, these data identify an essential role for ETS2 in macrophage inflammatory responses, which could explain why dysregulated ETS2 expression predisposes to disease. Indeed, differential expression of ETS2-regulated genes was observed in resting (M0) macrophages from patients with IBD stratified by rs2836882 genotype (matched for age, sex, therapy and disease activity) (Extended Data Fig. 5f ).
ETS2 coordinates macrophage inflammation
We next studied the effects of increasing ETS2 expression, as this is what drives disease risk. To do this, we optimized a method for controlled overexpression of target genes in primary macrophages through transfection of in vitro transcribed mRNA that was modified to minimize immunogenicity (Fig. 3a , Methods and Extended Data Fig. 3f ). Resting, non-activated macrophages were transfected with ETS2 mRNA or its reverse complement, thereby controlling for mRNA quantity, length and purine/pyrimidine composition (Fig. 3b ). After transfection, cells were exposed to low-dose lipopolysaccharide to initiate a low-grade inflammatory response that could potentially be amplified (Fig. 3a ). We found that overexpressing ETS2 increased pro-inflammatory cytokine secretion, while IL-10 was again less affected (Extended Data Fig. 3g ). To better characterize this response, we performed RNA-seq and re-examined the inflammatory pathways that required ETS2 . Notably, all of these pathways—including macrophage activation, cytokine production, ROS production, phagocytosis and migration—were induced in a dose-dependent manner by ETS2 overexpression, with greater enrichment of every pathway when more ETS2 mRNA was transfected (Fig. 3c ). This shows that ETS2 is both necessary and sufficient for inflammatory responses in human macrophages, consistent with being a central regulator of effector functions, with dysregulation directly linked to disease.
a , Experimental schematic for studying the effects of ETS2 overexpression. The diagram was created using BioRender. b , ETS2 mRNA levels in transfected ( n = 8) or untransfected (from a separate experiment) macrophages. Data are mean ± s.e.m. CPM, counts per million. c , fGSEA analysis of differentially expressed genes between ETS2 -overexpressing and control macrophages. Results shown for pathways downregulated by ETS2 disruption. The dot size denotes the unadjusted P value (two-sided), the colour denotes NES and the border colour denotes the quantity of transfected mRNA. d , fGSEA analysis of a Crohn’s disease intestinal macrophage signature in ETS2 -overexpressing macrophages (versus control). FDR P -value, two-sided (top). Heat map of the relative expression of leading-edge genes after ETS2 overexpression (500 ng mRNA; bottom). e , Enrichment of macrophage signatures from patients with the indicated diseases in ETS2 -overexpressing macrophages (versus control). The colour denotes the disease category, the numbers denote the NES and the dashed line denotes FDR = 0.05. The Crohn’s disease signature is from a different study to that shown in d . AS, ankylosing spondylitis. f , SNPsea analysis of genes tagged by 241 IBD SNPs within ETS2 -regulated genes (red) and known IBD pathways (black). Significant pathways (Bonferroni-corrected P < 0.05) are indicated by hash symbols (#).
ETS2 has a key pathogenic role in IBD
To test whether ETS2 contributes to macrophage phenotypes in disease, we compared the effects of overexpressing ETS2 in resting macrophages with a single-cell RNA-seq (scRNA-seq) signature from intestinal macrophages in Crohn’s disease 31 . ETS2 overexpression induced a transcriptional state that closely resembled disease macrophages, with core (leading edge) enrichment of most signature genes, including several therapeutic targets (Fig. 3d ). Similar enrichment was observed with myeloid signatures from other chr21q22-associated diseases and, to a lesser extent, from active bacterial infection, but not for signatures from influenza and tumour macrophages, suggesting that ETS2 was not simply inducing generic activation (Fig. 3e ).
Given the central role of ETS2 in inflammatory macrophages and the importance of these cells in disease, we hypothesized that other genetic associations would also implicate this pathway. A major goal of GWAS was to identify disease pathways, but this has proven to be challenging due to a paucity of confidently identified causal genes and variants 9 . To determine whether the macrophage ETS2 pathway was enriched for disease genetics, we focused on IBD as this has more GWAS hits than any other chr21q22-associated disease. Encouragingly, a network of 33 IBD-associated genes in intestinal mucosa was previously found to be enriched for predicted ETS2 motifs 32 . Examining the genes that were consistently downregulated in ETS2 -edited macrophages (adjusted P ( P adj ) < 0.05 for both gRNAs), we identified over 20 IBD-risk-associated genes, including many thought to be causal at their respective loci 3 , 33 (Extended Data Table 1 ). These included genes that are known to affect macrophage biology (such as SP140 , LACC1 , CCL2 , CARD9 , CXCL5 , TLR4 , SLAMF8 and FCGR2A ) and some that are highly expressed in macrophages but not linked to specific pathways (such as ADCY7 , PTPRC , TAGAP , PTAFR and PDLIM5 ). A polygenic risk score comprising these variants associated with features of more severe IBD across 18,249 patients, including earlier disease onset, increased the need for surgery, and stricturing or fistulating complications in Crohn’s disease (Extended Data Fig. 6a–h ). To better test the enrichment of IBD GWAS hits in ETS2-mediated inflammation, and compare this with known disease pathways, we used SNPsea 34 —a method to identify pathways affected by disease loci. In total, 241 IBD loci were tested for enrichment in 7,658 GO Biological Pathways and 2 overlapping lists of ETS2-regulated genes (either those downregulated by ETS2 disruption or upregulated by ETS2 overexpression). Statistical significance was computed using 5 million matched null SNP sets, and pathways implicated by IBD genetics were extracted for comparison. Notably, IBD-associated SNPs were more significantly enriched in the macrophage ETS2 pathway than in many IBD pathways, with not a single null SNP set being more enriched in either ETS2-regulated gene list (Fig. 3f and Extended Data Fig. 6i ). SNPs associated with primary sclerosing cholangitis (PSC), ankylosing spondylitis and Takayasu’s arteritis were also enriched in ETS2-target genes (Extended Data Fig. 6j ). Collectively, this suggests that macrophage ETS2 signalling has a central role in multiple inflammatory diseases.
ETS2 has distinct inflammatory effects
We next investigated how ETS2 might control such diverse macrophage functions. Studying ETS2 biology is challenging because no ChIP-grade antibodies exist, precluding direct identification of its transcriptional targets. We therefore first used a guilt-by-association approach to identify genes that were co-expressed with ETS2 across 67 human macrophage activation conditions (comprising 28 stimuli and various durations of exposure) 16 . This identified PFKFB3 —encoding the rate-limiting enzyme of glycolysis—as the most highly co-expressed gene, with HIF1A also highly co-expressed (Fig. 4a ). Together, these genes facilitate a ‘glycolytic switch’ that is required for myeloid inflammatory responses 35 . We therefore hypothesized that ETS2 might control inflammation through metabolic reprogramming—a possibility supported by OXPHOS genes being negatively correlated with ETS2 (Fig. 4a ) and upregulated after ETS2 disruption (Fig. 2f ). To assess the metabolic consequences of disrupting ETS2 , we quantified label incorporation from 13 C-glucose in edited and unedited TPP macrophages using gas chromatography coupled with mass spectrometry (GC–MS). Widespread modest reductions in labelled and total glucose metabolites were detected after ETS2 disruption (Fig. 4b and Extended Data Fig. 7a–c ). This affected both glycolytic and tricarboxylic acid (TCA) cycle metabolites, with significant reductions in lactate, a hallmark of anaerobic glycolysis, and succinate, a key inflammatory metabolite 36 . These results are consistent with glycolytic suppression, with reductions in TCA metabolites being due to reduced flux into TCA and increased consumption by mitochondrial OXPHOS 37 . To determine whether metabolic changes accounted for ETS2-mediated inflammatory effects, we treated ETS2 -edited macrophages with roxadustat—a HIF1α stabilizer that promotes glycolysis. This had the predicted effect on glycolysis and OXPHOS genes, but did not rescue the effects of ETS2 disruption, either transcriptionally or functionally (Fig. 4c and Extended Data Fig. 7d,e ). Thus, while disrupting ETS2 impairs macrophage glycometabolism, this does not fully explain the differences in inflammation.
a , Genes co-expressed with ETS2 across 67 monocyte/macrophage activation conditions. The dotted lines denote FDR-adjusted P < 0.05. b , The effect of ETS2 disruption on glucose metabolism. The colour denotes median log 2 -transformed fold change in label incorporation from 13 C-glucose in ETS2 -edited versus unedited cells. The bold black border denotes P < 0.05 (Wilcoxon matched-pairs, two-sided). n = 6. Sec., secreted. c , fGSEA analysis of differentially expressed genes between ETS2 -edited and unedited macrophages that were treated with roxadustat or vehicle. Results shown for pathways downregulated by ETS2 disruption. d , Enrichment heat maps of macrophage ETS2 CUT&RUN peaks (IDR cut-off 0.01, n = 2) in 4 kb peak-centred regions from ATAC–seq (accessible chromatin), H3K4me3 ChIP–seq (active promoters) and H3K27ac ChIP–seq (active regulatory elements). e , Functional annotations of ETS2-binding sites (using gene coordinates and TPP macrophage H3K27ac ChIP–seq data). f , ETS2 motif enrichment in CUT&RUN peaks (hypergeometric P value, two-sided). g , ETS2 binding, chromatin accessibility (ATAC–seq) and regulatory activity (H3K27ac) at selected loci. h , Intersections between genes with ETS2 peaks in their core promoters or cis -regulatory elements and genes upregulated (Up) or downregulated (Dn) after ETS2 editing (KO) or overexpression (OE). The vertical bars denote the size of overlap for lists indicated by connected dots in the bottom panel. The horizontal bars denote the percentage of gene list within intersections. i , ETS2 binding, PU.1 binding, chromatin accessibility and enhancer activity at chr21q22. Predicted ETS2-binding sites (red) and PU.1-binding sites (purple) shown below. The dashed line is positioned at rs2836882.
We therefore revisited whether we could directly identify ETS2-target genes. As ChIP–seq involves steps that can alter protein epitopes and prevent antibody binding (such as fixation) we tested whether any anti-ETS2 antibodies might work for cleavage under targets and release using nuclease (CUT&RUN), which does not require these steps. One antibody identified multiple significantly enriched genomic regions (peaks), of which 6,560 were reproducibly detected across two biological replicates with acceptable quality metrics 38 (Fig. 4d ). These peaks were mostly located in active regulatory regions (90% in promoters or enhancers; Fig. 4d,e ) and were highly enriched for both a canonical ETS2 motif (4.02-fold versus global controls; Fig. 4f ) and for motifs of known ETS2 interactors, including FOS, JUN and NF-κB 39 (Extended Data Fig. 7f ). After combining the biological replicates to improve peak detection, we identified ETS2 binding at genes involved in multiple inflammatory functions, including NCF4 (ROS production), NLRP3 (inflammasome activation) and TLR4 (bacterial pattern recognition) (Fig. 4g ). Overall, 48.3% (754 out of 1,560) of genes dysregulated after ETS2 disruption and 50.3% (1,078 out of 2,153) of genes dysregulated after ETS2 overexpression contained an ETS2-binding peak within their core promoter or cis -regulatory elements (Fig. 4h ). Notably, ETS2 targets included HIF1A , PFKFB3 and other glycolytic genes (such as GPI , HK2 and HK3 ), consistent with the observed metabolic changes being directly induced as part of this complex inflammatory programme. Notably, we also detected ETS2 binding at the chr21q22 enhancer (Fig. 4i ). This is consistent with reports that PU.1 and ETS2 can interact synergistically 40 , and suggests that ETS2 might contribute to the activity of its own enhancer. Indeed, manipulating ETS2 expression altered enhancer activity in a manner consistent with positive autoregulation (Extended Data Fig. 7g–i ). Together, these data implicate ETS2 as a central regulator of monocyte and macrophage inflammatory responses that is able to direct a multifaceted effector programme and create a metabolic environment that is permissive for inflammation.
Targeting the ETS2 pathway in disease
To assess how ETS2 affects macrophage heterogeneity in diseased tissue, and whether this could be targeted therapeutically, we examined intestinal scRNA-seq data from patients with Crohn’s disease and healthy control individuals 41 . Within myeloid cells, seven clusters were detected and identified using established markers and/or previous literature (Fig. 5a,b ). Inflammatory macrophages (cluster 1, expressing CD209, CCL4, IL1B and FCGR3A) and inflammatory monocytes (cluster 2, expressing S100A8/A9, TREM1, CD14 and MMP9) were expanded in disease, as previously described 42 , and expressed ETS2 and ETS2-regulated genes more highly than other clusters, including tissue-resident macrophages (cluster 0, expressing C1QA, C1QB, FTL and CD63) and conventional dendritic cells (cluster 5, expressing CLEC9A, CADM1 and XCR1) (Fig. 5a,b and Extended Data Fig. 8a ). Using spatial transcriptomics, a similar increase in inflammatory macrophages was observed in PSC liver tissue, with these cells being closely apposed to cholangiocytes—the main target of pathology (Fig. 5c–e ). Notably, expression of ETS2-regulated genes was higher the closer macrophages were to cholangiocytes (Fig. 5f and Extended Data Fig. 8b ). Indeed, using bulk RNA-seq data, we found that the transcriptional footprint of ETS2 was detectable in affected tissues from multiple chr21q22-associated diseases (Extended Data Fig. 8c ).
a , Myeloid cell clusters in intestinal scRNA-seq from Crohn’s disease and health (top). Middle, scaled expression of ETS2-regulated genes (downregulated by ETS2 disruption). Bottom, the source of cells (disease or health). b , Scaled expression of selected genes. c , Spatial transcriptomics of PSC and healthy liver. n = 4. The images show representative fields of view (0.51 mm × 0.51 mm) with cell segmentation and semisupervised clustering. The main key (left and middle below images) denotes InSituType cell types; clusters a–e (far right key) are unannotated cell populations. Hep., hepatocyte; LSECs, liver sinusoidal endothelial cells; non-inflamm. macs, non-inflammatory macrophages. d , The number of macrophages within the indicated distances of cholangiocytes. e , The distance from cholangiocytes to the nearest macrophage. Data are shown as Tukey box and whisker plots. Statistical analysis was performed using two-tailed Mann–Whitney U -tests. Data in d and e are from 10,532 PSC and 13,322 control cholangiocytes. f , Scaled expression of ETS2-regulated genes in 21,067 PSC macrophages at defined distances from cholangiocytes (excluding genes used to define macrophage subsets). g , Classes of drugs that phenocopy ETS2 disruption (from the NIH LINCS database). h , fGSEA results for NIH LINCS drug signatures. Significant MEK inhibitor signatures are coloured by molecule. i , The log 2 [fold change] of differentially expressed genes after chr21q22 enhancer deletion, plotted against their fold change after MEK inhibition. The percentages indicate the proportion of upregulated (red) and downregulated genes (blue). The coloured points (blue or red) were differentially expressed after MEK inhibition (FDR < 0.1). j , fGSEA of differentially expressed genes between MEK-inhibitor-treated and control TPP macrophages. Results are shown for pathways downregulated by ETS2 disruption. The dot size denotes the unadjusted P value (two-sided) and the colour denotes the NES. k , IBD biopsy cytokine release with PD-0325901, infliximab or vehicle control. l , GSVA enrichment scores for chr21q22-downregulated genes in IBD biopsies after MEK inhibition. m , GSVA enrichment scores of a biopsy-derived molecular inflammation score (bMIS). Data are mean ± 95% CI ( f and l ) and mean ± s.e.m. ( k and m ). Statistical analysis was performed using two-sided paired t -tests. n = 10 ( k ), n = 9 ( l ). ** P < 0.01, *** P < 0.001, **** P < 0.0001.
We next examined whether this pathway could be targeted pharmacologically. Specific ETS2 inhibitors do not exist and structural analyses indicate that there is no obvious allosteric inhibitory mechanism 43 . We therefore used the NIH LINCS database to identify drugs that might modulate ETS2 activity 7 . This contains over 30,000 differentially expressed gene lists from cell lines exposed to around 6,000 small molecules. Using fGSEA, 906 signatures mimicked the effect of disrupting ETS2 ( P adj < 0.05), including several approved IBD therapies. The largest class of drugs was MEK inhibitors (Fig. 5g ), which are licensed for non-inflammatory human diseases (such as neurofibromatosis). This result was not due to a single compound, but rather a class effect with multiple MEK1/2 inhibitors downregulating ETS2-target genes (Fig. 5h ). This made biological sense, as MEK1/2, together with several other targets identified, are known regulators of ETS-family transcription factors (Fig. 5g ). Some of these compounds have shown benefit in animal colitis models 44 , although this is often a poor indicator of clinical efficacy, as several IBD treatments are ineffective in mice and many compounds that improve mouse models are ineffective in humans 45 . To test whether MEK inhibition abrogates ETS2-driven inflammation in human macrophages, we treated TPP macrophages with PD-0325901, a selective non-ATP competitive MEK inhibitor. Potent anti-inflammatory activity was observed that phenocopied the effects of disrupting ETS2 or the chr21q22 enhancer (Fig. 5i,j and Extended Data Fig. 9a–c ). To further assess the therapeutic potential, we cultured intestinal biopsies from active, untreated IBD with either a MEK inhibitor or a negative or positive control ( Methods ). MEK inhibition reduced inflammatory cytokine release to similar levels as infliximab (an anti-TNF antibody that is widely used for IBD; Fig. 5k ). Moreover, ETS2-regulated gene expression was reduced (Fig. 5l and Extended Data Fig. 9d ) and there was improvement in a transcriptional inflammation score 46 (Fig. 5m ). Together, these data show that targeting an upstream regulator of ETS2 can abrogate pathological inflammation in a chr21q22-associated disease, and may be useful therapeutically.
Arguably the greatest challenge in modern genetics is to translate the success of GWAS into a better understanding of disease. Here, by studying a pleiotropic disease locus, we identify a central regulator of human macrophage inflammation and a pathogenic pathway that is potentially druggable. These findings also provide clues to the gene–environment interactions at this locus, highlighting a potential role for ETS2 in macrophage responses to bacteria. This would provide a balancing selection pressure that might explain why the risk allele remains so common (frequency of around 75% in Europeans and >90% in Africans) despite first being detected in archaic humans over 500,000 years ago (Extended Data Fig. 10 ).
Although ETS2 was reported to have pro-inflammatory effects on individual genes 24 , 25 , the full extent of its inflammatory programme—with effects on ROS production, phagocytosis, glycometabolism and macrophage activation—was unclear. Moreover, without direct proof of ETS2 targets, nor studies in primary human cells, it was difficult to reconcile reports of anti-inflammatory effects at other genes 23 , 26 . By systematically characterizing the effects of ETS2 disruption and overexpression in human macrophages, we identify an essential role in inflammation, delineate the mechanisms involved and show how ETS2 can induce pathogenic macrophage phenotypes. Increased ETS2 expression may also contribute to other human pathology. For example, Down’s syndrome (trisomy 21) was recently described as a cytokinopathy 47 , with basal increases in multiple inflammatory cytokines, including several ETS2 targets (such as IL-1β, TNF and IL-6). Whether the additional copy of ETS2 contributes to this phenotype is unknown, but warrants further study.
Blocking individual cytokines is a common treatment strategy in inflammatory disease 14 , but emerging evidence suggests that targeting several cytokines at once may be a better approach 48 . Blocking ETS2 signalling through MEK1/2 inhibition affects multiple cytokines, including TNF and IL-23, which are targets of existing therapies, and IL-1β, which is linked to treatment resistance 49 and not directly modulated by other small molecules (such as JAK inhibitors). However, long-term MEK inhibitor use may not be ideal owing to the physiological roles of MEK in other tissues, with multiple side-effects having been reported 50 . Targeting ETS2 directly—for example, through PROTACs—or selectively delivering MEK inhibitors to macrophages through antibody–drug conjugates could overcome this toxicity, and provide a safer means of blocking ETS2-driven inflammation.
In summary, using an intergenic GWAS hit as a starting point, we have identified a druggable pathway that is both necessary and sufficient for human macrophage inflammation. Moreover, we show how genetic dysregulation of this pathway—through perturbation of pioneer factor binding at a critical long-range enhancer—predisposes to multiple diseases. This highlights the considerable, yet largely untapped, opportunity to resolve disease biology from non-coding genetic associations.
Analysis of existing data relating to chr21q22
IBD GWAS summary statistics 3 were used to perform multiple causal variant fine-mapping using susieR 51 , with reference minor allele and LD information calculated from 503 European samples from 1000 Genomes phase 3 (ref. 52 ). All R analyses used v.4.2.1. Palindromic SNPs (A/T or C/G) and any SNPs that did not match by position or alleles were pruned before imputation using the ssimp equations reimplemented in R. This did not affect any candidate SNP at chr21q22. SuSiE fine-mapping results were obtained for ETS2 (identifier ENSG00000157557 or ILMN_1720158) in monocyte/macrophage datasets from the eQTL Catalogue 53 . Co-localization analyses were performed comparing the chr21q22 IBD association with summary statistics from other chr21q22-associated diseases 3 , 4 , 5 , 6 and monocyte/macrophage eQTLs 54 , 55 , 56 , 57 , 58 to determine whether there was a shared genetic basis for these different associations. This was performed using coloc (v.5.2.0) 59 using a posterior probability of H4 (PP.H4.abf) > 0.5 to call co-localization.
Raw H3K27ac ChIP–seq data from primary human immune cells were downloaded from Gene Expression Omnibus (GEO series GSE18927 and GSE96014 ) and processed as described previously 60 (code provided in the ‘Code availability’ section).
Processed promoter-capture Hi-C data 61 from 17 primary immune cell types were downloaded from OSF ( https://osf.io/u8tzp ) and cell type CHiCAGO scores for chr21q22-interacting regions were extracted.
Monocyte-derived macrophage differentiation
Leukocyte cones from healthy donors were obtained from NHS Blood and Transplant (Cambridge Blood Donor Centre, Colindale Blood Centre or Tooting Blood Donor Centre). Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation (Histopaque 1077, Sigma-Aldrich) and monocytes were positively selected using CD14 Microbeads (Miltenyi Biotec). Macrophage differentiation was performed either using conditions that model chronic inflammation (TPP) 16 : 3 days GM-CSF (50 ng ml −1 , Peprotech) followed by 3 days GM-CSF, TNF (50 ng ml −1 , Peprotech), PGE 2 (1 μg ml −1 , Sigma-Aldrich) and Pam 3 CSK4 (1 μg ml −1 , Invivogen); or, to produce resting (M0) macrophages: 6 days M-CSF (50 ng ml −1 , Peprotech). All cultures were performed at 37 °C under 5% CO 2 in antibiotic-free RPMI1640 medium containing 10% FBS, GlutaMax and MEM non-essential amino acids (all Thermo Fisher Scientific). Cells were detached using Accutase (BioLegend).
Identifying a model of chronic inflammatory macrophages
Human monocyte/macrophage gene expression data files ( n = 314) relating to 28 different stimuli with multiple durations of exposure (collectively comprising 67 different activation conditions) were downloaded from the GEO ( GSE47189 ) and quantile normalized. Data from biological replicates were summarized to the median value for every gene. Gene set variation analysis 62 (using the GSVA package in R) was performed to identify the activation condition that most closely resembled CD14 + monocytes/macrophages from active IBD using disease-associated lists of differentially expressed genes 63 .
CRISPR–Cas9 editing of primary human monocytes
gRNA sequences were designed using CRISPick and synthesized by IDT (Supplementary Table 3 ). Alt-R CRISPR–Cas9 negative control crRNA 1 (IDT) was used as a non-targeting control. Cas9–gRNA ribonucleoproteins were assembled as described previously 60 and nucleofected into 5 × 10 6 monocytes in 100 μl nucleofection buffer (Human Monocyte Nucleofection Kit, Lonza) using a Nucleofector 2b (Lonza, program Y-001). After nucleofection, monocytes were immediately transferred into 5 ml of prewarmed culture medium in a six-well plate, and differentiated into macrophages under TPP conditions. The editing efficiency was quantified by PCR amplification of the target region in extracted DNA. All primer sequences are provided in Supplementary Table 3 . The editing efficiency at the chr21q22 locus was measured by quantification of amplified fragments (2100 Bioanalyzer, Agilent) as previously described 60 . The editing efficiency for individual gRNAs was assessed using the Inference of CRISPR Edits tool 64 (ICE, Synthego).
PrimeFlow RNA assay
RNA abundance was quantified by PrimeFlow (Thermo Fisher Scientific) in chr21q22-edited and unedited (NTC) cells on days 0, 3, 4, 5 and 6 of TPP differentiation. Target probes specific for ETS2 (Alexa Fluor 647), BRWD1 (Alexa Fluor 568) and PSMG1 (Alexa Fluor 568) were used according to the manufacturer’s instructions. Data were collected using FACS Diva software and analysed using FlowJo v10 (BD Biosciences).
Overlapping oligonucleotides containing 114 nucleotides of genomic sequence were designed to tile the region containing chr21q22 candidate SNPs (99% credible set) at 50 bp intervals. Six technical replicates were designed for every genomic sequence, each tagged by a unique 11-nucleotide barcode. Additional oligonucleotides were included to test the expression-modulating effect of every candidate SNP in the 99% credible set. Allelic constructs were designed as described previously 60 and tagged by 30 unique 11-nucleotide barcodes. Positive and negative controls were included as described previously 60 . 170-nucleotide oligonucleotides were synthesized as part of a larger MPRA pool (Twist Biosciences) containing the 16-nucleotide universal primer site ACTGGCCGCTTCACTG, 114-nucleotide variable genomic sequence, KpnI and XbaI restriction sites (TGGACCTCTAGA), an 11-nucleotide barcode and the 17-nucleotide universal primer site AGATCGGAAGAGCGTCG. Cloning into the MPRA vector was performed as described previously 60 . A suitable promoter for the MPRA vector (RSV) was identified by testing promoter activities in TPP macrophages. The MPRA vector library was nucleofected into TPP macrophages (5 µg vector into 5 × 10 6 cells) in 100 μl nucleofection buffer (Human Macrophage Nucleofection Kit, Lonza) using a Nucleofector 2b (program Y-011). To ensure adequate barcode representation, a minimum of 2 × 10 7 cells was nucleofected for every donor ( n = 8). After 24 h, RNA was extracted and sequencing libraries were made from mRNA or DNA input vector as described previously 60 . Libraries were sequenced on the Illumina HiSeq2500 high-output flow-cell (50 bp, single-end reads). Data were demultiplexed and converted to FASTQ files using bcl2fastq and preprocessed as previously described using FastQC 60 . To identify regions of enhancer activity, a paired t -test was first performed to identify genomic sequences that enhanced transcription and a sliding-window analysis (300 bp window) was then performed using the les package in R. Expression-modulating variants were identified using QuASAR-MPRA 65 , as described previously 60 .
Publicly available PU.1 ChIP–seq datasets from human macrophages were downloaded from GEO, and BAM files were examined (IGV genome browser) to identify heterozygous samples (that is, files containing both A and G allele reads at chr21:40466570; hg19). Two suitable samples were identified ( GSM1681423 and GSM1681429 ) and used for a Bayesian analysis of allelic imbalances in PU.1 binding (implemented in the BaalChIP package 66 in R) with correction for biases introduced by overdispersion and biases towards the reference allele.
Allele-specific PU.1 ChIP genotyping
A 100 ml blood sample was taken from five healthy rs2836882 heterozygotes (assessed by Taqman genotyping; Thermo Fisher Scientific). All of the participants provided written informed consent. Ethical approval was provided by the London–Brent Regional Ethics Committee (21/LO/0682). Monocytes were isolated from PBMCs using CD14 Microbeads (Miltenyi Biotec) and differentiated into inflammatory macrophages using TPP conditions 16 . After differentiation, macrophages were detached and cross-linked for 10 min in fresh medium containing 1% formaldehyde. Cross-linking was quenched with glycine (final concentration 0.125 M, 5 min). Nucleus preparation and shearing were performed as described previously 60 with 10 cycles sonication (30 s on/30 s off, Bioruptor Pico, Diagenode). PU.1 was immunoprecipitated overnight at 4 °C using a polyclonal anti-PU.1 antibody (1:25; Cell Signaling) using the SimpleChIP Plus kit (Cell Signaling). The ratio of rs2836882 alleles in the PU.1-bound DNA was quantified in duplicate by TaqMan genotyping (assay C 2601507_20). A standard curve was generated using fixed ratios of geneblocks containing either the risk or non-risk allele (200-nucleotide genomic sequence centred on rs2836882; Genewiz).
PU.1 MPRA ChIP–seq
The MPRA vector library was transfected into TPP macrophages from six healthy donors. Assessment of PU.1 binding to SNP alleles was performed as described previously 60 , with minimal sonication (to remove contaminants without chromatin shearing). Immunoprecipitation was performed as described above. Sequencing libraries were prepared as for MPRA and sequenced on the MiSeq system (50 bp, single-end reads).
ATAC–seq analysis
ATAC–seq in ETS2 -edited and unedited TPP macrophages was performed using the Omni-ATAC protocol 67 with the following modifications: the cell number was increased to 75,000 cells; the cell lysis time was increased to 5 min; the volume of Tn5 transposase in the transposition mixture was doubled; and the duration of the transposition step was extended to 40 min. Amplified libraries were cleaned using AMPure XP beads (Beckman Coulter) and sequenced on the NovaSeq6000 system (100 bp paired-end reads). Data were processed as described previously 68 . Differential ATAC–seq analysis was performed as described previously using edgeR and TMM normalization 69 . Allele-specific ATAC–seq analysis was performed in 16 heterozygous monocyte datasets from healthy controls and patients with ankylosing spondylitis 70 and in 2 deeply sequenced heterozygous TPP macrophage samples. For these analyses, sequencing reads at rs2836882 were extracted from preprocessed data using splitSNP ( https://github.com/astatham/splitSNP ) (see the ‘Code availability’ section).
H3K27ac ChIP–seq
H3K27ac ChIP–seq was performed as described previously 60 using an anti-H3K27ac antibody (1:250, Abcam) or an isotype control (1:500, rabbit IgG, Abcam). Sequencing libraries from TPP macrophages from major and minor allele homozygotes at rs2836882 (identified through the NIHR BioResource, n = 4) were sequenced on the HiSeq4000 system (50 bp, single-end reads). Sequencing libraries from ETS2 -edited and unedited TPP macrophages ( n = 3) or resting M0 macrophages overexpressing ETS2 or control mRNA ( n = 3) were sequenced on the NovaSeq6000 system (100 bp, paired-end reads). Raw data were processed, quality controlled and analysed as described previously using the Burrows-Wheeler Aligner 60 . Unpaired differential ChIP–seq analysis, to compare rs2836882 genotypes, was performed using MEDIPS 71 by dividing the 560 kb region around rs2836882 (chr21:40150000–40710000, hg19) into 5 kb bins. Paired differential ChIP–seq analyses, to assess the effect of perturbing ETS2 expression on enhancer activity, were performed using edgeR with TMM normalization 69 , 72 (with donor as covariate). Genome-wide analyses used consensus MACS2 peaks. Superenhancer activity was evaluated using Rank-Ordering of Super-Enhancers (ROSE). Chr21q22-based analyses used the enhancer coordinates that exhibited allele-specific activity (chr21:40465000–40470000, hg19). Code is provided for all data analysis (see the ‘Code availability’ section).
Assays of macrophage effector functions
Flow cytometry.
Expression of myeloid markers was assessed using flow cytometry (BD LSRFortessa X-20) with the following panel: CD11b PE/Dazzle 594 (BioLegend), CD14 evolve605 (Thermo Fisher Scientific), CD16 PerCP (BioLegend), CD68 FITC (BioLegend), Live/Dead Fixable Aqua Dead Cell Stain (Thermo Fisher Scientific) and Fc Receptor Blocking Reagent (Miltenyi). All antibodies were used at a dilution of 1:40; Live/Dead stained was used at 1:400 dilution. Data were collected using FACS Diva and analysed using FlowJo v.10 (BD Biosciences).
Cytokine quantification
Supernatants were collected on day 6 of TPP macrophage culture and frozen. Cytokine concentrations were quantified in duplicate by electrochemiluminescence using assays (Meso Scale Diagnostics, DISCOVERY WORKBENCH v.4.0).
Phagocytosis
Phagocytosis was assessed using fluorescently labelled Zymosan particles (Green Zymosan, Abcam) according to the manufacturer’s instructions. Cells were seeded at 10 5 cells per well in 96-well round-bottom plates. Cytochalasin D (10 μg ml −1 , Thermo Fisher Scientific) was used as a negative control. Phagocytosis was quantified by flow cytometry, and a phagocytosis index was calculated (the proportion of positive cells multiplied by their mean fluorescence intensity).

Extracellular ROS production
Extracellular ROS production was quantified using the Diogenes Enhanced Superoxide Detection Kit (National Diagnostics) according to the manufacturer’s protocol. Cells were seeded at a density of 10 5 cells per well and prestimulated with PMA (200 ng ml −1 , Sigma-Aldrich).
Western blotting
Western blotting was performed as described previously 73 using the following primary antibodies: mouse anti-gp91phox (1:2,000), mouse anti-p22phox (1:500; both Santa Cruz), rabbit anti-C17ORF62/EROS (1:1,000; Atlas), mouse anti-vinculin (Sigma-Aldrich). Loading controls were run on the same gel. Secondary antibodies were as follows: goat anti-rabbit IgG-horseradish or goat anti-mouse IgG-horseradish peroxidase (both 1:10,000; Jackson Immuno). Chemiluminescence was recorded on the ChemiDoc Touch imager (Bio-Rad) after incubation of the membrane with ECL (Thermo Fisher Scientific) or SuperSignal West Pico PLUS (Thermo Fisher Scientific) reagent. Densitometry analysis was performed using ImageJ.
RNA-seq analysis
RNA was isolated from macrophage lysates (AllPrep DNA/RNA Micro Kit, Qiagen) and sequencing libraries were prepared from 10 ng RNA using the SMARTer Stranded Total RNA-Seq Kit v2 Pico Input Mammalian (Takara) according to the manufacturer’s instructions. Libraries were sequenced on either the NextSeq 2000 (50 bp paired-end reads: CRISPR, roxadustat and PD-0325901 experiments) or NovaSeq 6000 (100 bp paired-end reads: overexpression experiments) system and preprocessed using MultiQC. Reads were trimmed using Trim Galore (Phred score 24) and filtered to remove reads <20 bp. Ribosomal reads (mapping to human ribosomal DNA complete repeating unit; GenBank: U13369 .1 ) were removed using BBSplit ( https://sourceforge.net/projects/bbmap/ ). Reads were aligned to the human genome (hg38) using HISAT2 (ref. 74 ) and converted to BAM files, sorted and indexed using SAMtools 75 . Gene read counts were obtained using the featureCounts program 76 from Rsubread using the GTF annotation file for GRCh38 (v.102). Differential expression analysis was performed in R using limma 77 with voom transformation and including donor as a covariate. Differential expression results are shown in Supplementary Tables 1 and 2 .
GSEA was performed using fGSEA 78 in R with differentially expressed gene lists ranked by t -statistic. Gene sets were obtained from GO Biological Pathways (MSigDB), experimentally derived based on differential expression analysis or sourced from published literature 31 , 42 , 70 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 . Specific details of disease macrophage signatures (Fig. 3f ) are provided as source data. GO pathways shown in Figs. 2 – 5 are as follows: GO:0002274, GO:0042116, GO:0097529, GO:0006909, GO:0071706, GO:0032732, GO:0032755, GO:0032757, GO:2000379, GO:0009060, GO:0006119 and GO:0045649. Statistical significance was calculated using the adaptive multilevel split Monte Carlo method.
IBD BioResource recall-by-genotype study
IBD patients who were rs2836882 major or minor allele homozygotes ( n = 11 of each) were identified through the NIHR IBD BioResource. Patients were matched for age, sex, treatment and disease activity, and all provided written informed consent. Ethical approval was provided by the London–Brent Regional Ethics Committee (21/LO/0682). A 50 ml blood sample was taken from all patients and M0 monocyte-derived macrophages were generated as described. After 6 days, cells were collected, lysed and RNA was extracted. Quantitative PCR analysis of a panel of ETS2-regulated genes was performed in triplicate after reverse transcription (SuperScript IV VILO, Thermo Fisher Scientific) using the Quantifast SYBR Green PCR kit (Qiagen) on the Roche LightCycler 480. Primer sequences are provided in Supplementary Table 3 and PPIA and RPLP0 were used as housekeeping genes. Expression values for each gene ( \({2}^{\Delta {c}_{T}}\) ) were scaled to a minimum 0 and maximum 1 to enable intergene comparison.
In vitro transcription
The cDNA sequence for ETS2 (NCBI Reference Sequence Database NM005239.5 ) preceded by a Kozak sequence was synthesized and cloned into a TOPO vector. This was linearized and a PCR amplicon generated, adding a T7 promoter and an AG initiation sequence (Phusion, NEB). A reverse complement (control) amplicon was also generated. These amplicons were used as templates for in vitro transcription using the HiScribe T7 mRNA Kit with CleanCap Reagent AG kit (NEB) according to the manufacturer’s instructions, but with substitution of N1-methyl-pseudouridine for uridine and methylcytidine for cytidine (both Stratech) to minimize non-specific cellular activation by the transfected mRNA. mRNA was purified using the MEGAclear Kit (Thermo Fisher Scientific) and polyadenylated using an Escherichia coli poly(A) polymerase (NEB) before further clean-up (MEGAclear), quantification and analysis of the product size (NorthernMax-Gly gel, Thermo Fisher Scientific). For optimizing overexpression conditions, GFP mRNA was produced using the same method. All primer sequences are provided in Supplementary Table 3 .
mRNA overexpression
Lipofectamine MessengerMAX (Thermo Fisher Scientific) was diluted in Opti-MEM (1:75 v/v), vortexed and incubated at room temperature for 10 min. IVT mRNA was then diluted in a fixed volume of Opti-MEM (112.5 µl per transfection), mixed with an equal volume of diluted Lipofectamine MessengerMAX and incubated for a further 5 min at room temperature. The transfection mix was then added dropwise to 2.5 × 10 6 M0 macrophages (precultured for 6 days in a six-well plate in antibiotic-free RPMI1640 macrophage medium containing M-CSF (50 ng ml −1 , Peprotech), with medium change on day 3). For GFP overexpression, cells were detached using Accutase 18 h after transfection and GFP expression was measured using flow cytometry. For ETS2 /control overexpression, either 250 ng or 500 ng mRNA was transfected and low-dose LPS (0.5 ng ml −1 ) was added 18 h after transfection, and cells were detached using Accutase 6 h later. Representative ETS2 expression in untransfected macrophages was obtained from previous data ( GSE193336 ). Differential H3K27ac ChIP–seq analysis in ETS2 -overexpressing macrophages was performed using 500 ng RNA transfection (see the ‘Code availability’ section).
Plink1.9 ( https://www.cog-genomics.org/plink/1.9/ ) was used to calculate a polygenic risk score (PRS) for patients in the IBD BioResource using 22 ETS2-regulated IBD-associated SNPs ( β coefficients from a previous study 3 ). Linear regression was used to compare PRSs with age at diagnosis, and logistic regression to estimate the effect of PRSs on IBD subphenotypes, including anti-TNF primary non-response (PNR), CD behaviour (B1 versus B2/B3), perianal disease and surgery. For variables with more than two levels (for example, CD location or UC location), ANOVA was used to investigate the relationship with PRS. For analyses of age at diagnosis, anti-TNF response and surgery, IBD diagnosis was included as a covariate.
Pathway analysis of 241 IBD-associated GWAS hits 3 was performed using SNPsea v.1.0.4 (ref. 34 ). In brief, linkage intervals were defined for every lead SNP based on the furthest correlated SNPs ( r 2 > 0.5 in 1000 Genomes, European population) and were extended to the nearest recombination hotspots with recombination rate > 3 cM per Mb. If no genes were present in this region, the linkage interval was extended up- and downstream by 500 kb (as long-range regulatory interactions usually occur within 1 Mb). Genes within linkage intervals were tested for enrichment within 7,660 pathways, comprising 7,658 GO Biological Pathways and two lists of ETS2-regulated genes (either those significantly downregulated after ETS2 disruption with gRNA1 or those significantly upregulated after ETS2 overexpression, based on a consensus list obtained from differential expression analysis including all samples and using donor and mRNA quantity as covariates). The analysis was performed using a single score mode: assuming that only one gene per linkage interval is associated with the pathway. A null distribution of scores for each pathway was performed by sampling identically sized random SNP sets matched on the number of linked genes (5,000,000 iterations). A permutation P value was calculated by comparing the score of the IBD-associated gene list with the null scores. An enrichment statistic was calculated using a standardized effect size for the IBD-associated score compared to the mean and s.e.m. of the null scores. Gene sets relating to the following IBD-associated pathways were extracted for comparison: NOD2 signalling (GO:0032495), integrin signalling (GO:0033627, GO:0033622), TNF signalling (GO:0033209, GO:0034612), intestinal epithelium (GO:0060729, GO:0030277), Th17 cells (GO:0072539, GO:0072538, GO:2000318), T cell activation (GO:0046631, GO:0002827), IL-10 signalling (GO:0032613, GO:0032733) and autophagy (GO:0061919, GO:0010506, GO:0010508, GO:1905037, GO:0010507). SNPs associated with PSC 5 , 87 , ankylosing spondylitis 4 , 87 , Takayasu arteritis 6 , 88 , 89 and schizophrenia 90 (as a negative control) were collated from the indicated studies and tested for enrichment in ETS2-regulated gene lists.
ETS2 co-expression
Genes co-expressed with ETS2 across 67 human monocyte/macrophage activation conditions (normalized data from GSE47189 ) were identified using the rcorr function in the Hmisc package in R.
13 C-glucose GC–MS
ETS2 -edited or unedited TPP macrophages were generated in triplicate for each donor and on day 6, the medium was removed, cells were washed with PBS, and new medium with labelled glucose was added. Labelled medium was as follows: RPMI1640 medium, no glucose (Thermo Fisher Scientific), 10% FBS (Thermo Fisher Scientific), GlutaMax (Thermo Fisher Scientific), 13 C-labelled glucose (Cambridge Isotype Laboratories). After 24 h, a timepoint selected from a time-course to establish steady-state conditions, the supernatants were snap-frozen and macrophages were detached by scraping. Macrophages were washed three times with ice-cold PBS, counted, resuspended in 600 µl ice-cold chloroform:methanol (2:1, v/v) and sonicated in a waterbath (3 times for 8 min). All of the extraction steps were performed at 4 °C as previously described 91 . The samples were analysed on the Agilent 7890B-7000C GC–MS system. Spitless injection (injection temperature of 270 °C) onto a DB-5MS (Agilent) was used, using helium as the carrier gas, in electron ionization mode. The initial oven temperature was 70 °C (2 min), followed by temperature gradients to 295 °C at 12.5 °C per min and to 320 °C at 25 °C per min (held for 3 min). The scan range was m / z 50–550. Data analysis was performed using in-house software MANIC (v.3.0), based on the software package GAVIN 92 . Label incorporation was calculated by subtracting the natural abundance of stable isotopes from the observed amounts. Total metabolite abundance was normalized to the internal standard (scyllo-inositol 91 ).
Roxadustat in TPP macrophages
ETS2- edited or unedited TPP macrophages were generated as described previously. On day 5 of culture, cells were detached (Accutase) and replated at a density of 10 5 cells per well in 96-well round-bottom plates in TPP medium containing roxadustat (FG-4592, 30 μM). After 12 h, cells were collected for functional assays and RNA-seq as described.
CUT&RUN
Precultured TPP macrophages were collected and processed immediately using the CUT&RUN Assay kit (Cell Signaling) according to the manufacturer’s instructions but omitting the use of ConA-coated beads. In brief, 5 × 10 5 cells per reaction were pelleted, washed and resuspended in antibody binding buffer. Cells were incubated with antibodies: anti-ETS2 (1:100, Thermo Fisher Scientific) or IgG control (1:20, Cell Signaling) for 2 h at 4 °C. After washing in digitonin buffer, cells were incubated with pA/G-MNase for 1 h at 4 °C. Cells were washed twice in digitonin buffer, resuspended in the same buffer and cooled for 5 min on ice. Calcium chloride was added to activate pA/G-MNase digestion (30 min, 4 °C) before the reaction was stopped and cells incubated at 37 °C for 10 min to release cleaved chromatin fragments. DNA was extracted from the supernatants using spin columns (Cell Signaling). Library preparation was performed using the NEBNext Ultra II DNA Library Prep Kit according to a protocol available at protocols.io ( https://doi.org/10.17504/protocols.io.bagaibse ). Size selection was performed using AMPure XP beads (Beckman Coulter) and the fragment size was assessed using the Agilent 2100 Bioanalyzer (High Sensitivity DNA kit). Indexed libraries were sequenced on the NovaSeq 6000 system (100 bp paired-end reads). Raw data were analysed using guidelines from the Henikoff laboratory 93 . In brief, paired-end reads were trimmed using Trim Galore and aligned to the human genome (GRCh37/hg19) using Bowtie2. BAM files were sorted, merged (technical and, where indicated, biological replicates), resorted and indexed using SAMtools. Picard was used to mark unmapped reads and SAMtools to remove these, re-sort and re-index. Bigwig files were created using the deepTools bamCoverage function. Processed data were initially analysed using the nf-core CUT&RUN pipeline v.3.0, using CPM normalization and default MACS2 parameters for peak calling. This analysis yielded acceptable quality metrics (including an average FRiP score of 0.23) but there was a high number of peaks with low fold enrichment (<4) over the control. More stringent parameters were therefore applied for peak calling (--qvalue 0.05 -f BAMPE --keep-dup all -B --nomodel) and we applied an irreproducible discovery rate (IDR; cut-off 0.001) to identify consistent peaks between replicates, implemented in the idr package in R (see the ‘Code availability’ section). Enrichment of binding motifs for ETS2 and other transcription factors expressed in TPP macrophages (cpm > 0.5) within consensus IDR peaks was calculated using TFmotifView 94 using global genomic controls. The overlap between consensus IDR peaks and the core promoter (−250bp to +35 bp from the transcription start site) and/or putative cis -regulatory elements of ETS2-regulated genes was assessed using differentially expressed gene lists after ETS2 disruption (gRNA1) or ETS2 overexpression (based on a consensus across mRNA doses, as described earlier). Putative cis -regulatory elements were defined as shared interactions (CHiCAGO score > 5) in monocyte and M0 and M1 macrophage samples from publicly available promoter-capture Hi-C data 61 . Predicted ETS2- and PU.1-binding sites were identified at the rs2836882 locus (chr21:40466150–40467450) using CisBP 95 (database 2.0, PWMs log odds motif model, default settings).
Intestinal scRNA-seq
Raw count data from colonic immune cells 41 (including healthy controls and Crohn’s disease) were downloaded from the Single Cell Portal ( https://singlecell.broadinstitute.org/single_cell ). Myeloid cell data were extracted for further analysis using the cell annotation provided. Raw data were preprocessed, normalized and variance-stabilized using Seurat (v.4) 96 . PCA and UMAP clustering was performed and clusters annotated using established markers and/or previous literature. Marker genes were identified using the FindAllMarkers function. Modular expression of ETS2-regulated genes (downregulated after ETS2 editing, gRNA1) was measured using the AddModuleScore function.
Spatial transcriptomics
Formalin-fixed paraffin-embedded sections (thickness, 5 μm) were cut from two PSC liver explants and two controls (healthy liver adjacent to tumour metastases), baked overnight at 60 °C and prepared for CosMx according to manufacturer’s instructions using 15 min target retrieval and 30 min protease digestion. Tissue samples were obtained through Tissue Access for Patient Benefit (TAP-B, part of the UCL-RFH Biobank) under research ethics approval: 16/WA/0289 (Wales Research Ethics Committee 4). One case and one control were included on each slide. The Human Universal Cell Characterization core panel (960 genes) was used, supplemented with 8 additional genes to improve identification of cells of interest: CD1D , EREG , ETS2 , FCN1 , G0S2 , LYVE1 , MAP2K1 , MT1G . Segmentation was performed using the CosMx Human Universal Cell Segmentation Kit (RNA), Human IO PanCK/CD45 Kit (RNA) and Human CD68 Marker, Ch5 (RNA). Fields of view (FOVs) were tiled across all available regions (221 control, 378 PSC) and cyclic fluorescence in situ hybridization was performed using the CosMx SMI (Nanostring) system. Data were preprocessed on the AtoMx Spatial Informatics Platform, with images segmented to obtain cell boundaries, transcripts assigned to single cells, and a transcript by cell count matrix was obtained 97 . Expression matrices, transcript coordinates, polygon coordinates, FOV coordinates and cell metadata were exported, and quality control, normalization and cell-typing were performed using InSituType 98 —an R package developed to extract all the information available in every cell’s expression profile. A semi-supervised strategy was used to phenotype cells, incorporating the Liver Human Cell Atlas reference matrix. Spatial analysis of macrophage phenotypes was performed according to proximity from cholangiocytes (anchor cell type). Radius and nearest-neighbour analyses were performed using PhenoptR ( https://akoyabio.github.io/phenoptr/ ) with macrophage distribution from cholangiocytes binned in 100 µm increments up to 500 µm. Nearest-neighbour analysis was performed to determine the distance from cholangiocytes to the nearest inflammatory and non-inflammatory macrophage and vice versa.
To generate overlay images, raw transcript and image (morphology 2D) data were exported from AtoMx. Overlays of selected ETS2-target genes ( CXCL8 , S100A9 , CCL2 , CCL5 ) and fluorescent morphology markers were generated using napari (v.0.4.17, https://napari.org/stable/index.html ) on representative FOVs: FOV287 (PSC with involved duct), FOV294 (PSC background liver) and FOV55 (healthy liver).
Chr21q22 disease datasets
Publicly available raw RNA-seq data from the affected tissues of chr21q22-associated diseases (and controls from the same experiment) were downloaded from the GEO: IBD macrophages ( GSE123141 ), PSC liver ( GSE159676 ), ankylosing spondylitis synovium ( GSE41038 ). Reads were trimmed, filtered and aligned as described earlier. For each disease dataset, a ranked list of genes was obtained by differential expression analysis between cases and controls using limma with voom transformation. For IBD macrophages, only IBD samples with active disease were included. fGSEA using ETS2-regulated gene lists was performed as described.
LINCS signatures
A total of 31,027 lists of downregulated genes after exposure of a cell line to a small molecule was obtained from the NIH LINCS database 7 (downloaded in January 2021). These were used as gene sets for fGSEA (as described) with a ranked list of genes obtained by differential expression analysis between ETS2 -edited and unedited TPP macrophages (gRNA1) using limma with voom transformation and donor as a covariate. Drug classes for gene sets with FDR-adjusted P < 0.05 were manually assigned on the basis of known mechanisms of action.
MEK inhibition in TPP macrophages
TPP macrophages were generated as described previously. On day 4 of culture, PD-0325901 (0.5 μM, Sigma-Aldrich) or vehicle (DMSO) was added. Cells were collected on day 6 and RNA was extracted and sequenced as described.
Colonic biopsy explant culture
During colonoscopy, intestinal mucosal biopsies (6 per donor) were collected from ten patients with IBD (seven patients with ulcerative colitis, three patients with Crohn’s disease). All had endoscopically active disease and were not receiving immunosuppressive or biologic therapies. All biopsies were collected from a single inflamed site. All patients provided written informed consent. Ethical approval was provided by the London–Brent Regional Ethics Committee (21/LO/0682). Biopsies were collected into Opti-MEM and, within 1 h, were weighed and placed in pairs onto a Transwell insert (Thermo Fisher Scientific), designed to create an air–liquid interface 99 , in a 24-well plate. Each well contained 1 ml medium and was supplemented with either DMSO (vehicle control), PD-0325901 (0.5 μM) or infliximab (10 μg ml −1 ; MSD). Medium was as follows: Opti-MEM I (Gibco), GlutaMax (Thermo Fisher Scientific), 10% FBS (Thermo Fisher Scientific), MEM non-essential amino acids (Thermo Fisher Scientific), 1% sodium pyruvate (Thermo Fisher Scientific), 1% penicillin–streptomycin (Thermo Fisher Scientific) and 50 μg ml −1 gentamicin (Merck). After 18 h, the supernatants and biopsies were snap-frozen. The supernatant cytokine concentrations were quantified using the LEGENDplex Human Inflammation Panel (BioLegend). RNA was extracted from biopsies and libraries were prepared as described earlier ( n = 9, RNA from one donor was too degraded). Sequencing was performed on the NovaSeq 6000 system (100 bp paired-end reads). Data were processed as described earlier and GSVA was performed for ETS2-regulated genes and biopsy-derived signatures of IBD-associated inflammation 46 .
Chr21q22 genotypes in archaic humans
Using publicly available genomes from seven Neanderthal individuals 100 , 101 , 102 , 103 , one Denisovan individual 104 , and one Neanderthal and Denisovan F1 individual 105 , genotypes were called at the disease-associated chr21q22 candidate SNPs from the respective BAM files using bcftools mpileup with base and mapping quality options -q 20 -Q 20 -C 50 and using bcftools call -m -C alleles, specifying the two alleles expected at each site in a targets file (-T option). From the resulting .vcf file, the number of reads supporting the reference and alternative alleles was extracted and stored in the ‘DP4’ field.
Inference of Relate genealogy at rs2836882
Genome-wide genealogies, previously inferred for samples of the Simons Genome Diversity Project 106 using Relate 107 , 108 ( https://reichdata.hms.harvard.edu/pub/datasets/sgdp/ ), were downloaded from https://www.dropbox.com/sh/2gjyxe3kqzh932o/AAAQcipCHnySgEB873t9EQjNa?dl=0 . Using the inferred genealogies, the genealogy at rs2836882 (chr21:40466570) was plotted using the TreeView module of Relate.
Data presentation
The following R packages were used to create figures: GenomicRanges 109 , EnhancedVolcano 110 , ggplot2 (ref. 111 ), gplots 112 , karyoploteR 113 .
Statistical methodology
Statistical methods used in MPRA analysis, fGSEA and SNPsea are described above. For other analyses, comparison of continuous variables between two groups was performed using Wilcoxon matched-pairs tests (paired) or Mann–Whitney U -tests (unpaired) for nonparametric data or a t -tests for parametric data. Comparison against a hypothetical value was performed using Wilcoxon signed-rank tests for nonparametric data or one-sample t -tests for parametric data. A Shapiro–Wilk test was used to confirm normality. Two-sided tests were used as standard unless a specific hypothesis was being tested. Sample sizes are provided in the main text and figure captions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets produced in this study are accessible at the following repositories: MPRA (GEO: GSE229472 ), RNA-seq data of ETS2 or chr21q22-edited TPP macrophages (EGA: EGAD00001011338 ), RNA-seq data of ETS2 overexpression (EGA: EGAD00001011341 ), RNA-seq data of MEK-inhibitor-treated TPP macrophages (EGA: EGAD00001011337 ), H3K27ac ChIP–seq data in TPP macrophages (EGA: EGAD00001011351 ), ATAC–seq and H3K27ac ChIP–seq data in ETS2 -overexpressing or -edited macrophages (EGA: EGAD50000000154 ), ETS2 CUT&RUN data (EGA: EGAD00001011349 ), biopsy RNA-seq data (EGA: EGAD00001011333 ). MetaboLights: Metabolomics (MTBLS7665). The counts table for CosMx is provided at Zenodo ( https://zenodo.org/records/10707942 ) 114 . The phenotype and genotype data used for the PRS analysis are available on application to the IBD Bioresource ( https://www.ibdbioresource.nihr.ac.uk/ ). Source data are provided with this paper.
Code availability
Code to reproduce analyses are available at GitHub ( https://github.com/JamesLeeLab/chr21q22_manuscript ; https://github.com/chr1swallace/ibd-ets2-analysis ; https://github.com/qzhang314/PRS_IBD_subpheno ) 114 . Final code is deposited at Zenodo ( https://zenodo.org/records/10707942 ).
Miller, F. W. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 80 , 102266 (2023).
Article CAS PubMed Google Scholar
Dowden, H. & Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 18 , 495–496 (2019).
de Lange, K. M. et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49 , 256–261 (2017).
Article PubMed PubMed Central Google Scholar
International Genetics of Ankylosing Spondylitis Consortium et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45 , 730–738 (2013).
Article Google Scholar
Ji, S. G. et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 49 , 269–273 (2017).
Ortiz-Fernandez, L. et al. Identification of susceptibility loci for Takayasu arteritis through a large multi-ancestral genome-wide association study. Am. J. Hum. Genet. 108 , 84–99 (2021).
Stathias, V. et al. LINCS Data Portal 2.0: next generation access point for perturbation-response signatures. Nucleic Acids Res. 48 , D431–D439 (2020).
Harrison, R. K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 15 , 817–818 (2016).
Claussnitzer, M. et al. A brief history of human disease genetics. Nature 577 , 179–189 (2020).
Article CAS PubMed PubMed Central ADS Google Scholar
King, E. A., Davis, J. W. & Degner, J. F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 15 , e1008489 (2019).
Cader, M. Z. et al. C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nat. Immunol. 17 , 1046–1056 (2016).
Article CAS PubMed PubMed Central Google Scholar
Murthy, A. et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 506 , 456–462 (2014).
Article CAS PubMed ADS Google Scholar
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155 , 934–947 (2013).
Park, M. D., Silvin, A., Ginhoux, F. & Merad, M. Macrophages in health and disease. Cell 185 , 4259–4279 (2022).
Kugathasan, S. et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat. Genet. 40 , 1211–1215 (2008).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40 , 274–288 (2014).
Kuo, D. et al. HBEGF + macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 11 , eaau8587 (2019).
Melnikov, A. et al. Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay. Nat. Biotechnol. 30 , 271–277 (2012).
Nerlov, C. & Graf, T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 12 , 2403–2412 (1998).
Minderjahn, J. et al. Mechanisms governing the pioneering and redistribution capabilities of the non-classical pioneer PU.1. Nat. Commun. 11 , 402 (2020).
Martinez, L. A. Mutant p53 and ETS2, a tale of reciprocity. Front. Oncol. 6 , 35 (2016).
Wei, G. et al. Activated Ets2 is required for persistent inflammatory responses in the motheaten viable model. J. Immunol. 173 , 1374–1379 (2004).
Zhao, J., Huang, K., Peng, H. Z. & Feng, J. F. Protein C-ets-2 epigenetically suppresses TLRs-induced interleukin 6 production in macrophages. Biochem. Biophys. Res. Commun. 522 , 960–964 (2020).
Chung, S. W., Chen, Y. H. & Perrella, M. A. Role of Ets-2 in the regulation of heme oxygenase-1 by endotoxin. J. Biol. Chem. 280 , 4578–4584 (2005).
Quinn, S. R. et al. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J. Biol. Chem. 289 , 4316–4325 (2014).
Ma, X. et al. Ets2 suppresses inflammatory cytokines through MAPK/NF-κB signaling and directly binds to the IL-6 promoter in macrophages. Aging 11 , 10610–10625 (2019).
Aperlo, C., Pognonec, P., Stanley, E. R. & Boulukos, K. E. Constitutive c-ets2 expression in M1D + myeloblast leukemic cells induces their differentiation to macrophages. Mol. Cell. Biol. 16 , 6851–6858 (1996).
Henkel, G. W. et al. PU.1 but not ets-2 is essential for macrophage development from embryonic stem cells. Blood 88 , 2917–2926 (1996).
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P. & Malik, A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20 , 1126–1167 (2014).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25 , 771–784 (2015).
Martin, J. C. et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 178 , 1493–1508 (2019).
Peloquin, J. M. et al. Characterization of candidate genes in inflammatory bowel disease-associated risk loci. JCI Insight 1 , e87899 (2016).
Sazonovs, A. et al. Large-scale sequencing identifies multiple genes and rare variants associated with Crohn’s disease susceptibility. Nat. Genet. 54 , 1275–1283 (2022).
Slowikowski, K., Hu, X. & Raychaudhuri, S. SNPsea: an algorithm to identify cell types, tissues and pathways affected by risk loci. Bioinformatics 30 , 2496–2497 (2014).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112 , 645–657 (2003).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496 , 238–242 (2013).
Shiratori, R. et al. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci. Rep. 9 , 18699 (2019).
Landt, S. G. et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 22 , 1813–1831 (2012).
Basuyaux, J. P., Ferreira, E., Stehelin, D. & Buttice, G. The Ets transcription factors interact with each other and with the c-Fos/c-Jun complex via distinct protein domains in a DNA-dependent and -independent manner. J. Biol. Chem. 272 , 26188–26195 (1997).
Sevilla, L. et al. Bcl-XL expression correlates with primary macrophage differentiation, activation of functional competence, and survival and results from synergistic transcriptional activation by Ets2 and PU.1. J. Biol. Chem. 276 , 17800–17807 (2001).
Kong, L. et al. The landscape of immune dysregulation in Crohn’s disease revealed through single-cell transcriptomic profiling in the ileum and colon. Immunity 56 , 444–458 (2023).
Chapuy, L. et al. Two distinct colonic CD14 + subsets characterized by single-cell RNA profiling in Crohn’s disease. Mucosal Immunol. 12 , 703–719 (2019).
Newman, J. A., Cooper, C. D., Aitkenhead, H. & Gileadi, O. Structural insights into the autoregulation and cooperativity of the human transcription factor Ets-2. J. Biol. Chem. 290 , 8539–8549 (2015).
Liu, H. et al. ERK differentially regulates Th17- and T reg -cell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 43 , 1716–1726 (2013).
Koboziev, I., Karlsson, F., Zhang, S. & Grisham, M. B. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm. Bowel Dis. 17 , 1229–1245 (2011).
Article PubMed Google Scholar
Argmann, C. et al. Biopsy and blood-based molecular biomarker of inflammation in IBD. Gut 72 , 1271–1287 (2023).
Malle, L. et al. Autoimmunity in Down’s syndrome via cytokines, CD4 T cells and CD11c + B cells. Nature 615 , 305–314 (2023).
Feagan, B. G. et al. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol. Hepatol. 8 , 307–320 (2023).
Friedrich, M. et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27 , 1970–1981 (2021).
Klesse, L. J. et al. The use of MEK inhibitors in neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist 25 , e1109–e1116 (2020).
Zou, Y., Carbonetto, P., Wang, G. & Stephens, M. Fine-mapping from summary data with the “sum of single effects” model. PLoS Genet. 18 , e1010299 (2022).
1000 Genomes Consortium. A map of human genome variation from population-scale sequencing. Nature 467 , 1061–1073 (2010).
Kerimov, N. et al. A compendium of uniformly processed human gene expression and splicing quantitative trait loci. Nat. Genet. 53 , 1290–1299 (2021).
Fairfax, B. P. et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science 343 , 1246949 (2014).
Quach, H. et al. Genetic adaptation and Neandertal admixture shaped the immune system of human populations. Cell 167 , 643–656 (2016).
Chen, L. et al. Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell 167 , 1398–1414 (2016).
Nedelec, Y. et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 167 , 657–669 (2016).
Alasoo, K. et al. Shared genetic effects on chromatin and gene expression indicate a role for enhancer priming in immune response. Nat. Genet. 50 , 424–431 (2018).
Wallace, C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet. 17 , e1009440 (2021).
Bourges, C. et al. Resolving mechanisms of immune-mediated disease in primary CD4 T cells. EMBO Mol. Med. 12 , e12112 (2020).
Javierre, B. M. et al. Lineage-specific genome architecture links enhancers and non-coding disease variants to target gene promoters. Cell 167 , 1369–1384 (2016).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14 , 7 (2013).
Peters, J. E. et al. Insight into genotype-phenotype associations through eQTL mapping in multiple cell types in health and immune-mediated disease. PLoS Genet. 12 , e1005908 (2016).
Conant, D. et al. Inference of CRISPR edits from Sanger trace data. CRISPR J. 5 , 123–130 (2022).
Kalita, C. A. et al. QuASAR-MPRA: accurate allele-specific analysis for massively parallel reporter assays. Bioinformatics 34 , 787–794 (2018).
de Santiago, I. et al. BaalChIP: Bayesian analysis of allele-specific transcription factor binding in cancer genomes. Genome Biol. 18 , 39 (2017).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14 , 959–962 (2017).
Calderon, D. et al. Landscape of stimulation-responsive chromatin across diverse human immune cells. Nat. Genet. 51 , 1494–1505 (2019).
Reske, J. J., Wilson, M. R. & Chandler, R. L. ATAC-seq normalization method can significantly affect differential accessibility analysis and interpretation. Epigenet. Chromatin 13 , 22 (2020).
Article CAS Google Scholar
Brown, A. C. et al. Comprehensive epigenomic profiling reveals the extent of disease-specific chromatin states and informs target discovery in ankylosing spondylitis. Cell Genom. 3 , 100306 (2023).
Lienhard, M., Grimm, C., Morkel, M., Herwig, R. & Chavez, L. MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics 30 , 284–286 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 , 139–140 (2010).
Randzavola, L. O. et al. EROS is a selective chaperone regulating the phagocyte NADPH oxidase and purinergic signalling. eLife 11 , e76387 (2022).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37 , 907–915 (2019).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 , 2078–2079 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30 , 923–930 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 , e47 (2015).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Qing, G. et al. Single-cell RNA sequencing revealed CD14 + monocytes increased in patients with Takayasu’s arteritis requiring surgical management. Front. Cell Dev. Biol. 9 , 761300 (2021).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178 , 714–730 (2019).
Gao, K. M. et al. Human nasal wash RNA-Seq reveals distinct cell-specific innate immune responses in influenza versus SARS-CoV-2. JCI Insight 6 , e152288 (2021).
Yang, Q. et al. The interaction of macrophages and CD8 T cells in bronchoalveolar lavage fluid is associated with latent tuberculosis infection. Emerg. Microbes Infect. 12 , 2239940 (2023).
Reyes, M. et al. An immune-cell signature of bacterial sepsis. Nat. Med. 26 , 333–340 (2020).
Mulder, K. et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54 , 1883–1900 (2021).
Cassetta, L. et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35 , 588–602 (2019).
Zernecke, A. et al. Integrated single-cell analysis-based classification of vascular mononuclear phagocytes in mouse and human atherosclerosis. Cardiovasc. Res. 119 , 1676–1689 (2023).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48 , 510–518 (2016).
Renauer, P. A. et al. Identification of susceptibility loci in IL6, RPS9/LILRB3, and an intergenic locus on chromosome 21q22 in Takayasu arteritis in a genome-wide association study. Arthritis Rheumatol. 67 , 1361–1368 (2015).
Terao, C. et al. Genetic determinants and an epistasis of LILRA3 and HLA-B*52 in Takayasu arteritis. Proc. Natl Acad. Sci. USA 115 , 13045–13050 (2018).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604 , 502–508 (2022).
Bussi, C. et al. Lysosomal damage drives mitochondrial proteome remodelling and reprograms macrophage immunometabolism. Nat. Commun. 13 , 7338 (2022).
Behrends, V., Tredwell, G. D. & Bundy, J. G. A software complement to AMDIS for processing GC-MS metabolomic data. Anal. Biochem. 415 , 206–208 (2011).
Meers, M. P., Bryson, T. D., Henikoff, J. G. & Henikoff, S. Improved CUT&RUN chromatin profiling tools. eLife 8 , e46314 (2019).
Leporcq, C. et al. TFmotifView: a webserver for the visualization of transcription factor motifs in genomic regions. Nucleic Acids Res. 48 , W208–W217 (2020).
Weirauch, M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158 , 1431–1443 (2014).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184 , 3573–3587 (2021).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40 , 1794–1806 (2022).
Danaher, P. et al. InSituType: likelihood-based cell typing for single cell spatial transcriptomics. Preprint at bioRxiv https://doi.org/10.1101/2022.10.19.512902 (2022).
Vadstrup, K. et al. Validation and optimization of an ex vivo assay of intestinal mucosal biopsies in Crohn’s disease: reflects inflammation and drug effects. PLoS ONE 11 , e0155335 (2016).
Prufer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505 , 43–49 (2014).
Article PubMed ADS Google Scholar
Prufer, K. et al. A high-coverage Neandertal genome from Vindija Cave in Croatia. Science 358 , 655–658 (2017).
Article PubMed PubMed Central ADS Google Scholar
Hajdinjak, M. et al. Reconstructing the genetic history of late Neanderthals. Nature 555 , 652–656 (2018).
Mafessoni, F. et al. A high-coverage Neandertal genome from Chagyrskaya Cave. Proc. Natl Acad. Sci. USA 117 , 15132–15136 (2020).
Meyer, M. et al. A high-coverage genome sequence from an archaic Denisovan individual. Science 338 , 222–226 (2012).
Slon, V. et al. The genome of the offspring of a Neanderthal mother and a Denisovan father. Nature 561 , 113–116 (2018).
Mallick, S. et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 538 , 201–206 (2016).
Speidel, L., Forest, M., Shi, S. & Myers, S. R. A method for genome-wide genealogy estimation for thousands of samples. Nat. Genet. 51 , 1321–1329 (2019).
Speidel, L. et al. Inferring population histories for ancient genomes using genome-wide genealogies. Mol. Biol. Evol. 38 , 3497–3511 (2021).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9 , e1003118 (2013).
Blighe, K. et al. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. Bioconductor https://doi.org/10.18129/B9.bioc.EnhancedVolcano (2023).
Hadley, W. Ggplot2 (Springer, 2016).
Warnes, G. et al. gplots: various R programming tools for plotting data. CRAN https://CRAN.R-project.org/package=gplots (2022).
Gel, B. & Serra, E. karyoploteR: an R/Bioconductor package to plot customizable genomes displaying arbitrary data. Bioinformatics 33 , 3088–3090 (2017).
Stankey, C. T. et al. Data for ‘A disease-associated gene desert directs macrophage inflammation through ETS2’. Zenodo https://doi.org/10.5281/zenodo.10707942 (2024).
Download references
Acknowledgements
We thank the members of the Lee laboratory, K. Slowikowski and A. Kaser for discussions; G. Stockinger, C. Vinuesa, C. Swanton, R. Patani and C. Reis e Sousa for reading the manuscript; C. Cheshire and the staff at the Francis Crick Institute Advanced Sequencing Facility and Flow Cytometry STP for technical support; L. Lucaciu for help with patient recruitment; RFH PITU nurses for assistance obtaining infliximab; the members of Tissue Access for Patient Benefit (TAP-B) for providing liver samples; NIHR BioResource volunteers for their participation; and the NIHR BioResource centres, NHS Blood and Transplant, and NHS staff for their contributions. This work was supported by Crohn’s and Colitis UK (M2018-3), the Wellcome Trust (Sir Henry Wellcome Fellowship to L.S., 220457/Z/20/Z; Investigator Award to P.S., 217223/Z/19/Z; Senior Fellowship to C.W., WT220788; Clinical Research Career Development Fellowship to M.Z.C., 222056/Z/20/Z; Wellcome-Beit Prize Clinical Career Development Fellowship to D.C.T., 206617/A/17/A; and Intermediate Clinical Fellowship to J.C.L., 105920/Z/14/Z), and the Francis Crick Institute, which receives its core funding from Cancer Research UK (CC2219, FC001595), the UK Medical Research Council (CC2219, FC001595) and the Wellcome Trust (CC2219, FC001595). L.M.H. is supported by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health Charité (Clinician-Scientist Program); A.J.C. by the Medical Research Council (MR/V029711/1); A.L. by a Lord Kelvin/Adam Smith Leadership Grant; A.H.S. by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH, R01:AR070148); N.B.J. by Cancer Research UK (C55370/A25813); T.Z. by the Chinese Scholarship Council (202308060128); A.Q. by the NIHR UCLH/UCL BRC; J.C.K. by Versus Arthritis (program grant, 20773), Janssen Oxford Translational fellowships and NIHR Oxford BRC; P.S. by the European Molecular Biology Organisation, the Vallee Foundation and the European Research Council (852558); C.W. by the Medical Research Council (MC UU 00002/4), GSK, MSD and the NIHR Cambridge BRC (BRC-1215-20014); and D.C.T. by the Sidharth Burman endowment. J.C.L. is a Lister Institute Prize Fellow. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Experimental schematics in Figs. 1d , 2a and 3a and Extended Data Figs. 3a , 4a,b,e and 7g,h were created using BioRender. For the purpose of open access, the authors have applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission.
Open Access funding provided by The Francis Crick Institute.
Author information
These authors contributed equally: C. T. Stankey, C. Bourges, L. M. Haag
Authors and Affiliations
Genetic Mechanisms of Disease Laboratory, The Francis Crick Institute, London, UK
C. T. Stankey, C. Bourges, T. Turner-Stokes, A. P. Piedade, I. Papa, E. C. Parkes & J. C. Lee
Department of Immunology and Inflammation, Imperial College London, London, UK
C. T. Stankey, T. Turner-Stokes & L. O. Randzavola
Washington University School of Medicine, St Louis, MO, USA
C. T. Stankey
Division of Gastroenterology, Infectious Diseases and Rheumatology, Charité–Universitätsmedizin Berlin, Berlin, Germany
Department of Gastroenterology, Royal Free Hospital, London, UK
C. Palmer-Jones, A. P. Rochford, C. D. Murray & J. C. Lee
Institute for Liver and Digestive Health, Division of Medicine, University College London, London, UK
C. Palmer-Jones, F. Saffioti, D. Thorburn, A. P. Rochford, C. D. Murray & J. C. Lee
Metabolomics STP, The Francis Crick Institute, London, UK
M. Silva dos Santos & J. I. MacRae
Genomics of Inflammation and Immunity Group, Human Genetics Programme, Wellcome Sanger Institute, Hinxton, UK
Wolfson Wohl Cancer Centre, School of Cancer Sciences, University of Glasgow, Glasgow, UK
A. J. Cameron, A. Legrini, T. Zhang, C. S. Wood & N. B. Jamieson
NanoString Technologies, Seattle, WA, USA
F. N. New & P. Divakar
Ancient Genomics Laboratory, The Francis Crick Institute, London, UK
L. Speidel & P. Skoglund
Genetics Institute, University College London, London, UK
Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK
A. C. Brown & J. C. Knight
The Sheila Sherlock Liver Centre, Royal Free Hospital, London, UK
A. Hall, F. Saffioti & D. Thorburn
Department of Cellular Pathology, Royal Free Hospital, London, UK
A. Hall & A. Quaglia
Cambridge Institute of Therapeutic Immunology and Infectious Disease, University of Cambridge, Cambridge, UK
W. Edwards, M. Z. Cader, C. Wallace & D. C. Thomas
Department of Internal Medicine, Division of Rheumatology, Marmara University, Istanbul, Turkey
H. Direskeneli
Systemic Autoimmunity Branch, NIAMS, National Institutes of Health, Bethesda, MD, USA
P. C. Grayson
Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, China
Division of Rheumatology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA
P. A. Merkel
Division of Epidemiology, Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, Philadelphia, PA, USA
Department of Physiology, Istanbul University, Istanbul Faculty of Medicine, Istanbul, Turkey
G. Saruhan-Direskeneli
Division of Rheumatology, Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
A. H. Sawalha
Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
Lupus Center of Excellence, University of Pittsburgh, Pittsburgh, PA, USA
Department of Immunology, University of Pittsburgh, Pittsburgh, PA, USA
Department of Biomedical and Clinical Sciences, Milan University, Milan, Italy
E. Tombetti
Internal Medicine and Rheumatology, ASST FBF-Sacco, Milan, Italy
UCL Cancer Institute, London, UK
Chinese Academy of Medical Sciences Institute, Nuffield Department of Medicine, University of Oxford, Oxford, UK
J. C. Knight
NIHR Comprehensive Biomedical Research Centre, Oxford, UK
Experimental Histopathology STP, The Francis Crick Institute, London, UK
M. Green & E. Nye
Department of Medicine, University of Cambridge, Cambridge, UK
M. Z. Cader & D. C. Thomas
MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, UK
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: J.I.M., N.B.J., P.S., M.Z.C., C.W., D.C.T. and J.C.L. Methodology: C.T.S., C.B., M.S.d.S., M.G., E.N., J.I.M., C.W. and J.C.L. Software: C.B., M.S.d.S., Q.Z., A.J.C., A.L., T.Z., C.S.W., L.S., J.I.M., N.B.J., P.S., C.W. and J.C.L. Investigation: C.T.S., C.B., L.M.H., T.T.-S., A.P.P., I.P., M.S.d.S., L.O.R., A.C.B., E.C.P., W.E., M.G., C.D.M. and J.C.L. Resources: C.T.S., C.B., C.P.-J., A.H., F.S., A.Q., D.T., A.P.R., C.D.M. and J.C.L. Formal analysis: C.T.S., C.B., M.S.d.S., Q.Z., A.J.C., A.L., T.Z., F.N.N., L.S., P.D., C.W. and J.C.L. Writing—original draft: C.T.S., C.B. and J.C.L. Writing—review and editing: all of the authors. Funding acquisition: J.C.L. Supervision: J.C.K., J.I.M., N.B.J., P.S., C.W., D.C.T. and J.C.L.
Corresponding author
Correspondence to J. C. Lee .
Ethics declarations
Competing interests.
C.T.S., C.B. and J.C.L. are listed as co-inventors on a patent application related to this work. C.W. holds a part-time position at GSK. GSK had no role in the design or conduct of this study. F.N.N. and P.D. are employees and shareholders of NanoString Technologies. NanoString had no role in the design or conduct of this study. The other authors declare no competing interests.
Peer review
Peer review information.
Nature thanks Joachim Schultze and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 colocalisation between genetic associations at chr21q22..
a . Example comparison of genetic associations at chr21q22: IBD and ETS2 eQTL in unstimulated monocytes. Plot adapted from locuscomparer. b . Tukey box-and-whisker plot depicting ETS2 expression stratified by rs2836882 genotype in unstimulated monocytes (AA, n = 39; AG, n = 142; GG, n = 233) 54 . P -value is as reported in index study. c . Radar plot of representative colocalization results for the indicated genetic associations compared to IBD. Posterior probability of independent causal variants, PP.H3, dark blue; posterior probability of shared causal variant, PP.H4, light blue. PP.H4 > 0.5 was used to call colocalisation (denoted by dashed line). Labels are coloured according to class of data (indicated in the key). Asterisks denote colocalisation. Data sources are: IBD 3 , PSC 5 , AS 4 , Takayasu Arteritis 6 , BLUEPRINT 56 , Fairfax 54 , Quach 55 , Nedelec 57 , Alasoo 58 .
Extended Data Fig. 2 CRISPR-Cas9 editing of the chr21q22 locus and ETS2 in monocytes.
a . Cas9 gRNAs were designed to flank the chr21q22 enhancer region at the indicated sites. b . Representative bioanalyzer trace of PCR-amplified target region following monocyte CRISPR/Cas9 editing with an equimolar mix of RNPs containing 5′ and 3′ chr21q22 gRNAs. Example editing efficiency calculation shown. c . Editing efficiency at the chr21q22 locus. Mean enhancer deletion: 42.4% (n = 11). d . Location and sequence of gRNAs used to disrupt ETS2 . e . ETS2 editing efficiency. gRNA1 (mean), 89.7% (n = 31); gRNA2 (mean), 78.6% (n = 14). f . ETS2 expression (relative to NTC) following CRISPR/Cas9 editing, measured by qPCR (housekeeping gene PPIA ; equivalent results with other housekeeping genes; n = 10). g . Viability following monocyte nucleofection with Cas9 RNPs and macrophage differentiation. Mean values: NTC, 97.9%; gRNA1: 98.3%; gRNA2, 98.6% (n = 6). h . Expression of myeloid lineage markers following ETS2 editing and TPP differentiation (n = 5). Gating strategy shown in Supplementary Information Fig. 2 . i . GSVA enrichment scores for 67 different monocyte/macrophage activation conditions to identify stimuli that phenocopy CD14+ monocytes/macrophages from IBD patients. j . Chromatin accessibility in ETS2-edited versus unedited inflammatory macrophages (n = 3). k . Enhancer activity (H3K27ac) in ETS2-edited versus unedited inflammatory macrophages (n = 3). P values calculated using edgeR (two-sided) in j , k . Red points denote adjusted P -value (P adj ) < 0.1, grey points NS. Error bars are mean±SEM in c , e - h . * P < 0.05. NTC: non-targeting control.
Extended Data Fig. 3 Optimization of MPRA and mRNA overexpression in primary human macrophages.
a . Schematic of MPRA. A library of oligonucleotides (each containing a genomic sequence and unique barcode, separated by restriction enzyme sites) is cloned into a pGL4.10 M cloning vector. A promoter and reporter gene are inserted using directional cloning. The resulting plasmids are transfected into primary human macrophages (TPP) and RNA is extracted after 24 h. Barcode abundance in cellular mRNA and input DNA library are quantified by high-throughput sequencing, and mRNA barcode counts are normalized to corresponding counts in DNA library to assess expression-modulating activity. b . Identification of suitable promoters for MPRA in TPP macrophages. TPP macrophages were transfected with reporter vectors, each with GFP expression under the control of a different promoter. GFP expression was quantified by flow cytometry after 24 h. c . Adapted MPRA vector for use in primary human macrophages, containing RSV promoter. d . Heatmap showing pairwise correlation of expression-modulating activity of all constructs between donors. e . Principal component analysis of element counts (sum of barcodes tagging same genomic sequence) in mRNA from TPP macrophages (n = 8 donors; red) and four replicates of DNA vector (black). f . Primary human macrophages (M0) were transfected with different quantities of GFP mRNA using Lipofectamine MessengerMAX. GFP expression was quantified by flow cytometry 18 h after transfection. g . Cytokine secretion following ETS2 overexpression. Plot shows relative cytokine concentrations in macrophage supernatants ( ETS2 relative to control) following transfection with 500 ng mRNA (n = 11). Error bars are mean±SEM. One-sample t -test (two-tailed) * P < 0.05, ** P < 0.01. The diagram in a was created using BioRender.
Extended Data Fig. 4 Molecular effects of allelic variation at rs2836882 .
a . Schematic of PU.1 ChIP-genotyping assay to assess allele-specific PU.1 binding at rs2836882 in human macrophages. b . Schematic of standard curve generation by TaqMan genotyping various pre-defined ratios of risk and non-risk containing DNA sequences. c . Standard curve generated using different allelic ratios of 200-nt DNA geneblocks centred on either the major (risk) or minor (non-risk) rs2836882 allele. d . Allele-specific PU.1 binding at rs2836882 in TPP macrophages (one-sample t -test, two-sided, n = 5). Error bars represent mean±95%CI. e . Schematic of PU.1 MPRA-ChIP assay to assess allele-specific PU.1 binding at individual SNPs within chr21q22 enhancer. f . Allele-specific PU.1 binding at SNPs within chr21q22 enhancer in TPP macrophages. Data represents the allelic ratio of normalized PU.1 binding for constructs centred on the SNP allele from the MPRA library (fixed-effects meta-analysis of QuASAR-MPRA results, two-sided, n = 6). Box represents median (IQR), whiskers represent minima and maxima. g . Allele-specific ATAC-seq reads at rs2836882 in two deeply sequenced heterozygous TPP macrophage datasets (left: 154.7 million non-duplicate paired-end reads, right: 165.4 million non-duplicate paired-end reads). h . H3K27ac ChIP-seq data from risk (red) or non-risk (blue) allele homozygotes at rs2836882 (n = 4). i . Rank Ordering of Super-Enhancers (ROSE) analysis of H3K27ac ChIP-seq data from TPP macrophages from major (left) and minor (right) allele homozygotes. Dashed line denotes inflection point of curve, with enhancers above this point being denoted as super-enhancers. Red points indicate rs2836882 -containing chr21q22 enhancer. SE, super-enhancer. The diagrams in a , b and e were created using BioRender.
Extended Data Fig. 5 Functional effects of the chr21q22 enhancer.
a . Extracellular ROS production by unedited (NTC), chr21q22-edited, and ETS2 g1-edited TPP macrophages, quantified by chemiluminescence. Points represent relative area under curve for edited versus unedited cells (Wilcoxon signed-rank test, two-sided; n = 6). b . Cytokine secretion from inflammatory macrophages following deletion of the chr21q22 enhancer. Heatmap shows relative cytokine concentrations in the supernatants of chr21q22-edited TPP macrophages versus unedited (NTC) cells (Wilcoxon signed rank test, one-sided; n = 7). c . Representative flow cytometry histograms demonstrating phagocytosis of fluorescently-labelled zymosan particles by chr21q22-edited and unedited (NTC) TPP macrophages. d . Phagocytosis index for unedited and chr21q22-edited TPP macrophages, calculated as proportion of positive cells multiplied by mean fluorescence intensity of positive cells. Plot shows relative phagocytosis index for chr21q22-edited cells versus unedited cells (Wilcoxon signed-rank test two-sided; n = 7). e . Enrichment of differentially-expressed genes following deletion of the disease-associated chr21q22 locus (upregulated genes, top; downregulated genes, bottom) in ETS2 -edited versus unedited macrophages. P adj , FDR-adjusted P -value (two-sided). f . Tukey box-and-whisker plot depicting quantitative PCR of selected ETS2-target genes in resting (M0) macrophages from minor and major allele homozygote IBD patients (n = 22, expression normalized to PPIA and scaled to minimum 0, maximum 1). Mann-Whitney test (one-sided). * P < 0.05, ** P < 0.01, *** P < 0.001.
Extended Data Fig. 6 Polygenic Risk Score of 22 ETS2-regulated IBD-associated genes.
a . Summary of IBD BioResource cohorts used for PRS analysis. b . Association between PRS and age at diagnosis. c . Association between PRS and extent of ulcerative colitis (E1, proctitis; E2, left-sided; E3, extensive colitis). d . Association between PRS and Crohn’s disease location (L1, ileal; L2, colonic; L3, ileocolonic). L2 is associated with a milder disease phenotype. e . Association between PRS and perianal involvement in Crohn’s disease. f . Association between PRS and Crohn’s disease behaviour (B1, inflammatory; B2, stricturing; B3, fistulating). B2 and B3 represent more aggressive, complicated forms of Crohn’s disease. g . Association between PRS and response to anti-TNFα in Crohn’s disease and ulcerative colitis (PR, primary responder; PNR, primary non-responder). h . Association between PRS and need for surgery in Crohn’s disease and ulcerative colitis. Overall, higher PRS was associated with: earlier age at diagnosis, ileal or ileocolonic forms of Crohn’s disease, B2/B3 Crohn’s disease behaviour, and increased need for surgery in IBD. Analysis in b performed using linear regression. Analyses in c - h performed using logistic regression (with diagnosis as covariate in g and h ). SNPs included in PRS are listed in Extended Data Table 1 . i . Plot of enrichment statistic (standardized effect size) against statistical significance from SNPsea analysis of genes tagged by 241 IBD SNPs within ETS2 -regulated genes (red) and known IBD pathways (black). j . SNPsea analyses of SNPs associated with PSC, ankylosing spondylitis, Takayasu’s arteritis or Schizophrenia (negative control) within lists of ETS2-regulated genes–either upregulated by ETS2 overexpression, downregulated by ETS2 disruption, or downregulated following chr21q22 deletion (all FDR < 0.05). Dashed line denotes P < 0.05.
Extended Data Fig. 7 Effects of modulating ETS2.
a and b . Changes in total metabolite abundance ( a ) and percentage of label incorporation from 13 C-glucose ( b ) following ETS2 editing in TPP macrophages (n = 6). Colour depicts median log2 fold-change in ETS2 -edited macrophages relative to unedited macrophages (transfected with non-targeting control RNPs; NTC). Bold black border indicates P < 0.05 (Wilcoxon signed rank test, two-sided). c . Heatmap summarizing metabolic changes following ETS2 disruption. Colour depicts median log2 fold-change in ETS2 g1-edited cells relative to unedited cells (Wilcoxon signed rank test, two-sided, * P < 0.05). d . Phagocytosis index in unedited (NTC) and ETS2 -edited TPP macrophages treated with roxadustat (ROX) or vehicle. Phagocytosis index is calculated as proportion of positive cells multiplied by mean fluorescence intensity of positive cells (488 nm channel). Data normalized to phagocytosis index in unedited cells (n = 5). e . Extracellular ROS production by unedited (NTC) and ETS2 -edited TPP macrophages treated with ROX or vehicle – quantified using a chemiluminescence assay. Data represent log2 fold-change of area under curve (AUC) normalized to unedited (NTC) TPP macrophages (n = 5). f . TFmotifView enrichment results for motifs of transcription factors expressed in TPP macrophages (CPM > 0.5) within ETS2 CUT&RUN peaks. Results shown for all significantly enriched transcription factors (Bonferroni P value < 0.05, two-sided) with motifs in more than 10% peaks. g . Schematic of experiment to assess how ETS2 disruption affects the activity of the chr21q22 ETS2 enhancer in inflammatory (TPP) macrophages. h . Schematic of experiment to assess how ETS2 overexpression affects the activity of the chr21q22 ETS2 enhancer in resting (M0) macrophages. i . Normalized H3K27ac ChIP-seq read counts (edgeR fitted values) from chr21:40,465,000-40,470,000 in experiments depicted in g (left) and h (right) (edgeR P values, two-sided, n = 3 for each). Error bars in d and e represent mean±SEM. The diagrams in g and h were created using BioRender.
Extended Data Fig. 8 The transcriptional signature of ETS2 is detectable in affected tissues from chr21q22-linked diseases.
a . ETS2 expression in scRNA-seq clusters of myeloid cells from Crohn’s disease and healthy controls (upper panel). Relative contributions of single cells from Crohn’s disease or healthy controls to individual clusters (same UMAP dimensions as for combined analysis). b . Overlay of CosMx morphology 2D image data and raw transcripts of selected ETS2 target genes. Fluorescent morphology markers alone (top row), CXCL8 (cyan) and S1009A (yellow) transcripts (middle row), CCL5 (cyan) and CCL2 (yellow) transcripts (bottom row). Columns are representative examples of PSC with diseased ducts (left), PSC with uninflamed background liver (centre), and healthy liver (right). Size marker (white) on every field of view (FOV) denotes 50 µm. c . Gene set enrichment analysis (fGSEA) of genes downregulated following chr21q22 enhancer deletion or ETS2 disruption (gRNA1 or gRNA2) within intestinal macrophages from patients with active IBD (compared to control intestinal macrophages, n = 20; left), ankylosing spondylitis synovium (compared to control synovium, n = 15; centre), and PSC liver biopsies (compared to control liver biopsies, n = 17; right). P adj , FDR-adjusted P -value (two-sided).
Extended Data Fig. 9 Effect of MEK1/2 inhibition on ETS2- regulated genes.
a - c . Gene set enrichment analysis (fGSEA) in MEK1/2 inhibitor-treated TPP macrophages showing enrichment of gene sets upregulated (upper panel) or downregulated (lower panel) following ETS2 or chr21q22 editing (MEK1/2 inhibited using PD-0325901, 0.5 µM). Gene sets obtained from differential gene expression analysis (limma using voom transformation) following ETS2 disruption with gRNA1 ( a ), gRNA2 ( b ), or following chr21q22 deletion ( c ). d . fGSEA in intestinal biopsies from IBD patients showing enrichment of gene sets downregulated following ETS2 or chr21q22 editing in MEK inhibitor-treated biopsies. Upregulated gene sets were not enriched. e . Proportion and pathway analysis of MEK inhibitor-induced differentially expressed genes that have no evidence for being ETS2 targets in macrophages (incorporating differential expression from knockout or overexpression experiments and promoter / regulatory element binding from ETS2 CUT&RUN). P adj , FDR-adjusted P -value (two-sided).
Extended Data Fig. 10 Geographic distribution and history of rs2836882 .
a . rs2836882 allele frequency in modern global populations (data from 1000 Genomes Project, plotted using Geography of Genetic Variants browser: https://popgen.uchicago.edu/ggv/ ). b . Genotypes of candidate SNPs at chr21q22 (99% credible set) in archaic humans (Neanderthals and Denisovans). Colour depicts the proportion of reads containing ALT alleles, with a value close to 0 consistent with a homozygous REF (risk) genotype, a value close to 1 consistent with a homozygous ALT (non-risk) genotype, and an intermediate value indicating a potential heterozygous genotype. Number in each cell indicates the number of reads at that SNP in the indicated sample. Putative causal variant highlighted in red. c . Inferred genealogy of the age of the rs2836882 polymorphism – analysed using Relate. The diagram in a was created using the Geography of Genetic Variants browser.
Supplementary information
Supplementary figures.
Supplementary Fig. 1: uncropped Western blots from Fig. 2d. Two lanes were run for each sample: one lane to blot for vinculin and the NADPH oxidase components gp91phox, gp65 and p22phox, and one lane to blot for vinculin and the chaperone protein EROS. After transfer, the membranes were cut to blot for individual targets. Supplementary Fig. 2: example gating strategy. Example gating strategy for MPRA and macrophage phenotyping. Macrophages were gated by FSC-A/SSC-A and singlets were gated by FSC-A/FSC-H. Live cells were gated (and viability was quantified) using Live/Dead Fixable Aqua Dead Cell Stain.
Reporting Summary
Supplementary tables.
Supplementary Table 1: differentially expressed genes in primary macrophages after ETS2 or chr21q22 CRISPR–Cas9 editing. Supplementary Table 2: differentially expressed genes in primary macrophages after ETS2 overexpression. Supplementary Table 3: the primers and gRNA sequences used in this study.
Peer Review File
Source data, source data fig. 1, source data fig. 2, source data fig. 3, source data fig. 4, source data fig. 5, source data extended data fig. 1, source data extended data fig. 2, source data extended data fig. 3, source data extended data fig. 4, source data extended data fig. 5, source data extended data fig. 6, source data extended data fig. 7, source data extended data fig. 8, source data extended data fig. 9, source data extended data fig. 10, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Stankey, C.T., Bourges, C., Haag, L.M. et al. A disease-associated gene desert directs macrophage inflammation through ETS2. Nature 630 , 447–456 (2024). https://doi.org/10.1038/s41586-024-07501-1
Download citation
Received : 17 April 2023
Accepted : 01 May 2024
Published : 05 June 2024
Issue Date : 13 June 2024
DOI : https://doi.org/10.1038/s41586-024-07501-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Sexual Hookup Culture: A Review
Justin r. garcia.
The Kinsey Institute for Research in Sex, Gender, and Reproduction, Indiana University, Bloomington
Chris Reiber
Graduate Program in Biomedical Anthropology, Department of Anthropology, Binghamton University
Sean G. Massey
Women’s Studies Program, Binghamton University
Ann M. Merriwether
Departments of Psychology and Human Development, Binghamton University
“Hookups,” or uncommitted sexual encounters, are becoming progressively more engrained in popular culture, reflecting both evolved sexual predilections and changing social and sexual scripts. Hook-up activities may include a wide range of sexual behaviors, such as kissing, oral sex, and penetrative intercourse. However, these encounters often transpire without any promise of, or desire for, a more traditional romantic relationship. A review of the literature suggests that these encounters are becoming increasingly normative among adolescents and young adults in North America, representing a marked shift in openness and acceptance of uncommitted sex. We reviewed the current literature on sexual hookups and considered the multiple forces influencing hookup culture, using examples from popular culture to place hooking up in context. We argue that contemporary hookup culture is best understood as the convergence of evolutionary and social forces during the developmental period of emerging adulthood. We suggest that researchers must consider both evolutionary mechanisms and social processes, and be considerate of the contemporary popular cultural climate in which hookups occur, in order to provide a comprehensive and synergistic biopsychosocial view of “casual sex” among emerging adults today.
There’s a stranger in my bed There’s a pounding in my head Glitter all over the room Pink flamingos in the pool I smell like a minibar DJ’s passed out in the yard Barbies on the barbeque Is this a hickey or a bruise —Last Friday Night (T.G.I.F.) ( Perry, Gottwald, Martin, & McKee, 2011 )
Popular media representations of sexuality demonstrate the pervasiveness of a sexual hookup culture among emerging adults. The themes of books, plots of movies and television shows, and lyrics of numerous songs all demonstrate a permissive sexuality among consumers. As an example, the lyrics above, from the chart-topping pop song Last Friday Night (T.G.I.F.) by singer–songwriter Katy Perry highlight someone’s Friday night partying, presumably including casual sex, alcohol, and a piecemeal memory of the nights events. Research on media portrayals of sexual behavior has documented this pattern as well. In a 2005 Kaiser Family Foundation report about sex on television, media was highlighted as the primary basis for emerging adults’ opinions about sex, consistent with their result of 77% of prime-time television programs containing some sexual content ( Kunkel, Eyal, Finnerty, Biely, & Donnerstein, 2005 ). In terms of a more permissive uncommitted sexual content, 20% of sexual intercourse cases involved characters who knew each other but were not in a relationship, and another 15% involved characters having sex after just meeting ( Kunkel et al., 2005 ). Other studies have shown that college students believe their peers are substantially more sexually permissive than was actually the case ( Chia & Gunther, 2006 ; Reiber & Garcia, 2010 ). These incorrect beliefs of peer sexual norms are in part influenced by students’ perceptions of media and the influence of media on peers ( Chia & Gunther, 2006 ). Popular culture is simultaneously representing aspects of actual contemporary sexual behavior and providing sexual scripts for emerging adults. In the current review, we examine and explore these patterns in sexual hookups.
Hooking up— brief uncommitted sexual encounters among individuals who are not romantic partners or dating each other— has taken root within the sociocultural milieu of adolescents, emerging adults, and men and women throughout the Western world. Over the past 60 years, the prioritization of traditional forms of courting and pursuing romantic relationships has shifted to more casual “hookups” ( Bogle, 2007 , 2008 ). Among heterosexual emerging adults of both sexes, hookups have become culturally normative. Dating for courting purposes has decreased (but certainly not disappeared) and sexual behavior outside of traditional committed romantic pair-bonds has become increasingly typical and socially acceptable ( Bogle, 2007 , 2008 ). In one sample of undergraduate college students, both men and women had nearly double the number of hookups compared to first dates ( Bradshaw, Kahn, & Saville, 2010 ). Most notably, individuals of both sexes are willing to openly discuss the topic and advertise their acceptance and experiences of hooking up.
Sexual hookups are most comprehensively understood in an interdisciplinary framework that combines multiple levels of analyses. In this review, we consider how aspects of sexual popular culture reflect both the biological reproductive motive, social–sexual scripts, and how individuals adaptively, facultatively, respond to their environment. The evolutionary biological and sociocultural paradigms produce parallel, sometimes interacting, and sometimes contradictory, patterns of explanation. The emergence of sexual hookup culture provides a case of human social behavior through which to explore the relationship and possible interaction between evolved mating psychology and cultural context.
Cultural Shifts in Dating
Hookup culture has emerged from more general social shifts taking place during the last century. As early as the 1920s, with the rise of automobile use and novel entertainment venues throughout North America, traditional models of courting under parental supervision began to fade ( Bailey, 1988 ; Stinson, 2010 ). An increase in “dating” during this period gave way to a more permissive peer-influenced social–sexual script ( Bailey, 1988 ; Stinson, 2010 ). With the invention of visual media, images of erotic sex began finding their way into popular culture ( Black, 1994 ; Doherty, 1999 ). In opposition to this, censorship laws established during the 1930s and lasting until the late 1960s limited depictions of erotic life in film, including depictions of uncommitted sex ( Herbert & McKernan, 1996 ; Robertson, 2001 ; Vieira, 1999 ). Young adults became even more sexually liberated in the 1960s, with the rise of feminism, growth of college party events, widespread availability of birth control (condoms and oral contraceptives), and deposing of parental expectations as central to mating and marriage ( Laumann, Gagnon, Michael, & Michaels, 1994 ; Stinson, 2010 ). Again in opposition, many health care providers in the 1960s denied oral contraceptives to single, unmarried, women ( Coontz, 2005 ). Throughout American history, young adults were told, and at least publicly endorsed, that sexual behavior should only occur in the context of a marital union.
Representation of Hookups in Popular Culture
Contemporary popular culture is now ripe with examples that depict and often encourage sexual behavior, including premarital and uncommitted sex. Popular media, including television, has become a source of sex education, filled with (inaccurate) portrayals of sexuality ( Kunkel et al., 2005 ; Strasburger, 2005 ; Ward, 2003 ). Many popular representations suggest uncommitted sex, or hookups, can be both biophysically and emotionally enjoyable and occur without “strings.” Recent entertainment media have highlighted uncommitted sexual encounters and the more-common-than-not experimentation with this type of behavior. The film Hooking Up , released in 2009, details the chaotic romantic and sexual lives of adolescent characters. The film No Strings Attached , released in 2011 and staring Natalie Portman and Ashton Kutcher, features the uncommitted element of uncommitted sex, as two friends attempt to negotiate a sexual, yet nonromantic, component of their relationship. Popular television shows often portray hooking up as acceptable, entertaining, and perfectly sensible. The hit British series Skins , which began in 2007, and was remade in North America in 2011, often highlights the uncommitted sexual exploits of adolescents. The popular reality show Jersey Shore , which started its run in 2009, glorifies hookups among strangers, acquaintances, friends, and former partners. Popular pro-hookup same-sex representations have also emerged in television series like Queer as Folk and The L-Word . Several popular books on hookups have hit the shelves, with unscientific yet racy claims. These include, The Happy Hook-Up: A Single Girl’s Guide to Casual Sex ( Sherman & Tocantins, 2004 ), The Hookup Handbook: A Single Girl’s Guide to Living It Up ( Rozler & Lavinthal, 2005 ), Hooking Up: A Girl’s All-Out Guide to Sex and Sexuality ( Madison, 2006 ), Making the Hook-Up: Edgy Sex With Soul ( Riley, 2010 ), and 11 Points Guide to Hooking Up: Lists and Advice About First Dates, Hotties, Scandals, Pickups, Threesomes, and Booty Calls ( Greenspan, 2011 ).
Operationalizing “Hookups”
Hookups may include any sexual behavior in a seemingly uncommitted context. Nearly all hookups involve kissing; 98% of undergraduate respondents in one study reported kissing within a hookup ( Fielder & Carey, 2010a ). Other behaviors are less ubiquitous. In another study, a combined 81% of undergraduate respondents engaged in some form of hookup behavior, with 58% having engaged in sexual touching above the waist and 53% below the waist, 36% performed oral sex, 35% received oral sex, and 34% engaged in sexual intercourse in the context of a hookup ( Reiber & Garcia, 2010 ). Research has found minimal gender differences in terms of hookup behaviors. The term hookup focuses on the uncommitted nature of a sexual encounter rather than focus on what behaviors “count.” The ambiguity of this term may allow individuals to adaptively manipulate others’ perceptions of their sexual behavior.
Operational definitions of hookups differ among researchers. Hookups may be characterized as a form of “casual sex” or “uncommitted sexual encounter.” Hatfield, Hutchison, Bensman, Young, and Rapson (in press) define casual sex as “outside of a ‘formal’ relationship (dating, marriage, etc.), without a ‘traditional’ reason (such as love, procreation, or commitment) for doing so” (p. 3). Paul, McManus, and Hayes (2000) omitted the possibility of hooking up with previous partners or friends, by defining a hookup as “a sexual encounter, usually only lasting one night, between two people who are strangers or brief acquaintances. Some physical interaction is typical but may or may not include sexual intercourse” (p. 79). Using a broad situational definition, Garcia and Reiber (2008) told participants “a hook-up is a sexual encounter between people who are not dating or in a relationship, and where a more traditional romantic relationship is NOT an explicit condition of the encounter” (p. 196). Lewis, Granato, Blayney, Lostutter, and Kilmer (2011) used a more behaviorally specific definition, in which hooking up was defined as a “event where you were physically intimate (any of the following: kissing, touching, oral sex, vaginal sex, anal sex) with someone whom you were not dating or in a romantic relationship with at the time and in which you understood there was no mutual expectation of a romantic commitment” (p. 4). Glenn and Marquardt (2001) used an explicitly heteronormative definition for participants: a hook-up is “when a girl and a guy get together for a physical encounter and don’t necessarily expect anything further” (p. 82).
Friends With Benefits
On the surface, hookups are slightly different from more protracted mutual exchange arrangements for uncommitted sex, like those often referred to with colloquialisms such as “friends with benefits” (FWBs), “booty calls,” or “fuck-buddies” ( Jonason, Li, & Richardson, 2011 ). In terms of popular public discourse, Urban Dictionary defines FWBs as “two friends who have a sexual relationship without being emotionally involved. Typically two good friends who have casual sex without a monogamous relationship or any kind of commitment” ( Friends with benefits, 2003 ) and also “a safe relationship, that mimics a real partnership but is void or greatly lacking jealousy and other such emotions that come with a serious relationship” ( Friends with benefits, 2005 ). Yet, popular culture representations (e.g., The film Friends with Benefits , released in 2011 staring Mila Kunis and Justin Timberlake) suggest FWB partnerships may not truly be void of romantic elements.
FWB relationships represent a unique variation of hooking up worthy of more research attention, which it is beginning to generate. In one study, 60% of 125 undergraduates reported having a FWB relationship at some point in their lives ( Bisson & Levine, 2009 ). Of those who had engaged in a FWB experience, 98.7% were with an opposite sex partner and 1.3% with a same-sex partner. Much like in the movie of the same name, a common concern of participants describing their FWB relationships was the potential formation of unanticipated romantic feelings. At the time of the survey, 35.8% stayed friends but stopped having sex with their most recent FWB partner, 28.3% were maintaining an FWB relationship, 25.9% ended their relationship or friendship, and 9.8% initiated a romantic relationship ( Bisson & Levine, 2009 ). Because these situations represent a greater entanglement of friendship, trust, and emotional comfort, FWBs are distinct from notions of hooking up in some aspects. Namely, hookup scenarios do not implicitly include a friendship relationship component as a condition.
Hooking Up as Contemporary Casual Sex
There are also a large number of colloquial expressions used to describe uncommitted sexual behavior, including labels like “no strings attached” (NSA) sex, “casual encounters,” and “one-night stands.” It is important to explore whether, and in what context, these phrases (e.g., NSA) are really interchangeable with “hookups.” Hookups are different from infidelity situations (extrapair copulations), in which an individual engages in sex with an extrarelational partner, but is still functionally committed to the relationship partner. However, some sexual subcultures with open relationships actually allow extrarelationship casual sex without considering it to be a betrayal. For instance, the frequency of open relationships among gay men, where extrarelational casual sex is permissible, has been estimated as high as 60% ( Hoff & Beougher, 2010 ). In a sample of 2027 gay men from Australia, although 15% had no sexual relationship at time of the survey, 30% of men had a “regular” monogamous relationship partner, 23% had a casual sex partner, and 32% had both a regular (open relationship) partner and casual sex ( Zablotska, Frankland, Prestage, Down, & Ryan, 2008 ). In these cases, some extrapair encounters may constitute uncommitted hookups, albeit not among “singles.”
Across gender, ethnicity, or sexual orientation, nearly all adult Americans experience sexual activity, including sex beyond the context of a marital union ( Finer, 2007 ; Garcia & Kruger, 2010 ; Herbenick et al., 2010 ). It is important to note that uncommitted sex and one-night stands have been studied outside the current “hookup culture” frame ( Boswell & Spade, 1996 ; Cates, 1991 ; Hatfield et al., in press ; Maticka-Tyndale, 1991 ). Uncommitted sexual encounters became a topic of particular scientific interest beginning in the mid 20th century ( Ellis, 1958 ; Kinsey, Pomeroy, & Martin, 1948 ; Kinsey, Pomeroy, Martin, & Gebhard, 1953 ), and especially during the sexual liberation period of the 1960s and 1970s ( Altman, 1971 , 1982 ). Attention to causal sexual encounters among men who have sex with men also emerged as an area of study during the AIDS epidemic in the 1980s until today. Yet, this larger casual sex literature has remained largely disjointed from investigations of “hookups.” Research (especially from a public health perspective) on brief uncommitted sexual behaviors outside of traditional relationships extends well beyond heterosexual collegiate populations, including same-sex sexual behaviors among men who have sex with men. These complementary literatures and approaches should be integrated into the future study of hookup behavior, because the study of human sexuality must consider the vast range of variation and potential in human sexual behaviors.
A case in point, findings from the National Survey of Sexual Health and Behavior identified a much higher rate of American men and women who had ever engaged in same-sex sexual behavior compared to those who identify with a homosexual orientation (see Herbenick et al., 2010 , for a detailed account of same-sex and opposite sex sexual behavior in the United States by age group). This raises an important, but as of yet unanswered, question: If a proportion of heterosexual Americans have at some point engaged in at least one same-sex sexual encounter, is the context of such a scenario a hookup? Although speculative, it seems most probable that many such encounters are sexual experiments and uncommitted, but investigations of how this relates to the larger hookup culture are sorely lacking.
Frequency of Hooking Up
A vast majority of today’s young adults, from a wide range of college student populations studied so far, report some personal “casual” sexual experience ( Bogle, 2008 ; England, Shafer, & Fogarty, 2007 ; Fielder & Carey, 2010a ; Fisher, Worth, Garcia, & Meredith, 2012 ; Garcia & Reiber, 2008 ; Welsh, Grello, & Harper, 2006 ; Gute & Eshbaugh, 2008 ; Hatfield et al., in press ; Lambert, Kahn, & Apple, 2003 ; Lewis et al., 2011 ; Paul et al., 2000 ). The most recent data suggest that between 60% and 80% of North American college students have had some sort of hookup experience. This is consistent with the view of emerging adulthood (typical college age) as a period of developmental transition ( Arnett, 2000 ), exploring and internalizing sexuality and romantic intimacy, now including hookups ( Stinson, 2010 ).
Although much of the current research has been done on college campuses, among younger adolescents, 70% of sexually active 12–21 year olds reported having had uncommitted sex within the last year ( Grello, Welsh, Harper, & Dickson, 2003 ). Similarly, in a sample of seventh, ninth, and 11th graders, 32% of participants had experienced sexual intercourse and 61% of sexually experienced teenagers reported a sexual encounter outside the context of a dating relationship; this represents approximately one fifth of the entire sample ( Manning, Giordano, & Longmore, 2006 ).
Hookup Venues
Among college students, hookups have been reported in a variety of college settings. One study of students’ perceptions of hookups reported that 67% occur at parties, 57% at dormitories or fraternity houses, 10% at bars and clubs, 4% in cars, and 35% at any unspecified available place ( Paul & Hayes, 2002 ). In addition to college campus locations, spring break and holidays have been a time many individuals, particularly emerging adults, will purposely plan to experiment or engage in uncommitted sexual activity and other high-risk behaviors ( Josiam, Hobson, Dietrich, & Smeaton, 1998 ). In a study of Canadian college students on spring break, of those explicitly planning to participate in casual sex, 61% of men and 34% of women engaged in intercourse within a day of meeting a partner ( Maticka-Tyndale, Herold, & Mewhinney, 1998 ). This is echoed in another more recent report, where regardless of relationship status, approximately 30% of participants had sex with someone they met on spring break ( Sönmez et al., 2006 ). Such settings may help facilitate a preexisting desire for hookups (i.e., playful atmosphere and presence of alcohol).
More generally, in a sample of sexually experienced men and women, participants indicated a variety of settings where they met someone with whom they had casual sex: 70% at a party, 56% at a singles bar, 43% while away on vacation, 28% at a dance, 7% while away on business, and 5% on a blind date ( Herold & Mewhinney, 1993 ). In addition to sharing common social venues with heterosexuals, gay men and other men who have sex with men have an expanded array of venues in which hookups may occur. Research specifically sampling gay men and other men who have sex with men have similarly found bars to be common places for gay men to meet, socialize, and find others for casual sexual encounters ( Mustanski, Lyons, & Garcia, 2011 ). Although uncommitted sex among gay men occurs in a variety of locations, antigay prejudice and structural heterosexism can limit the availability of supportive and safe options for connecting with other men ( Harper, 2007 ). Consequently, more anonymous, sometimes public, spaces have been an alternative for some gay men. In a sample of 508 gay and bisexual men in college (all under the age of 30), nearly one third admitted to meeting partners in anonymous places (i.e., bathhouses, restrooms, gyms, bookstores, movies, parks, the street, or other public places) ( Seage et al., 1997 ). Public cruising areas, Internet cruising networks, and bathhouses are somewhat popular venues (although by no means archetypal) for explicitly initiating uncommitted sex among men who have sex with men ( Binson et al., 2001 ). These are not findings that seem to be prevalent among lesbians and women who have sex with women or among heterosexual hookups.
Theoretical Frameworks for Hookup Research
An interdisciplinary biopsychosocial model can synthesize traditionally disconnected theoretical perspectives and provide a more holistic understanding of hookup culture. Hatfield et al. (in press) state that
while many scholars emphasize cultural factors and others emphasize evolutionary factors, increasingly most take a cultural and biopsychosocial approach—pointing out that it is the interaction of culture, social context, personal experience, and biological factors that shape young people’s attitudes and willingness to participate in casual sexual encounters. Which of these factors prove to be most important depends on culture, personality, gender, and social context. (pp. 3– 4)
Some empirical studies of hookup behavior have also advocated multifactorial approaches ( Eshbaugh & Gute, 2008 ; Garcia & Reiber, 2008 ).
Evolutionary and social models often generate parallel hypotheses about uncommitted sex, although “each addresses a different level of analysis” ( Fisher et al., 2012 , p. 47). Using two midlevel theories, Fisher et al. (2012) explained that “parental investment theory is an example of an ultimate level of explanation, while social role theory is an example of a proximate level, although each leads to the same prediction” (p. 47). They argued that evolution may be most helpful in exploring the reproductive motive, and sexual scripts may be useful in exploring the cultural discourse agenda. That is, evolutionary biology influences why emerging adults engage in uncommitted sex and the way young men and women react to these encounters (ultimate level explanations). At the same time, social roles and sexual scripts influence how emerging adults navigate their desires in a particular socio-cultural context (proximate level explanations). For instance, that religiosity (religious feelings and attendance at religious services) was related to lower frequency of engaging in intercourse during a hookup encounter ( Penhollow, Young, & Bailey, 2007 ) may be envisioned as an adaptive sociocultural constraint. Or, that high degrees of closeness to peer social networks and peer communication about hookups was associated with more sexual hookups ( Holman & Sillars, 2012 ) may be considered as a facultative response to adaptively react to peer expectations and local norms.
It is important to point out that many sociocultural theorists disagree with the idea that culture offers only a proximate level explanation for human sexual behavior. However, it is not the goal of this review to resolve this debate. Instead, we attempt to articulate better the multitude of factors that shape the rich variety of human sexuality to enhance understanding of uncommitted sex among emerging adults. In the next two sections, we will introduce both evolutionary and social script views of uncommitted sex, to simultaneously consider the influence of each on hookup culture.
Evolution and “Short-Term” Sexual Behavior
Human evolutionary behavioral studies attempts to explain sexual behavior by understanding our evolutionary history and how this may influence behavioral patterns in a given environment. There are several different midlevel evolutionary or biological theories about the nature of human sexual behavior. These theories seek to understand the way evolutionary pressures influence human sexual propensities, variation, and, in some cases, sex differences. This logic is based on the premise that, compared to asexual reproduction, sexual reproduction is quite costly. Sexually reproducing organisms pay many costs, including the time, energy, and resources spent in finding and attracting mates—tasks that are unnecessary for asexual reproducers ( Daly, 1978 ). Offsetting the costs of sexual reproduction in large-bodied organisms is the benefit sexual reproduction provides against easy colonization by parasites and pathogens ( Van Valen, 1973 ). Sexual reproduction scrambles up genes, creating genotypes that are novel environments and forcing the parasites and pathogens to begin anew in their quest to exploit the host. Thus, large-bodied organisms with long lifespans generally benefit evolutionarily from sexual reproduction despite its substantial costs.
Sexual reproduction is characterized by sexes— generally male and female—whose evolutionary best interests differ because their potential reproductive rates differ ( Clutton-Brock & Parker, 1992 ). In humans, producing a viable offspring, from gestation through lactation, takes females longer than it takes males. The sex with the faster potential reproductive rate— generally males— can benefit by attempting to co-opt the reproductive effort of multiple members of the opposite sex. However, the sex with the slower potential reproductive rate— generally females—will be operationally in short supply relative to the sex with the faster potential reproductive rate, simply because it takes them longer to complete a reproductive venture.
According to evolutionary theorists, this discrepancy in reproductive rate between the sexes sets up general predictions about sex-specific mating behaviors ( Bateman, 1948 ; Clutton-Brock & Parker, 1992 ; Trivers, 1972 ). Males are predicted to compete for access to the reproductive potential of the slower sex; this generates expectations of psychological and physical adaptations in males that enhance their chances of success, including aggression and an array of physical features (e.g., large size, musculature, physical weaponry like antlers) that would assist them in competing with other males for access to females. Females are predicted to be choosy concerning their mates because they invest more in each offspring, and they stand to lose more if they make a poor reproductive choice. Relative parental investment costs are thought to be the arbiters of mating behaviors ( Trivers, 1972 ). Thus in sex role reversed species where males provide a majority of parental support, it is females that are then expected to compete more for mates and be more indiscriminate in their mating ( Alcock, 2005 ). Generally, females choose mates on the basis of whatever is most important to the success of the reproductive venture—at the least, good genes for the offspring, but often for particular resources with which to provision offspring, protection, and/or apparent willingness to assist in parenting. Because females choose males on the basis of critical features and resources, males are expected to compete with other males to acquire and display these features and resources. This provides a basic framework with which to begin, and in humans we expect complex cognitive processes to be overlaid on it.
In terms of applying this logic to human sexual behavior and in particular sexual hookups, uncommitted sex has most often been interpreted in evolutionary terms as a fitness-enhancing short-term mating strategy ( Buss, 1998 ; Buss & Schmitt, 1993 ). In this view—sexual strategies theory—men prefer as many mates as possible, including short-term sexual encounters that can potentially maximize reproductive output. Men will attempt to mate with a maximum number of partners (sexual variety), consent to sex more quickly than women, and provide minimal resources to any but long-term partners, only conceding to a long-term relationship for the purposes of enhancing offspring vitality ( Symons, 1979 ; Buss, 1998 ). Also in this view, women are expected to prefer long-term relationships to extract a maximum amount of resources from mates. Women will engage in short-term sex when it is typically viewed as an infidelity to obtain better quality genes for offspring ( Gangestad & Thornhill, 1997 ). That is, sexual strategies theory (a midlevel theory within the larger evolutionary metatheoretical framework) does allow for both men and women to engage in long-term and short-term sexual behaviors, but for sex-specific evolutionary reasons ( Buss & Schmitt, 1993 ; Schmitt et al., 2003 ). In Petersen and Hyde’s (2010) thorough meta-analytic review of gender differences in sexuality research (834 individual studies and 7 national data sets, across 87 countries), men and women are more similar than different in a majority of sexual behaviors. The exceptions, yielding the greatest effect sizes, included men’s greater permissiveness toward casual sex behavior and casual sex attitudes. This mirrors an earlier review finding that gender differences in attitudes toward casual sex were some of the most pronounced differences of all sexual behaviors ( Oliver & Hyde, 1993 ).
In measuring propensities for nonrelational sex, a variety of studies conducted within North America have demonstrated that men consistently have higher sociosexuality scores than women ( Schmitt, 2005 ). Research on sociosexuality has suggested individual differences in disposition toward engaging in sexual behavior and exhibitionism, with some individuals more permissive (unrestricted) and some nonpermissive (restricted) about sexual frequency ( Simpson & Gangestad, 1992 ). Individuals with more permissive sociosexuality rate physical attraction as more important than other characteristics in a potential partner ( Simpson & Gangestad, 1992 ). Several scholars have argued that the degree to which evolution shapes mating behaviors, including sociosexuality, will be contingent on particular environmental conditions ( Frayser, 1985 ; Low, 2000 ; Schmitt, 2005 ). To support the idea that sociosexuality is likely a combination of evolved sex-specific mating strategies and social structural factors, in a study of over 200,000 participants from 53 nations, Lippa (2009) demonstrated that although consistent sex differences emerged, gender equality and economic development tended to predict the magnitude of sex differences in sociosexuality (more permissive). Similarly, Wood and Eagly (2002) have endorsed a biosocial model for understanding sex differences cross-culturally that takes into account multiple levels of analyses, including biological constraints alongside social and economic constraints.
In support of evolved sexual strategies, in a cross-cultural study of 16,288 individuals across 52 nations, Schmitt et al. (2003) showed that on average men self-report a greater desire for sexual partner variety than women, regardless of relationship status (married or single) or sexual orientation (heterosexual or homosexual). Using the short-term seeking measure (asking participants on a 7-point scale whether they are actively seeking a short-term mate), they reported that, in North America, relatively more men (65.2%) than women (45.4%) fall into the category of seeking short-term mates in any way (any score above 1 on the scale). Of note, using the cross-cultural responses of those who are single (excluding those currently involved in a relationship), 79.3% of men and 64.0% of women reported seeking a short-term mate in some way. Evolutionary-inclined researchers have often used these findings to point to the adaptive nature of sex-specific mating strategies (see Schmitt, 2005 ). These data demonstrate fairly modest relative sex differences in propensities toward sex beyond a committed relationship—which are indeed important to document. Yet, a cross-cultural sex difference of 15.3% in number of single men and single women interested in seeking a short-term mate does not necessarily reveal discreet sex-specific (short-term) mating strategies per se. This is especially true considering that, compared to males, the relative risks of sexual behavior are higher for females: unintended pregnancy, increased transmission of disease, and greater susceptibility to sexual violence. Although there is a reasonable proportional difference between sexes, there are still nearly two thirds of unpartnered women interested in uncommitted sex and over one fifth of unpartnered men who are not interested in this activity. In short, there is significant overlap between the sexes and significant variation within the sexes. All things considered, the simplest expectation is that evolutionary processes will result in both men and women desiring both sex and pair-bonding. Extrarelational sex is part of the human mating repertoire, as is pair-bonding. Individuals have competing sexual and relational motivations at any given time, which should be expected to go in one direction or the other, depending on an individual’s environmental context.
The popularity of hooking up among both men and women presents a problem for approaching human sexuality purely from the perspective of sexual strategies theory. That both men and women are engaging in this behavior at such high rates is not consistent with the model. Homosexual relationships also presents a quandary for sexual strategies theory. Although the proportion of gay men in open relationships seems to support the theory (i.e., males are more sexually eager), the expectation that males should mate-guard their partners to prevent sexual infidelity cannot simultaneously coexist with such prevalence of open relationships among gay men.
Several evolutionary scholars have started to question the ability of sexual strategies theory to accurately reflect patterns of short-term sex in a shifting ecological context, and they have proposed alternative evolutionary approaches ( Gangestad & Simpson, 2000 ; Li & Kenrick, 2006 ; Garcia & Reiber, 2008 ; Fisher, 2011 ; Pedersen, Putcha-Bhagavatula, & Miller, 2011 ). For instance, Li and Kenrick (2006) have pointed to the benefits of using an evolutionary economic model of tradeoffs to understand sex differences in willingness to engage in short-term sex, and sex similarities in prioritization of short-term partners. Using biological and cross-cultural evidence, Fisher (1992 , 2011 ) has argued human possess a dual reproductive strategy of social monogamy (serial or long-term) and clandestine adultery. Pedersen et al. (2011) applied attachment fertility theory and demonstrated relatively few sex differences, arguing that predictions from sexual strategies theory are not consistent with their data. In their comparison of theoretical models, they found that attachment fertility theory
posits that short-term mating and other forms of mating outside of pair-bonds are natural byproducts of a suite of attachment and care-giving mechanisms… selected for in human evolutionary history to ultimately enable men and women to seek, select, create, and maintain a pair-bond… pointing to an increasingly coherent picture of the underlying biological and chemical systems involved… that generally operate similarly for men and women. ( Pedersen et al., 2011 , p. 639)
If humans possess a fairly flexible sexual repertoire, yet pair-bonding is essential, this sets the stage for a conflict between competing motivational drives that are fine tuned to particular environments.
In accordance with an evolutionary model, the simplest, most general prediction is that men will be relatively more competitive and sexually eager, and that women will be relatively choosier. Further, in accordance with an evolutionary model emphasizing pair-bonding, both men and women will have competing motivational drives for sexual engagement and pair-bond formation. This might assume that penetrative sexual intercourse between fertile men and women entails a sizable risk of reproduction for females—an assumption that simply no longer applies to humans in the 21st century. In contemporary industrialized cultures, pleasurable sexual behaviors can be divorced from reproduction and used for other purposes, including social standing and simple enjoyment, among others. Contraception and reproductive technologies allow women greater control over reproduction, but this should not be enough to completely overwrite millions of years of evolutionary pressure to shape certain aspects of mating psychology. Rather, in these contemporary conditions, those who use contraception to optimize their reproductive output may well be evolutionarily favored. Women could, for example, use contraception to control the timing of pregnancies in ways that maximize the chance of success, or ensure parentage by favored males over lesser-quality mates. And males too may be able to control siring a child and the cross-culture expectation of fatherhood (see Gray & Anderson, 2010 , for a review on evolution and fatherhood). Thus, contraception is simply an additional feature of the environment of reproduction, and males and females are expected to attempt to manipulate it in their own favor. Psychological adaptations that support the “choosy female” strategy are still evident, even when individuals choose to engage in nonreproductive sexual behavior. However, the ability to divorce sex from reproduction should allow for less discrepancy between males and females in willingness to engage in uncommitted sex and negotiations of both sexual and romantic desires. Clearly, the evolved reproductive motive involves both sexes desiring sex and desiring pair-bonds, but having different ways of obtaining each and different prioritizations for each.
Sexual Scripts and Uncommitted Sex
Sexual script theory suggests that our sexual behaviors are dictated by a set of “scripts” that are used to organize and interpret sexual encounters into understandable conventions ( Simon & Gagnon, 1986 ). Scripts, particularly gender-normative ones, dictate behaviors, such as who does what and when in context (e.g., men ask women on a date, men pay the bill on a first date, men initiate sex after date). The most widely produced and promoted cultural sexual scripts are heterosexual in nature and include those focused on male roles ( Kim et al., 2007 ; Tolman, 2006 ; Ward, 1995 ). For men, sex is portrayed as central to male identity, men prefer nonrelational sex, and men are active sexual agents. Women are portrayed as sexual objects, sexually passive compared to men, and women act as sexual gatekeepers. Sexual script theory is generally vague when it comes to origins, focusing more on descriptions of scripts. Wiederman (2005) , Phillips (2000) , and Jhally (2007) have argued that scripts are not only sexualized but also gendered, with underlying sexual messages being noticeably different for men and women. Many researchers ( Jhally, 2007 ; Kim et al., 2007 ; Phillips, 2000 ; Ward, 1995 ) have favored culture and subculture environment elements such as popular media (i.e., television, films, magazines) as the origin of gendered sexual scripts. But this does little to explain why the media industry produces these scripts in the first place. It is not by accident that consumer behavior can be well-explained by those products most salient to human survival and reproduction, and why messages of love and sex are among the most producible ( Saad, 2007 ). But, on their own, both the evolutionary perspective and the social scripts perspective have thus far been inadequate in fully unpacking the origin of sexual messages, their propagation, and their social retention. Without identifying a primary, hierarchal, origin, it is likely that media is reflecting actual behavioral change in a circular way—media is a reflection of our evolutionary penchants, further exaggerated and supported by the presumption that it is popular.
Images of a polymorphous sexuality that decenters the reproductive motive and focuses instead on sexual pleasure are consistently appearing in popular media. In music lyrics, for example, although opera arias and art songs have contained messages about reproduction and mating for more than 400 years, it is contemporary music lyrics where an erotic uncommitted sexuality has predominated ( Hobbs & Gallup, 2011 ). Some popular portrayals go against the popular trend, such as American Idol star Kelly Clarkson’s Billboard Hot 100 song “I Do Not Hook Up,” released in 2009, cowritten and covered under the title “Hook Up” by American singer–songwriter Katy Perry. Other representations celebrate sexual liberation, such as Kylie Minogue’s “All the Lovers” and Madonna’s frequent reversal of male sexual dominance ( Guilbert, 2002 ). Hobbs and Gallup (2011) performed a content analysis of song lyrics from Billboard’s Top Ten charts for Country, Pop, and R&B. They found that of 174 different songs in the Top Ten lists from 2009, 92% contained messages about reproduction or mating, with the best-selling songs containing more such messages than less-successful songs: “the ubiquitous presence of these reproductive themes is a reflection of evolved properties in the human psyche, where people are voting with their pocket books and listener preferences are driving the lyrics” ( Hobbs & Gallup, 2011 , p. 404). It seems plausible that sexual scripts in popular entertainment media are exaggerated examples of behaviors that are taken to an extreme for the purposes of media sensationalism and activation of core guttural interests.
Conflicting gendered scripts may contribute to mixed perceptions and expectations of hookups. In a detailed qualitative study of girls’ first sexual experiences, Phillips (2000) made the case that conflicting media discourse messages make it difficult for women to navigate sexual initiation. The first sexual experiences described by the 30 participants were almost all quite negative (and, in some cases, horrific). Girls receive conflicting messages about being a “good girl” and a “pleasing woman,” but also a “together woman.” A “together woman” is agentic and experienced, such as the character Samantha from Sex in the City , who is sexually assertive and displays a strong, almost stereotypically masculine desire discourse. Many women find the discrepant messages difficult to navigate: to be a good girl, to be a “Samantha,” or to try and be both. Messages often portray the sexually assertive woman as a woman who has extreme difficulty in being genuine and having a meaningful romantic relationship. Psychoanalytic analysis views this conflict as the Madonna–whore dichotomy, where women face challenges in being viewed as both a sexually expressive being and a maternal committed being, and at the same time their romantic or sexual partners face challenges with categorizing women as one or the other ( Welldon, 1988 ). Presumably, these same conflicting discourse messages can make it difficult for individuals to psychologically navigate hookups, including sexual decision-making.
There seems to be inconsistency in the scripts pertaining to the casualness and emotional investment in causal sexual encounters. An example of this disconnect is presented by Backstrom, Armstrong, and Puentes (2012) , whose study examined the responses of 43 college women who described their difficulties in their negotiations of cunnilingus, such as desiring it in a hookup or not desiring it in a relationship. As another example, a qualitative study of men’s hookup scripts also displayed inconsistency in casualness ( Epstein, Calzo, Smiler, & Ward, 2009 ). Men easily described stereotypic hookups and FWBs as nonrelational and noncommitted, and in an oppositional fashion compared to romantic committed “dating-esque” relationships. Yet, in interviews, participants also expressed distinct discomfort with these extrarelational scripts. Men voiced alternative definitions that highlighted emotional connection and the potential for committed romantic relationships.
While contrary to no-strings attached hookup discourse, these alternative romance and commitment-oriented scripts are not surprising. Similar discourse messages are present in other aspects of popular media. This is consistent with Phillips’s (2000) conclusion that media messages are contradictory. In addition to media focused on casual sex, emerging adults have simultaneously been fed a Disney film diet with romantic relational scripts in which men and women live happily ever after, as heterosexual love conquers all ( Tanner, Haddock, Zimmerman, & Lund, 2003 ). It is curious that, although purporting to regale the audience with nonrelational sex, the previously mentioned films Friends with Benefits and No Strings Attached also highlight this; in the end, couples in both movies actually end up in seemingly monogamous romantic relationships. Although the evolutionary reproductive motives produce contradictory motivations, for both short-term sex and long-term commitment, some media scripts apparently do the same.
Hookups as More Than “Just Sex”
Despite the high prevalence of uncommitted sexual behavior, emerging adults often have competing nonsexual interests. In a study of 681 emerging adults, 63% of college-aged men and 83% of college-aged women preferred, at their current stage of life or development, a traditional romantic relationship as opposed to an uncommitted sexual relationship ( Garcia, Reiber, Merriwether, Heywood, & Fisher, 2010 ). Although there is a proportional sex difference, note that a substantial majority of both sexes would prefer a romantic relationship, despite their particular developmental stage of emerging adulthood. In another survey of 500 students who all had experiences with hookups, 65% of women and 45% of men reported that they hoped their hookup encounter would become a committed relationship, with 51% of women and 42% of men reporting that they tried to discuss the possibility of starting a relationship with their hookup partner ( Owen & Fincham, 2011 ). The gender differences observed are modest, and point to the convergence of gender roles in hookup culture; even though there are some gender differences, it should not be ignored that the curves overlap significantly.
Just as the discourse of hooking up is often in conflict with itself, individuals often self-identify a variety of motivations for hooking up. In one investigation of the concomitant motivations for hookups, Garcia and Reiber (2008) found that while 89% of young men and women reported that physical gratification was important, 54% reported emotional gratification and 51% reported a desire to initiate a romantic relationship; there were no sex differences in the responses. That a substantial portion of individuals reported emotional and romantic motivations appears to be in apparent conflict with the sexual strategies framework discussed earlier, which predicts significant sex differences. However, this is not in conflict with an evolutionary pair-bond hypothesis, which suggests that humans desire both sex and romantic intimacy ( Garcia & Reiber, 2008 ). Indeed, some hookups turn into romantic relationships. Paik (2010a) found that individuals in relationships that start as hookups or FWBs report lower average relationship satisfaction. However, this varied as a function of whether the participants initially wanted a relationship. If individuals were open to a serious committed relationship initially, relationship satisfaction was just as high as those who did not engage in (initially) uncommitted sexual activity prior to starting a relationship ( Paik, 2010a ). The entanglement of more intimate and emotional aspects with sex is something the romantic comedy movies mentioned earlier highlight.
Again in seeming contrast to the sex-specific mating strategies, contemporary hookup behavior involves a high degree of female sexual assertiveness for sexual desire and pleasure. In another study of self-reported motivations for hooking up, which included 118 female first-semester students, 80% indicated sexual desire, 58% spontaneous urge, 56% perceived attractiveness of the partner, 51% intoxication, 33% willingness of the partner, and 29% desire to feel attractive or desirable ( Fielder & Carey, 2010a ). Contrary to some media messages, individuals do not appear to be engaging in truly no-strings attached sex. Competing interests at multiple levels result in young adults having to negotiate multiple desires, and multiple social pressures. Again, the most fruitful explanation is that both men and women have competing sexual and romantic interests, with tremendous individual differences in such desires.
Not all sexual subcultures necessarily experience casual sex in the same “singles” context. As such, the simultaneous motivations for sex and romance may appear different. Beyond heterosexual hookups, casual sex (not necessarily referred to as “hookups”) has been reported to be a normative sexual script among men who have sex with men. Despite the existence of casual sex and open relationships among gay men, there is also a strong desire for romantic and companionate attachment ( Clarke & Nichols, 1972 ). Early ethnography by Cory (1951 ; also known as Edward Sagarin) described sections of gay culture as being “brought together, driven by the sensual impulse, seeking new forms and new partners for the love of the flesh, hoping to find excitement and satisfaction…” (p. 115). The origins of these pro-sex scripts have been theorized to be due to a subculture focused on male sexuality ( Mealey, 2000 ). Another explanation is the social relegation of gay men to the status of “deviant,” limiting access to socially sanctioned relationship scripts. However, discourse surrounding monogamy in gay relationships does demonstrate simultaneous desires for sexual variety and commitment, representing a kaleidoscope of issues about trust, love, and sexual behavior ( Worth, Reid, & McMillan, 2002 ). Because same-sex relationships are naturally removed from the reproductive motive, it may be possible that part of the larger hookup culture is borrowed from sexual subcultures involving greater emphasis on the positive erotic.
Hookup Culture and Sexual Risk
The negative consequences of hookups can include emotional and psychological injury, sexual violence, sexually transmitted infections, and/or unintended pregnancy. Despite various health risks, in a qualitative study of 71 college students (39 women and 32 men), nearly half of participants were unconcerned with contracting a sexually transmitted infection from penetrative intercourse during a hookup, and a majority were unconcerned about diseases in hookups that included fellatio or cunnilingus ( Downing-Matibag & Geisinger, 2009 ). Most students reported not considering or realizing their own health risks during hookups, particularly those that occurred within their own community such as with someone else on their own college campus. Compounding disease risks, individuals involved in hookups are more likely to have concurrent sexual partners ( Paik, 2010b ). In a sample of 1,468 college students, among the 429 students who had engaged in oral sex, anal sex, or vaginal intercourse in their most recent hookup, only 46.6% reported using a condom ( Lewis et al., 2011 ). Although, in Paul et al.’s (2000) study, conducted nearly a decade earlier, of those hookups that included sexual intercourse, a higher, yet still too low, 81% of participants reported using a condom. Among women in their first semester of college, Fielder and Carey (2010a) reported that condoms were used for 0% of oral sex hookups, and only 69% of vaginal sex hookups. Health-based hookup research like this may lead to programs for correcting misperceptions of sexual risk and sexual norms to ultimately restore individual locus of control over sexual behavior, reproductive rights, and healthy personal decision-making.
Prevalence of Alcohol and Drugs
In addition to sexual risk-taking, in terms of low condom use, another issue of concern involving hookups is the high comorbidity with substance use. As part of a larger study, in a sample of several thousand individuals aged 15–25, men and women who had used marijuana or cocaine in the last 12 months were also more likely than nonusers to have had nonmonogamous sex in the past 12 months ( van Gelder, Reefhuis, Herron, Williams, & Roeleveld, 2011 )—although an operational definition for these presumably uncommitted partnerships was not discussed. More specifically, in one study of undergraduate students, 33% of those reporting uncommitted sex indicated their motivation was “unintentional,” likely due to alcohol and other drugs ( Garcia & Reiber, 2008 ). In Fielder and Carey’s (2010a) study among 118 first-semester female college students, participants reported that 64% of uncommitted sexual encounters follow alcohol use, with a median consumption of 3 alcoholic drinks. Similarly, another study employing a web-based survey found that nearly 61% of undergraduate students used alcohol, with an average of 3.3 alcoholic drinks, during their most recent hookup ( Lewis et al., 2011 ). Further, in a study based on 71 interviews with college students, nearly 80% indicated that alcohol was involved in initiating their most recent hookup, with 64% attributing the progression and extent of the hookup to alcohol ( Downing-Matibag & Geisinger, 2009 ). Alcohol use has also been associated with type of hookup: greatest alcohol use was associated with penetrative sexual hookups, less alcohol use with nonpenetrative hookups, and least amount of alcohol use among those who did not hookup ( Owen, Fincham, & Moore, 2011 ). In one study of men and women who had engaged in an uncommitted sexual encounter that included vaginal, anal, or oral sex, participants reported their intoxication levels: 35% were very intoxicated, 27% were mildly intoxicated, 27% were sober, and 9% were extremely intoxicated ( Fisher et al., 2012 ). Alcohol and drug use drastically increases the overall risks of sexual activity ( Abbey, Ross, McDuffie, & McAuslan, 1996 ). Alcohol may also serve as an excuse, purposely consumed as a strategy to protect the self from having to justify hookup behavior later ( Paul, 2006 ). This paints a picture very different from popular representations of alcohol and substance use in hookups, which are often handled with a detached air of humor. For instance, the interactive book Hookups & Hangovers: A Journal ( Chronicle Books, 2011 ) is playfully described by the publisher: “here to help piece together all the hilarious and humiliating details of last night’s party. Playful prompts—including ‘Where did I wake up?’ and ‘So drunk, I can’t believe I…’ as well as space to rate your hookups and hangovers—make this guided journal the perfect accessory for the morning after.” These findings raise several concerns about the occurrence of hookups and the psychological impact such behaviors have on the individuals involved.
Although alcohol and drugs are likely a strong factor, it is still largely unclear what role individual differences play in shaping decisions to engage in hookups. In a sample of 394 young adults, the strongest predictor of hookup behavior was having previously hooked up—those who engaged in penetrative sex hookups were approximately 600% more likely than others to repeat this over the course of a university semester ( Owen et al., 2011 ). Other factors may include media consumption, personality, and biological predispositions. Garcia, MacKillop, et al. (2010) demonstrated an association between the dopamine D4 receptor gene polymorphism (DRD4 VNTR) and uncommitted sexual activity among 181 young men and young women. Although genotypic groups in this study did not vary in terms of overall number of sexual partners, individuals with a particular “risk-taking” variant of the dopamine receptor D4 gene (DRD4 VNTR; also associated with substance abuse) were shown to have a higher likelihood of having uncommitted sexual encounters (including infidelity and one-night stands)— however, no sex differences were observed. This suggests that biological factors that contribute to motivating the different contexts of sexual behavior for both men and women may be fairly sexually monomorphic ( Garcia, Reiber, et al., 2010 ). This may, in some cases, point to fairly stable individual differences.
Hookup Culture and Psychological Well-Being
The discrepancy between behaviors and desires, particularly with respect to social–sexual relationships, has dramatic implications for physical and mental health. Despite widespread allure, uncommitted sexual behavior has been shown to elicit a pluralistic ignorance response promoting individuals to engage in behaviors regardless of privately feeling uncomfortable with doing so ( Lambert et al., 2003 ; Reiber & Garcia, 2010 ). Individuals overestimate others’ comfort with hookups and assign variable meanings to those behaviors ( Lambert et al., 2003 ; Reiber & Garcia, 2010 ). Misperception of sexual norms is one potential driver for people to behave in ways they do not personally endorse. In a replication and extension of Lambert et al.’s study (2003) , Reiber and Garcia (2010) found that 78% of individuals overestimated others’ comfort with many different sexual behaviors, with men particularly overestimating women’s actual comfort with a variety of sexual behaviors in hookups.
Hookup scenarios may include feelings of pressure and performance anxiety. In Paul et al.’s (2000) study on hookups, 16% of participants felt pressured during their typical hookup. In this sample, 12% of participants felt out of control when penetrative intercourse was not involved while 22% percent felt out of control when sexual intercourse took place. Note that this study asked participants about typical hookups, and although this was informative for general patterns, it does not capture specific factors influencing specific individual scenarios. That is, it is unclear how one might rate a “typical” hookup if, for instance, one instance involved sexual coercion and regret while other hookup experiences before and/or after such an event were consenting and more enjoyable. In a multiethnic sample of 109 women, hookup scripts were compared to rape scripts, and, even though hookup scripts contained psychological consequences such as shame, a majority did not presume sexual assault ( Littleton, Tabernik, Canales, & Backstrom, 2009 ). Further, in a qualitative study that asked 187 participants to report their feelings after a typical hookup, 35% reported feeling regretful or disappointed, 27% good or happy, 20% satisfied, 11% confused, 9% proud, 7% excited or nervous, 5% uncomfortable, and 2% desirable or wanted ( Paul & Hayes, 2002 ). However, this same study found that feelings differed during compared to after hookups: during a typical hookup, 65% of participants reported feeling good, aroused, or excited, 17% desirable or wanted, 17% nothing in particular or were focused on the hookup, 8% embarrassed or regretful, 7% nervous or scared, 6% confused, and 5% proud ( Paul & Hayes, 2002 ). Just as multiple motivations can be in conflict, and multiple discourse messages can be in conflict, individuals’ affective reactions during and after a hookup can be in conflict.
An individual history of hookup behavior has been associated with a variety of mental health factors. In a recent study of 394 young adults followed across a university semester, those participants with more depressive symptoms and greater feelings of loneliness who engaged in penetrative sex hookups subsequently reported a reduction in both depressive symptoms and feelings of loneliness ( Owen et al., 2011 ). At the same time, those participants who reported less depressive symptoms and fewer feelings of loneliness who engaged in penetrative sex hookups subsequently reported an increase in both depressive symptoms and feelings of loneliness ( Owen et al., 2011 ). In another study, among 291 sexually experienced individuals, those who had the most regret after uncommitted sex also had more symptoms of depression than those who had no regret ( Welsh et al., 2006 ). However, in the same sample, women’s but not men’s degree of depressive symptoms increased with number of previous sex partners within the last year ( Welsh et al., 2006 ). In the first study to investigate the issue of self-esteem and hookups, both men and women who had ever engaged in an uncommitted sexual encounter had lower overall self-esteem scores compared to those without uncommitted sexual experiences ( Paul et al., 2000 ). The potential causal direction of the relationship between self-esteem and uncommitted sex is yet unclear ( Paul et al., 2000 ; Fielder & Carey, 2010b ).
Hookups can result in guilt and negative feelings. In a study of 169 sexually experienced men and women surveyed in singles bars, when presented with the question “I feel guilty or would feel guilty about having sexual intercourse with someone I had just met,” 32% of men and 72% of women agreed with the statement ( Herold & Mewhinney, 1993 ). The percentage of women expressing guilt was more than twice that of men. This is consistent with a classic study by Clark and Hatfield (1989) , which demonstrated that men are much more likely than women to accept casual sex offers from attractive confederates. Conley (2011) replicated and extended this finding, demonstrating that, under certain conditions of perceived comfort, the gender differences in acceptance of casual sex is diminished. In a study of 333 men and 363 women on a college campus, in deliberate hookup situations women had more thoughts of worry and vulnerability than men ( Townsend & Wasserman, 2011 ). Moreover, as number of sex partners increased, marital thoughts decreased, for both sexes ( Townsend & Wasserman, 2011 ).
Qualitative descriptions of hookups reveal relative gender differences in terms of feelings afterward, with women displaying more negative reactions than men ( Paul & Hayes, 2002 ). This is also consistent with earlier work demonstrating a gender difference, with women generally identifying more emotional involvement in seemingly “low investment” (i.e., uncommitted) sexual encounters than men ( Townsend, 1995 ). Moreover, in a study of 140 (109 female, 31 male) first-semester undergraduates, women, but not men, who had engaged in penetrative intercourse during a hookup showed higher rates of mental distress ( Fielder & Carey, 2010b ). Possibly contributing to findings on gender differences in thoughts of worry, in a sample of 507 undergraduate students, more women than men leaned toward a relationship outcome following a hookup. Only 4.4% of men and 8.2% of women (6.45% of participants) expected a traditional romantic relationship as an outcome, while 29% of men and 42.9% of women (36.57% of participants) ideally wanted such an outcome ( Garcia & Reiber, 2008 ). It is possible that regret and negative consequences result from individuals attempting to negotiate multiple desires. It is likely that a substantial portion of emerging adults today are compelled to publicly engage in hookups while desiring both immediate sexual gratification and more stable romantic attachments.
Not all hookup encounters are necessarily wanted or consensual. Individuals occasionally consent to engage in a sexual act but do not necessarily want sex ( Peterson & Muehlenhard, 2007 ). In a sample of 178 college students, participants noted that a majority of their unwanted sex occurred in the context of hookups: 77.8% during a hookup, 13.9% in an ongoing relationship, and 8.3% on a date ( Flack et al., 2007 ). Similarly, in a sample of 761 women students, approximately 50% of women reported at least one experience of unwanted sex ( Hill, Garcia, & Geher, 2012 ). Of those women, 70% experienced unwanted sex in the context of a hookup and 57% in the context of a committed romantic relationship ( Hill et al., 2012 ). Even more worrisome, a proportion of hookups also involve nonconsensual sex. In a study by Lewis et al. (2011) , 86.3% of participants portrayed their most recent hookup experience as one they wanted to have, while 7.6% indicated that their most recent hookup was an experience they did not want to have or to which they were unable to give consent. Unwanted and nonconsensual sexual encounters are more likely occurring alongside alcohol and substance use.
Hookup Regret
A number of studies have included measures of regret with respect to hookups, and these studies have documented the negative feelings men and women may feel after hookups. In a large web-based study of 1,468 undergraduate students, participants reported a variety of consequences: 27.1% felt embarrassed, 24.7% reported emotional difficulties, 20.8% experienced loss of respect, and 10% reported difficulties with a steady partner ( Lewis et al., 2011 ). In another recent study conducted on a sample of 200 undergraduate students in Canada, 78% of women and 72% of men who had uncommitted sex (including vaginal, anal, and/or oral sex) reported a history of experiencing regret following such an encounter ( Fisher et al., 2012 ). A vast majority of both sexes indicated having ever experienced regret. There were few sex differences in reasons for regret, and better quality sex reduced the degree of regret reported ( Fisher et al., 2012 ). It appears the method of asking participants whether and when they had experienced regret (i.e., ever, last hookup, or typical hookup) produces a sex difference, but in terms of categorical presence, it is most emerging adults who have experienced a kaleidoscope of reactions. This is consistent with Stinson’s (2010) message of sexual development requiring experimentation, including trial and error, and good feelings and bad feelings.
On average, both men and women appear to have higher positive affect than negative affect following a hookup. Those with positive attitudes toward hookups and approval of sexual activity show the greatest positive affect ( Lewis et al., 2011 ). However, there are also negative consequences experienced by both sexes. In a study of 270 sexually active college-aged students, 72% regretted at least one instance of previous sexual activity ( Oswalt, Cameron, & Koob, 2005 ). In a report of 152 female undergraduate students, 74% of women had either a few or some regrets from uncommitted sex: 61% had a few regrets, 23% had no regrets, 13% had some regrets, and 3% had many regrets ( Eshbaugh & Gute, 2008 ). Further, categorical presence of uncommitted sex in a female’s sexual history was related to higher overall regret scores from sexual activity, although regret due to lack of commitment was not specifically addressed. Two types of sexual encounters were particularly predictive of sexual regret: engaging in penetrative intercourse with someone known less than 24 hours and engaging in penetrative intercourse with someone only once. Among a sample of 1,743 individuals who had experienced a previous one-night stand, Campbell (2008) showed that most men and women have combinations of both positive and negative affective reactions following this event. Using evolutionary theory to predict responses of regret, Campbell (2008) showed that men had stronger feelings of being “sorry because they felt they used another person” whereas women had stronger feelings of “regret because they felt used.” Again, both men and women had experienced some sexual regret, but the frequency and intensity of negative reactions appeared to vary by sex, with women more negatively impacted from some hookup experiences.
There are substantial individual differences in reactions to hookups not accounted for by gender alone. Among a subsample of 311 young adults with hookup experience, when asked to generally characterize the morning after a hookup encounter, 82% of men and 57% of women were generally glad they had done it ( Garcia & Reiber, 2008 ). The gap between men and women is notable, and demonstrates an average sex difference in affective reactions. Yet, this finding also conflicts with a strict sexual strategies model because more than half of women were glad they engaged in a hookup (and they were not in the context of commandeering extrapartner genes for offspring). With respect to scripts, although presumably being sexually agentic (e.g., the “Samantha”), only slightly more than half of women were actually generally glad they had hooked up, suggesting these encounters may not truly be pleasurable for all. Similarly, in a study of 832 college students, 26% of women and 50% of men reported a positive emotional reaction following a hookup, and 49% of women and 26% of men reported a negative reaction (the remainders for each sex had a mix of both positive and negative reactions; Owen et al., 2010 ). These findings accord with the social sexual double standard creating greater pressure for women ( Crawford & Popp, 2003 ; Fisher et al., 2012 ). Although the direction of the sex differences is in agreement with the evolutionary model, that nearly a quarter of women report primarily positive reactions is inconsistent with a truly sex-specific short-term mating psychology and with discourse messages of uncommitted sex being simply pleasurable. Also inconsistent with both of these theoretical models is that a quarter of men experience negative reactions. Taken alone, neither a biological nor social model is sufficient to explain these individual differences.
Some research has considered the interactions of sex and individual differences in predicting hookup behavior. The Mating Intelligence Scale, designed to measure an individual’s cognitive abilities in the evolutionary domain of mating (see Geher & Kaufman, 2011 ), was used to assess hookup behavior in a sample of 132 college students. Young men higher in mating intelligence were more likely than others to have hooked up with strangers, acquaintances, and friends; while young women higher in mating intelligence were only more likely than others to have had more hookup experiences with acquaintances ( O’Brien, Geher, Gallup, Garcia, & Kaufman, 2009 ). The authors proposed that given the potential risks and costs of sex to females, sex with strangers would be disadvantageous; and because women do not generally report having sexual motives toward opposite sex friends ( Bleske-Rechek & Buss, 2001 ), women with high mating intelligence were likely striking the optimal balance, whereas men high in mating intelligence were obtaining maximum sexual encounters ( O’Brien et al., 2009 ). In this regard, there are sex differences in cognitive processes, but one cannot necessarily presume that the sexes vary fundamentally in their behavioral potentials; rather, they vary in their decision-making, consistent with other evolutionary models.
It is still unclear the degree to which hookups may result in positive reactions, and whether young men and young women are sexually satisfied in these encounters. Fine (1988) has argued that sex negativity is even more pronounced for women and the possibility of desire seems to be missing from the sexual education of young women. Armstrong, England, and Fogarty (2009) addressed sexual satisfaction in a large study of online survey responses from 12,295 undergraduates from 17 different colleges. Because cunnilingus often facilitates women’s orgasm, participants were asked about oral sex rates and orgasm in their most recent hookup and most recent relationship sexual event. In this study, men reported receiving oral sex both in hookups and in relationships much more than women. In first-time hookups, 55% included only men receiving oral sex, 19% only women receiving oral sex, and 27% both mutually receiving; in last relationship sexual activity, 32% included only men receiving oral sex, 16% included only women receiving oral sex, and 52% included both mutually receiving. In both contexts, men also reached orgasm more often than women. In first time hookups, 31% of men and 10% of women reached orgasm; in last relationship sexual activity, 85% of men and 68% of women reached orgasm. Armstrong et al. (2009) concluded with an important message:
A challenge to the contemporary sexual double standard would mean defending the position that young women and men are equally entitled to sexual activity, sexual pleasure, and sexual respect in hookups as well as relationships. To achieve this, the attitudes and practices of both men and women need to be confronted. Men should be challenged to treat even first hookup partners as generously as the women they hook up with treat them. (p. 377)
Taken together, this points to a need for further and more diverse attention to the impact of hookups on the physical and mental health of individuals, as recommended by Heldman and Wade (2010) . Further, more attention is needed on potential positive aspects of hooking up, such as promoting sexual satisfaction and mutual comfort and enjoyment (see Armstrong et al., 2009 ).
Hookups are part of a popular cultural shift that has infiltrated the lives of emerging adults throughout the Westernized world. The past decade has witnessed an explosion in interest in the topic of hookups, both scientifically and in the popular media. Research on hookups is not seated within a singular disciplinary sphere; it sits at the crossroads of theoretical and empirical ideas drawn from a diverse range of fields, including psychology, anthropology, sociology, biology, medicine, and public health. The growth of our understanding of the hookup phenomenon is likely predicated on our ability to integrate these theoretical and empirical ideas into a unified whole that is capable of explaining the tremendous variety in human sexual expression.
Both evolutionary and social forces are likely facilitating hookup behavior, and together may help explain the rates of hookups, motivations for hooking up, perceptions of hookup culture, and the conflicting presence and lack of sex differences observed in various studies. Several scholars have suggested that shifting life-history patterns may be influential in shaping hookup patterns. In the United States, age at first marriage and first reproduction has been pushed back dramatically, while at the same time age at puberty has dropped dramatically, resulting in a historically unprecedented time gap where young adults are physiologically able to reproduce but not psychologically or socially ready to “settle down” and begin a family and child rearing ( Bogle, 2007 ; Garcia & Reiber, 2008 ).
Together, the research reviewed here can help us better understand the nature of uncommitted sex today. It is worth noting, however, that several shortcomings in our knowledge continue to impede the understanding of hookup behavior. Both the historical transformations that have resulted in the reordering of sexual scripts and the demise of romantic courting among emerging adults remain mysterious ( Bogle, 2007 ; Heldman & Wade, 2010 ). Second, recall bias may affect individuals’ reports of previous romantic and sexual engagements; previous partners may be viewed as less desirable when individuals perceive their current partner as superior, thus creating a dissonance effect (see Geher et al., 2005 ). Much of the research asking participants about previous hookup relationships may therefore be biased due to recall. Third, there exists a vast and rich literature on men who have sex with men (MSM), specifically addressing casual sex and cruising among this population, and typically focused on sexual health and HIV prevention (see van Kesteren, Hospers, & Kok, 2007 ). The literature reviewed here primarily focuses on heterosexual hookups among emerging adults, with some researchers not controlling for sexual orientation (some purposefully) and others restricting to exclusively heterosexual samples. Future hookup research should venture into the MSM literature to explore patterns of casual sex among these populations to understand other sexual subcultures where uncommitted sexual behavior is prevalent. Moreover, there exists little published literature on the hookup patterns among lesbians and women who have sex with women. Last, the cross-cultural data provide a unique understanding of sexual behavior and romantic attachments; some societies engage in sex for pleasure and others for procreation (see Hatfield & Rapson, 2005 ; Gray & Garcia, 2013 ). Westernized culture often views sex as something for pleasure and fun (despite the frequency of behavioral patterns such as using the sexual “missionary” position and reduced female sexual stimulation), which dramatically influences our sexual perceptions, purposes, and pleasures ( Hatfield & Rapson, 2005 ; Gray & Garcia, 2013 ).
Understanding hookups during the critical stage of late adolescent development and young adulthood is paramount for protecting and promoting healthy sexuality and healthy decision-making among emerging adults. Of the varied experiences and health risks young men and young women will experience, perhaps none are as pervasive and widely experienced as engagement in and desire for romantic attachments and experiences with sexual activity. Indeed, cross-cultural anthropological literature suggests men and women will go to extreme lengths for love and sex ( Fisher, 1992 ; Hatfield & Rapson, 2005 ; Jankowiak & Paladino, 2008 ).
This review suggests that uncommitted sex, now being explored from a variety of disciplinary and theoretical perspectives, is best understood from a biopsychosocial perspective that incorporates recent research trends in human biology, reproductive and mental health, and sexuality studies. Both popular scripts and predictions from evolutionary theory suggest that a reproductive motive may influence some sexual patterns, such as motivation and regret following uncommitted sex. However, patterns of casual sex among gay men highlight inadequacies of the reproductive motive and suggest that further theorizing is necessary before a satisfactory evolutionarily informed theory can be established. Further, the findings that a majority of both men and women are motivated to engage in hookups, but often desire a more romantic relationship, is also consistent with a more nuanced evolutionary biopsychosocial perspective that takes into account social context and the cross-cultural and biological centrality of the pair-bond ( Fisher, 1992 ; Jankowiak & Fischer, 1992 ; Pedersen et al., 2011 ; Gray & Garcia, 2013 ). Hookups, although increasingly socially acceptable, may leave more “strings” than public discourse would suggest.
Acknowledgments
JRG is supported in part by the National Institute of Child Health and Human Development, National Institutes of Health (Grant T32HD049336). We thank Melanie Hill for valuable discussion and feedback on an earlier draft of this review. We also thank Maryanne Fisher and Catherine Salmon for helpful editorial feedback.
Contributor Information
Justin R. Garcia, The Kinsey Institute for Research in Sex, Gender, and Reproduction, Indiana University, Bloomington.
Chris Reiber, Graduate Program in Biomedical Anthropology, Department of Anthropology, Binghamton University.
Sean G. Massey, Women’s Studies Program, Binghamton University.
Ann M. Merriwether, Departments of Psychology and Human Development, Binghamton University.
- Abbey A, Ross LT, McDuffie D, McAuslan P. Alcohol and dating risk factors for sexual assault among college women. Psychology of Women Quarterly. 1996; 20 :147–169. doi: 10.1111/j.1471-6402.1996.tb00669.x. [ CrossRef ] [ Google Scholar ]
- Alcock J. Animal behavior: An evolutionary approach. 8. Sunderland, MA: Sinauer Associates; 2005. [ Google Scholar ]
- Altman D. Homosexual: Oppression and liberation. New York, NY: Outerbridge & Dienstfrey; 1971. [ Google Scholar ]
- Altman D. The homosexualization of America: The Americanization of the homosexual. New York, NY: St Martin’s; 1982. [ Google Scholar ]
- Armstrong EA, England P, Fogarty ACK. Orgasm in college hookups and relationships. In: Risman BJ, editor. Families as they really are. New York, NY: Norton; 2009. pp. 362–377. [ Google Scholar ]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000; 55 :469–480. doi: 10.1037/0003-066X.55.5.469. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Backstrom L, Armstrong EA, Puentes J. Women’s negotiations of cunnilingus in college hookups and relationships. Journal of Sex Research. 2012; 49 :1–12. doi: 10.1080/00224499.2011.585523. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bailey BL. From front porch to back seat: Courtship in twentieth century America. Baltimore, MD: Johns Hopkins University Press; 1988. [ Google Scholar ]
- Bateman AJ. Intra-sexual selection in. Drosophila Heredity. 1948; 2 :349–368. doi: 10.1038/hdy.1948.21. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Binson D, Woods WJ, Pollack L, Paul J, Stall R, Catania JA. Differential HIV risk in bathhouses and public cruising areas. American Journal of Public Health. 2001; 91 :1482–1486. doi: 10.2105/AJPH.91.9.1482. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bisson MA, Levine TR. Negotiating a friends with benefits relationship. Archives of Sexual Behavior. 2009; 38 :66–73. doi: 10.1007/s10508-007-9211-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Black GD. Hollywood censored. Cambridge, MA: Cambridge University Press; 1994. [ Google Scholar ]
- Bleske-Rechek AL, Buss DM. Opposite-sex friendship: Sex differences and similarities in initiation, selection, and dissolution. Personality and Social Psychology Bulletin. 2001; 27 :1310–1323. doi: 10.1177/01461672012710007. [ CrossRef ] [ Google Scholar ]
- Bogle KA. The shift from dating to hooking up in college: What scholars have missed. Sociology Compass. 2007; 1/2 :775–788. [ Google Scholar ]
- Bogle KA. Hooking up: Sex, dating, and relationships on campus. New York, NY: New York University Press; 2008. [ Google Scholar ]
- Boswell AA, Spade JZ. Fraternities and collegiate rape culture: Why are some fraternities more dangerous places for women? Gender & Society. 1996; 10 :133–147. doi: 10.1177/089124396010002003. [ CrossRef ] [ Google Scholar ]
- Bradshaw C, Kahn AS, Saville BK. To hook up or date: Which gender benefits? Sex Roles. 2010; 62 :661–669. doi: 10.1007/s11199-010-9765-7. [ CrossRef ] [ Google Scholar ]
- Buss DM. Sexual strategies theory: Historical origins and current status. Journal of Sex Research. 1998; 35 :19–31. doi: 10.1080/00224499809551914. [ CrossRef ] [ Google Scholar ]
- Buss DM, Schmitt DP. Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review. 1993; 100 :204–232. doi: 10.1037/0033-295X.100.2.204. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Campbell A. The morning after the night before: Affective reactions to one-night stands among mated and unmated women and men. Human Nature. 2008; 19 :157–173. doi: 10.1007/s12110-008-9036-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cates W. Teenagers and sexual risk taking: The best of times and the worst of times. Journal of Adolescent Health. 1991; 12 :84–94. doi: 10.1016/0197-0070(91)90449-V. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chia SC, Gunther AC. How media contribute to misperceptions of social norms about sex. Mass Communication & Society. 2006; 9 :301–320. doi: 10.1207/s15327825mcs0903_3. [ CrossRef ] [ Google Scholar ]
- Chronicle Books. Hook-ups & hangovers: A journal. San Francisco, CA: Chronicle Books; 2011. [ Google Scholar ]
- Clark RD, Hatfield E. Gender differences in receptivity to sexual offers. Journal of Psychology & Human Sexuality. 1989; 2 :39–55. doi: 10.1300/J056v02n01_04. [ CrossRef ] [ Google Scholar ]
- Clarke L, Nichols J. I have more fun with you than anybody. New York, NY: St. Martin’s; 1972. [ Google Scholar ]
- Clutton-Brock TH, Parker GA. Potential reproductive rates and the operation of sexual selection. Quarterly Review of Biology. 1992; 67 :437–456. doi: 10.1086/417793. [ CrossRef ] [ Google Scholar ]
- Conley TD. Perceived proposer personality characteristics and gender differences in acceptance of casual sex offers. Journal of Personality and Social Psychology. 2011; 100 :309–329. doi: 10.1037/a0022152. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coontz S. Marriage, a history: How love conquered marriage. New York, NY: Penguin Books; 2005. [ Google Scholar ]
- Cory DW. The homosexual in America. New York, NY: Greenberg; 1951. [ Google Scholar ]
- Crawford M, Popp D. Sexual double standards: A review and methodological critique of two decades of research. Journal of Sex Research. 2003; 40 :13–26. doi: 10.1080/00224490309552163. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Daly M. The cost of mating. American Naturalist. 1978; 112 :771–774. doi: 10.1086/283319. [ CrossRef ] [ Google Scholar ]
- Doherty T. Pre-code Hollywood. New York, NY: Columbia University Press; 1999. [ Google Scholar ]
- Downing-Matibag TM, Geisinger B. Hooking up and sexual risk taking among college students: A health belief model perspective. Qualitative Health Research. 2009; 19 :1196–1209. doi: 10.1177/1049732309344206. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eliot J, Stilwell M. All the Lovers [Recorded by Kylie Minogue] On Aphrodite [CD] Parlophone 2010 [ Google Scholar ]
- Ellis A. Sex without guilt. New York, NY: Lyle Stuart; 1958. [ Google Scholar ]
- England P, Shafer EF, Fogarty ACK. Hooking up and forming romantic relationships on today’s college campuses. In: Kimmel MS, Aronson A, editors. The gendered society reader. 3. New York, NY: Oxford University Press; 2007. pp. 531–547. [ Google Scholar ]
- Epstein M, Calzo JP, Smiler AP, Ward LM. “Anything from making out to having sex”: Men’s negotiations of hooking up and friends with benefits scripts. Journal of Sex Research. 2009; 46 :414–424. doi: 10.1080/00224490902775801. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eshbaugh EM, Gute G. Hookups and sexual regret among college women. The Journal of Social Psychology. 2008; 148 :77–89. doi: 10.3200/SOCP.148.1.77-90. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fielder RL, Carey MP. Prevalence and characteristics of sexual hookups among first-semester female college students. Journal of Sex & Marital Therapy. 2010a; 36 :346–359. doi: 10.1080/0092623X.2010.488118. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fielder RL, Carey MP. Predictors and consequences of sexual “hookups” among college students: A short-term prospective study. Archives of Sexual Behavior. 2010b; 39 :1105–1119. doi: 10.1007/s10508-008-9448-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fine M. Sexuality, schooling and adolescent females: The missing discourse of desire. Harvard Educational Review. 1988; 58 :29–53. [ Google Scholar ]
- Finer LB. Trends in premarital sex in the United States, 1954 –2003. Public Health Reports. 2007; 122 :73–78. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fisher HE. Anatomy of love: The natural history of monogamy, adultery, and divorce. New York, NY: W. W. Norton & Company; 1992. [ Google Scholar ]
- Fisher HE. Serial monogamy and clandestine adultery: Evolution and consequences of the dual human reproductive strategy. In: Roberts SC, editor. Applied Evolutionary Psychology. New York, NY: Oxford University Press; 2011. [ CrossRef ] [ Google Scholar ]
- Fisher ML, Worth K, Garcia JR, Meredith T. Feelings of regret following uncommitted sexual encounters in Canadian university students. Culture, Health & Sexuality. 2012; 14 :45–57. doi: 10.1080/13691058.2011.619579. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Flack WF, Daubman KA, Caron ML, Asadorian JA, D’Aureli NR, Gigliotti SN, Stine ER. Risk factors and consequences of unwanted sex among university students: Hooking up, alcohol, and stress response. Journal of Interpersonal Violence. 2007; 22 :139–157. doi: 10.1177/0886260506295354. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frayser SG. Varieties of sexual experience: An anthropological perspective on human sexuality. New Haven, CT: HRAF Press; 1985. [ Google Scholar ]
- Friends with benefits. Urbandictionary.com. 2003 Retrieved from http://www.urbandictionary.com/define.php?term-friends%20with%20benefits .
- Friends with benefits. Urbandictionary.com. 2005 Retrieved from http://www.urbandictionary.com/define.php?term-friends%20with%20benefits .
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences. 2000; 23 :573–587. doi: 10.1017/S0140525X0000337X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gangestad SW, Thornhill R. The evolutionary psychology of extra-pair sex: The role of fluctuating asymmetry. Evolution and Human Behavior. 1997; 18 :69–88. doi: 10.1016/S1090-5138(97)00003-2. [ CrossRef ] [ Google Scholar ]
- Garcia JR, Kruger DJ. Unbuckling in the Bible Belt: Conservative sexual norms lower age at marriage. The Journal of Social, Evolutionary, and Cultural Psychology. 2010; 4 :206–214. [ Google Scholar ]
- Garcia JR, MacKillop J, Aller EL, Merriwether AM, Wilson DS, Lum JK. Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PLoS ONE. 2010; 5 :e14162. doi: 10.1371/journal.pone.0014162. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garcia JR, Reiber C. Hook-up behavior: A biopsychosocial perspective. The Journal of Social, Evolutionary, and Cultural Psychology. 2008; 2 :192–208. [ Google Scholar ]
- Garcia JR, Reiber C, Merriwether AM, Heywood LL, Fisher HE. Touch me in the morning: Intimately affiliative gestures in uncommitted and romantic relationships. Paper presented at the Annual Conference of the NorthEastern Evolutionary Psychology Society; New Paltz, NY. 2010a. Mar, [ Google Scholar ]
- Geher G, Bloodworth R, Mason J, Stoaks C, Downey HJ, Renstrom KL, Romero JF. Motivational underpinnings of romantic partner perceptions: Psychological and physiological evidence. Journal of Personal and Social Relationships. 2005; 22 :255–281. doi: 10.1177/0265407505050953. [ CrossRef ] [ Google Scholar ]
- Geher G, Kaufman SB. Mating intelligence. In: Sternberg RJ, Kaufman SB, editors. The Cambridge handbook of intelligence. Cambridge, England: Cambridge University Press; 2011. pp. 603–620. [ Google Scholar ]
- Glenn N, Marquardt E. Hooking up, hanging out, and hoping for Mr Right: College women on dating and mating today. New York, NY: Institute for American Values; 2001. [ Google Scholar ]
- Gray PB, Anderson KG. Fatherhood: Evolution and human paternal behavior. Cambridge, MA: Harvard University Press; 2010. [ Google Scholar ]
- Gray PB, Garcia JR. Darwin’s bedroom: Evolution and human sexual behavior. 2013. Manuscript submitted for publication. [ Google Scholar ]
- Greenspan S. 11 points guide to hooking up: Lists and advice about first dates, hotties, scandals, pickups, threesomes, and booty calls. New York, NY: Skyhorse Publishing; 2011. [ Google Scholar ]
- Grello CM, Welsh DP, Harper MS, Dickson JW. Dating and sexual relationship trajectories and adolescent functioning. Adolescent & Family Health. 2003; 3 :103–112. [ Google Scholar ]
- Guilbert G-C. Madonna as Postmodern Myth: How One Star’s Self-Construction Rewrites Sex, Gender, Hollywood and the American Dream. Jefferson, NC: McFarland; 2002. [ Google Scholar ]
- Gute G, Eshbaugh EM. Personality as a predictor of hooking up among college students. Journal of Community Health Nursing. 2008; 25 :26–43. doi: 10.1080/07370010701836385. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harper GW. Sex isn’t that simple: Culture and context in HIV prevention interventions for gay and bisexual male adolescents. American Psychologist. 2007; 62 :803–819. doi: 10.1037/0003-066X.62.8.806. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hatfield E, Hutchison ESS, Bensman L, Young DM, Rapson RL. Cultural, social, and gender influences on casual sex: New developments. In: Turner Jan M, Mitchell Andrew D., editors. Social psychology: New developments. Hauppauge, NY: Nova Science; in press. [ Google Scholar ]
- Hatfield E, Rapson RL. Love and sex: Cross-cultural perspectives. Lanham, MD: University Press of America; 2005. [ Google Scholar ]
- Heldman C, Wade L. Hook-up culture: Setting a new research agenda. Sexuality Research and Social Policy. 2010; 7 :323–333. doi: 10.1007/s13178-010-0024-z. [ CrossRef ] [ Google Scholar ]
- Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behavior in the United States: Results from a national probability sample of men and women ages 14 –94. Journal of Sexual Medicine. 2010; 7 :255–265. doi: 10.1111/j.1743-6109.2010.02012.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Herbert S, McKernan L. Who’s who of Victorian cinema. London, England: British Film Institute; 1996. [ Google Scholar ]
- Herold ES, Mewhinney DK. Gender differences in casual sex in AIDS prevention: A survey of dating bars. Journal of Sex Research. 1993; 15 :502–516. [ Google Scholar ]
- Hill M, Garcia JR, Geher G. Women having sex when they don’t want to: Exploring the occurrence of unwanted sex in the context of hook-ups. 2012. Manuscript submitted for publication. [ Google Scholar ]
- Hobbs DR, Gallup GG. Song as a medium for embedded reproductive messages. Evolutionary Psychology. 2011; 9 :390– 416. [ PubMed ] [ Google Scholar ]
- Hoff CC, Beougher SC. Sexual agreements among gay male couples. Archives of Sexual Behavior. 2010; 39 :774–787. doi: 10.1007/s10508-008-9393-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holman A, Sillars A. Talk about “hooking up”: The influence of college student social networks on nonrelationship sex. Health Communication. 2012; 27 :205–216. doi: 10.1080/10410236.2011.575540. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jankowiak WR, Fischer EF. A cross-cultural perspective on romantic love. Ethnology. 1992; 31 :149–155. doi: 10.2307/3773618. [ CrossRef ] [ Google Scholar ]
- Jankowiak WR, Paladino T. Desiring sex, longing for love: A tripartite conundrum. In: Jankowiak WR, editor. Intimacies: Love and sex across cultures. New York, NY: Columbia University Press; 2008. pp. 1–36. [ Google Scholar ]
- Jhally S. Dreamworlds 3: Desire, sex & power in music video [DVD] Northampton, MA: Media Education Foundation; 2007. [ Google Scholar ]
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: Sexual but more emotional than one-night stands. Journal of Sex Research. 2011; 48 :486–495. doi: 10.1080/00224499.2010.497984. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Josiam BM, Hobson JSP, Dietrich UC, Smeaton G. An analysis of the sexual, alcohol and drug related behavioural patterns of students on spring break. Tourism Management. 1998; 19 :501–513. doi: 10.1016/S0261-5177(98)00052-1. [ CrossRef ] [ Google Scholar ]
- Kim JL, Sorsoli CL, Collins K, Zylbergold BA, Schooler D, Tolman DL. From sex to sexuality: Exposing the heterosexual script on primetime network television. Journal of Sex Research. 2007; 44 :145–157. doi: 10.1080/00224490701263660. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia, PA: W. B. Saunders; 1948. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kinsey AC, Pomeroy WB, Martin CE, Gebhard PH. Sexual behavior in the human female. Philadelphia, PA: W. B. Saunders; 1953. [ Google Scholar ]
- Kunkel D, Eyal K, Finnerty K, Biely E, Donnerstein E. Sex on TV 4. Menlo Park, CA: Henry J. Kaiser Family Foundation; 2005. [ Google Scholar ]
- Lambert TA, Kahn AS, Apple KJ. Pluralistic ignorance and hooking up. Journal of Sex Research. 2003; 40 :129–133. doi: 10.1080/00224490309552174. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laumann E, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: Sexual practices in the United States. Chicago, IL: University of Chicago Press; 1994. [ Google Scholar ]
- Lewis MA, Granato H, Blayney JA, Lostutter TW, Kilmer JR. Predictors of hooking up sexual behavior and emotional reactions among U.S. college students. Archives of Sexual Behavior. 2011 doi: 10.1007/s10508-011-9817-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li NP, Kenrick DT. Sex similarities and differences in preferences for short-term mates: What, whether, and why. Journal of Personality and Social Psychology. 2006; 90 :468–489. doi: 10.1037/0022-3514.90.3.468. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lippa RA. Sex differences in sex drive, sociosexuality, and height across 53 nations: testing evolutionary and social structural theories. Archives of Sexual Behavior. 2009; 38 :631– 651. [ PubMed ] [ Google Scholar ]
- Littleton H, Tabernik H, Canales EJ, Backstrom T. Risky situation or harmless fun? A qualitative examination of college women’s bad hook-up and rape scripts. Sex Roles. 2009; 60 :793–804. doi: 10.1007/s11199-009-9586-8. [ CrossRef ] [ Google Scholar ]
- Low BS. Why sex matters. Princeton, NJ: Princeton University Press; 2000. [ Google Scholar ]
- Madison A. Hooking up: A girl’s all-out guide to sex and sexuality. New York, NY: Prometheus Books; 2006. [ Google Scholar ]
- Manning WS, Giordano PC, Longmore MA. Hooking up: The relationship contexts of “nonrelationship” sex. Journal of Adolescent Research. 2006; 21 :459–483. doi: 10.1177/0743558406291692. [ CrossRef ] [ Google Scholar ]
- Maticka-Tyndale E. Sexual scripts and AIDS prevention: Variations in adherence to safer-sex guidelines by heterosexual adolescents. Journal of Sex Research. 1991; 28 :45–66. doi: 10.1080/00224499109551594. [ CrossRef ] [ Google Scholar ]
- Maticka-Tyndale E, Herold ES, Mewhinney DM. Casual sex on spring break: Intentions and behaviors of Canadian students. Journal of Sex Research. 1998; 35 :254–264. doi: 10.1080/00224499809551941. [ CrossRef ] [ Google Scholar ]
- Mealey L. Sex differences: Developmental and evolutionary strategies. London, England: Academic Press; 2000. [ Google Scholar ]
- Mustanski B, Lyons T, Garcia SC. Internet use and sexual health of young men who have sex with men: A mixed-methods study. Archives of Sexual Behavior. 2011; 40 :289–300. doi: 10.1007/s10508-009-9596-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Brien DT, Geher G, Gallup AC, Garcia JR, Kaufman SB. Self-perceived Mating Intelligence predicts sexual behavior in college students: Empirical validation of a theoretical construct. Imagination, Cognition and Personality. 2009; 29 :341–362. doi: 10.2190/IC.29.4.e. [ CrossRef ] [ Google Scholar ]
- Oliver MB, Hyde JS. Gender differences in sexuality: A meta-analysis. Psychological Bulletin. 1993; 114 :29–51. doi: 10.1037/0033-2909.114.1.29. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oswalt SB, Cameron KA, Koob JJ. Sexual regret in college students. Archives of Sexual Behavior. 2005; 34 :663–669. doi: 10.1007/s10508-005-7920-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Owen J, Fincham FD. Young adults’ emotional reactions after hooking up encounters. Archives of Sexual Behavior. 2011; 40 :321–330. doi: 10.1007/s10508-010-9652-x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Owen J, Fincham FD, Moore J. Short-term prospective study of hooking up among college students. Archives of Sexual Behavior. 2011; 40 :331–341. doi: 10.1007/s10508-010-9697-x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Owen JJ, Rhoades GK, Stanley SM, Fincham FD. “Hooking up” among college students: Demographic and psychosocial correlates. Archives of Sexual Behavior. 2010; 39 :653–663. doi: 10.1007/s10508-008-9414-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paik A. “Hookups,” dating, and relationship quality: Does the type of sexual involvement matter? Social Science Research. 2010a; 39 :739–753. doi: 10.1016/j.ssresearch.2010.03.011. [ CrossRef ] [ Google Scholar ]
- Paik A. The contexts of sexual involvement and concurrent sexual partnerships. Perspectives on Sexual and Reproductive Health. 2010b; 42 :33–42. doi: 10.1363/4203310. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paul EL. Beer goggles, catching feelings, and the walk of shame: The myths and realities of the hookup experience. In: Kirkpatrick DC, Duck S, Foley MK, editors. Relating difficulty: The processes of constructing and managing difficult interaction. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. pp. 141–160. [ Google Scholar ]
- Paul EL, Hayes KA. The casualties of “casual” sex: A qualitative exploration of the phenomenology of college students’ hookups. Journal of Social and Personal Relationships. 2002; 19 :639–661. doi: 10.1177/0265407502195006. [ CrossRef ] [ Google Scholar ]
- Paul EL, McManus B, Hayes A. “Hook-ups”: Characteristics and correlates of college students’ spontaneous and anonymous sexual experiences. Journal of Sex Research. 2000; 37 :76–88. doi: 10.1080/00224490009552023. [ CrossRef ] [ Google Scholar ]
- Pedersen WC, Putcha-Bhagavatula A, Miller LC. Are men and women really that different? Examining some of sexual strategies theory (SST)’s key assumptions about sex-distinct mating mechanisms. Sex Roles. 2011; 64 :629–643. doi: 10.1007/s11199-010-9811-5. [ CrossRef ] [ Google Scholar ]
- Penhollow T, Young M, Bailey W. Relationship between religiosity and “hooking up” behavior. American Journal of Health Education. 2007; 38 :338–345. [ Google Scholar ]
- Perry K, Gottwald L, Martin M, McKee B. On Teenage dream [CD] Vol. 2010 New York, NY: Capitol Records; 2011. Last Friday night (T.G.I.F.) [Recorded by K. Perry] [ Google Scholar ]
- Petersen JL, Hyde JS. A meta-analytic review of research on gender differences in sexuality, 1993–2007. Psychological Bulletin. 2010; 136 :21–38. doi: 10.1037/a0017504. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Peterson ZD, Muehlenhard CL. Conceptualizing the “wantedness” of women’s consensual and nonconsensual sexual experiences: Implications for how women label their experiences with rape. Journal of Sex Research. 2007; 44 :72– 88. [ PubMed ] [ Google Scholar ]
- Phillips LM. Flirting with danger. New York, NY: New York University Press; 2000. [ Google Scholar ]
- Reiber C, Garcia JR. Hooking up: Gender differences, evolution, and pluralistic ignorance. Evolutionary Psychology. 2010; 8 :390–404. [ PubMed ] [ Google Scholar ]
- Reitman I, Medjuck J, Clifford J, Reitman I. No Strings Attached [Motion picture] United States: Paramount Pictures; [ Google Scholar ]
- Riley C, editor. Making the hook-up: Edgy sex with soul. Berkeley, CA: Cleis Press; 2010. [ Google Scholar ]
- Robertson P. Film facts. New York, NY: Billboard Books; 2001. [ Google Scholar ]
- Rozler J, Lavinthal A. The hookup handbook: A single girl’s guide to living it up. New York, NY: Simon Spotlight Entertainment; 2005. [ Google Scholar ]
- Saad G. The evolutionary bases of consumption. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. [ Google Scholar ]
- Schmitt DP. Sociosexuality from Argentina to Zimbabwe: A 48-nation study of sex, culture, and strategies of human mating. Behavioral and Brain Sciences. 2005; 28 :247–275. doi: 10.1017/S0140525X05000051. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schmitt DP, Alcalay L, Allik J, Ault L, Austers I, Bennett KL, Zupanéié A. Universal sex differences in the desire for sexual variety: Tests from 52 nations, 6 continents, and 13 islands. Journal of Personality and Social Psychology. 2003; 85 :85–104. doi: 10.1037/0022-3514.85.1.85. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seage GR, Mayer KH, Lenderking WR, Wold C, Gross M, Goldstein R, Holmberg S. HIV and hepatitis B infection and risk behavior in young gay and bisexual men. Public Health Reports. 1997; 112 :158–167. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shafer M, Glotzer L, Zucker J, Gluck W, Gluck W. Friends with Benefits [Motion picture] United States: Sony Pictures; [ Google Scholar ]
- Sherman AJ, Tocantins N. The happy hook-up: A single girl’s guide to casual sex. Berkeley, CA: Ten Speed Press; 2004. [ Google Scholar ]
- Siegel J, Rowland M, Scordia V, Scordia V. Hooking Up [Motion picture] United States: Morbid Mind Productions; 2009. [ Google Scholar ]
- Simon W, Gagnon JH. Sexual scripts: Permanence and change. Archives of Sexual Behavior. 1986; 15 :97–120. doi: 10.1007/BF01542219. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simpson JA, Gangestad SW. Sociosexuality and romantic partner choice. Journal of Personality. 1992; 60 :31–51. doi: 10.1111/j.1467-6494.1992.tb00264.x. [ CrossRef ] [ Google Scholar ]
- Sönmez S, Apostolopoulos Y, Yu CH, Yang S, Mattila AS, Yu LC. Binge drinking and casual sex on spring-break. Annals of Tourism Research. 2006; 33 :895–917. doi: 10.1016/j.annals.2006.06.005. [ CrossRef ] [ Google Scholar ]
- Stinson RD. Hooking up in young adulthood: A review of factors influencing the sexual behavior of college students. Journal of College Student Psychotherapy. 2010; 24 :98–115. doi: 10.1080/87568220903558596. [ CrossRef ] [ Google Scholar ]
- Strasburger VC. Adolescents, sex, and the media: Ooooo, baby, baby—A Q&A. Adolescent Medicine Clinics. 2005; 16 :269–288. doi: 10.1016/j.admecli.2005.02.009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Symons D. The evolution of human sexuality. New York, NY: Oxford University Press; 1979. [ Google Scholar ]
- Tanner LR, Haddock SA, Zimmerman TS, Lund LK. Images of couples and families in Disney feature-length animated films. American Journal of Family Therapy. 2003; 31 :355–373. doi: 10.1080/01926180390223987. [ CrossRef ] [ Google Scholar ]
- Tolman DL. In a different position: Conceptualizing female adolescent sexuality development within compulsory heterosexuality. New Directions for Child and Adolescent Development. 2006; 112 :71–89. doi: 10.1002/cd.163. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Townsend JM. Sex without emotional involvement: An evolutionary interpretation of sex differences. Archives of Sexual Behavior. 1995; 24 :173–206. doi: 10.1007/BF01541580. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Townsend JM, Wasserman TH. Sexual hookups among college students: Sex differences in emotional reactions. Archives of Sexual Behavior. 2011; 40 :1173–1181. doi: 10.1007/s10508-011-9841-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Trivers R. Parental investment and sexual selection. In: Camp-bell B, editor. Sexual selection and the descent of man: 1871–1971. Chicago, IL: Aldine Press; 1972. pp. 136–179. [ Google Scholar ]
- van Gelder MMHJ, Reefhuis J, Herron AM, Williams ML, Roeleveld N. Reproductive health characteristics of marijuana and cocaine users: Results from the 2002 National Survey of Family Growth. Perspectives on Sexual and Reproductive Health. 2011; 43 :164–172. doi: 10.1363/4316411. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Kesteren NMC, Hospers HJ, Kok G. Sexual risk behavior in HIV-positive men who have sex with men: A literature review. Patient Education and Counseling. 2007; 65 :5–20. doi: 10.1016/j.pec.2006.09.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Valen L. A new evolutionary law. Evolutionary Theory. 1973; 1 :1–30. [ Google Scholar ]
- Vieira M. Sin in soft focus. New York, NY: Harry Abrams; 1999. [ Google Scholar ]
- Ward LM. Talking about sex: Common themes about sexuality in the prime-time television programs children and adolescents view most. Journal of Youth and Adolescence. 1995; 24 :595–615. doi: 10.1007/BF01537058. [ CrossRef ] [ Google Scholar ]
- Ward LM. Understanding the role of entertainment media in the sexual socialization of American youth: A review of empirical research. Developmental Review. 2003; 23 :347–388. doi: 10.1016/S0273-2297(03)00013-3. [ CrossRef ] [ Google Scholar ]
- Welldon EV. Mother, Madonna, whore: The idealization and denigration of motherhood. London, England: Free Association Books; 1988. [ Google Scholar ]
- Wells G, Perry K, DioGuardi K. On All I Ever Wanted [CD] RCA. 2009. I Do Not Hook Up [Recorded by Kelly Clarkson] [ Google Scholar ]
- Welsh DP, Grello CM, Harper MS. No strings attached: The nature of casual sex in college students. Journal of Sex Research. 2006; 43 :255–267. doi: 10.1080/00224490609552324. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wiederman MW. The gendered nature of sexual scripts. The Family Journal. 2005; 13 :496–502. doi: 10.1177/1066480705278729. [ CrossRef ] [ Google Scholar ]
- Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychological Bulletin. 2002; 128 :699–727. doi: 10.1037/0033-2909.128.5.699. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Worth H, Reid A, McMillan K. Somewhere over the rainbow: Love, trust and monogamy in gay relationships. Journal of Sociology. 2002; 38 :237–253. doi: 10.1177/144078302128756642. [ CrossRef ] [ Google Scholar ]
- Zablotska I, Frankland A, Prestage G, Down I, Ryan D. Gay Community Periodic Survey: Sydney February 2008. Sydney, Australia: National Centre in HIV Social Research, The University of New South Wales; 2008. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
A noncausal relation between casual sex in adolescence and early adult depression and suicidal ideation: A longitudinal discordant twin study. Journal of Sex Research, 52, 770-780. 10.1080/00224499.2014.942413 [Google Scholar] *Dubé S, Lavoie F, Blais M, & Hébert M (2017a).
This finding is in line with prior research, confirming that while single people may have greater opportunities for casual sex with a greater number of partners, people in a relationship have more frequent sex [14-16]. Interestingly, people that reported being in a casual relationship(s) reported a higher frequency of sex and more sexual ...
Introduction. Gender differences in attitudes toward casual sex have been widely studied. It has been reported that between 44 and 75% of young adults between the ages of 18 and 25 have experienced at least one casual sexual encounter within their lives (Flack et al., 2016; Lyons et al., 2014; Maticka-Tyndale et al., 2003).Casual sex, also known as a hookup or one-night stand, can be described ...
This was especially pronounced in those who expressed a preference for casual sex encounters over romantic relationships: in a casual sex context these participants were about 1.5 times as likely to cuddle, 1.5 times as likely to spend the night and cuddle, and nearly 5 times as likely to engage in foreplay with a partner.
Casual sexual relationships and experiences (CSREs) are common and emotionally significant occurrences. ... J Sex Res. 2021 Oct;58(8):1069-1084. doi: 10.1080/00224499.2020.1821163. Epub 2020 Sep 29. ... 3 Nebraska Center for Research on Children, Youth, Families and Schools, University of Nebraska- Lincoln. PMID: 32991206
Casual sex, also referred to as a hookup, has been associated with a range of negative emotional outcomes for women, including regret, anxiety, depression and social stigma. However, it has been argued that it is the nature of the sexual motivation, not gender that influences the emotional outcome. This study was designed to ascertain what motivates people to have casual sex, what emotional ...
1 Introduction. In the last decade there has been an abundance of research on the topic of casual sex, often fueled by debates as to whether the phenomenon represents the "new" state of romantic and sexual experience in the developed world, and often peppered with concerns about sexual "risk.". The focus of much of the research on ...
Casual sexual relationships and experiences (CSREs) are common and emotionally significant occurrences for adolescents and adults. ... Search calls for papers Journal Suggester Open access publishing ... The Journal of Sex Research Volume 58, 2021 - Issue 8. Submit an article Journal homepage. 3,963 Views 18 ...
The decline in casual sexual activity could have both positive and negative consequences for young adults and broader society. On one hand, less frequent casual sex may lead to fewer unwanted pregnancies, sexually transmitted diseases, and mental health problems ( Arnett 2018; Bersamin et al. 2014; Townsend et al. 2020 ).
SUBMIT PAPER. Close Add email alerts. You are adding the following journal to your email alerts ... Resnick M. D., Neumark-Sztainer D. (2009). Casual sex and psychological health among young adults: Is having "friends with benefits" emotionally damaging? Perspectives on Sexual ... Journal of Sex Research, 48, 486-495. doi:10.1080/00224499 ...
Using longitudinal and weekly diary methodologies, this study examined the moderating influence of sociosexuality, a stable personality orientation toward casual sex, on psychological well-being (self-esteem, life satisfaction, depression, and anxiety) following penetrative (oral, vaginal, or anal) casual sex among single undergraduates.
Previous research has conflated different types of casual sex, potentially obscuring patterns that may vary across categories. Using data from two large online community samples, we examined whether differences in attachment orientation predict experiences in casual sex encounters (i.e., One-Night Stand, Booty Call, Fuck Buddies, Friends With Benefits). We construed these encounters as ranging ...
Previous research has conflated different types of casual sex, potentially obscuring patterns that may vary across categories. Using data from two large online community samples, we examined whether differences in attachment orientation predict experiences in casual sex encounters (i.e., One-Night Stand, Booty Call, Fuck Buddies, Friends With Benefits).
Thus, we extended our study to other underexplored indicators of CSREs among adolescent casual sex research, such as suicidal ideation, low self-esteem, and consumption of alcohol or drugs. Second, we used a longitudinal design to better understand the direction of the link between psychological well-being and CSREs (e.g., Deutsch & Slutske ...
casual sex and well-being may, in fact, be due to having sex in general rathe r than ca sual sex in pa rticu lar (Gre llo et al., 2003 ; Monahan & Lee, 2008 ).
The Psychology of Casual Sex A new study predicts when hookups lead to happiness versus despair. Posted February 24, 2022 ... Journal of Sex Research, 51(2), 131-144.
We examined (N = 281) the role of love styles and personality in people's choice to engage in serious and/or various kinds of casual relationships (i.e., one-night stands, booty-calls, and friends with benefits) within the last year.Men were more eager than women were to engage in all types of casual relationships, however, love styles and personality traits were more important in accounting ...
Indeed, research suggests that this orgasm gap may account for the gender difference in people's attitudes about casual sex. Psychologist Jennifer Piemonte of the University of Michigan and ...
Engagement in casual sex (or hooking up) is generally feared to have negative well-being consequences; however, empirical evidence is inconclusive, pointing toward potential moderators. Using self-determination theory (SDT), we hypothesized that well-being following hookups would depend on the type and level of motivation for hooking up. A university-wide sample of 528 undergraduates completed ...
Sexual hook-up culture. With more emerging adults having casual sex, researchers are exploring psychological consequences of such encounters. By Justin R. Garcia, The Kinsey Institute for Research in Sex, Gender, and Reproduction, Indiana University, Bloomington; and Chris Reiber, Sean G. Massey, and Ann M. Merriwether, Binghamton University, State University of New York
The goal of this paper is to examine the associations of CSRE partner type (casual dating, friends with benefits, booty calls, and one-night stands), with short-term outcomes of CSREs, including positive and negative evaluations, plans for future encounters with the same partner, and plans for future CSREs in general. CSRE Type.
While most areas studied do not show sex differences in regret, the sexual domain does (Roese et al., 2006).Roese and colleagues (2006) found a moderate (d ≈ 0.40) sex difference in regret related to sexual inaction, with men regretting more than women having missed sexual opportunities. There were few other sex differences in regret toward friends and family, and none of these sex ...
The papers of talk show host and sex therapist Ruth Westheimer have been acquired by the Library of Congress and are now opening for research in the Library's Manuscript Division. Westheimer became a household name as "Dr. Ruth" in the 1980s, filling radio airwaves, television screens, and bookshelves with advice on sex and relationships ...
ETS2 is an ETS-family transcription factor and proto-oncogene 21, but its exact role in human macrophages is unclear, with previous studies using either cell lines or complex mouse models and ...
a. Economics is a large and growing field in academia, especially if you include business schools. So there are just a lot of economists out there doing work and publishing papers. They will branch out into non-economics topics sometimes. b. Economics is also pretty open to research on non-academic topics. You don't always see that in other ...
Hookups may be characterized as a form of "casual sex" or "uncommitted sexual encounter." Hatfield, Hutchison, Bensman, Young, and Rapson (in press) define casual sex as "outside of a 'formal' relationship (dating, marriage, etc.), without a 'traditional' reason (such as love, procreation, or commitment) for doing so" (p. 3).