Module 11: Schizophrenia Spectrum and Other Psychotic Disorders
Case studies: schizophrenia spectrum disorders, learning objectives.
- Identify schizophrenia and psychotic disorders in case studies

Case Study: Bryant
Thirty-five-year-old Bryant was admitted to the hospital because of ritualistic behaviors, depression, and distrust. At the time of admission, prominent ritualistic behaviors and depression misled clinicians to diagnose Bryant with obsessive-compulsive disorder (OCD). Shortly after, psychotic symptoms such as disorganized thoughts and delusion of control were noticeable. He told the doctors he has not been receiving any treatment, was not on any substance or medication, and has been experiencing these symptoms for about two weeks. Throughout the course of his treatment, the doctors noticed that he developed a catatonic stupor and a respiratory infection, which was identified by respiratory symptoms, blood tests, and a chest X-ray. To treat the psychotic symptoms, catatonic stupor, and respiratory infection, risperidone, MECT, and ceftriaxone (antibiotic) were administered, and these therapies proved to be dramatically effective. [1]
Case Study: Shanta
Shanta, a 28-year-old female with no prior psychiatric hospitalizations, was sent to the local emergency room after her parents called 911; they were concerned that their daughter had become uncharacteristically irritable and paranoid. The family observed that she had stopped interacting with them and had been spending long periods of time alone in her bedroom. For over a month, she had not attended school at the local community college. Her parents finally made the decision to call the police when she started to threaten them with a knife, and the police took her to the local emergency room for a crisis evaluation.
Following the administration of the medication, she tried to escape from the emergency room, contending that the hospital staff was planning to kill her. She eventually slept and when she awoke, she told the crisis worker that she had been diagnosed with attention-deficit/hyperactive disorder (ADHD) a month ago. At the time of this ADHD diagnosis, she was started on 30 mg of a stimulant to be taken every morning in order to help her focus and become less stressed over the possibility of poor school performance.
After two weeks, the provider increased her dosage to 60 mg every morning and also started her on dextroamphetamine sulfate tablets (10 mg) that she took daily in the afternoon in order to improve her concentration and ability to study. Shanta claimed that she might have taken up to three dextroamphetamine sulfate tablets over the past three days because she was worried about falling asleep and being unable to adequately prepare for an examination.
Prior to the ADHD diagnosis, the patient had no known psychiatric or substance abuse history. The urine toxicology screen taken upon admission to the emergency department was positive only for amphetamines. There was no family history of psychotic or mood disorders, and she didn’t exhibit any depressive, manic, or hypomanic symptoms.
The stimulant medications were discontinued by the hospital upon admission to the emergency department and the patient was treated with an atypical antipsychotic. She tolerated the medications well, started psychotherapy sessions, and was released five days later. On the day of discharge, there were no delusions or hallucinations reported. She was referred to the local mental health center for aftercare follow-up with a psychiatrist. [2]
Another powerful case study example is that of Elyn R. Saks, the associate dean and Orrin B. Evans professor of law, psychology, and psychiatry and the behavioral sciences at the University of Southern California Gould Law School.
Saks began experiencing symptoms of mental illness at eight years old, but she had her first full-blown episode when studying as a Marshall scholar at Oxford University. Another breakdown happened while Saks was a student at Yale Law School, after which she “ended up forcibly restrained and forced to take anti-psychotic medication.” Her scholarly efforts thus include taking a careful look at the destructive impact force and coercion can have on the lives of people with psychiatric illnesses, whether during treatment or perhaps in interactions with police; the Saks Institute, for example, co-hosted a conference examining the urgent problem of how to address excessive use of force in encounters between law enforcement and individuals with mental health challenges.
Saks lives with schizophrenia and has written and spoken about her experiences. She says, “There’s a tremendous need to implode the myths of mental illness, to put a face on it, to show people that a diagnosis does not have to lead to a painful and oblique life.”
In recent years, researchers have begun talking about mental health care in the same way addiction specialists speak of recovery—the lifelong journey of self-treatment and discipline that guides substance abuse programs. The idea remains controversial: managing a severe mental illness is more complicated than simply avoiding certain behaviors. Approaches include “medication (usually), therapy (often), a measure of good luck (always)—and, most of all, the inner strength to manage one’s demons, if not banish them. That strength can come from any number of places…love, forgiveness, faith in God, a lifelong friendship.” Saks says, “We who struggle with these disorders can lead full, happy, productive lives, if we have the right resources.”
You can view the transcript for “A tale of mental illness | Elyn Saks” here (opens in new window) .
- Bai, Y., Yang, X., Zeng, Z., & Yang, H. (2018). A case report of schizoaffective disorder with ritualistic behaviors and catatonic stupor: successful treatment by risperidone and modified electroconvulsive therapy. BMC psychiatry , 18(1), 67. https://doi.org/10.1186/s12888-018-1655-5 ↵
- Henning A, Kurtom M, Espiridion E D (February 23, 2019) A Case Study of Acute Stimulant-induced Psychosis. Cureus 11(2): e4126. doi:10.7759/cureus.4126 ↵
- Modification, adaptation, and original content. Authored by : Wallis Back for Lumen Learning. Provided by : Lumen Learning. License : CC BY: Attribution
- A tale of mental illness . Authored by : Elyn Saks. Provided by : TED. Located at : https://www.youtube.com/watch?v=f6CILJA110Y . License : Other . License Terms : Standard YouTube License
- A Case Study of Acute Stimulant-induced Psychosis. Authored by : Ashley Henning, Muhannad Kurtom, Eduardo D. Espiridion. Provided by : Cureus. Located at : https://www.cureus.com/articles/17024-a-case-study-of-acute-stimulant-induced-psychosis#article-disclosures-acknowledgements . License : CC BY: Attribution
- Elyn Saks. Provided by : Wikipedia. Located at : https://en.wikipedia.org/wiki/Elyn_Saks . License : CC BY-SA: Attribution-ShareAlike
- A case report of schizoaffective disorder with ritualistic behaviors and catatonic stupor: successful treatment by risperidone and modified electroconvulsive therapy. Authored by : Yuanhan Bai, Xi Yang, Zhiqiang Zeng, and Haichen Yangcorresponding. Located at : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5851085/ . License : CC BY: Attribution

Schizophrenia case studies: putting theory into practice
This article considers how patients with schizophrenia should be managed when their condition or treatment changes.
DR P. MARAZZI/SCIENCE PHOTO LIBRARY
Treatments for schizophrenia are typically recommended by a mental health specialist; however, it is important that pharmacists recognise their role in the management and monitoring of this condition. In ‘ Schizophrenia: recognition and management ’, advice was provided that would help with identifying symptoms of the condition, and determining and monitoring treatment. In this article, hospital and community pharmacy-based case studies provide further context for the management of patients with schizophrenia who have concurrent conditions or factors that could impact their treatment.
Case study 1: A man who suddenly stops smoking
A man aged 35 years* has been admitted to a ward following a serious injury. He has been taking olanzapine 20mg at night for the past three years to treat his schizophrenia, without any problems, and does not take any other medicines. He smokes 25–30 cigarettes per day, but, because of his injury, he is unable to go outside and has opted to be started on nicotine replacement therapy (NRT) in the form of a patch.
When speaking to him about his medicines, he appears very drowsy and is barely able to speak. After checking his notes, it is found that the nurses are withholding his morphine because he appears over-sedated. The doctor asks the pharmacist if any of the patient’s prescribed therapies could be causing these symptoms.
What could be the cause?
Smoking is known to increase the metabolism of several antipsychotics, including olanzapine, haloperidol and clozapine. This increase is linked to a chemical found in cigarettes, but not nicotine itself. Tobacco smoke contains aromatic hydrocarbons that are inducers of CYP1A2, which are involved in the metabolism of several medicines [1] , [2] , [3] . Therefore, smoking cessation and starting NRT leads to a reduction in clearance of the patient’s olanzapine, leading to increased plasma levels of the antipsychotic olanzapine and potentially more adverse effects — sedation in this case.
Patients who want to stop, or who inadvertently stop, smoking while taking antipsychotics should be monitored for signs of increased adverse effects (e.g. extrapyramidal side effects, weight gain or confusion). Patients who take clozapine and who wish to stop smoking should be referred to their mental health team for review as clozapine levels can increase significantly when smoking is stopped [3] , [4] .
For this patient, olanzapine is reduced to 15mg at night; consequently, he seems much brighter and more responsive. After a period on the ward, he has successfully been treated for his injury and is ready to go home. The doctor has asked for him to be supplied with olanzapine 15mg for discharge along with his NRT.
What should be considered prior to discharge?
It is important to discuss with the patient why his dose was changed during his stay in hospital and to ask whether he intends to start smoking again or to continue with his NRT. Explain to him that if he wants to begin, or is at risk of, smoking again, his olanzapine levels may be impacted and he may be at risk of becoming unwell. It is necessary to warn him of the risk to his current therapy and to speak to his pharmacist or mental health team if he does decide to start smoking again. In addition, this should be used as an opportunity to reinforce the general risks of smoking to the patient and to encourage him to remain smoke-free.
It is also important to speak to the patient’s community team (e.g. doctors, nurses), who specialise in caring for patients with mental health disorders, about why the olanzapine dose was reduced during his stay, so that they can then monitor him in case he does begin smoking again.
Case 2: A woman with constipation
A woman aged 40 years* presents at the pharmacy. The pharmacist recognises her as she often comes in to collect medicine for her family. They are aware that she has a history of schizophrenia and that she was started on clozapine three months ago. She receives this from her mental health team on a weekly basis.
She has visited the pharmacy to discuss constipation that she is experiencing. She has noticed that since she was started on clozapine, her bowel movements have become less frequent. She is concerned as she is currently only able to go to the toilet about once per week. She explains that she feels uncomfortable and sick, and although she has been trying to change her diet to include more fibre, it does not seem to be helping. The patient asks for advice on a suitable laxative.
What needs to be considered?
Constipation is a very common side effect of clozapine . However, it has the potential to become serious and, in rare cases, even fatal [5] , [6] , [7] , [8] . While minor constipation can be managed using over-the-counter medicines (e.g. stimulant laxatives, such as senna, are normally recommended first-line with stool softeners, such as docusate, or osmotic laxatives, such as lactulose, as an alternative choice), severe constipation should be checked by a doctor to ensure there is no serious bowel obstruction as this can lead to paralytic ileus, which can be fatal [9] . Symptoms indicative of severe constipation include: no improvement or bowel movement following laxative use, fever, stomach pain, vomiting, loss of appetite and/or diarrhoea, which can be a sign of faecal impaction overflow.
As the patient has been experiencing this for some time and is only opening her bowels once per week, as well as having other symptoms (i.e. feeling uncomfortable and sick), she should be advised to see her GP as soon as possible.
The patient returns to the pharmacy again a few weeks later to collect a prescription for a member of their family and thanks the pharmacist for their advice. The patient was prescribed a laxative that has led to resolution of symptoms and she explains that she is feeling much better. Although she has a repeat prescription for lactulose 15ml twice per day, she says she is not sure whether she needs to continue to take it as she feels better.
What advice should be provided?
As she has already had an episode of constipation, despite dietary changes, it would be best for the patient to continue with the lactulose at the same dose (i.e. 15ml twice daily), to prevent the problem occurring again. Explain to the patient that as constipation is a common side effect of clozapine, it is reasonable for her to take laxatives before she gets constipation to prevent complications.
Pharmacists should encourage any patient who has previously had constipation to continue taking prescribed laxatives and explain why this is important. Pharmacists should also continue to ask patients about their bowel habits to help pick up any constipation that may be returning. Where pharmacists identify patients who have had problems with constipation prior to starting clozapine, they can recommend the use of a prophylactic laxative such as lactulose.
Case 3: A mother is concerned for her son who is talking to someone who is not there
A woman has been visiting the pharmacy for the past 3 months to collect a prescription for her son, aged 17 years*. In the past, the patient has collected his own medicine. Today the patient has presented with his mother; he looks dishevelled, preoccupied and does not speak to anyone in the pharmacy.
His mother beckons you to the side and expresses her concern for her son, explaining that she often hears him talking to someone who is not there. She adds that he is spending a lot of time in his room by himself and has accused her of tampering with his things. She is not sure what she should do and asks for advice.
What action can the pharmacist take?
It is important to reassure the mother that there is help available to review her son and identify if there are any problems that he is experiencing, but explain it is difficult to say at this point what he may be experiencing. Schizophrenia is a psychotic illness which has several symptoms that are classified as positive (e.g. hallucinations and delusions), negative (e.g. social withdrawal, self-neglect) and cognitive (e.g. poor memory and attention).
Many patients who go on to be diagnosed with schizophrenia will experience a prodromal period before schizophrenia is diagnosed. This may be a period where negative symptoms dominate and patients may become isolated and withdrawn. These symptoms can be confused with depression, particularly in younger people, though depression and anxiety disorders themselves may be prominent and treatment for these may also be needed. In this case, the patient’s mother is describing potential psychotic symptoms and it would be best for her son to be assessed. She should be encouraged to take her son to the GP for an assessment; however, if she is unable to do so, she can talk to the GP herself. It is usually the role of the doctor to refer patients for an assessment and to ensure that any other medical problems are assessed.
Three months later, the patient comes into the pharmacy and seems to be much more like his usual self, having been started on an antipsychotic. He collects his prescription for risperidone and mentions that he is very worried about his weight, which has increased since he started taking the newly prescribed tablets. Although he does not keep track of his weight, he has noticed a physical change and that some of his clothes no longer fit him.
What advice can the pharmacist provide?
Weight gain is common with many antipsychotics [10] . Risperidone is usually associated with a moderate chance of weight gain, which can occur early on in treatment [6] , [11] , [12] . As such, the National Institute for Health and Care Excellence recommends weekly monitoring of weight initially [13] . As well as weight gain, risperidone can be associated with an increased risk of diabetes and dyslipidaemia, which must also be monitored [6] , [11] , [12] . For example, the lipid profile and glucose should be assessed at 12 weeks, 6 months and then annually [12] .
The pharmacist should encourage the patient to attend any appointments for monitoring, which may be provided by his GP or mental health team, and to speak to his mental health team about his weight gain. If he agrees, the pharmacist could inform the patient’s mental health team of his weight gain and concerns on his behalf. It is important to tackle weight gain early on in treatment, as weight loss can be difficult to achieve, even if the medicine is changed.
The pharmacist should provide the patient with advice on healthy eating (e.g. eating a balanced diet with at least five fruit and vegetables per day) and exercising regularly (e.g. doing at least 150 minutes of moderate-intensity activity or 75 minutes of vigorous-intensity activity per week), and direct him to locally available services. The pharmacist can record the adverse effect on the patient’s medical record, which will help flag this in the future and thus help other pharmacists to intervene should he be prescribed risperidone again.
*All case studies are fictional.
Useful resources
- Mind — Schizophrenia
- Rethink Mental Illness — Schizophrenia
- Mental Health Foundation — Schizophrenia
- Royal College of Psychiatrists — Schizophrenia
- NICE guidance [CG178] — Psychosis and schizophrenia in adults: prevention and management
- NICE guidance [CG155] — Psychosis and schizophrenia in children and young people: recognition and management
- British Association for Psychopharmacology — Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology
About the author
Nicola Greenhalgh is lead pharmacist, Mental Health Services, North East London NHS Foundation Trust
[1] Chiu CC, Lu ML, Huang MC & Chen KP. Heavy smoking, reduced olanzapine levels, and treatment effects: a case report. Ther Drug Monit 2004;26(5):579–581. doi: 10.1097/00007691-200410000-00018
[2] de Leon J. Psychopharmacology: atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv 2004;55(5):491–493. doi: 10.1176/appi.ps.55.5.491
[3] Mayerova M, Ustohal L, Jarkovsky J et al . Influence of dose, gender, and cigarette smoking on clozapine plasma concentrations. Neuropsychiatr Dis Treat 2018;14:1535–1543. doi: 10.2147/NDT.S163839
[4] Ashir M & Petterson L. Smoking bans and clozapine levels. Adv Psychiatr Treat 2008;14(5):398–399. doi: 10.1192/apt.14.5.398b
[5] Young CR, Bowers MB & Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull 1998;24(3):381–390. doi: 10.1093/oxfordjournals.schbul.a033333
[6] Taylor D, Barnes TRE & Young AH. The Maudsley Prescribing Guidelines in Psychiatry . 13th edn. London: Wiley Blackwell; 2018
[7] Oke V, Schmidt F, Bhattarai B et al . Unrecognized clozapine-related constipation leading to fatal intra-abdominal sepsis — a case report. Int Med Case Rep J 2015;8:189–192. doi: 10.2147/IMCRJ.S86716
[8] Hibbard KR, Propst A, Frank DE & Wyse J. Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics 2009;50(4):416–419. doi: 10.1176/appi.psy.50.4.416
[9] Medicines and Healthcare products Regulatory Agency. Clozapine: reminder of potentially fatal risk of intestinal obstruction, faecal impaction, and paralytic ileus. 2020. Available from: https://www.gov.uk/drug-safety-update/clozapine-reminder-of-potentially-fatal-risk-of-intestinal-obstruction-faecal-impaction-and-paralytic-ileus (accessed April 2020)
[10] Leucht S, Cipriani A, Spineli L et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3
[11] Bazire S. Psychotropic Drug Directory . Norwich: Lloyd-Reinhold Communications LLP; 2018
[12] Cooper SJ & Reynolds GP. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016;30(8):717–748. doi: 10.1177/0269881116645254
[13] National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 2014. Available from: https://www.nice.org.uk/guidance/cg178 (accessed April 2020)
You might also be interested in…

Boots UK shuts online mental health service to new patients

Eating disorders: identification, treatment and support

Mental health considerations in older women
- Dermatology
- Gastroenterology
- Geriatric Medicine and Gerontology
- Gynecology and Obstetrics
- Heart and Vascular
- Neurology and Neurosurgery
- Ophthalmology
- Orthopaedics
- Otolaryngology–Head and Neck Surgery
- Physical Medicine and Rehabilitation
- Plastic and Reconstructive Surgery
- Psychiatry and Behavioral Sciences
- Pediatric Specialties
- Pediatric Diabetes and Endocrinology
- Pediatrics Florida
- Pediatric Gastroenterology and GI Surgery
- Pediatric Heart
- Pediatrics Maryland/DC
- Pediatric Neurology & Neurosurgery
- Pediatric Orthopaedics
- Physician Affiliations
- Health Care Technology
- High-Value Health Care
- Clinical Research Advancements
- Precision Medicine Excellence
- Patient Safety
Case Study Illustrates How Schizophrenia Can Often Be Overdiagnosed

Share Fast Facts
Study shows how schizophrenia can often be over diagnosed. Learn how. Click to Tweet
Study author Russell Margolis, director of the Johns Hopkins Schizophrenia Center, answers questions on misdiagnosis of the condition and reiterates the importance of thorough examination.
It’s not uncommon for an adolescent or young adult who reports hearing voices or seeing things to be diagnosed with schizophrenia, but using these reports alone can contribute to the disease being overdiagnosed, says Russell Margolis , clinical director of the Johns Hopkins Schizophrenia Center.
Many clinicians consider hallucinations as the sine qua non, or essential condition, of schizophrenia, he says. But even a true hallucination might be part of any number of disorders — or even within the range of normal. To diagnose a patient properly, he says, “There’s no substitute for taking time with patients and others who know them well. Trying to [diagnose] this in a compressed, shortcut kind of way leads to error.”
A case study he shared recently in the Journal of Psychiatric Practice illustrates the problem. Margolis, along with colleagues Krista Baker, schizophrenia supervisor at Johns Hopkins Bayview Medical Center, visiting resident Bianca Camerini, and Brazilian psychiatrist Ary Gadelha, described a 16-year-old girl who was referred to the Early Psychosis Intervention Clinic at Johns Hopkins Bayview for a second opinion concerning the diagnosis and treatment of suspected schizophrenia.
The patient made friends easily but had some academic difficulties. Returning to school in eighth grade after a period of home schooling, she was bullied, sexually groped and received texted death threats. She then began to complain of visions of a boy who harassed her, as well as three tall demons. The visions waxed and waned in relation to stress at school. The Johns Hopkins consultants determined that this girl did not have schizophrenia (or any other psychotic disorder), but that she had anxiety. They recommended psychotherapy and viewing herself as a healthy, competent person, instead of a sick one. A year later, the girl reported doing well: She was off medications and no longer complained of these visions.
Margolis answers Hopkins Brain Wise ’s questions.
Q: How are anxiety disorders mistaken for schizophrenia?
A: Patients often say they have hallucinations, but that doesn’t always mean they’re experiencing a true hallucination. What they may mean is that they have very vivid, distressing thoughts — in part because hallucinations have become a common way of talking about distress, and partly because they may have no other vocabulary with which to describe their experience.
Then, even if it is a true hallucination, there are features of the way psychiatry has come to be practiced that cause difficulties. Electronic medical records are often designed with questionnaires that have yes or no answers. Sometimes, whether the patient has hallucinations is murky, or possible — not yes or no. Also, one can’t make a diagnosis based just on a hallucination; the diagnosis of disorders like schizophrenia is based on a constellation of symptoms.
Q: How often are patients in this age range misdiagnosed?
A: There’s no true way to know the numbers. Among a very select group of people in our consultation clinic where questions have been raised, about half who were referred to us and said to have schizophrenia or a related disorder did not. That is not generalizable.
Q: Why does that happen?
A: There is a lack of attention to the context of symptoms and other details, and there’s also a tendency to take patients literally. If a patient complains about x, there’s sometimes a pressure to directly address x. In fact, that’s not appropriate medicine. It is very important to pay attention to a patient’s stated concerns, but to place these concerns in the bigger picture. Clinicians can go too far in accepting at face value something that needs more exploration.
Q: What lessons do you hope to impart by publishing this case?
A: I want it to be understood that the diagnosis of schizophrenia has to be made with care. Clinicians need to take the necessary time and obtain the necessary information so that they’re not led astray. Eventually, we would like to have more objective measures for defining our disorders so that we do not need to rely totally on a clinical evaluation.
Learn more about Russell Margolis’ research regarding the challenges of diagnosing schizophrenia .
- About Johns Hopkins Medicine
- Contact Johns Hopkins Medicine
- Centers & Departments
- Maps & Directions
- Find a Doctor
- Patient Care
- Terms & Conditions of Use
- Privacy Statement
Connect with Johns Hopkins Medicine
Join Our Social Media Communities >
Clinical Connection
- Otolaryngology—Head and Neck Surgery
- Contact Johns Hopkins
© The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. All rights reserved.
Privacy Policy and Disclaimer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 February 2022
Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics
- Christoph U. Correll ORCID: orcid.org/0000-0002-7254-5646 1 , 2 , 3 ,
- Amber Martin 4 ,
- Charmi Patel 5 ,
- Carmela Benson 5 ,
- Rebecca Goulding 6 ,
- Jennifer Kern-Sliwa 5 ,
- Kruti Joshi 5 ,
- Emma Schiller 4 &
- Edward Kim ORCID: orcid.org/0000-0001-8247-6675 7
Schizophrenia volume 8 , Article number: 5 ( 2022 ) Cite this article
15k Accesses
51 Citations
14 Altmetric
Metrics details
- Schizophrenia
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature review of English-language CPGs and synthesized current recommendations for the acute and maintenance management with antipsychotics. Searches for schizophrenia CPGs were conducted in MEDLINE/Embase from 1/1/2004–12/19/2019 and in guideline websites until 06/01/2020. Of 19 CPGs, 17 (89.5%) commented on first-episode schizophrenia (FES), with all recommending antipsychotic monotherapy, but without agreement on preferred antipsychotic. Of 18 CPGs commenting on maintenance therapy, 10 (55.6%) made no recommendations on the appropriate maximum duration of maintenance therapy, noting instead individualization of care. Eighteen (94.7%) CPGs commented on long-acting injectable antipsychotics (LAIs), mainly in cases of nonadherence (77.8%), maintenance care (72.2%), or patient preference (66.7%), with 5 (27.8%) CPGs recommending LAIs for FES. For treatment-resistant schizophrenia, 15/15 CPGs recommended clozapine. Only 7/19 (38.8%) CPGs included a treatment algorithm.
Similar content being viewed by others

A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials?

Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials
The efficacy and heterogeneity of antipsychotic response in schizophrenia: A meta-analysis
Introduction.
Schizophrenia is an often debilitating, chronic, and relapsing mental disorder with complex symptomology that manifests as a combination of positive, negative, and/or cognitive features 1 , 2 , 3 . Standard management of schizophrenia includes the use of antipsychotic medications to help control acute psychotic episodes 4 and prevent relapses 5 , 6 , whereas maintenance therapy is used in the long term after patients have been stabilized 7 , 8 , 9 . Two main classes of drugs—first- and second-generation antipsychotics (FGA and SGA)—are used to treat schizophrenia 10 . SGAs are favored due to the lower rates of adverse effects, such as extrapyramidal effects, tardive dyskinesia, and relapse 11 . However, pharmacologic treatment for schizophrenia is complicated because nonadherence is prevalent, and is a major risk factor for relapse 9 and poor overall outcomes 12 . The use of long-acting injectable (LAI) versions of antipsychotics aims to limit nonadherence-related relapses and poor outcomes 13 .
Patient treatment pathways and treatment choices are determined based on illness acuity/severity, past treatment response and tolerability, as well as balancing medication efficacy and adverse effect profiles in the context of patient preferences and adherence patterns 14 , 15 . Clinical practice guidelines (CPG) serve to inform clinicians with recommendations that reflect current evidence from meta-analyses of randomized controlled trials (RCTs), individual RCTs and, less so, epidemiologic studies, as well as clinical experience, with the goal of providing a framework and road-map for treatment decisions that will improve quality of care and achieve better patients outcomes. The use of clinical algorithms or other decision trees in CPGs may improve the ease of implementation of the evidence in clinical practice 16 . While CPGs are an important tool for mental health professionals, they have not been updated on a regular basis like they have been in other areas of medicine, such as in oncology. In the absence of current information, other governing bodies, healthcare systems, and hospitals have developed their own CPGs regarding the treatment of schizophrenia, and many of these have been recently updated 17 , 18 , 19 . As such, it is important to assess the latest guidelines to be aware of the changes resulting from consideration of updated evidence that informed the treatment recommendations. Since CPGs are comprehensive and include the diagnosis as well as the pharmacological and non-pharmacological management of individuals with schizophrenia, a detailed comparative review of all aspects of CPGs for schizophrenia would have been too broad a review topic. Further, despite ongoing efforts to broaden the pharmacologic tools for the treatment of schizophrenia 20 , antipsychotics remain the cornerstone of schizophrenia management 8 , 21 . Therefore, a focused review of guideline recommendations for the management of schizophrenia with antipsychotics would serve to provide clinicians with relevant information for treatment decisions.
To provide an updated overview of United States (US) national and English language international guidelines for the management of schizophrenia, we conducted a systematic literature review (SLR) to identify CPGs and synthesize current recommendations for pharmacological management with antipsychotics in the acute and maintenance phases of schizophrenia.
Systematic searches for the SLR yielded 1253 hits from the electronic literature databases. After removal of duplicate references, 1127 individual articles were screened at the title and abstract level. Of these, 58 publications were deemed eligible for screening at the full-text level, from which 19 were ultimately included in the SLR. Website searches of relevant organizations yielded 10 additional records, and an additional three records were identified by the state-by-state searches. Altogether, this process resulted in 32 records identified for inclusion in the SLR. Of the 32 sources, 19 primary CPGs, published/issued between 2004 and 2020, were selected for extraction, as illustrated in the PRISMA diagram (Fig. 1 ). While the most recent APA guideline was identified and available for download in 2020, the reference to cite in the document indicates a publication date of 2021.
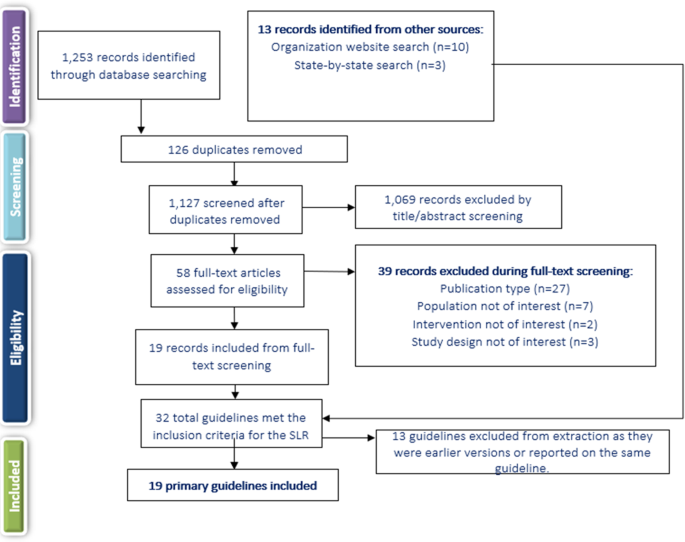
SLR systematic literature review.
Of the 19 included CPGs (Table 1 ), three had an international focus (from the following organizations: International College of Neuropsychopharmacology [CINP] 22 , United Nations High Commissioner for Refugees [UNHCR] 23 , and World Federation of Societies of Biological Psychiatry [WFSBP] 24 , 25 , 26 ); seven originated from the US; 17 , 18 , 19 , 27 , 28 , 29 , 30 , 31 , 32 three were from the United Kingdom (British Association for Psychopharmacology [BAP] 33 , the National Institute for Health and Care Excellence [NICE] 34 , and the Scottish Intercollegiate Guidelines Network [SIGN] 35 ); and one guideline each was from Singapore 36 , the Polish Psychiatric Association (PPA) 37 , 38 , the Canadian Psychiatric Association (CPA) 14 , the Royal Australia/New Zealand College of Psychiatrists (RANZCP) 39 , the Association Française de Psychiatrie Biologique et de Neuropsychopharmacologie (AFPBN) from France 40 , and Italy 41 . Fourteen CPGs (74%) recommended treatment with specific antipsychotics and 18 (95%) included recommendations for the use of LAIs, while just seven included a treatment algorithm Table 2 ). The AGREE II assessment resulted in the highest score across the CPGs domains for NICE 34 followed by the American Psychiatric Association (APA) guidelines 17 . The CPA 14 , BAP 33 , and SIGN 35 CPGs also scored well across domains.
Acute therapy
Seventeen CPGs (89.5%) provided treatment recommendations for patients experiencing a first schizophrenia episode 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 41 , but the depth and focus of the information varied greatly (Supplementary Table 1 ). In some CPGs, information on treatment of a first schizophrenia episode was limited or grouped with information on treating any acute episode, such as in the CPGs from CINP 22 , AFPBN 40 , New Jersey Division of Mental Health Services (NJDMHS) 32 , the APA 17 , and the PPA 37 , 38 , while the others provided more detailed information specific to patients experiencing a first schizophrenia episode 14 , 18 , 19 , 23 , 24 , 28 , 33 , 34 , 35 , 36 , 39 , 41 . The American Association of Community Psychiatrists (AACP) Clinical Tips did not provide any information on the treatment of schizophrenia patients with a first episode 29 .
There was little agreement among CPGs regarding the preferred antipsychotic for a first schizophrenia episode. However, there was strong consensus on antipsychotic monotherapy and that lower doses are generally recommended due to better treatment response and greater adverse effect sensitivity. Some guidelines recommended SGAs over FGAs when treating a first-episode schizophrenia patient (RANZCP 39 , Texas Medication Algorithm Project [TMAP] 28 , Oregon Health Authority 19 ), one recommended starting patients on an FGA (UNHCR 23 ), and others stated specifically that there was no evidence of any difference in efficacy between FGAs and SGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , Singapore guidelines 36 ), or did not make any recommendation (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , Schizophrenia Patient Outcomes Research Team [PORT] 30 , 31 ). The BAP 33 and WFBSP 24 noted that while there was probably no difference between FGAs and SGAs in efficacy, some SGAs (olanzapine, amisulpride, and risperidone) may perform better than some FGAs. The Schizophrenia PORT recommendations noted that while there seemed to be no differences between SGAs and FGAs in short-term studies (≤12 weeks), longer studies (one to two years) suggested that SGAs may provide benefits in terms of longer times to relapse and discontinuation rates 30 , 31 . The AFPBN guidelines 40 and Florida Medicaid Program guidelines 18 , which both focus on use of LAI antipsychotics, both recommended an SGA-LAI for patients experiencing a first schizophrenia episode. A caveat in most CPGs was that physicians and their patients should discuss decisions about the choice of antipsychotic and that the choice should consider individual patient factors/preferences, risk of adverse and metabolic effects, and symptom patterns 17 , 18 , 19 , 22 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 41 .
Most CPGs recommended switching to a different monotherapy if the initial antipsychotic was not effective or not well tolerated after an adequate antipsychotic trial at an appropriate dose 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 32 , 33 , 35 , 36 , 39 . For patients initially treated with an FGA, the UNHCR recommended switching to an SGA (olanzapine or risperidone) 23 . Guidance on response to treatment varied in the measures used but typically required at least a 20% improvement in symptoms (i.e. reduction in Positive and Negative Syndrome Scale or Brief Psychiatric Rating Scale scores) from pre-treatment levels.
Several CPGs contained recommendations on the duration of antipsychotic therapy after a first schizophrenia episode. The NJDMHS guidelines 32 recommended nine to 12 months; CINP 22 recommended at least one year; CPA 14 recommended at least 18 months; WFSBP 25 , the Italian guidelines 41 , and NICE 34 recommended 1 to 2 years; and the RANZCP 39 , BAP 33 , and SIGN 35 recommended at least 2 years. The APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy.
Twelve guidelines 14 , 18 , 22 , 24 , 28 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 (63.2%) discussed the treatment of subsequent/multiple episodes of schizophrenia (i.e., following relapse). These CPGs noted that the considerations guiding the choice of antipsychotic for subsequent/multiple episodes were similar to those for a first episode, factoring in prior patient treatment response, adverse effect patterns and adherence. The CPGs also noted that response to treatment may be lower and require higher doses to achieve a response than for first-episode schizophrenia, that a different antipsychotic than used to treat the first episode may be needed, and that a switch to an LAI is an option.
Several CPGs provided recommendations for patients with specific clinical features (Supplementary Table 1 ). The most frequently discussed group of clinical features was negative symptoms, with recommendations provided in the CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , APA 17 , and NJDMHS guidelines; 32 negative symptoms were the sole focus of the guidelines from the PPA 37 , 38 . The guidelines noted that due to limited evidence in patients with predominantly negative symptoms, there was no clear benefit for any strategy, but that options included SGAs (especially amisulpride) rather than FGAs (WFSBP 24 , CINP 22 , AFPBN 40 , SIGN 35 , NJDMHS 32 , PPA 37 , 38 ), and addition of an antidepressant (WFSBP 24 , UNHCR 23 , SIGN 35 , NJDMHS 32 ) or lamotrigine (SIGN 35 ), or switching to another SGA (NJDMHS 32 ) or clozapine (NJDMHS 32 ). The PPA guidelines 37 , 38 stated that the use of clozapine or adding an antidepressant or other medication class was not supported by evidence, but recommended the SGA cariprazine for patients with predominant and persistent negative symptoms, and other SGAs for those with full-spectrum negative symptoms. However, the BAP 33 stated that no recommendations can be made for any of these strategies because of the quality and paucity of the available evidence.
Some of the CPGs also discussed treatment of other clinical features to a limited degree, including depressive symptoms (CINP 22 , UNHCR 23 , CPA 14 , APA 17 , and NJDMHS 32 ), cognitive dysfunction (CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , and NJDMHS 32 ), persistent aggression (CINP 22 , WFSBP 24 , CPA 14 , AFPBN 40 , NICE 34 , SIGN 35 , BAP 33 , and NJDMHS 32 ), and comorbid psychiatric diagnoses (CINP 22 , RANZCP 39 , BAP 33 , APA 17 , and NJDMHS 32 ).
Fifteen CPGs (78.9%) discussed treatment-resistant schizophrenia (TRS); all defined it as persistent, predominantly positive symptoms after two adequate antipsychotic trials; clozapine was the unanimous first choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 . However, the UNHCR guidelines 23 , which included recommendations for treatment of refugees, noted that clozapine is only a reasonable choice in regions where white blood cell monitoring and specialist supervision are available, otherwise, risperidone or olanzapine are alternatives if they had not been used in the previous treatment regimen.
There were few options for patients who are resistant to clozapine therapy, and evidence supporting these options was limited. The CPA guidelines 14 therefore stated that no recommendation can be given due to inadequate evidence. Other CPGs discussed options (but noted there was limited supporting evidence), such as switching to olanzapine or risperidone (WFSBP 24 , TMAP 28 ), adding a second antipsychotic to clozapine (CINP 22 , NICE 34 , TMAP 28 , BAP 33 , Florida Medicaid Program 18 , Oregon Health Authority 19 , RANZCP 39 ), adding lamotrigine or topiramate to clozapine (CINP 22 , Florida Medicaid Program 18 ), combination therapy with two non-clozapine antipsychotics (Florida Medicaid Program 18 , NJDMHS 32 ), and high-dose non-clozapine antipsychotic therapy (BAP 33 , SIGN 35 ). Electroconvulsive therapy was noted as a last resort for patients who did not respond to any pharmacologic therapy, including clozapine, by 10 CPGs 17 , 18 , 19 , 22 , 24 , 28 , 32 , 35 , 36 , 39 .
Maintenance therapy
Fifteen CPGs (78.9%) discussed maintenance therapy to various degrees via dedicated sections or statements, while three others referred only to maintenance doses by antipsychotic agent 18 , 23 , 29 without accompanying recommendations (Supplementary Table 2 ). Only the Italian guideline provided no reference or comments on maintenance treatment. The CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 recommended keeping patients on the same antipsychotic and at the same dose on which they had achieved remission. Several CPGs recommended maintenance therapy at the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , and TMAP 28 ). The CPA 14 and SIGN 35 defined the lower dose as 300–400 mg chlorpromazine equivalents or 4–6 mg risperidone equivalents, and the Singapore guidelines 36 stated that the lower dose should not be less than half the original dose. TMAP 28 stated that given the relapsing nature of schizophrenia, the maintenance dose should often be close to the original dose. While SIGN 35 recommended that patients remain on the same antipsychotic that provided remission, these guidelines also stated that maintenance with amisulpride, olanzapine, or risperidone was preferred, and that chlorpromazine and other low-potency FGAs were also suitable. The BAP 33 recommended that the current regimen be optimized before any dose reduction or switch to another antipsychotic occurs. Several CPGs recommended LAIs as an option for maintenance therapy (see next section).
Altogether, 10/18 (55.5%) CPGs made no recommendations on the appropriate duration of maintenance therapy, noting instead that each patient should be considered individually. Other CPGs made specific recommendations: Both the Both BAP 33 and SIGN 35 guidelines suggested a minimum of 2 years, the NJDMHS guidelines 32 recommended 2–3 years; the WFSBP 25 recommended 2–5 years for patients who have had one relapse and more than 5 years for those who have had multiple relapses; the RANZCP 39 and the CPA 14 recommended 2–5 years; and the CINP 22 recommended that maintenance therapy last at least 6 years for patients who have had multiple episodes. The TMAP was the only CPG to recommend that maintenance therapy be continued indefinitely 28 .
Recommendations on the use of LAIs
All CPGs except the one from Italy (94.7%) discussed the use of LAIs for patients with schizophrenia to some extent. As shown in Table 3 , among the 18 CPGs, LAIs were primarily recommended in 14 CPGs (77.8%) for patients who are non-adherent to other antipsychotic administration routes (CINP 22 , UNHCR 23 , RANZCP 39 , PPA 37 , 38 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , TMAP 28 , NJDMHS 32 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ). Twelve CPGs (66.7%) also noted that LAIs should be prescribed based on patient preference (RANZCP 39 , CPA 14 , AFPBN 40 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , Schizophrenia PORT 30 , 31 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ).
Thirteen CPGs (72.2%) recommended LAIs as maintenance therapy 18 , 19 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 . While five CPGs (27.8%), i.e., AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and the Florida Medicaid Program 18 recommended LAIs specifically for patients experiencing a first episode. While the CPA 14 did not make any recommendations regarding when LAIs should be used, they discussed recent evidence supporting their use earlier in treatment. Five guidelines (27.8%, i.e., Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ) noted that evidence around LAIs was not sufficient to support recommending their use for first-episode patients. The AFPBN guidelines 40 also stated that LAIs (SGAs as first-line and FGAs as second-line treatment) should be more frequently considered for maintenance treatment of schizophrenia. Four CPGs (22.2%, i.e., CINP 22 , UNHCR 23 , Italian guidelines 41 , PPA guidelines 37 , 38 ) did not specify when LAIs should be used. The AACP guidelines 29 , which evaluated only LAIs, recommended expanding their use beyond treatment for nonadherence, suggesting that LAIs may offer a more convenient mode of administration or potentially address other clinical and social challenges, as well as provide more consistent plasma levels.
Treatment algorithms
Only Seven CPGs (36.8%) included an algorithm as part of the treatment recommendations. These included decision trees or flow diagrams that map out initial therapy, durations for assessing response, and treatment options in cases of non-response. However, none of these guidelines defined how to measure response, a theme that also extended to guidelines that did not include treatment algorithms. Four of the seven guidelines with algorithms recommended specific antipsychotic agents, while the remaining three referred only to the antipsychotic class.
LAIs were not consistently incorporated in treatment algorithms and in six CPGs were treated as a separate category of medicine reserved for patients with adherence issues or a preference for the route of administration. The only exception was the Florida Medicaid Program 18 , which recommended offering LAIs after oral antipsychotic stabilization even to patients who are at that point adherent to oral antipsychotics.
Benefits and harms
The need to balance the efficacy and safety of antipsychotics was mentioned by all CPGs as a basic treatment paradigm.
Ten CPGs provided conclusions on benefits of antipsychotic therapy. The APA 17 and the BAP 33 guidelines stated that antipsychotic treatment can improve the positive and negative symptoms of psychosis and leads to remission of symptoms. These CPGs 17 , 33 as well as those from NICE 34 and CPA 14 stated that these treatment effects can also lead to improvements in quality of life (including quality-adjusted life years), improved functioning, and reduction in disability. The CPA 14 and APA 17 guidelines noted decreases in hospitalizations with antipsychotic therapy, and the APA guidelines 17 stated that long-term antipsychotic treatment can also reduce mortality. The UNHCR 23 and the Italian 41 guidelines noted that early intervention increased positive outcomes. The WFSBP 24 , AFPBN 40 , CPA 14 , BAP 33 , APA 17 , and NJDMHS 32 affirmed that relapse prevention is a benefit of continued/maintenance treatment.
Some CPGs (WFSBP 24 , Italian 41 , CPA 14 , and SIGN 35 ) noted that reduced risk for extrapyramidal adverse effects and treatment discontinuation were potential benefits of SGAs vs. FGAs.
The risk of adverse effects (e.g., extrapyramidal, metabolic, cardiovascular, and hormonal adverse effects, sedation, and neuroleptic malignant syndrome) was noted by all CPGs as the major potential harm of antipsychotic therapy 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 . These adverse effects are known to limit long-term treatment and adherence 24 .
This SLR of CPGs for the treatment of schizophrenia yielded 19 most updated versions of individual CPGs, published/issued between 2004 and 2020. Structuring our comparative review according to illness phase, antipsychotic type and formulation, response to antipsychotic treatment as well as benefits and harms, several areas of consistent recommendations emerged from this review (e.g., balancing risk and benefits of antipsychotics, preferring antipsychotic monotherapy; using clozapine for treatment-resistant schizophrenia). On the other hand, other recommendations regarding other areas of antipsychotic treatment were mostly consistent (e.g., maintenance antipsychotic treatment for some time), somewhat inconsistent (e.g., differences in the management of first- vs multi-episode patients, type of antipsychotic, dose of antipsychotic maintenance treatment), or even contradictory (e.g., role of LAIs in first-episode schizophrenia patients).
Consistent with RCT evidence 43 , 44 , antipsychotic monotherapy was the treatment of choice for patients with first-episode schizophrenia in all CPGs, and all guidelines stated that a different single antipsychotic should be tried if the first is ineffective or intolerable. Recommendations were similar for multi-episode patients, but factored in prior patient treatment response, adverse effect patterns, and adherence. There was also broad consensus that the side-effect profile of antipsychotics is the most important consideration when making a decision on pharmacologic treatment, also reflecting meta-analytic evidence 4 , 5 , 10 . The risk of extrapyramidal symptoms (especially with FGAs) and metabolic effects (especially with SGAs) were noted as key considerations, which are also reflected in the literature as relevant concerns 4 , 45 , 46 , including for quality of life and treatment nonadherence 47 , 48 , 49 , 50 .
Largely consistent with the comparative meta-analytic evidence regarding the acute 4 , 51 , 52 and maintenance antipsychotic treatment 5 effects of schizophrenia, the majority of CPGs stated there was no difference in efficacy between SGAs and FGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , and Singapore guidelines 36 ), or did not make any recommendations (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , and Schizophrenia PORT 30 , 31 ); three CPGs (BAP 33 , WFBSP 24 , and Schizophrenia PORT 30 , 31 ) noted that SGAs may perform better than FGAs over the long term, consistent with a meta-analysis on this topic 53 .
The 12 CPGs that discussed treatment of subsequent/multiple episodes generally agreed on the factors guiding the choices of an antipsychotic, including that the decision may be more complicated and response may be lower than with a first episode, as described before 7 , 54 , 55 , 56 .
There was little consensus regarding maintenance therapy. Some CPGs recommended the same antipsychotic and dose that achieved remission (CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 ) and others recommended the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , TMAP 28 , CPA 14 , and SIGN 35 ). This inconsistency is likely based on insufficient data as well as conflicting results in existing meta-analyses on this topic 57 , 58 , 59 .
The 15 CPGs that discussed TRS all used the same definition for this condition, consistent with recent commendations 60 , and agreed that clozapine is the primary evidence-based treatment choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , reflecting the evidence base 61 , 62 , 63 . These CPGs also agreed that there are few options well supported by evidence for patients who do not respond to clozapine, with a recent meta-analysis of RCTs showing that electroconvulsive therapy augmentation may be the most evidence-based treatment option 64 .
One key gap in the treatment recommendations was how long patients should remain on antipsychotic therapy after a first episode or during maintenance therapy. While nine of the 17 CPGs discussing treatment of a first episode provided a recommended timeframe (varying from 1 to 2 years) 14 , 22 , 24 , 32 , 33 , 34 , 35 , 39 , 41 , the APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy. Similarly, six of the 18 CPGs discussing maintenance treatment recommended a specific duration of therapy (ranging from two to six years) 14 , 22 , 25 , 32 , 39 , while as many as 10 CPGs did not point to a firm end of the maintenance treatment, instead recommending individualized decisions. The CPGs not stating a definite endpoint or period of maintenance treatment after repeated schizophrenia episodes or even after a first episode of schizophrenia, reflects the different evidence types on which the recommendation is based. The RCT evidence ends after several years of maintenance treatment vs. discontinuation supporting ongoing antipsychotic treatment; however, naturalistic database studies do not indicate any time period after which one can safely discontinue maintenance antipsychotic care, even after a first schizophrenia episode 8 , 65 . In fact, stopping antipsychotics is associated not only with a substantially increased risk of hospitalization but also mortality 65 , 66 , 67 . In this sense, not stating an endpoint for antipsychotic maintenance therapy should not be taken as an implicit statement that antipsychotics should be discontinued at any time; data suggest the contrary.
A further gap exists regarding the most appropriate treatment of negative symptoms, such as anhedonia, amotivation, asociality, affective flattening, and alogia 1 , a long-standing challenge in the management of patients with schizophrenia. Negative symptoms often persist in patients after positive symptoms have resolved, or are the presenting feature in a substantial minority of patients 22 , 35 . Negative symptoms can also be secondary to pharmacotherapy 22 , 68 . Antipsychotics have been most successful in treating positive symptoms, and while eight of the CPGs provided some information on treatment of negative symptoms, the recommendations were generally limited 17 , 22 , 23 , 24 , 32 , 33 , 35 , 40 . Negative symptom management was a focus of the PPA guidelines, but the guidelines acknowledged that supporting evidence was limited, often due to the low number of patients with predominantly negative symptoms in clinical trials 37 , 38 . The Polish guidelines are also one of the more recently developed and included the newer antipsychotic cariprazine as a first-line option, which although being a point of differentiation from the other guidelines, this recommendation was based on RCT data 69 .
Another area in which more direction is needed is on the use of LAIs. While all but one of the 19 CPGs discussed this topic, the extent of information and recommendations for LAI use varied considerably. All CPGs categorized LAIs as an option to improve adherence to therapy or based on patient preference. However, 5/18 CPGs (27.8%) recommended the use of LAI early in treatment (at first episode: AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and Florida Medicaid Program 18 ) or across the entire illness course, while five others stated there was not sufficient evidence to recommend LAIs for these patients (Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ). The role of LAIs in first-episode schizophrenia was the only point where opposing recommendations were found across CPGs. This contradictory stance was not due to the incorporation of newer data suggesting benefits of LAIs in first episode and early-phase patients with schizophrenia 70 , 71 , 72 , 73 , 74 in the CPGs recommending LAI use in first-episode patients, as CPGs recommending LAI use were published between 2005 and 2020, while those opposing LAI use were published between 2011 and 2020. Only the Florida Medicaid CPG recommended LAIs as a first step equivalent to oral antipsychotics (OAP) after initial OAP response and tolerability, independent of nonadherence or other clinical variables. This guideline was also the only CPG to fully integrate LAI use in their clinical algorithm. The remaining six CPGs that included decision tress or treatment algorithms regarded LAIs as a separate paradigm of treatment reserved for nonadherence or patients preference rather than a routine treatment option to consider. While some CPGs provided fairly detailed information on the use of LAIs (AFPBN 40 , AACP 29 , Oregon Health Authority 19 , and Florida Medicaid Program 18 ), others mentioned them only in the context of adherence issues or patient preference. Notably, definitions of and means to determine nonadherence were not reported. One reason for this wide range of recommendations regarding the placement of LAIs in the treatment algorithm and clinical situations that prompt LAI use might be due to the fact that CPGs generally favor RCT evidence over evidence from other study designs. In the case of LAIs, there was a notable dissociation between consistent meta-analytic evidence of statistically significant superiority of LAIs vs OAPs in mirror-image 75 and cohort study designs 76 and non-significant advantages in RCTs 77 . Although patients in RCTs comparing LAIs vs OAPs were less severely ill and more adherent to OAPs 77 than in clinical care and although mirror-image and cohort studies arguably have greater external validity than RCTs 78 , CPGs generally disregard evidence from other study designs when RCT evidence exits. This narrow focus can lead to disregarding important additional data. Nevertheless, a most updated meta-analysis of all 3 study designs comparing LAIs with OAPs demonstrated consistent superiority of LAIs vs OAPs for hospitalization or relapse across all 3 designs 79 , which should lead to more uniform recommendations across CPGs in the future.
Only seven CPGs included treatment algorithms or flow charts to guide LAI treatment selection for patients with schizophrenia 17 , 18 , 19 , 24 , 29 , 35 , 40 . However, there was little commonality across algorithms beyond the guidance on LAIs mentioned above, as some listed specific treatments and conditions for antipsychotic switches, while others indicated that medication choice should be based on a patient’s preferences and responses, side effects, and in some cases, cost effectiveness. Since algorithms and flow charts facilitate the reception, adoption and implementation of guidelines, future CPGs should include them as dissemination tools, but they need to reflect the data and detailed text and be sufficiently specific to be actionable.
The systematic nature in the identification, summarization, and assessment of the CPGs is a strength of this review. This process removed any potential bias associated with subjective selection of evidence, which is not reproducible. However, only CPGs published in English were included and regardless of their quality and differing timeframes of development and publication, complicating a direct comparison of consensus and disagreement. Finally, based on the focus of this SLR, we only reviewed pharmacologic management with antipsychotics. Clearly, the assessment, other pharmacologic and, especially, psychosocial interventions are important in the management of individuals with schizophrenia, but these topics that were covered to varying degrees by the evaluated CPGs were outside of the scope of this review.
Numerous guidelines have recently updated their recommendations on the pharmacological treatment of patients with schizophrenia, which we have summarized in this review. Consistent recommendations were observed across CPGs in the areas of balancing risk and benefits of antipsychotics when selecting treatment, a preference for antipsychotic monotherapy, especially for patients with a first episode of schizophrenia, and the use of clozapine for treatment-resistant schizophrenia. By contrast, there were inconsistencies with regards to recommendations on maintenance antipsychotic treatment, with differences existing on type and dose of antipsychotic, as well as the duration of therapy. However, LAIs were consistently recommended, but mainly suggested in cases of nonadherence or patient preference, despite their established efficacy in broader patient populations and clinical scenarios in clinical trials. Guidelines were sometimes contradictory, with some recommending LAI use earlier in the disease course (e.g., first episode) and others suggesting they only be reserved for later in the disease. This inconsistency was not due to lack of evidence on the efficacy of LAIs in first-episode schizophrenia or the timing of the CPG, so that other reasons might be responsible, including possibly bias and stigma associated with this route of treatment administration. Lastly, gaps existed in the guidelines for recommendations on the duration of maintenance treatment, treatment of negative symptoms, and the development/use of treatment algorithms whenever evidence is sufficient to provide a simplified summary of the data and indicate their relevance for clinical decision making, all of which should be considered in future guideline development/revisions.
The SLR followed established best methods used in systematic review research to identify and assess the available CPGs for pharmacologic treatment of schizophrenia with antipsychotics in the acute and maintenance phases 80 , 81 . The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including use of a prespecified protocol to outline methods for conducting the review. The protocol for this review was approved by all authors prior to implementation but was not submitted to an external registry.
Data sources and search algorithms
Searches were conducted by two independent investigators in the MEDLINE and Embase databases via OvidSP to identify CPGs published in English. Articles were identified using search algorithms that paired terms for schizophrenia with keywords for CPGs. Articles indexed as case reports, reviews, letters, or news were excluded from the searches. The database search was limited to CPGs published from January 1, 2004, through December 19, 2019, without limit to geographic location. In addition to the database sources, guideline body websites and state-level health departments from the US were also searched for relevant CPGs published through June 2020. A manual check of the references of recent (i.e., published in the past three years), relevant SLRs and relevant practice CPGs was conducted to supplement the above searches and ensure and the most complete CPG retrieval.
This study did not involve human subjects as only published evidence was included in the review; ethical approval from an institution was therefore not required.
Selection of CPGs for inclusion
Each title and abstract identified from the database searches was screened and selected for inclusion or exclusion in the SLR by two independent investigators based on the populations, interventions/comparators, outcomes, study design, time period, language, and geographic criteria shown in Table 4 . During both rounds of the screening process, discrepancies between the two independent reviewers were resolved through discussion, and a third investigator resolved any disagreement. Articles/documents identified by the manual search of organizational websites were screened using the same criteria. All accepted studies were required to meet all inclusion criteria and none of the exclusion criteria. Only the most recent version of organizational CPGs was included for data extraction.
Data extraction and synthesis
Information on the recommendations regarding the antipsychotic management in the acute and maintenance phases of schizophrenia and related benefits and harms was captured from the included CPGs. Each guideline was reviewed and extracted by a single researcher and the data were validated by a senior team member to ensure accuracy and completeness. Additionally, each included CPG was assessed using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool. Following extraction and validation, results were qualitatively summarized across CPGs.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of the SLR are available from the corresponding author upon request.
Correll, C. U. & Schooler, N. R. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 16 , 519–534 (2020).
Article PubMed PubMed Central Google Scholar
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1 , 15067 (2015).
Article PubMed Google Scholar
Millan, M. J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15 , 485–515 (2016).
Article CAS PubMed Google Scholar
Huhn, M. et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 , 939–951 (2019).
Article CAS PubMed PubMed Central Google Scholar
Kishimoto, T., Hagi, K., Nitta, M., Kane, J. M. & Correll, C. U. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18 , 208–224 (2019).
Leucht, S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379 , 2063–2071 (2012).
Carbon, M. & Correll, C. U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 16 , 505–524 (2014).
Correll, C. U., Rubio, J. M. & Kane, J. M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17 , 149–160 (2018).
Emsley, R., Chiliza, B., Asmal, L. & Harvey, B. H. The nature of relapse in schizophrenia. BMC Psychiatry 13 , 50 (2013).
Correll, C. U. & Kane, J. M. Ranking antipsychotics for efficacy and safety in schizophrenia. JAMA Psychiatry 77 , 225–226 (2020).
Kane, J. M. & Correll, C. U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 12 , 345–357 (2010).
Kane, J. M., Kishimoto, T. & Correll, C. U. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12 , 216–226 (2013).
Correll, C. U. et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J. Clin. Psychiatry 77 , 1–24 (2016).
Remington, G. et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 62 , 604–616 (2017).
American Psychiatric Association. Treatment recommendations for patients with schizophrenia. Am. J. Psychiatry 161 , 1–56 (2004).
Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines, Field, M. J., & Lohr, K. N. (Eds.). Clinical Practice Guidelines: Directions for a New Program. (National Academies Press (US), 1990).
American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia , 3rd edn. (2021). https://doi.org/10.1176/appi.books.9780890424841 .
Florida Medicaid Drug Therapy Management Program. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults . (2020). Available at: https://floridabhcenter.org/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf .
Mental Health Clinical Advisory Group, Oregon Health Authority. Mental Health Care Guide for Licensed Practitioners and Mental Health Professionals. (2019). Available at: https://sharedsystems.dhsoha.state.or.us/DHSForms/Served/le7548.pdf .
Krogmann, A. et al. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 24 , 38–69 (2019).
Fountoulakis, K. N. et al. The report of the joint WPA/CINP workgroup on the use and usefulness of antipsychotic medication in the treatment of schizophrenia. CNS Spectr . https://doi.org/10.1017/S1092852920001546 (2020).
Leucht, S. et al. CINP Schizophrenia Guidelines . (CINP, 2013). Available at: https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf .
Ostuzzi, G. et al. Mapping the evidence on pharmacological interventions for non-affective psychosis in humanitarian non-specialised settings: A UNHCR clinical guidance. BMC Med. 15 , 197 (2017).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 13 , 318–378 (2012).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry 14 , 2–44 (2013).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int. J. Psychiatry Clin. Pract. 21 , 82–90 (2017).
Moore, T. A. et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J. Clin. Psychiatry 68 , 1751–1762 (2007).
Texas Medication Algorithm Project (TMAP). Texas Medication Algorithm Project (TMAP) Procedural Manual . (2008). Available at: https://jpshealthnet.org/sites/default/files/inline-files/tmapalgorithmforschizophrenia.pdf .
American Association of Community Psychiatrists (AACP). Clinical Tips Series, Long Acting Antipsychotic Medications. (2017). Accessed at: https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view .
Buchanan, R. W. et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36 , 71–93 (2010).
Kreyenbuhl, J. et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr. Bull. 36 , 94–103 (2010).
New Jersey Division of Mental Health Services. Pharmacological Practice Guidelines for the Treatment of Schizophrenia . (2005). Available at: https://www.state.nj.us/humanservices/dmhs_delete/consumer/NJDMHS_Pharmacological_Practice_Guidelines762005.pdf .
Barnes, T. R. et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 34 , 3–78 (2020).
National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. (2014). Available at: http://www.nice.org.uk/guidance/cg178 .
Scottish Intercollegiate Guidelines Network (SIGN). Management of Schizophrenia: A National Clinical Guideline . (2013). Available at: https://www.sign.ac.uk/assets/sign131.pdf .
Verma, S. et al. Ministry of Health Clinical Practice Guidelines: Schizophrenia. Singap. Med. J. 52 , 521–526 (2011).
CAS Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 1. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 1. 53 , 497–524 (2019).
Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 2. 53 , 525–540 (2019).
Galletly, C. et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust. N. Z. J. Psychiatry 50 , 410–472 (2016).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13 , 340 (2013).
De Masi, S. et al. The Italian guidelines for early intervention in schizophrenia: development and conclusions. Early Intervention Psychiatry 2 , 291–302 (2008).
Article Google Scholar
Barnes, T. R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 25 , 567–620 (2011).
Correll, C. U. et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74 , 675–684 (2017).
Galling, B. et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16 , 77–89 (2017).
Pillinger, T. et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 , 64–77 (2020).
Rummel-Kluge, C. et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 38 , 167–177 (2012).
Angermeyer, M. C. & Matschinger, H. Attitude of family to neuroleptics. Psychiatr. Prax. 26 , 171–174 (1999).
CAS PubMed Google Scholar
Dibonaventura, M., Gabriel, S., Dupclay, L., Gupta, S. & Kim, E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12 , 20 (2012).
McIntyre, R. S. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J. Clin. Psychiatry 70 (Suppl 3), 5–11 (2009).
Tandon, R. et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: a worldwide, cross-sectional, web-based survey. Ann. Gen. Psychiatry 19 , 42 (2020).
Zhang, J. P. et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 16 , 1205–1218 (2013).
Zhu, Y. et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 4 , 694–705 (2017).
Kishimoto, T. et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol. Psychiatry 18 , 53–66 (2013).
Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174 , 927–942 (2017).
Samara, M. T., Nikolakopoulou, A., Salanti, G. & Leucht, S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr. Bull. 45 , 639–646 (2019).
Zhu, Y. et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 27 , 835–844 (2017).
Bogers, J. P. A. M., Hambarian, G., Michiels, M., Vermeulen, J. & de Haan, L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. A systematic review and meta-analysis. Schizophr. Bull. Open 46 , Suppl 1 S326 (2020).
Tani, H. et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology 45 , 887–901 (2020).
Uchida, H., Suzuki, T., Takeuchi, H., Arenovich, T. & Mamo, D. C. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr. Bull. 37 , 788–799 (2011).
Howes, O. D. et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174 , 216–229 (2017).
Masuda, T., Misawa, F., Takase, M., Kane, J. M. & Correll, C. U. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry 76 , 1052–1062 (2019).
Siskind, D., McCartney, L., Goldschlager, R. & Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 209 , 385–392 (2016).
Siskind, D., Siskind, V. & Kisely, S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can. J. Psychiatry 62 , 772–777 (2017).
Wang, G. et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J. Psychiatr. Res. 105 , 23–32 (2018).
Tiihonen, J., Tanskanen, A. & Taipale, H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am. J. Psychiatry 175 , 765–773 (2018).
Taipale, H. et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res . 197 , 274–280 (2018).
Taipale, H. et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 19 , 61–68 (2020).
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19 (Suppl 1), 38–52 (2014).
PubMed Google Scholar
Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 , 625–639 (2018).
Kane, J. M. et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry 77 , 1217–1224 (2020).
Schreiner, A. et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr. Res. 169 , 393–399 (2015).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72 , 822–829 (2015).
Tiihonen, J. et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 168 , 603–609 (2011).
Zhang, F. et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr. Dis. Treat. 11 , 657–668 (2015).
Kishimoto, T., Nitta, M., Borenstein, M., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J. Clin. Psychiatry 74 , 957–965 (2013).
Kishimoto, T. et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr. Bull. 44 , 603–619 (2018).
Kishimoto, T. et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr. Bull. 40 , 192–213 (2014).
Kane, J. M., Kishimoto, T. & Correll, C. U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 66 , S37–41 (2013).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8 , 387–404 (2021).
Cook, D. J., Mulrow, C. D. & Haynes, R. B. Systematic reviews: synthesis of best evidence for clinical decisions. Ann. Intern. Med. 126 , 376–380 (1997).
Higgins, J. P. T. & Green, S. Cochrane Collaboration Handbook for Systematic Reviews of Interventions . (The Cochrane Collaboration and John Wiley & Sons, Ltd, 2008).
Download references
Acknowledgements
This study received financial funding from Janssen Scientific Affairs.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
The Zucker Hillside Hospital, Department of Psychiatry, Northwell Health, Glen Oaks, NY, USA
- Christoph U. Correll
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, NY, USA
Charité Universitätsmedizin Berlin, Department of Child and Adolescent Psychiatry, Berlin, Germany
Evidera, Waltham, MA, USA
Amber Martin & Emma Schiller
Janssen Scientific Affairs, LLC, Titusville, NJ, USA
Charmi Patel, Carmela Benson, Jennifer Kern-Sliwa & Kruti Joshi
Goulding HEOR Consulting Inc., Vancouver, BC, Canada
Rebecca Goulding
Biohaven Pharmaceuticals, New Haven, CT, USA
You can also search for this author in PubMed Google Scholar
Contributions
C.C., A.M., R.G., C.P., C.B., K.J., J.K.S., E.S. and E.K. contributed to the conception and the design of the study. A.M., R.G. and E.S. conducted the literature review, including screening, and extraction of the included guidelines. All authors contributed to the interpretations of the results for the review; A.M. and C.C. drafted the manuscript and all authors revised it critically for intellectual content. All authors gave their final approval of the completed manuscript.
Corresponding author
Correspondence to Christoph U. Correll .
Ethics declarations
Competing interests.
C.C. has received personal fees from Alkermes plc, Allergan plc, Angelini Pharma, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Inc, Janssen Pharmaceutica/Johnson & Johnson, LB Pharma International BV, H Lundbeck A/S, MedAvante-ProPhase, Medscape, Neurocrine Biosciences, Noven Pharmaceuticals, Inc, Otsuka Pharmaceutical Co, Inc, Pfizer, Inc, Recordati, Rovi, Sumitomo Dainippon Pharma, Sunovion Pharmaceuticals, Inc, Supernus Pharmaceuticals, Inc, Takeda Pharmaceutical Company Limited, Teva Pharmaceuticals, Acadia Pharmaceuticals, Inc, Axsome Therapeutics, Inc, Indivior, Merck & Co, Mylan NV, MedInCell, and Karuna Therapeutics and grants from Janssen Pharmaceutica, Takeda Pharmaceutical Company Limited, Berlin Institute of Health, the National Institute of Mental Health, Patient Centered Outcomes Research Institute, and the Thrasher Foundation outside the submitted work; receiving royalties from UpToDate; and holding stock options in LB Pharma. A.M., R.G., and E.S. were all employees of Evidera at the time the study was conducted on which the manuscript was based. C.P., C.B., K.J., J.K.S., and E.K. were all employees of Janssen Scientific Affairs, who hold stock/shares, at the time the study was conducted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Correll, C.U., Martin, A., Patel, C. et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophr 8 , 5 (2022). https://doi.org/10.1038/s41537-021-00192-x
Download citation
Received : 26 February 2021
Accepted : 02 November 2021
Published : 24 February 2022
DOI : https://doi.org/10.1038/s41537-021-00192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Psychosis superspectrum ii: neurobiology, treatment, and implications.
- Roman Kotov
- William T. Carpenter
- Katherine G. Jonas
Molecular Psychiatry (2024)
Sex Differences Between Female and Male Individuals in Antipsychotic Efficacy and Adverse Effects in the Treatment of Schizophrenia
- Megan Galbally
- Karen Wynter
- Susanna Every-Palmer
CNS Drugs (2024)
Antipsychotic Discontinuation through the Lens of Epistemic Injustice
- Helene Speyer
- Lene Falgaard Eplov
Community Mental Health Journal (2024)
Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery
- Celso Arango
- Andrea Fagiolini
BMC Psychiatry (2023)
Machine learning methods to predict outcomes of pharmacological treatment in psychosis
- Lorenzo Del Fabro
- Elena Bondi
- Paolo Brambilla
Translational Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Editorial Policy
- Privacy and Cookie Policy

- What is Schizophrenia?
- Modern Treatments
- Who Is At Risk?
- Facts and Figures
Information Sheets
- Schizophrenia and dangerous behaviour
- How is schizophrenia diagnosed?
- A brief history of schizophrenia
- Recovery strategies
- Disclosure – telling other people about your schizophrenia
- Can you recover from schizophrenia?
- Coping with stress
- Managing medication
- Preparing for relapses
- Coping with side effects of medication
- Schizophrenia and diet
- Organising your time
- Self monitoring your schizophrenia
- Understanding voices
- Coping with voices
- Understanding negative symptoms
- Treatments for negative symptoms
- Self help for negative symptoms
- Help from the Jobcentre
- Writing a CV (resumé)
- What kind of work can I do?
- Sources of help for job searching
- Volunteering
- Work experience
- Job Interview techniques
- Holding down a job
- Case study: Martin’s story
- Information for carers
- Self Help for Paranoia
- Treatments for Paranoia in Schizophrenia
- Understanding Paranoia
- Information for doctors and health workers
- Staying out of debt
- Dealing with existing debt
- Schizophrenia and Physical Health
- Exercise and Schizophrenia
- Schizophrenia and street drugs
- Schizophrenia and Alcohol
- Depression and Schizophrenia
- What can be done about depression in schizophrenia
- Labelling in Schizophrenia
- Schizophrenia and Language
- Stigma in Schizophrenia
- The Anti-Psychiatry Movement
- Cognitive Symptoms of Schizophrenia
- Schizophrenia: A Christian Perspective
- Religious and Spiritual Delusions in Schizophrenia
- Sleep problems
- The Science of Sleep in Schizophrenia
- Richard Dadd
- Ivor Gurney
- Daniel McNaghten
- Henry J Deutschendorf
- Dr William Chester Minor
- Recent Developments in the Treatment of Schizophrenia
- Guidance for Journalists
Get Involved
If you would like to get involved with Living with Schizophrenia’s work then please leave your details.
We are always looking for people to write about their experiences of schizophrenia, to contribute ideas and tips and oversee our work.
Leave your email and location and details of how schizophrenia has affected you and we will be in touch.
Case Study: Schizophrenia and Work: Martin’s Story
Martin had been out of work for several years following a prolonged psychotic episode which began when he was studying at university. He desperately wanted to get into work but found that employers treated his prolonged absence “on the sick” with suspicion. He thought that if he could do a period of work experience that would show prospective employers that he was capable of working again but he was afraid that if he did it might affect his benefits.
So Martin made an appointment to see the Disability Employment Advisor at the Jobcentre to discuss his plans. She was understanding and helpful and explained that a work placement would not affect his benefits as long as it was done as part of the Jobcentre’s own scheme. She also told him that the scheme would pay his travel-to work expenses while he was on the placement.
Job-searching
Next Martin researched local employers using the internet and the local press, looking for companies that might have vacancies in the sort of clerical and administrative work he thought he could do. Then he called the companies by ‘phone and speaking to the person on the switchboard checked that he had the correct postal address for them and asked the name of the person in charge of recruiting. It is vital to be able to write to a named person rather than just the Human Resources Manager.
Martin had already spent a lot of time on his CV so now he compiled a covering letter to go with it. It took him about a month to work up his CV and covering letter using books that he got from the local library. He also managed to get advice from a local back-to-work scheme recommended by the Disability Employment Advisor at the Jobcentre. Martin knew that it was essential that his letter and CV had the maximum impact.
Martin sent his CV and letter off to six employers and then waited about a week before calling them up on the ‘phone. He asked to speak to the person he had written to but if the person on the switchboard asked the reason for his call he simply said that he was calling to follow up a letter he had written.
After approaching about 20 employers in this way he finally found one who said there could be an opening for work experience in a couple of months time. So over the next three months Martin kept in touch with the company by ‘phone once a month just to let them know that he was still keen on coming to work for them.
The interview
Finally the company asked him in for an interview. Before going to the interview Martin prepared really well in advance by researching the company well and trying to anticipate the sorts of questions he would be asked. He also went to the local library and took out some books on interview techniques and managed to get on a one day course on interview skills that the Jobcentre had told him about. This included a mock interview which he found particularly useful.
The day of the interview arrived and Martin was very nervous but he was up early and washed and dressed. To be sure of being on time he left an hour early and checked out the location of the office. Then he went to Starbucks for a coffee while he waited. This gave him an opportunity to flick through his notes and prepare on some of the answers he had been working on. He made sure that he was punctual and well groomed and did his best to present himself well at the interview.
Despite being really well prepared walking through the front door of the office was one of the hardest things that he had done for years. But the receptionist was polite and could not have been more helpful. She made him feel welcome and even offered him a coffee (which he declined).
The Human Resources Manager who interviewed Martin was very professional but quickly put him at his ease. He asked questions about his education at school, his hobbies and pastimes and his qualifications and then came the bit that Martin had been dreading when the HR Manager asked him why he had dropped out of college. Martin explained that he had had a breakdown caused by too much stress while he was at college. He went on to explain that although it was a bad breakdown it was behind him now and that with the help of his family and friends and his doctor he had been able to make a really strong recovery. He also explained that in some ways the experience had made him a stronger person and that he had matured as a result of it.
As the end of the interview approached Martin was sure that he had flunked it but the interviewer told him that he had been successful and asked him to start on Monday. Martin was delighted to be offered a period of three months unpaid work experience during which he would work for two days a week at their local office doing clerical and administrative work.
Martin was walking on air when he left the office. All his hard work had been worth it.
The next day Martin called the Disability Employment Advisor at the local Jobcentre to tell them about the offer and see how his benefits would be affected. She confirmed that his benefits wouldn’t be affected as long as he only worked for 16 hours a week.
The placement
For the next three months Martin worked hard at his placement. He made sure that he got all the basics right: being punctual and well groomed every day. At work he was helpful and got on well with the other workers. Although he was very shy at first he soon learned the importance of making small talk with his colleagues and building good working relationships.
As the end of his placement approached Martin wondered if he would be offered a permanent position. He asked the HR Manager about this but sadly he was told that there were no permanent vacancies at that time so when the end of his placement came Martin had mixed feelings. On the one hand he was disappointed that the work experience had not turned into a permanent job but on the other hand he had had three months experience in the workplace and had something to put on his CV to demonstrate to other employers that he could work. And most importantly he had that all important reference from a well respected local employer.
But that isn’t quite the end of the story. Martin continued searching for a job without success for another six months but continued to keep in touch with the HR Manager he had worked for during his work experience. One day he saw in the local press that they were advertising for a clerical assistant so he called them and explained that he was still jobsearching and would be available for this position. The HR Manager was very pleased to hear from him and said that he would call him back. The next day Martin got a call asking him to go in for an interview straight away and was offered the job.
Martin called the Jobcentre Plus helpline and found out what benefits he would be entitled to while he was working and was pleased to find out that he would be better off in work.
Martin has now been employed in his new job for two years and is delighted to be living an independent lifestyle free of the benefits culture he was in before. It has had its difficulties though. For instance Martin found that his illness had left him emotionally very sensitive and that he found it difficult to cope if his work was criticised. But he knew that this was something he had to learn to live with and gradually he managed to learn new social skills that helped him to cope better and at the same time helped him in other areas of his life.
Martin has enjoyed the structure that the new job has brought to his life. He enjoys the work and the social contact that the job entails. He has made new friends and above all his self-esteem has grown vastly. Now when people ask him what he does for a living he no longer has to say that he is unemployed.
Some Key Points from Martin’s Story:
- Research the local job market really well
- Before writing to a firm call to check the postal address.
- Find out the name of the person in charge of recruitment. Writing to a named person makes sure your letter gets read.
- You can’t spend enough time preparing your CV and cover letter. Get as much help as you can from books, the library etc.
- When making follow up calls avoid Mondays and Fridays as these are busy days for people in business. Similarly don’t call too early in the morning or after 3.30 pm and don’t call around lunchtime.
- When making follow up calls be prepared for few false starts but use these to develop your technique. Treat the first half a dozen calls as practice calls.
- Don’t pester firms with too frequent follow up calls. Once every three weeks is about right.
- Be prepared for disappointment and don’t feel let down by it.
- Before going for an interview research the firm really well. Google and Google News and the local press are useful sources.
- It is perfectly normal to be nervous at an interview. Try to minimise the nerves by making sure you have planned and prepared well and getting a good night’s sleep beforehand.
- At the interview you may be asked about your illness. Be honest but there is no need to disclose your diagnosis at this stage unless you are asked directly: a broad brush explanation such as “a breakdown” is sufficient.

LWS Speaking Out
Read what Living with Schizophrenia has to say about topical issues in mental health
Schizophrenia and Cancer
Out of area placements, schizophrenia and heat related deaths, schizophrenia is a major cause of homelessness, shortage of doctors in the nhs, speaking out archives.

Living with schizophrenia was set up by people who have direct personal experience of the condition using their own personal funds and relies on donations to continue its work. We do not get grants from any public body or commercial organisation: we rely on people like you supporting our work.
Subscribe to our mailing list
View our Privacy and Cookie Policy .
Living with Schizophrenia is a trading style of LWS (UK) CIC a Community Interest Company registered in England no. 7492057 Copyright © 2024 Living With Schizophrenia . All rights reserved. Web Design by Priority Pixels . Terms of use , Privacy and Cookie Policy , Website acceptable use policy
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Understanding Schizophrenia: A Case Study

Schizophrenia is characterized mainly, by the gross distortion of reality, withdrawal from social interaction, disorganization and fragmentation of perception, thoughts and emotions. Insight is an important concept in clinical psychiatry, a lack of insight is particularly common in schizophrenia patient. Previous studies reported that between 50-80% of patients with schizophrenia do not believe, they have a disorder. By the help of psychological assessment, we can come to know an individual's problems especially in cases, where patient is hesitant or has less insight into illness. Assessment is also important for the psychological management of the illness. Knowing the strengths and weaknesses of that particular individual with psychological analysis tools can help to make better plan for the treatment. The present study was designed to assess the cognitive functioning, to elicit severity of psychopathology, understanding diagnostic indicators, personality traits that make the individual vulnerable to the disorder and interpersonal relationship in order to plan effective management. Schizophrenia is a chronic disorder, characterized mainly by the gross distortion of reality, withdrawal from social interaction, and disorganization and fragmentation of perception, thought and emotion. Approximately, 1% world population suffering with the problem of Schizophrenia. Both male and female are almost equally affected with slight male predominance. Schizophrenia is socioeconomic burden with suicidal rate of 10% and expense of 0.02-1.65% of GDP spent on treatment. Other co-morbid factors associated with Schizophrenia are diabetes, Obesity, HIV infection many metabolic disorders etc. Clinically, schizophrenia is a syndrome of variables symptoms, but profoundly disruptive, psychopathology that involves cognition, emotion, perception, and other aspects of behavior. The expression of these manifestations varies across patients and over the time, but the effect of the illness is always severe and is usually long-lasting. Patients with schizophrenia usually get relapse after treatment. The most common cause for the relapse is non-adherent with the medication. The relapse rate of schizophrenia increases later time on from 53.7% at 2 years to
Related Papers
Bangladesh Journal of Psychiatry
Luna Krasota Nur Laila
Schizophrenia is a chronic psychiatric illness with high rate of relapse which is commonly associated with noncompliance of medicine, as well as stress and high expressed emotions. The objective of the study was to determine the factors of relapse among the schizophrenic patients attending in outpatient departments of three tertiary level psychiatric facilities in Bangladesh. This was a cross sectional study conducted from July, 2001 to June, 2002. Two hundred patients including both relapse and nonrelapse cases of schizophrenia and their key relatives were included by purposive sampling. The results showed no statistically significant difference in terms of relapse with age, sex, religion, residence, occupation and level of education (p>0.05), but statistically significant difference was found with marital status and economic status (p<0.01). The proportion of non-compliance was found to be 80% and 14%, of high expressed emotion was 17% and 2% and of the occurrence of stressf...
Ashok Kumar Patel
BMC Psychiatry
Bonginkosi Chiliza
Revista Brasileira de Psiquiatria
Helio Elkis
OBJECTIVES: The heterogeneity of clinical manifestations in schizophrenia has lead to the study of symptom clusters through psychopathological assessment scales. The objective of this study was to elucidate clusters of symptoms in patients with refractory schizophrenia which may also help to assess the patients' therapeutical response. METHODS: Ninety-six treatment resistant patients were evaluated by the anchored version Brief Psychiatric Rating Scale (BPRS-A) as translated into Portuguese. The inter-rater reliability was 0.80. The 18 items of the BPRS-A were subjected to exploratory factor analysis with Varimax rotation. RESULTS: Four factors were obtained: Negative/Disorganization, composed by emotional withdrawal, disorientation, blunted affect, mannerisms/posturing, and conceptual disorganization; Excitement, composed of excitement, hostility, tension, grandiosity, and uncooperativeness, grouped variables that evoke brain excitement or a manic-like syndrome; Positive, compo...
Nicholas Tarrier
Annals of Clinical and Laboratory Research
James Mwaura
Sou Agarwal
Schizophrenia Bulletin
Joseph Goldberg
International journal of mental health nursing
Inayat ullah Shah
Despite a large body of research evaluating factors associated with the relapse of psychosis in schizophrenia, no studies in Pakistan have been undertaken to date to identify any such factors, including specific cultural factors pertinent to Pakistan. Semistructured interviews and psychometric measures were undertaken with 60 patients diagnosed with schizophrenia (49 male and 11 female) and their caregivers at four psychiatric hospitals in the Peshawar region in Pakistan. Factors significantly associated with psychotic relapse included treatment non-adherence, comorbid active psychiatric illnesses, poor social support, and high expressed emotion in living environments (P < 0.05). The attribution of symptoms to social and cultural values (97%) and a poor knowledge of psychosis by family members (88%) was also prevalent. In addition to many well-documented factors associated with psychotic relapse, beliefs in social and cultural myths and values were found to be an important, and p...
Octavian Vasiliu
RELATED PAPERS
Jurnal Niara
Bunga Utami
Energy Conversion and Management
Nelson Rodriguez
Dr. Uday Kumar Umesan
David Pecknold
Sinais de Cena
José Oliveira Barata
2019 15th International Conference on eScience (eScience)
Spiros Koulouzis
Engr. MD. ANISUR RAHMAN ~ TiTU, M.Sc(2nd)., FIEB
Australasian Road Safety Conference, 1st, 2015, Gold Coast, Queensland, Australia
Percival tavares da silva
james olayi
International journal of community medicine and public health
deepak gurung
Environmental and Experimental Botany
Pia Parolin
JURING (Journal for Research in Mathematics Learning)
Arnida Sari
Experimental Cell Research
Ruth Gotian
买美国爱荷华大学毕业证 iowa毕业证书研究生学位证书学历认证报告原版一模一样
Remote Sensing
hicham bahi
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
CASE REPORT article
Early-onset schizophrenia with predominantly negative symptoms: a case study of a drug-naive female patient treated with cariprazine.

- Institute of Genomic Medicine and Rare Disorders, Semmelweis University, Budapest, Hungary
Schizophrenia is a chronic and severe mental disorder characterized by positive, negative, and cognitive symptoms. Negative symptoms are usually present from the prodromal phase; early diagnosis and management of negative symptoms is a major health concern since an insidious onset dominated by negative symptoms is associated with a worse outcome. Antipsychotic medications, which are effective for treating positive symptoms, are generally ineffective for treating negative or cognitive symptoms. We present a 23-year-old woman showing severe symptoms at her first visit to our department. The patient’s parents reported that their daughter had experienced several years of psychosocial decline and putative psychiatric symptoms, but no medical attention had been previously sought; as such, the diagnosis of schizophrenia with predominantly negative symptoms was very much delayed. Early onset of schizophrenia, longer duration of untreated psychosis, and severe negative symptoms, which have limited treatment options, suggested a poor prognosis. We initiated monotherapy with cariprazine, a novel antipsychotic that has recently been proven efficacious in treating schizophrenia with predominantly negative symptoms. This report describes a 52-week cariprazine treatment regimen and follows the patient’s impressive clinical improvement confirmed by PANSS and CGI scores, and psychological tests.
Schizophrenia is a severe, chronic, and heterogeneous mental disorder that often has debilitating long-term outcomes. Its lifetime prevalence rate is estimated to be approximately 1% worldwide in the adult population ( Lehman et al., 2010 ). Onset generally occurs in late adolescence or early adulthood, with an average age of 18 years for men and 25 years for women. 1 The term early-onset schizophrenia (EOS) is used to refer to patients who are diagnosed with the disorder before this age. EOS is a severe, frequently disabling, and chronic condition with a prevalence approaching 0.5% in those younger than 18 years ( Hafner and Van der Heiden, 1997 ).
Schizophrenia is accompanied by a distortion of personality that affects fundamental mental and social functions, making everyday life extremely difficult for patients. Clinical symptoms are often classified in three main domains: positive symptoms, such as hallucinations, delusions, suspiciousness/persecution; negative symptoms, such as emotional withdrawal, blunted affect, and passive social withdrawal; and cognitive symptoms, such as impaired perception, learning, thinking, and memorizing. EOS may be accompanied by greater symptom severity, premorbid developmental impairment, ‘soft’ neurological signs (eg, clumsiness, motor incoordination), and a higher rate of substance abuse ( Hsiao and McClellan, 2008 ; Clemmensen et al., 2012 ; Immonen et al., 2017 ). Accordingly, diagnosis of EOS is often difficult and frequently delayed since onset is more commonly insidious than acute, which makes it difficult to differentiate EOS from underlying cognitive deficits, premorbid functional impairment, or other abnormalities ( Russell, 1994 ; Bartlet, 2014 ). Given this common delay in recognition of the disorder, the duration of untreated psychosis is often very long, further contributing to a poor outcome ( Penttila et al., 2014 ).
Although various hypotheses have been developed, the etiopathogenesis of schizophrenia and EOS is not fully understood ( McGuffin, 2004 ; Klosterkotter et al., 2011 ). 2 Among the rising and falling neurochemical theories, the dopamine hypothesis has remained a primary hypothesis guiding the treatment of schizophrenia. There are four dopaminergic pathways in the human brain: the mesolimbic, the mesocortical, the tuberoinfundibular, and the nigrostriatal. Positive symptoms of schizophrenia are associated with the hyperdopaminergic state of D 2 receptors in the mesolimbic area, while negative and cognitive symptoms are believed to be related to the hypodopaminergic dysregulation of the prefrontal cortex ( Stahl, 2003 ).
Negative symptoms of schizophrenia, which affect up to 60% of patients with schizophrenia ( Rabinowitz et al., 2013 ), form a complex clinical constellation of symptoms that challenge both diagnosis and treatment. By definition, negative symptoms mean the absence of normal functions. Negative symptoms are classified by their etiology as primary negative symptoms, which are core features of the disease itself, and secondary negative symptoms, which are consequences of positive symptoms, antipsychotic treatment, depression or extrapyramidal side effects. Five constructs have been accepted by general consensus as key aspects of negative symptoms: blunted affect, alogia, anhedonia, asociality, and avolition ( Marder and Galderisi, 2017 ). Patients with predominant negative symptoms lose their motivation, cannot function at school or work, and their interpersonal relationships severely decay. Due to impaired daily functioning and social amotivation, they may need constant care.
Although early intervention is associated with improvement in negative symptoms ( Boonstra et al., 2012 ), this may be challenging since negative symptoms develop slowly and may be difficult to detect or differentiate from other clinical features ( Kirkpatrick et al., 2001 ; Galderisi et al., 2018 ). Moreover, a more insidious onset predicts poorer outcome and more severe negative symptoms ( Kao and Liu, 2010 ; Immonen et al., 2017 ; Murru and Carpiniello, 2018 ). Diagnosis of patients with predominantly negative symptoms (lacking manifest psychotic signs) is often delayed, resulting in a longer duration of untreated psychosis. The length of untreated psychosis is closely related to poorer functional outcome ( Perkins et al., 2005 ).
Negative symptoms have traditionally had minimal response to antipsychotic treatment. First-generation antipsychotics are effective in treating positive symptoms, but negative symptom improvement is only evident when symptoms are secondary to positive symptoms. It was initially hoped that second-generation antipsychotics would target both positive and negative symptoms, but efficacy data have been disappointing. This was a large meta-analysis where only four second-generation drugs (amisulpride, risperidone, olanzapine, and clozapine) resulted to be more efficacious than first-generation antipsychotics in the overall change of symptoms, including positive and negative symptoms. The other examined second-generation antipsychotics were only as efficacious as first-generation antipsychotic agents ( Leucht et al., 2009 ). These studies were mainly conducted in patients with general symptoms of schizophrenia, therefore a secondary effect on negative symptoms could not be ruled out. Therefore negative symptom improvement cannot be considered a core component of atypicality ( Veerman et al., 2017 ). Previous studies have demonstrated that no drug had a beneficial effect on negative symptoms when compared to another drug ( Arango et al., 2013 ; Millan et al., 2014 ; Fusar-Poli et al., 2015 ), meaning that head to head comparisons of different agents among each other did not result in superiority of one drug to another. The latest comparison ( Krause et al., 2018 ) evaluated all studies that have been performed in the negative symptom population so far, and has found that amisulpride claimed superiority only to placebo, olanzapine was superior to haloperidol, but only in a small trial (n = 35), and cariprazine outperformed risperidone in a large well-controlled trial.
Hence cariprazine emerged as an agent of particular interest in regard to negative symptoms. Cariprazine is a dopamine D 3 /D 2 receptor partial agonist and serotonin 5-HT 1A receptor partial agonist. It has been hypothesized that cariprazine is the only antipsychotic that can block D 3 receptors in the living brain, thereby exhibiting functions that are related to D 3 blockade (e.g., improvement of negative symptoms) ( Stahl, 2016 ). In that large clinical trial including 460 patients with predominant negative symptoms and stable positive symptoms of schizophrenia, cariprazine was significantly more effective than risperidone in improving negative symptoms and patient functioning ( Nemeth et al., 2017 ).
Case Description
The 23-year-old female patient visited the Institute of Rare Diseases at our university with her parents. They had suspected for a long time that something was wrong with their daughter, but this was the first time they had asked for medical help. The patient was quiet and restrained since she did not speak much, her parents told us her story instead. Initially, the patient had done very well in a bilingual secondary school and was socially active with friends and peers. At the age of 15 years, her academic performance started to deteriorate, with her first problems associated with difficulty learning languages and memorizing. Her school grades dropped, and her personality started to gradually change. She became increasingly irritated, and was verbally and physically hostile toward her classmates, resorting to hitting and kicking at times. She was required to repeat a school year and subsequently dropped out of school at the age of 18 because she was unable to complete her studies. During these years, her social activity greatly diminished. She lived at home with her parents, did not go out with friends, or participate in relationships. Most of the time she was silent and unsociable, but occasionally she had fits of laughter without reason. Once the patient told her mother that she could hear the thoughts of others and was probably hearing voices as well. Slowly, her impulse-control problems faded; however, restlessness of the legs was quite often present.
Our patient’s medical history was generally unremarkable. She lacked neurological or psychiatric signs. She had a tonsillectomy and adenotomy at age 7 years. Epilepsy was identified in the patient’s family history (father’s uncle). On physical examination, there were no signs of internal or neurological disease; body mass index was 21.5 (normal weight).
During the first psychiatric interview and examination, we found that our patient was alert and vigilant, but had trouble relating due to decreased integrity of consciousness. Her attention could be aroused or partially directed, and she had difficulty keeping a target idea. Autopsychic and allopsychic orientations were preserved. Longer thinking latencies and slowed movement responses were observed, sometimes with even cataleptic impressions. Cognitive functions, such as thinking, memory, and concept formation, were severely impaired, and we were unable to carry out some of our neurocognitive tests -such as the Addenbrooke’s Cognitive Examination ( Hsieh et al., 2013 ), the Toulouse-Pieron attention test (Kanizsa G1951), Bells test ( Gauthier et al., 1989 ) and the Trail Making Test- because of the patient’s denial of symptoms and refusal to cooperate.
She often looked aside and laughed frequently, suggesting the presence of perceptual disturbances, but she denied her symptoms when asked. In contrast to the periodic inappropriate laughing, apathy and anhedonia were markedly present. During the examination, the patient could not recall anything she would do or even think of with pleasure. According to the heteroanamnesis, she lost her interest in activities she used to like, did not go out with friends anymore, and showed no signs of joy or intimacy towards her family members either.
Along with the affective hyporesponsiveness, amotivation and a general psychomotor slowing were observed. Hypobulia, void perspectives, and lack of motivation were explored. Parental statements indicated that the patient’s social activity had continued to diminish, and her appearance and personal physical hygiene had deteriorated. When we initiated a conversation, the patient was negativistic and agitated. Her critical thinking ability was reduced, which led to inappropriate behavior (she, e.g., unexpectedly stood up and left the room while the examination was still ongoing). Considering her status, she was admitted to the clinic after her first visit.
After several differential diagnostic tests were performed (e.g., routine diagnostic laboratory parameters, immune serological analyses, electroencephalogram, magnetic resonance imaging, genetic testing), all the possible common and rare disorders, such as Huntington’s disease, Niemann Pick C disease, mitochondrial disorders, and autoimmune diseases, were ruled out.
At first contact, to differentiate the symptoms and severity of putative schizophrenia, we mapped the positive, negative, and general symptoms, as well as a clinical impression, using the Positive and Negative Syndrome Scale (PANSS), the Scale for Assessment of Negative Symptoms, and the Clinical Global Impressions-Severity (CGI-S) ( Groth-Marnat, 2009 ).
The patient had a very high PANSS total score, which corresponded to being considered “severely ill” or “among the most severely ill’ on the CGI-S ( Leucht et al., 2005 ). The PANSS score was derived dominantly from the negative items of the scale. Overall, her negative symptoms fulfilled criteria for predominantly negative symptoms, meaning that positive symptomatology was reduced, while negative symptoms were more explicit and dominated the clinical picture ( Riedel et al., 2005 ; Olie et al., 2006 ; Mucci et al., 2017 ). Baseline rating scale sores are presented in Table 1 .
Table 1 Summary of symptom scale scores at the time of admission to the hospital.
The diagnosis of EOS with predominantly negative symptoms was given and treatment with the antipsychotic agent cariprazine was initiated. The patient was hospitalized for 2 weeks following her arrival at the clinic. Cariprazine was started at the dose of 1.5 mg/day and titrated up to 4.5 mg/day over a 2-week period: the patient received 1.5 mg/day for the first 3 days, 3 mg/day from day 4 to day 12, and eventually 4.5 mg/day from day 13 onward. During these 2 weeks, which were spent in hospital, the patient’s explicit negative symptoms such as poverty of speech, psychomotor retardation, poor eye contact, and affective nonresponsiveness improved; however, delusions and hallucinatory perceptions did not fade significantly.
Two weeks after discharge, we saw the patient for her first outpatient visit. Significant clinical improvement was observed. The patient calmly cooperated during the examination, with no signs of agitation. She was oriented to time, place, and self, attention could be drawn and directed, and she was able to keep a target idea and change the subject. Although according to the family, perceptual disturbances were still present, laughing with no reason and looking aside were much less frequent, and restlessness of the legs had stopped; these symptoms were not observed during the examination. Psychomotoric negativism had improved greatly, the patient was more communicative, and she paid more attention to the activities of family members. The pace of speech was close to normal: the thinking latencies and slowed movement responses as observed at admission were not seen anymore. The patient had adequate reaction time to questions asked and could focus in the interview. Mild obstipation and somnolence in the evening were her main complaints. Apart from some tick-like eye closures, there was no pathological finding during physical and neurological examination. At this point, cariprazine was reduced to 3 mg per day.
At her second outpatient visit, which occurred 8 weeks after treatment initiation, further improvement was observed. According to her mother, the patient was more active and open at home. Neurological examination found that the alternating movements of her fingers were slightly slowed. Cariprazine 3 mg/day was continued with concomitant anticholinergic medication.
At the third outpatient visit, which occurred 16 weeks after the first contact, the patient’s overall symptoms, including cognitive functions, such as memory and abstract thinking, as well as functions in activities of daily living, had improved remarkably. She had started to participate in the family’s daily life, even taking responsibility for some household duties; further, she went to the hairdresser for the first time in years, a step forward from her previous state of self-neglect. She was probably still having auditory hallucinations, which she considered natural, and some extrapyramidal symptom (EPS)-like ruminating movements, like to-and-fro swinging of her trunk, were observed. She did not look aside any more and tics were no longer present. Compared with previous visits overall, she was very relaxed, retained eye contact, cooperated, and communicated adequately during the interview. She started to develop insight into her condition, and she told us that her “thoughts were not healthy.” At the last two visits, the synkinesis of the arms was reduced.
After 16 weeks of treatment, the patient’s PANSS Negative Subscale Score and PANSS factor score for negative symptoms (PANSS-FSNS) score were reduced by 44.44% and 41.31%, respectively. Recent studies have demonstrated that linking the percentage improvement of PANSS with CGI-S and -Improvement (CGI-I) scores shows that a 25–50% reduction of PANSS scores corresponds to clinically meaningful change ( Correll et al., 2011 ; Fusar-Poli et al., 2015 ). In acutely ill patients with predominantly positive symptoms who are more likely to respond well to treatment, the 50% cutoff would be a more clinically meaningful criterion; however, since even slight improvement might represent a clinically significant effect in a patient with atypical schizophrenia, the use of 25% cutoff is justified ( Correll et al., 2011 ; Fusar-Poli et al., 2015 ).
In this regard, the 44.44% (change from baseline: −20) and 41.31% (change from baseline: −19) improvement demonstrated on PANSS Negative Symptom subscale and PANSS-FSNS, respectively, are considered a clearly clinically relevant change. Beyond the impaired synkinesis and alternating movement of the arms and fingers, there were no other treatment-related physical dysfunctions. Change from baseline on the PANSS and CGI scales are shown over the course of treatment in Table 2 .
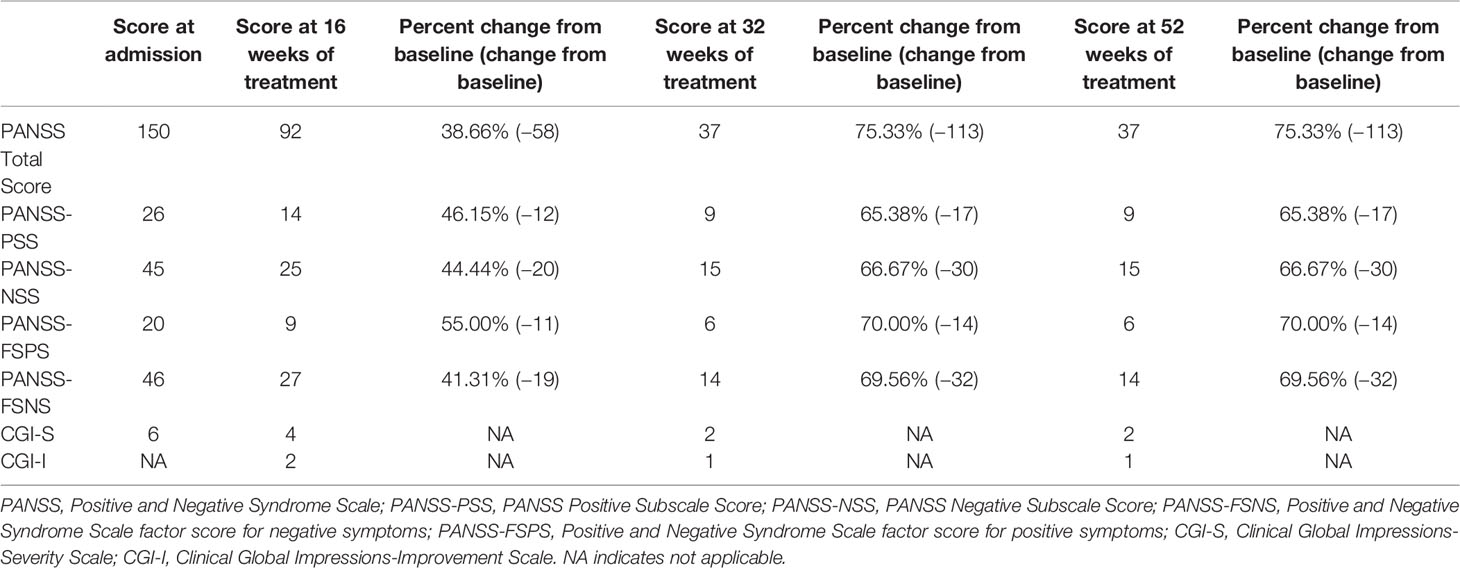
Table 2 Summary of symptom scale scores at weeks 16, 32, and 52.
Since our patient’s symptoms demonstrated strong improvement and tolerability was favorable, cariprazine therapy was continued. Improvement in both negative and positive symptoms was maintained over the course of treatment. At her later visits (32 and 52 weeks), PANSS total score was reduced to a level that was close to the minimum, and the decrease in negative symptom scores was considerable (PANSS-NSS=66.67% and PANS-FSNS=70.00% at both time points). The patient’s progress was also reflected in clinical and functional measurements, with the CGI-S score reduced to 2 (borderline mentally ill) and a CGI-I score of 1 (very much improved) indicating notable improvement.
Cariprazine has demonstrated broad spectrum efficacy in the treatment of positive and negative symptoms of schizophrenia. In a field where no treatment is available for difficult-to-treat negative symptoms, this case is unique and may have important implications for schizophrenia treatment. Despite experiencing approximately 8 years of untreated symptoms and functional impairment associated with predominantly negative symptom EOS, our 23-year-old female patient showed considerable symptomatic and functional improvement after several weeks of treatment with cariprazine. Given that the duration of untreated negative symptoms is associated with worse functional outcomes ( Boonstra et al., 2012 ), the remarkable improvement seen in this case shows how valuable cariprazine could be for patients with similar symptom presentations. Although it is not possible to generalize the observations and findings of this single case, it has the novelty of detecting a potential effect of cariprazine in a drug-naïve patient with marked negative symptoms of early-onset schizophrenia. To our knowledge, no cariprazine-related data has been published in this type of patients. A single case study is obviously far from being predictive for the efficacy of a drug, however, the results seen with this case are promising. With a dose recommended for patients with negative symptoms, our patient’s clinical condition, including positive, negative, and cognitive symptoms, as well as social functioning have improved notably, with the effect maintained for over 12 months. Generally, cariprazine has been well tolerated, with mild EPS observed after 8 weeks, but no metabolic, cardiac, or other side effects.
This case report suggests that the management of patients with EOS and prominent negative symptoms is achievable in everyday practice with cariprazine. More real-world clinical experience is needed to support this finding.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.

Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported from Research and Technology Innovation Fund by the Hungarian National Brain Research Program (KTIA_NAP_ 2017-1.2.1-NKP-2017-00002). Editorial support for this case report was supported by funding from Gedeon Richter. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
We are thankful to the patient and her family for giving us the opportunity to share her story in the form of a publication. Also, we acknowledge editorial assistance was provided by Carol Brown, MS, ELS, of Prescott Medical Communications Group, Chicago, Illinois, USA, a contractor of Gedeon Richter plc.
- ^ http://www.schizophrenia.com/szfacts.htm
- ^ https://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
Arango, C., Garibaldi, G., Marder, S. R. (2013). Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr. Res. 150 (2-3), 346–352. doi: 10.1016/j.schres.2013.07.026
PubMed Abstract | CrossRef Full Text | Google Scholar
Bartlet, J. (2014). Childhood-onset schizophrenia: what do we really know? Health Psychol. Behav. Med. 2 (1), 735–747. doi: 10.1080/21642850.2014.927738
Boonstra, N., Klaassen, R., Sytema, S., Marshall, M., De Haan, L., Wunderink, L., et al. (2012). Duration of untreated psychosis and negative symptoms–a systematic review and meta-analysis of individual patient data. Schizophr. Res. 142 (1-3), 12–19. doi: 10.1016/j.schres.2012.08.017
Clemmensen, L., Vernal, D. L., Steinhausen, H. C. (2012). A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 12, 150. doi: 10.1186/1471-244X-12-150
Correll, C. U., Kishimoto, T., Nielsen, J., Kane, J. M. (2011). Quantifying clinical relevance in the treatment of schizophrenia. Clin. Ther. 33 (12), B16–B39. doi: 10.1016/j.clinthera.2011.11.016
Fusar-Poli, P., Papanastasiou, E., Stahl, D., Rocchetti, M., Carpenter, W., Shergill, S., et al. (2015). Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 41 (4), 892–899. doi: 10.1093/schbul/sbu170
Galderisi, S., Mucci, A., Buchanan, R. W., Arango, C. (2018). Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5 (8), 664–677. doi: 10.1016/S2215-0366(18)30050-6
Gauthier, L., Dehaut, F., Joanette, Y. (1989). A quantitative and qualitative test for visual neglect. Int. J. Clin. Neuropsychol. 11 (2), 49–54. doi: 10.1016/j.neuropsychologia.2010.04.018
CrossRef Full Text | Google Scholar
Groth-Marnat, G. (2009). “Handbook of Psychological Assessment”. Fifth ed. (Hoboken, NJ: Wiley).
Google Scholar
Hafner, H., Van der Heiden, W. (1997). Epidemiology of schizophrenia. Can. J. Psychiatry 42 (2), 139–151. doi: 10.1177/070674379704200204
Hsiao, R., McClellan, J. (2008). Substance abuse in early onset psychotic disorders. J. Dual Diagn. 4 (1), 87–99. doi: 10.1300/J374v04n01_06
Hsieh, S., Schubert, S., Hoon, C., Mioshi, E., Hodges, J. R. (2013). Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr. Cognit. Disord. 36 (3-4), 242–250. doi: 10.1159/000351671
Immonen, J., Jaaskelainen, E., Korpela, H., Miettunen, J. (2017). Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv Psychiatry 11 (6), 453–460. doi: 10.1111/eip.12412
Kao, Y. C., Liu, Y. P. (2010). Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry 10, 63. doi: 10.1186/1471-244X-10-63
Kirkpatrick, B., Buchanan, R. W., Ross, D. E., Carpenter, J. (2001). A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 58 (2), 165–1671. doi: 10.1001/archpsyc.58.2.165
Klosterkotter, J., Schultze-Lutter, F., Bechdolf, A., Ruhrmann, S. (2011). Prediction and prevention of schizophrenia: what has been achieved and where to go next? World Psychiatry 10 (3), 165–174. doi: 10.1007/s00406-018-0869-3
Krause, M., Zhu, Y., Huhn, M., Schneider-Thoma, J., Bighelli, I., Nikolakopoulou, A., et al. (2018). Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 (7), 625–639. doi: 10.1007/s00406-018-0869-3
Lehman, A. F., Lieberman, J. A., Dixon, L. B., McGlashan, T. H., Miller, A. L., Perkins, D. O., et al. (2010). “Practice guideline for the treatment of patients with schizophrenia”, 2nd ed. American Psychiatric Association. Avaialble at: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf .
Leucht, S., Kane, J. M., Kissling, W., Hamann, J., Etschel, E., Engel, R. R. (2005). What does the PANSS mean? Schizophr. Res. 79 (2-3), 231–238. doi: 10.1016/j.schres.2005.04.008
Leucht, S., Corves, C., Arbter, D., Engel, R. R., Li, C., Davis, J. M. (2009). Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373 (9657), 31–41. doi: 10.1016/S0140-6736(08)61764-X
Marder, S. R., Galderisi, S. (2017). The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16 (1), 14–24. doi: 10.1002/wps.20385
McGuffin, P. (2004). Nature and nurture interplay: schizophrenia. Psychiatr. Prax 31 Suppl 2, S189–S193. doi: 10.1055/s-2004-834565
Millan, M. J., Fone, K., Steckler, T., Horan, W. P. (2014). Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 24 (5), 645–692. doi: 10.1016/j.euroneuro.2014.03.008
Mucci, A., Merlotti, E., Ucok, A., Aleman, A., Galderisi, S. (2017). Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res. 186, 19–28. doi: 10.1016/j.schres.2016.05.014
Murru, A., Carpiniello, B. (2018). Duration of untreated illness as a key to early intervention in schizophrenia: A review. Neurosci. Lett. 669, 59–67. doi: 10.1016/j.neulet.2016.10.003
Nemeth, G., Laszlovszky, I., Czobor, P., Szalai, E., Szatmari, B., Harsanyi, J., et al. (2017). Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389 (10074), 1103–1113. doi: 10.1016/S0140-6736(17)30060-0
Olie, J. P., Spina, E., Murray, S., Yang, R. (2006). Ziprasidone and amisulpride effectively treat negative symptoms of schizophrenia: results of a 12-week, double-blind study. Int. Clin. Psychopharmacol. 21 (3), 143–151. doi: 10.1097/01.yic.0000182121.59296.70
Penttila, M., Jaaskelainen, E., Hirvonen, N., Isohanni, M., Miettunen, J. (2014). Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 205 (2), 88–94. doi: 10.1192/bjp.bp.113.127753
Perkins, D. O., Gu, H., Boteva, K., Lieberman, J. A. (2005). Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am. J. Psychiatry 162 (10), 1785–1804. doi: 10.1176/appi.ajp.162.10.1785
Rabinowitz, J., Werbeloff, N., Caers, I., Mandel, F. S., Stauffer, V., Menard, F., et al. (2013). Negative symptoms in schizophrenia–the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr. Res. 150 (2-3), 334–338. doi: 10.1016/j.schres.2013.06.023
Riedel, M., Muller, N., Strassnig, M., Spellmann, I., Engel, R. R., Musil, R., et al. (2005). Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 255 (6), 432–437. doi: 10.1007/s00406-005-0622-6
Russell, ,. A. T. (1994). The Clinical Presentation of Childhood-Onset Schizophrenia. Schizophr. Bull. 20 (4), 631–646. doi: 10.1093/schbul/20.4.631
Stahl, S. M. (2003). Describing an atypical antipsychotic: Receptor binding and its role in pathophysiology. Prim Care Companion J. Clin. Psychiatry 5 (Suppl 3), 9–13.
Stahl, S. M. (2016). Mechanism of action of cariprazine. CNS Spectr. 21 (2), 123–127. doi: 10.1017/S1092852916000043
Veerman, S. R. T., Schulte, P. F. J., de Haan, L. (2017). Treatment for Negative Symptoms in Schizophrenia: A Comprehensive Review. Drugs 77 (13), 1423–1459. doi: 10.1007/s40265-017-0789-y
Keywords: cariprazine, schizophrenia, negative symptoms, early-onset schizophrenia, second-generation antipsychotic
Citation: Molnar MJ, Jimoh IJ, Zeke H, Palásti Á and Fedor M (2020) Early-Onset Schizophrenia With Predominantly Negative Symptoms: A Case Study of a Drug-Naive Female Patient Treated With Cariprazine. Front. Pharmacol. 11:477. doi: 10.3389/fphar.2020.00477
Received: 24 October 2019; Accepted: 26 March 2020; Published: 23 April 2020.
Reviewed by:
Copyright © 2020 Molnar, Jimoh, Zeke, Palásti and Fedor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Judit Molnar, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10216771

The Patient Journey of Schizophrenia in Mental Health Services: Results from a Co-Designed Survey by Clinicians, Expert Patients and Caregivers
Mauro emilio percudani.
1 Department of Mental Health and Addiction Services, Niguarda Hospital, 20162 Milan, Italy
Rosaria Iardino
2 Fondazione The Bridge, 20156 Milan, Italy
Matteo Porcellana
Jacopo lisoni.
3 Department of Mental Health and Addiction Services, ASST Spedali Civili of Brescia, 25123 Brescia, Italy
Luisa Brogonzoli
4 Research Department, Fondazione The Bridge, 20156 Milan, Italy
Stefano Barlati
5 Department of Clinical and Experimental Sciences, University of Brescia, 25123 Brescia, Italy
Antonio Vita
Associated data.
The data presented in this study are available on request from the corresponding author.
Background: The Patient Journey Project aims to collect real-world experiences on schizophrenia management in clinical practice throughout all the phases of the disorder, highlighting virtuous paths, challenges and unmet needs. Methods: A 60-item survey was co-designed with all the stakeholders (clinicians, expert patients and caregivers) involved in the patient’s journey, focusing on three areas: early detection and management , acute phase management and long-term management/continuity of care . For each statement, the respondents expressed their consensus on the importance and the degree of implementation in clinical practice. The respondents included heads of the Mental Health Services (MHSs) in the Lombardy region, Italy. Results: For early diagnosis and management , a strong consensus was found; however, the implementation degree was moderate-to-good. For acute phase management , a strong consensus and a good level of implementation were found. For long-term management/continuity of care , a strong consensus was found, but the implementation level was slightly above the cut-off, with 44.4% of the statements being rated as only moderately implemented. Overall, the survey showed a strong consensus and a good level of implementation. Conclusions: The survey offered an updated evaluation of the priority intervention areas for MHSs and highlighted the current limitations. Particularly, early phases and chronicity management should be further implemented to improve the patient journey of schizophrenia patients.
1. Introduction
Schizophrenia is a severe mental disorder characterized by a debilitating progression in most cases. Despite its etiology not being completely understood, schizophrenia seems to result from a complex interaction of biological, genetic and environmental factors [ 1 ]. According to the DSM-5 criteria [ 2 ], clinical features of schizophrenia comprise positive (delusions and hallucinations), negative (anhedonia, avolition, alogia, asociality, blunted affect), and disorganization (formal thought disorder, disorganized behavior) symptoms [ 1 , 3 , 4 ]. Moreover, cognitive impairment represents a further core feature [ 1 ]: in particular, impaired neuro-cognitive functioning exhibited the strongest association with poor psychosocial functioning [ 5 , 6 ].
Despite a low prevalence of ~1%, about 26.3 million people are currently living with schizophrenia [ 7 ]. Indeed, schizophrenia is ranked among the leading causes of disability worldwide [ 8 , 9 ]. Slightly more common in men [ 1 ], schizophrenia seems to present with a highly variable yet definitively chronic course in almost 60% of cases [ 10 ] and low recovery rates [ 11 ], negatively affecting subjective well-being, quality of life and psychosocial functioning [ 1 , 3 ].
Furthermore, people living with schizophrenia present with a low life expectancy due to the occurrence of other psychiatric disorders (i.e., depression, anxiety, substance misuse) [ 12 ] and somatic comorbidities (i.e., metabolic syndrome, diabetes, cardiovascular, respiratory and infectious diseases) [ 13 , 14 ]. Therefore, achieving common psychosocial milestones is uncommon for people living with schizophrenia [ 15 ]. This places great socioeconomic burdens on health care systems, mainly due to the indirect costs (i.e., unemployment, social support and hospitalization during crises) [ 16 ]. Indeed, while the annual amount ranges from USD 94 million to USD 102 billion [ 17 ], great costs also indirectly result from the increased family burdens and the reduced quality of life for relatives and caregivers [ 18 ].
Moreover, great burdens are also derived from the so-called dual diagnosis condition, which is when patients affected by severe psychiatric disorders suffer from concomitant substance use disorders (SUD). Indeed, data from an Italian study showed that the management of comorbid SUD in patients with schizophrenia is increasingly complex, highlighting an urgent need to optimize the management of this difficult-to-treat condition by considering several factors (i.e., treatment efficacy, tolerability, metabolic effect sides) and, according to a multidisciplinary approach, throughout all the phases of both disorders [ 19 ].
It, therefore, seems obvious that the management of patients with schizophrenia is complicated by the various and multifaceted elements that have to deal not only with the course of the disease but also with the different phases of the patient’s life. To address these challenges, mental health care systems (MHS) and providers have to offer updated treatment plans to take care of the individual suffering from schizophrenia throughout all the phases of what may amount to an ideal patient’s journey, with the aim to increase the functional outcomes and reduce the risk of chronicity [ 20 ], particularly focusing on the clinical features (i.e., negative symptoms and cognitive impairment) linked to poor functional outcomes [ 21 ]. Indeed, a previous Delphi study explored the consensus of Italian experts, psychiatrists and trainees in psychiatry and showed high consensus on several components of schizophrenia care, including early recognition, personalization, integration of care, assessment standardization and the management of somatic and psychiatric comorbidities [ 20 ].
Thus, the patient’s journey has to focus on particular moments throughout all the phases of the disorder, which are the early detection , the acute phase management , and the long-term management/continuity of care , with an eye open to the personalization of this path. However, as we believe that the patient’s journey should not be built based solely on a clinician-oriented perspective, this ideal path should consider the opinions and the needs of all the stakeholders involved in the care of patients with schizophrenia.
Therefore, the purpose of this Patient Journey Project is to perform a survey to share evidence-based information and real-world experiences on schizophrenia management, with the specific aim of involving all the stakeholders (i.e., clinicians, expert patients, caregivers and family members) engaged in the planning of the ideal path of care for patients with schizophrenia. In the manuscript, the ideal and virtuous pathways of care, as well as the challenges, barriers and difficulties in their implementation, will be taken into account. Taking care of the schizophrenic patient implies, on the one hand, the necessity for highly specialized care and, on the other hand, the need for multidisciplinary skills distributed throughout the community healthcare setting. The idea to plan a patient’s journey in schizophrenia through the organization of a codified path of care and management, giving specific assistance and improving adherence to pharmacological and psychosocial treatments, may represent a response to the complexity of schizophrenia, with the ultimate goal to achieve recovery in this population.
Thus, we first provide an up-to-date scenario of the current knowledge and existing matters on three main themes of early detection and its management , acute phase management and long-term management/continuity of care . Then, we will discuss the results of the survey to examine the unresolved needs outlined by the stakeholders and identify possible gaps and areas to be further implemented throughout the three phases of the patient’s journey while also proposing solutions and recommendations.
1.1. Early Detection and Management
Regarding early management, MHSs should implement structural plans to improve treatment outcomes and reduce the worst consequences of a full-blown psychotic disorder [ 22 ]. In order to foster an adequate treatment plan for adolescent help-seekers with mental disorders, several factors should be considered, including the family milieu, environmental (i.e., illicit substances abuse) or traumatic exposures, the duration of untreated psychosis (DUP), the onset/course of the disorder and its clinical manifestations (i.e., negative symptoms, social and non-social cognitive impairments), social functioning and quality of life, concomitant psychiatric/physical comorbidities, as well as resilience and internalized stigma levels [ 23 ].
As schizophrenia generally occurs in late adolescence/early adulthood, this life stage represents an essential period towards which the efforts of MHSs must be directed to identify the early signs of a forthcoming disorder and to prevent further clinical deterioration [ 24 ]. Indeed, according to the neurodevelopmental hypothesis of schizophrenia [ 25 ], adolescence represents an essential moment for brain maturation [ 26 ], during which several stressing factors, both biological (disturbed pruning, altered myelinization, exposure to cannabis or other illicit drugs and genetic load) and psychosocial (increased academic demands and responsibilities, social stress and social deprivation), could negatively impact normal brain development significantly earlier than the illness onset [ 27 ]. Thus, the Ultra-High Risk (UHR) model [ 28 , 29 , 30 ], including the Attenuated Psychotic Symptoms (APS) , Brief Limited and Intermittent Psychotic Symptoms BLIPS , and the Genetic Risk and Deterioration factor definitions, was developed to classify at-risk individuals in a prodromal phase of a psychotic disorder, with the aim to delay and prevent the onset of a full disorder, further reducing the impact of unfavorable factors (e.g., the duration of untreated illness (DUI) and the DUP) that could deteriorate psychosocial functioning and quality of life [ 28 ]. However, the recognition of subtle psychotic symptoms in at-risk subjects is frequently made by family and teachers rather than healthcare professionals. In this context, MHSs should also conceive preventative measures to identify at-risk adolescents through public awareness campaigns, social media, public events and community works to educate those who are part of the individual social context (e.g., academic milieu, general practitioners and local public health authorities) on the early manifestations of the disorder. Thus, assuming that the timing of treatment of the first episode of psychosis (FEP) is a crucial factor in determining the prognosis, several international programs [ 31 , 32 , 33 , 34 , 35 ], including Italian ones [ 36 , 37 ], have been developed to target both at-risk help-seekers or FEP patients, demonstrating the effectiveness of these interventions at improving symptom severity, retention rates and treatment adherence as well as the quality of life and psychosocial functioning [ 38 ]. The crucial point is to deliver a tailored intervention addressing the patient’s needs through a team-based multidisciplinary approach that involves psychiatrists, psychologists, nurses, social care workers, general practitioners (GPs) and families and comprises providing pharmacological, psychological, psychoeducation, psychosocial interventions, rehabilitation, family therapy and supported employment interventions [ 39 ]. Moreover, given the feasibility of receiving psychosocial and/or pharmacological treatments aimed at reducing the perceived distress at the early stages, it seems that early detection/intervention services could result in considerable cost-savings for national health systems, reducing hospitalization rates and improving employment outcomes [ 40 ].
Although the transition rate to full-blown psychosis is relatively low (20–35% over 2 years), clinicians and national legislators are called upon to appropriately respond to the adolescent population transitioning from the Child and Adolescent Mental Health Services (CAMHS) to Adult Mental Health Services (AMHS) [ 41 ], in order to reduce the personal, familiar and societal costs and burdens. However, several barriers prevent optimal collaboration between these services, hindering a real understanding of personal and family needs as well as spreading stigma towards AMHS [ 42 , 43 ]. In Italy, the National Action Plan for Mental Health [ 44 ] provided recommendations to develop innovative plans, creating multidisciplinary teams that involve CAMHS and AMHS together with families, educational facilities and the environmental context to share all information and recommendations on the clinical course of the disorder at an individual level. Conversely, bridging the gap in the transition phase and providing continuity of care, the Italian Partnership for Psychosis Prevention (ITAPP) project included five national Clinical High Risk for Psychosis (CHR-P) academic centers aimed at serving both adolescents and young adults with multidisciplinary and integrated interventions [ 45 ].
In this scenario, a central point of early management should be to promote simplified access to MHSs, promoting close connections between the AMHS, CAMHS, GPs and other parties operating in the social and health network of the patient, providing multi-professional interventions within the family to address patient’s physical and psychosocial needs through psychoeducational, psychotherapeutic and rehabilitative interventions [ 46 ].
Regarding therapeutic management, international guidelines [ 24 , 47 , 48 ] advise professionals to provide interventions addressing all the needs of the patient and their family, especially in the case of at-risk conditions (e.g., psychological support; cognitive behavioral therapy (CBT) and family-oriented interventions; continuous physical health assessments promoting well-being and healthy diet, including physical activity, smoking and psychoactive substance misuse cessation; education and employment support), where pharmacological intervention is highly recommended during an acute crisis, particularly with FEP [ 46 ].
1.2. Acute Phase Management
While long-term antipsychotic treatments prevent the relapse of the disorder both after a first episode and in the case of a chronic course [ 49 , 50 ], delaying time to hospitalization, especially in the early phases of the disorder [ 51 ], the management of the acute phase of schizophrenia should consider an adequate antipsychotic therapy [ 46 ] and, if recommended, a hospital admission, thus avoiding involuntary hospitalization where possible [ 46 , 52 ]. However, the patient’s journey usually begins with an acute crisis that, especially in the case of FEP, is generally followed by immediate hospitalization. Thus, as involvement in early diagnosis programs is usually difficult, the implementation of outpatient services should be pursued, preventing acute crises and involuntary treatments [ 46 ].
When hospitalization occurs, given the recognized efficacy of both first- (FGAs) and second-generation antipsychotics (SGAs) during the acute phase [ 53 ], clinicians should provide personalized treatments after careful assessment of the ratio between the benefits derived from symptom control and the risk of adverse events due to the chosen drug [ 20 , 54 ]. Moreover, the international guidelines advise a careful evaluation of the patient’s clinical presentation (i.e., symptom severity, suicidal risk, agitation/aggressiveness and psychiatric comorbidities, including substance abuse disorders) and of somatic comorbidities to maximize treatment adherence, tolerability and efficacy [ 55 , 56 ]. A feasible option is to receive long-acting injectable (LAI) antipsychotic treatments and clozapine for treatment resistance, as they are associated with higher reductions in hospitalization rates when compared to oral antipsychotics [ 57 ] and a reduced risk of relapse and recurrence [ 58 ]. However, as prolonged inpatient treatment of the general psychiatric and forensic populations and the use of coercive treatments (i.e., forced physical seclusion, restraint, forced medication treatments) [ 59 , 60 , 61 , 62 ] currently represent unethical methods [ 46 ], national MHSs are claimed to have developed effective intervention programs that might integrate the family and psychoeducational interventions during the acute phases, improving therapeutic alliance and treatment adherence [ 63 ], reducing those factors associated with longer hospitalization stays (e.g., the DUP) [ 64 ] while always ensuring the continuity of care with community setting services [ 46 ].
1.3. Long-Term Management/Continuity of Care
As the main goals of this phase are to prevent relapses and recurrences and to maintain remission and achieve recovery, in November 2014, the Italian Ministry of Health provided a clear definition of the long-term management phase, firmly recommending the implementation of the integration and continuity of care services, endorsing the application of multi-professional community-based interventions through the definition of the so-called Individual Treatment Plan [ 46 , 65 ]. If the initial suggestion is to avoid drug discontinuation through LAI treatments [ 66 , 67 ] and to carefully manage substance abuse disorders [ 65 ], then further recommendations should concern the application of non-pharmacological evidence-based interventions, such as psychoeducation for patients and their families [ 68 ], problem-oriented therapy, CBT for resistant-positive symptoms [ 69 ], cognitive remediation for cognitive impairment [ 70 ], social skills training [ 71 ], interventions to improve job skills and employment support [ 72 ], thus favoring increased patient awareness and insight, autonomy and social inclusion [ 20 , 46 , 65 , 73 ]. To achieve these goals, the involvement of caregivers and patients in shared decision-making on pharmacotherapy and personal needs are essential elements to improve the patient’s quality of care [ 46 , 74 , 75 ]. Furthermore, GPs’ involvement is of pivotal interest, especially in softening the physical comorbidity burdens [ 46 , 65 ]. Moreover, in cases of serious psychosocial functioning impairments, a rehabilitation program should be planned, even via admission into residential or semi-residential facilities [ 46 , 65 ]. In Italy, these interventions are provided according to community-based integrated health and social care services referring to the cost-effective model of case management, whereas additional approaches, such as assertive community treatment and intensive case management, have been found to be similarly effective [ 76 , 77 ].
2. Materials and Methods
2.1. survey construction.
As we were interested in building up a survey with a multidisciplinary approach, attentive to both clinicians’ and patients’ needs, a scientific board was defined to structure the survey statements. In this phase, the scientific board included social researchers, psychologists and psychiatrists. This phase concerned a desk research design to review the existing Italian regulatory sources, guidelines and best practices on the management of mental frailties and schizophrenia [ 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 ]. The scientific board identified three areas of interest: early detection and management , acute phase management , and long-term management/continuity of care , which were considered the most significant areas in the ideal journey of a patient with schizophrenia. Then, to create the survey, the scientific board identified a list of possible statements according to the Italian regulatory sources, guidelines and best practices.
After this, the scientific board shared the list of possible survey statements with 8 representatives of 4 patients’ and caregivers’ associations (Coplotta, Diversamente, Anpis Puglia, Club Itaca Milano) and with 3 expert peer supporter patients (aka, ESP patients). ESP patients are patients diagnosed with schizophrenia according to the ongoing classification for mental disorders and are trained at the regional level through a dedicated class to be recognized as expert peer supporters. We deemed it necessary to include ESP patients and caregivers, given their increasingly recognized relevance to patient engagement [ 86 ] and their empowerment in clinical and institutional settings. Moreover, this sharing was essential to strengthening our multidisciplinary approach, as we considered it of strategic importance to include all the stakeholders involved in an ideal patient’s journey [ 87 ]. In order to do so, a semi-structured one-on-one interview was conducted by one clinician and one social researcher with the ESP patients and patient and caregiver association representatives, with the purpose of collecting real-life evidence and relating what had emerged from the guidelines and best practices with the unmet needs still present in the management of schizophrenia.
From this interview, the scientific board was able to construct the statements that composed the present survey, focusing on specific themes that were considered of interest by clinicians, patient and caregiver association representatives and ESP patients. For early detection and management , we focused on services accessibility, continuity of care, multidisciplinary evaluation of patients’ needs, rehabilitation, psychoeducational and psychotherapeutic interventions, and drug treatment safety and appropriateness. For acute phase management , we investigated the experience of hospitalization, the prevention and decrease in commitment and forced treatment and physical restraints, and linkage to local and outpatient services. For long-term management/continuity of care , we focused on individual treatment plans, psychoeducational interventions, continuity in drug treatment, awareness of the patient’s physical health, recovery and social integration interventions, social and job support, and residential and semi-residential interventions.
Thus, following a thorough validation process carried out by clinicians, ESP patients and caregivers, we finally formulated a series of statements: 17 on early detection and management , 16 on acute phase management and 27 on long-term management/continuity of care . Indeed, the survey finally comprised a 60-statement questionnaire built on the three main areas of interest. To answer the survey, the respondents had to express agreement or disagreement with the 60 statements on a 5-point Likert scale. Each statement was analyzed according to 2 subscales: the first subscale assessed the importance of the statement , from (1) “of no importance” to (5) “extremely important”; the second subscale assessed the degree of implementation of each statement in real-life clinical practice, from (1) “not implemented at all” to (5) “extremely implemented”.
The survey was deployed with the CAWI (computer-assisted web interviewing) method by using a web program created and developed to manage research, surveys and customer satisfaction studies.
2.2. Participants
The survey was sent to all the heads of the mental health departments (MHDs) in the Lombardy region, Italy, as an initial sample of this research. Thus, the respondents included psychiatrists only, working as heads of mental health departments (MHDs) in the Lombardy region, Italy, regardless of whether they worked in academic or non-academic settings. No patients, caregivers or other stakeholders completed the survey.
The survey was sent to 45 heads of the Lombardy MHDs, aiming to reach at least half of the responses with adequate territorial representativity: this aim was successfully achieved with 25 responses, with a 55.5% response rate. The survey was available online from 22 September 2021 to 20 January 2022, and it received the support of the Lombardy Directors of Psychiatry Steering Group and the Regional Division of the Italian Psychiatry Association.
We supported the submission of the survey with two rounds of recalls.
2.3. Survey Aims
The purpose of this survey was to evaluate how the heads of MHDs could consider important topics on managing schizophrenia throughout all phases of the disorder, according to their knowledge, best practices guidelines and national regulatory sources. This was possible by analyzing the importance of the statement subscale.
Then, by analyzing the degree of implementation subscale and the existing gaps between available knowledge/guidelines and real-life management, the survey aimed to assess how much of the available knowledge and best practices guidelines are currently applied in the Lombardy MHDs in the situation of real-life management and how MHDs could further implement the codified care paths for patients with schizophrenia.
2.4. Statistical Analyses
The analysis of the results comprised a general review of responses, as well as an assessment of the consensus level on the importance of the statement and degree of implementation and of the existing gaps between the guidelines and real-life clinical practice of managing schizophrenia, according to the points of view of the heads of Lombardy MHDs.
Regarding the importance of the statement , a strong consensus was defined when rated as (4): “important” or above, whereas a low consensus was defined when rated as (3) “quite important”. Regarding the degree of implementation , the results were reported by combining the degree of implementation in 3 groups according to the mean scores for each item in the three areas of interest. A good level of implementation was defined for a score rated as (4) “properly implemented” or above; moderate levels of implementation were rated as (3) “enough implemented”; and poor levels of implementation were rated as (2) “slightly implemented” or below.
To quantify the consensus level on the importance of the statement and degree of implementation , we derived a mean score for each subscale for the three areas of interest and a total score. Moreover, the mode and median values were calculated. Adopting only descriptive statistical analyses, the appropriate analyses were calculated using the IBM ® SPSS Statistics Version 20 software. There were no a priori assumptions made. For the interpretation of the results, we primarily focused on the mean score values.
Additionally, we assessed the gap between the importance of the statement and the degree of implementation in order to identify which items should be further implemented with additional mental health care programs. The existence of the gap was defined when two conditions were satisfied: if the items of the importance of the statement subscale were above a score of 4, and if the items of the degree of implementation subscale underwent a score of 4. As the gap was considered when the degree of implementation subscale underwent a score of 4, we implicitly considered those statements showing moderate levels of implementation, rated as (3) “enough implemented” or below.
The respondents included 25 heads of MHDs from 17 different territorial social healthcare zones of the Lombardy region: Bergamo Ovest (2), Brianza, Garda, Lecco, Lodi, Mantova, Melegnano, Martesana (2), Niguarda Milano (2), Nord Milano, Ovest Milanese (2), Papa Giovanni Bergamo, Pavia (2), Rhodense, Santi Paolo and Carlo Milano (2), Spedali Civili Brescia (3), Valcamonica and Valle Olona.
No missing data was found, as all 25 heads of the MHDs who responded to the survey filled in all the statements.
The total results on the importance of the statement and degree of implementation are summarized in Table 1 and Table 2 .
Importance of statement and degree of implementation (mean scores).
AMHS, Adult Mental Health Services; CAMHS, Child and Adolescent Mental Health Services; GPs, general practitioners.
Importance of statement and degree of implementation (mean scores, medians, mode and standard deviations).
SD, standard deviations.
Regarding the first subscale, assessing the importance of the statement , a strong consensus emerged for several statements of the survey.
Considering the early detection and management , a strong consensus was found for all 17 statements, especially on “promotion of projects and protocols with CAMHS to promote and facilitate access to AMHS”, “to assure the continuity of care between CAMHS and AMHS”, “to create a personalized project with continuous and intensive contacts in community mental health services”, “to keep continuous and intensive contacts with family members”, “need of multidisciplinary assessment of patient’s clinical and psychosocial problems”, “to deliver a team-based multidisciplinary approach involving different healthcare professionals”, “to provide psychoeducation support”, “to provide evidence-based rehabilitation interventions”, “to provide work and study support interventions in case of moderate/severe psychosocial functioning impairment”, “to provide adequate pharmacological treatment for dosage and duration” and “to pay attention to the safety of pharmacological treatments”.
In the acute phase management , a strong consensus was found for all 16 statements, especially on “to improve accessibility to community mental health services”, “to avoid the use of physical restraint”, “to organize educational programs in order to minimize the need of physical restraint”, “to start as soon as possible an antipsychotic treatment”, “to identify the minimum effective dosage”, “to take care of safety of pharmacological treatment through an early monitoring of side effects”, “maintenance of pharmacological treatment for at least two years at discharge”, “to ensure a continuity of care with community MHS”, “ to ensure intensive contacts with community MHS after discharge” and “to review the ongoing treatment plans, when an hospitalization occurs, through a collaboration between inpatient and outpatient healthcare services”.
In the long-term management/continuity of care , a strong consensus was obtained for 26 out 27 statements, especially on “to provide continuous and multidisciplinary-based treatment to promote full psychosocial recovery”, “to define an individual treatment plan and to identify a case manager”, “to take care of family members”, “to provide psychoeducational and psychotherapeutic treatments for patients”, to provide psychoeducational treatments for family members”, “to carefully assess and treat substance abuse disorders conjointly with dedicated addiction services”, “to evaluate physical health conjointly with GPs”, “to monitor patient’s lifestyle in collaboration with GPs”, “to offer clozapine in case of treatment-resistance”, “to offer LAI antipsychotic treatment in case of frequent relapses and poor adherence”, “to maintain regular contact with patients that interrupted drug treatment”, “to re-contact patients who interrupted the contact with outpatients mental health service”, “to promote peer support groups oriented to recovery and social inclusion”, “to monitor adverse outcomes of the patients being cared for (death, suicide)”, “to provide psychosocial interventions and work placement support”, “to provide evidenced-based rehabilitation and resocialization interventions either in community and day-care facilities” and “to provide rehabilitation programs in residential facilities in case of serious psychosocial functioning impairment aiming for the patient’s return at home”. Only one statement (e.g., “to offer psychotherapeutic treatments for patients’ relatives and family members”) was ranked as (3) “quite important”.
The second subscale of the survey assessed the degree of implementation .
Good levels of implementation were found on 8 out of 17 statements (47% of the sample) for the early detection and management area, on 14 out of 16 statements for the acute phase management area (87.50% of the sample), and on 15 out of 27 statements for the long-term management/continuity of care area (55.6% of the sample).
Poor levels of implementation were found on 1 out of 17 statements of early detection and management (6% of the sample), while no statements were rated as poorly implemented neither for acute phase management nor long-term management/continuity of care .
Figure 1 shows the number of items (and the percentage of the total items for each of the three thematic areas) divided according to the degree of implementation.

Degree of implementation. The figure shows the number of items (and the percentage of the total items for each of the three thematic areas) divided according to the level of implementation. The results of the degree of implementation subscale are subdivided into 3 groups according to the mean scores for each item in the three areas of interest. Good level of implementation was defined for a score rated as (4) “properly implemented” or above; moderate levels of implementation was rated as (3) “enough implemented”; poor levels of implementation was rated as (2) “slightly implemented” or below.
Focusing on each item of the early detection and management , good levels of implementation were especially found on “to provide adequate pharmacological treatment for dosage and duration” and “to pay attention to the safety of pharmacological treatments”.
Focusing on each item of the acute phase management , good levels of implementation were especially found on “to start as soon as possible an antipsychotic treatment”, “to take care of safety of pharmacological treatment through an early monitoring of side effects”, “maintenance of pharmacological treatment for at least two years at discharge” and “to ensure a continuity of care with community MHS”.
Focusing on each item of the long-term management/continuity of care , good levels of implementation were especially found on “to define an individual treatment plan and to identify a case manager”, “to offer clozapine in case of treatment-resistance” and “to offer LAI antipsychotic treatment in case of frequent relapses and poor adherence”.
On the other hand, poor levels of implementation were found in the early detection and management phase, on “promoting projects and protocols with GPs aimed at prevention”.
Then, we considered the gap between the importance of the statement and the degree of implementation by considering those statements showing moderate levels of implementation or below.
Considering the early detection and management , moderate levels of implementation were found on 8 out of 17 statements (47% of the sample), especially on the “promotion of projects and protocols with CAMHS to promote and facilitate access to AMHS”, “to assure the continuity of care between CAMHS and AMHS”, “to use internationally validated assessment tools”, “to assess family burdens and their needs”, “to deliver multidisciplinary support for family members”, “to provide home interventions”, “to provide psychotherapy interventions” and “to provide work and study support interventions in case of moderate/severe psychosocial functioning impairment”. Figure 2 summarizes the mean scores of the importance of the statement , degree of implementation , and the gap between these subscales for the items related to early detection and management .

The gap between the importance of the statement and the degree of implementation in the early detection and management area. Figure legend: the abscissa axis represents items (0–17) of the early detection and management ; the ordinate axis represents the 5-point Likert scale anchor points: deep gray for importance of the statement (from (1) “of no importance” to (5) “extremely important”) and light gray for degree of implementation (from (1) “not implemented at all” to (5) “extremely implemented”).
Considering the acute phase management , moderate levels of implementation were found on 2 out of 16 statements (12.50% of the sample), especially on the “need of acute inward admission when an acute decompensation occurs” and on the “need to limit pharmacological restraint”. Figure 3 summarizes the mean scores of the importance of the statement , degree of implementation , and the gap between these subscales for the items related to acute phase management .

The gap between the importance of the statement and the degree of implementation in the acute phase management area. Figure legend: the abscissa axis represents items (18–33) of the acute phase management ; the ordinate axis represents the 5-point Likert scale anchor points: deep gray for importance of the statement (from (1) “of no importance” to (5) “extremely important”) and light gray for degree of implementation (from (1) “not implemented at all” to (5) “extremely implemented”).
Concerning the long-term management/continuity of care , moderate levels of implementation were found on 12 out of 27 statements (44.4% of the sample), especially on “to take care of family members”, “to provide psychoeducational and psychotherapeutic treatments for patients and for family members”, “to evaluate physical health conjointly with GPs”, “to monitor patient’s lifestyle in collaboration with GPs”, “to promote peer support groups oriented to recovery and social inclusion”, “to promote the integration of expert in peer support”, “to promote the role of the expert in peer support in improving efficacy of treatments” and “to promote rehabilitation programs in semi-residential facilities for patients with a good level of autonomy”. Figure 4 summarizes the mean scores of the importance of the statement , degree of implementation , and the gap between these subscales for the items related to the long-term management/continuity of care .

The gap between the importance of the statement and the degree of implementation in the long-term management/continuity of care area. Figure legend: the abscissa axis represents items (34–60) of the long-term management/continuity of care area; the ordinate axis represents the 5-point Likert scale anchor points: deep gray for importance of the statement (from (1) “of no importance” to (5) “extremely important”) and light gray for degree of implementation (from (1) “not implemented at all” to (5) “extremely implemented”).
Figure 5 summarizes the overall mean scores of the importance of the statement , degree of implementation , and the gap between these subscales for the three areas of interest.

The gap between the importance of the statement and the degree of implementation (mean scores). The figure summarizes the overall mean scores of the importance of the statement , degree of implementation , and the gap between these subscales for the three areas of interest and the total score. Deep gray for importance of the statement (from (1) “of no importance” to (5) “extremely important”) and light gray for degree of implementation (from (1) “not implemented at all” to (5) “extremely implemented”).
For early diagnosis and management , while a strong consensus was found (mean score = 4.63), the level of implementation was found to be slightly below the cut-off (mean score = 3.97), being rated as moderate-to-good. Therefore, we can assume that only a minor gap emerged in this area of interest.
For the acute phase management , a strong consensus (mean score = 4.69) and a good level of implementation (mean score = 4.30) were found.
For long-term management/continuity of care , while a strong consensus was found (mean score = 4.62), the level of implementation was found to be slightly above the cut-off (mean score = 4.02).
Overall, the survey found a strong consensus (mean score = 4.63) and a good level of implementation (mean score = 4.07) for the analyzed statements.
4. Discussion
Schizophrenia represents a leading cause of disability worldwide. Indeed, people living with schizophrenia present with poor quality of life, insufficient psychosocial functioning, increased unemployment levels, social isolation and a reduced life expectancy due to excess mortality and morbidity. Thus, early recognition and appropriate management are needed to reduce the risk of chronicity and comorbidity, especially in the case of dual diagnosis. Thus, the personalization and integration of pharmacological and psychosocial interventions, as well as the accurate identification and management of psychiatric and somatic comorbidities, can significantly improve the mental and physical health of patients living with schizophrenia, thus promoting recovery [ 73 ]. In this scenario, both at a national and local level, MHSs should identify the strengths and weaknesses of their interventions in order to promote constant updating of the organization and implementation of the MHS. Indeed, a previous Delphi study found several weaknesses (i.e., lack of time, human resources and training) as the main barriers and challenges to the translation of knowledge into clinical practice [ 20 ]. Moreover, as healthcare utilization (HCU) databases can reveal the strengths and weaknesses of the national care system by representing a useful tool in the routine assessment of mental healthcare quality [ 79 ], a recent multi-regional Italian investigation based on the HCU databases found that to reduce regional variability, Italian MHSs should improve the accessibility to psychosocial interventions and the quality of care for newly taken-in-care patients, focusing on somatic health and mortality [ 88 ].
Thus, with the Patient Journey Project, we built a survey considering three macro areas ( early detection , acute phase management and long-term management/continuity of care ), with the contribution of all the stakeholders involved in the planning of the ideal clinical path of care, including clinicians, expert patients, caregivers and family members, in order to describe the current evidence and to discuss the unmet needs of the care management of schizophrenia according to Lombardy MHD heads’ point of views. Thus, covering these macro areas, we will discuss the consensus on the importance of many areas of intervention, analyze the areas with good or low levels of implementation, and also discuss the gaps between the importance of the statements and their implementation in Lombardy’s psychiatric services.
Regarding early detection and management , the survey highlighted a strong consensus concerning the importance of promoting shared protocols to facilitate access from CAMHS to AMHS, assuring continuity of care between child and adult services, and also keeping continuous and intensive contact with family members. However, the survey found that these protocols are only implemented enough among the Lombardy MHDs: these findings suggest further developing structured plans favoring the transition and continuity of care from CAMHS to AMHS. Indeed, while national and international guidelines strongly recommend this approach, in Lombardy, only a few examples are available in clinical practice. Among them, the mental health department of the territorial social healthcare zones of Melegnano and Martesana conducted a four-year follow-up study to verify the continuity of care effectiveness for adolescent patients aged between 16 and 19 who were transitioning from CAMHS to AMHS. Of the 93 users, most cases (54.8%) needed to be managed by a multidisciplinary team in order to have an effective transition. Moreover, when a dedicated team was arranged, the success of the transition was achieved in 86% of the cases; therefore, 88.6% of the patients were still followed one year after their transition from CAMHS to AMHS [ 89 ]. These results highlighted that an effective transition from one service to another is possible if this process is adequately structured and planned and possibly delivered by a specialized and dedicated team.
Moreover, the creation of an individualized project—one that involves continuous and intensive contact between community mental health services and the patient and their family—represents another core need that is properly implemented within the Lombardy MHDs. In this light, the need for a multidisciplinary assessment of a patient’s clinical and psychosocial problems by a team-based multidisciplinary approach is another crucial aspect to consider during the early phases of the patient’s journey, especially with the aim of providing work and study support interventions in case of severe psychosocial functioning impairment. Nevertheless, while the survey showed a strong consensus for these statements and good levels of implementation , in Lombardy, only a few pilot projects have been carried out in public MHSs, including the Ambulatorio Spazio Giovani [ 90 ] and Centro Giovani “Ponti” [ 91 ], with the aim of protecting the mental health of young adults, thus suggesting that further efforts are urgently required to bridge this gap.
In addition, while a good consensus was found regarding the importance of employing internationally validated assessment tools during the early detection phase, the implementation level among MHDs was considerably low. Thus, a further suggestion is that practitioners should be routinely trained on the application of these tools, including the Comprehensive Assessment of At-Risk Mental States (CAARMS) that assesses both the psychopathology and identification of individuals at high risk for psychosis [ 92 ], the Structured Interview for Prodromal Symptoms/Structured Interview for Psychosis-Risk Syndromes (SIPS) [ 93 ], and the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) , which is more generally aimed at an early diagnosis of both psychotic and anxious/affective disorders in youths [ 94 ]. The standardization of assessment tools represents an unresolved matter in psychiatry, as the diagnostic evaluation could be extremely heterogeneous, complicating the evaluation of the psychopathological needs of subjects in a prodromal phase. To this extent, the application of shared protocols could reduce this heterogeneity, and through the incorporation of all the information from family members or the real-life context, it might represent a timely intervention that avoids further diagnostic and therapeutic delays and clinical deterioration.
In line with national and international guidelines and recommendations [ 24 , 46 , 47 , 48 , 73 ] relating to therapeutic interventions, a strong consensus and good levels of implementation were both observed in the present survey regarding the provision of adequate pharmacological treatment in terms of the congruent dosage and duration, with a special focus on safety and tolerability profiles. Conversely, several non-pharmacological interventions (i.e., home-based interventions, psychotherapy, psychoeducation, rehabilitation and work/study support interventions) were found to be of great importance in the survey. However, important gaps emerged regarding their implementation in real-world clinical practice among the Lombardy MHDs, as these interventions were at a moderate level of implementation.
Moreover, while satisfactory consensus emerged regarding GPs’ involvement during the early detection and management phase, severe discordance was found regarding its implementation in clinical practice. Nevertheless, some reports highlighted that GPs considered themselves as the first line of contact with cases experiencing acute symptoms of psychosis, providing support and psychoeducation to the patient’s family, thus not limiting their action to somatic comorbidity management [ 95 , 96 ]. Although primary care for psychotic patients depends on the GP’s personal resources, as the quality of collaboration between GPs and MHSs represents another key factor in moderating the GP’s engagement in the patient’s journey [ 97 ], further efforts are needed to develop shared plans and connections between GPs and mental health providers to improve the quality of care among adolescents with a constraining psychotic disorder as well as during long-term management [ 98 ].
Finally, the survey found that topics concerning the family’s involvement (i.e., the assessment of family burden and the multidisciplinary support provided to family members) are only at moderate levels of implementation among Lombardy MHDs. These points emphasize the need to further realize plans to reduce the family burdens of those young patients who are in the early stages of the disease. A suggestion could be to promote further connections with GPs, AMHSs and family members through public awareness campaigns, social media and public events.
Summing up, as we found moderate-to-good levels of implementation in early diagnosis and management , we could assume that further efforts are needed to improve the patient’s journey during the early phases: in particular, the Lombardy MHDs should focus on the transition from CAMHS to AMHS and on the involvement of GPs and family members to increase the efficacy of preventive actions.
On the other hand, the survey showed an overall strong consensus for all the statements dedicated to acute phase management . Notably, a great consensus was reached regarding the provision of adequate antipsychotic treatments in terms of their immediate availability during a crisis while also taking into consideration the safety and tolerability profiles of the prescribed drugs. As international recommendations suggested [ 55 , 56 , 73 ], prior to choosing an antipsychotic, a clear recommendation is to evaluate the clinical presentation and the somatic comorbidities to further improve clinical efficacy, tolerability and long-term adherence.
Furthermore, attention should be paid when an acute admission occurs. Indeed, hospitalization should be conceived not only as a strategy to manage the psychopathological crisis through a timely antipsychotic treatment at the minimum effective dose but also as a background in which diagnostic assessment of somatic problems should be required and guaranteed through a feasible collaboration with other medical disciplines [ 99 ], while also considering the safety of the pharmacological treatments through the early monitoring of side effects.
In addition, participants in the survey found a strong consensus and quite satisfactory implementation levels regarding the role of acute admission, ensuring a rapid continuity of care with outpatient services. Thus, acute hospitalization could represent a crucial framework during which a re-evaluation of treatment plans should be recommended, especially in those cases of poor clinical and psychosocial outcomes and refractory symptoms, anticipating the unmet needs of the patients, caregivers and outpatient community services [ 44 , 46 , 98 ]. Similarly, the survey found a satisfactory consensus on the theme appropriateness of hospitalization to manage the acute phase of a psychotic episode, emphasizing how difficult it is for the MHSs to adequately implement outpatient care systems to prevent acute hospitalization. Indeed, this practice was found to be only enough implemented among Lombardy MHDs, indicating that MHSs should develop flexible strategies aimed at preventing emergencies through the implementation of outpatient and community-based services, ensuring continuity of care. Accordingly, these plans should consider proper community-based interventions for medium-to-long-term periods, involving all the figures already active in the treatment process, including GPs, local mental health providers, the patients and their relatives [ 44 , 98 ]. With a view to reconsidering the hospital-community relationship, a suggestion could be to avoid inappropriate admissions, especially in those cases of social problems or when odd behaviors that are not primarily attributable to psychopathological frameworks are present [ 99 ]. Moreover, to ensure continuity of care, a great consensus and good implementation levels were found regarding the need to provide quick and intensive contact with community mental health services following hospital discharge and on the maintenance of pharmacological treatment in the follow-up.
Moreover, a strong consensus was reached regarding the suggestion to avoid coercive treatments during hospitalization, including involuntary admissions and physical/pharmacological restraints, with a good level of implementation to obtain this. Notably, the Lombardy MHD heads have expressly suggested the adoption of specific protocols aimed at limiting, preventing and managing the possible applications of physical restraint measures [ 98 ]. Therefore, great importance is given to educational programs, where meta-analytic evidence suggests that when educational programs are provided for mental health workers, the use of physical restraint is significantly reduced [ 99 ]. Moreover, the duration and frequency of education produced a stronger effect in reducing the use of physical restraints, thus suggesting that a continuous education program, especially involving nurses, should be provided to prevent physical and pharmacological restraint [ 98 , 99 , 100 ]. Nevertheless, while participants in the survey reported a good consensus in limiting the use of pharmacological restraints, only moderate levels of implementation were found: these results are not surprising since several factors seem to be associated with increased levels of restraints, including sociodemographic factors (i.e., male gender, younger age, foreign ethnicity) [ 101 , 102 ], the type of hospitalization (e.g., compulsory admissions) [ 103 , 104 ], type of diagnosis (e.g., substance abuse, psychotic or affective disorders) [ 103 , 104 ] and environmental factors (e.g., the presence of male staff, night time, a seasonal trend with frequent pharmacological coercion during spring and mechanical coercion during summer) [ 101 , 103 , 104 , 105 ].
Finally, the survey highlighted a strong consensus for all the statements regarding long-term management/continuity of care , except for the provision of psychotherapy treatment for family members. In particular, a high consensus was achieved regarding the need to provide continuous and multidisciplinary-based treatment to promote full psychosocial recovery, define an individual treatment plan, identify a case manager, provide psychoeducational and psychotherapy treatments, and carefully assess concomitant substance abuse disorders conjointly with addiction services, as well as offer LAI treatments to reach great adherence, offer clozapine in case of treatment resistance, and provide rehabilitation programs in residential facilities for serious psychosocial functioning impairment. These results are expected, as the Lombardy operating model for people living with schizophrenia gave particular attention to long-term management, especially in those cases of high clinical complexity, and always with the aim of ensuring continuity of care through various outpatient services and the activation of familiar, social and environmental resources. This is possible with the creation of a multi-disciplinary community-based intervention through the definition of the so-called individual treatment plan and the application of the case management approach [ 44 , 46 , 98 ]: starting with a systematic analysis of the patient’s complex needs, both at a psychopathological and psychosocial level, through a multi-professional team-based evaluation. This operating model has made it possible to improve the quality and management of psychiatric assistance, thus reducing interventions in emergency situations and allowing for the adequate planning of future treatment paths to achieve recovery [ 99 ].
Nevertheless, the survey also found several gaps between the importance of statements and the degree of implementation in clinical practice for long-term management/continuity of care . Indeed, while the level of implementation was found to be slightly above the cut-off, almost 45% of the statements (that is, 12/27) were rated as enough implemented . Specifically, moderate levels of implementation were found for psychoeducational and psychotherapeutic treatments for patients and family members, collaborations with GPs who monitor the patient’s physical health and lifestyle, the provision of peer support groups together with the collaboration of experts and multi-professional teams to improve treatment efficacy, achieve recovery and social inclusion, and the provision of rehabilitation plans in semi-residential facilities, including social housing. These problems have already been emphasized in the Definizione dei percorsi di cura da attivare nei Dipartimenti di Salute Mentale per i Disturbi schizofrenici, i Disturbi dell’umore e i Disturbi gravi di personalità agenda [ 65 ], where it was recommended to further manage these issues. After almost a decade, the Lombardy MHDs must clearly provide further indications for developing and implementing adequate interventions to respond to these structural problems. While evidenced-based rehabilitation programs in residential facilities were found to be adequately improved in the survey, one suggestion is to rethink the role of semi-residential facilities, the so-called day centers. In fact, semi-residential structures are designated to solve therapeutic-rehabilitative functions, including pharmacological intervention to prevent and contain hospitalization [ 65 ]. Moreover, day centers are placed in the community context. A multi-professional team can help the patient experiment and learn skills or strategies to implement psychosocial functioning, including self-care, daily life activities and interpersonal relationships, while also giving employment advice [ 44 , 46 , 106 , 107 ]. In this context, the day centers could be a feasible solution in supporting an effective continuity of care in the community setting, counterbalancing the phenomenon of interminable psychiatric residency by favoring rehabilitation, socialization and social reintegration, thanks to a strong link with primary and secondary community networks [ 107 ], thus also reducing the high levels of familiar expressed emotions [ 107 ]. Moreover, when engaged in day center activities, ESP patients could be of pivotal importance in promoting peer support to improve recovery, social inclusion and treatment efficacy.
In summary, the present survey offers an updated evaluation of the intervention areas of priority importance for MHD heads and also covers the current limitations of the Lombardy MHSs that affect various elements of the journey of patients with schizophrenia. In particular, the moment of transition from CAMHs to AMHs during the early phase and the management of chronicity (with particular mention to the psychological support for family members, the provision of semi-residential interventions, the monitoring of patients’ lifestyles and physical health in collaboration with their GPs and the integration of experts in peer support in a multi-professional team) represent the current unmet needs to which the Lombardy MHSs must respond to further improve the patient’s journey of living with schizophrenia.
Thus, several issues could represent new areas for future interventions. Firstly, as aerobic exercise seems to improve cognitive functioning among people with schizophrenia [ 108 , 109 ] and physical activity and a person’s well-being represent key moderators of primary prevention and clinical treatment [ 110 ], MHSs should develop systematic plans to improve lifestyle and physical health, particularly focusing on tobacco smoking cessation, healthy diets and the promotion of sleep-focused interventions [ 111 ]. This can be achieved by encouraging further integration of the MHSs into primary care settings and providing supervised exercise to people with schizophrenia [ 110 ].
Secondly, current evidence suggests that only an integrated approach involving pharmacotherapy and psychosocial interventions could improve outcomes in schizophrenia to achieve recovery. Thus, the efficacy of several psychosocial interventions, including approaches such as cognitive rehabilitation [ 70 ], CBT [ 111 ], social skill training [ 71 ], illness self-management training and supported employment [ 112 ], is supported by substantial evidence in many outcomes measures, and therefore, a further suggestion for the Lombardy MHDs is to systematically integrate these evidence-based approaches into the daily management of people living with schizophrenia [ 113 ]. Moreover, as family intervention and psychoeducation seem to reduce the number of relapse events and hospitalizations [ 114 , 115 , 116 , 117 ], MHDs should further promote and integrate family care approaches in the long-term management of patients with schizophrenia through the participation of relatives in such psychoeducational programs, thus helping to build a proper family emotional environment that will benefit the caregiver–patient relationship and improve patient functioning [ 117 ].
Thirdly, peer support is an essential component of the patient’s journey for achieving personal recovery that MHDs should improve on in daily clinical practice: based on the rationale that people who have experienced mental illness [ 118 ] are qualified to provide support and hope to others dealing with similar challenges, peer support programs with ESP patients focus on helping others to become active participants in their recovery process, breaking out of the passive role of the patient [ 113 , 119 ]. Moreover, by becoming involved in the recovery process of others, peers who provide support represent one of the best examples of community integration, increasing personal autonomy and self-worth [ 115 ]. Lastly, an important factor for people living with schizophrenia is a personal participation in the definition of their care pathway [ 73 ]. Indeed, patients require autonomy and participation in treatment decisions, including active participation in the so-called shared decision-making (SDM) process in treatment choice [ 74 ]. Current evidence has provided good preliminary data on SDM as a method to improve mental health services, including guideline-concordant care, attendance and retention in treatment, and satisfaction with health care, thus representing a promising strategy to improve collaboration between clinicians and patients in achieving recovery [ 120 ]. Notably, there is evidence that SDM is feasible and time-comparable to the usual care in psychiatric and primary care settings when considering both acute [ 121 ] and long-term drug treatment choices [ 75 ]. Clinicians should evaluate the patient’s preferences, expectations and concerns towards the development of a personalized treatment strategy in order to reduce the patient’s perception of being forced to receive a drug [ 75 ], thus achieving greater involvement in the treatment process and increasing knowledge about the illness [ 74 ].
Lastly, another suggestion could be to improve the family’s support and the patient’s engagement with cultural and ethical practices. Indeed, it was suggested that the perceived role of family bonds and religiosity–spirituality could positively influence clinical outcomes, such as treatment maintenance-adherence and mortality [ 122 , 123 ]: this could be partly linked to improved health practices, increased social contacts and reduced stigma levels [ 122 ]. Thus, an appropriate analysis of these psychosocial factors, including cultural and ethnic milieu, could be helpful to further improve the patient’s journey.
In this project, some limitations are to be addressed. First, the results cover only the Lombardy MHDs; thus, they are not representative of the entire national territory and may not be generalizable to other countries. Second, the survey involved only specialists in adult psychiatry, not child neuropsychiatrists, GPs and other mental health workers. Therefore, our study does not cover the views of all the stakeholders involved in this complex field.
5. Conclusions
Despite these limitations, the present survey, co-designed by clinicians, expert patients and caregivers, offers an updated evaluation of the areas of intervention of priority importance for the patient’s journey with schizophrenia in MHSs, also covering several current critical issues. Particularly, the moment of transition from child to adult services in the early phase of the disorder and the management of chronicity represent the unmet needs to which a response is needed to improve the patient’s journey when living with schizophrenia.
Acknowledgments
The authors would like to thank all patient association members who contributed to the project.
Funding Statement
It is an unconditional support of the Angelini Pharmaceutical Company.
Author Contributions
Conceptualization, M.E.P., S.B. and A.V.; Data curation, M.P., J.L., L.B., S.B. and A.V.; Formal analysis, J.L.; Funding acquisition, R.I. and L.B.; Investigation, R.I., L.B. and S.B.; Methodology, M.E.P., M.P., L.B., S.B. and A.V.; Project administration, R.I. and L.B.; Supervision, M.E.P., R.I., M.P., J.L., L.B., S.B. and A.V.; Validation, M.E.P., M.P., J.L., S.B. and A.V.; Visualization, A.V.; Writing—original draft, J.L.; Writing—review and editing, M.P., S.B. and A.V. All authors conceived the project, discussed the results, and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This survey study aimed to collect different stakeholder opinions, and therefore, did not involve the sharing of sensitive data. Consequently, this study did not require ethical approval. All experts involved in the project were informed of the study’s objectives and the possibility of publishing the results in a peer-reviewed article. Participation was voluntary by invitation and participants did not receive any compensation or benefits for participating in the survey. Participants, by accessing the secure online survey platform using their credentials, gave their consent to participate in the survey by clicking on the appropriate button to submit the completed questionnaire. All survey results are anonymous and presented in aggregate form.
Informed Consent Statement
No personal or medical data were collected; therefore, no consent for publication is required.
Data Availability Statement
Conflicts of interest.
Rosaria Iardino has participated in pharmaceutical industry-sponsored symposia and projects for Angelini. Luisa Brogonzoli has participated in pharmaceutical industry-sponsored symposia and projects for Angelini. Martina Sacchi has participated in pharmaceutical industry-sponsored projects for Angelini. Stefano Barlati, Mauro Emilio Percudani, Jacopo Lisoni, Matteo Porcellana and Antonio Vita declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Medications to Treat Schizophrenia
Types, Precautions, and Alternative Treatments
How They Work
- Second Generation
- First Generation
- Injectables
Effectiveness
- Alternatives
The first line of pharmacological treatment for schizophrenia is antipsychotic medication. However, antipsychotic medications are ineffective in more than 30% of people with the disorder. This is referred to as treatment-resistant schizophrenia.
Medications to treat schizophrenia come in three forms:
- Second-generation antipsychotics
- First-generation antipsychotics
- Long-acting injectable antipsychotics
This article discusses the three types of antipsychotic medications to treat schizophrenia, including how they work and side effects they may cause.
Dean Mitchell / Getty Images
For people with schizophrenia, antipsychotic medications can help treat psychosis symptoms like hallucinations and delusions, which are at the core of the disorder.
Unfortunately, antipsychotics are not effective for treating other common symptoms of schizophrenia, like social withdrawal, or cognitive problems that involve learning and attention.
While antipsychotics do not cure schizophrenia, they help control symptoms and, when taken long-term, can help prevent future episodes of psychosis .
Antipsychotics work by blocking dopamine in the brain. Dopamine is a brain chemical that, when overactive, is thought to play a part in psychosis.
With the exception of long-acting injectables, antipsychotic medication is usually taken in pill form, but some are available in dissolving tablets, suppositories, or liquid form.
Antipsychotics should be started as soon as possible after symptoms appear.
Second-Generation Antipsychotics
Second-generation antipsychotics (sometimes called atypical antipsychotics) are the first treatment choice for most professionals treating schizophrenia.
In addition to blocking dopamine, second-generation antipsychotics also affect another brain chemical called serotonin .
Second-Generation Antipsychotics vs. First-Generation Antipsychotics
While second-generation and first-generation antipsychotics work about equally well, second-generation antipsychotics tend to have milder movement-related side effects than first-generation antipsychotics.
Types of second-generation antipsychotics include:
- Abilify (aripiprazole)
- Fanapt (iloperidone)
- Geodon (ziprasidone)
- Invega (paliperidone)
- Latuda (lurasidone)
- Risperdal; Risvan (risperidone)
- Saphris (asenapine)
- Seroquel (quetiapine)
- Zyprexa (olanzapine)
Clorazil (clozapine) is a second-generation antipsychotic, but it is typically only used when other antipsychotics are ineffective or when a person has suicidal ideation. It has an increased risk of lowered white blood cells, so people taking Clorazil will usually have their white blood cell count monitored.
Side Effects
Side effects for second-generation antipsychotics include:
- Blurred vision
- Seizures (rarely)
- Weight gain
- Movement effects (such as tremor, agitation, stiffness)
- Sedation (sleepiness, low energy)
- Decreased sex drive and function
- Missed periods
- Discharge from breasts
- Higher risk of diabetes
First-Generation Antipsychotics
First-generation antipsychotics (sometimes called typical antipsychotics) are older medications, which were first developed during the 1950s.
While they can work well, they carry a higher risk of side effects, including a serious long-term side effect, tardive dyskinesia (TD), an involuntary movement disorder in which people may experience random movements in their muscles, eyes, tongue, jaw, and lips.
For this reason, first-generation antipsychotics are usually only prescribed when second-generation antipsychotics have not been effective or cannot be used.
Types of first-generation antipsychotics include:
- Haldol (haloperidol)
- Calypta (lumateperone)
- Prolixin (fluphenazine)
- Thorazine (chlorpromazine)
- Trilafon (perphenazine)
- Stelazine (trifluoperazine)
Side effects of first-generation antipsychotics vary depending on the drug, but can include:
- Constipation
- Emotional blunting
- Stuffy nose
- Breast tenderness
- Liquid discharge from breasts
- Muscle stiffness or spasms
- Tardive dyskinesia (TD)
Long-Acting Injectable Antipsychotics
Long-acting injectable antipsychotics are an option for people who struggle with taking pills or sticking to a regular medication schedule.
These medications can help reduce:
- Hospitalizations
- Emergency room visits
- Intentional or accidental overdose
Dosage (including how often it is administered) varies by drug. They are typically administered every two to four weeks but can be given every six or eight weeks, depending on the drug and the individual.
Some injectables require oral supplementation initially when treatment begins. Also, some injectables need to be refrigerated.
The long-acting injectable antipsychotics include:
- Risperdal Consta (risperidone microspheres)
- Invega Sustenna (paliperidone palmitate)
- Invega Trinza (paliperidone palmitate)
- Zyprexa Relprevv (olanzapine pamoate)
- Aristada (aripiprazole lauroxil)
- Rykindo; Risvan (risperidone)
Side effects for long-acting injectable antipsychotics may include:
- Injection-site redness, pain, or swelling
- Increase in cholesterol
- Increase in blood pressure
- Heart problems
- Type 2 diabetes
- Irregular menstrual cycles
- Problems having sex
- Restlessness
- Muscle spasms
- Tardive dyskinesia
Although rare, long-acting injectable antipsychotics are associated with a risk of neuroleptic malignant syndrome. This life-threatening condition may begin with a high fever, and changes in heart or breathing patterns. If you have these symptoms, see a healthcare provider right away.
How Long Does It Take for Medication to Work?
Antipsychotic medication can work within a few days to help a person with acute psychosis calm down and clear confusion, but for full effect, it can take up to four to six weeks.
Precautions and Contraindications
In addition to side effects, there are some things that affect the use of antipsychotics.
Other Medications
Some medications can cause side effects when taken with antipsychotics, while others, like antacids, can affect absorption. To limit the risk of any adverse effects, it's important to let your healthcare provider know all the medications you are taking.
Smoking can make the body break down antipsychotics faster. People who smoke heavily may require more medication.
Tell your healthcare provider if the amount you smoke changes.
Coffee can slow down how long it takes the body to break down antipsychotics. Tell your healthcare provider if the amount of coffee you drink changes.
Antipsychotics can increase the effects of alcohol, making one drink have the effects of two or three drinks.
Therefore, it's important to limit alcohol while taking such medications. If you're struggling with alcohol misuse, let your healthcare provider know, as there are resources available to help.
Street/Illicit Drugs
Drugs such as marijuana, cocaine, and amphetamines can cause symptoms to reoccur or worsen. They can also interfere with medication and worsen side effects.
For some people, antipsychotics can cause sleepiness or sedation. It is best to avoid driving—or anything else that requires alertness—until you know how the medication affects you.
Pregnancy and Breastfeeding
Antipsychotics may cause irregular periods and/or false pregnancy tests.
Antipsychotics are considered relatively safe during pregnancy and breastfeeding but may cause the baby to have temporary breathing difficulties and/or withdrawal symptoms (e.g., restlessness, feeding problems) if taken in high doses close to delivery.
Antipsychotics do pass into breastmilk and may cause the baby to be drowsy, depending on the dose.
These risks and side-effects are often manageable and may be a better choice than living with unmedicated schizophrenia/psychosis.
People who are or are planning to be pregnant or breastfeeding should discuss the benefits versus risks with their care provider.
Age can play a role in the use of antipsychotics.
Children and teens are more likely to experience side effects from these medications, as are people older than 60.
People older than 60 may also be more sensitive to the medication and require lower doses. Older adults are more likely to be taking other medications, which may cause interactions with antipsychotics. Antipsychotics may also create a higher risk of falls.
Antipsychotic use in older adults has been associated with an increased risk of stroke, and should only be used if other treatments are not an option.
Some antipsychotics come in forms that contain substances known to affect people with allergies or dietary restrictions , such as:
- Coconut oil
- Vegetable oil
Tell your healthcare provider if you have any allergies at all, not just medication allergies.
Talk to Your Healthcare Provider
It is important to be honest with your healthcare provider about any medications or substances you are taking. This includes:
- Prescribed medication
- Over-the-counter (OTC) medications
- Herbal/natural supplements
- Street/illicit drugs
The effectiveness of antipsychotics in the treatment of schizophrenia depends on a number of factors and varies among people.
The most effective treatment for schizophrenia is a multidisciplinary approach including:
- Psychological treatment
- Social support
People with schizophrenia who are taking antipsychotics report a higher quality of life than those who are not but also experience side effects. For those who report symptom relief and manageable side effects, longer-term maintenance therapy using antipsychotics is suggested.
While it may take months for antipsychotics to reach maximum effect, how a person responds within the first few weeks is considered highly predictive of how they will respond long-term.
It takes at least four weeks at a therapeutic dose to determine the effectiveness of a treatment, and unless a person is experiencing an unmanageable adverse reaction or side effects, healthcare providers usually give a trial of four weeks or longer before suggesting a different medication or treatment.
Sometimes a combination of medications is used to treat schizophrenia.
Treatment for schizophrenia works best when started as early as possible and is approached as a team effort between the person with schizophrenia, medical and therapeutic specialists, and support people.
Adherence to treatment (including taking medication as prescribed) can be difficult for people with schizophrenia. It is important to work with your healthcare provider to find strategies for treatments that work for you and ways to make them successful.
Other Treatments for Schizophrenia
In people whose psychosis symptoms do not respond to antipsychotics, non-pharmacologic therapies like talk therapy can help manage symptoms. Adjunctive (additional) therapies are also beneficial for people using antipsychotics to enhance their treatment or address concurrent problems.
Adjunctive treatments for schizophrenia include the following:
Antidepressant medications are prescribed to treat "negative symptoms" (loss of feeling or behaviors) such as social withdrawal, poverty of speech ( alogia ), and inability to experience joy ( anhedonia ). Commonly prescribed antidepressants include selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants.
Mood stabilizers are medications prescribed to people with schizophrenia to reduce mood swings, aggression, or agitation. Some types of mood stabilizers, like lithium , reduce the risk of suicide.
Cognitive behavioral therapy (CBT) is a form of talk therapy that helps manage "positive symptoms" (those abnormally present) such as delusions, hallucinations, and disorganized speech. It is also effective for treating common concurrent disorders, like depression, anxiety, and substance abuse.
Family interventions are effective tools for preventing relapses of psychosis. Sessions may involve educating patients and family members about schizophrenia, finding ways to improve communication and problem-solving skills, and ultimately lowering family stress.
Social skill training helps people with schizophrenia develop skills needed to operate in society. Sessions may cover such skills as conversing with people, making friends and dating, interacting with healthcare providers, or dealing with social situations that involve drugs or alcohol.
Electroconvulsive therapy (ECT) involves sending electronic signals to a person's brain through electrodes placed on their head. The person is sedated with general anesthesia throughout the procedure. ECT aims to reduce the chances of relapse, particularly in people who do not respond to drug treatments. It is also an effective treatment for catatonia (a group of symptoms involving lack of movement and communication).
Hospitalization may be needed during a severe psychotic episode, or if they show signs of wanting to hurt themselves or others. They may also be hospitalized if they have severe side effects from a medication, or other problems related to substance abuse.
Antipsychotic medications are the first-line treatment for people with schizophrenia, although they are not effective in 30% of people with the disorder. These drugs can reduce delusions, hallucinations, and other symptoms of psychosis, but other therapies, like talk therapy, ECT, or antidepressant medications may be needed to address co-occurring symptoms.
Antipsychotics are linked to numerous side effects, like drowsiness, dry mouth, and potentially serious side effects like tardive dyskinesia. To reduce the risk of side effects, your healthcare provider may ask you to make some lifestyle changes, like avoiding alcohol, caffeine, and cigarettes.
Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options . P T . 2014;39(9):638-645.
Northwestern. Antipsychotic drugs work differently than scientists believed .
Stępnicki P, Kondej M, Kaczor A. Current concepts and treatments of schizophrenia . Molecules . 2018 Aug;23(8):2087. doi:10.3390/molecules23082087
Wunderink L. Personalizing antipsychotic treatment: evidence and thoughts on individualized tailoring of antipsychotic dosage in the treatment of psychotic disorders . Ther Adv Psychopharmacol . 2019 Apr;9(1):2045125319836566. doi:10.1177/2045125319836566
Fernández-Miranda J, Díaz-Fernández S, López-Muñoz F. The use of second-generation antipsychotics in patients with severe schizophrenia in the real world: The role of the route of administration and dosage—a 5-year follow up . Biomedicines. 2022 Dec;11(1):42. doi:10.3390/biomedicines11010042
Grajales D, Ferreira V, Valverde Á. Second-generation antipsychotics and dysregulation of glucose metabolism: Beyond weight gain . Cells . 2019;8(11):1336. doi:10.3390/cells8111336
Paribello P, Manchia M, Zedda M, Pinna F, Carpiniello B. Leukocytosis associated with clozapine treatment: A case series and systematic review of the literature . Medicine (Kaunas) . 2021 Aug;57(8):816. doi:10.3390/medicina57080816
The Centre For Addiction and Mental Health. Antipsychotic medication .
Mount Sinai. Schizophrenia .
Edinoff A, Wu N, deBoisblanc C. Lumateperone for the treatment of schizophrenia . Psychopharmacol Bull . 2020 Sep;50(4):32-59.
Kishimoto T, Sanghani S, Russ M, et al. Indications for and use of long-acting injectable antipsychotics: consideration from an inpatient setting . Int Clin Psychopharmacol . 2017 May;32(3):161-168. doi:10.1097/YIC.0000000000000165
Zolezzi M, Abouelhassan R, Eltorki Y, Haddad P, Norrizadeh M. Long-acting injectable antipsychotics: A systematic review of their non-systemic adverse effect profile . Neuropsychiatr Dis Treat . 2021 Jun;17(1):1917-1926. doi:10.2147/NDT.S309768
Kaiser Permanente. Long-acting injectable antipsychotic medications .
Yartsev A, Peisah C. Caffeine-clozapine interaction associated with severe toxicity and multiorgan system failure: a case report . BMC Psychiatr . 2021 Apr;21(1):192. doi:10.1186/s12888-021-03199-x
Cheng C. Interaction between psychotropic medications and alcohol: Perceptions among patients attending an adult mental health day hospital program . Can J Hosp Pharm . 2018 Feb;71(1):7-13.
American Family Physician. Schizophrenia .
Long Y, Wu Q, Yang Y. Early non-response as a predictor of later non-response to antipsychotics in schizophrenia: a randomized trial . BMC Med . 2023 Jul;21(1):263. doi:10.1186/s12916-023-02968-7
Kane J, Agid O, Baldwin M, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia . J Clin Psychiatr . 2019 Mar;80(2):18com12123. doi:10.4088/JCP.18com12123
Cerveri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: Update and proposal of a clinical algorithm . Neuropsychiatr Dis Treat . 2019 Jun;15(1):1525–1535. doi:10.2147/NDT.S201726
Puranen A, Koponen M, Lähteenvuo M, Tanskanen A, Tiihonen J, Taipale H. Real-world effectiveness of mood stabilizer use in schizophrenia . Acta Psychiatrica Scandinavica . 2023 Mar;147(3):257-266. doi:10.1111/acps.13498
Kart A, Özdel K, Türkçapar M. Cognitive behavioral therapy in treatment of schizophrenia . Noro Pscyhiatr Ars . 2021 Sep;58(1):61-65. doi:10.29399/npa.27418
Hahlweg K, Baucom DH. Family therapy for persons with schizophrenia: neglected yet important . Eur Arch Psychiatry Clin Neurosci. 2023;273(1):819–824. doi:10.1007/s00406-022-01393-w
Browne J, Mueser K, Pratt S. Social skills training for persons with schizophrenia . In: Social Skills Across the Lifespan. 2020;1(1):329-342. doi:10.1016/B978-0-12-817752-5.00017-2
Grover S, Sahoo S, Rabha A, Koirala R. ECT in schizophrenia: a review of evidence . ACTA Neuropsychiatr . 2019 Jun;31(3):115-127. doi:10.1017/neu.2018.32
Kaiser Permanente. Schizophrenia: When hospital care is needed .
By Heather Jones Jones is a freelance writer with a strong focus on health, parenting, disability, and feminism.
- Open access
- Published: 29 May 2024
The associations of psychopathology and metabolic parameters with serum bilirubin levels in patients with acute-episode and drug-free schizophrenia: a 5-year retrospective study using an electronic medical record system
- Yinghan Tian 1 , 2 , 4 na1 ,
- Cheng Yang 1 , 2 , 4 na1 ,
- Lewei Liu 1 , 2 , 4 na1 ,
- Xin Zhao 4 ,
- Haojie Fan 1 , 2 , 4 ,
- Lei Xia 1 , 2 , 4 &
- Huanzhong Liu 1 , 2 , 3
BMC Psychiatry volume 24 , Article number: 403 ( 2024 ) Cite this article
151 Accesses
Metrics details
The oxidative system plays an important role in the pathogenesis of schizophrenia. Inconsistent associations were found between hyperbilirubinemia and psychopathology as well as glycolipid metabolism in patients with schizophrenia at different episodes. This current study aimed to examine these associations in patients with acute-episode and drug-free (AEDF) schizophrenia.
This is a retrospective study using 5 years of data from May 2017 to May 2022 extracted from the electronic medical record system of Chaohu Hospital of Anhui Medical University. Healthy controls (HCs) from the local medical screening center during the same period were also included. Participants’ data of the bilirubin levels [total bilirubin (TB), conjugated bilirubin (CB), unconjugated bilirubin (UCB)], glycolipid metabolic parameters and the score of the Brief Psychiatric Rating Scale (BPRS) were collected.
A total of 1468 case records were identified through the initial search. After screening, 89 AEDF patients and 100 HCs were included. Compared with HCs, patients had a higher CB level, and lower levels of glycolipid metabolic parameters excluding high density lipoprotein-cholesterol (HDL-C) (all P < 0.001). Binary logistic regression analyses revealed that high bilirubin levels in the patients were independently associated with higher total and resistance subscale scores of BPRS, a higher HDL-C level, and lower total cholesterol and triglyceride levels (all P < 0.05).
Bilirubin levels are elevated in patients with AEDF schizophrenia. Patients with high bilirubin levels have more severe psychopathology and relatively optimized glycolipid metabolism. In clinical practice, regular monitoring of bilirubin levels in this patient population should be carried out.
Peer Review reports
Introduction
Approximately 1% of the worldwide population is affected by schizophrenia [ 1 ], while the China Mental Health Survey showed that the lifetime prevalence of schizophrenia in China is 0.6% [ 2 ]. Schizophrenia is often combined with hyperbilirubinemia, which is usually considered a sign of liver or hemolytic diseases [ 3 , 4 ]. The prevalence of hyperbilirubinemia is about 25% in patients with schizophrenia, which is 2–3 times higher than in the general population [ 5 ].
Bilirubin is the end product of heme metabolism in human red blood cells, and comes in two forms: conjugated bilirubin (CB) and unconjugated bilirubin (UCB) [ 6 ]. On the one hand, a study reported that bilirubin had a strong antioxidant effect at a concentration below 100 nanomoles per liter (nM), which can protect nerve cells from oxidative stress damage [ 7 ]. On the other hand, high bilirubin levels can exhibit significant pro-oxidant and neurotoxic effects, ultimately leading to neuronal necrosis or apoptosis [ 8 , 9 ]. The dysfunction of the oxidative system may be involved in the pathogenesis of schizophrenia [ 10 ]. In both animals and humans, higher bilirubin levels have been shown to be strongly associated with a greater risk of schizophrenia and more severe psychiatric symptoms. For example, an animal study showed that Gunn rats, a model of hyperbilirubinemia, have remarkably schizophrenic-like behaviors [ 11 ], and a cohort study with 21-year follow-up found that newborns with hyperbilirubinemia had an increased risk of developing schizophrenia in adulthood [ 12 ]. Moreover, Miyaoka et al. reported that levels of biopyrrins, oxidative products of bilirubin, are positively correlated with the score of Brief Psychiatric Rating Scale (BPRS) in patients with chronic schizophrenia [ 13 ]. Additionally, previous studies found that there were significant differences in UCB levels among three groups (schizophrenia > schizoaffective disorder > bipolar disorder), which indicates that UCB may be a potential biomarker in schizophrenia [ 14 , 15 , 16 ]. Therefore, given the complex mechanisms of action of bilirubin, it is important to reveal the characteristics of bilirubin and its relationship with psychopathology in patients with schizophrenia.
In the general population, bilirubin levels were negatively correlated with total cholesterol (TC), triglyceride (TG), and fasting blood glucose (FBG) levels, which have been repeatedly confirmed in previous meta-analysis, cohort study, and national survey [ 17 , 18 , 19 ]. Similarly, in patients with schizophrenia, CB level was negatively correlated with the levels of TG and FBG, which has been shown to be a valid predictor of risk for metabolic syndrome (MetS) [ 20 ].
The differences in bilirubin levels in patients with schizophrenia across studies may be due to the heterogeneity of the subjects studied (e.g. gender, intake of antipsychotics, and different periods of illness). For example, in a previous cross-sectional study, bilirubin levels were higher in males with schizophrenia than in females [ 21 ]. Christian et al. found that patients with schizophrenia had significantly lower bilirubin levels after 2 and 4 weeks of antipsychotic treatments [ 22 ]. Moreover, both case-control and prospective studies showed that patients with acute-phase schizophrenia had higher bilirubin levels than those with remission-phase schizophrenia [ 23 , 24 ]. Therefore, this current study aimed to examine (1) the levels of bilirubin in patients with acute-episode and drug-free (AEDF) schizophrenia and their associations with psychopathology, and (2) the associations between bilirubin levels and glycolipid metabolism parameters in patients with AEDF schizophrenia.
Study design and participants
This is a retrospective study using 5 years of data from May 2017 to May 2022 extracted from the electronic medical record system of Chaohu Hospital of Anhui Medical University. Patients were included, if they met the following criteria: (1) Han Chinese, aged between 18 and 65 years; (2) diagnosed as schizophrenia, based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) using a structured Clinical Interview, and were in an acute-episode as defined by BPRS score ≥ 40 [ 25 ]; (3) without antipsychotic treatments for the last 1 month [ 26 ]. Patients were excluded who had (1) any other psychiatric disorders; (2) organic brain diseases, hypertension, diabetes, hyperlipidemia, immune system diseases, hepatic diseases or other serious physical diseases; (3) or were pregnant or breastfeeding women; (4) recently used any anti-inflammatory drugs. The HCs were recruited from the local medical examination in the same period, who were Han Chinese, aged between 18 and 65 years, and had no personal or family history of psychiatric diseases.
In accordance with the Declaration of Helsinki, all participants and their guardians signed an electronic informed consent via smartphone. The protocol of this retrospective study was approved by the Medical Ethics Committee of Chaohu Hospital of Anhui Medical University on 13 October 2022 (202210-kyxm-015).
Data collection measurements
All participants’ socio-demographic and clinical data were collected through the electronic medical record system, including age (years), sex (male and female), age of onset (years), duration of illness (months). Body mass index (BMI) was calculated as weight (kg)/height (m) 2 . Blood samples from patients were collected between 06:00 and 07:00 AM after an overnight fast. And bilirubin [total bilirubin (TB), CB, UCB], and glycolipid metabolism parameters [TC, TG, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), FBG] were measured. Psychiatric symptoms of the patients included in this study were assessed using the Chinese version of the 18-item BPRS [ 27 , 28 ]. Each item was evaluated on a seven-point scale, ranging from “0 = not present” to “7 = extremely severe”, with a higher total score indicating more severe symptoms [ 29 ]. The scale consists of five subscales: affect subscale (items 1, 2, 5, and 9), negative symptoms subscale (items 3, 13, 16, and 18), positive symptoms subscale (items 4, 8, 12, and 15), resistance subscale (items 10, 11, and 14), and activation subscale (items 6, 7, and 17) [ 30 ].
Statistical analysis
The continuous and categorical variables were described as Mean ± standard deviation (SD) and frequency distributions (%), respectively. The Kolmogorov–Smirnov one-sample test was used to test the normal distribution of continuous variables. Patients were divided into low and high serum bilirubin groups using the 50th percentile of total TB, CB, and UCB, respectively [ 20 ]. Socio-demographic and clinical characteristics were compared between groups using independent samples t-test, Mann-Whitney U test, and Chi-square test as appropriate. Binary logistic regression models (Forward: LR) were used to examine the independent correlates associated with bilirubin levels, with bilirubin levels (categorical) as the dependent variables, and the variables which were significant in univariate analyses ( P < 0.05) as the independent variables, as well as age and sex as the control variables. The correlations between bilirubin levels (after ln-transformed) and other clinical data, as well as metabolic parameters in the patients group were examined with Pearson or Spearman correlation analyses. Then multivariate linear regression models (Forward: LR) were used to examined any significant correlations ( P < 0.05) in correlation analyses. Statistical Product and Service Solutions (SPSS) version 23.0 (SPSS incorporated, Chicago, Illinois, United States of America) was used for statistical analyses. The P - values were set as two-tailed α = 0.05.
Case record selection and participant characteristics
A total of 1,468 potentially relevant case records were initially identified (Fig. 1 ). After screening according to the inclusion (1,328 excluded) and exclusion (51 excluded) criteria, 89 patients with AEFD schizophrenia were included. And 100 HCs from the local medical examination were also included. In this study, the mean age of patients was 38.92 years (SD = 13.75), and 42.7% were male. The mean age of HCs was 35.35 years (SD = 6.95), and 42.0% were male. There were significant differences in BMI between groups (all P < 0.001) (Table 1 ).
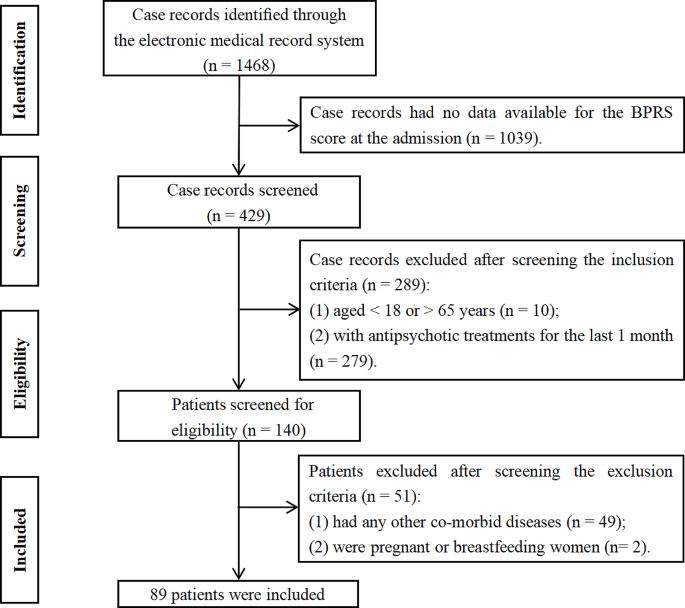
Flowchart of the case records selection
Comparisons between patients with schizophrenia and healthy controls
As shown in Table 1 , the patients with schizophrenia had a higher CB level, and lower levels of TC, TG, LDL-C, and FBG than HCs. Analysis of covariance (ANCOVA) showed that differences in levels of CB (F (1,189) = 20.223, P < 0.001), TC (F (1,189) = 10.380, P = 0.002), TG (F (1,189) = 6.648, P = 0.011), LDL-C (F (1,189) = 18.098, P < 0.001), and FBG (F (1,189) = 6.053, P = 0.015), between the two groups remained significant after controlling for BMI.
Comparisons between patients with low and high bilirubin levels
As shown in Table 2 , the patients in the high TB group had a lower TG level, and a higher total score of BPRS, and higher scores on negative symptoms and resistance subscales than those in the low TB group (all P < 0.05). The patients in the high CB group had lower levels of TC, TG, LDL-C, a higher total score of BPRS, and scores on resistance and activation subscales than those in the low CB group (all P < 0.05). In addition, the patients in the high UCB group had a lower TG level, a higher HDL-C level, and a higher total score of BPRS, and a higher resistance subscale score than those in low UCB group (all P < 0.05).
Factors associated with high bilirubin levels (categorical)
Binary logistic regression (model 1: BPRS total score, or model 2: BPRS subscale scores involved in the regression models, respectively) analyses revealed that the high TB level (categorical) was significantly associated with a lower TG level [model 1: Odds Ratio (OR) = 0.25, 95% Confidence Interval (CI) = 0.07–0.90; model 2: OR = 0.25, 95% CI = 0.07–0.87], a higher total score of BPRS (OR = 1.09, 95% CI = 1.03–1.16) and a higher resistance subscale score (OR = 1.17, 95% CI = 1.04–1.32). The high CB level was significantly associated with a lower TC level, a higher total score of BPRS and a higher resistance subscale score (all P < 0.05). And the high UCB level was significantly associated with a higher HDL-C level, a higher total score of BPRS, and a higher resistance subscale score (all P < 0.05) (Table 3 ).
Independent correlates of bilirubin levels (continuous)
There were several correlations between socio-demographic and clinical variables with bilirubin levels (continuous) in patients (Table 1 S). Multivariate linear regression analyses showed that the TB level was negatively associated with levels of TG ( β = -0.26, P = 0.025), and was positively associated with resistance subscale score ( β = 0.05, P < 0.001). The CB level was negatively associated with levels of TG and FBG, and was positively associated with total score of BPRS (all P < 0.05). The UCB level was negatively associated with TG level, and was positively associated with resistance subscale score (all P < 0.05) (Table 2 S).
In this study, we examined the peripheral bilirubin levels and their associations with psychopathology and glycolipid metabolism parameters in patients with AEDF schizophrenia. Consistent with the findings of two recent studies conducted in patients with acute-episode schizophrenia, we found that patients with AEDF schizophrenia had a higher CB level than HCs [ 23 , 31 ]. This may be due to the fact that patients with acute onset schizophrenia are in a state of high oxidative stress, and sustained oxidative stress induces rupture of the erythrocyte cell membrane, which increases serum bilirubin levels [ 32 ]. Since CB is formed by the conversion of UCB in the liver, a significant elevated CB level may reflect an increased overall bilirubin level. In turn, elevated UCB levels induce reactive oxygen species (ROS) and nitric oxide (NO) production by neuroglia cells, leading to further enhancement of oxidative stress [ 33 ]. In a previous study, drug-naive, first-episode patients with schizophrenia had significantly higher levels of UCB, compared to HCs [ 34 ]. A new insight suggests that we can distinguish schizophrenia from schizoaffective and bipolar disorders based on the degree of UCB elevation [ 35 , 36 ]. However, there were no differences in UCB levels between patients with schizophrenia and HCs in this study. On the contrary, in a recent study, Huang et al. reported that there was no statistical difference in bilirubin levels between patients with first-episode schizophrenia and HCs [ 37 ]. Even more, some studies conducted in different countries (South Korea, China, and Norway) found varying degrees of reduction in serum bilirubin levels in patients with schizophrenia compared with HCs [ 21 , 38 , 39 ]. In this case, reduced bilirubin levels may indicate a defect in the antioxidant defense system of patients with schizophrenia or a sustained oxidative depletion during the chronic course of this disease [ 40 , 41 ].
In this study, we also found some associations between bilirubin and psychopathology in patients with AEDF schizophrenia. First, bilirubin levels were positively associated with the total score of BPRS. A clinical study found that increased oxidative metabolites of bilirubin are correlated with higher scores of BPRS in schizophrenia, and higher scores of Hamilton depression scale (HAMD) in depression [ 42 ]. These findings suggest that higher bilirubin levels are associated with exacerbated psychotic states, possibly as a result of bilirubin-induced neurotoxic effects and pro-inflammatory responses, as well as alterations in brain structures. For example, high bilirubin levels result in a rapid increase in extracellular glutamate concentrations, which ultimately leads to neuronal and oligodendrocyte damage [ 43 ]. In addition, by inducing the secretion of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), bilirubin provokes mitochondrial swelling, and inhibits the formation of myelin, which aggravates the patient’s psychiatric symptoms [ 44 , 45 , 46 ]. Neuroimaging studies found that patients with schizophrenia combined with hyperbilirubinemia have an enlarged cerebrospinal fluid cavity and enhanced signal in areas such as the frontotemporal cortex and the limbic system and basal ganglia [ 47 , 48 , 49 ].
Second, we found that bilirubin levels were positively associated with the BPRS resistance subscale score, which is usually defined by hostility, uncooperativeness and suspiciousness [ 30 ]. One Portuguese study [ 24 ] found that a positive association between UCB level with disturbing and aggressive behaviors in patients with schizophrenia and schizoaffective, which bring a new insight into the novel role of UCB level as a biomarker for psychomotor agitation. This was confirmed by the improvement in psychomotor agitation with decreasing bilirubin levels during a subsequent treatment. In patients with hyperbilirubinemia, elevated oxygen radicals or oxidative stress markers are associated with an increased risk of aggressive behaviors [ 50 , 51 ]. Sustained oxidative stress can lead to reduced neuronal membrane fluidity, ion channel inactivation and demyelination, which may result in brain connectivity and impulse control disorders [ 52 , 53 ]. A meta-analysis and the United States National Survey showed that the prevalence of aggressive behavior in patients with schizophrenia and bipolar disorder was 33.3% and 12.2%, respectively, both much higher than in the general population [ 54 , 55 ]. Therefore, it is necessary to monitor bilirubin levels, which can objectively and accurately assess the risk of aggression and hostility in patients with severe mental illness. Third, a study conducted in unmedicated patients with schizophrenia found that bilirubin levels were positively correlated with the excitement component score of Positive and Negative Syndrome Scale (PANSS) [ 22 ]. Similarly, we also found a significant correlation between a higher CB level and a higher activation subscale score of BPRS, which is usually defined by excitement, tension, and mannerisms–posturing. However, the correlation disappeared after adjusting for the other BPRS subscale scores. Our interpretation is that the high CB level may be an indicator of a higher activation score, but may not be independent of the other BPRS subscale scores. In clinical evaluation, the use and dissemination of biomarkers that can provide a mode of clinical monitoring are the future frontiers in the evaluation of schizophrenia and related spectrum disorders. In therapy, anti-inflammatory drugs were considered an effective and safe adjunctive therapy for improving the symptoms of schizophrenia [ 56 ]. For example, as an anti-inflammatory drug, minocycline can reduce oxidative stress and protect the brain from bilirubin neurotoxicity [ 57 ].
Overall, we also found that bilirubin levels were negatively associated with levels of FBG, TG, and TC, and were positively associated with HDL-C level in patients with AEDF schizophrenia. Of note, we observed differences in glycolipid metabolic parameters in patients with different hyperbilirubinemia subtypes. Specifically, in the logistic regression analysis model, patients with a higher TB level had a lower TG level, those with a higher CB level had a lower TC level, and those with a higher UCB level had a higher HDL-C level; In the linear regression model, CB, but not UCB levels, were negatively correlated with the FBG level. The differences were also observed in the general population and schizophrenia patients in previous cross-sectional and prospective studies [ 58 , 59 ]. Bilirubin is a lipid-soluble substance, and different types of bilirubin bind differently to albumin. Compared to UCB, CB may be more readily separated from albumin to protect patients from metabolism disorders [ 17 ]. Regarding the mechanism of action of bilirubin on glycolipid metabolism, it has been found that bilirubin can reduce glucose and lipid accumulation by increasing insulin sensitivity and intracellular glucose uptake, and activating the aryl hydrocarbon receptor (AhR) signaling pathway [ 60 , 61 ]. A meta-analysis found that the overall prevalence of MetS in patients with schizophrenia was 32.5%, and the prevalence in unmedicated patients was also as high as 20.2% [ 62 ]. Notably, the risk of cardiovascular death in patients with MetS was 1.6 times higher than in those without, and 3.2 times higher than in the general population [ 63 , 64 ]. As a valid predictor of MetS risk, bilirubin has potential use in monitoring and assessing the risk of cardiovascular events and death in patients with schizophrenia.
In this study, there are several limitations. First, due to methodological limitations, including cross-sectional study design, no patients with medicated schizophrenia or other severe mental disorders (e.g. schizoaffective and bipolar disorders), we were unable to make comparisons of bilirubin levels between subgroups [ 65 ]. Second, factors potentially associated with bilirubin levels, such as smoking, the use/abuse of caffeine, and pro re nata treatment (e.g. psychotherapy and counseling intervention) before blood sampling, were not examined. Finally, we have to acknowledge the importance of excluding pseudo- or organic schizophrenia, such as Gilbert syndrome by genetic testing, encephalitis by cerebrospinal fluid testing, and encephalic anomaly by electroencephalography and magnetic resonance imaging [ 24 , 66 ]. Therefore, our results should be considered preliminary.
In summary, patients with AEDF schizophrenia had higher bilirubin levels, especially CB level, compared with HCs. Patients with high bilirubin levels have an exacerbated psychotic state with high risk of aggression, hostility and excitement; and have relatively optimized glycolipid metabolism with lower TG, TC and FBG levels and higher HDL-C levels. In clinical practice, regular monitoring of bilirubin levels is necessary to objectively and comprehensively assess psychopathology and the risk of glycolipid metabolism disorders in patients with schizophrenia. Given that our findings are concerning but preliminary, longitudinal studies are needed to further explore whether management of peripheral bilirubin could improve the prognosis of this patient population.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Hasan A, Falkai P, Lehmann I, Gaebel W, Schizophrenia. Dtsch Arztebl Int. 2020;117(24):412–19.
PubMed PubMed Central Google Scholar
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–24.
Article PubMed Google Scholar
Hamoud AR, Weaver L, Stec DE, Hinds TD. Jr. Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol Metab. 2018;29(3):140–50.
Article CAS PubMed PubMed Central Google Scholar
Zipursky A, Bhutani VK, Odame I. Rhesus disease: a global prevention strategy. Lancet Child Adolesc Health. 2018;2(7):536–42.
Müller N, Schiller P, Ackenheil M. Coincidence of schizophrenia and hyperbilirubinemia. Pharmacopsychiatry. 1991;24(6):225–8.
Vítek L, Jirásková A, Malíková I, Dostálová G, Eremiášová L, Danzig V, et al. Serum bilirubin and markers of oxidative stress and inflammation in a healthy Population and in patients with various forms of atherosclerosis. Antioxid (Basel). 2022;11(11):2118.
Article Google Scholar
Doré S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96(5):2445–50.
Article PubMed PubMed Central Google Scholar
Bortolussi G, Shi X, Ten Bloemendaal L, Banerjee B, De Waart DR, Baj G, et al. Long-Term effects of Biliverdin Reductase a Deficiency in Ugt1(-/-) mice: impact on Redox Status and Metabolism. Antioxid (Basel). 2021;10(12):2029.
Article CAS Google Scholar
Bianco A, Dvořák A, Capková N, Gironde C, Tiribelli C, Furger C, et al. The extent of Intracellular Accumulation of Bilirubin determines its Anti- or pro-oxidant effect. Int J Mol Sci. 2020;21(21):8101.
Lambert B, Semmler A, Beer C, Voisey J. Pyrroles as a potential biomarker for oxidative stress disorders. Int J Mol Sci. 2023;24(3):2712.
Hayashida M, Hashioka S, Hayashida K, Miura S, Tsuchie K, Araki T, et al. Low serum levels of fibroblast growth factor 2 in Gunn rats: a Hyperbilirubinemia Animal Model of schizophrenic symptoms. CNS Neurol Disord Drug Targets. 2020;19(7):503–08.
Article CAS PubMed Google Scholar
Dalman C, Cullberg J. Neonatal hyperbilirubinaemia–a vulnerability factor for mental disorder? Acta Psychiatr Scand. 1999;100(6):469–71.
Miyaoka T, Ieda M, Hashioka S, Wake R, Furuya M, Liaury K, et al. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2015;69(11):693–8.
Pommerening Dornelles E, Gama Marques J, Ouakinin S. Unconjugated bilirubin and schizophrenia: a systematic review. CNS Spectr. 2019;24(6):577–88.
Gama Marques J, Pedro I, Ouakinin S. Unconjugated bilirubin and acute psychosis: a five years retrospective observational and controlled study in patients with schizophrenia, schizoaffective and bipolar disorders. Int J Psychiatry Clin Pract. 2019;23(4):281–85.
Gama-Marques J, Tinoco I, Pedro I, Leote F, Silva R, Ouakinin S. Unconjugated bilirubin and acute schizophrenia: a probable correlation? Actas Esp De Psiquiatria. 2017;45(2):79–88.
Google Scholar
Liang C, Yu Z, Bai L, Hou W, Tang S, Zhang W, et al. Association of Serum Bilirubin with metabolic syndrome and non-alcoholic fatty liver disease: a systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2022;13:869579.
Zhang X, Meng Z, Li X, Liu M, Ren X, Zhu M, et al. The association between total bilirubin and serum triglyceride in both sexes in Chinese. Lipids Health Dis. 2018;17(1):217.
Seyed Khoei N, Wagner KH, Sedlmeier AM, Gunter MJ, Murphy N, Freisling H. Bilirubin as an indicator of cardiometabolic health: a cross-sectional analysis in the UK Biobank. Cardiovasc Diabetol. 2022;21(1):54.
Karadag F, Sengul CB, Enli Y, Karakulah K, Alacam H, Kaptanoglu B, et al. Relationship between serum bilirubin levels and metabolic syndrome in patients with Schizophrenia Spectrum disorders. Clin Psychopharmacol Neurosci. 2017;15(2):153–62.
Pae CU, Paik IH, Lee C, Lee SJ, Kim JJ, Lee CU. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50(1):54–6.
Widschwendter CG, Rettenbacher MA, Kemmler G, Edlinger M, Baumgartner S, Fleischhacker WW, et al. Bilirubin concentration correlates with positive symptoms in patients with schizophrenia. J Clin Psychiatry. 2016;77(4):512–6.
Lu Z, Wen T, Wang Y, Kan W, Xun G. Peripheral non-enzymatic antioxidants in patients with schizophrenia: a case-control study. BMC Psychiatry. 2020;20(1):241.
Gama Marques J, Ouakinin S. Clinical profile in schizophrenia and schizoaffective spectrum: relation with unconjugated bilirubin in a prospective and controlled study with psychopathological and psychosocial variables. CNS Spectr. 2020;25(6):782–89.
El Kissi Y, Samoud S, Mtiraoui A, Letaief L, Hannachi N, Ayachi M, et al. Increased Interleukin-17 and decreased BAFF serum levels in drug-free acute schizophrenia. Psychiatry Res. 2015;225(1–2):58–63.
Zhou X, Wang X, Li R, Yan J, Xiao Y, Li W, et al. Neutrophil-to-lymphocyte ratio is independently Associated with severe psychopathology in Schizophrenia and is changed by Antipsychotic Administration: a large-scale cross-sectional retrospective study. Front Psychiatry. 2020;11:581061.
Overall JE, Gorham DR. The brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812.
Zhang M, Wang Z. Brief Psychiatric Rating Scale Chinese version (BPRS-CR) application notes. Shanghai Archives Psychiatry. 1983;03:130–33.
Overall JE, Hollister LE, Pichot P. Major psychiatric disorders. A four-dimensional model. Arch Gen Psychiatry. 1967;16(2):146–51.
Shafer A. Meta-analysis of the brief psychiatric rating scale factor structure. Psychol Assess. 2005;17(3):324–35.
Rasool M, Malik A, Saleem S, Ashraf MAB, Khan AQ, Waquar S, et al. Role of oxidative stress and the identification of biomarkers Associated with thyroid dysfunction in Schizophrenics. Front Pharmacol. 2021;12:646287.
Glen AI, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, Morse-Fisher N, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994;12(1):53–61.
Silva SL, Vaz AR, Barateiro A, Falcão AS, Fernandes A, Brito MA, et al. Features of bilirubin-induced reactive microglia: from phagocytosis to inflammation. Neurobiol Dis. 2010;40(3):663–75.
Wang S, Yuan X, Pang L, Song P, Jia R, Song X. Establishment of an assistive diagnostic model for schizophrenia with oxidative stress biomarkers. Front Pharmacol. 2023;14:1158254.
Marques JG. Unconjugated bilirubin in Schizophrenia/Schizoaffective Disorder Spectrum: an overlooked and underestimated biomarker candidate. Prim Care Companion CNS Disord. 2020;22(2):1902525.
Pradeep JR, Acharya MS, Radhakrishnan R, Srinivasan K. Elevated unconjugated bilirubin in Schizophrenia compared to bipolar affective disorder. Prim Care Companion CNS Disord. 2019;21(4):19m02448.
Huang K, Tang Y, Chen Z, Ding S, Zeng H, Zhao Y, et al. Comparison of hematological parameters between First-Episode Schizophrenia and Anti-NMDAR Encephalitis. Front Cell Dev Biol. 2022;10:895178.
Xu H, Wei Y, Zheng L, Zhang H, Luo T, Li H, et al. Relation between unconjugated bilirubin and peripheral biomarkers of inflammation derived from complete blood counts in patients with Acute Stage of Schizophrenia. Front Psychiatry. 2022;13:843985.
Solberg DK, Refsum H, Andreassen OA, Bentsen H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 2019;31(4):202–12.
Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63(1):68–78.
Yin XL, Jia QF, Zhang GY, Zhang JP, Shirao T, Jiang CX, et al. Association between decreased serum TBIL concentration and immediate memory impairment in schizophrenia patients. Sci Rep. 2019;9(1):1622.
Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, et al. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15(3):249–52.
Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front Pharmacol. 2012;3:88.
Vodret S, Bortolussi G, Jašprová J, Vitek L, Muro AF. Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1 (-/-) mouse model. J Neuroinflammation. 2017;14(1):64.
Feng J, Li M, Wei Q, Li S, Song S, Hua Z. Unconjugated bilirubin induces pyroptosis in cultured rat cortical astrocytes. J Neuroinflammation. 2018;15(1):23.
Vaz AR, Falcão AS, Scarpa E, Semproni C, Brites D. Microglia susceptibility to free bilirubin is age-dependent. Front Pharmacol. 2020;11:1012.
Wake R, Miyaoka T, Tsuchie K, Kawakami K, Nishida A, Inagaki T, et al. Abnormalities in MRI signal intensity in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia. Aust N Z J Psychiatry. 2009;43(11):1057–69.
Miyaoka T, Yasukawa R, Mihara T, Mizuno S, Yasuda H, Sukegawa T, et al. Fluid-attenuated inversion-recovery MR imaging in schizophrenia-associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). Eur Psychiatry. 2005;20(4):327–31.
Miyaoka T, Seno H, Itoga M, Inagaki T, Horiguchi J. Structural brain changes in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome): a planimetric CT study. Schizophr Res. 2001;52(3):291–3.
Tobore TO. On the neurobiological role of oxidative stress in Alcohol-Induced Impulsive, aggressive and suicidal behavior. Subst Use Misuse. 2019;54(14):2290–303.
Patki G, Atrooz F, Alkadhi I, Solanki N, Salim S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett. 2015;584:308–13.
Gorlova A, Svirin E, Pavlov D, Cespuglio R, Proshin A, Schroeter CA, et al. Understanding the role of oxidative stress, neuroinflammation and abnormal myelination in Excessive Aggression Associated with Depression: recent input from mechanistic studies. Int J Mol Sci. 2023;24(2):915.
Coccaro EF, Lee R, Gozal D. Elevated plasma oxidative stress markers in individuals with intermittent explosive disorder and correlation with aggression in humans. Biol Psychiatry. 2016;79(2):127–35.
Corrigan PW, Watson AC. Findings from the National Comorbidity Survey on the frequency of violent behavior in individuals with psychiatric disorders. Psychiatry Res. 2005;136(2–3):153–62.
Li W, Yang Y, Hong L, An FR, Ungvari GS, Ng CH, et al. Prevalence of aggression in patients with schizophrenia: a systematic review and meta-analysis of observational studies. Asian J Psychiatr. 2020;47:101846.
Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49(14):2307–19.
Zhang F, Chen L, Jiang K. Neuroinflammation in Bilirubin Neurotoxicity. J Integr Neurosci. 2023;22(1):9.
Li XH, Lin HY, Guan LY, Peng H, Wen MM, Cao YQ, et al. Direct bilirubin levels and risk of metabolic syndrome in healthy Chinese men. Biomed Res Int. 2017;2017:9621615.
Fu J, Wang Q, Zhang L, Liu J, Wang G. Serum bilirubin level is increased in metabolically healthy obesity. Front Endocrinol (Lausanne). 2021;12:792795.
Vitek L, Hinds TD Jr., Stec DE, Tiribelli C. The physiology of bilirubin: health and disease equilibrium. Trends Mol Med. 2023;29(4):315–28.
Bates EA, Kipp ZA, Martinez GJ, Badmus OO, Soundarapandian MM, Foster D, et al. Suppressing hepatic UGT1A1 increases plasma bilirubin, lowers plasma urobilin, reorganizes kinase signaling pathways and lipid species and improves fatty liver disease. Biomolecules. 2023;13(2):252.
Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–18.
Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19(1):195.
Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry. 2017;211(3):175–81.
Gama Marques J, Arantes-Gonçalves F. A perspective on a possible relation between the psychopathology of the Schizophrenia/Schizoaffective Spectrum and Unconjugated Bilirubin: a longitudinal protocol study. Front Psychiatry. 2018;9:146.
Marques JG. Organic psychosis causing secondary Schizophrenia in one-Fourth of a cohort of 200 patients previously diagnosed with primary Schizophrenia. Prim Care Companion CNS Disord. 2020;22(2):19m02549.
Download references
Acknowledgements
Not applicable.
This study was supported by the National Clinical Key Specialty Project Foundation (P. R. China), and the Natural Science Foundation of Anhui Province, China (Number: 2108085MH275) and the Scientific Research Project of Anhui Higher Education Institutions (Number: 2022AH050671).
Author information
Yinghan Tian, Cheng Yang and Lewei Liu contributed equally to the work.
Authors and Affiliations
Department of Psychiatry, Chaohu Hospital of Anhui Medical University, 64 Chaohu North Road, Hefei, 238000, Anhui Province, P. R. China
Yinghan Tian, Cheng Yang, Lewei Liu, Haojie Fan, Lei Xia & Huanzhong Liu
Anhui Psychiatric Center, Anhui Medical University, Hefei, Anhui Province, P. R. China
Anhui Provincial Key Laboratory for Brain Bank Construction and Resource Utilization, Anhui Medical University, Hefei, Anhui Province, P. R. China
Huanzhong Liu
Department of Psychiatry, School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, Anhui Province, P. R. China
Yinghan Tian, Cheng Yang, Lewei Liu, Xin Zhao, Haojie Fan & Lei Xia
You can also search for this author in PubMed Google Scholar
Contributions
HL and LX were responsible for the design and direction of the study. YT, CY, LL, XZ and HF were responsible for the collection, analysis and interpretation of the data. Drafting of the manuscript was done by YT. HL and LX were responsible for critical revision of the manuscript. All the authors revised the final version for publication.
Corresponding authors
Correspondence to Lei Xia or Huanzhong Liu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tian, Y., Yang, C., Liu, L. et al. The associations of psychopathology and metabolic parameters with serum bilirubin levels in patients with acute-episode and drug-free schizophrenia: a 5-year retrospective study using an electronic medical record system. BMC Psychiatry 24 , 403 (2024). https://doi.org/10.1186/s12888-024-05862-5
Download citation
Received : 20 October 2023
Accepted : 23 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s12888-024-05862-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Schizophrenia
- Psychopathology
- Glycolipid metabolism
- Oxidative stress
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]

TerXT: Combination of Xanomeline and Trospium Prodrugs for Schizophrenia
Sam Clark, MD, PhD, the founder and CEO of Terran Biosciences shared all you need to know about the development of their schizophrenia treatment, TerXT.

CLINICAL CONVERSATIONS
Terran has announced the development of TerXT, a combination of xanomeline and trospium for the long-acting treatment of schizophrenia. 1 To help share more on the announcement, Psychiatric Times sat down with Sam Clark, MD, PhD, the founder and CEO of Terran Biosciences.
PT : Tell us a little about TerXT in your own words.
Sam Clark, MD, PhD: TerXT is a novel fixed-dose combination therapeutic that consists of a prodrug of xanomeline and a prodrug of trospium. It was designed to enable longer-acting treatments for schizophrenia than would otherwise be possible with the older non-prodrug versions of xanomeline and trospium. Xanomeline and trospium were first developed in the 1990s and 1960s respectively, and in recent clinical trials they were tested as a fixed-dose combination given twice daily, which demonstrated safety and efficacy in treating schizophrenia. Terran was able to use a prodrug approach to overcome inherent pharmacokinetic and physical property limitations of the older forms of xanomeline and trospium with the goal of creating longer-acting combinations of the novel prodrugs, both for once-daily oral administration (“TerXT”) and also long-acting intramuscular injection with multi-month duration (“TerXT LAI”).
PT : What about TerXT sets it apart from other long-acting treatment options for schizophrenia? Can you share more on its mechanism of action, as well as its safety/efficacy data thus far?
Clark: Xanomeline is an agonist of the muscarinic M1 and M4 receptors. As a modulatory neurotransmitter, the muscarinic system is involved in modulating dopaminergic signaling. While activation of the M1/M4 receptors in the brain has an antipsychotic effect, activation of these receptors in the rest of the body leads to gastrointestinal adverse effects such as nausea and vomiting. To cancel out these peripheral effects, xanomeline is combined with trospium, which is a peripherally-restricted muscarinic receptor blocker. Trospium does not cross the blood brain barrier and thus only cancels out the effects of xanomeline in the rest of the body. This muscarinic mechanism is exciting as it represents the first truly novel antipsychotic mechanism in over 50 years.
The older non-prodrug twice-daily oral combination of xanomeline and trospium (called “KarXT”) has already passed 2 phase 3 studies with an excellent safety and efficacy profile and is currently under FDA review for approval, with a PDUFA date scheduled for September 26 of this year. There were 2 phase 3 trials conducted in adult schizophrenia patients, EMERGENT-2 with 252 patients and EMERGENT-3 with 256 patients. Patients were randomized 1:1 to receive KarXT (flexible dose) or placebo twice daily for 5 weeks. The primary endpoint measured was the change in the Positive and Negative Syndrome Scale (PANSS) total score from baseline. In both studies KarXT was found to be well-tolerated and efficacious, with EMERGENT-2 showing a 9.6-point decline and EMERGENT-3 showing a 8.4-point decline in PANSS total scores from baseline.
What differentiates TerXT from KarXT is that TerXT utilizes a dual prodrug approach, and includes both a prodrug of xanomeline and a prodrug of trospium. In vitro and in vivo safety studies with TerXT have shown an excellent safety profile to date. Prodrug strategies have been widely used in the industry to improve older antipsychotics including aripiprazole lauroxil (Aristada®) and iloperidone palmitate (Invega Halfyera®) to make long-acting injectables with multi-month durations. Additionally, prodrug versions of antipsychotics are often approved via the 505(b)(2) accelerated regulatory pathway.
PT : What is the 505(b)(2) regulatory pathway? How does it differ from other approval processes? What makes TerXT a good candidate?
Clark: The FDA 505(b)(2) regulatory pathway is designed for the approval of drugs that have the same active ingredient as an FDA approved product, and allows the drug developer to reference all of the completed safety and efficacy studies of the approved drug as to not have to repeat those studies. The sponsor then must simply conduct bridging pharmacokinetic and safety studies to show bioequivalence with the approved drug, typically avoiding the need for large phase 2 and phase 3 clinical efficacy studies that are required in a standard 505(b)(1) process.
If the current non-prodrug form of xanomeline/trospium is approved on the 505(b)(1) pathway later this year, the FDA would grant a period of data exclusivity lasting approximately 5 years. After this period, TerXT would a good candidate for the 505(b)(2) pathway because it contains prodrugs of the active compounds. As mentioned previously, this prodrug approach is common, and antipsychotic prodrugs have been approved on the 505(b)(2) pathway many times before.
PT : Why should clinicians be excited about this treatment option? How do you anticipate it will change patient outcomes?
Clark: A twice-daily oral antipsychotic may pose a challenge for patients with schizophrenia as medication compliance is a known issue, and we believe clinicians would be excited by having access to improved formulations if available. The gold standard for oral antipsychotics is once-daily dosing, which we hope to achieve with TerXT. Additionally, multi-month duration is becoming the standard for long-acting injectable antipsychotics, which we hope to achieve with TerXT LAI. In comparison to the older twice-daily xanomeline/trospium, we believe longer-acting prodrug forms could potentially improve ease of dosing, increase compliance, and reduce psychotic episodes due to noncompliance.
PT : What are the next steps for TerXT?
Clark: After the approval of the older xanomeline/trospium therapeutic later this year, Terran plans to follow the well-established playbook for antipsychotic prodrugs, moving TerXT into pharmacokinetic bridging studies and toward a potential 505(b)(2) pathway to approval.
PT : Thank you!
Dr Clark is the founder and CEO of Terran Biosciences.
1. Terran Biosciences announces development of TerXT, a combination of xanomeline and trospium prodrugs for the treatment of schizophrenia, and intends to pursue accelerated 505(b)(2) approval pathway. News release. May 20, 2024. https://terranbiosciences.com/TerXT

Why Clinicians Should Be Excited About Austedo XR

Treating ‘Morally Objectionable’ Patients

A Critical Step Forward: A Clinician’s Thoughts on MDMA-Assisted Therapy

More Than Postpartum Depression: Addressing Maternal Mental Health Through the Life Cycle

MDMA-Assisted Therapy for PTSD: A Conversation With Amy Emerson, CEO of Lykos Therapeutics

The Cutting Edge of Medicine: An Interview With Alejandro Alva, MD
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


IMAGES
VIDEO
COMMENTS
The patient was diagnosed with schizophrenia by a psychiatrist and was prescribed Risperdal. Implications: This case study reinforces the importance of a thorough patient interview by physical therapists to rule out non-musculoskeletal disorders. Patients seeking neuromusculoskeletal assessment and treatment may have undiagnosed primary or ...
Schizophrenia is a chronic and severe mental disorder characterized by distortions in thinking, perception, emotions, language, sense of self, and behaviour. This report presents the role of clinical pharmacists in the management of a patient diagnosed with schizophrenia with symptoms of paranoia. A gainfully employed young African male adult reported to be roaming around town moving from one ...
Case Study: Bryant. Thirty-five-year-old Bryant was admitted to the hospital because of ritualistic behaviors, depression, and distrust. At the time of admission, prominent ritualistic behaviors and depression misled clinicians to diagnose Bryant with obsessive-compulsive disorder (OCD). Shortly after, psychotic symptoms such as disorganized ...
Case study 1: A man who suddenly stops smoking. A man aged 35 years* has been admitted to a ward following a serious injury. He has been taking olanzapine 20mg at night for the past three years to treat his schizophrenia, without any problems, and does not take any other medicines. He smokes 25-30 cigarettes per day, but, because of his ...
Introduction. Schizophrenia is a chronic severe mental illness with heterogeneous clinical profile and debilitating course. Research shows that clinical features, severity of illness, prognosis, and treatment of schizophrenia vary depending on the age of onset of illness.[1,2] Hence, age-specific research in schizophrenia has been emphasized.Although consistency has been noted in ...
Mental Health Clinician (2012) 1 (8): 191-195. Clinical pearls based on the treatment of a patient with schizophrenia who had stabbed a taxi cab driver are discussed in this case study. Areas explored include the pharmacokinetics of fluphenazine decanoate, strategies to manage clozapine-associated agranulocytosis, and approaches to addressing ...
The patient had been healthy and studying for a doctoral degree when she began having symptoms of psychosis. Her first symptom was a belief that "people were talking about her" as part of a ...
A schizophrenic patient and his family were provided with a nine month multi-component behavioural intervention programme as part of a controlled study. The patient was at high risk of relapse according to the High EE status of his parents. Multiple outcome measures were used to assess the efficacy of the programme.
Worse still, the nature of the health care system does not allow family members to be actively involved in the care of those living with schizophrenia. In contrast, the several case studies described from developing countries illustrate that although psychiatrists may indicate in patient records that one meets criteria for schizophrenia ...
A single case study is obviously far from being predictive for the efficacy of a drug, however, the results seen with this case are promising. With a dose recommended for patients with negative symptoms, our patient's clinical condition, including positive, negative, and cognitive symptoms, as well as social functioning have improved notably ...
This case study describes the cognitive-behavioral therapy (CBT) of a married adult male diagnosed with paranoid schizophrenia. "Michael" was initially oriented to CBT for psychosis (CBTp) in a partial hospital program at McLean Hospital in Belmont, Massachusetts. Michael was then followed as an outpatient over 30 weekly sessions of CBTp.
Researchers evaluated a case study and treatment of a patient who was diagnosed with schizophreniform disorder who presented at an emergency department testing positive for COVID-19.
Schizophrenia is considered one of the most severe psychiatric disorders (5). It is often associated with significant neurocognitive and social cognition deficits (6-8), daily functional impairment for many, high levels of internalized stigma (9, 10), and poor real-world outcomes (11-13). In this context, case reports and case series of ...
Introduction. The goal of this guideline is to improve the quality of care and treatment outcomes for patients with schizophrenia, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (American Psychiatric Association 2013).Since publication of the last full practice guideline (American Psychiatric Association 2004) and guideline watch (American Psychiatric ...
A case study he shared recently in the Journal of Psychiatric Practice illustrates the problem.Margolis, along with colleagues Krista Baker, schizophrenia supervisor at Johns Hopkins Bayview Medical Center, visiting resident Bianca Camerini, and Brazilian psychiatrist Ary Gadelha, described a 16-year-old girl who was referred to the Early Psychosis Intervention Clinic at Johns Hopkins Bayview ...
chemistry and structure of the brain, are identified as causes of schizophrenia. (6) There are seven. subtypes of schizophrenia, classified according to their symptoms. (1) Paranoid schizophrenia ...
Taipale, H. et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20 ...
Case reports provide insight into the differential diagnosis, overlapping diagnoses and the increased complexity (such as treating schizophrenia and obsessive-compulsive disorder), decision-making, and clinical management of unusual cases as a valuable educational tool.Schizophrenia is a severe mental disorder, often associated with ...
Case Study: Schizophrenia and Work: Martin's Story. Martin had been out of work for several years following a prolonged psychotic episode which began when he was studying at university. He desperately wanted to get into work but found that employers treated his prolonged absence "on the sick" with suspicion. He thought that if he could do ...
Case Illustration 2. YS is a 61-year-old woman with a medical history of schizophrenia diagnosed in her early 20s. After a decade of having very limited contact with family and experiencing homelessness as a result of discontinuing of her antipsychotic medication, YS was hospitalized and restarted on an antipsychotic, after which she resumed contact with her family.
Understanding Schizophrenia: A Case Study. Schizophrenia is characterized mainly, by the gross distortion of reality, withdrawal from social interaction, disorganization and fragmentation of perception, thoughts and emotions. Insight is an important concept in clinical psychiatry, a lack of insight is particularly common in schizophrenia patient.
Negative symptoms of schizophrenia, which affect up to 60% of patients with schizophrenia ... To our knowledge, no cariprazine-related data has been published in this type of patients. A single case study is obviously far from being predictive for the efficacy of a drug, however, the results seen with this case are promising. ...
Electronic database searches were conducted to identify case reports or series evaluating the efficacy of C/M-ECT in patients with schizophrenia or schizoaffective disorder. Results C/M-ECT treatment span varied from 3 months to 36 years ( Median = 30 months; M = 43.9 months; SD = 63.0) and was effective in maintaining remission for most ...
Schizophrenia is a serious mental illness that affects about 3.9 million people in the United States and 24 million people worldwide. 1 The symptoms are typically categorized into 3 general categories: positive (delusions, disorganized speech, or hallucinations), negative (lack of pleasure, enjoyment, or motivation), and cognitive (impaired executive function). 2 The annual economic burden of ...
Indeed, data from an Italian study showed that the management of comorbid SUD in patients with schizophrenia is increasingly complex, highlighting an urgent need to optimize the management of this difficult-to-treat condition by considering several factors (i.e., treatment efficacy, tolerability, metabolic effect sides) and, according to a ...
Approximately one-third of patients with schizophrenia do not receive meaningful benefits from first-line antipsychotics ().When this occurs despite two or more adequate treatment episodes with different antipsychotics, it is termed treatment-resistant schizophrenia ().Treatment-resistant schizophrenia is associated with reduced quality of life and substantial economic cost to the individual ...
Medications to treat schizophrenia come in three forms: Second-generation antipsychotics. First-generation antipsychotics. Long-acting injectable antipsychotics. This article discusses the three types of antipsychotic medications to treat schizophrenia, including how they work and side effects they may cause.
Background The oxidative system plays an important role in the pathogenesis of schizophrenia. Inconsistent associations were found between hyperbilirubinemia and psychopathology as well as glycolipid metabolism in patients with schizophrenia at different episodes. This current study aimed to examine these associations in patients with acute-episode and drug-free (AEDF) schizophrenia. Methods ...
CLINICAL CONVERSATIONS. Terran has announced the development of TerXT, a combination of xanomeline and trospium for the long-acting treatment of schizophrenia. 1 To help share more on the announcement, Psychiatric Times sat down with Sam Clark, MD, PhD, the founder and CEO of Terran Biosciences. PT: Tell us a little about TerXT in your own words.. Sam Clark, MD, PhD: TerXT is a novel fixed ...
Clozapine is a second generation antipsychotic Food and Drug Administration-approved for treatment-resistant schizophrenia and reducing suicidal behavior in patients with schizophrenia and schizoaffective disorder. 1 Clozapine is also considered a treatment option for patients with bipolar disorder, tardive dyskinesia, and psychosis associated with Parkinson disease. 2 However, clozapine ...