Home — Essay Samples — Nursing & Health — Public Health Issues — Vaccination

Essays on Vaccination
Vaccines essay topics and outline examples, essay title 1: "the vital role of vaccines in public health: debunking myths and upholding science".
Thesis Statement: Vaccines are a cornerstone of public health, and it is crucial to dispel misinformation and emphasize the overwhelming scientific evidence supporting their safety and efficacy.
Essay Outline:
- Introduction
- The History and Impact of Vaccines
- Common Vaccine Myths and Misconceptions
- Scientific Evidence Supporting Vaccines
- Vaccine Safety and Adverse Effects
- The Importance of Herd Immunity
- Addressing Vaccine Hesitancy
Essay Title 2: "Vaccination Mandates: Balancing Individual Rights with Public Health"
Thesis Statement: While respecting individual rights is essential, vaccination mandates are a legitimate measure to safeguard public health and prevent outbreaks of vaccine-preventable diseases.
- The Concept of Vaccination Mandates
- Individual Rights and Autonomy
- Public Health Concerns and Disease Prevention
- Legal and Ethical Considerations
- Case Studies of Vaccine Mandates
- Opposition and Challenges to Mandates
Essay Title 3: "The Impact of Vaccine Disinformation on Public Health: A Global Challenge"
Thesis Statement: The proliferation of vaccine disinformation poses a significant threat to public health, and addressing this challenge is vital to ensure widespread vaccine acceptance and disease control.
- The Spread and Impact of Vaccine Disinformation
- Factors Contributing to Vaccine Hesitancy
- The Role of Social Media and Online Platforms
- Countering Vaccine Disinformation Efforts
- Global Initiatives and Collaborations
- Case Studies on Successful Interventions
The Benefits of Vaccination
The issues surrounding vaccination and its importance, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Arguments About The Importance of Making Vaccinations Mandatory
Vaccination – the greatest invention of all times, my research of advantages and disadvantages of vaccination, the use of vaccination – a choice for every one, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Problem of The Vaccine War in The World
The ethical theories and issues surrounding vaccination in america, the effectiveness of vacciness in preventing illnesses and infectious diseases, the importance of vaccines to prevent infectious diseases, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Advantages and Disadvantages of The Various Types of Vaccines
Chickenpox: history, symptoms and treatment, the importance of increasing hpv vaccination in children, why is vaccination of human papillomavirus significant, debate on vaccination and autism, impact of media on parents' acceptance of immunization, the use of vaccines in modern medicine and the vaccination delimma, legal and ethical issues about the mmr vaccine, an argument in favor of using vaccines, the urgent need for a vaccine against zika virus, report on the measles disease and vaccination, yellow fever disease - what problems are caused by mosquitoes, chasing polio eradication: vaccine development, the examination of human sciences in connection to the effectiveness of vaccines, the different types of vaccines, vaccine types, should vaccinations be mandatory: future safety for children, should parents vaccinate their child, should vaccines be required to attend public school, why you should get vaccinated: a persuasive discussion.
Vaccination, also known as immunization, is a medical procedure that involves the administration of a vaccine to stimulate the immune system and provide protection against specific infectious diseases. It is a preventive measure designed to enhance the body's natural defenses by introducing harmless fragments of the disease-causing agent or weakened or inactivated forms of the pathogen.
The mechanism of vaccination involves introducing a weakened or inactivated form of a disease-causing agent, such as a virus or bacterium, into the body. This prompts the immune system to recognize and respond to the pathogen. When a vaccine is administered, it stimulates the immune system to produce an immune response, similar to what would happen during a natural infection. The immune system recognizes the foreign antigens present in the vaccine and mounts a defense by producing antibodies and activating immune cells. These immune responses help the body develop immunity against the specific pathogen. Vaccination can also involve the use of genetically engineered proteins or pieces of the pathogen to stimulate an immune response. These components are known as antigens and can be derived from the outer coats of viruses or the cell walls of bacteria. By introducing these harmless components of the pathogen into the body, vaccines help the immune system recognize and remember the specific pathogen. This way, if the individual is later exposed to the actual disease-causing agent, their immune system can mount a rapid and effective response to neutralize or eliminate the pathogen, preventing the development of the disease or reducing its severity.
1. Inactivated Vaccines 2. Live Attenuated Vaccines 3. Subunit, Recombinant, and Conjugate Vaccines 4. mRNA Vaccines 5. Viral Vector Vaccines
The origin of vaccination can be traced back to ancient times, although the concept was not fully understood at the time. The practice of vaccination, as we know it today, began with the discovery of immunization against smallpox by Edward Jenner in the late 18th century. Jenner, an English physician, observed that milkmaids who had contracted cowpox, a much milder disease, seemed to be protected against smallpox. In 1796, he conducted an experiment where he took material from a cowpox sore and inoculated it into an eight-year-old boy named James Phipps. Afterward, Jenner exposed the boy to smallpox, but he did not develop the disease. This groundbreaking experiment led to the development of the smallpox vaccine. The term "vaccination" itself comes from the Latin word "vacca," meaning cow, as the original smallpox vaccine was derived from cowpox. Jenner's work paved the way for the development of vaccines against other infectious diseases, and vaccination quickly became a widely accepted method for preventing and controlling the spread of deadly diseases.
Public opinion on vaccination varies across different societies and individuals. Overall, vaccination has been widely accepted and supported by the majority of the population, recognizing its significant role in preventing and controlling infectious diseases. Vaccines have been instrumental in eradicating or significantly reducing the impact of diseases such as smallpox, polio, measles, and more. However, there are also pockets of skepticism and opposition towards vaccination, driven by various factors such as misinformation, fear, religious beliefs, or concerns about vaccine safety. This has led to the emergence of anti-vaccine movements and vaccine hesitancy in some communities. Public opinion on vaccination is influenced by various factors, including access to accurate information, trust in healthcare professionals and scientific research, cultural and religious beliefs, personal experiences, and the influence of social media and other communication channels. Efforts to promote vaccination and address vaccine hesitancy involve public health campaigns, education, and communication strategies to provide accurate information about vaccines, address concerns, and emphasize the importance of vaccination in protecting individual and public health.
1. Disease prevention 2. Herd immunity 3. Public health impact 4. Safety and effectiveness 5. Global impact
1. Vaccine safety concerns 2. Personal freedom and choice 3. Misinformation and skepticism 4. Religious or philosophical objections 5. Perception of low disease risk
1. According to the World Health Organization (WHO), vaccines prevent between 2-3 million deaths worldwide every year. 2. Smallpox is the only disease that has been totally eradicated through vaccination. 3. Vaccines have significantly reduced the global burden of infectious diseases. For instance, measles deaths decreased by 73% worldwide between 2000 and 2018. 4. The influenza vaccine helps reduce the risk of severe illness and hospitalization. In the United States, annual flu vaccination prevented an estimated 7.5 million flu illnesses during the 2019-2020 season. 5. The average vaccine takes around 10-15 years of research and development before it is widely available.
The topic of vaccination is of paramount importance when considering the impact it has had on public health. Writing an essay about vaccination provides an opportunity to explore the profound significance of this medical intervention. Vaccination has played a pivotal role in preventing and controlling infectious diseases, saving countless lives worldwide. By delving into the subject, one can highlight the historical development of vaccines, their mechanisms of action, and the scientific evidence supporting their effectiveness. Furthermore, examining the topic of vaccination allows for an exploration of the public health implications, including the concept of herd immunity and the role of vaccination in disease eradication efforts. It also provides a platform to address the various arguments surrounding vaccine hesitancy and vaccine refusal, shedding light on the importance of accurate information, education, and communication. Moreover, the essay can delve into the ethical considerations surrounding vaccination policies, such as balancing individual autonomy with the collective responsibility for public health. By exploring these aspects, one can foster a deeper understanding of the challenges, controversies, and potential solutions in promoting vaccination uptake.
1. American Academy of Pediatrics. (2018). Immunization information for parents. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/default.aspx 2. Centers for Disease Control and Prevention. (2021). Vaccines & immunizations. https://www.cdc.gov/vaccines/index.html 3. Gust, D. A., Darling, N., Kennedy, A., & Schwartz, B. (2008). Parents with doubts about vaccines: Which vaccines and reasons why. Pediatrics, 122(4), 718-725. https://doi.org/10.1542/peds.2007-0538 4. Larson, H. J., de Figueiredo, A., Xiahong, Z., Schulz, W. S., Verger, P., Johnston, I. G., Cook, A. R., Jones, N. S., & the SAGE Working Group on Vaccine Hesitancy. (2016). The state of vaccine confidence 2016: Global insights through a 67-country survey. EBioMedicine, 12, 295-301. https://doi.org/10.1016/j.ebiom.2016.08.042 5. MacDonald, N. E., Hesitancy SAGE Working Group. (2015). Vaccine hesitancy: Definition, scope and determinants. Vaccine, 33(34), 4161-4164. https://doi.org/10.1016/j.vaccine.2015.04.036 6. Offit, P. A., Quarles, J., Gerber, M. A., Hackett, C. J., & Marcuse, E. K. (2002). Addressing parents' concerns: Do vaccines cause allergic or autoimmune diseases? Pediatrics, 110(6), 1113-1116. https://doi.org/10.1542/peds.110.6.1113 7. Omer, S. B., Salmon, D. A., Orenstein, W. A., deHart, M. P., & Halsey, N. (2009). Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. New England Journal of Medicine, 360(19), 1981-1988. https://doi.org/10.1056/NEJMsa0806477 8. Smith, P. J., Humiston, S. G., Parnell, T., Vannice, K. S., & Salmon, D. A. (2011). The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Reports, 126(Suppl 2), 135-146. https://doi.org/10.1177/00333549111260S219 9. World Health Organization. (2019). Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 10. World Health Organization. (2021). Immunization coverage. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
Relevant topics
- Eating Disorders
- Drug Addiction
- Childhood Obesity
- Teenage Pregnancy
- Birth Control
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Intermittent Fasting
Pre-Exposure Prophylaxis (PrEP)
The importance of vaccinations.
Last Updated August 2023 | This article was created by familydoctor.org editorial staff and reviewed by Deepak S. Patel, MD, FAAFP, FACSM
Related Topics
Childhood vaccines: what they are and why your child needs them, immunization schedules, preventive services for healthy living.
Name (required)
Mail (will not be published) (required)
Gimme a site! Just a username, please.
Remember Me
There has been confusion and misunderstandings about vaccines. But vaccinations are an important part of family and public health. Vaccines prevent the spread of contagious, dangerous, and deadly diseases. These include measles, polio, mumps, chicken pox, whooping cough, diphtheria, HPV, and COVID-19.
The first vaccine discovered was the smallpox vaccine. Smallpox was a deadly illness. It killed 300 million to 500 million people around the world in the last century. After the vaccine was given to people, the disease was eventually erased. It’s the only disease to be completely destroyed. There are now others close to that point, including polio.
When vaccination rates decline, cases of preventable diseases go up. This has been happening in recent years with measles. As of July 7, 2023, the Centers for Disease Control has been notified of 18 confirmed cases in 12 U.S. jurisdictions. That may not seem like a lot but compare it with just 3 cases during the same time in 2022. By the end of 2022, there were 121 cases. Almost all those cases could have been prevented with vaccines.
What are vaccines?
A vaccine (or immunization) is a way to build your body’s natural immunity to a disease before you get sick. This keeps you from getting and spreading the disease.
For some vaccines, a weakened form of the disease germ is injected into your body. This is usually done with a shot in the leg or arm. Your body detects the invading germs (antigens) and produces antibodies to fight them. Those antibodies then stay in your body for a long time. In many cases, they stay for the rest of your life. If you’re ever exposed to the disease again, your body will fight it off without you ever getting the disease.
Some illnesses, like strains of cold viruses, are fairly mild. But some, like COVID-19, smallpox or polio, can cause life-altering changes. They can even result in death. That’s why preventing your body from contracting these illnesses is very important.
How does immunity work?
Your body builds a defense system to fight foreign germs that could make you sick or hurt you. It’s called your immune system. To build up your immune system, your body must be exposed to different germs. When your body is exposed to a germ for the first time, it produces antibodies to fight it. But that takes time, and you usually get sick before the antibodies have built up. But once you have antibodies, they stay in your body. So, the next time you’re exposed to that germ, the antibodies will attack it, and you won’t get sick.
Path to improved health
Everyone needs vaccines. They are recommended for infants, children, teenagers, and adults. There are widely accepted immunization schedules available. They list what vaccines are needed, and at what age they should be given. Most vaccines are given to children. It’s recommended they receive 12 different vaccines by their 6th birthday. Some of these come in a series of shots. Some vaccines are combined so they can be given together with fewer shots.
The American Academy of Family Physicians (AAFP) believes that immunization is essential to preventing the spread of contagious diseases. Vaccines are especially important for at-risk populations such as young children and older adults. The AAFP offers vaccination recommendations, immunization schedules , and information on disease-specific vaccines.
Being up to date on vaccines is especially important as children head back to school. During the 2021 school year, state-required vaccines among kindergarteners dropped from 95% to 94%. In the 2021-2022 year it fell again to 93%. Part of this was due to disruptions from the COVID-19 pandemic.
Is there anyone who can’t get vaccines?
Some people with certain immune system diseases should not receive some types of vaccines and should speak with their health care providers first. There is also a small number of people who don’t respond to a particular vaccine. Because these people can’t be vaccinated, it’s very important everyone else gets vaccinated. This helps preserve the “herd immunity” for the vast majority of people. This means that if most people are immune to a disease because of vaccinations, it will stop spreading.
Are there side effects to vaccines?
There can be side effects after you or your child get a vaccine. They are usually mild. They include redness or swelling at the injection site. Sometimes children develop a low-grade fever. These symptoms usually go away in a day or two. More serious side effects have been reported but are rare.
Typically, it takes years of development and testing before a vaccine is approved as safe and effective. However, in cases affecting a global, public health crisis or pandemic, it is possible to advance research, development, and production of a vaccine for emergency needs. Scientists and doctors at the U.S. Food and Drug Administration (FDA) study the research before approving a vaccine. They also inspect places where the vaccines are produced to make sure all rules are being followed. After the vaccine is released to the public, the FDA continues to monitor its use. It makes sure there are no safety issues.
The benefits of their use far outweigh any risks of side effects.
What would happen if we stopped vaccinating children and adults?
If we stopped vaccinating, the diseases would start coming back. Aside from smallpox, all other diseases are still active in some part of the world. If we don’t stay vaccinated, the diseases will come back. There would be epidemics, just like there used to be.
This happened in Japan in the 1970s. They had a good vaccination program for pertussis (whooping cough). Around 80% of Japanese children received a vaccination. In 1974, there were 393 cases of whooping cough and no deaths. Then rumors began that the vaccine was unsafe and wasn’t needed. By 1976, the vaccination rate was 10%. In 1979, there was a pertussis epidemic, with more than 13,000 cases and 41 deaths. Soon after, vaccination rates improved, and the number of cases went back down.
Things to consider
There have been many misunderstandings about vaccines. There are myths and misleading statements that spread on the internet and social media about vaccines. Here are answers to 5 of the most common questions/misconceptions about vaccines.
Vaccines do NOT cause autism.
Though multiple studies have been conducted, none have shown a link between autism and vaccines. The initial paper that started the rumor has since been discredited.
Vaccines are NOT too much for an infant’s immune system to handle.
Infants’ immune systems can handle much more than what vaccines give them. They are exposed to hundreds of bacteria and viruses every day. Adding a few more with a vaccine doesn’t add to what their immune systems are capable of handling.
Vaccines do NOT contain toxins that will harm you.
Some vaccines contain trace amounts of substances that could be harmful in a large dose. These include formaldehyde, aluminum, and mercury. But the amount used in the vaccines is so small that the vaccines are completely safe. For example, over the course of all vaccinations by the age of 2, a child will take in 4mg of aluminum. A breast-fed baby will take in 10mg in 6 months. Soy-based formula delivers 120mg in 6 months. In addition, infants have 10 times as much formaldehyde naturally occurring in their bodies than what is contained in a vaccine. And the toxic form of mercury has never been used in vaccines.
Vaccines do NOT cause the diseases they are meant to prevent.
This is a common misconception, especially about the flu vaccine. Many people think they get sick after getting a flu shot. But flu shots contain dead viruses—it’s impossible to get sick from the shot but mild symptoms can occur because the vaccine may trigger an immune response, which is normal. Even with vaccines that use weakened live viruses, you could experience mild symptoms similar to the illness. But you don’t actually have the disease.
We DO still need vaccines in the U.S., even though infection rates are low.
Many diseases are uncommon in the U.S. because of our high vaccination rate. But they haven’t been eliminated from other areas of the world. If a traveler from another country brings a disease to the U.S., anyone who isn’t vaccinated is at risk of getting that disease. The only way to keep infection rates low is to keep vaccinating.
Questions to ask your doctor
- Why does my child need to be vaccinated?
- What are the possible side effects of the vaccination?
- What do I do if my child experiences a side effect from the vaccine?
- What happens if my child doesn’t get all doses of the recommended vaccines? Will he or she be able to go to daycare or school?
- We missed a vaccination. Can my child still get it late?
- Are there new vaccines that aren’t on the immunization schedules for kids?
- What should I do if I don’t have health insurance, or my insurance doesn’t cover vaccinations?
- What vaccinations do I need as an adult?
- Why do some people insist they became sick after getting the flu vaccine?
Centers for Disease Control and Prevention: Vaccines & Immunizations
Last Updated: August 10, 2023
This article was contributed by familydoctor.org editorial staff.
Copyright © American Academy of Family Physicians
This information provides a general overview and may not apply to everyone. Talk to your family doctor to find out if this information applies to you and to get more information on this subject.
Related Articles
Most schools and daycares require certain childhood vaccines, but they’re also good for your child’s health.
Certain immunizations are important at every age to reduce illness, hospitalization, or death.
A preventive service might be a test, an immunization or vaccine, or advice from your doctor. These services can…
Advertisement

familydoctor.org is powered by

Visit our interactive symptom checker
REVIEW article
Impact of vaccines; health, economic and social perspectives.

- 1 Department of Zoology, University of Oxford, Oxford, United Kingdom
- 2 Department of Paediatric Infectious Diseases, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom
- 3 Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, United States
In the 20th century, the development, licensing and implementation of vaccines as part of large, systematic immunization programs started to address health inequities that existed globally. However, at the time of writing, access to vaccines that prevent life-threatening infectious diseases remains unequal to all infants, children and adults in the world. This is a problem that many individuals and agencies are working hard to address globally. As clinicians and biomedical scientists we often focus on the health benefits that vaccines provide, in the prevention of ill-health and death from infectious pathogens. Here we discuss the health, economic and social benefits of vaccines that have been identified and studied in recent years, impacting all regions and all age groups. After learning of the emergence of SARS-CoV-2 virus in December 2019, and its potential for global dissemination to cause COVID-19 disease was realized, there was an urgent need to develop vaccines at an unprecedented rate and scale. As we appreciate and quantify the health, economic and social benefits of vaccines and immunization programs to individuals and society, we should endeavor to communicate this to the public and policy makers, for the benefit of endemic, epidemic, and pandemic diseases.
Introduction
“The impact of vaccination on the health of the world’s peoples is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” ( Plotkin and Mortimer, 1988 ).
The development of safe and efficacious vaccination against diseases that cause substantial morbidity and mortality has been one of the foremost scientific advances of the 21st century. Vaccination, along with sanitation and clean drinking water, are public health interventions that are undeniably responsible for improved health outcomes globally. It is estimated that vaccines have prevented 6 million deaths from vaccine-preventable diseases annually ( Ehreth, 2003 ). By 2055, the earth’s population is estimated to reach almost 10 billion ( United Nations Department of Economic and Social Affairs, 2019 ), a feat that in part is due to effective vaccines that prevent disease and prolong life expectancy across all continents. That said, there is still much to be done to ensure the financing, provision, distribution, and administration of vaccines to all populations, in particular those which are difficult to reach, including those skeptical about their protective value and those living in civil disruption. Agencies including the World Health Organization (WHO), United Nations Children’s Fund (UNICEF), Gavi, the Vaccine Alliance, The Bill & Melinda Gates Foundation, and the Coalition for Epidemic Preparedness Initiative (CEPI), with their multiple funding streams have been instrumental in expanding vaccine benefits to all. These importance of these organizations in global co-operation and participation was essential in the setting of the 2019 global pandemic of SARS-CoV-2, in light of the health and economic impact of COVID-19 on societies in high-, middle- and low-income countries. This review will highlight the benefits of vaccinations to society from the perspectives of health, economy, and social fabric ( Figure 1 ), which need to be considered in the overall assessment of impact to ensure that vaccines are prioritized by those making funding decisions.
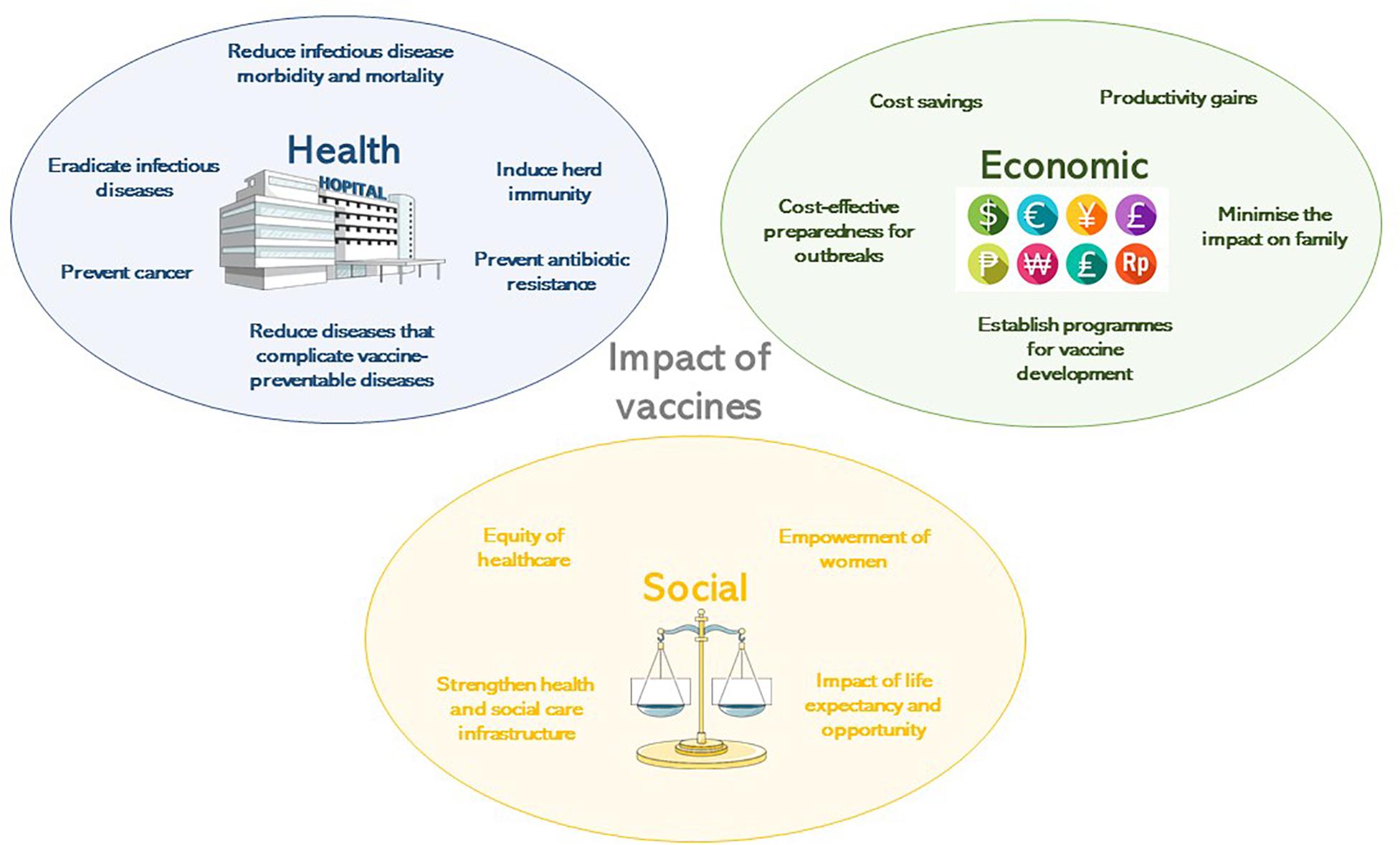
Figure 1. The impact of vaccines according to their health, economic or social benefit.
Brief History of Vaccine Development
Human use of preparations to prevent specific infections have been described since 1500 AD, beginning in China ( Needham, 2000 ) where smallpox was prevented by variolation, which is the introduction of material from scabs into the skin. In 1796 in the United Kingdom, Edward Jenner observed the immunity to smallpox of milkmaids having previously had natural infection with cowpox ( Jenner, 1798 ). He determined that inoculating small amounts of pus from the lesions of cowpox, presumably containing a virus related to vaccinia, into susceptible hosts rendered them immune to smallpox. The vaccine against smallpox was developed in 1798. The next phase of scientific developments involving the manipulation of infectious agents to extract suitable vaccine antigens took almost a century of research. Louis Pasteur’s work with attenuation by oxygen or heat led to live-attenuated chicken cholera, inactivated anthrax and live-attenuated rabies vaccines at the turn of the 20th century ( Pasteur, 1880 , 1881 , 1885 ). Alternative methods of attenuation using serial passage of Mycobacterium bovis led to the live Bacille Calmette-Guerin (BCG) ( Calmette, 1927 ) vaccine, still in use today for the prevention of tuberculosis. Serial passage was also used in the development of yellow fever vaccines ( Theiler and Smith, 1937a ) which are grown in chicken embryo tissues ( Theiler and Smith, 1937b ). Whole cell killed bacterial vaccines were developed when methods to treat and kill bacteria through heat or chemicals were established and whole cell typhoid, cholera and pertussis vaccines resulted at the end of the 19th Century. In 1923, Alexander Glenny and Barbara Hopkins developed methods to inactivate bacterial toxins with formaldehyde, leading to the diphtheria and tetanus toxoid vaccines ( Glenny and Hopkins, 1923 ).
Advances in virus culture in vitro allowed viral pathogens to be studied in greater detail and attenuation methods due to cultivation in artificial conditions led to the live oral polio, measles, rubella, mumps and varicella virus vaccines. In the 1960’s at the Walter Reed Army Institute of Research, vaccines were developed using capsular polysaccharides ( Gold and Artenstein, 1971 ; Artenstein, 1975 ), of encapsulated organisms including meningococci and later pneumococci ( Austrian, 1989 ) and Haemophilus influenzae type b (Hib) ( Anderson et al., 1972 ). To protect against multiple serotype variants of polysaccharide capsules, polyvalent vaccines were developed and later conjugated to carrier proteins to enhance their efficacy in infants in particular by recruiting T-cell mediated help to induce memory B-cells ( Schneerson et al., 1980 ). Vaccines made solely from proteins were rare, with the exception of the toxoid vaccines, but the acellular pertussis vaccine containing five protein antigens, was developed to mitigate the unwanted effects of the whole cell vaccine ( Sato and Sato, 1999 ).
The end of the 20th century marked a revolution in molecular biology and provided insights into microbiology and immunology allowing a greater understanding of pathogen epitopes and host responses to vaccination. Molecular genetics and genome sequencing has enabled the development of vaccines against RNA viruses possessing multiple variants of epitopes, such as the live and inactivated influenza vaccines ( Maassab and DeBorde, 1985 ) and live rotavirus vaccines ( Clark et al., 2006 ). DNA manipulation and excision allowed the use of surface antigen for hepatitis B viral vectors ( Plotkin, 2014 ). The human papilloma virus (HPV) vaccine benefits from enhanced immunogenicity due to the formation of virus-like particles by the L1 antigen of each virus contained in the vaccine ( Kirnbauer et al., 1992 ). Bacterial genome sequencing has provided in depth analysis of meningococcal antigens, to identify potential proteins for meningococcal B vaccines ( Serruto et al., 2012 ).
Vaccine development was tested in 2020 when a novel coronavirus, SARS-CoV-2, emerged from China causing a severe acute respiratory illness, which subsequently spread globally. Within 5 months of the discovery of this virus (7th January 2020) ( Zhu et al., 2020 ) and person-person transmission ( Chan et al., 2020 ), 5,697,334 cases had been identified, with orders of magnitude likely not measured and almost no country escaped the pandemic. Owing to the previous advances in vaccinology, by 8th April 2020, there were 73 vaccine candidates under pre-clinical investigation ( Thanh Le et al., 2020 ). Of these, six were in Phase 1 or 1/2 trials and one was in Phase 2/3 trials by 28th May 2020. The rapidity of this response demonstrated the ability to harness existing technologies including: RNA vaccine platforms (NCT04283461), DNA vaccine platforms (NCT04336410), recombinant vector vaccines (NCT04313127, NCT04324606) and adjuvants. The regulation, manufacturer and distribution of these vaccines will require expedition given the global public health need, from a period of many years to a matter of months. The efficacy and health impact of these vaccines is yet to be established, but if they are effective, then vaccines need to be made available for all global regions affected by SARS-CoV-2. The funding of this endeavor will prove challenging in a global context of national social and economic lockdown and massive government borrowing, but the justification for this provision will be through the multiple benefits to society that will need healthy citizens to rebuild economies in the decades post-COVID-19.
The history of vaccination is not complete without describing the public health intervention that led to the routine use of these vaccines for children globally. The Expanded Program of Immunization (EPI) was founded by WHO in 1974 with the aim of providing routine vaccines to all children by 1990 ( World Health Assembly, 1974 ). In 1977, global policies for immunization against diphtheria, pertussis, tetanus, measles, polio, and tuberculosis were set out. The EPI includes hepatitis B, Hib, and pneumococcal vaccines in many areas and by 2017, 85% of the world’s children (12–23 months of age) received diphtheria, pertussis, tetanus, and measles vaccines ( World Bank, 2019 ).
Health Benefits of Vaccination
Reduction in infectious diseases morbidity and mortality.
The most significant impact of vaccines has been to prevent morbidity and mortality from serious infections that disproportionately affect children. Vaccines are estimated to prevent almost six million deaths/year and to save 386 million life years and 96 million disability-adjusted life years (DALYs) globally ( Ehreth, 2003 ). The traditional measures of vaccine impact include: vaccine efficacy, the direct protection offered to a vaccinated group under optimal conditions e.g., trial settings; or vaccine effectiveness, the direct and indirect effect of vaccines on the population in a real-life setting ( Wilder-Smith et al., 2017 ). Providing a numerical measure of vaccine impact therefore involves estimating the extent of morbidity and mortality prevented. In the United States in 2009, amongst an annual birth cohort vaccinated against 13 diseases it was estimated that nearly 20 million cases of disease and ∼42,000 deaths were prevented ( Zhou et al., 2009 ). Infectious diseases that accounted for major mortality and morbidity in the early 20th century in the United States all showed over a 90% decline in incidence by 2017 from the pre-vaccine peak incidence ( Roush and Murphy, 2007 ), due to high vaccine uptake of over 90% for the DTaP (diphtheria, tetanus, and acellular pertussis), MMR (measles, mumps, and rubella) and polio vaccines ( World Health Organisation, 2019a ; Table 1 ). A similar pattern of infectious diseases reduction was seen across other high-income countries, demonstrating the efficacy of vaccines when available and accessible.
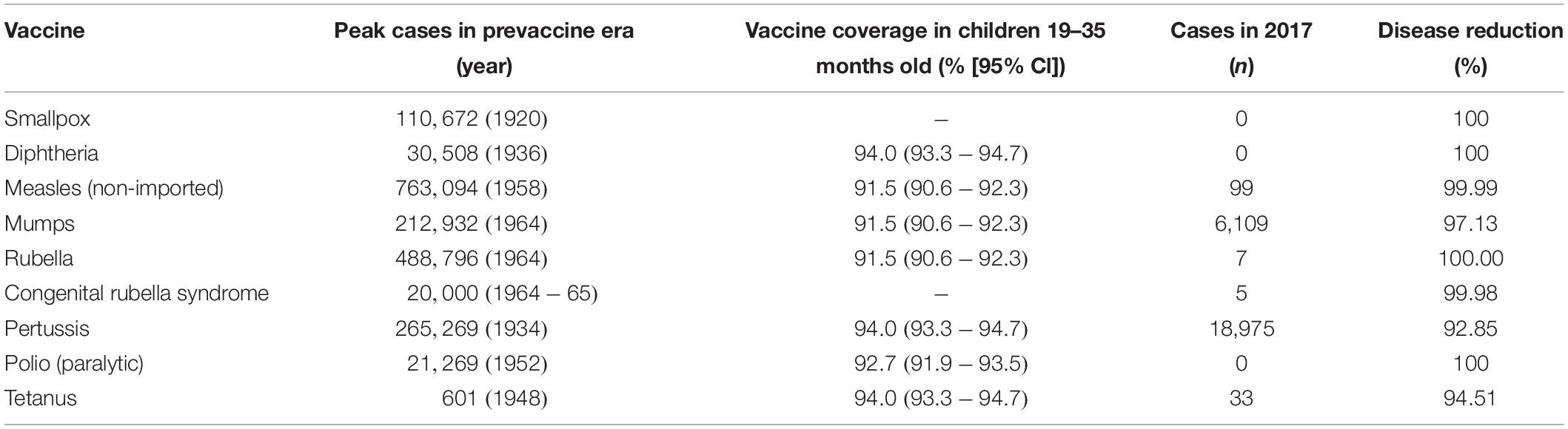
Table 1. Vaccine impact in United States comparing the incidence of diseases prior to the implementation of vaccine ( Roush and Murphy, 2007 ), described as the pre-vaccine era and the vaccine coverage ( Hill et al., 2017 ) and disease incidence ( Centers for Disease Control and Prevention, 2017 ) in 2017, as reported by the Centers for Disease Control and Prevention.
Globally, the provision of vaccines is more challenging in many low- and middle- income countries (LMIC), as evidenced by the failure to make the EPI vaccines available to every child by 1990, irrespective of setting ( Keja et al., 1988 ). Central to this is limited financial resources, but other barriers to vaccine introduction include: underappreciation of the value of vaccines locally/regionally though insufficient relevant data on disease burden, vaccine efficacy, or cost-effectiveness; inadequate healthcare infrastructure for vaccine handling, storage, programmatic management, and disease surveillance; and lack of global, regional or local policy-making and leadership ( Munira and Fritzen, 2007 ; Hajjeh, 2011 ). In 2018, the global uptake of three doses of DTaP reached 86% which corresponded to 116,300,000 infants ( World Health Organisation, 2019a ). The vaccine coverage is, however, variable between low-, middle- and high-income countries because of a combination of economic and political circumstances as well as variable access to non-governmental support from Gavi, the Vaccine Alliance ( Turner et al., 2018 ; Figure 2 ). Nevertheless, there has been a decrease in the global burden of diseases caused by vaccine-preventable pathogens ( Figure 3 ) enabling healthier lives for many millions of children. A further benefit following vaccination, is the evidence that although vaccines may not always prevent an infection, for example VZV or pertussis, a milder disease course may follow ( Andre et al., 2008 ; Bonanni et al., 2015 ).
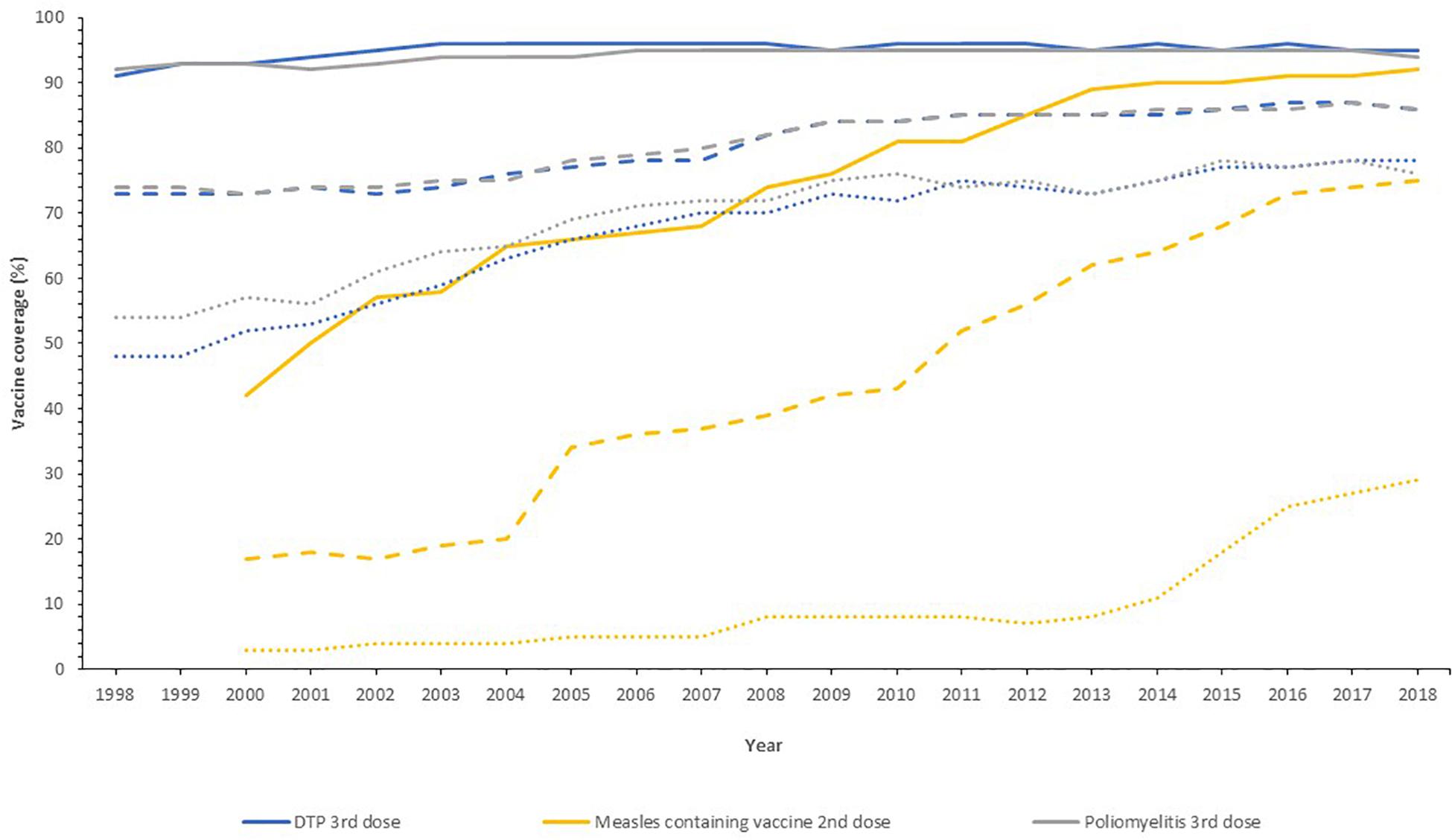
Figure 2. Vaccine uptake across different regions defined by economic status by the World Bank into high- (solid line), middle- (dashed line), and low-income countries (dotted line) for the past 20 years. Data from the World Health Organization and UNICEF dataset “Coverage Estimates Series” ( World Health Organization [WHO] and United Nations Children’s Fund [UNICEF], 2019 ).
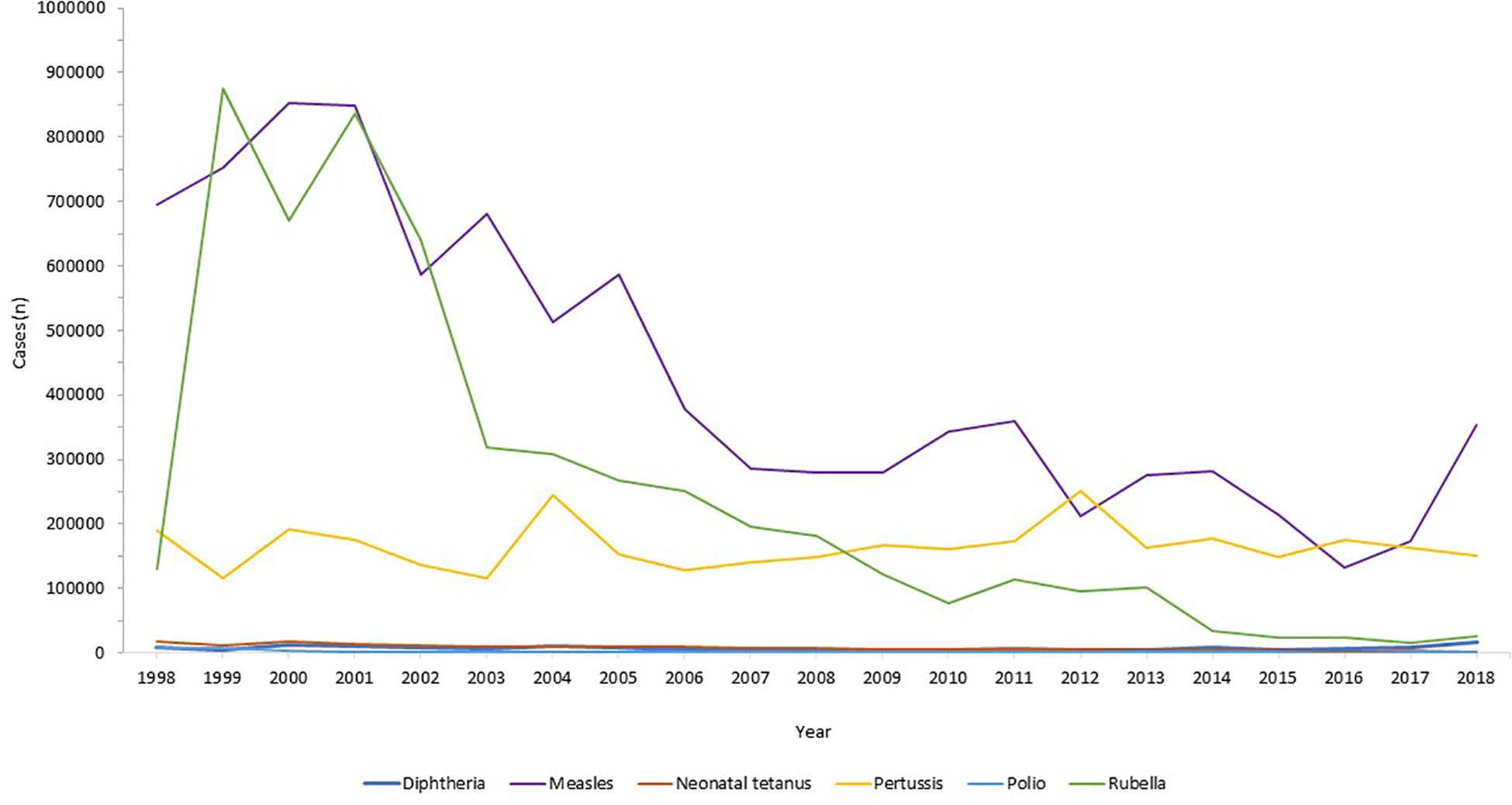
Figure 3. Reduction in infectious diseases globally. Across all world regions, data from the WHO, for the last 20 years showing the control of diphtheria and tetanus and the decline in rubella and congenital rubella syndrome (data not shown). Data from the World Health Organization dataset “Reported cases of vaccine-preventable diseases” ( World Health Organisation, 2019c ).
Eradication of Infectious Diseases
Global disease eradication can be achieved for pathogens that are restricted to human reservoirs. For eradication of infectious diseases, high levels of population immunity are required globally, to ensure no ongoing transmission in our well-connected world ( Andre et al., 2008 ). Furthermore, surveillance systems must be in place to monitor the decline in disease, with accurate and reliable diagnostic testing to monitor ongoing cases. At the time of writing, the only infectious disease that has been eradicated in humans by vaccination is smallpox. This disease had afflicted humans for millenia, with the earliest evidence found in Egyptian mummies from 1000 BC ( Geddes, 2006 ). Jenner’s successful development of the smallpox vaccine using vaccinia virus ( Jenner, 1798 ) led to the ultimate eradication of the disease through ring vaccination as announced by the World Health Assembly in 1980 ( Strassburg, 1982 ), which was an historic public health achievement. The second example of eradication was of the rinderpest virus in livestock, an infection that indirectly led to human loss of life through loss of agriculture leading to humanitarian crises through famine and poverty. Rinderpest virus infects cattle, buffalo and numerous other domestic species, with widespread disease affecting large parts of Africa and Europe in the 19th century ( Roeder et al., 2013 ). The Plowright tissue culture rinderpest vaccine, developed during the 1950s, was used for mass vaccination campaigns, alongside other public health measures, leading to eradication in 2011 ( Morens et al., 2011 ).
The next infection targeted for eradication is wild polio virus. This devastating paralytic disease routinely afflicted children and adults in both industrialized and developing settings, prior to the development of vaccines. Two polio vaccines, the inactivated polio vaccine (IPV) and the live-attenuated oral polio vaccine (OPV) became available in 1955 and 1963, respectively ( Plotkin, 2014 ), both able to protect against all three wild types of polio virus. Both vaccines have been used globally, with live-attenuated OPV much cheaper and easier to administer but carrying the risk of causing circulating vaccine-derived poliovirus (cVDPV) owing to back-mutation and re-acquisition of neurovirulence. Hence, due to its safety IPV was preferred in industrialized regions and those where the polio incidence was low. In 1998, the Global Polio Eradication Initiative, the largest public-private partnership led by national governments in partnership with the WHO, Rotary International, United States Centers for Disease Control and Prevention (CDC), and UNICEF was launched with the aim of global polio eradication by 2000. Although this target was not met due to lack of funding, political will, and competing health initiatives, there was a 99% reduction in polio incidence by 2000 ( Lien and Heymann, 2013 ). By 2003, there were only six endemic countries with new cases: Egypt, Niger, India, Nigeria, Afghanistan, and Pakistan, of which only the latter four had new cases by 2005. Eradication in India was problematic due to the high birth rates and poor sanitation amongst densely populated regions with marginalized communities and high population mobility ( Thacker et al., 2016 ). India was declared polio free in 2014. Wild polio virus type 2 was eradicated in 2015, the last case of wild type 3 was in 2012 and eradication announced in 2019, with wild type 1 virus remaining in two countries, Pakistan and Afghanistan ( World Health Organisation, 2019b ). In 2019, Nigeria was declared 3 years free of wild polio, the last country in Africa to declare any cases. In the first 6 months of 2020, there were 51 and 17 cases of wild type 1 polio reported in Pakistan and Afghanistan respectively ( Global Polio Eradication Initiative, 2019 ). Ongoing programs to roll out universal vaccination in both countries remain hindered by armed conflict, political instability, remote communities and underdeveloped infrastructure. The risk of the OPV recipients developing cVDPV disease, with transmission through the faeco-oral route to cause outbreaks of vaccine-derived paralytic poliomyelitis remains a concerning obstacle in the eradication process, requiring intensive surveillance.
Herd Immunity
The overriding health benefit perceived by most vaccine recipients is their personal, direct, protection. The added value of vaccination, on a population level, is the potential to generate herd immunity. Where a sufficiently high proportion of the population are vaccinated, transmission of the infecting agent is halted thereby protecting the unvaccinated, who may be those too young, too vulnerable, or too immunosuppressed to receive vaccines. Highly successful vaccination programs have been in place as part of the routine EPI, against encapsulated bacteria that are carried asymptomatically in the oropharynx but that can invade and cause septicemia and meningitis in all age groups. Vaccines against Neisseria meningitidis ( Gold and Artenstein, 1971 ), Streptococcus pneumoniae ( Austrian, 1989 ), and Hib ( Anderson et al., 1972 ) were developed in the 1960s, 1970s, and 1980s, respectively, using their polysaccharide capsules as vaccine antigens, which successfully induced protective immunity (direct protection). Conjugation of these polysaccharides to carrier proteins in the 1990s improved their efficacy by not only ensuring a T cell response and immune memory, but by reducing acquisition of pharyngeal carriage of these organisms, thus providing indirect protection and thereby preventing ongoing transmission ( Pollard et al., 2009 ). This was first observed in national carriage studies in the United Kingdom in 1999–2001 during a mass vaccination campaign against serogroup C N. meningitidis ( Maiden et al., 2008 ) and was a major contributing factor to the declining disease thereafter.
Herd (population) immunity requires high levels of vaccine uptake, to limit the number of unvaccinated people and the opportunity for pathogen transmission between them. The proportion of a given population required to induce herd immunity through vaccination is lower for the bacterial infections and conjugate polysaccharide vaccines, as their basic reproductive number (R 0 ) is lower than viral infections like measles, varicella or polio ( Table 2 ). Measles virus can cause devastating disease ranging from acute presentations with pneumonia or encephalitis, to immune amnesia and long-term complications such as subacute sclerosing panencephalitis ( Mina et al., 2015 , 2019 ; Moss, 2017 ; Petrova et al., 2019 ). The live-attenuated measles vaccine is highly efficacious and the first dose is recommended at 9–12 months of age. To protect those who cannot receive live vaccines (younger infants, pregnant women, the immunosuppressed) from acquiring measles in the community, at least 93–95% of the population is required to be vaccinated with two doses in order to interrupt measles virus transmission. In many countries in Europe and in the United States, this level of vaccination uptake is falling ( Wise, 2018 ), due to a combination of reduced accessibility to health services and vaccine misinformation. As a result, some countries, including the United Kingdom and United States, where elimination of measles had been declared have had a resurgence of disease ( Wise, 2019 ). For high-risk individuals who are unable to be vaccinated, herd immunity represents a life-saving protection strategy against many infections. An alternative strategy, cocooning, has been employed with limited success for pertussis and influenza ( Grizas et al., 2012 ), where their close/household contacts are vaccinated to prevent transmission.
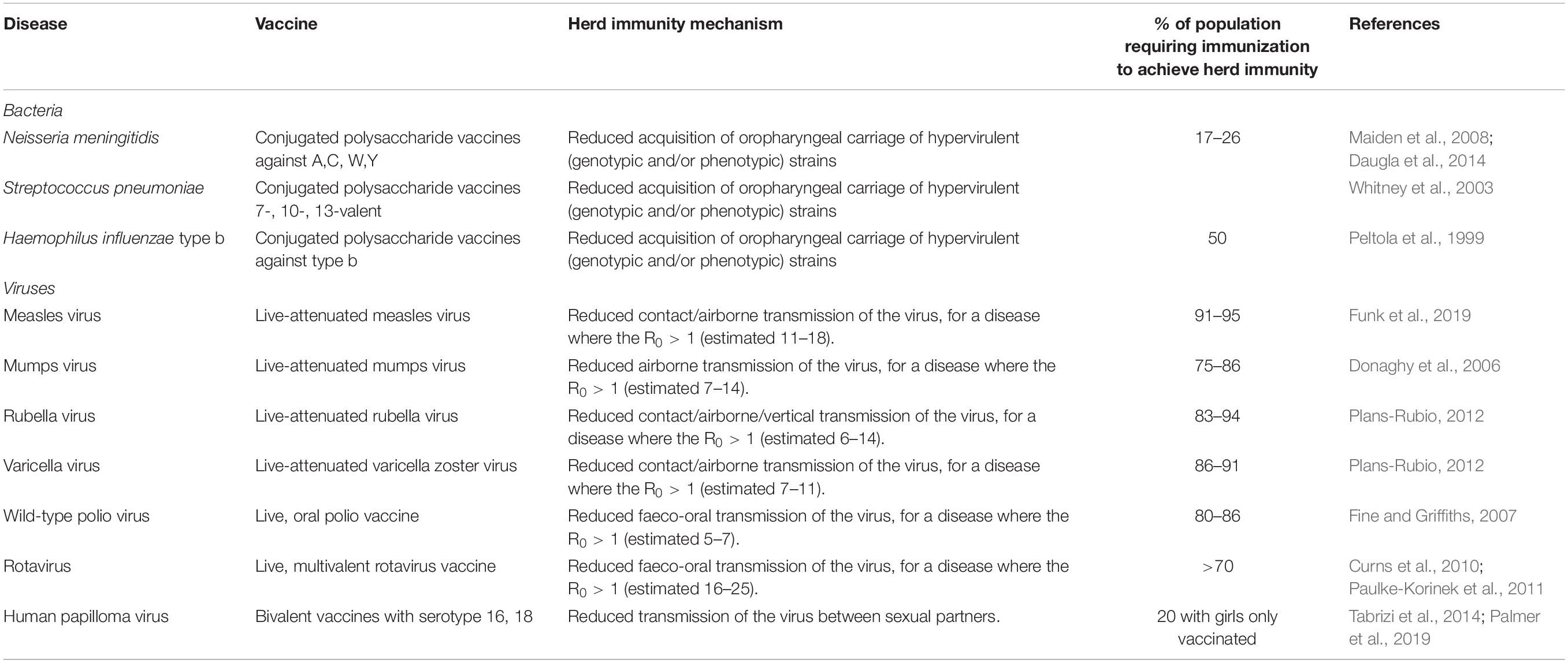
Table 2. Vaccines with the potential to induce herd immunity, with the infectious agent, vaccine type, and thresholds of population vaccination needed for herd immunity ( Peltola et al., 1999 ; Whitney et al., 2003 ; Donaghy et al., 2006 ; Fine and Griffiths, 2007 ; Maiden et al., 2008 ; Curns et al., 2010 ; Paulke-Korinek et al., 2011 ; Plans-Rubio, 2012 ; Daugla et al., 2014 ; Tabrizi et al., 2014 ; Funk et al., 2019 ; Palmer et al., 2019 ).
Herd immunity has been observed for gastrointestinal infections with vaccines against cholera (oral cholera vaccine) and rotavirus (oral rotavirus vaccines). Early adopters of rotavirus vaccines included the United States (2006) and Austria (2007) where there were dramatic reductions in disease observed in the vaccinated infant cohort, and also in the older age groups of children and adults ( Curns et al., 2010 ; Paulke-Korinek et al., 2011 ), suggesting that the reduction in disease and shedding of virus in the stool stopped transmission to healthy household contacts. For the OPV, herd protection may also be induced through vaccine virus shedding and spread to unvaccinated people ( Fine and Griffiths, 2007 ).
Reduction in Secondary Infections That Complicate Vaccine-Preventable Diseases
Vaccines can prevent diseases beyond the specific infection they are designed to target. Infections with pathogens, in particular viruses, can predispose to the acquisition of other bacterial infections. For example, influenza virus infection, both seasonal and pandemic, is frequently complicated by bacterial pneumonia and acute otitis media (OM), and infrequently Aspergillus pneumonia/pneumonitis. During the influenza pandemic of 1918–19, secondary bacterial bronchopneumonia with S. pneumoniae, Streptococcus pyogenes , H. influenzae , and Staphylococcus aureus identified at autopsy, likely contributed to the excess mortality observed amongst healthy children and adults ( Morens and Fauci, 2007 ). Influenza vaccinations can be beneficial in preventing these complications and also morbidity including acute OM in children; a systematic review demonstrated influenza vaccine efficacy against OM of 51% (21–70%) ( Manzoli et al., 2007 ). Further, there is evidence that inactivated influenza vaccines administered to pregnant women can reduce the hospital admission with acute respiratory illnesses in their infants up to 6 months of age ( Regan et al., 2016 ). Amongst pregnant, HIV-negative women in South Africa, infants (<3 months) were protected against hospitalization with all-cause lower respiratory tract infections with a vaccine efficacy of 43% ( p = 0.05), including primary viral and secondary bacterial causes ( Nunes et al., 2017 ). Additionally, in children pneumococcal conjugate vaccines were observed to reduce the incidence of influenza-associated hospital admissions in United States ( Simonsen et al., 2011 ), Spain ( Dominguez et al., 2013 ), and South Africa ( Madhi et al., 2004 ; Abadom et al., 2016 ), through the prevention of secondary bacterial infections following primary influenza infection.
The introduction of the live-attenuated measles vaccine in the 1970s was observed to reduce both measles and non-measles mortality in children ( Aaby et al., 2003 ). Measles causes severe pneumonia, encephalitis, and the long-term sequel of subacute sclerosing panencephalitis ( Moss, 2017 ), but the decline in mortality was not limited to preventing these alone ( Aaby et al., 2003 ). Mathematical modeling of vaccination and immunological research demonstrated that measles causes an immunological amnesia, eliminating B cell populations and thus immune memory, leaving measles survivors susceptible to all the infective agents they had previously developed immunity against; it is estimated to take 3 years for immune recovery to occur ( Mina et al., 2015 ).
Prevention of Cancer
Historically, vaccines were developed against very severe infections with major morbidity and mortality from acute disease. As non-communicable diseases, including cancer, become the most frequent causes of death in industrialized countries and some developing countries, vaccines are being used to prevent these too, when the infectious agents are involved in carcinogenesis. Hepatitis B prevalence is high in regions of East Asia, sub-Saharan Africa, and the Pacific Islands. Chronic hepatitis B infection can lead to liver cirrhosis and hepatocellular carcinoma ( Bogler et al., 2018 ). Vertical transmission of hepatitis B is problematic as 70–90% of babies born to HbsAg and HbeAg positive mothers will become infected without prophylaxis administered to babies; with ∼90% of infants developing chronic hepatitis ( Borgia et al., 2012 ; Gentile and Borgia, 2014 ). The chronic hepatitis B carriage status of mothers is routinely checked at the start of pregnancy, in order to assess the need to vaccinate the infant after birth. The use of both hepatitis B vaccine, containing hepatitis B surface antigen, and immunoglobulin containing hepatitis B antibody can be used to minimize vertical transmission, with evidence from a 20-year-long study in Thailand demonstrating 100% prevention of transmission ( Poovorawan et al., 2011 ).
The sexually transmitted HPV is responsible for genital tract and oropharyngeal infections as a precursor to causing oncological disease affecting the cervix, vagina, vulva, penis, anal tract, and pharynx in both men and women. Cervical cancer is the fourth most common cancer globally, with 528,000 new cases annually and peak incidence in young women aged 25–34 years ( Ferlay et al., 2012 ). The HPV serotypes 16 and 18 carry a high-risk for cervical cancer ( Wang et al., 2018 ) and vaccination against these specific serotypes has been available since 2006 through bivalent (16 and 18), quadrivalent (6, 11, 16, and 18), and nonavalent (6, 11, 16, 18, 31, 33, 45, 52, 58) vaccines, which are now available to individuals from the age of 9 years ( Gupta et al., 2017 ). A vaccination program started in the United Kingdom in 2008, and at the time of writing over 10.5 million doses had been given to girls ( Public Health England, 2018 ), with the aim of preventing primary infection with HPV. The vaccine coverage was 83.8% for 13–14 year old girls in England in 2017/18 ( Public Health England, 2019 ). In July 2018, the vaccine was approved for use in boys ( Public Health England, 2019 ). After a decade of use, there has been an observed decline in the genital infections caused by serotypes 16 and 18 ( Public Health England, 2018 ), with further time needed to observe the fall in cervical cancer incidence. However, the incidence of pre-invasive cervical diseases has been reduced by 79–89% in Scottish women over 20 who were vaccinated with bivalent HPV vaccine when aged 12–13 years, with evidence of herd protection ( Palmer et al., 2019 ), offering a promising outlook for the reduction of cervical cancer in the future. An additional benefit of HPV vaccines, is their impact on neonatal morbidity and mortality, through the reduction in surgical treatment of cervical neoplasias, and the related preterm births and complications ( Soergel et al., 2012 ).
Preventing Antibiotic Resistance
The rise in antimicrobial resistance (AMR) is a universal threat. The use of antibiotics in humans, exposes the bacteria that reside in our microbiota to selection pressures resulting in the development of AMR. As the bacteria constituting the host microbiota are frequently responsible for invasive diseases such as: meningitis, pneumonia, urinary tract, or abdominal infections, the risk of developing infections that are difficult or eventually impossible to treat is fast becoming a reality ( Brinkac et al., 2017 ). In regions where resistant pathogens are circulating at high frequency, such as India or regions of Europe ( Logan and Weinstein, 2017 ), patients will be faced with choosing between having elective surgical procedures or chemotherapy for malignancy, and the risk of acquiring potentially untreatable, multi-drug resistant bacterial infections ( Liu et al., 2016 ). Vaccination is crucial in mitigating this risk, by preventing people from developing viral and bacterial infections in the first instance, and therefore reducing the antibiotic burden to which their microbiota are exposed. The development of AMR in bacteria is a cumulative process with frequent, repeated exposure to broad spectrum antibiotics as a major driver. Children and the elderly who are at particular risk of infection can benefit from vaccines against common primary and secondary infections such as: pneumonia (prevented by PCV, PPSV, influenza, and measles vaccines), OM (PCV, Hib, and measles vaccines), cellulitis secondary to VZV (VZV vaccine), and typhoid fever (typhoid vaccine) which alleviates the need for antibiotics being prescribed or bought ( Kyaw et al., 2006 ; Palmu et al., 2014 ). The extent to which vaccination contributes to antimicrobial stewardship was highlighted by its inclusion in vaccine cost-effectiveness analyses as part of national United Kingdom policy ( Bonanni et al., 2015 ).
Economic Benefits
Cost savings.
Vaccines are highly beneficial on a population level and also cost-effective ( Shearley, 1999 ) in comparison to other public health interventions ( Bloom et al., 2005 ). Government departments are required to perform systematic economic analyses of vaccines and vaccine programs to justify their purchase in view of pressure on public and private finances globally, this was exacerbated by the 2008 financial crash. A vaccination program has clear direct costs including: vaccine purchase, infrastructure to run the program and maintain the cold chain, and healthcare/administration personnel. Governments, sometimes supported by charities and non-governmental organizations, invest in these with the intention of improving health. The reduction in morbidity and mortality associated with successful vaccine programs, through a combination of direct and indirect protection, has led to reduced incidence of diseases and their associated treatments and healthcare costs ( Deogaonkar et al., 2012 ). This potentially leads to economic growth, with less money spent owing to the costs averted through fewer medical tests, procedures, treatments and less time off work by patients/parents. Additionally, the use of combination vaccines e.g., DTaP/IPV/Hib/HepB provides protection against an increased number of diseases, with no additional infrastructure costs i.e. the same number of injections per child within existing immunization programs.
The cost-effectiveness analyses of vaccination programs demonstrate that they are overwhelmingly worth the investment, with most programs costing less than $50 per life gained, orders of magnitude less than prevention of diseases like hypertension ( Ehreth, 2003 ; Bloom et al., 2005 ). The returns on investment in vaccines, given their increasing provision through Gavi, have been estimated at 12–18% ( Bloom et al., 2005 ), but this is likely an underestimate. The monetary advantages of vaccination programs are important both to industrialized nations, such as the United States which obtains a net economic benefit of $69 billion, but also in 94 LMIC where investment of $34 billion, resulted in savings of $586 billion from the direct illness costs ( Ozawa et al., 2016 ; Orenstein and Ahmed, 2017 ). The net economic impact of eradication of disease has been estimated for both smallpox and polio. For smallpox, the eradication costs were over 100 million USD, but there are cost savings of 1.35 billion USD annually, with elimination of polio estimated to save 1.5 billion USD annually ( Barrett, 2004 ; Bloom et al., 2005 ). A less well-considered economic saving, not captured in cost-effectiveness or cost-benefit analyses, is from the prevention of long-term morbidity following acute infections ( Bloom et al., 2005 ), for example hearing impairment following pneumococcal meningitis or limb amputation following meningococcal disease, along with broader productivity gains ( Deogaonkar et al., 2012 ), which could have a major impact on LMIC adoption of vaccine programs.
Productivity Gains
The relationship between health and the economy is bidirectional, whereby economic growth enables funding in investments that improve health; and a healthy population contributes to and enhances an economy. These benefits of vaccinations and other public health interventions including sanitation, clean water, and antibiotics, are important for social as well as economic reasons. It has been suggested that the economic impact of vaccines should be considered more broadly than just the averted healthcare costs from prevented illness episodes and associated carer costs ( Deogaonkar et al., 2012 ; Barnighausen et al., 2014 ; Bonanni et al., 2015 ; Gessner et al., 2017 ; Wilder-Smith et al., 2017 ). Bärnighausen et al. (2011) , set out a framework to consider productivity gains measured by: outcome and behavior; community health and economic externalities; risk reduction; and health gains. Healthy children demonstrate improved educational attainment at school through better attendance and better cognitive performance ( Barham and Calimeria, 2008 ; Bloom et al., 2011 ; Deogaonkar et al., 2012 ). The impact of hearing loss from mumps, rubella or pneumococcal infections, or visual impairment from measles may require specific educational support, whereas the cognitive deficits from those childhood infections may require substantial remedial input. As more children survive to adulthood, a larger adult workforce is available, who when healthy can work for longer and more productively both physically and mentally ( Bloom and Canning, 2000 ; Bloom et al., 2005 ); though to date this has been observed largely following other health improvements, not vaccination specifically ( Jit et al., 2015 ). As a result of vaccination healthy and economically successful populations have lower fertility rates and smaller families ( Sah, 1991 ; Andre et al., 2008 ). With improved health and therefore life expectancy, there is a wider effect on families who may choose to invest more money in their future, for example to enhance their education or through savings ( Jit et al., 2015 ). Overall, vaccine programs should be viewed as an investment in human capital, providing enduring impact on economies worldwide.
Minimizing the Impact on Family
The economic impact of adult illness is evident from loss of productivity and pay for the duration of the illness and recovery period. The impact of childhood illness falls primarily on their adult carers, generally parents. In most industrialized regions, two-parent families are reliant on both parents undertaking at least part-time or full-time work. Therefore, when a child is unwell with childhood illnesses, which may or may not necessitate admission to hospital, the parent will invariably have to forego their paid employment to care for the child. In seven European countries one parent or carer required time off work in 39–91% of rotavirus gastroenteritis cases ( Van der Wielen et al., 2010 ). This loss of productivity in the parental workforce tends to disproportionately affect women, but loss of either parental attendance at work reduces overall employer productivity and in the short-term is rarely replaced. This argument was made for the impact of chicken pox on children, whereby the exclusion from school mandates parental caring at home for a period until the lesions are crusted over. VZV vaccines are estimated to have had a similar impact as rotavirus vaccine in United States studies ( Lieu et al., 1994 ). In many regions, mothers are still the primary carers, spending their days at home caring for children and maintaining the household; in these settings, the impact on this unpaid work is harder to determine.
It is of paramount importance to quantify and include productivity gains and the wider effects in analyses of impact for vaccines with only moderate efficacy, as calculated using traditional metrics. Vaccines such as the RTS,S/AS01 malaria vaccine, CYD-TDV dengue vaccine and rotavirus vaccine used in LMIC all have limited ability to broadly protect populations over a long duration but the public health benefits were important in vaccine implementation decisions in those countries ( Wilder-Smith et al., 2017 ). This suggests a paradigm for alternative regulatory requirements with a focus on public health outcomes ( Gessner et al., 2017 ).
Cost-Effective Preparedness for Outbreaks
As human populations grow and their use of the finite land resources increases, we are in increasingly close association with other living creatures, voluntarily or involuntarily. This interaction with natural reservoirs of potential infectious diseases increases the risk of zoonotic transmission of new infectious pathogens e.g., SARS, MERS-CoV, or known infectious pathogens with increased virulence e.g., influenza. Emerging infectious diseases disproportionately affect developing regions, where health infrastructure and surveillance are likely to be less well-established and robust. There were 1,307 epidemics of infectious diseases between 2011 and 2017, which cumulatively cost $60 billion annually to manage ( GHRF Commission, 2016 ). The unpredictability of outbreaks was highlighted by the Ebola epidemic in Western African countries of Liberia, Sierra Leone, and Guinea in 2014, which occurred in a period when public health was supposedly at its most advanced in recent history. However, a catalog of areas including: outbreak planning infrastructure; disease surveillance; local health services; escalation to international agencies were found to be lacking ( GHRF Commission, 2016 ). Although the WHO received criticism for its lack of escalation, in reality the global and interconnected infrastructure to prevent such epidemics taking lives and devastating societies is insufficient at the present time. The Zika virus epidemic in Latin America in 2015, first observed through an unexpectedly high incidence of microcephaly amongst newborns in Brazil’s northern regions ( Heukelbach et al., 2016 ), provide another example of how epidemics can have lasting impact, with the virus causing significant neurological damage to surviving infants ( Russo et al., 2017 ). The SARS-CoV-2 pandemic which began in 2019, was, at the time of writing, the largest infectious disease pandemic since the influenza pandemic of 1918/9. This global public health crisis highlighted stark societal inequalities persistent in many high-, middle- and low-income countries with direct and indirect impact on health outcomes from this infection. The cost-effectiveness of a vaccine in this setting was unquestionable, with economies and societies shut down for months in early 2020 and likely again in future. As it is not feasible or practical to be able to predict the location or nature of the next emerging threat, investment of an estimated $4.5 billion/year in healthcare systems could help speed up responses to infectious epidemics by prompt identification of the agent and effective control measures to limit the spread and consequences of disease ( GHRF Commission, 2016 ). The importance of this planning within the political landscape and the ongoing threat that infectious disease pose, may be appreciated more widely after 2020.
Establishing Programs for Vaccine Development
One effective infection control method is the use of vaccines in the course of an epidemic to halt transmission and to induce immunity to those as yet unaffected. The cost of vaccine development is a major challenge as there is little incentive for industry to invest in the design, testing and manufacture of vaccines that may never be needed, have a limited market, and, as previously eluded to, may be required in LMIC which cannot afford the upfront costs as an epidemic unfolds. The estimated costs for funding the development of infectious diseases vaccines for epidemics through phase 2a clinical trials are a minimum of $2.8-3.7 billion ( Gouglas et al., 2018 ). The CEPI alliance was established at the Davos World Economic Forum in 2017 as a global partnership between public, private and philanthropic organizations. In response to the conclusion that “a coordinated, international, and intergovernmental plan was needed to develop and deploy new vaccines to prevent future epidemics,” CEPI have identified the most important known global infectious threats and invested in the development of vaccines, stockpiling, and policies to allow equitable access to these ( Plotkin, 2017 ). Further, the establishment of research and development infrastructure pipelines will allow production of suitable vaccine candidates within 16 weeks of identification of a new pathogen antigen. The broader aims including: improving global epidemic responses; capacity building; and global regulation of outbreak management strategies are also within the remit of CEPI’s work. It is these types of preparedness plans that assisted vaccine development and global health collaborations to address the COVID-19 pandemic, though many regions of high-, middle-, and low-income countries alike were slow or resistant to pre-empt and prepare for this type of infectious disease threat.
Social Benefits
Equity of healthcare.
As a result of the combined effects of poverty, malnutrition, poor hygiene and sanitation, overcrowding, discrimination and poorer access to health-care, the underprivileged in society are disproportionately afflicted by infectious diseases. Over the 20th century, it has become a moral standpoint and a human right for every individual to be provided with access to safe vaccines. The provision of vaccination as part of the EPI on a national and international scale ( World Health Assembly, 1974 ) acted as a great leveler to start reducing the impact of infectious diseases to all, regardless of other disadvantages. Over the 15 years of the EPI, the vaccine coverage in developing countries increased from 5% to ∼80% ( Levine and Robins-Browne, 2009 ). The EPI was revolutionary for its time, an ambitious public health program that aimed to improve children’s life chances despite the country and situation in which they were born. The administration of vaccines by UNICEF was deemed so important that there have been at least seven ceasefires in civil conflicts to allow this to happen ( Hotez, 2001 ).
The impact of vaccines on the inequity of those living in poverty is marked. A study of over 16,000 children during the phased introduction of the measles vaccine in Bangladesh in 1982, demonstrated improved health outcome equity when measured by under-5 mortality ( Bishai et al., 2003 ). Further, modeling of the impact of the rotavirus vaccine in India across social strata, which are closely aligned to wealth, suggested that the vaccine program would provide the poor with both health and financial benefits ( Verguet et al., 2013 ). Including such equity impact in the health economic modeling of vaccines would allow policy decisions to be targeted to the most vulnerable in society ( Riumallo-Herl et al., 2018 ). Additional cost-effective benefits observed after the implementation of combined public health initiatives ( Deogaonkar et al., 2012 ; Gessner et al., 2017 ) include provision of vaccines, facilitation of healthcare, reduction of indoor air pollution and improvement of nutrition to prevent childhood pneumonia ( Niessen et al., 2009 ).
Strengthening Health and Social Care Infrastructure
To provide the EPI universally to infants and children, a significant degree of healthcare infrastructure is required ranging from primary care to public health. An example of the multiple facets of a successful vaccine program were outlined in the Mission Indradhanush in India, which planned to make life-saving vaccines available to all children and pregnant women by 2020 through programs with (i) national, (ii) state, (iii) district, and (iv) block/urban level input ( Hinman and McKinlay, 2015 ). National programs require governments to provide financial resources and set out policy for implementation. States needed to obtain the vaccines and to store them appropriately whilst eligible children were identified through public health messaging and outreach. Districts and urban areas recruited staff trained in vaccine delivery and communication to administer vaccines and to provide the aftercare where required. Establishing this degree of nationwide infrastructure to reach those in urban and rural areas, provides the basis for the provision of other health and social care services for all members of the community, in particular improving maternal and infant mortality in developing regions and in the elderly in industrialized regions ( Shearley, 1999 ). Public health infrastructure and personnel could be used to promote other important messages and health education ( Shearley, 1999 ), relating to malnutrition, hygiene and sanitation and preventable diseases such as malaria and HIV infection. Global drivers are also key, as demonstrated by the establishment of the EPI in 1974, when all countries were directed to provide these vaccines, thereby developing their primary- and public health-care infrastructure, with benefit beyond the vaccine program. Vaccination contributes to the UN Millennium Development Goals and later Sustainable Development Goals for achievement by 2030. Gavi, the Vaccine Alliance, has been an important provider of funds, vaccines and support for countries whose gross national income per capita was <£1000/year ( Hinman and McKinlay, 2015 ). The partnerships forged through the development of vaccine programs in LMIC, can be long-lasting and beneficial through other health and social care endeavors ( Shearley, 1999 ).
Impact of Life Expectancy and Opportunity
Vaccination programs provide a degree of social mobility, as poverty and the associated ill-health and mortality from infectious diseases are no longer the determinants of one’s life chances. Vaccine recipients have the potential for improved life-expectancy largely demonstrated by, but not confined to, infants and children ( Andre et al., 2008 ). It has become increasingly recognized that an aging population goes through the process of immunosenescence ( Fulop et al., 2017 ), and increased incidence and severity of infectious diseases. In many countries, therefore, older people are offered vaccines to prevent infections with high mortality and morbidity, including the influenza, pneumococcal, herpes zoster, and pertussis vaccines ( Bonanni et al., 2015 ). These prevent the development of pneumonia, admission to hospital and the subsequent associated risks of death from cardiac failure, as observed in Sweden ( Christenson et al., 2004 ).
The global and interconnected world of the 21st century provides opportunity to discover new cultures, new environments and their resident microbes. The safety of global travel has been greatly enhanced by the availability of vaccines that provide protection against organisms that are different to those in a person’s home setting. Movement of people may be through necessity when fleeing war and conflict, in the search of better life opportunities, or for leisure purposes. For mass movements of refugees vaccines are crucial to the aid and relief efforts to support these individuals ( Hermans et al., 2017 ), as measles and cholera can be highly problematic in refugee camps. Global mass cultural or religious gatherings, such as the Hajj pilgrimage ( Yezli et al., 2018 ) or the Chinese New Year ( Chen et al., 2018 ) have been implicated in the spread of meningococcal disease outbreaks. Pre-travel vaccines offer the optimal level of protection for those with scheduled travel plans and include protection against: yellow fever, hepatitis A and B, rabies, Japanese encephalitis, tick-borne encephalitis, typhoid, and cholera.
Empowerment of Women
The empowerment of women is both a driver and effect of vaccination programs. The degree of education, literacy and independence of girls and women varies considerably across the world and within countries. Where women have the information and autonomy to make health-related decision for their children, childhood immunization rates improve. In a study in Bihar State in rural India involving an empowerment program, where participating women were educated about health and hygiene, there was a higher rate of DTP, measles and BCG vaccination in their children compared to the non-participants in the villages running the program ( Janssens, 2011 ). Further, this information and autonomy served to improve the rates of vaccination in children of non-participants in the villages running the program compared to control villages not running the education program, through social or formal ongoing dialogue within the village community. A separate public health initiative in Haryana, India conducted between 2005 and 2012 to reduce maternal and child health inequalities, involved improving access and provision of health resources to rural areas, the poor in society, women and children. One significant outcome of this initiative was the equitable provision of immunizations to girls and boys, despite the male-favored disparity prior to starting the public health initiative ( Gupta et al., 2016 ).
By improving infant and childhood mortality from infection, more children will survive to adulthood with the potential to have productive and healthy lives. This has led to healthy and economically secure women having fewer children and less peripartum morbidity and mortality ( Sah, 1991 ; Shearley, 1999 ). Thus, women are able to spend more time with their children and on their development ( Shearley, 1999 ) as well as their own education and contribution to the workforce. The strategy of maternal vaccination has demonstrated great success at preventing diseases that afflict infants too young to be vaccinated against pertussis, influenza and tetanus ( Marchant et al., 2017 ). Factors influencing the uptake of maternal vaccination include women’s previous experiences with healthcare and vaccines, so it is crucial to provide the access and support required to enable them to make informed choices during their pregnancy ( Wilson et al., 2019 ).
The impact of vaccines is broad and far-reaching, though not consistently quantifiable, analyzed or communicated. Traditionally, the perceived benefits of vaccination were to reduce morbidity and mortality from infections, and those remain the drivers for the innovation of new vaccines, in particular in preparation for outbreaks or against infections that afflict the most disadvantaged in society. However, an increasing appreciation for the economic and social effects of vaccines is being included in the development and assessment of vaccine programs, potentially realizing a greater benefit to society and resulting in wider implementation. There remain challenges to delivering vaccines to all children and vulnerable people worldwide, in particular those in communities that are difficult to reach geographically, politically and culturally and these challenges can only be overcome with the continued commitment and dedication to this endeavor on an international, national and individual scale.
Author Contributions
SP conceptualized and designed the study. CR prepared the manuscript and figures. CR and SP contributed to literature search and revision and review of the final manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
SP consults for many major vaccine manufacturers and biotechnology companies but this article was unfunded.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aaby, P., Bhuiya, A., Nahar, L., Knudsen, K., de Francisco, A., and Strong, M. (2003). The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Intern. J. Epidemiol. 32, 106–116.
Google Scholar
Abadom, T. R., Smith, A. D., Tempia, S., Madhi, S. A., Cohen, C., and Cohen, A. L. (2016). Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: a case-population study. Vaccine 34, 5649–5655.
Anderson, P., Peter, G., Johnston, R. B. Jr., Wetterlow, L. H., and Smith, D. H. (1972). Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae , type b. J. Clin. Invest. 51, 39–44.
Andre, F. E., Booy, R., Bock, H. L., Clemens, J., Datta, S. K., John, T. J., et al. (2008). Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 86, 140–146.
Artenstein, M. S. (1975). Control of meningococcal meningitis with meningococcal vaccines. Yale J. Biol. Med. 48, 197–200.
Austrian, R. (1989). Pneumococcal polysaccharide vaccines. Rev. Infect. Dis. 11, (Suppl. 3), S598–S602.
Barham, T., and Calimeria, L. (2008). Long-term Effects of Family Planning and Child Health Interventions on Adolescent Cognition: Evidence from Matlab in Bangladesh. Boulder, CO: Univeristy of Colorado.
Barnighausen, T., Bloom, D. E., Cafiero-Fonseca, E. T., and O’Brien, J. C. (2014). Valuing vaccination. Proc. Nat. Acad. Sci. U.S.A. 111, 12313–12319.
Bärnighausen, T., Bloom, D. E., Canning, D., Friedman, A., Levine, O. S., O’Brien, J., et al. (2011). Rethinking the benefits and costs of childhood vaccination: the example of the Haemophilus influenzae type b vaccine. Vaccine 29, 2371–2380.
Barrett, S. (2004). Eradication versus control: the economics of global infectious disease policies. Bull. World Health Organ. 82, 683–688.
Bishai, D., Koenig, M., and Ali Khan, M. (2003). Measles vaccination improves the equity of health outcomes: evidence from Bangladesh. Health Econ. 12, 415–419.
Bloom, D. E., and Canning, D. (2000). Policy forum: public health. The health and wealth of nations. Science 287:1207.
Bloom, D. E., Canning, D., and Seiguer, E. (2011). The Effect of Vaccination on Children’s Physical and Cognitive Development in the Philippines. Boston, MA: Harvard School of Public Health.
Bloom, D. E., Canning, D., and Weston, M. (2005). The value of vaccination. World Econ. 6, 15–16.
Bogler, Y., Wong, R. J., and Gish, R. G. (2018). “Epidemiology and natural history of chronic hepatitis B virus infection,” in Hepatitis B Virus and Liver Disease , eds J.-H. Kao and D.-S. Chen (Berlin: Springer), 63–89.
Bonanni, P., Picazo, J. J., and Remy, V. (2015). The intangible benefits of vaccination - what is the true economic value of vaccination? J. Mark. Access Health Policy 3:10.3402/jmahv3.26964.
Borgia, G., Carleo, M. A., Gaeta, G. B., and Gentile, I. (2012). Hepatitis B in pregnancy. World J. Gastroenterol. 18, 4677–4683.
Brinkac, L., Voorhies, A., Gomez, A., and Nelson, K. E. (2017). The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 74, 1001–1008.
Calmette, A. (1927). La Vaccination Preìventive Contre La Tuberculose par le “BCG,”. Paris: Masson.
Centers for Disease Control and Prevention (2017). Reported Cases Of Notifiable Diseases And Rates Per 100,000, Excluding U.S. Territories, United States, 2017. Atlanta: Centers for Disease Control and Prevention.
Chan, J. F., Yuan, S., Kok, K. H., To, K. K., Chu, H., Yang, J., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523.
Chen, M., Rodrigues, C. M. C., Harrison, O. B., Zhang, C., Tan, T., Chen, J., et al. (2018). Invasive meningococcal disease in Shanghai, China from 1950 to 2016: implications for serogroup B vaccine implementation. Sci. Rep. 8:12334.
Christenson, B., Hedlund, J., Lundbergh, P., and Ortqvist, A. (2004). Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur. Resp. J. 23, 363–368.
Clark, H. F., Offit, P. A., Plotkin, S. A., and Heaton, P. M. (2006). The new pentavalent rotavirus vaccine composed of bovine (strain WC3) -human rotavirus reassortants. Pediatr. Infect. Dis. J. 25, 577–583.
Curns, A. T., Steiner, C. A., Barrett, M., Hunter, K., Wilson, E., and Parashar, U. D. (2010). Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J. Infect. Dis. 201, 1617–1624.
Daugla, D. M., Gami, J. P., Gamougam, K., Naibei, N., Mbainadji, L., Narbe, M., et al. (2014). Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 383, 40–47.
Deogaonkar, R., Hutubessy, R., van der Putten, I., Evers, S., and Jit, M. (2012). Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health 12:878. doi: 10.1186/s12916-017-0911-878
CrossRef Full Text | Google Scholar
Dominguez, A., Castilla, J., Godoy, P., Delgado-Rodriguez, M., Saez, M., Soldevila, N., et al. (2013). Benefit of conjugate pneumococcal vaccination in preventing influenza hospitalization in children: a case-control study. Pediatr. Infect. Dis. J. 32, 330–334.
Donaghy, M., Cameron, J. C., and Friederichs, V. (2006). Increasing incidence of mumps in Scotland: options for reducing transmission. J. Clin. Virol. 35, 121–129.
Ehreth, J. (2003). The global value of vaccination. Vaccine 21, 596–600.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2012). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int. J. Cancer 136, E359–E386.
Fine, P. E., and Griffiths, U. K. (2007). Global poliomyelitis eradication: status and implications. Lancet 369, 1321–1322.
Fulop, T., Larbi, A., Dupuis, G., Le Page, A., Frost, E. H., Cohen, A. A., et al. (2017). Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8:1960. doi: 10.3389/fmicb.2018.01960
PubMed Abstract | CrossRef Full Text | Google Scholar
Funk, S., Knapp, J. K., Lebo, E., Reef, S. E., Dabbagh, A. J., Kretsinger, K., et al. (2019). Combining serological and contact data to derive target immunity levels for achieving and maintaining measles elimination. BMC Med. 17:180. doi: 10.1186/s12889-019-6655-180
Geddes, A. M. (2006). The history of smallpox. Clin. Dermatol. 24, 152–157.
Gentile, I., and Borgia, G. (2014). Vertical transmission of hepatitis B virus: challenges and solutions. Intern. J. Women Health 6, 605–611.
Gessner, B. D., Kaslow, D., Louis, J., Neuzil, K., O’Brien, K. L., Picot, V., et al. (2017). Estimating the full public health value of vaccination. Vaccine 35, 6255–6263.
GHRF Commission (2016). Commission on a Global Health Risk Framework for the Future). The Neglected Dimension of Global Security A Framework to Counter Infectious Disease Crises. Commission on Global Health Risk Framework for the Future. Available online at: https://nam.edu/wp-content/uploads/2016/01/Neglected-Dimension-of-Global-Security.pdf (September 24, 2019).
Glenny, A. T., and Hopkins, B. E. (1923). Diphtheria toxoid as an immunising agent. Br. J. Exp. Pathol. 4, 283–288.
Global Polio Eradication Initiative (2019). Polio this Week, Wild Poliovirus Type 1 And Circulating Vaccine-Derived Poliovirus Cases. Available online at: http://polioeradication.org/polio-today/polio-now/this-week/ (accessed June 25, 2020).
Gold, R., and Artenstein, M. S. (1971). Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull. World Health Organ. 45, 279–282.
Gouglas, D., Thanh, Le, T., Henderson, K., Kaloudis, A., Danielsen, T., et al. (2018). Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Health 6:e001386-96.
Grizas, A. P., Camenga, D., and Vazquez, M. (2012). Cocooning: a concept to protect young children from infectious diseases. Curr. Opin. Pediatr. 24, 92–97.
Gupta, G., Glueck, R., and Patel, P. R. (2017). HPV vaccines: global perspectives. Hum. Vacc. Immunotherap. 13, 1–4.
Gupta, M., Angeli, F., Bosma, H., Rana, M., Prinja, S., Kumar, R., et al. (2016). Effectiveness of multiple-strategy community intervention in reducing geographical, socioeconomic and gender based inequalities in maternal and child health outcomes in Haryana, India. PLoS One 11:e0150537. doi: 10.1371/journal.pone.0150537
Hajjeh, R. (2011). Accelerating introduction of new vaccines: barriers to introduction and lessons learned from the recent Haemophilus influenzae type B vaccine experience. Philos. Trans. R. Soc. B. 366, 2827–2832.
Hermans, M. P. J., Kooistra, J., Cannegieter, S. C., Rosendaal, F. R., Mook-Kanamori, D. O., and Nemeth, B. (2017). Healthcare and disease burden among refugees in long-stay refugee camps at Lesbos. Greece. Eur. J. Epidemiol. 32, 851–854.
Heukelbach, J., Alencar, C. H., Kelvin, A. A., de Oliveira, W. K., and Pamplona de Goes, C. L. (2016). Zika virus outbreak in Brazil. J. Infect. Dev. Countr. 10, 116–120.
Hill, H. A., Elam-Evans, L. D., Yankey, D., Singleton, J. A., and Kang, Y. (2017). Vaccination coverage among children aged 19-35 Months - United States, 2016. MMWR Morb. Mort. Wkly Rep. 66, 1171–1177.
Hinman, A. R., and McKinlay, M. A. (2015). Immunization equity. Vaccine 33, (Suppl. 4), D72–D77.
Hotez, P. J. (2001). Vaccines as instruments of foreign policy. The new vaccines for tropical infectious diseases may have unanticipated uses beyond fighting diseases. EMBO Rep. 2, 862–868.
Janssens, W. (2011). Externalities in program evaluation: the impact of a Women’s empowerment program on immunization. J. Eur. Econ. Assoc. 9, 1082–1113.
Jenner, E. (1798). An Inquiry Into The Causes And Effects Of The Variolæ Vaccinæ, A Disease Discovered In Some Of The Western Counties Of England, Particularly Gloucestershire, And Known By The Name Of The Cow Pox. London: Sampson Low.
Jit, M., Hutubessy, R., Png, M. E., Sundaram, N., Audimulam, J., Salim, S., et al. (2015). The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 13:209. doi: 10.1186/s12916-017-0911-209
Keja, K., Chan, C., Hayden, G., and Henderson, R. H. (1988). Expanded programme on immunization. World Health Stat. Q. 41, 59–63.
Kirnbauer, R., Booy, F., Cheng, N., Lowy, D. R., and Schiller, J. T. (1992). Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Nat. Acad. Sci. U.S.A. 89, 12180–12184.
Kyaw, M. H., Lynfield, R., Schaffner, W., Craig, A. S., Hadler, J., Reingold, A., et al. (2006). Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae . New Engl. J. Med. 354, 1455–1463.
Levine, M. M., and Robins-Browne, R. (2009). Vaccines, global health and social equity. Immunol. Cell Biol. 87, 274–278.
Lien, G., and Heymann, D. L. (2013). The problems with polio: toward eradication. Infect. Dis. Ther. 2, 167–174.
Lieu, T. A., Cochi, S. L., Black, S. B., Halloran, M. E., Shinefield, H. R., Holmes, S. J., et al. (1994). Cost-effectiveness of a routine varicella vaccination program for US children. JAMA 271, 375–381.
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168.
Logan, L. K., and Weinstein, R. A. (2017). The epidemiology of carbapenem-resistant Enterobacteriaceae : the impact and evolution of a global menace. J. Infect. Dis. 215(Suppl._1), S28–S36.
Maassab, H. F., and DeBorde, D. C. (1985). Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine 3, 355–369.
Madhi, S. A., Klugman, K. P., and Vaccine Trialist, G. (2004). A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10, 811–813.
Maiden, M. C., Ibarz-Pavon, A. B., Urwin, R., Gray, S. J., Andrews, N. J., Clarke, S. C., et al. (2008). Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197, 737–743.
Manzoli, L., Schioppa, F., Boccia, A., and Villari, P. (2007). The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr. Infect. Dis. J. 26, 97–106.
Marchant, A., Sadarangani, M., Garand, M., Dauby, N., Verhasselt, V., Pereira, L., et al. (2017). Maternal immunisation: collaborating with mother nature. Lancet. Infect. Dis. 17, e197–e208. doi: 10.1016/S1473-3099(17)30229-3
Mina, M. J., Kula, T., Leng, Y., Li, M., de Vries, R. D., Knip, M., et al. (2019). Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 366, 599–606.
Mina, M. J., Metcalf, C. J., de Swart, R. L., Osterhaus, A. D., and Grenfell, B. T. (2015). Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 348, 694–699.
Morens, D. M., and Fauci, A. S. (2007). The 1918 influenza pandemic: insights for the 21st century. J. Infect. Dis. 195, 1018–1028.
Morens, D. M., Holmes, E. C., Davis, A. S., and Taubenberger, J. K. (2011). Global rinderpest eradication: lessons learned and why humans should celebrate too. J. Infect. Dis. 204, 502–505.
Moss, W. J. (2017). Measles. Lancet 390, 2490–2502.
Munira, S. L., and Fritzen, S. A. (2007). What influences government adoption of vaccines in developing countries? A policy process analysis. Soc. Sci. Med. 65, 1751–1764.
Needham, J. (2000). Science and Civilisation in China: Volume 6, Biology and Biological Technology. Cambridge: Cambridge University Press.
Niessen, L. W., ten Hove, A., Hilderink, H., Weber, M., Mulholland, K., and Ezzati, M. (2009). Comparative impact assessment of child pneumonia interventions. Bull. World Health Organ. 87, 472–480.
Nunes, M. C., Cutland, C. L., Jones, S., Downs, S., Weinberg, A., Ortiz, J. R., et al. (2017). Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin. Infect. Dis. 65, 1066–1071.
Orenstein, W. A., and Ahmed, R. (2017). Simply put: vaccination saves lives. Proc. Nat. Acad. Sci. U.S.A. 114, 4031–4033.
Ozawa, S., Clark, S., Portnoy, A., Grewal, S., Brenzel, L., and Walker, D. G. (2016). Return On investment from childhood immunization in low- and middle-income countries, 2011-20. Health Aff. 35, 199–207.
Palmer, T., Wallace, L., Pollock, K. G., Cuschieri, K., Robertson, C., Kavanagh, K., et al. (2019). Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12-13 in Scotland: retrospective population study. BMJ 365:l1161.
Palmu, A. A., Jokinen, J., Nieminen, H., Rinta-Kokko, H., Ruokokoski, E., Puumalainen, T., et al. (2014). Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: a double-blind, cluster randomised phase 3-4 trial. Lancet Infect. Dis. 14, 205–212.
Pasteur, L. (1880). De l’attenuation du virus du choléra des poules. Comptes Rendus De l’Acad. Sci. 91, 673–680.
Pasteur, L. (1881). Sur la vaccination charbonneuse. Comptes Rendus De l’Acad. Sci. 92, 1378–1383.
Pasteur, L. (1885). Mèthode pour prévenir la rage apres morsure. Comptes Rendus De l’Acad. Sci. 101, 765–772.
Paulke-Korinek, M., Kundi, M., Rendi-Wagner, P., de Martin, A., Eder, G., Schmidle-Loss, B., et al. (2011). Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 29, 2791–2796.
Peltola, H., Aavitsland, P., Hansen, K. G., Jonsdottir, K. E., Nokleby, H., and Romanus, V. (1999). Perspective: a five-country analysis of the impact of four different Haemophilus influenzae type b conjugates and vaccination strategies in Scandinavia. J. Infect. Dis. 179, 223–229.
Petrova, V. N., Sawatsky, B., Han, A. X., Laksono, B. M., Walz, L., Parker, E., et al. (2019). Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci. Immunol. 4:eaay6125.
Plans-Rubio, P. (2012). Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum. Vacc. Immunotherap. 8, 184–188.
Plotkin, S. (2014). History of vaccination. Proc. Natl. Acad. Sci. U.S.A. 111, 12283–12287.
Plotkin, S. A. (2017). Vaccines for epidemic infections and the role of CEPI. Hum. Vacc. Immunotherap. 13, 2755–2762.
Plotkin, S. A., and Mortimer, E. A. (1988). Vaccines. Philadelphia, PA: Saunders.
Pollard, A. J., Perrett, K. P., and Beverley, P. C. (2009). Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9, 213–220.
Poovorawan, Y., Chongsrisawat, V., Theamboonlers, A., Leroux-Roels, G., Kuriyakose, S., Leyssen, M., et al. (2011). Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral Hepat. 18, 369–375.
Public Health England (2018). Public Health Matters Ten Years On Since The Start Of The HPV Vaccine Programme – What Impact Is It Having? London: Public Health England.
Public Health England (2019). Human Papillomavirus (HPV) Vaccination Coverage In Adolescent Females In England: 2017/18. London: Public Health England.
Regan, A. K., de Klerk, N., Moore, H. C., Omer, S. B., Shellam, G., and Effler, P. V. (2016). Effect of maternal influenza vaccination on hospitalization for respiratory infections in newborns: a retrospective cohort study. Pediatr. Infect. Dis. J. 35, 1097–1103.
Riumallo-Herl, C., Chang, A. Y., Clark, S., Constenla, D., Clark, A., Brenzel, L., et al. (2018). Poverty reduction and equity benefits of introducing or scaling up measles, rotavirus and pneumococcal vaccines in low-income and middle-income countries: a modelling study. BMJ Glob. Health 3:e000613.
Roeder, P., Mariner, J., and Kock, R. (2013). Rinderpest: the veterinary perspective on eradication. Philos. Trans. R. Soc. B 368:20120139.
Roush, S. W., and Murphy, T. V. (2007). Vaccine-preventable disease table working G. historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298, 2155–2163.
Russo, F. B., Jungmann, P., and Beltrao-Braga, P. C. B. (2017). Zika infection and the development of neurological defects. Cell. Microbiol. 19: 12744.
Sah, R. K. (1991). The effects of child-mortality changes on fertility choice and parental welfare. J. Polit. Econ. 99, 582–606.
Sato, Y., and Sato, H. (1999). Development of acellular pertussis vaccines. Biologicals 27, 61–69.
Schneerson, R., Barrera, O., Sutton, A., and Robbins, J. B. (1980). Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 152, 361–376.
Serruto, D., Bottomley, M. J., Ram, S., Giuliani, M. M., and Rappuoli, R. (2012). The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30, (Suppl. 2), B87–B97.
Shearley, A. E. (1999). The societal value of vaccination in developing countries. Vaccine 17, (Suppl. 3), S109–S112.
Simonsen, L., Taylor, R. J., Young-Xu, Y., Haber, M., May, L., and Klugman, K. P. (2011). Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio 2:e00309-10.
Soergel, P., Makowski, L., Schippert, C., Staboulidou, I., Hille, U., and Hillemanns, P. (2012). The cost efficiency of HPV vaccines is significantly underestimated due to omission of conisation-associated prematurity with neonatal mortality and morbidity. Hum. Vacc. Immunother. 8, 243–251.
Strassburg, M. A. (1982). The global eradication of smallpox. Am. J. Infect. Control 10, 53–59.
Tabrizi, S. N., Brotherton, J. M., Kaldor, J. M., Skinner, S. R., Liu, B., Bateson, D., et al. (2014). Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect.ous Dis. 14, 958–966.
Thacker, N., Vashishtha, V. M., and Thacker, D. (2016). Polio eradication in India: the lessons learned. Pediatrics 138:e20160461.
Thanh Le, T., Andreadakis, Z., Kumar, A., Gomez Roman, R., Tollefsen, S., Saville, M., et al. (2020). The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 19, 305–306.
Theiler, M., and Smith, H. H. (1937a). The effect of prolonged cultivation in vitro upon the pathogenicity of yellow fever virus. J. Exp. Med. 65, 767–786.
Theiler, M., and Smith, H. H. (1937b). The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 65, 787–800.
Turner, H. C., Thwaites, G. E., and Clapham, H. E. (2018). Vaccine-preventable diseases in lower-middle-income countries. Lancet Infect. Dis. 18, 937–939.
United Nations Department of Economic and Social Affairs (2019). Population Division. World Population Prospects 2019. New York, NY: United Nations Department of Economic and Social Affairs.
Van der Wielen, M., Giaquinto, C., Gothefors, L., Huelsse, C., Huet, F., Littmann, M., et al. (2010). Impact of community-acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL study. BMC Fam. Pract. 11:22. doi: 10.1186/s12916-017-0911-22
Verguet, S., Murphy, S., Anderson, B., Johansson, K. A., Glass, R., and Rheingans, R. (2013). Public finance of rotavirus vaccination in India and Ethiopia: an extended cost-effectiveness analysis. Vaccine 31, 4902–4910.
Wang, X., Huang, X., and Zhang, Y. (2018). Involvement of human papillomaviruses in cervical cancer. Front. Microbiol. 9:2896. doi: 10.3389/fmicb.2018.02896
Whitney, C. G., Farley, M. M., Hadler, J., Harrison, L. H., Bennett, N. M., Lynfield, R., et al. (2003). Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. New Engl. J. Med. 348, 1737–1746.
Wilder-Smith, A., Longini, I., Zuber, P. L., Barnighausen, T., Edmunds, W. J., Dean, N., et al. (2017). The public health value of vaccines beyond efficacy: methods, measures and outcomes. BMC Med. 15:138. doi: 10.1186/s12916-017-0911-8
Wilson, R., Paterson, P., and Larson, H. J. (2019). Strategies to improve maternal vaccination acceptance. BMC Public Health 19:342. doi: 10.1186/s12889-019-6655-y
Wise, J. (2018). Child vaccination rates drop in England as MMR uptake falls for fourth year. BMJ 362:k3967.
Wise, J. (2019). MMR vaccine: johnson urges new impetus to increase uptake as UK loses measles-free status. BMJ 366:l5219.
World Bank (2019). Immunization, DPT (% of Children Ages 12-23 Months). Washington, DC: World Bank.
World Health Assembly (1974). The expanded programme on immunization: the 1974 resolution by the world health assembly. Assign. Child. 1985, 87–88.
World Health Organisation (2019a). Immunization Coverage. Geneva: World Health Organisation.
World Health Organisation (2019b). Polio Eradication. Geneva: World Health Organisation.
World Health Organisation (2019c). Reported Cases of Selected Vaccine Preventable Diseases (VPDs). Geneva: World Health Organisation.
World Health Organization [WHO], United Nations Children’s Fund [UNICEF] (2019). WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) 1980-2018. Geneva: WHO.
Yezli, S., Gautret, P., Assiri, A. M., Gessner, B. D., and Alotaibi, B. (2018). Prevention of meningococcal disease at mass gatherings: lessons from the Hajj and Umrah. Vaccine 36, 4603–4609.
Zhou, F., Shefer, A., Wenger, J., Messonnier, M., Wang, L. Y., Lopez, A., et al. (2009). Economic evaluation of the routine childhood immunization program in the United States. Pediatrics 133, 577–585.
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 382, 727–733.
Keywords : immunization, vaccines, infectious diseases, infection, children, health economics
Citation: Rodrigues CMC and Plotkin SA (2020) Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 11:1526. doi: 10.3389/fmicb.2020.01526
Received: 09 April 2020; Accepted: 12 June 2020; Published: 14 July 2020.
Reviewed by:
Copyright © 2020 Rodrigues and Plotkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanley A. Plotkin, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
MANDATORY VACCINATION: WHY WE STILL GOT TO GET FOLKS TO TAKE THEIR SHOTS
Ben Balding
Class of 2006
April 27, 2006
This paper is submitted in satisfaction of the Food and Drug Law course paper and the Harvard Law School 3L Written Work Requirement
Vaccination is widely considered one of the greatest medical achievements of modern civilization. Childhood diseases that were commonplace less than a generation ago are now increasingly rare because of vaccines. In order to be effective at eliminating communicable diseases, vaccines must be administered to sufficient levels of persons in the community. Because of this, public health officials have mandated vaccination for certain diseases as a condition to school attendance. The overwhelming effectiveness of vaccination programs may lead individuals to ignore the benefits of vaccination and focus more on the risk of side effects. Moreover, some have criticized the coercive nature of these programs. These objections may lead to an unacceptably high number of exemptions, which can compromise vaccination programs and leave the population susceptible to outbreaks.
This paper explores vaccination programs with an eye toward greater public safety without ignoring the reality of a small but committed group of vaccine critics. The paper begins with a discussion of the historical development of mandatory vaccination policies and the issues posed by exemptions. It then addresses some of these issues in the context of vaccine safety. It also seeks solution by framing the discussion in economic terms. It concludes by recommending stricter enforcement of mandatory requirements for most vaccines and greater dissemination of information on the continued importance of vaccination.
TABLE OF CONTENTS
Introduction.
Vaccination is widely considered one of the greatest medical achievements of modern civilization. Childhood diseases that were commonplace less than a generation ago are now increasingly rare because of vaccines. The smallpox vaccine has eradicated a disease that was responsible for centuries of outbreaks and had a 30% fatality rate. [1] Physical handicaps resulting from polio can still be observed on some of those who were children before Jonas Salk developed a vaccine in 1955. Formerly common childhood diseases are now rarely observed. Even ear infections may soon be prevented by vaccination. [2] The widespread success of vaccinations has led one medical report to comment that “[n]ext to clean water, no single intervention has had so profound an effect on reducing mortality from childhood diseases as has the widespread introduction of vaccines.” [3]
The story of modern vaccination begins with Edward Jenner’s development of the vaccine for smallpox, one of the most feared diseases in recent history. At first, vaccination was optional and not everyone chose to vaccinate. [4] In time, states would allow municipalities to mandate vaccination in time of outbreak in order to protect the public from epidemics. [5] A further step was taken when states imposed smallpox vaccination as a prerequisite for attending public schools. [6] These requirements were amended in time as new vaccines were developed. [7] At some point actual outbreaks and epidemics ceased to be the trigger for mandatory vaccination, and prevention became the overriding justification. [8] Most states today require vaccination for a multitude of childhood diseases, including measles, diphtheria, pertussis, polio, and now even chickenpox. [9]
Because of the success and the mandatory nature of vaccination, most people would probably not consider vaccination an optional method of medical treatment. For most parents, the “decision” to vaccinate is equivalent to the “decision” to feed one’s child. [10] Typically, a doctor informs parents of the school vaccination schedule and the parents consent to having their child vaccinated. Since the vaccination schedule usually corresponds to the scheduled doctor visits for infants, full compliance with mandatory vaccination schedules is typically not a problem and can usually be substantially accomplished by age two. [11]
For some parents, however, vaccination is no routine matter. [12] From the time of Jenner’s smallpox vaccine, vaccination has had its critics. [13] In the two centuries since that time, many different types of objections have been raised. Some have questioned the scientific qualifications of mass immunization. [14] Others have focused on the personal liberty interests at stake and have objected to the paternalistic nature of government imposition of what is viewed as a personal medical choice. [15] Still others have opposed vaccination for personal or religious reasons. [16]
Today, some parents raise similar objections. The idea that a potentially harmful substance is being placed directly into the bloodstream raises a red flag for some. Additionally, the decline of many diseases for which vaccination is still mandated may make some parents skeptical of the continued wisdom of subjecting a child to a vaccine, even if the vaccine is considered extremely safe. This skepticism grows when some point to the correlation between vaccinations and conditions such as SIDS and autism. Whether or not such a correlation is scientifically significant, many parents simply wonder if it is wise to vaccinate against a disease unlikely to afflict their child if any chance exists that the vaccine will cause autism, SIDS, or any other side effect. [17]
Since the efficacy of a particular vaccine corresponds directly with the percentage of a given population that has been vaccinated, proponents of mandatory vaccination have sought to convince those with reservations about vaccines that vaccination is the right choice. The Center for Disease Control has attempted to allay possible reservations parents may have with vaccinations by rebutting some of the commonly held fears about vaccines. [18] The CDC has pointed out, for example, that most adverse effects from vaccines are “minor and temporary, such as a sore arm or mild fever.” [19] Because vaccination often involves the introduction of a harmful live (although seriously weakened) organism into the patient, vaccination can never be 100% safe. Serious side effects usually occur only between one per thousands to one per millions of doses, while some serious reactions and death occur so rarely that accurate risk assessment is difficult. [20] The CDC has also responded to many of the other concerns raised about the need for vaccination, and the FDA continually works to ensure vaccine safety and efficacy, but many still harbor reservations toward vaccination.
This paper will endeavor to discuss some of the most common objections to vaccination programs in general while trying to shed light on the veracity and tenability of these objections. Part I will discuss the nature of mandatory vaccination programs in this country; both scientific and historical issues will play a key part in this discussion. Part II will describe the role of the FDA and other governmental bodies in the overall vaccination picture. Part III will attempt to utilize multiple analytical tools in search of possible solutions to the dangers posed by those who may attempt to opt out of vaccination programs. It will first examine vaccination through the lens of an old television show episode. It will then adopt an economic analytical framework to discuss the balance between individual and general welfare in the context of vaccination. Part IV will conclude with some observations on how the goal of greater public health might be achieved without completely neglecting the concerns of many in the community regarding the prudence of using a medical technique that by definition relies on a degree of coercion.
I. MANDATORY VACCINATION
Historical background.
Jenner’s smallpox vaccine led to the research and development of vaccines for other widespread and epidemic diseases. The twentieth century saw the development of vaccines for such diseases as polio, diphtheria, tetanus, pertussis, measles, and others. [21] As with the smallpox vaccine, many of these vaccines soon found their way into vaccination programs mandated by the government, albeit through a somewhat different pathway.
Mandatory smallpox vaccination programs typically arose through state police power legislation authorizing municipalities to deal with outbreaks. [22] Typically, when a local municipality decided that the threat of outbreak was sufficient to exercise this authority it would require vaccination of everyone in the community (with a possible exception for individuals who could demonstrate uncommonly high health risks from receiving the vaccine, although this exception sometimes applied only to children) and fine and/or quarantine those who refused to be vaccinated. [23] When other diseases became preventable by vaccination, outbreak ceased to be the trigger for mandatory vaccination. Rather, because of their cost-efficiency and their ability to reduce and ultimately eliminate disease, vaccination programs became an important part of general public health policy. [24]
Most of the time, vaccination programs are accomplished through the dual efforts of national entities (which tend to develop and recommend vaccines) and state legislatures and local boards of health (which usually implement these recommended vaccines through vaccination programs). [25] It is not entirely accurate to refer to this as “mandatory vaccination,” as typically individual states will not criminally punish parents for not vaccinating their children or forcefully subject individuals to vaccination. [26] Instead, states typically condition school enrollment on proof of vaccination. [27] Though it may be a high price to pay, home schooling is usually an available means parents have if they wish to bypass these vaccination requirements. Moreover, most states grant exemptions to vaccination requirements for religious reasons and some even grant exemptions for philosophical reasons (in addition, every state exempts from school vaccination requirements individuals who cannot be vaccinated for medical reasons). [28]
The connection between school enrollment and vaccination programs may now seem obvious. Public health officials, faced with a means of protecting the general population from the harmful disease smallpox, realized that mass vaccination could lead to a sufficient level of immunity to eliminate the risk of outbreak, even for those in the community unable to vaccinate (because of medical reasons, for instance). [29] Because of the concept of herd immunity, public health officials considering the proper utilization of vaccines were dealing with a medical procedure quite out of the ordinary. Since vaccination itself does not typically provide 100% immunity to a disease, vaccinated individuals can still contract the disease. [30] Yet because of herd immunity, if a sufficient level of vaccination within a population is attained, the entire population will no longer be susceptible to the disease. In this way, vaccination came to be viewed not only as a personal medical choice but also as a step taken to improve the overall health of the population.
With the rise of public schooling in the mid- to late-nineteenth century, cities decided to condition public school attendance on smallpox vaccination. [31] By the latter part of the century, many states had adopted this practice. [32] Such a policy makes sense when one considers the increased risk of infectious disease in public areas like cities in general and schools in particular. By mandating vaccination for school attendance, of course, the state would eventually have ensured the vaccination of the entire population by the time the initially vaccinated generation became the oldest living one.
These vaccination schemes have faced challenges, both legal and social, throughout their existence. [33] The reasons for such challenges have ranged from personal liberty interests to doubts about the efficacy of vaccines. [34] State courts in the nineteenth century typically upheld both the enactment of mandatory vaccination programs and the delegation of power to local authorities. [35] More importantly for the future of mandatory vaccination policy, two important Supreme Court decisions in the early part of the twentieth century affirmed the power of state governments both to mandate vaccination and to delegate a broad degree of authority to local municipalities and health boards to carry out particular vaccination programs.
Judicial Approval
In 1905 the Court held in Jacobson v. Massachusetts [36] that the general police power of states is broad enough to overcome a Due Process claim brought by an individual who claimed his personal liberty interests were unconstitutionally invaded by the mandatory smallpox vaccination program in question. [37] In an opinion by Justice Harlan, the Court ruled that the constitutional guarantee of liberty “does not import an absolute right in each person, to be, at all times and in all circumstances wholly free from restraint.” [38]
This case still represents the initial constitutional basis of most mandatory vaccination legislation. Many states still provide for the governor or a public health official to mandate vaccination for all in the event of an outbreak. [39] Individuals who cannot vaccinate for health reasons or who refuse to vaccinate may be quarantined in order to protect the population in some states. [40] These laws gained greater relevance following the terrorist attacks of 9/11 and the increased public concerns regarding bioterrorism. For the most part, however, mandatory vaccination laws in the name of outbreak control have given way to vaccination requirements as a prerequisite for school attendance.
The issue of school vaccination came before the Court nearly two decades after Jacobson . In Zucht v. King [41] , the plaintiff challenged a general grant of authority from Texas to local boards of health to condition school entry on proof of vaccination. [42] To differentiate the case from Jacobson , the plaintiff noted that the San Antonio ordinances mandated vaccination even in the absence of evidence of outbreak. [43] The Court, speaking this time through Justice Brandeis, upheld the validity of the ordinances as well as the broad grant of authority to local health boards. [44] On the issue of the state’s power to mandate vaccination, he merely cited Jacobson : “[l]ong before this suit was instituted, Jacobson v. Massachusetts...had settled that it is within the police power of a state to provide for compulsory vaccination.” [45] As for entrusting a broad degree of authority on local health officials, he noted that Jacobson and other cases had affirmed that a state may “delegate to a municipality authority to determine under what conditions health regulations shall become operative.” [46] This delegation includes the permission to vest municipal officials with “broad discretion in matters affecting the application and enforcement of a health law.” [47] In summary, the Court found that these ordinances were valid assignments of “that broad discretion required for the protection of the public health.” [48] The language of the opinion emphasizes the importance of the public health as the key justification for mandatory vaccination.
Zucht , along with Jacobson , thus became the legal foundation for the mandatory vaccination laws of the twentieth century. Modern school vaccination laws and policies have grown from early mandatory smallpox vaccination laws:
The early successes of school vaccination laws against most political, legal, and social challenges helped lay the foundation for modern immunization statutes. Since the introduction of smallpox vaccination policies in the mid-to-late 1800s, states have amended them to include additional diseases as new vaccines become available. [49]
Though various amendments and additions have been made to mandatory vaccination laws throughout their history, the past half century has experienced the true culmination of mandatory vaccination policy. Public health officials have been able to institute a scheme for near-universal vaccination:
Many existing school vaccination laws were enacted in response to the transmission of measles in schools in the 1960s and 1970s. State legislatures at that time were influenced by the significantly lower incidence rates of measles among school children in states that strictly enforced vaccination requirements and school exclusions in outbreak situations without significant community opposition. Rather than having health departments require immunization in emergency conditions, legislatures acted to prevent disease by mandatory immunization as a condition of enrollment or attendance in schools or licensed day care facilities. [50]
Moreover, states have not been completely left to implement the recommended immunization schedule. [51] Though school requirements are still a state matter, national public health officials are typically able to enact their recommendations through federally funded immunization plans. [52] These plans require states to implement and enforce federally recommended immunization requirements before the states can receive federal funds. [53] The current recommended vaccination schedule appears below.
Recommended Childhood and Adolescent Immunization Schedule [54]
Challenges and concessions.
While school vaccination requirements have been credited with bringing about the control and elimination of many devastating childhood diseases, critics have continued to voice concerns and raise legal and political challenges to the entire process of mandatory vaccination.
Personal Liberty Concerns
One key argument against mandatory school vaccination has always focused on government intrusion into what is considered a personal medical choice. [55] Just as the government cannot force a person to have surgery to repair a torn ligament, for example, the government should not be able to force parents to vaccinate their children if the parents believe that vaccination is not the best medical decision. One prominent critic of mandatory vaccination has stated her organization’s goal as simply providing parents with choices: “[w]e believe that health care consumers should have the right to choose the type of preventive health care that they want to use – including choosing whether to use one, ten, or no vaccines.” [56] Other objections along similar bases argue that mandatory vaccination violates the medical ethic of informed consent or even that school district control over mandatory vaccination policies amounts to the unlawful practice of medicine without a license. [57]
The typical counterargument given by the public health officials is to point out that one’s decision to vaccinate, unlike one’s decision whether to undergo surgery, affects the health of others in the community. [58] To allow parents the right to choose not to vaccinate is to infringe on the ability of other parents to raise their children in a society free of certain deadly diseases. From a legal standpoint, Jacobson still seems to have settled the issue that at least under some circumstances, the government may force an individual to receive a vaccination.
Although public health officials have the legal authority to mandate vaccination for the public health under Jacobson, they should be very mindful of the personal liberty concerns just stated. Those with such views often cling to them vigorously. [59] As certain vaccine-preventable diseases decline, such concerns become even stronger. For this reason, it is important for public health officials to support their mandatory vaccination programs with justifiable arguments rather than simply citing legal precedent or historical tradition in support of their exercise of power. Fortunately for public health officials, the benefits provided by vaccination programs can be utilized to justify the existence of such programs.
Safety Accountability Concerns
A variation on the consumer choice challenge to mandatory school vaccination requirements tends to accuse the public health community of conspiring with or at least willfully acquiescing to powerful vaccine manufacturers at the expense of citizens. [60] Mandatory programs, the argument goes, eliminate any accountability from vaccine manufacturers that the free market might otherwise provide. [61] Both the safety and efficacy of vaccines fail to improve because manufacturers do not have to respond to consumer concerns. [62] Mandatory programs thus prevent better vaccines. A prominent critic of these programs has stated that if mandatory vaccination programs are ended, “we will have the ability to put economic pressure on the drug companies and on the health agencies to do a better job with vaccine safety and efficacy.” [63]
The strength of this argument lies in its apparent lack of hostility toward vaccines per se. Given the historical success of vaccination in eradicating smallpox and in reducing or eliminating the risk of other childhood diseases, any critique of mandatory vaccination programs that focuses on the use of vaccines generally is likely to be dismissed by those in the field of public health. By focusing on the economic drawbacks inherent in a mandatory vaccination program and how those drawbacks can negatively affect the quality of vaccines, this argument may gain more traction. Indeed, all sides of this debate claim to desire both safer and more effective vaccines.
The response to this argument, I would imagine, would be to emphasize the drawbacks of opening up the “market” in this case. Because vaccination programs depend on a sufficient percentage of the community being vaccinated, complete consumer choice carries with it problems that might be absent in a standard market. As for vaccine quality, FDA regulation is in place to ensure a sufficient level of safety and efficacy to accomplish the goals of vaccination. [64] The pressure faced by vaccine manufacturers to obtain and maintain FDA approval should provide a check sufficient to guarantee proper vaccine quality. If not, the answer should be to raise FDA standards, rather than to jettison the entire mandatory vaccination process and with it the likelihood of maintaining a sufficient level of immunity among the population.
This response might be unacceptable to those concerned. If the connection between public health officials entrusted with implementing the mandatory vaccination schedule and FDA regulators entrusted with ensuring the safety and efficacy of vaccines is seen as too close, proposing higher FDA standards as a solution may not allay concerns. The independence and integrity of FDA is therefore critical in this arena, just as it is in other areas of public health.
Concern of Unknown Risks
In what may be a combination of the two challenges previously discussed, many individuals challenge vaccine programs because of a lack of information about vaccines. [65] Many people, for example, legitimately question the wisdom of forced vaccination before long-term effects of a vaccine are studied. One website that purports “to provide a wide range of news and views on vaccination and vaccination policy” has summarized this challenge to vaccines simply as opposing the idea of “a parent, any parent, being forced to do something that has even a remote chance of harming their child.” [66] Since long-term (ten or more years down the road) and low-risk (on the order of one-per-million or less, for example) side effects may truly be unknown, this concern does present a challenge for public health officials. [67]
Unfortunately, even the best studies are unable to fully determine all long-term consequences of vaccination. In addition, “[t]here is no such thing as a ‘perfect’ vaccine which protects everyone who receives it AND is entirely safe for everyone.” [68] Therefore, it is true that mandatory vaccination probably forces some parents to inject their children with a substance that will cause some unknown harm.
As with the other objections to mandatory vaccination, however, this objection suffers from a critical flaw. Mandatory school vaccination requirements are not justified solely on the benefit they provide to the recipient. Instead, it is the benefit they provide to the community as a whole by ensuring a sufficient level of vaccination to prevent outbreak that justifies their intrusive nature on individual medical decision-making. [69] For this reason, if public health officials did not enact the mandatory vaccination program, they would be forcing on parents a system that had at least a “remote chance of harming their child.” [70] Because the decision to enact a community-wide vaccination program must be made at the general level if it is to be made at all, and because some children will undoubtedly suffer some health consequences regardless of which policy is chosen, individuals will always be able to raise this argument against mandatory vaccination programs.
A better critique of these programs would focus on whether mandatory vaccination causes more overall harm than a voluntary system; that is, is it better when viewed at the general, rather than the individual, level? Ironically, the very success of vaccination programs in reducing the incidence of once-prominent diseases has led some to ignore the overall and continuing benefit of community vaccination (herd immunity). [71] But for parents to decry the “remote chance” of harm from vaccination while ignoring the very real chance of outbreak in an under-vaccinated population is to reframe the issue entirely.
Other Concerns
Other challenges to vaccination laws have cited strongly held religious or philosophical positions against vaccination in general. Such challenges require a different type of response from public health officials; often the options are limited to overriding such objections and excluding children of parents adhering to such positions from public schools (which is constitutionally permissible under Jacobson and its progeny) or creating exemptions to vaccination requirements (which is detrimental to the overall goals of mandatory vaccination if a sufficient number of exemptors exist). Reactions to such religious and philosophical concerns vary from state to state, with a general trend toward greater accommodation of objectors.
In response to these and other challenges to mandatory vaccination laws, states have enacted various exemptions to vaccination requirements for school entry. Actual enforcement varies by state.
All states provide exemptions for those with medical risks associated with vaccines. [72] If certain contraindications indicate a likelihood of harm from a particular vaccine, the exemption will be allowed. [73] Because such cases are rare and exemptions relatively easy to enforce, there usually is very little risk of compromising the efficacy of the overall vaccination program by granting these exemptions. [74] The ability to grant medical exemptions while still maintaining sufficient levels of vaccination to provide community-wide immunity is one of the great accomplishments of the vaccination system. [75]
In addition to medical exemptions, almost every state grants religious exemptions for those with sincere religious beliefs opposing vaccination. [76] Individual states tend to vary with regard to the level of religious conviction necessary to obtain a religious exemption. Such exemptions reflect the sometimes uneasy balance between mandatory vaccination programs and First Amendment Free Exercise rights, even though the Supreme Court has validated the right of states to mandate vaccination without providing for such exemptions. [77] West Virginia, for example, does not provide religious exemptions. [78]
Some religious exemption statutes have spurred challenges on Establishment Clause grounds by those who claim they favor organized or recognized religions over the sincerely held religious views of others. [79] These challenges, if successful, would lead to the invalidation of many religious exemption statutes. Rather than decrease the number of religious exemptors, however, this may actually lead to more religious exemptors. The political climate of our day, along with the experience of a few states already (such as Arkansas), suggests that legislatures may respond to invalidation of religious exemption statutes that require adherence to an organized religion by drafting more general and expansive religious exemption statutes. [80] By subjugating religion to compulsory vaccination, courts may actually be helping to bring about a system with even more religious exemptors, thereby harming the very vaccination programs to which religious objections had been subordinated. [81]
Philosophical
The possibility that some parents who strongly oppose vaccination for other than religious reasons has led to other means of exempting from mandatory vaccination programs. In some states, people may avoid vaccination requirements by way of philosophical exemptions. [82] In California, for example, a parent need only “submit a letter or affidavit stating that the immunization is contrary to his or her beliefs” to exempt their child from vaccination requirements. [83] “Where available, parents are taking advantage of such exemptions with growing regularity; and in states offering both exemptions, the number of philosophical exemptions far exceeds the number of religious and medical exemptions.” [84]
States without philosophical exemptions, moreover, are often lax with their enforcement of religious exemptions. [85] Because of this, parents in these states can usually submit insincere affidavits purporting to object to vaccination for religious reasons and local health officials, unconcerned with delving into the sincerity of such affidavits, will widely grant exemptions. [86] In most states, therefore, persistent parents can usually find some way to exempt their children from vaccination requirements. If all else fails and vaccination is still regarded as unacceptable to the parent, the option of home schooling may provide a final avenue of evading these school vaccination requirements.
Dangers of Widespread Exemptions
The ease with which non-medical exemptions can typically be obtained has raised concerns among many that the benefits of widespread immunization are being compromised. [87] Because of the nature of medical exemptions, unvaccinated persons in a community with only medical exemptions would be expected to be few and dispersed. Herd immunity can be attained, and protection is ensured for both the vaccinated majority and the unvaccinated few. [88] Broadly granted philosophical and religious exemptions make herd immunity more difficult to attain and increase the risk to the community. This risk is exacerbated by the fact that many of those who apply for such exemptions “will cluster together in one geographic area.” [89] This cluster effect tends to increase the likelihood of serious outbreaks:
Recent studies have shown that clusters of exemptors, who are significantly more susceptible to contracting vaccine preventable illnesses, pose an increased risk of spread of diseases not only to their unimmunized peers, but also to the surrounding, largely vaccinated population. [90]
Given that many childhood diseases seem to be in decline, exemptors may fail to realize the continued value of vaccination. As the mumps outbreak in Iowa makes clear, however, vaccination programs take time and are at risk if vaccination rates fall. Other diseases are still prevalent in other parts of the world, and outbreaks can still occur in this country due to the prevalence of international travel. Ever though measles is rarely observed in the US, for example, the World Health Organization has reported that nearly 900,000 measles-related deaths occurred in developing countries in 1999. [91] Until diseases are eradicated globally, it may be necessary to continue vaccination.
Because many of the aforementioned risks are frequently underappreciated by those who seek exemptions, some have suggested a combination of stricter enforcement of exemption requirements and increased public knowledge of the reasons underlying childhood vaccination requirements. [92] Knowledge is indeed essential to the resolution of this problem. The easier it is to obtain an exemption, the less likely individuals are to understand and appreciate the importance of widespread participation to the success of a vaccination program. Greater public appreciation of the need for such participation (even for diseases that seem to be in retreat), along with greater information on the safety of vaccines can go a long way toward increasing public health in this area. [93]
Partial Exemptors – A Modern Phenomenon
The availability of exemptions has led to other interesting developments in the vaccination debate. Recently, for example, challenges have been raised against the need for mandatory chickenpox and hepatitis B vaccines. Diseases such as these, which are either not greatly feared (chickenpox) or transmitted primarily through voluntary rather than involuntary contact (hepatitis B), do not fit neatly into the typical justification for mandatory vaccination. [94] Nevertheless, public health officials have decided that recently-developed vaccines for these diseases should be placed on the recommended schedule. This has given rise to a significant number of partial exemptors – those who are not opposed to vaccination requirements per se, but who oppose particular vaccines on the schedule. Such a position may not have been comprehended by those who drafted the religious and philosophical exemptions, which seem to assume that a parent’s opposition is to vaccination generally, rather than to a specific vaccine. [95]
Because the religious exemption is usually constructed to apply to those who oppose vaccination generally because of sincere religious beliefs, would-be partial exemptors have difficulty fulfilling their optimal desires. In states without a philosophical objection, parents must choose either to accept the entirety of the recommended schedule of vaccines or to obtain a religious exemption for all vaccinations. [96] Parents who live in states with a philosophical exemption are much more able to tailor their objection to those vaccines with which they disagree. [97]
From the standpoint of a public health official, this presents two possible worlds. In the world with traditional religious exemptors but no philosophical exemptors, overall percentages of vaccinations would be relatively equal from vaccine to vaccine, and higher vaccination rates would be obtained for diseases associated with more objectionable vaccines at the expense of lower vaccination rates for diseases associated with less objectionable vaccines. [98] By contrast, in the world with philosophical exemptors, the public health official would observe higher vaccination rates for the less objectionable vaccines and lower vaccination rates for the more objectionable vaccines. [99]
The difference between these two worlds can have far-reaching implications. If parents are forced to make the all-or-nothing choice, a significant enough number could choose to forego vaccines (including some which they would otherwise accept) that herd immunity is lost, even for less objectionable vaccinations. On the other hand, a significant enough number could accept the more objectionable vaccinations to bring about herd immunity for those diseases. Though the public health official might prefer a world in which neither religious nor philosophical exemptions exist, such a world may not be possible. Therefore, the official should determine which of the two possible worlds provides a greater overall level of safety for the society. In addition, potential public reaction to a vaccine should cause the public health official to consider the ramifications the addition of a vaccine to the schedule will have on those vaccines already on the schedule.
Because partial exemptors have the potential to sway the balance between herd immunity and vulnerability, public health officials must take account of their concerns. Unlike in years past, today the development of a new vaccine presents public health officials with a choice that can affect other vaccines on the recommended schedule. Though the possibility for a chickenpox- and Hepatitis-B-free nation may seem tempting, officials should now consider the possible consequences of mandating such “borderline” vaccines. Parents who might otherwise vaccinate according to the old schedule might have second thoughts about the new vaccines on the schedule and seek means of avoiding the new requirements. If no means exist for avoiding the new vaccines other than complete exemption on religious grounds, parents who would subsequently pursue such exemptions would bring about a lower level of immunization for older diseases.
Studies may be necessary in the above situation to determine whether herd immunity status could be in jeopardy for those diseases for which vaccines are already on the schedule. While one solution might be to provide parents with greater ability to tailor their individual vaccination desires, such a solution would undermine the efficacy of newly scheduled vaccines. In addition, greater levels of flexibility in vaccination choice would undermine public understanding of the community-based nature of vaccination. I think it might be worth sacrificing the efficacy of the newer vaccines in order to maintain that of the more established ones. The public might be willing to suffer the possibility of chickenpox outbreaks, for example, in order to prevent an even minor epidemic of diphtheria or the measles.
Again, information should play a key role in the resolution of this issue. Many of the websites urging parents to carefully consider the vaccination decision do not inform parents that their decision to vaccinate may affect the overall health of the community. [100] The CDC, for its part, does urge parents to take note of this concern. [101] The very persons who most need to know of this concern (those seeking exemptions), however, are often those most likely to distrust CDC publications. For supposed citizen-oriented websites to urge individuals to make vaccination choices without considering how such decisions affect the community is irresponsible, especially given the scientific stability of the concept of herd immunity.
II. THE ROLE OF THE FEDERAL GOVERNMENT
Some of the problems posed to vaccination programs by exemptors and others could be partially solved through greater public awareness of the stringent safety and efficacy testing done on vaccines before they may enter the market. This section summarizes the role of FDA in the context of vaccination programs. In addition, this section will discuss other ways in which the federal government gets involved in the vaccination issue, concluding with a brief synopsis of the no-fault compensation scheme enacted pursuant to the National Childhood Vaccine Injury Act of 1986. [102]
FDA Regulation
Though state governments determine which vaccinations are mandatory for school attendance, the federal government plays a key role in vaccination. Perhaps most importantly, the federal government regulates the safety and effectiveness of all vaccines. The FDA’s Center for Biologics Evaluation and Research (CBER) is charged with this critical task. [103] The role of CBER ranges from pre-approval testing of potential vaccines to facility inspection to continued oversight and sampling after approval. [104] Regulation of vaccines can be more stringent than for other biologics or drugs. [105] Even after a vaccine is licensed, for example, FDA oversight is prevalent. [106] Since vaccines are derived from living organisms and are particularly susceptible to contamination and other environmental factors, manufacturers usually must submit samples of each vaccine lot for testing before release. [107]
Before a vaccine can even be licensed for distribution and use, it must go through an extensive testing process relatively similar to that of drugs and other biologics. [108] First, a new vaccine must be tested for safety on animals. [109] The vaccine manufacturer next must file an Investigational New Drug application (IND) with the FDA. [110] Studies are then undertaken to ensure safety before any human testing takes place. [111] In addition, the IND must describe the studies intended for humans. [112]
Once these initial steps are completed, proposed vaccines must undergo three phases of clinical trials, in which the vaccine is tested on humans. [113] Phase 1 testing looks only for very serious or very common problems. [114] A small number of subjects (usually less than 100) are closely monitored, usually for only a few months. [115] Testing expands in Phase 2 to begin evaluating efficacy, as well as to further test safety. [116] Phase 2 trials can last up to two years and typically include hundreds of subjects. [117] The final stage of testing, Phase 3, further studies safety and effectiveness. [118] Thousands of people may be involved in this stage of testing, and if successful it can lead to application for FDA licensing. [119]
Once the clinical trials are completed, the FDA can examine the results of the tests to determine whether the vaccine is safe and effective enough to be placed on the market. [120] At any point in the process, the FDA may halt ongoing studies if safety concerns require such action. [121] The FDA also reviews the data from the studies and inspects the manufacturing facility. [122] At this point the vaccine may be licensed.
As stated above, the FDA’s role in protecting the safety and effectiveness of vaccines does not end at the licensing stage. [123] Before any vaccines from a particular lot can be released, the manufacturer must typically submit samples for potency, safety, and purity testing. [124] Periodic facility inspections also continue for the duration of the license. [125] Furthermore, formal post-market studies may be conducted in order to identify problems that would not show up in pre-market clinical testing. [126] These tests are referred to as Phase 4 tests and are not mandatory, but can help identify problems that may only occur very infrequently. [127] Post-marketing surveillance programs are important because manufacturers are “never going to be able to do studies big enough to detect risks that might happen at a level of one in 100,000 or one in 1 million.” [128]
The Vaccine Adverse Event Reporting System (VAERS) is another valuable tool in identifying problems with a vaccine once it has been approved for the market. [129] VAERS was developed following Congress’s enactment of the National Childhood Vaccine Injury Act of 1986 and has become a very useful tool for identifying possible adverse effects that would otherwise escape detection. [130] VAERS allows anyone to report a problem that may be associated with any vaccine. [131]
It is important to keep in mind that VAERS is simply a reporting system. Experts and others use the data in VAERS to attempt to determine whether a vaccine actually causes a particular adverse effect, but the events that VAERS documents are not all caused by vaccines. It is therefore easy to understand why VAERS encourages doctors and others to report any adverse event that may be related to a vaccine. “VAERS is designed to detect signals or warnings that there might be a problem rather than to answer questions about what caused the adverse event.” [132] It is important to keep these facts in mind when looking at VAERS data, as many of the adverse effects may be completely unrelated to the vaccine in question. Often the effects are correlated with, but unrelated to, vaccination simply because many of the problems reported are those usually associated with events happening during the vaccination period (the first few years of life). [133]
Used correctly, VAERS can lead to useful studies and the discovery of potentially rare adverse effects. [134] VAERS can also be used to monitor individual lots of a vaccine. [135] Unfortunately, by encouraging individuals to report any adverse effect that may possibly have been caused by a vaccine, VAERS can provide ammunition for those claiming a definite link between a vaccine and a particular adverse effect, even if the data is silent on whether such a link exists. [136] While VAERS is in place to help identify actual risks associated with vaccines, these risks cannot be accurately assessed solely on the basis of reported incidents of adverse effects. [137]
The real value of VAERS lies in the testing and hypotheses that are developed in response to the data that has been reported. Because of the serious adverse effects already occurring during the typical vaccination period, it will often be easy and convenient to point to the correlation between vaccines and reported adverse events. Lost in the picture is the foundational proposition that VAERS is, at its core, a data collection system. To forego scientific inquiry and point instead to simple correlation may be convenient, but it is unwise. [138]
The recent public discussion surrounding the use of thimerosal as a preservative in vaccines helps to illustrate the importance of the FDA and other factors in furthering the goals of vaccine safety and public confidence in the entire safety regulatory process. Thimerosal is a mercury-containing organic compound that for many years has been used as a preservative in vaccines to help prevent contamination with microbes that could potentially be fatal. [139] Recently, fears that mercury at very low levels may be toxic to the brain have raised concern among many in the public about allowing the use of thimerosal in vaccines. [140] Many began to fear a connection between thimerosal and autism. [141] Standard FDA testing of lots, as well as studies measuring the amount of mercury contained in the standard immunization schedule versus accepted safe amounts, did not lead to safety concerns sufficient to pull thimerosal from the market. [142] Though one committee (the Immunization Safety Review Committee, commissioned by the Institute of Medicine) concluded that a theoretical link between thimerosal and autism was biologically plausible, most health experts continue to assert that there simply is no scientific evidence of a link between the two. [143]
During this time period FDA performed additional tests to verify or refute the supposed link between thimerosal and autism. [144] In 1999, FDA performed a comprehensive study and review of thimerosal use in vaccines for children. This review revealed no risk from thimerosal use, other than “local hypersensitivity reactions.” [145] Indeed, none of the standard safety protocols in place suggested or required that FDA pull thimerosal from the market. This is not to say, however, that no risk existed. As is clear from the foregoing summary of FDA vaccine approval, not all adverse effects will be known from clinical trials. [146] It may take years or longer to assess some of the risks of vaccines, including the risk of thimerosal as a preservative. [147]
Continued public concern over the safety of thimerosal caused FDA to begin to work with vaccine manufacturers in order to reduce or eliminate thimerosal from vaccines as a precautionary measure. [148] About this time, the American Academy of Pediatrics and the Public Health Service urged the removal of thimerosal from vaccines. [149] Today, with the exception of the inactivated influenza vaccine, all recommended childhood vaccines are either thimerosal free or contain only trace amounts of the compound. [150] Even though the risk may not have been as great as feared by the public or even existent at all, if the new vaccines are equally effective, the elimination of thimerosal from vaccines can probably be seen as a safety improvement, albeit at the expense of the added research and development needed to create the new thimerosal-free vaccines.
Rather than quell the existing safety concerns, this action led many of those who had decried the use of thimerosal to accuse FDA of participating in a cover-up to protect vaccine manufacturers. [151] Government agencies, for their part, continue to claim that vaccines with thimerosal are as safe as thimerosal-free vaccines, suggesting that the added development may have been superfluous. [152] While this may be so, the availability and now prevalence of thimerosal-free vaccines does provide the scientific and medical community with a new means of assessing the possible autism-causing effects of thimerosal. Namely, since thimerosal is suspected to cause autism within the first few years of life (the routine vaccination calendar), those who were vaccinated in the years since thimerosal-free vaccines have comprised the overwhelming majority of vaccines (that is, those born after 2001) would be expected to experience lower incidences of autism than the groups vaccinated with thimerosal-containing vaccines. [153]
In spite of the potentially costly decision to encourage the development of thimerosal-free vaccines when there is no sufficient safety concern to pull thimerosal from the market, FDA and other government officials have had little success in assuaging the fears and concerns of thimerosal critics. [154] Scientific arguments often fail to persuade, either because they are inconclusive or because of a perceived bias favoring vaccine manufacturers. [155] To back up their own arguments, thimerosal critics rarely point to scientific studies. [156] Instead, their reasoning seems to stem more from anecdotal evidence and comparison of thimerosal (which contains ethyl-mercury) to methyl-mercury-containing fish. [157] Representative Dan Burton (R-Indiana), a key supporter of the fight against thimerosal, explained that his belief in the toxicity of thimerosal stemmed from a personal episode: “[m]y grandson received nine shots in one day, seven of which contained thimerosal, which is 50 percent mercury as you know, and he became autistic a short time later.” [158] Others point to the rise in autism rates in the past twenty years and put the onus on the medical community to prove that this rise is not due to thimerosal. [159]
The response of health officials has been to ask why the burden should be placed on them to disprove a link between thimerosal and autism; cell phones, ultrasound, or diet soda could just as easily be the culprit. [160] Indeed, the typical response to those charging vaccination with causing many of the adverse effects occurring in life’s first few years is to point out that usually such accusations are based on nothing more than the temporal proximity of the vaccine and the illness. Some have suggested that the rates of autism may be on the rise not because of thimerosal, but because of generally more accurate diagnosis of the affliction. [161] In the past, an autistic child may have been wrongfully diagnosed with other mental disorders. [162] Figures showing a correlation between the rise in autism and the drop in other diagnosed mental disorders bolster such assertions, and suggest that vaccination may simply be a convenient scapegoat. [163]
As the thimerosal issue makes clear, vaccines often provoke strong feelings amongst various segments of the population. [164] Proper consideration of public reaction to its actions is a delicate aspect of FDA regulation of vaccine safety. To complicate matters further, one can easily imagine an equally vehement response and similar claims of conspiracy had the FDA not worked to reduce thimerosal from vaccines as a precautionary measure. Indeed, public confidence in the safety of vaccines is often influenced by factors outside the typical FDA calculus. Though FDA must act in the interests of the general safety regardless of public opinion, it may sometimes be necessary for FDA to consider public opinion, at least when exercising discretionary oversight. After all, the entire VAERS system is to a large extent dependant on public cooperation. Nevertheless, when the choice is between FDA popularity and doing what is right for the safety of Americans, the FDA should not allow itself to be swayed by a misinformed public.
Vaccine Injury Compensation Program
Congressional reaction to safety concerns goes beyond the adverse reporting system VAERS. The National Childhood Vaccine Injury Act of 1986, which created VAERS, also created a no-fault compensation scheme for people injured or killed by vaccines as an alternative to the traditional tort system. [165] This system was intended to efficiently and rapidly compensate those who are actually injured by vaccines while maintaining an environment in which further vaccine research and safety improvement could exist. The situation giving rise to this compensation program sounds remarkably similar to the more recent concerns surrounding thimerosal:
In the early 1980's, reports of harmful side effects following the DTP (diphtheria, tetanus, pertussis) vaccine posed major liability concerns for vaccine companies and health care providers, and caused many to question the safety of the DTP vaccine. Parents began filing many more lawsuits against vaccine companies and health care providers. Vaccination rates among children began to fall and many companies that develop and produce vaccines decided to leave the marketplace, creating significant vaccine shortages and a real threat to the Nation’s health. [166]
Funding for the no-fault compensation scheme initially came from Congressional grants of federal tax dollars totaling $110 million per year. [167] Since October 1, 1988, funding has proceeded from the Vaccine Injury Compensation Trust Fund, which is funded by a $0.75 excise tax on all doses of vaccines covered under the program. [168]
One may wonder what makes vaccines worthy of an alternative dispute resolution system. Perhaps it is the result of the power of the vaccine manufacturing lobby or simply an attempt by Congress to pass some legislation in the face of strong public sentiment. Although these reasons may appear plausible, it seems more likely to me that the Act created this no-fault compensation scheme because of the mandatory nature of vaccination. For those injured by other medical devices or drugs, the traditional tort system or medical insurance seem the proper means of addressing the issue. When people are told to undertake a medical procedure they may not agree with because it helps further a public goal, however, it may make sense to have a system in place whereby they can obtain relief quickly if harmed by the procedure. Moreover, because certain vaccines may be closely associated with particular adverse effects, the efficiency of a no-fault scheme may trump the standard fact-finding processes of the legal system. The government has chosen to enact such a no-fault scheme, and err on the side of compensation.
III. ANALYTICAL MEANS OF ADDRESSING THE ISSUE
The concerns and problems raised in the context of mandatory vaccination programs do not readily suggest a simple answer. In examining the issue, I came across two particularly useful tools for analyzing the problem. The first comes from an old episode of The Andy Griffith Show in which a local farmer refused to accept a vaccination from the local nurse. In addition to providing substantial entertainment to the viewer, the characters can be viewed metaphorically to represent the various parties in the mandatory vaccination debate. The episode’s solution, in turn, sheds some light on the current debate.
This section will also utilize the analytical framework of economic analysis. Though not as enjoyable a topic as The Andy Griffith Show, economic theory helps to reshape the vaccination discussion and greatly facilitates the process of assessing the various positions.
“We got to get folks to take their shots” – Sheriff Andy Taylor [169]
The Andy Griffith Show addressed the concept of popular resistance to universal vaccination over forty years ago. In “The County Nurse,” Sheriff Andy Taylor confronted a local nurse who was trying to bring everyone up to date on their tetanus shots. Not surprisingly, at least to Andy, many of the mountain farmers had not been inoculated. The naïve nurse would soon discover the reason for the low vaccination rate.
Rafe Hollister, one of the leading farmers in Mayberry, had little use for modern medicine or doctors in general. “We don’t need any nurse, nobody gets sick up here.” [170] Thermometers? “I know when I got a fever, I’m hot.” [171] Stethoscopes? “I know my heart’s beating, I’m alive ain’t I?” [172] But his strongest objection was saved for vaccinations: “I ain’t never been jabbed and I ain’t fixin’ to be.” [173] Such were the views that the nurse was up against in her attempt to achieve 100% vaccination rates.
Rafe Hollister
Rafe Hollister’s reasons for opposing vaccination went beyond his desire to avoid getting “jabbed.” He was a farmer who lived off the land, and when he got sick he let his body fight the sickness naturally. His daddy had lived to the age of hundred and he aimed to do the same. [174] The concept of a vaccination was certainly something foreign to him, as was the idea that a health official could force him to do anything. Even in the wake of the nurse’s impassioned plea to accept a shot that could someday save his life, he retorted simply, “I done alright before you come around and I’m doing alright now.” [175]
Although the county nurse was not acting pursuant to a mandatory vaccination program, under the circumstances her attempts to get Rafe inoculated were pretty forceful. The nurse was accompanied by the local sheriff to Rafe’s farm to try to convince him to take the shot, and when he refused, the sheriff and nurse continued to attempt to make him acquiesce. When Deputy Barney Fife heard of Rafe’s stubbornness, he insisted the nurse return to Rafe’s farm with him to force Rafe to take the shot. After all, boasted the deputy, “Rafe Hollister’s like a child and he’s gotta be treated like one...I’ll make him take his shot.” [176] When the deputy arrived at Rafe’s farm yelling that he was forcing Rafe to accept the vaccination, Rafe decided to fight the mandatory vaccination by drawing his rifle and forcing the deputy to leave the farm.
In a classic manifestation of the early spirit of the television series, Sheriff Andy Taylor finally convinced Rafe to take the shot through a little reverse psychology. Andy began by facetiously praising Rafe’s refusal to take the shot as stemming from Rafe’s desire for immortality. Namely, by refusing to take the shot, Rafe was sure to become the impetus for all the other townspeople not to neglect to take their shots. Unfortunately for Rafe, this heroic stature would only be achieved posthumously, as he will have succumbed to a violent and painful death from tetanus. As Andy explained to Rafe, someday, after getting cut by a rusty saw or bitten by an animal, without the shot he’ll “be a cinch to go.” [177] Eschewing the chance to be a dead hero, Rafe finally took the shot.
Sheriff Andy Taylor
Vaccination has changed the modern world. Indeed, it has led to the elimination or significant decline of many diseases that once posed significant and potentially deadly health risks. Public health officials in the United States have managed to institute a program that, though subject to variations on a state by state basis, essentially mandates certain vaccinations as a requirement for school attendance. While these vaccination programs are touted by most public health officials, a significant number of people oppose mandatory vaccination. The County Nurse episode helps illuminate the perspectives of the various sides of the issue, as well as one possible solution.
The nurse herself represents the public health officials. Though she is not implementing a mandatory vaccination program, her stated goal is to inoculate 100% of the population. [178] As mentioned above, she has the assistance of local law enforcement and she is quite persistent. Rafe Hollister, the stubborn farmer, represents those within the community who oppose or resist mandatory vaccination programs. His reasons initially rest on a general reluctance to stray from natural medicine. In this way he represents the contingent of society that scientists and medical researchers will always find difficult to convince of any developments in the medical field. In many ways, he is comparable to the plaintiff in Jacobson . Andy and Barney can be seen as the arms of the state that are entrusted with carrying out the general vaccination plan. Their varying styles can be seen as varying state requirements and enforcement options for vaccination.
Though these comparisons may seem elementary and of little value, the character development that the characters undertake during the episode greatly increases the episode’s usefulness as a surrogate for real world concerns and issues. Rafe resists the shot initially not only because he distrusts medicine in general, but also because he resents the idea that a county nurse can make him do anything. Many who resist mandatory vaccination schemes do so because of personal liberty concerns; they do not want the government to tell them what to do, especially in the context of personal medical decisions. Just as Rafe’s stance becomes more vehement the harder the nurse attempts to convince him, many who oppose mandatory vaccination see the persistence of the medical community as evidence of blind adherence to a potentially dangerous system, or worse yet as an active promotion of the special interests of the vaccine manufacturers. [179] The episode does not paint the nurse in this way at all, however. Rather, after seeing how strongly Rafe opposes vaccination, the nurse passionately pleads with him to reconsider. Her stance truly seems to stem from a genuine concern that he not suffer the potentially terrible effects of the disease. [180] As before, he refuses; this seems to illustrate that the stance of some may be so strong that they will never accept vaccination on the basis of arguments advanced by government officials.
Barney Fife’s insistence that Rafe accept the shot demonstrates the lack of understanding among many in the government and in the general population as to the vehemence with which those opposing mandatory vaccination hold to their views. His paternalistic stand only serves to exacerbate the situation with Rafe. Indeed, Barney Fife helps to illustrate that there cannot be a one-way solution to the issue of mandatory vaccination.
Andy Taylor’s method of convincing, which eventually carried the day, may not be very conducive to real-world implementation. After all, it is unrealistic to think that reverse psychology will convince those currently opposed to vaccination programs to change their minds. What I think is important to notice, however, is the role information can play in this issue. Andy finally convinces Rafe Hollister to take his shot after describing the horrible effects of the disease and how likely Rafe is to contract it. Similarly, any solution to the issue of mandatory vaccination holdouts must rely on increased information dissemination. That the information in the episode came from a trustworthy source may also have been crucial, which seems to imply that public health officials may need to work more closely with local personnel in order to obtain higher vaccination rates.
Because this episode deals with the vaccine for tetanus, a non-communicable disease, the usual community-based arguments in favor of vaccination do not enter the equation. Extra-personal consequences of Rafe’s decision to vaccinate do exist, however. Most importantly, as the unofficial leader of the farming community, his decision will be followed by the other farmers. This is shown both in Andy’s assurances to the nurse that Rafe is the most important of the farmers to convince on the issue and later, after Rafe has decided to get the shot, in his promise to the nurse that all she has to do is come with him and he’ll get all the farmers to take their shots. Perhaps those parents who support vaccination can help bring about higher vaccination rates by being more vocal and persistent with their neighbors who oppose vaccination programs.
Economic Analysis
Economic analysis [181] provides a useful theoretical basis for evaluating the competing sides of the vaccination debate. Arguments regarding the wisdom of the current vaccination policy can often be recast as economic questions involving a cost-benefit analysis.
When an epidemic breaks out, for example, the benefits of vaccination (protection from the disease both for the individual and for society through herd immunity) seem more clearly to outweigh the costs (potential side effects of the vaccine, decreased ability of the immune system to defend the body from variant strands of the disease, or personal or religious objection). Vaccination rates would, therefore, be expected to be highest during such epidemics. Consequently, those few who continue to oppose vaccination during such epidemics would be expected to do so for only the strongest reasons. This is due to the fact that in economic terms, the opponent of vaccination would have to believe that the benefits of vaccination still do not outweigh the costs, even during an epidemic. This might stem from a relative undervaluation of the benefits of vaccination (perhaps due to a belief that contracting the disease would not be so bad) or a relative overvaluation of the costs of vaccination (possibly due to the greater cost to the conscience of the personal or religious opponent of vaccination) or some combination of both. Medical exemptions directly illustrate this cost-benefit analysis: for a person likely to suffer serious side effects from a vaccine, the cost of vaccination is much greater than the cost to the average individual. Even in a time of epidemic, therefore, vaccination might not be rational for such an individual.
This economic analysis of vaccination is well illustrated by the facts of Jacobson v. Massachusetts [182] , the first Supreme Court case addressing the constitutionality of mandatory vaccination legislation. The case involved a Massachusetts statute allowing local authorities to mandate vaccination for smallpox if necessary for the public health and safety. [183] Subsequently, and upon a determination that smallpox was “prevalent to some extent” and “continues to increase,” the city of Cambridge passed a mandatory vaccination ordinance. [184] This ordinance represented the economic determination that the benefit of mandatory vaccination outweighed the cost of supplying vaccines, finding and prosecuting holdouts (such as Jacobson), and the decreased liberty of individuals to be permitted to decide whether to vaccinate.
Jacobson subsequently challenged his prosecution under the ordinance by claiming it to be an unconstitutional denial of his liberty under the 14th Amendment (as well as in violation of the Preamble and the “spirit” of the Constitution, arguments that were summarily dismissed). [185] In economic terms, this may simply indicate that he viewed the cost of accepting a forced vaccination (perhaps of any kind, in any circumstance) as greater than any possible benefit. A closer look at his arguments, however, suggests that he may have performed a more detailed cost-benefit analysis. One can easily convert the various arguments he attempted to advance into economic costs. Among these arguments were the likelihood of vaccination to bring about “serious and permanent injury” and occasional death, the inability of an individual to assess the risk of vaccination in a particular case, and the potential impurity of vaccines and inability to test such impurity, among others. [186] At the very least, it would appear that Jacobson attributed a greater than average cost to vaccination.
The statute also provided that ordinances mandating vaccination provide an exception for “children who present a certificate, signed by a registered physician, that they are unfit subjects for vaccination.” [187] This reflects the state’s determination that the cost of forcing vaccination upon those more likely to suffer adverse side effects outweighed the benefit of completely universal vaccination. Given the determination that near-universal vaccination was required to provide the desired benefit, one would expect that the state expected to grant relatively few medical exemptions (or at least few enough not to seriously compromise the goal of providing protection against smallpox through vaccination).
In rejecting Jacobson’s liberty challenge to the ordinance, the Court endorsed the concept that the State’s cost-benefit analysis can supersede that of the individual, at least in the area of public health. The Court’s decision, in fact, makes irrelevant any individual cost-benefit analysis in the face of a comprehensive mandatory vaccination program.
Various vaccination-related developments in the century since Jacobson can also be cast in an economic analytical framework. Certainly the benefit from vaccination disappears when a disease has been eradicated, which explains why the smallpox vaccine is no longer mandated. Any cost greater than zero (the likely benefit of smallpox vaccination at this point, barring of course a reintroduction of the disease using laboratory samples) will suffice to outweigh this benefit. [188] The success of vaccination policies, however, may lead to an undervaluation of the benefit of continuing to vaccinate due to the lack of visible instances of the disease. [189] This problem may be compounded when vaccines are mandated for diseases which are not associated with high mortality rates, such as chickenpox. A further complication to the cost-benefit analysis arises when assessing vaccination policy for diseases such as Hepatitis B, which is spread typically through voluntary contact. In such a case, an individual who feels highly unlikely to engage in the behavior giving rise to the risk of the disease might rationally see very minimal benefit from vaccination, while the state may view widespread vaccination as the most cost-effective method of dealing with the disease. [190]
Altruism and Free Riding
Given the continuing policy of vaccinating for diseases that have become relatively rare in recent decades, one might expect individual cost-benefit analyses to increasingly come into conflict with the societal policy. Several factors, however, serve to counteract this possibility. Perhaps most significantly, it is likely that many parents defer on the question of vaccination and accept the cost-benefit analysis of the state (communicated to the individual through the vaccination schedule and through doctor’s recommendations) as their own. Along the same lines, many individuals might not strongly consider the pros and cons involved in vaccinating; if the possibility exists for contracting a disease, and a vaccination is available, the decision may already be made. [191] A third possibility implicates a factor that I have not yet mentioned in relation to the individual cost-benefit analysis: altruism.
Some have proposed that altruism may bridge the gap between incompatible cost-benefit analyses of states and individuals. [192] Whereas typical medical decisions affect only the patient making the decision, it is pointed out, medical decisions regarding vaccine-preventable diseases usually implicate outside interests. [193] A patient thinking only of his own interests may forego vaccination if he feels the risk from vaccination outweighs the personal benefit. Altruism, it is argued, may present a separate benefit for such an individual. [194] Though the individual may not consider the risk of contracting the disease high enough by itself to justify vaccination, he may still vaccinate in order to help accomplish the public goal of eliminating the threat of an epidemic. Public health officials hope that comprehensive vaccination will produce herd immunity. [195] Thus the individual who may otherwise forego vaccination might undertake it in order to “do his part” for the community at large. Individuals who cannot vaccinate are particularly dependent on this sort of altruistic behavior, as they often have no other protection from the disease. [196]
Working against this altruistic behavior is the temptation of individuals to enjoy the benefit conferred on them by herd immunity without undertaking the cost of being vaccinated personally. [197] This is widely referred to as “free riding,” and greatly undermines the goal of comprehensive vaccination. Since herd immunity is supposed to create a level of protection sufficient for even those few who are not vaccinated, a small number of free riders might not pose a significant problem. As described earlier in this paper, comprehensive vaccination programs are designed to work even though some members of society cannot be vaccinated. [198] The problem arises when the number of free riders becomes sufficiently high to compromise the ability of the society to achieve herd immunity. Since the average citizen (one with no greater reason to avoid vaccination than any other member of society) could always choose to free ride if immunization were voluntary, herd immunity might never be achieved. This is one of the key arguments advanced in support of government mandated vaccinations. [199]
Ex Ante Versus Ex Post
The concepts of altruism and especially free riding emphasize the importance of ex ante (before the fact) versus ex post (after the fact) decision making in the context of vaccination. One of the main benefits of economic analysis is that it requires decisions to be justified ex ante. Public health officials, for example, are faced with the decision of whether to mandate vaccination for a particular disease at a time when all adverse effects cannot be known. They must weigh the possible consequences of allowing a disease to continue against the possible known and unknown adverse effects of a vaccine that may have just entered the market. When this decision is made properly, the benefit of the vaccination program will have outweighed the cost. The benefit is manifested in lower or no occurrences of the disease, while the cost is seen most directly in those children who have actually experienced adverse effects as a result of the vaccine. If the benefit is greater than the cost from an ex ante perspective, to the economist there should be no second-guessing of the vaccination program. [200]
The economist, of course, is not the parent. Parents who decry mandatory vaccination as the cause of their child’s adverse reaction are typically viewing the situation ex post. That the program has been implemented assumes that the sum of these adverse reactions was an acceptable alternative to non-implementation, and should therefore not be allowed to undermine public confidence in the program. When one surveys the landscape of the vaccination issue, however, objections are usually of the ex post variety. Since it is harder to appreciate the absence of an epidemic than the presence of a child suffering a vaccine-related injury, it is easy to look at the issue solely ex post. In the interests of public safety, such reasoning should be avoided.
This is not to imply that all critics of mandatory vaccination are on unsound theoretical footing. In fact, those whose objections are marked by a distrust of the government authorities in charge of implementing vaccination programs can be seen as questioning only the ex ante judgment of the officials. If this is so, they are actually on firmer ground than those who object to the programs because they feel their child was harmed by the vaccine. Ex ante critiques are valuable because they can bring about change in the system at a time when it can still prove useful.
The National Vaccine Injury Compensation Program represents a theoretically sound program under these criteria. Economically, it represents the idea that some of the costs of mandatory vaccination programs known only ex post will be compensated by all those who share the benefits ex ante. The excise tax, paid ex ante by all who receive the vaccine, is used to compensate anyone who experiences certain adverse effects ex post. This is simply an example of the government distributing the costs of the vaccination program across the spectrum of those who receive the benefit, rather than an ex post complaint by those on whom the costs have fallen.
Other Issues
The modern trend toward more widely-granted exemptions represents government acquiescence toward a certain degree of free riding. Should such exemptions proliferate too widely, herd immunity may indeed be lost and a recalculation of the cost-benefit analysis of individuals will be necessary. In the face of a greater potential to contract disease, the benefit of vaccination grows significantly, while the cost of accepting the vaccine remains the same. Likewise, from the standpoint of the government, the cost of allowing widespread exemptions will eventually overtake the benefit of permitting such exemptions if that cost suddenly includes serious risk of epidemic.
The risks associated with non-vaccination can be illustrated through a rather simplified mathematical example. [201] Suppose a school with 1,000 students is exposed to a measles outbreak. 990 of the students have received all of their measles shots, and so are fully immunized. Suppose further that the measles vaccine is 99% effective; that is, it produces complete immunity in 99% of patients. [202] Therefore, 10 out of the 990 who have been fully immunized will be susceptible to the disease. In addition, all 10 of the 1,000 students who had not been fully immunized will be susceptible to measles. Therefore, 20 out of 1,000 students will get the disease. Although the number of infected students who were vaccinated is equal to the number who were not, this example demonstrates that vaccination can be very effective even if it sometimes does not produce immunity in an individual. If no one had been vaccinated, 980 more students would probably have caught the measles. It is also important to note that this example assumes an epidemic; in reality, herd immunity would probably be attained at this level of inoculation and none of the 1,000 students would have caught the disease.
IV. CONCLUSION
Vaccines have immeasurably improved our quality of life. They have led to the eradication of deadly diseases like smallpox and the near elimination of diseases such as diphtheria, polio, and measles. Outbreaks of vaccine-preventable diseases, such as mumps, are infrequent and are also quite newsworthy on the rare occasion that they do occur. And people like Rafe Hollister can survive a run-in with a rusty saw or an animal bite.
The life-saving benefits of vaccination often overshadow the vast economic and personal benefits it has helped provide. Jonas Salk’s cure for polio has spared generations from a life hindered by the devastating physical handicaps of that terrible affliction. Children no longer must miss vast stretches of school to overcome a debilitating battle with pertussis (although there is no doubt that some children lament this decline in excused absences from school). Parents no longer have to spend restless hours worrying as their children suffer the body’s natural response to disease. In economic terms, this translates directly into fewer missed hours of work and less administrative difficulty, leading to a generally more productive society.
For all the benefits of vaccines, of course, it is important not to ignore the costs. The National Vaccine Injury Compensation Program is one way of dealing with the economic costs of vaccination, but this may provide little solace to the parent of a child who has been injured by a vaccine for a disease that is seemingly in decline. Side effects with very low probability will sometimes occur; though from a community-wide view this possibility is acceptable, for the individual who experiences the adverse effect the vaccination may not have been the best medical decision. Many who view natural immunity as a rite of passage for children might not desire a means of bypassing the disease entirely.
Some may accuse public health officials of dreaming for an unreachable day when all diseases are controlled by vaccination. Zeal on the part of public health officials, however, should not overshadow the actual benefits of vaccination generally. Soon may come the day when diphtheria, like smallpox, will be eradicated globally. At that point, it can be removed from the vaccination schedule and future generations will reap the benefits of vaccination while undertaking none of the costs.
This prospect, I think, sheds light on the ultimate solution to vaccination issues that have been discussed in this paper. Highly communicable and especially terrible diseases should continue on the vaccination schedule until they are virtually eliminated. The eventual elimination of these scourges will someday make vaccination unnecessary, and the costs of vaccination will drop to zero. Until that time, officials should seek stricter enforcement of the mandatory vaccination laws and should tighten down on non-medical exemptions. At the same time, information campaigns should be considered in the interest of reminding the public of the continued importance and relevance of vaccine programs. Though risks are unavoidable when dealing with vaccines, parents should constantly be reminded that immunity depends on a high level of cooperation. This will hopefully keep immunization rates high, at least for the most harmful diseases.
Meanwhile, public health officials may be wise to consider an alternate stance toward somewhat less-important vaccines such as Hepatitis B and varicella (chickenpox). [203] With such diseases it may be worthwhile to wait longer before placing the vaccines on the recommended schedule. This will undoubtedly make herd immunity more difficult if not impossible to attain, while simultaneously announcing to parents that undertaking the vaccine in question is a personal medical decision. Most of those who choose to vaccinate (and accept the risk of adverse effects from these newer vaccines) will still acquire immunity. Without a mandatory program in place, however, one would still expect to see regular occurrences of the disease. Given the relatively high likelihood of outbreak under these circumstances, a percentage of those who vaccinate will probably get the disease. They will likely turn to those who did not vaccinate at all and see them as the cause of the outbreak. In time, social pressures may lead to greater vaccination rates, and the time may be ripe for greater acceptance of mandatory vaccination for the disease.
One significant benefit to this approach lies in its natural tendency to point out to parents the importance of receiving the more important vaccines. When some vaccines are mandatory and others are not, the distinction between the two types of vaccines is impossible to neglect. It would hopefully make parents think more carefully before attempting to gain an insincere exemption. This approach would fail to satisfy those who want parents to have the option to choose “one, ten, or no vaccines,” [204] but it would at least allow an element of choice for some vaccines while hopefully maintaining a sufficient level of immunization for the more important vaccines. It is also important to remember that parents with serious reservations about any vaccines will usually have the option of home schooling. Overall, this approach might have the advantage of winning over those who only partially object to the vaccination schedule, thus helping bring about a greater chance of herd immunity for diseases associated with less objectionable vaccines.
Vaccination certainly is unique among medical treatments, both for its incredible potential and its coercive nature. It is unfortunate that questionable evidence has led many concerned parents to question the wisdom of vaccination programs that still serve important goals. Given the importance of public support for the achievement of these goals, however, public health officials must account for sometimes questionable concerns in determining vaccination policy. Greater information dissemination, combined with more sharply drawn (and potentially vaccine-specific) guidelines, can hopefully further the important goals of vaccination policy.
[1] Center for Disease Control, “Smallpox Disease Overview,” at http://www.bt.cdc.gov/agent/smallpox/overview/disease-facts.asp (last visited April 27, 2006).
[2] GlaxoSmithKline is currently developing an ear infection vaccine and plans to seek regulatory approval shortly. Jessica Said, “Vaccine Could End Children’s Ear Infections,” CNN online article, March 3, 2006 (on file with author).
[3] Institute of Medicine. CP Howson, et al. eds. Adverse Effects of Pertussis and Rubella Vaccines. Washington, DC: National Academy Press; 1991, at 1.
[4] James G. Hodge, Jr. and Lawrence O. Gostin, School Vaccination Requirements: Historical, Social, and Legal Perspectives , 90 Ky. L. J. 831, 867 (2001).
[5] See, e.g., Jacobson v. Massachusetts , 197 U.S. 11 (1905).
[6] Hodge and Gostin, supra note 4, at 867.
[7] Id. at 868.
[8] “Rather than having health departments require immunization in emergency conditions, legislatures acted to prevent disease by mandatory immunization as a condition of enrollment or attendance in schools or licensed day care facilities.” Id.
[9] See id. ; see also infra Part I (chart describing the current recommended vaccination schedule).
[10] The Center for Disease Control has gone so far as to suggest that “to have a medical intervention as effective as vaccination in preventing disease not use it would be unconscionable.” Center for Disease Control, National Immunization Program publication, “Six Common Misconceptions About Vaccination and How to Respond to Them,” at http://www.cdc.gov/nip/publications/6mishome.htm (last visited April 27, 2006) (hereinafter “Six Common Misconceptions”).
[11] Center for Disease Control, National Immunization Program publication, “Ten Things You Need to Know about Immunizations,” at http://www.cdc.gov/nip/publications/fs/gen/shouldknow.htm (last visited April 27, 2006).
[12] This is not to imply that parents who vaccinate without carefully considering the pros and cons of vaccination are in the wrong. The health and safety of a child is of paramount importance to most parents, and every parent must make decisions that affect the welfare of the child. Most parents approach such decisions with a sincere desire to promote the child’s best interests, and this desire is no different in the context of vaccination.
[13] “Despite its utility, vaccination has provoked popular resistance from the beginning.” Hodge and Gostin, supra note 4, at 834.
[14] “Some opponents express valid scientific objections about effectiveness or need for mass vaccinations; some fear harmful effects arising from the introduction of foreign particles into the human body; and others worry that vaccination actually transmits, rather than prevents, disease, or weakens the immune system.” Id.
[15] See, e.g. , Jacobson v. Massachusetts , 197 U.S. 11 (1905) (constitutional challenge to government mandated smallpox vaccination); “Six Common Misconceptions,” supra note 10 (“[s]ome see mandatory vaccination as interference by the government into what they believe should be a personal choice”).
[16] “Six Common Misconceptions,” supra note 10.
[17] A more detailed explanation of this subject appears in Part I of this paper.
[21] See, e.g. , “Ten Things You Need to Know about Immunizations,” supra note 11.
[22] Angie A. Welborn, “Mandatory Vaccinations: Precedent and Current Laws,” CRS Report for Congress, at http://www.fas.org/sgp/crs/RS21414.pdf (last updated Jan. 18, 2005).
[23] For a typical scenario of public health response to outbreak, see the facts of Jacobson v. Massachusetts , 197 U.S. 11 (1905).
[24] Hodge and Gostin, supra note 4, at 833-34.
[25] Id. at 867-68.
[26] Id. at 833.
[29] This level of immunity is often referred to as “herd immunity,” the concept that not everyone in a population must be vaccinated in order for the entire population to be protected. Abi Berger, “How Does Herd Immunity Work?” 319 BMJ 1466 (1999). “As long as a sufficient number of children are immunised against each disease for which there is a vaccine, protection against that disease will be conferred on everybody.” Id. Also, the level of vaccination necessary to attain herd immunity increases as the infectivity of the disease increases. Id. Highly infectious diseases, therefore, require higher levels of immunity for herd immunity to occur. Id. The concept of herd immunity will arise throughout this paper, with particular emphasis in Part III.
[30] This is evidenced by the fact that in time of outbreak, the vaccinated population can still be susceptible to the disease, although usually the vaccinated population is far less susceptible to the disease than the unvaccinated population. Vaccines typically produce the desired antibody in an individual around 90% of the time, with actual percentages varying from vaccine to vaccine. Some vaccines, moreover, lose their efficacy and require boosters. These concepts will be further developed throughout this paper.
[31] Hodge and Gostin, supra note 4, at 850-51.
[32] Id. at 851.
[33] Id. at 834.
[34] Id. at 834-35.
[35] See, e.g. , Duffield v. Sch. Dist. , 29 A. 742 (Penn. 1894).
[36] 197 U.S. 11 (1905).
[38] Id. at 26.
[39] Welborn, supra note 22.
[41] 260 U.S. 174 (1922).
[42] Id. at 175.
[43] Id. (“[t]he bill charges that there was then no occasion for requiring vaccination” and that the ordinances “in effect, mak[e] vaccination compulsory”).
[45] Id. at 176.
[48] Id. at 177.
[49] Hodge and Gostin, supra note 4, at 867-68.
[50] Id. at 868.
[51] The schedule of immunizations is published by the Center for Disease Control, and follows the recommendations of the Advisory Committee on Immunizations Practices, the American Academy of Pediatrics’ Committee on Infectious Diseases, and the American Academy of Family Physicians. Id.
[52] Id. at 869.
[54] Based on chart publicized by Center for Disease Control, approved by Advisory Committee on Immunization Practices, American Academy of Pediatrics, American Academy of Family Physicians, available at http://www.cispimmunize.org/IZSchedule_2006.pdf (last visited April 27, 2006).
[55] Indeed, the law in Jacobson was challenged for this reason.
[56] Statement of Barbara Fisher, founder of National Vaccine Information Center, quoted in Neenyah Ostrom, “First Do No Harm,” at http://www.chronicillnet.org/online/Fisher.html (last visited April 27, 2006).
[57] K.N.O.W. Vaccines, Vaccine Awareness of Florida fact sheet, at http://www.know-vaccines.org/vaccine_fact.html (last visited April 27, 2006).
[58] The most direct way in which this occurs surrounds the concept of herd immunity, as discussed elsewhere throughout this paper. If a sufficient number of persons in the community does not vaccinate, herd immunity may be unattainable and others may be put at risk.
[59] See, e.g. , the discussion in Part III involving The Andy Griffith Show.
[60] See Statement of Barbara Fisher, quoted in Ostrom, supra note 56. See also “Autism and Vaccines: Activists Wage a Nasty Campaign to Silence Scientists,” Wall Street Journal, February 16, 2004, at http://www.opinionjournal.com/forms/printThis.html?id=110004700 (last visited April 27, 2006) (citing vaccination critics who had accused the vaccination-defending writers of “having an ‘industry profit promoting agenda’”).
[61] See Statement of Barbara Fisher, quoted in Ostrom, supra note 56.
[64] See the discussion in Part II regarding vaccine safety.
[65] See, e.g. , “Six Common Misconceptions,” supra note 10.
[66] Mission Statement of Vaccination News website, at http://www.vaccinationnews.com (last visited April 27, 2006).
[67] As the discussion in Part II on vaccine safety demonstrates, pre-licensing testing for very rare adverse effects cannot take place if vaccines are ever to reach the market. Phase 4 post-licensing testing does exist, but may take years to discover extremely rare adverse effects.
[68] World Health Organization Immunization Safety page, “Adverse Events Following Immunization,” at http://www.who.int/immunization_safety/aefi/en/ (last visited April 27, 2006).
[69] As the recent mumps outbreak in Iowa demonstrates, not everyone who receives a vaccine develops immunity to the disease. For this reason, the success of vaccination depends on a sufficient level of vaccination in the community. When a significant percentage of the population has not received the vaccine, an outbreak can occur and even threaten some of those who have been vaccinated. See David Pitt, “Iowa Mumps Epidemic Continues to Broaden,” Associated Press, April 13, 2006, at http://www.breitbart.com/news/2006/04/13/D8GVGL600.html (last visited April 27, 2006). See also the above discussion of the history of vaccination.
[70] Mission Statement of Vaccination News website, supra note 66.
[71] See, e.g. , Ross D. Silverman, “No More Kidding Around: Restructuring Non-Medical Childhood Immunization Exemptions to Ensure Public Health Protection,” 12 Annals Health L. 277, 278-79 (2003).
[A]s risks of contracting many deadly and crippling diseases continue to decline to near negligible levels, and rates of childhood immunization continue to reach record levels, the public today places greater attention on the relative weaknesses and dangers of immunizations, and the systems through which they are administered.
[72] Hodge and Gostin, supra note 4, at 874.
[73] Usually this requires physician certification. Id.
[74] Indeed, the CDC itself presupposes the existence of medical exemptors in any broad mandatory vaccination program. See “Six Common Misconceptions,” supra note 10 (noting that the mandatory vaccination program can work to protect even those few who cannot vaccinate because of the possibility of adverse medical reactions).
[76] Hodge and Gostin, supra note 4, at 874.
[77] Jacobson v. Massachusetts , 197 U.S. 11 (1905). See also Employment Division v. Smith , 494 U.S. 872 (1990) (permitting neutral laws of general applicability that incidentally affect religion); Boone v. Boozman , 217 F.Supp.2d 938 (E.D. Ark. 2002) (“constitutionally-protected free exercise of religion does not excuse an individual from compulsory immunization...the right to free exercise of religion and parental rights are subordinated to society’s interest in protecting against the spread of disease”).
[78] W. Va. Code Sec. 16-3-4 (2004).
[79] See, e.g. , Boone v. Boozman , 217 F.Supp.2d 938 (E.D. Ark. 2002). The challenged Arkansas immunization statute exempted “individuals for whom ‘immunization conflicts with the religious tenets and practices of a recognized church or religious denomination of which [they are] an adherent or member.’” The statute was struck down under the Establishment Clause using the test laid out in Lemon v. Kurtzman , 403 U.S. 602 (1971). 217 F.Supp.2d at 950. The Arkansas legislature subsequently amended the exemption generally to allow for religious or philosophical objections without regard to recognized churches. Ark. Code Sec. 6-18-702(d).
[80] See Silverman, supra note 71, at 290-93.
[81] See id.
[82] Hodge and Gostin, supra note 4, at 874.
[83] Cal. Health and Safety Code Sec. 120365 (2003).
[84] Silverman, supra note 71, at 284.
[85] Id. at 285.
[87] See id.
[88] Recall that for those unable to vaccinate for medical reasons, herd immunity provides the only protection from the disease. See “Six Common Misconceptions,” supra note 10.
[89] Silverman, supra note 71, at 285.
[90] Id. The recent mumps outbreak may directly demonstrate this. Officials have pointed out that vaccination only confers immunity on 95% of patients, and of those affected in the recent outbreak, 25% have been vaccinated. See Pitt, supra note 69. The strong implication is that the 75% of those inflicted who were not vaccinated have put the entire community at risk.
[91] Center for Disease Control, National Immunization Program publication, “What Would Happen If We Stopped Vaccinations?” at http://www.cdc.gov/nip/publications/fs/gen/WhatIfStop.htm (last visited April 27, 2006).
[92] Silverman, supra note 71, at 293.
[93] Silverman suggests that eliminating philosophical and religious exemptions would do more harm than good. This approach, he believes, “would exacerbate feelings of animosity and skepticism toward vaccination and the public health system in general.” Id. at 293. On this score he is probably correct, and I agree that wider knowledge, at the very least, is a better initial response to this problem.
[94] Incidentally, it is worth mentioning that of the more longstanding vaccines, the tetanus vaccine stands out as unique. Tetanus is a very harmful disease with about a 20% fatality rate. “What Would Happen If We Stopped Vaccinations?” supra note 91. What makes it unique in the vaccine schedule is that tetanus is not contagious. That is, herd immunity is not attainable and cannot be used to justify mandatory tetanus vaccination. The reason for the general acceptance of the tetanus vaccine seems to stem both from the high risk of the disease and the fact that tetanus can only be prevented by immunization. In addition, the tetanus vaccine for infants has been combined with the vaccines for diphtheria and pertussis. On strictly public health grounds, however, the status of the tetanus shot on the compulsory vaccination schedule comes closest to government fiat of individual health decisions.
[95] Because medical risks may vary from vaccine to vaccine, and thus the justification for such exemptions remains even if the risk is to some but not all vaccines, medical exemptions are somewhat outside the scope of this discussion.
[96] Sean Coletti, Taking Account of Partial Exemptors in Vaccination Law, Policy, and Practice , 36 Conn. L. Rev. 1341, 1344 (2004).
[98] This follows directly from the all-or-nothing nature of the vaccination decision in this world.
[99] Again, this follows directly from the nature of the decision.
[100] See, e.g. , National Vaccine Information Center, at http://www.nvic.org (last visited April 27, 2006) (urging parents to consider eight questions before vaccinating, none of which inform parents of the effect their decision may have on others).
[101] See “Six Common Misconceptions,” supra note 10.
[102] 42 U.S.C. §§ 300aa-1 to 300aa-34.
[103] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research, “Vaccine Product Approval Process,” updated July 27, 2002, at http://www.fda.gov/cber/vaccine/vacappr.htm (last visited April 27, 2006) (hereinafter “Vaccine Product Approval Process”).
[104] See id.
[105] See, e.g. , Isadora Stehlin, “How FDA Works to Ensure Vaccine Safety,” FDA Consumer magazine (December 1995), at http://www.fda.gov/fdac/features/095_vacc.html (last visited April 27, 2006).
[106] “Licensing of a vaccine is only the beginning of FDA’s oversight.” Id.
[109] “Vaccine Product Approval Process,” supra note 103.
[112] Stehlin, supra note 105.
[115] Id. ; “Vaccine Product Approval Process,” supra note 103.
[116] Stehlin, supra note 105.
[118] “Vaccine Product Approval Process,” supra note 103.
[121] Id. ; Stehlin, supra note 105.
[123] Indeed, the National Immunization Program has confidently pointed to the FDA’s role in continued oversight of vaccines:
FDA would recall a lot of vaccine at the first sign of problems. There is no benefit to either the FDA or the manufacturer in allowing unsafe vaccine to remain on the market. The American public would not tolerate vaccines if they did not have to conform to the most rigorous safety standards. The mere fact that a vaccine lot [is] still in distribution says that the FDA considers it safe.
“Six Common Misconceptions,” supra note 10.
[124] “Vaccine Product Approval Process,” supra note 103.
[127] Stehlin, supra note 105.
[128] So states Susan Ellenberg, Ph.D., director of CBER’s division of biostatistics and epidemiology. Id.
[129] “Vaccine Product Approval Process,” supra note 103.
[130] Stehlin, supra note 105.
[136] See, e.g. , “Six Common Misconceptions,” supra note 10 (“[o]nly some of the reported health conditions are side effects related to vaccines. A certain number of VAERS reports of serious illnesses or death do occur by chance alone among persons who have been recently vaccinated”).
[137] “VAERS reports have many limitations since they often lack important information, such as laboratory results, used to establish a true association with the vaccine.” Id.
[138] “In summary, scientists are not able to identify a problem...based on VAERS reports alone without scientific analysis of other factors and data.” Id.
[139] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research, “Thimerosal in Vaccines,” at http://www.fda.gov/Cber/vaccine/thimerosal.htm (last updated Sept. 6, 2005).
[141] See, e.g. , Gardiner Harris and Anahad O’Connor, “On Autism’s Cause, It’s Parents vs. Research,” New York Times, June 25, 2005, at http://www.nytimes.com/2005/06/25/science/25autism.html (last visited April 27, 2006) (reporting the ongoing tension between parents of autistic children and the medical community over the use of thimerosal in vaccines).
[142] See, e.g. , Center for Disease Control, National Immunization Program publication, “Mercury and Vaccines (Thimerosal),” at http://www.cdc.gov/nip/vacsafe/concerns/thimerosal/default.htm (last visited April 27, 2006) (studies have failed to find any association between exposure to thimerosal in vaccines and autism); “On Autism’s Cause, It’s Parents vs. Research,” supra (noting that the amount of ethyl mercury in each childhood vaccine was once about the same as the amount of methyl mercury, a more toxic compound, found in an average tuna sandwich).
[143] “Thimerosal in Vaccines,” supra note 139.
[146] Stehlin, supra note 105.
[149] “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[150] “Thimerosal in Vaccines,” supra note 139; see also “On Autism’s Cause, It’s Parents vs. Research,” supra note 141 (“[b]y 2001, no vaccine routinely administered to children in the United States had more than half a microgram of mercury – about what is found in an infant’s daily supply of breast milk”).
[151] “Autism and Vaccines: Activists Wage a Nasty Campaign to Silence Scientists,” Wall Street Journal editorial, February 16, 2004, at http://www.opinionjournal.com/forms/printThis.html?id=110004700 (last visited April 27, 2006).
[152] “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[153] Indeed, one recent study has suggested that neurological disorders have decreased with the removal of thimerosal from most vaccines. See David A. Geier and Mark R. Geier, “Early Downward Trends in Neurodevelopmental Disorders Following Removal of Thimerosal-Containing Vaccines,” 11 J. Am. Physicians and Surgeons 8 (2006). This study should be taken with a grain of salt, however, as the Geiers are widely known thimerosal critics. Years before this study, Dr. Mark Geier called thimerosal use in vaccines the world’s “greatest catastrophe that’s ever happened, regardless of cause.” “On Autism’s Cause, It’s Parents vs. Research,” supra note 141. A witness in many vaccine cases, a judge once ruled that he was “a professional witness in areas for which he has no training, expertise and experience.” Id. Scientists have criticized his prior studies and even called his methods “voodoo science.” Id.
[155] See id.
[156] See “The Politics of Autism: Lawsuits and Emotion vs. Science and Childhood Vaccines,” Wall Street Journal editorial, Dec. 29, 2003, at http://www.opinionjournal.com/forms/printThis.html?id=110004487 (last visited April 27, 2006) (characterizing the position of thimerosal critics as “scientifically untenable”).
[157] See generally “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[161] “The Politics of Autism,” supra note 156.
[162] Id. ; “Study: Autism Rise from Labeling, Not Epidemic,” April 3, 2006, at http://www.cnn.com/2006/EDUCATION/04/03/health.autism.reut/index.html (last visited April 27, 2006) (noting rise in diagnosed cases of autism since 1994 is correlated with fall in diagnosed cases of mental retardation and learning disabilities).
[163] The Politics of Autism,” supra note 156.
[164] See, e.g. , “Six Common Misconceptions,” supra note 10 (noting that many anti-vaccine publications claim vaccines are unsafe on the basis of sheer numbers of reports to VAERS without noting that many of them may not represent actual vaccine side-effects).
[165] National Vaccine Information Center, “The Vaccine Injury Compensation Program,” at http://www.909shot.com/Issues/Comp_Summary.htm (last visited April 27, 2006).
[166] Center for Disease Control, National Vaccine Program Office, Vaccine Fact Sheets, “National Vaccine Injury Compensation Program,” at http://www.hhs.gov/nvpo/factsheets/fs_tableIV_doc1.htm (last visited April 27, 2006).
[167] See National Vaccine Injury Compensation Program, at http://www.hrsa.gov/vaccinecompensation/ (last visited April 27, 2006).
[169] The Andy Griffith Show: The County Nurse (CBS television broadcast, March 19, 1962).
[177] Id. That is, there will be a high probability of death.
[179] See the discussion above in Part I of this paper.
[180] For example, she begs Rafe to consider his family and what his decision could mean to them. She literally appears to be on the verge of tears as he refuses.
[181] In utilizing the theoretical framework of economic analysis, it is useful to keep in mind a few foundational concepts. First, a policy or program (in this case mandatory vaccination) is desirable if the overall benefit to society as a whole outweighs the cost of the program, where benefits and costs include both monetary and non-monetary factors. Second, individuals making rational choices regarding vaccination will vaccinate when the benefits of vaccination outweigh the risks or costs of non-vaccination to the individual. This decision-making process can be skewed by externalities, such as an unforeseeable decrease in the effectiveness of a vaccine due to a reduction in vaccination by others unknown to the individual at the time of the decision.
[182] 197 U.S. 11 (1905).
[183] Id. at 12.
[185] Id. at 13, 22.
[186] Id. at 36.
[187] Id. at 12.
[188] As the CDC itself explains, “[e]ven one serious adverse effect in a million doses of vaccine cannot be justified if there is no benefit from the vaccination.” “Six Common Misconceptions,” supra note 10.
[189] In Japan in the 1970s, for instance, pertussis vaccination coverage fell from 80% to 20%, leading to an outbreak in 1979 resulting in 13,000 cases and 41 deaths. “What Would Happen If We Stopped Vaccinations?” supra note 91.
[190] Judge Richard Posner has suggested that this difference between sexually transmitted diseases and air- and water-borne diseases may imply a lesser imperative to eliminate sexually transmitted diseases:
[T]he externality created by sexually transmitted diseases is smaller than in the case of other contagious diseases. Sexually transmitted disease is spread primarily by voluntary contact, implying (to the economist) that a person is compensated...for assuming the risk of contracting the disease. Hence the number of cases of sexually transmitted diseases may be closer to the optimum than in the usual air-borne or water-borne or insect-borne epidemics.
Posner, Economic Analysis of Law 162. (6th Ed. 2003).
[191] Additionally, if vaccination rates are high, these individuals may assume that those in society who have already made the choice to vaccinate have performed a similar cost-benefit analysis. These individuals choose to vaccinate based simply on vaccination rates in the community. See John C. Hershey et al., The Roles of Altruism, Free Riding, and Bandwagoning in Vaccination Decisions , 59 Organizational Behavior and Human Processes 177, 178 (1994).
[192] See, e.g. , id. (behavioral survey studying various factors individuals use to make vaccination decisions).
[194] See id. at 178 (“[i]f a patient believes vaccination is in his own best interests, then he has two reasons to vaccinate. One is selfish, in that he will improve his own well being. The other is altruistic, in that he can improve the health prospects of those around him who might otherwise become infected if he is not vaccinated himself”).
[195] The concept of herd immunity is discussed in Part I. Note that “[i]n economic terms, herd immunity is a positive externality of vaccination. Altruistic individuals who recognize and value this externality may undergo vaccination partly to help others in addition to themselves.” Id. See also Berger, supra note 29 (“‘[h]erd immunity’...is the concept that not everybody in a population has to be immunised to protect everyone in that population. As long as a sufficient number of children are immunised against each disease for which there is a vaccine, protection against that disease will be conferred on everybody”).
[196] The CDC has pointed to this as one of the two most important reasons to vaccinate:
There is a small number of people who cannot be vaccinated (because of severe allergies to vaccine components, for example), and a small percentage of people don’t respond to vaccines. These people are susceptible to disease, and their only hope of protection is that people around them are immune and cannot pass disease along to them. A successful vaccination program, like a successful society, depends on the cooperation of every individual to ensure the good of all.
[197] In economic terms, “[w]idening vaccine use decreases each individual’s benefit from being vaccinated, but leaves unchanged each individual’s risk from the vaccination itself.” Hershey, supra note 191, at 178.
[198] “Six Common Misconceptions, supra note 10.
[199] Hershey, supra note 191, at 178.
[200] Suppose, for sake of example, that a vaccination program, if implemented, would save ten lives out of a thousand that would otherwise have perished without the program. Unfortunately, the vaccine will randomly cause death to five persons out of a thousand. From an ex ante perspective, the vaccination program should be implemented as it will save five lives overall. Concerns or complaints from those five persons who die (or their estates) represent ex post objections, and, though unfortunate, should not affect evaluations of the soundness of the program.
[201] This mathematical explanation is a slight variation of that found at CDC, “Six Common Misconceptions,” supra note __.
[202] Note that no vaccine is 100% effective, and vaccination efficacy rates for most childhood vaccinations range from 85 to 95%. Id. As stated in an earlier section, herd immunity is relied upon to protect those who do not develop full immunity from the vaccine.
[203] Given that these particular vaccines are already on the schedule, I think it would be unwise to remove them now. My analysis applies to comparable vaccines that may arise in the future – vaccines for those communicable diseases that do not pose relatively significant health risks. The definition of such diseases, of course, would be a matter of debate. Vaccines for noncommunicable diseases like ear infections would also fall within this rubric.
[204] Statement of Barbara Fisher, quoted in Ostrom, supra note 56.

The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies (2013)
Chapter: 7 conclusions and recommendations.
Conclusions and Recommendations
COMMITTEE RESPONSE TO ITS STATEMENT OF TASK
This final chapter highlights selected findings and conclusions and presents recommendations for each section of the committee’s statement of task. The preceding chapters, especially Chapter 6 , include many assessments that may be construed as the committee’s preferences among the alternatives presented but that fall short of formal recommendations.
Vaccine safety is critically important, but a determination of safety is ultimately a value judgment. For example, some might believe that a serious adverse event that occurs once in 1 million doses is “safe enough” relative to the benefit of preventing a serious disease, whereas others may consider that risk unacceptably high. The committee did not set a specific numerical target or goal for what should be considered “safe enough.” Instead, the committee made a judgment based on the literature that failed to link adverse effects to schedule exposures or multiple immunizations, concluding that there is no evidence that the schedule is not safe.
The committee recognized that final decisions about research studies must await knowledge of further evidence, including biological plausibility and/or epidemiological evidence, feasibility, cost, and the exact circumstances of stakeholder concerns, before the planning and conduct of specific research projects. In turn, the committee believes that it would be inappropriate to make unqualified recommendations without this knowledge. The committee notes that stakeholder concerns may be used to drive a search for scientific evidence (biological or epidemiological), although such concerns would not be sufficient motivation to embark
on costly clinical research, such as new randomized controlled trials or cohort studies.
The committee thus decided to make five general recommendations. Three recommendations focus on improvements to understanding stakeholder concerns, harmonizing research methods, and sequencing the process for selecting research questions. Two recommendations focus on research methods, including randomized controlled trials and data systems that would enable ongoing and improved observational studies.
Statement of Task (Part I): Review scientific findings and stakeholder concerns related to the safety of the recommended childhood immunization schedule.
Summary of Stakeholder Concerns
The committee’s findings and conclusions about stakeholder concerns are presented in Chapter 4 . Although the committee identified the concerns of some parents about the number, frequency, and timing of immunizations in the overall immunization schedule, the committee did not find in its literature review that clinicians, public health personnel, or policy makers have similar safety concerns. Among the latter groups, the childhood immunization schedule is considered to be among the most effective and safe of the public interventions available to prevent serious disease and death. However, although health care professionals have much information about individual vaccines, they have much less information about the effects of administration of multiple vaccines at a single visit or the timing of the immunizations. Additionally, the cited concerns of health care professionals include efficacy of certain vaccines as well as appropriate delivery and communication regarding the recommended childhood immunization schedule.
Although the 2010 National Vaccine Plan addresses the need to provide health care providers with more timely, accurate, and transparent information about the benefits and risks of vaccines, the plan does not specifically address strategies to assist providers with questions about the safety of the immunization schedule (HHS, 2010). The committee concluded that parents and health care professionals would benefit from more comprehensive and detailed information with which to address parental concerns about the safety of the immunization schedule. Such information should clearly address vaccine-preventable diseases, the risks and benefits of immunizations, and the safety of the immunization schedule.
The committee’s literature review highlighted the lack of high-quality evidence supporting stakeholder concerns (the priority stakeholders are listed in Box 4-1 ) about the immunization schedule. In its role to ensure
vaccine safety, the federal government has already prioritized the engagement of stakeholders in multiple activities, as detailed in the 2010 National Vaccine Plan and implementation efforts, as well as the Centers for Disease Control and Prevention’s Immunization Safety Office scientific agenda (CDC, 2011; HHS, 2010). However, an effective national vaccine program will require more complete information on stakeholder concerns about the safety of the immunization schedule, the severity of vaccine-preventable diseases, individual- and population-level immunization rates, vaccine efficacy, and the delivery and supply of vaccines recommended in the childhood immunization schedule. Improved communication between public health authorities and parents requires improvements to the clarity of the information provided, as well as the building of trust and the use of a systematic approach to elicit public concerns. Further research into the type of questions that parents seek to answer by the use of the scientific methods of social, behavioral, and decision science is indicated.
On the basis of the committee’s literature review and public testimony, the committee strongly endorses the need for research to understand the public’s knowledge, beliefs, and concerns about vaccines and vaccine-preventable diseases in particular, which is a key strategy in the 2010 National Vaccine Plan (HHS, 2010). It must be acknowledged that the methods used in most immunization studies do not permit a detailed analysis of the impact of parental concerns on the decision to immunize their children. Although the committee found that the largest safety concerns exist among a subset of parents, the concerns of multiple stakeholders should be included as part of the efforts of the National Vaccine Program Office (NVPO). For example, health care providers have much knowledge about individual vaccines but less information about the effects of administering multiple vaccines at a single visit or the timing of the immunizations.
Recommendation 4-1: The committee recommends that the National Vaccine Program Office systematically collect and assess evidence regarding public confidence in and concerns about the entire childhood immunization schedule, with the goal to improve communication with health care professionals, and between health care professionals and the public regarding the safety of the schedule.
Summary of Scientific Findings
The committee’s findings and conclusions about the safety of the immunization schedule on the basis of the information in the scientific literature are presented in Chapter 5 . The committee encountered two major issues. First, the concept of the immunization “schedule” is not well developed in the scientific literature. Most vaccine research focuses on the health outcomes associated with single immunizations or combinations of vaccines
administered at a single visit. Even though each new vaccine is evaluated in the context of the overall immunization schedule that existed at the time of review, individual elements of the schedule are not evaluated once it is adjusted to accommodate a new vaccine. Key elements of the immunization schedule—for example, the number, frequency, timing, order, and age at the time of administration of vaccines—have not been systematically examined in research studies.
The second major issue that the committee encountered during the review of the scientific literature was uncertainty over whether the scientific literature has addressed all health outcomes and safety concerns. The committee could not determine whether its list of health outcomes was complete or whether a more comprehensive system of surveillance might identify other outcomes of potential safety significance. In addition, the conditions of concern to some stakeholders, such as immunological, neurological, and developmental problems, are illnesses and conditions for which the etiology, in general, is not well understood. Further research on these conditions may clarify their etiologies.
Finally, the committee found that evidence from assessments of health outcomes in potentially susceptible subpopulations of children who may have an increased risk of adverse reactions to vaccines (such as children with a family history of autoimmune disease or allergies or children born prematurely) was limited and is characterized by uncertainty about the definition of populations of interest and definitions of exposures and outcomes. Most children who experience an adverse reaction to immunization have a preexisting susceptibility. Some predispositions may be detectable prior to vaccination; others, at least with current technology and practice, are not (IOM, 2012, p. 82).
In summary, to consider whether and how to study the safety and health outcomes of the entire childhood immunization schedule, the field needs valid and accepted metrics of the entire immunization schedule (the “exposure”) and clearer definitions of health outcomes linked to stakeholder concerns (the “outcomes”) in rigorous research that will ensure validity and generalizability.
Recommendation 5-1: To improve the utility of studies of the entire childhood immunization schedule, the committee recommends that the National Vaccine Program Office develop a framework that clarifies and standardizes definitions of
- key elements of the schedule ,
- relevant health outcomes, and
- populations that are potentially susceptible to adverse events.
Statement of Task (Part II): Identify potential research approaches, methodologies, and study designs that could inform this question, including an assessment of the potential strengths and limitations of each approach, methodology, and design, as well as the financial and ethical feasibility of doing them.
Summary of Methodological Issues
The committee’s findings and conclusions about research approaches are presented in Chapter 6 . The committee parsed the phrase “this question” in Part 2 of the statement of task into four broad research questions in Box 7-1 .
The committee then discussed general research approaches with the potential to answer these questions: ongoing research with data from existing data systems, research with enhanced data from existing data systems, prospective observational studies, and randomized controlled trials. The committee also recognized that to advance the knowledge about the safety
BOX 7-1 Leading Research Questions of Interest to Select Stakeholders
- How do child health outcomes compare between those who receive no vaccinations and those who receive the full currently recommended immunization schedule?
- How do child health outcomes compare between (a) those who receive the full currently recommended immunization schedule and (b) those who omit specific vaccines?
- For children who receive the currently recommended immunization schedule, do short- or long-term health outcomes differ for those who receive fewer immunizations per visit (e.g., when immunizations are spread out over multiple occasions), or for those who receive their immunizations at later ages but still within the recommended ranges?
- Do potentially susceptible subpopulations—for example, children from families with a history of allergies or autoimmune diseases— who may experience adverse health consequences in association with immunization with the currently recommended immunization schedule exist?
of the immunization schedule, certain enhancements to the research infrastructure will be needed, as detailed in Chapter 6 .
The committee recognizes that the establishment of priorities for research will be a challenge. Thus, the committee proposes a process for setting priorities that recognizes stakeholder concerns and establishes these priorities on the basis of epidemiological and other evidence (based on formal systematic reviews), biological plausibility, and feasibility.
Before the U.S. Department of Health and Human Services (HHS) initiates further research on the entire immunization schedule through its agencies—most notably CDC, FDA, the National Institutes of Health, and NVPO—the biological plausibility of the association of a particular outcome with an aspect of the immunization schedule must be thoroughly reviewed. Along these lines, previous IOM vaccine safety committees have assessed the mechanisms by which vaccines potentially cause adverse events by identifying and evaluating the clinical and biological evidence (from human, animal, and in vitro studies) for individual vaccines. Furthermore, the recent IOM Committee to Review Adverse Effects of Vaccines developed categories for a mechanistic assessment of the weight of the evidence. Each assessment considers clinical information from case reports and clinical and experimental evidence from other sources (IOM, 2012).
Recommendation 6-1: The committee recommends that the Department of Health and Human Services incorporate study of the safety of the overall childhood immunization schedule into its processes for setting priorities for research, recognizing stakeholder concerns, and establishing the priorities on the basis of epidemiological evidence, biological plausibility, and feasibility.
The decision to initiate further studies should be based on an evaluation of three considerations that the committee identified through its review of stakeholder concerns and scientific findings:
- epidemiological evidence of potential adverse health outcomes associated with elements of the immunization schedule (such as postmarketing signals or indications of elevated risk from observational studies);
- biological plausibility supporting hypotheses linking specific aspects of the immunization schedule with particular adverse health outcomes; and
- concern about the immunization schedule’s safety expressed by stakeholders, which should initiate efforts to explore the two previous considerations.
The committee acknowledges the evidence that reducing vaccine coverage is associated with increases in vaccine-preventable disease and found only inconsistent and anecdotal evidence to imply that the recommended immunization schedule is not safe. Furthermore, existing systems for the detection of adverse events provide confidence that the existing childhood immunization schedule is safe, and the committee recognizes that the federal government invests considerable resources to ensure vaccine safety. Nevertheless, some stakeholders have suggested that further work is warranted, such as a comparison of vaccinated children with unvaccinated children or children receiving immunizations on alternative immunization schedules.
The committee supports the National Vaccine Advisory Committee Safety Working Group statement that “the strongest study design, a prospective, randomized clinical trial that includes a study arm receiving no vaccine or vaccine not given according to the current recommended schedule, would be unethical and therefore cannot be done” (NVAC, 2009, p. 38). In Chapter 6 , the committee presents the formidable ethical and feasibility problems associated with the conduct of randomized controlled trials of children who receive all recommended immunizations and children who receive none of them and randomized controlled trials of children who receive all recommended immunizations and children who receive the recommended immunization on an alternative schedule. There are very low observed rates of adverse events with vaccination, which is another factor sffecting feasibility of a randomized controlled trial. Because of these problems, the committee concludes that a randomized controlled trial comparing the recommended schedule with any alternative schedule would be unethical and infeasible and could increase the risk of vaccine-preventable diseases in individuals and in the community.
Furthermore, the committee found that a trial of a modified version of the ACIP schedule—one that would disperse the timing of vaccinations so that children are visiting health care professionals more often but receiving fewer shots at each visit—would be ethical; however, it would add substantial costs to both parents and providers and, moreover, may be unacceptable to insurers if its effectiveness—measured as a decreased rate of adverse safety outcomes—was negligible. This modified schedule would provide immunizations within the time intervals approved by ACIP and would address the concern about immunization with too many vaccines at one office visit, but the committee did not view this option to be feasible for study.
In light of the ethical and feasibility requirements and the available evidence, the committee concludes that new randomized controlled trials of the childhood immunization schedule are not justified at this time.
Recommendation 6-2: The Department of Health and Human Services should refrain from initiating randomized controlled trials of the childhood
immunization schedule that compare safety outcomes in fully vaccinated children with those in unvaccinated children or those vaccinated by use of an alternative schedule.
The committee also reviewed opportunities to study groups that choose not to vaccinate their children by use of a prospective cohort study design. However, such a study would not conclusively reveal differences in health outcomes between unimmunized and fully immunized children for two main reasons. First, the sample populations often suggested for study (such as some religious populations) may be too small to adequately power such a comparative analysis, particularly for very rare adverse health outcomes. Such a study would also need to account for the many confounding variables that separate these naturally occurring unimmunized populations from the average U.S. child, including lifestyle factors and genetic variables.
The committee finds that secondary analyses of existing systems are more promising approaches to examination of the research questions that the committee identified in future studies of the childhood immunization schedule. The Vaccine Safety Datalink (VSD) is a useful collaborative project that could conduct both postmarketing surveillance and longer-term targeted research. The ability to augment routinely collected administrative data in VSD with data from parent interviews and reviews of medical records for a selected study population is an important strength.
VSD is currently the best available system for studying the safety of the immunization schedule in the United States. VSD should strive to improve the generalizability of its data to the U.S. population as a whole by enhancing the quality of its demographic information and by expanding its scope to include more diversity in its study populations. Secondary analyses with data from other existing databases (that might be modeled on VSD) could be a feasible, ethical, and cost-effective means of investigating several research questions that the committee identified. The committee recognizes that the commitment to VSD studies by the managed care organizations currently receiving funding through VSD needs to be sustained to continue to build on existing efforts. The committee concludes that VSD is a valuable component of the federal research infrastructure and will be the best-suited source of data for studying the childhood immunization schedule. Its utility will be expanded with the addition of more detailed demographic data and family medical histories.
Recommendation 6-3: The committee recommends that the Department of Health and Human Services (HHS) and its partners continue to fund and support the Vaccine Safety Datalink project to study the safety of the recommended immunization schedule. Furthermore, HHS should
consider expanding the collaboration with new health plan members and enhancing the data to improve its utility and generalizability.
CONCLUDING OBSERVATIONS
The committee’s efforts to identify priorities for recommended research studies did not reveal a base of evidence suggesting that the childhood immunization schedule is linked to autoimmune diseases, asthma, hypersensitivity, seizures or epilepsy, child developmental disorders, learning disorders or developmental disorders, or attention deficit or disruptive behavior disorders. While the committee found that there is no scientific evidence to justify the majority of safety concerns, perceptions dictate parental support and actions. Therefore further study of the full immunization schedule as well as further study to understand stakeholder perceptions and how they are formed may help improve awareness and education efforts. Stakeholder concerns should be one of the elements used to drive searches for scientific evidence, but these concerns alone, absent epidemiological or biological evidence, do not warrant the initiation of new high-cost randomized controlled trials. The committee concludes that data from existing data systems may be used to conduct observational studies and offer the best means for ongoing research efforts of the immunization schedule’s safety.
The committee found no significant evidence to imply that the recommended immunization schedule is not safe. Furthermore, existing surveillance and response systems have identified adverse events known to be associated with vaccination. The federal immunization research infrastructure is strong. A key component is the VSD project, which with ongoing support will be able to feasibly address the committee’s identified key research questions. Although the committee concludes that protection of children from vaccine-preventable diseases is of higher importance than testing of alternative immunization schedules without epidemiological or biological evidence indicating a safety problem, VSD should continue to examine the health outcomes of people who choose alternative schedules.
Looking to the future, the committee supports the work of the federal research infrastructure in ensuring that stakeholders are involved in all stages of development, implementation, evaluation, and dissemination of the immunization schedule. As electronic medical records become more commonly used, they may provide an opportunity to capture complete immunization data linked with hospital discharge records that will be useful to future studies. Further, the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) program may have the capability to monitor rare adverse events potentially associated with the childhood immunization schedule. Initiatives such as the National Children’s Study also hold promise; it
will be one of the most comprehensive research efforts focused on studying children’s health and development.
The childhood immunization schedule may become more complex over time as scientific advances are made and new vaccines are developed. Feasible research approaches to study potential adverse health outcomes will emerge only with a sustained and substantial federal commitment to research on vaccine safety.
CDC (Centers for Disease Control and Prevention). 2011. Immunization Safety Office scientific agenda. Atlanta, GA: Immunization Safety Office, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention.
HHS (Department of Health and Human Services). 2010. 2010 National Vaccine Plan. Washington, DC: Department of Health and Human Services.
IOM (Institute of Medicine). 2012. Adverse effects of vaccines: Evidence and causality . Washington, DC: The National Academies Press.
NVAC (National Vaccine Advisory Committee). 2009. Recommendations on the Centers for Disease Control and Prevention Immunization Safety Office draft 5-year scientific agenda. Washington, DC: National Vaccine Advisory Committee.
Vaccines are among the most safe and effective public health interventions to prevent serious disease and death. Because of the success of vaccines, most Americans today have no firsthand experience with such devastating illnesses as polio or diphtheria. Health care providers who vaccinate young children follow a schedule prepared by the U.S. Advisory Committee on Immunization Practices. Under the current schedule, children younger than six may receive as many as 24 immunizations by their second birthday. New vaccines undergo rigorous testing prior to receiving FDA approval; however, like all medicines and medical interventions, vaccines carry some risk.
Driven largely by concerns about potential side effects, there has been a shift in some parents' attitudes toward the child immunization schedule. The Childhood Immunization Schedule and Safety identifies research approaches, methodologies, and study designs that could address questions about the safety of the current schedule.
This report is the most comprehensive examination of the immunization schedule to date. The IOM authoring committee uncovered no evidence of major safety concerns associated with adherence to the childhood immunization schedule. Should signals arise that there may be need for investigation, however, the report offers a framework for conducting safety research using existing or new data collection systems.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
How to Write About Coronavirus in a College Essay
Students can share how they navigated life during the coronavirus pandemic in a full-length essay or an optional supplement.
Writing About COVID-19 in College Essays

Getty Images
Experts say students should be honest and not limit themselves to merely their experiences with the pandemic.
The global impact of COVID-19, the disease caused by the novel coronavirus, means colleges and prospective students alike are in for an admissions cycle like no other. Both face unprecedented challenges and questions as they grapple with their respective futures amid the ongoing fallout of the pandemic.
Colleges must examine applicants without the aid of standardized test scores for many – a factor that prompted many schools to go test-optional for now . Even grades, a significant component of a college application, may be hard to interpret with some high schools adopting pass-fail classes last spring due to the pandemic. Major college admissions factors are suddenly skewed.
"I can't help but think other (admissions) factors are going to matter more," says Ethan Sawyer, founder of the College Essay Guy, a website that offers free and paid essay-writing resources.
College essays and letters of recommendation , Sawyer says, are likely to carry more weight than ever in this admissions cycle. And many essays will likely focus on how the pandemic shaped students' lives throughout an often tumultuous 2020.
But before writing a college essay focused on the coronavirus, students should explore whether it's the best topic for them.
Writing About COVID-19 for a College Application
Much of daily life has been colored by the coronavirus. Virtual learning is the norm at many colleges and high schools, many extracurriculars have vanished and social lives have stalled for students complying with measures to stop the spread of COVID-19.
"For some young people, the pandemic took away what they envisioned as their senior year," says Robert Alexander, dean of admissions, financial aid and enrollment management at the University of Rochester in New York. "Maybe that's a spot on a varsity athletic team or the lead role in the fall play. And it's OK for them to mourn what should have been and what they feel like they lost, but more important is how are they making the most of the opportunities they do have?"
That question, Alexander says, is what colleges want answered if students choose to address COVID-19 in their college essay.
But the question of whether a student should write about the coronavirus is tricky. The answer depends largely on the student.
"In general, I don't think students should write about COVID-19 in their main personal statement for their application," Robin Miller, master college admissions counselor at IvyWise, a college counseling company, wrote in an email.
"Certainly, there may be exceptions to this based on a student's individual experience, but since the personal essay is the main place in the application where the student can really allow their voice to be heard and share insight into who they are as an individual, there are likely many other topics they can choose to write about that are more distinctive and unique than COVID-19," Miller says.
Opinions among admissions experts vary on whether to write about the likely popular topic of the pandemic.
"If your essay communicates something positive, unique, and compelling about you in an interesting and eloquent way, go for it," Carolyn Pippen, principal college admissions counselor at IvyWise, wrote in an email. She adds that students shouldn't be dissuaded from writing about a topic merely because it's common, noting that "topics are bound to repeat, no matter how hard we try to avoid it."
Above all, she urges honesty.
"If your experience within the context of the pandemic has been truly unique, then write about that experience, and the standing out will take care of itself," Pippen says. "If your experience has been generally the same as most other students in your context, then trying to find a unique angle can easily cross the line into exploiting a tragedy, or at least appearing as though you have."
But focusing entirely on the pandemic can limit a student to a single story and narrow who they are in an application, Sawyer says. "There are so many wonderful possibilities for what you can say about yourself outside of your experience within the pandemic."
He notes that passions, strengths, career interests and personal identity are among the multitude of essay topic options available to applicants and encourages them to probe their values to help determine the topic that matters most to them – and write about it.
That doesn't mean the pandemic experience has to be ignored if applicants feel the need to write about it.
Writing About Coronavirus in Main and Supplemental Essays
Students can choose to write a full-length college essay on the coronavirus or summarize their experience in a shorter form.
To help students explain how the pandemic affected them, The Common App has added an optional section to address this topic. Applicants have 250 words to describe their pandemic experience and the personal and academic impact of COVID-19.
"That's not a trick question, and there's no right or wrong answer," Alexander says. Colleges want to know, he adds, how students navigated the pandemic, how they prioritized their time, what responsibilities they took on and what they learned along the way.
If students can distill all of the above information into 250 words, there's likely no need to write about it in a full-length college essay, experts say. And applicants whose lives were not heavily altered by the pandemic may even choose to skip the optional COVID-19 question.
"This space is best used to discuss hardship and/or significant challenges that the student and/or the student's family experienced as a result of COVID-19 and how they have responded to those difficulties," Miller notes. Using the section to acknowledge a lack of impact, she adds, "could be perceived as trite and lacking insight, despite the good intentions of the applicant."
To guard against this lack of awareness, Sawyer encourages students to tap someone they trust to review their writing , whether it's the 250-word Common App response or the full-length essay.
Experts tend to agree that the short-form approach to this as an essay topic works better, but there are exceptions. And if a student does have a coronavirus story that he or she feels must be told, Alexander encourages the writer to be authentic in the essay.
"My advice for an essay about COVID-19 is the same as my advice about an essay for any topic – and that is, don't write what you think we want to read or hear," Alexander says. "Write what really changed you and that story that now is yours and yours alone to tell."
Sawyer urges students to ask themselves, "What's the sentence that only I can write?" He also encourages students to remember that the pandemic is only a chapter of their lives and not the whole book.
Miller, who cautions against writing a full-length essay on the coronavirus, says that if students choose to do so they should have a conversation with their high school counselor about whether that's the right move. And if students choose to proceed with COVID-19 as a topic, she says they need to be clear, detailed and insightful about what they learned and how they adapted along the way.
"Approaching the essay in this manner will provide important balance while demonstrating personal growth and vulnerability," Miller says.
Pippen encourages students to remember that they are in an unprecedented time for college admissions.
"It is important to keep in mind with all of these (admission) factors that no colleges have ever had to consider them this way in the selection process, if at all," Pippen says. "They have had very little time to calibrate their evaluations of different application components within their offices, let alone across institutions. This means that colleges will all be handling the admissions process a little bit differently, and their approaches may even evolve over the course of the admissions cycle."
Searching for a college? Get our complete rankings of Best Colleges.
10 Ways to Discover College Essay Ideas

Tags: students , colleges , college admissions , college applications , college search , Coronavirus
2024 Best Colleges

Search for your perfect fit with the U.S. News rankings of colleges and universities.
College Admissions: Get a Step Ahead!
Sign up to receive the latest updates from U.S. News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
Ask an Alum: Making the Most Out of College
You May Also Like
Premedical programs: what to know.
Sarah Wood May 21, 2024

How Geography Affects College Admissions
Cole Claybourn May 21, 2024

Q&A: College Alumni Engagement
LaMont Jones, Jr. May 20, 2024

10 Destination West Coast College Towns
Cole Claybourn May 16, 2024

Scholarships for Lesser-Known Sports
Sarah Wood May 15, 2024

Should Students Submit Test Scores?
Sarah Wood May 13, 2024

Poll: Antisemitism a Problem on Campus
Lauren Camera May 13, 2024

Federal vs. Private Parent Student Loans
Erika Giovanetti May 9, 2024

14 Colleges With Great Food Options
Sarah Wood May 8, 2024

Colleges With Religious Affiliations
Anayat Durrani May 8, 2024

Should COVID-19 vaccines be mandatory? Two experts discuss
Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Disclosure statement
Alberto Giubilini receives funding from the Arts and Humanities Research Council/UK Research and Innovation (AHRC/UKRI) and has previously received funding from the Wellcome Trust.
Vageesh Jain is affiliated with Public Health England under an honorary contract as a speciality registrar.
University College London provides funding as a founding partner of The Conversation UK.
University of Oxford provides funding as a member of The Conversation UK.
View all partners

To be properly protective, COVID-19 vaccines need to be given to most people worldwide. Only through widespread vaccination will we reach herd immunity – where enough people are immune to stop the disease from spreading freely. To achieve this, some have suggested vaccines should be made compulsory , though the UK government has ruled this out . But with high rates of COVID-19 vaccine hesitancy in the UK and elsewhere , is this the right call? Here, two experts to make the case for and against mandatory COVID-19 vaccines.
Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
COVID-19 vaccination should be mandatory – at least for certain groups. This means there would be penalties for failure to vaccinate, such as fines or limitations on freedom of movement.
The less burdensome it is for an individual to do something that prevents harm to others, and the greater the harm prevented, the stronger the ethical reason for mandating it.
Being vaccinated dramatically reduces the risk of seriously harming or killing others. Vaccines such as the Pfizer , AstraZeneca or Moderna ones with 90-95% efficacy at preventing people from getting sick are also likely to be effective at stopping the virus from spreading, though possibly to a lower degree. Such benefits would come at a very minimal cost to individuals.
Lockdown is mandatory. Exactly like mandatory vaccination, it protects vulnerable people from COVID-19. But, as I have argued in detail elsewhere, unlike mandatory vaccination, lockdown entails very large individual and societal costs. It is inconsistent to accept mandatory lockdown but reject mandatory vaccination. The latter can achieve a much greater good at a much smaller cost.
Also, mandatory vaccination ensures that the risks and burdens of reaching herd immunity are distributed evenly across the population. Because herd immunity benefits society collectively, it’s only fair that the responsibility of reaching it is shared evenly among society’s individual members.
Of course, we might achieve herd immunity through less restrictive alternatives than making vaccination mandatory – such as information campaigns to encourage people to be vaccinated. But even if we reach herd immunity, the higher the uptake of vaccines, the lower the risk of falling below the herd immunity threshold at a later time. We should do everything we can to prevent that emergency from happening – especially when the cost of doing so is low.
Fostering trust and driving uptake by making people more informed is a nice narrative, but it’s risky. Merely giving people information on vaccines does not always result in increased willingness to vaccinate and might actually lower confidence in vaccines. On the other hand, we’ve seen mandatory vaccination policies in Italy recently successfully boost vaccine uptake for other diseases.
Mandatory seatbelt policies have proven very successful in reducing deaths from car accidents, and are now widely endorsed despite the (very small) risks that seatbelts entail. We should see vaccines as seatbelts against COVID-19. In fact, as very special seatbelts, which protect ourselves and protect others.

Vageesh Jain, NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Mandatory vaccination does not automatically increase vaccine uptake. An EU-funded project on epidemics and pandemics, which took place several years before COVID-19, found no evidence to support this notion. Looking at Baltic and Scandinavian countries, the project’s report noted that countries “where a vaccination is mandatory do not usually reach better coverage than neighbour or similar countries where there is no legal obligation”.
According to the Nuffield Council of Bioethics, mandatory vaccination may be justified for highly contagious and serious diseases. But although contagious, Public Health England does not classify COVID-19 as a high-consequence infectious disease due to its relatively low case fatality rate.
COVID-19 severity is strongly linked with age, dividing individual perceptions of vulnerability within populations. The death rate is estimated at 7.8% in people aged over 80, but at just 0.0016% in children aged nine and under. In a liberal democracy, forcing the vaccination of millions of young and healthy citizens who perceive themselves to be at an acceptably low risk from COVID-19 will be ethically disputed and is politically risky.
Public apprehensions for a novel vaccine produced at breakneck speed are wholly legitimate. A UK survey of 70,000 people found 49% were “very likely” to get a COVID-19 vaccine once available. US surveys are similar . This is not because the majority are anti-vaxxers.
Despite promising headlines, the trials and pharmaceutical processes surrounding them have not yet been scrutinised. With the first trials only beginning in April , there is limited data on long-term safety and efficacy. We don’t know how long immunity lasts for. None of the trials were designed to tell us if the vaccine prevents serious disease or virus transmission.
To disregard these ubiquitous concerns would be counterproductive. As a tool for combating anti-vaxxers – estimated at around 58 million globally and making up a small minority of those not getting vaccinated – mandatory vaccines are also problematic. The forces driving scientific and political populism are the same . Anti-vaxxers do not trust experts, industry and especially not the government. A government mandate will not just be met with unshakeable defiance, but will also be weaponised to recruit others to the anti-vaxxer cause.
In the early 1990s, polio was endemic in India , with between 500 and 1,000 children getting paralysed daily. By 2011, the virus was eliminated. This was not achieved through legislation. It was down to a consolidated effort to involve communities, target high-need groups, understand concerns, inform, educate, remove barriers, invest in local delivery systems and link with political and religious leaders.
Mandatory vaccination is rarely justified. The successful roll-out of novel COVID-19 vaccines will require time, communication and trust. We have come too far, too fast, to lose our nerve now.
- Mandatory vaccination
- Coronavirus
- Vaccine hesitancy
- Coronavirus insights

Research Fellow

Senior Research Fellow - Women's Health Services

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health
Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:

Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional persuasive essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and place your ' write my essay online ' request today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading

- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- Free Sample Papers
- Free Ebooks
- NCERT Notes
- NCERT Syllabus
- NCERT Books
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- NCERT solutions for Class 9
- NCERT solutions for Class 8
- NCERT Solutions for Class 7
- JEE Main 2024
- MHT CET 2024
- JEE Advanced 2024
- BITSAT 2024
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Main Question Paper
- JEE Main Cutoff
- JEE Main Advanced Admit Card
- JEE Advanced Admit Card 2024
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- KCET Result
- MAH MBA CET Exam
- View All Management Exams
Colleges & Courses
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2024
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2024
- CAT Percentile Predictor 2023
- CAT 2023 College Predictor
- CMAT 2024 Answer Key
- TS ICET 2024 Hall Ticket
- CMAT Result 2024
- MAH MBA CET Cutoff 2024
- Download Helpful Ebooks
- List of Popular Branches
- QnA - Get answers to your doubts
- IIM Fees Structure
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- NEET Rank Predictor
- DNB PDCET College Predictor
- NEET Result 2024
- NEET Asnwer Key 2024
- NEET Cut off
- NEET Online Preparation
- Download Helpful E-books
- Colleges Accepting Admissions
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top NLUs Colleges in India
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow
Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- Sample Papers
- Compare Law Collages
- Careers360 Youtube Channel
- CLAT Syllabus 2025
- CLAT Previous Year Question Paper
- NID DAT Exam
- Pearl Academy Exam
Predictors & Articles
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- NID DAT Syllabus 2025
- NID DAT 2025
- Design Colleges in India
- Top NIFT Colleges in India
- Fashion Design Colleges in India
- Top Interior Design Colleges in India
- Top Graphic Designing Colleges in India
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Mumbai
- Top Interior Design Colleges in Bangalore
- NIFT Result 2024
- NIFT Fees Structure
- NIFT Syllabus 2025
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- IPU CET BJMC
- JMI Mass Communication Entrance Exam
- IIMC Entrance Exam
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India
Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- MCA Colleges in India
- BCA Colleges in India
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- RUHS Pharmacy Admission Test
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- NCHMCT JEE 2024
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
Diploma Colleges
- Top Diploma Colleges in Maharashtra
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA Application Form 2024
- UPSC IAS Application Form 2024
- CDS Application Form 2024
- CTET Admit card 2024
- HP TET Result 2023
- SSC GD Constable Admit Card 2024
- UPTET Notification 2024
- SBI Clerk Result 2024
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- CUET PG 2024
- IGNOU B.Ed Admission 2024
- DU Admission 2024
- UP B.Ed JEE 2024
- LPU NEST 2024
- IIT JAM 2024
- IGNOU Online Admission 2024
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET DU Cut off 2024
- IGNOU Date Sheet
- CUET Mock Test 2024
- CUET Admit card 2024
- CUET Result 2024
- CUET Participating Universities 2024
- CUET Previous Year Question Paper
- CUET Syllabus 2024 for Science Students
- E-Books and Sample Papers
- CUET Exam Pattern 2024
- CUET Exam Date 2024
- CUET Cut Off 2024
- CUET Exam Analysis 2024
- IGNOU Exam Form 2024
- CUET 2024 Exam Live
- CUET Answer Key 2024
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
Importance Of Vaccination Essay
A vaccination is a treatment that increases immunity to a specific illness. It is a biologically produced item that includes typical components resembling a disease-causing bacteria, generated from weak or dead versions of the microbe. It aids in immune system stimulation, identifies invasive bacteria as foreign invaders, and helps eradicate them so that the immune system can detect and eradicate any microorganism it encounters. Here are a few sample essays on ‘Importance Of Vaccination’.
100 Words Essay On Importance Of Vaccination
Vaccinations are a critical aspect of modern medicine, designed to protect individuals from harmful illnesses. They work by introducing a small, harmless dose of a microbe or its components, such as a protein or toxin, into the body. This exposure triggers the immune system to recognize and respond to the invader, allowing it to quickly identify and eliminate the pathogen if encountered again in the future.
One of the main reasons why vaccination is so important is that it helps to prevent the spread of infectious diseases. By providing immunity to a specific illness, vaccinations can help to reduce the number of people who become sick from that disease. This not only benefits the individuals who are vaccinated, but also those around them, particularly those who are unable to receive the vaccine due to underlying health conditions or other reasons.
Vaccination also plays a crucial role in herd immunity. Herd immunity is achieved when a large percentage of a population is vaccinated, making it difficult for a disease to spread. This protects not only the vaccinated individuals but also those who are unable to be vaccinated, such as newborns and people with certain medical conditions.
In conclusion, vaccinations are a safe and effective way to protect ourselves and our communities from harmful illnesses. They are a crucial tool in the fight against the spread of infectious diseases and help to ensure the health and well-being of all individuals.
200 Words Essay On Importance Of Vaccination
To prevent hazardous infections, vaccination is a simple, secure, reliable method that can be applied before you are exposed to them. As a result, your immune system is boosted, and your body's natural defences to infection are reinforced. Vaccines train your immune system to produce antibodies when exposed to a disease. A vaccine, however, does not cause an illness or increase your risk of contracting it since it only contains dead or weakened versions of bacteria or viruses.
How It Works
Natural defences of your body work together with vaccines to create immunity. Your immune system reacts when you receive a vaccination . It recognizes the bacterium or virus that is causing the invasion. Generated antibodies Proteins called antibodies are naturally created by the immune system to combat disease. In the future, if you are exposed to the pathogen, your immune system can quickly wipe it out before you get sick.
To receive the vaccine, individuals must regularly check with their local and state health departments. When the opportunity presents itself, they must take advantage of it. Some persons with specific immune system problems shouldn't have particular vaccines, and they should first see their doctors. Additionally, a small percentage of people do not react to a certain vaccine. It's crucial that everyone else have vaccinations because these people cannot be immunized. The great majority of people's "herd immunity" is preserved as a result. This implies that a disease will stop spreading if the majority of people are immune to it as a result of vaccination.
500 Words Essay On Importance Of Vaccination
The primary purpose of vaccinations is to protect by identifying and combating diseases like viruses or bacteria. Measles, polio, tetanus, diphtheria, meningitis, influenza, typhoid, and cervical cancer are among the deadly illnesses that can be avoided with vaccination . The substance used for immunization is the vaccine. The vaccine is made from weakened or dead microorganisms and contains components comparable to those found in the microbe that uses one of its toxins or surface proteins to cause the disease. The vaccine aids in boosting the immune system's ability to recognize and eliminate foreign objects. The Smallpox vaccine was the first to be developed.
Secure And Reliable
Vaccines are the best defence against a potentially fatal, preventable, and contagious disease. Although vaccines are among the safest medical medicines on the market, some precautions should be taken. People can make decisions regarding vaccinations with the help of precise information on the benefits and potential adverse effects of vaccines.
Do Vaccines Work?
Most vaccines provide immunity in 90–100% of cases. At the same time, improved sanitation and hygiene can undoubtedly contribute to preventing disease and the microorganisms that cause conditions to remain. As long as bacteria exist, people will continue to get sick.
You can see that once a vaccine is approved, the number of cases of diseases that can be prevented by vaccination begins to decline. Every year, vaccines save millions of lives. The number of people in the same community is protected from diseases when a specific area of a city or town is vaccinated against a contagious disease since the likelihood of an outbreak is reduced. The concept of immunity deals with preventing infectious diseases like rabies, measles, mumps, influenza, and pneumococcal disease.
History Of Vaccination
Before the first vaccines, humans were injected against smallpox in China and other places using cowpox, a practice known as variolation. This practice was copied in the west. The first mention of variolation as a treatment for smallpox dates back to China in the 10th century.
In 1796, a physician named Edward Jenner from Berkeley, Gloucestershire, England, tested the theory that someone with cowpox would be resistant to smallpox. To test the idea, he gave cowpox vesicles from a milkmaid named Sarah Nelmes to an eight-year-old boy named James Phipps. Two months later, he gave the child a smallpox injection, but smallpox did not manifest. There was a lot of interest in Jenner's 1798 Inquiry into the Causes and Effects of the Variolae Vaccine.
He distinguished between "real" and "false" cowpox (which did not give the desired effect). He created an "arm-to-arm" technique to spread the vaccine from a vaccinated person's pustules. Smallpox contamination delayed early attempts at confirmation, but by 1801, his paper had been translated into six other languages, and more than 100,000 people had received vaccinations, despite controversy in the medical field and religious opposition to the use of animal products. The term "vaccination" was created by surgeon Richard Dunning and was first used in his 1800 book ‘Some notes on immunization’.
Applications for Admissions are open.

Aakash iACST Scholarship Test 2024
Get up to 90% scholarship on NEET, JEE & Foundation courses

ALLEN Digital Scholarship Admission Test (ADSAT)
Register FREE for ALLEN Digital Scholarship Admission Test (ADSAT)

JEE Main Important Physics formulas
As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

PW JEE Coaching
Enrol in PW Vidyapeeth center for JEE coaching

PW NEET Coaching
Enrol in PW Vidyapeeth center for NEET coaching

JEE Main Important Chemistry formulas
As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
Download Careers360 App's
Regular exam updates, QnA, Predictors, College Applications & E-books now on your Mobile
Certifications
We Appeared in
Skip to content
News & views: Vaccine Communication (Part 2): The Message and the Messenger
Published on May 23, 2024
Vaccine Update for Healthcare Professionals
Editor’s note: This is the second of a two-part series related to vaccine communication. It is based on a presentation originally developed by Charlotte Moser for the Wilkes University Kimball Lectureship. Part 1 focused on the recipient of a vaccine-related message, and part 2 focuses on the message and the messenger.
Rhetoric is defined by the Cambridge Dictionary as “speech or writing intended to be effective and influence people.” Aristotle’s "Theory of Persuasion" speaks to the necessary components for delivering this influence:
- Logos or lines of proof
- Pathos or evocation of emotion
- Ethos or credibility of source
The first two of these address aspects of communication addressed in part 1 , further highlighting the importance of considering the recipient in any communication. In part 2, we will describe a communication theory that offers a framework for delivering a message while considering the individual nature of and complex milieu surrounding its recipient. Then, we will turn our attention to the third component of Aristotle’s theory, ethos, or the credibility of the messenger.
A framework for considering how messages can affect change
In part 1, we considered an array of factors that shape an individual’s receipt of a message. You might have even wondered how we can possibly deliver effective messages in light of those considerations. A theory of attitude change developed in the early to mid-1960s by Sherif and Hovland can help. Known as “Social Judgement Theory,” the authors described the cognitive structure of a person’s attitudes and how this structure affects message evaluation. Sherif and Hovland described three components of attitude structure:
- Latitude of acceptance – the range of ideas and messages about a topic with which the individual agrees
- Latitude of rejection – the range of ideas and messages about a topic with which the individual disagrees
- Latitude of noncommitment – ideas and messages about the topic that do not have a positive or negative effect
For example, if someone is presented with a series of statements about vaccines and asked to sort them based on their agreement or disagreement, the sorted statements would provide a framework of the individual’s vaccine attitude structure. If the individual was then asked which idea most aligns with their views, that would be considered their “anchor statement.”
According to Sherif and Hovland, if a topic is extremely relevant to the individual, it is more difficult to change their attitudes. To account for this, the theory refers to one’s “ego involvement.” As vaccine communicators and administrators, most of us are likely to have a high ego involvement related to vaccines. So, too, would people who demonstrate against vaccines. Both groups would have less malleable attitudes than many others in the population.
So how does knowing a person’s attitude structure and ego involvement help with message delivery? According to Sherif and Hovland, when a person evaluates a message, they will judge it based on their existing attitudes and then adjust their thinking accordingly. For example, a message that falls within a person’s latitude of acceptance will be accepted and the person will adjust their thinking to accommodate the new information or message. However, if the message falls within their latitude of rejection, not only will it be unacceptable, but it may also make them “dig in” on their pre-existing ideas. Said another way, a disagreeable message could have a boomerang effect. According to the authors of the theory, the biggest opportunity to change attitude is to deliver a message that is as far from one’s anchor statement as possible, but which still falls within their latitude of acceptance because it will require the biggest adjustment to their thinking. Unfortunately, but important for communicators, this explains why a message does not typically cause a large-scale about-face. Rather, it suggests that a series of small changes will accumulate over time to generate overarching change. And while this is not what a communicator (or clinician) wants to hear, it provides an understanding that any positive change to one’s attitudes should be considered good progress.
Messengers, the ecosystem and trust
Another important component of communication is the messenger. We witnessed this during COVID-19 vaccine rollout, where in some places, community partners were more effective messengers than public health officials. So, what do we know about this?
As described, Aristotle’s theory of persuasion focuses on the credibility and perceived competence of the messenger. Today, we talk a lot about trust. Trust takes time to build, but sometimes we are asked — or are asking others — to trust in the absence of time. This is where credibility and perceived competence become important surrogates. In 2022, the Ad Council Research Institute conducted a study in which participants were asked what makes someone trustworthy. Among 2,500 participants, 65% to 70% indicated that people who demonstrate honesty, consistency of message, lack of bias, and an acknowledgement of both sides of an issue are considered more trustworthy. In contrast, people who are endorsed or paid by companies, influencers, government officials, and popular national or local voices do not enjoy the same level of trust. Importantly, both the Ad Council survey and longitudinal surveys by the Pew Research Center found that medical professionals and scientists are among the most trusted occupational groups; however, the fragility of this trust was also apparent. Among Ad Council survey respondents, the percent indicating trust varied based on political leanings and geography (urban, suburban, rural). Likewise, the Pew data varied across time. When asked about the likelihood of various types of professionals to act in the best interest of the public, the highest percentage of confidence among Pew respondents came during 2019 and 2020, but data captured at the end of 2021 suggested some erosion in that confidence (albeit confidence was still greater for these professionals than many others).
Another aspect of trust relates to how a message is being used. Trusted messengers vary depending on whether someone is becoming aware of a topic, learning more about it, or making a decision using the information. To describe this, the Ad Council created a model, called the “trusted messenger ecosystem,” in which a series of concentric circles represent each use with decision-making requiring the most trust and subject awareness requiring the least. As described by the authors, “Broader reach helps drive initial awareness, but for becoming more and remaining informed, Americans generally trust messengers with either a close personal connection or expert credentials” (p. 15). If you’ve watched “Meet the Fockers,” it’s those in the “circle of trust” that most influence decision making. However, all is not lost when influence is low. According to the Ad Council report, individuals outside of the most trusted circle can still exert influence in one of two ways. First, they can work to exact change by influencing others who have more personal influence. Community messengers during COVID-19 vaccine rollout are an example of this approach. Second, as relationships develop, some people may gain influence. For example, over time someone in an individual’s social media network may gain more influence, particularly if the online interactions reinforce or enhance a more personal relationship.
Summing up part 2
The complexity of communication around a topic like vaccines is the result of an array of factors. Messages and messengers will be received differently based on the who is assessing them. Likewise, these assessments are not static. A message may become more or less palatable over time as an individual’s topical framework changes, and the perceived value of particular messengers can also change based on the intended use for information as well as changes to existing relationships between the messenger and the recipient. However, understanding the personal nature of message evaluation and the trusted messenger ecosystem can help communicators develop appropriate messages, determine who is the best messenger for their delivery, and hopefully, remove some of the frustration that can result when opinions are not dramatically changed by a particular message.
Part 1 of this series demonstrated the complex considerations around message recipients and the importance of placing them at the center of any communication strategy. Part 2 described considerations related to the message and the messenger — again placing the recipient at the center. Hopefully, by considering both discussions, you will be positioned to deliver the right message at the right time by the right person, and if a message is not received as intended, maybe you will at least be less frustrated and have a way to start sorting out the “why.”
References (Part 2)
Feldmann D, Foleno T, Thompson-Kuhn C, et al. The 2022 Trusted Messenger Study. 2022. Accessed May 16, 2024.
Griffin E, Ledbetter A, Sparks G. (2015). Social Judgement Theory in A First Look at Communication Theory , Ninth Edition, McGraw Hill Education.
Kennedy B, Tyson A, Funk C. (2022, Feb. 15). Americans’ Trust in Scientists, Other Groups Declines. Pew Research Center. Accessed May 16, 2024.
Sherif M, Hovland CI. (1961). Social judgement: Assimilation and contrast effects in communication and attitude change. Yale University Press.
Contributed by: Charlotte A. Moser, MS, Paul A. Offit, MD
Categories: Vaccine Update May 2024 , News and Views About Vaccines
Materials in this section are updated as new information and vaccines become available. The Vaccine Education Center staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family's personal health. You should not use it to replace any relationship with a physician or other qualified healthcare professional. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult your physician or, in serious cases, seek immediate assistance from emergency personnel.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Effect of the HPV...
Effect of the HPV vaccination programme on incidence of cervical cancer and grade 3 cervical intraepithelial neoplasia by socioeconomic deprivation in England: population based observational study
Linked editorial.
HPV vaccine: the key to eliminating cervical cancer inequities
- Related content
- Peer review
- Milena Falcaro , senior statistician 1 ,
- Kate Soldan , scientist and epidemiologist 2 ,
- Busani Ndlela , cancer information analyst 3 ,
- Peter Sasieni , professor of cancer epidemiology 1
- 1 Centre for Cancer Screening, Prevention and Early Diagnosis, Wolfson Institute of Population Health, Queen Mary University of London, London EC1M 6BQ, UK
- 2 Blood Safety, Hepatitis, Sexually Transmitted Infections and HIV Division, UK Health Security Agency (UKHSA), London, UK
- 3 National Disease Registration Service (NDRS), NHS England, London, UK
- Correspondence to: P Sasieni p.sasieni{at}qmul.ac.uk (or @petersasieni on X)
- Accepted 27 March 2024
Objectives To replicate previous analyses on the effectiveness of the English human papillomavirus (HPV) vaccination programme on incidence of cervical cancer and grade 3 cervical intraepithelial neoplasia (CIN3) using 12 additional months of follow-up, and to investigate effectiveness across levels of socioeconomic deprivation.
Design Observational study.
Setting England, UK.
Participants Women aged 20-64 years resident in England between January 2006 and June 2020 including 29 968 with a diagnosis of cervical cancer and 335 228 with a diagnosis of CIN3. In England, HPV vaccination was introduced nationally in 2008 and was offered routinely to girls aged 12-13 years, with catch-up campaigns during 2008-10 targeting older teenagers aged <19 years.
Main outcome measures Incidence of invasive cervical cancer and CIN3.
Results In England, 29 968 women aged 20-64 years received a diagnosis of cervical cancer and 335 228 a diagnosis of CIN3 between 1 January 2006 and 30 June 2020. In the birth cohort of women offered vaccination routinely at age 12-13 years, adjusted age standardised incidence rates of cervical cancer and CIN3 in the additional 12 months of follow-up (1 July 2019 to 30 June 2020) were, respectively, 83.9% (95% confidence interval (CI) 63.8% to 92.8%) and 94.3% (92.6% to 95.7%) lower than in the reference cohort of women who were never offered HPV vaccination. By mid-2020, HPV vaccination had prevented an estimated 687 (95% CI 556 to 819) cervical cancers and 23 192 (22 163 to 24 220) CIN3s. The highest rates remained among women living in the most deprived areas, but the HPV vaccination programme had a large effect in all five levels of deprivation. In women offered catch-up vaccination, CIN3 rates decreased more in those from the least deprived areas than from the most deprived areas (reductions of 40.6% v 29.6% and 72.8% v 67.7% for women offered vaccination at age 16-18 and 14-16, respectively). The strong downward gradient in cervical cancer incidence from high to low deprivation in the reference unvaccinated group was no longer present among those offered the vaccine.
Conclusions The high effectiveness of the national HPV vaccination programme previously seen in England continued during the additional 12 months of follow-up. HPV vaccination was associated with a substantially reduced incidence of cervical cancer and CIN3 across all five deprivation groups, especially in women offered routine vaccination.
Introduction
Human papillomavirus (HPV) comprises a family of viruses, a subset of which are responsible for virtually all cervical and some anogenital and oropharyngeal cancers. 1 More than 100 countries worldwide have introduced prophylactic HPV vaccination as part of routine immunisation schedules. 2 One important outcome yet to be reported is whether vaccination has reduced or increased the inequalities seen for cervical disease in the UK and elsewhere.
In England, the national HPV vaccination programme started in 2008 using the bivalent Cervarix vaccine to prevent infections due to HPV types 16 and 18, which are estimated to cause around 80% of all cervical cancers in the UK. 3 Vaccination was offered routinely to 12-13 year old (school year 8) girls and as part of a catch-up campaign to those aged <19 years. 4 In September 2012 the programme switched to the quadrivalent vaccine (Gardasil), which additionally protects against HPV types 6 and 11 (responsible for genital warts), and in 2019 the programme was extended to 12-13 year old boys. Those who are eligible but not vaccinated can receive the vaccine free of charge from their general practitioner until their 25th birthday. 5
The introduction and implementation of HPV immunisation in this way means that noticeable discontinuities exist in the proportion of women vaccinated by date of birth, enabling a rigorous evaluation of the effectiveness of the programme. 6 For example, women born in August 1990 are unlikely to have received HPV vaccination, whereas among those born in the year from 1 September 1990 nearly 70% have received at least one dose of the vaccine.
Findings on the early effect of national HPV vaccination programmes have been encouraging. A wealth of real world evidence for the effect of vaccination on HPV prevalence exists 7 8 9 10 11 and evidence is growing for its effectiveness in reducing high grade cervical intraepithelial neoplasia (CIN) 12 13 14 15 and cervical cancer in vaccinated women. 14 16 17 18 19 For instance, we found that in England rates of grade 3 CIN (CIN3) and of cervical cancer were greatly reduced among those who were offered HPV vaccination, and that the magnitude of the reduction was greatest in the cohorts with the highest uptake and younger age at vaccination. 14 We estimated that by mid-2019 the immunisation programme had prevented cervical cancer in nearly 450 women and CIN3 in around 17 000 women.
Along with preventing ill health, a key aim of the NHS is to reduce health inequalities. 20 To this end, we investigated whether the effect of immunisation against HPV has resulted in a reduction in inequalities in cervical disease or a widening. Concern has been expressed that if the uptake of HPV vaccination is lower in those at greatest risk of cervical cancer, as has been seen in the US, 21 this could accentuate health inequalities. One study found that the introduction of HPV immunisation in England might initially have increased inequities in HPV related cancer incidence among ethnic minority groups because of the differential effect of herd protection in subpopulations with dissimilar vaccination coverage. 22 Previous studies have suggested that white people have a higher awareness of HPV and acceptance of the immunisation 23 and that vaccination uptake is lower in women from ethnic minority groups and more deprived areas. 24 Using data on HPV vaccination coverage by local area, however, a study found little variation by deprivation score in women offered routine vaccination (83% v 86% for most and least deprived areas, respectively) and only a small negative correlation between deprivation and vaccine uptake in those offered catch-up vaccination (47% v 53% for most and least deprived areas, respectively). 25 A full understanding of the effect of HPV vaccination across different socioeconomic groups is complicated by the poor uptake of cervical screening observed among younger women in the most deprived areas, leading to lower rates of screen detected cervical cancer and CIN3 at age 25 years compared with women in less deprived areas. 26 27
We replicated results from an analysis of population based cancer registry data to evaluate if the high vaccination effectiveness seen previously continued during an additional year of follow-up. The combined data were also used to investigate the effect of the vaccination programme by socioeconomic deprivation.
To represent socioeconomic deprivation, we used the index of multiple deprivation, a small area measure based on several domains of deprivation, such as income, employment, and health. The index is determined by using a standard statistical geographical unit, called lower super output area, which divides England into small areas of similar sized populations (on average about 1500 residents, or 650 households). 28 The lower super output areas are then ranked from the most to the least deprived and divided into five equal groups. The first and fifth groups correspond to the 20% most deprived and 20% least deprived lower super output areas in England, respectively.
We retrieved the records of all women aged 20-64 years resident in England with a diagnosis of invasive cervical cancer (ICD-10 (international classification of diseases, 10th revision) code C53) or CIN3 (ICD-10 code D06) between 1 January 2006 and 30 June 2020. These records are stored in the database managed by NHS England’s National Disease Registration Service, 29 and for each patient included information on index of multiple deprivation derived from the patient’s home postcode at the time of diagnosis. To convert these counts into rates, we used mid-year estimates of the female population for England by single year of age, calendar year (January 2006 to June 2020), and index of multiple deprivation (five groups). These estimates were retrieved from multiple tables publicly available on the website of the UK’s Office for National Statistics (ONS). 30 The supplementary material provides more details about the index of multiple deprivation versions used by the National Disease Registration Service and ONS, along with information on how we derived the population estimates required in our statistical analysis.
Statistical analysis
We separately analysed incidence rates of cervical cancer and CIN3 by using extensions of our previously described age-period-cohort Poisson model. 14 31 32 Data on women with cancer or CIN3 were aggregated by single month of age, calendar time (period), and date of birth (cohort). We derived the corresponding population risk time by subdividing the mid-year ONS population estimates into one month intervals for age, period, and cohort. For the analysis of the effectiveness by deprivation, we further split both the data on women with cancer or CIN3 and the population estimates by deprivation group (fifths). We then used the population risk time as the denominator for calculating rates (formally, the subdivided population estimates were log transformed and included in the Poisson regression model as an offset). Confidence intervals were computed using robust standard errors. 33 34
The code for the analysis was written and tested on synthetic data (extending the Simulacrum dataset) 35 by a statistician (MF) at King’s College London and then run on the real dataset by an analyst (BN) at the National Disease Registration Service.
We started by considering a core model where we included the main effects for age, period, and birth cohort, along with selected age by cohort and age by period interactions (see supplementary table S1). The interaction terms were included to account for variations in screening policy and historical events that affected cervical cancer rates. Specifically, we defined seven birth cohorts to capture differences in the age at first invitation to screening and the school years in which HPV vaccination was offered (see table 1 ). We added terms for seasonality and for events that may have affected registrations for cervical cancer and CIN3, such as the covid-19 lockdown, the “Jade Goody effect,” 36 37 and the 2019 cervical screening awareness campaign. In our previous paper, 14 we used several similar regression models to study the sensitivity of results to the precise way in which we adjusted for potential confounding factors. Because we found that the estimates of the cohort specific incidence rate ratios changed little across the various models, here we report on only a single model adjustment for confounders.
Characteristics of the birth cohorts
- View inline
Using the core model described, we investigated if the high effectiveness of the HPV immunisation programme reported previously 14 continued during an additional 12 months of follow-up. To do this we split the main effect of each cohort offered vaccination into two subgroup effects depending on whether the data related to the periods 1 January 2006 to 30 June 2019 or 1 July 2019 to 30 June 2020; this approach corresponded to adding three cohort by period interaction terms.
To evaluate the impact of socioeconomic deprivation on incidences of cervical cancer and CIN3, we extended the core model by adding main effects for deprivation and deprivation by cohort interactions. Specifically, we allowed the effect of each deprivation level to vary between unvaccinated women (cohorts 1-4) and those offered vaccination (cohorts 5-7), but we assumed it was otherwise constant within these two groups. We did not include further interactions between deprivation and other covariates as they were not of primary interest in this analysis. Using the fitted Poisson regression models, we made “what if” predictions by changing the value of one or more predictors and by leaving the others as observed. In this way it was possible to compare what happened (factual scenario) with what would have happened under an alternative (counterfactual) scenario.
We also carried out a sensitivity analysis where the effects of these deprivation by cohort interactions were allowed to vary across the three different groups offered vaccination (ie, we used 15 terms instead of five). For cervical cancer, owing to small numbers in cohort 7, we fitted a reduced model where the effects of these interactions were constrained to be the same for cohorts 6 and 7.
All analyses were performed in Stata, version 17. 38
Patient and public involvement
Patient and public involvement contributors were not formally involved in this research. We did, however, engage with Cancer Research UK (CRUK), Jo’s Cervical Cancer Trust, and the HPV Coalition on the importance of these analyses and the dissemination of the results. This included taking part in a video produced by ITN Business for World Cancer Day 2023, writing a piece for the 20th anniversary of the creation of CRUK, and engaging with international media about our research findings on the effect of the English HPV vaccination programme. We have also discussed the research and a draft of this paper with individual patients, journalists, and patient and public involvement representatives linked to broader research programmes.
Table 1 lists the characteristics of the birth cohorts included in the study. We defined the different cohorts so that each cohort is homogeneous in terms of the age women would have been offered HPV vaccination (if at all) and the age at which they would have first been invited for cervical screening.
Overall, there were 231.1 million women years of observation between 1 January 2006 and 30 June 2020 on women aged 20-64 years in England. During this time, 29 968 women received a diagnosis of invasive cervical cancer and 335 228 a diagnosis of CIN3 ( table 2 ). Observations between 1 July 2019 and 30 June 2020 have not been reported previously. With these additional 12 months of follow-up, there are, in the routine vaccination group (cohort 7), about twice the number of diagnoses compared with the same group in our previous study (we now have 13 v 7 previously for cervical cancer, 109 v 49 for CIN3; see supplementary table S2).
Summary statistics of study population
Our previously published findings on the effect of the national HPV vaccination were largely confirmed with the new data ( table 3 , also see supplementary table S3). The analysis showed that the previously observed low rates of disease and the estimated high effectiveness of the immunisation programme continued during the additional 12 months of follow-up (diagnoses in July 2019 to June 2020) among women born since 1 September 1990. In particular, the estimated effects of vaccination for that later period in cohort 7 (those born since 1 September 1995) imply a reduction in incidence of 83.9% (95% confidence interval (CI) 63.8% to 92.8%) for cervical cancer and 94.3% (92.6% to 95.7%) for CIN3 ( table 3 ). The relative risk reduction estimates for the earlier period are not identical to those reported previously because we also had new data for the unvaccinated cohorts that affected the baseline rates.
Estimated relative risk reductions (percentages) in incidence of invasive cervical cancer and CIN3 in the three cohorts offered HPV vaccination compared with the most recent unvaccinated cohort
Supplementary table S4 shows the full estimates from modelling the effects of vaccination in different levels of socioeconomic deprivation, with summary results reported in table 4 , table 5 , and table 6 . The highest incidence rates for invasive cervical cancer were observed among women living in the most deprived areas (first fifth) but, while in the reference unvaccinated group there was a strong downward gradient moving from women in the most deprived areas to those in the least deprived, little difference was found between the second and fifth fifths of deprivation in the groups offered vaccination. In both the reference and the vaccination cohorts the highest rates of CIN3 occurred in those from the most deprived areas, but no clear trend was observed among the other four fifths of deprivation (see supplementary tables S5 and S6).
Estimated number of invasive cervical cancers and CIN3s predicted and prevented by mid-2020 in the three cohorts of women offered HPV vaccination
Estimated cohort specific numbers of invasive cervical cancers predicted and prevented by mid-2020 among women in the least and most deprived areas
Estimated cohort specific numbers of CIN3 predicted and prevented by mid-2020 among women in the least and most deprived areas
Overall, our model estimated that 687 (95% CI 556 to 819) cervical cancers and 23 192 (22 163 to 24 220) CIN3s had been prevented by the vaccination programme up to mid-2020 among young women in England ( table 4 ). The greatest numbers for cervical cancer were prevented in women in the most deprived areas (192 and 199 for first and second fifths, respectively) and the fewest in women in the least deprived fifth (61 cancers prevented). The number of women with CIN3 prevented was high across all deprivation groups but greatest among women living in the more deprived areas: 5121 and 5773 for first and second fifths, respectively, compared with 4173 and 3309 in the fourth and fifth fifths, respectively. When we looked at the corresponding cohort specific figures ( table 5 and table 6 ), we noticed differences between the cohorts, particularly for CIN3. In all three cohorts offered vaccination the numbers and rates of prevented cervical cancers were much higher in women from the most deprived areas than least deprived areas ( table 5 ). The proportion of women with prevented cervical cancer in each cohort was, however, similar between the first and fifth fifths of deprivation. For CIN3 ( table 6 ), the results were more complicated. In women offered vaccination at age 16-18 years (cohort 5), the proportion of cervical cancers prevented was substantially less in those from the most deprived areas (29.6%) compared with those from the least deprived areas (40.6%). An inequality still existed in cohorts 6 and 7, but it was greatly reduced (67.7% v 72.8% in cohort 6 and 95.3% v 96.1% in cohort 7).
In England, the social-class gradient for cervical cancer is one of the steepest of any cancers: women in the most deprived fifth have had double the risk of those in the least deprived fifth. 39 40 Some of this results from differences in exposure to HPV and risk of an infection becoming persistent, 41 but differential uptake of cervical screening has also been an important factor. Previous research has highlighted the need for new engagement strategies to improve attendance for cervical screening among young women living in more socially deprived areas. 42 Encouragingly, the coverage of HPV vaccination has been (at least for the routine campaign and before the covid-19 pandemic) uniformly high. 43 It is, however, important to investigate whether immunisation—including the indirect effects achieved by high uptake—is helping to reduce health inequalities.
Using population based cancer registrations updated to mid-2020, which provided information on about twice the expected number of cancers in women offered HPV vaccination aged 12-13 years than in our previous analysis, we were able to show that the high vaccination effectiveness seen previously was confirmed with more recent data. The largest differences between the old and the new data were found for cohort 6 (the catch-up group offered the vaccine at age 14-16 years): for cervical cancer the estimated effectiveness increased, whereas for CIN3 it decreased. The reasons behind these differences are unclear. The results for cohorts 6 and 7 in the new data are more in keeping with what we would have expected given that the proportion of disease caused by HPV types 16 and 18 is greater for invasive cancer than for CIN3.
We also investigated the effect of the HPV immunisation programme by socioeconomic deprivation. Overall, we found that the programme was associated with a substantial reduction in the expected number of women with cervical cancers and CIN3 in all fifths of deprivation. For cervical cancer before vaccination, the downward gradient with decreasing deprivation was strong. In all cohorts offered vaccination, the highest rate was still seen among women living in the most deprived areas, but little difference was observed between women living in the second to fifth deprived areas. For CIN3, similar patterns were observed for the reference unvaccinated group and the three cohorts offered vaccination, but rates were greatly reduced in all fifths of deprivation in the latter. When we compared women in the most deprived areas with those in the least deprived areas in terms of percentage of disease averted, we observed differences across the cohorts for CIN3, with women in the least deprived areas in the older catch-up cohort (vaccine offered at age 16-18 years) having a greater proportion of averted CIN3s after HPV immunisation than women in the most deprived area (40.6% v 29.6%). The same, although to a much less extent, was observed for the younger catch-up cohort (72.8% v 67.7%). For invasive cervical cancer, we found no evidence of a less beneficial impact (in terms of percentage of cases averted) of the vaccination in women living in the most deprived areas; in fact, especially for the older catch-up cohort, the percentage was slightly higher in women in the most deprived areas compared with those in the least deprived areas.
The observed incidences of cervical cancer and CIN3 depend on three key factors: the intensity of exposure to HPV infections (including age at first exposure), the uptake of cervical screening, and HPV vaccination coverage. It is therefore difficult to disentangle the effects of these three drivers on the index of multiple deprivation specific rates with the data at hand. The health inequality in CIN3 in cohort 5 might result from the lower vaccination coverage among women in the most deprived areas since at age 16-18 years when they became eligible for vaccination more of those from the most deprived fifth may not have been in school or, for other reasons, may have missed the offer of HPV immunisation. These observations are consistent with previous understanding that higher uptake of catch-up vaccination was associated, although not as strongly as in some countries, with lower deprivation. 25 It is, however, reassuring that cohorts 6 and 7 showed little inequality in relative reductions in cancer (as in vaccination coverage).
However, since the UK has recently announced a change to a one dose schedule for routine HPV vaccination, ensuring this change achieves high coverage (including in the birth cohorts currently with lower coverage owing to covid-19 related interruption to schooling, and to immunisation services) is important to maintain the effects we have seen on cervical disease and on inequalities. Further investigations could be carried out in the future to check for any effect on cancer incidence caused by covid-19, gender neutral vaccination (since 2019), a change in the type of vaccine used, or reduced dose schedules.
Strengths and limitations of this study
Our analysis has several strengths. Our study provides direct evidence for the effect of a public health intervention (such as HPV vaccination) on cancer rates by deprivation. We used high quality data from population based cancer registries and were able to investigate the extent of socioeconomic inequalities in cohorts offered vaccination and whether the effectiveness of the HPV immunisation continued in an additional year of follow-up. The code for the analysis was written and tested using simulated data and an independent analyst later ran the code on the real dataset, guaranteeing reliable and robust results and preserving patient confidentiality.
The main limitations of our study are that it was observational and individual level data on vaccination status were not available. However, previous published research 14 provided detailed information on potential confounding factors and the best way to adjust for these in the analysis. Additionally, the discontinuities in vaccine uptake with date of birth makes this study powerful and less prone to biases from unobserved confounders than an analysis based on individual level data on HPV vaccination status.
Women born after 1 September 1999 were offered the Gardasil vaccine from 1 September 2012. As these women were at most aged 20 years and 10 months at the end of the study follow-up (30 June 2020), it is not yet possible with the data available to compare the effectiveness of the programme among those offered Cervarix and those offered Gardasil. This additional comparative analysis will become feasible with a longer follow-up on the recipients of Gardasil.
Policy implications
We found that the high effectiveness of the national HPV immunisation continued in the additional year of follow-up (July 2019 to June 2020). This is encouraging as it validates the previously published results and further supports consideration of more limited cervical screening for cohorts with high vaccination coverage aged 12-13 years. Moreover, although women living in the most deprived areas are still at higher risk of cervical cancer than those in less deprived areas, the HPV vaccination programme is associated with substantially lowered rates of disease across all fifths of socioeconomic deprivation. For cervical cancer, this has led to the levelling-up of the rates across the second to fifth fifths of deprivation so that the strong downward gradient observed in the reference unvaccinated cohort is no longer present in the cohorts offered vaccination. For CIN3, in the older catch-up cohorts women living in the least deprived areas seem to have benefited more from vaccination than those living in the most deprived areas, but the rates were still greatly reduced in all socioeconomic groups. Cervical screening strategies for women offered vaccination should carefully consider the differential effect both on rates of disease and on inequalities that are evident among women offered catch-up vaccination.
Conclusions
The HPV vaccination programme in England has not only been associated with a substantial reduction in incidence of cervical neoplasia in targeted cohorts, but also in all socioeconomic groups. This shows that well planned and executed public health interventions can both improve health and reduce health inequalities.
What is already known on this topic
In England, immunisation against human papillomavirus (HPV) has been associated with greatly reduced incidence rates of cervical cancer and grade 3 cervical intraepithelial neoplasia (CIN3) up to June 2019, especially among women offered routine vaccination at age 12-13 years
The social-class gradient for cervical cancer incidence has been one of the steepest of any cancers
Concern has been raised that HPV vaccination could least benefit those at highest risk of cervical cancer
What this study adds
The high effectiveness of vaccination against HPV seen previously continued during an additional year of follow-up, from July 2019 to June 2020
The English HPV vaccination programme was associated with substantially lower rates of cervical cancer and CIN3 in all fifths of socioeconomic deprivation, although the highest rates remained among women in the most deprived areas
For cervical cancer, the strong downward gradient from high to low deprivation observed in the reference unvaccinated cohort was no longer present among those offered vaccination
Ethics statements
Ethical approval.
Not required as the study used aggregated data from the National Disease Registration Service as well as publicly available information from the Office for National Statistics website.
Data availability statement
The cancer registry data analysed for this paper are securely held by the National Disease Registration Service (NDRS). Requests to access the data can be made through NHS England’s DARS service ( https://digital.nhs.uk/services/data-access-request-service-dars ). The Simulacrum ( https://simulacrum.healthdatainsight.org.uk/ ) is a synthetic dataset developed by Health Data Insight and derived from anonymous cancer data provided by NHS England’s NDRS. Mid-year population estimates are freely downloadable from the Office for National Statistics website ( https://www.ons.gov.uk/ ).
Acknowledgments
We thank Alejandra Castañon (LCP Health Analytics), Marta Checchi (UK Health Security Agency), and Lucy Elliss-Brookes (NHS England) for helpful comments on the study protocol, and Kwok Wong (NHS England) for contributing to the quality assurance of the data extraction code.
Contributors: PS had the original idea. He is the guarantor. MF and PS conceptualised the study and prepared the study protocol, which was subsequently reviewed by the other co-authors. MF wrote and tested the Stata code (checked by PS) for the data analysis and drafted the manuscript. BN extracted the dataset and ran the Stata code on it. All authors critically reviewed and approved the final submitted version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by Cancer Research UK (grant No C8162/A27047). The funder had no role in the study design or in the collection, analysis, interpretation of data, writing of the report or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare support from Cancer Research UK for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of this research will be disseminated through the media, blogs and scientific meetings and will inform the design and implementation of interventions to reduce health inequalities. We will also work with others to produce information for the public to support human papillomavirus immunisation and cervical screening programmes and, if the opportunity arises, to contribute summary data for an international meta-analysis of similar studies.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- ↵ IARC. Human papillomaviruses. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 90. 2007.
- ↵ World Health Organization (WHO). Global Market Study: HPV 2022 https://cdn.who.int/media/docs/default-source/immunization/mi4a/who-mi4a-global-market-study-hpv.pdf?sfvrsn=649561b3_1&download=true .
- Cuschieri K ,
- Hibbitts S ,
- ↵ Public Health England (PHE). Human Papillomavirus (HPV) vaccine coverage in England, 2008/09 to 2013/14. A review of the full six years of the three-dose schedule: Public Health England (PHE); 2015. https://www.gov.uk/government/publications/human-papillomavirus-hpv-immunisation-programme-review-2008-to-2014 ; accessed 6 January 2021.
- ↵ UK Health Security Agency. HPV vaccination: guidance for healthcare practitioners (version 6) 2022 [updated April 2022]. https://www.gov.uk/government/publications/hpv-universal-vaccination-guidance-for-health-professionals ; accessed 24 August 2022.
- Lévesque LE ,
- Kaufman JS ,
- Brisson M ,
- HPV Vaccination Impact Study Group
- Thomas SL ,
- Tabrizi SN ,
- Brotherton JM ,
- Kaldor JM ,
- Markowitz LE ,
- Steinau M ,
- Hernandez-Aguado JJ ,
- Sánchez Torres DA ,
- Martínez Lamela E ,
- Lehtinen M ,
- Lagheden C ,
- Luostarinen T ,
- Falcaro M ,
- Castañon A ,
- Wallace L ,
- Pollock KG ,
- Elfström KM ,
- Skorstengaard M ,
- Thamsborg LH ,
- Dillner J ,
- Dehlendorff C ,
- Belmonte F ,
- ↵ NHS. The NHS long term plan 2019. https://www.longtermplan.nhs.uk/ ; accessed 24 August 2022.
- Johnson HC ,
- Lafferty EI ,
- Roberts SA ,
- Stretch R ,
- Sheridan A ,
- Pappas-Gogos G ,
- Douglas E ,
- McLennan D ,
- Henson KE ,
- Elliss-Brookes L ,
- Coupland VH ,
- ↵ Office for National Statistics. https://www.ons.gov.uk/ ; accessed 24 October 2022.
- Carstensen B
- Sasieni P ,
- ↵ Huber P. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the 5th Berkeley Symposium on Mathematical Statistics and Probability: University of California Press, 1967:221-33.
- Health Data Insight
- Lancucki L ,
- Patnick J ,
- Castanon A ,
- Thomson CS ,
- UK Association of Cancer Registries
- ↵ Cancer Research UK. Cervical Cancer Incidence Statistics 2015. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence ; accessed 14 March 2023.
- Currin LG ,
- Linklater KM ,
- Rahman MA ,
- Paranjothy S
- ↵ UK Health Security Agency (UKHSA). HPV vaccine uptake 2023. https://www.gov.uk/government/collections/vaccine-uptake#hpv-vaccine-uptake ; accessed 12 March 2023.
- Share full article
Advertisement
Supported by
Letter of Recommendation
What I’ve Learned From My Students’ College Essays
The genre is often maligned for being formulaic and melodramatic, but it’s more important than you think.

By Nell Freudenberger
Most high school seniors approach the college essay with dread. Either their upbringing hasn’t supplied them with several hundred words of adversity, or worse, they’re afraid that packaging the genuine trauma they’ve experienced is the only way to secure their future. The college counselor at the Brooklyn high school where I’m a writing tutor advises against trauma porn. “Keep it brief , ” she says, “and show how you rose above it.”
I started volunteering in New York City schools in my 20s, before I had kids of my own. At the time, I liked hanging out with teenagers, whom I sometimes had more interesting conversations with than I did my peers. Often I worked with students who spoke English as a second language or who used slang in their writing, and at first I was hung up on grammar. Should I correct any deviation from “standard English” to appeal to some Wizard of Oz behind the curtains of a college admissions office? Or should I encourage students to write the way they speak, in pursuit of an authentic voice, that most elusive of literary qualities?
In fact, I was missing the point. One of many lessons the students have taught me is to let the story dictate the voice of the essay. A few years ago, I worked with a boy who claimed to have nothing to write about. His life had been ordinary, he said; nothing had happened to him. I asked if he wanted to try writing about a family member, his favorite school subject, a summer job? He glanced at his phone, his posture and expression suggesting that he’d rather be anywhere but in front of a computer with me. “Hobbies?” I suggested, without much hope. He gave me a shy glance. “I like to box,” he said.
I’ve had this experience with reluctant writers again and again — when a topic clicks with a student, an essay can unfurl spontaneously. Of course the primary goal of a college essay is to help its author get an education that leads to a career. Changes in testing policies and financial aid have made applying to college more confusing than ever, but essays have remained basically the same. I would argue that they’re much more than an onerous task or rote exercise, and that unlike standardized tests they are infinitely variable and sometimes beautiful. College essays also provide an opportunity to learn precision, clarity and the process of working toward the truth through multiple revisions.
When a topic clicks with a student, an essay can unfurl spontaneously.
Even if writing doesn’t end up being fundamental to their future professions, students learn to choose language carefully and to be suspicious of the first words that come to mind. Especially now, as college students shoulder so much of the country’s ethical responsibility for war with their protest movement, essay writing teaches prospective students an increasingly urgent lesson: that choosing their own words over ready-made phrases is the only reliable way to ensure they’re thinking for themselves.
Teenagers are ideal writers for several reasons. They’re usually free of preconceptions about writing, and they tend not to use self-consciously ‘‘literary’’ language. They’re allergic to hypocrisy and are generally unfiltered: They overshare, ask personal questions and call you out for microaggressions as well as less egregious (but still mortifying) verbal errors, such as referring to weed as ‘‘pot.’’ Most important, they have yet to put down their best stories in a finished form.
I can imagine an essay taking a risk and distinguishing itself formally — a poem or a one-act play — but most kids use a more straightforward model: a hook followed by a narrative built around “small moments” that lead to a concluding lesson or aspiration for the future. I never get tired of working with students on these essays because each one is different, and the short, rigid form sometimes makes an emotional story even more powerful. Before I read Javier Zamora’s wrenching “Solito,” I worked with a student who had been transported by a coyote into the U.S. and was reunited with his mother in the parking lot of a big-box store. I don’t remember whether this essay focused on specific skills or coping mechanisms that he gained from his ordeal. I remember only the bliss of the parent-and-child reunion in that uninspiring setting. If I were making a case to an admissions officer, I would suggest that simply being able to convey that experience demonstrates the kind of resilience that any college should admire.
The essays that have stayed with me over the years don’t follow a pattern. There are some narratives on very predictable topics — living up to the expectations of immigrant parents, or suffering from depression in 2020 — that are moving because of the attention with which the student describes the experience. One girl determined to become an engineer while watching her father build furniture from scraps after work; a boy, grieving for his mother during lockdown, began taking pictures of the sky.
If, as Lorrie Moore said, “a short story is a love affair; a novel is a marriage,” what is a college essay? Every once in a while I sit down next to a student and start reading, and I have to suppress my excitement, because there on the Google Doc in front of me is a real writer’s voice. One of the first students I ever worked with wrote about falling in love with another girl in dance class, the absolute magic of watching her move and the terror in the conflict between her feelings and the instruction of her religious middle school. She made me think that college essays are less like love than limerence: one-sided, obsessive, idiosyncratic but profound, the first draft of the most personal story their writers will ever tell.
Nell Freudenberger’s novel “The Limits” was published by Knopf last month. She volunteers through the PEN America Writers in the Schools program.
Jump to navigation
Forgotten Spaces: Ecocriticism Social Justice, and the U.S. South (Collection of Essays)
The U.S. South is often a forgotten space within ecocritical discussions, yet it provides fruitful ground for thinking about environmental issues. In 2019, in the first edited collection of essays on the topic, Zachary Vernon notes that focusing attention on this bioregion might help “provide a way out of the limitations of thinking too locally or too globally,” and it might inspire a group of stakeholders to come to the table as well (7). One problem with ecocritical approaches is the long history of representing the U.S. South as an “internal other in the national imagination: colonized, subordinate, primitive, developmentally arrested, or even regressive” (Watson 254). Another issue is that both the environmental humanities and Southern studies have frequently been white spaces. This proposed anthology convenes a conversation about the U.S. South and environmental issues with an eye towards social justice. We seek theoretically-sophisticated essays attentive to intersections between race, class, gender, and sexuality within the U.S. South to round out our proposed collection. Interdisciplinary environmental research from a variety of frameworks and disciplines is welcome, including literature, film, art, history, popular culture, public memory, sociology, political science, and geography.
Questions to consider:
- Why does the U.S. South seem like a forgotten space within ecocritical discussions?
- How do we reach across entrenched divides and academic silos to engage in cross-disciplinary engagement with ecocritical concerns about the South?
- What entanglements might we find between race, environment, gender, sexuality, class, and social justice?
- How have artists, writers, activists, and cultural workers of color engaged with representing the environment, and what might their creative labor contribute to wider discussions beyond the academy?
- How are rural and urban environments represented in the U.S. South? How are they represented from outside?
- What constitutes the commons in the South? Was there ever really a Southern commons?
- How are public parks, museums, and recreation areas curated in the South, and what might we learn about entanglements between race and the environment through attending to these spaces?
- What is the history of traveling southward or leaving the South? What kinds of cultural constructions represent the region as a place to return to or escape from?
- How might we interrogate Donna Haraway’s phrase “the plantationocene” to consider the vexed history of work, nature, and captivity in Southern spaces?
- How might we consider Settler colonialism, genocide, and Indian Removal within an ecocritical framework? How has a legacy of Settler colonialist violence in the South impacted the environment?
- Can indigenous practices, beliefs, and cultural production be mobilized towards a Southern ecocriticism?
- What are the many varieties of experience within different souths?
Other possible topics:
- Climate change and its impact on southern spaces. Southern climate diaspora.
- Hurricanes, floods, tornados. Natural disasters and social justice.
- Disaster capitalism and southern spaces.
- Sacrifice zones. Industrial pollution.
- Carceral, military, and/or institutional Southern spaces.
- Queer ecology and queer ecological souths.
- Global approaches to environment and the U.S. South.
- Animals and animality in southern cultural productions. Domestic/wild/wilding.
- Southern megacities and the built environment in the U.S. South.
- Race and nature in the South.
- White supremacy and public spaces.
We seek MLA-formatted essays from 4,000-7,000 words. Please submit abstracts of 250-500 words by July 15, 2024. Notification of acceptance will be made by Aug. 1, 2024. And final essays will be due October 15, 2024. We will be submitting the proposal, table of contents, and sample essays to academic presses by Aug. 1, 2024.
Send abstracts and questions to: Katie Simon, Georgia College and State University, [email protected] and Catherine Bowlin, Elon University, [email protected]

What does John Green's book of essays say about the Indy 500? About the Indianapolis nod
Author John Green is no stranger to Indianapolis and the Indy 500, which is Sunday at the Indianapolis Motor Speedway.
Green has many works in his back pocket, including several with nods to Indianapolis. It seems fitting to revisit some of the mentions as we wait for drivers to start their engines.
The IndyStar has several guides to get fans ready for the Greatest Spectacle in Racing including a printable starting lineup , how to tune in to the race from outside the racetrack and what people can bring to the Indianapolis Motor Speedway.
Start the day smarter. Get all the news you need in your inbox each morning.
What to know about John Green and the Indy 500:
What does John Green's book of essays say about the Indy 500?
In " The Anthropocene Reviewed ," Green writes essays reviewing different topics from Halley's Comet to Diet Dr Pepper and even the Indianapolis 500, the IndyStar previously reported.
He wrote the Indy 500 review during the pandemic.
“I wanted to write about my experience of suddenly being unable to go to the race, and how it felt to go through all the same rituals that I always go through on that Sunday, and to bike to the race as I always do and to arrive at an empty Speedway, with the gates locked shut."
"It can be hard at times because we have to get used to a new normal to be able to reflect on how much has been lost in the last year and a half," he said. "And obviously the loss of fans at the speedway wasn't one of the big losses, but it was a loss. One loss among billions. For me, it was a way to feel that."
But people don't have to feel that loss again as they can attend the race on Sunday.
The book, which was released in 2021, is his first work of nonfiction and is inspired by his podcast of the same name where he also published monthly reviews.
'The Anthropocene Reviewed': John Green's new nonfiction book finds wonder in Diet Dr Pepper, Indianapolis 500
What John Green books mention Indianapolis?
"The Fault in Our Stars" and "Turtles All the Way Down" are both situated in Indianapolis.
In the latter, there are many references to the city, including:
- White River
- Pogue's Run
- Michigan Road mansion
- Applebee’s at 86th and Ditch
- IU Health North Hospital
- The Indianapolis Star
- The Indianapolis Prize
- Juan Solomon Park
Others are reading: John Green’s ‘Turtles’ at home in Indianapolis
Is John Green from Indianapolis?
Not originally.
In his webpage , Green states that he grew up in Orlando. He moved to Indianapolis in 2007 when his wife got a job at the Indianapolis Museum of Art, the IndyStar previously reported.
John Green on TikTok: Author still can't stop talking about how great Indianapolis is
How to watch 'Turtles All the Way Down'
The movie adaptation is now available streaming on Max .
When is the 2024 Indy 500?
This year's Indy 500 race is on Sunday at the Indianapolis Motor Speedway.
David Lindquist, Rachel Fradette and Ethan May contributed to this article.
This article originally appeared on Louisville Courier Journal: What does John Green's book of essays say about the Indy 500? About the Indianapolis nod

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ther Adv Vaccines Immunother

Comprehensive literature review on COVID-19 vaccines and role of SARS-CoV-2 variants in the pandemic
Charles yap.
School of Medicine, National University of Ireland, Galway, Ireland
Abulhassan Ali
Amogh prabhakar, akul prabhakar, ying yi lim, pramath kakodkar.
School of Medicine, National University of Ireland, Galway, University Road, Galway H91 TK33, Ireland
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although the current preventive measures are primarily socially distancing by maintaining a 1 m distance, it is supplemented using facial masks and other personal hygiene measures. However, the induction of vaccines as primary prevention is crucial to eradicating the disease to attempt restoration to normalcy. This literature review aims to describe the physiology of the vaccines and how the spike protein is used as a target to elicit an antibody-dependent immune response in humans. Furthermore, the overview, dosing strategies, efficacy, and side effects will be discussed for the notable vaccines: BioNTech/Pfizer, Moderna, AstraZeneca, Janssen, Gamaleya, and SinoVac. In addition, the development of other prominent COVID-19 vaccines will be highlighted alongside the sustainability of the vaccine-mediated immune response and current contraindications. As the research is rapidly expanding, we have looked at the association between pregnancy and COVID-19 vaccinations, in addition to the current reviews on the mixing of vaccines. Finally, the prominent emerging variants of concern are described, and the efficacy of the notable vaccines toward these variants has been summarized.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there have been devasting effects in other sectors contributing to our economy. This has plunged the global economies toward deep recession and has racked up a debt of approximately 19.5 trillion USD. 2
Immune protection in COVID-19 infection can be conceptualized as a spectrum wherein sterile immunity is at the end of positive spectrum. This is followed by transient infection (<3 days) and asymptomatic infection (~1 week). The negative spectrum of immune protection includes patients who are symptomatic, or hospitalized, or admitted to the intensive care unit for multiorgan support. The extreme end of the negative spectrum of immune protection is encompassed by case fatality. The vaccine will intervene prior to the viral insult and stabilize the population at the positive end of the spectrum of the immune protection. It will also prevent the perpetuating cycle of infection and reinfection via variants of SARS-CoV-2 virus in those who have achieved prior convalescence. One study by Dan et al. showed that in patients infected with COVID-19, immunological memory to SARS-CoV-2 remained intact for up to 6 months. 3 Unfortunately, there is no long-term data on the duration of protected immunity against SARS-CoV-2 in patients after convalescence. Therefore, these patients may also require vaccination but the current priority for vaccination can be stretched relative to the unaffected population.
While the ideal goal of the COVID-19 vaccine roll-out is to instill a global herd immunity; it is important to remember that this goal may never be reached. Furthermore, additional goals of vaccination may be to reduce mortality and stress on healthcare systems by reducing the cases of admitted patients. Various countries have already approved COVID-19 vaccines for human use, and more are expected to be licensed in the upcoming year. It is important that these vaccines are safe, efficacious, and can be deployed on a large scale. It is also prudent to eliminate the concerns of both the scientific and general community regarding its effectiveness, side-effects, and dosing strategies.
Historically, the process of vaccine manufacturing and clinical trials required approximately 10 years, but due to the burden of this disease, various observational studies were expedited so that all crucial information regarding the vaccine pharmacokinetics, pharmacodynamics, dosing, efficacy, and adverse events can be collected within a short period of time. Furthermore, there is a need to provide a compilation of accredited and appraised scientific literature on each of these approved vaccines with an aim to instill public health knowledge and vaccine literacy to members of the scientific and general community. A section dedicated to COVID-19 vaccines and pregnancy is also included in the penultimate section of this review.
Finally, the emergence of the SARS-CoV-2 viral variants of concern (VOC) has attained increased replication, transmission, and infectivity warranting exploration of these genomic mutations as their phenotypes. Hence, the final section of this review will aim to clarify the jargon, highlight the vaccine efficacy (VE) against VOCs, and eliminate any misinformation regarding these variants.
Vaccine physiology
The global burden of the pandemic requires an efficacious vaccine that elicits a lasting protective immune response against SARS-CoV-2. This will be an essential armament for the prevention and mitigation of the downstream morbidity and mortality caused by SARS-CoV-2 infection. As of July 20, 2021, there are approximately 108 vaccines in clinical development and 184 vaccines in pre-clinical development with several vaccines being distributed globally. 4
The technologies employed in the vaccine synthesis and development aim to trigger the adaptive immune system and elicit memory cells that will protect the body from subsequent infections. These technologies may be mRNA-based vaccines such as the Moderna and Pfizer/BioNTech, inactivated virus vector vaccines, DNA vaccines, and numerous other technologies. 5
Due to the urgent implementation of vaccine development, the most obvious target will be the robust proteins expressed on the surface of the virus. Therefore, these technologies target molecular expression of the trimeric SARS-CoV-2 spike (S) glycoprotein. These targets could include its mRNA, DNA, full S1 subunit, or fusion subunits. The S protein is a major component of the virus envelope, it is vital for viral fusion, receptor binding, and virus-entry through recognition of host-cellular receptor. The S protein comprises of two main functional units, the S1 subunit, which contains the receptor-binding domain (RBD) and the S2 subunit which is responsible for virus fusion with the host-cell membrane. 6 The choice to proceed with S protein as the target was reinforced when a study by Dan et al. confirmed that in 169 patients infected with SARS-CoV-2, spike-specific immunoglobulin G (IgG) remained stable for over 6 months. 3 In addition, both spike-specific CD4+ T-cells (CD137+ and OX40+) and spike-specific CD8+ T-cells (CD69+ and CD137+) were present at the 6-month post-convalescence period, but their subpopulations exhibited a steady decline with a half-life of 139 days and 225 days, respectively. 3
There are subtle differences in the mechanism by which the different vaccine products interact within host cells to induce immunity. Many successful vaccines of the 20 century utilized the target proteins directly such as the tetanus and pertussis vaccine. A summary of the major types of vaccines and their mechanism of action are shown in Figure 1 .

Summary of major vaccine types and their mechanism of action.
DNA, deoxyribonucleic acid; HPV, Human papillomavirus; mRNA, messenger ribonucleic acid; MMR, Measles, Mumps, and Rubella; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Historically, vaccines usually contained adjuvants which are protein sensitizers that heighten the migratory and sampling response of antigen presenting cells (APCs). Interestingly, the current mRNA vaccines are engineered to code for their own sensitizing protein alongside the S-protein epitopes. Therefore, these new mRNA vaccines usually do not contain any adjuvants. In addition, the mRNA vaccines utilize lipid nanoparticles to deliver the genetic material of a viral S-protein. Contrastingly, vaccines such as the AstraZeneca vaccine may employ a chimpanzee adenovirus vector to carry the DNA genome of the S-protein to the host-cell. 7 Once undergoing the processes of transcription and translation into proteins, these are trafficked and expressed on the host cell surface wherein the adaptive immune system mounts a response via the major histocompatibility complex (MHC) molecules ( Figure 2 ).

Mechanism of induction of immunity through vaccination.
APC, antigen presenting cells; DNA, deoxyribonucleic acid; MHC, major histocompatibility complex; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There are two types of MHC molecules, the first one that will be discussed is the MHC-II, which is found exclusively on APC: these comprise of B-cells, macrophages, and dendritic cells in the lymph nodes. Once the S-protein antigen is presented at the cell surface of the MHC-II molecules, the naïve helper T-cell’s (Th Cells) T-cell receptor (TCR) complex will interact with this antigen leading to activation of CD4+ Th cells. This activation is perpetuated by a secondary activation signal with B7 on the APC recognizing the CD28 on the Th cell which triggers the proliferation of Th cells that can recognize the S-protein antigen. Activated CD4+ Th cells then secrete numerous cytokines, namely interleukin (IL)-2 which activates CD8+ cytotoxic T-cells (Tc cell) and trigger clonal expansion of B-cells in memory B-cells and plasma cells. The cytokines IL-4 and IL-5 facilitate B-cell isotype switching and maturation to plasma cells; promoting secretion of IgG antibodies against S-protein. 8 Formation of antibodies allows the immune system to direct an immune response against cells expressing the S-protein of the virus. The second process involves MHC-I, which activates CD8+ Naïve Tc cells through TCR complex interaction with processed endogenously synthesized S-protein expressed on MHC-I. MHC-I is expressed in all nucleated cells, APCs, and platelets and require a second activation signal provided by IL-2 from activated CD4+ Th cells. This activates CD8+ Tc cells which can mount a cytotoxic response against SARS-CoV-2-infected cells through two mechanisms of apoptosis. The first mechanism is the secretion of perforin which create pores to allow granzyme to enter the targeted cell, thus activating apoptosis. The second mechanism is via the expression of FasL, which binds Fas on target cells and induces apoptosis. 8 A crucial part of this process is the stimulation of memory T-cells and memory B-cells. Importantly, while the SARS-CoV-2 vaccine’s lasting effect is still being researched in the context of the pandemic, theoretically these should provide lasting immunity and allow the immune system to mount a faster and more effective response should a vaccinated individual encounter the virus in the future.
Current prominent COVID-19 vaccines
Biontech/pfizer.
The BNT162b2 COVID-19 vaccine developed by BioNTech and Pfizer is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion membrane-anchored SARS-CoV-2 full-length spike protein. 9 It was the first vaccine approved by the US Food and Drug Association (FDA) and now it has been approved in many other countries. 10 The BNT162b2 COVID-19 vaccine may be stored at standard refrigerator temperatures prior to use, but it requires very cold temperatures for long-term storage and shipping (−70°C) to maintain the stability of the lipid nanoparticle. In a phase-1 trial, it was compared to another vaccine candidate BNT162b1, and it was found to have a milder systemic side-effect profile with a similar antibody response. 11 Therefore, it was pushed forward to a blinded phase-2/3 clinical study. 9 In total, 43,548 participants were randomized to receive either two doses of the BNT162b2 vaccine (n = 21,720) or a placebo (n = 21,728) 21 days apart. The participant ages ranged from 16 to 91 years, 35.1% of participants were classified as having obesity and comorbidities within participants included HIV, malignancy, diabetes, and vascular diseases. 9 Based on the results of the study, 7 days after the second BNT162b2 dose, the VE was 95% (95% confidence interval (CI), 90.3–97.6) with only eight observed cases of COVID-19 in the vaccine recipients and 162 cases in the placebo recipients. 9 The efficacy remained consistent across subgroups characterized by age, sex, race, ethnicity, body mass index (BMI), and comorbidities (generally 90–100%). 9 Although there were 10 cases of severe COVID-19 with onset after the first dose, only one occurred in a vaccine recipient and nine in placebo recipients. Like the phase-1 trial results, the safety profile remained favorable with the most common local reaction being mild-to-moderate pain at the injection site while the most common systemic symptoms were fatigue and headache (reported in ⩾50%). 9 In both the vaccine and placebo group, the incidence of severe adverse events did not differ significantly (0.6% and 0.5%, respectively) and no deaths occurred related to the vaccine. As indicated by the manufacturer’s information, contraindications for use include hypersensitivity to the active substance or any of the excipients. 12 These studies show that the mRNA-vaccine BNT162b2 is safe and effective in protecting against COVID-19. However, further investigations are needed to confirm long-term safety and to establish safety and efficacy for populations not included in this study.
The mRNA-1273 vaccine, developed by Moderna, relies on mRNA technology to encode prefusion stabilized SARS-CoV-2 spike protein. It is the second COVID-19 vaccine to receive emergency use approval by the US FDA, and it is given as two 100-µg doses intramuscularly into the deltoid muscle, 28 days apart. 13 Storage of the vaccine is done at temperatures between −25°C to −15°C for long-term storage, 2°C to 8°C for 30 days, or 8°C to 25°C for up to 12 hours. Results from the COVE phase-3 trial showed that the mRNA-1273 vaccine was effective at preventing COVID-19 illness in persons 18 years of age or older. A total of 30,420 participants aged 18 years or older were randomized 1:1 to receive either two doses of the vaccine or a placebo, 28 days apart. 14 The mean age of the participants was 51.4 years, and enrollment was adjusted for equal representation of racial and ethnic minorities. In the trial, symptomatic COVID-19 illness occurred in 11 participants within the vaccine group versus 185 participants within the placebo group, showing a 94.1% (95% CI, 89.3–96.8%) efficacy of the vaccine. Efficacy was similar across age, sex, race, and ethnicity as well as in patients with and without risk factors for severe disease (e.g. chronic lung disease, cardiac disease, and severe obesity). Importantly, a secondary endpoint for determining the efficacy of the vaccine in preventing severe COVID-19 was also used. All 30 participants with severe COVID-19 were in the placebo group, indicating a 100% efficacy of no hospital admissions. 14 Regarding the side effects of the vaccine, adverse events at the injection site and systemic adverse events occurred more commonly with the mRNA-1273 group compared to the placebo. The most common local reaction was mild to moderate pain at the injection site (75%). The most common systemic symptoms were fatigue, myalgia, arthralgia, and headache (50%). 14 The overall incidence of serious adverse events did not differ significantly between groups and no deaths occurred in relation to the vaccine. While this vaccine is already being administered, further investigations are still necessary to establish safety and efficacy profiles for populations not included in this study as well as to assess its long-term effects. Current contraindications of the mRNA-1273 vaccine include any persons with known allergy to polyethylene glycol (PEG), another mRNA vaccine component or polysorbate. 15
AstraZeneca
The Oxford and AstraZeneca ChAdOx1 COVID-19 vaccine uses a chimpanzee adenovirus vector to deliver the genetic sequence of a full-length spike protein of SARS-CoV-2 into host cells. 16 The storage for the ChAdOx1 vaccine is favorable, as it may be refrigerated at 2°C–8°C for 6 months. Pooled analysis of four ongoing clinical studies was used to assess efficacy, safety, and immunogenicity of the ChAdOx1 vaccine: COV001 (phase 1/2), COV002 (phase 2/3), COV003 (phase 3), and COV005 (phase 1/2). 17 Across the four studies participants over 18 were randomized to receive either the vaccine or a control (meningococcal group A, C, W, or saline). ChAdOx1 vaccine recipients received two standard doses (SDs) of the vaccine (SD/SD cohort) except for a subset in the COV002 trial who received a half lower dose (LD) followed by an SD (LD/SD cohort). 17 In the four studies, there was a total 23,848 participants, all of whom were used for gathering safety data; only 11,636 participants from the COV002 and COV003 trials were included in the primary efficacy analysis. 17 Of the 11,636 participants in the efficacy analysis, 2741 were in the LD/SD cohort, 88% were between 18 and 55 years old, and comorbidities present included cardiovascular disease, respiratory disease, and diabetes. 17 The results show that in the intended dosing regimen (SD/SD cohort), the VE was 62.1% (95% CI, 41.0–75.7) ⩾14 days after the second injection for symptomatic COVID-19 (27 cases vs 71 cases respectively). 17 In the group that received an LD (LD/SD cohort), the VE was 90.0% (95% CI, 67.4–97.0; 3 cases vs 30 cases, respectively) while across the two dosing regimens the overall efficacy was 70.4% (95.8% CI, 54.8–80.6;30 cases vs 101 cases, respectively). 17 The higher efficacy observed in the LD/SD cohort can be attributed to this group having a longer dosing interval between the two doses in comparison to the SD/SD cohort. Regarding safety, most of the adverse events were mild-moderate with the most frequently reported being injection site pain/tenderness, fatigue, headache, malaise, and myalgia. 18 About 175 serious adverse events were noted, only three of which were possibly linked to intervention: transverse myelitis 14 days after second dose, haemolytic anemia in a control recipient and fever >40°C in a participant still masked to group allocation. One contraindication for use of the vaccine is hypersensitivity to any of its components. In very rare cases, AstraZeneca has been associated internationally with venous thromboembolic events with thrombocytopenia with current estimates being 10–15 cases per million vaccinated patients. 19 This adverse event has been termed thrombosis with thrombocytopenia syndrome (TTS). In summary, these studies demonstrate that the AstraZeneca ChAdOx1 vaccine has a good efficacy and side-effect profile. Limitations include that less than 4% of participants were >70, no one over 55 got the mixed-dose regimen (LD/SD cohort), and those with comorbidities were a minority. Additional investigations are required to analyze long-term effects and assess efficacy and safety in populations not included or underrepresented.
Janssen COVID-19 vaccine
The Janssen (Johnson & Johnson) COVID-19 vaccine, developed by Janssen Pharmaceutical in Netherlands. It is a single-dose intramuscular (IM) vaccine that contains a recombinant, replication incompetent human adenovirus (Ad26) vector encoding the spike protein of SARS-CoV-2 in the stabilized conformation. 20 It can be stored between 2°C and 8°C for up to 6 hours or at room temperature for a duration of 2 hours. The ENSEMBLE Phase-3 trial (n = 43,783) is a randomized, double-blind, placebo-controlled study which included participants ⩾18 years. Efficacy assessment was performed at day 14 and 28. The primary outcome only included moderate and severe (hospitalization and death) infection. Overall, the VE in the moderate to severe cohort was 66.9% (95% CI: 59.0–73.4) at 14 days and 66.1% (95% CI: 55.0–74.8) at 28 days. 20 In the severe cohort, the VE was 76.7% (95% CI: 54.6–89.1) and 85.4% (95% CI: 54.2–96.9) at day 14 and 28 days, respectively. 20 At the time of the study, 96.4% of the strains in the United States, 96.4% were identified as the Wuhan-H1 variant D614G. The VE in the United States for the moderate to severe cohort was 74.4% (95% CI: 65.0–81.6) and 72.0% (95% CI: 58.2–81.7) at 14 days and 28 days, respectively. 20 In the US severe cohort, the VE was 78.0% (95% CI: 33.1–94.6) and 85.9% (95% CI: −9.4 to 99.7) at day 14 and 28 days, respectively. 20 Alternatively, 94.5% of the strains in South Africa were identified as beta variant. The VE in South Africa for the moderate to severe cohort was 52.0% (95% CI: 30.3–67.4) and 64.0% (95% CI: 41.2–78.7) at 14 days and 28 days, respectively. 20 In the South African severe cohort, the VE was 73.1% (95% CI: 40.0–89.4) and 81.7% (95% CI: 46.2–95.4) at day 14 and 28 days, respectively. 20 In Brazil, 69.4% of the strains were identified as P.2 lineage variant and 30.6% were identified as Wuhan-H1 variant D614G. The VE in Brazil for the moderate to severe cohort was 66.2% (95% CI: 51.0–77.1) and 68.1% (95% CI: 48.8–80.7) at 14 days and 28 days, respectively. 20 In the Brazilian severe cohort, the VE was 81.9% (95% CI: 17.0–98.1) and 87.6% (95% CI: 7.8–99.7) at day 14 and 28 days, respectively. 20 The most common localized solitary adverse reaction was the injection site pain (48.6%). Conversely, the most common systemic adverse reactions included headache, fatigue, myalgia, and nausea. 20 In the post authorization phase, adverse reaction included anaphylaxis, thrombosis with thrombocytopenia, Guillain Barré syndrome, and capillary leak syndrome. 20 Overall, the data demonstrate that the Janssen vaccine has a good efficacy and side-effect profile.
Sputnik V or Gam-COVID-Vac, developed by the Gamaleya Institute, is a recombinant human adenovirus-based vaccine that uses two different vectors (rAd26 and rAd5) to carry the gene encoding for the spike protein of SARS-CoV-2. Only one vector (rAd26) is given at dose 1 and the other (rAd5) at dose 2. This strategy prevents immunity against the vector. It can be stored as either a liquid at −18°C, or it can be freeze-dried and stored at 2°C to 8°C. 21 Regarding the safety and efficacy of the vaccine, both were evaluated in a randomized, double-blind phase-3 trial performed in Moscow, Russia. In the trial, a total of 21,977 participants aged 18 years or older were randomized in a 3:1 ratio to the vaccine or placebo groups. Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian (98.5%). 21 From 21 days after the first dose of the vaccine, efficacy against symptomatic COVID-19 illness was 91.6% (95% CI, 85.6–95.2%) with 16 confirmed cases of COVID-19 in the vaccine group and 62 confirmed in the placebo group. 21 There were also 20 cases of moderate to severe COVID-19 infection confirmed in the placebo group at least 21 days after the first dose and 0 in the vaccine group, indicating a VE of 100% against moderate to severe infection. 21 The most common adverse effects in both groups were flu-like illness, injection site reactions, headaches, and asthenia, with the majority being grade 1 (94.0%). 21 Serious adverse events were also reported in both the vaccine group and placebo group, but they were deemed not to be associated with the vaccination. Further investigations are still needed to determine the duration of protection of the vaccine and to determine the safety and efficacy of the vaccine in populations not included in the study (e.g. children, adolescents, and pregnant and lactating women).
CoronaVac is an inactivated vaccine developed by SinoVac Biotech containing inactivated SARS-CoV-2. 22 The vaccine can be stored at 2°C to 8°C for up to 3 years making it an attractive option for development. Two phase-1/2 clinical trials assessed the safety, tolerability, and immunogenicity of the CoronaVac vaccine. 22 , 23 The first study (18–59 years old included only) placed 744 participants in either a vaccine or placebo group where they were further divided based on vaccination schedule and dosage (3 and 6 μg). In the second study (⩾60 years old included only), 422 participants were randomized to receive two doses of CoronaVac or placebo 28 days apart and then further divided based on dosage amount only (3 and 6 μg for phase 1; 1.5, 3, and 6 μg for phase 2). Safety results from both trials show a favorable side-effect profile with most symptoms being transient and of mild severity. The most common adverse effect was injection site pain; others included fatigue and fever. In the 18–59 years old study, one serious adverse event of acute hypersensitivity was possibly related to vaccination. 22 No serious adverse events were associated with the vaccine or placebo in the ⩾60-year-old study. Between the dosage amounts in both studies, the tolerability was consistent and the immunogenicity was also similar for the 3 and 6 μg doses (less in 1.5 μg). 23 Multiple phase-3 trials have also taken place to determine the effectiveness of CoronaVac in countries, such as Brazil, Indonesia, and Turkey. In the Brazil trial, 252 cases of COVID-19 were recorded from roughly 9200 health care workers, with 167 in the placebo group and 85 in the vaccine group. 24 The reported efficacy of the vaccine in preventing mild and severe COVID-19 infection was 50.4%. In comparison, the Turkey trial reported that the vaccine was 83.5% effective at preventing symptomatic infection based on 29 COVID-19 cases among 1,322 volunteers while results from the Indonesia trial found that the vaccine was 65.3% effective at preventing symptomatic infection based on 25 COVID-19 cases among 1,600 people. 24 Some reasons for the lower efficacy of CoronaVac in the Brazil trial may include increased likelihood of exposure to the virus since participants were healthcare workers, and insufficient time for participants to reach peak immunity since the doses were administered only 2 weeks apart. 24 The phase-3 SinoVac study in Chile showed the VE 14 days post second dose to prevent symptomatic COVID-19 (67%, 95% CI: 65–69%), hospital admission (85%, 95% CI: 83–87%), intensive care unit (ICU) admission (89%, 95%CI: 84–92%) and death (80%, 95%CI: 73–86%). 25 The Phase-3 SinoVac trial in Brazil showed an overall VE against symptomatic COVID-19 (50.7%, 95% CI: 35.9–62%), moderate cases requiring hospitalization (83.7%, 95% CI: 58–93.7%), and severe cases requiring hospitalization (100%, 95%CI: 56.4–100%). 26 As with any vaccine, a contraindication for CoronaVac is anaphylaxis to it or to one of its constituents.
Other prominent COVID-19 vaccines
Due to the disease burden of SARS-CoV-2, the development and manufacturing of COVID-19 vaccines has been occurring at a remarkable pace which has not been seen before. There are many emerging vaccines with different mechanisms of actions that will be briefly explored. Bharat Biotech, an Indian company, has designed the inactivated COVID-19 vaccine Covaxin (BBV152). Once inside the body, the inactivated viruses can initiate an immune response through the interaction of surface proteins with APCs. Phase-1/2 trials showed no serious side effects and phase-3 trials are currently underway. 27 The state-owned Chinese company Sinopharm has also made an inactivated COVID-19 vaccine called BBIBP-CorV. The Sinopharm phase-3 trial showed that the VE in symptomatic cases for the WIV04 strain-based vaccine (72.8, 95% CI: 58.1–82.4%) and HB02 strain-based vaccine (78.1 95% CI: 64.8–86.3%). 28 , 29 It is approved in Bahrain, U.A.E, and China. NVX-CoV2373 is another promising vaccine produced by Novavax. It is a protein subunit vaccine made by assembling SARS-CoV-2 spike proteins into nanoparticles. A phase-3 trial in the United Kingdom displayed an efficacy rate of 89.3%; however, a phase-2 trial in South Africa had an efficacy just under 50%. 28 This discrepancy is thought to arise because of a new variant in South Africa. Other emerging vaccines include CoVLP produced by Medicago which uses the plant N. benthamiana to create virus-like particles that mimic SARS-CoV-2, CVnCoV produced by CureVac which is an mRNA vaccine, Convidecia produced by CanSino Biologics which is adenovirus based (Ad5), Ad26.COV2.S produced by Johnson & Johnson which is also adenovirus based (Ad26), and ZF2001 created by Anhui Zhifei Longcom which is a protein subunit vaccine. Even though highly effective, COVID-19 vaccines are already in use, it is still important to have a range of vaccines such as those listed above to bring the pandemic under control. Having a diverse profile ensures that vaccines will work for individuals from all ethnic backgrounds and with various underlying health conditions. 30 Getting the virus under control will also require doses for a large proportion of the world. To meet this requirement as soon as possible, having multiple vaccines will help in maximizing the volume of doses that can be produced. In addition, there are many technical issues such as cold storage and transportation, cost, and dosing of certain vaccines that arise when trying to vaccinate remote populations. For example, both the Pfizer-BioNTech and Moderna vaccines are expensive and transported at temperatures of −70°C and −20°C making it difficult to access many locations all at once. Since most vaccines require two doses spaced a few weeks apart, it can be challenging for individuals without regular access to healthcare as well. 30 Such considerations highlight the importance of having a range of single-dose vaccines and vaccines without the need for cold storage. A summary of efficacy, prominent side effects and storage recommendations for all the notable COVID-19 vaccines are shown in Table 1 .
Summary of vaccine efficacy, dosing strategy, and side-effects of different COVID-19 vaccines.
CI, confidence interval; COVID-19, coronavirus disease 2019; IM, intramuscular.
Post-vaccination contagion
With the endurance of the COVID-19 vaccine still being heavily researched, a chief concern is the sustainability of the vaccine-mediated immune response. This is important in the consideration of whether vaccinated individuals could still contract, transmit, or be carriers of SARS-CoV-2 virus. Vaccinated individuals currently may not understand the rationale behind why social restriction rules still apply to them. Most COVID-19 mRNA vaccines require at least 3 weeks to mount an immunological response and create the required antibodies and proliferate accessory cells of the adaptive immune system of the appropriate recognition repertoire. 50 This may be particularly relevant in the context of travel, as the World Health Organization (WHO) states that a proof of vaccination should not exempt international travelers from complying with social restrictions and risk-reduction measures. 51
Contraindications for COVID-19 vaccines
All vaccines are contraindicated in cases of documented hypersensitivity to the active substance or any of the excipients. There are a set of general guidelines relative to patients which must be adhered to until further information is provided; predominantly regarding groups such as pregnant or lactating women and immunodeficient patients. The Centers for Disease Control and Prevention (CDC) considers absolute contraindications to patients who have had severe anaphylactic reactions to a previous dose of an mRNA COVID-19 vaccine or PEG, a component of the vaccine. Moreover, immediate allergic reactions of any severity to polysorbate are also a significant contraindication. Importantly, there are many precautions which are not classified as contraindications but must be considered, such as patients who have had allergic reactions to any vaccine or injectable therapy. In the cases of patients with a precaution to the vaccine, they should be counseled on the benefits and risks, but are not contraindicated from vaccination. 15 In the instance of patients with autoimmune diseases, there is currently insubstantial data regarding the efficacy of the vaccine; however, current guidelines suggest that individuals with autoimmune conditions may take the vaccine if they do not have any absolute contraindications. In the case of patients with HIV, limited data from COVID-19 mRNA vaccination trials suggest that they can receive the vaccine barring any contraindications.
COVID-19 vaccines and pregnancy
Prior to discussing the relationship between the current vaccines for COVID-19 and pregnancy, it is crucial to gain an insight of the relationship between pregnancy and COVID-19 itself. Adhikari et al. showed that there was no difference in the frequency of Caesarean section, pre-eclampsia, preterm births, and abnormal fetal cardiotocography in pregnant women with and without SARS-CoV-2 infection. In addition, examination of the placenta revealed were no abnormalities, which were initially suspected due to the cross-matching between the SARS-CoV-2 spike protein and the placental synctyin-1 protein. 52 Similarly, there was no association found between COVID-19 and first-trimester spontaneous abortions. 53 A systematic review and meta-analysis revealed that COVID-19 leads to higher preterm deliveries (odds ratio (OR): 3.01, 95% CI: 1.16–7.85) and an increase in the ICU admission rates (OR: 71.63, 95% CI: 9.81–523.06) in pregnant women. 54
Pregnancy remained an exclusion criterion for all the COVID-19 vaccine trial; therefore, the efficacy of the COVID-19 vaccines in pregnant women is unavailable. However, given the effectiveness of the influenza vaccines elucidated in a meta-analysis conducted by Quach et al ., it can be hypothesized that the effects of pregnancy on the vaccine would be minimal, but more data would be needed for confirmation. 55 Pfizer’s animal studies revealed antibodies in the maternal rats, fetus, and offspring, in addition to no effects on fertility pregnancy or fetal development. 56 A similar study was conducted with the Moderna vaccine which led the US FDA to conclude that the vaccine did not have any adverse effects on female reproduction, fetal development, or postnatal development. 34 Furthermore, the Oxford-AstraZeneca vaccine animal studies are still pending. However, as a precaution, the National Immunization Advisory Committee (NIAC) has recommended for the two-dose schedule to not commence before 14 weeks of gestation and to be completed by week 33 of gestation. This precaution reduces any potential associations with miscarriage or pre-term birth. 57
Despite the exclusion of pregnancy in the preliminary stages of the trials, 23 Pfizer, 13 Moderna, and 21 AstraZeneca subjects became pregnant after enrolment into the trial. Among this cohort, there was one miscarriage part of the Pfizer control group, no miscarriages part of the Pfizer vaccine group, one miscarriage part of the Moderna control group, no miscarriages part of the Moderna vaccine group, three miscarriages part of the AstraZeneca control group, and two miscarriages part of the AstraZeneca vaccine group. While these preliminary numbers support the current guidelines regarding the vaccines being safe in pregnancy, it is crucial to be aware of the ongoing studies as new data emerges.
The CDC v-safe COVID-19 Pregnancy Study explored the effect of mRNA vaccine (Pfizer-BioNTech or Moderna) on the pregnancy. The pregnancy loss within those with a completed pregnancy included a spontaneous abortion (<20 weeks) rate of 12.6% (104 out of 827) and stillbirth (⩾20 weeks) incidence of 0.1% (1 out of 725). 58 The neonatal outcomes within the live birth infant cohort showed preterm birth (<37 weeks) incidence at 9.4% (60 out of 636), small for gestational age incidence of 3.2% (23 out of 724), and congenital anomalies were seen in 2.2% (16 out of 724). 58 No neonatal deaths were observed in this study.
Vaccine dosing strategies
Limited vaccine resources have caused some governments to extend the date of the second dose beyond the recommended manufacturer date. On December 30, NHS England had made the decision to prioritize the administration of the first doses, and to extend the second doses of the vaccine to the end of 12 weeks, rather than the recommended 3–4 weeks as shown in the clinical phase-3 trial. Pfizer-BioNTech at the time had no data to support this decision, and thus stated that the safety and efficacy of the vaccine had not been evaluated on different dosing schedules, and importantly, the second dose should not be administered later than 42 days. 59
Newly accrued evidence might warrant changes in the landscape of this vaccination program. Estimation of the effectiveness of the Pfizer-BioNTech after a single dose from the primary data from Israeli population (n = 500,000) showed that from day 0 to day 8 post–vaccination, the likelihood of contracting COVID-19 infection doubled. 60 This result may appear counterintuitive, but it takes 3 weeks for the vaccine to instill efficacy during which this real-world population could have not maintained the stringent public health measures which lead to the increased incidence in COVID-19 in this time-period. Then from day 8 to day 21 the incidence of COVID-19 declined and at day 21 the vaccine effectiveness was documented at 91%. 60 This efficacy was seen to stabilize at 90% for the duration of the study (9 weeks), and the authors of this study extrapolate this stability up to 6 months. 60 This concludes that the single dose of Pfizer-BioNTech is highly protective from day 21 onwards and supports the NHS England’s vaccination policy for extending gaps between the doses. The data from the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) trial in the Scottish population revealed that a single dose of Pfizer (n = 650,000) and Oxford-AstraZeneca (n = 490,000) vaccines resulted in a decline in hospitalization at 4 weeks by 84% and 94%, respectively. 61
However, the trials for the Oxford-AstraZeneca vaccine included varied spacing schedules between doses. The findings from these trials displayed that a greater space between the first and second dose provided a superior immune response. This is supported by a combined trial between a UK and Brazil study, which demonstrated a higher VE 14 days after a second dose in patients who had greater than 6 weeks between their first and second dose than patients who had less than 6 weeks by 53.4%. 17 , 62
It was also proposed that to meet the supply shortage that vaccine dose can be halved. Half-dose of Moderna vaccine (50ug) was in a phase-IIa trial. Immune response in the half-dose group compared to those that received a full dose were the same. Therefore, this dosing strategy is supported from an immunogenicity perspective. It is reasonable to infer that the immunogenicity would translate to immune protection, but unfortunately no clinical trial has validated the immune protection for this dosing strategy.
SARS-CoV-2 genome mutations
Mutations are changes in the SARS-CoV-2 viral genome that occur naturally over time. These mutations from the parent SARS-CoV-2 virus create variants. A certain amount of genetic variation is expected as SARS-CoV-2 replicates as such it is important to monitor circulating viral variants to collate key mutations. Fortunately, coronaviruses have a slower rate of mutation of 1 to 2 nucleotides per month. 63 These definitions become complicated when environmental factors apply selective pressures on these variants that enable them to express distinct phenotypes that may facilitate viral fitness. This ability of a variant to express distinct phenotypes is termed as a strain. A compilation of beneficial lineage defining mutations can create a strain that has a higher transmission rate or induce severe disease. This raises the question: will the current vaccines or convalescent immunity from a non-variant SARS-CoV-2 infection provide adequate immunological protection against these new variants?
Coronaviruses mutate spontaneously via antigenic drift. This process typically utilizes the virus-specific transcription regulatory network (TRN) sequence to initiate the change, resulting in a new mRNA sequence virus being formed. Homologous and genetic recombination allows for the virus to gain more ecological features and has been speculated to be the reason why SARS-CoV-2 was zoonotic in origin. 64 A variant of the original SARS-CoV-2 virus with a D614G substitution in the spike protein encoding gene emerged in early February 2020, and by June 2020, D614G became the dominant form of the virus circulating globally. 65 Studies have shown that the D614G mutation resulted in increased infectivity and transmissibility. 66 Since then, there have been many viral lineages to note, most notable VOC include the B.1.1.7/20I/501.Y.V1 variant that was first detected in the United Kingdom in October 2020, the B.1.351/20 H/501Y.V2 variant that was detected in South Africa in December 2020, and the Lineage P.1. (B.1.1.28.1) variant that was detected in Tokyo in January 2021 but is believed to have originated from Brazil.
Currently, there exists two open-source real-time software tools to analyze and assign nomenclature of genetic variations in the SARS-CoV-2 virus: Nextstrain and PANGOLIN. 64 , 67 Both refer to the GISAID (Global Initiative on Sharing All Influenza Data) genomic database but have slight differences with regards to their nomenclature to describe various lineages of the virus. The COVID-19 Genomics UK Consortium has also developed CoV-GLUE, an open-source browser application that allows for easy referral of all sequenced SARS-CoV-2 genetic replacements, insertions, and deletions. 68 Therefore, sequencing every local infection will yield a repository to track the development of new mutations and variants.
Notable mutation drivers in the SARS-CoV-2 genome
Before diving deeper into these variants, it is important to understand the physical alteration in the S-protein at a molecular level and the perceived functional advantages that the SARS-CoV-2 gains. Table 2 highlights some of the notable S-protein mutations as they evolve amid the pandemic.
Summary of the physical and functional alterations of S-protein due to notable amino acid substitutions.
RBD, receptor-binding domain; VOC, variants of concern.
Notable emerging VOC
Newly emerged variants of SARS-CoV-2 have now become VOC which can be attributed to their new ability of increased transmission and infectivity. Therefore, it is important to collate the data on the mutations they acquired, the extend of spread, and the efficacy of different vaccines to create a repository for further analysis ( Table 3 ).
Summary of data on features, acquired cluster of S-protein mutations, and vaccine efficacy studies for the major COVID-19 variants of concern.
CI, confidence interval; COVID-19, coronavirus disease 2019; VOC, variants of concern.
There are more variants emerging as the pandemic progresses, but it is important to note that there is still a myriad of available vaccines in our armamentarium that are adequately efficacious in the performed neutralization assays as well as the real-world data. Furthermore, while vaccines induce the antibody-dependent immunity, they can also stimulate other components of the adaptative immune system such as the Memory B-cells, CD8+ Tc cells, and CD4+ Th cells to mount their own response against the viral variants. This can compensate for the reduction in neutralization rate by the vaccine induced antibodies. Interestingly, the adaptative immune system can proliferate libraries of memory B-cells with mutated antibody repertoires that can predict viral variants. Therefore, it is prudent to commence vaccinations in accordance with the local public health bodies. This combined with the continued implementation of public health measures until target level of herd immunity is acquired can lead toward mitigating the prevalence and incidence of COVID-19 variants.
This review highlighted the current available vaccines and candidates being rolled out amid the ongoing prevention measures and summarized the documented findings with regards to their efficacies, side-effects, and storage requirements. An overview of the physiology of immunogenic responses against the disease provided by the more prominent vaccines were discussed, alongside questions regarding the implementation of vaccines; heterologous prime-boosting, vaccine contraindications, dosing strategies, side effects, and the presence of SARS-CoV-2 mutations and variants.
There are still many unanswered questions that need to be addressed with regards to antibodies produced in individuals including their impact on the clinical course and severity of the disease, how long will they remain in the body to protect from the disease, and if what we have is enough to deal with newly emerging variants. Studies on these topics are rapidly being conducted and published on a global scale, and scientific communities are working on the clock to produce as much information to bring us a better understanding on how to deal with this disease.
For this global pandemic to end, it is imperative that people are vaccinated as quickly as possible until herd immunity can be achieved. One aspect of achieving this, in the face of vaccine hesitancy, is to address the lack of community understanding on how vaccines work, the risks, and the factors that keep this area of research volatile and distribution policies ever-changing. In addition, it is important to remain cautious about the information being released and to trust the accredited sources and experts, rather than the aberrant rumors being spread through social media. Nonetheless, the COVID-19 vaccines have shown to be highly promising and we recommend for everyone that is eligible to take the vaccine at the correct dosing interval when they are given the chance as this would potentiate a positive trend toward pandemic resolution.
Authors’ contributions: CY, AA, Amogh P, Akul P, AP performed acquisition and curation of the data; CY, AA, Amogh P, Akul P, AP, YYL and PK analyzed the data, performed interpretation of the data, and wrote of the original draft; YYL and PK performed the critical revision; All authors have read and approved the final manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.

Contributor Information
Charles Yap, School of Medicine, National University of Ireland, Galway, Ireland.
Abulhassan Ali, School of Medicine, National University of Ireland, Galway, Ireland.
Amogh Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Akul Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Aman Pal, School of Medicine, National University of Ireland, Galway, Ireland.
Ying Yi Lim, School of Medicine, National University of Ireland, Galway, Ireland.
Pramath Kakodkar, School of Medicine, National University of Ireland, Galway, University Road, Galway H91 TK33, Ireland.
If any body is a beach body, any book is a beach read. Try on these books this summer.
Just like any body can be a beach body, any book can be a beach read.
When you’re packing a travel bag this summer and mulling over the Beach Reads ! display at your local independent bookstore , stop and ask yourself: What do I really want to read? What do I enjoy reading?
The category “beach read,” as best as anyone can tell, came into fashion in the 1990s, according to The Guardian. It’s a marketing trick, not a mandate.
As a marketing term, it’s successful because it’s aspirational. We see ourselves on a beach, relaxed and lazily reading that fun book with the bright cover, one that looks nothing like spreadsheets or reports, a book that entertains but doesn’t ask too much.
But not everyone relaxes the same way.
Check out: USA TODAY's weekly Best-selling Booklist
Maybe you really do want to spend time with the light contemporary fiction, steamy romance, or compulsive thriller that generally gets labeled “beach read.” Totally fine. We’ve got some suggestions for you.
On the other hand, lazy days and long flights mean vacation can be a perfect time to tackle the books you’ve always meant to read. Classics, essays, literary fiction — if you’re a reader who considers heavy reading light work, we’ve got some less conventional recommendations, too.
Is it a body on the beach? Yes: Beach body. Is it a book on a beach? Yes: Beach read.
Find your next read USA TODAY's Best-selling booklist
Smart romance
"The Other Side of Disappearing," Kate Clayborn (Kensington, pp 432.. Out now)
What does “smart romance” mean? This book gave me a definition: a romance in which a happy-ever-after ending happens but doesn’t feel required because the characters all had emotional growth. Here, Clayborn sends a true crime podcast producer and a tough-as-nails older sister on a road trip that will change their lives.
More like this: "Summer Romance," Annabel Monaghan; "When I Think of You," Myah Ariel; "Funny Story," Emily Henry
Literary Larks
"Martyr!," Kaveh Akbar (Penguin Random House, pp.352, out now)
Akbar is a poet, and you can see that in the lyrical writing of his debut novel. The story dips in and out of time and memory and points of view, always twisting around the idea of love. Fun and touching and a little weird, this book is made for hot summer nights.
More like this: "Help Wanted," Adelle Waldman ; "Come and Get It," Kiley Reid; "Family Meal," Bryan Washington
Literary Adventures
"James," Percival Everett (Doubleday, pp 320, out now)
Consider this retelling of "Huck Finn" your summer reading assignment. Told from the perspective of clever and compassionate Jim, the dangerous Mississippi River raft trip includes familiar stops and characters (no need to read the original), but is sharper and comes with higher stakes as our hero tries to reunite his family.
More like this: "The Vaster Wilds ," Lauren Groff; "Lies & Weddings", Kevin Kwan; "Lone Women," Victor Lavalle
"While We Were Burning," Sara Koffi (Penguin, pp. 304, out now)
Unreliable narrators and blurry relationship boundaries make this story, examining race and class in Memphis, especially twisty.
More like this : "First Lie Wins," Ashley Elston; "A Line in The Sand," Kevin Powers; "Bright Young Women," Jessica Knoll
"The Count of Monte Cristo," Alexander Dumas (Penguin, pp. 1,276, out now)
Don’t be intimidated by size. Many classics, including this one, were written in installments, which means short chapters and built-in cliffhangers. And no matter the time period, people are the same, loving and scheming and struggling. Think of this classic revenge story like your latest binge watch.
More like this : "Their Eyes Were Watching God," Zora Neale Hurston; "Anna Karenina," Leo Tolstoy; "Jane Eyre," Charlotte Bronte
"Bite by Bite ," Aimee Nezhukumatathil (HarperCollins, pp. 224, out now)
Essay collections are excellent vacation reads, able to be picked up and put down without interrupting a narrative. Each of these short essays is a perfect little bite, exploring the ways food sparks memory and meaning in our lives.
More like this: "Divine Might," Natalie Haynes; "The Comfort of Crows," Margaret Renkl; "A Praise Song for Kitchen Ghosts," Crystal Wilkinson
"There’s Always This Year," Hanif Abdurraqib (Random House, pp. 352 out now)
If you want nonfiction that requires you to go a little deeper, Abdurraqib delivers. This is a book about basketball. It’s also about belonging and grief, ambition and America. And all of it is delivered in a structure that perfectly, brilliantly mimics a basketball game. Everything comes down to the final two minutes.
More like this: "A Map of Future Ruins," Lauren Markham; "Grief Is for People," Sloane Crosley; "This Is What It Sounds Like," Susan Rogers & Ogi Ogas
Hillary Copsey is the book advisor at The Mercantile Library in Cincinnati, Ohio.

IMAGES
VIDEO
COMMENTS
Conclusion; Essay Title 2: "Vaccination Mandates: Balancing Individual Rights with Public Health" Thesis Statement: While respecting individual rights is essential, vaccination mandates are a legitimate measure to safeguard public health and prevent outbreaks of vaccine-preventable diseases.
Introduction. Childhood vaccines save an estimated 2-3 million lives worldwide every year, which has contributed substantially to the reduction in global infant mortality rate from 65 per 1,000 live births in 1990 to 29 in 2018. 1, 2 Vaccines are found to be the most cost-effective approach for reducing childhood disease burden, especially when compared with interventions such as clean water ...
An additional aspect of vaccines many parents are troubled with is the increase in suggested vaccines for young children. "Today, the CDC recommends that children receive vaccines for 10 diseases — plus the flu vaccine — by age 6, which can mean up to 37 separate shots. That compares to five vaccines for the same age group in 1995 ...
Vaccines prevent the spread of contagious, dangerous, and deadly diseases. These include measles, polio, mumps, chicken pox, whooping cough, diphtheria, HPV, and COVID-19. The first vaccine discovered was the smallpox vaccine. Smallpox was a deadly illness. It killed 300 million to 500 million people around the world in the last century.
Simply put: Vaccination saves lives. See "Emerging infectious diseases: A proactive approach" in volume 114 on page 4055. Few measures in public health can compare with the impact of vaccines. Vaccinations have reduced disease, disability, and death from a variety of infectious diseases. For example, in the United States, children are ...
A vaccination program started in the United Kingdom in 2008, and at the time of writing over 10.5 million doses had been given to girls (Public Health England, 2018), with the aim of preventing primary infection with HPV. The vaccine coverage was 83.8% for 13-14 year old girls in England in 2017/18 (Public Health England, 2019).
CONCLUSION. Vaccines have immeasurably improved our quality of life. They have led to the eradication of deadly diseases like smallpox and the near elimination of diseases such as diphtheria, polio, and measles. Outbreaks of vaccine-preventable diseases, such as mumps, are infrequent and are also quite newsworthy on the rare occasion that they ...
Summary of Stakeholder Concerns. The committee's findings and conclusions about stakeholder concerns are presented in Chapter 4.Although the committee identified the concerns of some parents about the number, frequency, and timing of immunizations in the overall immunization schedule, the committee did not find in its literature review that clinicians, public health personnel, or policy ...
This chapter presents the results obtained from the priority assessment system described in the preceding chapters. Potential health benefits and potential vaccine expenditures have been calculated for each of the 29 vaccine projects.**The analysis covers 29 vaccine projects directed against 19 diseases. In some cases, there may be more than one promising approach; also, various vaccine ...
Vaccines and immunization. Immunization is a global health and development success story, saving millions of lives every year. Vaccines reduce risks of getting a disease by working with your body's natural defences to build protection. When you get a vaccine, your immune system responds. We now have vaccines to prevent more than 20 life ...
Students can choose to write a full-length college essay on the coronavirus or summarize their experience in a shorter form. To help students explain how the pandemic affected them, The Common App ...
25 October 2022. Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love. We've gathered the latest expert information to answer some of the most common questions about COVID-19 vaccines.
Here, two experts to make the case for and against mandatory COVID-19 vaccines. Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford. COVID-19 ...
Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others. A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Importance Of Vaccination Essay. A vaccination is a treatment that increases immunity to a specific illness. It is a biologically produced item that includes typical components resembling a disease-causing bacteria, generated from weak or dead versions of the microbe. It aids in immune system stimulation, identifies invasive bacteria as foreign ...
Vaccination Essay. Sort By: Page 1 of 50 - About 500 essays. Good Essays. Vaccination Of Vaccination And Vaccination. 1585 Words; 7 Pages; Vaccination Of Vaccination And Vaccination. Vaccination is widely considered one of mankind's utmost medical achievements. Diseases that were not long ago commonplace in society are now increasingly rare ...
Conclusion. Restatement of thesis: Research shows that the benefits of vaccination outweigh the risks because vaccines can prevent serious illness and disease in individuals, vaccinations can also prevent widespread outbreaks of diseases in populations and the side effect of vaccinations, though occasionally serious, are very rare.
High rates of COVID-19 vaccination were observed in this pregnant population. Of the 351 respondents, 82% had received at least one dose of the COVID 19-vaccination. This increased compared to estimates of 15% in June 2021 which were obtained from the hospital's electronic health record. Conclusions
Vaccinations in Children. Nicole Stacy ENG 111 Essay #4 Today‚ nearly 40% of American parents refuse to vaccinate their children due to a variety of unfounded fears. Vaccinations against diseases should be mandatory‚ without exception‚ for all children of the U.S. who wish to attend school.
In conclusion, this systematic review summarized the results of clinical trials related to the COVID-19 vaccine, showing that most vaccines had a good safety and effectiveness. It is believed that with the widespread vaccination of COVID-19, it is possible to control and end the global pandemic of COVID-19.
Step 1: Return to your thesis. To begin your conclusion, signal that the essay is coming to an end by returning to your overall argument. Don't just repeat your thesis statement —instead, try to rephrase your argument in a way that shows how it has been developed since the introduction. Example: Returning to the thesis.
As vaccine communicators and administrators, most of us are likely to have a high ego involvement related to vaccines. So, too, would people who demonstrate against vaccines. Both groups would have less malleable attitudes than many others in the population. ... Conclusion. Part 1 of this series demonstrated the complex considerations around ...
Mr. Conniff is the author of "Ending Epidemics: A History of Escape From Contagion." The fight to eradicate polio has been long and difficult. It's been nearly 50 years since vaccines ...
Conclusions The high effectiveness of the national HPV vaccination programme previously seen in England continued during the additional 12 months of follow-up. HPV vaccination was associated with a substantially reduced incidence of cervical cancer and CIN3 across all five deprivation groups, especially in women offered routine vaccination.
May 14, 2024. Most high school seniors approach the college essay with dread. Either their upbringing hasn't supplied them with several hundred words of adversity, or worse, they're afraid ...
We seek MLA-formatted essays from 4,000-7,000 words. Please submit abstracts of 250-500 words by July 15, 2024. Notification of acceptance will be made by Aug. 1, 2024. And final essays will be due October 15, 2024. We will be submitting the proposal, table of contents, and sample essays to academic presses by Aug. 1, 2024.
In " The Anthropocene Reviewed ," Green writes essays reviewing different topics from Halley's Comet to Diet Dr Pepper and even the Indianapolis 500, the IndyStar previously reported. Continue ...
Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian (98.5%). 21 From 21 days after the first dose of the vaccine, ... Conclusion. This review highlighted the current available vaccines and candidates being ...
My father's side of the family spoke Mandarin, but my father did not. So, my sister and I never learned. We spent a lifetime clumsily sounding out the 谢谢 and 再见 of our heritage language ...
Essays "Bite by Bite ," Aimee Nezhukumatathil (HarperCollins, pp. 224, out now) Essay collections are excellent vacation reads, able to be picked up and put down without interrupting a narrative.