- Chemistry Concept Questions and Answers
- Boyles law Questions

Boyles Law Questions
Boyle’s law is also referred to as Boyle–Mariotte law or Mariotte’s law. It tells us about the behaviour of gases. Boyle’s law states that the pressure is inversely proportional to the volume of the gas at constant pressure.
P ∝ 1 / V
Boyles Law Chemistry Questions with Solutions
Q1. Suppose P, V, and T represent the gas’s pressure, volume, and temperature, then the correct representation of Boyle’s law is
- V is inversely proportional to T (at constant P)
- V inversely proportional to P (at constant T)
Answer: (b), If P, V, and T represent the gas’s pressure, volume, and temperature, then the correct representation of Boyle’s law is V inversely proportional to P (at constant T).
V ∝ 1 / P
Q2. What is the nature of Boyle’s Law’s pressure vs volume (P vs V) graph?
- Straight Line
- Rectangular Hyperbola
- None of the above
Answer: (b), The nature of Boyle’s Law’s pressure vs volume (P vs V) graph is a rectangular hyperbola.
Q3. What is the nature of Boyle’s Law’s pressure-volume vs pressure (PV vs P) graph?
- Straight-line parallel to the P axis
- Straight-line parallel to the PV axis
- Straight-line parallel to the V axis
Answer: (a), The nature of Boyle’s Law’s pressure-volume vs pressure (PV vs P) graph is a straight line parallel to the P axis.
Q4. Which of the following quantity is kept constant in Boyle’s law?
- Gas mass only
- Gas Temperature only
- Gas Mass and Gas Pressure
- Gas Mass and Gas Temperature
Answer: (d), In Boyle’s law, the mass of the gas its temperature are kept constant.
Q5. Boyle’s law is valid only for
- Ideal gases
- Non-ideal gases
- Light Gases
- Heavy Gases
Answer: (a), Boyle’s law is valid only for ideal gases.
Q6. What is Boyle’s law?
Answer: Boyle’s law depicts the relationship between the pressure, volume, and temperature of a gas. It states that the pressure of a gas is inversely proportional to its volume at a constant temperature.
Q7. How is Boyle’s law used in everyday life?
Answer: Boyle’s law can be observed in our everyday life. Filling air in the bike tire is one of the significant applications of Boyle’s law. While pumping air into the tyre, the gas molecules inside the tire are compressed and packed closer together. It increases the pressure exerted on the walls of the tyre.
Q8. What is Boyle’s temperature?
Answer: Boyle’s temperature is the temperature at which the real and non-ideal gases behave like an ideal gas over a broad spectrum of pressure. It is related to the Van der Waal’s constant a, b as TB = a / Rb
Q9. Differentiate between Boyle’s law and Charle’s law.
Q10. Match the following gas laws with the equation representing them.
Q11. A helium balloon has a volume of 735 mL at ground level. The balloon is transported to an elevation of 5 km, where the pressure is 0.8 atm. At this altitude, the gas occupies a volume of 1286 mL. Assuming that the temperature is constant, what was the ground level pressure?
Answer: Given
Initial Volume (V 1 ) = 735 mL
Final Pressure (P 2 ) = 0.8 atm
Final Volume (V 2 ) = 1286 mL
To Find: Initial Pressure (P 1 ) = ?
We can calculate the initial pressure of the gas using Boyle’s law.
P 1 V 1 = P 2 V 2
P 1 X 735 = 0.8 X 1286
P 1 = 1028.8 / 735
P 1 = 1.39 ≈ 1.4 atm
Hence the ground level pressure is 1.4 atm.
Q12. A sample of oxygen gas has a volume of 225 mL when its pressure is 1.12 atm. What will the volume of the gas be at a pressure of 0.98 atm if the temperature remains constant?
Initial Volume (V 1 ) = 225 mL
Initial Pressure (P 1 ) = 1.12 atm
Final Pressure (P 2 ) = 0.98 atm
To Find: Final Volume (V 2 ) = ?
We can calculate the final volume of the gas using Boyle’s law.
1.12 X 225 = 0.98 X V 2
252 = 0.98 X V 2
252 / 0.98 = V 2
V 2 = 257.14 mL ≈ 257mL
Hence the final volume of the gas at pressure of 0.98 atm is equivalent to 257 mL.
Q13. An ideal gas occupying a 2.0 L flask at 760 torrs is allowed to expand to a volume of 6,000 mL. Calculate the final pressure
Initial Volume (V 1 ) = 2 L
Initial Pressure (P 1 ) = 760 torrs
Final Volume (V 2 ) = 6000 mL = 6 L
To Find: Final Pressure (P 2 ) = ?
We can calculate the final pressure of the gas using Boyle’s law.
760 X 2 = P 2 X 6
1520 = P 2 X 6
P 2 = 1520 / 6
P 2 = 253.33 torrs ≈ 253 torrs
Hence the final pressure of the gas at volume of 6 L is equivalent to 253 torrs.
Q14. A gas occupies a volume of 1 L and exerts a pressure of 400 kPa on the walls of its container. What would be the pressure exerted by the gas if it is completely transferred into a new container having a volume of 3 litres (assuming that the temperature and amount of the gas remain the same.)?
Initial Volume (V 1 ) = 1 L
Initial Pressure (P 1 ) = 400 kPa
Final Volume (V 2 ) = 3 L
400 X 1 = P 2 X 3
P 2 = 400 / 3
P 2 = 133.33 ≈ 133 kPa
Hence the final pressure of the gas at of volume 3 L is equivalent to 133 kPa.
Q15. A gas exerts a pressure of 3 kPa on the walls of container 1. When container one is emptied into a 10 litre container, the pressure exerted by the gas increases to 6 kPa. Find the volume of container 1. Assume that the temperature and amount of the gas remain the same.
Initial Pressure (P 1 ) = 3 kPa
Final Volume (V 2 ) = 10 L
Final Pressure (P 2 ) = 6 kPa
To Find: Initial Volume (V 1 ) = ?
We can calculate the initial volume of the gas using Boyle’s law.
3 X V 1 = 6 X 10
3 X V 1 = 60
V 1 = 60 / 3
Hence the initial volume of the gas at pressure of 3 kPa is equivalent to 20 L.
Practise Questions on Boyle’s Law
Q1. A gas is initially in a 5 L piston with a pressure of 1 atm. What is the new volume if the pressure changes to 3.5 atm by moving the piston down?
Q2. A balloon of volume 0.666 L at 1.03atm is placed in a pressure chamber where the pressure becomes 5.68atm. Determine the new volume.
Q3. A gas in a 30.0 mL container is at a pressure of 1.05 atm and is compressed to a volume of 15.0 mL. What is the new pressure of the container?
Q4. If a gas occupies 3.60 litres at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm?
Q5. A gas occupies 12.3 litres at a pressure of 40.0 mmHg. What is the volume when the pressure is increased to 60.0 mmHg?
Click the PDF to check the answers for Practice Questions. Download PDF
Recommended Videos
Boyle’s law.

Ideal Gas Equation definitions, derivation

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Boyle's Law Explained With Example Problem
Volume's inversely proportional to pressure if temperature's constant
Dan Brownsword / Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Boyle's gas law states that the volume of a gas is inversely proportional to the pressure of the gas when the temperature is held constant. Anglo-Irish chemist Robert Boyle (1627–1691) discovered the law and for it he is considered the first modern chemist. This example problem uses Boyle's law to find the volume of gas when pressure changes.
Boyle's Law Example Problem
- A balloon with a volume of 2.0 L is filled with a gas at 3 atmospheres. If the pressure is reduced to 0.5 atmospheres without a change in temperature, what would be the volume of the balloon?
Since the temperature doesn't change, Boyle's law can be used. Boyle's gas law can be expressed as:
- P i V i = P f V f
- P i = initial pressure
- V i = initial volume
- P f = final pressure
- V f = final volume
To find the final volume, solve the equation for V f :
- V f = P i V i /P f
- V i = 2.0 L
- P i = 3 atm
- P f = 0.5 atm
- V f = (2.0 L) (3 atm) / (0.5 atm)
- V f = 6 L / 0.5 atm
- V f = 12 L
The volume of the balloon will expand to 12 L.
More Examples of Boyle's Law
As long as the temperature and number of moles of gas remain constant, Boyle's law means doubling the pressure of a gas halves its volume. Here are more examples of Boyle's law in action:
- When the plunger on a sealed syringe is pushed, the pressure increases and the volume decreases. Since the boiling point is dependent on pressure, you can use Boyle's law and a syringe to make water boil at room temperature.
- Deep-sea fish die when they're brought from the depths to the surface. The pressure decreases dramatically as they are raised, increasing the volume of gases in their blood and swim bladder. Essentially, the fish pop.
- The same principle applies to divers when they get "the bends." If a diver returns to the surface too quickly, dissolved gases in the blood expand and form bubbles, which can get stuck in capillaries and organs.
- If you blow bubbles underwater, they expand as they rise to the surface. One theory about why ships disappear in the Bermuda Triangle relates to Boyle's law. Gases released from the seafloor rise and expand so much that they essentially become a gigantic bubble by the time they reach the surface. Small boats fall into the "holes" and are engulfed by the sea.
Walsh C., E. Stride, U. Cheema, and N. Ovenden. " A combined three-dimensional in vitro–in silico approach to modelling bubble dynamics in decompression sickness ." Journal of the Royal Society Interface , vol. 14, no. 137, 2017, pp. 20170653, doi:10.1098/rsif.2017.0653
- Examples of Solids, Liquids, and Gases
- The Formula for Boyle's Law
- Boyle's Law Definition in Chemistry
- Gases Study Guide
- Boyle's Law: Worked Chemistry Problems
- Charles' Law Example Problem
- Avogadro's Law Example Problem
- Gases - General Properties of Gases
- Ideal Gas Example Problem: Partial Pressure
- Henry's Law Example Problem
- Ideal Gas Law Test Questions
- Gay-Lussac's Gas Law Examples
- The Formula for the Combined Gas Law
- How to Calculate the Density of a Gas
- The Combined Gas Law in Chemistry
- Pressure Definition and Examples
HIGH SCHOOL
- ACT Tutoring
- SAT Tutoring
- PSAT Tutoring
- ASPIRE Tutoring
- SHSAT Tutoring
- STAAR Tutoring
GRADUATE SCHOOL
- MCAT Tutoring
- GRE Tutoring
- LSAT Tutoring
- GMAT Tutoring
- AIMS Tutoring
- HSPT Tutoring
- ISAT Tutoring
- SSAT Tutoring
Search 50+ Tests
Loading Page
math tutoring
- Elementary Math
- Pre-Calculus
- Trigonometry
science tutoring
Foreign languages.
- Mandarin Chinese
elementary tutoring
- Computer Science
Search 350+ Subjects
- Video Overview
- Tutor Selection Process
- Online Tutoring
- Mobile Tutoring
- Instant Tutoring
- How We Operate
- Our Guarantee
- Impact of Tutoring
- Reviews & Testimonials
- Media Coverage
- About Varsity Tutors
High School Chemistry : Using Boyle's Law
Study concepts, example questions & explanations for high school chemistry, all high school chemistry resources, example questions, example question #11 : gases and gas laws.

Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:

Use the given volumes and the initial pressure to solve for the final pressure.


Example Question #1 : Using Boyle's Law
What law is the following formula?

Charles's law
Boyle's law
Combined gas law
Ideal gas law
Gay-Lussac's law
Boyle's law relates the pressure and volume of a system, which are inversely proportional to one another. When the parameters of a system change, Boyle's law helps us anticipate the effect the changes have on pressure and volume.

Example Question #13 : Gases And Gas Laws

To solve this question we will need to use Boyle's law:

We are given the final pressure and volume, along with the initial volume. Using these values, we can calculate the initial pressure.

Example Question #14 : Gases And Gas Laws
The graph depicted here represents which of the gas laws?

The graph shows that there is an inverse relationship between the volume and pressure of a gas, when kept at a constant temperature. This was described by Robert Boyle and can be represented mathematically as Boyle's law:

Gay-Lussac's law shows the relationship between pressure and temperature. Charles's law shows the relationship between volume and temperature. Hund's rule (Hund's law) is not related to gases, and states that electron orbitals of an element will be filled with single electrons before any electrons will form pairs within a single orbital.

We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.

A gas is initially in a 5L piston with a pressure of 1atm.
If pressure changes to 3.5atm by moving the piston down, what is new volume?

Use Boyle's Law:

Plug in known values and solve for final volume.

Use Boyle's law and plug in appropriate parameters:

Boyle's Law is:

Notice the answer has 3 significant figures.

Report an issue with this question
If you've found an issue with this question, please let us know. With the help of the community we can continue to improve our educational resources.
DMCA Complaint
If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing the information described below to the designated agent listed below. If Varsity Tutors takes action in response to an Infringement Notice, it will make a good faith attempt to contact the party that made such content available by means of the most recent email address, if any, provided by such party to Varsity Tutors.
Your Infringement Notice may be forwarded to the party that made the content available or to third parties such as ChillingEffects.org.
Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially misrepresent that a product or activity is infringing your copyrights. Thus, if you are not sure content located on or linked-to by the Website infringes your copyright, you should consider first contacting an attorney.
Please follow these steps to file a notice:
You must include the following:
A physical or electronic signature of the copyright owner or a person authorized to act on their behalf; An identification of the copyright claimed to have been infringed; A description of the nature and exact location of the content that you claim to infringe your copyright, in \ sufficient detail to permit Varsity Tutors to find and positively identify that content; for example we require a link to the specific question (not just the name of the question) that contains the content and a description of which specific portion of the question – an image, a link, the text, etc – your complaint refers to; Your name, address, telephone number and email address; and A statement by you: (a) that you believe in good faith that the use of the content that you claim to infringe your copyright is not authorized by law, or by the copyright owner or such owner’s agent; (b) that all of the information contained in your Infringement Notice is accurate, and (c) under penalty of perjury, that you are either the copyright owner or a person authorized to act on their behalf.
Send your complaint to our designated agent at:
Charles Cohn Varsity Tutors LLC 101 S. Hanley Rd, Suite 300 St. Louis, MO 63105
Or fill out the form below:
Contact Information
Complaint details.


- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Boyle’s Law – Definition, Formula, Example

Boyle’s law or Mariotte’s law states that pressure of an ideal gas is inversely proportional to volume under conditions of constant mass and temperature. When the gas volume increases, pressure decreases. When the volume decreases, pressure increases. Boyle’s law takes its name from chemist and physicist Robert Boyle , who published the law in 1862.
Boyle’s law states that the absolute pressure of an ideal gas is inversely proportional to its volume under conditions of constant mass and temperature.
Boyle’s Law Formula
There are three common formulas for Boyle’s law:
P ∝ 1/V PV = k P 1 V 1 = P 2 V 2
P is absolute pressure, V is volume, and k is a constant.
Graphing Boyle’s Law

The graph of volume versus pressure has a characteristic downward curved shape that shows the inverse relationship between pressure and volume. Boyle used the graph of experimental data to establish the relationship between the two variables.
Richard Towneley and Henry Power described the relationship between the pressure and volume of a gas in the 17th century. Robert Boyle experimentally confirmed their results using a device constructed by his assistant, Robert Hooke. The apparatus consisted of a closed J-shaped tube. Boyle poured mercury into the tube, decreasing the air volume and increasing its pressure. He used different amounts of mercury, recording air pressure and volume measurements, and graphed the data. Boyle published his results in 1662. Sometimes the gas law is called the Boyle-Mariotte law or Mariotte’s law because French physicist Edme Mariotte independently discovered the law in 1670.
Examples of Boyle’s Law in Everyday Life
There are examples of Boyle’s law in everyday life:
- The bends : A diver ascends to the water surface slowly to avoid the bends. As a diver rises to the surface, the pressure from the water decreases, which increases the volume of gases in the blood and joints. Ascending too quickly allows these gases to form bubbles, blocking blood flow and damaging joints and even teeth.
- Air bubbles : Similarly, air bubbles expand as they rise up a column of water. If you have a tall glass, you can watch bubble expand in volume as pressure decreases. One theory about why ships disappear in the Bermuda Triangle relates to Boyle’s law. Gases released from the seafloor rise and expand so much that they essentially become a gigantic bubble by the time they reach the surface. Small boats fall into the bubbles and are engulfed by the sea.
- Deep-sea fish : Deep-sea fish die if you bring them up to the surface. As outside pressure drops, the volume of gas within their swim bladder increases. Essentially, the fish blow up or pop.
- Syringe : Depressing the plunger on a sealed syringe decreases the air volume inside it and increases its pressure. Similarly, if you have a syringe containing a small amount of water and pull back on the plunger, the volume of air increases, but it’s pressure decreases. The pressure drop is enough to boil the water within the syringe at room temperature.
- Breathing: The diaphragm expands the volume of the lungs, causing a pressure drop that allows outside air to rush into the lungs (inhalation). Relaxing the diaphragm reduces the volume of the lungs, increasing the gas pressure within them. Exhaling occurs naturally to equalize pressure.
Boyle’s Law Example Problem
For example, calculate the final volume of a balloon if it has a volume of 2.0 L and pressure of 2 atmospheres and the pressure is reduced to 1 atmosphere. Assume temperature remains constant.
P 1 V 1 = P 2 V 2 (2 atm)(2.0 L) = (1 atm)V 2 V 2 = (2 atm)(2.0 L)/(1 atm) V 2 = 4.0 L
It’s a good idea to check your work to make sure the answer makes sense. In this example, the balloon pressure decreased by a factor of two (halved). The volume increased and doubled. This is what you expect from an inverse proportion relationship.
Most of the time, homework and test questions require reasoning rather than math. For example, if volume increases by a factor of 10, what happens to pressure? You know increasing volume decreases pressure by the same amount. Pressure decreases by a factor of 10.
See another Boyle’s law example problem .
- Fullick, P. (1994). Physics . Heinemann. ISBN 978-0-435-57078-1.
- Holton, Gerald James (2001). Physics, The Human Adventure: From Copernicus to Einstein and Beyond . Rutgers University Press. ISBN 978-0-8135-2908-0.
- Tortora, Gerald J.; Dickinson, Bryan (2006). ‘Pulmonary Ventilation’ in Principles of Anatomy and Physiology (11th ed.). Hoboken: John Wiley & Sons, Inc. pp. 863–867.
- Walsh, C.; Stride, E.; Cheema, U.; Ovenden, N. (2017). “A combined three-dimensional in vitro–in silico approach to modelling bubble dynamics in decompression sickness.” Journal of the Royal Society Interface . 14(137). doi: 10.1098/rsif.2017.0653
- Webster, Charles (1965). “The discovery of Boyle’s law, and the concept of the elasticity of air in seventeenth century”. Archive for the History of Exact Sciences . 2(6) : 441–502.
Related Posts

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.4: Boyle’s Law: Pressure and Volume
- Last updated
- Save as PDF
- Page ID 182712
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Learn what is meant by the term gas laws .
- Learn and apply Boyle’s Law.
When seventeenth-century scientists began studying the physical properties of gases, they noticed some simple relationships between some of the measurable properties of the gas. Take pressure ( P ) and volume ( V ), for example. Scientists noted that for a given amount of a gas (usually expressed in units of moles [ n ]), if the temperature ( T ) of the gas was kept constant, pressure and volume were related: as one increases, the other decreases. As one decreases, the other increases. This means that pressure and volume are inversely related .
There is more to it, however: pressure and volume of a given amount of gas at constant temperature are numerically related. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:
\[P × V = \text{ constant at constant n and T} \nonumber \]
If either volume or pressure changes while amount and temperature stay the same, then the other property must change so that the product of the two properties still equals that same constant. That is, if the original conditions are labeled \(P_1\) and \(V_1\) and the new conditions are labeled \(P_2\) and \(V_2\), we have
\[P_1V_1 = \text{constant} = P_2V_2 \nonumber \]
where the properties are assumed to be multiplied together. Leaving out the middle part, we have simply
\[P_1V_1 = P_2V_2 \text{ at constant n and T} \nonumber \]
This equation is an example of a gas law. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. This particular gas law is called Boyle's Law , after the English scientist Robert Boyle, who first announced it in 1662. Figure \(\PageIndex{1}\) shows two representations of how Boyle’s Law works.
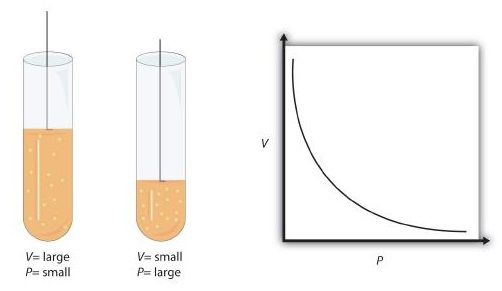
Boyle’s Law is an example of a second type of mathematical problem we see in chemistry—one based on a mathematical formula. Tactics for working with mathematical formulas are different from tactics for working with conversion factors. First, most of the questions you will have to answer using formulas are word-type questions, so the first step is to identify what quantities are known and assign them to variables. Second, in most formulas, some mathematical rearrangements (i.e., algebra) must be performed to solve for an unknown variable. The rule is that to find the value of the unknown variable, you must mathematically isolate the unknown variable by itself and in the numerator of one side of the equation. Finally, units must be consistent. For example, in Boyle’s Law there are two pressure variables; they must have the same unit. There are also two volume variables; they also must have the same unit. In most cases, it won’t matter what the unit is, but the unit must be the same on both sides of the equation.
Example \(\PageIndex{1}\)
A sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 L. Its pressure changes to 1.93 atm. What is the new volume if temperature and amount are kept constant?
Exercise \(\PageIndex{1}\)
If P 1 = 334 torr, V 1 = 37.8 mL, and P 2 = 102 torr, what is V 2 ?
As mentioned, you can use any units for pressure and volume, but both pressures must be expressed in the same units, and both volumes must be expressed in the same units.
Example \(\PageIndex{2}\):
A sample of gas has an initial pressure of 722 torr and an initial volume of 88.8 mL. Its volume changes to 0.663 L. What is the new pressure?
Exercise \(\PageIndex{2}\)
If V 1 = 456 mL, P 1 = 308 torr, and P 2 = 1.55 atm, what is V 2 ?
- The behavior of gases can be modeled with gas laws.
- Boyle’s Law relates the pressure and volume of a gas at constant temperature and amount.
V 1 / T 1 = V 2 / T 2
V 1 T 2 = V 2 T 1
(2.00 L) / 294.0 K) = (1.00 L) / (x) cross multiply to get: 2x = 293 x = 147.0 K Converting 147.0 K to Celsius, we find -126.0 °C, for a total decrease of 147.0 °C, from 21.0 °C to -126.0 °C.
(600.0 mL) / (293.0) = (x) / (333.0 K) x = 682 mL
(900.0 mL) / (300.0 K) = (x) / (405.0 K) x = 1215 mL
(60.0 mL) / (306.0 K) = (x) / (278.00 K) Cross multiply to get: 306x = 16680 x = 54.5 mL The volume decreases by 5.5 mL.
In cross-multiplied form, it is this: V 1 T 2 = V 2 T 1 V 2 = (V 1 T 2 ) / T 1 1 x = [(300.0 mL) (283.0 K)] / 290.0 K
In cross-multiplied form, it is this: V 1 T 2 = V 2 T 1 V 2 = (V 1 ) [T 2 / T 1 ] x = (1.00 L) [(606.0 K) / (273.0 K)] x = 2.22 L
(6.00 L) / (300.0 K) = (x) / (423.0 K) or (6.00 L) (423.0 K) = (x) (300.0 K)
V 2 = (V 1 ) [T 2 / T 1 ] x = (400.0 mL) [(400.0 K) / (498.0 K) x = 321 mL
(400.0 mL) / (498.0 K) = (x) / (400.0 K)
(8.00 L) / (483.0 K) = (x) / (250.0 K) Note how you can have a negative Celsius temperature, but not a negative Kelvin temperature.
V 1 / T 1 = V 2 / T 2 x / 588 K = 852 mL / 725 K (x) (725 K) = (852 mL) (588 K) x = 691 mL
2.05 L / 278 K = V 2 / 294 K Calculate V 2 . The volume that "escapes" is V 2 minus 2.05 L

IMAGES
VIDEO
COMMENTS
1) Let us use a ratio and proportion to estimate the pressure required for water to boil at 88 °C: 100 °C is to 101.3 kPa as 88 °C is to x. x = 89.144 kPa. 2) Now, we can solve the problem using Boyle's Law: P 1 V 1 = P 2 V 2. (101.3) (2.0) = (88.144) (x) x = 2.27 L. The balloon will not burst.
Boyle's Law Practice Problems Boyle's Law states: P 1 V 1 = P 2 V 2 1. If a gas at 75.0 °C occupies 13.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm? 2. A gas occupies 21.56 L at 71.00 atm. What will be the volume of this gas if the pressure becomes 35.00 atm? 3.
1. V. 1. = P. 2. V. 2. For the following problems calculate the value which the question asks for. Be sure to follow the proper steps and show all your work (1) Convert values (temp) to correct units if necessary and list variables (2) write the law (3) solve the law for desired variable (4) plug in the numbers with the correct units and (5 ...
Solve the following problems. 1. According to the graph, when the pressure of a gas sample is decreased what happens to the volume? 2. The gas in a 600 mL balloon has a pressure of 1.20 atm. ... Boyle's Law Name _____ Worksheet Boyle's Law 0 10 20 30 40 50 60 0 0.5 1 1.5 2 2.5 3 3.5 Pressure (atm) Volume (mL) Created Date:
Boyle's Law Practice Problems. Boyle's Law. Name_______________. You decide to climb to the tops of some of the tallest mountains. Before you are about to leave on your epic journey, a friend gives you a balloon with a volume of 800.0 cm3 that was inflated under standard atmospheric pressure. You climbed two different mountains with this ...
PROBLEM 7.2.1.11 7.2.1. 11. A high altitude balloon is filled with 1.41 × 10 4 L of hydrogen at a temperature of 21 °C and a pressure of 745 torr. What is the volume of the balloon at a height of 20 km, where the temperature is -48 °C and the pressure is 63.1 torr? Answer. Click here to see a video solution.
Boyle's Law Problem 1 Solution Plug into the formula and solve 0.98 (2.0) = 2.5 V 2 (0.98)(2.0) = (V 2) (2.5) 0.78 L = V 2. 13 Freon-12, CCl 2 F 2, is used in refrigeration systems. What is the new volume (L) of a 8.0 L sample of Freon gas initially at 550 mm Hg after its pressure is changed to
Figure 11.4.1 11.4. 1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second ...
In gas laws, temperatures must always be expressed in kelvins. 6.3 Gas Laws - Boyle's and Charles' Laws is shared under a license and was authored, remixed, and/or curated by LibreTexts. The behavior of gases can be modeled with gas laws. Boyle's law relates a gas's pressure and volume at constant temperature and amount.
Gas Law Worksheet #2 (Dalton's Law and Ideal Gas Law) Dalton's Law: PT = P1 + P2 + P3 + . . . 1. Determine the total pressure of a gas mixture that contains oxygen at a pressure of 150.mmHg, nitrogen at 350.mmHg pressure, and helium at a pressure of 200.mmHg. 2. A gas mixture containing oxygen, nitrogen, and carbon dioxide has a pressure of ...
1. A sample of oxygen gas occupies a volume of 250. mL at 101.3 kPa. What volume will it occupy at 152 kPa? 2. A sample of carbon dioxide occupies a volume of 3.50 L at 125 kPa. What pressure will the gas exert if the volume were decreased to 2.00 L? 3. A 2.0 liter container of nitrogen had a pressure of 3.2 atm.
WORKSHEET: BOYLE'S LAW WS #1 - Pressure & Volume (OBJECTIVE 4) Algebra Review: 1) Solve the following equations for X, show your algebra steps! 12 = 3X X = 7X = 57 X = 11X = 27.7 X = Boyles Law Problems: SHOW ALL WORK; INCLUDE UNITS IN YOUR ANSWERS! EQUATION: P1V1 = P2V2 ANSWERS: 1) Solve for the unknown variable:
P final = 1/z x V initial. Boyle's Law describes the relationship between pressure and volume of a gas when mass and temperature are held constant. (NASA) Example Problem. For example, calculate the final volume of a gas if the pressure of a 4.0 L sample is changed from 2.5 atm to 5.0 atm. You calculate z = P final /P initial. z = 5.0 / 2.5.
Gas Mass and Gas Temperature. Answer: (d), In Boyle's law, the mass of the gas its temperature are kept constant. Q5. Boyle's law is valid only for. Ideal gases. Non-ideal gases. Light Gases. Heavy Gases. Answer: (a), Boyle's law is valid only for ideal gases.
Boyle's gas law states that the volume of a gas is inversely proportional to the pressure of the gas when the temperature is held constant. Anglo-Irish chemist Robert Boyle (1627-1691) discovered the law and for it he is considered the first modern chemist. This example problem uses Boyle's law to find the volume of gas when pressure changes.
Problem #17: A gas occupies 25.3 mL at a pressure of 790.5 mmHg. Determine the volume if the pressure is reduced to 0.804 atm. Problem #18: A sample of gas has a volume of 12.0 L and a pressure of 1.00 atm. If the pressure of gas is increased to 2.00 atm, what is the new volume of the gas?
Correct answer: Explanation: Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's Law Example Problem. For example, calculate the final volume of a balloon if it has a volume of 2.0 L and pressure of 2 atmospheres and the pressure is reduced to 1 atmosphere. Assume temperature remains constant. P 1 V 1 = P 2 V 2. (2 atm) (2.0 L) = (1 atm)V 2.
Charles's Law Problems. 1. A gas sample at 40.0 C occupies a volume of 2.32 L. If the temperature is raised to 75.0 C, what will the volume be, assuming the pressure remains constant? 2. A gas at 89 C occupies a volume of 0.67 L. At what Celsius temperature will the volume increase to 1.12 L? 3.
PROBLEM \(\PageIndex{3}\) One way to state Boyle's law is "All other things being equal, the pressure of a gas is inversely proportional to its volume." (a) What is the meaning of the term "inversely proportional?" (b) What are the "other things" that must be equal? Answer a . The pressure of the gas increases as the volume ...
Figure 11.4.1 11.4. 1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second ...
Solution: Write Charles Law and substitute values in: V 1 / T 1 = V 2 / T 2. x / 588 K = 852 mL / 725 K. (x) (725 K) = (852 mL) (588 K) x = 691 mL. Note the large °C values, trying to get you to forget to add 273. Remember, only Kelvin temperatures are allowed in the calculations. Bonus Problem: An open "empty" 2 L plastic pop container, which ...