

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
Top 12 Clinical Research Organisations (CROs) in 2023

- Author Company: PharmiWeb.Jobs
- Author Name: Lucy Walters
- Author Email: [email protected]
- Author Website: https://www.pharmiweb.jobs/
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world , highlighting the companies driving this considerable growth and accelerating research and development across the globe.
Recognised as the world’s largest and most comprehensive CRO powered by Healthcare Intelligence, ICON provides outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. ICON has a global network of offices in 46 countries, working on specialities including Medical Devices, Therapeutics, Government and Public Health Solutions, Clinical Research Services, Commercialisation and Outcomes, and more.
With approximately 70,000 employees, IQVIA is a leading provider of advanced analytics, technology solutions and clinical research services to the life sciences industry. Operating in more than 100 countries, IQVIA’s Clinical Research solutions include Protocol Design, Phase I Trials, Phase IIb / III Trials, Site Identification, Patient Recruitment and Global Laboratories.
With more than 20,000 employees globally, Parexel is one of the world’s largest CROs and provides contract research, consulting, and medical communications services to the worldwide pharmaceutical, biotechnology, and medical device industries. Parexel provides the full range of Phase I to IV clinical development services, with global study locations in Baltimore, US, London, UK, Los Angeles, US, and Berlin, Germany.
Syneos Health
Syneos Health is the only fully integrated biopharmaceutical solutions organisation built to accelerate customer success, with 29,000+ employees working across more than 110 countries. The company’s Clinical Development services include Decentralized Solutions, Bioanalytical Solutions, Early Phase, Phase II–III, Phase IIIb – IV, Real World and Late Phase, and Medical Device and Diagnostics. In 2022, Syneos Health was the winner of the Life Sciences Award for Most Innovative CRO.
Labcorp Drug Development
With more than 70,000 employees, Labcorp is a leading global life sciences company with unparalleled diagnostics and drug development capabilities. The company is driven to help medical, biotech, and pharmaceutical companies transform ideas into innovations, and supports clinical trial activities in approximately 100 countries. Labcorp plans to spin-off its clinical development business, to be named Fortrea, in mid-2023.
Part of Thermo Fisher Scientific, PPD is a leading global CRO with 130,000+ employees, working to make the world healthier, cleaner, and safer. The company offers a broad range of clinical development and analytical services, helping to accelerate medicines from early development through regulatory approval and market access. PPD works with a broad range of therapeutic areas, including Biosimilar Development, Cell & Gene Therapy, Immunology and Rheumatology, Infectious Diseases, Women’s Health, and more.
In 2022, Medpace celebrated 30 years of accelerating the development of medical therapeutics, with approximately 5,000 employees working across 41 countries. The company provides medical and regulatory leadership and guidance throughout the clinical development life cycle, along with efficient execution of studies around the world, from Early Phase to Late Phase. Medpace also earned the 2022 CRO Leadership Awards for Capability, Compatibility, Expertise, Quality, and Reliability.
Charles River Laboratories
With 20,000+ employees worldwide, Charles River Laboratories provides essential products and services that help pharmaceutical and biotechnology companies, government agencies and leading academic institutions across the globe to accelerate their research and drug development efforts. The company currently operates more than 110 facilities across 20+ countries, working on therapeutic areas including COVID-19, Cardiovascular Disease, Immunology, Infectious Diseases, and Rare Diseases.
WuXi AppTec
WuXi AppTec is a global company providing a broad portfolio of R&D and manufacturing services to help pharmaceutical and healthcare companies across the globe advance discoveries and deliver ground-breaking treatments. The company currently has more than 45,000 employees globally, including 42,000 scientists. WuXi Clinical, a wholly owned subsidiary of WuXi AppTec, is a global CRO providing a comprehensive range of clinical development services for the development of pharmaceuticals, biologics, and medical devices.
CTI is one of the 20 largest CROs in the world, with associates in 60+ countries across six continents, including 12 locations in Europe. The company is the highest-ranked CRO in the world after being named a winner in all 5 core categories of the 2022 CRO Leadership Award. CTI provides its partners with a full range of clinical services, across therapeutic areas including Rare / Orphan Diseases, Regenerative Medicine / Gene Therapy, Immunology, Transplantation, and Hematology / Oncology.
Worldwide Clinical Trials
With approximately 3,000 employees and operating in more than 60 countries, Worldwide Clinical Trials is a global, midsize CRO providing top-performing bioanalytical and Phase I-IV clinical development services to biotechnology and pharmaceutical companies. The company’s major therapeutic focus areas include Cardiovascular, Metabolic, Neuroscience, Oncology, and Rare Diseases. In 2022, they were recognised for excellence and honoured in 5 categories in the CRO Leadership Awards.
PSI CRO is a privately-owned, full-service CRO operating globally, with 27,000+ employees in more than 60 countries. Committed to being the best CRO in the world, the company specialises in the planning and execution of global pivotal registration clinical trials, supporting trials across multiple countries and continents. PSI was named a 2022 CRO Leadership Award Champion in the categories of Compatibility, Quality, and Reliability.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
Top 28 Companies in Pharmaceutical Clinical Trials

The pharmaceutical clinical trials industry plays an indispensable role across the global healthcare spectrum. Made of numerous companies, their primary responsibility involves conducting research, managing clinical trials, and ensuring the safety and efficacy of medical treatments. These corporations frequently collaborate with pharmaceutical and medical device manufacturers, healthcare organizations, and research institutes. As innovation intensifies and international healthcare challenges elevate, the pharmaceutical clinical trials industry is set to register unprecedented expansion, while contributing towards the introduction of advanced medical treatments and therapies.
Top 28 Pharmaceutical Clinical Trials Companies
- Website: parexel.com
- Headquarters: Newton, Massachusetts, United States
- Founded: 1982
- Headcount: 10001+
- Latest funding type: Acquired
Parexel is a global company that offers services and solutions in the clinical research and healthcare industry. They provide expertise in various areas such as clinical trial management, regulatory outsourcing, medical monitoring, and consulting. They work with pharmaceutical companies, medical device manufacturers, and other healthcare organizations to support the development and advancement of new medicines and therapies.
2. Worldwide Clinical Trials
- Website: worldwide.com
- Headquarters: Research Triangle Park, North Carolina, United States
- Founded: 1986
- Headcount: 1001-5000
Worldwide.com is a global company specializing in clinical trial services and real-world evidence. With over 35 years of experience, they offer a range of services including clinical monitoring, data management, drug safety, patient recruitment, and project management. They focus on therapeutic areas such as cardiovascular, metabolic, neuroscience, oncology, and rare diseases. Their innovative lab services and personalized approach make them a trusted partner in the pharmaceutical industry.
- Website: medpace.com
- Headquarters: Cincinnati, Ohio, United States
- Founded: 1992
- Latest funding type: Ipo
Medpace is a full-service Contract Research Organization (CRO) that accelerates the advancement of safe and effective medical therapeutics. With a global reach, Medpace offers a wide range of services including clinical trial management, patient recruitment, and drug safety monitoring.
4. Premier Research
- Website: premier-research.com
- Headquarters: Morrisville, North Carolina, United States
- Founded: 1989
- Latest funding type: Series Unknown
Premier Research is a clinical research organization that helps innovative biotech and specialty pharma companies transform ideas and breakthrough science into new medical treatments. They offer a range of services, including product development consulting, global regulatory consulting, nonclinical studies, quality assurance, and project management.
5. Novotech
- Website: novotech-cro.com
- Headquarters: Sydney, Nsw, Australia
- Founded: 1996
- Latest funding type: Debt Financing
Novotech is a leading Asia Pacific biotech specialist CRO that offers cost competitive solutions for clinical research. They provide a full range of services including medical and regulatory consulting, patient recruitment, site selection, and clinical operations. With expertise in infectious diseases, vaccines, and biometrics, Novotech offers high-quality research and data management. They have a large homogenous population and locations in various countries.
6. Clinipace
- Website: clinipace.com
- Founded: 2003
- Headcount: 501-1000
Clinipace is a global CRO that specializes in clinical research and development. They provide personalized solutions to biotech and pharmaceutical companies, offering expertise in clinical technology, analytics, regulatory and strategic product development. With a focus on collaboration and control, Clinipace guides their clients through the entire clinical development program.
7. Crown Bioscience
- Website: crownbio.com
- Headquarters: San Diego, California, United States
- Founded: 2006
Crown Bioscience is a Contract Research Organization (CRO) that empowers customers to improve human health. They engage scientific talent, uphold quality standards, and pursue innovation to accelerate and de-risk drug development. Their services include biomarker discovery, efficacy testing, DMPK, and pharmacology.
8. Veeda Clinical Research Limited
- Website: veedacr.com
- Headquarters: Ahmedabad, Gujarat, India
- Founded: 2004
- Latest funding type: Private Equity
Veeda Clinical Research is a company that provides quality research solutions for sponsors and regulatory authorities. They conduct early phase clinical trials in healthy volunteers and patients, specializing in ophthalmology trials. Additionally, they offer bioequivalence and bioavailability studies, drug discovery and development services, clinical endpoint phase III studies, and safety monitoring services.
9. Quanticate
- Website: quanticate.com
- Headquarters: Hitchin, Hertfordshire, United Kingdom
- Founded: 1994
- Headcount: 201-500
Quanticate is a global biometric Clinical Research Organization (CRO) offering statistical and data expertise. They help pharmaceutical and biotech companies bring their drugs to market by providing fast and reliable access to expert biometric teams. Their services include biostatistics, statistical programming, clinical data management, and biostatistical consultancy.
10. Nucleus Network
- Website: nucleusnetwork.com.au
- Headquarters: Melbourne, Victoria, Australia
Nucleus Network is a company that offers clinical research services focusing on early phase and vaccine trials. They provide state-of-the-art facilities, experienced staff, and innovative approaches to help pharmaceutical and biotech companies accelerate their drug development processes.
11. Richmond Pharmacology
- Website: richmondpharmacology.com
- Headquarters: London, London, United Kingdom
- Founded: 2001
- Headcount: 51-200
Richmond Pharmacology is a Clinical Research Organisation that conducts early phase clinical trials in a wide range of medical conditions with patients and healthy volunteers. They work with international pharmaceutical and biotech organizations to drive innovation in the field of clinical research.
12. Integrium, LLC
- Website: integrium.com
- Headquarters: Tustin, California, United States
- Founded: 1998
Integrium is a full-service Clinical Proof of Concept (PoC) firm specializing in various therapeutic areas, including cardiovascular, metabolic disease, and dermatology research. We assist pharmaceutical companies in designing and executing clinical trials to achieve their objectives on time and with expected quality. With cutting-edge technology and unparalleled services, we play a crucial role in advancing new medical advancements. Our team of experts works collaboratively with clients, investigators, and vendors to ensure the successful implementation of clinical trials.
13. Mithra Biotechnology Inc.
- Website: mithracro.com
- Headquarters: 新北市 New Taipei City, Taiwan, Taiwan
- Founded: 1988
MithraCro is a biotechnology company specializing in pharmaceutical analysis services, with expertise in bioequivalence testing, clinical trial services, drug metabolism research, and protein analysis.
14. Pearl Therapeutics
- Website: devprobiopharma.com
- Headquarters: Morristown, New Jersey, United States
- Founded: 2020
- Headcount: 11-50
DevPro Biopharma is a clinical research and development accelerator that specializes in transforming molecules into medicines. They offer comprehensive services to assist in the development of pharmaceutical products.
15. Almac Group
- Website: almacgroup.com
- Headquarters: Craigavon, County Armagh, United Kingdom
- Founded: 1968
- Headcount: 5001-10000
- Latest funding type: Grant
Almac Group provides a range of services in the pharmaceutical industry, including storage and distribution, product launch and distribution, API development and manufacture, clinical trial assays, genomic services, clinical testing, and clinical supply chain management. They offer flexible solutions to meet the needs of their clients and ensure the successful launch and distribution of pharmaceutical products. Almac Group is committed to exceptional quality and customer satisfaction.
16. 4B Technologies, Ltd
- Website: 4btechnologies.com
- Headquarters: 苏州市, Jiangsu, China
- Founded: 2017
- Latest funding type: Series B
4B Technologies is a biopharmaceutical company with a leadership team composed of elite professionals from various fields. They are dedicated to developing innovative and effective treatments for neurological diseases.
17. TechnoDerma Medicines
- Website: tkskin.com
- Headquarters: Xuzhou, Jiangsu, China
- Founded: 2014
- Latest funding type: Series A
Techonderma Medicines, Inc. is a private clinical-stage biopharmaceutical company focused on developing innovative therapies for various skin conditions.
- Website: caidya.com
Caidya is a clinical research organization (CRO) that offers personalized services and expertise in various therapeutic areas. They specialize in conducting regional or multinational studies using efficient operational models, local market knowledge, and advanced technologies. Caidya is committed to transparency, clinical development expertise, and personalized experiences for their clients.
19. Novum Pharmaceutical Research Services
- Website: novumprs.com
- Headquarters: Pittsburgh, Pennsylvania, United States
- Founded: 1972
Novum is a global clinical research organization (CRO) with a focus on delivering high-quality research outcomes and exceptional customer satisfaction. With nearly fifty years of experience, Novum offers a comprehensive suite of services in early clinical development, bioanalytical testing, and phase II-IV clinical trial management. The company's dedicated and skilled team, extensive training programs, and global resources enable efficient project optimization and streamline timelines and budgets.
20. PharPoint Research, Inc.
- Website: pharpoint.com
- Headquarters: Durham, North Carolina, United States
- Founded: 2007
PharPoint Research provides drug development solutions to clients of all sizes. Contact us to speak with a representative about how PharPoint can help with your current or upcoming study needs.
21. NC Coast Clinical Research Initiative
- Website: nccoastclinicalresearch.com
- Headquarters: United States
- Founded: 2012
NC Coast Clinical Research is a consortium of 60+ CROs and 100+ clinical research support companies, providing comprehensive integrated drug development, laboratory and lifecycle management services.
22. Quinta-analytica
- Website: quinta.cz
- Headquarters: Praha 10, Hlavni Mesto Praha, Czechia
- Founded: 1997
Quinta is an innovative and trusted world leader in pharmaceutical analysis, R&D, and clinical testing in both human and veterinary medicinal products.
23. LINK Medical Research
- Website: linkmedical.eu
- Headquarters: Oslo, Oslo, Norway
- Founded: 1995
LINK Medical Research is a leading contract research organization (CRO) in Northern Europe. They offer a strategic partnership and provide highly competent teams and evidence-based documentation for superior clinical outcomes. From early phase development to post-marketing, they offer expert guidance through every stage of product development. With a well-integrated local presence in the Nordics, UK, and Germany, they make the journey to market simple and cost-effective.
24. Medlab Clinical Ltd
- Website: medlab.co
- Headquarters: Botany, New South Wales, Australia
Medlab.co is a company that specializes in drug delivery technology. They have a proprietary NanoCelle® platform with 57 patents worldwide. They offer partnering opportunities, clinical trials, and a range of products. Medlab.co is committed to transforming medicine and improving patient outcomes.
25. Clexio Biosciences
- Website: clexio.com
- Headquarters: Petach Tikva, Israel, Israel
- Founded: 2018
Clexio Biosciences is a multi-asset company dedicated to developing novel therapies for patients suffering from neurological and psychiatric conditions.
26. Suzhou Abogen Biosciences
- Website: abogenbio.com
- Headquarters: 苏州市, 江苏省, China
- Founded: 2019
- Latest funding type: Series C
Abogenbio is a biotechnology company focused on innovative drug development and research. With a team culture of openness and collaboration, we strive to bring hope and healing to patients through the creation of groundbreaking medicines. We are dedicated to fostering a fearless and accountable environment where partners can thrive and pave the way for a brighter future.
27. Abond CRO Inc.
- Website: abondcro.com
- Headquarters: Allendale, Michigan, United States
- Founded: 1974
ABOND is a full-service CRO that offers consulting, clinical operations, data management, and statistical analysis. Our commitment to honesty, focus, and responsibility sets us apart. We provide reliable outcomes and are always answerable for our work.
28. Novatek Pharmaceuticals
- Website: novatekpharmaceuticals.com
Novatek Pharmaceuticals Inc. is a startup based in Houston, TX that provides scientific guidance and conducts clinical research for immunomodulatory drugs related to cancers and infectious diseases. They are currently raising Series A for clinical trials and pipeline development.
Want to find more pharmaceutical clinical trials companies?
If you want to find more companies that offer comprehensive clinical trial services and research solutions you can do so with Inven . This list was built with Inven and there are hundreds of companies like these globally.
With Inven you'll also get to know the company's:
- Ownership: Which of these are private equity backed? Which are family-owned?
- Contact data: Who are the owners and CEO's? What are their emails and phone numbers?
- Financials: How do these companies perform financially? What are their revenues and profit margins?
...and a lot more!
Find companies 10x faster with Inven

Keep on reading
More articles.

The leading clinical research organizations
We've ranked the top 10 clinical research organizations.
UNBIASED RESEARCH RANKINGS
HIGHEST STANDARDS REQUIRED
PROPRIETARY CRITERIA SYSTEM

What people are saying: #Passionate #Innovative #Dedicated
Parexel is a clinical research organization that provides biopharmaceutical services and solutions. They have expertise in clinical development, translational medicine, and early phase research. Parexel has over 10,000 employees in 50 countries. Parexel has been recognized by Frost & Sullivan with the 2022 Global Customer Value Leadership Award. Parexel's services are designed to help pharmaceutical and medical device companies bring new treatments and therapies to market. Parexel's clinical development services include phase I-IV clinical trials, biostatistics, clinical data management, clinical supply chain management, decentralized clinical trials, medical monitoring and consulting, medical writing, patient sensors, pharmacovigilance, and risk-based quality management. The company's expanded access services provide access to investigational treatments for patients with serious or life-threatening conditions who are not eligible for clinical trials. Their outsourcing services include functional services provider and strategic partnerships.Parexel's team of experts has the knowledge and experience to help bring new treatments and therapies to market.

IQVIA clinical research
What people are saying: #Involved #ResearchOriented #PatientFriendly
IQVIA clinical research is a company that provides information on clinical trials and patient communities. They also have a section on their website where you can register for updates on new clinical trials and community events. The company has a strong focus on helping to expand meningitis research and they are currently recruiting participants for a new clinical study. They are also seeking volunteers to help advance respiratory syncytial virus (RSV) research. In addition, they are working on a clinical study to help find a way to speed up recovery from COVID-19 and reduce the need for hospital care. Overall, IQVIA clinical research is a company that is heavily involved in a variety of research studies in order to help improve medical treatments and find new cures for diseases.

What people are saying: #Comprehensive #Quality #Dedicated
Medpace is a full-service CRO that provides support for drug, biologic, and medical device programs. They have a global reach and offer services including clinical monitoring, biostatistics and data sciences, regulatory affairs, and more. They are dedicated to making the complex seamless and strive to provide the highest level of quality and service.

Syneos Health
What people are saying: #Quality #Experienced #Committed
Syneos Health is a biopharmaceutical company that provides solutions for clinical development, commercialization, and consulting. The company has a wide range of services and products that cater to the needs of their clients. They have a team of experienced professionals that work together to provide the best possible service. The company is committed to providing the highest quality of care to their clients and patients. They have a wide range of experience in different therapeutic areas, which allows them to provide the best possible care to their patients.

Charles River Laboratories
What people are saying: #Experienced #Dedicated #Successful
Charles River Laboratories is one of the top clinical research organizations in the United States. They offer a variety of services to help pharmaceutical and biotechnology companies develop and commercialize their products. These services include preclinical research, clinical research, and manufacturing support. Charles River Laboratories has a long history of helping companies develop new drugs and therapies. They have a team of experienced scientists and clinicians who are dedicated to helping their clients succeed. They offer a wide range of services, from preclinical research to clinical research to manufacturing support. They have a proven track record of success, and they are a trusted partner for many companies. If you're looking for a top clinical research organization to help you develop and commercialize your products, Charles River Laboratories is a great choice.

What people are saying: #Transparent #Innovative #Unique
Caidya is a leading clinical research organization that provides full-service support and vast therapeutic expertise to its clients. The company has a personalized approach to clinical research and offers a unique combination of global experience and knowledge sharing. Caidya's clinical research services are designed to connect you with unrivaled access to specialist knowledge and resources. The company's cutting-edge clinical technology integrates effortlessly to create an ecosystem that meets the specific needs of your study. Caidya's Clarity platform synthesizes data from different systems to create a near real-time view of your trial. This holistic view allows integrated teams within Caidya to transparently share information and data, generating insights to inform your decisions.

What people are saying: #Healthy #Helpful #Compensated
ICON is a clinical research company that is looking for healthy volunteers to participate in studies. The company has locations in Lenexa, KS and Salt Lake City, UT. Participation in a study includes 1 screening visit, 1 stay of 11 nights (12 days) and 1 follow-up visit. Compensation for participation is up to $6400. ICON is a great opportunity for people who want to make some extra money and help out with medical research.

What people are saying: #Trusted #Innovative #Leading
PPD is one of the top clinical research organizations in the United States. They offer a wide range of services, from drug development to clinical trials. They have a team of experienced professionals who are dedicated to helping their clients succeed.

CTI Clinical Trial and Consulting
What people are saying: #Experienced #FullService #Comprehensive
CTI Clinical Trial and Consulting is a full-service CRO that specializes in clinical research and consulting services. They offer a wide range of services including regulatory development, clinical project management, clinical monitoring, biometrics, quality assurance, and real world evidence. They also have a research center where you can participate in clinical trials. The range of services offered by CTI Clinical Trial and Consulting is excellent. They seem to be a one-stop shop for all your clinical research needs. We also like that they have a research center where you can participate in clinical trials. This is a great way to get involved in the latest medical research and to potentially help develop new treatments for various diseases.

Advanced Clinical
What people are saying: #Comprehensive #CustomerFocused #AwardWinning
Advanced Clinical is a top-tier contract research organization that has provided clinical research services to the pharmaceutical and biotech industry for many years. Their global reach and comprehensive solutions make them a perfect partner for companies launching clinical trials. Their focus on satisfaction and customer service is evident in their high employee retention rate and repeat business rate. Their award-winning culture and commitment to excellence make them a great choice for anyone looking for a clinical research partner.
Clinical Research Organizations: What should you be looking for?
The website's mission is to provide accurate, timely, and reliable rankings of clinical research organizations. We aim to provide users with a comprehensive and up-to-date list of the top clinical research organizations so they can make informed decisions about which ones to use. We also provide detailed descriptions of each organization so users can learn more about their work.
Are Clinical Research Organizations (CRO) worth it?
Yes, it is worth hiring clinical research organizations. Here are three reasons why: 1. Clinical research organizations have the experience and expertise to conduct clinical trials effectively. They know how to design trials that meet regulatory requirements, recruit patients, and collect data. 2. Clinical research organizations can help you save time and money. They can manage all aspects of the clinical trial process, so you can focus on other aspects of your business. 3. Clinical research organizations can provide valuable insights into the clinical trial process. They can help you understand the data, identify potential problems, and make decisions about the best course of action.
What to look for when hiring Clinical Research Organizations (CROs)?
Using a Clinical Research Organizations can be a great way to get the medical care that you need. However, there are a lot of questions that you may have about using one. That is why we have provided FAQs for customers interested in using a Clinical Research Organizations. We want to make sure that you have all the information that you need so that you can make the best decision for your medical care.
What are the specific research needs of your organization?
The first step in finding the right Clinical Research Organization (CRO) for your needs is to identify the specific research needs of your organization. These needs will vary depending on the type of research you are conducting, the size and scope of your project, and your budget. Once you have a clear understanding of your research needs, you can begin to research different CROs and compare their services. When considering a CRO, it is important to look at their experience in conducting the type of research you need. Make sure to ask about their success rates and customer satisfaction levels. You should also inquire about their pricing structures and what type of services are included in their fees. It is also important to consider the size of the CRO and their ability to handle your project. Make sure they have the staff and resources necessary to complete your project on time and within budget. Finally, you should also consider the location of the CRO. If your project requires frequent travel, you may want to choose a CRO that is located near your site. If you are conducting research that requires special expertise, you may want to choose a CRO that is located near a research university or hospital.
What is the size and scope of your organization?
Next, you need to understand the size and scope of your organization, and thereby understand the size and scope of the CRO you need to hire. Is the CRO a large, full-service clinical research organization that offers a wide range of services to its clients? Does the CRO have a global reach and is it able to provide its services to clients in a number of different countries? Does the CRO have a large team of experienced and qualified staff who are able to provide a high level of service to its clients? Is the CRO accredited by a number of different bodies and is it able to offer its services to a wide range of clients?
What are the your clinical research priorities?
Finally, you need to understand your organization's clinical research priorities. Are they to develop new treatments for diseases and conditions, to improve the safety and efficacy of existing treatments, or to increase our understanding of the underlying causes of disease? This will help you select a CRO that aligns with your needs.
Key Takeaways about Clinical Research Organizations
When choosing a clinical research organization, it is important to consider the size of the organization, its focus, its location, and its resources. The best clinical research organizations are those that are able to provide the most comprehensive services and the most experienced staff. They should also be able to offer the latest technology and facilities. Top Clinical Research Organizations is dedicated to providing objective rankings of clinical research organizations. We believe that our rankings will help you choose the best organization for your needs. We are committed to providing the most accurate and up-to-date information possible.
Frequently Asked Questions
What is a clinical research organization (cro).
A clinical research organization (CRO) is a company that provides services to pharmaceutical and biotechnology companies to help them outsource and conduct clinical trials. A CRO manages all aspects of a clinical trial, from start to finish, and works with a variety of stakeholders, including investigators, research sites, and patients.
CROs are an important part of the drug development process, as they provide expertise and resources that allow companies to efficiently and effectively conduct clinical trials. Without CROs, many new drugs and treatments would not be possible.
What services do CROs provide?
CROs provide a variety of services to support clinical research, including study design, data management and analysis, regulatory affairs support, and clinical trial management. CROs also provide a variety of other services, such as market research, project management, and training.
What are the benefits of working with a CRO?
There are many benefits of working with a clinical research organization (CRO). CROs can provide expertise and resources that Sponsors may not have internally, and can therefore help to increase the efficiency and quality of clinical trials. In addition, working with a CRO can help to reduce costs associated with clinical trials. CROs also have experience working with a variety of different Sponsors, and can therefore provide valuable insights and perspectives.
What are the risks of working with a CRO?
The risks of working with a CRO include the potential for conflicts of interest, the potential for data manipulation, and the potential for data fraud.
How do CROs ensure the safety of trial participants?
There are a number of ways that clinical research organizations (CROs) ensure the safety of trial participants.
First, CROs typically have staff members who are responsible for monitoring the safety of participants throughout the course of a study. These staff members may be nurses, doctors, or other health care professionals. They typically have experience in managing clinical trials and are familiar with the potential risks and side effects of the drugs or treatments being studied.
Second, CROs typically have established procedures for reporting and managing adverse events. These procedures ensure that any potential safety concerns are promptly identified and addressed.
Third, CROs typically work closely with the sponsor of a clinical trial (usually a pharmaceutical company) to ensure that the sponsor is aware of any safety concerns that arise during the course of the trial. The sponsor is ultimately responsible for the safety of the participants, and the CRO ensures that the sponsor is kept informed of any safety issues that arise.
Fourth, CROs typically conduct regular audits of their clinical trials to ensure that they are being conducted in accordance with good clinical practices and that the rights and safety of participants are being protected.
Finally, CROs typically have insurance policies in place that protect participants in case of any unforeseen events that may occur during the course of a trial.
Overall, the procedures and policies that CROs have in place help to ensure the safety of participants in clinical trials.
How do CROs ensure the quality of data collected during a clinical trial?
CROs ensure the quality of data collected during a clinical trial by following Good Clinical Practices (GCPs).
GCPs are a set of international standards that ensure the quality, safety, and integrity of data collected during a clinical trial. All CROs must adhere to these standards in order to conduct clinical trials.
What happens if a CRO is unable to meet its contractual obligations?
If a CRO is unable to meet its contractual obligations, it may be required to pay damages to the sponsor. The sponsor may also be able to terminate the contract and seek a new CRO.
What is the role of the sponsor in a clinical trial?
What are the responsibilities of the principal investigator in a clinical trial.
The principal investigator (PI) is responsible for the overall conduct of the clinical trial. This includes ensuring that the trial is conducted in accordance with the protocol, applicable regulations, and standard operating procedures. The PI is also responsible for the safety of the trial participants and for ensuring that the data collected are of high quality.
The PI works with the sponsor, other investigators, and the research team to ensure that the clinical trial is carried out correctly and that the data collected are of high quality. The PI is also responsible for the safety of the trial participants.
How are clinical trial sites selected?
When selecting a clinical trial site, sponsors and contract research organizations (CROs) consider a number of factors.
The most important factor is usually the site’s experience and success in conducting similar trials.
Other important factors can include the site’s patient population, geographic location, and facilities.
How are clinical trial subjects recruited?
There are a number of ways that clinical trial subjects can be recruited. One common method is through advertisements in newspapers or other media outlets. Another common method is through word-of-mouth, where people who know about the clinical trial spread the word to others who might be interested. Additionally, clinical trial subjects can be recruited through doctors or other medical professionals, as well as through patient advocacy groups.
How is informed consent obtained from clinical trial subjects?
Informed consent is obtained from clinical trial subjects by providing them with information about the trial in a way that is easy for them to understand. This includes information about the purpose of the trial, the procedures that will be used, the risks and benefits of participating, and their right to withdraw from the trial at any time. Informed consent is not simply a form that subjects must sign; it is a process of communication between the researcher and the subject that should ensure that the subject understands the trial and is willing to participate.
Top 15 Clinical Research Companies: Leaders in Medical Innovation
What’s on this page:

In the healthcare industry, the number of contract research organizations in the US has reached 2,823 in 2023. This marks a subtle but significant increase of 0.9% compared to the previous year.
This increase signals a vital trend: the growing complexity of finding the best clinical research companies in a crowded field. These organizations aren’t just businesses; they’re important in advancing medicine and developing drugs and therapies.
With such an important task, choosing the right company becomes essential. In this guide, we’ve looked closely at many companies along with their strengths and weaknesses and made a list of the top clinical research organizations.
By the end, you’ll know which company is the best fit for your needs.
Quick List of Top 15 Clinical Research Companies
Here is a quick overview of the best companies of clinical research:
- IQVIA: Best for data-driven insights and advanced analytics in healthcare research.
- ICON: Best for comprehensive clinical development services and therapeutic expertise.
- Parexel: Best for global biopharmaceutical services, emphasizing regulatory and clinical trial excellence.
- Syneos Health: Best for integrated biopharmaceutical solutions and clinical-commercial capabilities.
- PPD: Best for drug development services with innovative, technology-enhanced trial strategies.
- Labcorp: Best for comprehensive clinical testing and diagnostics services with global reach.
- Medpace: Best for expertise in clinical research and regulatory affairs for pharmaceutical companies.
- Charles River Laboratories: Best for preclinical research and development services, including animal testing and research models.
- PRA Health Sciences: Best for clinical trial expertise and integrated solutions for biopharmaceutical development.
- AdvanCell: Best for innovative cell and tissue-based research solutions for life science industries.
- Dynata: Best for data-driven insights and market research services for informed decision-making.
- Covance: Best for end-to-end drug development solutions, from preclinical to post-marketing.
- MedNet: Best for technology solutions and eClinical platforms for streamlined clinical trials.
- Fisher Clinical Services Inc: Best for global logistics and supply chain services for clinical trial materials.
- Worldwide Clinical Trials: Best for specialized CRO offering personalized clinical research solutions.
3 Best Clinical Research Organizations: Comparison Chart
Here’s a comparison table to highlight the key features and differences among the best companies of clinical research. This table aims to provide a quick overview of each company’s unique strengths and areas of expertise in the pharmaceutical and healthcare research sector.
| Data-driven insights, advanced analytics | Healthcare research, Data analytics | Technology-driven healthcare research services | |
| Comprehensive clinical development, expertise | Clinical development, Therapeutic research | Clinical trial and development services | |
| Regulatory expertise, clinical trial excellence | Biopharmaceutical services | Global clinical trial management |
3 Top Clinical Research Organization List For Advanced Medical Discoveries

Now, we’ll explore the top clinical research organizations (CROs) dedicated to advancing medical discoveries. Let’s jump into the details of these exceptional organizations.
IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Why is IQVIA among the best? Their strength lies in their vast database and advanced technology, which enable them to analyze complex healthcare data efficiently. This leads to a better understanding of diseases, more effective treatments, and faster drug development.
IQVIA’s work is essential because it speeds up the process of bringing new medicines to the market, ultimately benefiting patients worldwide. In short, IQVIA is a key catalyst in advancing global healthcare.

About IQVIA
- Founding Team: Dennis Gillings
- Founding Year: 1982
- Company Size: 86,000
Features of IQVIA
IQVIA, a prominent player in the life sciences sector, is dedicated to advancing healthcare through connected intelligence. Here are some key features of IQVIA in the world of clinical research:
Innovative Clinical Development
IQVIA is reimagining clinical development by intelligently connecting data, technology, and analytics. This approach leads to faster decision-making and reduced risk, enabling the delivery of life-changing therapies more quickly.
Efficient Payment Systems for Clinical Trials
They have simplified the process of paying sites involved in clinical trials. IQVIA offers the capability to make payments within 30 days, even in challenging locations. This significantly reduces the administrative burden of managing clinical trial payments by up to 90%.
Decentralized Trials Expertise
The company has conducted over 500 studies in more than 75 countries, covering over 30 indications using decentralized trial methodologies. This demonstrates their capability in managing complex, multinational clinical trials.
Global Reach and Impact
With a presence in various regions, including Australia, New Zealand, the Middle East, and Africa, IQVIA’s global footprint allows it to drive healthcare innovations worldwide.
AI and Technology Integration
The company is at the forefront of integrating AI and other technologies in healthcare. Their Healthcare-grade AI promises precision, speed, scale, trust, and reliability, essential for advancing health and improving patient outcomes.
- Extensive, reliable healthcare data enhances market research quality.
- Utilizes AI and machine learning for advanced healthcare insights.
- Specialized focus yields a deep understanding of healthcare dynamics.
- Broad international presence enables diverse and large-scale studies.
- Offers advanced tools for insightful healthcare data analysis.
- Advanced tools can be challenging to use without training.
- Handling sensitive health data raises privacy and security issues.
Our Review of IQVIA
IQVIA, a prominent player in the healthcare and life sciences industry, presents a mixed bag of strengths and weaknesses. On the positive side, we appreciate IQVIA’s extensive expertise in data analytics and healthcare consulting.
Their comprehensive research and analysis have undoubtedly driven valuable insights and innovations in the sector. Moreover, their global presence allows for diverse perspectives and access to critical healthcare data.
However, we must also acknowledge some shortcomings. IQVIA’s services can be prohibitively expensive for smaller organizations, limiting accessibility. Additionally, the sheer volume of data can sometimes lead to information overload, making it challenging to extract actionable insights.
ICON is a prominent company in the field of clinical research, playing a significant role in advancing medical science. They specialize in designing and conducting clinical trials for new medicines and treatments.
The work of ICON is crucial because they help determine the safety and effectiveness of these potential medical breakthroughs. They are considered one of the best in clinical research due to their high standards of accuracy, reliability, and ethical practices.
ICON’s expertise ensures that the clinical trials they manage are conducted efficiently and effectively, leading to faster approval of new treatments. This directly impacts patient care, as it allows quicker access to new, potentially life-saving medicines.
In essence, ICON’s contribution is vital in driving forward medical innovations.
- Founding Team: John Climax and Ronan Lambe
- Founding Year: 1990
- Company Size: 41,160
Features of ICON
Here are some of the key features of ICON in clinical research:
Diverse Clinical and Scientific Operations
ICON offers a wide range of clinical and scientific operations services, ensuring comprehensive support for various aspects of clinical trials. This includes everything from study design to execution and data analysis.
Decentralized Clinical Trial Solutions
They provide end-to-end services, operational models, and technology to deliver customized solutions for decentralized clinical trials. This approach is increasingly important in today’s clinical research landscape, offering flexibility and efficiency.
Specialized Therapeutic Areas
ICON has expertise across multiple therapeutic areas including cardiovascular, central nervous system, endocrine & metabolic disorders, infectious diseases, internal medicine & immunology, oncology, and more. This broad expertise allows them to handle a wide range of clinical research projects.
Innovative Solutions for Biotech
ICON provides full-service outsourcing and flexible support customized to the specific needs of biotech companies. This includes due diligence and asset valuation, which are critical for biotech firms navigating the complex landscape of drug development.
Advanced Medical Imaging Solutions
Their expert medical imaging solutions support all stages of clinical research, improving decision-making, increasing efficiency, and reducing trial costs.
- Decades of expertise ensure high-quality clinical research.
- Offers wide-reaching capabilities for multi-regional clinical studies.
- Deep understanding of global regulations enhances compliance and efficiency.
- Invests in new technologies for more efficient trial processes.
- Broad range of specialties contributes to comprehensive service offerings.
- Managing multi-regional trials can lead to logistical challenges.
- Rapid growth may strain resources and affect service quality.
Our Review of ICON
When we researched ICON, we found both commendable aspects and areas for improvement. On the positive side, we appreciate their commitment to clinical research and their global presence, which allows for diverse study options. Their experienced team and advanced technology contribute to reliable data collection and analysis.
However, there are some drawbacks to consider. We have noticed occasional delays in project timelines, which can be frustrating. Additionally, the cost of their services tends to be on the higher side, making it a potential barrier for smaller research endeavors.
Parexel is a globally recognized company in clinical research, known for its important role in developing new medical treatments. They are one of the biggest clinical research organizations. Parexel conducts clinical trials, crucial steps in testing the safety and effectiveness of new drugs.
The work of Parexel is essential because it bridges the gap between medical research and the availability of new treatments to patients. One of the reasons they stand out as one of the best in this field is their rigorous approach to research.
Their commitment to quality and their global network also enables diverse and large-scale studies, setting them apart from others in the field. These strengths allow Parexel to deliver reliable and valuable data, accelerating the process of bringing new, effective medicines to the market.
Simply put, Parexel is a key player in transforming medical research into real-world health solutions.
About Parexel
- Founding Team: Josef von Rickenbach and Anne B. Sayigh
- Company Size: 18,900
Features of Parexel
Parexel, a global biopharmaceutical services organization, offers a range of features in clinical research. Here are some key aspects of their approach:
Patient-Centric Approach
Parexel emphasizes a patient-first strategy in their clinical trials. This approach results in deeper and more relevant insights for trial design and execution. This ensures that the trials are more aligned with patient needs and experiences.
Innovative Trial Designs
Parexel employs innovative trial designs to optimize trials for maximum impact. This includes advanced modeling and simulation to predict drug effects ahead of time, which can save time, money, and resources.
Regulatory Compliance and Market Access
Parexel designs studies and endpoints with market access in mind, ensuring that they satisfy global regulations. This approach helps in getting treatments to patients safely and quickly.
Patient Advocacy and Engagement
The company includes patient advocates in their council, using their experiences to improve trial designs. This inclusion demonstrates their commitment to understanding and incorporating patient perspectives in clinical research.
Focus on Speed and Precision
Parexel aims to design neuroscience trials with speed and precision, utilizing the right experts and specializations. This focus is crucial in delivering effective treatments on time.
- Provides advanced technology and analytics for efficient data management.
- Extensive network provides global insights with regional knowledge.
- Expertise in navigating complex regulatory environments worldwide.
- Broad experience across various therapeutic areas ensures versatile solutions.
- Focuses on patient engagement for more effective trial outcomes.
- Rapid expansion can lead to challenges in resource management.
- Concentration in specific areas could pose risks in market shifts.
Our Review of Parexel
Parexel is a notable player in the field of clinical research and pharmaceutical services. We’ve thoroughly analyzed their offerings and found both strengths and areas that need improvement.
On the positive side, Parexel excels in its commitment to innovation and technology. We appreciate their continuous efforts to simplify clinical trials and drug development processes, making them more efficient.
However, we also noticed some downsides. Communication with clients could be more transparent, with clearer updates on project progress. Additionally, there’s room for improvement in terms of ensuring consistency in service quality across different projects.
Other 12 Companies of Clinical Research

In the world of clinical research, beyond the well-known names, there are 12 other companies making significant contributions. Let’s explore their vital role in advancing healthcare.
1. Syneos Health
Syneos Health helps develop medicines by managing clinical trials for new drugs. They’re essential because they ensure medicines are safe and effective. Syneos Health stands out in clinical research for its comprehensive services and global reach, making drug development smoother and faster.
About Syneos Health
- Founding Team: Colin Shannon
- Founding Year: 1980
- Company Size: 28,000
PPD is a group that tests new drugs to see if they’re good and safe. This is crucial for getting new treatments to people. They stand out for their thorough research and global reach.
- Founding Team: Fred Eshelman
- Founding Year: 1985
- Company Size: 40,000+3
Labcorp does important tests and research for health. They’re needed because they help find out if new treatments are good. They’re among the best for their big labs and fast results.
About Labcorp
- Founding Team: Matthew Benger
- Founding Year: 1978
- Company Size: 75,5000
Medpace focuses on making sure new health treatments are safe. This is key for better medicine. They’re a top choice because of their focus on quality and detail in research.
About Medpace
- Founding Team: August Troendle
- Founding Year: 1992
- Company Size: 5,400
5. Charles River Laboratories
Charles River Laboratories tests drugs and does research to help pets and people stay healthy. They’re essential for safe, new treatments. Their expertise makes them a leader in the field.
About Charles River Laboratories
- Founding Team: Henry Foster
- Founding Year: 1947
- Company Size: 21,400
6. PRA Health Science
PRA Health Science works on finding out if new medicines are safe. This helps everyone get better treatments. They’re known for their excellent research and care in studies.
About PRA Health Science
- Founding Year: 1976
- Company Size: 17,000+
7. AdvanCell
AdvanCell specializes in new treatments, checking if they’re safe and working. Their work is vital for progress in medicine. They’re recognized for their innovation in research.

About AdvanCell
- Founding Team: Andrew Adamovich
Dynata gathers data for health studies. They’re needed for understanding what works in healthcare. They’re a top name for their accurate and wide-reaching data collection.
About Dynata
- Founding Team: Mike Petrullo
- Founding Year: 1940
- Company Size: 5000-10000
Covance helps with drug tests and research to fight diseases. Their role is key for new treatments. They’re celebrated for their comprehensive services and global impact.
About Covance
- Founding Team: Fred Cummings
- Founding Year: 1981
- Company Size: 50,000
MedNet provides software for managing clinical trials. This helps in making research easier and faster. They’re among the best for their tech solutions in research.
About MedNet
- Founding Team: John “Rob” Robertson
- Founding Year: 1996
- Company Size: 51-200
11. Fisher Clinical Services Inc.
Fisher Clinical Services Inc. manages the logistics of clinical trials, ensuring that treatments are tested efficiently. Their work is crucial for the progress of medicine, and they are renowned for their reliability and global network.
About Fisher Clinical Services Inc.
- Founding Team: John Pickering
- Founding Year: 1989
12. Worldwide Clinical Trials
Worldwide Clinical Trials conducts essential research to evaluate new medical treatments. Their work is critical for advancing healthcare. They are distinguished by their global expertise and commitment to innovation in clinical research.
About Worldwide Clinical Trials
- Founding Team: Neal Cutler
- Founding Year: 1986
- Company Size: 3,147
What To Consider When Choosing the Best Clinical Research Companies?
Choosing the right clinical research company (CRC) is crucial for the success of any clinical trial. Here’s a detailed guide on what to consider:

Expertise and Specialization
Always ensure the CRC has expertise in your specific therapeutic area. Companies with experience in similar drug trials or medical devices can better navigate the complexities of your project.
Regulatory Compliance
The CRC must adhere to regulatory guidelines like FDA (US) , EMA (Europe), and others. Check their track record in meeting these standards to avoid compliance issues.
Reputation and Track Record
You should research the company’s history. Look for testimonials, case studies, and reviews from past clients. A company with a strong reputation is likely to deliver quality results.
Project Management Capabilities
Effective project management is key. Assess their ability to manage timelines, budgets, and communication. A CRC that provides transparent, regular updates is preferable.
Patient Recruitment Strategies
Patient recruitment can be challenging. Evaluate their strategies for participant recruitment and retention. Consider their demographic reach and methods for ensuring a diverse participant pool.
Data Management and Analysis
The CRC should have strong systems for data collection, management, and analysis. Ask about their use of Electronic Data Capture (EDC) systems and how they handle data security and confidentiality.
Cost and Financial Terms
Get a clear understanding of the cost structure. Consider the value for money rather than just the lowest cost. Ensure there are no hidden fees and clarify what is included in the quoted price.
How Heartbeat AI Can Help You Get the Best List of Clinical Research Organizations?
Heartbeat AI, with its advanced features, can significantly assist in identifying the best list of clinical research organizations (CROs). Here’s how its various features contribute to this process:

Data Analysis and Processing
Heartbeat AI excels in analyzing vast amounts of data. When it comes to selecting CROs, it can process and analyze information from numerous sources, including past performance records, clinical trial reports, and regulatory compliance data. This thorough analysis helps in identifying CROs with a proven track record of success and reliability.
Machine Learning Algorithms
These algorithms enable Heartbeat AI to learn from historical data and improve its recommendations over time. By understanding trends and patterns in the successful execution of clinical trials, it can better predict which CROs are likely to meet your specific needs.
Predictive Analytics
Heartbeat AI uses predictive models to forecast future trends and outcomes based on historical data. This can be invaluable in predicting the success rate of CROs in upcoming projects, thus aiding in making more informed choices.
Customization and Personalization
The AI can be customized to your specific requirements. If you’re focusing on a specific therapeutic area or clinical trial phase, Heartbeat AI can prioritize specialized CROs in these fields.
Real-time Data Updates
The healthcare and pharmaceutical landscapes are constantly changing. Heartbeat AI’s ability to integrate and analyze real-time data ensures that the recommendations are based on the most current information available.
Integration with External Databases
Heartbeat AI can integrate with various external databases and platforms. This enables it to pull in comprehensive information about CROs from diverse sources, enhancing the accuracy of its recommendations.
Claim $500 of Free Data
Summing up, we’ve explored the best clinical research companies, diving into their features, strengths, weaknesses, and more. Clinical research is vital in healthcare; it’s key for advancing medical knowledge and developing new treatments.
With this guide, you’re equipped to find the right clinical research company that meets your specific needs. Whether it’s for innovative therapies, drug development, or medical advancements, choosing the right partner is crucial. This guide serves as a valuable resource to help you make an informed decision in the complex world of clinical research.
Frequently Asked Question
What services do companies of clinical research offer.
Clinical research organizations offer a wide range of services, including protocol development, patient recruitment, data collection and analysis, regulatory compliance, and more.
What is the role of a clinical research coordinator?
A clinical research coordinator is responsible for managing various aspects of a clinical trial, including patient recruitment, data collection, and ensuring compliance with protocols.
What is informed consent in clinical research?
Informed consent is the process by which participants in a clinical trial are fully informed about the study’s purpose, risks, and benefits. They voluntarily agree to participate based on this information.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms
De-risking clinical development of precision medicines in oncology
Strategies for commercialising oncology treatments for young adults, icon innovation.
Our experience, knowledge and technological advancements converge to shape a better future for healthcare.
Applying AI to manage the risks and costs of postmarketing requirements
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Considerations for clinical trial design focused on obesity treatments.
The triad of trust: Navigating real-world healthcare data integration
Uncover how reshaping data and integration strategies can empower your organisation.
Ensuring the validity of clinical outcomes assessment data
Important considerations and best practices for effective COA implementation.
Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk.
Featured Solutions
Blended solutions
Bespoke, seamless solutions to meet unique sponsor challenges.
Outcome Measures
A multi-faceted approach to identifying, selecting, and implementing evidence-based measures that matter to patients.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Specialty Laboratory Solutions
Supporting precision medicine programs across all phases of drug diagnostic co-development.
Survival analysis for immature data: A review of techniques and lessons learned
17 September 2024. Register today.
Panel discussion: Immunogenicity assessment of therapeutic proteins
18 September 2024. Register today.
Medicare Part D Price Negotiation update
20 September 2024. Register today.
ICON Insights
- 09 Sep 2024
Navigating the impacts of the Inflation Reduction Act (IRA): Strategic considerations for pharma
Medicare Part D Price Negotiations update – Widespread impact on the industry
- 04 Sep 2024
Utilising connected sensors to power diverse and equitable clinical trials
- 02 Sep 2024
Outcome Measures: Driving forward patient-centered clinical trials
- 28 Aug 2024
Enabling a new way to select fit-for-purpose COAs to simplify clinical protocols
- 19 Aug 2024
Enhancing diversity in clinical trials with AI and human expertise
- 14 Aug 2024
The rise of precision outcome measures
Understanding China's 2024 NRDL: Key changes and their impact
The race to develop combination vaccines – and the importance of indirect effects
What’s happening in icon, how can we help.
- Cell and Gene Therapies
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions
- Regulatory Intelligence
Learn how our solutions can help you advance your oncology drug development. Meet with our team at the European Society of Medical Oncology (ESMO) Congress at booth 522 in Barcelona, Spain, on Sept. 13-17.
We’ve been named a leader in digital solutions and services by ISG for the fourth straight year, affirming our commitment to our customers and patients around the world.
Our new GMP lab building in Middleton, Wisconsin, will enhance services in new and emerging therapeutic areas and add 350 new jobs to the region.
It’s time for a partner who drives you forward. Let’s devise strategies that position you to meet key milestones, faster.
Drive your drug development program forward. Partner with a leading provider of global clinical research solutions .

Unrivaled therapeutic expertise honed over decades
Deep expertise that covers more than 20 therapeutic areas, from established drug markets to cutting-edge R&D, to perform studies with exceptional quality.
Solutions tailored to accelerate your development
Dependable project management and delivery, underpinned by a global network, integrated data ecosystem and people-first approach.

Comprehensive laboratory services that transform drug development
Laboratory services built on innovation and award-winning technologies to enhance clinical trial performance and regulatory success.
Bring your life-saving therapies to the world
Transformative strategies that empower you to achieve key milestones faster. Because every moment counts in clinical research.

PPD FSP Solutions
The biotech mindset, optimized study planning, insights hub.
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Delivery models
- Operational excellence
- Medical affairs
- Building patient insights into assets, profile and claims
- Portfolio optimization
- Asset valuation and indication prioritization
- Early evidence review
- Model-based drug development
- Integrated development strategy and planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Operationalize post-trial access with a proven platform approach
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Real World Evidence
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Gain an advantage through FSP
- Leveraging AI and digital in clinical development

Parexel Biotech provides the end-to-end capabilities you’ll need to succeed. We're fast, flexible, and dedicated to impacting patient lives.

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing precision oncology
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- Gene therapy: are high costs and manufacturing complexities impeding progress?
- New FDA draft guidance: Implications for simplifying interchangeability for biosimilars in the US
- Key implications for nonclinical development: FDA guidance on human gene therapy products incorporating human genome editing
Latest Report

New Medicines, Novel Insights: Advancing precision oncology

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Leadership team
- Global reach
- Our DE&I strategy
- Compliance, tax & privacy
- Meet us at an event
- Board of Directors
- Our ESG strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Cookies and other web tracking
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- Data Privacy Framework
- Supplier Diversity Policy
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
- Rare Diseases
- Inflammation & Immunology
Cell & Gene Therapy
When austin was 3 years old, his parents realized something was wrong..
DMD weakens the muscles until the patient can't walk and greatly shortens their lifespan. There's no cure.
After multiple falls, concussions, and a broken arm, they discovered he had Duchenne muscular dystrophy, a condition diagnosed in 20,000 children globally each year.
Now she was the one in need of care — joining the 3.8 million women in the US and many other around the world impacted by breast cancer.
Through clinical trials, however, Austin found treatment to slow the disease and live a better life.
Today, he's a college student, co-founder of the nonprofit one rare, and a member of our patient advisory council — helping us better meet the needs of rare disease patients., lives can change when you design rare disease trials with care and precision..
- Become a study of choice in patient communities
- Expedite startup and accelerate timelines
- Satisfy global regulations to get your treatment to market safely and quickly
- Find the right experts, focused on the right indications
What we do, we do
Our Experts
Our rare disease specialists collaborate to help get your treatment to patients like Austin faster.
MORE EXPERTS
Stacy Hurt, M.H.A., M.B.A.
Chief Patient Officer
Chris Learn, Ph.D., P.M.P.
Senior Vice Presiden...
Angela Qu, M.D., Ph.D.
Wyatt Gotbetter
Martin Roessner
Corporate Vice Presi...
Peter Kiely, M.D.
Vice President, Tech...
Steve Winitsky, M.D.
Rachel Smith
With 10+ years of experience in rare diseases and cell and gene therapy trials, Rachel leads our global, cross-functional Center of Excellence for Rare Diseases. She supports strategies to deliver your projects effectively, ensuring the patient perspective is included every step of the way.

Executive Director, Rare Disease Center of Excellence
"We need to develop products that meet the needs of the patient. That’s why we work so closely with them, focusing on improving their quality of life."
Our diverse rare disease experiences ensure you get the expertise you need, no matter the indication.
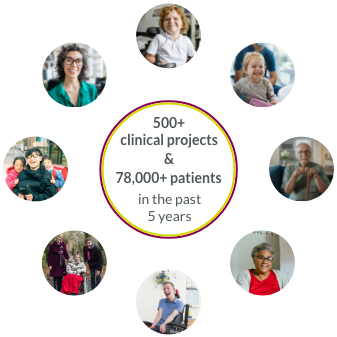
Our collaboration with rare disease clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Middle East & Africa

Our field-leading genetic specialists customize your endpoints by age, genotype, and phenotype for better data.
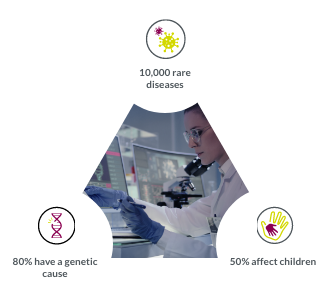
Our global regulatory team helps keep everything running smoothly, with expertise in breakthrough therapy designations and more.

And our patient-first approach results in deeper, more relevant insights for trial design and execution.
BENEFITS MAY INCLUDE
What can we do to help you change patient lives?
See rare diseases capabilities Visit all therapeutic areas See rare diseases career openings
Discover other patient stories
Sara's only symptom was fatigue. But after some routine tests, she was diagnosed with breast cancer.
Tina had lost her father to Crohn's disease. Now she faced her own diagnosis — and it derailed her life.
One night, while watching TV with her husband, Robyn felt a lump in her neck. As a doctor, she knew the risk.
While running a triathlon, Andrea stumbled and realized something was wrong. She thought it was just an injury. But it was much more.
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Austin.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.

Top 10 Companies Sponsoring Clinical Trials
Clinical trials are critical for advancing medical research and developing new treatments and therapies. These trials require substantial resources, and leading companies play a crucial role by sponsoring and supporting these endeavors. Several prominent companies have recognized the importance of clinical trials and have actively contributed to their success.
Pharmaceutical giants like Pfizer, Johnson & Johnson, and Novartis are among the leading companies sponsoring clinical trials. These companies invest significant financial resources and scientific expertise to bring new drugs and treatments to the market. By sponsoring clinical trials, they gain valuable insights into the efficacy and safety of their products, which helps them obtain regulatory approvals and ensure patient well-being. These companies often collaborate with academic institutions, healthcare organizations, and research centers to conduct large-scale trials and engage diverse patient populations.
Biotechnology companies such as Amgen, Gilead Sciences, and Moderna also play a vital role in sponsoring clinical trials. With a focus on innovative therapies and cutting-edge research, biotech companies contribute to advancing medical science. They often specialize in developing novel drugs and therapies, particularly in areas with unmet medical needs. By sponsoring clinical trials, these companies not only test the effectiveness of their experimental treatments but also support the scientific community in discovering new possibilities for disease management and prevention.
Medical device manufacturers like Medtronic, Abbott Laboratories, and Philips are another significant group of companies that sponsor clinical trials. They design and produce devices such as pacemakers, imaging systems, and surgical instruments. These companies sponsor clinical trials to evaluate the safety and efficacy of their devices in real-world settings. Through these trials, they gather data on device performance, patient outcomes, and usability, ensuring that their products meet the highest standards and address the needs of healthcare professionals and patients.
The importance of leading companies sponsoring clinical trials cannot be overstated. Their financial investments, research capabilities, and industry knowledge enable the successful completion of trials, leading to improved medical interventions and better patient care. Additionally, their involvement fosters collaborations between academia, healthcare providers, and industry, creating a rich ecosystem for scientific progress. By supporting clinical trials, these companies contribute to the growth of medical knowledge, the development of innovative treatments, and the overall improvement of global healthcare.
1. Pfizer: A Leader in Clinical Research
When it comes to clinical research, one name that stands out is Pfizer . They're making groundbreaking contributions through their involvement in clinical trials. Pfizer, a global pharmaceutical giant, has been at the forefront of clinical research for decades. They're driven by a passion for innovation and a commitment to improving lives through medical advancements. With their extensive resources and expertise, Pfizer has successfully conducted numerous notable clinical trials.
One remarkable example is the clinical trial that led to the development of the Pfizer-BioNTech COVID-19 vaccine . This breakthrough vaccine revolutionized the fight against the pandemic and offered hope to millions worldwide. It's a shining testament to Pfizer's dedication to tackling global health challenges head-on.
2. Johnson & Johnson: Advancing Healthcare through Trials
When it comes to advancing healthcare through clinical trials, Johnson & Johnson is dedicated to making a positive impact on people's lives by driving innovation through their commitment to clinical research. Johnson & Johnson is a renowned pharmaceutical and medical device company with a rich history of groundbreaking contributions to the world of healthcare. Their commitment to clinical research is unwavering, and they continuously strive to develop innovative solutions that address unmet medical needs.
One of the key areas where Johnson & Johnson has made a significant impact is in the field of oncology. Through their clinical trials, they have pioneered breakthrough treatments and therapies for various types of cancer . Their dedication to improving cancer care has offered hope and better outcomes for patients fighting this formidable disease.
In addition to oncology, Johnson & Johnson has been involved in numerous other clinical trials, exploring treatments for cardiovascular diseases, neurological conditions, and infectious diseases, to name just a few. Their diverse portfolio of trials showcases their wide-ranging expertise and commitment to advancing healthcare across various therapeutic areas.
3. Novartis: Pioneering Breakthroughs in Medicine
When it comes to pioneering breakthroughs in medicine, Novartis is at the forefront of clinical trials, driving innovation and transforming the lives of patients worldwide.
Novartis is a global healthcare company with a relentless commitment to improving patient outcomes through its involvement in clinical trials. They have made significant contributions to the development of innovative therapies and treatments across various therapeutic areas.
In the realm of oncology, Novartis has been a game-changer. They have conducted groundbreaking clinical trials that have revolutionized cancer care. Through their research, they have introduced targeted therapies and immunotherapies that are changing the way we fight cancer, offering new hope and improved survival rates for patients.
They have also made notable advancements in areas such as cardiovascular diseases, neurology, ophthalmology , and rare genetic disorders. Their commitment to diverse therapeutic areas demonstrates their dedication to addressing unmet medical needs and improving the lives of patients with various conditions.
4. Merck & Co.: A Legacy of Clinical Excellence
Merck & Co . has a long-standing commitment to advancing healthcare and has left an indelible mark on the world of clinical trials. Merck & Co. has been a driving force in the pharmaceutical industry for decades. Their involvement in clinical trials spans a wide range of therapeutic areas, from infectious diseases to cardiovascular health and beyond. With a legacy built on scientific rigor and a dedication to improving patient lives, Merck & Co. continues to make significant contributions to medical advancements.
One notable example of their groundbreaking research is the development of the human papillomavirus (HPV) vaccine . Through meticulous clinical trials, Merck & Co. brought this life-saving vaccine to the world, helping to prevent HPV-related cancers and protect countless lives. This achievement exemplifies their unwavering commitment to disease prevention and public health.
Merck & Co.'s commitment to clinical excellence and its transformative trials have made a lasting impact on global health.
5. Eli Lilly and Company: Transforming Lives through Research
Eli Lilly and Company 's dedication to clinical research is unwavering, and they have made significant contributions to the world of healthcare.
Are you curious about clinical trials?
One remarkable example of their impact is their work in the field of mental health. Eli Lilly and Company has conducted groundbreaking trials that have led to the development of medications for conditions such as depression , anxiety, and schizophrenia. Their research has provided hope to individuals and families affected by these challenging conditions.
Beyond mental health , Eli Lilly and Company has also made significant advancements in areas such as diabetes , oncology, and immunology. Through their clinical trials, they have introduced life-changing treatments that have the potential to save lives and improve the quality of life for countless patients.
6. Bristol-Myers Squibb: Advancing Cancer and Immunotherapy Research
When it comes to advancing cancer and immunotherapy research, Bristol-Myers Squibb (BMS) has a strong focus on these critical areas of healthcare and are committed to making significant strides in the fight against cancer.
Bristol-Myers Squibb has dedicated considerable resources and expertise to the field of oncology. They are driven by a passion to improve the lives of cancer patients and develop innovative treatments that target the disease at its core. Through its extensive research efforts, BMS has become a trailblazer in the fight against cancer.
BMS is also at the forefront of immunotherapy research, which harnesses the power of the immune system to combat cancer. Their trials focus on developing therapies that activate the body's own defense mechanisms to recognize and destroy cancer cells. This cutting-edge approach has revolutionized cancer treatment and provided new hope for patients.
When it comes to key clinical trials, BMS has numerous noteworthy examples such as the trial involving the development of a groundbreaking immunotherapy drug that targets specific proteins found on cancer cells. This therapy has shown remarkable efficacy in treating certain types of cancer, improving patient outcomes, and extending survival rates.
7. Sanofi: Innovating Healthcare through Clinical Trials
When it comes to innovating healthcare through clinical trials, Sanofi is committed to pushing the boundaries of medical research and developing life-changing treatments.
Sanofi has a deep-rooted commitment to clinical research and drug development. They believe in the power of scientific exploration to transform healthcare and improve patient outcomes. With its strong focus on research and innovation, Sanofi continues to make significant contributions to the medical world.
One area where Sanofi has made notable strides is in the field of rare diseases. Through their clinical trials, they have developed groundbreaking therapies that address the unmet medical needs of individuals with rare and complex conditions. These treatments have brought new hope and improved quality of life to patients who previously had limited options.
But Sanofi's impact extends beyond rare diseases. They have also conducted noteworthy clinical trials in areas such as cardiovascular health, diabetes, and immunology. Their dedication to diverse therapeutic areas demonstrates their commitment to improving healthcare across a wide spectrum of conditions.
8. AstraZeneca: Revolutionizing Medicine through Collaborative Trials
When it comes to revolutionizing medicine through collaborative trials, AstraZeneca is a standout. They believe in the power of partnerships and collaborative research to drive medical advancements.
AstraZeneca takes a unique approach to clinical research by fostering collaboration. They understand that by working together with scientists, healthcare professionals, and research institutions, they can accelerate the pace of medical breakthroughs. Through their collaborative trials, AstraZeneca aims to tackle complex diseases and develop innovative solutions that improve patient lives.
One area where AstraZeneca has made significant strides is in the field of respiratory medicine. Through their clinical trials, they have developed groundbreaking treatments for conditions such as asthma and chronic obstructive pulmonary disease (COPD). These treatments have provided relief and improved quality of life for millions of individuals affected by respiratory disorders.
AstraZeneca has also conducted notable clinical trials in areas such as cardiovascular health, oncology, and autoimmune diseases. Their commitment to diverse therapeutic areas demonstrates their dedication to addressing a wide range of medical needs.
9. AbbVie: Leading the Way in Biopharmaceutical Research
AbbVie is dedicated to pushing the boundaries of biopharmaceutical research and clinical trials scientific innovation and developing transformative treatments.
AbbVie has a strong focus on biopharmaceutical research, utilizing the power of biotechnology to develop cutting-edge therapies. Their commitment to scientific excellence is unwavering, and they continuously strive to address unmet medical needs and improve patient outcomes. Through their clinical trials, AbbVie is shaping the future of medicine.
One area where AbbVie has made significant strides is in the field of immunology. Their clinical trials have resulted in breakthrough treatments for autoimmune diseases such as rheumatoid arthritis and psoriasis . These therapies have provided much-needed relief and improved the quality of life for patients living with these chronic conditions.
AbbVie has also conducted notable clinical trials in areas such as oncology, neuroscience, and gastroenterology . Their diverse portfolio of trials demonstrates their commitment to tackling a wide range of medical challenges and improving healthcare across various therapeutic areas.
10. Gilead Sciences: Innovators in Infectious Disease and Beyond
When it comes to innovating in the field of infectious disease research and beyond, Gilead Sciences is a name that stands out. They are dedicated to tackling some of the most challenging health issues of our time.
Gilead Sciences has made significant contributions to infectious disease research, particularly in areas like HIV/AIDS and viral hepatitis. They have developed groundbreaking treatments that have revolutionized the lives of patients affected by these diseases. Gilead Sciences' relentless pursuit of scientific innovation has brought new hope to millions worldwide.
One key area where Gilead Sciences has made an impact is in the treatment of HIV/AIDS . Through their clinical trials, they have developed antiretroviral therapies that have transformed HIV/AIDS from a fatal disease to a manageable chronic condition. Their commitment to research and development has saved countless lives and improved the quality of life for individuals living with HIV/AIDS.
Gilead Sciences have also conducted key clinical trials in other therapeutic areas such as oncology, respiratory diseases, and liver diseases. Their diverse portfolio of trials showcases their dedication to addressing a wide range of health challenges.
The top 10 companies we've explored in this article play a pivotal role in advancing medical research and driving innovation in healthcare. From Pfizer to AstraZeneca, these companies are at the forefront of clinical trials, pushing the boundaries of scientific discovery and improving patient outcomes.
Their research has led to groundbreaking advancements in various therapeutic areas, from oncology to infectious diseases, immunology, and rare genetic disorders. Through their transformative trials, they have developed life-saving treatments, innovative therapies, and preventive interventions that have transformed the lives of countless individuals worldwide.

VIEW ALL FEATURED COMPANIES

- Health, Pharma & Medtech ›
- Pharmaceutical Products & Market
Clinical trials – statistics & facts
How much do pharma companies spend on r&d, the complexities of clinical studies, drug developers seek approval, key insights.
Detailed statistics
Pharmaceuticals: cost of drug development in the U.S. since 1975
Total global pharmaceutical R&D spending 2014-2030
Number of drugs in the R&D pipeline worldwide 2001-2024
Editor’s Picks Current statistics on this topic
Pharmaceuticals
Total number of registered clinical studies worldwide 2000-2024
Industry & Market
CRO market size worldwide forecast 2032
Biotechnology
Top companies by COVID-19 treatment vaccines in development June 2022
Further recommended statistics
R&d overview.
- Premium Statistic Total global pharmaceutical R&D spending 2014-2030
- Premium Statistic Industry sectors - expenditure on research and development 2022
- Premium Statistic Top pharmaceutical R&D projects based on net present value May 2024
- Basic Statistic Research and development expenditure: U.S. pharmaceutical industry 1995-2023
- Premium Statistic Research and development in European pharmaceutical industry by country 2022
- Premium Statistic Total clinical research funding by National Institutes for Health 2013-2025
Total global pharmaceutical R&D spending 2014-2030
Total global spending on pharmaceutical research and development from 2014 to 2030 (in billion U.S. dollars)
Industry sectors - expenditure on research and development 2022
Percentage of spending on research and development of total revenue in 2022, by industrial sector
Top pharmaceutical R&D projects based on net present value May 2024
Selected top pharmaceutical R&D projects based on net present value (NPV) as of May 2024 (in billion U.S. dollars)
Research and development expenditure: U.S. pharmaceutical industry 1995-2023
Research and development expenditure of total U.S. pharmaceutical industry from 1995 to 2023 (in billion U.S. dollars)
Research and development in European pharmaceutical industry by country 2022
Pharmaceutical research and development spending in selected European countries in 2022 (in million euros)
Total clinical research funding by National Institutes for Health 2013-2025
Total clinical research funding by the National Institutes for Health (NIH) from FY 2013 to FY 2025 (in million U.S. dollars)
Top R&D companies
- Basic Statistic Global top pharmaceutical companies based on R&D spending 2026
- Basic Statistic Top 50 global pharmaceutical and biotech companies by R&D intensity in 2022
- Basic Statistic Top pharmaceutical companies in R&D spending growth 2022
- Premium Statistic Leading global contract research organizations based on revenue 2023
- Basic Statistic Roche: participation of patients in clinical trials 2009-2017
Global top pharmaceutical companies based on R&D spending 2026
Global top 10 pharmaceutical companies based on projected R&D spending in 2026 (in billion U.S. dollars)
Top 50 global pharmaceutical and biotech companies by R&D intensity in 2022
World's top 50 pharmaceutical and biotechnology companies based on R&D intensity in 2022
Top pharmaceutical companies in R&D spending growth 2022
World's top 50 pharmaceutical and biotechnology companies based on R&D spending growth in 2022
Leading global contract research organizations based on revenue 2023
Leading global contract research organizations (CROs) based on 2023 revenue (in million U.S. dollars)
Roche: participation of patients in clinical trials 2009-2017
Number of patients who took part in clinical trials for pharmaceutical company Roche from 2009 to 2017*
Clinical studies
- Basic Statistic Increase in clinical trials' complexity 2001-2015
- Premium Statistic Clinical trial success rates by therapeutic area 2020
- Basic Statistic Number of registered clinical studies by location worldwide 2024
- Basic Statistic Percent of registered clinical studies worldwide by location 2024
- Basic Statistic Share of recruiting clinical studies worldwide by location 2024
- Premium Statistic Total number of registered clinical studies worldwide 2000-2024
- Basic Statistic Total number of registered clinical studies with posted results worldwide 2008-2024
Increase in clinical trials' complexity 2001-2015
Increase in clinical trials' complexity between 2001-2005 and 2011-2015
Clinical trial success rates by therapeutic area 2020
Clinical trial success rates by therapeutic area as of 2020*
Number of registered clinical studies by location worldwide 2024
Number of registered clinical studies worldwide by location as of April 2024
Percent of registered clinical studies worldwide by location 2024
Percentage of registered clinical studies worldwide by location as of April 2024
Share of recruiting clinical studies worldwide by location 2024
Percentage of registered recruiting clinical studies worldwide by location as of April 2024
Total number of registered clinical studies worldwide since 2000 (as of April 2024)
Total number of registered clinical studies with posted results worldwide 2008-2024
Total number of registered clinical studies with posted results worldwide since 2008 (as of April 2024)
Participation
- Basic Statistic Top clinical trial participant countries worldwide 2015-19, by share
- Basic Statistic Clinical trial participants U.S. vs. rest of world by therapy area 2015-2019
- Basic Statistic Clinical trial participants gender share worldwide 2015-19, by geographic location
- Basic Statistic Clinical trial participants ethnicity share worldwide 2015-19, by geographic location
Top clinical trial participant countries worldwide 2015-19, by share
Top 20 clinical trial participant countries worldwide in 2015-2019, by share of participants
Clinical trial participants U.S. vs. rest of world by therapy area 2015-2019
Number of clinical trial participants in the U.S. and rest of the world in 2015-2019, by therapeutic area (in 1,000s)
Clinical trial participants gender share worldwide 2015-19, by geographic location
Gender share of clinical trial participants worldwide in 2015-2019, by geographic location
Clinical trial participants ethnicity share worldwide 2015-19, by geographic location
Ethnicity share of clinical trial participants worldwide in 2015-2019, by geographic location
Costs and market
- Basic Statistic Pharmaceuticals: cost of drug development in the U.S. since 1975
- Premium Statistic R&D expenditure of new therapeutic drugs 2009-2018
- Basic Statistic Estimated clinical trial cost per patient by therapeutic class 2015-2017
- Basic Statistic Estimated clinical trial cost per drug by therapeutic class 2015-2017
- Basic Statistic CRO market size worldwide forecast 2032
- Premium Statistic Global pharma CRO market size 2015-2024, by pre-clinical, clinical and discovery
Cost of developing a drug in the U.S. from the 1970s until today (in million U.S. dollars)*
R&D expenditure of new therapeutic drugs 2009-2018
Mean and median R&D expenditure on new drugs by therapeutic area between 2009 and 2018* (in million U.S. dollars)
Estimated clinical trial cost per patient by therapeutic class 2015-2017
Estimated clinical trial cost per patient by therapeutic area in 2015-2017* (in U.S. dollars)
Estimated clinical trial cost per drug by therapeutic class 2015-2017
Estimated clinical trial cost per drug by therapeutic area in 2015-2017* (in million U.S. dollars)
Global contract research organization (CRO) market in 2022 and a forecast for 2032 (in billion U.S. dollars)
Global pharma CRO market size 2015-2024, by pre-clinical, clinical and discovery
Global pharmaceutical CRO market size from 2015 to 2024, by pre-clinical, clinical and discovery (in billion U.S. dollars)
Approvals, launches, setbacks
- Premium Statistic Pharmaceutical industry - number of new substances 1998-2023
- Premium Statistic Number of novel drugs approved annually by CDER 2008-2023
- Basic Statistic Key measurements of U.S. CDER drug approvals in 2023
- Basic Statistic FDA first premarket approvals for medtech products granted 2005-2022
- Premium Statistic Projection of top 2024 pharma and biotech launches by revenue 2028
- Basic Statistic Number of unsuccessful Alzheimer’s drugs in development in the U.S. 1998-2017
Pharmaceutical industry - number of new substances 1998-2023
Number of new chemical or biological entities developed between 1998 and 2023, by region of origin
Number of novel drugs approved annually by CDER 2008-2023
Total number of novel drugs approved by CDER from 2008 to 2023
Key measurements of U.S. CDER drug approvals in 2023
Percentage of drugs approved by the U.S. Center for Drug Evaluation and Research (CDER) in 2023 that met select key measurements
FDA first premarket approvals for medtech products granted 2005-2022
Number of first premarket approvals (PMA/HDE) granted by the FDA for medtech products from 2005 to 2022
Projection of top 2024 pharma and biotech launches by revenue 2028
Leading biotech and pharma product launches in 2024 and revenue forecasts for 2028 (in billion U.S. dollars)
Number of unsuccessful Alzheimer’s drugs in development in the U.S. 1998-2017
Number of unsuccessful Alzheimer’s drugs in development in the U.S. from 1998 to 2017
- Premium Statistic Top companies by COVID-19 treatment vaccines in development June 2022
- Basic Statistic Number of COVID-19 drugs in development worldwide by phase June 2022
- Basic Statistic Number of COVID-19 treatment vaccine trials worldwide by phase June 2022
- Basic Statistic Number of COVID-19 treatment vaccine trials worldwide by type June 2022
Leading companies by number of COVID-19 drugs and vaccines in development as of June 3, 2022
Number of COVID-19 drugs in development worldwide by phase June 2022
Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of June 3, 2022, by phase
Number of COVID-19 treatment vaccine trials worldwide by phase June 2022
Number of coronavirus (COVID-19) clinical trials for drugs and vaccines worldwide as of June 3, 2022, by phase
Number of COVID-19 treatment vaccine trials worldwide by type June 2022
Number of coronavirus (COVID-19) clinical trials for drugs and vaccines worldwide as of June 3, 2022, by type*
Further reports
Get the best reports to understand your industry.
- Pharmaceutical trends in Europe
- Generics and biosimilars
Mon - Fri, 9am - 6pm (EST)
Mon - Fri, 9am - 5pm (SGT)
Mon - Fri, 10:00am - 6:00pm (JST)
Mon - Fri, 9:30am - 5pm (GMT)
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Contract Research Organizations in Russia
Featured cros.

Smooth Drug Development
Smooth Drug Development offers a full range of clinical development services in Europe and Asia (Baltic States, Belarus, Bulgaria, Hungary, Germany, Moldova, Russia, Serbia, Spain, Slovakia, India, Kazakhstan, Uzbekistan). It includes project managem...
ACROSS Global in Russia

E-mail: [email protected]
Web: www.across.global
Synergy Research Group Russia
Address: 33/2, Usacheva St., Moscow, 119048
Phone: +74956004445
Local, small- and mid-size Contract Research Organizations in Russia
We are a full-service clinical CRO with a relentless mission to complete your clinical trial on time. Backed by our strong operational strategy and leadership, a devoted team of medical professionals in each country and connections to prominent medic... View full profile
- United States
At Atlant Clinical, we work hand-in-hand with the world’s leading biotech and pharmaceutical companies. Our preferred supplier status with some of the biggest names in the industry confirms we have the insight and daily experience to manage your proj... View full profile
International logistics of temperature-sensitive cargoes in the field of health care and clinical research. We have been working for over 13 years and our employees have a regular training program Your shipment will be in the right place on time. We... View full profile
A Contract Research Organization that specializes in organization and conducting of international and local clinical trials in Russia. Here you can find information about our services, contact details, history and experience. We will familiarize you... View full profile
Congenix is an independent contract research organization offering its clients a full range of services, covering the organization and implementation of clinical trials on phases I to IV in the Russian Federation and former Soviet countries. Our miss... View full profile
- United Kingdom
We go beyond testing, inspecting and certifying products; we are a Total Quality Assurance provider to industries worldwide. Through our global network of state-of-the-art facilities and industry-leading technical expertise we provide innovative and... View full profile
IPHARMA is a contract research organization focusing on conducting clinical trials and registration of investigational drugs and medical devices in Russia and EAEU. IPHARMA is a member of ChemRar High-Tech Center group of companies and an accredited... View full profile
Modern Medical Innovations is a new CRO in Russia with highly experienced and motivated team. We work across all EAEU countries (Kazakhstan, Armenia, Belarus). Our employees have extensive experience and expertise in the field of clinical research, r... View full profile
RusClinic CRO is a consulting company that has been successfully providing its services in the Russian pharmaceutical and medical markets for more than 20 years. RusCliniC CRO company was created by a team of professionals with extensive experience i... View full profile
Global Contract Research Organizations in Russia
ACROSS Global is a unique, full-service, comprehensive alliance of qualified CROs and Specialist Service Providers dedicated to providing a professional, cost-effective, focused, and seamless service to the pharmaceutical, biopharma and medical devic... View full profile
- Bosnia & Herzegovina
- Congo, DR of
- Philippines
- Saudi Arabia
- South Africa
- South Korea
- Switzerland
- United Arab Emirates
With 15 years of experience, we have defined a process that consistently achieves success for our clients.RESEARCH that uncovers the real needs and delivers actionable insights.At the heart of any healthcare challenge lies a compelling narrative. To... View full profile
A global, full-service contract research organization. We take your trials personally. dMed Global, a full-service Clinical Contract Research Organization (CRO) based in Shanghai, China and Clinipace Incorporated, a full-service Clinical CRO with hea... View full profile
- Czech Republic
Cromos Pharma is a US-based, international contract research organization delivering fully integrated clinical research solutions, in all trial phases, across a wide range of therapeutic indications. Our expert team, comprised of 95% MDs, has extensi... View full profile
Established in 1995, Dokumeds has been expanding its coverage and services significantly over 25 years of operations. Gradual geographical and operational coverage expansion correlated with company staff growth and lowering of employee turnover to an... View full profile
- Netherlands
Emergo by UL is a leading regulatory consulting firm specializing in global medical device and IVD compliance. Our comprehensive solution is designed to help you achieve and maintain regulatory and commercial success. With a presence on six continent... View full profile
As a provider of comprehensive Phase I through IV clinical trial management, clinical pharmacology, patient access solutions and other enabling services, Fortrea partners with emerging and large biopharma and medical device and diagnostic companies t... View full profile
- New Zealand
Global Clinical Trials, LLC is a full-service CRO with offices and operations in the United States, Europe and Asia. Our professional staff have been sharing their unique experience and utilizing established contacts with the key opinion leaders (KOL... View full profile
Since our foundation in Dublin, Ireland in 1990, our mission has been to help our clients to accelerate the development of drugs and devices that save lives and improve quality of life. We are a global provider of consulting, and outsourced developme... View full profile
IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, m... View full profile
MB Quest was founded in Moscow in 1997 as one of the first international CROs to work in Russia. We provide Sponsors with a full range of clinical trial services in Russia, Ukraine, Belarus, Georgia, and Kazakhstan. Our offices are located in the lar... View full profile
Our unique global partnering philosophy emphasizes an uncompromising commitment to clinical research and to the highest level of ethical standards and performance in our jobs. We are selective about the projects we engage in because we are devoted to... View full profile
OCT is one of the leading Eastern European contract research organizations. Since 2005, we have conducted hundreds of clinical trials in Eastern Europe, in both CEE and the CIS. Our team of 200+ professionals provides a full range of high-quality CRO... View full profile
For over 35 years, PAREXEL has proven to be a trusted partner for the complex development journey required of biopharmaceutical and medical device companies. We’re also an astute guide, able to simplify that journey for our clients, so safe new produ... View full profile
PPD is a leading global contract research organization providing comprehensive, integrated drug development, laboratory and lifecycle management services. Our clients and partners include pharmaceutical, biotechnology, medical device, academic and go... View full profile
Our mission is to be the best CRO in the world as measured by our employees, clients, investigators, and vendors. Our teams work tirelessly to ensure that we deliver on time and on budget. You will always know what's going on with your study when you... View full profile
SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex... View full profile
Life sciences services from SGS – optimize your development timelines to get medicines and medical devices to market quickly and safely. There is no other area of business that is more heavily regulated than the development, testing and distribution... View full profile
- Burkina Faso
- Cote d'Ivoire
- Dominican Republic
- El Salvador
- Equatorial Guinea
- Papua New Guinea
- Saint Lucia
- Trinidad & Tobago
- Turkmenistan
Smooth Drug Development offers a full range of clinical development services in Europe and Asia (Baltic States, Belarus, Bulgaria, Hungary, Germany, Moldova, Russia, Serbia, Spain, Slovakia, India, Kazakhstan, Uzbekistan). It includes project managem... View full profile
Syneos Health (Nasdaq:SYNH) is the only fully integrated biopharmaceutical solutions organization purpose-built to accelerate customer success. We lead with a product development mindset, strategically blending clinical development, medical affairs a... View full profile
X7 Research is a new generation CRO providing a vast range of services in the EU, CIS, USA and Asia. X7 Research is a CRO of just the right size, where the clients’ needs are always first priority. As a result, 100% of our initial customers return to... View full profile
List of CROs by location
- Verona Pharma-stock
- News for Verona Pharma
Verona Pharma’s Growth Prospects Bolstered by Clinical Advancements and Regulatory Milestones
Verona Pharma ( VRNA – Research Report ), the Healthcare sector company, was revisited by a Wall Street analyst today. Analyst Ram Selvaraju from H.C. Wainwright reiterated a Buy rating on the stock and has a $36.00 price target.
Ram Selvaraju has given his Buy rating due to a combination of factors surrounding Verona Pharma’s recent clinical and regulatory advancements. Verona Pharma’s partner, Nuance Pharma, has successfully completed patient enrollment for the Phase 3 trial of Ohtuvayre™ in China, which is a positive step towards expanding the drug’s global footprint. Additionally, the ongoing U.S. launch of Ohtuvayre following FDA approval and the anticipated issuance of a unique J-code will likely enhance the drug’s market penetration and reimbursement prospects. These developments present a strong case for Verona Pharma’s growth potential, thus justifying the Buy rating.
Furthermore, the presentation of additional clinical data analyses at a major respiratory disorders conference underscores the clinical value of Ohtuvayre, which is the first novel inhaled treatment mechanism for COPD in over two decades. The drug’s dual inhibition action and its impact on patient outcomes, including reductions in exacerbation rates, are significant. Verona Pharma is also broadening the potential utility of ensifentrine with new clinical initiatives, such as the planned Phase 2 trials for a fixed-dose combination therapy for COPD and another trial for non-cystic fibrosis bronchiectasis. These initiatives suggest a robust pipeline that could further solidify the company’s position in the respiratory treatment market.
Selvaraju covers the Healthcare sector, focusing on stocks such as Genmab, BridgeBio Pharma, and Annovis Bio. According to TipRanks , Selvaraju has an average return of 0.8% and a 40.80% success rate on recommended stocks.
TipRanks tracks over 100,000 company insiders, identifying the select few who excel in timing their transactions. By upgrading to TipRanks Premium, you will gain access to this exclusive data and discover crucial insights to guide your investment decisions. Begin your TipRanks Premium journey today.
Verona Pharma (VRNA) Company Description:
Verona Pharma Plc operates as a clinical-stage biopharmaceutical company, which engages in the development and commercialization of therapeutics for the treatment of respiratory diseases. Its lead product candidate, ensifentrine, has the potential to be the first therapy for the treatment of respiratory diseases that combines bronchodilator and anti-inflammatory activities in one compound. The company was founded by Michael J. A. Walker and Clive P. Page on February 24, 2005 and is headquartered in London, the United Kingdom.
Read More on VRNA:
- Verona Pharma to Present Additional Analyses of Phase 3 ENHANCE Studies in COPD at ERS International Congress 2024
- Verona Pharma management to meet virtually with Piper Sandler
- Biotech Alert: Searches spiking for these stocks today
- Verona Pharma Reports Second Quarter 2024 Financial Results and Provides Corporate Update
- Verona Pharma to Present at 44th Annual Canaccord Growth Conference
Verona Pharma News MORE
Related Stocks
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 September 2024
Post-approval evidence generation: a shared responsibility for healthcare
- Ali Abbasi 1 ,
- Donna Rivera 1 ,
- Lesley H. Curtis 1 &
- Robert M. Califf 1
Nature Medicine ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Clinical trials
- Drug development
- Scientific community
- Translational research
Post-approval evidence generation is essential for high-quality clinical care and should be a shared priority for clinicians, health systems, payors, and the medical products industry, as well as the FDA and federal agencies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

21 US Code § 355 (d).
Fanaroff, A. C., Califf, R. M., Windecker, S., Smith, S. C. Jr. & Lopes, R. D. JAMA 321 , 1069–1080 (2019).
Article PubMed PubMed Central Google Scholar
US Food and Drug Administration. https://go.nature.com/4e5igl9 (12 January 2016).
21 US Code § 355(o)(3) and (c).
21 US Code § 356(c).
Cooke, N. J. & Hilton, M. L. (eds.) Enhancing the Effectiveness of Team Science (National Academies Press, 2015).
Abbasi, A. B., Curtis, L. H., Fleisher, L. A. & Califf, R. M. JAMA 332 , 412–417 (2024).
Article PubMed Google Scholar
Mackay, C. B., Antonelli, K. R., Bruinooge, S. S., Saint Onge, J. M. & Ellis, S. D. Cancer 123 , 2893–2900 (2017).
Office of the Assistant Secretary for Planning and Evaluation. https://go.nature.com/4e5K34V (31 October 2023).
The Reagan-Udall Foundation for the Food and Drug Administration. https://go.nature.com/3MqItik (14 November 2023).
US Food and Drug Administration. https://go.nature.com/43MUG74 (5 February 2023).
US Food and Drug Administration. https://go.nature.com/4dMDYL2 (2 May 2024).
Sweet, K. L. et al. Clin. Cancer Res. 29 , 2179–2183 (2023).
Article CAS PubMed PubMed Central Google Scholar
Mangoni, A. A. et al. BMC Rheumatol. 3 , 10 (2019).
Pulte, E. D. et al. Oncologist 27 , 149–157 (2022).
Bhatnagar, V. et al. Blood 130 , 4352 (2017).
Google Scholar
Centers for Disease Control and Prevention. https://go.nature.com/3XoaQE6 (21 September 2022).
Download references
Acknowledgements
The authors acknowledge J. Woodcock, P. Marks and P. Stein for their comments on this manuscript.
Author information
Authors and affiliations.
US Food and Drug Administration, Silver Spring, Maryland, USA
Ali Abbasi, Donna Rivera, Lesley H. Curtis & Robert M. Califf
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Robert M. Califf .
Ethics declarations
Competing interests.
A.A. is a resident physician at the University of California, San Francisco, is the recipient of an NIH T32 training grant (5T32CA251070-04) on assignment at the US Food and Drug Administration (FDA) and has no conflicts of interest related to this manuscript to disclose; he reports past consulting income from LeMaitre and MIS Technologies, LLC. D.R. is an employee of the FDA and has no conflicts of interest to report. L.H.C. is a faculty member at Duke University who is on assignment at the FDA and has no conflicts of interest related to this manuscript to disclose; she reports past consulting income from Regeneron Pharmaceuticals, the NFL Players Association and Boehringer Ingelheim and institutional grants from GlaxoSmithKline and Novartis. R.M.C. is an employee of the FDA and has no conflicts of interest related to this manuscript to disclose; prior to his appointment to the FDA as Commissioner for Food and Drugs, he was an employee of and held equity in Verily Life Sciences and Google Health (Alphabet) and served on boards of directors for Cytokinetics, Centessa, Clinetic, Keystone Symposia, the Critical Path Institute (C-Path), the Clinical Research Forum and One Fifteen.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Abbasi, A., Rivera, D., Curtis, L.H. et al. Post-approval evidence generation: a shared responsibility for healthcare. Nat Med (2024). https://doi.org/10.1038/s41591-024-03241-x
Download citation
Published : 13 September 2024
DOI : https://doi.org/10.1038/s41591-024-03241-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
INVITRO is a provider of full Central laboratory services and Clinical trial management services for Phase I-IV.
INVITRO Laboratory also performs Local laboratory services for International clinical trials. Because of our broad experience and specialized expertise, we’re in unique position to support safety laboratory tests as an alternative to the different site bases.

Local laboratory services are offered for Russia, Belarus and Kazakhstan
INVITRO Clinical Research can use its own Courier logistics service for pick-up and delivery of samples to the laboratory.
Due to the absence of custom between these countries, we are one of the small groups of organization with the capability and expertise to conduct Laboratory services and development projects for either local or global clinical trials.
All the tests for Clinical trials are performed in the Moscow laboratory.
It is stocked with high-tech equipment and located in a space of 3 500 sqm including:
- 365 work days per year, including monitoring and registration of biological samples around the clock
- Quality assurance of laboratory services in accordance with ISO 9001, ISO 15189 certificates
- Validated methodologies, logistical and data management support, reliable and flexible reporting systems and cohesive quality control, which are critical to meet the requirements of your organization and regulatory agencies.
Our Project teams perform ongoing monitoring and proactive communications to provide high-quality outcomes. Protocol specifications are built in during database setup to provide customized reports and sophisticated result flagging for monitoring. Our secure Internet-based, remote data access system allows members of the project team to review lab results in real time. INVITRO scientists, technologists and project managers can assist with protocol development to provide the most appropriate tests for each study, and all INVITRO staff is trained and certified accordingly. INVITRO Laboratory offers the flexibility required for quick and customized responses to unique client needs for any size project.
We strongly believe that a collaborative relationship between INVITRO Laboratory and Clinical Investigators is fundamental study success.
We provide:
- Design of study-specific manual
- Lab Kit manufacturing in accordance with GLP requirements
- Study-specific requisition forms and courier documents
- Biomaterial transportation in compliance with IATA requirements
- Telephone assistance
- Specimen handling
- Long-Term Storage and frozen specimen tracking
- Personal Account of clinical trials – an opportunity to use a tool that allows you to monitor the results of laboratory research, query, order a courier and lab kits online and have access to laboratory documents and project -specific documents
INVITRO clinical research
- Patient service
- Project Management
- Request for proposal
Invitro Clinical Research
INVITRO Clinical Research has supported clinical study teams at pharma companies and CROs facing the challenges of recruiting study subjects
www.invitro-cr.com
- General Information
- Quality Assurance
- Request for Proposal
Legal Disclaimer
- Privacy Policy
- Cookie Policy

IMAGES
VIDEO
COMMENTS
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn. Syneos Health.
1. IQVIA. Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research. The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world, highlighting the companies driving this considerable growth and accelerating research and development across the globe.. ICON. Recognised as the world's largest and most comprehensive CRO powered by ...
10. Nucleus Network. Nucleus Network is a company that offers clinical research services focusing on early phase and vaccine trials. They provide state-of-the-art facilities, experienced staff, and innovative approaches to help pharmaceutical and biotech companies accelerate their drug development processes. 11.
iNGENū CRO. iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec... 💻 Website ↗ 📞 +1300 633 226 View all details.
A clinical research organization (CRO) is a company that provides services to pharmaceutical and biotechnology companies to help them outsource and conduct clinical trials. A CRO manages all aspects of a clinical trial, from start to finish, and works with a variety of stakeholders, including investigators, research sites, and patients.
Who we are. Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals ...
Chanice Henry. 03/27/2018. Pharma IQ showcases the top 10 Contract Research Organizations across the Pharma IQ network. Much of the world's population is in desperate need of better medical care. This need is a large driving force behind drug discovery's mission to uncover new medicines. The drug discovery process is complex and stubborn.
1. IQVIA. IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Clinical study. A research study involving human volunteers (also called participants) that is intended to add to medical knowledge. There are two types of clinical studies: Clinical trial. ClinicalTrials.gov identifier (NCT number) The unique identification code given to each clinical study upon at ClinicalTrials.gov.
LabCorp (Covance), with 15.05 billion USD (2023) and 75000 employees (Labcorp, 2024). In February 2015, Labcorp completes its $6 billion purchase of Covance, Inc., creating the world's leading health care diagnostics company. The combination of Covance's drug development leadership and Labcorp's medical testing expertise builds the market ...
ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, biotechnology and medical device industries.
The PPD™ clinical research business of Thermo Fisher Scientific is a global contract research organization (CRO) delivering clinical expertise for your product's success. ... A model intentionally built to serve the needs of small- to mid-sized biotech and pharma companies. Simplify the journey. Optimized Study Planning.
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical ...
Biotechnology companies such as Amgen, Gilead Sciences, and Moderna also play a vital role in sponsoring clinical trials. With a focus on innovative therapies and cutting-edge research, biotech companies contribute to advancing medical science. They often specialize in developing novel drugs and therapies, particularly in areas with unmet ...
Since September 27, 2007, results of Lilly clinical trials have been presented on for compounds that are approved for use in humans. Since January 2019, results of all Lilly-registered clinical trials are provided on either 30 days after the approval of the medicine (Phase 1) or within one year after a trial has completed (Phases 2-4). Lilly ...
Well-financed startup Tome is winding down operations just as two new companies, Borealis Biosciences and GondolaBio, are launching. Meanwhile, in the midst of already tense relations with China, House lawmakers raise the alarm about U.S. companies working with the country's military on trials. August 28, 2024. ·.
PSI CRO has received 2024 CRO Leadership Awards in the categories of Expertise, Quality, Compatibility, and Reliability in the Overall (combined Big and Small Pharma) respondent group. This is the seventh consecutive year that PSI has received leadership awards presented by Clinical Leader and Life Science Connect based on research conducted by ...
List of clinical research organizations in the US. 1. IQVIA. IQVIA is a globally renowned medical research companies in USA that stands out for its exceptional contributions to the healthcare and pharmaceutical industries. As one of the leading providers of advanced analytics, technology solutions, and contract research services, IQVIA plays a ...
Premium Statistic Global pharma CRO market size 2015-2024, by pre-clinical, clinical and discovery Costs and market Basic Statistic Pharmaceuticals: cost of drug development in the U.S. since 1975
IPHARMA LLC. IPHARMA is a contract research organization focusing on conducting clinical trials and registration of investigational drugs and medical devices in Russia and EAEU. IPHARMA is a member of ChemRar High-Tech Center group of companies and an accredited... View full profile. View locations.
Verona Pharma (VRNA - Research Report), the Healthcare sector company, was revisited by a Wall Street analyst today. Analyst Ram Selvaraju from H.C. Wainwright reiterated a Buy rating on the ...
INVITRO Clinical Research has supported clinical study teams at pharma companies and CROs facing the challenges of recruiting study subjects. INVITRO offers targeted strategic planning, own database using and well trained in matters of patient recruitment staff will help your study teams accelerate timelines, maximize budgets and exceed ...
The OCE is interested in collaborating with patients, trial investigators, pharmaceutical companies, government agencies and international healthcare policy groups to evaluate opportunities and ...
Laboratory. INVITRO is a provider of full Central laboratory services and Clinical trial management services for Phase I-IV. INVITRO Laboratory also performs Local laboratory services for International clinical trials. Because of our broad experience and specialized expertise, we're in unique position to support safety laboratory tests as an ...