Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 21, Issue 1
- What is a case study?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Roberta Heale 1 ,
- Alison Twycross 2
- 1 School of Nursing , Laurentian University , Sudbury , Ontario , Canada
- 2 School of Health and Social Care , London South Bank University , London , UK
- Correspondence to Dr Roberta Heale, School of Nursing, Laurentian University, Sudbury, ON P3E2C6, Canada; rheale{at}laurentian.ca
https://doi.org/10.1136/eb-2017-102845
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is it?
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research. 1 However, very simply… ‘a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units’. 1 A case study has also been described as an intensive, systematic investigation of a single individual, group, community or some other unit in which the researcher examines in-depth data relating to several variables. 2
Often there are several similar cases to consider such as educational or social service programmes that are delivered from a number of locations. Although similar, they are complex and have unique features. In these circumstances, the evaluation of several, similar cases will provide a better answer to a research question than if only one case is examined, hence the multiple-case study. Stake asserts that the cases are grouped and viewed as one entity, called the quintain . 6 ‘We study what is similar and different about the cases to understand the quintain better’. 6
The steps when using case study methodology are the same as for other types of research. 6 The first step is defining the single case or identifying a group of similar cases that can then be incorporated into a multiple-case study. A search to determine what is known about the case(s) is typically conducted. This may include a review of the literature, grey literature, media, reports and more, which serves to establish a basic understanding of the cases and informs the development of research questions. Data in case studies are often, but not exclusively, qualitative in nature. In multiple-case studies, analysis within cases and across cases is conducted. Themes arise from the analyses and assertions about the cases as a whole, or the quintain, emerge. 6
Benefits and limitations of case studies
If a researcher wants to study a specific phenomenon arising from a particular entity, then a single-case study is warranted and will allow for a in-depth understanding of the single phenomenon and, as discussed above, would involve collecting several different types of data. This is illustrated in example 1 below.
Using a multiple-case research study allows for a more in-depth understanding of the cases as a unit, through comparison of similarities and differences of the individual cases embedded within the quintain. Evidence arising from multiple-case studies is often stronger and more reliable than from single-case research. Multiple-case studies allow for more comprehensive exploration of research questions and theory development. 6
Despite the advantages of case studies, there are limitations. The sheer volume of data is difficult to organise and data analysis and integration strategies need to be carefully thought through. There is also sometimes a temptation to veer away from the research focus. 2 Reporting of findings from multiple-case research studies is also challenging at times, 1 particularly in relation to the word limits for some journal papers.
Examples of case studies
Example 1: nurses’ paediatric pain management practices.
One of the authors of this paper (AT) has used a case study approach to explore nurses’ paediatric pain management practices. This involved collecting several datasets:
Observational data to gain a picture about actual pain management practices.
Questionnaire data about nurses’ knowledge about paediatric pain management practices and how well they felt they managed pain in children.
Questionnaire data about how critical nurses perceived pain management tasks to be.
These datasets were analysed separately and then compared 7–9 and demonstrated that nurses’ level of theoretical did not impact on the quality of their pain management practices. 7 Nor did individual nurse’s perceptions of how critical a task was effect the likelihood of them carrying out this task in practice. 8 There was also a difference in self-reported and observed practices 9 ; actual (observed) practices did not confirm to best practice guidelines, whereas self-reported practices tended to.
Example 2: quality of care for complex patients at Nurse Practitioner-Led Clinics (NPLCs)
The other author of this paper (RH) has conducted a multiple-case study to determine the quality of care for patients with complex clinical presentations in NPLCs in Ontario, Canada. 10 Five NPLCs served as individual cases that, together, represented the quatrain. Three types of data were collected including:
Review of documentation related to the NPLC model (media, annual reports, research articles, grey literature and regulatory legislation).
Interviews with nurse practitioners (NPs) practising at the five NPLCs to determine their perceptions of the impact of the NPLC model on the quality of care provided to patients with multimorbidity.
Chart audits conducted at the five NPLCs to determine the extent to which evidence-based guidelines were followed for patients with diabetes and at least one other chronic condition.
The three sources of data collected from the five NPLCs were analysed and themes arose related to the quality of care for complex patients at NPLCs. The multiple-case study confirmed that nurse practitioners are the primary care providers at the NPLCs, and this positively impacts the quality of care for patients with multimorbidity. Healthcare policy, such as lack of an increase in salary for NPs for 10 years, has resulted in issues in recruitment and retention of NPs at NPLCs. This, along with insufficient resources in the communities where NPLCs are located and high patient vulnerability at NPLCs, have a negative impact on the quality of care. 10
These examples illustrate how collecting data about a single case or multiple cases helps us to better understand the phenomenon in question. Case study methodology serves to provide a framework for evaluation and analysis of complex issues. It shines a light on the holistic nature of nursing practice and offers a perspective that informs improved patient care.
- Gustafsson J
- Calanzaro M
- Sandelowski M
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
Read the full text or download the PDF:
- Open access
- Published: 27 June 2011
The case study approach
- Sarah Crowe 1 ,
- Kathrin Cresswell 2 ,
- Ann Robertson 2 ,
- Guro Huby 3 ,
- Anthony Avery 1 &
- Aziz Sheikh 2
BMC Medical Research Methodology volume 11 , Article number: 100 ( 2011 ) Cite this article
807k Accesses
1157 Citations
42 Altmetric
Metrics details
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we reflect on the different types of case study design, the specific research questions this approach can help answer, the data sources that tend to be used, and the particular advantages and disadvantages of employing this methodological approach. The paper concludes with key pointers to aid those designing and appraising proposals for conducting case study research, and a checklist to help readers assess the quality of case study reports.
Peer Review reports
Introduction
The case study approach is particularly useful to employ when there is a need to obtain an in-depth appreciation of an issue, event or phenomenon of interest, in its natural real-life context. Our aim in writing this piece is to provide insights into when to consider employing this approach and an overview of key methodological considerations in relation to the design, planning, analysis, interpretation and reporting of case studies.
The illustrative 'grand round', 'case report' and 'case series' have a long tradition in clinical practice and research. Presenting detailed critiques, typically of one or more patients, aims to provide insights into aspects of the clinical case and, in doing so, illustrate broader lessons that may be learnt. In research, the conceptually-related case study approach can be used, for example, to describe in detail a patient's episode of care, explore professional attitudes to and experiences of a new policy initiative or service development or more generally to 'investigate contemporary phenomena within its real-life context' [ 1 ]. Based on our experiences of conducting a range of case studies, we reflect on when to consider using this approach, discuss the key steps involved and illustrate, with examples, some of the practical challenges of attaining an in-depth understanding of a 'case' as an integrated whole. In keeping with previously published work, we acknowledge the importance of theory to underpin the design, selection, conduct and interpretation of case studies[ 2 ]. In so doing, we make passing reference to the different epistemological approaches used in case study research by key theoreticians and methodologists in this field of enquiry.
This paper is structured around the following main questions: What is a case study? What are case studies used for? How are case studies conducted? What are the potential pitfalls and how can these be avoided? We draw in particular on four of our own recently published examples of case studies (see Tables 1 , 2 , 3 and 4 ) and those of others to illustrate our discussion[ 3 – 7 ].
What is a case study?
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the central tenet being the need to explore an event or phenomenon in depth and in its natural context. It is for this reason sometimes referred to as a "naturalistic" design; this is in contrast to an "experimental" design (such as a randomised controlled trial) in which the investigator seeks to exert control over and manipulate the variable(s) of interest.
Stake's work has been particularly influential in defining the case study approach to scientific enquiry. He has helpfully characterised three main types of case study: intrinsic , instrumental and collective [ 8 ]. An intrinsic case study is typically undertaken to learn about a unique phenomenon. The researcher should define the uniqueness of the phenomenon, which distinguishes it from all others. In contrast, the instrumental case study uses a particular case (some of which may be better than others) to gain a broader appreciation of an issue or phenomenon. The collective case study involves studying multiple cases simultaneously or sequentially in an attempt to generate a still broader appreciation of a particular issue.
These are however not necessarily mutually exclusive categories. In the first of our examples (Table 1 ), we undertook an intrinsic case study to investigate the issue of recruitment of minority ethnic people into the specific context of asthma research studies, but it developed into a instrumental case study through seeking to understand the issue of recruitment of these marginalised populations more generally, generating a number of the findings that are potentially transferable to other disease contexts[ 3 ]. In contrast, the other three examples (see Tables 2 , 3 and 4 ) employed collective case study designs to study the introduction of workforce reconfiguration in primary care, the implementation of electronic health records into hospitals, and to understand the ways in which healthcare students learn about patient safety considerations[ 4 – 6 ]. Although our study focusing on the introduction of General Practitioners with Specialist Interests (Table 2 ) was explicitly collective in design (four contrasting primary care organisations were studied), is was also instrumental in that this particular professional group was studied as an exemplar of the more general phenomenon of workforce redesign[ 4 ].
What are case studies used for?
According to Yin, case studies can be used to explain, describe or explore events or phenomena in the everyday contexts in which they occur[ 1 ]. These can, for example, help to understand and explain causal links and pathways resulting from a new policy initiative or service development (see Tables 2 and 3 , for example)[ 1 ]. In contrast to experimental designs, which seek to test a specific hypothesis through deliberately manipulating the environment (like, for example, in a randomised controlled trial giving a new drug to randomly selected individuals and then comparing outcomes with controls),[ 9 ] the case study approach lends itself well to capturing information on more explanatory ' how ', 'what' and ' why ' questions, such as ' how is the intervention being implemented and received on the ground?'. The case study approach can offer additional insights into what gaps exist in its delivery or why one implementation strategy might be chosen over another. This in turn can help develop or refine theory, as shown in our study of the teaching of patient safety in undergraduate curricula (Table 4 )[ 6 , 10 ]. Key questions to consider when selecting the most appropriate study design are whether it is desirable or indeed possible to undertake a formal experimental investigation in which individuals and/or organisations are allocated to an intervention or control arm? Or whether the wish is to obtain a more naturalistic understanding of an issue? The former is ideally studied using a controlled experimental design, whereas the latter is more appropriately studied using a case study design.
Case studies may be approached in different ways depending on the epistemological standpoint of the researcher, that is, whether they take a critical (questioning one's own and others' assumptions), interpretivist (trying to understand individual and shared social meanings) or positivist approach (orientating towards the criteria of natural sciences, such as focusing on generalisability considerations) (Table 6 ). Whilst such a schema can be conceptually helpful, it may be appropriate to draw on more than one approach in any case study, particularly in the context of conducting health services research. Doolin has, for example, noted that in the context of undertaking interpretative case studies, researchers can usefully draw on a critical, reflective perspective which seeks to take into account the wider social and political environment that has shaped the case[ 11 ].
How are case studies conducted?
Here, we focus on the main stages of research activity when planning and undertaking a case study; the crucial stages are: defining the case; selecting the case(s); collecting and analysing the data; interpreting data; and reporting the findings.
Defining the case
Carefully formulated research question(s), informed by the existing literature and a prior appreciation of the theoretical issues and setting(s), are all important in appropriately and succinctly defining the case[ 8 , 12 ]. Crucially, each case should have a pre-defined boundary which clarifies the nature and time period covered by the case study (i.e. its scope, beginning and end), the relevant social group, organisation or geographical area of interest to the investigator, the types of evidence to be collected, and the priorities for data collection and analysis (see Table 7 )[ 1 ]. A theory driven approach to defining the case may help generate knowledge that is potentially transferable to a range of clinical contexts and behaviours; using theory is also likely to result in a more informed appreciation of, for example, how and why interventions have succeeded or failed[ 13 ].
For example, in our evaluation of the introduction of electronic health records in English hospitals (Table 3 ), we defined our cases as the NHS Trusts that were receiving the new technology[ 5 ]. Our focus was on how the technology was being implemented. However, if the primary research interest had been on the social and organisational dimensions of implementation, we might have defined our case differently as a grouping of healthcare professionals (e.g. doctors and/or nurses). The precise beginning and end of the case may however prove difficult to define. Pursuing this same example, when does the process of implementation and adoption of an electronic health record system really begin or end? Such judgements will inevitably be influenced by a range of factors, including the research question, theory of interest, the scope and richness of the gathered data and the resources available to the research team.
Selecting the case(s)
The decision on how to select the case(s) to study is a very important one that merits some reflection. In an intrinsic case study, the case is selected on its own merits[ 8 ]. The case is selected not because it is representative of other cases, but because of its uniqueness, which is of genuine interest to the researchers. This was, for example, the case in our study of the recruitment of minority ethnic participants into asthma research (Table 1 ) as our earlier work had demonstrated the marginalisation of minority ethnic people with asthma, despite evidence of disproportionate asthma morbidity[ 14 , 15 ]. In another example of an intrinsic case study, Hellstrom et al.[ 16 ] studied an elderly married couple living with dementia to explore how dementia had impacted on their understanding of home, their everyday life and their relationships.
For an instrumental case study, selecting a "typical" case can work well[ 8 ]. In contrast to the intrinsic case study, the particular case which is chosen is of less importance than selecting a case that allows the researcher to investigate an issue or phenomenon. For example, in order to gain an understanding of doctors' responses to health policy initiatives, Som undertook an instrumental case study interviewing clinicians who had a range of responsibilities for clinical governance in one NHS acute hospital trust[ 17 ]. Sampling a "deviant" or "atypical" case may however prove even more informative, potentially enabling the researcher to identify causal processes, generate hypotheses and develop theory.
In collective or multiple case studies, a number of cases are carefully selected. This offers the advantage of allowing comparisons to be made across several cases and/or replication. Choosing a "typical" case may enable the findings to be generalised to theory (i.e. analytical generalisation) or to test theory by replicating the findings in a second or even a third case (i.e. replication logic)[ 1 ]. Yin suggests two or three literal replications (i.e. predicting similar results) if the theory is straightforward and five or more if the theory is more subtle. However, critics might argue that selecting 'cases' in this way is insufficiently reflexive and ill-suited to the complexities of contemporary healthcare organisations.
The selected case study site(s) should allow the research team access to the group of individuals, the organisation, the processes or whatever else constitutes the chosen unit of analysis for the study. Access is therefore a central consideration; the researcher needs to come to know the case study site(s) well and to work cooperatively with them. Selected cases need to be not only interesting but also hospitable to the inquiry [ 8 ] if they are to be informative and answer the research question(s). Case study sites may also be pre-selected for the researcher, with decisions being influenced by key stakeholders. For example, our selection of case study sites in the evaluation of the implementation and adoption of electronic health record systems (see Table 3 ) was heavily influenced by NHS Connecting for Health, the government agency that was responsible for overseeing the National Programme for Information Technology (NPfIT)[ 5 ]. This prominent stakeholder had already selected the NHS sites (through a competitive bidding process) to be early adopters of the electronic health record systems and had negotiated contracts that detailed the deployment timelines.
It is also important to consider in advance the likely burden and risks associated with participation for those who (or the site(s) which) comprise the case study. Of particular importance is the obligation for the researcher to think through the ethical implications of the study (e.g. the risk of inadvertently breaching anonymity or confidentiality) and to ensure that potential participants/participating sites are provided with sufficient information to make an informed choice about joining the study. The outcome of providing this information might be that the emotive burden associated with participation, or the organisational disruption associated with supporting the fieldwork, is considered so high that the individuals or sites decide against participation.
In our example of evaluating implementations of electronic health record systems, given the restricted number of early adopter sites available to us, we sought purposively to select a diverse range of implementation cases among those that were available[ 5 ]. We chose a mixture of teaching, non-teaching and Foundation Trust hospitals, and examples of each of the three electronic health record systems procured centrally by the NPfIT. At one recruited site, it quickly became apparent that access was problematic because of competing demands on that organisation. Recognising the importance of full access and co-operative working for generating rich data, the research team decided not to pursue work at that site and instead to focus on other recruited sites.
Collecting the data
In order to develop a thorough understanding of the case, the case study approach usually involves the collection of multiple sources of evidence, using a range of quantitative (e.g. questionnaires, audits and analysis of routinely collected healthcare data) and more commonly qualitative techniques (e.g. interviews, focus groups and observations). The use of multiple sources of data (data triangulation) has been advocated as a way of increasing the internal validity of a study (i.e. the extent to which the method is appropriate to answer the research question)[ 8 , 18 – 21 ]. An underlying assumption is that data collected in different ways should lead to similar conclusions, and approaching the same issue from different angles can help develop a holistic picture of the phenomenon (Table 2 )[ 4 ].
Brazier and colleagues used a mixed-methods case study approach to investigate the impact of a cancer care programme[ 22 ]. Here, quantitative measures were collected with questionnaires before, and five months after, the start of the intervention which did not yield any statistically significant results. Qualitative interviews with patients however helped provide an insight into potentially beneficial process-related aspects of the programme, such as greater, perceived patient involvement in care. The authors reported how this case study approach provided a number of contextual factors likely to influence the effectiveness of the intervention and which were not likely to have been obtained from quantitative methods alone.
In collective or multiple case studies, data collection needs to be flexible enough to allow a detailed description of each individual case to be developed (e.g. the nature of different cancer care programmes), before considering the emerging similarities and differences in cross-case comparisons (e.g. to explore why one programme is more effective than another). It is important that data sources from different cases are, where possible, broadly comparable for this purpose even though they may vary in nature and depth.
Analysing, interpreting and reporting case studies
Making sense and offering a coherent interpretation of the typically disparate sources of data (whether qualitative alone or together with quantitative) is far from straightforward. Repeated reviewing and sorting of the voluminous and detail-rich data are integral to the process of analysis. In collective case studies, it is helpful to analyse data relating to the individual component cases first, before making comparisons across cases. Attention needs to be paid to variations within each case and, where relevant, the relationship between different causes, effects and outcomes[ 23 ]. Data will need to be organised and coded to allow the key issues, both derived from the literature and emerging from the dataset, to be easily retrieved at a later stage. An initial coding frame can help capture these issues and can be applied systematically to the whole dataset with the aid of a qualitative data analysis software package.
The Framework approach is a practical approach, comprising of five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) , to managing and analysing large datasets particularly if time is limited, as was the case in our study of recruitment of South Asians into asthma research (Table 1 )[ 3 , 24 ]. Theoretical frameworks may also play an important role in integrating different sources of data and examining emerging themes. For example, we drew on a socio-technical framework to help explain the connections between different elements - technology; people; and the organisational settings within which they worked - in our study of the introduction of electronic health record systems (Table 3 )[ 5 ]. Our study of patient safety in undergraduate curricula drew on an evaluation-based approach to design and analysis, which emphasised the importance of the academic, organisational and practice contexts through which students learn (Table 4 )[ 6 ].
Case study findings can have implications both for theory development and theory testing. They may establish, strengthen or weaken historical explanations of a case and, in certain circumstances, allow theoretical (as opposed to statistical) generalisation beyond the particular cases studied[ 12 ]. These theoretical lenses should not, however, constitute a strait-jacket and the cases should not be "forced to fit" the particular theoretical framework that is being employed.
When reporting findings, it is important to provide the reader with enough contextual information to understand the processes that were followed and how the conclusions were reached. In a collective case study, researchers may choose to present the findings from individual cases separately before amalgamating across cases. Care must be taken to ensure the anonymity of both case sites and individual participants (if agreed in advance) by allocating appropriate codes or withholding descriptors. In the example given in Table 3 , we decided against providing detailed information on the NHS sites and individual participants in order to avoid the risk of inadvertent disclosure of identities[ 5 , 25 ].
What are the potential pitfalls and how can these be avoided?
The case study approach is, as with all research, not without its limitations. When investigating the formal and informal ways undergraduate students learn about patient safety (Table 4 ), for example, we rapidly accumulated a large quantity of data. The volume of data, together with the time restrictions in place, impacted on the depth of analysis that was possible within the available resources. This highlights a more general point of the importance of avoiding the temptation to collect as much data as possible; adequate time also needs to be set aside for data analysis and interpretation of what are often highly complex datasets.
Case study research has sometimes been criticised for lacking scientific rigour and providing little basis for generalisation (i.e. producing findings that may be transferable to other settings)[ 1 ]. There are several ways to address these concerns, including: the use of theoretical sampling (i.e. drawing on a particular conceptual framework); respondent validation (i.e. participants checking emerging findings and the researcher's interpretation, and providing an opinion as to whether they feel these are accurate); and transparency throughout the research process (see Table 8 )[ 8 , 18 – 21 , 23 , 26 ]. Transparency can be achieved by describing in detail the steps involved in case selection, data collection, the reasons for the particular methods chosen, and the researcher's background and level of involvement (i.e. being explicit about how the researcher has influenced data collection and interpretation). Seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, help readers to judge the trustworthiness of the case study report. Stake provides a critique checklist for a case study report (Table 9 )[ 8 ].
Conclusions
The case study approach allows, amongst other things, critical events, interventions, policy developments and programme-based service reforms to be studied in detail in a real-life context. It should therefore be considered when an experimental design is either inappropriate to answer the research questions posed or impossible to undertake. Considering the frequency with which implementations of innovations are now taking place in healthcare settings and how well the case study approach lends itself to in-depth, complex health service research, we believe this approach should be more widely considered by researchers. Though inherently challenging, the research case study can, if carefully conceptualised and thoughtfully undertaken and reported, yield powerful insights into many important aspects of health and healthcare delivery.
Yin RK: Case study research, design and method. 2009, London: Sage Publications Ltd., 4
Google Scholar
Keen J, Packwood T: Qualitative research; case study evaluation. BMJ. 1995, 311: 444-446.
Article CAS PubMed PubMed Central Google Scholar
Sheikh A, Halani L, Bhopal R, Netuveli G, Partridge M, Car J, et al: Facilitating the Recruitment of Minority Ethnic People into Research: Qualitative Case Study of South Asians and Asthma. PLoS Med. 2009, 6 (10): 1-11.
Article Google Scholar
Pinnock H, Huby G, Powell A, Kielmann T, Price D, Williams S, et al: The process of planning, development and implementation of a General Practitioner with a Special Interest service in Primary Care Organisations in England and Wales: a comparative prospective case study. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). 2008, [ http://www.sdo.nihr.ac.uk/files/project/99-final-report.pdf ]
Robertson A, Cresswell K, Takian A, Petrakaki D, Crowe S, Cornford T, et al: Prospective evaluation of the implementation and adoption of NHS Connecting for Health's national electronic health record in secondary care in England: interim findings. BMJ. 2010, 41: c4564-
Pearson P, Steven A, Howe A, Sheikh A, Ashcroft D, Smith P, the Patient Safety Education Study Group: Learning about patient safety: organisational context and culture in the education of healthcare professionals. J Health Serv Res Policy. 2010, 15: 4-10. 10.1258/jhsrp.2009.009052.
Article PubMed Google Scholar
van Harten WH, Casparie TF, Fisscher OA: The evaluation of the introduction of a quality management system: a process-oriented case study in a large rehabilitation hospital. Health Policy. 2002, 60 (1): 17-37. 10.1016/S0168-8510(01)00187-7.
Stake RE: The art of case study research. 1995, London: Sage Publications Ltd.
Sheikh A, Smeeth L, Ashcroft R: Randomised controlled trials in primary care: scope and application. Br J Gen Pract. 2002, 52 (482): 746-51.
PubMed PubMed Central Google Scholar
King G, Keohane R, Verba S: Designing Social Inquiry. 1996, Princeton: Princeton University Press
Doolin B: Information technology as disciplinary technology: being critical in interpretative research on information systems. Journal of Information Technology. 1998, 13: 301-311. 10.1057/jit.1998.8.
George AL, Bennett A: Case studies and theory development in the social sciences. 2005, Cambridge, MA: MIT Press
Eccles M, the Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG): Designing theoretically-informed implementation interventions. Implementation Science. 2006, 1: 1-8. 10.1186/1748-5908-1-1.
Article PubMed Central Google Scholar
Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, Sheikh A: Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005, 365 (9456): 312-7.
Sheikh A, Panesar SS, Lasserson T, Netuveli G: Recruitment of ethnic minorities to asthma studies. Thorax. 2004, 59 (7): 634-
CAS PubMed PubMed Central Google Scholar
Hellström I, Nolan M, Lundh U: 'We do things together': A case study of 'couplehood' in dementia. Dementia. 2005, 4: 7-22. 10.1177/1471301205049188.
Som CV: Nothing seems to have changed, nothing seems to be changing and perhaps nothing will change in the NHS: doctors' response to clinical governance. International Journal of Public Sector Management. 2005, 18: 463-477. 10.1108/09513550510608903.
Lincoln Y, Guba E: Naturalistic inquiry. 1985, Newbury Park: Sage Publications
Barbour RS: Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ. 2001, 322: 1115-1117. 10.1136/bmj.322.7294.1115.
Mays N, Pope C: Qualitative research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mason J: Qualitative researching. 2002, London: Sage
Brazier A, Cooke K, Moravan V: Using Mixed Methods for Evaluating an Integrative Approach to Cancer Care: A Case Study. Integr Cancer Ther. 2008, 7: 5-17. 10.1177/1534735407313395.
Miles MB, Huberman M: Qualitative data analysis: an expanded sourcebook. 1994, CA: Sage Publications Inc., 2
Pope C, Ziebland S, Mays N: Analysing qualitative data. Qualitative research in health care. BMJ. 2000, 320: 114-116. 10.1136/bmj.320.7227.114.
Cresswell KM, Worth A, Sheikh A: Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Med Inform Decis Mak. 2010, 10 (1): 67-10.1186/1472-6947-10-67.
Article PubMed PubMed Central Google Scholar
Malterud K: Qualitative research: standards, challenges, and guidelines. Lancet. 2001, 358: 483-488. 10.1016/S0140-6736(01)05627-6.
Article CAS PubMed Google Scholar
Yin R: Case study research: design and methods. 1994, Thousand Oaks, CA: Sage Publishing, 2
Yin R: Enhancing the quality of case studies in health services research. Health Serv Res. 1999, 34: 1209-1224.
Green J, Thorogood N: Qualitative methods for health research. 2009, Los Angeles: Sage, 2
Howcroft D, Trauth E: Handbook of Critical Information Systems Research, Theory and Application. 2005, Cheltenham, UK: Northampton, MA, USA: Edward Elgar
Book Google Scholar
Blakie N: Approaches to Social Enquiry. 1993, Cambridge: Polity Press
Doolin B: Power and resistance in the implementation of a medical management information system. Info Systems J. 2004, 14: 343-362. 10.1111/j.1365-2575.2004.00176.x.
Bloomfield BP, Best A: Management consultants: systems development, power and the translation of problems. Sociological Review. 1992, 40: 533-560.
Shanks G, Parr A: Positivist, single case study research in information systems: A critical analysis. Proceedings of the European Conference on Information Systems. 2003, Naples
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/11/100/prepub
Download references
Acknowledgements
We are grateful to the participants and colleagues who contributed to the individual case studies that we have drawn on. This work received no direct funding, but it has been informed by projects funded by Asthma UK, the NHS Service Delivery Organisation, NHS Connecting for Health Evaluation Programme, and Patient Safety Research Portfolio. We would also like to thank the expert reviewers for their insightful and constructive feedback. Our thanks are also due to Dr. Allison Worth who commented on an earlier draft of this manuscript.
Author information
Authors and affiliations.
Division of Primary Care, The University of Nottingham, Nottingham, UK
Sarah Crowe & Anthony Avery
Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK
Kathrin Cresswell, Ann Robertson & Aziz Sheikh
School of Health in Social Science, The University of Edinburgh, Edinburgh, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarah Crowe .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
AS conceived this article. SC, KC and AR wrote this paper with GH, AA and AS all commenting on various drafts. SC and AS are guarantors.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Crowe, S., Cresswell, K., Robertson, A. et al. The case study approach. BMC Med Res Methodol 11 , 100 (2011). https://doi.org/10.1186/1471-2288-11-100
Download citation
Received : 29 November 2010
Accepted : 27 June 2011
Published : 27 June 2011
DOI : https://doi.org/10.1186/1471-2288-11-100
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case Study Approach
- Electronic Health Record System
- Case Study Design
- Case Study Site
- Case Study Report
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
Journal of Medical Case Reports
In the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge. Prof Michael Kidd AO, Editor-in- Chief

Join the Editorial Board
We are recruiting Associate Editors to join our Editorial Board. Learn more about the role and how to apply here !

- Meet the Editors
Get to know the Editors behind Journal of Medical Case Reports !

megaflopp / Getty Images / iStock
Requirements for case reports submitted to JMCR
• Patient ethnicity must be included in the Abstract under the Case Presentation section.
• Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Report of the Month
Superior mesenteric vein thrombosis due to covid-19 vaccination.
Vaccines have made a significant contribute to sowing the spread of the COVID-19 infection. However, side effects of the vaccination are beginning to appear, and one of which, thrombosis, is a particular problem as it can cuase serious complications. While cases of splanchnic venous thrombosis (SVT) after ChAdOx1 nCoV-19 vaccinations have been reported, cases of SVT mRNA-1273 vaccines are rare.
In this case report, clinicians describe a patient presenting with superior mesentric vein thrombosis following a COVID-19 vaccination, and examine the relationship between the mRNA-1273 vaccines and intestinal ischemia.
- Most accessed
Dysphasia: metastatic prostate cancer to the leptomeninges: a case report
Authors: Diana Jodeh, Milan Terzic, Evan Wenig, Amit Amin and Tareq Al Baghdadi
Reperfusion injury case following cervical fusion with OPLL: a case report and literature review
Authors: Assil Mahamid, Sana Zahalka, David Maman, Liad Alfandari, Amit Keren and Eyal Behrbalk
Triple immunostaining demonstrates the possible existence of segregated-nucleus-containing atypical monocytes in human primary myelofibrosis bone marrow: a case report
Authors: Shunsuke Homma, Toshie Ogasawara, Michie Suga, Yoshiyasu Nakamura, Katsuya Takenaka, Shoko Marshall, Kiyotaka Kawauchi, Naoki Mori, Hajime Kuroda, Naoya Nakamura, Yohei Miyagi and Atsuko Masunaga
Dramatic improvement of drug-resistant epilepsy following cerebral infarction: a case report
Authors: Wankiun Lee, Daeyoung Kim, Jae-Moon Kim and Eun Young Kim
Congenital partial diaphragmatic eventration presenting with Chilaiditi’s sign: a case report
Authors: B. Haluk Güvenç and Kemal Rasa
Most recent articles RSS
View all articles
An itchy erythematous papular skin rash as a possible early sign of COVID-19: a case report
Authors: Alice Serafini, Peter Konstantin Kurotschka, Mariabeatrice Bertolani and Silvia Riccomi
Red ear syndrome precipitated by a dietary trigger: a case report
Authors: Chung Chi Chan and Susmita Ghosh
How to choose the best journal for your case report
Authors: Richard A. Rison, Jennifer Kelly Shepphird and Michael R. Kidd
The Erratum to this article has been published in Journal of Medical Case Reports 2017 11 :287
COVID-19 with repeated positive test results for SARS-CoV-2 by PCR and then negative test results twice during intensive care: a case report
Authors: Masafumi Kanamoto, Masaru Tobe, Tomonori Takazawa and Shigeru Saito
Triphallia: the first cadaveric description of internal penile triplication: a case report
Authors: John Buchanan, Madeleine Gadd, Rose How, Edward Mathews, Andre Coetzee and Karuna Katti
Most accessed articles RSS
A Guide to Writing and Using Case Reports
This thematic series, published in 2016, provides a valuable resource for clinicians who are considered producing a case report. It comprises of a special editorial series of guides on writing, reviewing and using case reports.

Aims and scope
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports.
Case reports should show one of the following:
- Unreported or unusual side effects or adverse interactions involving medications
- Unexpected or unusual presentations of a disease
- New associations or variations in disease processes
- Presentations, diagnoses and/or management of new and emerging diseases
- An unexpected association between diseases or symptoms
- An unexpected event in the course of observing or treating a patient
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients.
Article accesses
Throughout 2022, articles were accessed from the journal website more than 4.17 million times; an average of over 11 ,400 accesses per day.
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Peer Review Mentoring Scheme
The Editors at Journal of Medical Case Reports endorse peer review mentoring of early career researchers.
If you are a senior researcher or professor and supervise an early career researcher with the appropriate expertise, we invite you to co-write and mentor them through the peer review process. Find out how to express your interest in the scheme here .
Call for Papers
The Journal of Medical Case Reports is calling for submissions to our Collection on COVID-19 – a look at the past, present and future of the pandemic . Guest Edited by Dr. Jean Karl Soler, The Family Practice Malta, Malta
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 0.9 5-year Journal Impact Factor: N/A Source Normalized Impact per Paper (SNIP): 0.591 SCImago Journal Rank (SJR): 0.304
Speed 2023 Submission to first editorial decision (median days): 33 Submission to acceptance (median days): 148
Usage 2023 Downloads: 4,048,208 Altmetric mentions: 2,745
- More about our metrics

- Follow us on Twitter
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Case Study Research Method in Psychology
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews).
The case study research method originated in clinical medicine (the case history, i.e., the patient’s personal history). In psychology, case studies are often confined to the study of a particular individual.
The information is mainly biographical and relates to events in the individual’s past (i.e., retrospective), as well as to significant events that are currently occurring in his or her everyday life.
The case study is not a research method, but researchers select methods of data collection and analysis that will generate material suitable for case studies.
Freud (1909a, 1909b) conducted very detailed investigations into the private lives of his patients in an attempt to both understand and help them overcome their illnesses.
This makes it clear that the case study is a method that should only be used by a psychologist, therapist, or psychiatrist, i.e., someone with a professional qualification.
There is an ethical issue of competence. Only someone qualified to diagnose and treat a person can conduct a formal case study relating to atypical (i.e., abnormal) behavior or atypical development.
Famous Case Studies
- Anna O – One of the most famous case studies, documenting psychoanalyst Josef Breuer’s treatment of “Anna O” (real name Bertha Pappenheim) for hysteria in the late 1800s using early psychoanalytic theory.
- Little Hans – A child psychoanalysis case study published by Sigmund Freud in 1909 analyzing his five-year-old patient Herbert Graf’s house phobia as related to the Oedipus complex.
- Bruce/Brenda – Gender identity case of the boy (Bruce) whose botched circumcision led psychologist John Money to advise gender reassignment and raise him as a girl (Brenda) in the 1960s.
- Genie Wiley – Linguistics/psychological development case of the victim of extreme isolation abuse who was studied in 1970s California for effects of early language deprivation on acquiring speech later in life.
- Phineas Gage – One of the most famous neuropsychology case studies analyzes personality changes in railroad worker Phineas Gage after an 1848 brain injury involving a tamping iron piercing his skull.
Clinical Case Studies
- Studying the effectiveness of psychotherapy approaches with an individual patient
- Assessing and treating mental illnesses like depression, anxiety disorders, PTSD
- Neuropsychological cases investigating brain injuries or disorders
Child Psychology Case Studies
- Studying psychological development from birth through adolescence
- Cases of learning disabilities, autism spectrum disorders, ADHD
- Effects of trauma, abuse, deprivation on development
Types of Case Studies
- Explanatory case studies : Used to explore causation in order to find underlying principles. Helpful for doing qualitative analysis to explain presumed causal links.
- Exploratory case studies : Used to explore situations where an intervention being evaluated has no clear set of outcomes. It helps define questions and hypotheses for future research.
- Descriptive case studies : Describe an intervention or phenomenon and the real-life context in which it occurred. It is helpful for illustrating certain topics within an evaluation.
- Multiple-case studies : Used to explore differences between cases and replicate findings across cases. Helpful for comparing and contrasting specific cases.
- Intrinsic : Used to gain a better understanding of a particular case. Helpful for capturing the complexity of a single case.
- Collective : Used to explore a general phenomenon using multiple case studies. Helpful for jointly studying a group of cases in order to inquire into the phenomenon.
Where Do You Find Data for a Case Study?
There are several places to find data for a case study. The key is to gather data from multiple sources to get a complete picture of the case and corroborate facts or findings through triangulation of evidence. Most of this information is likely qualitative (i.e., verbal description rather than measurement), but the psychologist might also collect numerical data.
1. Primary sources
- Interviews – Interviewing key people related to the case to get their perspectives and insights. The interview is an extremely effective procedure for obtaining information about an individual, and it may be used to collect comments from the person’s friends, parents, employer, workmates, and others who have a good knowledge of the person, as well as to obtain facts from the person him or herself.
- Observations – Observing behaviors, interactions, processes, etc., related to the case as they unfold in real-time.
- Documents & Records – Reviewing private documents, diaries, public records, correspondence, meeting minutes, etc., relevant to the case.
2. Secondary sources
- News/Media – News coverage of events related to the case study.
- Academic articles – Journal articles, dissertations etc. that discuss the case.
- Government reports – Official data and records related to the case context.
- Books/films – Books, documentaries or films discussing the case.
3. Archival records
Searching historical archives, museum collections and databases to find relevant documents, visual/audio records related to the case history and context.
Public archives like newspapers, organizational records, photographic collections could all include potentially relevant pieces of information to shed light on attitudes, cultural perspectives, common practices and historical contexts related to psychology.
4. Organizational records
Organizational records offer the advantage of often having large datasets collected over time that can reveal or confirm psychological insights.
Of course, privacy and ethical concerns regarding confidential data must be navigated carefully.
However, with proper protocols, organizational records can provide invaluable context and empirical depth to qualitative case studies exploring the intersection of psychology and organizations.
- Organizational/industrial psychology research : Organizational records like employee surveys, turnover/retention data, policies, incident reports etc. may provide insight into topics like job satisfaction, workplace culture and dynamics, leadership issues, employee behaviors etc.
- Clinical psychology : Therapists/hospitals may grant access to anonymized medical records to study aspects like assessments, diagnoses, treatment plans etc. This could shed light on clinical practices.
- School psychology : Studies could utilize anonymized student records like test scores, grades, disciplinary issues, and counseling referrals to study child development, learning barriers, effectiveness of support programs, and more.
How do I Write a Case Study in Psychology?
Follow specified case study guidelines provided by a journal or your psychology tutor. General components of clinical case studies include: background, symptoms, assessments, diagnosis, treatment, and outcomes. Interpreting the information means the researcher decides what to include or leave out. A good case study should always clarify which information is the factual description and which is an inference or the researcher’s opinion.
1. Introduction
- Provide background on the case context and why it is of interest, presenting background information like demographics, relevant history, and presenting problem.
- Compare briefly to similar published cases if applicable. Clearly state the focus/importance of the case.
2. Case Presentation
- Describe the presenting problem in detail, including symptoms, duration,and impact on daily life.
- Include client demographics like age and gender, information about social relationships, and mental health history.
- Describe all physical, emotional, and/or sensory symptoms reported by the client.
- Use patient quotes to describe the initial complaint verbatim. Follow with full-sentence summaries of relevant history details gathered, including key components that led to a working diagnosis.
- Summarize clinical exam results, namely orthopedic/neurological tests, imaging, lab tests, etc. Note actual results rather than subjective conclusions. Provide images if clearly reproducible/anonymized.
- Clearly state the working diagnosis or clinical impression before transitioning to management.
3. Management and Outcome
- Indicate the total duration of care and number of treatments given over what timeframe. Use specific names/descriptions for any therapies/interventions applied.
- Present the results of the intervention,including any quantitative or qualitative data collected.
- For outcomes, utilize visual analog scales for pain, medication usage logs, etc., if possible. Include patient self-reports of improvement/worsening of symptoms. Note the reason for discharge/end of care.
4. Discussion
- Analyze the case, exploring contributing factors, limitations of the study, and connections to existing research.
- Analyze the effectiveness of the intervention,considering factors like participant adherence, limitations of the study, and potential alternative explanations for the results.
- Identify any questions raised in the case analysis and relate insights to established theories and current research if applicable. Avoid definitive claims about physiological explanations.
- Offer clinical implications, and suggest future research directions.
5. Additional Items
- Thank specific assistants for writing support only. No patient acknowledgments.
- References should directly support any key claims or quotes included.
- Use tables/figures/images only if substantially informative. Include permissions and legends/explanatory notes.
- Provides detailed (rich qualitative) information.
- Provides insight for further research.
- Permitting investigation of otherwise impractical (or unethical) situations.
Case studies allow a researcher to investigate a topic in far more detail than might be possible if they were trying to deal with a large number of research participants (nomothetic approach) with the aim of ‘averaging’.
Because of their in-depth, multi-sided approach, case studies often shed light on aspects of human thinking and behavior that would be unethical or impractical to study in other ways.
Research that only looks into the measurable aspects of human behavior is not likely to give us insights into the subjective dimension of experience, which is important to psychoanalytic and humanistic psychologists.
Case studies are often used in exploratory research. They can help us generate new ideas (that might be tested by other methods). They are an important way of illustrating theories and can help show how different aspects of a person’s life are related to each other.
The method is, therefore, important for psychologists who adopt a holistic point of view (i.e., humanistic psychologists ).
Limitations
- Lacking scientific rigor and providing little basis for generalization of results to the wider population.
- Researchers’ own subjective feelings may influence the case study (researcher bias).
- Difficult to replicate.
- Time-consuming and expensive.
- The volume of data, together with the time restrictions in place, impacted the depth of analysis that was possible within the available resources.
Because a case study deals with only one person/event/group, we can never be sure if the case study investigated is representative of the wider body of “similar” instances. This means the conclusions drawn from a particular case may not be transferable to other settings.
Because case studies are based on the analysis of qualitative (i.e., descriptive) data , a lot depends on the psychologist’s interpretation of the information she has acquired.
This means that there is a lot of scope for Anna O , and it could be that the subjective opinions of the psychologist intrude in the assessment of what the data means.
For example, Freud has been criticized for producing case studies in which the information was sometimes distorted to fit particular behavioral theories (e.g., Little Hans ).
This is also true of Money’s interpretation of the Bruce/Brenda case study (Diamond, 1997) when he ignored evidence that went against his theory.
Breuer, J., & Freud, S. (1895). Studies on hysteria . Standard Edition 2: London.
Curtiss, S. (1981). Genie: The case of a modern wild child .
Diamond, M., & Sigmundson, K. (1997). Sex Reassignment at Birth: Long-term Review and Clinical Implications. Archives of Pediatrics & Adolescent Medicine , 151(3), 298-304
Freud, S. (1909a). Analysis of a phobia of a five year old boy. In The Pelican Freud Library (1977), Vol 8, Case Histories 1, pages 169-306
Freud, S. (1909b). Bemerkungen über einen Fall von Zwangsneurose (Der “Rattenmann”). Jb. psychoanal. psychopathol. Forsch ., I, p. 357-421; GW, VII, p. 379-463; Notes upon a case of obsessional neurosis, SE , 10: 151-318.
Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Money, J., & Ehrhardt, A. A. (1972). Man & Woman, Boy & Girl : The Differentiation and Dimorphism of Gender Identity from Conception to Maturity. Baltimore, Maryland: Johns Hopkins University Press.
Money, J., & Tucker, P. (1975). Sexual signatures: On being a man or a woman.
Further Information
- Case Study Approach
- Case Study Method
- Enhancing the Quality of Case Studies in Health Services Research
- “We do things together” A case study of “couplehood” in dementia
- Using mixed methods for evaluating an integrative approach to cancer care: a case study
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
What Is a Case Study?
Weighing the pros and cons of this method of research
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Cara Lustik is a fact-checker and copywriter.
:max_bytes(150000):strip_icc():format(webp)/Cara-Lustik-1000-77abe13cf6c14a34a58c2a0ffb7297da.jpg)
Verywell / Colleen Tighe
- Pros and Cons
What Types of Case Studies Are Out There?
Where do you find data for a case study, how do i write a psychology case study.
A case study is an in-depth study of one person, group, or event. In a case study, nearly every aspect of the subject's life and history is analyzed to seek patterns and causes of behavior. Case studies can be used in many different fields, including psychology, medicine, education, anthropology, political science, and social work.
The point of a case study is to learn as much as possible about an individual or group so that the information can be generalized to many others. Unfortunately, case studies tend to be highly subjective, and it is sometimes difficult to generalize results to a larger population.
While case studies focus on a single individual or group, they follow a format similar to other types of psychology writing. If you are writing a case study, we got you—here are some rules of APA format to reference.
At a Glance
A case study, or an in-depth study of a person, group, or event, can be a useful research tool when used wisely. In many cases, case studies are best used in situations where it would be difficult or impossible for you to conduct an experiment. They are helpful for looking at unique situations and allow researchers to gather a lot of˜ information about a specific individual or group of people. However, it's important to be cautious of any bias we draw from them as they are highly subjective.
What Are the Benefits and Limitations of Case Studies?
A case study can have its strengths and weaknesses. Researchers must consider these pros and cons before deciding if this type of study is appropriate for their needs.
One of the greatest advantages of a case study is that it allows researchers to investigate things that are often difficult or impossible to replicate in a lab. Some other benefits of a case study:
- Allows researchers to capture information on the 'how,' 'what,' and 'why,' of something that's implemented
- Gives researchers the chance to collect information on why one strategy might be chosen over another
- Permits researchers to develop hypotheses that can be explored in experimental research
On the other hand, a case study can have some drawbacks:
- It cannot necessarily be generalized to the larger population
- Cannot demonstrate cause and effect
- It may not be scientifically rigorous
- It can lead to bias
Researchers may choose to perform a case study if they want to explore a unique or recently discovered phenomenon. Through their insights, researchers develop additional ideas and study questions that might be explored in future studies.
It's important to remember that the insights from case studies cannot be used to determine cause-and-effect relationships between variables. However, case studies may be used to develop hypotheses that can then be addressed in experimental research.
Case Study Examples
There have been a number of notable case studies in the history of psychology. Much of Freud's work and theories were developed through individual case studies. Some great examples of case studies in psychology include:
- Anna O : Anna O. was a pseudonym of a woman named Bertha Pappenheim, a patient of a physician named Josef Breuer. While she was never a patient of Freud's, Freud and Breuer discussed her case extensively. The woman was experiencing symptoms of a condition that was then known as hysteria and found that talking about her problems helped relieve her symptoms. Her case played an important part in the development of talk therapy as an approach to mental health treatment.
- Phineas Gage : Phineas Gage was a railroad employee who experienced a terrible accident in which an explosion sent a metal rod through his skull, damaging important portions of his brain. Gage recovered from his accident but was left with serious changes in both personality and behavior.
- Genie : Genie was a young girl subjected to horrific abuse and isolation. The case study of Genie allowed researchers to study whether language learning was possible, even after missing critical periods for language development. Her case also served as an example of how scientific research may interfere with treatment and lead to further abuse of vulnerable individuals.
Such cases demonstrate how case research can be used to study things that researchers could not replicate in experimental settings. In Genie's case, her horrific abuse denied her the opportunity to learn a language at critical points in her development.
This is clearly not something researchers could ethically replicate, but conducting a case study on Genie allowed researchers to study phenomena that are otherwise impossible to reproduce.
There are a few different types of case studies that psychologists and other researchers might use:
- Collective case studies : These involve studying a group of individuals. Researchers might study a group of people in a certain setting or look at an entire community. For example, psychologists might explore how access to resources in a community has affected the collective mental well-being of those who live there.
- Descriptive case studies : These involve starting with a descriptive theory. The subjects are then observed, and the information gathered is compared to the pre-existing theory.
- Explanatory case studies : These are often used to do causal investigations. In other words, researchers are interested in looking at factors that may have caused certain things to occur.
- Exploratory case studies : These are sometimes used as a prelude to further, more in-depth research. This allows researchers to gather more information before developing their research questions and hypotheses .
- Instrumental case studies : These occur when the individual or group allows researchers to understand more than what is initially obvious to observers.
- Intrinsic case studies : This type of case study is when the researcher has a personal interest in the case. Jean Piaget's observations of his own children are good examples of how an intrinsic case study can contribute to the development of a psychological theory.
The three main case study types often used are intrinsic, instrumental, and collective. Intrinsic case studies are useful for learning about unique cases. Instrumental case studies help look at an individual to learn more about a broader issue. A collective case study can be useful for looking at several cases simultaneously.
The type of case study that psychology researchers use depends on the unique characteristics of the situation and the case itself.
There are a number of different sources and methods that researchers can use to gather information about an individual or group. Six major sources that have been identified by researchers are:
- Archival records : Census records, survey records, and name lists are examples of archival records.
- Direct observation : This strategy involves observing the subject, often in a natural setting . While an individual observer is sometimes used, it is more common to utilize a group of observers.
- Documents : Letters, newspaper articles, administrative records, etc., are the types of documents often used as sources.
- Interviews : Interviews are one of the most important methods for gathering information in case studies. An interview can involve structured survey questions or more open-ended questions.
- Participant observation : When the researcher serves as a participant in events and observes the actions and outcomes, it is called participant observation.
- Physical artifacts : Tools, objects, instruments, and other artifacts are often observed during a direct observation of the subject.
If you have been directed to write a case study for a psychology course, be sure to check with your instructor for any specific guidelines you need to follow. If you are writing your case study for a professional publication, check with the publisher for their specific guidelines for submitting a case study.
Here is a general outline of what should be included in a case study.
Section 1: A Case History
This section will have the following structure and content:
Background information : The first section of your paper will present your client's background. Include factors such as age, gender, work, health status, family mental health history, family and social relationships, drug and alcohol history, life difficulties, goals, and coping skills and weaknesses.
Description of the presenting problem : In the next section of your case study, you will describe the problem or symptoms that the client presented with.
Describe any physical, emotional, or sensory symptoms reported by the client. Thoughts, feelings, and perceptions related to the symptoms should also be noted. Any screening or diagnostic assessments that are used should also be described in detail and all scores reported.
Your diagnosis : Provide your diagnosis and give the appropriate Diagnostic and Statistical Manual code. Explain how you reached your diagnosis, how the client's symptoms fit the diagnostic criteria for the disorder(s), or any possible difficulties in reaching a diagnosis.
Section 2: Treatment Plan
This portion of the paper will address the chosen treatment for the condition. This might also include the theoretical basis for the chosen treatment or any other evidence that might exist to support why this approach was chosen.
- Cognitive behavioral approach : Explain how a cognitive behavioral therapist would approach treatment. Offer background information on cognitive behavioral therapy and describe the treatment sessions, client response, and outcome of this type of treatment. Make note of any difficulties or successes encountered by your client during treatment.
- Humanistic approach : Describe a humanistic approach that could be used to treat your client, such as client-centered therapy . Provide information on the type of treatment you chose, the client's reaction to the treatment, and the end result of this approach. Explain why the treatment was successful or unsuccessful.
- Psychoanalytic approach : Describe how a psychoanalytic therapist would view the client's problem. Provide some background on the psychoanalytic approach and cite relevant references. Explain how psychoanalytic therapy would be used to treat the client, how the client would respond to therapy, and the effectiveness of this treatment approach.
- Pharmacological approach : If treatment primarily involves the use of medications, explain which medications were used and why. Provide background on the effectiveness of these medications and how monotherapy may compare with an approach that combines medications with therapy or other treatments.
This section of a case study should also include information about the treatment goals, process, and outcomes.
When you are writing a case study, you should also include a section where you discuss the case study itself, including the strengths and limitiations of the study. You should note how the findings of your case study might support previous research.
In your discussion section, you should also describe some of the implications of your case study. What ideas or findings might require further exploration? How might researchers go about exploring some of these questions in additional studies?
Need More Tips?
Here are a few additional pointers to keep in mind when formatting your case study:
- Never refer to the subject of your case study as "the client." Instead, use their name or a pseudonym.
- Read examples of case studies to gain an idea about the style and format.
- Remember to use APA format when citing references .
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach . BMC Med Res Methodol . 2011;11:100.
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach . BMC Med Res Methodol . 2011 Jun 27;11:100. doi:10.1186/1471-2288-11-100
Gagnon, Yves-Chantal. The Case Study as Research Method: A Practical Handbook . Canada, Chicago Review Press Incorporated DBA Independent Pub Group, 2010.
Yin, Robert K. Case Study Research and Applications: Design and Methods . United States, SAGE Publications, 2017.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Overview of clinical study designs
Seonwoo kim.
- Author information
- Article notes
- Copyright and License information
Correspondence to: Seonwoo Kim Academic Research Service Headquarters, LSK Global PS, 97 Toegye-ro, Jung-gu, Seoul 04535, Korea Email: [email protected]
Received 2023 Mar 28; Revised 2023 May 11; Accepted 2023 May 11; Collection date 2024 Mar.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/4.0/ ).
The goal of a clinical study is to determine the factors associated with a disease and to assess the efficacy and safety of an investigational drug, procedure, or device. Since clinical study designs vary due to unique requirements of individual studies, the aims of this report are to educate researchers on the different types of studies and to assist researchers in choosing the optimal study type to fulfill their individual requirements. Clinical studies are classified into the two main types, observational studies and clinical trials, depending on the presence or absence of an intervention. Observational studies include case-control studies, cohort studies, and cross-sectional studies. Case-control and cohort studies may be prospective or retrospective, and case-control studies may be nested or not. Clinical trials may be pragmatic and may be controlled or noncontrolled; randomized or nonrandomized; open label or blinded; and parallel, crossover, or factorial. These observational and clinical trial designs are reviewed. Each type of clinical study has advantages and disadvantages. Therefore, researchers must consider these in choosing the design best suited for achieving their study objectives.
Keywords: Clinical study, Observational study, Clinical trial, Study design, Bias
INTRODUCTION
Clinical studies are medical studies of groups of individuals. The goals of clinical studies are to determine associated disease factors and to assess the efficacy and safety of an investigational drug, procedure, or device for preventing, diagnosing, and treating disease. Clinical studies may test for the long-term effects or cost-effectiveness of an investigational treatment. There are two main types of clinical study, observational studies and clinical trials. In observational studies, investigators gather information on broad characteristics. For example, investigators may collect data through medical exams or questionnaires on the effects of lifestyle on cognitive health. Observational studies provide valuable information and may assist in identifying topics for clinical trials. Clinical trials test the safety and efficacy of medical, surgical, or behavioral interventions in individuals. Clinical study results have clinical, public, and economic impacts and need to be well-planned to provide valid study results.
Since study designs vary due to unique individual requirements, choosing the optimal study design is important. The aims of this article are to educate researchers on the different study designs and to assist those researchers in choosing optimal designs for fulfilling their research needs.
OBSERVATIONAL STUDY DESIGNS
Observational studies are those in which groups of individuals are monitored or outcomes are measured without manipulation or intervention to affect the result. Observational studies include case-control, cohort, and cross-sectional studies. Advantages and disadvantages of each type of observational study are listed in Table 1 .
Advantages and disadvantages of a case-control study, cohort study, case-control study within a defined cohort, and cross-sectional study
Case-control study
Case-control studies compare groups, such as subjects with a disease or condition under study (cases) to subjects without the disease or condition (controls). Investigators study the medical or lifestyle histories of those in each group to determine factors that may be associated with the disease or condition ( Fig. 1 ). If a factor is found more commonly in the cases than in the controls, the investigator may hypothesize that the exposure is linked to the disease. For example, in the investigation of risk factors for depression in intensive care unit (ICU) patients, the patients with depression were defined as cases; and sex, age, length of ICU stay, and individual medications were considered as risk factors associated with depression [ 1 ].
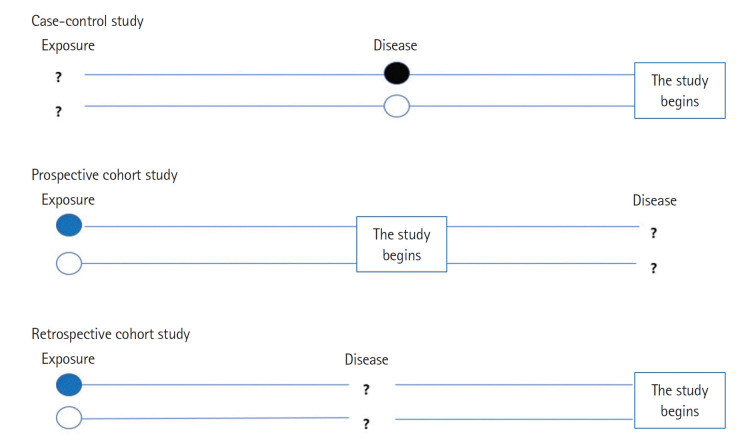
Case-control study, prospective cohort study, and retrospective cohort study.
Advantages and disadvantages
The main advantages of a case-control study are low cost and low time consumption. The case-control approach allows for study of rare diseases that require lengthy study periods. The case-control study design allows assessment of multiple factors at once.
A disadvantage is that bias is inherent in case-control studies. Case-control studies have the potential for recall bias, the increased likelihood that those with the outcome will recall and report exposures more frequently than those without the outcome. Recall bias may lead to conclusion of false associations between exposure and disease.
One of the aspects that is often overlooked is the selection of cases and controls. Appropriate selection of cases and controls to obtain a meaningful and scientifically sound conclusion is important and can be achieved by matching. Matching assists in risk factor or etiological identification that cannot be explained by other differences between the groups. Thus, choosing a control group that bolsters the strength of the case-control study and enhances the researcher’s ability to find valid potential correlations between exposures and disease states is important.
In addition to bias, the investigator must recognize the potential for confounding factors that result when a variable that is not being accounted for is related to both the exposure and the outcome. The potential for confounding is another disadvantage of case-control studies.
Cohort study
Cohort studies are a type of longitudinal study, an approach that follows study participants over time. Specifically, cohort studies recruit and follow study participants who share common characteristics. Baseline information on the individual cohort members are gathered first to get detailed picture of the cohort. Then, investigators collect data from different time points in the study. Investigators compare the development of disease between two groups, the exposed and the nonexposed groups, generated from the baseline information. Also, by comparing data from the follow-up points, investigators can evaluate the effects of factors on health.
Prospective cohort study and retrospective cohort study
Cohort studies can be prospective or retrospective. A prospective cohort study investigates an event, for example, a disease, yet to occur in the group. A retrospective study investigates an event that has already occurred ( Fig. 1 ).
Prospective cohort studies require recruitment of groups of participants to follow over time to gather new data. For exposuredisease correlations, investigators follow the participants from presence of exposure to development of disease. One prospective cohort study of syncope prognosis based on emergency department (ED) diagnosis generated a cohort from a group of adult patients with ED visits for syncope. The patients were followed for 30 days to investigate the frequency of serious outcomes and determine the factors associated with the outcome [ 2 ].
Retrospective cohort studies involve analysis of preexisting data. For exposure-disease investigations, retrospective cohort studies identify populations with and without an exposure based on past records and then assess disease development by the time of study. For example, to determine the effects of three aspects of care provided by primary physicians (physician specialty, continuity of care, and comprehensiveness of care) on patient use of the ED, investigators created a retrospective cohort of adults aged 18 years and older using provincial administrative databases that covered a 3-year span. The primary care variable and covariables were measured during an initial baseline period (the first 2 years of the study); visits to the ED for the primary outcome were measured during the last year of the study [ 3 ].
One of the advantages of cohort studies is their effectiveness in establishing cause and effect. Cohorts are usually large, allowing investigators to draw relatively confident conclusions regarding the links between risk factors and disease. In many cases, because participants are often free of disease at the commencement of the study, cohort studies are particularly useful at identifying the timelines over which behaviors contribute to disease development. Another advantage is that investigators can collect a wide variety of data in cohort studies that can be used in multiple ways. A study on the impact of smoking, for example, might reveal links with multiple types of diseases. Investigators can also compare degrees of risk among risk factors.
Like case-control studies, cohort studies are subject to bias. Some of the biases observed with cohort studies include selection bias and information bias. Selection bias results when exposure is linked to study participation. Individuals with an exposure may refuse to participate in the study at a higher rate than those without the exposure. Selection bias also occurs when the exposed are lost to follow-up. Because of selection bias, interpretation of associations between exposures and outcomes is difficult. Information bias occurs when the data in past records are inaccurate in the evaluation of exposure status creating interpretation difficulties. Causal inference problems also result in prospective cohort studies when participants who are aware of their participation in a cohort alter their behavior during follow-up. In addition to bias, disadvantages of prospective cohort studies include their time consumption and expense compared to case-control studies. Retrospective cohort studies are more pragmatic; the use of historical data decreases time and expense requirements. However, retrospective approaches increase the risk of bias in sampling of the cohort due to missing data. Retrospective cohort studies are also weakened by the data fields available not being designed with the study in mind.
Case-control studies based within a defined cohort
This type of study combines some of the features of a cohort study with those of a case-control study design. When a defined cohort is embedded in a case-control study design, all baseline information is collected before the onset of disease, and the cohort is followed until onset of disease. One of the advantages of this design is the elimination of recall bias as the information regarding risk factors is collected before onset of disease. Case-control studies based within a defined cohort can be further classified into nested case-control studies and case-cohort studies.
Nested case-control study
This type of study design involves the selection of several controls for each case, typically from those still under observation at the time when the case developed the disease. A nested case-control study consists of a defined cohort with suspected risk factors and assignment of controls within the cohort to cases, subjects who develop the disease [ 4 ]. Over a period, cases and controls are identified and followed as per the study protocol. Hence, the case and control are matched in time and length of follow-up ( Fig. 2A ). When this study design is implemented, controls may become cases. The procedures for sampling in a nested case-control study follow. Select all those who become cases. Select controls randomly from those still at risk at time of case development (risk set), selection of five controls typically maximizes efficiency. Controls are time-matched to cases. Individuals can be controls more than once, and an individual selected as a control may later become a case. In the matching process, additional matching on confounders is often involved. One such study examined the association between incident injury after prescription opioid initiation and subsequent risk of opioid-related adverse events (ORAEs). The nested case-control study was conducted in a cohort of individuals 65 years and older. This assessment was of the association of prescription opioid use with recency of injury among older patients. ORAE cases were identified as patients who became inpatients or outpatients with a diagnosis code for opioid misuse, dependence, or poisoning. Using 1:4 matching, controls were randomly selected using incidence density sampling with matching criteria that included the year of cohort entry date and a disease risk score [ 5 ].
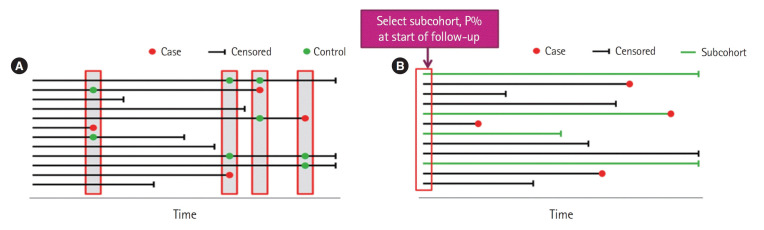
Case-control study based within a defined cohort. (A) Nested case-control study. Controls are time-matched to the cases. (B) Case-cohort study. Subcohort is not time-matched to the cases.
Nested case-control studies have some limitations. When more than one disease outcome is considered, a strict implementation of the nested case-control design requires selection of a new set of controls for each distinct disease outcome. Estimation of risk is not possible because the at-risk period is unknown. We can estimate rate, however, if the size and fraction of each risk set are known; but this is not a trivial matter, especially if there are time-dependent effects.
Case-cohort study
Case-cohort study designs were proposed as an alternative to the nested case-control study design. This design requires only selection of a subcohort random sample and all cases. Cases are defined as those participants of the cohort who developed the disease of interest, but controls are identified before the cases develop ( Fig. 2B ). Controls are randomly chosen from all cohort participants regardless of disease of interest status, allowing for early collection of baseline data. Case-cohort studies are similar to nested case-control studies; the main difference between the two study types is the way in which controls are chosen. A case-cohort study was conducted to examine the association between the risk factors and hospitalization in a cohort of dog bite victims requiring ED visits. The risk factors included infection, complicated injury, host defense abnormality, number of previous evaluations for the injury, and anatomic location of the bite. The case-cohort design was chosen because cases could be identified in a well-defined administrative cohort, medical record review was required for each study patient, and the risk ratio was the effect measure of interest. Cases were cohort members who were admitted as inpatients directly from the ED. From the cohort, a simple random sample was selected for the subcohort comparison group. Some patients were included into both subcohort and case groups [ 6 ].
Compared to the nested case-control studies, a major advantage of the case-cohort design is the ability to study several disease outcomes using the same subcohort. For example, investigators interested in determining if smoking is a risk factor for both diabetes and lung cancer would require two control groups with a nested case-control design, while a case-cohort design only requires one subcohort. Unlike the nested case-control study, the case-cohort study can estimate rate or risk, since the measurement in the subcohort can be observed for any time up to variable event onset.
A case-cohort study has some limitations. Information bias can be increased when the subcohort is established after baseline. With much censoring, the subcohort becomes “thin” and may not be representative of the cohort. Also, statistical analysis is more complicated than with a nested case-control study.
Cross-sectional study
A cross-sectional study is a type of observational study that involves data collected at a defined time; a cross-sectional study analyzes data from a population, or a representative subset, at a specific point in time. These studies are often used to assess the prevalence of acute or chronic conditions but cannot be used to answer questions about the causes of disease or the results of interventions. That is, cross-sectional data cannot be used to infer causality because temporality is not known. Cross-sectional studies may involve special data collection, including questions about the past, but often rely on data originally collected for other purposes.
The use of routinely collected data allows large cross-sectional studies to be conducted at little or no expense. A natural progression has been suggested from cross-sectional studies of routinely collected data that suggest hypotheses, to case-control studies that test these hypotheses more specifically, to more costly and time-consuming cohort studies and trials that provide stronger evidence.
Temporal association cannot be established as the information is collected at the same time point. If a study involves a questionnaire, the investigator can ask questions about onset of symptoms or risk factors in relation to onset of disease. The prevalence of a disease can be determined; the incidence cannot. Cross-sectional studies are not suited for studying rare diseases and are susceptible to biases such as nonresponse bias and recall bias.
CLINICAL TRIAL
A clinical trial is a prospective study of the effects of interventions or manipulations of interest. Since this type of study can provide the most convincing demonstration of evidence of causality, the design requires meticulous planning and resources to provide an accurate result.
General considerations
When designing a clinical trial, selecting a representative population that assures generalizability to the target population is of paramount importance, as is selection of appropriate endpoints. Endpoints need to be well-defined, reproducible, clinically relevant, and achievable. The types of endpoints are continuous, ordinal, nominal, and time-to-event; and the endpoint is typically classified as primary, secondary, or tertiary. An ideal endpoint is a purely clinical outcome; for example, cure or survival, and clinical trials can be long and expensive. Surrogate endpoints may be biologically related to the ideal endpoint and need to be reproducible, easily measured, related to the clinical outcome, affected by treatment, and occur earlier than the clinical outcome.
Controlled vs. noncontrolled trials
Clinical trials are divided into controlled versus noncontrolled clinical trials depending on the presence or absence of a control group for the investigational treatment of interest.
Uncontrolled trials
Uncontrolled trials are often used in the early phases of drug research, phases I and II, to determine pharmacokinetic properties or to investigate tolerated dose ranges. Uncontrolled trials can also be useful to study side effects, biochemical changes in long-term therapies, tolerance, interaction, or efficacy of drugs. Uncontrolled trials produce higher estimates of the mean effect than those obtained in a controlled trial since, by not having a control group acting as a reference, uncontrolled trials can induce erroneous impressions of the investigated drug [ 7 ]. Sine these trials generate bias, the results of uncontrolled trials are considered less valid than those of controlled trials.
Controlled trials
The design of these trials includes at least one treatment group that is compared with a control group. The control group receives placebo or another active treatment. Both groups are studied simultaneously, except when the control group is derived from historical data or when some adaptive designs are used. Controlled trials are the most common in clinical phase III. Controlled trials allow the participant’s outcome to be discriminated from an outcome caused by other factors, such as the natural history of the disease or the expectations of the participant or the investigator.
Common controls are placebo control, active treatment control, control with dose comparison, and historical control. Particular care is required when attempting to use placebo control and historical control.
Placebo control
Placebo is defined as “an inert or innocuous substance used especially in a controlled experiment testing the efficacy of another substance (such as a drug)” [ 8 ]. This is especially useful if the outcome measured is subjective and should only be used if no permanent harm (death or irreversible morbidity) occurs by delaying available active treatment for the duration of the trial. The ethics of placebo-controlled studies is complex and continues to create a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “the benefits, risks, burdens, and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances: where no proven intervention exists; the use of placebo, or no intervention, is acceptable; or where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the participants who receive any intervention less effective than the best proved one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention. Extreme care must be taken to avoid abuse of this option” [ 9 ]. Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Active treatment control
This design involves comparing a new drug with a standard drug or comparing the combination of new and standard therapies versus standard therapy alone. This design is more ethical than the placebo control, provided that approved drugs are available for the disease under study.
Control with dose comparison
Different doses or regimens of the same treatment are used as the active arm and control arm. The purpose is to establish a relationship between the dose and the efficacy and safety of the intervention. This design can include active and placebo groups in addition to the different dose groups. The design may be ineffective if the therapeutic range of the drug is not known.
Historical control (external and nonconcurrent)
In this design, the information from the controls is not obtained during the study but is from subjects who were treated at an earlier time or in a different setting. This design has an advantage when studying rare conditions in which difficulty arises in generating a sample size. This design is also cost-effective and time-saving. However, the design has many disadvantages. Randomization and blinding are not possible, and the comparability of the current intervention with the historical control is difficult due to the differences in baseline characteristics of the subjects. The comparability problem can be addressed to some extent by statistical methods, but the information obtained may not be accurate or reliable and may lack uniformity and/or completeness.
Randomized vs. nonrandomized clinical trials
Clinical trials are randomized or nonrandomized based on the method used to allocate a participant to a treatment or control group.
Randomized clinical trials
A randomized clinical trial involves randomizing participants with similar characteristics to one of two or multiple groups, the group(s) that receives the intervention/experimental therapy and the other group(s) that received the placebo or standard of care. Randomization is typically performed using a computer software package. Hence, we can measure the outcomes and efficacy of the intervention or experimental therapy being studied without bias as participants with similar baseline characteristics have been randomized to their respective groups. Randomized controlled trials are the gold standard for clinical study. However, this study design is generally not applicable to rare and serious disease processes due to the ethics involved in treating affected individuals with a placebo.
Nonrandomized trials
A nonrandomized trial involves an approach of selecting controls without randomization, usually allocation of participants into groups by the investigator. This may also result from selection of participants and controls based on day of the week presentation or assignment to a particular clinician. This type of participant and control selection becomes predictable. Therefore, there is bias introduced that can impact the validity of the results.
Open-label vs. blind trials
Clinical trials are divided into open-label versus blind trial based on participants’ or investigators’ awareness of the treatment group to which participants have been allocated.
Open-label trials
Certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention. In this case, open-label trials are planned in which both trial participants and investigators know the group assignment of the participants.
Blind trials
This is a method used in clinical trials to reduce the risk of intentional or unintentional bias. There are three forms of blinding: single, double, and triple blind. In a single-blind study, only the participants do not know their group assignment until the trial is over. In double-blind studies, both the study participants and the investigator are unaware of the group to which subjects were allocated. Double-blind studies are typically used in clinical trials to test the safety and efficacy of drugs. In triple-blind studies, participants, investigators, and data analysts are unaware of the group allocation. Those who are directly or indirectly involved in the trial, such as caregivers and data recorders, should also be blinded to the group allocation of the trial participant in order to increase the effect of blinding.
Parallel, crossover, and factorial design trials
Based on the treatment structure, clinical trial designs are classified into parallel, crossover, and factorial designs. A summary of the advantages and disadvantages of each design is provided in Table 2 .
Advantages and disadvantages of parallel, crossover, factorial design trials
Parallel design trials
A parallel design of a clinical trial is a design in which two or more groups of participants receive different interventions. Participants are assigned to one of the treatment arms at the beginning of the trial and continue in that arm throughout the length of the trial ( Fig. 3 ). This is the most common clinical trial design.
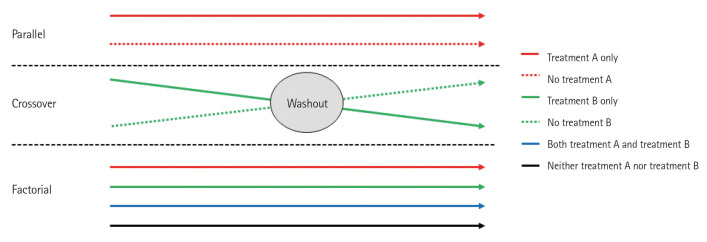
Parallel design trial, crossover design trial, and factorial design trial.
Parallel design has two advantages over the crossover design described later. All other conditions being the same, the duration of the study is shorter and the visits required are fewer, which results in a study that is less burdensome for the participant. The statistical analysis requires fewer assumptions, which, if not verified, would reduce the reliability of the conclusions. The weakness of the parallel design is that it requires a larger sample size than the crossover design.
Crossover design trial
The crossover clinical trial is a design in which all participants receive the same two or more treatments, but the order of receipt depends on the group of assignment. Hence, in this type of design, there are two groups that undergo the same intervention/experiment at different time periods. That is, each group serves as a control while the other is undergoing the intervention/experiment. A “washout” period is recommended in order to eliminate residual effects of the intervention or experiment (carryover effect) when the experiment group transitions to the control group, or vice versa ( Fig. 3 ).
The main advantage of the crossover design is that each subject acts as a their own control. Therefore, a smaller number of subjects is required in comparison to parallel group studies because of removing of participant variation in this way. This type of trial can only be considered when the disease persists for a relatively long period. Hence, crossover trials are mostly used in studying chronic diseases. The main disadvantage is that the carryover effect may be aliased (confounded) with direct treatment effects as they cannot be estimated separately.
Factorial trial
In a factorial trial, two or more intervention comparisons are carried out simultaneously. For example, participants may be randomized to receive aspirin or placebo and randomized to receive a behavioral intervention or standard care. This factorial trial has two factors, each of which has two levels; there are called 2×2 factorial trials ( Fig. 3 ). Whenc designing a factorial trial, the main intention of investigators is to achieve “two trials for the price of one”; and the assumptions are that the effects of the different active interventions are independent, and that there is no interaction (no synergy or antagonism) between the treatments. The interaction effect between the two treatments can be tested by a proper methodology. Since a 2×2 factorial trial can be seen as two trials addressing different questions, it is important that both parts of the trial are reported as if they were part of a two-arm parallel group trial. Thus, in the example given, we would expect to view the results for aspirin versus placebo, including all participants regardless of whether they had behavioral intervention or standard care, and likewise of the behavioral intervention. An evaluation of the interaction between the two treatments based on the factorial design may also be available.
The factorial design allows investigators to obtain evidence about efficacy from fewer patients than would be needed if treatment A and B were individually tested in two separate trials. The main disadvantage is the difficulty of experimenting with more than one factor or level. A factorial design must be planned meticulously, as an error in one of the levels, or in the general operationalization, will jeopardize a vast amount of work.
Pragmatic clinical trial design
A classical clinical trial may not be adequately reflective of practice because the trial may have been optimized to determine intervention efficacy. Because such trials were also performed with a relatively small size of highly selected participants at sites with experienced investigators, the trials could overestimate benefits and underestimate harm of the intervention. These concerns create the need for more pragmatic trials designed to demonstrate the actual effectiveness of the intervention in more generalized settings. Trial design can be more pragmatic when considering four domains: the study population, the setting of the trial, operationalization of the intervention, and the outcome measures [ 10 , 11 ]. In order to provide the comprehensive evaluation of comparative clinical effects of 0.9% saline and balanced crystalloids across the full spectrum of diseases typical for hospitalized adults, a pragmatic trial was conducted among noncritically ill adults who were subsequently hospitalized outside an ICU. This trial was designed to consider broad eligibility criteria, large sample size, study procedures that included routine care, and execution of the trial by clinical personnel [ 12 , 13 ].
The different types of clinical studies are used for different reasons. Selecting the best design for a given study is critical to a successful outcome. In terms of the quality of evidence, a clinical trial is superior to an observational study. Observational studies are, however, conducted much more frequently than clinical trials. Ethical considerations and cost are main reasons that observational studies are frequently employed. A case-control study is a valuable tool for exploring risk factors for rare diseases or when other types of study are not feasible. Investigators explore possible associations between exposure and disease through case-control studies, and data from case-control studies can provide a focus for future studies. Then, through cohort studies or clinical trials, the evidence of an association between exposure and disease can be increased. Cohort studies are often complex, large, and long in duration. However, with careful planning and implementation, cohort studies are valuable in providing healthcare evidence. To reduce cost and achieve the same goal as a cohort study, nested case-control and case-cohort study are alternatives. These types of studies are based on large cohorts and can be useful in “big data” analysis [ 14 ]. Nested case-control and case-cohort study designs are efficient in terms of cost and can be used to evaluate the relationship between exposure and disease. Compared to a nested case-control design, the case-cohort design is more efficient and allows an investigator to study several disease outcomes using the same random sample [ 15 ]. While there are some advantages in observational studies, biases are inherent and should be addressed. Recently, as studies using “big data” have become possible, well-designed historical control studies have increased. In clinical trials, appropriate control group selection is vital. The clinical trial study should be planned so that those involved in the study, including participants, are blinded to the maximum extent possible. The classical trial designs are parallel, crossover, and factorial designs. In addition, although not applicable to all diseases or clinical trials, new methodologies such as adaptive designs can shorten the duration of a clinical trial. Investigators should also consider pragmatic clinical trials that are more efficient, patient-centered, and empirical and are conducted in order to provide more valuable clinical and policymaking information.
Capsule Summary
What is already known
Multiple study designs exist to help determine the factors associated with a disease and to assess the efficacy and safety of an intervention.
What is new in the current study
In this paper we review multiple study designs and discuss their advantages and disadvantages.
Conflicts of interest
The author has no conflicts of interest to declare.
The author received no financial support for this study.
Data availability
Data sharing is not applicable as no new data were created or analyzed in this study.
- 1. Abdelmonem S, Hadi GA, Fayed A, El Sayed I, Mogazy K. A case control study of risk factors for depression in intensive care unit patients. Int J Crit Care Emerg Med. 2019;5:077. [ Google Scholar ]
- 2. Toarta C, Mukarram M, Arcot K, et al. Syncope prognosis based on emergency department diagnosis: a prospective cohort study. Acad Emerg Med. 2018;25:388–96. doi: 10.1111/acem.13346. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. McCusker J, Tousignant P, Borges Da Silva R, et al. Factors predicting patient use of the emergency department: a retrospective cohort study. CMAJ. 2012;184:E307–16. doi: 10.1503/cmaj.111069. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Ernster VL. Nested case-control studies. Prev Med. 1994;23:587–90. doi: 10.1006/pmed.1994.1093. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Wei YJ, Chen C, Cheng TD, Schmidt SO, Fillingim RB, Winterstein AG. Association of injury after prescription opioid initiation with risk for opioid-related adverse events among older Medicare beneficiaries in the United States: a nested case-control study. PLoS Med. 2022;19:e1004101. doi: 10.1371/journal.pmed.1004101. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Rhea S, Weber DJ, Poole C, Cairns C. Risk factors for hospitalization after dog bite injury: a case-cohort study of emergency department visits. Acad Emerg Med. 2014;21:196–203. doi: 10.1111/acem.12312. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Nair B. Clinical trial designs. Indian Dermatol Online J. 2019;10:193–201. doi: 10.4103/idoj.IDOJ_475_18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Merriam-Webster Dictionary. Placebo [Internet]. Merriam-Webster; c2023 [cited 2023 Mar 21]. Available from: https://www.merriam-webster.com/dictionary/placebo .
- 9. World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–63. doi: 10.1056/NEJMra1510059. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Sepehrvand N, Alemayehu W, Ezekowitz JA. Pragmatic vs explanatory trials: reply. JAMA Cardiol. 2020;5:488. doi: 10.1001/jamacardio.2019.6114. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–28. doi: 10.1056/NEJMoa1711586. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Self WH, Semler MW, Wanderer JP, et al. Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial. Trials. 2017;18:178. doi: 10.1186/s13063-017-1923-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J Hepatol. 2018;68:476–84. doi: 10.1016/j.jhep.2017.10.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Kim S. Case-cohort studies vs nested case-cohort studies. Datum. 2016;22(1) [ Google Scholar ]
- View on publisher site
- PDF (409.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

How to Write a Case Study in Research? (with Examples)

A case study is a powerful tool in research and education, offering deep insights into complex phenomena through the lens of a single subject or group. Case studies zoom in to the details of a specific situation, examining context, history, and behavior to reveal certain patterns and highlight practical applications of theories.
A case study in research can be used when:
- atypical or abnormal behavior or development is observed,
- an unexplained outcome to treatment is found, or
- an emerging disease or condition occurs.
Thus, case studies enable researchers and practitioners to analyze specific instances, identify trends, and obtain learnings that can inform broader decision-making and problem-solving. In this post, you will learn all about case studies, including how to conduct a case study , limitations and benefits of a case study , and case study methods .
What is a Case Study ?
Case study definition : A case study is an in-depth or intensive study of a person, group, or event. Case studies involve deep analyses of an individual or group to identify patterns and are used across fields such as psychology and medicine to draw broad conclusions. They are descriptive studies based on qualitative data such as observations, interviews, questionnaires, clinical notes.
Here are some historically significant case study examples:
The curious case of Phineas Gage: This is arguably the most cited case study in psychology, which sheds light on how different areas of the brain affect personality and cognition. While working as a construction foreman on a railroad, Phineas Gage was involved in an accident in which a rod impaled his brain. Gage survived the physical trauma, but his personality and his ability to learn new skills were altered. This case report was crucial to research on brain function, memory, and personality.
Anna O and the talking cure : Anna O (pseudonym) was a German woman who was one of the first patients to undergo psychoanalysis. Her case inspired many of the theories of Freud and other prominent psychologists of the time to mitigate the symptoms of depression through “talk therapy.” This case study is still cited as a reason psychologists believe that psychotherapy, or talk therapy, can be helpful to many patients.
When to Do a Case Study ?
The case study design may be chosen by a researcher in the following situations:
Need for deep understanding: When you require detailed insights about a specific situation, and you want to understand complex relationships and processes.
Resource constraints: When you have limited time and finances available for research and/or limited access to large sample sizes.
Research context: Certain real-world phenomena need to be studied in their natural context, especially when you cannot control variables.
Accordingly, the types of research questions best suited for a case study are exploratory (e.g., when investigating a new or poorly understood phenomenon, emerging conditions, adverse reactions to treatments, new methods of treatment) or involve unique situations (e.g., rare or exceptional cases, atypical behavior, breakthrough events). See Table 1 for developing a case study from a research question.
The case study design might be for a single case study, such as for unique cases or when studying a representative or typical case, or multiple case studies (a case series, comparative analysis, or to identify patterns across different contexts). Note that a case study is not recommended if you require statistical generalization or broad population-level insights.

How Long Should a Case Study Be?
Case studies are structured very differently from research articles (see “ How to write a case study in Research ” below and the case study template in Figure 1). However, as a general guideline, note that case studies might range from 500 to 1,500 words. The word count would depend on factors such as the target journal’s specifications, case type, and study discipline. Case reports also have a limit on the number of references to be cited. Remember, you must always check the target journal for word and reference limits before submission.
How to Write a Case Study in Research
Let’s delve into how to conduct a case study and write one. First, you need to understand how to create a case study .
Before writing
- On the basis of your research problem and research question , select the case that you want to study.
- Perform an in-depth literature review to develop a relevant theoretical framework, wherein you aim to demonstrate, expand upon, or challenge an existing theory in your field.
- Collect the data, which will typically be qualitative in nature. Data collection, therefore, will be collected by direct observations , interviews , or analysis of primary and secondary sources of information. Be as thorough as you can at this step.
- Analyze the case, highlighting key facts and problems, identify key problems and their causes and impacts, and explore potential solutions.
Drafting and writing your case study
The structure of the case report may vary—some follow the format of scientific papers, while others adopt a narrative style for a deeper exploration.
- State the key problem and present a concise thesis under an “Introduction” or “Background” section. Provide background, facts, and evidence of research.
- Describe the specific case, group, or event.
- 3. Provide specific solutions, suggest strategies for implementing the solution and, if needed, additional
- Discuss the case, including the strengths and limitations of the study. Summarize the outcome of your analysis and highlight specific strategies for implementing the proposed solution.
When describing and analyzing a case, be sure to include contextual details, link findings to existing literature and theory, and discuss broader implications. For medical case reports, follow the CARE guidelines (EQUATOR) to ensure completeness and transparency. Please also refer to the CARE Checklist of information to include when writing a case report. Finally, check the target journal requirements for word count and formatting guidelines. See Figure 1 for a case study template .

Figure 1. Case study template
Table 1. From research question to case study : Some fictional examples
Real case study examples (published):
- Baker et al. (2024) Enhanced family-based treatment for an adolescent with binge-eating disorder: A case report. Cognitive and Behavioral Practice . 31(2), 272–282.
- da Silva et al. (2024) Impacts of oil palm monocultures on freshwater ecosystems in the Amazon: a case study of dragonflies and damselflies (Insecta: Odonata). Aquatic Science 87, 1.
- Sakamoto et al. (2024) Online gaming reduces psychological distress in a patient with schizophrenia: A case report. PCN Reports. 3(3), e70015.

What Are the Benefits of a Case Study ?
On the topic of the case study , a quote by Ivy Mckenzie comes to mind: “The physician is concerned [unlike the naturalist]…with a single organism, the human subject, striving to preserve its identity in adverse circumstances .”
A physician’s meticulous documentation of an unusual or rare condition might not only help the patient but also revolutionize current understanding of the disorder and lead to a revision of treatment protocols. In fact, clinicians and psychologists are often encouraged to publish more case studies documenting the methods they use.
Let’s look at some more benefits of a case study :
- They can be published quickly.
- They are suitable under situations of time and budget crunches.
- They are appropriate to study phenomena in their natural context
- They allow detailed investigation into situations that would otherwise be impractical to perform using another study design.
- They are sometimes used in therapy to guide the best course of treatment.
What Are the Limitations of a Case Study ?
Case studies provide critical information and galvanize further research; however, there are some caveats. The following are the limitations of a case study :
- A case study is not definitive proof of a theory and cannot demonstrate cause and effect.
- Case studies with insufficient or incorrect information or based on a flawed premise can harm future research.
- Ethical issues may arise if the reported patients have not provided consent for publication of their case or are not treated with dignity and respect.
- If a patient declines to provide consent, the case report cannot be written or published.
- A case study cannot necessarily be generalized to the larger population.
- A case study might be impossible or difficult to replicate.
- Case reports can lead to bias.
Key Takeaways
- A case study in research is an in-depth or intensive study of a person, group, or event using qualitative data. It is used to examine complex phenomena through detailed analysis of specific instances. Case studies are particularly valuable in psychology, medicine, and other fields for drawing broader conclusions
- Case study methods involve data collection through direct observations, interviews, and analyses of primary and secondary sources.
- Case reports are typically 500–1,500 words long. You may develop single case studies (for unique cases) or multiple case studies (case series, for comparative analysis). Where applicable, be sure to follow specific guidelines (e.g., CARE guidelines for medical cases).
- A case study design is best used when deep understanding is needed, time and resources are limited, and the natural context must be preserved. It is suited to studying exploratory research questions, unique situations, emerging conditions, and atypical behavior.
- Writing case studies involves the following steps:
- Pre-writing phase:
– Select appropriate case based on research problem
– Conduct literature review
– Collect thorough qualitative data
– Analyze case to identify key problems
- Writing phase:
– State key problem and thesis in introduction
– Describe specific case/event
– Provide solutions and implementation strategies
– Discuss strengths and limitations
– Link findings to existing literature
- The advantages of case studies are that they permit quick writing and publication, are cost-effective, are suitable for natural context study, and are extremely valuable for rare or unusual cases. The limitations of case studies, however, are that they cannot prove cause and effect, may not be generalizable, are difficult to replicate, are prone to bias, and require patient consent in medical cases.
Frequently Asked Questions
What is a case study in research, why are case studies important in research, what are the key components of a case study, what is the difference between case studies and case series.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 22+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$25 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- PhD Qualifying Exam: Tips For Success
- Academic Editing: How to Self-Edit Academic Text With Paperpal
- How to Write the First Draft of a Research Paper with Paperpal?
How to Write an Academic Paragraph (Step-by-Step Guide)
Boost your earnings with paperpal’s newly launched affiliate program, you may also like, how to cite in apa format (7th edition):..., how to write your research paper in apa..., how to choose a dissertation topic, how to write a phd research proposal, research funding basics: what should a grant proposal..., how to write the first draft of a..., mla works cited page: format, template & examples, academic editing: how to self-edit academic text with..., measuring academic success: definition & strategies for excellence.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to present patient...
How to present patient cases
- Related content
- Peer review
- Mary Ni Lochlainn , foundation year 2 doctor 1 ,
- Ibrahim Balogun , healthcare of older people/stroke medicine consultant 1
- 1 East Kent Foundation Trust, UK
A guide on how to structure a case presentation
This article contains...
-History of presenting problem
-Medical and surgical history
-Drugs, including allergies to drugs
-Family history
-Social history
-Review of systems
-Findings on examination, including vital signs and observations
-Differential diagnosis/impression
-Investigations
-Management
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1
The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the patient’s condition and further management can be planned accordingly. 2 To give a high quality presentation you need to take a thorough history. Consultants make decisions about patient care based on information presented to them by junior members of the team, so the importance of accurately presenting your patient cannot be overemphasised.
As a medical student, you are likely to be asked to present in numerous settings. A formal case presentation may take place at a teaching session or even at a conference or scientific meeting. These presentations are usually thorough and have an accompanying PowerPoint presentation or poster. More often, case presentations take place on the wards or over the phone and tend to be brief, using only memory or short, handwritten notes as an aid.
Everyone has their own presenting style, and the context of the presentation will determine how much detail you need to put in. You should anticipate what information your senior colleagues will need to know about the patient’s history and the care he or she has received since admission, to enable them to make further management decisions. In this article, I use a fictitious case to show how you can structure case presentations, which can be adapted to different clinical and teaching settings (box 1).
Box 1: Structure for presenting patient cases
Presenting problem, history of presenting problem, medical and surgical history.
Drugs, including allergies to drugs
Family history
Social history, review of systems.
Findings on examination, including vital signs and observations
Differential diagnosis/impression
Investigations
Case: tom murphy.
You should start with a sentence that includes the patient’s name, sex (Mr/Ms), age, and presenting symptoms. In your presentation, you may want to include the patient’s main diagnosis if known—for example, “admitted with shortness of breath on a background of COPD [chronic obstructive pulmonary disease].” You should include any additional information that might give the presentation of symptoms further context, such as the patient’s profession, ethnic origin, recent travel, or chronic conditions.
“ Mr Tom Murphy is a 56 year old ex-smoker admitted with sudden onset central crushing chest pain that radiated down his left arm.”
In this section you should expand on the presenting problem. Use the SOCRATES mnemonic to help describe the pain (see box 2). If the patient has multiple problems, describe each in turn, covering one system at a time.
Box 2: SOCRATES—mnemonic for pain
Associations
Time course
Exacerbating/relieving factors
“ The pain started suddenly at 1 pm, when Mr Murphy was at his desk. The pain was dull in nature, and radiated down his left arm. He experienced shortness of breath and felt sweaty and clammy. His colleague phoned an ambulance. He rated the pain 9/10 in severity. In the ambulance he was given GTN [glyceryl trinitrate] spray under the tongue, which relieved the pain to 5/10. The pain lasted 30 minutes in total. No exacerbating factors were noted. Of note: Mr Murphy is an ex-smoker with a 20 pack year history”
Some patients have multiple comorbidities, and the most life threatening conditions should be mentioned first. They can also be categorised by organ system—for example, “has a long history of cardiovascular disease, having had a stroke, two TIAs [transient ischaemic attacks], and previous ACS [acute coronary syndrome].” For some conditions it can be worth stating whether a general practitioner or a specialist manages it, as this gives an indication of its severity.
In a surgical case, colleagues will be interested in exercise tolerance and any comorbidity that could affect the patient’s fitness for surgery and anaesthesia. If the patient has had any previous surgical procedures, mention whether there were any complications or reactions to anaesthesia.
“Mr Murphy has a history of type 2 diabetes, well controlled on metformin. He also has hypertension, managed with ramipril, and gout. Of note: he has no history of ischaemic heart disease (relevant negative) (see box 3).”
Box 3: Relevant negatives
Mention any relevant negatives that will help narrow down the differential diagnosis or could be important in the management of the patient, 3 such as any risk factors you know for the condition and any associations that you are aware of. For example, if the differential diagnosis includes a condition that you know can be hereditary, a relevant negative could be the lack of a family history. If the differential diagnosis includes cardiovascular disease, mention the cardiovascular risk factors such as body mass index, smoking, and high cholesterol.
Highlight any recent changes to the patient’s drugs because these could be a factor in the presenting problem. Mention any allergies to drugs or the patient’s non-compliance to a previously prescribed drug regimen.
To link the medical history and the drugs you might comment on them together, either here or in the medical history. “Mrs Walsh’s drugs include regular azathioprine for her rheumatoid arthritis.”Or, “His regular drugs are ramipril 5 mg once a day, metformin 1g three times a day, and allopurinol 200 mg once a day. He has no known drug allergies.”
If the family history is unrelated to the presenting problem, it is sufficient to say “no relevant family history noted.” For hereditary conditions more detail is needed.
“ Mr Murphy’s father experienced a fatal myocardial infarction aged 50.”
Social history should include the patient’s occupation; their smoking, alcohol, and illicit drug status; who they live with; their relationship status; and their sexual history, baseline mobility, and travel history. In an older patient, more detail is usually required, including whether or not they have carers, how often the carers help, and if they need to use walking aids.
“He works as an accountant and is an ex-smoker since five years ago with a 20 pack year history. He drinks about 14 units of alcohol a week. He denies any illicit drug use. He lives with his wife in a two storey house and is independent in all activities of daily living.”
Do not dwell on this section. If something comes up that is relevant to the presenting problem, it should be mentioned in the history of the presenting problem rather than here.
“Systems review showed long standing occasional lower back pain, responsive to paracetamol.”
Findings on examination
Initially, it can be useful to practise presenting the full examination to make sure you don’t leave anything out, but it is rare that you would need to present all the normal findings. Instead, focus on the most important main findings and any abnormalities.
“On examination the patient was comfortable at rest, heart sounds one and two were heard with no additional murmurs, heaves, or thrills. Jugular venous pressure was not raised. No peripheral oedema was noted and calves were soft and non-tender. Chest was clear on auscultation. Abdomen was soft and non-tender and normal bowel sounds were heard. GCS [Glasgow coma scale] was 15, pupils were equal and reactive to light [PEARL], cranial nerves 1-12 were intact, and he was moving all four limbs. Observations showed an early warning score of 1 for a tachycardia of 105 beats/ min. Blood pressure was 150/90 mm Hg, respiratory rate 18 breaths/min, saturations were 98% on room air, and he was apyrexial with a temperature of 36.8 ºC.”
Differential diagnoses
Mentioning one or two of the most likely diagnoses is sufficient. A useful phrase you can use is, “I would like to rule out,” especially when you suspect a more serious cause is in the differential diagnosis. “History and examination were in keeping with diverticular disease; however, I would like to rule out colorectal cancer in this patient.”
Remember common things are common, so try not to mention rare conditions first. Sometimes it is acceptable to report investigations you would do first, and then base your differential diagnosis on what the history and investigation findings tell you.
“My impression is acute coronary syndrome. The differential diagnosis includes other cardiovascular causes such as acute pericarditis, myocarditis, aortic stenosis, aortic dissection, and pulmonary embolism. Possible respiratory causes include pneumonia or pneumothorax. Gastrointestinal causes include oesophageal spasm, oesophagitis, gastro-oesophageal reflux disease, gastritis, cholecystitis, and acute pancreatitis. I would also consider a musculoskeletal cause for the pain.”
This section can include a summary of the investigations already performed and further investigations that you would like to request. “On the basis of these differentials, I would like to carry out the following investigations: 12 lead electrocardiography and blood tests, including full blood count, urea and electrolytes, clotting screen, troponin levels, lipid profile, and glycated haemoglobin levels. I would also book a chest radiograph and check the patient’s point of care blood glucose level.”
You should consider recommending investigations in a structured way, prioritising them by how long they take to perform and how easy it is to get them done and how long it takes for the results to come back. Put the quickest and easiest first: so bedside tests, electrocardiography, followed by blood tests, plain radiology, then special tests. You should always be able to explain why you would like to request a test. Mention the patient’s baseline test values if they are available, especially if the patient has a chronic condition—for example, give the patient’s creatinine levels if he or she has chronic kidney disease This shows the change over time and indicates the severity of the patient’s current condition.
“To further investigate these differentials, 12 lead electrocardiography was carried out, which showed ST segment depression in the anterior leads. Results of laboratory tests showed an initial troponin level of 85 µg/L, which increased to 1250 µg/L when repeated at six hours. Blood test results showed raised total cholesterol at 7.6 mmol /L and nil else. A chest radiograph showed clear lung fields. Blood glucose level was 6.3 mmol/L; a glycated haemoglobin test result is pending.”
Dependent on the case, you may need to describe the management plan so far or what further management you would recommend.“My management plan for this patient includes ACS [acute coronary syndrome] protocol, echocardiography, cardiology review, and treatment with high dose statins. If you are unsure what the management should be, you should say that you would discuss further with senior colleagues and the patient. At this point, check to see if there is a treatment escalation plan or a “do not attempt to resuscitate” order in place.
“Mr Murphy was given ACS protocol in the emergency department. An echocardiogram has been requested and he has been discussed with cardiology, who are going to come and see him. He has also been started on atorvastatin 80 mg nightly. Mr Murphy and his family are happy with this plan.”
The summary can be a concise recap of what you have presented beforehand or it can sometimes form a standalone presentation. Pick out salient points, such as positive findings—but also draw conclusions from what you highlight. Finish with a brief synopsis of the current situation (“currently pain free”) and next step (“awaiting cardiology review”). Do not trail off at the end, and state the diagnosis if you are confident you know what it is. If you are not sure what the diagnosis is then communicate this uncertainty and do not pretend to be more confident than you are. When possible, you should include the patient’s thoughts about the diagnosis, how they are feeling generally, and if they are happy with the management plan.
“In summary, Mr Murphy is a 56 year old man admitted with central crushing chest pain, radiating down his left arm, of 30 minutes’ duration. His cardiac risk factors include 20 pack year smoking history, positive family history, type 2 diabetes, and hypertension. Examination was normal other than tachycardia. However, 12 lead electrocardiography showed ST segment depression in the anterior leads and troponin rise from 85 to 250 µg/L. Acute coronary syndrome protocol was initiated and a diagnosis of NSTEMI [non-ST elevation myocardial infarction] was made. Mr Murphy is currently pain free and awaiting cardiology review.”
Originally published as: Student BMJ 2017;25:i4406
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed
- ↵ Green EH, Durning SJ, DeCherrie L, Fagan MJ, Sharpe B, Hershman W. Expectations for oral case presentations for clinical clerks: opinions of internal medicine clerkship directors. J Gen Intern Med 2009 ; 24 : 370 - 3 . doi:10.1007/s11606-008-0900-x pmid:19139965 . OpenUrl CrossRef PubMed Web of Science
- ↵ Olaitan A, Okunade O, Corne J. How to present clinical cases. Student BMJ 2010;18:c1539.
- ↵ Gaillard F. The secret art of relevant negatives, Radiopedia 2016; http://radiopaedia.org/blog/the-secret-art-of-relevant-negatives .
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies
Jay l goldstein, byron cryer.
- Author information
- Article notes
- Copyright and License information
Correspondence: Jay L Goldstein, Department of Medicine, North Shore University HealthSystem, 2650 Ridge Avenue, Evanston, IL 60201, USA, Tel +1 847 570 2510; +1 847 570 2615, Fax +1 847 570 2905, Email [email protected]
Collection date 2015.
The full terms of the License are available at http://creativecommons.org/licenses/by-nc/3.0/ . Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective anti-inflammatory and analgesic agents and are among the most commonly used classes of medications worldwide. However, their use has been associated with potentially serious dose-dependent gastrointestinal (GI) complications such as upper GI bleeding. GI complications resulting from NSAID use are among the most common drug side effects in the United States, due to the widespread use of NSAIDs. The risk of upper GI complications can occur even with short-term NSAID use, and the rate of events is linear over time with continued use. Although gastroprotective therapies are available, they are underused, and patient and physician awareness and recognition of some of the factors influencing the development of NSAID-related upper GI complications are limited. Herein, we present a case report of a patient experiencing a gastric ulcer following NSAID use and examine some of the risk factors and potential strategies for prevention of upper GI mucosal injuries and associated bleeding following NSAID use. These risk factors include advanced age, previous history of GI injury, and concurrent use of medications such as anticoagulants, aspirin, corticosteroids, and selective serotonin reuptake inhibitors. Strategies for prevention of GI injuries include anti-secretory agents, gastroprotective agents, alternative NSAID formulations, and nonpharmacologic therapies. Greater awareness of the risk factors and potential therapies for GI complications resulting from NSAID use could help improve outcomes for patients requiring NSAID treatment.
Keywords: side effects, ulcer, GI bleed, NSAID, gastrointestinal
A 53-year-old otherwise healthy female was admitted to the emergency department following two bouts of hematemesis and a single melenic stool. She denied abdominal pain or discomfort and reported no personal or family history of gastric ulcer. The patient reported being prescribed naproxen 500 mg twice daily for the 2 days prior for an ankle sprain. On examination, the patient was hypotensive in the supine position, with a blood pressure of 90/30 mmHg, and was tachycardic, with a heart rate of 130 beats per minute. Abdominal examination was benign without tenderness. Hemoglobin was 10.2 g/dL and hematocrit was 33.4%; all other evaluated laboratory values were within normal limits. Endoscopy revealed a 1×1 cm hemorrhagic gastric ulcer in the antrum with a visible vessel ( Figure 1 ), which was cauterized at the time of endoscopy. Biopsies of the antrum and body were negative for Helicobacter pylori . Cautery was successful, and the patient was treated with an intravenous proton-pump inhibitor (PPI) and remained hospitalized for observation and to evaluate for rebleeding. During hospitalization, the patient was transitioned to an oral PPI. After 2 days without evidence of rebleeding and with the patient’s vital signs returning to normal, she was discharged home with an oral PPI. Her naproxen was not continued.
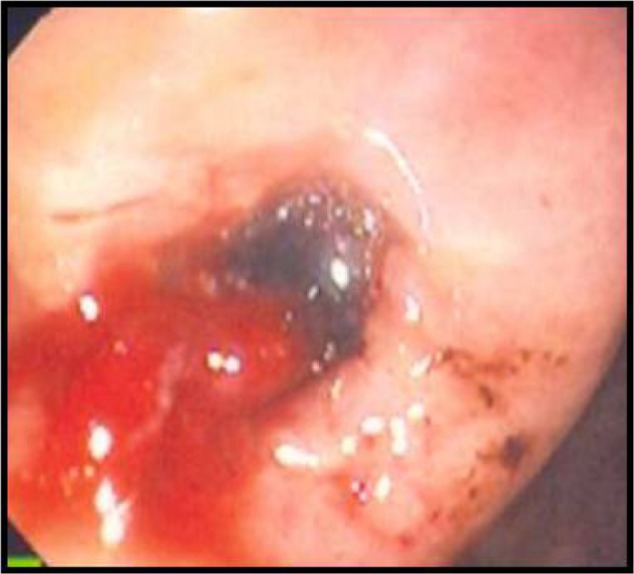
Endoscopic image of a 1×1 cm hemorrhagic gastric ulcer in the antrum with a visible vessel revealed by endoscopy.
Note: Endoscopy is from a 53-year-old woman presenting to the emergency department following two bouts of hematemesis and a melenic stool.
Nonsteroidal anti-inflammatory drug use and incidence of complications
Prevalence of nonsteroidal anti-inflammatory drug use.
Adequate pain management is a widespread clinical concern, and both prescription and over-the-counter (OTC) nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used for pain relief. 1 NSAID use may also be increasing, as indicated by a 2010 National Health Interview Survey (NHIS) that found that 12.8% of adults in the United States were taking NSAIDs at least three times a week for 3 months, representing an increase of over 40% compared with the results of a similar interview in 2005. 2 This volume of use and the increase represent substantial concerns, which are compounded by the results of telephone surveys indicating that up to 26% of OTC NSAID users take more than the recommended dose. 1 , 3
NSAID-associated gastrointestinal complications
NSAID use has been associated with cardiovascular (CV), renal, and gastrointestinal (GI) complications, and certain patients are at increased risk. NSAID use results in small but consistent increases in the risk of CV events such as myocardial infarction, affected in part by dose and potency of cyclooxygenase-2 (COX-2) inhibition. 4 , 5 NSAID use has also long been associated with kidney disease, 6 resulting in both acute and chronic impairments in kidney function. 7 These complications prompted the US Food and Drug Administration (FDA) to release a scientific statement in 2005 emphasizing “the importance of using the lowest effective dose for the shortest duration possible if treatment with an NSAID is warranted for an individual patient.” 8 , 9
More common than CV and renal complications associated with NSAID use are NSAID-related GI events, which are among the most common drug-related side effects in the United States 10 , 11 and occur in both prescription and OTC NSAID users. These complications include bleeding gastric or duodenal ulcers and, to a lesser extent, obstructions and perforations. 12 In a retrospective analysis of a rheumatoid arthritis patient database published in 2000, OTC ibuprofen and naproxen users had a relative risk for serious GI complications of approximately 3.5 compared with NSAID nonusers, 13 and it is estimated that 1%–2% of continuous NSAID users experience a clinically significant upper GI event per year. 14 – 18 These findings represent a significant clinical concern, as patients taking NSAIDs experience a relative risk of upper GI bleeding and perforations of up to 4.7 compared with nonusers. 19
GI complications are generally thought to be mediated primarily through inhibition of mucosal cyclooxygenase-1 (COX-1) and resultant suppression of prostaglandin production. 20 However, COX-2 inhibition and other mechanisms, such as changes in the bacterial microbiome in the gut or the generation of free radicals, are also possibly involved. 21 , 22 There is some question regarding the extent to which differential inhibition of COX-1 and -2 affects the GI risks associated with various NSAIDs. NSAIDs exhibit differential COX-1 and -2 inhibition and have been associated with different risks of GI and CV adverse events that vary among patients, 20 , 23 but data sufficient to justify differences in labeling among NSAIDs in the United States have not been established. 24 , 25
In contrast to the acute effects of NSAIDs on the GI tract, there is some evidence that NSAID use may reduce risks of GI cancers, including gastric, pancreatic, and colorectal cancers. 26 For example, several studies have found that nonaspirin NSAID treatments are associated with decreased risk of gastric cancer 27 , 28 and, in the case of celecoxib, increased regression of precancerous gastric lesions compared with placebo. 29 However, further study is needed to better characterize these potentially protective effects.
Upper GI complications
It is often noted that potentially serious GI complications commonly develop with no clinical warning symptoms suggestive of ulcers or bleeding. However, although NSAID users report increased frequency of various symptoms including reflux, belching, bloating, and/or nausea compared with nonusers, 30 these symptoms do not reliably indicate the presence of significant upper GI mucosal injury, 31 which includes ulcers, bleeding, perforation, obstruction, and extensive erosions. A prospective observational study found that bleeding complications occurred without typical ulcer symptoms (epigastric pain or dyspepsia) in up to 80% of affected patients. 32 In addition, a meta-analysis of studies performed before 1997 and from 1997–2008 found that, while the overall mortality rate from GI bleeds has fallen since the mid-1990s, NSAID users with upper GI bleeding or perforation exhibit a higher mortality rate from these injuries compared with nonusers with comparable clinical scenarios. 33 Endoscopic techniques are frequently used to manage peptic ulcer bleeding, but little research has been done to investigate whether NSAID use impacts the rate of successful hemostasis following endoscopic therapy. A retrospective study of only 76 patients found no association between NSAIDs and failure of endoscopy therapy for the treatment of gastric ulcer-associated bleeding, but the sample size was small. 34 Interestingly, a randomized controlled trial of 224 patients who developed ulcer complications following NSAID use found that use of a COX-2 selective NSAID (celecoxib) with no effect on platelet aggregation was associated with a lower risk of recurrent bleeding compared with PPI and nonselective NSAID co-therapy; however, both therapies conferred a significant risk of rebleeding. 35 These results led to a recommendation by the American College of Physicians that patients with previous ulcer bleeding who require an NSAID be treated with “the combination of a PPI and a COX-2 inhibitor.” 36
Lower GI complications
The rate of lower GI complications resulting from NSAID use has not been as widely documented as that of upper GI damage, but such complications have been recognized for decades. 37 These injuries include bleeding in the large and small bowel, strictures of the small bowel, or exacerbation of existing illnesses such as inflammatory bowel disease. 38 While the incidence of lower GI injury associated with NSAID use is somewhat lower than that of upper GI injury, results from a 2003 prospective study of rheumatoid arthritis patients found that 0.9% of patients taking naproxen 500 mg twice daily developed serious lower GI complications over a 1-year period, 39 and a 2005 retrospective study of health records in Spain found that, while the greater incidence of upper GI events results in more fatalities overall, upper and lower GI events have similar mortality rates. 40 Video capsule endoscopy assessment of the small bowel has allowed clinicians to better quantify NSAID-related small intestinal mucosal injury, 41 as shown by a study that found that healthy volunteers who received either naproxen 500 mg twice daily and omeprazole 20 mg once daily or celecoxib 200 mg twice daily for 2 weeks exhibited significantly more mucosal breaks compared with those receiving placebo. 42 Slow-release diclofenac also resulted in new small intestinal mucosal injury compared with baseline after 7 days of use in almost three-quarters of healthy volunteers assessed via capsule endoscopy. 43
It is hypothesized that asymptomatic small bowel mucosal injury may lead to occult blood loss over time, resulting in decreases in hemoglobin levels. Results from the CONDOR (celecoxib versus omeprazole and diclofenac in patients with Osteoarthritis and Rheumatoid arthritis) study, which compared celecoxib 200 mg twice daily with diclofenac slow-release 75 mg twice daily plus omeprazole (a PPI) 20 mg once daily in arthritis patients at high risk of upper GI complications, support this concept. In that study, investigators found that, while upper GI events did not differ among treatment groups, use of diclofenac and omeprazole resulted in 3.4% of patients exhibiting a ≥2.0 g/L decrease in hemoglobin over approximately 6 months in the absence of overt bleeding. 44 , 45 This finding suggests that GI blood loss may have been more related to slow occult bleeding. 46 Together, these studies suggest that lower bowel injuries should be taken into account when considering the risks of NSAID use and when considering managing long-term risk.
Risks of NSAID-associated GI injuries over time
The risk of NSAID-associated GI complications is dose dependent and remains linear over time, based on the results of randomized controlled trials. 14 , 15 The 6-month Misoprostol Ulcer Complication Outcomes Safety Assessment (MUCOSA) study, which investigated the effects of misoprostol coadministration on GI complications associated with NSAID use, and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study, which compared the GI risks of naproxen with rofecoxib, both found that the cumulative incidence of upper GI events in the nonselective NSAID treatment arms was linear over time ( Figure 2 ). 14 , 15 , 47 In addition, two 6-month, double-blind, prospective, randomized clinical trials investigating lower GI injury following NSAID use found that the rate of patients meeting the endpoint of a decrease in hemoglobin >2 g/dL was roughly constant over time. 47 Confirming these results in a clinical practice setting was the Gastrointestinal Randomized Event and Safety Open-label Non-steroidal anti-inflammatory drug Study (GI-REASONS), a randomized, prospective, open-label study comparing celecoxib with nonselective NSAIDs in osteoarthritis (OA) patients, which also found that the cumulative incidence of clinically significant upper and lower GI events was roughly linear over the 6-month trial period. 18 Because GI complications associated with NSAID use are dose related and can occur at any time following exposure, 32 several international societies, including the National Institute for Health and Care Excellence (NICE) in the United Kingdom, the American College of Gastroenterology, and the American College of Rheumatology (ACR), have recommended identification of risk factors and prophylaxis independent of the presence or absence of symptoms in patients with moderate-to-high risk of GI complications ( Table 1 ). 48 – 50
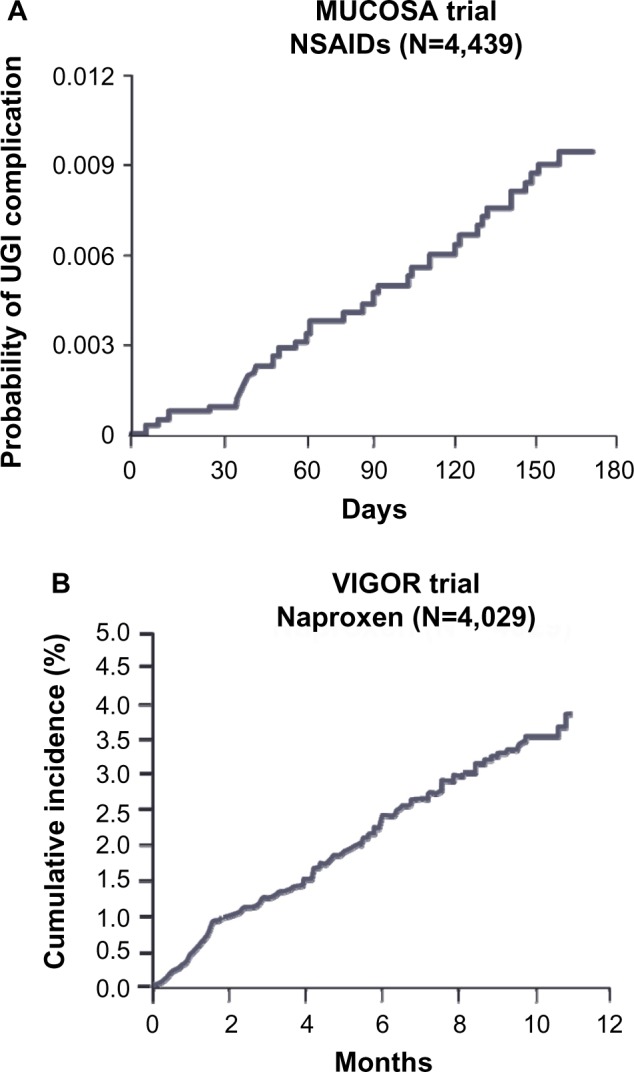
Incidence of UGI complications with increasing duration of NSAIDs in the MUCOSA and VIGOR trials.
Notes: The MUCOSA trial ( A ) evaluated the effects of misoprostol- co-administration with a variety of nonselective NSAIDs (eg, naproxen, ibuprofen, diclofenac, and others) on gastrointestinal complication rates. Reproduced from Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med . 1995;123(4): 241–249. 14 The VIGOR trial ( B ) compared the gastrointestinal effects of naproxen and rofecoxib overtime. Reproduced from N Engl J Med, Bombardier C, Laine L, Reicin A, et al; VIGOR Study Group, Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group . 2000;343(21):1520–1528. Copyright ©2000 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. 15 In both trials, the incidence of upper gastrointestinal complications with NSAIDs was began early, linear, constant, and was present for the duration of NSAID exposure.
Abbreviations: MUCOSA, Misoprostol Ulcer Complication Outcomes Safety Assessment; NSAID, nonsteroidal anti-inflammatory drug; UGI, upper gastrointestinal; VIGOR, Vioxx Gastrointestinal Outcomes Research.
Characteristics of patients with an elevated risk for NSAID-associated gastrointestinal complications
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
Risk factors for NSAID-associated GI injury
A variety of patient characteristics are associated with increased risk for NSAID-related GI complications ( Table 1 ). Patients with a history of GI injury are at higher risk for GI complications following NSAID use, 14 , 51 and patients with renal failure who are on hemodialysis also exhibit increased risk of GI bleeding with NSAID use. 52 Age is an important factor, with risk increasing with increasing age. As the absolute risk varies by age, a threshold of risk based on age is often suggested to be >60 years old. 53 , 54 Patients taking high-dose NSAIDs and those taking NSAIDs with aspirin, even at low, CV-prevention doses (≤325 mg/day), have elevated risks of GI events. 55 Certain medications also increase the risk of GI injury when used concurrently with NSAIDs. For example, use of oral corticosteroids coadministered with NSAIDs is associated with an increase in the rate of GI complications as much as twofold compared with patients taking NSAIDs alone. 55 Anticoagulants have been found to substantially increase the risk for elderly patients of developing ulcer bleeding when used with NSAIDs. 56 Additionally, a Danish study of prescription and hospitalization records of patients ages 16 to 105 years found that anticoagulants and nonsalicylate NSAIDs taken concurrently increased upper GI bleeding more than anticoagulants taken with aspirin or acetaminophen. 58 Furthermore, the increased risk of ulcer bleeding due to anticoagulant use may not be mitigated by gastroprotective agents. 59 Selective serotonin reuptake inhibitors also increase risk of upper GI complications when used with NSAIDs, as several studies have shown that concurrent selective serotonin reuptake inhibitor and NSAID use results in a greater increase in the incidence of GI bleeding than the sum of their independent effects would suggest. 61 – 64 These results suggest that caution should be used when considering prescribing NSAIDs to patients using these agents.
The limited awareness of risk factors results in many patients receiving inadequate preventative therapies. For example, a study of veterans prescribed NSAIDs over a 1-year period showed that nearly three-quarters (73%) of the patients with risk factors for NSAID-related upper GI injury were not receiving appropriate gastroprotective therapy based on evidence-based guidelines. 65 In fact, prescription practices may frequently be inappropriate when a patient’s GI and CV history are considered, according to results from a Spanish National Health System study conducted in 2011, which found that 74% of OA patients with elevated risk for GI and CV NSAID-related side effects were receiving nonselective NSAIDs or COX-2 selective NSAIDs. 66 These data indicate that not only do NSAIDs represent heightened risks to some patients, but that awareness of the risk factors and of the use of preventative therapy for NSAID-related upper GI injury could be improved.
Approaches to the prevention of GI injuries from NSAIDs
Ppis and histamine-2 (h2) receptor antagonists.
Coadministration of NSAIDs with PPIs is a well-documented and effective, although underutilized, approach to reduce endoscopic damage and control dyspeptic symptoms associated with the use of NSAIDs ( Table 2 ). 65 , 67 – 69 Infrequent side effects associated with PPIs have occurred; these may include an increased chance of pneumonia compared with nonusers, 12 , 70 hypomagnesemia, 71 and increased incidence of spine and hip fractures, 72 as well as an increased chance of contracting Clostridium difficile -associated diarrhea compared with PPI-naïve patients. 73 H2 receptor antagonists (H 2 RAs), which inhibit acid secretion, have also been evaluated for reducing NSAID-associated complications. A meta-analysis of 14 trials found that H 2 RAs (eg, famotidine and ranitidine) were protective at high doses, but, at commonly prescribed doses, they reduced the risk of duodenal but not gastric ulcers. 82 This was demonstrated in a 24-week study of patients requiring chronic NSAID treatment, which showed that coadministration of high-dose (80 mg/day) famotidine with ibuprofen (2,400 mg/day) resulted in significantly fewer gastroduodenal ulcers compared with ibuprofen alone. 76 Rare side effects associated with H 2 RA use include increased risk of pneumonia. 68
Gastroprotective strategies to prevent gastrointestinal complications associated with NSAID use
Only effective as a protective agent at 300 mg twice daily for ranitidine, ≥40 mg twice daily for nizatidine, and 80 mg total daily dose for famotidine.
Abbreviations: COX, cyclooxygenase; NSAID, nonsteroidal anti-inflammatory drug.
Misoprostol
Co-prescribed misoprostol, a synthetic prostaglandin used to counteract the prostaglandin-inhibitory actions of NSAIDs, decreases NSAID-related upper GI complications by approximately 40% compared with NSAIDs alone. 14 , 83 The single-tablet combination therapy of diclofenac sodium–misoprostol was found to be an effective gastroprotective therapy measured by cost per life-year gained in a study in the Netherlands. 84 Of note is that the single-tablet formulation may improve patient adherence, as nonadherence with multiple-pill therapeutic regimens increases the risk of GI events. 69 , 85 , 86 Unfortunately, misoprostol is not always well tolerated due to diarrhea and abdominal pain, preventing continued use; lower doses of misoprostol with a lesser frequency of side effects may also be less effective at preventing GI events. 74 Misoprostol is an abortifacient, contraindicating its use in women who are pregnant or may become pregnant ( Table 2 ). 75 Rebamipide is a cytoprotective anti-ulcer drug that stimulates prostaglandin production. At a dose of 100 mg three times daily (TID), it was found to be significantly more effective in reducing rates of endoscopic gastric or duodenal ulcer compared with misoprostol 200 mg TID in the Study of NSAID-induced GI Toxicity Prevention by Rebamipide and Misoprostol (STORM), a multicenter, 12-week, randomized controlled trial of patients using NSAIDs. Rebamipide is not approved for use in the United States. 87
COX-2 inhibitors
COX-2 selective inhibitors are associated with less risk of GI injury than nonselective NSAIDs due to lesser inhibition of COX-1, which is involved in the maintenance of gastric mucosa integrity. COX-2 selective inhibitor use was associated with a decrease in the risk of symptomatic ulcers and clinically significant ulcer complications compared with nonselective NSAIDs, according to a 2007 meta-analysis. 88 In the CONDOR study, while efficacy was similar, fewer patients treated with the COX-2 selective inhibitor celecoxib experienced reductions in hemoglobin or withdrew from the study due to adverse GI events compared with those treated with diclofenac plus omeprazole. 44 , 45 However, use of COX-2 inhibitors at therapeutic doses may increase CV risks. 77 Following an FDA warning about the CV risks of COX-2 inhibitors and all nonselective NSAIDs, and the voluntary withdrawals of rofecoxib and valdecoxib from the market, 8 , 89 prescriptions for COX-2 inhibitors decreased by 12% and those for nonselective NSAIDs increased by 47% between 2003 and 2005. However, this trend was not accompanied by an increase in prescriptions of gastroprotective co-therapies. 90
Enteric-coated NSAIDs
While at least one study found that enteric-coated NSAIDs reduce upper GI events, 91 most data indicate that enteric-coated NSAIDs do not reduce incidence of upper GI events compared with other formulations. 92 – 94 Interestingly, attempts to reduce upper GI symptoms through use of slow-release and enteric-coated formulations may hypothetically increase lower GI complications. 95
Topical NSAIDs
Because NSAID-associated GI complications are dose dependent, development of formulations that lower systemic exposure while providing efficacious pain relief may reduce GI injury. Topical NSAID formulations can produce higher concentrations of drug in local tissue with very low systemic exposure as measured via plasma concentrations, 96 and use of topical NSAIDs may be associated with fewer GI events ( Table 2 ). 97 – 101 Although topical NSAID formulations have been shown to be effective in treatment of acute pain, 78 and for short-term use in treating chronic pain, there are conflicting results regarding whether topical NSAIDs provide effective long-term pain relief. 102 , 103 Further study is necessary to determine the long-term benefits and risks of topical NSAID use.
Lower-dose NSAID formulations
New formulations of NSAIDs may reduce risks of adverse events by using lower doses while providing effective analgesia ( Table 2 ). There is some evidence that some NSAIDs, such as diclofenac, could provide effective pain relief at lower doses than are currently used, assuming 80% inhibition of COX-2 is necessary for therapeutic efficacy. 51 This would hypothetically provide effective pain relief with an improved GI safety profile due to lessened inhibition of COX-1. 20
A diclofenac potassium liquid-filled capsule 104 using a formulation designed to deliver diclofenac more rapidly than conventional tablets was approved by the FDA in 2009. 105 , 106 Absorption of the liquid-filled capsules is faster than that of diclofenac potassium immediate-release tablets, and the capsules produce greater pain relief compared with placebo at lower doses of diclofenac (25 mg four times daily) than are generally used; 79 , 105 , 107 however, it is unclear whether they produce more rapid or effective pain relief than other diclofenac formulations. Lower-dose capsules that contain finely milled, rapidly absorbed NSAID particles may also provide analgesia at lower systemic doses. 80 , 81 Low-dose diclofenac capsules (18 mg or 35 mg TID for mild-to-moderate pain) and indomethacin capsules (20 mg TID or 40 mg two times daily or TID) containing fine-milled particles have been approved by the FDA for treatment of mild-to-moderate acute pain in adults 25 , 108 and have been found to provide effective relief of acute, postoperative pain in Phase III studies. 80 , 81 Additionally, low-dose diclofenac (35 mg TID) has been shown to provide effective treatment of OA pain in a 12-week study and has been approved by the FDA for management of OA-related pain. 109 A low-dose naproxen capsule was also found to effectively relieve postoperative dental pain in a Phase II study. 110
Eradicating H. pylori may decrease GI risks in some NSAID users, which could reduce worldwide incidence of NSAID-related GI injury, as H. pylori affects up to 50% of the worldwide population. 111 One systematic literature review found that H. pylori eradication in infected patients was as effective as the use of PPIs in preventing GI complications due to NSAID use; 57 however, another found that, although H. pylori eradication reduces risk, PPIs provided superior ulcer prevention. 112 While it is unclear whether H. pylori eradication is as effective as other strategies, it may provide benefit to some patients.
Nonpharmacologic therapies
Another possibility for reducing the incidence of NSAID-associated GI complications is to reduce NSAID use through adoption of alternative therapies. While assessment of their effectiveness is challenging, therapies such as acupuncture and physical therapy/exercise may provide relief for some patients. While some randomized controlled trials have found acupuncture to be more effective for OA pain relief than sham treatments, a meta-analysis of eleven studies published between 1994 and 2006 found sufficiently heterogeneous results that the authors were unable to draw firm conclusions regarding acupuncture’s efficacy. 113 While significant results have been found for use of acupuncture, particularly for knee OA, 114 the effect is generally small, and larger studies are needed. 115 Exercise and physical therapy may also provide pain relief, as they have been found to improve pain and function in knee OA, 116 may delay the need for surgical intervention, 117 and may reduce the need for medication. 118 Because of these results, the ACR has issued guidelines strongly recommending exercise for knee OA. 50 Unfortunately, the effect of exercise on knee OA may be short-term, and the extent of functional improvement is unclear. 119 While many approaches for prevention of NSAID-associated GI injury show effectiveness in some studies, practical considerations prevent their universal use.
Cost-effectiveness
The direct cost of preventative strategies to patients and payers and the absolute patient risk for GI complications are the key factors that influence cost-effectiveness. The relative cost of preventing a single complication is high in low-risk populations and is the basis of recommendations from the ACR and other health authorities that indicate that low-risk patients should not receive gastroprotective therapies. 49 , 120 , 121 The picture becomes more complicated in patients with higher risk for GI injury; as risk increases, the associated costs of prescribing such therapies is increasingly offset by the escalating cumulative costs associated with the adverse events. Net costs/savings are driven by the additional procurement costs of the preventative therapy and the expected frequency of adverse events based on risk. 122 Many studies have tried to evaluate cost-effectiveness, but it is difficult to make decisions based on finances given that the cost of PPIs has dramatically declined and that PPIs are now available OTC. Additionally, cost-effectiveness studies do not always adequately take into account the impact of injury on quality of life. An economic model examining PPI use in three large randomized trials, weighted by quality of life, found that use of PPIs with either COX-2 selective inhibitors or nonselective NSAIDs was cost-effective in OA patients, even those at low risk of GI events, with the caveat that the mean age of participants was above 60 years and thus these patients may not be considered to be objectively low risk. 123 These studies suggest that the economic picture of how to most cost-effectively decrease NSAID-associated GI injury is not yet clear and that, together, studies considering different risk pools are necessary to determine optimal management of patient subpopulations.
Balancing risks
The CV risks associated with NSAID use have received increased attention recently, such that the American Heart Association released a statement declaring that NSAID use should be “coupled with the realization that effective pain relief may come at the cost of a small but real increase in risk for cardiovascular or cerebrovascular complications.” 9 In February 2014, the FDA reviewed the data surrounding the CV complications associated with NSAID use and determined that there were insufficient data to distinguish CV toxicity among individual NSAIDs, including COX-2 selective inhibitors and naproxen, and that this class of agents was associated with an increased risk of ischemic events. 25 This attention to the risks associated with NSAIDs and the potential differences among specific NSAIDs represents a growing awareness about the complications associated with this class of drugs.
GI mucosal injury associated with use of NSAIDs is a serious clinical concern, and studies suggest that the rate of complications does not decrease with duration of use. There are several strategies and NSAID drug product formulations that may be associated with decreased GI risk, but there is no one therapy that will provide optimal pain relief and decrease risk for all patients. Also, although nonpharmacological therapies have promise, often they have been inadequately studied compared with pharmacological therapies. Meanwhile, the high cost of GI events to the health care system and to patients’ quality of life necessitates improvement in the risk–benefit profile of NSAIDs or development of alternative medications or therapies. In addition, the CV and renal side effects of NSAIDs must be considered alongside reducing the risk of GI complications. Optimally, developments in pain management will focus on tailoring therapies to the individual patient. Also, in addition to development of new therapies, improvements in patient and provider education and patient adherence are necessary to improve outcomes. Greater awareness of the short-term GI risks of NSAIDs, including potential overuse of OTC NSAIDs and more frequent use of gastroprotection, might have prevented the ulcer in the patient described in the case study at the beginning of this article.
Acknowledgments
Editorial support for this manuscript was provided by Jill See, PhD, and Colville Brown, MD, of AlphaBioCom LLC (King of Prussia, PA, USA). Funding for editorial support was provided by Iroko Pharmaceuticals, LLC (Philadelphia, PA, USA).
JLG has served as an advisory board attendee for Iroko Pharmaceuticals, LLC. BC has served as a consultant for Iroko Pharmaceuticals, LLC; Ritter Pharmaceuticals; Sanofi Pharmaceuticals; Sandoz Pharmaceuticals; and Sucampo, Inc. The authors report no other conflicts of interest in this work.
- 1. Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J Rheumatol. 2005;32(11):2218–2224. [ PubMed ] [ Google Scholar ]
- 2. Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23(1):43–50. doi: 10.1002/pds.3463. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Goldstein JL, Lefkowith JB. Public misunderstanding of nonsteroidal antiinflammatory drug (NSAID)-mediated gastrointestinal (GI) toxicity: a serious potential health threat. Gastroenterology. 1998;114:A136. [ Google Scholar ]
- 4. Capone ML, Tacconelli S, Rodriguez LG, Patrignani P. NSAIDs and cardiovascular disease: transducing human pharmacology results into clinical read-outs in the general population. Pharmacol Rep. 2010;62(3):530–535. doi: 10.1016/s1734-1140(10)70310-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. García Rodríguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol. 2008;52(20):1628–1636. doi: 10.1016/j.jacc.2008.08.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. doi: 10.18433/j3vw2f. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol. 2006;2(2):80–91. doi: 10.1038/ncpneph0076. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Public Health Advisory – FDA Announces Important Changes and Additional Warnings for COX-2 Selective and Non-Selective Non-Steroidal AntiInflammatory Drugs (NSAIDs) [webpage on the Internet] Silver Spring, MD: US Food and Drug Administration; 2005. [Accessed October 10, 2014]. [updated August 16, 2013; accessed October 10, 2014]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm . [ Google Scholar ]
- 9. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA, American Heart Association Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Butt JH, Barthel JS, Moore RA. Clinical spectrum of the upper gastrointestinal effects of nonsteroidal anti-inflammatory drugs. Natural history, symptomatology, and significance. Am J Med. 1988;84(2A):5–14. doi: 10.1016/0002-9343(88)90248-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Fries J. Toward an understanding of NSAID-related adverse events: the contribution of longitudinal data. Scand J Rheumatol Suppl. 1996;102:3–8. doi: 10.3109/03009749609097225. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. de Jager CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941–949. doi: 10.1111/apt.12069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Singh G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: a view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am J Ther. 2000;7(2):115–121. doi: 10.1097/00045391-200007020-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123(4):241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Bombardier C, Laine L, Reicin A, et al. VIGOR Study Group Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520–1528. doi: 10.1056/NEJM200011233432103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. doi: 10.1001/jama.284.10.1247. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Laine L, Bombardier C, Hawkey CJ, et al. Stratifying the risk of NSAID-related upper gastrointestinal clinical events: results of a double-blind outcomes study in patients with rheumatoid arthritis. Gastroenterology. 2002;123(4):1006–1012. doi: 10.1053/gast.2002.36013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Cryer B, Li C, Simon LS, Singh G, Stillman MJ, Berger MF. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol. 2013;108(3):392–400. doi: 10.1038/ajg.2012.467. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343(8900):769–772. doi: 10.1016/s0140-6736(94)91843-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96(13):7563–7568. doi: 10.1073/pnas.96.13.7563. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73(Suppl 1):136–150. doi: 10.1159/000089789. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Bruno A, Tacconelli S, Patrignani P. Variability in the response to non-steroidal anti-inflammatory drugs: mechanisms and perspectives. Basic Clin Pharmacol Toxicol. 2014;114(1):56–63. doi: 10.1111/bcpt.12117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Celebrex® (celecoxib) [package insert] New York, NY: Pfizer; 2008. [ Google Scholar ]
- 25. O’Riordan M. FDA Advisory Panel Votes Against CV Safety Claim for Naproxen [webpage on the Internet] New York, NY: Medscape; 2014. [Accessed May 14, 2014]. Available from: http://www.medscape.com/viewarticle/820470 . [ Google Scholar ]
- 26. Sahin IH, Hassan MM, Garrett CR. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett. 2014;345(2):249–257. doi: 10.1016/j.canlet.2013.09.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Lindblad M, Lagergren J, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):444–450. doi: 10.1158/1055-9965.EPI-04-0467. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Sørensen HT, Friis S, Nørgård B, et al. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88(11):1687–1692. doi: 10.1038/sj.bjc.6600945. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Wong BC, Zhang L, Ma JL, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61(6):812–818. doi: 10.1136/gutjnl-2011-300154. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3. doi: 10.1186/ar4175. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987;28(5):527–532. doi: 10.1136/gut.28.5.527. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 1996;156(14):1530–1536. [ PubMed ] [ Google Scholar ]
- 33. Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol. 2009;9:41. doi: 10.1186/1471-230X-9-41. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Siva R, Al Zubaidi G, Masoud AK, Nihar M. Predictive factors for failure of endoscopic management therapy in peptic ulcer bleeding. Saudi J Gastroenterol. 2002;8(1):17–21. [ PubMed ] [ Google Scholar ]
- 35. Lai KC, Chu KM, Hui WM, et al. Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med. 2005;118(11):1271–1278. doi: 10.1016/j.amjmed.2005.04.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Barkun AN, Bardou M, Kuipers EJ, et al. International Consensus Upper Gastrointestinal Bleeding Conference Group International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Langman MJ, Morgan L, Worrall A. Use of anti-inflammatory drugs by patients admitted with small or large bowel perforations and haemorrhage. Br Med J (Clin Res Ed) 1985;290(6465):347–349. doi: 10.1136/bmj.290.6465.347. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Bjorkman D. Nonsteroidal anti-inflammatory drug-associated toxicity of the liver, lower gastrointestinal tract, and esophagus. Am J Med. 1998;105(5A):17S–21S. doi: 10.1016/s0002-9343(98)00276-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124(2):288–292. doi: 10.1053/gast.2003.50054. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Lanas A, Perez-Aisa MA, Feu F, et al. Investigators of the Asociación Española de Gastroenterología (AEG) A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol. 2005;100(8):1685–1693. doi: 10.1111/j.1572-0241.2005.41833.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Chan FK, Cryer B, Goldstein JL, et al. A novel composite endpoint to evaluate the gastrointestinal (GI) effects of nonsteroidal antiinflammatory drugs through the entire GI tract. J Rheumatol. 2010;37(1):167–174. doi: 10.3899/jrheum.090168. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG, Investigators Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3(2):133–141. doi: 10.1016/s1542-3565(04)00619-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128(5):1172–1178. doi: 10.1053/j.gastro.2005.03.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376(9736):173–179. doi: 10.1016/S0140-6736(10)60673-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Kellner HL, Li C, Essex MN. Celecoxib and diclofenac plus omeprazole are similarly effective in the treatment of arthritis in patients at high GI risk in the CONDOR Trial. Open Rheumatol J. 2013;7:96–100. doi: 10.2174/1874312901307010096. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Bowen B, Yuan Y, James C, Rashid F, Hunt RH. Time course and pattern of blood loss with ibuprofen treatment in healthy subjects. Clin Gastroenterol Hepatol. 2005;3(11):1075–1082. doi: 10.1016/s1542-3565(05)00605-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Goldstein JL, Chan FK, Lanas A, et al. Haemoglobin decreases in NSAID users over time: an analysis of two large outcome trials. Aliment Pharmacol Ther. 2011;34(7):808–816. doi: 10.1111/j.1365-2036.2011.04790.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Conaghan PG, Dickson J, Grant RL, Guideline Development Group Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336(7642):502–503. doi: 10.1136/bmj.39490.608009.AD. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Lanza FL, Chan FK, Quigley EM, Practice Parameters Committee of the American College of Gastroenterology Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi: 10.1038/ajg.2009.115. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Van Hecken A, Schwartz JI, Depré M, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000;40(10):1109–1120. [ PubMed ] [ Google Scholar ]
- 52. Jankovic SM, Aleksic J, Rakovic S, et al. Nonsteroidal antiinflammatory drugs and risk of gastrointestinal bleeding among patients on hemodialysis. J Nephrol. 2009;22(4):502–507. [ PubMed ] [ Google Scholar ]
- 53. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991;91(3):213–222. doi: 10.1016/0002-9343(91)90118-h. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Hernández-Díaz S, Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160(14):2093–2099. doi: 10.1001/archinte.160.14.2093. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Garcia Rodríguez LA, Hernández-Díaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001;3(2):98–101. doi: 10.1186/ar146. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med. 1993;153(14):1665–1670. [ PubMed ] [ Google Scholar ]
- 57. Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007;11(51):iii–iv. 1–164. doi: 10.3310/hta11510. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Johnsen SP, Sørensen HT, Mellemkjoer L, et al. Hospitalisation for upper gastrointestinal bleeding associated with use of oral anticoagulants. Thromb Haemost. 2001;86(2):563–568. [ PubMed ] [ Google Scholar ]
- 59. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Investigators of the Asociación Española de Gastroenterología (AEG) Effect of anti-secretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102(3):507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Masclee GM, Valkhoff VE, van Soest EM, et al. Cyclo-oxygenase-2 inhibitors or nonselective NSAIDs plus gastroprotective agents: what to prescribe in daily clinical practice? Aliment Pharmacol Ther. 2013;38(2):178–189. doi: 10.1111/apt.12348. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. de Abajo FJ, Rodríguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319(7217):1106–1109. doi: 10.1136/bmj.319.7217.1106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. de Jong JC, van den Berg PB, Tobi H, de Jong-van den Berg LT. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55(6):591–595. doi: 10.1046/j.0306-5251.2002.01770.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27(1):31–40. doi: 10.1111/j.1365-2036.2007.03541.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Helin-Salmivaara A, Huttunen T, Grönroos JM, Klaukka T, Huupponen R. Risk of serious upper gastrointestinal events with concurrent use of NSAIDs and SSRIs: a case-control study in the general population. Eur J Clin Pharmacol. 2007;63(4):403–408. doi: 10.1007/s00228-007-0263-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Abraham NS, El-Serag HB, Johnson ML, et al. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129(4):1171–1178. doi: 10.1053/j.gastro.2005.08.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011;9:38. doi: 10.1186/1741-7015-9-38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Smalley W, Stein CM, Arbogast PG, Eisen G, Ray WA, Griffin M. Underutilization of gastroprotective measures in patients receiving nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2002;46(8):2195–2200. doi: 10.1002/art.10425. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Sturkenboom MC, Burke TA, Tangelder MJ, Dieleman JP, Walton S, Goldstein JL. Adherence to proton pump inhibitors or H2-receptor antagonists during the use of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2003;18(11–12):1137–1147. doi: 10.1046/j.1365-2036.2003.01795.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Goldstein JL, Howard KB, Walton SM, McLaughlin TP, Kruzikas DT. Impact of adherence to concomitant gastroprotective therapy on non-steroidal-related gastroduodenal ulcer complications. Clin Gastroenterol Hepatol. 2006;4(11):1337–1345. doi: 10.1016/j.cgh.2006.08.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310–319. doi: 10.1503/cmaj.092129. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405–413. doi: 10.1111/j.1365-2036.2012.05201.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526. doi: 10.1016/j.amjmed.2011.01.007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001–1010. doi: 10.1038/ajg.2012.179. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Rostom A, Dube C, Wells G, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;(4):CD002296. doi: 10.1002/14651858.CD002296. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99(Suppl 2):S160–S167. doi: 10.1016/j.ijgo.2007.09.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Laine L, Kivitz AJ, Bello AE, Grahn AY, Schiff MH, Taha AS. Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers. Am J Gastroenterol. 2012;107(3):379–386. doi: 10.1038/ajg.2011.443. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098. doi: 10.1371/journal.pmed.1001098. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010;(6):CD007402. doi: 10.1002/14651858.CD007402.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Lissy M, Scallion R, Stiff DD, Moore K. Pharmacokinetic comparison of an oral diclofenac potassium liquid-filled soft gelatin capsule with a diclofenac potassium tablet. Expert Opin Pharmacother. 2010;11(5):701–708. doi: 10.1517/14656561003614773. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Gibofsky A, Silberstein S, Argoff C, Daniels S, Jensen S, Young CL. Lower-dose diclofenac submicron particle capsules provide early and sustained acute patient pain relief in a phase 3 study. Postgrad Med. 2013;125(5):130–138. doi: 10.3810/pgm.2013.09.2693. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Altman R, Daniels S, Young CL. Indomethacin submicron particle capsules provide effective pain relief in patients with acute pain: a phase 3 study. Phys Sportsmed. 2013;41(4):7–15. doi: 10.3810/psm.2013.11.2031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Rostom A, Wells G, Tugwell P, Welch V, Dubé C, McGowan J. The prevention of chronic NSAID induced upper gastrointestinal toxicity: a Cochrane collaboration metaanalysis of randomized controlled trials. J Rheumatol. 2000;27(9):2203–2214. [ PubMed ] [ Google Scholar ]
- 83. Bocanegra TS, Weaver AL, Tindall EA, et al. Diclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or hip: a randomized, placebo controlled trial. Arthrotec Osteoarthritis Study Group. J Rheumatol. 1998;25(8):1602–1611. [ PubMed ] [ Google Scholar ]
- 84. Al MJ, Maniadakis N, Grijseels EW, Janssen M. Costs and effects of various analgesic treatments for patients with rheumatoid arthritis and osteoarthritis in the Netherlands. Value Health. 2008;11(4):589–599. doi: 10.1111/j.1524-4733.2007.00303.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Sturkenboom MC, Burke TA, Dieleman JP, Tangelder MJ, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford) 2003;42(Suppl 3):iii23–31. doi: 10.1093/rheumatology/keg495. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Lanas A, Polo-Tomás M, Roncales P, Gonzalez MA, Zapardiel J. Prescription of and adherence to non-steroidal anti-inflammatory drugs and gastroprotective agents in at-risk gastrointestinal patients. Am J Gastroenterol. 2012;107(5):707–714. doi: 10.1038/ajg.2012.13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Park SH, Cho CS, Lee OY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: a randomized, multicenter, controlled trial-STORM STUDY. J Clin Biochem Nutr. 2007;40(2):148–155. doi: 10.3164/jcbn.40.148. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Rostom A, Muir K, Dubé C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5(7):818–828. 828. e1–e5. doi: 10.1016/j.cgh.2007.03.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. US Food and Drug Administration . FDA issues public health advisory on Vioxx as its manufacturer voluntarily withdraws the product [press release] Silver Spring, MD: US Food and Drug Administration; 2004. [Accessed October 2, 2014]. [September 30] [updated April 2, 2013; cited October 10, 2014]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108361.htm . [ Google Scholar ]
- 90. Greenberg JD, Fisher MC, Kremer J, et al. CORRONA Investigators The COX-2 inhibitor market withdrawals and prescribing patterns by rheumatologists in patients with gastrointestinal and cardiovascular risk. Clin Exp Rheumatol. 2009;27(3):395–401. [ PubMed ] [ Google Scholar ]
- 91. Caldwell JR, Roth SH. A double blind study comparing the efficacy and safety of enteric coated naproxen to naproxen in the management of NSAID intolerant patients with rheumatoid arthritis and osteoarthritis. Naproxen EC Study Group. J Rheumatol. 1994;21(4):689–695. [ PubMed ] [ Google Scholar ]
- 92. Le Loët X, Dreiser RL, Le Gros V, Febvre N. Therapeutic equivalence of diclofenac sustained-released 75 mg tablets and diclofenac enteric-coated 50 mg tablets in the treatment of painful osteoarthritis. Int J Clin Pract. 1997;51(6):389–393. [ PubMed ] [ Google Scholar ]
- 93. Khong TK, Downing ME, Ellis R, Patchett I, Trayner J, Miller AJ. The efficacy and tolerability of enteric and non-enteric coated naproxen tablets: a double-blind study in patients with osteoarthritis. Curr Med Res Opin. 1991;12(8):540–546. doi: 10.1185/03007999109111664. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Bellamy N, Beaulieu A, Bombardier C, et al. Efficacy and tolerability of enteric-coated naproxen in the treatment of osteoarthritis and rheumatoid arthritis: a double-blind comparison with standard naproxen followed by an open-label trial. Curr Med Res Opin. 1992;12(10):640–651. doi: 10.1185/03007999209111531. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Davies NM. Sustained release and enteric coated NSAIDs: are they really GI safe? J Pharm Pharm Sci. 1999;2(1):5–14. [ PubMed ] [ Google Scholar ]
- 96. Rolf C, Engström B, Beauchard C, Jacobs LD, Le Liboux A. Intra-articular absorption and distribution of ketoprofen after topical plaster application and oral intake in 100 patients undergoing knee arthroscopy. Rheumatology (Oxford) 1999;38(6):564–567. doi: 10.1093/rheumatology/38.6.564. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Martens M. Efficacy and tolerability of a topical NSAID patch (local action transcutaneous flurbiprofen) and oral diclofenac in the treatment of soft-tissue rheumatism. Clin Rheumatol. 1997;16(1):25–31. doi: 10.1007/BF02238759. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31(10):2002–2012. [ PubMed ] [ Google Scholar ]
- 99. Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143(3):238–245. doi: 10.1016/j.pain.2009.03.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Brewer AR, Pierchala LA, Yanchick JK, Magelli M, Rovati S. Gastrointestinal tolerability of diclofenac epolamine topical patch 1.3%: a pooled analysis of 14 clinical studies. Postgrad Med. 2011;123(4):168–176. doi: 10.3810/pgm.2011.07.2316. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Peniston JH, Gold MS, Wieman MS, Alwine LK. Long-term tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Clin Interv Aging. 2012;7:517–523. doi: 10.2147/CIA.S35416. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Lin J, Zhang W, Jones A, Doherty M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. BMJ. 2004;329(7461):324. doi: 10.1136/bmj.38159.639028.7C. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Rother M, Lavins BJ, Kneer W, Lehnhardt K, Seidel EJ, Mazgareanu S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis. 2007;66(9):1178–1183. doi: 10.1136/ard.2006.065128. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Zipsor® (diclofenac potassium) [package insert] Newport, KY: Xanodyne Pharmaceuticals, Inc; 2009. [ Google Scholar ]
- 105. Daniels SE, Baum DR, Clark F, Golf MH, McDonnell ME, Boesing SE. Diclofenac potassium liquid-filled soft gelatin capsules for the treatment of postbunionectomy pain. Curr Med Res Opin. 2010;26(10):2375–2384. doi: 10.1185/03007995.2010.515478. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Hersh EV, Levin LM, Adamson D, et al. Dose-ranging analgesic study of Prosorb diclofenac potassium in postsurgical dental pain. Clin Ther. 2004;26(8):1215–1227. doi: 10.1016/s0149-2918(04)80033-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Kowalski M, Stoker DG, Bon C, Moore KA, Boesing SE. A pharmacokinetic analysis of diclofenac potassium soft-gelatin capsule in patients after bunionectomy. Am J Ther. 2010;17(5):460–468. doi: 10.1097/MJT.0b013e3181aa3eda. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Bello AE. DUEXIS(®) (ibuprofen 800 mg, famotidine 26.6 mg): a new approach to gastroprotection for patients with chronic pain and inflammation who require treatment with a nonsteroidal anti-inflammatory drug. Ther Adv Musculoskelet Dis. 2012;4(5):327–339. doi: 10.1177/1759720X12444710. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Zorvolex Highlights of Prescribing Information. [Accessed August 29, 2014]. Available from: https://www.zorvolex.com/wp-content/uploads/2013/12/Zorvolex_Approved_PI.pdf .
- 110. Young CL, Strand V, Altman R, Daniels S. A phase 2 study of naproxen submicron particle capsules in patients with post-surgical dental pain. Adv Ther. 2013;30(10):885–896. doi: 10.1007/s12325-013-0057-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Czinn SJ. Helicobacter pylori infection: detection, investigation, and management. J Pediatr. 2005;146(Suppl 3):S21–S26. doi: 10.1016/j.jpeds.2004.11.037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Vergara M, Catalán M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21(12):1411–1418. doi: 10.1111/j.1365-2036.2005.02444.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Manheimer E, Linde K, Lao L, Bouter LM, Berman BM. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146(12):868–877. doi: 10.7326/0003-4819-146-12-200706190-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2006;45(11):1331–1337. doi: 10.1093/rheumatology/kel207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977. doi: 10.1002/14651858.CD001977.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Thomas KS, Muir KR, Doherty M, Jones AC, O’Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ. 2002;325(7367):752. doi: 10.1136/bmj.325.7367.752. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132(3):173–181. doi: 10.7326/0003-4819-132-3-200002010-00002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85(12):1301–1317. [ PubMed ] [ Google Scholar ]
- 119. Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–1117. doi: 10.3899/jrheum.090058. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Scheiman JM. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S5. doi: 10.1186/ar4177. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [No authors listed] [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Rahme E, Joseph L, Kong SX, Watson DJ, LeLorier J. Cost of prescribed NSAID-related gastrointestinal adverse events in elderly patients. Br J Clin Pharmacol. 2001;52(2):185–192. doi: 10.1046/j.1365-2125.2001.00348.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Latimer N, Lord J, Grant RL, O’Mahony R, Dickson J, Conaghan PG, National Institute for Health and Clinical Excellence Osteoarthritis Guideline Development Group Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538. doi: 10.1136/bmj.b2538. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (669.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. Crossref
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research.1 However, very simply… 'a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units'.1 A case study has also been described as an intensive, systematic investigation of a ...
A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are sometimes also used. Case studies are ...
Case 33-2024: A 71-Year-Old Woman with Confusion, Aphasia, and a Brain Mass. I. Arrillaga-Romany and OthersN Engl J Med 2024;391:1529-1538. A 71-year-old woman with a history of myasthenia gravis ...
Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study ...
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5), the ...
Clinical Case Studies (CCS), peer-reviewed & published bi-monthly electronic only, is the only journal devoted entirely to innovative psychotherapy case studies & presents cases involving individual, couples, & family therapy.The easy-to-follow case presentation format allows you to learn how interesting & challenging cases were assessed & conceptualized, & how treatment followed such ...
Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition ...
Differences between published case studies can make it difficult for researchers to define and understand case study as a methodology. Experienced qualitative researchers have identified case study research as a stand-alone qualitative approach (Denzin & Lincoln, 2011b). Case study research has a level of flexibility that is not readily offered ...
Challenges Faced and Lessons Learned from Our Trial of VTE Prophylaxis. G. Le Gal and D. MottierNEJM Evid 2023;2 (9) In this Clinical Trials Case Study, the authors describe the challenges faced and lessons learned conducting a trial of venous thromboembolism prophylaxis among hospitalized older adults. Clinical Trials Case Study.
In the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge.
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Clinical case reports are the first-line evidence in medical literature as they present original observations. This article provides detailed guidance on how to identify, write, and publish a case report.
General components of clinical case studies include: background, symptoms, assessments, diagnosis, treatment, and outcomes. Interpreting the information means the researcher decides what to include or leave out. A good case study should always clarify which information is the factual description and which is an inference or the researcher's ...
tive use of case studies for your students (Agency for Healthcare Research and Quality, 2019):• Settin. the Stage: Place students in groups of 2-4 using whatever method you feel will be productive. Provide a timeframe for completion of the case study while ensuring support for learning and psychological safety.
Study design. There are four basic clinical studies: case series, case-control studies, cohort studies, and randomized clinical trials. 2 The case report or case series is probably the weakest method of deriving clinical data. Case series are usually retrospective reviews that list the clinical findings of patients with a specific disease.
A case study is an in-depth study of one person, group, or event. In a case study, nearly every aspect of the subject's life and history is analyzed to seek patterns and causes of behavior. Case studies can be used in many different fields, including psychology, medicine, education, anthropology, political science, and social work.
Clinical Case Reports is an open access journal aiming to improve global health outcomes by conveying important best practice messages and sharing clinical knowledge with medical case reports, clinical images, and procedural videos. As part of Wiley's Forward Series, this journal offers a streamlined, faster publication experience with a ...
Case Studies. Solving medical case studies plays a crucial role in developing and refining clinical skills, ultimately contributing to becoming a better clinician. Here are a few ways in which working on case studies can enhance clinical competence: 1. Diagnostic Reasoning: Medical case studies provide an opportunity to practice diagnostic ...
A case study is one of the most commonly used methodologies of social research. This article attempts to look into the various dimensions of a case study research strategy, the different epistemological strands which determine the particular case study type and approach adopted in the field, discusses the factors which can enhance the effectiveness of a case study research, and the debate ...
Clinical studies are classified into the two main types, observational studies and clinical trials, depending on the presence or absence of an intervention. Observational studies include case-control studies, cohort studies, and cross-sectional studies. Case-control and cohort studies may be prospective or retrospective, and case-control ...
Real case study examples (published): . Baker et al. (2024) Enhanced family-based treatment for an adolescent with binge-eating disorder: A case report. Cognitive and Behavioral Practice. 31(2), 272-282.; da Silva et al. (2024) Impacts of oil palm monocultures on freshwater ecosystems in the Amazon: a case study of dragonflies and damselflies (Insecta: Odonata).
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1 The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the ...
A prospective observational study found that bleeding complications occurred without typical ulcer symptoms (epigastric pain or dyspepsia) in up to 80% of affected patients.32 In addition, a meta-analysis of studies performed before 1997 and from 1997-2008 found that, while the overall mortality rate from GI bleeds has fallen since the mid ...