- Open access
- Published: 27 June 2011

The case study approach
- Sarah Crowe 1 ,
- Kathrin Cresswell 2 ,
- Ann Robertson 2 ,
- Guro Huby 3 ,
- Anthony Avery 1 &
- Aziz Sheikh 2
BMC Medical Research Methodology volume 11 , Article number: 100 ( 2011 ) Cite this article
782k Accesses
1038 Citations
37 Altmetric
Metrics details
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we reflect on the different types of case study design, the specific research questions this approach can help answer, the data sources that tend to be used, and the particular advantages and disadvantages of employing this methodological approach. The paper concludes with key pointers to aid those designing and appraising proposals for conducting case study research, and a checklist to help readers assess the quality of case study reports.
Peer Review reports
Introduction
The case study approach is particularly useful to employ when there is a need to obtain an in-depth appreciation of an issue, event or phenomenon of interest, in its natural real-life context. Our aim in writing this piece is to provide insights into when to consider employing this approach and an overview of key methodological considerations in relation to the design, planning, analysis, interpretation and reporting of case studies.
The illustrative 'grand round', 'case report' and 'case series' have a long tradition in clinical practice and research. Presenting detailed critiques, typically of one or more patients, aims to provide insights into aspects of the clinical case and, in doing so, illustrate broader lessons that may be learnt. In research, the conceptually-related case study approach can be used, for example, to describe in detail a patient's episode of care, explore professional attitudes to and experiences of a new policy initiative or service development or more generally to 'investigate contemporary phenomena within its real-life context' [ 1 ]. Based on our experiences of conducting a range of case studies, we reflect on when to consider using this approach, discuss the key steps involved and illustrate, with examples, some of the practical challenges of attaining an in-depth understanding of a 'case' as an integrated whole. In keeping with previously published work, we acknowledge the importance of theory to underpin the design, selection, conduct and interpretation of case studies[ 2 ]. In so doing, we make passing reference to the different epistemological approaches used in case study research by key theoreticians and methodologists in this field of enquiry.
This paper is structured around the following main questions: What is a case study? What are case studies used for? How are case studies conducted? What are the potential pitfalls and how can these be avoided? We draw in particular on four of our own recently published examples of case studies (see Tables 1 , 2 , 3 and 4 ) and those of others to illustrate our discussion[ 3 – 7 ].
What is a case study?
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the central tenet being the need to explore an event or phenomenon in depth and in its natural context. It is for this reason sometimes referred to as a "naturalistic" design; this is in contrast to an "experimental" design (such as a randomised controlled trial) in which the investigator seeks to exert control over and manipulate the variable(s) of interest.
Stake's work has been particularly influential in defining the case study approach to scientific enquiry. He has helpfully characterised three main types of case study: intrinsic , instrumental and collective [ 8 ]. An intrinsic case study is typically undertaken to learn about a unique phenomenon. The researcher should define the uniqueness of the phenomenon, which distinguishes it from all others. In contrast, the instrumental case study uses a particular case (some of which may be better than others) to gain a broader appreciation of an issue or phenomenon. The collective case study involves studying multiple cases simultaneously or sequentially in an attempt to generate a still broader appreciation of a particular issue.
These are however not necessarily mutually exclusive categories. In the first of our examples (Table 1 ), we undertook an intrinsic case study to investigate the issue of recruitment of minority ethnic people into the specific context of asthma research studies, but it developed into a instrumental case study through seeking to understand the issue of recruitment of these marginalised populations more generally, generating a number of the findings that are potentially transferable to other disease contexts[ 3 ]. In contrast, the other three examples (see Tables 2 , 3 and 4 ) employed collective case study designs to study the introduction of workforce reconfiguration in primary care, the implementation of electronic health records into hospitals, and to understand the ways in which healthcare students learn about patient safety considerations[ 4 – 6 ]. Although our study focusing on the introduction of General Practitioners with Specialist Interests (Table 2 ) was explicitly collective in design (four contrasting primary care organisations were studied), is was also instrumental in that this particular professional group was studied as an exemplar of the more general phenomenon of workforce redesign[ 4 ].
What are case studies used for?
According to Yin, case studies can be used to explain, describe or explore events or phenomena in the everyday contexts in which they occur[ 1 ]. These can, for example, help to understand and explain causal links and pathways resulting from a new policy initiative or service development (see Tables 2 and 3 , for example)[ 1 ]. In contrast to experimental designs, which seek to test a specific hypothesis through deliberately manipulating the environment (like, for example, in a randomised controlled trial giving a new drug to randomly selected individuals and then comparing outcomes with controls),[ 9 ] the case study approach lends itself well to capturing information on more explanatory ' how ', 'what' and ' why ' questions, such as ' how is the intervention being implemented and received on the ground?'. The case study approach can offer additional insights into what gaps exist in its delivery or why one implementation strategy might be chosen over another. This in turn can help develop or refine theory, as shown in our study of the teaching of patient safety in undergraduate curricula (Table 4 )[ 6 , 10 ]. Key questions to consider when selecting the most appropriate study design are whether it is desirable or indeed possible to undertake a formal experimental investigation in which individuals and/or organisations are allocated to an intervention or control arm? Or whether the wish is to obtain a more naturalistic understanding of an issue? The former is ideally studied using a controlled experimental design, whereas the latter is more appropriately studied using a case study design.
Case studies may be approached in different ways depending on the epistemological standpoint of the researcher, that is, whether they take a critical (questioning one's own and others' assumptions), interpretivist (trying to understand individual and shared social meanings) or positivist approach (orientating towards the criteria of natural sciences, such as focusing on generalisability considerations) (Table 6 ). Whilst such a schema can be conceptually helpful, it may be appropriate to draw on more than one approach in any case study, particularly in the context of conducting health services research. Doolin has, for example, noted that in the context of undertaking interpretative case studies, researchers can usefully draw on a critical, reflective perspective which seeks to take into account the wider social and political environment that has shaped the case[ 11 ].
How are case studies conducted?
Here, we focus on the main stages of research activity when planning and undertaking a case study; the crucial stages are: defining the case; selecting the case(s); collecting and analysing the data; interpreting data; and reporting the findings.
Defining the case
Carefully formulated research question(s), informed by the existing literature and a prior appreciation of the theoretical issues and setting(s), are all important in appropriately and succinctly defining the case[ 8 , 12 ]. Crucially, each case should have a pre-defined boundary which clarifies the nature and time period covered by the case study (i.e. its scope, beginning and end), the relevant social group, organisation or geographical area of interest to the investigator, the types of evidence to be collected, and the priorities for data collection and analysis (see Table 7 )[ 1 ]. A theory driven approach to defining the case may help generate knowledge that is potentially transferable to a range of clinical contexts and behaviours; using theory is also likely to result in a more informed appreciation of, for example, how and why interventions have succeeded or failed[ 13 ].
For example, in our evaluation of the introduction of electronic health records in English hospitals (Table 3 ), we defined our cases as the NHS Trusts that were receiving the new technology[ 5 ]. Our focus was on how the technology was being implemented. However, if the primary research interest had been on the social and organisational dimensions of implementation, we might have defined our case differently as a grouping of healthcare professionals (e.g. doctors and/or nurses). The precise beginning and end of the case may however prove difficult to define. Pursuing this same example, when does the process of implementation and adoption of an electronic health record system really begin or end? Such judgements will inevitably be influenced by a range of factors, including the research question, theory of interest, the scope and richness of the gathered data and the resources available to the research team.
Selecting the case(s)
The decision on how to select the case(s) to study is a very important one that merits some reflection. In an intrinsic case study, the case is selected on its own merits[ 8 ]. The case is selected not because it is representative of other cases, but because of its uniqueness, which is of genuine interest to the researchers. This was, for example, the case in our study of the recruitment of minority ethnic participants into asthma research (Table 1 ) as our earlier work had demonstrated the marginalisation of minority ethnic people with asthma, despite evidence of disproportionate asthma morbidity[ 14 , 15 ]. In another example of an intrinsic case study, Hellstrom et al.[ 16 ] studied an elderly married couple living with dementia to explore how dementia had impacted on their understanding of home, their everyday life and their relationships.
For an instrumental case study, selecting a "typical" case can work well[ 8 ]. In contrast to the intrinsic case study, the particular case which is chosen is of less importance than selecting a case that allows the researcher to investigate an issue or phenomenon. For example, in order to gain an understanding of doctors' responses to health policy initiatives, Som undertook an instrumental case study interviewing clinicians who had a range of responsibilities for clinical governance in one NHS acute hospital trust[ 17 ]. Sampling a "deviant" or "atypical" case may however prove even more informative, potentially enabling the researcher to identify causal processes, generate hypotheses and develop theory.
In collective or multiple case studies, a number of cases are carefully selected. This offers the advantage of allowing comparisons to be made across several cases and/or replication. Choosing a "typical" case may enable the findings to be generalised to theory (i.e. analytical generalisation) or to test theory by replicating the findings in a second or even a third case (i.e. replication logic)[ 1 ]. Yin suggests two or three literal replications (i.e. predicting similar results) if the theory is straightforward and five or more if the theory is more subtle. However, critics might argue that selecting 'cases' in this way is insufficiently reflexive and ill-suited to the complexities of contemporary healthcare organisations.
The selected case study site(s) should allow the research team access to the group of individuals, the organisation, the processes or whatever else constitutes the chosen unit of analysis for the study. Access is therefore a central consideration; the researcher needs to come to know the case study site(s) well and to work cooperatively with them. Selected cases need to be not only interesting but also hospitable to the inquiry [ 8 ] if they are to be informative and answer the research question(s). Case study sites may also be pre-selected for the researcher, with decisions being influenced by key stakeholders. For example, our selection of case study sites in the evaluation of the implementation and adoption of electronic health record systems (see Table 3 ) was heavily influenced by NHS Connecting for Health, the government agency that was responsible for overseeing the National Programme for Information Technology (NPfIT)[ 5 ]. This prominent stakeholder had already selected the NHS sites (through a competitive bidding process) to be early adopters of the electronic health record systems and had negotiated contracts that detailed the deployment timelines.
It is also important to consider in advance the likely burden and risks associated with participation for those who (or the site(s) which) comprise the case study. Of particular importance is the obligation for the researcher to think through the ethical implications of the study (e.g. the risk of inadvertently breaching anonymity or confidentiality) and to ensure that potential participants/participating sites are provided with sufficient information to make an informed choice about joining the study. The outcome of providing this information might be that the emotive burden associated with participation, or the organisational disruption associated with supporting the fieldwork, is considered so high that the individuals or sites decide against participation.
In our example of evaluating implementations of electronic health record systems, given the restricted number of early adopter sites available to us, we sought purposively to select a diverse range of implementation cases among those that were available[ 5 ]. We chose a mixture of teaching, non-teaching and Foundation Trust hospitals, and examples of each of the three electronic health record systems procured centrally by the NPfIT. At one recruited site, it quickly became apparent that access was problematic because of competing demands on that organisation. Recognising the importance of full access and co-operative working for generating rich data, the research team decided not to pursue work at that site and instead to focus on other recruited sites.
Collecting the data
In order to develop a thorough understanding of the case, the case study approach usually involves the collection of multiple sources of evidence, using a range of quantitative (e.g. questionnaires, audits and analysis of routinely collected healthcare data) and more commonly qualitative techniques (e.g. interviews, focus groups and observations). The use of multiple sources of data (data triangulation) has been advocated as a way of increasing the internal validity of a study (i.e. the extent to which the method is appropriate to answer the research question)[ 8 , 18 – 21 ]. An underlying assumption is that data collected in different ways should lead to similar conclusions, and approaching the same issue from different angles can help develop a holistic picture of the phenomenon (Table 2 )[ 4 ].
Brazier and colleagues used a mixed-methods case study approach to investigate the impact of a cancer care programme[ 22 ]. Here, quantitative measures were collected with questionnaires before, and five months after, the start of the intervention which did not yield any statistically significant results. Qualitative interviews with patients however helped provide an insight into potentially beneficial process-related aspects of the programme, such as greater, perceived patient involvement in care. The authors reported how this case study approach provided a number of contextual factors likely to influence the effectiveness of the intervention and which were not likely to have been obtained from quantitative methods alone.
In collective or multiple case studies, data collection needs to be flexible enough to allow a detailed description of each individual case to be developed (e.g. the nature of different cancer care programmes), before considering the emerging similarities and differences in cross-case comparisons (e.g. to explore why one programme is more effective than another). It is important that data sources from different cases are, where possible, broadly comparable for this purpose even though they may vary in nature and depth.
Analysing, interpreting and reporting case studies
Making sense and offering a coherent interpretation of the typically disparate sources of data (whether qualitative alone or together with quantitative) is far from straightforward. Repeated reviewing and sorting of the voluminous and detail-rich data are integral to the process of analysis. In collective case studies, it is helpful to analyse data relating to the individual component cases first, before making comparisons across cases. Attention needs to be paid to variations within each case and, where relevant, the relationship between different causes, effects and outcomes[ 23 ]. Data will need to be organised and coded to allow the key issues, both derived from the literature and emerging from the dataset, to be easily retrieved at a later stage. An initial coding frame can help capture these issues and can be applied systematically to the whole dataset with the aid of a qualitative data analysis software package.
The Framework approach is a practical approach, comprising of five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) , to managing and analysing large datasets particularly if time is limited, as was the case in our study of recruitment of South Asians into asthma research (Table 1 )[ 3 , 24 ]. Theoretical frameworks may also play an important role in integrating different sources of data and examining emerging themes. For example, we drew on a socio-technical framework to help explain the connections between different elements - technology; people; and the organisational settings within which they worked - in our study of the introduction of electronic health record systems (Table 3 )[ 5 ]. Our study of patient safety in undergraduate curricula drew on an evaluation-based approach to design and analysis, which emphasised the importance of the academic, organisational and practice contexts through which students learn (Table 4 )[ 6 ].
Case study findings can have implications both for theory development and theory testing. They may establish, strengthen or weaken historical explanations of a case and, in certain circumstances, allow theoretical (as opposed to statistical) generalisation beyond the particular cases studied[ 12 ]. These theoretical lenses should not, however, constitute a strait-jacket and the cases should not be "forced to fit" the particular theoretical framework that is being employed.
When reporting findings, it is important to provide the reader with enough contextual information to understand the processes that were followed and how the conclusions were reached. In a collective case study, researchers may choose to present the findings from individual cases separately before amalgamating across cases. Care must be taken to ensure the anonymity of both case sites and individual participants (if agreed in advance) by allocating appropriate codes or withholding descriptors. In the example given in Table 3 , we decided against providing detailed information on the NHS sites and individual participants in order to avoid the risk of inadvertent disclosure of identities[ 5 , 25 ].
What are the potential pitfalls and how can these be avoided?
The case study approach is, as with all research, not without its limitations. When investigating the formal and informal ways undergraduate students learn about patient safety (Table 4 ), for example, we rapidly accumulated a large quantity of data. The volume of data, together with the time restrictions in place, impacted on the depth of analysis that was possible within the available resources. This highlights a more general point of the importance of avoiding the temptation to collect as much data as possible; adequate time also needs to be set aside for data analysis and interpretation of what are often highly complex datasets.
Case study research has sometimes been criticised for lacking scientific rigour and providing little basis for generalisation (i.e. producing findings that may be transferable to other settings)[ 1 ]. There are several ways to address these concerns, including: the use of theoretical sampling (i.e. drawing on a particular conceptual framework); respondent validation (i.e. participants checking emerging findings and the researcher's interpretation, and providing an opinion as to whether they feel these are accurate); and transparency throughout the research process (see Table 8 )[ 8 , 18 – 21 , 23 , 26 ]. Transparency can be achieved by describing in detail the steps involved in case selection, data collection, the reasons for the particular methods chosen, and the researcher's background and level of involvement (i.e. being explicit about how the researcher has influenced data collection and interpretation). Seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, help readers to judge the trustworthiness of the case study report. Stake provides a critique checklist for a case study report (Table 9 )[ 8 ].
Conclusions
The case study approach allows, amongst other things, critical events, interventions, policy developments and programme-based service reforms to be studied in detail in a real-life context. It should therefore be considered when an experimental design is either inappropriate to answer the research questions posed or impossible to undertake. Considering the frequency with which implementations of innovations are now taking place in healthcare settings and how well the case study approach lends itself to in-depth, complex health service research, we believe this approach should be more widely considered by researchers. Though inherently challenging, the research case study can, if carefully conceptualised and thoughtfully undertaken and reported, yield powerful insights into many important aspects of health and healthcare delivery.
Yin RK: Case study research, design and method. 2009, London: Sage Publications Ltd., 4
Google Scholar
Keen J, Packwood T: Qualitative research; case study evaluation. BMJ. 1995, 311: 444-446.
Article CAS PubMed PubMed Central Google Scholar
Sheikh A, Halani L, Bhopal R, Netuveli G, Partridge M, Car J, et al: Facilitating the Recruitment of Minority Ethnic People into Research: Qualitative Case Study of South Asians and Asthma. PLoS Med. 2009, 6 (10): 1-11.
Article Google Scholar
Pinnock H, Huby G, Powell A, Kielmann T, Price D, Williams S, et al: The process of planning, development and implementation of a General Practitioner with a Special Interest service in Primary Care Organisations in England and Wales: a comparative prospective case study. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). 2008, [ http://www.sdo.nihr.ac.uk/files/project/99-final-report.pdf ]
Robertson A, Cresswell K, Takian A, Petrakaki D, Crowe S, Cornford T, et al: Prospective evaluation of the implementation and adoption of NHS Connecting for Health's national electronic health record in secondary care in England: interim findings. BMJ. 2010, 41: c4564-
Pearson P, Steven A, Howe A, Sheikh A, Ashcroft D, Smith P, the Patient Safety Education Study Group: Learning about patient safety: organisational context and culture in the education of healthcare professionals. J Health Serv Res Policy. 2010, 15: 4-10. 10.1258/jhsrp.2009.009052.
Article PubMed Google Scholar
van Harten WH, Casparie TF, Fisscher OA: The evaluation of the introduction of a quality management system: a process-oriented case study in a large rehabilitation hospital. Health Policy. 2002, 60 (1): 17-37. 10.1016/S0168-8510(01)00187-7.
Stake RE: The art of case study research. 1995, London: Sage Publications Ltd.
Sheikh A, Smeeth L, Ashcroft R: Randomised controlled trials in primary care: scope and application. Br J Gen Pract. 2002, 52 (482): 746-51.
PubMed PubMed Central Google Scholar
King G, Keohane R, Verba S: Designing Social Inquiry. 1996, Princeton: Princeton University Press
Doolin B: Information technology as disciplinary technology: being critical in interpretative research on information systems. Journal of Information Technology. 1998, 13: 301-311. 10.1057/jit.1998.8.
George AL, Bennett A: Case studies and theory development in the social sciences. 2005, Cambridge, MA: MIT Press
Eccles M, the Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG): Designing theoretically-informed implementation interventions. Implementation Science. 2006, 1: 1-8. 10.1186/1748-5908-1-1.
Article PubMed Central Google Scholar
Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, Sheikh A: Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005, 365 (9456): 312-7.
Sheikh A, Panesar SS, Lasserson T, Netuveli G: Recruitment of ethnic minorities to asthma studies. Thorax. 2004, 59 (7): 634-
CAS PubMed PubMed Central Google Scholar
Hellström I, Nolan M, Lundh U: 'We do things together': A case study of 'couplehood' in dementia. Dementia. 2005, 4: 7-22. 10.1177/1471301205049188.
Som CV: Nothing seems to have changed, nothing seems to be changing and perhaps nothing will change in the NHS: doctors' response to clinical governance. International Journal of Public Sector Management. 2005, 18: 463-477. 10.1108/09513550510608903.
Lincoln Y, Guba E: Naturalistic inquiry. 1985, Newbury Park: Sage Publications
Barbour RS: Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ. 2001, 322: 1115-1117. 10.1136/bmj.322.7294.1115.
Mays N, Pope C: Qualitative research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mason J: Qualitative researching. 2002, London: Sage
Brazier A, Cooke K, Moravan V: Using Mixed Methods for Evaluating an Integrative Approach to Cancer Care: A Case Study. Integr Cancer Ther. 2008, 7: 5-17. 10.1177/1534735407313395.
Miles MB, Huberman M: Qualitative data analysis: an expanded sourcebook. 1994, CA: Sage Publications Inc., 2
Pope C, Ziebland S, Mays N: Analysing qualitative data. Qualitative research in health care. BMJ. 2000, 320: 114-116. 10.1136/bmj.320.7227.114.
Cresswell KM, Worth A, Sheikh A: Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Med Inform Decis Mak. 2010, 10 (1): 67-10.1186/1472-6947-10-67.
Article PubMed PubMed Central Google Scholar
Malterud K: Qualitative research: standards, challenges, and guidelines. Lancet. 2001, 358: 483-488. 10.1016/S0140-6736(01)05627-6.
Article CAS PubMed Google Scholar
Yin R: Case study research: design and methods. 1994, Thousand Oaks, CA: Sage Publishing, 2
Yin R: Enhancing the quality of case studies in health services research. Health Serv Res. 1999, 34: 1209-1224.
Green J, Thorogood N: Qualitative methods for health research. 2009, Los Angeles: Sage, 2
Howcroft D, Trauth E: Handbook of Critical Information Systems Research, Theory and Application. 2005, Cheltenham, UK: Northampton, MA, USA: Edward Elgar
Book Google Scholar
Blakie N: Approaches to Social Enquiry. 1993, Cambridge: Polity Press
Doolin B: Power and resistance in the implementation of a medical management information system. Info Systems J. 2004, 14: 343-362. 10.1111/j.1365-2575.2004.00176.x.
Bloomfield BP, Best A: Management consultants: systems development, power and the translation of problems. Sociological Review. 1992, 40: 533-560.
Shanks G, Parr A: Positivist, single case study research in information systems: A critical analysis. Proceedings of the European Conference on Information Systems. 2003, Naples
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/11/100/prepub
Download references
Acknowledgements
We are grateful to the participants and colleagues who contributed to the individual case studies that we have drawn on. This work received no direct funding, but it has been informed by projects funded by Asthma UK, the NHS Service Delivery Organisation, NHS Connecting for Health Evaluation Programme, and Patient Safety Research Portfolio. We would also like to thank the expert reviewers for their insightful and constructive feedback. Our thanks are also due to Dr. Allison Worth who commented on an earlier draft of this manuscript.
Author information
Authors and affiliations.
Division of Primary Care, The University of Nottingham, Nottingham, UK
Sarah Crowe & Anthony Avery
Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK
Kathrin Cresswell, Ann Robertson & Aziz Sheikh
School of Health in Social Science, The University of Edinburgh, Edinburgh, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarah Crowe .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
AS conceived this article. SC, KC and AR wrote this paper with GH, AA and AS all commenting on various drafts. SC and AS are guarantors.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Crowe, S., Cresswell, K., Robertson, A. et al. The case study approach. BMC Med Res Methodol 11 , 100 (2011). https://doi.org/10.1186/1471-2288-11-100
Download citation
Received : 29 November 2010
Accepted : 27 June 2011
Published : 27 June 2011
DOI : https://doi.org/10.1186/1471-2288-11-100
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case Study Approach
- Electronic Health Record System
- Case Study Design
- Case Study Site
- Case Study Report
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Writing a case report...
Writing a case report in 10 steps
- Related content
- Peer review
- Victoria Stokes , foundation year 2 doctor, trauma and orthopaedics, Basildon Hospital ,
- Caroline Fertleman , paediatrics consultant, The Whittington Hospital NHS Trust
- victoria.stokes1{at}nhs.net
Victoria Stokes and Caroline Fertleman explain how to turn an interesting case or unusual presentation into an educational report
It is common practice in medicine that when we come across an interesting case with an unusual presentation or a surprise twist, we must tell the rest of the medical world. This is how we continue our lifelong learning and aid faster diagnosis and treatment for patients.
It usually falls to the junior to write up the case, so here are a few simple tips to get you started.
First steps
Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline and work timeframe, and discuss the order in which the authors will be listed. All listed authors should contribute substantially, with the person doing most of the work put first and the guarantor (usually the most senior team member) at the end.
Getting consent
Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and keep a copy because you will need it later for submission to journals.
Information gathering
Gather all the information from the medical notes and the hospital’s electronic systems, including copies of blood results and imaging, as medical notes often disappear when the patient is discharged and are notoriously difficult to find again. Remember to anonymise the data according to your local hospital policy.
Write up the case emphasising the interesting points of the presentation, investigations leading to diagnosis, and management of the disease/pathology. Get input on the case from all members of the team, highlighting their involvement. Also include the prognosis of the patient, if known, as the reader will want to know the outcome.
Coming up with a title
Discuss a title with your supervisor and other members of the team, as this provides the focus for your article. The title should be concise and interesting but should also enable people to find it in medical literature search engines. Also think about how you will present your case study—for example, a poster presentation or scientific paper—and consider potential journals or conferences, as you may need to write in a particular style or format.
Background research
Research the disease/pathology that is the focus of your article and write a background paragraph or two, highlighting the relevance of your case report in relation to this. If you are struggling, seek the opinion of a specialist who may know of relevant articles or texts. Another good resource is your hospital library, where staff are often more than happy to help with literature searches.
How your case is different
Move on to explore how the case presented differently to the admitting team. Alternatively, if your report is focused on management, explore the difficulties the team came across and alternative options for treatment.
Finish by explaining why your case report adds to the medical literature and highlight any learning points.
Writing an abstract
The abstract should be no longer than 100-200 words and should highlight all your key points concisely. This can be harder than writing the full article and needs special care as it will be used to judge whether your case is accepted for presentation or publication.
Discuss with your supervisor or team about options for presenting or publishing your case report. At the very least, you should present your article locally within a departmental or team meeting or at a hospital grand round. Well done!
Competing interests: We have read and understood BMJ’s policy on declaration of interests and declare that we have no competing interests.

Writing a Case Report
This page is intended for medical students, residents or others who do not have much experience with case reports, but are planning on writing one.
What is a case report? A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are commonly of the following categories :
- Rare diseases
- Unusual presentation of disease
- Unexpected events
- Unusual combination of diseases or conditions
- Difficult or inconclusive diagnosis
- Treatment or management challenges
- Personal impact
- Observations that shed new light on a disease or condition
- Anatomical variations
It is important that you recognize what is unique or interesting about your case, and this must be described clearly in the case report.
Case reports generally take the format of :
1. Background
2. Case presentation
3. Observations and investigation
4. Diagnosis
5. Treatment
7. Discussion
Does a case report require IRB approval?
Case reports typically discuss a single patient. If this is true for your case report, then it most likely does not require IRB approval because it not considered research. If you have more than one patient, your study could qualify as a Case Series, which would require IRB review. If you have questions, you chould check your local IRB's guidelines on reviewing case reports.
Are there other rules for writing a case report?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health
While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply to any case report. This includes answering questions like: Do I need written HIPAA authorization to publish a case report? When do I need IRB review of a case report? What qualifies as a patient identifier?
How do I get started?
1. We STRONGLY encourage you to consult the CARE Guidelines, which provide guidance on writing case reports - https://www.care-statement.org/
Specifically, the checklist - https://www.care-statement.org/checklist - which explains exactly the information you should collect and include in your case report.
2. Identify a case. If you are a medical student, you may not yet have the clinical expertise to determine if a specific case is worth writing up. If so, you must seek the help of a clinician. It is common for students to ask attendings or residents if they have any interesting cases that can be used for a case report.
3. Select a journal or two to which you think you will submit the case report. Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things. Journals may also charge publication fees (see Is it free to publish? below)
4. Obtain informed consent from the patient (see " Do I have to obtain informed consent from the patient? " below). Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal.
Once you've identified the case, selected an appropriate journal(s), and considered informed consent, you can collect the required information to write the case report.
How do I write a case report?
Once you identify a case and have learned what information to include in the case report, try to find a previously published case report. Finding published case reports in a similar field will provide examples to guide you through the process of writing a case report.
One journal you can consult is BMJ Case Reports . MSU has an institutional fellowship with BMJ Case Reports which allows MSU faculty, staff and students to publish in this journal for free. See this page for a link to the journal and more information on publishing- https://lib.msu.edu/medicalwriting_publishing/
There are numerous other journals where you can find published case reports to help guide you in your writing.
Do I have to obtain informed consent from the patient?
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible). Please consider this as well.
If required, it is recommended you obtain informed consent before the case report is written.
An example of a case report consent form can be found on the BMJ Case Reports website, which you can access via the MSU library page - https://casereports.bmj.com/ . Go to "Instructions for Authors" and then "Patient Consent" to find the consent form they use. You can create a similar form to obtain consent from your patient. If you have identified a journal already, please consult their requirements and determine if they have a specific consent form they would like you to use.
Seek feedback
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before.
Selecting a journal
Aside from BMJ Case Reports mentioned above, there are many, many journals out there who publish medical case reports. Ask your mentor if they have a journal they would like to use. If you need to select on your own, here are some strategies:
1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/
a. Do a search for a topic, disease or other feature of your case report
b. When the results appear, on the left side of the page is a limiter for "article type". Case reports are an article type to which you can limit your search results. If you don't see that option on the left, click "additional filters".
c. Review the case reports that come up and see what journals they are published in.
2. Use JANE - https://jane.biosemantics.org/
3. Check with specialty societies. Many specialty societies are affiliated with one or more journal, which can be reviewed for ones that match your needs
4. Search through individual publisher journal lists. Elsevier publishes many different medical research journals, and they have a journal finder, much like JANE ( https://journalfinder.elsevier.com/ ). This is exclusive to Elsevier journals. There are many other publishers of medical journals for review, including Springer, Dove Press, BMJ, BMC, Wiley, Sage, Nature and many others.
Is it free to publish ?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option". It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. MSU-CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing
*A more thorough discussion on finding a journal, publication costs, predatory journals and other publication-related issues can be found here: https://research.chm.msu.edu/students-residents/finding-a-journal
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med . 2:38-43. doi: 10.7453/gahmj.2013.008
Riley DS, Barber MS, Kienle GS, AronsonJK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. 2017. CARE guidelines for case reports: explanation and elaboration document . J Clin Epidemiol . 89:218-234. doi: 10.1016/j.jclinepi.2017.04.026
Guidelines to writing a clinical case report. 2017. Heart Views . 18:104-105. doi: 10.4103/1995-705X.217857
Ortega-Loubon C, Culquichicon C, Correa R. The importance of writing and publishing case reports during medical education. 2017. Cureus. 9:e1964. doi: 10.7759/cureus.1964
Writing and publishing a useful and interesting case report. 2019. BMJ Case Reports. https://casereports.bmj.com/pages/wp-content/uploads/sites/69/2019/04/How-to-write-a-Case-Report-DIGITAL.pdf
Camm CF. Writing an excellent case report: EHJ Case Reports , Case of the Year 2019. 2020. European Heart Jounrnal. 41:1230-1231. https://doi.org/10.1093/eurheartj/ehaa176
*content developed by Mark Trottier, PhD
Improving case study research in medical education: a systematised review
Affiliations.
- 1 Rural Clinical School, Faculty of Health, University of Tasmania, Burnie, Tasmania, Australia.
- 2 Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Queensland, Australia.
- PMID: 29178211
- DOI: 10.1111/medu.13469
Context: Case study research (CSR) is a research approach that guides holistic investigation of a real phenomenon. This approach may be useful in medical education to provide critical analyses of teaching and learning, and to reveal the underlying elements of leadership and innovation. There are variations in the definition, design and choice of methods, which may diminish the value of CSR as a form of inquiry.
Objectives: This paper reports an analysis of CSR papers in the medical education literature. The review aims to describe how CSR has been used and how more consistency might be achieved to promote understanding and value.
Methods: A systematised review was undertaken to quantify the number of CSR articles published in scholarly medical education journals over the last 10 years. A typology of CSR proposed by Thomas and Myers to integrate the various ways in which CSR is constructed was applied.
Results: Of the 362 full-text articles assessed, 290 were excluded as they did not meet the eligibility criteria; 76 of these were titled 'case study'. Of the 72 included articles, 50 used single-case and 22 multi-case design; 46 connected with theory and 26 were atheoretical. In some articles it was unclear what the subject was or how the subject was being analysed.
Conclusions: In this study, more articles titled 'case study' failed than succeeded in meeting the eligibility criteria. Well-structured, clearly written CSR in medical education has the potential to increase understanding of more complex situations, but this review shows there is considerable variation in how it is conducted, which potentially limits its utility and translation into education practice. Case study research might be of more value in medical education if researchers were to follow more consistently principles of design, and harness rich observation with connection of ideas and knowledge to engage the reader in what is most interesting.
© 2017 John Wiley & Sons Ltd and The Association for the Study of Medical Education.
Publication types
- Biomedical Research* / standards
- Education, Medical
- Evidence-Based Medicine* / methods
- Evidence-Based Medicine* / standards

- Policy & Compliance
- Clinical Trials
NIH Definition of Clinical Trial Case Studies
The case studies provided below are designed to help you identify whether your study would be considered by NIH to be a clinical trial. Expect the case studies and related guidance to evolve over the upcoming year. For continuity and ease of reference, case studies will retain their original numbering and will not be renumbered if cases are revised or removed.
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
See this page for more information about the NIH definition of a clinical trial.
General Case Studies
Institute or center specific case studies.
The study involves the recruitment of research participants who are randomized to receive one of two approved drugs. It is designed to compare the effects of the drugs on the blood level of a protein.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, one of two drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the drugs on the level of the protein in the participants’ blood.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a protein, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition Y to receive a drug that has been approved for another indication. It is designed to measure the drug’s effects on the level of a biomarker associated with the severity of condition Y.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the approved drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the drug’s effect on the level of the biomarker.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a biomarker, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition X to receive investigational compound A. It is designed to assess the pharmacokinetic properties of compound A.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, compound A.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how the body interacts with compound A
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, pharmacokinetic properties, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess safety and determine the maximum tolerated dose of the drug.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to assess safety and determine the maximum tolerated dose of the investigational drug.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, safety and maximum tolerated dose, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive a chronic disease management program. It is designed to assess usability and to determine the maximum tolerated dose of the chronic disease program (e.g., how many in-person and telemedicine visits with adequate adherence).
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the chronic disease management program.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine the maximum tolerated dose of the program to obtain adequate adherence.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, tolerable intensity and adequate adherence of the intervention, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive either an investigational drug or a placebo. It is designed to evaluate the efficacy of the investigational drug to relieve disease symptoms.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the participants’ symptoms.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, relief of symptoms, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess whether there is a change in disease progression compared to baseline. There is no concurrent control used in this study.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the subject’s disease progression.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, disease progression, is a health-related outcome.
The study involves the recruitment of research participants with disease X to test an investigational in vitro diagnostic device (IVD). It is designed to evaluate the ability of the device to measure the level of an antibody in blood.
- Are the participants prospectively assigned to an intervention? No, in this context the IVD would not be considered an intervention. The IVD is being used to test its ability to measure antibody levels, but not to test its effects on any health-related biomedical or behavioral outcomes.
The study involves the recruitment of research participants with disease X to be evaluated with an investigational in vitro diagnostic device (IVD). The study is designed to evaluate how knowledge of certain antibody levels impacts clinical management of disease.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, measurement of an antibody level, with the idea that knowledge of that antibody level might affect clinical management.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how knowledge of the level of an antibody might inform treatment.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being measured, how blood antibody levels inform treatment, is a health-related outcome.
The study involves the recruitment of healthy volunteers who will be randomized to different durations of sleep deprivation (including no sleep deprivation as a control) and who will have stress hormone levels measured. It is designed to determine whether the levels of stress hormones in blood rise in response to different durations of sleep deprivation.
- Does the study involve human participants? Yes, the healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, different durations of sleep deprivation followed by a blood draw.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of different durations of sleep deprivation on stress hormone levels.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, stress hormone levels, is a health-related biomedical outcome.
The study involves the analysis of de-identified, stored blood samples and de-identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? No, the study does not involve human participants because only de-identified samples and information are used.
The study involves the analysis of identifiable, stored blood samples and identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? Yes, patients are human participants because the blood and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, secondary research with biospecimens or health information is not a clinical trial.
The study involves the recruitment of a healthy volunteers whose blood is drawn for genomic analysis. It is designed to identify the prevalence of a genetic mutation in the cohort and evaluate potential association between the presence of the mutation and the risk of developing a genetic disorder.
- Are the participants prospectively assigned to an intervention? No, sample collection (blood draw) is not an intervention in this context.
Physicians report that some patients being treated with drug A for disease X are also experiencing some improvement in a second condition, condition Y. The study involves the recruitment of research participants who have disease X and condition Y and are being treated with drug A. The participants are surveyed to ascertain whether they are experiencing an improvement in condition Y.
- Are the participants prospectively assigned to an intervention? No, participants are not prospectively assigned to receive an intervention as they are receiving drugs as part of their clinical care. The surveys are being used for measurement, not to modify a biomedical or behavioral outcome.
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years.
- Are the participants prospectively assigned to an intervention? No, there is no intervention. The therapies are prescribed as part of clinical care; they are not prospectively assigned for the purpose of the study. The study is observational.
The study involves the recruitment of research participants with disease X vs. healthy controls and comparing these participants on a range of health processes and outcomes including genomics, biospecimens, self-report measures, etc. to explore differences that may be relevant to the development of disease X.
- Are the participants prospectively assigned to an intervention? No, the measures needed to assess the outcomes are not interventions in this context, as the study is not intended to determine whether the measures modify a health-related biomedical or behavioral outcome.
The study involves the recruitment of healthy volunteers for a respiratory challenge study; participants are randomized to receive different combinations of allergens. The study evaluates the severity and mechanism of the immune response to different combinations of allergens introduced via inhalation.
- Does the study involve human participants? Yes, healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to randomly selected combinations of allergens.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of different combinations of allergens on the immune response in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates the severity and mechanism of the immune reaction to allergens, which are health-related biomedical outcomes.
The study involves the recruitment of research participants with Alzheimer’s disease (AD) to evaluate the effects of an investigational drug on memory, and retention and recall of information.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the drug on participants’ memory.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates memory, and retention and recall of information in the context of AD.
The study involves the recruitment of individuals to receive a new behavioral intervention for sedentary behavior. It is designed to measure the effect of the intervention on hypothesized differential mediators of behavior change.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the intervetion on mediators of behavior change.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, mediators of behavior change, are behavioral outcomes relevant to health.
The study involves the recruitment of patients with disease X to be evaluated with a new visual acuity task. It is designed to evaluate the ability of the new task to measure visual acuity as compared with the gold standard Snellen Test
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, the new visual acuity test.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is designed to evaluate the ability of the new visual acuity test to measure visual acuity as compared to the gold standard Snellen Test, but not to modify visual acuity.
The study involves the recruitment of research participants with CHF who were hospitalized before or after implementation of the Medicare incentives to reduce re-hospitalizations. Morbidity, mortality, and quality of life of these participants are evaluated to compare the effects of these Medicare incentives on these outcomes.
- Are the participants prospectively assigned to an intervention? No, the intervention (incentives to reduce re-hospitalization) were assigned by Medicare, not by the research study.
The study involves the recruitment of healthcare providers to assess the extent to which being provided with genomic sequence information about their patients informs their treatment of those patients towards improved outcomes.
- Does the study involve human participants? Yes, both the physicians and the patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to receive genomic sequence information, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on the treatment they provide to their patients.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the extent to which providing specific information to physicians informs the treatment of patients, is a health-related outcome.
The study involves the recruitment of research participants with a behavioral condition to receive either an investigational behavioral intervention or a behavioral intervention in clinical use. It is designed to evaluate the effectiveness of the investigational intervention compared to the intervention in clinical use in reducing the severity of the obsessive compulsive disorder.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, either the investigational intervention or an intervention in clinical use.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate whether the investigational intervention is as effective as the standard intervention, at changing behavior.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the interventions’ effectiveness in reducing the severity of the condition, is a health-related behavioral outcome.
The study involves the recruitment of physicians who will be randomly assigned to use a new app or an existing app, which cues directed interviewing techniques. The study is designed to determine whether the new app is better than the existing app at assisting physicians in identifying families in need of social service support. The number of community service referrals will be measured.
- Does the study involve human participants? Yes, both the physicians and the families are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to use one of two apps, which are the interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on social service support referral for families.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the number of referrals, is a health-related outcome.
The study involves the recruitment of parents to participate in focus groups to discuss topics related to parental self-efficacy and positive parenting behaviors. It is designed to gather information needed to develop an intervention to promote parental self-efficacy and positive parenting behaviors.
- Does the study involve human participants? Yes, the parents are human participants.
- Are the participants prospectively assigned to an intervention? No, a focus group is not an intervention.
The study involves the recruitment of healthy volunteers to test a new behavioral intervention. It is designed to evaluate the effect of a meditation intervention on adherence to exercise regimens and quality of life to inform the design of a subsequent, fully-powered trial.
- Does the study involve human participants? Yes, study participants are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the intervention on adherence, and quality of life.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, adherence and quality of life are health-related outcomes.
A study will test the feasibility a mobile phone app designed to increase physical activity. A group of sedentary individuals will use the app for a week while their interactions with the app are monitored. The number of interactions with the app will be measured, as well as any software issues. Participants will also complete a survey indicating their satisfaction with and willingness to use the app, as well as any feedback for improvement. The app’s effect on physical activity, weight, or cardiovascular fitness will not be evaluated.
- Does the study involve human participants? Yes, sedentary individuals will be enrolled.
- Are the participants prospectively assigned to an intervention? The participants will interact with the app for a week.
- Is the study designed to evaluate the effect of the intervention on the participants? No. While the participants’ interactions are monitored (steps or heart rate may be recorded in this process), the study is NOT measuring the effect of using the app ON the participant. The study is only measuring the usability and acceptability of the app, and testing for bugs in the software. The effect on physical activity is NOT being measured.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A
The study involves the recruitment of healthy family members of patients hospitalized for disease X to test two CPR training strategies. Participants will receive one of two training strategies. The outcome is improved CPR skills retention.
- Does the study involve human participants? Yes, family members of patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to one of two CPR educational strategies.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of educational strategies on CPR skills.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, retention of CPR skills is a health-related behavioral outcome.
The study involves the recruitment of research participants in three different communities (clusters) to test three CPR training strategies. The rate of out-of- hospital cardiac arrest survival will be compared.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive one of three types of CPR training, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of different CPR training strategies on patient survival rates post cardiac arrest.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, out-of-hospital cardiac arrest survival is a health-related outcome.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. The accuracy of the two food monitoring methods in measuring energy intake will be assessed.
- Does the study involve human participants? Yes, children are human participants.
- Are the participants prospectively assigned to an intervention? No, in this context the monitoring methods would not be considered an intervention. The study is designed to test the accuracy of two monitoring methods, but not to test the effect on any health-related biomedical or behavioral outcomes.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. Changes to eating behavior will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to two food monitoring methods.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether using the monitoring methods changes eating behavior.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, eating behavior is a health-related outcome.
A study involves the recruitment of children at two schools to monitor eating behavior. Children’s food choices will be monitored using a remote food photography method. Food consumption and the accuracy of food monitoring methods will be assessed.
- Does the study involve human participants? Yes, the children participating in this study are human participants.
- Are the participants prospectively assigned to an intervention? No, not in this context. The study involves observing and measuring eating behavior, but not modifying it. This is an observational study.
A study involves the recruitment of children at two schools to evaluate their preferences for graphics and colors used in healthy food advertisements. Children will be presented with multiple health advertisements and their preferences for graphics and colors will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to see different advertisements.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the advertisements.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? No, preferences are not health-related biomedical or behavioral outcomes.
The study involves ambulatory patients who have new-onset stable angina and who are recruited from community practices. They are randomized to undergo CT angiography or an exercise stress test of the doctor’s choice. To keep the trial pragmatic, the investigators do not prescribe a protocol for how physicians should respond to test results. The study is designed to determine whether the initial test (CT angiography or stress test) affects long-term rates of premature death, stroke, or myocardial infarctions.
- Are the participants prospectively assigned to an intervention? Yes, the participants are randomized to undergo CT angiography or an exercise stress test.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether the initial test done affects long-term rates of certain clinical events.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, premature death, stroke, and myocardial infarction are health-related biomedical outcomes.
The study involves patients who present with stable angina to community practices. As part of their routine care some of their physicians refer them for CT angiography, while others refer them for exercise stress tests. The study is designed to see whether or not there's an association between the type of test that is chosen and long-term risk of death, stroke, or myocardial infarction.
- Are the participants prospectively assigned to an intervention? No, the intervention is not prospectively assigned by the investigators. Rather, the intervention, in this case diagnostic study, occurs as part of routine clinical care.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Does the study involve human participants? Yes.
- Are the participants prospectively assigned to an intervention? No, not in this context. Antipsychotic medications are given as part of clinical care, not as part of a prospective, approved research protocol.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. As part of the research protocol, all participants will be prescribed the same dose of the antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Are the participants prospectively assigned to an intervention? Yes, although participants are all receiving antipsychotic medication as part of their standard medical care, the dose of the antipsychotic medication is determined by the research protocol, rather than individual clinical need.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of a dose of antipsychotic medication on brain function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, brain function measured by imaging is a health-related outcome.
The study involves recruitment of healthy volunteers who will wear a thermal compression device around their legs. This pilot study is designed to examine preliminary performance and safety of a thermal compression device worn during surgery. Investigators will measure core temperature, comfort, and presence of skin injury in 15-minute intervals.
- Are the participants prospectively assigned to an intervention? Yes, participants are assigned to wear a thermal compression device.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the thermal compression device on participant core temperature, comfort, and presence of skin injury.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, participant core temperature, comfort, and presence of skin injury are health-related biomedical outcomes.
The study involves collection of data on hospitalizations for various acute illnesses among people who live close to a border between two states that have recently implemented different laws related to public health (e.g. smoking regulations, soda taxes). The investigators want to take advantage of this “natural experiment” to assess the health impact of the laws.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? No, the interventions were assigned by state laws and state of residence, not by the research study.
The study involves recruitment of healthy volunteers to engage in working memory tasks while undergoing transcranial magnetic stimulation (TMS) to induce competing local neuronal activity. The study is measuring task performance to investigate the neural underpinnings of working memory storage and processing.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive TMS stimulation protocols during a working memory task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of local TMS stimulation on working memory performance and oscillatory brain activity in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates working memory processes, which are health-related biomedical outcomes.
The study involves recruitment of healthy volunteers to engage in a social valuation task while dopamine tone in the brain is manipulated using tolcapone, an FDA-approved medication. The study aims to understand the role of dopamine in social decision-making and to search for neural correlates of this valuation using fMRI.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive tolcapone during a social valuation task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of modulating dopamine tone on social decision-making. Although this study uses an FDA-approved drug to modulate dopamine tone, the goal of this intervention is to understand the role of dopamine in a fundamental phenomenon (social valuation), and not to study the mechanism of action of the drug or its clinical effects.
The career development candidate proposes to independently lead a study to test a new drug A on patients with disease X. Patients will be randomized to a test and control group, with the test group receiving one dose of drug A per week for 12 months and controls receiving placebo. To assess presence, number, and type of any polyps, a colonoscopy will be performed. To assess biomarkers of precancerous lesions, colon mucosal biopsies will be collected. Complete blood count will be measured, and plasma will be stored for potential biomarker evaluation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drug A or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drug A and placebo on the presence and type of polyps.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the presence and type of polyps, is a health-related biomedical outcome.
Ancillary Study to Case Study #42b: Some types of drug A being evaluated in Case Study #42a have been reported to impact renal function. An internal medicine fellow performs an ancillary study where stored plasma from Case Study #42a will be evaluated for multiple biomarkers of renal function.
- Does the study involve human participants? Yes, patients are human participants because the plasma and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention occurs as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that is not an independent clinical trial.
Ancillary Study to Case Study #42a: An internal medicine fellow designs an independent ancillary trial where a subset of patients from the parent trial in Case Study #42a will also receive drug B, based on the assumption that a two-drug combination will work significantly better than a single drug at both improving renal function and reducing polyps. The test subjects will be evaluated for renal function via plasma clearance rates at 6 and 12 months after initiation of drugs A and B.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drugs A and B.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drugs A and B on renal function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, renal function, is a health-related biomedical outcome.
A group of healthy young adults will perform a Go/No-Go task while undergoing fMRI scans. The purpose of the study is to characterize the pattern of neural activation in the frontal cortex during response inhibition, and the ability of the participant to correctly withhold a response on no-go
- Does the study involve human participants? Yes, healthy young adults will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a Go/No-Go task, which involves different levels of inhibitory control.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the Go/No-Go task on neural activation in the frontal cortex. The study will measure inhibitory control and the neural systems being engaged. In this study, the Go/No-Go task is the independent variable, and behavioral performance and the associated fMRI activations are the dependent variables.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the neural correlates of inhibitory control and behavioral performance are health-related biomedical outcomes.
A group of adolescents will participate in a longitudinal study examining changes in executive function over the course of a normal school year. Color naming performance on the standard version of the Stroop test will be obtained. All measures will be compared at multiple time points during the school year to examine changes in executive function. The purpose is to observe changes in executive function and to observe if differences exist in the Stroop effect over the course of the school year for these adolescents.
- Does the study involve human participants? Yes, adolescents will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? No, there is no intervention in this study and no independent variable manipulated. The adolescents are not prospectively assigned to an intervention, but instead the investigator will examine variables of interest (including the Stroop test) over time. The Stroop effect is used as a measurement of point-in-time data.
- Is the study designed to evaluate the effect of the intervention on the participants? No, there is no intervention. Performance on the Stroop test is a well-established measure of executive function and the test is not providing an independent variable of interest here. It is not being used to manipulate the participants or their environment. The purpose is simply to obtain a measure of executive function in adolescents over the course of the school year.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A. No effect of an intervention is being evaluated.
A group of participants with social anxiety will perform an experimentally manipulated Stroop test. In this variant of the Stroop test, the stimuli presented are varied to include emotional and neutral facial expressions presented in different colors. Participants are instructed to name the colors of the faces presented, with the expectation that they will be slower to name the color of the emotional face than the neutral face. The purpose of the study is to examine the degree to which participants with social anxiety will be slower to process emotional faces than neutral faces.
- Does the study involve human participants? Yes, participants with social anxiety will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a modified Stroop test using different colored emotional/neutral faces to explore emotional processing in people with social anxiety. Note that the independent variable is the presentation of emotional vs neutral faces.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of emotional valence (i.e. emotional faces) on participant response time to name the color. The purpose is to determine whether the response time to emotional faces is exaggerated for people with social anxiety as compared to neutral faces. Note that the response time to name the colors is the dependent variable in this study.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the processing of emotional information is a health-related biomedical outcome.
The study involves healthy volunteers and compares temporal SNR obtained with a new fMRI pulse sequence with that from another sequence.
- Are the participants prospectively assigned to an intervention? No, in this context the different pulse sequences would not be considered an intervention. The pulse sequences are not being used to modify any biomedical or behavioral outcome; rather the investigator is comparing performance characteristics of the two pulse sequences.
The study is designed to demonstrate that a new imaging technology (e.g. MRI, PET, ultrasound technologies, or image processing algorithm) is equivalent to, or has better sensitivity/specificity than a standard of care imaging technology. Aim one will use the new imaging technology and the gold standard in ten healthy volunteers. Aim Two will use the new imaging technology and the gold standard before and after a standard care procedure in ten patients. In both aims the performance of the new technology will be compared to the gold standard. No clinical care decisions will be made based on the use of the device in this study.
- Does the study involve human participants? YES. Aim one will study ten healthy volunteers, and aim two will study ten patient volunteers.
- Are the participants prospectively assigned to an intervention? Yes, participants will be prospectively assigned to be evaluated with a new imaging technology and the gold standard technology.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is not measuring the effect of the technologies ON the human subjects. The study is determining if the new technology is equivalent or better than the gold standard technology. No effect on the participant is being measured.
An investigator proposes to add secondary outcomes to an already funded clinical trial of a nutritional intervention. The trial is supported by other funding, but the investigator is interested in obtaining NIH funding for studying oral health outcomes. Participants in the existing trial would be assessed for oral health outcomes at baseline and at additional time points during a multi-week dietary intervention. The oral health outcomes would include measures of gingivitis and responses to oral health related quality of life questionnaires. Oral fluids would be collected for analysis of inflammatory markers and microbiome components.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention (and the administration of the intervention) occur as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that leverages an already existing clinical trial.
The goal of the project is to use functional neuroimaging to distinguish patients with temporomandibular disorders (TMD) who experience TMD pain through centralized pain processes from those with TMD related to peripheral pain. Pain processing in a study cohort of TMD patients and healthy controls will be measured through functional magnetic resonance neuroimaging (fMRI) following transient stimulation of pain pathways through multimodal automated quantitative sensory testing (MAST QST). TMD patients will receive study questionnaires to better correlate the extent to which TMD pain centralization influences TMD prognosis and response to standard of care peripherally targeted treatment (prescribed by physicians, independently of the study).
- Are the participants prospectively assigned to an intervention? No, not in this context. The transient stimulation of pain pathways and the fMRI are being performed to measure and describe brain activity, but not to modify it.
An investigator proposes to perform a study of induced gingivitis in healthy humans, to study microbial colonization and inflammation under conditions of health and disease. During a 3-week gingivitis induction period, each study participant will use a stent to cover the teeth in one quadrant during teeth brushing. A contralateral uncovered quadrant will be exposed to the individual's usual oral hygiene procedures, to serve as a control. Standard clinical assessments for gingivitis will be made and biospecimens will be collected at the point of maximal induced gingivitis, and again after normal oral hygiene is resumed. Biospecimens will be assessed for microbial composition and levels of inflammation-associated chemokines.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, abstaining from normal oral hygiene for a portion of the mouth, to induce gingivitis.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the induced gingivitis on microbial composition and levels of inflammatory chemokines in oral samples.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the microbial composition and chemokine levels in oral samples are health-related biomedical outcomes.
The study will enroll older adults with hearing loss, comparing the effectiveness of enhanced hearing health care (HHC) to usual HHC. In addition to routine hearing-aid consultation and fitting, participants randomized to enhanced HCC will be provided patient-centered information and education about a full range of hearing assistive technologies and services. Study outcomes include the utilization of technology or services, quality of life, communication abilities, and cognitive function.
- Does the study involve human participants? Yes, the study enrolls older adults with hearing loss.
- Are the participants prospectively assigned to an intervention? Yes, participants are randomized to receive enhanced HCC or usual HCC interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study will evaluate enhanced HCC’s effectiveness in modifying participant behavior and biomedical outcomes.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, rate of technology/service utilization is a behavioral outcome and quality of life, communications, and cognition are biomedical outcomes that may be impacted by the interventions.
The study involves the recruitment of obese individuals who will undergo a muscle biopsy before and after either exercise training or diet-induced weight loss. Sarcolemmal 1,2-disaturated DAG and C18:0 ceramide species and mitochondrial function will be measured. Levels will be correlated with insulin sensitivity.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to either exercise training or a diet.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the interventions on muscle metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, muscle metabolism/signaling is a health-related outcome.
The study involves the recruitment of participants with type 2 diabetes who will undergo a muscle biopsy before and after a fast to measure acetylation on lysine 23 of the mitochondrial solute carrier adenine nucleotide translocase 1 (ANT1). Levels will be related to rates of fat oxidation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to undergo a fast.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the fast on molecular parameters of metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, metabolism is a health-related outcome.
Insulin-resistant and insulin-sensitive nondiabetic adults who have a parent with type 2 diabetes will be followed over time to understand the role of mitochondrial dysfunction in the development of diabetes. Oral glucose tolerance tests will be performed annually to measure insulin sensitivity and glycemic status. Participants will also undergo a brief bout of exercise, and mitochondrial ATP synthesis rates will be measured by assessing the rate of recovery of phosphocreatine in the leg muscle, using 31P magnetic resonance spectroscopy.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to an intervention; the OGTT and 31P MRS are measures.
Participants with chronic kidney disease will be recruited to receive one of two drug agents. After 6 weeks of therapy, subjects will undergo vascular function testing and have measures of oxidative stress evaluated in their plasma and urine. Results of the function testing and the oxidative stress biomarkers will be related to drug treatment.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive two different drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function is a health-related outcome.
Participants with Autosomal Dominant Polycystic Kidney Disease will be recruited to receive an oral curcumin therapy or placebo and the participants will undergo vascular function testing, renal imaging to assess kidney size, and assessment of oxidative stress biomarkers in urine and plasma after an ascorbic acid challenge. Changes in these outcomes will be related to oral therapy.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive medication or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function and kidney size.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function and kidney size are health-related outcomes.
Kidney transplant recipients will be recruited to undergo an experimental imaging procedure at several timepoints up to 4 months post-transplantation. Output from the images will be related to pathological assessments of the transplant as well as clinical measures of renal function.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to receive an intervention. They undergo transplantation as part of their routine clinical care. The imaging procedure is a measure and not an intervention.
The study proposes the development of a novel probe to assess clearance of a nutritional metabolite in a given disease state. The probe is a GMP grade, deuterated, intravenously administered tracer and clearance is assessed by mass spectrometry analysis of serial blood draws. Participants will either receive a micronutrient supplement or will receive no supplementation. The clearance rate of the probe will be compared in the two groups, to understand the performance of the probe.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive either a micronutrient supplement or nothing.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention is being used to assess the performance of the probe and is not looking at an effect on the participant.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive a controlled diet for three days.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention (controlled diet) is being used to minimize exogenous dietary sources of oxalate in the participants prior to the labeled tracer infusion. The study will not be evaluating the effect of the diet on the participants.
This page last updated on: April 28, 2021
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
Case Study Research Method in Psychology
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews).
The case study research method originated in clinical medicine (the case history, i.e., the patient’s personal history). In psychology, case studies are often confined to the study of a particular individual.
The information is mainly biographical and relates to events in the individual’s past (i.e., retrospective), as well as to significant events that are currently occurring in his or her everyday life.
The case study is not a research method, but researchers select methods of data collection and analysis that will generate material suitable for case studies.
Freud (1909a, 1909b) conducted very detailed investigations into the private lives of his patients in an attempt to both understand and help them overcome their illnesses.
This makes it clear that the case study is a method that should only be used by a psychologist, therapist, or psychiatrist, i.e., someone with a professional qualification.
There is an ethical issue of competence. Only someone qualified to diagnose and treat a person can conduct a formal case study relating to atypical (i.e., abnormal) behavior or atypical development.
Famous Case Studies
- Anna O – One of the most famous case studies, documenting psychoanalyst Josef Breuer’s treatment of “Anna O” (real name Bertha Pappenheim) for hysteria in the late 1800s using early psychoanalytic theory.
- Little Hans – A child psychoanalysis case study published by Sigmund Freud in 1909 analyzing his five-year-old patient Herbert Graf’s house phobia as related to the Oedipus complex.
- Bruce/Brenda – Gender identity case of the boy (Bruce) whose botched circumcision led psychologist John Money to advise gender reassignment and raise him as a girl (Brenda) in the 1960s.
- Genie Wiley – Linguistics/psychological development case of the victim of extreme isolation abuse who was studied in 1970s California for effects of early language deprivation on acquiring speech later in life.
- Phineas Gage – One of the most famous neuropsychology case studies analyzes personality changes in railroad worker Phineas Gage after an 1848 brain injury involving a tamping iron piercing his skull.
Clinical Case Studies
- Studying the effectiveness of psychotherapy approaches with an individual patient
- Assessing and treating mental illnesses like depression, anxiety disorders, PTSD
- Neuropsychological cases investigating brain injuries or disorders
Child Psychology Case Studies
- Studying psychological development from birth through adolescence
- Cases of learning disabilities, autism spectrum disorders, ADHD
- Effects of trauma, abuse, deprivation on development
Types of Case Studies
- Explanatory case studies : Used to explore causation in order to find underlying principles. Helpful for doing qualitative analysis to explain presumed causal links.
- Exploratory case studies : Used to explore situations where an intervention being evaluated has no clear set of outcomes. It helps define questions and hypotheses for future research.
- Descriptive case studies : Describe an intervention or phenomenon and the real-life context in which it occurred. It is helpful for illustrating certain topics within an evaluation.
- Multiple-case studies : Used to explore differences between cases and replicate findings across cases. Helpful for comparing and contrasting specific cases.
- Intrinsic : Used to gain a better understanding of a particular case. Helpful for capturing the complexity of a single case.
- Collective : Used to explore a general phenomenon using multiple case studies. Helpful for jointly studying a group of cases in order to inquire into the phenomenon.
Where Do You Find Data for a Case Study?
There are several places to find data for a case study. The key is to gather data from multiple sources to get a complete picture of the case and corroborate facts or findings through triangulation of evidence. Most of this information is likely qualitative (i.e., verbal description rather than measurement), but the psychologist might also collect numerical data.
1. Primary sources
- Interviews – Interviewing key people related to the case to get their perspectives and insights. The interview is an extremely effective procedure for obtaining information about an individual, and it may be used to collect comments from the person’s friends, parents, employer, workmates, and others who have a good knowledge of the person, as well as to obtain facts from the person him or herself.
- Observations – Observing behaviors, interactions, processes, etc., related to the case as they unfold in real-time.
- Documents & Records – Reviewing private documents, diaries, public records, correspondence, meeting minutes, etc., relevant to the case.
2. Secondary sources
- News/Media – News coverage of events related to the case study.
- Academic articles – Journal articles, dissertations etc. that discuss the case.
- Government reports – Official data and records related to the case context.
- Books/films – Books, documentaries or films discussing the case.
3. Archival records
Searching historical archives, museum collections and databases to find relevant documents, visual/audio records related to the case history and context.
Public archives like newspapers, organizational records, photographic collections could all include potentially relevant pieces of information to shed light on attitudes, cultural perspectives, common practices and historical contexts related to psychology.
4. Organizational records
Organizational records offer the advantage of often having large datasets collected over time that can reveal or confirm psychological insights.
Of course, privacy and ethical concerns regarding confidential data must be navigated carefully.
However, with proper protocols, organizational records can provide invaluable context and empirical depth to qualitative case studies exploring the intersection of psychology and organizations.
- Organizational/industrial psychology research : Organizational records like employee surveys, turnover/retention data, policies, incident reports etc. may provide insight into topics like job satisfaction, workplace culture and dynamics, leadership issues, employee behaviors etc.
- Clinical psychology : Therapists/hospitals may grant access to anonymized medical records to study aspects like assessments, diagnoses, treatment plans etc. This could shed light on clinical practices.
- School psychology : Studies could utilize anonymized student records like test scores, grades, disciplinary issues, and counseling referrals to study child development, learning barriers, effectiveness of support programs, and more.
How do I Write a Case Study in Psychology?
Follow specified case study guidelines provided by a journal or your psychology tutor. General components of clinical case studies include: background, symptoms, assessments, diagnosis, treatment, and outcomes. Interpreting the information means the researcher decides what to include or leave out. A good case study should always clarify which information is the factual description and which is an inference or the researcher’s opinion.
1. Introduction
- Provide background on the case context and why it is of interest, presenting background information like demographics, relevant history, and presenting problem.
- Compare briefly to similar published cases if applicable. Clearly state the focus/importance of the case.
2. Case Presentation
- Describe the presenting problem in detail, including symptoms, duration,and impact on daily life.
- Include client demographics like age and gender, information about social relationships, and mental health history.
- Describe all physical, emotional, and/or sensory symptoms reported by the client.
- Use patient quotes to describe the initial complaint verbatim. Follow with full-sentence summaries of relevant history details gathered, including key components that led to a working diagnosis.
- Summarize clinical exam results, namely orthopedic/neurological tests, imaging, lab tests, etc. Note actual results rather than subjective conclusions. Provide images if clearly reproducible/anonymized.
- Clearly state the working diagnosis or clinical impression before transitioning to management.
3. Management and Outcome
- Indicate the total duration of care and number of treatments given over what timeframe. Use specific names/descriptions for any therapies/interventions applied.
- Present the results of the intervention,including any quantitative or qualitative data collected.
- For outcomes, utilize visual analog scales for pain, medication usage logs, etc., if possible. Include patient self-reports of improvement/worsening of symptoms. Note the reason for discharge/end of care.
4. Discussion
- Analyze the case, exploring contributing factors, limitations of the study, and connections to existing research.
- Analyze the effectiveness of the intervention,considering factors like participant adherence, limitations of the study, and potential alternative explanations for the results.
- Identify any questions raised in the case analysis and relate insights to established theories and current research if applicable. Avoid definitive claims about physiological explanations.
- Offer clinical implications, and suggest future research directions.
5. Additional Items
- Thank specific assistants for writing support only. No patient acknowledgments.
- References should directly support any key claims or quotes included.
- Use tables/figures/images only if substantially informative. Include permissions and legends/explanatory notes.
- Provides detailed (rich qualitative) information.
- Provides insight for further research.
- Permitting investigation of otherwise impractical (or unethical) situations.
Case studies allow a researcher to investigate a topic in far more detail than might be possible if they were trying to deal with a large number of research participants (nomothetic approach) with the aim of ‘averaging’.
Because of their in-depth, multi-sided approach, case studies often shed light on aspects of human thinking and behavior that would be unethical or impractical to study in other ways.
Research that only looks into the measurable aspects of human behavior is not likely to give us insights into the subjective dimension of experience, which is important to psychoanalytic and humanistic psychologists.
Case studies are often used in exploratory research. They can help us generate new ideas (that might be tested by other methods). They are an important way of illustrating theories and can help show how different aspects of a person’s life are related to each other.
The method is, therefore, important for psychologists who adopt a holistic point of view (i.e., humanistic psychologists ).
Limitations
- Lacking scientific rigor and providing little basis for generalization of results to the wider population.
- Researchers’ own subjective feelings may influence the case study (researcher bias).
- Difficult to replicate.
- Time-consuming and expensive.
- The volume of data, together with the time restrictions in place, impacted the depth of analysis that was possible within the available resources.
Because a case study deals with only one person/event/group, we can never be sure if the case study investigated is representative of the wider body of “similar” instances. This means the conclusions drawn from a particular case may not be transferable to other settings.
Because case studies are based on the analysis of qualitative (i.e., descriptive) data , a lot depends on the psychologist’s interpretation of the information she has acquired.
This means that there is a lot of scope for Anna O , and it could be that the subjective opinions of the psychologist intrude in the assessment of what the data means.
For example, Freud has been criticized for producing case studies in which the information was sometimes distorted to fit particular behavioral theories (e.g., Little Hans ).
This is also true of Money’s interpretation of the Bruce/Brenda case study (Diamond, 1997) when he ignored evidence that went against his theory.
Breuer, J., & Freud, S. (1895). Studies on hysteria . Standard Edition 2: London.
Curtiss, S. (1981). Genie: The case of a modern wild child .
Diamond, M., & Sigmundson, K. (1997). Sex Reassignment at Birth: Long-term Review and Clinical Implications. Archives of Pediatrics & Adolescent Medicine , 151(3), 298-304
Freud, S. (1909a). Analysis of a phobia of a five year old boy. In The Pelican Freud Library (1977), Vol 8, Case Histories 1, pages 169-306
Freud, S. (1909b). Bemerkungen über einen Fall von Zwangsneurose (Der “Rattenmann”). Jb. psychoanal. psychopathol. Forsch ., I, p. 357-421; GW, VII, p. 379-463; Notes upon a case of obsessional neurosis, SE , 10: 151-318.
Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Money, J., & Ehrhardt, A. A. (1972). Man & Woman, Boy & Girl : The Differentiation and Dimorphism of Gender Identity from Conception to Maturity. Baltimore, Maryland: Johns Hopkins University Press.
Money, J., & Tucker, P. (1975). Sexual signatures: On being a man or a woman.
Further Information
- Case Study Approach
- Case Study Method
- Enhancing the Quality of Case Studies in Health Services Research
- “We do things together” A case study of “couplehood” in dementia
- Using mixed methods for evaluating an integrative approach to cancer care: a case study
Related Articles
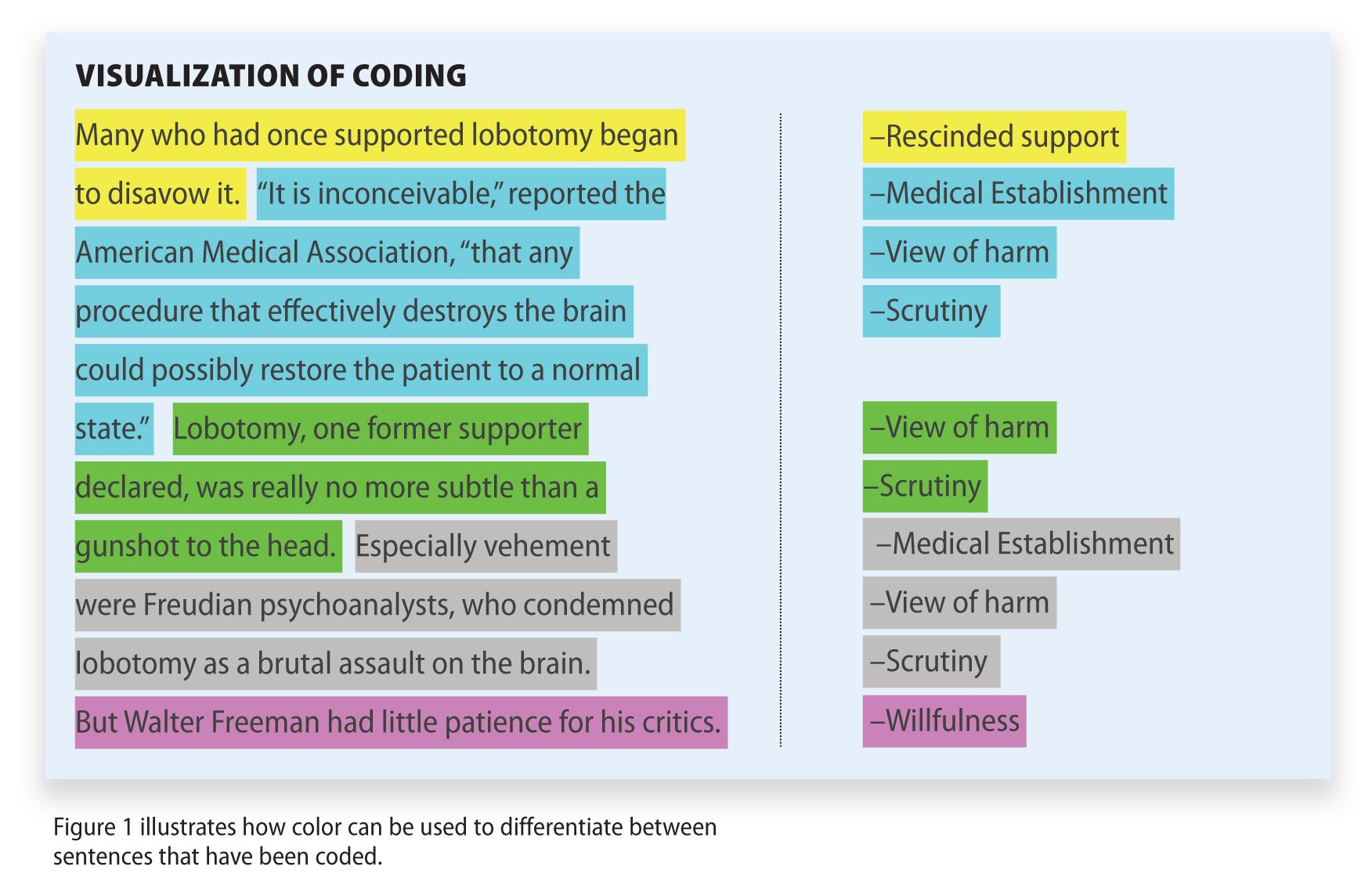
Research Methodology
Qualitative Data Coding

What Is a Focus Group?
Cross-Cultural Research Methodology In Psychology

What Is Internal Validity In Research?

Research Methodology , Statistics
What Is Face Validity In Research? Importance & How To Measure

Criterion Validity: Definition & Examples
Editor's Choice: How AI Will Enhance Imaging Access and Analysis
- Kidney Donor and Recipient Outcomes May 23, 2024 Original Investigation Hypertension and Kidney Function After Living Kidney Donation Amit X. Garg, MD, PhD; Jennifer B. Arnold, BSc; Meaghan S. Cuerden, PhD; et al Editorial Prospectively Examining Outcomes After Living Kidney Donation: Informing the Altruist Elizabeth C. Lorenz, MD; Wolfgang C. Winkelmayer, MD, MPH, ScD
- Original Investigation Kidney Transplant Outcomes From Deceased Donors Who Received Dialysis Yumeng Wen, MD, PhD; Sherry G. Mansour, MD, MSc; Nityasree Srialluri, MD, MS, MHS; et al Editorial Expanding the Overton Window in Deceased Kidney Donor Eligibility—Enough to Make a Difference? Xingxing S. Cheng, MD, MS; Colin R. Lenihan, MB BCh BAO, PhD
- Audio Outcomes After Living Kidney Donation Amit X. Garg, MD, PhD, and Elizabeth C. Lorenz, MD, with host JAMA Associate Editor Wolfgang C. Winkelmayer, MD, MPH, ScD
Just Published
- Shingles Vaccination in Medicare Part D After Inflation Reduction Act Elimination of Cost Sharing Dima M. Qato, PharmD, MPH, PhD; et al. Research Letter online first Dima M. Qato, PharmD, MPH, PhD; et al.
- Hypertension and Kidney Function After Living Kidney Donation Amit X. Garg, MD, PhD; et al. Original Investigation online first free access has multimedia Amit X. Garg, MD, PhD; et al. Editorial
- Kidney Transplant Outcomes From Deceased Donors Who Received Dialysis Yumeng Wen, MD, PhD; et al. Original Investigation online first free access has multimedia Yumeng Wen, MD, PhD; et al. Editorial
- Krill Oil for Knee Osteoarthritis Laura L. Laslett, PhD; et al. Original Investigation online first has active quiz Laura L. Laslett, PhD; et al.
- Dispensing of Glucagon-Like Peptide-1 Receptor Agonists to Adolescents and Young Adults, 2020-2023 Joyce M. Lee, MD, MPH; et al. Research Letter online first Joyce M. Lee, MD, MPH; et al.
- Heard but Excluded: A Language Manifesto Rose L. Molina, MD, MPH; et al. Viewpoint online first Rose L. Molina, MD, MPH; et al.
- The Other Side of the Curtain Paige Stevens, MD A Piece of My Mind online first Paige Stevens, MD
- Expanding the Overton Window in Deceased Kidney Donor Eligibility Xingxing S. Cheng, MD, MS; et al. Editorial online first free access has multimedia Xingxing S. Cheng, MD, MS; et al.
- Prospectively Examining Outcomes After Living Kidney Donation Elizabeth C. Lorenz, MD; et al. Editorial online first free access has multimedia Elizabeth C. Lorenz, MD; et al.
- Advancing Access to Cell and Gene Therapies in Medicaid Joseph T. Kannarkat, MPP; et al. Viewpoint online first Joseph T. Kannarkat, MPP; et al.
- Federal Trade Commission Actions on Prescription Drugs, 2000-2022 C. Joseph Ross Daval, JD; et al. Special Communication online first has active quiz has multimedia C. Joseph Ross Daval, JD; et al. Editorial
- Blood Testing for Phosphatidylethanol Areej Mazhar, DO; et al. JAMA Diagnostic Test Interpretation online first has active quiz Areej Mazhar, DO; et al.
- Causal Inference and Effects of Interventions From Observational Studies in Medical Journals Issa J. Dahabreh, MD, ScD; et al. Special Communication online first free access has active quiz Issa J. Dahabreh, MD, ScD; et al. Editor's Note
- A 3-Year-Old With Gingival Hemorrhage and Musculoskeletal Pain Khanh Trinh, DMD; et al. JAMA Clinical Challenge online first has active quiz Khanh Trinh, DMD; et al.
- The Women’s Health Initiative Randomized Trials and Clinical Practice JoAnn E. Manson, MD, DrPH; et al. Review online first has active quiz JoAnn E. Manson, MD, DrPH; et al.
Latest from the USPSTF
- USPSTF Recommendation: Screening for Breast Cancer
- USPSTF Recommendation: Primary Care Interventions to Prevent Child Maltreatment
- USPSTF Recommendation: Screening for Speech and Language Delay and Disorders
- 80,600 Views USPSTF Recommendation: Screening for Breast Cancer
- 38,359 Views Causal Inference and Effects of Interventions From Observational Studies in Medical Journals
- 31,430 Views Krill Oil for Knee Osteoarthritis
- 29,492 Views The Women’s Health Initiative Randomized Trials and Clinical Practice
- 27,208 Views Mortality in Patients Hospitalized for COVID-19 vs Influenza in Fall-Winter 2023-2024
- 23,800 Views Acetaminophen for Prevention and Treatment of Organ Dysfunction
- 22,517 Views Associations of Milestone Ratings and Certification Examination Scores With Patient Outcomes
- 22,148 Views Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer
- 21,119 Views Why the Bird Flu Outbreak in Dairy Cows Matters
- 21,082 Views Survival Benefit Associated With Participation in Clinical Trials of Anticancer Drugs
- 726 Citations Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients
- 717 Citations Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization
- 631 Citations Pancreatic Cancer
- 623 Citations Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals
- 523 Citations Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults
- 448 Citations Association Between IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19
- 414 Citations Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by Omicron and Delta Variants
- 383 Citations Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination
- 382 Citations Association of COVID-19 mRNA Vaccination With Hospitalizations and Disease Severity
- 378 Citations Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: population-based, nested case-control study
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Raphael S. Peter
- ORCID record for Alexandra Nieters
- ORCID record for Siri Göpel
- ORCID record for Uta Merle
- ORCID record for Jürgen M. Steinacker
- ORCID record for Peter Deibert
- ORCID record for Birgit Friedmann-Bette
- ORCID record for Andreas Niess
- ORCID record for Barbara Müller
- ORCID record for Claudia Schilling
- ORCID record for Gunnar Erz
- ORCID record for Roland Giesen
- ORCID record for Veronika Götz
- ORCID record for Karsten Keller
- ORCID record for Philipp Maier
- ORCID record for Lynn Matits
- ORCID record for Sylvia Parthé
- ORCID record for Martin Rehm
- ORCID record for Jana Schellenberg
- ORCID record for Ulrike Schempf
- ORCID record for Mengyu Zhu
- ORCID record for Hans-Georg Kräusslich
- ORCID record for Dietrich Rothenbacher
- ORCID record for Winfried V. Kern
- For correspondence: [email protected]
- Info/History
- Supplementary material
- Preview PDF
Objective To assess risk factors for persistence vs improvement and to describe clinical characteristics and diagnostic evaluation of subjects with post-acute sequelae of COVID-19/post-COVID-19 syndrome (PCS) persisting for more than one year.
Design Nested population-based case-control study.
Setting Comprehensive outpatient assessment, including neurocognitive, cardiopulmonary exercise, and laboratory testing in four university health centres in southwestern Germany (2022).
Participants PCS cases aged 18 to 65 years with (n=982) and age and sex-matched controls without PCS (n=576) according to an earlier population-based questionnaire study (six to 12 months after acute infection, phase 1) consenting to provide follow-up information and to undergo clinical diagnostic assessment (phase 2, another 8.5 months [median] after phase 1).
Main outcome measures Relative frequencies of symptoms and health problems and distribution of symptom scores and diagnostic test results between persistent cases and controls. Additional analysis included predictors of changing case or control status over time with adjustments for potentially confounding variables.
Results At the time of clinical examination (phase 2), 67.6% of the initial cases (phase 1) remained cases, whereas 78.5% of the controls continued to report no health problems related to PCS. In adjusted analyses, predictors of improvement among cases were mild acute index infection, previous full-time employment, educational status, and no specialist consultation and not attending a rehabilitation programme. Among controls, predictors of new symptoms or worsening with PCS development were an intercurrent secondary SARS-CoV-2 infection and educational status. At phase 2, persistent cases were less frequently never smokers, had higher values for BMI and body fat, and had lower educational status than controls. Fatigue/exhaustion, neurocognitive disturbance, chest symptoms/breathlessness and anxiety/depression/sleep problems remained the predominant symptom clusters, and exercise intolerance with post-exertional malaise for >14 h (PEM) and symptoms compatible with ME/CFS (according to Canadian consensus criteria) were reported by 35.6% and 11.6% of persistent cases, respectively. In adjusted analyses, significant differences between persistent cases and stable controls (at phase 2) were observed for neurocognitive test performances, scores for perceived stress and subjective cognitive disturbances, symptoms indicating dysautonomia, depression and anxiety, sleep quality, fatigue, and quality of life. In persistent cases, handgrip strength, maximal oxygen consumption, and ventilator efficiency were significantly reduced. However, there were no differences in measures of systolic and diastolic cardiac function, in the level of pro-BNP blood levels or other laboratory measurements (including complement activity, serological markers of EBV reactivation, inflammatory and coagulation markers, cortisol, ACTH and DHEA-S serum levels). Screening for viral persistence (based on PCR in stool samples and SARS-CoV-2 spike antigen levels in plasma in a subgroup of the cases) was negative. Sensitivity analyses (pre-existing illness/comorbidity, obesity, PEM, medical care of the index acute infection) revealed similar findings and showed that persistent cases with PEM reported more pain symptoms and had worse results in almost all tests.
Conclusions This nested population-based case-control study demonstrates that the majority of PCS cases do not recover in the second year of their illness, with patterns of reported symptoms remaining essentially similar, nonspecific and dominated by fatigue, exercise intolerance and cognitive complaints. We found objective signs of cognitive deficits and reduced exercise capacity likely to be unrelated to primary cardiac or pulmonary dysfunction in some of the cases, but there was no major pathology in laboratory investigations. A history of PEM >14 h which was associated with more severe symptoms as well as with more objective signs of disease may be a pragmatic means to stratify cases for disease severity.
What is already known on this topic Self-reported health problems following SARS-CoV-2 infection have commonly been described and may persist for months. They typically include relatively non-specific complaints such as fatigue, exertional dyspnoea, concentration or memory disturbance and sleep problems. The incidence of this post-COVID-19 syndrome (PCS) is varying and associated with sociodemographic variables, pre-existing disease and comorbidities, the severity of the acute SARS-CoV-2 index infection, and some other factors. The long-term prognosis is unknown and may differ for different symptoms or symptom clusters. Evidence of measurable single or multiple organ dysfunction and pathology and their correlation with self-reported symptoms in patients with non-recovery from PCS for more than a year have not been well described.
What this study adds The study describes the severity of the index infection, lower educational status, no previous full-time employment, and (need for) specialist consultation or a rehabilitation programme (the latter probably due to reverse causation) as factors for non-recovery from PCS, and found no major changes in symptom clusters among PCS cases persisting for more than a year. After a comprehensive medical evaluation of cases and controls and adjusted analyses, objective signs of organ dysfunction and pathology among persistent PCS cases correlated with self-reported symptoms, were detected more often among cases with longer lasting post-exertional malaise, and included both reduced physical exercise capacity (diminished handgrip strength, maximal oxygen consumption and ventilatory efficiency), and reduced cognitive test performances while there were no differences in the results of multiple laboratory investigations after adjustment for possible confounders.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This work was funded by the Baden-Wuerttemberg Federal State Ministry of Science and Art (grant number MR/S028188/1).
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Ethical approval was obtained from the Ethics Committee of the University of Freiburg, Engelberger Strasse 21, D-79106 Freiburg/Germany (#21/1484_1), the Ethics Committee of the Medical Faculty of Heidelberg University, Alte Glockengiesserei 11/1, D-69115 Heidelberg/Germany (#S-846/2021), the Ethics Committee at the Medical Faculty of the Eberhard-Karls-University and at the University Hospital of Tuebingen, Gartenstrasse 47, D-72074 Tuebingen/Germany (#845/2021BO2), and the Ethic Committee of the University of Ulm, Oberberghof 7, D-89081 Ulm/Germany (#337/21).
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
↵ * Co-first authors
↵ # Joint senior authors
↵ † Additional members of the EPILOC Phase 2 Study Group (in alphabetic order): Parwez Aidery (Tübingen), Daniel Bizjak (Ulm), Stefanie Bunk (Tübingen), Nadine Conzelmann (Tübingen), Stefanie Döbele (Tübingen), Lisamaria Eble (Ulm), Melanie Greibich (Heidelberg), Beate Grüner (Ulm), Lucas John (Ulm), Gerhard Kindle (Freiburg), Oliver Krumnau (Freiburg), Jessica Langel (Heidelberg), Nisar Malek (Tübingen), Moritz Munk (Ulm), Stefanie Pfau (Freiburg), Stephan Prettin (Freiburg), Hardy Richter (Tübingen), Siegbert Rieg (Freiburg), Cynthia Stapornwongkul (Freiburg), Sabine Tuma-Kellner (Heidelberg), Kay Winkert (Ulm).
Data Availability
All data produced in the present study are available upon reasonable request to the authors.
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Infectious Diseases (except HIV/AIDS)
- Addiction Medicine (324)
- Allergy and Immunology (632)
- Anesthesia (168)
- Cardiovascular Medicine (2401)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (381)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (850)
- Epidemiology (11799)
- Forensic Medicine (10)
- Gastroenterology (705)
- Genetic and Genomic Medicine (3769)
- Geriatric Medicine (350)
- Health Economics (637)
- Health Informatics (2409)
- Health Policy (940)
- Health Systems and Quality Improvement (905)
- Hematology (342)
- HIV/AIDS (787)
- Infectious Diseases (except HIV/AIDS) (13347)
- Intensive Care and Critical Care Medicine (769)
- Medical Education (369)
- Medical Ethics (105)
- Nephrology (401)
- Neurology (3524)
- Nursing (199)
- Nutrition (529)
- Obstetrics and Gynecology (681)
- Occupational and Environmental Health (669)
- Oncology (1835)
- Ophthalmology (539)
- Orthopedics (221)
- Otolaryngology (287)
- Pain Medicine (234)
- Palliative Medicine (66)
- Pathology (447)
- Pediatrics (1038)
- Pharmacology and Therapeutics (426)
- Primary Care Research (424)
- Psychiatry and Clinical Psychology (3190)
- Public and Global Health (6184)
- Radiology and Imaging (1291)
- Rehabilitation Medicine and Physical Therapy (751)
- Respiratory Medicine (832)
- Rheumatology (380)
- Sexual and Reproductive Health (373)
- Sports Medicine (324)
- Surgery (406)
- Toxicology (50)
- Transplantation (172)
- Urology (147)
Association Between Exposure to Multiple Toxic Metals in Follicular Fluid and the Risk of PCOS Among Infertile Women: The Mediating Effect of Metabolic Markers
- Published: 24 May 2024
Cite this article

- Xin Wang 1 , 3 , 4 ,
- Ying Zhang 1 , 3 , 4 ,
- Jie Peng 1 , 3 , 4 ,
- Hua Zhang 1 , 3 , 4 ,
- Tingting Jiang 2 ,
- Zhikang Zhang 1 , 3 , 4 ,
- Tao Yin 1 , 3 , 4 ,
- Xun Su 1 , 3 , 4 ,
- Tao Zhang 5 ,
- Lingchao Shen 1 , 3 , 4 ,
- Shitao He 2 ,
- Xiaolei Wang 1 , 3 , 4 ,
- Danyang Li 1 , 3 , 4 ,
- Xinyu Yue 1 , 3 , 4 ,
- Duoxu Ji 1 ,
- Dongyang Zhang 2 ,
- Rui Dong 2 ,
- Weiwei Zou 1 , 3 , 4 ,
- Dan Liang 1 , 3 , 4 ,
- Yajing Liu 1 , 3 , 4 ,
- Yinan Du 6 ,
- Zhiguo Zhang 1 , 3 , 4 ,
- Yunxia Cao 1 , 3 , 4 ,
- Chunmei Liang 1 , 2 , 3 , 4 &
- Dongmei Ji 1 , 3 , 4
Explore all metrics
Polycystic ovary syndrome (PCOS) severely affects women's fertility and accompanies serious metabolic disturbances, affecting 5%-20% of women of reproductive age globally. We previously found that exposure to toxic metals in the blood raised the risk of PCOS, but the association between exposure to toxic metals and the risk of PCOS in the follicular fluid, the microenvironment for oocyte growth and development in females, and its effect on metabolism has not been reported. This study aimed to evaluate the associations between the concentrations of cadmium (Cd), mercury (Hg), barium (Ba) and arsenic (As) in FF and the risk of PCOS, and to explore the mediating effect of metabolic markers in FF on the above relationship. We conducted a case–control study, including 557 women with PCOS and 651 controls. Ba, Cd, Hg and As levels in FF were measured by ICP-MS, metabolites levels in FF was measured by LC–MS/MS among 168 participants randomly selected from all the participants. Logistic regression models were used to assess the association of a single metal level with the PCOS risk, and linear regression models were used to assess the relationships of a single metal level with clinical phenotype parameters and metabolites levels. Combined effect of metals mixture levels on the risk of PCOS were assessed via weighted quantile sum (WQS) regression and bayesian kernel machine regression (BKMR). Medication analysis was performed to explore the role of metabolic markers on the relationship of toxic metals levels with the risk of PCOS. The exposure levels of Cd, Hg, Ba and As in FF were all positively and significantly associated with the PCOS risk (with respect to the highest vs. lowest tertile group: OR = 1.57, 95% CI = 1.17 ~ 2.12 for Cd, OR = 1.69, 95% CI = 1.22 ~ 2.34 for Hg, OR = 1.76, 95% CI = 1.32 ~ 2.34 for Ba, OR = 1.42, 95% CI = 1.05 ~ 1.91 for As). In addition, levels of metal mixture also significantly correlated with the risk of PCOS, Cd level contributed most to it. Moreover, we observed significant positive relationships between Cd level and LH (β = 0.048, 95% CI = 0.002 ~ 0.094), T (β = 0.077, 95% CI = 0.029 ~ 0.125) and HOMA-IR value (β = 0.060, 95% CI = 0.012 ~ 0.107), as well as Hg level with LH, FSH/LH ratio and TC. Furthermore, we revealed that estrone sulfate, LysoPE 22:6 and N-Undecanoylglycine were significantly and positively mediating the association between Cd level and the risk of PCOS (with mediated proportion of 0.39, 0.24 and 0.35, respectively), and between Hg level and the risk of PCOS (with mediated proportion of 0.29, 0.20 and 0.46, respectively). These highly expressed metabolites significantly enriched in the fatty acid oxidation, steroid hormone biosynthesis and glycerophospholipids metabolism, which may explain the reason why the levels of Cd and Hg in FF associated with the phenotype of PCOS. Ba and As in FF was not found the above phenomenon. Our results suggested that exposure to multiple toxic metals (Cd, Hg, Ba and As) in FF associated with the increased risk of PCOS, Cd was a major contributor. Levels of Cd and Hg in FF significantly associated with the phenotype of PCOS. The above association may result from that Cd and Hg in FF related with the disturbance of fatty acid oxidation, steroid hormone biosynthesis and the glycerophospholipids metabolism.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Blood trace element concentrations in polycystic ovary syndrome: systematic review and meta-analysis.


Assessment Between Follicular Fluid Multiple Element Concentrations and Hormonal Profiles of Women with Polycystic Ovary Syndrome

Association of Serum Heavy Metals and Trace Element Concentrations with Reproductive Hormone Levels and Polycystic Ovary Syndrome in a Chinese Population
Data availability.
No datasets were generated or analysed during the current study.
Abbreviations
Polycystic ovary syndrome
Follicular fluid
Weighted quantile sum
Bayesian kernel machine regression
Luteinizing hormone
Fistula-stimulating hormone
Assisted reproductive technology
Body mass index
Limits of detection
Quality control
Geometric means
Restricted cubic spline
Confidence interval
Serum sex hormones
Progesterone
Testosterone
Homeostasis model assessment of insulin resistance
Homeostasis model assessment of β-cell function
Triglyceride
Total cholesterol
High-density lipoprotein
Low-density lipoprotein.
Tricarboxylic acid cycle
Variable Important for the Projection
Standard deviation
Carson SA, Kallen AN (2021) Diagnosis and management of infertility: a review. JAMA 326(1):65–76. https://doi.org/10.1001/jama.2021.4788
Article CAS PubMed PubMed Central Google Scholar
Kazemi M, Kim JY, Wan C et al (2022) Comparison of dietary and physical activity behaviors in women with and without polycystic ovary syndrome: a systematic review and meta-analysis of 39 471 women. Hum Reprod Update 28(6):910–955. https://doi.org/10.1093/humupd/dmac023
Article PubMed PubMed Central Google Scholar
Sadeghi HM, Adeli I, Calina D et al (2022) Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J MolSci 23(2). https://doi.org/10.3390/ijms23020583
Bloom MS, Fujimoto VY, Steuerwald AJ et al (2012) Background exposure to toxic metals in women adversely influences pregnancy during in vitro fertilization (IVF). Reprod Toxicol 34(3):471–481. https://doi.org/10.1016/j.reprotox.2012.06.002
Article CAS PubMed Google Scholar
Rattan S, Zhou C, Chiang C et al (2017) Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 233(3):R109-r129. https://doi.org/10.1530/joe-17-0023
Zhou C, Zhang X, Chen Y et al (2019) Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ Pollut 255(Pt 1):113194. https://doi.org/10.1016/j.envpol.2019.113194
Merlo E, Schereider IRG, Simões MR et al (2019) Mercury leads to features of polycystic ovary syndrome in rats. Toxicol Lett 312:45–54. https://doi.org/10.1016/j.toxlet.2019.05.006
Kravchenko J, Darrah TH, Miller RK et al (2014) A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health 36(4):797–814. https://doi.org/10.1007/s10653-014-9622-7
Chen L, Zhao Y, Liu F et al (2022) Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med 20(1):207. https://doi.org/10.1186/s12916-022-02403-3
Chen P, Luo Q, Lin Y et al (2022) Arsenic exposure during juvenile and puberty significantly affected reproductive system development of female SD rats. Ecotoxicol Environ Saf 242:113857. https://doi.org/10.1016/j.ecoenv.2022.113857
Chen Y, Sun Y, Zhao A et al (2022) Arsenic exposure diminishes ovarian follicular reserve and induces abnormal steroidogenesis by DNA methylation. Ecotoxicol Environ Saf 241:113816. https://doi.org/10.1016/j.ecoenv.2022.113816
Basuino L, Silveira CF Jr (2016) Human follicular fluid and effects on reproduction. JBRA Assist Reprod 20(1):38–40. https://doi.org/10.5935/1518-0557.20160009
Article PubMed Google Scholar
Qiao J, Feng HL (2011) Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 17(1):17–33. https://doi.org/10.1093/humupd/dmq032
Brinca AT, Ramalhinho AC, Sousa  et al (2022) Follicular fluid: a powerful tool for the understanding and diagnosis of polycystic ovary syndrome. Biomedicines 10(6). https://doi.org/10.3390/biomedicines10061254
Engström KS, Vahter M, Johansson G et al (2010) Chronic exposure to cadmium and arsenic strongly influences concentrations of 8-oxo-7,8-dihydro-2’-deoxyguanosine in urine. Free Radic Biol Med 48(9):1211–1217. https://doi.org/10.1016/j.freeradbiomed.2010.02.004
Sun F, Pan XF, Hu Y et al (2023) Metal exposure during early pregnancy and risk of gestational diabetes mellitus: mixture effect and mediation by phospholipid fatty acids. Environ Sci Technol 57(37):13778–13792. https://doi.org/10.1021/acs.est.3c04065
Zhao S, Yang X, Xu Q et al (2023) Association of maternal metals exposure, metabolites and birth outcomes in newborns: A prospective cohort study. Environ Int 179:108183. https://doi.org/10.1016/j.envint.2023.108183
(2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1): 41-47. https://doi.org/10.1093/humrep/deh098
Shen L, Liang C, Li D et al (2023) The association between exposure to multiple toxic metals and the risk of endometriosis: Evidence from the results of blood and follicular fluid. Sci Total Environ 855:158882. https://doi.org/10.1016/j.scitotenv.2022.158882
Li X, Huang Y, Xing Y et al (2020) Association of urinary cadmium, circulating fatty acids, and risk of gestational diabetes mellitus: A nested case-control study in China. Environ Int 137:105527. https://doi.org/10.1016/j.envint.2020.105527
Liang C, Zhang Z, Cao Y et al (2022) Exposure to multiple toxic metals and polycystic ovary syndrome risk: Endocrine disrupting effect from As, Pb and Ba. Sci Total Environ 849:157780. https://doi.org/10.1016/j.scitotenv.2022.157780
Carrico C, Gennings C, Wheeler DC et al (2015) Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120. https://doi.org/10.1007/s13253-014-0180-3
Bobb JF, Valeri L, Claus Henn B et al (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508. https://doi.org/10.1093/biostatistics/kxu058
Gleason KM, Valeri L, Shankar AH et al (2020) Stunting and lead: using causal mediation analysis to better understand how environmental lead exposure affects cognitive outcomes in children. J Neurodev Disord 12(1):39. https://doi.org/10.1186/s11689-020-09346-x
Joham AE, Norman RJ, Stener-Victorin E et al (2022) Polycystic ovary syndrome. Lancet Diabetes Endocrinol 10(9):668–680. https://doi.org/10.1016/s2213-8587(22)00163-2
Al Wattar BH, Teede H, Garad R et al (2020) Harmonising research outcomes for polycystic ovary syndrome: an international multi-stakeholder core outcome set. Hum Reprod 35(2):404–412. https://doi.org/10.1093/humrep/dez272
Butts CD, Bloom MS, McGough A et al (2021) Variability of essential and non-essential trace elements in the follicular fluid of women undergoing in vitro fertilization (IVF). Ecotoxicol Environ Saf 209:111733. https://doi.org/10.1016/j.ecoenv.2020.111733
Kruger PC, Bloom MS, Arnason JG et al (2012) Trace elements in human follicular fluid: development of a sensitive multielement ICP-MS method for use in biomonitoring studies. J Anal At Spectr 27(8). https://doi.org/10.1039/c2ja30053b
Ren S, Song C, Ye S et al (2022) The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci Total Environ 806(Pt 2):150322. https://doi.org/10.1016/j.scitotenv.2021.150322
Palomar A, Gonzalez-Martin R, Quiñonero A et al (2023) Bioaccumulation of non-essential trace elements detected in women's follicular fluid, urine, and plasma is associated with poor reproductive outcomes following single euploid embryo transfer: a pilot study. Int J Mol Sci 24(17). https://doi.org/10.3390/ijms241713147
Wu S, Wang M, Deng Y et al (2020) Associations of toxic and essential trace elements in serum, follicular fluid, and seminal plasma with In vitro fertilization outcomes. Ecotoxicol Environ Saf 204:110965. https://doi.org/10.1016/j.ecoenv.2020.110965
Chen H, Teng Y, Lu S et al (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512–513:143–153. https://doi.org/10.1016/j.scitotenv.2015.01.025
Dong F, Li J, Lei WL et al (2020) Chronic cadmium exposure causes oocyte meiotic arrest by disrupting spindle assembly checkpoint and maturation promoting factor. Reprod Toxicol 96:141–149. https://doi.org/10.1016/j.reprotox.2020.06.009
Qu J, Wang Q, Sun X et al (2022) The environment and female reproduction: Potential mechanism of cadmium poisoning to the growth and development of ovarian follicle. Ecotoxicol Environ Saf 244:114029. https://doi.org/10.1016/j.ecoenv.2022.114029
Abudawood M, Tabassum H, Alanazi AH et al (2021) Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci Rep 11(1):22935. https://doi.org/10.1038/s41598-021-02120-6
Kirmizi DA, Baser E, Turksoy VA et al (2020) Are heavy metal exposure and trace element levels related to metabolic and endocrine problems in polycystic ovary syndrome? Biol Trace Elem Res 198(1):77–86. https://doi.org/10.1007/s12011-020-02220-w
Margetaki K, Vafeiadi M, Kampouri M et al (2021) Associations of exposure to cadmium, antimony, lead and their mixture with gestational thyroid homeostasis. Environ Pollut 289:117905. https://doi.org/10.1016/j.envpol.2021.117905
Hong H, Xu J, He H et al (2022) Cadmium perturbed metabolomic signature in pancreatic beta cells correlates with disturbed metabolite profile in human urine. Environ Int 161:107139. https://doi.org/10.1016/j.envint.2022.107139
da Costa CS, Oliveira TF, Freitas-Lima LC et al (2021) Subacute cadmium exposure disrupts the hypothalamic-pituitary-gonadal axis, leading to polycystic ovarian syndrome and premature ovarian failure features in female rats. Environ Pollut 269:116154. https://doi.org/10.1016/j.envpol.2020.116154
Barregård L, Lindstedt G, Schütz A et al (1994) Endocrine function in mercury exposed chloralkali workers. Occup Environ Med 51(8):536–540. https://doi.org/10.1136/oem.51.8.536
Cho HW, Kim SH, Park MJ (2020) An association of blood mercury levels and hypercholesterolemia among Korean adolescents. Sci Total Environ 709:135965. https://doi.org/10.1016/j.scitotenv.2019.135965
Zhang C, Xu L, Zhao Y et al (2022) Changes in serum heavy metals in polycystic ovary syndrome and their association with endocrine, lipid-metabolism, inflammatory characteristics and pregnancy outcomes. Reprod Toxicol 111:20–26. https://doi.org/10.1016/j.reprotox.2022.05.002
Ma Y, Gong YJ, Xu QQ et al (2018) Molecular mechanism of mercuric chloride inhibiting progesterone secretion in ovarian granulosa cells of laying hens. J Anim Physiol Anim Nutr (Berl) 102(6):1533–1542. https://doi.org/10.1111/jpn.12955
Jiang T, Hu Y, He S et al (2022) Exposure to multiple toxic metals and the risk of early embryonic arrest among women undergoing assisted reproductive techniques. Environ Res 211:113072. https://doi.org/10.1016/j.envres.2022.113072
Vergara-Gerónimo CA, León Del Río A, Rodríguez-Dorantes M et al (2021) Arsenic-protein interactions as a mechanism of arsenic toxicity. Toxicol Appl Pharmacol 431:115738. https://doi.org/10.1016/j.taap.2021.115738
Hwang S, Hood RB, Hauser R et al (2022) Using follicular fluid metabolomics to investigate the association between air pollution and oocyte quality. Environ Int 169:107552. https://doi.org/10.1016/j.envint.2022.107552
Jia C, Xu H, Xu Y et al (2019) Serum metabolomics analysis of patients with polycystic ovary syndrome by mass spectrometry. Mol Reprod Dev 86(3):292–297. https://doi.org/10.1002/mrd.23104
Qi X, Yun C, Sun L et al (2019) Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 25(8):1225–1233. https://doi.org/10.1038/s41591-019-0509-0
Yoost JL, Ruley M, Smith K et al (2022) Diagnostic value of bile acids and fibroblast growth factor 21 in women with polycystic ovary syndrome. Womens Health Rep (New Rochelle) 3(1):803–812. https://doi.org/10.1089/whr.2022.0060
Sanchez TR, Hu X, Zhao J et al (2021) An atlas of metallome and metabolome interactions and associations with incident diabetes in the Strong Heart Family Study. Environ Int 157:106810. https://doi.org/10.1016/j.envint.2021.106810
Sun J, Fang R, Wang H et al (2022) A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ Int 158:106941. https://doi.org/10.1016/j.envint.2021.106941
Chen X, Lu T, Wang X et al (2020) Metabolic alterations associated with polycystic ovary syndrome: A UPLC Q-Exactive based metabolomic study. Clin Chim Acta 502:280–286. https://doi.org/10.1016/j.cca.2019.11.016
Sriraman V, Modi SR, Bodenburg Y et al (2008) Identification of ERK and JNK as signaling mediators on protein kinase C activation in cultured granulosa cells. Mol Cell Endocrinol 294(1–2):52–60. https://doi.org/10.1016/j.mce.2008.07.011
Li C, Wang B, Lu X et al (2022) Maternal exposure to cadmium from puberty through lactation induces abnormal reproductive development in female offspring. Ecotoxicol Environ Saf 242:113927. https://doi.org/10.1016/j.ecoenv.2022.113927
Yu L, Liu M, Wang Z et al (2021) Correlation between steroid levels in follicular fluid and hormone synthesis related substances in its exosomes and embryo quality in patients with polycystic ovary syndrome. Reprod Biol Endocrinol 19(1):74. https://doi.org/10.1186/s12958-021-00749-6
Yang ZY, Ye M, Xing YX et al (2022) Changes in the mitochondria-related nuclear gene expression profile during human oocyte maturation by the IVM technique. Cells 11(2). https://doi.org/10.3390/cells11020297
Download references
Acknowledgements
The authors are grateful to the healthcare professionals at the First Afflicted Hospital of Anhui Medical University, for enrolling subjects and sampling follicular fluid. The authors are also grateful to the Scientific Research Center in Preventive Medicine, School of Public Health, Anhui Medical University for the technical contribution of our experiments.
This work was supported by the National Natural Science Foundation of China (NSFC-81971455, NSFC-82173532, NSFC-U20A20350), the National Key Research and Development Program of China (2021YFC2700901), the Excellent Youth Program for Research in Universities of Anhui Education Department (2022AH020072) and the Key Project of Scientific Research Fund of Anhui Institute of Translational Medicine (2021zhyx-B12), Distinguished Young Scholar Project of Anhui Colleges (gxgnfx2020087), the Innovation and Entrepreneurial Projects for Students of Anhui Medical University (S202210366001).
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, the First Affiliated Hospital of Anhui Medical University, No 218 Jixi Road, Hefei, 230022, Anhui, China
Xin Wang, Ying Zhang, Jie Peng, Hua Zhang, Zhikang Zhang, Tao Yin, Xun Su, Lingchao Shen, Xiaolei Wang, Danyang Li, Xinyu Yue, Duoxu Ji, Weiwei Zou, Dan Liang, Yajing Liu, Zhiguo Zhang, Yunxia Cao, Chunmei Liang & Dongmei Ji
School of Public Health, Anhui Medical University, No 81 Meishan Road, Hefei, 230032, Anhui, China
Tingting Jiang, Shitao He, Dongyang Zhang, Rui Dong & Chunmei Liang
NHC Key Laboratory of Study On Abnormal Gametes and Reproductive Tract (Anhui Medical University), No 81 Meishan Road, Hefei, 230032, Anhui, China
Xin Wang, Ying Zhang, Jie Peng, Hua Zhang, Zhikang Zhang, Tao Yin, Xun Su, Lingchao Shen, Xiaolei Wang, Danyang Li, Xinyu Yue, Weiwei Zou, Dan Liang, Yajing Liu, Zhiguo Zhang, Yunxia Cao, Chunmei Liang & Dongmei Ji
Key Laboratory of Population Health Across Life Cycle (Anhui Medical University), Ministry of Education of the People’s Republic of China, No 81 Meishan Road, Hefei, 230032, Anhui, China
Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China
School of Basic Medical Sciences, Anhui Medical University, No 81 Meishan Road, Hefei, 230032, Anhui, China
You can also search for this author in PubMed Google Scholar
Contributions
Xin Wang: Writing-original draft, Investigation, Visualization. Ying Zhang: Formal analysis, Investigation. Jie Peng: Resources. Hua Zhang: Software. Tingting Jiang: Methodology. Zhikang Zhang: Data curation, Validation. Tao Yin: Methodology, Formal analysis. Xun Su: Data curation. Tao Zhang : Software. Lingchao Shen: Investigation. Shitao He: Methodology. Xiaolei Wang: Resources. Danyang Li : Software. Xinyu Yue : Formal analysis. Duoxu Ji: Resources. Dongyang Zhang: Visualization. Rui Dong : Methodology. Weiwei Zou: Conceptualization. Dan Liang: Conceptualization. Yajing Liu: Supervision. Yinan Du: Project administration, Zhiguo Zhang : Supervision. Yunxia Cao: Project administration, Supervision, Funding acquisition. Chunmei Liang: Funding acquisition, Supervision, Writing – review & editing. Dongmei Ji: Funding acquisition, Methodology, Writing – review & editing. All authors have read and approved the final version of the manuscript.
Corresponding authors
Correspondence to Yunxia Cao , Chunmei Liang or Dongmei Ji .
Ethics declarations
Ethics approval and consent to participate.
The study was approved granted approval from the Bioethics Committee at the First Affiliated Hospital, Anhui Medical University (No. PJ20220723). All patients received a full explanation of the controlled ovarian hyperstimulation regimen and provided written signed informed consent. Moreover, all donor patients of follicular fluid signed the written informed consent.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Wang, Ying Zhang, Jie Peng and Hua Zhang are the equal first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 2178 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Wang, X., Zhang, Y., Peng, J. et al. Association Between Exposure to Multiple Toxic Metals in Follicular Fluid and the Risk of PCOS Among Infertile Women: The Mediating Effect of Metabolic Markers. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04236-y
Download citation
Received : 21 March 2024
Accepted : 14 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1007/s12011-024-04236-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metals exposure
- Joint effect
- Metabolic marker
- Mediation analysis
- Find a journal
- Publish with us
- Track your research
This paper is in the following e-collection/theme issue:
Published on 23.5.2024 in Vol 8 (2024)
Barriers to Implementing Registered Nurse–Driven Clinical Decision Support for Antibiotic Stewardship: Retrospective Case Study
Authors of this article:

Original Paper
- Elizabeth R Stevens 1 , MPH, PhD ;
- Lynn Xu 1 , MPH ;
- JaeEun Kwon 1 , MPP ;
- Sumaiya Tasneem 1 , MPH ;
- Natalie Henning 1 , MPH ;
- Dawn Feldthouse 1 , RN-BC, MSN ;
- Eun Ji Kim 2 , MSc, MS, MD ;
- Rachel Hess 3, 4 , MS, MD ;
- Katherine L Dauber-Decker 2 , PhD ;
- Paul D Smith 5 , MD ;
- Wendy Halm 6, 7 , DNP ;
- Pranisha Gautam-Goyal 2 , MD ;
- David A Feldstein 6 , MD ;
- Devin M Mann 1, 8 , MS, MD
1 Department of Population Health, New York University Grossman School of Medicine, New York, NY, United States
2 Northwell, New Hyde Park, NY, United States
3 Department of Population Health Sciences, University of Utah, Salt Lake City, UT, United States
4 Department of Internal Medicine, University of Utah, Salt Lake City, UT, United States
5 Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
6 Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
7 University of Wisconsin-Madison School of Nursing, Madison, WI, United States
8 Department of Medicine, New York University Langone, New York, NY, United States
Corresponding Author:
Elizabeth R Stevens, MPH, PhD
Department of Population Health
New York University Grossman School of Medicine
180 Madison Ave
New York, NY, 10016
United States
Phone: 1 6465012558
Email: [email protected]
Background: Up to 50% of antibiotic prescriptions for upper respiratory infections (URIs) are inappropriate. Clinical decision support (CDS) systems to mitigate unnecessary antibiotic prescriptions have been implemented into electronic health records, but their use by providers has been limited.
Objective: As a delegation protocol, we adapted a validated electronic health record–integrated clinical prediction rule (iCPR) CDS-based intervention for registered nurses (RNs), consisting of triage to identify patients with low-acuity URI followed by CDS-guided RN visits. It was implemented in February 2022 as a randomized controlled stepped-wedge trial in 43 primary and urgent care practices within 4 academic health systems in New York, Wisconsin, and Utah. While issues were pragmatically addressed as they arose, a systematic assessment of the barriers to implementation is needed to better understand and address these barriers.
Methods: We performed a retrospective case study, collecting quantitative and qualitative data regarding clinical workflows and triage-template use from expert interviews, study surveys, routine check-ins with practice personnel, and chart reviews over the first year of implementation of the iCPR intervention. Guided by the updated CFIR (Consolidated Framework for Implementation Research), we characterized the initial barriers to implementing a URI iCPR intervention for RNs in ambulatory care. CFIR constructs were coded as missing, neutral, weak, or strong implementation factors.
Results: Barriers were identified within all implementation domains. The strongest barriers were found in the outer setting, with those factors trickling down to impact the inner setting. Local conditions driven by COVID-19 served as one of the strongest barriers, impacting attitudes among practice staff and ultimately contributing to a work infrastructure characterized by staff changes, RN shortages and turnover, and competing responsibilities. Policies and laws regarding scope of practice of RNs varied by state and institutional application of those laws, with some allowing more clinical autonomy for RNs. This necessitated different study procedures at each study site to meet practice requirements, increasing innovation complexity. Similarly, institutional policies led to varying levels of compatibility with existing triage, rooming, and documentation workflows. These workflow conflicts were compounded by limited available resources, as well as an implementation climate of optional participation, few participation incentives, and thus low relative priority compared to other clinical duties.
Conclusions: Both between and within health care systems, significant variability existed in workflows for patient intake and triage. Even in a relatively straightforward clinical workflow, workflow and cultural differences appreciably impacted intervention adoption. Takeaways from this study can be applied to other RN delegation protocol implementations of new and innovative CDS tools within existing workflows to support integration and improve uptake. When implementing a system-wide clinical care intervention, considerations must be made for variability in culture and workflows at the state, health system, practice, and individual levels.
Trial Registration: ClinicalTrials.gov NCT04255303; https://clinicaltrials.gov/ct2/show/NCT04255303
Introduction
Antibiotic resistance is a major public health risk, with more than 35,000 deaths each year in the United States due to antibiotic-resistant bacterial infections [ 1 , 2 ]. Overprescribing and misuse of antibiotics for upper respiratory infections (URIs) remain the most significant combined factors causing antibiotic resistance [ 3 , 4 ]. In the United States, up to 50% of all outpatient antibiotic prescriptions for URIs are inappropriate [ 5 , 6 ].
An estimated 80%-90% of antibiotic prescribing occurs in outpatient settings, such as doctors’ offices, urgent care facilities, and emergency departments [ 7 - 9 ]. From 1996 to 2010, 72% of adult patients in primary care with a diagnosis of acute bronchitis received antibiotics contrary to guideline recommendations against antibiotic treatment, and prescription rates actually increased during this time frame [ 10 ]. Patients with sore throats received antibiotics 61% of the time when the prevalence of group A streptococcus, the only clear indication for antibiotics, was only 10% in adults [ 11 ].
By providing real-time evidence-based data to assist providers (physicians, nurse practitioners, and physician assistants) in estimating the likelihood of a patient having either pneumococcal pneumonia or group A streptococcus, electronic health record (EHR)–integrated clinical prediction rules (iCPRs) can help address prescriber-level barriers to antibiotic stewardship and reduce antibiotic prescribing for URIs in primary care [ 12 - 15 ]. Indeed, CPRs have already been validated to successfully distinguish between viral and bacterial respiratory infections [ 16 - 18 ].
While potentially effective, there is low uptake of the iCPR tools among physicians in primary care practices, thus indicating implementation barriers to antibiotic stewardship iCPRs among physicians [ 19 ]. This outcome is consistent with other literature, indicating that physicians perceive antibiotic stewardship as onerous and would require substantial assistance to change their antibiotic prescribing behaviors [ 20 ]. Due to these limitations associated with the physician-driven iCPR implementation model, such as “alert fatigue” and time constraints [ 21 , 22 ], the iCPR intervention was adapted so antibiotic stewardship tasks could be delegated to other qualified members of the medical team.
A registered nurse (RN)–driven implementation model of iCPR for low-acuity URIs has the potential to be an effective alternative to the physician-driven implementation model. RNs have demonstrated equivalent symptom resolution compared to physicians when using protocols to improve ambulatory care across a number of chronic diseases [ 23 ] as well as the treatment of acute minor illnesses [ 24 , 25 ]. Therefore, the iCPR intervention was adapted for RNs to include the identification of patients with low-acuity URI followed by clinical decision support (CDS)–guided RN visits. The intervention was implemented in February 2022 as a stepped-wedge trial in primary and urgent care practices within 4 academic health systems in New York, Wisconsin, and Utah [ 26 ].
Despite a seemingly straightforward URI clinical workflow, the RN-driven iCPR intervention encountered significant barriers early on during implementation. While these issues were pragmatically addressed as they arose during study implementation, a systematic assessment of the barriers to implementation is needed to better understand and address these barriers. The CFIR (Consolidated Framework for Implementation Research) [ 27 ] has been widely used to guide the systematic assessment of multilevel implementation contexts to identify contextual determinants of implementation success [ 28 ]. Using the updated CFIR as a guide [ 29 ], we sought to identify and categorize the barriers experienced during the implementation of the RN-driven antibiotic stewardship “iCPR3” intervention.
We performed a retrospective case study, collecting quantitative and qualitative data from expert interviews, study surveys, routine check-ins with practice personnel, and chart reviews over the first year of implementation of the iCPR3 intervention. We used the updated CFIR [ 29 ] to characterize the initial barriers to adapting a URI iCPR intervention for RNs in ambulatory care.
Ethical Considerations
The study protocol and procedures were approved by the NYU Langone Health institutional review board, which served as the study’s single institutional review board (NYULH Study: i19-01222). Informed consent was received from all participants. Documentation of consent was waived for this study. All study data reported in this manuscript are deidentified. Compensation was not provided for participation.
Study Intervention
The study intervention consists of triage followed by an in-person iCPR–guided RN visit for patients with low-acuity URIs ( Figure 1 ). RNs perform telephone triage (or in urgent care, an RN or medical assistant performs a similar assessment through a rooming protocol) for patients reporting cough or sore throat symptoms to assess acuity, need for primary care, urgent care, or ED visit, and appropriateness for an in-person iCPR–guided RN visit. In the urgent care setting, the assessment is dichotomous as either a need for a provider visit or appropriateness for an iCPR-guided RN visit. The triage tool consists of a prepopulated note template integrated into the EHR system designed to document patient symptoms and their severity and determine the most appropriate level of care. Triage algorithms were based on institutional triage resources for decisions about ED or urgent care visits, primary care visits, and home care [ 30 ].
Patients triaged as low acuity and appropriate for an RN visit are invited for an in-person RN visit that replaces the standard of care provider visit. During the RN visit, guided by iCPR tools, the RN evaluates a patient to determine their risk of bacterial infections of strep pharyngitis (sore throat) or pneumonia (cough). Prepopulated note template EHR tools lead RNs through a focused history and physical examination. Once an RN completes the patient history and physical examination, they use an iCPR tool specific to cough or sore throat to calculate the risk of bacterial infection based on the patient’s vitals, symptoms, and pertinent history [ 26 ]. The iCPR tools are informed by the CPRs [ 17 , 18 , 31 ] used in the iCPR1 and iCPR2 studies [ 12 , 19 ], which were validated in prior studies among patients with acute respiratory illnesses [ 17 , 18 , 31 ]. The CPRs are integrated into the EHR, and upon completion of the calculator, the level of risk with an approximate probability of having either strep pharyngitis or pneumonia is displayed. After completion of the risk calculator, the RN is linked by the EHR to an order set specific to the level of risk, along with relevant patient education.

Setting and Participants
The iCPR3 intervention study was implemented in February 2022, as a randomized controlled stepped-wedge trial, in 43 primary and urgent care practices associated with 4 academic medical centers including 2 in New York, 1 in Wisconsin, and 1 in Utah. To be eligible for participation, a practice must include general internal medicine, family medicine, or urgent care practices. Furthermore, practices must have at least 1 RN full-time equivalent capable of performing triage within the EHR and in-person RN visits.
For this case study, purposive sampling was used to select experts with key knowledge and insight on study implementation from members of the research team and study practices. This sample included research study team members engaged in the implementation of the iCPR3 study (ie, research coordinators, research assistants, and investigators) and study practice personnel (ie, RNs, RN or practice managers, and providers) from each study site (academic medical center). At least 2 research study team members per site participated in semistructured interviews, with those experts determining which practice personnel to include in their data collection. All RNs participating in the iCPR3 intervention were included in study acceptability surveys and routine implementation check-ins with study staff.
Of note, approximately 8 months into iCPR3 implementation, 1 New York–based study site withdrew from the intervention study due to limited practice recruitment and insurmountable barriers to implementing the intervention. Interviews were still performed with site personnel and their comments are included in these analyses.
Data Collection
A semistructured interview guide containing questions based on the 5 domains of CFIR [ 27 , 29 ] was developed. The CFIR constructs supported the research team in defining topics for the interviews and ensured that all major domains in the framework that influence implementation were addressed. Interview questions did not explicitly name or ask participants to name the CFIR domains or constructs. The interviews were performed via in-depth email interviews [ 32 ], in which research study team interviewees were asked questions to identify which of the 48 CFIR constructs were perceived as current barriers to iCPR3 implementation and provided detailed descriptions of the identified barriers and strategies that have already been used by the iCPR3 research study team.
Surveys and Routine Check-Ins
The perspectives of practice personnel were incorporated into the case study based on notes from surveys; individual interviews; or written feedback from RNs, providers, and RN and practice managers collected over the implementation period as routine intervention study procedures. As this was a pragmatic study, study staff routinely elicited informal feedback from practice personnel throughout intervention implementation to identify barriers and improve intervention implementation.
At 6 and 12 months post-RN visit implementation, participating RNs completed a short survey that asked about burnout, job satisfaction, and comfort levels with performing tasks related to treating patients reporting cough and sore throat. The survey also collected information on ease of use of the EHR tools as well as feedback on elements of the intervention, such as training, and recommendations.
Chart Review
Clinical workflows and EHR note templates (triage and RN visits) use in the first 12 months of implementation were collected via chart review. A subset of EHR template uses initiated was evaluated for appropriateness and completeness. To determine the total number of potential patients in a practice eligible for triage template use, patients with visits resulting in a diagnosis code for cough or sore throat were documented ( International Classification of Diseases-10 [ ICD-10 ] codes: R05, R07.0, J20.9, J06.9, and J18.9). The EHR records related to the visit were reviewed to determine patient eligibility for triage and document the workflow leading to the patient visit (ie, how the appointment was scheduled, by whom, and whether appointment notes were present).
CFIR Domains and Constructs
The CFIR was used to retrospectively describe the implementation process of the iCPR3 intervention to identify determinants in this process. Only the determinants relevant to the iCPR3 intervention implementation process were described. The CFIR is composed of 48 constructs sorted into 5 major domains including innovation, outer setting, inner setting, individuals, and implementation process [ 27 , 29 ]. Operationalization of CFIR domains for this study are shown in Table 1 .
a iCPR3: integrated clinical prediction rule 3.
b RN: registered nurse.
Data Coding and Analysis
Insights gathered from the surveys, chart reviews, and formal and informal check-ins with study practice personnel helped inform research study team members’ responses to the semistructured interview guide. The written responses and notes collected from the email interviews were analyzed using techniques of qualitative content analysis, inspired by a deductive-directed approach, deemed applicable because the data were analyzed in light of an existing framework [ 33 ]. The analysis was performed by 3 authors (ERS, LX, and JK) in a stepwise interactive process. The first step in the analysis, after reading all transcripts, notes, and written responses to obtain an understanding of the whole, was to develop initial coding nodes and subnodes based on the domains and constructs of the CFIR [ 29 ].
In the second step, units of analysis, such as sentences or sections of thought, were deductively coded into the nodes and subnodes. Third, the coded text was rated based on the recommended method described by the authors of CFIR, Damschroder and Lowery [ 34 ]. In the rating process, a consensus process was used to assign a rating to each construct obtained from each study site. The ratings reflected the positive or negative influence and the strength of each construct that emerged based on the coded text. When all constructs obtained from all study sites were rated, we compared and compiled ratings for each construct across study sites. Constructs were coded as missing, not distinguishing between positive or negative implementation factors (0), or weakly (+1/–1), or strongly (+2/–2) distinguishing low from high implementation factors ( Table 2 ).
a CFIR: Consolidated Framework for Implementation Research.
b iCPR3: integrated clinical prediction rule intervention.
Barriers and facilitators to implementation were identified within the CFIR domains and constructs and are presented within the frame of CFIR domains including innovation, outer and inner settings, individuals, and implementation process ( Table 3 ).
c Construct lettering and numbers correspond with Damschroder et al [ 27 ].
d Only constructs applicable to the iCPR implementation are cited.
e –2: strong negative influence; –1: weak negative influence; 0: neutral influence; 0 (mix): mixed positive and negative influences, which balanced each other; +1: weak positive influence; +2: strong positive influence; missing: not asked or miscoded.
Outer Setting
Local conditions , primarily driven by the COVID-19 pandemic, served as one of the strongest barriers to implementation as COVID-19 impacted nearly every aspect of implementation from changes in workflows and staffing availability to patient volume and URI care protocols. There were observed changes to URI care protocols including shifts from in-office care to telehealth and redirection to urgent care, driven by COVID-19–testing requirements and hesitancy from both patients and practices to have on-site care. Furthermore, COVID-19 affected local attitudes among practice staff as health issues and burnout led to staff shortages, turnover, and shifting of responsibilities. These barriers were further compounded by regional nursing shortages and financial incentives that drew RNs out of primary care practices.
Policies and laws, such as state regulatory laws and institutional policies, also had a strong impact on the study procedures and implementation. RN scope-of-practice varied by state and between institutions. Wisconsin has existing RN delegation protocols, allowing for more clinical autonomy among RNs than at institutions in New York and Utah. This required additional training and modification to the RN visit portion of the intervention at institutions, where RNs had a more limited scope of practice and could not function autonomously. For example, the New York sites were required to adopt a “co-visit” structure to ensure that providers could oversee RN visits. This created additional scheduling constraints and complexity, as well as an unanticipated burden for providers. Multimedia Appendix 1 shows the analysis of performance measurement pressure and the innovation construct.
Inner Setting
Within the construct of structural characteristics , work infrastructure served as a strong barrier to the intervention implementation as practices across institutions experienced staff changes, RN shortages and turnover, and competing responsibilities that all hindered their ability to effectively participate in the study. Notably, at practices with only 1 RN, implementation was negatively impacted as clinic participation was dependent on 1 individual, whereas at other practices, study responsibilities were distributed across multiple RNs. Within the culture construct, a norm of limited deliverer centeredness , related to the prioritization of the needs and desires of RNs, served as a barrier to the implementation of this RN-focused intervention. As patient ( recipient centeredness ) and provider preferences were prioritized over RN activities, the innovation activities that would have been performed by the RNs were overridden. For example, to ensure patient autonomy, if a patient preferred to see a provider, they were not scheduled for an RN visit even if they were eligible. Similarly, at most institutions (except those with more RN autonomy), RNs tended to defer to providers in terms of preference and final decisions. Therefore, if the provider preferred seeing a patient themselves, the patient, even if eligible for an RN visit, would not be seen by an RN.
Overall, relational connections , specifically the RN-provider dynamic, negatively affected implementation. RNs in the study did not always have open bidirectional communication with providers, thus limiting the self-efficacy of RNs to explain or justify intervention-related activities. As observed within the culture construct, many practices had limited deliverer centeredness , typically deferring to providers to make final decisions, and therefore RNs were hesitant to push these boundaries or make decisions that were contrary to a provider’s preferences. In particular, some sites mentioned some practices having poor relationships among practice staff, even requiring team-building training in some instances. On the other hand, this was less of a barrier at practices, where RNs had more clinical autonomy or had developed stronger relationships within the practice.
Communications culture within practices served as a barrier to effectively implementing aspects of the study; for example, some practices did not have a culture of communicating with patients prior to visits in the form of triage or lacked formalized documentation as information was often conveyed informally (eg, verbal, secure chat message, and free-text note). In some practices, a strong communication system between RNs (ie, a chat channel used by most RNs) served as a facilitator to innovation implementation by allowing RNs to support and answer each other’s questions.
The intervention’s compatibility , or lack thereof, with existing workflows was a strong barrier to implementation, as the necessary intervention-specific workflow adaptions required great effort on the part of the practice if not already in place (eg, front desk forwarding eligible patients for triage, RNs performing triage after appointments had been scheduled, and filling out EHR note templates as opposed to free text). As the new study workflow required changes to the status quo, tension for change also served as a barrier since practices perceived little anticipated benefit from the study as compared to the difficulty of change. Relatedly, relative priority of the intervention was a strong barrier as competing clinical responsibilities and the voluntary nature of the study meant staff would not prioritize study-related tasks.
Overall, there was a lack of incentive systems in place related to study activities, which hindered RN participation. While gift card incentives for RNs performing triage were used, these tended to incentivize the same RNs already using the tools as opposed to encouraging new RNs to participate. Additionally, at institutions where RNs were unable to bill for visits and did not receive any other recognition for their efforts, this lack of incentives was a strong barrier to participation. One institution was able to reduce the influence of this barrier by providing incentives to RNs through continuing education credits, an employee recognition fund, and paid time for training.
Multimedia Appendix 1 presents the analyses of physical infrastructure, IT infrastructure, access to knowledge and information, available resources, learning-centeredness, and mission alignment.
Individuals: Characteristics Subdomain
Both capability and motivation were barriers to implementation. As these tools were new to many of the participating RNs, they were less confident in their skills and required continuous feedback, training, and support. In addition, RNs were not motivated to participate in the study largely due to competing priorities, lack of a strong incentive, and COVID-19–related stress and burnout. Opportunity was also a strong barrier, as RNs did not have many opportunities to use the innovation tools. Conflicting responsibilities, staff shortages, workflow barriers, patient volume, and patient eligibility were observed as contributors to this barrier.
Multimedia Appendix 1 shows the analyses of roles subdomain constructs high-level leaders, mid-level leaders, opinion leaders, innovation deliverers, innovation recipients, implementation facilitators, implementation leaders, and implementation team members. Multimedia Appendix 1 shows the analyses of the implementation process domain constructs assessing context and assessing needs, innovation deliverers, doing, planning and tailoring strategies, teaming, engaging the innovation deliverers, reflecting and evaluating, and adapting.
Principal Findings
This case study identified numerous barriers to the successful implementation of iCPR3, an RN-driven antibiotic stewardship intervention. Many of the identified barriers are consistent with those observed in other interventions that sought to alter nursing responsibilities and workflows within primary care [ 35 , 36 ]. The most impactful barriers were noted within the outer setting, and these conditions were observed to influence the inner setting constructs. The effects of COVID-19 served as an overarching barrier that impacted nearly all implementation constructs, shifting the culture and conditions at many participating practices as well as decreasing the capacity of practices to engage in activities perceived as nonessential. These barriers, however, were less prevalent within clinics that had previously established workflows with patient care within the RN role description. Takeaways from this study can be applied to support integration and improve uptake during the implementation of other RN delegation protocols involving CDS tools into existing workflows.
Policies impacting innovation deliverers’ (RNs) clinical autonomy at both the state and institutional levels need to be considered when developing RN delegation protocols as they can impact implementation depending on compatibility with existing workflows. As a multisite study with implementation spanning 3 states, the differing state regulatory laws and institutional policies dictating RN scope-of-practice had a substantial impact on the compatibility of the iCPR3 implementation at each site. This was evident in the higher rate of RN visits occurring in practices in Wisconsin compared to New York. At the Wisconsin study site, there were established delegation protocols for RNs to see patients with minimal provider supervision. In contrast, for the 2 New York study sites, a more complex “covisit” design was developed, which involved joint scheduling of the iCPR3 RN visit followed immediately by a visit for the provider to see the patient and confirm the RN plan of care. The addition of a provider visit component increased the intervention’s dependency on already limited provider availability, thus inhibiting the ability to schedule the iCPR3 RN visits even when a patient was appropriate and willing and an RN was available to conduct the visit. As observed in other RN delegation protocols, considerations for local regulations must be made when assessing the viability of implementing these types of interventions [ 36 ].
Consideration of practice-level culture and work infrastructure is also essential for the successful implementation of an intervention that includes RN delegation protocols. This implementation study revealed impactful differences in existing workflow expectations that affected RN capability and intervention complexity. One unexpected barrier was the influence of practice personnel who were part of the local workflow but were not directly involved in the implementation of the iCPR tools. For example, at one institution, successful implementation of the intervention was reliant on administrative staff to forward patients reporting cough and sore throat to participating RNs for triage. Implementation planning with greater efforts to clarify practice-level workflows, identifying potential stakeholders early on, and engaging these personnel who ultimately support the innovation deliverers can support a successful implementation.
Similarly, when delegating provider tasks to RNs, it is important to secure provider buy-in early on in the implementation process, even with a seemingly RN-focused intervention. Consistent with previous research demonstrating the importance of RN-provider relationships in job satisfaction [ 37 ], this study showed that power dynamics between providers and RNs can serve as a barrier to RN intervention engagement. With a culture of deference to providers, many RNs did not want to overstep these boundaries and would not engage with the intervention if there was any perceived resistance from practice providers. Barriers experienced due to this power structure were further compounded when poor relations existed between RNs and providers. Furthermore, as seen in other clinical academic partnerships, future implementation efforts would benefit from more active engagement of leadership at all levels [ 38 ].
Future clinical delegation interventions may also need to consider alternate care mechanisms to account for unexpected shifts in clinic workflows. Due to the timing of the implementation, one of the largest observed barriers to implementation was the COVID-19 pandemic, which amplified nearly all other barriers and created additional unique challenges. As an intervention specifically designed for in-person care, the shift toward telemedicine driven by the pandemic [ 39 ] had a particularly negative impact on implementation. One study institution piloted a program to divert all patients with URI to telemedicine visits with a centrally employed nurse practitioner, which bypassed all potential points of intervention for the iCPR study. Further diverting potentially eligible patients away from primary care practices was the increased popularity of urgent care centers [ 40 ], which served as an expedient solution for patients with URI seeking to avoid long wait times at many primary care practices. Incorporating alternate care mechanisms to provide agility in the intervention may support the success of study implementation. Similarly, integrating CDS tools with existing EHR tools and templates can help minimize changes in workflow, thereby allowing interventions to be resilient in the face of unforeseen events.
As observed in this case study, the pandemic also directly impacted practice staff and their ability to participate in activities beyond the essential, including research. Practices across all study institutions experienced nursing staff shortages due to RNs themselves being sick, covering for others who are sick, or leaving the practice altogether, thus resulting in a redistribution of responsibilities. An increased workload, along with outside stressors, led to increased reported stress and burnout among practice staff [ 41 ], making it difficult for them to view the study as a daily priority. The voluntary nature of the study and these conflicting responsibilities greatly reduced the opportunity for RNs to use the innovation and participate fully. This was particularly evident in practices that required greater workflow modifications. Practices with existing expectations of note documentation and template use facilitated implementation; however, in other practices, the lack of RN familiarity with these EHR functions required the creation of additional training and workflow modification efforts, as well as a greater perceived effort burden on the part of RNs.
Future implementation should consider the value of face-to-face communication in encouraging engagement and team building during the implementation process [ 42 ]. In addition to its impact at the institutional and practice level, the effects of COVID-19 hindered the implementation process itself, especially early in the planning phase by limiting in-person interactions and creating communication barriers [ 43 ]. With nearly all communication occurring remotely, interactions to collect practice workflow information and engage stakeholders were perceived as less efficient, requiring additional follow-up meetings and hindering the development of relationships of the study team with leadership and innovation deliverers. When in-person practice visits by the research team became feasible, an improvement in practice responsiveness and innovation uptake was observed [ 42 ].
This study had several limitations. First, the use of an emailed in-depth interview hindered the study team’s ability to probe respondents for further information at the moment, potentially limiting the collection of further details that may have impacted the interpretation of interview responses. However, the emailed format increased the feasibility of conducting a long interview and created an opportunity for study sites to compile perspectives from multiple team members, thus improving the richness of information provided. Second, the reported barriers and facilitators were self-reported and not directly observed and are therefore based on the perceptions of the study site research teams. Similarly, as the data collection was primarily retrospective, it may be subject to recall bias. We attempted to mitigate this by conducting semistructured interviews during the implementation process. Finally, this analysis was performed prior to the completion of implementation at all sites and analysis of the primary intervention effectiveness outcomes. Therefore, it was not possible to link perceived implementation constructs to intervention outcome measures, and additional implementation construct influences may have been missed.
Conclusions
Both between and within health care systems, significant variability exists in workflows for patient intake and triage. Even in a relatively straightforward clinical workflow, seemingly nuanced workflow and culture differences appreciably impacted successful intervention adoption. Barriers to intervention adoption existed within multiple constructs and domains. When implementing a system-wide clinical care intervention, stakeholders should consider the variability in workflow policy and culture at the health system, practice, and individual levels, as well as create accommodations for changing care patterns.
Acknowledgments
This work was funded by NIAID 5R01AI108680 (PI Mann). Generative AI was not used in the writing of this manuscript.
Data Availability
The data sets used and analyzed during this study contain personally identifiable information and are therefore not made publicly available. Data are available from the corresponding author upon reasonable request.
Authors' Contributions
ERS wrote the original draft and contributed to conceptualization, methodology, data collection, and analysis; LX contributed to writing the original draft, methodology, data collection, and analysis; JK contributed to writing the original draft, methodology, data collection, and analysis; ST contributed to data collection and project administration; NH contributed to data collection and writing editing; DF contributed to data collection and writing editing; EJK contributed to data collection and writing editing; RH contributed to data collection and writing editing; KLD-D contributed to data collection and writing editing; PDS contributed to data collection and writing editing; WH contributed to data collection and writing editing; PG-G contributed to data collection and writing editing; DAF contributed to the conceptualization, funding acquisition, and writing editing; DMM contributed to the conceptualization, funding acquisition, and writing editing. All authors reviewed and approved the final manuscript draft.
Conflicts of Interest
None declared.
Consolidated Framework for Implementation Research domain barrier analysis results.
- Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention. 2019. URL: https://www.hhs.gov/sites/default/files/michael-craig-cdc-talk-thursday-am-508.pdf [accessed 2024-04-10]
- Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903-3910. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894-896. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nadimpalli ML, Chan CW, Doron S. Antibiotic resistance: a call to action to prevent the next epidemic of inequality. Nat Med. 2021;27(2):187-188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Havers F, Thaker S, Clippard JR, Jackson M, McLean HQ, Gaglani M, et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012-2013 influenza season. Clin Infect Dis. 2014;59(6):774-782. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Duffy E, Ritchie S, Metcalfe S, Van Bakel B, Thomas MG. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther. 2018;43(1):59-64. [ CrossRef ] [ Medline ]
- Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Swedres-Svarm. URL: https://www.sva.se/media/jzdlctnk/rapport_swedres-svarm_2018.pdf [accessed 2024-04-10]
- Hopkins S, Muller-Pebody B, Guy R, Gerver S, Ironmonger D, Puleston R, et al. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2010 to 2014: Report 2015. UK National Archives. 2015. URL: https://webarchive.nationalarchives.gov.uk/ukgwa/20181130125613/https://www.gov.uk/government/publications/english-survei llance-programme-antimicrobial-utilisation-and-resistance-espaur-report [accessed 2024-04-10]
- Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med. 2014;174(1):138-140. [ FREE Full text ] [ CrossRef ] [ Medline ]
- McGinn TG, McCullagh L, Kannry J, Knaus M, Sofianou A, Wisnivesky JP, et al. Efficacy of an evidence-based clinical decision support in primary care practices: a randomized clinical trial. JAMA Intern Med. 2013;173(17):1584-1591. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Linder JA, Schnipper JL, Tsurikova R, Yu T, Volk LA, Melnikas AJ, et al. Documentation-based clinical decision support to improve antibiotic prescribing for acute respiratory infections in primary care: a cluster randomised controlled trial. Inform Prim Care. 2009;17(4):231-240. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gonzales R, Anderer T, McCulloch CE, Maselli JH, Bloom FJ, Graf TR, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Keller SC, Tamma PD, Cosgrove SE, Miller MA, Sateia H, Szymczak J, et al. Ambulatory antibiotic stewardship through a human factors engineering approach: a systematic review. J Am Board Fam Med. 2018;31(3):417-430. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fine AM, Nizet V, Mandl KD. Large-scale validation of the centor and McIsaac scores to predict group a streptococcal pharyngitis. Arch Intern Med. 2012;172(11):847-852. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Heckerling PS, Tape TG, Wigton RS, Hissong KK, Leikin JB, Ornato JP, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113(9):664-670. [ CrossRef ] [ Medline ]
- McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158(1):75-83. [ FREE Full text ] [ Medline ]
- Mann D, Hess R, McGinn T, Richardson S, Jones S, Palmisano J, et al. Impact of clinical decision support on antibiotic prescribing for acute respiratory infections: a cluster randomized implementation trial. J Gen Intern Med. 2020;35(Suppl 2):788-795. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Zetts RM, Garcia AM, Doctor JN, Gerber JS, Linder JA, Hyun DY. Primary care physicians' attitudes and perceptions towards antibiotic resistance and antibiotic stewardship: a national survey. Open Forum Infect Dis. 2020;7(7):ofaa244. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Phansalkar S, van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489-493. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R, et al. HITEC Investigators. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shaw RJ, McDuffie JR, Hendrix CC, Edie A, Lindsey-Davis L, Williams JW. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions. Department of Veterans Affairs. 2013. URL: https://www.care innovations.org/wp-content/uploads/Nurse-Managed-Protocols-in-Adults-with-Chronic-Disease_VA.pdf [accessed 2024-04-10]
- Iglesias B, Ramos F, Serrano B, Fàbregas M, Sánchez C, García MJ, et al. A randomized controlled trial of nurses vs. doctors in the resolution of acute disease of low complexity in primary care. J Adv Nurs. 2013;69(11):2446-2457. [ CrossRef ] [ Medline ]
- Shum C, Humphreys A, Wheeler D, Cochrane MA, Skoda S, Clement S. Nurse management of patients with minor illnesses in general practice: multicentre, randomised controlled trial. BMJ. 2000;320(7241):1038-1043. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stevens ER, Agbakoba R, Mann DM, Hess R, Richardson SI, McGinn T, et al. Reducing prescribing of antibiotics for acute respiratory infections using a frontline nurse-led EHR-integrated clinical decision support tool: protocol for a stepped wedge randomized control trial. BMC Med Inform Decis Mak. 2023;23(1):260. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2016;11:72. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Briggs JK. Telephone Triage Protocols for Nurses, Fifth Edition. Philadelphia, PA. Wolters Kluwer; 2015.
- Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239-246. [ CrossRef ] [ Medline ]
- Fritz RL, Vandermause R. Data collection via in-depth email interviewing: lessons from the field. Qual Health Res. 2018;28(10):1640-1649. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. [ CrossRef ] [ Medline ]
- Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the Consolidated Framework for Implementation Research (CFIR). Implement Sci. 2013;8:51. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Karimi-Shahanjarini A, Shakibazadeh E, Rashidian A, Hajimiri K, Glenton C, Noyes J, et al. Barriers and facilitators to the implementation of doctor-nurse substitution strategies in primary care: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2019;4(4):CD010412. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Busca E, Savatteri A, Calafato TL, Mazzoleni B, Barisone M, Dal Molin A. Barriers and facilitators to the implementation of nurse's role in primary care settings: an integrative review. BMC Nurs. 2021;20(1):171. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Galletta M, Portoghese I, Carta MG, D'Aloja E, Campagna M. The effect of nurse-physician collaboration on job satisfaction, team commitment, and turnover intention in nurses. Res Nurs Health. 2016;39(5):375-385. [ CrossRef ] [ Medline ]
- Baptiste DL, Whalen M, Goodwin M. Approaches for establishing and sustaining clinical academic partnerships: a discursive review. J Clin Nurs. 2022;31(3-4):329-334. [ CrossRef ] [ Medline ]
- Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27(7):1132-1135. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ayers A. As COVID turns endemic, investors remain bullish on urgent care growth. J Urgent Care Med. 2022;16(10):35-39. [ FREE Full text ]
- Restauri N, Sheridan AD. Burnout and posttraumatic stress disorder in the coronavirus disease 2019 (COVID-19) pandemic: intersection, impact, and interventions. J Am Coll Radiol. 2020;17(7):921-926. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lederman O. Hacking innovation—group dynamics in innovation teams. MIT Libraries. 2015. URL: https://dspace.mit.edu/handle/1721.1/101790 [accessed 2024-04-10]
- Mourad M, Bousleiman S, Wapner R, Gyamfi-Bannerman C. Conducting research during the COVID-19 pandemic. Semin Perinatol. 2020;44(7):151287. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by A Mavragani; submitted 30.11.23; peer-reviewed by R Hillard; comments to author 21.02.24; revised version received 13.03.24; accepted 15.03.24; published 23.05.24.
©Elizabeth R Stevens, Lynn Xu, JaeEun Kwon, Sumaiya Tasneem, Natalie Henning, Dawn Feldthouse, Eun Ji Kim, Rachel Hess, Katherine L Dauber-Decker, Paul D Smith, Wendy Halm, Pranisha Gautam-Goyal, David A Feldstein, Devin M Mann. Originally published in JMIR Formative Research (https://formative.jmir.org), 23.05.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Formative Research, is properly cited. The complete bibliographic information, a link to the original publication on https://formative.jmir.org, as well as this copyright and license information must be included.

- Graduate Education
- Graduate Student Experience
Current Graduate Student Resources
Academic resources.
The Graduate Education Office, in collaboration with other university entities and our SOM academic programs, provides resources for academic success including: University Policies, School of Graduate Studies Policy and Program Academic Specific Resources for Postbaccalaureate, Masters and Doctoral Education.
Browse student academic resources.
Wellness Resources
A student's time in graduate school encompasses not only academics, but self-growth and establishing a community that supports and encourages development in all aspects of life. Explore our Student Experience page for: Wellness Resources, University Division of Student Affairs and the SOM Diversity, Equity and Inclusive Excellence (DEIE).
View our wellness resources for graduate students.
- India Today
- Business Today
- Reader’s Digest
- Harper's Bazaar
- Brides Today
- Cosmopolitan
- Aaj Tak Campus
- India Today Hindi
'Poor design, misleading': ICMR calls out BHU study on Covaxin's side effects
Icmr's director criticised the study about covaxin's side effects, citing its poor methodology and design, and clarified that the article misleadingly "acknowledges" india's apex medical research body..
Listen to Story

- ICMR director criticised BHU's study on Covaxin's side effects
- He said that the study misleadingly "acknowledges" ICMR
- The data collection method of the study was highly prone to bias, he added
The Indian Council of Medical Research (ICMR), the country's apex body of medical research, has called out Banaras Hindu University (BHU) for releasing a paper on the long-term side effects of Covaxin.
Dr Rajiv Bahl, Director General of ICMR, criticised the study, citing its poor methodology and design, and clarified that the article misleadingly "acknowledges" ICMR.
The director of the medical research body has also written to the authors of the study and the editor of the journal in which it was published about incorrectly mentioning ICMR, even though the body did not offer any financial or technical support for the paper .
Dr Bahl pointed out that the study lacked a control group of unvaccinated individuals, which is crucial for comparing the rates of adverse events between vaccinated and unvaccinated groups.
Therefore, the reported events in the study cannot be attributed to the Covid-19 vaccination .

He highlighted several critical flaws in the study, which undermine its credibility.
He noted that the study failed to provide background rates of observed events in the population, making it impossible to assess changes in the incidence of events post-vaccination.
The baseline information of study participants was missing.
As per ICMR, the study tool used was inconsistent with the definition of AESI provided in the reference, and the data collection method was highly prone to bias.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Res Notes

The clinical case report: a review of its merits and limitations
Trygve nissen.
1 Department of Clinical Medicine, University of Tromsø, N-9038 Tromsø, Norway
2 Division of General Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
3 Division of Addictions and Specialized Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. Some scholars point to its limited value for medical progress, while others assert that the genre is undervalued. We aimed to present the various points of view regarding the merits and limitations of the case report genre. We searched Google Scholar, PubMed and select textbooks on epidemiology and medical research for articles and book-chapters discussing the merits and limitations of clinical case reports and case series.
The major merits of case reporting were these: Detecting novelties, generating hypotheses, pharmacovigilance, high applicability when other research designs are not possible to carry out, allowing emphasis on the narrative aspect (in-depth understanding), and educational value. The major limitations were: Lack of ability to generalize, no possibility to establish cause-effect relationship, danger of over-interpretation, publication bias, retrospective design, and distraction of reader when focusing on the unusual.
Conclusions
Despite having lost its central role in medical literature in the 20th century, the genre still appears popular. It is a valuable part of the various research methods, especially since it complements other approaches. Furthermore, it also contributes in areas of medicine that are not specifically research-related, e.g. as an educational tool. Revision of the case report genre has been attempted in order to integrate the biomedical model with the narrative approach, but without significant success. The future prospects of the case report could possibly be in new applications of the genre, i.e. exclusive case report databases available online, and open access for clinicians and researchers.
Throughout history the clinical case report and case report series have been integral components of medical literature [ 1 ]. The case report genre held a strong position until it was sidelined in the second half of the 20 th century [ 2 , 3 ]. New methodologies for research articles paved the way for evidence-based medicine. Editors had to make space for these research articles and at the same time signaled less enthusiasm for publishing case reports [ 4 ]. This spurred some heated debates in medical journals as readers were worried that the traditional case report was in jeopardy [ 5 , 6 ]. Those who welcomed the new trend with fewer case reports being published pointed mainly to their low quality and inclination to emphasize mere curiosa [ 7 - 9 ]. Some of the proponents of the genre claimed that the case report had been and still was indispensible for furthering medical knowledge and that it was unique in taking care of the detailed study of the individual patient as opposed to the new research methods with their “…nomothetic approach [taking] precedence…” [ 5 ]. Still, the case report got a low ranking on the evidence hierarchy. After a decline in popularity a new interest for the case report emerged, probably beginning in the late 1990s [ 2 ]. A peer-reviewed ‘Case reports’ section was introduced in the Lancet in 1995 [ 10 ]. In 2007, the first international, Pubmed-listed medical journal publishing only case reports was established [ 11 , 12 ]. In the following years, several similar journals, for the most part online and open-access, have been launched.
The present debate is not so much focused on whether case reporting is obsolete or not. Some of the discussions after the turn of the century have been about adapting the case report genre to new challenges. One example is the suggestion of incorporating the narrative, i.e. “… stressing the patient’s story”, in the case report [ 13 ]. The authors termed their initiative “The storied case report”. Their endeavor was not met with success. In analyzing the causes for this, they wondered if “… junior trainees find it too hard to determine what is relevant and senior trainees find it too hard to change their habits” [ 13 ]. A similar attempt was done when the editors of the Journal of Medical Case Reports in 2012 encouraged authors to include the patients’ perspectives by letting patients describe their own experiences [ 14 ].
Notwithstanding, we feel there is much to be gained from having an ongoing discussion highlighting the indications and contraindications for producing case reports. This can to some degree be facilitated by getting an understanding of the merits and limitations of the genre. The objective of this article is to present the merits and limitations of case reports and case series reports.
We adopted Taber’s Cyclopedic Medical Dictionary’s definition of the case report : “A formal summary of a unique patient and his or her illness, including the presenting signs and symptoms, diagnostic studies, treatment course and outcome” [ 15 ]. A case report consists of one or two cases, most often only one. The case series or case series report usually consists of three to ten cases [ 16 ]. (In the following we use the term case report to denote both case reports and case series report). Case reports are most often naturalistic and descriptive. Sometimes, however, they can be prospective and experimental.
As literature specifically dealing with the case report genre seemed harder to elicit from the databases than the vast amount of particular case reports, we performed iterative searches. We searched Google Scholar and PubMed using the search terms ‘case report(s)’, ‘case series’, ‘case series report(s)’, ‘case reporting’ in various combinations with ‘clinical’, ‘medical’, ‘anecdotal’, ‘methodology’, ‘review’, ‘overview’, ‘strengths’, ‘weaknesses’, ‘merits’, and ‘limitations’. Further references were identified by examining the literature found in the electronic searches. We also consulted major textbooks on epidemiology [ 17 , 18 ], some scholars of medical genres [ 19 , 20 ] and a monograph on case reporting by the epidemiologist M. Jenicek [ 16 ]. We delimited our review to the retrospective, naturalistic, and descriptive case report, also labeled the “traditional” or “classic” case report, and case series including such reports. Thus we excluded other types, such as the planned, qualitative case study approach [ 21 ] and simulated cases [ 22 - 24 ]. Finally, we extracted the relevant data and grouped the merits and limitations items in rank order with the items we judged to be the most important first.
New observations
The major advantage of case reporting is probably its ability to detect novelties [ 16 ]. It is the only way to present unusual, uncontrolled observations regarding symptoms, clinical findings, course of illness, complications of interventions, associations of diseases, side effects of drugs, etc. In short, anything that is rare or has never been observed previously might be important for the medical community and ought to be published. A case report might sensitize readers and thus facilitate detection of similar or identical cases.
Generating hypotheses
From a single, or preferably several single case reports or a case series, new hypotheses could be formulated. These could then be tested with formal research methods that are designed to refute or confirm the hypotheses, i.e. comparative (observational and experimental) studies.
There are numerous examples of new discoveries or major advancements in medicine that started with a case report or, in some cases, as humbly as a letter to the editor. The first concern from the medical community about the devastating side effect of thalidomide, i.e. the congenital abnormalities, appeared as a letter to the editor in the Lancet in 1961 [ 25 ]. Soon thereafter, several case reports and case series reports were published in various journals. Case reporting is thus indispensable in drug safety surveillance (pharmacovigilance) [ 26 ].
Sometimes significant advancements in knowledge have come not from what researchers were pursuing, but from “accidental discoveries”, i.e. by serendipity. The story of Alexander Fleming’s discovery of penicillin in 1928 is well known in the medical field [ 27 ]. Psychiatry has profited to a large degree from this mode of advancing medical science as many of the drugs used for mental disorders have been discovered serendipitously [ 27 ]. One notable example is the discovery of the effect of lithium on manic episodes in patients with manic-depressive disorder [ 28 ]. A more recent discovery is the successful treatment of infantile hemangiomas with systemic propranolol. This discovery was published, as a case series report, in the correspondence section in New England Journal of Medicine [ 29 ]. However, the evidence for the effect of this treatment is still preliminary, and several randomized trials are under way [ 30 , 31 ].
Clear and operational entities are prerequisites for doing medical research. Descriptions must come before understanding. Clinical observations that lead to new disorders being described are well suited for case reporting. The medical literature is replete with case-based articles describing new diseases and syndromes. One notable example is the first description of neurasthenia by G. Beard in Boston Medical and Surgical Journal in 1869 [ 32 ].
Researching rare disorders
For rare disorders randomized controlled trials (RCTs) can be impossible to run due to lack of patients to be enrolled. Research on drug treatment and other kinds of interventions must therefore be based on less rigorous methodologies, among them case series and case reports. This would be in accordance with the European Commission’s recommendation to its members to improve health care for those with rare disorders [ 33 ].
Solving ethical constraints
Case reporting can be valuable when ethical constraints prohibit experimental research. Take as an example the challenge of how to manage the side effects of accidental extravasation of cytotoxic drugs. As RCTs on humans seem unethical in this clinical situation the current guidelines rest on small observational studies, case reports and animal studies [ 34 ]. Or another example: Physical restraint is sometimes associated with sudden, unexpected death. The cause or causes for this are to some degree enigmatic, and it is hard to conceive of a controlled study that could be ethical [ 35 , 36 ]. Case reports and case series being “natural experiments” might be the only evidence available for guiding clinical practice.
In-depth narrative case studies
Case reporting can be a way of presenting research with an idiographic emphasis. As contrasted to nomothetic research, an idiographic approach aims at in-depth understanding of human phenomena, especially in the field of psychology and psychiatry. The objective is not generalizable knowledge, but an understanding of meaning and intentionality for an individual or individuals. Sigmund Freud’s case studies are relevant examples. This usage of case reports borders on qualitative research. Qualitative studies, although developed in the social sciences, have become a welcome contribution within health sciences in the last two decades.
Educational value
Clinical medical learning is to a large degree case-based. Typical case histories and vignettes are often presented in textbooks, in lectures, etc. Unusual observations presented as published case reports are important as part of doctors’ continuing medical education, especially as they demonstrate the diversity of manifestations both within and between medical diseases and syndromes [ 37 , 38 ]. Among the various medical texts, the case report is the only one that presents day-to-day clinical practice, clinicians’ diagnostic reasoning, disease management, and follow-up. We believe that some case reports that are written with the aim of contributing to medical knowledge turn out to be of most value educationally because the phenomena have already been described elsewhere. Other case reports are clearly primarily written for educational value [ 37 ]. Some journals have regular sections dedicated to educational case reports, e.g. The Case Records of the Massachusetts General Hospital in the New England Journal of Medicine and the Clinical Case Conference found in the American Journal of Psychiatry.
The cost of doing a case report is low compared to planned, formal studies. Most often the necessary work is probably done in the clinical setting without specific funding. Larger studies, for instance RCTs, will usually need an academic setting.
Fast publication
The time span from observation to publication can be much shorter than for other kinds of studies. This is obviously a great advantage as a case report can be an important alert to the medical community about a serious event. The unexpected side effects of the sedative-antinauseant thalidomide on newborn babies is a telling story. The drug had been prescribed during pregnancy to the babies’ mothers. After the first published observation of severe abnormalities in babies appeared as a letter to the editor of the Lancet in December 16 th , 1961 [ 25 ], several case reports and series followed [ 39 , 40 ]. It should be mentioned though that the drug company had announced on December 2 nd , 1961, i.e. two weeks before the letter from McBride [ 25 ], that it would withdraw the drug form the market immediately [ 41 ].
Flexible structure
Riaz Agha, editor of the International Journal of Surgery Case Reports suggests that the case report, with its less rigid structure is useful as it “… allows the surgeon(s) to discuss their diagnostic approach, the context, background, decision-making, reasoning and outcomes” [ 42 ]. Although the editor is commenting on the surgical case report, the argument can be applied for the whole field of clinical medicine. It should be mentioned though, that other commentators have argued for a more standardized, in effect more rigid, structure [ 43 ].
Clinical practice can be changed
Case reporting can lead to or contribute to a change in clinical practice. A drug might be withdrawn from the market. Or a relabeling might change the attitude to and treatment of a condition. During Word War I the shell shock syndrome was labeled and described thoroughly in several articles in the Lancet , the first of them appearing in February 1915 [ 44 ]. The author was the British captain and military doctor Charles S. Myers. Before his efforts to bring good care and treatment to afflicted soldiers there had been a common misconception that many of these dysfunctional soldiers were malingerers or cowards.
Exercise for novice researchers
The case report format is well suited for young doctors not yet trained as researchers. It can be an opportunity for a first exercise in authoring an article and a preparation for a scientific career [ 37 , 45 , 46 ].
Communication between the clinical and academic fields
Articles authored by clinicians can promote communication between practicing clinicians and academic researchers. Observations published can generate ideas and be a trigger for further studies. For instance, a case series consisting of several similar cases in a short period can make up the case-group for a case–control study [ 47 ]. Clinicians could do the observation and publish the case series while the case–control study could be left to the academics.
Entertainment
Some commentators find reading case reports fun. Although a rather weak argument in favor of case reporting, the value of being entertained should not be dismissed altogether. It might inspire physicians to spend more time browsing and reading scientific literature [ 48 ].
Studying the history of medicine
Finally, we present a note on a different and unintended aspect of the genre. The accumulated case reports from past eras are a rich resource for researching and understanding medical history [ 49 , 50 ]. A close study of old case reports can provide valuable information about how medicine has been practiced through the centuries [ 50 , 51 ].
Limitations
No epidemiological quantities.
As case reports are not chosen from representative population samples they cannot generate information on rates, ratios, incidences or prevalences. The case or cases being the numerator in the equation, has no denominator. However, if a case series report consists of a cluster of cases, it can signal an important and possibly causal association, e.g. an epidemic or a side effect of a newly marketed drug.
Causal inference not possible
Causality cannot be inferred from an uncontrolled observation. An association does not imply a cause-effect relationship. The observation or event in question could be a mere coincidence. This is a limitation shared by all the descriptive studies [ 47 ]. Take the thalidomide tragedy already mentioned as an example; Unusual events such as congenital malformations in some of the children born to mothers having taken a specific drug during pregnancy does not prove that the drug is the culprit. It is a mere hypothesis until further studies have either rejected or confirmed it. Cause-effect relationships require planned studies including control groups that to the extent possible control for chance, bias and confounders [ 52 ].
Generalization not possible
From the argument above, it follows that findings from case reports cannot be generalized. In order to generalize we need both a cause-effect relationship and a representative population for which the findings are valid. A single case report has neither. A case series, on the other hand, e.g. many “thalidomide babies” in a short time period, could strengthen the suspicion of a causal relationship, demanding further surveillance and research.
Publication bias could be a limiting factor. Journals in general favor positive-outcome findings [ 53 ]. One group of investigators studying case reports published in the Lancet found that only 5% of case reports and 10% of case series reported treatment failures [ 54 ]. A study of 435 case reports from the field of dentistry found that in 99.1%, the reports “…clearly [had] a positive outcome and the intervention was considered and described as successful by the authors” [ 55 ].
Overinterpretation
Overinterpretation or misinterpretation is the tendency or temptation to generalize when there is no justification for it. It has also been labeled “the anecdotal fallacy” [ 56 ]. This is not a shortcoming intrinsic to the method itself. Overinterpretation may be due to the phenomenon of case reports often having an emotional appeal on readers. The story implicitly makes a claim to truth. The reader might conclude prematurely that there is a causal connection. The phenomenon might be more clearly illustrated by the impact of the clinician’s load of personal cases on his or her practice. Here exemplified by a young doctor’s confession: “I often tell residents and medical students, ‘The only thing that actually changes practice is adverse anecdote.’” [ 57 ].
Emphasis on the rare
As case reporting often deals with the rare and atypical, it might divert the readers’ attention from common diseases and problems [ 58 ].
Confidentiality
Journals today require written informed consent from patients before publishing case reports. Both authors and publishers are responsible for securing confidentiality. A guarantee for full confidentiality is not always possible. Despite all possible measures taken to preserve confidentiality, sometimes the patient will be recognized by someone. This information should be given to the patient. An adequately informed patient might not consent to publication. In 1995 in an Editorial in the British Journal of Psychiatry one commentator, Isaac Marks, feared that written consent would discourage case reports being written [ 59 ]. Fortunately, judged form the large number of reports being published today, it seems unlikely that the demand for consent has impeded their publication.
Other methodological limitations
Case reports and series are written after the relevant event, i.e. the observation. Thus, the reports are produced retrospectively. The medical record might not contain all relevant data. Recall bias might prevent us from getting the necessary information from the patient or other informants such as family members and health professionals.
It has also been held against case reporting that it is subjective. The observer’s subjectivity might bias the quality and interpretation of the observation (i.e. information bias).
Finally, the falsification criterion within science, which is tested by repeating an experiment, cannot be applied for case reports. We cannot design another identical and uncontrolled observation. However, unplanned similar “experiments” of nature can be repeated. Several such observations can constitute a case series that represents stronger indicative evidence than the single case report.
The major advantages of case reporting are the ability to make new observations, generate hypotheses, accumulate scientific data about rare disorders, do in-depth narrative studies, and serve as a major educational tool. The method is deficient mainly in being unable to deliver quantitative data. Nor can it prove cause-effect relationship or allow generalizations. Furthermore, there is a risk of overinterpretation and publication bias.
The traditional case report does not fit easily into the qualitative-quantitative dichotomy of research methods. It certainly shares some characteristics with qualitative research [ 16 ], especially with regard to the idiographic, narrative perspective – the patient’s “interior world” [ 60 ] – that sometimes is attended to. Apart from “The storied case report” mentioned in the Background-section, other innovative modifications of the traditional case report have been tried: the “evidence-based case report” [ 61 ], the “interactive case report” [ 62 ] and the “integrated narrative and evidence based case report” [ 63 ]. These modifications of the format have not made a lasting impact on the way case reports in general are written today.
The method of case reporting is briefly dealt with in some textbooks on epidemiology [ 17 , 18 ]. Journals that welcome case reports often put more emphasis on style and design than on content in their ‘instruction to authors’ section [ 64 ]. As a consequence, Sorinola and coworkers argue for more consensus and more consistent guidance on writing case reports [ 64 ]. We feel that a satisfactory amount of guidance concerning both style and content now exists [ 12 , 16 , 65 , 66 ]. The latest contribution, “The CARE guidelines”, is an ambitious endeavor to improve completeness and transparency of reports [ 66 ]. These guidelines have included the “Patient perspective” as an item, apparently a bit half-heartedly as this item is placed after the Discussion section, thus not allowing this perspective to influence the Discussion and/or Conclusion section. We assume this is symptomatic of medicine’s problem with integrating the biomedical model with “narrative-based medicine”.
In recent years the medical community has taken an increased interest in case reports [ 2 ], especially after the surge of online, exclusive case report journals started in 2007 with the Journal of Medical Case Reports (which was the first international, Pubmed-listed medical journal publishing only case reports) as the first of this new brand. The climate of skepticism has been replaced by enthusiasm and demand for more case reports. A registry for case reports, Cases Database, was founded in 2012 [ 67 ]. On the condition that it succeeds in becoming a large, international database it could serve as a register being useful for clinicians at work as well as for medical research on various clinical issues. Assuming Pamela P. Powell’s assertion that “[a]lmost all practicing physicians eventually will encounter a case worthy of being reported” [ 60 ] is valid, there should be no shortage of potential cases waiting to be reported and filed in various databases, preferably online and open access.
Limitations of this review
There are several limitations to this study. It is a weakness that we have not been able to review all the relevant literature. The number of publications in some way related to case reports and case report series is enormous, and although we have attempted to identify those publications relevant for our purpose (i.e. those that describe the merits and limitations of the case report genre), we might have missed some. It was difficult to find good search terms for our objective. Still, after repeated electronic searches supplemented with manual searches in reference lists, we had a corpus of literature where essentially no new merits or limitations emerged.
As we point out above, the ranking of merits and limitations represents our subjective opinion and we acknowledge that others might rank the importance of the items differently.
The perspective on merits and limitations of case reporting has been strictly medical. As a consequence we have not analyzed or discussed the various non-medical factors affecting the publication of case reports in different medical journals [ 2 ]. For instance, case reports are cited less often than other kinds of medical research articles [ 68 ]. Thus they can lower a journal’s impact factor, potentially making the journal less attractive. This might lead some high-impact journals to publish few or no case reports, while other journals have chosen to specialize in this genre.
Before deciding on producing a case report or case series based on a particular patient or patients at hand, the observant clinician has to determine if the case report method is the appropriate article type. This review could hopefully assist in that judgment and perhaps be a stimulus to the continuing debate in the medical community on the value of case reporting.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
TN contributed to the conception, drafting, and revision of the article. RW contributed to the conception, drafting, and revision of the article. Both authors approved the final manuscript.
Acknowledgements
There was no specific funding for this study.
- Cabán-Martinez AJ, Beltrán WF. Advancing medicine one note at a time: the educational value in clinical case reports. BMC Res Notes. 2012; 5 :293. doi: 10.1186/1756-0500-5-293. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The recent history of the clinical case report: a narrative review. JRSM Sh Rep. 2012; 3 :87. doi: 10.1258/shorts.2012.012046. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The history of the case report: a selective review. JRSM Open. 2014; 5 :4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G. Fare the well – the Editor’s last words. Br J Psychiatry. 2003; 182 :465–466. doi: 10.1192/bjp.182.6.465. [ CrossRef ] [ Google Scholar ]
- Williams DDR. In defence of case of the case report (Letter) Br J Psychiatry. 2004; 184 :84. doi: 10.1192/bjp.184.1.84. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enoch MD. Case reports (Letter) Br J Psychiatry. 2005; 185 :169. [ PubMed ] [ Google Scholar ]
- Nahum AM. The clinical case report: “Pot boiler” or scientific literature? Head Neck Surg. 1979; 1 :291–292. doi: 10.1002/hed.2890010402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doherty M. What value case reports? (Editorial) Ann Rheum Dis. 1994; 53 :1–2. doi: 10.1136/ard.53.1.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Procopio M. Publication of case reports. Br J Psychiatry. 2005; 187 :91. [ PubMed ] [ Google Scholar ]
- Bignall J, Horton R. Learning from stories – Lancet’s case reports. Lancet. 1995; 346 :1256. [ PubMed ] [ Google Scholar ]
- Kidd M, Hubbard C. Introducing Journal of Medical Case Reports. J Med Case Rep. 2007; 1 :1. doi: 10.1186/1752-1947-1-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rison RA. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes. J Med Case Reports. 2013; 7 :239. doi: 10.1186/1752-1947-7-239. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayoumi AM, Kopplin PA. The storied case report. CMAJ. 2004; 171 :569–570. doi: 10.1503/cmaj.1031503. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kidd MR, Saltman D. Case reports at the vanguard of the 21 st century medicine (Editorial) J Med Case Rep. 2012; 6 :156. doi: 10.1186/1752-1947-6-156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venes D. Taber’s Cyclopedic Medical Dictionary. 21. Philadelphia: F.A. Davis Company; 2009. [ Google Scholar ]
- Jenicek M. Clinical case reporting in evidence-based medicine. Oxford: Butterworth Heinemann; 1999. [ Google Scholar ]
- Fletcher RH, Fletcher SW. Clinical epidemiology: The essentials. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [ Google Scholar ]
- Sackett DL, Brian Haynes R, Tugwell P. Clinical epidemiology. Boston: Little, Brown and Company; 1985. [ Google Scholar ]
- Berkencotter C. Patient tales. Case histories and the uses of narrative in psychiatry. Columbia: University of South Carolina Press; 2008. [ Google Scholar ]
- Hunter KM. Doctors’ stories. The narrative structure of medical knowledge. Princeton: Princeton University Press; 1991. [ Google Scholar ]
- Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011; 11 :100. doi: 10.1186/1471-2288-11-100. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Kvalvik AM, Hynnekleiv T. Attitudes to coercion at two Norwegian psychiatric units. Nord J Psychiatry. 2011; 65 :133–137. doi: 10.3109/08039488.2010.513068. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Myklebust LH, Bratlid T. Psychologists and coercion: decisions regarding involuntary psychiatric admission and treatment in a group of Norwegian psychologists. Nord J Psychiatry. 2007; 61 :433–437. doi: 10.1080/08039480701773139. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaker E, Knudsen A, Wynn R, Lund A. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001; 19 :103–106. doi: 10.1080/028134301750235330. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McBride WG. Thalidomide and congenital abnormalities. (Letter) Lancet. 1961; 278 :1358. [ Google Scholar ]
- Chakra CAN, Pariente A, Pinet M, Nkeng L, Moore N, Moride Y. Case series in drug safety. A review to determine characteristics and quality. Drug Saf. 2010; 33 :1081–1088. doi: 10.2165/11539300-000000000-00000. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006; 8 :335–344. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949; 2 :349–352. [ PubMed ] [ Google Scholar ]
- Léauté-Labrèze C, de la Roque Dumas E, Hubiche T, Boralevi F. Propranolol for severe hemangiomas of infancy. (Letter to the editor) N Engl J Med. 2008; 358 :2649–2651. doi: 10.1056/NEJMc0708819. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A. Godt nok dokumentert? (Sufficiently documented?) (Editorial) Tidsskr Nor Legeforen. 2010; 130 :1806. [ PubMed ] [ Google Scholar ]
- U.S. National Institutes of Health. Studies found for the search 'propanolol and hemangiomas' in the ClinicalTrials.gov database. Accessed on 22 April, 2014 at: http://www.clinicaltrials.gov/ct2/results?term=propanolol+and+hemangiomas&Search=Search .
- Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869; 80 :217–221. doi: 10.1056/NEJM186904290801301. [ CrossRef ] [ Google Scholar ]
- Taylor DM. Developing a strategy for the management of rare disorders. BMJ. 2012; 344 :e2417. doi: 10.1136/bmj.e2417. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bjånes TK. Lokale bivirkninger ved parenteral administrering av legemidler. (Localized side effects caused by parenteral administration of drugs) Tidsskr Nor Legeforen. 2011; 131 :472–474. [ PubMed ] [ Google Scholar ]
- Nissen T, Rørvik P, Haugslett L, Wynn R. Physical restraint and near death of a psychiatric patient. J Forensic Sci. 2013; 58 :259–262. doi: 10.1111/j.1556-4029.2012.02290.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R. Coercion in psychiatric care: clinical, legal, and ethical controversies. Int J Psychiatry Clin Pract. 2006; 10 :247–251. doi: 10.1080/13651500600650026. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lundh A, Christensen M, Jørgensen AW. International or national publication of case reports. Dan Med Bul. 2011; 58 :A4242. [ PubMed ] [ Google Scholar ]
- Grønli O, Wynn R. Normocalcemic hyperparathyroidism and treatment resistant depression. Psychosomatics. 2013; 54 :493–497. doi: 10.1016/j.psym.2012.10.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward SP. Thalidomide and congenital abnormalities. BMJ. 1962; 2 (5305):646–647. doi: 10.1136/bmj.2.5305.646. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coodin FJ, Uchida CM, Murphy CH. Phocomelia: report of three cases. CMAJ. 1962; 87 :735–739. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heyman DJ. Managing director of the Destillers Company Ltd. (Letter) Lancet. 1961; 278 :1262. [ Google Scholar ]
- Agha R, Rosin RD. Time for a new approach to case reports. (Editorial) Int J Surg Case Rep. 2010; 1 :1–3. doi: 10.1016/j.ijscr.2010.04.001. doi:10.1016/j.ijscr.2010.04.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aronson JA. Anecdotes as evidence. (Editorial) BMJ. 2003; 326 :1346. doi: 10.1136/bmj.326.7403.1346. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Myers CS. A contribution to the study of the shell shock. Being an account of three cases of loss of memory, vision, smell and taste, admitted into the Duchess of Westminster’s War Hospital, Le Touquet. Lancet. 1915; 185 :316–320. doi: 10.1016/S0140-6736(00)52916-X. [ CrossRef ] [ Google Scholar ]
- Morris BA. The importance of case reports. (Letter to the Editor) CMAJ. 1989; 141 :875–876. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rison RA. Neurology case reports: a call for all. J Med Case Rep. 2011; 5 :113. doi: 10.1186/1752-1947-5-113. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002; 359 :145–149. doi: 10.1016/S0140-6736(02)07373-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S. Anecdotes in medicine - 15 years of Lancet case reports. Lancet. 2010; 376 :1448–1449. doi: 10.1016/S0140-6736(10)60624-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rose JC, Corn M. Dr. E. and other patients: new lessons from old case reports. J Hist Med Allied Sci. 1984; 39 :3–32. doi: 10.1093/jhmas/39.1.3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. Practice versus theory: tenth-century case histories from Islamic Middle East. Soc Hist Med. 2000; 13 :293–306. doi: 10.1093/shm/13.2.293. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. The case history in Medieval Islamic medical literature: Tajarib and Mujarrabat as source. Med Hist. 2010; 54 :195–214. doi: 10.1017/S0025727300006712. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Google Scholar ]
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991; 337 :867–872. doi: 10.1016/0140-6736(91)90201-Y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albrecht J, Meves A, Bigby M. Case reports and case series from the Lancet had significant impact on medical literature. J Clin Epidemiol. 2005; 58 :1227–1232. doi: 10.1016/j.jclinepi.2005.04.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oliveira GJ, Leles CR. Critical appraisal and positive outcome bias in case reports published in Brazilian dental journals. J Dent Educ. 2006; 70 :869–874. [ PubMed ] [ Google Scholar ]
- Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice. 1998; 4 :147–155. doi: 10.1111/j.1365-2753.1998.tb00081.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stuebe AM. Level IV evidence – adverse anecdote and clinical practice. N Engl J Med. 2011; 365 :8–9. doi: 10.1056/NEJMp1102632. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffman JR. Rethinking case reports. West J Med. 1999; 170 :253–254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G, Fahy T, Russell G, Healy D, Marks I, Tantam D, Dimond B. Case reports and confidentiality. (Editorial) Br J Psychiatry. 1995; 166 :555–558. doi: 10.1192/bjp.166.5.555. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Powell PP. The single-case report in medical literature: the ‘Elephant man’ serves as an exellent example. J Am Med Writers Assoc. 1990; 5 :9–12. [ Google Scholar ]
- Glasziou P. Evidence based case report: twenty year cough in a non-smoker. BMJ. 1998; 316 :1660–1661. doi: 10.1136/bmj.316.7145.1660. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harker N, Montgomery A, Fahey T. Interactive case report. Treating nausea and vomiting during pregnancy: case outcome. BMJ. 2004; 328 :276. doi: 10.1136/bmj.328.7434.276. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reis S, Hermoni D, Livingstone P, Borkan J. Integrated narrative and evidence based case report: case report of paroxysmal atrial fibrillation and anticoagulation. BMJ. 2002; 325 :1018–1020. doi: 10.1136/bmj.325.7371.1018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorinola O, Olufowobi O, Coomarasamy A, Khan KS. Instructions to authors for case reporting are limited: a review of a core journal list. BMC Med Educ. 2004; 4 :4. doi: 10.1186/1472-6920-4-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rossor MN. In: How to write a paper. 4. Hall GM, editor. London: Blackwell Publishing; 2008. How to write a case report; pp. 71–75. [ Google Scholar ]
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. CARE group. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013; 7 :223. doi: 10.1186/1752-1947-7-223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BioMed Central. Cases Database. Accessed on 22 April, 2014 at: http://www.casesdatabase.com .
- Mason RA. The case report – an endangered species? (Editorial) Anesthesia. 2001; 56 :99–102. doi: 10.1046/j.1365-2044.2001.01919.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the ...
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...
It is best to simply tell the story and let the outcome speak for itself. With these points in mind, let's begin the process of writing the case study: Title page: Title: The title page will contain the full title of the article. Remember that many people may find our article by searching on the internet.
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we ...
The popularity of case study research methodology in Health Services Research (HSR) has grown over the past 40 years. 1 This may be attributed to a shift towards the use of implementation research and a newfound appreciation of contextual factors affecting the uptake of evidence-based interventions within diverse settings. 2 Incorporating context-specific information on the delivery and ...
Empirical case studies typically enable dynamic understanding of complex challenges and provide evidence about causal mechanisms and the necessary and sufficient conditions (contexts) for intervention implementation and effects. This is essential evidence not just for researchers concerned about internal and external validity, but also research ...
Revised on November 20, 2023. A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are ...
The aim of this article is to introduce family medicine researchers to case study research, a rigorous research methodology commonly used in the social and health sciences and only distantly related to clinical case reports. The article begins with an overview of case study in the social and health sciences, including its definition, potential applications, historical background and core ...
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
A case study is one of the most commonly used methodologies of social research. This article attempts to look into the various dimensions of a case study research strategy, the different epistemological strands which determine the particular case study type and approach adopted in the field, discusses the factors which can enhance the effectiveness of a case study research, and the debate ...
A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Abstract. Context: Case study research (CSR) is a research approach that guides holistic investigation of a real phenomenon. This approach may be useful in medical education to provide critical analyses of teaching and learning, and to reveal the underlying elements of leadership and innovation. There are variations in the definition, design ...
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years. Case #13b.
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews). The case study research method originated in clinical medicine (the case history, i.e., the patient's personal history). In psychology, case studies are ...
The Women's Health Initiative Randomized Trials and Clinical Practice. JoAnn E. Manson, MD, DrPH; et al. Review. online firsthas active quiz. Read More. View. Explore the latest in medicine including the JNC8 blood pressure guideline, sepsis and ARDS definitions, autism science, cancer screening guidelines, and.
A hospital-based case-control study was conducted among the cohort of patients admitted for respiratory pathology at the Hospital Universitario Central de Asturias-Spain (HUCA) during 2016-2019. ... The study was approved by the Hospital Board of Directors and the Clinical Research Ethics Committee of Asturias, Spain (ref. 123/19). Open Research.
Objective: To assess risk factors for persistence vs improvement and to describe clinical characteristics and diagnostic evaluation of subjects with post-acute sequelae of COVID-19/post-COVID-19 syndrome (PCS) persisting for more than one year. Design: Nested population-based case-control study. Setting: Comprehensive outpatient assessment, including neurocognitive, cardiopulmonary exercise ...
Origins of case study research. Case study is a research design that involves an intensive and holistic examination of a contemporary phenomenon in a real-life setting. 1-3 It uses a variety of methods and multiple data sources to explore, describe or explain a single case bounded in time and place (ie, an event, individual, group, organisation or programme).
Study Design and Study Participants. The workflow of our work is described as Fig. 1A. After obtaining research ethics approval, we recruited patients from the Reproductive Center of the First Affiliated Hospital of Anhui Medical University for a case-control study about PCOS.
Methods: We performed a retrospective case study, collecting quantitative and qualitative data regarding clinical workflows and triage-template use from expert interviews, study surveys, routine check-ins with practice personnel, and chart reviews over the first year of implementation of the iCPR intervention. ... Interactive Journal of Medical ...
Isaac Anaya, Student Success Specialist . Graduate Education Office Sears Tower, TG01-C 10900 Euclid Ave. Cleveland, Ohio 44106 Phone: 216.368.6052 Email: [email protected]
The clinical case report has been an integral part of medical literature throughout history. The oldest example of a preserved clinical case in medical literature is a text from an ancient Egyptian papyrus dating from the 16 th to the 17 th dynasty, 1600 BC, addressing the management of dislocated jawbone. From Hippocratic case histories ...
This approach can be done using existing data sets or new ones, leveraging the insights that a real-world dataset can provide to clinical researchers to generate new ideas for study. Real-world ...
ICMR's director criticised the study about Covaxin's side effects, citing its poor methodology and design, and clarified that the article misleadingly "acknowledges" India's apex medical research body. Listen to Story ICMR director criticised BHU's study on Covaxin's side effects He said that the ...
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. ... Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice ...