- Search Menu
- Sign in through your institution
- Advance articles
- Author Guidelines
- Submission Site
- Open Access
- Reasons to publish
- About Human Molecular Genetics
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, recent advances in understanding phenotypes associated with ds, recent advances in therapy and future prospects, acknowledgements.
- < Previous
Down syndrome—recent progress and future prospects
- Article contents
- Figures & tables
- Supplementary Data
Frances K. Wiseman, Kate A. Alford, Victor L.J. Tybulewicz, Elizabeth M.C. Fisher, Down syndrome—recent progress and future prospects, Human Molecular Genetics , Volume 18, Issue R1, 15 April 2009, Pages R75–R83, https://doi.org/10.1093/hmg/ddp010
- Permissions Icon Permissions
Down syndrome (DS) is caused by trisomy of chromosome 21 (Hsa21) and is associated with a number of deleterious phenotypes, including learning disability, heart defects, early-onset Alzheimer's disease and childhood leukaemia. Individuals with DS are affected by these phenotypes to a variable extent; understanding the cause of this variation is a key challenge. Here, we review recent research progress in DS, both in patients and relevant animal models. In particular, we highlight exciting advances in therapy to improve cognitive function in people with DS and the significant developments in understanding the gene content of Hsa21. Moreover, we discuss future research directions in light of new technologies. In particular, the use of chromosome engineering to generate new trisomic mouse models and large-scale studies of genotype–phenotype relationships in patients are likely to significantly contribute to the future understanding of DS.
Down syndrome (DS) is caused by trisomy of human chromosome 21 (Hsa21). Approximately 0.45% of human conceptions are trisomic for Hsa21 ( 1 ). The incidence of trisomy is influenced by maternal age and differs between populations (between 1 in 319 and 1 in 1000 live births are trisomic for Hsa21) ( 2 – 6 ). Trisomic fetuses are at an elevated risk of miscarriage, and people with DS have an increased risk of developing several medical conditions ( 7 ). Recent advances in medical treatment and social inclusion have significantly increased the life expectancy of people with DS. In economically developed countries, the average life span of people who are trisomic for Hsa21 is now greater than 55 years ( 8 ). In this review, we will discuss novel findings in the understanding of DS and highlight future important avenues of research.
Mouse models of Hsa21 trisomy and monosomy. Hsa21 (orange) is syntenic with regions of mouse chromosomes 16 (Mmu16, blue), 17 (Mmu 17, green) and 10 (Mmu10, grey). The Tc1 mouse model carries a freely segregating copy of Hsa21, which has two deleted regions, such that the model is trisomic for the majority of genes on Hsa21. The Dp1Yu, Ts65Dn, Ts1Cje and Ts1Rhr mouse models contain an additional copy of regions of mouse chromosome 16 that are syntenic with Hsa21, such that they are trisomic for a proportion of Hsa21 genes. The Ms1Rhr mouse model contains a deletion of a region of Mmu16; the Ms1Yah mouse model contains a deletion of a region of Mmu10. Hence, these models are monosomic for the genes in these deleted Hsa21 syntenic segments.
Box 1: What is a gene?
The definition of a gene has shifted over the past 100 years since it was first coined by Wilhelm Johannsen in 1909, based on the ideas of Mendel, de Vries, Correns and Tschermak. Their original theoretical definition of the gene being ‘the smallest unit of genetic inheritance’ remains the cornerstone of our understanding; however, the definition has grown with our knowledge of molecular biology. The gene has recently been defined as ‘a union of genomic sequences encoding a coherent set of potentially overlapping functional products’ ( 133 ). Splicing generates multiple transcripts from one gene. Moreover, exons from genes previously considered to be separate may be spliced together to generate novel transcripts ( 9 ). How to classify these fusion transcripts is a significant challenge. In addition, alternative transcription start sites that generate novel 5′ untranslated regions continue to be discovered, even for well-characterized genes ( 134 ). Although many of these novel transcripts are rare and their functional importance is not understood, our definition of a gene must encompass the observed diversity of the genome.
Trisomy of Hsa21 is associated with a small number of conserved features, occurring in all individuals, including mild-to-moderate learning disability, craniofacial abnormalities and hypotonia in early infancy ( 17 ). Although these phenotypes are always found in people with DS, the degree to which an individual is affected varies. Additionally, trisomy of Hsa21 is also associated with variant phenotypes that only affect some people with DS, including atrioventricular septal defects (AVSDs) in the heart, acute megakaryoblastic leukaemia (AMKL) and a decrease in the incidence of some solid tumours. This phenotypic variation is likely to be caused by a combination of environmental and genetic causes. Genetic polymorphisms in both Hsa21 and non-Hsa21 genes may account for much of this variation. Genome-wide association studies to identify these polymorphisms constitute a promising strategy to gain novel insights into the pathology of DS.
A central goal of DS research is to understand which of the genes on Hsa21, when present in three copies, lead to each of the different DS-associated phenotypes, and to elucidate how increased expression leads to the molecular, cellular and physiological changes underlying DS pathology. Two distinct approaches are being taken to address these issues. First, genomic association studies, such as that recently published by Lyle et al ( 18 )., may point to genes that play an important role in pathology. Secondly, a number of animal models of Hsa21 trisomy have been generated. Recent advances in chromosome engineering have led to the establishment of mice trisomic for different sets of mouse genes syntenic to Hsa21, and a mouse strain, Tc(Hsa21)1TybEmcf (Tc1), carrying most of Hsa21, as a freely segregating chromosome (Fig. 1 ) ( 19 – 27 ). These strains are being used both to map dosage-sensitive genes on Hsa21 and to understand pathological mechanisms. Here, we review recent advances in the understanding of DS-associated phenotypes and the development of therapeutic strategies to treat them.
Development
Trisomy of Hsa21 has a significant impact on the development of many tissues, most notably the heart and the brain. A recent paper has suggested that trisomy of the Hsa21 genes, dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1A ( DYRK1A ) and regulator of calcineurin 1 ( RCAN1 ), may have an impact on the development of multiple tissues ( 28 ). DYRK1A is a priming kinase that facilitates the further phosphorylation of numerous proteins by other kinases (Fig. 2 ) ( 29 – 38 ). It is up-regulated in a number of tissues from people with DS ( 39 , 40 ). RCAN1 is a regulator of the protein phosphatase calcineurin ( 41 ). Crabtree and colleagues hypothesized that trisomy of these two genes may act synergistically to alter signalling via the NFAT family of transcription factors ( 28 ). In an independent study, increased DYRK1A gene dosage was shown to decrease the expression level of RE1-silencing transcription factor ( REST ) ( 42 ). As REST is required both to maintain pluripotency and to facilitate neuronal differentiation, a perturbation in REST expression may alter the development of many cell types. Indeed, over-expression of DYRK1A in some animal models is associated with a number of phenotypes, including heart defects and abnormal learning and memory ( 28 , 33 , 43 – 45 ). However, not all animal models that over-express DYRK1A exhibit these defects, suggesting that polymorphisms or differences in the expression of other genes influence the outcome of DYRK1A trisomy ( 24 ).
Phosphorylation targets of DYRK1A. The Hsa21-encoded kinase DYRK1A has been shown to phosphorylate a multitude of targets, which have been implicated in a number of biological processes and DS-associated phenotypes, including endocytosis and AD.
Trisomy of Hsa21 is associated with a reduction in brain volume, the size of the hippocampus and cerebellum being particularly affected ( 46 – 49 ). A similar phenotype is also observed in the Ts65Dn model ( 50 ). Recent studies have started to elucidate the developmental mechanisms underlying these important phenotypes. Trisomic granule cell precursors from the cerebellum have a reduced mitogenic response to the morphogen sonic hedgehog ( 51 ). This was shown to underlie the reduced number of cerebellar granular cells observed in the Ts65Dn mouse model of DS. Hypocellularity in the hippocampus also has a developmental origin ( 52 , 53 ). Abnormalities in cell-cycle length, apoptosis and neocortical neurogenesis have been shown to contribute to this phenotype ( 53 – 55 ). The reduced level of neurogenesis in Ts65Dn adult hippocampus can be ameliorated by treatment with the anti-depressant fluoxetine, which is a serotonin reuptake inhibitor ( 56 ). Fluoxetine may promote neurogenesis via a number of potential mechanisms, including a direct effect on serotonin levels or via an indirect effect on behaviour. Whether this drug has similar effect during embryonic development has yet to be determined.
Ts65Dn pups exhibit a delay in attaining several developmental milestones, such as forelimb grip and the righting reflex, mimicking the developmental delay observed in babies with DS ( 57 ). A recent report has demonstrated that treatment of Ts65Dn embryos with two neuroprotective peptides reduced the delay in achieving a number of sensory and motor developmental milestones during early post-natal development ( 58 ).
People with DS exhibit craniofacial dysmorphology, including a mandible of reduced size. This phenotype is also observed in the Ts65Dn and Tc1 models ( 26 , 59 ). In the Ts65Dn model, craniofacial dysmorphology is present from early post-natal development and may be related to specific changes in bone development ( 60 , 61 ). The small mandible in people with DS may be caused by migration and proliferation defects in mandible precursor (neural crest) cells in the developing embryo, related to an altered response to sonic hedgehog ( 62 ).
Learning and memory
All people with DS have a mild-to-moderate learning disability. Over-expression of a number of Hsa21 genes, including DYRK1A, synaptojanin 1 and single-minded homologue 2 (SIM2), results in learning and memory defects in mouse models, suggesting that trisomy of these genes may contribute to learning disability in people with DS ( 43 , 45 , 63 , 64 ). In addition, trisomy of neuronal channel proteins, such as G-protein-coupled inward-rectifying potassium channel subunit 2 ( GIRK2 ), may also influence learning in people with DS ( 65 – 67 ). Recent work has demonstrated that trisomy of a segment of mouse chromosome 16 ( Mmu16 ) containing 33 genes including DYRK1A , GIRK2 and SIM2 was necessary, but not sufficient for the hippocampal-based learning deficits in the Ts65Dn mouse model ( 68 ). These data indicate that trisomy of multiple Hsa21 genes is required for the deficits in learning associated with DS. Moreover, Hsa21 trisomy may independently impact on multiple learning pathways.
Recent work on the Tc1 transchromosomic mouse model of DS has examined in detail the learning pathways affected by trisomy of Hsa21 ( 26 , 69 ). The Tc1 transchromosomic model exhibits abnormalities in short-term but not in long-term hippocampal-dependent learning. The learning deficits are correlated with specific abnormalities in long-term potentiation (LTP) in the dentate gyrus of the hippocampus. LTP is an electrophysiological process proposed to be the cellular basis of learning and memory ( 70 ). These data provide insight into which learning mechanisms may be affected by Hsa21 trisomy and can be used to further understand their genetic cause. Structural abnormalities may contribute to these deficits in learning and memory. Indeed, a correlation between specific synaptic abnormalities in the hippocampus of the Ts(16C-tel)1Cje (Ts1Cje) mouse and a defect in LTP has been reported ( 71 ). Moreover, a recent paper has demonstrated an alteration in the amounts of a number of synaptic components in the hippocampus of the Ts65Dn mouse ( 72 ).
Alzheimer's disease
People with DS have a greatly increased risk of early-onset Alzheimer's disease (AD). By the age of 60, between 50 and 70% of the people with DS develop dementia ( 73 – 77 ). The known AD risk factor amyloid precursor protein ( APP ) is encoded on Hsa21. Trisomy of APP is likely to make a significant contribution to the increased frequency of dementia in people with DS. Indeed, triplication of a short segment of Hsa21 that includes APP in people without DS has been recently shown to be associated with early-onset AD. A number of features of neurodegeneration have been observed in mouse models of DS ( 78 – 86 ). Loss of basal forebrain cholinergic neurons (BFCNs) occurs early in AD and also is observed in the Ts65Dn mouse model ( 87 ). Degeneration of BFCNs in Ts65Dn mice is dependent on trisomy of APP and is mediated by the effect of increased APP expression of retrograde axonal transport ( 83 ).
Hsa21 genes other than APP may also contribute to the early onset of AD in people with DS ( 33 , 34 , 40 , 88 – 97 ). Indeed, the Ts1Cje mouse model, which is not trisomic for APP , exhibits tau hyperphosphorylation, an early sign of AD ( 98 ). Recent evidence suggests that trisomy of DYRK1A may contribute to the development of AD in people with DS. DYRK1A can phosphorylate Tau at a key priming site that permits its hyperphosphorylation ( 33 , 36 , 40 , 95 ). DYRK1A may also influence the alternative splicing of Tau and the phosphorylation of APP ( 34 , 99 ). A reduction in the level of protein phosphatase 2A and a decrease in the activity of α-secretase in the brains of people with DS have also been reported, both of which may contribute to AD in this population ( 94 , 100 ). Further studies are required to determine the identity of the trisomic genes that contribute to these phenotypes.
Heart defects
Trisomy of Hsa21 is associated with a number of congenital heart defects, the most common being AVSD that occurs in ∼20% of the people with DS ( 101 ). Mutations in the non-Hsa21 CRELD1 gene may contribute to the development of AVSD in DS ( 102 ). CRELD1 has also been linked to AVSDs by mapping the deletion breakpoints, on chromosome 3, in people with 3p-syndrome. Further studies are required to determine the identity of other genes that are important for heart development in people with DS. A number of Hsa21 trisomy mouse models exhibit heart defects similar to those observed in DS, suggesting that trisomy of one or more of the approximately 100 genes common to these models influences development of the heart ( 22 , 26 , 103 , 104 ).
Leukaemia and cancer
DS increases the risk of developing AMKL and acute lymphoblastic leukaemia (ALL). Approximately 10% of the DS newborns present with a transient myeloproliferative disorder (TMD), characterized by a clonal population of megakaryoblasts in the blood. This transient disease usually spontaneously resolves; however, 10–20% of the DS patients with TMD develop AMKL before 4 years of age (reviewed in 105 ). The development of TMD requires both trisomy 21 and mutations in the transcription factor GATA1 ( 106 , 107 ). It is likely that further mutations are required for TMD to develop into AMKL. The GATA1 mutations found in TMD and AMKL always have the same effect, causing translation to initiate at the second ATG of the coding region, leading to the production of a shorter protein, termed GATA1s. Trisomy of Hsa21 on its own, even in the absence of GATA1s, leads to an expansion of the megakaryocyte-erythroid progenitor population in fetal livers from human DS abortuses ( 108 , 109 ). These data suggest that trisomy of Hsa21 perturbs hematopoiesis, making megakaryocyte-erythroid progenitors susceptible to the effects of GATA1s, thereby promoting development of TMD. Several groups have reported the presence of mutations in Janus Kinase 3 ( JAK3 ) in a small proportion of TMD/AMKL patients ( 110 – 115 ). It was suggested that JAK3 inhibitors could be used as a therapy ( 111 , 114 ). However, both loss- and gain-of-function mutations have been found, so this may not be a viable treatment. Stem cell factor/KIT signalling has recently been demonstrated to stimulate TMD blast cell proliferation, and inhibitors of this pathway may be a treatment for severe TMD ( 116 ).
Attempts have been made to model these disorders in mice with a view to establishing which genes on Hsa21 need to be present in three copies in order to induce disease. A study of the Ts65Dn mouse model showed that it developed a late-onset myeloproliferative disorder, but did not develop leukaemia ( 117 ). It may be that the Ts65Dn model is not trisomic for the relevant dosage-sensitive genes required for the development of AMKL or that the expression of a mutant form of GATA1 will be required to increase the frequency of leukaemogenesis in this mouse model of DS.
The genetic events involved in DS-ALL are less well understood than those in DS-AMKL. A number of studies have reported DS-ALL cases with chromosomal abnormalities, gain-of-function mutations in JAK2 and submicroscopic deletions of genes including ETV6 , CDKN2A and PAX5 ( 118 – 121 ).
Although the incidence of leukaemia and cancer of the testis are increased in DS, the risk of developing most solid tumours is reduced ( 122 , 123 ). Crossing mouse models of DS with mice heterozygous for the Apc min mutation reduced the number of tumours, which would normally accumulate in this model of colon cancer ( 124 ). Protection against the development of tumours required three copies of the Hsa21 ‘proto-oncogene’ Ets2 , suggesting that in this context, Ets2 may be acting as a tumour suppressor ( 124 ).
Hypertension
People with DS have been reported to have a reduced incidence of hypertension ( 125 , 126 ). Trisomy of the Hsa21 microRNA hsa-miR-155 may contribute to this ( 12 ). Hsa-miR-155 is proposed to specifically target one allele of the type-1 angiotensin II receptor ( AGTR1 ) gene, resulting in its under-expression, which may contribute to a reduced risk of hypertension. Further studies are required to validate this hypothesis and determine whether other genes may also protect people with DS against hypertension.
Recent interest in therapy for people with DS has focused on pharmacological treatment to enhance cognition. A number of compounds have been shown to improve learning in the Ts65Dn mouse model. Chronic treatment with picrotoxin or pentylenetetrazole improved hippocampal-based learning and LTP deficits in Ts65Dn mice, even after treatment had ceased ( 127 ). These compounds reduce gamma-aminobutyric acid-mediated inhibition in the hippocampus and are proposed to improve cognition by releasing normal learning from excess inhibition. Learning in Ts65Dn mice is also improved by the non-competitive N-methyl-D-aspartic acid receptor (NMDAR) antagonist, memantine ( 128 ). Memantine partially inhibits the opening of the NMDAR and is proposed to counter the effect of trisomy of RCAN1 on the function of the receptor. Further studies and clinical trials are required to further investigate the potential of these drugs to improve cognition in people who have DS.
To develop new therapeutic targets, it is necessary to determine the identity of genes that contribute to DS phenotypes. This requires a precise and standardized definition of phenotype. Ideally, these measurements should be formulated into a standardized protocol that can be applied at multiple centres, to permit sufficiently large numbers of samples for meaningful analysis to be collected. This can be facilitated by a carefully designed and curated biobank of detailed phenotypic data alongside DNA and tissue samples from participating individuals. These collections can then be used for both candidate gene and genome-wide analyses, by different investigators, permitting the identification of both dosage-sensitive trisomic Hsa21 and non-Hsa21 genes that contribute to DS phenotypes. Pooling of large data sets has led to recent important findings in the study of schizophrenia, diabetes and obesity, illustrating the importance of large-scale collaboration ( 129 – 132 ). The careful collection of additional patient data will add much to our current understanding of DS.
As recent progress demonstrates, mouse models can be used in parallel with data collected from people with DS to test genetic associations, to explore biological mechanisms and to trial therapies. In addition to the long-standing Ts65Dn and Ts1Cje models, the newly developed mouse strains such as Tc1, Dp1Yu and Ts1Rhr have generated a range of models with distinct sets of trisomic genes (Fig. 1 ) ( 19 – 27 ). Furthermore, the crossing of these strains with mice-bearing deletions of chromosomal segments syntenic to Hsa21, such as Ms1Yah and Ms1Rhr (Fig. 1 ), will allow systematic mapping and eventually identification of the dosage-sensitive genes causing DS-associated pathology.
DS was once thought to be an intractable condition because of the genetic complexity underlying it. Here, we have described recently reported breakthroughs in the understanding of Hsa21 trisomy, illustrating that research efforts in this field are making significant strides to understand and to develop treatments for the debilitating aspects of the syndrome. Many issues vital to the health and well-being of people with DS remain to be studied, making this an important and exciting time for Hsa21 trisomy research.
V.L.J.T. and K.A.A. are funded by the UK Medical Research Council, the EU, the Leukaemia Research Fund and the Wellcome Trust; F.K.W. and E.M.C.F. are funded by the UK Medical Research Council, the Wellcome Trust and the Fidelity Foundation.
We thank Roger Reeves, Dalia Kasperaviciute, Olivia Sheppard and Matilda Haas for advice on the manuscript and we thank Ray Young for help with preparation of the figures. We apologize to the many authors whose work we were unable to cite owing to space limitations.
Conflict of Interest statement . None declared.
Hassold T. Abruzzo M. Adkins K. Griffin D. Merrill M. Millie E. Saker D. Shen J. Zaragoza M. Human aneuploidy: incidence, origin, and etiology Environ. Mol. Mutagen. 1996 28 167 175
Google Scholar
O'Nuallain S. Flanagan O. Raffat I. Avalos G. Dineen B. The prevalence of Down syndrome in County Galway Ir. Med. J. 2007 100 329 331
Carothers A.D. Hecht C.A. Hook E.B. International variation in reported livebirth prevalence rates of Down syndrome, adjusted for maternal age J. Med. Genet. 1999 36 386 393
Canfield M.A. Honein M.A. Yuskiv N. Xing J. Mai C.T. Collins J.S. Devine O. Petrini J. Ramadhani T.A. Hobbs C.A. et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001 Birth Defects Res. A Clin. Mol. Teratol. 2006 76 747 756
Murthy S.K. Malhotra A.K. Mani S. Shara M.E. Al Rowaished E.E. Naveed S. Alkhayat A.I. Alali M.T. Incidence of Down syndrome in Dubai, UAE Med. Princ. Pract. 2007 16 25 28
Wahab A.A. Bener A. Teebi A.S. The incidence patterns of Down syndrome in Qatar Clin. Genet. 2006 69 360 362
Morris J.K. Wald N.J. Watt H.C. Fetal loss in Down syndrome pregnancies Prenat. Diagn. 1999 19 142 145
Glasson E.J. Sullivan S.G. Hussain R. Petterson B.A. Montgomery P.D. Bittles A.H. The changing survival profile of people with Down's syndrome: implications for genetic counselling Clin. Genet. 2002 62 390 393
Birney E. Stamatoyannopoulos J.A. Dutta A. Guigo R. Gingeras T.R. Margulies E.H. Weng Z. Snyder M. Dermitzakis E.T. Thurman R.E. et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project Nature 2007 447 799 816
Gardiner K. Costa A.C. The proteins of human chromosome 21 Am. J. Med. Genet. C Semin. Med. Genet. 2006 142C 196 205
Kuhn D.E. Nuovo G.J. Martin M.M. Malana G.E. Pleister A.P. Jiang J. Schmittgen T.D. Terry A.V. Jr Gardiner K. Head E. et al. Human chromosome 21-derived miRNAs are overexpressed in Down syndrome brains and hearts Biochem. Biophys. Res. Commun. 2008 370 473 477
Sethupathy P. Borel C. Gagnebin M. Grant G.R. Deutsch S. Elton T.S. Hatzigeorgiou A.G. Antonarakis S.E. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes Am. J. Hum. Genet. 2007 81 405 413
Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function Cell 2004 116 281 297
Prandini P. Deutsch S. Lyle R. Gagnebin M. Delucinge V.C. Delorenzi M. Gehrig C. Descombes P. Sherman S. Dagna B.F. et al. Natural gene-expression variation in Down syndrome modulates the outcome of gene–dosage imbalance Am. J. Hum. Genet. 2007 81 252 263
Ait Yahya-Graison E. Aubert J. Dauphinot L. Rivals I. Prieur M. Golfier G. Rossier J. Personnaz L. Creau N. Blehaut H. et al. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes Am. J. Hum. Genet. 2007 81 475 491
Sultan M. Piccini I. Balzereit D. Herwig R. Saran N.G. Lehrach H. Reeves R.H. Yaspo M.L. Gene expression variation in Down's syndrome mice allows prioritization of candidate genes Genome Biol. 2007 8 R91
Antonarakis S.E. Lyle R. Dermitzakis E.T. Reymond A. Deutsch S. Chromosome 21 and Down syndrome: from genomics to pathophysiology Nat. Rev. Genet. 2004 5 725 738
Lyle R. Bena F. Gagos S. Gehrig C. Lopez G. Schinzel A. Lespinasse J. Bottani A. Dahoun S. Taine L. et al. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21 Eur. J. Hum. Genet. 2008 advance online publication 12 November 2008; doi: 10.1038/ejhg.2008.214
Adams D.J. Biggs P.J. Cox T. Davies R. van der W.L. Jonkers J. Smith J. Plumb B. Taylor R. Nishijima I. et al. Mutagenic insertion and chromosome engineering resource (MICER) Nat. Genet. 2004 36 867 871
Brault V. Besson V. Magnol L. Duchon A. Herault Y. Cre/loxP-mediated chromosome engineering of the mouse genome Handb. Exp. Pharmacol. 2007 178 29 48
Duchon A. Besson V. Pereira P.L. Magnol L. Herault Y. Inducing segmental aneuploid mosaicism in the mouse through targeted asymmetric sister chromatid event of recombination Genetics 2008 180 51 59
Li Z. Yu T. Morishima M. Pao A. LaDuca J. Conroy J. Nowak N. Matsui S. Shiraishi I. Yu Y.E. Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities Hum. Mol. Genet. 2007 16 1359 1366
Tybulewicz V.L. Fisher E.M. New techniques to understand chromosome dosage: mouse models of aneuploidy Hum. Mol. Genet. 2006 15 Spec no. 2 R103 R109
Olson L.E. Richtsmeier J.T. Leszl J. Reeves R.H. A chromosome 21 critical region does not cause specific down syndrome phenotypes Science 2004 306 687 690
Brault V. Pereira P. Duchon A. Herault Y. Modeling chromosomes in mouse to explore the function of genes, genomic disorders, and chromosomal organization PLoS Genet. 2006 2 e86
O'Doherty A. Ruf S. Mulligan C. Hildreth V. Errington M.L. Cooke S. Sesay A. Modino S. Vanes L. Hernandez D. et al. An aneuploid mouse strain carrying human chromosome 21 with down syndrome phenotypes Science 2005 309 2033 2037
Besson V. Brault V. Duchon A. Togbe D. Bizot J.C. Quesniaux V.F. Ryffel B. Herault Y. Modeling the monosomy for the telomeric part of human chromosome 21 reveals haploinsufficient genes modulating the inflammatory and airway responses Hum. Mol. Genet. 2007 16 2040 2052
Arron J.R. Winslow M.M. Polleri A. Chang C.P. Wu H. Gao X. Neilson J.R. Chen L. Heit J.J. Kim S.K. et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21 Nature 2006 441 595 600
de Graaf K. Hekerman P. Spelten O. Herrmann A. Packman L.C. Bussow K. Muller-Newen G. Becker W. Characterization of cyclin L2, a novel cyclin with an arginine/serine-rich domain: phosphorylation by DYRK1A and colocalization with splicing factors J. Biol. Chem. 2004 279 4612 4624
de Graaf K. Czajkowska H. Rottmann S. Packman L.C. Lilischkis R. Luscher B. Becker W. The protein kinase DYRK1A phosphorylates the splicing factor SF3b1/SAP155 at Thr434, a novel in vivo phosphorylation site BMC Biochem. 2006 7 7
Adayev T. Chen-Hwang M.C. Murakami N. Wang R. Hwang Y.W. MNB/DYRK1A phosphorylation regulates the interactions of synaptojanin 1 with endocytic accessory proteins Biochem. Biophys. Res. Commun. 2006 351 1060 1065
Kim E.J. Sung J.Y. Lee H.J. Rhim H. Hasegawa M. Iwatsubo T. Min d.S. Kim J. Paik S.R. Chung K.C. Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation J. Biol. Chem. 2006 281 33250 33257
Ryoo S.R. Jeong H.K. Radnaabazar C. Yoo J.J. Cho H.J. Lee H.W. Kim I.S. Cheon Y.H. Ahn Y.S. Chung S.H. et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease J. Biol. Chem. 2007 282 34850 34857
Ryoo S.R. Cho H.J. Lee H.W. Jeong H.K. Radnaabazar C. Kim Y.S. Kim M.J. Son M.Y. Seo H. Chung S.H. et al. Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: evidence for a functional link between Down syndrome and Alzheimer's disease J. Neurochem. 2008 104 1333 1344
Huang Y. Chen-Hwang M.C. Dolios G. Murakami N. Padovan J.C. Wang R. Hwang Y.W. Mnb/Dyrk1A phosphorylation regulates the interaction of dynamin 1 with SH3 domain-containing proteins Biochemistry 2004 43 10173 10185
Woods Y.L. Cohen P. Becker W. Jakes R. Goedert M. Wang X. Proud C.G. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase Biochem. J. 2001 355 609 615
Aranda S. Alvarez M. Turro S. Laguna A. de la L.S. Sprouty2-mediated inhibition of fibroblast growth factor signaling is modulated by the protein kinase DYRK1A Mol. Cell. Biol. 2008 28 5899 5911
Gwack Y. Sharma S. Nardone J. Tanasa B. Iuga A. Srikanth S. Okamura H. Bolton D. Feske S. Hogan P.G. et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT Nature 2006 441 646 650
Dowjat W.K. Adayev T. Kuchna I. Nowicki K. Palminiello S. Hwang Y.W. Wegiel J. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome Neurosci. Lett. 2007 413 77 81
Liu F. Liang Z. Wegiel J. Hwang Y.W. Iqbal K. Grundke-Iqbal I. Ramakrishna N. Gong C.X. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome FASEB J. 2008 22 3224 3233
Fuentes J.J. Genesca L. Kingsbury T.J. Cunningham K.W. Perez-Riba M. Estivill X. de la L.S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways Hum. Mol. Genet. 2000 9 1681 1690
Canzonetta C. Mulligan C. Deutsch S. Ruf S. O'Doherty A. Lyle R. Borel C. Lin-Marq N. Delom F. Groet J. et al. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome Am. J. Hum. Genet. 2008 83 388 400
Altafaj X. Dierssen M. Baamonde C. Marti E. Visa J. Guimera J. Oset M. Gonzalez J.R. Florez J. Fillat C. et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome Hum. Mol. Genet. 2001 10 1915 1923
Martinez D.L. Altafaj X. Gallego X. Marti E. Estivill X. Sahun I. Fillat C. Dierssen M. Motor phenotypic alterations in TgDyrk1a transgenic mice implicate DYRK1A in Down syndrome motor dysfunction Neurobiol. Dis. 2004 15 132 142
Ahn K.J. Jeong H.K. Choi H.S. Ryoo S.R. Kim Y.J. Goo J.S. Choi S.Y. Han J.S. Ha I. Song W.J. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects Neurobiol. Dis. 2006 22 463 472
Weis S. Weber G. Neuhold A. Rett A. Down syndrome: MR quantification of brain structures and comparison with normal control subjects AJNR Am. J. Neuroradiol. 1991 12 1207 1211
Aylward E.H. Habbak R. Warren A.C. Pulsifer M.B. Barta P.E. Jerram M. Pearlson G.D. Cerebellar volume in adults with Down syndrome Arch. Neurol. 1997 54 209 212
Pearlson G.D. Breiter S.N. Aylward E.H. Warren A.C. Grygorcewicz M. Frangou S. Barta P.E. Pulsifer M.B. MRI brain changes in subjects with Down syndrome with and without dementia Dev. Med. Child Neurol. 1998 40 326 334
Aylward E.H. Li Q. Honeycutt N.A. Warren A.C. Pulsifer M.B. Barta P.E. Chan M.D. Smith P.D. Jerram M. Pearlson G.D. MRI volumes of the hippocampus and amygdala in adults with Down's syndrome with and without dementia Am. J. Psychiatry 1999 156 564 568
Aldridge K. Reeves R.H. Olson L.E. Richtsmeier J.T. Differential effects of trisomy on brain shape and volume in related aneuploid mouse models Am. J. Med. Genet. A 2007 143A 1060 1070
Roper R.J. Baxter L.L. Saran N.G. Klinedinst D.K. Beachy P.A. Reeves R.H. Defective cerebellar response to mitogenic Hedgehog signaling in Down syndrome mice Proc. Natl Acad. Sci. USA 2006 103 1452 1456
Lorenzi H.A. Reeves R.H. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development Brain Res. 2006 1104 153 159
Guidi S. Bonasoni P. Ceccarelli C. Santini D. Gualtieri F. Ciani E. Bartesaghi R. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome Brain Pathol. 2008 18 180 197
Contestabile A. Fila T. Ceccarelli C. Bonasoni P. Bonapace L. Santini D. Bartesaghi R. Ciani E. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice Hippocampus 2007 17 665 678
Clark S. Schwalbe J. Stasko M.R. Yarowsky P.J. Costa A.C. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome Exp. Neurol. 2006 200 256 261
Holtzman D.M. Santucci D. Kilbridge J. Chua-Couzens J. Fontana D.J. Daniels S.E. Johnson R.M. Chen K. Sun Y. Carlson E. et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome Proc. Natl Acad. Sci. USA 1996 93 13333 13338
Toso L. Cameroni I. Roberson R. Abebe D. Bissell S. Spong C.Y. Prevention of developmental delays in a Down syndrome mouse model Obstet. Gynecol. 2008 112 1242 1251
Richtsmeier J.T. Baxter L.L. Reeves R.H. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice Dev. Dyn. 2000 217 137 145
Hill C.A. Reeves R.H. Richtsmeier J.T. Effects of aneuploidy on skull growth in a mouse model of Down syndrome J Anat. 2007 210 394 405
Parsons T. Ryan T.M. Reeves R.H. Richtsmeier J.T. Microstructure of trabecular bone in a mouse model for Down syndrome Anat. Rec. (Hoboken.) 2007 290 414 421
Roper R.J. Vanhorn J.F. Cain C.C. Reeves R.H. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog Mech. Dev 2008 Published online 21 November, doi: 10.1016/j.mod.2008.11.002
Voronov S.V. Frere S.G. Giovedi S. Pollina E.A. Borel C. Zhang H. Schmidt C. Akeson E.C. Wenk M.R. Cimasoni L. et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome Proc. Natl Acad. Sci. USA 2008 105 9415 9420
Meng X. Peng B. Shi J. Zheng Y. Chen H. Zhang J. Li L. Zhang C. Effects of overexpression of Sim2 on spatial memory and expression of synapsin I in rat hippocampus Cell Biol. Int. 2006 30 841 847
Best T.K. Cho-Clark M. Siarey R.J. Galdzicki Z. Speeding of miniature excitatory post-synaptic currents in Ts65Dn cultured hippocampal neurons Neurosci. Lett. 2008 438 356 361
Best T.K. Siarey R.J. Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current J. Neurophysiol. 2007 97 892 900
Harashima C. Jacobowitz D.M. Witta J. Borke R.C. Best T.K. Siarey R.J. Galdzicki Z. Abnormal expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in hippocampus, frontal cortex, and substantia nigra of Ts65Dn mouse: a model of Down syndrome J. Comp. Neurol. 2006 494 815 833
Olson L.E. Roper R.J. Sengstaken C.L. Peterson E.A. Aquino V. Galdzicki Z. Siarey R. Pletnikov M. Moran T.H. Reeves R.H. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice Hum. Mol. Genet. 2007 16 774 782
Morice E. Andreae L.C. Cooke S.F. Vanes L. Fisher E.M. Tybulewicz V.L. Bliss T.V. Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome Learn. Mem. 2008 15 492 500
Bliss T.V. Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path J. Physiol. 1973 232 331 356
Belichenko P.V. Kleschevnikov A.M. Salehi A. Epstein C.J. Mobley W.C. Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype–phenotype relationships J. Comp. Neurol. 2007 504 329 345
Belichenko P.V. Kleschevnikov A.M. Masliah E. Wu C. Takimoto-Kimura R. Salehi A. Mobley W.C. Excitatory–inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of down syndrome J. Comp. Neurol. 2008 512 453 466
Holland A.J. Hon J. Huppert F.A. Stevens F. Incidence and course of dementia in people with Down's syndrome: findings from a population-based study J. Intellect. Disabil. Res. 2000 44 138 146
Holland A.J. Hon J. Huppert F.A. Stevens F. Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down's syndrome Br. J. Psychiatry 1998 172 493 498
Janicki M.P. Dalton A.J. Prevalence of dementia and impact on intellectual disability services Ment. Retard. 2000 38 276 288
Johannsen P. Christensen J.E. Mai J. The prevalence of dementia in Down syndrome Dementia 1996 7 221 225
Lai F. Williams R.S. A prospective study of Alzheimer disease in Down syndrome Arch. Neurol. 1989 46 849 853
Granholm A.C. Sanders L.A. Crnic L.S. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome Exp. Neurol. 2000 161 647 663
Granholm A.C. Ford K.A. Hyde L.A. Bimonte H.A. Hunter C.L. Nelson M. Albeck D. Sanders L.A. Mufson E.J. Crnic L.S. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome Physiol. Behav. 2002 77 371 385
Hunter C.L. Bimonte H.A. Granholm A.C. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory Behav. Brain Res. 2003 138 121 131
Hunter C.L. Bachman D. Granholm A.C. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome Ann. Neurol. 2004 56 675 688
Necchi D. Lomoio S. Scherini E. Axonal abnormalities in cerebellar Purkinje cells of the Ts65Dn mouse Brain Res. 2008 1238 181 188
Salehi A. Delcroix J.D. Belichenko P.V. Zhan K. Wu C. Valletta J.S. Takimoto-Kimura R. Kleschevnikov A.M. Sambamurti K. Chung P.P. et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration Neuron 2006 51 29 42
Cooper J.D. Salehi A. Delcroix J.D. Howe C.L. Belichenko P.V. Chua-Couzens J. Kilbridge J.F. Carlson E.J. Epstein C.J. Mobley W.C. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion Proc. Natl Acad. Sci. USA 2001 98 10439 10444
Seo H. Isacson O. Abnormal APP, cholinergic and cognitive function in Ts65Dn Down's model mice Exp. Neurol. 2005 193 469 480
Holtzman D.M. Li Y. Chen K. Gage F.H. Epstein C.J. Mobley W.C. Nerve growth factor reverses neuronal atrophy in a Down syndrome model of age-related neurodegeneration Neurology 1993 43 2668 2673
Mann D.M. Yates P.O. Marcyniuk B. Ravindra C.R. Pathological evidence for neurotransmitter deficits in Down's syndrome of middle age J. Ment. Defic. Res. 1985 29 125 135
Porta S. Serra S.A. Huch M. Valverde M.A. Llorens F. Estivill X. Arbones M.L. Marti E. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration Hum. Mol. Genet. 2007 16 1039 1050
Ermak G. Morgan T.E. Davies K.J. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease J. Biol. Chem. 2001 276 38787 38794
Ermak G. Davies K.J. DSCR1(Adapt78)—a Janus gene providing stress protection but causing Alzheimer's disease? IUBMB Life 2003 55 29 31
Ermak G. Harris C.D. Battocchio D. Davies K.J. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta FEBS J. 2006 273 2100 2109
Lee J.H. Chulikavit M. Pang D. Zigman W.B. Silverman W. Schupf N. Association between genetic variants in sortilin-related receptor 1 (SORL1) and Alzheimer's disease in adults with Down syndrome Neurosci. Lett. 2007 425 105 109
Kimura R. Kamino K. Yamamoto M. Nuripa A. Kida T. Kazui H. Hashimoto R. Tanaka T. Kudo T. Yamagata H. et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease Hum. Mol. Genet. 2007 16 15 23
Liang Z. Liu F. Iqbal K. Grundke-Iqbal I. Wegiel J. Gong C.X. Decrease of protein phosphatase 2A and its association with accumulation and hyperphosphorylation of tau in Down syndrome J. Alzheimers Dis. 2008 13 295 302
Park J. Yang E.J. Yoon J.H. Chung K.C. Dyrk1A overexpression in immortalized hippocampal cells produces the neuropathological features of Down syndrome Mol. Cell. Neurosci. 2007 36 270 279
Wegiel J. Dowjat K. Kaczmarski W. Kuchna I. Nowicki K. Frackowiak J. Mazur K.B. Wegiel J. Silverman W.P. Reisberg B. et al. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome Acta Neuropathol. 2008 116 391 407
Shi J. Zhang T. Zhou C. Chohan M.O. Gu X. Wegiel J. Zhou J. Hwang Y.W. Iqbal K. Grundke-Iqbal I. et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of Tau in Down syndrome J. Biol. Chem. 2008 283 28660 28669
Shukkur E.A. Shimohata A. Akagi T. Yu W. Yamaguchi M. Murayama M. Chui D. Takeuchi T. Amano K. Subramhanya K.H. et al. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for Down syndrome Hum. Mol. Genet. 2006 15 2752 2762
Nistor M. Don M. Parekh M. Sarsoza F. Goodus M. Lopez G.E. Kawas C. Leverenz J. Doran E. Lott I.T. et al. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain Neurobiol. Aging 2007 28 1493 1506
Freeman S.B. Bean L.H. Allen E.G. Tinker S.W. Locke A.E. Druschel C. Hobbs C.A. Romitti P.A. Royle M.H. Torfs C.P. et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project Genet. Med. 2008 10 173 180
Maslen C.L. Babcock D. Robinson S.W. Bean L.J. Dooley K.J. Willour V.L. Sherman S.L. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome Am. J. Med. Genet. A 2006 140 2501 2505
Moore C.S. Postnatal lethality and cardiac anomalies in the Ts65Dn Down syndrome mouse model Mamm. Genome 2006 17 1005 1012
Williams A.D. Mjaatvedt C.H. Moore C.S. Characterization of the cardiac phenotype in neonatal Ts65Dn mice Dev. Dyn. 2008 237 426 435
Izraeli S. Rainis L. Hertzberg L. Smooha G. Birger Y. Trisomy of chromosome 21 in leukemogenesis Blood Cells Mol. Dis. 2007 39 156 159
Groet J. McElwaine S. Spinelli M. Rinaldi A. Burtscher I. Mulligan C. Mensah A. Cavani S. Dagna-Bricarelli F. Basso G. et al. Acquired mutations in GATA1 in neonates with Down's syndrome with transient myeloid disorder Lancet 2003 361 1617 1620
Wechsler J. Greene M. McDevitt M.A. Anastasi J. Karp J.E. Le Beau M.M. Crispino J.D. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome Nat. Genet. 2002 32 148 152
Chou S.T. Opalinska J.B. Yao Y. Fernandes M.A. Kalota A. Brooks J.S. Choi J.K. Gewirtz A.M. Danet-Desnoyers G.A. Nemiroff R.L. et al. Trisomy 21 enhances human fetal erythro-megakaryocytic development Blood 2008 112 4503 4506
Tunstall-Pedoe O. Roy A. Karadimitris A. de la F.J. Fisk N.M. Bennett P. Norton A. Vyas P. Roberts I. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations Blood 2008 112 4507 4511
Malinge S. Ragu C. Della-Valle V. Pisani D. Constantinescu S.N. Perez C. Villeval J.L. Reinhardt D. Landman-Parker J. Michaux L. et al. Activating mutations in human acute megakaryoblastic leukemia Blood 2008 112 4220 4226
Sato T. Toki T. Kanezaki R. Xu G. Terui K. Kanegane H. Miura M. Adachi S. Migita M. Morinaga S. et al. Functional analysis of JAK3 mutations in transient myeloproliferative disorder and acute megakaryoblastic leukaemia accompanying Down syndrome Br. J. Haematol. 2008 141 681 688
Klusmann J.H. Reinhardt D. Hasle H. Kaspers G.J. Creutzig U. Hahlen K. van den Heuvel-Eibrink M.M. Zwaan C.M. Janus kinase mutations in the development of acute megakaryoblastic leukemia in children with and without Down's syndrome Leukemia 2007 21 1584 1587
Kiyoi H. Yamaji S. Kojima S. Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults Leukemia 2007 21 574 576
Walters D.K. Mercher T. Gu T.L. O'Hare T. Tyner J.W. Loriaux M. Goss V.L. Lee K.A. Eide C.A. Wong M.J. et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia Cancer Cell 2006 10 65 75
De Vita S. Mulligan C. McElwaine S. Dagna-Bricarelli F. Spinelli M. Basso G. Nizetic D. Groet J. Loss-of-function JAK3 mutations in TMD and AMKL of Down syndrome Br. J. Haematol. 2007 137 337 341
Toki T. Kanezaki R. Adachi S. Fujino H. Xu G. Sato T. Suzuki K. Tauchi H. Endo M. Ito E. The key role of stem cell factor/KIT signaling in the proliferation of blast cells from Down syndrome-related leukemia Leukemia 2008 advance online publication 2 October 2008; doi: 10.1038/leu.2008.267
Kirsammer G. Jilani S. Liu H. Davis E. Gurbuxani S. Le Beau M.M. Crispino J.D. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome Blood 2008 111 767 775
Forestier E. Izraeli S. Beverloo B. Haas O. Pession A. Michalova K. Stark B. Harrison C.J. Teigler-Schlegel A. Johansson B. Cytogenetic features of acute lymphoblastic and myeloid leukemias in pediatric patients with Down syndrome: an iBFM-SG study Blood 2008 111 1575 1583
Malinge S. Ben Abdelali R. Settegrana C. Radford-Weiss I. Debre M. Beldjord K. Macintyre E.A. Villeval J.L. Vainchenker W. Berger R. et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia Blood 2007 109 2202 2204
Kearney L. Gonzalez D.C. Yeung J. Procter J. Horsley S.W. Eguchi-Ishimae M. Bateman C.M. Anderson K. Chaplin T. Young B.D. et al. A specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukaemia Blood 2008 prepublished online 16 October 2008, doi:10.1182/blood-2008-08-170928
Bercovich D. Ganmore I. Scott L.M. Wainreb G. Birger Y. Elimelech A. Shochat C. Cazzaniga G. Biondi A. Basso G. et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome Lancet 2008 372 1484 1492
Hasle H. Pattern of malignant disorders in individuals with Down's syndrome Lancet Oncol. 2001 2 429 436
Yang Q. Rasmussen S.A. Friedman J.M. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study Lancet 2002 359 1019 1025
Sussan T.E. Yang A. Li F. Ostrowski M.C. Reeves R.H. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down's syndrome Nature 2008 451 73 75
Morrison R.A. McGrath A. Davidson G. Brown J.J. Murray G.D. Lever A.F. Low blood pressure in Down's syndrome, a link with Alzheimer's disease? Hypertension 1996 28 569 575
Draheim C.C. McCubbin J.A. Williams D.P. Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome Am. J. Ment. Retard. 2002 107 201 211
Fernandez F. Morishita W. Zuniga E. Nguyen J. Blank M. Malenka R.C. Garner C.C. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome Nat. Neurosci. 2007 10 411 413
Costa A.C. Scott-McKean J.J. Stasko M.R. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test Neuropsychopharmacology 2008 33 1624 1632
Saxena R. Voight B.F. Lyssenko V. Burtt N.P. de Bakker P.I. Chen H. Roix J.J. Kathiresan S. Hirschhorn J.N. Daly M.J. et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels Science 2007 316 1331 1336
Loos R.J. Lindgren C.M. Li S. Wheeler E. Zhao J.H. Prokopenko I. Inouye M. Freathy R.M. Attwood A.P. Beckmann J.S. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity Nat. Genet. 2008 40 768 775
O'Donovan M.C. Craddock N. Norton N. Williams H. Peirce T. Moskvina V. Nikolov I. Hamshere M. Carroll L. Georgieva L. et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up Nat. Genet. 2008 40 1053 1055
Stefansson H. Rujescu D. Cichon S. Pietilainen O.P. Ingason A. Steinberg S. Fossdal R. Sigurdsson E. Sigmundsson T. Buizer-Voskamp J.E. et al. Large recurrent microdeletions associated with schizophrenia Nature 2008 455 232 236
Gerstein M.B. Bruce C. Rozowsky J.S. Zheng D. Du J. Korbel J.O. Emanuelsson O. Zhang Z.D. Weissman S. Snyder M. What is a gene, post-ENCODE? History and updated definition Genome Res. 2007 17 669 681
Denoeud F. Kapranov P. Ucla C. Frankish A. Castelo R. Drenkow J. Lagarde J. Alioto T. Manzano C. Chrast J. et al. Prominent use of distal 5’ transcription start sites and discovery of a large number of additional exons in ENCODE regions Genome Res. 2007 17 746 759
- down syndrome
- chromosomes
- chromosomes, human, pair 21
- engineering
- mental processes
- animal model
- learning disabilities
- cognitive ability
- childhood leukemia
- alzheimer disease, early onset
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2083
- Print ISSN 0964-6906
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 11 June 2015
“Down syndrome: an insight of the disease”
- Ambreen Asim 1 ,
- Ashok Kumar 1 ,
- Srinivasan Muthuswamy 1 ,
- Shalu Jain 1 &
- Sarita Agarwal 1
Journal of Biomedical Science volume 22 , Article number: 41 ( 2015 ) Cite this article
166k Accesses
172 Citations
16 Altmetric
Metrics details
Down syndrome (DS) is one of the commonest disorders with huge medical and social cost. DS is associated with number of phenotypes including congenital heart defects, leukemia, Alzeihmer’s disease, Hirschsprung disease etc. DS individuals are affected by these phenotypes to a variable extent thus understanding the cause of this variation is a key challenge. In the present review article, we emphasize an overview of DS, DS-associated phenotypes diagnosis and management of the disease. The genes or miRNA involved in Down syndrome associated Alzheimer’s disease, congenital heart defects (AVSD), leukemia including AMKL and ALL, hypertension and Hirschprung disease are discussed in this article. Moreover, we have also reviewed various prenatal diagnostic method from karyotyping to rapid molecular methods - MLPA, FISH, QF-PCR, PSQ, NGS and noninvasive prenatal diagnosis in detail.
Introduction
Down syndrome is one of the most leading causes of intellectual disability and millions of these patients face various health issues including learning and memory, congenital heart diseases(CHD), Alzheimer’s diseases (AD), leukemia, cancers and Hirschprung disease(HD). The incidence of trisomy is influenced by maternal age and differs in population (between 1 in 319 and 1 in 1000 live births) [ 1 - 5 ]. DS has high genetic complexity and phenotype variability [ 6 - 8 ]. Trisomic fetuses are at elevated risk of miscarriages and DS people have increased incidence of developing several medical conditions [ 9 ]. Recent advancement in medical treatment with social support has increased the life expectancy for DS population. In developed countries, the average life span for DS population is 55 years [ 10 ].
Various conditions associated with Downs’s syndrome with its causative genes.
Human Chromosome 21
DS complex phenotype results from dosage imbalance of genes located on human chromosome 21(Hsa 21). The genetic nature of DS together with the relatively small size of Hsa 21 encouraged scientist to concentrate efforts towards the complete characterization of this chromosome in the past few years. The length of 21q is 33.5 Mb [ 11 ] and 21 p is 5–15 Mb [ 12 ]. A total 225 genes was estimated when initial sequence of 21q was published [ 11 ]. Hsa 21 has 40.06% repeat content out of which the repeat content of SINE’s, LINE’s, and LTR are 10.84%, 15.15%, 9.21% respectively. The Table 1 given below highlights some of the genes present on chromosome 21.
Features of DS
There are various conserved features occurring in all DS population, including learning disabilities, craniofacial abnormality and hypotonia in early infancy [ 13 ]. Some people of DS are affected by variant phenotypes including atrioventricular septal defects (AVSD) in heart, leukemia’s (both acute megakaryoblastic leukemia(AMKL) and acute lymphoblastic leukemia(ALL)), AD and HD. DS individual have variety of physical characteristics like a small chin, slanted eye, poor muscle tone, a flat nasal bridge, a single crease of the palm and a protuding due to small mouth and large tongue [ 14 ]. Other features includes big toe, abnormal pattern of fingerprint and short fingers.
Genetics of the disease
The most common cause of having a DS babies is presence extra copy chromosome 21 resulting in trisomy. The other causes can be Robertsonian translocation and isochromosomal or ring chromosome. Ischromosome is a term used to describe a condition in which two long arms of chromosome separate together rather than the long and short arm separating together during egg sperm development. Trisomy 21 (karyotype 47, XX, + 21 for females and 47, XY, + 21 for males) is caused by a failure of the chromosome 21 to separate during egg or sperm development. In Robertsonian translocation which occurs only in 2-4% of the cases, the long arm of the chromosome 21 is attached to another chromosome (generally chromosome 14). While mosaicism deals with the error or misdivision occurs after fertilization at some point during cell division. Due to this people with mosaic DS have two cell lineages which contribute to tissues and organs of individuals with Mosacism (one with the normal number of chromosomes, and other one with an extra number 21) [ 15 ].
Genotype-phenotype correlation
Gene dosage imbalance hypothesis states that DS patients have an increased dosage or copy number of genes on Hsa 21 that may lead to an increase in gene expression [ 13 - 15 ]. This hypothesis has been extended to include the possibility that specific genes or subsets of genes may control specific DS phenotypes [ 16 ]. Amplified developmental instability hypothesis states that a non-specific dosage of a number of trisomic genes leads to a genetic imbalance that causes a great impact on the expression and regulation of many genes throughout the genome [ 13 , 14 ]. Another hypothesis known as critical region hypothesis was also added to this list. Phenotypic analyses was done on individuals with partial trisomy for Hsa21 identified that only one or a few small chromosomal regions, termed “Down syndrome critical regions” (DSCR) a region of 3.8-6.5 Mb on 21q21.22, with approximately 30 genes responsible for the majority of DS phenotypes [ 15 , 16 ]. Previously a region of 1.6 to 2.5 Mb was recognised as sufficient cause for DS pehnotype [17, 18]. The sequencing of Hsa 21 proved to be an important factor in the progression of DS research [ 19 ] and led to further insight into genotype-phenotype correlations associated with DS and precise characterizations of DSCR regions [ 13 ]. A “critical region” within 21q22 was believed to be responsible for several DS phenotypes including craniofacial abnormalities, congenital heart defects of the endocardial cushions, clinodactyly of the fifth finger and mental retardation [ 20 ].
Dual-specificity tyrosine phosphorylation-regulated kinase (DYRK1A) and regulator of calcineurin 1 (RCAN1), Down syndrome cell adhesion molecule (DSCAM) has been suggested to play a critical role in the developing brain and has also been identified as a candidate gene for the increased risk of CHD in DS individuals [ 21 , 22 ]. DSCAM is a critical factor in neural differentiation, axon guidance, and the establishment of neural networks and it has been suggested that the disruption of these processes contributes to the DS neurocognitive phenotype [ 22 ]. Based on thorough analyses of studies on humans and DS mouse models, it is evident that there is not a single critical region of genes sufficient to cause all DS phenotypes. Alternatively, it is likely that there are multiple critical regions or critical genes contributing to a respective phenotype or group of phenotypes associated with DS [ 23 ].
Various clinical conditions associated to Down syndrome
The various clinical conditions associated with DS are Alzheimer’s disease, heart defects, leukemia, hypertension and gastrointestinal problems (Figure 1 ). The molecular pathogenesis mechanism of these DS related phenotype must be studied along with its causative agents in order to have a better understanding of the disease. Below are some DS related phenotype discussed in detail which are as follows:
Neurological problems
DS patients have greatly increased risk of early onset AD. After the age of 50, the risk of developing dementia increases in DS patients up to 70% [ 23 - 27 ]. There are various genes reported to cause early onset AD. Some of the genes described in the current literature are APP (amyloid precursor protein), BACE2 (beta secretase 2), PICALM (Phosphatidylinositol binding clathrin assembly protein) and APOE(Apolipoprotein E) etc. APP is an integral membrane protein which is concentrated in synapse of neurons and trisomy of this protein is likely to make significant contribution to the increased frequency of dementia in DS individuals. The triplication of Hsa 21 along with APP in people without DS has been recently shown to be associated with early onset AD. A tetranucleotide repeat, ATTT , in intron 7 of the amyloid precursor protein has been associated with the age of onset of AD in DS in a preliminary study [ 28 ]. Various mouse models are used to observe degeneration of basal forebrain cholinergic neurons (BFCNs). Ts65Dn mice is dependent on trisomy of APP expression of retrograde axonal transport [ 29 ]. Studies have also revealed that BACE2 which encodes enzyme beta secretase 2 is also involved in AD. APP and BACE 2 genes are located on chromosome 21. The current data on DS support the association of haplotypes in BACE2 with AD [ 30 ]. Besides APP and BACE2 genes, other genes like PICALM and APOE are also found to be associated with the age of onset of Alzheimer’s dimentia in DS [ 31 ].
Cardiac problems
The incidence of CHD in newborn babies with DS is up to 50% [ 32 ]. Endocardial cushion defect also called as atrioventricular cushion defect is most common form which affects up to 40% of the patients. Ventricular septal defect (VSD) is also present in these population which affects up to 35% of the patients [ 33 ]. The essential morphological hallmark of an AVSD is the presence of a common atrioventricular junction as compared to the separate right and left atrioventricular junction in the normal heart. Other morphological features include defects of the muscular and membranous atrioventricular septum and an ovoid shape of the common atrioventricular junction. There is disproportion of outlet and inlet dimensions of the left ventricle, with the former greater than the latter as compared to the normal heart where both dimensions are similar [ 34 ]. While in case of VSD, the defect lies in ventricular septum of the heart due to which some of the blood from the left ventricle leaks into the right ventric leading to pulmonary hypertension. Mutation in non Hsa 21 CRELD1 (Cysteine rich EGF like domain1) gene contributes to the development of AVSD in DS [ 35 ]. CRELD1 is located on chromosome 3p25. It encodes a cell surface protein that functions as cell adhesion molecule and is expressed during cardiac cushion development. CRELD1 gene contains 11 exons spanning approximately 12 kb [ 36 ]. To the present, two specific genetic loci for AVSD have been identified. One was AVSD 1 locus present on chromosome 1p31-p21 [ 37 ]. Other locus was present on chromosome 3p25 and the corresponding gene was CRELD1 [ 36 , 38 ]. Maslen et al . in [ 33 ] have identified two heterozygous missense mutation (p.R329C and p.E414K) with two subjects in DS and AVSD. They have recruited 39 individual of DS with complete AVSD and have found the same mutations. In the same study, DNA of 30 individual of trisomy without CHD was studied for both mutations, no such mutation was identified [ 35 ]. R329C which was originally reported in an individual with sporadic partial AVSD and now it is also detected in individual of DS with AVSD. Interestingly, with the same mutation (p.R329C), the severity of heart defect was greater in patients of DS with AVSD. Thus, identification of CRELD 1 mutation in 2/39 individual (5.1%) of DS with complete AVSD suggests the defects in CRELD 1 contribute to pathogenesis of AVSD in context with trisomy 21.
Hematological problems
Patients with DS display a unique spectrum of malignancies, which include leukemia’s as well as solid tumors. The first report of leukemia in a DS patient occurred in 1930 [ 39 ] and the first systematic study in 1957 [ 40 ]. Studies indicate that patients with DS have a 10–20 fold increased relative risk of leukemia, with a cumulative risk of 2% by age 5 and 2.7% by age 30 [ 41 ]. They constitute approximately 2% of all pediatric acute lymphoblastic leukemia(ALL) and approximately 10% of pediatric acute myeloid leukemia (AML). Leukemogenesis of acute megakaryoblastic leukemia (AMKL) in DS patients is associated with the presence of somatic mutations involving GATA 1 gene (or also called as GATA-binding factor 1) [ 42 ]. GATA 1 is a chromosome X- linked transcription factor which is essential for erythoid and megakaryocytic differentiation. Because of these GATA 1 mutations, there is a production of shorter GATA 1 protein thereby leading to uncontrolled proliferation of immature megakaryocytes [ 42 , 43 ]. On the other hand, acquired gain of function mutation in Janus Kinase 2 gene are present in approximately 30% of cases with ALL in DS [ 44 , 45 ].
Hypertension
People with DS have been reported to have a reduced incidence of hypertension [ 46 , 47 ]. Trisomy of the Hsa21 microRNA hsa-miR-155 contributes to this [ 48 ]. Hsa-miR-155 is proposed to specifically target one allele of the type-1 angiotensin II receptor (AGTR1) gene, resulting in it’s under- expression, which contribute to a reduced risk of hypertension. Further studies are required to validate this hypothesis and determine whether other genes may also protect people with DS against hypertension.
Gastrointestinal problems
DS patients constitute ~12% of all cases of HD. Duodenal stenosis (DST) and imperforate anus (IA) are 260 and 33 times more likely to occur DS [ 23 , 49 ]. HD is a form of low intestinal obstruction caused by the absence of normal myenteric ganglion cells in a segment of the colon [ 50 ]. In HD children, the absence of ganglion cells results in the failure of the distal intestine to relax normally. Peristaltic waves do not pass through the aganglionic segment and there is no normal defecation, leading to functional obstruction. Abdominal distention, failure to pass meconium, enterocolitis and bilious vomiting are the predominant signs and symptoms and appear within a few days after birth. Infants with duodenal atresia or DST present with bilious vomiting early in the neonatal period. If left untreated, it will result in severe dehydration and electrolyte imbalance. IA is a birth defects in which the rectum is malformed and it is associated with an increased incidence of some other specific anomalies as well, together being called the VACTERL association: vertebral anomalies, anal atresia, cardiovascular anomalies, tracheoesophageal fistula, esophageal atresia, renal and limb defects.
Alterations of approximately 10 non Hsa21 genes have been linked to this disease [ 51 ]. Several researches have shown that HD contain the DSCAM gene which is expressed in neural crest that give rise to enteric nervous system [ 49 ]. Overlapping critical region was described both for DST and IA [ 51 ]. No other Hsa21 genes have been implicated so far.
Diagnostic methods
Prevention of DS depends upon offering prenatal diagnosis to high risk pregnancies via amniocentesis and chorionic villus sampling (CVS). Amniocentesis and CVS are quite reliable but offers risk of miscarriage of between 0.5 to 1% [ 52 ]. Based soft markers like small or no nasal bone, large ventricles and nuchal fold thickness, the risk of DS for fetus can be identified through ultrasound generally at 14 to 24 weeks of gestation [ 53 ]. Increased fetal nuchal translucency indicates an increased risk of DS [ 54 ]. The other methods used for prenatal diagnosis in which traditional cytogenic analysis is still widely used in different countries. However some rapid molecular assays-FISH(fluorescent in situ hybridization), QF-PCR (quantitative fluorescence PCR), and MLPA(multiplex probe ligation assay)- also used for prenatal diagnosis.
Routine karyotyping
Cytogenetic analysis of metaphase karyotype remains the standard practice to identify not only trisomy 21, but also all other aneuploidies and balanced translocations. Details on diagnostic methods with advantages and disadvantages are mentioned in Table 2 .
Rapid aneuploidy testing methods
Over the past 10 years however, several other methods have been developed and used for the rapid detection of trisomy 21, either in fetal life or after birth. The most widely used is FISH of interphase nuclei, using Hsa 21-specific probes or whole-Hsa 21 [ 55 ]. An alternative method that is now widely used in some countries is QF-PCR, in which DNA polymorphic markers (microsatellites) on Hsa 21 are used to determine the presence of three different alleles [ 56 ]. This method relies on informative markers and the availability of DNA. Rapid diagnosis by PCR-based methods using polymorphic STR markers may reduce these difficulties using conventional approach. Using STR markers method we can detect trisomy in 86.67% cases with only two markers. Using more number of markers can further increase the reliability of the test. Simultaneously parental origin of the nondysjunction can also be detected [ 57 , 58 ]. Additional method to measure copy number of DNA sequences include MLPA [ 59 ] which was first introduced in 2002 as a method of relative quantification in DNA. MLPA offers various advantages like – a very short time for diagnosis (2–4 days), effectiveness, simplicity and relatively low costs. It is based on hybridization and PCR method and is divided into four steps: DNA denaturation, hybridization of probe to the complementary target sequence, probe ligation and PCR amplification. And finally capillary electrophoresis of PCR amplified products is carried out. However MLPA is unable to exclude low level placental and true mosaicism [ 60 ].
Advancement in the diagnosis
A recent method, termed paralogous sequence quantification (PSQ), uses paralogous sequences to quantify the Hsa 21 copy number. PSQ is a PCR based method for the detection of targeted chromosome number abnormalities termed paralogous sequence quantification (PSQ), based on the use of paralogous genes. Paralogous sequences have a high degree of sequence identity, but accumulate nucleotide substitutions in a locus specific manner. These sequence differences, which are termed as paralogous sequence mismatches (PSMs), can be quantified using pyrosequencing technology, to estimate the relative dosage between different chromosomes. PSQ is a robust, easy to interpret, and easy to set up method for the diagnosis of common aneuploidies, and can be performed in less than 48 h, representing a competitive alternative for widespread use in diagnostic laboratories. The sequencing is quantitatively done by using pyrosequencing [ 61 ]. Finally, comparative genomic hybridization (CGH) on BAC chips can be used for the diagnosis of full trisomy or monosomy, and for partial (segmental) aneuploidies [ 62 , 63 ].
Noninvasive Prenatal diagnosis
Fetal cells in maternal ciruculation: Ever since the discovery of presence of fetal lymphocytes in maternal blood was made in 1969, the investigators are trying to develop genetics-based noninvasive prenatal diagnostics (NIPD) [ 64 ]. Despite several advantages offered by this approach, the use of fetal cells for NIPD has never reached clinical implementation because of their paucity (on the order of a few cells per milliliter of maternal blood) and concerns of fetal cell persistence in the maternal circulation between pregnancies.
Cell free fetal DNA from maternal serum: This novel approach was proposed in 1997. Cell-free fetal DNA constitutes between 5% and 10% of the total DNA in maternal plasma and increases during gestation and rapidly clears from the circulation post delivery. Several clinical applications based on the analysis of cell-free fetal DNA have been developed like determining fetal Rh D status in Rh D-negative women [ 65 ], sex in sex-linked disorders [ 66 , 67 ], and detection of paternally inherited autosomal recessive and dominant mutations [ 68 ]. However, there remains the outstanding challenge of the use of cell-free fetal DNA for the detection of chromosomal aneuploidy, in particular trisomies 21, 18, and 13. Several approaches have been adopted like the origin of circulating cell-free fetal DNA is primarily the placenta, whereas maternal cell-free DNA is derived from maternal leukocytes [ 69 ]. The approach includes studying differences in genomic DNA methylation between the placenta and paired maternal leukocytes, investigators have characterized placenta-specific epigenetic markers [ 70 ] and also finding of circulating cell-free placenta-derived mRNA allowed the identification of placenta-specific mRNA production [ 71 ].
The concept of digital PCR was also introduced to serve the same purpose. In digital PCR, individual fetal and maternal circulating cell-free DNA fragments are amplified under limiting-dilution conditions and the total number of chromosome 21 amplifications (representing maternal plus fetal contributions) divided by the number of reference chromosome amplifications should yield a ratio indicating an over- or underrepresentation of chromosome 21.
Although the digital PCR approach is conceptually solid, the low percentage of cell-free fetal DNA in the maternal plasma sample requires the performance of thousands of PCRs to generate a ratio with statistical confidence. This can be overcome by using of multiple target amplifications and enrichment of cell-free fetal DNA which are still under research trail.
Next recent method added to the list is next generation sequencing (NGS) which is based on the principle of clonally amplified DNA templates (or, most recently, single DNA molecules) are sequenced in a massively parallel fashion within a flow cell [ 72 , 73 ]. NGS provides digital quantitative information, in which each sequence read is a countable “sequence tag” representing an individual clonal DNA template or a single DNA molecule. This quantification allows NGS to expand the digital PCR concept of counting cell-free DNA molecules.
Fan et al. and Chiu et al. in 2008 described noninvasive detection of trisomy 21 by NGS [ 74 ]. Both groups extracted cell-free DNA from maternal plasma samples from both euploid and trisomy pregnancies. DNA from each sample was sequenced on the Illumina Genome Analyzer, and each sequence read was aligned to the reference human genome. Chiu et al. build on their earlier work with the Illumina Genome Analyzer and demonstrate noninvasive NGS-based trisomy 21 detection with the sequencing-by-ligation approach on the Life Technologies SOLiD platform [ 75 ]. Cell-free DNA was extracted from 15 pregnant women, 5 of whom carried trisomy 21 fetuses and it was clonally amplified by emulsion PCR, and sequenced in 1 chamber of an 8-chamber SOLiD slide. This process yielded a median of 59 × 10 6 50-base reads per sample. A median of 12 × 10 6 reads (or 21%) were each aligned uniquely to one location of the reference human genome (with masking of repeat regions), for a coverage of approximately 20% of the haploid human genome. For each trisomy 21 case, the chromosome 21 z score value indicated a 99% chance of a statistically significant difference from the chromosome 21 z scores for the controls. As reported earlier with the Illumina Genome Analyzer, a nonuniform distribution of aligned sequence reads was observed with the SOLiD data.
The current time for sample processing, sequencing, and data interpretation in experienced hands is 5 to 8 days for the Genome Analyzer and SOLiD platforms respectively with the cost of approximately $700 – $1000 per sample. Going forward, one can expect streamlining and automation of technical processes and data analysis, coupled with reduced sequencing costs.
Ultimately, reduced sequencing costs and turnaround times could pave the way for NGS-based NIPD to be considered as an alternative to serum biomarker screening, which,while cost-effective remains prone to false positives. Forty years after the discovery of circulating fetal cells, the vision of NIPD appears clearer and closer.
Management of the disease
One of the hallmarks of DS is the variability in the way that the condition affects people with DS. With the third 21st chromosome existing in every cell, it is not surprising to find that every system in the body is affected in some way. However, not every child with DS has the same problems or associated conditions. Parents of children with DS should be aware of these possible conditions so they can be diagnosed and treated quickly and appropriately. The goal of the study is to point out the most common problems of which parents should be aware.
Timely surgical treatment of cardiac defects during first 6 months of life may prevent from serious complications. Congenital cataracts occur in about 3% of children and must be extracted soon after birth to allow light to reach the retina. A balance diet and regular exercise are needed to maintain appropriate weight. Feeding problems and failure to thrive usually improve after cardiac surgery. A DS child should have regular check up from various consultants. These include:
Clinical geneticist - Referral to a genetics counseling program is highly desirable
Developmental pediatrician
Cardiologist - Early cardiologic evaluation is crucial for diagnosing and treating congenital heart defects, which occur in as many as 60% of these patients
Pediatric pneumonologist -Recurrent respiratory tract infections are common in patients with DS
Ophthalmologist
Neurologist/Neurosurgeon – As many as 10% of patients with DS have epilepsy; therefore, neurologic evaluation may be needed
Orthopedic specialist
Child psychiatrist - A child psychiatrist should lead liaison interventions, family therapies, and psychometric evaluations
Physical and occupational therapist
Speech-language pathologist
Audiologist
DS or Trisomy 21, being the most common chromosomal abnormality among live born infants, is associated with a number of congenital malformations. Several theories have been put forward to increase our understanding in phenotype and genotype correlation. A “critical region” within 21q22 was believed to be responsible for several DS phenotypes including craniofacial abnormalities, congenital heart defects of the endocardial cushions, clinodactyly of the fifth finger and mental retardation and several other features. The primary goal of this review is to unravel the common genes involved in DS associated phenotypes, including APP, BACE2, PICALM, APOE, GATA 1, JAK 2, CRELD 1 and DSCAM. This reviews also provides the detailed description on the application of techniques to prenatal diagnosis in DS. Rapid aneuploidy testing has been introduced in mid 1990’s in the form of FISH where testing can be done on uncultured amniocytes. Within a couple of years, MLPA and QF-PCR has been added in the list of rapid aneuploidy testing. The other methods includes: NGS for cell free fetal DNA screening for maternal plasma. Except ,FISH, MLPA and QF-PCR other method are not commercialized for aneuploidy diagnosis due to their running cost, labor intensive protocol and complex data analysis. Since various clinical conditions are associated with DS, hence the management of these patients requires an organized multidisciplinary approach and continuous monitoring of these patients which has been discussed in this review article.
O’ Nuallain S, Flanagan O, Raffat I, Avalos G, Dineen B. The prevalence of Down syndrome in County Galway. Ir Med. 2009;100:329–31.
Google Scholar
Carothers AD, Hecht CA, Hook EB. International variation in reported live birth prevalence rates of Down syndrome, adjusted for maternal age. J Med Genet. 1999;36:386–93.
Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States. Birth Defects Res A Clin Mol Teratol. 2006;76:747–56.
Murthy SK, Malhotra AK, Mani S, Shara ME, Al Rowaished EE, Naveed S, et al. Incidence of Down syndrome in Dubai. Med Princ Pract. 2007;16:25–8.
Article PubMed Google Scholar
Wahab AA, Bener A, Teebi AS. The incidence patterns of Down syndrome in Qatar. Clin Genet. 2006;69:360–2.
Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethoré MO et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med. 2009;11:611–6.
Gardiner KJ. Molecular basis of pharmacotherapies for cognition in Down syndrome. Trends Pharmacol Sci. 2010;31:66–73.
Article CAS PubMed Central PubMed Google Scholar
Prandini P, Deutsch S, Lyle R, Gagnebin M, Delucinge Vivier C, Delorenzi M et al. Natural gene-expression variation in Down syndrome modulates the outcome of gene-dosage imbalance. Am J Hum Genet. 2007;81:252–63.
Morris JK, Wald NJ, Watt HC. Fetal loss in Down syndrome pregnancies. Prenat Diagn. 1999;19:142–5.
Article CAS PubMed Google Scholar
Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH, et al. The changing survival profile of people with Down’s syndrome: implications for genetic counselling. Clin Genet. 2002;62:390–3.
Lyle R, Bena F, Gagos S, Gehrig C, Lopez G, Schinzel A, et al. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur J Hum Genet. 2009;17:454–66.
Ermak G, Harris CD, Battocchio D, Davies KJ. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta. FEBS J. 2006;273:2100–9.
Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and Down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5:725–38.
Sinet PM, Theopile D, Rahmani Z, Chettouch Z, Blovin JL, Prier M, et al. Mapping of Down syndrome phenotype on chromosome 21 at the molecular level. Biomed Pharmacother. 1994;48(5–6):247–52.
Epstein CJ, Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. p. 1223–56.
Pritchard MA, Kola I. The "gene dosage effect" hypothesis versus the "amplified developmental instability" hypothesis in Down syndrome. J Neural Transm Suppl. 1999;57:293–303.
CAS PubMed Google Scholar
Patterson D. Genetic mechanisms involved in the phenotype of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:199–206.
Shapiro BL. Down syndrome–a disruption of homeostasis. Am J Med Genet. 1983;14:241–69.
Delabar JM, Theophile D, Rahmani Z, Chettouh Z, Blouin JL, Prieur M, et al. Molecular mapping of twenty-four features of Down syndrome on chromosome 21. Eur J Hum Genet. 1993;1:114–24.
Ohira M, Ichikawa H, Suzuki E, Iwaki M, Suzuki K, Saito-Ohara F, et al. A 1.6-Mb P1-based physical map of the Down syndrome region on chromosome 21. Genomics. 1996;33:65–74.
Korbel JO, Tirosh-Wagner T, Urban AE, Chen XN, Kasowski M, Dai L, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci U S A. 2009;106:12031–6.
Niebuhr E. Down's syndrome. The possibility of a pathogenetic segment on chromosome no. 21. Humangenetik. 1974;21:99–101.
Holland AJ, Hon J, Huppert FA, Stevens F. Incidence and course of dementia in people with Down’s syndrome: findings from a population-based study. J Intellect Disabil Res. 2000;44:138–46.
Holland AJ, Hon J, Huppert FA, Stevens F, Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down’s syndrome. Br J Psychiatry. 1998;172:493–8.
Janicki MP, Dalton AJ. Prevalence of dementia and impact on intellectual disability services. Ment Retard. 2000;38:276–88.
Johannsen P, Christensen JE, Mai J. The prevalence of dementia in Down syndrome. Dementia. 1996;7:221–5.
Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–53.
EL Jones, CG Ballard, VP Prasher, M Arno, S Tyrer, B Moore et al. An Intron 7 polymorphism in APP affects the age of Dimentia in Down Syndrome. Int J Alzheimers Dis. 2011;5. doi:10.4061/2011/929102
Salehi A, Delcroix JD, Belichenko PV, Zhan KWC, Valletta JS, Takimoto-Kimura, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42.23.
Myllykangas L, Wavrant –De Vrieze F, Polvikoski T, Notkola IL, Sulkava R, Niinisto L. Chromosome 21 BACE2 haplotype associates with Alzheimer's disease: a two-stage study. J Neurol Sci. 2005;236(1–2):17–24.
Jones EL, Mok K, Hanney M, Harold D, Sims R, Williams J. Evidence that PICALM affects age at onset of Alzheimer’s dementia in Down syndrome. Neurobiol Aging. 2013;34(10):244.
Article Google Scholar
Urbano R. Health issues among person with down syndrome. 2012.
Maslen CL, Babcock D, Robinson SW, Bean LJ, Dooley KJ, Willour VL, et al. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome. Am J Med Genet. 2006;140:2501–5.
Craig B. Atrioventricular septal defect: from fetus to adult. Heart. 2006;92(12):1879–85.
Article PubMed Central PubMed Google Scholar
Rup PA, Fouad GT, Egelston CA, Reifsteck CA, Oslon SB, Knosp WM, et al. Identification, genomic organization and mRNA expression of CRELD1, the founding member of a unique family of matricellular proteins. Gene. 2002;293:47–57.
Sheffield VC, Pierpont ME, Nishimura D, Beek JS, Burns TL, Berg MA, et al. Identification of a complex congenital heart defect susceptibility locus by using DNA pooling and shared segment analysis. Hum Mol Genet. 1997;6:117–21.
Priestley MD, Water J, Maliszewska C, Latif F, Maher ER. Detailed mapping of a congenital heart disease gene in chromosome 3p25. J Med Genet. 2000;37:581–7.
Robinsom SW, Morris CD, Goldmuntz E, Reller MD, Jones MA, Maslen CL, et al. Missense mutation in CRELD1 are associated with cardiac atrioventricular septal defects. Am J Hum Genet. 2003;72:1047–52.
Brewster HF, Cannon HE. Acute lymphatic leukemia: Report of a case in eleventh month mongolina idiot. New Orleans Med Surg J. 1930;82:872–3.
Krivit W, Good RA. Simultaneous occurrence of mongolism and leukemia; report of a nationwide survey. AMA J Dis Child. 1957;94:289–93.
Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–9.
Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32(1):148–52.
Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16(13):3965–73.
Kearney L, Gonzalez De Castro D, Yeung J, Procter J, Horsley SW, Eguchi-Ishimae M, et al. Specific JAK2 mutation (JAK2R683) andmultiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood. 2009;113(3):646–8.
Morrison RA, McGrath A, Davidson G, Brown JJ, Murray GD, Lever AF. Low blood pressure in Down’s syndrome, a link with Alzheimer’s disease? Hypertension. 1996;28:569–75.
Draheim CC, McCubbin JA, Williams DP. Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome. Am J Ment Retard. 2002;107:201–11.
Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81:405–13.
Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77:431–8.
Berrocal T, Lamas M, Gutiérrez J. Congenital Anomalies of the Small Intestine, Colon, and Rectum. Radiographics Radiol Bras. 1999;19:1219–36.
Article CAS Google Scholar
Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14.
Tabor A, Alfirevic Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther. 2010;27(1):1–7.
Reena MS, Pisani PO Conversano F, Perrone E, Casciaro E, Renzo GCD et al. Sonographic markers for early diagnosis of fetal malformations. World J Radiol. 2013;10:356-371.
Agathokleous M, Chaveeva P, Poon LC, Kosinski P, Nicolaides KH. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstetrics Gynecol. 2013;41(3):247–61.
F.D. Malone, M.E D'Alton. First trimester sonographic screenign for Down syndrome. Obstetrics and Gynecology. 2003;102:1066-79.
Kuo WL. Detection of aneuploidy involving chromosomes 13, 18, or 21, by fluorescence in situ hybridization (FISH) to interphase and metaphase amniocytes. Am J Hum Genet. 1991;49:112–9.
CAS PubMed Central PubMed Google Scholar
Shalu J, Sarita A, Inusha P, Parag T, Shubha P. Diagnosis of Down Syndrome and Detection of Origin of Nondisjunction by Short Tandem Repeat Analysis. Genetic Test Mol Biomark. 2010;14:4.
Shalu J, Inusha P, Shubha RP, Sarita A. Multiplex quantitative fluorescent polymerase chain reaction for detection of aneuploidies. Genet Test Mol Biomarker. 2012;16:624–7.
Armour JA, Sismani C, Patsalis PC, Cross G. Measurement of locus copy number by hybridisation with amplifiable probes. Nucleic Acids Res. 2000;28:605–9.
Slater HR, Bruno DL, Ren H, Pertile M, Schouten JP, Choo KH. Rapid, high throughput prenatal detection of aneuploidy using a novel quantitative method (MLPA). J Med Genet. 2003;40:907–12.
Van Veghel-Plandsoen M, Wouters C, Kromosoeto J. Multiplex ligation-dependent probe amplification is not suitable for detection of low-grade mosaicism. Eur J Hum Genet. 2011;19:1009–12.
S Deutsch, U Choudhury, SE Antonarakis. Detection of trisomy 21 and other aneuploidies by paralogous gene quantification. J Med Genet (in the press).
Ishkanian AS, Malloff CA, Watson SK, DeLeeuw RJ, Chi B, Coe BP. A tiling resolution DNA microarray with complete coverage of the human genome. Nature Genet. 2004;36:299–303.
Snijders AM, Nowak N, Segraves R, Blackwood S, Brown N, Conroy J et al. Assembly of microarrays for genome- wide measurement of DNA copy number. Nature Genet. 2001;29:263–4.
Walknowska J, Conte FA, Grumbach MM. Practical and theoretical implications of fetal- maternal lymphocyte transfer. Lancet. 1969;1:1119–22.
Daniels G, Finning K, Martin P, Massey E. Noninvasive prenatal diagnosis of fetal blood group phenotypes: current practice and future prospects. Prenat Diagn. 2009;29:101–7.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7.
Costa JM, Benachi A, Gautier E. New strategy for prenatal diagnosis of X-linked disorders. N Engl J Med. 2002;346:1502.
Wright CF, Burton H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update. 2009;15:139–51.
Alberry M, Maddocks D, Jones M, Abdel Hadi M, Abdel-Fattah S, Avent N, et al. Free fetal DNA in maternal plasma in anembryonic pregnancies: confirmation that the origin is the trophoblast. Prenat Diagn. 2007;27:415–8.
Chim SS, Jin S, Lee TY, Lun FM, Lee WS, Chan LY, et al. Systematic search for placental DNA-methylation markers on chromosome 21: toward a maternal plasma-based epigenetic test for fetal trisomy 21. Clin Chem. 2008;54:500–11.
Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;24:133–41.
Voelkerding KV, Dames SA, Durtschi JD. Next-generation sequencing: from basic research to diagnostics. Clin Chem. 2009;55:641–58.
Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105:16266–71.
Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;105:20458–63.
Chiu RWK, Sun H, Akolekar R, Clouser C, Lee C, McKernan K, et al. Maternal plasma DNA analysis with massively parallel sequencing by ligation for noninvasive prenatal diagnosis of trisomy 21. Clin Chem. 2010;56:459–63.
Download references
Author information
Authors and affiliations.
Department of Medical Genetics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, 226014, India
Ambreen Asim, Ashok Kumar, Srinivasan Muthuswamy, Shalu Jain & Sarita Agarwal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarita Agarwal .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contribution
AA drafting of the manuscript, AS and MS, did substantial contribution in finalising, correcting and critical review of manuscript, KA and JS contributed in review and collection of articles. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/ .
The Creative Commons Public Domain Dedication waiver ( https://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Asim, A., Kumar, A., Muthuswamy, S. et al. “Down syndrome: an insight of the disease”. J Biomed Sci 22 , 41 (2015). https://doi.org/10.1186/s12929-015-0138-y
Download citation
Received : 28 May 2014
Accepted : 22 April 2015
Published : 11 June 2015
DOI : https://doi.org/10.1186/s12929-015-0138-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Down Syndrome
- Imperforate Anus
- Illumina Genome Analyzer
- Endocardial Cushion
- Down Syndrome Patient
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Your Privacy on DSE sites
We use cookies to provide essential functionality and to analyse how our sites are used.
Down Syndrome Research and Practice
Down Syndrome Research and Practice is a peer-reviewed journal focused on Down syndrome research. It was published by Down Syndrome Education International in partnership with the University of Portsmouth from 1992 to 2009.
- Research and Practice
- News and Update
Presentations
- Research Forum
- Online courses
- See and Learn
Legal information
- Cookies Policy
- Privacy Policy
- Terms of Use
- Terms of sale
- DSE Client ID
- Access RLI Online
- Subscriptions
- English (Australia)
- English (Canada)
- English (India)
- English (Ireland)
- English (Malaysia)
- English (New Zealand)
- English (Philippines)
- English (Singapore)
- English (South Africa)
- English (United Kingdom)
- English (United States)
Neuropathological findings in Down syndrome, Alzheimer’s disease and control patients with and without SARS-COV-2: preliminary findings
- Original Paper
- Open access
- Published: 27 May 2024
- Volume 147 , article number 92 , ( 2024 )
Cite this article
You have full access to this open access article

- Ann-Charlotte E. Granholm ORCID: orcid.org/0000-0002-9685-7599 1 ,
- Elisabet Englund 2 ,
- Anah Gilmore 1 ,
- Elizabeth Head 3 , 4 ,
- William H. Yong 3 ,
- Sylvia E. Perez 5 ,
- Samuel J. Guzman 6 ,
- Eric D. Hamlett 7 &
- Elliott J. Mufson 5
396 Accesses
2 Altmetric
Explore all metrics
The SARS-CoV-2 virus that led to COVID-19 is associated with significant and long-lasting neurologic symptoms in many patients, with an increased mortality risk for people with Alzheimer’s disease (AD) and/or Down syndrome (DS). However, few studies have evaluated the neuropathological and inflammatory sequelae in postmortem brain tissue obtained from AD and people with DS with severe SARS-CoV-2 infections. We examined tau, beta-amyloid (Aβ), inflammatory markers and SARS-CoV-2 nucleoprotein in DS, AD, and healthy non-demented controls with COVID-19 and compared with non-infected brain tissue from each disease group (total n = 24). A nested ANOVA was used to determine regional effects of the COVID-19 infection on arborization of astrocytes (Sholl analysis) and percent-stained area of Iba-1 and TMEM 119. SARS-CoV-2 antibodies labeled neurons and glial cells in the frontal cortex of all subjects with COVID-19, and in the hippocampus of two of the three DS COVID-19 cases. SARS-CoV-2-related alterations were observed in peri-vascular astrocytes and microglial cells in the gray matter of the frontal cortex, hippocampus, and para-hippocampal gyrus. Bright field microscopy revealed scattered intracellular and diffuse extracellular Aβ deposits in the hippocampus of controls with confirmed SARS-CoV-2 infections. Overall, the present preliminary findings suggest that SARS-CoV-2 infections induce abnormal inflammatory responses in Down syndrome.
Similar content being viewed by others
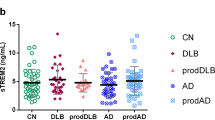
Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease
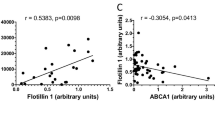
Abundance of Nef and p-Tau217 in Brains of Individuals Diagnosed with HIV-Associated Neurocognitive Disorders Correlate with Disease Severance

Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease
Avoid common mistakes on your manuscript.
SARS-CoV-2 is a coronavirus that gave rise to COVID-19, which spread rapidly throughout the world, resulting in the death of more than 6.9 million people during the pandemic of 2019–21 [ 16 ]. Neurologic side effects have been reported in numerous patients both with SARS-CoV-2 infections and its predecessors, SARS (severe acute respiratory syndrome) and MERS (Middle Eastern Respiratory Syndrome) [ 23 , 46 , 78 ]. Viruses enter the central nervous system (CNS) either through hematogenous transport, the blood–brain barrier (BBB), or retrograde axonal transport [ 81 ]. Since SARS, MERS, and SARS-Cov-2 are respiratory viruses that enter the body via aerosol droplets, access to the CNS could be via nerve terminals located in the upper respiratory tract, via olfactory mucosa, olfactory bulb and olfactory tract [ 7 , 61 ]. However, whether this is the main pathway for CNS-invasion of SARS-CoV-2 is an issue that remains under-investigated. The primary cellular receptor for SARS-CoV-2 is the Angiotensin-converting enzyme 2 (ACE2) receptor [ 51 ]. A study reported that neurologic symptoms were exclusively found in patients with a moderate or high expression of ACE2 in peri-vascular cells, while similar symptoms were not detected in individuals without peri-vascular ACE2 [ 8 ].
Furthermore, viral particles of SARS-CoV-2 were discovered in postmortem cerebral spinal fluid (CSF) and brain gray matter [ 34 , 41 , 55 , 61 , 63 , 75 ]. Several different clinical–pathologic aspects observed in patients with COVID-19 such as neurologic symptoms, frontotemporal hypoperfusion, frontal slowed EEG and frontal hypometabolism on 18 F-FDG-PET imaging, suggesting that SARS-CoV-2 initially accumulates in the frontal lobes [ 30 , 77 ]. An FDG-PET study showed that patients with cortical cognitive dysfunction associated with COVID-19 displayed hypometabolism in the frontal cortex, anterior cingulate, insula and caudate nucleus [ 48 ]. Furthermore, transcriptomics revealed SARS-CoV-2 viral activity in frontal cortex of COVID-19 patients [ 24 ]. Although the ability of the SARS-CoV-2 virus to enter the CNS may underlie the loss of smell and taste, headaches, fatigue, nausea, dizziness, and delirium reported by people that recovered from COVID-19 [ 27 , 77 ], little is known about the pathogenesis of SARS-CoV-2 in the brain of people with neurodegenerative disorders.
Interestingly, the SARS-CoV-2 virus is associated with an exaggerated immune response, long-lasting neurologic symptoms, and an increased mortality risk for people with Alzheimer’s disease (AD, [ 35 ]) and/or Down syndrome (DS, [ 56 ]). Down syndrome is a developmental genetic condition associated with the most prevalent type of intellectual disability caused by complete or partial trisomy of human chromosome 21 (Chr 21) that affects more than 350,000 people in the USA alone [ 84 ]. Due to improved healthcare, individuals with DS have experienced a significant increase in average life span the last few decades but suffer from an increased incidence of early onset AD and associated amyloid [ 37 ], phospho-tau [ 38 , 57 , 72 ] and neurotransmitter cellular dysfunction [ 4 , 11 , 28 , 32 , 49 , 54 ] compared to the general population [ 36 , 79 , 80 ]. Moreover, adults with DS have an estimated fourfold increased risk for COVID-19 hospitalization and a tenfold increased risk for COVID-19-related mortality [ 13 ]. This led the Centers for Disease Control (CDC) to place persons with DS on the priority list for COVID-19 vaccination, both in Europe and the USA. The increased vulnerability to COVID-19 in people with DS might be due to several factors: a) sensitivity to pulmonary infections due to anatomic and medical comorbidities [ 42 ], b) the presence of the gene encoding for transmembrane serine protease 2 (TMPRSS2), a protein involved in spike protein priming after cell entry [ 39 ] on Chr 21, c) increased inflammatory response in individuals with DS, possibly due to the observation that four of the six known genes encoding interferon receptors are located on Chr 21 [ 12 , 18 , 22 , 73 ], and d) secretion of excessive amounts of exosomes [ 33 ] by which SARS-CoV-2 virus spreads [ 31 ]. Finally, persons with DS have a dysfunctional antibody response to viral respiratory infections [ 42 ], possibly increasing vulnerability to COVID-19. Despite these findings, there is a paucity of data on the neuropathological effect(s) and inflammatory reaction of the brain in people with DS after COVID-19. A single trisomy 21 case was listed in a study of the neuropathology of patients with COVID-19 from a German cohort showing widespread astrocytosis throughout the brain including the frontal cortex, but the study did not provide a detailed description of the DS case [ 59 ]. Others have reported microglial abnormalities in frontal cortex of patients with AD and COVID-19 [ 29 ], in the temporal and parietal cortex in elders with COVID-19 with dementia [ 66 ] and activated inflammatory signaling and oxidative overload in lateral cortex in patients with Covid [ 67 ]. It is generally thought that there is also an increased mortality rate for COVID-19 in patients with AD [ 83 ]. Moreover, SARS-CoV-2 infection induces AD tau pathology and increases AD plasma biomarkers [ 19 , 21 ]. However, to date there are no studies that have compared glial pathology in the brain of individuals with COVID-19 and AD, DS, or DS-AD comorbidities. To our knowledge, the present study is the first in-depth analysis of glial pathology in postmortem brains of people with DS with or without pre-existing AD, patients with AD and aged controls that succumbed to the SARS-CoV-2 virus infection. Here, we provide evidence showing an abnormal microglial and astrocytic neuroinflammatory response as well as the presence of SARS-CoV-2 nucleoprotein antibodies in postmortem human frontal cortex and to a lesser degree in hippocampal tissue obtained from individuals with AD, DS, or controls with or without COVID-19.
Materials and methods
Demographic information.
We examined brain tissue from three different clinical cohorts obtained from donations or clinical autopsies from 7 individuals with DS obtained from the Down Syndrome Biobank consortium (DSBC), a multisite brain bank consortium, a clinical autopsy cohort of 9 cases with or without COVID-19 from Lund University (Dr. Elisabet Englund), and 8 cases from the Medical University of South Carolina (MUSC) Brain bank in Charleston (Dr. Eric Hamlett). Table 1 details the demographic data for the 12 confirmed COVID-19 and 12 non-COVID-19 AD, DS and non-demented healthy control (CTRL) cases examined. The collection consisted of 10 females and 14 males with an average age of 58 years (range from 31 to 96 years) at the time of death . AD pathology varied across the different diagnostic groups: three AD non-COVID-19, four AD COVID-19 + , five non-COVID-19 and five COVID-19 + controls, one DS and two DS-AD without COVID-19 and three DS with COVID-19. Only one of the DS with COVID-19 reported dementia prior to the SARS-CoV-2 infection (case 24; DS COVID-19 recovered (DS COVID + r). Controls were carefully selected based on age, gender, and postmortem interval (PMI) to match the COVID cases and were collected by the MUSC brain bank. All individuals with DS COVID + cases in this study were treated in the intensive care unit for severe COVID and two of the three cases died from COVID-related complications. Case #24 was a 50-year-old male who had severe COVID, treated at the ICU, after which he recovered and was COVID negative (PCR test) and returned to an assisted living facility. During the subsequent year, his AD symptoms worsened, and the patient passed away one year following the COVID-19 infection. All DS cases with COVID were confirmed with a PCR test. Among the Swedish cases, all but one of the 8 COVID-19-positive cases were identified with nasopharyngeal PCR — either during hospital care or postmortem prior to autopsy. One COVID case was not tested but was later found positive—it was primarily sent for prion disease diagnostics due to suspected Creutzfeldt-Jakob’s disease. In this cohort symptoms varied extensively, likley due to various comorbidities. Six of eight cases were in-hospital patients, treated in the ICU or within the department of infectious disease, while one came to the emergency department as an unexpected cardiac arrest, and one died at home.
The AD COVID + and Control-COVID + cases were collected at Lund University, the DS COVID + cases were collected at University of Colorado and the DS-AD, Control, and AD cases without COVID were collected at the MUSC brain bank in Charleston and the DSBC. These collaborating sites use harmonized brain bank collection protocols and adhere to the NIH-AA brain banking protocols [ 6 , 40 ].
Tissue preparation
Brain pathology assessment was performed on paraffin-embedded cortical and limbic tissue as well as the medulla and spinal cord using a harmonized procedure based upon the NIA-AA protocol [ 6 ] with a few modifications at each site (Lund University, MUSC, UC Irvine, and CU Anschutz). The cases from DSBC were obtained under existing IRBs at each site and using a standard operating procedure [ 3 ]. Brains were carefully removed, and a standard assessment was conducted of the gross anatomy including regional atrophy and inspection of major blood vessels for vascular disease to document potential comorbidities or previous central events of importance for diagnosis. Brains were photographed from multiple surfaces and sliced in 1 cm coronal slices using a plexiglass brain jig to produce uniform coronal slices at each site [ 3 ]. The slices were placed on a cutting board and photographed prior to microdissection for freezing (left hemisphere) and immersion fixation (right hemisphere) for microscopic analysis. Right hemisphere slices were fixed in 4% paraformaldehyde for 72 h and transferred to a cryoprotectant solution consisting of 30% sucrose in phosphate buffered saline (PBS) with sodium azide according to a harmonized protocol [ 3 ]. Data regarding time and date of death, date of birth, potential clinical diagnoses, PMI , wet weight of the entire brain, and any special antemortem circumstances such as anxiety or pain were recorded. All cases were assigned a randomly generated global unified identifier (GUID). A neuropathology report was conducted on 19 standard brain regions consistent with a modified NIA-AA protocol [ 3 , 43 ]. Dissected fixed brain regions were paraffin embedded [ 65 ], cut at 5-microns and stained for hematoxylin and eosine (H&E), silver impregnation, and immunohistochemistry using alpha-synuclein, amyloid, phospho-tau, TDP43, and other specialty stains according to NIH protocols. For more details regarding the harmonized protocols in the DSBC consortium, see [ 3 ]. The cohort from Sweden underwent a clinical diagnostic analysis [ 10 , 44 ] as performed in every-day diagnostic autopsies (700 investigated cases per year). Briefly, the AD cases included patients who had a clinical diagnosis of Alzheimer-related dementia in the clinic followed by a neuropathological diagnosis as outlined in the NIA-AA protocol, see e.g., [ 10 ]. One laboratory (Barrow Neurological Institute) also cut 40-micron thick sections from fixed cryoprotected slabs on a sliding microtome for immunohistochemistry [ 65 ].
Immunostaining for the SARS-CoV-2 nucleoprotein
An anti-SARS-CoV-2 nucleoprotein antibody was used to detect the virus in postmortem brain tissue. The antibody (Catalog # 40,143-V08B; NP_828858.1; Met1-Ala422, Sino Biological (Nordic Biosite)/40143-T62) was produced in rabbit immunized with purified, recombinant SARS-CoV Nucleoprotein and used at a dilution of 1:2000. The staining was conducted using the Roche Ventana pathology laboratory staining system with Ultra View Universal AP (alkaline Phosphatase) Red Fast Red B as the chromophore. Positive/negative controls included staining of PCR-verified/excluded SARS-CoV-2 in paraffin-embedded fixed placental tissue (Fig. 1 , a1 and b1, respectively). Sections were counterstained with Hematoxylin. The nucleoprotein antibodies were tested against an anti-double-stranded RNA antibody [J2] (ab288755, Abcam), and tested for cross-reactivity with herpes simplex virus (HSV) 1 and 2, and cytomegaloviruses (CMV). The staining for the SARS-CoV-2 virus was conducted for all the cases at Lund University. Confirmatory studies included immunostaining with an anti-spike protein SARS-CoV-2 antibody (Proteintech, Rosemont, IL).

SARS-CoV-2 nucleoprotein immunostaining and H&E histochemistry. a – c SARS-CoV-2 nucleoprotein immunostaining (red stain) in positive (a1) and negative (b1) controls, AD and DS cases. a1 is a positive control (human placenta) and b1 is a negative control (human placenta). a2 is a micrograph from the dentate gyrus of a 37-year-old negative DS control (case #18 in Table 1 ). a3 and a4: SARS-CoV-2 immunoreactivity in neurons and glia cells (arrows in a3 and a4) in the frontal cortex in a 31-year-old DS COVID + case. The a3 inset shows a glial cell displaying punctate SARS-CoV-2 nucleoprotein cytoplasmic staining in a DS COVID + case. a4 displays SARS-Cov-2-positive neurons and microglia in layers II-III of frontal cortex from 50-year-old male with confirmed COVID infection a year before death but negative at death (DS COVID + r ). a2–4 shows SARS-CoV-2 nucleoprotein immunostaining in the hippocampus of a 35-year-old who died from acute COVID (b2), the 31-year-old DS COVID + case (b3), and the 50-year-old who recovered before death (DS COVID + r , b4). Note the lack of nucleoprotein immunoreactivity in the dentate gyrus granule cell layer (DGL) of the hippocampus in the 35-year-old case (b2) compared to the strong positive staining observed in the 31-year-old acute DS COVID case (b3) and the recovered case (b4), suggesting a temporal spread of the virus from frontal cortex to the hippocampus. The inset in b3 shows strong punctate SARS-CoV-2 labeling in a CA1 neuron in the hippocampus. Arrows in b3 and b4 mark glial cells labeled with the nucleoprotein antibodies. c1–c3: SARS-CoV-2 nucleoprotein labeling in cortex of AD COVID + cases from autopsy at Lund University. Note a strongly positive microglial cell in c1, and arrows pointing to positive glial cells in c2 and c3. C2 depicts positive cells (red labeling) in Layer I of the frontal cortex. Since this layer is devoid of neurons, positive cells in this layer represent glial cells only. d and e H&E hippocampal staining showing vascular abnormalities including microbleeds (d1, arrows) and extravasation of erythrocytes (d2 and d3) and vacuolization (arrow d2) as well as inflammatory infiltrates (d3) and neurodegenerative changes including dying and pyknotic neuronal cell bodies (d4) in all three DS COVID + and the DS COVID + r (d4) cases. e1–e3: H&E staining shows vascular abnormalities including focal congestion-like vein dilatation (e1, e3) and mild rupture of the vein wall with limited perifocal bleeding (e2) in the frontal cortex of a control COVID + and an AD COVID + case, respectively. Sections shown in panels a–c were counterstained with hematoxylin. Scale bar in a3 represents 25 microns in a1-3; scale bar in a4 represents 15 microns; scale bar in b4 represents 15 microns for b1-4; scale bar in c3 represents 15 microns for c1–3; scale bar in d3 represents 50 microns for d1–3; scale bar in d4 represents 20 microns and scale bar in e3 represents 200 microns for e1-3
Neuropathologic and clinical diagnostic evaluation
Neuropathological staging was conducted according to the Alzheimer’s disease neuropathologic change (ADNC) protocol, considering Braak, CERAD, and Thal diagnostic scoring [ 3 , 6 , 9 , 43 ]. For cases obtained from Lund University (AD + COVID and Control + COVID), the diagnosis of AD and of vascular pathology, as well as the exclusion of such pathology in the controls and other pathology, was established in adherence with internationally accepted criteria for AD [ 15 , 40 , 60 , 76 ]. The diagnostic strategy was corroborated by the extensive clinical information available in these cases (see Table 1 ), as described in a previous publication [ 44 ].
Immunohistochemical staining
Standard H & E and immunohistochemistry using primary antibodies directed against alpha-synuclein (cat: AB5038, Millipore, Darmstadt, Germany, 1:250), Aβ 1–42 (Abcam, cat. AB5078P, 1:100), and phospho-Tau (AT8; cat: MN1020, Thermo Fisher Scientific, Waltham, MA, USA, 1:250) was performed. Endogenous peroxidase activity was blocked by a 7:2:1 ratio of TBS, MeOH, and 3% hydrogen peroxide (H 2 O 2 ), respectively, followed by incubation with 100 mM sodium metaperiodate. Pretreatment with heat and 0.05% citraconic anhydride for 10 min were used to enhance p-Tau and amyloid immunostaining. Sections were blocked with TBS containing 0.25% Triton-X and 10% normal serum for 1 h and incubated overnight with the primary antibody. After washing in PBS, sections were incubated with biotin-conjugated secondary antibodies, washed in PBS, incubated with streptavidin–horseradish peroxidase (HRP) complex, and visualized using a 1 mg/mL 3′,3′-diaminobenzidine (DAB) solution containing 0.02% H 2 O 2 , dehydrated, and cover-slipped using Permount mounting medium. Immunohistochemical controls included deleting either the primary or secondary antibodies resulting in no detectable reactivity. Immunohistochemical stains from the cases obtained from each brain bank were performed together in the same batch to avoid inter-batch variabilities in staining.
The tissue sections shown in Fig. 3 were obtained from additional 1 cm brain slices fixed in 4% paraformaldehyde for 48 h, cryoprotected in graded concentrations of sucrose, sectioned on a freezing sliding microtome at 40-micron thickness and immunostained at Barrow Neurological Institute. All other sections were generated from 5-micron paraffin-embedded blocks obtained on a microtome and were stained at CU Anschutz Medical Campus. This manuscript is the result of a collaboration between several different laboratories with slightly different protocols.
Glial staining and analysis
Two to three sections per brain region were immunostained with antibodies directed against glial fibrillary acidic protein (GFAP, rabbit polyclonal, Abcam, Boston, MA, cat. # EPR1034Y, 1:500) to visualize astrocytes, a pan-microglial marker Iba-1 (rabbit polyclonal, WAKO, Richmond, VA, cat. # 013–27691, 1:1,000) and a transmembrane protein 119 (TMEM119) specifically expressed by resident microglia (rabbit polyclonal, Abcam, Waltham, Boston, MA, cat. # ab185333, 1:100), but not macrophages [ 5 ], following the above immunohistochemistry protocol. Iba-1 and TMEM119 immunostained sections were in addition counterstained with hematoxylin. All sections included in the quantification were stained at CU Anschutz Medical Campus and parallel staining with the same antibodies were also conducted at Barrow Neurological Institute.
Three images of each region of interest (ROI) were captured on each section using a Nikon Optiphot microscope for image analysis at 20X magnification. The ROIs examined included gray matter of the middle frontal gyrus (Brodmann area 46), hippocampal dentate gyrus and CA1 subfield, and the para-hippocampal gyrus. For each ROI, three images of the underlying white matter were captured for comparison with gray matter. Images used for quantification were taken at the same magnification and light intensity.
Sholl analysis
Since the complex morphologic structure of astrocytes is crucial to their situational function in brain, we determined group differences in peri-vascular astrocytic processes and branching using the Fiji ImageJ software package with the Sholl analysis plugin [ 25 , 71 ]. This assessment was performed by an unbiased investigator blinded to the groups. Sholl is an open-source software used for morphometric analysis of astrocytic processes by quantifying length, number, and intersections at concentric spheres originating from the soma [ 74 ]. The first 25 astrocytes in the vicinity of a blood vessel in gray matter displaying a visible nucleus and at least one process stained for GFAP were analyzed per section and per region by an investigator blinded to the identity of each sample. Morphologic complexity of the astrocytes was calculated based on the number of intersections at each radial distance from the starting point, including sum of intersections, mean intersections, and ramification index determined by the Sholl plugin software [ 25 , 71 ]. The ramification index is the ratio between the maximum number of intersections of the processes with the circles and the number of the primary processes.
Iba-1 immunostaining was evaluated by an unbiased and blinded investigator using the Fiji ImageJ software system (Version 2.3.0/1.53q) according to a semiquantitative previously validated and published protocol [ 14 ]. First, the color channels were split to isolate the DAB-reaction product from background staining. The threshold was adjusted to include only immunostained cells, and % area stained was assessed. Measurements were taken on three random areas per section of the dentate gyrus, CA1, and the white matter adjacent to the hippocampal formation.
Statistical analysis
Brain tissue from each case was stained 2–4 different times, allowing multiple sections to be evaluated per brain and ROI. In addition, repeated staining batches ensured that all groups were represented in each staining batch to avoid inter-batch variability in staining density between groups. A nested two-way ANOVA was used, where one factor (individual sections in this example) was nested within another factor (infection/diagnosis) due to the limited access to COVID-19-infected brains from each group in the study. We used a nested design to determine the morphologic complexity of single astrocytes [ 1 ]. Nested designs yield clusters of observations that are not considered independent and require special considerations in terms of statistical analysis [ 1 ]. Valid and efficient subgroup analyses were performed using nested case–control data according to previously published protocols [ 20 ]. This nested case–control design provides an unbiased estimate of the effects of SARS-CoV-2 neuroinflammation in several samples of each brain region, using several different parameters obtained from the Sholl analysis of astrocytes, and the density of two different microglial markers. Previous work has shown that subgroup analyses provide unbiased estimates of the effects in a population, when sampling different brain regions and different distinct neuronal populations from the same individual as carried out in the current study [ 20 ]. Sex, age, and PMI were considered in the analyses, but none altered the statistical outcomes.
Ethical considerations
Postmortem consents were obtained from the next of kin for all brain donations used in the study, either via the DSBC consortium or the Carroll Campbell Jr. Neuropathology Laboratory (CCNL) brain bank at the Medical University of South Carolina (MUSC). For the Swedish cohort, ethical permission was given from the regional ethical authorities, nr 2020–02369. The application was submitted since the intention of the primary examination (autopsy) was a clinical diagnostic investigation.
SARS-CoV-2 nucleoprotein and H&E staining
Positive (Fig. 1 a1) and negative (Fig. 1 b1) immunostaining for the SARS-CoV-2 nucleoprotein showed significant staining in the placenta from a PCR-confirmed patient (Fig. 1a1) and a PCR-confirmed negative placenta (Fig. 1 b1). SARS-CoV-2 nucleoprotein immunoreactivity appeared granular within glial cells (Fig. 1a3) and neurons (Fig. 1 a3, a4,) in frontal cortex of all three DS COVID-19 + cases. In the hippocampus, two of the three DS COVID + cases, the 50-year-old (Fig. 1 b4) and the 31-year-old (Fig. 1 b3) were positive for SARS-CoV-2, but not the 35 YO case with DS and COVID + (Fig. 1 b2). SARS-CoV-2 nucleoprotein immunoreactivity was not observed in the frontal cortex (not shown) or hippocampus in individuals with DS without (Fig. 1 a2) COVID-19 infection. Interestingly, the strongest nucleoprotein immunostaining was seen in both the frontal cortex (Fig. 1 a4) and hippocampus (Fig. 1b4) of the DS case that died 12 months after contracting COVID-19 but tested negative for the virus at the time of death (DS COVID + r). SARS-CoV-2 nucleoprotein immunolabeling was also observed in all AD and control COVID-19 + cases (Fig. 1 c1-3). A microglial cell in gray matter is shown with strong nucleoprotein immunostaining in an AD COVID + case in Fig. 1 c1. The sections were counterstained with hematoxylin (blue). Pink granular labeling represents SARS-CoV-2 nucleoprotein immunostaining.
Brain histopathology
Vascular abnormalities including microbleeds and extravasation of erythrocytes (Fig. 1 d1-2) as well as inflammatory infiltrates and deposits or calcification of the external wall of the blood vessels (Fig. 1 d3) were observed in the hippocampus and frontal cortex in all three DS COVID + patients. This was confirmed by the neuropathology report for the cases, where the two DS cases with acute COVID-19 were found to have mild CAA and microcalcifications, respectively (see Table 1 ). Abnormal neuronal morphology with pyknotic cell bodies (Fig. d 4 ) was frequently observed in DS COVID + cases. Focal blood vessel abnormalities were often seen in both gray and white matter in the AD COVID + and Control-COVID + cases, with scattered aggregates of congestion-like dilated veins (Fig. 1 e1 and e3), rupture of the vein wall (Fig. 1 e2) and limited perifocal bleeding (Fig. 1 e2). Some vascular abnormalities were associated with tissue edema (Fig. 1 e2). Such vascular alterations were not seen in the non-COVID cases.
p-Tau and amyloid immunostaining
All AD and DS cases with a Braak stage of V-VI exhibited p-Tau (AT8) NFT-like immunostaining in the frontal cortex (Fig. 2 a1), hippocampus (Fig. 2 a2), and para-hippocampal gyrus (not shown). In the two AD cases with Braak stage III and IV all-tau pathology was observed in limbic structures. In addition, several (Fig. 2 a3) to occasional (Fig. 2 a4) AT8 positive neurons and neuropil threads were found in the para-hippocampal gyrus but not in the hippocampal formation or frontal cortex in two control COVID + cases (71 and a 65-years-old, see Table 1 ).

AT8 and Aβ1-42 immunostaining. a Images showing AT8 positive NFTs in the frontal cortex (a1) and hippocampus (a2-a4). a1-a2. AD COVID + cases contained frequent NFTs (dark brown staining) and lightly labeled neurons with an intact morphology (i.e., pre-tangles) in frontal cortex and hippocampus (arrows), respectively, compared to a few scattered NFTs in control COVID + cases (a3, a4). Note numerous AT8 positive neuropil threads in the hippocampus of a 71-year-old control COVID + case (Fig. 2 a3) compared to a rare NFT observed in the CA1 of a 65-year-old patient with COVID (Fig. 2 a4). b and c Aβ1-42 (brown) immunostaining in AD, DS, and control cases. b1. Aβ immunostaining was not observed in DG of the hippocampus of a young person with DS (37-year-old) without COVID-19. b2–b4. Images showing an occasional Aβ positive blood vessel in the frontal cortex (b2) of a DS COVID + case, and numerous fibrillar and diffuse amyloid plaques in the AD COVID + cases (b3 and 4). c . Frontal cortex diffuse Aβ (c1-c2) in DS COVID + cases compared to neuritic plaques in the DS COVID + r case (c3) and a DS-AD COVID- case (c4). The DS-AD case depicted in c4 was a 66-year-old male with DS, while the DS COVID + cases in c1 and c2 were 31 and 35 years old, respectively, and the DS COVID + r case in c3 was 50 years old. d Weak to moderate deposits of diffuse (d2, age 37 and d3, age 71 years) and intracellular (d1, age 33, d2 age 37 (arrows) and d4, age 65 (arrows) Aβ1-42 immunostaining were seen in the CA1 and dentate gyrus of the hippocampus in 5 out of 5 control COVID + cases (d1-4). All sections in panels b-d were counterstained with Hematoxylin. The scale bar in b1 represents 50 microns for a1, a3, b1, b3, c1-c4, and scale bar in b4 = 20 microns in b4, a2, and a4
Aβ-positive plaque pathology was not observed in the dentate gyrus (DG) of the hippocampus of a young person with DS (37-year-old) without COVID-19 (Fig. 2 b1), which had a Thal Phase score of 3 with mild cortical atrophy (Table 1 ). An occasional Aβ positive blood vessel in the frontal cortex (Fig. 2 b2) as well as diffuse amyloid plaques (Fig. Fig. 2 c1-2) were observed in the 31 YO and the 35 YO cases with DS and COVID-19. On the contrary, frequent fibrillar and diffuse amyloid plaques were observed in the 50 YO male who passed away with severe dementia one year after COVID-19 (DS COVID + r, Fig. 2 c3). The frequency of amyloid plaques in this 50 YO case was comparable to that seen in DS-AD cases without COVID infections (Fig. 2 c4). Finally, we found diffuse amyloid plaques in the neuropil (Fig. 2 d2 and 3) and intra-neuronal (d4, arrows and Fig. 2 d1, inset) Aβ labeling in the hippocampus of control COVID + cases, respectively. Intraneuronal and extracellular Aβ immunostaining was observed in all young (Case #13, 16 and 17) and the two older (Case# 14,15) Control-COVID + cases (see Table 1 ).
GFAP astrocytic immunostaining
GFAP immunostaining revealed different astrocytic morphologies between cases with and without COVID-19 (Figs. 3 and 4 ). Representative images of GFAP immunostaining in the frontal cortex from a 31-year-old male with DS who died from severe COVID-19 (Fig. 3 a and c), compared to the frontal cortex from an individual with DS without dementia and no COVID-19 infection (Fig. 3 b and d). Note the extensive dense branching of individual astrocytic processes in COVID + (Fig. 3 a and c) compared to shorter and less dense GFAP stained processes surrounding the bifurcation of a blood vessel in a DS COVID-(Fig. 3 b and d) case.
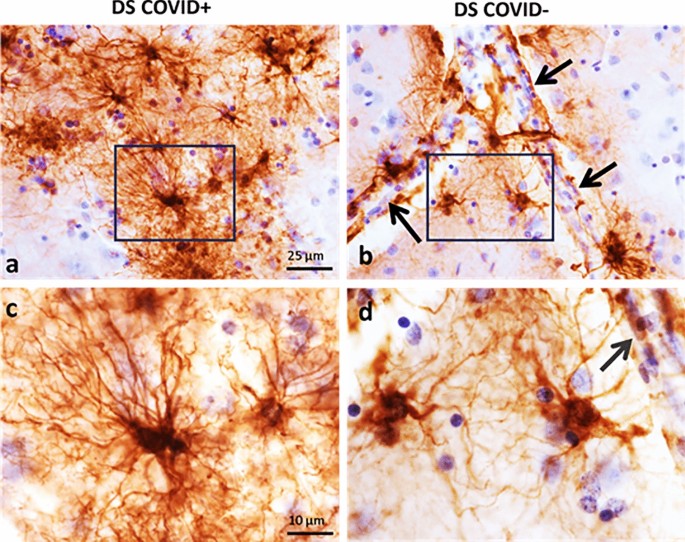
GFAP immunostaining in the frontal cortex. Low ( a and b ) and high ( c and d ) magnification photomicrographs showing GFAP positive astrocytes in the frontal cortex gray matter of a 31-year-old DS COVID + ( a , c ) compared to a 37-year-old DS COVID- ( b , d ) case. Panels c and d show a higher magnification image of the boxed area in a and b displaying an increase in the length and number of GFAP positive processes in a DS COVID + ( C ) compared to a COVID- ( d ) DS case. Black arrows in b and d indicate the close apposition of GFAP processes with the vascular wall. All sections were counterstained with H&E. The scale bar in a = 25 µm applies to b , and bar in c = 10 µm, which applies to d

GFAP immunostaining in the frontal cortex ( a ) and hippocampus ( b ) across cases. Frontal cortex (a1-8) and hippocampal (b1-8) images showing differences in morphology between GFAP labeled peri-vascular astrocytes (brown) in DS COVID + compared to other cases. GFAP positive glia exhibited long processes with prominent end-feet in close apposition to the vascular wall (arrows) in DS COVID + cases (a2,3 and b2,3) compared to smaller cell bodies and fewer slender branched processes in the frontal cortex (a1, arrows) and hippocampus (b1) in control non-COVID cases. AD (a5, b8) and DS-AD (b5) COVID- cases displayed GFAP positive astrocytes with thicker but shorter processes. Control COVID + (a6, a7, b6) and AD COVID + (a8, b7) cases exhibit reduced GFAP astrocytic activation and occasionally displayed an apoptotic or necrotic cell (a8, b7). The scale bar in a5 represents 20 microns for all panels except for a8, where the scale bar represents 10 microns
Similar astrocytic morphologies were observed in frontal cortex (Fig. 4 a2-4) and hippocampus (Fig. 4 b2-4) in DS COVID-19 + cases. Control non-COVID-19 cases (Control COVID-) showed star-shaped peri-vascular astrocytes with small end-feet extending onto the vascular wall in the frontal cortex and hippocampus (Fig. 4 a1 and b1). AD and DS-AD COVID-19 negative (COVID-) cases exhibited an increase in the density of GFAP labeled cells, with shorter, stubbier processes indicative of an inflammatory glial-induced response to the AD pathology (Fig. 4 a5, b5, b8). By contrast, AD COVID + and DS COVID + cases displayed elongated astrocytic processes and a significant increase in the number of processes extending from each cell within the gray matter of the frontal cortex (Fig. 4 a2-4,a5) and hippocampus (Fig. 4 b2-4, b7). This was particularly evident adjacent to the vascular wall in the gray matter in both regions. Moreover, peri-vascular astrocytes also extended enlarged end-feet, particularly in the DS COVID + cases, sometimes surrounding the vascular wall (Fig. 4 a2 and a3, arrows). Glial processes extended long distances reaching the vascular wall, where prominent end-feet lined the lumen, resembling a protective wall of end-feet known as glia limitans . This pattern of astrocyte processes and prominent end-feet on the vascular wall resembles that seen in encephalitis [ 69 ], but not usually observed in AD or DS brain tissue. In some areas of gray matter, GFAP staining appeared punctate, and an occasional degenerating cell body was seen mainly in the AD COVID + cases (Fig. 4 a8 and b7). The DS recovered from COVID-19 case (Fig. 4 a4 and b4) displayed astrocytic morphologies like that seen in the hippocampus and frontal cortex of AD COVID- (Fig. 4a5) and DS-AD COVID- (Fig. 4 b5) cases, suggesting that acute SARS-CoV-2 infection gives rise to the abnormal astrocyte morphology observed in DS COVID + and AD COVID + cases, which does not linger months after recovery from acute infection.
Sholl analysis of GFAP immunostained sections revealed an increase in arbor complexity in peri-vascular astrocytes in DS and AD COVID-19 positive cases. The ramification index for peri-vascular astrocytes in the gray matter of hippocampus is shown in Fig. 5 a. The ramification index is the ratio between the maximum number of the intersections of the astrocytic processes and the number of primary astrocytic processes [ 25 ]. The average hippocampal ramification index in the control COVID- was 29.6 ± 5.6, the AD COVID- was 44.2 ± 15.2, the DS COVID- was 41.1 ± 16.7, the control COVID + was 36.7 ± 4.2, the AD COVID + was 47.1 ± 18.3, and the DS COVID + was 84.2 ± 0.8. The highest average ramification index was observed in the DS- COVID + compared to the other groups, with nearly a threefold higher average in the control COVID- cases. The overall ramification index in the hippocampus between groups had a p value of 0.007 (nested one-way ANOVA F, dfd 5.745, 5, 11, where F is the F distribution, dfn is the degrees of freedom of the numerator, and dfd is the degrees of freedom of the denominator). A post-hoc analysis (Tukey’s method) revealed a significantly higher average ramification index in DS COVID + compared to non-COVID control ( p = 0.004) cases, and between the DS COVID + vs. DS COVID- ( p = 0.04) group. Finally, DS COVID + cases showed a significantly higher ramification index compared to the control COVID + group ( p = 0.01). Although sex, age, and PMI were considered as covariates, statistical outcomes were not changed. Whether access to a larger group of DS COVID cases would alter the present statistical outcomes remains to be determined.

Sholl analysis of differences in astrocytic morphology across groups. a Analysis revealed a significantly higher ramification index for peri-vascular astrocytes in the gray matter in DS COVID + group compared to DS COVID- (Tukey test, p = 0.04, n = 24) and control non-COVID (Tukey test, p = 0.004) and COVID + (Tukey test, p = 0.01) groups. b Ending radius measurements of peri-vascular astrocytic processes were significantly greater in DS COVID + compared to control COVID + (Tukey test, p = 0.0007), AD COVID- (Tukey test, p = 0.002), and DS COVID- (Tukey test, p = 0.001) groups. c . Analysis of the sum of intersections in the hippocampal gray matter revealed significant differences between groups (Nested ANOVA, p = < 0.0001) with greater numbers of astrocytic intersections in the DS COVID + group. The Tukey post-hoc test revealed significant differences between control COVID- vs. DS COVID + ( p < 0.0001), AD COVID- vs. DS COVID + ( p < 0.0001), DS COVID- vs. DS COVID + ( p < 0.0001), control COVID + vs. DS COVID + ( p < 0.0001), and AD COVID + vs. DS COVID + ( p = 0.0006). d Nested ANOVA analysis revealed significant differences in the sum of intersections only for frontal cortex gray matter between groups; Tukey post hoc test revealed a greater sum of intersections in DS COVID + compared AD COVID-, p = 0.02) and DS COVID- ( p = 0.01) groups
We also measured the ending radius of astrocytic processes. The DS COVID + exhibited the longest extension of astrocytic peri-vascular processes (mean length of 58 ± 7.8 μm) compared to the control COVID- (mean length of 35 ± 6 μm) group. AD COVID + group also exhibited greater astrocytic process length (mean of 46 ± 10 μm). Statistical analysis revealed an overall significant difference between groups (Fig. 5 b, p = 0.0003; F, DFn, Dfd 6.509, 5, 31). Post-hoc group comparisons using the Tukey’s multiple comparisons test revealed significant differences between control COVID + and DS COVID + ( p = 0.0007), AD COVID- and DS COVID + ( p = 0.002), and DS COVID- and the DS COVID + ( p = 0.001) groups, suggesting that the COVID-19 infection induced a significant lengthening of peri-vascular astrocytic processes, particularly in DS compared to control COVID- and AD COVID- cases.
A key measure of astrocytic complexity is the sum number of branch points of astrocytes. The average number of intersections was highest in the DS COVID + cohort, with an average sum of intersections of 23,125 ± 1391 compared to an average 3042 ± 1000 intersections in the control COVID- group. Sholl analysis of the sum intersections of GFAP positive astrocytes within the hippocampus revealed a significant difference between groups (Fig. 5 c p = < 0.0001; F, DFn, Dfd: 13.18, 5, 31). Post-hoc analysis revealed a statistical significance between control COVID- vs. DS COVID + ( p < 0.0001), AD COVID- vs. DS COVID + ( p < 0.0001), DS COVID- vs. DS COVID + ( p < 0.0001), control COVID + vs. DS COVID + ( p < 0.0001), and AD COVID + vs. the DS COVID + ( p = 0.0006) groups.
We next measured morphologic alterations to astrocytes in the gray matter of the frontal cortex using Sholl analysis (Fig. 5 d). Although differences in astrocyte morphology were observed between the groups in the frontal cortex (see Figs .3 and 4 a), Sholl analysis revealed only a statistically significant change in the sum of intersections (Fig. 5 d) ( p = 0.007; F, DFn, Dfd 4.484, 5, 19). The average values for sum of intersections in the frontal cortex was 4270 for the control COVID- group, 5760 ± 871.6 for the AD COVID-, 5499 ± 2023 for the DS COVID, 9214 ± 2640 for the control COVID + , 8313 ± 1875 for the AD COVID + and 12,129 ± 1296 for the DS COVID + group. Post-hoc Tukey analysis revealed significantly higher numbers of intersections in the DS COVID + compared to the AD COVID- ( p = 0.02) and DS COVID- ( p = 0.01) groups in the frontal cortex. This analysis revealed the highest number of astrocyte branch points in DS COVID + , followed by control COVID + and then AD COVID + cases in this brain region (Fig. 5 d), with more than a 100% increase in the average number of branch points in the DS COVID + compared to the non-COVID control group. Interestingly, both the control COVID + and the AD COVID + groups showed elevated average branch points compared to the control COVID- cases, suggesting a common feature of astrocytic morphology after SARS-CoV-2 infection.
Iba-1 and TMEM119 microglial immunostaining
We examined the morphology of microglial cells in the hippocampus and frontal cortex using antibodies against Iba-1 that recognize microglia and peripheral immune cells (Figs. 6 and 9 ) and TMEM119 (Figs. 8 and 9 ), which only label microglia [ 26 ]. Qualitative observations revealed fewer Iba-1 immunostained cells in gray and white matter in both frontal cortex and hippocampus (Fig. 6 a2, a6, b2, b6) in DS COVID + cases compared to the other groups. The loss of Iba-1-ir microglia in the gray matter of DS COVID + (Fig. 6 a2) seen in both the 31-year-old and the 35-year-old DS COVID + cases was conspicuous compared to the DS 37-year-old age-matched individual without COVID infection (Fig. 6 a1). A similar loss of Iba-1 immunostaining was observed in the white matter (Fig. 6 a6 and b6), but not to the same extent as observed in gray matter. Most groups showed a comparable density of Iba-1 positive microglia in white matter, except for an increase in the white matter of the DS-AD COVID- case (Fig. 6 a8) compared to the other groups. Higher magnification microscopy revealed focal groups of activated Iba-1-ir microglia in the DS COVID + (Fig. 9 b) group and numerous small, rounded Iba-1-ir cell bodies in the gray matter (Fig. 9 b inset and c), compared to the more common branched microglial morphology observed in control COVID- brains (Fig. 9 a).

Hippocampal Iba-1 immunostaining in the dentate granule cell layer (DGL) and white matter between COVID + and COVID- cases. a . Images showing a reduction in Iba-1-ir cells in DGL (a1-a4) and white matter (a5-a8) in DS COVID + (a2, a6) compared to DS COVID- (a1, a5) and DS-AD COVID- (a4, a8) cases, while intermediate Iba-1 cell immunostaining was seen in the DS COVID + r (a3, a7) case. b . Images showing reduced Iba-1 positive cells in the DGL (b1-b4) and white matter (b5-b8) in a DS COVID + (b2) compared to control COVID + (b1), AD COVID + (b3) and control COVID- (b4) case. The DS COVID + case depicted in a2 and a6 was 31 years old, and the DS COVID + case depicted in B2 and B6 was 35 years old, respectively, while the DS COVID- case in A1 and A5 was 37 years old, and the DS COVID + r case depicted in a3 and a7 was 50 years old. The DS COVID- case shown in a1 and a5 was 37-years old. All sections were counterstained with Hematoxylin. Scale bar in b2 = 50 µm applies to all panels
To quantify the loss of Iba-1 and TMEM119 immunoreactivity in the hippocampus, we used a nested ANOVA analysis, where one factor (individual sections in this example) was nested within another factor (infection/diagnosis), to explore whether the percent area covered by Iba-1 positive microglia differed in the gray matter (Fig. 7 a) or in white matter (Fig. 7 b) between groups. The average percent area covered by Iba-1 positive microglia in the hippocampal gray matter was 2.5 ± 0.8 percent in control COVID-, 6.3 ± 4 percent in AD COVID-, 2.1 ± 0.1 percent in DS COVID-, 0.9 ± 0.9 percent in control COVID + , 1.9 ± 1.2 percent in AD COVID + , and 0.6 ± 0.3 percent in DS COVID + cases. We found a statistically significant difference between groups in hippocampal gray matter (nested ANOVA ( p < 0.0001; F, DFn, Dfd = 24.60, 5, 53). Post-hoc analysis Tukey test showed significant group differences between the control COVID- and AD COVID- ( p < 0.0001), AD COVID- and DS COVID- ( p < 0.001), AD COVID- and control COVID + ( p < 0.0001), and AD COVID- compared to DS COVID + ( p < 0.0001) groups. These findings suggest that the COVID-19 infection affected Iba-1 labeled immune cells in the gray matter in both the AD and the DS cases, with the greatest loss in the DS COVID + cases, which displayed an approximately fourfold lower percentage of staining compared to the control COVID- group. Whether similar statistical differences between microglia densities would be found in larger cohorts of similar patient groups remains to be seen.

Analysis of Iba-1 and TMEM119 immunostaining density in hippocampal dentate gyrus and white matter. a Percent area of Iba-1 immunostaining was significantly lower in DS COVID + , DS COVID-, control COVID-, and control COVID + in hippocampal gray matter compared to AD COVID- cases (Tukey test, p < 0.0001 for all groups in comparison to the AD COVID- group, n = 20). b Measurements of the percent area of Iba-1 staining in the white matter was significantly greater in AD COVID- compared to DS COVID-, control COVID + , AD COVID + , DS COVID + groups (Tukey–Kramer test, p < 0.0001 for all groups). c Percent immunostaining in hippocampal white matter as examined using a nested ANOVA analysis. Nested one-way ANOVA, p value 0.0003 p value summary***, F , DFd, Dfd 6.2, 5, 40. Significance: DS COVID vs. AD COVID p = 0.0076, DS COVID vs. AD p = 0.0006, DS COVID vs. Ctrl, p = 0.0041, Ctrl-COVID vs. AD: p = 0.0279
In the hippocampal white matter (Fig. 7 b), the overall percent area covered by Iba-1 positive cells was also significantly different between the groups following a nested ANOVA analysis where individual sections are nested within groups (infection vs. diagnosis; p = 0.005, F, DFn, Dfd = 5.627, 5, 14). Post-hoc Tukey test revealed significant differences between the AD COVID- vs. control COVID + ( p = 0.02), AD COVID- vs. AD COVID + ( p = 0.04) and AD COVID- vs. DS COVID + ( p = 0.002) groups. The average percent area covered by Iba-1 positive cells in the white matter was 3.9 ± 1.7 percent in control COVID-, 5.98 ± 5 percent in AD COVID-, 3.3 ± 1.1 percent in DS COVID-, 1.8 ± 0.9 percent in control COVID + , 2.75 ± 0.4 percent in AD COVID + , and 1.2 ± 0.8 percent in DS COVID + cases.
There was also a reduction in the density of TMEM119 stained microglia in the CA1 pyramidal cell layer (Fig. 8 a2) and DG (Fig. 8 a3) of the hippocampus in the DS COVID + and control COVID + (Fig. 8 b2) groups. A nested ANOVA revealed an overall significant difference between the groups ( p = 0.0003; F, DFn, and Dfd were 6.2, 5 and 40, respectively, see Fig. 7 c). However, no significant difference between the groups was found in terms of percent staining for TMEM119 in white matter of the same cases. A greater number of TMEM119-positive cells were found in the white matter than in the gray matter in all groups (Fig. 8 ). TMEM119-positive microglia displayed rounded, smaller cell bodies with few observable processes in DS COVID + (Fig. 9 e) or a resting-type morphology with longer, slender processes, mostly in control COVID- or in DS COVID- (Fig. 9 d and f, respectively) cases. In the hippocampal gray matter of the DS COVID + cases, TMEM119-positive rounded cell types were more prevalent than those with a resting-type morphology (Fig. 9 e), suggesting activation of remaining microglia in response to the SARS-CoV-2 infection. Focal groups of activated microglia were observed in both gray and white matter in the DS COVID + (Fig. 9 b), and sometimes in AD COVID + and control COVID + cases (data not shown).
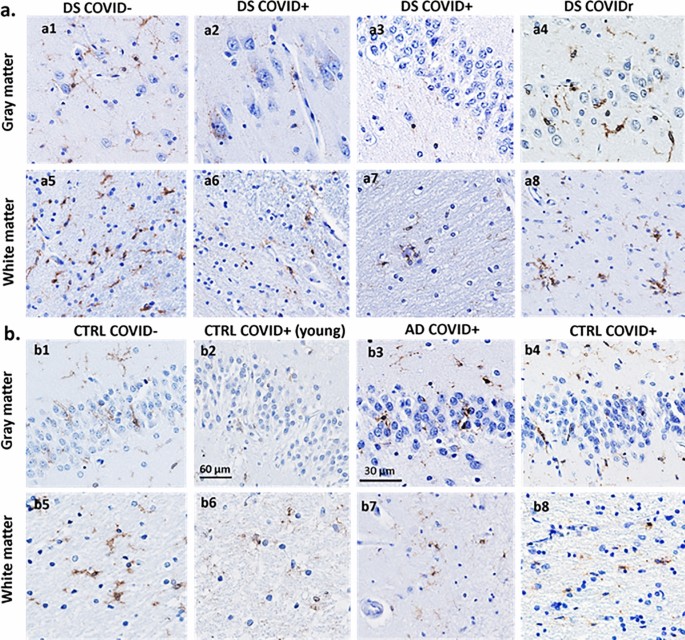
Microglial TMEM119 immunoreactivity in gray and white matter of the hippocampus. a Images showing a reduction in TMEM119-ir cells in the CA1 region (a2) and the DGL (a3) and white matter (a6, a7) in DS COVID + (a2, a3, a6, a7) compared to a DS COVID- (a1, a5) and DS COVID + r (a4, a8) case. b Images showing less TMEM119 immunoreactivity in the DGL (b2) and white matter (b6) of the hippocampus in a young control COVID + (33-year-old) compared to control COVID- (59-year-old) (b1, b5), AD COVID + (71-year-old) (b3, b7) and an older control COVID + (71-year-old) (b4, b8) case. All sections were counterstained with Hematoxylin. The scale bar in b3 represent 30 microns in a2, a3, a4, and b3, and the scale bar in b2 represents 60 microns for the rest of the panels

Morphologic features of Iba-1 ( a–c ) and TMEM119 ( d – f ) positive hippocampal microglia in control COVID- and DS COVID + cases. Microglial cells stained for Iba-1( b , c ) and TMEM119 ( e ) displayed a rounded appearance with few processes (arrows in c and e, and inset in b ), resembling the morphology observed in phagocytic microglial cells, in DS COVID + ( b , c , e ) compared to more elongated cell bodies and extensive processes seen in control COVID- ( a , d ) and DS COVID- ( f ) cases. Clusters of Iba-1 immunostained microglial cells were occasionally observed in white matter ( b ) in DS COVID + cases. All sections were counterstained with Hematoxylin. The scale bar in d represents 25 microns for all images
This is the first systematic study comparing the neuropathological effects of SARS-CoV-2 infections in postmortem brain tissue obtained from patients with DS, AD, and non-demented healthy controls compared to non-COVID counterparts. SARS-CoV-2 nucleoprotein immunostaining, using a nucleoprotein antibody against the virus [ 75 ] demonstrated viral nucleoprotein in neurons and microglial cells in gray matter of the frontal cortex and hippocampus of two of the three DS cases with confirmed SARS-CoV-2 infection. GFAP immunostained astrocytes exhibited a significant increase in length of arbors, as well as a significant increase in the numbers of branch points on astrocytic processes in DS COVID + and AD COVID + compared to the other groups, particularly in peri-vascular astrocytes. These findings suggest that SARS-CoV-2 infection gives rise to a significant alteration in peri-vascular astrocyte morphology, consisting of an overall lengthening of processes and a significant increase in branch points in individuals with DS COVID + and AD-COVID + post infections, as well as an increase in the size and number of vascular end-feet. In addition, there was a reduction of Iba-1 and TMEM119 stained microglial cells in gray matter but to a lesser extent in white matter in the frontal cortex and hippocampus.
SARS-CoV-2 nucleoprotein displayed a granular appearance within neurons and glial cells in the frontal cortex and hippocampus in SARS-CoV-2 infected DS cases. Recently, it was shown that both neuron-derived and astrocyte-derived exosomes obtained from patients with COVID-19 contain both SARS-CoV-2 Spike protein 1 and nucleocapsid (N) proteins, suggesting that exosomes play a role in transport and/or cellular uptake of the virus [ 64 ]. A recent study demonstrated that exosomes from SARS-CoV-2 infected lungs reach the brain parenchyma, which affects AD associated neuronal gene regulatory networks in frontal cortex, temporal cortex and hippocampus in AD accelerating neurodegeneration implicating an importance of brain exosomes in disease progression [ 2 ]. To confirm exosomal content of viral particles future studies will undertake sub-cellular co-labeling of the nucleoprotein with vesicle markers in DS and AD. We found SARS-CoV-2 weak to moderate nucleoprotein immunostaining in the frontal cortex of all three DS cases with COVID-19, but only in the hippocampus in two of the three DS cases—the case that had the infection 12 months prior to death, and the patient that passed away acutely from severe COVID-19. However, whether this coronavirus enters the central and/or peripheral nervous system to directly affect blood vessels, neurons, and glial cells (see [ 27 ]) or are secondary to the cytokine storm [ 22 ] remains controversial. In this regard, it has been shown that the BBB is dysregulated in COVID-19 + which serves as a potential entry route for SARS-CoV-2 to the brain [ 51 ]. These investigators also demonstrated SARS-CoV-2 in the basolateral compartment of the BBB using a trans-well assay after apical infection in vitro, suggesting active replication and transcellular transport of the virus across the BBB perhaps by exosomes [ 2 ], or via other systems.
In a recent study, 41 autopsy cases with confirmed COVID-19 showed areas from each brain with hypoxic/ischemic changes, which were either global or focal with large and small infarcts, many of which were hemorrhagic [ 75 ]. The presence of viral RNA and protein, using quantitative reverse-transcriptase PCR, and immunocytochemistry with primers, probes and antibodies directed against the spike and nucleoprotein regions, found low but detectable viral RNA levels in brain tissue but were unable to detect immunohistochemical evidence of viral particles [ 75 ]. On the other hand, another autopsy study provided evidence for peri-vascular hemosiderin-laden macrophages and hypoxic-ischemic changes in neurons [ 52 ], which we also observed (data not shown). Although immunostaining for SARS-CoV-2 viral spike and nucleoprotein was seen in a single brain, PCR revealed SARS-CoV-2 RNA in all brains [ 52 ]. In another autopsy study, confocal imaging of sections stained for fluorescence, RNAscope, and immunohistochemistry demonstrated extracellular SARS-CoV-2 virions but failed to show viral particles in the brain parenchyma or olfactory bulb [ 50 ]. However, whether the patients examined had AD and/or DS was not reported. This is important because individuals with DS and/or AD have a deficient BBB and an altered brain-immune system [ 53 , 58 ]. These deficiencies likely would affect the invasiveness of a virus or other pathogens representing a potential factor for the increased mortality to COVID-19 found in these two patient groups. Other autopsy studies have shown similar findings as those presented here in the AD brain post COVID-19 infection. For example, Reiken and collaborators found inflammatory activation in the brain of COVID-19 patients, as well as activation of pathways related to tau phosphorylation—indicating that AD pathology may be accelerated by the viral infection [ 67 ]. In addition, Poloni and colleagues showed that patients with a pre-existing neurocognitive syndrome suffered from delirium after contracting COVID-19, which was related to hyperactivation of microglia in the brainstem and hippocampus [ 66 ]. These novel findings together with the present observations support the need to explore DS and/or AD-related COVID-19 pathology across a wide range of clinical–pathologic cases.
A recent study provides additional information regarding the neuro-invasiveness of the SARS-CoV-2 virus and its variants [ 17 ]. Investigators infected golden hamsters with the original Wuhan SARS-CoV-2 strain, and with the Gamma, Delta and Omicron/BA.1 viral strains and demonstrated that all viral variants were neuro-invasive and were retrogradely or anterogradely transported along axons in the brain [ 17 ]. de Melo et al. [ 17 ] showed neuro-invasiveness of all three viral variants studied to date. It is important to consider whether differences in staining protocols, fixation, or PMI affects staining for the virus, its spike protein or nucleoprotein antibodies in brain.
The findings reported here indicate that astrocytes around the brain vasculature display an intense immune response, particularly in those with DS that died from a SARS-Cov-2 infection or severe COVID-19-related complications. Astrocytic end-feet surrounding blood vessels play an important role in viral neuropathogenesis [ 70 ]. This was especially evident in the DS brains with COVID-19, where peri-vascular astrocytes presented with significantly enhanced vascular end-feet, which enveloped the entire lining of the vascular wall of a single cell (Fig. 4 , arrows). Astrocytes are thought to represent a viral reservoir in the brain [ 47 ], which is supported by images showing infection in the brain of the long-term DS COVID survivor (Fig. 1 ) . The current data suggest an increased immune response in the brain of those with DS and COVID-19, which may explain the more severe complications observed in the clinic in individuals with DS. Interestingly, microglia express ACE2 receptors [ 45 ] which may make them especially vulnerable to a SARS-CoV-2 viral attack. Although direct infection of SARS-CoV-2 virus into microglial cells in vitro induces a microglial inflammatory response followed by cell death [ 45 ], conclusive evidence of this occurring in postmortem brain in humans remains to be demonstrated but is partially supported by the findings reported in the current study.
Many of the COVID-19 brains investigated here showed either a greater or reduced accumulation of hemosiderin-laden macrophages in areas of vascular injury in the frontal cortex and hippocampus. To differentiate macrophage from microglia cells, we used an Iba-1 antibody that recognizes both macrophages and microglia and an antibody against TMEM119 that is specific to brain-derived microglial cells [ 26 , 68 ]. We found that both markers were reduced in the gray matter in the frontal cortex and hippocampus in DS COVID + cases, whereas others reported increases in the number of nodes of activated microglia labeled with Iba-1 and alterations in BBB integrity in postmortem brains of patients with AD and COVID-19 [ 29 ]. Although these previous findings suggest that COVID-19 infection in AD increases the number of microglia in the brain, this report did not differentiate between gray and white matter, nor did it include the distribution of GFAP positive astrocytes. Here, we observed similar focal accumulations of Iba-1 positive microglial cells, particularly in the white matter underlying the hippocampus (see Fig. 9 b). Although anecdotal reports suggest that neurodegenerative disease is accelerated by COVID-19 disease, the consequences of SARS-CoV-2 infections were not systematically examined in the brain of people with DS, adding to the novelty of the present report.
Although neuro-invasion of SARS-CoV-2 viral particles into brain is still debated, human autopsy and animal model studies indicate that the virus has significant effects on brain function, leading to neurologic symptoms and long-term disabilities at all ages [ 27 ]. Autopsy studies have revealed significant cardiovascular involvement, including large vessel strokes, hemorrhagic microbleeds, extravasation and BBB disruption, and peripheral T-cell and macrophage invasion in the parenchyma [ 82 ] caused either by direct viral attack of glial cells and neurons or indirectly via a massive cytokine storm resulting from acute infection [ 62 ]. The current study provides novel information regarding effects of this virus in the brain of people with DS without dementia compared to patients with AD or controls that succumbed to COVID-19.
This initial investigation has some limitations. The present study contains a small number of cases. The DS group consisted of three cases with a history of COVID-19, which were younger (50, 31, and 35 years old) compared to the age when AD pathology normally occurs in individuals with DS. Another limitation concerns the lack of a greater number of age-matched DS controls. Moreover, the relatively low number of COVID + controls ( n = 5) hinder the ability to exclude the potential effects of other clinical covariates (e.g., hypoxia or seizures) (see Table 1 ) upon the interpretation of the present findings. The low number of cases is partially due to the almost complete cessation of autopsies for research purposes during the pandemic, which hopefully will be remedied by the continued expansion of the DSBC.
Conclusions
The current preliminary observations presented here demonstrate a long-term viral presence as well as significant glial pathology in the brain of cases with DS and COVID-19 but to a lesser extent in patients with AD or controls with COVID-19. Although neuro-invasion of SARS-CoV-2 viral particles into brain is still debated, human autopsy and animal model studies indicate that the virus has significant effects on brain function, leading to neurologic symptoms and long-term disabilities at all ages. The current findings provide neuropathological data related to the long-term effects of the SARS-CoV-2 virus on brain inflammatory responses and AD-related pathology, which may play a role in cases with long-COVID and its neurologic symptoms. The present findings need to be expanded upon and confirmed in a larger controlled cohort of cases.
Availability of data and materials
The materials will be available to other research groups after publication. Brain tissues from the cases utilized in the current study can be available via the Down Syndrome Biobank Consortium, DSBC.
Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S (2014) A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci 17:491–496. https://doi.org/10.1038/nn.3648
Article CAS PubMed Google Scholar
Ahmed S, Paramasivam P, Kamath M, Sharma A, Rome S, Murugesan R (2021) Genetic exchange of lung-derived exosome to brain causing neuronal changes on COVID-19 infection. Mol Neurobiol 58:5356–5368. https://doi.org/10.1007/s12035-021-02485-9
Article CAS PubMed PubMed Central Google Scholar
Aldecoa I, Barroeta I, Carroll SL, Fortea J, Gilmore A, Ginsberg SD et al (2024) Down syndrome biobank consortium: a perspective. Alzheimers Dement 20:2262–2272. https://doi.org/10.1002/alz.13692
Article PubMed PubMed Central Google Scholar
Alldred MJ, Lee SH, Petkova E, Ginsberg SD (2015) Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer’s disease (AD). Brain Struct Funct 220:2983–2996. https://doi.org/10.1007/s00429-014-0839-0
Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB et al (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113:E1738-1746. https://doi.org/10.1073/pnas.1525528113
Bharadwaj R, Cimino PJ, Flanagan ME, Latimer CS, Gonzalez-Cuyar LF, Juric-Sekhar G et al (2018) Application of the condensed protocol for the NIA-AA guidelines for the neuropathological assessment of Alzheimer’s disease in an academic clinical practice. Histopathology 72:433–440. https://doi.org/10.1111/his.13345
Article PubMed Google Scholar
Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R (2020) Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 11:1555–1562. https://doi.org/10.1021/acschemneuro.0c00210
Bocci M, Oudenaarden C, Saenz-Sarda X, Simren J, Eden A, Sjolund J et al (2021) Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int J Mol Sci. https://doi.org/10.3390/ijms222111622
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278. https://doi.org/10.1016/0197-4580(95)00021-6
Brunnstrom H, Englund E (2009) Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psychiatry 17:664–670. https://doi.org/10.1097/jgp.0b013e3181a6516e
Cardenas AM, Ardiles AO, Barraza N, Baez-Matus X, Caviedes P (2012) Role of tau protein in neuronal damage in Alzheimer’s disease and down syndrome. Arch Med Res 43:645–654. https://doi.org/10.1016/j.arcmed.2012.10.012
Chung H, Green PHR, Wang TC, Kong XF (2021) Interferon-driven immune dysregulation in down syndrome: a review of the evidence. J Inflamm Res 14:5187–5200. https://doi.org/10.2147/JIR.S280953
Clift AK, Coupland CAC, Keogh RH, Hemingway H, Hippisley-Cox J (2020) COVID-19 mortality risk in down syndrome: results from a cohort study of 8 million adults. Ann Intern Med. https://doi.org/10.7326/M20-4986
Crowe AR, Yue W (2019) Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. https://doi.org/10.21769/BioProtoc.3465
Custodio N, Montesinos R, Lira D, Herrera-Perez E, Bardales Y, Valeriano-Lorenzo L (2017) Mixed dementia: a review of the evidence. Dement Neuropsychol 11:364–370. https://doi.org/10.1590/1980-57642016dn11-040005
de Lusignan S, Lopez Bernal J, Zambon M, Akinyemi O, Amirthalingam G, Andrews N et al (2020) Emergence of a novel coronavirus (COVID-19): Protocol for extending surveillance used by the royal college of general practitioners research and surveillance centre and public health England. JMIR Public Health Surv 6:e18606. https://doi.org/10.2196/18606
Article Google Scholar
de Melo GD, Perraud V, Alvarez F, Vieites-Prado A, Kim S, Kergoat L et al (2023) Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat Commun 14:4485. https://doi.org/10.1038/s41467-023-40228-7
De Toma I, Dierssen M (2021) Network analysis of down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci Rep 11:1930. https://doi.org/10.1038/s41598-021-81451-w
DeKosky ST, Kochanek PM, Valadka AB, Clark RSB, Chou SH, Au AK et al (2021) Blood biomarkers for detection of brain injury in COVID-19 patients. J Neurotrauma 38:1–43. https://doi.org/10.1089/neu.2020.7332
Delcoigne B, Stoer NC, Reilly M (2018) Valid and efficient subgroup analyses using nested case-control data. Int J Epidemiol 47:841–849. https://doi.org/10.1093/ije/dyx282
Di Primio C, Quaranta P, Mignanelli M, Siano G, Bimbati M, Scarlatti A et al (2023) Severe acute respiratory syndrome coronavirus 2 infection leads to Tau pathological signature in neurons. PNAS Nexus 2:pgad282. https://doi.org/10.1093/pnasnexus/pgad282
Espinosa JM (2020) Down syndrome and COVID-19: a perfect storm? Cell Rep Med 1:100019. https://doi.org/10.1016/j.xcrm.2020.100019
Fotuhi M, Mian A, Meysami S, Raji CA (2020) Neurobiology of COVID-19. J Alzheimers Dis 76:3–19. https://doi.org/10.3233/JAD-200581
Gagliardi S, Poloni ET, Pandini C, Garofalo M, Dragoni F, Medici V et al (2021) Detection of SARS-CoV-2 genome and whole transcriptome sequencing in frontal cortex of COVID-19 patients. Brain Behav Immun 97:13–21. https://doi.org/10.1016/j.bbi.2021.05.012
Garcia-Segura LM, Perez-Marquez J (2014) A new mathematical function to evaluate neuronal morphology using the Sholl analysis. J Neurosci Methods 226:103–109. https://doi.org/10.1016/j.jneumeth.2014.01.016
Gonzalez Ibanez F, Picard K, Bordeleau M, Sharma K, Bisht K, Tremblay ME (2019) Immunofluorescence staining using IBA1 and TMEM119 for microglial density, morphology and peripheral myeloid cell infiltration analysis in mouse brain. J Vis Exp. https://doi.org/10.3791/60510
Granholm AC (2023) Long-term effects of SARS-CoV-2 in the brain: clinical consequences and molecular mechanisms. J Clin Med. https://doi.org/10.3390/jcm12093190
Granholm AC, Sanders LA, Crnic LS (2000) Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol 161:647–663. https://doi.org/10.1006/exnr.1999.7289
Griggs E, Trageser K, Naughton S, Yang EJ, Mathew B, Van Hyfte G et al (2022) Molecular and cellular similarities in the brain of SARS-CoV-2 and Alzheimer’s disease individuals. bioRxiv. https://doi.org/10.1101/2022.11.23.517706
Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H et al (2021) (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-021-05215-4
Gurunathan S, Kang MH, Kim JH (2021) Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front Immunol 12:716407. https://doi.org/10.3389/fimmu.2021.716407
Hamlett ED, Boger HA, Ledreux A, Kelley CM, Mufson EJ, Falangola MF et al (2016) Cognitive impairment, neuroimaging, and Alzheimer neuropathology in mouse models of down syndrome. Curr Alzheimer Res 13:35–52
Hamlett ED, LaRosa A, Mufson EJ, Fortea J, Ledreux A, Granholm AC (2019) Exosome release and cargo in down syndrome. Dev Neurobiol 79:639–655. https://doi.org/10.1002/dneu.22712
Harapan BN, Yoo HJ (2021) Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J Neurol. https://doi.org/10.1007/s00415-021-10406-y
Hardan L, Filtchev D, Kassem R, Bourgi R, Lukomska-Szymanska M, Tarhini H et al (2021) COVID-19 and Alzheimer’s disease: a literature review. Medicina (Kaunas). https://doi.org/10.3390/medicina57111159
Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K et al (2015) Down syndrome and Alzheimer’s disease: common pathways, common goals. Alzheimers Dement 11:700–709. https://doi.org/10.1016/j.jalz.2014.10.007
Head E, Garzon-Rodriguez W, Johnson JK, Lott IT, Cotman CW, Glabe C (2001) Oxidation of Abeta and plaque biogenesis in Alzheimer’s disease and Down syndrome. Neurobiol Dis 8:792–806. https://doi.org/10.1006/nbdi.2001.0431
Head E, Lott IT, Wilcock DM, Lemere CA (2016) Aging in down syndrome and the development of Alzheimer’s disease neuropathology. Curr Alzheimer Res 13:18–29
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(271–280):e278. https://doi.org/10.1016/j.cell.2020.02.052
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Ibrahim Fouad G (2021) The neuropathological impact of COVID-19: a review. Bull Natl Res Cent 45:19. https://doi.org/10.1186/s42269-020-00478-7
Illouz T, Biragyn A, Iulita MF, Flores-Aguilar L, Dierssen M, De Toma I et al (2021) Immune dysregulation and the increased risk of complications and mortality following respiratory tract infections in adults with down syndrome. Front Immunol 12:621440. https://doi.org/10.3389/fimmu.2021.621440
Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V et al (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71:765–775. https://doi.org/10.1002/ana.22628
Javanshiri K, Waldo ML, Friberg N, Sjovall F, Wickerstrom K, Haglund M et al (2018) Atherosclerosis, hypertension, and diabetes in Alzheimer’s disease, vascular dementia, and mixed dementia: prevalence and presentation. J Alzheimers Dis 65:1247–1258. https://doi.org/10.3233/JAD-180644
Jeong GU, Lyu J, Kim KD, Chung YC, Yoon GY, Lee S et al (2022) SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol Spectr 10:e0109122. https://doi.org/10.1128/spectrum.01091-22
Jha NK, Ojha S, Jha SK, Dureja H, Singh SK, Shukla SD et al (2021) Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: a review on neurological impairments and manifestations. J Mol Neurosci. https://doi.org/10.1007/s12031-020-01767-6
Jorgacevski J, Potokar M (2023) Immune functions of astrocytes in viral neuroinfections. Int J Mol Sci. https://doi.org/10.3390/ijms24043514
Kas A, Soret M, Pyatigoskaya N, Habert MO, Hesters A, Le Guennec L et al (2021) The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging 48:2543–2557. https://doi.org/10.1007/s00259-020-05178-y
Kelley CM, Powers BE, Velazquez R, Ash JA, Ginsberg SD, Strupp BJ et al (2014) Maternal choline supplementation differentially alters the basal forebrain cholinergic system of young-adult Ts65Dn and disomic mice. J Comp Neurol 522:1390–1410. https://doi.org/10.1002/cne.23492
Khan M, Clijsters M, Choi S, Backaert W, Claerhout M, Couvreur F et al (2022) Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron 110(3919–3935):e3916. https://doi.org/10.1016/j.neuron.2022.11.007
Article CAS Google Scholar
Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M et al (2022) The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep 17:307–320. https://doi.org/10.1016/j.stemcr.2021.12.011
Lebrun L, Absil L, Remmelink M, De Mendonca R, D’Haene N, Gaspard N et al (2023) SARS-Cov-2 infection and neuropathological findings: a report of 18 cases and review of the literature. Acta Neuropathol Commun 11:78. https://doi.org/10.1186/s40478-023-01566-1
Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G (2018) Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 135:311–336. https://doi.org/10.1007/s00401-018-1815-1
Lott IT (2012) Neurological phenotypes for down syndrome across the life span. Prog Brain Res 197:101–121. https://doi.org/10.1016/B978-0-444-54299-1.00006-6
Maiese A, Manetti AC, Bosetti C, Del Duca F, La Russa R, Frati P et al (2021) SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol 31:e13013. https://doi.org/10.1111/bpa.13013
Majithia M, Ribeiro SP (2022) COVID-19 and Down syndrome: the spark in the fuel. Nat Rev Immunol 22:404–405. https://doi.org/10.1038/s41577-022-00745-w
Mann DM, Esiri MM (1989) The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci 89:169–179
Martini AC, Gross TJ, Head E, Mapstone M (2022) Beyond amyloid: Immune, cerebrovascular, and metabolic contributions to Alzheimer disease in people with Down syndrome. Neuron 110:2063–2079. https://doi.org/10.1016/j.neuron.2022.04.001
Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C et al (2020) Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. https://doi.org/10.1016/S1474-4422(20)30308-2
McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH et al (2016) Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med 14:129. https://doi.org/10.1186/s12916-016-0676-5
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R et al (2021) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. https://doi.org/10.1038/s41593-020-00758-5
Murta V, Villarreal A, Ramos AJ (2020) Severe acute respiratory syndrome coronavirus 2 impact on the central nervous system: are astrocytes and microglia main players or merely bystanders? ASN Neuro 12:1759091420954960. https://doi.org/10.1177/1759091420954960
Pajo AT, Espiritu AI, Apor A, Jamora RDG (2021) Neuropathologic findings of patients with COVID-19: a systematic review. Neurol Sci. https://doi.org/10.1007/s10072-021-05068-7
Peluso MJ, Deeks SG, Mustapic M, Kapogiannis D, Henrich TJ, Lu S et al (2022) SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol 91:772–781. https://doi.org/10.1002/ana.26350
Perez SE, Miguel JC, He B, Malek-Ahmadi M, Abrahamson EE, Ikonomovic MD et al (2019) Frontal cortex and striatal cellular and molecular pathobiology in individuals with Down syndrome with and without dementia. Acta Neuropathol 137:413–436. https://doi.org/10.1007/s00401-019-01965-6
Poloni TE, Medici V, Moretti M, Visona SD, Cirrincione A, Carlos AF et al (2021) COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol 31:e12997. https://doi.org/10.1111/bpa.12997
Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR (2022) Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement 18:955–965. https://doi.org/10.1002/alz.12558
Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T et al (2016) TMEM119 marks a subset of microglia in the human brain. Neuropathology 36:39–49. https://doi.org/10.1111/neup.12235
Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263. https://doi.org/10.1038/nrn3898
Soung A, Klein RS (2018) Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol Med 24:950–962. https://doi.org/10.1016/j.molmed.2018.09.001
Srinivasan A, Srinivasan A, Ferland RJ (2020) AutoSholl allows for automation of Sholl analysis independent of user tracing. J Neurosci Methods 331:108529. https://doi.org/10.1016/j.jneumeth.2019.108529
Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ et al (2000) Temporal accrual of complement proteins in amyloid plaques in Down’s syndrome with Alzheimer’s disease. Am J Pathol 156:489–499. https://doi.org/10.1016/S0002-9440(10)64753-0
Sullivan KD, Lewis HC, Hill AA, Pandey A, Jackson LP, Cabral JM et al (2016) Trisomy 21 consistently activates the interferon response. Elife. https://doi.org/10.7554/eLife.16220
Tavares G, Martins M, Correia JS, Sardinha VM, Guerra-Gomes S, Das Neves SP et al (2017) Employing an open-source tool to assess astrocyte tridimensional structure. Brain Struct Funct 222:1989–1999. https://doi.org/10.1007/s00429-016-1316-8
Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK et al (2021) COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 144:2696–2708. https://doi.org/10.1093/brain/awab148
Thal DR, Grinberg LT, Attems J (2012) Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47:816–824. https://doi.org/10.1016/j.exger.2012.05.023
Toniolo S, Di Lorenzo F, Scarioni M, Frederiksen KS, Nobili F (2021) Is the frontal lobe the primary target of SARS-CoV-2? J Alzheimers Dis 81:75–81. https://doi.org/10.3233/JAD-210008
Verstrepen K, Baisier L, De Cauwer H (2020) Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg 120:1051–1060. https://doi.org/10.1007/s13760-020-01412-4
Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM (1985) Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology 35:957–961
Wisniewski KE, Wisniewski HM, Wen GY (1985) Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 17:278–282. https://doi.org/10.1002/ana.410170310
Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L et al (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87:18–22. https://doi.org/10.1016/j.bbi.2020.03.031
Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP et al (2021) Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595:565–571. https://doi.org/10.1038/s41586-021-03710-0
Yu Y, Travaglio M, Popovic R, Leal NS, Martins LM (2021) Alzheimer’s and Parkinson’s diseases predict different COVID-19 outcomes: a UK biobank study. Geriatrics (Basel). https://doi.org/10.3390/geriatrics6010010
Zigman WB (2013) Atypical aging in down syndrome. Dev Disabil Res Rev 18:51–67. https://doi.org/10.1002/ddrr.1128
Download references
Acknowledgements
We would like to acknowledge donors and family members as well as the autopsy technicians, dieners, and pathologists at the different sites who carried out the autopsies. We would also like to thank all members of the Down syndrome Biobank Consortium (DSBC), who contributed high-quality brain tissues for research studies both in the USA and Europe.
ACG was supported by the BrightFocus Foundation (Grant no. CA2018010) and research grants from the NIH (R01AG071228-02, R01AG070153, and R01AG061566). EH was supported by P30AG066519. Funding to EJM and SEP: RF1AG081286, PO1AG14449, Arizona Alzheimer’s Consortium and Barrow Neurological Institute. EE was funded by Region Skane, Sweden 2022–2024 and by the Trolle-Wachtmeister Foundation grant 20221216. EDH was supported by the Alzheimer’s Association (Grant no. AARG-22–974669) and the Infectious Diseases Society of America (Grant no. 00128).
Author information
Authors and affiliations.
Department of Neurosurgery, University of Colorado Anschutz Medical Campus, Research Complex II, Aurora, CO, USA
Ann-Charlotte E. Granholm & Anah Gilmore
Division of Pathology, Department of Clinical Sciences, Lund University, Lund, Sweden
Elisabet Englund
Department of Pathology and Laboratory Medicine, University of California Irvine, Irvine, CA, USA
Elizabeth Head & William H. Yong
Department of Neurology, University of California Irvine, Irvine, CA, USA
Elizabeth Head
Department of Translational Neuroscience and Neurology, Barrow Neurological Institute, Phoenix, AZ, USA
Sylvia E. Perez & Elliott J. Mufson
Department of Pathology, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Samuel J. Guzman
Department of Pathology and Laboratory Medicine, Medical University of South Carolina, Charleston, SC, USA
Eric D. Hamlett
You can also search for this author in PubMed Google Scholar
Contributions
ACG, SEP, and EJM conceptualized the manuscript and designed the study. ACG and EJM carried out staining and AG developed staining methods. EE contributed postmortem cases and designed the SARS-CoV-2 immunostaining. WY and EH contributed cases and neuropathological staging of some cases. EDH contributed cases and neuropathological staging. SG also contributed cases and neuropathological staging.
Corresponding author
Correspondence to Ann-Charlotte E. Granholm .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Ethical approval and consent to participate
A statement on ethics approval and consent.
Consent for publication
The data presented here does not contain any personal identification items and all data are de-identified.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Granholm, AC.E., Englund, E., Gilmore, A. et al. Neuropathological findings in Down syndrome, Alzheimer’s disease and control patients with and without SARS-COV-2: preliminary findings. Acta Neuropathol 147 , 92 (2024). https://doi.org/10.1007/s00401-024-02743-9
Download citation
Received : 20 February 2024
Revised : 11 May 2024
Accepted : 12 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s00401-024-02743-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Corona viruses
- Neurologic symptoms
- Alzheimer’s disease
- Glial cells
- Down syndrome
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 29 May 2024
Cerebrovascular disease emerges with age and Alzheimer’s disease in adults with Down syndrome
- Patrick Lao 1 , 2 ,
- Natalie Edwards 1 ,
- Lisi Flores-Aguilar 3 ,
- Mohamad Alshikho 1 ,
- Batool Rizvi 4 ,
- Dana Tudorascu 5 ,
- H. Diana Rosas 6 ,
- Michael Yassa 4 ,
- Bradley T. Christian 7 ,
- Mark Mapstone 4 ,
- Benjamin Handen 5 ,
- Molly E. Zimmerman 8 ,
- Jose Gutierrez 2 ,
- Donna Wilcock 9 ,
- Elizabeth Head 3 , 10 &
- Adam M. Brickman 1 , 2
Scientific Reports volume 14 , Article number: 12334 ( 2024 ) Cite this article
54 Accesses
1 Altmetric
Metrics details
- Diseases of the nervous system
Adults with Down syndrome have a genetic form of Alzheimer’s disease (AD) and evidence of cerebrovascular disease across the AD continuum, despite few systemic vascular risk factors. The onset and progression of AD in Down syndrome is highly age-dependent, but it is unknown at what age cerebrovascular disease emerges and what factors influence its severity. In the Alzheimer’s Biomarker Consortium-Down Syndrome study (ABC-DS; n = 242; age = 25–72), we estimated the age inflection point at which MRI-based white matter hyperintensities (WMH), enlarged perivascular spaces (PVS), microbleeds, and infarcts emerge in relation to demographic data, risk factors, amyloid and tau, and AD diagnosis. Enlarged PVS and infarcts appear to develop in the early 30s, while microbleeds, WMH, amyloid, and tau emerge in the mid to late 30s. Age-residualized WMH were higher in women, in individuals with dementia, and with lower body mass index. Participants with hypertension and APOE-ε4 had higher age-residualized PVS and microbleeds, respectively. Lifespan trajectories demonstrate a dramatic cerebrovascular profile in adults with Down syndrome that appears to evolve developmentally in parallel with AD pathophysiology approximately two decades prior to dementia symptoms.
Similar content being viewed by others

Evidence against a temporal association between cerebrovascular disease and Alzheimer’s disease imaging biomarkers
Accelerated functional brain aging in pre-clinical familial Alzheimer’s disease

Distinct spatiotemporal subtypes of amyloid deposition are associated with diverging disease profiles in cognitively normal and mild cognitive impairment individuals
Introduction.
Although typically considered a disorder that affects early life intellectual and physical development, Down syndrome is also associated with increased risk for Alzheimer’s disease (AD) in later life. The link between Down syndrome and AD was first uncovered in the 1940s 1 and subsequently attributed to the triplication of the 21st chromosome 2 , which contains the amyloid precursor protein gene ( APP ). With chromosome 21 trisomy comes increased production and aggregation of beta-amyloid protein, one of the primary pathological features of AD 3 , 4 , 5 , 6 . Indeed, individuals with Down syndrome overproduce beta-amyloid protein from birth 7 and by the time they are in their 40 s, most have the full pathological features of AD. Today, Down syndrome is considered a “genetic form” of AD, together with fully penetrant, autosomal dominant familial genetic mutations 8 , 9 , 10 , 11 , 12 , 13 , 14 .
Understanding the emergence and progression of AD-related features across the adult lifespan in Down syndrome is critical for two reasons. First, significant recent advances in medical care for individuals with Down syndrome have resulted in an average lifespan that has nearly doubled since the 1980s 15 . As a result, people with Down syndrome are typically living into their 60 s and almost all will suffer from AD dementia within their lifetimes 15 . Thus, AD represents an emerging public health crisis in this population and the time course, risk and pathogenic factors, and potential prevention or treatment targets need to be identified to mitigate its impact. Second, because the biological and clinical progression of AD among individuals with Down syndrome are very similar to late onset AD, the study of AD in this genetically at-risk population has great potential to provide insight into pathogenesis, course, and prevention or treatment strategies for the neurotypical population as well. With advances in care of medical conditions, improved social integration, and a large segment of the Down syndrome population entering older adulthood, AD threatens the health economics and quality of life of an aging society.
There is significant debate about the causes of AD. The field has embraced a single pathogenic pathway, in which accumulation of amyloid leads to tau pathology, subsequent neurodegeneration, and associated cognitive and functional decline. This “amyloid cascade hypothesis” has informed both diagnostic frameworks 12 , 13 , 14 and primary treatment strategies for AD 16 . However, emerging evidence suggests that the clinical course and, possibly, the pathogenesis of AD are multiply determined 17 . Notably, the vast majority of people who die with symptomatic AD have evidence of significant cerebrovascular disease 18 , 19 . Cerebrovascular disease contributes to risk, onset, and clinical course of AD 20 , 21 and recent studies show elevated severity of cerebrovascular disease among individuals with autosomal dominant forms of AD 22 , 23 . Supported by animal experiments that suggest that cerebrovascular disease gives rise to AD pathological features 24 and genetic studies linking vascular factors to AD prevalence 25 , emerging evidence suggests that cerebrovascular disease is a core feature of AD.
Despite having low rates of systemic vascular risk factors, such as hypertension, we previously showed that individuals with Down syndrome have increased magnetic resonance imaging (MRI) markers of cerebrovascular disease, including white matter hyperintensities (WMH), enlarged perivascular spaces (PVS), cerebral microbleeds, and infarcts, which increase as a function of clinical AD diagnosis and its antecedent clinical conditions 26 and may be mediated in part by inflammatory processes that result in neurodegeneration 27 , 28 . Notably, even in individuals without clinical symptoms of AD, we observed a significant degree of cerebrovascular changes, suggesting that they emerged earlier in life and may precede or coincide with the emergence of classical AD pathophysiology. Consistent with work in late onset and autosomal dominant AD, these findings highlight the centrality of cerebrovascular disease to the presentation of AD and possibly to its pathogenesis.
Like other genetically deterministic forms of AD, the development of AD pathological features and subsequent symptoms in Down syndrome is age-dependent and follows a somewhat prescribed pattern, highlighting that AD is a developmental component of Down syndrome. Despite our initial observations of an association between cerebrovascular disease and AD diagnosis 26 , it is unclear at what age cerebrovascular features typically arise in adults with Down syndrome and how the temporal evolution of cerebrovascular disease compares with other AD biomarkers, like amyloid and tau. In the current study, we examined the age “inflection point” at which MRI-based cerebrovascular disease markers emerge in adults with Down syndrome, characterizing them relative to the temporality of amyloid and tau biomarkers, measured with positron emission tomography (PET). We also examined vascular and AD-related factors that could explain a greater-than-expected cerebrovascular biomarker for a given age.
Older age (F(2,239) = 40.3, p < 0.001), greater amyloid burden (F(2,170) = 82.3, p < 0.001), and greater tau burden (Braak I/II: F(2,141) = 47.5, p < 0.001; Braak III/IV: F(2,141) = 55.9, p -value < 0.001; Braak V/VI: F(2,141) = 27.2, p < 0.001) were associated with more advanced AD-related diagnostic group from Cognitively-Stable to MCI-DS and AD dementia, highlighting the strong age-dependency of disease-related factors in this population (Table 1 ). The proportion of participants with hyperlipidemia was higher in those with AD dementia compared with those characterized as Cognitively-Stable; BMI was lower in those with MCI-DS compared with those characterized as cognitively-stable; and obstructive sleep apnea was common in all diagnostic groups (Table 1 ). Despite low frequencies of traditional vascular risk factors including hypertension (6.2%) and diabetes (5.4%), WMH volume (F(2,239) = 9.8, p < 0.001), enlarged PVS scores (F(2,167) = 5.4, p = 0.005), and the presence of infarcts (χ 2 (2) = 7.2, p = 0.03), but not the presence of microbleeds (χ 2 (2) = 4.9, p = 0.09) were associated with more advanced diagnostic group, as reported previously in a subset of the older participants 26 . Figure 1 displays representative images from each MRI modality.

Representative MRI scans for white matter hyperintensity volume, enlarged perivascular spaces, microbleeds, and infarcts across the lifespan of adults with Down syndrome.
Piecewise left-null regression models were fit to each cerebrovascular biomarker across the lifespan to test the hypothesis that each cerebrovascular disease biomarker emerges at a given age, reflecting disease progression in adults with DS (Fig. 2 ). Enlarged PVS and the presence of infarcts were the biomarkers showing the earliest age-associated increase at 31 and 32 years old, respectively. Global WMH inflected at 35 years old. Regionally, frontal and parietal WMH inflected at 35 years old, while occipital WMH inflected later at 41 years old. In relation to traditional AD biomarkers, global amyloid and tau burden in early Braak regions, measured with molecular positron emission tomography (PET) imaging, inflected at 35 years old, after enlarged PVS scores. Then, tau burden in middle and late Braak regions inflected at 39 and 37 years old, respectively, after WMH.

Piecewise left-null regressions of ( A ) cerebrovascular biomarkers, ( B ) regional white matter hyperintensity volume, and ( C ) traditional AD biomarkers against age across the lifespan in adults with Down syndrome. Inflection point estimates, their 95% confidence interval, and their p -value are displayed at the top.
We examined the association of age-residualized cerebrovascular biomarker levels, which reflect the extent to which the biomarker severity deviates from the value predicted by age, with demographic variables, vascular risk factors, amyloid and tau burden, and AD diagnosis (Table 2 ). Women had larger residuals compared with men for global WMH, driven by parietal, temporal, and occipital WMH. APOE-ε4 carriers had larger residuals compared with non-carriers for the presence of microbleeds. Individuals with hypertension had larger residuals compared with those without hypertension for enlarged PVS scores. Greater BMI was associated with lower age-residualized global WMH, driven by temporal WMH. Individuals with AD dementia had larger residuals compared with those who were cognitively-stable for global WMH, driven by parietal, temporal, and occipital WMH.
We found that markers of cerebrovascular disease emerge in adults with Down syndrome within the same timeframe as amyloid and tau pathology and prior to the onset of AD clinical symptoms. The temporal ordering of inflection points suggests that enlarged PVS and infarcts develop in the early 30 s, while microbleeds, WMH, amyloid, and tau develop in the mid to late 30 s. Several demographic factors, vascular risk factors, and AD diagnosis were associated with a greater amount of cerebrovascular biomarkers for a given age. Therefore, vascular risk factors may exacerbate the extent of cerebrovascular disease, but they are not necessary for cerebrovascular disease to emerge across the lifespan in adults with Down syndrome.
Potential pathways between specific markers of cerebrovascular disease and AD have been studied in adults without Down syndrome 29 and animal models 30 . Enlarged PVS generally capture impaired clearance glymphatic mechanisms due to chronic exposure to toxins like soluble beta-amyloid and may contribute to further amyloid deposition in the parenchyma 31 . Microbleeds among people with Down syndrome reflect amyloid deposits in the vasculature (i.e., cerebral amyloid angiopathy) that weaken the vascular endothelium and may potentiate further downstream vascular dysfunction 32 . White matter hyperintensities are associated with inflammation and white matter demyelination due to small vessel disease or disruption 33 . Infarcts reflect ischemic lesions in larger vessels; they may not be mechanistically related to amyloid and tau, but may contribute to downstream neurodegeneration and cognition 34 .
Based on previous mechanistic work, chronic exposure to soluble amyloid may impair perivascular clearance, leading to downstream amyloid deposition in the parenchyma and in the vasculature. Further, small vessel disease may be one initiator of tau phosphorylation, perhaps through an inflammatory response to damage in small vessels that upregulates kinase activity 24 , 28 , 30 . Our findings were consistent with this expected temporality. We observed the earliest inflection points at age 31 for enlarged PVS and, surprisingly, infarcts, demonstrating that these vascular abnormalities are among the earliest biomarker changes observed in Down syndrome. Amyloid deposition increased in the parenchyma (amyloid PET) and in the vasculature (microbleeds) at ages 35 and 36 years, respectively. Increased tau deposition in early Braak regions was also observed at 35 years, which is consistent with previous studies showing tau deposition in adults with Down syndrome who were amyloid-negative but accumulating amyloid over time 35 , suggesting that the emergence of amyloid and tau pathology are tightly linked together in time 36 , 37 . Tau deposition in middle and late Braak regions were later at 39 and 37 years, respectively. To investigate this somewhat unexpected finding, we ran a sensitivity analysis excluding one individual with a very low late Braak SUVR (< 0.5) at an older age, which may have biased the age trajectory after the inflection point lower and pushed the inflection point earlier; however, the estimated age inflection point in late Braak regions was very similar (37.5 [29.1, 45.9]). Longitudinal within-subject data in adults with Down syndrome demonstrated that middle Braak regions accumulate tau prior to late Braak regions according to the amyloid cascade 36 White matter hyperintensities emerged at 35 years old across the brain. The temporal ordering observed is consistent with previous work in late onset AD that demonstrated that greater WMH are associated with tau burden in middle and late Braak regions, but not early Braak regions 38 . The estimated inflection point for microbleeds was not significant, similar to a previous study that did not show an increase across diagnostic groups in adults with Down syndrome 26 and may be due to methodological limitations (e.g., GRE/SWI being more susceptible to motion artifacts than other MRI sequences, and motion artifacts being more common in older individuals with more advanced disease). Alternatively, microbleeds may start to manifest even earlier than the ages represented in these studies. Indeed, pathological studies of adults with Down syndrome suggest a particularly profound vascular amyloid profile 39 .
Regionally, parietal WMH, which may be more specific to AD compared with other lobar WMH, emerged at 35 years old, approximately 18 years prior to the average age of symptom onset in adults with Down syndrome 15 . Strikingly, in autosomal dominant AD, posteriorly distributed WMH emerged as early as 22 years before estimated onset of symptoms 22 , 23 . The majority of enlarged PVS were observed in the cortex (5.4 [5.0, 5.9]), while approximately one third of enlarged PVS were observed in the basal ganglia (2.6 [2.4, 2.8]), which have been associated with hypertension in adults without Down syndrome 40 . Global enlarged PVS score was strongly associated with cortical PVS (R 2 = 0.89) and moderately associated with basal ganglia PVS (0.51), while cortical PVS and basal ganglia PVS scores were less correlated with each other (0.21). Cortical PVS emerged at age 31 [16.9, 45.0], while basal ganglia PVS emerged at age 42 [28.3, 54.9]. In relation to microbleeds, 1 participant had deep microbleed(s) in the absence of lobar microbleed(s) and 14 participants had lobar microbleed(s) in absence of deep microbleed(s). In relation to infarcts, 9 participants had deep infarct(s) in the absence of lobar infarct(s), 16 participants had lobar infarct(s) in absence of deep infarct(s), and 1 participant had both. Microbleeds outside of cortical lobes and infarcts in deep, subcortical structures were not common enough in adults with DS to reliably model regionally specific age trajectories.
Compared with men, women had greater global WMH than expected for participant age, particularly in parietal, temporal, and occipital lobes, which may be another contributor to age-specific sex differences in AD risk in adults with DS 41 . Individuals with the APOE-ε4 allele had a greater likelihood of having microbleeds than expected for age, suggesting that even in the context of amyloid precursor protein overproduction due to triplication of the 21st chromosome in Down syndrome, APOE-ε4 can still affect disease progression 42 . While hypertension was not common in study participants, it was associated with PVS scores that were greater than expected for participant age, suggesting a role of hemodynamics for clearance through the perivascular space. Potential mechanisms for the enlargement of PVS include atherosclerosis, arteriolosclerosis, and elastin dysfunction, which reduces the pliability and increases pulsatility 31 . However, autopsy studies demonstrated low prevalence of atherosclerosis and arteriosclerosis in adults with Down syndrome 39 , leaving the possibility that hypertension likely affects enlarged PVS through mechanisms related to vessel elasticity in this study. Hyperlipidemia was common but was not associated with cerebrovascular disease for a given age; hyperlipidemia may not operate as a vascular risk factor in the absence of metabolic disease (e.g., diabetes) in adults with DS. Lower BMI was associated with greater global WMH, highlighting a role of other disease related processes that affect diet, exercise, and weight 43 . While WMH may reflect Wallerian degeneration to some degree in advanced stages of late onset AD, tau burden was not associated with greater than expected WMH volume for participant age in adults with Down syndrome across the lifespan, and WMH age inflection preceded later stage tau deposition, suggesting that cerebrovascular biomarkers are upstream of advanced tau pathology. Greater age-residualized global WMH, driven by parietal, occipital, and temporal lobe WMH, was associated with a diagnosis of AD dementia. Small vessel disease in posterior brain regions is a consistent cerebrovascular process in AD pathogenesis in adults with Down syndrome 26 , adults with autosomal dominant AD 22 , 23 , and adults with late onset AD 20 , 21 . Therefore, these four cerebrovascular biomarkers may represent unique biological mechanisms, each with their own influence on disease pathogenesis and course 29 , 30 . Ongoing longitudinal data collection in ABC-DS will support investigations into individual-level inflection points as well as the shape and rates of these cerebrovascular disease biomarker trajectories.
This study has some limitations, including the lack of pathological validation, using temporal ordering of cross-sectional events to infer change, and the lack of correction for multiple comparisons. Autopsy studies can identify individual plaques and tangle within specific cell layers, but amyloid and tau PET scans indicate when the amount of pathology is above the limit of detection at a spatial resolution of 2 mm. However, PET imaging is the in vivo gold standard for comparison against histopathology 12 . Similarly, only a proportion of microbleeds are detected on MRI with current imaging parameter 44 and subtle cerebral blood flow changes likely precede the formation of WMH and infarcts 45 , 46 . Therefore, our results may be most relevant to later manifestations of pathology that can be captured with neuroimaging. Nonetheless, we used conventional radiology tools that can inform clinical evaluation by establishing normative expectations for the measured pathologies for an individual given their age. Future work will include the identification, validation, and incorporation of biofluidic measures of vascular function 47 , 48 and comparison to autopsy data that could provide more mechanistic context for the radiological markers studied here. Conclusions about temporal ordering of the emergence of radiological abnormalities were derived with methods that are similar to those used in studies of autosomal dominant AD 49 , 50 . In both cases, there is a nearly 100% likelihood of AD incidence in the context of overproduction and/or altered metabolism of amyloid pathology with similar variability around age of dementia onset 15 . Multiple comparison correction was not performed as our primary interest was in characterizing the natural history of markers of cerebrovascular disease among adults with Down syndrome (i.e., inflection point models). Given the relevance of these findings to therapeutic intervention strategies in adults with Down syndrome, we wanted to minimize Type 2 statistical error (i.e., false negative) to inform future mechanistic studies of any potentially relevant pathways (i.e., age-residualized models), although we recognize the possibility of inflated Type 1 error (i.e., false positive) as a limitation. Still, our findings converge with cross-sectional literature in autosomal dominant AD, showing an early and reliable increase in WMH 22 , 23 , enlarged PVS 51 , microbleeds 52 , and infarcts 53 , which were later confirmed longitudinally 54 , 55 . Further, in late onset AD, APOEε-4 was associated with microbleeds 56 , women had faster rates of deep WMH progression 57 , and enlarged PVS were dependent on arterial hemodynamics 58 and were associated with hypertension in a spatially-dependent manner 59 . Replication studies are needed in larger, longitudinal, and external datasets. Ongoing longitudinal data collection in ABC-DS will support investigations into individual-level inflection points, which may be earlier or later compared to the group estimate, as well as the shape and rates of these cerebrovascular disease biomarker trajectories.
Furthering our understanding of AD pathogenesis with respect to cerebrovascular disease is additionally important, particularly in adults with Down syndrome, because of the common incidence of edema or hemorrhage amyloid-related imaging abnormalities (ARIA) that emerge as a results of current anti-amyloid antibody therapeutics 16 . There is evidence of cerebrovascular disease on the group level in adults with Down syndrome along the AD continuum, but some older adults have relatively low burden. Even in the absence of existing microbleeds, which are currently the only known MRI risk factor for ARIA, adults with Down syndrome may still be at increased risk because MRI only captures a proportion of the microbleeds detected at autopsy 39 , 52 . Future research should study the extent to which other visible cerebrovascular lesions on MRI may be used to predict who is at risk for developing ARIA. Individuals with Down syndrome may be at particular risk for these side effects in anti-amyloid therapeutics. In the absence of approved pharmacological treatments for AD in adults with Down syndrome, modifiable factors like sleep 60 , 61 , diet and exercise 62 , and leisure activity 63 , 64 may be potential therapeutic avenues.
MRI imaging data across the lifespan demonstrate a dramatic cerebrovascular profile in adults with Down syndrome that appears to evolve developmentally in parallel with AD pathophysiology approximately two decades prior to dementia symptoms. This work joins an emerging literature that incorporates cerebrovascular disease into our understanding of AD pathogenesis and progression and highlights new avenues towards our understanding of the cause of AD, therapeutic and preventative strategies, and safety outcomes in this unique population. Future work should emphasize the potential role of cerebrovascular pathologies in AD, beyond the way by which they impact downstream neurodegeneration and cognitive impairment as simple comorbidities as they may precede and contribute to AD pathology.
Clinical characterization
Adults with Down syndrome from the multisite Alzheimer’s Biomarker Consortium-Down Syndrome study 65 (ABC-DS; n = 242; age = 25–72, 45 ± 10; 43% women) underwent MRI, amyloid PET, and tau PET under the Neurodegeneration in Aging Down Syndrome (NiAD) study and the Biomarkers of Alzheimer’s Disease in Down Syndrome (ADDS) study protocols. The studies under which data were collected were approved by the institutional review boards at participating institutions (i.e., University of Pittsburgh, Columbia University Irving Medical Center, The New York State Institute for Basic Research in Developmental Disabilities/New York State Psychiatric Institute, Harvard Medical School, University of Wisconsin-Madison, University of Cambridge, University of California, Irvine), performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from participants and/or their legal guardian or legally authorized representative. Every participant gave assent prior to any study-related procedure.
Clinical diagnoses were assigned by a consensus panel that included clinicians with expertise in the assessment of adults with Down syndrome 66 . One of four AD consensus diagnoses was assigned to each participant based on the results from neuropsychological testing, clinical chart reviews, and interviews with knowledgeable informants, with additional consideration of health history, functional and vocational abilities, and neuropsychiatric symptoms. Results from neuroimaging or other biomarker studies were not considered in the diagnostic formulation. A diagnosis of “cognitively-stable” (CS) indicated no evidence of clinically significant cognitive decline beyond preclinical intellectual functioning and age. A diagnosis of “mild cognitive impairment-Down syndrome” (MCI-DS) indicated evidence of cognitive decline over time beyond preclinical intellectual functioning and age, but insufficient to suggest dementia. A diagnosis of “AD dementia” indicated clear evidence of substantial cognitive and functional decline of breadth and severity greater than MCI-DS, with a high degree of confidence. Eleven participants in the neuroimaging sample (4.2%) were excluded based on complications or concerns unrelated to neurodegenerative disorders (e.g., severe sensory loss, new psychiatric diagnosis).
Neuroimaging acquisition and analysis
Participants were scanned on 3 T MRI and PET scanners, following protocols put forth by the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Participants underwent a high-resolution T1-weighted anatomical scan (repetition time [TR]/echo time [TE]/inversion time [TI] = 2,300/2.96/900 ms, voxel size = 1 × 1 × 1 mm 3 ), a T2-weighted fluid-attenuated inversion recovery (FLAIR) scan (TR/TE/TI = 5,000/386/1,800 ms, voxel size = 0.4 × 0.4 × 0.9mm 3 ), and a T2*-weighted gradient echo (GRE) scan (TR/TE = 650/20 ms, voxel size = 0.8 × 0.8 × 4 mm 3 ) or susceptibility-weighted image (SWI; TR/TE = 27/20 ms, voxel size = 0.9 × 0.9 × 1.5 mm 3 ). Participants underwent amyloid PET with [ 11 C]PiB (15 mCi, 50–70 min post-injection scan, 5 min frames) or [ 18 F]Florbetapir (AV45; 10 mCi, 80–100 min post-injection scan, 5 min frames); participants also underwent tau PET with [ 18 F]Flortaucipir (AV1451; 10 mCi, 75–105 min post-injection, 5 min frames). All PET data were corrected for attenuation, detection dead time, scanner normalization, scatter, and radioactive decay.
Magnetic resonance imaging scans were analyzed for cerebrovascular disease with analytic pipelines developed in-house. For white matter hyperintensities , total and lobar (frontal, temporal, parietal, occipital) WMH volumes were semi-automatically segmented from T2-weighted FLAIR scans. Images were interpolated to a standard matrix in MNI152 space (256 × 256 × 256; 1 mm 3 ), skull stripped, bias field corrected, and intensity normalized (0–255). Percentile thresholds initiated a Gaussian mixture model, separating dark/bright and bright/brightest intensities; estimated percentile thresholds were then relaxed by an intensity of 10 to account for variations in FLAIR quality. Roberts edge detection removed any hyperintense labels from non-white matter before visual inspection. Enlarged perivascular spaces (PVS) were visually inspected on T1-weighted scans and classified as hypointensities across 13 brain regions, rated from 0 to 2 based on FLAIR characteristics (hyperintense ring), and combined into a global score ranging from 0 (no enlarged PVS in any region) to 26 (most severe enlarged PVS in each region) 67 , 68 . We developed an algorithm 58 based on anatomical location, appearance on FLAIR, and size to determine the most likely underlying pathology of a given lesion. A hyperintense FLAIR rim around a T1 void is by far the single most important determining factor to distinguish enlarged PVS from infarcts, although the need for a FLAIR rim is lower in areas in which enlarged PVS rarely exist (such as the brain stem or the upper basal ganglia). Microbleeds were visually rated as hypointense round or ovoid lesions on GRE or SWI, surrounded at least halfway by parenchyma with a “blooming” effect and no hyperintensity on accompanying T1-weighted or FLAIR scans to distinguish them from iron or calcium deposits, bone, or vessel flow voids. Due to a skewed distribution (i.e., if present, most scans had 1 microbleed), microbleeds were scored across the whole brain as present or not present. Infarcts were visually rated on T2-weighted FLAIR scans as discrete hypointense lesions greater than 5 mm with a partial or complete hyperintense ring, confirmed on T1 scans as hypointense areas, and scored across the whole brain as present or not present due to a skewed distribution. Amyloid PET with [ 11 C]PiB or [ 18 F]Florbetapir were harmonized into the centiloid scale using reported formulas available on Global Alzheimer’s Association Information Network (GAAIN; http://www.gaain.org ) 69 , 70 . Tau PET with [ 18 F]Flortaucipir was quantified as standard uptake value ratio (SUVR, 80–100 min post-injection, FreeSurfer-defined cerebellar gray matter reference region) in Braak I/II, Braak III/IV, and Braak V/VI for early, middle, and late tau burden. Biomarkers were available in 242 participants for WMH, 182 participants for enlarged PVS score, 140 participants for microbleeds, and 237 participants for infarcts; 215 participants for amyloid PET, and 175 participants for tau PET.
Participants were genotyped for APOE (rs429358 and rs7412) with the Kompetitive allele-specific polymerase chain reaction genotyping system (LGC Genomics; Berlin, Germany). For these analyses, individuals with at least one copy of the APOE-ε4 allele were classified as APOE-ε4 carriers.
Statistical analysis
Age, amyloid burden, and tau burden were compared across clinical diagnostic groups to support the use of chronological age as disease progression in adults with Down syndrome, while demographic characteristics (i.e., sex, premorbid intellectual developmental disability, APOE status) and vascular risk factors (i.e., hypertension, diabetes, hyperlipidemia, body mass index (BMI), and obstructive sleep apnea (OSA)) were compared across clinical diagnostic groups to assess potential covariates. Vascular risk factors were reported by participants or their informants as part of their health history (“Do you currently have or have you ever had a diagnosis of [disease]”) or obtained from medical/health records. Information was aggregated across sources and binarized as “yes” or “no”. Body mass index was objectively measured and used continuously. Cerebrovascular biomarkers were fit with piece-wise, left null regression models against age to estimate the age inflection point at which these cerebrovascular markers emerge, adjusting for study protocol (i.e., NiAD vs ADDS). The Davies test 71 was used to determine if the slope after the estimated inflection point was different from the slope before the estimated inflection point (i.e., zero). General linear models were used for continuous variables (i.e., WMH, enlarged PVS) and logistic regression models were used for dichotomous variables (i.e., the presence of microbleeds, the presence of infarcts). Log transformations for skewed variables (i.e., WMH volume) and multisite harmonization methods (e.g., ComBat 72 ) were explored, and led to minimal improvements in model fits with similar results; therefore, untransformed WMH volumes were used and study was included as a simple covariate in reported models. Further, the interpretation of the inflection point as the age at which cerebrovascular biomarkers visibly emerge on MRI is preserved in comparison to the age at which a transformed variable deviates from zero.
As amyloid precursor protein is overexpressed from birth in adults with Down syndrome, age can reasonably represent disease duration and cross-sectional models can provide pseudo-longitudinal trajectories of cerebrovascular biomarker development 11 , 15 . Amyloid chronicity 73 has also been shown to represent disease progression beyond chronological age, but would limit our sample size to those with amyloid PET. To determine the emergence of cerebrovascular disease relative to classical AD biomarkers, including amyloid and tau, we also fit left null regression models with age for amyloid PET Centiloids and tau PET SUVRs.
Traditional vascular risk factors, including hypertension and diabetes type 2, are lower in adults with Down syndrome compared with adults without Down syndrome; however, some vascular risk factors are present at similar or higher rates, including hyperlipidemia, high BMI, and OSA. The residuals from the piecewise left-null regression against age (i.e., higher or lower biomarker level than expected for a given age) were fit against demographic data, vascular risk factors, amyloid and tau burden, and AD diagnosis (Cognitively-Stable (reference group), MCI-DS, AD dementia) to determine their influence on the development of each cerebrovascular disease biomarker. All statistics were run in R v4.2.2.
Data availability
ABC-DS is committed to providing rapid public access to all clinical, cognitive and biomarker (fluid and imaging) data, without embargo, and access to the biological samples by qualified scientific investigators. ABC-DS data are transferred to the Laboratory of Neuro Imaging (LONI), for harmonization, documentation and de-identification; biospecimen samples are transferred and managed by the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD) and an ABC-DS biospecimen bank. As of May 2021, data from the first and second waves of longitudinal data are available for requests. Qualified investigators can submit requests for access to data and samples ( https://pitt.co1.qualtrics.com/jfe/form/SV_cu0pNCZZlrdSxUN ), and all requests will be reviewed by ABC-DS investigators and NIH staff. Approved data requests will be managed by the ABC-DS Biostatistics and Data Management Core for access to the clinical, cognitive, and neuroimaging data listed below. Upon approval and availability of biospecimen samples, NCRAD will distribute DNA, plasma and serum, and the ABC-DS biospecimen bank will distribute CSF. LONI will store the associated data for access by approved investigators ( https://ida.loni.usc.edu/collaboration/access/appLicense.jsp;jsessionid=AC572158DA02C57FD870AE42D137FFF0 ).
Jervis, G. A. Early senile dementia in mongoloid idiocy. Am. J. Psychiatry 105 , 102–106 (1948).
Article CAS PubMed Google Scholar
Glenner, G. G., Wong, C. W., J. B. & communications. Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122 , 1131–1135 (1984).
Beyreuther, K. & Masters, C. L. Amyloid precursor protein (APP) and ΒZA4 amyloid in the etiology of Alzheimer’s disease: Precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1 , 241–251 (1991).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12 , 383–388 (1991).
Selkoe, D. J. The molecular pathology of Alzheimer’s disease. Neuron 6 , 487–498 (1991).
Hardy, J. A. & Higgins, G. A. J. S. Alzheimer’s disease: The amyloid cascade hypothesis. Science 256 , 184–185 (1992).
Article ADS CAS PubMed Google Scholar
Oyama, F. et al. Down’s syndrome: Up-regulation of β-amyloid protein precursor and τ mRNAs and their defective coordination. J. Neurochem. 62 (1062), 1066 (1994).
Google Scholar
Rafii, M. S. et al. The AT (N) framework for Alzheimer’s disease in adults with Down syndrome. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 12 , e12062 (2020).
Hartley, S. L. et al. AT (N) biomarker profiles and Alzheimer’s disease symptomology in Down syndrome. Alzheimer’s Dement. 20 (1), 366–375 (2023).
Article Google Scholar
Fortea, J. et al. Alzheimer’s disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol. 20 (930), 942 (2021).
Boerwinkle, A. H. et al. Comparison of amyloid burden in individuals with Down syndrome versus autosomal dominant Alzheimer’s disease: A cross-sectional study. Lancet Neurol. 22 (55), 65 (2023).
Jack, C. R. Jr. et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 , 535–562. https://doi.org/10.1016/j.jalz.2018.02.018 (2018).
Article PubMed Google Scholar
Hampel, H. et al. Developing the ATX (N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 17 , 580–589 (2021).
Workgroup, A. s. A. Revised criteria for diagnosis and staging of Alzheimer's disease. (2023).
Iulita, M. F. et al. Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Netw. Open 5 , e2212910–e2212910 (2022).
Article PubMed PubMed Central Google Scholar
Cummings, J. J. D. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s disease therapeutics. Drugs 83 (7), 569–576 (2023).
Article CAS PubMed PubMed Central Google Scholar
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396 , 413–446 (2020).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134 , 171–186. https://doi.org/10.1007/s00401-017-1717-7 (2017).
Barnes, L. L. et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 85 , 528–534. https://doi.org/10.1212/wnl.0000000000001834 (2015).
Article ADS PubMed PubMed Central Google Scholar
Brickman, A. M. et al. Reconsidering harbingers of dementia: Progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol. Aging 36 , 27–32 (2015).
Lao, P. J. et al. Amyloid, cerebrovascular disease, and neurodegeneration biomarkers are associated with cognitive trajectories in a racially and ethnically diverse, community-based sample. Neurobiol. Aging 117 , 83–96 (2022).
Lee, S. et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 79 , 929–939. https://doi.org/10.1002/ana.24647 (2016).
Schoemaker, D. et al. White matter hyperintensities are a prominent feature of autosomal dominant Alzheimer’s disease that emerge prior to dementia. Alzheimer’s Res. Ther. 14 , 89 (2022).
Article CAS Google Scholar
Laing, K. K. et al. Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun. 2 (2), fcaa132 (2020).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603 (885), 892 (2022).
ADS Google Scholar
Lao, P. J. et al. Alzheimer-related cerebrovascular disease in Down syndrome. Ann. Neurol. 88 , 1165–1177 (2020).
Moni, F. et al. Probing the proteome to explore potential correlates of increased Alzheimer’s-related cerebrovascular disease in adults with Down syndrome. Alzheimer’s Dement. 18 (1744), 1753 (2022).
Edwards, N. C. et al. Cerebrovascular disease drives Alzheimer plasma biomarker concentrations in adults with Down syndrome. Medrxiv. https://doi.org/10.1101/2023.11.28.23298693 (2023).
Zlokovic, B. V. J. N. R. N. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12 (723), 738 (2011).
Scheffer, S. et al. Vascular hypothesis of Alzheimer disease: Topical review of mouse models. Arterioscler. Thromb. Vasc. Biol. 41 (1265), 1283 (2021).
Gouveia-Freitas, K. & Bastos-Leite, A. J. J. N. Perivascular spaces and brain waste clearance systems: Relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology 63 (1581), 1597 (2021).
van Dalen, J. W. et al. Cortical microinfarcts detected in vivo on 3 Tesla MRI: Clinical and radiological correlates. Stroke 46 , 255–257. https://doi.org/10.1161/strokeaha.114.007568 (2015).
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 11 , 157–165 (2015).
Vermeer, S. E. et al. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 348 , 1215–1222 (2003).
Zammit, M. D. et al. Neurofibrillary tau depositions emerge with subthreshold cerebral beta-amyloidosis in Down syndrome. NeuroImage Clin. 31 , 102740 (2021).
Zammit, M. D. et al. Characterizing the emergence of amyloid and tau burden in Down syndrome. Alzheimer’s Dement. 20 (1), 388–398 (2023).
Article MathSciNet Google Scholar
Tudorascu, D. et al. Relationship of amyloid beta and neurofibrillary tau deposition in Neurodegeneration in Aging Down Syndrome (NiAD) study at baseline. Transl. Res. Clin. Interv. 6 , e12096 (2020).
Royse, S. K. et al. Unhealthy white matter connectivity, cognition, and racialization in older adults. Alzheimer’s Dement. 20 (3), 1483–1496 (2023).
Head, E. et al. Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta Neuropathol. Commun. 5 (1), 9 (2017).
Charidimou, A. et al. White matter perivascular spaces: An MRI marker in pathology-proven cerebral amyloid angiopathy?. Neurology 82 , 57–62 (2014).
Mhatre, P. G. et al. The association between sex and risk of Alzheimer’s disease in adults with Down syndrome. J. Clin. Med. 10 , 2966 (2021).
Schupf, N. et al. Onset of dementia is associated with apolipoprotein E ε4 in Down’s syndrome. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 40 , 799–801 (1996).
Fleming, V. et al. Weight loss and Alzheimer’s disease in Down Syndrome. J. Alzheimer’s Dis. 91 (3), 1215–1227 (2023).
Article MathSciNet CAS Google Scholar
Jolink, W. M. et al. Histopathology of cerebral microinfarcts and microbleeds in spontaneous intracerebral hemorrhage. Transl. Stroke Res. 14 (174), 184 (2023).
Wardlaw, J. M., Valdes Hernandez, M. C. & Munoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.114.001140 (2015).
Lao, P. et al. White matter regions with low microstructure in young adults are associated with white matter hyperintensities in late life. bioRxiv , 517763, doi: https://doi.org/10.1101/517763 (2019).
Wilcock, D. et al. MarkVCID cerebral small vessel consortium: I Enrollment, clinical, fluid protocols. Alzheimer’s Dement. 17 (704), 715 (2021).
Foley, K. E. & Wilcock, D. M. Soluble biomarkers of cerebrovascular pathologie. Stroke 55 , 801–811 (2024).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367 , 795–804. https://doi.org/10.1056/NEJMoa1202753 (2012).
Johnson, E. C. et al. 2023 Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer’s disease. Nat. Med. 29 , 1979 (1988).
Littau, J. L. et al. Evidence of beta amyloid independent small vessel disease in familial Alzheimer’s disease. Brain Pathol. 32 , e13097 (2022).
Carmona-Iragui, M. et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer’s disease. Alzheimer’s Dement. 13 , 1251–1260 (2017).
Tang, M. et al. Neurological manifestations of autosomal dominant familial Alzheimer’s disease: A comparison of the published literature with the dominantly inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol. 15 , 1317–1325 (2016).
Joseph-Mathurin, N. et al. Longitudinal accumulation of cerebral microhemorrhages in dominantly inherited Alzheimer disease. Neurology 96 , e1632–e1645 (2021).
Shirzadi, Z. et al. Etiology of white matter hyperintensities in autosomal dominant and sporadic Alzheimer disease. JAMA Neurol. 80 , 1353–1363 (2023).
Ingala, S. et al. The relation between APOE genotype and cerebral microbleeds in cognitively unimpaired middle-and old-aged individuals. Neurobiol. Aging 95 (104), 114 (2020).
Van Den Heuvel, D. et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology 63 (1699), 1701 (2004).
Gutierrez, J. et al. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The Northern Manhattan Study. J. Hypertens. 33 , 2115 (2015).
Charidimou, A. et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88 , 1157–1164 (2017).
Lao, P. et al. Obstructive sleep apnea, cerebrovascular disease, and amyloid in older adults with Down syndrome across the Alzheimer’s continuum. Sleep Adv. 3 (1), 130 (2022).
Cody, K. A. et al. Association of sleep with cognition and beta amyloid accumulation in adults with Down syndrome. Neurobiol. Aging 93 , 44–51 (2020).
Kenshole, A. V., Gallichan, D., Pahl, S. & Clibbens, J. Lifestyle factors and Alzheimer’s disease in people with Down syndrome. J. Appl. Res. Intell. Disabil. 30 , 58–66 (2017).
Mihaila, I. et al. Leisure activity and caregiver involvement in middle-aged and older adults with Down syndrome. Intell. Dev. Disabil. 55 , 97–109 (2017).
Mihaila, I. et al. Leisure activity brain β-amyloid, and episodic memory in adults with Down Syndrome. Dev. Neurobiol. 79 , 738–749 (2019).
Handen, B. L. et al. The Alzheimer’s biomarker consortium-Down syndrome: Rationale and methodology. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 12 , e12065 (2020).
Krinsky-McHale, S. J. et al. Promising outcome measures of early Alzheimer’s dementia in adults with Down syndrome. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 12 , e12044 (2020).
Gutierrez, J. et al. Brain perivascular spaces as biomarkers of vascular risk: Results from the northern manhattan study. Am. J. Neuroradiol. 38 , 862–867 (2017).
Gutierrez, J., Rundek, T., Ekind, M., Sacco, R. L. & Wright, C. B. Perivascular spaces are associated with atherosclerosis: An insight from the Northern Manhattan study. Am. J. Neuroradiol. 34 , 1711–1716 (2013).
Klunk, W. E. et al. The Centiloid Project standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11 (1), 1–15. https://doi.org/10.1016/j.jalz.2014.07.003 (2015).
Tudorascu, D. L. et al. The use of Centiloids for applying [(11)C]PiB classification cutoffs across region-of-interest delineation methods. Alzheimer’s Dement. 10 , 332–339. https://doi.org/10.1016/j.dadm.2018.03.006 (2018).
Davies, R. B. Hypothesis testing when a nuisance parameter is present only under the alternative: Linear model case. Biometrika 64 (2), 247–254 (2002).
Orlhac, F. et al. A guide to ComBat harmonization of imaging biomarkers in multicenter studies. J. Nucl. Med. 63 , 172–179 (2022).
Koscik, R. L. et al. Amyloid duration is associated with preclinical cognitive decline and tau PET. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 12 , 63–72 (2020).
Download references
Acknowledgements
The Alzheimer’s Biomarkers Consortium-Down Syndrome (ABC-DS) is funded by the National Institute on Aging and the National Institute for Child Health and Human Development (U01 AG051406, U01 AG051412, U19 AG068054). The work contained in this publication was also supported through the following National Institutes of Health Programs: The Alzheimer’s Disease Research Centers Program (P50 AG008702, P30 AG062421, P50 AG16537, P50 AG005133, P50 AG005681, P30 AG062715, and P30 AG066519), the Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Program (U54 HD090256, U54 HD087011, and P50 HD105353), the National Center for Advancing Translational Sciences (UL1 TR001873, UL1 TR002373, UL1 TR001414, UL1 TR001857, UL1 TR002345), the National Centralized Repository for Alzheimer Disease and Related Dementias (U24 AG21886), and DS-Connect® (The Down Syndrome Registry) supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). In Cambridge, UK this research was supported by the NIHR Cambridge Biomedical Research Centre and the Windsor Research Unit, CPFT, Fulbourn Hospital Cambridge, UK. The authors are grateful to the ABC-DS study participants, their families and care providers, and the ABC-DS research and support staff for their contributions to this study. This manuscript has been reviewed by ABC-DS investigators for scientific content and consistency of data interpretation with previous ABC-DS study publications. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the CPFT, the NIHR or the UK Department of Health and Social Care. Alzheimer’s Biomarker Consortium- Down Syndrome (ABC-DS) Investigators: Howard J. Aizenstein, MD PhD; Beau M. Ances, MD PhD; Howard F. Andrews, PhD; Karen Bell, MD; Rasmus M. Birn, PhD; Adam M. Brickman, PhD; Peter Bulova, MD; Amrita Cheema, PhD; Kewei Chen, PhD; Bradley T. Christian, PhD; Isabel Clare, PhD; Lorraine Clark, PhD; Ann D. Cohen, PhD; John N. Constantino, MD; Eric W. Doran, MS; Anne Fagan, PhD; Eleanor Feingold, PhD; Tatiana M. Foroud, PhD; Benjamin L. Handen, PhD; Jordan Harp, PhD; Sigan L. Hartley, PhD; Elizabeth Head, PhD; Rachel Henson, MS; Christy Hom, PhD; Lawrence Honig, MD; Milos D. Ikonomovic, MD; Sterling C Johnson, PhD; Courtney Jordan, RN; M. Ilyas Kamboh, PhD; David Keator, PhD; William E. Klunk, MD PhD; Julia K. Kofler, MD; William Charles Kreisl, MD; Sharon J. Krinsky-McHale, PhD; Florence Lai, MD; Patrick Lao, PhD; Charles Laymon, PhD; Joseph Hyungwoo Lee, PhD; Ira T. Lott, MD; Victoria Lupson, PhD; Mark Mapstone, PhD; Chester A. Mathis, PhD; Davneet Singh Minhas, PhD; Neelesh Nadkarni, MD; Sid O’Bryant, PhD; Melissa Parisi, MD PhD; Deborah Pang, MPH; Melissa Petersen, PhD; Julie C. Price, PhD; Margaret Pulsifer, PhD; Michael S. Rafii, MD PhD, Eric Reiman, MD; Batool Rizvi, MS; Herminia Diana Rosas, MD; Laurie Ryan, PhD; Frederick Schmitt, PhD; Nicole Schupf, PhD; Wayne P. Silverman, PhD; Dana L. Tudorascu, PhD; Rameshwari Tumuluru, MD; Benjamin Tycko, MD PhD; Badri Varadarajan, PhD; Desiree A. White, PhD; Michael A. Yassa, PhD; Shahid Zaman, MD PhD; Fan Zhang, PhD. AVID Pharmaceuticals supplied AV1451, but did not provide financial support for this study nor did it have a role in designing the study or interpreting the results.
This work was supported by US National Institutes of Health grants RF1 AG079519, U19 AG068054, U01 AG051412, U01 AG051406, and R00 AG065506.
Author information
Authors and affiliations.
Gertrude H. Sergievsky Center and Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, College of Physicians and Surgeons, Columbia University, 630 West 168th Street, PS Box 16, New York, NY, 10032, USA
Patrick Lao, Natalie Edwards, Mohamad Alshikho & Adam M. Brickman
Department of Neurology, College of Physicians and Surgeons, Columbia University, New York, NY, 10032, USA
Patrick Lao, Jose Gutierrez & Adam M. Brickman
Department of Pathology and Laboratory Medicine, University of California, Irvine, Irvine, CA, 92697, USA
Lisi Flores-Aguilar & Elizabeth Head
Department of Neurology, University of California, Irvine, Irvine, CA, 92697, USA
Batool Rizvi, Michael Yassa & Mark Mapstone
Department of Psychiatry, University of Pittsburgh Medical Center, Pittsburgh, PA, 15213, USA
Dana Tudorascu & Benjamin Handen
Department of Neurology, Massachusetts General Hospital, Harvard Medical Center, Boston, MA, 02114, USA
H. Diana Rosas
Waisman Center, University of Wisconsin-Madison, Madison, WI, 53705, USA
Bradley T. Christian
Department of Psychology, Fordham University, Bronx, NY, 10458, USA
Molly E. Zimmerman
Departments of Neurology and Anatomy, Cell Biology, and Physiology, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis, IN, USA
Donna Wilcock
Department of Neurobiology and Behavior, University of California, Irvine, Irvine, CA, 92697, USA
Elizabeth Head
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the drafting of the work. P.J.L. contributed to conception, design, analysis, and interpretation of the work. N.E., L.F.A., and B.R. contributed to interpretation of the work. M.A. and D.T. contributed to analysis of the work. D.T., H.D.R., M.Y., B.T.C., M.M., B.H., M.E.Z., J.G., D.W., E.H., and A.M.B. contributed to the design, acquisition, and interpretation of the work. E.H. and A.M.B. additionally contributed to the conception of the work.
Corresponding author
Correspondence to Adam M. Brickman .
Ethics declarations
Competing interests.
Michael A. Yassa has received consulting fees from Eisai Cognito Therapeutics, LLC, CuraSen Therapeutics, Inc, and Enthorin Therapeutics, LLC. Adam Brickman receives compensation for consultation to Cognition Therapeutics and Cognito Therapeutics and for his role on the Scientific Advisory Board of CogState. He is an inventor a patent for white matter hyperintensity quantification (US Patent US9867566B2) and serves on a Data Safety Monitoring Board for University of Illinois, Urbana-Champaign. All other authors do not have competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lao, P., Edwards, N., Flores-Aguilar, L. et al. Cerebrovascular disease emerges with age and Alzheimer’s disease in adults with Down syndrome. Sci Rep 14 , 12334 (2024). https://doi.org/10.1038/s41598-024-61962-y
Download citation
Received : 13 February 2024
Accepted : 13 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1038/s41598-024-61962-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
MINI REVIEW article
Development of down syndrome research over the last decades–what healthcare and education professionals need to know.

- 1 Division of Obstetrics and Feto-Maternal Medicine, Department of Obstetrics and Gynecology, Medical University of Vienna, Vienna, Austria
- 2 Research Unit Developmental Psychology, Department of Developmental and Educational Psychology, Faculty of Psychology, University of Vienna, Vienna, Austria
Down syndrome (DS) is the most prevalent neurodevelopmental disorder, with a known genetic cause. Besides facial dysmorphologies and congenital and/or acquired medical conditions, the syndrome is characterized by intellectual disability, accelerated aging, and an increased likelihood of an early onset Alzheimer's disease in adulthood. These common patterns of DS are derived from the long-held standard in the field of DS research, that describes individuals with DS as a homogeneous group and compares phenotypic outcomes with either neurotypical controls or other neurodevelopmental disorders. This traditional view has changed, as modern research pinpoints a broad variability in both the occurrence and severity of symptoms across DS, arguing for DS heterogeneity and against a single “DS profile.” Nevertheless, prenatal counseling does not often prioritize the awareness of potential within-group variations of DS, portraying only a vague picture of the developmental outcomes of children with DS to expectant parents. This mini-review provides a concise update on existent information about the heterogeneity of DS from a full-spectrum developmental perspective, within an interdisciplinary context. Knowledge on DS heterogeneity will not only enable professionals to enhance the quality of prenatal counseling, but also help parents to set targeted early interventions, to further optimize daily functions and the quality of life of their children.
Introduction
Down syndrome (DS) is the most common neurodevelopmental disorder with known genetic causes, and an incidence of 1 in 691 live births ( 1 ). This suggests that ~417,000 people with DS live in Europe ( 2 ). Currently, an expansive menu of prenatal diagnostic methods for DS is spreading worldwide, advancing the diagnosis of DS from postnatal to prenatal ( 3 ). Giving an expectant parent a fetal diagnosis of DS provides them with 2 options: keeping or terminating their pregnancy, following the lack of a cure ( 4 ).
Prenatal counseling is crucial for providing parents with an accurate picture of DS so that informed decisions can be made in the context of their own beliefs and values ( 3 ). Although studies are still examining the nature of DS, portraying the expected neurodevelopmental outcomes of affected children remains challenging. Indeed, retrospective studies indicate that parents felt that the information received during prenatal counseling was inaccurate, outdated, and unbalanced, and either too negative or too optimistic ( 5 – 7 ). Without appropriate professional training or updated professional development regarding the individual variability in outcomes associated with DS, prenatal counselors might present expectant parents with inaccurate information or impressions. Therefore, expectant parents may not receive the level of information needed. Accordingly, all professionals working with families affected by DS must be aware of the most current scientific research regarding the heterogeneity of phenotypic outcomes ( 8 ).
This mini-review closes an existent literature gap by providing a concise update on the available information on within-group variations in the DS phenotype of infants, children, and adolescents for professionals. First, a gross outline of DS research is given, focusing on the significant paradigm shift from a group- to an individual-level approach. Second, the current knowledge on significant within-group variations of DS in cognitive, behavioral, emotional, and olfactory functioning is summarized. Finally, the review concludes by arguing that only an interdisciplinary approach allows for the description of realistic individual DS profiles. The scope of this review is to further increase the awareness on DS heterogeneity concerning developmental outcomes.
A Paradigm Shift in DS Research: From a Group- to Individual-Level Approach
DS research dates back to 1866, when the English physician John Langdon Down systematically described the syndrome for the first time ( 9 , 10 ). In addition to intellectual disability (ID), he chronicled a distinct physical phenotype of individuals with DS, conjecturing that they were “born to the same family” (page 9) ( 10 , 11 ). The century following his pioneering work was filled with publications of diverse medical case studies documenting a range of physical traits and medical comorbidities, leading to various etiologies ( 10 , 11 ).
Almost 100 years later, the French pediatrician and cytogeneticist, Jérôme Lejeune, identified the genetic basis of DS in 1959 as an extra copy of all or part of chromosome 21 ( 10 , 12 ). The discovery of “trisomy 21” paved the way for further research, to elucidate genotype-phenotype-relationships ( 13 , 14 ). Since its original description, classical DS research has analyzed the syndrome's phenotypes relative to neurotypicals and/or other neurodevelopmental disorders, hence providing group-level data that have advanced our basic knowledge of DS ( 8 ). It is characterized by both typical physical features that make the syndrome “instantly recognizable” (page 8) and ID ( 11 ). Common appearance includes craniofacial dysmorphologies, short stature, low muscle tone, and a proportionally large tongue. Additionally, medical comorbidities, such as sleep apnea, visual and/or hearing problems, congenital heart defects, and altered behavioral, hematopoietic, endocrine, gastrointestinal, neurological, and musculoskeletal conditions, are linked to DS ( 10 ).
Most of these medical problems are treatable with pharmacotherapy and/or surgical interventions. Therefore, among the key focuses in recent DS research is the widespread field of neurocognition, associating DS with weaknesses in motor ability, auditory processing, verbal short-term memory, and expressive language. However, relative strengths in visuospatial processing, receptive language, and some aspects of social functioning have been reported ( 15 – 18 ). Further, DS is associated with accelerated aging and an increased likelihood of the early onset of Alzheimer's disease (AD) ( 18 ).
Although the generalizability of the characteristics of DS has been questioned repeatedly in the history of DS research, the group-level approach is a long-held standard ( 19 , 20 ). However, this traditional view has changed, following a growing number of studies, which pinpoint significant within-group variations across individuals with DS at many levels of description. Pioneer studies have launched this paradigm shift, from a group to an individual-level approach, by highlighting significant individual differences in genetics, cell biology, brain research, and subsequently, parts of cognitive research on DS [see ( 8 )]. These studies suggest that this heterogeneity may be continued in DS phenotypes ( 8 ). The following review aims to supplement the prevailing knowledge about the variability of the developmental outcomes of DS by addressing this issue from an interdisciplinary and applied science perspective, as this practical information may be the most useful for professionals to pass to expectant parents.
Infants, Children, and Adolescents With DS: Variability in Developmental Outcomes
Acquisition of developmental milestones.
Generally, it was assumed that infants and children with DS reached developmental milestones in the same linear fashion as their non-DS peers, but at later chronological ages. This view is too simplistic, as the age of acquiring milestones among infants and children with DS is reported to vary significantly ( 21 , 22 ). For example, the mean age at the onset of babbling is ~15 months, with an interindividual variability of 10 months. Similarly, sphincter control is acquired by DS children at an approximate age of 44 months, with 22 months of interindividual variability ( 22 ). Notably, Locatelli et al. suggested that the age at which developmental milestones are reached influences the subsequent development of diverse cognitive domains significantly ( 21 , 22 ).
Intellectual Disability (ID)
ID, defined by an intelligence quotient (IQ) score of <70, is reported to be universal in the DS population. However, this construct presents in DS with large interindividual variability ( 23 ). The majority of individuals with DS fall within the severe (IQ 20–35) to mild (IQ 50–69) range of ID. However, some cases reach IQ scores equivalent to children without ID ( 14 , 24 ). Research on the developmental trajectories of cognitive function in neurotypicals shows that IQ is a construct that remains relatively stable and consistent across ages. A slight decline was observed only in older adults ( 14 ). Conversely, DS research has identified a linear decline in IQ scores as development progresses, starting in the first year of life (i.e., cognitive gains do not keep pace with chronological age). Notably, single IQ levels and the degree of cognitive decline vary across the DS group ( 14 ).
Language is another cognitive domain that generates significant differences among individuals with DS. DS is associated with weaknesses in expressive language and a relative strength in the receptive language ( 18 ). The available literature reports developmental delays in both language domains, becoming apparent no later than age five, yet with wide individual differences ( 25 , 26 ). Regarding vocabulary acquisition and growth, longitudinal studies reported an existing continuum, ranging from non-verbal children to those with a vocabulary close to the normal range ( 27 , 28 ). Children with DS use gestures as a means of communication, which has been positively associated with the development of spoken vocabulary ( 29 ). Nevertheless, significant individual variability in the extent to which this “gestural advantage” is used has been demonstrated by empirical data ( 30 ). All within-group differences in language development persist into adulthood ( 26 ).
Memory and learning deficits are universal characteristics of DS and are known to become more pronounced as development progresses ( 14 ). In classical DS research, the findings of affected memory domains are mixed, suggesting underlying variability ( 18 ). Indeed, scientific data demonstrate that there are individual differences in both implicit and explicit memory ( 8 , 31 ). Regarding the latter, significant within-group variations are described for short-term verbal and long-term visual memory ( 8 ). Individuals with DS often show deficits in processing local detail. Therefore, classical DS literature claims that individuals with DS were “global processors.” However, this preference for global over local processing does not always occur in the DS population. Therefore, individuals with DS cannot be simply categorized into one of these processing styles ( 32 ).
Executive Function (EF)
EF encompasses a range of cognitive processes involved in goal-oriented behavior, and is a domain in which individuals with DS are shown to have pronounced difficulties ( 33 ). The areas of working memory, attention, planning, and inhibition are considered particularly challenging for individuals with DS; emotional control is considered a relative strength ( 34 , 35 ). However, significant individual differences in EF across the DS group have become evident ( 33 , 36 ). Within-group variations in auditory attention have been identified via electrophysiological measurement among toddlers with DS, data that also predict differences in language abilities as development progresses ( 37 ). Patterns of executive dysfunction appear to be relatively consistent across development until adulthood ( 23 , 34 ).
Adaptive Behavior (AB)
Children and adolescents with DS are known to be severely impaired in AB, which subsumes behavioral skills that enable them to function independently in their everyday life ( 23 , 38 ). Generally, AB encompasses 4 domains: socialization, communication, daily living, and motor skills ( 23 ). Significant within-group variations were apparent for all the 4 domains. For example, DS has been associated with sociability, friendliness, affection, empathy, good competence in forming relationships, and high tendency to smile ( 39 ). Yet, children and adolescents with DS are also considered stubborn, to show little accommodation to social partners, and approach strangers inappropriately ( 40 ). Some individuals with DS have even deficits in socialization to the extent of a comorbid diagnosis of autism ( 41 ).
Maladaptive Behavior (MB) and Psychiatric Comorbidities
MB encompasses a range of behaviors that impede an individual's activities of daily living or the ability to adjust to and participate in particular settings ( 23 ). Approximately 1/4 to 1/3 of individuals with DS exhibit clinically significant levels of maladaptive behavioral concerns ( 42 – 44 ). This behavioral construct is another domain that yields significant within-group differences ( 21 , 23 , 45 ). More difficulties with “anxious-depressed” symptoms are observed among adolescents than younger children with DS ( 23 ). Children with DS often exhibit externalizing behavior ( 46 ). The manifestation of MB is significantly higher when neurobehavioral disorders are concomitant ( 47 – 49 ). According to the available literature, the manifestation of psychiatric features, including autism, depression, and the attention-deficit/hyperactivity disorder, vary significantly, between 6 and >50% ( 42 , 44 , 50 , 51 ). Channell et al. underscored within-group differences in the behavioral domain by subtyping a >300-person DS group, hence identifying a separate “behavioral” class as described in Table 1 ( 23 ).
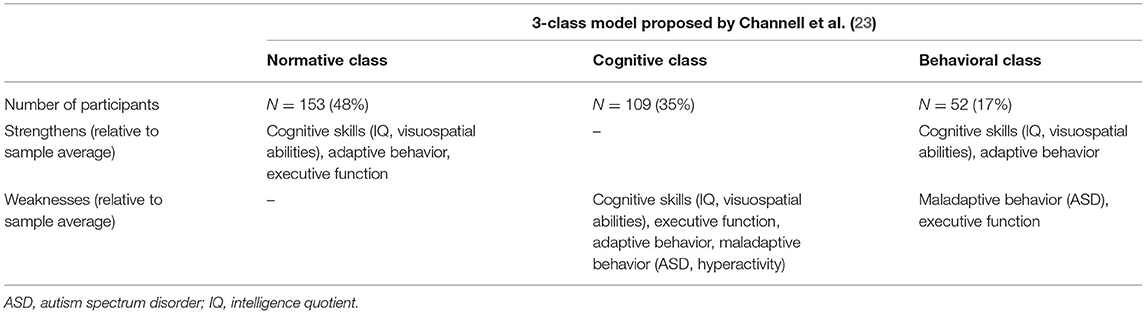
Table 1 . Characterization of the 3-class model of individuals with DS ( N = 314; 6–25 years) based on the variability observed in cognitive and behavioral measures, identified by Channell et al. ( 23 ) using a latent profile analysis.
Emotional Functioning
The emotional profiles of individuals with DS have remained underexplored, which could be attributed to the assumed stereotype of high sociability in this population ( 52 , 53 ). Available literature provides variable data about whether children and adolescents have difficulties in emotional functioning ( 52 ). Whereas, some studies negate differences in identifying basic emotion in faces between DS and non-DS groups, other scientific reports indicate that children and adolescents with DS have impairments in this emotional skill [see Roch et al. ( 52 )] ( 54 – 57 ). Deficits in recognizing facial expressions were not generalized to all emotions, but mostly to fear ( 52 , 58 ). Other studies report impairments in determining feelings, including surprise, anger, and neutral expression ( 40 , 58 – 61 ). Some studies pinpoint problems in ascertaining negative emotions ( 40 ). Moreover, an inability to distinguish between fear and sadness is another atypical pattern that has been reported among some individuals ( 58 ). Most of these deficits are identified during infancy and childhood. Therefore, a negative impact on the subsequent development of interpersonal relationships is discussed ( 52 ). As previously mentioned, studies have exclusively gathered data at the group level. Moreover, further research should examine whether inconsistencies in findings across studies can be attributed to underlying within-group variations.
Olfactory Functioning
The number of studies on olfactory function among patients with DS is limited and relatively out of date ( 62 – 69 ). Historical studies have described olfactory deficits in the DS population for many years ( 62 , 63 , 65 , 70 ). Because rhinologic pathologies have been ruled out by studies showing nasal function in DS as comparable to controls, central-nervous causes are suggested ( 64 ). More recently, Cecchini et al. described olfactory function as severely impaired among adults with DS ( 71 ). They found a positive correlation between odor identification and cognition ( 71 ). To date, the largest study, which included people with DS and under 18 years, described a minimal impairment of olfactory functioning among children and adolescents (9–17 years), which became pronounced in young adulthood (18–29 years) and was the lowest in adulthood (30–50 years) ( 72 ). Of the three groups, DS, IQ, and age-matched controls, significant within-group differences were evident only in the DS group ( 72 ). However, large and detailed analyses of olfactory function in light of within-group variations among children and adolescents with DS are still lacking. Odor identification deficits are considered a valid non-invasive early marker of AD. Therefore, future research on whether olfactory dysfunction can help to ascertain the subset of children and adolescents with DS that will later develop AD is warranted.
Alzheimer's Disease (AD)
Although the issue of AD appears outside the scope of this review, the following considerations must be made when the heterogeneity of DS is discussed with expectant parents from a full-spectrum developmental perspective. Owing to a shared genetic predisposition, individuals with DS have an increased likelihood of developing early onset AD in adulthood ( 18 ). Prevalence rates of dementia among the DS population vary significantly in the literature, from 8 to 100% ( 18 , 73 ). Recent brain research has identified Alzheimer's plaques among some children with DS, that is, as early as 8 years of age, whereas some DS brains show no plaques until early adulthood ( 14 , 26 ). Although AD neuropathology occurs in virtually all individuals with DS over the age of 30, only a subset of people develop clinical symptoms of dementia ( 26 , 74 , 75 ). Hence, it is apparent that the widespread interindividual variability, typical for DS, is a pivotal feature not only during development, but also during aging ( 26 ). Aging is part of the continuous lifespan development. Accordingly, some authors argue that AD should be considered a disease that occurs during development, rather than aging ( 76 ).
Extrinsic Influencing Factors of Developmental Outcomes of Infants, Children, and Adolescents With DS
Medical comorbidities.
In addition to cognitive limitations, parents must be informed that there is a list of medical comorbidities associated with DS. Some of them, including congenital heart defects (CHD), seizures, visual and/or hearing impairments, autism, and sleep disruptions, are known to moderate cognitive functioning ( 18 ). Analogous to neurodevelopmental outcomes, both the occurrence and expression of congenital and/or acquired medical complications are variable ( 18 ). For example, 41–56% of infants with DS are born with a CHD, with an atrioventricular septal defect that occurs between 31 and 61% being the most common form ( 77 , 78 ). Cognition, gross motor skills, and language are significantly worse among infants with DS and CHD, relative to peers without CHD, in some, but not in all related studies ( 79 – 81 ). For example, Alsaied et al. showed that children with DS and CHD, who undergo cardiac surgery during their first year, have no significant differences in neurodevelopmental outcomes at preschool and school age. However, as infants and toddlers, they were prone to poorer outcomes in receptive, expressive, and composite language compared to children with DS without CHD, suggesting that deleterious effects may be dependent on clinical management ( 82 ).
Home Environment
Another variable that affects the observed variability of DS phenotypes, which is influenced by the expectant parents, is the home environment. According to Karmiloff-Smith et al., the genetic syndrome changes the family context in terms of parent-child-interactions ( 8 ). D'Souza et al. demonstrated that parental depression, a disease linked to difficulties in responding to the child in a sensitive and consistent manner, explained deficits in expressive language development among children between 8 and 48 months of age with DS ( 83 ). Similarly, there is evidence that vocabulary development among children with DS is influenced by how parents respond to their children's communication. Deckers et al. argued that mothers with a higher level of education had a better ability to fine-tune their communication with their children with DS ( 28 ). Further demographic factors, including socioeconomic status, neighborhood demographics, and the availability of therapeutic resources, modulate the developmental outcomes of DS effectively ( 84 , 85 ). These data demonstrate that only an interdisciplinary approach that considers psychological, physical, and social parameters will enable professionals to accurately inform expectant parents on how the DS phenotype will be expressed in each individual.
Although DS has been examined for a long time, that is 155 years, it is still one of the least understood genetic ID syndromes. The most significant reason for this is the high degree of phenotypic variability observed in the DS population, an issue that professionals are often unaware of when discussing the diagnosis with expectant parents. However, DS research has advanced from a group to an individual-level approach, attempting to acknowledge within-group differences at many levels of basic science ( 8 ). To expand on this wealth of data, this mini-review has shed light on the available information on individual variability in the developmental outcomes of infants, children, and adolescents with DS from an applied science perspective, which will enhance the quality of prenatal counseling. Diverse developmental domains, including cognition, behavior, and emotional and olfactory functioning, have been discussed.
The evaluation of developmental outcomes from a full-spectrum perspective, however, must not only address different developmental domains, but also the change of phenotypes over time ( 86 ). Outcome variables are not completely intact or impaired uniformly throughout development, but manifest as variations at an early state, that may be magnified with age, ending up as either a strength or a weakness. Therefore, parents should be made aware that early development can be considered a critical window of opportunity to set adequate phenotype-specific interventions before deficits become severely pronounced ( 87 ). Thus, the maximization of individual potential is possible. In addition to psychological factors, other influencing variables must be considered by parents when the variability of DS phenotypes is discussed. According to Karmiloff-Smith who states that having a neurodevelopmental disorder changes both the social environment and physical status, only an interdisciplinary research approach can successfully describe valid profiles of individuals with DS ( 8 ).
The most convincing argument for emphasizing individual variability among DS groups and discussing them with expectant parents are both an average life expectancy of 60 years combined with an early onset of Alzheimer's disease in the DS population ( 18 ). Focusing on individual differences in the development of DS may be the best approach for exploring the risk and protective factors of AD ( 88 , 89 ).
Modern DS research shows that developmental heterogeneity has become increasingly validated ( 23 ). Moving forward, these up-to-date data must be disseminated under the supervision of professionals so that prenatal counseling can be optimized in quality, hence allowing parents to gain realistic expectations about the future of their children. Thus, more targeted treatments and interventions can be set to improve the daily function and quality of life.
Author Contributions
KW and SH designed the paper. KW did the literature research and wrote the manuscript. SH provided intellectual input and critically revised the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. (2010) 88:1008–16. doi: 10.1002/bdra.20735
PubMed Abstract | CrossRef Full Text | Google Scholar
2. de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in Europe. Eur J Hum Genet. (2021) 29:402–10. doi: 10.1038/s41431-020-00748-y
3. Minear MA, Alessi S, Allyse M, Michie M, Chandrasekharan S. Noninvasive prenatal genetic testing: current and emerging ethical, legal, and social issues. Annu Rev Genomics Hum Genet. (2015) 16:369–98. doi: 10.1146/annurev-genom-090314-050000
4. Guedj F, Bianchi DW. Noninvasive prenatal testing creates an opportunity for antenatal treatment of Down syndrome. Prenat Diagn. (2013) 33:614–8. doi: 10.1002/pd.4134
5. Skotko BG, Kishnani PS, Capone GT, Group DSDS. Prenatal diagnosis of Down syndrome: how best to deliver the news. Am J Med Genet A . (2009) 149A:2361–7. doi: 10.1002/ajmg.a.33082
6. Zerres K, Rudnik-Schöneborn S, Holzgreve W. Do non-invasive prenatal tests promote discrimination against people with Down syndrome? What should be done? J Perinat Med. (2021) 49:965–71. doi: 10.1515/jpm-2021-0204
7. Skotko BG. With new prenatal testing, will babies with Down syndrome slowly disappear? Arch Dis Child. (2009) 94:823–6. doi: 10.1136/adc.2009.166017
8. Karmiloff-Smith A, Al-Janabi T, D'Souza H, Groet J, Massand E, Mok K, et al. The importance of understanding individual differences in Down syndrome. F1000Res . (2016) 5:389. doi: 10.12688/f1000research.7506.1
9. Down JL. Observations on an ethnic classification of idiots. Ment Retard . (1995) 33:54–6.
Google Scholar
10. Hickey F, Hickey E, Summar KL. Medical update for children with Down syndrome for the pediatrician and family practitioner. Adv Pediatr. (2012) 59:137–57. doi: 10.1016/j.yapd.2012.04.006
11. D W. Downs: The History of a Disability. Oxford: Oxford University Press (2011).
12. Lejeune J, Gautier M, Turpin R. Study of somatic chromosomes from 9 mongoloid children. C R Hebd Seances Acad Sci. (1959) 248:1721–2.
PubMed Abstract | Google Scholar
13. Patterson D, Costa AC. Down syndrome and genetics - a case of linked histories. Nat Rev Genet. (2005) 6:137–47. doi: 10.1038/nrg1525
14. Lukowski AF, Milojevich HM, Eales L. Cognitive functioning in children with down syndrome: current knowledge and future directions. Adv Child Dev Behav. (2019) 56:257–89. doi: 10.1016/bs.acdb.2019.01.002
15. Daunhauer LA, Fidler DJ. The down syndrome behavioral phenotype: implications for practice and research in occupational therapy. Occup Ther Health Care. (2011) 25:7–25. doi: 10.3109/07380577.2010.535601
16. Jarrold C, Baddeley AD. Short-term memory for verbal and visuospatial information in down's syndrome. Cogn Neuropsychiatry. (1997) 2:101–22. doi: 10.1080/135468097396351
17. Jarrold C, Baddeley AD, Phillips C. Down syndrome and the phonological loop: the evidence for, and importance of, a specific verbal short-term memory deficit. Downs Syndr Res Pract. (1999) 6:61–75. doi: 10.3104/reviews.97
18. Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. (2015) 169:135–49. doi: 10.1002/ajmg.c.31439
19. Desai SS. Down syndrome: a review of the literature. Oral Surg Oral Med Oral. (1997) 84:279–85. doi: 10.1016/S1079-2104(97)90343-7
20. Hodapp RM. Studying interactions, reactions, and perceptions: can genetic disorders serve as behavioral proxies? J Autism Dev Disord. (2004) 34:29–34. doi: 10.1023/B:JADD.0000018071.02942.00
21. van Gameren-Oosterom HB, Fekkes M, Buitendijk SE, Mohangoo AD, Bruil J, Van Wouwe JP. Development, problem behavior, and quality of life in a population based sample of eight-year-old children with Down syndrome. PLoS ONE. (2011) 6:e21879. doi: 10.1371/journal.pone.0021879
22. Locatelli C, Onnivello S, Antonaros F, Feliciello A, Filoni S, Rossi S, et al. Is the age of developmental milestones a predictor for future development in down syndrome? Brain Sci. (2021) 11:655. doi: 10.3390/brainsci11050655
23. Channell MM, Mattie LJ, Hamilton DR, Capone GT, Mahone EM, Sherman SL, et al. Capturing cognitive and behavioral variability among individuals with Down syndrome: a latent profile analysis. J Neurodev Disord. (2021) 13:16. doi: 10.1186/s11689-021-09365-2
24. Edgin JO. Cognition in Down syndrome: a developmental cognitive neuroscience perspective. Wiley Interdiscip Rev Cogn Sci. (2013) 4:307–17. doi: 10.1002/wcs.1221
25. Guralnick MJ. Involvement with peers: comparisons between young children with and without Down's syndrome. J Intellect Disabil Res. (2002) 46(Pt5):379–93. doi: 10.1046/j.1365-2788.2002.00405.x
26. Thomas MSC, Ojinaga Alfageme O, D'Souza H, Patkee PA, Rutherford MA, Mok KY, et al. A multi-level developmental approach to exploring individual differences in Down syndrome: genes, brain, behaviour, and environment. Res Dev Disabil. (2020) 104:103638. doi: 10.1016/j.ridd.2020.103638
27. Zampini L, D'Odorico L. Communicative gestures and vocabulary development in 36-month-old children with Down's syndrome. Int J Lang Commun Disord. (2009) 44:1063–73. doi: 10.1080/13682820802398288
28. Deckers SRJM, Van Zaalen Y, Van Balkom H, Verhoeven L. Predictors of receptive and expressive vocabulary development in children with Down syndrome. Int J Speech Lang Pathol. (2019) 21:10–22. doi: 10.1080/17549507.2017.1363290
29. Özçalişkan S, Adamson LB, Dimitrova N, Baumann S. Early gesture provides a helping hand to spoken vocabulary development for children with autism, Down syndrome and typical development. J Cogn Dev. (2017) 18:325–37. doi: 10.1080/15248372.2017.1329735
30. Kaat-van den Os D, Volman C, Jongmans M, Lauteslager P. Expressive vocabulary development in children with down syndrome: a longitudinal study. J Policy Pract Intellect Disabil. (2016) 14:311–8. doi: 10.1111/jppi.12212
CrossRef Full Text | Google Scholar
31. Vicari S. Implicit versus explicit memory function in children with Down and Williams syndrome. Downs Syndr Res Pract. (2001) 7:35–40. doi: 10.3104/reports.112
32. D'Souza D, Booth R, Connolly M, Happé F, Karmiloff-Smith A. Rethinking the concepts of 'local or global processors': evidence from Williams syndrome, Down syndrome, and Autism Spectrum Disorders. Dev Sci. (2016) 19:452–68. doi: 10.1111/desc.12312
33. Tungate AS, Conners FA. Executive function in Down syndrome: a meta-analysis. Res Dev Disabil. (2021) 108:103802. doi: 10.1016/j.ridd.2020.103802
34. Loveall SJ, Conners FA, Tungate AS, Hahn LJ, Osso TD. A cross-sectional analysis of executive function in Down syndrome from 2 to 35 years. J Intellect Disabil Res. (2017) 61:877–87. doi: 10.1111/jir.12396
35. Lee NR, Anand P, Will E, Adeyemi EI, Clasen LS, Blumenthal JD, et al. Everyday executive functions in Down syndrome from early childhood to young adulthood: evidence for both unique and shared characteristics compared to youth with sex chromosome trisomy (XXX and XXY). Front Behav Neurosci. (2015) 9:264. doi: 10.3389/fnbeh.2015.00264
36. Grealish KG, Price AM, Stein DS. Systematic review of recent pediatric down syndrome neuropsychology literature: considerations for regression assessment and monitoring. J Dev Behav Pediatr. (2020) 41:486–95. doi: 10.1097/DBP.0000000000000800
37. D'Souza D, D'Souza H, Johnson MH, Gliga T, Kushnerenko E, Scerif G, et al. Are early neurophysiological markers of ASD syndrome-specific? A cross-syndrome comparison. In: Paper presented at the XIX Biennial International Conference on Infant Studies. Berlin: International Society on Infant Studies (2014).
38. de Weger C, Boonstra FN, Goossens J. Differences between children with Down syndrome and typically developing children in adaptive behaviour, executive functions and visual acuity. Sci Rep. (2021) 11:7602. doi: 10.1038/s41598-021-85037-4
39. Fidler DJ. The emerging Down syndrome behavioral phenotype in early childhood. Infants Young Child. (2005) 18:86–103. doi: 10.1097/00001163-200504000-00003
40. Porter MA, Coltheart M, Langdon R. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. (2007) 45:2839–49. doi: 10.1016/j.neuropsychologia.2007.05.006
41. Glennon JM, Karmiloff-Smith A, Thomas MSC. Syndromic autism: progressing beyond current levels of description. Rev J Autism Dev Disord. (2017) 4:321–7. doi: 10.1007/s40489-017-0116-2
42. Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Ment Retard Dev Disabil Res Rev. (2007) 13:272–8. doi: 10.1002/mrdd.20159
43. Coe DA, Matson JL, Russell DW, Slifer KJ, Capone GT, Baglio C, et al. Behavior problems of children with Down syndrome and life events. J Autism Dev Disord. (1999) 29:149–56. doi: 10.1023/A:1023044711293
44. Roizen NJ, Patterson D. Down's syndrome. Lancet. (2003) 361:1281–9. doi: 10.1016/S0140-6736(03)12987-X
45. van Gameren-Oosterom HB, Fekkes M, van Wouwe JP, Detmar SB, Oudesluys-Murphy AM, Verkerk PH. Problem behavior of individuals with Down syndrome in a nationwide cohort assessed in late adolescence. J Pediatr. (2013) 163:1396–401. doi: 10.1016/j.jpeds.2013.06.054
46. Dykens EM, Shah B, Sagun J, Beck T, King BH. Maladaptive behaviour in children and adolescents with Down's syndrome. J Intellect Disabil Res. (2002) 46(Pt6):484–92. doi: 10.1046/j.1365-2788.2002.00431.x
47. Capone GT, Grados MA, Kaufmann WE, Bernad-Ripoll S, Jewell A. Down syndrome and comorbid autism-spectrum disorder: characterization using the aberrant behavior checklist. Am J Med Genet A. (2005) 134:373–80. doi: 10.1002/ajmg.a.30622
48. Carter JC, Capone GT, Gray RM, Cox CS, Kaufmann WE. Autistic-spectrum disorders in Down syndrome: further delineation and distinction from other behavioral abnormalities. Am J Med Genet B Neuropsychiatr Genet. (2007) 144B:87–94. doi: 10.1002/ajmg.b.30407
49. Ji NY, Capone GT, Kaufmann WE. Autism spectrum disorder in Down syndrome: cluster analysis of Aberrant Behaviour Checklist data supports diagnosis. J Intellect Disabil Res. (2011) 55:1064–77. doi: 10.1111/j.1365-2788.2011.01465.x
50. Siegel MS, Smith WE. Psychiatric features in children with genetic syndromes: toward functional phenotypes. Pediatr Clin North Am. (2011) 58:833–64. doi: 10.1016/j.pcl.2011.06.010
51. Capone G, Goyal P, Ares W, Lannigan E. Neurobehavioral disorders in children, adolescents, and young adults with Down syndrome. Am J Med Genet C Semin Med Genet. (2006) 142C:158–72. doi: 10.1002/ajmg.c.30097
52. Roch M, Pesciarelli F, Leo I. How individuals with down syndrome process faces and words conveying emotions? Evidence From a Priming Paradigm. Front Psychol. (2020) 11:692. doi: 10.3389/fpsyg.2020.00692
53. Pitcairn TK, Wishart JG. Reactions of young children with Down's syndrome to an impossible task. Br J Dev Psychol. (1994) 12:485–9. doi: 10.1111/j.2044-835X.1994.tb00649
54. Turk J, Cornish K. Face recognition and emotion perception in boys with fragile-X syndrome. J Intellect Disabil Res. (1998) 42:490–9. doi: 10.1046/j.1365-2788.1998.4260490.x
55. Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J Autism Dev Disord. (1999) 29:57–66. doi: 10.1023/A:1025970600181
56. Goldman KJ, Shulman C, Bar-Haim Y, Abend R, Burack JA. Attention allocation to facial expressions of emotion among persons with Williams and Down syndromes. Dev Psychopathol. (2017) 29:1189–97. doi: 10.1017/S0954579416001231
57. Martínez-Castilla P, Burt M, Borgatti R, Gagliardi C. Facial emotion recognition in Williams syndrome and Down syndrome: a matching and developmental study. Child Neuropsychol. (2015) 21:668–92. doi: 10.1080/09297049.2014.945408
58. Wishart JG, Cebula KR, Willis DS, Pitcairn TK. Understanding of facial expressions of emotion by children with intellectual disabilities of differing aetiology. J Intellect Disabil Res. (2007) 51(Pt7):551–63. doi: 10.1111/j.1365-2788.2006.00947.x
59. Hippolyte L, Barisnikov K, Van der Linden M. Face processing and facial emotion recognition in adults with Down syndrome. Am J Ment Retard. (2008) 113:292–306. doi: 10.1352/0895-8017(2008)113[292:FPAFER]2.0.CO;2
60. Kasari C, Freeman SF, Hughes MA. Emotion recognition by children with Down syndrome. Am J Ment Retard. (2001) 106:59–72. doi: 10.1352/0895-8017(2001)106<0059:ERBCWD>2.0.CO;2
61. Wishart JG, Pitcairn TK. Recognition of identity and expression in faces by children with Down syndrome. Am J Ment Retard. (2000) 105:466–79. doi: 10.1352/0895-8017(2000)105 <0466:ROIAEI>2.0.CO;2
62. Murphy C, Jinich S. Olfactory dysfunction in Down's Syndrome. Neurobiol Aging. (1996) 17:631–7. doi: 10.1016/0197-4580(96)00008-5
63. Hemdal P, Corwin J, Oster H. Olfactory identification deficits in Down's syndrome and idiopathic mental retardation. Neuropsychologia. (1993) 31:977–84. doi: 10.1016/0028-3932(93)90152-P
64. Chen MA, Lander TR, Murphy C. Nasal health in Down syndrome: a cross-sectional study. Otolaryngol Head Neck Surg. (2006) 134:741–5. doi: 10.1016/j.otohns.2005.12.035
65. Warner MD, Peabody CA, Berger PA. Olfactory deficits and Down's syndrome. Biol Psychiatry. (1988) 23:836–9. doi: 10.1016/0006-3223(88)90073-X
66. Zucco GM, Negrin NS. Olfactory deficits in Down subjects: a link with Alzheimer disease. Percept Mot Skills. (1994) 78:627–31. doi: 10.2466/pms.1994.78.2.627
67. McKeown DA, Doty RL, Perl DP, Frye RE, Simms I, Mester A. Olfactory function in young adolescents with Down's syndrome. J Neurol Neurosurg Psychiatry. (1996) 61:412–4. doi: 10.1136/jnnp.61.4.412
68. Wetter S, Murphy C. Individuals with Down's syndrome demonstrate abnormal olfactory event-related potentials. Clin Neurophysiol. (1999) 110:1563–9. doi: 10.1016/S1388-2457(99)00086-3
69. Sliger M, Lander T, Murphy C. Effects of the ApoE epsilon4 allele on olfactory function in Down syndrome. J Alzheimers Dis. (2004) 6:397–402; discussion 43–9. doi: 10.3233/JAD-2004-6407
70. Brousseau K, Brainerd MG. A Study of the Physical and Mental Characteristics of Mongoloid Imbeciles. Baltimore: Williams and Wilkins (1928). doi: 10.1097/00000441-192808000-00020
71. Cecchini MP, Viviani D, Sandri M, Hähner A, Hummel T, Zancanaro C. Olfaction in people with down syndrome: a comprehensive assessment across four decades of age. PLoS ONE. (2016) 11:e0146486. doi: 10.1371/journal.pone.0146486
72. Nijjar RK, Murphy C. Olfactory impairment increases as a function of age in persons with Down syndrome. Neurobiol Aging. (2002) 23:65–73. doi: 10.1016/S0197-4580(01)00263-9
73. Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. (2007) 13:237–46. doi: 10.1002/mrdd.20163
74. Head E, Silverman W, Patterson D, Lott IT. Aging and down syndrome. Curr Gerontol Geriatr Res. (2012) 2012:412536. doi: 10.1155/2012/412536
75. Head E, Powell D, Gold BT, Schmitt FA. Alzheimer's disease in down syndrome. Eur J Neurodegener Dis. (2012) 1:353–64.
76. Karmiloff-Smith A. What Can Studying Babies With Down Syndrome Possibly Tell Us About Alzheimer's Disease in Adults? UCSD Dart Neuroscience Seminar. Retrieved from: https://tdlcucsdedu/research/DNS/videos/Karmiloff-Smithmp4 (accessed August 28, 2019).
77. Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. (1998) 77:431–8. doi: 10.1002/(SICI)1096-8628(19980605)77:5<431::AID-AJMG15>3.0.CO;2-J
78. Freeman SB, Bean LH, Allen EG, Tinker SW, Locke AE, Druschel C, et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. (2008) 10:173–80. doi: 10.1097/GIM.0b013e3181634867
79. Visootsak J, Mahle WT, Kirshbom PM, Huddleston L, Caron-Besch M, Ransom A, et al. Neurodevelopmental outcomes in children with Down syndrome and congenital heart defects. Am J Med Genet A. (2011) 155A:2688–91. doi: 10.1002/ajmg.a.34252
80. Visootsak J, Huddleston L, Buterbaugh A, Perkins A, Sherman S, Hunter J. Influence of CHDs on psycho-social and neurodevelopmental outcomes in children with Down syndrome. Cardiol Young. (2016) 26:250–6. doi: 10.1017/S1047951115000062
81. Startin CM, D'Souza H, Ball G, Hamburg S, Hithersay R, Hughes KMO, et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J Neurodev Disord. (2020) 12:4. doi: 10.1186/s11689-019-9306-9
82. Alsaied T, Marino BS, Esbensen AJ, Anixt JS, Epstein JN, Cnota JF. Does congenital heart disease affect neurodevelopmental outcomes in children with down syndrome? Congenit Heart Dis. (2016) 11:26–33. doi: 10.1111/chd.12322
83. D'Souza H, Lathan A, Karmiloff-Smith A, Mareschal D. Down syndrome and parental depression: A double hit on early expressive language development. Res Dev Disabil. (2020) 100:103613. doi: 10.1016/j.ridd.2020.103613
84. Cebula KR, Moore DG, Wishart JG. Social cognition in children with Down's syndrome: challenges to research and theory building. J Intellect Disabil Res. (2010) 54:113–34. doi: 10.1111/j.1365-2788.2009.01215.x
85. Moore DG, Oates JM, Hobson RP, Goodwin J. Cognitive and social factors in the development of infants with Down syndrome. Downs Syndr Res Pract. (2002) 8:43–52. doi: 10.3104/reviews.129
86. Karmiloff-Smith A, Grant J, Berthoud I, Davies M, Howlin P, Udwin O. Language and Williams syndrome: how intact is “intact”? Child Dev. (1997) 68:246–62. doi: 10.1111/j.1467-8624.1997.tb01938.x
87. Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. (1998) 2:389–98. doi: 10.1016/S1364-6613(98)01230-3
88. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. (2019) 15:135–47. doi: 10.1038/s41582-018-0132-6
89. Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher EMC, et al. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. (2019) 76:152–60. doi: 10.1001/jamaneurol.2018.3616
Keywords: Down syndrome, trisomy 21, developmental outcome, phenotypic heterogeneity, Alzheimer's disease, medical comorbidities, social environment, prenatal counseling
Citation: Windsperger K and Hoehl S (2021) Development of Down Syndrome Research Over the Last Decades–What Healthcare and Education Professionals Need to Know. Front. Psychiatry 12:749046. doi: 10.3389/fpsyt.2021.749046
Received: 28 July 2021; Accepted: 22 November 2021; Published: 14 December 2021.
Reviewed by:
Copyright © 2021 Windsperger and Hoehl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefanie Hoehl, stefanie.hoehl@univie.ac.at
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- Get Press Releases

- Media Contacts
- News Releases
- Photos & B-Roll Downloads
- VUMC Facts and Figures
- Credo Award
- DAISY Award
- Elevate Team Award
- Health, Yes
- Employee Spotlight
- Five Pillar Leader Award
Patient Spotlight
- Pets of VUMC
- Tales of VUMC Past
- All Voice Stories
Explore by Highlight
- Education & Training
- Growth & Finance
- Leadership Perspectives
- VUMC People
Explore by Topic
- Emergency & Trauma
- Genetics & Genomics
- Health Equity
- Health Policy
- Tech & Health
- Women's Health
Explore by Location
- Monroe Carell Jr. Children’s Hospital at Vanderbilt
- Vanderbilt Bedford County Hospital
- Vanderbilt Health One Hundred Oaks
- Vanderbilt Health Affiliated Network
- Vanderbilt-Ingram Cancer Center
- Vanderbilt Kennedy Center
- Vanderbilt Psychiatric Hospital
- Vanderbilt Wilson County Hospital
- Vanderbilt Stallworth Rehabilitation Hospital
- Vanderbilt Tullahoma-Harton Hospital
- Vanderbilt University Hospital

- Photos & B-Roll Downloads
Featured Story

A gunshot to the head. A long recovery. Then a wedding.
May 30, 2024, study to prevent alzheimer’s disease in people with down syndrome seeks participants.
Participants may be able to join this study if they have Down syndrome, are between 35 and 50 years old and have a study partner willing to attend all study visits.

The Vanderbilt Center for Cognitive Medicine will be launching the first study aimed at preventing Alzheimer’s disease in individuals with Down Syndrome, the ABATE Study. This clinical trial is testing whether a vaccine may reduce the levels of an abnormal protein called amyloid that accumulates in the brain of people who develop Alzheimer’s disease including people with Down Syndrome.
Virtually all people affected with Down Syndrome show Aβ-related neuropathological changes by age 40. In subjects with DS, Aβ deposits are visible in the cerebral cortex as early as their 30s. There is currently no cure for Aβ-related cognitive decline in DS. Current therapeutic options are considered to be mostly symptomatic and do not modify the course of the disease. There is, therefore, an unmet medical need for more effective treatments for individuals with DS experiencing Aβ-related cognitive decline.
For more information, contact the Vanderbilt Center for Cognitive Medicine at 615-936-4997.
Related Articles

September 30, 2021
Project seeks to treat alzheimer’s in people with down syndrome.
A joint project between the Vanderbilt Kennedy Center and the Vanderbilt Center for Cognitive Medicine will help deliver Alzheimer’s disease therapies and treatments to people with Down syndrome (DS).
By Emily Stembridge

January 10, 2013
Kennedy center science day set for jan. 15.
The latest science relevant to autism, Down syndrome and post-traumatic stress disorder will be discussed Tuesday, Jan. 15, during Science Day 2013, sponsored by the Vanderbilt Kennedy Center for Research on Human Development.
By Bill Snyder

January 9, 2024
Vumc receives $13 million grant to coordinate multisite clinical trial for rett syndrome treatment.
Vanderbilt University Medical Center received a $13 million Department of Defense grant to lead a multisite clinical trial that will evaluate repurposed FDA-approved drugs as treatment options for patients with Rett syndrome.
By Danny Bonvissuto
A mystery illness stole their kids’ personalities. These moms fought for answers.
Their children’s decline was precipitous and dramatic, with patients losing function in days or weeks, including the ability to talk, move or take care of themselves.

Before Sara Smythe began to disappear, she was thriving.
The youngest of four sisters, Sara was born with Down syndrome and lived the life of an active teen. At 13, the Toledo student was heading to middle school and loved soccer and swim practice, took dance and karate classes, and was a Girl Scout.
But in 2011, everything changed in a matter of weeks. Sara morphed from a sociable teen to a person who stopped talking and engaging with other people, and, at her worst, had full-blown catatonia.
Sara’s doctors were at a loss, but her mother, Eileen Quinn, wasn’t giving up. She embarked on what would become a 13-year quest, harnessing the power of a mother’s love to push the scientific community to pay attention to the mysterious regressions that some young people with Down syndrome were experiencing.
“I think people just might have a bias that, well, this person already has a disability, so it’s not as important,” Quinn said. “It was just devastating to think that I had lost Sara. I mean, this kid who made us laugh out loud every single day was totally gone. And there was just a shell left.”
As she searched for treatments for her daughter, Quinn , a developmental pediatrician at the University of Toledo, spoke at medical conferences and co-wrote research to spread the word. Through social media, Quinn galvanized other parents who also had seen their once-thriving children disappear. Quinn’s advocacy, combined with the efforts of another mother whose daughter had regressed, helped spur the first clinical trial of treatments for the regression disorder.
“She really gets a lot of credit shaking the consciousness of the community and saying, ‘There’s something new out there that we really need to target,’” said Brian Skotko , the director of the Down syndrome program at Massachusetts General Hospital and an associate professor at Harvard Medical School.
A mystery condition that could hold clues to aging
Regression symptoms in patients with Down syndrome were identified as early as 1946 , but the condition was often misdiagnosed as either early-onset Alzheimer’s disease or late-onset autism.
Its effects can be devastating. When someone with Down syndrome regresses, the decline can be precipitous and dramatic, with patients losing function in days or weeks, including the ability to talk, move or take care of themselves. Some, like Sara, can enter a catatonic state or suffer from hallucination and depersonalization, leaving loved ones desperately searching for answers and help.
For decades, there hadn’t been a formal diagnosis for patients experiencing regression symptoms. In fact, parents say doctors often would dismiss concerns, saying the regression was just a normal part of Down syndrome.
“I think people just might have a bias that, well, this person already has a disability, so it’s not as important.” — Eileen Quinn, Sara Smythe's mother
It was only in 2022 that the condition was finally given an official name: Down syndrome regression disorder, or DSRD. Though its prevalence is difficult to measure, DSRD is believed to affect between 1 to 5 percent of people with Down syndrome.
Many believe a better understanding of Down syndrome, which occurs in approximately 1 in 800 births and is caused by an extra chromosome 21, could lead to advances in our overall understanding of cancer, autoimmune conditions and longevity. Remarkably, people with Down syndrome rarely develop hypertension as they age and almost never develop most solid tumor cancers . But they are far more likely to develop Alzheimer’s disease earlier in life. About 30 percent of people with Down syndrome develop dementia in their 50s.
Scientists who study Down syndrome say people with the condition live rich and fulfilling lives, but they also experience “a very atypical form of aging,” said Joaquin Espinosa , executive director of the Linda Crnic Institute for Down Syndrome at the University of Colorado Anschutz Medical Campus. For researchers, “that could be super-rich to understand aging in general.”
Galvanizing mothers
In 2013, before DSRD had been named or widely acknowledged by the medical community, Quinn joined a panel discussion of the Down Syndrome Medical Interest Group , which focused on the mysterious regression clinicians had been seeing in some patients with Down syndrome. Quinn presented her own daughter as a case study, including videos she had taken to document Sara’s decline. She noticed that their story brought tears to the eyes of some of those in the audience.
After the panel appearance, other families began to contact Quinn, desperate to talk with someone who understood the pain and isolation of watching a loved one regress. One of the first to reach out was Linda Roan of Boulder, Colo. Her daughter, Miah Yager, was 20 when she underwent a similar regression in 2013.
Get Well+Being tips straight to your inbox

Gregarious and friendly, Miah had a natural talent for acting and had played a lead role as Lucy in the musical “You’re a Good Man, Charlie Brown.” Right after she graduated high school in 2012, Miah gave a speech at Purdue University as the youth keynote speaker for her church. The crowd gave her a standing ovation.
But in 2013, during her first semester at the College of Charleston , Miah began to disconnect from the outside world. Her friends noticed she was staying up for nights on end, obsessively copying notes and textbooks and missing appointments and social outings.
By Thanksgiving break, something was clearly amiss. The usually sociable Miah was not interested in being with her family and was suffering from the delusion that she was living in one of her favorite TV shows, “Pretty Little Liars .”
Miah didn’t return to school, and began what would become the first of many treatment attempts by her doctors. Over 18 months, psychotropic medications helped stabilize her, but it didn’t last. Miah regressed again and was no longer able to speak or answer questions. Roan had to feed her daughter by hand, rubbing her daughter’s cheeks to help her chew.
“I was like a bulldog trying to find some treatment and care for her,” Roan said.
Meanwhile, more and more families were reaching out to Quinn. In 2016, Quinn started a Facebook group called Regression in Down Syndrome . The private online community is now almost 2,000 strong, made up of family members — mostly mothers — seeking advice and trying to help their sons and daughters.
As more members joined the group, parents sought support, swapped tips and shared information about physicians and a variety of potential treatments, including psychotropic drugs and brain stimulation treatments. Some of the treatments helped for a while, but for many, including Miah, long-term success remained elusive.
‘It’s like they wake up’
In April 2020, at the start of the coronavirus pandemic, Jonathan Santoro , director of the neuroimmunology program at Children’s Hospital Los Angeles, for the first time saw a patient with Down syndrome who had inexplicably regressed.
He ordered a lumbar puncture, which collects cerebrospinal fluid, and the test revealed proteins that suggested the patient’s blood-brain barrier had broken down, indicating the potential for inflammation in his brain. Santoro remembers running down the hallway to tell the patient’s father that he found something.
For Santoro, a trained immunologist and neurologist, it was an important clue. People with Down syndrome were already known to have a much higher rate of autoimmune disorders such as thyroid disorders, skin conditions and celiac disease. Santoro recalled thinking: “How is this not something autoimmune? This is exactly like so many of the other diseases I treat.”
He and his team eventually treated that first patient with a standard immunotherapy called intravenous immunoglobulin, or IVIg, with a goal of resetting the immune system. After a combination of treatments, the patient dramatically improved. “Two weeks later, he’s running down the hall and hugging me and talking, and it’s like meeting a different person,” Santoro said.
“How is this not something autoimmune? This is exactly like so many of the other diseases I treat.” — Jonathan Santoro, associate professor of neurology and pediatrics at the University of Southern California’s Keck School of Medicine in Los Angeles
When Santoro and his colleagues later followed 82 patients for up to a year after ending treatment with IVIg, they found that more than half remained well .
“When these treatments do work, it’s like they wake up,” Santoro said.
Santoro, who spearheaded the 2022 paper defining DSRD, said he believes that the regression symptoms had historically been downplayed by the medical community because of a bias many doctors may have about people with Down syndrome and intellectual disability.
“If you brought your perfectly healthy teenage son or daughter in and they were not sleeping, not eating, catatonic and hallucinating, we would admit them to the hospital and do a full workup,” Santoro said. “And we weren’t doing that with individuals with Down syndrome.”
A fortuitous connection
In October 2020, Santoro found Quinn’s Facebook group and asked to post about his research into the root causes of the strange regression disorder. Since then, hundreds of families have reached out.
Santoro and his team say they now have evaluated over 500 patients from all over the world, and many had abnormal brain scans or immune systems in overdrive, leading to inflammation that appeared to have affected their brains, possibly triggering the regression.
One of the patients Santoro treated was Miah. Unfortunately, the IVIg treatment he prescribed only helped a little, possibly because at that time, Miah already had been in regression for about seven years.
But the connection proved fortuitous in another way. After working with Santoro, Roan remembered meeting another researcher who had taken an interest in her daughter’s case and also told her he suspected the immune system may have played a role in her regression.
It was Espinosa, now executive director of the Linda Crnic Institute for Down Syndrome. He was researching immune system changes in Down syndrome as well as a more targeted form of immunotherapy.
Roan discovered that Espinosa and Santoro didn’t know of each other’s complementary research. She thought, “Oh, my gosh, these guys need to talk,” and introduced them over email.
“We hit it off right away,” Espinosa said. “So finally I found a partner who understands what we’re seeing.”
An immune system attacking the brain
In earlier studies , Espinosa and his colleagues had found that the stark uptick in immune activity in people with Down syndrome may lie on the extra copy of chromosome 21 that causes the condition. The chromosome contains important immune system genes for receptors that detect interferon, which allows our immune system to fight off viral infections.
But because people with Down syndrome have an extra chromosome 21, they have extra copies of these immune genes — which appear to dysregulate and ramp up the immune system’s response . The result, Espinosa said, is a body that acts like it’s “constantly trying to fight a virus that is not there.”
Now, researchers believe that people with DSRD may have a hyperactive immune system that’s mistakenly attacking the brain.
When Santoro and Espinosa began talking as a result of Roan’s introduction, they discovered they had both been working on different forms of immunotherapy treatments for Down syndrome patients. Santoro’s research focused on IVIg. Espinosa had been studying tofacitinib, an immunosuppressant drug that targets the interferon pathway and has been approved by the Food and Drug Administration to treat other autoimmune disorders , including rheumatoid arthritis, psoriatic arthritis and ulcerative colitis. Though Espinosa was testing tofacitinib for autoimmune skin conditions that afflict patients with Down syndrome, the treatment had also shown promise in patients with DSRD, including Miah.
To compare the treatments side by side, the researchers designed a trial pitting lorazepam, a standard psychiatric drug, against each of the two immunotherapies. In 2023, Santoro and Espinosa, along with Elise Sannar , a child psychiatrist at Children’s Hospital Colorado, established the first randomized control clinical trial of potential treatments for DSRD.
The researchers are working together to collect as many biological specimens as possible to understand both what causes DSRD as well as what biological signatures could help predict the most effective treatment for each patient.
Espinosa credits Miah and Roan for this collaboration. “It was really the story of this participant and this mom that brought us all together,” he said.
Roan now works with Espinosa at the Crnic Institute as the clinical trial community liaison, a role in which she draws on her experience as a parent and caregiver to explain the research and treatments to other families.
“My hope is — and I believe this to be true — that no families are going to have to go through what my family, what Miah’s been through,” Roan said.
Spreading the word about DSRD
Santoro and Espinosa want to create more awareness of DSRD so doctors and patients can seek treatment sooner. They’ve conducted workshops with the Global Down Syndrome Foundation and the National Down Syndrome Society . To help, Quinn continues to offer resources through the Facebook group and has held webinars and podcasts with Santoro to help spread the word.
The rapid progress in research and awareness about DSRD reached the small town of Eagle River, Alaska. That’s where 27-year-old Darci Owens was living when she began to regress.
Owens, who has Down syndrome, was the first athlete in Alaska to become a certified coach through Special Olympics and traveled with the Seattle Seahawks for fundraising events. She hosted a cooking show on Facebook called “ Dining With Darci ,” teaching Special Olympics athletes how to make healthy meals and snacks, including fish tacos made from Alaska halibut, her favorite recipe on the show.
But on April 11, 2022, Darci’s life changed. That week, she became mute and would not eat. She could no longer run without falling, and quickly developed psychosis, which meant the once-independent athlete had to be fed, bathed and dressed.
Her mother, Dana Owens, a manager of therapy services at a hospital, found Quinn’s Facebook group as well as Santoro’s research on DSRD. Dana got in touch with Santoro and flew to Children’s Hospital Los Angeles with Darci for testing. Santoro recommended IVIg and provided Darci’s doctors in Alaska with detailed protocols for treatment.
With a combination of IVIg, electroconvulsive therapy and lorazepam, Darci, now 28, emerged from the depths of regression and catatonia. Most, but not all, of her symptoms have now abated, and she is continuing to undergo treatments. “But overall, oh, such vast improvement from where we were,” Dana Owens said.
The road ahead for Miah
Miah, now 31, started on tofacitinib in 2021 as part of Espinosa’s clinical trial on autoimmune skin conditions. Though the tofacitinib only partially helped her skin condition, Miah’s regression symptoms improved.
In November 2023, Miah was focused on rehearsing for a musical, “You’re a Good Man, Charlie Brown” — the same one she had starred in 12 years prior. “I’ve been practicing my play a ton, so that’s fun,” Miah said over a plate of chicken tacos at the taqueria next door to the dress rehearsal, where she danced and sang in her role as Sally, the titular character’s younger sister.
But DSRD has taken its toll.
“Her memory is pretty much wiped out, like, she has no memory — not even just of DSRD, but even the good times back to high school,” Roan said. Miah does not remember that she had performed in this musical before as its star.
Miah still experiences flare-ups and periods of relapse in which she feels “fuzzy” and “heat” in her head, as she describes it. Last fall, Miah said she still felt a “medium heat” but she continues to improve. She is training to be a waitress, a job in which she enjoys getting “to meet new people every day,” and lives five nights a week with two roommates who give her extra support.
“She’s never gotten fully 100 percent back to the person that she was prior, but she’s pretty darn close, and she’s doing well,” Roan said.
A formal diagnosis for Sara
In June 2023, Quinn and Sara flew to see Santoro, who officially diagnosed Sara with DSRD. Sara received her first dose of IVIg in mid-October that year. Like Miah, Sara hasn’t returned to who she was before regression, but she is improving.
Recently, Sara was part of a performance for an ice-skating program called Gliding Stars. Sara smiled during the performance, which she hadn’t done in the 10 years she had previously participated, Quinn said. Sara, now 26, recently graduated with a certificate from the University of Toledo transition program for people with disabilities. She has also become more social and recently attended the wedding of a friend from grade school.
While the improvements aren’t dramatic, they are significant. The immunotherapy has given Sara back “joy and the capacity to show it,” Quinn said.
About this story
Photo editing by Maya Valentine. Design editing by Chelsea Conrad. Copy editing by Matt Schnabel.
Read more from Well+Being
Well+Being shares news and advice for living well every day. Sign up for our newsletter to get tips directly in your inbox.
Diabetes, air pollution and alcohol consumption could be the biggest risk factors for dementia .
Dairy vs. plant milk : Which is better?
Why is my gas so smelly ? Gender, diet and plane rides can play a role.
Quitting can be a superpower that helps your mental health. Here’s how to quit smarter .
Our relationships with pets can be strong and uncomplicated.

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Down syndrome.
Faisal Akhtar ; Syed Rizwan A. Bokhari .
Affiliations
Last Update: August 8, 2023 .
- Continuing Education Activity
Down syndrome (trisomy 21) is a genetic disorder caused by the presence of all or a portion of a third chromosome 21. Patients typically present with mild to moderate intellectual disability, growth retardation, and characteristic facial features. This activity reviews the evaluation and management of Down syndrome and explains the role of the interprofessional team in improving care for patients with this condition.
- Describe the etiology of Down syndrome.
- Identify atrial septal defects as the most common cardiac abnormalities in patients with Down syndrome.
- Summarize the use of ultrasound, amniocentesis, and chorionic villus sampling in the prenatal diagnosis of Down syndrome.
- Outline the importance of collaboration and communication among the interprofessional team to enhance the delivery of care and improve outcomes for patients affected by Down syndrome.
- Introduction
Down syndrome was first described by an English physician, John Langdon Down, in 1866, but its association with chromosome 21 was established almost 100 years later by Dr. Jerome Lejeune in Paris. It is the presence of all or part of the third copy of chromosome 21 that causes Down syndrome, the most common chromosomal abnormality occurring in humans. [1] It is also found that the most frequently occurring live-born aneuploidy is trisomy 21, which causes this syndrome. [2]
The majority of patients with Down syndrome have an extra copy of chromosome 21. There are different hypotheses related to the genetic basis of Down syndrome and the association of different genotypes with the phenotypes. Among them is gene dosage imbalance, in which there is an increased dosage or number of genes of Hsa21, which results in increased gene expansion. [3] . It further includes the possibility of association of different genes with different phenotypes of Down syndrome. The other popular hypothesis is the amplified development instability hypothesis, according to which the genetic imbalance created by a number of trisomic genes results in a greater impact on the expression and regulation of many genes. [3]
The critical region hypothesis is also well-known in this list. Down syndrome critical regions (DSCR) are a few chromosomal regions that are associated with partial trisomy for Has21. DSCR on 21q21.22 is responsible for many clinical features of Down syndrome. [3] [4] After a thorough study of different analyses, it became clear that a single critical region gene cannot cause all the phenotypical features associated with trisomy 21, rather it is more evident that multiple critical regions or critical genes have a role to play in this phenomenon. [5]
- Epidemiology
The incidence of Down syndrome increases with maternal age, and its occurrence varies in different populations (1 in 319 to 1 in 1000 live births) [6] [7] . It is also known that the frequency of Down syndrome fetuses is quite high at the time of conception, but about 50% to 75% of these fetuses are lost before term. The occurrence of other autosomal trisomy is much more common than the 21, but the postnatal survival is very poor as compared to Down syndrome. This high percentage of survival of patients with trisomy 21 is thought to be a function of a small number of genes on chromosome 21 called Hsa21, which is the smallest and least dense of the autosomes. [8]
- Pathophysiology
An extra copy of chromosome 21 is associated with Down syndrome, which occurs due to the failure of chromosome 21 to separate during gametogenesis, resulting in an extra chromosome in all the body cells. Robertsonian translocation and isochromosome or ring chromosome are the other 2 possible causes of trisomy 21. Isochromosome is a condition when 2 long arms separate together instead of the long and short arms while in Robertsonian translocation. This occurs in 2% to 4% of the patients. The long arm of chromosome 21 is attached to another chromosome, mostly chromosome 14. In mosaicism, there are 2 different cell lines because of the error of division after fertilization. [6]
- History and Physical
Clinical Features
Different clinical conditions are associated with Down syndrome as different systems are affected by it. These patients have a wide array of signs and symptoms like intellectual and developmental disabilities or neurological features, congenital heart defects, gastrointestinal (GI) abnormalities, characteristic facial features, and abnormalities. [9]
Congenital Cardiac Defects (CHD)
Congenital cardiac defects are by far the most common and leading cause associated with morbidity and mortality in patients with Down syndrome, especially in the first 2 years of life. Though different suggestions have been made about the geographical as well as seasonal variation in the occurrence of different types of congenital cardiac defects in trisomy 21, so far none of the results have been conclusive. [10]
The incidence of CHD in babies born with Down syndrome is up to 50%. The most common cardiac defect associated with Down syndrome is an atrioventricular septal defect (AVSD), and this defect makes up to 40% of the congenital cardiac defects in Down syndrome. [6] It is said to be associated with the mutation of the non-Hsa21 CRELD1 gene [6] [11] The second most common cardiac defect in Down syndrome is a ventricular septal defect (VSD), which is seen in about 32% of the patients with Down syndrome. Together with AVSD, these account for more than 50% of congenital cardiac defects in patients with Down syndrome. [6] [11]
The other cardiac defects associated with trisomy 21 are secundum atrial defect (10%), tetralogy of Fallot (6%), and isolated PDA (4%), while about 30% of the patients have more than one cardiac defect. There is geographical variation in the prevalence of the cardiac defect in Down syndrome, with VSD being the most common in Asia and secundum type ASD in Latin America. The reason behind this difference in the prevalence of different types of CHD in different regions is still unclear, and many factors such as regional proximity have been found to contribute. [6]
Because of such a high prevalence of CHD in patients with Down syndrome, it has been recommended that all patients get an echocardiogram within the first few weeks of life.
Gastrointestinal (GI) Tract Abnormalities
Patients with trisomy 21 have many structural and functional disorders related to the GI tract. Structural defects can occur anywhere from the mouth to anus, and it has been found that certain defects like duodenal and small bowel atresia or stenosis, annular pancreas, imperforate anus, and Hirschsprung disease occur more commonly in these patients as compared to the general population. [1]
About 2% of patients with Down syndrome have Hirschsprung disease while 12% of patients with Hirschsprung disease have Down syndrome. [1] [6] Hirschsprung disease is a form of functional lower intestinal obstruction in which the neural cells fail to migrate to the distal segment of the rectum resulting in an aganglionic segment which does not have normal peristalsis resulting in failure of normal defecation reflex causing a functional obstruction. [12] The infant usually presents with signs and symptoms related to intestinal obstruction. Duodenal atresia and imperforate anus usually present in the neonatal period.
Apart from the structural defects patients with Down syndrome, patients are also prone to many other GI disorders like gastroesophageal reflux (GERD), chronic constipation, intermittent diarrhea, and celiac disease. Since there is a strong association of celiac disease with Down syndrome being present in about 5% of these patients, it is recommended to do yearly screening of celiac disease. Once diagnosed, these patients will have to remain on a gluten-free diet for the rest of life. [13]
Hematologic Disorders
There are several hematological disorders associated with Down syndrome. The hematological abnormalities in a newborn with Down syndrome (HANDS) constitute neutrophilia, thrombocytopenia, and polycythemia, which are seen in 80%, 66% and 34% of Down syndrome babies respectively. [14] [15] [16] HANDS is usually mild and resolves within the first thr3e weeks of life. [14] [15] [16]
The other disorder that is quite specific to Down syndrome is a transient myeloproliferative disorder, which is defined as detection of blast cells in younger than 3 month old babies with Down syndrome. It is characterized by the clonal proliferation of megakaryocytes and is detected during the first week of life and is resolved by 3 months of life. It is also known as transient abnormal myelopoiesis or transient leukemia and is known to be present in about 10% of patients with Down syndrome. If this occurs in the fetus, it can cause spontaneous abortion. [17] [18]
Patients with Down syndrome are 10-times more at risk of developing leukemia, [19] which constitute about 2% of all pediatric acute lymphoblastic leukemia and 10% of all pediatric acute myeloid leukemia. Thirty percent of Down syndrome patients with acute lymphoblastic leukemia have an association with function mutation in Janus Kinase 2 gene. [20]
About 10% of patients with chronic myeloid leukemia (TML) develop leukemogenesis of acute megakaryoblastic leukemia (AMKL) before the age of 4 years. AMKL is associated with GATA1 gene which is an X-linked transcriptor factor leading to an uncontrolled proliferation of immature megakaryocytes. [21]
Neurologic Disorders
Trisomy of Hsa21 has associated with reduced brain volume especially hippocampus and cerebellum. [22] Hypotonia is the hallmark of babies with Down syndrome and is present in almost all of them. It is defined as decreased resistance to passive muscle stretch and is responsible for delayed motor development in these patients. [23] . Because of hypotonia Down syndrome patients have joint laxity that causes decreased gait stability and increased energy requirement for physical exertion. [24] . These patients are prone to decreased bone mass and increased risk of fractures due to the low level of physical activity [25] , while the ligamentous laxity predisposes these patients to atlantoaxial subluxation. [26]
Five percent to 13% of children with Down syndrome have seizures [27] , out of that, 40% will have seizures before their first birthday, and in these cases, the seizures are usually infantile spasms. [28] Down syndrome children with infantile spasm do respond better to antiepileptics as compared to other kids with the same, and therefore, early intervention and treatment improve the developmental outcome. [27]
Lennox-Gestaut syndrome is also seen to be more prevalent in children with Down syndrome when it does occur, has a late onset, and is associated with reflex seizures along with an increased rate of EEG abnormalities. [29]
Forty percent of patients with Down syndrome develop tonic-clonic or myoclonic seizures in their first 3 decades. [28] Dementia occurs more commonly in patients older than 45 years of age with Down syndrome [30] , and about 84% are more prone to develop seizures. [31] The seizures in these patients are related to the rapid decline in their cognitive functions. [32]
The risk of developing early-onset Alzheimer disease is significantly high in patients with Down syndrome with 50% to 70% of patients developing dementia by the age of 60 years. [33] Amyloid precursor protein (APP), which is known to be associated with increased risk for the Alzheimer disease is found to be encoded on Hsa21, and trisomy of this protein is likely to be responsible for increased frequency of dementia in people with Down syndrome. Recent studies have shown that triplication of APP is associated with increased risk of early-onset Alzheimer disease even in the normal population. [34]
Nearly all the patients with Down syndrome have mild to moderate learning disability. Trisomy of multiple genes including DYRK1A, synaptojanin 1, and single-minded homolog 2 (SIM2) have been found to cause learning and memory defects in mice, which suggests the possibility that the overexpression of these genes may likely be causing the learning disability in people with Down syndrome. [35]
Endocrinological Disorders
Thyroid gland dysfunction is most commonly associated with Down syndrome. Hypothyroidism can be congenital or acquired at any time during life. [25] The newborn screening program in New York has reported an increased incidence of congenital hypothyroidism in babies with Down syndrome as compared to the others. [36] The anti-thyroid autoantibodies were found in 13% to 34% of patients with Down syndrome who had acquired hypothyroidism, and the concentration of these antibodies increased after 8 years of life. [25] . About half of the patients with Down syndrome have been shown to have subclinical hypothyroidism with elevated TSH and normal thyroxine levels. [37] Hyperthyroidism is much less frequent in patients with Down syndrome as compared to hypothyroidism, although the rate of it still exceeds the incidence of hyperthyroidism in the general pediatric population. [38]
Abnormalities in sexual development are also noted to be significant with delayed puberty in both genders. In girls, primary hypogonadism presents as delay in menarche or adrenarche, while in boys it can manifest as cryptorchidism, ambiguous genitalia, micropenis, small testes, low sperm count, and scanty growth of axillary and pubic hair. [25]
The insulin-like growth factor is also said to be responsible for the delay in skeletal maturation and short stature in patients with Down syndrome. [25]
Musculoskeletal Disorders
Children with Down syndrome are at an increased risk of reduced muscle mass because of hypotonia increased ligamentous laxity which causes retardation of gross motor skills and can result in joint dislocation. [39] These patients also have vitamin D deficiency due to several factors like inadequate exposure to sunlight, inadequate intake of vitamin D, malabsorption secondary to celiac disease, increased breakdown because of anticonvulsant therapy, among other factors. These factors increase the risk of decreased bone mass in children with Down syndrome and predispose them to recurrent fractures. [40]
Refractive Errors and Visual Abnormalities
Ocular and orbital anomalies are common in children with Down syndrome. These include blepharitis (2-7%), keratoconus (5-8%), cataract (25% to 85%), retinal anomalies (0% to 38%), strabismus (23% to 44%), amblyopia (10% to 26%), nystagmus (5% to 30%), refractive errors (18% to 58%), glaucoma (less than 1%), iris anomalies (38% to 90%) and optic nerve anomalies (very few cases).
The ocular anomalies, if left untreated, can significantly affect the lives of these patients. Therefore, all the patients with Down syndrome should have an eye exam is done during the first 6 months of life and then annually. [41]
Otorhinolaryngological ( ENT) Disorders
Ear, nose, and throat problems are also quite common in patients with Down syndrome. The anatomical structure of the ear in Down syndrome patients predisposes them to hearing deficits. Hearing loss is usually conductive because of impaction of cerumen and middle ear pathologies, including chronic middle ear effusion due to the small Eustachian tube, acute otitis media, and eardrum perforation. These patients usually require pressure equalization tubes for the treatment.
The sensorineural hearing loss has also been associated with Down syndrome because of the structural abnormalities in the inner ears such as narrow internal auditory canals. [42]
There are different methods used for the prenatal diagnosis of Down syndrome. Ultrasound, between 14 and 24 weeks of gestation, can be used as a tool for diagnosis based on soft markers like increased nuchal fold thickness, small or no nasal bone, and large ventricles. [43] Nuchal translucency (NT) is detected by ultrasound and is caused by a collection of fluid under the skin behind the fetal neck. It is done between 11 and 14 weeks of gestation. Other causes of this finding include Other causes are trisomy 13 (Patau syndrome), trisomy 18 (Edwards syndrome), and Turner syndrome. Amniocentesis and chorionic villus sampling have widely been used for the diagnosis, but there is a small risk of miscarriages between 0.5% to 1%. [44]
Several other methods have also been developed and are used for the rapid detection of trisomy 21 both during fetal life and after birth. The FISH of interphase nuclei is most commonly used by either using Hsa21-specific probes or the whole of the Hsa21. [45] Another method that is currently being used is QF-PCR, in which the presence of 3 different alleles is determined by using DNA polymorphic markers. [46] The success of this method depends upon the informative markers and the presence of DNA. It has been found that up to 86.67% of cases of Down syndrome can be identified by using the STR marker method. [47]
A relatively new method called paralogue sequence quantification (PSQ) uses the paralogue sequence on the Hsa21 copy number. It is a PCR-based method that uses the paralogue genes to detect the targeted chromosome number abnormalities, which is known as paralogue sequence quantification. [48]
There are non-invasive prenatal diagnostic methods that are being studied to be used for the diagnosis of Down syndrome prenatally. These are based on the presence of fetal cells in the maternal blood and the presence of cell-free fetal DNA in the maternal serum. [49]
Cell-free fetal DNA makes up 5% to 10% of the maternal plasma, and it increases during pregnancy and clears after delivery. Though this method has been used to determine fetal Rh status in Rhive women [50] , sex in sex-linked disorders [51] , and for the detection of paternally inherited autosomal recessive and dominant traits, [52] its use for the detection of chromosomal aneuploidy, especially the trisomy is still a challenge.
Few other recent methods like digital PCR and next-generation sequencing (NGS) are also being developed for the diagnosis of Down syndrome. [53]
- Treatment / Management
The management of patients with Down syndrome is multidisciplinary. Newborns with suspicion of Down syndrome should have a karyotyping done to confirm the diagnosis. The family needs to be referred to the clinical geneticist for the genetic testing and counseling of both parents.
Parental education is one of the foremost aspects regarding the management of Down syndrome, as parents need to be aware of the different possible conditions associated with it so that they can be diagnosed and treated appropriately. Treatment is basically symptomatic, and complete recovery is not possible.
These patients should have their hearing and vision assessed, and as they are more prone to have cataracts, timely surgery is required. Thyroid function tests should be done on a yearly basis and, if deranged, should be managed accordingly.
A balanced diet, regular exercise, and physical therapy are needed for optimum growth and weight gain, although feeding problems improve after cardiac surgery.
Cardiac referral should arranged for all the patients regardless of the clinical signs of congenital heart disease. If present, this should be corrected within the first 6 months of life to ensure optimum growth and development of the child.
Other specialties involved include a developmental pediatrician, pediatric pulmonologist, gastroenterologist, neurologist, neurosurgeon, orthopedic specialist, child psychiatrist, physical and occupational therapist, speech and language therapist, and audiologist.
- Differential Diagnosis
- Congenital hypothyroidism
- Mosaic trisomy 21 syndrome
- Partial trisomy 21(or 21q duplication)
- Robertsonian trisomy 21
- Zellweger syndrome or other peroxisomal disorders
With the recent advances in the medical practice, development of surgical techniques for the correction of congenital disabilities, and improvement in general care, there has been a tremendous increase in the survival of infants and life expectancy of patients with Down syndrome. A Birmingham (United Kingdom) study done almost 60 years ago showed that 45% of infants survived the first year of life, and only 40% would be alive at 5 years. [54] A later study conducted about 50 years after that showed that 78% of patients with Down syndrome plus a congenital heart defect survived for 1 year, while the number went up to 96% in patients without the anomalies. [55] This rise in the life expectancy of these patients should continue to rise significantly because of the developments in medical science. Healthcare facilities aim to provide proper and timely management to these patients and to help them to have a fulfilled and productive life. [56]
- Enhancing Healthcare Team Outcomes
The management of patients with Down syndrome is an interprofessional endeavor. Newborns with suspicion of Down syndrome should have a karyotyping done to confirm the diagnosis. The family needs to be referred to the clinical geneticist for the genetic testing and counseling of both parents.
Because almost every organ system is involved, the child needs to be seen by the ophthalmologist, orthopedic surgeon, cardiologist, dermatologist, gastroenterologist, physical therapist, mental health nurse, ENT surgeon, and behavior specialist.
While life span has increased over the past 3 decades, these individuals still have a shorter life expectancy compared to healthy individuals.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
A cropped photo of the eyes of a baby with Down Syndrome. Brushfield spots are visible between the inner and outer circle of the iris. Contributed by Wikimedia Commons, Szymon Tomczak (Public Domain)
"This photograph depicts a newborn with the genetic disorder Down Syndrome, due to the presence of an extra 21st chromosome." Contributed by The Centers for Disease Control and Prevention -- ID# 2634/Dr. Godfrey P. Oakley (Public Domain)
Karyotype for trisomy Down syndrome: Notice the three copies of chromosome 21 Contributed by The National Human Genome Research Institute, Human Genome Project
A drawing of the facial features of Down syndrome Contributed by the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities (Public Domain)
Disclosure: Faisal Akhtar declares no relevant financial relationships with ineligible companies.
Disclosure: Syed Rizwan Bokhari declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Akhtar F, Bokhari SRA. Down Syndrome. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. [Genet Med. 2009] Review The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethoré MO, Delabar JM, Mobley WC. Genet Med. 2009 Sep; 11(9):611-6.
- Review Down syndrome-associated periodontitis: a critical review of the literature. [Compend Contin Educ Dent. 2012] Review Down syndrome-associated periodontitis: a critical review of the literature. Frydman A, Nowzari H. Compend Contin Educ Dent. 2012 May; 33(5):356-61.
- Review Cognitive and medical features of chromosomal aneuploidy. [Handb Clin Neurol. 2013] Review Cognitive and medical features of chromosomal aneuploidy. Hutaff-Lee C, Cordeiro L, Tartaglia N. Handb Clin Neurol. 2013; 111:273-9.
- Down syndrome and the molecular pathogenesis resulting from trisomy of human chromosome 21. [J Biomed Res. 2010] Down syndrome and the molecular pathogenesis resulting from trisomy of human chromosome 21. Ruparelia A, Wiseman F, Sheppard O, Tybulewicz VL, Fisher EM. J Biomed Res. 2010 Mar; 24(2):87-99.
- Review Chances and Challenges of New Genetic Screening Technologies (NIPT) in Prenatal Medicine from a Clinical Perspective: A Narrative Review. [Genes (Basel). 2021] Review Chances and Challenges of New Genetic Screening Technologies (NIPT) in Prenatal Medicine from a Clinical Perspective: A Narrative Review. Bedei I, Wolter A, Weber A, Signore F, Axt-Fliedner R. Genes (Basel). 2021 Mar 29; 12(4). Epub 2021 Mar 29.
Recent Activity
- Down Syndrome - StatPearls Down Syndrome - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Down syndrome (DS) is a birth defect with huge medical and social costs, caused by trisomy of whole or part of chromosome 21. It is the most prevalent genetic disease worldwide and the common genetic cause of intellectual disabilities appearing in about 1 in 400-1500 newborns. Although the syndrome had been described thousands of years before ...
Recent advances in medical care have increased life expectancy and improved the quality of life for people with Down syndrome (DS). These advances are the result of both pre-clinical and clinical research but much about DS is still poorly understood. In 2020, the NIH announced their plan to update their DS research plan and requested input from ...
A Paradigm Shift in DS Research: From a Group- to Individual-Level Approach. DS research dates back to 1866, when the English physician John Langdon Down systematically described the syndrome for the first time (9, 10).In addition to intellectual disability (ID), he chronicled a distinct physical phenotype of individuals with DS, conjecturing that they were "born to the same family" (page ...
Down's syndrome is the most common autosomal abnormality worldwide, affecting around 1 in 1000 live births (World Health Organization, 2018).In 2011, it was estimated that there were 37 000 people with the condition in England and Wales, with a population prevalence of 0.66 per 1000 (Wu and Morris, 2013).Down's syndrome accounts for one-third of cases of severe learning disability.
N Engl J Med 2020;382: 2344 - 2352. DOI: 10.1056/NEJMra1706537. VOL. 382 NO. 24. Down syndrome, also called Down's syndrome (DS), is the most common chromosomal condition associated with ...
Down syndrome (DS) is caused by trisomy of chromosome 21 (Hsa21) and is associated with a number of deleterious phenotypes, including learning disability, heart defects, early-onset Alzheimer's disease and childhood leukaemia. Individuals with DS are affected by these phenotypes to a variable extent; understanding the cause of this variation is ...
Individuals with Down syndrome have seen improvements in quality of life and life expectancy in recent decades due to health-care and research advancements, along with greater societal participation. However, there are persistent disparities in health outcomes stemming from insufficient awareness and training about Down syndrome among physicians, professionals, families, and other stakeholders ...
Down syndrome (DS) is a genetic disorder caused by trisomy 21, the presence of a supernumerary chromosome 21, which results in physical and neurocognitive alterations. This Primer reviews the ...
Down syndrome, the most common chromosomal condition (approximately 1 in 700 births), is associated with an increased risk of common autoimmune diseases, including rheumatic diseases 1. For ...
Down syndrome (DS) is one of the commonest disorders with huge medical and social cost. DS is associated with number of phenotypes including congenital heart defects, leukemia, Alzeihmer's disease, Hirschsprung disease etc. DS individuals are affected by these phenotypes to a variable extent thus understanding the cause of this variation is a key challenge. In the present review article, we ...
1 Sep 2022. 4:10 PM ET. By Emily Underwood. A hormone that boosts memory in mice may hold promise for adults with Down syndrome. Halfpoint/iStock. Share: New research with mice—and a small human trial—raises the prospect of treatments that could improve learning difficulties in people with Down syndrome. Though still preliminary, the work ...
Down syndrome, also known as trisomy 21, is a genetic disorder caused by an extra copy of around 200 genes from chromosome 21. Individuals with Down syndrome show developmental defects that affect ...
We are pleased to present this Special Research Topic on advancements in modeling both developmental and age-related changes in Down Syndrome, including Alzheimer's disease (AD) in Down syndrome (DS-AD). AD is characterized by a progressive deterioration of memory and other neural functions, resulting in impairments to decision-making, behavior ...
Provocative new findings suggest a surprising cause of Down syndrome: cells linked to aging. Neural progenitor cells derived from stem cells of a person with Down syndrome. Courtesy Hiruy Meharena ...
Down Syndrome Research and Practice. Down Syndrome Research and Practice is a peer-reviewed journal focused on Down syndrome research. It was published by Down Syndrome Education International in partnership with the University of Portsmouth from 1992 to 2009. Volume 1. Issue 1.
The SARS-CoV-2 virus that led to COVID-19 is associated with significant and long-lasting neurologic symptoms in many patients, with an increased mortality risk for people with Alzheimer's disease (AD) and/or Down syndrome (DS). However, few studies have evaluated the neuropathological and inflammatory sequelae in postmortem brain tissue obtained from AD and people with DS with severe SARS ...
Adults with Down syndrome have a genetic form of Alzheimer's disease (AD) and evidence of cerebrovascular disease across the AD continuum, despite few systemic vascular risk factors. The onset ...
Down Syndrome. Down syndrome is a chromosomal abnormality characterized by the presence of an extra copy of genetic material on the 21st chromosome, either in whole (Trisomy 21) or in part (such ...
Down syndrome (DS) is the most common genomic disorder of intellectual disability and is caused by trisomy of Homo sapiens chromosome 21 (HSA21). The eponym of the syndrome is from Down, who described the clinical aspects of the syndrome in 1866 (REF. 1).The DS phenotype involves manifestations that affect multiple bodily systems, in particular the musculoskeletal, neurological and ...
The majority of patients with Down syndrome (56%) had a psychiatric disorder, and attention deficit and hyperactivity disorder was the most common diagnosis. Conclusion. Clinicians should not overlook the need for psychiatric assessment, early diagnosis, and collaboration between pediatricians and child psychiatrists, which are crucial during ...
In Hendrix et al.'s (2021) paper, the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study reported early findings from a natural history study of adults with DS in the USA. The LIFE-DSR study consists of 11 sites, who are collectively recruiting 270 individuals with DS over the age of 25.
DS research dates back to 1866, when the English physician John Langdon Down systematically described the syndrome for the first time (9, 10). In addition to intellectual disability (ID), he chronicled a distinct physical phenotype of individuals with DS, conjecturing that they were "born to the same family" (page 9) ( 10 , 11 ).
This article is a part of a study entitled explaining the process of managing a Down syndrome child in the family, approved by the research council and ethics committee of Bushehr University of Medical Sciences, which was approved by the ethics code IR.BPUMS.REC.1401.135.
Participants may be able to join this study if they have Down syndrome, are between 35 and 50 years old and have a study partner willing to attend all study visits. ... Jan. 15, during Science Day 2013, sponsored by the Vanderbilt Kennedy Center for Research on Human Development. By Bill Snyder . January 9, 2024
Its effects can be devastating. When someone with Down syndrome regresses, the decline can be precipitous and dramatic, with patients losing function in days or weeks, including the ability to ...
Down syndrome was first described by an English physician, John Langdon Down, in 1866, but its association with chromosome 21 was established almost 100 years later by Dr. Jerome Lejeune in Paris. It is the presence of all or part of the third copy of chromosome 21 that causes Down syndrome, the most common chromosomal abnormality occurring in humans.[1] It is also found that the most ...