
Consulte los artículos y contenidos publicados en éste medio, además de los e-sumarios de las revistas científicas en el mismo momento de publicación
Esté informado en todo momento gracias a las alertas y novedades
Acceda a promociones exclusivas en suscripciones, lanzamientos y cursos acreditados
Estamos experimentado problemas con el acceso a las cuentas de usuarios. Disculpe la molestias
- I have forgotten my password

The GE Portuguese Journal of Gastroenterology is the official publicatio of the Portuguese Society of Gastroenterology, Portuguese Society of Digestive Endoscopy and the Portuguese Association for the Study of the Liver. The GE Portuguese Journal of Gastroenterology publishes original manuscripts on on Gastroenterology, Digestive Endoscopy, Hepatology and related matters. Review articles, clinical cases, images, letters to the editor and other articles are also published and included in the structure of the journal (such as recommendations, articles on gastroenterology clinical practice and notifications of meetings of scientific societies). Articles must be written in English.
An 84-year-old woman presented with a 2-day history of jaundice, fever and abdominal pain. Physical examination showed scleral icterus and right upper quadrant tenderness without inspiratory arrest at palpation (absent Murphy's sign). Laboratory workup revealed leukocytosis (12.4 × 10 3 μL), elevated C-reactive protein (8.3 mg/dL) and cholestasis (bilirubin 5.4 mg/dL, alkaline phosphatase 893 U/L, gamma-glutamyl transferase 1143 U/L) with elevated liver enzymes (aspartate aminotransferase 231 U/L, alanine aminotransferase 178 U/L). Abdominal ultrasound demonstrated a scleroatrophic gallbladder with cholelithiasis and an impacted large gallstone in the common bile duct with dilated common and intrahepatic bile ducts.
We performed an endoscopic retrograde cholangiopancreatography (ERCP) that clearly showed common hepatic duct compression by a large gallstone (20 mm) impacted in the cystic duct ( Fig. 1 ), compatible with the diagnosis of Mirizzi syndrome. Successful biliary decompression was performed by internal stenting ( Fig. 2 ) with subsequent patient referral to surgery (cholecystectomy plus closure of the fistula).
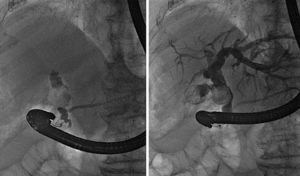
ERCP: cholangiography.
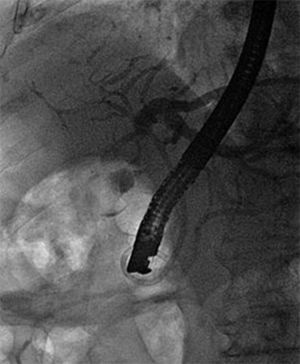
ERCP: internal stenting.
The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. 1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the laboratory evaluation elevations in the serum concentrations of alkaline phosphatase and bilirubin. 2
The Mirizzi syndrome is part of the differential diagnosis of obstructive jaundice and therefore the diagnostic approach usually begins with ultrasonography complemented by ERCP or magnetic resonance cholangiography.
A useful classification system takes into account the presence and extent of a cholecystobiliary fistula, due to erosion of the anterior or lateral wall of the common bile duct by impacted stones. 3
Surgery is the mainstay of therapy for Mirizzi syndrome. 4 ERCP treatment can be effective as a temporizing measure before surgery and can be definitive treatment for unsuitable surgical candidates.
The authors declare that no experiments were performed on humans or animals for this study.
The authors declare that they have followed the protocols of their work center on the publication of patient data.
The authors declare that no patient data appear in this article.
- Subscribe to our newsletter
- Send to a friend
- Export reference

- Current Issue
- Supplements
- Aims and scope
- Most often read
- All metrics
- Call for papers
- Artículos más leídos
- Colecciones
- Fondo editorial
- Más información
- Download PDF
- Bibliography
- Get new issue alerts Get alerts
- Submit a Manuscript

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Ayurveda management of jaundice: A case study
Sinha, Nitu; Ojha, Nisha Kumari
Department of Kaumarbhritya, National Institute of Ayurveda, Deemed To Be University (De Novo), Jaipur, Rajasthan, India
Address for correspondence: Dr. Nitu Sinha, Department of Kaumarbhritya, National Institute of Ayurveda, Deemed to Be University (De Novo), Jaipur 302002, Rajasthan, India. E-mail: [email protected]
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- Infographic
Jaundice is a yellow discoloration of the body’s tissues from excess bilirubin (hyperbilirubinemia). According to the Ayurveda perspective, the clinical presentation of jaundice can be correlated with that of the disease Kamala . We present a case of a 10-year-old female patient who attained the outpatient department with complaints of anorexia, poor appetite, general weakness, abdominal pain, and hard stools since 10 days. The examination revealed yellow discoloration of the sclera, oral mucosa, skin, urine, and hard yellowish stool, and the blood investigation revealed elevated level of serum bilirubin, serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, and alkaline phosphatase. The case was managed with Tab Liv 52 DS, 1 tab twice a day and Tab Nirocil, 1 tab twice daily before food with lukewarm water . Mridu Virechana (mild purgation) was done using a combination of Avipatikar Churna 2 gm twice a day and Kutki Churna (powder of Picrorhiza kurrooa ) 1 gm twice a day with lukewarm water for 4 weeks. These medications effectively improved the clinical symptoms, and the appetite increased, the patient became more active, and the icterus decreased. The patient also started passing normal stool and urine. complete blood count, liver function test , and urine examination revealed that the values were within the reference range. This combination can be utilized for symptoms of Koshthashrita Kamala .

Introduction
The clinical features of jaundice are abdominal pain, nausea, and fatigue. Some clinical features are also explained in the case of Kamala . [ 1 ] So, based on common characteristics and pathology, Kamala (Jaundice) can be correlated with jaundice. Jaundice, also known as hyperbilirubinemia, is a yellow discoloration of the body tissue resulting from the accumulation of an excess of bilirubin. [ 2 ] In Ayurveda, Jaundice is associated with the Pitta Dosha because a person with Pandu Roga (anemia) may acquire jaundice if they continue to consume Pitta vitiating diet. [ 3 ] According to Acharya Sushrut, jaundice is a distinct illness that a further complication of anemia may also cause. [ 4 ] Ayurvedic classics have mentioned the treatment of jaundice and its complications. The present case study reports the treatment of a 10-year-old female child with jaundice.
Patient Information
A 10-year-old female patient visited Kaumarbhritya outpatient department, National Institute of Ayurveda, Jaipur on August 16, 2022 with complaints of yellowish discoloration of the body, anorexia, poor appetite, generalized weakness, abdominal pain, and hard stool since 10 days. There was no history of diarrhea/blood transfusions/contact with a patient with jaundice, or surgery. The patient had taken allopathic medication [tablet of ofloxacin 200 mg, one tablet twice a day for 7 days, a liver tonic, 5 mL thrice a day for 15 days, a syrup of Ornithine and Aspartate, 5 mL thrice a day for 15 days, a lactulose syrup, 20 mL twice a day for 15 days, Paracetamol (500 mg) syrup 5 mL Si Opus Sit (means as and when required)] since August 7, 2022. When symptoms were not relieved, the patient’s guardian brought her to take Ayurvedic treatment.
Clinical Finding
On examination, the child looked ill, weak, pale, hemodynamically stable, afebrile, well-oriented, and responding to commands. Her sclera was deep yellow; her skin was dry, lusterless, with a yellowish hue. The pulse was 86 per minute, regular, and the blood pressure was within normal range. Per abdominal examination revealed tenderness in the right upper quadrant, and the liver was palpable 1.5 cm below the costal margin, soft, with normal peristaltic sounds. There were no cutaneous markers like spider naevi, ascites, or palpable lymph nodes. On systemic examination, the respiratory sounds were bilaterally equal, clear chest, no added sound present, and no obvious deformity, and S1 and S2 were audible, with no murmur. Gastrointestinal tract findings, such as decreased appetite and constipation, were present, and the patient also reported yellowish discoloration of urine. Other systems were normal.
Personal history revealed that the child lost appetite with significantly less food intake and one kg weight loss since the onset of the illness. Her bowel habit was once on alternate days and 6–7 times per day urinary outputs. Sleep was slightly disturbed, mild irritability and on and off aversion and off aversion to food.
Ayurveda clinical assessment revealed, Nadi (pulse): 82/min; Mala (stool): yellowish, constipated; Mutra (urine): yellowish; Jihwa (tongue): Ishat Saam (slightly whitish coated); Agni (digestive fire): Mandagni ; Shabda (speech): normal; skin and eyes were slightly yellowish.
The patient was normal before 2 months of manifestation of illness. Symptoms such as anorexia, poor appetite, generalized weakness, abdominal pain, and hard stool started manifesting and increased gradually. The patient had taken allopathic medicines from August 7, 2022, to August 15, 2022, but had not felt satisfactory relief. The patient came for Ayurvedic treatment on August 16, 2022, and continued until September 27, 2022. The total duration of the treatment was 6 weeks [ Table 1 ].

Diagnostic Assessment
The diagnosis was made as jaundice based on clinical manifestations such as yellowish discoloration of skin and sclera of the eyes due to high levels of serum bilirubin; and the laboratory investigation, that is, liver function test [total bilirubin (5.41 mg/dL), indirect bilirubin (1.96 mg/dL), direct bilirubin (3.45 mg/dL), serum glutamic-oxaloacetic transaminase (SGOT) (1098.76 U/L), serum glutamic-pyruvic transaminase (SGPT) (1175.84 U/L), alkaline phosphate (628.07 U/L)]. According to Ayurveda, clinical signs such as yellowish eyes, urine, skin, weakness, and yellowish stool, described by Charaka, were present in this case. Thus, based on these symptoms, the diagnosis of Koshthashrita Kamala was confirmed.
Therapeutic Intervention
Deepana and Pachana (increasing appetite and digestion) were done by Tablet Liv 52 DS (1 tablet twice a day after food with lukewarm water) and Tablet Nirocil (1 tablet twice a day before food with lukewarm water). Both formulations have hepatoprotective action. Mild Virechana was done by Avipatikara Churna (2 gm twice a day before food and Kutaki Churna (1 gm twice a day before food) with lukewarm water for 4 weeks. The total duration of intervention was 4 weeks [ Table 2 ].

During this period, the patient was advised to avoid the Pitta Dosha vitiating diet, such as fried and fatty food, and take old rice ( Oryza sativa ), barley ( Hordeum vulgare ), Godhuma (Wheat), carbohydrate-rich diet bread, rice, potato, yam, custard, sugarcane juice, pulses such as Adhaki (Red gram- Cajanuscajan ), Kulattha (horse gram), and Mudga (green gram), Leafy vegetables such as lettuce and spinach, and fruits such as orange, watermelon, apple, Jambu ( Syzygium cumini ), Kapitha ( Feronia limonia ), grapes, pears, carrots, and beets.
Follow-up and Outcomes
Follow-up was done twice in the interval of 7 days. In the second follow-up, the patient has come 2 days before the follow-up period. So, the patient was kept only on oral medication for 12 days. Thus, after 12 days of treatment, the investigations including complete blood count, liver function test, and urine examination of the patient were done, and the liver function test showed improvement. During each follow-up, improvement was found clinically and by laboratory investigations. There is no recurrence of symptoms during follow-up. Medications were taken until September 14, 2022, but the subsequent investigation was done on September 27, 2022.
Clinically, icterus and yellowish discoloration of urine decreased. There was relief in the symptoms such as loss of appetite, weakness, and a reduction in serum bilirubin levels, SGOT, SGPT, alkaline phosphatase, and PT INR (Prothrombin Time) was obtained in laboratory parameters criteria [ Table 3 ]. Still, the child has not been cured entirely at 2nd follow-up. After 4 weeks of intervention, the child was cured completely and looked normal on clinical examination. The interventions have shown a positive effect on improving appetite and taste and reducing yellow discoloration of skin and urine [ Figure 1 ].

Jaundice occurs due to the excessive breaking of RBC, leading to the accumulation of bilirubin in the blood. According to Ayurveda, excessive Pitta must be excreted from the body; therefore, Pittavirechana is significant in managing Kamala . The liver has a vital role in the excretion of this bilirubin. Therefore, the Pittavirechana and hepatoprotective properties of the drug will be helpful. The given Ayurvedic medications have the quality to remove Pittadushti and treat Kamala (Jaundice).
The main ingredient of Avipattikara Churna is Nishotha ( Operculina turpethum ) which is mild purgative. [ 5 ] It increases gastric motility, enhances gastric secretion, and relieves hyperacidity and constipation. [ 6 ] It also has Triphala Churna as an ingredient, which is a mild laxative. Triphala is the most effective to pacify Pitta Dosha , which was found as a cause of liver disorders in the studied case. All the drug of Avipattikara Churna has Deepana - Pachanga (increase appetite and digestion) property which can improve digestion and metabolism and prevents Ama formation. Avipattikara Churna contains sugar, which also has Pitta pacifying properties. [ 7 ] Kutaki Churna is a strong purgative, liver stimulating, and Pittasaraka (helps excrete Pitta ). [ 8 ] It is helpful in liver problems, spleen disorders, and fever. [ 9 ] It also has hepato-protective, antioxidant, anti-inflammatory, anticancer, immunomodulator, anti-ulcerative colitis, and antimicrobial actions. [ 10 ]
Tab Liv 52 DS is one of the formulations that has been frequently utilized to strengthen the liver. It contains Himsra ( Capparis spinosa ), Kasani ( Cichorium intybus ), Mandur Bhasma , Kakamachi ( Solanum nigrum ), Arjuna ( Terminalia arjuna ), Kasamarda ( Cassia occidentalis ), Biranjasipha ( Achillea millefolium ), Jhavuka ( Tamarix gallica ). It has digestive, liver-stimulating, anti-hepatotoxic, and antioxidant properties. [ 11-14 ] Tab Nirocil contains Bhumyamalaki ( Phyllanthus niruri ), which also has purgative, appetizer, liver stimulating, blood purifying, hepatoprotective, and Balya (provide strength) action [ 15-17 ] [ Table 4 ].

The preferred treatment principle was improving digestive metabolism and mild therapeutic purgation. At first instance, the child was planned with alleviation of vitiated Pitta , anti-inflammatory, clearing obstruction, and increased digestive fire treatment. Virechana was difficult considering Amavastha of disease and the reduced strength of the child. So, the first 3 days of treatment resulted in the relief of acute pain in the abdomen and improved digestive fire, which was indicated by reduced abdominal pain, increased hunger, easy evacuation of bowels, and considerable relief from the initial complaints. Further effectiveness of this step was supported by a reduction in bilirubin level and liver enzyme level (SGOT, SGPT, Alkaline phosphatase) observed in liver function test. Tablet Liv 52 DS and Tablet Nirocil were beneficial for reducing the liver enzyme level. The child’s general health was gradually improved. The clinical absence of jaundice symptoms was observed during a follow-up evaluation. The absence of pigment and bile salts in urine tests further supported this assertion. 1 kg weight gain and decreased yellowishness of the body were also noted during the treatment.
Along with hepatoprotective medicines, Virechana plays a vital role in treating jaundice. This case report reveals that Ayurveda medications are beneficial for managing jaundice in children.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. The consent for publication of picture and clinical information was obtained from the patient and the parents. The patient and parents understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Cited Here |
- Google Scholar
- PubMed | CrossRef |
Jaundice; Koshthashrit Kamala ; Virechana
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Effect of ayurvedic treatment in intractable palmoplantar psoriasis: a case..., kshara sutra</em>: boon for anal fistulae with scrotal extension: a case series', 'hanifa nasreen; sherkhane, rahul', 'journal of research in ayurvedic sciences', 'july-september 2023', '7', '3' , 'p 159-165');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link">interception of fistulous tract and application of kshara sutra : boon for anal..., altmetric attention analysis of ayurveda and covid-19 scholarly publications: a ..., a case study on the management of hemiplegia (intra cranial hemorrhage) through ..., effect of ayurveda interventions in the management of venous stasis dermatitis....
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Obstructive Jaundice Mimicking Pancreatic Cancer: An Unusual Presentation of Autoimmune Pancreatitis
Affiliations.
- 1 General Surgery, Peoples Friendship University of Russia, Moscow, RUS.
- 2 Internal Medicine, American University of Antigua, Osbourn, ATG.
- 3 Medicine and Surgery, University of Benin, Benin, NGA.
- 4 Medicine, Shadan Institute of Medical Sciences, Hyderabad, IND.
- 5 General Surgery, Jimma University, Jimma, ETH.
- 6 Internal Medicine, University of Ghana Medical Centre, Accra, GHA.
- PMID: 37900366
- PMCID: PMC10600591
- DOI: 10.7759/cureus.45970
Autoimmune pancreatitis (AIP) is an uncommon variant of chronic pancreatitis characterized by inflammatory changes within the pancreatic tissue triggered by autoimmune mechanisms. It is known to mimic pancreatic cancer due to its similar clinical and radiological presentations. We underline a case of a 55-year-old male who presented with weight loss, jaundice, and pruritus. Radiological imaging suggested a pancreatic mass, raising suspicion of malignancy. However, subsequent evaluation, absence of parenchymal tissue and lymphoplasmacytic cells on endoscopic ultrasound-guided biopsy, and elevated serum immunoglobulin G4 level resulted in the diagnosis of AIP. Our case emphasizes that AIP should be included in the differential diagnosis of obstructive jaundice, especially when clinical and radiological findings are inconclusive for pancreatic cancer.
Keywords: autoimmune pancreatitis; endoscopic ultrasound; immunosuppressive therapy; obstructive jaundice; pancreatic cancer.
Copyright © 2023, Ogunlaja et al.
PubMed Disclaimer
Conflict of interest statement
The authors have declared that no competing interests exist.
Figure 1. Abdominal ultrasonography reveals a dilated…
Figure 1. Abdominal ultrasonography reveals a dilated pancreatic duct wall (a), and CT abdomen reveals…
Figure 2. A tissue biopsy shows fibrocollagenous…
Figure 2. A tissue biopsy shows fibrocollagenous tissue with scattered lymphoplasmacytic infiltrates containing neutrophils and…
Similar articles
- Autoimmune Pancreatitis Presenting as Obstructive Jaundice Mimicking Pancreatic Cancer: A Case Report. Suvvari TK, Godavari ST, Sanapala P, Panchagnula S, Godavari SKAN. Suvvari TK, et al. Cureus. 2023 Apr 21;15(4):e37947. doi: 10.7759/cureus.37947. eCollection 2023 Apr. Cureus. 2023. PMID: 37228559 Free PMC article.
- Autoimmune chronic pancreatitis. Lin LF, Huang PT, Ho KS, Tung JN. Lin LF, et al. J Chin Med Assoc. 2008 Jan;71(1):14-22. doi: 10.1016/S1726-4901(08)70067-4. J Chin Med Assoc. 2008. PMID: 18218555
- Type 1 Autoimmune Pancreatitis Masquerading as Pancreatic Head Carcinoma. Agboola AA, Mohamed KH, Syed M, Shiwlani S, Butt R, Reza RR, Haseeb M, Nasir H. Agboola AA, et al. Cureus. 2023 Oct 22;15(10):e47471. doi: 10.7759/cureus.47471. eCollection 2023 Oct. Cureus. 2023. PMID: 38022068 Free PMC article.
- Localized Autoimmune Pancreatitis: Report of a Case Clinically Mimicking Pancreatic Cancer and a Literature Review. Cao Z, Tian R, Zhang T, Zhao Y. Cao Z, et al. Medicine (Baltimore). 2015 Oct;94(42):e1656. doi: 10.1097/MD.0000000000001656. Medicine (Baltimore). 2015. PMID: 26496272 Free PMC article. Review.
- Strategy to differentiate autoimmune pancreatitis from pancreas cancer. Takuma K, Kamisawa T, Gopalakrishna R, Hara S, Tabata T, Inaba Y, Egawa N, Igarashi Y. Takuma K, et al. World J Gastroenterol. 2012 Mar 14;18(10):1015-20. doi: 10.3748/wjg.v18.i10.1015. World J Gastroenterol. 2012. PMID: 22416175 Free PMC article. Review.
- Autoimmune pancreatitis presenting as obstructive jaundice mimicking pancreatic cancer: a case report. Suvvari TK, Godavari ST, Sanapala P, Panchagnula S, Godavari SK. Cureus. 2023;15:0. - PMC - PubMed
- Autoimmune pancreatitis masquerading as carcinoma head of pancreas: a case report and review of literature. Gill M, Brar K, Godara R, Bhargava S, Sachdeva B, Sen R, Jain P. Ann Med Surg (Lond) 2019;45:82–85. - PMC - PubMed
- Focal mass-forming autoimmune pancreatitis mimicking pancreatic cancer: which strategy? Hedfi M, Charfi M, Nejib FZ, et al. https://pubmed.ncbi.nlm.nih.gov/31729749/ Tunis Med. 2019;97:731–735. - PubMed
- Basyal B, Pawan KC. Treasure Island: StatPearls; 2023. Autoimmune Pancreatitis. - PubMed
- The diagnostic challenges of autoimmune pancreatitis. Papp K, Angst E, Seidel S, Flury-Frei R, Hetzer FH. Case Rep Gastroenterol. 2015;9:56–61. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The constellation of risk factors and paraneoplastic syndromes in cholangiocarcinoma: integrating the endocrine panel amid tumour-related biology (a narrative review).

Simple Summary
1. introduction, 2. cca-associated risk factors and potential contributors, 2.1. chronic biliary diseases, 2.2. chronic liver conditions, 2.3. digestive ailments, 2.4. parasitic infections, 2.5. lifestyle influence, 2.6. environmental exposure, 2.7. genetic and epigenetic (potential) interplay, 3. metabolic and endocrine interferences in cca development, 3.1. non-alcoholic fatty liver disease (nafld), 3.2. obesity, 3.3. type 2 diabetes mellitus, 3.4. vitamin d status, 3.5. glucagon-like peptide 1 receptor (glp-1r), 3.6. galanin system, 3.7. sex hormone therapy, 4. paraneoplastic syndrome in ccas, 4.1. dermatological features have been found as followings, 4.1.1. acanthosis, 4.1.2. alopecia, 4.1.3. dermatomyositis, 4.1.4. porokeratosis, 4.1.5. necrotic migratory erythema, 4.1.6. persistent erythema multiform, 4.1.7. sweet syndrome, 4.1.8. bazex syndrome, 4.1.9. erythema, 4.1.10. pityriasis, 4.1.11. lupus, 4.1.12. leser–trelat sign, 4.1.13. porphyria, 4.2. neurological paraneoplastic elements, 4.3. renal findings, 4.4. haematological manifestations, 4.5. humoral manifestations, 5. discussion, 5.1. imagery tools to help the clinical assessment, 5.2. is there a place for endocrine considerations in ccas, 5.3. current limits and further expansion, 6. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest, abbreviations.
| ANCA | anti-neutrophilic cytoplasmic antibodies |
| ATF2 | activating transcription factor-2 |
| CCA | cholangiocarcinoma |
| CA 19-9 | carbohydrate antigen 19-9 |
| CI | confidence interval |
| CEUS | contrast-enhanced ultrasound |
| dCCA | distal cholangiocarcinoma |
| DNA | deoxyribonucleic acid |
| DDP4 | dipeptidyl peptidase 4 inhibitors |
| ENDSs | electronic nicotine delivery systems |
| ERK | extracellular signal-related kinase |
| CCAs | extrahepatic cholangiocarcinoma |
| GLP-1R | glucagon-like peptide 1 receptor |
| GAL-R | galanin receptors |
| G-CSF | granulocyte-colony stimulation factor |
| HBV | hepatitis B virus chronic infection |
| HVC | hepatitis C virus chronic infection |
| iCCA | intrahepatic cholangiocarcinoma |
| JNK | c-Jun N-terminal kinase |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NOX1 | KNOTTED-like homebox |
| NOX | NADPH oxidase |
| pCCA | perihilar cholangiocarcinoma |
| PSC | primary sclerosing cholangitis |
| PTHrP | parathyroid hormone-related protein |
| PTHLH | parathyroid hormone-like hormone |
| PAN | polyarteritis nodosa |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| S1PR2 | sphingosine-1 phosphate receptor 2 |
| TGF-α | tumour growth factor-alpha |
| TNF-α | tumour necrosis factor-alpha |
| TAUS | transabdominal ultrasound |
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019 , 39 (Suppl. S1), 19–31. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dar, F.S.; Abbas, Z.; Ahmed, I.; Atique, M.; Aujla, U.I.; Azeemuddin, M.; Aziz, Z.; Bhatti, A.B.H.; Bangash, T.A.; Butt, A.S.; et al. National guidelines for the diagnosis and treatment of hilar cholangiocarcinoma. World J. Gastroenterol. 2024 , 30 , 1018–1042. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023 , 13 , 233. [ Google Scholar ] [ CrossRef ]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021 , 7 , 65. [ Google Scholar ] [ CrossRef ]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022 , 76 , 1109–1121. [ Google Scholar ] [ CrossRef ]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020 , 17 , 557–588. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vithayathil, M.; Khan, S.A. Current epidemiology of cholangiocarcinoma in Western countries. J. Hepatol. 2022 , 77 , 1690–1698. [ Google Scholar ] [ CrossRef ]
- WHO World Health Organization (WHO). Mortality Database Health Statistics and Information Systems. 2019. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 10 June 2024).
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022 , 27 , 100737. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barner-Rasmussen, N.; Pukkala, E.; Jussila, A.; Färkkilä, M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: A population-based study in Finland. Scand. J. Gastroenterol. 2020 , 55 , 74–81. [ Google Scholar ] [ CrossRef ]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013 , 58 , 2045–2055. [ Google Scholar ] [ CrossRef ]
- Bergquist, A.; Weismüller, T.J.; Levy, C.; Rupp, C.; Joshi, D.; Nayagam, J.S.; Montano-Loza, A.J.; Lytvyak, E.; Wunsch, E.; Milkiewicz, P.; et al. Impact on follow-up strategies in patients with primary sclerosing cholangitis. Liver Int. 2023 , 43 , 127–138. [ Google Scholar ] [ CrossRef ]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017 , 12 , e0186643. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Söreide, K.; Körner, H.; Havnen, J.; Söreide, J.A. Bile duct cysts in adults. Br. J. Surg. 2004 , 91 , 1538–1548. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020 , 72 , 95–103. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jang, M.H.; Lee, Y.J.; Kim, H. Intrahepatic cholangiocarcinoma arising in Caroli’s disease. Clin. Mol. Hepatol. 2014 , 20 , 402–405. [ Google Scholar ] [ CrossRef ]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consensus statement. HPB 2015 , 17 , 691–699. [ Google Scholar ] [ CrossRef ]
- Kim, H.J.; Kim, J.S.; Joo, M.K.; Lee, B.J.; Kim, J.H.; Yeon, J.E.; Park, J.J.; Byun, K.S.; Bak, Y.T. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J. Gastroenterol. 2015 , 21 , 13418–13431. [ Google Scholar ] [ CrossRef ]
- Cai, H.; Kong, W.T.; Chen, C.B.; Shi, G.M.; Huang, C.; Shen, Y.H.; Sun, H.C. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: A meta-analysis of observational studies. BMC Cancer 2015 , 15 , 831. [ Google Scholar ] [ CrossRef ]
- Palmer, W.C.; Patel, T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J. Hepatol. 2012 , 57 , 69–76. [ Google Scholar ] [ CrossRef ]
- Chang, J.S.; Tsai, C.R.; Chen, L.T. Medical risk factors associated with cholangiocarcinoma in Taiwan: A population-based case-control study. PLoS ONE 2013 , 8 , e69981. [ Google Scholar ] [ CrossRef ]
- Welzel, T.M.; Graubard, B.I.; El–Serag, H.B.; Shaib, Y.H.; Hsing, A.W.; Davila, J.A.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based case-control study. Clin. Gastroenterol. Hepatol. 2007 , 5 , 1221–1228. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012 , 12 , 289. [ Google Scholar ] [ CrossRef ]
- Li, M.; Li, J.; Li, P.; Li, H.; Su, T.; Zhu, R.; Gong, J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: A meta-analysis and systematic review. J. Gastroenterol. Hepatol. 2012 , 27 , 1561–1568. [ Google Scholar ] [ CrossRef ]
- Zhang, H.; Zhu, B.; Zhang, H.; Liang, J.; Zeng, W. HBV Infection Status and the Risk of Cholangiocarcinoma in Asia: A Meta-Analysis. BioMed Res. Int. 2016 , 2016 , 3417976. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, H.; Hu, B.; Zhou, Z.Q.; Guan, J.; Zhang, Z.Y.; Zhou, G.W. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: Evidence from a systematic review and meta-analysis of 16 case-control studies. World J. Surg. Oncol. 2015 , 13 , 161. [ Google Scholar ] [ CrossRef ]
- Ralphs, S.; Khan, S.A. The role of the hepatitis viruses in cholangiocarcinoma. J. Viral Hepat. 2013 , 20 , 297–305. [ Google Scholar ] [ CrossRef ]
- Yaziji, N.; Martin, L.; Hillon, P.; Favre, J.P.; Henninger, J.F.; Piard, F. Cholangiocarcinoma arising from biliary micro-hamartomas in a man suffering from hemochromatosis. Ann. Pathol. 1997 , 17 , 346–349. [ Google Scholar ] [ PubMed ]
- Fernandez Pelaez, J.M.; Sanchez Martin, E.; Tirado Miranda, R.; Navarro Martinez, A.; Alamillo, S.A. Hemochromatosis and hilar cholangiocarcinoma: Report of a case. Rev. Esp. Enferm. Dig. 2000 , 92 , 474–475. [ Google Scholar ]
- Di Stefano, F.; Verna, N.; Balatsinou, L.; Schiavone, C.; Di Gioacchino, M. Genetic hemochromatosis with normal transferrin saturation in a man with cholangiocarcinoma and yellow nail syndrome. J. Gastroenterol. Hepatol. 2003 , 18 , 1221–1222. [ Google Scholar ] [ CrossRef ]
- Sulpice, L.; Rayar, M.; Boucher, E.; Pele, F.; Pracht, M.; Meunier, B.; Boudjema, K. Intrahepatic cholangiocarcinoma: Impact of genetic hemochromatosis on outcome and overall survival after surgical resection. J. Surg. Res. 2013 , 180 , 56–61. [ Google Scholar ] [ CrossRef ]
- Morcos, M.; Dubois, S.; Bralet, M.P.; Belghiti, J.; Degott, C.; Terris, B. Primary liver carcinoma in genetic hemochromatosis reveals a broad histologic spectrum. Am. J. Clin. Pathol. 2001 , 116 , 738–743. [ Google Scholar ] [ CrossRef ]
- Nkontchou, G.; Tran Van Nhieu, J.; Ziol, M.; Tengher, I.; Mahmoudi, A.; Roulot, D.; Bourcier, V.; Ganne Carrie, N.; Grando-Lemaire, V.; Trinchet, J.C.; et al. Peripheral intrahepatic cholangiocarcinoma occurring in patients without cirrhosis or chronic bile duct diseases: Epidemiology and histopathology of distant nontumoral liver in 57 White patients. Eur. J. Gastroenterol. Hepatol. 2013 , 25 , 94–98. [ Google Scholar ] [ CrossRef ]
- Pfeiffenberger, J.; Mogler, C.; Gotthardt, D.N.; Schulze-Bergkamen, H.; Litwin, T.; Reuner, U.; Hefter, H.; Huster, D.; Schemmer, P.; Członkowska, A.; et al. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015 , 35 , 1615–1622. [ Google Scholar ] [ CrossRef ]
- Angele-Martinez, C.; Goodman, C.; Brumaghim, J. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014 , 6 , 1358–1381. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kamamoto, Y.; Makiura, S.; Sugihara, S.; Hiasa, Y.; Arai, M. The inhibitory effect of copper on DL-ethionine carcinogenesis in rats. Cancer Res. 1973 , 33 , 1129–1135. [ Google Scholar ]
- Wilkinson, M.L.; Portmann, B.; Williams, R. Wilson’s disease and hepatocellular carcinoma: Possible protective role of copper. Gut 1983 , 24 , 767–771. [ Google Scholar ] [ CrossRef ]
- Huai, J.P.; Ding, J.; Ye, X.H.; Chen, Y.P. Inflammatory bowel disease and risk of cholangiocarcinoma: Evidence from a meta-analysis of population-based studies. Asian Pac. J. Cancer Prev. 2014 , 15 , 3477–3482. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016 , 22 , 4794–4801. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011 , 19 , 349–359. [ Google Scholar ] [ CrossRef ]
- Saich, R.; Chapman, R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J. Gastroenterol. 2008 , 14 , 331–337. [ Google Scholar ] [ CrossRef ]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007 , 4 , e201. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schwartz, D.A. Helminths in the induction of cancer: Opisthorchis viverrini , Clonorchis sinensis and cholangiocarcinoma. Trop. Geogr. Med. 1980 , 32 , 95–100. [ Google Scholar ] [ PubMed ]
- Shin, H.R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010 , 101 , 579–585. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012 , 100B , 341–370. [ Google Scholar ]
- Shin, H.R.; Oh, J.K.; Lim, M.K.; Shin, A.; Kong, H.J.; Jung, K.W.; Won, Y.J.; Park, S.; Park, S.J.; Hong, S.T. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J. Korean Med. Sci. 2010 , 25 , 1011–1016. [ Google Scholar ] [ CrossRef ]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014 , 21 , 301–308. [ Google Scholar ] [ CrossRef ]
- Marahatta, S.B.; Punyarit, P.; Bhudisawasdi, V.; Paupairoj, A.; Wongkham, S.; Petmitr, S. Polymorphism of glutathione S-transferase omega gene and risk of cancer. Cancer Lett. 2006 , 236 , 276–281. [ Google Scholar ] [ CrossRef ]
- Songserm, N.; Promthet, S.; Sithithaworn, P.; Pientong, C.; Ekalaksananan, T.; Chopjitt, P.; Parkin, D.M. MTHFR polymorphisms and Opisthorchis viverrini infection: A relationship with increased susceptibility to cholangiocarcinoma in Thailand. Asian Pac. J. Cancer Prev. 2011 , 12 , 1341–1345. [ Google Scholar ]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The liver cancer pooling project. Br. J. Cancer 2018 , 118 , 1005–1012. [ Google Scholar ] [ CrossRef ]
- Makiuchi, T.; Sobue, T.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Inoue, M.; Tsugane, S. Smoking, Alcohol Consumption, and Risks for Biliary Tract Cancer and Intrahepatic Bile Duct Cancer. J. Epidemiol. 2019 , 29 , 180–186. [ Google Scholar ] [ CrossRef ]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol. Res. Health 2006 , 29 , 245–254. [ Google Scholar ]
- Ye, X.H.; Huai, J.P.; Ding, J.; Chen, Y.P.; Sun, X.C. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: A meta-analysis. World J. Gastroenterol. 2013 , 19 , 8780–8788. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jinga, M.; Jurcuţ, C.; Vasilescu, F.; Becheanu, G.; Stancu, S.H.; Ciobaca, L.; Mircescu, G.; Jinga, V. A rare case of digestive hemorrhage in an elderly patient: Diagnosis and treatment difficulties. Rom. J. Morphol. Embryol. 2012 , 53 (Suppl. S3), 831–834. [ Google Scholar ] [ PubMed ]
- Huang, Y.; You, L.; Xie, W.; Ning, L.; Lang, J. Smoking and risk of cholangiocarcinoma: A systematic review and meta-analysis. Oncotarget 2017 , 8 , 100570–100581. [ Google Scholar ] [ CrossRef ]
- Staretz, M.E.; Murphy, S.E.; Patten, C.J.; Nunes, M.G.; Koehl, W.; Amin, S.; Koenig, L.A.; Guengerich, F.P.; Hecht, S.S. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab. Dispos. 1997 , 25 , 154–162. [ Google Scholar ]
- Park, J.H.; Hong, J.Y.; Han, K. Association between Smoking Cessation and the Risk of Cholangiocarcinoma and Ampulla of Vater Cancer: A Nationwide Cohort Study. Liver Cancer 2023 , 12 , 457–466. [ Google Scholar ] [ CrossRef ]
- Granata, S.; Vivarelli, F.; Morosini, C.; Canistro, D.; Paolini, M.; Fairclough, L.C. Toxicological Aspects Associated with Consumption from Electronic Nicotine Delivery System (ENDS): Focus on Heavy Metals Exposure and Cancer Risk. Int. J. Mol. Sci. 2024 , 25 , 2737. [ Google Scholar ] [ CrossRef ]
- Gallagher, K.P.; Vargas, P.A.; Santos-Silva, A.R. The use of E-cigarettes as a risk factor for oral potentially malignant disorders and oral cancer: A rapid review of clinical evidence. Med. Oral. Patol. Oral. Cir. Bucal 2024 , 29 , e18–e26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Muthumalage, T.; Noel, A.; Thanavala, Y.; Alcheva, A.; Rahman, I. Challenges in current inhalable tobacco toxicity assessment models: A narrative review. Tob. Induc. Dis. 2024 , 22 , 102. [ Google Scholar ] [ CrossRef ]
- de Martel, C.; Plummer, M.; Franceschi, S. Cholangiocarcinoma: Descriptive epidemiology and risk factors. Gastroenterol. Clin. Biol. 2010 , 34 , 173–180. [ Google Scholar ] [ CrossRef ]
- Yamamoto, Y.; Chikawa, J.; Uegaki, Y.; Usuda, N.; Kuwahara, Y.; Fukumoto, M. Histological type of Thorotrast-induced liver tumors associated with the translocation of deposited radionuclides. Cancer Sci. 2010 , 101 , 336–340. [ Google Scholar ] [ CrossRef ]
- Khan, S.A.; Toledano, M.B.; Taylor-Robinson, S.D. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 2008 , 10 , 77–82. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, W.T.; Lin, Y.J.; Li, C.Y.; Tsai, P.J.; Yang, C.Y.; Liou, S.H.; Wu, T.N. Cancer attributable to asbestos exposure in shipbreaking workers: A matched-cohort study. PLoS ONE 2015 , 10 , e0133128. [ Google Scholar ] [ CrossRef ]
- Boulanger, M.; Morlais, F.; Bouvier, V.; Galateau-Salle, F.; Guittet, L.; Marquignon, M.F.; Paris, C.; Raffaelli, C.; Launoy, G.; Clin, B. Digestive cancers and occupational asbestos exposure: Incidence study in a cohort of asbestos plant workers. Occup. Environ. Med. 2015 , 72 , 792–797. [ Google Scholar ] [ CrossRef ]
- Brandi, G.; Di Girolamo, S.; Farioli, A.; de Rosa, F.; Curti, S.; Pinna, A.D.; Ercolani, G.; Violante, F.S.; Biasco, G.; Mattioli, S. Asbestos: A hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control 2013 , 24 , 911–918. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, J.; Kim, H.; Park, J.S. Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma. Life 2024 , 14 , 698. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kerdkumthong, K.; Nanarong, S.; Roytrakul, S.; Pitakpornpreecha, T.; Tantimetta, P.; Runsaeng, P.; Obchoei, S. Quantitative proteomics analysis reveals possible anticancer mechanisms of 5′-deoxy-5′-methylthioadenosine in cholangiocarcinoma cells. PLoS ONE 2024 , 19 , e0306060. [ Google Scholar ] [ CrossRef ]
- Deng, S.; Lu, X.; Wang, X.; Liang, B.; Xu, H.; Yang, D.; Cui, G.; Yonemura, A.; Paine, H.; Zhou, Y.; et al. Overexpression of TBX3 suppresses tumorigenesis in experimental and human cholangiocarcinoma. Cell Death Dis. 2024 , 15 , 441. [ Google Scholar ] [ CrossRef ]
- Prabhakar, N.; Chiang, H.; Nabrinsky, E.; Eklund, J. Report of Cholangiocarcinoma with Transheterozygous BRCA1 and BRCA2 Co-mutation. Cureus 2024 , 16 , e60767. [ Google Scholar ] [ CrossRef ]
- Zhang, Q.; Zhou, J.; Zhai, D.; Jiang, Q.; Yang, M.; Zhou, M. Gut microbiota regulates the ALK5/NOX1 axis by altering glutamine metabolism to inhibit ferroptosis of intrahepatic cholangiocarcinoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2024 , 1870 , 167152. [ Google Scholar ] [ CrossRef ]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.I.; Alexiu-Toma, O.A.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balkan J. Med. Genet. 2023 , 26 , 21–26. [ Google Scholar ] [ CrossRef ]
- Anghel, D.; Ciobîcă, L.M.; Stanciu, S.M.; Jurcuț, C.V.; Stoicescu, G.D.; Răduță, I.A.; Coca, A. Ankylosing spondylitis and cardiovascular risk—Case report. Rom. J. Mil. Med. 2016 , 119 , 39–42. [ Google Scholar ]
- Ionescu, O.P.; Stanciu, S.M.; Ciobîcă, M.L. Atherosclerosis in rheumatoid arthritis—The importance of imaging testing. Rom. J. Mil. Med. 2020 , 123 , 26–31. [ Google Scholar ]
- Zeng, L.; You, G.; Tanaka, H.; Srivatanakul, P.; Ohta, E.; Viwatthanasittiphong, C.; Matharit, M.; Chenvidhya, D.; Jedpiyawongse, A.; Tanaka, M.; et al. Combined effects of polymorphisms of DNA-repair protein genes and metabolic enzyme genes on the risk of cholangiocarcinoma. Jpn. J. Clin. Oncol. 2013 , 43 , 1190–1194. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ko, K.H.; Kim, N.K.; Yim, D.J.; Hong, S.P.; Park, P.W.; Rim, K.S.; Kim, S.; Hwang, S.G. Polymorphisms of 5,10-methylenetetrahydrofolate reductase (MTHFR C677T) and thymidylate synthase enhancer region (TSER) as a risk factor of cholangiocarcinoma in a Korean population. Anticancer Res. 2006 , 26 , 4229–4233. [ Google Scholar ]
- Kinoshita, M.; Kubo, S.; Tanaka, S.; Takemura, S.; Nishioka, T.; Hamano, G.; Ito, T.; Tanaka, S.; Ohsawa, M.; Shibata, T. The association between non-alcoholic steatohepatitis and intrahepatic cholangiocarcinoma: A hospital based case-control study. J. Surg. Oncol. 2016 , 113 , 779–783. [ Google Scholar ] [ CrossRef ]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017 , 17 , 149. [ Google Scholar ] [ CrossRef ]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D'Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020 , 12 , 3182. [ Google Scholar ] [ CrossRef ]
- Parsi, M.A. Obesity and cholangiocarcinoma. World J. Gastroenterol. 2013 , 19 , 457–462. [ Google Scholar ] [ CrossRef ]
- Ciobîcă, L.M.; Sârbu, I.; Stanciu, S.M.; Coca, A. Behçet disease—Case presentation. Rom. J. Mil. Med. 2016 , 119 , 43–46. [ Google Scholar ] [ CrossRef ]
- Li, J.S.; Han, T.J.; Jing, N.; Li, L.; Zhang, X.H.; Ma, F.Z.; Liu, J.Y. Obesity and the risk of cholangiocarcinoma: A meta-analysis. Tumour Biol. 2014 , 35 , 6831–6838. [ Google Scholar ] [ CrossRef ]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023 , 15 , 485. [ Google Scholar ] [ CrossRef ]
- Jing, W.; Jin, G.; Zhou, X.; Zhou, Y.; Zhang, Y.; Shao, C.; Liu, R.; Hu, X. Diabetes mellitus and increased risk of cholangiocarcinoma: A meta-analysis. Eur. J. Cancer Prev. 2012 , 21 , 24–31. [ Google Scholar ] [ CrossRef ]
- Chaiteerakij, R.; Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Mettler, T.A.; Fredericksen, Z.S.; Kim, W.R.; Gores, G.J.; Roberts, R.O.; Olson, J.E.; et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology 2013 , 57 , 648–655. [ Google Scholar ] [ CrossRef ]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015 , 350 , g7607. [ Google Scholar ] [ CrossRef ]
- Rahman, S.U.; Sana, M.K.; Tahir, Z.; Ali, A.; Shah, P.A. Paraneoplastic syndromes in cholangiocarcinoma. World J. Hepatol. 2020 , 12 , 897–907. [ Google Scholar ] [ CrossRef ]
- Zhou, H.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014 , 86 , 62–68. [ Google Scholar ] [ CrossRef ]
- Casadei-Gardini, A.; Filippi, R.; Rimini, M.; Rapposelli, I.G.; Fornaro, L.; Silvestris, N.; Aldrighetti, L.; Aimar, G.; Rovesti, G.; Bartolini, G.; et al. Effects of Metformin and Vitamin D on Clinical Outcome in Cholangiocarcinoma Patients. Oncology 2021 , 99 , 292–299. [ Google Scholar ] [ CrossRef ]
- Powała, A.; Żołek, T.; Brown, G.; Kutner, A. Structure and the Anticancer Activity of Vitamin D Receptor Agonists. Int. J. Mol. Sci. 2024 , 25 , 6624. [ Google Scholar ] [ CrossRef ]
- Zhao, S.; Qian, F.; Wan, Z.; Chen, X.; Pan, A.; Liu, G. Vitamin D and major chronic diseases. Trends Endocrinol. Metab. 2024; online ahead of print . [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bird, R.P. Vitamin D and cancer. Adv. Food Nutr. Res. 2024 , 109 , 92–159. [ Google Scholar ] [ CrossRef ]
- Chiang, K.C.; Yeh, C.N.; Lin, K.J.; Su, L.J.; Yen, T.C.; Pang, J.H.; Kittaka, A.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; et al. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a Chemical-Induced animal model. Oncotarget 2014 , 5 , 3849–3861. [ Google Scholar ] [ CrossRef ]
- Kennedy, L.; Baker, K.; Hodges, K.; Graf, A.; Venter, J.; Hargrove, L.; Harris, R.; Harnish, E.; Meng, F.; Francis, H. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth. Dig. Liver Dis. 2013 , 45 , 316–322. [ Google Scholar ] [ CrossRef ]
- Sookprasert, A.; Pugkhem, A.; Khuntikeo, N.; Chur-in, S.; Chamadol, N.; Prawan, A.; Janeklang, S.; Vaeteewoottacharn, K.; Kukongviriyapan, V.; Pairojkul, C.; et al. Evaluation of efficacy, safety and tolerability of high dose-intermittent calcitriol supplementation to advanced intrahepatic cholangiocarcinoma patients-a pilot study. Asian Pac. J. Cancer Prev. 2012 , 13 , 161–167. [ Google Scholar ]
- Chiang, K.C.; Yeh, T.S.; Huang, C.C.; Chang, Y.C.; Juang, H.H.; Cheng, C.T.; Pang, J.S.; Hsu, J.T.; Takano, M.; Chen, T.C.; et al. MART-10 represses cholangiocarcinoma cell growth and high vitamin D receptor expression indicates better prognosis for cholangiocarcinoma. Sci. Rep. 2017 , 7 , 43773. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chiang, K.C.; Yeh, C.N.; Huang, C.C.; Yeh, T.S.; SPang, J.H.; Hsu, J.T.; Chen, L.W.; Kuo, S.F.; Kittaka, A.; Chen, T.C.; et al. 25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1alpha,25(OH)2D3. Int. J. Mol. Sci. 2016 , 17 , 1326. [ Google Scholar ] [ CrossRef ]
- Trakoonsenathong, R.; Kunprom, W.; Aphivatanasiri, C.; Yueangchantuek, P.; Pimkeeree, P.; Sorin, S.; Khawkhiaw, K.; Chiu, C.F.; Okada, S.; Wongkham, S.; et al. Liraglutide exhibits potential anti-tumor effects on the progression of intrahepatic cholangiocarcinoma, in vitro and in vivo. Sci. Rep. 2024 , 14 , 13726. [ Google Scholar ] [ CrossRef ]
- Ueda, P.; Wintzell, V.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; et al. Use of incretin-based drugs and risk of cholangiocarcinoma: Scandinavian cohort study. Diabetologia 2021 , 64 , 2204–2214. [ Google Scholar ] [ CrossRef ]
- Huber, S.; Fitzner, T.; Feichtinger, R.G.; Hochmann, S.; Kraus, T.; Sotlar, K.; Kofler, B.; Varga, M. Galanin System in the Human Bile Duct and Perihilar Cholangiocarcinoma. Cells 2023 , 12 , 1678. [ Google Scholar ] [ CrossRef ]
- Petrescu, A.D.; Grant, S.; Williams, E.; Frampton, G.; Parks, N.; Blaney, H.; Davies, M.; John, R.; Reinhart, E.H.; McMillin, M.; et al. Coordinated Targeting of Galanin Receptors on Cholangiocytes and Hepatic Stellate Cells Ameliorates Liver Fibrosis in Multidrug Resistance Protein 2 Knockout Mice. Am. J. Pathol. 2020 , 190 , 586–601. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Falkenstetter, S.; Leitner, J.; Brunner, S.M.; Rieder, T.N.; Kofler, B.; Weis, S. Galanin System in Human Glioma and Pituitary Adenoma. Front. Endocrinol. 2020 , 11 , 155. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J. Med. Life 2020 , 13 , 449–453. [ Google Scholar ] [ CrossRef ]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Clavicular Malignancies: A Borderline Surgical Management. Medicina 2022 , 58 , 910. [ Google Scholar ] [ CrossRef ]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union. 2016 , 51 , 476–480. [ Google Scholar ]
- Rubino, J.G.; Flemming, J.A. Menopausal hormone therapy and risk of biliary tract cancers: Addressing the ant, not the elephant in the room. Hepatology 2022 , 75 , 243–245. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kamani, M.; Akgor, U.; Gültekin, M. Review of the literature on combined oral contraceptives and cancer. Ecancermedicalscience 2022 , 16 , 1416. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, Y.; Jiang, L.; Li, F.; Li, Q.; Yuan, S.; Huang, S.; Fu, Y.; Yan, X.; Chen, J.; Li, H.; et al. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022 , 22 , 546. [ Google Scholar ] [ CrossRef ]
- Zhang, G.Q.; Chen, J.L.; Luo, Y.; Mathur, M.B.; Anagnostis, P.; Nurmatov, U.; Talibov, M.; Zhang, J.; Hawrylowicz, C.M.; Lumsden, M.A.; et al. Menopausal hormone therapy and women’s health: An umbrella review. PLoS Med. 2021 , 18 , e1003731. [ Google Scholar ] [ CrossRef ]
- Li, F.; Chen, Q.; Yang, Y.; Li, M.; Zhang, L.; Yan, Z.; Zhang, J.; Wang, K. ESR1 as a recurrence-related gene in intrahepatic cholangiocarcinoma: A weighted gene coexpression network analysis. Cancer Cell Int. 2021 , 21 , 225. [ Google Scholar ] [ CrossRef ]
- Kaewlert, W.; Sakonsinsiri, C.; Namwat, N.; Sawanyawisuth, K.; Ungarreevittaya, P.; Khuntikeo, N.; Armartmuntree, N.; Thanan, R. The Importance of CYP19A1 in Estrogen Receptor-Positive Cholangiocarcinoma. Horm. Cancer 2018 , 9 , 408–419. [ Google Scholar ] [ CrossRef ]
- Lu, C.; Miao, J.; Li, M.; Zheng, Q.; Xu, F.; Pan, Y.; Wang, Y.; Yang, Z.; Xia, X.; Zhu, H.; et al. Characterization of the Estrogen Response Helps to Predict Prognosis and Identify Potential Therapeutic Targets in Cholangiocarcinoma. Front. Oncol. 2022 , 12 , 870840. [ Google Scholar ] [ CrossRef ]
- Jackson, S.S.; Pfeiffer, R.M.; Gabbi, C.; Anderson, L.; Gadalla, S.M.; Koshiol, J. Menopausal hormone therapy and risk of biliary tract cancers. Hepatology 2022 , 75 , 309–321. [ Google Scholar ] [ CrossRef ]
- Petrick, J.L.; McMenamin, Ú.C.; Zhang, X.; Zeleniuch-Jacquotte, A.; Wactawski-Wende, J.; Simon, T.G.; Sinha, R.; Sesso, H.D.; Schairer, C.; Rosenberg, L.; et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: Results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br. J. Cancer 2020 , 123 , 316–324. [ Google Scholar ] [ CrossRef ]
- Khalid, S.; Laput, G.; Khorfan, K.; Roytman, M. Development of Liver Cancers as an Unexpected Consequence of Anabolic Androgenic Steroid Use. Cureus 2023 , 15 , e34357. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pothuri, V.S.; Anzelmo, M.; Gallaher, E.; Ogunlana, Y.; Aliabadi-Wahle, S.; Tan, B.; Crippin, J.S.; Hammill, C.W. Transgender Males on Gender-Affirming Hormone Therapy and Hepatobiliary Neoplasms: A Systematic Review. Endocr. Pract. 2023 , 29 , 822–829. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fadahunsi, O.O.; Ibitoye, B.O.; Adisa, A.O.; Alatise, O.I.; Adetiloye, V.A.; Idowu, B.M. Diagnostic accuracy of ultrasonography in adults with obstructive jaundice. J. Ultrason. 2020 , 20 , e100–e105. [ Google Scholar ] [ CrossRef ]
- Costa, M.; Valente, A.; Freire Coelho, A.; Meireles, S.; Barbosa, M. Acanthosis Nigricans Manifesting as a Paraneoplastic Syndrome Associated With Cholangiocarcinoma. Cureus 2023 , 15 , e35853. [ Google Scholar ] [ CrossRef ]
- Suchonwanit, P.; McMichael, A.J. Alopecia in Association with Malignancy: A Review. Am. J. Clin. Dermatol. 2018 , 19 , 853–865. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Antoniou, E.; Paraskeva, P.; Smyrnis, A.; Konstantopoulos, K. Alopecia: A common paraneoplastic manifestation of cholangiocarcinoma in humans and animals. BMJ Case Rep. 2012 , 2012 , bcr2012006217. [ Google Scholar ] [ CrossRef ]
- Ciobîcă, M.L.; Ionescu, O.P.; Săndulescu, B.A. Osteoporosis and the fracture risk in systemic lupus erythematosus. Rom. J. Mil. Med. 2020 , 123 , 341–347. [ Google Scholar ]
- Suh, K.J.; Park, J.K.; Cho, S.; Park, H.; Baek, H.W.; Lee, K.; Lee, D.S.; Lee, K.H. Dermatomyositis in a Patient with Cholangiocarcinoma Detected by an [(18)F]-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Scan. Cancer Res. Treat. 2016 , 48 , 848–852. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nistor, C.E.; Staden, R.S.; Dumitru, A.V.; Stanciu Găvan, C. A Screening Test for Early Diagnosis of Microcellular Bronchopulmonary Cancer-Pilot Study. J. Clin. Med. 2019 , 9 , 76. [ Google Scholar ] [ CrossRef ]
- Sotoodian, B.; Mahmood, M.N.; Salopek, T.G. Clinical and Dermoscopic Features of Pigmented Disseminated Superficial Actinic Porokeratosis: Case Report and Literature Review. J. Cutan. Med. Surg. 2018 , 22 , 229–231. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chiyomaru, K.; Takai, T.; Ohashi, A.; Nishigori, C. Necrolytic migratory erythema with cholangiocarcinoma: Pseudoglucagonoma syndrome. Eur. J. Dermatol. 2010 , 20 , 238–239. [ Google Scholar ] [ CrossRef ]
- Tzovaras, V.; Liberopoulos, E.N.; Zioga, A.; Pavlidis, N.; Elisaf, M. Persistent erythema multiforme in a patient with extrahepatic cholangiocarcinoma. Oncology 2007 , 73 , 127–129. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Nitipir, C.; Slavu, C.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Management of Lung Cancer Presenting with Solitary Bone Metastasis. Medicina 2022 , 58 , 1463. [ Google Scholar ] [ CrossRef ]
- Chacko, A.M.; Carrero, G.; Akhouri, S. A Case Report of Erythema Multiforme Secondary to Atorvastatin Use. Cureus 2024 , 16 , e52175. [ Google Scholar ] [ CrossRef ]
- Cohen, P.R.; Holder, W.R.; Tucker, S.B.; Kono, S.; Kurzrock, R. Sweet syndrome in patients with solid tumors. Cancer 1993 , 72 , 2723–2731. [ Google Scholar ] [ CrossRef ]
- Shinojima, Y.; Toma, Y.; Terui, T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br. J. Dermatol. 2006 , 155 , 1103–1104. [ Google Scholar ] [ CrossRef ]
- Lemaire, C.C.; Portilho, A.L.C.; Pinheiro, L.V.; Vivas, R.A.; Britto, M.; Montenegro, M.; Rodrigues, L.F.F.; Arruda, S.; Lyra, A.C.; Cavalcante, L.N. Sweet syndrome as a paraneoplastic manifestation of cholangiocarcinoma: A case report. World J. Clin. Cases 2020 , 8 , 4122–4127. [ Google Scholar ] [ CrossRef ]
- Holzgruber, J.; Oberneder-Popper, J.; Guenova, E.; Hötzenecker, W. Acrokeratosis Paraneoplastica (Bazex Syndrome): A Case Report. Case Rep. Dermatol. 2022 , 14 , 307–312. [ Google Scholar ] [ CrossRef ]
- Ciuche, A.; Nistor, C.; Motaş, C.; Horvat, T. Minimally invasive surgery in the treatment of malignant pleuro-pericardial effusions. Chirurgia 2012 , 107 , 206–212. [ Google Scholar ] [ PubMed ]
- Stone, S.P.; Buescher, L.S. Life-threatening paraneoplastic cutaneous syndromes. Clin. Dermatol. 2005 , 23 , 301–306. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Boyd, A.S.; Neldner, K.H.; Menter, A. Erythema gyratum repens: A paraneoplastic eruption. J. Am. Acad. Dermatol. 1992 , 26 Pt 1 , 757–762. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nistor, C.E.; Găvan, C.S.; Ciritel, A.A.; Nemes, A.F.; Ciuche, A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis-A 10 Year Retrospective Observational Study. Medicina 2022 , 58 , 718. [ Google Scholar ] [ CrossRef ]
- Liau, M.M.; Long, V.; Yang, S.S. Erythema gyratum repens: A paraneoplastic eruption. BMJ Case Rep. 2016 , 2016 , bcr2016214665. [ Google Scholar ] [ CrossRef ]
- Bar-Ilan, E.; Gat, A.; Sprecher, E.; Zeeli, T. Paraneoplastic pityriasis rubra pilaris: Case report and literature review. Clin. Exp. Dermatol. 2017 , 42 , 54–57. [ Google Scholar ] [ CrossRef ]
- Opneja, A.; Mahajan, S.; Kapoor, S.; Marur, S.; Yang, S.H.; Manno, R. Unusual Paraneoplastic Presentation of Cholangiocarcinoma. Case Rep. Med. 2015 , 2015 , 806835. [ Google Scholar ] [ CrossRef ]
- Morgenthau, A.; Almudaires, A. Klatskin's cholangiocarcinoma presenting with the sign of Leser-Trelat. BMJ Case Rep. 2019 , 12 , e232507. [ Google Scholar ] [ CrossRef ]
- Scully, C.; Barrett, W.A.; Gilkes, J.; Rees, M.; Sarner, M.; Southcott, R.J. Oral acanthosis nigricans, the sign of Leser-Trélat and cholangiocarcinoma. Br. J. Dermatol. 2001 , 145 , 506–507. [ Google Scholar ] [ CrossRef ]
- Sökmen, M.; Demirsoy, H.; Ersoy, O.; Gökdemir, G.; Akbayir, N.; Karaca, C.; Ozdil, K.; Kesici, B.; Calişkan, C.; Yilmaz, B. Paraneoplastic porphyria cutanea tarda associated with cholangiocarcinoma: Case report. Turk. J. Gastroenterol. 2007 , 18 , 200–205. [ Google Scholar ] [ PubMed ]
- Schmidt, S.L.; Schmidt, J.J.; Tolentino, J.C.; Ferreira, C.G.; de Almeida, S.A.; Alvarenga, R.P.; Simoes, E.N.; Schmidt, G.J.; Canedo, N.H.; Chimelli, L. Cholangiocarcinoma associated with limbic encephalitis and early cerebral abnormalities detected by 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography-positron emission tomography: A case report. J. Med. Case Rep. 2016 , 10 , 200. [ Google Scholar ] [ CrossRef ]
- Bruhnding, A.; Notch, D.; Beard, A. Anti-Yo positive paraneoplastic cerebellar degeneration in the setting of cholangiocarcinoma. J. Clin. Neurosci. 2017 , 36 , 71–72. [ Google Scholar ] [ CrossRef ]
- Normand, G.; Jolivot, A.; Rabeyrin, M.; Hervieu, V.; Valette, P.J.; Scoazec, J.Y.; Gougon, J.M.; Juillard, L.; Dumortier, J. Paraneoplastic fibrillary glomerulonephritis associated with intrahepatic cholangiocarcinoma: When diagnosis of a rare kidney disease leads to successful hepatic cancer treatment. Clin. Res. Hepatol. Gastroenterol. 2017 , 41 , e8–e11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Solans-Laqué, R.; Bosch-Gil, J.A.; Pérez-Bocanegra, C.; Selva-O'Callaghan, A.; Simeón-Aznar, C.P.; Vilardell-Tarres, M. Paraneoplastic vasculitis in patients with solid tumors: Report of 15 cases. J. Rheumatol. 2008 , 35 , 294–304. [ Google Scholar ]
- Hatzis, G.S.; Papachristodoulou, A.; Delladetsima, I.K.; Moutsopoulos, H.M. Polyarteritis nodosa associated with cholangiocarcinoma. Lupus 1998 , 7 , 301–306. [ Google Scholar ] [ CrossRef ]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012 , 9 , e1001275. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jeon, H.K.; Kim, D.U.; Baek, D.H.; Ha, D.W.; Lee, B.E.; Ryu, D.Y.; Cheong, J.H.; Kim, G.H.; Song, G.A.; Jang, A.L. Venous thromboembolism in patients with cholangiocarcinoma: Focus on risk factors and impact on survival. Eur. J. Gastroenterol. Hepatol. 2012 , 24 , 444–449. [ Google Scholar ] [ CrossRef ]
- Blum, M.F.; Ma, V.Y.; Betbadal, A.M.; Bonomo, R.A.; Raju, R.R.; Packer, C.D. Trousseau's Syndrome in Cholangiocarcinoma: The Risk of Making the Diagnosis. Clin. Med. Res. 2016 , 14 , 53–59. [ Google Scholar ] [ CrossRef ]
- Zahidin, M.A.; Iberahim, S.; Hassan, M.N.; Zulkafli, Z.; Mohd Noor, N.H. Clinical and Laboratory Diagnosis of Antiphospholipid Syndrome: A Review. Cureus 2024 , 16 , e61713. [ Google Scholar ] [ CrossRef ]
- Samadian, S.; Estcourt, L. Recurrent thrombo-embolic episodes: The association of cholangiocarcinoma with antiphospholipid syndrome. Postgrad. Med. J. 1999 , 75 , 45–46. [ Google Scholar ] [ CrossRef ]
- Ham, H.; Kim, H.Y.; Seo, K.J.; Lee, S.L.; Kim, C.W. Cholangiocarcinoma with a paraneoplastic leukemoid reaction mimicking a pyogenic liver abscess. Korean J. Intern. Med. 2015 , 30 , 110–113. [ Google Scholar ] [ CrossRef ]
- Sohda, T.; Shiga, H.; Nakane, H.; Watanabe, H.; Takeshita, M.; Sakisaka, S. Cholangiocellular carcinoma that produced both granulocyte-colony-stimulating factor and parathyroid hormone-related protein. Int. J. Clin. Oncol. 2006 , 11 , 246–249. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xi, L.F.; Jin, Y.; Li, J.T. Intrahepatic sarcomatoid cholangiocarcinoma: A case report of the youngest patient on record and a review of the condition's characteristics. Front. Surg. 2022 , 9 , 963952. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Raza, A.; Houk, L.; Yousaf, W.; Smiley, D.; Coberly, L. Unusual para-neoplastic manifestation of cholangiocarcinoma. J. Gastrointest. Cancer 2013 , 44 , 228–230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- González Amores, Y.; Hernando Rebollar, S.; Casado Bernabeu, A. Lupus as a paraneoplastic manifestation of cholangiocarcinoma. Rev. Esp. Enferm. Dig. 2016 , 108 , 292. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jenkins, D.; McPherson, T. Paraneoplastic subacute cutaneous lupus erythematosus associated with cholangiocarcinoma. Australas. J. Dermatol. 2016 , 57 , e5–e7. [ Google Scholar ] [ CrossRef ]
- Erdinc, B.; Ramachandran, P.; Yadav, R.; Sahni, S.; Joseph, G. Cholangiocarcinoma Presenting as Humoral Hypercalcemia of Malignancy: A Case Report and Literature Review. Cureus 2019 , 11 , e6481. [ Google Scholar ] [ CrossRef ]
- Harsch, I.A.; Konturek, P.C. Humoral Hypercalcemia in a Patient with Cholangiocellular Carcinoma—Effective Therapy with Denosumab. Am. J. Case Rep. 2019 , 20 , 1325–1330. [ Google Scholar ] [ CrossRef ]
- Ozawa, N.; Doi, S.; Tsujikawa, T.; Mabuchi, M.; Kajiyama, Y.; Sato, K.; Kikuchi, K.; Takahashi, M.; Kawamoto, M.; Yasuda, I. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai Zasshi 2017 , 114 , 1285–1292. [ Google Scholar ]
- Chauhan, A.; Likasitwatanakul, P.; Ahmed, A.; Sibley, S.D. A Case of Fibroblast Growth Factor Receptor Fusion-Positive Intrahepatic Cholangiocarcinoma With Humoral Hypercalcemia of Malignancy. Cureus 2024 , 16 , e58741. [ Google Scholar ] [ CrossRef ]
- Meegada, S.; Eisen, R.; Coons, G.; Verma, R. Intrahepatic Cholangiocarcinoma Associated with High Procalcitonin, Hypercalcemia, Polycythemia and Leukocytosis. Cureus 2020 , 12 , e6587. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Baiocchi, L.; Sato, K.; Ekser, B.; Kennedy, L.; Francis, H.; Ceci, L.; Lenci, I.; Alvaro, D.; Franchitto, A.; Onori, P.; et al. Cholangiocarcinoma: Bridging the translational gap from preclinical to clinical development and implications for future therapy. Expert. Opin. Investig. Drugs 2021 , 30 , 365–375. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology 2018 , 288 , 7–13. [ Google Scholar ] [ CrossRef ]
- Razumilava, N.; Gores, G.J.; Lindor, K.D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011 , 54 , 1842–1852. [ Google Scholar ] [ CrossRef ]
- Liu, G.J.; Wang, W.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Chen, L.D.; Wang, Z.; Liang, J.Y.; Huang, Y.; et al. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer 2015 , 4 , 241–252. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Olthof, S.C.; Othman, A.; Clasen, S.; Schraml, C.; Nikolaou, K.; Bongers, M. Imaging of Cholangiocarcinoma. Visc. Med. 2016 , 32 , 402–410. [ Google Scholar ] [ CrossRef ]
- Pfrepper, C. Paraneoplastic Thromboembolism and Thrombophilia: Significance in Visceral Medicine. Visc. Med. 2020 , 36 , 280–287. [ Google Scholar ] [ CrossRef ]
- Tai, N.; Inoue, D. Bone and calcium metabolism associated with malignancy. Malignancy-associated hypercalcemia. Clin. Calcium 2018 , 28 , 1503–1508. [ Google Scholar ]
- Nistor, C.E.; Stanciu-Găvan, C.; Vasilescu, F.; Dumitru, A.V.; Ciuche, A. Attitude of the surgical approach in hyperparathyroidism: A retrospective study. Exp. Ther. Med. 2021 , 22 , 959. [ Google Scholar ] [ CrossRef ]
- Ashihara, N.; Nakajima, K.; Nakamura, Y.; Kobayashi, M.; Shirahata, K.; Maeda, C.; Uehara, T.; Gomi, D.; Ito, N. Denosumab is Effective for Controlling Serum Calcium Levels in Patients with Humoral Hypercalcemia of Malignancy Syndrome: A Case Report on Parathyroid Hormone-related Protein-producing Cholangiocarcinoma. Intern. Med. 2016 , 55 , 3453–3457. [ Google Scholar ] [ CrossRef ]
- Tang, J.; Liao, Y.; He, S.; Shi, J.; Peng, L.; Xu, X.; Xie, F.; Diao, N.; Huang, J.; Xie, Q.; et al. Autocrine parathyroid hormone-like hormone promotes intrahepatic cholangiocarcinoma cell proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J. Transl. Med. 2017 , 15 , 238. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kane, J.F.; Johnson, R.W. e-Evaluating the Role of PTHrP in Breast Cancer. Cancers 2023 , 15 , 2670. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mc Donald, D.; Drake, M.T.; Crowley, R.K. Treatment of hypercalcaemia of malignancy in adults. Clin. Med. 2023 , 23 , 503–507. [ Google Scholar ] [ CrossRef ]
- El-Hajj Fuleihan, G.; Clines, G.A.; Hu, M.I.; Marcocci, C.; Murad, M.H.; Piggott, T.; Van Poznak, C.; Wu, J.Y.; Drake, M.T. Treatment of Hypercalcemia of Malignancy in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2023 , 108 , 507–528. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nistor, C.; Ciuche, A.; Constantinescu, I. Emergency surgical tracheal decompression in a huge retrosternal goiter. Acta Endocrinol. 2017 , 13 , 370–374. [ Google Scholar ] [ CrossRef ]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020 , 43 , 885–899. [ Google Scholar ] [ CrossRef ]
- Benner, B.J.M.; Alsma, J.; Feelders, R.A. Hyponatraemia and hyperpigmentation in primary adrenal insufficiency. BMJ Case Rep. 2019 , 12 , e227200. [ Google Scholar ] [ CrossRef ]
- Nistor, C.E.; Pantile, D.; Gavan, C.S.; Ciuche, A. Pneumothorax on COVID-19 patients-retrospective clinical observations. Rom. J. Leg. Med. 2022 , 30 , 112–116. [ Google Scholar ] [ CrossRef ]
- Zhou, X.; Huang, T.; Pan, H.; Du, A.; Wu, T.; Lan, J.; Song, Y.; Lv, Y.; He, F.; Yuan, K. Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma. PLoS ONE 2024 , 19 , e0300441. [ Google Scholar ] [ CrossRef ]
- Wiwanitkit, V. Thioredoxin reductase-1 and incidence of COVID-19 among cholangiocarcinoma patients in tropical endemic area. J. Cancer Res. Ther. 2023 , 19 , 845. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, H.; Li, J.; Xiao, W.; Zhang, Y.; Lv, Y.; Yu, X.; Zheng, J. The Therapeutic Potential of Galectin-3 in the Treatment of Intrahepatic Cholangiocarcinoma Patients and Those Compromised with COVID-19. Front. Mol. Biosci. 2021 , 8 , 666054. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sharma, S.; Pavuluri, S.; Srinivasan, K.; Ghouse, M. Thrombotic Microangiopathy in a Patient with COVID-19 Infection and Metastatic Cholangiocarcinoma. J. Hematol. 2021 , 10 , 83–88. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bhatia, S.; Gokhale, P.; Katte, T.; Acharya, S.; Rasalkar, A.A.; Vidapanakal, S.; Manas, R.; Chinnam, S.; Narayanan, P.; Shettihalli, A.K.; et al. Assessing the Vulnerability of Cancer Patients for COVID-19. ACS Omega 2022 , 7 , 35735–35742. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Conditions | Key Elements | Reference Numbers |
|---|---|---|
| Chronic biliary diseases | Primary sclerosing cholangitis | [ , , ] |
| Bile ducts cysts or choledochal cysts (including Caroli’s disease) | [ , , , ] | |
| Hepatholithiasis | [ ] | |
| Chronic liver conditions | Cirrhosis | [ , , , , , ] |
| Hepatitis B and C virus chronic infections | [ , , , , , ] | |
| Hemochromatosis | [ , , , , , , ] | |
| Wilson’s disease | [ , , , ] | |
| Digestive ailments | Inflammatory bowel disease (ulcerative colitis, Crohn’s disease) Chronic pancreatitis Duodenal or gastric ulcer | [ , , , , ] |
| Parasitic infections (liver fluke) | Opisthorchis viverrini or Clonorchis sinensis | [ , , , , , , ] |
| Lifestyle influence | Chronic alcohol consumption | [ , ] |
| Cigarette smoking | [ , , ] | |
| Environmental exposure | Thorotrast | [ , ] |
| Asbestos | [ , , ] | |
| Genetic and epigenetic considerations | BRCA to TBX3, p53 | [ , , ] |
| Metabolic and endocrine interferences | Non-alcoholic fatty liver disease | [ , ] |
| Obesity | [ , , , ] | |
| Type 2 diabetes mellitus | [ , , , , , , ] | |
| Vitamin D deficiency | [ , ] | |
| Modulation of glucagon-like peptide 1 receptor | [ ] | |
| Modulation of galanin system | [ , ] | |
| Sex hormone therapy (oestrogens in adult females) | [ , , ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Ciobica, M.-L.; Sandulescu, B.-A.; Chicea, L.-M.; Iordache, M.; Groseanu, M.-L.; Carsote, M.; Nistor, C.; Radu, A.-M. The Constellation of Risk Factors and Paraneoplastic Syndromes in Cholangiocarcinoma: Integrating the Endocrine Panel Amid Tumour-Related Biology (A Narrative Review). Biology 2024 , 13 , 662. https://doi.org/10.3390/biology13090662
Ciobica M-L, Sandulescu B-A, Chicea L-M, Iordache M, Groseanu M-L, Carsote M, Nistor C, Radu A-M. The Constellation of Risk Factors and Paraneoplastic Syndromes in Cholangiocarcinoma: Integrating the Endocrine Panel Amid Tumour-Related Biology (A Narrative Review). Biology . 2024; 13(9):662. https://doi.org/10.3390/biology13090662
Ciobica, Mihai-Lucian, Bianca-Andreea Sandulescu, Liana-Maria Chicea, Mihaela Iordache, Maria-Laura Groseanu, Mara Carsote, Claudiu Nistor, and Ana-Maria Radu. 2024. "The Constellation of Risk Factors and Paraneoplastic Syndromes in Cholangiocarcinoma: Integrating the Endocrine Panel Amid Tumour-Related Biology (A Narrative Review)" Biology 13, no. 9: 662. https://doi.org/10.3390/biology13090662
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 24 August 2024
Total laparoscopic radical resection of hilar cholangiocarcinoma: preliminary experience of a single center
- Yusheng Du 1 ,
- Ji Wang 1 ,
- Hongqin Ma 1 ,
- Wenxing Zhao 1 &
- Ying Li 1
BMC Surgery volume 24 , Article number: 241 ( 2024 ) Cite this article
48 Accesses
Metrics details
The aim of this study was to describe our preliminary experience in the procedure of laparoscopic radical resection of hilar cholangiocarcinoma and to evaluate its feasibility, safety, and clinical efficacy.
A retrospective analysis was conducted on 44 patients with hilar cholangiocarcinoma who underwent laparoscopic surgery at our hospital from August 2019 to September 2023. Clinical data were collected from these patients, including 13 cases of Bismuth type I, 17 cases of Bismuth type II, 5 cases of Bismuth type IIIa, and 9 cases of Bismuth type IIIb.
Laparoscopic radical resection of hilar cholangiocarcinoma was successfully performed in 38 patients (86.3%). Among the remaining patients, 3 required vascular reconstruction to complete radical surgery and were converted to laparotomies, while 3 others underwent T-tube drainage only due to unresectable metastases. The median operation time was 285 min (range, 190–450), and the median estimated blood loss was 360 mL (range, 260–1200). The postoperative hospital stay duration was 14.3 ± 3.6 days. No perioperative mortality was observed. Postoperative pathological examination revealed negative microscopic margins (R0) in 39 cases and positive microscopic margins (R1) in 2 cases. Postoperative complications occurred in 8 patients (18.1%), with 4 cases (9.0%) of Grade I, 3 cases (6.8%) of Grade II, 1 case (2.2%) of Grade IIIa, and no Grade IIIb or IV complications. The median overall survival for patients who underwent radical R0 resection was 30.4 months (range, 5.3–43.6). The Disease-free survival rates were 73.6% at 1 year, 61.2% at 2 years, and 40.1% at 3 years.
Total laparoscopic radical resection of hilar cholangiocarcinoma can be performed safely, feasibly, and effectively by experienced surgeons after an accurate preoperative evaluation.
Peer Review reports
Introduction
Hilar cholangiocarcinoma, a malignant biliary epithelial tumor originating from the common hepatic duct, left and right hepatic ducts, and their junctions, accounts for approximately 40–60% of all cholangiocarcinomas [ 1 ]. This tumor is characterized by a low early diagnosis rate and a poor prognosis. Radical resection remains the only effective treatment for hilar cholangiocarcinoma, with a 5-year survival rate ranging from 20 to 40% following radical surgery [ 2 , 3 , 4 ]. However, traditional open surgery is highly invasive and poses greater risks for elderly patients with comorbid underlying diseases and poor cardiopulmonary function, resulting in many patients being deemed unsuitable for surgery. Therefore, it is imperative to explore a less invasive radical surgical approach that has a reduced impact on cardiopulmonary function in clinical practice to improve this situation. This paper aims to analyze a case series and summarize the surgical experience of total laparoscopic radical resection for hilar cholangiocarcinoma, with the goal of providing surgeons with a better understanding of this novel technique.
Materials and methods
A retrospective study was conducted, approved and adopted by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethics No.XYFY2019-KL051-01). Informed consent was obtained from all patients. Clinical data were collected from 44 patients who underwent laparoscopic surgery for hilar cholangiocarcinoma at the Department of General Surgery, Affiliated Hospital of Xuzhou Medical University, from August 2019 to September 2023. The study population consisted of 29 males and 15 females, with a mean age of 69.3 ± 7.8 years and a mean BMI of 24.5 ± 2.8 kg/m 2 . Among the patients4 had diabetes, 3 had coronary artery disease, 6 had hypertension, and one had previously undergone laparoscopic cholecystectomy. The median preoperative total bilirubin level was 189.7 µmol/L (range: 91.9-347.9 µmol/L), and the median preoperative direct bilirubin level was 164.3 µmol/L (range: 75.8-208.5 µmol/L). All patients were diagnosed with hilar cholangiocarcinoma combined with obstructive jaundice by color Doppler ultrasound, computed tomography (CT), and magnetic resonance cholangiopancreatography (MRCP) before surgery. For patients considered preoperatively for possible combined extensive hepatectomy, 3D image reconstruction imaging was performed, and the residual liver volume was assessed. Endoscopic nasobiliary drainage (ENBD) was performed in one patient to attenuate jaundice due to severe jaundice (347.9 µmol/L) and cholangitis. Two patients with severe jaundice required a period of preoperative nutritional support due to poor nutrition and underwent percutaneous transhepatic cholangial drainage (PTCD). The American Society of Anesthesiologists (ASA) classification was as follows: 15 cases were Grade I, 27 cases were Grade II, and 2 cases were Grade III. According to the Bismuth classification, there were 13 cases of Bismuth type I, 17 cases of Bismuth type II, 5 cases of Bismuth type IIIa, and 9 cases of Bismuth type IIIb. No obvious operative contraindications or distant metastases were found on preoperative examination (details in Table 1 ).
Surgical procedures
Surgical position and exploration.
The patient was placed in the supine and Trendelenburg positions. A 5-port laparoscopic approach was employed, with the primary operator positioned on the patient’s left side. An observation port was established using a 10-mm trocar placed under the umbilicus, while a 12-mm trocar, serving as the main operation port, was inserted under the left anterior axillary costal margin. Two 5-mm trocars were placed in the midclavicular line of the middle abdomen and the symmetrical position of the right abdomen as auxiliary operation ports, and an additional 12-mm trocar was positioned in the right abdomen. Upon entering the abdominal cavity, a thorough exploration was conducted to confirm the absence of implantation metastasis. Subsequently, the hilar plate was exposed and meticulously dissected to assess the tumor’s location, size, texture, and the presence of enlarged surrounding lymph nodes, facilitating an initial evaluation of the feasibility of radical surgery.
Resection of cholangiocarcinoma
The gastrocolic ligament was opened to clear the Group 8 lymph nodes of the common hepatic artery (CHA) along the upper edge of the pancreas. The anterior wall fascia of the hepatoduodenal ligament was incised to strip the proper hepatic artery (PHA) and remove the Group 12 lymph nodes on the left portal vein (PV). The right gastric artery was then resected by dissociating towards the porta hepatis and the stripped PHA, followed by the stripping of the left hepatic artery (LHA) and right hepatic artery (RHA). By lifting the PHA and dissecting the right wall of the PV, the posterior wall of the PV was stripped. The lymphatic adipose tissue on the left side of the hepatoduodenal ligament was pulled from behind the PV to the right side, ensuring complete removal of the lymphatic adipose tissue along with the tumor. The common bile duct was resected along the upper edge of the duodenum and pancreas until the pancreatic segment, and the lymph nodes behind the pancreatic head (Group 13) were cleared using a Kocher incision. An anastomosis stapler was used to resect the common bile duct along the upper edge of the pancreas, and the margin was subjected to fast-frozen pathology. The gallbladder was retrogradely stripped from the gallbladder bed and resected towards the hilar region along the bile duct. The left and right hepatic gallbladder and caudate bile duct were separated at least 1 cm above the tumor, with the margin undergoing fast-frozen pathology. Additionally, the liver tissue surrounding the bile duct was removed to achieve radical resection of hilar cholangiocarcinoma (Fig. 1 A). In cases with multiple bile duct openings at the upper cutting edge of the bile duct stump, rapid freezing pathology was mandatory. Following confirmation of negative pathological reports, the anastomosis operation was performed.
Hepatectomy
In instances where intraoperative exploration or rapid pathology suggested tumor invasion of one side of the intrahepatic bile duct, a combined hepatectomy was necessary to achieve radical surgery. The specific method, using combined left hepatectomy as an example, involved dissecting the portal blood vessels and branches, followed by the disconnection of the LHA (Fig. 2 A) and the left branch of the PV (Fig. 2 B).

Surgical field of view after hilar cholangiocarcinoma (Bismuth type I) A Hepatoduodenal ligament after lymph node dissection. B Portal vein naked 360°

Surgical field of view during radical operation of hilar cholangiocarcinoma combined with left hepatectomy (Bismuth type IIIb) A The left hepatic artery is being cutting and closing. B The Left portal vein is being cutting and closing. C The left liver is being cutting and closing
The perihepatic ligament of the left liver was dissected, exposing the hepatic vein at the second hepatic hilar. The peritoneum was dissected anterior to the inferior hepatic vena cava, and the short hepatic veins were gradually dissected from the bottom up, clipped, and dissected, separating the liver from the posterior hepatic vena cava. The liver parenchyma was transected using an ultrasonic knife combined with an electric hook along the left hepatic ischemic zone, and the intrahepatic vessels were sequentially ligated. The left hepatic vein was dissected using an anastomosis stapler (Fig. 2 C), completing the left hepatectomy and caudate lobectomy. Throughout the procedure, meticulous hemostasis was ensured, and the remaining liver section was carefully examined, with any missing vessel or bile duct sections being sutured.
Gastrointestinal reconstruction
Roux-en-Y hepaticojejunostomy was performed for gastrointestinal reconstruction. Approximately 15–20 cm away from the Treitz ligament, the jejunum was dissected using an anastomosis stapler. Following closure of the distal jejunum, the hepatic duct-jejunum anastomosis was carried out after lifting the jejunum to the hilar region over the back of the colon. In cases where the tumor location was high, multiple lumens would be present in the broken end of the hepatic duct after cutting off the left and right hepatic ducts. In such instances, hilar cholangioplasty was performed prior to bilioenteric anastomosis (Fig. 3 A). The size of the anastomosis was determined by the cross-sectional diameter of the bile duct after shaping. An incision was made in the distal jejunum, matching the size of the mesangial limbus. Using 4 − 0 VOL-C, a continuous full-thickness suture of the hepatic duct and jejunum was performed in the order from left to right, starting with the first wall and then the front wall (Fig. 3 B-C). In cases where the opening of the hepatic duct was too far apart, multiple hepatic duct jejunostomies were required (Fig. 3 D).

Roux-en-Y anastomosis of hepatic duct and jejunum A Left and right hepatic duct reshaping. B Hepatic jejunostomy after perihepatic resection. C Hepatic jejunostomy after combined left hepatectomy. D Two hepatic duct jejunostomy
Postoperative management
Postoperative management was conducted in accordance with the principles of Enhanced Recovery After Surgery (ERAS) [ 5 ]. Briefly, the patients were encouraged to engage in off-bed activities on the first postoperative day and were allowed to consume an appropriate amount of water. The liquid diet was reintroduced as soon as possible, guided by the individualized target-oriented fluid infusion principle. Additionally, conventional anti-infection measures, somatostatin, acid suppression, and nutritional support therapy were implemented. Albumin and plasma were supplemented as necessary. Abdominal CT scans were performed 3 to 5 days following surgery, and drainage tubes were removed in patients with no significant surgical area effusion or bile leakage. For patients with distinct peritoneal effusions, ultrasound-guided puncture drainage was employed.
Data collection and statistical analysis
The following data were collected and analyzed: average operation time, intraoperative blood loss, intraoperative transfusion requirement, time to first postoperative bowel movement, average postoperative hospitalization duration, postoperative pathological grading and staging, number of dissected lymph nodes, negative margin rate (including the radial margin, hepatic and duodenal margin of the resected bile duct, and cut surface of the liver), and postoperative complications (assessed using the Clavien–Dindo classification [ 6 ].
Follow-up was not preplanned but was generally conducted at intervals of 3–6 months. It included comprehensive physical examinations, laboratory testing, and computed tomography when necessary. If patient survival status was unknown at the time of data collection, it was verified through telephone communication.
Statistical analysis was performed using SPSS 23.0 software. Measurement data with a normal distribution are presented as mean ± standard deviation (x ± s), while measurement data with a skewed distribution are presented as median (range). Count data are expressed as absolute numbers.
Laparoscopic radical resection of hilar cholangiocarcinoma was ultimately performed in 38 (86.3%) patients. The remaining patients included 3 who required vascular reconstruction to complete radical surgery and thus converted to laparotomy, and 3 who underwent T-tube drainage only due to unresectable metastases. Among the patients who underwent laparoscopic radical resection, 13 underwent perihilar resection, 11 underwent perihilar resection combined with caudate lobectomy, 11 underwent left hemi hepatectomy combined with caudate lobectomy, and 6 underwent right hemi hepatectomy combined with caudate lobectomy. Among the 17 patients who underwent combined hemihepatectomy, the transected ends of the right or left hepatic ducts were reshaped and subsequently anastomosed with the jejunum. Similarly, for the 22 patients who underwent perihilar hepatectomy or perihilar hepatectomy combined with caudate lobe resection, the bile ducts at the hepatic hilum required reshaping prior to the anastomosis with the jejunum.The median operation time was 285 min (range, 190–450), and the median estimated blood loss was 360 mL (range, 260–1200). Postoperative pathological examination revealed negative microscopic margins (R0) in 39 cases and positive microscopic margins (R1) in 2 cases. The number of lymph nodes harvested ranged from 6 to 17 per case, with a median of 8. According to the American Joint Committee on Cancer (AJCC) eighth edition staging system for hilar cholangiocarcinoma, 14 cases were classified as stage I, 17 as stage II, 10 as stage III, and 3 as stage IV (Table 2 ). The mean time to the first postoperative flatus was 2.7 ± 0.3 days, and the average postoperative hospital stay was 14.3 ± 3.6 days. Postoperative complications occurred in 8 patients (18.1%), with 4 cases (9.0%) classified as Grade I, 3 cases (6.8%) as Grade II, and 1 case (2.2%) as Grade IIIa according to the Clavien–Dindo complication grading system. No Grade IIIb or IV complications were observed. The main complications included pulmonary infection (1 case), postoperative abdominal infections (3 cases), postoperative hepatic insufficiency (1 case), and Grade A biliary fistulas (3 cases). The patient with a Grade IIIa complication had preoperative diabetes mellitus and hypoalbuminemia and developed persistent hyperthermia on the sixth postoperative day. Abdominal culture showed multidrug-resistant bacterial infection with a positive fungal culture. The patient was discharged on the 23rd postoperative day with a normal white blood cell count and no significant effusion on repeat CT. All other patients with complications were cured after symptomatic treatment and discharged from the hospital. No perioperative deaths or severe postoperative complications, such as bleeding, liver failure, or cardiovascular or cerebrovascular events, were observed (Table 3 ).
As of December 10, 2023, 35 of 44 patients (79.5%) were followed up.The median overall survival for patients who underwent radical R0 resection was 30.4 months (range, 5.3–43.6). The Disease-free survival rates were 73.6% at 1 year, 61.2% at 2 years, and 40.1% at 3 years.
Laparoscopic hepatectomy (LH) has gradually expanded in hepatobiliary surgery since Reich’s first successful LH in 1991 [ 7 ]. However, laparoscopy is more often used for exploratory assessment of surgical feasibility in managing hilar cholangiocarcinoma, avoiding unnecessary open surgery [ 8 ]. The porta hepatis’s special location and the region’s complex structures make tumor resection difficult and complex, requiring extensive experience in open surgery and laparoscopic techniques. Consequently, only a few larger hepatobiliary and pancreatic centers in China and abroad have performed experimental procedures for this purpose [ 9 , 10 , 11 ]. It is worth investigating the availability of minimally invasive surgery for patients with hilar cholangiocarcinoma. Our institute has explored laparoscopic radical resection of hilar cholangiocarcinoma based on numerous laparoscopic resections of the digestive tract and pancreatic tumors, with satisfactory clinical efficacy [ 12 , 13 ].
Feasibility of laparoscopic radical resection of hilar cholangiocarcinoma
The surgeon and team’s laparoscopic experience play a crucial role.
Laparoscopic surgery differs from open surgery due to the lack of longitudinal depth and tactile sensation. Simultaneously, the difficult and complex anatomical relationships of the surgery itself for hilar cholangiocarcinoma make this procedure highly demanding for the surgeon and team. Our team can now routinely perform laparoscopic radical gastric cancer surgery, laparoscopic pancreaticoduodenectomy, and laparoscopic hemihepatectomy. Through these procedures, we have mastered the critical techniques required in radical laparoscopic surgery for hilar cholangiocarcinomas, such as “naked vascularization of the hilum,” “laparoscopic biliary-enteric anastomosis,” and laparoscopic hemihepatectomy, which has significantly accelerated our learning curve for this procedure. The surgeon’s extensive laparoscopic experience plays a vital role in managing intraoperative bleeding and accidental injury, reducing the rate of conversion from laparoscopy to open surgery, and ensuring the operation’s safety.
We believe that for a highly challenging operation such as laparoscopic hilar cholangiocarcinoma, a smooth and safe learning curve requires the above-mentioned laparoscopic technical reserve, as well as the close cooperation of anesthesiologists and nursing teams. It is recommended to select suitable type 1 or partial type 2 cases in the initial stage, because these patients are relatively less difficult to operate. Because if serious complications occur early in the development of a new technology, it is a fatal blow to the confidence of the surgeon.
Evaluation of the assistance of preoperative imaging
Advanced imaging techniques can accurately determine tumor staging, classification, and resectability preoperatively, which is crucial for surgical success. MRCP displays the complete hepatic hilum mass, intrahepatic bile duct distribution, and invasion extent of the left and right hepatic ducts [ 14 ]. Three-dimensional CT reconstruction is essential for assessing vascular variation and the relationship between tumors and blood vessels. For patients potentially undergoing extensive hepatectomy, the residual liver volume should be evaluated before surgery. These factors guide surgical planning. All patients underwent enhanced CT and MRCP, followed by careful assessment to determine the preset upper edge. Additionally, the feasibility of safely performing R0 resection laparoscopically was judged, ensuring a safe and thorough operation while saving time.
Understanding and development of the scope of surgery
Radical R0 resection of hilar cholangiocarcinoma requires complete mass resection, negative margins, and clearance of potentially invaded surrounding tissues [ 15 ]. The previously prevalent theory of enlarged hepatectomy positively impacts tumor radical resection. However, it increases the risk of liver failure due to insufficient postoperative residual liver function, failing to improve prognosis and increasing risk [ 16 ]. Recently, many scholars have proposed ‘perihepatic resection’ under the guidance of precision hepatic surgery, which retains functional liver tissue while radically resecting tumor lesions [ 17 ]. This view aligns with our goal of radical laparoscopic surgery. We routinely handle the hepatoduodenal ligament during surgery to expose the hepatic artery, portal vein, and extrahepatic bile ducts, accurately localizing the tumor and its relationship with arterial and portal vein branches. Intraoperative fast frozen pathological examination confirms resection margins, including secondary bile ducts, caudate lobe bile ducts, and liver tissues.
For Bismuth type I and some type II cases, R0 resection is achievable after the above procedure. However, if preoperative examination or intraoperative exploration detects tumor invasion of the left (right) hepatic duct or caudate lobe hepatic duct, combined or extended hepatectomy is performed strictly according to standardized consensus [ 1 ].
Safety and clinical effect of this surgery
In this study, 39 out of 44 patients (88.6%) successfully underwent radical R0 resection, with most procedures (37/39) performed laparoscopically. The operation time and intraoperative blood loss were comparable to, or slightly better than, data from open surgery in most local and international centers in recent years [ 7 , 8 , 9 , 10 , 11 ]. Regarding postoperative complications, no cases of postoperative bleeding, perioperative death, or severe cardiopulmonary complications were observed. According to the Clavien–Dindo postoperative complication grading system, only one patient had a grade of 3 or higher, and this patient was successfully discharged after aggressive nutritional support, anti-infection, and other nonsurgical treatments.
The median age of the 44 patients in this study was 69.3 years, with the oldest patient being 80 years old, which is higher than previously reported in China [ 8 , 9 ]. All patients recovered well after surgery, suggesting that the upper age limit for inclusion in this procedure can be relaxed. The minimally invasive nature of laparoscopic surgery may provide an opportunity for patients of advanced age and poor cardiopulmonary function to experience better postoperative recovery and a reduced incidence of postoperative cardiopulmonary-related complications.
Experiences and problems
Preoperative biliary drainage (pbd) strategy.
Undoubtedly, PBD can significantly reduce the risk of liver resection in patients with hyperbilirubinemia. However, there remains ongoing debate about the benefits and drawbacks of PBD for perioperative and long-term survival in patients with hilar cholangiocarcinoma (HCCA) [ 18 ]. Guidelines from Europe and the United States recommend PBD before HCCA surgery, but there are no clear recommendations on the specific drainage route or protocol [ 19 ]. Our approach is to advocate for PBD in patients with cholangitis, prolonged biliary obstruction, poor nutritional status, serum total bilirubin > 200 µmol/L, and when extensive liver resection is needed with a residual liver volume of less than 40%. Currently, there is no uniform standard for the duration of preoperative biliary drainage. It is generally believed that the duration of drainage should be preferably more than 3 weeks, with a serum total bilirubin level below 50 µmol/L, and the liver function should be basically restored to normal.
Lymph node clearance
As shown by data, lymph node metastasis is a significant indicator of poor prognosis in hilar cholangiocarcinoma. The eighth edition of AJCC recommends routine dissection of lymph nodes located in the hilar, cystic duct, common bile duct, hepatic artery, pancreaticoduodenum, and near the portal vein [ 20 ]. Lymph node dissection in the hepatoduodenal ligament is a challenging and critical aspect of radical resection due to the presence of important blood vessels and the risk of uncontrollable bleeding during the dissection process.
Incomplete clearance of the site can lead to the remaining cancer cells in the hepatoduodenal ligament tissue becoming a high-risk factor for the recurrence of cholangiocarcinoma after resection19 [ 21 ]. Laparoscopy offers advantages in this regard, as it can be performed perpendicular to the visceral surface of the liver. The 30-degree lens provides a wide and deep field of vision, allowing for flexible rotation in the hilar region. The ultrasonic knife can be operated in close proximity to the blood vessel wall under close-sight operation. Additionally, laparoscopy facilitates the identification and clipping of small blood vessels supplying the tumor during lymph node dissection in the hilar area, minimizing unnecessary bleeding and ensuring a clear operation field and smooth progress of the operation. These advantages enable a more detailed and secure’skeletonization’ operation of the hepatoduodenal ligament, which is associated with a high risk of haemorrhage. Although lymph node dissection behind the pancreatic head was previously considered to pose a risk of postoperative pancreatic leakage, our experience in laparoscopic pancreaticoduodenectomy suggests that a ‘Kocher incision’ can be utilized to expose the posterior segment of the common bile duct, allowing for the clearance of posterior pancreatic lymph nodes [ 13 ]. If the tumor is closely related to the blood vessels and the preoperative assessment indicates possible resection, a complete dissection and subsequent nondestructive blocking clip are recommended before proceeding with other relevant interventions.
Hepaticojejunostomy anastomosis
Hepaticojejunostomy anastomosis is another critical step in this operation, as the quality of the anastomosis is generally associated with the risk of postoperative biliary fistula. The fundamental principle of hepatic duct reconstruction is the mucosa-to-mucosa anastomosis of the hepatic duct to the jejunum [ 11 , 22 ]. The main challenge arises from the deep location of the high bile duct and its proximity to the main branches of the portal vein, necessitating extra caution during the procedure. The magnification provided by laparoscopic surgery enables the operator to better control suture spacing, avoiding issues such as missed stitches and inadvertent injury to surrounding vessels, ultimately enhancing the quality of the anastomosis. It is recommended that the residual bile duct should not be excessively stripped, with 3–5 mm being an appropriate length. For patients undergoing PTCD before surgery, the catheter is generally visible after biliary duct incision, and the drainage tube is slightly retracted visually to avoid suture of the catheter during anastomosis, and then pulled out about 4 weeks after surgery. Dilated bile duct can be an advantage when constructing the anastomosis, in cases of significantly dilated and flexible bile ducts, a single layer of continuous epithelialization using a 4 − 0 or 5 − 0 barbed suture can be employed. However, if the bile duct is edematous and fragile, a 5 − 0 PDS II suture is preferable to prevent tissue tearing. When addressing bile duct severed ends, type III bile duct confluence is low, and adjacent bile ducts can be assembled and shaped into a single duct, reducing both the complexity of the anastomosis and the likelihood of biliary fistula formation. If the opening distance remains too excessive after rectification, separate anastomoses may be necessary. In situations where the bile duct openings are large and difficult to reshape, where excessive tension is present after reshaping, or where the bile duct wall is weak and prone to tearing during suturing, a pelvic anastomosis utilizing the Glisson sheath or the tough tissue of the porta hepatis is recommended.
Determination of negative hepatic duct margins
The “skeletonization” of the hepatoduodenal ligament, high-quality bile-intestinal anastomosis, and even combined hemihepatectomy no longer pose insurmountable technical barriers for surgeons with extensive laparoscopic experience. However, ensuring negative hepatic duct margins remains a challenge. Negative hepatic duct margins are of paramount importance for achieving R0 resection [ 23 ].
In open surgery, the texture of the bile duct and surrounding tissues can be visualized to provide a comprehensive view of the incisional margin. Even so, accurate determination by palpation can be difficult, relying on preoperative imaging and intraoperative frozen section pathology. Laparoscopic surgery, on the other hand, relies more heavily on intraoperative frozen section pathology to determine margins, requiring a higher level of pathology expertise in the hospital and greater demands on the surgeon to remove the bile duct stump during surgery. Ensuring the integrity of the anterior and posterior walls of the bile ducts and marking the upper and lower ends before sending the specimen for pathological examination, with a margin of at least 3 mm, is crucial. The magnification provided by the laparoscope can help distinguish small bile ducts and the specific location of tumor invasion, offering certain advantages. Future technological advances (e.g., 3D printing) may better address this issue [ 24 ].
Limitations of this study
This study is a single-center retrospective analysis with a small sample size, so the conclusions drawn are one-sided to a certain extent. Therefore, prospective studies with large sample size and long follow-up time are still needed in the later stage to further verify the findings. Additionally, the role of neoadjuvant chemotherapy, targeted therapy, and immunotherapy for patients with resectable hilar cholangiocarcinoma was not addressed. Despite these shortcomings, this minimally invasive surgery is friendly for elderly patients with liver insufficiency, and laparoscopic surgery also has advantages in amplification for lymph node dissection and high bilioenteric anastomosis. The most important thing is that none of the patients in this study had serious complications or died after surgery, and the safety of this operation can be preliminarily confirmed.
Laparoscopic hilar cholangiocarcinoma is a challenging procedure, and our limited experience suggests that it can be performed safely by selecting the appropriate cases for surgeons with extensive laparoscopic experience, which warrants further exploration to confirm its clinical efficacy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Endoscopic nasobiliary drainage
Ercutaneous transhepatic cholangial drainage
Computed tomography
Magnetic resonance cholangiopancreatography
The American Society of Anaesthesiologists classification
Common hepatic artery
Proper hepatic artery
Hepatic artery
American Joint Committee on Cancer
Laparoscopic hepatectomy
Total bilirubin
Direct bilirubin
Body mass index
Interquartile range
Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M. Vauthey JN. hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):691–9.
Kovalenko YA, Zharikov YO, Konchina NA, Gurmikov BN, Marinova LA, Zhao AV. Perihilar cholangiocarcinoma: a different concept for radical resection. Surg Oncol. 2020;33:270–5.
Article PubMed Google Scholar
Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, Blumgart LH, Busch OR, D’Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, van Gulik TM, Jarnagin WR. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2016;27(4):753.
Article CAS PubMed PubMed Central Google Scholar
Buettner S, van Vugt JLA, Gaspersz MP, Coelen RJS, Roos E, Labeur TA, Margonis GA, Ethun CG, Maithel SK, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick BA, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, IJzermans JNM, van Gulik TM, Pawlik TM, Groot Koerkamp B. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB (Oxford). 2017;19(8):735–40.
Article PubMed PubMed Central Google Scholar
Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, Wellge EB, Kunzler F, Besselink MG, Asbun H, Scott MJ, Dejong CHC, Vrochides D, Aloia T, Izbicki JR, Demartines N. Guidelines for Perioperative Care for Pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg. 2019;44(7):2056–84.
Article Google Scholar
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Reich H, McGlynn F, J DeCaprio,R Budin. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–8.
CAS PubMed Google Scholar
Zhang Y, Dou C, Wu W, Liu J, Jin L, Hu Z, Zhang C. Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc. 2020;34(10):4382–7.
Chen Y, Xu Y, Zhang Y. Current status of laparoscopic radical hilar cholangiocarcinoma in Mainland China. Biosci Trends. 2020;14(3):168–73.
Sucher R, Scheuermann U, Seehofer D. Total laparoscopic resection of Hilar Cholangiocarcinoma Type 3b: applying a parachute technique for Hepaticojejunostomy. Ann Surg Oncol. 2021;28(4):2030–4.
Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic right hepatectomy with Roux-en-Y hepaticojejunostomy for fight-sided intraductal papillary mucinous neoplasm of the bile duct. Ann Surg Oncol. 2014;21(6):1841–3.
Du Y, Wang J, Li Y, Ma HQ, Liu L, Zhu YX, Zhao WX. Clinical application of a modified pancreatojejunostomy technique for laparoscopic pancreaticoduodenectomy. HPB (Oxford). 2019;21(10):1336–43.
Wang J, Ma HQ, Liu L, Zhu YX, Zhao WX. Total laparoscopic pancreaticoduodenectomy: a report of 111 cases in a single center. Chin J Gen Surg. 2019;34(12):1035–9.
Google Scholar
Park MJ, Kim YK, Lim S, Rhim H, Lee WJ. Hilar cholangiocarcinoma: value of adding DW imaging to gadoxetic acid-enhanced MR imaging with MR cholangiopancreatography for preoperative evaluation. Radiology. 2014;270(3):768–76.
Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250(2):210–8.
Ramos Rubio E. Radical surgery for hilar cholangiocarcinoma (klatskin tumor). Cir Esp. 2007;82(1):11–5.
Noji T, Tsuchikawa T, Okamura K, Shichinohe T, Tanaka E, Hirano S. Surgical outcome of hilar plate resection: extended hilar bile duct resection without hepatectomy. J Gastrointest Surg. 2014;18(6):1131–7.
Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The Clinical Management of Cholangiocarcinoma in the United States and Europe: a Comprehensive and evidence-based comparison of guidelines. Ann Surg Oncol. 2021;28(5):2660–74.
Ramanathan R, Borrebach J, Tohme S, Tsung A. Preoperative biliary drainage is Associated with increased complications after Liver Resection for Proximal Cholangiocarcinoma. J Gastrointest Surg. 2018;22(11):1950–7.
Gaspersz MP, Buettner S, van Vugt JLA, de Jonge J, Polak WG, Doukas M, Ijzermans JNM, Koerkamp BG, Willemssen FEJA. Evaluation of the New American Joint Committee on Cancer Staging Manual 8th Edition for Perihilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24(7):1612–8.
Yoshida T, Matsumoto T, Sasaki A, Morii Y, Shibata K, Ishio T, Kitano S. Lymphatic spread differs according to tumor location in extrahepatic bile duct cancer. Hepatogastroenterology. 2003;50(49):17–20.
PubMed Google Scholar
Kadaba RS, Bowers KA, Khorsandi S, Hutchins RR, Abraham AT, Sarker SJ, Bhattacharya S, Kocher HM. Complications of biliary-enteric anastomoses. Ann R Coll Surg Engl. 2017;99(3):210–5.
Article CAS PubMed Google Scholar
Feng F, Cao X, Liu X, Qin J, Zhang S, Li Q, Liu J. Laparoscopic resection for Bismuth type III and IV hilar cholangiocarcinoma: how to improve the radicality without direct palpation. J Surg Onco. 2019;120(8):1379–85.
Zhang J, Guo X, Wang H, Zhang J, Liu P, Qiao Q, Wang X. The application of three-Dimensional visualization in preoperative evaluation of Portal Vein Invasion in Hilar Cholangiocarcinoma. Cancer Manag Res. 2020;12:9297–302.
Download references
Author information
Authors and affiliations.
Department of General Surgery, Affiliated Hospital of Xuzhou Medical University, #99 Huai Xi Road, Jiangsu, Xuzhou, 221002, P. R. China
Yusheng Du, Ji Wang, Li Liu, Hongqin Ma, Wenxing Zhao & Ying Li
You can also search for this author in PubMed Google Scholar
Contributions
W.X.Z and Y.L proposed the study. Y.S.D designed and wrote the article. All authors contributed to literature searching, data collecting and interpretation of the study. Y.L and W.X.Z supervised the study and revised the article. All authors have reviewed and approved the final article.
Corresponding authors
Correspondence to Wenxing Zhao or Ying Li .
Ethics declarations
Human ethics and consent to participate.
This study was approved and adopted by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethics No.XYFY2019-KL051-01). All patients provided informed consent.
Competing interests
The authors declare no competing interests.

Disclosures
Yusheng Du, Ji Wang, Li Liu, Hongqin Ma, Wenxing Zhao, Ying Li have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Du, Y., Wang, J., Liu, L. et al. Total laparoscopic radical resection of hilar cholangiocarcinoma: preliminary experience of a single center. BMC Surg 24 , 241 (2024). https://doi.org/10.1186/s12893-024-02533-w
Download citation
Received : 29 May 2024
Accepted : 14 August 2024
Published : 24 August 2024
DOI : https://doi.org/10.1186/s12893-024-02533-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hilar cholangiocarcinoma
- Laparoscopy
- Hepaticojejunostomy
BMC Surgery
ISSN: 1471-2482
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 8, August 2024 (In Progress)
- Volume 2024, Issue 7, July 2024
- Bariatric Surgery
- Breast Surgery
- Cardiothoracic Surgery
- Colorectal Surgery
- Colorectal Surgery, Upper GI Surgery
- Gynaecology
- Hepatobiliary Surgery
- Interventional Radiology
- Neurosurgery
- Ophthalmology
- Oral and Maxillofacial Surgery
- Otorhinolaryngology - Head & Neck Surgery
- Paediatric Surgery
- Plastic Surgery
- Transplant Surgery
- Trauma & Orthopaedic Surgery
- Upper GI Surgery
- Vascular Surgery
- Author Guidelines
- Submission Site
- Open Access
- Reasons to Submit
- About Journal of Surgical Case Reports
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case report, conflict of interest statement, type i choledochal cyst. total laparoscopic resection and roux-en-y reconstruction to two separated ducts.
- Article contents
- Figures & tables
- Supplementary Data
Natalia Reyes, Camila Sotomayor, Martín Inzunza, Eduardo Briceño, Eduardo Viñuela, Jorge Martínez, Nicolás Jarufe, Type I choledochal cyst. Total laparoscopic resection and Roux-en-Y reconstruction to two separated ducts, Journal of Surgical Case Reports , Volume 2024, Issue 8, August 2024, rjae543, https://doi.org/10.1093/jscr/rjae543
- Permissions Icon Permissions
A choledochal cyst is a rare condition that requires surgical treatment to prevent complications, such as obstructive jaundice, cyst rupture, cholangitis, and the risk of malignancy. Complete cyst excision is considered the best option, as it reduces the risk of inflammation and the development of cholangiocarcinoma. Therefore, cholecystectomy and complete cyst resection followed by reconstruction with a Roux-en-Y hepaticojejunostomy is the treatment of choice. We present a case (with video) that shows the complete resection of a type I choledochal cyst with Roux-en-Y reconstruction of two separate ducts since the right posterior duct reached the cyst independently. The laparoscopic approach offers all the advantages of mini-invasive surgery and better visualization of the structures; however, biliary reconstruction to fine ducts implies a surgical challenge that requires high training in mini-invasive surgery.
Choledochal cyst (CC) is a rare congenital pathology that causes dilation of the extrahepatic, intrahepatic biliary tree, or both. Its incidence varies between 1 in every 13 000 and 2 000 000 live births [ 1 ], with type I CC representing 80%–90% of these cases [ 2 ]. Symptoms appear in 84.5% of patients, with abdominal pain and jaundice being the most prevalent. Complications include cholelithiasis, cholangitis, spontaneous rupture, pancreatitis, and cholangiocarcinoma [ 3 ].
Complete resection of type I CC involves removing the entire dilated extrahepatic biliary tree from the confluence to the intrapancreatic common bile duct to eliminate the risk of malignant transformation. Reconstruction is generally performed using Roux-en-Y hepaticojejunostomy (RYHJ). The association between an anatomical variant of the right posterior hepatic duct (RPHD) and CC is rare, with few documented cases in the medical literature [ 4 ].
We present the case of a patient with a type I CC resolved by laparoscopic surgery, with complete resection of the cyst and Roux-en-Y reconstruction of two separate ducts since the right posterior duct reached the cyst independently. Our goal is to contribute to the limited evidence regarding this rare case, as a few published cases in the medical literature have undergone this surgical technique treatment.
The present case corresponds to a 45-year-old woman with a history of recurrent episodes of recurrent abdominal pain and jaundice, which has required sporadic hospitalizations, during which she underwent endoscopic retrograde cholangiopancreatography with a bile duct stent placed.
A magnetic resonance cholangiopancreatography revealed the presence of a large 5 cm CC, classified as type I according to the Todani classification, accompanied by significant lithiasis of up to 3 cm inside and cholelithiasis. An anatomical variant was identified in which the RPHD drained directly into the CC.
The surgical team performed a complete resection of the CC, accompanied by reconstruction using two independent enterotomies and anastomoses, given the considerable distance between the right anterior and posterior bile ducts. The common hepatic duct (CHD) was dissected distally until the intrapancreatic bile duct of normal caliber was reached. Proximally, dissection continued to the confluence of the right anterior and left ducts. Two separate hepaticojejunostomy were performed, first to the right posterior duct and then to the CHD (see Video 1 ).
The patient showed a favorable recovery with no signs of biliary fistula, allowing the drain to be removed, and the patient to be discharged 5 days postoperatively. Follow-up revealed no stenosis of the hepaticojejunostomy, and the patient reported no significant discomfort.
CC is a rare pathology in adults. Although mainly diagnosed during childhood, 20%–25% of patients are diagnosed in adulthood. It affects 0.1% of the population and is four times more common in women [ 5 ]. The incidence of malignant transformation to cholangiocarcinoma is 3%–5%, increasing from 0.7% in the first decade to over 14% after 20 years [ 1 ]. Therefore, early diagnosis and cyst resection surgery are essential.
The evolution of surgical treatment has progressed from cyst-enteral anastomosis, associated with symptom recurrence and malignancy risk, to complete cyst resection with biliodigestive reconstruction via open or laparoscopic approaches [ 6 ].
Currently, minimally invasive approaches are used to treat CC, as laparoscopy provides better visualization and more precise manipulation. Zhen et al. compared the open and laparoscopic techniques, finding that laparoscopy resulted in a shorter hospital stays (MD = −1.93; 95%CI = −2.51 to −1.36; P < .00001) and faster recovery of intestinal function (MD = −0.94; 95%CI = −1.33 to −0.55; P < .00001), with no significant differences in postoperative complications (RR = 1.04; 95%CI = 0.66–1.62; P = .88). Although the laparoscopic group had longer operative times (MD = 56.57; 95%CI = 32.20–80.93; P < .00001), it had lower rates of red blood cell transfusion (RR = 0.20; 95%CI = 0.11–0.38; P < .00001) and intestinal obstruction (RR = 0.17, 95%CI = 0.04–0.77; P = .02) [ 7 ].
The laparoscopic technique represents a surgical challenge that requires advanced experience and skills from the surgical team.
The standard biliodigestive reconstruction technique is RYHJ. However, studies have defended hepaticoduodenostomy as a more physiological tension-free technique, faster to perform, and with potential for future endoscopic interventions. Ai et al. identified that patients undergoing biliodigestive reconstruction with hepaticoduodenostomy had a shorter hospital stay (MD = −0.40; P = .02), a shorter operative time (MD = −59.54; P < .00001), and a lower incidence of adhesive intestinal obstruction (OR = 0.20; P = .02) than those undergoing hepaticojejunostomy. However, it was associated with a higher incidence of gastritis (OR = 6.24; P = .002) [ 8 ]. Howell et al. [ 9 ] reported that patients undergoing hepaticojejunostomy had a lower readmission rate compared to those undergoing hepaticoduodenostomy (4.0% vs. 10.5%, OR = 0.34, CI [0.12, 0.79], P = .02), the causes of readmission being pancreatitis, surgical site infection, abdominal pain, and cholangitis.
Regarding the reconstruction technique of the anatomical variant of the RPHD, there are two approaches depending on the distance between the CHD and the RPHD: ductoplasty and double hepaticojejunostomy [ 4 ]. Ligation or failure to identify the RPHD during surgery can lead to complications such as cholangitis, liver abscess, liver atrophy, cirrhosis, biliary fistula, or biliary peritonitis [ 10 ].
There is little evidence comparing both techniques, the majority being in the pediatric population. Diao et al. [ 11 ] evaluated laparoscopic treatment for aberrant hepatic duct (AHD) in children with CC, identifying that the AHD was frequently confused with adhesions, causing bile leaks in the postoperative period. Xie et al. [ 12 ], when comparing robotic versus laparoscopic surgery for CC in children with AHDs, found that of a total of 22 patients, the majority required ductoplasty and only two required double hepaticojejunostomy in both groups, with no significant differences in postoperative complications. Bile leak is one of the most worrying complications associated with this procedure [ 4 ].
In this case, the technique of choice was separate hepaticojejunostomy, given the distance between both ducts, as the evidence suggests. It is essential to mention that to perform this procedure, a specialized team with the experience and skills necessary to perform fine anastomoses laparoscopically was required, and adequate training was necessary to achieve good results.
It is important to note that little additional published evidence resembles this case. We hope to contribute to medical knowledge to manage these challenging cases effectively.
None declared.
Brown ZJ , Baghdadi A , Kamel I , et al. Diagnosis and management of choledochal cysts . HPB (Oxford) 2023 ; 25 : 14 – 25 . https://doi.org/10.1016/j.hpb.2022.09.010 .
Google Scholar
Abbas HM , Yassin NA , Ammori BJ . Laparoscopic resection of type I choledochal cyst in an adult and Roux-en-Y hepaticojejunostomy: a case report and literature review . Surg Laparosc Endosc Percutan Tech 2006 ; 16 : 439 – 44 . https://doi.org/10.1097/01.sle.0000213768.70923.99 .
Pakkala AK , Nekarakanti PK , Nagari B , et al. An audit of complicated choledochal cysts- 15-years' experience at a tertiary care center . Langenbecks Arch Surg 2023 ; 408 : 212 . https://doi.org/10.1007/s00423-023-02952-y .
Wen Z , Cheng W , Liang Q , et al. Laparoscopic Management of choledochal cysts associated with aberrant hepatic ducts . J Laparoendosc Adv Surg Tech A 2019 ; 29 : 1060 – 6 . https://doi.org/10.1089/lap.2019.0026 .
Ulas M , Polat E , Karaman K , et al. Management of choledochal cysts in adults: a retrospective analysis of 23 patients . Hepatogastroenterology 2012 ; 59 : 1155 – 9 . https://doi.org/10.5754/hge10827 .
Yuan H , Dong G , Zhang N , et al. Minimally invasive strategy for type I choledochal cyst in adult: combination of laparoscopy and choledochoscopy . Surg Endosc 2021 ; 35 : 1093 – 100 . https://doi.org/10.1007/s00464-020-07473-z .
Zhen C , Xia Z , Long L , et al. Laparoscopic excision versus open excision for the treatment of choledochal cysts: a systematic review and meta-analysis . Int Surg 2015 ; 100 : 115 – 22 . https://doi.org/10.9738/INTSURG-D-14-00165.1 .
Ai C , Wu Y , Xie X , et al. Roux-en-Y hepaticojejunostomy or hepaticoduodenostomy for biliary reconstruction after resection of congenital biliary dilatation: a systematic review and meta-analysis . Surg Today 2023 ; 53 : 1 – 11 . https://doi.org/10.1007/s00595-021-02425-z .
Howell TC , Beckhorn CB , Antiel RM , et al. Contemporary trends in choledochal cyst excision: an analysis of the pediatric national surgical quality improvement program . World J Surg 2024 ; 48 : 967 – 77 . https://doi.org/10.1002/wjs.12128 .
Sofi AA , Alaradi OH , Abouljoud M , Nawras AT . Aberrant right hepatic duct draining into the cystic duct: clinical outcomes and management . Gastroenterol Res Pract 2011 ; 2011 :458915. https://doi.org/10.1155/2011/458915 .
Diao M , Li L , Cheng W . Laparoscopic management for aberrant hepatic duct in children with choledochal cysts . Surg Endosc 2019 ; 33 : 2376 – 80 . https://doi.org/10.1007/s00464-019-06807-w .
Xie X , Li K , Xiang B . Robotic versus laparoscopic surgery for choledochal cyst in children with aberrant hepatic ducts: a retrospective study . Asian J Surg 2023 ; 46 : 4186 – 90 . https://doi.org/10.1016/j.asjsur.2022.11.006 .
Supplementary data
Email alerts, citing articles via, affiliations.
- Online ISSN 2042-8812
- Copyright © 2024 Oxford University Press and JSCR Publishing Ltd
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.10(11); 2021 Nov
Etiology of obstructive jaundice and its correlation with the ethnic population of Sikkim
Karma d. bhutia.
1 Department of Intermediate Reference Laboratory, S.T.N.M. Hospital, Gangtok, Sikkim, India
Tsella Lachungpa
2 Department of Radiology, S.T.N.M. Hospital, Gangtok, Sikkim, India
Sangey C. Lamtha
3 Department of Gastroenterology, S.T.N.M. Hospital, Gangtok, Sikkim, India
The aim of the study was to find out the etiology of obstructive jaundice and its correlation with the ethnic population of Sikkim.
Material and Method:
The data of patients with obstructive jaundice admitted under the Department of Gastroenterology was collected retrospectively from March 2019 till February 2020. There were a total of 73 patients of obstructive jaundice patients, the benign etiology was found to be more common than malignant etiology.
The male-to-female ratio in our study was 0.35:1. The most common etiology of benign cause of obstructive jaundice was choledocholithiasis (95.83%) followed by common bile duct stricture (3.07%), Mirizzi syndrome (1.53%). The most common causes of malignant obstructive jaundice were carcinoma of gall bladder (62.5%) followed by carcinoma of periampullary region (12.5%), cholangiocarcinoma (12.5%), carcinoma of head of pancreas (12.5%).
Conclusions:
The most common etiology of obstructive jaundice in this study was choledocholithiasis. There was no any correlation of obstructive jaundice with ethnic population of Sikkim.
Introduction
Obstructive Jaundice is a common problem that occurs when there is an obstruction to the passage of conjugated bile from liver cells to intestine.[ 1 ] Endoscopic retrograde cholangiopancreatography (ERCP) has become the one of treatment modality for patients with obstructive jaundice because of its therapeutic capabilities. The success rate of ERCP for treatment is highly variable ranging from 50% to 96% depending on the operator, endoscopic aspect, disease severity, and anatomical abnormality.[ 2 , 3 ] Jaundice due to biliary obstruction may be caused by a heterogeneous group of diseases that include both benign and malignant conditions.[ 4 ] The common etiologies of obstructive jaundice have been reported to vary from one center to another and from one individual to another.[ 4 , 5 ] The morbidity and mortality related to obstructive jaundice depends upon the causes of obstruction.[ 6 ] There are some studies on the etiology of obstructive jaundice but none from the state of Sikkim. Lack of literature lead to this study. This study was done to find out the etiologies of obstructive jaundice among the ethnic population of Sikkim, India.
As per first colonial census of Sikkim 1891, there were thirteen ethnic races in the Kingdom of Sikkim groups namely Chettri, Subba, Bhutia, Tamang, Gurung, Biswakarma (BK) and others.[ 7 ]
Material and Method
The data of the admitted patients of obstructive jaundice was collected retrospectively from the department of gastroenterology, Sir Thutop Namgyal Memorial Hospital (S.T.N.M.), Socheygang a tertiary care referral center from March 2019 till February 2020. There were a total of 73 patients of obstructive jaundice during that period. Patients of hepatocellular jaundice, prehepatic jaundice and young patients with age less than 15 years were excluded. All patients have undergone complete blood counts, liver function tests, kidney function test, prothrombin time with internationalized ratio, hepatitis B surface antigen, anti-HCV antibody test, retrovirus test. Diagnosis of obstructive jaundice etiology was made with ultrasound of whole abdomen, magnetic resonance cholangiography (MRCP) and contrast-enhanced computed tomography (CECT) whole abdomen. ERCP was done in these patients only as therapeutic procedure.
Of 73 cases, 2 patients of advanced malignancy refused further interventions (one patient of gall bladder malignancy and other of carcinoma of head of pancreas), 3 cases of large common bile duct stone about 2 cm in size on MRCP, underwent open laparotomy and common bile duct exploration and therapeutic ERCP procedures was done in only 69 cases. 8 cases of ERCP developed mild to moderate post ERCP pancreatitis despite using rectal diclofenac 100 mg suppository and they were managed conservatively. Pancreatic plastic stent was placed in 11 cases due to repeated pancreatic duct cannulation, while biliary plastic stent was placed in 63 cases. Self-expanding biliary metal stent was placed in 6 patients, four patients of gall bladder malignancy, one each of periampullary carcinoma and cholangiocarcinoma [ Table 1 ].
Details of therapeutic procedures
| Total patients | Total ERCP | Biliary plastic stents | Pancreatic stents | Biliary metal stents | Patient refused further treatment | Post ERCP pancreatitis | Open Surgery |
|---|---|---|---|---|---|---|---|
| 73 | 69 | 63 | 11 | 6 | 2 | 8 | 2 |
In our study, the male-to-female ratio was 0.35:1 [ Table 2 ]. The female patients were more in number as compared to male patients. The age group of the patients ranged from 23 years to 80 years with benign disease while age group from 40 years old to 73 years old patients were found with malignant disease. The most common etiology among benign causes was Choledocholithiasis (95.83%) followed by common bile duct stricture (3.07%), mirrizzi syndrome type 1 (1.53%). The most common malignant causes was carcinoma of gall bladder (62.50%) followed by periampullary cancer (12.5%), cholangiocarcinoma (12.5%), carcinoma of head of pancreas (12.5%). Among the ethnic group, the obstructive jaundice was common among Chettri ethnic group in both benign as well as malignant cause compared with the rest of other ethnic groups. But this difference with other ethnic groups was not found to be statistically significant [ Table 3 ].
| Total number of patients | Total number of Male patients | Total number of Female patients | Sex ratio (M:F) |
|---|---|---|---|
| 73 | 19 | 54 | 0.35 :1 |
Etiology of obstructive jaundice and its correlation with ethnic population of Sikkim
| Etiology | Chettri | Gurung | Tamang | Subba | Bhutia | BK | Others | Total | Statistically significance |
|---|---|---|---|---|---|---|---|---|---|
| Choledocholithiasis (95.38%) | 17 | 3 | 8 | 9 | 9 | 6 | 10 | 62 | =0.4295 Not significant, R/R=0.5846 |
| Common bile duct stricture (3.07%) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | |
| Mirizzi Syndrome (1.53%) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Carcinoma of Gall Bladder (62.5%) | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 5 | |
| Periampullary carcinoma (12.5%) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Cholangiocarcinoma (12.5%) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Carcinoma of pancreas (12.5%) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 23 | 5 | 9 | 9 | 10 | 7 | 10 | 73 |
Obstructive jaundice is characterized by presence of jaundice, itching, pain abdomen, vomiting, fever or cholangitis, in some cases depending upon the etiology, weight loss, clay color stool. The treatment and prognosis depends upon the etiology and level of biliary obstruction. The occurrence of the most frequent cholesterol stones is connected with the manner and place of living, nourishment, and sex.[ 8 ] The benign etiology of obstructive jaundice are choledocholithiasis, common bile duct strictures, mirrizzi syndrome, impacted parasites in common bile duct, chronic pancreatitis while the malignant causes are carcinoma gall bladder, carcionoma of pancreas, hilar metastasis, periampullary carcinoma, cholangiocarcionoma.[ 9 ]
This study was done to find out the most common etiology of obstructive jaundice both benign and malignant cause prevalent in the state of Sikkim. This study was also done to guide the primary care physicians to know the commonest etiology of obstructive jaundice in their day-to-day practice and also for early referral to tertiary care center for treatment and management of such patients.
Mangam et al .[ 10 ] concluded in their study that males (54.71%) had more obstructive jaundice compare to females (45.28%). Gill HS et al .[ 11 ] in their study, they found that the incidence of gall stones was more common in female than male. Kotwal et al .[ 12 ] in their study concluded gallstones are common in Sikkim and North Bengal among dyspeptics and majority of these stones were cholesterol stones. The gallstones was more common in females and in patients with normal weight. In our study also female had more common bile duct stones than males. Ahsan Ali Laghari et al .[ 13 ] in their study among 50 patients of Obstructive jaundice, males (62%) were more common than females (38%). Jaundice was the most common presentation and majority of patients had benign etiology 31 patients and 19 patients had malignant etiology of obstructive jaundice.
Björnsson et al .[ 14 ] in their study, they found pancreatic cancer and cholangiocarcinoma were the most common cause of obstructive jaundice. The age group among the malignant obstruction was ranging 61 years to 81 years. Shalini et al .[ 15 ] also found in their study carcinoma of head of pancreas (66.7%) was the most common cause of overall obstructive jaundice and choledocholithiasis (33.3%) was the common cause among benign disease. Lindberg et al .[ 16 ] studied 64 cases of bile duct obstruction and observed gallstones disease in 29 patients, pancreatitis in 1 patient, sclerosing cholangitisin 2 patients, pancreatic carcinoma in 18 patients, bile duct carcinoma in nine patients, and gall bladder carcinoma in five patients. Kajal Kumar Patra et al .[ 17 ] found that the most common age group among obstructive jaundice was between 31 years to 70 years and the most common etiology was choledocholithiasis followed by carcinoma of head of pancreas. However, in our study, the most common etiology was choledocholithiasis while the most common malignant etiology was carcinoma of gall bladder in association with gallstones.
Few studies related to ethnicity and gallbladder disease were done. Comparisons across studies suggest that the highest risk of gallstones occurs among American Indians with progressively lower risk among whites, blacks, and some Asian groups.[ 18 ] Mexican American women also have a higher prevalence of gallstones than U.S. Hispanic women.[ 19 ] In this study, there was no any correlation of ethnicity with obstructive jaundice.
In India, gallbladder carcinoma (GBC) is most prevalent in northern and northeastern states of Uttar Pradesh, Bihar, Orissa, West Bengal, and Assam.[ 20 ] GBC is two times higher in women than men and is the leading digestive cancer in women in northern Indian cities.[ 21 ] Six Cancer registries of the Indian Council of Medical Research (1990–96) show a 10 times lower incidence of GBC per 100 000 in South India compared with the North, the age-adjusted incidence rate for females being 0.8 in Chennai in the south and 8.9 in Delhi in the north.[ 22 ] Gallstones were said to play a major role.[ 23 ] Other risk factors are obesity, multiparity, and chronic infections.[ 24 ]
In this study, the key points to highlight were that the benign cause was more common than malignant cause among obstructive jaundice prevalent in the state of Sikkim. Choledocholithiaisis was the most common etiology among benign etiology and while gall bladder carcinoma was the commonest malignant etiology. Both the conditions require early diagnosis and management of such cases.
Overall the most common etiology of obstructive jaundice was choledocholithiasis while gall bladder carcinoma was the most common malignant cause in Sikkim which lies in the eastern Himalayan region of India. Our study also concluded that there was no any association of ethnicity with obstructive jaundice in the state of Sikkim.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Carcinoma esophagus with small bowel metastasis: a case report with review of literature
- Case report - Complication
- Published: 27 August 2024
Cite this article

- Vigneshwaran Chandran 1 ,
- Kannan Periasamy ORCID: orcid.org/0000-0001-7440-0996 1 nAff4 ,
- Lileswar Kaman 2 ,
- Kirti Gupta 3 &
- Uma Nahar 3
Small intestinal metastasis is extremely rare and only 13 cases have been reported till date and almost all such patients have presented with intestinal obstruction. The 5-year overall survival for metastatic esophageal cancer is as low as 5% while the patients with small intestinal metastasis have a median survival of only 3 months (range 1–12 months) despite undergoing radical resection of the small bowel. We present a case of a male in his 50’s who presented with difficulty in swallowing for 4 months. On evaluation, he was found to have squamous cell carcinoma in the mid thoracic esophagus. He underwent radical chemo-radiation up to 60 Gy in 25 fractions over 5 weeks. One week after completion of treatment he presented with ileal obstruction and omental nodules and surgical resection and evaluation of the respective ileal segment and omental biopsy revealed a metastatic squamous cell carcinoma. The patient expired 3 months post-surgery. Carcinoma esophagus with small bowel metastasis has a very grave prognosis that patients rarely survive beyond 1 year despite undergoing resection. Hence it is imperative to consider a small bowel metastasis when such patients present with clinical features of intestinal obstruction for early diagnosis and aggressive management.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data availability
The patient’s medical file is available in PGIMER, Chandigarh. Dr. Kannan Periasamy has got full access to the medical records of the patient and can be made available on request.
Ferlay J, Ervik M, Lam F et al (2024) Global cancer observatory: cancer today (version 1.1). International Agency for Research on Cancer, Lyon, France. Available from https://gco.iarc.who.int/today . Accessed 24 Aug 2024
Google Scholar
Bray F, Laversanne M, Sung H et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263
Article PubMed Google Scholar
Quint LE, Hepburn LM, Francis IR et al (1995) Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer 76(7):1120–1125
Article CAS PubMed Google Scholar
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Napier KJ, Scheerer M, Misra S (2014) Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 6(5):112–120
Article PubMed PubMed Central Google Scholar
Datta A, Periasamy K, Khosla D et al (2021) Synchronous splenic metastases from squamous cell carcinoma of oesophagus: a case report and review of literature. Clin J Gastroenterol 14(2):410–414
Wang M, Patel J, Casey TT et al (1985) Metastatic squamous cell carcinoma from the esophagus occurring as small bowel obstruction. South Med J 78(7):884–886
Idelevich E, Kashtan H, Mavor E et al (2006) Small bowel obstruction caused by secondary tumors. Surg Oncol 15(1):29–32
Schottenfeld D, Beebe-Dimmer JL, Vigneau FD (2009) The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 19(1):58–69
Ono R, Ogino H, Kawachi J et al (2018) Small intestinal metastases from esophageal carcinoma presenting as a perforation: a case report and review of the literature. Int J Surg Case Rep 48:104–108
Matthew RM, Singh S, Viswanathan PN et al (1999) Esophageal carcinoma with spread to mesenteric and iliac lymph nodes. Indian J Gastroenterol 18(3):125
CAS PubMed Google Scholar
Williams DJ (1988) Metastatic oesophageal squamous carcinoma in a small bowel neurofibroma. Jpn J Surg 18(1):110–113
Yamada T, Yagi S, Tatsuzawa Y et al (1996) Small intestinal metastasis from esophageal carcinoma associated with small intestinal obstruction: report of a case. Surg Today 26(10):800–802
Neve RS, Qureshi SS, Mistry RC (2005) Ileal metastases from oesophageal carcinoma causing intestinal obstruction. J Postgrad Med 51(1):74–75
Sreenarasimhaiah J, Hoang MP (2005) Esophageal squamous cell carcinoma with metastasis to the ampulla. Gastrointest Endosc 62(2):310–311
Lindenmann J, Gollowitsch F, Matzi V et al (2005) Occult solitary submucosal jejunal metastasis from esophageal carcinoma. World J Surg Oncol 16(3):44
Article Google Scholar
Arulraj P, Damodaran V, Raman ML et al (2005) Small bowel metastases from esophageal and oropharyngeal cancers. Indian J Gastroenterol 24(3):116–118
Dasari BV, Lee J, Reid D et al (2009) Ileocecal intussusception due to isolated metastasis from primary esophageal adenocarcinoma. South Med J 102(4):419–421
Horio T, Aiko S, Kadoma Y et al (2009) Solitary jejunal metastasis from esophageal squamous cell carcinoma, a case report. J Natl Def Med 34(1):23–29
Yamana I, Takeno S, Maki K et al (2013) Synchronous solitary small intestinal metastasis from esophageal squamous cell carcinoma: report of a case. Esophagus 10:264–267
Chino O, Makuuchi H, Ozawa S et al (2015) Small intestinal metastasis from esophageal squamous cell carcinoma presenting with perforated peritonitis. Tokai J Exp Clin Med 40(2):63–68
PubMed Google Scholar
Morinaga T, Horio K, Shimada K et al (2017) A case report about small intestinal metastasis from esophageal carcinoma combined with bowel obstruction. Biomed J Sci Tech Res. https://doi.org/10.26717/BJSTR.2017.01.000536
Sun JM, Shen L, Shah MA et al (2021) KEYNOTE-590 Investigators Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398(10302):759–771
Download references
The authors did not receive support from any organization for the submitted work.
Author information
Kannan Periasamy
Present address: Department of Radiation Oncology, JIPMER, Puducherry, India
Authors and Affiliations
Department of Radiotherapy and Oncology, PGIMER, Chandigarh, India
Vigneshwaran Chandran & Kannan Periasamy
Department of General Surgery, PGIMER, Chandigarh, India
Lileswar Kaman
Department of Histopathology, PGIMER, Chandigarh, India
Kirti Gupta & Uma Nahar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kannan Periasamy .
Ethics declarations
Conflict of interest.
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Not required for case report by our institution.
Consent to participate
Written informed consent was obtained from the patient’s relative.
Consent for publication
The participant’s relative has consented to the submission of the case report to the journal.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Chandran, V., Periasamy, K., Kaman, L. et al. Carcinoma esophagus with small bowel metastasis: a case report with review of literature. Int Canc Conf J (2024). https://doi.org/10.1007/s13691-024-00717-y
Download citation
Received : 24 November 2023
Accepted : 30 July 2024
Published : 27 August 2024
DOI : https://doi.org/10.1007/s13691-024-00717-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Esophageal cancer
- Small intestinal metastasis
- Radiotherapy
- Chemotherapy
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 28 August 2024
Effect of Tiotropium on eye findings in the treatment of chronic obstructive pulmonary disease
- Hayriye Bektaş Aksoy ORCID: orcid.org/0000-0002-4390-5198 1 &
- Hakan Koç ORCID: orcid.org/0000-0003-1241-1686 2
BMC Pulmonary Medicine volume 24 , Article number: 418 ( 2024 ) Cite this article
Metrics details
Chronic obstructive pulmonary disease (COPD) is a persistent, chronic inflammatory disease of the lungs. Tiotropium, used in the treatment of COPD, is a muscarinic receptor antagonist that provides long-acting bronchodilation. Our study aimed to investigate the effects of Tiotropium on anterior chamber parameters.
The study was conducted as an observational cross-sectional and prospectively between October 2023 and April 2024. Patients were examined in three groups: Group 1 consisted of untreated COPD patients; Group 2 consisted of healthy volunteers similar age and gender, and Group 3 included COPD patients receiving Tiotropium 18 mcg via HandiHaler. Anterior chamber parameters, intraocular pressure values, and photopic-mesopic pupil diameters were measured at the initial visit for Group 1 and Group 2 patients, and at the third month of treatment for Group 3 patients.
Thirty-six patients were included in each group in the study. No significant differences were observed in ocular findings between the patient and control groups. In COPD patients receiving Tiotropium, narrowing of angle parameters, an increase in photopic-mesopic pupil diameters, and intraocular pressure were observed at the third month of treatment.
This study is the first research that investigate the effects of Tiotropium on anterior chamber parameters, pupil diameters, and intraocular pressure in COPD treatment. In conclusion, patients with COPD receiving Tiotropium therapy for three months showed narrowing in angle parameters, an increase in intraocular pressure, and photopic-mesopic pupil diameter; however, no patients developed drug-induced acute angle closure glaucoma.
Trial registration
An independent ethics committee approved the study (Giresun EAH KEAK 2023/180 and 9.10.2023/02) which was performed in accordance with the Declaration of Helsinki, Guidelines for Good Clinical Practice. The study was conducted as prospectively, observational case–control. The Clinical Trial Number obtained for the study is NCT06525051 and was taken on 2024–07-29.
Peer Review reports
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by persistent obstruction of the airways and chronic respiratory symptoms, often with progressive features [ 1 ]. The basic pathophysiological events in the course of COPD can be defined as airflow limitation, air trapping, changes in lung parenchyma, gas exchange abnormalities, excessive mucus secretion, and vascular changes [ 2 ].
There are five types of muscarinic receptors in the airways (M1-M5). M3 receptors mediate the bronchoconstrictor effect of acetylcholine in the airways. Therefore, antimuscarinic drugs that selectively block M3 receptors are considered ideal agents in the treatment of chronic airway disorders [ 3 ].
Bronchodilator drugs are used initially for pharmacotherapy in COPD. Short-acting bronchodilators are used for rapid symptom relief, while long-acting bronchodilators provide more prolonged symptom control. One of the long-acting bronchodilator drugs is inhaled antimuscarinics and the first member of this drug class is Tiotropium. Inhaled antimuscarinic drugs have been used in the treatment of COPD for many years and have a bronchodilator effect lasting more than 24 h [ 4 ]. Tiotropium reduces exacerbations of the disease and related hospitalizations and improves quality of life by providing effective symptom control.
Inhaled antimuscarinic agents have a broad therapeutic range and are well tolerated [ 5 ]. The most common side effects of Tiotropium include dry mouth, metallic taste in the mouth, and ocular side effects such as visual disturbances, mydriasis, increased intraocular pressure, narrow-angle glaucoma, and cataracts [ 6 ].
This study aims to investigate the effects of Tiotropium, a long-acting bronchodilator used in the treatment of COPD, on anterior chamber parameters.
Materials and methods
The study was conducted as prospectively, observational case–control at the Chest Diseases and Ophthalmology Clinic of Giresun Training and Research Hospital between October 2023 and April 2024 after obtaining local Ethics Committee approval (Giresun EAH KEAK 2023/180 and 9.10.2023/02). The Clinical Trial Number obtained for the study is NCT06525051 and was taken on 2024–07-29. Patients diagnosed with COPD and included in Group A according to the GOLD 2024 guidelines, along with healthy volunteers, were enrolled. Patients diagnosed with COPD, with mMRC scale 0–1, CAT score < 10, who had never experienced an exacerbation before or had a mild severity exacerbation once that not required hospitalization were considered as Group A [ 1 ].
Three groups were formed for the study. Group 1 consisted of COPD patients not receiving treatment, Group 2 consisted of healthy volunteers for similar age and gender, and Group 3 consisted of COPD patients of the same age and gender with receiving Tiotropium 18 mcg Handihaler. Anterior chamber parameters, intraocular pressure values and photopic-mesopic pupil diameters were measured at the initial visit for Group 1 and Group 2 patients, and at the third month of treatment for Group 3 patients.
Patients under 18 years of age, pregnant women, those who did not provide written consent, patients diagnosed with COPD who were using inhaled and/or systemic steroids, those using long-acting inhaled beta-2 agonists alone, and those using combined long-acting beta-2 agonists and long-acting anticholinergics, patients with comorbidities receiving medication (e.g., diabetes mellitus, hypertension), those diagnosed with or suspected of glaucoma, those using topical medication, those with shallow anterior chambers, those had previously undergone eyelid, refractive, or intraocular surgery, those with corneal diseases altering the corneal surface, those using contact lenses, and those with chronic eye diseases were excluded from the study.
Anterior segment optical coherence tomography (AS-OCT) was used to measure anterior chamber and angle parameters, corneal topography was utilized to assess anterior chamber depth, pupil diameter measurements in mesopic and photopic conditions, and intraocular pressure (IOP) was calculated using Goldmann applanation tonometry. Each patient underwent a comprehensive ophthalmic evaluation, including testing of best-corrected visual acuity, slit-lamp biomicroscopy, and fundoscopic examination.
Anterior segment optical coherence tomography (AS-OCT)
Ocular measurements were performed using the AS-OCT device (NIDEK RS-3000, NIDEK Co. Ltd, Japan). All measurements were conducted by the same AS-OCT operator, who was blinded to the treatment. Measurements were obtained for nasal and temporal quadrants (180° and 0° meridians) using the ACA line until adequate centering and quality were achieved.
The width of anterior chamber angle (ACA) was measured by calculating the angle between the posterior corneal angle and the tangent iris line. After manually identifying the apex of the iris recess and scleral protrusion, the width of ACA was analyzed using standard parameters. Angle opening distances (AOD), measured at 750 mm (AOD 750) and 500 mm (AOD 500), were measured as the perpendicular distances from the anterior surface of the iris to the scleral spur at 750 mm and 500 mm, respectively. Trabecular iris space area (TISA) was defined as the trapezoidal area (TISA 750 or 500) limited by the vertical distance between the anterior iris surface, the inner corneo-scleral wall, and the scleral spur, for either AOD 750 or 500 (Fig. 1 ). Intraocular pressure measurements were performed around 10:00 a.m. by the same researcher using the same Goldmann applanation tonometer, without any manipulation of the eyelids or with negligible manipulation. Angle parameters measured with anterior segment OCT were obtained by the same researcher, using the same device, at the same times of day and under similar lighting conditions.
Anterior segment OCT image of angle parameters. ACA Anterior Chamber Angle, TISA Trabecular Iris Space Area, AOD Angle Opening Distance
Corneal topography
Corneal topography (Topcon Aladdin Corneal Topography, Japan) was used to evaluate anterior chamber depth and pupil diameter in mesopic and photopic conditions. Pupil diameters measured with corneal topography were obtained by the same researcher, using the same device, at the same times of day and under similar lighting conditions.
Goldmann applanation tonometry
In addition, intraocular pressure was measured using Goldman Applanation tonometry. All measurements were conducted by the same researcher around 10 a.m., using the same tonometer device, without any manipulation or negligible manipulation of the eyelids. Applanation was performed by rotating the dial on the Goldman tonometer previously set to 10 mm Hg. The procedure was repeated after one minute. If the difference between the first and second readings was greater than 2 mm Hg, a third reading was performed. The average of the data obtained from two readings was taken, and if a third reading was needed, the median was used instead.
Statistical analysis
Statistical analysis was performed using IBM SPSS v23. The normality distribution of quantitative data was assessed using the Shapiro–Wilk test. Independent samples t-test was used for normally distributed quantitative data comparison. The Pearson Chi-square test was used for qualitative data comparison. Data were presented as mean ± SD, minimum—maximum, and n (%). A p -value of < 0.05 was considered statistically significant.
Thirty-six patients were included in each group in the study. Healthy volunteers matched for similar age and gender were included as the control group. There were no significant differences in age and gender between all three groups. The demographic characteristics, smoking status, mMRC score, CAT score, exacerbation history in the last year and spirometry results of COPD patients not using any bronchodilator (Group 1) and those using only Tiotropium (Group 3) are presented in Table 1 . Group 1 consisted of 58.3% ( n = 21) active smokers, while 52.8% ( n = 19) of group 3 were ex-smokers. All patients in group 3 had an mMRC score of 1. %47.2 ( n = 17) of group 1 did not experience any exacerbations of COPD in the last year, whereas all of group 3 experienced one exacerbation in the last year. There were no statistically significant differences observed between group 1 and group 3 in terms of smoking status, CAT score and spirometry findings. In group 3, parameters of mMRC score and COPD exacerbation in the last year were found to be statistically significant (p < 0.001).
No significant differences were observed in ocular findings between COPD patient group and healthy volunteers matched for age and gender (Table 2 ). According to the ocular measurements at the third month, a decrease was observed in ACD, ACA T, ACA N, AOD 500 T, AOD 500 N, AOD 750 T, AOD 750 N, TISA 500 T, TISA 500 N, TISA 750 T and TISA 750 N values, while an increase was observed in photopic diameter, mesopic diameter, and IOP values. Statistically significant differences were observed in ocular measurements between patients receiving Tiotropium therapy (Group-3) and those not receiving any bronchodilator therapy (Group-1) (Table 2 ).
After three months of Tiotropium therapy in patients diagnosed with COPD; narrowing in angle parameters, an increase in photopic-mesopic pupil diameters and intraocular pressure were observed.
Chronic inflammation and hypoxia seen in COPD can lead to ocular changes such as choroidal thinning, thinning of the retinal nerve fiber layer, retinal arterial hypoxia, increased retinal vein diameter, and subfoveal choroidal thickening [ 7 ].
In a study evaluating the effects of anticholinergic drugs in stable COPD, it was reported that accidental application of any anticholinergic drug directly to the eye during inhalation could cause pupil dilation and blurred vision, potentially exacerbating acute glaucoma [ 8 ]. These effects may occur when the drug is administered using a metered-dose inhaler (MDI) with eyes open, a poorly fitting mask with a nebulizer inhaler, or when inhaled in HandiHaler form [ 8 ]. Francis et al. reported a statistically significant increase in intraocular pressure in COPD patients following inhaler Tiotropium therapy [ 9 ]. Similarly, Verma et al. reported an increase in intraocular pressure after inhaled Tiotropium therapy [ 10 ]. In our study, we included healthy volunteers to investigate if HandiHaler Tiotropium has a more pronounced effect on the anterior chamber without influencing anterior chamber parameters, pupil diameter, and IOP in COPD. Tasli et al. reported no statistically significant difference in anterior chamber depth between COPD patients and healthy volunteers in their study comparing anterior segment parameters between the two groups [ 11 ]. In this study, we evaluated not only anterior chamber depth but also all angle parameters (AOD500, AOD750, TISA500, TISA750), anterior chamber angle (ACA N, ACA T), pupil diameters (mesopic, photopic), and intraocular pressure (IOP) between COPD patients not using any bronchodilator (Group 1) and healthy volunteers (Group 2). We found no statistically significant differences in any of these parameters between the two groups. The lack of significant differences in anterior chamber and angle parameters between Group 1 and Group 2 may be attributed to several factors. Patients in Group 1 did not use medications (such as steroids) that could potentially affect eye parameters. Additionally, both groups of patients did not have comorbidities (such as hypertension, diabetes mellitus, or glaucoma) that could lead to pathological findings in the eyes, as per exclusion criteria. These factors likely contributed to the absence of significant differences observed in the eye parameters between the two groups.
A case of unilateral angle closure glaucoma in the right eye of a patient using Tiotropium for COPD was reported by Oksuz et al. They speculated that the shorter axial length and narrower anterior chamber angle in the patient's right eye, as well as the dilation of the pupil caused by the Tiotropium treatment, contributed to the development of angle closure glaucoma. They hypothesized that the reason for the absence of angle closure glaucoma in the left eye was likely due to the wider anterior chamber angle resulting from the patient's previous cataract surgery in the left eye. They also suggested that patients receiving Tiotropium therapy should be warned about the inadvertent exposure of the eyes to Tiotropium dry powder with compromised capsule integrity, which could potentially lead to acute angle closure glaucoma, especially in high-risk individuals [ 12 ].
An analysis of Tiotropium found that neither the HandiHaler nor nebulized forms caused glaucoma development or worsening of preexisting glaucoma when compared to the placebo group [ 13 ]. Another study found that short- and long-acting antimuscarinic drugs (Ipratropium and Tiotropium) in MDI form did not cause a significant increase in intraocular pressure in COPD patients [ 14 ].
In our study, although narrowing in angle parameters, an increase in photopic-mesopic pupil diameter, and intraocular pressure values were observed in COPD patients receiving Tiotropium therapy via HandiHaler, none of the patients developed acute angle closure glaucoma due to the treatment. There were no ocular or systemic side effects potentially leading to discontinuation of the medication were observed in patients receiving Tiotropium therapy. We hypothesize that the increase in both mesopic and photopic pupil diameters could be attributed to anticholinergic effects of Tiotropium. The resulting partial mydriasis may cause peripheral iris crowding into the iridocorneal angle. This tissue crowding could partially obstruct aqueous humor outflow through the trabecular meshwork, leading to angle narrowing and a potential increase in intraocular pressure.
Since patients identified with narrow angles during anterior segment examinations were excluded from our study, the results allow for certain conjectures to be made regarding individuals with narrow angles. The findings suggest that reductions in angle parameters observed in patients using HandiHaler Tiotropium could be particularly clinically significant for those with narrow angles. In patients with narrow angles, HandiHaler Tiotropium may potentially lead to angle closure glaucoma. In patients with glaucoma, HandiHaler Tiotropium should be used cautiously due to its potential to increase intraocular pressure by causing pupil dilation, which can reduce the passage of aqueous humor through the iridocorneal angle. Similarly, when planning intraocular anterior segment surgery, potential changes in angle parameters, intraocular pressure, and pupil diameter due to HandiHaler Tiotropium should be considered.
This study is the first research that investigate the effects of HandiHaler Tiotropium on anterior chamber parameters, pupil diameters, and intraocular pressure in the treatment of COPD. It suggests that prior to commencing Tiotropium treatment for stable COPD, patients should undergo a detailed ophthalmological examination, and particular caution should be exercised in receiving of Tiotropium therapy in patients with narrow anterior chamber angles. Furthermore, due to the risk of exposure to the powdered form of Tiotropium when the capsule integrity is compromised, it should be specifically emphasized to patients that the medication should be used without compromising the capsule integrity.
Limitations of our study include the relatively small sample size, short-term follow-up, and the use of only the HandiHaler form of the drug.
In conclusion, patients receiving Tiotropium therapy via HandiHaler for three months showed narrowing in angle parameters, an increase in intraocular pressure, and photopic-mesopic pupil diameter; however, no patients developed drug-induced acute angle closure glaucoma.
Although acute angle closure glaucoma was not observed in patients using Tiotropium for three months, the changes in angle parameters raise concerns about the careful use of Tiotropium therapy, especially in patients with narrow angles. The reliability of our study could be further supported by larger patient cohorts and more comprehensive studies with longer follow-up periods.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Venkatesan P. GOLD COPD report: 2024 update. Lancet Respir Med. 2024;12(1):15–6. https://doi.org/10.1016/S2213-2600(23)00461-7 .
Article PubMed Google Scholar
Celli B, Fabbri L, Criner G, Martinez FJ, Mannino D, Vogelmeier C, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med. 2022;206(11):1317–25. https://doi.org/10.1164/rccm.202204-0671PP .
Article PubMed PubMed Central Google Scholar
Chakkamadathil JK. Newer therapeutic options in chronic obstructive pulmonary disease. J Adv Lung Health. 2023;3(1):5–12. https://doi.org/10.4103/jalh.jalh_23_22 .
Article Google Scholar
Cazzola M, Centanni S, Donner C. Anticholinergic agents. Pulm Pharmacol Ther. 1998;11(5–6):381–92.
Article CAS PubMed Google Scholar
Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. https://doi.org/10.1124/pr.111.004580 .
Shah BK, Singh B, Wang Y, Xie S, Wang C. Mucus hypersecretion in chronic obstructive pulmonary disease and its treatment. Mediators Inflamm. 2023. https://doi.org/10.1155/2023/8840594 .
Vaes AW, Spruit MA, Theunis J, Goswami N, Vanfleteren LE, Franssen FM, et al. Looking into the eye of patients with chronic obstructive pulmonary disease: an opportunity for better microvascular profiling of these complex patients. Acta Ophthalmol. 2018;96(6):539–49. https://doi.org/10.1111/aos.13765 .
Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533(1–3):36–9. https://doi.org/10.1016/j.ejphar.2005.12.072 .
Francis F, Manoj DK, Rajani M, Muhammad Shafeek K, Padmanabhan KV. Effect of inhaled tiotropium on intraocular pressure in patients of chronic obstructive pulmonary disease. Int J Adv Res. 2023;11(6):490–6. https://doi.org/10.21474/IJAR01/17093 .
Verma AM, Alam N, Sahu PK, Narang S, Maroof KA. Effect of inhaled tiotropium on intraocular pressure in patients of chronic obstructive pulmonary disease. Int J Sci Res. 2019;8(10):65–6. https://doi.org/10.36106/ijsr .
Taşlı N, Ölmez H, Uğurlu A, Uçak T, İçel E, Karakurt Y, et al. Assessment of anterior segment parameters and specular microscopy findings in patients with COPD. Erciyes Med J. 2020;42(1):66–70.
Google Scholar
Oksuz H, Tamer C, Akoglu S, Duru M. Acute angle-closure glaucoma precipitated by local tiotropium absorption. Pulm Pharmacol Ther. 2007;20(6):627–8.
Tashkin D. The safety of anticholinergic bronchodilators for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015;14(11):1759–72. https://doi.org/10.1517/14740338.2015.1093621 .
Prashant Y, Sourabh P, Aditya G, Anand K, Sudhir C. Effect of inhaled anticholinergic drugs on intraocular pressure in chronic obstructive pulmonary disease patients. Indian J Basic Appl Med Res. 2015;4(2):458–64.
Download references
Acknowledgements
The authors would like to thank the included patients.
Not applicable.
Author information
Authors and affiliations.
Department of Chest Diseases, Giresun University Faculty of Medicine, Giresun, Turkey
Hayriye Bektaş Aksoy
Department of Ophthalmology, Giresun University Faculty of Medicine, Giresun, Turkey
You can also search for this author in PubMed Google Scholar
Contributions
HBA: Study concept design, data acquisition/ analysis, manuscript drafting/revision/editing, literature review, statistics. HK: Study concept design, data acquisition/analysis, literature review. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Hayriye Bektaş Aksoy .
Ethics declarations
Ethics approval and consent to participate.
Giresun Training and Research Hospital Clinical Research and Ethics Committee approved this study (KEAK 2023/180 and 9.10.2023/02). Informed consent was obtained from all subjects.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Aksoy, H.B., Koç, H. Effect of Tiotropium on eye findings in the treatment of chronic obstructive pulmonary disease. BMC Pulm Med 24 , 418 (2024). https://doi.org/10.1186/s12890-024-03240-1
Download citation
Received : 10 May 2024
Accepted : 20 August 2024
Published : 28 August 2024
DOI : https://doi.org/10.1186/s12890-024-03240-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anterior chamber angle
- Anterior chamber depth
- Intraocular pressure
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Dr. Akash Gupta (Pediatrics): A 16-year-old boy was admitted to this hospital in autumn because of jaundice and abnormal results on liver-function tests. The patient had been well until 4 days ...
ERCP: internal stenting. The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. 1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the laboratory ...
How to cite this article: Duan B, Zhao X, Fan S, Zhou L, Zhang X. A rare case report of obstructive jaundice caused by mucus-producing cholangiocarcinoma. Medicine. 2022;101:3 (e28478). This study was approved by the Ethics Committee of the Binzhou Medical University Hospital. The patient provided consent for the publication of her case.
In a study of 66 patients with primary ... RL, O'Neill, J, Ghishan, FK. Fibrosing pancreatitis -- an obscure cause of painless obstructive jaundice: a case report and review of the literature. ...
The patient was admitted to the hospital because of obstructive jaundice caused by mucus-producing cholangiocarcinoma. We found a few reports of obstructive jaundice caused by mucus-producing biliary duct tumors in the past ten years, although the mucus around the tumor does not fill the bile duct tree. [ 6, 7] Previous reports on intrahepatic ...
Case 34-2019: A 16-Year-Old Boy with Jaundice. Dr. Akash Gupta (Pediatrics): A 16-year-old boy was admitted to this hospital in au-tumn because of jaundice and abnormal results on liver-function ...
Abstract. Obstructive jaundice (OJ) is a common problem in daily clinical practice. However, completely understanding the pathophysiological changes in OJ remains a challenge for planning current and future management. The effects of OJ are widespread, affecting the biliary tree, hepatic cells, liver function, and causing systemic complications.
An unusual finding of obstructive jaundice—a case report and review of the literature Darren Fernandes, Darren Fernandes ... This case study presents a 54-year-old British Caucasian female patient, admitted with a 1-week history of generalized abdominal pain. Ultrasound scan of the abdomen showed a collapsed and abnormal image of the gallbladder.
In a study conducted in our hospital with 60 cases of clinical obstructive jaundice, it was found that the sensitivity of detecting the presence and level of obstruction was almost same 100% vs ...
Obstructive jaundice is strictly defined as a condition. occurring due to a block in the pathway between the site of. conjugation of bile in liver cells and the entry of bile into the. duodenum ...
y from March 2019 till February 2020. There were a total of 73 patients of obstructive jaundice patients, the benign etiology was found to be more common than malignant etiology. Results: The male-to-female ratio in our study was 0.35:1. The most common etiology of benign cause of obstructive jaundice was choledocholithiasis (95.83%) followed by common bile duct stricture (3.07%), Mirizzi ...
Abstract. Case 28 introduces a 71-year-old man with a 2-day history of diffuse pruritus. The patient states that he was well until approximately 2 days ago, when he first noted mild itching, principally on his trunk. The pruritus has progressed so that it is now severe and diffuse. His sleep last night was disturbed, and he is having difficulty ...
At present, 'obstructive' jaundice in particular is included within the NICE Referral Guidelines for Suspected Cancer. 7 It would help GPs to formulate a plan for investigation if the various underlying causes of jaundice in primary care settings were identified, and their relative importance established. This study aimed to do that, using ...
Obstructive Jaundice[4] is due to obstruction of bile flow, its cause may lie anywhere between the hepatocyte and ... 244-250 Case Study ISSN 2454-2229 World Journal of Pharmaceutical and Life Sciences www.wjpls.org SJIF Impact Factor: 6.129 Corresponding Author: Dr. Malleshwar Rao Final Year Kayachikitsa PG Scholar, Taranath Government ...
ERCP: internal stenting. (0.1MB). The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. 1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the ...
A Case Study on Obstructive Jaundice - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. Sample Case study on Obstructive Jaundice
Extrahepatic obstructive jaundice ... This was not the case for other studies, 14,26 where many cases received either temporary drainage of bile or definitive surgery. The delay to access health care in our centre explains late presentation with complications at admissions. Only one case of biliary atresia, for example, presented in time for a ...
Here is a case of obstructive jaundice, with a h/o cholecystectomy 20y back and H/O ERCP 5y back, treated successfully with Ayurvedic medications and some diet modifications for about 42 days. A ...
A 29-year-old man was admitted to the hospital because of anemia, jaundice, fatigue, and diffuse body aches. He had returned from a 3-month trip to North Africa 1 month before presentation. A diagn...
Abstract. Jaundice is a yellow discoloration of the body's tissues from excess bilirubin (hyperbilirubinemia). According to the Ayurveda perspective, the clinical presentation of jaundice can be correlated with that of the disease Kamala. We present a case of a 10-year-old female patient who attained the outpatient department with complaints ...
obstructive jaundice case study. Mrs doaa Mohamed ahmed a 35 year old female patient complaining of ri... View more. Course. General surgery (USUS07) 51 Documents. Students shared 51 documents in this course. ... .calcular obstructive jaundice- 1 Date with: female patient, 35 years, fertile , right upper quadrant pain of acute onset and 15 days ...
Purpose Early diagnosis of biliary atresia (BA) is critical for best outcomes, but is challenged by overlapping clinical manifestations with other causes of obstructive jaundice in neonates. We evaluate the performance of the modified Simple BA Scoring System (SBASS) in diagnosing BA. Methods We performed a prospective, cross-sectional study on infants with cholestatic jaundice (June 2021 ...
We underline a case of a 55-year-old male who presented with weight loss, jaundice, and pruritus. Radiological imaging suggested a pancreatic mass, raising suspicion of malignancy. However, subsequent evaluation, absence of parenchymal tissue and lymphoplasmacytic cells on endoscopic ultrasound-guided biopsy, and elevated serum immunoglobulin ...
Typically, jaundice is also seen when cancer is at a more advanced stage. But depending on where a tumor is located, it could be an early sign, too. Even a small tumor on the head of the pancreas can cause scarring and lead to obstruction. What jaundice looks like. A lot of people think of yellow skin or eyes when you first mention jaundice.
Also, a meta-analysis of five cohort studies and five case-control studies reported a small, positive association between obesity and CCA . ... O.I.; Adetiloye, V.A.; Idowu, B.M. Diagnostic accuracy of ultrasonography in adults with obstructive jaundice. J. Ultrason. 2020, 20, e100-e105. [Google Scholar]
The aim of this study was to describe our preliminary experience in the procedure of laparoscopic radical resection of hilar cholangiocarcinoma and to evaluate its feasibility, safety, and clinical efficacy. A retrospective analysis was conducted on 44 patients with hilar cholangiocarcinoma who underwent laparoscopic surgery at our hospital from August 2019 to September 2023.
A choledochal cyst is a rare condition that requires surgical treatment to prevent complications, such as obstructive jaundice, cyst rupture, cholangitis, and the risk of malignancy. Complete cyst excision is considered the best option, as it reduces the risk of inflammation and the development of cholangiocarcinoma.
The common etiologies of obstructive jaundice have been reported to vary from one center to another and from one individual to another.[4,5] The morbidity and mortality related to obstructive jaundice depends upon the causes of obstruction. There are some studies on the etiology of obstructive jaundice but none from the state of Sikkim.
Small intestinal metastasis is extremely rare and only 13 cases have been reported till date and almost all such patients have presented with intestinal obstruction. The 5-year overall survival for metastatic esophageal cancer is as low as 5% while the patients with small intestinal metastasis have a median survival of only 3 months (range 1-12 months) despite undergoing radical resection of ...
Chronic obstructive pulmonary disease (COPD) is a persistent, chronic inflammatory disease of the lungs. Tiotropium, used in the treatment of COPD, is a muscarinic receptor antagonist that provides long-acting bronchodilation. Our study aimed to investigate the effects of Tiotropium on anterior chamber parameters. The study was conducted as an observational cross-sectional and prospectively ...