- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries

Clinical Research Associate (CRA)
This role-based course provides the practical know-how to monitor clinical research sites effectively.
About this Course
A foundational course for individuals considering a career as a clinical research associate (CRA). The course provides broad training on the ethical, regulatory, and practical aspects of monitoring a research site. The modules explain how recent developments in clinical trial organization and conduct have changed the role of CRAs. In addition, the course details the communication and relationships among CRAs, sponsors, contract research organizations (CROs), clinical research coordinators (CRCs), and principal investigators.
Course Preview:
Language Availability: English
Suggested Audiences: Clinical Research Associates (CRAs), Clinical Research Coordinators (CRCs), Contract Research Organizations (CROs), Faculty, Principal Investigators (PIs), Research Administrators, Sponsors, Students
Organizational Subscription Price: $675 per year/per site for government and non-profit organizations; $750 per year/per site for for-profit organizations Independent Learner Price: $99 per person
Course Content
" role="button"> scientific concepts and research design.
This module explores how scientific principles inform study design and protocol development. It defines what qualifies as a research question and study hypothesis, lists the components of rigorous clinical research, notes some common weaknesses of different protocol designs, details the central elements of a study protocol, explores how to interpret protocols, and delineates the CRA’s role in monitoring a study site’s implementation of a protocol.
Recommended Use: Required ID (Language): 21178 (English) Author(s): Jessica Rosenbluth, MS - Syneos Health
" role="button"> Ethical and Participant Safety Considerations
This module examines the relevant national and international principles of participant protection in clinical research. It explains the ethical issues involved when working with vulnerable populations and describes the additional safeguards that should be in place to protect such subjects. The module also details the role of the study monitor in ensuring protection of participant rights in an ongoing clinical study.
Recommended Use: Required ID (Language): 21179 (English) Author(s): Natasha Williams, BSc - Narra Consulting LLC
" role="button"> Investigational Products Development and Regulation
This module explores the conception, development, testing, and approval of biomedical products and details the key stages of a biomedical product’s lifecycle. It defines the regulatory pathways for drugs, biologics, and medical devices, while describing how sponsors work with the U.S. Food and Drug Administration to gain approval for product marketing. The module provides CRAs with a working knowledge of the biomedical product lifecycle necessary to facilitate interactions between clinical research sponsors and sites.
Recommended Use: Required ID (Language): 21180 (English) Author(s): Robert Michalik, JD, RAC - RegulatoryPro Consulting
" role="button"> Clinical Study Operations (Good Clinical Practice)
This module provides an overview of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6(R2) guideline, with a particular focus on sections that are most relevant to the work of a CRA. The module describes how a CRA’s monitoring activities help a site comply Good Clinical Practice (GCP). The module concludes with introduction to the proposed changes found in the E6(R3) update of the ICH guideline.
Recommended Use: Required ID (Language): 21181 (English) Author(s): Chinonso Singleton, MS - LIBRE, LLC
" role="button"> Understanding the Clinical Monitoring Plan
This module covers the roles and responsibilities of study monitors and details a monitoring plan's creation, purpose, and contents. It outlines risk-based and remote monitoring approaches and contrasts these with an earlier traditional model for monitoring. The module also focuses on the essential components of a monitor’s communication with investigators, study staff, and sponsors.
Recommended Use: Required ID (Language): 21182 (English) Author(s): Natasha Williams, BSc - Narra Consulting LLC
" role="button"> Implementing the Monitoring Plan
This module describes the preparation for and conduct of different site visits. It identifies the central actors involved and the common interactions among them. The module also details the key components of a visit follow up.
Recommended Use: Required ID (Language): 21183 (English) Author(s): Natasha Williams, BSc - Narra Consulting LLC
" role="button"> Emerging Clinical Trial Methods and Technologies
This module defines the purpose and use of emerging technologies for study management and data collection. It describes how these technologies enhance risk-based monitoring (RBM). In addition, the module discusses how decentralized clinical trial (DCT) modules are shaping the future of clinical trial conduct.
Recommended Use: Required ID (Language): 21184 (English) Author(s): Natasha Williams, BSc - Narra Consulting LLC
" role="button"> Data Management and Informatics
This module defines the core aspects of data management, describes quality clinical trial data, defines data-related milestones, and connects data processes to related systems and software. The module also outlines selected data-handling processes and provides CRAs with information on operational activities to maintain data quality at their assigned clinical trial sites.
Recommended Use: Required ID (Language): 21185 (English) Author(s): Chinonso Singleton, MS - LIBRE, LLC
" role="button"> Leadership, Communication, Professionalism, and Teamwork
This module describes some principles and practices of leadership, communication, professionalism, and teamwork related to clinical research. It describes how a CRA can leverage soft skills to effectively manage stakeholder relationships in a clinical trial.
Recommended Use: Required ID (Language): 21186 (English) Author(s): Clareece West, BSc - West-Fenn Enterprise, LLC
" role="button"> Who should take the Clinical Research Associate course?
Learners who wish to gain foundational knowledge of clinical research site monitoring should take this course to prepare for a career as a clinical research associate (CRA). The course is ideal for clinical research coordinators (CRCs) looking to transition to becoming CRAs and for organizations looking to onboard new CRAs.
Suggested audiences include new Clinical Research Associates, Clinical Research Coordinators, Contract Research Organizations, Faculty, Principal Investigators, Research Administrators, Sponsors, and Students.
" role="button"> Why should someone take the Clinical Research Associate course?
This role-based course provides the practical know-how to monitor clinical research sites effectively. It provides broad training on the ethical, regulatory, and practical aspects of monitoring a research site. The modules explain how recent developments in clinical trial organization and conduct have changed the role of CRAs. In addition, the course details the communication and relationships among CRAs, sponsors, clinical research coordinators (CRCs), and principal investigators.
" role="button"> How does the Clinical Research Associate course complement other CITI Program Courses?
Clinical Research Associate is a helpful complement to CITI Program’s Clinical Research Coordinator (CRC) Foundations and Clinical Research Coordinator (CRC) Advanced courses. It provides the next set of tools required for research monitors. CRCs looking to become CRAs will find that Clinical Research Associate is essential to making this professional transition.
" role="button"> What are the required and supplemental modules?
The modules for the Clinical Research Associate course are:
- Scientific Concepts and Research Design
- Ethical and Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (Good Clinical Practice)
- Understanding the Clinical Monitoring Plan
- Implementing the Monitoring Plan
- Emerging Clinical Trial Methods and Technologies
- Data Management and Informatics, and
- Leadership, Communication, Professionalism, and Teamwork
The course is designed to be completed sequentially through all nine modules (we recommend they are set as “required”).
" role="button"> Is this course eligible for continuing medical education credits?
The course does not currently have CE/CME credits available.
" role="button"> How long will the course take a learner to complete?
This course consists of nine modules. Each module contains detailed content, a quiz, images, supplemental materials, and case studies.
Modules vary in length, and learners may require different amounts of time to complete them based on their familiarity and knowledge of the topic. As a rule of thumb, modules can take about 30 to 45 minutes to complete, which means it could take around 4.5 to 5.5 hours to complete.
Related Content
CRC courses focus on key topics essential to the conduct of clinical research. They are specifically tailored to the needs of clinical research coordinators.
Discusses the importance of CRA soft skills in building productive relationships with sites and sponsors.
Discusses the process and content of a CRA’s interim monitoring visit.
Details ways to produce productive partnerships between clinical research associates and clinical research coordinators.
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| BUY_NOW | This cookie is set to transfer purchase details to our learning management system. | |
| CART_COUNT | This cookie is set to enable shopping cart details on the site and to pass the data to our learning management system. | |
| cookielawinfo-checkbox-advertisement | 1 year | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Advertisement". |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| JSESSIONID | session | Used by sites written in JSP. General purpose platform session cookies that are used to maintain users' state across page requests. |
| PHPSESSID | session | This cookie is native to PHP applications. The cookie is used to store and identify a users' unique session ID for the purpose of managing user session on the website. The cookie is a session cookies and is deleted when all the browser windows are closed. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| XSRF-TOKEN | session | The cookie is set by Wix website building platform on Wix website. The cookie is used for security purposes. |
| Cookie | Duration | Description |
|---|---|---|
| bcookie | 2 years | This cookie is set by linkedIn. The purpose of the cookie is to enable LinkedIn functionalities on the page. |
| lang | session | This cookie is used to store the language preferences of a user to serve up content in that stored language the next time user visit the website. |
| lidc | 1 day | This cookie is set by LinkedIn and used for routing. |
| pll_language | 1 year | This cookie is set by Polylang plugin for WordPress powered websites. The cookie stores the language code of the last browsed page. |
| Cookie | Duration | Description |
|---|---|---|
| _gat | 1 minute | This cookies is installed by Google Universal Analytics to throttle the request rate to limit the colllection of data on high traffic sites. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | This cookie is installed by Google Analytics. The cookie is used to calculate visitor, session, campaign data and keep track of site usage for the site's analytics report. The cookies store information anonymously and assign a randomly generated number to identify unique visitors. |
| _gat_UA-33803854-1 | 1 minute | This is a pattern type cookie set by Google Analytics, where the pattern element on the name contains the unique identity number of the account or website it relates to. It appears to be a variation of the _gat cookie which is used to limit the amount of data recorded by Google on high traffic volume websites. |
| _gat_UA-33803854-7 | 1 minute | This is a pattern type cookie set by Google Analytics, where the pattern element on the name contains the unique identity number of the account or website it relates to. It appears to be a variation of the _gat cookie which is used to limit the amount of data recorded by Google on high traffic volume websites. |
| _gcl_au | 3 months | This cookie is used by Google Analytics to understand user interaction with the website. |
| _gid | 1 day | This cookie is installed by Google Analytics. The cookie is used to store information of how visitors use a website and helps in creating an analytics report of how the website is doing. The data collected including the number visitors, the source where they have come from, and the pages visted in an anonymous form. |
| _hjAbsoluteSessionInProgress | 30 minutes | No description available. |
| _hjFirstSeen | 30 minutes | This is set by Hotjar to identify a new user’s first session. It stores a true/false value, indicating whether this was the first time Hotjar saw this user. It is used by Recording filters to identify new user sessions. |
| _hjid | 1 year | This cookie is set by Hotjar. This cookie is set when the customer first lands on a page with the Hotjar script. It is used to persist the random user ID, unique to that site on the browser. This ensures that behavior in subsequent visits to the same site will be attributed to the same user ID. |
| _hjIncludedInPageviewSample | 2 minutes | No description available. |
| _hjIncludedInSessionSample | 2 minutes | No description available. |
| _hjTLDTest | session | No description available. |
| _uetsid | 1 day | This cookies are used to collect analytical information about how visitors use the website. This information is used to compile report and improve site. |
| BrowserId | 1 year | This cookie is used for registering a unique ID that identifies the type of browser. It helps in identifying the visitor device on their revisit. |
| CFID | session | This cookie is set by Adobe ColdFusion applications. This cookie is used to identify the client. It is a sequential client identifier, used in conjunction with the cookie "CFTOKEN". |
| CFTOKEN | session | This cookie is set by Adobe ColdFusion applications. This cookie is used to identify the client. It provides a random-number client security token. |
| CONSENT | 16 years 5 months 4 days 4 hours | These cookies are set via embedded youtube-videos. They register anonymous statistical data on for example how many times the video is displayed and what settings are used for playback.No sensitive data is collected unless you log in to your google account, in that case your choices are linked with your account, for example if you click “like” on a video. |
| vuid | 2 years | This domain of this cookie is owned by Vimeo. This cookie is used by vimeo to collect tracking information. It sets a unique ID to embed videos to the website. |
| Cookie | Duration | Description |
|---|---|---|
| bscookie | 2 years | This cookie is a browser ID cookie set by Linked share Buttons and ad tags. |
| IDE | 1 year 24 days | Used by Google DoubleClick and stores information about how the user uses the website and any other advertisement before visiting the website. This is used to present users with ads that are relevant to them according to the user profile. |
| MUID | 1 year 24 days | Used by Microsoft as a unique identifier. The cookie is set by embedded Microsoft scripts. The purpose of this cookie is to synchronize the ID across many different Microsoft domains to enable user tracking. |
| test_cookie | 15 minutes | This cookie is set by doubleclick.net. The purpose of the cookie is to determine if the user's browser supports cookies. |
| VISITOR_INFO1_LIVE | 5 months 27 days | This cookie is set by Youtube. Used to track the information of the embedded YouTube videos on a website. |
| YSC | session | This cookies is set by Youtube and is used to track the views of embedded videos. |
| yt-remote-connected-devices | never | These cookies are set via embedded youtube-videos. |
| yt-remote-device-id | never | These cookies are set via embedded youtube-videos. |
| Cookie | Duration | Description |
|---|---|---|
| _app_session | 1 month | No description available. |
| _gfpc | session | No description available. |
| _uetvid | 1 year 24 days | No description available. |
| _zm_chtaid | 2 hours | No description available. |
| _zm_csp_script_nonce | session | No description available. |
| _zm_cta | 1 day | No description |
| _zm_ctaid | 2 hours | No description available. |
| _zm_currency | 1 day | No description available. |
| _zm_mtk_guid | 2 years | No description available. |
| _zm_page_auth | session | No description available. |
| _zm_sa_si_none | session | No description |
| _zm_ssid | session | No description available. |
| AnalyticsSyncHistory | 1 month | No description |
| BNI_persistence | 4 hours | No description available. |
| BrowserId_sec | 1 year | No description available. |
| CookieConsentPolicy | 1 year | No description |
| cred | No description available. | |
| f | never | No description available. |
| L-veVQq | 1 day | No description |
| li_gc | 2 years | No description |
| owner_token | 1 day | No description available. |
| PP-veVQq | 1 hour | No description |
| renderCtx | session | This cookie is used for tracking community context state. |
| RL-veVQq | 1 day | No description |
| twine_session | 1 month | No description available. |
| UserMatchHistory | 1 month | Linkedin - Used to track visitors on multiple websites, in order to present relevant advertisement based on the visitor's preferences. |
| web_zak | past | No description |
| wULrMv6t | No description | |
| zm_aid | past | No description |
| zm_haid | past | No description |
Average Senior Clinical Research Associate (CRA) Salary at Novartis
The average salary for a Senior Clinical Research Associate (CRA) is $120,316 in 2024
Featured Content
What do senior clinical research associate (cra)s do.
Senior clinical research associates (CRAs) handle the day-to-day operations of the clinical investigative phase of drug development. They are in charge of laboratory and research duties to reach all the organizational objectives. They need to have strong analytical aptitudes and pay strict attention to detail during all clinical research. They also handle experiment inquiries and take assessment notes on a frequent basis. Most senior clinical research associates are in charge of keeping …Read more
Common Health Benefits for a Senior Clinical Research Associate (CRA)
Gender breakdown for senior clinical research associate (cra)s.
FAQs About Senior Clinical Research Associate (CRA)s
What is the highest pay for senior clinical research associate (cra)s.
Our data indicates that the highest pay for a Senior Clinical Research Associate (CRA) is $NaN / year

What is the lowest pay for Senior Clinical Research Associate (CRA)s?
Our data indicates that the lowest pay for a Senior Clinical Research Associate (CRA) is $NaN / year
How can Senior Clinical Research Associate (CRA)s increase their salary?
Increasing your pay as a Senior Clinical Research Associate (CRA) is possible in different ways. Change of employer: Consider a career move to a new employer that is willing to pay higher for your skills. Level of Education: Gaining advanced degrees may allow this role to increase their income potential and qualify for promotions. Managing Experience: If you are a Senior Clinical Research Associate (CRA) that oversees more junior Senior Clinical Research Associate (CRA)s, this experience can increase the likelihood to earn more.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Sign up for alerts
- NEWS FEATURE
- 31 May 2024
- Correction 06 June 2024
Top ten biopharma deals of 2024

- Monica Hoyos Flight 0
Monica Hoyos Flight is a science communications consultant, writer and editor.
You can also search for this author in PubMed Google Scholar
Credit: Witthaya Prasongsin/Getty
You have full access to this article via your institution.
The biggest Q1 deal, with a total value of $4.1 billion, was announced in January when Argo Biopharmaceutical, a privately held biotechnology company based in Shanghai, granted Novartis exclusive rights to develop and commercialize two clinical-stage RNA-interference candidates targeting cardiovascular diseases.
Novartis is paying Argo $185 million upfront for rights to a phase 1/2a prospect outside of China and other nearby territories, and global rights to a phase 1 program, with an option to license candidates for up to two additional cardiovascular targets.
Since it was founded in 2021, Argo has been using its small-interfering RNA (siRNA) platform technology known as RADS (RNA molecules with superior Activity, Durability, and Safety) to build a pipeline of over 20 siRNA drug candidates for several indications, including cardiovascular diseases, rare diseases, central nervous system (CNS) diseases, and metabolic diseases. Three of its five most advanced candidates are in the cardiovascular disease space.
Drugs based on the RADS platform can silence target genes involved in disease pathogenesis over long periods of time so patients may only require one injection per year. This deal will bolster Novartis’ therapeutics pipeline for cardiovascular disease.
Boehringer Ingelheim GmbH also bets big on siRNA therapies. In a deal worth $2 billion, Suzhou Ribo Life Science (Ribo) and Ribocure Pharmaceuticals granted Boehringer exclusive global rights to develop and commercialize siRNA therapies using Ribo’s RIBO-GalSTAR platform for the treatment of non-alcoholic or metabolic dysfunction-associated steatohepatitis (NASH/MASH).
The RIBO-GalSTAR platform enables the development of siRNA therapeutics that can target disease-causing genes specifically in liver cells, potentially leading to new treatment options that prevent NASH progression and restore liver function.
Companies growing their oncology portfolio
Four of the top ten highest value deals last quarter (Table 1) are in the cancer field; three involve technology platforms to discover new cancer therapies and one will advance the development of Umoja Biopharma’s in-situ-generated chimeric antigen receptor (CAR) T cell therapies.
Roche paid MOMA Therapeutics, a US-based biotechnology company focused on targeting the molecular machines that underlie human disease, $66 million up front and potential milestone payments and royalties exceeding $2 billion for exclusive, worldwide rights to develop cancer therapies using MOMA’s proprietary KnowledgeBase platform.
MOMA’s platform is being used to target ATPases—a difficult-to-drug class of enzymes involved in intracellular energy transfer—by exploiting the dependence of these proteins on coordinated, stepwise changes in structural states to carry out their function. The five-year deal with Roche aims to identify and develop novel drug targets involved in promoting cancer cell growth and survival.
Gilead Sciences has signed a research partnership with clinical-stage oncology company Merus to discover two new antibody-based drugs against T cells that are capable of binding three targets at once, using Merus’ Triclonics platform. Merus will receive $56 million upfront cash and $25 million in equity, and is eligible for up to $1.5 billion in potential milestone payments and royalties.
By binding to multiple targets, bispecific and trispecific antibodies can have more than one mechanism of action and potentially elicit more robust and specific anti-tumor immune responses. To date, Merus has advanced seven bispecific antibodies to the clinic; its lead cancer drug candidate petosemtamab, which targets epidermal growth factor receptor (EGFR) and the leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5), has shown encouraging activity in patients with advanced head and neck cancer.
Merck KGaA is also investing in discovery platform technology to expand its oncology portfolio. By partnering with Caris Discovery, the therapeutic research arm of Caris Life Sciences (Caris), Merck KGaA seeks to accelerate the discovery and development of first-in-class antibody‒drug conjugates (ADCs) for cancer patients. In a deal worth $1.4 billion, Merck KGaA, will receive an exclusive global license to develop, manufacture, and commercialize ADC therapeutics against selected targets for the treatment of cancer discovered using Caris’ proprietary ADAPT (Adaptive Dynamic Artificial Polyligand Targeting) platform, artificial intelligence (AI) and machine-learning capabilities.
AbbVie’s deal with Umoja Biopharma also made the top ten list. Umoja is eligible for up to $1.44 billion for granting AbbVie an exclusive, worldwide option to license the development and commercialization of its UB-VV111 CAR T cell therapy using the VivoVec gene delivery platform for the treatment of hematologic cancers. The companies will additionally develop up to 4 additional CAR-T cell therapies for AbbVie-selected targets.
Umoja’s VivoVec platform uses lentiviral particles to deliver CAR payloads directly to human T cells, enabling them to manufacture their own cancer-targeting CARs in vivo. This approach has the potential to expand the patient populations that could benefit from CAR T cell approaches as it eliminates the complexity, time, and expense of modifying T cells ex vivo and delivering them to patients.
An AI-first approach to drug discovery
In Q1 of 2024, both Eli Lilly and Novartis signed agreements with the London-based drug discovery company Isomorphic Labs, to gain rights to its next-generation AlphaFold model and AI technology.
Following upfront payments of $45 million and $37.5 million from Eli Lilly and Novartis, respectively, Isomorphic will apply its virtual screens and small-molecule design approaches to develop small-molecule therapies against undisclosed targets. The company will be eligible for up to $1.7 billion in performance-based milestone payments from Eli Lilly and up to $1.2 billion from Novartis.
Since its release in 2020, Google DeepMind’s AlphaFold—a freely available AI system developed to predict the three-dimensional (3D) structure of proteins from their amino-acid sequences—has drastically accelerated understanding of protein interactions. Working in collaboration with Google DeepMind, Isomorphic is developing a more powerful AlphaFold model with improved accuracy that also covers small molecules and nucleic acids, and will aid the rational design of novel therapeutics.
The R&D partnership landscape
After spiking in 2020‒2021, the number of partnerships between biopharma therapeutics and platform companies has remained steady with between 100 and 150 deals being signed per quarter (Fig. 1). The total value of these deals has increased from just under $110 billion in 2018 and 2019 to over $160 billion in 2020‒2022, with a record $178.4 billion in 2023.
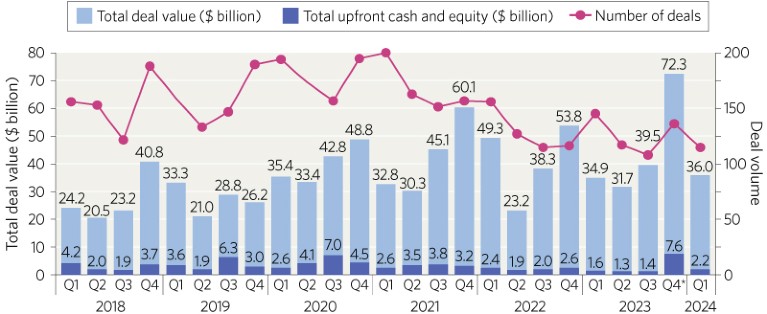
Fig. 1 | Research and development partnerships between biopharma therapeutics and platform companies. Source: DealForma database.
The total deal value in Q1 of 2024 ($36 billion) was slightly higher than in Q1 of 2023 ($34.9 billion). These values are dwarfed by the total for Q4 of 2023 ($72.3 billion), which included a mega-deal between Merck and Daiichi Sankyo to develop and commercialize three cancer drugs worth $22 billion.
Interestingly, the average upfront payments seem to be increasing after they dipped in 2021 and 2022—when they were $67.75 million and $54.25 million, respectively, compared to $88.5 million in 2020—as in 2023 the average upfront cash and equity was $83.5 million. This trend looks set to continue in 2024 as the Q1 average upfront cash and equity was $58 million compared to $41 million in the same quarter last year. These data highlight the eagerness of biopharma companies to expand their pipelines by making use of platform technologies to identify new drug candidates and improve the delivery of existing ones.
Table 1 | Top ten research and development partnerships by value for year-to-date as of 10 April 2024
Licensor and licensee | Announced | Total deal value (upfront payment) | Synopsis |
|---|---|---|---|
Argo Biopharmaceutical, Novartis | January 2024 | $4,165 million ($185 million) | Argo Biopharmaceutical granted Novartis exclusive, worldwide rights to develop a phase 1 RNA-based therapy with an option for up to two additional therapies for the treatment of cardiovascular diseases. Additionally, Novartis has exclusive, worldwide rights (excluding China) for a phase 1/2a therapy. Argo will receive $185 million up front and is eligible for up to $3.98 billion in milestones and royalties. |
MOMA Therapeutics, Roche | January 2024 | $2,066 million ($66 million) | MOMA Therapeutics granted Roche exclusive, rights to develop therapies using its KnowledgeBase platform for the treatment of cancer. MOMA will be responsible for development activities through confirmation while Roche will be responsible for further activities. MOMA will receive $66 million up front and is eligible for up to $2 billion in development and commercial milestones, plus tiered royalties. |
Suzhou Ribo Life Science, Boehringer Ingelheim | January 2024 | $2,000 million (undisclosed) | Ribo Life Science and Ribocure granted Boehringer exclusive, worldwide rights to develop small interfering RNA (siRNA) therapies using Ribo’s RIBO-GalSTAR platform for the treatment of nonalcoholic or metabolic dysfunction-associated steatohepatitis (NASH/MASH). Ribo will receive an undisclosed upfront payment and is eligible for up to $2 billion in development milestones plus tiered royalties. |
Isomorphic Labs, Eli Lilly | January 2024 | $1,745 million ($45 million) | Isomorphic Labs granted Eli Lilly exclusive rights to its AlphaFold and AI technology to develop small molecule therapies for multiple undisclosed targets selected by Eli Lilly. Isomorphic will apply its virtual screens and ab initio design approaches, among others. Isomorphic will receive $45 million up front and is eligible for up to $1.7 billion in performance-based milestone payments, plus royalties. |
Merus, Gilead Sciences | March 2024 | $1,581 million ($81 million) | Gilead Sciences partnered with Merus to develop two antibody-based trispecific T cell engagers using Merus’ Triclonics platform for the treatment of cancer. Gilead has an excusive, worldwide option to license the development of resulting therapies while Merus has the option to discover a third program. |
Neomorph, Novo Nordisk | February 2024 | $1,460 million (undisclosed) | Neomorph granted Novo Nordisk exclusive, worldwide rights to develop molecular glue degrader based therapies for cardiometabolic and rare diseases. Neomorph will lead discovery activities against selected targets, with Novo Nordisk having the right to pursue further development. Neomorph will receive upfront and milestone payments, R&D funding, and is eligible for further milestones up to $1.46 billion. |
Umoja Biopharma, AbbVie | January 2024 | $1,440 million (undisclosed) | Umoja Biopharma granted AbbVie an option to license its lead in vivo CAR-T cell therapy program UB-VV111 using the VivoVec gene delivery platform for the treatment of hematologic cancers. The companies will develop up to 4 additional CAR-T cell therapies for AbbVie-selected targets. |
Caris Life Sciences, Merck KGaA | April 2024 | $1,400 million (undisclosed) | Caris Life Sciences granted Merck exclusive, worldwide rights to develop antibody-drug conjugates (ADCs) against selected targets for the treatment of cancer discovered using Caris’ AI based orthogonal multi-omics tech. Caris will receive an upfront payment, R&D funding and up to $1.4 billion in milestones and royalties. |
Voyager Therapeutics, Novartis | January 2024 | $1,300 million ($100 million) | Voyager granted Novartis rights to develop gene therapies using its TRACER capsids and IP for the treatment of Huntington’s disease (HD) and spinal muscular atrophy (SMA). Voyager will receive $20 million upfront and $80 million in equity by granting 2,145,002 common shares. For SMA, Voyager is eligible for up to $200 million in milestones (for HD $225 million) and $400 million in sales milestones (for HD $375 million). |
Isomorphic Labs, Novartis | January 2024 | $1,238 million ($38 million) | Isomorphic Labs granted Novartis exclusive rights to its AlphaFold model and AI technology platform to discover small molecule therapeutics against three undisclosed targets. Isomorphic will receive $37.5million up front, research funding and up to $1.2 billion in milestone payments. |
Source: DealForma database.
doi: https://doi.org/10.1038/d43747-024-00027-5
Updates & Corrections
Correction 06 June 2024 : The article has been corrected to clarify the details of Umoja Biopharma's partnership with AbbVie, specifically that AbbVie’s license is only for Umoja Biopharma's CD19 asset UB-VV111 rather than any/all developed by Umoja Biopharma.
Reprints and permissions
Faculty Positions in School of Engineering, Westlake University
The School of Engineering (SOE) at Westlake University is seeking to fill multiple tenured or tenure-track faculty positions in all ranks.
Hangzhou, Zhejiang, China
Westlake University
High-Level Talents at the First Affiliated Hospital of Nanchang University
For clinical medicine and basic medicine; basic research of emerging inter-disciplines and medical big data.
Nanchang, Jiangxi, China
The First Affiliated Hospital of Nanchang University
Professor/Associate Professor/Assistant Professor/Senior Lecturer/Lecturer
The School of Science and Engineering (SSE) at The Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen) sincerely invites applications for mul...
Shenzhen, China
The Chinese University of Hong Kong, Shenzhen (CUHK Shenzhen)
Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUST
Job Opportunities: Leading talents, young talents, overseas outstanding young scholars, postdoctoral researchers.
Wuhan, Hubei, China
School of Optical and Electronic Information, Huazhong University of Science and Technology
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
May 31, 2024 , by W. Kimryn Rathmell, M.D., Ph.D., and Shaalan Beg, M.D.

Greater use of technologies that can increase participation in cancer clinical trials is just one of the innovations that can help overcome some of the bottlenecks holding up progress in clinical research.
Thanks to advances in technology, data science, and infrastructure, the pace of discovery and innovation in cancer research has accelerated, producing an impressive range of potential new treatments and other interventions that are being tested in clinical studies . The extent of the innovative ideas that might help people live longer, improve our ability to detect cancer early, or otherwise transform care is staggering.
Our understanding of tumor biology is also evolving, and those gains in knowledge are being translated into the continued discovery of targets for potential interventions and the development of novel types of treatments. Some of these therapies are producing unprecedented clinical responses in studies, including in traditionally difficult-to-treat cancers.
These advances have contributed to a record number of Food and Drug Administration (FDA) approvals in recent years with, arguably, the most notable approvals being those for drugs that can be used for any cancer, regardless of where it is in the body .
In some instances, the activity of new agents has been so profound that clinical investigators are having to rethink their criteria for implementation in patient care and their definitions of treatment response.
For example, although HER2 has been a known therapeutic target in breast cancer for many decades, the new antibody-drug conjugates (ADCs) that target HER2 have proven to be vastly more effective than the original HER2-targeted therapies. This has forced researchers to rethink fundamental questions about how these ADCs are used in patient care: Can they be effective in people whose tumors have lower expression of HER2 than we previously thought was needed ? And, if so, do we need to redefine how we classify HER2-positive cancer?
As more innovative therapies like ADCs hit the clinic at a far more rapid cadence than ever before, the research community is being inundated with such fundamentally important questions.
However, the remarkable progress we're experiencing with novel new therapies is tempered by a critical bottleneck: the clinical research infrastructure can’t be expected to keep pace in this new landscape.
Currently, many studies struggle to enroll enough participants. At the same time, there are patients who don’t have ready access to studies from which they might benefit. Furthermore, ideas researchers have today for studies of innovative new interventions might not come to fruition for 2 or 3 years, or even longer—years that people with cancer don’t have.
The key to overcoming this bottleneck is to invite innovation to help reshape our clinical trials infrastructure. And here’s how we plan to accomplish that.
Testing Innovation in Cancer Clinical Trials
A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial .
This innovative study ushered in novel ways of recruiting participants and involving oncologists at centers big and small. And NCI-MATCH has spawned several successor studies that are incorporating and building on its innovations and achievements.
An innovation that emerged from the COVID pandemic was the increase of remote work, even in the clinical trials domain. Indeed, staffing shortages have caused participation in NCI-funded trials to decline. In response, NCI is piloting a Virtual Clinical Trials Office to offer remote support staff to participating study sites. This support staff includes research nurses, clinical research associates, and data specialists, all of whom will help NCI-Designated Cancer Centers and community practices engaged in clinical research activities.
Such technology-enabled services can allow us to reimagine how clinical trials are designed and run. This includes developing technologies and processes for remotely identifying clinical trial participants, shipping medications to participants at home, having imaging performed in the health care settings where our patients live, and empowering local physicians to participate in clinical trials.
We also need mechanisms to test and implement innovations in designing and conducting clinical studies.
The Pragmatica-Lung Cancer Treatment Trial , an innovative phase 3 study launched by NCI’s National Clinical Trials Network (NCTN) , was designed to be easy to launch, enroll participants, and interpret its results.
NCI recently established Clinical Trials Innovation Unit (CTIU) to pressure test a variety of innovations. The CTIU, which includes leadership from FDA and NCTN, is already working on future innovations, including those that will streamline data collection and apply novel approaches to clinical studies, all with the goal of making them less burdensome to run and easier for patients to participate.
Data-Driven Solutions
The era of data-driven health care is here, providing still more opportunities to transform cancer clinical research.
The emergence of artificial intelligence (AI) solutions, large language models, and informatics brings real potential for wholesale changes in how we match patients to clinical studies, assess side effects, and monitor events like disease progression.
Recognizing this potential, NCI is offering funding opportunities and other resources that will fuel the development of AI tools for clinical research, allow us to carefully test their usefulness, and ultimately deploy them across the oncology community.
Creating Partnerships and Expanding Health Equity
To be sure, none of this will be, or can be, done by NCI alone. All these innovations require partnerships. We will increase our engagement with partners in the public- and private-sectors, including other government agencies and nonprofits.
That includes high-level engagement with the Office of the National Coordinator for Health Information Technology (ONC), with input from FDA, Centers for Medicare & Medicaid Services, and Centers for Disease Control and Prevention.

Dr. W. Kimryn Rathmell, M.D., Ph.D.
NCI Director
One example of such a partnership is the USCDI+ Cancer program . Conducted under the auspices of the ONC, this program will further the aims of the White House's reignited Cancer Moonshot SM by encouraging the adoption and utilization of interoperable cancer health IT standards, providing resources to support cancer-specific use cases, and promoting alignment between federal partners.
And just as importantly, the new partnerships we create must include those with patients, advocates, and communities in ways we have never considered before.
A central feature of this community engagement must involve intentional efforts to expand health equity, to create study designs that are inclusive and culturally appropriate. Far too many marginalized communities and populations today are further harmed by studies that fail to provide findings that apply to their unique situations and needs.
Very importantly, the future will require educating our next generation of clinical investigators and empowering them with the tools that enable new ways of managing clinical studies. By supporting initiatives spearheaded by FDA and professional groups like the American Society of Clinical Oncology, NCI is making it easier for community oncologists to participate in clinical trials and helping clarify previously misunderstood regulatory requirements.
These efforts must also ensure that we have a clinical research workforce that is representative of the people it is intended to serve. Far too many structural barriers have prevented this from taking place in the past, and it’s time for that to change.
Expanding our capacity doesn’t mean doing more of the same, it means challenging ourselves to work differently. This will let us move forward to a new state, one in which clinical research is integrated in everyday practice. It is only with more strategic partnerships and increased inclusivity that we can open the doors to seeing clinical investigation in new ways, with new standards for success.
A Collaborative Effort

Shaalan Beg, M.D.
Senior Advisor for Clinical Research
To make the kind of progress we all desire, we have to recognize that our clinical studies system needs to evolve.
There was a time when taking years to design, launch, and complete a clinical trial was acceptable. It isn’t acceptable anymore. We are in an era where we have the tools and the research talent to make far more rapid progress than we have in the past.
And we can do that by engaging with many different communities and stakeholders in unique and dynamic ways—making them partners in our effort to end cancer as we know it.
Together, our task is to capitalize on this work so we can move faster and enable cutting-edge research that benefits as many people as possible.
We also know that there are more good ideas in this space, and part of this transformation includes grass roots efforts to drive systemic change. So, we encourage you to share your ideas on how we can transform clinical research. Because achieving this goal can’t be done by any one group alone. We are all in this together.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
- Introduction
- Conclusions
- Article Information
The number of patients screened was not collected.
Association between risk of the primary outcome (composite of cardiopulmonary hospitalization or death) per patient-week and mean influenza-like illness (ILI) activity over time.
Weekly odds of the primary outcome (composite of cardiopulmonary hospitalization or death) by month (referenced to July), after adjusting for influenza-like illness, treatment, and state.
Trial Protocol
eTable. Association of Weekly Odds of Primary and Secondary Outcomes With Influenza-Like Illness Activity by Time
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Hegde SM , Claggett BL , Udell JA, et al. Temporal Association Among Influenza-Like Illness, Cardiovascular Events, and Vaccine Dose in Patients With High-Risk Cardiovascular Disease : Secondary Analysis of a Randomized Clinical Trial . JAMA Netw Open. 2023;6(9):e2331284. doi:10.1001/jamanetworkopen.2023.31284
Manage citations:
© 2024
- Permissions
Temporal Association Among Influenza-Like Illness, Cardiovascular Events, and Vaccine Dose in Patients With High-Risk Cardiovascular Disease : Secondary Analysis of a Randomized Clinical Trial
- 1 Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
- 2 Women’s College Hospital and Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, Ontario, Canada
- 3 Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison
- 4 Brown University, The Warren Alpert Medical School, Providence, Rhode Island
- 5 Kaiser Permanente Division of Research, Northern California, Oakland
- 6 Department of Medicine, University of Wisconsin-Madison
- 7 Mount Sinai Heart, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, New York
- 8 National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Bethesda, Maryland
- 9 Department of Medicine, University of Minnesota, Minneapolis
- 10 VA Minneapolis Health Care System, US Department of Veterans Affairs, Minneapolis, Minnesota
Question Does high-dose compared with standard-dose influenza vaccine reduce cardiopulmonary events during periods of high, local influenza activity?
Findings In this secondary analysis of the INVESTED randomized clinical trial of 3094 participants from US sites with available weekly Centers for Disease Control and Prevention–reported influenza-like illness (ILI) activity, ILI was temporally associated with cardiopulmonary and cardiovascular (CV) hospitalizations. However, high-dose compared with standard-dose influenza vaccine did not significantly reduce occurrence of the primary outcome.
Meaning The findings indicate that influenza activity was temporally associated with CV events in patients with high-risk CV disease, and temporal CV risk was not significantly reduced by a higher influenza vaccine dose.
Importance Influenza-like illness (ILI) activity has been associated with increased risk of cardiopulmonary (CP) events during the influenza season. High-dose trivalent influenza vaccine was not superior to standard-dose quadrivalent vaccine for reducing these events in patients with high-risk cardiovascular (CV) disease in the Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial.
Objective To evaluate whether high-dose trivalent influenza vaccination is associated with benefit over standard-dose quadrivalent vaccination in reducing CP events during periods of high, local influenza activity.
Design, Setting, and Participants This study was a prespecified secondary analysis of INVESTED, a multicenter, double-blind, active comparator randomized clinical trial conducted over 3 consecutive influenza seasons from September 2016 to July 2019. Follow-up was completed in July 2019, and data were analyzed from September 21, 2016, to July 31, 2019. Weekly Centers for Disease Control and Prevention (CDC)–reported, state-level ILI activity was ascertained to assess the weekly odds of the primary outcome. The study population included 3094 patients with high-risk CV disease from participating centers in the US.
Intervention Participants were randomized to high-dose trivalent or standard-dose quadrivalent influenza vaccine and revaccinated for up to 3 seasons.
Main Outcomes and Measures The primary outcome was the time to composite of all-cause death or CP hospitalization within each season. Additional measures included weekly CDC-reported ILI activity data by state.
Results Among 3094 participants (mean [SD] age, 65 [12] years; 2309 male [75%]), we analyzed 129 285 person-weeks of enrollment, including 1396 composite primary outcome events (1278 CP hospitalization, 118 deaths). A 1% ILI increase in the prior week was associated with an increased risk in the primary outcome (odds ratio [OR], 1.14; 95% CI, 1.07-1.21; P < .001), CP hospitalization (OR, 1.13; 95% CI, 1.06-1.21; P < .001), and CV hospitalization (OR, 1.12; 95% CI, 1.04-1.19; P = .001), after adjusting for state, demographic characteristics, enrollment strata, and CV risk factors. Increased ILI activity was not associated with all-cause death (OR, 1.00; 95% CI, 0.88-1.13; P > .99). High-dose compared with standard-dose vaccine did not significantly reduce the primary outcome, even when the analysis was restricted to weeks of high ILI activity (OR, 0.88; 95% CI, 0.65-1.20; P = .43). Traditionally warmer months in the US were associated with lower CV risk independent of local ILI activity.
Conclusions and Relevance In this secondary analysis of a randomized clinical trial, ILI activity was temporally associated with increased CP events in patients with high-risk CV disease, and a higher influenza vaccine dose did not significantly reduce temporal CV risk. Other seasonal factors may play a role in the coincident high rates of ILI and CV events.
Trial Registration ClinicalTrials.gov Identifier: NCT02787044
Influenza has been associated with increased risk of cardiopulmonary (CP) events, including myocardial infarction (MI) and heart failure (HF). 1 - 8 Proposed mechanisms that suggest an association between influenza infection and cardiovascular (CV) risk include induction of systemic effects via immune stimulation and inflammation that can provoke plaque rupture, increased metabolic demand, adrenergic surge, hypoxia, hypercoagulability, and direct myocardial toxic effects. 9 , 10 Seasonal outbreaks of influenza occur primarily in winter months when transmission may be more favorable, and CV events demonstrate a similar temporal pattern. 11 Time-series analyses have also demonstrated an association between influenza and CV events in observational and retrospective studies. 12 , 13 Influenza vaccine may be involved in reducing adverse CV outcomes, as suggested by observational and randomized clinical trials. 14 - 19
The Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial assessed the efficacy of high-dose trivalent or standard-dose quadrivalent influenza vaccine in North American participants with high-risk CV disease during the 2016 to 2019 influenza seasons. 20 The primary end point was time to first CP hospitalization or all-cause death in each season. High-dose vaccine was not superior to standard-dose vaccine for the primary end point, which assessed benefit from 2 weeks following vaccination through the end of July of each influenza season. 21 Whether treatment efficacy may be different during periods of high influenza-like illness (ILI) activity is not known. In this analysis, we compared locally defined ILI activity, as provided by the Centers for Disease Control and Prevention (CDC), with outcomes in INVESTED and assessed whether high-dose compared with standard-dose influenza vaccine modified the events in association with locally determined ILI activity.
This secondary analysis of a randomized clinical trial was a prespecified analysis of the INVESTED trial, a pragmatic, randomized, double-blind, active comparator trial conducted at 157 participating centers in the US and Canada from September 2016 to July 2019. Follow-up was completed in July 2019. Patients with high-risk CV disease previously hospitalized for either MI in the past 12 months or HF in the past 24 months were randomized to high-dose or standard-dose influenza vaccine. The protocol and primary results have been previously published ( Supplement 1 and Figure 1 ). 21 This study was a preplanned analysis of the parent trial and follows the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline for randomized studies. 22 Ethics committees at each enrolling site approved the protocol or the review was ceded to a central institutional review board. All participants provided written informed consent in accordance with established guidelines.
Influenza-like illness was defined based on the percentage of visits to sentinel clinicians for fever (temperature ≥37.8 °C) and a cough and/or a sore throat without a known cause other than influenza as reported to the CDC US Outpatient Influenza-Like Illness Surveillance Network. Therefore, ILI could have included any respiratory pathogen presenting with these symptoms. We included CDC data from 108 US sites and territories; sites from Florida and Canada were not included due to unavailable weekly ILI data. Race categories included Asian, Black, First Nations or American Indian, White, and other (Native Hawaiian, Pacific Islander, >1 race, chooses not to report, does not know, and not available or missing), and ethnicity categories included Hispanic or Latino, non-Hispanic or non-Latino, and other (chooses not to report, does not know, and not available or missing). Race and ethnicity were ascertained by self-report.
Data were analyzed from September 21, 2016, to July 31, 2019. We compared publicly available, weekly, CDC-reported, state-level ILI with the weekly occurrence of the primary outcome (composite of all-cause death or CP hospitalization) and secondary outcomes (death, CV hospitalization, and pulmonary hospitalization) among US participants from September 2016 to July 2019 using logistic regression models. Weekly ILI activity from 0 to 9 weeks prior to (lag 0 to lag 9) the outcome of interest was considered to assess the temporal association between exposure and outcome. Model 1 was adjusted for state, demographics (age, self-reported sex, and race) and enrollment strata (history of MI, history of hospitalization for HF). Model 2 was additionally adjusted for CV risk factors (diabetes, body mass index >30 [calculated as weight in kilograms divided by height in meters squared], kidney impairment, current smoker, peripheral arterial disease, ischemic stroke, hypertension, hyperlipidemia, asthma, chronic obstructive pulmonary disease, percutaneous coronary intervention, coronary artery bypass graft, atrial fibrillation, and implantable cardioverter-defibrillator).
To assess the treatment outcome, we used logistic regression models to evaluate the weekly odds of the primary outcome for the high-dose compared with standard-dose vaccine treatment group adjusting for state to account for possible regional differences. In a sensitivity analysis, we analyzed the association between ILI activity and outcomes (1) only during months with typically higher influenza activity (September 15th to May 15th of each enrolling season) and (2) during weeks with higher ILI activity (>2 SDs above a state’s mean ILI activity). Last, we compared weekly ILI with outcomes adjusting for state, vaccine treatment assignment, and month, referenced to the month of July.
Analyses were performed using Stata, version 14 (StataCorp LLC), and statistical significance was set at 2-sided P < .05. P values were not adjusted for multiple testing.
In this sample of 3094 US participants (mean [SD] age, 65 [12] years; 781 [25.2%] were female, 2309 [74.6%] were male, and 4 participants did not report sex [0.1%]). Of these, 30 (1.0%) were Asian, 690 (22.3%) Black, 12 (0.4%) First Nations or American Indian, 226 (7.3%) Hispanic or Latino, 2832 (91.5%) non-Hispanic or non-Latino, 2238 (72.3%) White, 124 (4.0%) other race, and 36 (1.2%) other ethnicity. Baseline characteristics and concomitant therapies were well balanced between treatment groups ( Table 1 ). The majority of enrolled participants (2352 [76.0%]) had an HF qualifying event, 672 (21.7%) had prior HF hospitalization, and 375 (12.1%) had a prior MI event. The sample associated 129 285 person-weeks of enrollment with 1396 composite primary outcome events (1278 CP hospitalizations, 118 deaths), 322 deaths, 1141 CV hospitalizations, and 183 pulmonary hospitalizations. A lag of 1 week was chosen for the logistic regression analyses relating ILI activity to the primary and secondary outcomes via forward stepwise selection models (eTable in Supplement 2 ). Mean ILI activity over time, as reported by the CDC and risk of the primary outcome over time are shown in Figure 2 . Mean ILI activity was the highest in the 2017 to 2018 season.
A 1% ILI increase in the prior week was associated with an increased risk in the primary outcome of composite of CP hospitalization or all-cause death (odds ratio [OR], 1.14; 95% CI, 1.07-1.21; P < .001), CP hospitalization (OR, 1.13; 95% CI, 1.06-1.21; P < .001), and CV hospitalization (OR, 1.12; 95% CI, 1.04-1.19; P = .001) after adjusting for state, demographic characteristics, enrollment strata, and CV risk factors ( Table 2 ). No association was found between increased ILI activity and pulmonary hospitalizations (OR, 1.18; 95% CI, 0.99-1.40; P = .06). Increased ILI activity was not associated with all-cause death (OR, 1.00; 95% CI, 0.88-1.13; P > .99).
Warmer months (July, August, and September) were associated with a lower risk of events even after adjusting for ILI ( Figure 3 ). Figure 3 demonstrates the weekly odds of the primary outcome by month (referenced to July) after adjusting for ILI, vaccine treatment assignment, and state.
Evaluation of the weekly odds of the primary outcome by treatment yielded similar results to the primary results from the INVESTED trial. High-dose was not superior to standard-dose vaccine in its association with risk of the primary outcome (CP hospitalization or death) in the prespecified primary analysis after adjusting for state (OR, 1.07; 95% CI, 0.95-1.20; P = .25), when restricting analysis to months with typically higher influenza activity (OR, 1.09; 95% CI, 0.97-1.24; P = .15), or during weeks of high ILI activity (OR, 0.88; 95% CI, 0.65-1.20; P = .43) in this temporal analysis.
In this secondary analysis of the INVESTED randomized clinical trial, ILI was temporally associated with CP and CV hospitalization but not with all-cause death in patients with high-risk CV disease. A higher dose of trivalent influenza vaccine compared with standard-dose quadrivalent influenza vaccine did not modify this association, even after restricting the analysis to months with typically higher influenza activity or weeks in which ILI activity was high. Warmer months in the US were associated with lower CV risk independent of local ILI activity.
The primary end point of INVESTED was designed to capture CP events that occurred during the broad influenza season, but not necessarily during times of high influenza activity, because we hypothesized that if influenza infection led to CV and pulmonary events, the events might occur even several months after a primary infection. Nevertheless, we anticipated the possibility that modification of these events might be more temporally associated with influenza infection. This prespecified analysis was designed to assess whether high-dose influenza vaccine would provide greater benefit than standard-dose vaccine during times of high influenza activity. That we did not observe a benefit in this analysis suggests that in a high-risk population, the higher dose of vaccine was not associated with modification of events that were extremely likely. However, the benefit of any influenza vaccine compared with placebo in this population cannot be determined from this analysis. Based on other data comparing vaccination with placebo, it is likely that influenza vaccination with either dose would have more substantial benefits over placebo in patients with high risk. 23 Moreover, as the influenza virus is not the only circulating virus during the winter months, it is possible that other respiratory viruses may contribute to the association between time and CV events.
In patients with high-risk CV disease, ILI activity was temporally associated with CP and CV hospitalizations. Although a similar OR for pulmonary-related hospitalizations was present, there was no association with increased ILI activity, which may in part be attributed to the small number of events (n = 183) and underscores the relatively higher occurrence of CV (n = 1141) compared with pulmonary events in this sample. Furthermore, individual-level ILI or infection was not assessed in this sample, so the true incidence of influenza is not known. Nevertheless, the temporal association with CV events supported prior findings of emergency department visits in New York City of the association of ILI with CV mortality and in observational data in an older cohort of the association of ILI with MI and HF events. 12 , 13 This association remained when the analysis focused on months with typically higher influenza activity and weeks with high ILI activity.
Although all participants received influenza vaccine, the temporal association between ILI activity and CV events remained. Additional analysis demonstrated that warmer months were associated with lower risk of CV events after accounting for ILI activity and vaccine dose, suggesting that other seasonal factors may also play a role in the seasonal variation in CV events. Vaccine effectiveness was not associated with a modification in the seasonality of ILI and CV events in another analysis of New York state data. 24 Unmeasured environmental factors, such as temperature, absolute and relative humidity, and wind speed, have been associated with influenza outbreaks and CV events with some variation by influenza subtypes and may explain some of the seasonal variation. 25 - 29 Registry data from Sweden have similarly demonstrated the impact of seasonal environmental factors, which attenuated the association between higher incidence of MI and higher incidence of influenza. 30
Several limitations of this analysis should be noted. Inferences about the association between patient-level influenza illness or other respiratory viruses and subsequent events could not be made, as the respiratory influenza infection status of participants was not assessed in this study. State-level ILI activity may not have represented regional-level or patient-level ILI activity. Only states with available ILI activity were included in this analysis, which limited generalizability to participants, excluding those in Florida and Canada. That we did not randomize patients to placebo prevented us from drawing conclusions about the impact of any influenza vaccination on the temporal association between ILI activity and CV events. Also, the difference in valence between the high-dose trivalent influenza vaccine and the standard-dose quadrivalent vaccine may have contributed to the absence of outcome modification of the vaccine dose, with the standard-dose vaccine providing additional coverage for the influenza B/Yamagata strain. Changes in seasonal temperature, humidity, weather patterns, and other environmental factors were not accounted for in this analysis. Additionally, the trial had limited power for this prespecified secondary analysis.
In this secondary analysis of the INVESTED randomized clinical trial evaluating the efficacy of high-dose trivalent vaccine compared with standard-dose quadrivalent vaccine in reducing CP events among patients with high-risk CV disease in the US, influenza activity was temporally associated with an increasing risk of CP events, yet a higher-dose influenza vaccine did not significantly reduce temporal CV risk. Other seasonal factors may also play a role in the higher rate of CV events associated with high rates of influenza. Further studies and additional measures are needed to understand additional factors influencing the temporal association between influenza and CV events and to assess new strategies to reduce events.
Accepted for Publication: July 22, 2023.
Published: September 14, 2023. doi:10.1001/jamanetworkopen.2023.31284
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Hegde SM et al. JAMA Network Open .
Corresponding Author: Orly Vardeny, PharmD, MS, VA Minneapolis Health Care System, US Department of Veterans Affairs, 1 Veterans Dr, Minneapolis, MN 55417 ( [email protected] ).
Author Contributions: Drs Hegde and Claggett had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Hegde, Udell, Kim, Joseph, Farkouh, A. S. Bhatt, Tattersall, D. L. Bhatt, Solomon, Vardeny.
Acquisition, analysis, or interpretation of data: Hegde, Claggett, Udell, Kim, Joseph, Peikert, Tattersall, D. L. Bhatt, Cooper, Vardeny.
Drafting of the manuscript: Hegde, Vardeny.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Hegde, Claggett, Kim.
Obtained funding: Kim, Solomon.
Administrative, technical, or material support: Hegde, Kim, Tattersall.
Supervision: Claggett, Kim, Joseph, Tattersall, Solomon, Vardeny.
Conflict of Interest Disclosures: Dr Hegde reported receiving funding from Bristol Myers Squibb (BMS) to the Brigham and Women’s Hospital for core echocardiographic laboratory services outside the submitted work. Dr Udell reported receiving grants from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, and Sanofi and receiving personal fees from GlaxoSmithKline and Novartis outside the submitted work. Dr Joseph reported receiving grants from the National Heart, Lung, and Blood Institute for research during the conduct of the study and receiving grants from Amgen, Kowa, and Alnylam outside the submitted work. Dr Farkouh reported receiving grants from Novartis and Novo Nordisk, funding from the AstraZeneca Data and Safety Monitoring Board service, and personal fees from Otitopic outside the submitted work. Dr Peikert reported receiving grants from the German Research Foundation outside the submitted work. Dr A. S. Bhatt reported receiving personal fees from Sanofi outside the submitted work. Dr D. L. Bhatt reported serving on the advisory boards of Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, PracticeUpdate Cardiology for Elsevier, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; on the boards of directors of Angiowave (with stock options), Boston VA Research Institute, BMS (with stock options), DRS.LINQ (with stock options), High Enroll (with stock options), the Society of Cardiovascular Patient Care, and TobeSoft; as the inaugural chair of the American Heart Association (AHA) Quality Oversight Committee; as a consultant of Broadview Ventures and Hims; on the Data Monitoring Committee of Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute) for the PORTICO trial funded by St Jude Medical (now Abbott), Boston Scientific (chair) for the PEITHO trial, Cleveland Clinic (including for the ExCEED trial) funded by Edwards, Contego Medical (chair) for the PERFORMANCE 2 trial, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine for the ENVISAGE trial funded by Daiichi Sankyo and for the ABILITY-DM trial funded by Concept Medical, Novartis, Population Health Research Institute, and Rutgers University for the National Institutes of Health–funded MINT trial; as chair of the American College of Cardiology Accreditation Oversight Committee, the Arnold and Porter law firm (work related to Sanofi/BMS clopidogrel litigation), and the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute); on the Clinical Trial Steering Committee of the RE-DUAL PCI trial funded by Boehringer Ingelheim, the Canadian Medical and Surgical Knowledge Translation Research Group, and the Duke Clinical Research Institute (including for the PRONOUNCE trial funded by Ferring Pharmaceuticals); on the AEGIS-II Executive Committee funded by CSL Behring; as editor in chief of the Harvard Heart Letter for Belvoir Publications and of the Journal of Invasive Cardiology for HMP Global; as a guest editor and associate editor of the Journal of the American College of Cardiology ; as cochair of K2P for interdisciplinary curriculum; on the Continuing Medical Education Steering Committee of Medtelligence/ReachMD and WebMD; as course director of the Comprehensive Review of Interventional Cardiology for Oakstone continuing medical education; on the Operations, Publications, and Steering Committees of the Population Health Research Institute for the COMPASS trial; as a USA national coleader funded by Bayer; as chief medical editor of Cardiology Today’s Intervention for Slack Publications; as secretary/treasurer of the Society of Cardiovascular Patient Care; on the Steering Committee of Wiley; as deputy editor of Clinical Cardiology ; on the Registry Steering Committee (chair) of the National Cardiovascular Data Registry ACTION; on the Research and Publications Committee (chair) of the Veterans Affairs Clinical Assessment Reporting and Tracking; as a site coinvestigator of Abbott, Biotronik, Boston Scientific, Central States Industrial, Endotronix, St Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; and as a trustee of the American College of Cardiology; performing unfunded research for FlowCo and Takeda; receiving honoraria from the American College of Cardiology as senior associate editor of the Clinical Trials and News website and ACC.org; speaker honorarium for an AHA lecture for CSL Behring; research funding from Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, BMS, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Eli Lilly & Company, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; royalties from Elsevier as editor of Braunwald’s Heart Disease ; and having a patent with sotagliflozin assigned to Brigham and Women’s Hospital assigned to Lexicon with no income received; and consulting for Cowen and Company, MJH Life Sciences, Piper Sandler. Dr Solomon reported receiving grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Eli Lilly & Company, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and Us2.ai grants to the Brigham and Women’s Hospital and personal fees from Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi Sankyo, GSK, Eli Lilly & Company, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Valo for consulting outside the submitted work. Dr Vardeny reported receiving personal fees from Sanofi-Pasteur outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by grants U01HL130163 and U01HL130204 from the NHLBI (Drs Kim, Solomon, and Vardeny). Additional funding was provided by Sanofi Pasteur (Dr Solomon).
Role of the Funder/Sponsor: Sanofi Pasteur had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the US government, the NHLBI, the NIH, or the US Department of Health and Human Services.
Data Sharing Statement: See Supplement 3 .
Additional Information: Dr Cooper is retired from the NHLBI, NIH. Vaccines were provided by Sanofi Pasteur.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
COMMENTS
Summary. -Planning, execution, and interpretation of clinical trials research, data collection activities and clinical operations. May interact with investigational sites, clinical consultants, Contract Research Organizations and other vendors. Collaborates with Country medical/clinical colleagues, global clinical teams and directs activities ...
90 Novartis Clinical Research Associate jobs. Search job openings, see if they fit - company salaries, reviews, and more posted by Novartis employees.
See how it works. The estimated total pay range for a Clinical Research Associate at Novartis is $117K-$159K per year, which includes base salary and additional pay. The average Clinical Research Associate base salary at Novartis is $125K per year. The average additional pay is $11K per year, which could include cash bonus, stock, commission ...
The estimated average pay for Clinical Research Associate at this company in the United States is $71,306 per year, which is 22% below the national average. Disclaimer Indeed estimates the pay amounts by analyzing the available public or private data and pay grades across nearby locations, similar companies, reviews, resumes, similar roles and ...
Novartis. East Hanover, NJ. Be an early applicant. 2 weeks ago. You've viewed all jobs for this search. Today's top 3 Novartis Clinical Research Associate jobs in United States. Leverage your ...
Novartis. Today's top 82 Novartis Clinical Research jobs in United States. Leverage your professional network, and get hired. New Novartis Clinical Research jobs added daily.
Reviews from Novartis employees about working as a Clinical Research Associate at Novartis. Learn about Novartis culture, salaries, benefits, work-life balance, management, job security, and more.
100,000. That's how many patients participate in our clinical trials at any given time. Global Clinical Operations (GCO) touches patients' lives every day acting as a link between science and medicine. Envision the impact you could have! #GCOThe Senior CRA performs monitoring activities related to initiation, conduct (recruitment, quality data collection) and timely completion of Phase I ...
Central Clinical Research Associate (Former Employee) - Hyderabad, Andhra Pradesh - December 14, 2016. I completely and thoroughly enjoyed working for Novartis. I joined a completely new team and our work was just a concept; so I got the opportunity to develop a team and innovate.
The average salary for a Clinical Research Associate (CRA) at Novartis is $96,288 in 2024. Visit PayScale to research clinical research associate (cra) salaries by city, experience, skill ...
The Associate Clinical Research Medical Director provides clinical strategic and tactical leadership in the country to support GDD trials and selected NIBR trials, clinical development plans which ...
The average Senior Clinical Research Associate base salary at Novartis is $150K per year. The average additional pay is $16K per year, which could include cash bonus, stock, commission, profit sharing or tips. The "Most Likely Range" reflects values within the 25th and 75th percentile of all pay data available for this role.
Novartis Compensation and Benefit Summary: The pay range for this position at commencement of employment is expected to be between $112,800- $169,200 annually; however, w hile salary ranges are effective from 1/1/24 through 12/31/24, fluctuations in the job market may necessitate adjustments to pay ranges during this period.
The average Research Associate base salary at Novartis is $81K per year. The average additional pay is $6K per year, which could include cash bonus, stock, commission, profit sharing or tips. The "Most Likely Range" reflects values within the 25th and 75th percentile of all pay data available for this role. Glassdoor salaries are powered by ...
Experienced Clinical Research Associate with a demonstrated history of working in the pharmaceuticals industry. Skilled in Electronic Data Capture (EDC), Institutional Review Board (IRB), Oncology ...
A foundational course for individuals considering a career as a clinical research associate (CRA). The course provides broad training on the ethical, regulatory, and practical aspects of monitoring a research site. The modules explain how recent developments in clinical trial organization and conduct have changed the role of CRAs.
The average salary for a Senior Clinical Research Associate (CRA) at Novartis is $120,316 in 2024. Visit PayScale to research senior clinical research associate (cra) salaries by city, experience ...
January 2024. $1,300 million ($100 million) Voyager granted Novartis rights to develop gene therapies using its TRACER capsids and IP for the treatment of Huntington's disease (HD) and spinal ...
Testing Innovation in Cancer Clinical Trials. A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial. This innovative study ushered in novel ways of recruiting participants and involving ...
Research & Development Development Spain. Key Responsibilities: Your responsibilities include but are not limited to:• Frontline liaison between Novartis and sites to ensure successful collaboration, meeting Novartis expectation on milestone and deliverables with true ownership mindset.•. Manages assigned study sites, conducting phase I-IV ...
Sr. Clinical Research Associate @ Novartis | MPH, GCP · Senior Clinical Research Associate Consultant with over 10 years skilled in Oncology, Medical Devices, CNS, Vaccines, Endocrinology ...
Key Points. Question Does high-dose compared with standard-dose influenza vaccine reduce cardiopulmonary events during periods of high, local influenza activity?. Findings In this secondary analysis of the INVESTED randomized clinical trial of 3094 participants from US sites with available weekly Centers for Disease Control and Prevention-reported influenza-like illness (ILI) activity, ILI ...
Clinical Research Associate. Jul 2018 - Aug 2021 3 years 2 months. -Conduct site qualification, initiation, routine monitoring and study closeout visits for investigational drug and device Phase 1 ...