Case Study Based Questions on Biomolecules – Chapter 14 Class 12
Case Study Based Questions on Biomolecules
1. Read the given passage and answer the questions that follow Monosaccharides containing an aldehyde group are called aldoses while those containing a keto group are called ketoses. All monosaccharides containing five and six carbon atoms have cyclic structures, furanose (five-membered) and pyranose (six-membered). During ring formation, C 1 aldoses and C 2 in ketoses becomes chiral and hence all these monosaccharides exist in two forms called the α-anomer and β-anomer while C 1 and C2 are called glycosidic or anomeric carbon atoms. In contrast, stereoisomers, which differ in configuration at any other chiral carbon other than the glycosidic carbon are called epimers. Two molecules of the same or different monosaccharides combine together through glycosidic linkage to form disaccharides Que 1. Which of the following compounds show furanose structures?

2. Read the given passage and answer the questions that follow The sequence of bases in mRNA is read in serial order in groups of three at a time. Each triplet of nucleotides (having a specific sequence of bases) is known as codon. Each codon specifies one amino acid. Many amino acids have more than one codons. The amino acids are brought to mRNA by another type of RNA called tRNA each amino acid has at least one corresponding tRNA. At one end of the tRNA is a trinucleotide base sequence on mRNA. Que 1. Which of the following nitrogen bases is not present in RNA? a) Thymine b) Adenine c) Guanine d) Cytosine Ans 1. (a) Que 2. Each triplet of nucleotides is called a) Anticodon b) Codon c) mRNA d) tRNA Ans 2. (b) Que 3. Each codon specifies a) 1 amino acid b) 2 amino acids c) 3 amino acids d) None of these Ans 3. (a)
Case Study based questions on Biomolecules
3. Read the given passage and answer the questions that follow Proteins are the most abundant biomolecules of the living system. The chief sources of proteins are milk, cheese, pulses, fish, meat, peanuts, etc. They are found in every part of the body and form a fundamental basis of the structure and functions of life. These are also required for the growth and maintenance of the body. The word protein is derived from the Greek word, ‘proteios’ meaning ‘primary’ or of ‘prime importance’. Chemically, proteins are the polymers in which the monomeric units are the α-amino acids. Amino acids contain an amino (-NH 2 ) and carboxylic (-COOH) functional groups. Depending upon the relative position of the amino group with respect to the carboxylic group, the amino acids can be classified as α, β, and γ-amino acids. Amino acids which are synthesised by the body are called non-essential amino acids. On the other hand, those amino acids which cannot be synthesized in the human body and are supplied in the form of diet (because they are required for proper health and growth) are called essential amino acids. Que 1. Amino acids show amphoteric behavior. Why? a) They have an amino group b) They have a carboxylic group c) Both (a) and (b) d) none of the above Ans 1. (c) Que 2. The name of linkage joining two amino acids a) Hydrogen bonding b) Peptide linkage c) Amino linkage d) Imino joints Ans 2. (b) Que 3. What are polypeptides? a) 10 < α-amino acids joined together b) amino acids joined together c) 20 < β-amino acids joined together d) None of the above Ans 3. (a)
Que 4. What type of bonding helps in stabilizing the α-helix structure of proteins? a) Peptide linkage b) Hydrogen bonding c) Amino linkage d) Van der waals force Ans 4. (b)
4. Read the given passage and answer the questions that follow
Biomolecules are complex molecules that build up living organisms and are required for their growth, maintenance, and ability to reproduce. Carbohydrates are polyhydroxy aldehydes and ketones which are major sources of energy. Monosaccharides are simple sugars that cannot be hydrolyzed. Oligosaccharides, on hydrolysis, give 2 to 10 molecules of monosaccharides. Polysaccharides like starch and cellulose on hydrolysis give a large number of molecules of glucose a-glucose and b-glucose (Anomers). Proteins are complex nitrogenous polymers of amino acids connected through peptide bonds. The sequence in which amino acids are linked is called Primary structure. Secondary structures are of 2 types a-helix in globular proteins and b-pleated structure in fibrous proteins involving H-bonds. The tertiary structure has H-bonds, disulphide linkage, ionic bonding, and van der Waals’ forces. Insulin is a hormone for the metabolism of glucose, has a quarternary structure. Denaturation of protein destroys a secondary and tertiary structure, loss of biological activity but primary structure remaining the same. Enzymes are highly specific, work at specific pH, moderate temperature, and catalyze biochemical reactions. Hormones perform specific functions and are secreted by endocrine glands. Vitamins are essential for a healthy body. A, D, E, K are fat-soluble vitamins. Vitamin C and B1, B2, B6 are water-soluble. B 12 is neither water nor fat-soluble. Nucleic acids are polymers of nucleotides. RNA consists of m-RNA, t-RNA, r-RNA. RNA has Adenine, Cytosine, Uracil, and Guanine. It helps in protein synthesis. It cannot replicate. DNA contains deoxyribose, A, C, G, and Thymine. It transfers genetic characteristics. DNA has a double helix structure and undergoes replication. Que 1. Name a disaccharide which on hydrolysis give glucose and galactose. Ans 1. Lactose. Que 2. What type of protein is albumin? Ans 2. Globular protein. Que 3. Name one non-reducing sugar. Ans 3. Sucrose Que 4. Which one is the complementary base of cytosine in one strand of DNA to that in another strand of DNA? Ans 4. Guanine. Que 5. Which linkage by which nucleotides are joined together between 5′ and 3′ atoms of pentose sugar? Ans 5. Phosphodiester linkage. Que 6. Which vitamin helps in the coagulation of blood? Ans 6. Vitamin K. Que 7. Which enzyme can dissolve blood clots to prevent a heart attack? Ans 7. Streptokinase

Related Posts

Solid State Chemistry Class 12 Notes PDF Download

CBSE Sample Paper Session 2022-23 (Chemistry) PDF

Haloalkanes and Haloarenes Class 12 – Questions & Answers
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.

Class 12 Chemistry Case Study Questions Chapter 14 Biomolecules
- Post author: studyrate
- Post published:
- Post category: Class 12 / 12 board
- Post comments: 0 Comments
In Class 12 Boards there will be Case studies and Passage Based Questions will be asked, So practice these types of questions. Study Rate is always there to help you. Free PDF Downloads of CBSE Class 12 Chemistry Chapter 14 Biomolecules Case Study and Passage-Based Questions with Answers were Prepared Based on the Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Case Study Questions Biomolecules to know their preparation level.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 12 Chemistry Paper, There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Biomolecules Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 14 Biomolecules
Case Study/Passage-Based Questions
Case Study 1: Monosaccharides containing an aldehyde group are called aldoses while those containing a keto group are called ketoses. All monosaccharides containing five and six carbon atoms have cyclic structures, furanose (five-membered) and pyranose (six-membered). During ring formation, C 1 aldoses and C 2 in ketoses become chiral and hence all these monosaccharides exist in two forms called the α-anomer and β-anomer while C 1 and C2 are called glycosidic or anomeric carbon atoms. In contrast, stereoisomers, which differ in configuration at any other chiral carbon other than the glycosidic carbon are called epimers. Two molecules of the same or different monosaccharides combine together through glycosidic linkage to form disaccharides
Que 1. Which of the following compounds show furanose structures?
a) Mannose b) Galactose c) Fructose
Answer:c) Fructose
Que 2. What is the relation between two molecules? (a) Enantiomers (b) Epimers (c) Functional groups (d) Anomers
Answer:(b) Epimers
Que 3. In disaccharides, the linkage connecting monosaccharide units is called (a) Glycogen linkage (b) Nucleoside linkage (c) Glycosidic linkage (d) Peptide linkage.
Answer:(c) Glycosidic linkage
Case Study 2: Proteins are the most abundant biomolecules in the living system. The chief sources of proteins are milk, cheese, pulses, fish, meat, peanuts, etc. They are found in every part of the body and form a fundamental basis of the structure and functions of life. These are also required for the growth and maintenance of the body. The word protein is derived from the Greek word, ‘proteins’ meaning ‘primary’ or of ‘prime importance’. Chemically, proteins are polymers in which the monomeric units are the α-amino acids. Amino acids contain amino (-NH2) and carboxylic (-COOH) functional groups. Depending upon the relative position of the amino group with respect to the carboxylic group, the amino acids can be classified as α, β, and γ-amino acids. Amino acids which are synthesized by the body are called non-essential amino acids. On the other hand, those amino acids which cannot be synthesized in the human body and are supplied in the form of diet (because they are required for proper health and growth) are called essential amino acids. Que 1. Amino acids show amphoteric behavior. Why? a) They have an amino group b) They have a carboxylic group c) Both (a) and (b) d) none of the above
Answer:c) Both (a) and (b)
Que 2. The name of linkage joining two amino acids a) Hydrogen bonding b) Peptide linkage c) Amino linkage d) Imino joints
Answer:b) Peptide linkage
Que 3. What are polypeptides? a) 10 < α-amino acids joined together b) amino acids joined together c) 20 < β-amino acids joined together d) None of the above
Answer: a) 10 < α-amino acids joined together
Que 4. What type of bonding helps in stabilizing the α-helix structure of proteins? a) Peptide linkage b) Hydrogen bonding c) Amino linkage d) Van der waals force
Answer: b) Hydrogen bonding
Case Study 3: When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix gets uncoiled and protein loses its biological activity. This is called the denaturation of protein. The denaturation causes change in secondary and tertiary structures but primary structures remain intact. Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk, and formation of cheese when an acid is added to milk.
(i) Mark the wrong statement about denaturation of proteins
| (a) The primary structure of the protein does not change |
| (b) Globular proteins are converted into fibrous proteins. |
| (c) Fibrous proteins are converted into globular proteins. |
| (d) The biological activity of the protein is destroyed. |
Answer:(c) Fibrous proteins are converted into globular proteins.
(ii) Which structure(s) of proteins remains(s) intact during denaturation process?
| (a) Both secondary and tertiary structures | (b) Primary structure only |
| (c) Secondary structure only | (d) Tertiary structure only |
Answer: (b) Primary structure only
(iii) Cheese is a
| (a) globular protein | (b) conjugated protein |
| (c) denatured protein | (d) derived protein |
Answer: (c) denatured protein
(iv) Secondary structure of protein refers to
| (a) mainly denatured proteins and structure of prosthetic groups |
| (b) three-dimensional structure, especially the bond between amino acid residues that are distant from each other in the polypeptide chain |
| (c) linear sequence of amino acid residues in the polypeptide chain |
| (d) regular folding patterns of continuous portions of the polypeptide chain |
Answer: (d) regular folding patterns of continuous portions of the polypeptide chain
Hope the information shed above regarding Case Study and Passage Based Questions for Class 12 Chemistry Chapter 14 Biomolecules with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 12 Chemistry Biomolecules Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible. By Team Study Rate

You Might Also Like
[pdf] download class 12 mathematics previous year questions pdf for cbse class 12 maths, class 12 physics case study questions chapter 7 alternating current, class 12 physics assertion reason questions pdf download, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Topper Combo Flashcards
- Contains the Latest NCERT in just 350 flashcards.
- Colourful and Interactive
- Summarised Important reactions according to the latest PYQs of NEET(UG) and JEE
No thanks, I’m not interested!
Not Able To Find Desired Paper or Worksheet SEARCH
Find papers & worksheets search, case study questions for class 12 chemistry chapter 14 biomolecules.

- (0) Comments
- 10 Downloads
Related Papers
Click to view more related papers, display_name = "class 11" && $paper->display_name = "class 12") { // echo $paper->display_name." questions papers and worksheets"; } //else { // echo $paper->display_name." sample papers and previous year papers"; //} //>, important questions, mcq's, ncert solutions - class 12 chemistry.
Get here all the Important questions for Class 12 Chemistry chapter wise as free PDF download. Here you will get Extra Important Questions with answers, Numericals and Multiple Choice Questions (MCQ's) chapter wise in Printable format. Solving Chapter wise questions is one of the best ways to prepare for the examination. Students are advised to understand the concepts and theories of Chemistry properly before the exam. You can easily find 1 Mark, 2 marks, 3 marks, and 5 marks questions from each chapter of Class 12 Chemistry and prepare for exam more effectively. These preparation material for Class 12 Chemistry , shared by teachers, parents and students, are as per latest NCERT and CBSE Pattern syllabus and assure great success in achieving high score in Final CBSE Board Examinations.
Latest MCQ's and Important Questions for CBSE Class 12 Chemistry
class 12 chemistry chapter 1 important questions with answers class 12 chemistry chapter 2 important questions with answers class 12 chemistry chapter 3 important questions with answers class 12 chemistry chapter 4 important questions with answers class 12 chemistry chapter 5 important questions with answers class 12 chemistry chapter 6 important questions with answers class 12 chemistry chapter 7 important questions with answers class 12 chemistry chapter 8 important questions with answers class 12 chemistry chapter 9 important questions with answers class 12 chemistry chapter 10 important questions with answers class 12 chemistry chapter 11 important questions with answers class 12 chemistry chapter 12 important questions with answers class 12 chemistry chapter 13 important questions with answers class 12 chemistry chapter 14 important questions with answers class 12 chemistry chapter 15 important questions with answers class 12 chemistry chapter 16 important questions with answers mcqs of chemistry class 12 chapter 1 mcqs of chemistry class 12 chapter 2 mcqs of chemistry class 12 chapter 3 mcqs of chemistry class 12 chapter 4 mcqs of chemistry class 12 chapter 5 mcqs of chemistry class 12 chapter 6 mcqs of chemistry class 12 chapter 7 mcqs of chemistry class 12 chapter 8 mcqs of chemistry class 12 chapter 9 mcqs of chemistry class 12 chapter 10 mcqs of chemistry class 12 chapter 11 mcqs of chemistry class 12 chapter 12 mcqs of chemistry class 12 chapter 13 mcqs of chemistry class 12 chapter 14 mcqs of chemistry class 12 chapter 15 mcqs of chemistry class 12 chapter 16 The Solid State Class 12 Case Study Questions Solutions Class 12 Case Study Questions Notes Electrochemistry Class 12 Case Study Questions Chemical Kinetics Class 12 Case Study Questions Surface Notes Class 12 Case Study Questions General Principles and Processes of Isolation of Elements Class 12 Case Study Questions The p-Block Elements Class 12 Case Study Questions The d and f Block Elements Class 12 Case Study Questions Coordination Compounds Class 12 Case Study Questions Haloalkanes and Haloarenes Class 12 Case Study Questions Alcohols, Phenols and Ethers Class 12 Case Study Questions Aldehydes, Ketones and Carboxylic Acids Class 12 Case Study Questions Amines Class 12 Case Study Questions Biomolecules Class 12 Case Study Questions Polymers Class 12 Case Study Questions Chemistry in Everyday Life Class 12 Case Study Questions
Total Papers :
| Class 12 Chemistry Marks Distribution | |
|---|---|
| Units | Marks |
| Solid State | 23 |
| Solutions | |
| Electrochemistry | |
| Chemical Kinetics | |
| Surface Chemistry | |
| General Principles and Processes of Isolation of Elements | 19 |
| p- Block Elements | |
| d - and f- Block Elements | |
| Coordination Compounds | |
| Haloalkanes and Haloarenes | 28 |
| Alcohols, Phenols and Ethers | |
| Aldehydes, Ketones and Carboxylic Acids | |
| Organic Compounds containing Nitrogen | |
| Biomolecules | |
| Polymers | |
| Chemistry in Everyday Life | |
| Total | 70 |
CBSE Class 12 Chemistry Syllabus
- Solid State
- Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- General Principles and Processes of Isolation of Elements
- p-Block Elements
- d- and f-Block Elements
- Coordination Compounds
- Haloalkanes and Haloarenes.
- Alcohols, Phenols and Ethers
- Aldehydes, Ketones and Carboxylic Acids
- Organic compounds containing Nitrogen
- Biomolecules
- Chemistry in Everyday life
Unit II: Solutions 15 Periods
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult's law, colligative properties - relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van't Hoff factor.
Unit III: Electrochemistry 18 Periods
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch's Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit IV: Chemical Kinetics 15 Periods
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit VIII: d and f Block Elements 18 Periods
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first-row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids - Electronic configuration, oxidation states and comparison with lanthanoids.
Unit IX: Coordination Compounds 18 Periods
Coordination compounds - Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner's theory, VBT, and CFT; structure and stereoisomerism, the importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit X: Haloalkanes and Haloarenes. 15 Periods Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of - dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers 14 Periods
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids 15 Periods
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Amines 14 Periods
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules 18 Periods
Carbohydrates - Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins - Elementary idea of - amino acids, peptide bond, polypeptides, proteins, structure of proteins - primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes. Hormones - Elementary idea excluding structure.
Vitamins - Classification and functions. Nucleic Acids: DNA and RNA.
Structure of CBSE Chemistry Sample Paper for Class 12 Science is
| Type of Question | Marks per Question | Total No. of Questions | Total Marks |
|---|---|---|---|
| Very Short Answer Type Questions | 1 | 5 | 5 |
| Short Answer Type Questions - 1 | 2 | 5 | 10 |
| Short Answer Type Questions - 2 | 3 | 12 | 36 |
| Value Based Type Questions | 4 | - | 4 |
| Long Answer Type Questions | 3 | 5 | 15 |
| Total | 26 | 70 | |
For Preparation of exams students can also check out other resource material
CBSE Class 12 Chemistry Sample Papers
CBSE Class 12 Chemistry Worksheets
CBSE Class 12 Chemistry Question Papers
CBSE Class 12 Chemistry Test Papers
CBSE Class 12 Chemistry Revision Notes
Question Bank of Other Subjects of Class 12
Importance of Question Bank for Exam Preparation?
There are many ways to ascertain whether a student has understood the important points and topics of a particular chapter and is he or she well prepared for exams and tests of that particular chapter. Apart from reference books and notes, Question Banks are very effective study materials for exam preparation. When a student tries to attempt and solve all the important questions of any particular subject , it becomes very easy to gauge how much well the topics have been understood and what kind of questions are asked in exams related to that chapter.. Some of the other advantaging factors of Question Banks are as follows
- Since Important questions included in question bank are collections of questions that were asked in previous exams and tests thus when a student tries to attempt them they get a complete idea about what type of questions are usually asked and whether they have learned the topics well enough. This gives them an edge to prepare well for the exam.Students get the clear idea whether the questions framed from any particular chapter are mostly either short or long answer type questions or multiple choice based and also marks weightage of any particular chapter in final exams.
- CBSE Question Banks are great tools to help in analysis for Exams. As it has a collection of important questions that were asked previously in exams thereby it covers every question from most of the important topics. Thus solving questions from the question bank helps students in analysing their preparation levels for the exam. However the practice should be done in a way that first the set of questions on any particular chapter are solved and then solutions should be consulted to get an analysis of their strong and weak points. This ensures that they are more clear about what to answer and what can be avoided on the day of the exam.
- Solving a lot of different types of important questions gives students a clear idea of what are the main important topics of any particular chapter that needs to focussed on from examination perspective and should be emphasised on for revision before attempting the final paper. So attempting most frequently asked questions and important questions helps students to prepare well for almost everything in that subject.
- Although students cover up all the chapters included in the course syllabus by the end of the session, sometimes revision becomes a time consuming and difficult process. Thus, practicing important questions from Question Bank allows students to check the preparation status of each and every small topic in a chapter. Doing that ensures quick and easy insight into all the important questions and topics in each and every individual. Solving the important questions also acts as the revision process.
Question Bank of Other Classes
To Prepare better for CBSE paperclass; ?> " title="Download Free CBSE Papers">Ribblu.com brings to you all the previous years papers & worksheets of subject; ?//> for CBSE paperclass; ?>. This CBSE paper and worksheet can be instrumental in students achieving maximum marks in their exams. These Papers and worksheets help students gain confidence and make them ready to face their school examinations. These Papers and worksheets school wise, covers important concepts from an examination perspective. Students and parents can download all the available papers & worksheets directly in the form of PDF. One can use these papers and worksheets to get extensive practice and familiarise themselves with the format of the question paper.
You can help other users
Be the first to write comment .
Upload papers and the more your paper get downloaded the more you earn the points
You may send papers on email [email protected] along with userid
- Downloaded by: Partha Sarathy
Rules and regulations for uploads
| 1. | The uploaded material should be original and not duplicated. |
| 2. | It should be clear, legible and genuine. The file type should be pdf for multiple pages and jpg is allowed only for single page document. Use apps like “cam scanner” for mobile capture, crop and edit photos and save it as pdf. One file should be complete in all aspects like one full question paper, Notes of a complete topic. File name should be self explanatory (eg. CBSE 10th 2012 hindi question paper by – ‘Name of the uploader’) |
| 3. | No copyright violations allowed. |
| 4. | Points and coupons will be given at the sole discretion of Ribblu. |
| 5. | Ribblu admin has the power to reject, remove, alter, approve, accept any material that is uploaded by the user without consent of owner. |
Write your comment
Report this paper, how to earn points.
Upload Papers / Worksheets and Earn 50 Points.
The uploaded material should be original paper or worksheet of any school. Check out some videos on how to upload papers on ribblu
Rate & Review your school and Earn 25 Points.
Review any school that you may be knowing and once your review is approved, you will be credited with 25 points.
Answer on question posted on JustAsk and earn 15 points.
JustAsk is a platform where you can help others to find answers of any question. Share your Knowledge. Answer questions and once approved you will earn 15 points
Complete your profile and earn upto 25 Points.
Edit and complete your user profile and earn points. The more details you submit, the more points you will earn.
Download Ribblu Mobile App and you will (Earn 20 Points) (one time only)
CBSE Schools
- CBSE Schools In Delhi
- CBSE Schools In Noida
- CBSE Schools In Greater Noida
- CBSE Schools In Faridabad
- CBSE Schools In Ghaziabad
- CBSE Schools In Gurgaon
- CBSE Schools In Mumbai
- CBSE Schools In Pune
- CBSE Schools In Bangalore
- CBSE Schools In Hyderabad
- CBSE Schools In Kolkata
- CBSE Schools In Chennai
- CBSE Schools In Patna
- CBSE Schools In Meerut
- CBSE Schools In Kanpur
- CBSE Schools In Indore
- CBSE Schools In Ludhiana
- CBSE Schools In Dehradun
Top Schools
- Schools In Delhi
- Schools In Noida
- Schools In Greater Noida
- Schools In Faridabad
- Schools In Ghaziabad
- Schools In Gurgaon
- Schools In Mumbai
- Schools In Pune
- Schools In Bangalore
- Schools In Hyderabad
- Schools In Kolkata
- Schools In Chennai
- Schools In Patna
- Schools In Meerut
- Schools In Kanpur
- Schools In Indore
- Schools In Ludhiana
- Schools In Dehradun
Other Schools
- Pre Nursery Schools In Noida
- Day Boarding Schools In Noida
- Pre Nursery Schools In Gurgaon
- Pre Nursery Schools In Delhi
- Play Schools In Delhi
- Day Boarding Schools In Delhi
CBSE Papers
- CBSE Class 1 Sample Papers
- CBSE Class 2 Sample Papers
- CBSE Class 3 Sample Papers
- CBSE Class 4 Sample Papers
- CBSE Class 5 Sample Papers
- CBSE Class 6 Sample Papers
- CBSE Class 7 Sample Papers
- CBSE Class 8 Sample Papers
Paper Categories
- Question Bank
- Question Papers
- Revision Notes
- Sample Papers
- Test Papers
- CBSE Class 9 Sample Papers
- CBSE Class 10 Sample Papers
- CBSE Class 11 Sample Papers
- CBSE Class 12 Sample Papers

CBSE Expert
CBSE Class 12 Chemistry Case Study Questions PDF
Case studies play a pivotal role in CBSE Class 12 Chemistry, as they enable students to apply theoretical knowledge to real-life scenarios. CBSE Class 12 Chemistry Case Study Questions PDF section introduces the significance of case studies in enhancing analytical skills and understanding complex chemical reactions.
Case studies challenge students to think critically, analyze experimental data, and devise problem-solving strategies. They provide a deeper understanding of chemical principles and their practical applications, fostering a holistic learning experience. Familiarize yourself with the structure of case study questions to streamline your preparation. Each case study presents a unique chemical problem, encouraging students to identify relevant concepts and devise accurate solutions.
Table of Contents
Class 12 Chemistry Case Study Questions
CBSE Class 12 Chemistry question paper will have case study questions too. These case-based questions will be objective type in nature. So, Class 12 Chemistry students must prepare themselves for such questions. First of all, you should study NCERT Textbooks line by line, and then you should practice as many questions as possible.

Chapter-wise Solved Case Study Questions for Class 12 Chemistry
| Click Below | |
|---|---|
Class 12 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year’s exams. The solutions have been designed in a manner to help the grade 12 students understand the concepts and also easy-to-learn solutions.
Tips to Excel in CBSE Class 12 Chemistry Examinations
Excel in your Chemistry exams with these practical tips.
A. Regular Practice with Case Studies
Consistent practice with case study questions enhances your ability to tackle complex problems. Dedicate time to solving various case studies to build confidence.
B. Understanding Analytical Skills
Develop strong analytical skills to approach case studies logically. Break down complex problems into simpler components and analyze them step-by-step.
C. Time Management Strategies
Allocate sufficient time for each case study during the exam. Practice time management in mock tests to complete the paper within the stipulated time.
Best Books for Class 12 Chemistry
Strictly as per the new term-wise syllabus for Board Examinations to be held in the academic session 2024 for class 12 Multiple Choice Questions based on new typologies introduced by the board- Stand-Alone MCQs, MCQs based on Assertion-Reason Case-based MCQs. Include Questions from CBSE official Question Bank released in April 2024 Answer key with Explanations What are the updates in the book: Strictly as per the Term wise syllabus for Board Examinations to be held in the academic session 2024. Chapter-wise -Topic-wise Multiple choice questions based on the special scheme of assessment for Board Examination for Class 12th Chemistry.

Mastering CBSE Class 12 Chemistry case study questions is crucial for excelling in the exams. Embrace case studies as a valuable learning tool, and with practice, you’ll ace your Chemistry exams with confidence.
Benefits of Utilizing the CBSE Class 12 Chemistry Case Study PDF
- Enhanced Learning Experience : The case study PDF offers practical examples and scenarios, making the learning process engaging and relatable for students.
- Application of Theoretical Concepts : It enables students to apply theoretical knowledge to practical situations, honing their problem-solving and analytical skills.
- Real-World Relevance : By connecting classroom learning to real-life applications, students can grasp the practical significance of chemistry in various industries.
- Critical Thinking Development : Analyzing case studies encourages students to think critically and make informed decisions based on chemical principles.
- Exam Preparation : Exposure to case studies aids in better preparation for chemistry examinations by providing a comprehensive understanding of the subject.
The CBSE Class 12 Chemistry case study PDF brings a refreshing perspective to the world of education. By intertwining theoretical knowledge with practical applications, it equips students to face real-world challenges with confidence. The diverse case studies provide invaluable insights, encouraging students to explore chemistry beyond the classroom and make a positive impact on society.
What is the CBSE Class 12 Chemistry case study PDF?
The CBSE Class 12 Chemistry case study PDF is a curated document by CBSE, presenting real-life applications of chemistry concepts for students to understand the subject’s practical relevance.
How does the case study PDF benefit students?
The case study PDF enhances the learning experience, fosters critical thinking, promotes application-based learning, and prepares students for examinations.
Are the case studies diverse in content?
Yes, the case studies cover various branches of chemistry, including organic, inorganic, physical, environmental, and analytical chemistry.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
- New QB365-SLMS
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships
Class 12th Chemsitry - Biomolecules Case Study Questions and Answers 2022 - 2023
By QB365 on 08 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Biomolecules, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Biomolecules case study questions with answer key.
12th Standard CBSE
Final Semester - June 2015

Case Study

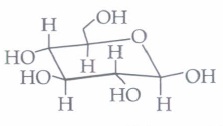
| -furanose | -pyranose |
(iv) The term anomers of glucose refers to

(ii) In aqueous solutions, amino acids mostly exist as
(iii) Amino acids are least soluble
(iv) The \(pK_{a_{1}}\) and \(pK_{a_{2}}\) of an amino acid are 2.3 and 9.7 respectively. The isoelectric point of the amino acid is
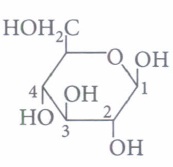
(ii) Which of the following monosaccharides, is the majority found in the human body?
(iii) Monosaccharides contain
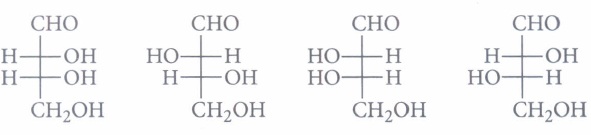
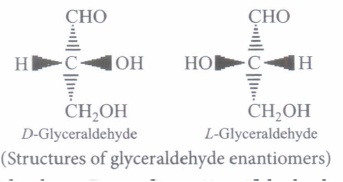
Read the passage given below and answer the following questions: Carbohydrates are polyhydroxy aldehydes and ketones and those compounds which on hydrolysis give such compounds are also carbohydrates. The carbohydrates which are not hydrolysed are called monosaccharides. Monosaccharides with aldehydic group are called aldose and those which free ketonic groups are called ketose. Carbohydrates are optically active. Number of optical isomers = 2 n Where n = number of asymmetric carbons. Carbohydrates are mainly synthesised by plants during photosynthesis. The monosaccharides give the characteristic reactions of alcohols and carbonyl group (aldehydes and ketones). It has been found that these monosaccharides exist in the form of cyclic structures. In cyclization, the -OH groups (generally C 5 or C 4 in aldohexoses and C 5 or C 6 in ketohexoses) combine with the aldehyde or keto group. As a result, cyclic structures of five or six membered rings containing one oxygen atom are formed, e.g., glucose forms a ring structure. Glucose contains one aldehyde group, one 1 o alcoholic group and four 2 o alcoholic groups in its open chain structure. The following questions are multiple choice questions. Choose the most appropriate answer: (i) First member of ketos sugar is
(ii) In CH 2 OHCHOHCHOHCHOHCHOHCHO, the number of optical isomers will be
(iii) Some statements are given below: 1. Glucose is aldohexose. 2. Naturally occurring glucose is dextrorotatory. 3. Glucose contains three, chiral centres. 4. Glucose contains one 1 o alcoholic group and four 2 o alcoholic groups. Among the above, correct statements are
(iv) Which of the following reactions of glucose can be explained only by its cyclic structure?
Read the passage given below and answer the following questions: When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogenbonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein. The denaturation causes change in secondary and tertiary structures but primary structures remains intact. Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk, formation of cheese when an acid is added to milk. The following questions are multiple choice questions. Choose the most appropriate answer: (i) Mark the wrong statement about denaturation of proteins
(ii) Which structure(s) of proteins remains(s) intact during denaturation process?
(iii) Cheese is a
(iv) Secondary structure of protein refers to
Read the passage given below and answer the following questions: The sequence of bases along the DNA and RNA chain establishes its primary structure which controls the specific properties of the nucleic acid. An RNA molecule is usually a single chain of ribose-containing nucleotide. On the basis of X-ray analysis of DNA, J.D., Watson and F.H.C. crick (shared noble prize in 1962) proposed a three dimensional secondary structure for DNA. DNA molecule is a long and highly complex, spirally twisted, double helix, ladder like structure. The two polynucleotide chains or strands are linked up by hydrogen bonding between the nitrogeneous base molecules of their nucleotide monomers. Adenine (purine) always links with thymine (pyrimidine) with the help of two hydrogen bonds and guanine (purine) with cytosine (pyrimidine) with the help of three hydrogen bonds. Hence, the two strands extend in opposite directions, i.e., are antiparallel and complimentary. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement. (i) Assertion : DNA molecules and RNA molecules are found in the nucleus of a cell. Reason : There are two types of nitrogenous bases, purines and pyrimidines. Adenine (A) and guanine (G) are substituted purines; cytosine (C), thymine (T) and uracil (U) are substituted pyrimidines (ii) Assertion: In both DNA and RNA, heterocyclic base and phosphate ester linkages are at C-1 ' and C-5 ' respectively of the sugar molecule. Reason: Nucleotides and nucleosides mainly differ from each other in presence of phosphate units. (iii) Assertion: The backbone ofRNA molecule is a linear chain consisting of an alternating units of a heterocylic base, D-ribose and a phosphate. Reason: The segment of DNA which acts as the instruction manual for the synthesis of protein is ribose. (iv) Assertion: The double helical structure of DNA was proposed by Emil Fischer. Reason: A nucleoside is an N-glycoside of heterocyclic base.
Read the passage given below and answer the following questions: Proteins are high molecular mass complex biomolecules of amino acids. The important proteins required for our body are enzymes, hormones, antibodies, transport proteins, structural proteins, contractile proteins etc. Except for glycine, all a-amino acids have chiral carbon atom and most of them have L-configuration. The amino acids exists as dipolar ion called zwitter ion, in which a proton goes from the carboxyl group to the amino group. A large number of a-amino acids are joined by peptide bonds forming polypeptides. The pep tides having very large molecular mass (more than 10,000) are called proteins. The structure of proteins is described as primary structure giving sequence of linking of amino acids; secondary structure giving manner in which polypeptide chains are arranged and folded; tertiary structure giving folding, coiling or bonding polypeptide chains producing three dimensional structures and quaternary structure giving arrangement of sub-units in an aggregate protein molecule. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement. (i) Assertion: All amino acids are optically active. Reason: Amino acids contain asymmetric carbon atoms. (ii) Assertion: In \(\alpha \) -helix structure, intramolecular H-bonding takes place whereas in \(\beta \) -pleated structure, intermolecular H-bonding takes place. Reason: An egg contains a soluble globular protein called albumin which is present in the white part. (iii) Assertion: Secondary structure of protein refers to regular folding patterns of continuos portions of the polypeptide chain. Reason: Out of 20 amino acids, only 12 amino acids can be synthesised by human body. (iv) Assertion: The helical structure of protein is stabilised by intramolecular hydrogen bond between -NH and carbonyl oxygen. Reason: Sanger's reagent is used for the identification of N-terminal amino acid of peptide chain.
Read the passage given below and answer the following questions: Glucose is known as dextrose because it occurs in nature as the optically active dextrorotatory isomer. It is essential constituent of human blood. The blood normally contains 65 to 110 mg of glucose per 100 mL (hence named Blood sugar). The level may be much higher in diabetic persons. The urine of diabetic persons also contain considerable amount of glucose. In combined form, it occurs in cane sugar and polysaccharides such as starch and cellulose. Glucose has an aldehyde group (-CHO), one primary alcoholic group (-CH 2 OH) and four secondary alcoholic groups (-CHOH) in their structure. Due to the presence five hydroxyl groups (-OH), glucose acetylation. Glucose also undergoes oxidation with mild oxidising agents like bromine water as well as with strong oxidising agents like nitric acid. Since glucose is readily oxidised, it acts as a strong reducing agent and reduces Tollen's reagent and Fehling solution. Glucose exists in two crystalline forms: \(\alpha \) -D-glucose and \(\beta \) -Dglucose undergoes If either of the two forms is dissolved in water and allowed to stand, the specific rotation of the solution changes gradually, until a constant value is obtained. This change is called mutarotation. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement. (i) Assertion: A diabetic person carries a packet of glucose with him always. Reason: Glucose increases the blood sugar level almost instantaneously. (ii) Assertion: On oxidation with nitric acid, glucose as well as gluconic acid both yield saccharic acid. Reason : The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free -CHO group. (iii) Assertion : Glucose reacts with acetyl chloride to form pentaacetyl glucose. Reason: The formation of pentaacetyl derivative confirms the presence of five -OH groups in glucose. (iv) Assertion: A certain compound gives negative test with ninhydrin and positive test with Benedict's solution, the compound is an amino acid. Reason: Glucose is a monosaccharide.
Biomolecules are complex molecules which build up living organisms and required for their growth, maintenance and ability to reproduce. Carbohydrates are polyhydroxy aldehydes and ketones which are major sources of energy. Monosachharides are simple sugars which cannot be hydrolysed. Oligosachharide, on hydrolysis give 2 to 10 molecules of monosachharides. Polysachharides like starch and cellulose on hydrolysis give large number of molecules of glucose \(\alpha\) -glucose and \(\beta\) -glucose (Anomers). Proteins are complex nitrogeneous polymers of amino acids connected through peptide bonds. The sequence in which amino acids are linked is called Primary structure. Secondary structures are of 2 types \(\alpha\) -helix in globular proteins and \(\beta\) -pleated structure in fibrous proteins involving H-bonds. Tertiary structure has H-bonds, disulphide linkage, ionic bonding and van der Waals' forces. Insulin is hormone for metabolism of glucose, has quarternary structure. Denaturation of protein destroys secondary and tertiary structure, loss of biological activity but primary structure remaining the same. Enzymes are highly specific, work at specific pH, moderate temperature and catalyse biochemical reactions. Hormones perform specific functions and secreated by endocrine glands. Vitamins are essential for healthy body. A, D, E, K are fat soluble vitamins. Vitamin C and B 1 , B 2 , B 6 are water soluble. B 12 is neither water, nor fat soluble. Nucleic acids are polymer of nucleotides. RNA consist of m-RNA, t-RNA, r-RNA. RNA has Adenine, Cytosine, Uracil and Guanine. It helps in protein synthesis. It cannot replicate. DNA contains deoxyribose, A, C, G and Thymine. It transfers genetic characteristics. DNA has double helix structure and undergoes replication. (a) Name a disaccharide which on hydrolysis give glucose and galactose. (b) What type of protein is albumin? (c) Name one non-reducing sugar. (d) Which one is complementary base of cytosine in one strand of DNA to that in other strand of DNA? (e) Which linkage by which nucleotide are joined together between 5' and 3' atoms of pentose sugar? (f) Which vitamin helps in coagulation of blood? (g) Which enzyme can dissolve blood clots to prevent heart attack?
Living system are made up of complex molecules called Biomolecules. Carbohydrate, proteins, enzymes, nucleic acids, lipids, hormones ATP, DNA and RNA play an important role in our daily life. Carbohydrates provide us energy. Protein help in growth and maintenance of body. Nucleic acids, RNA helps in protein synthesis, DNA helps in transfer of genetic characteristics. Fat are source of energy and protect our vital organs. (a) Why are carbohydrates optically active? (b) Name two acidic amino acids. (c) Name a protein which has quarternary structure. (d) What are products of hydrolysis of fats? (e) What is role of glycerol in shaving creams?
Table shows carbohydrates and artificial sweeteners and their relative sweetness. Study the table and answer the questions based on table and related concepts.
| Lactose | 16 |
| Maltose | 32 |
| Galactose | 32 |
| Glucose | 74 |
| Sucrose | 100 |
| Fructose | 173 |
| Saccharine | 500 time than sugar |
| Aspartame | 160 times than sugar |
| Alitame | 2000 times than sugar |
| Sucralose | 650 times than sugar |
(a) Which is sweetest sugar and why? (b) What is difference between glucose and fructose? (c) Why are artificial sweetener better than sugar for diabetic patients? (d) What is limitation of Alitame? (e) What happens when glucose is treated with conc. HNO 3 ? (f) What are non-reducing sugar? Select the nonreducing sugar from the table? (g) Which sugar is present in milk? What are its product of hydrolysis? (h) Classify the sugars given in table into monosachharides and disachharides. Glucose, Lactose, Sucrose, Maltose, Fructose, Galactose. (i) Which artificial sweetener is most suitable for making sweets for diabetic patients. (j) Which artificial sweetener is added in diet coke and pepsi? What is its limitation?
*****************************************
Exam Style Practice Questions and Study Notes- Improves Your Grade 2x
Course Now Available IBDP, MYP, AP, iGCSE, A-Level, DSAT & KS 1-4
IBDP HL & SL
Practice questions and notes.
200K skills
Practice Questions & Notes
10 K skills
Advance Placement
Practice questions & notes , cie as & a level, digital sat , digital sat practice questions , digital sat mock tests, national curriculum, our most popular courses.
Get access to comprehensive study material, detail explanation and lots of practice papers
What Our Students Have to Say
What makes iitianacademy unique, exam style questions.
IITianAcademy has Exam Style Questions for each and every topics of the Course. It helps students practice questions for each topics , hence makes you ready for actual exam and also assessments exams in between. Topic wise – exam style question will help to check if you need to revise the topic again or you are good to go.
Past Papers
Past papers are available with detailed step by Step Solutions. Questions are modified as will in some places for making it little different so that students can have them to test preparedness for actual exam. It will help in identifying the weak area in advance and hence modify study plan to improve in this area.
Revision Notes
Revision notes at IITianAcademy are prepared by Alumni Student of the Couse, Teachers and Subject Matter Experts. Technology is used everywhere , to make it more Students friendly. Utmost focus is given on making notes to enhance understanding of the topics. These notes not only enhance your memory but also help in identifying knowledge gap and filling it.
Time bound Online Practice Exam
For Multiple Choice Questions , IITianAcademy has developed a tool to create exam style environment to test your speed , accuracy and understanding of the course. We have tried to simulate exam conditions to test your preparedness . It will not only help improve your time management but also identify area of improvement and hence will Boost Your Confidence
- School Solutions
- Star Program
- NCERT Solutions Class 12 Maths
- NCERT Solutions Class 12 Physics
- NCERT Solutions Class 12 Chemistry
- NCERT Solutions Class 12 Biology
- NCERT Solutions Class 12 Commerce
- NCERT Solutions Class 12 Economics
- NCERT Solutions Class 12 Accountancy
- NCERT Solutions Class 12 English
- NCERT Solutions Class 12 Hindi
- NCERT Solutions Class 11 Maths
- NCERT Solutions Class 11 Physics
- NCERT Solutions Class 11 Chemistry
- NCERT Solutions Class 11 Biology
- NCERT Solutions Class 11 Commerce
- NCERT Solutions Class 11 Accountancy
- NCERT Solutions Class 11 English
- NCERT Solutions Class 11 Hindi
- NCERT Solutions Class 11 Statistics
- NCERT Solutions Class 10 Maths
- NCERT Solutions Class 10 Science
- NCERT Solutions Class 10 English
- NCERT Solutions Class 10 Hindi
- NCERT Solutions Class 10 Social Science
- NCERT Solutions Class 9 Maths
- NCERT Solutions Class 9 Science
- NCERT Solutions Class 9 English
- NCERT Solutions Class 9 Hindi
- NCERT Solutions Class 9 Social Science
- NCERT Solutions Class 8 Maths
- NCERT Solutions Class 8 Science
- NCERT Solutions Class 8 English
- NCERT Solutions Class 8 Hindi
- NCERT Solutions Class 8 Social Science
- NCERT Solutions Class 7 Maths
- NCERT Solutions Class 7 Science
- NCERT Solutions Class 7 English
- NCERT Solutions Class 7 Hindi
- NCERT Solutions Class 7 Social Science
- NCERT Solutions Class 6 Maths
- NCERT Solutions Class 6 Science
- NCERT Solutions Class 6 English
- NCERT Solutions Class 6 Hindi
- NCERT Solutions Class 6 Social Science
- NCERT Solutions Class 5 Maths
- NCERT Solutions Class 5 English
- NCERT Solutions Class 5 EVS
- NCERT Solutions Class 4 Maths
- NCERT Solutions Class 4 English
- NCERT Solutions Class 4 EVS
- NCERT Solutions Class 4 Hindi
- NCERT Solutions Class 3 Maths
- NCERT Solutions Class 3 English
- NCERT Solutions Class 3 EVS
- NCERT Solutions Class 3 Hindi
- NCERT Solutions Class 2 Maths
- NCERT Solutions Class 2 English
- NCERT Solutions Class 2 Hindi
- NCERT Solutions Class 1 Maths
- NCERT Solutions Class 1 English
- NCERT Solutions Class 1 Hindi
- NCERT Books Class 12
- NCERT Books Class 11
- NCERT Books Class 10
- NCERT Books Class 9
- NCERT Books Class 8
- NCERT Books Class 7
- NCERT Books Class 6
- NCERT Books Class 5
- NCERT Books Class 4
- NCERT Books Class 3
- NCERT Books Class 2
- NCERT Books Class 1
- Important Questions Class 12
- Important Questions Class 11
- Important Questions Class 10
- Important Questions Class 9
- Important Questions Class 8
- Important Questions Class 7
- important questions class 6
- CBSE Class 12 Revision Notes
- CBSE Class 11 Revision Notes
- CBSE Class 10 Revision Notes
- CBSE Class 9 Revision Notes
- CBSE Class 8 Revision Notes
- CBSE Class 7 Revision Notes
- CBSE Class 6 Revision Notes
- CBSE Class 12 Syllabus
- CBSE Class 11 Syllabus
- CBSE Class 10 Syllabus
- CBSE Class 9 Syllabus
- CBSE Class 8 Syllabus
- CBSE Class 7 Syllabus
- CBSE Class 6 Syllabus
- CBSE Class 5 Syllabus
- CBSE Class 4 Syllabus
- CBSE Class 3 Syllabus
- CBSE Class 2 Syllabus
- CBSE Class 1 Syllabus
- CBSE Sample Question Papers For Class 12
- CBSE Sample Question Papers For Class 11
- CBSE Sample Question Papers For Class 10
- CBSE Sample Question Papers For Class 9
- CBSE Sample Question Papers For Class 8
- CBSE Sample Question Papers For Class 7
- CBSE Sample Question Papers For Class 6
- CBSE Sample Question Papers For Class 5
- CBSE Sample Question Papers For Class 4
- CBSE Sample Question Papers For Class 3
- CBSE Sample Question Papers For Class 2
- CBSE Sample Question Papers For Class 1
- CBSE Previous Year Question Papers Class 12
- CBSE Previous Year Question Papers Class 10
- Extra Questions For Class 8 Maths
- Extra Questions For Class 8 Science
- Extra Questions For Class 9 Maths
- Extra Questions For Class 9 Science
- Extra Questions For Class 10 Maths
- Extra Questions For Class 10 Science
- NEET 2021 Question Paper
- NEET 2020 Question Paper
- NEET 2019 Question Paper
- NEET 2018 Question Paper
- NEET 2017 Question Paper
- NEET 2016 Question Paper
- NEET 2015 Question Paper
- NEET Physics Questions
- NEET Chemistry Questions
- NEET Biology Questions
- NEET Sample Papers
- NEET Physics Syllabus
- NEET Chemistry Syllabus
- NEET Biology Syllabus
- NEET Mock Test
- NEET Eligibility Criteria
- JEE Main 2021 Question Paper
- JEE Main 2020 Question Paper
- JEE Main 2019 Question Paper
- JEE Main 2018 Question Paper
- JEE Main 2017 Question Paper
- JEE Main 2016 Question Paper
- JEE Main 2015 Question Paper
- JEE Main Sample Papers
- JEE Main Physics Syllabus
- JEE Main Chemistry Syllabus
- JEE Main Maths Syllabus
- JEE Main Physics Questions
- JEE Main Chemistry Questions
- JEE Main Maths Questions
- JEE main revision notes
- JEE Main Mock Test
- JEE Advanced Physics Questions
- JEE Advanced Chemistry Questions
- JEE Advanced Maths Questions
- JEE Advanced 2021 Question Paper
- JEE Advanced 2020 Question Paper
- JEE Advanced 2019 Question Paper
- JEE Advanced 2018 Question Paper
- JEE Advanced 2017 Question Paper
- JEE Advanced 2016 Question Paper
- JEE Advanced 2015 Question Paper
- JEE Advanced Physics Syllabus
- JEE Advanced Chemistry Syllabus
- JEE Advanced Maths Syllabus
- JEE Advanced Mock Test
- ISC Class 12 Syllabus
- ISC Class 11 Syllabus
- ICSE Class 10 Syllabus
- ICSE Class 9 Syllabus
- ICSE Class 8 Syllabus
- ICSE Class 7 Syllabus
- ICSE Class 6 Syllabus
- ISC Sample Question Papers for Class 12
- ISC Sample Question Papers for Class 11
- ICSE Sample Question Papers for Class 10
- ICSE Sample Question Papers for Class 9
- ICSE Sample Question Papers for Class 8
- ICSE Sample Question Papers for Class 7
- ICSE Sample Question Papers for Class 6
- ICSE Class 10 Revision Notes
- ICSE Class 9 Revision Notes
- ISC Important Questions for Class 12
- ISC Important Questions for Class 11
- ICSE Important Questions for Class 10
- ICSE Important Questions for Class 9
- ICSE Important Questions for Class 8
- ICSE Important Questions for Class 7
- ICSE Important Questions for Class 6
- ISC Class 12 Question Paper
- ICSE Class 10 Question Paper
- Maharashtra Board Syllabus
- Maharashtra Board Sample Question Paper
- Maharashtra Board Previous Year Question Paper
- AP Board Syllabus
- AP Board Sample Question Paper
- AP Board Previous Year Question Paper
- Tamilnadu Board Syllabus
- Tamilnadu Board Sample Question Paper
- Tamilnadu Board Previous Year Question Paper
- Telangana Board Syllabus
- Telangana Board Sample Question Paper
- Telangana Board Previous Year Question Paper
- Karnataka Board Syllabus
- Karnataka Board Sample Question Paper
- Karnataka Board Previous Year Question Paper
- Examination Full Forms
- Physics Full Forms
- Chemistry Full Forms
- Biology Full Forms
- Educational Full Form
- CUET Eligibility Criteria
- CUET Exam Pattern
- CUET Cutoff
- CUET Syllabus
- CUET Admit Card
- CUET Counselling
- CUET Previous Year Question Papers
- CUET Application Form
- CUET Sample Papers
- CUET Exam Centers
- CUET Exam Dates
- CUET Results
- Physics Formulas
- Chemistry Formulas
- Math Formulas
- Algebra Formulas
- Geometry Formulas
- Trigonometry Formulas
- Subscription
CBSE Class 12 Chemistry Revision Notes Chapter 14
Home » CBSE » CBSE Class 12 Chemistry Revision Notes Chapter 14

- CBSE Important Questions
- Important Questions Class 6
- CBSE Previous Year Question Papers
- CBSE Revision Notes
- CBSE Syllabus
- CBSE Extra Questions
- CBSE Sample Papers
- ISC & ICSE Syllabus
- ICSE Syllabus Class 9
- ICSE Syllabus Class 8
- ICSE Syllabus Class 7
- ICSE Syllabus Class 6
- ICSE Syllabus Class 10
- ICSE Question Paper
- ICSE Sample Question Papers
- ISC Sample Question Papers For Class 12
- ISC Sample Question Papers For Class 11
- ICSE Sample Question Papers For Class 10
- ICSE Sample Question Papers For Class 9
- ICSE Sample Question Papers For Class 8
- ICSE Sample Question Papers For Class 7
- ICSE Sample Question Papers For Class 6
- ICSE Revision Notes
- ICSE Important Questions
- ISC Important Questions For Class 12
- ISC Important Questions For Class 11
- ICSE Important Questions For Class 10
- ICSE Important Questions For Class 9
- ICSE Important Questions For Class 8
- ICSE Important Questions For Class 7
- ICSE Important Questions For Class 6
- Maharashtra board
- Rajasthan-Board
- Andhrapradesh Board
- AP Board syllabus
- Telangana Board
- Tamilnadu Board
- Tamilnadu Sample Question Paper
- Tamilnadu Syllabus
- Tamilnadu Previous Year Question Paper
- NCERT Solutions Class 12
- NCERT Solutions Class 10
- NCERT Solutions Class 11
- NCERT Solutions Class 9
- NCERT Solutions Class 8
- NCERT Solutions Class 7
- NCERT Solutions Class 6
- NCERT Solutions Class 5
- NCERT Solutions Class 4
- NCERT Solutions Class 3
- NCERT Solutions Class 2
- NCERT Solutions Class 1
- JEE Main Question Papers
- JEE Main Syllabus
- JEE Main Questions
- JEE Main Revision Notes
- JEE Advanced Question Papers
- JEE Advanced Syllabus
- JEE Advanced Questions
- JEE Advanced Sample Papers
- NEET Question Papers
- Neet 2021 Question Paper
- Neet 2020 Question Paper
- Neet 2019 Question Paper
- Neet 2018 Question Paper
- Neet 2017 Question Paper
- Neet 2016 Question Paper
- Neet 2015 Question Paper
- NEET Syllabus

Class 12 Chemistry Chapter 14 Notes: Biomolecules
This chapter deals with Biomolecules, which comprise a biological living system that sustains and reproduces itself. Furthermore, a living system comprises non-living atoms and molecules. Biochemistry is the study of what goes on chemically within a particular living ecosystem. Most noteworthy, living organisms consist of various complex biomolecules, including nucleic acids, lipids, carbohydrates, proteins, etc.
Quick Links
Biomolecules describe the molecules living things require to build body parts and maintain the biochemical processes required for life functions. These biomolecules may be divided into either organic or inorganic compounds. Organic compounds are compounds containing carbon which are found in living things. Know more details about these in Class 12 Chemistry Chapter 14 Notes: Biomolecules, available at the Extramarks’ website.
As stated in the Chemistry Class 12 Chapter 14 Notes, proteins and carbohydrates are essential elements of our food. Furthermore, these biomolecules interact with one another and are the molecular logic of life processes. Moreover, mineral salts and vitamins play a crucial role in various essential functions of organisms.
Thus, biomolecules Class 12 Notes contain all the information you need. The complex organic substances, which combine in a specific manner to produce living systems and maintain them, are called biomolecules. Biomolecules are the branch of Chemistry that studies chemical reactions in living organisms.
Key Topics covered in Class 12 Chemistry Chapter 14 Notes
This unit will discuss the structure and functions of some biomolecules. The structure and function of biomolecules inside the living organism are studied in Biochemistry. Living systems comprise several complex biomolecules like carbohydrates, proteins, enzymes, lipids, vitamins, hormones, nucleic acids, and compounds for storing and exchanging energy like ATP.
Carbohydrates:
The term carbohydrate is a combination of the “hydrates of carbon”. They are also known as “Saccharides”, a derivation of the Greek word “Sakcharon”, which means sugar. The definition of carbohydrates in Chemistry is shown as follows:
“Optically active polyhydroxy aldehydes or ketones or substances formed during hydrolysis are known as carbohydrates”.
Few of the most common carbohydrates that we come across in our daily lives are in the form of sugars. These sugars can be in the form of Glucose, Sucrose, Fructose, Cellulose, Maltose etc.
The general formula for carbohydrates is C x (H 2 O) y . Although, it must be considered that this is just a standard formula. Carbohydrates are also termed saccharides. Few of the carbohydrates which are sweet to taste are also called Sugars. There are several exceptions to this.
Let us take a look at acetic acid, which is CH 3 COOH. Although this will fit the standard formula of carbohydrates, i.e. Cx(H 2 O)y, we mention that acetic acid is not a carbohydrate.
Formaldehyde also falls under this category of this general formula but is also not a carbohydrate. And on the other hand, Rhamnose (C 6 H 12 O 6 ) is a carbohydrate but does not follow the general formula. Students can refer to the Class 12 Chemistry Chapter 14 Notes to get more information on this topic.
Classification of Carbohydrates:
The main classification of carbohydrates is done based on hydrolysis. This classification is as follows:
- Monosaccharides are the more straightforward form of carbohydrates that cannot be hydrolyzed into a more straightforward unit of polyhydroxy aldehyde or ketone called monosaccharides. Approximately twenty monosaccharides are known to occur in nature. For example, glucose and fructose. Their general formula is (CH2O)n. Some examples are glucose, Ribose, etc.
- Oligosaccharides : Carbohydrates that, upon hydrolysis yield, two to ten smaller units or monosaccharides are called oligosaccharides. They are a large category and further divided into various subcategories.
- Disaccharides : A further classification of oligosaccharides, gives two units of different or the same monosaccharides on hydrolysis. One example, sucrose on hydrolysis gives one molecule of glucose and fructose each. In contrast, maltose on hydrolysis gives two molecules of only glucose.
- Trisaccharides : Carbohydrates that on hydrolysis give three molecules of monosaccharides, identical or different. An example is Raffinose.
- Tetrasaccharides : As the name suggests, this carbohydrate on hydrolysis gives four molecules of monosaccharides. Stachyose is an example.
- Polysaccharides : The last and final category of carbohydrates. These give a very large number of monosaccharides when they go through hydrolysis. These carbohydrates are not sweet and are also known as non-sugars. Some common examples are starch, glycogen etc.
Importance of Carbohydrates:
As discussed in Class 12 Chemistry Chapter 14 Notes , carbohydrates are essential for life on the planet. So lets take a more detailed look at the necessity of carbohydrates.
- They are responsible for storing chemical energy in living organisms. You must hear it all the time when athletes carbo-load before a game. This is to provide them with extra energy. They are also an essential constituent for supporting tissues in plants and even in some animals.
- Photosynthesis is a process by which plants use solar energy to produce food for themselves. . With this process, plants fix CO 2 and synthesize carbohydrates. Let us take a look at the chemical reaction involved during photosynthesis.
x(CO 2 ) + y(H 2 O) + Solar energy ⇒ Cx (H 2 O)y + O 2
- So carbohydrates, due to photosynthesis, are plants’ repositories of solar energy. When plants or animals metabolize these carbohydrates, this energy is released. The metabolizing equation shown below is just the reverse of the photosynthesis equation.
Cx (H 2 O)y + O 2 ⇒ x(CO 2 ) + y(H 2 O) + Energy
Sugar and Non Sugars :
Monosaccharides and oligosaccharides are crystalline solids that are soluble in water and sweet to taste, collectively known as sugars. The polysaccharides are amorphous, insoluble in water, tasteless, and are known as non-sugars.
Reducing and non-reducing carbohydrates:
Tollen reagents are termed reducing carbohydrates. The carbohydrates containing free aldehyde or ketone group can reduce Fehling’s solution. Many monosaccharides, whether aldose or ketose, are reduced in behaviour. The carbohydrates in which the reducing part are not free can’t reduce Fehling’s solution, and tollens reagents are termed as non-reducing carbohydrates. Many polysaccharides like starch, cellulose, glycogen etc., are non-reducing carbohydrates.
Monosaccharides :
These are the most straightforward carbohydrates that can’t be hydrolyzed into more minor compounds. They are called aldose or ketose, depending upon whether they have an aldehyde or ketone group. Based on the number of carbon atoms present, they are called triose, tetrose etc. All monosaccharides are sweet-smelling crystalline, water-soluble and can diffuse through cell membranes. Students are advised to refer to Class 12 Chemistry Chapter 14 Notes, available on the Extramarks’ website, for a more detailed explanation of the Monosaccharides.
It occurs in nature in free and in combination forms. Glucose is present in sweet fruits and honey. Ripe grapes contain approx 20% of glucose.
The most abundant monosaccharide in nature is, in fact, glucose. We can find glucose in fruits, honey, starch, and sugarcane. We obtain a large amount of the energy in our bodies from glucose through the foods we eat. It is aldohexose, which means it has a total of six carbon atoms in its molecule. Its chemical formula is C6H12O6.
We get glucose mostly from two sources – starch and sucrose. Let us now look at how we can prepare glucose from these two sources. Students may refer to the Class 12 Chemistry Chapter 14 Notes to learn about glucose and its functions.
Preparation of Glucose :
- From Starch :
On a large commercial scale, glucose is mostly prepared from hydrolysis of starch by boiling it with dilute H2SO4 at 393 K under high pressure. This is the commercial way for the preparation of glucose. The chemical reaction is as follows:
(C 6 H 10 O 5 ) + n (H 2 O) ————-> n C 6 H 12 O 6
Starch Glucose
- From Sucrose :
Another way to prepare glucose, with fructose as a by-product, is to boil sucrose in diluted HCl or H2SO4 in an alcoholic solution. The chemical reaction for this is as below:
C 12 H 22 O 11 + H 2 O ————> C 6 H 12 O 6 + C 6 H 12 O 6
Sucrose Glucose + Fructose
For example, glucose is considered to be an aldohexose while fructose is ketohexose. Both of them have six carbon atoms. The simplest monosaccharide is triose (n=3), such as Glyceraldehyde and Dihydroxyacetone. They are optically active and have one or more asymmetric carbon.
Structure of Glucose :
Glucose is an aldohexose that is the monomer of many more significant carbohydrates like starch, cellulose etc.
Configuration:
All naturally occurring monosaccharides belong to the D—series, the OH group at their penultimate C-atom.
The cyclic structure of glucose :
It was proposed that glucose form a six-membered ring in which OH at C5 can add to the CHO group and form a cyclic hemiacetal structure. This explains the absence of the CHO group and also the existence of glucose in alpha and beta anomeric forms as:
The two cyclic hemiacetal forms of glucose vary only in the configuration of the hydroxyl group at C1, known as anomeric carbon. The corresponding alpha and beta forms are called anomers. It is observed that alpha and beta forms of glucose are not minor images of each other and hence are not enantiomers.
Fructose :
Fructose is an important ketohexose. The hydrolysis of sucrose obtains it. Based on molecular weight determination, elemental analysis, and various reactions, its molecular formula is found to be C 6 H 12 O 6 , and its open-chain structure can be written as:
Fructose is a ketonic monosaccharide, primarily found in fructose in plants and their fruits, flowers and root vegetables, earning it the moniker of fruit sugar. It is also present abundantly in corn syrup and honey. Usually, fructose and glucose bonds to form a disaccharide called sucrose. Fructose was first discovered by French chemist Augustin – Pierre Debrunfaut.
The chemical formula of fructose is also C6H12O6, but the bonding of fructose is very different from the bonding of glucose. Fructose has a cyclic structure which is an intramolecular hemiacetal. Fructose has its carbonyl group at its number two carbon (a ketone function group). In its cyclic format, it (typically) forms a five-member ring which we call a Furanose ring with an analogy of the compound furan.
- Oligosaccharides:
These carbohydrates, on hydrolysis, give 2 to 9 molecules of monosaccharides.
They are further of few types :
Disaccharides (C 12 H 22 O 11 ) :
On hydrolysis, they give two molecules of monosaccharides held together by Glycosidic linkage.
example: Sucrose.
Trisaccharides (C 18 H 32 O 16 ) :
On hydrolysis, they form three molecules of monosaccharides.
example: Raffinose.
Tetra-saccharides: (C 24 H 42 O 21 ) :
Such as stachyose which gives four monosaccharides on hydrolysis.
- Polysaccharides:
These are the carbohydrates which, on hydrolysis, yield more than nine monosaccharides molecules.
example: Starch.
Mutarotation:
Glucose exists in two forms: i.e. alpha –D glucose with a specific rotation of 112 degrees and beta D-glucose with a specific rotation of +19 degrees. However, when either of these two forms is dissolved in water and allowed to stand, it gets converted into the same equilibrium mixture of alpha and beta forms with a small amount of open-chain form having a specific rotation of 52.7 degrees. As a result, equilibrium, the specific rotation of freshly prepared solution of alpha glucose, decreases from +112 degrees to 52.7 degrees while that for beta glucose increases from +19 to 52.7 degrees. This phenomenon of change in specific rotation of optically active compounds with time to an equilibrium value is known as Mutarotation.
The alpha D (+) glucose and beta (+) glucose differ in configuration at C-1 carbon, and the compounds differing in configuration at C-1 are called Anomers.
- Disaccharide :
The disaccharides are the combination of two units of monosaccharides. They give the corresponding monomers on hydrolysis with dilute acids or specific enzymes.
C 12 H 22 O 11 + H 2 O → C 6 H 12 O 6 + C 6 H 12 O 6
In disaccharides, the two monosaccharides’ units are connected by an oxide linkage formed by the loss of water molecules and the linkage is called glycosidic linkage.
These topics are complex and many students find it difficult to understand. So students can refer to the step-by-step detailed chapter notes given in our Class 12 Chemistry Chapter 14 Notes to get more clarity on these concepts.
Sucrose :
A glycosidic linkage between C1 of α -glucose and C2 of β -fructose holds these two monosaccharides together. Sucrose is a non-reducing sugar due to the reducing groups of glucose and fructose involved in forming glycosidic bonds.
It is developed by the glycosidic linkage between the C1 of one glucose unit and the C4 of another. With acid or enzyme treatment, maltose is hydrolyzed into two molecules. That is alpha D-glucose. Since one of the glucose units exists in hemiacetal form, it is a reducing sugar.
It is made up of any molecule and a molecule of galactose. The units are linked together. Lactose is found in milk, also termed milk sugar. It is developed by the glycosidic linkage between C1 of alpha D- galactose unit and C4 of beta D- glucose unit. Lactose is a reducing sugar.
The sweetness of sugars :
- All the monosaccharides and disaccharides are sweet and hence also known as sugars.
- Sucrose is given a sweet value of 100. The sweetness of other sugars is compared with the value of sucrose.
- The sweetness of fructose is -173, invert sugar 130, sucrose 100, glucose 74, galactose 32, maltose 32 and lactose 16.
- The monosaccharides and disaccharides are reducing agents due to hemiacetal and hemiketal forms, which quickly change into aldehydic in the alkaline medium.
- Although fructose doesn’t contain any aldehydic group yet, it gives Tollen’s reagent test and Fehling’s solution test because, under the primary conditions of the reagent, the fructose gets converted into the mixture of glucose and mannose, both of which contain the aldehydic group.
- This is called the Lobry De Bruyn Van Eikensten rearrangement.
Students may refer to the CBSE Class 12 Chemistry Chapter 14 Notes and other study materials available at the Extramarks’ website to learn more about the above phenomena.
- The alpha and beta glucose react with one ethanol molecule to form the corresponding methyl glucosides.
- When glucose is treated with methanol in the presence of HCl, the hemiacetal form changes to acetal form.
Polysaccharides are long-chain polymers composed of many monosaccharide units joined together by glycosidic linkages. They primarily show as food storage or structural materials. For example, starch, cellulose, glycogen etc.
Starch(C 6 H 10 O 5 ) n :
Plants’ primary storage polysaccharide is starch. It is the essential source of nutrition for a human being. Cereals, rice, roots, tubers, and some vegetables have a high starch content. Starch is a polymer of alpha D+ glucose composed of two components, i.e. Amylose and Amylopectin.
- Amylose is a water-soluble component which accounts for about 15-20% of starch.
- Amylose is a long unbranched chain containing 15- 20% of starch.
- It is a straight-chain polysaccharide containing α -D-(+)-glucose units held together by a C1-C4 glycosidic linkage.
Amylopectin:
- Amylopectin is a branched chain of polysaccharides.
- It is insoluble in water.
- It constitutes approximately 80-85% of starch.
- It is a branched-chain polymer of α-D-glucose units with a chain formed by C1-C4 glycosidic linkage, whereas branching occurs by C1-C6 glycosidic linkage.
Cellulose :
- It is a straight-chain of polysaccharides composed of only a beta D glucose unit.
- Cellulose is found mainly in all plants, constituting 50% of total organic matter in living beings.
- Cotton is pure cellulose.
- Cellulose is a linear polymer of beta D-glucose. The chains are arranged to form bundles and linked together by hydrogen bonds between glucose molecules of adjacent organic solvents. It slowly passes into the solution when treated with concentrated sulphuric acid in the cold.
- When diluted with water, this solution gives the starch-like substance amyloid, parchment paper.
- On boiling with water, it is hydrolyzed into D-glucose. Cellulose gives many useful products when treated with chemicals like rayon, gum, cotton etc. Cellulose is directly used in making cloth and paper.
Students should visit the Extramarks’ website to access the Class 12 Chemistry Chapter 14 Notes and get more in depth study notes on this chapter.
- The structure of Glycogen is similar to amylopectin, with more branching than in amylopectin.
- It is also called animal starch.
- In the body, carbohydrates are stored as glycogen, and when the body needs glucose, enzymes break the glycogen down to glucose.
- Glycogen is present in the liver, muscle and brain.
- In glycogen, there are about 25 glucose units. Its structure is the same as amylopectin, a condensation polymer of alpha glucose.
Tests for carbohydrates:
For this, a Molisch test is performed. Molisch reagent, a 10% alcoholic solution of alpha naphthol, is added to an aqueous solution of carbohydrates, followed by concentrated sulphuric along the sides of the tube. As a result, a violet ring is formed at the junction of two layers.
Importance of carbohydrates:
Carbohydrates are essential for life in plants and animals; carbohydrates are stored in plants as starch and in animals as glycogen. Carbohydrates are the primary source of energy (except cellulose). These carbohydrates store energy for the functioning of living organisms. They are used as raw materials in producing textiles, papers, lacquers, breweries, etc.
Proteins are higher in molecular weight, complex bio-polymers of alpha-amino acids found in all living organisms. They occur in all parts of the body and form the fundamental basis of the structure and functions of life. The term ‘Protein’ is derived from the Greek word ‘Protein’, which means ‘Primary importance’.
Proteins are the amplest biomolecules of the living cell. The primary sources of proteins are milk, cheese, pulses, peanuts, fish etc. All living systems comprise biomolecules with high molecular mass, called amino acids.
Amino acids :
These acids are the building block units of proteins. These are the organic compounds which contain amino as well as carboxyl functional groups known as amino acids. Depending upon the relative position of the amino group concerning the COOH group, amino acids are Classified into alpha-beta, gamma delta and so on. Hydrolysis of proteins gives only alpha-amino acids represented as:
- The above unit may be connected to any carbon atom other than the -COOH group.
- The amino acids can also be classified based on their need and availability in the human body .
Essential Amino Acids :
These acids cannot be synthesised in our bodies and are essential amino acids that must be taken through diet. We must depend on food sources to obtain these amino acids. Some of the primary essential Amino Acids are as below:
- Isoleucine.
- Methionine.
- Phenylalanine.
- Tryptophan.
- Histidine (conditionally essential).
Non-Essential Amino acids :
These acids are synthesised in our bodies by themselves. So we need not rely on external sources for them. They are produced in our bodies and also obtained from protein breakdowns.
Properties of Amino Acids:
We have seen the overall structure and types of amino acids. Based on this information, we can arrive at the properties of amino acids.
- Each amino acid has an acidic and fundamental group, as seen from its structure. Because of this reason they behave similar to salts.
- They exist as dipolar ions.
- Any amino acid in the dry state exists in crystalline form.
- The NH2 group exists as a cation, and the COOH group exists as an anion. This dipolar ion has a unique name, “Zwitterions’.
- In an aqueous solution, alpha-amino acids exist in equilibrium between a cationic form, an anionic form and a dipolar ion.
- The isoelectric point(IEP) is the pH point at which the concentration of zwitterions is the highest, and the concentration of cationic and anionic forms is equal.
- This specific point is definite for every α-amino acid.
- They are generally water-soluble and also have high melting points.
Structure of proteins:
- Amino acids exist as a zwitterion, which is dipolar.
- The fundamental nature of the zwitterion is due to the -COO – ion.
- The acidic character of the zwitterion is due to the -NH 3 + group.
Peptide linkage :
When two or more amino acids are attached, the resulting -CO-NH- link is termed peptide linkage of the peptide bond.
Peptides :
When amino acids are joined together by amides bonds, they form larger molecules called peptides and proteins.
Students are advised to visit the Extramarks’ website and access the Class 12 Chemistry Chapter 14 Notes, which are prepared by the subject experts team of Extramarks. These are most reliable study notes and lakhs of students have trusted our study solutions for their board exam preparation.
Polypeptides :
A dipeptide contains two amino acids linked by one peptide linkage. A tripeptide contains three amino acids linked by two peptides linkage and so on. When the number of few amino acids is more than ten, the products are known as polypeptides. Students can visit the Extramarks’ website and access the NCERT solutions Class 12 Chemistry Chapter 14 Notes for a more detailed explanation of the Polypeptides.
Classification of amino acids:
Amino acids can be Classified as:
- Neutral amino acids : These amino acids contain an equal number of amino and carboxyl groups. For example, glycine, alanine, valine, etc.
- Acidic, neutral acids : These amino acids contain more carboxyl groups than amino groups, examples include aspartic acid, asparagine, and glutamic acid, which contain two carboxylic (-COOH ) groups and one -NH 3 amine group.
- Essential amino acids : These consist of more amino groups than carboxyl groups, examples are lysine, arginine, and histidine.
Classification of Proteins:
Based on molecular shape, proteins are classified into two types:
- Fibrous protein : When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, a fibre-like structure is formed, known as fibrous protein. Such proteins are insoluble in water. For example, keratin, myosin etc.
- Globular protein : Globular proteins form when the polypeptide chains coil around to give a spherical shape. Such proteins are usually soluble in water. For example., Insulin, albumin etc.
Primary, Secondary, Tertiary and Quaternary Structure of Proteins:
Primary structure of proteins :
Proteins are linked in one or more polypeptide chains, known as the primary structure of proteins. Each polypeptide is a protein with amino acids linked with each in a specific sequence, and it is the sequence of amino acids. It is said to be the primary structure of that protein.
Secondary structure of proteins :
The secondary structure refers to the shape in which a long polypeptide chain can exist. The folding and rearrangement of polypeptide chains give the shape or conformation of the protein. Secondary structure can be of alpha-helix or beta-pleated sheet structure.
Tertiary structure :
This protein structure represents the overall folding of the polypeptide chains due to the folding, bending, and coiling, resulting in three-dimensional structures. It gives rise to two main molecular shapes, namely fibrous and globular.
Quaternary structure :
Many proteins exist by grouping two or more polypeptide chains referred to as sub-units. These polypeptide chains are called the sub-units, and the spatial arrangement of these subunits concerning each other is known as quaternary structure.
Denaturation of proteins :
The loss in the biological activity of a protein due to the unfolding of globules and uncoiling of helix is termed denaturation of protein. During denaturation, secondary and tertiary structures vanish, but primary structures remain intact. The coagulation of egg white on steaming is a typical example of denaturation. Proteins can be denatured (physical and biological changes), but there is no chemical variation in the protein structure.
Denaturation can arise due to various factors, such as changes in temperature, pH, or specific chemical agents. Refer to Class 12 Chemistry Chapter 14 Notes and download the Extramarks’ app to learn more about this and other related concepts.
A colloidal solution of protein which works as a biological catalyst is known as an enzyme. Many enzymes are globular proteins. Zymase, invertase, lactase, maltase emulsion, urease, pepsin trypsin alpha-amylase etc., are examples of enzymes.
Enzymes are biological catalysts which catalyse chemical reactions in living organisms. For example, hydrolysis of maltose is catalysed by maltase.
C 12 H 22 O 11 → 2C 6 H 12 O 6
Maltose Glucose
Mechanism of enzyme action:
The mechanism is given as follows:
- The enzyme (E) binds to the substrate(s)
- Product formation
- Products released from the above complex.
The enzymes process best at an optimum temperature range of 298 K to 313 K. Their activity decreases with a decrease or increase in temperature and stops at 273 K.
To master Chemistry, students may refer to various study materials based on CBSE solutions and also check out our Class 12 Chemistry Chapter 14 Notes to ace their examinations, all available on Extramarks’ website.
Vitamins are organic compounds essential for the average growth of life for animals, some bacteria and microorganisms. These are the biomolecules which are not produced by the body and hence, need to be supplied in small amounts for necessary biological functions. Vitamins are an essential dietary factor.
A, B, C, D, E, & K vitamins are present in various food forms.
Classification of vitamins :
Vitamins are classified into two categories:
- Water-soluble vitamins : Water-soluble vitamins are vitamin B and C complex etc. These vitamins need to be transferred to the body from time to time.
- Fat-soluble vitamins : Vitamins only soluble in fat are called fat-soluble vitamins. A(Retinol), D(calciferol), E(Tocopherol), and K(Phylloquinone) vitamins are soluble in fat.
Particular vitamins are responsible for certain essential functions. Let us have a brief look at them.
- Vitamin A : Required to enable night vision in humans. Cells require Vitamin A for the transfusion of light.
- Vitamin B : Necessary for creating serotonin, myelin, dopamine and epinephrine. It also lowers cholesterol levels.
- Vitamin C : Increases the immune system and helps fatigued muscles.
- Vitamin D : The formation of RNA needs Vitamin D. It also helps bones absorb calcium to stay healthy and strong and reduces the risk of fractures
- Vitamin E has antioxidant properties that help our bodies get rid of free radicals and assist in the formation of red blood cells .
- Vitamin K : Essential in creating some crucial proteins , Various important vitamins, their sources and their deficiency diseases.
| Name of vitamins | sources | Deficiency diseases |
| Vitamin A | Fish liver oil, carrots, butter and milk | Xerophthalmia, Night blindness |
| Vitamin B1 | Yeast, milk, cereals, green veggies | Beri Beri |
| Vitamin B2 | egg white, milk, liver | Cheilosis |
| Vitamin B6 | Yeast, milk, cereals and grams | convulsions |
| Vitamin B12 | Meat, fish, egg and curd | Pernicious |
| Vitamin C | Citrus fruits, amla | scurvy |
| Vitamin D | Exposure to sunlight, fish | Rickets |
| Vitamin E | Vegetable oils like wheat germ oil | Increased fragility of RBC and muscular weakness |
| Vitamin K | Green leafy vegetables | Increased blood clotting time |
Nucleic acids :
As described in the Class 12 Chemistry Chapter 14 Notes, the particles in the nucleus of the biological cell responsible for heredity are called chromosomes which are made up of proteins and other biomolecules called nucleic acids. Nucleic acids are polymers which are present in all human bodies.
- Nucleic acids play an essential role in the development and reproduction of every life form.
- Nucleic acid contains the elements carbon-oxygen, nitrogen and phosphorus.
- They have nucleotides as their repeating units.
Two types of nucleic acids:
- DNA (Deoxyribonucleic acid).
- RNA (Ribonucleic acid).
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are two major types of nucleic acids. DNA and RNA are responsible for inheriting and transmitting specific characteristics from one generation to the other.
Deoxyribonucleic Acid (DNA):
- Chemically, DNA comprises a pentose sugar, phosphoric acid and a few cyclic bases containing nitrogen.
- The sugar unit present in DNA molecules is β-D-2-deoxyribose.
- The cyclic bases that have nitrogen-containing in them are Adenine (A), guanine (G), thymine (T) and cytosine(C).
- These bases and their configuration in the molecules of DNA play an essential role in storing information from one generation to the next.
- DNA has a double-strand helical structure in which the strands complement each other.
Ribonucleic Acid (RNA):
- The RNA molecule is also composed of phosphoric acid, a pentose sugar and a few cyclic bases containing nitrogen.
- RNA has β-D-ribose in it as the sugar unit.
- The heterocyclic bases available in RNA are Adenine (A), guanine (G), cytosine(C) and uracil (U).
- In RNA, the fourth base varies from that of DNA.
- The RNA commonly consists of a single strand which sometimes folds back; that results in a double helix structure.
There are three different types of RNA molecules, each having a specific function:
- Messenger RNA (mRNA).
- Ribosomal RNA (rRNA).
- Transfer RNA (t-RNA).
To learn more about the above three RNA molecules, students are recommended to visit the Extramarks’ website and refer to the Class 12 Chemistry Chapter 14 Notes.
Chemical composition of nucleic acids:
Nucleotides contain three chemical components:
- A heterocyclic base.
- A five-carbon sugar moiety.
- A phosphate group.
Structure of Nucleic acids:
- Nitrogen-containing heterocyclic base : Purines and pyrimidines are two types of heterocyclic bases. For example, Adenine and guanine are purines. Cytosine, thymine, and uracil are pyrimidines.
- Sugars : The two types of sugars are RNA and DNA.
iii. Phosphate group : Nucleotides are joined by these linkages.
- Nucleoside : A nucleoside unit is produced when a nitrogen base is attached to a sugar molecule.
Base + sugar = nucleoside.
- Nucleotide : When a nitrogen base is attached to a sugar molecule and phosphate, the unit forms a nucleotide.
Base+Sugar+phosphate → nucleotide.
The primary structure is based on how sugar, phosphate, and bases are linked in nucleic acids.
Secondary structure :
Watson and Crick described the double helix structure of DNA. The nucleotides in each strand are connected by phosphate ester bonds and the bases of one strand by hydrogen bonds. Adenine pairs with thymine through two double hydrogen bonds. In contrast, cytosine pairs with guanine through triple hydrogen bonds.
The two strands of DNA are always complementary to each other; i.e., if on one side there is Purine, then on another side at the same position, Pyrimidine is present. For example, if the base sequence on the strand is ACTCGCCA, then on the other strand, the sequence will be complementary: TGAGCGGT.
The biological function of nucleic acids:
A few of the biological functions of nucleic acids, as per the Class 12 Chemistry Chapter 14 Notes, are:
- Nucleic acids are responsible for transmitting inherent characteristics from parent to offspring. They are also responsible for the synthesis of protein in our bodies.
- DNA fingerprinting is a method that is used by forensic experts to determine paternity. This method is also used for the identification of criminals. It has also played a significant role in biological evolution and genetics studies.
- Replication : It is the characteristics of a bio-molecule to synthesize different molecules. For example, DNA has a specific property to replicate itself.
- Protein synthesis : Genetic information collected in DNA in a specific base sequence is expressed in the form of a specific base sequence.
- Gene and genetic code : Every segment of DNA molecule that codes for a specific protein or a polypeptide, known as the relationship between nucleotides triplets and the amino acids, is called the genetic code. This is what forms gene and genetic code.
- Mutation : A chemical process in a DNA molecule leads to the synthesis of proteins with a changed amino acid sequence. Radiation, viruses or chemical agents cause these changes. Special enzymes replicate most changes in DNA in the cell, but if there is a failure to repair by the enzymes, then it can cause mutation.
Hormones are molecules which act as intracellular messengers. These are constructed by endocrine glands in the body and are poured directly into the bloodstream, which transports them to the site of action.
Hormones have various functions in the body. They help to adjust the balance of biological activities in the body. Testosterone is the primary sex hormone developed in males and progesterone in females.
Hormones are chemical compounds which are produced in ductless glands in the body. Because of their function, hormones are also termed chemical messengers.
To understand more about the Hormones and its functions, students are advised to visit the Extramarks’ website and access the Class 12 Chemistry Chapter 14 Notes.
Key Features of Class 12 Chemistry Chapter 14 Notes
Extramarks is the best website for students to enhance their board preparation. The platform is trusted by lakhs of students and teachers across the country. CBSE Class 12 Chemistry Chapter 14 Notes can be used as study reference material and also as quick revision notes. These are beneficial for a quick revision for competitive examinations like board exams, JEE Mains, NEET, etc. Class 12 Chemistry Chapter 14 Notes can also be used for offline revision.
Below are few of the important features for students to study from Extramarks study materials:
- The study materials at Extramarks are well prepared with comprehensive coverage of all topics from NCERT textbook and NCERT exemplar.
- The notes are prepared pointwise and in a simple format to help students study and understand long/complex concepts in a short period.
- The notes are prepared by subject experts with decades of teaching experience. The chapter notes cover all topics with detailed explanations and also include a set of important questions and formulas for quick reference.
Class 12 Chemistry Chapter 14 Notes : Exercise & Answer Solutions
Class 12 Chemistry Chapter 14 Notes includes questions and answer solutions which are based on NCERT books. Every exercise and solution is compiled to add more value for the students while revising the chapter. Students may refer to various study materials such as revision notes, past years’ questions papers, and essential questions and learn more about the chapter concepts from the Extramarks’ website.
NCERT Exemplar Class 12 Chemistry
All solutions and problems are given to help students prepare for their final examinations. These NCERT Exemplar book questions are a little more complex and cover every concept introduced in Class 12 Chemistry chapters.
Students will fully understand the concepts covered in each chapter by practising this NCERT Exemplar for Class 12 Chemistry. Exemplars provide the best solutions to challenges that students might face in board exams. They cover all the topics taught in each class and provide the most outstanding practising materials or worksheets for students. These questions from exemplars strictly follow the latest CBSE guidelines and board examinations pattern.
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
FAQs (Frequently Asked Questions)
1. can the chapter 14 chemistry class 12 notes be used as revision notes.
Yes, Chemistry Chapter 14 Class 12 Notes can be used as revision notes as it gives all the information students need to understand the chapter in a succinct manner.
2. How important are the CBSE Class 12 Chemistry Chapter 14 Notes?
The Chemistry Class 12 Chapter 14 CBSE solutions help students understand all the concepts and give a detailed explanation of every reaction and mechanism that falls under the chapter. It allows students to study for the Examinations without depending on someone to explain. Students may refer to CBSE solutions Class 12 Chemistry Chapter 14 Notes on the Extramarks’ website.
3. What are Biomolecules, as per the solutions given in Class 12 Chemistry Chapter 14 Notes?
We all know that a living organism indeed sustains and reproduces itself. Moreover, a living system comprises both molecules and non-living atoms. Furthermore, Biochemistry also deals with what goes on chemically within a specific living system. Most Noteworthy, living organisms also consist of different complex biomolecules. In addition, these bio-molecules include nucleic acids, lipids, proteins, and carbohydrates. Carbohydrates and proteins are the most fundamental and required elements of our food.
Moreover, these biomolecules join and exist in the molecular logic of life processes. Furthermore, vitamins and mineral salts play a crucial role in various essential functions of organisms. Hence, Class 12 Chemistry Chapter 14 Notes: Biomolecules contain all the necessary information you need to understand them completely.
CBSE Related Links

Fill this form to view question paper
Otp verification.
- NCERT Solutions
- NCERT Class 12
- NCERT 12 Chemistry
- Chapter 14: Biomolecules
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
Ncert solutions for class 12 chemistry chapter 14 – free pdf download.
* According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 10.
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules are intended for students of Class 12 to write their Class 12 Chemistry board examination successfully. The NCERT Solutions for Class 12 Chemistry deals with the topic of Biomolecules and contains questions and solutions on topics such as saccharides, glycogen, cellulose, starch and more. To become well-versed in these topics, access these solutions.
The NCERT Solutions for Class 12 Chemistry is developed by expert subject teachers according to the latest CBSE Syllabus 2023-24 and its guidelines. These solutions are written in simple language for ease of comprehension. Get the free PDF of NCERT Solutions for Class 12 Chemistry Chapter 14 from the link below.
carouselExampleControls112

Previous Next
Class 12 Chemistry NCERT Solutions Chapter 14 Biomolecules – Important Questions
Q 1. What are monosaccharides?
Monosaccharides , known as simple sugars, comprise one sugar unit that cannot be further broken down into simple sugars.
We can classify a monosaccharide on the basis of the number of carbon atoms and the functional group present in them. The monosaccharide, which contains an aldehyde group, is termed an aldose, and those which have a keto group are called ketoses. Depending on the number of carbon atoms present in a monosaccharide, it is further classified as trioses, tetroses, pentoses, hexoses and heptoses. For example, we can call an aldose which contains 3 carbon atoms as aldotriose and a keto which contains 3 carbon atoms as ketotriose.
Q 2. What are reducing sugars?
Those type of carbohydrates which reduces Fehling’s solution and Tollen’s reagent are termed as reducing sugars.
Q 3. Write two main functions of carbohydrates in plants.
The two main functions of carbohydrates in a plant are as follows:
(a) Polysaccharides like starch act as storage molecules.
(b) Cellulose is used to build the cell wall, and it is a polysaccharide.
Q 4. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Monosaccharides: 2-deoxyribose, galactose, ribose, fructose
Disaccharides: lactose, maltose
Q 5. What do you understand by the term glycosidic linkage?
The linkage which forms by the loss of water between two monosaccharide units through an oxygen atom is known as glycosidic linkage.
For example, in a sucrose molecule, two monosaccharide units, \(\begin{array}{l}\alpha\end{array} \) -glucose and \(\begin{array}{l}\beta\end{array} \) – fructose, are joined together by a glycosidic linkage.
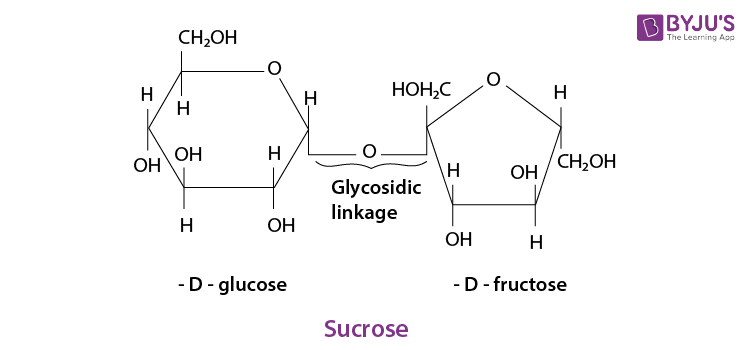
Q 6. What is glycogen? How is it different from starch?
Glycogen, also termed animal starch, is found only in animals. It is a polysaccharide.
Both glycogen and starch are the main sources of glucose that provides energy to humans that are later converted into carbohydrates.
They differ in structure. Starch comprises a chain and a branched compound, whereas glycogen is composed of a single molecule and is branched.
Q 7. What are the hydrolysis products of (i) sucrose and (ii) lactose?
(i) The hydrolysis of sucrose will give one molecule of \(\begin{array}{l}\alpha\end{array} \) -D glucose and one molecule of \(\begin{array}{l}\beta\end{array} \) –D fructose.
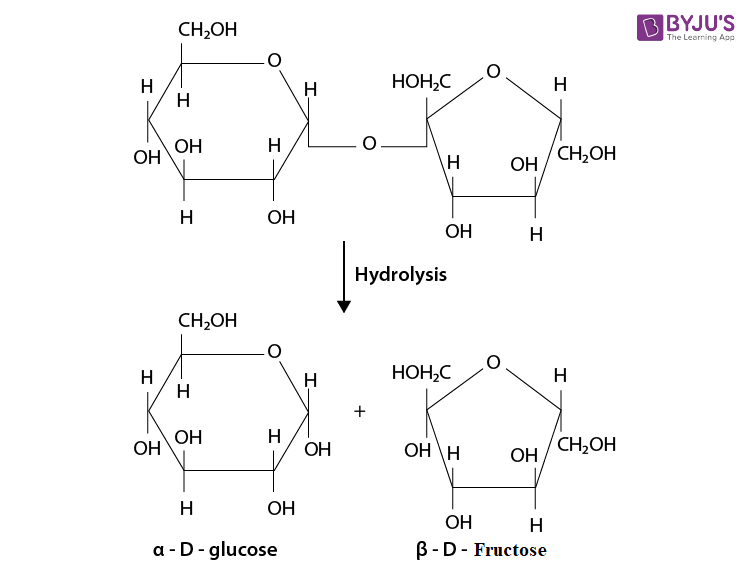
(ii) On hydrolysis of lactose, it will give \(\begin{array}{l}\beta\end{array} \) -D-galactose and \(\begin{array}{l}\beta\end{array} \) -D-glucose.
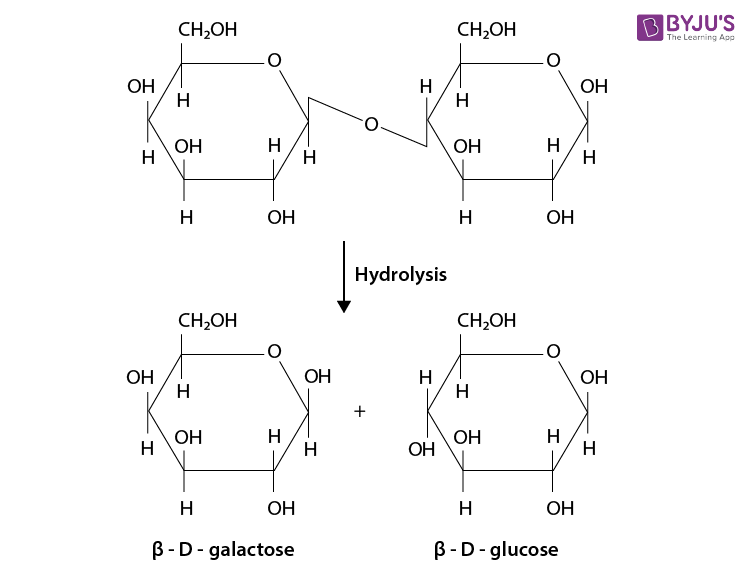
Q 8. What is the basic structural difference between starch and cellulose?
Starch consists of two components – amylopectin and amylose. Amylose have a longer linear chain of \(\begin{array}{l}\alpha\end{array} \) – D −(+)−glucose units joined by C1−C4 glycosidic linkage ( \(\begin{array}{l}\alpha \end{array} \) -link).
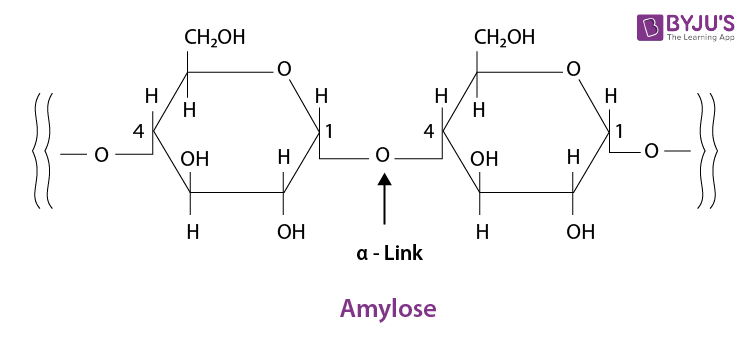
While amylopectin is a branched-chain polymer of \(\begin{array}{l}\alpha\end{array} \) -D-glucose units, in which the chain is formed by C1−C4 glycosidic linkage, and the branching occurs by C1−C6 glycosidic linkage.
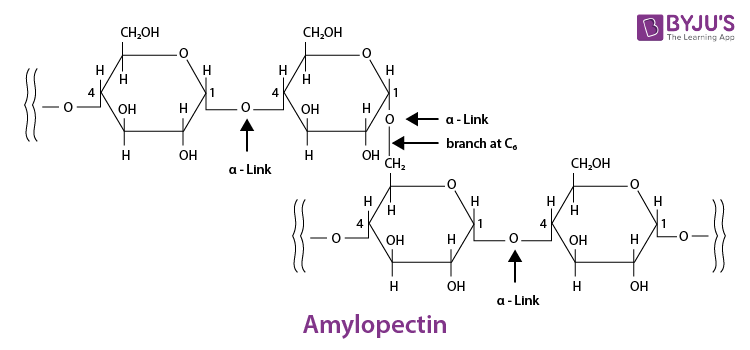
The cellulose is a straight-chain polysaccharide of \(\begin{array}{l}\beta\end{array} \) – D-glucose units joined by C1−C4 glycosidic linkage ( \(\begin{array}{l}\beta\end{array} \) – link).
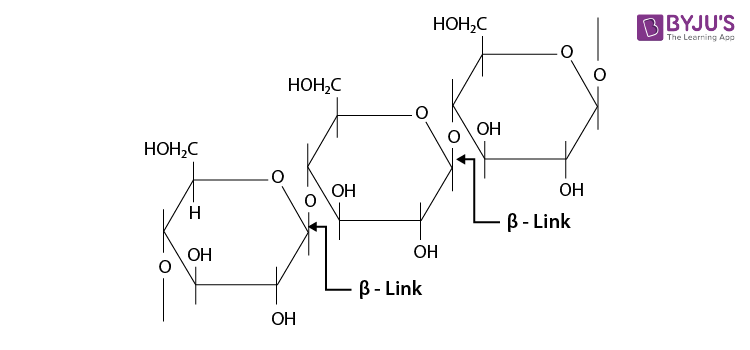
Q 9. What happens when D-glucose is treated with the following reagents? (i) HI (ii) Bromine water (iii) HNO 3
(i) After heating a D-glucose with HI for a long time, n-hexane is formed.
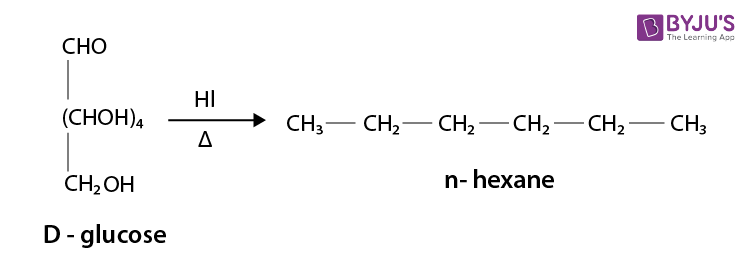
(ii) After treating a D-glucose with \(\begin{array}{l}Br _{2}\end{array} \) water, D-gluconic acid is produced.
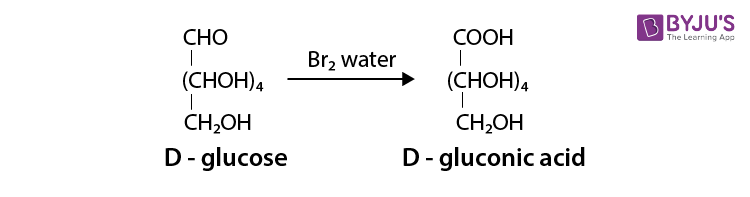
(iii) After treating with \(\begin{array}{l}HNO _{3}\end{array} \) , D-glucose gets oxidised to give saccharic acid.
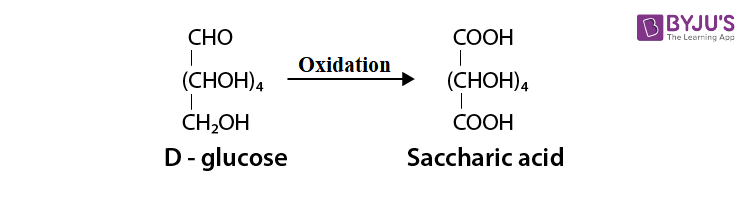
Q 10. Enumerate the reactions of D-glucose, which cannot be explained by its open chain structure.
(i) The pentaacetate of glucose does not react with hydroxylamine. This shows that a free −CHO group is not present in glucose.
(ii) Aldehydes form the hydrogen sulphite additional product by giving 2,4 – DNP test, Schiff’s test and react with \(\begin{array}{l}NaHSO_{4}\end{array} \) . But glucose does not undergo these reactions.
(iii) Glucose is available in two crystalline forms \(\begin{array}{l}\alpha\end{array} \) and \(\begin{array}{l}\beta\end{array} \) . The \(\begin{array}{l}\alpha\end{array} \) form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K, and the \(\begin{array}{l}\beta\end{array} \) form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour can’t be explained by the open chain structure of glucose.
Q 11. What are essential and non-essential amino acids? Give two examples of each type.
The amino acids, which are required by the human body, are called essential amino acids, but these cannot be produced inside the human body. They must be taken from any external source like food, for example, leucine and valine.
The amino acids which are required by the human body and can be produced inside the body are called non–essential amino acids, for example, glycine and alanine.
Q 12. Define the following as related to proteins (I) Primary structure, (ii) Peptide linkage, (iii) Denaturation.
(i) Primary Structure
We can refer to the specific sequence in which various amino acids are present if we talk about the primary structure of the protein, like the sequence of linkage between amino acids in a polypeptide chain.
The amino acids are arranged in a different sequence in each protein. A little difference in the sequence of the arrangements will create a completely different protein.
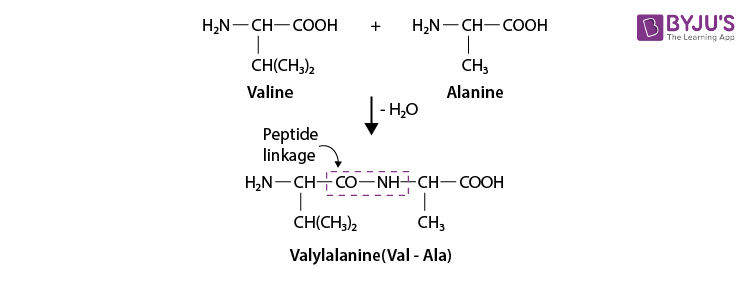
(ii) Peptide Linkage
A peptide linkage is an amide which is formed by the elimination of a water molecule between the –COOH group of one molecule of amino acid and \(\begin{array}{l}-NH _{2}\end{array} \) group of another molecule of the amino acid.
(iii) Denaturation
A protein has a unique 3-dimensional structure and a unique biological activity inside a biological system. In these types of circumstances, proteins are called native proteins. Whenever we put a native protein into a physical change like a change in temperature or any chemical change like a change in pH, then there its H-bonds are disturbed or changed.
This result in the unfolding of the globules ad uncoils the helix. And the consequences of this change are that the protein results in the loss of its biological activity. This loss of biological activity by the protein is called denaturation. During this process, no changes are encountered in the primary structure, whereas tertiary and secondary structures will be destroyed.
An example of denaturation proteins is the coagulation of egg white when an egg is boiled.
Q 13. What are the common types of the secondary structure of proteins?
Secondary structures of proteins are of two types:
(a) \(\begin{array}{l}\alpha\end{array} \) – helix structure
(b) \(\begin{array}{l}\beta\end{array} \) – pleated sheet structures.
\(\begin{array}{l}\alpha\end{array} \) – helix structure
In this structure, the −NH group of an amino acid residue forms an H-bond with the g roup of the adjacent turn of the right–handed screw ( \(\begin{array}{l}\alpha\end{array} \) – helix ).
\(\begin{array}{l}\beta\end{array} \) – pleated sheet structures.
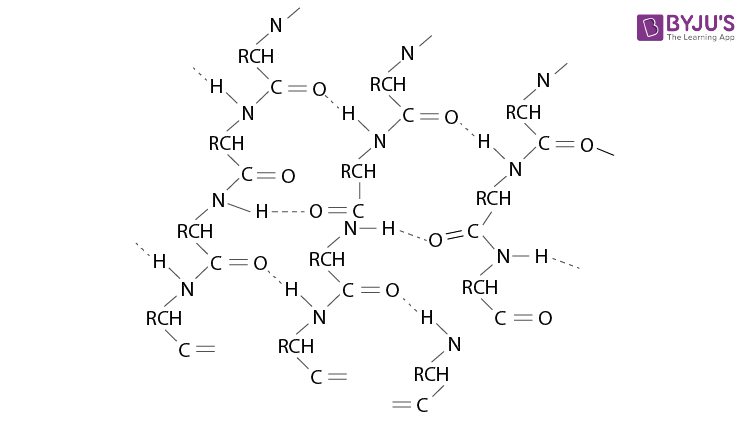
This structure is called so because it looks like the pleated folds of drapery. In this structure, the peptide chains are laid side by side after stretching out near the maximum extension. The intermolecular hydrogen bond keeps the peptide chain together.
Q 14. What type of bonding helps in stabilising the α-helix structure of proteins?
The H-bonds formed between the −NH group of each amino acid residue and the g roup of the adjacent turns of the \(\begin{array}{l}\alpha\end{array} \) helix help stabilise the helix.
Q 15. Differentiate between globular and fibrous proteins.
| The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. | 1 | It is a fibre-like structure formed by the polypeptide chain. These are the proteins which are held together by strong hydrogen and disulphide bonds. | |
| It is usually soluble in water. | 2. | It is usually not soluble in water. | |
| Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair, collagen in tendons, and myosin in muscles. | 3. | All enzymes are globular proteins. Some hormones, such as insulin, are also globular proteins. |
Q 16. How do you explain the amphoteric behaviour of amino acids?
In the presence of water or an aqueous solution, the carboxyl group of an amino acid can lose a proton, and the amino group can accept a proton to give a dipolar ion known as a zwitterion.
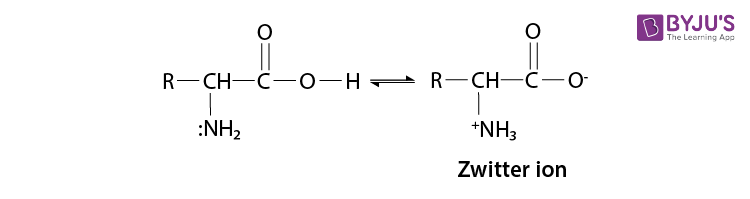
Therefore, the amino acid can act both as an acid and as a base in the presence of zwitterionic form.
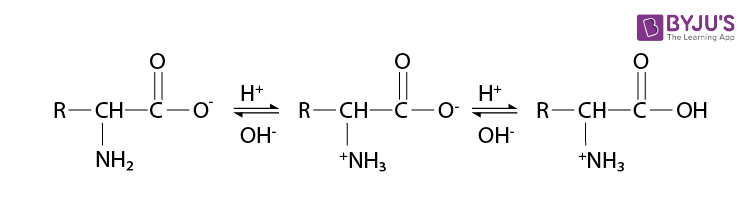
So, the amino acid show amphoteric behaviour.
Q 17. What are enzymes?
The protein that catalyses the biological reactions are called enzymes. They are very particular in nature, and for some specific substrates, they catalyse particular reactions.
The enzymes are named after a particular reaction or, in common bases, they are named after a particular class of substrate.
For example, maltase is the enzyme which is used to catalyse the hydrolysis of maltose into glucose.
Also, oxidoreductase enzymes are those which are used to catalyse the oxidation of one substrate with the simultaneous reaction of another substrate.
The name of an enzyme ends with “-ase”
Q 18. What is the effect of denaturation on the structure of proteins?
The outcome of denaturation, helixes get uncoiled, and globules get unfolded. There would be no change in the primary structure of the protein, while the secondary and the tertiary structure gets destroyed. We can say that the secondary and the tertiary–structured proteins are changed into primary–structured proteins. Also, because of the loss of secondary and tertiary structure, the enzymes lose their activity.
Q 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
We can classify vitamins on the basis of solubility in water or fat into two categories.
(a) Water-soluble vitamins: Vitamins which are soluble in water come in the category.
For example, B group vitamins ( \(\begin{array}{l}B_{1}, B_{2}, B_{12}, \; etc.\end{array} \) ) and vitamin C.
(b) Fat-soluble vitamins: Those vitamins which are soluble only in fat, not in the water, come under this group. For example, Vitamins A, D, E, and K.
However, biotin or Vitamin H is neither soluble in water nor in fat.
The vitamin which is responsible for the coagulation of blood is Vitamin K.
Q 20. Why are Vitamin A and Vitamin C essential to us? Give their important sources.
These two vitamins are essential to us because the deficiency of these two vitamins causes us harmful diseases like xerophthalmia (which hardens the cornea of the eye) and night blindness. While the deficiency of Vitamin C causes scurvy (bleeding gums).
The sources of these two vitamins are given below:
Vitamin A: Carrots, fish liver oil, milk and butter.
Vitamin C: Amla, citrus fruits and green leafy vegetables.
Q 21. What are nucleic acids? Mention their two important functions.
It is a molecule which is found as one of the constituents of chromosomes which is found in the nuclei of all living cells.
Nucleic acid can be categorised into two categories: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
Nucleic acids are long-chain polymers of nucleotides, so they are also known as polynucleotides.
(i) It is responsible for heredity. In heredity, there is a transfer of inherent characters from one generation to another. This process is held by the DNA.
(ii) The protein cell synthesis is held by nucleic acid (both RNA and DNA). Protein synthesis is majorly done by the various RNA molecules in a cell, while DNA contains the message for the synthesis of a specific protein.
Q 22. What is the difference between a nucleoside and a nucleotide?
A nucleotide is formed by the combination of all three basic components of nucleic acids, i.e. base, pentose sugar, and phosphoric acid).
Therefore, Nucleotide = Base + Sugar + Phosphoric acid
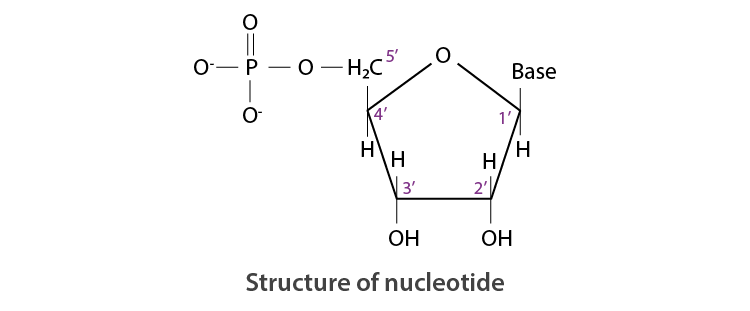
On the other hand, a nucleoside is formed by the attachment of a base to the 1’ position of the sugar.
Nucleoside = Sugar + Base
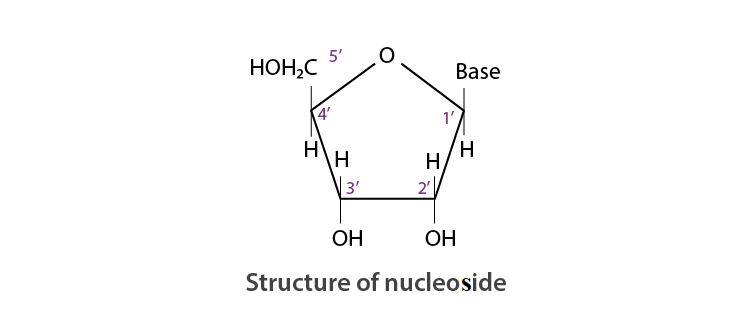
Q 23. The two strands in DNA are not identical but complementary. Explain.
In the helical structure of DNA, the hydrogen bond holds the two strands between specific pairs of bases. Adenine forms a hydrogen bond with thymine, while cytosine forms a hydrogen bond with guanine. As a result, the two strands act as complementary to each other.
Q 24. Write the important structural and functional differences between DNA and RNA.
The difference between DNA and RNA, on the basis of their functions, is as follows:
| 1 | DNA is the chemical basis of heredity. | 1 | RNA is not responsible for heredity. |
The differences on the basis of their structures are as follows:
| The sugar moiety in DNA molecules is -D-2 deoxyribose. | The sugar moiety in RNA molecules is -D-ribose. | ||
| Bases are Adenine(A), Guanine(G), Cytosine(C) and Thymine(T). | The bases are Adenine(A), Guanine(G), Cytosine(C), and Uracil(U). | ||
| The helical structure of DNA is double-stranded. | The helical structure of RNA is single-stranded. | ||
What are the different types of RNA found in the cell?
(i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii) Transfer RNA (t-RNA)
Biomolecules are the organic compounds present as essential constituents of the living organism in different cells. Biomolecules include carbohydrates, proteins, enzymes, vitamins and nucleic acids. To help students to get familiar with the topics, we have provided a downloadable form of solutions for Chapter 14 in PDF. This PDF will help students to score good marks. The biomolecules solutions PDF provided here has answers to the textbook questions along with other Chemistry Class 12 Important Questions , worksheets, MCQs, HOTS (Higher Order Thinking Skills) and exemplary questions prepared by experts at BYJU’S.
Class 12 NCERT Solutions for Chemistry Chapter 14 Biomolecules
Life is made up of chemicals, living beings are constituted of chemicals known as biomolecules, such as carbohydrates, vitamins, lipids, proteins and nucleic acids. These biomolecules interact with each other and constitute the molecular logic of life processes. In addition, some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms. The structures and functions of some of these biomolecules are discussed in NCERT Solutions for Class 12 under Chemistry Chapter 14.
Class 12 Chemistry Chapter 14 Biomolecules is formulated as per the CBSE Syllabus for 2023-24. After studying this chapter, students will be able to explain the characteristics of biomolecules like carbohydrates, proteins and nucleic acids and hormones; classify carbohydrates, proteins, nucleic acids and vitamins on the basis of their structures; explain the difference between DNA and RNA and describe the role of biomolecules in bio-system.
Subtopics of Class 12 Chemistry Chapter 14 – Biomolecules
- Carbohydrates
- Nucleic Acids
The NCERT Chemistry Solutions for Class 12 Chemistry have been designed in such a way that they enable students to tackle chemistry questions effectively. Using these solutions, students can understand difficult concepts and perform better in the board exams. They can use NCERT Solutions for Class 12 Chemistry Chapter 14 PDF for their reference and be well-versed in all the topics before the board exam. Students can perform well in the board exams by going through and solving the NCERT Solutions. The solutions, which include important questions and sample papers, will help them get a broader idea about the different types of questions along with their difficulty level as well as the marking scheme.
Why Opt for BYJU’S?
BYJU’S NCERT Solutions for Chemistry covers all the chapters in general. So, students can benefit from not just one chapter but all the chapters’ NCERT Solutions for Class 12 Chemistry. Students also get an additional advantage as they can learn NCERT or CBSE-based Class 12 Chemistry chapters and topics within their comfort zone. They can access the materials right from their homes or from anywhere. Students can also download and use our free Learning App. BYJU’S also provides experienced subject experts who can clarify all their doubts while guiding them to learn the subject and its concepts in a more structured manner.
In case a student faces problems while referring to NCERT Class 12 Chemistry Solutions , they can approach the BYJU’S support team to clear all their doubts. Apart from Chemistry, students can also submit all their queries regarding other subjects, including Physics, Maths and Biology. And that is not just it. BYJU’S also keeps track of the progress that students make. Besides, feedback is provided at regular intervals.
Frequently Asked Questions on NCERT Solutions for Class 12 Chemistry Chapter 14
Explain the difference between glycogen and starch covered in chapter 14 of ncert solutions for class 12 chemistry., what are biomolecules and their types discussed in chapter 14 of ncert solutions for class 12 chemistry, can students rely on the ncert solutions for class 12 chemistry chapter 14 at byju’s, leave a comment cancel reply.
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Category: Case Study Based Questions for Class 12 Chemistry
Join our Telegram Channel for Free PDF Download
Case Study Questions for Class 12 Chemistry Chapter 15 Polymers
Case study questions for class 12 chemistry chapter 12 aldehydes, ketones and carboxylic acids, case study questions for class 12 chemistry chapter 4 chemical kinetics, case study questions for class 12 chemistry chapter 9 coordination compounds, case study questions for class 12 chemistry chapter 10 haloalkanes and haloarenes, case study questions for class 12 chemistry chapter 11 alcohols, phenols and ethers, case study questions for class 12 chemistry chapter 13 amines, case study questions for class 12 chemistry chapter 14 biomolecules, case study questions for class 12 chemistry chapter 8 the d- and f-block elements, case study questions for class 12 chemistry chapter 7 the p-block elements, case study questions for class 12 chemistry chapter 5 surface chemistry, case study questions for class 12 chemistry chapter 3 electrochemistry, case study questions for class 12 chemistry chapter 2 solutions, case study questions for class 12 chemistry chapter 1 the solid state.

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
NCERT Solutions For Class 12 Chemistry Chapter 14 Biomolecules
September 25, 2020 by phani
NCERT Solutions For Class 12 Chemistry Chapter 14 Biomolecules
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules :
| 14 | Biomolecules |
| 14.1 | Carbohydrates |
| 14.2 | Proteins |
| 14.3 | Enzymes |
| 14.4 | Vitamins |
| 14.5 | Nucleic Acids |
| 14.6 | Hormones |
NCERT INTEXT QUESTIONS
14.1. Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain. Ans: The .solubility of a solute in a given solvent follows the rule ‘ Like dissolves like’.Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water. As a result of this extensive intermoleeular H-bonding, glucose and sucrose are soluble in water.On the other hand, benzene and cyclohexane do not contain -OH bonds and hence do not form H-bonds with water. Moreover, they are non-polar molecules and hence do not dissolve in polar water molecules.
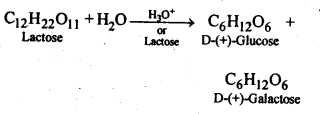
14.4. The melting points and solubility in water of a-amino acids are generally higher than those of corresponding haloacids. Explain. Ans: a-amino acids as we all know, are dipolar in nature (\(\overset { + }{ N }\)H 3 -CHR-COO – ) and have strong dipolar interactions. As a result, these are high melting solids. These are also involved in intermolecular hydrogen bonding with the molecules of water and are therefore, water soluble. On the contrary, the haloacids RCH(X)COOH are not dipolar like a-amino acids. Moreover, only the carboxyl group of haloacids are involved in hydrogen bonding with the molecules of water and not the halogen atoms. These have therefore, comparatively less melting points and are also soluble in water to smaller extent.
14.5. Where does the water present in the egg go after boiling the egg? Ans: When egg is boiled, proteins first undergo denaturation and then coagulation and the water present in the egg gets absorbed in coagulated protein, probably through H- bonding
14.6. Why cannot Vitamin C be stored in our body? Ans: Vitamin C cannot be stored in the body because it is water soluble and is, therefore, easily excreted in urine.
14.7. Which products would be formed when a nucleotide from DNA containing thymine is hydrolysed? Ans: Upon hydrolysis, nucleotide from DNA would form 2-deoxyribose and phosphoric acid along-with thymine.
14.8. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA? Ans: A DNA molecule has two strands in which the four complementary bases pair each other, i.e., cytosine (C) always pair with guanine (G) while thymine (T) always pairs with adenine (A). Thus, when a DNA molecule is hydrolysed, the molar amounts of cytosine is always equal to that of guanine and that of adenine is always equal to thymine.In RNA, there is no relationship between the quantities of four bases (C, G, A and U) obtained, therefore, the base pairing principle, i.e. A pairs with U and C pairs with G is not followed. Therefore, unlike DNA, RNA has a single strand.
NCERT EXERCISES
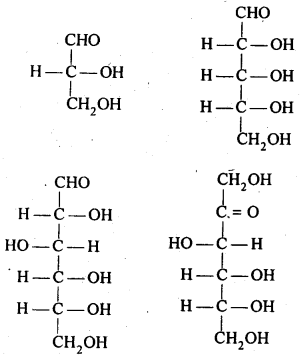
14.2. What are reducing sugars? Ans: Reducing sugars are those which can act as reducing agents. They contain in them a reducing group which may be aldehydic (-CHO) or ketonic (>C=0) group. The characteristic reactions of reducing sugars are with Tollen’s reagent and Fehling solution. Non-reducing sugars donot give these reactions. For example, glucose, fructose, lactose etc. are reducing sugars. Sucrose is regarded as a non-reducing sugar because both glucose and fructose are linked through their aldehydic and ketonic groups by glycosidic linkage. Since these groups are not free, sucrose is a non-reducing sugar.
14.3. Write two main functions of carbohydrates in plants. Ans: Two major functions of carbohydrates in plants are following (a)Structural material for plant cell walls: The polysaccharide cellulose acts as the chief structural material of the plant cell walls. (b)Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and act as the reserve food material for the tiny plant till it is capable of making its own food by photosynthesis.
14.4. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose. Ans: Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose. Disaccharides: Maltose and lactose.
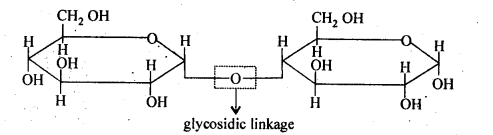
14.6. What is glycogen? How is it different from starch? Ans: The carbohydrates are stored in animal body as glycogen. It is also called animal starch and its structure is similar toamylopectin which means that it is a branched chain polymer of α-D-glucose units in which the chain is formed by C 1 – C 4 glycosidic linkage whereas branching occurs by the formation of C 1 – C 6 glycosidic linkage. One main difference between glycogen and amylopectin is the length of the chain. In amylopectin, the chain consists of 20 – 25 α – D – glucose molecules whereas in glycogen, there are 10 -14 molecules of α – D – glucose present. Glycogen is more branched than amylopectin. It is present mainly in liver, muscles and also in brain. Glycogen gets converted into glucose when the body needs it with the help of certain enzymes present in the body. Glycogen has also been found to be present in yeast and fungi.
Starch is a major source of carbohydrates which are very much essential to the human body since they supply energy to the body. It occurs as granules mainly in seeds, fruits, tubers and also in the roots of the plants. The chief commercial sources of starch are wheat, maize, rice, potatoes etc.
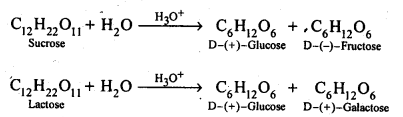
14.10. Enumerate the reactions of D-glucose which cannot be explained with open chain structure. (C.B.S.E. Delhi 2008, C.B.S.E. Sample Paper 2011) Ans: Open chain structure of D-glucose contains a free aldehydic group (- CHO). However, it does not give the following reactions:
- D(+) glucose does not react with 2, 4 D.N.P.
- D(+) glucose does not react with NaHSO 3 .
- D(+) glucose does not restore the pink colour to Schiff’s reagent.
- Penia acetyl glucose formed by carrying acetylation with acetic anhydride does not react with hydroxyl amine (NH 2 OH) which is the characteristic reaction of all aldehydes.
- D( +) glucose is found to exist in two different crystalline forms which are named as α and β. Both these forms have actually been isolated. For example, α form with m.p. 419 K is obtained by the crystallisation of the saturated solution of glucose prepared at 303 K. Similarly, β-form with m.p. 423 K is isolated by carrying out the crystallisation of the saturated solution of glucose prepared at 371 K. Apart from that the a-form has a specific rotation (α) equal to + 112° while the β- form has specific rotation (α) equal to + 19°.
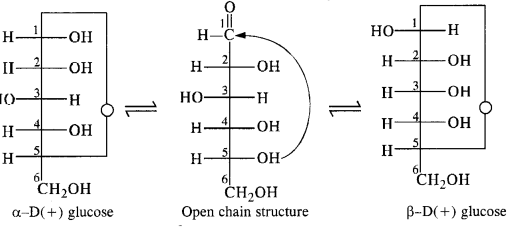
14.11. What are essential and non-essential amino acids? Give two examples of each type. Ans: α-Amino acids which are needed for good health and proper growth of human beings but are not synthesized by the human body are called- essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, α-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid etc.
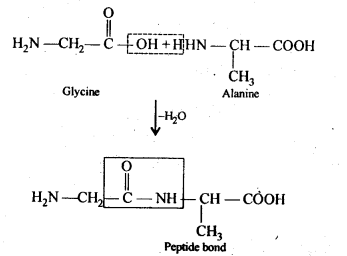
14.13. What are the common types of secondary structure of proteins? Ans: Secondary structure of protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
- α-helix structure
- β-pleated sheet structure.
Secondary Structure of Proteins: The long, flexible peptide chains of proteins are folded into the relatively rigid regular conformations called the secondary structure. It refers to the conformation which the polvpeptide chains assume as a result of hydrogen bonding between the > C= O and > N-H groups of different peptide bonds. The type of secondary structure a protein will acquire, in general depends upon the size of the R-group. If the size of the R-groups are quite large, the protein will acquire ct-helix structure. If on the other hand, the size of the R-groups are relatively smaller, the protein will acquire a β – flat sheet structure.
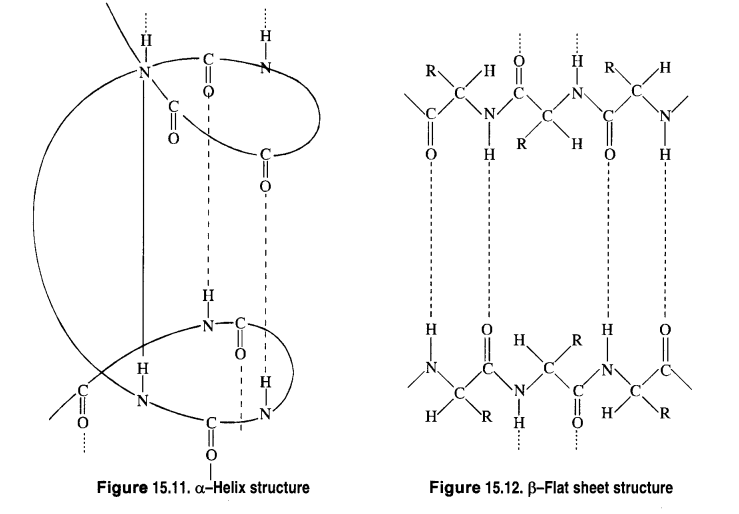
(b) β—Flat sheet or β—Pleated sheet structure: If R-groups are relatively small, the peptide chains lie side by side in a zig zag manner with alternate R-groups on the same side situated at fixed distances apart. The two such neighbouring chains are held together by intermolecular hydrogen bonds. A number of such chains can be inter-bonded and this results in the formation of a flat sheet structure These chains may contract or bend a little in order to accommodate moderate sized R-groups. As a result, the sheet bends into parallel folds to form pleated sheet structure known as β – pleated sheet structure. These sheets are then stacked one above the other like the pages of a book to form a three dimensional structure. The protein fibrion in silk fibre has a β – pleated sheet structure. The characteristic mechanical properties of silk can easily be explained on the basis of its β – sheet structure. For example, silk is non-elastic since stretching leads to pulling the peptide covalent bonds. On the other hand, it can be bent easily like a stack of pages because during this process, the sheets slide over each other.
14.14. What types of bonding helps in stabilising the α-helix structure of proteins? Ans: α-helix structure of proteins is stabilised through hydrogen bonding. (a) α -Helix structure. If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group of one amino acid unit and the > N- H group of the fourth amino acid unit within the same chain. As such the polypeptide chain coils up into a spiral structure called right handed a—helix structure. This type of structure is adopted by most of the fibrous structural proteins such as those present in wool, hair and muscles. These proteins are elastic i.e., they can be stretched. During this process, the weak hydrogen bonds causing the α-helix are broken. This tends to increase the length of the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.
14.15: Differentiate between globular and fibrous proteins. Ans. (i) Fibrous proteins: These proteins consist of linear thread like molecules which tend to lie side by side (parallel) to form fibres. The polypeptide chains in them are held together usually at many points by hydrogen bonds and some disulphide bonds. As a result,intermolecular forces of attraction are very’ strong and hence fibrous proteins are insoluble in water. Further, these proteins are stable to moderate changes in temperature and pH. Fibrous proteins serve as the chief structural material of animal tissues.For example, keratin in skin, hair, nails and wool, collagen in tendons, fibrosis in silk and myosin in muscles. (ii) Globular proteins: The polypeptide chain in these proteins is folded around itself in such a way so as to give the entire protein molecule an almost spheroidal shape. The folding takes place in such a manner that hydrophobic (non-polar) parts are pushed inwards and hydrophilic (polar) parts are pushed outwards. As a result, water molecules interact strongly with the polar groups and hence globular protein are water soluble. As compared to fibrous proteins, these are very sensitive to small changes of temperature and pH. This class of proteins include all enzymes, many hormones such as insulin from pancreas, thyroglobulin from thyroid gland, etc.
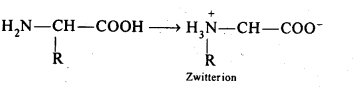
14.17. What are enzymes? Ans: Enzymes are biological catalyst. Each biological reaction requires a different enzyme. Thus, as compared to conventional catalyst enzymes are very specific and efficient in their action. Each type of enzyme has its own specific optimum conditions of concentration, pH and temperature at which it works best.
14.18. What is the effect of denaturation on the structure of proteins? Ans: Denaturation of proteins is done either by change in temperature (upon heating) or by bringing a change in the pH of the medium. As a result, the hydrogen bonding is disturbed and the proteins lose their biological activity i.e., their nature changes. During the denaturation, both the tertiary and secondary structures of proteins are destroyed while the primary structures remain intact.
14.19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood. Ans: Vitamins are classified into two groups depending upon their solubility in water or fat: (i) Water soluble vitamins: These include vitamin B-complex (B 1 , B 2 , B 5 , i.e., nicotinic acid,B 6 , B 12 , pantothenic acid, biotin, i.e., vitamin H and folic acid) and vitamin C. (ii) Fat soluble vitamins: These include vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues. Vitamin K is responsible for coagulation of blood.
14.20. Why are vitamin A and vitamin C essential to us? Give their important sources. Ans: Vitamin A is essential for us because its deficiency causes xerophthalmia (hardening of cornea of eye) and night blindness. Sources: Fish liver oil, carrots, butter, milk, etc. Vitamin C is essential for us because its deficiency causes scurvy (bleeding of gums) and pyorrhea (loosening and bleeding of teeth). Sources: Citrous fruits, amla, green leafy vegetables etc.
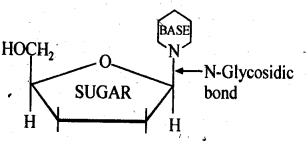
14.23. The two strands in DNA are not identical but are complementary. Explain. Ans: The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (cytosine) through three H-bonds, (i.e.,C = G) and between A (adenine) and T (thiamine) through two H-bonds (i.e., A = T). Due to this base -pairing principle, the sequence of bases in one strand automatically fixes the sequence of bases in the other strand. Thus, the two strands are complimentary and not identical.
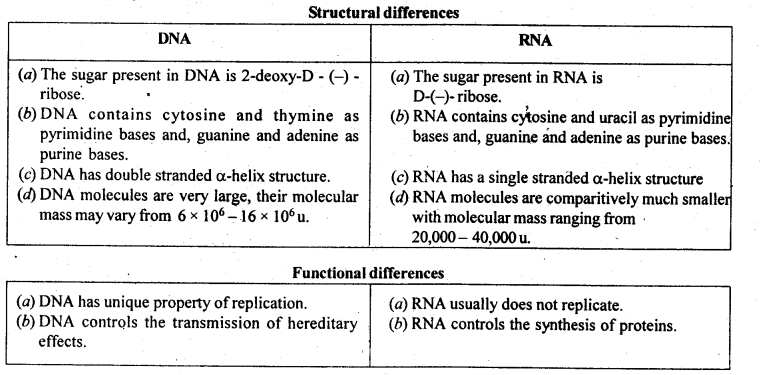
14.25. What are the different types of RNA found in the cell? Ans: There are three types of RNA: (a) Ribosomal RNA (r RNA) (b) Messenger RNA (m RNA) (c) Transfer RNA (t RNA)
More Resources for CBSE Class 12:
- CBSE Class 12 Maths
- RD Sharma class 12 Solutions
- CBSE Class 12th English Flamingo
- CBSE Class 12th English Vistas
- CBSE Class 12 Accountancy
- CBSE Sample Papers For Class 12
NCERT Solutions Maths Physics Chemistry Biology Science
Free Resources
NCERT Solutions
Quick Resources
- Skip to main content
- Skip to secondary menu
- Skip to primary sidebar
- Skip to footer
Learn Insta
RD Sharma Solutions , RS Aggarwal Solutions and NCERT Solutions
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
June 18, 2021 by Prasanna
Class 12 Chemistry NCERT Solutions Chapter 14 provides a detailed insight into the concepts related to the chapter “Biomolecules”. Chemistry is an important subject and the students need to be thorough with its concepts if they are preparing for boards or NEET and JEE.
NCERT Solutions also contains solutions to the questions provided in the textbook. The students can use these solutions to answer in the exam and score well.
| CBSE | |
| NCERT | |
| Class 12 | |
| Chemistry | |
| Chapter 14 | |
| Biomolecules | |
| 33 | |
Biomolecules is a very important chapter and requires detailed understanding of the concepts. This topic is often asked in the examination and the students need to be thorough with the concepts.
The students can learn the structure, properties and classification of various biomolecules such as carbohydrates, nucleic acids, etc. These concepts are also taught in further studies. Therefore, the students need to be thorough with the basics.
NCERT INTEXT QUESTIONS
Question 1. Glucose and sucrose are soluble in water but cyclohexane and benzene (simple six-membered ring compounds) are insoluble in water. Explain. Answer: Both glucose (C 6 H 12 O 6 ) and sucrose (C 1 2 H 22 O 11 ) are organic compounds and are expected to be insoluble in water. But quite surprisingly, they readily dissolve in water. This is due to the presence of a number of OH groups (five in the case of glucose and eight in sucrose) which are of polar nature. These are involved in the intermolecular hydrogen bonding with the molecules of H 2 O (water). As a result, both of them readily dissolve in water. Benzene (C 6 H 6 ) and cyclohexane (C 6 H 12 ) are hydrocarbons which don’t have any polar group. They, therefore, don’t dissolve in water since there is hardly any scope of hydrogen bonding in their molecules with those of H 2 O (water).
Question 2. What are the expected products of hydrolysis of lactose? Answer: The hydrolysis of lactose (disaccharide) can be done either with dilute HC1 or with enzyme emulsin. D-glucose and D-galactose are the products of hydrolysis. Both of them are monosaccharides with the molecular formula C6Hi 2 0 6 .
Question 3. How do you explain the absence of an aldehydic group in the pentaacetate of D-glucose? Answer: Glucose, as we know is an aldohexose and it is expected to give the characteristic reactions of the aldehydic group e.g., action with NH 2 OH, HCN, Tollen’s reagent, Fehling reagent etc. However, the pentadactyl glucose formed by the acylation of glucose with acetic anhydride does not give these reactions.
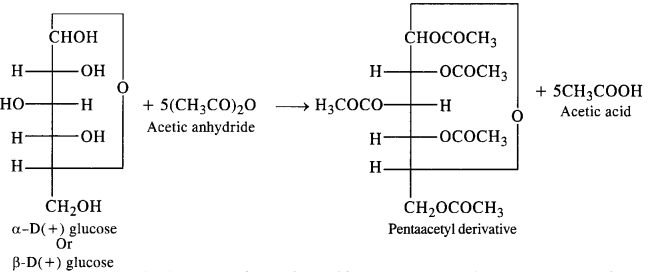
Question 4. The melting points and solubility of amino acids in water are generally higher than those of corresponding haloacids. Explain. Answer: The amino acids exist as zwitter ions, H 3 N + — CHR-COO-. Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are higher than corresponding haloacids which do not have salt-like character. Due to their salt-like character, amino acids interact strongly with water. As a result, their solubility in water is higher than corresponding haloacids which do not have a salt-like character.
Question 5. Where does the water in the egg go after boiling the egg? Answer: Upon boiling the egg, denaturation of globular protein present in it occurs. Water present probably gets either absorbed or adsorbed during denaturation and disappears.
Question 6. Explain why vitamin C can not be stored in the body. Answer: Vitamin C is mainly ascorbic acid which is water-soluble. It is readily excreted through urine and cannot be stored in the body.
Question 7. What products would be formed when a nucleotide from DNA containing thymine is hydrolysed? Answer: The products obtained are 2-deoxy-D-ribose, phosphoric acid, and thymine.
Question 8. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained? What does this fact suggest about the structure of RNA? Answer: As we know, a molecule of DNA has a double-strand structure, and the four complementary bases pair each other. Cytosine (C) always pairs up with guanine (G) while thymine (T) is paired up with adenine (A). Because of the presence of the double-strand structure, when a molecule of DNA is hydrolysed, in each pair the molar ratio of the bases remains the same. However, this is not seen when RNA is subjected to hydrolysis. This suggests that RNA has not a double-strand structure like DNA. It exists as a single strand.
NCERT Exercises
Question 1. What are monosaccharides? Answer: Monosaccharides are carbohydrates which cannot be hydrolysed to smaller molecules. Their general formula is (CH 2 O) n where n = 3 → 7. These are of two types. Those which contain an aldehyde (-CHO) group are called aldose and those which contain a keto (C = 0) group are called ketose. They are further classified as triose, tetrose, pentose etc. according to the no. of carbon atoms present (3, 4, 5 respectively).
Question 2. What are reducing sugars? Answer: Carbohydrates which reduce Fehling’s solution to red precipitate of Cu 2 0 or Tollen’s reagent to metallic Ag are called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except sucrose are reducing sugars. Thus, D – (+) – glucose, D-(-)-fructose, D – (+) – maltose and D – (+) – lactose are reducing sugars.
Question 3. Write two major functions of carbohydrates in plants. (C.B.S.E. Delhi 2008) Answer:
- Structural material for plant cell walls: The polysaccharides cellulose acts as the chief structural material of the plant’s cell walls.
- Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and acts as the reserve food material for the tiny plant till its capable of making food on its own by photosynthesis.
Question 4. Classify the following into monosaccharides and disaccharides: Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose. Answer: Monosaccharides: Ribose, 2-deoxyribose, galactose, fructose. Disaccharides: Maltose, lactose.
Question 5. What do you understand by the term glycosidic linkage? Answer: Glycosidic linkage is used to link different monosaccharides in disaccharides and polysaccharides through an oxygen atoms. For example, the glycosidic linkage is present in sucrose, lactose, maltose, etc. These are all disaccharides.
Question 6. What is glycogen? How is it different from starch? Answer:
- The carbohydrates are stored in the animal body as glycogen. It is present in the liver, muscles, and brain. Enzymes break the glycogen down to glucose when the body needs glucose.
- Glycogen is more highly branched than amylopectin (starch) glycogen chain consists of 10-14 glucose units, whereas amylopectin (starch) glycogen chain consists of 20-25 glucose units.
Question 7. What are the hydrolysis products of starch and lactose? Answer: Starch upon hydrolysis gives α – D(+) glucose which is the constituent of both amylose and amylopectin. Lactose upon hydrolysis gives galactose and glucose. Upon hydrolysis, cellulose gives only D(+) glucose. This means that only D(+) glucose units are present in cellulose but unlike starch these are -D(+) glucose molecules and not a – D(+) glucose molecules. The X – ray analysis has shown that there are large linear chains of 3 – D( +) glucose molecules lying side by side in the form of bundles held together by hydrogen bonding in the neighbouring hydroxyl groups.
Question 8. What is the basic structural difference between starch and cellulose? Answer: Starch is not a single component. It consists of amylose and amylopectin. In contrast, cellulose is a single compound. Amylose is a linear polymer of α – D glucose while cellulose is a linear polymer of β -D glucose. In amylose, C1 – C4 α- glycosidic linkage is present, whereas in cellulose C1 – C4 β- glycosidic linkage is present. Amylopectin has a highly branched structure.
Question 10. Enumerate the reactions of D-glucose which cannot be explained by its open-chain structure. Answer: The following reactions of D- glucose cannot be explained by its open-chain structure. 1. D – the glucose does not undergo certain characteristic reactions of aldehydes. For example, glucose does not form NaHSO 3 addition product, aldehyde-ammonia adduct, 2, 4, DNP derivative and does not respond to Schiff’s reagent test.
2. Glucose reacts with NH 2 OH to form an oxime but glucose pentaacetate does not. This implies that the aldehyde group is absent in glucose pentaacetate.
3. D (+) – Glucose exists in two stereoisomeric forms ie. α – glucose and β- glucose, α – D (+) – glucose is obtained when a concentrated aqueous or alcoholic solution is crystallised at 303K. It has a melting point of 419K and has a specific rotation of +111° in a freshly prepared aqueous solution. However when glucose is crystallised from the water above 371 K β – D (+) glucose is obtained.
4. Both α – D glucose and β – D glucose undergoes mutarotation in an aqueous solution.
Question 11. What are essential and non-essential amino acids? Answer: α – Amino acids which are needed for the health and growth of human beings but are not synthesised by the human body are called essential amino acids. For example, valine, leucine phenylalanine etc. On the other hand α – amino acids which are needed for the health and growth of human beings and are synthesised by the human body are called non-essential amino acids. For example glycine, alanine, aspartic acid etc.
Question 12. Define the following as related to proteins (i) Peptide linkage (ii) Primary structure (iii) Denaturation. Answer: (i) Peptide Linkage: Proteins are the polymers of a-amino acids which are connected to each other by peptide bond or peptide linkage. Chemically, peptide linkage is an amide formed between -COOH group and -NH2 group. The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other This results in the elimination of a water molecule and formation of a peptide bond -CO-NH-. The product of the reaction is called a dipeptide because it is made up of two amino acids. For example, when carboxyl group of glycine combines with the amino group of alanine we get a dipeptide, glycylalanine.
(ii) Primary Structure: Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein. Any change in this primary structure i.e.; the sequence of amine acids creates a different protein.
(iii) Denaturation: Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to physical change like change in temperature or chemical change in pH, the hydrogen bonds are disturbed. Due to this; globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein. During denaturation 2° and 3° structures are destroyed but 1° structure remains intact. The coagulation of egg white on boiling is a common example of denaturation. Another example is the curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.
Question 13. What are the common types of secondary structures of proteins? Answer: Secondary structure of a protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
- α-helix structure
- β-pleated sheet structure.
Secondary Structure of Proteins: The long, flexible peptide chains of proteins are folded into the relatively rigid regular conformations called the secondary structure. It refers to the conformation which the polypeptide chains assume as a result of hydrogen bonding between the > C= O and > N-H groups of different peptide bonds. The type of secondary structure a protein will acquire, in general, depends upon the size of the R-group. If the size of the R-groups are quite large, the protein will acquire a ct-helix structure. If on the other hand, the size of the R-groups are relatively smaller, the protein will acquire a β – flat sheet structure.
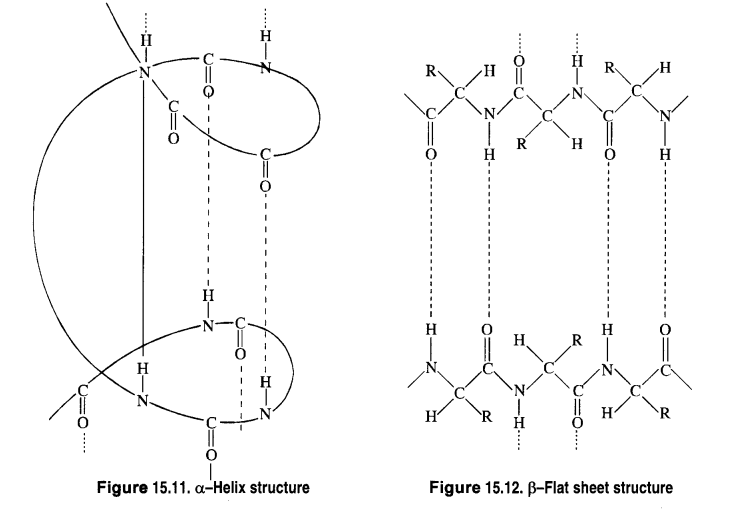
(b) β—Flat sheet or β—Pleated sheet structure: If R-groups are relatively small, the peptide chains lie side by side in a zig zag manner with alternate R-groups on the same side situated at fixed distances apart. The two such neighbouring chains are held together by intermolecular hydrogen bonds. A number of such chains can be inter-bonded and this results in the formation of a flat sheet structure These chains may contract or bend a little in order to accommodate moderate-sized R-groups. As a result, the sheet bends into parallel folds to form a pleated sheet structure known as β – pleated sheet structure. These sheets are then stacked one above the other like the pages of a book to form a three-dimensional structure. The protein fibrion in silk fibre has a β – pleated sheet structure. The characteristic mechanical properties of silk can easily be explained on the basis of its β – sheet structure. For example, silk is non-elastic since stretching leads to pulling the peptide covalent bonds. On the other hand, it can be bent easily like a stack of pages because, during this process, the sheets slide over each other.
Question 14. What type of bonding helps in stabilising the α-helix structure of proteins? Answer: The α-helix structure of proteins is stabilized by intramolecular H-bonding between C = O of one amino acid residue and the N – H of the fourth amino acid residue in the chain. This causes the polypeptide chain to coil up into a spiral structure called the right-handed α- helix structure.
Question 15. Differentiate between globular proteins and fibrous proteins. (Jharkhand Board 2014; C.B.S.E. Delhi 2015) Answer:
| 1. Polypeptide chains are arranged as coils. | 1.Polypeptide chains run parallel to each other. |
| 2. They have a spherical shape. | 2. They have a thread-like structure. |
| 3. These are water-soluble. | 3. These are insoluble in water. |
| 4. These are sensitive to a small change in temperature and pH. | 4. These are not affected by a small change in temperature and pH. |
| 5. They possess biological activity. | 5. They don’t have any biological activity but serve as the chief structural material of animal tissues. |
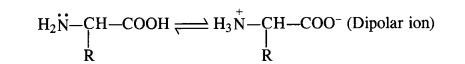
Question 18. What is the effect of denaturation on the structure of proteins? Answer: As a result of denaturation, globules get unfolded and helixes get uncoiled. Secondary and tertiary structures of the protein are destroyed, but the primary structures remain unaltered. It can be said that during denaturation, secondary and tertiary – structured proteins get converted into primary – structured proteins. Also, as the secondary and tertiary structures of a protein are destroyed, the enzyme loses its activity.
Question 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood? (C.B.S.E. Outside Delhi 2015) Answer: On the basis of their solubility in water or fat, vitamins are classified into two groups: 1. Fat-soluble vitamins: Vitamins that are soluble in fat and oils, but not in the water, belong to their group. For example Vitamins A, D, E, and K.
2. Water-soluble vitamins: Vitamins that are soluble in water belong to their group. For example, B group vitamins (B 1 , B 2 , B 6 , B 12 , etc) and vitamin C. However, biotin or vitamin H is neither soluble in water nor in fat. Vitamin K is responsible for the coagulation of blood.
Question 20. Why are vitamin A and vitamin C essential to us? Mention their sources. Answer: vitamin A: Soluble in water but insoluble in oils and fas. Destroyed by cooking or prolonged exposure to air. it increases the resistance of the body towards diseases. Maintains healthy skin and helps in the healing of cuts and abrasions. It is available in
vitamin C: Soluble in oils and fats but insoluble in water, stable to heat. Promotes growth and improves vision. ¡t also increases resistance to disease. It is available in Citrus fruits (oranges, lemon, grapefruit, lime, etc.), amia, cabbage. guava etc.
Question 21. What are nucleic acids? Mention their two important functions. Answer: Nucleic acids are biologically important polymers which are present in all living cells.
- Nucleic acids play a vital role in the transmission of heredity characteristics.
- Nucleic acids help in the biosynthesis of proteins.
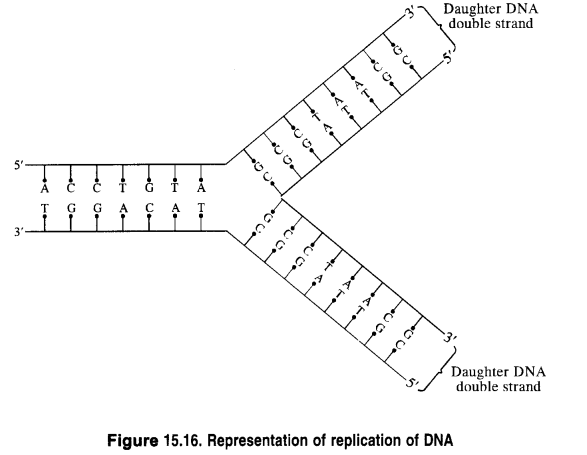
Question 22. What is the difference between a nucleoside and a nucleotide? Answer: A nucleoside contains a pentose sugar and base (purine or pyrimidine) while in the nucleotide, a phosphoric acid component is also present. Arrangement of constituents in Nucleic Acids. These are, intact, three building blocks in nucleic acid. A combination ol base and sugar are known as a nucleoside. Similarly, base, sugar, and phosphates from nucleotides while nucleic acids are polynucleotides which means that these are the polymers of nucleotides.
Question 23. The two strands in DNA are not identical but are complementary. Explain. Answer: In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms a hydrogen bond with guanine, while adenine forms a hydrogen bond with thymine. As a result, the two strands are complementary to each other.
Question 24. Write the important structural and functional differences between DNA and RNA. Answer:
| 1. The pentose sugar present in RNA is D-ribose | 1. The pentose sugar present in DNA is D-2-deoxyribose |
| 2. RNA contains cytosine and uracil as pyrimidine bases and guanine and adenine as purine bases. | 2. DNA contains cytosine and thymine as pyrimidine bases and guanine and adenine as purine bases. |
| 3. It is a single chain of polynucleotides. | 3. It is a double chain of polynucleotides. |
| 4. It is formed by DNA and cannot replicate itself. | 4. It can replicate itself. |
| 5. Its molecule is relatively short with low molecular mass. | 5. Its molecule is relatively long with a high molecular mass. |
| 6. It regulates protein synthesis. | 6. It controls structure, metabolism, differentiation and transfer the characters from one generation to the other. |
| 7. It is an essential genetic material of plant viruses. | 7. It is an essential genetic material of eukaryotic cells. |
Question 25. What are the different types of RNA found in the cell? Answer:
- Messenger RNA (m – RNA)
- Ribosomal RNA (r – RNA)
- Transfer RNA (t – RNA)
We hope the NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules help you. If you have any query regarding NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules, drop a comment below and we will get back to you at the earliest.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Important Question
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- Question Bank
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- Toppers Notes
- Most Repeated Question
- Diagram Based Question
- Study Planner
- JEE Previous Year Paper
- JEE Mock Test
- JEE Crash Course
- JEE Sample Papers
- Important Info
- SRM-JEEE Previous Year Paper
- SRM-JEEE Mock Test
- VITEEE Previous Year Paper
- VITEEE Mock Test
- BITSAT Previous Year Paper
- BITSAT Mock Test
- Manipal Previous Year Paper
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- AP EAMCET Mock Test
- COMEDK Previous Year Paper
- COMEDK Mock Test
- GUJCET Previous Year Paper
- GUJCET Mock Test
- KCET Previous Year Paper
- KCET Mock Test
- KEAM Previous Year Paper
- KEAM Mock Test
- MHT CET Previous Year Paper
- MHT CET Mock Test
- TS EAMCET Previous Year Paper
- TS EAMCET Mock Test
- WBJEE Previous Year Paper
- WBJEE Mock Test
- AMU Previous Year Paper
- AMU Mock Test
- CUSAT Previous Year Paper
- CUSAT Mock Test
- AEEE Previous Year Paper
- AEEE Mock Test
- UPSEE Previous Year Paper
- UPSEE Mock Test
- CGPET Previous Year Paper
- BCECE Previous Year Paper
- JCECE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- Verbal Ability & Reading Comprehension
- Logical Reasoning & Data Interpretation
- CAT Mock Test
- CAT Important Question
- CAT Vocabulary
- CAT English Grammar
- MBA General Knowledge
- CAT Mind Map
- CAT Study Planner
- CMAT Mock Test
- SRCC GBO Mock Test
- SRCC GBO PYQs
- XAT Mock Test
- SNAP Mock Test
- IIFT Mock Test
- MAT Mock Test
- CUET PG Mock Test
- CUET PG PYQs
- MAH CET Mock Test
- MAH CET PYQs
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
Chemistry PYP Chapter-Wise || Biomolecules || Biomolecules

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

One Last Step...

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


IMAGES
VIDEO
COMMENTS
Ans 3. (a) Case Study based questions on Biomolecules. 3. Read the given passage and answer the questions that follow. Proteins are the most abundant biomolecules of the living system. The chief sources of proteins are milk, cheese, pulses, fish, meat, peanuts, etc. They are found in every part of the body and form a fundamental basis of the ...
There is Case Study Questions in class 12 Chemistry in session 2020-21. For the first time, the board has introduced the case study questions in the board exam. The first two questions in the board exam question paper will be based on Case Study and Assertion & Reason. The first question will have 5 MCQs … Continue reading Case Study Questions for Class 12 Chemistry Chapter 14 Biomolecules
In Class 12 Boards there will be Case studies and Passage Based Questions will be asked, So practice these types of questions. Study Rate is always there to help you. Free PDF Downloads of CBSE Class 12 Chemistry Chapter 14 Biomolecules Case Study and Passage-Based Questions with Answers were Prepared Based on the Latest Exam Pattern.
The PDF file of the Biomolecules Case Study for Class 12 Chemistry with Solutions is a very important study resource that can help students better prepare for the exam and boost conceptual learning. The solutions are in the hint manner as well as contain full examples too, refer to the link to access the Case Study on Biomolecules Class 12 ...
CBSE 12th Standard Chemistry Subject Biomolecules Case Study Questions 2021 Answer Keys. (i) (d): DNA occurs in nucleus of the cell while RNA is found mainly in cytoplasm of the cell. Nucleosides contain only sugar and a base whereas nucleotides contain sugar, base and a phosphate group as well.
471 Views. 10 Downloads. Case Study Questions for Class 12 Chemistry Chapter 14 Biomolecules Case Study Based Questions Class 12 Chemistry Passage Based Questions and Answers Class 12 Chemistry Important Questions for Class 12 Chemistry in PDF Format Free PDF Download Class 12 Chemistry Study Material Class 12 Chemistry CBSE 2022.
This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year's exams. The solutions have been designed in a manner to help the grade 12 ...
Class 12th Chemsitry - Biomolecules Case Study Questions and Answers 2022 - 2023 - Complete list of 12th Standard CBSE question papers, syllabus, exam tips, study material, previous year exam question papers, centum tips, formula, answer keys, solutions etc.. ... Class 12th Chemistry - Solution Case Study Questions and Answers 2022 - 2023 Click ...
Free Question Bank for 12th Class Chemistry Biomolecules / जैव-अणु Case Based (MCQs) - Biomolecules. Customer Care : 6267349244. Toggle navigation 0 ... Class XII) The RNA which carries the genetic information from DNA for protein synthesis is . A)
By QB365 on 08 Sep, 2022 . QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Biomolecules, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
In Part-I & II you got acquainted to the Carbohydrates, Proteins, Amino acids and Peptides along with their various types.This Class 12 Chemistry: Chapter- Biomolecules, Part-III, explains you about the Enzymes, Nucleic Acids and Vitamins, their various types and structures.These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12 th Chemistry.
Q.4. Assertion : At isoelectric point, the amino group does not migrate under the influence of electric field. Reason : At isoelectric point, amino acid exists as a zwitterion. Answer. a. Q.5. Assertion : Vitamin D cannot be stored in our body. Reason : Vitamin D is fat soluble vitamin and is excreted from the body in urine.
Q21. The activation energy for the acid catalysed hydrolysis of sucrose is 6.22 kJ mol -1, while the activation energy is only 2.15 kJ mol -1 when the enzyme sucrase catalyses hydrolysis. Explain. Answer: Enzymes reduce the magnitude of activation energy by providing an alternative path in the hydrolysis of sucrose.
Ans. Examples of biomolecules -. carbohydrates, proteins, Nucleic acids, Lipids, enzymes etc. 2.What are carbohydrates? Ans. Carbohydrates are optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis. 3.Give one example of each- Monosaccharide, disaccharide and polysaccharide.
Class 12 Chemistry Case Study Questions for Term 1 exam includes The Solid State, The P block elements, Haloalkanes and Haloarenes, Biomolecules, etc. Questions for all these chapters are given in the PDF file that are available here for free to download. Term 1 exam is about to be held in November-December this year.
As stated in the Chemistry Class 12 Chapter 14 Notes, proteins and carbohydrates are essential elements of our food. Furthermore, these biomolecules interact with one another and are the molecular logic of life processes. Moreover, mineral salts and vitamins play a crucial role in various essential functions of organisms.
👉Previous Video: https://www.youtube.com/watch?v=qnAqoAOZ_vU👉Next Video:===== ️📚👉 Watch Full Free Cours...
RT Exemplar Solutions for Class 12ChemistryChapter 14 - Biomolec. the liver of animals? Amy. ose Cellulose Amylopectin GlycogenAns: Correct option is (b).In humans and animals, glycogen. is mostly synthesized and stored in the liver and skeletal muscle cells. Glycogen accounts for around 5-6% of the liver's fresh weight, and an ad.
NCERT S olutions for Class 12 Chemistry Chapter 14 Biomolecules- It is fascinating to know that the living systems (like humans, plants, animals) are made up of non-living atoms and molecules. These molecules can be very complex as well as simple in nature and are simply known as biomolecules for eg. Carbohydrates, Proteins, Lipids and Nucleic acids, etc.
The NCERT Solutions for Class 12 Chemistry is developed by expert subject teachers according to the latest CBSE Syllabus 2023-24 and its guidelines. These solutions are written in simple language for ease of comprehension. Get the free PDF of NCERT Solutions for Class 12 Chemistry Chapter 14 from the link below.
April 7, 2021 May 6, 2021 Manju Kaushik Leave a Comment on Case Study Questions for Class 12 Chemistry Chapter 14 Biomolecules Case Study Questions for Class 12 Chemistry Chapter 14 Biomolecules March 31, 2021 May 6, 2021 Physics Gurukul Leave a Comment on Case Study Questions for Class 12 Chemistry Chapter 8 The d- and f-Block Elements
14.1. Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain. Ans: The .solubility of a solute in a given solvent follows the rule ' Like dissolves like'.Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water.
June 18, 2021 by Prasanna. Class 12 Chemistry NCERT Solutions Chapter 14 provides a detailed insight into the concepts related to the chapter "Biomolecules". Chemistry is an important subject and the students need to be thorough with its concepts if they are preparing for boards or NEET and JEE. NCERT Solutions also contains solutions to ...
Chemistry PYP Chapter-Wise || Biomolecules || Biomolecules. FREE PRIME MEMBERSHIP. HD PDF DOWNLOAD PDF . ... CBSE Case Study; CBSE Previous Year Papers; Free Books and Solutions. Free Books and Solutions. ... CBSE Class 12 Study Material; CBSE Class 10 Study Material; CBSE Class 12 Syllabus; CBSE Class 10 Syllabus; GATE.