We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


34: Tuberculosis
David Cluck
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Patient presentation.
- Full Chapter
- Supplementary Content
Chief Complaint
“I have a cough that won’t go away.”
History of Present Illness
A 63-year-old male presents to the emergency department with complaints of cough/shortness of breath which he attributes to a “nagging cold.” He states he fears this may be something worse after experiencing hemoptysis for the past 3 days. He also admits to waking up in the middle of the night “drenched in sweat” for the past few weeks. When asked, the patient denies ever having a positive PPD and was last screened “several years ago.” His chart indicates he was in the emergency department last week with similar symptoms and was diagnosed with community-acquired pneumonia and discharged with azithromycin.
Past Medical History
Hypertension, dyslipidemia, COPD, atrial fibrillation, generalized anxiety disorder
Surgical History
Appendectomy at age 18
Family History
Father passed away from a myocardial infarction 4 years ago; mother had type 2 DM and passed away from a ruptured abdominal aortic aneurysm
Social History
Retired geologist recently moved from India to live with his son who is currently in medical school in upstate New York. Smoked ½ ppd × 40 years and drinks 6 to 8 beers per day, recently admits to drinking ½ pint of vodka “every few days” since the passing of his wife 6 months ago.
Sulfa (hives); penicillin (nausea/vomiting); shellfish (itching)
Home Medications
Albuterol metered-dose-inhaler 2 puffs q4h PRN shortness of breath
Aspirin 81 mg PO daily
Atorvastatin 40 mg PO daily
Budesonide/formoterol 160 mcg/4.5 mcg 2 inhalations BID
Clonazepam 0.5 mg PO three times daily PRN anxiety
Lisinopril 20 mg PO daily
Metoprolol succinate 100 mg PO daily
Tiotropium 2 inhalations once daily
Venlafaxine 150 mg PO daily
Warfarin 7.5 mg PO daily
Physical Examination
Vital signs.
Temp 100.8°F, P 96, RR 24 breaths per minute, BP 150/84 mm Hg, pO 2 92%, Ht 5′10″, Wt 56.4 kg
Slightly disheveled male in mild-to-moderate distress
Normocephalic, atraumatic, PERRLA, EOMI, pale/dry mucous membranes and conjunctiva, poor dentition
Bronchial breath sounds in RUL
Cardiovascular
NSR, no m/r/g
Soft, non-distended, non-tender, (+) bowel sounds
Genitourinary
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Emerg Infect Dis
- v.8(11); 2002 Nov
Two Cases of Pulmonary Tuberculosis Caused by Mycobacterium tuberculosis subsp. canetti
Jean miltgen.
* Hôpital d’instruction des armées Laveran, Marseille, France
Marc Morillon
Jean-louis koeck.
† Hôpital d’instruction des armées Val de Grâce, Paris, France
Anne Varnerot
‡ Institut Pasteur, Paris, France
Jean-François Briant
Gilbert nguyen, denis verrot, daniel bonnet, véronique vincent.
We identified an unusual strain of mycobacteria from two patients with pulmonary tuberculosis by its smooth, glossy morphotype and, primarily, its genotypic characteristics. Spoligotyping and restriction fragment length polymorphism typing were carried out with the insertion sequence IS6110 patterns. All known cases of tuberculosis caused by Mycobacterium canetti have been contracted in the Horn of Africa.
The Mycobacterium tuberculosis complex includes the following mycobacteria, which are characterized by a slow growing rate: M. tuberculosis, M. africanum, M. bovis, and M. microti ( 1 ). In recently published reports of two cases of lymphatic node tuberculosis (TB), the strains were recognized as belonging to a new taxon of M. tuberculosis ( 2 , 3 ). These isolates were characterized by a highly particular growing pattern, and the colonies appeared smooth and glossy. A complete genetic study of these strains led to their integration into the M. tuberculosis complex. This strain, identified as M. tuberculosis subsp. canetti or, more simply, M. canetti, was first isolated in 1969 by Georges Canetti from a French farmer. The strain was preserved at the Pasteur Institute where its antigenic pattern was studied extensively. We report two cases of pulmonary TB caused by this strain. The two patients had also lived in East Africa.
In September 1998, a 36-year-old male soldier in the French Foreign Legion with hemoptysis was sent back to France from Djibouti. He expectorated bloody sputum after running and on a few other occasions. His medical history was not unusual. When the patient was hospitalized, 2 weeks after the initial symptoms, he began to experience progressive fatigue. He did not experience fever, weight loss, night sweats, anorexia, cough, dyspnea, or chest pain, and did not produce sputum.
Results of the clinical examination were normal. The Mantoux test, performed with 10 IU of purified tuberculin (Aventis-Pasteur-MSD, Lyon, France), yielded a maximum transverse diameter of induration of 15 mm. Laboratory values were normal ( Table ). The chest X-ray showed a triangular consolidation of the left upper lobe with blurred limits and small cavitary lesions. No other contiguous mediastinohilar anomalies were visible. A computed tomographic scan confirmed the cavitary syndrome: three excavated nodular images showed radiating spicules within a micronodular infiltrate. Bronchoscopy showed a moderate inflammation of airway mucosa, especially in the left upper lobe. Biopsy specimens exhibited nonspecific inflammation.
A bronchial washing smear from the left upper lobe was positive for acid-fast bacilli. Serologic tests for HIV-1 and HIV-2 were negative. No evidence of disease was found elsewhere; the patient did not experience bone pain. Results of neurologic and ophthalmologic examinations were normal; no lymphadenopathy or hepatosplenomegaly were found and the genitalia were normal. Auscultation revealed no pericardial fremitus; no ascitic fluid was detected. The urinary sediment contained <1,000 red blood cells/L and <5,000 leukocytes/L. Antituberculosis chemotherapy was begun with four drugs: rifampicin, isoniazid, ethambutol, and pyrazinamide. Cultures revealed a strain identified as M. tuberculosis subsp. canetti that was susceptible to all primary antituberculous drugs. Therefore, rifampicin and isoniazid were continued for 3 more months for a total treatment period of 6 months. The patient’s response to treatment was favorable, and he remained asymptomatic.
A 55-year-old male soldier in the French Foreign Legion, who returned from Djibouti, was hospitalized in September 1999 after his chest x-ray showed abnormal findings. He was a nurse and had been occasionally in charge at the Djibouti Hospital for 2 years. His medical history was unremarkable. Eight months before he returned to France, he experienced asthenia, anorexia, and a weight loss of 3 kg. The symptoms resolved spontaneously after 2 months, and he had been asymptomatic since then. He had no history of cough, sputum production, hemoptysis, dyspnea, fever, or night sweats.
Results of a clinical examination and of laboratory studies were normal ( Table ), except for hypereosinophilia. Serologic tests for schistosomiasis, hydatidosis, distomiasis, amebiasis, toxocariasis, and trichinosis were negative, and parasites were not found in stool samples. Thoracic radiographs performed when he came back from Djibouti showed parenchymal consolidation of the right upper lobe with small cavities. Sputum was not produced. A gastric aspirate smear was negative for acid-fast bacilli, and a bronchial aspiration smear was positive for acid-fast bacilli. HIV serology was negative, and no other site of the infection was found. Drug therapy was initiated with rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months. Cultures of bronchial aspirates were positive within 14 days; later, cultures of two gastric aspirates were positive for acid-fast bacilli. An M. tuberculosis subsp. canetti isolate was identified, which was susceptible to all primary antituberculous drugs. The treatment was then extended for 4 months with rifampicin and isoniazid. The patient's response to treatment was favorable.
The following methods were used to identify the etiologic agent. First, the samples were decontaminated with N-acetyl-L-cysteine/NaOH. Acid-fast bacilli were detected by auramine staining, the positive smears also were stained with Ziehl-Nielsen stain. The samples were then seeded onto Löwenstein-Jensen and Coletsos slants and also into a liquid system, the BBL Mycobacterial Growth Indicator Tube (MGIT, BD Diagnostic Systems, Sparks, MD).
The mycobacteria were identified by using a specific DNA probe (Gen-Probe, Gen-Probe Incorporated, San Diego, CA) and by performing the usual biochemical tests (nitrate reduction, 68°C catalase resistance, niacin production).
The Pasteur Institute of Paris used two methods for typing: restriction fragment length polymorphism (RFLP) analysis and spoligotyping. In RFLP analysis, after digestion of the M. tuberculosis strain's genomic DNA with PvuII restriction enzyme and agarose gel migration, the DNA was transferred on a membrane, according to the Southern method, and then hybridized with an insertion sequence IS6110 probe ( 4 ). In the spoligotyping method, after DNA direct repeat amplification, the labeled polymerase chain reaction product was used as a probe to hybridize with 43 synthetic spacer oligonucleotides (DNA sequences derived from the direct repeat [DR] region of M. tuberculosis, H37Rv and M. bovis BCG P3), which were attached to a carrier membrane ( 5 ). The sensitivity to antituberculous drugs was determined by the indirect proportion method.
MGIT results were positive for the two cultures in 9 and 12 days, respectively. On Löwenstein-Jensen slants, the cultures were positive in 12 and 14 days, respectively. The white, smooth, and glossy colonies were characteristic of M. tuberculosis subsp. canetti ( Figure 1 ). The two strains had the same phenotypic and genotypic pattern; 68°C catalase was negative, and they reduced nitrate, as do other M. tuberculosis species, but they did not produce niacin. The DNA probe, Gen-Probe, confirmed that these strains belonged to the M. tuberculosis complex.

Colony morphology on Löwenstein-Jensen slants, showing M. canetti and M. tuberculosis strains. (A) Colonies of M. tuberculosis are rough, thick, wrinkled, have an irregular margin, and are faintly buff-colored. (B) M. canetti exhibits smooth, white and glossy colonies.
These strains contained two copies of IS6110. Spoligotyping showed that they shared only 2 of the 43 oligonucleotides reproducing the spacer DNA sequences of M. tuberculosis, H37Rv and M. bovis BCG P3. This profile is characteristic of M. tuberculosis subsp. canetti ( Figure 2 ).

(A) IS6110 hybridization patterns of PvuII-digested genomic DNA. Lane 1, Mycobacterium tuberculosis Mt 14323 (reference strain). Lane 2, M. canetti strain NZM 217/94. Lanes 3 and 4, the strains isolated from French legionnaires with pulmonary tuberculosis (TB). (B) Spoligotyping patterns. Lane 1, M. tuberculosis H37Rv (reference strain). Lane 2, M. canetti strain NZM 217/94. Lanes 3 and 4, the strains isolated from French legionnaires with pulmonary TB.
In 1997, van Soolingen reported a case of lymph node TB in a 2-year-old Somali child on the child’s arrival in the Netherlands in 1993 ( 2 ). In 1998, Pfyffer described abdominal lymphatic TB in a 56-year-old Swiss man (who lived in Kenya) with stage C2 HIV infection ( 3 ). These strains of M. canetti (So93 from the Somali child and NZM 217/94 from the Swiss man) have been studied extensively. In culture they grow faster than other strains in the M. tuberculosis complex. The So93 strain expands by one rough colony for every 500 smooth colonies. They appear smooth, white, and glossy because of the high amount of lipooligosaccharides in the membrane ( 6 ); the So93 rough colonies lack this amount ( 2 ).
Two copies of the IS6110 insertion sequence were found in the NZM 217/94 and So93 genome. This fingerprint matched none of the 5,000 other strains preserved in the laboratory of van Soolingen (Bilthoven, the Netherlands) ( 2 ). The strains we observed also showed two copies of IS6110.
So93, NZM 217/94, and our two strains share only 2 of 43 identical repeated sequences that have been observed by spoligotyping. Study of the IS6110 RFLP patterns and of the spacer DNA sequences of the DR locus confirmed that M. tuberculosis, M. bovis, M. africanum, M. microti, and M. canetti represent a closely related group of mycobacteria that are clearly distinct from other mycobacterial species. In the M. tuberculosis complex, M. canetti appears to be the most divergent strain ( 2 ).
We believe that this is the first published report of pulmonary disease caused by M. canetti. Our two cases confirm that M. canetti is able to involve lungs, like any other other member of the M. tuberculosis complex and is able to affect immunocompetent subjects. The clinical features of these two pulmonary cases of TB caused by M. canetti are not specific.
TB caused by M. canetti appears to be an emerging disease in the Horn of Africa. A history of a visit to the region should cause this strain to be considered promptly. As travel to this area becomes more frequent, and mycobacterial identification techniques improve, the number of diagnosed cases will likely increase.
Acknowledgments
We thank Michel Fabre for the photographs and Jan Eskandari for his translation of this article.
Dr. Miltgen is assistant head of the Pneumology Department at the Hôpital d’Instruction des Armées of Marseilles, where he specializes in tropical diseases.
Suggested citation for this article: Miltgen J, Morrillon M, Koeck J-L, Varnerot A, Briant J-F, Nguyen G. Two cases of pulmonary tuberculosis caused by Mycobacterium tuberculosis subsp. canetti. Emerg Infect Dis [serial online] 2002 Nov [date cited]. Available from http://www.cdc.gov/ncidod/EID/vol8no11/02-0017.htm
Case Report: Pulmonary tuberculosis and raised transaminases without pre-existing liver disease- Do we need to modify the antitubercular therapy?
Sanjeev Gautam Roles: Data Curation, Writing – Original Draft Preparation Keshav Raj Sigdel Roles: Conceptualization, Supervision, Writing – Review & Editing Sudeep Adhikari Roles: Writing – Original Draft Preparation, Writing – Review & Editing Buddha Basnyat Roles: Conceptualization, Supervision, Writing – Review & Editing Buddhi Paudyal Roles: Supervision, Writing – Review & Editing Jiwan Poudel Roles: Writing – Review & Editing Ujjwol Risal Roles: Writing – Review & Editing
This article is included in the Oxford University Clinical Research Unit (OUCRU) gateway.
tuberculosis, transaminitis, standard ATT, liver friendly regimen
Revised Amendments from Version 1
The changes have been made in the revised manuscript as suggested by the reviewers. The concerns of reviewers regarding limited workup of the patient for liver disease has been addressed in the manuscript. This is a single case report and further studies are required to make a firm recommendation for management of pulmonary tuberculosis with transaminitis without pre-existing liver disease. We have acknowledged the fact in the revised manuscript.
See the authors' detailed response to the review by Vivek Neelakantan See the authors' detailed response to the review by Neesha Rockwood See the authors' detailed response to the review by Prajowl Shrestha and Ashesh Dhungana
Tuberculosis is the biggest infectious disease killer in the world 1 , and is endemic in Nepal with the national prevalence at 416 cases per 100000 population 2 . Pulmonary tuberculosis is the most common form. In Nepal, tuberculosis prevalence is more in productive age group (25–64 years) and men. Poverty, malnutrition, overcrowding, immunocompromised state like HIV infection, alcohol, smoking, air pollution, diabetes and other comorbidities are important risk factors for acquiring the disease 3 . Though under-reported, involvement of liver with tuberculosis is encountered often in clinical practice in endemic areas like Nepal.Liver can be involved; a) diffusely as a part of disseminated miliary tuberculosis or as primary miliary tuberculosis of liver, or b) focal involvement as hepatic tuberculoma or abscess, as was classified by Reed in 1990 4 . The biochemical pattern of liver function abnormality in these forms of extrapulmonary tuberculosis is cholestatic (predominantly raised alkaline phosphatase and gamma-glutamyltranspeptidase) rather than hepatocellular (predominantly raised transaminases) 5 , 6 . The hepatocellular pattern of liver injury is seen in cases with pre-existing liver disease including hepatotoxic drug use, which are unrelated to tuberculosis 7 , 8 .
As per national protocol of Nepal, any patient with tuberculosis receives combination antitubercular therapy (ATT) including four drugs; Isoniazid (H), Rifampicin (R), Pyrazinamide (Z) and Ethambutol (E) for initial 2 months popularly known as HRZE. This is followed by 4 months of two drugs; HR. Treatment is given under Directly Observed Treatment Short- Course (DOTS) to improve the patient compliance which could otherwise be compromised owing to lower socioeconomic status of patients, longer duration of treatment and side effects 9 . Patients with extrapulmonary hepatic tuberculosis are treated with full dose of standard ATT 5 , 6 . But three out of the four drugs (H, R and Z) are hepatotoxic 7 . So the patients having pre-existing liver disease usually require liver-friendly modified regimens to protect the liver but they may be suboptimal for eradicating underlying tuberculosis 8 . The protocol of Nepal does not warrant baseline investigations except chest X-ray and sputum smear microscopy to be done routinely before prescribing ATT in programmatic setting 9 . However in hospital setting like our case, baseline blood investigations including liver function tests are usually done before starting treatment even in absence of features suggesting liver injury and therapy modified accordingly.
Here we present a case of pulmonary tuberculosis with predominant transaminitis but there was no feature of pre-existing liver disease nor a history of hepatotoxic drug use. The liver injury was attributed to the pulmonary tuberculosis itself, and treated with standard first line ATT which led to resolution of liver function abnormalities.
Case presentation
A 33 year old Newar housewife from Kathmandu, Nepal, with no known comorbidity, presented to Patan Hospital Emergency Department in November, 2019 with a history of cough with occasional sputum production over the previous 20 days and low grade fever for 10 days. There was no history of chest pain, difficulty breathing, headache, vomiting, altered mentation, abdominal pain, yellowish discoloration of eyes, burning urine, hair loss, photosensitivity, joint pain, or rash but she had decreased appetite and weight loss. There was no past history of tuberculosis or jaundice. She did not consume alcohol or any drugs including acetaminophen, aflatoxin or herbal products. Her father-in-law had been diagnosed with pulmonary tuberculosis five years earlier, but there was no family history of liver disease.
Initial examination showed temperature of 101 o F with pulse of 110 beats/minute and respiratory rate of 26 breaths/minute. There was diffuse fine crepitation on the left side on auscultation of the chest. There was no lymphadenopathy, icterus, peripheral edema or wheezes. Neck veins were not distended. Liver and spleen were not palpable, and abdomen examination was normal.
Laboratory parameters with normal ranges in parenthesis are as follow:
Complete blood count before transfusion: white cell count 7.8 (4–10) × 10 9 /L; neutrophils 80%; lymphocytes 16%; monocytes 4%; red blood cells 3.6 (4.2–5.4) × 10 12 /L; haemoglobin 10.6 (12–15) g/dL; platelets 410 (150–400) × 10 9 /L.
Biochemistry: random blood sugar 126 (65–110) mg/dL; urea 39 (17–45) mg/dL; creatinine 1.1 (0.8–1.3) mg/dL; sodium 138 (135–145) mmol/L and potassium 4 (3.5–5) mmol/L.
Chest X-ray ( Figure 1 ) showed thick walled cavitating lesions in the left upper lobe and patchy infiltrates in left middle and lower zones. There were hyperinflated lung fields with blunting of left costophrenic angle. Sputum smear examination showed 3+ acid fast bacilli. Sputum Gene Xpert was positive for Rifampicin sensitive tubercle bacilli. A diagnosis of pulmonary tuberculosis was made, and planned for starting ATT.
Figure 1. Chest X-ray showing thick walled cavitating lesions in the left upper lobe and patchy infiltrates in left middle and lower zones.
Liver function test was performed as baseline workup before starting treatment which showed the following results (with normal ranges in parenthesis): bilirubin total 1.1 (0.1–1.2) mg/dL and direct 0.5 (0–0.4) mg/dL; alanine transaminase 308 (5–30) units/L; aspartate transaminase 605 (5–30) units/L; alkaline phosphatase 149 (50–100) IU/L; gamma-glutamyltranspeptidase66 (9–48) units/L. The raised transaminases led us to perform further workup for liver disease. There was no clinical evidence of chronic liver disease or portal hypertension. Liver synthetic functions were as following;albumin 3.5 (3.5–5) g/dL; total protein 6.5 (6–8.3) g/dL; prothrombin time 14 (11–13.5) s. Serologies for HIV, HBsAg, Hepatitis C virus (HCV), Hepatitis A virus (HAV) and Hepatitis E virus (HEV) were nonreactive. Testing for other hepatotropic viruses was not done because of unavailability of the tests. Neurological examinations and the slit lamp examination of eye were normal. Ultrasound of the abdomen showed a normal sized liver with smooth outline and echotexture. However fibroscan, upper gastrointestinal endoscopy, abdominal CT scan and liver biopsy were not done due to financial constraints of the patient.
She was admitted to the respiratory isolation unit. At first there was some hesitation in starting the full treatment for her pulmonary tuberculosis because of her liver function tests. But taking into consideration her presentation and laboratory findings, we opted for the full treatment rather than a modified TB regimen. We started standard four drugs ATT based on her weight as per national TB guidelines which included three tablets of HRZE given once daily with each tablet containing 75 mg isoniazid (H), 150 mg rifampicin (R), 400 mg pyrazinamide (Z) and 275 mg ethambutol (E). This led to improvement in her clinical status. She was closely observed for possible worsening of her liver disease due to the hepatotoxic antitubercular drugs. Providentially, at 1 week after starting treatment, she was afebrile and continuing to improve and her liver function test showed a total bilirubin of 0.7 mg/dl, aspartate transaminase of 40 IU/L and alanine transaminase of 62 IU/L.
She was discharged with advice to follow up in 1 month. At 1 month follow up she had no symptoms and therefore no further tests were done. At 2 months, she was still asymptomatic and her sputum smear was negative for acid fast bacilli. Her liver function test showed a total bilirubin of 0.6 mg/dl, aspartate transaminase of 30 IU/L and alanine transaminase of 35 IU/L. She was switched to3 tablets of HR to be taken for 4 months.
Our patient with pulmonary tuberculosis had predominantly raised transaminases (hepatocellular pattern)during the initial presentation, with only modest elevation in alkaline phosphatase and gamma glutamyltranspeptidase. The workup for liver disease could not be performed completely because of resource limitation. Looking for clinical evidences by history and examination, and performing liver function tests, abdominal ultrasound and serology for common hepatotropic viruses are usually considered sufficient in our limited setup. We perform further tests only if the initial workup hints towards another etiology. There were no clinical features of chronic liver disease or portal hypertension. She had no risk factors for liver disease such as family history, alcohol, drugs, toxins, features suggesting autoimmune or metabolic liver diseases. Her viral hepatitis serologies were negative. Ultrasound also showed normal liver architecture and size.Though incomplete, the initial workup led us to believe that she had no pre-existing liver injury.
Patients with extrapulmonary hepatic tuberculosis as classified by Reed (diffuse or focal)usually present withnonspecific symptoms like abdominal pain, jaundice, fever, night sweats, fatigue, weight loss and hepatomegaly. They havecholestatic pattern of liver function abnormality with normal transaminases, increased protein- albumin gap owing to raised serum globulin. Hepatic imaging with ultrasound or CT scan reveal abnormalities in 76 and 88% cases respectively. Liver biopsy and demonstration of caseating granuloma and mycobacterial culture remain gold standard for diagnosing hepatic tuberculosis 4 – 6 . Following points in our patient precluded making the diagnosis of hepatic tuberculosis; a) absence of abdominal symptoms and hepatomegaly; b) predominantly raised transaminases (hepatocellular pattern) and normal protein- albumin gap; and c) normal ultrasound finding (though CT and biopsy were not done).
There is another classification schema, given by Levine in 1990 which has incorporated additional entity under hepatic tuberculosis which is ‘pulmonary tuberculosis withliver involvement’ 10 . In the absence of obvious pre-existing liver disease or drug and the presence of active cavitary tuberculosis in lungs, we attributed the transaminitisin our patient to the pulmonary tuberculosis itself. In our anecdotal experience, we have found many such patients though we do not have any formal data to back this up. They are often managed with modified liver-friendly antitubercular regimens for fear of increasing the hepatotoxicity and causing acute liver failure with the use of standard regimen. Few case reports are available in literature reporting the use of the modified regimens 11 , 12 . We believe such cases are underreported, and firm guidelines have not been established to guide clinicians in these cases. Given this, many clinicians in low-middle income countries, including Nepal, who have been treating tuberculosis patients tend to be skeptical in using full doses of first line ATT in such patients and tend to use a modified regimen. However, this practice may potentially lead to under-treatment and therefore increase fatality 13 . The use of modified regimen may also increase the risk of developing drug-resistant tuberculosis because of exclusion of more potent drugs 14 . Though there was some hesitation at first in our case, we soon started treatment with the standard ATT in our patient with close monitoring. This we believe led to the resolution of liver injury, evidenced by the normalization of transaminases.
However, acknowledging that the patient may develop drug induced liver injury (DILI) with the hepatotoxic antitubercular drugs, we should monitorsuch patients closely in an inpatient basis to look forclinical deterioration or any feature suggesting liver failureand liver function test repeated regularly. Though there is no firm recommendation for when to repeat the tests, patient should not be discharged till there is significant improvement in the transaminases level. The close monitoring is important in those with higher risks for developing DILI associated with ATT such as elderly, females, alcohol consumers, the malnourished and those with genetic susceptibility like slow acetylators 7 . Such monitoring is even more important in our setup because there are possibilities of missing occult hepatic diseases owing tolimited workup.Our patient had improving transaminases evidenced till 2 months follow up.
Though limited by incomplete investigations, we concluded pulmonary tuberculosis as the cause for transaminitis in our patient, and the normalization of transaminases after starting the standard dose of ATT further supports this conclusion. We believe pulmonary TB presenting with transaminitis is a common problem and that treatment may often be compromised because of decreased dosing of ATT.We further aim to perform case series study to explore the magnitude of problem and reach specific conclusions.
When treating a tuberculosis patient with transaminitis, it is important to look for any possibility of pre-existing liver disease or drug use. If none is found, then the use of standard ATT from the beginning with close inpatient monitoring of the patient may be essential for optimal management of tuberculosis, and this may help resolve any liver injury caused by the tuberculosis. This is a single case report, so further case series or cohort studies would be helpful to reach some conclusion and provide concrete recommendations.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Data availability
Underlying data.
All data underlying the results are available as part of the article and no additional source data are required.
- 1. https://www.who.int/news-room/fact-sheets/detail/tuberculosis . .
- 2. https://nepalntp.gov.np/wp-content/uploads/2020/03/NEPAL-NATIONAL-TB-PREVALENCE-SURVEY-BRIEF-March-24-2020.pdf . .
- 3. https://nepalntp.gov.np/wp-content/uploads/2020/04/NTP-Annual-Report-2075-76-2018-19.pdf .
- 4. Reed DH, Nash AF, Valabhji P: Radiological diagnosis and management of a solitary tuberculous hepatic abscess. Br J Radiol. 1990; 63 (755): 902–4. PubMed Abstract | Publisher Full Text
- 5. Hickey AJ, Gounder L, Moosa MY, et al. : A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015; 15 : 209. PubMed Abstract | Publisher Full Text | Free Full Text
- 6. Wu Z, Wang WL, Zhu Y, et al. : Diagnosis and treatment of hepatic tuberculosis: report of five cases and review of literature. Int J Clin Exp Med. 2013; 6 (9): 845–50. PubMed Abstract | Free Full Text
- 7. Ramappa V, Aithal GP: Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013; 3 (1): 37–49. PubMed Abstract | Publisher Full Text | Free Full Text
- 8. Sonika U, Kar P: Tuberculosis and liver disease: management issues. Trop Gastroenterol. 2012; 33 (2): 102–6. PubMed Abstract | Publisher Full Text
- 9. https://nepalntp.gov.np/wp-content/uploads/2019/10/National-Tuberculosis-Management-Guidelines-2019_Nepal.pdf . .
- 10. Levine C: Primary macronodular hepatic tuberculosis: US and CT appearances. Gastrointest Radiol. 1990; 15 (4): 307–9. PubMed Abstract | Publisher Full Text
- 11. Sanchez-Codez M, Hunt WG, Watson J, et al. : Hepatitis in children with tuberculosis: a case report and review of the literature. BMC Pulm Med. 2020; 20 (1): 173. PubMed Abstract | Publisher Full Text | Free Full Text
- 12. Shastri M, Kausadikar S, Jariwala J, et al. : Isolated hepatic tuberculosis: An uncommon presentation of a common culprit. Australas Med J. 2014; 7 (6): 247–50. PubMed Abstract | Publisher Full Text | Free Full Text
- 13. Essop AR, Posen JA, Hodkinson JH, et al. : Tuberculosis hepatitis: a clinical review of 96 cases. Q J Med. 1984; 53 (212): 465–77. PubMed Abstract
- 14. Kumar N, Kedarisetty CK, Kumar S, et al. : Antitubercular therapy in patients with cirrhosis: challenges and options. World J Gastroenterol. 2014; 20 (19): 5760–72. PubMed Abstract | Publisher Full Text | Free Full Text
Comments on this article Comments (0)
Open peer review.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tuberculosis, Interventional Pulmonology, ILD
- Respond or Comment
- COMMENT ON THIS REPORT
Reviewer Expertise: History of Tuberculosis, Global Health, South and Southeast Asia, Medical Humanities
Is the background of the case’s history and progression described in sufficient detail?
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Is the case presented with sufficient detail to be useful for other practitioners?
Reviewer Expertise: Management of HIV/tuberculosis; PK/PD for tuberculosis
- Author Response 16 Oct 2020 Sudeep Adhikari , Internal Medicine, Patan Academy of Health Sciences, Lalitpur, Nepal 16 Oct 2020 Author Response We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. The title needs to convey that ... Continue reading We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. The title needs to convey that this is new onset transaminitis attributed to tuberculosis prior to commencement of anti tubercular therapy Response: Title has been modified. Thank you Background Please outline the baseline work up for TB in programmatic setting in Nepal. Do all patients have baseline LFTs done? Response: Programmatic setting does not require any baseline workup except sputum smear and in chest Xray. But in hospital setting, we perform baseline blood investigations like blood counts, renal function, electrolytes and liver function tests before starting treatment, so that treatment can be modified accordingly. Changes have been made in the background section. Case presentation Has this patient had normal LFTs recorded prior to current TB presentation? Response: No prior LFT testing was done by the patient Mention drug history incl. paracetamol, aflatoxin exposure, family history of liver disease. Text has been modified to state the fact. Thank you The liver screen is incomplete - e.g. autoimmune and inherited liver disease, paracetamol levels, ferritin, occult hep B. Also liver fibrosis assessment. Baseline GGT and clotting needs to be given. Please give a table of ALT, AST, GGT, Alk phos, Albumin and clotting during first 2 weeks of treatment (and any further tests during 6 months of treatment) Of note, there is no clear evidence of disseminated miliary TB on CXR. Abdominal or liver CT was not done. Hence, no comment can be made regarding liver/splenic micronodular abscesses. It appears no mycobacterial blood cultures were taken to assess for bacteraemia. Nor was a liver biopsy done. All relevant negatives and limitations should be mentioned. Response: The liver disease screen is incomplete as the reviewer pointed out. However, because of resource limitation and financial constraints, it is not usually possible to perform full liver disease screening in Nepal. So we usually opt for limited screen, and rely more on history, physical examination and initial limited investigations. Then we perform further tests only if the initial workup hints towards another etiology. Text has been modified to include investigations and other limitations. When was she discharged? Response: She was discharged after 1 week after becoming afebrile and improvement in transaminases. Considering she was never symptomatic from the point of view of GI/hepatobiliary system, was there no further blood work to monitor LFTs since discharge? Response: LFT was repeated first at 1 week before discharge, then at 2 months follow up after discharge. Discussion: Briefly explain classifications of liver TB e.g. Reed, Alvarez, Levine. The authors do not clearly say what clinical, biochemical, radiological and histopathological presentation would be seen with disseminated TB involving liver. This case does not clearly illustrate steps to confirm a transaminitis secondary disseminated miliary TB. Response: Text has been modified to include further discussion. Thank you The implications of this case report would have greater impact and relevance for practitioners in the context of a case series or retrospective cohort and the authors should consider doing this. Response: Yes we hope further case reports and case series would be published in future, and we look forward to do case series and we have included this notion in the ms. Thank you. Further discussion is needed of considerations such as potentiated toxicity with pharmacogenomic factors e.g. slow acetylators, the need for individualized monitoring e.g. therapeutic drug monitoring. Response: Further discussion has been added. Unfortunately such therapeutic drug monitoring is not widely available in Nepal. How long should these patients have monitoring of their LFTs? Is there are risk of paradoxical reactions? Unlike Drug induced liver injury, there is no specific monitoring protocols for patients described in our case. The monitoring should be done till the deranged tests normalize and patient becomes clinically well. We have not encountered paradoxical reactions so far. We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. The title needs to convey that this is new onset transaminitis attributed to tuberculosis prior to commencement of anti tubercular therapy Response: Title has been modified. Thank you Background Please outline the baseline work up for TB in programmatic setting in Nepal. Do all patients have baseline LFTs done? Response: Programmatic setting does not require any baseline workup except sputum smear and in chest Xray. But in hospital setting, we perform baseline blood investigations like blood counts, renal function, electrolytes and liver function tests before starting treatment, so that treatment can be modified accordingly. Changes have been made in the background section. Case presentation Has this patient had normal LFTs recorded prior to current TB presentation? Response: No prior LFT testing was done by the patient Mention drug history incl. paracetamol, aflatoxin exposure, family history of liver disease. Text has been modified to state the fact. Thank you The liver screen is incomplete - e.g. autoimmune and inherited liver disease, paracetamol levels, ferritin, occult hep B. Also liver fibrosis assessment. Baseline GGT and clotting needs to be given. Please give a table of ALT, AST, GGT, Alk phos, Albumin and clotting during first 2 weeks of treatment (and any further tests during 6 months of treatment) Of note, there is no clear evidence of disseminated miliary TB on CXR. Abdominal or liver CT was not done. Hence, no comment can be made regarding liver/splenic micronodular abscesses. It appears no mycobacterial blood cultures were taken to assess for bacteraemia. Nor was a liver biopsy done. All relevant negatives and limitations should be mentioned. Response: The liver disease screen is incomplete as the reviewer pointed out. However, because of resource limitation and financial constraints, it is not usually possible to perform full liver disease screening in Nepal. So we usually opt for limited screen, and rely more on history, physical examination and initial limited investigations. Then we perform further tests only if the initial workup hints towards another etiology. Text has been modified to include investigations and other limitations. When was she discharged? Response: She was discharged after 1 week after becoming afebrile and improvement in transaminases. Considering she was never symptomatic from the point of view of GI/hepatobiliary system, was there no further blood work to monitor LFTs since discharge? Response: LFT was repeated first at 1 week before discharge, then at 2 months follow up after discharge. Discussion: Briefly explain classifications of liver TB e.g. Reed, Alvarez, Levine. The authors do not clearly say what clinical, biochemical, radiological and histopathological presentation would be seen with disseminated TB involving liver. This case does not clearly illustrate steps to confirm a transaminitis secondary disseminated miliary TB. Response: Text has been modified to include further discussion. Thank you The implications of this case report would have greater impact and relevance for practitioners in the context of a case series or retrospective cohort and the authors should consider doing this. Response: Yes we hope further case reports and case series would be published in future, and we look forward to do case series and we have included this notion in the ms. Thank you. Further discussion is needed of considerations such as potentiated toxicity with pharmacogenomic factors e.g. slow acetylators, the need for individualized monitoring e.g. therapeutic drug monitoring. Response: Further discussion has been added. Unfortunately such therapeutic drug monitoring is not widely available in Nepal. How long should these patients have monitoring of their LFTs? Is there are risk of paradoxical reactions? Unlike Drug induced liver injury, there is no specific monitoring protocols for patients described in our case. The monitoring should be done till the deranged tests normalize and patient becomes clinically well. We have not encountered paradoxical reactions so far. Competing Interests: none Close Report a concern Reply -->
- The authors describe a patient with pulmonary tuberculosis and raised liver enzymes (Transaminases). They do mention that the patient did not have any preexisting liver disease or drug use, but do not mention how they systematically ruled out other causes of tansaminitis (other non A-E viral hepatitis like GBV, Hep G, EBV,TT virus and other tropical infections).
- “In this report, we gave full dose standard antitubercular drugs, and the liver injury resolved evidenced by normalization of transaminases”. The authors should make their message more clear to “presence of transamnitis with no obvious common underlying etiology may not warrant a modification of standard antitubercular regimen”
- Evaluation of patient: Diagnostic work-up is incomplete and should also include evaluation for degree of hepatocellular injury. Prothrombin time is not mentioned, GGT levels not mentioned, Serum globulin levels not mentioned (hepatic TB has inverted albumin to globulin ration), imaging was limited (only USG performed, CT not done), liver biopsy not performed. More investigations should have been performed to rule out underlying chronic liver diseases: Fibroscan, upper GI endoscopy for Portal HTN)
- Figure 1: The quality of the image is suboptimal. The entire bony cage is not visible. Right costophrenic angle is not visible. Finding of hyperinflated lung fields and blunting of left CP angle are not described. Did the patient have underlying obstructive airway disease? If so did she also have pulmonary hypertension?. Could hepatic congestion due to RHF explain the raised liver enzymes?
- Follow up: The patient improved significantly at 1 month follow up. It would be desirable to have a complete follow up of the patient with evaluation of liver enzymes at least once during treatment as patient initially also did not have any liver specific symptoms.
- The authors argue that the patient did not have underlying liver disease or liver involvement due to tuberculosis as there was no features of Granuloma and cholestatic pattern of liver enzyme elevation. To ascribe the transamnitis to be caused by TB would be an arbitrary statement especially in the absence of liver biopsy.
- The authors mention that they have found many patients of pulmonary TB to have predominant transamnitis and without any preexisting liver disease in their experience. Such statement is not backed by any formal data.
- The authors conclude that pulmonary TB was the cause of transamnitis in their patient, not hepatic TB or underlying liver disease. In the absence of complete workup, such strong conclusions should not be made.
- Author Response 16 Oct 2020 Sudeep Adhikari , Internal Medicine, Patan Academy of Health Sciences, Lalitpur, Nepal 16 Oct 2020 Author Response We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: The title ... Continue reading We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: The title is unclear as to whether the transaminitis in the index patient is caused by tuberculosis (as proposed by the authors in the subsequent report) or a consequence of other coexisting condition. The title needs to be rephrased as to impart a message that the authors want to convey Response: Title has been modified to make our message clearer. Thank you Abstract: The authors describe a patient with pulmonary tuberculosis and raised liver enzymes (Transaminases). They do mention that the patient did not have any preexisting liver disease or drug use, but do not mention how they systematically ruled out other causes of tansaminitis (other non A-E viral hepatitis like GBV, Hep G, EBV,TT virus and other tropical infections). ○ References: DOI: 10.5812/hepatmon.188651; Alter HJ, Bradley DW. Non-A, non-B hepatitis unrelated to the hepatitis C virus (non ABC) Semin Liver Dis 1995; 15:110-1202; Current Opinion in Infectious Diseases: October 2002 - Volume 15 - Issue 5 - p 529-5343. Testing of other viruses were not done due to unavailability.Since raised transaminases resolved after starting ATT we assumed tuberculosis was most likely explanation. “In this report, we gave full dose standard antitubercular drugs, and the liver injury resolved evidenced by normalization of transaminases”. The authors should make their message more clear to “presence of transaminitis with no obvious common underlying etiology may not warrant a modification of standard antitubercular regimen” Response: Text has been modified to make our message clearer. Thank you Case report Evaluation of patient: Diagnostic work-up is incomplete and should also include evaluation for degree of hepatocellular injury. Prothrombin time is not mentioned, GGT levels not mentioned, Serum globulin levels not mentioned (hepatic TB has inverted albumin to globulin ration), imaging was limited (only USG performed, CT not done), liver biopsy not performed. More investigations should have been performed to rule out underlying chronic liver diseases: Fibroscan, upper GI endoscopy for Portal HTN) Response: The liver disease screen is incomplete as the reviewers pointed out. However, because of resource limitation and financial constraints, it is not usually possible to perform full liver disease screening in Nepal. So we usually opt for limited screen, and rely more on history, physical examination and initial limited investigations. Then we perform further tests only if the initial workup hints towards another etiology. Text has been modified to include further lab reports. The limitations have been acknowledged. Figure 1: The quality of the image is suboptimal. The entire bony cage is not visible. Right costophrenic angle is not visible. Finding of hyperinflated lung fields and blunting of left CP angle are not described. Did the patient have underlying obstructive airway disease? If so did she also have pulmonary hypertension?. Could hepatic congestion due to RHF explain the raised liver enzymes? Response: There was no history of underlying lung disease. Although echocardiography was not done hepatic congestion due to RHF is unlikely as there was no suggestive history and examination findings and patient responded without any diuretics or fluid restrictions. Follow up: The patient improved significantly at 1 month follow up. It would be desirable to have a complete follow up of the patient with evaluation of liver enzymes at least once during treatment as patient initially also did not have any liver specific symptoms. Response: Text has been modified to include follow up reports. LFT was repeated first at 1 week before discharge, then at 2 months follow up after discharge. Discussion: The authors argue that the patient did not have underlying liver disease or liver involvement due to tuberculosis as there was no features of Granuloma and cholestatic pattern of liver enzyme elevation. To ascribe the transaminitis to be caused by TB would be an arbitrary statement especially in the absence of liver biopsy. Response: Imaging and further investigations were limited, so hepatic tuberculosis could not be ruled out with certainty especially without biopsy of liver. However this would not make much difference. Because even if the diagnosis of hepatic tuberculosis had been considered in our patient, the management would be the same, i.e. with standard ATT as we did in our patient. The point we wanted to make here is that modification in treatment may not be required in the absence of pre-existing liver disease. Text has been modified to include the limitations. Thank you The authors mention that they have found many patients of pulmonary TB to have predominant transaminitis and without any preexisting liver disease in their experience. Such statement is not backed by any formal data. Response: We need more data but unfortunately not much is being published from low middle income countries even for endemic diseases like tuberculosis. There is no formal data to back up our claim, and this has been acknowledged as limitation. The authors conclude that pulmonary TB was the cause of transaminitis in their patient, not hepatic TB or underlying liver disease. In the absence of complete workup, such strong conclusions should not be made. Response: Due to rapid resolution of raised transaminases after starting ATT, preexisting liver disease would be unlikely cause. Although hepatic tb is a possibility, it was our opinion that standard dose ATT can be safely started in the patient and further imaging would not be cost effective in terms of treatment. We agree with the reviewer that the strong conclusions are not justified due to incomplete workup and this section has been toned down and modified to highlight the limitations. Thank you Conclusion The authors recommend use of full dose standard ATT for patients with transaminitis and no underlying liver disease. This recommendation should not be made based on a single case report. Response: We agree. Text have been modified to include our limitations. Thank you. Opinion: The case report describes a common scenario especially in low income countries while treating patients with tuberculosis. The availability of resources limit the diagnostic workup of such patients in our settings. I opine that this case report is suitable for indexing with modifications. Response: Thank you We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: The title is unclear as to whether the transaminitis in the index patient is caused by tuberculosis (as proposed by the authors in the subsequent report) or a consequence of other coexisting condition. The title needs to be rephrased as to impart a message that the authors want to convey Response: Title has been modified to make our message clearer. Thank you Abstract: The authors describe a patient with pulmonary tuberculosis and raised liver enzymes (Transaminases). They do mention that the patient did not have any preexisting liver disease or drug use, but do not mention how they systematically ruled out other causes of tansaminitis (other non A-E viral hepatitis like GBV, Hep G, EBV,TT virus and other tropical infections). ○ References: DOI: 10.5812/hepatmon.188651; Alter HJ, Bradley DW. Non-A, non-B hepatitis unrelated to the hepatitis C virus (non ABC) Semin Liver Dis 1995; 15:110-1202; Current Opinion in Infectious Diseases: October 2002 - Volume 15 - Issue 5 - p 529-5343. Testing of other viruses were not done due to unavailability.Since raised transaminases resolved after starting ATT we assumed tuberculosis was most likely explanation. “In this report, we gave full dose standard antitubercular drugs, and the liver injury resolved evidenced by normalization of transaminases”. The authors should make their message more clear to “presence of transaminitis with no obvious common underlying etiology may not warrant a modification of standard antitubercular regimen” Response: Text has been modified to make our message clearer. Thank you Case report Evaluation of patient: Diagnostic work-up is incomplete and should also include evaluation for degree of hepatocellular injury. Prothrombin time is not mentioned, GGT levels not mentioned, Serum globulin levels not mentioned (hepatic TB has inverted albumin to globulin ration), imaging was limited (only USG performed, CT not done), liver biopsy not performed. More investigations should have been performed to rule out underlying chronic liver diseases: Fibroscan, upper GI endoscopy for Portal HTN) Response: The liver disease screen is incomplete as the reviewers pointed out. However, because of resource limitation and financial constraints, it is not usually possible to perform full liver disease screening in Nepal. So we usually opt for limited screen, and rely more on history, physical examination and initial limited investigations. Then we perform further tests only if the initial workup hints towards another etiology. Text has been modified to include further lab reports. The limitations have been acknowledged. Figure 1: The quality of the image is suboptimal. The entire bony cage is not visible. Right costophrenic angle is not visible. Finding of hyperinflated lung fields and blunting of left CP angle are not described. Did the patient have underlying obstructive airway disease? If so did she also have pulmonary hypertension?. Could hepatic congestion due to RHF explain the raised liver enzymes? Response: There was no history of underlying lung disease. Although echocardiography was not done hepatic congestion due to RHF is unlikely as there was no suggestive history and examination findings and patient responded without any diuretics or fluid restrictions. Follow up: The patient improved significantly at 1 month follow up. It would be desirable to have a complete follow up of the patient with evaluation of liver enzymes at least once during treatment as patient initially also did not have any liver specific symptoms. Response: Text has been modified to include follow up reports. LFT was repeated first at 1 week before discharge, then at 2 months follow up after discharge. Discussion: The authors argue that the patient did not have underlying liver disease or liver involvement due to tuberculosis as there was no features of Granuloma and cholestatic pattern of liver enzyme elevation. To ascribe the transaminitis to be caused by TB would be an arbitrary statement especially in the absence of liver biopsy. Response: Imaging and further investigations were limited, so hepatic tuberculosis could not be ruled out with certainty especially without biopsy of liver. However this would not make much difference. Because even if the diagnosis of hepatic tuberculosis had been considered in our patient, the management would be the same, i.e. with standard ATT as we did in our patient. The point we wanted to make here is that modification in treatment may not be required in the absence of pre-existing liver disease. Text has been modified to include the limitations. Thank you The authors mention that they have found many patients of pulmonary TB to have predominant transaminitis and without any preexisting liver disease in their experience. Such statement is not backed by any formal data. Response: We need more data but unfortunately not much is being published from low middle income countries even for endemic diseases like tuberculosis. There is no formal data to back up our claim, and this has been acknowledged as limitation. The authors conclude that pulmonary TB was the cause of transaminitis in their patient, not hepatic TB or underlying liver disease. In the absence of complete workup, such strong conclusions should not be made. Response: Due to rapid resolution of raised transaminases after starting ATT, preexisting liver disease would be unlikely cause. Although hepatic tb is a possibility, it was our opinion that standard dose ATT can be safely started in the patient and further imaging would not be cost effective in terms of treatment. We agree with the reviewer that the strong conclusions are not justified due to incomplete workup and this section has been toned down and modified to highlight the limitations. Thank you Conclusion The authors recommend use of full dose standard ATT for patients with transaminitis and no underlying liver disease. This recommendation should not be made based on a single case report. Response: We agree. Text have been modified to include our limitations. Thank you. Opinion: The case report describes a common scenario especially in low income countries while treating patients with tuberculosis. The availability of resources limit the diagnostic workup of such patients in our settings. I opine that this case report is suitable for indexing with modifications. Response: Thank you Competing Interests: none Close Report a concern Reply -->
- Author Response 16 Oct 2020 Sudeep Adhikari , Internal Medicine, Patan Academy of Health Sciences, Lalitpur, Nepal 16 Oct 2020 Author Response We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: ... Continue reading We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: Drug-resistant TB is a hot topic in medicine today. Research on TB treatment-associated transaminitis would further the existing scholarly understanding on drug-resistant TB. A close keyword search on PubMed revealed only 17 hit on TB AND transaminitis. For this reason, the case report is potentially publishable but suffers from several drawbacks in its current draft that must be remedied before the contribution is re-refereed. But the title itself is unclear. “With” is repeated twice. It is not clear to a medical historian what transaminitis is. A better framing of the title is urgently needed. A cogent argument can be organised around the title. What is four-drugs therapy? It is clear to medical practitioners but not clear to the larger scholarly community. Avoid jargons in the title. 1) What is transaminitis? Why not provide a brief explanation at the start so that the general reader can follow the rest of the article. 2) The two levels of transaminitis: (a) pre-existing liver disease leads to transaminitis. (b) Hepatoxicity of anti-TB drugs. This needs to be made explicit in the very beginning. The authors have not been explicit about the two levels of transaminitis and that is where the problem begins. “While encountering such patients, it is important to differentiate if the patient had pre-existing liver disease or if the present infection with tuberculosis has impacted on the liver, as the approach to management differs given the hepatotoxicity associated with first line drugs.” Rewrite this sentence. Make more explicit. Response: Title and text have been modified to make our message clearer. Thank you Summary and Abstract: The writers present a case of pulmonary TB with transaminitis without pre-existing liver damage. The therapeutic regimen of the authors included anti-TB drugs and liver injury resolved evidenced by normalization of transaminase. The abstract merits rewriting for clarity. Response: Abstract has been modified to make our message clearer Background: Needs to sketch out the larger socio-economic picture of TB patients in Nepal. Medicine for whom? Response: Background has been modified to make it clear. The socioeconomic picture of TB patients in Nepal has been highlighted. In Nepal, tuberculosis prevalence is more in productive age group (25-64 years) and men. Poverty, malnutrition, overcrowding, immunocompromised state like HIV infection, alcohol, smoking, air pollution, diabetes and other comorbidities are important risk factors for acquiring the disease. All patients diagnosed with TB receive treatment as per the national protocol which has been mentioned in the text. Case Presentation: How do you define compliance with TB treatment? How socio-economic factors militate against the successful completion of treatment? Response: To improve the treatment compliance, treatment of TB is done under DOTS program all over Nepal, which stands for ‘Directly Observed Treatment Short Course’. Otherwise the compliance would be compromised owing to the lower socioeconomic status of patients, longer duration of therapy and side effects of drugs. Specific quote from the report: “In our anecdotal experience, we have found many patients with pulmonary tuberculosis, similarly to subject of this case report, present with predominant transaminitis and without pre-existing liver disease or drugs-use. They are often managed with modified liver-friendly antitubercular regimens for fear of increasing the hepatotoxicity and causing acute liver failure with the use of standard regimen. Few case reports are available in literature reporting the use of the modified regimens. We believe such cases are underreported, and firm guidelines have not been established to guide clinicians in these cases. Given this, many clinicians in low-middle income countries, including Nepal, who have been treating tuberculosis patients tend to be skeptical in using full doses of first line ATT in such patients and tend to use a modified regimen. However, this practice may potentially lead to undertreatment and therefore increase fatality9. Though there was some hesitation at first in our case, we soon started treatment with the standard ATT in our patient with close monitoring. This we believe led to the resolution of liver injury, evidenced by the normalization of transaminases.” What is your sample size? Unclear. What guidelines can be established with the aid of the study?" Make explicit and elaborate Response: Our opinion is that there are cases of pulmonary tb with some hepatic involvement, which are sometimes being managed with modified regimen when it can be safely managed with standard regimen. Unfortunately very few published research is available from low middle income countries. Text has been edited to include limitation of evidence. This is a case report only and further research is needed. Conclusion: The report lacks an inevitable conclusion. The conclusion needs to point to the “so what” question? So, what are the implications of this study? What protocols could be devised? How does this case history further medical practitioners’ as well as policymakers’ understanding of drug-resistant TB? Response: Being a case report and paucity of previous research, we have toned down our previous strong conclusions, also as suggested above by another reviewer. We hope that further reports will be published. The use of modified drug regimen excluding more potent drugs, instead of standard regimen may promote drug resistance, and how far such practices are prevalent can be another area of further study. Other points: Please make sure that the manuscript is thoroughly copyedited for legibility of prose, clarity of argument, and grammar. The social context of TB in Nepal merits attention (alcoholism is a contributing factor). You need to compare transaminitis with case studies from other countries. Carefully refer to the PLoS Response: Text has been revised to correct errors. Social context of TB in Nepal has been mentioned in the text. Thank you Murphy, Richard A., Vincent C. Marconi, Rajesh T. Gandhi, Daniel R. Kuritzkes, and Henry Sunpath. "Coadministration of lopinavir/ritonavir and rifampicin in HIV and tuberculosis co-infected adults in South Africa." PLoS One 7, no. 9 (2012): e447931. Sarda, Pawan, S. K. Sharma, Alladi Mohan, Govind Makharia, Arvind Jayaswal, R. M. Pandey, and Sarman Singh. "Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity." Indian Journal of Medical Research 129, no. 1 (2009): 642. How does your case differ from the 2 references mentioned above? Response: As compared to first article our patient did not have HIV. As compared to second article, our patient did not have antituberculous drug induced hepatotoxicity but liver injury likely due to tuberculosis itself. Thank you We would like to whole-heartedly thank you for spending so much time and effort to improve our manuscript. Here below is the point by point response. Title: Drug-resistant TB is a hot topic in medicine today. Research on TB treatment-associated transaminitis would further the existing scholarly understanding on drug-resistant TB. A close keyword search on PubMed revealed only 17 hit on TB AND transaminitis. For this reason, the case report is potentially publishable but suffers from several drawbacks in its current draft that must be remedied before the contribution is re-refereed. But the title itself is unclear. “With” is repeated twice. It is not clear to a medical historian what transaminitis is. A better framing of the title is urgently needed. A cogent argument can be organised around the title. What is four-drugs therapy? It is clear to medical practitioners but not clear to the larger scholarly community. Avoid jargons in the title. 1) What is transaminitis? Why not provide a brief explanation at the start so that the general reader can follow the rest of the article. 2) The two levels of transaminitis: (a) pre-existing liver disease leads to transaminitis. (b) Hepatoxicity of anti-TB drugs. This needs to be made explicit in the very beginning. The authors have not been explicit about the two levels of transaminitis and that is where the problem begins. “While encountering such patients, it is important to differentiate if the patient had pre-existing liver disease or if the present infection with tuberculosis has impacted on the liver, as the approach to management differs given the hepatotoxicity associated with first line drugs.” Rewrite this sentence. Make more explicit. Response: Title and text have been modified to make our message clearer. Thank you Summary and Abstract: The writers present a case of pulmonary TB with transaminitis without pre-existing liver damage. The therapeutic regimen of the authors included anti-TB drugs and liver injury resolved evidenced by normalization of transaminase. The abstract merits rewriting for clarity. Response: Abstract has been modified to make our message clearer Background: Needs to sketch out the larger socio-economic picture of TB patients in Nepal. Medicine for whom? Response: Background has been modified to make it clear. The socioeconomic picture of TB patients in Nepal has been highlighted. In Nepal, tuberculosis prevalence is more in productive age group (25-64 years) and men. Poverty, malnutrition, overcrowding, immunocompromised state like HIV infection, alcohol, smoking, air pollution, diabetes and other comorbidities are important risk factors for acquiring the disease. All patients diagnosed with TB receive treatment as per the national protocol which has been mentioned in the text. Case Presentation: How do you define compliance with TB treatment? How socio-economic factors militate against the successful completion of treatment? Response: To improve the treatment compliance, treatment of TB is done under DOTS program all over Nepal, which stands for ‘Directly Observed Treatment Short Course’. Otherwise the compliance would be compromised owing to the lower socioeconomic status of patients, longer duration of therapy and side effects of drugs. Specific quote from the report: “In our anecdotal experience, we have found many patients with pulmonary tuberculosis, similarly to subject of this case report, present with predominant transaminitis and without pre-existing liver disease or drugs-use. They are often managed with modified liver-friendly antitubercular regimens for fear of increasing the hepatotoxicity and causing acute liver failure with the use of standard regimen. Few case reports are available in literature reporting the use of the modified regimens. We believe such cases are underreported, and firm guidelines have not been established to guide clinicians in these cases. Given this, many clinicians in low-middle income countries, including Nepal, who have been treating tuberculosis patients tend to be skeptical in using full doses of first line ATT in such patients and tend to use a modified regimen. However, this practice may potentially lead to undertreatment and therefore increase fatality9. Though there was some hesitation at first in our case, we soon started treatment with the standard ATT in our patient with close monitoring. This we believe led to the resolution of liver injury, evidenced by the normalization of transaminases.” What is your sample size? Unclear. What guidelines can be established with the aid of the study?" Make explicit and elaborate Response: Our opinion is that there are cases of pulmonary tb with some hepatic involvement, which are sometimes being managed with modified regimen when it can be safely managed with standard regimen. Unfortunately very few published research is available from low middle income countries. Text has been edited to include limitation of evidence. This is a case report only and further research is needed. Conclusion: The report lacks an inevitable conclusion. The conclusion needs to point to the “so what” question? So, what are the implications of this study? What protocols could be devised? How does this case history further medical practitioners’ as well as policymakers’ understanding of drug-resistant TB? Response: Being a case report and paucity of previous research, we have toned down our previous strong conclusions, also as suggested above by another reviewer. We hope that further reports will be published. The use of modified drug regimen excluding more potent drugs, instead of standard regimen may promote drug resistance, and how far such practices are prevalent can be another area of further study. Other points: Please make sure that the manuscript is thoroughly copyedited for legibility of prose, clarity of argument, and grammar. The social context of TB in Nepal merits attention (alcoholism is a contributing factor). You need to compare transaminitis with case studies from other countries. Carefully refer to the PLoS Response: Text has been revised to correct errors. Social context of TB in Nepal has been mentioned in the text. Thank you Murphy, Richard A., Vincent C. Marconi, Rajesh T. Gandhi, Daniel R. Kuritzkes, and Henry Sunpath. "Coadministration of lopinavir/ritonavir and rifampicin in HIV and tuberculosis co-infected adults in South Africa." PLoS One 7, no. 9 (2012): e447931. Sarda, Pawan, S. K. Sharma, Alladi Mohan, Govind Makharia, Arvind Jayaswal, R. M. Pandey, and Sarman Singh. "Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity." Indian Journal of Medical Research 129, no. 1 (2009): 642. How does your case differ from the 2 references mentioned above? Response: As compared to first article our patient did not have HIV. As compared to second article, our patient did not have antituberculous drug induced hepatotoxicity but liver injury likely due to tuberculosis itself. Thank you Competing Interests: none Close Report a concern Reply -->
Reviewer Status
Alongside their report, reviewers assign a status to the article:
Reviewer Reports
- Vivek Neelakantan , Independent Medical Historian, Mumbai, India
- Prajowl Shrestha , Bir Hospital, Kathmandu, Nepal Ashesh Dhungana , Bir Hospital, Kathmandu, Nepal
- Neesha Rockwood , University of Cape Town, London, UK; University of Cape Town, Cape Town, South Africa; University of Colombo, Colombu, Sri Lanka
Comments on this article
All Comments (0)
Competing Interests Policy
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
- Within the past 4 years, you have held joint grants, published or collaborated with any of the authors of the selected paper.
- You have a close personal relationship (e.g. parent, spouse, sibling, or domestic partner) with any of the authors.
- You are a close professional associate of any of the authors (e.g. scientific mentor, recent student).
- You work at the same institute as any of the authors.
- You hope/expect to benefit (e.g. favour or employment) as a result of your submission.
- You are an Editor for the journal in which the article is published.
- You expect to receive, or in the past 4 years have received, any of the following from any commercial organisation that may gain financially from your submission: a salary, fees, funding, reimbursements.
- You expect to receive, or in the past 4 years have received, shared grant support or other funding with any of the authors.
- You hold, or are currently applying for, any patents or significant stocks/shares relating to the subject matter of the paper you are commenting on.
Stay Updated
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Wellcome Open Research
Already registered? Sign in
Not now, thanks
Are you a Wellcome-funded researcher?
If you are a previous or current Wellcome grant holder, sign up for information about developments, publishing and publications from Wellcome Open Research.
We'll keep you updated on any major new updates to Wellcome Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here .
If you still need help with your Google account password, please click here .
You registered with F1000 via Facebook, so we cannot reset your password.
If you still need help with your Facebook account password, please click here .
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
- Research article
- Open access
- Published: 17 January 2019
Bacteriologically confirmed extra pulmonary tuberculosis and treatment outcome of patients consulted and treated under program conditions in the littoral region of Cameroon
- Teyim Pride Mbuh ORCID: orcid.org/0000-0002-0593-6831 1 , 3 ,
- Irene Ane-Anyangwe 1 ,
- Wandji Adeline 3 ,
- Benjamin D. Thumamo Pokam 2 ,
- Henry Dilonga Meriki 1 &
- Wilfred Fon Mbacham 4
BMC Pulmonary Medicine volume 19 , Article number: 17 ( 2019 ) Cite this article
7777 Accesses
22 Citations
1 Altmetric
Metrics details
Extra-pulmonary tuberculosis (EPTB) is defined as any bacteriologically confirmed or clinically diagnosed case of TB involving organs other than the lungs. It is frequently a diagnostic and therapeutic challenge with paucity of data available. The aim of this study was to assess the prevalence of bacteriologically confirmed EPTB; to determine the most affected organs and to evaluate the therapeutic outcome of EPTB patients treated under program conditions in the littoral region of Cameroon.
A descriptive cross-sectional laboratory-based epidemiological survey was conducted from January 2016 to December 2017 and 109 specimens from 15 of the 39 diagnosis and treatment centers in the littoral region were obtained.
Two diagnostic methods (Gene Xpert MTB and culture (LJ and MGIT) were used for EPTB diagnosis. Determine HIV1/2 and SD Biolinewere used for HIV diagnosis. Confirmed EPTB cases were treated following the national tuberculosis guide.
The prevalence of bacteriologically confirmed EPTB was 41.3% (45). All 45 cases were sensitive to rifampicin. Males were predominately more infected [26 (57.8%)] likewise the age group 31–45 years with 15 (33.3%) cases. The overall prevalence for HIV was 33.6% (36). HIV infection was present in 28.9% (13) of patients with EPTB. The most affected sites with EPTB were: Lymph nodes (66.5%), pleural cavity (15.6%), abdominal organs (11.1%), neuromeningeal (2.2%), joints (2.2%) and heart (2.2%). Overall, 84.4% of the study participants had a therapeutic success with males responding better 57.9% ( p = 0.442). Therapeutic success was better (71.7%) in HIV negative EPTB patients ( p = 0.787).
The prevalence of bacteriologically confirmed EPTB patients treated under program conditions in the littoral region of Cameroon is high with a therapeutic success of 84.4% and the lymph nodes is the most affected site.
Peer Review reports
Tuberculosis (TB) is a leading cause of morbidity and mortality worldwide, accounting for about 9.6 million new cases and 1.5 million deaths annually [ 1 ].
Pulmonary TB represents about 70% of all cases of TB and is the most contagious form TB and remains the main target for TB control [ 2 , 3 ]. Extra-pulmonary tuberculosis (EPTB) defined as any bacteriologically confirmed or clinically diagnosed case of TB involving organs other than the lungs. The description of a form of EPTB is a function of its location affecting the pleura, lymph nodes, abdomen, genitourinary tract, skin, joints and bones, meninges.
EPTB represents 15 to 30% of all forms of tuberculosis [ 4 , 5 ], and is frequently a diagnostic and therapeutic challenge. It is a common opportunistic infection in people living with HIV/AIDS and other immunocompromised states [ 6 ]. The immune suppression status of these persons results in dissemination of the bacteria form the lungs to other organs. There is a paucity of data on bacteriological diagnosis and therapeutic evaluation on EPTB in Cameroon.
The objective of this study was to assess the prevalence of bacteriologically confirmed EPTB; to determine the most affected organs and to evaluate the therapeutic outcome of EPTB patients treated under program conditions in the littoral region of Cameroon.
We conducted a descriptive cross-sectional hospital-based epidemiological survey from January 2016 to December 2017. A non-probabilistic accidental sampling method was used to obtain 145 non sputum specimens and 145 venous blood in dry tubes from 80 male and 65 female participants suspected for EPTB and consulting 15 of the 39 diagnostic and treatment centers (DTCs) in the Littoral region of Cameroon. The littoral region was chosen because it harbours about 20% of all TB diagnosed cases in Cameroon and also because it is the economic head quarter of Cameroon and Douala the regional head quarter is the largest city in Cameroon with a population of 1,338,082 people. It harbors many referral diagnostic and treatment institutions that pulls many persons from the regions in search for better health service. A total of 36 participants were rejected due to incomplete request forms. 109 (57 male and 52 female) participants were finally retained and analyzed for this study. Verbal Consent of participant was sort during clinical consultation. For participants younger than 15 years; consent was obtained from parent/legal representative. Authorizations for this study were obtained from the permanent secretary for national tuberculosis control program and ethical clearance was obtained from the University of Douala’s ethical review board.
EPTB specimens (plural fluid, cerebrospinal fluid, synovial fluid, ascites and lymph node aspirate) obtained from participants were aliquoted and analyzed on Gene Xpert MTB/RIF. This system integrates and automates sample processing, nucleic acid amplification, and detection of the target sequences. The primers in the XpertMTB/RIF assay amplify a portion of the rpoB gene containing the 81 base pair “core” region. The probes are able to differentiate between the conserved wild-type sequence and mutations in the core region that are associated with rifampicin resistance. The aliquoted specimen was decontaminated using 3% NaOH and the pellet inoculated on MGIT and grown in BACTEC 960 for 42 days. Positive MGIT samples were further subjected to Gene Xpert MTB/RIF for confirmation and Rifampicin drug susceptibility testing. A positive diagnosis for EPTB was declared when direct Gene XpertMTB/RIF and or culture results were positive.
Bacteriologically confirmed EPTB cases were treated following the national tuberculosis guide which recommended that new cases should be treated for 6 months consisting of 2 months of daily rifampicin (R), isoniazide (H), pyrazinamide (Z) and ethambutol (E) (intensive phase), followed by 4 months of daily RH (2RHZE/4RH). Retreatment cases [relapses, treatment failure cases, defaulters) should be treated for 2 month with RHZE and streptomycin (S), and a third month without S (intensive phase), followed by 5 months of RHE daily (2RHZES/1RHZE/5RHE). At the end of treatment, treatment outcome was categorized as cured, completed treatment, lost to follow up, failure and died based on the standard criteria [ 7 ].
HIV screening was done by testing serum from coagulated blood collected in dry tubes on Determine HIV 1/2 strip test . Positive samples were confirmed on rapid SD BiolineHIV 1/2 3.0 cassettes.
Data was analyzed using SPSS version 16.0 software (Statistical Package for Social Science). Percentage accuracy, sensitivity, specitivity, positive predictive values, and negative predictive values were manually calculated as follows:
(%) Accuracy = number of correct results / total number of results X 100,
(%) Sensitivity = number of true positive results/ number of true-positive plus false-negative results X 100,
(%) Specificity = number of true negative results/ number of true-negative plus false positive results X 100.
(%) Positive Predictive Value = number of true-positive results/ number of true-positive plus false-positive results X 100.
(%) Negative Predictive Value = number of true negative results / number of true-negative results plus false negative results X 100; and statistically significant was set at p < 0.05.
Table 1 shows the socio-demographic characteristics of 109 study participants. The median age of study participant was 40.0 ± 19.23 years with females being relatively younger (35.5 ± 20.0 years) than their male counterparts 45.0 ± 18.3 years). Thirty six (33.6%) of the study population were infected with HIV-1. The prevalence of bacteriologically confirmed EPTB in the study was 45 (41.3%), and all the subjects were sensitive to rifampicin. Of the 45 cases, a male predominance [26 (57.8%)] was observed ( P = 0.336). The EPTB/HIV co-infection rate was 13 (28.9%). The most affected age group was that of 35–51 years with 15 (33.3%) cases recorded followed by that of 18–34 years with 14 (31.1%). Those who had no treatment history for TB represented 66.6% of the study population as illustrated on Table 2 .
The most affected site were the lymph nodes 31/109 (68.9%) and there was a statistically significant different between the positivity of lymph node aspirates and all other EPTB specimens tested [ p = 0.001, CI 55.4–82.4, OR=] this was closely followed by the pleural cavity 7 (15.6%) as illustrated on Fig. 1 . Based on both diagnostic methods used in our study, the overall prevalence of extra pulmonary tuberculosis was 45 (41.3%). There was a positive discordance in 7 cases, and negative discordance in 4 cases as illustrated in Table 3 .
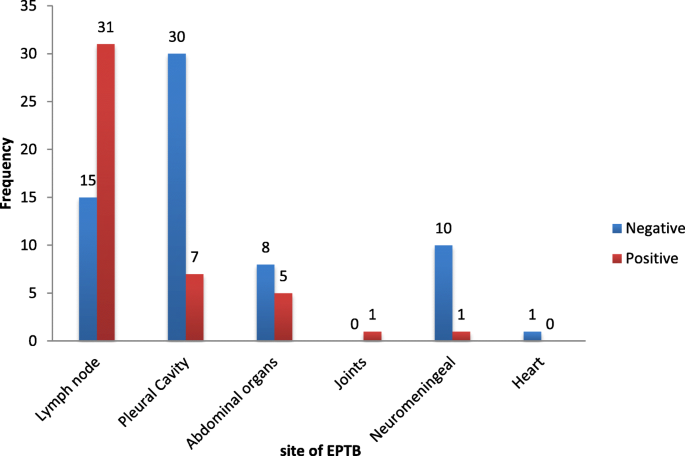
Prevalence of extra pulmonary tuberculosis according to the organ sites affected
By observing the treatment outcome of the studied participants, we found that 38 (84.4%) cases have completed the treatment course. Five (11.1%) cases died before completing the course and were all EPTB/HIV co-infected. Two (4.2%) cases were loss to follow up and no defaulter or relapse cases were recorded.
Overall, 84.4% of these participants had a therapeutic success with males responding better 22 (57.9%) as oppose to females16 (42.1%) ( p = 0.442). Therapeutic success [27(71.7%)] was better in HIV negative EPTB patients compared to HIV positive [11(28.1)] patients ( p = 0.787). With respect to site, treatment completion rate was observed as follows: lymph node 25 (83.3%), pleural effusion 7 (100%), abdominal organs 4(80%), joints 1(100%), neuromeningeal 1(100%) and the heart 0(0%) as shown on Table 4 .
Discussion and conclusion
This study was aimed at determining the prevalence of bacteriologically confirmed EPTB; to determine the most affected organs and to evaluate the treatment outcome of EPTB patients. Though the sampling method was non-probabilistic (not give all those visiting the study sites equal chances of participating in our study), we still thing these findings are of epidemiological importance.
The prevalence of bacteriologically confirm EPTB in this study was high (41.3%). This finding is similar to those of Luma [ 8 ], were they found a prevalence of EPTB to be 42.9% amongst patients registered for anti-TB treatment in the general hospital of Douala in the same country. This was however higher than the 23.2% reported by Yone et al., [ 9 ] in Yaoundé-Cameroon. This high prevalence of EPTB reported in our study could be partially justified by the elevated (33.6%) prevalence of HIV in our study participant. HIV has be known to facilitates the dissemination of Mycobacterium tuberculosis out of the lungs and the reactivation of infection in extra-pulmonary organs [ 10 ]. Bacteriological confirmation of EPTB is a very challenging issue in most national tuberculosis programs because of the pauci-bacillary nature of the disease, the apportioning of the sample for various diagnostic tests resulting in non-uniform distribution of microorganisms, the difficulty to obtain an adequate sample, the variable clinical presentation, and need for invasive procedures to secure appropriate sample [ 11 ], lack of laboratory facilities in the resource-limited settings and the lack of an efficient sample processing technique universally applicable on all types of extra-pulmonary samples [ 12 ]. All these limitations cause poor contribution of bacteriological techniques in the establishment of EPTB diagnosis [ 13 ].The male gender was predominantly more represented than the female gender. Those aged from 35 to 51 years old were also the most infected. These findings are similar to those of Kiran [ 14 ] in Morocco who found a significant male predilection (59.3%). The relative higher incidence in males could be attributed to more exposure of the male gender to the external environment for their jobs especially as they are the principal bread winners in resource limited counties.
We found that lymph node involvement was the most common site of infection. This was different from recent studies [ 8 , 15 ] where they found that the pleural and bones/joints were the most common site affected respectively. Our findings where however in line with other studies [ 15 , 16 , 17 ], who also reported lymph node involvement as the most affected EPTB site. This findings could be partially justified by the fact that we worked with several health facilities contrary to the Luma’s study who worked in a single health facility [ 8 ].
Even though culture is considered the gold standard in TB diagnostics, growth on solid culture media requires four to six weeks. This delay would negatively affect patient care. To overcome this problem, we opted for automated cartridge-based molecular nucleic acid amplification (NAA) techniques, which offer a rapid diagnosis of life-threatening disease such as TB meningitis with a turnaround time of 24 h. This method is said to be very sensitive as it can detect as few as 10 mycobacteria [ 13 ]. There was a positive discordance in 7 cases, and negative discordance in 4 cases with MGIT cultures. Other studies [ 12 , 14 , 18 ], however report very varying sensitivity and specificity of molecular nucleic acid amplification techniques in comparison with culture.
EPTB treatment success rate in this study was high and identical to the nationwide pulmonary TB therapeutic success rate of 75 to 84% reported in 2006 to 2015 [ 19 , 20 ]. This could be attributed to the fact that we assigned a staff to call our patients very regularly to ensure that they all complied to their treatment even when they no longer felt sick; thought this is not always feasible in real life setting. This finding was in line with the stop TB strategy united nations millennium development goals to cure at least 85% of sputum smear-positive TB patients [ 21 ].
This study established that treatment success of EPTB patients co-infected with HIV was lower (28.1%) compared to TB-HIV negative patients (71.7%) and this is in line with Atekem, et al [ 22 ] in the South West region of Cameroon, but contrary to that of Mekonnenet al. [ 23 ] in North Eastern Ethiopia. Therapeutic success in this study was relatively high because we assigned a staff to call our patients very regularly to ensure that they all complied to their treatment even when they no longer felt sick. All the patients who died in this study were HIV positively co-infected, but the numbers were too small for a proper analysis. We thing that these patients may not have been compliant to their treatment and turned to neglect the regimen as they thought having HIV is the end of life. Furthermore, a weaken immune system may justify the death cases recorded in these HIV positive cases.There was no significant different between males and females and across the different age groups.
Abbreviations
Confidence interval
Extra-pulmonary tuberculosis
Human immune deficiency virus
Lowenstein Jensen media
Minimum Growth Inhibition Tube
Mycobacterium tuberculosis / rifampicin
Negative predictive value
Positive predictive value
Tuberculosis
Raviglione M, Sulis G. Tuberculosis 2015: burden, challenges and strategy for control and elimination. Infect Dis Rep. 2016;8(2):33–7.
Article Google Scholar
WHO. Global tuberculosis report. 2015;20:1–4. http://www.who.int/iris/handle/10665/191102 .
WHO. Tuberculosis annual report. In 2013. p. 1–37.
Ramirez-Lapausa M, Menendez-Saldana A. AN-A. Extrapulmonary tuberculosis : an overview. Rev Esp Sanid Penit. 2015;17:3–11.
Article CAS PubMed Central Google Scholar
Pollett S, Banner P, O’Sullivan MVN, Ralph AP. Epidemiology, diagnosis and management of extra-pulmonary tuberculosis in a low-prevalence country: a four year retrospective study in an Australian tertiary infectious diseases unit. PLoS One. 2016;11(3).
WHO. Global tuberculosis report. France; 2017. p. 63–97.
NTP-Cameroon. National Tuberculosis Strategic Plan 2010–2014 [Internet]. Yaounde; 2014. p. 9. Available from: http://www.nationalplanningcycles.org/sites/default/files/planning_cycle_repository/cameroon/nationaltuberculosisstrategicplan2010.pdf .
Namme LH, Marie-Solange D, Bertrand MN, Elvis T, Achu JH, Christopher K. Extrapulmonary tuberculosis and HIV coinfection in patients treated for tuberculosis at the Douala general hospital in Cameroon year. Ann Trop Med public Heal. 2013;06(01):100–4.
Yone EW, Kengne AP, Moifo B, Kuaban C. Prevalence and determinants of extrapulmonary involvement in patients with pulmonary tuberculosis in a Sub-Saharan African country: A cross-sectional study. Scand J Infect Dis. 2013;45(2):104–11.
Article PubMed Central Google Scholar
Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer FBP. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7.
Purohit M, Mustafa T. Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource-constrained setting: State of the art, challenges and the need. J Clin Diagnostic Res. 2015;9(4):1–6.
Manjunath N, Shankar P, Rajan L, Bhargava A, Saluja SS. Evaluation of a polymerase chain reaction for the diagnosis of tuberculosis. Tubercle. 1991;72(1):21–7.
Maurya AK, Kant S, Nag VL, Kushwaha RADT. Trends of anti-tuberculosis drug resistance pattern in new cases and previously treated cases of extrapulmonary tuberculosis cases in referral hospitals in northern India. J Postgr Med. 2012;58(3):185–9.
Article CAS Google Scholar
Chawla K, Johar R, Shashidhar Vishwanath CM. Role of PCR in the diagnosis of pulmonary and extra-pulmonary tuberculosis. National Journal of laboratory medicine. Natl J Lab Med. 2015;4(4):43–6.
CAS Google Scholar
Skoura E, Zumla A, Bomanji J. Imaging in extrapulmonary tuberculosis. Int J Infect Dis [Internet] 2015;32:87–93. Available from: https://doi.org/10.1016/j.ijid.2014.12.007
Al-Otaibi F, Hazmi MM EI. Extra-pulmonary tuberculosis in Saudi Arabia. Indian J Pathol Microbiol. 2010;53(2):227–31.
Maji A, Misra R, Mondal AK, Kumar D, Bajaj D, Singhal A, et al. Expression profiling of lymph nodes in tuberculosis patients reveal inflammatory milieu at site of infection. Sci Rep. 2015;5(April):1–10. Available from: https://doi.org/10.1038/srep15214
Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai MSK. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44:435–46.
Noeske J, Yakam AN, Foe JA. Epid emiologie de la tuberculose au Cameroun telle qu ’ elle est ´ ee ´ dans les donn ees ´ de noti fi cation , 2006 – 2014 re fl et. Int J Tuberc Lung Dis. 2016;20(11):1489–94.
CDC. C D C division of global HIV & TB Cameroon country profile strategic focus. 2018. p. 2018.
WHO. The Stop TB Strategy: Building on and enhancing DOTS to meet the TB-related Millennium Development Goals. 2006.
Atekem KA, Tanih NF, Ndip RNNL. Evaluation of the tuberculosis control program in south West Cameroon: factors affecting treatment outcomes. Int J Mycobacteriol. 2018;7:137–42.
Mekonnen D, Derbie ADE. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes. 2015;8:666.
Download references
Acknowledgements
Special thanks to the permanent secretary of the National Tuberculosis Program in Cameroon who permitted us to work in 15 of the 39 diagnostic and treatment centers in the Littoral region.
We also wish to thank all the staffs of the 15 diagnostic and treatment centers who assisted us with specimen collection drug administration and treatment monitoring.
No funding was obtained for this study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
Department of Microbiology and Parasitology, Faculty of Science, University of Buea, Buea, Cameroon
Teyim Pride Mbuh, Irene Ane-Anyangwe & Henry Dilonga Meriki
Department of Medical Laboratory Science, Faculty Health Sciences, University of Buea, Buea, Cameroon
Benjamin D. Thumamo Pokam
Tuberculosis Reference Laboratory, Douala, Littoral Region, Cameroon
Teyim Pride Mbuh & Wandji Adeline
Laboratory for Public Health Research Biotechnologies, Biotechnology Centre, University of Yaoundé, Yaoundé, Cameroon
Wilfred Fon Mbacham
You can also search for this author in PubMed Google Scholar
Contributions
TPM, IAA, WA and WFM participated in conception and design of the study. TPM collected the data. TPM, BTB and HDM analysis and interpretation of the data and also drafted the article. All authors revised and approved the final version.
Corresponding author
Correspondence to Teyim Pride Mbuh .
Ethics declarations
Ethics approval and consent to participate.
Verbal Consent of participant was sort during clinical consultation and for participant younger than 15 years, consent was obtained from parent/legal representative. Ethical clearance was obtained from the institutional ethics committee for research on humans of the University of Douala with ref. N o : 1257 IEC-Udo/02/2016/T.
Consent for publication
Not applicable
Competing interests
I testify on behalf of all co-authors that our article submitted to the Journal BMC pulmonary medicine that:
➣This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
➣The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mbuh, T.P., Ane-Anyangwe, I., Adeline, W. et al. Bacteriologically confirmed extra pulmonary tuberculosis and treatment outcome of patients consulted and treated under program conditions in the littoral region of Cameroon. BMC Pulm Med 19 , 17 (2019). https://doi.org/10.1186/s12890-018-0770-x
Download citation
Received : 17 August 2018
Accepted : 19 December 2018
Published : 17 January 2019
DOI : https://doi.org/10.1186/s12890-018-0770-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gene xpert MTB/RIF
- Extra pulmonary TB
- Treatment outcome
- Littoral region Cameroon
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 May 2024
Multiple pulmonary sclerosing pneumocytoma, based on a study of 36 cases worldwide
- Jianwei Wang 1 ,
- Jiong Guo 1 ,
- Shunqi Li 1 &
- Weidong Zhang 1 , 2
Scientific Reports volume 14 , Article number: 12242 ( 2024 ) Cite this article
98 Accesses
1 Altmetric
Metrics details
- Oncogenesis
- Respiratory tract diseases
- Risk factors
- Surgical oncology
To analyze the clinical characteristics and to improve clinicians’ understanding of multiple pulmonary sclerosing pneumocytoma (PSP) patients. A total of 36 PSP patients with multiple tumor characteristics were identified from the literature search. They were compared with 43 solitary PSP patients diagnosed and treated in our hospital in the past 5 years. Thus, the pathogenesis, clinical symptoms, diagnosis methods, treatment strategies, and prognosis of pulmonary sclerosing pneumocytoma (PSP) patients with multiple tumors were explored. Patients with multiple PSP are mostly distributed in Asia (88.89%) and are females (83.33%). PSP can be located in any one lobe (19.44%), or grow across ipsilateral lobes (44.44%), or even, bilateral lobes (36.11%). It can be accompanied by metastasis (9.09%) and is prone to misdiagnosis (27.78%). Compared with solitary PSP, the occurrence age of multiple PSP was younger (mean ± standard deviation [SD]: 40.36 ± 18.12: 51.28 ± 12.74 years), but there was no significant difference in sex, tumor size (mean ± SD: 43.54 ± 46.18: 30.56 ± 17.62 mm), or symptoms. Individualized surgical resection is required for treatment, including pneumonectomy (17.65%), lobectomy (23.53%), subpulmonary lobectomy (38.24%), or combined lobectomy (5.88%). Multiple PSP is relatively rare. Surgical resection within a limited time should be the main treatment for such patients. The prognosis of patients with multiple PSP is generally good, but inappropriate diagnosis and treatment plans may lead to poor prognosis.
Similar content being viewed by others
Pneumonitis associated with pembrolizumab plus chemotherapy for non-squamous non-small cell lung cancer
Clinical course and risk factors for development and progression of interstitial lung disease in primary Sjögren’s syndrome

Long-term clinical course and outcome in patients with primary Sjögren syndrome-associated interstitial lung disease
Introduction.
In 2015, the World Health Organization officially named primary pulmonary sclerosing hemangioma as pulmonary sclerosing pneumocytoma (PSP), and classified it into pulmonary adenoma 1 . Most scholars believe 2 , 3 PSP to mostly occur in Asian women. Its overall incidence is low and shows solitary nodules with single, quasi-round, and occasional calcification on imaging. Few cases of multiple and metastatic PSP have been reported. However, in recent years, several literature works have reported PSP with multiple characteristics. In 1980, Indian scholar Joshi 4 first reported a 40-year-old female patient with multiple pulmonary nodules in both lungs, and later, confirmed it as multiple PSP. Since then, at least 35 patients with multiple PSP have been reported by scholars worldwide 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 .
The location of multiple PSP in these patients differs: some are located in one lung lobe, some in multiple lobes of the unilateral chest, and some grow across the chest in bilateral multiple lobes. Compared with general PSP, multiple growth is a special manifestation of PSP, and their etiology, diagnosis, and treatment have certain specificities. A total of 36 PSP patients with multiple growth were searched through the network, and the medical records of 43 solitary PSP patients who underwent surgery in our hospital in the past five years were collected. In this study, the clinical characteristics of these two types of patients are compared and analyzed. In addition, the clinical characteristics of multiple PSP patients and the appropriate diagnosis and treatment methods are explored to provide clinical diagnosis and treatment references for more medical workers.
Materials and methods
“Pulmonary sclerosing hemangioma, multiple” and “pulmonary sclerosing pneumocytoma, multiple” are considered as the keywords, respectively, through the China National Knowledge Infrastructure, Wanfang, Google Scholar, and PubMed databases. A total of 36 cases of PSP patients with multiple characteristics are retrieved and these characteristics are summarized in Table 1 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 .
Simultaneously, 43 cases of solitary PSP in patients from our hospital were used as the control group. The differences between solitary and multiple PSP were compared by analyzing their general characteristics, symptoms, treatment methods, tumor characteristics, and other information.
Data presentation
Numerical variables of normal distribution were displayed as mean ± standard deviation, and those of nonnormal distribution were displayed as median (interquartile range). Categorical variables were displayed as frequency (percentage).
Ethics approval and informed consent
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Henan Provincial Chest Hospital. Written informed consent has been provided by our hospital patients to have the case details. And another part of patients’ information in this article comes from the references.
Results and discussion
In 1956, Liebow and Hubbell 5 first described the predecessor of PSP: pulmonary sclerosing hemangioma. In 2015, the World Health Organization formally renamed it as PSP 1 . It is composed of “two cell types, four patterns”, that is, two cell kinds of cuboidal surface cells and rounded cells, and four histology areas of papillary, solid, sclerosing, and hemorrhagic areas. PSP is a rare disease; especially, in those patients with multiple lesions, it is easy to be misdiagnosed as metastatic tumor, pulmonary tuberculosis, and other diseases 5 , 6 , 7 , 8 , 9 , 10 .
Table 1 indicates that since the first detection of multiple PSP patients in 1980, only a small number of subsequent cases have been reported. Among them, the number of reported cases in the past five years has been higher, accounting for more than half (52.78%). Most of them are distributed in Asian countries (88.89%), such as China (47.22%), Japan (25.00%), South Korea (8.33%), India (8.33%), and also, in Europe and America (11.11%), which is similar to the solitary PSP patients. There is no significant difference between the two groups 11 , 12 , 13 . Multiple PSP mostly occurs in women (83.33%) within the age range of 16 to 73 years (median [interquartile range]: 37 [25, 54.5]; mean: 40.36 years), of which, a relatively large proportion is of the age group 21–30 years (27.78%). Compared with the solitary PSP group, those patients in the multiple PSP group were younger (mean: 40.36:51.28 years). Most of the patients (64.71%) were accidentally found by physical examination, while a small number sought medical attention because of symptoms such as “cough (29.41%)”, “hemoptysis (14.71%)”, chest tightness (14.71%)”, “chest pain (5.88%)”, and “fever (8.82%)”. During the hospital visit, a computed tomography (CT) examination of the chest revealed multiple pulmonary nodules that were diagnosed as multiple PSP. There was no significant difference in the occurrence probability of various symptoms between the multiple PSP group and the solitary PSP group. This means multiple PSP did not have specific symptoms (Fig. 1 ).

The symptom of multiple PSP and single PSP.
Patients with multiple PSP generally require a CT examination of the chest for its detection. Imaging analysis of 36 patients with multiple PSP revealed the maximum lesion diameter to range from 6 to 226 mm (median [interquartile range]: 27.5 [20, 50] mm; mean ± SD: 43.54 ± 46.18 mm). This is larger than the average diameter of solitary PSP patients (mean: 43.54:30.56 mm), but there is no statistical difference between the two groups. With regard to the distribution of tumor locations, some scholars 14 classified them into five different subtypes, such as “Type I: a dominant SP (Sclerosing pneumocytoma) with satellite nodules that occur in one lobe (multi local); Type II: SPs that are distinct, separate fractions in the same love; Type III: SP occurring in different ipsilateral lobes; Type IV: SP occurring independently in central lobes and Type V: SP occurring bilaterally in all lobes”. After analysis, this classification method is considered to be relatively cumbersome and to have no clinical guiding significance in diagnosis and treatment. Therefore, the distribution of tumor locations is simplified into three types: I, multiple PSP located in a single lung lobe; II, multiple PSP located in multiple lung lobes of the unilateral chest; and III, multiple PSP located in multiple lung lobes of the bilateral chest (Figs. 2 , 3 ). An analysis revealed that type II multiple PSP was more, approximately 44.44%, followed by type III multiple PSP (36.11%), whereas type I multiple PSP was relatively less (19.44%). This classification method can better guide the clinical treatment strategy of multiple PSP. An analysis of the imaging findings of multiple PSP revealed 8.33% of patients to have lymph node metastasis or even extrapulmonary metastasis 15 , which was significantly higher than the lymph node metastasis rate of solitary PSP 17 , 18 , 19 , 20 .

The site of multiple PSP.

The subtypes of multiple PSP. Type I: multiple PSP located in a single lung lobe; Type II: multiple PSP located in multiple lung lobes of the unilateral chest; Type III: multiple PSP located in multiple lung lobes of the bilateral chest.
Figure 4 demonstrates that multiple PSP is not image specific; hence, multiple PSP diagnosis by a CT examination of the chest alone is difficult. Some patients with a previous history of malignant tumors might first consider the diagnosis of pulmonary metastases when they find multiple round nodules in the lungs. They may give up treatment at this time, but some reports 6 , 7 , 8 suggest that multiple PSP can be diagnosed by puncture or surgical biopsy. Patients with multiple pulmonary nodules, who seek medical attention due to fever, can be misdiagnosed to have pulmonary tuberculosis by inexperienced doctors. Even if their tuberculosis-related tests are negative, they might still receive unnecessary anti-tuberculosis treatment 5 , 9 . However, some reports 21 imply that patients with multiple PSP complicated with pulmonary tuberculosis may also exist. For patients who have multiple round pulmonary nodules with a previous history of malignant tumors, the time of discovery of pulmonary nodules is important. Similar to most scholars’ reports 14 , 15 , 16 , the diagnosis of multiple PSP is mainly based on the pathological diagnosis of postoperative resected specimens. The accuracy rate of percutaneous lung biopsy pathology and intraoperative rapid pathology diagnosis is low. Therefore, for multiple round pulmonary nodules which are difficult to be diagnosed, the authors propose to promptly perform a minimally invasive surgery for nodule resection and pathological diagnosis.

Chest CT of multiple PSP. This is a rare case of multiple PSP. The patient’s physical examination revealed the presence of multiple nodules in both lungs. A chest CT scan confirmed the existence of a total of 4 circular pulmonary nodules, distributed as follows: 1 in the upper lobe of the right lung, 1 in the lower lobe of the right lung, 1 in the upper lobe of the left lung, and 1 in the lower lobe of the left lung. The largest nodule, measuring 26 mm in diameter, was surgically excised from the left upper lobe and subjected to pathological analysis, which confirmed the diagnosis of PSP. Consequently, the final diagnosis was determined to be multiple PSP, and the patient is currently undergoing follow-up care.
It is still controversial whether multiple nodules of multiple PSP are multiple primary lesions or metastatic lesions. Many scholars 23 , 24 , 25 , 26 have conducted relevant research in the past, but so far there is no meaningful analysis result. And Jung 28 discovered all germline variations in the two PSP nodules to be the same. Whereas somatic cell variations were mutually exclusive when he conducted somatic mutation sequencing and pathway analysis of nodule lesions in a patient with multiple PSP. Therefore, Jung perceived that they had different origins and belonged to multicentric lesions. The authors discovered only 8.33% of patients with multiple PSP to have lymph node metastasis (refer Table 1 ). The death of a patient with multiple PSP was reported 15 . Multiple lesions gradually enlarged and fused into a huge mass during the 10-year follow-up. In this study, multiple PSP is considered as multiple primary lesions, but these are only conjectures at present. Sufficient pathological and genetic analysis data to confirm this is still scarce.
In accordance with the current literature data 28 , 29 , multiple PSP is rare and grows slowly; but, it is not a malignant tumor, and the prognosis is mostly good. Although the prognosis of most multiple PSP is good, there are still some reports 6 , 30 that suggest the growth of multiple PSP over one lung lobe or one side of the chest, which even compresses the surrounding blood vessels, trachea, and heart, leading to death 15 , 29 . There are also some reports 8 , 20 that imply the relief of symptoms (fever, hemoptysis) in patients only after the complete removal of multiple nodules. An analysis of the existing literature data suggests lobectomy for multiple PSP located in a single lung lobe (type I); For patients with multiple PSP located in multiple lung lobes of the unilateral chest (type II), the largest tumor should be removed, or if the tumor is large and close to fusion, total pneumonectomy should be considered; For patients with multiple PSP located in multiple lung lobes of the bilateral chest (type III), if the tumors are small, they can be observed. However, for larger tumors, individualized treatment options such as lobectomy, multi-site wedge resection, stereotactic radiotherapy, and radiofrequency ablation can be chosen. For patients undergoing surgical treatment, lymph node dissection can also be considered. The evaluation of lymph node metastasis has a certain guiding significance for prognosis. The aforementioned treatments still lack medical evidence because of the less number of patients with multiple PSP and the availability of long-term follow-up clinical data. Hence, continuous study on this matter is required to confirm them.
This study was subject to certain limitations. Notably, the sample size of patients with multiple PSP was relatively small, and their distribution was widely dispersed. Consequently, the authors were only able to analyze the characteristics of PSP patients with malignancy based on globally reported data. While some conclusions were drawn, the absence of multicenter clinical research verification remains a notable deficiency. Nevertheless, the authors maintain that our research findings can still serve as a valuable reference for clinicians and offer potential avenues for future investigation.
Multiple PSP is a rare type of PSP that occurs mostly in middle-aged women in Asia. PSP can be located in any lobe of the lung, and can grow across the lobe of the lung, or even across the chest. Most scholars consider multiple PSP to be multiple primary lesions, but their pathogenesis is still unknown. Its treatment should be individualized according to the location of multiple nodules, but larger lesions should be promptly removed. The prognosis of patients with multiple PSP is generally good, but if not treated in time, especially for patients with large tumors or multiple PSP with metastasis, it can lead to serious consequences and even death.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
Computed tomography
Pulmonary sclerosing hemangioma
- Pulmonary sclerosing pneumocytoma
Sclerosing pneumocytoma
Travis, W. D. et al. The 2015 World Health Organization Classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 10 (9), 1243–1260 (2015).
Article PubMed Google Scholar
Chen, B. et al. Pulmonary sclerosing hemangioma: A unique epithelial neoplasm of the lung (report of 26 cases). World J. Surg. Oncol. 11 , 85 (2013).
Article PubMed PubMed Central Google Scholar
Lin, H., Yao, H. & Peng, F. CT image morphology features of pulmonary sclerosing hemangiomas. Chin. Ger. J. Clin. Oncol. 10 (1), 19–23 (2011).
Article Google Scholar
Joshi, K., Shankar, S. K., Gopinath, N., Kumar, R. & Chopra, P. Multiple sclerosing haemangiomas of the lung. Postgrad. Med. J. 56 (651), 50–53 (1980).
Article CAS PubMed PubMed Central Google Scholar
Liebow, A. A. & Hubbell, D. S. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 9 , 53–75 (1956).
Article CAS PubMed Google Scholar
Shin, S. Y., Kim, M. Y., Lee, H. J., Oh, S. Y. & Jang, S. J. Clustered pulmonary sclerosing pneumocytoma in a young man: A case report. Clin. Imaging 38 (4), 532–535 (2014).
Lizarraga Madrigal, D. et al. Pulmonary sclerosing pneumocytomas mimicking lung cancer. Cureus 15 (4), e37395 (2023).
PubMed PubMed Central Google Scholar
Soumil, V. J. et al. Multiple sclerosing hemangiomas of the lung. Asian Cardiovasc. Thorac. Ann. 12 (4), 357–359 (2004).
Schiergens, T. S. et al. Pulmonary sclerosing hemangioma in a 21-year-old male with metastatic hereditary non-polyposis colorectal cancer: Report of a case. World J. Surg. Oncol. 9 , 62 (2011).
Zhou, L. et al. Pulmonary sclerosing hemangioma with a rare symptom: A case report and review of the literature. Mol. Clin. Oncol. 6 (2), 221–224 (2017).
Jiang, L., Sun, L. & Liu, L. Benign tumor behaves malignantly: A case report of bilateral multiple pulmonary sclerosing pneumocytoma. Int. J. Clin. Exp. Pathol. 10 (8), 8735–8740 (2017).
Hu, A. M. et al. Preoperative diagnosis in 46 cases of pulmonary sclerosing hemangioma. Chin. Med. J. (Engl.) 129 (11), 1377–1378 (2016).
Fan, X. et al. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol. Ther. 19 (1), 13–19 (2018).
Pal, P. & Chetty, R. Multiple sclerosing pneumocytomas: A review. J. Clin. Pathol. 73 (9), 531–534 (2020).
Zhang, W. et al. Clinical characteristics of malignant pulmonary sclerosing pneumocytoma based on a study of 46 cases worldwide. Cancer Manag. Res. 14 , 2459–2467 (2022).
Zhang, Y. et al. CT-based radiomics for differentiating peripherally located pulmonary sclerosing pneumocytoma from carcinoid. Med. Phys. https://doi.org/10.1002/mp.17037 (2024).
Zhang, Y., Ran, C. & Li, W. Central and peripheral pulmonary sclerosing pneumocytomas: Multi-phase CT study and comparison with Ki-67. Radiol. Oncol. 57 (3), 310–316 (2023).
Miyagawa-Hayashino, A., Tazelaar, H. D., Langel, D. J. & Colby, T. V. Pulmonary sclerosing hemangioma with lymph node metastases: Report of 4 cases. Arch. Pathol. Lab. Med. 127 (3), 321–325 (2003).
Devouassoux-Shisheboran, M., Hayashi, T., Linnoila, R. I., Koss, M. N. & Travis, W. D. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am. J. Surg. Pathol. 24 (7), 906–916 (2000).
Adachi, Y. et al. Pulmonary sclerosing hemangioma with lymph node metastasis: A case report and literature review. Oncol. Lett. 7 (4), 997–1000 (2014).
Luo, H. et al. Multiple sclerosing hemangioma of the right lung in a 23-year-old female patient: A case report and review of the literature. Mol. Clin. Oncol. 12 (3), 263–267 (2020).
Wang, P. et al. A case of pulmonary sclerosing pneumocytoma complicated with pulmonary tuberculosis. Chin. J. Infect. Dis. 37 (8), 3 (2019).
Google Scholar
Wang, X., Ng, C. S., Shi, X. & Yin, W. Characteristics of metastatic and nonmetastatic pulmonary sclerosing pneumocytomas: A clinicopathological study of 68 cases and 15 reported metastatic cases. Lab. Investig. 103 (7), 100135 (2023).
Yang, C. H. & Lee, L. Y. Pulmonary sclerosing pneumocytoma remains a diagnostic challenge using frozen sections: A clinicopathological analysis of 59 cases. Histopathology 72 (3), 500–508 (2018).
Komatsu, T., Fukuse, T., Wada, H. & Sakurai, T. Pulmonary sclerosing hemangioma with pulmonary metastasis. Thorac. Cardiovasc. Surg. 54 (5), 348–349 (2006).
Martini, N. & Melamed, M. R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 70 (4), 606–612 (1975).
Maeda, R. et al. Bilateral multiple sclerosing hemangiomas of the lung. Gen. Thorac. Cardiovasc. Surg. 57 (12), 667–670 (2009).
Jung, S. H. et al. Whole-exome sequencing identifies recurrent AKT1 mutations in sclerosing hemangioma of lung. Proc. Natl. Acad. Sci. U.S.A. 113 (38), 10672–10677 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Hishida, T. et al. Multiple sclerosing hemangiomas with a 10-year history. Jpn. J. Clin. Oncol. 35 (1), 37–39 (2005).
Lee, S. T., Lee, Y. C., Hsu, C. Y. & Lin, C. C. Bilateral multiple sclerosing hemangiomas of the lung. Chest 101 (2), 572–573 (1992).
Teng, X. & Teng, X. First report of pulmonary sclerosing pneomucytoma with malignant transformation in both cuboidal surface cells and stromal round cells: A case report. BMC Cancer 19 (1), 1154 (2019).
Download references
Author information
Authors and affiliations.
Department of Cardiothoracic Surgery, Henan Provincial Chest Hospital (Chest Hospital of Zhengzhou University), Zhengzhou, Henan, China
Pan He, Jianwei Wang, Jiong Guo, Shunqi Li & Weidong Zhang
Henan Provincial Chest Hospital, Room 1, Weiwu Road, Zhengzhou, 450003, Henan, China
Weidong Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Pan He, Jianwei Wang, Jiong Guo, Shunqi Li and Weidong Zhang wrote the main manuscript text. Pan He, Jianwei Wang and Weidong Zhang analyzed the data. Pan He and Weidong Zhang prepared Figs. 1 , 2 , 3 , 4 and Table 1 . All authors reviewed the manuscript.

Corresponding author
Correspondence to Weidong Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
He, P., Wang, J., Guo, J. et al. Multiple pulmonary sclerosing pneumocytoma, based on a study of 36 cases worldwide. Sci Rep 14 , 12242 (2024). https://doi.org/10.1038/s41598-024-63185-7
Download citation
Received : 09 October 2023
Accepted : 27 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1038/s41598-024-63185-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Multiple tumor
- Treatment and diagnosis
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Open access
- Published: 01 June 2024
Development and validation of a preoperative CT‑based radiomics nomogram to differentiate tuberculosis granulomas from lung adenocarcinomas: an external validation study
- Liping Yang 1 ,
- Zhiyun Jiang 2 na1 ,
- Jinlong Tong 3 ,
- Qing Dong 5 &
- Kezheng Wang 1
BMC Cancer volume 24 , Article number: 670 ( 2024 ) Cite this article
Metrics details
An accurate and non-invasive approach is urgently needed to distinguish tuberculosis granulomas from lung adenocarcinomas. This study aimed to develop and validate a nomogram based on contrast enhanced-compute tomography (CE-CT) to preoperatively differentiate tuberculosis granuloma from lung adenocarcinoma appearing as solitary pulmonary solid nodules (SPSN).
This retrospective study analyzed 143 patients with lung adenocarcinoma (mean age: 62.4 ± 6.5 years; 54.5% female) and 137 patients with tuberculosis granulomas (mean age: 54.7 ± 8.2 years; 29.2% female) from two centers between March 2015 and June 2020. The training and internal validation cohorts included 161 and 69 patients (7:3 ratio) from center No.1, respectively. The external testing cohort included 50 patients from center No.2. Clinical factors and conventional radiological characteristics were analyzed to build independent predictors. Radiomics features were extracted from each CT-volume of interest (VOI). Feature selection was performed using univariate and multivariate logistic regression analysis, as well as the least absolute shrinkage and selection operator (LASSO) method. A clinical model was constructed with clinical factors and radiological findings. Individualized radiomics nomograms incorporating clinical data and radiomics signature were established to validate the clinical usefulness. The diagnostic performance was assessed using the receiver operating characteristic (ROC) curve analysis with the area under the receiver operating characteristic curve (AUC).
One clinical factor (CA125), one radiological characteristic (enhanced-CT value) and nine radiomics features were found to be independent predictors, which were used to establish the radiomics nomogram. The nomogram demonstrated better diagnostic efficacy than any single model, with respective AUC, accuracy, sensitivity, and specificity of 0.903, 0.857, 0.901, and 0.807 in the training cohort; 0.933, 0.884, 0.893, and 0.892 in the internal validation cohort; 0.914, 0.800, 0.937, and 0.735 in the external test cohort. The calibration curve showed a good agreement between prediction probability and actual clinical findings.
The nomogram incorporating clinical factors, radiological characteristics and radiomics signature provides additional value in distinguishing tuberculosis granuloma from lung adenocarcinoma in patients with a SPSN, potentially serving as a robust diagnostic strategy in clinical practice.
Peer Review reports
Introduction
The differentiation between peripheral lung cancer and tuberculosis granuloma is still a challenging issue [ 1 ]. Lung tuberculosis manifested as nodular or mass is easily misdiagnosed as peripheral lung cancer [ 2 ]. However, the treatment options and clinical prognosis between lung cancer and tuberculosis are completely distinct. Radical surgical resection is the first choice for the former, while the latter tends to be treated with anti-tuberculosis drugs [ 3 ]. Misdiagnosis will cause unnecessary treatment and financial burden, especially when the diagnosis of lung adenocarcinoma is delayed. Patients might lose the best chance for treatment, leading to uncontrollable tumor progression and poorer prognosis [ 4 ]. Therefore, finding an accurate and non-invasive approach to differentiate lung cancer from tuberculosis is of great significance, which undoubtedly has become an important topic for radiologists and clinicians.
The contrast enhanced-compute tomography (CE-CT) is widely applied to distinguish different lung diseases mainly dependent on morphological features, such as speculation, contour, and border definition, which have shown improved diagnostic performance [ 5 ]. Unfortunately, nodular/mass pulmonary tuberculosis granuloma usually appears as round or irregular mass shadows in CT images, and its edges may be lobed or manifested as a spicule sign [ 6 ]. As a result of non-specific clinical and radiological manifestations, diagnostic accuracy is closely related to the radiologists’ knowledge level and working experience. Despite the rapid development of CT-guided biopsy technology, a series of adverse complications severely restrict the wide application of this operation for pathological diagnosis [ 7 ].
Radiomics is a promising and non-invasive method that can extract automatic high-throughput quantitative features from images [ 8 ]. It captures relationships between image voxels that the naked eye of physicians may not perceive-even experienced radiologists, which can contribute to the diagnostic and predictive accuracy of the disease. Therefore, this study was aimed to develop and validate a radiomics nomogram based on preoperative CE-CT images to differentiate tuberculosis granuloma from lung adenocarcinoma presenting as solid nodules or masses.
Materials and methods
Study population.
The retrospective research protocol was reviewed, approved, and overseen by the institutional review board of Harbin Medical University Cancer Hospital and The Fourth Affiliated Hospital of Harbin Medical University, and the need for written informed consent was waived. The enrollment flowchart of this study was displayed in Fig. 1 . Detailed inclusion criteria were listed as follows: (a) solitary and solid nodules or masses, which may contain cavities or vacuoles and do not exhibit a ground glass density; (b) histological diagnosis confirmed by surgical resection; and (c) available preoperative chest CT images (within 4 weeks prior to surgery). Detailed exclusion criteria were described as follows: (a) ambiguous pathological diagnosis from insufficient tissue samples; (b) subsolid pulmonary nodules, including non-solid nodules and partly solid nodules; (c) patients with a history of surgery, radiotherapy, or chemotherapy; (d) patients with a history of other malignant tumors and (e) substandard image quality, such as motion artifacts.

The flow diagram of this study
Image acquisition and image analysis
All chest CT images were obtained from Discovery CT 750 HD (GE Medical Systems, USA). Scans were acquired from the thoracic inlet to the level of the bilateral adrenal glands during deep inspiration breath-hold. Detailed scanning parameters were as follows: tube voltage 100-140 kV, tube current 350-550 mA, slice thickness 3 mm, reconstruction interval 3 mm, matrix size 512 × 512, and field of view 450 mm. Non-ionic contrast media (Iohexeol, 350 mg/ml, GE, Boston, USA) was administered at a rate of 3.0-3.5 ml/s and 1.2 ml/kg to all patients, as standardized protocol. Arterial phase (AP) images were scanned around 30s post-injection of contrast media. Two experienced radiologists who both has 10-year practicing experience in chest disease diagnosis, blinded to the pathological results, independently evaluated the CT images. The details of the evaluation of subjective CT findings were displayed in Supplementary Materials (Supplementary data 1).
Tumor segmentation and radiomics feature extraction
Three steps were adopted to preprocess the CT images before feature extraction. Firstly, all images were resampled to a uniform voxel size of 1 mm × 1 mm × 1 mm using linear interpolation to minimize the influence of different layer thicknesses. Secondly, the continuous images were converted into discrete values based on the gray-scale discretization process (bin width = 25). Finally, the Laplacian of Gaussian and wavelet image filters were used to eliminate the mixed noise in the image digitization process to obtain low- or high-frequency features. Axial CT Digital Imaging and Communications in Medicine images were applied for tumor segmentation. The tumor lesion was delineated on axial CT images using ITK-SNAP software (version 3.6.0, www.itksnap.org ). Radiomics features were extracted from each CT-derived volume of interest (VOI) by applying dedicated AK software (Artificial Intelligence Kit, GE Healthcare), which complies with image biomarker standardization initiative guidelines [ 9 ]. A total of 851 radiomics features were extracted from each VOIs, and the classification of various features was listed in Supplementary Materials (Supplementary data 2).
Radiomics feature selection and model establishment
Intra- and inter-class correlation coefficients (ICCs) were calculated to evaluate the intra- and inter-observer reproducibility. Two readers drew the volumes of interest (VOIs) on 40 randomly selected CT images (20 cases of tuberculosis granulomas and 20 cases of lung adenocarcinomas). Reader 1 repeated the segmentations two weeks later. An ICC greater than 0.80 indicated good agreement with feature extraction. The VOI segmentation for the remaining cases was performed by Reader 1. After the intra- and inter-operator agreement evaluation, radiomics features with ICC > 0.80 were selected for further analysis. Next, the feature selection was carried out by using a step-by-step selection method. Firstly, univariate logistic regression analysis was applied to select features with P -value < 0.05 for the subsequent analysis. Secondly, multivariate logistic regression analysis was utilized to choose features closely related to pulmonary nodules classification. Finally, the most informative features were retained using the least absolute shrinkage and selection operator (LASSO) method. LASSO regression shrinks the coefficient estimates toward zero, with the degree of shrinkage dependent on an additional parameter, alpha. To determine the optimal values for alpha, a 10-time cross-validation was used, and we chose alpha via the minimum criteria and a value of ln (alpha)= -2.1 was chosen.
Model building and evaluation of predictive models
A clinical model for predicting pulmonary nodule classification was developed using univariable and multivariable logistic regression analysis. The following candidate predictors were considered: tumor size, location, cavity, vacuole, spicule, satellite lesions, calcification, lobulation, pleural indentation, and air bronchogram. Furthermore, a nomogram was created based on both clinical-radiological parameters and the combined radiomics signature. The diagnostic performance of the different models was evaluated in the training and testing sets by assessing sensitivity, specificity, and accuracy. The calibration curve was employed to assess the agreement between the nomogram’s prediction results and actual clinical findings. Decision curve analysis (DCA) was used to validate the clinical usefulness of the radiomics nomogram.
Statistical analysis
Statistical analysis for the present study was conducted using the IPM Statistics program (V 2.1.0.R), R (version 3.5.1), and Python (version 3.5.6). Categorical variables were compared using either a chi-squared test or Fisher’s exact test, with P < 0.05 denoting a significant difference. The receiver operating characteristic (ROC) curve was constructed to evaluate the discriminative performance of each model. A two-sided P value < 0.001 was considered statistically significant.
Clinical characteristics of patients
Patient demographics and CT characteristics of all patients were presented in Table 1 . A total of 280 patients from the two centers between March 2015 to June 2020 were enrolled in this study. The training and internal validation cohorts included 161 and 69 patients from center No.1, respectively. The external test cohort included 50 patients from center No.2. In the univariate regression analysis, the classification of solitary pulmonary nodules had significant associations with sex, age, mean pack-years, spiculation, air bronchogram, maximum diameter, enhanced-CT value, CEA, CA125 and CA199 (both P < 0.001). In the multivariate regression analysis, only enhanced-CT value and CA125 were demonstrated to be independent predictors (both P < 0.001). The results of univariate and multivariate regression analysis were displayed in Table 2 .
Intra and inter-observer reproducibility of feature extraction
The intra-observer ICC ranged from 0.815 to 0.940, and inter-observer ICCs ranged from 0.720 to 0.906. Therefore, a favorable intra- and inter-observer reproducibility of radiomics feature extraction was observed in our study.
Feature selection and model building
The clinical model was developed based on multivariable logistic regression, and it identified enhanced-CT value (odds ratio [OR], 2.389; 95% confidence interval [CI], 1.756–3.247; P < 0.001) and CA125 (OR, 1.312; 95% CI, 1.134–1.518; P < 0.001) as independent predictors. Initially, 850 radiomics features were extracted from each VOI of the CT image. All obtained radiomics features underwent preprocessing and standardization using the z-score approach. Subsequently, 720 radiomics features were selected based on a repeatability standard of ICC ≥ 0.80. Univariate and multivariate logistic regression analyses were further performed to reduce the dimensions of these features. Then, 9 features were selected as the most predictive subset to construct radiomics model after LASSO (Fig. 2 A, B). The selected radiomics features and corresponding coefficients were listed in Table 3 . The nomogram was created to differentiate tuberculosis granulomas from lung adenocarcinomas, incorporating two factors (enhanced-CT value and CA125) and the radiomics signature (namely rad-score).

Selection of significant parameters in radiomics features in the training cohort and definition of linear predictor. (a) Ten time cross-validation for tuning parameter selection in the LASSO model. (b) LASSO
Rad-score = 0.5014 − 0.03942*original_firstorder_10Percentile + 20.82*original_glcm_MaximumProbability-3.425*original_shape_Flatness + 2.369*wavelet.HHH_glcm_MCC-1.049*wavelet.HHL_firstorder_Mean-28.89*wavelet.HHL_firstorder_Median-30.42*wavelet.HHL_glcm_Imc1 + 0.1982*wavelet.LHH_firstorder_Skewness + 0.0000008524*wavelet.LLH_ngtdm_Complexity.
Performance of three prediction models
The ROC curve was employed to assess the performance of different predictive models in the training, internal validation, and external testing cohorts. The area under the curve (AUC), sensitivity, specificity, and accuracy were calculated. All results pertaining to diagnostic efficacy are presented in Table 4 . The ROC curves are displayed in Figs. 3 , 4 and 5 .

ROC of the clinical model in the training, internal validation and external testing cohorts
ROC of the radiomics model in the training, internal validation and external testing cohorts

ROC of the nomogram in the training, internal validation and external testing cohorts
Clinical prediction model
The clinical prediction model incorporated independent clinic-radiological predictors (enhanced-CT value and CA125). In the training cohort, this model exhibited an area under the curve (AUC) of 0.804 (95% CI 0.746–0.862) with sensitivity, specificity, and accuracy of 0.736, 0.789, and 0.761, respectively. When applied to the internal validation cohort, the model yielded an AUC of 0.803 (95% CI 0.684–0.922) with sensitivity, specificity, and accuracy of 0.750, 0.881, and 0.826, respectively. Upon validation in the external test cohort, the model yielded an AUC of 0.597 (95% CI 0.432–0.763) with sensitivity, specificity, and accuracy of 0.812, 0.441, and 0.560, respectively.
Radiomics prediction model
The radiomics prediction model was built based on nine significant radiomics features. In the training cohort, this model exhibited an area under the curve (AUC) of 0.876 (95% CI 0.828–0.924) with sensitivity, specificity, and accuracy of 0.843, 0.826, and 0.835, respectively. When applied to the internal validation cohort, the model yielded an AUC of 0.931 (95% CI 0.859-1.000) with sensitivity, specificity, and accuracy of 0.964, 0.878, and 0.913, respectively. Upon validation in the external test cohort, the model yielded an AUC of 0.877 (95% CI 0.783–0.970) with sensitivity, specificity, and accuracy of 0.937, 0.835, and 0.800, respectively.
Development and validation of the nomogram
The nomogram is presented in Fig. 6 A and satisfactory prediction performance was obtained. In the training cohort, the nomogram exhibited an area under the curve (AUC) of 0.903 (95% CI 0.861–0.945) with sensitivity, specificity, and accuracy of 0.901, 0.807, and 0.857, respectively. Applied in the internal validation cohort, the model yielded AUC of 0.933 (95% CI 0.874–0.992) with sensitivity, specificity, and accuracy of 0.893, 0.892, and 0.884 respectively. Validated in the external test cohort, the model yielded AUC of 0.914 (95% CI 0.838–0.990) with sensitivity, specificity, and accuracy of 0.937, 0.835, and 0.800 respectively. Calibration curves (Fig. 6 B, C) indicated that the predicted probabilities of the nomogram were closely aligned with the actual clinical observation in both the training and external testing cohorts. The decision curve of nomogram demonstrated a higher net benefit for the differentiation between lung cancer and tuberculosis than the clinical model and the radiomics model (Fig. 6 D). This suggests that the results predicted by our nomogram demonstrated favorable clinical usefulness, and a representative case was displayed in Fig. 7 .

Nomogram for differentiation between tuberculosis granulomas and lung adenocarcinomas based on training cohort and the model evaluation of calibration curve. (A) Radiomics nomogram based on clinical characteristics and Radscore. The calibration curves were used to evaluate the consistency between the probability of nomogram prediction and the actual clinical observation in the training (B) and external validation (C) cohorts. (D) DCA for the classification of solitary pulmonary nodules for each model. X-axis represents the threshold probability and Y-axis represents the net benefit. The red curve represents the nomogram. The blue curve represents the clinical model. The red curve represents the radiomics model. The green curve represents the nomogram

A-C : lung window of axial thin-section enhanced chest CT images in a 62-year-old male with proven diagnosis of pulmonary tuberculosis. (A) Chest CT image shows, in the right superior lobe, a consolidative opacity. (B) The same image showing the consolidative opacity after radiomic volumetric segmentation (in orange). The rad-score calculated by nomogram was 0.76, meanwhile we collected the enhanced-CT value (54.9) and the value of CA125 (19.54). The predicted risk value (0.88) was higher than the cut-off value (0.71), which indicated that a benign lesion. (C) The lesion was confirmed on pathological diagnosis as a pulmonary tuberculosis (hematoxylin and eosin, ×400). D-E: In a 58-year-old male, CT scan shows an irregular solid nodule in the left upper lobe. (D) Chest CT image shows, in the left superior lobe, a consolidative opacity. (E) The same image showing the consolidative opacity after radiomic volumetric segmentation (in orange). We used the same method to obtain the rad-score (0.15), the enhanced-CT value (33.3) and CA125 (8.31). The predicted risk value was 0.62 (< 0.71), indicating the lesion was malignant. (F) The lesion was confirmed on pathological diagnosis as a lung adenocarcinoma (hematoxylin and eosin, ×400)
Comparison of different prediction models
Figure 8 demonstrates the ROC curves of clinical model, radiomics model and nomogram in the training cohort, internal validation cohort and external test cohort, respectively. The nomogram demonstrated the optimal discriminative power for pulmonary nodules classification among the three indicators, with improvements in the AUC from 0.804 for the clinical model to 0.903 ( P < 0.05, DeLong’s test) in the training cohort, from 0.803 for the clinical model to 0.933 ( P < 0.05, DeLong’s test) in the internal validation cohort, and from 0.597 for the clinical model to 0.914 ( P < 0.05, DeLong’s test) in the external test cohort. However, the performance of nomogram model did not differ from that of the radiomics model (AUCs 0.903 vs. 0.876; P = 0.760) in the training cohort, (AUCs 0.933 vs. 0.931; P = 0.940) in the internal validation cohort, (AUCs 0.914 vs. 0.877; P = 0.740) in the external test cohort. In contrast, the radiomics model yield a better predictive performance than the clinical model, with improvements in the AUC from 0.803 for the clinical model to 0.931 ( P < 0.05, DeLong’s test) in the internal validation cohort, from 0.597 for the clinical model to 0.877 ( P < 0.05, DeLong’s test) in the external test cohort, while the performance of radiomics model did not differ from that of the clinical model (AUCs 0.804 vs. 0.876; P = 0.180) in the training cohort.
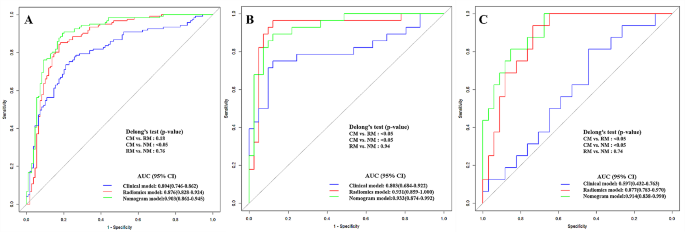
DeLong ROC curves of the three prediction models
The current investigation successfully developed a CE-CT radiomics-based nomogram that integrated the radiomics signature and clinical risk indicators to differentiate tuberculosis granulomas from lung adenocarcinomas prior to operation. Our results showed that the enhanced-CT value, CA125 and radiomics signature were significant predictors in differentiating between tuberculosis granulomas and lung adenocarcinomas. The radiomics nomogram is a non-invasive, easy-to-use, personalized approach with excellent performance to preoperatively differentiate tuberculosis granulomas from lung adenocarcinomas, yielding a superior performance with an AUC of 0.903 in the training cohort, 0.933 in the testing cohort, and 0.914 in the external validation cohort, as compared to the clinical or radiomics model alone. This combined radiomics nomogram is conductive to avoid unnecessary surgeries or repeat CT examinations in patients with SPSN.
There is a growing interest in using radiomics for expeditious diagnosis of non-invasive pulmonary CT images among radiologists, which could potentially improve clinical diagnoses. In this study, radiomics features extracted from arterial phase CT images were applied to enhance the differential diagnosis of lung adenocarcinoma and tuberculosis granuloma. The integrated model demonstrated good predictive performance, with a higher predictive ability than the clinical or radiomics model alone. It is noteworthy that the nomogram presented superior diagnostic efficiency and exhibited high prediction ability for the external data. The nomogram is not dependent on the nodules’ conditions, indicating its potential as a non-invasive diagnostic method with a high degree of accuracy for clinicians to obtain results quickly and reliably.
In this study, a clinical model was established that incorporated demographic information and subjective CE-CT findings. By using enhanced-CT value and CA125 as the independent factors, the clinical model achieved a relatively low AUC (0.804 in the training cohort, 0.803 in the internal validation cohort, and 0.597 in the external testing cohort) for differentiating tuberculosis granulomas from lung adenocarcinomas. Therefore, the results confirmed that some CT imaging features did not demonstrate significant correlations with the pathological classification of solid pulmonary nodules. In contrast, the radiomics model yielded better performance in the training cohort (AUC = 0.876), internal validation cohort (AUC = 0.931), and external testing cohort (AUC = 0.877). The radiomics approach can extract large amounts of high-throughput macroscopic features from CT images to quantify lesion information. Thus, the radiomics model enables the extraction of high-dimensional information that is valuable for differentiating tuberculosis granulomas from lung adenocarcinomas in patients with solitary pulmonary nodules compared with the subjective findings model.
The nomogram was developed based on two independent predictors and rad-score. This research explored several significant clinical and radiological characteristics contributing to the differential diagnosis of solitary pulmonary nodules. Univariate analysis showed that six clinical factors (sex, age, mean pack-years, CEA, CA125 and CA199) and three radiological characteristics (spiculated sign, maximum diameter, and enhanced-CT value) were statistically different between the tuberculosis granuloma and lung adenocarcinoma groups. Consistent with the radiologists’ experience, lung adenocarcinoma is more likely to have spiculated sign due to the spread of malignant cells in the pulmonary interstitial. Pathology has demonstrated that spicules’ formation tends to be related to fibrous tissue proliferation or tumor cell infiltration [ 10 ]. Previous study has demonstrated that spiculated sign was more likely to be identified as a sign of malignancy on multivariate analysis for screening solitary pulmonary nodules [ 11 ]. Although a lobulated shape is often a feature of malignant lesions, it is not an exclusive characteristic, as it can also be observed in benign nodules. The occurrence of a lobulated shape in tuberculosis granuloma was high in our study (119/121). On multivariate analysis, the enhanced-CT value and CA125 remained highly significant in relation to the classification of solitary pulmonary nodules. However, the shape of the lesion (spiculated) was found to only weakly predict the possibility of lung adenocarcinoma. The identification of the spiculated sign on CT images by radiologists was just subjective, and inter-reader variability cannot be ignored. Wang et al. expounded dynamic enhanced CT scanning indicating the value of differentiating lung cancer and pulmonary tuberculosis, in which the enhanced-CT value of adenocarcinoma in the arterial phase is higher than that of tuberculosis (59.27 ± 41.58 vs. 38.88 ± 23.58, P < 0.001) [ 1 ]. Some studies have shown that the enhancement of tuberculoma was none or mild, and the enhancement peak value were lower than that of lung cancer [ 12 ]. This is in agreement with the result of this study. In addition, there was a significant difference in CA125 value between adenocarcinoma and tuberculosis ( P < 0.001). Specifically, the value of CA125 in adenocarcinoma was generally higher than that in tuberculosis, which was different from the findings of previous study [ 12 ]. The increase of the CA125 value in tuberculosis was not prominent, which might be related to the degree or stage of cases enrolled.
In the current study, the 9 core radiomics features were finally retained. Among these radiomics features, Wavelet transform can cover the entire frequency domain and reduce the correlation between different extracted features [ 13 ]. The first-order features describe the distribution of voxel intensities in images. The GLCM features quantify the second-order joint probabilities of images which quantifies the intensity distribution of the gray level at a given offset to extract information about tone homogeneity, linear connection, contrast, and boundaries adjacent to gray zones, as well as complicacy of distribution [ 14 ]. The GLRLM features describe gray-level runs in an image. Skewness, as one of the simple parameters, represents the asymmetric distribution of gray levels in the histogram that describes the heterogeneity of lesions [ 15 ]. Tuberculous granuloma comprises macrophages, including T lymphocytes, B lymphocytes, dendritic cells, fibroblasts, and extracellular matrix components [ 16 ]. The above features describe the patterns or spatial distribution of voxel intensities within the ROIs, which serve as recognized parameters to capture tumor heterogeneity [ 17 ]. Indirectly, our findings confirmed that the selected features were all closely related to high-dimensional space information that can hardly be understood by naked-eye examination, which may potentially assist in the differential diagnosis.
Several limitations should be considered in this study. Firstly, this study was a retrospective analysis, and selection bias was inevitable. Secondly, the strict inclusion and exclusion criteria resulted in a limited sample size for this study, we did not include GGO nodules. In the future, we will try to include GGO lesions in subsequent studies. Thirdly, this study contained only one external validation, and multi-center collaboration is imperative for us to collect more data to improve the prediction capacity. Fourthly, due to technical limitations, although the intra-nodular radiomics features on Lung CT Images were not extracted, the most important features representing the characteristics of lesions were analyzed.
Conclusions
In summary, the developed nomogram, which integrates clinical risk factors and radiomics features, demonstrates robust classification performance prior to surgery. Its visualization and interpretability suggest that it has the potential to serve as a valuable and user-friendly tool for personalized decision-making in clinical treatment strategy management.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Receiver Operating Characteristics
Area Under the Curve
Contrast-Enhanced Compute Tomography
Intra- and Inter-Class Correlation coefficients
Volume of Interest
Least Absolute Shrinkage and Selection Operator
Decision Curve Analysis
Confidence Interval
Wang XL, Shan W. Application of dynamic CT to identify lung cancer, pulmonary tuberculosis, and pulmonary inflammatory pseudotumor. Eur Rev Med Pharmacol Sci. 2017;21(21):4804–9.
PubMed Google Scholar
Cardinale L, Nika L, Teti M, Dalpiaz G, Larici AR, Rea G. La Tubercolosi polmonare nella diagnostica per immagini: Il Grande mimo [Pulmonary Tuberculosis in diagnostic imaging: the great mime]. Recenti Prog Med. 2018;109(4):220–5. https://doi.org/10.1701/2896.29193 .
Article PubMed Google Scholar
Prabhakar B, Shende P, Augustine S. Current trends and emerging diagnostic techniques for lung cancer. Biomed Pharmacother. 2018;106:1586–99. https://doi.org/10.1016/j.biopha.2018.07.145 .
Article CAS PubMed Google Scholar
Tiberi S, du Plessis N, Walzl G, Vjecha MJ, Rao M, Ntoumi F, Mfinanga S, Kapata N, Mwaba P, McHugh TD, Ippolito G, Migliori GB, Maeurer MJ, Zumla A. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis. 2018;18(7):e183–98. https://doi.org/10.1016/S1473-3099(18)30110-5 .
Li CR, Li YZ, Li YM, Zheng YS. Dynamic and contrast enhanced CT imaging of lung carcinoma, pulmonary tuberculoma, and inflammatory pseudotumor. Eur Rev Med Pharmacol Sci. 2017;21(7):1588–92.
Lin JZ, Zhang L, Zhang CY, Yang L, Lou HN, Wang ZG. Application of Gemstone Spectral computed Tomography Imaging in the characterization of Solitary Pulmonary nodules: preliminary result. J Comput Assist Tomogr. 2016;40(6):907–11. https://doi.org/10.1097/RCT.0000000000000469 .
Kalanjeri S, Abbasi A, Luthra M, Johnson JC. Invasive modalities for the diagnosis of peripheral lung nodules. Expert Rev Respir Med. 2021;15(6):781–90. https://doi.org/10.1080/17476348.2021.1913059 .
Yang X, He J, Wang J, Li W, Liu C, Gao D, Guan Y. CT-based radiomics signature for differentiating solitary granulomatous nodules from solid lung adenocarcinoma. Lung Cancer. 2018;125:109–14. https://doi.org/10.1016/j.lungcan.2018.09.013 .
Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, El Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Götz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegård A, Maier-Hein KH, Morin O, Müller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers RJHM, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Löck S. The image Biomarker Standardization Initiative: standardized quantitative Radiomics for High-Throughput Image-based phenotyping. Radiology. 2020;295(2):328–38. https://doi.org/10.1148/radiol.2020191145 .
Wang S, Lin D, Yang X, Zhan C, Zhao S, Luo R, Wang Q, Tan L. Clinical significance of PET/CT uptake for peripheral clinical N0 non-small cell lung cancer. Cancer Med. 2020;9(7):2445–53. https://doi.org/10.1002/cam4.2900 .
Article PubMed PubMed Central Google Scholar
Qi L, Lu W, Yang L, Tang W, Zhao S, Huang Y, Wu N, Wang J. Qualitative and quantitative imaging features of pulmonary subsolid nodules: differentiating invasive adenocarcinoma from minimally invasive adenocarcinoma and preinvasive lesions. J Thorac Dis. 2019;11(11):4835–46. https://doi.org/10.21037/jtd.2019.11.35 .
Sharma M, Sandhu MS, Gorsi U, Gupta D, Khandelwal N. Role of digital tomosynthesis and dual energy subtraction digital radiography in detection of parenchymal lesions in active pulmonary tuberculosis. Eur J Radiol. 2015;84(9):1820–7. https://doi.org/10.1016/j.ejrad.2015.05.031 .
Wang ZZ, Yong JH. Texture analysis and classification with linear regression model based on wavelet transform. IEEE Trans Image Process. 2008;17(8):1421–30. https://doi.org/10.1109/TIP.2008.926150 .
Dinčić M, Todorović J, Nešović Ostojić J, Kovačević S, Dunđerović D, Lopičić S, Spasić S, Radojević-Škodrić S, Stanisavljević D, Ilić AŽ. The fractal and GLCM textural parameters of chromatin may be potential biomarkers of papillary thyroid carcinoma in Hashimoto’s thyroiditis specimens. Microsc Microanal. 2020;26(4):717–30. https://doi.org/10.1017/S1431927620001683 .
Defeudis A, De Mattia C, Rizzetto F, Calderoni F, Mazzetti S, Torresin A, Vanzulli A, Regge D, Giannini V. Standardization of CT radiomics features for multi-center analysis: impact of software settings and parameters. Phys Med Biol. 2020;65(19):195012. https://doi.org/10.1088/1361-6560/ab9f61 .
Hosseini MP, Hosseini A, Ahi K. A review on machine learning for EEG Signal Processing in Bioengineering. IEEE Rev Biomed Eng. 2021;14:204–18. https://doi.org/10.1109/RBME.2020.2969915 .
Reis SP, Sutphin PD, Singal AG, Grzybowski R, Fisher S, Ball C, Xi Y, Grewal S, Kalva SP. Tumor enhancement and heterogeneity are Associated with Treatment response to drug-eluting bead chemoembolization for Hepatocellular Carcinoma. J Comput Assist Tomogr. 2017;41(2):289–93. https://doi.org/10.1097/RCT.0000000000000509 .
Download references
Acknowledgements
The authors would like thank professor Thelma R. Gower for her selfless and valuable assistance.
This research was funded by Provincial Key Research and Development Program of Heilongjiang Province (No. GA21C001), Distinguished Young Scientist Funding of Harbin Medical University Affiliated Tumor Hospital (JCQN2019-02), Key Project of Harbin Medical University Cancer Hospital Climbing Funding (PDYS2024-03), Key Innovation Technology Project Harbin Medical University Cancer Hospital Innovation Technology Funding (CXJSZD-2023-04), Heilongjiang Province’s “unveiling and leading” technology research and development project (2022ZXJ03C01) and Haiyan Funding of Harbin Medical University Cancer Hospital (No. JJMS2023-05). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Zhiyun Jiang have contributed equally to this work.
Authors and Affiliations
Department of PET-CT, Harbin Medical University Cancer Hospital, Harbin, China
Liping Yang & Kezheng Wang
Medical Imaging Department, Harbin Medical University Cancer Hospital, Harbin, China
Zhiyun Jiang
Medical Imaging Department, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, China
Jinlong Tong
Department of Pathology, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, China
Department of Thoracic Surgery, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, China
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Liping Yang, Zhiyun Jiang; Data curation: Liping Yang, Zhiyun Jiang; Formal analysis: Zhiyun Jiang; Funding acquisition: Liping Yang, Kezheng Wang; Investigation: Jinlong Tong; Methodology: Qing Dong; Project administration: Nan Li; Resources: Zhiyun Jiang, Jinlong Tong; Software: Liping Yang, Zhiyun Jiang; Supervision: Qing Dong, Kezheng Wang; Validation: Zhiyun Jiang; Visualization: Jinlong Tong, Nan Li; Writing-original draft: Liping Yang, Zhiyun Jiang ; Writing-review & editing: Qing Dong, Kezheng Wang.
Corresponding authors
Correspondence to Qing Dong or Kezheng Wang .
Ethics declarations
Ethics approval and consent to participate.
The retrospective study was approved by the Institutional Review Board of “Harbin Medical University Cancer Hospital” and “The Fourth Affiliated Hospital of Harbin Medical University” and was granted a waiver of written informed consent for use of data. Institutional Review Board of “Harbin Medical University Cancer Hospital” and “The Fourth Affiliated Hospital of Harbin Medical University” waived informed consent from all patients due to retrospective nature of the study. All methods were performed in accordance with the relevant guidelines and regulations. Research involving human participants, human material, or human data have been performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yang, L., Jiang, Z., Tong, J. et al. Development and validation of a preoperative CT‑based radiomics nomogram to differentiate tuberculosis granulomas from lung adenocarcinomas: an external validation study. BMC Cancer 24 , 670 (2024). https://doi.org/10.1186/s12885-024-12422-3
Download citation
Received : 29 November 2023
Accepted : 23 May 2024
Published : 01 June 2024
DOI : https://doi.org/10.1186/s12885-024-12422-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung adenocarcinoma
- Tuberculosis granuloma
- Computed tomography
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Case Report
- Open access
- Published: 27 May 2024
A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient
- Wei Fu 1 , 2 na1 ,
- Zi Wei Deng 3 na1 ,
- Pei Wang 1 ,
- Zhen Wang Zhu 1 ,
- Zhi Bing Xie 1 ,
- Yong Zhong Li 1 &
- Hong Ying Yu 1
BMC Infectious Diseases volume 24 , Article number: 533 ( 2024 ) Cite this article
294 Accesses
1 Altmetric
Metrics details
Hepatitis B virus (HBV) infection can cause liver failure, while individuals with Acquired Immunodeficiency Virus Disease (AIDS) are highly susceptible to various opportunistic infections, which can occur concurrently. The treatment process is further complicated by the potential occurrence of immune reconstitution inflammatory syndrome (IRIS), which presents significant challenges and contributes to elevated mortality rates.
Case presentation
The 50-year-old male with a history of chronic hepatitis B and untreated human immunodeficiency virus (HIV) infection presented to the hospital with a mild cough and expectoration, revealing multi-drug resistant pulmonary tuberculosis (MDR-PTB), which was confirmed by XpertMTB/RIF PCR testing and tuberculosis culture of bronchoalveolar lavage fluid (BALF). The patient was treated with a regimen consisting of linezolid, moxifloxacin, cycloserine, pyrazinamide, and ethambutol for tuberculosis, as well as a combination of bictegravir/tenofovir alafenamide/emtricitabine (BIC/TAF/FTC) for HBV and HIV viral suppression. After three months of treatment, the patient discontinued all medications, leading to hepatitis B virus reactivation and subsequent liver failure. During the subsequent treatment for AIDS, HBV, and drug-resistant tuberculosis, the patient developed disseminated cryptococcal disease. The patient’s condition worsened during treatment with liposomal amphotericin B and fluconazole, which was ultimately attributed to IRIS. Fortunately, the patient achieved successful recovery after appropriate management.
Enhancing medical compliance is crucial for AIDS patients, particularly those co-infected with HBV, to prevent HBV reactivation and subsequent liver failure. Furthermore, conducting a comprehensive assessment of potential infections in patients before resuming antiviral therapy is essential to prevent the occurrence of IRIS. Early intervention plays a pivotal role in improving survival rates.
Peer Review reports
HIV infection remains a significant global public health concern, with a cumulative death toll of 40 million individuals [ 1 ]. In 2021 alone, there were 650,000 deaths worldwide attributed to AIDS-related causes. As of the end of 2021, approximately 38 million individuals were living with HIV, and there were 1.5 million new HIV infections reported annually on a global scale [ 2 ]. Co-infection with HBV and HIV is prevalent due to their similar transmission routes, affecting around 8% of HIV-infected individuals worldwide who also have chronic HBV infection [ 3 ]. Compared to those with HBV infection alone, individuals co-infected with HIV/HBV exhibit higher HBV DNA levels and a greater risk of reactivation [ 4 ]. Opportunistic infections, such as Pneumocystis jirovecii pneumonia, Toxoplasma encephalitis, cytomegalovirus retinitis, cryptococcal meningitis (CM), tuberculosis, disseminated Mycobacterium avium complex disease, pneumococcal pneumonia, Kaposi’s sarcoma, and central nervous system lymphoma, are commonly observed due to HIV-induced immunodeficiency [ 5 ]. Tuberculosis not only contributes to the overall mortality rate in HIV-infected individuals but also leads to a rise in the number of drug-resistant tuberculosis cases and transmission of drug-resistant strains. Disseminated cryptococcal infection is a severe opportunistic infection in AIDS patients [ 6 ], and compared to other opportunistic infections, there is a higher incidence of IRIS in patients with cryptococcal infection following antiviral and antifungal therapy [ 7 ]. This article presents a rare case of an HIV/HBV co-infected patient who presented with MDR-PTB and discontinued all medications during the initial treatment for HIV, HBV, and tuberculosis. During the subsequent re-anti-HBV/HIV treatment, the patient experienced two episodes of IRIS associated with cryptococcal infection. One episode was classified as “unmasking” IRIS, where previously subclinical cryptococcal infection became apparent with immune improvement. The other episode was categorized as “paradoxical” IRIS, characterized by the worsening of pre-existing cryptococcal infection despite immune restoration [ 8 ]. Fortunately, both episodes were effectively treated.
A 50-year-old male patient, who is self-employed, presented to our hospital in January 2022 with a chief complaint of a persistent cough for the past 2 months, without significant shortness of breath, palpitations, or fever. His medical history revealed a previous hepatitis B infection, which resulted in hepatic failure 10 years ago. Additionally, he was diagnosed with HIV infection. However, he ceased taking antiviral treatment with the medications provided free of charge by the Chinese government for a period of three years. During this hospital visit, his CD4 + T-cell count was found to be 26/μL (normal range: 500–1612/μL), HIV-1 RNA was 1.1 × 10 5 copies/ml, and HBV-DNA was negative. Chest computed tomography (CT) scan revealed nodular and patchy lung lesions (Fig. 1 ). The BALF shows positive acid-fast staining. Further assessment of the BALF using XpertMTB/RIF PCR revealed resistance to rifampicin, and the tuberculosis drug susceptibility test of the BALF (liquid culture, medium MGIT 960) indicated resistance to rifampicin, isoniazid, and streptomycin. Considering the World Health Organization (WHO) guidelines for drug-resistant tuberculosis, the patient’s drug susceptibility results, and the co-infection of HIV and HBV, an individualized treatment plan was tailored for him. The treatment plan included BIC/TAF/FTC (50 mg/25 mg/200 mg per day) for HBV and HIV antiviral therapy, as well as linezolid (0.6 g/day), cycloserine (0.5 g/day), moxifloxacin (0.4 g/day), pyrazinamide (1.5 g/day), and ethambutol (0.75 g/day) for anti-tuberculosis treatment, along with supportive care.
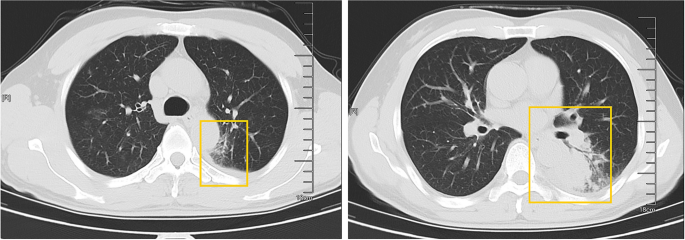
The patient’s pulmonary CT scan shows patchy and nodular lesions accompanied by a small amount of pleural effusion, later confirmed to be MDR-PTB
Unfortunately, after 3 months of follow-up, the patient discontinued all medications due to inaccessibility of the drugs. He returned to our hospital (Nov 12, 2022, day 0) after discontinuing medication for six months, with a complaint of poor appetite for the past 10 days. Elevated liver enzymes were observed, with an alanine aminotransferase level of 295 IU/L (normal range: 0–40 IU/L) and a total bilirubin(TBIL) level of 1.8 mg/dL (normal range: 0–1 mg/dL). His HBV viral load increased to 5.5 × 10 9 copies/ml. Considering the liver impairment, elevated HBV-DNA and the incomplete anti-tuberculosis treatment regimen (Fig. 2 A), we discontinued pyrazinamide and initiated treatment with linezolid, cycloserine, levofloxacin, and ethambutol for anti-tuberculosis therapy, along with BIC/TAF/FTC for HIV and HBV antiviral treatment. Additionally, enhanced liver protection and supportive management were provided, involving hepatoprotective effects of medications such as glutathione, magnesium isoglycyrrhizinate, and bicyclol. However, the patient’s TBIL levels continued to rise progressively, reaching 4.4 mg/dL on day 10 (Fig. 3 B). Suspecting drug-related factors, we discontinued all anti-tuberculosis medications while maintaining BIC/TAF/FTC for antiviral therapy, the patient’s TBIL levels continued to rise persistently. We ruled out other viral hepatitis and found no significant evidence of obstructive lesions on magnetic resonance cholangiopancreatography. Starting from the day 19, due to the patient’s elevated TBIL levels of 12.5 mg/dL, a decrease in prothrombin activity (PTA) to 52% (Fig. 3 D), and the emergence of evident symptoms such as abdominal distension and poor appetite, we initiated aggressive treatment methods. Unfortunately, on day 38, his hemoglobin level dropped to 65 g/L (normal range: 120–170 g/L, Fig. 3 A), and his platelet count decreased to 23 × 10 9 /L (normal range: 125–300 × 10 9 /L, Fig. 3 C). Based on a score of 7 on the Naranjo Scale, it was highly suspected that “Linezolid” was the cause of these hematological abnormalities. Therefore, we had to discontinue Linezolid for the anti-tuberculosis treatment. Subsequently, on day 50, the patient developed recurrent fever, a follow-up chest CT scan revealed enlarged nodules in the lungs (Fig. 2 B). The patient also reported mild dizziness and a worsening cough. On day 61, the previous blood culture results reported the growth of Cryptococcus. A lumbar puncture was performed on the same day, and the cerebrospinal fluid (CSF) opening pressure was measured at 130 mmH 2 O. India ink staining of the CSF showed typical encapsulated yeast cells suggestive of Cryptococcus. Other CSF results indicated mild leukocytosis and mildly elevated protein levels, while chloride and glucose levels were within normal limits. Subsequently, the patient received a fungal treatment regimen consisting of liposomal amphotericin B (3 mg/kg·d −1 ) in combination with fluconazole(600 mg/d). After 5 days of antifungal therapy, the patient’s fever symptoms were well controlled. Despite experiencing bone marrow suppression, including thrombocytopenia and worsening anemia, during this period, proactive symptom management, such as the use of erythropoietin, granulocyte colony-stimulating factor, and thrombopoietin, along with high-calorie dietary management, even reducing the dosage of liposomal amphotericin B to 2 mg/kg/day for 10 days at the peak of severity, successfully controlled the bone marrow suppression. However, within the following week, the patient experienced fever again, accompanied by a worsened cough, increased sputum production, and dyspnea. Nevertheless, the bilirubin levels did not show a significant increase. On day 78 the patient’s lung CT revealed patchy infiltrates and an increased amount of pleural effusion (Fig. 2 C). The CD4 + T-cell count was 89/μL (normal range: 500–700/μL), indicating a significant improvement in immune function compared to the previous stage, and C-reactive protein was significantly elevated, reflecting the inflammatory state, other inflammatory markers such as IL-6 and γ-IFN were also significantly elevated. On day 84, Considering the possibility of IRIS, the patient began taking methylprednisolone 30 mg once a day as part of an effort to control his excessive inflammation. Following the administration of methylprednisolone, the man experienced an immediate improvement in his fever. Additionally, symptoms such as cough, sputum production, dyspnea, and poor appetite gradually subsided over time. A follow-up lung CT showed significant improvement, indicating a positive response to the treatment. After 28 days of treatment with liposomal amphotericin B in combination with fluconazole, liposomal amphotericin B was discontinued, and the patient continued with fluconazole to consolidate the antifungal therapy for Cryptococcus. Considering the patient’s ongoing immunodeficiency, the dosage of methylprednisolone was gradually reduced by 4 mg every week. After improvement in liver function, the patient’s anti-tuberculosis treatment regimen was adjusted to include bedaquiline, contezolid, cycloserine, moxifloxacin, and ethambutol. The patient’s condition was well controlled, and a follow-up lung CT on day 117 indicated a significant improvement in lung lesions (Fig. 2 D).
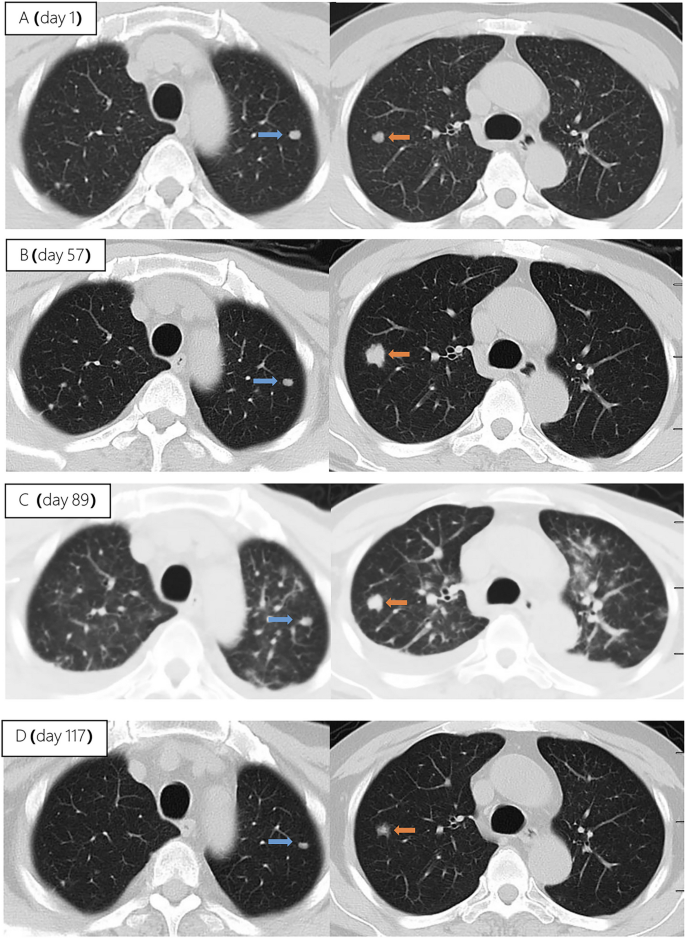
Upon second hospitalization admission ( A ), nodular lesions were already present in the lungs, and their size gradually increased after the initiation of ART ( B , C ). Notably, the lung lesions became more pronounced following the commencement of anti-cryptococcal therapy, coinciding with the occurrence of pleural effusion ( C ). However, with the continuation of antifungal treatment and the addition of glucocorticoids, there was a significant absorption and reduction of both the pleural effusion and pulmonary nodules ( D )
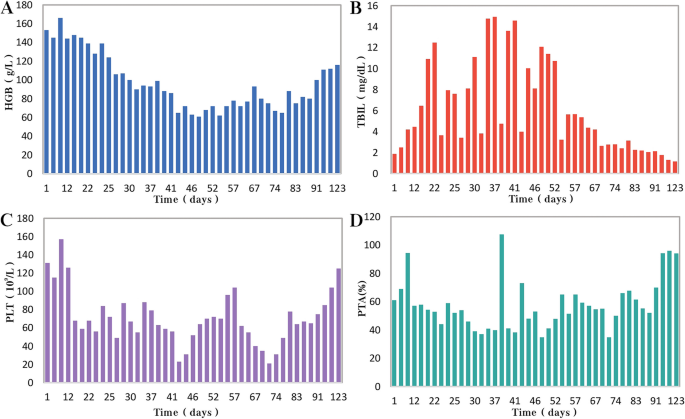
During the patient's second hospitalization, as the anti-tuberculosis treatment progressed and liver failure developed, the patient’s HGB levels gradually decreased ( A ), while TBIL levels increased ( B ). Additionally, there was a gradual decrease in PLT count ( C ) and a reduction in prothrombin activity (PTA) ( D ), indicating impaired clotting function. Moreover, myelosuppression was observed during the anti-cryptococcal treatment ( C )
People living with HIV/AIDS are susceptible to various opportunistic infections, which pose the greatest threat to their survival [ 5 ]. Pulmonary tuberculosis and disseminated cryptococcosis remain opportunistic infections with high mortality rates among AIDS patients [ 9 , 10 ]. These infections occurring on the basis of liver failure not only increase diagnostic difficulty but also present challenges in treatment. Furthermore, as the patient’s immune function and liver function recover, the occurrence of IRIS seems inevitable.
HIV and HBV co-infected patients are at a higher risk of HBV reactivation following the discontinuation of antiviral drugs
In this case, the patient presented with both HIV and HBV infections. Although the HBV DNA test was negative upon admission. However, due to the patient’s self-discontinuation of antiretroviral therapy (ART), HBV virologic and immunologic reactivation occurred six months later, leading to a rapid increase in viral load and subsequent hepatic failure. Charles Hannoun et al. also reported similar cases in 2001, where two HIV-infected patients with positive HBsAg experienced HBV reactivation and a rapid increase in HBV DNA levels after discontinuing antiretroviral and antiviral therapy, ultimately resulting in severe liver failure [ 11 ]. The European AIDS Clinical Society (EACS) also emphasize that abrupt discontinuation of antiviral therapy in patients co-infected with HBV and HIV can trigger HBV reactivation, which, although rare, can potentially result in liver failure [ 12 ].
Diagnosing disseminated Cryptococcus becomes more challenging in AIDS patients with liver failure, and the selection of antifungal medications is significantly restricted
In HIV-infected individuals, cryptococcal disease typically manifests as subacute meningitis or meningoencephalitis, often accompanied by fever, headache, and neck stiffness. The onset of symptoms usually occurs approximately two weeks after infection, with typical signs and symptoms including meningeal signs such as neck stiffness and photophobia. Some patients may also experience encephalopathy symptoms like somnolence, mental changes, personality changes, and memory loss, which are often associated with increased intracranial pressure (ICP) [ 13 ]. The presentation of cryptococcal disease in this patient was atypical, as there were no prominent symptoms such as high fever or rigors, nor were there any signs of increased ICP such as somnolence, headache, or vomiting. The presence of pre-existing pulmonary tuberculosis further complicated the early diagnosis, potentially leading to the clinical oversight of recognizing the presence of cryptococcus. In addition to the diagnostic challenges, treating a patient with underlying liver disease, multidrug-resistant tuberculosis, and concurrent cryptococcal infection poses significant challenges. It requires considering both the hepatotoxicity of antifungal agents and potential drug interactions. EACS and global guideline for the diagnosis and management of cryptococcosis suggest that liposomal amphotericin B (3 mg/kg·d −1 ) in combination with flucytosine (100 mg/kg·d −1 ) or fluconazole (800 mg/d) is the preferred induction therapy for CM for 14 days [ 12 , 14 ]. Flucytosine has hepatotoxicity and myelosuppressive effects, and it is contraindicated in patients with severe liver dysfunction. The antiviral drug bictegravir is a substrate for hepatic metabolism by CYP3A and UGT1A1 enzymes [ 15 ], while fluconazole inhibits hepatic enzymes CYP3A4 and CYP2C9 [ 16 ]. Due to the patient's liver failure and bone marrow suppression, we reduced the dosage of liposomal amphotericin B and fluconazole during the induction period. Considering the hepatotoxicity of fluconazole and its interaction with bictegravir, we decreased the dosage of fluconazole to 600 mg/d, while extending the duration of induction therapy to 28 days.
During re-antiviral treatment, maintaining vigilance for the development of IRIS remains crucial
IRIS refers to a series of inflammatory diseases that occur in HIV-infected individuals after initiating ART. It is associated with the paradoxical worsening of pre-existing infections, which may have been previously diagnosed and treated or may have been subclinical but become apparent due to the host regaining the ability to mount an inflammatory response. Currently, there is no universally accepted definition of IRIS. However, the following conditions are generally considered necessary for diagnosing IRIS: worsening of a diagnosed or previously unrecognized pre-existing infection with immune improvement (referred to as “paradoxical” IRIS) or the unmasking of a previously subclinical infection (referred to as “unmasking” IRIS) [ 8 ]. It is estimated that 10% to 30% of HIV-infected individuals with CM will develop IRIS after initiating or restarting effective ART [ 7 , 17 ]. In the guidelines of the WHO and EACS, it is recommended to delay the initiation of antiviral treatment for patients with CM for a minimum of 4 weeks to reduce the incidence of IRIS. Since we accurately identified the presence of multidrug-resistant pulmonary tuberculosis in the patient during the early stage, we promptly initiated antiretroviral and anti-hepatitis B virus treatment during the second hospitalization. However, subsequent treatment revealed that the patient experienced at least two episodes of IRIS. The first episode was classified as “unmasking” IRIS, as supported by the enlargement of pulmonary nodules observed on the chest CT scan following the initiation of ART (Fig. 2 A). Considering the morphological changes of the nodules on the chest CT before antifungal therapy, the subsequent emergence of disseminated cryptococcal infection, and the subsequent reduction in the size of the lung nodules after antifungal treatment, although there is no definitive microbiological evidence, we believe that the initial enlargement of the lung nodules was caused by cryptococcal pneumonia. As ART treatment progressed, the patient experienced disseminated cryptococcosis involving the blood and central nervous system, representing the first episode. Following the initiation of antifungal therapy for cryptococcosis, the patient encountered a second episode characterized by fever and worsening pulmonary lesions. Given the upward trend in CD4 + T-cell count, we attributed this to the second episode of IRIS, the “paradoxical” type. The patient exhibited a prompt response to low-dose corticosteroids, further supporting our hypothesis. Additionally, the occurrence of cryptococcal IRIS in the lungs, rather than the central nervous system, is relatively uncommon among HIV patients [ 17 ].
Conclusions
From the initial case of AIDS combined with chronic hepatitis B, through the diagnosis and treatment of multidrug-resistant tuberculosis, the development of liver failure and disseminated cryptococcosis, and ultimately the concurrent occurrence of IRIS, the entire process was tortuous but ultimately resulted in a good outcome (Fig. 4 ). Treatment challenges arose due to drug interactions, myelosuppression, and the need to manage both infectious and inflammatory conditions. Despite these hurdles, a tailored treatment regimen involving antifungal and antiretroviral therapies, along with corticosteroids, led to significant clinical improvement. While CM is relatively common among immunocompromised individuals, especially those with acquired immunodeficiency syndrome (AIDS) [ 13 ], reports of disseminated cryptococcal infection on the background of AIDS complicated with liver failure are extremely rare, with a very high mortality rate.
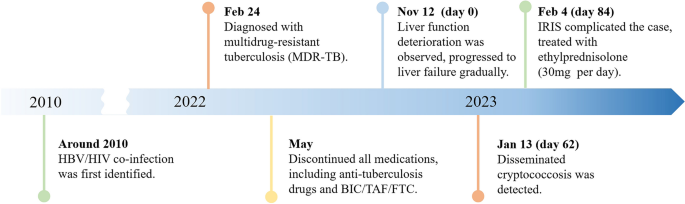
A brief timeline of the patient's medical condition progression and evolution
Through managing this patient, we have also gained valuable insights. (1) Swift and accurate diagnosis, along with timely and effective treatment, can improve prognosis, reduce mortality, and lower disability rates. Whether it's the discovery and early intervention of liver failure, the identification and treatment of disseminated cryptococcosis, or the detection and management of IRIS, all these interventions are crucially timely. They are essential for the successful treatment of such complex and critically ill patients.
(2) Patients who exhibit significant drug reactions, reducing the dosage of relevant medications and prolonging the treatment duration can improve treatment success rates with fewer side effects. In this case, the dosages of liposomal amphotericin B and fluconazole are lower than the recommended dosages by the World Health Organization and EACS guidelines. Fortunately, after 28 days of induction therapy, repeat CSF cultures showed negative results for Cryptococcus, and the improvement of related symptoms also indicates that the patient has achieved satisfactory treatment outcomes. (3) When cryptococcal infection in the bloodstream or lungs is detected, prompt lumbar puncture should be performed to screen for central nervous system cryptococcal infection. Despite the absence of neurological symptoms, the presence of Cryptococcus neoformans in the cerebrospinal fluid detected through lumbar puncture suggests the possibility of subclinical or latent CM, especially in late-stage HIV-infected patients.
We also encountered several challenges and identified certain issues that deserve attention. Limitations: (1) The withdrawal of antiviral drugs is a critical factor in the occurrence and progression of subsequent diseases in patients. Improved medical education is needed to raise awareness and prevent catastrophic consequences. (2) Prior to re-initiating antiviral therapy, a thorough evaluation of possible infections in the patient is necessary. Caution should be exercised, particularly in the case of diseases prone to IRIS, such as cryptococcal infection. (3) There is limited evidence on the use of reduced fluconazole dosage (600 mg daily) during antifungal therapy, and the potential interactions between daily fluconazole (600 mg) and the antiviral drug bictegravir and other tuberculosis medications have not been extensively studied. (4) Further observation is needed to assess the impact of early-stage limitations in the selection of anti-tuberculosis drugs on the treatment outcome of tuberculosis in this patient, considering the presence of liver failure.
In conclusion, managing opportunistic infections in HIV patients remains a complex and challenging task, particularly when multiple opportunistic infections are compounded by underlying liver failure. Further research efforts are needed in this area.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
Hepatitis B virus
Acquired immunodeficiency virus disease
Immune reconstitution inflammatory syndrome
Human immunodeficiency virus
Multi-drug resistant pulmonary tuberculosis
Bronchoalveolar lavage fluid
Bictegravir/tenofovir alafenamide/emtricitabine
Cryptococcal meningitis
World Health Organization
Computed tomography
Total bilirubin
Cerebrospinal fluid
European AIDS Clinical Society
Intracranial pressure
Antiretroviral therapy
Prothrombin activity
Bekker L-G, Beyrer C, Mgodi N, Lewin SR, Delany-Moretlwe S, Taiwo B, et al. HIV infection. Nat Rev Dis Primer. 2023;9:1–21.
Google Scholar
Data on the size of the HIV epidemic. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-the-size-of-the-hiv-aids-epidemic?lang=en . Accessed 3 May 2023.
Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, Noubiap JJ. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:2799–806.
Article Google Scholar
McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antivir Ther. 2007;12(Suppl 3):H3-13.
Article CAS PubMed Google Scholar
Kaplan JE, Masur H, Holmes KK, Wilfert CM, Sperling R, Baker SA, et al. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: an overview. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21 Suppl 1:S12-31.
Article CAS Google Scholar
Bamba S, Lortholary O, Sawadogo A, Millogo A, Guiguemdé RT, Bretagne S. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS Lond Engl. 2012;26:1039–41.
Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61.
Article PubMed PubMed Central Google Scholar
Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, Moodley P, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49:1424–32.
Obeagu E, Onuoha E. Tuberculosis among HIV patients: a review of Prevalence and Associated Factors. Int J Adv Res Biol Sci. 2023;10:128–34.
Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748–55.
Manegold C, Hannoun C, Wywiol A, Dietrich M, Polywka S, Chiwakata CB, et al. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;32:144–8.
EACS Guidelines | EACSociety. https://www.eacsociety.org/guidelines/eacs-guidelines/ . Accessed 7 May 2023.
Cryptococcosis | NIH. 2021. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/cryptococcosis . Accessed 6 May 2023.
Chang CC, Harrison TS, Bicanic TA, Chayakulkeeree M, Sorrell TC, Warris A, et al. Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect Dis. 2024;10:S1473-3099(23)00731-4.
Deeks ED. Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs. 2018;78:1817–28.
Article CAS PubMed PubMed Central Google Scholar
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Shelburne SA, Darcourt J, White AC, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40:1049–52.
Download references
Acknowledgements
We express our sincere gratitude for the unwavering trust bestowed upon our medical team by the patient throughout the entire treatment process.
This work was supported by the Scientific Research Project of Hunan Public Health Alliance with the approval No. ky2022-002.
Author information
Wei Fu and Zi Wei Deng contributed equally to this work.
Authors and Affiliations
Center for Infectious Diseases, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Wei Fu, Pei Wang, Zhen Wang Zhu, Ye Pu, Zhi Bing Xie, Yong Zhong Li & Hong Ying Yu
Department of Tuberculosis, The First Affiliated Hospital of Xinxiang Medical University, XinXiang, Henan, China
Department of Clinical Pharmacy, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Zi Wei Deng
You can also search for this author in PubMed Google Scholar
Contributions
WF and ZWD integrated the data and wrote the manuscript, YHY contributed the revision of the manuscript, PW and YP provided necessary assistance and provided key suggestions, ZWZ, YZL and ZBX contributed data acquisition and interpretation for etiological diagnosis. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Hong Ying Yu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of the Hunan University of Medicine General Hospital (HYZY-EC-202306-C1), and with the informed consent of the patient.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fu, W., Deng, Z.W., Wang, P. et al. A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient. BMC Infect Dis 24 , 533 (2024). https://doi.org/10.1186/s12879-024-09431-9
Download citation
Received : 30 June 2023
Accepted : 24 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12879-024-09431-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver failure
- Disseminated cryptococcal disease
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Pulmonary tuberculosis_CS - Free download as Word Doc (.doc), PDF File (.pdf), Text File (.txt) or read online for free. This case study examines a 26-year-old female patient diagnosed with pulmonary tuberculosis. The patient presented with complaints of cough, dizziness, and shortness of breath. Her medical history and current symptoms are reviewed.
CASE STUDY PULMONARY TUBERCULOSIS - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or view presentation slides online.
1) The document is a case study on a 73-year old female patient named C.C.N. who was admitted to Cebu North General Hospital due to easy fatigability and was found to have pulmonary tuberculosis. 2) C.C.N. lives with her family in Mandaue City near a busy road and reports a history of respiratory illnesses. She is undergoing directly observed treatment for her active pulmonary TB infection. 3 ...
Pulmonary tuberculosis (PTB) is a widespread, infectious disease caused by Mycobacterium tuberculosis that typically attacks the lungs. It can spread through the air when people with active TB infection cough or sneeze. Most infections are latent and asymptomatic, but about 10% eventually progress to active disease. The patient presented with difficulty breathing, fatigue, and chest pain. He ...
This case study examines a 24-year-old Filipino man admitted to the hospital with pulmonary tuberculosis, pneumothorax, and hydrothorax. He had a history of tuberculosis 6 years prior but relapsed due to smoking and drinking. He presented with cough, blood-tinged sputum, weight loss, night sweats, and difficulty breathing. His family history included deaths from heart attack, tuberculosis, and ...
Tuberculosis continues tobe a major cause of death. It is a preventable and curable disease. Themost commonbacteria likely to cause a cystic pneumoniaare Staphylococcusaureus, streptococcuspneumonia and Escherichiacoli. The cystic pulmonary tuberculosis is exceptional and shoulddefinitized be from other cystic lung disease.Wepresent a case report of immunocompetent patient with acute ...
Abstract. Pulmonary tuberculosis is a contagious, airborne infection which primarily attacks the lungs, if the therapeutic regimen was not complied it can lead to MDR (Multi drug resistant ...
Introduction. Tuberculosis (TB) is caused by strains of Mycobacterium tuberculosis (M. Tuberculosis). TB is a primarily pulmonary infection spread by airborne droplet transmission. The development and spread of drug-resistant strains of M. tuberculosis greatly jeopardize TB control efforts. In the US, 91 cases of MDR-TB were reported in 2014.
TB case study.docx - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. This document outlines a case study on tuberculosis. It begins with objectives and an introduction defining tuberculosis, its causes, and prevalence worldwide and in the Philippines. It describes the types of tuberculosis ...
Introduction. Pulmonary tuberculosis (PTB), accounting for almost 50% of all new tuberculosis (TB) cases reported globally in 2021, continues to pose a serious threat to global health due to its high morbidity and mortality [].Some immunocompromised states, including those produced by aging, chronic diseases (such as HIV/AIDS and diabetes mellitus), and immunosuppressive therapy, may ...
The annual medians of incidence rates per 100 000 inhabitants were 7.5 for PTB and 3.1 for ETB. The study included 136 PTB cases and 57 ETB cases, because a reported ETB case in 2014 suffered a reactivation in 2016. The follow-up rate was 98.5% for PTB, and 100% for ETB, with loss of 2 patients in the PTB group.
This report describes three cases of tumour-like pulmonary tuberculosis: two patients had stage C3 human immunodeficiency virus (HIV) infection (with uncontrolled HIV-1 in one case) and one patient was immunocompetent. All patients initially presented with general and respiratory symptoms, with radiological findings simulating lung carcinoma.
Case Study. A 44-year-old man presented to the TB Clinic with symptoms of progressive shortness of breath and cough with greenish sputum production. His sputum test results showed that he had atypical TB ( Mycobacterium Avium Complex MAC infection ). He was HIV negative at this time. Past history revealed that he was in good health till 1991 ...
Background Some medical conditions may increase the risk of developing pulmonary tuberculosis (PTB); however, no systematic study on PTB-associated comorbidities and comorbidity clusters has been undertaken. Methods A nested case-control study was conducted from 2013 to 2017 using multi-source big data. We defined cases as patients with incident PTB, and we matched each case with four event ...
The annual medians of incidence rates per 100 000 inhabitants were 7.5 for PTB and 3.1 for ETB. The study included 136 PTB cases and 57 ETB cases, because a reported ETB case in 2014 suffered a reactivation in 2016. The follow-up rate was 98.5% for PTB, and 100% for ETB, with loss of 2 patients in the PTB group. Figure 1.
Read chapter 34 of Infectious Diseases: A Case Study Approach online now, exclusively on AccessPharmacy. AccessPharmacy is a subscription-based resource from McGraw Hill that features trusted pharmacy content from the best minds in the field.
TB CASE STUDY #1. 31-year-old caucasian male presented to the Emergency Department (ED) after experiencing gross hemoptysis. He had a 2 month history of productive cough, a 25 pound weight loss, night sweats, and fatigue. A CXR revealed bilateral cavitary infiltrates.
This strain, identified as M. tuberculosis subsp. canetti or, more simply, M. canetti, was first isolated in 1969 by Georges Canetti from a French farmer. The strain was preserved at the Pasteur Institute where its antigenic pattern was studied extensively. We report two cases of pulmonary TB caused by this strain.
Clinical and radiological manifestations of tuberculosis (TB) are heterogeneous, and differential diagnosis can include both benign and malignant diseases (e.g., sarcoidosis, metastatic diseases, and lymphoma). Diagnostic dilemmas can delay appropriate therapy, favoring Mycobacterium tuberculosis transmission.We report on a case of TB in an immunocompetent, Somalian 22-year-old boy admitted in ...
Tuberculosis is the biggest infectious disease killer in the world 1, and is endemic in Nepal with the national prevalence at 416 cases per 100000 population 2. Pulmonary tuberculosis is the most common form. In Nepal, tuberculosis prevalence is more in productive age group (25-64 years) and men. Poverty, malnutrition, overcrowding ...
and tuberculosis and HIV co-infection burdens in the world. 6. We aimed to estimate the prevalence of bacteriologically confirmed pulmonary tuberculosis among people aged . 15 years or older in South Africa and improve the understanding of tuberculosis epidemiology for evidence-based control efforts. Methods. Study design and participants
Extra-pulmonary tuberculosis (EPTB) is defined as any bacteriologically confirmed or clinically diagnosed case of TB involving organs other than the lungs. It is frequently a diagnostic and therapeutic challenge with paucity of data available. The aim of this study was to assess the prevalence of bacteriologically confirmed EPTB; to determine the most affected organs and to evaluate the ...
According to the WHO Global tuberculosis report 2020, Extra pulmonary Involvement can occur in isolation or along with a pulmonary cases focus as in the case of patients with multiple tuberculosis ...
PSP is a rare disease; especially, in those patients with multiple lesions, it is easy to be misdiagnosed as metastatic tumor, pulmonary tuberculosis, and other diseases 5,6,7,8,9,10.
Background An accurate and non-invasive approach is urgently needed to distinguish tuberculosis granulomas from lung adenocarcinomas. This study aimed to develop and validate a nomogram based on contrast enhanced-compute tomography (CE-CT) to preoperatively differentiate tuberculosis granuloma from lung adenocarcinoma appearing as solitary pulmonary solid nodules (SPSN). Methods This ...
1 1 Title: Diabetes mellitus is associated with a shared hyper-inflammatory immune 2 response in melioidosis and tuberculosis patients: an observational case-control study 3 4 Authors 5 Patpong Rongkard1,2, Barbara Kronsteiner1,2, Clare Eckold3, Kemajittra Jenjaroen2, Suchintana 6 Chumseng2, Parinya Chamnan4, Mohammad Ali1,2,11, Emanuele Marchi5, Direk
The 50-year-old male with a history of chronic hepatitis B and untreated human immunodeficiency virus (HIV) infection presented to the hospital with a mild cough and expectoration, revealing multi-drug resistant pulmonary tuberculosis (MDR-PTB), which was confirmed by XpertMTB/RIF PCR testing and tuberculosis culture of bronchoalveolar lavage ...