Your session is about to expire
Clinical research project management: back to basics.
Clinical trials require care and precision regarding planning, coordination, and collaboration. The stakes are high, with participants’ health and well-being and significant investments of both time and money on the line. That’s why clinical research project managers are necessary – to ensure coordinated and collaborative efforts between numerous departments, teams, and vendors that adhere to the study protocol as well as regulatory and ethical standards. In this article, we will take an in-depth look at the basics of clinical research project management.

What is project management in clinical research?
Clinical trials involve several complex, dynamic parts with different boundaries/areas of responsibility, and personnel with specific skill sets and qualifications. As a consequence, successful clinical trials require organizers who are adept at project management.
Clinical trials can be thought of as large-scale, complex projects with multiple milestones and parallel workstreams, including:
- Study and protocol design
- Study startup
- Site activation
- Recruitment
- Documentation (promotional material, informed consent forms, case report forms, protocol documents, surveys, etc.)
- IRB and IEC approval
- Site management
- Investigational product distribution and management of study materials
- Laboratories (analyses, lab tests, imaging, etc.)
- Reporting and regulatory affairs
Why is project management in clinical research important?
Clinical research involves multiple stakeholders. Project management can essentially be thought of as monitoring progress and keeping everyone involved on the same page. Project management is critical for the success of a clinical trial; it helps the different teams stay on schedule, adhere to protocol, and communicate amongst one another, as well as meet the trial objectives/targets, maintain quality standards, stay within budget, and follow regulatory requirements.
The following are three major reasons why project management in clinical research is important:
Timeline management
Project management ensures the delivery of clinical trial objectives and sub-objectives within the allocated time and budget. This is important because nearly 85% of all clinical trials experience delays. [1] Project management techniques can allow investigators to plan ahead if it looks like a deadline is not going to be met, so they can reallocate resources and priorities to accelerate the process, or otherwise notify teams responsible for tasks that would be affected by the resultant delay and adjust the timelines.
Streamline and facilitate communications
It is important for the various stakeholders involved in a clinical trial to be on the same page. Keeping all of these actors updated and facilitating communication amongst them is another task of the project management team. Lack of communication in clinical trials can have negative consequences on the quality of research. [2] An efficient project manager or management team can streamline communication and collaboration between multiple teams and departments, further increasing the transparency of the individual but interdependent operations.
Quality control (QC)
Quality control is another important aspect of clinical research project management. Quality standards are often stringent, but this is designed to protect the safety of participants and the general population. Clinical trials that fail to adhere to or meet quality standards will not be considered to have provided sufficient evidence on the effectiveness and/or safety of an investigational drug. Researchers and investigators tend to be under a lot of pressure as trials are often on tight budgets and timelines, but it is important that quality not be sacrificed in order to meet other objectives.
Project management helps sponsors/investigators manage all objectives and sub-tasks in a clinical trial while still prioritizing adherence to quality and regulatory standards. Part of the task of the project management team may even be to define internal quality standards for specific tasks, objectives, and/or teams.
What does a clinical research project manager do?
A clinical research project manager coordinates with other departments, teams, and personnel involved in the clinical trial to ensure the organized completion of clearly defined tasks. They also manage external vendors such as central laboratories or technology providers. Project managers will monitor the progress of all tasks and objectives to keep the study on track according to its protocol, including timeline and budget, and also communicate with stakeholders such as the sponsor to keep them up to date.
What is the clinical research project manager responsible for?
The responsibilities of a clinical research project manager depend on the specifics of the trial and its complexity, but they generally include the following:
- Planning : This includes general planning of the trial, including the internal organization between departments/teams, how tasks should be executed in order to comply with regulations, how many and which sites to involve, whether external tools/solutions need to be contracted, etc.
- Budgeting : Making sure that the study’s resources are allocated appropriately to the different teams/tasks, within the overall study budget, also providing room for unexpected costs or delays.
- Vendor identification/selection : Negotiation with vendors, technology providers, and suppliers.
- Scheduling : Scheduling the objectives/sub-tasks of the clinical trial and monitoring activities to make sure they are completed on time.
- Liaising : Acting as a central point of contact for members of the project team and sponsors.
- Task delegation : Assigning tasks to team members and updating them about their responsibilities, as well as deadlines and expectations.
What are the key topics included in a clinical research project plan?
The project manager may organize all of the above-mentioned tasks and responsibilities into a document or repository referred to as a clinical research project plan. This plan would formally outline standard protocols for aspects of the clinical research project management, such as:
- Timeline : The timeline should clearly outline specific tasks for each team/department, including their expected initiation and completion dates, and the project manager will ensure tasks and teams are on track. Clinical trials often get delayed, so it is useful to have protocols in place regarding how to deal with potential delays. [3]
- Budget : Often related to unexpected delays, it is not uncommon for projects to end up over budget. The project plan should clearly define budgets, both for teams and for individual tasks, and should outline how deviations from budgets should be dealt with.
- Stakeholder management : The project management plan should outline the content and dates of formal reports for keeping stakeholders updated about the trial’s progress.
- Documentation : The project plan should outline how documentation should be collected, organized, stored, and verified in order to ensure compliance with laws as well as ethical and clinical standards as established by the WHO and ICH guidelines for Clinical Good Practice.
- Site management : Although site monitoring is usually a separate responsibility in clinical trials, the project plan may include instructions and guidelines for individual study sites regarding adherence to protocol, tasks, and timelines. In addition, sites should have clear guidelines on who to contact in the case of any problems, questions, or adverse events that may arise during the trial.
- Data management : The project plan should specify protocol for the collection, secure storage, management, validation, and cleaning of subject information and trial data, in accordance with quality standards and applicable regulations. Proper data management ties in closely with quality assurance, and sound results require healthy data.
Tips for successful clinical research project planning and management
Here are 4 specific tips and ideas for maximizing the efficacy of project management functions in clinical research.
1. Plan with flexibility
Delays can be hard to avoid, especially in the recruitment stage, and they are costly to sponsors. Nonetheless, proper consideration of these potential delays in the timeline (i.e., allowing for some degree of flexibility) can make the difference between the delays simply setting the trial back a few weeks, or ending in the entire trial being canceled.
While delays aren’t ideal, proper planning can allow the sponsors to absorb these delays without them leading to completely missed deadlines and/or cancellation of the trial; in the end, cancellation likely represents a much more significant waste of resources than delays. The same logic can be applied to flexibility in budgeting, as delays may imply additional costs; if these are less unexpected, they can be better absorbed within the trial budget without setting it entirely off track.
2. Identify possible risks and establish mitigation strategies
Perform a thorough analysis of the protocol and utilize specialist knowledge in the fields of medicine and clinical research management to identify and create a list of risks that could arise throughout the clinical trial. Planning ahead of time will allow sponsors to respond rapidly to these risk factors and mitigate them, without having to perform lengthy analyses and coming up with mitigation strategies when it may be too late.
Some potential risks to consider include recruitment delays or low accrual, adverse events, patient dropouts, protocol breaches, problems with study drug supply or distribution, technical failures (of software systems, medical equipment, etc.), and data integrity issues, to name a few. Start with the risks that pose the greatest threat to the integrity of the study, i.e., those which would result in it being canceled, stopped, or rendering the results unusable.
3. Use project management tools
Constantly reviewing all aspects of the clinical trial is a daunting task, so the use of specialized and customizable software solutions can be helpful. There are many such solutions available, from general project management tools to dedicated clinical trial management systems ( CTMS ). These tools can be of significant help in managing, organizing, and overviewing all of the aspects of project management discussed previously, acting as a sort of central dashboard as well as a “safeguard” for the project management plan and tasks.
4. Leverage data automation tools and functions
Similarly to the previous point, data management is another aspect of clinical trials (and clinical trial project management) that can benefit greatly from the assistance of technological tools. Lots of data management functions, including organization, cleanup, and validation, can be streamlined or even completely automated through data processing tools, which are sometimes integrated directly into CTMS or other clinical trial monitoring solutions. The benefits of healthy data include enhanced regulatory compliance and faster progression to data analysis and results once the study data has been collected. Data can also be improved at the source through the use of electronic reporting/collection/recording methods such as:
- Electronic patient-reported outcomes ( ePRO )
- Electronic trial master files ( eTMF )
- Electronic clinical outcome assessments ( eCOA )
- Electronic case report forms ( eCRF )
Conclusions
Clinical research project management is a vital function for keeping the numerous separate yet highly interconnected parts involved in a clinical trial operating in coordination and on track with protocol, budget, timelines, and regulations. There are numerous strategies and tools that can facilitate clinical trial project management tasks and help improve clinical trial quality and speed while still ensuring patient safety and regulatory compliance.
Other Trials to Consider
SAD Cohorts 1 to 2: Participants receiving ECC4703
Vutrisiran + vutrisiran (helios-a), insulin icodec, apalutamide, esym app usage, theater/pilot testing, popular categories.
Stroke Clinical Trials 2024
Malaria Clinical Trials 2024
Chronic Lymphocytic Leukemia Clinical Trials 2024
Clinical Trials in Tampa, FL
Paid Clinical Trials in San Diego, CA
Smoking Cessation Clinical Trials 2024
Familial Hypercholesterolemia Clinical Trials 2024
Keloid Clinical Trials 2023
Domestic Violence Clinical Trials 2023
Acute Myeloid Leukemia Clinical Trials 2023
Popular guides.

Project Management In Clinical Trials: Practical 2024 Guide
If you’re new to project management in clinical trials, this guide will help you understand what it means to be a project manager on a clinical trial, and then provide some practical tips to help you plan and manage your clinical trial effectively.
- Struggling to effectively manage timelines and budgets in clinical trial projects?
- Feeling overwhelmed by the complexity of regulatory requirements and documentation?
- Unsure how to navigate communication challenges between stakeholders?
This article is for:
- Clinical trial project managers seeking practical strategies for streamlining processes and maximizing efficiency.
- Researchers and coordinators looking to enhance their understanding of project management principles in the context of clinical trials.
- Pharmaceutical and biotech professionals interested in improving trial outcomes through effective project management techniques.
Project Management in Clinical Trials: How Does It Work?
As a Project Manager, you may need to be prepared to manage client expectations from day one and will be responsible for managing resources and relationships with internal and external parties. As a Project Manager, your main job function is to get projects completed on time.
The importance of your role means that you will have regular contact with senior stakeholders across all functions in an organization and therefore be highly visible within your organization. You will ensure your team is focused on building long-term relationships that benefit both parties; being able to build good relationships quickly will allow you a high level of engagement with key stakeholders.
Good project management in clinical trials requires a detailed understanding of business procedures, being aware of commercial and strategic priorities, and an awareness of key performance indicators and trends. The key areas of expertise for clinical trial project managers are communication, relationships, influence, planning, attention to detail, and following instructions.
Strong teamwork skills are also essential when working on projects as well as people management skills. You will need to be adaptable to a rapidly changing environment with diverse cultures; you will also be required to negotiate successfully with stakeholders at all levels.
Being an effective project manager in clinical trials is a challenging but fulfilling role. As well as being rewarded with great job satisfaction, you may find your salary increases significantly as you gain experience. However, like any other profession, there are challenges associated with being a clinical trial project manager and you will need to be emotionally resilient when dealing with change.
Common problems include:
- Rapidly changing priorities and unrealistic expectations of resources
- Internal conflict between team members
- Dealing with stressful situations on a daily basis such as late deliveries or looming deadlines
- Having limited influence over projects due to numerous stakeholders who have input on projects being assigned to your team
You will need to be able to organize tasks based on deadlines, manage staff resource allocation, and work with your team members to ensure projects are completed on time. It is essential that you listen to client requirements when managing your project. Your team will look to you for guidance in difficult situations and as such, it is vital that you have excellent communication skills. You will also be required to report project status information throughout a project lifecycle as well as performance against specific objectives, these metrics can be used by management in evaluating staff effectiveness.
Tips for a Clinical Project Manager
Clinical project manager is one of those jobs that, like teaching or parenting, requires a different set of skills and approaches. Among other things, it requires project management.
So what makes managing clinical research projects different from any other sort of project?
Well, in order to begin answering that question let’s first look at what makes any sort of project management different from regular life. In short, it is knowing your resources and being able to apply them towards meeting goals.
Here are some things that a clinical project manager should keep in mind:
- Having a solid budget and a clear schedule for every step of your project.
- Having effective methods for team communication, as well as leadership from above.
- Working with expert subject matter consultants to make sure that your plans are effective and reliable.
There are many other tips for being a good clinical project manager, but these are some of the biggest in terms of avoiding common pitfalls or conflicts in project management methodology. If you want to be successful in your job, know what resources you have access to and how they can be used most effectively.

Don’t forget to consider tips that are specific to project management in clinical trials. These might be more limited, but they can be equally important for your team. For example, working with regulatory bodies is a critical part of any clinical trial, but understanding how to navigate their complex structure and meet their expectations can be a challenge for even experienced project managers.
Good project management for clinical trials is an essential part of a successful research process. Having a clear plan from start to finish, as well as qualified personnel, can help you to avoid common pitfalls and make sure that your project comes in on time and within budget. And there are many more tips and tricks that you can use when you need to work with regulatory bodies or coordinate teams of expert consultants. Remember that just because something seems different doesn’t mean it has to be overwhelming. Use good project management techniques to keep your focus on what matters most: getting results!
How to run effective meetings with your team members
One of the most important aspects of project management for clinical trials are effective meetings. No matter how efficient your team members are, if they’re not meeting regularly, then it’s going to be difficult to keep projects on schedule and ensure that everyone knows what’s going on at all times. Effective meetings help to solve problems quickly, identify potential issues before they become major roadblocks and keep everyone moving forward with their work. The key is to hold effective meetings consistently, meetings are only useful if they’re regularly scheduled and if people feel comfortable discussing what needs to be discussed.
Pick a meeting location and schedule that works for everyone on your team. Meetings are only useful if they’re easy to get to and scheduled at a time when everyone can attend. Try to avoid scheduling too many meetings all at once as it can become overwhelming. Likewise, try not to schedule them at times when people are likely to be focused on work that isn’t directly related. People need time in their day to focus on other tasks and don’t want unrelated matters distracting them from what they need to be doing.
Follow these tips to help keep your meetings effective:
- Make sure that everyone is on time for meetings.
- If someone is late, you might need to reschedule so that they can come and discuss whatever it was.
- Be sure to start and end meetings on time.
- It’s also a good idea to have a meeting go-round. It is a great way to make sure that everyone has had an opportunity to talk about their topics during meetings. How often you do go-rounds depends on how many people are in your meetings. It is best not to waste too much time talking over each other just because there are more people present.
Keeping your team informed about the clinical trial
Keeping a clinical trial team informed on a daily basis is key to success. There’s just too much going on during these types of projects to not communicate regularly with your team members and update them on all their responsibilities and deliverables. A good project manager will be able to keep stakeholders informed with reports and will keep team members informed with notes or individual emails.
As new information comes in, the project manager can quickly and easily relay it to everyone involved in a timely manner. That type of communication helps ensure you aren’t wasting time answering basic questions again and again or waiting for important feedback from someone who isn’t aware of what’s happening.
An example of an effective way to keep stakeholders informed is with regular emails and reports. Project managers who are taking part in a clinical trial should receive updates and relevant information on a daily basis, so they can distribute it to their teams as needed. This keeps everyone informed while reducing back-and-forth communication and helps reduce confusion.
As a clinical project manager, you may also be asked to help keep patients informed about their participation in a trial. This type of communication with patients is generally conducted through phone calls or emails and depends on how long they have been participating in that trial. For more information on your role as a project manager when it comes to patient communication and retention, check out some helpful resources from Clinical Trials Arena .
Project management principles in clinical trials
Just like in any other business, planning and management are key to a successful clinical trial. A good manager should set clear goals, organize resources and delegate tasks so that all team members are clear on their responsibilities. They should also facilitate communication among team members, look for opportunities to make improvements, and be proactive with regards to potential issues.
We know what you’re thinking: your scientific skills have little to do with project management—but there’s truth in that statement only if you think of management as supervising every aspect of your study yourself. Think of it instead as simply facilitating effective teamwork so everyone involved is aware of what their role is and how they can best contribute toward achieving success.

A good project manager will also recognize that there are different types of clinical trials, each with their own set of challenges. For example, a drug study will have its own specific regulatory requirements and an investigation into new medical devices may involve equipment and electronic record keeping.
The clinical trial project manager should discuss these factors at length with their team so that everyone involved knows what to expect and is prepared for any hiccups along the way. This will ultimately lead to a smoother process overall—especially if you’re conducting your research over several years—and allow you to make better use of valuable time and resources.
It is important to always think ahead. It might be tempting to take a day off when you’re tired, but doing so can really set you back in your work. For example, if you’re a clinical project manager and decide not to come into work one day because of fatigue, that could leave your team shorthanded for a couple of days—which may mean that testing has to be postponed for weeks or months. Not only does that mean your research will take longer than anticipated, but also you may find yourself running up against regulatory deadlines later on down the line which are much harder to meet with a short timeframe. The Project Management Institute has a page dedicated to clinical trial information for additional information.
What is a clinical trial project plan?
The specific deliverables and timelines of a project plan are based on what an individual project manager expects to accomplish. A single clinical trial can take years, so having concrete milestones and deliverables with exact timing is essential for project management in clinical trials. Project managers will often develop detailed, line-by-line schedules that map out a year or more of tasks and related deadlines. Major milestones can include everything from identifying sites and recruiting participants to regulatory submission requirements or final approval of study results by ethics committees. Project managers in clinical trials will work closely with other members of research teams—from technicians performing experiments to doctors who conduct examinations—to ensure their efforts remain on track.
Many project managers in clinical trials will also develop detailed budgets for their projects, mapping out how much time and money is being spent on each step of research. Tracking costs from day one can help a project manager avoid major costs down the line. Even simple errors can end up costing hundreds of thousands or even millions of dollars over an extended project timeline.
A detailed budget will help an experienced project manager identify opportunities to save money while still keeping their project on schedule and within its budget, ensuring that study efforts don’t experience any major bottlenecks. As with any other kind of project management work, it is vital to ensure that your clinical trial has assigned resources at every step.
You can also see why project management in clinical trials is needed: multiple research groups, vast amounts of money and costly resources, many moving parts. And because clinical trial projects are often funded by pharmaceutical companies or other entities with a financial stake in study results, having a designated project manager with expertise in study design and patient recruitment will ensure that everyone on your team is working to improve your end product: better drugs for patients. By hiring an experienced project manager for your clinical trial—or doing everything yourself if you have enough time and expertise—you can ensure that all study efforts move along smoothly, ending with actionable results for drug companies looking to bring safe new treatments to market.
How do you get into clinical trials project management?
When looking for a job in clinical trials project management, it is best to apply to organizations within your geographical area as you may then be able to take on part time work while you are learning on-the-job. Once you have gained experience and achieved success with projects, you can apply for full time jobs with other organizations.
Alternatively, you may wish to work for yourself or set up your own company if there is a gap in the market for your services; however, running your own business does require additional skills. If becoming self employed interests you, be aware that it will require commitment and hard work to succeed but being self employed allows flexibility so if working away from home is important to you then taking on additional freelance projects could suit.
Another way to gain experience is to do internships or clinical research assistant work. Aspiring project managers in clinical trials will find that a lot of trial management jobs require some previous experience and it can be difficult for new graduates to find roles but internships give students an opportunity to try out roles within clinical trials without making a long term commitment; they also make it easier for employers who may not otherwise take on new graduates to take them on as interns are short term and low-risk commitments.
If you would like to be a project manager in clinical trials, then you should consider your strengths and abilities. Consider what subjects you enjoyed studying at school and university and think about what skills or qualities you have that could make you a good project manager in clinical trials.
Think about what personal characteristics would help in your role; for example, extroverts may find it easier to liaise with other departments, whereas conscientious people may perform better at planning. Keep an eye out for job adverts on job boards or visit healthcare organizations to ask if they are looking for project managers. This is also a good way of meeting people who work within clinical trials and gaining more contacts.
Get a Free Project Plan for Your Study

Related Posts

Centralized Monitoring In Clinical Trials: How It Works + 5 Key Benefits

Pragmatic Clinical Trials Common Challenges & Best Practices

New Ways To Overcome Clinical Trial Recruitment Challenges

ABOUT MOSIO
Mosio is a two-way text messaging company specializing in mobile solutions for research. Our award winning Patient Engagement Platform enables researchers to engage and retain study participants through text message alerts, reminders, surveys and interactive TextChat features. Read more about us .
Meet our Team Press Careers Blog
Privacy Policy
OVERVIEW CAPABILITIES REMIND (Text Reminders) RETAIN (Engagement) REPORT (Data Collection)
ePRO and eDiaries Text Message Surveys Automated Messaging Appointment Reminders Google and Outlook Calendar Reminders Medication Reminders Interactive TextChat Text Message Alerts Retention and Engagement Study Team Reminders Custom REDCap Integration REDCap Direct COVID Contact Tracing
HELPFUL LINKS
Login Support System Status Video Guides Pricing Get a Free Project Plan Developers
WHO WE HELP
Overview Use Cases University Researchers Behavioral Researchers Pharmaceutical and Biotech Medical Device Manufacturers Social Researchers Children’s Hospital Researchers Public Health Departments CROs REDCap Admins Developers Clinical Study Teams IT and Compliance Departments
CONTACT INFORMATION
TEXT US USA / Canada: 425-559-9993
TALK TO US 877-667-4699 / 425-559-9993
OFFICE 8001 14th Ave NE, Suite A Seattle, WA 98115
© 2024 Mosio. All Rights Reserved.
- Virtual Office Hours
- Video Guides
- Benefits & Features
- Alerts & Reminders
- Appointment Reminders
- Automated Messaging
- ePRO and Data Collection
- REDCap Integration
- SMS Surveys
- Two-Way Text Messaging
- Universities & Hospitals
- REDCap Users
- Behavioral Research
- Ecological Momentary Assessment
- Children’s Hospital Research
- Pharmaceutical
- Public Health
- Social Researchers
- Studies Published
- Completed Studies
- Knowledge Base
- Webinar Sign Up
- REDCAP DIRECT
- GET A QUOTE
- (425) 559-9993
Clinical Trial Templates to Start Your Clinical Research
By Kate Eby | May 13, 2019
- Share on Facebook
- Share on LinkedIn
Link copied
In this article, you will find everything you need to start your clinical research trials, with easy-to-understand guidance and terminology, 26 adaptable templates, and project plans in Microsoft Word, Excel, Project, and SharePoint formats.
Included on this page, you'll find details on what a research protocol is, project management for clinical trials , research compliance templates , and post-clinical study research documentation and templates
What Is the Research Protocol?
All clinical research starts with the research protocol , a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization. With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance standards with the Food and Drug Administration (FDA) and clinical study best practices.

Download Research Protocol Template - Word
The full research protocol includes the following sections and topics:
- Title Pages: These pages provide general information about the protocol, including name, number, version number and date, trial phase, investigational product name, investigational new drug (IND) number, sponsor (or principal investigator in academia), funding organization, medical monitor, and coordinating center. The pages include the principal investigator’s signature (or sponsor), as well as site-specific information, such as the agreement, and protocol details. They also detail the study team and site, particularly in the case of multiple teams and sites.
- Objectives: List the study’s primary and secondary objectives.
- Background Information: Describe the problem under study and priority. Include the medical and scientific rationale that justifies researching the problem. Include data from other studies relevant to this proposed research. Include the name and description of the proposed intervention, including the dosage, route of administration, period, and frequency of intervention.
- Study Design: Describe the methodology and how it will answer the study question. This should include the type of study, primary and secondary outcome(s), population, sample size, study location, period of enrollment and follow-up, intervention and route of administration, randomization (as necessary), and any other relevant protocol information.
- Selection and Exclusion of Subjects: Provide statements describing how the participants must meet all the inclusion and exclusion criteria, and list the criteria. Clearly define the study population. For example, list the demographic criteria, required laboratory data, any prior therapies allowed or disallowed, ability to understand and meet all study requirements, if contraception is necessary, exclusion criteria such as specific health status, use of excluded drugs, cancer status, and chemical dependency status.
- Study Enrollment Procedures: Describe the methods and procedures for identifying and enrolling subjects, how they are documented, how consent is obtained, and any randomization procedures.
- Study Intervention, Duration, and Route of Administration: This section should describe each intervention and duration, as well as how each is administered. List expected adverse effects and dose escalation, if applicable. Discuss how the intervention is acquired, stored, and disposed of, as well as documentation for intervention accountability. In addition, note the medications restricted, allowed, and required, along with the extent to which these medications are tracked and documented.
- Study Procedures: This section includes a study evaluation schedule (presented as a chart) and explanations of the required assessments, what each period is, and any special considerations or instructions necessary. These should match what is available in the column headers of the chart above, and they should include information on the screening or baseline assessments, randomization, blinding, follow-up visits, and final assessments.
- Safety Assessment: List any expected adverse events, and how these could be managed. Mention any toxicities seen in earlier IND studies here. Also, include safety measures as identified in laboratory findings, methods and timing for safety parameters based on the risk profile, definitions for adverse events (AE) and serious adverse events (SAE) and laboratory values used to identify their possibility, timeframes for reporting and collecting information on AEs and SAEs, the reporting system, how you will follow up on AEs, and the specific guidelines for independent monitoring.
- Intervention Discontinuation: List criteria for intervention discontinuation and how you could meet them. Also list possible reasons for discontinuation, any modifications to the schedule should it be discontinued, duration of follow-up, any temporary discontinuation criteria, or any evaluations should participants be temporarily or permanently discontinued from the study.
- Statistical and Analytical Considerations: Include primary and secondary statistical hypotheses, why you chose the study design, the primary and secondary outcome measures, and the validity and reliability of these measures. Also discuss sample size and randomization, treatment assignment procedures, how you define the population, any interim analyses, primary and secondary outcome analyses, the statistical methods you use to consider any necessary intervention effect between groups, and if necessary, the expected positive within group correlations among different study arms.
- Data Collection: Detail how you will gather the data, the required forms, how to keep these forms confidential, and what source data to expect. Note site responsibility for data collection and management, and (if necessary) the responsibilities of the coordinating center.
- Quality Assurance: Describe training for study staff, whether there is a control committee and their required practices, any quality control metrics, how you will identify and document protocol deviations, how you will assure protocol compliance, and the schedule for reviews. If you have a manual of procedures (MOP), reference it here.
- Participants Rights: Include references to the Institutional Review Board (IRB) requirements, informed consent documents, procedures for participant confidentiality, and study discontinuation requirements.
- Committees: List any committees associated with the study, along with their roles.
- Publication: Outline the requirements and procedures for publication.
- References: List any citations referenced in this protocol.
- Supplements/Appendices: Include any additional documentation.
To track every aspect of the proposed research for each participant, create a case report form (CRF) that you can use in both paper and electronic formats. With CRFs, you can collect and analyze data for analysis, and then generate a conclusion for your study. For more information on the distinct phases of clinical trials, see “ Understanding the Phases of Clinical Trials .”
Concept Protocol Template
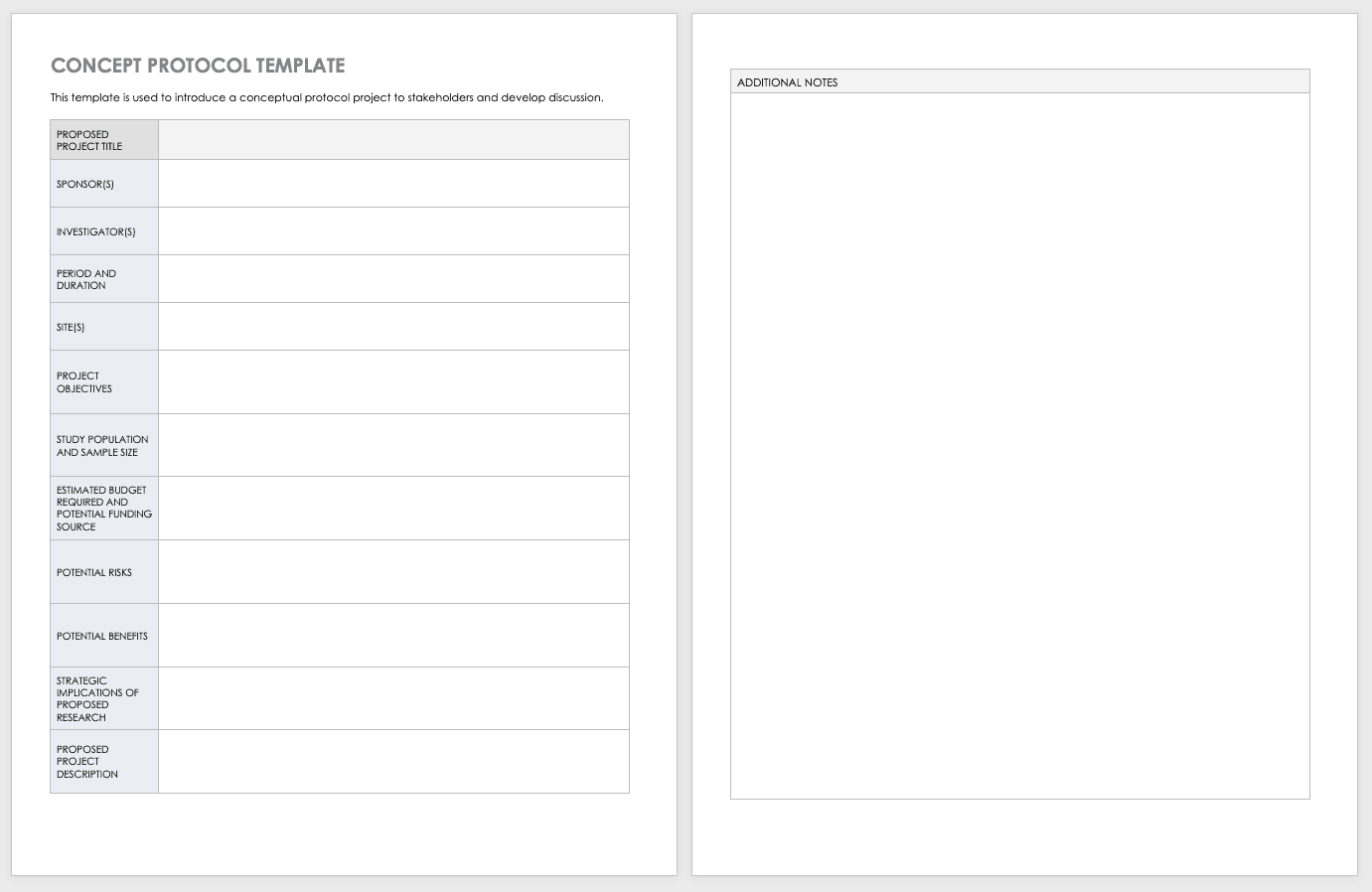
Before you start your full protocol, consider putting together a concept protocol. A concept protocol helps you introduce an abstract project to stakeholders and encourage discussion around the proposed project.
Download Concept Protocol Template for Clinical Research
Phase 1 Clinical Trial Protocol Template

For nonclinical research or clinical trials that are Phase 0 or Phase 1, use this free template. Phase 1 or nonclinical trials do not require the same amount of detail as a full study protocol.
Download Phase 1 Clinical Trial Protocol Template - Word
Research Compliance Templates
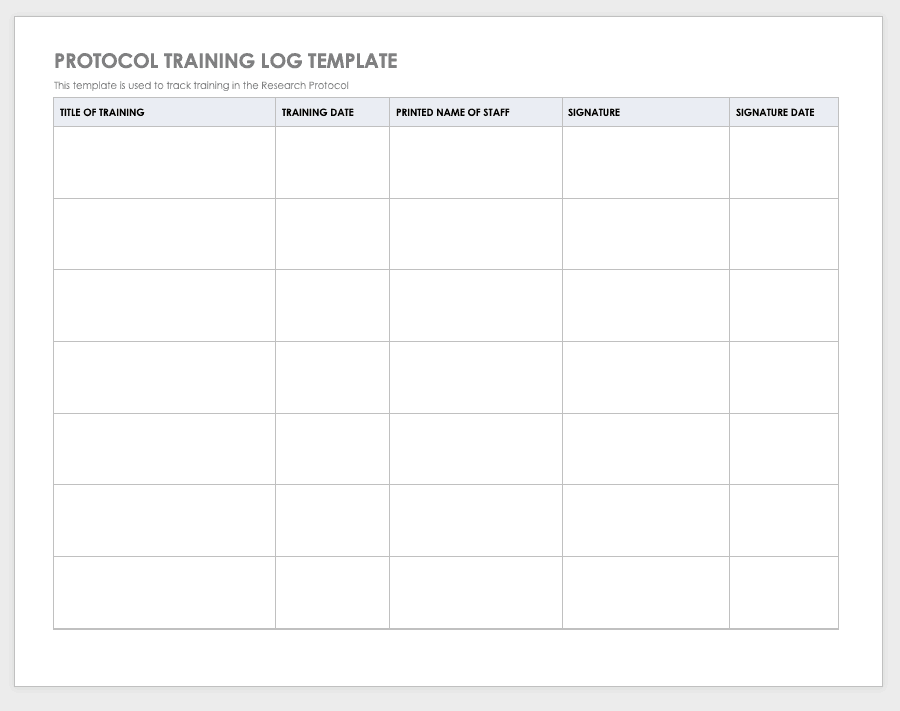
By training staff members on the research protocol, you’ll help them meet compliance standards and understand the purpose and details of the study. Use a training log to record all training that the site study staff completes, signing the log entry for verification.
Download Protocol Training Log Template
Excel | Word | PDF | Smartsheet
Protocol Deviation Template
Protocol deviations are inadvertent or unplanned changes or noncompliance with the research protocol. These events do not increase risk or decrease benefit, nor do they impinge on participants’ safety or rights. They do not compromise study data, but you should capture the deviation for reference.
Download Protocol Deviation Log Template
Excel | Word | PDF
Delegation of Authority Log Template
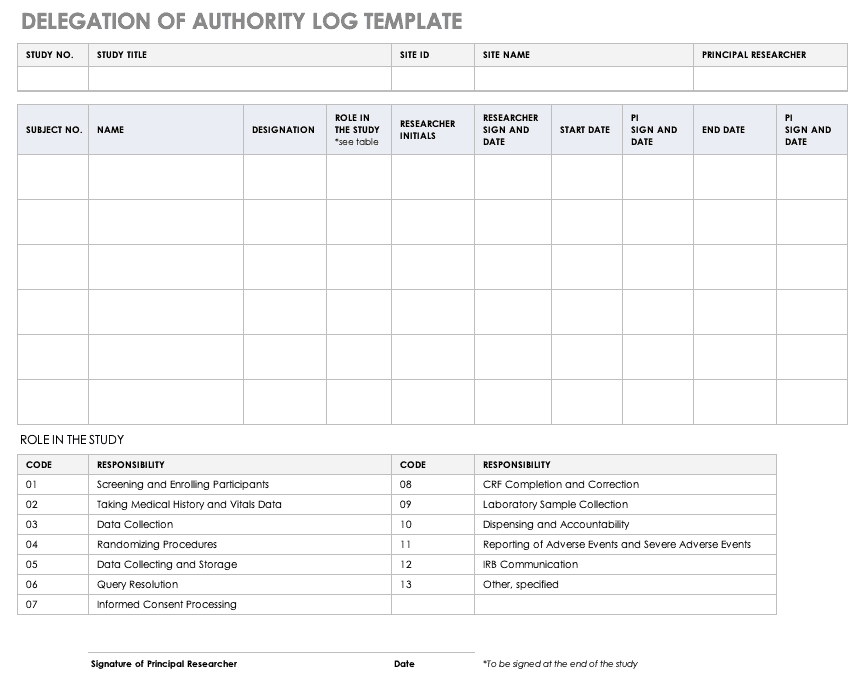
Once you’ve trained your staff and figured out their roles and responsibilities, the principal investigator must delegate authority. The delegation of authority log should be filled out and signed prior to the study’s start.
Download Delegation of Authority Log Template
Site Selection Visit Form Template
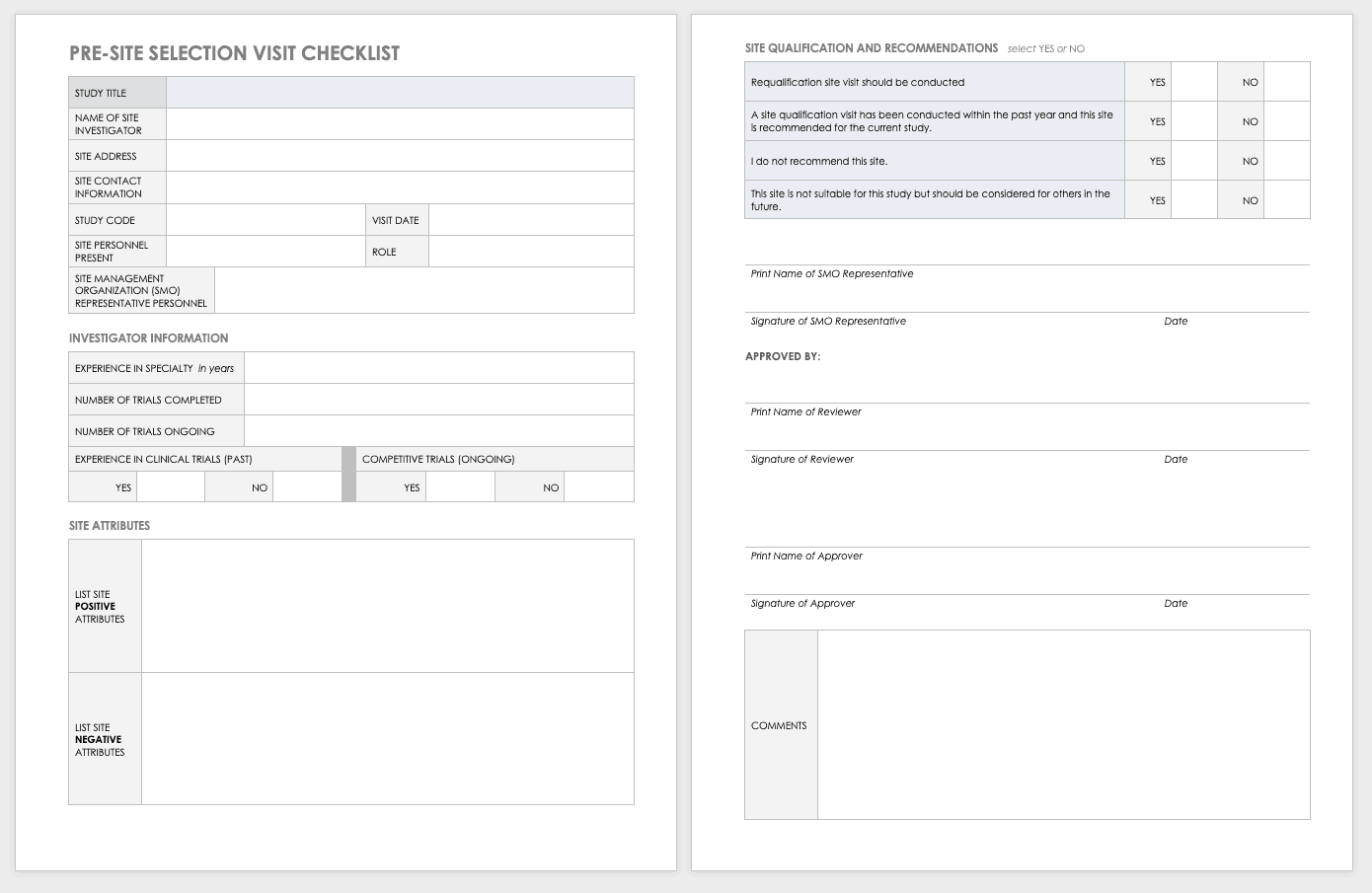
The sponsor must perform a site visit to determine its suitability as part of a multisite study. This means taking a tour to determine whether the site has the capabilities to meet the sponsor’s goals.
Download Site Selection Visit Form Template
Word | PDF | Smartsheet
Study Site Initiation Checklist
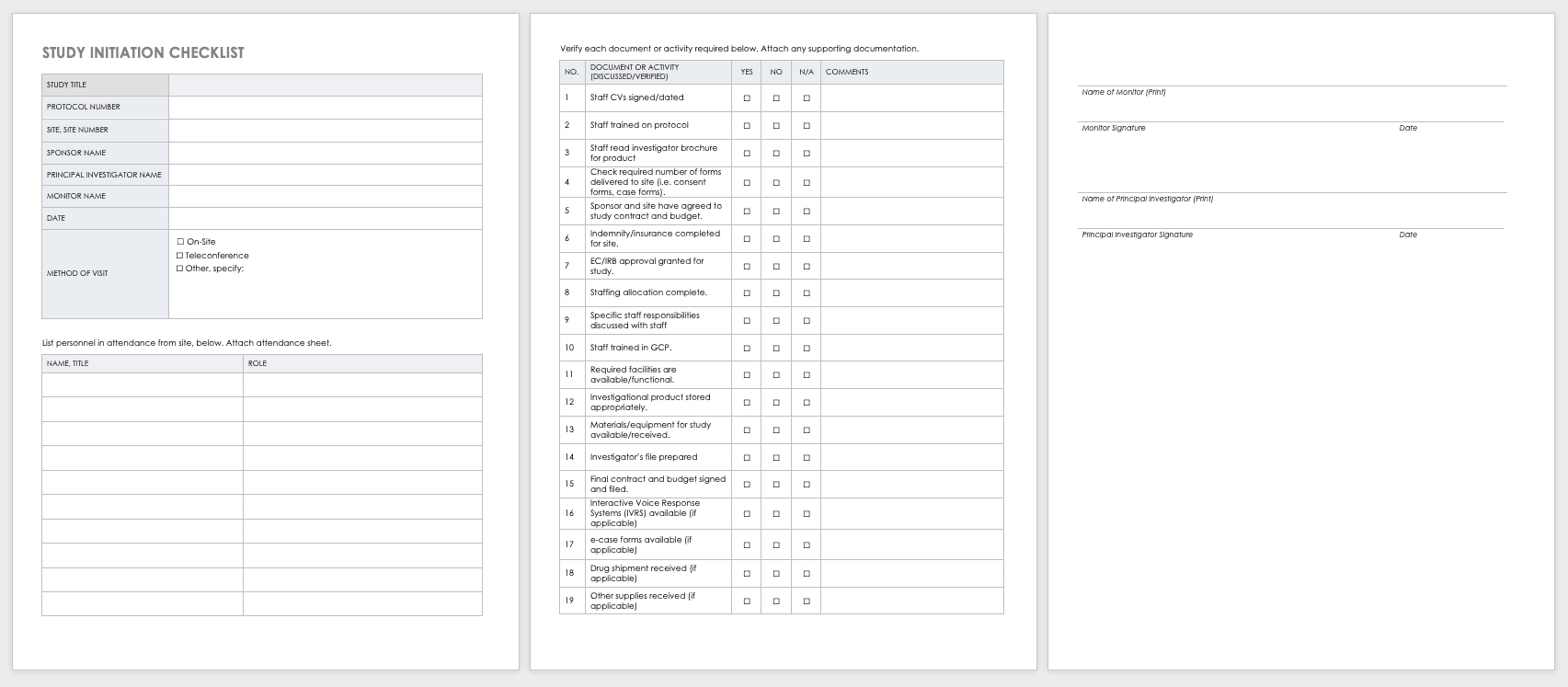
Teams must also perform an inspection to determine if a site has the appropriate staff, training, equipment, and supplies to be part of a multisite trial.
Download Study Site Initiation Checklist
Project Management for Clinical Trials, Practices, Templates, and Documents
Clinical trials are big projects. If the organization is not used to planning and wants to conduct clinical research, it must hire a project manager and work with senior leadership to introduce planning into the organization.
Together, they should develop the main goals and define their limits and the terms of success. They should set out a strategy for which tasks and sets of tasks to perform and in what manner. Test any planning tools or software before the trials start. When possible, use templates to ensure consistency and best practices.
Once the trial starts, evaluate your systems with standardized metrics. The project manager can track study deviations and apply corrective actions. Use the lessons learned from past and current projects to help guide future projects. Employing consistent tools gives you the opportunity to draw from a reservoir of data.
Clinical research can cost billions of dollars and years of time, resources, and effort. As
such, project management best practices and methodologies are critical to the success of a clinical trial, according to experts .
Many software systems are available to manage clinical trials. When very specialized, these are referred to as clinical trial management systems (CTMSs). However, other platforms can also manage clinical trials and may already be embedded with your information technology. Regardless of the platform you use, you should have full project management functionality, such as planning and reporting modules, as well as the ability to track participant contact information, deadlines, and milestones.
You may want to consider the following project management documents for your clinical research.
Project Management Plan (PMP) for Clinical Trials
A PMP delineates and acts as an agreed-upon document of scope, responsibilities, and guidance. You can use it throughout the project to help stay on track. Every clinical trial has difficult milestones, but a good project management plan can help you sidestep some of the regular issues.
You have many PMP software platforms to choose from, but regardless of your ultimate decision, your PMP must focus on protocol adherence, subject care, and service quality, along with how to achieve each standard. Here are the sections you should include in your PMP for a clinical trial:
- Project Objectives: This is an outline of the research objectives for the study, your quantifying standards, and your goals.
- Background and Strategic Context: By documenting background and context, you establish a foundation for decisions and discussion to follow.
- Study Governance: The governance covers the roles and responsibilities in the project, encouraging open communication, sharing, and accountability.
- Stakeholder Management Plan: This plan details how the staff and investigators will collaborate and effectively communication with stakeholders. This could include (as per the roles and responsibilities) regular emails, newsletters, consultation, oversight, training, and documentation.
- Scope: This document delineates assumptions, constraints, and deliverables (and their expected dates).
- Project Risk Assessment: This document helps you prepare for risks and decide on the risk profile.
Clinical Research Project Activity List
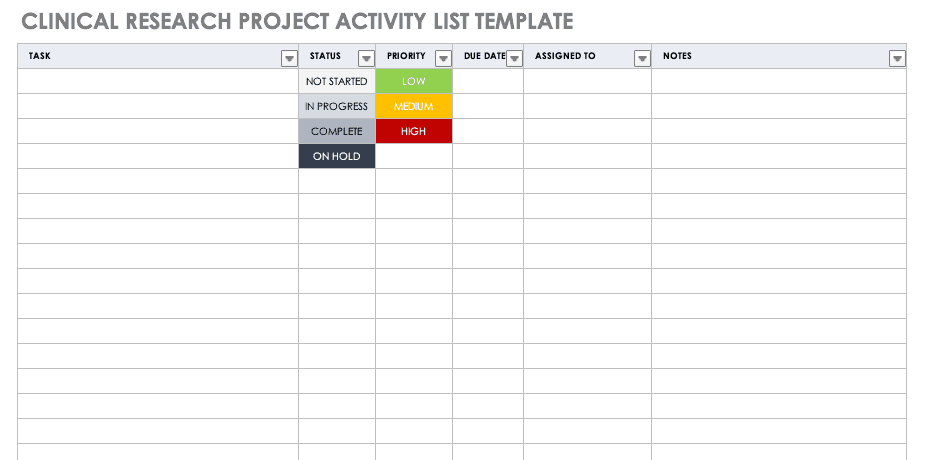
A project activity list is an itemized documentation of all the activities scheduled as part of the project. This list should be very detailed, including the status and priority of the task, when it is due, and to whom it is assigned.
Download Clinical Research Project Activity List Template
Excel | Smartsheet
Clinical Trial Timeline Template
A timeline enables you and your staff to track each major portion or milestone of your clinical trial. Your timeline should include these steps:
- Choose Research Questions and Study Design: Research always begins with questions. Your research question will determine how you design your study.
- Choose Outcomes: The outcomes for any trial are dependent on many factors, including scope, health conditions under study, target population, type of intervention. One resource to help develop outcomes is Core Outcome Measures in Effectiveness Trials (COMET) . This database details core outcome sets for comparison in clinical trials.
- Prospectively Register the Trial: Whether you are working through the FDA, World Health Organization (WHO), or another national agency, study transparency is critical. Prospective registration of trials is recommended. One resource for registration is the ISRCTN registry .
- Obtain Ethics Approval: Any trial involving human participants must go through an ethics review to safeguard the subjects’ rights, safety, well-being, and dignity. There are many options for institutional review, including through a university or a private or governmental organization. Without this step, research cannot commence.
- Prospectively Publish Protocol and Analysis Plan: Before a clinical trial, you must complete some pilot research. When you publish the research leading up to a clinical trial, along with the protocol and analysis for the trial itself, you increase transparency and accountability of the research.
- Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the plan early and allowing potential participants to review it, you help improve recruitment and retention during the trial.
- Recruitment and Retention: Recruitment is getting the right people to take part in your trial, and retention is about keeping their interest and trust. A source of unending frustration for researchers, recruitment and retention can make or break a trial.
- Identify and Manage Trial Sites and Staff: This process is not as straightforward as it is often thought to be. Study coordinators must use feasibility checklists to choose sites and figure out how to get bring on staff who have the bandwidth to recruit for the study.
- Data Collection: The methods for collecting data are critical to any study. Advance planning and structure help you stay organized, comprehensive, and transparent so that your study can have a seamless analysis and solid conclusions.
- Data analysis: Flaws in analysis can generate poor, biased, or erroneous outcomes. In advance, researchers should consider patient blinding, randomization procedures, and sequence generation.
- Findings dissemination: Some researchers recommend threading all research on a trial topic. One resource for this is CrossRef , a database that links similar research. Regardless, the point of research is to capitalize on scientific progress and move it along. By having a plan to disseminate your results, you ensure that others capitalize on your research and move the knowledge forward.
Use this free template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

Download Clinical Trial Timeline Template
For a different perspective, add your project details to this free template so you can view your timeline visually.
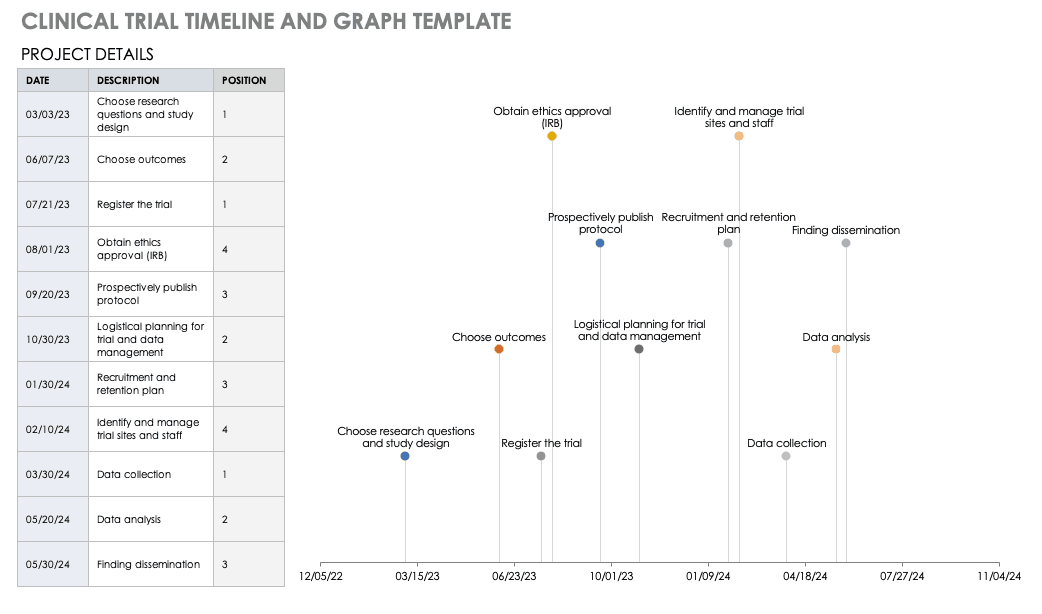
Download Trial Timeline and Graph Template
Microsoft Project Management for Clinical Trials
First released in 1985, Project is a well-respected Microsoft product for project management. Microsoft Project was not traditionally available as a part of Office Suites, a package of programs for professionals and professional organizations. However, Microsoft recently included it as a part of the Windows 2016 suite.
Microsoft Project Management has the following features:
- Built-in templates
- Project portfolio management
- IT management
- Presentations
- Out-of-the-box reports
- Multiple timelines
- Real-time reporting
- Dependency management
- Priority assignment
- Lean management
- Gantt charts/project mapping
- Calendar views
- Setting baselines/KPIs
- Project budgeting
- Issue tracking
- Task creation
- Resource management
- Cloud access
Microsoft Project has built-in templates that you can apply to clinical trial management.
Microsoft SharePoint for Clinical Trials
SharePoint is a collaboration platform that is integrated with Microsoft Office. SharePoint manages and stores documents , and it enables multiple users to access the documents via their own site or a standardized Microsoft site. A subscription to Microsoft Office 365’s SharePoint does not require a server, but customization options are limited; the flexible authentication and authorization systems are built in.
SharePoint Server, available in Standard or Enterprise versions, can be developed as either
virtual or hosted services in a business’s IT department. SharePoint Server enables the organization to control the SharePoint features available to staff, and you can scale it to meet different numbers of users.
Windows SharePoint Services 3.0 is a Microsoft-hosted version that comes with Microsoft Office. Microsoft provides a template in SharePoint for Clinical Trials: Clinical Trial Initiation and Management application template for Windows SharePoint Services 3.0 . You can download and add this template to your SharePoint Services, which enables you to create the following:
- Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget.
- Protocol Documents: This includes additional documents relative to your study.
- Calendar: Track milestones in the project.
- Threaded Document Discussions: Team members can start and track discussions within documents.
- Task Creation and Assignment: You can create and assign tasks to users, who receive email notifications.
- Archiving: You can move documents or groups of documents to archive status, keeping them but not making them visible.
The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint. To download this template, you will need access to SharePoint Server 3.0.
Clinical Research Budget Plan Template
In many instances, you set the clinical trial budget after much negotiation with a sponsor. Other times, you need to build a budget before the sponsor is even on board, as a way to convince them of the project’s feasibility. The key cost drivers for any clinical research project are the following:
- Patient Grants: These include the costs for screening failures, baseline patient measurements, and procedural costs.
- Site Costs: This covers any expenses associated with the site, such as start-up fees, IRB fees, storage fees, and site management costs.
- Non-Patient Costs: This includes consultation fees, monitoring board fees, and any medical device costs.
- Labor Costs: You must account for all the staff required for the project and their full-time equivalency (FTE).
- Site Management: These costs include pre-study visits, initiation fees, monitoring, and close-out fees.
- Miscellaneous: These include investigator meetings, any technology needs, and ad hoc travel.
- Unexpected Costs: These are costs resulting from protocol amendments, value added tax (VAT), delays, and inflation.
Before you start putting together your research budget, you must gather the following:
- Schedule of assessments from the protocol
- Standard institutional fees from your institution, if applicable
- Evaluation and procedural costs
- Staff allocation and their hourly rates
- Indirect cost rate
- Subject compensation costs
- Data storage fee estimate
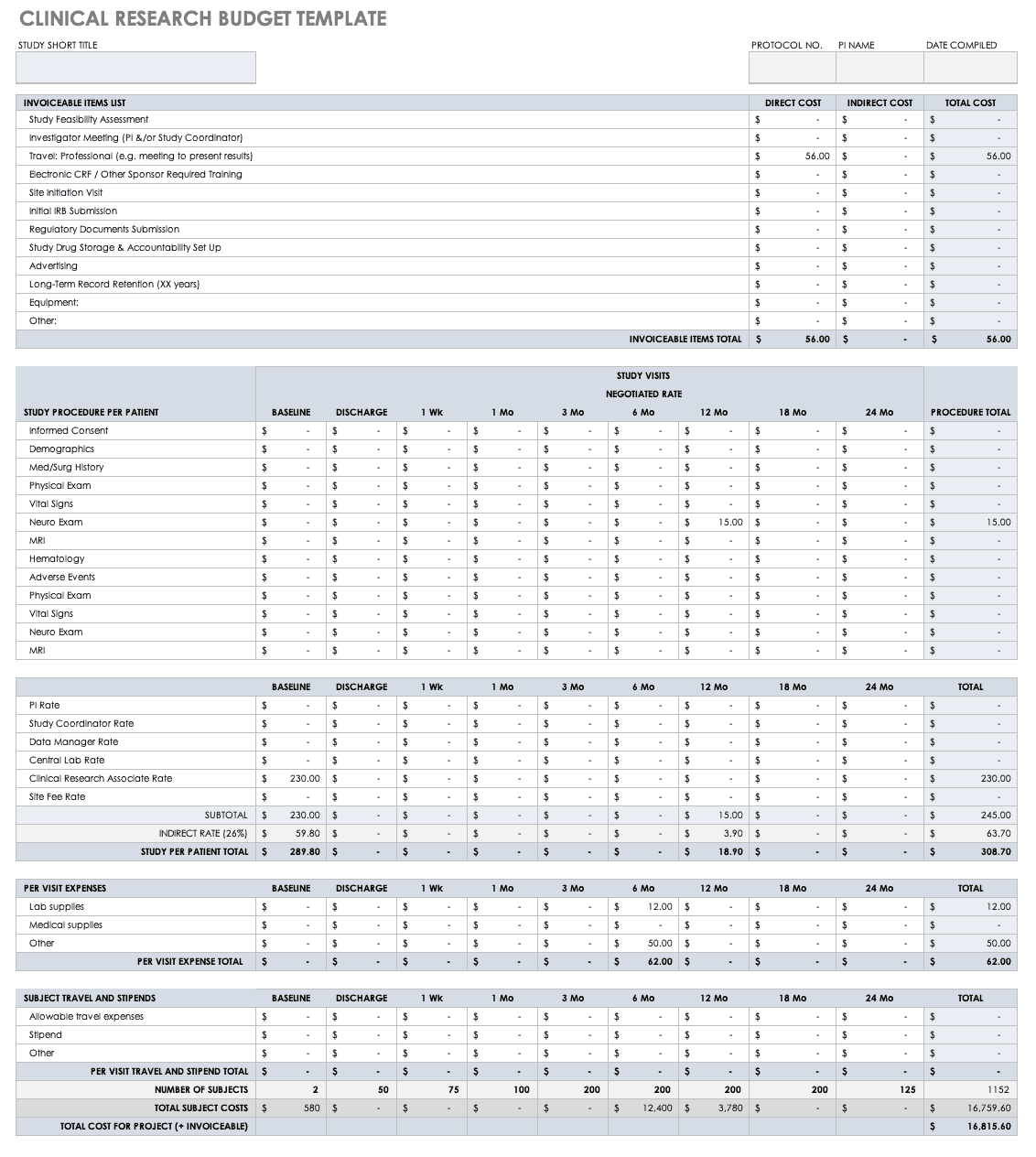
Put together your own clinical trial budget with this free clinical research budget template.
Download Clinical Research Budget Template - Excel
Clinical Research Tracking Log Templates
Clinical research requires scrupulous planning, a well-developed team, regulatory adherence, and above all, excellent documentation. It is therefore critical for clinical trial project managers to have a completed scope of work and to develop all the forms and templates before the trial begins. Some of these documents are for planning, and some, like those included below, are for operational purposes.
Regulatory Binder Checklist

Strong clinical practice thrives with a regulatory binder checklist. This checklist keeps track of all paper versions of essential regulatory study documents. Each document should also include any electronic locations. This document should be regularly updated, customized for unique studies, and stored in reverse chronological order.
Download Regulatory Binder Checklist
Clinical Study Document Tracking Log
It is important to not only track all paperwork related to a clinical trial, but also be able to locate it easily between various staff and sites. A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved. You should also keep copies of the documents with the log. Use this free template to develop your own clinical study document tracking log. You can also adapt the log for specific correspondence, such as documents relating to FDA or IRB submissions, but it should not be mixed with regulatory documentation.
Download Clinical Study Document Tracking Log
Data and Safety Monitoring Plan (DSMP) Template
Before you can undertake a study, you must develop a DSMP for how to keep participants safe and how to secure data and ensure accuracy. The DSMP has several sections:
- The study purpose
- An adherence statement
- Any protocol amendments
- Multisite agreements
- A plan for subject privacy
- Confidentiality during adverse event reporting
- Expected risks
- Adverse events, unanticipated problems, and serious adverse events: how they are defined, their relation to the study, expectations, severity grading, and reporting procedures in single-site and multisite trials, and whether they are IND or non-IND studies
- Events of special interest
- Pregnancy reporting
- Rules to halt the study for participants
- Quality control and quality assurance
- Subject accrual and compliance
- Sample size justification
- Stoppage rules
- Monitoring committee designation
- Safety review plan
- Study report plan for independent monitors
- Plan to submit reports from onsite monitoring and audits
- Data handling and record keeping
- Informed consent
- Reporting changes in study status

Create your own data and safety monitoring plan using this free template. It lays out each section so you can specify them for your research. The principal investigator should sign and date this document once it is complete so that it may be filed.
Download Data and Safety Monitoring Plan Template - Word
Research Communication Plan Template
A communication plan should describe how you will converse with internal and external stakeholders during your project. Your communication plan should include a brief overview of your project and a breakdown of the messages you need to get out. You should adapt the messages for different audiences and define who will deliver these messages. The messages should include the following:
- The purpose and benefits of the research
- The known effectiveness of the intervention, or (if the intervention is under study) the disclosure that the effectiveness is unknown
- How participants will be protected
- The risks and benefits of participating

Develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and threats.
Download Clinical Trial Communication Plan Template - Word
Participant Management in Clinical Trials Using Templates
A few main documents help ensure that your participants are tracked and well-cared for before and during your research study.
Enrollment Log for Clinical Trials Template
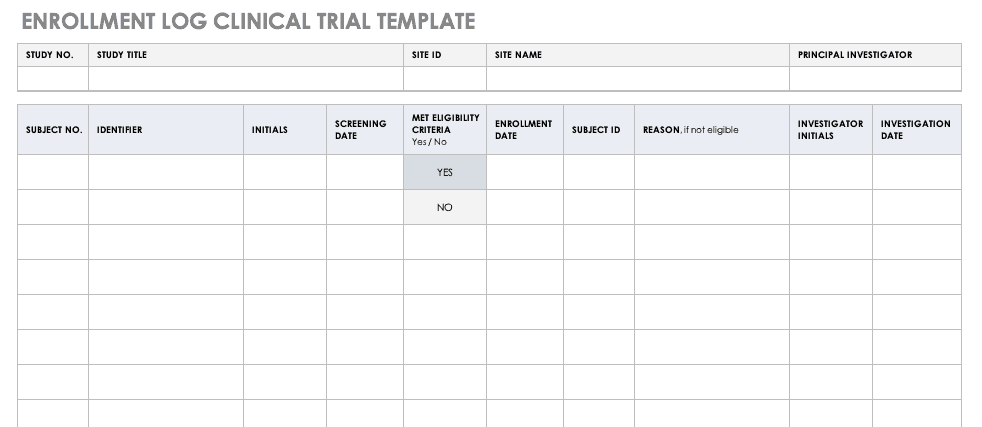
This log keeps track of everyone that has been enrolled for participation in your study. This does not mean that they have met the eligibility requirements or have been otherwise screened, but it is a record that they have signed up to be admitted.
Download Enrollment Log for Clinical Trials Template
Informed Consent Form Templates
Informed consent is the central tenet of ethical research with human subjects. The consent process typically involves a researcher delineating what is involved in the study, its risks and benefits, what a participant’s duties entail, and answering any questions they have. Before you perform any research, make sure the informed consent document is signed and the participant receives a copy, unless the informed consent document has been waived by an institutional review board (IRB). Federal regulations 45 CFR 46.116 govern what you must provide in the informed consent process in the United States.
To prepare informed consent documentation, researchers must do the following:
- Use plain, easily understandable language no higher than an 8th-grade reading level.
- Tailor documents to the potential population.
- Avoid technical jargon.
- Use the second or third person (you/he/she) to present study details.
- Include a statement of agreement.
- Ensure that the consent document is consistent with information in the IRB application.
These templates assist the principal investigator in the design of their informed consent forms (ICFs). You can adapt them to accommodate the details of any study and include both the information sheet and the consent form. Modify each section with the appropriate description described in italics. Use the general template for any type of research.
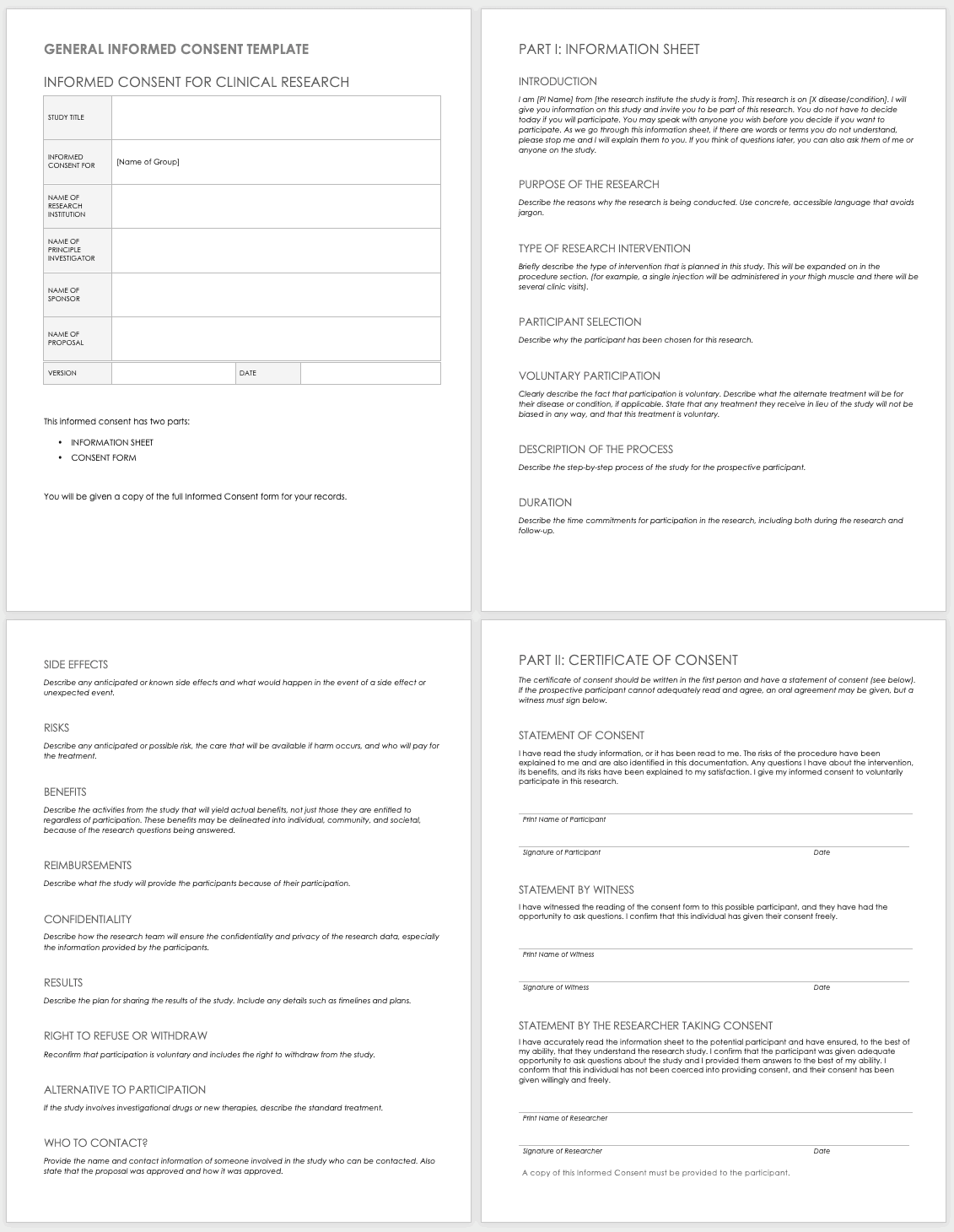
Download General Informed Consent Template - Word
Use the clinical trial template for medical research.

Download Informed Consent for Clinical Trials Template - Word
Eligibility Criteria (Inclusion/Exclusion) Checklist
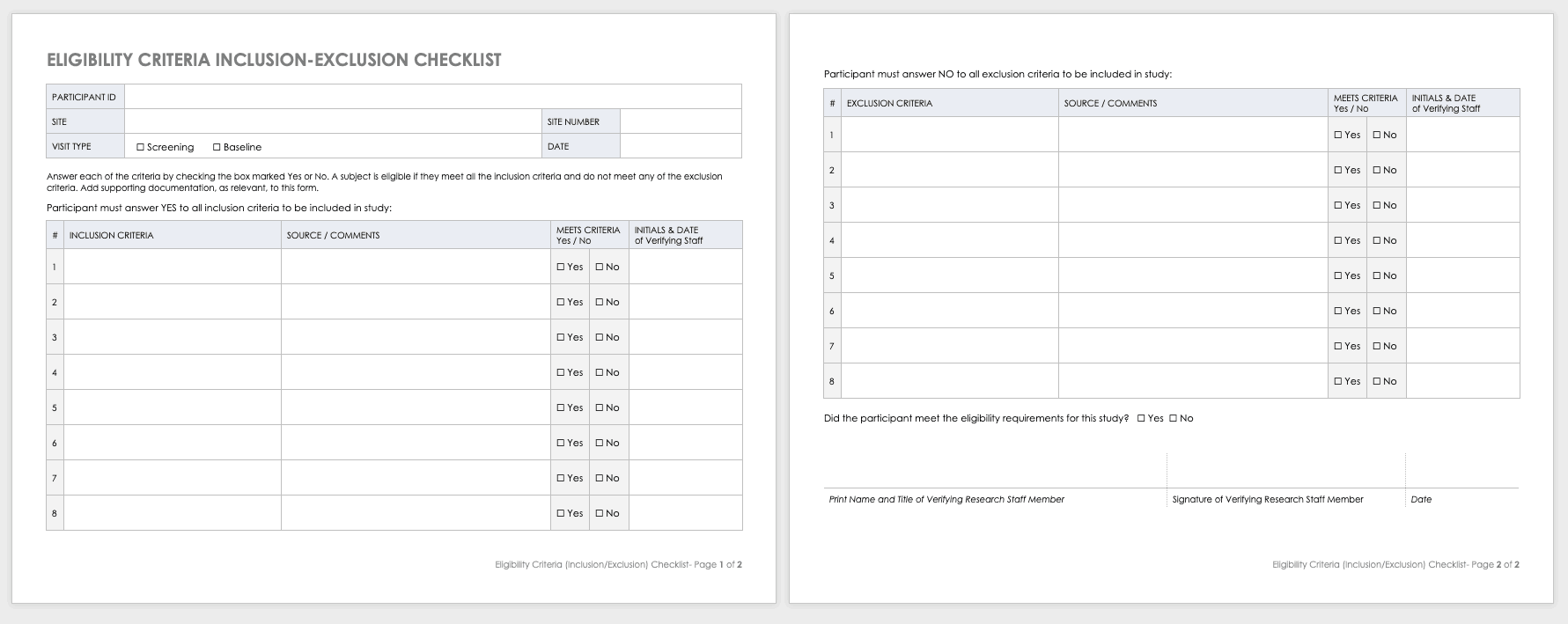
Eligibility criteria are an essential part of clinical trials. They define the population under investigation.
Inclusion criteria are the standards that participants must meet to enroll in the study. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes.
Exclusion criteria specify the characteristics that disqualify participants from taking part in the research. For example, in the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range.
Download Eligibility Checklist Inclusion-Exclusion Template
Concomitant Medication Log Template
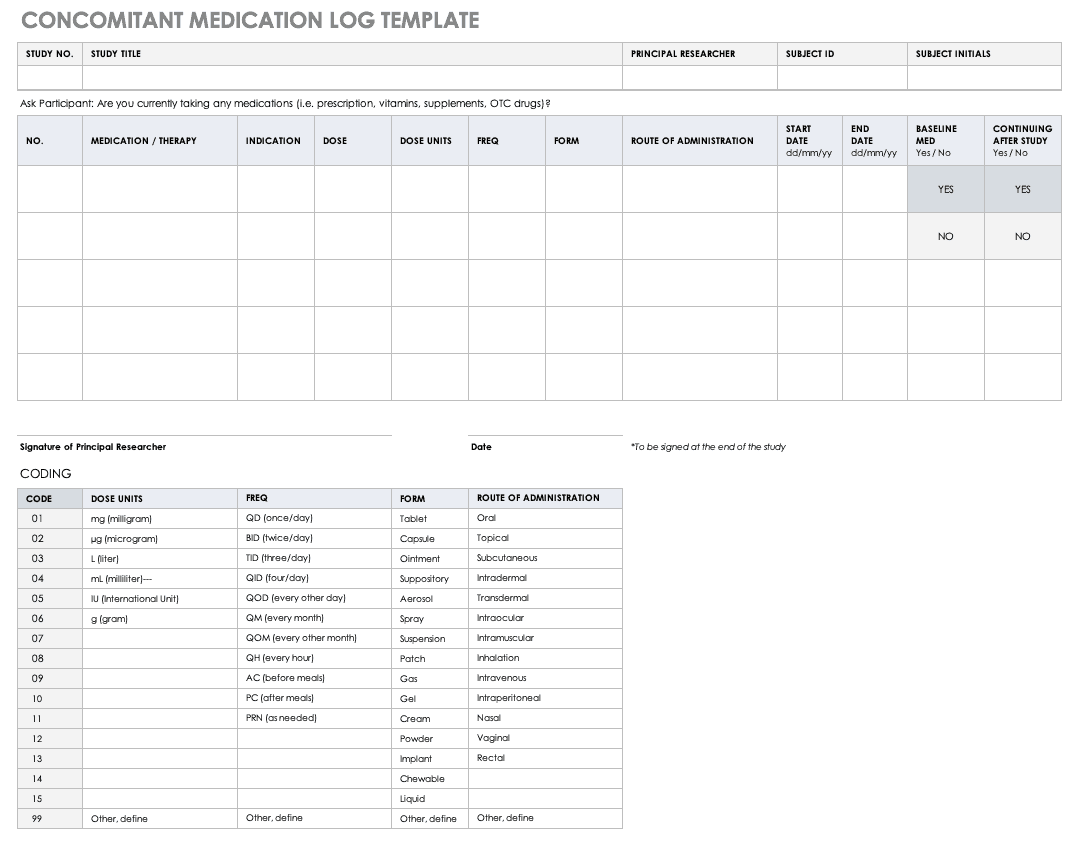
Properly documenting any medications that participants are taking is imperative to understanding the reactions occurring in their bodies, as well as what could spur adverse and severe adverse events during the study. Fill out a concomitant medication log for every participant and account for everything participants take, even seemingly innocuous items like multivitamins.
Download Concomitant Medication Log Template
Excel | Word | PDF
Adverse Event Form
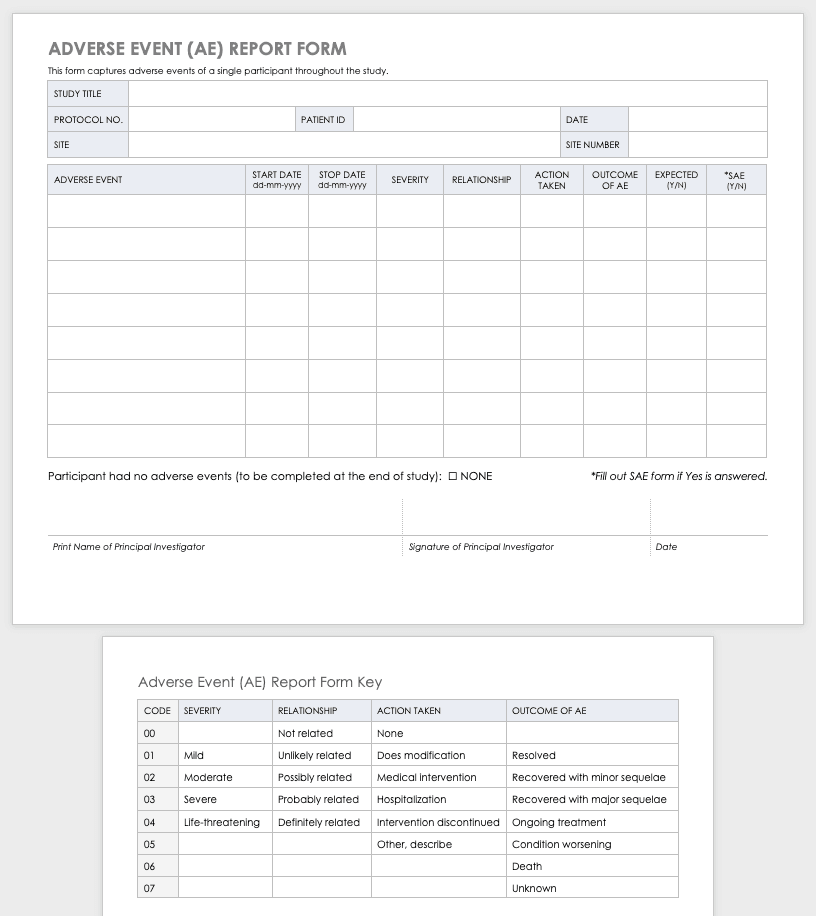
Clinical research can result in complications for the participants and trigger an adverse or severe adverse event. An adverse or severe adverse event is when participants in a clinical trial have negative medical symptoms that can be shown in laboratory or physical testing. Each participant in a clinical trial should have an adverse event log that tracks any adverse events through the duration of the study.
Download Adverse Event Form Template
Severe Adverse Event Form

A severe adverse event (SAE) is a special case of an adverse event in which the outcomes are acute. Examples of SAEs include death, life-threatening complications, or anything leading to immediate hospitalization, physical disability, or congenital abnormalities. Log SAEs in the AE form, but fill out an additional SAE form.
Download Severe Adverse Event Form Template
Word | PDF | Smartsheet
Post-Clinical Study Research Documentation and Templates
After you complete or terminate a clinical trial, you should prepare several additional documents. Here are some examples of this documentation:
- Investigational Product Accountability Log: You generally provide an accountability log to the authorities that tracks drug products to show product disposition and accountability per participant. It also helps you track the drug product stock and any imbalance at the end of the study.
- Investigational Product Destruction: Due to regulations governing the proper disposition of investigational products in clinical research, you must properly dispose of products left at the end of a study (as evidenced by the product accountability log). This form describes and ensures that you have properly handled any leftover products.
- Close-out Checklist/Report: A study close-out checklist and report helps ensure that you complete all closing procedures, archive the paperwork, and resolve electronic data.
Clinical Study Summary Report Template

Assemble the summary report at the end of a study to get results into the sponsor’s or public’s hands while you complete the full report. A summary report is typically about 2-3 page-long document that encompasses the highlights from the trial.
Download Study Summary Report Template - Word
Clinical Study Report (Full) Template

The full clinical study report (CSR) encompasses all aspects and details of the research you’ve conducted. It is not a sales or marketing tool; instead, it is a scientific report details the methodology and shows scientific rigor.
Download Clinical Study Report Template - Word
Public Links and Resources for Clinical Trials
The following are publicly available resources, tools, and links for clinical trial practitioners and principal investigators:
- PROMIS : Patient-Reported Outcomes Measurement Information System (PROMIS) software gives clinicians health status patient measures that are physical, mental, and social patient-reported metrics. Funded by the National Institutes of Health (NIH), PROMIS can be used in clinical trials as measures of conditions and disease and as a comparison to the general population. The measures in PROMIS are free to administer on paper, by computer (computer adaptive tests), or with an app. The computer adaptive tests may be conducted on REDCap , Assessment Center , or Epic .
- REDCap: REDCap (Research Electronic Data Capture) is an electronic data capture system that works on browsers to develop research databases. It was developed at Vanderbilt University to support clinical research data collection and is a free resource to nonprofit organizations. It is limited to organizations joining the REDCap consortium and is not open-source or available for commercial use.
- Good Clinical Practice (GCP) Training: GCP is an international quality standard designed for use by staff involved in clinical trials. The guidelines for this are from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). These regulate the ethical guidelines, documentation, record keeping, training, facilities, technology, and inspections. The purpose of these guidelines is to keep clinical trials scientifically rigorous and to delineate the roles and responsibilities of research staff. The National Institutes of Health administers training for GCP.
- Quality Management Study-wide Review Tool: Developed by the NIH, this review tool is for PIs and study teams to manage their quality reviews, and may be customized for unique studies.
- Quality Management Subject Review Tool: Also developed by the NIH, this review tool provides study teams the structure for review of participant data, and may be customized for the unique study. This should be developed in concert with the DSMP.
- AccrualNet: AccrualNet is sponsored by the National Cancer Institute (NCI), and offers advice and training to staff on how to recruit study participants.
- Regulatory Education for Industry (REdI): The FDA offers a Clinical Investigator Training Course for researchers conducting investigational new drug (IND) or device exemption (IDE) studies.
- ResearchMatch: Available to volunteers and researchers affiliated with the NIH Clinical and Translational Science Award (CTSA) program, this site helps match prospective participants with specific studies.
- Grant Policies and Guidance: The NIH and National Center for Complementary and Integrative Health (NCCIH) offer links to many resources that are policy- and grant-specific to the NIH and NCCIH, updated regularly.
- Protocol Amendments: The NIH and NCCIH offer regularly updated guidance for NIH policy and protocol changes.
- Clinical Terms of Award for Human Subjects Research: The NIH and NCCIH offer guidance for clinical trial grant awardees for compliance.
- NIH Single IRB (sIRB) Policy for Multisite Research: The NIH offers a FAQ page for multisite research that includes policy, contract and application information, responsibilities, exceptions, and costs.
- Dictionary of Cancer Terms: The National Cancer Institute (NCI) offers a dictionary of cancer terms for researchers and laypersons. You can add this dictionary to your website as a widget.
- Informed Consent FAQs: The U.S. Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP) offer a FAQ page about informed consent for researchers and lay persons.\Informed Consent Language (ICL) Database: The National Comprehensive Cancer Network (NCCN) offers a database to help write informed consents. This database is specific to medical conditions and different risk language.
Improve Clinical Trial Research with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.
Project Management: Introduction to Tools and Templates
By: melissa harris, mpa, ccrp director of interventional resources & clinical trials unit pennington biomedical research center at lsu.
Abstract: Project management involves many complex components and moving parts. Prior to initiating a clinical trial, various types of project tools and templates can be used to successfully plan and execute a clinical trial. This article highlights tools that are readily available for project management, including Microsoft Excel, Access, Visio, Outlook, and SharePoint, as well as Web-based applications. Monitoring progress through various tracking mechanisms ensures successful clinical trial execution from recruitment through retention and follow-up.
Project Management
The project management life cycle for clinical trials is comprised of:
- Study start-up
- Team management
- Clinical assessment
- Intervention.
The project management tools covered in this article are described in relation to the project management life cycle. Examples are from clinical trials in academia; however, the tools can be used in any research setting.
At any given time, the project manager is shuffling plates and trying not to drop one. Project management can be considered similar to riding a bike. The project manager should be able to get on the bike or project and do the same thing on each ride, or in this case, from research project to research project. Unfortunately, in clinical research, the bike is “on fire,” the project manager is “on fire,” and everybody working on the clinical trial is “on fire.” This article provides tools to help douse the fire and continue to move forward on the research project.
There are many components of a study, from the research idea through analyzing the data and publishing results (Table 1). The most difficult parts of a project are study start-up and keeping the study going when recruitment is not going well. The project manager and study team try to complete the first four components of a study (research idea, protocol, grant, and institutional review board review) in as compacted an amount of time as possible. Sometimes this requires having a very strong foundation. Tools and processes can be rotated from study to study, enabling the project team to move through the cycle fairly quickly.
The clinical trial lifespan includes:
- Trial initiation and timeline management
- Creating and managing the budget
- Protocol/consent preparations, institutional review board (IRB) submissions, and revisions
- Development of processes and the manual of procedures (MOP)
- Liaison for contracts, subawards, and community partnerships
- Identifying and managing resources (staffing)
- Training/certification plans and tracking
- Ongoing communication and clinical trial oversight.
Tools help project managers calm the chaos of clinical trials. There are many project management tools, including:
- Microsoft Office Excel, Word, Access, Outlook, and SharePoint
Office 365 is not covered in this article, outside of Outlook, however, Office 365 offers a multitude of online apps.
Tools for Study Start-up
Tools for study start-up include organizational charts, timelines, and process flows (Table 2). A research program organizational chart documents a clear chain of command. It can create camaraderie and outlines responsibilities so that staff know to whom to report. The organizational chart also identifies people who have specialized positions, such as blinded staff. Visio is an easy tool to use in developing organizational charts.
Timelines provide milestone time points for various stages of start-up to be completed. They are crucial and should be revised constantly to align with the pace of the study. When developing a timeline, the author starts with the expected date of the first randomization or site activation and works backwards. The timeline maps the amount of time necessary for each step involved in study start-up. The timeline establishes clear goals for study staff so that each staff member knows the due date for assigned tasks.
Process flows, also known as flowcharts, allow a process and the steps in the process to be viewed at a glance. The author uses flowcharts for all extensive processes within a protocol, investigator brochure, or manual of procedures. It is much more efficient to refer to a flowchart when working with a study participant or working on other study tasks than to have to pull out a large document and search for the necessary information.
Cross-functional flowcharts, also known as swim lanes, demonstrate the process steps in sequential order and show who does each task. Decision channels (yes/no) can also be included in a cross-functional flowchart.
The cycle of implementing the project and maintaining it is extremely important. Implementation must constantly be re-evaluated to determine whether it is working. A project may be working; however, it could be more efficient. Without monitoring, the project manager will never know if project efficiency could be improved. Monitoring includes identifying inefficiencies, assessing the cost-benefit ratios, and monitoring expenses against the budget.
Tools for Team Management
Team management is a major component of running a clinical trial. The author spends much of her day reading emails from people who are updating her on the work that they are doing on a study. This makes it difficult for her to accomplish her tasks for that day. Regular team meetings are an important form of communication. Team meetings can minimize the need for many emails. They can be done electronically, through teleconferences, Web conferences, online reporting systems, etc.
Effective team management also requires ongoing communication with internal affiliates (other departments) and external affiliates (community partners). A 10-minute telephone call twice a month may be sufficient to communicate with internal and external affiliates. Ongoing communication on the study’s outcomes/progress is also necessary with regulators, funders, and other external affiliates.
Document libraries, calendars, and action items are good tools for team management (Table 3). Document libraries provide a central location for all departmental or project-specific files. They may be housed on shared drives such as Google drives or Dropbox. Since these shared drives do not comply with the Health Insurance Portability and Accountability Act, some universities do not allow their use. Universities often use tools such as SharePoint, OneDrive, and Basecamp, which staff can access from anywhere. A document library automatically backs up the documents every night.
Basecamp allows the project manager to set up study teams and provide different levels of access to documents for different team members. Assignments, schedules, and bookmarking of certain materials can also be done using Basecamp. In SharePoint, the project manager can create folders and list documents. SharePoint and Basecamp both track edits to documents.
Project managers and study staff use calendars, such as Outlook, extensively for scheduling appointments or responsibilities, participant scheduling, and study-specific calendars. Appointment reminder alerts are a key benefit of calendars. The author maintains a personal calendar and a department calendar to oversee staff activities via a central destination to book participant visits and other study related meetings.
The department calendar is color-coded so that people can easily see the type of visit: green for assessment visits, purple for remote data monitoring, and yellow for phone call visits to name a few examples. Red indicates something important, such as not scheduling participants for visits requiring online RedCap surveys on a day that Internet access will be shut off or when the center will be closed. Calendars also show when staff will be out of the office. Patient identifiers and notes can also be put into calendars so that staff can reference the invite for patient information.
Action items are a key component of team management. Pennington Biomedical Research Center does action items with the Interventional Resources Unit for administrative activities and study specific tasks for every study. Action items clarify tasks to be completed by members of the study team. Each action item is associated with a responsible person and the deadline. If study team members cannot meet their deadlines, they need to notify the author because her deadlines are contingent on team members meeting their deadlines. Action items also increase accountability by providing clear expectations.
Using SharePoint, the responsible staff member can update action items as she/he completes them so that the author does not to have to receive emails documenting this. SharePoint can also send notifications, emailing a staff member when she/he is assigned to a task. SharePoint can be used to prioritize tasks. This author has experienced a major challenge with Generation X and Z team members is who may have difficulty prioritizing. In the author’s experience, these generations may more often work on the last task assigned to them instead of the most important task.
Tools for Recruiting
Recruitment is the costliest part of clinical trials. Table 4 highlights tools for recruitment:
- Advertising timeline
- Recruitment goal tracking
- Recruitment budget tracking
- Participant flow diagram
- Enrollment predictions.
The author develops an advertising timeline that is separate from the overall study timeline. The advertising timeline has recruiting and advertising tasks, with color coding for tasks that have been completed, and yield rates of completed events. Pennington Biomedical Research Center does a great deal of community-based recruitment. The yield rates (number of participants randomized) of completed events show the most effective recruitment methods for each quarter or year. This enables staff to repeat the most effective recruitment methods.
Tracking recruitment goals is very helpful. Many of the clinical trials conducted at Pennington Biomedical Research Center are funded by the National Institutes of Health (NIH) or another government agency or department. These trials have quarterly recruitment goals. The author usually uses more aggressive goals than the NIH’s goals, since it is easier to recruit participants earlier in the grant when the project is novel and exciting to potential study participants that may be reached during the recruitment process.
Achieving recruitment goals requires providing the study team with clear expectations. Tracking enables project managers to assess monthly/quarterly randomization goals to see when the clinical research site was most successful and to identify effective recruitment methods that can be used again.
Tracking spending on recruitment is also helpful. The most expensive recruitment methods, such as television and radio advertising, may not be providing the most participants. Tracking spending and sources of participants enables the project manager to assess the cost effectiveness of advertising campaigns and adjust them as needed. Pennington Biomedical Research Center has different departments for recruitment and advertising. The departments have designated budgets over the study year yet coordinate marketing and outreach activities to maximize recruitment reach.
The participant flow diagram is one of the author’s most important tools. A participant flow diagram tracks what is happening in real time, allowing project managers to see where participants are in any part of the study flow. It also lets study team members see when potential research participants and enrolled participants are lost due to exclusionary criteria or dropouts. A participant flow diagram documents the ratio of phone screens to randomization and the number of participants in the pipeline.
Screening yields can also be reviewed through the participant flow diagram. Pennington Biomedical Research Center always assesses why the clinical research site is losing potential subjects. This sometimes enables the project manager to make changes. For example, by tracking screening yields, Pennington Biomedical Research Center has found that people were being excluded from a study in the phone screen because they did not understand a question. In response to this problem, the question was clarified. Enrollment predictions can also be done with a participant flow diagram, and the pending pipeline can be assessed. Finally, retention rates can be assessed with the same diagram by looking at the number of completed, anticipated, and pending visits at each follow-up time point. This could allow for the project manager to identify whether a particular follow-up visit is problematic in return rates for study participants. This could lead to more intensive staff contact for said visits to work to improve these rates for future visit windows.
Tools for Clinical Assessment
Electronic case report forms (CRFs), visit scheduling, and visit windows are tools for clinical assessment (Table 5). The world is moving toward electronic CRFs. Some sites and PIs may be reluctant to eliminate paper especially with specific clinical trial populations such as the elderly. However, technology is moving clinical and research practices towards paperless data entry. In this author’s experience, many industry and pharmaceutical clinical trials are paperless or at least using electronic data capture options in many of their trials.
RedCap, built at Vanderbilt University, is a secure Web application for electronic data capture. Various levels of access can be set up for different staff members. Participant self-reported forms captured via surveys are part of RedCap. These surveys can be sent by email. Rather than call participants to collect information such as adverse events and weight, RedCap can send out automated emails on a timer to collect this information. Pennington Biomedical Research Center sets these up in advance and only has to contact participants when they do not complete the surveys.
Visit scheduling windows can be set in various electronic platforms. Pennington Biomedical Research Center uses Outlook or Sharepoint for visit schedules for some trials. The visit schedules show the start and stop time, preventing double booking of staff. If the visit includes laboratory testing, the system can send an alert to the laboratory with an appointment reminder. RedCap also does visit scheduling. The visit schedule can be printed for study participants or for the study folder.
Tools for Intervention
Real-time data capture, adherence and compliance reporting, and retention tracking are tools for intervention (Table 6). Pennington Biomedical Research Center does many large multi-site exercise clinical trials or trials with many participants. In one study, 300 participants came to the center three times a week. Instead of writing all of the exercise prescriptions and data capture on paper, staff created the Exercise Database for Intervention (EDIN) to capture the exercise data in real-time using laptops on rolling carts. iPads can also be used to collect data in real time.
With real-time data capture, the data are automatically entered into a website or clinical trial management system. Real-time data capture also allows compliance reports to be generated instantly.
Other data capture tools include Fitabase, heart rate monitors, and body trace scales. When Fitbits are used in a study, its Fitabase can be used to look at data for all participants together. Fitabase provides more data than the data that are available on the app. Participants do need to sync their Fitbits in order for researchers to use Fitabase.
PolarÔ Heart Rates Monitor and Zephyr can be used to monitor heart rates, including monitoring the heart rates of a group of people at once. Body trace scales are sent home with the participants, where they transmit weight wirelessly to Pennington Biomedical Research Center. Study staff can review trends and share individual data with each participant.
Adherence and compliance reporting is necessary because it is important for participants to stay in the study and to comply with the intervention. Project managers and study teams need to monitor compliance. Pennington Biomedical Research Center extracts adherence and compliance information and puts it in a format that will resonate with investigators. Staff create monthly or weekly participant compliance reports depending on the speed of the study. These reports can show what is happening between groups or within a group.
Since all of the information is in the system, staff can generate reports for participants such as score cards or report cards. Participants often enjoy receiving these reports. If a participant is not doing something well, this is an opportunity for study staff to discuss any challenges and ways to overcome those challenges.
In order to facilitate intervention retention, Pennington Biomedical Research Center does case assessment to identify thresholds for adherence or compliance. Any participant who reaches the threshold for poor compliance is assigned to a study staff member who acts as a case manager and troubleshoots problems.
Staff also assess reasons for poor compliance to identify trends. They adjust screening and/or retention methods based on assessment results.
The author is often asked how Pennington Biomedical Research Center tracks contacts with research participants. It is important to know why participants miss visits and the number of times that study staff call them. Clinical research sites must have a retention/participant contact system in place such as a SharePoint list. Pennington Biomedical Research Center’s retention/participant contact system lets the author see the visit window and when to call. She assigns a study staff member to call participants on the specified dates.
Take-Home Messages
Clinical research sites should not rely solely on the successes or failures of past programs or models. It is often necessary to tailor tools to a specific study. In order to be successful over time, clinical research sites must establish:
- A strong infrastructure
- Clear operational procedures
- A variety of tools to monitor research programs, study teams, and research participants.
Continual evaluation and revision of the research program is necessary. If something works very well, keep doing it. If something does not work, reevaluate it and shift to a more effective strategy. Project managers and study staff must be willing to adapt and change. Some study staff members may require more micro-management than others. The author uses electronic platforms to manage study staff, which is less confronting than managing them face to face.
Project managers should create a versatile study team that matches the needs of the research program and has a great deal of information. Team members will be different. Some may be very technologically savvy while others may not be technologically savvy.
Increasing efficiencies by saving minutes a day does matter. This can reduce staff burden, burnout, and turnover. Project managers and study staff should work smarter, not harder.
Study Components
- Choose a topic
- Create a hypothesis
- Develop a plan
- Submit a grant for funding
- Submit the protocol for approval
- Market the study to the target population
- Screen potential participants by telephone
- Orient participants
- Obtain informed consent
- Inclusion/exclusion criteria
- Compliance assessment
- Perform initial assessments
- Conduct study group
- Monitor progress
- Test outcomes for changes
- Prove/disprove hypothesis
- Assess outcomes
- Publish findings
- Outlines chain of command
- Outlines responsibilities
- Helpful for all study staff to appreciate where they fall and where other’s fall
- Provides pre-identified time points for completion of various stages of start-up
- Establishes clear goals for study staff
- Ever-changing with the pace of the study
- Also known as flowcharts
- Allows process and steps to be viewed at a glance
- Identifies actions within a process in a sequential order
- Provides specifics of process steps with relevant “if, then” scenarios
- Central location for all departmental or project-specific files
- Increases accessibility
- Increases dissemination of information
- Archived history of all documents, processes, data, etc.
- Document security with automatic nightly backups
- Staff scheduling for designated appointments or responsibilities
- Participant scheduling for appointments or procedures
- Study-specific calendar for meetings, visits, etc.
- Clear assignment of staff to participant visits
- Appointment reminder alerts
- Ease of identifying other staff members’ availability
- Reduces double booking staff and appointments
- Color coding to easily identify appointments
- Clarify tasks to be completed by the study team
- Provide study team pending action items with associated deadlines
- Assign actions items to designated staff
- Increase accountability through clear expectations
Tools for Recruitment
- Timeline of recruitment and advertisement events
- Yield rates of completed events
- Identify and track study recruitment requirements
- Assess monthly/quarterly randomization goals
- Monitor progress
- Adjust advertising campaigns as needed
- Identify and track study recruitment budget
- Assess cost effectiveness of advertising campaigns
- Determines screening yields
- Assess where participants are lost due to dropouts or exclusionary criteria
- Determine ratio of phone screens to randomization
- Stage of the process for pending participants
- Management of N in each arm of the trial
- Follow-up visit completion rates
- Assess pending pipeline
- Use throughput rates to predict enrollment
- Predict quantity needed to reach goals
- Paperless electronic data capture systems
- Participant self-reported forms captured via surveys
- Auto-generated timed survey requests for completion
- Calculated visit windows for follow-up testing
- Tracking scheduled and actual visit dates
- Pending visit reports
Tools for Intervention
- Real-time data
- Easily accessible compliance reports
- Assess participant compliance monthly
- Identify discrepancies and any areas of concern
- Generating reports for participants
- Tracking participants’ attendance and reasons by the individual, group, cohort, month, etc.
- Tracking retention procedures
- Tracking contact attempts during dropout recovery
2 thoughts on “Project Management: Introduction to Tools and Templates”
where is the tools template?
Hey, fellow readers! I just finished reading the article on project management tools and templates, and I couldn’t resist leaving a comment here. First off, I want to thank the author for putting together such a comprehensive and informative piece. As someone who’s relatively new to the project management world, this article was like a goldmine of practical tips and resources.
The way the author explained various project management tools, from Gantt charts to PERT diagrams, was incredibly helpful. I always found these concepts a bit overwhelming, but the article managed to break them down into digestible chunks, making it easier for me to understand their applications. The best part is that they provided links to free templates and software, which is a lifesaver for anyone on a budget.
Moreover, the insights on how to choose the right tools for different types of projects were enlightening. Understanding that not all projects are the same and tailoring our approach accordingly is crucial for success. I’ve already bookmarked this article for future reference and plan to explore the recommended tools further.
Lastly, I want to express my gratitude for the tips on how to collaborate effectively with teams using these tools. As a project manager, fostering good communication and collaboration is essential, and the author’s suggestions will undoubtedly prove invaluable in my journey.
Great job on this article! I can’t wait to dive deeper into project management armed with these newfound knowledge and resources. Keep up the fantastic work, and I’ll be eagerly awaiting more insightful pieces from this blog. Cheers!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
33RD ANNUAL CONFERENCE
ACHIEVING EXCELLENCE IN CLINICAL RESEARCH :
FORGING STRATEGIC COLLABORATIONS
September 27 to 29
COUNTDOWN TO ANNUAL CONFERENCE
Join us for expert-led sessions, interactive workshops, a peer-driven poster program, an engaging exhibit program and unparalleled networking opportunities!
Connect with us:
What to know about project management for clinical trials.

Completing any multi-part task requires organization, coordination, and discipline — and, of course, clinical trials are no exeption. Planning a research study, launching a trial, and keeping things running smoothly requires knowledge and expertise, which is why clinical trial project managers are so vital to the process.
From creating a plan, communicating updates, calculating risks, and addressing any mistakes that arise , solid project management is a necessity to ensure medical research is allowed to move forward. Here, we’ll discuss the role of the clinical trial project manager, as well as tips for developing project plans, stakeholder involvement, communication, IRB submission, and summarizing lessons learned.
What is project management in clinical trials?
The Project Management Institute (PMI) defines project management as the "application of knowledge, skills, tools, and techniques to project activities to meet the project requirements." Project management combines expertise in scope, time, cost, quality, risk management, communication, and stakeholder management in order to move through the five basic phases of any project :
- Project initiation. This phase involves developing an idea, understanding the necessity of the project, and identifying the key decision-makers
- Project planning. This is the phase for making a plan and outlining the work required, including prioritization, budget, schedule, and resources
- Project execution. This is where tasks are distributed by informing all teams of their responsibilities and deadlines
- Project monitoring. This entails implementing project tracking to compare the current project status and progress with the original plan, adjusting as needed
- Project closure. The final phase, where project managers reflect on project success and key learnings for next time
When concerning clinical trials, project management brings all of these phases together to ensure set up, enrollment, operations, and reporting are all done smoothly and effectively.
Roles of a clinical trial project manager
A clinical trial project manager may have different responsibilities specific to each trial, but in general, they will be tasked with vendor selection, budget oversight, IRB submissions, report creation, and meeting planning, all of which are detailed below.
Vendor selection: Because conducting a clinical trial requires many different elements, outside vendors will often be brought in to provide expertise in certain aspects of a study. This is most common for specialized elements such as Interactive Web Response Systems (IWRS) , electronic patient-reported outcome (ePRO) technology, and clinical trial patient recruitment . Often, it will be the project manager's job to vet these vendors and assist with comparing options.
Timeline and budget oversight: Though every study begins with a specific timeline and budget, nearly 80% of all clinical trials are delayed due to difficulties in patient recruitment, which causes many to exceed their budget. In these situations, a project manager can leverage their expertise to hold the trial team accountable for the time and money spent on the study, in addition to managing expectations should these elements begin to change.
IRB submissions: Any patient-facing materials involved in research studies must be reviewed and submitted for Institutional Review Board (IRB) approval, and gathering these materials is often the responsibility of the clinical trial project manager. Because every IRB is different, the project manager will need to look at previous submissions and any templates that are available to ensure the study's particular IRB requirements are met.
Report generation: As part of tracking the progress of a trial, clinical trial project managers should regularly generate and distribute reports on various aspects of a study’s progress. These reports can often be automated so they are not a time-consuming task, but they do play an essential role in keeping key stakeholders looped in on the progress of the research.
Meeting coordination: Occasionally, the trial’s key stakeholders may need to meet to review a trial’s progress and address any roadblocks. The project manager will likely be charged with planning and leading these meetings to ensure that all details are covered and relevant updates are provided.
Tips for effective project management in clinical trials
Create a detailed project plan
One of the best ways to circumvent delays and issues in a clinical trial is to create a detailed project plan before the study launches. This plan should include a timeline of milestones, key dates, a task schedule, and any other relevant pieces that can keep the project on track. Some people may prefer pen and paper for this, but there are also many online resources available to help project managers keep track of details and stay on schedule.
Anticipate risk management demands
Every project will have some level of risk, so it’s wise to acknowledge what points of contention may arise and plan accordingly. A few examples of risks associated with clinical trials include:
- Long wait times for IRB approval
- Delays in patient recruitment
- Turnover among site staff
- Changes to the trial protocol
Before the project starts, it can be helpful to come together as a team to discuss potential risks that may arise, share past experiences, and determine how they can be prevented or handled if they do arise.
Understand IRB requirements
In most studies, the project manager will also be tasked with gathering materials and submitting them for approval from the Institutional Review Board (IRB). Every IRB will vary on its guidelines, but IRB administrators should be able to answer any questions to ensure a smooth and efficient process.
Even if the responsibility of IRB submission is a task for the project manager, it can be helpful to enlist additional team members to provide a second set of eyes before the materials are submitted. It is also wise to create a checklist of elements that should be included in the IRB packet to ensure nothing gets left out.
Foster open communication between sites and sponsors
Another important communication piece for project managers is to share feedback from sites back with the sponsor of the trial. Communication is particularly important in relation to sharing updates with sponsors about recruitment or screening challenges the site may be facing.
Communication tips for project management
One of the most important jobs of a clinical trial project manager is to ensure clear and effective communication with multiple stakeholders. To manage this communication from the onset, it can be useful to create a list of stakeholders, the updates they'll need, and how often they should be informed.
To manage this communication, the RACI project management method can provide a helpful framework to organize stakeholders into four categories based on their involvement in the project and communication needs.
- Responsible: The responsible party is the main point person for communication – this is the stakeholder who does the actual work of this part of the project. For example, when submitting outreach material to the IRB, the person responsible for creating the material may be the lead on the marketing team or the contact at a clinical trial recruitment company.
- Accountable: The accountable person is generally the manager of the responsible party and may wish to be involved in only some of the updates related to the project. Generally, if the responsible party needs approval from their manager, they should do so before sharing updates more broadly.
- Consulted: The consulted party would be any additional stakeholders who should weigh in on a project. The responsible or accountable party can generally help project managers determine who should be involved in the consultation.
- Informed: These are people who are simply kept up-to-date on the progress of the project at appropriate intervals.
A paper on managing clinical trials published by the National Institutes of Health also mentions the importance of keeping the investigators themselves in the loop about a trial, stating, "Investigators need to feel valued and part of an inclusive team answering an important clinical question, so providing regular feedback that ensures they feel involved must be central to a trial's communication strategy." Because the investigators may also have busy clinical practices in addition to being part of the trial, it’s important to respect their time while making them feel involved and informed.
Project managers’ roles after a clinical trial
Project management is an ever-evolving skill and there are lessons to be learned from even the most successful project execution. It’s important that project managers evaluate each trial after it's complete in order to analyze trends and plan for the next one while key learnings are still top of mind. As Hubspot puts it, "a productive project post-mortem is a chance to fully unpack a project's trajectory and dig deeper into why things unfolded the way they did.”
Hosting a project post-mortem meeting involving key stakeholders is advisable. Sending a pre-meeting questionnaire can streamline the process of gathering thoughts on what went well, what didn't, and what could be done better next time. The agenda should include a recap of the project's goals and a review of the results so that any discrepancies can be addressed and everyone is able to come up with actionable takeaways for the future.
If you're interested in learning more about how Antidote begins the clinical trial recruitment process and manages the project throughout, download our recruitment template below.
Download now

Get our latest blog posts in your inbox

Clinical Trial Project Management Plan Template

What is a Clinical Trial Project Management Plan?
A clinical trial project management plan is a strategy that combines project management and clinical trial processes. It helps clinical trial project managers and teams to plan and execute clinical trials with greater efficiency and accuracy. This plan includes focus areas, objectives, KPIs, and related projects that contribute to the overall goal of the clinical trial.

What's included in this Clinical Trial Project Management Plan template?
- 3 focus areas
- 6 objectives
Each focus area has its own objectives, projects, and KPIs to ensure that the strategy is comprehensive and effective.
Who is the Clinical Trial Project Management Plan template for?
This plan is best suited for project managers and teams that are preparing for or managing clinical trials. It is an effective tool to streamline clinical trials processes, increase efficiency and data accuracy, and identify and reduce potential risks.
1. Define clear examples of your focus areas
A focus area is a broad category that outlines the main goal of a clinical trial project management plan. Examples of focus areas include project management, quality assurance, and risk management. Each focus area should be broken down into more specific objectives and related projects.
2. Think about the objectives that could fall under that focus area
Objectives are the specific goals that are being targeted in each focus area. For example, an objective in the project management focus area might be to streamline clinical trial processes. An objective in the quality assurance focus area might be to ensure data accuracy.
3. Set measurable targets (KPIs) to tackle the objective
KPIs (Key Performance Indicators) are measurable targets that are set to tackle objectives. For example, for the objective of streamlining clinical trial processes, you might set a KPI to decrease clinical trial setup time. The initial KPI might be 30 days, and the target KPI might be 20 days.
4. Implement related projects to achieve the KPIs
Projects are the actionable steps that are taken to achieve KPIs. For example, to achieve the KPI of decreasing clinical trial setup time, you might create standardized processes. To achieve the KPI of increasing data accuracy, you might implement data validation checks.
5. Utilize Cascade Strategy Execution Platform to see faster results from your strategy
Cascade Strategy Execution Platform is a complete solution for clinical trial strategies enabling project management and quality assurance. Cascade's features help project managers and teams to streamline processes, increase efficiency, and ensure compliance with regulatory standards, resulting in faster and more successful clinical trials.

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
Effective Project Management of Clinical Trials
Feature article.
During medical school or post graduate training, we receive some formal training in designing and running complex programs like research projects or clinical trials. For those interested in clinical research, we then spend years learning how to ask good research questions, draft research protocols, apply correct research methodology and write successful grant applications.
Following this path, I was lucky to secure a grant and start a pilot clinical trial involving 30 patients. I thought I had all the right tools, time and resources to successfully conduct this project. However, I soon realized the painful reality of managing a clinical trial – it is incredibly challenging! I encountered multiple early failures like slow recruitment, lack of team coordination and overall inefficiency. It dawned on me that at no point during my clinical career had I received formal training in project management. A project management expert helped me navigate this challenge. In this article, I share my insights on project management not only for clinical trials but also for any type of project requiring the coordination of a team and resources around a specific objective.
Pearl #1: Project management of clinical trials is a science with valuable tools at your disposal. We tend to think the day-to-day business of managing a clinical trial is not the "scientific part." However, project management is a science and has well-defined evidence-based methods. Recognizing this is the first step in successfully managing clinical trials. Learning to harness the power of these tools is the second step. Using tools like chunking , GANTT charts , and resource allocation will organize and simplify the flow of your trial.
Pearl #2: Focus more on human aspects as compared to technical aspects. There are two major aspects of any large project: the technical and the human. As trained scientists, we tend to take the rational approach: "If I have two research nurses available and I am paying for 60% of their full time equivalent from my grant, then they will work whole-heartedly for three days per week on my project and I will finish this project in three months."
The root cause of inefficiency in my clinical trial was not technical, it was me ignoring the "human-side." I was fixated on productivity but overlooked the people working with me – my team. I realized that regardless of the resources and planning, it is the human element that drives successful project management.
- Grow the Team Leader: Building a better team always starts with building a better team leader. Inspiring your team with your hard work, work ethic, compassion, empathy and leading by example can ensure a long-lasting relationship even in challenging times. "Leading means that others willingly follow you – not because they have to, not because they are paid to, but because they want to." – Simon Sinek.
- Know your team: Ask, "What motivates my team to conduct research?" "What is their background, their perspective?" Have an honest conversation and get to know the person behind the title. For example, what motivates those trainees to volunteer their 80-hour work week and sacrifice their weekends to your project? What sparks the interest of your research nurses? Is it just a job requirement or are they passionate about the science? What are your team members' expectations? Answers to these questions will help you build a motivated, hard-working team. "A team is not a group of people that work together. A team is a group of people that trust each other." – Simon Sinek.
- Rapport building: Showing interest in your team member's life not only at work but also outside work helps to create a stronger relationship. While we have multiple project meetings where we discuss progress, timelines and feedback, taking an interest in the person behind the job is essential. Asking about their family, hobbies and how their weekend was is enough to create a connection. A big part of being a scientist is being a good friendly human being first.
- Feedback: It is important to get feedback from team members and always get their input on how to improve workflow and identify gaps between your expectations and their perception. It provides the opportunity to look at things from their perspective and realize their challenges.
Conclusion Project management coaching made me realize that although I was technically competent, I was ignoring the human element of my clinical trial. What did I change? I got to know my team members as individuals. I discovered what motivated each one of them and we worked together to align their roles in light of their strengths and interests. They were helpful in suggesting several quality improvements to the project and our overall workflow and efficiency was much better. I went from frazzled to effectively managing my first clinical trial.
In the end, real life is a lot messier than a mathematical equation. To successfully move a project, program or trial forward, designing it is not enough: plan using the right tools, keep your key stakeholders motivated and informed, and channel your energy into growing a solid team.

Tweet #ACCFIT

This article was co-authored by Zain Asad, MD, Fellow in Training (FIT) at the University of Oklahoma in Oklahoma City, OK, and Kat Niewiadomska, PhD, ACC Member Leadership Development associate.
You must be logged in to save to your library.
Jacc journals on acc.org.
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
Home / Insights / Project Management Framework and Tools in Clinical Trials
- Clinical Trial Design
- Feasibility
- Site Identification
- Data Privacy
- Decentralized Trials
- Patient Engagement
- Publication Planning
- Medical Information

Project Management Framework and Tools in Clinical Trials
September 29, 2020
Project management’s maturity as a discipline has had wide-reaching effects across most industries.
It’s changed the way people manufacture products, develop software, plan company strategies — and even how clinical trials are conducted.
Here, we explore how the evolution of project management has impacted clinical research, and what aspects of project management trial leaders need to know to be successful.
Meet Your Clinical Project Manager
Because clinical trials have so many stakeholders and moving parts, it makes sense that someone should take responsibility at a project level.
As Genesis Research Services Business and Clinical Research Manager Dom Bailey points out, this person — the clinical project manager — is responsible for explicitly laying out the project objectives, scope and stakeholder management plan.
Additionally, the project manager will work with a CRO’s leadership team to ensure things like sufficient recruitment numbers and that the necessary project management tools are in place.
Dalfoni Banerjee at 3Sixty Pharma Solutions says a good clinical project manager must be able to plan and establish key metrics, communicate very well, and remain oriented on the project’s goals while maintaining flexibility to accommodate any unexpected changes.
For trial leaders, perhaps the most important trait in a project manager is their communication skills. “Nothing is worse than working for an out-of-touch project manager—someone who is lost in the project plan or the study metrics, or making assignments without listening or making any kinds of assessments or mid-course corrections,” Jim Moat at IMARC Research writes.
“Someone with a balanced view of the big picture and the smaller, but important, details, is bound to lead a more successful project and team.”
Moat underscores how important relationships are in managing a trial, which means a good PM knows how to listen and is willing to spend time with research staff to understand realities on the ground.
Zain Asad, M.D., and Kat Niewiadomska, Ph.D. , write for the American College of Cardiology that their own experience with project management made them better team members. The PM perspective allowed them to see how each person complemented others and fit into a cohesive team.
The Rising Influence of Agile Project Management in Clinical Trials
Nearly 20 years ago, the Agile Manifesto laid out a new framework for developing software by making the production process iterative and dynamic.
Today, that methodology has found purchase across industries, and its principles are nudging clinical project management into new directions.
Most clinical project managers apply agile frameworks in an effort to cut costs and improve efficiencies. That said, aspects of agile such as iterative development and regular opportunities for stakeholder feedback create other opportunities in clinical trials.
As Syneos Health notes, an agile approach to clinical trial management can:
- Break up siloed teams and facilitate cross-functional cooperation.
- Upend linear business processes and replace those with opportunities to innovate.
- Center stakeholder needs.
- Facilitate “early and proactive dialogue with regulators to support expedited pathways for bringing new products or innovation to market.”
As promising as that all sounds, though, agile’s uptake has been stifled on a few fronts. In a 2018 dissertation for Harrisburg University of Science and Technology, Jitendrakumar Narola surveyed nearly two dozen healthcare IT professionals about their experience with agile projects in clinical trials.
Narola’s research found that many respondents were hesitant to embrace an agile approach to project management because they didn’t feel sufficiently familiar with the framework, or because they weren’t sure how compatible it would be with FDA regulations.
Further, agile transformations are more fundamental undertakings than simply tweaking how a project is managed. “[A]gile methodology is not applicable neither to all parts of a study nor to all kinds of studies, but striving for an agile transformation in an organization as a whole will pave the way in making clinical trials as ‘adaptive’ as possible,” researchers Katarina B. Pavlović, Ivana Berić and Ljiljana Berezljev write in the European Project Management Journal.
This, of course, speaks to a larger movement among researchers who lean on adaptive trial designs to make data collection and analysis concurrent during an in-progress trial.

The Tools Shaping Clinical Project Management
The fundamental tasks of project management are the same, no matter the industry or the project.
A clinical project manager will specifically need a tool to help with:
- Creating the work breakdown structure.
- Analyzing and mitigating risks.
- Planning budgets.
- Determining milestones and key metrics to track.
This is the kind of work that can be done in a clinical trial management system (CTMS). A CTMS should give you space to organize an electronic trial master file (eTMF), which is absolutely crucial in what Jim Moat at IMARC describes as “telling the story of your study.”
“Ensure your trial master file is kept current and complete,” he writes. “ … Make judicious use of notes-to-file and memos to document unusual steps taken in a given situation. As we say at IMARC, ‘you’ve done the work; now write it down to take credit for your work.’”
Further, ensure your CTMS allows you to share documents (or at least integrate with a tool like Microsoft SharePoint , which facilitates document collaboration), and that it provides a place to track timelines and milestones.
From there, a project manager might opt for a separate project management platform that accommodates their preferred methodology. For example, a tool like Asana or Microsoft Project Manager can help visualize timelines, budgets and tasks.
Or, if the project manager needs to organize work in a Kanban-style workflow, tools like Trello or MeisterTask can help visualize those tasks.
Finally, a project manager needs to be able to host and lead meetings, sometimes with remote staff. In such cases, a conferencing tool like Zoom or GoToMeeting would be useful.
With the tools above and a framework in place, a clinical project manager has what they need to try to steer a clinical trial toward its goals within budget and within expected timelines. Of course, no projects go exactly as planned, so transparency and communication go a long way to getting a trial back on track in the face of unexpected changes.
For clinical trial leaders, having this kind of perspective into the project-management aspects of a trial will be useful in marshalling resources and leading teams as needed.
Want to stay up to date with our news?
Related posts, streamlining clinical trial operations: how integrated technology solutions drive efficiency, leveraging advanced technologies for clinical trial success, clinical trials: how automation is reducing time to market, clinical trial financial management: strategies for budgeting and payments, exploring digital tech and ai in clinical research, data integrity in clinical trials: the role of advanced edc systems, related products, protect patient safety in early phase trials with esource solutions, streamlining clinical trials: the impact of automation in the monitoring and reporting process, anju’s ta scan selected by leading pharma company to enhance clinical research, patient-centric clinical trials: leveraging technology for enhanced participant engagement, updated fda race and diversity guidance open for comments, how patient-centricity is becoming a cliche and the crucial tool in anju’s arsenal, clinical trial costs keep rising: how to fight back, outsourcing data management in 2024: challenges and benefits, the benefits of big data in drug development, time for clinical trials to deliver on patient-centric care promises, the importance of diversity in clinical trials: a comprehensive analysis, scope orlando 2024: the summit for clinical ops executives, conference preview: ismpp europe 2024, overcoming data management challenges in self-reporting clinical trials, medical research and publishing has exploded: how are you managing knowledge, collaborating with key opinion leaders in early clinical trial development: navigating risks and rewards, how medical affairs teams can optimize product life cycle, anju welcomes atherion bioresearch as the newest cro partner, achieving data compliance in decentralized trials, revolutionizing clinical research: the role of generative ai, ta scan’s diversity module won best of show award at scope europe 2023, community pharmacies and clinical trial enrollment, the regulations clinical researchers should track in 2024, anju showcases life science solutions at scdm, scope europe 2023: 5 unmissable sessions and insights for clinical operations professionals, preview: 6 sessions at dia medical information conference, clinical trial compliance auditing: challenges and tools, fda guidance on umbrella trials can help cros choose the best software, trialmaster: the right edc solution for oncology trials [white paper], how automated site visit reports improve clinical trials, how medical affairs teams can boost stakeholder engagement, how patient voices power pharmaceutical innovation, rare disease clinical trials: challenges and opportunities researchers face, clinical data analysis: 3 most important frontiers right now, the fda race and ethnicity diversity plan: how to get researchers prepared, video interview: advancing clinical trials with advanced technology, 3 tips to perform a disease landscaping analysis, etmf for alcoa-c compliance in clinical trials, medical publishing: how the right tools maximize your deliverable’s reach, human analysis crucial in ai-driven data management, insights into the 2023 asco annual meeting, how to improve the process of adverse event reporting, our most anticipated events at dia masc 2023, outsourcing clinical trials: how to build successful partnerships, building opinion leader relationships in a post-pandemic world, how the right tools make clinical data integration seamless, you’ve collected your medical affairs data. now what, laurence birch interview: adaptive life sciences tech, ta scan won scope best of show award, the future of decentralized trials, scope 2023 conference preview, configurability and customization: key features in an etmf solution, why cro staff need robust digital literacy skills, using ai to formulate clinical trial research questions, tracking relationships: understanding kpis that measure kol engagement, understanding security and role-based access in an etmf platform, decentralized trials: combating bias and reaching diverse patients, in a data-rich landscape, a single source of truth is key, how medical affairs teams can advocate for patient-centric healthcare, accurate clinical trial forecasting and budgeting strategies, retaining trial participants: adherence and dropout mitigation, how third party data can improve clinical trials, discussing clinical trial performance metrics with stakeholders, the role of medical affairs in combating misinformation, medical affairs kpis: how to create a framework for measuring success, data interoperability and sharing: practical considerations, understanding roles and needs of stakeholders in decentralized trials, top kpis for measuring medical affairs success in 2022, automation, analytics, ai and the future of trial monitoring, how a real-time etmf dashboard helps keep a trial on track, ease of implementation and customization spell etmf success, world-class edc for world-class cancer research center, 5 skills that tomorrow’s data managers will need to succeed, the emerging role of medical science liaisons on medical affairs teams, global oncology community leveraging technology for clinical trials, understanding which kols are key for you, what are the responsibilities of a clinical data manager and how have they evolved, why your etmf needs role-based access, mike keens on clinical trials podcast, what to look for in an etmf, why an etmf it’s time for a specialized digital solution, data analysis on research for multiple sclerosis day, understanding cross-border enrollment for international clinical trials, chronic obstructive pulmonary disease: latest clinical data, lightening the load: reducing patient burden in clinical trials, partnering with patient advocacy groups benefits pharma and patients, ai and clinical intelligence in oncology research, machine learning and kol networks: the future of medical affairs, data analysis on latest clinical research for parkinson’s day, understanding the value of blockchain for clinical trial data, benefits and challenges in virtual clinical trials, clinical trial data privacy: what trial managers need to know, working with disparate data sources to improve your trials, future of decentralized trials: anju evp’s predictions, how historical data and controls improve clinical trials, clinical trial & therapy results: anju’s pubstrat max, impact of cybersecurity and data privacy on medical affairs teams, 6th european clinical research conference: presentations, overcoming accessibility challenges in international clinical trials, how ai and machine learning are reshaping clinical trials, managing multiple sources within the clinical data ecosystem, the post-pandemic future of medical information call centers, enhancing trial planning with screening and randomization, how to empower medical science liaisons with data, and why you should, what challenges face researchers with direct-to-patient trials, choosing a medical affairs crm platform: key considerations, 5 strategies to engage digital opinion leaders, digital opinion leaders in post-covid medical affairs, getting the most out of your edc software, how technology is moving decentralized clinical trials forward, understanding hhs’ proposed rule changes to hipaa, the role of electronic data capture in decentralized trials, decentralized clinical trials: post-covid future, 5 ways to improve the security of clinical trial data, patient privacy and cybersecurity in remote trials, healthcare cyberattacks and clinical data management, understanding risk-based clinical quality management technology, anju’s adaptive tech solutions for clinical trials and medical affairs, the benefits of standardized, machine-readable clinical data, how to use clinical oversight solutions effectively, ai and ml in clinical data management, diverse clinical trials: a necessity for personalized medicine, how to measure the effectiveness of medical information distribution, covid-19’s impact on data collection in trials, how more data can lead to shorter clinical trials, early-stage decentralized clinical trial site selection: key factors, how to best ensure accuracy in coding for clinical trials, how medical science liaisons should engage with key opinion leaders, how electronic data capture software facilitates cleaner trial data, maximizing decentralized trials with adaptive systems, what is the role of synthetic data in early-phase clinical trials, best practices of medical affairs publication planning, what five key features should a trial director look for in an edc, how medical affairs teams can improve their digital communications, 4 ways an irms helps medical affairs manage and share insights, 4 lessons cros can take away from the covid-19 vaccine trials, understanding the changing face of early phase clinical trials, covid-19 vaccine’s impact on the role of medical affairs, new shifts in the medical affairs ecosystem, covid-19 vaccine development enables cros to optimize processes, collaboration opportunities between regulators and medical affairs, how big data and ai are changing the role of medical affairs & pharma, cros’ guide to data protection officers in clinical trials, three ways decentralized trials bolster patient recruitment, pandemic-driven medical affairs and scientific insights management, the continuing rise of decentralized clinical trials, how do we solve pharma’s data dysfunction conundrum, data protection in medical affairs: basic cybersecurity, fda guidance and clinical trial innovation: who’s setting the pace, public health misinformation: what can medical affairs teams do, legal questions shaping clinical research, how medical affairs can support a product against new competition, trust-building in a pandemic: collaborative strategies for ma teams, anju innovation forum day 3 highlights, anju innovation forum day 2 highlights, what happens when medical affairs intersects with politics, international research collaboration: what tools can help, the mental health impact of covid-19 and how ma teams can help, how to manage patient fear regarding clinical trial risks, building a strong foundation for medical affairs leadership, covid-19: clear, accessible science’s importance, 10 medical affairs conferences in late 2020 and early 2021, dtrial forum in nanjing, china hosts feng cheng as speaker, insights on using synthetic data for clinical trial leaders, maintain remote professional relationships: 3 tips for medical affairs, how will covid-19 impact clinical trials in the long term, why medical affairs must lead a patient-centric approach to r&d, research during a pandemic: trial leadership supporting their teams, medical affairs leads covid-19 response, what extra support should clinical research teams offer participants, drug labeling trends: making language accessible to patients, how do clinical trial recruitment incentives bias research outcomes, 5 things medical affairs can do to support late life cycle drugs, publicizing research: reach audiences and combat misinformation, the role of nurse navigators in oncology trials, the importance of user experience in life sciences software, covid: the role of medical affairs teams in public health, informed site selection is mission critical in post covid-19 era, the state of clinical research during the covid-19 pandemic, support for awareness campaigns: good for pharma and patients, anju’s ta scan analysis of covid-19 update, how medical affairs can amplify the patient voice, the right tool for edc, promoting value-based clinical trials in medical affairs, how to create social media content to engage clinical trials patients, science behind the comms: how ma teams add value to messaging, adaptations of contract research organizations in virtual trials, uncovering predatory journals and conferences: best practices, how medical affairs can support pharma marketing, understanding attrition: gleaning insights from patients who quit, analysis performed by anju via ta scan on covid-19, decentralized trials: bridging distances in covid-19, medical affairs provides credible consumer information, avoiding 5 pitfalls in clinical trial design, clinical trial data sharing: medical affairs’ role, when a direct-to-patient trial model is the right choice, how to prevent systemic bias in clinical trials, top 2020 conferences cros should not miss, 10 global medical affairs conferences to attend in 2020, insights from ismpp eu, global clinical trials: managing patients and meeting compliance, patient-centric approach in clinical trial design: involve patients early, does your clinical trial need a chief patient experience officer, how medical affairs teams can help get off-label drugs to patients, virtual reality in clinical research: potential and challenges, how medical affairs can use tech to provide medical information letters, insights from dia 2019 micc, experimental design: new therapies are being developed to treat lifelong illness, best practices for managing phase transition in adaptive trials, understanding fda 483: how to run a compliant trial every time, current event analysis: brexit, clinical trials and regulations, changing stakeholder relationships in clinical trials, automated clinical data exchange for collaboration in clinical trials, clinical research as care option for better outcomes, enhancing virtual bedside manner in clinical trials, evolution of clinical trials: faster than ever, cbi’s medical publications forum insights, evolving needs for cros: understanding fda expectations, how cros benefit from continual improvement analysis and benchmarking, attracting and developing clinical research associates, how to engage primary care providers in clinical trials and connect with patients who don’t have them, enhancing ehr use for efficient clinical trials, clinical trial under-enrollment: how to prevent it, do no harm: how to mitigate risk in child and fih clinical trials, tech skills and digital natives in demand as clinical trial industry evolves, how to foster positive relationships with patient advocates — and why it’s important, more than reduced costs: the benefits of remote monitoring in clinical trials, how tech can streamline communication between clinical trial sites, sponsors and cros, how to simplify the clinical trial regulatory submission process, price check: how to cut costs in clinical trials, patients as partners: the value of post-trial communication, bias in clinical trial incentives, the ugly truth about paper diaries, expert hire: profiles of 5 top clinical research recruitment agencies, is there a place for integrative medicine approaches in clinical trials, what to consider when adopting new technology in clinical trials, precision medicine: its impact on clinical trials and orphan drugs, 21st century cures act and informed consent in trials, how clinical trial organizations can convey trust and credibility, unified data platform’s impact on clinical trial procedures, forbes ranks virtual trials as a top 5 digital health technology trend, a global approach: clinical trials in emerging markets, how to achieve ctms best practices: train your staff, achieving transparency: why and how to share trial results data, 2019 exhibitions and events: important healthcare it conferences, rare disease clinical trials: how to improve participation and outcomes, geriatric research: why your study should include more seniors, women’s representation in clinical trials: beyond equality, the next step: using ai to formulate clinical trial research questions, trial eligibility criteria: the problem with current exclusion strategies, voice tech and wearables’ emerging role in clinical trials, mental health clinical trials: controversies, challenges, solutions, why time management is essential for clinical trials, how ctm software can improve site selection for clinical trials, how to build flexible clinical trial design — and how software can help, what google’s entrance into healthcare data means for clinical trials, how to improve trial site relationships for more efficient trials, engagement and adherence: how technology is keeping trial patients in the loop, resolving patient reimbursement challenges in clinical trials, overcoming language and culture barriers in multinational clinical trials, how trial managers can increase diversity in clinical trials, social media for clinical trial recruitment, the requirements of an effective electronic data capture system (edcs), how to improve patient retention in clinical trials, how gdpr is affecting clinical trials, how to create an effective risk-based monitoring plan, how risk-based monitoring can improve clinical trial operations, how to prepare a successful nda submission, researchers whose work may lead to cancer treatment breakthroughs, designing patient-friendly trials for site performance, 10 reasons why we must integrate emr and edc, covid-19’s transformation of the clinical trial industry ecosystem.
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
Project Management
According to the Business Dictionary (2010), ‘project management’ is organisation of work within time, budget and cost limits . A bad project manager, therefore, can be defined as a manager who is unable to arrange project work according to some or all of these constraints. The reasons for this may be a PM’s lack of experience/knowledge or skills and poor communication between concerned parties (which is also due to insufficient training, SOPs, lack of experience or inability to account for previous failures and success).
As highlighted by Rettig, R.A. (2000, p.129), the importance of project management and oversight is crucial in the environment of prevailing commercial approach in pharmaceutical industry. Though it may be safer for Pharma to manage their own R&D projects internally, it becomes more time- and cost-effective to outsource clinical trial and pre-approval activities to specialized CROs. However, it is true that quality of project management services delivered by CROs is often unsatisfactory due to different reasons (Bryde, D.J. and Joby, R., 2007). Among other reasons for such outcome the authors cite the view of British Standards Institute (2003), according to which project management is a service that must be of an appropriate quality, i.e. must satisfy all participating parties. However, projects (even very similar in many aspects) are also unique and, thus, require individual approach based on previous experience.
Indeed, reasons for failure of a project and most significant elements of project success can be defined as symmetrical antipodes . If one would define what a bad project manager could do wrong to cause a project failure, it is possible to mention instead what a good project manager would do to make a project successful. The most important elements of a successful project management are Quality Assurance and project planning at each step. So, to avoid problems in project management the following must be taken into account by project management team (or a PM):
Houston, S.M. & Bove, L.A. (2007):
- Consistent methodology
- Definition of a project by limits of time, cost and performance (OR by scope, cost and time)
EA RHA, 1993 cited in Caan, W., Wright, J. & Hampton-Matthews, S. (1997, p.468):
- Team with sufficient knowledge and training
- Support and back-up of the organisation
Wycoff, 1991 cited in Caan, W., Wright, J. & Hampton-Matthews, S. (1997, p.469):
- Knowledge that you, as a PM, can achieve/facilitate achievement of the project goals
- Planning: a clear picture for all participants to describe the stage of the project, how a certain goal was achieved and where/how to move further to reach further goals
- Utilization of project management frameworks, such as PRINCE (EA RHA, 1993 cited in Caan, W., Wright, J. & Hampton-Matthews, S., 1997, p.469)
- Assessment and re-assessment of risks = development and subsequent adjustment of the initial project plan
Besides, a good project manager would ask him/her-self the following questions and make the answers clear to other team members (Caan, W., Wright, J. & Hampton-Matthews, S., 1997):
– Why certain steps are maid = what result is expected
– What is required for goals achievement
– Who should do what and when: Development of the Communication Plan
– Whether everything needed is in place to start
– When and whether the project is completed
– What lessons have been learnt from this project
– What can be done to improve further performance
So, to make a project successful a PM must: be experienced, have appropriate team and support, plan accordingly, communicate, make corrections, learn from previous mistakes/success, implement gained knowledge.
References:
Bryde, D.J. and Joby, R. (2007) ‘Importance of project and project management deliverables in clinical trials’ , R&D Management , 37(4), pp. 363-377.
Business Dictionary (2010) Project Management [Online]. Available from: http://www.businessdictionary.com/definition/project-management.html .
Caan, W., Wright, J. & Hampton-Matthews, S. (1997) ‘Start as you mean to go on: Project management for beginners’, Journal of Mental Health , October, 6(5), pp. 467-472.
Houston, S.M. & Bove, L.A. (2007) Project Management for Healthcare Informatics , Springer (New York), Chapter 1 ‘Project Management Process’, pp. 1-14 .
Rettig, R.A. (2000) ‘The Industrialisation of Clinical Research’, Health Affairs , Vol 19, Issue 2, pp. 129-146.
Clinical Research News
Upcoming clinical trials.
- Medacta International SA Recruiting A Post-Market, Retrospective Study on 3D Metal Tibial and Femoral Cones Total Knee Arthroplasty United States
- Ayşe Demirtaş Recruiting The Effect of Abdominal Massage and Warm Application on Excretory Activity in Patients Undergoing Total Knee Replacement With Spinal Anesthesia (Abdominal) Total Knee Replacement Turkey
- Alexion Pharmaceuticals, Inc. Not yet recruiting Efficacy, Safety, Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Ravulizumab in Chinese Adults Participants With Paroxysmal Nocturnal Hemoglobinuria (PNH) Paroxysmal Nocturnal Hemoglobinuria | PNH China
- JDeinum Not yet recruiting HT-ENDO: A Multiomics-based Biomarker for the Diagnosis of Endocrine Hypertension: a Pragmatic, Diagnostic, Randomized, Outcome-based Trial (HT-ENDO) Hypertension
- Second Affiliated Hospital, School of Medicine,... Not yet recruiting Effect of Nasal Spray Dexmedetomidine on Emergence Delirium Prevention in Total Hip Replacement Delirium
- Assiut University Not yet recruiting Prevalence and Prognostic Effects of Thyroid Abnormalities in Patients With Acute Coronary Syndrome. Thyroid Dysfunction
- University of Connecticut Eunice Kennedy Shriver National Institute of Child Health and Human Development... Recruiting A Novel Program Using Ride-on Toys to Improve Upper Extremity Function in Children With Hemiplegia Cerebral Palsy | Hemiplegia | Children, Only United States
- Sunnybrook Health Sciences Centre Not yet recruiting The HEROES Trial: Hyperbaric Oxygen Therapy for Endometriosis-Related Pain (HEROES) Endometriosis-related Pain Canada
- Ain Shams University Not yet recruiting The Effect of Probiotics vs Activated Charcoal in the Management of CKD Patients Suffering From Uremic Pruritus. Chronic Kidney Disease | Uremic Pruritus
- Temple University Not yet recruiting Improving Participation in Universal School Meals (USM) Obesity, Childhood | Food Deprivation United States
- AstraZeneca Not yet recruiting Effects of AZD5004 in Adults Who Are Living With Obesity or Overweight With at Least 1 Weight-related Comorbidity (VISTA) Obesity or Overweight United States, Germany, United Kingdom, Taiwan, Australia, Canada
- AstraZeneca Not yet recruiting Efficacy, Safety, and Tolerability of Once Daily Oral Administration of AZD5004 Versus Placebo for 26 Weeks in Adults With Type 2 Diabetes Mellitus. (SOLSTICE) Diabetes Mellitus, Type 2 United States, Germany, United Kingdom, Hungary, Spain, Slovakia, Japan, Canada, Poland
Clinical Research Jobs
Sponsors and Collaborators
- Assiut University
- GlaxoSmithKline
- National Cancer Institute (NCI)
- Cairo University
- AstraZeneca
- Assistance Publique - Hôpitaux de Paris
- Mayo Clinic
- M.D. Anderson Cancer Center
- Novartis Pharmaceuticals
- National Institute of Allergy and Infectious Diseases (NIAID)
- Massachusetts General Hospital
- National Taiwan University Hospital
- Boehringer Ingelheim
- Hoffmann-La Roche
- Merck Sharp & Dohme LLC
- Stanford University
- University of California, San Francisco
- Eli Lilly and Company
- Duke University
- View Full List
Medical Conditions
- Breast Cancer
- Hypertension
- Prostate Cancer
- HIV Infections
- Coronary Artery Disease
- Heart Failure
- Diabetes Mellitus, Type 2
- Colorectal Cancer
- Lung Cancer
- Schizophrenia
- Cardiovascular Diseases
- Atrial Fibrillation
Drug Interventions
- Cyclophosphamide
- Carboplatin
- Dexamethasone
- Gemcitabine
- Capecitabine
- Bevacizumab
- Pembrolizumab
- Methotrexate
- Oxaliplatin
- Dexmedetomidine
- Fludarabine
- Search Menu
Sign in through your institution
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Numismatics
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Greek and Roman Papyrology
- Late Antiquity
- Religion in the Ancient World
- Social History
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Agriculture
- History of Education
- History of Emotions
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Acquisition
- Language Variation
- Language Families
- Language Evolution
- Language Reference
- Lexicography
- Linguistic Theories
- Linguistic Typology
- Linguistic Anthropology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Religion
- Music and Culture
- Music and Media
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Legal System - Costs and Funding
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Restitution
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Oncology
- Medical Toxicology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Neuroscience
- Cognitive Psychology
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Strategy
- Business History
- Business Ethics
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Social Issues in Business and Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Systems
- Economic Methodology
- Economic History
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Management of Land and Natural Resources (Social Science)
- Natural Disasters (Environment)
- Pollution and Threats to the Environment (Social Science)
- Social Impact of Environmental Issues (Social Science)
- Sustainability
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- Ethnic Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Political Behaviour
- Political Economy
- Political Institutions
- Politics and Law
- Politics of Development
- Public Administration
- Public Policy
- Qualitative Political Methodology
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Disability Studies
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
21 Research project management
- Published: February 2016
- Cite Icon Cite
- Permissions Icon Permissions
What is a project? - Stage 1: Definition: defining and agreeing what the project is about - Stage 2: Planning: planning how the project will be conducted - Stage 3: Implementation and control: running the project - Stage 4: Close out: delivery and the end of the project
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
| Month: | Total Views: |
|---|---|
| May 2023 | 6 |
| July 2023 | 1 |
| August 2023 | 2 |
| September 2023 | 3 |
| November 2023 | 3 |
| December 2023 | 4 |
| February 2024 | 1 |
| April 2024 | 2 |
| May 2024 | 3 |
| June 2024 | 1 |
| August 2024 | 1 |
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Introduction to Clinical Research Project Management
Clinical research is pivotal in advancing medical knowledge and improving patient care. It involves the systematic investigation of new treatments, interventions, and healthcare practices. However, conducting clinical research requires careful planning, coordination, and management. This is where clinical research project management comes into play.

Defining Clinical Research Project Management
Clinical research project management is a specialized discipline that focuses on planning, organizing, and overseeing the various aspects of a clinical research study. It involves managing a wide range of tasks, including study protocol development, participant recruitment, data collection, monitoring, regulatory compliance, budgeting, and timeline management.
Project management plays a crucial role in clinical research, ensuring the successful execution of studies and the generation of reliable and meaningful results.
Stay informed about our upcoming in-person gatherings and educational webinars. Visit our clinical research events calendar to learn more and register for events.
The importance of project management in clinical research can be understood from the following perspectives:
Study Quality and Integrity
Effective project management helps ensure the quality and integrity of clinical research studies. Project managers work closely with researchers to develop robust study protocols, establish standardized procedures, and implement quality control measures. They ensure that the research is conducted in accordance with scientific standards, minimizing bias and increasing the reliability and validity of research findings.
Participant Safety and Ethics
Clinical research involves human participants, and their safety and well-being are of paramount importance. Project managers play a vital role in safeguarding participants’ rights and ensuring ethical conduct throughout the study. They ensure adherence to ethical guidelines, obtain necessary regulatory approvals, and implement protocols to protect participants from potential risks or harm.
Timely Execution
Clinical research projects often have strict timelines and deadlines. Effective project management ensures that studies progress according to the planned schedule, avoiding unnecessary delays. Project managers develop comprehensive project plans, identify critical milestones, and coordinate activities to keep the research on track. Timely execution is essential for the availability of research outcomes and potential interventions promptly.
Resource Optimization
Clinical research projects require various resources, including personnel, funding, equipment, and facilities. Project managers are vital in resource management, ensuring that resources are allocated efficiently and utilized optimally. They identify resource requirements, secure the necessary funding, coordinate personnel allocation, and make informed decisions to optimize resource utilization, ultimately maximizing the efficiency and cost-effectiveness of the research project.
Risk Management
Clinical research projects are subject to various risks and challenges impacting the study’s progress and outcomes. Project managers proactively identify potential risks, assess their potential impact, and develop risk mitigation strategies. They implement measures to prevent risks or address them promptly if they arise. Effective risk management minimizes disruptions and ensures that studies proceed smoothly, thereby safeguarding the integrity of the research.
Collaboration and Communication
Clinical research involves collaboration among multiple stakeholders, including researchers, study coordinators, ethics committees, regulatory authorities, sponsors, and participants. Project managers serve as a central point of contact, facilitating effective communication and collaboration among these stakeholders. They ensure that all team members are well-informed, aligned with project goals, and working cohesively towards the successful execution of the research project.
Regulatory Compliance
Clinical research is subject to a complex regulatory environment, with strict guidelines and requirements. Project managers are responsible for ensuring compliance with applicable regulations and obtaining necessary approvals and permits. They stay updated with regulatory changes, coordinate with regulatory authorities, and ensure that the research adheres to ethical and legal standards. Compliance with regulations is crucial for the credibility and acceptance of research findings.
Stakeholder Management
Successful clinical research project management involves engaging and managing relationships with various stakeholders. Project managers collaborate with researchers, sponsors, ethics committees, regulatory authorities, and participants, among others. Effective stakeholder management builds trust, facilitates cooperation, and enhances the overall success of the research project. It also sets the foundation for future collaborations and research endeavors.
Project management is vital in clinical research to ensure study quality, participant safety, timely execution, resource optimization, risk management, collaboration, regulatory compliance, and stakeholder management. By applying project management principles and methodologies, project managers contribute significantly to the successful execution of clinical research studies, ultimately advancing medical knowledge and improving patient care.
Clinical Research Project Managers (CRPM) was established to bring together clinical research professionals who oversee activities in clinical research that utilize project management tools or methodologies to assist them in achieving project deliverables on time and on budget. CRPM aims to connect members of the clinical research project management (CRPM) community through online groups, in-person meet-ups, monthly webinars, and retreats that target the growth of CRPM’s network throughout the clinical research industry.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Project Management Best Practices™
For employers looking to expand project management skills across the entire study team, this training program delivers practical, hands-on training specific to clinical research..
This is an intensive two-day course which provides detailed and practical guidance on the different project management skills used by study sponsors and Contract Research Organizations (CROs), so helps you decide how to apply these skills in your situation. The course is specifically designed for clinical research project management, with examples and exercises developed using pharmaceutical research scenarios. A key component of this course is that you will learn how to take control of your clinical trials, to manage and direct them, rather than simply tracking what is happening.
Attendees new to project management will receive an introduction to the project management of clinical trials, whereas those more familiar with project management will receive a boost to their knowledge and further their understanding of how sponsor and CRO companies operate.
Learning Objectives
- Define project management and understand the differences between the role of project management and a senior clinical role.
- Apply measurement and control to project timelines, tasks and staff resourcing.
- Describe different management methods and how they operate to achieve project control.
- Cite aspects of project management from budget management to operational delivery.
- Properly document your project management systems and procedures.
Clinical research professionals interested in enhancing project management skills or moving into a project management role.
Not a member?
Find out what The Global Health Network can do for you. Register now.
Member Sites A network of members around the world. Join now.
- 1000 Challenge
- ODIN Wastewater Surveillance Project
- CEPI Technical Resources
- Global Health Research Management
- UK Overseas Territories Public Health Network
- Global Malaria Research
- Global Outbreaks Research
- Sub-Saharan Congenital Anomalies Network
- Global Pathogen Variants
- Global Health Data Science
- AI for Global Health Research
- MRC Clinical Trials Unit at UCL
- Virtual Biorepository
- Epidemic Preparedness Innovations
- Rapid Support Team
- The Global Health Network Africa
- The Global Health Network Asia
- The Global Health Network LAC
- Global Health Bioethics
- Global Pandemic Planning
- EPIDEMIC ETHICS
- Global Vector Hub
- Global Health Economics
- LactaHub – Breastfeeding Knowledge
- Global Birth Defects
- Antimicrobial Resistance (AMR)
- Human Infection Studies
- EDCTP Knowledge Hub
- CHAIN Network
- Brain Infections Global
- Research Capacity Network
- Global Research Nurses
- ZIKAlliance
- TDR Fellows
- Global Health Coordinators
- Global Health Laboratories
- Global Health Methodology Research
- Global Health Social Science
- Global Health Trials
- Zika Infection
- Global Musculoskeletal
- Global Pharmacovigilance
- Global Pregnancy CoLab
- INTERGROWTH-21ˢᵗ
- East African Consortium for Clinical Research
- Women in Global Health Research
- Coronavirus
Research Tools Resources designed to help you.
- Site Finder
- Process Map
- Global Health Training Centre
- Resources Gateway
- Global Health Research Process Map
- About This Site
Downloadable Templates and Tools for Clinical Research
Welcome to global health trials' tools and templates library. please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. please click on the orange text to download each template., the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use them. to share your own templates and sops, or comment on these, please email [email protected]. we look forward to hearing from you.
These templates and tools are ordered by category, so please scroll down to find what you need.
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
|
| |
|
| ||
|
|
| |
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
|
|
|
|
| |
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
| /td>< |
| |
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
To share your own templates and SOPs, or comment on these, please email [email protected]. We look forward to hearing from you!
- Webinar on community engagement in clinical research involving pregnant women
- Free Webinar: Science, technology and innovation for upskilling knowledge-based economies in Africa
- Open Public Consultation on “Strengthened cooperation against vaccine preventable diseases”
Trial Operations Trial Management Ethics and Informed Consent Resources Trial Design Data Management and Statistics
training
This is Degena Bahrey Tadesse from Tigray, Ethiopia. I am new for this web I am assistant professor in Adult Health Nursing Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award
I have learned lot..Thanks..
i was wondering why there is no SOP on laboratory procedures ?
Hi, Can you provide me the SOP for electronic signatures in Clinical trial
Do you have an "SOP for Telephonic site selection visit". Kindly Share on my registered mail ID
Thank you for sharing the resources. It is very kind of you.
Hi These tolls are very useful! Thank you
Do you have a task and responsability matrix template for clinical trial managment ? Best
I am very much happy to find myself here as a clinician
Dear Getrude
We have a free 14-module course on research ethics on our training centre; you'll receive a certificate if you complete all the modules and quizzes. You can take it in your own time. Just visit 'Training centre' in the tabs above, then 'short courses'.
Kind regards The Editorial Team
need modules on free online gcp course on research ethics
Estimados: me parece excelente el aporte que han hecho dado que aporta. por un lado a mejorar la transparencia del trabajo como a facilitar el seguimiento y supervisión de los mismos. Muchas gracias por ello
We also have an up to date list of global health events available here: https://globalhealthtrials.tghn.org/community/training-events/
Dear Nazish
Thank you, I am glad you found the seminars and the training courses useful. We list many training events (all relevant to Global Health, and as many of them as possible are either free or subsidised) on the 'community' web pages above. Keep an eye on those for events and activities which you can get involved with. Also, if you post an 'introduction' on the introduction group stating where you are from and your research interests, we can keep you updated of relevant local events.
Thanks so much. These are very helpful seminars. Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help.
Hi Nazish, and welcome to the Network. The items here are downloadable templates for you to use; it sounds like you may be seeking lectures and eLearning courses? If so - no problem! You can find free seminars with sound and slides here: https://globalhealthtrainingcentre.tghn.org/webinars/ , and you can find free, certified eLearning courses here: https://globalhealthtrials.tghn.org/elearning . Certificates are awarded for the eLearning courses for those scoring over 80% in the quiz at the end of each course. If you need anything else, do ask! Kind regards The Editorial Team
Hi, I am new to this website and also to the Clinical Research Industry for that matter I only am able to see the PDF of these courses, just wanted to know are these audio lectures and also happen to have audio clips that go with the pdf?
This site is impeccable and very useful for my job!!!!
Thank you for your kind comments.
Fantastic resources
I am delighted you found this website. I earlier introduced it to you because of your prolific interest in health care information and resource sharing....
Please Sign in (or Register ) to view further.
Useful Resources
Related articles.
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts BY Jai K Das
- 5 ways statistics can fool you—Tips for practicing clinicians BY Jai K Das
- How to prepare for a job interview and predict the questions you’ll be asked BY The Editorial Team
- Preparing for and Executing a Randomised Controlled Trial of Podoconiosis Treatment in Northern Ethiopia BY Henok Negussie, Thomas Addissie, Adamu Addissie, Gail Davey
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control BY WHO/ TDR
Most popular tags
- Archive (303)
- archive (104)
- data sharing (70)
- sharing (63)
- training (49)
- malaria (30)
- ACT consortium (25)
- informed consent (7)
- data management (6)
- trial management (6)
- careers (5)
- guidelines (5)
- monitoring (5)
- workshop (5)
- administration (4)
- clinical research (4)

An Overview of Project Management in Clinical Trials

Clinical trial project management is an indispensable part of keeping tests on track and budget, but project managers aren’t always given the resources they need to succeed. Effective and well-structured project management is the support for a successful Clinical Trial . Project management includes many multiple elements and moving parts. Before initiating a clinical trial, various project tools and templates can be used to plan and execute a clinical trial successfully.
Unique characteristics of Worldwide Clinical Trials Project Management include:
- Preparation of study materials such as the case report forms, protocol, and informed consent records
- Fastening essential documentation for IRB and Ethics Committee approval
- Communications and Frequent reports and to sustain you apprised of data accrual and quality on a site-by-site basis also to assist in keeping the study team on track
- Practice and support through initial encounters with the FDA and other regulatory bodies
- Pre-investigational visits to train site staff on the study materials, protocol, applicable regulations, and Good Clinical Exercise
The Tools Shaping Clinical Project Management
The fundamental tasks of project management are the same, no matter the industry or the project.
A clinical project manager will specifically need a tool to help with:
- Creating the work breakdown structure
- Analyzing and mitigating risks
- Planning budgets
- Determining milestones and key metrics to track
With the tools mentioned earlier and a framework in place, a clinical project manager has what they require to undertake to drive a clinical trial toward its aims within estimates and within exacted timelines. No projects go as thought, so effective communication and transparency go a long way to making a trial back on the path in the light of sudden fluctuations.
Having this sort of aspect into a trial’s project-management elements will help marshal resources and leading teams as needed for clinical trial leaders.
Begin with a project plan
Designing a plan before a new trial boat is the best way to evade stoppages once the trial begins. In extension to adding a timeline of breakthroughs, your goal should also outline staff members accountable for each part of your project, communication features to be shared with the team, and a risk assessment that pleads for potential roadblocks.
List out possible risks and create a plan to inscribe them.
Of course, you will aim to avoid any patient recruitment delays, retention issues, or other difficulties during your trial. Just in case, think about creating a risk evaluation plan along with your more comprehensive project plan.
Challenges to thinking include:
- Institutional Review Board (IRB) impediments
- Staff turnover
- Protocol changes
- Recruitment delays
Generate a plan for stakeholder engagement
Including crucial stakeholders for every part of your project can assist ensure you’re getting buy-in from the best people and keeping everyone updated.
Plan time to study
As so much coordination and communication are going on, it can be hard to get time in your schedule to examine your work so far and obtain everything is on track.
Observe and analyze your results
During the project, monitor decisions and look out for inabilities that crop up early on.
Master latest skills with in-depth learning from Sollers certification/Master’s program . Take the next step to accomplishing your professional success.
Related posts

Specialist researcher holding microscope slide analyzing blood sample working at coronavirus vaccine development during virus examination in microbiology hospital laboratory. Biochemistry experiment
In what ways have health professionals played a vital role in drug testing?

Why Autonomous Clinical Trials are the Best Chance of Success?

How Adaptive AI Boosts Clinical Trials Today?
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.11(2); 2019 Feb

Planning and Conducting Clinical Research: The Whole Process
Boon-how chew.
1 Family Medicine, Universiti Putra Malaysia, Serdang, MYS
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question. Engagement with clinical experts, experienced researchers, relevant stakeholders of the research topic, and even patients can enhance the research question’s relevance, feasibility, and efficiency. Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental or non-experimental, sampling or census, and time features of the variables to be studied. The ultimate aims of research reporting are to present findings succinctly and timely. Concise, explicit, and complete reporting are the guiding principles in clinical studies reporting.
Introduction and background
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices. There are many ways a clinical research's findings can become invalid or less impactful including ignorance of previous similar studies, a paucity of similar studies, poor study design and implementation, low test agent efficacy, no predetermined statistical analysis, insufficient reporting, bias, and conflicts of interest [ 1 - 4 ]. Scientific, ethical, and moral decadence among researchers can be due to incognizant criteria in academic promotion and remuneration and too many forced studies by amateurs and students for the sake of research without adequate training or guidance [ 2 , 5 - 6 ]. This article will review the proper methods to conduct medical research from the planning stage to submission for publication (Table (Table1 1 ).
a Feasibility and efficiency are considered during the refinement of the research question and adhered to during data collection.
| Concept | Research Idea | Research Question | Acquiring Data | Analysis | Publication | Practice |
| Actions | Relevant clinical problem or issue | Primary or secondary | Measuring | Prespecified | Writing skills | Guidelines |
| Literature review | Quantitative or qualitative | Measuring tool | Predetermined | Guidelines | Protocol | |
| Conceptual framework | Causal or non-causal | Measurement | Exploratory allowed | Journal selection | Policy | |
| Collaboration with experts | Feasibility | Feasibility | Strength and direction of the effect estimate | Response to reviewers’ comments | Change | |
| Seek target population’s opinions on the research topic | Efficiency | Efficiency | ||||
| Theoretical Design | Data Collection Design | Statistical design | ||||
| Domain (external validity) | Experimental or non-experimental | Data cleaning | ||||
| Valid (confounding minimized) | Sampling or census | Outlier | ||||
| Precise (good sample size) | Time features | Missing data | ||||
| Pilot study | Descriptive | |||||
| Inferential | ||||||
| Statistical assumptions | ||||||
| Collaboration with statistician |
Epidemiologic studies in clinical and medical fields focus on the effect of a determinant on an outcome [ 7 ]. Measurement errors that happen systematically give rise to biases leading to invalid study results, whereas random measurement errors will cause imprecise reporting of effects. Precision can usually be increased with an increased sample size provided biases are avoided or trivialized. Otherwise, the increased precision will aggravate the biases. Because epidemiologic, clinical research focuses on measurement, measurement errors are addressed throughout the research process. Obtaining the most accurate estimate of a treatment effect constitutes the whole business of epidemiologic research in clinical practice. This is greatly facilitated by clinical expertise and current scientific knowledge of the research topic. Current scientific knowledge is acquired through literature reviews or in collaboration with an expert clinician. Collaboration and consultation with an expert clinician should also include input from the target population to confirm the relevance of the research question. The novelty of a research topic is less important than the clinical applicability of the topic. Researchers need to acquire appropriate writing and reporting skills from the beginning of their careers, and these skills should improve with persistent use and regular reviewing of published journal articles. A published clinical research study stands on solid scientific ground to inform clinical practice given the article has passed through proper peer-reviews, revision, and content improvement.
Systematic literature reviews
Systematic literature reviews of published papers will inform authors of the existing clinical evidence on a research topic. This is an important step to reduce wasted efforts and evaluate the planned study [ 8 ]. Conducting a systematic literature review is a well-known important step before embarking on a new study [ 9 ]. A rigorously performed and cautiously interpreted systematic review that includes in-process trials can inform researchers of several factors [ 10 ]. Reviewing the literature will inform the choice of recruitment methods, outcome measures, questionnaires, intervention details, and statistical strategies – useful information to increase the study’s relevance, value, and power. A good review of previous studies will also provide evidence of the effects of an intervention that may or may not be worthwhile; this would suggest either no further studies are warranted or that further study of the intervention is needed. A review can also inform whether a larger and better study is preferable to an additional small study. Reviews of previously published work may yield few studies or low-quality evidence from small or poorly designed studies on certain intervention or observation; this may encourage or discourage further research or prompt consideration of a first clinical trial.
Conceptual framework
The result of a literature review should include identifying a working conceptual framework to clarify the nature of the research problem, questions, and designs, and even guide the latter discussion of the findings and development of possible solutions. Conceptual frameworks represent ways of thinking about a problem or how complex things work the way they do [ 11 ]. Different frameworks will emphasize different variables and outcomes, and their inter-relatedness. Each framework highlights or emphasizes different aspects of a problem or research question. Often, any single conceptual framework presents only a partial view of reality [ 11 ]. Furthermore, each framework magnifies certain elements of the problem. Therefore, a thorough literature search is warranted for authors to avoid repeating the same research endeavors or mistakes. It may also help them find relevant conceptual frameworks including those that are outside one’s specialty or system.
Conceptual frameworks can come from theories with well-organized principles and propositions that have been confirmed by observations or experiments. Conceptual frameworks can also come from models derived from theories, observations or sets of concepts or even evidence-based best practices derived from past studies [ 11 ].
Researchers convey their assumptions of the associations of the variables explicitly in the conceptual framework to connect the research to the literature. After selecting a single conceptual framework or a combination of a few frameworks, a clinical study can be completed in two fundamental steps: study design and study report. Three study designs should be planned in sequence and iterated until satisfaction: the theoretical design, data collection design, and statistical analysis design [ 7 ].
Study designs
Theoretical Design
Theoretical design is the next important step in the research process after a literature review and conceptual framework identification. While the theoretical design is a crucial step in research planning, it is often dealt with lightly because of the more alluring second step (data collection design). In the theoretical design phase, a research question is designed to address a clinical problem, which involves an informed understanding based on the literature review and effective collaboration with the right experts and clinicians. A well-developed research question will have an initial hypothesis of the possible relationship between the explanatory variable/exposure and the outcome. This will inform the nature of the study design, be it qualitative or quantitative, primary or secondary, and non-causal or causal (Figure (Figure1 1 ).

A study is qualitative if the research question aims to explore, understand, describe, discover or generate reasons underlying certain phenomena. Qualitative studies usually focus on a process to determine how and why things happen [ 12 ]. Quantitative studies use deductive reasoning, and numerical statistical quantification of the association between groups on data often gathered during experiments [ 13 ]. A primary clinical study is an original study gathering a new set of patient-level data. Secondary research draws on the existing available data and pooling them into a larger database to generate a wider perspective or a more powerful conclusion. Non-causal or descriptive research aims to identify the determinants or associated factors for the outcome or health condition, without regard for causal relationships. Causal research is an exploration of the determinants of an outcome while mitigating confounding variables. Table Table2 2 shows examples of non-causal (e.g., diagnostic and prognostic) and causal (e.g., intervention and etiologic) clinical studies. Concordance between the research question, its aim, and the choice of theoretical design will provide a strong foundation and the right direction for the research process and path.
| Research Category | Study Title |
| Diagnostic | Plasma Concentration of B-type Natriuretic Peptide (BNP) in the Diagnosis of Left Ventricular Dysfunction |
| The Centor and McIsaac Scores and the Group A Streptococcal Pharyngitis | |
| Prognostic | The Apgar Score and Infant Mortality |
| SCORE (Systematic COronary Risk Evaluation) for the Estimation of Ten-Year Risk of Fatal Cardiovascular Disease | |
| Intervention | Dexamethasone in Very Low Birth Weight Infants |
| Bariatric Surgery of Obesity in Type 2 Diabetes and Metabolic Syndrome | |
| Etiologic | Thalidomide and Reduction Deformities of the Limbs |
| Work Stress and Risk of Cardiovascular Mortality |
A problem in clinical epidemiology is phrased in a mathematical relationship below, where the outcome is a function of the determinant (D) conditional on the extraneous determinants (ED) or more commonly known as the confounding factors [ 7 ]:
For non-causal research, Outcome = f (D1, D2…Dn) For causal research, Outcome = f (D | ED)
A fine research question is composed of at least three components: 1) an outcome or a health condition, 2) determinant/s or associated factors to the outcome, and 3) the domain. The outcome and the determinants have to be clearly conceptualized and operationalized as measurable variables (Table (Table3; 3 ; PICOT [ 14 ] and FINER [ 15 ]). The study domain is the theoretical source population from which the study population will be sampled, similar to the wording on a drug package insert that reads, “use this medication (study results) in people with this disease” [ 7 ].
| Acronym | Explanation |
| P = | Patient (or the domain) |
| I = | Intervention or treatment (or the determinants in non-experimental) |
| C = | Comparison (only in experimental) |
| O = | Outcome |
| T = | Time describes the duration of data collection |
| F = | Feasible with the current and/or potential available resources |
| I = | Important and interesting to current clinical practice and to you, respectively |
| N = | Novel and adding to the existing corpus of scientific knowledge |
| E = | Ethical research conducted without harm to participants and institutions |
| R = | Relevant to as many parties as possible, not only to your own practice |
The interpretation of study results as they apply to wider populations is known as generalization, and generalization can either be statistical or made using scientific inferences [ 16 ]. Generalization supported by statistical inferences is seen in studies on disease prevalence where the sample population is representative of the source population. By contrast, generalizations made using scientific inferences are not bound by the representativeness of the sample in the study; rather, the generalization should be plausible from the underlying scientific mechanisms as long as the study design is valid and nonbiased. Scientific inferences and generalizations are usually the aims of causal studies.
Confounding: Confounding is a situation where true effects are obscured or confused [ 7 , 16 ]. Confounding variables or confounders affect the validity of a study’s outcomes and should be prevented or mitigated in the planning stages and further managed in the analytical stages. Confounders are also known as extraneous determinants in epidemiology due to their inherent and simultaneous relationships to both the determinant and outcome (Figure (Figure2), 2 ), which are usually one-determinant-to-one outcome in causal clinical studies. The known confounders are also called observed confounders. These can be minimized using randomization, restriction, or a matching strategy. Residual confounding has occurred in a causal relationship when identified confounders were not measured accurately. Unobserved confounding occurs when the confounding effect is present as a variable or factor not observed or yet defined and, thus, not measured in the study. Age and gender are almost universal confounders followed by ethnicity and socio-economic status.

Confounders have three main characteristics. They are a potential risk factor for the disease, associated with the determinant of interest, and should not be an intermediate variable between the determinant and the outcome or a precursor to the determinant. For example, a sedentary lifestyle is a cause for acute coronary syndrome (ACS), and smoking could be a confounder but not cardiorespiratory unfitness (which is an intermediate factor between a sedentary lifestyle and ACS). For patients with ACS, not having a pair of sports shoes is not a confounder – it is a correlate for the sedentary lifestyle. Similarly, depression would be a precursor, not a confounder.
Sample size consideration: Sample size calculation provides the required number of participants to be recruited in a new study to detect true differences in the target population if they exist. Sample size calculation is based on three facets: an estimated difference in group sizes, the probability of α (Type I) and β (Type II) errors chosen based on the nature of the treatment or intervention, and the estimated variability (interval data) or proportion of the outcome (nominal data) [ 17 - 18 ]. The clinically important effect sizes are determined based on expert consensus or patients’ perception of benefit. Value and economic consideration have increasingly been included in sample size estimations. Sample size and the degree to which the sample represents the target population affect the accuracy and generalization of a study’s reported effects.
Pilot study: Pilot studies assess the feasibility of the proposed research procedures on small sample size. Pilot studies test the efficiency of participant recruitment with minimal practice or service interruptions. Pilot studies should not be conducted to obtain a projected effect size for a larger study population because, in a typical pilot study, the sample size is small, leading to a large standard error of that effect size. This leads to bias when projected for a large population. In the case of underestimation, this could lead to inappropriately terminating the full-scale study. As the small pilot study is equally prone to bias of overestimation of the effect size, this would lead to an underpowered study and a failed full-scale study [ 19 ].
The Design of Data Collection
The “perfect” study design in the theoretical phase now faces the practical and realistic challenges of feasibility. This is the step where different methods for data collection are considered, with one selected as the most appropriate based on the theoretical design along with feasibility and efficiency. The goal of this stage is to achieve the highest possible validity with the lowest risk of biases given available resources and existing constraints.
In causal research, data on the outcome and determinants are collected with utmost accuracy via a strict protocol to maximize validity and precision. The validity of an instrument is defined as the degree of fidelity of the instrument, measuring what it is intended to measure, that is, the results of the measurement correlate with the true state of an occurrence. Another widely used word for validity is accuracy. Internal validity refers to the degree of accuracy of a study’s results to its own study sample. Internal validity is influenced by the study designs, whereas the external validity refers to the applicability of a study’s result in other populations. External validity is also known as generalizability and expresses the validity of assuming the similarity and comparability between the study population and the other populations. Reliability of an instrument denotes the extent of agreeableness of the results of repeated measurements of an occurrence by that instrument at a different time, by different investigators or in a different setting. Other terms that are used for reliability include reproducibility and precision. Preventing confounders by identifying and including them in data collection will allow statistical adjustment in the later analyses. In descriptive research, outcomes must be confirmed with a referent standard, and the determinants should be as valid as those found in real clinical practice.
Common designs for data collection include cross-sectional, case-control, cohort, and randomized controlled trials (RCTs). Many other modern epidemiology study designs are based on these classical study designs such as nested case-control, case-crossover, case-control without control, and stepwise wedge clustered RCTs. A cross-sectional study is typically a snapshot of the study population, and an RCT is almost always a prospective study. Case-control and cohort studies can be retrospective or prospective in data collection. The nested case-control design differs from the traditional case-control design in that it is “nested” in a well-defined cohort from which information on the cohorts can be obtained. This design also satisfies the assumption that cases and controls represent random samples of the same study base. Table Table4 4 provides examples of these data collection designs.
| Data Collection Designs | Study Title |
| Cross-sectional | The National Health and Morbidity Survey (NHMS) |
| The National Health and Nutrition Examination Survey (NHANES) | |
| Cohort | Framingham Heart Study |
| The Malaysian Cohort (TMC) project | |
| Case-control | A Case-Control Study of the Effectiveness of Bicycle Safety Helmets |
| Open-Angle Glaucoma and Ocular Hypertension: the Long Island Glaucoma Case-Control Study | |
| Nested case-control | Nurses' Health Study on Plasma Adipokines and Endometriosis Risk |
| Physicians' Health Study Plasma Homocysteine and Risk of Myocardial Infarction | |
| Randomized controlled trial | The Women’s Health Initiative |
| U.K. Prospective Diabetes Study | |
| Cross-over | Intranasal-agonist in Allergic Rhinitis Published in the Allergy in 2000 |
| Effect of Palm-based Tocotrienols and Tocopherol Mixture Supplementation on Platelet Aggregation in Subjects with Metabolic Syndrome |
Additional aspects in data collection: No single design of data collection for any research question as stated in the theoretical design will be perfect in actual conduct. This is because of myriad issues facing the investigators such as the dynamic clinical practices, constraints of time and budget, the urgency for an answer to the research question, and the ethical integrity of the proposed experiment. Therefore, feasibility and efficiency without sacrificing validity and precision are important considerations in data collection design. Therefore, data collection design requires additional consideration in the following three aspects: experimental/non-experimental, sampling, and timing [ 7 ]:
Experimental or non-experimental: Non-experimental research (i.e., “observational”), in contrast to experimental, involves data collection of the study participants in their natural or real-world environments. Non-experimental researches are usually the diagnostic and prognostic studies with cross-sectional in data collection. The pinnacle of non-experimental research is the comparative effectiveness study, which is grouped with other non-experimental study designs such as cross-sectional, case-control, and cohort studies [ 20 ]. It is also known as the benchmarking-controlled trials because of the element of peer comparison (using comparable groups) in interpreting the outcome effects [ 20 ]. Experimental study designs are characterized by an intervention on a selected group of the study population in a controlled environment, and often in the presence of a similar group of the study population to act as a comparison group who receive no intervention (i.e., the control group). Thus, the widely known RCT is classified as an experimental design in data collection. An experimental study design without randomization is referred to as a quasi-experimental study. Experimental studies try to determine the efficacy of a new intervention on a specified population. Table Table5 5 presents the advantages and disadvantages of experimental and non-experimental studies [ 21 ].
a May be an issue in cross-sectional studies that require a long recall to the past such as dietary patterns, antenatal events, and life experiences during childhood.
| Non-experimental | Experimental |
| Advantages | |
| Quick results are possible | Comparable groups |
| Relatively less costly | Hawthorne and placebo effects mitigated |
| No recall bias | Straightforward, robust statistical analysis |
| No time effects | Convincing results as evidence |
| Real-life data | |
| Disadvantages | |
| Observed, unobserved, and residual confounding | Expensive |
| Time-consuming | |
| Overly controlled environment | |
| Loss to follow-up | |
| Random allocation of potentially harmful treatment may not be ethically permissible | |
Once an intervention yields a proven effect in an experimental study, non-experimental and quasi-experimental studies can be used to determine the intervention’s effect in a wider population and within real-world settings and clinical practices. Pragmatic or comparative effectiveness are the usual designs used for data collection in these situations [ 22 ].
Sampling/census: Census is a data collection on the whole source population (i.e., the study population is the source population). This is possible when the defined population is restricted to a given geographical area. A cohort study uses the census method in data collection. An ecologic study is a cohort study that collects summary measures of the study population instead of individual patient data. However, many studies sample from the source population and infer the results of the study to the source population for feasibility and efficiency because adequate sampling provides similar results to the census of the whole population. Important aspects of sampling in research planning are sample size and representation of the population. Sample size calculation accounts for the number of participants needed to be in the study to discover the actual association between the determinant and outcome. Sample size calculation relies on the primary objective or outcome of interest and is informed by the estimated possible differences or effect size from previous similar studies. Therefore, the sample size is a scientific estimation for the design of the planned study.
A sampling of participants or cases in a study can represent the study population and the larger population of patients in that disease space, but only in prevalence, diagnostic, and prognostic studies. Etiologic and interventional studies do not share this same level of representation. A cross-sectional study design is common for determining disease prevalence in the population. Cross-sectional studies can also determine the referent ranges of variables in the population and measure change over time (e.g., repeated cross-sectional studies). Besides being cost- and time-efficient, cross-sectional studies have no loss to follow-up; recall bias; learning effect on the participant; or variability over time in equipment, measurement, and technician. A cross-sectional design for an etiologic study is possible when the determinants do not change with time (e.g., gender, ethnicity, genetic traits, and blood groups).
In etiologic research, comparability between the exposed and the non-exposed groups is more important than sample representation. Comparability between these two groups will provide an accurate estimate of the effect of the exposure (risk factor) on the outcome (disease) and enable valid inference of the causal relation to the domain (the theoretical population). In a case-control study, a sampling of the control group should be taken from the same study population (study base), have similar profiles to the cases (matching) but do not have the outcome seen in the cases. Matching important factors minimizes the confounding of the factors and increases statistical efficiency by ensuring similar numbers of cases and controls in confounders’ strata [ 23 - 24 ]. Nonetheless, perfect matching is neither necessary nor achievable in a case-control study because a partial match could achieve most of the benefits of the perfect match regarding a more precise estimate of odds ratio than statistical control of confounding in unmatched designs [ 25 - 26 ]. Moreover, perfect or full matching can lead to an underestimation of the point estimates [ 27 - 28 ].
Time feature: The timing of data collection for the determinant and outcome characterizes the types of studies. A cross-sectional study has the axis of time zero (T = 0) for both the determinant and the outcome, which separates it from all other types of research that have time for the outcome T > 0. Retrospective or prospective studies refer to the direction of data collection. In retrospective studies, information on the determinant and outcome have been collected or recorded before. In prospective studies, this information will be collected in the future. These terms should not be used to describe the relationship between the determinant and the outcome in etiologic studies. Time of exposure to the determinant, the time of induction, and the time at risk for the outcome are important aspects to understand. Time at risk is the period of time exposed to the determinant risk factors. Time of induction is the time from the sufficient exposure to the risk or causal factors to the occurrence of a disease. The latent period is when the occurrence of a disease without manifestation of the disease such as in “silence” diseases for example cancers, hypertension and type 2 diabetes mellitus which is detected from screening practices. Figure Figure3 3 illustrates the time features of a variable. Variable timing is important for accurate data capture.

The Design of Statistical Analysis
Statistical analysis of epidemiologic data provides the estimate of effects after correcting for biases (e.g., confounding factors) measures the variability in the data from random errors or chance [ 7 , 16 , 29 ]. An effect estimate gives the size of an association between the studied variables or the level of effectiveness of an intervention. This quantitative result allows for comparison and assessment of the usefulness and significance of the association or the intervention between studies. This significance must be interpreted with a statistical model and an appropriate study design. Random errors could arise in the study resulting from unexplained personal choices by the participants. Random error is, therefore, when values or units of measurement between variables change in non-concerted or non-directional manner. Conversely, when these values or units of measurement between variables change in a concerted or directional manner, we note a significant relationship as shown by statistical significance.
Variability: Researchers almost always collect the needed data through a sampling of subjects/participants from a population instead of a census. The process of sampling or multiple sampling in different geographical regions or over different periods contributes to varied information due to the random inclusion of different participants and chance occurrence. This sampling variation becomes the focus of statistics when communicating the degree and intensity of variation in the sampled data and the level of inference in the population. Sampling variation can be influenced profoundly by the total number of participants and the width of differences of the measured variable (standard deviation). Hence, the characteristics of the participants, measurements and sample size are all important factors in planning a study.
Statistical strategy: Statistical strategy is usually determined based on the theoretical and data collection designs. Use of a prespecified statistical strategy (including the decision to dichotomize any continuous data at certain cut-points, sub-group analysis or sensitive analyses) is recommended in the study proposal (i.e., protocol) to prevent data dredging and data-driven reports that predispose to bias. The nature of the study hypothesis also dictates whether directional (one-tailed) or non-directional (two-tailed) significance tests are conducted. In most studies, two-sided tests are used except in specific instances when unidirectional hypotheses may be appropriate (e.g., in superiority or non-inferiority trials). While data exploration is discouraged, epidemiological research is, by nature of its objectives, statistical research. Hence, it is acceptable to report the presence of persistent associations between any variables with plausible underlying mechanisms during the exploration of the data. The statistical methods used to produce the results should be explicitly explained. Many different statistical tests are used to handle various kinds of data appropriately (e.g., interval vs discrete), and/or the various distribution of the data (e.g., normally distributed or skewed). For additional details on statistical explanations and underlying concepts of statistical tests, readers are recommended the references as cited in this sentence [ 30 - 31 ].
Steps in statistical analyses: Statistical analysis begins with checking for data entry errors. Duplicates are eliminated, and proper units should be confirmed. Extremely low, high or suspicious values are confirmed from the source data again. If this is not possible, this is better classified as a missing value. However, if the unverified suspicious data are not obviously wrong, they should be further examined as an outlier in the analysis. The data checking and cleaning enables the analyst to establish a connection with the raw data and to anticipate possible results from further analyses. This initial step involves descriptive statistics that analyze central tendency (i.e., mode, median, and mean) and dispersion (i.e., (minimum, maximum, range, quartiles, absolute deviation, variance, and standard deviation) of the data. Certain graphical plotting such as scatter plot, a box-whiskers plot, histogram or normal Q-Q plot are helpful at this stage to verify data normality in distribution. See Figure Figure4 4 for the statistical tests available for analyses of different types of data.

Once data characteristics are ascertained, further statistical tests are selected. The analytical strategy sometimes involves the transformation of the data distribution for the selected tests (e.g., log, natural log, exponential, quadratic) or for checking the robustness of the association between the determinants and their outcomes. This step is also referred to as inferential statistics whereby the results are about hypothesis testing and generalization to the wider population that the study’s sampled participants represent. The last statistical step is checking whether the statistical analyses fulfill the assumptions of that particular statistical test and model to avoid violation and misleading results. These assumptions include evaluating normality, variance homogeneity, and residuals included in the final statistical model. Other statistical values such as Akaike information criterion, variance inflation factor/tolerance, and R2 are also considered when choosing the best-fitted models. Transforming raw data could be done, or a higher level of statistical analyses can be used (e.g., generalized linear models and mixed-effect modeling). Successful statistical analysis allows conclusions of the study to fit the data.
Bayesian and Frequentist statistical frameworks: Most of the current clinical research reporting is based on the frequentist approach and hypotheses testing p values and confidence intervals. The frequentist approach assumes the acquired data are random, attained by random sampling, through randomized experiments or influences, and with random errors. The distribution of the data (its point estimate and confident interval) infers a true parameter in the real population. The major conceptual difference between Bayesian statistics and frequentist statistics is that in Bayesian statistics, the parameter (i.e., the studied variable in the population) is random and the data acquired is real (true or fix). Therefore, the Bayesian approach provides a probability interval for the parameter. The studied parameter is random because it could vary and be affected by prior beliefs, experience or evidence of plausibility. In the Bayesian statistical approach, this prior belief or available knowledge is quantified into a probability distribution and incorporated into the acquired data to get the results (i.e., the posterior distribution). This uses mathematical theory of Bayes’ Theorem to “turn around” conditional probabilities.
The goal of research reporting is to present findings succinctly and timely via conference proceedings or journal publication. Concise and explicit language use, with all the necessary details to enable replication and judgment of the study applicability, are the guiding principles in clinical studies reporting.
Writing for Reporting
Medical writing is very much a technical chore that accommodates little artistic expression. Research reporting in medicine and health sciences emphasize clear and standardized reporting, eschewing adjectives and adverbs extensively used in popular literature. Regularly reviewing published journal articles can familiarize authors with proper reporting styles and help enhance writing skills. Authors should familiarize themselves with standard, concise, and appropriate rhetoric for the intended audience, which includes consideration for journal reviewers, editors, and referees. However, proper language can be somewhat subjective. While each publication may have varying requirements for submission, the technical requirements for formatting an article are usually available via author or submission guidelines provided by the target journal.
Research reports for publication often contain a title, abstract, introduction, methods, results, discussion, and conclusions section, and authors may want to write each section in sequence. However, best practices indicate the abstract and title should be written last. Authors may find that when writing one section of the report, ideas come to mind that pertains to other sections, so careful note taking is encouraged. One effective approach is to organize and write the result section first, followed by the discussion and conclusions sections. Once these are drafted, write the introduction, abstract, and the title of the report. Regardless of the sequence of writing, the author should begin with a clear and relevant research question to guide the statistical analyses, result interpretation, and discussion. The study findings can be a motivator to propel the author through the writing process, and the conclusions can help the author draft a focused introduction.
Writing for Publication
Specific recommendations on effective medical writing and table generation are available [ 32 ]. One such resource is Effective Medical Writing: The Write Way to Get Published, which is an updated collection of medical writing articles previously published in the Singapore Medical Journal [ 33 ]. The British Medical Journal’s Statistics Notes series also elucidates common and important statistical concepts and usages in clinical studies. Writing guides are also available from individual professional societies, journals, or publishers such as Chest (American College of Physicians) medical writing tips, PLoS Reporting guidelines collection, Springer’s Journal Author Academy, and SAGE’s Research methods [ 34 - 37 ]. Standardized research reporting guidelines often come in the form of checklists and flow diagrams. Table Table6 6 presents a list of reporting guidelines. A full compilation of these guidelines is available at the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network website [ 38 ] which aims to improve the reliability and value of medical literature by promoting transparent and accurate reporting of research studies. Publication of the trial protocol in a publicly available database is almost compulsory for publication of the full report in many potential journals.
| No. | Reporting Guidelines and Checklists | |
| CONSORT - CONsolidated Standards Of Reporting Trials | ||
| A 25-item checklist for reporting of randomized controlled trials. There are appropriate extensions to the CONSORT statement due to variations in the standard trial methodology such as different design aspects (e.g., cluster, pragmatic, non-inferiority and equivalence trials), interventions (e.g., herbals) and data (e.g., harms, including the extension for writing abstracts) | ||
| SPIRIT - Standard Protocol Items: Recommendations for Interventional Trials | ||
| A 33-item checklist for reporting protocols for randomized controlled trials | ||
| COREQ - COnsolidated criteria for REporting Qualitative research | ||
| A 32-item checklist for reporting qualitative research of interviews and focus groups | ||
| STARD - STAndards for the Reporting of Diagnostic accuracy studies | ||
| A 25-item checklist for reporting of diagnostic accuracy studies | ||
| PRISMA - Preferred Reporting Items for Systematic reviews and Meta-Analyses | ||
| A 27-item checklist for reporting of systematic reviews | ||
| PRISMA-P - Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocols | ||
| A 17-item checklist for reporting of systematic review and meta-analysis protocols | ||
| MOOSE - Meta-analysis Of Observational Studies in Epidemiology | ||
| A 35-item checklist for reporting of meta-analyses of observational studies | ||
| STROBE - STrengthening the Reporting of OBservational studies in Epidemiology | ||
| For reporting of observational studies in epidemiology | ||
| Checklist for cohort, case-control and cross-sectional studies (combined) | ||
| Checklist for cohort studies | ||
| Checklist for case-control studies | ||
| Checklist for cross-sectional studies | ||
| Extensions of the STROBE statement | ||
| STROME-ID - STrengthening the Reporting Of Molecular Epidemiology for Infectious Diseases | ||
| A 42-item checklist | ||
| STREGA - STrengthening the REporting of Genetic Associations | ||
| A 22-item checklist for reporting of gene-disease association studies | ||
| CHEERS - Consolidated Health Economic Evaluation Reporting Standards | ||
| A 24-item checklist for reporting of health economic evaluations | ||
Graphics and Tables
Graphics and tables should emphasize salient features of the underlying data and should coherently summarize large quantities of information. Although graphics provide a break from dense prose, authors must not forget that these illustrations should be scientifically informative, not decorative. The titles for graphics and tables should be clear, informative, provide the sample size, and use minimal font weight and formatting only to distinguish headings, data entry or to highlight certain results. Provide a consistent number of decimal points for the numerical results, and with no more than four for the P value. Most journals prefer cell-delineated tables created using the table function in word processing or spreadsheet programs. Some journals require specific table formatting such as the absence or presence of intermediate horizontal lines between cells.
Decisions of authorship are both sensitive and important and should be made at an early stage by the study’s stakeholders. Guidelines and journals’ instructions to authors abound with authorship qualifications. The guideline on authorship by the International Committee of Medical Journal Editors is widely known and provides a standard used by many medical and clinical journals [ 39 ]. Generally, authors are those who have made major contributions to the design, conduct, and analysis of the study, and who provided critical readings of the manuscript (if not involved directly in manuscript writing).
Picking a target journal for submission
Once a report has been written and revised, the authors should select a relevant target journal for submission. Authors should avoid predatory journals—publications that do not aim to advance science and disseminate quality research. These journals focus on commercial gain in medical and clinical publishing. Two good resources for authors during journal selection are Think-Check-Submit and the defunct Beall's List of Predatory Publishers and Journals (now archived and maintained by an anonymous third-party) [ 40 , 41 ]. Alternatively, reputable journal indexes such as Thomson Reuters Journal Citation Reports, SCOPUS, MedLine, PubMed, EMBASE, EBSCO Publishing's Electronic Databases are available areas to start the search for an appropriate target journal. Authors should review the journals’ names, aims/scope, and recently published articles to determine the kind of research each journal accepts for publication. Open-access journals almost always charge article publication fees, while subscription-based journals tend to publish without author fees and instead rely on subscription or access fees for the full text of published articles.
Conclusions
Conducting a valid clinical research requires consideration of theoretical study design, data collection design, and statistical analysis design. Proper study design implementation and quality control during data collection ensures high-quality data analysis and can mitigate bias and confounders during statistical analysis and data interpretation. Clear, effective study reporting facilitates dissemination, appreciation, and adoption, and allows the researchers to affect real-world change in clinical practices and care models. Neutral or absence of findings in a clinical study are as important as positive or negative findings. Valid studies, even when they report an absence of expected results, still inform scientific communities of the nature of a certain treatment or intervention, and this contributes to future research, systematic reviews, and meta-analyses. Reporting a study adequately and comprehensively is important for accuracy, transparency, and reproducibility of the scientific work as well as informing readers.
Acknowledgments
The author would like to thank Universiti Putra Malaysia and the Ministry of Higher Education, Malaysia for their support in sponsoring the Ph.D. study and living allowances for Boon-How Chew.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The materials presented in this paper is being organized by the author into a book.
How to Plan and Design a Clinical Research Project
Cite this chapter.

- A. A. Zin 5 ,
- A. Gullo 6 &
- W. A. Zin 7
1866 Accesses
Scientific research is in fact the systematic process of collecting and analyzing data in order to increase the available knowledge on a specific field of interest. To start with it is mandatory that a protocol be established and understood by all personnel involved in the research. This protocol must be approved by an Institutional Review Board (IRB) before the research starts off. The research protocol is a formal written document specifying the study design and the manner in which it will be conducted. It is the blueprint of the study and serves as a guideline throughout the implementation and analysis phases. It details procedures to be followed yielding valid results. The research protocol fulfils scientific, ethical, and organizational requirements so that the study may be conducted efficiently and according to the plan, thus standardizing the procedures for research personnel to follow. The purpose of the study and the setting will determine which professionals will be consulted about the development of the research protocol. Subject area experts, epidemiologists, and/or statisticians can be included.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Similar content being viewed by others

The Research Protocol
Steps of a research study: from research question to publication.

Introduction to Clinical Research Concepts, Essential Characteristics of Clinical Research, Overview of Clinical Research Study Designs
Suggested reading.
Clayton D, Hills M (1993) Statistical models in epidemiology. Oxford University Press, United Kingdom
Google Scholar
Christensen LB (1997) Experimental methodology. Allyn and Bacon, United States of America
Daly LE, Bourke GJ (2000) Interpretation and uses of medical statistics. Blackwell Science, United Kingdom
Book Google Scholar
Hulley S, Cummings S, Browner W et al (2007) Designing clinical research. Lippincott Williams & Wilkins, United States of America
Kirkwood BR, Sterne JAC (2003) Medical Statistics. Blackwell Science, United Kingdom
Leedy PD (1997) Practical research planning and design. Prentice Hall, United States of America
Download references
Author information
Authors and affiliations.
Fernandes Figueira Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
Department and School of Anesthesia and Intensive Care, Catania University-Hospital, Catania, Italy
Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department and School of Anesthesia and Intensive Care, Catania School of Medicine and University-Hospital, Catania, Italy
Antonino Gullo
Department of Anesthesiology, Keck Medical School, Los Angeles, CA, USA
Philip D. Lumb
Department of Critical Care Medicine, Hospital Centro Medico de Caracas, Caracas, Venezuela
World Federation of Critical Care Nurses C/-Nursing Administration, Gold Coast Health, Southport, Queensland, Australia
Ged F. Williams
Rights and permissions
Reprints and permissions
Copyright information
© 2009 Springer-Verlag Italia
About this chapter
Zin, A.A., Gullo, A., Zin, W.A. (2009). How to Plan and Design a Clinical Research Project. In: Gullo, A., Lumb, P.D., Besso, J., Williams, G.F. (eds) Intensive and Critical Care Medicine. Springer, Milano. https://doi.org/10.1007/978-88-470-1436-7_41
Download citation
DOI : https://doi.org/10.1007/978-88-470-1436-7_41
Publisher Name : Springer, Milano
Print ISBN : 978-88-470-1435-0
Online ISBN : 978-88-470-1436-7
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Project Manager: Clinical Practice & Research Partnerships
Job summary.
The Michigan Medicine Quality Department is seeking a skilled individual to provide support for the Michigan Program on Value Enhancement (MPrOVE). The Michigan Program on Value Enhancement (MPrOVE) is a multi-faceted strategic initiative designed to implement and evaluate innovative projects that improve the value of care at Michigan Medicine (MM) and research the implementation of novel ideas around value and right-sizing care. MPrOVE is jointly supported by the MM Quality Department and the Institute for Healthcare Policy & Innovation (IHPI).
This Project Manager will directly lead multiple MPrOVE projects that build research practice partnerships to improve patient care. These will include collaborative quality improvement and research projects aimed to reduce low-value care and bridge the gap between health services research and clinical operations at Michigan Medicine. This role will coordinate a team of physician researchers, clinicians, statisticians, analysts, and other stakeholders to coordinate the implementation and evaluation of impactful projects across the health system. This work is designed to accelerate the application of innovative research methods to facilitate the identification, assessment, and de-implementation of low-value services at MM and beyond.
Depending on the skills and interests of the individual who takes this position, there will be flexibility regarding additional opportunities to support value-based project and research activities.
This is a full-time position working approximately 8:30am-5:00pm, Monday through Friday.
This position will be eligible for a remote first approach to work location, however, there are some required in-person meetings which occur approximately once per week (4 times per month). Flexible work opportunities are determined at the discretion of the hiring department. Flexible work agreements are reviewed annually and are subject to change dependent on the business needs of the team throughout the course of employment.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Why Join Michigan Medicine?
Michigan Medicine is one of the largest health care complexes in the world and has been the site of many groundbreaking medical and technological advancements since the opening of the U-M Medical School in 1850. Michigan Medicine is comprised of over 30,000 employees and our vision is to attract, inspire, and develop outstanding people in medicine, sciences, and healthcare to become one of the world’s most distinguished academic health systems. In some way, great or small, every person here helps to advance this world-class institution. Work at Michigan Medicine and become a victor for the greater good.
What Benefits can you Look Forward to?
- Excellent medical, dental and vision coverage effective on your very first day
- 2:1 Match on retirement savings
Responsibilities*
Project Management (60%)
- Translate leadership priorities and goals into actionable project deliverables for specific MPrOVE projects. This includes tying MPrOVE projects to the health system priorities framework.
- Independently lead projects that connect health system leaders with researchers to achieve impact. This will include conducting technical project work (such as project planning, intervention design, and quality improvement efforts), leading stakeholder coordination (facilitating meetings as well as collaborative decision-making), and developing research dissemination content in partnership with faculty (such as abstracts, presentations, and manuscripts).
- Independently produce detailed timelines and resource plans, define requirements, create project documentation, report status to team and stakeholders through project summaries and progress tracking, track and solve issues with team members and work with faculty to complete necessary activities that drive progress.
- Draft, edit, and format communications, documents, and reports using Word, Excel and PowerPoint for use in a variety of venues with diverse audiences including senior leaders and external stakeholders.
- Manage project resources, including budgets, staff time, faculty time, and stakeholder capital, to design, implement, and evaluate impactful value-based quality improvement projects.
- Build and sustain working relationships across different levels of the organization, including connections throughout all levels of the Quality Department, MM, and IHPI, as well as establishing connections within key clinical divisions involved in specific projects.
- Devise innovative solutions to any problems that may arise during the course of the project, and take appropriate actions to resolve without waiting for direction.
Research Responsibilities (25%)
- Work collaboratively with faculty members, analysts, and data teams to develop evaluation and research approaches.
- Cooperate with physician faculty, PhD researchers, analysts, and other partners to plan and execute surveys, qualitative interviews, mixed-methods projects, and/or research projects on value of care at MM.
- Collect or direct other team members to organize and analyze medical chart data (the electronic health record; EPIC/MiChart)..
- Assist with IRB requirements.
- Coordinate the development of collaborative academic products and research presentations.
Coordinate MPrOVE Learner Program (10%)
- In coordination with other MPrOVE staff and leaders, work to develop and coordinate the MPrOVE Learner Program, which will actively engage students and learners from multiple disciplines, particularly those interested in value, de-implementation, and research.
- Tasks will include coordination of learning/training sessions, actively engaging with students, and at times facilitating open dialogue among learners about projects, emerging research, and additional topics related to value and research.
Additional Activities and Tasks as Needed (5%)
- As needed, provide high-level project management expertise leading to the advancement of the MPrOVE Program.
- Other duties as assigned.
Required Qualifications*
- Bachelor's degree.
- 5+ years of relevant experience such as quality improvement, projects related to health services data/research, or quality of care in adult or pediatric populations.
- 3+ years of experience working directly with clinical operations team leaders and physician faculty.
- Ability to consistently meet deadlines for multiple projects and stakeholders in a fast-paced environment with competing tasks and priorities.
- Strong skills related to collaboration, active listening, interpersonal communication, meeting facilitation.
- Detail oriented with strong organizational, communication, and advanced problem-solving skills.
- Experience with research methods such as surveys, qualitative interviews, or analytic approaches.
- Experience with quality improvement, implementation science, healthcare evaluation and health services research.
- Experience with the academic research process including protocol development, data collection and management, and dissemination of academic products.
Desired Qualifications*
- Advanced technical skills and expertise related to quality improvement, implementation science, or health services research.
- Familiarity with value-based care, research practice partnerships, or translational research .
Modes of Work
Positions that are eligible for hybrid or mobile/remote work mode are at the discretion of the hiring department. Work agreements are reviewed annually at a minimum and are subject to change at any time, and for any reason, throughout the course of employment. Learn more about the work modes .
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.

- Clinical Study Resources
Project Management: Guides to the Basics
Project management tools and principals are used successfully in business to manage goals and objectives to meet expectations of deliverables: product quality, timelines and cost. We in research know how complicated it is to get from “idea” to completed study publication. Experience shows us project management skills and tools can help streamline the research process and use your time more effectively through a planned, methodical approach; a little like applying the scientific method to study start-up, recruitment, study conduct and closure!
Our four modules (15 minutes a day) provide an introduction to the basics of project management with skills, tools and local resources you can use immediately.
Additional Project Management Resources
If, after finishing our Project Management: Guides to the Basics series, you want to learn more about the discipline of project management, consider these courses:
Project Management for Clinical Research Professionals – ACRP
Project Management Certificate Program – DIA
Project Management Certificate Program – the Pharmaceutical Education & Research Institute
Project Management Professional Certification – The Project Management Institute
Postgraduate Certificate in Clinical Trial Management – Parexel
30-Hour Clinical Project Management Fundamentals Certification Program – Barnett International

Working together, we can reimagine medicine to improve and extend people’s lives.
Senior Clinical Development Medical Director - Renal
About the role.
Major accountabilities:
Providing clinical leadership and strategic medical input for all clinical deliverables in the assigned project or section of a clinical program
Leading development of clinical sections of trial and program level regulatory documents
Driving execution the assigned clinical program and/or clinical trial in partnership with global line functions, assigned Global Trial Directors (GTDs), and regional/country medical associates, where applicable
Supporting (Senior) Global Program Clinical Head (GPCH) in ensuring overall safety of the molecule for the assigned section, and may act as a core member of the Safety Management Team (SMT), supporting overall program safety reporting in collaboration with Patient Safety colleagues
Supporting the Clinical Development Head (CDH) by providing medical input into Clinical Development Plan (CDP), Integrated Development Plan (IDP) and Clinical Trial Protocol (CTP) reviews, and contributing to/driving development of disease clinical standards for new disease areas
As a medical expert, supporting the (Sr.) GPCH or CDH in interactions with external and internal stakeholders and decision boards
May work with BR (Novartis Biomedical Research/ Translational Medical Sciences) to drive transition of pre-PoC (Proof of Concept) projects to DDP (Development Decision Point) and with BD&L (Business Development & Licensing) including target identification and due diligences together with other medical matters, as needed.
Minimum Requirements:
MD or equivalent medical degree is required in addition to advanced knowledge and clinical training in medical/scientific area; Clinical practice experience 4 years (including residency) and board certification or eligibility in disease area preferred
Minimum of 7 years of experience in clinical research or drug development
Experience in an academic clinical research or industry environment spanning clinical activities in Phases I through IV required. • 2 years of contribution to and accomplishment in all aspects of conducting clinical trials (e.g., planning, executing, reporting and publishing) in a global/matrix environment in pharmaceutical industry required.
Working knowledge of disease area is required, with proven ability to interpret, discuss and present efficacy and safety data relating to clinical trial(s) and proven ability to understand and interpret basic and clinical scientific research reports
Demonstrated ability to establish effective scientific partnerships with key stakeholders
Working knowledge of GCP, clinical trial design, statistics, and regulatory and clinical development processes
Previous global people management experience is preferred, though this may include management in a matrix environment.
Why Novartis: Helping people with disease and their families takes more than innovative science. It takes a community of smart, passionate people like you. Collaborating, supporting and inspiring each other. Combining to achieve breakthroughs that change patients’ lives. Ready to create a brighter future together? https://www.novartis.com/about/strategy/people-and-culture
Join our Novartis Network: Not the right Novartis role for you? Sign up to our talent community to stay connected and learn about suitable career opportunities as soon as they come up: https://talentnetwork.novartis.com/network
Benefits and Rewards: Read our handbook to learn about all the ways we’ll help you thrive personally and professionally: https://www.novartis.com/careers/benefits-rewards
EEO Statement:
The Novartis Group of Companies are Equal Opportunity Employers who are focused on building and advancing a culture of inclusion that values and celebrates individual differences, uniqueness, backgrounds and perspectives. We do not discriminate in recruitment, hiring, training, promotion or other employment practices for reasons of race, color, religion, sex, national origin, age, sexual orientation, gender identity or expression, marital or veteran status, disability, or any other legally protected status. We are committed to fostering a diverse and inclusive workplace that reflects the world around us and connects us to the patients, customers and communities we serve.
Accessibility & Reasonable Accommodations
The Novartis Group of Companies are committed to working with and providing reasonable accommodation to individuals with disabilities. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the application process, or to perform the essential functions of a position, please send an e-mail to [email protected] or call +1(877)395-2339 and let us know the nature of your request and your contact information. Please include the job requisition number in your message.


IMAGES
VIDEO
COMMENTS
The following are three major reasons why project management in clinical research is important: Timeline management. Project management ensures the delivery of clinical trial objectives and sub-objectives within the allocated time and budget. This is important because nearly 85% of all clinical trials experience delays. [1]
Learn how to plan, execute and close out a clinical trial with guidance, templates and tools. This module covers project management activities, documentation, human subject protection, and quality assurance.
The key areas of expertise for clinical trial project managers are communication, relationships, influence, planning, attention to detail, and following instructions. Strong teamwork skills are also essential when working on projects as well as people management skills. You will need to be adaptable to a rapidly changing environment with ...
Project Management Plan (PMP) for Clinical Trials; Clinical Research Project Activity List; Clinical Trial Timeline Template; ... Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the ...
Learn how to use various tools and templates to plan and execute a clinical trial, from study start-up to team management. Find examples of Microsoft Office, Visio, RedCap, Basecamp, and more.
CRPM. June 1, 2023. Clinical research is pivotal in advancing medical knowledge and improving patient care. It involves the systematic investigation of new treatments, interventions, and healthcare practices. However, conducting clinical research requires careful planning, coordination, and management. This is where clinical research project ...
From creating a plan, communicating updates, calculating risks, and addressing any mistakes that arise, solid project management is a necessity to ensure medical research is allowed to move forward. Here, we'll discuss the role of the clinical trial project manager, as well as tips for developing project plans, stakeholder involvement ...
A clinical trial project management plan is a strategy that combines project management and clinical trial processes. It helps clinical trial project managers and teams to plan and execute clinical trials with greater efficiency and accuracy. This plan includes focus areas, objectives, KPIs, and related projects that contribute to the overall ...
All because of poor project management practices. Containing costs in a clinical trial is a real struggle. The process and discipline of project management will help. A recent survey1 demonstrates the impact of project management. At organizations that place a high priority on creating a culture that recognizes the importance of project ...
Recognizing this is the first step in successfully managing clinical trials. Learning to harness the power of these tools is the second step. Using tools like chunking, GANTT charts, and resource allocation will organize and simplify the flow of your trial. Pearl #2: Focus more on human aspects as compared to technical aspects.
National Institut e of Dental and Craniofacial Research Clinical Researcher Toolkit, Planning & Start -up Templates: Clinical Data Management Plan, Manual of Operations, Monitoring plan, Quality Management, Safety Monitoring • Coverage Analysis . o CITI program module (if available through your institution)
As Genesis Research Services Business and Clinical Research Manager Dom Bailey points out, this person — the clinical project manager — is responsible for explicitly laying out the project objectives, scope and stakeholder management plan. Additionally, the project manager will work with a CRO's leadership team to ensure things like ...
As highlighted by Rettig, R.A. (2000, p.129), the importance of project management and oversight is crucial in the environment of prevailing commercial approach in pharmaceutical industry. Though it may be safer for Pharma to manage their own R&D projects internally, it becomes more time- and cost-effective to outsource clinical trial and pre ...
A clinical research trial is a good example of a project. This involves a group of people (a clinical project team) working together to try to answer a specific medical question (e.g. does this compound work in a specified group of patients with a certain disease?), aiming to obtain the answer within a set time frame and budget.
Defining Clinical Research Project Management. Clinical research project management is a specialized discipline that focuses on planning, organizing, and overseeing the various aspects of a clinical research study. It involves managing a wide range of tasks, including study protocol development, participant recruitment, data collection ...
Project Management Best Practices™. For employers looking to expand project management skills across the entire study team, this training program delivers practical, hands-on training specific to clinical research. This is an intensive two-day course which provides detailed and practical guidance on the different project management skills ...
Data management plan : CRF template -generic malaria : CRF template generic : CRF tracking template : ... Research Project Award. Joshy Thomas 31 Oct 2017. I have learned lot..Thanks.. ... Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help. The Editorial Team 28 Apr 2014.
The clinical project manager is a professional who applies the definition of project management to the field of clinical research to ensure that all stages of a clinical trial are properly managed, that the objectives of the trial are achieved on time, on budget, and according to the GCP, and that the safety of the subjects participating in the ...
The fundamental tasks of project management are the same, no matter the industry or the project. A clinical project manager will specifically need a tool to help with: Creating the work breakdown structure. Analyzing and mitigating risks. Planning budgets. Determining milestones and key metrics to track. With the tools mentioned earlier and a ...
We in research know how complicated it is to get from "idea" to completed study publication. Our four modules (15 minutes a day) provide an introduction to the basics of clinical trial project management with skills, tools and local resources you can use immediately.
Abstract. The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice ...
To start with it is mandatory that a protocol be established and understood by all personnel involved in the research. This protocol must be approved by an Institutional Review Board (IRB) before the research starts off. The research protocol is a formal written document specifying the study design and the manner in which it will be conducted.
As needed, provide high-level project management expertise leading to the advancement of the MPrOVE Program. Other duties as assigned. Required Qualifications* Bachelor's degree. 5+ years of relevant experience such as quality improvement, projects related to health services data/research, or quality of care in adult or pediatric populations.
If, after finishing our Project Management: Guides to the Basics series, you want to learn more about the discipline of project management, consider these courses: Project Management for Clinical Research Professionals - ACRP. Project Management Certificate Program - DIA. Project Management Certificate Program - the Pharmaceutical ...
Major accountabilities: Providing clinical leadership and strategic medical input for all clinical deliverables in the assigned project or section of a clinical program Leading development of clinical sections of trial and program level regulatory documents Driving execution the assigned clinical program and/or clinical trial in partnership with global line functions, assigned Global Trial ...