Loading metrics
Open Access
Collection Review
Collection Review articles synthesize in narrative form the best available evidence on a topic. Submission of Collection Review articles is by invitation only, and they are only published as part of a PLOS Collection as agreed in advance by the PLOS Medicine Editors.
See all article types »

The prevention and treatment of Plasmodium vivax malaria
* E-mail: [email protected]
Affiliations Shoklo Malaria Research Unit-Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Headington, Oxford, United Kingdom
Affiliations Shoklo Malaria Research Unit-Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand, Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- Cindy S. Chu,
- Nicholas J. White

Published: April 23, 2021
- https://doi.org/10.1371/journal.pmed.1003561
- Reader Comments
Citation: Chu CS, White NJ (2021) The prevention and treatment of Plasmodium vivax malaria. PLoS Med 18(4): e1003561. https://doi.org/10.1371/journal.pmed.1003561
Copyright: © 2021 Chu, White. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors received no specific funding for this work.
Competing interests: We have read the journal’s policy and the authors of this manuscript have the following competing interests: NJW is an Academic Editor on PLOS Medicine’s editorial board.
Abbreviations: ACT, artemisinin combination therapy; FST, fluorescent spot test; G6PDd, glucose-6-phosphate dehydrogenase deficiency; IPTi, intermittent presumptive treatment for infants; IPTp, intermittent presumptive treatment for pregnant women; IRS, indoor residual spraying; ITN, insecticide-treated net; LLIN, long-lasting net; PART, presumptive antirelapse therapy; PBO, piperonyl butoxide; PCR, polymerase chain reaction; PvAMA, P . vivax apical membrane antigen; PvCSP, P . vivax circumsporozoite protein; PvDBP, P . vivax Duffy binding protein; PvMSP, P . vivax merozoite surface protein; qPCR, quantitative polymerase chain reaction; RDT, rapid diagnostic test; SMC, seasonal malaria chemoprevention; uPCR, ultrasensitive PCR
Summary points
- The worldwide burden of Plasmodium vivax malaria has more than halved from an estimated 17.3 to 6.5 million cases between 2010 and 2019. This resulted from increased deployment of conventional malaria control measures (rapid diagnostic tests, effective antimalarial treatment, vector control) and significant global investment in malaria elimination. There is no generally available P . vivax vaccine, nor is there likely to be one in the near future.
- The latest-generation RDTs used for P . vivax diagnosis have sensitivities comparable to microscopy. Ultrasensitive PCR methods which can detect parasite densities as low as 28/ml have revealed a much higher prevalence of asymptomatic P . vivax infection in malaria endemic regions than previously estimated.
- Chloroquine remains an effective schizonticide for vivax malaria, except in Indonesia, Sabah and Papua New Guinea where there is high-level chloroquine resistance. Artemisinin combination therapies are effective alternative schizonticides that unify the treatment of all malarias. Relapses contribute significantly to the burden of P . vivax infections. Prevention of relapse requires “radical cure” with an 8-aminoquinoline (primaquine daily for 7 to 14 days, or single-dose tafenoquine). These drugs cause oxidant haemolysis in G6PD deficiency, so safe use requires G6PD testing. Overall, the risks of primaquine haemolysis have been overemphasised and the benefits of relapse prevention underappreciated.
- Qualitative screening tests for G6PD deficiency (detecting <30% normal enzyme activity) are adequate for screening before giving primaquine for radical cure, but a quantitative point of care G6PD test to detect <70% normal enzyme activity is needed for tafenoquine.
- Radical curative efficacy depends on the total 8-aminoquinoline dose given; higher primaquine doses (7 mg base/kg rather than 3.5 mg base/kg) are required in parts of Southeast Asia and Oceania. The currently recommended dose of tafenoquine (300 mg) is sub-optimal. In low transmission settings, elimination of vivax malaria is possible with current tools.
Introduction
Plasmodium vivax is more geographically dispersed than Plasmodium falciparum , with transmission occurring over a wider range of temperatures than for P . falciparum [ 1 ], and at latitudes as far as 64° North [ 2 ]. P . vivax is the predominant cause of human malaria in Asia and the Asia-Pacific regions where, with large populations and a declining incidence of P . falciparum infections, over 80% of the global P . vivax burden occurs [ 3 ]. P . vivax is also prevalent in the horn of Africa, Madagascar, and parts of Central and South America, but it has been eradicated from Europe, Russia, North America, and most of the Middle East [ 2 , 4 ].
Clinical management of P . vivax malaria relies on clinical suspicion, a reliable blood diagnostic, and access to efficacious effective schizonticidal (blood stage) and hypnozoiticidal (radical cure) drug regimens. Currently, P . vivax malaria is treated with chloroquine or artemisinin combination therapy (ACT) for the blood stage infection. While chloroquine has been standard treatment for vivax malaria for some 70 years, the emergence and global spread of chloroquine resistance in P . falciparum has meant that different treatments are now required for P . falciparum and the other human malarias. Use of ACTs (except artesunate-sulfadoxine-pyrimethamine) allows again a unified treatment for all malaria, and it provides a safety net in case of misdiagnosis or an unidentified mixed infection with P . falciparum . Despite declining susceptibility, chloroquine is still effective for P . vivax infection in most of the world, except for Indonesia, Sabah and Oceania where resistance is high grade [ 5 , 6 ]. Chloroquine is substantially less expensive than ACTs, although a unified treatment provides significant operational cost-savings.
Historically, P . vivax has been considered benign, although severe infections may sometimes occur [ 7 – 10 ]. Concomitant illness or chronic disease may contribute to the severity of P . vivax infection [ 11 , 12 ]. The management of severely ill patients with P . vivax malaria is similar to that of P . falciparum malaria [ 13 ]. P. vivax malaria in pregnancy is associated with miscarriage and low birth weight [ 14 , 15 ].
The great challenge for P . vivax malaria treatment is how to prevent relapses. These result from dormant liver stage parasites (hypnozoites) that activate weeks or months after the primary infection to cause a recurrent P . vivax infection (relapse). The intervals to and frequency of symptomatic relapses vary geographically and depend also on age and the intensity of transmission [ 16 – 18 ]. Frequent P . vivax infections are a major cause of morbidity in vivax malaria, particularly in young children in whom repeated relapse may cause severe anaemia, malnutrition, and growth delay [ 19 , 20 ]. Thus, an important goal of treatment is to prevent relapse. This requires radical curative treatment with an 8-aminoquinoline in addition to treatment of the blood stage infection. The 8-aminoquinoline drugs cause haemolysis in patients with glucose-6-phosphate dehydrogenase deficiency (G6PDd), and this risk of dangerous iatrogenic haemolysis has limited their use substantially. In general, the harm caused by frequent relapse, particularly in young children, has been under appreciated, whereas the risks of haemolysis have been overemphasised, with a net result that primaquine radical cure has been underused.
Diagnosis of Plasmodium vivax malaria
Microscopy and rapid diagnostic tests..
For over a century, the gold standard for diagnosing P . vivax malaria has been the microscopy observation of asexual parasites on a thick or thin blood film. Accurate microscopy requires trained laboratory staff supported with adequate laboratory supplies and well-maintained microscopes, which are not always available in resource-limited settings. The limit of detection for expert microscopy is about 50 parasites/uL which coincides approximately with the pyrogenic density [ 21 ]. The diagnostic sensitivity depends on the slide quality and the experience of the microscopist. Thus under field conditions, microscopy may miss some symptomatic cases because of the low parasite densities in P . vivax infections, but overall, it is suitably sensitive and specific for clinical diagnosis. Rapid diagnostic tests (RDTs) have been developed which detect antigens common to all malarias (e.g., aldolase) or those specific to P . vivax (e.g., Pv LDH). First-generation RDTs had lower sensitivities for P . vivax parasitaemia [ 22 ] and could give false positive results [ 23 ] with high-density P . falciparum infections. The sensitivity and specificity of P . vivax RDTs have both been improved significantly. From 2008 to 2018, the proportion of P . vivax , P . falciparum , and pan-malaria RDTs meeting the current WHO performance threshold for procurement increased from approximately 20% to 90% [ 24 ]. During that decade, over 2.3 billion RDTs have been sold [ 25 ]. Access to reliable point-of-care diagnostic tools has allowed for early diagnosis and improved tremendously the ability to treat confirmed rather than suspected P . vivax infections.
Polymerase chain reaction (PCR) detection and genotyping.
PCR allows low densities of malaria parasites to be detected reliably in blood samples. For low-volume blood samples such as filter paper blood spots, lower limits of reliable detection are typically in the range of 1 to 10 parasites/uL. In recent years, high-volume ultrasensitive PCR methods (uPCR) have been developed which amplify a larger amount of DNA, and these provide lower limits of detection around 28 parasites per mL (based on genus) with a second species-specific probe based on the 18S RNA gene [ 26 ]. These ultrasensitive methods allow detection of the majority of all infected people in endemic areas [ 27 ]. Most of these malaria-infected people are asymptomatic although, as parasite densities fluctuate over time, transmissible densities of gametocytes occur intermittently, and they are therefore a source of transmission [ 28 ]. PCR identification of infection is therefore an important component of epidemiological or clinical research assessment for submicroscopic recurrences, but it should not be used in disease diagnosis and management as it is too sensitive. That is, it will detect asymptomatic individuals who often have another reason for their febrile illness (reduced specificity).
Molecular genotyping is not needed for clinical management, although it is useful in clinical trials where, together with time-to-event information, it can help distinguish relapse from reinfection [ 29 ]. The interpretation of parasite genotyping is not as straightforward as it is for P . falciparum infections as relapses can be with genotypes similar or identical to the initial infection, or they can be with completely different genotypes resulting from heterologous hypnozoite activation [ 30 , 31 ]. Nevertheless, combining genotype comparison with time-to-event information does allow probabilistic differentiation between relapses and reinfections.
Insecticide-treated nets.
Insecticide-treated nets (ITNs) are the mainstay of malaria control in most of the malaria-endemic world. Between 2017 and 2019, over 25,000,000 ITNs were distributed annually in malaria-endemic regions outside sub-Saharan Africa [ 4 ]. The effectiveness of ITNs depends on many factors which include distribution, coverage, adherence, maintenance, vector biting patterns, and levels of insecticide resistance [ 32 ]. Unsurprisingly ITNs are less effective if the anopheline vectors bite when people are not under or near their bed nets [ 33 , 34 ]. Most of the main vectors in SE Asia exhibit exophilic crepuscular biting patterns which decrease the effectiveness of ITNs in preventing malaria. In Papua New Guinea, anopheline vectors changed their behaviour to bite earlier after the introduction of bed nets [ 35 ]. Moreover, ITNs have no direct impact against relapse, which is often the main cause of vivax malaria (and once relapse proportions exceed 50%, relapses become the main cause of P . vivax infections). Despite these limitations, ITNs do provide partial benefit and are an adjunct method to prevent primary P . vivax infections and interrupt transmission [ 36 ]. Long-lasting nets (LLINs), in which the insecticide persists for the natural life of the net, are a substantial advance. There is rising concern about increasing pyrethroid resistance. Progress against pyrethroid resistance has been made in the forms of a new pyrrole insecticide, chlorfenapyr [ 37 , 38 ], and a chemical synergist which prevents insects from detoxifying pyrethroids, piperonyl butoxide (PBO) [ 39 ].
Insecticide use.
The use of insecticides for malaria prevention focuses mainly on indoor residual spraying (IRS) and long-lasting ITNs, although there are a variety of other approaches. Organochlorides, organophosphates, carbamates, and pyrethroids are recommended by WHO for IRS. From 2000 to 2009, over 3,200 metric tonnes of insecticide were used against malaria vectors in P . vivax endemic regions [ 40 ]. Widespread insecticide use for agricultural treatments and large-scale malaria control programmes create intense selection pressure on vector populations to develop insecticide resistance [ 41 , 42 ]. Resistance to organochlorides and pyrethroids has been detected in the Americas, Asia, SE Asia, and the southern Pacific [ 42 , 43 ] where P . vivax is endemic, though even within a single country, resistance patterns may vary [ 44 ]. The full extent of insecticide resistance is not well characterised because routine monitoring in anopheline vector populations is not performed in all countries.
There is no generally available P . vivax vaccine, and none is on the near horizon. In contrast to P . falciparum , where hundreds of clinical trials and nearly 20 preclinical trials are registered, only 1 clinical trial and 2 preclinical trials are registered for P . vivax [ 45 ]. Potential vaccine strategies for P . vivax are irradiated sporozoites [ 46 ] and the blood stage antigens P . vivax Duffy binding protein (PvDBP), P . vivax merozoite surface proteins (PvMSP1, PvMSP3α, PvMSP9), P . vivax apical membrane antigen (PvAMA1), and the liver stage antigen P . vivax circumsporozoite protein (PvCSP) [ 47 ]. Transmission-blocking antigens are also potential targets. Phase I human trials have been conducted with PvCSP [ 48 ] and PvDBP [ 49 ] and asexual stage antigen Pv25 [ 50 , 51 ]. Currently, a single Phase I P . vivax vaccine trial (of a viral vectored PvDBP vaccine) is listed on the global WHO malaria clinical vaccine projects “Rainbow tables” (Trial NCT01816113) [ 45 ]. Vaccine development for P . vivax faces different challenges than with P . falciparum . P . vivax has greater genetic diversity [ 52 ] and cannot be maintained reliably in continuous cultures in vitro.
Chemoprophylaxis.
Chemoprophylaxis is recommended to protect travellers from nonendemic areas entering a malaria-endemic area. Recommendations are largely focused on P . falciparum , the more dangerous malaria parasite, both to prevent severe disease, and also because nearly all the drugs which work against P . falciparum (either in pre-erythrocytic or “causal” prophylaxis, or in blood stage “suppressive” prophylaxis) are also effective against P . vivax . Intermittent presumptive treatment for infants and pregnant women (IPTi and IPTp) and seasonal malaria chemoprevention (SMC) are interventions which aim to prevent P . falciparum blood stage infections in populations that live in areas of moderate to high P . falciparum transmission. These are typically areas where P . vivax is either uncommon or absent. The only suppressive chemoprophylactic strategy specifically targeting P . vivax for endemic populations is a weekly chloroquine dose of 5 mg base/kg (300 mg base adult dose) given during pregnancy and lactation [ 53 ] as 8-aminoquinolines are contraindicated in this population ( Table 1 ). This is seldom used, probably because of insufficient cost-effectiveness where incidence is low. But the cost-effectiveness balance does favour chloroquine prophylaxis to suppress relapse in pregnancy in women following an incident tropical (frequent relapse) P . vivax infection—and then it should be given. The role of IPTp with dihydroartemisinin-piperaquine in parts of Indonesia and Papua New Guinea, where transmission and resistance to chloroquine are both high, is under investigation with promising results in clinical trials [ 54 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1003561.t001
As countries eliminate malaria, the infections they encounter will increasingly be in travellers arriving from endemic areas. Suppressive chemoprophylaxis with drugs effective against P . falciparum has been the primary approach for malaria prevention (e.g., the same regimens apply to P . vivax ), although they do not prevent the establishment of hypnozoites. The 8-aminoquinolines (primaquine and tafenoquine) have good activities against the pre-erythrocytic stages of all malarias, as well as blood stage activities against P . vivax , and so can be used for chemoprophylaxis. These drugs prevent later relapses as they kill hypnozoites (whereas IPTi or SMC with schizonticides only delay multiplication of the blood stage). Atovaquone-proguanil is also considered to provide highly effective causal prophylaxis against P . vivax but it does not prevent relapses. Recent observations in travellers showed a similar rate of P . vivax infections (40% to 50%) up to 1 year after returning to a nonendemic area following either atovaquone-proguanil, mefloquine, or doxycycline prophylaxis. This compares with a rate of only 1% to 2% in travellers who had received primaquine [ 55 ]. This suggests that while atovaquone is active against the developing hypnozoite, it is not active against the metabolically inert hypnozoite more than 3 days postinoculation [ 56 ]. The drugs, which act primarily on the liver stage (pre-erythrocytic) infection (i.e., causal prophylaxis), have the substantial advantage that they can be stopped within days of leaving the endemic area, whereas the blood stage suppressive drugs (mefloquine, tetracyclines, chloroquine) need to be continued for 1 month after leaving to capture late emerging blood stage infections. To ensure that relapses from P . vivax and Plasmodium ovale are prevented presumptive antirelapse therapy (PART) with a radical curative dose of 8-aminoquinolines (primaquine and tafenoquine) may be given following departure from an endemic area to persons receiving suppressive chemoprophylaxis ( Table 1 ). The timing of the PART dose is important as it should be started while blood schizonticide concentrations remain above the mean inhibitory concentration. Thus, PART can be started within the final 2 weeks of doxycycline and mefloquine, and the final week of atovaquone-proguanil suppressive chemoprophylactic regimens.
Treatment of acute Plasmodium vivax malaria
Blood stage treatment..
Since 1947, chloroquine has been the treatment of choice for blood stage infections with P . vivax . The standard treatment regimen is 25 mg of base equivalent/kg divided over 3 days and given either as 10 mg/kg initially followed either by 10 mg/kg at 24 hours and 5 mg/kg at 48 hours, or by 3 doses of 5 mg/kg 12 hourly [ 53 ]. Various other ways of giving this 25 mg/kg treatment dose have been recommended, and chloroquine may be administered safely over 36 hours provided that individual doses do not exceed 10 mg/kg. Although lower doses are effective, there is no reason to depart from this standard regimen in most regions. Absorption of chloroquine by mouth is reliable even when patients are prostrate [ 62 ], and there is seldom need for parenteral treatment. Indeed, parenteral formulations of chloroquine are no longer generally available, so those patients who do require initial parenteral treatment for severe P . vivax malaria should be given intravenous or intramuscular artesunate until they can take oral medication reliably [ 13 ]. ACTs are also highly effective (except for artesunate-sulfadoxine-pyrimethamine) and provide a slightly more rapid clinical and parasitological response than chloroquine [ 63 – 67 ]. The post-treatment suppression of early relapses depends on the elimination kinetics of the ACT partner drug—mefloquine, piperaquine, pyronaridine, and amodiaquine ACTs provide approximately similar durations of suppression compared with chloroquine, whereas artemether-lumefantrine provides a significantly shorter period of suppression [ 68 ]. Both chloroquine and ACTs are generally well tolerated antimalarial treatments. The weight-adjusted schizonticidal treatment regimens are similar in pregnant and lactating women [ 54 ]. Paediatric formulations are available for artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine, and pyronaridine-artesunate, and the doses are similar to those recommended for falciparum malaria. Child-friendly formulations and smaller-dose tablets are needed for primaquine.
Primaquine and tafenoquine both have significant blood stage activities but as the therapeutic response is significantly slower than with chloroquine or ACTs, they should not be used as monotherapies for blood stage infections [ 69 , 70 ]. Nevertheless, the asexual stage activity of 8-aminoquinolines does contribute significantly to the treatment efficacy and prevents resistance to standard schizonticides by providing a combination treatment for the blood stage infection. P . vivax gametocytes are considered as sensitive as the asexual stages to antimalarial drugs [ 71 ] so, unlike P . falciparum , specific gametocytocidal treatment is not necessary.
High fevers can be managed with standard doses of paracetamol, and vomiting can be treated with antiemetics. If patients show signs of severe vivax malaria, then the management is exactly the same as for severe falciparum malaria [ 13 ]. Uncomplicated mixed infections with P . falciparum and P . vivax should be treated with an ACT and a radical curative regimen [ 53 ].
Antimalarial drug resistance.
Chloroquine resistance in P . vivax has emerged more slowly than for P . falciparum . The mechanism of resistance and its molecular basis have proved elusive. Evidence of chloroquine resistance was detected first in a traveller returning from Papua New Guinea over 30 years ago [ 5 , 6 ]. Chloroquine resistance has been defined as a patent P . vivax infection in the presence of chloroquine + desethychloroquine levels >100 ng/mL in whole blood or >10 ng/mL in plasma [ 72 ]. Since then, chloroquine resistance has risen to high levels in Indonesia and Oceania ( Fig 1 ) and so ACTs are used instead of chloroquine in this region. Dihydroartemisinin-piperaquine, artesunate-mefloquine, artemether-lumefantrine, and artesunate-pyronaridine are all very effective treatments of vivax malaria [ 62 – 65 ]. While amodiaquine is also more effective than chloroquine against resistant P . vivax , it is not as well tolerated as dihydroartemisinin-piperaquine, and resistance in P . falciparum is widespread in P . vivax -endemic regions. Amodiaquine-containing regimens are not therefore recommended for chloroquine-resistant P . vivax infections [ 73 – 74 ]. Despite evidence for slowly rising chloroquine resistance in P . vivax , chloroquine still remains effective throughout most malaria-endemic areas [ 65 , 75 – 79 ]. As the concomitant use of primaquine (for radical cure) provides significant independent asexual stage activity, it may mask low-level chloroquine resistance [ 80 – 82 ]. The lack of a molecular marker of chloroquine resistance and the limited availability of in vitro testing in vivax malaria [ 83 ] means that the epidemiology of chloroquine resistance is not well described. Antifol resistance occurs readily in P . vivax through mutations in Pvdhfr which are readily identified. Antifol resistance is geographically widespread [ 84 , 85 ]. P . vivax is also intrinsically relatively resistant to sulphonamides. This is why the artesunate-sulfadoxine-pyrimethamine ACT should not be used for P . vivax treatment.
The data were exported from the WWARN Vivax Surveyor tool at https://www.wwarn.org/tracking-resistance/vivax-surveyor . Evidence for chloroquine resistance comes from P . vivax clinical trials published from 1985–2019. Some trials are from different years in the same area; this figure does not specify the change in chloroquine resistance pattern over time. CQS : Chloroquine sensitive, Category 1: very suggestive of resistance, Category 2: suggestive of resistance, Category 3 : potential evidence of resistance.
https://doi.org/10.1371/journal.pmed.1003561.g001
Plasmodium vivax relapse.
Relapses of P . vivax and P . ovale are the recurrent blood stage infections which originate from hypnozoites in the liver. Relapses occur with periodic regularity ( Fig 2 ); short latency phenotypes prevalent in tropical climes relapse at approximately 3- to 4-week intervals. These intervals are prolonged by slowly eliminated antimalarial drugs. Longer latency phenotypes may relapse 8 to 9 months after a primary infection [ 86 ]. The proportion of infections which relapse varies between 20% and 100% depending on location and transmission intensity.
The temporal patterns of P . vivax relapse in different “strains.” The red arrow indicates the infective mosquito bite which leads to the primary infection. The blue triangles represent patent P . vivax infections; the largest triangle is the primary infection. The proportions of successive relapses decline, and there is an increasing probability that the relapses are oligosymptomatic or asymptomatic. The translucent blue triangles are P . vivax infections which may sometimes occur. The short latency frequent relapse pattern (typified by the “Chesson strain”) is prevalent across tropical areas. The intermediate phenotype may occur in South Asia. The long latency phenotype (typified by the Madagascar, St Elizabeth, and McCoy strains) is found in Central America, North Africa, and central Asia, while the long latency “hibernans” phenotype, which was prevalent in Northern Europe and Russia, is still found in North Korea.
https://doi.org/10.1371/journal.pmed.1003561.g002
The multiplication of the blood stage infection of early relapses is suppressed by slowly eliminated antimalarials (e.g., chloroquine) prescribed for the previous clinical P . vivax episode. As a consequence, in tropical areas, relapses become patent 3–6 weeks after artemether-lumefantrine and 5–7 weeks after chloroquine, whereas they occur around 3 weeks after artesunate or quinine treatment [ 76 ].
Antirelapse (radical cure) treatment
Primaquine..
For over 60 years, the mainstay of antirelapse treatment has been a 14-day primaquine regimen. Divergent policies and practices, concerns over haemolysis, and the unavailability of G6PD testing have contributed to low rates of primaquine uptake for radical cure in malaria-endemic countries [ 87 ].
The radical curative efficacy of primaquine depends on the total dose given. There have been relatively few randomised trials in which the radical curative efficacy of primaquine has been assessed with a necessary ≥4-month follow-up (1 year is preferable). In South America, 7-day regimens are used. The radical curative efficacy of the 7-day low-dose (total 3.5 mg base/kg) primaquine regimen was approximately 80% [ 88 – 92 ]. In SE Asia and Oceania, higher doses are needed for maximum efficacy [ 93 ]. The most commonly recommended higher dose is a total of 7 mg base/kg given over 14 days (adult dose 30 mg/day), although most countries still recommend the lower dose (3.5 mg base/kg total dose; adult dose 15 mg/day for 14 days or 30 mg/day for 7 days). Potentially poor adherence to the 14-day treatment reduces the effectiveness of radical cure. Recent studies show that shortening the course of the higher-dose treatment to 7 days (adult dose 60 mg/day) is efficacious and safe in G6PD normal patients, although significant haemolysis may occur in heterozygote females with intermediate G6PD activity who have tested normal with standard qualitative G6PD screening tests [ 94 , 95 ] (see below). G6PD quantitative tests may be needed for this short-course high-dose treatment. For patients who test as G6PD deficient, a weekly primaquine dose (0.75 mg base/kg/dose) given for 8 weeks has been recommended, although the safety of this dose has only been assessed in a few populations [ 53 , 96 – 98 ].
In early clinical trials, it was observed that chloroquine potentiated the radical curative efficacy of primaquine [ 99 ]. The mechanism underlying this relationship and its clinical significance remain unclear. Radical curative efficacy is similar when primaquine is paired with chloroquine or an ACT, and radical cure rates over 95% can be achieved after supervised dosing [ 29 , 94 , 95 ]. Primaquine requires metabolism to its bioactive metabolites by the liver isoenzyme CYP2D6 [ 100 , 101 ]. Primaquine failure may therefore occur in patients with CYP2D6 mutations associated with a poor or intermediate metaboliser phenotype. Overall, adherence likely plays a greater role in the failure of primaquine to prevent relapse. Resistance to primaquine has been reported, but there is no conclusive evidence of acquired resistance in liver stages.
Primaquine is contraindicated in pregnancy, is not recommended in infants <6 months, and has also been withheld from lactating women, although a recent study shows that the dose ingested by the breastfeeding infant is very small [ 57 ] and therefore very unlikely to exert any biological effect. This restriction may well be lifted. Primaquine is generally well tolerated. It may cause abdominal pain if larger doses (over 0.5 mg/kg) are taken on an empty stomach. This is lessened if given with food. The main adverse effect of the 8-aminoquinolines is dose-dependent oxidant haemolysis in G6PD deficiency. Primaquine-induced methaemoglobinaemia is usually mild, but it can be severe in NADH methaemoglobin reductase deficiency.
Haemolysis in G6PD deficiency.
G6PD deficiency is the most common human enzymopathy. Gene frequencies average 8% to 10% across much of the tropical world [ 102 ]. The risk of haemolysis in G6PD deficiency is the main obstacle to the use of 8-aminoquinolines for radical cure. The enzyme deficiency is X-linked so males are either normal or deficient (hemizygotes), whereas women can be either normal, or fully (homozygotes) or partially deficient (heterozygotes). On average, at a population level, female heterozygotes have half their red cells which are normal and half which are G6PD deficient but, because of random X-inactivation (Lyonisation), these proportions vary substantially between individuals, thus causing wide variations in the degree of enzyme deficiency among female heterozygotes. Both primaquine and tafenoquine cause predictable dose-related haemolysis in G6PD-deficient individuals [ 103 , 104 ]. Haemolysis disproportionately affects older red blood cells because G6PD activity declines as red blood cells age [ 105 ]. There are approximately 200 different polymorphic G6PD deficiency genotypes [ 106 ]. The majority confer reduced enzyme activity, although the degree of deficiency varies substantially among the different genotypes. The extent of haemolysis depends on the dosage of drug and the genotype. In Africa, the less severe A-genotype is prevalent, whereas in Asia, the common genotypes confer greater enzyme deficiency. The most commonly used screen for G6PD deficiency is the qualitative G6PD fluorescent spot test (FST), which detects the naturally fluorescent NADPH. The FST detects <30% of normal G6PD activity reliably (deficient blood does not fluoresce) so it identifies the male hemizygotes, female homozygotes, and those heterozygotes with G6PD activity near the 30% threshold. The FST may not be feasible at lower-level healthcare settings as it requires electricity, trained laboratory staff, and quality control. New qualitative lateral flow G6PD RDTs are available. These can be performed where the FST cannot. These RDTs are an important support tool for diagnosing G6PD deficiency, and the safe prescription of primaquine as P . vivax radical cure is scaled up. Patients who have a test result indicating G6PD deficiency should not receive the standard primaquine regimen. Instead, in areas where the less severe G6PD variants (e.g., G6PD A-) are prevalent, they should receive a once weekly 0.75 mg base/kg dose for 8 weeks [ 53 , 96 ]. The safety of the weekly regimen has not been established in patients with G6PD variants that are severe (e.g., G6PD Mediterranean). However, a threshold for phenotypic severity has not been defined. Even with FST or RDT screening, there may still be significant haemolysis in G6PD heterozygote females who have slightly more than 30% of G6PD normal erythrocytes. Such females would be considered as G6PD normal and potentially given primaquine. Unfortunately, in many regions, G6PD testing is unavailable so the practitioner treating vivax malaria is faced with a dilemma—to give radical cure “at risk,” or to err on the side of safety and not give it. In this difficult choice, the substantial morbidity of recurrent P . vivax malaria, particularly in young children who may be ill every second month, has often been underappreciated [ 107 ]. Importantly, in gauging the risk, it is important to note that G6PD deficiency protects against P . vivax malaria, so at a population level, the risk of haemolysis in patients is lower than would be predicted from prevalence surveys in healthy individuals [ 108 ]. In a recent study in Afghanistan of Pashtun patients (in whom the main G6PD variant is the “severe” Mediterranean variant), the prevalence of G6PD deficiency in men (i.e., hemizygotes) presenting with vivax malaria was 4 times lower than in the healthy population [ 109 ]. The result of these various uncertainties has been a diverse array of National treatment recommendations and practices for radical cure. Many countries do recommend giving primaquine “at risk,” yet despite over 60 years of widespread use, the total number of documented fatalities from haemolysis is only fifteen [ 110 , 111 ]. The likely reason for this low number is that primaquine can be stopped as soon as there are symptoms or clinical evidence of substantial acute haemolysis (e.g., lassitude, exertional dyspnoea, haemoglobinuria, or abdominal discomfort) and, because primaquine and its oxidant metabolites are rapidly eliminated, the anaemia is limited. This probably explains why mass treatments with primaquine without G6PD testing, even in areas where G6PD Mediterranean was the main deficiency genotype, were not apparently associated with serious toxicity [ 110 ].
Tafenoquine.
Tafenoquine is a slowly eliminated 8-aminoquinoline (terminal elimination half-life of approximately 12 days) which allows single-dose treatment. As with primaquine, tafenoquine is generally well tolerated and abdominal discomfort is lessened if given with food. During the long development of tafenoquine, concerns were raised about the potential for vortex keratopathy, electrocardiograph QT prolongation, and psychiatric reactions. The clinical evidence to date has largely resolved these issues [ 112 – 115 ] although, in the product labelling, tafenoquine for causal prophylaxis is considered contraindicated in persons with psychiatric symptoms. Being a single-dose treatment, tafenoquine addresses a major limitation of the 7- or 14-day primaquine regimen, the potential for poor adherence. However, this advantage is at the expense of a substantial risk of haemolytic toxicity in G6PD deficiency as, once tafenoquine is taken, it cannot be “stopped” if there is drug-induced haemolysis. A meta-analysis of the multicountry phase 3 studies showed that tafenoquine had a 6-month radical curative efficacy similar to low-dose primaquine (0.25 mg/kg/day for 14 days) in South America (67% versus 66%, respectively) and the horn of Africa (75% versus 80%, respectively), but the efficacy of the currently recommended 300 mg dose was significantly inferior in the SE Asian sites (74% versus 93%, respectively) [ 116 , 117 ]. These are disappointing radical curative efficacies and suggest that higher tafenoquine doses are probably needed in SE Asia and Oceania where P . vivax relapse rates are high, and also in South America where both radical cure regimens performed poorly [ 118 ]. Tafenoquine efficacy did not appear to be affected significantly by CYP2D6 polymorphisms in the Phase III trials [ 119 ], but more data are needed to confirm these early findings.
As described, tafenoquine’s advantage in being slowly eliminated (terminal half-life approximately 12 days), and thus effective in a single dose, is also its Achilles heel. If given to an individual who is G6PD deficient, the drug exposure and resulting haemolysis will persist until all the susceptible erythrocytes have been destroyed. Some female heterozygotes, who would be undetected by the standard screens, could still theoretically lose up to 70% of their red cells. This is why the higher G6PD activity threshold (>70% G6PD activity) has been chosen for tafenoquine eligibility. The gold standard method for G6PD quantitation is spectrophotometry requiring relatively expensive and sophisticated laboratory techniques, so it is not feasible in nearly all healthcare settings. Thus, for safe use of tafenoquine, new quantitative point-of-care G6PD tests have been developed. Studies to validate G6PD quantitative tests are completed [ 120 , 121 ] and under regulatory review. More extensive field testing is ongoing to assess the performance of these quantitative G6PD tests in field settings under “real world” conditions and the rates of incorrect test results or interpretations.
The current exclusions to tafenoquine apply to approximately 25% of the population in endemic areas, depending on the G6PD allelic frequency, and fertility and lactation rates [ 122 ]. Approving its use in lactating females would substantially improve potential population coverage. Tafenoquine has received regulatory approval in the United States of America, Australia, Brazil, and Thailand [ 116 , 117 ]. Results from the first trial assessing tafenoquine use in children (2 to 15 years) with P . vivax malaria show that dosing regimens in 4 weight bands were safe and efficacious and reached the target AUC 0-∞ [ 60 ]. As a result, current restrictions on use in children will likely be lifted. Tafenoquine has usually been coadministered with chloroquine. In a recently completed clinical trial in Indonesian soldiers, dihydroartemisinin-piperaquine coadministered with tafenoquine (300 mg dose) was shown to be no different than dihydroartemisinin-piperaquine alone [ 58 ] in preventing recurrence of P . vivax malaria. These data prompted a product label change to Krintafel (the US regulatory approved form of tafenoquine for treatment) in early 2020 [ 59 ]. Now, only chloroquine is recommended to be the partner drug to tafenoquine for P . vivax malaria treatment. If followed this would prevent the use of tafenoquine in areas with chloroquine-resistant P . vivax parasites where national malaria programmes recommend ACTs for vivax malaria. Clearly, further clinical trials are needed to identify the correct dose of tafenoquine for radical cure [ 118 ] and resolve this partner drug uncertainty.
How can we identify hypnozoite carriers?
There is currently no test which identifies people with hypnozoites who may later relapse. Living or working in an area of P . vivax transmission is obviously a risk factor, and having had vivax malaria without radical treatment is clearly the major risk. Pregnant women cannot receive 8-aminoquinolines so are at significant risk if they have vivax malaria during pregnancy. If primaquine could be allowed during lactation (as breast milk excretion is minimal [ 57 ]), this should result in a recommendation to give radical cure after delivery. PCR, particularly high-volume uPCR, identifies the majority of asymptomatic P . vivax infections. Serology can also identify recent infection, although is not widely available.
Assessment of therapeutic responses.
As in falciparum malaria, the treatment responses in P . vivax infections can be assessed by measurement of symptom resolution, fever clearance, and parasite clearance. The lower parasite densities in P . vivax mean that parasite rate clearance estimates are more difficult to assess, although sequestration is not a confounder in measuring parasite clearance rates because all parasite stages are in the circulation [ 123 ]. Quantitative polymerase chain reaction (qPCR) estimation of parasite clearance rates has been used successfully in human challenge experiments but is not widely available [ 124 ]. These parasite clearance estimates based on qPCR are not confounded by gametocytaemia (as they are in falciparum malaria) because P . vivax gametocyte clearance parallels asexual stage clearance after a blood schizonticide [ 71 ]. The major challenge in therapeutic assessment is distinguishing relapse, recrudescence, and reinfection, and therefore identifying early resistance. Both chloroquine and the ACT partner drugs are eliminated slowly so that suppressive blood concentrations persist for weeks after treatment [ 118 ]. Lumefantrine provides the shortest period of suppression and mefloquine the longest of the currently deployed ACTs. In tropical areas, the first sign of chloroquine resistance in vivo, well before slowing of parasite clearance, is the failure to suppress the first relapse [ 72 , 125 ]. This may not be evident unless patients are followed for 2 months in comparative studies ( Fig 3A ). As the level of resistance increases, the interval shortens and the first relapse becomes patent within 28 days ( Fig 3B ). Chloroquine should suppress relapse emergence within 28 days so recurrence within this period, whether from relapse or reinfection, still indicates resistance [ 125 ]. If a recurrent infection occurs within 28 days of starting chloroquine for the previous vivax infection, an ACT (such as dihydroartemisinin-piperaquine, artemether-lumefantrine, or artesunate-mefloquine) should be prescribed. Resistance can be confirmed by measuring the whole blood chloroquine level at the time of recurrent parasitaemia [ 93 ]. With higher levels of resistance ( Fig 3C ), recrudescence preempts the relapse. At this stage, parasite genotyping is informative as these early recurrences will all be of the same genotype as the initial infection [ 29 ].
The vertical axis shows the total number of parasites in the body in acute vivax malaria. The light grey shaded area is the blood chloroquine concentration profile. The limit of microscopy detection is approximately 50 parasites/uL. In each panel, chloroquine-sensitive parasites are shown by green lines and marked S. The relapses emerge from the liver approximately 2 weeks after starting treatment. There is uncertainty whether chloroquine eliminates or temporarily suppresses the first relapse (in most cases it suppresses but does not eliminate [ 118 ]) so dotted lines representing both scenarios are shown. Resistant parasites causing relapse and recrudescence are marked R. Upper panel (A): When there are low levels of resistance, the blood stage infection is cleared usually by the schizonticide, and the first relapse is suppressed until the drug levels fall below the mean inhibitory concentration (MIC) (e.g., in this illustrated example, chloroquine levels above the MIC are maintained until day 28, and relapse parasitaemia becomes patent 2 weeks later) [ 125 ]. Recrudescence is very unlikely (occurring only in those patients with low drug levels) and in patients with relapse, the recrudescence would not be detected because the relapse appears first. Middle panel (B): When resistance to chloroquine is at an intermediate level, the blood stage infection clears, but the first relapse becomes patent before 28 days. If a relapse occurs, it would still preempt any recrudescence. Lower panel (C): With high levels of resistance, the blood stage infection recrudesces before the relapse parasitaemia becomes patent.
https://doi.org/10.1371/journal.pmed.1003561.g003
Frequent recurrent P . vivax malaria causes substantial preventable morbidity. Better diagnosis, easier G6PD testing, and use of ACTs as an alternative to chloroquine are improving the clinical management of P . vivax malaria, but the challenge now is to make these more widely available. Primaquine radical cure is under used. Tafenoquine provides single-dose radical cure, but the optimum dose and method of using tafenoquine safely still need to be determined. The tools needed for P . vivax elimination are available. Malaria elimination activities conducted in remote settings [ 126 – 128 ] have been successful in reducing P . vivax incidence considerably, but continuing to sustained elimination will require substantial commitment and resources.
Acknowledgments
We would like to thank Jade D. Rae for her generous help with the mapping for Fig 1 .
- View Article
- PubMed/NCBI
- Google Scholar
- 4. World Health Organization. World malaria Report 2020. Geneva; 2020. Available from: https://www.who.int/publications/i/item/9789240015791
- 25. World Health Organization. World Malaria Report 2019. Geneva; 2019. Available from: https://www.who.int/publications-detail/world-malaria-report-2019
- 32. Stewart T, Marchand RP. Factors that affect the success and failure of insecticide treated net programs for malaria control in SE Asia and the Western Pacific. World Heal Organ. Geneva; 2003. Available from: http://www.who.int/malaria/publications/atoz/itn_r62.pdf
- 42. World Health Organization. Global report on insecticide resistance in malaria vectors: 2010–2016. Geneva;2018. Available from: https://www.who.int/malaria/publications/atoz/9789241514057/en/
- 45. World Health Organization. WHO malaria vaccine Rainbow tables. In: who.int [Internet]. 2020 [cited 7 Aug 2020]. Available from: http://www.who.int/immunization/research/development/Rainbow_tables/en/
- 53. World Health Organization. Guidelines for the treatment of malaria, 3rd Edition. Geneva: World Health Organization; 2015. Available from: https://apps.who.int/iris/handle/10665/162441
- 59. Centers for Disease Control and Prevention. Change in Krintafel (tafenoquine) label [Internet]. 2020 [cited 30 Jul 2020]. Available from: https://www.cdc.gov/malaria/new_info/2020/tafenoquine_2020.html
- 110. Recht J, Ashley EA, White NJ. Safety of 8-aminoquinoline antimalarial medicines. World Health Organization. Geneva: World Health Organization; 2014. Available from: https://www.who.int/malaria/publications/atoz/9789241506977/en/
- Parasitic Diseases
- Protozoan Infections
- Parasitology
Malaria: An Overview
- 16:3339-3347
- CC BY-NC 3.0

- Addis Ababa University

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Thu Huyen Thi Tran
- Bui Thi Thu Hien
- Nguyen Thi Lan Dung
- Nguyen Dang Ton
- Jae-Won Choi
- Yeon-Jun Kim
- Jung Hoon Kang
- Parasitol Res

- Sarah Alansari
- Mouayad Abdulghani
- Malik Zakaullah
- Rambabu Vadlamudi
- Anju Ranjit

- BIOORG MED CHEM LETT
- Udhav V. Mhetre

- Syed Sarfaraz Ali

- Bushra Khalid
- Natasha Hassan
- Noor Ul Ain Soomra

- Laurent Rénia
- Ganesh Tadepalli

- Ashok Kumar Pannu

- Melkamu Adigo Shibeshi

- Corine Karema
- Abigail Sidibe
- Allison Tatarsky

- Julie-Anne Tangena

- Maria Devine

- Dominique RICHARD-LENOBLE
- Martin DANIS
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Open access
- Published: 06 October 2022
Severe malaria
- Nicholas J. White 1 , 2
Malaria Journal volume 21 , Article number: 284 ( 2022 ) Cite this article
27k Accesses
47 Citations
8 Altmetric
Metrics details
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice. Approximately one third of children diagnosed with severe malaria have another condition, usually sepsis, as the cause of their severe illness. But these children have a high mortality, contributing substantially to the number of deaths attributed to ‘severe malaria’. Simple well-established tests, such as examination of the thin blood smear and the full blood count, improve the specificity of diagnosis and provide prognostic information in severe malaria. They should be performed more widely. Early administration of artesunate and broad-spectrum antibiotics to all children with suspected severe malaria would reduce global malaria mortality.
Severe malaria is important. It is a major cause of preventable childhood death in tropical countries. This large number of avoidable deaths justifies the substantial global investments in malaria control and elimination. But severe malaria is increasingly overlooked by the international agencies, donors and policy makers who determine the direction and support for global malaria initiatives.
Severe malaria, or ague, was recognized long before discovery of the malaria parasite by Laveran in 1880. The Cinchona bark arrived in Europe nearly four hundred years ago providing, for the first time, a potential cure for the pervasive and dangerous illness that then affected most of the inhabited world. But, as today, the specificity of the clinical diagnosis of febrile illnesses was poor. Torti recognized that only some fevers could be cured by the bark [ 1 ]. Even after the malaria parasite was identified first in 1880, severe forms such as algid malaria (shock), haemorrhagic or gastrointestinal malaria bore an uncertain relationship to Plasmodium infection, as did the notorious “blackwater fever”. Until the 1980s, the majority of research on severe malaria was conducted in adults. It derived largely from war-time experiences in the military, or observations from colonial medical services. Specific anti-malarial treatment comprised the parenteral administration of quinine and, from the 1950s, chloroquine. When they became available, renal replacement therapies for adult patients with acute renal failure could also save lives [ 2 ].
Soon after Laveran’s discovery of the causative parasite, the pathological processes underlying severe malaria were elucidated by the great Italian malariologists Marchiafava and Bignami. They considered, correctly, that the sequestration of parasitized erythrocytes in the microvasculature, causing microcirculatory dysfunction, was the key pathological event in “malignant tertian” (severe falciparum) malaria [ 3 , 4 ]. Beginning in the 1960s, coincident with the emergence of immunology as a discipline, and continuing to this day, various novel theories of severe malaria pathogenesis were proposed. These were often derived from observations in a murine “model” of cerebral malaria, which was fundamentally different to the human infection [ 2 , 5 ]. These new theories spawned a long succession of putative adjuvant therapies for severe malaria. Unfortunately, none of these therapies worked, and several were harmful [ 2 , 4 , 5 ].
1985 WHO meeting
Before 1985, there was no standard definition of severe malaria. Cerebral malaria was defined as unrousable coma (no localizing response to a painful stimulus). After publication of the Glasgow Coma Scale (GCS) in 1976 [ 6 ], this level of coma became a GCS less than 11. In 1985 an “informal meeting” was convened by the Malaria Action Programme of the World Health Organization (WHO). It was held in the Institute for Medical Research in Kuala Lumpur where, decades before, Field and colleagues had conducted seminal studies on the diagnosis, pathology and prognosis of severe malaria. The WHO meeting had the objective of reviewing available information on severe falciparum malaria, standardizing the definition, and advising on management [ 7 ]. The resulting document, which derived heavily from studies in Thailand conducted in the previous five years [ 8 ], provided a definition of severe malaria which is broadly similar to that used today, but with the following exceptions.
hyperparasitaemia was defined as > 5% parasitaemia (today this is 10%)
after a convulsion, coma had to persist for 6 h (now 30 min),
severe anaemia was defined as a haematocrit < 20% (now < 15%),
jaundice (total bilirubin > 50 µmol/L) alone was a criterion (today this requires a parasite density > 100,000/uL as well),
‘fluid, electrolyte or acid–base disturbances requiring intravenous therapy’ was a criterion (today more specific criteria have been instituted: either a venous plasma lactate > 5 mmol/L, arterial pH < 7.25, or a plasma bicarbonate < 15 mmol/L is required).
Hyperpyrexia (> 39 °C), vomiting of oral treatment and haemoglobinuria were also included – none of which today are considered defining criteria.
These definitions and descriptions have been generally referred to and referenced as “WHO definitions” although each successive version of the severe malaria review contains a disclaimer that the contents are the opinions of experts, and not those of the WHO itself.
1988 WHO meeting
In 1988 a second informal WHO meeting was held to update the recommendations and to incorporate recent observations in African children with cerebral malaria [ 9 ]. For the definition of severe malaria, hyperparasitaemia, jaundice, and hyperpyrexia were “dropped”, the haematocrit criterion was reduced to 15% and, after some debate, a requirement for a concomitant parasitaemia of > 10,000/µL was added to the severe anaemia criterion. Acidaemia or acidosis were defined as above, and repeated generalized convulsions (more than two observed within 24 h despite cooling) was added as a criterion. In this second meeting, the readily evaluated Blantyre Coma scale [ 10 ] was endorsed as the method to assess the level of consciousness in children.
1995 WHO meeting
The third WHO meeting was held in Geneva in December 1995 to incorporate further experience from clinical research in African children [ 10 – 12 ]. This meeting resulted in a broader, more inclusive and pragmatic, definition of severe malaria in children centred around prostration and respiratory distress (acidotic breathing) [ 13 ].
The hyperparasitaemia threshold was changed to 4% in low transmission settings, and to 20% in high transmission settings. The newly added “prostrate” criterion was very broad. It included many children with acute malaria who had no other signs of severity. This substantially expanded definition of severe malaria therefore encompassed a larger proportion of all children with acute malaria (and so it had a lower case-specific mortality). The new inclusive definition ensured a high proportion of at-risk children would be managed appropriately (i.e. it had high diagnostic sensitivity), but it had low specificity in identifying potentially fatal infections. In clinical research use of the broader inclusion criteria obviously resulted in overall “better outcomes” as more children with a good prognosis were included within the broader definition of severe malaria. Recognizing the disparities with the earlier criteria some investigators continued with the stricter (i.e. more specific) earlier severe malaria criteria [ 9 ] in their clinical research studies (Table 1 ).
2013 WHO meeting
The most recent WHO meeting on severe malaria was convened in 2013, again in Geneva [ 2 ]. By 2013, large prospective series of patients with severe malaria had been studied in Asia and Africa. These studies provided a much larger evidence base than for previous meetings. Many of the data came from randomized controlled trials [ 14 – 21 ]. The key therapeutic advance was the replacement of quinine by artesunate, which had been shown to reduce mortality by between one fifth and one third in very large randomized controlled trials [ 18 , 19 ]. The definitions of severe malaria, and components of the definitions, could now be associated with mortalities [ 22 – 26 ] (which were falling globally as artemisinin combination treatments were rolled out and parenteral artesunate was replacing quinine as first line parenteral treatment) [ 2 , 27 ]. The 2013 “WHO” meeting recognized both the requirements of a definition for practitioners, for whom sensitivity in recognizing potentially severe malaria and thus inclusiveness takes priority, with the contrasting needs of epidemiology and research studies where specificity is more important. The most recent research definition is shown in Table 2 [ 2 ].
Prostration was not included in the ‘research” definition, convulsions were “dropped”, the acidosis criterion was refined, the jaundice criterion was reintroduced with a parasite density > 100,000/µL, and the hyperparasitaemia criterion was changed (again!). In addition, severe Plasmodium vivax and Plasmodium knowlesi infections were reviewed specifically, and slightly modified definitions for severe malaria with these infections were proposed [ 2 ].
The meaning of severe malaria
Strictly speaking, severe malaria is malaria with an increased risk of death at the time of assessment compared to everyone else in that community with malaria illness. How much higher this risk should be (i.e. the lower threshold for the increase in mortality) has not been agreed upon. Mortality varies substantially as it depends on the infection, the host, the circumstances and the treatment. For the same admission severity, outcomes in well-equipped intensive care units (ICUs) with well-trained staff are better than in peripheral health centres. However, tertiary ICUs often receive the very sickest patients, often after long delays in referral – with consequently high mortalities. A frail and debilitated patient may die from a malaria infection that would be regarded as mild in a younger and fitter person. The high mortality of imported malaria (both P. falciparum and P. vivax ) in elderly travellers, and of malariatherapy (all species) in neurosyphilis, testifies to the lethal potential of acute malaria illness, whichever the infecting parasite species, in frail or debilitated persons [ 28 , 29 ]. In contrast, most people with malaria illness in endemic areas are either children or young adults without underlying conditions (although in Southern Africa HIV prevalence is high, and the untreated coinfection predisposes to severe malaria [ 30 ]). Acknowledging that this is an oxymoron, the “uncomplicated falciparum malaria” mortality of orally treated patients ranges from 1 in 10,000 to 1 in 1,000 if effective anti-malarial drugs are being used. Many factors affect this risk. Severe malaria usually has a mortality well over 5%, and therefore represents a > 50 fold increase in the risk of death. In general, as with many infections, mortality in malaria is proportional to the total number of infecting organisms (biomass) in the body. In non-immune adults mortality increases steeply as peripheral blood parasite densities rise over 100,000/µL [ 31 ]. This corresponds approximately to total parasite numbers within the blood of over 10 12 . If severe malaria was defined as clinical and laboratory measures which are associated with > 5% mortality, then the current thresholds would conform, except for the anaemia criterion (see below) which would require a threshold of 3 g/dL rather than 5 g/dL.
Malaria parasite densities
Malaria is traditionally diagnosed by microscopy examination of a peripheral blood smear. Unfortunately, this diagnostic skill is being lost in many places as microscopy is replaced by the more ‘convenient’, but less informative, rapid diagnostic tests. In malaria microscopy, the parasites are speciated and their numbers counted. The result is reported either as the number of parasitized erythrocytes in a stained thin smear or, in a thick film, as the number of parasites seen in a fixed volume or while counting a certain number of white blood cells (usually 200 or 500). The old semi-quantitative ‘cross’ system, in which density is graded from + to + + + + , is no longer recommended. The thin film should be used for high parasite densities (> 0.2% parasitaemia).
In falciparum malaria the parasite count can be misleading. This is because after approximately 12 to 16 h (depending on core temperature) of intraerythrocytic parasite growth (i.e. one quarter to one third of the asexual life cycle) Plasmodium falciparum infected erythrocytes begin to stick (“cytoadhere”) to vascular endothelium. By 20 h the majority have cytoadhered. This “sequestration” is the fundamental pathological process in falciparum malaria [ 2 , 3 ]. It occurs in all P. falciparum infections, although the tissue distribution of sequestration varies between patients. As a result, the parasite densities measured in blood films (reflecting circulating parasites only) variably underestimate the total malaria parasite biomass [ 32 – 34 ]. Nevertheless, the mortality of falciparum malaria is still proportional approximately to the peripheral blood parasite density. Among several factors, the relationship between peripheral blood parasite density and mortality depends on the prevailing intensity of transmission and thus the levels of “immunity” or “premunition”. Field showed in Kuala Lumpur (a generally low transmission area from the 1930s to the 1950s) that the mortality of falciparum malaria in adults with little or no immunity increased markedly when parasite densities rose above 100,000/µL [ 31 ] (Fig. 1 ). There is, therefore, a non-linear relationship between mortality and parasite densities. In a low transmission setting on the Thailand-Myanmar border, where the P. falciparum entomological inoculation rate was approximately 0.5/year, the mortality of children with > 4% P. falciparum parasitaemia (circa 200,000/µL) was 3% [ 35 , 36 ]. In that location a 3% mortality was thirty times higher than the mortality in patients with lower parasite densities, but it was five times lower than in patients who fulfilled the strict WHO definition of severe falciparum malaria [ 9 ]. As the predominant stage of parasite development determines the proportion of the parasite biomass that circulates, some patients with severe falciparum malaria have relatively low parasite densities because most of the malaria parasites are sequestered [ 32 – 34 ]. Others may have low parasite densities because they have already received anti-malarial drugs before assessment. On the other hand, a synchronous infection may have recently undergone schizogony and merozoite release resulting in a high parasite density with a predominance of young ring stage parasites. In this latter case most of the parasites in the body are circulating, and relatively few are still sequestered. Provided the patient receives an artemisinin derivative the prognosis is good. In children in areas of higher transmission, P. falciparum peripheral blood parasite densities over 200,000/uL may be tolerated with relatively few symptoms. Thus, the prognostic value of parasitaemia depends on the epidemiological setting and, overall, it is poor.
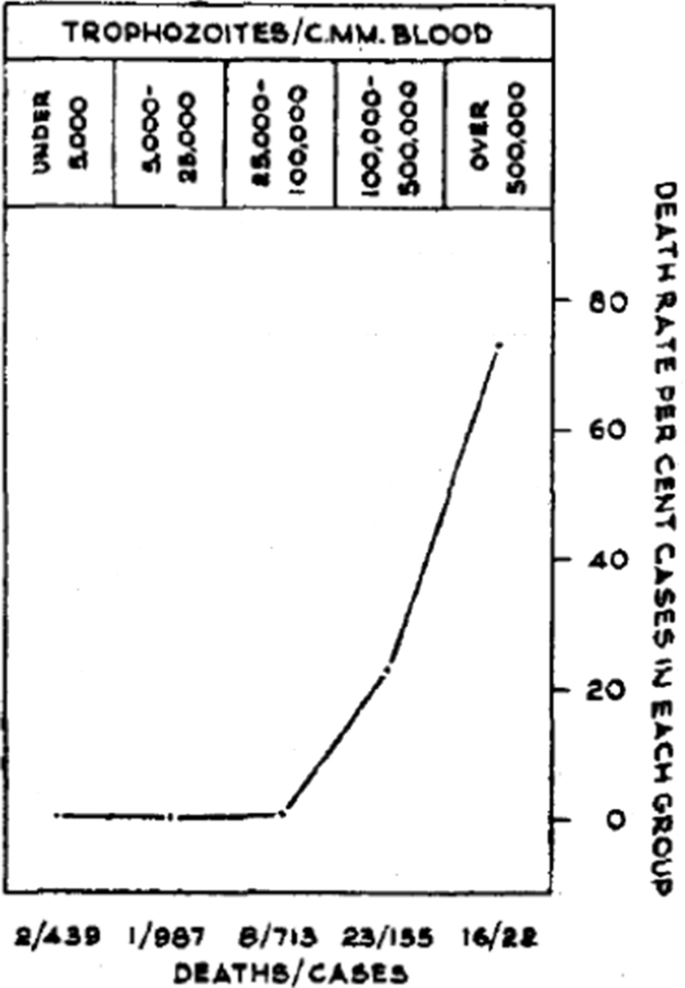
Relationship between peripheral blood parasite density and outcome in patients with acute falciparum malaria studied by Field and colleagues in Kuala Lumpur over 70 years ago [ 31 ]
Factors associated with mortality
The three main clinical presentations of severe malaria in children are coma, metabolic acidosis (usually manifest by an acidotic or “Kussmaul’s” breathing pattern, and commonly termed “respiratory distress”) and anaemia [ 2 , 10 – 13 , 22 – 26 ]. None of these are specific for malaria. These clinical presentations are major manifestations in adults too, although severe anaemia is less common. In contrast many adult patients present with acute kidney injury often accompanied by jaundice [ 37 ]. As noted earlier, there is no agreed threshold mortality threshold to define severe malaria. Among the different syndromes included in the current definition, the lowest case specific mortality is associated with malarial anaemia which can be below 1% [ 38 ]. This is still higher (by a factor of 10–100) than in uncomplicated malaria, but it is substantially lower than the mortalities associated with coma, severe metabolic acidosis, pulmonary oedema or acute renal failure (8–50%) [ 2 , 26 ]. The low mortality of severe anaemia with malaria is explained by the low sequestered parasite biomass and the inclusion, within the definition of severe malaria, of children with chronic anaemia (often as a result of repeated malaria attacks) and either incidental parasitaemia or a concomitant, otherwise uncomplicated, malaria illness. This is a very common presentation in high transmission settings where it is usually the main reason for blood transfusion in young children. The current “WHO” severe anaemia criterion requires an accompanying parasite density of 10,000/µL [ 2 ]. Densities in this range are often found in asymptomatic children, so may be incidental to the anaemia rather than causal. Even if causal the anaemia may result from a chronic process in which the parasite numbers are in a quasi-steady state, controlled by the immune response, and are very unlikely to increase further. If the parasite density requirement in the criterion for “severe anaemia” was raised it would be more specific for acute malaria but, even at higher densities, acute case specific mortalities do not rise above 5% until admission haemoglobin concentrations fall below 3 g/dL. However, it is still very important to recognize children admitted to hospital with severe malaria anaemia as a high risk group. These anaemic children have a high post-discharge mortality [ 39 – 41 ]). Furthermore they may not recover fully from their anaemia for 2–3 months after discharge. Thus, the overall mortality associated with severe malaria anaemia is significantly greater than appreciated from the acute admission [ 39 , 40 ].
The clinical syndromes
Neurological dysfunction.
The most characteristic syndrome of severe falciparum malaria is unrousable coma or cerebral malaria [ 2 , 42 ]. This diffuse, symmetrical, reversible encephalopathy may occur at any age (Fig. 2 ). The main differential diagnoses are bacterial meningoencephalitis, viral encephalitis and, in some areas, toxic encephalopathy. Cerebral malaria occurs typically in people with little or no immunity, so it is seldom seen in residents of areas of high stable malaria transmission where severe anaemia in the first years of life predominates as the manifestation of severe malaria (Fig. 3 ). The outcome of cerebral malaria depends on access to treatment and intensive care, and the degree of associated vital organ dysfunction. ‘Pure’ cerebral malaria (i.e. without other vital organ dysfunction) has approximately half the mortality of patients with coma and other organ dysfunction i.e. renal impairment, pulmonary oedema, jaundice, metabolic acidosis, or hypoglycaemia. Overall, the treated mortality of cerebral malaria in the “quinine era” was approximately 20% in adults and 12–15% in children. These mortalities have been reduced by about one third by parenteral artesunate treatment [ 17 – 20 ]. Falciparum malaria is specifically associated with convulsions, even in otherwise uncomplicated infections. The seizures are usually generalized, and they may herald the onset of coma. Although most children make a full recovery, cerebral malaria in children is associated with significant neurodevelopmental sequelae; stroke, cognitive impairment and an increased risk of epilepsy [ 42 ]. It is very important to distinguish the causal relationship between convulsions in malaria and cerebral malaria and later cognitive impairment and epilepsy, from pre-morbid conditions which may present, sometimes for the first time, as neurological dysfunction in acute malaria (and thus be misdiagnosed as cerebral malaria). Otherwise, the adverse impact of cerebral malaria on long-term neurological outcomes will be overestimated. The specificity of the diagnosis of cerebral malaria is improved by clinical and laboratory examination (see below). For example, demonstration of malaria retinopathy is highly specific for cerebral malaria as the cause of coma [ 44 ]. Severe anaemia has also been associated with neurocognitive deficits [ 45 ]. There is no evidence that severe malaria causes permanent damage to other vital organs.
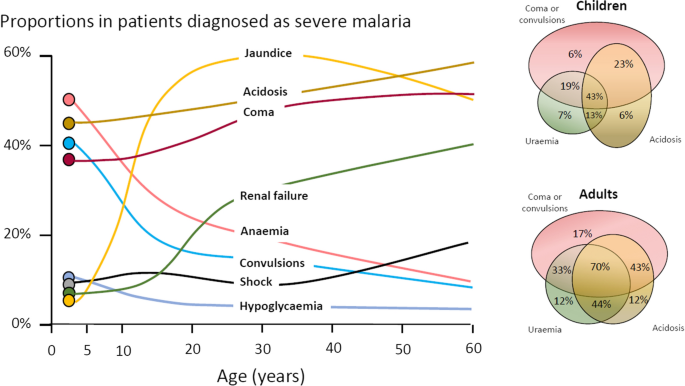
Overlap of clinical syndromes and mortalities in adults and children with severe falciparum malaria. These proportions are derived from prospective studies in SouthEast Asia and Africa of adults and children with severe falciparum malaria conducted or coordinated by the Mahidol Oxford Research Unit over the past 40 years [ 26 ]
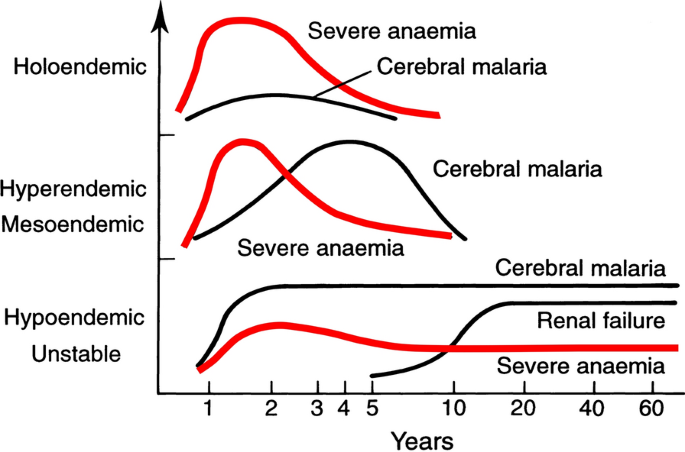
Approximate age relationships for the major clinical manifestations of severe falciparum malaria in relation to the intensity of transmission [ 53 ]. Holoendemic in this illustration approximates to a sustained entomological inoculation rate > 10 per year or a parasite rate (prevalence) in children of 0.5, and hypoendemic refers to an average entomological inoculation rate ≤ 1 year
Acidosis, kidney injury
Metabolic acidosis is a grave sign in both adults and children with severe malaria, [ 2 , 24 , 47 – 49 ] (Fig. 2 ), unless it results from very severe anaemia only, where the prognosis is better [ 38 ]. Lactate (reflecting lactic acid) accumulation is an important component of the malaria acidosis. Other organic acids, mainly of gut origin, are also significant contributors [ 46 , 50 ]. Lactic acidosis is often accompanied by hypoglycaemia reflecting anaerobic glycolysis and impaired hepatic gluconeogenesis [ 47 – 49 ]. Impaired renal function is an important manifestation of severity in younger children, but acute kidney injury (AKI) requiring renal replacement therapies is almost confined to older children and adults [ 2 , 37 , 51 ] (Figs. 2 , 3 ). The fulminant form of AKI, often associated with multiple vital organ dysfunction, is associated with a poor prognosis. In contrast the sub-acute presentation, in which plasma or serum creatinine rises steadily as the patient otherwise recovers, has a good prognosis. A period of renal replacement therapy (preferably haemofiltration or haemodialysis [ 52 ]) may be required, but there is always full recovery of renal function in survivors. The ‘hepatorenal’ combination of jaundice and renal failure became a more common presentation of severe malaria relative to cerebral malaria in Southeast Asia over the past four decades -the prognosis is worse than with AKI alone. Renal dysfunction in malaria can be misattributed in much the same way that neurological dysfunction following malaria can be overdiagnosed. In many tropical regions chronic kidney disease is common, particularly in older adults, and renal impairment may become evident for the first time during hospitalization for malaria. This may be causally attributed to malaria by mistake, and so a diagnosis of malaria nephropathy is made incorrectly. Concomitant anaemia and acidosis may also be ascribed incorrectly to malaria rather than chronic renal disease. In these misattributed cases, renal imaging, if available, often reveals small kidneys, or nephrolithiasis and hydronephrosis, and there may be biochemical or radiological evidence of metabolic bone disease.
Severe anaemia
The definitions of anaemia in malaria vary widely [ 53 ]. The most common classification—used in higher malaria transmission settings- is based on haemoglobin concentrations. In patients with acute malaria haemoglobin (Hb) concentrations between 8 g/dL and 11 g/dL are considered as mild anaemia, Hb between 5 g/dl and 8 g/dL is considered moderate, and Hb < 5 g/dL is defined as severe anaemia [ 53 ]. Unfortunately, despite their simplicity, the point of care measurements of haemoglobin concentrations, which are necessary to ensure appropriate use of blood transfusions, are often unavailable [ 54 ]. In sub-Saharan Africa the Hb ≤ 5 g/dL threshold is used widely as an indication for blood transfusion in children with malaria (whereas Hb ≤ 4 g/dL is often used for other causes of anaemia) (Fig. 4 ). The recent finding, in a large randomized trial, that children with fever (> 37.5 °C) were harmed by higher blood transfusion volumes (30 mL/kg versus 20 mL/kg) whereas children without fever benefited [ 55 – 57 ], has forced a reconsideration of blood transfusion guidelines for African children with severe anaemia [ 58 ] (Fig. 4 ). In low transmission settings an Hb ≤ 7 g/dL has been used as a transfusion indicator [ 2 ]. There is no evidence to support this threshold. Anaemia is the main severe manifestation of malaria in areas of high transmission, where it is largely confined to young children [ 59 ] (Fig. 2 ). Severe anaemia, as a criterion of severe malaria, encompasses a spectrum of aetiologies with several different, but often overlapping, pathological processes which are still not well understood [ 53 ]. At one end of the disease spectrum is an acute illness in patients with high parasite biomass infections and rapid destruction of parasitized and unparasitized red cells. The unparasitized cells comprise the majority of erythrocytes lost. Haemolysis is sometimes sufficient to result in haemoglobinuria (blackwater fever). However, malaria is not the only cause of blackwater fever, which, after over 120 years of investigation, still remains a puzzle [ 60 – 64 ]. Massive haemolysis may occur in any epidemiological setting. At the other end of the disease spectrum, in settings of high transmission or poor access to treatment, are patients (usually young children) with chronic anaemia and incidental parasitaemia. Repeated or untreated malaria infections resulting in shortened erythrocyte survival and protracted dyserythropoeisis are important contributors to this chronic, or acute on chronic, syndrome [ 52 ].
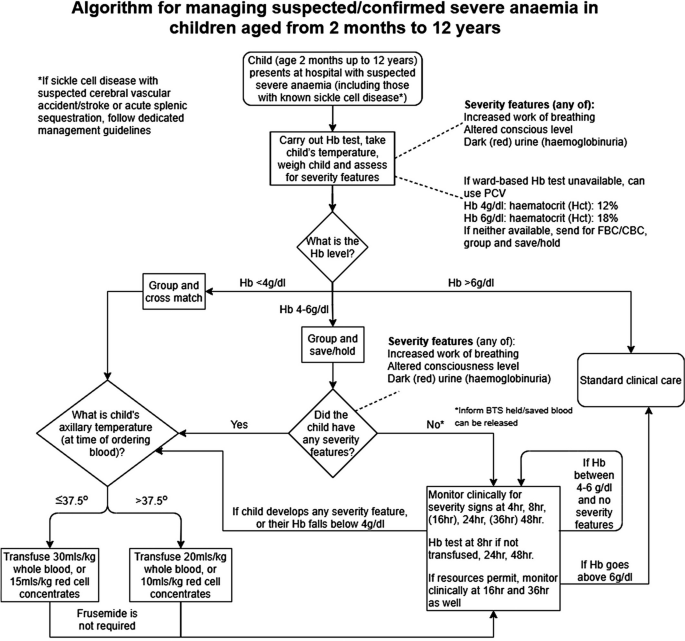
Proposed algorithm for managing suspected/confirmed severe anaemia in African children aged from 2 months to 12 years [ 58 ]
Improved malaria control reduces the frequency of malaria infections and thus the prevalence of severe anaemia [ 59 , 65 ]. As in chronic inflammatory conditions, malaria is associated with iron deficiency [ 66 ]. Other common causes of anaemia in malaria endemic regions are nutritional deficiencies, hookworm, bacterial infections and haemoglobinopathies. Bacterial infections are also associated with acute anaemia presentations [ 67 ]. At presentation to hospital the short-term prognosis of severe anaemia is relatively good as the anaemia is mainly chronic and partially compensated (by the right shifted oxygen dissociation curve). If blood transfusion can be given promptly then the acute mortality is low but, in higher malaria transmission settings, hospitalization for severe anaemia identifies children who are at increased risk of subsequent death. Approximately 5% will die within 6 months. Post-discharge anti-malarial chemoprophylaxis provides temporary protection, which suggests that recurrent malaria is a major contributor to this high mortality [ 40 , 41 ]. The prognosis of children hospitalized with severe anaemia is much better than for the other severe manifestations of falciparum malaria but, because of the longer-term impact, and because it is so common in high transmission settings, the adverse impact at a population level is substantial [ 59 ]. Deaths from malaria overall are positively correlated with transmission intensity [ 59 ], and the direct or indirect consequences of severe anaemia are major contributors to this relationship.
Other complications
Pulmonary oedema (ARDS) carries a very high mortality in falciparum malaria- even with positive pressure ventilation. It often occurs after the other severe manifestations have become evident. Pulmonary oedema results from increased pulmonary capillary permeability. Pulmonary oedema may also occur in vivax malaria, where the prognosis is much better [ 2 ]. Liver dysfunction is usual in severe malaria [ 68 ] although liver failure, as in viral or toxic hepatic injury, never occurs [ 2 ]. Profound thrombocytopenia is associated with an increased mortality in severe malaria, but it is not an independent risk factor and, contrary to some reports, it is not regarded as a criterion of severe malaria [ 69 ]. Although thrombocytopenia is usual in all malarias and coagulation indices are often abnormal in severe illness, significant bleeding (if present, usually from the stomach) and clinically significant coagulopathy are unusual in severe malaria. Overall, the probability of death from severe falciparum malaria depends on the extent and degree of vital organ dysfunction and the access to appropriate treatment [ 2 , 70 ]. Secondary bacterial infection is a potentially lethal complication, particularly in African children. Approximately 6% of children diagnosed with severe malaria have concomitant bacteraemia [ 71 ]. In adults the incidence is much lower (1%) [ 72 ]. Misdiagnosis (see below) is common [ 73 ], as it is difficult to differentiate between severe malaria with concomitant bacteraemia and a primary bacterial infection with incidental parasitaemia [ 74 , 75 ].
Pathophysiology of severe falciparum malaria
Similar to some primate malaria parasites ( P. fragile, P. coatneyi ), but unlike the other human malaria parasites, P. falciparum causes the infected erythrocyte to cytoadhere to vascular endothelium after the first third of the asexual blood cycle [ 2 ]. Severe falciparum malaria results from the extensive sequestration of erythrocytes containing these mature parasite forms in the microvasculature of vital organs [ 2 , 3 , 76 , 77 ] (Fig. 5 ). The microvascular obstruction by highly metabolically active cells, consequent cellular dysfunction, and the liberation of large quantities of bioactive haem are considered the main pathological processes in severe falciparum malaria [ 70 , 76 – 79 ]. There are secondary consequences on vascular function, permeability, tone and on cellular transport. Thus, vital organ dysfunction depends on the extent and the location of parasitized erythrocyte sequestration. The extent of sequestration is heterogeneous, even at a microvascular level [ 80 ]. Magnetic resonance cerebral imaging in paediatric cerebral malaria shows a variety of different patterns. The brain is usually swollen, with restricted diffusion and variable evidence of oedema [ 81 ]. Isolated restricted white matter diffusion is associated with a better prognosis, while oedema is associated with a worse prognosis and an increased risk of sequelae [ 82 , 83 ]. The sequestered static red blood cells occupy space and cause cerebral engorgement [ 3 , 4 ] which contributes to raised intracranial pressure. Cytoadherent parasitised erythrocytes are not the only contributors to disease severity. Very high parasitaemias caused by non-sequestering malaria parasites cause severe malaria across the animal kingdom, and the simian parasite P. knowlesi is potentially lethal in humans – but these parasites do not cause cerebral malaria [ 2 , 84 ]. At very high parasite densities, erythrocyte dysfunction contributes to aggregation and impaired microcirculatory flow and oxygen delivery without cytoadherence. The precise causes of acute kidney injury and acute pulmonary oedema in severe malaria are unclear. Despite extensive research and much speculation over many years, there is little evidence for a primary immunopathological process in severe malaria, or for a final common pathological pathway with bacterial sepsis involving pro-inflammatory cytokine release. As described earlier, the pathobiology of severe malaria has been rich ground for hypothesis and speculation, often fueled by observations in a murine model, which is readily studied in the laboratory but has very little similarity to the human disease [ 5 ]. Observations in the murine ‘model’ have led to a long list of putative adjuvant interventions -all of which have proved either ineffective or harmful. This emphasizes the importance of distinguishing causal pathological processes in malaria from their consequences. From a clinical and operational perspective, it is essential to distinguish causal processes in severe malaria [ 70 ] from those processes in other severe infections with which severe malaria is very often confused (notably bacterial infections). The implications of misdiagnosis on operational disease management and pathobiology understanding are discussed below.
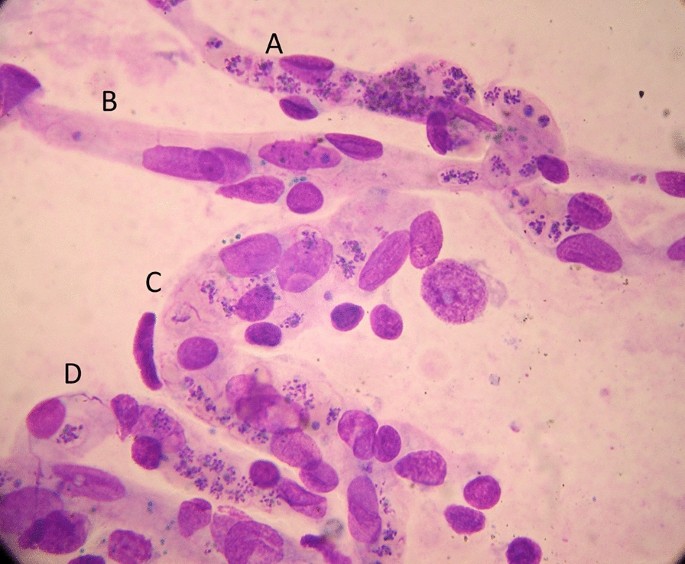
Brain smear from fatal cerebral malaria. The vessels ( A , C and D ) are packed with red cells containing P. falciparum schizonts (many of which are disrupted) and malaria pigment (haemozoin). Vessel segment B , by contrast, contains mainly unparasitized erythrocytes
The diagnosis of severe falciparum malaria
Severe malaria is a medical emergency. Appropriate immediate management is life-saving. An initial brief clinical examination assessing vital signs, peripheral perfusion, respiratory pattern, anaemia, jaundice and level of consciousness, and confirming the absence of rash should be followed rapidly by a blood smear or RDT confirmation [ 2 ]. In a low transmission setting, or with imported malaria, the diagnosis is straightforward. The results of a thin blood film or RDT can be available within minutes of taking a blood sample. Treatment should not be delayed if the blood results take longer than this. Microscopy examination of thin and thick blood smears provides both diagnostic and prognostic information; the parasite count, the parasite stage of development and the presence of neutrophil ingested pigment all have prognostic value and are readily assessed [ 2 , 32 , 33 , 85 – 87 ]. If the parasitaemia is high, the thin film assessment can take less than one minute. The RDT does not provide this quantitative prognostic information. In addition, the Pf HRP2 based RDTs can remain positive for days or weeks following a previous infection [ 88 ]. On the other hand, RDTs are useful in excluding a mixed P. falciparum infection in a patient with a blood slide diagnosis of vivax, malariae or ovale malaria [ 89 ], and they provide a diagnosis in patients who have received treatment with artemisinins several days previously and who are still severely ill (but have cleared their parasitaemia). This is common in adults presenting with acute kidney injury, which may take days or weeks to recover fully. In a low transmission setting, finding malaria parasites in the peripheral blood (by microscopy or RDT) is highly specific for malaria as the cause of illness. PCR diagnosis and speciation has proved very valuable in epidemiological studies, but PCR has no role in the acute diagnosis of severe malaria in endemic areas. It is too slow to be reported and it is too sensitive. PCR detects a higher proportion of people with previously asymptomatic (i.e. incidental) parasitaemia and therefore results in even more misdiagnosis of severe malaria.
At higher levels of transmission, the diagnosis of malaria as the cause of the presenting illness is much more difficult. The prevalence of microscopy or RDT detectable parasitaemia in apparently healthy individuals increases with transmission intensity, so the possibility of ascribing malaria incorrectly as the cause of illness rises too [ 90 ]. In sub-Saharan Africa a high proportion of apparently healthy children have detectable malaria parasitaemia. So how can severe illness caused by malaria parasites be distinguished from severe illness caused by something else with coincident parasitaemia? Good clinical examination is important but diagnostic uncertainty often persists. Other sites and sources of infection should be sought. In unconscious patients a lumbar puncture should be performed to exclude bacterial meningoencephalitis. Sequestration can be seen in-vivo by skilled indirect ophthalmoscopy along with other changes termed “malaria retinopathy” which have high specificity for cerebral malaria [ 44 , 92 – 94 ]. The buccal or rectal microcirculations can be visualized by direct orthogonal polarized light imaging [ 76 , 95 ]. In fatal cases sequestration can be demonstrated in the capillaries and venules of the brain in a post-mortem needle biopsy [ 80 , 96 , 97 ] (Fig. 5 ). But none of these specialist techniques are available in most places where severe malaria is managed. However, most hospitals and many health centres do have microscopes, and many centres now can perform full blood counts. Brief microscopy examination of a stained thin blood film provides valuable diagnostic and prognostic information [ 85 – 87 ]. The blood count is also informative (see below). Point of care blood glucose and lactate measurement is very important, particularly in unconscious or obtunded patients.
The immediate management of severe malaria
The outcomes of severe malaria and of severe sepsis are critically dependent on rapid access to health care and immediate treatment. Delays in giving artesunate and antibiotics are potentially lethal. Sadly, additional delays may still occur after the patient has reached hospital. Any patient suspected of having severe malaria should be treated as such [ 2 ].
Pre-referral
Severe malaria often presents initially far from the health centre or hospital. Referral for medical care can take hours, or sometimes days. At the community level, where giving parenteral drugs is not possible, pre-referral treatment of severe malaria with rectal artesunate reduces mortality by about 25% [ 98 ]. This community-based intervention has been very slow to be deployed, and now the WHO has recommended that it be stopped [ 99 ]. This recent WHO moratorium followed preliminary analysis of a large sequential observational study (“CARAMAL”) in Nigeria, Uganda and the Democratic Republic of the Congo [ 100 ]. Mortality reportedly increased after rectal artesunate was deployed, attributed to delays in the referral of severely ill children. However, there are serious concerns over the design of the study, potential major confounders, the accuracy of the diagnosis, and particularly—the causal interpretation of the results [ 101 ]. The CARAMAL study identified important problems with the referral of severely ill children, but it should not be used to evaluate the effectiveness of pre-referral rectal artesunate. The WHO moratorium appears to be a mistake. Rectal artesunate should be deployed to counter lethal delays in the referral of severe malaria. There are no pre-referral rectal antibiotic formulations unfortunately.
Health centre or hospital
At the level of the health centre or hospital in an area of higher malaria transmission (i.e. most of sub-Saharan Africa), the difficulty in distinguishing malaria from sepsis in children means that both parenteral anti-malarials (i.e. artesunate 3 mg/kg stat for children < 20 kg and 2.4 mg/kg for larger patients) and broad-spectrum antibiotics should be given together as soon as the diagnosis is suspected [ 2 , 73 ]. The most widely used empirical antibiotic treatment of severe sepsis is parenteral ceftriaxone. Administration of antibiotics should not be delayed. The drugs are very safe. Giving anti-malarials initially does no harm if the infection turns out to be bacterial or viral, and giving antibiotics does no harm if the infection is severe malaria only. Immediate administration of parenteral artesunate and broad-spectrum antibiotics to a child suspected of having severe malaria is the single most important life-saving intervention .
In low transmission settings where misdiagnosis is much less likely, it is reasonable in adults to treat only for severe malaria unless there is evidence for concomitant bacterial sepsis. However, antibiotics should be given to all adult patients with a very high parasitaemia (> 20%) [ 72 ], and should be given immediately if there is any unexplained clinical deterioration.
The misdiagnosis of severe malaria
Misdiagnosis of severe malaria is common. Its impact is underestimated. Misdiagnosis can result in incorrect treatment [ 73 ] and it dilutes and distorts genetic, epidemiology, burden of disease, long term impact, pathophysiology and therapeutic studies. In areas of higher transmission (e.g. Sub-Saharan Africa, Oceania), children are often diagnosed as having severe malaria because the blood test is “positive” but, in fact, they have another infection (often bacterial sepsis) causing their severe illness [ 90 ]. As severe bacterial infections have a higher mortality than severe malaria, and require antibiotic treatment, it is essential that both are treated immediately.
The relationship between malaria and bacterial infections is complex [ 71 – 75 , 102 – 109 ]. Severe malaria predisposes to bacterial infections. In a large prospective series of Vietnamese adults with strictly defined severe falciparum malaria (in whom diagnostic specificity for severe malaria is very high), the overall incidence of concomitant septicaemia (identified by positive blood culture) was 1.1% [ 72 ]. Hyperparasitaemia was a risk factor for bacteraemia; in patients with > 20% parasitemia the prevalence of concomitant bacteremia was 5.2%, whereas it was eight times lower (0.65%) in patients with lower parasitaemias. Concomitant bacteraemia is much more frequent in African children diagnosed with severe malaria. Approximately 6% of children hospitalized with a diagnosis of severe falciparum malaria in Africa are also bacteraemic [ 71 ]. As blood cultures are insensitive (but more specific- at least for most organisms) in diagnosis, the true proportion is likely to be much higher. Recent probabilistic assessments based on platelet and white blood cell counts, and also a quantitative parasite biomass indicator (plasma P f HRP2) [ 110 , 111 ] measured in large prospective studies of severe malaria in children, suggest that approximately one third of children diagnosed as having severe malaria in leading research centres actually had another condition (likely mainly sepsis) as the main cause of their illness [ 108 , 109 ] (Fig. 6 ). These probabilistic assessments were validated by comparing the prevalences of sickle cell trait (HbAS), which provides strong protection against severe malaria, between the two groups. The prevalence of HbAS was substantially lower in children with ‘true’ severe malaria than it was in those with a different cause of severe illness. Even for the relatively specific syndrome diagnosed as cerebral malaria, a post-mortem examination study, conducted in a leading research centre in Malawi, revealed a different pathology in one quarter of cases [ 112 ].
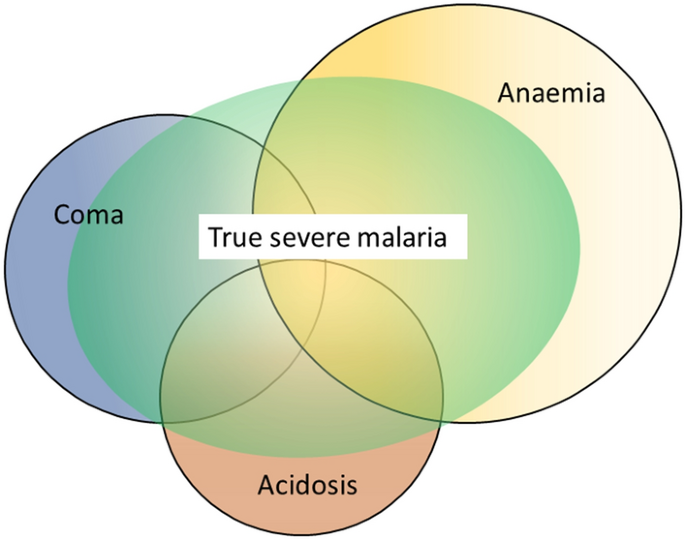
Misdiagnosis of severe falciparum malaria in African children -approximate relationships. [ 108 , 109 ]
The substantial overdiagnosis of severe malaria cannot be ignored in epidemiology, burden of disease, pathophysiology, genetic association and treatment studies. In the large evaluation of African children who had been admitted to leading research centres with a diagnosis of severe malaria (described above), mortality was higher in the likely misdiagnosed group, presumably because most had sepsis [ 108 , 109 ]. This suggests that malaria attributable mortality in African children may have been overestimated. If it has indeed been overestimated then the benefits of the substantial investments in malaria control measures and the provision of effective drugs (i.e. ACTs) have been underestimated [ 113 ]. Progress in reducing the number of deaths from severe malaria may have been better than estimated currently. The high rates of misdiagnosis, even in expert research centres, should be also accomodated by those formulating treatment guidelines and policies for severe malaria. Prompt, or preferably pre-referral, antibiotics must be given together with artesunate. Misdiagnosis also probably explains the difference in mortality reduction with artesunate compared with quinine in adults and children in Asia (where diagnostic specificity is high) compared with children in Africa (where diagnostic specificity is lower) (22.5%) [ 5 , 18 , 19 ]. In Asia the mortality reduction was 35% compared with 22.5% in African children (Fig. 7 ).
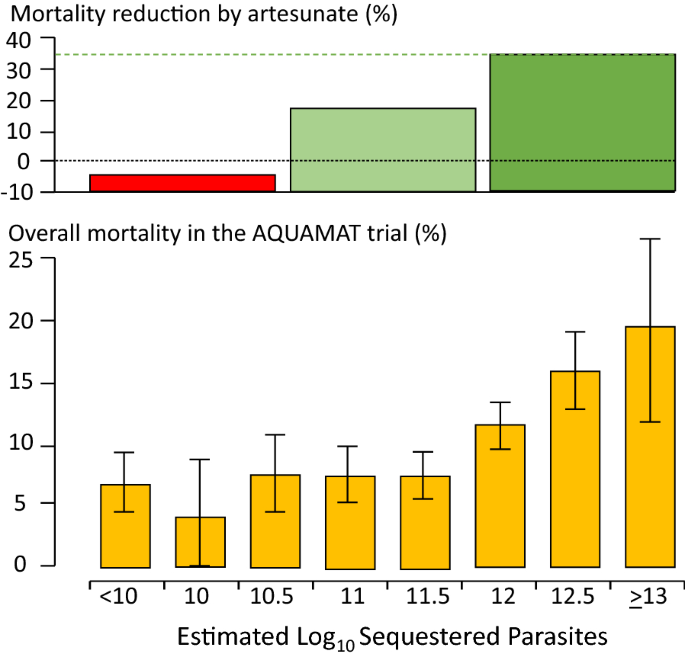
Relationship between estimated parasite biomass and mortality [ 4 , 110 ] in the large randomized controlled trial which compared artesunate and quinine in African children with severe malaria (AQUAMAT) [ 19 ]. The upper panel divides the patients into tertiles by treatment effect (reduction in mortality by artesunate). The mortality reduction in the preceding randomized controlled trial (SEAQUAMAT) which compared artesunate and quinine in Southeast Asia (where the diagnosis of severe malaria is more specific) is shown for comparison [ 18 ] (upper green dashed line). There was no treatment benefit from artesunate in patients in the lowest tertile of parasite biomass (red), likely corresponding to patients with another cause of severe illness (probably sepsis) and incidental parasitaemia [ 108 , 109 ]. The lower panel shows the corresponding relationship between mortality in the AQUAMAT study and the estimated total parasite numbers in the body derived from the admission plasma Pf HRP2 concentration [ 110 ]
Thus, it seems that some of the children with bacteraemia who are diagnosed as having severe malaria may genuinely have a high parasite biomass and extensive sequestration predisposing to bacterial sepsis—but the remainder have a primary bacterial infection and incidental or concomitant malaria. The interaction is complicated further as severe malarial anaemia predisposes to bacterial sepsis, and patients with uncomplicated malaria may have concomitant sepsis. At a population level, as malaria is controlled, the prevalence of sepsis declines (and so does the apparent protective benefit of HbAS against bacterial infections) pointing to the important contribution of malaria to bacterial sepsis, both concomitantly and sequentially [ 104 ]. It is very likely that the same problem of misdiagnosis occurs with Plasmodium vivax. In endemic areas low density chronic P. vivax parasitaemia is common, and so it is not unusual for severely ill patients to have incidental low-density infections, particularly if PCR is used for parasite detection.
The consequences of severe malaria
Children who are admitted with severe malaria anaemia have a high mortality in the months following admission [ 39 – 41 ]. This can be reduced by giving effective antimalarial prophylaxis, which indicates that repeated malaria infection is associated with death. Seizures and coma are associated with neurological deficit in surviving children [ 42 , 114 ]. The deficit is evident immediately following recovery in approximately 10% of children following cerebral malaria [ 115 ]. In two thirds of these cases the clinical picture is of stroke (suggesting a large cerebral vessel territory has been compromised). While many children recover fully, other deficits and behavioural and mental health problems often become apparent -particularly with detailed psychomotor and behavioural evaluation [ 42 , 114 , 116 – 118 ]. Epilepsy is increasingly recognized. These later onset epileptic, psychomotor and behavioural abnormalities may result from cerebral malaria, but they may also be pre-morbid conditions revealed by acute malaria [ 44 ].
Implications for the assessment and treatment of patients diagnosed with severe falciparum malaria
Overall, the consensus definitions of severe malaria described generally as “WHO criteria” have worked well to identify patients at risk and to inform research studies. From a practical case management perspective, specificity in the diagnosis is not as important as recognition that severe malaria could be the cause of the severe illness, and thus starting life-saving treatment with artesunate as soon as possible [ 2 ]. A new simple to administer artesunate formulation is under development. In children with suspected severe malaria in higher transmission settings parenteral broad-spectrum antibiotics should also be given immediately in all cases. As delay in receiving artesunate is a major contributor to death, it is important that referral to a facility capable of managing the sick patient should be as rapid as possible. Pre-referral rectal artesunate should be given to all children with suspected severe malaria [ 2 , 98 ]. The WHO moratorium [ 99 ] on rectal artesunate will hopefully soon be lifted [ 101 ]. Pre-referral antibiotic formulations should be developed.
For patients needing respiratory support, artificial ventilation has improved in recent years as the dangers of high inflation pressures have become evident [ 119 ]. Unfortunately, ventilators and trained staff are often unavailable in the areas where severe malaria is common. Otherwise, apart from the replacement of quinine by artesunate, the overall recommended management of severe malaria has changed relatively little over the past few decades. Aggressive fluid management (as in sepsis) [ 76 , 120 ], high volume (30 mL/kg) blood transfusions (in febrile children)[ 56 ], mannitol to reduce brain swelling [ 121 , 122 ], and unproven adjuvant therapies [ 5 ] have all proved harmful. Studies to optimize blood transfusion and fluid management are ongoing, but the general consensus is returning back to more cautious fluid management in severe malaria [ 7 , 123 ]. Evidence to date does not support red cell concentrates over whole blood in immediate management [ 57 ]. The optimum prevention and treatment of convulsions still remains uncertain. In a large randomized trial, conducted in a centre without access to artificial ventilation, seizure prevention by full dose prophylactic phenobarbitone increased mortality because of respiratory depression [ 16 ]. In a small trial levetiracetam proved safer [ 124 ], and may well become the anticonvulsant of choice, as it is in other settings, although more evidence is needed. Fosphenytoin was ineffective [ 125 ]. Renal replacement should start early in adults, blood glucose should be tested frequently and hypoglycaemia treated promptly [ 2 ]. Studies are ongoing to determine if paracetamol could attenuate renal injury in severe malaria [ 51 ]. If broad spectrum antibiotics have not been started (e.g. in adults in low transmission settings) there should be a low threshold for giving them if the patient deteriorates [ 2 ] -particularly in hyperparasitaemic patients [ 72 ].
From a research or epidemiology perspective, the low specificity of the current definition of severe malaria in African children is a challenge (Fig. 6 ). It has diluted therapeutic evaluations and distorted pathophysiology interpretations and genetic association studies. Most of the techniques to improve the specificity of diagnosis (notably indirect ophthalmoscopy or other methods of visualizing the microcirculation, or measurement of parasite biomass indicators such as plasma Pf HRP2 (Fig. 7 ) or plasma Pf DNA concentrations) are not readily available [ 91 – 95 , 110 , 111 , 126 ]—although simple dilution of a plasma sample and testing (by eye) with a Pf HRP2 RDT is not too difficult [ 127 ]. Importantly, the time-honoured peripheral thin blood smear does contain valuable information. Sadly, it is underused as a diagnostic and as a prognostic tool, and in many centres has been supplanted by the malaria rapid test, which, as currently used, does not provide prognostic information. In blood slides with parasitaemias over 0.5% the stage of parasite development can be easily and rapidly evaluated by microscopy. For any parasite density, finding > 50% tiny rings carries a relatively good prognosis whereas if > 20% parasites contain visible malaria pigment the prognosis is worse [ 85 ]. The proportion of neutrophils containing malaria pigment is also a very useful and readily assessed both for diagnosis and for prognostic assessment [ 86 , 87 ]. Most health facilities have at least one microscope – but sadly it is often old, fungus infested and accompanied by dirty slides, waterlogged methanol and outdated unfiltered stains. Malaria microscopy is well established but it is not well supported, and it is not prioritized in current malaria control funding. Hospital and health centres managing severe malaria should support good microscopy as an essential diagnostic and prognostic measure. Blood counts are valuable too. The haemoglobin concentration or haematocrit guides blood transfusion. The differential white count provides diagnostic information. Although severe malaria may be accompanied by leukocytosis, finding a high neutrophil count (often with toxic granules) together with lymphopenia points to bacterial sepsis. Thrombocytopenia is usual in severe malaria, but not in sepsis. In the recent large probabilistic assessments of severe malaria in African children, the combination of a platelet count of ≤ 150,000/μl and a plasma Pf HRP2 concentration of ≥ 1000 ng/ml had an estimated sensitivity of 74% and specificity of 93% in identifying true severe falciparum malaria [ 109 ] (Table 3 ). Future studies of severe malaria should always include differential blood counts, platelet counts and, preferably, a parasite biomass indicator. The anaemia criterion to define severe malaria should be reviewed.
Severe malaria caused by other malaria species
Plasmodium knowlesi, with its quotidian cycle, can sometimes cause fulminant infections in humans [ 84 , 128 , 129 ]. It does not sequester markedly so the parasite count is a good guide to biomass. P. knowlesi infections do not cause coma (cerebral malaria) but they can cause the other potentially lethal manifestations of severe malaria. Morphologically the younger P. knowlesi parasites resemble P. falciparum , whereas the older forms are often mistaken for Plasmodium malariae . Indeed any P. malariae parasitaemia over 1% should be regarded as P. knowlesi until proved otherwise. Uncomplicated P. vivax infections in a non-immune subject are often worse than uncomplicated P. falciparum malaria infections, causing high fever, weakness, malaise and sometimes rigors and prostration. Some of these vivax malaria illnesses warrant hospital admission. In the past 20 years there has been a marked increase in the number of reports of “severe” vivax malaria, mainly from India [ 130 – 132 ]. In some of the reports, the basis for the classification has been thrombocytopenia, which is not generally regarded as a criterion for severe malaria. Some patients hospitalized with P. vivax malaria die, particularly if they are old or debilitated [ 133 , 134 ]. P. vivax may sometimes cause acute pulmonary oedema-although the prognosis is better than in severe falciparum malaria [ 133 , 135 ]. But severe vivax malaria is overdiagnosed for the same reasons that severe falciparum malaria is overdiagnosed. Incidental parasitaemias are found in patients with severe anaemia or vital organ dysfunction and a causal relationship is inferred. In low transmission settings (i.e. most P. vivax endemic areas) Plasmodium vivax can cause severe illness, but the proportion of symptomatic cases which develop life-threatening illness is substantially less than for P. falciparum infections. However recurrent infections with P. vivax in areas of high transmission, such as the island of New Guinea, are associated with severe anaemia and substantial mortality both in the acute phase and over the longer term [ 136 – 138 ]. Further large and detailed cohort studies of hospitalized P. vivax infections would help clarify the prognostic associations and risk factors. But overall, the mortality of acute P. vivax infections is substantially lower than that of P. falciparum infections.
Conclusions
The apparent lack of progress in reducing the global death toll from malaria despite substantial investment suggests that we should reexamine the evidence, and review the current strategies to prevent and treat severe malaria [ 113 ]. The mortality of this common but frequently misdiagnosed syndrome can and should be reduced. Severe malaria deserves more attention.
Availability of data and materials
Review—individual trial data from trials conducted by MORU can be requested from the MORU data access committee.
Torti F. Therapeutice specialis ad febres quasdam perniciosas, (Venice 1712)
World Health Organization. Severe Malaria. Trop Med Int Health. 2014;19(Suppl 1):1–131.
Google Scholar
Marchiafava E, Bignami A. On summer-autumn malarial fevers. In: Marchiafava E, editor. Two monographs on malaria and the parasites of malarial fevers (translated from the first Italian edition by JH Thompson). London: New Sydenham Society; 1894. p. 1–232.
White NJ, Turner GD, Dondorp AM, Day NP. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208:192–8.
Article CAS PubMed PubMed Central Google Scholar
White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26:11–5.
Article PubMed PubMed Central Google Scholar
Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–4.
Article CAS PubMed Google Scholar
World Health Organization Malaria Action Programme. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1986;80(suppl):1–50.
Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, et al. Dexamethasone proves deleterious in cerebral malaria A double-blind trial in 100 comatose patients. N Engl J Med. 1982;306:313–9.
World Health Organization Division of Control of Tropical Diseases. Severe and complicated malaria. 2nd edn. Trans R Soc Trop Med Hyg 1990;2:1‑65.
Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Quart J Med. 1989;71:441–59.
CAS PubMed Google Scholar
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404.
Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87.
World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1-90.
Article Google Scholar
Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83.
van Hensbroek MB, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–7.
Article PubMed Google Scholar
Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–6.
The Artemether-Quinine Meta-analysis Study Group. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–50.
Dondorp A, Nosten F, Stepniewska K, Day N, White NJ; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005; 366: 717–25.
Dondorp AM, Fanello CE, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57.
Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 2010;9:97.
Kremsner PG, Taylor T, Issifou S, Kombila M, Chimalizeni Y, Kawaza K, et al. A simplified intravenous artesunate regimen for severe malaria. J Infect Dis. 2012;205:312–9.
Taylor T, Olola C, Valim C, Agbenyega T, Kremsner P, Krishna S, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–22.
Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health. 2006;11:115–24.
English M, Waruiru C, Amukoye E, Murphy S, Crawley J, Mwangi I, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55:521–4.
Imbert P, Sartelet I, Rogier C, Ka S, Baujat G, Candito D. Severe malaria among children in a low seasonal transmission area, Dakar, Senegal: influence of age on clinical presentation. Trans R Soc Trop Med Hyg. 1997;91:22–4.
von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–90.
WHO. Guidelines for the treatment of malaria. Geneva, World Health Organization, 2015.
Swellengrebel NH, de Buck A. Malaria in The Netherlands: Scheltema & Holkema Ltd. Amsterdam, 1938.
Snounou G, Pérignon JL. Malariotherapy–insanity at the service of malariology. Adv Parasitol. 2013;81:223–55.
Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56.
Field JW. Blood examination and prognosis in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1949;43:33–48.
White NJ, Chapman D, Watt G. The effects of multiplication and synchronicity on the vascular distribution of parasites in falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:590–7.
Kingston HW, Ghose A, Plewes K, Ishioka H, Leopold SJ, Maude RJ, et al. Disease severity and effective parasite multiplication rate in falciparum malaria. Open Forum Infect Dis. 2017;4:169.
Intharabut B, Kingston HW, Srinamon K, Ashley EA, Imwong M, Dhorda M, et al. Artemisinin resistance and stage dependency of parasite clearance in falciparum malaria. J Infect Dis. 2019;219:1483–9.
Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE, Maelankirri L, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11.
Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62.
Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TTH, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15:874–80.
Brand NR, Opoka RO, Hamre KE, John CC. Differing causes of lactic acidosis and deep breathing in cerebral malaria and severe malarial anemia may explain differences in acidosis-related mortality. PLoS ONE. 2016;11: e0163728.
Phiri KS, Calis JC, Faragher B, Nkhoma E, Ng’oma K, Mangochi B, et al. Long term outcome of severe anaemia in Malawian children. PLoS ONE. 2008;3: e2903.
Kwambai TK, Dhabangi A, Idro R, Opoka R, Watson V, Kariuki S, et al. Malaria chemoprevention in the post-discharge management of severe anemia. N Engl J Med. 2020;383:2242–54.
Kwambai TK, Mori AT, Nevitt S, van Eijk AM, Samuels AM, Robberstad B, et al. Post-discharge morbidity and mortality in children admitted with severe anaemia and other health conditions in malaria-endemic settings in Africa: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:474–83.
Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40.
Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994;88:426–8.
Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux ME, Kaplan PW, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–4.
Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59:336–44.
Herdman MT, Sriboonvorakul N, Leopold SJ, Douthwaite S, Mohanty S, Hassan MM, et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit Care. 2015;19: e317.
White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–6.
White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, et al. Hypoglycaemia in African children with severe malaria. Lancet. 1987;11:708–11.
Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988;319:1040–7.
Leopold SJ, Ghose A, Allman EL, Kingston HWF, Hossain A, Dutta AK, et al. Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J Infect Dis. 2019;219:1766–76.
Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69–77.
Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902.
White NJ. Anaemia and malaria. Malar J. 2018;17: e371.
Uyoga S, George EC, Bates I, Olupot-Olupot P, Chimalizeni Y, Molyneux EM, et al. Point-of-care haemoglobin testing in African hospitals: a neglected essential diagnostic test. Br J Haematol. 2021;193:894–901.
Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. N Engl J Med. 2019;381:407–19.
Maitland K, Olupot-Olupot P, Kiguli S, Chagaluka G, Alaroker F, Opoka RO, et al. Transfusion volume for children with severe anemia in Africa. N Engl J Med. 2019;381:420–31.
George EC, Uyoga S, M'baya B, Kyeyune Byabazair D, Kiguli S, Olupot-Olupot P et al. Whole blood versus red cell concentrates for children with severe anaemia: a secondary analysis of the transfusion and treatment of African children (TRACT) trial. Lancet Glob Health. 2022;10:e360-e368
Maitland K, Kiguli S, Olupot-Olupot P, Opoka RO, Chimalizeni Y, Alaroker F, et al. Transfusion management of severe anaemia in African children: a consensus algorithm. Br J Haematol. 2021;193:1247–59.
Paton RS, Kamau A, Akech S, Agweyu A, Ogero M, Mwandawiro C, et al. Malaria infection and severe disease risks in Africa. Science. 2021;373:926–31.
Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274–81.
Bodi JM, Nsibu CN, Longenge RL, Aloni MN, Akilimali PZ, Tshibassu PM, et al. Blackwater fever in Congolese children: a report of clinical, laboratory features and risk factors. Malar J. 2013;12:205.
Olupot-Olupot P, Engoru C, Uyoga S, Muhindo R, Macharia A, Kiguli S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in Eastern Uganda. Clin Infect Dis. 2017;64:939–46.
Opoka RO, Waiswa A, Harriet N, John CC, Tumwine JK, Karamagi C. Blackwater fever in Ugandan children with severe anemia is associated with poor post-discharge outcomes: a prospective cohort study. Clin Infect Dis. 2020;70:2247–54.
Conroy AL, Hawkes MT, Leligdowicz A, Mufumba I, Starr MC, Zhong K, et al. Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: pathophysiology and prognostic significance. BMC Med. 2022;20:221.
Kamau A, Paton RS, Akech S, Mpimbaza A, Khazenzi C, Ogero M, et al. Malaria hospitalisation in East Africa: age, phenotype and transmission intensity. BMC Med. 2022;20: e28.
Muriuki JM, Mentzer AJ, Mitchell R, Webb EL, Etyang AO, Kyobutungi C, et al. Malaria is a cause of iron deficiency in African children. Nat Med. 2021;27:653–8.
Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–99.
Pukrittayakamee S, Looareesuwan S, Keeratithakul D, Davis TM, Teja-Isavadharm P, Nagachinta B, et al. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharmacol. 1997;52:487–93.
Hanson J, Phu NH, Hasan MU, Charunwatthana P, Plewes K, Maude RJ, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: a retrospective analysis. BMC Med. 2015;13:97.
Leopold SJ, Watson JA, Jeeyapant A, Simpson JA, Phu NH, Hien TT, et al. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLoS Med. 2019;16: e1002858.
Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med. 2014;12:31.
Phu NH, Day NPJ, Tuan PQ, Mai NTH, Chau TTH, Chuong LV, et al. Concomitant bacteremia in adults with severe falciparum malaria. Clin Infect Dis. 2020;71:e465–70.
PubMed PubMed Central Google Scholar
White NJ, Watson JA, Uyoga S, Williams TN, Maitland KM. Substantial misdiagnosis of severe malaria in African children. Lancet. 2022;400:807.
Aung NM, Nyein PP, Kyi MM, Hanson J. Bacterial coinfection in adults with severe malaria. Clin Infect Dis. 2021;72:535–6.
White NJ.Reply to Aung, et al. Clin Infect Dis. 2021;72:536–8.
Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206:571–9.
Ishioka H, Ghose A, Charunwatthana P, Maude R, Plewes K, Kingston H, et al. Sequestration and red cell deformability as determinants of hyperlactatemia in falciparum Malaria. J Infect Dis. 2016;213:788–93.
Plewes K, Kingston HWF, Ghose A, Maude RJ, Herdman MT, Leopold SJ, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17:313.
Kingston HWF, Ghose A, Rungpradubvong V, Satitthummanid S, Herdman MT, Plewes K, et al. Cell-free hemoglobin is associated with increased vascular resistance and reduced peripheral perfusion in severe malaria. J Infect Dis. 2020;221:127–37.
Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, et al. A quantitative analysis of the microvascular sequestration of malaria parasite in the human brain. Am J Pathol. 1999;155:395–410.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–37.
Moghaddam SM, Birbeck GL, Taylor TE, Seydel KB, Kampondeni SD, Potchen MJ. Diffusion-weighted MR imaging in a prospective cohort of children with cerebral malaria offers insights into pathophysiology and prognosis. AJNR Am J Neuroradiol. 2019;40:1575–80.
CAS PubMed PubMed Central Google Scholar
Kampondeni S, Seydel KB, Zhang B, Small DS, Birbeck GL, Hammond CA, et al. Amount of brain edema correlates with neurologic recovery in pediatric cerebral malaria. Pediatr Infect Dis J. 2020;39:277–82.
Anstey NM, Grigg MJ, Rajahram GS, Cooper DJ, William T, Kho S, et al. Knowlesi malaria: Human risk factors, clinical spectrum, and pathophysiology. Adv Parasitol. 2021;113:1–43.
Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43.
Phu NH, Day NPJ, Diep TS, Ferguson DJP, White NJ. Intraleukocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:197–9.
Srinamon K, Watson JA, Silamut K, Intharabut B, Phu NH, Diep PT, et al. The prognostic and diagnostic value of intraleukocytic malaria pigment: an individual patient data pooled meta-analysis of 32,000 patients with severe falciparum malaria in Africa and Asia. Nat Comm, in press.
Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–26.
Mayxay M, Pukritrayakamee S, Chotivanich K, Imwong M, Looareesuwan S, White NJ. Identification of cryptic coinfection with Plasmodium falciparum in patients presenting with vivax malaria. Am J Trop Med Hyg. 2001;65:588–92.
Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212.
Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7.
Maude RJ, Beare NA, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2009;103:665–71.
Seydel KB, Fox LL, Glover SJ, Reeves MJ, Pensulo P, Muiruri A, et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis. 2012;206:309–18.
Barrera V, MacCormick IJC, Czanner G, Hiscott PS, White VA, Craig AG, et al. Neurovascular sequestration in paediatric P. falciparum malaria is visible clinically in the retina. Elife. 2018;7:e32208.
Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84.
Raja RN. Post-mortem examination in cerebral malaria: a new simple method of demonstrating parasites in the capillaries of the brain. Ind Med Gaz. 1922;57:298–9.
Milner DA Jr, Valim C, Luo R, Playforth KB, Kamiza S, Molyneux ME, et al. Supraorbital postmortem brain sampling for definitive quantitative confirmation of cerebral sequestration of Plasmodium falciparum parasites. J Infect Dis. 2012;205:1601–6.
Gomes MF, Faiz MA, Gyapong JO, Warsame M, Agbenyega T, Babiker A, et al. Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet. 2009;373:557–66.
WHO. The use of rectal artesunate as a pre- referral treatment for severe P. falciparum malaria. Geneva, World Health Organization, 2022.
Brunner NC, Omoluabi E, Awor P, Okitawutshu J, Tshefu Kitoto A, Signorell A, et al. Prereferral rectal artesunate and referral completion among children with suspected severe malaria in the Democratic Republic of the Congo. Nigeria and Uganda BMJ Glob Health. 2022;7: e008346.
Watson JA, Warsame M, Peto TJ, Onyamboko M, Fanello C, Dondorp AM, et al. Stopping prereferral rectal artesunate - a grave error. BMJ Glob Health. 2022;7: e010006.
Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–21.
Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–6.
Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Ndila C, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–23.
Nichols C, Cruz Espinoza LM, von Kalckreuth V, Aaby P, Tayeb M, Ali M, et al. Bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis. 2015;61(4):S372–9.
Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis. 2016;62(1):S23-31.
Bruneel F, Tubach F, Mira JP, Houze S, Gibot S, Huisse MG, et al. Imported falciparum malaria in adults: host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016;42:1588–96.
Watson JA, Ndila CM, Uyoga S, Macharia A, Nyutu G, Mohammed S, et al. Improving statistical power in severe malaria genetic association studies by augmenting phenotypic precision. Elife. 2021;10: e69698.
Watson JA, Uyoga S, Wanjiku P, Makale J, Nyutu GM, Mturi N, et al. Improving the diagnosis of severe malaria in African children using platelet counts and plasma Pf HRP2 concentrations. Sci Transl Med. 2022;14:5040.
Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, Mtove G, White LJ, Olaosebikan R, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 2012;9: e1001297.
Hendriksen IC, White LJ, Veenemans J, Mtove G, Woodrow C, Amos B, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207:351–61.
Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5.
White NJ, Day NPJ, Ashley EA, Smithuis FM, Nosten FH. Have we really failed to roll back malaria? Lancet. 2022;399:799–800.
John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9.
Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–43.
Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO, et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184.
Brim R, Mboma S, Semrud-Clikeman M, Kampondeni S, Magen J, Taylor T, et al. Cognitive Outcomes and Psychiatric Symptoms of Retinopathy-Positive Cerebral Malaria: Cohort Description and Baseline Results. Am J Trop Med Hyg. 2017;97:225–31.
Boivin MJ, Mohanty A, Sikorskii A, Vokhiwa M, Magen JG, Gladstone M. Early and middle childhood developmental, cognitive, and psychiatric outcomes of Malawian children affected by retinopathy positive cerebral malaria. Child Neuropsychol. 2019;25:81–102.
Dondorp AM, Hoang MNT, Mer M, Dünser MW, Mohanty S, Nakibuuka J, Management of severe malaria and severe dengue in resource-limited settings., et al. 9. In: Dondorp AM, Dünser MW, Schultz MJ, editors., et al., Sepsis Management in Resource-limited Settings [Internet]. Cham (CH): Springer; 2019. p. 2019.
Chapter Google Scholar
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95.
Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J. 2007;6:138.
Mohanty S, Mishra SK, Patnaik R, Dutt AK, Pradhan S, Das B, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349–55.
Ishioka H, Plewes K, Pattnaik R, Kingston HWF, Leopold SJ, Herdman MT, et al. Associations Between Restrictive Fluid Management and Renal Function and Tissue Perfusion in Adults With Severe Falciparum Malaria: A Prospective Observational Study. J Infect Dis. 2020;221:285–92.
Birbeck GL, Herman ST, Capparelli EV, Dzinjalamala FK, Abdel Baki SG, Mallewa M, et al. A clinical trial of enteral Levetiracetam for acute seizures in pediatric cerebral malaria. BMC Pediatr. 2019;19:399.
Gwer SA, Idro RI, Fegan G, Chengo EM, Mpoya A, Kivaya E, et al. Fosphenytoin for seizure prevention in childhood coma in Africa: a randomized clinical trial. J Crit Care. 2013;28:1086–92.
Imwong M, Woodrow CJ, Hendriksen IC, Veenemans J, Verhoef H, Faiz MA, et al. Plasma concentration of parasite DNA as a measure of disease severity in falciparum malaria. J Infect Dis. 2015;211:1128–33.
Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J. 2015;14:362.
Rajahram GS, Cooper DJ, William T, Grigg MJ, Anstey NM, Barber BE. Deaths from Plasmodium knowlesi malaria: case series and systematic review. Clin Infect Dis. 2019;69:1703–11.
Barber BE, Grigg MJ, Cooper DJ, van Schalkwyk DA, William T, Rajahram GS, et al. Clinical management of Plasmodium knowlesi malaria. Adv Parasitol. 2021;113:45–76.
Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481.
Kojom Foko LP, Arya A, Sharma A, Singh V. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect. 2021;82:231–46.
Phyo AP, Dahal P, Mayxay M, Ashley EA. Clinical impact of vivax malaria: a collection review. PLoS Med. 2022;19: e1003890.
Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax : clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201.
Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67-74.
Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria?: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8: e3071.
Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo JR, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua. Indonesia BMC Med. 2014;12:217.
Patriani D, Arguni E, Kenangalem E, Dini S, Sugiarto P, Hasanuddin A, et al. Early and late mortality after malaria in young children in Papua. Indonesia BMC Infect Dis. 2019;19:922.
Dini S, Douglas NM, Poespoprodjo JR, Kenangalem E, Sugiarto P, Plumb ID, et al. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18:28.
Download references
Acknowledgements
I am a Wellcome Trust Principal Fellow (093956/Z/10/C). I am very grateful to my colleagues in the Mahidol Oxford Research Unit and associated research programmes for all their advice and help.
NJW is a Wellcome Trust Principal Fellow (093956/Z/10/C).
Author information
Authors and affiliations.
Mahidol Oxford Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Nicholas J. White
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
The author wrote read and approved the final manuscript.
Corresponding author
Correspondence to Nicholas J. White .
Ethics declarations
Ethics approval and consent to participate.
Not relevant.
Consent for publication
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
White, N.J. Severe malaria. Malar J 21 , 284 (2022). https://doi.org/10.1186/s12936-022-04301-8
Download citation
Received : 12 August 2022
Accepted : 23 September 2022
Published : 06 October 2022
DOI : https://doi.org/10.1186/s12936-022-04301-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Collection 25 April 2022
Malaria research toward disease elimination
Malaria is a life-threatening febrile illness caused by Plasmodium spp. parasites that are transmitted to humans by infected female Anopheles mosquitoes. According to WHO, 241 million cases occurred in 2020, killing an estimated 627,000 people. Children and pregnant women are the most vulnerable groups accounting for more than two-thirds of malaria-related deaths. In 2015 the World Health Assembly provided a global strategy aiming to reduce the malaria burden and mortality rates by at least 90% in 35 countries and to prevent a resurgence in malaria-free countries by 2030. To reach this goal, in October 2021, WHO approved the broad use of the world’s first vaccine against a human parasitic disease for young children living in endemic areas to reduce deadly severe malaria risk.
On the occasion of World Malaria Day 2022, themed ‘Harness innovation to reduce the malaria disease burden and save lives’ the infectious disease team at Nature Communications has curated a collection of research articles to shed light on recent progress in malaria research.
Madlen Luckner
Senior Editor, Nature Communications
Plasmodium spp. and vector biology
Tackling malaria transmission at a single cell level in an endemic setting in sub-saharan africa.
Studying malaria transmission biology using scRNA-sequencing provides information on within-host strain diversity and transcriptional states. Here, we comment on our collaborative efforts at establishing single-cell capacities in sub-Saharan Africa and the challenges encountered in Mali’s endemic setting.
- Antoine Dara
- Sunil Kumar Dogga
- Mara K. N. Lawniczak
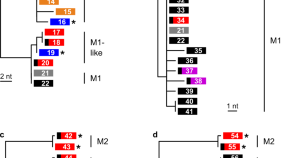
Zoonotic origin of the human malaria parasite Plasmodium malariae from African apes
Plasmodium malariae is a cause of malaria in humans and related species have been identified in non-human primates. Here, the authors use genomic analyses to establish that human P. malariae arose from a host switch of an ape parasite whilst a species infecting New World monkeys can be traced to a reverse zoonosis.
- Lindsey J. Plenderleith
- Paul M. Sharp
Functional genomics of RAP proteins and their role in mitoribosome regulation in Plasmodium falciparum
The function of RNA-binding domain abundant in Apicomplexans (RAP) protein family members is largely unknown. Here, using high-throughput functional genomics, including metabolomics, Hollin et al . characterize two RAP proteins that are essential for Plasmodium falciparum survival and control mitochondrial rRNAs.
- Thomas Hollin
- Steven Abel
- Karine G. Le Roch
Composition and stage dynamics of mitochondrial complexes in Plasmodium falciparum
Applying complexome profiling, Evers et al. unravel the composition of mitochondrial oxidative phosphorylation complexes in P. falciparum asexual and sexual blood stages. Abundance of these complexes differs between both stages, supporting the hypothesis that a mitochondrial metabolic switch is central to gametocyte development and functioning.
- Felix Evers
- Alfredo Cabrera-Orefice
- Taco W. A. Kooij
A single-cell atlas of Plasmodium falciparum transmission through the mosquito
Here the authors use single-cell RNA-seq to profile the transmission stages of the human malaria parasite Plasmodium falciparum as it progresses through the Anopheles mosquito. They highlight unique patterns of gene usage throughout this development and identify potential pleiotropic genes that function at multiple life cycle stages.
- Eliana Real
- Virginia M. Howick
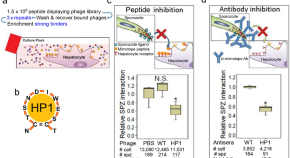
Plasmodium sporozoite phospholipid scramblase interacts with mammalian carbamoyl-phosphate synthetase 1 to infect hepatocytes
After transmission of Plasmodium sporozoites from infected mosquitoes, parasites first infect hepatocytes. Here, Cha et al. identify a sporozoite ligand (phospholipid scramblase) and the hepatocytic receptor (carbamoyl-phosphate synthetase 1) as relevant for hepatocyte invasion and show that an antibody to hepatocyte-binding peptide 1 (HP1), which structurally mimics the sporozoite ligand, partially protects mice from infection.
- Sung-Jae Cha
- Min-Sik Kim
- Marcelo Jacobs-Lorena
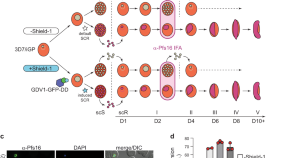
CRISPR/Cas9-engineered inducible gametocyte producer lines as a valuable tool for Plasmodium falciparum malaria transmission research
During each replication cycle of P. falciparum in the human bloodstream, a small proportion of parasites commits to sexual development and differentiates into transmission-relevant gametocytes. Applying CRISPR-based genome editing, Boltryk et al. engineer P. falciparum lines with sexual commitment rates of 75% to promote future studies on gametocyte biology.
- Sylwia D. Boltryk
- Armin Passecker
- Till S. Voss
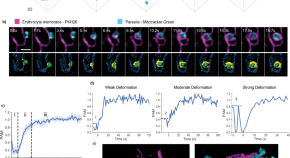
4D analysis of malaria parasite invasion offers insights into erythrocyte membrane remodeling and parasitophorous vacuole formation
Here, Geoghegan, Evelyn et al. provide a lattice light-sheet microscopy based 4D imaging pipeline to quantitatively investigate Plasmodium spp. invasion and show that the nascent parasitophorous vacuole is predominantly formed from host’s erythrocyte membrane and undergoes continuous remodeling throughout invasion.
- Niall D. Geoghegan
- Cindy Evelyn
- Kelly L. Rogers
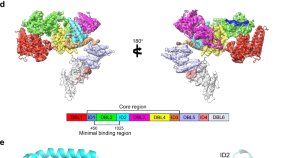
Cryo-EM reveals the architecture of placental malaria VAR2CSA and provides molecular insight into chondroitin sulfate binding
In placental malaria, interactions between parasite protein VAR2CSA and human glycosaminoglycan chondroitin sulfate A (CS) sequesters infected red blood cells in the placenta. Here, the authors provide cryo-EM structures of VAR2CSA and placental CS, identifying molecular interactions that could guide design of placental malaria vaccines.
- Kaituo Wang
- Robert Dagil
- Ali Salanti
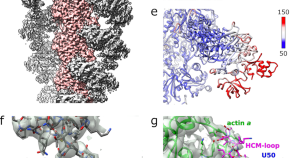
The actomyosin interface contains an evolutionary conserved core and an ancillary interface involved in specificity
Plasmodium falciparum moves by an atypical process called gliding motility which comprises of atypical myosin A (PfMyoA) and filaments of the dynamic and divergent PfActin-1 (PfAct1). Here authors present the cryo-EM structure of PfMyoA bound to filamentous PfAct1 stabilized with jasplakinolide and provide insights into the interactions that are required for the parasite to produce the force and motion required for infectivity.
- Julien Robert-Paganin
- Xiao-Ping Xu
- Dorit Hanein
20S proteasomes secreted by the malaria parasite promote its growth
Plasmodium falciparum secretes extracellular vesicles (EVs) while growing inside red blood cells (RBCs). Here the authors show that these EVs contain assembled and functional 20S proteasome complexes that remodel the cytoskeleton of naïve human RBCs, priming the RBCs for parasite invasion.
- Neta Regev-Rudzki
Malaria parasites both repress host CXCL10 and use it as a cue for growth acceleration
The chemokine CXCL10 is associated with pathogenesis of cerebral malaria in Plasmodium falciparum infection. Here the authors show that P. falciparum produces extracellular vesicles laden with RNAs that are taken up by monocytes resulting in a RIG-I and HUR-1 mediated mechanism of inhibition of CXCL10 protein translation.
- Yifat Ofir-Birin
- Hila Ben Ami Pilo
Structural basis of LAIR1 targeting by polymorphic Plasmodium RIFINs
RIFINs are Plasmodium surface antigens that suppress the immune response by binding inhibitory receptors such as LAIR1. Here, Xu et al . characterize the interaction between RIFIN-variable 2 domain and a LAIR1 domain and identify LAIR1-binding RIFINs in several Plasmodium species.
- Peter D. Kwong
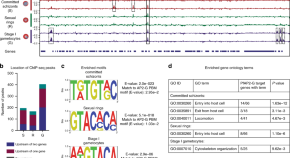
Dissecting the role of PfAP2-G in malaria gametocytogenesis
The transcription factor PfAP2-G is a key determinant of sexual commitment in Plasmodium falciparum . Here, Josling et al. define the transcriptional regulatory network of PfAP2-G by identifying its DNA binding sites genome-wide, which vary depending on the route of sexual conversion and rely on interactions with the PfAP2-I transcription factor.
- Gabrielle A. Josling
- Timothy J. Russell
- Manuel Llinás
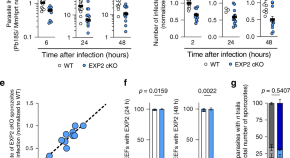
Plasmodium translocon component EXP2 facilitates hepatocyte invasion
While the role of Plasmodium EXP2 protein as translocon component of blood stage parasites is established, its functional role in liver stage parasites remains unclear. Here, Mello-Vieira et al. reveal that EXP2 pore-forming activity induces hepatocyte membrane repair and hence is critical for hepatocyte invasion.
- João Mello-Vieira
- Francisco J. Enguita
- Maria M. Mota
Co-option of Plasmodium falciparum PP1 for egress from host erythrocytes
Plasmodium protein phosphatase PP1 is essential for the asexual proliferation of malaria parasites. Here the authors show that PP1 regulates egress of parasites from host red blood cells, integrating parasite intrinsic pathways with environmental signals for release into the bloodstream.
- Aditya S. Paul
- Alexandra Miliu
- Manoj T. Duraisingh
Malaria parasites regulate intra-erythrocytic development duration via serpentine receptor 10 to coordinate with host rhythms
The mechanism underlying periodicity of Plasmodium ’s intra-erythrocytic developmental cycle (IDC) is unclear. Here, Subudhi et al. show that serpentine receptor 10 (SR10) plays a role in regulating the schedule of the IDC in line with the timing of host daily rhythms.
- Amit K. Subudhi
- Aidan J. O’Donnell
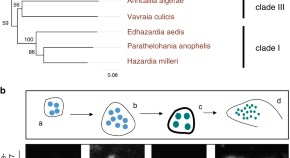
A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes
Mircobial symbionts of mosquitoes can affect transmission of human pathogens. Here, Herren et al . identify a microsporidian symbiont in Anopheles gambiae that impairs transmission without affecting mosquito fecundity or survival.
- Jeremy K. Herren
- Lilian Mbaisi
- Steven P. Sinkins
PfCERLI1 is a conserved rhoptry associated protein essential for Plasmodium falciparum merozoite invasion of erythrocytes
Rhoptries are essential organelles for invasion of erythrocytes by Plasmodium . Here, the authors characterize the rhoptry-associated protein CERLI1 using quantitative super-resolution microscopy, showing that it is important for parasite invasion and secretion of rhoptry proteins including vaccine antigens.
- Benjamin Liffner
- Sonja Frölich
- Danny W. Wilson
Adaptation of Plasmodium falciparum to humans involved the loss of an ape-specific erythrocyte invasion ligand
Here, Proto et al. show that human infective Plasmodium falciparum isolates contain an inactivating mutation in the erythrocyte invasion associated gene PfEBA165 , while homologues of ape-infective Laverania species are intact, and that expression of intact PfEBA165 is incompatible with parasite growth in human erythrocytes.
- William R. Proto
- Sasha V. Siegel
- Julian C. Rayner
Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development
Plasmodium growth is adapted to the reproductive cycle of mosquitoes, but underlying mechanisms are unclear. Here, Lampe et al. show that the blood-meal induced miR-276 balances the termination of the mosquito amino acid catabolism and egg development, providing nutrients for Plasmodium sporozoite development.
- Marius Jentzsch
- Elena A. Levashina
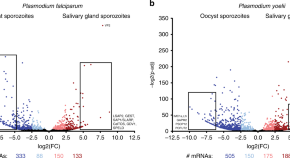
Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites
Here, the authors report transcriptomes and proteomes of oocyst sporozoite and salivary gland sporozoite stages in rodent-infectious Plasmodium yoelii parasites and human infectious Plasmodium falciparum parasites and define two waves of translational repression during sporozoite maturation.
- Scott E. Lindner
- Kristian E. Swearingen
- Stefan H. I. Kappe

Plasmodium vivax transcriptomes reveal stage-specific chloroquine response and differential regulation of male and female gametocytes
Plasmodium vivax biology is not well understood, due to a lack of in vitro culture systems and difficulties associated with studying clinical blood samples. Here, Kim et al. use gene expression profiles from P. vivax infected patient blood and show stage-specific chloroquine response and differential regulation of male and female gametocytes.
- Jean Popovici
- David Serre
An essential contractile ring protein controls cell division in Plasmodium falciparum
Schizogony is essential for blood stage infection of Plasmodium parasites and produces several daughter cells. Here, Rudlaff et al. identify PfCINCH and interacting proteins as essential components of the basal complex required to establish daughter cell boundaries.
- Rachel M. Rudlaff
- Stephan Kraemer
- Jeffrey D. Dvorin
Plasmodium myosin A drives parasite invasion by an atypical force generating mechanism
Here, Robert-Paganin et al. show that myosin A from Plasmodium falciparum is critical for red blood cell invasion and that non-canonical interactions and regulated phosphorylation are important for force generation during parasite invasion.
- James P. Robblee
- Anne Houdusse
Prevention strategies and immunology
Malaria in 2022: increasing challenges, cautious optimism.
Malaria cases and deaths remain unacceptably high and are resurgent in several settings, though recent developments inspire optimism. This includes the approval of the world’s first malaria vaccine and results from novel vaccine candidates and trials testing innovative combinatorial interventions.
- Prasanna Jagannathan
- Abel Kakuru

Mark-release-recapture experiment in Burkina Faso demonstrates reduced fitness and dispersal of genetically-modified sterile malaria mosquitoes
Release of genetically-modified sterile mosquitoes is a potential method of malaria control but has yet to be tested in the field. Here, the authors perform a mark-release-recapture experiment and show that genetically-modified mosquitoes have reduced survival and dispersal compared to wild-types.
- Franck Adama Yao
- Abdoul-Azize Millogo
- Abdoulaye Diabaté
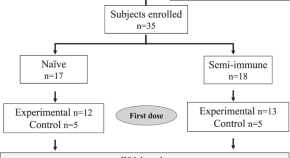
Randomized clinical trial to assess the protective efficacy of a Plasmodium vivax CS synthetic vaccine
In this phase 2 clinical trial, the authors assess protective efficacy of a Plasmodium vivax circumsporozoite vaccine in naïve and semi-immune individuals from controlled human malaria infection as well as antibody and IFN-γ response to vaccination.
- Myriam Arévalo-Herrera
- Xiomara Gaitán
- Sócrates Herrera
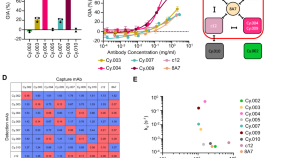
Heterotypic interactions drive antibody synergy against a malaria vaccine candidate
Antibodies can have synergistic effects, but mechanisms are not well understood. Here, Ragotte et al . identify three antibodies that bind neighbouring epitopes on CyRPA, a malaria vaccine candidate, and show that lateral interactions between the antibodies slow dissociation and inhibit parasite growth synergistically.
- Robert J. Ragotte
- David Pulido
- Simon J. Draper
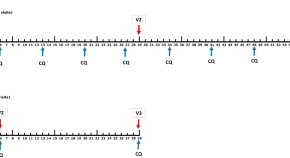
Heterologous protection against malaria by a simple chemoattenuated PfSPZ vaccine regimen in a randomized trial
In this placebo-controlled trial, 10/13 malaria naïve subjects immunized with a simplified regimen of chemoattenuated P. falciparum sporozoites, PfSPZ-CVac, show sterile protection from heterologous malaria challenge. Immunization was well tolerated and induced high levels of anti-PfCSP antibodies.
- Zita Sulyok
- Rolf Fendel
- Peter G. Kremsner
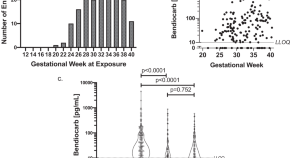
Exposure to pesticides in utero impacts the fetal immune system and response to vaccination in infancy
Control of mosquito populations using pesticides is important for malaria elimination, but effects of pesticides on humans aren’t well understood. Here, Prahl et al. show in a cohort of pregnant Ugandan women and their infants that household spraying with bendiocarb affects the fetal immune system and response to vaccination in infancy.
- Pamela Odorizzi
- Margaret E. Feeney
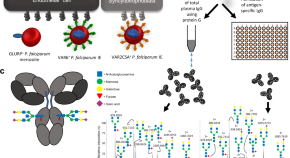
Afucosylated Plasmodium falciparum -specific IgG is induced by infection but not by subunit vaccination
Here, Larsen et al. describe differences in Fc fucosylation of P. falciparum PfEMP1-specific IgG produced in response to natural infection versus VAR2CSA-type subunit vaccination, which leads to differences in the ability to induce FcγRIIIa-dependent natural killer cell degranulation.
- Mads Delbo Larsen
- Mary Lopez-Perez
- Gestur Vidarsson
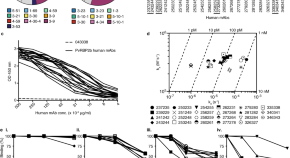
Naturally acquired blocking human monoclonal antibodies to Plasmodium vivax reticulocyte binding protein 2b
Plasmodium vivax reticulocyte binding protein 2b (PvRBP2b) is important for invasion of reticulocytes and PvRBP2b antibodies correlate with protection. Here, Chan et al. isolate and characterize anti-PvRBP2b human monoclonal antibodies and describe mechanisms by which these antibodies inhibit invasion.
- Li-Jin Chan
- Anugraha Gandhirajan
- Wai-Hong Tham
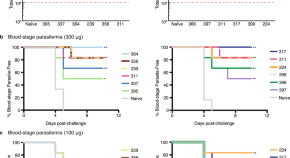
Structural and biophysical correlation of anti-NANP antibodies with in vivo protection against P. falciparum
The most advanced P. falciparum circumsporozoite protein (PfCSP)-based malaria vaccine confers partial protection. Here, Pholcharee et al. present crystal structures, binding affinities/kinetics, and in vivo protection of 8 anti-NANP antibodies to understand in vivo protection of PfCSP-targeting antibodies.
- Tossapol Pholcharee
- Ian A. Wilson
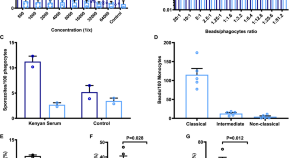
Mechanisms and targets of Fcγ-receptor mediated immunity to malaria sporozoites
Antibodies plays critical roles in the adaptive immune response to infectious agents including malaria. Here the authors defined antibody interactions with -Fcγ-receptors expressed on immune cells with sporozoites of Plasmodium falciparum, and identified specific target epitopes of antibodies.
- Gaoqian Feng
- Bruce D. Wines
- James G. Beeson
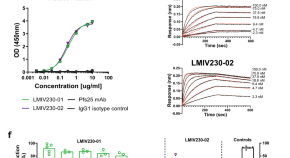
A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes
Vaccines that interrupt malaria transmission will be important tools for malaria elimination. Here the authors identify a human monoclonal antibody from Pfs230 vaccinated individuals that blocks transmission of Plasmodium falciparum to mosquitoes in a complement-dependent manner and reacts with gamete surface.
- Camila H. Coelho
- Wai Kwan Tang
- Patrick E. Duffy
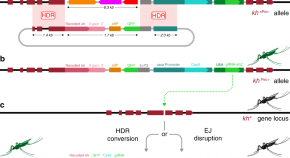
Efficient population modification gene-drive rescue system in the malaria mosquito Anopheles stephensi
Gene drives may be impeded by the generation of resistant alleles following NHEJ. Here the authors develop a recoded gene-drive rescue system for the malaria mosquito, Anopheles stephensi, that targets the drive to the kynurenine hydroxylase gene for negative selection against mutated alleles.
- Adriana Adolfi
- Valentino M. Gantz
- Anthony A. James
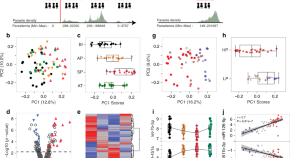
Integrative genomic analysis reveals mechanisms of immune evasion in P . falciparum malaria
Here, the authors identify signatures of miRNA expression differentiation associated with Plasmodium falciparum infection and parasitemia in a longitudinal pediatric cohort in Burkina Faso. In particular, expression of several miRNAs known to promote lymphocyte cell death is affected during infection.
- Mame Massar Dieng
- Aïssatou Diawara
- Youssef Idaghdour
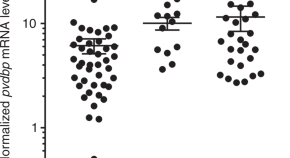
Amplification of Duffy binding protein-encoding gene allows Plasmodium vivax to evade host anti-DBP humoral immunity
Duffy binding protein (DBP) of Plasmodium vivax is important for invasion and is a potential vaccine candidate. Here, the authors show that PvDBP gene amplification protects P vivax in vitro against invasion inhibitory human monoclonal antibodies and is associated to infection of patients with PvDBP binding inhibitory antibodies.
- Camille Roesch
- Benoit Witkowski
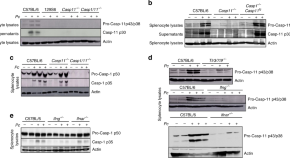
Caspase-8 mediates inflammation and disease in rodent malaria
Inflammasome activation plays a role in malaria pathogenesis, but details aren’t well understood. Here, the authors show that caspase-8 is a central mediator of systemic inflammation in rodent malaria and that monocytes from malaria patients express active caspases-1, -4 and -8.
- Larissa M. N. Pereira
- Patrícia A. Assis
- Ricardo T. Gazzinelli
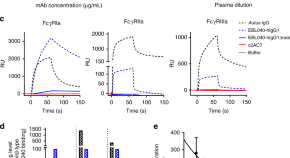
A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates
Proof of protection against blood-stage P. falciparum malaria by a single immunological mechanism has been elusive. Here, using engineered anti-PfRH5 chimeric monoclonal antibodies in non-human primates, the authors show that high levels of merozoite-neutralizing antibodies can achieve protection.
- Alexander D. Douglas
- G. Christian Baldeviano
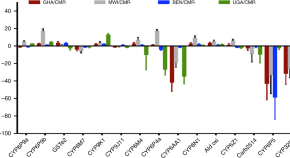
Cis -regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus
Bed nets treated with insecticides have been instrumental in reducing malaria mortality, but insecticide resistance is on the rise. Here, Mugenzi et al. identify genetic variants in the P450 gene CYP6P9b of Anopheles funestus that associate with insecticide resistance and develop a PCR-based diagnostic assay to help identify pyrethroid-resistant strains.
- Leon M. J. Mugenzi
- Benjamin D. Menze
- Charles S. Wondji
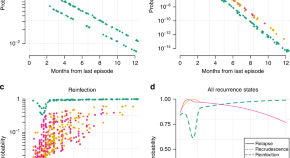
Resolving the cause of recurrent Plasmodium vivax malaria probabilistically
Relapse, reinfection and recrudescence can all cause recurrent infection after treatment of Plasmodium vivax malaria in endemic areas, but are difficult to distinguish. Here the authors show that they can be differentiated probabilistically and thereby demonstrate the high efficacy of primaquine treatment in preventing relapse.
- Aimee R. Taylor
- James A. Watson
- Nicholas J. White
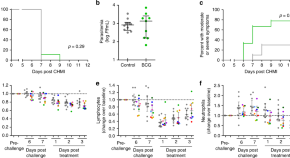
Outcomes of controlled human malaria infection after BCG vaccination
Immune activation induces long-term alterations of setpoints, impacting responses to subsequent unrelated stimuli. Here the authors show that volunteers vaccinated with BCG respond to controlled human malaria infection with increased clinical symptoms and an inverse correlation between immune activation markers and parasitemia.
- L. Charlotte J. de Bree
- Robert W. Sauerwein
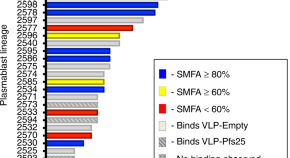
Potent antibody lineage against malaria transmission elicited by human vaccination with Pfs25
Pfs25 is a transmission-blocking vaccine candidate for Plasmodium . Here, McLeod et al. analyze the antibody response to Pfs25 in sera from a clinical trial evaluating a Pfs25 vaccine candidate, identify a potent transmission-blocking antibody and determine recognized epitopes on Pfs25.
- Brandon McLeod
- Kazutoyo Miura
- Jean-Philippe Julien
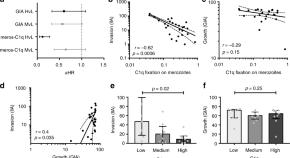
Targets of complement-fixing antibodies in protective immunity against malaria in children
Antibodies against Plasmodium falciparum merozoites that fix complement can inhibit blood-stage replication. Here, Reiling et al. show that complement-fixing antibodies strongly correlate with protective immunity in children, identify the merozoite targets, and predict antigen combinations that should result in strong protection.
- Linda Reiling
- Michelle J. Boyle

Innate immunity limits protective adaptive immune responses against pre-erythrocytic malaria parasites
Here, Minkah et al . show that, while immunization with replication-competent Plasmodium parasites can confer sterile protection against infection, it also induces a type I interferon response that adversely affects anti-malaria immunity by affecting numbers of protective hepatic CD8 T cells and CD8 T cell function.
- Nana K. Minkah
- Brandon K. Wilder
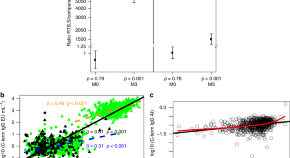
Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy
RTS,S/AS01E has been tested in a phase 3 malaria vaccine trial and has shown partial efficacy in children and infants. Here, the authors analyze IgG concentration and avidity to CSP in ~1000 participants and show that IgG avidity to the C-terminus of CSP is significantly associated with vaccine-mediated protection.
- Carlota Dobaño
- Hèctor Sanz
- Claudia Daubenberger
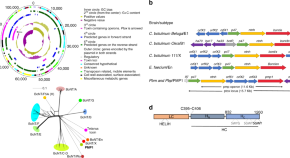
A neurotoxin that specifically targets Anopheles mosquitoes
So far identified clostridial neurotoxins target vertebrates. Here, Contreras et al. isolate the clostridial-like neurotoxin PMP1 from Paraclostridium bifermentans strains and show that it selectively targets anopheline mosquitoes by targeting mosquito syntaxin.
- Estefania Contreras
- Geoffrey Masuyer
- Sarjeet S. Gill
Dual RNA-seq identifies human mucosal immunity protein Mucin-13 as a hallmark of Plasmodium exoerythrocytic infection
Host-parasite interactions during the exoerythrocytic stage of Plasmodium infection remains poorly understood. Using dual RNA-Seq, the authors show that human mucosal immunity protein mucin-13 is upregulated during Plasmodium hepatic-stage infection and marks infected cells independent of tested Plasmodium species.
- Gregory M. LaMonte
- Pamela Orjuela-Sanchez
- Elizabeth A. Winzeler
Case control strategies and drug development
Distinct kinetics of antibodies to 111 Plasmodium falciparum proteins identifies markers of recent malaria exposure
Serological markers of recent Plasmodium falciparum infection could be useful to estimate incidence. Here, the authors identify a combination of five serological markers to detect exposure to infection within the previous three months with >80% sensitivity and specificity.
- Victor Yman
- Anna Färnert
Preclinical characterization and target validation of the antimalarial pantothenamide MMV693183
Here, de Vries et al. perform a pre-clinical characterization of the antimalarial compound MMV693183: the compound targets acetyl-CoA synthetase, has efficacy in humanized mice against Plasmodium falciparum infection, blocks transmission to mosquito vectors, is safe in rats, and pharmacokinetic-pharmacodynamic modeling informs about a potential oral human dosing regimen.
- Laura E. de Vries
- Patrick A. M. Jansen
- Koen J. Dechering
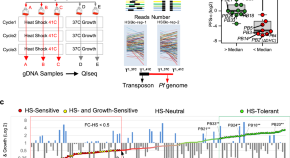
The apicoplast link to fever-survival and artemisinin-resistance in the malaria parasite
Repeating fever is a hallmark of malaria. Here, a large-scale forward genetic screen in malaria-causing Plasmodium falciparum identifies genes associated with parasite tolerance to host fever, including apicoplast targeted isoprenoid biosynthesis—sharing features with artemisinin resistance.
- Chengqi Wang
- John H. Adams
Magneto-optical diagnosis of symptomatic malaria in Papua New Guinea
Here Arndt et al. establish rotating-crystal magneto-optical detection (RMOD) as a near-point-of-care diagnostic tool for malaria detection and report a sensitivity and specificity of 82% and 84%, respectively, as validated by analyzing a clinical population in a high transmission setting in Papua New Guinea.
Artemisinin-resistant K13 mutations rewire Plasmodium falciparum ’ s intra-erythrocytic metabolic program to enhance survival
The emergence and spread of artemisinin resistance has compromised antimalarial efficacy. Here, Mok et al. apply quantitative transcriptomics, proteomics, and metabolomics to provide evidence that K13 mutations alter multiple aspects of the parasite’s intra-erythrocytic development to enhance survival following artemisinin treatment.
- Barbara H. Stokes
- David A. Fidock
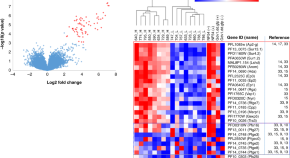
The transcriptome of circulating sexually committed Plasmodium falciparum ring stage parasites forecasts malaria transmission potential
Malaria gametocytes are sexual-stage parasites transmitted from mammalian host’s blood back to their insect vector. Here, Prajapati et al. identify gametocyte-committed ring-stage biomarkers allowing to forecast malaria transmission potential.
- Surendra K. Prajapati
- Ruth Ayanful-Torgby
- Kim C. Williamson
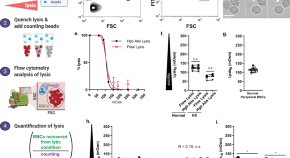
Plasmodium vivax infection compromises reticulocyte stability
During Plasmodium intra-erythrocytic developmental, parasites compromise the structural integrity of host red-blood cells. Here, Clark et al. develop a flow cytometric osmotic stability assay to show that P. vivax infection destabilizes host reticulocytes, which are less stable than P. falciparum-infected normocytes.
- Martha A. Clark
- Usheer Kanjee
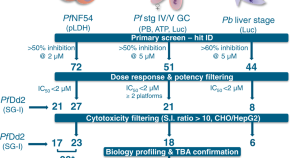
Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box
Here, Reader et al. screen the Medicines for Malaria Venture Pandemic Response Box in parallel against Plasmodium asexual and liver stage parasites, stage IV/V gametocytes, gametes, oocysts and as endectocides. They identify two potent transmission-blocking drugs: a histone demethylase inhibitor ML324 and the antitubercular SQ109.
- Janette Reader
- Mariëtte E. van der Watt
- Lyn-Marié Birkholtz
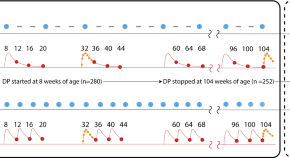
Identifying an optimal dihydroartemisinin-piperaquine dosing regimen for malaria prevention in young Ugandan children
Intermittent preventive treatment with dihydroartemisinin-piperaquine (DP) is protective in children against malaria. Here, the authors analyze plasma drug concentration, malaria incidence, and drug resistance markers from a clinical trial in Uganda and determine the optimal DP dosing regimen.
- Erika Wallender
- Ali Mohamed Ali
- Rada M. Savic
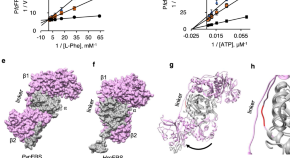
Structural basis of malaria parasite phenylalanine tRNA-synthetase inhibition by bicyclic azetidines
Bicyclic azetidine inhibitors are promising antimalarials that target the Plasmodium cytosolic phenylalanine tRNAsynthetase (cFRS). Here, Sharma et al. provide the biochemical and structural basis of its mechanism using co-crystal structure of Pv cFRS with BRD1389.
- Manmohan Sharma
- Nipun Malhotra
- Amit Sharma
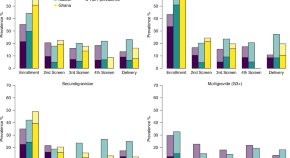
Modelling the incremental benefit of introducing malaria screening strategies to antenatal care in Africa
Plasmodium falciparum infection in pregnancy is a major cause of adverse pregnancy outcomes. Here, the authors combine performance estimates of standard rapid diagnostic tests with modelling to assess whether screening at antenatal visits improves upon current intermittent preventative therapy.
- Patrick G. T. Walker
- Matt Cairns
- Feiko O. ter Kuile
The impact of antimalarial resistance on the genetic structure of Plasmodium falciparum in the DRC
The genome of the malaria parasite Plasmodium falciparum contains a record of past evolutionary forces. Here, using 2537 parasite sequences from the Democratic Republic of the Congo, the authors demonstrate how drug pressure and human movement have shaped the present-day parasite population.
- Robert Verity
- Ozkan Aydemir
- Jonathan J. Juliano
Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood
Reliable plasmonic biosensors with high throughput and ease of use are highly sought after. Here, the authors report a plasmon-enhanced fluorescence antibody-aptamer biosensor based on a gold nanoparticle array, and demonstrate its use for effective specific detection of a malaria marker, at femtomolar level, in whole blood.
- Antonio Minopoli
- Bartolomeo Della Ventura
- Raffaele Velotta
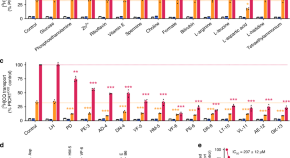
The natural function of the malaria parasite’s chloroquine resistance transporter
Plasmodium falciparum chloroquine resistance transporter (PfCRT) mediates multidrug resistance, but its natural function remains unclear. Here, Shafik et al. show that PfCRT transports host-derived peptides of 4-11 residues but not other ions or metabolites, and that drug-resistance-conferring PfCRT mutants have reduced peptide transport.
- Sarah H. Shafik
- Simon A. Cobbold
- Rowena E. Martin
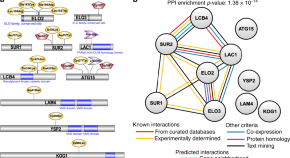
Pan-active imidazolopiperazine antimalarials target the Plasmodium falciparum intracellular secretory pathway
Imidazolopiperazines (IZPs) are a class of compounds under clinical development for malaria, but their mechanism of action is unclear. Here, the authors show that IZPs inhibit the parasite’s secretory pathway, affecting protein trafficking and export.
- Frances Rocamora
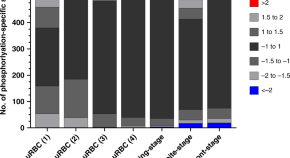
Analysis of erythrocyte signalling pathways during Plasmodium falciparum infection identifies targets for host-directed antimalarial intervention
Plasmodium infection activates signaling pathways in a-nucleated erythrocytes. Here, Adderley et al. use a comprehensive antibody microarray to show that infection extensively modulates host cell signalling and that the host receptor tyrosine kinase c-MET supports Plasmodium falciparum proliferation.
- Jack D. Adderley
- Simona John von Freyend
- Christian Doerig
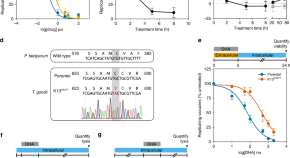
Genetic screens reveal a central role for heme metabolism in artemisinin susceptibility
Artemisinin (ART) resistance poses a problem for malaria elimination. Here, the authors perform genome-wide CRISPR screens in Toxoplasma gondii and identify that the putative transporter Tmem14c and mitochondrial heme metabolism, through mitochondrial protease DegP2, affect ART susceptibility.
- Clare R. Harding
- Saima M. Sidik
- Sebastian Lourido
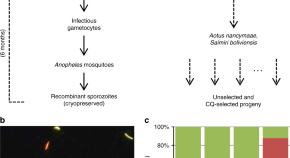
Plasmodium vivax chloroquine resistance links to pvcrt transcription in a genetic cross
Here, a cross of Plasmodium vivax malaria parasites links a chloroquine resistance (CQR) phenotype to a 76 kb region of chromosome 1 and greater expression of pvcrt , an ortholog of the Plasmodium falciparum CQR transporter gene.
- Juliana M. Sá
- Sarah R. Kaslow
- Thomas E. Wellems
Hemozoin-catalyzed precipitation polymerization as an assay for malaria diagnosis
Methods to diagnose malaria are of interest but can be costly or not sensitive enough to detect low levels of parasitemia. Here the authors report an ultrasensitive method by using hemozoin (a biomarker of all Plasmodium species) to catalyse the polymerization of N-isopropylacrylamide.
- Omar Rifaie-Graham
- Jonas Pollard
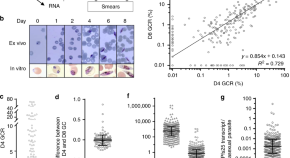
Plasmodium falciparum sexual differentiation in malaria patients is associated with host factors and GDV1-dependent genes
Here, the authors quantify early gametocyte-committed ring (gc-ring) stage Plasmodium falciparum parasites in 260 malaria patients 10 days before maturation to transmissible stage V gametocytes, and show that the ratio of circulating gc-rings is positively correlated with parasitemia and negatively correlated with body temperature.
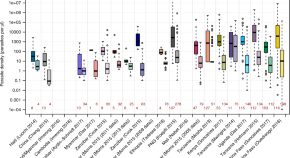
The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density
The role of subpatent infections for malaria transmission and elimination is unclear. Here, Slater et al . analyse several malaria datasets to quantify the density, detectability, course of infection and infectiousness of subpatent infections.
- Hannah C. Slater
- Amanda Ross
- Lucy C Okell
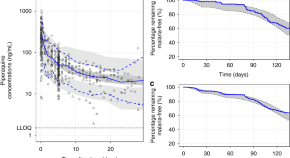
Optimal dosing of dihydroartemisinin-piperaquine for seasonal malaria chemoprevention in young children
Seasonal malaria chemoprevention provides substantial benefit for young children, but resistance to used drugs will likely develop. Here, Chotsiri et al. evaluate the use of dihydroartemisinin-piperaquine as a regimen in 179 children, and population-based simulations suggest that small children would benefit from a higher and extended dosage.
- Palang Chotsiri
- Issaka Zongo
- Joel Tarning
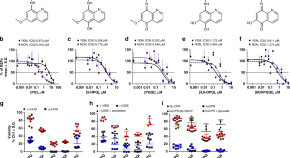
Antimalarial activity of primaquine operates via a two-step biochemical relay
Primaquine (PQ) is a widely used anti-malaria drug, but its mechanism of action is unclear. Here, Camarda et al. show that PQ’s activity against liver and sexual Plasmodium stages depends on generation of hydroxylated-PQ metabolites (OH-PQm), which, undergoing further reactions, results in production of H 2 O 2 .
- Grazia Camarda
- Piyaporn Jirawatcharadech
- Giancarlo A. Biagini
Surveillance and risk assessment
Learnings from thailand in building strong surveillance for malaria elimination.
On the cusp of Plasmodium falciparum ( Pf ) elimination, Thailand is accelerating towards zero malaria by 2024. This commentary reviews the heart of its success—effective surveillance—and what else may be needed to reach zero on time.
- Jui A. Shah
A broader perspective on the economics of malaria prevention and the potential impact of SARS-CoV-2
Economic evaluations of public health interventions to prevent malaria should consider the adoption of wider perspectives and the inclusion of non-health impacts, particularly economic development outcomes, such as education. This is especially relevant in malaria elimination settings and in the context of the current SARS-CoV-2 pandemic.
- Elisa Sicuri
- Francesco Ramponi
- Francisco Saúte
Solar geoengineering could redistribute malaria risk in developing countries
Solar geoengineering, an emergency climate intervention, could shift one billion people back into areas of malaria risk. Regional tradeoffs and potential adverse outcomes point to the need for health sector planning with Global South leadership.
- Colin J. Carlson
- Rita Colwell
- Christopher H. Trisos
The neglected role of relative humidity in the interannual variability of urban malaria in Indian cities
Climate conditions and urbanization can be major drivers of vector-borne infections. Here the authors demonstrate that an often-neglected climate variable, humidity, is an important factor for malaria epidemics in two urban areas in India.
- M. Santos-Vega
- P. P. Martinez
The epidemiology of Plasmodium vivax among adults in the Democratic Republic of the Congo
Plasmodium vivax generally accounts for a low proportion of malaria cases in Africa, but population-level data on the distribution of infections is limited. Here, the authors use data from the Democratic Republic of the Congo and show that the prevalence is low (~3%) and diffusely spread.
- Nicholas F. Brazeau
- Cedar L. Mitchell
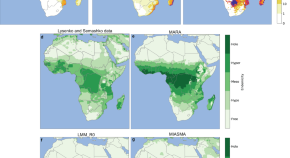
Impact of an accelerated melting of Greenland on malaria distribution over Africa
Release of freshwater into the oceans as a result of ice sheet melting could impact the distribution of climate-sensitive diseases. Here, the authors show that a rapid ice sheet melting in Greenland could cause an emergence of malaria in Southern Africa whilst transmission risks in West Africa may decline.
- Alizée Chemison
- Gilles Ramstein
- Cyril Caminade
Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections
In this longitudinal study of an incident (new infections) and chronic (asymptomatic infections) cohort of Plasmodium falciparum infection in children in Burkina Faso, the authors show higher gametocyte production and mosquito infectivity in chronic infections.
- Aissata Barry
- John Bradley
- Teun Bousema
Predicting the public health impact of a malaria transmission-blocking vaccine
Malaria transmission-blocking vaccines are in development, but roll-out strategies have not been assessed. Here, the authors show that transmission-blocking activity is likely to be higher in the field than in laboratory conditions, and that school-aged children are an important group to target.
- Joseph D. Challenger
- Daniela Olivera Mesa
- Thomas S. Churcher
Genotyping cognate Plasmodium falciparum in humans and mosquitoes to estimate onward transmission of asymptomatic infections
Asymptomatic malaria infections contribute to transmission. Here, Sumner et al. infer participant-to-mosquito transmission by sampling naturally-fed mosquitoes from households in Western Kenya and find that asymptomatic infections more than double the odds of transmission to a mosquito compared to symptomatic infections.
- Kelsey M. Sumner
- Elizabeth Freedman
- Steve M. Taylor
Distinctive genetic structure and selection patterns in Plasmodium vivax from South Asia and East Africa
The genetic diversity of Plasmodium vivax strains in South Asia isn’t well described. Here, the authors sequence P. vivax from returning UK travelers and establish South Asian isolates as subpopulation distinct from East African and South East Asian isolates.
- Ernest Diez Benavente
- Emilia Manko
- Taane G. Clark
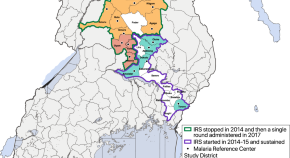
The impact of stopping and starting indoor residual spraying on malaria burden in Uganda
Indoor residual spraying (IRS) of insecticide is one of the primary malaria vector control initiatives, but implementation is limited. Here, the authors show that discontinuation of IRS in Uganda was associated with increased malaria incidence, and introduction of IRS was associated with decreased incidence.
- Jane F. Namuganga
- Adrienne Epstein
- Isabel Rodriguez-Barraquer
Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000-2020
Insecticide treated nets (ITNs) are an important part of malaria control in Africa and WHO targets aim for 80% coverage. This study estimates the spatio-temporal access and use of ITNs in Africa from 2000-2020, and shows that both metrics have improved over time but access remains below WHO targets.
- Amelia Bertozzi-Villa
- Caitlin A. Bever
- Samir Bhatt

Incorporating hydrology into climate suitability models changes projections of malaria transmission in Africa
Prior studies mapping climatologically suitable areas for malaria transmission have used relatively simple thresholds for precipitation. Here the authors show that when models incorporate hydrological processes a more complex pattern of malaria suitability emerges in Africa and future shifts in suitability are more pronounced.
- M. W. Smith
- C. J. Thomas
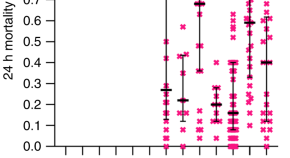
Decreased bioefficacy of long-lasting insecticidal nets and the resurgence of malaria in Papua New Guinea
Malaria prevalence in Papua New Guinea has risen in recent years after almost a decade of decline. In this study, the authors demonstrate that long-lasting insecticidal nets used in the country that were manufactured since 2013 have significantly reduced bioefficacy.
- Rebecca Vinit
- Lincoln Timinao
- Stephan Karl
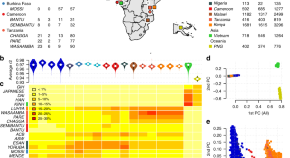
Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania
Four genome-wide associated loci are currently known for malaria susceptibility. Here, the authors expand on earlier work by combining data from 11 malaria-endemic countries and additional population sequencing informing an African-enriched imputation reference panel, with findings including a previously unreported association on chromosome 6.
- Malaria Genomic Epidemiology Network
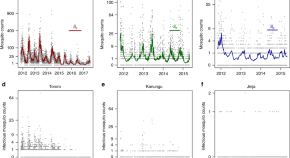
Pareto rules for malaria super-spreaders and super-spreading
Investigating malaria transmission at three sites in Uganda, the authors identify super-spreaders and show that super-spreading is more prominent at low-intensity transmission, and that seasonality and environmental stochasticity have a greater influence on super-spreading.
- Laura Cooper
- Su Yun Kang
- David L. Smith
Genomic structure and diversity of Plasmodium falciparum in Southeast Asia reveal recent parasite migration patterns
Understanding genomic variation in Plasmodium falciparum parasites and inferring migration patterns can guide malaria elimination strategies. Using genome-wide data for 1722 parasites collected from 54 districts, the authors use identity-by-descent approaches to estimate regional parasite migration and spread of artemisinin drug resistance.
- Amol C. Shetty
- Christopher G. Jacob
- Tracking Resistance to Artemisinin Collaboration (TRAC)
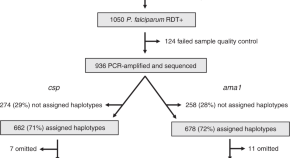
High-resolution micro-epidemiology of parasite spatial and temporal dynamics in a high malaria transmission setting in Kenya
Here, Nelson et al . use amplicon next-generation sequencing of two P. falciparum polymorphic gene regions to investigate the genetic similarity of parasite populations across time and space in a pediatric cohort in Kenya. They identify both micro- and macro-scale structuring of malaria parasites in this high-transmission setting, which could inform future intervention strategies.
- Cody S. Nelson
- Wendy P. O’Meara
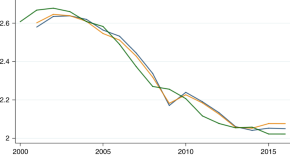
The economics of malaria control in an age of declining aid
Foreign aid is necessary to control tropical diseases in endemic countries. Here the authors outline the steps taken to control malaria in Africa since 2000 and present an economic model to propose that US$25−30 per capita will be needed to avoid a disease trap.
- Eric Maskin
- Célestin Monga
- Jean-Claude Berthélemy
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Analysis of Global Research on Malaria and Plasmodium vivax
José antonio garrido-cardenas.
1 Department of Biology and Geology, University of Almeria, 04120 Almeria, Spain; se.lau@anedracj (J.A.G.-C.); moc.liamtoh@irbec_epep (J.C.-C.); se.lau@asemc (C.M.-V.)
José Cebrián-Carmona
Lilia gonzález-cerón.
2 Regional Center for Public Health Research, National Institute of Public Health, Tapachula, Chiapas 30700, Mexico; xm.psni@laznogl
Francisco Manzano-Agugliaro
3 Department of Engineering, University of Almeria, CeiA3, 04120 Almeria, Spain
Concepción Mesa-Valle
Background : Malaria is one of the infectious diseases of greatest interest to the scientific community and of greatest concern to international health authorities. Traditionally, the focus has been on Plasmodium falciparum , the parasite that causes the most severe form of the disease in Africa. However, in the last twenty years, the Plasmodium vivax parasite, responsible for a large number of cases in Latin America, the Middle East, South and Southeast Asia, the Horn of Africa, and Oceania, has also generated enormous interest due, among other things, to the published evidence that it can cause severe malaria. Methods : In this paper, the international scientific publication on malaria and P. vivax has been analyzed using the Scopus database to try to define global trends in this field of study. Results : It has been shown that events such as the emergence of resistance to certain drugs can break a trend. The important role of non-malaria-endemic countries such as the USA or Switzerland in malaria research is also evident. Conclusions : International cooperation will be essential for the eradication of the disease. Moreover, in this sense, the general vision given by the bibliometric analysis of malaria caused by P. vivax is fundamental to paint the picture regarding the current situation and encourage international cooperation and control efforts.
1. Introduction
Malaria is a disease that has affected the human population since ancient times. Today, it remains one of the infectious diseases with the highest morbidity and mortality rates, with 219 million estimated cases occurred worldwide in 2017, according to WHO, and half a million people dying each year worldwide, many of them children under five [ 1 ]. Because of this, a series of international programs have been initiated that aim to reduce and eradicate malaria, such as malERA, a research agenda for malaria elimination and eradication [ 2 ]. Human malaria can be caused by different species of the Plasmodium parasite. P. falciparum and P. vivax are the most important, with more than 95% of the cases diagnosed in the world, but there are others such as P. malariae , P. knowlesi , P. ovale wallikeri , and P. ovale curtisi [ 3 ].
P. falciparum is the most severe form of the disease in Africa, where more than 90 percent of all malaria cases occur. For this reason, this is the best-characterized species [ 4 ]. However, in recent years, the infections caused by P. vivax are increasing in significance because of evidence for severe malaria P. vivax infection [ 5 ]. In addition, P. vivax has recently received a large amount of attention, as it is the species with the largest geographical distribution, being reported fundamentally in Latin America, the Middle East, South and Southeast Asia, the Horn of Africa and Oceania [ 6 ]. This attention received by P. vivax is not only scientific, as illustrated by the increase in the number of published articles on this subject, but has also increased interest at the health level, trying to improve the diagnosis of malaria by these species or the specific treatments. The last factor that makes P. vivax a parasite of growing interest is its very hard elimination, with a high number of symptomatic relapses in malaria patients [ 7 ].
The malaria parasite is transmitted by a female Anopheles mosquito, inoculating sporozoites into the human host [ 8 ]. The sporozoites reach human liver cells, where they transform to give rise to another form called the merozoite. The merozoite reaches the erythrocytes, through the bloodstream, and multiples to produce new merozoites. Some of the merozoites released after the breakage of the erythrocytes are transformed into gametocytes. If the Anopheles mosquito bites an infected individual, then this is how the merozoites re-enter it. Inside the mosquito, in its midgut, sexual reproduction takes place, generating zygotes and developing into oocysts. These, as they grow and break, release sporozoites that invade the mosquito’s salivary glands. The parasite will then in the proper form to infect a new individual [ 9 ]. A fundamental difference in the life cycle of P. vivax is that it can successfully finish its development cycle in the mosquito at lower temperatures and faster than P. falciparum .
The complexity of the parasite’s life cycle, its great genetic variability, and the numerous mechanisms it can develop to avoid the host’s immune response, make it very difficult to find a vaccine to suppress human malaria [ 10 , 11 ]. In addition, the biology of P. vivax , as opposed to P. falciparum, makes it harder to control and eliminate and is the main reason for the higher vectorial capacity of P. vivax. Other reasons are related to the presence of hypnozoites (latent hepatic forms), which lead to multiple relapses and low parasites densities, which in turn makes diagnosis difficult and delays treatments [ 12 ]. On the other hand, there are no suitable experimental models for the analysis of a hypothetical vaccine. Despite this, finding a vaccine to eradicate malaria caused by P. vivax has become a fundamental objective for the WHO and the scientific communities around the world.
Bibliometric analyses provide fundamental scientific tools allowing for objective quantification of a scientific fact. These show the level of current knowledge in a scientific field by the compilation of data obtained from bibliographic databases. Bibliometrics facilitate comprehension and elaborates a real image of the research activity. In this study, the analysis of the international scientific publication on malaria and P. vivax has been raised to establish worldwide trends. It is essential for defining the research lines in malaria and creating synergies in order to achieve the WHO objective of eradicating malaria [ 13 ].
2. Materials and Methods
The evolution of the electronic age has led to the development of numerous scientific databases on the World Wide Web, which offer search facilities on a particular issue. Among them, some allow the opportunity to analyze citations from published works. The main scientific databases covering medical terms are PubMed, Scopus, Web of Science, and Google Scholar. PubMed focuses essentially on medicine and biomedical sciences, while Scopus, Web of Science, and Google Scholar cover most scientific fields. Usually, bibliometric studies are based on the keywords indexed by the published works. The main differences between these databases are PubMed (no limit), Scopus (30), Web of Science (15), and Google Scholar (no limit). However, in indexed journals and conference proceedings, there is usually a limit of about 6 keywords per published work so the limit of 15 is considered adequate for bibliometric works. However, PubMed’s search function, which is designed to search for medical documents, are exceptional and offer a service that other search engines do not. In this database, the search results can only be sorted by general characteristics such as publication date or author, and therefore, this is not very useful to get an overview of a topic [ 14 ]. Nowadays, mainly Web of Knowledge and Scopus allow large-scale downloads of bibliographic information from indexed publications and so most of the worldwide bibliometric works are based on one of these two databases. Scopus is the database that indexes the largest number of publications and conference proceedings when compared to the other three databases mentioned. Elsevier’s Scopus database is currently the largest abstract and citation database of peer-reviewed literature, and although another database, the Web of Science (WoS), is also available, it lists fewer titles whereas Scopus lists 84% of WoS titles compared to only 54% of Scopus tiles listed by WoS [ 15 , 16 ].
Scopus is usually considered the largest abstract and citation database of peer-reviewed scientific literature in the world. It contains more than 35,000 titles belonging to more than 10,000 publishers. For this reason, in order to analyze who, how, where, and what is being researched in a given scientific field, the most commonly used option is to use this database. Thus, it is common to find bibliometric works in many scientific fields using Scopus [ 17 , 18 , 19 ]. In short, Scopus is the most effective search engine and provides an overview of the subject. For extensive and in-depth research in the area of life sciences and closely related topics, PubMed should be considered as well [ 14 ]. For this reason, Scopus was the database of choice for this analysis.
In this work, a full search of the Elsevier Scopus database was conducted using TITLE-ABS-KEY (malaria and vivax ) as the search query. This resulted in 11,166 documents being obtained between 1916 and 2018, the last full year from Scopus database. It should be noted that varying the search criteria, or subsequent modifications of Scopus, can give significantly different results. That keywords entered by the author or publisher may not strictly conform to the subject matter of the articles should also be considered. Notwithstanding the previous, Scopus is considered a valid option for this type of analysis. In keyword analysis, terms with identical meanings were grouped together (e.g., Plasmodium vivax and P. vivax ), and terms that do not contribute to this analysis were discarded (e.g., article). The aspects that have been studied are the progression in the number of publications per year, the distribution of publications by institutions and by country, and the keywords ( Figure 1 ). For the detection of scientific communities, understood as the set of nodes connected to each other in a complex network, the software tool VOSviewer [ 20 ] ( http://www.vosviewer.com/ ) was used. This software has been used to create graphs in which each institution is represented by a node and the connections between two nodes represent the collaboration between the two institutions represented.

Methodology for searching the different analyses.
3. Results and Discussion
3.1. progression of scientific output.
The search returned 11,166 documents. Figure 2 shows the evolution trend of the number of documents on Plasmodium vivax and malaria since the first article was published. As shown in Figure 2 , the first article published on this is dated in 1916 [ 21 ]. This early entry in the first article shows the strong interest that the scientific community has placed on malaria over the years.

The trend of the number of publications in malaria and Plasmodium vivax , from the years 1916–2018.
The maximum number of annual publications was obtained in 2014, reaching a value of 646. The growth in the number of publications presents an exponential pattern, with an R 2 coefficient close to 1. There are two years in which the trend is interrupted, and therefore, the R 2 value is not even higher. These are the years 1946 and 1973. In these years, considering the trend line, the publications were higher than expected. The reason is that, on the one hand, in 1946, chloroquine was identified as a first-line blood schizontocide for P. vivax [ 22 ]. This led to an increased interest in malaria research and an increase in the publications. On the other hand, in 1973, there was intensification in the disease due to two reasons: the reduction in international aid programs from developed countries in the early 1970s, due to the lack of prospects for eradication; and the international economic crisis of 1973, which pushed up the price of insecticides. Between the years 2002–2010, the increase in the number of publications was three times greater than the whole previous period. This could be explained by the first malaria conference, Vivax Malaria Research: 2002 and Beyond, which took place in Bangkok, Thailand, 3–8 February 2002. This was a conference devoted entirely to Plasmodium vivax research and convened by the Multilateral Initiative on Malaria [ 23 ]. On the other hand, at the turn of the century, there was a general increase in funding for malaria control, and initiatives such as Roll Back Malaria, RBM, Partnership were born. This is a major worldwide platform for coordinated action towards a malaria-free world and is composed of international researchers, companies, and organizations.
3.2. Publication Distribution by Countries and Institutions
To get an overview of worldwide research on a specific subject, one of the aspects most considered is the study of publications by countries and institutions [ 24 ]. Note that when an article has several affiliations, the indexation implies that the article is attributed to each of them; therefore, the sum of articles by countries could be greater than the sum of articles obtained with the search term. Figure 3 shows the distribution by country of the scientific publication on malaria and P. vivax . In 159 countries, at least one article on P. vivax has been published, and 25 countries, with at least 150 publications, represent 75% of the total. Note that the same article may be signed by authors from different countries. Figure 4 shows the data on a world map with colors identifying the number of manuscripts that are published in each country. The United States of America, United Kingdom, and India, top the ranking with 2528, 1439, and 1383 publications, respectively. To normalize the results, the data have been referenced to the population of each country, based on the 2018 statistics obtained from the website [ 25 ] ( Table 1 ). In this case, it can be observed that there are 10 countries that publish at least 7 articles per million inhabitants. These are (ranked from highest to lowest): Switzerland, Australia, Papua New Guinea, United Kingdom, Netherlands, Thailand, Belgium, France, United States, and Sri Lanka.

Representation of the countries with the highest number of publications on malaria and P. vivax.

World map representing scientific publications by the intensity of color.
Leading countries in terms of scientific research and publication in terms of population and wealth cited.
| Country | Publications (N) | Population (P) (Mill. of Inhabitants) | N/P | GDP Per Capita |
|---|---|---|---|---|
| United States | 2528 | 329,093 | 7682 | 62,332 |
| UK | 1439 | 66,959 | 21,491 | 41,951 |
| India | 1383 | 1,368,738 | 1010 | 1965 |
| Thailand | 948 | 69,306 | 13,678 | 6984 |
| Australia | 773 | 25,089 | 30,812 | 56,920 |
| Brazil | 718 | 212,393 | 3381 | 8988 |
| France | 559 | 65,481 | 8537 | 42,684 |
| Switzerland | 427 | 8608 | 49,605 | 82,365 |
| China | 353 | 1,420,062 | 249 | 9476 |
| Colombia | 321 | 49,850 | 6439 | 6580 |
| Japan | 318 | 126,855 | 2507 | 39,975 |
| Indonesia | 315 | 269,536 | 1169 | 3729 |
| Spain | 299 | 46,441 | 6438 | 30,942 |
| South Korea | 295 | 51,339 | 5746 | 32,256 |
| Germany | 270 | 82,439 | 3275 | 48,872 |
| Netherlands | 256 | 17,133 | 14,942 | 53,114 |
| Italy | 215 | 59,217 | 3631 | 35,226 |
| Pakistan | 206 | 204,596 | 1007 | 1486 |
| Papua New Guinea | 205 | 8587 | 23,873 | 3028 |
| Malaysia | 198 | 32,454 | 6101 | 11,247 |
| Ethiopia | 192 | 110,136 | 1743 | 781 |
| Iran | 171 | 82,821 | 2065 | 5059 |
| Canada | 162 | 37,280 | 4345 | 46,513 |
| Sri Lanka | 158 | 21,019 | 7517 | 4425 |
| Belgium | 153 | 11,563 | 13,232 | 48,603 |
In relative terms, Switzerland very high scientific output is striking. This can be explained by two reasons. First, Switzerland must be considered an international power in innovation. It is no coincidence that the development of an effective malaria vaccine is taking place in the country. The pharmaceutical industry is well established in this country, and there are numerous public and private research and development institutions around this type of industry [ 26 ]. On the other hand, Switzerland is home to international agencies and institutions concerned with global health care. The Swiss Agency for Development and Cooperation (SDC), an agency of the Swiss federal administration responsible for coordinating cooperation and humanitarian aid activities, and the World Health Organization, whose headquarters are in Geneva, stand out in this regard.
Finally, Table 1 also reflects the relative wealth of each country based on the value of GDP per capita (IMF data). Thus, it can be observed that the 10 countries studied above are divided into two groups. Seven high-income countries (Switzerland, Australia, United Kingdom, Netherlands, Belgium, France, and United States), with more than 40,000 GDP per inhabitant, and three low-income countries (Papua New Guinea, Thailand, and Sri Lanka), with less than 7000. The motivations in one or the other case are different. While in the former, the interest is purely scientific, for the latter it is a question of survival. In these three countries, malaria is an endemic disease, although in terms of survival, there is very little malaria in Thailand, and Sri Lanka has eliminated all malaria.
Figure 5 shows the 13 institutions with at least 180 publications on malaria and P. vivax . Of these, four are from USA (Centers for Disease Control and Prevention, National Institutes of Health in Bethesda, National Institute of Allergy and Infectious Diseases, and Armed Forces Research Institute of Medical Sciences known as AFRIMS), three are from the UK (the University of Oxford, Nuffield Department of Clinical Medicine, and London School of Hygiene and Tropical Medicine), two are from Thailand (Mahidol University and Shoklo Malaria Research Unit), two are Brazilian (Fundacao Oswaldo Cruz and Universidade de Sao Paulo), and one is Indian (National Institute of Malaria Research of India). Note that there may be several affiliations within the same institution but the database considers them separately respecting the decision of the authors.

Main institutions in terms of scientific publication in malaria and P. vivax.
As mentioned above, these five countries along with Australia, are the most relevant in scientific publications on this topic. In addition, it is surprising that among these, there is also an institution from Papua New Guinea. This is the Papua New Guinea Institute of Medical Research, also known as PNGIMR, that has the support of the World Health Organization (WHO). Figure 6 shows a distribution by communities of the main institutions. It can be observed that most institutions are grouped into a cluster whose central element is the faculty of tropical medicine of the Mahidol University (Thailand). Each line of union between the nodes represents the relationships established between the institutions. Thus, the relations are quite complex, and that they are observed not only between the different elements of this large cluster but also with the other institutions of the two additional minority clusters. The two smaller clusters are made up of the Papua New Guinea Institute of Medical Research and the Swiss Tropical and Public Health Institute, in one, and the Medicines for Malaria Venture (MMV) in the other.

Distribution by communities of the main institutions.
3.3. Keyword Analysis
In the analysis of the keywords, if one of them does not contribute anything to the study then it must be eliminated, e.g., “article.” In the second place, all terms that refer to the same concept must be grouped together, e.g., “ Plasmodium vivax ” and “ P. vivax .” Figure 7 shows, using a word cloud, that the 32 keywords are in more than 1000 publications on malaria and P. vivax . In Figure 7 , the relative size of each word is directly proportional to the number of times the keyword is present in the analyzed documents. As expected, Plasmodium vivax , human, and malaria, with 9826, 9428, and 8572, respectively, stand out.

Word cloud with the main keywords.
Among the 32 keywords with more than 1000 presences in the analyzed articles are two drugs, chloroquine and primaquine, present in 2395 and 1682 documents, respectively. Furthermore, 11 other keywords related to drugs are among the 160 most used keywords. These are ranked in order of their importance: quinine, mefloquine, artemisinin, artesunate, pyrimethamine, doxycycline, fansidar (actually, the trade name for sulfadoxine/pyrimethamine), artemether plus benflumetol, sulfadoxine, proguanil, and amodiaquine. Figure 8 shows that since 1946, the evolution of malaria and P. vivax research in relation to the different drugs. As already mentioned, the most important drug as keyword is chloroquine. Although considering only the last 5 years, the relative importance of primaquine is similar. This is because the WHO indications are that, in areas where chloroquine maintains its efficacy, this must be the drug used against malaria caused by P. vivax [ 27 ]. On the other hand, primaquine has been shown to be highly effective in acting against hypnozoites, which are the predominant latent forms in P. vivax . Furthermore, until recently, it was the only approved hypnozoiticide. For these reasons, to avoid relapses caused by it, the administration of primaquine is appropriate although there are threats from incomplete compliance with standards and the development of tolerance, although the real problem in the supply of this drug is its potential toxicity due to the deficiency, in patients, of the enzyme G6PD [ 28 ]. Figure 8 also shows the relative importance of different drugs at any given time. For example, mefloquine had a relative maximum in 2004 and how, subsequently, it has been losing relevance due to its scarce further use. On the contrary, artemisinin—and its derivative artesunate—has a fundamental importance in recent years, being the most effective drug against all forms of multidrug-resistant P. falciparum [ 29 ]. Other drugs such as fansidar (sulfadoxine/pyrimethamine) or proguanil have practically no importance in recent publications on malaria and P. vivax , highlighting the trend in the use of these in the treatment of the disease.

Time progression of antimalarial drugs in the fight against malaria caused by P. vivax.
Also, it can be seen that the five species of Plasmodium that have been shown to cause malaria in humans ( P. vivax , P. falciparum , P. malariae , P. ovale, and P. knowlesi ) are among the 100 most used keywords. Finally, five countries are also present among the most important keywords: India, Thailand, Brazil, China, and Papua New Guinea. These are countries where malaria caused by P. vivax is endemic and they are at the focus of many efforts by the international scientific community to eliminate malaria.
4. Conclusions
Malaria is still one of the world’s major health problems today; both for its extent and for the priority consideration it has received from public and private organizations concerned with human health. For this reason, malaria is a topic that is increasingly published in scientific journals with impact factors. Moreover, within this theme, malaria caused by P. vivax is currently receiving special interest. The present study has shown that the growth in the number of publications is exponentially curved, demonstrating the enormous interest that P. vivax causes in the international scientific community. This trend is only interrupted by two moments in history when interest in malaria has broken the norm. Thus, it becomes clear how bibliometric analysis of a given subject allows fundamental facts or moments to be identified. In this specific case, it is the discovery of a drug, chloroquine, which proved to be useful in the fight against malaria, and the rebound in the number of malaria cases in the early 1970s due to the relaxation of the alert level in the international scientific community.
On the other hand, the most important countries in terms of scientific publication have been identified. In global terms, the USA, UK, and India stand out above the rest. However, when a more exhaustive analysis is carried out, and both the population and the wealth of the country are considered, it is observed that there are other countries of greater relative importance. Thus, Switzerland has been found to be the country that devotes the most relative effort to the fight against malaria. This is no coincidence. This country is a biotechnological benchmark and is home to many international public and private bodies that have been working to eliminate malaria for decades. The identification of countries such as Switzerland highlights the importance of combining technological, scientific, and political efforts of public and private initiatives in the fight against the disease. This union of efforts is evidenced by the study of the relationships between the most outstanding institutions and scientists in the field of malaria and P. vivax . In most cases, there are collaborations that dilute the borders between rich countries that are devoting efforts to fight malaria, such as the UK, and countries where the disease is a real public health problem, such as Thailand.
Bibliometric studies not only give an overview of the current state of a scientific issue but can help to understand policy decisions and shape future scientific research. For this reason, an analysis of the keywords has been carried out allowing us to identify the main sectors in which the greatest efforts are being focused on research on malaria and P. vivax . Of these, studies on antimalarials stand out. The elements that define the lines of international economic investment and objectives in research projects are the progression of trends, the recommendations of the WHO, the updating of studies on the effectiveness of drugs, and the existence of resistance to them. It is important to have a general view of the subject in order to focus on the strategies that are still valid and to open up new promising lines of research.
Author Contributions
Conceptualization, J.A.G.-C. and F.M.-A.; methodology, J.A.G.-C., J.C.-C., and C.M.-V.; formal analysis, J.A.G.-C. and F.M.-A.; investigation, J.A.G.-C., L.G.-C., and C.M.-V.; writing—original draft preparation, J.A.G.-C. and J.C.-C.; writing—review and editing, L.G.-C., F.M.-A., and C.M.-V.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.

IMAGES
COMMENTS
Post-discharge malaria chemoprevention (PDMC) is an intervention aimed at reducing morbidity and mortality in patients hospitalized with severe anaemia, with its effectiveness established in several clinical t... Céline Audibert and Hans Rietveld. Malaria Journal 2024 23:270. Research Published on: 6 September 2024.
Malaria is a mosquito-borne disease that is caused by Plasmodium parasites. Patients with malaria experience flu-like symptoms and, in severe cases, the disease can progress to neurological ...
Introduction. Recent World Health Organization (WHO) data provide insight into the evolving epidemiology of malaria globally. According to the World Malaria Report 2022, the number of total malaria cases increased again in 2021 (to 247 million), although case incidence remained stable following an increase from the preceding year [1••].Similarly, deaths due to malaria increased in 2020 ...
The 2023 WHO World malaria report. The estimatednumber ofglobalmalaria cases in 2022 exceeded pre-COVID-19 pandemic levels in 2019, according to WHO's 2023 World malaria report. Several threats to the malaria global response are highlighted in the report, including climate change. The report, an annual assessment of global trends in malaria ...
Malaria is resurging in many African and South American countries, exacerbated by COVID-19-related health service disruption. In 2021, there were an estimated 247 million malaria cases and 619 000 deaths in 84 endemic countries. Plasmodium falciparum strains partly resistant to artemisinins are entrenched in the Greater Mekong region and have emerged in Africa, while Anopheles mosquito vectors ...
he World Malaria Report, released December 2021, reflects the unique challenges facing the global malaria community. The report lays bare the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020. The numbers in the report tell two diferent stories for countries nearing elimination and countries ...
Great progress has been made in recent years to reduce the high level of suffering caused by malaria worldwide. Notably, the use of insecticide-treated mosquito nets for malaria prevention and the use of artemisinin-based combination therapy (ACT) for malaria treatment have made a significant impact. Nevertheless, the development of resistance to the past and present anti-malarial drugs ...
5.6eported malaria cases in R HBHI countries since 2018 and comparisons with estimated cases 48 6.vestments in malaria programmes and research In 52 6.1unding trends for malaria control and elimination 52F 6.2vestments in malaria-related R&D 56In 7.istribution and coverage of malaria prevention, diagnosis and treatment D 58
This phase 2 trial showed that a single subcutaneous dose of L9LS at the highest dose tested (300 mg) provided protective efficacy of 69.9% against P. falciparum infection and 77.4% against ...
Global Malaria Epidemiology. The number of total malaria cases globally increased in 2021 (from 245 million in 2020 to 247 million in 2021), with most of the increase occurring in Africa. However, case incidence remained stable from 2020 to 2021 (59 cases per 1000 population at risk) following an increase from 2019 (57 cases/1000 population) [1
In this Review, we provide a brief history of the use of population genomics in malaria research and discuss recent advances, focusing on parasites and vectors. We describe innovations for ...
Summary points. The worldwide burden of Plasmodium vivax malaria has more than halved from an estimated 17.3 to 6.5 million cases between 2010 and 2019. This resulted from increased deployment of conventional malaria control measures (rapid diagnostic tests, effective antimalarial treatment, vector control) and significant global investment in malaria elimination.
This paper aims to be a didactic material providing the reader with an overview of malaria. More importantly, using a system approach lens, we intend to highlight the debated topics and the multifaceted thematic aspects of malaria transmission mechanisms, while showing the control approaches used as well as the model supporting the dynamics of ...
PDF | Malaria is a global public health burden with an estimated 229 million cases reported worldwide in 2019. About 94% of the reported cases were... | Find, read and cite all the research you ...
Be bold. Malaria has plagued humans for millennia and has led to an unimaginable loss of life. Malaria has also had an important role in the geopolitics and evolutionary history of humans. The ...
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
Aims and Scope. Malaria Journal is aimed at the scientific community interested in malaria in its broadest sense. It is the only journal that publishes exclusively articles on malaria and, as such, it aims to bring together knowledge from the different specialties involved in this very broad discipline, from the bench to the bedside and to the ...
Abstract: Malaria transmission and prevalence involves a triangular web of interactions between man, vector, and the environment. Any meaningful effort in malaria control, elimination and or eradication should target weakening and or breaking the forces of interactions within the triangle. In sub-Saharan Africa, effective malaria control ...
Malaria is caused in humans by five species of single-celled eukaryotic Plasmodium parasites (mainly Plasmodium falciparum and Plasmodium vivax) that are transmitted by the bite of Anopheles spp ...
DOI: 10.1056/NEJMp2216703. In this documentary video from the New England Journal of Medicine, physicians and scientists from across the world discuss the epidemiology of malaria and outline key ...
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice.
Malaria is a life-threatening febrile illness caused by Plasmodium spp. parasites that are transmitted to humans by infected female Anopheles mosquitoes. According to WHO, 241 million cases ...
Background: Malaria is one of the infectious diseases of greatest interest to the scientific community and of greatest concern to international health authorities.Traditionally, the focus has been on Plasmodium falciparum, the parasite that causes the most severe form of the disease in Africa.However, in the last twenty years, the Plasmodium vivax parasite, responsible for a large number of ...