Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 28 May 2024

Prediabetes remission for type 2 diabetes mellitus prevention
- Andreas L. Birkenfeld ORCID: orcid.org/0000-0003-1407-9023 1 , 2 , 3 , 4 &
- Viswanathan Mohan ORCID: orcid.org/0000-0001-5038-6210 5 , 6
Nature Reviews Endocrinology volume 20 , pages 441–442 ( 2024 ) Cite this article
924 Accesses
2 Citations
55 Altmetric
Metrics details
- Medical research
- Pre-diabetes
Current guidelines for the delay and prevention of type 2 diabetes mellitus recommend for people with prediabetes to lose at least 7% of their body weight. Here, we advocate to use glycaemic remission as a goal of prevention in people with prediabetes and those who are at high risk for type 2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Nathan, D. M. et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 62 , 1319–1328 (2019).
Article PubMed PubMed Central Google Scholar
American Diabetes Association Professional Practice Committee. prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2024. Diabetes Care 47 (Suppl 1), S43–S51 (2024).
Article Google Scholar
Sandforth, A. et al. Mechanisms of weight loss-induced remission in people with prediabetes: a post-hoc analysis of the randomised, controlled, multicentre Prediabetes Lifestyle Intervention Study (PLIS). Lancet Diabetes Endocrinol. 11 , 798–810 (2023).
Article PubMed Google Scholar
Fritsche, A. et al. Different effects of lifestyle intervention in high- and low-risk prediabetes: results of the randomized controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 70 , 2785–2795 (2021).
Article CAS PubMed Google Scholar
Zhyzhneuskaya, S. V. et al. Time course of normalization of functional β-cell capacity in the diabetes remission clinical trial after weight loss in type 2 diabetes. Diabetes Care 43 , 813–820 (2020).
Tabák, A. G. et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373 , 2215–2221 (2009).
Jumpertz von Schwartzenberg, R., Vazquez Arreola, E., Sandforth, A. et al. Role of weight loss-induced prediabetes remission in the prevention of type 2 diabetes: time to improve diabetes prevention. Diabetologia https://doi.org/10.1007/s00125-024-06178-5 (2024).
Taylor, R. Type 2 diabetes and remission: practical management guided by pathophysiology. J. Intern. Med. 289 , 754–770 (2021).
Sathish, T. et al. Effect of conventional lifestyle interventions on type 2 diabetes incidence by glucose-defined prediabetes phenotype: an individual participant data meta-analysis of randomized controlled trials. Diabetes Care 46 , 1903–1907 (2023).
Wagner, R. et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 27 , 49–57 (2021).
Download references
Acknowledgements
The authors acknowledge the members of ‘The Prediabetes Group’, who are listed in the Supplementary Information document, for their assistance with reviewing this article. A.L.B. acknowledges the support of funding from the German Federal Ministry for Education and Research (01GI0925) via the German Center for Diabetes Research (DZD e.V.).
Author information
Authors and affiliations.
German Center for Diabetes Research (DZD), Neuherberg, Germany
Andreas L. Birkenfeld
Department of Internal Medicine IV, Diabetology, Endocrinology and Nephrology, Eberhard-Karls University Tübingen, Tübingen, Germany
Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen, Tübingen, Germany
Department of Diabetes, Life Sciences & Medicine Cardiovascular Medicine & Sciences, King’s College London, London, UK
Madras Diabetes Research Foundation, Chennai, India
Viswanathan Mohan
Dr. Mohan’s Diabetes Specialities Centre, Chennai, India
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andreas L. Birkenfeld .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Related links.
Diabetes mellitus: https://www.who.int/news-room/fact-sheets/detail/diabetes
World Health Organization’s global targets for noncommunicable diseases: https://www.who.int/publications/i/item/9789241506236
Supplementary information
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Birkenfeld, A.L., Mohan, V. Prediabetes remission for type 2 diabetes mellitus prevention. Nat Rev Endocrinol 20 , 441–442 (2024). https://doi.org/10.1038/s41574-024-00996-8
Download citation
Published : 28 May 2024
Issue Date : August 2024
DOI : https://doi.org/10.1038/s41574-024-00996-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
Public Health Approaches to Type 2 Diabetes Prevention: the US National Diabetes Prevention Program and Beyond
- Diabetes Epidemiology (E Selvin and K Foti, Section Editors)
- Open access
- Published: 05 August 2019
- Volume 19 , article number 78 , ( 2019 )
Cite this article
You have full access to this open access article

- Stephanie M. Gruss 1 ,
- Kunthea Nhim 1 ,
- Edward Gregg 2 ,
- Miriam Bell 1 ,
- Elizabeth Luman 1 &
- Ann Albright 1
29k Accesses
92 Citations
Explore all metrics
A Correction to this article was published on 27 June 2020
This article has been updated
Purpose of Review
This article highlights foundational evidence, translation studies, and current research behind type 2 diabetes prevention efforts worldwide, with focus on high-risk populations, and whole-population approaches as catalysts to global prevention.
Recent Findings
Continued focus on the goals of foundational lifestyle change program trials and their global translations, and the targeting of those at highest risk through both in-person and virtual modes of program delivery, is critical. Whole-population approaches (e.g., socioeconomic policies, healthy food promotion, environmental/systems changes) and awareness raising are essential complements to efforts aimed at high-risk populations.
Successful type 2 diabetes prevention strategies are being realized in the USA through the National Diabetes Prevention Program and elsewhere in the world. A multi-tiered approach involving appropriate risk targeting and whole-population efforts is essential to curb the global diabetes epidemic.
Similar content being viewed by others
Evidence and Challenges for Translation and Population Impact of the Diabetes Prevention Program

Diabetes Prevention Amongst South Asians: Current Evidence, Challenges, and a Way Forward
Lifestyle interventions for diabetes prevention in south asians: current evidence and opportunities.
Avoid common mistakes on your manuscript.
Introduction
Worldwide, it is estimated that 425 million adults (20–79 years) have diabetes, projected to reach 629 million by 2045 [ 1 •]. In the USA, about 30 million adults (18 years and older) have diabetes, or 12.2% of the adult population [ 2 ]. Diabetes was estimated to cost $727 billion in 2017 in health expenditures worldwide [ 1 ] and $327 billion in 2017 in total economic costs in the USA [ 3 •]. Increases in diabetes prevalence have led to increases in related complications, such as cardiovascular disease, visual impairment and vision loss, lower extremity amputations, end-stage renal disease, disability, and premature mortality [ 2 ].
The majority of diabetes is type 2, which generally follows a period of prediabetes, a condition where blood glucose levels are higher than normal, but not high enough for a type 2 diabetes diagnosis [ 2 ]. In the USA, risk factors for prediabetes and type 2 diabetes include being overweight or having obesity; having a racial/ethnic background that is African-American, Hispanic/Latino, American Indian, Asian American, or Pacific Islander; having a parent or sibling with diabetes; having hypertension; having a history of gestational diabetes; and living a sedentary lifestyle. An estimated 353 million adults worldwide [ 1 ]—84.1 million in the USA (33.9% of all adults) [ 2 ]—have prediabetes diabetes. Given the magnitude of these numbers, identifying those at risk and preventing or delaying onset of type 2 diabetes are critical to ending the pandemic.
Type 2 diabetes can be prevented or delayed through mitigation of modifiable risk factors, such as healthier eating, weight loss, and increased physical activity. Studies show lifestyle intervention as a sole modality, lifestyle intervention in combination with therapeutics, and whole-population approaches are all promising for type 2 diabetes prevention. Public health interventions using glycemic risk stratification to target individuals at “very high” risk (having obesity and impaired glucose tolerance (IGT)) and “high” risk (being overweight with IGT) have proven to significantly reduce conversion to type 2 diabetes [ 4 ]. Individuals in these categories have a fasting plasma glucose (FPG) > 100 mg/dL, HbA1c levels > 5.7%, and a 10-year diabetes incidence of 20–30% or more [ 5 ]. Individuals in lower risk tiers may be more appropriately targeted via risk counseling and whole-population strategies [ 5 , 6 , 7 ]. Population-level policies, systems, and environmental approaches, along with lifestyle intervention for those at high risk, are likely optimal to achieve the greatest level of impact [ 8 ].
The purpose of this review is to highlight the foundational and current research and translation studies which underlie high-risk population and whole-population strategies for type 2 diabetes prevention worldwide, with particular focus on the U.S. National Diabetes Prevention Program, as catalysts for global prevention efforts.
Worldwide Evidence for Type 2 Diabetes Prevention Through Lifestyle Change
Foundational research.
The scientific evidence for prevention through lifestyle changes is compelling based on a series of randomized control trials (RCTs), which found through intensive, structured, yearlong educational programs focused on moderate weight loss (5–7%), increasing self-efficacy around engagement in one’s health, and moderate increases in physical activity over time, it is possible to prevent or delay type 2 diabetes among those at very high and high risk. RCTs have been conducted in various settings among diverse racial and ethnic populations. Common elements include utilizing a group-based intervention and evaluating effectiveness in terms of increased physical activity and healthy eating, and improved clinical metrics, such as weight, body mass index (BMI), waist circumference, HbA1c, and blood glucose.
The Chinese Da Qing group-based RCT was the first and longest running of such studies. It began in 1986 [ 9 ] with a follow-up study conducted in 1997–2006. The study demonstrated a 43% incidence reduction in the intervention group after 14 years when compared to the control group. Type 2 diabetes was delayed by an average of 3.6 years [ 10 ], and the incidence of severe retinopathy and cardiovascular-related disease and events were reduced [ 10 , 11 ]. After 30 years, 577 adults with IGT were followed from the original trial; the intervention group had a median delay in type 2 diabetes incidence of 3.96 years, an average increase in life expectancy of 1.44 years, and fewer cardiovascular disease and all-cause deaths, reduced incidence of cardiovascular events, and lower incidence of microvascular complications compared to the control group [ 12 ].
The U.S. Diabetes Prevention Program (DPP) was a three-arm RCT, which began in 1996 [ 13 ••]. The study found that a lifestyle change intervention focused on a 5–7% weight loss and a moderate increase in physical activity over one year achieved a 58% relative risk reduction in type 2 diabetes, and that use of metformin achieved a 31% reduction, when compared to a placebo [ 13 ••]. The DPP used intensive one-on-one counseling with a minimum of 50% racially and ethnically diverse individuals across both male and female genders at high risk for type 2 diabetes (elevated plasma glucose of 95 to 125 mmol/L or fasting glucose of 7.8 to 11.0 mmol/L) [ 13 ••]. A follow-up study, the U.S. Diabetes Prevention Program Outcomes Study (DPPOS), reported a 34% reduction in type 2 diabetes incidence 10 years after the completion of the DPP trial for the lifestyle change intervention arm [ 14 ], and a 27% reduction after 15 years (18% for the metformin arm), compared to the placebo group [ 15 ]. Furthermore, the study found that lifestyle change was cost-effective compared to a placebo, and that cumulative quality-adjusted life years (QALYs) gained over a 3-year timeframe were greater for lifestyle (6.81) than either metformin (6.69) or a placebo (6.67) [ 16 ].
The Finnish Diabetes Prevention Study (Finnish DPS), which began in 2001 [ 17 ], tested lifestyle intervention in a community-based primary health care setting, and designed and implemented a high-risk screening assessment for type 2 diabetes that is used worldwide called the Finnish Type 2 Diabetes Risk Score [ 18 ]. The study demonstrated a risk reduction of 58%, as well as a legacy effect of 43% reduction in type 2 diabetes incidence three years after completion of the study. The study has also been successfully translated in Greece with similar results [ 19 ].
The Japanese DPP, conducted in 2005 in male participants with IGT > 140 mg/dL, found a cumulative 4-year incidence of diabetes of 9.3% in the control group, versus 3.0% in the intervention group [ 20 ]. The Indian DPP-1 trial, conducted in 2006, was a community-based RCT involving 531 subjects with IGT across three intervention arms (one was given advice on lifestyle modification (LSM), one was treated with metformin (MET), and another was given LSM plus MET) and one control arm. A relative risk reduction of 26–29% after 30 months was similar in all three intervention arms [ 21 ]. Additional 3-year results suggest that both metformin and lifestyle change were cost-effective in preventing type 2 diabetes [ 22 ].
Translational Research
Translational research, which examines how to best tailor key research findings into policy, program, or practice [ 23 ], further demonstrates that lifestyle interventions are feasible and effective in real-world settings [ 24 ]. A systematic review and meta-analysis of 28 US-based DPP translation studies found a mean weight loss of over 4% across 3797 high-risk participants and demonstrated cost-effectiveness in terms of program, materials, and staff costs [ 24 ].
The European DE-PLAN study (“Diabetes in Europe – Prevention using Lifestyle, Physical Activity, and Nutritional Intervention”), implemented in 17 countries, was a community-based 10-month translation of the Finnish DPS targeting those at high risk for type 2 diabetes [ 19 ]. DE-PLAN began in 2008 and used the Finnish Diabetes Risk Score to determine eligibility [ 19 ]. In 2018, study participants in Poland ( n = 175) with increased risk for type 2 diabetes received 10 months of lifestyle counseling sessions, physical activity, and self-efficacy sessions [ 25 ••]. Participants with a higher starting BMI and a history of increased glucose were more likely to achieve the goal weight loss of ≥ 5% of initial body weight compared to those with lower risk [ 25 ]. A UK study had similar findings, also determining adults with obesity and higher HbA1c levels (6–6.4%) not only met the weight loss outcomes of the US DPP trial but also gained more QALYs than those with lower risk; the intervention was also determined to be cost-saving [ 26 ].
The Australian Good Ageing in Lahti Region Lifestyle (GOAL) Implementation program, based on the Finnish DPS, found positive associations between changes in self-efficacy and dietary behaviors and improvement in waist circumference, cholesterol levels, triglycerides, diastolic blood pressure, and FPG in the lifestyle intervention arm compared to the control group [ 27 ]. GOAL was further scaled up with over 10,000 participants via the Melbourne DPS with significant improvements in cardiovascular risk factors, waist circumference, BMI, and weight loss [ 28 ].
Alternative modalities of lifestyle change program delivery, such as virtual program delivery and telehealth, have the potential to reach millions of people, even in remote areas. Shortly after publication of the 2002 DPP research study, Tate et al. (2003) conducted the first RCT of a yearlong Internet-based diabetes prevention lifestyle change weight loss program alone vs. one with the addition of e-behavioral counseling [ 29 ]. The group receiving e-behavioral counseling submitted calorie and exercise information and received weekly e-mail behavioral counseling and feedback from a counselor for 12 months and lost 4.8% of original body weight compared to 2.2% among those receiving the Internet program only [ 29 ]. A 2013 text messaging study in India showed that mobile technology can have an impact on clinical outcomes, with cumulative incidence of type 2 diabetes at 2 years of 18% in the text messaging counseling group vs. 27% in the control group [ 30 ], and a sustained reduction in incidence after 5 years [ 31 ]. Similar studies have been conducted (Supplemental Table 1 ) to determine the effectiveness of various forms of virtual delivery, including delivery via television, social networking sites, and online. These published studies on virtual delivery found ~ 4% to 10% weight loss after virtual implementation of a yearlong diabetes prevention lifestyle change program. A summary of results is included in Supplemental Table 1 .
The evidence is clear: lifestyle interventions can prevent or delay type 2 diabetes among various races, ethnicities, genders, and regions. Additionally, type 2 diabetes prevention program interventions continue to demonstrate cost-effectiveness/cost savings in real-world settings over time [ 32 , 33 ].
The US National DPP—a Case Study
Large-scale implementation of the US DPP trial’s lifestyle change program began in 2010, when Congress authorized the US Centers for Disease Control and Prevention (CDC) to establish and lead the National DPP in an effort to make the intervention broadly available to individuals at high risk [ 34 ]. The National DPP is a partnership of public and private organizations working to build a delivery system for the lifestyle intervention. It consists of four core elements: a trained workforce of lifestyle coaches; national quality standards supported by the CDC Diabetes Prevention Recognition Program (DPRP); a network of program delivery organizations sustained through coverage; and participant referral and engagement [ 35 ]. The National DPP lifestyle change program is based on evidence and key features of the US DPP trial that were shown to be successful: realistic weight loss goal after 12 months (minimum 5% of initial body weight), documentation of physical activity minutes (≥ 150 min per week), and attendance throughout the 12-month program, with an emphasis on self-efficacy that focuses on improving problem-solving skills, social supports, the use of built environments, and strategies to adapt to change [ 35 ]. The National DPP is the world’s largest translation of the US DPP study, having reached over 324,000 participants across > 3,000 organizations as of April 12, 2019 [ 36 ]. The DPRP is the quality assurance arm of the National DPP, developing evidence-based standards (DPRP Standards) and monitoring and evaluating participating organizations for fidelity and effectiveness of intervention delivery [ 37 ]. The DPRP Standards are updated every three years based on new dietary, physical activity, self-efficacy, delivery modality, and other type 2 diabetes prevention evidence. Through the DPRP, CDC awards either preliminary (based on participant attendance rather than outcomes) or full (meeting all DPRP Standards) recognition to successful organizations [ 37 ]. CDC recognition is widely accepted in the USA as an assurance of a quality type 2 diabetes prevention program and can result in insurance coverage for participants and reimbursement for program delivery organizations.
Public and private insurance coverage for type 2 diabetes prevention interventions is crucial to widespread adoption and participation in National DPP lifestyle change programs. Insurance coverage expands payment options, thereby reducing the burden of cost for participants. Currently, over 3.8 million public employees and dependents in 20 states have the National DPP lifestyle change program as a covered benefit and over 100 private employers and commercial plans include it as a covered benefit for their employees [ 38 ]. In 2018, the US Centers for Medicare & Medicaid Services (CMS) began coverage for eligible Medicare beneficiaries who participate in CDC-recognized programs and meet performance goals of the National DPP [ 40 ]. CMS provides reimbursement for participants meeting program goals such as 5% weight loss in organizations that have achieved either preliminary or full CDC recognition [ 39 ]. Eight states in the USA also provide Medicaid coverage for eligible beneficiaries [ 38 ].
Based on published translational research, feedback from stakeholder organizations, and gray literature scans (stakeholder materials and policies not found in peer-reviewed journals), CDC concluded that there was sufficient evidence to allow organizations delivering the National DPP lifestyle change program virtually to apply for CDC recognition. Thus, in order to expand availability and increase program participation (especially for those in rural or remote locations), the DPRP Standards were amended in January 2015 to allow online modes of delivery in addition to in-person. The Standards were amended again in February 2018 to also include telehealth and combination (in-person/virtual) delivery and new participant-level variables that include education level, enrollment source, and payer source. All virtual providers are held accountable to the same quality standards as in-person delivery organizations, including the provision of coaching services [ 37 ]. As of April 2019, CDC had 121 recognized virtual providers delivering the yearlong lifestyle change program to over 193,000 people [ 36 ].
Key Findings from In-Person National DPP Delivery in the USA
DPRP data from the first 4 years (February 2012–January 2016) of the National DPP, describing the experience of 14,747 participants who attended 4 or more sessions of the lifestyle change program in 220 organizations, showed an average weight loss of 4.2%, with 35.5% of participants achieving ≥ 5% weight loss [ 40 ]. Participants reported an average 152 min per week of physical activity, with 41.8% meeting the physical activity goal of 150 min per week. Participants with longer retention and greater participation in the program and who were more physically active were more likely to have higher weight loss [ 40 ].
Implementation of the National DPP: New Insights
Analysis sample.
To further assess National DPP implementation success, we examined recent DPRP data and analyzed new variables (education level, enrollment source, and payer source) to assess their relationship with participant outcomes. More than 297,000 participants attended one or more lifestyle change program sessions between February 2012 and January 2019. For the purpose of this new analysis, 143,489 eligible participants across all program modalities (in-person, online, distance learning, and combination) who completed the yearlong lifestyle change program and attended ≥ 3 sessions in the first 6 months (analyzed participants) were included in descriptive analyses. Of those, 29,069 eligible participants who started the program in 2018 and reported new variables (education level, enrollment source, and payer source) were included in the multivariable analysis (Fig. 1 ).
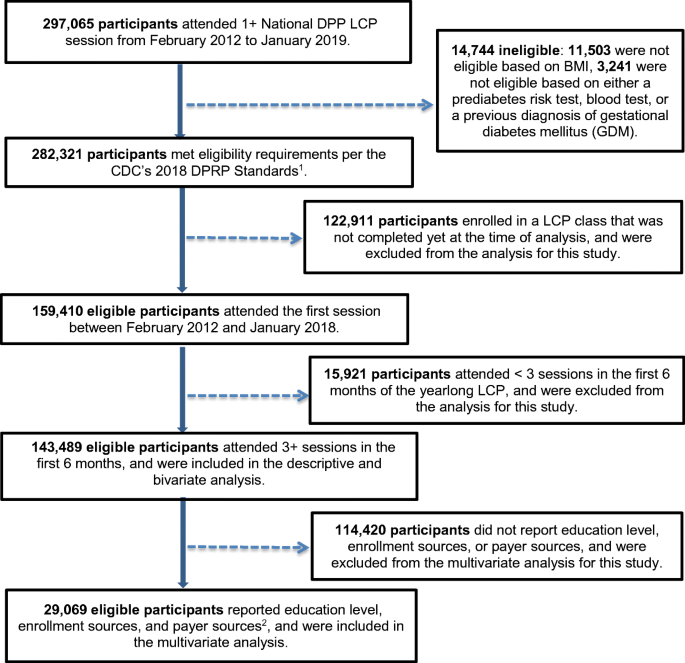
Flow chart for analysis sample. CDC = Centers for Disease Control and Prevention; DPRP = Diabetes Prevention Recognition Program; LCP = CDC-recognized lifestyle change program; National DPP = National Diabetes Prevention Program. 1 Participant eligibility was based on BMI (≥ 25 kg/m 2 , or ≥ 23 kg/m 2 if Asian American), a blood test indicating prediabetes, a CDC Prediabetes Screening Test or American Diabetes Association Type 2 Diabetes Risk Test, or a previous diagnosis of gestational diabetes mellitus. 2 Education level, enrollment sources, and payer sources were not collected before February 2018
Measures and Methods
Percent body weight change was the primary outcome of this analysis, calculated among participants with at least 2 documented body weights using the first (baseline) and last recorded weights. Average weight loss and physical activity minutes per week were calculated among participants who attended ≥ 3 sessions in the first 6 months and whose time from first session attended to last session attended was ≥ 9 months. CDC considers this threshold to be the minimum dose to begin seeing lifestyle and weight change that can impact type 2 diabetes [ 40 ].
Mantel-Haenszel chi-square tests of difference were used to assess bivariate associations between participants’ characteristics and duration of participation. Results were stratified by duration of participation (≥ 9 vs. < 9 months). Multiple logistic regression models were used to estimate the association between participants’ attendance and duration of participation and the likelihood of meeting the minimum 5% weight loss goal conditional on other factors. Adjusted odds ratios (AORs) in relation to a reference category were reported with their respective 95% confidence intervals (CIs). Results with p < 0.05 were considered statistically significant. All analyses were conducted using SAS, version 9.4.
Overall, about 60% of analyzed participants attended the lifestyle change via an online-only modality, 40% via an in-person only modality, and < 1% via distance learning only or combination modality (Table 1 ). About 40% of analyzed participants attended at least 17 lifestyle change sessions; 31% met the minimum 5% weight loss goal, and 45% met the average 150+ min per week of physical activity goal. Three quarters were females; over half were aged 45–64 years; over 60% were non-Hispanic whites; about half reported having 4 years of college or more; and over 70% had obesity (body mass index (BMI) ≥ 30 kg/m 2 ).
Weight loss success was significantly higher among those who attended ≥ 17 sessions (AOR 3.2), those who stayed in the program for ≥ 9 months (AOR 1.3), and those with ≥ 150 min of physical activity per week (AOR 1.7) (Table 2 ). Participants aged 45–64 and ≥ 65 years had 3.2 and 1.6 times, respectively, the odds of meeting the 5% weight loss goal than those aged 18–44. Females, non-Hispanic blacks, and those with obesity were slightly less likely to meet the 5% weight loss goal than males, non-Hispanic whites, and those overweight (BMI between 25 and 29.9 kg/m 2 ).
This analysis is subjected to some limitations. First, biometric data (weight and physical activity minutes) was self-reported either by CDC-recognized organizations or participants themselves. However, lifestyle coaches were provided guidance to use the same scale at each session for recording participants’ body weight to ensure consistency. Second, calculation of percent weight loss was based on first and last recorded weight. Participants who lost more weight might be more likely to stay in the program longer and continue to lose more weight by the end of the program than those who dropped out early. Third, CDC’s DPRP began requiring organizations to submit information on participants’ education level, enrollment source, and payer source in February 2018; thus, our analyses were limited to a small sample of participants who started the program in 2018 (~ 10% of the total National DPP participants), as the remaining participants had not yet had the opportunity to participate in the program for a year prior to our study. Lastly, the analysis only included eligible participants based on CDC’s DPRP Standards, so it may not be generalizable to ineligible participants who may also benefit from this program.
Participation in Lifestyle Change Intervention
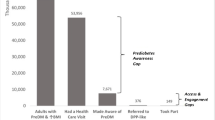
Successful expansion of lifestyle change interventions relies on sufficient enrollment and retention. A recent analysis of 2016–2017 U.S. National Health Interview Survey data showed that 73.5% of adults with overweight or obesity with diagnosed prediabetes and 50.6% of adults with overweight or obesity and elevated American Diabetes Association (ADA) risk scores reported receiving risk reduction advice or referrals to risk reduction activities from their health care providers [ 41 •]. Of those referred, only one third reported engagement in risk-reducing activities in the past year, and less than 3% reported participating in a type 2 diabetes prevention program. The key drivers for engagement in risk-reducing activities and programs included receiving advice from a health professional, having higher education, having insurance, being non-white race/ethnicity, and being middle aged. This study underscores the need for research from fields such as behavioral economics, human-centered design, and habit formation, to understand how to best engage people at high risk in type 2 diabetes prevention programs. Other approaches for systems change include the establishment of referral processes from clinical health care providers to community-based implementation programs, and media and marketing strategies to drive traffic to such programs. A recent study found that primary care providers in the USA who were aware of the National DPP lifestyle change program and the Prevent Diabetes STAT: Screen, Test, and Act Today™ Toolkit developed by CDC and the American Medical Association (AMA) were more likely to screen patients for prediabetes and make referrals to CDC-recognized organizations offering the National DPP. Those who used electronic health records were also more likely to screen, test, and refer [ 42 ].
Offering diabetes prevention programs via businesses and worksites could help lower employee health care costs and increase QALYs, and is an important systems approach still in need of broader consideration among employers [ 43 ]. Evidence-based programs within worksite wellness programs and health-based interventions within worksites are increasing in scope in the USA, and seem to be slowly gaining traction elsewhere in the world as well. To assist employers and insurers in determining the feasibility of providing the National DPP lifestyle change program or including it as part of their employees’ insurance benefits, CDC developed the Diabetes Prevention Impact Toolkit [ 43 ]. The toolkit helps estimate the cost per employee and the associated cost savings related to offering the National DPP lifestyle change program, including estimating the employee’s QALYs gained. A summary of recent peer-reviewed literature on employer-based diabetes prevention programs in the USA found that greater weight loss and maintenance of weight loss were achieved among worksites that implemented the National DPP lifestyle change program compared to worksites that implemented other interventions [ 44 ].
Another key approach to increasing participation focuses on raising awareness of both prediabetes as a serious condition and of type 2 diabetes prevention activities. This is a challenge, as many providers are not screening or testing patients for prediabetes when considering its risk factors (obesity, overweight, glycemic range, and cardiovascular risks) In the USA, CDC, ADA, and AMA partnered with the Ad Council to launch the nation’s first national public service campaign about prediabetes [ 45 ] which encourages people to take a short online test at DoIHavePrediabetes.org to learn their prediabetes risk. From the campaign’s launch on January 21, 2016, through March 31, 2019, 2.5 million people have completed the online risk test [ 46 ].
Whole-Population Approaches
Because of the widespread prevalence of type 2 diabetes and its risk factors, lifestyle change programs for the highest-risk people alone cannot sufficiently impact the diabetes pandemic without approaches that support prevention efforts across whole systems and communities. Larger-scale, population-wide prevention strategies, such as environmental, policy, cost reimbursement, and health marketing/awareness efforts, are therefore needed. Several whole-population approaches have been implemented in the USA. Many focus on socioeconomic policy involving nutrition regulation such as menu labeling; subsidies to increase the affordability of fruits and vegetables, particularly in rural or hard-to-reach areas [ 47 , 48 ]; sugar-sweetened beverage tax and decreased added sugars; and increased whole grains, fibers, nuts, and legumes and elimination of trans fats (or trans fatty acids) as recommended by the American Heart Association [ 49 ]. Globally, supported by WHO, clear nutrition “front-of-pack labeling” has been found to improve dietary habits and reduce cardiovascular complications [ 50 ].
A systematic review found strong evidence in Europe for implementing multiple policies simultaneously, including taxes on unhealthy food, subsidies for healthy food, trans fat elimination, and trade agreements with supportive countries who implement similar taxation and food policies [ 51 ••]. A 2014 systematic review of the evidence behind food taxation and food subsidies (government/local investments in healthy food) found that both should be implemented in tandem at a minimum rate of 10 to 15% to ensure the unhealthy foods are less accessible and healthy foods/beverages are more accessible to purchasers [ 52 ]. Similarly, Colchero et al. studied the effect of taxing sugar-sweetened beverages in stores in Mexico and found reductions in purchases of the taxed beverages associated with increases in purchases of untaxed beverages [ 53 ].
Environmental changes such as targeting the built environment and community planning efforts also show promise in reaching large populations. These approaches include the expansion or building of walking, biking, and hiking trials, and other “safe” routes [ 54 ]. A systematic review evaluating the effect of built environment policies on obesity-related outcomes across the USA, Canada, Chile, the UK, and New Zealand found that physical activity-related policies had a stronger impact when they involved improvements to transportation infrastructure. These improvements included creating more structural access such as building cycling lanes and park trails [ 55 ].
A possible limitation of whole-population approaches is that researchers have to rely largely on modeling studies or intermediate outcomes to determine impact. Short-term and longitudinal impact testing involving actual health and economic trends is more difficult, but critical to assessing intervention success. Also critical is understanding the contexts in which whole-population approaches are most effective.
To achieve large-scale type 2 diabetes prevention, interventions directed to both high-risk populations and the general population are necessary. There is strong evidence for the prevention of type 2 diabetes from RCTs and subsequent translation studies in which people at high risk engage in a structured lifestyle intervention that addresses nutrition, physical activity, and behavior change strategies resulting in a weight loss of ≥ 5%. Whole-population strategies also show promise in reaching large numbers of people and include multi-sector and multi-policy approaches, most successfully in combination. These approaches include taxation of unhealthy foods, enhancing the built environment, addressing food accessibility, offering worksite wellness, and raising awareness of prediabetes among health care providers and the general public.
Large-scale implementation of what has been proven to work, including alternate delivery approaches for type 2 diabetes prevention programs to reach disparate and geographically isolated populations, is needed. Continued examination of outcomes and program effectiveness is needed to refine global prevention efforts. Fortunately, there is much evidence worldwide that type 2 diabetes prevention or delay is attainable, and that prevention strategies can be adapted across cultures and environments. Success will require a combination of policy, systems, environmental, and health marketing/awareness approaches with effective interventions for high-risk populations and partnerships across sectors. Based on the data showing the impact of diabetes it warrants being prioritized to protect the public’s health around the world and curb the increasing burden of diabetes.

Change history
27 june 2020.
The sentence should read: ��� Average weight loss and physical activity minutes per week were calculated among participants who attended���������3 sessions in the first 6��months and whose time from first session attended to last session attended was���������9��months.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• International Diabetes Federation. Diabetes atlas 8 th edition 2017 global fact sheet. 2017. Available at https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html . Accessed April 14, 2019. Findings indicate the significant and costly economic and disease burden that diabetes has across the world.
Centers for Disease Control and Prevention. Diabetes 2017 report card. 2017. Available at https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf . Accessed April 14, 2019.
• American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28. https://doi.org/10.2337/dci18-0007 Findings indicate the significant and costly economic and productivity burden that diabetes has in the USA.
Article PubMed Central Google Scholar
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. https://doi.org/10.1056/nejm200105033441801.
Article CAS PubMed Google Scholar
Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. Br Med J (Clin Res Ed). 2011;343:d7163.
Article Google Scholar
Modesti PA, Galanti G, Cala P, Calabrese M. Lifestyle interventions in preventing new type 2 diabetes in Asian populations. Intern Emerg Med. 2016;11(3):375–84. https://doi.org/10.1007/s11739-015-1325-2 .
Article PubMed Google Scholar
Pearson-Stuttard J, Bandosz P, Rehm CD, Penalvo J, Whitsel L, Gaziano T, et al. Reducing US cardiovascular disease burden and disparities through national and targeted dietary policies: a modelling study. PLoS Med. 2017;14(6):e1002311. https://doi.org/10.1371/journal.pmed.1002311 .
Article PubMed PubMed Central Google Scholar
Konchak JN, Moran MR, O'Brien MJ, Kandula NR, Ackermann RT. The state of diabetes prevention policy in the USA following the Affordable Care Act. Curr Diab Rep. 2016;16(6):55. https://doi.org/10.1007/s11892-016-0742-6.
Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44.
Article CAS Google Scholar
Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–9. https://doi.org/10.1016/s0140-6736(08)60766-7 .
Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011;54(2):300–7. https://doi.org/10.1007/s00125-010-1948-9 .
Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019. https://doi.org/10.1016/S2213-8587(19)30093-2 .
•• Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. https://doi.org/10.1056/NEJMoa012512 Findings from this study demonstrate that diabetes incidence reduction in those at high risk for type 2 diabetes is possible with both lifestyle interventions and metformin use, but greater through lifestyle intervention overall.
Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. https://doi.org/10.1016/s0140-6736(09)61457-4 .
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. https://doi.org/10.1016/s2213-8587(15)00291-0 .
Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35(4):723–30. https://doi.org/10.2337/dc11-1468 .
The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34.
Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–9. https://doi.org/10.1016/s0140-6736(06)69701-8 .
Makrilakis K, Liatis S, Grammatikou S, Perrea D, Katsilambros N. Implementation and effectiveness of the first community lifestyle intervention programme to prevent type 2 diabetes in Greece. The DE-PLAN study. Diabet Med. 2010;27(4):459–65. https://doi.org/10.1111/j.1464-5491.2010.02918.x .
Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–62. https://doi.org/10.1016/j.diabres.2004.06.010 .
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97. https://doi.org/10.1007/s00125-005-0097-z .
Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost-effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within-trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care. 2007;30(10):2548–52. https://doi.org/10.2337/dc07-0150 .
Davidson A. Translational research: what does it mean? Anesthesiology. 2011;115(5):909–11. https://doi.org/10.1097/ALN.0b013e3182337a5e .
Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75. https://doi.org/10.1377/hlthaff.2011.1009 .
•• Gilis-Januszewska A, Piwonska-Solska B, Lindstrom J, Wojtowicz E, Tuomilehto J, Schwarz PEH, et al. Determinants of weight outcomes in type 2 diabetes prevention intervention in primary health care setting (the DE-PLAN project). BMC Public Health. 2018;18(1):97. https://doi.org/10.1186/s12889-017-4977-1 Findings from this study indicate that persons with a higher body mass index, increased glucose, and lower education (i.e., at higher risk for type 2 diabetes) achieved successful weight loss of 5% or more after a yearlong lifestyle change intervention as compared to those with lower risk for type 2 diabetes.
Thomas C, Sadler S, Breeze P, Squires H, Gillett M, Brennan A. Assessing the potential return on investment of the proposed UK NHS diabetes prevention programme in different population subgroups: an economic evaluation. Br Med J Open. 2017;7(8):e014953. https://doi.org/10.1136/bmjopen-2016-014953 .
Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health. 2007;7:249. https://doi.org/10.1186/1471-2458-7-249 .
Janus ED, Best JD, Davis-Lameloise N, Philpot B, Hernan A, Bennett CM, et al. Scaling-up from an implementation trial to state-wide coverage: results from the preliminary Melbourne Diabetes Prevention Study. Trials. 2012;13:152. https://doi.org/10.1186/1745-6215-13-152 .
Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833–6. https://doi.org/10.1001/jama.289.14.1833 .
Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1(3):191–8. https://doi.org/10.1016/s2213-8587(13)70067-6 .
Nanditha A, Snehalatha C, Raghavan A, Vinitha R, Satheesh K, Susairaj P, et al. The post-trial analysis of the Indian SMS diabetes prevention study shows persistent beneficial effects of lifestyle intervention. Diabetes Res Clin Pract. 2018;142:213–21. https://doi.org/10.1016/j.diabres.2018.05.042 .
Centers for Medicare & Medicaid Services. 2016 annual report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/downloads/tr2016.pdf . Accessed April 14, 2019.
Herman WH. The economics of diabetes prevention. Med Clin North Am. 2011;95(2):373–84, viii . https://doi.org/10.1016/j.mcna.2010.11.010 .
US Congress. H.R.4124 - Diabetes Prevention Act of 2009. Available at https://www.congress.gov/bill/111th-congress/house-bill/4124 . Accessed April 14, 2019.
Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med. 2013;44(4 Suppl 4):S346–51. https://doi.org/10.1016/j.amepre.2012.12.009 .
Centers for Disease Control and Prevention. Diabetes Prevention Recognition Program data dashboard. Accessed April 11, 2019.
Centers for Disease Control and Prevention. 2018 Diabetes Prevention Recognition Program standards and operating procedures. Available at https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf . Accessed April 14, 2019.
Centers for Disease Control and Prevention. Insurance coverage for the US National Diabetes Prevention Program State reported data. Accessed May 14, 2019.
Centers for Medicare & Medicaid Services. Medicare Diabetes Prevention Program (MDPP) expanded model. Available at https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/ . Accessed April 14, 2019.
Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–41. https://doi.org/10.2337/dc16-2099 .
• Ali MK, McKeever Bullard K, Imperatore G, Benoit SR, Rolka DB, Albright AL, et al. Reach and use of diabetes prevention services in the United States, 2016-2017. JAMA Netw Open. 2019;2(5):e193160. https://doi.org/10.1001/jamanetworkopen.2019.3160 Findings from this study offer the basis by which to assess future diabetes prevention lifestyle change programs and coverage for such programs, strategies for identification of prediabetes, and referral practices into such programs that lead to retention.
Nhim K, Khan T, Gruss SM, Wozniak G, Kirley K, Schumacher P, et al. Primary care providers’ prediabetes screening, testing, and referral behaviors. Am J Prev Med. 2018;55(2):e39–47. https://doi.org/10.1016/j.amepre.2018.04.017 .
Centers for Disease Control and Prevention. Diabetes prevention impact toolkit. Available at https://nccd.cdc.gov/toolkit/diabetesimpact . Accessed April 14, 2019.
Hafez D, Fedewa A, Moran M, O'Brien M, Ackermann R, Kullgren JT. Workplace interventions to prevent type 2 diabetes mellitus: a narrative review. Curr Diab Rep. 2017;17(2):9. https://doi.org/10.1007/s11892-017-0840-0.
Ad Council. Do I have prediabetes Available at DoIHavePrediabetes.org . Accessed April 14, 2019.
Centers for Disease Control and Prevention. Prediabetes campaign results report, April 2019 Accessed April 4, 2019.
Andreyeva T, Long MW, Brownell KD. The impact of food prices on consumption: a systematic review of research on the price elasticity of demand for food. Am J Public Health. 2010;100(2):216–22. https://doi.org/10.2105/ajph.2008.151415 .
Calancie L, Leeman J, Jilcott Pitts SB, Khan LK, Fleischhacker S, Evenson KR, et al. Nutrition-related policy and environmental strategies to prevent obesity in rural communities: a systematic review of the literature, 2002-2013. Prev Chronic Dis. 2015;12:E57. https://doi.org/10.5888/pcd12.140540 .
Olstad DL, Teychenne M, Minaker LM, Taber DR, Raine KD, Nykiforuk CI, et al. Can policy ameliorate socioeconomic inequities in obesity and obesity-related behaviours? A systematic review of the impact of universal policies on adults and children. Obes Rev. 2016;17(12):1198–217. https://doi.org/10.1111/obr.12457 .
Lichtenstein AH, Carson JS, Johnson RK, Kris-Etherton PM, Pappas A, Rupp L, et al. Food-intake patterns assessed by using front-of-pack labeling program criteria associated with better diet quality and lower cardiometabolic risk. Am J Clin Nutr. 2014;99(3):454–62. https://doi.org/10.3945/ajcn.113.071407 .
•• Hyseni L, Atkinson M, Bromley H, Orton L, Lloyd-Williams F, McGill R, et al. The effects of policy actions to improve population dietary patterns and prevent diet-related non-communicable diseases: scoping review. Eur J Clin Nutr. 2017;71(6):694–711. https://doi.org/10.1038/ejcn.2016.234 Findings are from a large, worldwide systematic and non-systematic review that highlights successful whole-population strategies for preventing and reducing non-communicable diseases.
Niebylski ML, Redburn KA, Duhaney T, Campbell NR. Healthy food subsidies and unhealthy food taxation: a systematic review of the evidence. Nutrition. 2015;31(6):787–95. https://doi.org/10.1016/j.nut.2014.12.010 .
Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. Br Med J (Clin Res Ed). 2016;352:h6704.
Google Scholar
Albright A, Devlin H, and Zhang X: Integrating Nutrition Therapy into Community-Based Diabetes Prevention Programs (pp 579). In: Franz MJ, Evert AB, editors. American Diabetes Association Guide to Nutrition Therapy for Diabetes, 3rd Edition; 2012.
Mayne SL, Auchincloss AH, Michael YL. Impact of policy and built environment changes on obesity-related outcomes: a systematic review of naturally occurring experiments. Obes Rev. 2015;16(5):362–75. https://doi.org/10.1111/obr.12269 .
Article CAS PubMed PubMed Central Google Scholar
Download references
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. No fınancial disclosures were reported by the authors of this paper.
Author information
Authors and affiliations.
Division of Diabetes Translation, Centers for Disease Control and Prevention, 4770 Buford Hwy., Mailstop F75, Atlanta, GA, 30341, USA
Stephanie M. Gruss, Kunthea Nhim, Miriam Bell, Elizabeth Luman & Ann Albright
Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK
Edward Gregg
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stephanie M. Gruss .
Ethics declarations
Conflict of interest.
Stephanie M. Gruss, Kunthea Nhim, Edward Gregg, Miriam Bell, Elizabeth Luman, and Ann Albright declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes Epidemiology
Electronic supplementary material
(DOCX 49 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Gruss, S.M., Nhim, K., Gregg, E. et al. Public Health Approaches to Type 2 Diabetes Prevention: the US National Diabetes Prevention Program and Beyond. Curr Diab Rep 19 , 78 (2019). https://doi.org/10.1007/s11892-019-1200-z
Download citation
Published : 05 August 2019
DOI : https://doi.org/10.1007/s11892-019-1200-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetes prevention
- National Diabetes Prevention Program
- Type 2 diabetes
- Diabetes policy
- Diabetes prevention program
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Epidemiol Glob Health
- v.10(1); 2020 Mar

Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted Trends
Moien abdul basith khan.
1 Department of Family Medicine, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
Muhammad Jawad Hashim
Jeffrey kwan king, romona devi govender, halla mustafa, juma al kaabi.
2 Department of Internal Medicine, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
The rising burden of type 2 diabetes is a major concern in healthcare worldwide. This research aimed to analyze the global epidemiology of type 2 diabetes. We analyzed the incidence, prevalence, and burden of suffering of diabetes mellitus based on epidemiological data from the Global Burden of Disease (GBD) current dataset from the Institute of Health Metrics, Seattle. Global and regional trends from 1990 to 2017 of type 2 diabetes for all ages were compiled. Forecast estimates were obtained using the SPSS Time Series Modeler. In 2017, approximately 462 million individuals were affected by type 2 diabetes corresponding to 6.28% of the world’s population (4.4% of those aged 15–49 years, 15% of those aged 50–69, and 22% of those aged 70+), or a prevalence rate of 6059 cases per 100,000. Over 1 million deaths per year can be attributed to diabetes alone, making it the ninth leading cause of mortality. The burden of diabetes mellitus is rising globally, and at a much faster rate in developed regions, such as Western Europe. The gender distribution is equal, and the incidence peaks at around 55 years of age. Global prevalence of type 2 diabetes is projected to increase to 7079 individuals per 100,000 by 2030, reflecting a continued rise across all regions of the world. There are concerning trends of rising prevalence in lower-income countries. Urgent public health and clinical preventive measures are warranted.
1. INTRODUCTION
Type 2 diabetes is recognized as a serious public health concern with a considerable impact on human life and health expenditures. Rapid economic development and urbanization have led to a rising burden of diabetes in many parts of the world [ 1 ]. Diabetes affects individuals’ functional capacities and quality of life, leading to significant morbidity and premature mortality [ 2 ]. Recently, concerns have been raised that more than one-third of the diabetes-related deaths occur in people under the age of 60 [ 3 ]. Increased consumption of unhealthy diets and sedentary lifestyles, resulting in elevated Body Mass Index (BMI) and fasting plasma glucose, have been blamed for these trends [ 4 ]. In particular, persons with higher BMI are more likely to have type 2 diabetes [ 5 ]. The aging of the human population is another contributor, as diabetes tends to affect older individuals [ 6 ]. The cost of diabetes care is at least 3.2 times greater than the average per capita healthcare expenditure, rising to 9.4 times in presence of complications [ 7 ]. Control of blood glucose, blood pressure, and other targets remains suboptimal for many patients [ 8 ]. This has been partly attributed to the lack of awareness and health promotion needed for diabetes control [ 9 ].
Unfortunately, the global epidemiology of diabetes has not been re-evaluated since the availability of recent high-quality data [ 10 ]. We found no studies providing global forecasts for the intermediate future, which would be a critical piece of information for health policymakers.
This research project examines the latest dataset of the Global Burden of Disease (GBD) to assess the burden of type 2 diabetes worldwide. The aim is to study the current global epidemiology of diabetes and highlight the current distribution of disease and emerging epidemiologic trends.
2. MATERIALS AND METHODS
We analyzed descriptive epidemiological data from the GBD dataset managed by the Institute of Health Metrics and Evaluation at the University of Washington, Seattle [ 11 ]. The GBD dataset is actively maintained and updated based on research data, epidemiology studies, and governmental publications from more than 100,000 sources. As a systematic public health project, it carefully builds models and statistical estimates for health loss due to illness, injury, and risk factors based on empirical data. GBD produces annual estimates of disease measures, such as prevalence, incidence, deaths, and Disability-Adjusted Life Years (DALYs). DALYs combine years of life lost due to premature death and years lived with disability, and are a more accurate reflection of human suffering resulting from a disease than prevalence or mortality alone.
We used the latest data refresh from GBD (the 2017 update). This dataset includes annual figures from 1990 to 2017 for type 2 diabetes in all countries and regions. We selected four world regions (Asia, Europe, America, and Africa) instead of other classification schemes based on economic development. All data were directly retrieved from GBD without any adjustments. Estimates were not age adjusted for differences in underlying population age distributions. Thus, the rates for different countries represent the actual burden on their respective health systems.
2.1. Statistical Data Analysis
Forecasting was conducted using IBM SPSS version 25 (IBM Corp., Armonk, NY, USA). The Time Series Modeler was used to develop a forecast model using the Expert Modeler option without any events. None of the observed values were marked as outliers.
Globally, an estimated 462 million individuals are affected by type 2 diabetes, corresponding to 6.28% of the world’s population ( Table 1 ). More than 1 million deaths were attributed to this condition in 2017 alone, ranking it as the ninth leading cause of mortality. This is an alarming rise when compared with 1990, when type 2 diabetes was ranked as the eighteenth leading cause of deaths. In terms of human suffering (DALYs), diabetes ranks as the seventh leading disease.
Disease burden of type 2 diabetes, 2017
| Global | 6059 | 751 |
| Europe | 8529 | 842 |
| Germany | 9091 | 820 |
| France | 6843 | 564 |
| Italy | 9938 | 1083 |
| Spain | 8796 | 773 |
| Netherlands | 11,344 | 924 |
| Switzerland | 10,040 | 815 |
| Sweden | 10,448 | 877 |
| Turkey | 6483 | 889 |
| Russia | 6865 | 740 |
| United Kingdom | 8663 | 644 |
| Asia | 5961 | 729 |
| China | 6262 | 635 |
| India | 4770 | 663 |
| Japan | 6737 | 553 |
| South Korea | 8835 | 1044 |
| Taiwan | 10,012 | 1294 |
| Saudi Arabia | 7661 | 623 |
| Iran | 7000 | 851 |
| Australia | 5235 | 593 |
| America | 7060 | 1036 |
| United States | 8911 | 1046 |
| Canada | 7095 | 829 |
| Brazil | 4240 | 780 |
| Africa | 3916 | 537 |
| South Africa | 7360 | 1374 |
The prevalence of type 2 diabetes shows a distribution pattern that matches socio-economic development ( Figure 1 ). Developed regions, such as Western Europe, show considerably higher prevalence rates that continue to rise despite public health measures ( Figure 2 ). The rate of increase does not appear to be slowing down.

Global distribution of diabetes mellitus type 2 prevalence. Note: Colors indicate prevalence rates per 100,000 population in 2017.

Trends in the prevalence of type 2 diabetes. Note: Forecast estimates using SPSS Time Series Modeler (Ljung Box Q, p = 0.16). Dotted lines indicate upper and lower confidence limits.
Remarkably, certain regions, such as Pacific Ocean island nations, are sustaining the highest prevalence of disease. These countries include Fiji (20,277 per 100,000), Mauritius (18,545), American Samoa (18,312), and Kiribati (17,432). Southeast Asian countries, such as Indonesia, Malaysia, Thailand, and Vietnam, have moved up the ranks in the last two decades. Owing to their large population sizes, China (88.5 million individuals with type 2 diabetes), India (65.9 million), and the US (28.9 million) retain the top spots as the countries with the greatest total number of individuals with this condition.
Males show a slightly higher prevalence than females (6219 compared with 5898 cases per 100,000), although this difference is within the margin of uncertainty. The age of onset of new diagnosis is also somewhat earlier among males and shows expected patterns of rising prevalence with increasing age, whereas the incidence peaks at 55–59 years ( Figure 3 ). There appears to be no major shift in the age distribution from 1990 to 2017.

Age distribution of diabetes mellitus type 2, worldwide. (A) Incidence vs. prevalence (both 2017). (B) Incidence in 1990 vs. 2017. p < 0.0001, chi-square test.
Even though it afflicts individuals later in life, type 2 diabetes ranks seventh among the leading causes of disability and years of life lost (DALYs). It has jumped ranks from nineteenth position in 1990, indicating a global transition in disease patterns toward noncommunicable diseases.
Statistical forecasting using a model based on the 1990–2017 data showed that global diabetes prevalence could increase to 7079 per 100,000 by 2030 and 7862 by 2040. This estimate for 2040 is flanked by an upper confidence limit of 9904 and a lower limit of 5821 per 100,000.
4. DISCUSSION
This study reports on the current trends in the global burden of diabetes with emphasis on the burden of human suffering. The high prevalence of type 2 diabetes worldwide continues to rise, and there are no signs of it stabilizing. A concerning finding is the rapidly rising burden in lower-income countries. These findings have implications for health policy planners, physicians, healthcare professionals, and the public.
The burden of suffering due to diabetes, as measured by DALYs, is increasing despite significant investment in clinical care and pharmaceutical research. This increase is in excess of population growth and aging. Notably, Western Europe has a rate of increase greater than that of global and Asian averages. Even with the high levels of clinical and public health expenditure, this region is losing the battle against diabetes. One explanation might be non-modifiable risk factors, such as age and family history [ 12 ]. However, factors like a highly processed, calorie-dense western diet and a sedentary lifestyle may also be contributing. Developed countries like Italy and the US endure the highest burdens of human suffering (DALYs) due to diabetes. Advanced economies in Asia, such as South Korea and Taiwan, are joining the ranks of these countries, based on GBD data. Thus, our findings support the correlation between diabetes and economic development [ 13 ]. We speculate that our current approach to diabetes management, which focuses on expensive oral medications and insulin, is not working. Lowering blood glucose levels is perhaps not sufficient by itself nor effective in reducing all-cause mortality among these patients.
Prevention of new cases of diabetes appears to be not working as well based on our findings from global data. Although research is ongoing to reduce the progression from metabolic syndrome and prediabetes to diabetes, most interventions being tried seem to be unsuccessful in affecting the incidence. According to our data, there is no evidence of a decrease in incidence. Alarmingly high incidence rates recorded in island nations in the Pacific region are an indication of the interaction between genetic predisposition and the effect of rapid nutritional change on these indigenous populations. Meanwhile, the sheer number of individuals with diabetes is testing health systems in China, India, and the US to the limit. Rapid urbanization and its effects on diet and lifestyle has been implicated [ 14 ]. These findings have direct implications for health systems planning and resource allocation. Clearly, hospital-based management and subspecialist care are not sustainable strategies. Resource allocation in healthcare budgets for prevention of diabetes needs to be comparable to expenditures on treatment. Strengthening of primary care and community restructuring for active lifestyles and healthy nutrition are perhaps more likely to be cost effective [ 15 ]. Sadly, the rising tide of type 2 diabetes is out pacing preventive efforts by a wide margin [ 16 ].
The rising incidence of type 2 diabetes at earlier ages warrants closer attention. Previous clinic-based studies have reported a high number of young adults being diagnosed with type 2 diabetes, most of whom are obese [ 17 ]. There appears to be an age gradient with early-onset type 2 diabetes patients (those younger than 45) showing more obesity, dyslipidemia, smoking, sedentary lifestyles, and low-grade inflammation [ 18 ]. In our study, although the incidence of diabetes in young adults has increased over the past decades, the rise is across all ages. Thus, there appears to be no clear indication that the age of onset of type 2 diabetes has shifted to younger age groups. In any case, rising life expectancy in many countries will lead to a substantially greater burden of diabetes in the elderly.
The main limitations of our study include reliance on secondary data, which in turn is affected by the accuracy of measurement, changes in case definition, and heterogeneity in study designs. Yet as GBD evolves and matures, its estimation techniques have become more accurate and reliable. These statistical estimates provide a more complete and continuous picture of disease epidemiology than relying on raw data from isolated studies [ 11 ]. Ultimately, the goal is to guide decision making in clinical care and public health policy.
5. CONCLUSION
Type 2 diabetes continues to increase in prevalence, incidence, and as a leading cause of human suffering and deaths. Despite significant investments in clinical care, research, and public health interventions, there appears to be no sign of reduction in the rate of increase. Certain regions of the world, such as Western Europe and island states in the Pacific, are experiencing a disproportionately high burden. This epidemic will require an urgent and unwavering commitment to aggressive solutions at national levels with public policies, public health funding, and economic incentives for local communities to start diabetes prevention programs. Healthy eating options need to be subsidized, and unhealthy foods need to be taxed or otherwise disincentivized. Healthcare organizations and individual healthcare providers from multiple disciplines (doctors, nurses, pharmacists, dieticians, and diabetes educators) must be given time and resources to collaborate as they educate and care for individual and groups of patients. Unless urgent measures are instituted to reduce unhealthy eating, sedentary lifestyles, rapid urbanization, and other factors related to economic development, the burden of diabetes is expected to continue rising.
ACKNOWLEDGMENT
We would like to thank the Institute of Health Metrics, Seattle for compiling global epidemiological statistics and allowing access to data.
Data availability statement: The data that support the findings of this study are openly available in Global Health Data Exchange by the Institute of Health Metrics at http://ghdx.healthdata.org/gbd-results-tool .
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
MK contributed to writing the manuscript including the literature review. MJH designed the study/basic concept, wrote sections of the manuscript, analyzed the data, and provided overall supervision of the study. JK wrote parts of the manuscripts, proofread, and provided insights into the interpretation. RDG revised the manuscript and provided additional interpretation of results. HM compiled data and wrote the table. JAK revised and proofread the manuscript and provided additional interpretation of results.
This study did not receive any external grants from government, private or commercial sources.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Diabetes Mellitus Review
- PMID: 27093761
Diabetes mellitus is a group of physiological dysfunctions characterized by hyperglycemia resulting directly from insulin resistance, inadequate insulin secretion, or excessive glucagon secretion. Type 1 diabetes (T1D) is an autoimmune disorder leading to the destruction of pancreatic beta-cells. Type 2 diabetes (T2D), which is much more common, is primarily a problem of progressively impaired glucose regulation due to a combination of dysfunctional pancreatic beta cells and insulin resistance. The purpose of this article is to review the basic science of type 2 diabetes and its complications, and to discuss the most recent treatment guidelines.
PubMed Disclaimer
Similar articles
- Lessons for pediatricians from the diabetes control and complications trial. Malone JI. Malone JI. Pediatr Ann. 1994 Jun;23(6):295-9. doi: 10.3928/0090-4481-19940601-08. Pediatr Ann. 1994. PMID: 8078706 Review. No abstract available.
- A desktop guide to Type 2 diabetes mellitus. European Diabetes Policy Group 1999. [No authors listed] [No authors listed] Diabet Med. 1999 Sep;16(9):716-30. Diabet Med. 1999. PMID: 10510947 No abstract available.
- [Management of diabetics in the outpatient department]. Mimura G, Ishikawa K, Higa S, Futema H. Mimura G, et al. Nihon Rinsho. 1990 Dec;48 Suppl:796-802. Nihon Rinsho. 1990. PMID: 2086959 Japanese. No abstract available.
- A 64-year-old man with adult-onset diabetes. Rubenstein AH. Rubenstein AH. JAMA. 1996 Sep 11;276(10):816-22. JAMA. 1996. PMID: 8769592 No abstract available.
- Treatment of non-insulin-dependent diabetes mellitus and its complications. A state of the art review. Ilarde A, Tuck M. Ilarde A, et al. Drugs Aging. 1994 Jun;4(6):470-91. doi: 10.2165/00002512-199404060-00004. Drugs Aging. 1994. PMID: 8075474 Review.
- Prevalence of nephropathy among diabetic patients in North American region: A systematic review and meta-analysis. Zahra S, Saleem MK, Ejaz KF, Akbar A, Jadoon SK, Hussain S, Ali AI, Ifty M, Jannati SZ, Armin F, Sarker D, Islam DZ, Khandker SS, Khan MS, Alvi S. Zahra S, et al. Medicine (Baltimore). 2024 Sep 20;103(38):e39759. doi: 10.1097/MD.0000000000039759. Medicine (Baltimore). 2024. PMID: 39312314 Free PMC article.
- Macrophage Activation Syndrome in Coinciding Pandemics of Obesity and COVID-19: Worse than Bad. Engin AB, Engin ED, Engin A. Engin AB, et al. Adv Exp Med Biol. 2024;1460:919-954. doi: 10.1007/978-3-031-63657-8_31. Adv Exp Med Biol. 2024. PMID: 39287877 Review.
- Paper integrated microfluidic contact lens for colorimetric glucose detection. Isgor PK, Abbasiasl T, Das R, Istif E, Yener UC, Beker L. Isgor PK, et al. Sens Diagn. 2024 Aug 5. doi: 10.1039/d4sd00135d. Online ahead of print. Sens Diagn. 2024. PMID: 39247807 Free PMC article.
- Function and expression of N-acetyltransferases 1 and 2 are altered in lymphocytes in type 2 diabetes and obesity. Paz-Rodríguez VA, Herrera-Vargas DJ, Turiján-Espinoza E, Martínez-Leija ME, Rivera-López E, Hernández-González O, Zavala-Reyes D, García-Hernández MH, Vargas-Morales JM, Milán-Segovia RDC, Portales-Pérez DP. Paz-Rodríguez VA, et al. Biochem Biophys Rep. 2024 May 3;38:101716. doi: 10.1016/j.bbrep.2024.101716. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38737726 Free PMC article.
- Influence of Diabetes Mellitus and Universal Adhesive Application Mode on the Bond Strength of Composite Resin to Dentine. Attia R, El-Bahrawy E, Shebl E, Rashed A, El-Husseiny F. Attia R, et al. J Clin Exp Dent. 2024 Apr 1;16(4):e416-e425. doi: 10.4317/jced.61328. eCollection 2024 Apr. J Clin Exp Dent. 2024. PMID: 38725826 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Ovid Technologies, Inc.
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
COMMENTS
Type 2 diabetes mellitus (T2DM) accounts for >90% of the cases of diabetes in adults. Resistance to insulin action is the major cause that leads to chronic hyperglycemia in diabetic patients. T2DM is the consequence of activation of multiple pathways and factors involved in insulin resistance and β-cell dysfunction.
1. Introduction. Diabetes Mellitus (DM) commonly referred to as diabetes, is a chronic disease that affects how the body turns food into energy .It is one of the top 10 causes of death worldwide causing 4 million deaths in 2017 , .According to a report by the International Diabetes Federation (IDF) , the total number of adults (20-79 years) with diabetes in 2045 will be 629 million from 425 ...
Abstract. Type 2 diabetes mellitus (DM) is a lifelong metabolic disease, characterized by hyperglycaemia which gradually leads to the development and progression of vascular complications. It is recognized as a global burden disease, with substantial consequences on human health (fatality) as well as on health-care system costs.
PubMed. ISI. Google Scholar. 3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of ...
Excerpt. Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. It may be due to impaired insulin secretion, resistance to peripheral actions of insulin, or both. According to the International Diabetes Federation (IDF), approximately 415 million adults between the ages of 20 to 79 years had diabetes ...
Type 2 diabetes accounts for more than 90% of patients with diabetes and leads to microvascular and macrovascular complications that cause profound psychological and physical distress to both patients and carers and put a huge burden on health-care systems. Despite increasing knowledge regarding risk factors for type 2 diabetes and evidence for ...
Abstract. Diabetes mellitus is a chronic heterogeneous metabolic disorder with complex pathogenesis. It is characterized by elevated blood glucose levels or hyperglycemia, which results from abnormalities in either insulin secretion or insulin action or both. Hyperglycemia manifests in various forms with a varied presentation and results in ...
The diagnosis of incident diabetes was based on an oral glucose tolerance test (OGTT). The overall risk reduction of T2D by the lifestyle interventions was 0.53 (95% CI 0.41; 0.67). Most of the trials aimed to reduce weight, increase physical activity, and apply a diet relatively low in saturated fat and high in fiber.
Methods. In this trial involving participants with type 2 diabetes of less than 10 years' duration who were receiving metformin and had glycated hemoglobin levels of 6.8 to 8.5%, we compared the ...
Diabetes mellitus is associated with an increased risk of various cancers, especially gastrointestinal cancers and female-specific cancers. Hospitalization and mortality from various infections ...
Herein, the search criteria were based on the screening of all the respected and available research and review articles in the literature about diabetes. The authors screened over 500 scientific articles from different databases like PubMed and Google Scholar. Fig. (1). Download: Download high-res image (182KB) Download: Download full-size image
This article provides an overview of the main types and common symptoms of diabetes, its acute and long-term complications and its management. It also outlines the nurse's role in diabetes care, which frequently includes assessing and empowering patients. Keywords: blood glucose; clinical; diabetes; diabetic foot ulcers; diabetic ketoacidosis ...
Pre-diabetes. Current guidelines for the delay and prevention of type 2 diabetes mellitus recommend for people with prediabetes to lose at least 7% of their body weight. Here, we advocate to use ...
DEFINITION OF DIABETES MELLITUS. Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Metabolic abnormalities in carbohydrates, lipids, and proteins result from the importance of insulin as an anabolic hormone.
Diabetes remains a substantial public health issue. Type 2 diabetes, which makes up the bulk of diabetes cases, is largely preventable and, in some cases, potentially reversible if identified and managed early in the disease course. However, all evidence indicates that diabetes prevalence is increasing worldwide, primarily due to a rise in obesity caused by multiple factors. Preventing and ...
Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number affected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). Early detection and proactive management are crucial for prevention and mitigation of microvascular and macrovascular complications and mortality burden. Access to novel therapies ...
Unfortunately, even today, diabetes is one of the most common chronic diseases in the country and worldwide. In the US, it remains as the seventh leading cause of death. Diabetes mellitus (DM) is a metabolic disease, involving inappropriately elevated blood glucose levels. DM has several categories, including type 1, type 2, maturity-onset ...
The chronic complications of type 2 diabetes are a major cause of mortality and disability worldwide [1, 2]. Clinical research is the main way to gain knowledge about long-term diabetic complications and reduce the burden of diabetes. This allows for designing effective programs for screening and follow-up and fine-targeted therapeutic ...
Purpose of Review This article highlights foundational evidence, translation studies, and current research behind type 2 diabetes prevention efforts worldwide, with focus on high-risk populations, and whole-population approaches as catalysts to global prevention. Recent Findings Continued focus on the goals of foundational lifestyle change program trials and their global translations, and the ...
Abstract. Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number affected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). Early detection and proactive management are crucial for prevention and mitigation of microvascular and macrovascular ...
Global and regional trends from 1990 to 2017 of type 2 diabetes for all ages were compiled. Forecast estimates were obtained using the SPSS Time Series Modeler. In 2017, approximately 462 million individuals were affected by type 2 diabetes corresponding to 6.28% of the world's population (4.4% of those aged 15-49 years, 15% of those aged ...
Journal of Diabetes Research is an open access journal that publishes articles related to type 1 and type 2 diabetes. Topics include etiology, pathogenesis, management, and prevention of diabetes, as well as associated complications such as nephropathy. As part of Wiley's Forward Series, this journal offers a streamlined, faster publication ...
Diabetes mellitus is a group of physiological dysfunctions characterized by hyperglycemia resulting directly from insulin resistance, inadequate insulin secretion, or excessive glucagon secretion. Type 1 diabetes (T1D) is an autoimmune disorder leading to the destruction of pancreatic beta-cells. Type 2 diabetes (T2D), which is much more common ...