
- Report Highlights
- EU-Summaries

- About the publications

Malaria status & challenges of the epidemic
- Level 1: Summary
- Level 2: Details
- 1. Introduction

Malaria is one of the most common infectious diseases and a great public health problem worldwide, particularly in Africa and south Asia. About three billion people are at risk of infection in 109 countries. Each year, there are an estimated 250 million cases of malaria leading to approximately one million deaths, mostly in children under five years of age. The organism that causes the most dangerous form of malaria is a microscopic parasite called Plasmodium falciparum .
This parasite is transmitted by mosquito species belonging to the Anopheles genus and only by females of those species.
There is growing international agreement on how best to use prevention and treatment methods that are available. The most effective prevention measures include the use of mosquito bed nets treated with long-lasting insecticides – to avoid the mosquito bites and to kill the mosquitoes – and spraying the inside walls of houses with similar insecticides to kill malaria-carrying mosquitoes. The most effective treatment for malaria consists in using a combination of several anti-malarial drugs, one of which is a derivative of artemisinin . Preventive treatment of pregnant women with anti-malarial drugs can also reduce the harmful effects of malaria both on the mother and on the unborn child.
Several international organisations have set up ambitious objectives for large-scale malaria control. The target set by the Word Health Organization ( WHO ) in 2005 is to offer malaria prevention and treatment services by 2010 to at least 80% of the people who need them. By doing so, it aims to reduce at least by half the proportion of people who become ill or die from malaria by 2010 and at least by three quarters by 2015 compared to 2005.
It is vital to monitor malaria trends to see if malaria control campaigns are being effective, and to make improvements.
The WHO World Malaria Report 2008 estimates the number of malaria cases and deaths for the period 2001-2006 in affected countries and investigates whether or not WHO recommendations are being implemented. It evaluates progress made against the disease it also describes the sources of funding and reviews the impact of malaria control programmes. The aim of the report is to support the development of effective national malaria control programmes.
- 2. Which strategies were adopted to prevent and treat malaria?
- 3. How many people were affected by malaria in 2006?
- 4. What is being done to prevent and treat malaria?
- 5. How much funding is allocated to malaria control?
- 6. How effective is malaria control?
- 7. Can malaria be completely eradicated?
- Accidental poisoning
- Acrylamide in food
- Acupuncture
- Agriculture
- Aids Epidemic
- Air Pollution Europe
- Air quality in Europe
- Allergenic fragrances
- Aluminium exposure
- Animal testing
- Antibiotic resistance
- Antibiotics Research
- Antimicrobial resistance
- Aquatic environment
- Arctic Climate Change
- Artificial Light
- Artificial Light and Health
- Aspartame Reevaluation
- Aspirin & Cancer
- Benzodiazepines
- Biodiversity
- Biological Diversity
- Biosecurity
- Bisphenol A
- CO 2 Capture & Storage
- Cancer rates and mortality, types and causes
- Chemical Mixtures
- Children & Screens
- Chlorine Sodium Hypochlorite
- Chlorpyrifos pesticide
- Chronic Diseases on Labour Practices
- Circular Economy
- Climate Change
- Climate Change Mitigation
- Climate impact of shale gas
- Climate impacts adaptation
- Dental Amalgams
- Dental Fillings
- Desertification
- Diet & Nutrition
- Ecosystem Change
- Effects of cannabis
- Electromagnetic Fields
- Electronic Cigarettes
- Endocrine Disruptors
- Endocrine disrupting properties of pesticides
- Endocrine disruptors risks
- Energy Saving Lamps
- Energy Technologies
- Epidemic diseases
- Estrogen-progestogen cancer risk
- Europe Green Deal
- Evaluation of endocrine disruptors
- Exposure to chemical mixtures
- Fisheries and aquaculture
- Fluorinated gases
- Food & Agriculture
- Food Wastage
- Forests & Energy
- Forests & agriculture land use
- Fukushima Consequences
- Fukushima accident
- Genetically Modified Crops
- Geothermal Energy
- Global Biodiversity Outlook 4
- Global Public Health Threats
- Global Warming
- Gluten intolerance
- Glyphosate and cancer
- Hazardous chemicals
- Health Effects of Electromagnetic Fields
- Health Environment Management
- Illicit drugs in Europe
- Impacts of a 4°C global warming
- India Millennium Development Goals
- Indonesian forests
- Indoor Air Quality
- Land Degradation and Desertification
- Lyme Disease
- Marine Litter
- Marine litter
- Mercury from dental amalgam
- Mercury in CFL
- Metal-on-Metal hip implants
- Methylene glycol
- Mineral extraction risks
- Multiple vaccinations
- Nano-silica
- Nanomaterials
- Nanotechnologies
- Neonicotinoids
- Nitrogen Dioxide
- Non-human primates
- Organic Food
- Ozone layer depletion
- Parabens used in cosmetics
- Particulate Matter
- Perfluorooctanoic acid (PFOA)
- Personal Music Players & Hearing
- Pesticides occupational risks
- Pharmaceuticals environment
- Phosphate resources
- Phthalates Comparison
- Phthalates in school supplies
- Poly brominated flame retardant decaBDE
- Power lines
- Psychoactive Drugs
- Radiological nuclear emergency
- Respiratory Diseases
- Safety of Cosmetics
- Safety of sunscreens
- Sand Extraction
- Security Scanners
- Silver Nanoparticles
- Single-use plastics
- Soils degradation
- Solar Energy
- State of the European Environment
- Static Fields
- Substitution of harmful chemicals
- Sulfaxoflor Pesticide
- Sunbeds & UV radiation
- Sustainable oceans
- Synthetic Biology
- Thorium nuclear fuel
- Tidal Energy
- Titanium dioxide nanoparticles
- Tooth Whiteners
- Transgenic salmon
- Tuberculosis
- Wastewater management
- Water Disinfectants
- Water Resources
- Water Resources Assessments
- Water resources
- Wind Resources
- X-Ray Full-Body Scanners

Get involved!
This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!
- Terms & Conditions

- Biology Article
- Common Diseases In Humans Malaria
Table of Contents
What is malaria, causes of malaria.
- Symptoms of Malaria
Malaria Life Cycle Diagram
- Prevention of Malaria
Malaria is a mosquito-borne infectious disease caused by various species of the parasitic protozoan microorganisms called Plasmodium . Malaria is a disease that man has battled with for a long time. The first evidence of this protozoan came from mosquitoes preserved in amber nearly 30 million years ago.
It is even thought to have brought the Roman Empire to its knees. Malaria was so prevalent during the Roman times that the disease is also called ‘Roman Fever’. Today, the credit for actually discovering the parasite is given to Charles Louis Alphonse Laveran, a French physician. He even won the Nobel Prize in 1907 for his findings.
Here, let’s learn more about malaria and the life cycle of malarial parasite with the help of a diagram.
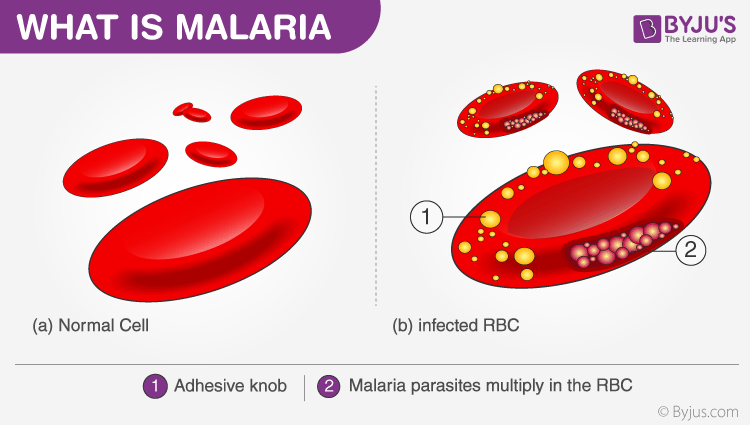
There are many factors that can cause malaria, such as –
- Bitten by a malarial vector (Anopheles stephensi)
- Use of shared and infected syringes.
- Organ transplantation.
- Transfusion.
- From an infected mother to her baby during birth.
Symptoms of malaria
Symptoms of malaria are exhibited within 7 to 18 days of being infected. Common symptoms include:
- Fever, fatigue, chills, vomiting, and headaches
- Diarrhoea, anaemia and muscle pain
- Profuse sweating and convulsions
- Bloody stools.
- In severe cases, malaria can be devastating; it can lead to seizures, coma and eventually, death.
Sir Ronald Ross and his study on the transmission of the disease helped carve the way for future scientists to effectively combat the disease. His deep research showed that specifically, the female Anopheles stephensi mosquito is the vector of the disease, and addressing this problem will prevent malaria and in turn, save countless lives.
Below is a labelled diagram of the malarial parasite to give you a better insight of their life cycle.
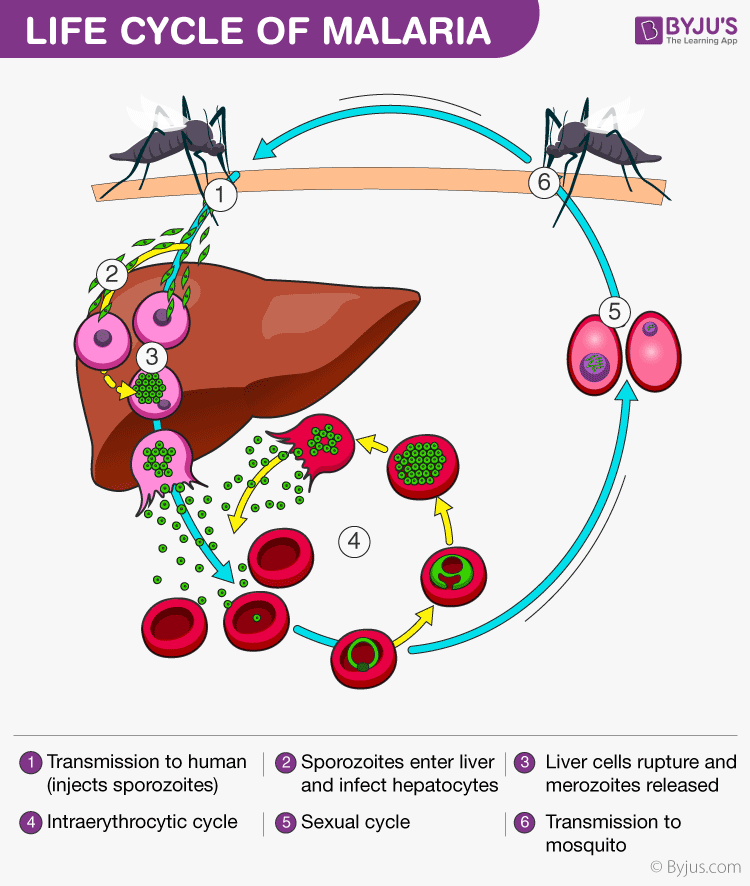
Malaria parasite exists in the form of a motile sporozoite. The vector of malaria i.e. the female Anopheles mosquito transmits the malarial sporozoites into the hosts. When an infected mosquito bites a human, the sporozoites are injected into the blood through the mosquito’s saliva.
The sporozoites travel into our body and accumulate in the liver. These parasites initially multiply within the liver, by damaging the liver and rupturing the blood cells in the body. Malaria kills by causing the destruction of the red blood cells in the host. The parasites reproduce asexually in the RBCs, bursting the cells and releasing more parasites to infect more cells. The rupture of red blood cells by the malaria parasite releases a toxin called hemozoin which causes the patient to experience a condition known as the chills.
When the female Anopheles mosquito bites an infected human, the parasites enter the mosquito’s body along the human blood it is drinking. It is inside the mosquito’s body that the actual development and maturing of the parasite happens. The parasites produced in the human body reach the intestine of the mosquito where the male and females cells fertilize each other to lead to the formation of a sporozoite. On maturing, the sporozoite breaks out the mosquito’s intestine and migrate to the salivary glands. Once they reach salivary glands, they wait till the mosquito bites another human and the process of infection and disease begins all over again. It is prudent however to observe that the complete development of the malaria parasite takes place in two different hosts; humans and mosquitoes.

Prevention of malaria
Malaria is one of the major causes of preventable death in the world today. It affects more than 500 million people worldwide and causes 1 to 2 million deaths every year. It is a tropical infectious disease and almost 90 per cent of the cases are from Sub-Saharan Africa.
There are two ways to deal with malaria – prevent the mosquito bite from happening (i.e. preventative steps) or attack the parasites once they have infected the body.
The first method advocates the use of mosquito nets and mosquito repellents such as permethrin to prevent mosquitoes from biting. The second form of treatment uses a chemical called Quinine present in the bark of a cinchona tree. A form of drug chloroquine has proven very effective against malaria even though it is not a vaccine.
To learn more about malaria or other related diseases, please visit BYJU’S.
Frequently Asked Questions
What is plasmodium.
Plasmodium is a genus of parasitic protozoans that are known to infect the red blood cells in mammals and causes an infectious disease called malaria. Some species of plasmodium such as P.ovale, P.vivax, P.malariae and P.knowlesi are known to cause malaria.
What are some symptoms of malaria?
The primary symptoms include fever, fatigue, vomiting, chills, headaches, anaemia and diarrhoea. Muscle pain, bloody stools and profuse sweating are also seen. In severe cases, it can lead to seizures, coma and eventually death.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
