
- How to submit Manuscripts
- How to propose Special Issue Topics
- Help Center
Research & Reviews : Journal of Herbal Science
Welcome to the Manuscript Submission site for
To begin, log in with your user ID and password.
If you are unsure about whether or not you have an account, or have forgotten your password, go to the Reset Password screen.
- Focus & Scope
- Instruction for Authors
- Journal Home
- Site Support

Create Account
Reset password.
Please enter your username or email address. You will receive a link to create a new password via email.
Identifiers
Linking ISSN (ISSN-L): 2278-2257
URL http://stmjournals.com/journals/RRJHS/Research-and-Reviews-Journal-of-Herbal-Science.html
Google https://www.google.com/search?q=ISSN+%222278-2257%22
Bing https://www.bing.com/search?q=ISSN+%222278-2257%22
Yahoo https://search.yahoo.com/search?p=ISSN%20%222278-2257%22
National Library of India http://nationallibraryopac.nvli.in/cgi-bin/koha/opac-search.pl?advsearch=1&idx=ns&q=2278-2257&weight_search=1&do=Search&sort_by=relevance
Resource information
Title proper: Research & reviews : journal of herbal science.
Country: India
Medium: Online
Record information
Last modification date: 29/05/2023
Type of record: Confirmed
ISSN Center responsible of the record: ISSN National Centre for India
downloads requested
Discover all the features of the complete ISSN records
Display mode x.
Labelled view
MARC21 view
UNIMARC view
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pharmacol
- PMC10569491
Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments
Sandra salm.
1 Institute of Pharmaceutical Biology, Goethe University, Frankfurt, Germany
2 Institute of General Practice, Goethe University, Frankfurt, Germany
Jochen Rutz
3 Department of Urology and Pediatric Urology, University Medical Center Mainz, Mainz, Germany
Marjan van den Akker
4 Department of Family Medicine, Care and Public Health Research Institute, Maastricht University, Maastricht, Netherlands
5 Department of Public Health and Primary Care, Academic Centre of General Practice, KU Leuven, Leuven, Belgium
Roman A. Blaheta
Beatrice e. bachmeier.
Marilena Gilca , Carol Davila University of Medicine and Pharmacy, Romania
Associated Data
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author.
Herbal medicines are becoming increasingly popular among patients because they are well tolerated and do not exert severe side effects. Nevertheless, they receive little consideration in therapeutic settings. The present article reviews the current state of research on the clinical benefits of herbal medicines on five indication groups, psychosomatic disorders, gynecological complaints, gastrointestinal disorders, urinary and upper respiratory tract infections. The study search was based on the database PubMed and concentrated on herbal medicines legally approved in Europe. After applying defined inclusion and exclusion criteria, 141 articles were selected: 59 for psychosomatic disorders (100% randomized controlled trials; RCTs), 20 for gynecological complaints (56% RCTs), 19 for gastrointestinal disorders (68% RCTs), 16 for urinary tract infections (UTI, 63% RCTs) and 24 for upper respiratory tract infections (URTI) (79% RCTs). For the majority of the studies, therapeutic benefits were evaluated by patient reported outcome measures (PROs). For psychosomatic disorders, gynecological complaints and URTI more than 80% of the study outcomes were positive, whereas the clinical benefit of herbal medicines for the treatment of UTI and gastrointestinal disorders was lower with 55%. The critical appraisal of the articles shows that there is a lack of high-quality studies and, with regard to gastrointestinal disorders, the clinical benefits of herbal medicines as a stand-alone form of therapy are unclear. According to the current state of knowledge, scientific evidence has still to be improved to allow integration of herbal medicines into guidelines and standard treatment regimens for the indications reviewed here. In addition to clinical data, real world data and outcome measures can add significant value to pave the way for herbal medicines into future therapeutic applications.
1 Introduction
Plant derived drugs have been used since humans have started treating physical and mental illnesses. They are part of Traditional Medicine in different cultures all over the world ( Yuan et al., 2016 ). Since then, medicine and treatment procedures have evolved and while in Traditional Medicine a holistic approach of life focusing on health and its maintenance was common philosophy, present Modern Medicine has a clear emphasis on unravelling the changes leading to disease and eradiating it ( Fries, 2019 ). Traditional medicine has a rigorous algorithm of identifying the root of the disease, which is based on traditional concepts, which, unfortunately, are considered obsolete nowadays, despite their practical longevity (e.g., acupuncture, ayurveda). The problem is that this traditional medical epistemology is not fully understood and science has limited tools to “translate” it into modern terms.
With the success of synthetic drugs along with the design of targeted therapies interfering specifically with the respective disease-related signaling pathways, herbal medicines have been eliminated from modern rational treatment strategies. The most important obstacles for the use in novel therapy strategies is that markers to measure clinical efficacy of herbal medicine have not been developed so far. Markers of efficacy of herbal drugs could also be useful to distinguish between patients who could benefit from a therapy with herbal medicines from those who will not. First preclinical studies already indicate that those markers or “signatures” (e.g., mRNA, miRNA) could be found in the future ( Bachmeier et al., 2007 ; Bachmeier et al., 2008 ; Bachmeier et al., 2009 ; Bachmeier et al., 2010 ; Killian et al., 2012 ; Kronski et al., 2014 ).
In the last years, more and more patients report on the perceived efficacy of herbal drugs and praise the absence of undesired side effects and the good tolerability.
The following section provides insights into the standard therapies of selected ailments for which herbal medicines may be a rational alternative.
1.1 Indications suitable for treatment with herbal medicines
Herbal medicines are in particular suitable for the treatment of non-life-threatening conditions for which knowledge from traditional use is available pointing to their clinical benefits in treating the respective ailment ( Wachtel-Galor and Benzie, 2011 ). This applies especially to psychosomatic disorders, gynecological complaints, and upper respiratory tract infections. However also for other diseases like gastrointestinal diseases, urinary tract infections herbal medicines have been clinically applied and—as we will show in this review—with some success.
Standard Care of psychosomatic disorders comprises the application of synthetic psychotropic drugs and psychotherapy ( Laux, 2021 ). Psychotropic drugs are used not only for the treatment of depressive disorders and anxiety, but also for sleep disorders, excitation and chronic pain ( Gründer and Benkert, 2012 ). However undesired adverse events having negative impact on quality of life can occur like, e.g., weight gain, sexual dysfunction, sedation, headache and tremor ( Grunze et al., 2017 ). In addition their use, in particular benzodiazepines, can lead to addiction and drug abuse ( Soyka and Mann, 2018 ) and interactions with other medication has to be taken into consideration especially in older multimorbid patients ( Burkhardt and Wehling, 2010 ). About 23% of all over 70-year-old people have psychosomatic disorders with about 40% requiring therapy ( Haupt and Vollmar, 2008 ). In this context herbal medicines represent an interesting alternative to avoid the above-mentioned problems with standard synthetic drugs. However, they do not belong to standard therapy-options and therefore are underrepresented in therapy-guidelines ( Bittel et al., 2022 ). Nevertheless they play an important role in self-medication of patients ( Stange, 2014 ) probably due to their favorable ratio between benefit and side-effects.
Gynecological complaints include, e.g., menopausal and premenstrual symptoms. According to the German medical guideline for post- and perimenopause, vasomotor symptoms of the peri- and post-menopause such as hot flushes and sweating should be treated with hormone therapy for menopause (hormone replacement therapy; HRT), if not contraindicated ( AWMF, 2020 ). The side effects of HRT include edema, joint pain, psychological symptoms or even thrombosis and breast cancer ( Maclennan et al., 2004 ). Herbal medicines, on the other hand, are characterized by a low risk of adverse events which increases patients’ adherence and in consequence prevents therapy discontinuations ( AWMF, 2020 ). Premenstrual syndrome (PMS) is characterized by recurring physical and psychological symptoms in the days before menstruation. There are currently no medical guidelines in German-speaking countries for the treatment of PMS. Systematic reviews on hormonal treatments (oral contraceptives, progesterone and estrogen) ( Ford et al., 2006 ; Lopez et al., 2007 ; Naheed et al., 2013 ; Kwan and Onwude, 2015 ) and acupuncture/acupressure ( Armour et al., 2018 ) point to ambiguous evidence. Treatment with serotonin reuptake inhibitors was shown to be effective but was associated with frequent side effects, e.g., nausea and asthenia ( Marjoribanks et al., 2013 ).
Gastrointestinal diseases include several conditions like irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), liver disease (hepatitis), and functional dyspepsia (FD).
Beside dietary changes, stress management and psychotherapy, severe cases of IBS and IBD require additional medication to reduce inflammation or to slow down the intestinal irritations. However patients often complain about the side effects of medical treatment like, e.g., dizziness or weight gain (particularly caused by steroids), or undesired fatigue, headache, and/or tiredness associated with the intake of methotrexate ( Feagan et al., 1995 ). Common types of hepatitis are viral hepatitis B and C. Antiviral therapy represents the treatment of choice to fight the virus caused disease. However, poor tolerability and significant adverse effects that include, for example, headaches, dizziness, depression, and irritability often lead to treatment discontinuation, further decreasing response rates ( Cornberg et al., 2002 ). FD is a common gastrointestinal disorder treated by proton pump inhibitors (PPI) or H2 receptor antagonist, and/or treatment with tricyclic antidepressants or prokinetic agents. As in all cases, adverse side effects may occur ranging from dizziness to the development of diabetes mellitus type 2 ( Yuan et al., 2021 ).
Urinary tract infections (UTI) with estimated 150 million cases worldwide each year reflect the most common outpatient infections ( Zavala-Cerna et al., 2020 ). Women are more susceptible than men with a lifetime incidence of 50%–60%. Application of antibiotics represents the standard treatment regimen to overcome the infection. However, serious side effects, predominantly exerted on the digestive system, may outweigh the benefits of this drug class. Most importantly, routine use of antibiotics bears the risk to trigger the selection of resistant strains. Hence, avoiding antibiotic treatment of UTI has gained high priority among the urologic community ( Jung et al., 2023 ). Lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH) requires a medical therapy which aims to reduce the BPH-related complications. A range of synthetic drugs is available to treat this condition. However, these have a range of side effects, including postural hypotension, dizziness, asthenia, abnormal ejaculation, intraoperative floppy iris syndrome (α1-blocker), or decreased libido, gynecomastia, and erectile dysfunction (5α-reductase inhibitors) ( Cheng et al., 2020 ). Due to this, patients often discontinue treatment.
The most common acute upper respiratory infections include bronchitis, rhinosinusitis and common cold. Common cold or acute viral rhinosinusitis is triggered by a viral infection/inflammation of the nose and by definition has a duration up to 10 days. According to Jaume and co-workers ( Jaume et al., 2020 ) the recommended therapy (mainly symptomatic) contains of paracetamol, NSAIDs, second-generation antihistamines to reduce symptoms the first 2 days; nasal decongestants with small effect in nasal congestion in adults; combination of analgesics and nasal decongestants; ipratropium bromide for reducing rhinorrhea; probiotics; zinc when administered the first 24 h after the onset of symptoms; nasal saline irrigations; and some herbal medicines. About 5% of adults have an episode of acute bronchitis each year. An estimated 90% of these seek medical advice for the same ( Saust et al., 2018 ). Acute bronchitis is caused by infection of the large airways commonly due to viruses and is usually self-limiting. Bacterial infection is uncommon. Still, often antibiotics are prescribed, despite lacking effectiveness ( Tanner and Karen Roddis, 2018 ). Most medical guidelines advice a “wait-and-see” policy, the use of antihistamines and cough medicines is discouraged.
1.2 Objectives
In the last decade we experienced a renaissance of herbal medicines with a rising demand especially for the treatment of the before-mentioned indications. This implicates that there is an urgent need for a scientific progress towards a rational phytotherapy, which will combine the benefits of “Modern Medicine” with the “Traditional Knowledge” on the therapeutic benefits of herbal medicines.
In order to create a basis of knowledge to build upon novel interdisciplinary research ideas towards the establishment of herbal medicines into rational therapeutic strategies, we extracted information from clinical studies. Thereby we aimed to get an overview on.
- - which herbal medicines have been studied so far for which ailment
- - which outcomes have been studied
- - what quality level (level of evidence) the published studies have
Answering these questions, we create a comprehensive critical picture of the current knowledge on clinical efficacy and benefits as well as on failures and possible adverse events. Based on the results of these studies we give recommendations for practitioners and patients.
2.1 Search strategy and selection of scientific reports
Information on the therapeutic use of herbal medicines in different ailments was collected from scientifically published articles by conducting a search in the database PubMed for each of the five indication groups according to the following inclusion and exclusion criteria.
2.1.1 Inclusion criteria
- 1. Herbal Medicine
- a.Psychosomatic symptoms (depressive disorder, sleeping disorders/insomnia, anxiety, cognitive impairment)
- b.Gynecological complaints (climactic symptoms, menstrual symptoms, premenstrual syndrome)
- c.Gastrointestinal disorders/dyspepsia
- d.Urinary tract infections
- e.Upper respiratory tract infections
- 3. Clinical Trial
- Exclusion criteria
- a. Reports in languages other than German or English language
- b. No full-text available
- c. Study protocols
- d. Traditional medicine (e.g., Traditional Chinese Medicine, Ayurveda, etc. ),
- e. Aroma therapy
- f. Dietary supplements
- g. Self-made extracts and preparations
- h. Adjuvant treatment with herbal medicine
- i. Herbal medicines without market access in the EU
- j. In vivo / in vitro studies (pre-clinical studies)
- k. Homeopathy
- l. Acupuncture/acupressure
- m. Children and youth (under the age of 18 years)
- n. Healthy volunteers
- o. Primary preventive interventions (incl. Pre-post-operative complaints)
- p. Predominant comorbidities
- q. Case studies/case reports
- r. Televised, internet-based or web-based trials
Reasons for exclusion criteria:
a, b: Authors should be able to read and understand the full text; c: clinical results should have been obtained from a study; d, e, f, g, h, i: selected in order to filter all available information on legally approved (in Europe in particular in Germany) herbal medicines or the respective standardized extract (HMPC Monographs of the European Medical Agency - EMA) only; j: preclinical evidence should be excluded; k, l: alternative naturopathic therapy forms should be excluded; m: children should be excluded due to different drug metabolism; n, o: healthy volunteers should be excluded in order to obtain information on clinical therapeutic benefits; p: predominant comorbidities should be excluded because they can affect the efficacy of the herbal drug in particular when co-administered with other drugs; q; clinical benefits from single cases are difficult to generalize; r: excluded for methodological reasons, e.g., data interpretation.
2.2 Data extraction and quality assessment of scientific reports
To get an overview on the characteristics of all included articles, a table was created for each indication group containing information on the publication, the study design, the population and treatment duration, the indication and the primary outcome, the herbal medicine and comparison treatment (comparator) as well as the results. Furthermore, we performed a quality assessment of the collected reports according to the following scoring method.
- • 1 point for an observational study or a pre-post observational comparison
- • 2 points for a clinical trial
- • 3 points for a randomized controlled trial plus 1 additional point for blinding
Thereby, a score between 1 and 4 was obtained indicating the quality for all scientific reports; respectively publications with the highest level of evidence (RCT + blinded) had a scoring value of four points (see Figures 1 – 5 ).

Numbers of studies and outcomes.

3.1 Psychosomatic disorders
A search for publications with the terms “psychosomatic disorder” and “herbal medicine” yielded only 64 results. Therefore, the search was extended with more specific terms (see inclusion criteria) yielding in 4.440 hits for depressive disorder, 1.907 hits for sleeping disorders, 2.380 hits for anxiety and 1.374 hits for cognitive impairment including Alzheimer’s disease. After eliminating all publications according to the exclusion criteria 59 publications remained. Among those, 39 studies were related to depressive disorders, 4 to sleeping disorders, 6 to anxiety and 10 to cognitive impairment and Alzheimer’s disease (neurological disorders). Most of them were double blind randomized controlled trials (quality group 4). For the treatment of depressive disorders predominantly Hypericum perforatum L (St. John’s Wort; SJW) was used and only few studies examined the clinical benefits of Rhodiola rosea L (Rosewood). Valeriana officinalis L (Valerian Root) and Humulus lupulus L (Hops) extracts were preferred for the treatment of sleeping disorders, while for anxietyextracts of Lavandula angustifolia (Lavender) were studied. Extracts of Ginkgo biloba L (Maidenhair Tree) were used in clinical studies with patients having neurological disorders (cognitive impairment and Alzheimer’s disease). Supplementary Table S1 provides an overview of the studies, their characteristics and results (see also Figure 1 ).
3.1.1 Depressive disorders
The use of herbal medicines in depressive disorders is well examined and in particular the clinical benefits of SJW are well supported by clinical studies of high quality. All 37 selected studies on the use of SJW in depressive disorders ranging from mild to severe forms have been double-blind randomized controlled trials (quality group 4). Study duration was predominantly between 4 and 8 weeks and only few studies examined the effects for longer time periods of up to 6 months. The majority of the studies reported positive therapeutic effects concerning Hamilton depression rating scale (HAMD) as primary outcome parameter and only 5 of them ( Shelton et al., 2001 ; Davidson et al., 2002 ; Bjerkenstedt et al., 2005 ; Moreno et al., 2006 ; Rapaport et al., 2011 ) did not demonstrate superiority as compared to placebo or pre-post.
In six studies (published predominantly before the year 2000) comparing SJW with tricyclic anti-depressive drugs the clinical benefits of the herbal drug in respect to placebo or in pre-post comparison was at least equal to the synthetic drug no matter if it was imipramine ( Vorbach et al., 1994 ; Vorbach et al., 1997 ; Philipp et al., 1999 ; Woelk, 2000 ), maprotiline ( Harrer et al., 1994 ) or amitriptyline ( Wheatley, 1997 ). However, with regards to tolerability, SJW was clearly superior to any of the tricyclic antidepressants.
The more recent studies compared the efficacy of SJW with the selective serotonin reuptake inhibitors (SSRI) paroxetine, sertraline, citalopram and fluoxetine. In most of the 18 studies the therapeutic benefits of SJW were at least equal to those of the SSRIs ( Harrer et al., 1999 ; Berger et al., 2000 ; Brenner et al., 2000 ; Friede et al., 2001 ; van Gurp et al., 2002 ; Bjerkenstedt et al., 2005 ; Gastpar et al., 2005 ; Szegedi et al., 2005 ; Anghelescu et al., 2006 ; Gastpar et al., 2006 ; Sarris et al., 2012 ). In two studies SJW was even superior to fluoxetine ( Fava et al., 2005 ) or paroxetine ( Seifritz et al., 2016 ) in reducing depressive symptoms. In one study the responders of a previous study were included in a further RCT testing the efficacy of SJW against citalopram. Here the numbers of patients with relapse was lower in the SJW group as compared to citalopram ( Singer et al., 2011 ). The results of one study indicated that SJW was less efficacious than both fluoxetine and placebo, however in this study the group on SJW had the lowest remission rates ( Moreno et al., 2006 ). In two studies no statistical differences in HAMD scores between SJW, placebo and citalopram ( Rapaport et al., 2011 ) or sertraline ( Davidson et al., 2002 ) could be found with adverse effects in the SJW and the SSRI groups.
In most of the above-mentioned studies, comparing the efficacy of SJW to standard therapy, a placebo group was included. However, in 13 studies SJW was tested exclusively against placebo whereby two of these studies examined the efficacy of different dosages of SJW extract ( Laakmann et al., 1998 ; Kasper et al., 2006 ). In these studies, the higher concentrations had the better clinical benefits. In a continuation study of the effect of SJW in long term treatment a higher dosage (1,200 mg/d) was not superior to the lower one (600 mg/d) ( Kasper et al., 2007 ). Interestingly the higher dosages were still well tolerated although mild adverse events related to gastrointestinal disorders were observed in a small portion of the patients ( Kasper et al., 2006 ). In only one of our selected studies SJW was not effective in comparison to placebo for the treatment of major depression but safe and well tolerated ( Shelton et al., 2001 ). In all other studies SJW was superior to placebo no matter if given in low ( Laakmann et al., 1998 ; Lecrubier et al., 2002 ; Randlov et al., 2006 ), medium ( Kasper et al., 2006 ; Kasper et al., 2007 ; Mannel et al., 2010 ) or in high ( Hansgen et al., 1994 ; Harrer et al., 1994 ; Sommer and Harrer, 1994 ; Kalb et al., 2001 ; Uebelhack et al., 2004 ; Kasper et al., 2006 ; Kasper et al., 2007 ; Kasper et al., 2008 ) dosages.
For the efficacy of Rhodiola rosea in treatment of depressive disorders only few studies were performed so far. Therefore, a clear conclusion cannot be drawn, especially as the outcomes are not homogenous. While one study investigating the efficacy of R. rosea against placebo and the SSRI sertraline reported on a statistically not-significant inferiority of the herbal medicine ( Mao et al., 2015 ) another study demonstrated clinical benefits concerning the symptoms of depression, insomnia, emotional instability and somatization against placebo. In this study two dosages of R. rosea were tested and the higher dose (680 mg/d) showed even positive effects on self-esteem ( Darbinyan et al., 2007 ).
3.1.2 Sleeping disorder
Interestingly the search for qualitatively high clinical studies (according to our inclusion and exclusion criteria) revealed only few studies. The majority of them investigated the efficacy of valerian alone ( Donath et al., 2000 ) or in combination with hops ( Koetter et al., 2007 ) compared to placebo ( Donath et al., 2000 ; Koetter et al., 2007 ) or to oxazepam ( Dorn, 2000 ; Ziegler et al., 2002 ). All studies reported clinical benefits, however while the one research group reported that valerian alone was efficacious against insomnia ( Donath et al., 2000 ) the other group reported on clinical benefits only in combination with hops ( Koetter et al., 2007 ). Both study designs were placebo-controlled. In comparison to oxazepam valerian was not inferior and both therapy options improved sleep quality (SF-B) in a similar fashion ( Dorn, 2000 ; Ziegler et al., 2002 ).
3.1.3 Anxiety
Herbal Medicines with lavender extracts were clinically studied for the treatment of anxiety. Between 2010 and 2019 six qualitatively high studies performed in Germany, Austria and Switzerland reported on the beneficial effects of lavender against symptoms of anxiety with improvements on the Hamilton anxiety rating (HAMA) scale as primary outcome ( Kasper et al., 2010 ; Woelk and Schlafke, 2010 ; Kasper et al., 2014 ; Kasper et al., 2015 ; Kasper et al., 2016 ; Seifritz et al., 2019 ) and all studies used the same extract (WS1265). Four of the 6 studies were performed by the same group, however the study design differed. In these studies the efficacy of lavender was either compared to placebo ( Anghelescu et al., 2006 ; Kasper et al., 2010 ; Kasper et al., 2016 ; Seifritz et al., 2016 ) and/or to paroxetine ( Kasper et al., 2014 ) and lorazepam ( Woelk and Schlafke, 2010 ). Overall, the lavender preparation was regarded as efficacious and safe.
3.1.4 Neurological disorders (cognitive impairment and Alzheimer)
We selected 10 studies investigating the efficacy of ginkgo biloba extract in the treatment of cognitive impairment and Alzheimer’s Disease (AD) with 8 of them testing against placebo ( Le Bars et al., 1997 ; Le Bars et al., 2002 ; Le Bars, 2003 ; van Dongen et al., 2003 ; Schneider et al., 2005 ; Napryeyenko et al., 2007 ; Gavrilova et al., 2014 ; Gschwind et al., 2017 ), one against rivastigmine ( Nasab et al., 2012 ) and one against donepezil ( Mazza et al., 2006 ). In three of the studies two different ginkgo extracts did not show superiority over placebo regarding the primary outcome. In detail 5 of the studies showed that extracts of ginkgo biloba lead to a decrease in NPI composite score ( Gavrilova et al., 2014 ) improved significantly ADAS-Gog and GERRI ( Le Bars et al., 1997 ; Le Bars et al., 2002 ; Le Bars, 2003 ), or the SKT test battery ( Napryeyenko et al., 2007 ) as outcome parameters. In three studies ginkgo extracts did not show superiority over placebo regarding the primary outcome parameters ADAS-cog ( Schneider et al., 2005 ), gait analyses ( Gschwind et al., 2017 ) or SKT test-battery ( van Dongen et al., 2003 ), whereby in one of these studies the primary outcome parameter ADAS-cog also declined in the placebo group rendering the results of the study inconclusive ( Schneider et al., 2005 ). With respect to the AD conventional medication rivastigmine, ginkgo biloba extract was inferior regarding the primary outcome parameters MMSE and SKT test-battery ( Nasab et al., 2012 ). Finally one study in which gingko biloba was more efficacious than placebo and equal to the second generation cholinesterase inhibitor donepezil ( Mazza et al., 2006 ) was heavily criticized by two other groups ( Corrao et al., 2007 ; Korczyn, 2007 ), making it difficult to estimate if the use of ginkgo containing herbal medicines are justified for the treatment of mild to moderate AD.
3.2 Gynecological complaints
Of 383 search hits, 20 articles met the inclusion criteria. Eleven studies were related to menopausal symptoms and nine to PMS. Most were double-blind randomized controlled trials or observational studies ( Figure 2 ). The studies on menopausal symptoms reported mainly positive results and the results concerning PMS were exclusively positive ( Figure 2 ). The tested phytopharmaceuticals contained Cimicifuga racemosa (L.) (Black cohosh) (10 studies) and Salvia officinalis (Sage) (1 study) for the treatment of menopausal symptoms and Vitex agnus-castus L (VAC, Chaste tree) (8 studies) and SJW (1 study) for PMS. Supplementary Table S2 provides an overview of the study characteristics and results.

3.2.1 Menopausal symptoms
In studies examining the clinical benefits of black cohosh for the treatment of menopausal symptoms, sample sizes ranged from n = 62 to n = 6,141. Treatment duration was between 12 weeks and 9 months. The herbal drug dosages ranged from 20 to 127.3 mg.
In comparison to HRT, the benefit-risk-balance points to significant non-inferiority and superiority of black cohosh ( Bai et al., 2007 ). In three other studies menopausal complaints improved overall, but differences between black cohosh and HRT were not significant ( Wuttke et al., 2003 ; Nappi et al., 2005 ; Friederichsen et al., 2020 ). The combination of black cohosh with SJW significantly reduced menopausal complaints and was superior to transdermal estradiol ( Briese et al., 2007 ). Independent of a high or low dose, menopausal complaints decreased significantly ( Liske et al., 2002 ; Drewe et al., 2013 ). Adverse events rates were lower in the low dose group ( Drewe et al., 2013 ) or similar to the high dose group ( Liske et al., 2002 ). Menopausal symptoms decreased significantly more for black cohosh compared to placebo ( Osmers et al., 2005 ). In another study with 62 participants, the difference between the symptom scores just approached significance ( Wuttke et al., 2003 ). Interestingly, this also applies to the comparison of conjugated estrogens and placebo. Adverse events rates did not differ significantly between black cohosh and placebo ( Wuttke et al., 2003 ; Osmers et al., 2005 ). Significant and clinically relevant reductions in menopausal symptoms ( Vermes et al., 2005 ) or higher quality of life ( Julia Molla et al., 2009 ) were observed after treatment with black cohosh compared to therapy start. Sage taken for 8 weeks significantly decreased the number of menopausal hot flushes from week to week ( Bommer et al., 2011 ). Observed treatment-related adverse events were mild and occurred in only one person. However, no comparison was made to another treatment or placebo.
3.2.2 Premenstrual syndrome
Eight studies dealt with the treatment of PMS with VAC. The sample sizes ranged from n = 43 to n = 1,634. Treatment duration was three cycles; Berger et al. (2000) added three subsequent cycles without treatment. The administered dosages ranged from 1.6 to 20 mg extract.
Results of studies comparing VAC with pyridoxine or placebo were similar. PMS symptom reduction was significantly more pronounced for VAC compared to pyridoxine ( Lauritzen et al., 1997 ) or placebo ( Schellenberg, 2001 ; Bachert et al., 2009 ; Barrett et al., 2010 ; Schellenberg et al., 2012 ). Rates of adverse events were similar between groups in each study ( Loch et al., 2000 ; Schellenberg, 2001 ; Barrett et al., 2010 ; Schellenberg et al., 2012 ). Schellenberg et al. (2012) compared a VAC reference dose to a lower and higher dose; the results were in favor for the reference dose compared to the low dose. No significant differences between the high and reference dose emerged. The number of participants with adverse events was slightly elevated for the high dose. In single-arm studies, symptoms of PMS significantly decreased after three cycles of VAC treatment ( Berger et al., 2000 ; Loch et al., 2000 ; Momoeda et al., 2014 ). Only mild PMS-like adverse events were observed. Berger et al. demonstrated a gradual symptom return after therapy completion ( Berger et al., 2000 ). PMS symptoms were significantly higher compared to the end of the treatment, but still 20% lower than at baseline.
A clinical study testing the efficacy of SJW in treating mild PMS ( Canning et al., 2010 ) demonstrated significant improvements in physical (e.g., food craving) and behavioral (e.g., confusion) symptoms compared to placebo. The effect on mood (e.g., irritability) and pain (e.g., cramps) was not significant.
3.3 Gastrointestinal disorders
A search for publications with the search terms “gastrointestinal disorder” and “herbal medicine” yielded a total of 19 results after applying the exclusion criteria. Of these, eight studies were related to hepatic disorders, three publications dealt with IBD, two studies focused on IBS, and six studies had been done on FD. Most of them were done in a double-blinded randomized controlled manner ( n = 12) ( Figure 3 ). Silybum marianum (L.) Gaertn (Silymarin, milk thistle) was used in patients suffering from a hepatic disease. Patients with IBD were treated with Artemisia absinthium L (wormwood) or Potentilla erecta (tormentil). The standardized extract STW 5 containing Iberis amara (bitter candytuft), Glycyrrhiza glabra L (Liquorice), Carum carvi L (caraway), Mentha × piperita (peppermint), Melissa officinalis L (lemon balm) , Matricaria chamomilla (chamomile) , Angelica archangelica (wild celery), Chelidonium majus (greater celandine) and milk thistle has been applied in IBS and FD. The same has been done with the standardized extract STW 5-II which in contrast to STW 5 is free of wild celery, greater celandine, and milk thistle. SJW has been used to treat patients suffering from IBS. A combination of the standardized extracts WS 1340 (peppermint oil) and WS 1520 (caraway oil) was used for patients with FD. Supplementary Table S3 and Figure 3 provide an overview of the study characteristics and results.

3.3.1 Hepatic disease
Trials on steatohepatitis, cirrhosis and different kinds of hepatitis ( n = 18) included patient cohorts ranging from 14 to 200 participants, all of them aged >18 years. Patients were treated with silymarin orally or intravenously ( Pares et al., 1998 ; Tanamly et al., 2004 ; Ferenci et al., 2008 ; Hawke et al., 2010 ; Fried et al., 2012 ; Adeyemo et al., 2013 ; Fathalah et al., 2017 ; Tanwar et al., 2017 ) with dosages ranging from 280 to 2,100 mg/day or 5–20 mg/kg/day, respectively. Six studies compared the HM group to a placebo group ( Pares et al., 1998 ; Tanamly et al., 2004 ; Hawke et al., 2010 ; Fried et al., 2012 ; Adeyemo et al., 2013 ; Tanwar et al., 2017 ). Silymarin did not reduce virus titers and/or serum alanine transaminase (ALT) in patients with Hepatitis C and non-alcoholic Steatohepatitis C, compared to placebo ( Adeyemo et al., 2013 ). The same observation has been made by others ( Hawke et al., 2010 ). Furthermore, the integration of silymarin into a PEGylated (Peg)-interferon based regimen did not improve the outcome of HCV patients in terms of HCV RNA suppression and Enhanced Liver Fibrosis score performance ( Tanamly et al., 2004 ). There was also no effect of silymarin on HCV patients who were previously unsuccessfully treated with interferon (multicenter, double-blind, placebo-controlled trial) ( Fried et al., 2012 ). Although HCV-patients reported to “feel better” after 12 months of silymarin therapy in a further study, symptoms and quality of life (QOL) scores did not differ between the silymarin and the placebo group ( Tanamly et al., 2004 ). Treatment with silymarin was also well tolerated over a period of 2 years. However, the course of liver cirrhosis in this patient cohort has not been improved ( Pares et al., 1998 ). Contrasting these results, dose escalating studies on HCV cirrhotic patients revealed positive effects of silymarin or silibinin (also milk thistle), in a way that high-dosed silymarin (1,050 mg/day) improved QOL and biochemical parameters of chronic HCV-decompensated cirrhotic patients with no serious adverse events ( Ferenci et al., 2008 ; Fathalah et al., 2017 ) compared to low-dosed silymarin (420 mg/day). Notably, silibinin exerted a dose-dependent antiviral effect on Peg-interferon/ribavirin non-responders ( Ferenci et al., 2008 ; Fathalah et al., 2017 ).
3.3.2 Inflammatory bowel disease (IBD)
Between 2007 and 2009, three clinical trials on CD or IBD have been conducted, two in Germany (quality groups 1 and 2) and one in the United States (quality group 4) ( Huber et al., 2007 ; Omer et al., 2007 ; Krebs et al., 2010 ). Patients were treated with wormwood or tormentil for 3–10 weeks. A total of 30 patients were treated with wormwood or placebo ( Omer et al., 2007 ; Krebs et al., 2010 ). In this context, wormwood decreased tumor necrosis factor alpha levels and the CD activity index score, whilst scores for IBD questionnaire and Hamilton depression scale have been improved, compared to the controls ( Omer et al., 2007 ; Krebs et al., 2010 ). Daily intake of tormentil reduced clinical activity index scores in all patients, however, during the wash out phase scores increased again. Tormentil has been proven to be safe for ulcerative colitis patients in dosages up to 3,000 mg/day ( Huber et al., 2007 ).
3.3.3 Irritable bowel syndrome (IBS)
Symptoms of IBS were treated with STW 5 and STW 5-II or SJW (both studies were quality group 4) ( Madisch et al., 2004b ; Saito et al., 2010 ). The clinical trial carried out by Madisch et al. compared the effects of the treatment group with those of bitter candytuft mono-extract and placebo. STW 5 and STW 5-II (60 drops/day over 4 weeks) significantly reduced the total abdominal pain and the IBS score compared to placebo and bitter candytuft mono-extract ( Madisch et al., 2004b ). The study carried out by Saito and others investigated the clinical efficacy of SJW pointing to a lower effect as compared to placebo ( Saito et al., 2010 ).
3.3.4 Functional dyspepsia (FD)
Six studies on patients suffering from FD were performed, including treatment with either a WS 1520/WS 1340 combination ( n = 3) ( Madisch et al., 1999 ; Rich et al., 2017 ; Storr and Stracke, 2022 ) or with STW 5 ( von Arnim et al., 2007 ) and/or STW 5-II ( n = 3) ( Rösch et al., 2002 ; Madisch et al., 2004a ). WS 1340/WS 1520 was documented to be a “valuable” ( Storr and Stracke, 2022 ) or an “effective” therapeutic regimen ( Rich et al., 2017 ), as it relieved pain and improved disease-specific QOL, compared to placebo. The primary outcome of WS 1340/WS 1520 was also proven to be comparable to the prokinetic agent cisapride ( Madisch et al., 1999 ).
It is to be noted that the use of cisapride has meanwhile be restricted by the EMA due to the risk of potentially life-threatening cardiac arrhythmia [ https://www.ema.europa.eu/en/medicines/human/referrals/cisapride ].
Similar results have been presented in the STW 5 and STW 5-II trials. The gastrointestinal symptom score was significantly lowered when compared to the placebo group ( Madisch et al., 2004a ; von Arnim et al., 2007 ), with a therapeutic response comparable to cisapride ( Rösch et al., 2002 ).
3.4 Urinary tract infection (UTI) and lower urinary tract symptoms (LUTS)
Initial search on herbal drugs in urologic clinical trials pointed to 263 manuscripts published between 1983 and 2022. Narrowing the search to “herbal medicine” (HM) 18 relevant publications were identified. One publication was nearly identical to another one and, therefore, has not been taken care of in this chapter, one article only reviewed former trials (16 publications remaining). All of them were related to lower urinary tract infection (UTI), or acute uncomplicated cystitis, respectively. Four different HM have been applied, either compared to placebo or guideline-based treatment ( n = 12).
3.4.1 Urinary tract infections (UTI)
Several studies investigated the standardized herbal extract BNO 1045 which contains Centaurium erythraea Rafin, herba (Centaury); Levisticum officinale Koch, radix (Lovage); and Rosmarinus officinalis L., folium (Rosemary). In two studies, the clinical benefits of BNO 1045 in preventing UTI in high-risk women undergoing urodynamic studies (UDS) ( Miotla et al., 2018 ) or urogynecological surgeries ( Wawrysiuk et al., 2022 ) was evaluated. High-risk women were defined as: age over 70, elevated postvoid residual urine>100 mL, recurrent UTI, pelvic organ prolapse (POP) ≥II in POP-Q scale, and neurogenic bladder. No statistical differences in UTI incidence were found between patients receiving antibiotics or BNO 1045. No superiority of antibiotics over BNO 1045 has been confirmed as well in a subsequent prospective study on postoperative UTI after midurethral sling surgery (MUS) ( Rechberger et al., 2020 ). In another study, an herbal mixture based on D-mannose, Arctostaphylos uva-ursi, Betula pendula, and Berberis aristata was compared to BNO 1045 in reducing symptoms of UTI after MUS ( Rechberger et al., 2022 ). The rationale was based on the EAU 2022 guidelines which recommended D-mannose as prophylaxis of UTI. In this context, BNO 1045 was proven to be similar effective, compared to the herbal mixture. The use of BNO 1045 has been documented here to be a potential and valuable alternative to antibiotics for UTI prevention. All four trials have been carried out in the same institution involving the same main investigators which were (partially) associated with the manufacturer of BNO 1045.
A randomized, double-blind, multicenter Phase III clinical trials compared the efficacy and of BNO 1045 to antibiotics concerning symptoms and recurrence rates in women with uncomplicated UTI. Based on the endpoints “UTI-recurrence” and “additional antibiotics use”, BNO 1045 was proven to be non-inferior to antibiotic treatment ( Wagenlehner et al., 2018 ). In a retrospective cohort study, data from outpatients in Germany with at least one diagnosis of acute cystitis or UTI and a prescription of either BNO 1045 or standard antibiotics were analyzed ( Holler et al., 2021 ). Compared to antibiotics, BNO 1045 was associated with significantly fewer recurrence rates of UTI and with reduced additional antibiotic prescription. BNO 1045 was propagated to be an effective and safe symptomatic treatment option for acute cystitis or UTI.
In an open-labeled, randomized, controlled trail the effect of BNO 1045 to prevent recurrences of cystitis in younger women was evaluated ( Sabadash and Shulyak, 2017 ). All patients received an antibacterial therapy, the test group was additionally treated with BNO 1045. The integration of BNO 1045 prevented bacteriuria and recurrent cystitis episodes more frequently (primary outcome), compared to the control group without BNO 1045. This may indicate superiority of the combination therapy. However, interpretation of the results of the study is limited due to the lack of blinding on both sides - patients and physicians. A further study without any involvement of the manufacturer (no conflicts of interest noted) included younger women with acute uncomplicated cystitis. All patients received the same therapy, the nonsteroidal anti-inflammatory drug ketoprofen in combination with BNO 1045 ( Kulchavenya, 2018 ). Quite interestingly, although the majority of the patients responded well to the therapy, the investigators also observed patients who only slightly responded, or did not respond to treatment at all. The authors concluded that uncomplicated cystitis might be cured by BNO 1045 instead of antibiotics which may be required only in minor cases. Still, the data seems to be over-interpreted, since patients were treated with both ketoprofen and BNO 1045 which does not allow to conclude to one drug alone.
Aside from BNO 1045, further herbal medicines have been investigated in clinical studies. Tablets with a standardized herbal extract containing Armoraciae rusticanae radix (Horseradish root) (80 mg) and Tropaeoli majoris herba (Nasturtium) (200 mg) have been applied to patients suffering from chronically recurrent UTI symptoms, with the result that recurrent UTI symptoms were less, compared to the placebo group ( Albrecht et al., 2007 ). However, a subsequent trial failed to demonstrate non-inferiority of this extract to antibiotics due to a poor recruitment rate ( Stange et al., 2017 ). Actually, no respective clinical trials with sufficient statistical power are underway.
3.4.2 Lower urinary tract symptoms LUTS
Clinical studies have also been conducted with an herbal medicine containing the standardized extracts WS 1473 Sabal serrulata Schult.f (Sabal fruit) (160 mg) and WS1031 Urtica dioica L (Urtica root) (120 mg). All studies were related to the treatment of lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH). The study protocols (placebo-controlled, double-blind, multicentric) were similar in all trials with the International Prostate Symptom Score (I-PSS), quality of life index, uroflow and sonographic parameters as the outcome measures for treatment efficacy. In one study ( Lopatkin et al., 2005 ) patients were randomized to either the herbal medicine (WS 1473 and WS1031) (treatment group) or placebo (control group) while in another study patients received either WS 1473 and 1031 or the α1-adrenoceptor antagonist tamsulosin ( Engelmann et al., 2011 ). A further study was based on the previous mentioned study ( Lopatkin et al., 2005 ), whereby all patients were offered participation in a further 48-week follow-up with WS 1473/1031 ( Lopatkin et al., 2007 ). Independent on the study design, it was concluded that WS 1473/1031 is superior to the placebo, and not inferior to tamsulosin in the treatment of LUTS. In a later re-evaluation of the data sets, WS 1473/1031 was shown to significantly improve nocturnal voiding frequency compared to placebo, with similar effects compared to tamsulosin or the 5α-reductase inhibitor finasteride ( Oelke et al., 2014 ). No further studies have been enrolled since then. However, a database search in 2022 including 3,000 private practices in Germany revealed a significant association between WS 1473/1031 prescription and reduced incidence of urinary incontinence and urinary retention compared to tamsulosin and tamsulosin/dutasteride (5α-reductase blocker), as well as reduced incidence of erectile dysfunction compared to dutasteride ( Madersbacher et al., 2023 ). In all four studies the manufacturer of the extract was involved.
One observational study was investigating the effectiveness of a standardized herbal extract containing a combination of Cucurbita pepo L (Marrow), Rhus aromatica bark (Fragrant sumac), and hops, in women with overactive bladder ( Gauruder-Burmester et al., 2019 ). Of the 113 patients included, nearly the half (61 patients) used concomitant medications (e.g., antihypertensive, levothyroxine, lipid/cholesterol lowering agents, low dose ASS, NSAIDS) within the frame of a routine clinical setting. Considering the noninterventional character of this study, the herbal combination was demonstrated to improve overactive bladder symptoms and quality of life. A controlled study has not yet been initiated.
3.5 Upper respiratory tract infections (URTI)
The search on herbal medicines for the indication Upper Respiratory Infections revealed 24 publications.
The most common indications studied for the effectiveness of herbal medications were sinusitis, viral acute Rhisosinusitis (ARS) and common cold (N = 13), bronchitis (N = 8), and less frequently on acute cough (N = 2) and Acute lower and upper tract respiratory infections (N = 1) and chronic rhinosinusitis (N = 1). Most of them (N = 18) were double-blind randomized placebo-controlled trials, there were also randomized controlled trials that compared herbal medication to other herbal medication (N = 2) or to antibiotics (N = 1). Other study designs involved prospective cohorts (N = 3) and one retrospective cohort.
3.5.1 Sinusitis/common cold and chronic rhinosinusitis
Studies on treatment of acute sinusitis and acute rhinosinusitis used a follow-up period between 7 and 14 days, with the (adapted) Sinusitis Severity Score (SSS) (N = 2), the Major Symptom Score (MSS) (N = 4), the Total Symptom Score (N = 1) and facial pain relief (N = 1) as primary endpoints. All studies reported significantly improvement of the intervention group over the placebo or control group.
The treatment of acute sinusitis and acute rhinosinusitis with Eps 7630 (standardized root extract of Pelargonium sidoides DC (Pelargonium) was studied in two double blind randomised placebo controlled trials ( Bachert et al., 2009 ; Dejaco et al., 2019 ) and in one prospective ( Perić et al., 2020 ), randomized, open-label, non-inferiority study comparing study medication to Amoxicillin All three studies reported a significant superiority resp. Non-inferiority for Eps 7630. The use of the standardized herbal extract BNO 1016 ( Primulae flos (Primrose), Gentiana lutea Ruiz and Pav. Ex G.Don (Yellow gentian), Rumicis herba (Sorrel), Sambuci flos (Elderflower) and verbenae herba (Vervain) was tested in two randomised placebo controlled trials ( Jund et al., 2015 ), one of which was blinded ( Jund et al., 2015 ). Both studies showed stronger impact on the symptom score for BNO 1016 compared to placebo. One more study tested BNO 1016 in a multicenter, prospective, open-label study comparing its effect to intranasal fluticasone furoate, with patients in both groups showing improvement ( Passali et al., 2015 ). ELOM-080 (standardized herbal drug preparation containing specially destilled oils from Eucalyptus (Eucalypt) and Citrus ×sinensis (Sweet orange) and Myrtus (Myrtle) and Citrus limon (L.) Osbeck (Lemon oil)) was evaluated once in a double blind randomised placebo controlled trial ( Federspil et al., 1997 ) and once in a prospective, non-interventional parallel-group trial where the control group received BNO 1016 ( Gottschlich et al., 2018 ). In both studies BNO 1016 showed superior results.
The use of extracts containing Echinacea for the treatment of common cold was positively tested in two studies, reporting on total number of facial tissues used in three to 7 days after intervention start ( Naser et al., 2005 ) and on the Total Daily Symptom Scores (TDSS) after 7 days ( Goel et al., 2004 ). No statistically significant differences were observed between treatment groups for the total symptom score (SS) after 14 days. In two other studies testing capsules/pills containing Echinacea angustifolia root and Echinacea purpurea root and E. purpurea herb there was no statistically significant difference between the intervention and placebo group concerning severity and duration of self-reported symptoms ( Barrett et al., 2002 ) or global severity ( Barrett et al., 2010 ).
In a double blind randomised placebo controlled trial BNO 1016 was tested for the treatment of chronic rhinosinusitis. The results reveal that the herbal drug was not superior over placebo regarding the Major Symptom Score (MSS) in week 8 and week 12 ( Palm et al., 2017 ).
3.5.2 Bronchitis
For bronchitis, nine studies were included, of which six were double-blind randomized placebo-controlled trials, testing EPs 7630 (N = 5) ( Matthys et al., 2003 ; Chuchalin et al., 2005 ; Matthys and Heger, 2007 ; Matthys et al., 2010 ; Kähler et al., 2019 ) or ELOM-080 (N = 1) ( Gillissen et al., 2013 ). The prospective observational studies included a standardized syrup of Hedera helix L (Ivy leaves) (N = 1) ( Fazio et al., 2009 ), pills with ethanolic Ivy-leaves dry extracts (N = 1) ( Hecker et al., 2002 ) and EPs 7630 (N = 1) ( Matthys and Heger, 2007 ).
Using a follow-up period of 7 days to 4 weeks, all but one (double-blinded placebo controlled trial) ( Matthys et al., 2003 ) reported positive effects of the study medication on either Bronchitis Severity Scores, change of symptoms and coughing frequency.
3.5.3 Acute cough
The treatment of acute cough with EA-575 (standardized extract from H. helix L.) was tested against placebo in one double blind randomized placebo controlled trial and reported a significantly better improvement of cough severity (CS) assessed by Visual Analogue Scale (VAS) in the intervention group after 1 week as compared to placebo ( Schaefer et al., 2016 ).
3.5.4 Acute lower and upper tract respiratory infections
We included one retrospective cohort study comparing people with acute lower and upper tract respiratory infections who were prescribed a phytopharmaceutical to those who were not prescribed such drugs. They found that extract EPs 7630 (description see 3.5.1) (odds ratio (OR) 0.49 [95% CI: 0.43–0.57]) and thyme extract (OR 0.62 [0.49–0.76]) compared to no phytopharmaceutical prescription exhibited the strongest decrease in antibiotics prescriptions among patients treated by general practitioners ( Martin et al., 2020 ).
4 Discussion
The aim of this review is to depict the current evidence for the therapeutic efficacy of herbal medicines. Therefore, we conducted a literature search with defined inclusion and exclusion criteria in particular to select information from clinical studies with high levels of evidence and legally approved (in Europe) herbal medicines. Certainly, life-threatening disease are not suitable for the treatment with herbal medicines. This is the reason why we limited our perspective on psychosomatic disorders, gynecological complaints, gastrointestinal disorders and common infectious diseases of the urinary and the upper respiratory tract. Additionally, we concentrated on clinical trials with adult patients. It is to be emphasized that respective studies using herbal drugs have also been done in children with psychosomatic diseases ( Verlaet et al., 2017 ; Schloss et al., 2021 ), IBS ( Menon et al., 2023 ), gastrointestinal disorders ( Michael et al., 2022 ), UTIs ( Ching, 2022 ), and URIs ( Mancak Karakus et al., 2023 ) to mention only some examples.
The use of herbal medicines in the treatment of psychosomatic disorders is widespread and accordingly a high number of clinical studies was available for our analysis. In our literature search, the term “psychosomatic disorders” has been chosen. This term has not been clearly defined but is related to diseases which involve both physical and psychological illness. In other words, the respective symptoms are caused by mental processes and not directly by a physical disorder. The hits we got are based on this “terminology”. In contrast, the term “mental illnesses” which also includes psychological or behavioral manifestations is strictly defined as “health conditions with changes in emotion, thinking or behavior” ( Stein et al., 2021 ). However, even this definition is problematic, since there are concerns about specific conditions, the discrimination between independent biological entities or value-laden social constructs, and the defined indicators of dysfunction ( Stein et al., 2021 ). Independent on these concerns, we did not apply this search term. Therefore, we cannot exclude that (very few) articles have not been discovered with our search strategy.
For the treatment of depressive disorders, St. John’s wort is well-established and the studies we selected were predominantly positive regarding improvement of symptoms. Concurrently, SJW is well tolerated and in the majority of the studies at least equal to conventional medication like tricyclic anti-depressants and selective serotonin reuptake inhibitors, which exhibit in part notable adverse events impacting patients’ quality of life of ( Voican et al., 2014 ; Jakobsen et al., 2017 ).
In contrast evidence for insomnia and anxiety was thinner. It would be worthwhile to study the use of herbal drugs as alternative medication for the treatment of sleeping disorders, as for elderly people or long term use conventional hypnotics are not always the best option ( Wortelboer et al., 2002 ; Cheng et al., 2020 ). All the studies we included were using valerian root extract alone or in combination with Humulus lupulus extract and showed positive effects on sleep without notable side effects. The few studies we selected for anxiety demonstrated efficacy of lavender extract (Lavandula angustifolia) and also here we had a homogenous picture of good efficacy along with good tolerability.
Several years ago, consistent beneficial effects of Ginkgo biloba for patients with cerebral insufficiency were proven in a systematic review ( Kleijnen and Knipschild, 1992 ). However, the methodologic quality of many trials was considered to be poor. Moreover, the studies entailed a heterogeneous collection of target health problems, ranging from overt dementia to noncognitive manifestations of brain dysfunction, such as vertigo and tinnitus. More recently, the results of several new Ginkgo biloba trials have been published, most of them focusing on dementia, and showing positive effects. Probably the most talked about is the trial of the North American EGb Study Group, which was published in the JAMA in 1997 and showed a modest improvement of the cognitive performance and the social functioning of the demented patients involved ( Le Bars et al., 1997 ), which is well in line with the studies we have collected.
In addition, menopausal symptoms and premenstrual syndrome are suitable for treatment with herbal medicines. In the here collected studies, no overall negative effects were observed and adverse events did not occur more frequently than in the comparison groups. A consistent picture emerged when comparing herbal treatment with synthetic drugs or placebo: while herbal drugs and treatment with, e.g., HRT or pyridoxine showed equal efficacy, herbal treatment was in general superior to placebo administration, except for one study.
Effective treatment of menopausal symptoms with black cohosh is supported with multiple study designs. Regardless of the study quality, there are no contradictory results.
The evidence for the treatment of PMS with VAC initially appears similar to that of black cohosh for menopausal symptoms. However, the sample sizes have been insufficient and there was a complete lack of comparisons of VAC with other therapies. Also of interest are the hints on the importance of the dose and continuous administration. A higher dosage did not have a higher efficacy compared to the standard dosage, but slightly more participants experienced adverse events ( Momoeda et al., 2014 ). This suggests a preference for the standard dosage of VAC. Continuous use of VAC is recommended, as it has been shown that symptoms increase significantly, even if they are still lower than before therapy ( Bachert et al., 2009 ).
However, further research is needed for both gynecological indications. Only one study each on sage for menopausal symptoms and SJW for premenstrual symptoms was found ( Lauritzen et al., 1997 ; Lauritzen et al., 1997 ; Adeyemo et al., 2013 ). The trend-setting results point to positive effects which have to be confirmed.
For gastrointestinal disorders herbal drugs were, at least partially, shown to be similar efficacious as the standard treatment. Selected, non-toxic plant derived natural compounds may, therefore, replace synthesized drugs which are associated with undesired negative side effects and the therapeutic potential of the compounds may depend on both the plant extract and the type of disease to be treated. Indeed, SJW was not efficacious in treating IBS, whereas WS 1340/WS 1520 and STW 5 and STW 5-II showed efficacy in both IBS and FD. Considering the broad spectrum of gastrointestinal complaints, therapy of severe liver disease may require more effort than treatment of moderate dyspepsia and, hence, herbal medicine may not replace standard therapy.
As no standard therapy has so far been established for FD ( Madisch et al., 2018 ) and IBS ( Lacy et al., 2021 ) the design of clinical studies is difficult, making it impossible to compare the phytodrug group with a “reference” cohort, and to finally assess the value of the phytodrugs.
Particular attention should be given to STW 5 containing greater celandine which has been related to liver and biliary tract disorders ( Zielińska et al., 2018 ). Therefore, careful preclinical examination of potential toxic properties of a compound of question is necessary before starting clinical trials.
Overall, most of the studies were well designed (multicenter, double-blind, placebo-controlled trials) with large cohorts. Considering the low side effects and often significant improvements, it might be useful to conduct further studies to either gain more detailed information about herbal medicine or to transfer the knowledge to diseases with a similar cluster of symptoms, so that distinct ailments might particularly benefit from herbal medicine ( Chey et al., 2015 ).
With respect to urinary tract infections (UTI), herbal medicines have been proven to be similar effective as antibiotics. Undoubtedly, the data encourages further research on herbal medicines as alternatives to antibiotics in acute lower uncomplicated UTI ( Wagenlehner et al., 2018 ). The use of herbal medicines has also been considered to be a good and safe alternative to perioperative antibiotic prophylaxis ( Miotla et al., 2018 ). However, whether herbal medicines may reduce or even replace antibiotics in future guideline-based regimen requires more prospective studies conducted on large groups of participants ( Wawrysiuk et al., 2022 ).
It is important to note in this context that one study discriminated between HM responders and non-responders ( Kulchavenya, 2018 ). This phenomenon is highly important, since it indicates that the application of HM in general might be restricted to a subset of patients. Unfortunately, no ongoing trials have been enrolled in this matter, and none of the publications cited here discussed the problem of acquired or innate resistance, at least from a theoretical point of view.
LUTS caused by BPH was treated differently than UTI, since the complications of BPH, namely, urinary incontinence, polyuria, urinary retention, and erectile dysfunction, have to be targeted. The clinical trials published so far point to the benefit of herbal medicines in reducing BPH symptoms. However, it is not clear yet whether the integration of herbal medicines may allow to reduce or even to avoid the use of standard medical therapeutics in this case.
Overall, several clinical studies conducted in the last years document a beneficial role of herbal medicines in the treatment of UTI and LUTS.
Upper Respiratory Infections (URIs) are a frequent cause of troublesome symptoms, that might be appropriately treated with herbal medicine. Most studies included in this paper evaluated herbal medicines for the treatment of acute bronchitis or common cold and acute sinusitis or rhinosinusitis.
The majority of the studies we included for the treatment of acute bronchitis tested P. sidoides against placebo and reported a statistically significant decrease of bronchitis symptoms and/severity. This is in line with the results of a systematic review and meta-analysis ( Agbabiaka et al., 2008 ), although a more recent systematic review judged that the evidence was of low quality ( Timmer et al., 2013 ). Evidence for other herbal medicines in the treatment of acute bronchitis was scarce.
For the treatment of common cold we found some indications of effectiveness of P. sidoides , Eucalyptus, sweet orange, myrtle and lemon oil (ELOM-080) and for Gentianae radix, Primulae flos, Sambuci flos, Rumicis herba and verbenae herba (BNO 1016). A recent systematic review with network meta-analysis, showed very little solid evidence of herbal medicine versus placebo for common cold, with only P. sidoides and Andrographis paniculata showing a reliable decrease of symptoms. Better results were found for herbal medicine versus placebo concerning health related quality of life (HRQoL) (in particular Spicae aetheroleum ) and for symptoms (Cineole and P. sidoides ) ( Hoang et al., 2023 ). A further systematic review reported on the efficacy of P. sidoides (liquid and tablet preparation) for the treatment of acute bronchitis, showing a positive results with, however, low evidence quality ( Timmer et al., 2013 ).
Although herbal medicines are considered to be safe in principle, this might not always be the case. Some herbal compounds are suspected to be carcinogenic and/or hepatotoxic. Herbal products have also been shown to inhibit and/or induce drug-metabolizing enzymes ( Moreira et al., 2014 ). This has to be taken into account, since herbal medicines are often used in combination with conventional drugs. In this context, preparations with SJW may reduce the efficacy of chemotherapy and of anticoagulants but enhance the one of certain consciousness-lowering agents (e.g., sedative medicines, antidepressants) ( Nicolussi et al., 2020 ; Scholz et al., 2021 ). Due to potential liver toxicity of chelidonium majus, preparations containing more than 2.5 mg daily dose of whole chelidonium alkaloids had to be withdrawn, and for all preparations with lower daily doses, their instruction leaflet must include warnings on liver toxicity ( Rosien, 2019 ). Therefore, the drug’s safety must always be carefully investigated and guaranteed by the producers and the regulatory authorities.
The analysis of the outcomes in the selected disorders reflects that herbal medicines are most efficacious for the treatment of URTI ( Figure 5 ), followed by gynecological complaints ( Figure 2 ) and psychosomatic disorders ( Figure 1 ). For the treatment of urological diseases ( Figure 4 ) in particular UTI and LUTS, we could select only 16 studies according to our strict inclusion/exclusion criteria and therefore more studies of high quality have to be performed to gain a better insight into the efficacy of herbal drugs for these ailments. Gastrointestinal diseases hold a special position as only the added value of the phytodrugs to the conventional therapy was tested. In addition, the number of studies we selected was small ( Figure 3 ), making it difficult to judge the efficacy of herbal drugs for this indication.

This report on the current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments has some limitations.
- 1. The literature search had to be restricted to Pubmed, because other relevant databases like e.g., EMBASE or CINAHL have not been accessible to the authors.
- 2. Further limitations are the small cohorts in some of the studies
- 3. Or that the results/outcomes of some studies have been re-analyzed from previous studies.
- 4. A general obstacle of data interpretation is that for some indications, in particular for gastrointestinal diseases, herbal medicines are predominantly co-administered with standard therapy, which makes it difficult to estimate the clinical benefit of the phytodrug alone.
5 Perspective
Our literature research gives insights into applied herbal medicines for selected indications, the study outcomes and their quality. Based on our results, we (the authors) provide an overview for patients and healthcare practitioners which extracts can be recommended for the treatment if which disorder/complaint ( Supplementary Table S1 ).
In this context we recommend in particular H. perforatum L. for depressive disorder, V. agnus castus L. for menstrual complaints, Cimicifica racemose (L.) for menopausal symptoms, a combination of I. amara L., M. chamomilla L., Mentha × piperita L., C. carvi L., G. glabra L. and M. officinalis L., for functional dyspepsia, a combination of C. erythraea , Levisticum officinale W.D.J.Koch and Rosmarinus officinalis L. for uncomlicated urinary tract infections, P. sidoides DC. for bronchitis and sinusitis and finally H. helix for cough ( Supplementary Table S1 ). These recommendations are based on studies with the highest levels of evidence (RCTs).
However, evidence for efficacy of herbal medicines is still not satisfying in order to integrate them in conventional medicine guidelines and standard treatment regimen, which is the reason why statutory health insurances do not reimburse the costs. In fact, herbal medicines are highly popular and accepted among patients, since their application is safe since they do not exert severe side-effects. Especially when conventional medical therapies fail due to undesired side effects having a negative impact on the quality of life, patients are willing to purchase herbal medicines at their own expense. Often doctors do not know about the self-medication activities of their patients and in consequence cannot monitor the treatment with herbal medicines and possible interactions with other drugs.
The discrepancy between available results from clinical research and the use of herbal medicines under everyday conditions shows that we need to perform more interdisciplinary research studies in the future in order to collect scientific sound evidence on their benefits. Clinical research can provide information on the efficacy of phytodrugs and the importance of genetic dispositions and metabolism as well as possible interactions with other medicines. For effectiveness under everyday conditions (from bedside to practice), methods of health services research are necessary. With the help of these, the outcomes of herbal medicines can be recorded from different perspectives, in particular those of the patients (patient-reported outcomes (PROs)). For longitudinal observations, analyses of health insurance and sales volume data are also relevant, using prescriptions and the over-the-counter sales to get a picture on the needs of the patients and the acceptance of phytotherapy by healthcare practitioners. In order to pave the way for the integration of herbal medicines into therapy guidelines and regimens, findings from clinical studies should be carefully evaluated for their transferability to everyday healthcare within the scope of health services research. This way could lead to novel rational efficacious therapy strategies with less side-effects and better compliance of the patients.
Data availability statement
Author contributions.
SS: Investigation, Formal analysis, Writing–Original Draft. JR: Investigation, Formal analysis, Writing–Original Draft, Visualization. MA: Methodology, Investigation, Formal analysis, Writing–Original Draft. RB: Investigation, Formal analysis, Writing–Original Draft. BB: Conceptualization, Methodology, Investigation, Formal analysis, Writing–Original Draft, Supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1234701/full#supplementary-material
- Adeyemo O., Doi H., Rajender Reddy K., Kaplan D. E. (2013). Impact of oral silymarin on virus- and non-virus-specific T-cell responses in chronic hepatitis C infection . J. Viral Hepat. 20 , 453–462. 10.1111/jvh.12050 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Agbabiaka T. B., Guo R., Ernst E. (2008). Pelargonium sidoides for acute bronchitis: A systematic review and meta-analysis . Phytomedicine 15 , 378–385. 10.1016/j.phymed.2007.11.023 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albrecht U., Goos K. H., Schneider B. (2007). A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections . Curr. Med. Res. Opin. 23 , 2415–2422. 10.1185/030079907X233089 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anghelescu I. G., Kohnen R., Szegedi A., Klement S., Kieser M. (2006). Comparison of Hypericum extract WS 5570 and paroxetine in ongoing treatment after recovery from an episode of moderate to severe depression: Results from a randomized multicenter study . Pharmacopsychiatry 39 , 213–219. 10.1055/s-2006-951388 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Armour M., Ee C. C., Hao J., Wilson T. M., Yao S. S., Smith C. A. (2018). Acupuncture and acupressure for premenstrual syndrome . Cochrane Database Syst. Rev. 8 , CD005290. 10.1002/14651858.CD005290.pub2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- AWMF (2020). Peri- and postmenopause – diagnosis and interventions. Guideline of the DGGG, SGGG and OEGGG (S3 level, AWMF registry No. 015-062, january 2020) . Marburg, Germany: Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. [ Google Scholar ]
- Bachert C., Schapowal A., Funk P., Kieser M. (2009). Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: A randomized, double-blind, placebo-controlled trial . Rhinology 47 , 51–58. [ PubMed ] [ Google Scholar ]
- Bachmeier B. E., Iancu C. M., Killian P. H., Kronski E., Mirisola V., Angelini G., et al. (2009). Overexpression of the ATP binding cassette gene ABCA1 determines resistance to Curcumin in M14 melanoma cells . Mol. Cancer 8 , 129. 10.1186/1476-4598-8-129 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bachmeier B. E., Mirisola V., Romeo F., Generoso L., Esposito A., Dell'eva R., et al. (2010). Reference profile correlation reveals estrogen-like trancriptional activity of Curcumin . Cell Physiol. Biochem. 26 , 471–482. 10.1159/000320570 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bachmeier B. E., Mohrenz I. V., Mirisola V., Schleicher E., Romeo F., Hohneke C., et al. (2008). Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB . Carcinogenesis 29 , 779–789. 10.1093/carcin/bgm248 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bachmeier B., Nerlich A. G., Iancu C. M., Cilli M., Schleicher E., Vene R., et al. (2007). The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice . Cell Physiol. Biochem. 19 , 137–152. 10.1159/000099202 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bai W., Henneicke-Von Zepelin H. H., Wang S., Zheng S., Liu J., Zhang Z., et al. (2007). Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: A randomized, double blind, parallel-controlled study versus tibolone . Maturitas 58 , 31–41. 10.1016/j.maturitas.2007.04.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett B., Brown R., Rakel D., Mundt M., Bone K., Barlow S., et al. (2010). Echinacea for treating the common cold: A randomized trial . Ann. Intern. Med. 153 , 769–777. 10.7326/0003-4819-153-12-201012210-00003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett B. P., Brown R. L., Locken K., Maberry R., Bobula J. A., D'alessio D. (2002). Treatment of the common cold with unrefined echinacea. A randomized, double-blind, placebo-controlled trial . Ann. Intern Med. 137 , 939–946. 10.7326/0003-4819-137-12-200212170-00006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berger D., Schaffner W., Schrader E., Meier B., Brattstrom A. (2000). Efficacy of Vitex agnus castus L. extract Ze 440 in patients with pre-menstrual syndrome (PMS) . Arch. Gynecol. Obstet. 264 , 150–153. 10.1007/s004040000123 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bittel M., Rakoczy T., Fröhlich A., Langhorst J. (2022). Phytotherapie bei psychischen Erkrankungen in medizinischen Leitlinien . Z. für Phytother. 43 , 112–120. 10.1055/a-1716-9651 [ CrossRef ] [ Google Scholar ]
- Bjerkenstedt L., Edman G. V., Alken R. G., Mannel M. (2005). Hypericum extract LI 160 and fluoxetine in mild to moderate depression: A randomized, placebo-controlled multi-center study in outpatients . Eur. Arch. Psychiatry Clin. Neurosci. 255 , 40–47. 10.1007/s00406-004-0532-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bommer S., Klein P., Suter A. (2011). First time proof of sage's tolerability and efficacy in menopausal women with hot flushes . Adv. Ther. 28 , 490–500. 10.1007/s12325-011-0027-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brenner R., Azbel V., Madhusoodanan S., Pawlowska M. (2000). Comparison of an extract of hypericum (li 160) and sertraline in the treatment of depression: A double-blind, randomized pilot study . Clin. Ther. 22 , 411–419. 10.1016/S0149-2918(00)89010-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Briese V., Stammwitz U., Friede M., Henneicke-Von Zepelin H. H. (2007). Black cohosh with or without St. John's wort for symptom-specific climacteric treatment-results of a large-scale, controlled, observational study . Maturitas 57 , 405–414. 10.1016/j.maturitas.2007.04.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burkhardt H., Wehling M. (2010). [ Difficulties in pharmacotherapy of the elderly ]. Der Internist 51 , 737–747. 10.1007/s00108-010-2582-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canning S., Waterman M., Orsi N., Ayres J., Simpson N., Dye L. (2010). The efficacy of Hypericum perforatum (St john's wort) for the treatment of premenstrual syndrome: A randomized, double-blind, placebo-controlled trial . CNS Drugs 24 , 207–225. 10.2165/11530120-000000000-00000 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheng B., Liu Y., Tian J., Gao R., Liu Y. (2020). Complementary and alternative medicine for the treatment of insomnia: An overview of scientific evidence from 2008 to 2018 . Curr. Vasc. Pharmacol. 18 , 307–321. 10.2174/1570161117666190506111239 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chey W. D., Kurlander J., Eswaran S. (2015). Irritable bowel syndrome: A clinical review . JAMA 313 , 949–958. 10.1001/jama.2015.0954 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ching C. B. (2022). Non-antibiotic approaches to preventing pediatric UTIs: A role for D-mannose, cranberry, and probiotics? Curr. Urol. Rep. 23 , 113–127. 10.1007/s11934-022-01094-w [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chuchalin A. G., Berman B., Lehmacher W. (2005). Treatment of acute bronchitis in adults with a pelargonium sidoides preparation (EPs 7630): A randomized, double-blind, placebo-controlled trial . Explore (New York, N.Y.) 1 , 437–445. 10.1016/j.explore.2005.08.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cornberg M., Wedemeyer H., Manns M. P. (2002). Treatment of chronic hepatitis C with PEGylated interferon and ribavirin . Curr. Gastroenterol. Rep. 4 , 23–30. 10.1007/s11894-002-0034-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Corrao S., Scaglione R., Calvo L., Licata G. (2007). Methodological matters on an alzheimer's dementia trial: Is a double-blind randomized controlled study design sufficient to draw strong conclusions on treatment? Reply to Dr mazza and colleagues . Eur. J. Neurology 14 , e11; author reply e12–e11. 10.1111/j.1468-1331.2007.01713.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darbinyan V., Aslanyan G., Amroyan E., Gabrielyan E., Malmstrom C., Panossian A. (2007). Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression . Nordic J. Psychiatry 61 , 343–348. 10.1080/08039480701643290 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davidson J. R. T., Gadde K. M., Fairbank J. A., Krishnan R. R., Califf R. M., Binanay C., et al. (2002). Effect of Hypericum perforatum (St John's wort) in major depressive disorder - a randomized controlled trial . Jama 287 , 1807–1814. 10.1001/jama.287.14.1807 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dejaco D., Bocian-Sobkowska J., Szymanski W., Zacke G., Hockner V., Riechelmann H. (2019). Tavipec in acute rhinosinusitis: A multi-centre, doubleblind, randomized, placebo-controlled, clinical trial . Rhinology 57 , 367–374. 10.4193/Rhin19.089 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donath F., Quispe S., Diefenbach K., Maurer A., Fietze I., Roots I. (2000). Critical evaluation of the effect of valerian extract on sleep structure and sleep quality . Pharmacopsychiatry 33 , 47–53. 10.1055/s-2000-7972 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dorn M. (2000). Efficacy and tolerability of baldrian versus oxazepam in non-organic and non-psychiatric insomniacs: A randomised, double-blind, clinical, comparative study . Forsch Komplementarmed Klass. Naturheilkd 7 , 79–84. 10.1159/000021314 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Drewe J., Zimmermann C., Zahner C. (2013). The effect of a Cimicifuga racemosa extracts Ze 450 in the treatment of climacteric complaints-an observational study . Phytomedicine 20 , 659–666. 10.1016/j.phymed.2013.02.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Engelmann U., Walther C., Bondarenko B., Funk P., Schläfke S. (2011). Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin . Arzneimittelforschung 56 , 222–229. 10.1055/s-0031-1296714 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fathalah W. F., Aziz M. A., Abou El Soud N. H., El Raziky M. E. (2017). High dose of silymarin in patients with decompensated liver disease: A randomized controlled trial . J. Interferon Cytokine Res. 37 , 480–487. 10.1089/jir.2017.0051 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fava M., Alpert J., Nierenberg A. A., Mischoulon D., Otto M. W., Zajecka J., et al. (2005). A double-blind, randomized trial of St John's wort, fluoxetine, and placebo in major depressive disorder . J. Clin. Psychopharmacol. 25 , 441–447. 10.1097/01.jcp.0000178416.60426.29 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fazio S., Pouso J., Dolinsky D., Fernandez A., Hernandez M., Clavier G., et al. (2009). Tolerance, safety and efficacy of Hedera helix extract in inflammatory bronchial diseases under clinical practice conditions: A prospective, open, multicentre postmarketing study in 9657 patients . Phytomedicine 16 , 17–24. 10.1016/j.phymed.2006.05.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Feagan B. G., Rochon J., Fedorak R. N., Irvine E. J., Wild G., Sutherland L., et al. (1995). Methotrexate for the treatment of crohn's disease. The North American crohn's study group investigators . N. Engl. J. Med. 332 , 292–297. 10.1056/NEJM199502023320503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Federspil P., Wulkow R., Zimmermann T. (1997). [ Effects of standardized Myrtol in therapy of acute sinusitis-results of a double-blind, randomized multicenter study compared with placebo ]. Laryngo- rhino- Otol. 76 , 23–27. 10.1055/s-2007-997381 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ferenci P., Scherzer T. M., Hofer H., Schniger-Hekele M., Steindl-Munda P. (2008). Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to antiviral combination therapy . J. Hepatology 48 , S28. 10.1016/s0168-8278(08)60065-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ford O., Mol B., Roberts H., Ford O. (2006). Progesterone for premenstrual syndrome . Cochrane Database Syst. Rev. 2012 , CD003415. 10.1002/14651858.CD003415.pub4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fried M. W., Navarro V. J., Afdhal N., Belle S. H., Wahed A. S., Hawke R. L., et al. (2012). Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: A randomized controlled trial . JAMA 308 , 274–282. 10.1001/jama.2012.8265 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friede M., Von Zepelin H. H. H., Freudenstein J. (2001). Differential therapy of mild to moderate depressive episodes (ICD-10 F 32.0; F 32.1) with St. John's wort . Pharmacopsychiatry 34 , S38–S41. 10.1055/s-2001-15459 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friederichsen L., Nebel S., Zahner C., Butikofer L., Stute P. (2020). Effect of CIMicifuga racemosa on metaBOLIC parameters in women with menopausal symptoms: A retrospective observational study (CIMBOLIC) . Archives Gynecol. Obstetrics 301 , 517–523. 10.1007/s00404-019-05366-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fries C. J. (2019). Healing health care: From sick care towards salutogenic healing systems . Soc. Theory and Health 18 , 16–32. 10.1057/s41285-019-00103-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gastpar M., Singer A., Zeller K. (2006). Comparative efficacy and safety of a once-daily dosage of hypericum extract STW3-VI and citalopram in patients with moderate depression: A double-blind, randomised, multicentre, placebo-controlled study . Pharmacopsychiatry 39 , 66–75. 10.1055/s-2006-931544 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gastpar M., Singer A., Zeller K. (2005). Efficacy and tolerability of hypericum extract STW3 in long-term treatment with a once-daily dosage in comparison with sertraline . Pharmacopsychiatry 38 , 78–86. 10.1055/s-2005-837807 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gauruder-Burmester A., Heim S., Patz B., Seibt S. (2019). Cucurbita pepo-rhus aromatica-humulus lupulus combination reduces overactive bladder symptoms in women - a noninterventional study . Planta Med. 85 , 1044–1053. 10.1055/a-0946-2280 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gavrilova S. I., Preuss U. W., Wong J. W., Hoerr R., Kaschel R., Bachinskaya N., et al. (2014). Efficacy and safety of ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: A randomized, placebo-controlled, double-blind, multi-center trial . Int. J. Geriatr. Psychiatry 29 , 1087–1095. 10.1002/gps.4103 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gillissen A., Wittig T., Ehmen M., Krezdorn H. G., Mey C. D. (2013). A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol® forte in acute bronchitis . Drug Res. 63 , 19–27. 10.1055/s-0032-1331182 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goel V., Lovlin R., Barton R., Lyon M. R., Bauer R., Lee T. D., et al. (2004). Efficacy of a standardized echinacea preparation (echinilin) for the treatment of the common cold: A randomized, double-blind, placebo-controlled trial . J. Clin. Pharm. Ther. 29 , 75–83. 10.1111/j.1365-2710.2003.00542.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gottschlich S., Röschmann K., Candler H. (2018). Phytomedicines in acute rhinosinusitis: A prospective, non-interventional parallel-group trial . Adv. Ther. 35 , 1023–1034. 10.1007/s12325-018-0736-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gründer G., Benkert O. (2012). Handbuch der Psychopharmakotherapie . Berlin, Heidelberg: Springer. [ Google Scholar ]
- Grunze A., Mago R., Grunze H. (2017). [ Side effects of psychotropic medication: Suggestions for clinical practice ]. DMW - Dtsch. Med. Wochenschr. 142 , 1690–1700. 10.1055/s-0043-110654 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gschwind Y., Bridenbaugh S., Reinhard S., Granacher U., Monsch A., Kressig R. (2017). Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: A randomised, double-blind, placebo-controlled exploratory study . Aging Clin. Exp. Res. 29 , 609–619. 10.1007/s40520-016-0699-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hansgen K. D., Vesper J., Ploch M. (1994). Multicenter double-blind study examining the antidepressant effectiveness of the hypericum extract LI 160 . J. Geriatr. Psychiatry Neurol. 7 ( 1 ), S15–S18. 10.1177/089198879400700106 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harrer G., Hubner W. D., Podzuweit H. (1994). Effectiveness and tolerance of the hypericum extract LI 160 compared to maprotiline: A multicenter double-blind study . J. Geriatr. Psychiatry Neurol. 7 ( 1 ), S24–S28. 10.1177/089198879400700108 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harrer G., Schmidt U., Kuhn U., Biller A. (1999). Comparison of equivalence between the St. John's wort extract LoHyp-57 and fluoxetine . Arzneimittelforschung-Drug Res. 49 , 289–296. 10.1055/s-0031-1300417 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haupt M., Vollmar H. C. (2008). “ Psychische Erkrankungen bei älteren Patienten ,” in Psychische erkrankungen in der Hausarztpraxis . Editors Schneider F, Niebling W. (Berlin, Heidelberg: Springer; ), 517–532. [ Google Scholar ]
- Hawke R. L., Schrieber S. J., Soule T. A., Wen Z. M., Smith P. C., Reddy K. R., et al. (2010). Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C . J. Clin. Pharmacol. 50 , 434–449. 10.1177/0091270009347475 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hecker M., Runkel F., Voelp A. (2002). [ Treatment of chronic bronchitis with ivy leaf special extract-multicenter post-marketing surveillance study in 1,350 patients ]. Forsch. Komplementarmedizin Klass. Naturheilkd. 9 , 77–84. 10.1159/000057269 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoang M. P., Seresirikachorn K., Chitsuthipakorn W., Snidvongs K. (2023). Herbal medicines for rhinosinusitis: A systematic review and network meta-analysis . Curr. Allergy Asthma Rep. 23 , 93–109. 10.1007/s11882-022-01060-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holler M., Steindl H., Abramov-Sommariva D., Wagenlehner F., Naber K. G., Kostev K. (2021). Treatment of urinary tract infections with Canephron ® in Germany: A retrospective database analysis . Antibiot. (Basel) 10 , 685. 10.3390/antibiotics10060685 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huber R., Ditfurth A. V., Amann F., Güthlin C., Rostock M., Trittler R., et al. (2007). Tormentil for active ulcerative colitis: An open-label, dose-escalating study . J. Clin. Gastroenterology 41 , 834–838. 10.1097/MCG.0b013e31804b2173 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jakobsen J. C., Katakam K. K., Schou A., Hellmuth S. G., Stallknecht S. E., Leth-Møller K., et al. (2017). Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis . BMC Psychiatry 17 , 58. 10.1186/s12888-016-1173-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jaume F., Valls-Mateus M., Mullol J. (2020). Common cold and acute rhinosinusitis: Up-to-Date management in 2020 . Curr. Allergy Asthma Rep. 20 , 28. 10.1007/s11882-020-00917-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Julia Molla M. D., Garcia-Sanchez Y., Romeu Sarri A., Perez-Lopez F. R. (2009). Cimicifuga racemosa treatment and health related quality of life in post-menopausal Spanish women . Gynecol. Endocrinol. 25 , 21–26. 10.1080/09513590802404005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jund R., Mondigler M., Stammer H., Stierna P., Bachert C. (2015). Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis . Acta oto-laryngologica 135 , 42–50. 10.3109/00016489.2014.952047 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jung N., Tometten L., Draenert R. (2023). Choosing Wisely internationally – helpful recommendations for antimicrobial stewardship! . Infection 51 , 567–581. 10.1007/s15010-023-02005-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kähler C., Derezinski T., Bocian-Sobkowska J., Keckeis A., Zacke G. (2019). Spicae aetheroleum in uncomplicated acute bronchitis: A double-blind, randomised clinical trial . Wien. Med. Wochenschr. 169 , 137–148. 10.1007/s10354-017-0612-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kalb R., Trautmann-Sponsel R. D., Kieser M. (2001). Efficacy and tolerability of Hypericum extract WS 5572 versus placebo in mildly to moderately depressed patients - a randomized double-blind multicenter clinical trial . Pharmacopsychiatry 34 , 96–103. 10.1055/s-2001-14280 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Anghelescu I., Dienel A. (2015). Efficacy of orally administered Silexan in patients with anxiety-related restlessness and disturbed sleep-A randomized, placebo-controlled trial . Eur. Neuropsychopharmacol. 25 , 1960–1967. 10.1016/j.euroneuro.2015.07.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Anghelescu I. G., Szegedi A., Dienel A., Kieser M. (2007). Placebo controlled continuation treatment with Hypericum extract WS 5570 after recovery from a mild or moderate depressive episode . Wien Med. Wochenschr 157 , 362–366. 10.1007/s10354-007-0441-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Anghelescu I. G., Szegedi A., Dienel A., Kieser M. (2006). Superior efficacy of St john's wort extract WS 5570 compared to placebo in patients with major depression: A randomized, double-blind, placebo-controlled, multi-center trial [ISRCTN77277298] . BMC Med. 4 , 14. 10.1186/1741-7015-4-14 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Gastpar M., Muller W. E., Volz H. P., Moller H. J., Dienel A., et al. (2010). Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of 'subsyndromal' anxiety disorder: A randomized, double-blind, placebo controlled trial . Int. Clin. Psychopharmacol. 25 , 277–287. 10.1097/YIC.0b013e32833b3242 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Gastpar M., Muller W. E., Volz H. P., Moller H. J., Schlafke S., et al. (2014). Lavender oil preparation Silexan is effective in generalized anxiety disorder - a randomized, double-blind comparison to placebo and paroxetine . Int. J. Neuropsychopharmacol. 17 , 859–869. 10.1017/S1461145714000017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Volz H. P., Dienel A., Schlafke S. (2016). Efficacy of Silexan in mixed anxiety-depression-A randomized, placebo-controlled trial . Eur. Neuropsychopharmacol. 26 , 331–340. 10.1016/j.euroneuro.2015.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kasper S., Volz H. P., Moller H. J., Dienel A., Kieser M. (2008). Continuation and long-term maintenance treatment with Hypericum extract WS 5570 after recovery from an acute episode of moderate depression-a double-blind, randomized, placebo controlled long-term trial . Eur. Neuropsychopharmacol. 18 , 803–813. 10.1016/j.euroneuro.2008.06.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Killian P. H., Kronski E., Michalik K. M., Barbieri O., Astigiano S., Sommerhoff C. P., et al. (2012). Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2 . Carcinogenesis 33 , 2507–2519. 10.1093/carcin/bgs312 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kleijnen J., Knipschild P. (1992). Ginkgo biloba . Lancet 340 , 1136–1139. 10.1016/0140-6736(92)93158-j [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koetter U., Schrader E., Kaufeler R., Brattstrom A. (2007). A randomized, double blind, placebo-controlled, prospective clinical study to demonstrate clinical efficacy of a fixed valerian hops extract combination (Ze 91019) in patients suffering from non-organic sleep disorder . Phytother. Res. 21 , 847–851. 10.1002/ptr.2167 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korczyn A. D. (2007). Comments on the article by mazza et al. Concerning ginkgo biloba and donepezil: A comparison in the treatment of alzheimer's dementia in a randomized placebo-controlled double-blind study . Eur. J. Neurol. 14 , e9; author reply e10. 10.1111/j.1468-1331.2007.01710.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Krebs S., Omer T. N., Omer B. (2010). Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease – a controlled clinical trial . Phytomedicine 17 , 305–309. 10.1016/j.phymed.2009.10.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kronski E., Fiori M. E., Barbieri O., Astigiano S., Mirisola V., Killian P. H., et al. (2014). miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2 . Mol. Oncol. 8 , 581–595. 10.1016/j.molonc.2014.01.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kulchavenya E. (2018). Acute uncomplicated cystitis: Is antibiotic unavoidable? Ther. Adv. Urol. 10 , 257–262. 10.1177/1756287218783644 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kwan I., Onwude J. L. (2015). Premenstrual syndrome . BMJ Clin. Evid. 2015 , 0806. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Laakmann G., Schule C., Baghai T., Kieser M. (1998). St. John's wort in mild to moderate depression: The relevance of hyperforin for the clinical efficacy . Pharmacopsychiatry 31 ( 1 ), 54–59. 10.1055/s-2007-979346 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lacy B. E., Pimentel M., Brenner D. M., Chey W. D., Keefer L. A., Long M. D., et al. (2021). ACG clinical guideline: Management of irritable bowel syndrome . Am. J. Gastroenterology 116 , 17–44. 10.14309/ajg.0000000000001036 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauritzen C., Reuter H. D., Repges R., Bohnert K. J., Schmidt U. (1997). Treatment of premenstrual tension syndrome with Vitex agnus castus controlled, double-blind study versus pyridoxine . Phytomedicine 4 , 183–189. 10.1016/S0944-7113(97)80066-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laux G. (2021). “ Psychopharmakotherapie ,” in Duale Reihe Psychiatrie, Psychosomatik und Psychotherapie . Editors Falkai P. L, Gerd L., Deister A, Möller H. J. 7 ed (New York: Thieme; ). [ Google Scholar ]
- Le Bars P. L. (2003). Response patterns of EGb 761 in alzheimer's disease: Influence of neuropsychological profiles . Pharmacopsychiatry 36 ( 1 ), S50–S55. 10.1055/s-2003-40456 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Le Bars P. L., Katz M. M., Berman N., Itil T. M., Freedman A. M., Schatzberg A. F. (1997). A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group . JAMA 278 , 1327–1332. 10.1001/jama.278.16.1327 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Le Bars P. L., Velasco F. M., Ferguson J. M., Dessain E. C., Kieser M., Hoerr R. (2002). Influence of the severity of cognitive impairment on the effect of the Ginkgo biloba extract EGb 761 ® in Alzheimer’s disease . Neuropsychobiology 45 , 19–26. 10.1159/000048668 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lecrubier Y., Clerc G., Didi R., Kieser M. (2002). Efficacy of St. John's wort extract WS 5570 in major depression: A double-blind, placebo-controlled trial . Am. J. Psychiatry 159 , 1361–1366. 10.1176/appi.ajp.159.8.1361 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liske E., Hanggi W., Henneicke-Von Zepelin H. H., Boblitz N., Wustenberg P., Rahlfs V. W. (2002). Physiological investigation of a unique extract of black cohosh (cimicifugae racemosae rhizoma): A 6-month clinical study demonstrates no systemic estrogenic effect . J. Womens Health Gend. Based Med. 11 , 163–174. 10.1089/152460902753645308 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Loch E. G., Selle H., Boblitz N. (2000). Treatment of premenstrual syndrome with a phytopharmaceutical formulation containing Vitex agnus castus . J. Womens Health Gend. Based Med. 9 , 315–320. 10.1089/152460900318515 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopatkin N., Sivkov A., Schlafke S., Funk P., Medvedev A., Engelmann U. (2007). Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms-long-term follow-up of a placebo-controlled, double-blind, multicenter trial . Int. Urol. Nephrol. 39 , 1137–1146. 10.1007/s11255-006-9173-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopatkin N., Sivkov A., Walther C., Schlafke S., Medvedev A., Avdeichuk J., et al. (2005). Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms-a placebo-controlled, double-blind, multicenter trial . World J. Urol. 23 , 139–146. 10.1007/s00345-005-0501-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopez L. M., Kaptein A., Helmerhorst F. M., Lopez L. (2007). Oral contraceptives containing drospirenone for premenstrual syndrome . Cochrane Database Syst. Rev. 15 , CD006586. 10.1002/14651858.CD006586.pub4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maclennan A. H., Broadbent J. L., Lester S., Moore V. (2004). Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes . Cochrane database Syst. Rev. 2004 , CD002978. 10.1002/14651858.CD002978.pub2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madersbacher S., Rieken M., Reuber K., Kostev K. (2023). Association between PRO 160/120 prescriptions and incidence of benign prostatic hyperplasia complications in Germany: A retrospective cohort study . Postgrad. Med. 135 , 149–154. 10.1080/00325481.2022.2149156 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madisch A., Andresen V., Enck P., Labenz J., Frieling T., Schemann M. (2018). The diagnosis and treatment of functional dyspepsia . Dtsch. Ärzteblatt Int. 115 , 222–232. 10.3238/arztebl.2018.0222 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madisch A., Heydenreich C. J., Wieland V., Hufnagel R., Hotz J. (1999). Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride - a multicenter, reference-controlled double-blind equivalence study . Arzneimittel-Forschung-Drug Res. 49 , 925–932. 10.1055/s-0031-1300528 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madisch A., Holtmann G., Mayr G., Vinson B., Hotz J. (2004a). Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial . Digestion 69 , 45–52. 10.1159/000076546 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madisch A., Holtmann G., Plein K., Hotz J. (2004b). Treatment of irritable bowel syndrome with herbal preparations: Results of a double-blind, randomized, placebo-controlled, multi-centre trial . Aliment. Pharmacol. Ther. 19 , 271–279. 10.1111/j.1365-2036.2004.01859.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mancak Karakus M., Tapisiz A., Mutlu Karakas N., Deniz M., Koca Caliskan U. (2023). Use of herbal tea/herbal preparations for children with symptoms of viral upper respiratory infections . Turk J. Pharm. Sci. 20 , 8–15. 10.4274/tjps.galenos.2022.65475 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mannel M., Kuhn U., Schmidt U., Ploch M., Murck H. (2010). St. John's wort extract LI160 for the treatment of depression with atypical features - a double-blind, randomized, and placebo-controlled trial . J. Psychiatr. Res. 44 , 760–767. 10.1016/j.jpsychires.2010.01.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mao J. J., Xie S. X., Zee J., Soeller I., Li Q. S., Rockwell K., et al. (2015). Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial . Phytomedicine 22 , 394–399. 10.1016/j.phymed.2015.01.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marjoribanks J., Brown J., O'brien P. M. S., Wyatt K. (2013). Selective serotonin reuptake inhibitors for premenstrual syndrome . Cochrane Database Syst. Rev. 2013 , CD001396. 10.1002/14651858.CD001396.pub3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Martin D., Konrad M., Adarkwah C. C., Kostev K. (2020). Reduced antibiotic use after initial treatment of acute respiratory infections with phytopharmaceuticals-a retrospective cohort study . Postgrad. Med. 132 , 412–418. 10.1080/00325481.2020.1751497 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matthys H., Eisebitt R., Seith B., Heger M. (2003). Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis. A randomised, double-blind, placebo-controlled trial . Phytomedicine Int. J. phytotherapy Phytopharm. 10 ( 4 ), 7–17. 10.1078/1433-187x-00308 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matthys H., Heger M. (2007). Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomised, double-blind, placebo-controlled, multicentre study . Curr. Med. Res. Opin. 23 , 323–331. 10.1185/030079906X167318 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matthys H., Lizogub V. G., Malek F. A., Kieser M. (2010). Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: A randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides . Curr. Med. Res. Opin. 26 , 1413–1422. 10.1185/03007991003798463 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazza M., Capuano A., Bria P., Mazza S. (2006). Ginkgo biloba and donepezil: A comparison in the treatment of alzheimer's dementia in a randomized placebo-controlled double-blind study . Eur. J. Neurol. 13 , 981–985. 10.1111/j.1468-1331.2006.01409.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Menon J., Thapa B. R., Kumari R., Puttaiah Kadyada S., Rana S., Lal S. B. (2023). Efficacy of oral psyllium in pediatric irritable bowel syndrome: A double-blind randomized control trial . J. Pediatr. Gastroenterol. Nutr. 76 , 14–19. 10.1097/MPG.0000000000003622 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Michael R., Bettina V., Eckehard L. (2022). Functional gastrointestinal disorders in children: Effectivity, safety, and tolerability of the herbal preparation STW-5 (Iberogast®) in general practice . Complement. Ther. Med. 71 , 102873. 10.1016/j.ctim.2022.102873 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miotla P., Wawrysiuk S., Naber K., Markut-Miotla E., Skorupski P., Skorupska K., et al. (2018). Should we always use antibiotics after urodynamic studies in high-risk patients? Biomed. Res. Int. 2018 , 1607425. 10.1155/2018/1607425 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Momoeda M., Sasaki H., Tagashira E., Ogishima M., Takano Y., Ochiai K. (2014). Efficacy and safety of Vitex agnus-castus extract for treatment of premenstrual syndrome in Japanese patients: A prospective, open-label study . Adv. Ther. 31 , 362–373. 10.1007/s12325-014-0106-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moreira D. D. L., Teixeira S. S., Monteiro M. H. D., De-Oliveira A. C. a. X., Paumgartten F. J. R. (2014). Traditional use and safety of herbal medicines1 . Rev. Bras. Farmacogn. 24 , 248–257. 10.1016/j.bjp.2014.03.006 [ CrossRef ] [ Google Scholar ]
- Moreno R. A., Teng C. T., Almeida K. M., Tavares Junior H. (2006). Hypericum perforatum versus fluoxetine in the treatment of mild to moderate depression: A randomized double-blind trial in a Brazilian sample . Braz J. Psychiatry 28 , 29–32. 10.1590/s1516-44462006000100007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Naheed B., O'brien P. M. S., Uthman O. A., O'mahony F., Naheed B. (2013). Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst. Rev. 3 , CD010503. 10.1002/14651858.CD010503.pub2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nappi R. E., Malavasi B., Brundu B., Facchinetti F. (2005). Efficacy of Cimicifuga racemosa on climacteric complaints: A randomized study versus low-dose transdermal estradiol . Gynecol. Endocrinol. 20 , 30–35. 10.1080/09513590400020922 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Napryeyenko O., Borzenko I., Grp G. N. S. (2007). Ginkgo biloba special extract in dementia with neuropsychiatric features - a randomised, placebo-controlled, double-blind clinical trial . Arzneimittelforschung-Drug Res. 57 , 4–11. 10.1055/s-0031-1296579 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nasab N. M., Bahrammi M. A., Nikpour M. R., Rahim F., Naghibis S. N. (2012). Efficacy of rivastigmine in comparison to ginkgo for treating Alzheimer's dementia . J. Pak Med. Assoc. 62 , 677–680. [ PubMed ] [ Google Scholar ]
- Naser B., Lund B., Henneicke-Von Zepelin H. H., Köhler G., Lehmacher W., Scaglione F. (2005). A randomized, double-blind, placebo-controlled, clinical dose-response trial of an extract of Baptisia, Echinacea and Thuja for the treatment of patients with common cold . Phytomedicine 12 , 715–722. 10.1016/j.phymed.2005.03.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nicolussi S., Drewe J., Butterweck V., Meyer Zu Schwabedissen H. E. (2020). Clinical relevance of St. John's wort drug interactions revisited . Br. J. Pharmacol. 177 , 1212–1226. 10.1111/bph.14936 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oelke M., Berges R., Schlafke S., Burkart M. (2014). Fixed-dose combination PRO 160/120 of sabal and urtica extracts improves nocturia in men with LUTS suggestive of BPH: Re-evaluation of four controlled clinical studies . World J. Urol. 32 , 1149–1154. 10.1007/s00345-014-1338-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Omer B., Krebs S., Omer H., Noor T. O. (2007). Steroid-sparing effect of wormwood (Artemisia absinthium) in crohn's disease: A double-blind placebo-controlled study . Phytomedicine 14 , 87–95. 10.1016/j.phymed.2007.01.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Osmers R., Friede M., Liske E., Schnitker J., Freudenstein J., Henneicke-Von Zepelin H. H. (2005). Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms . Obstet. Gynecol. 105 , 1074–1083. 10.1097/01.AOG.0000158865.98070.89 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Palm J., Steiner I., Abramov-Sommariva D., Ammendola A., Mitzenheim S., Steindl H., et al. (2017). Assessment of efficacy and safety of the herbal medicinal product BNO 1016 in chronic rhinosinusitis . Rhinology 55 , 142–151. 10.4193/Rhin16.103 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pares A., Planas R., Torres M., Caballeria J., Viver J. M., Acero D., et al. (1998). Effects of silymarin in alcoholic patients with cirrhosis of the liver: Results of a controlled, double-blind, randomized and multicenter trial . J. Hepatology 28 , 615–621. 10.1016/s0168-8278(98)80285-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Passali D., Loglisci M., Passali G. C., Cassano P., Rodriguez H. A., Bellussi L. M. (2015). A prospective open-label study to assess the efficacy and safety of a herbal medicinal product (Sinupret) in patients with acute rhinosinusitis . ORL J. Otorhinolaryngol. Relat. Spec. 77 , 27–32. 10.1159/000370123 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perić A., Gaćeša D., Barać A., Sotirović J., Perić A. V. (2020). Herbal drug EPs 7630 versus Amoxicillin in patients with uncomplicated acute bacterial rhinosinusitis: A randomized, open-label study . Ann. otology, rhinology, laryngology 129 , 969–976. 10.1177/0003489420918266 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Philipp M., Kohnen R., Hiller K. O. (1999). Hypericum extract versus imipramine or placebo in patients with moderate depression: Randomised multicentre study of treatment for eight weeks . Br. Med. J. 319 , 1534–1538. 10.1136/bmj.319.7224.1534 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Randlov C., Mehlsen J., Thomsen C. F., Hedman C., Von Fircks H., Winther K. (2006). The efficacy of St. John's Wort in patients with minor depressive symptoms or dysthymia - a double-blind placebo-controlled study . Phytomedicine 13 , 215–221. 10.1016/j.phymed.2005.11.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rapaport M. H., Nierenberg A. A., Howland R., Dording C., Schettler P. J., Mischoulon D. (2011). The treatment of minor depression with St. John's Wort or citalopram: Failure to show benefit over placebo . J. Psychiatric Res. 45 , 931–941. 10.1016/j.jpsychires.2011.05.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rechberger E., Rechberger T., Wawrysiuk S., Miotla P., Kulik-Rechberger B., Kuszka A., et al. (2020). A randomized clinical trial to evaluate the effect of canephron N in comparison to ciprofloxacin in the prevention of postoperative lower urinary tract infections after midurethral sling surgery . J. Clin. Med. 9 , 3391. 10.3390/jcm9113391 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rechberger E., Wrobel A., Kulik-Rechberger B., Miotla P., Rechberger T. (2022). Femistina versus Canephron as a prevention of urinary tract infections after midurethral sling surgery - non-inferiority study . Eur. J. Obstet. Gynecol. Reprod. Biol. 277 , 71–76. 10.1016/j.ejogrb.2022.08.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rich G., Shah A., Koloski N., Funk P., Stracke B., Köhler S., et al. (2017). A randomized placebo-controlled trial on the effects of Menthacarin, a proprietary peppermint- and caraway-oil-preparation, on symptoms and quality of life in patients with functional dyspepsia . Neurogastroenterol. Motil. 29 , e13132. 10.1111/nmo.13132 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rösch W., Vinson B., Sassin I. (2002). A randomised clinical trial comparing the efficacy of a herbal preparation STW 5 with the prokinetic drug cisapride in patients with Dysmotility type of functional dyspepsia . Z. für Gastroenterol. 40 , 401–408. 10.1055/s-2002-32130 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosien U. (2019). Iberogast® causes potentially life-threatening liver damages: Finally, warning notices are included in the use instructions by Bayer . Arzneiverordnung der Praxis 46 , 5–7. [ Google Scholar ]
- Sabadash M., Shulyak A. (2017). Canephron® N in the treatment of recurrent cystitis in women of child-bearing age: A randomised controlled study . Clin. Phytoscience 3 , 9. 10.1186/s40816-017-0046-7 [ CrossRef ] [ Google Scholar ]
- Saito Y. A., Rey E., Almazar-Elder A. E., Harmsen S. W., Zinsmeister A. R., Locke R. G., et al. (2010). A randomized, double-blind, placebo-controlled trial of St john's wort for treating irritable bowel syndrome . Am. J. Gastroenterology 105 , 170–177. 10.1038/ajg.2009.577 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sarris J., Fava M., Schweitzer I., Mischoulon D. (2012). St john's wort ( Hypericum perforatum ) versus sertraline and placebo in major depressive disorder: Continuation data from a 26-week RCT . Pharmacopsychiatry 45 , 275–278. 10.1055/s-0032-1306348 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saust L. T., Bjerrum L., Siersma V., Arpi M., Hansen M. P. (2018). Quality assessment in general practice: Diagnosis and antibiotic treatment of acute respiratory tract infections . Scand. J. Prim. Health Care 36 , 372–379. 10.1080/02813432.2018.1523996 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schaefer A., Kehr M. S., Giannetti B. M., Bulitta M., Staiger C. (2016). A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575®) vs. placebo in the treatment of adults with acute cough . Die Pharm. 71 , 504–509. 10.1691/ph.2016.6712 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schellenberg R. (2001). Treatment for the premenstrual syndrome with agnus castus fruit extract: Prospective, randomised, placebo controlled study . BMJ 322 , 134–137. 10.1136/bmj.322.7279.134 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schellenberg R., Zimmermann C., Drewe J., Hoexter G., Zahner C. (2012). Dose-dependent efficacy of the Vitex agnus castus extract Ze 440 in patients suffering from premenstrual syndrome . Phytomedicine 19 , 1325–1331. 10.1016/j.phymed.2012.08.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schloss J., Ryan K., Steel A. (2021). A randomised, double-blind, placebo-controlled clinical trial found that a novel herbal formula Urox® (Bedtime Buddy®) assisted children for the treatment of nocturnal enuresis . Phytomedicine 93 , 153783. 10.1016/j.phymed.2021.153783 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schneider L. S., Dekosky S. T., Farlow M. R., Tariot P. N., Hoerr R., Kieser M. (2005). A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer's type . Curr. Alzheimer Res. 2 , 541–551. 10.2174/156720505774932287 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scholz I., Liakoni E., Hammann F., Grafinger K. E., Duthaler U., Nagler M., et al. (2021). Effects of Hypericum perforatum (St John's wort) on the pharmacokinetics and pharmacodynamics of rivaroxaban in humans . Br. J. Clin. Pharmacol. 87 , 1466–1474. 10.1111/bcp.14553 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seifritz E., Hatzinger M., Holsboer-Trachsler E. (2016). Efficacy of Hypericum extract WS(®) 5570 compared with paroxetine in patients with a moderate major depressive episode - a subgroup analysis . Int. J. Psychiatry Clin. Pract. 20 , 126–132. 10.1080/13651501.2016.1179765 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seifritz E., Schlafke S., Holsboer-Trachsler E. (2019). Beneficial effects of Silexan on sleep are mediated by its anxiolytic effect . J. Psychiatr. Res. 115 , 69–74. 10.1016/j.jpsychires.2019.04.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shelton R. C., Keller M. B., Gelenberg A., Dunner D. L., Hirschfeld R., Thase M. E., et al. (2001). Effectiveness of St john's wort in major depression: A randomized controlled trial . JAMA 285 , 1978–1986. 10.1001/jama.285.15.1978 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singer A., Schmidt M., Hauke W., Stade K. (2011). Duration of response after treatment of mild to moderate depression with Hypericum extract STW 3-VI, citalopram and placebo: A reanalysis of data from a controlled clinical trial . Phytomedicine 18 , 739–742. 10.1016/j.phymed.2011.02.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sommer H., Harrer G. (1994). Placebo-controlled double-blind study examining the effectiveness of an hypericum preparation in 105 mildly depressed patients . J. Geriatr. Psychiatry Neurol. 7 ( 1 ), S9–S11. 10.1177/089198879400700104 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Soyka M., Mann K. (2018). “ Medikamentenabhängigkeit ,” in Entstehungsbedingungen - klinik - therapie (Stuttgart: Schattauer; ). [ Google Scholar ]
- Stange R. (2014). Beliebtheit und Akzeptanz von Phytopharmaka bei Publikum und Verordnern . Z. fur Phytother. 35 , 16–20. 10.1055/s-0033-1349783 [ CrossRef ] [ Google Scholar ]
- Stange R., Schneider B., Albrecht U., Mueller V., Schnitker J., Michalsen A. (2017). Results of a randomized, prospective, double-dummy, double-blind trial to compare efficacy and safety of a herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix with co-trimoxazole in patients with acute and uncomplicated cystitis . Res. Rep. Urol. 9 , 43–50. 10.2147/RRU.S121203 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stein D. J., Palk A. C., Kendler K. S. (2021). What is a mental disorder? An exemplar-focused approach . Psychol. Med. 51 , 894–901. 10.1017/S0033291721001185 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Storr M., Stracke B. (2022). Menthacarin for long-term treatment of functional dyspepsia – results from a clinical trial follow-up . Z. für Gastroenterol. 61 , 257–267. 10.1055/a-1823-1333 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Szegedi A., Kohnen R., Dienel A., Kieser M. (2005). Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St john's wort): Randomised controlled double blind non-inferiority trial versus paroxetine . BMJ 330 , 503. 10.1136/bmj.38356.655266.82 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tanamly M. D., Tadros F., Labeeb S., Makld H., Shehata M., Mikhail N., et al. (2004). Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: Study description and 12-month results . Dig. Liver Dis. 36 , 752–759. 10.1016/j.dld.2004.06.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tanner M., Karen Roddis J. (2018). Antibiotics for acute bronchitis . Nurs. Stand 32 , 41–43. 10.7748/ns.2018.e11123 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tanwar S., Trembling P. M., Hogan B. J., Srivastava A., Parkes J., Harris S., et al. (2017). Noninvasive markers of liver fibrosis: On-treatment changes of serum markers predict the outcome of antifibrotic therapy . Eur. J. Gastroenterology Hepatology 29 , 289–296. 10.1097/MEG.0000000000000789 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Timmer A., Günther J., Motschall E., Rücker G., Antes G., Kern W. V. (2013). Pelargonium sidoides extract for treating acute respiratory tract infections . Cochrane Database Syst. Rev. , CD006323. 10.1002/14651858.CD006323.pub3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Uebelhack R., Gruenwald J., Graubaum H. J., Busch R. (2004). Efficacy and tolerability of Hypericum extract STW 3-VI in patients with moderate depression: A double-blind, randomized, placebo-controlled clinical trial . Adv. Ther. 21 , 265–275. 10.1007/BF02850158 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Dongen M., Van Rossum E., Kessels A., Sielhorst H., Knipschild P. (2003). Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial . J. Clin. Epidemiol. 56 , 367–376. 10.1016/s0895-4356(03)00003-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Gurp G., Meterissian G. B., Haiek L. N., Mccusker J., Bellavance F. (2002). St john's wort or sertraline? Randomized controlled trial in primary care . Can. Fam. Physician 48 , 905–912. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Verlaet A. A., Ceulemans B., Verhelst H., Van West D., De Bruyne T., Pieters L., et al. (2017). Effect of Pycnogenol® on attention-deficit hyperactivity disorder (ADHD): Study protocol for a randomised controlled trial . Trials 18 , 145. 10.1186/s13063-017-1879-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vermes G., Banhidy F., Acs N. (2005). The effects of remifemin on subjective symptoms of menopause . Adv. Ther. 22 , 148–154. 10.1007/BF02849885 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Voican C. S., Corruble E., Naveau S., Perlemuter G. (2014). Antidepressant-Induced liver injury: A review for clinicians . Am. J. Psychiatry 171 , 404–415. 10.1176/appi.ajp.2013.13050709 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Von Arnim U., Peitz U., Vinson B., Gundermann K. J., Malfertheiner P. (2007). STW 5, a phytopharmacon for patients with functional dyspepsia: Results of a multicenter, placebo-controlled double-blind study . Am. J. Gastroenterol. 102 , 1268–1275. 10.1111/j.1572-0241.2006.01183.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vorbach E. U., Arnoldt K. H., Hubner W. D. (1997). Efficacy and tolerability of St. John's Wort extract LI 160 versus imipramine in patients with severe depressive episodes according to ICD-10 . Pharmacopsychiatry 30 , 81–85. 10.1055/s-2007-979524 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vorbach E. U., Hubner W. D., Arnoldt K. H. (1994). Effectiveness and tolerance of the hypericum extract LI 160 in comparison with imipramine: Randomized double-blind study with 135 outpatients . J. Geriatr. Psychiatry Neurol. 7 ( 1 ), S19–S23. 10.1177/089198879400700107 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wachtel-Galor S., Benzie I. F. F. (2011). “ Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs ,” in Herbal medicine: Biomolecular and clinical aspects . Editors Benzie I. F. F, Wachtel-Galor S. 2nd ed (Boca Raton, FL: CRC Press; ). [ PubMed ] [ Google Scholar ]
- Wagenlehner F. M., Abramov-Sommariva D., Holler M., Steindl H., Naber K. G. (2018). Non-antibiotic herbal therapy (BNO 1045) versus antibiotic therapy (fosfomycin trometamol) for the treatment of acute lower uncomplicated urinary tract infections in women: A double-blind, parallel-group, randomized, multicentre, non-inferiority phase III trial . Urol. Int. 101 , 327–336. 10.1159/000493368 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wawrysiuk S., Rechberger T., Kubik-Komar A., Kolodynska A., Naber K., Miotla P. (2022). Postoperative prevention of urinary tract infections in patients after urogynecological surgeries-nonantibiotic herbal (canephron) versus antibiotic prophylaxis (fosfomycin trometamol): A parallel-group, randomized, noninferiority experimental trial . Pathogens 12 , 27. 10.3390/pathogens12010027 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wheatley D. (1997). LI 160, an extract of St. John's Wort, versus amitriptyline in mildly to moderately depressed outpatients - a controlled 6-week clinical trial . Pharmacopsychiatry 30 , 77–80. 10.1055/s-2007-979523 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woelk H. (2000). Comparison of St john's wort and imipramine for treating depression: Randomised controlled trial . BMJ 321 , 536–539. 10.1136/bmj.321.7260.536 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woelk H., Schlafke S. (2010). A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder . Phytomedicine 17 , 94–99. 10.1016/j.phymed.2009.10.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wortelboer U., Cohrs S., Rodenbeck A., Rüther E. (2002). Tolerability of hypnosedatives in older patients . Drugs and Aging 19 , 529–539. 10.2165/00002512-200219070-00006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wuttke W., Seidlova-Wuttke D., Gorkow C. (2003). The Cimicifuga preparation BNO 1055 vs. Conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers . Maturitas 44 , S67–S77. 10.1016/s0378-5122(02)00350-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan H., Ma Q., Ye L., Piao G. (2016). The traditional medicine and modern medicine from natural products . Molecules 21 , 559. 10.3390/molecules21050559 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan J., He Q., Nguyen L. H., Wong M. C. S., Huang J., Yu Y., et al. (2021). Regular use of proton pump inhibitors and risk of type 2 diabetes: Results from three prospective cohort studies . Gut 70 , 1070–1077. 10.1136/gutjnl-2020-322557 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zavala-Cerna M. G., Segura-Cobos M., Gonzalez R., Zavala-Trujillo I. G., Navarro-Perez S. F., Rueda-Cruz J. A., et al. (2020). The clinical significance of high antimicrobial resistance in community-acquired urinary tract infections . Can. J. Infect. Dis. Med. Microbiol. 2020 , 2967260–2967267. 10.1155/2020/2967260 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ziegler G., Ploch M., Miettinen-Baumann A., Collet W. (2002). Efficacy and tolerability of valerian extract LI 156 compared with oxazepam in the treatment of non-organic insomnia-a randomized, double-blind, comparative clinical study . Eur. J. Med. Res. 7 , 480–486. [ PubMed ] [ Google Scholar ]
- Zielińska S., Jezierska-Domaradzka A., Wójciak-Kosior M., Sowa I., Junka A., Matkowski A. M. (2018). Greater celandine's ups and Downs−21 centuries of medicinal uses of chelidonium majus from the viewpoint of today's Pharmacology . Front. Pharmacol. 9 , 299. 10.3389/fphar.2018.00299 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

- Impact Factor
Top Publication Journals
- Business, Economics & Management
- Chemical & Material Sciences
- Engineering & Computer Science
- Health & Medical Sci
- Humanities, Literature & Arts
- Life Sciences & Earth Sciences
- Physics & Mathematics
- Social Sciences
- 216860 Articles
- 26214 Journals
Journal Impact Factor Report 2021
Submit journal for impact factor evaluation, submit your journal for indexing, journal impact factor report 2020, journal impact factor report 2018, journal impact factor list 2014 ( now online ), getting your journal indexed, 2012 impact factor list.

Research & Reviews: Journal of Herbal Science (RRJoHS)

URL: http://stmjournals.com/Journal-of-Herbal-Science.html
Keywords: Medicinal Botany, Herb-Drug Interaction, Phyto-constituents
ISSN: 2348-9553
EISSN: 2278-2257
Subject: Plant Sciences
Publisher: STM Journals
Country: India
Author Ratings

Share With Us
Journal areas list.
- Agriculture
- Biochemistry
- Biotechnology
- Business and Management
- Computer Science
- Environmental Sciences
Information
- Terms & Conditions
- Privacy Policy
Important Links
- Publisher Guidelines
- Journal Indexing
- Paper Indexing
- Impact Factor List

- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Focus Issue Archive
- Open Access Articles
- JAMIA Journal Club
- Author Guidelines
- Submission Site
- Open Access
- Call for Papers
- About Journal of the American Medical Informatics Association
- About the American Medical Informatics Association
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Dispatch Dates
- For Reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
- Introduction
- Related work
- Acknowledgments
- Author contributions
- Supplementary material
- Conflicts of interest
- Data availability
- < Previous
PresRecST: a novel herbal prescription recommendation algorithm for real-world patients with integration of syndrome differentiation and treatment planning
- Article contents
- Figures & tables
- Supplementary Data
Xin Dong, Chenxi Zhao, Xinpeng Song, Lei Zhang, Yu Liu, Jun Wu, Yiran Xu, Ning Xu, Jialing Liu, Haibin Yu, Kuo Yang, Xuezhong Zhou, PresRecST: a novel herbal prescription recommendation algorithm for real-world patients with integration of syndrome differentiation and treatment planning, Journal of the American Medical Informatics Association , Volume 31, Issue 6, June 2024, Pages 1268–1279, https://doi.org/10.1093/jamia/ocae066
- Permissions Icon Permissions
Herbal prescription recommendation (HPR) is a hot topic and challenging issue in field of clinical decision support of traditional Chinese medicine (TCM). However, almost all previous HPR methods have not adhered to the clinical principles of syndrome differentiation and treatment planning of TCM, which has resulted in suboptimal performance and difficulties in application to real-world clinical scenarios.
We emphasize the synergy among diagnosis and treatment procedure in real-world TCM clinical settings to propose the PresRecST model, which effectively combines the key components of symptom collection, syndrome differentiation, treatment method determination, and herb recommendation. This model integrates a self-curated TCM knowledge graph to learn the high-quality representations of TCM biomedical entities and performs 3 stages of clinical predictions to meet the principle of systematic sequential procedure of TCM decision making.
To address the limitations of previous datasets, we constructed the TCM-Lung dataset, which is suitable for the simultaneous training of the syndrome differentiation, treatment method determination, and herb recommendation. Overall experimental results on 2 datasets demonstrate that the proposed PresRecST outperforms the state-of-the-art algorithm by significant improvements (eg, improvements of P@5 by 4.70%, P@10 by 5.37%, P@20 by 3.08% compared with the best baseline).
The workflow of PresRecST effectively integrates the embedding vectors of the knowledge graph for progressive recommendation tasks, and it closely aligns with the actual diagnostic and treatment procedures followed by TCM doctors. A series of ablation experiments and case study show the availability and interpretability of PresRecST, indicating the proposed PresRecST can be beneficial for assisting the diagnosis and treatment in real-world TCM clinical settings.
Our technology can be applied in a progressive recommendation scenario, providing recommendations for related items in a progressive manner, which can assist in providing more reliable diagnoses and herbal therapies for TCM clinical task.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1527-974X
- Copyright © 2024 American Medical Informatics Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Latest Research Across NCFR's Journals
Keep up with the latest research from the three scholarly NCFR journals — Journal of Marriage and Family (JMF), Family Relations: Interdisciplinary Journal of Applied Family Science (FR), and Journal of Family Theory & Review (JFTR) — all of which publish early-view articles online. Below are the early-view articles published within the listed timeframe.
New Early-View Articles (May 12 – May 25)
Journal of Family Theory & Review
- Children's social–emotional learning as emotional labor: Recognizing children's contributions — Karina Ruiz
Family Relations
- Do more egalitarian men experience less union dissolution? A couple-level analysis ( open access ) — Liat Raz-Yurovich, Barbara S. Okun, Matanel Ben-Avi
- Family-building desires among adopted adolescents with lesbian, gay, and heterosexual parents ( open access ) — Abbie E. Goldberg, Lea Silvert, Rachel H. Farr
- Latent profiles of dyadic parent–child interaction and associations with triadic family interaction in early childhood ( open access ) — Johanna Lindstedt, Sari Ahlqvist-Björkroth, Niina Junttila, Riikka Korja
- Understanding marital stability through work–family experiences in proximal and distal contexts: Comparing United States and Japan — Chengfei Jiao, Joseph G. Grzywacz
- The educational resources and needs of mothers of preschool children from two socioeconomic levels ( open access ) — Senem Han-Uysal, Zeynep Kızıltepe, Sibel Akmehmet-Şekerler, Aylin Buran
Journal of Marriage and Family
- Youth's political identity and fertility desires — Heather M. Rackin, Christina M. Gibson-Davis
- Slow to launch: Young men's parental coresidence and employment outcomes — Asya Saydam, Kelly Raley
- Marriage, cohabitation, and institutional context: Household specialization among same-sex and different-sex couples ( open access ) — Chih-lan Winnie Yang
- Methods and theory for analyzing intensive longitudinal data in family research — Jennifer S. Barber, Tim Futing Liao
- Grandparents' and domestic helpers' childcare support: Implications for well-being in Asian families ( open access ) — Mioko Sudo, Petrina Hui Xian Low, Yena Kyeong, Michael J. Meaney, Michelle Z. L. Kee, Helen Chen, Birit F. P. Broekman, Ranjani Nadarajan, Anne Rifkin-Graboi, Henning Tiemeier, Peipei Setoh
NCFR member journal subscribers have access to the full text of articles through the NCFR website .
Explore our tips and strategies and submit your manuscript to one of NCFR's scholarly journals .
Sign up for email alerts for JMF , FR , and JFTR to get immediate notifications of NEW journal articles!
Family Science is a vibrant and growing discipline. Visit Family.Science to learn more and see how Family Scientists make a difference.
NCFR is a nonpartisan, 501(c)(3) nonprofit organization whose members support all families through research, teaching, practice, and advocacy.
Get the latest updates on NCFR & Family Science in our weekly email newsletter:
Connect with Us
National Council on Family Relations 661 LaSalle Street, Suite 200 Saint Paul, MN 55114 Phone: (888) 781-9331 [email protected] Terms & Conditions | Privacy Policy
© Copyright 2023 NCFR
This paper is in the following e-collection/theme issue:
Published on 31.5.2024 in Vol 26 (2024)
Use of Patient-Generated Health Data From Consumer-Grade Devices by Health Care Professionals in the Clinic: Systematic Review
Authors of this article:

- Sharon Guardado 1 , MSc ;
- Maria Karampela 1 , PhD ;
- Minna Isomursu 1 , PhD ;
- Casandra Grundstrom 2 , PhD
1 Faculty of Information Technology and Electrical Engineering, University of Oulu, Oulu, Finland
2 Department of Computer Science, Norwegian University of Science and Technology, Trondheim, Norway
Corresponding Author:
Sharon Guardado, MSc
Faculty of Information Technology and Electrical Engineering
University of Oulu
Pentti Kaiteran katu 1
Oulu, 90570
Phone: 358 504388396
Email: [email protected]
Background: Mobile health (mHealth) uses mobile technologies to promote wellness and help disease management. Although mHealth solutions used in the clinical setting have typically been medical-grade devices, passive and active sensing capabilities of consumer-grade devices like smartphones and activity trackers have the potential to bridge information gaps regarding patients’ behaviors, environment, lifestyle, and other ubiquitous data. Individuals are increasingly adopting mHealth solutions, which facilitate the collection of patient-generated health data (PGHD). Health care professionals (HCPs) could potentially use these data to support care of chronic conditions. However, there is limited research on real-life experiences of HPCs using PGHD from consumer-grade mHealth solutions in the clinical context.
Objective: This systematic review aims to analyze existing literature to identify how HCPs have used PGHD from consumer-grade mobile devices in the clinical setting. The objectives are to determine the types of PGHD used by HCPs, in which health conditions they use them, and to understand the motivations behind their willingness to use them.
Methods: A systematic literature review was the main research method to synthesize prior research. Eligible studies were identified through comprehensive searches in health, biomedicine, and computer science databases, and a complementary hand search was performed. The search strategy was constructed iteratively based on key topics related to PGHD, HCPs, and mobile technologies. The screening process involved 2 stages. Data extraction was performed using a predefined form. The extracted data were summarized using a combination of descriptive and narrative syntheses.
Results: The review included 16 studies. The studies spanned from 2015 to 2021, with a majority published in 2019 or later. Studies showed that HCPs have been reviewing PGHD through various channels, including solutions portals and patients’ devices. PGHD about patients’ behavior seem particularly useful for HCPs. Our findings suggest that PGHD are more commonly used by HCPs to treat conditions related to lifestyle, such as diabetes and obesity. Physicians were the most frequently reported users of PGHD, participating in more than 80% of the studies.
Conclusions: PGHD collection through mHealth solutions has proven beneficial for patients and can also support HCPs. PGHD have been particularly useful to treat conditions related to lifestyle, such as diabetes, cardiovascular diseases, and obesity, or in domains with high levels of uncertainty, such as infertility. Integrating PGHD into clinical care poses challenges related to privacy and accessibility. Some HCPs have identified that though PGHD from consumer devices might not be perfect or completely accurate, their perceived clinical value outweighs the alternative of having no data. Despite their perceived value, our findings reveal their use in clinical practice is still scarce.
International Registered Report Identifier (IRRID): RR2-10.2196/39389
Introduction
The term “mobile health” (mHealth) has been in use for nearly 2 decades to refer to the application of mobile technologies in delivering health services and collecting data pertinent to disease diagnosis, prevention, and management [ 1 , 2 ]. In the last decade, the scope of mHealth has expanded to include consumer-grade devices, such as smartphones, wearable, sensors, and other quasi-medical devices, while it increasingly targets specific health conditions, in addition to wellness [ 2 , 3 ]. Whereas medical-grade mobile devices require clinical evidence for certification, often requiring years to bring a device to the market [ 4 ], consumer-grade mobile devices evolving at a rapid pace, and open numerous possibilities through their capacity for ubiquitous data collection [ 5 ]. mHealth solutions have become integral to many people’s lives, serving as tools for tracking health and well-being. Research has found that mHealth solutions can benefit individuals in general by fostering moderate increases in physical activity [ 6 ] or by being a convenient tool for self-management of health issues [ 7 ]. For individuals with chronic diseases, mHealth solutions have been particularly effective in offering support for condition management, goal setting, and enhancing overall satisfaction [ 7 , 8 ]. In addition to supporting people’s efforts to manage their health, mHealth solutions also enable the collection of electronic patient-generated health data (PGHD), which can be used in the clinical context. PGHD refer to health-related data created, recorded, and gathered by and from patients outside of the clinical settings [ 9 , 10 ]. PGHD encompasses a broad range of data types from both passive and active sensing [ 1 , 11 ]. Passive data collection usually involves sensors that are connected to a mobile device that may be worn or embedded, limiting the patient’s participation to wearing, carrying, or activating the device [ 12 ]. Active data collection requires patients to manually enter information or interact with an external device such as a peak flowmeter, glucometer, or thermometer to generate information. These data are “patient-generated” since the patient has actively participated in collecting and recording [ 12 ]. It has been hypothesized that through both passively and actively collected PGHD, health care professionals (HCPs) could gain insights into patients’ activities, lifestyle, and physical condition to inform care decisions and personalize care approaches [ 13 ].
In countries with medium or high levels of digitalization, more than 56% of people appear willing to share their personal health data, even if the purpose of sharing them is not directly related to the improvement of their health [ 14 ]. Similarly, 46.3% of individuals who owned a wearable medical device indicated having shared data with a health provider in 2019 [ 15 ]. With mHealth solutions becoming increasingly accessible, it can be expected that more people may be interested in sharing their health data with HCPs if they believe that it could help them improve health care. However, a recent study found that although providers of mHealth solutions for chronic condition self-management encourage data sharing with HCPs, few solutions are designed to facilitate HCPs’ review of these data [ 4 ]. This issue, in combination with already known challenges such as interoperability, data privacy issues, data validity, and the added burden of reviewing [ 9 , 16 ], makes the use of PGHD in the clinic an unrealistic possibility for many HCPs.
HCPs might have different approaches and goals when deciding to ponder PGHD collected through nonmedical mobile devices. According to Nittas et al [ 17 ], when integrating PGHD into the care process, HCPs can take the supporter or the reviewer role. In the supporter role, they limit themselves to motivating patients to use mHealth, whereas in the reviewer role, HCPs assess PGHD to complement medical data. Taking the reviewer role implies an active stance, and though some might value PGHD’s contribution to care, this type of data may still be a new and unfamiliar source of information for some HCPs [ 18 ]. For PGHD for mobile devices to be feasible as a complementary tool in the clinical setting, their use should benefit both patients and HCPs. Though the adoption of mHealth solutions by patients supports their well-being and enables the availability of PGHD, such availability does not automatically equate to usefulness for HCPs. Despite the acceptance and adoption of mHealth solutions by HCPs being one of the most influential factors regarding the success of those solutions [ 19 , 20 ], there has not been significant research on the role HCPs are expected to take in the use of mHealth solutions [ 4 , 17 ] or on the concrete experiences and motivators of those willing to review PGHD.
The main objective of our review is to systematically analyze existing scientific literature to identify what types of PGHD and in what health conditions HCPs have been using PGHD from consumer-grade mobile devices, as well as further context information for their motivations to use these types of data as a complementary tool in the clinic.
To attain these objectives the proposed research questions for our review are as follows: (1) In what health conditions have PGHD from consumer-grade mobile been a suitable tool for HCP? (2) What types of PGHD have HCPs found useful in the care of chronic conditions? (3) What are the main motivations behind HCPs’ decision to review PGHD from consumer-grade devices?
Study Design
A systematic literature review (SLR) was selected as our main research method to comprehensively synthesize evidence and prior research on HCPs’ experiences reviewing PGHD from consumer-grade mobile devices to address our research questions. We wanted to follow a transparent and systematic method to inform further research on this topic. We adopted methodologies from the Guidelines for Performing Systematic Literature Reviews in Software Engineering [ 21 ] and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) [ 22 , 23 ], both of which provide reliable methodologies to perform SLRs in the fields of computer science and medicine, respectively ( Multimedia Appendix 1 [ 24 ]). We deemed it pertinent to combine methodological traditions from both computer science and medicine, as our research topic combines technical and care viewpoints and is interdisciplinary by nature [ 24 ].
To perform this SLR, we adhered to a systematic review protocol that was prepared before starting the searches and screening process. The protocol has been published elsewhere [ 25 ] and provides an ample description of the methods used in the search strategy and the inclusion and exclusion criteria used. The review adhered closely to the original protocol, with no significant deviations.
Search Strategy
Eligible studies were identified through comprehensive literature searches we conducted in bibliographical databases on health and biomedicine and information technology domains. The searched databases included PubMed, ACM Digital Library (including the ACM Guide to Computing Literature), IEEE Xplore, and Scopus. The searches were carried out in May 2022.
To ensure the identification of relevant papers, we constructed the search strategy in an iterative way [ 25 ]. The search string used for each database is available in Multimedia Appendix 2 . Based on the specific objectives of our review, and after conducting a pilot search in PubMed, we determined that the literature search should be constructed around 3 specific key topics: “patient-generated health data,” “health personnel,” and “mobile technologies.” We used the corresponding Medical Subject Headings (MeSH) and their possible variants to construct the final search query. Once the query had been tested, it was validated by a research librarian from the University of Oulu. After completing the electronic searches, we performed a supplementary hand search of the citations found within other SLRs and scoping reviews that were retrieved during the literature searches.
Eligibility Criteria
The defined eligibility criteria ( Table 1 ) aimed to include original papers that reported on the use of PGHD created via consumer-grade mHealth solutions by HCPs. PGHD reported in the studies should have been collected outside of the clinical setting, through either the patients’ use of mobile health apps or the wearable devices such as smartwatches, smart rings, fitness trackers, and similar wearable trackers; studies reporting on PGHD collected by HCPs during appointments or inside the clinical settings were excluded. Studies were limited to those involving consumer-grade devices to focus on, excluding those solely focusing on PGHD from medical-grade devices. The included papers report on the experiences of HCPs who have experience using PGHD in their clinical practice, as part of a stand-alone mHealth solution, by personal initiative, or for any other reasons. We excluded papers that focus solely on the perceptions or perspectives of HCPs as potential users of PGHD. Eligible publications were restricted to those accepted in peer-reviewed journals and conference proceedings written in the English language.
a PGHD: patient-generated health data.
b HCP: health care professional.
Though current consumer-grade mobile and wearable technologies started to become more accessible in the first half of the last decade, their impact on the health care scene started to become evident only years later. In 2013, it was acknowledged that only a few studies had assessed the impact of mobile apps in the health context, and all those studies referred to apps that had been created only for research purposes and were not available to the public at that time [ 26 ]. Therefore, we limited our search to papers with publication dates starting in 2013. Our search criteria did not delimit aspects such as the medical profile or specialties of the HCPs participating in the studies, or the health conditions treated, as we aimed to ascertain whether PGHD usage by HCPs would be more prevalent in the treatment of certain medical conditions or certain medical fields.
Selection Process
After the electronic search, the resulting papers were imported into Covidence (Veritas Health Innovation) for screening. The screening process was divided into 2 stages carried out independently by 2 researchers (SG and MK) with computer science backgrounds and previous research experience with mHealth and PGHD. Initially, the screening was limited to titles and abstracts. Before starting this stage, the reviewers completed a joint exercise to validate the review methodology and ensure that the inclusion and exclusion criteria were correctly understood. The disagreements that arose during the initial stage were all discussed and resolved between the 2 reviewers before starting the second screening stage. The second screening round included the review of the full text of all the preliminarily included papers.
Data Extraction
The relevant information of the included papers was collected using a structured data extraction form constructed in Covidence ( Multimedia Appendix 3 ). The most relevant data extracted for each paper included the professions of the participants; health conditions treated; mobile technologies used; the type of PGHD collected; and the channels used for visualization. In addition, to understand what motivated HCPs to review PGHD quotes related to their motivations and conclusions related to the topic were extracted from each study.
The data extraction task was completed by the 2 original reviewers (SG and MK) and 2 additional reviewers (CG and MI), all of whom have previous research experience with the topic of this review. Each paper was randomly assigned to be examined by 2 of the reviewers. Each reviewer performed the data extraction independently. Upon completion, the data extracted by both reviewers were compared. Discrepancies were resolved through discussion between the reviewers and a final consensus was reached in all cases.
Data Analysis and Synthesis
Quantitative and qualitative studies were included in this review. Due to the significant heterogeneity observed in the studies’ design, types of health conditions, types of PGHD, and types of mHealth solutions, methods such as meta-analysis or meta-synthesis were not deemed the most appropriate approach for the data synthesis. The extracted data were summarized using a combination of descriptive and narrative syntheses [ 27 ]. The descriptive analysis was conducted to summarize data from the different studies. This involved classifying the studies based on the type of mobile technologies used, health conditions treated, and the types of PGHD reviewed. This approach arranged the studies into more homogenous subgroups, which aided in synthesizing different types of data. The data related to the motivations of HCPs were examined using a thematic analysis, from which different categories were derived. For the narrative synthesis, similarities, and differences between the findings of different studies were identified. The analysis and synthesis comprised three major steps: (1) organization of the included studies, (2) descriptive analysis of the findings within studies, and (3) a narrative synthesis aiming at exploring interconnections between the studies.
Quality Assessment
The quality of the included studies was assessed in parallel to the data extraction process. From the checklists, the quality of studies proposed by Kitchenham and Charters [ 21 ] were assessed. As the included studies were both qualitative and quantitative, we selected the questions that were most appropriate for our specific research questions that were present in both the qualitative and quantitative checklists.
Upon assessment, all reviewers agreed that the included studies had credible findings; proper data collection methods; clear and coherent reporting; and clear links between data, interpretation, and conclusions ( Figure 1 ).

The single topic that produced some uncertainty during the quality assessment was the lack of clarity on whether some of the selected studies had explored enough diversity of perspective and context. This can likely be attributed to the fact that almost all included studies were performed in developed countries, predominantly in the United States or Europe, which is a typical setting for digital health studies. Hence, the findings of these investigations will provide the most accurate depiction of the state of health care systems in developed countries.

Ethical Considerations
The Ethics Committee of Human Sciences of the University of Oulu guidelines state that as no human or animal subjects were involved in the study, no separate ethics statement is required. However, the general ethical guidelines from the Finnish National Board on Research Integrity [ 28 ] guided the ethics of the study.
Our search across electronic databases and supplementary hand searches identified 1696 papers. Covidence automatically removed 374 duplicates. We screened 1322 titles and abstracts, resulting in 86 papers for full-text screening. Following the completion of this second screening stage, 18 papers met all the inclusion criteria. However, upon closer examination, it was observed that 2 pairs of papers ([ 29 , 30 ] and [ 31 , 32 ]) had similar authors and identical samples and methodologies. Each pair was merged into a single study for analysis, resulting in the final inclusion of 16 studies for our SLR ( Figure 2 ).
During full-text screening, papers were primarily excluded for focusing exclusively on PGHD from medical-grade devices (31/68, 46%); evaluating the usability of specific mHealth solutions, rather than PGHD use (27/68, 39%); lacking data collection from HCPs (9/68, 13%); and discussing potential rather than actual use of PGHD (1/68, 1%).

Characteristics of the Included Studies
We included studies spanning 2015-2021. Notably, more than two-thirds of the papers (11/16, 69%) were published in 2019 or later, indicating a growing interest in the topic both before and during the COVID-19 pandemic. The predominant location was North America (11/16, 69%), specifically the United States and Canada; within Europe (3/16, 19%), Sweden and the United Kingdom were the primary locations; and 1 study was conducted in Asia and 1 in a multicountry setting. The authors used diverse methodologies for data collection, with interviews (8/16, 50%) and mixed methods (4/16, 25%) being the most common. More comprehensive insights into the specific study designs and data collection methods are available in Table 2 . A complete summary of the included studies can be found in Multimedia Appendix 4 [ 29 , 30 , 32 - 46 ].
a HCP: health care professional.
b PGHD: patient-generated health data.
Medical Profiles and Specialties
Although some of the studies examined data collected from various stakeholders such as patients, researchers, hospital managers, or solution providers, our focus centered on data collected from HCPs. Collectively, the studies in our review had 355 HCPs as participants. Among the represented professions, physicians accounted for the largest number of participants, present in 81% (13/16) of the studies. While approximately half of those studies referred to physicians using a general term, the other half provided clear information about the medical specialties of the physicians. Nurses were the second most represented profession, participating in 62% (10/16) of the studies. Physiotherapists were the third most represented, participating in 38% (6/16) of the studies. Other health professions present were psychologists (3/16, 19%) and surgeons, dietitians, health coaches, and assistant practitioners, each mentioned in 12% (2/16) of the studies ( Table 2 ).
All the studies reported the medical specialties where PGHD was being used. Those specialties included geriatrics, anesthesiology, orthopedic surgery, gastroenterology, dietetics and nutrition, behavioral and clinical psychology, psychiatry, obstetrics and gynecology, infertility, endocrinology, internal medicine, family medicine, rehabilitation, pediatric nephrology, otorhinolaryngology, and audiology.
Health Conditions Treated
The studies examined a wide range of health conditions, classified according to the WHO International Classification of Diseases , Eleventh Revision ( ICD-11 ), into categories such as endocrine, nutritional, or metabolic diseases; mental, behavioral, or neurodevelopmental disorders; diseases of the nervous, circulatory respiratory, and digestive systems, and diseases of the musculoskeletal system or connective tissue. In addition, some studies reported the use of PGHD for other types of medical tasks including perioperative care and care of older adults.
The most cited health conditions for which PGHD from mobile devices were reviewed by HCPs were diabetes and obesity, each mentioned in at least 3 studies. A quarter of the studies did not address a specific health condition. In those cases, the contextual information provided was limited to medical specialties or professions ( Table 2 ).
Types of mHealth Solutions
Among the 16 included studies, 5 mentioned specific mHealth solutions patients had been using to self-manage their health condition. The remaining studies mentioned commercial mHealth solutions in general. In half of the studies, HCPs reported using PGHD derived from a combination of diverse mHealth solutions, which included 1 or multiple mobile health apps and wearable devices. The remaining half of the studies addressed the experience of HCPs using PGHD exclusively generated through mobile health apps installed in patients’ smartphones (4/16, 25%) or captured from wearable devices (4/16, 25%).
Types of PGHD
Various classifications of PGHD have been proposed in terms of purpose (self-use, behavior change, clinical use, and research), management of a condition (eg, diabetes, hypertension), data type (physiological, behavioral, or environmental), mode of data capture (using sensors, external devices, implanted devices, patient portals, web-based surveys, and manual entry), and whether the process is active, passive, or mixed [ 12 ]. In this study, we focused on classifying PGHD based on data types.
Physiological data were reviewed in all studies. In 7 of 16 studies, at least 3 different types of physiological data were collected. Weight was the most frequently mentioned physiological data, reported in 44% (7/16) of the studies, followed by mood (6/16, 38%) and vital signs (5/16, 31%). Other less commonly reviewed types of data were pain, blood glucose level, and other symptoms ( Table 3 ).
Behavioral data constituted the most used category of PGHD. More than 80% (13/16) of the studies indicated that HCPs had reviewed some form of behavioral data, although always in combination with physiological data. Physical activity seems to be the most reviewed type of PGHD produced by consumer-grade devices, with 75% of the studies reporting its use, followed by food intake (9/16, 56%), sleep quality or quantity (8/16, 50%), and medication adherence (6/16, 38%).
Only 12% (2/16) of the studies reported the use of environmental data, which were primarily collected through passive sensing, using wearables, whereas physiological and behavioral types of data were reported to be collected through either passive or active sensing or by a combination of both. For instance, certain types of PGHD, such as sleep, physical activity, or sedentariness, were collected through active sensing in some studies and through passive sensing in others.
Access to PGHD
Diverse channels for PGHD access were presented. Notably, 19% (3/16) of the papers did not describe the precise channels HCPs used to access PGHD. Dashboards or solution portals were used in 56% (9/16) of the studies. The second most common channel was the patient’s mobile device (5/16, 31%). In a few studies, HCPs accessed PGHD through integration with the electronic health record (EHR; 2/16, 12%), by email (2/16, 12%), or from patients’ verbal summaries of data from their mobile devices (1/16, 6%).
Motivation for Reviewing PGHD
Although not all studies cited the reasons behind HCPs’ willingness to review PGHD from consumer-grade devices, motivation for reviewing them centered into 3 main categories: benefits for the patient, supporting their clinical roles, and strengthening the patient-HCP relationship ( Figure 3 ). Key motivations that showed how PGHD supported HCPs included topics such as accessing additional data types, identifying health patterns, and reducing data collection workload.

Principal Findings
Our review underlines a growing interest in understanding the experiences of HCPs who are using PGHD in the clinic. We aimed to identify how PGHD from consumer-grade mobile devices have been used to assist them in clinical practice. HCPs, who were primarily physicians and nurses, shared their experience on the topic. The health conditions for which HCPs most resorted to PGHD were diabetes and obesity. We found that physiological data, such as weight, mood, and vital signs, and behavioral data, such as physical activity, food intake, and sleep quality, have been frequently used. HCPs had access to PGHD through different channels, such as web portals provided by the mHealth solutions or through integration with the EHR.
Previous reviews have explored the role of PGHD in facilitating prevention and health promotion [ 17 ], their use in clinical practice [ 18 ], and their effect on patient-clinician relationships [ 47 ]. However, those studies have concentrated on PGHD from medical-grade devices, which tend to be more accurate and more accepted in the medical community. PGHD created through consumer-grade mHealth solutions, although praised for their potential to transform health care, have typically not been deemed reliable or accurate enough for the clinical context [ 3 , 48 , 49 ]. Despite concerns over PGHD accuracy and reliability, HCPs recognized that their clinical value outweighs the absence of data [ 40 ]. This value comes with a caveat, as recent studies indicate that PGHD must be curated by HCPs to ascribe actionable clinical value, but even then, they can be treated as supplementary to data collected through clinically recognized standards such as through laboratory tests [ 50 , 51 ]. PGHD from consumer-grade solutions have been used by HCPs in the treatment of a wide variety of health conditions, although it seems common only in the care of diabetes, cardiovascular diseases, and obesity ( Table 2 ).
The most frequently used types of data (physical activity, food intake, sleep quantity, and weight) are highly associated with lifestyle health risks, implying that access to lifestyle-related data can provide valuable insights into the control of lifestyle-related diseases. Furthermore, patients having these conditions are more willing to share PGHD, therefore, fostering HCPs’ familiarity with those types of data [ 15 ]. Our findings reveal that PGHD’s use in clinical practice remains relatively scarce [ 29 , 36 , 42 ], pointing out a gap between their potential and their current use. This finding is in line with a recent study suggesting that in comparison with the expectations of policies related to the European Health Data Space, the prompting and reviewing of PGHD from consumer-grade devices seem still relatively rare [ 50 ]. It is plausible that these types of PGHD have been used by HCPs in practice, but research on the practicalities of this phenomenon has only increased in the last 5 years.
HCPs indicated that PGHD are useful in the identification of patterns, to support certain diagnoses, and for certain types of monitoring. For example, lifestyle diseases [ 36 ], irritable bowel syndrome [ 42 ], or infertility [ 37 ] requires long-term management or presents a high level of uncertainty. In these cases, PGHD can provide longitudinal insights into patients’ health between clinic visits or even before they start treatment, saving time in identifying patterns. It is worth noting that, although HCPs in those studies acknowledged the value of PGHD, they also indicated engaging with PGHD infrequently and only with a few specific data types, in comparison with the substantial amount of data some patients want to share. For patients with chronic diseases, knowing that HCPs are reviewing their PGHD can be a comfortable way to know that they are being monitored and can provide data at the right time to facilitate decision-making and early intervention [ 41 , 52 ].
Multiple types of data were collected in all the studies, which signifies that as more data are collected, the need for analytical strategies that can support HCPs in reviewing and analyzing the potential relationships between different categories of data will be higher. Most existing mHealth solutions for self-monitoring lack standardized formats and mechanisms for patients to control and share PGHD [ 40 , 45 ]. Support for HCPs’ data access and use requires standardization and, in some cases, EHR integration [ 44 , 45 ].
Limitations
We limited our inclusion to papers written in English. However, this approach may have excluded relevant papers from developing regions where English is not the primary language for scientific dissemination but where the interest and potential for mHealth solutions and PGHD are growing. Similarly, a gap in the current body of research regarding these topics in developing regions is highlighted, since all studies came from countries with high economic and digitalization levels.
PGHD is a relatively recent definition, and some relevant papers published prior to its official designation as a MeSH term may have employed alternative terminology to describe the same concept of PGHD used in our study.
The shift toward digital health solutions the COVID-19 pandemic potentiated may have modified HCPs’ perceptions of PGHD use. However, no studies explicitly examining this relationship were identified in our prior searches or a later search. Therefore, future research could explore whether the shift toward digital health has catalyzed the adoption of consumer-grade technologies and PGHD in clinical settings.
Conclusions
Despite skepticism regarding the reliability and accuracy of PGHD and the multiple challenges that they convey, our study highlights a noticeable shift toward recognizing their practical value in health care, particularly in managing chronic conditions such as diabetes, obesity, and cardiovascular diseases. Yet, their impact in supporting the clinical practice is not clear from the literature. Many HCPs in the study, predominantly physicians and nurses, showed interest in using PGHD in the clinical workflows, albeit with a cautious approach that considers them as supplementary to traditional clinical data only. While they acknowledged the benefit of reviewing PGHD for the patient-HCP relationship, it was also noted that only certain types of PGHD are truly deemed useful and even then, they are not regularly used by HCPs. The findings call for continued research and innovation in mHealth, with a focus on enhancing the reliability, usability, and clinical relevance of PGHD, which in return can foster a culture of trust and collaboration between patients and HCPs.
Acknowledgments
We would like to acknowledge the More Stamina Project research group for supporting the development of this work. We acknowledge the use of ChatGPT version 4 and Grammarly to identify improvements in the organization of our text and to improve the writing style in the Introduction and Discussion sections.
Data Availability
This literature review synthesizes findings from peer-reviewed journal papers and conference papers. Given the nature of this review, it does not generate new primary data; instead, it compiled and analyzed existing publications on the use of PGHD from mobile technologies by HCPs. The reviewed papers are all available in public scientific databases. The data extraction form and the extracted data are available in the Multimedia Appendix section. These resources aim to ensure the reproducibility of our methods and facilitate future research in this area.
Conflicts of Interest
None declared.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.
Search strategies for all searched databases.
Data extraction form.
Summary of the included studies.
- Sim I. Mobile devices and health. N Engl J Med. 2019;381(10):956-968. [ CrossRef ] [ Medline ]
- Istepanian RSH. Mobile health (m-Health) in retrospect: the known unknowns. Int J Environ Res Public Health. 2022;19(7):3747. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Omoloja A, Vundavalli S. Patient generated health data: benefits and challenges. Curr Probl Pediatr Adolesc Health Care. 2021;51(11):101103. [ CrossRef ] [ Medline ]
- Bradway M, Gabarron E, Johansen M, Zanaboni P, Jardim P, Joakimsen R, et al. Methods and measures used to evaluate patient-operated mobile health interventions: scoping literature review. JMIR Mhealth Uhealth. 2020;8(4):e16814. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dunn J, Runge R, Snyder M. Wearables and the medical revolution. Per Med. 2018;15(5):429-448. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mönninghoff A, Kramer JN, Hess AJ, Ismailova K, Teepe GW, Tudor Car L, et al. Long-term effectiveness of mHealth physical activity interventions: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2021;23(4):e26699. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vo V, Auroy L, Sarradon-Eck A. Patients' perceptions of mHealth apps: meta-ethnographic review of qualitative studies. JMIR Mhealth Uhealth. 2019;7(7):e13817. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Feng S, Mäntymäki M, Dhir A, Salmela H. How self-tracking and the quantified self promote health and well-being: systematic review. J Med Internet Res. 2021;23(9):e25171. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lavallee DC, Lee JR, Austin E, Bloch R, Lawrence SO, McCall D, et al. mHealth and patient generated health data: stakeholder perspectives on opportunities and barriers for transforming healthcare. Mhealth. 2020;6:8. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Figueiredo MC, Chen Y. Patient-generated health data: dimensions, challenges, and open questions. Found Trends Hum–Comput Interact. 2020;13(3):165-297. [ CrossRef ]
- Jim HSL, Hoogland AI, Brownstein NC, Barata A, Dicker AP, Knoop H, et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J Clin. 2020;70(3):182-199. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mars M, Scott RE. Electronic patient-generated health data for healthcare. In: Linwood SL, editor. Digital Health. Brisbane, Australia. Exon Publications; 2022:1-16.
- Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, et al. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol. 2017;15(1):e2001402. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Karampela M, Ouhbi S, Isomursu M. Connected health user willingness to share personal health data: questionnaire study. J Med Internet Res. 2019;21(11):e14537. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Turner K, Jo A, Wei G, Tabriz AA, Clary A, Jim HSL. Sharing patient-generated data with healthcare providers: findings from a 2019 national survey. J Am Med Inform Assoc. 2021;28(2):371-376. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gurupur VP, Wan TTH. Challenges in implementing mHealth interventions: a technical perspective. Mhealth. 2017;3:32. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nittas V, Lun P, Ehrler F, Puhan MA, Mütsch M. Electronic patient-generated health data to facilitate disease prevention and health promotion: scoping review. J Med Internet Res. 2019;21(10):e13320. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Demiris G, Iribarren SJ, Sward K, Lee S, Yang R. Patient generated health data use in clinical practice: a systematic review. Nurs Outlook. 2019;67(4):311-330. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jacob C, Sanchez-Vazquez A, Ivory C. Social, organizational, and technological factors impacting clinicians' adoption of mobile health tools: systematic literature review. JMIR Mhealth Uhealth. 2020;8(2):e15935. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. J Am Med Inform Assoc. 2016;23(1):212-220. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kitchenham B, Charters S. Guidelines for performing systematic literature reviews in software engineering. Researchgate. 2007. URL: https://www.researchgate.net/profile/Barbara-Kitchenham/publication/302924724_Guidelines_for_performing_Systematic_Literature_Reviews_in_Software_Engineering/links/61712932766c4a211c03a6f7/Guidelines-for-performing-Systematic-Literature-Reviews-in-Software-Engineering.pdf [accessed 2024-05-07]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535-b2535. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29, 2021;372:n71. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Karampela M, Ouhbi S, Isomursu M. Personal health data: a systematic mapping study. Int J Med Inform. 2018;118:86-98. [ CrossRef ] [ Medline ]
- Medina SG, Isomursu M. The use of patient-generated health data from consumer-grade mobile devices in clinical workflows: protocol for a systematic review. JMIR Res Protoc. 2023;12:e39389. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fiordelli M, Diviani N, Schulz PJ. Mapping mHealth research: a decade of evolution. J Med Internet Res. 2013;15(5):e95. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Evans D. Systematic reviews of interpretive research: interpretive data synthesis of processed data. Aust J Adv Nurs. 2002;20(2):22-26. [ FREE Full text ]
- Guidelines for ethical review in human sciences. Publications of the Finnish National Board on Research Integrity. 2019. URL: https://tenk.fi/sites/default/files/2023-11/RI_Guidelines_2023.pdf
- Alpert JM, Manini T, Roberts M, Kota NSP, Mendoza TV, Solberg LM, et al. Secondary care provider attitudes towards patient generated health data from smartwatches. NPJ Digit Med. 2020;3(1):27. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Alpert JM, Kota NSP, Ranka S, Mendoza TV, Solberg LM, Rashidi P, et al. A simulated graphical interface for integrating patient-generated health data from smartwatches with electronic health records: usability study. JMIR Hum Factors. 2020;7(4):e19769. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lindroth T, Islind AS, Steineck G, Lundin J. From narratives to numbers: data work and patient-generated health data in consultations. Stud Health Technol Inform. 2018;247:491-495. [ Medline ]
- Cerna K, Grisot M, Islind AS, Lindroth T, Lundin J, Steineck G. Changing categorical work in healthcare: the use of patient-generated health data in cancer rehabilitation. Comput Supported Coop Work. 2020;29(5):563-586. [ FREE Full text ] [ CrossRef ]
- Abdolkhani R, Gray K, Borda A, DeSouza R. Patient-generated health data management and quality challenges in remote patient monitoring. JAMIA Open. 2019;2(4):471-478. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Austin E, Lee JR, Amtmann D, Bloch R, Lawrence SO, McCall D, et al. Use of patient-generated health data across healthcare settings: implications for health systems. JAMIA Open. 2020;3(1):70-76. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chung CF, Cook J, Bales E, Zia J, Munson SA. More than telemonitoring: health provider use and nonuse of life-log data in irritable bowel syndrome and weight management. J Med Internet Res. 2015;17(8):e203. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cohen DJ, Keller SR, Hayes GR, Dorr DA, Ash JS, Sittig DF. Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project HealthDesign. JMIR Hum Factors. 2016;3(2):e26. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Costa Figueiredo M, Su HI, Chen Y. Using data to approach the unknown. Proc ACM Hum-Comput Interact. 2021;4(CSCW3):1-35. [ FREE Full text ] [ CrossRef ]
- Gupta A, Heng T, Shaw C, Gromala D, Leese J, Li L. Oh, I didn't do a good job: how objective data affects physiotherapist-patient conversations for arthritis patients. In: Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare. 2020. Presented at: Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare; May 18-20, 2020:156-165; Atlanta, GA, USA. [ CrossRef ]
- Holtz B, Vasold K, Cotten S, Mackert M, Zhang M. Health care provider perceptions of consumer-grade devices and apps for tracking health: a pilot study. JMIR Mhealth Uhealth. 2019;7(1):e9929. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kim B, Ghasemi P, Stolee P, Lee J. Clinicians and older adults' perceptions of the utility of patient-generated health data in caring for older adults: exploratory mixed methods study. JMIR Aging. 2021;4(4):e29788. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kim Y, Ji S, Lee H, Kim JW, Yoo S, Lee J. "My doctor is keeping an eye on me!": exploring the clinical applicability of a mobile food logger. 2016. Presented at: Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems; May 7-12, 2016:5620-5631; San Jose, CA. [ CrossRef ]
- Ng A, Kornfield R, Schueller SM, Zalta AK, Brennan M, Reddy M. Provider perspectives on integrating sensor-captured patient-generated data in mental health care. Proc ACM Hum Comput Interact. 2019;3(CSCW):1-25. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tendedez H, Ferrario MA, McNaney R. 'The issue with that sort of data?': clinicians' accountability concerns around COPD self-monitoring tools. 2019. Presented at: Companion Publication of the 2019 Conference on Computer Supported Cooperative Work and Social Computing; November 9-13, 2019:382-386; Austin, TX. [ CrossRef ]
- West P, Van Kleek M, Giordano R, Weal MJ, Shadbolt N. Common barriers to the use of patient-generated data across clinical settings. 2018. Presented at: Proceedings of the 2018 CHI Conference on Human Factors in Computing System; April 21-26, 2018:1-13; Montreal, QC. [ CrossRef ]
- Wu DTY, Xin C, Bindhu S, Xu C, Sachdeva J, Brown JL, et al. Clinician perspectives and design implications in using patient-generated health data to improve mental health practices: mixed methods study. JMIR Form Res. 2020;4(8):e18123. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zhu H, Colgan J, Reddy M, Choe EK. Sharing patient-generated data in clinical practices: an interview study. AMIA Annu Symp Proc. 2016;2016:1303-1312. [ FREE Full text ] [ Medline ]
- Lordon RJ, Mikles SP, Kneale L, Evans HL, Munson SA, Backonja U, et al. How patient-generated health data and patient-reported outcomes affect patient-clinician relationships: a systematic review. Health Informatics J. 2020;26(4):2689-2706. [ FREE Full text ] [ CrossRef ] [ Medline ]
- West P, Van Kleek M, Giordano R, Weal M, Shadbolt N. Information quality challenges of patient-generated data in clinical practice. Front Public Health. 2017;5:284. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Park JI, Lee HY, Kim H, Lee J, Shinn J, Kim HS. Lack of acceptance of digital healthcare in the medical market: addressing old problems raised by various clinical professionals and developing possible solutions. J Korean Med Sci. 2021;36(37):e253. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Haase CB, Ajjawi R, Bearman M, Brodersen JB, Risor T, Hoeyer K. Data as symptom: doctors' responses to patient-provided data in general practice. Soc Stud Sci. 2023;53(4):522-544. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gaveikaite V, Grundstrom C, Winter S, Schonenberg H, Isomursu M, Chouvarda I, et al. Challenges and opportunities for telehealth in the management of chronic obstructive pulmonary disease: a qualitative case study in Greece. BMC Med Inform Decis Mak. 2020;20(1):216. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by A Mavragani; submitted 26.05.23; peer-reviewed by CM Chu, P Dunn, A Brigden, C Baxter; comments to author 08.02.24; revised version received 05.04.24; accepted 11.04.24; published 31.05.24.
©Sharon Guardado, Maria Karampela, Minna Isomursu, Casandra Grundstrom. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 31.05.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Journal of Materials Chemistry A
Research progress on two-dimensional indium selenide crystals and optoelectronic devices.
Two-dimensional (2D) materials, with unique electronic properties, superior optoelectronic properties, and dangling-bond-free surfaces, have attracted significant attention and experienced rapid development both in fundamental science and for practical applications. Amid the plethora of 2D materials, indium selenide (InSe) has emerged as a promising candidate for future high-mobility optoelectronic devices. Nobel Prize laureate Andre Geim even describes it as "the 'golden middle' between silicon and graphene". Over the past decade, remarkable findings and progress have been made in the fabrication of 2D InSe crystals and their application in devices, motivating us to delve deeply into these forefront developments. In this review, the physical properties such as the crystalline structure, band structure and photoluminescence characteristics are discussed at first. Then, the advancement in terms of synthesis techniques, characteristics and synthesis schemes in the fabrication of 2D InSe are summarized. Subsequently, the mechanisms of optimized strategy and recent progress in field effect transistors (FETs) as well as photodetectors based on this material are summarized, also highlighting the promising applications of 2D InSe in sensors. Finally, an outlook, challenges, and potential future research directions in the fabrication of 2D InSe and its devices are presented.
- This article is part of the themed collection: Journal of Materials Chemistry A Recent Review Articles
Article information
Download citation, permissions.
D. Zheng, P. Chen, Y. Liu, X. Li, K. Liu, Z. Yin, R. Frisenda, Q. Zhao and T. Wang, J. Mater. Chem. A , 2024, Accepted Manuscript , DOI: 10.1039/D4TA01584C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

- STM Journals
Special Issues
- Conferences
- Editorial Board Members
- Reviewers Board Members
- Advisory Panel
- Indexing Bodies
- For Authors
- For Reviewers
- For Editors
- For Advisory Board
- Special Issue Guidelines
- Peer-Review Policy
- Manuscript Submission Guidelines
- Publication Ethics and Virtue
- Article Processing Charge
- Editorial Policy
- Advertising Policy
- STM Website and Link Policy
- Distribution and dessemination of Research
- Informed consent Policy
- DOI Payment
"Connect with colleagues and showcase your academic achievements."
"Unleashing the potential of your words"
"Explore a vast collection of books and broaden your horizons."
"Empower yourself with the knowledge and skills needed to succeed."
"Collaborate with like-minded professionals and share your knowledge."
"Learn from experts and engage with a community of learners."
- ICDR Group of Companies

- Training Programs

Research & Reviews : Journal of Herbal Science
ISSN: 2278-2257
Journal Menu
Journal menu, editor overview.
RRJOHS maintains an Editorial Board of practicing researchers from around the world, to ensure manuscripts are handled by editors who are experts in the field of study.
Article Processing Charges
Information
Research & Reviews : Journal of Herbal Science [ISSN : [837 show=125]] is here with an open access platform for our journals specifically for the special issues topics which are available online, immediately upon publication which leads to unlimited access anytime anywhere. This is made possible by an article-processing charge (APC) that covers the costs of turning a manuscript into a finished article, as well as the costs of hosting, distributing and promoting an article. Here's the details regarding the article processing charge (APC) which is applied to papers accepted after peer review.
Open Access
Its the normal publication process which is completed in 20 working days
APC Open Access [GOLD]
[837 show=1736] $
APC Open Access [Green]
[837 show=1737] $
Rapid Publication
Simple, fast and effective flexible move to get your paper published within 10 working days of manuscript submission
(Additional)
Expert, editorial advice within 4 days
Acceptance within next 1 day.
High quality Language editing and formatting along with plag check in another 3 days
Plagiarism check done with high standard tools such as Quetext, Drillbit and Turnitin in another 1 days
After all the process is completed the paper gets published
Specialist Publication
The publication gets done way more faster than expected within 5 working days with high quality standards.
For any further queries regarding APC, please write us at : [email protected] or submit your query on the Query Portal
For FAQs regarding APC, click here
Further Links:
- Article Processing Charges (APC) Information
- Open Access Information and Policy
- Information for Authors
- Accepted Methods of Payment
- Invoicing and APC FAQs

WEBSITE DISCLAIMER
Last updated: 2022-06-15
The information provided by STM Journals (“Company”, “we”, “our”, “us”) on https://journals.stmjournals.com / (the “Site”) is for general informational purposes only. All information on the Site is provided in good faith, however, we make no representation or warranty of any kind, express or implied, regarding the accuracy, adequacy, validity, reliability, availability, or completeness of any information on the Site.
UNDER NO CIRCUMSTANCE SHALL WE HAVE ANY LIABILITY TO YOU FOR ANY LOSS OR DAMAGE OF ANY KIND INCURRED AS A RESULT OF THE USE OF THE SITE OR RELIANCE ON ANY INFORMATION PROVIDED ON THE SITE. YOUR USE OF THE SITE AND YOUR RELIANCE ON ANY INFORMATION ON THE SITE IS SOLELY AT YOUR OWN RISK.
EXTERNAL LINKS DISCLAIMER
The Site may contain (or you may be sent through the Site) links to other websites or content belonging to or originating from third parties or links to websites and features. Such external links are not investigated, monitored, or checked for accuracy, adequacy, validity, reliability, availability, or completeness by us.
WE DO NOT WARRANT, ENDORSE, GUARANTEE, OR ASSUME RESPONSIBILITY FOR THE ACCURACY OR RELIABILITY OF ANY INFORMATION OFFERED BY THIRD-PARTY WEBSITES LINKED THROUGH THE SITE OR ANY WEBSITE OR FEATURE LINKED IN ANY BANNER OR OTHER ADVERTISING. WE WILL NOT BE A PARTY TO OR IN ANY WAY BE RESPONSIBLE FOR MONITORING ANY TRANSACTION BETWEEN YOU AND THIRD-PARTY PROVIDERS OF PRODUCTS OR SERVICES.
PROFESSIONAL DISCLAIMER
The Site can not and does not contain medical advice. The information is provided for general informational and educational purposes only and is not a substitute for professional medical advice. Accordingly, before taking any actions based on such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical advice.
Content published on https://journals.stmjournals.com / is intended to be used and must be used for informational purposes only. It is very important to do your analysis before making any decision based on your circumstances. You should take independent medical advice from a professional or independently research and verify any information that you find on our Website and wish to rely upon.
THE USE OR RELIANCE OF ANY INFORMATION CONTAINED ON THIS SITE IS SOLELY AT YOUR OWN RISK.
AFFILIATES DISCLAIMER
The Site may contain links to affiliate websites, and we may receive an affiliate commission for any purchases or actions made by you on the affiliate websites using such links.
TESTIMONIALS DISCLAIMER
The Site may contain testimonials by users of our products and/or services. These testimonials reflect the real-life experiences and opinions of such users. However, the experiences are personal to those particular users, and may not necessarily be representative of all users of our products and/or services. We do not claim, and you should not assume that all users will have the same experiences.
YOUR RESULTS MAY VARY.
The testimonials on the Site are submitted in various forms such as text, audio, and/or video, and are reviewed by us before being posted. They appear on the Site verbatim as given by the users, except for the correction of grammar or typing errors. Some testimonials may have been shortened for the sake of brevity, where the full testimonial contained extraneous information not relevant to the general public.
The views and opinions contained in the testimonials belong solely to the individual user and do not reflect our views and opinions.
ERRORS AND OMISSIONS DISCLAIMER
While we have made every attempt to ensure that the information contained in this site has been obtained from reliable sources, STM Journals is not responsible for any errors or omissions or the results obtained from the use of this information. All information on this site is provided “as is”, with no guarantee of completeness, accuracy, timeliness, or of the results obtained from the use of this information, and without warranty of any kind, express or implied, including, but not limited to warranties of performance, merchantability, and fitness for a particular purpose.
In no event will STM Journals, its related partnerships or corporations, or the partners, agents, or employees thereof be liable to you or anyone else for any decision made or action taken in reliance on the information in this Site or for any consequential, special or similar damages, even if advised of the possibility of such damages.
GUEST CONTRIBUTORS DISCLAIMER
This Site may include content from guest contributors and any views or opinions expressed in such posts are personal and do not represent those of STM Journals or any of its staff or affiliates unless explicitly stated.
LOGOS AND TRADEMARKS DISCLAIMER
All logos and trademarks of third parties referenced on https://journals.stmjournals.com / are the trademarks and logos of their respective owners. Any inclusion of such trademarks or logos does not imply or constitute any approval, endorsement, or sponsorship of STM Journals by such owners.
Should you have any feedback, comments, requests for technical support, or other inquiries, please contact us by email: [email protected] .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 31, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
Radio waves from mobile phones do not affect cognition: Study
by Australian Radiation Protection and Nuclear Safety Agency (ARPANSA)

A World Health Organization-commissioned systematic review finds that radio wave exposure from mobile phones does not affect learning, memory, attention span and other cognitive functions like coordination. The work is published in the journal Environment International .
Co-led by the Australian Radiation Protection and Nuclear Safety Agency's (ARPANSA) Health Impact Assessment Assistant Director, Associate Professor Ken Karipidis, the review addresses a long-held community concern.
"One of the motivations for this research was to assess effects on the brain because mobile phones are usually held close to the head during calls," A/Prof. Karipidis said. "One of the challenges of studying the effects of mobile phones on health is that it's hard to separate radiation exposure from behavioral effects from social media and gaming on our cognition. Overall, this systematic review found that radio wave exposure from mobile phones does not affect cognition."
The WHO systematic review was a collaboration between ARPANSA and Monash University. ARPANSA's Dr. Chris Brzozek and Dr. Masoumeh Sanagou also contributed to the study.
This review identified 3,945 papers for consideration, but only five studies were found to have appropriate methods and were included in the final analysis. The authors acknowledge that more high-quality research is needed to address all types of populations, radio wave exposures, and cognitive outcomes, particularly studies investigating environmental and occupational exposure in adults.
The WHO commissioned a series of systematic reviews in 2019 to help them undertake an updated health risk assessment of radio wave exposure. These reviews will be used to help inform a new Environmental Health Criterion monograph on radiofrequency electromagnetic fields (RF-EMF).
Explore further
Feedback to editors

Eye-tracking techniques could help primary care providers diagnose autism sooner, more accurately
9 hours ago
This self-powered sensor could make MRIs more efficient
10 hours ago

Not eating can hinder weight loss, study in fruit flies suggests
11 hours ago

Neuroscience research suggests ketones can enhance cognitive function and protect brain networks
12 hours ago
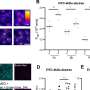
New research finds antidepressants may help deliver other drugs into the brain

Mediterranean diet tied to one-fifth lower risk of death in women

Scientists find new method to enhance efficacy of bispecific antibodies for solid tumors

Cardiomyocytes study discovers new way to regenerate damaged heart cells
13 hours ago
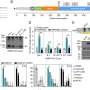
Study: The route into the cell influences the outcome of SARS-CoV-2 infection
Prenatal testing offers a window for finding a mother's cancer risk
Related stories.

More research needed on health effects of airborne ultrasound that's increasingly used in virtual reality tech
May 21, 2024

There's no evidence 5G is going to harm our health, so let's stop worrying about it
Aug 2, 2019
Exposure of European children to electromagnetic fields is below the maximum levels
May 30, 2018

Intranasal insulin may have pro-cognitive benefits
Jul 3, 2023

No link between mobile phones and brain cancer in the over-60s
Sep 17, 2019
Aussies getting less radiation from CT scans than 5 years ago
Feb 11, 2020
Recommended for you
Researchers identify a genetic cause of intellectual disability affecting tens of thousands
14 hours ago
Tackling the hurdle of tumor formation in stem cell therapies

Study finds use of in-hospital mortality as a sepsis quality metric may unfairly penalize safety-net hospitals

New avenues to developing personalized treatments for schizophrenia
May 30, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Research & Reviews: Journal of Herbal Science
- Other Journals
- For Readers
- For Authors
- For Librarians
- Announcements
- Author Guideline
- Referencing Pattern
- Editorial Board
- Publication Ethics & Malpractice Statement
Studies on Microbiological Assessment of Some Commercial Herbal Drugs and Spices
ABSTRACT The issue of quality, safety and efficacy of herbal formulations remains always of great concern while considering the genuineness and presence or absence of pathogens in medicinal plants. In the present study, five commercial herbal commodities used as drug and spice, viz. samples Capsicum frutescens L. Cuminum cyminum L., Piper longum L., Syzygium cumini (L.) Skeels and Terminalia chebula Retz., (Figure 1) were subjected to microbiological analysis. The findings from this study emphasized the need for constant quality assessment of herbal drugs or spices on sale in order to ensure the production of therapeutic products suitable for human consumption . Keywords: Herbal drugs, medicinal plant, microorganisms, spice
- There are currently no refbacks.

COMMENTS
About Journal. Research & Reviews : Journal of Herbal Science (RRJOHS): 2278-2257(e) is a peer-reviewed hybrid open-access journal launched in 2011 focused on the publication of current View Full Focus and Scope… view full focus and scope
Scientific Journal Impact Factor (SJIF): 6.488. Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee ...
Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work ...
A comprehensive web based literature review is employed in order to retrieve relevant information using international scientific databases including PubMed, Science direct, Web of Science, Google scholar. The review of selected 34 original published research articles were evaluated and summarized.
Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells and certain animal parts.
Dr. Ishwar Singh is an Assistant Professor in the Department of Botany at Hans Raj College, University of Delhi. He received his Ph.D. Degree in Botany from Delhi University in 2002. Then worked as a Lecturer/Senior Lecturer in M. B. (P.G.) College, Dadri (C.C.S. University, Meerut) for 9 Years. He is having 17 Years of Teaching and Research ...
Welcome to the Manuscript Submission site for. Research & Reviews : Journal of Herbal Science. To begin, log in with your user ID and password. If you are unsure about whether or not you have an account, or have forgotten your password, go to the Reset Password screen.
Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells and ...
The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells, and certain animal parts. Research & Reviews: Journal of Herbal Science [2278-2257(e)] is a peer-reviewed hybrid open-access journal launched in 2011 focused on the publication of current research work carried out under Herbal ...
The Journal of Herbal Medicine is a peer reviewed journal which aims to serve its readers as an authoritative resource on the profession and practice of herbal medicine. The content areas of the journal reflect the interests of Medical Herbalists and other health professionals interested in the …. View full aims & scope.
Pre-Review Considerations. Expertise: Ensure that you have the appropriate level of expertise in the subject area of the manuscript. If you are not an expert in the area, consider
Research & Reviews: Journal of Herbal Science Content Available in Online Subscription 2012-2021 * The editorial board is a dynamic place and may be changed by publisher any time without notice. Journals Report 2020 Download Subscribe Journal Journal No.: J0007 Submit Manuscript EDITORIAL BOARD ¾ R. Prakash Kumar, Malabar Botanical Garden ...
URL: stmjournals.com ... http://stmjournals.com/journals/RRJHS/Research-and-Reviews-Journal-of-Herbal-Science.html. Google: www.google.com/ ... https://www.google.com ...
Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells and certain animal parts.
1.1 Indications suitable for treatment with herbal medicines. Herbal medicines are in particular suitable for the treatment of non-life-threatening conditions for which knowledge from traditional use is available pointing to their clinical benefits in treating the respective ailment (Wachtel-Galor and Benzie, 2011).This applies especially to psychosomatic disorders, gynecological complaints ...
RRJoHS is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells and certain animal parts.
Research & Reviews: Journal of Herbal Science (RRJoHS) is focused towards the publication of current research work carried out under Herbal science. This journal covers all major fields of applications in Herbal Science. The scope of herbal medicine is sometimes extended to include fungal and bee products, as well as minerals, shells and ...
Herbal prescription recommendation (HPR) is a hot topic and challenging issue in field of clinical decision support of traditional Chin ... Journal of the American Medical Informatics Association, Volume 31, Issue 6, June 2024, ... It furthers the University's objective of excellence in research, scholarship, and education by publishing ...
May 30, 2024. Keep up with the latest research from the three scholarly NCFR journals — Journal of Marriage and Family (JMF), Family Relations: Interdisciplinary Journal of Applied Family Science (FR), and Journal of Family Theory & Review (JFTR) — all of which publish early-view articles online. Below are the early-view articles published ...
This research paper highlights potential benefits and possible risks associated with consumption of herbal products as jam, its organoleptic properties, shelf life and overall acceptability by the consumers. Keywords: Amla, pudina, basil, herbal jam, ascorbic acid, fruit pulp. Cite this Article Ranita Dey, Debasree Ghosh, Ina Mukherjee.
The need for a new review on masks was highlighted by a widely publicized polarization in scientific opinion. The masks section of a 2023 Cochrane review of non-pharmaceutical interventions was—controversially—limited to randomized controlled trials (RCTs).It was interpreted by the press and by some but not all of its own authors to mean that "masks don't work" and "mask mandates ...
Background: Mobile health (mHealth) uses mobile technologies to promote wellness and help disease management. Although mHealth solutions used in the clinical setting have typically been medical-grade devices, passive and active sensing capabilities of consumer-grade devices like smartphones and activity trackers have the potential to bridge information gaps regarding patients' behaviors ...
Two-dimensional (2D) materials, with unique electronic properties, superior optoelectronic properties, and dangling-bond-free surfaces, have attracted significant attention and experienced rapid development both in fundamental science and for practical applications. Amid the plethora of 2D materials, indium Journal of Materials Chemistry A Recent Review Articles
Chandrasekar R. A Comprehensive Review on Herbal Cosmetics in the Management of Skin Diseases Research Journal of Tropical and Cosmetic Sciences;Raipur. 2020, 11(1):32-44. Arun S.K. Formulation and Evaluation of Herbal Soap. World Journal of Pharmaceutical Research, 2023. 12(9),2136-2147. ISSN 2277-7105.
Information. Research & Reviews : Journal of Herbal Science [ISSN : [837 show=125]] is here with an open access platform for our journals specifically for the special issues topics which are available online, immediately upon publication which leads to unlimited access anytime anywhere. This is made possible by an article-processing charge (APC) that covers the costs of turning a manuscript ...
Overall, this systematic review found that radio wave exposure from mobile phones does not affect cognition." The WHO systematic review was a collaboration between ARPANSA and Monash University ...
ABSTRACT The issue of quality, safety and efficacy of herbal formulations remains always of great concern while considering the genuineness and presence or absence of pathogens in medicinal plants. In the present study, five commercial herbal commodities used as drug and spice, viz. samples Capsicum frutescens L. Cuminum cyminum L., Piper longum L., Syzygium cumini (L.) Skeels and Terminalia ...