Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Type 1 diabetes articles from across Nature Portfolio
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads to the inability to regulate blood sugar levels. Patients are usually treated by insulin-replacement therapy.
Latest Research and Reviews
Biological hypoxia in pre-transplant human pancreatic islets induces transplant failure in diabetic mice
- Hiroyuki Kato
- Mayra Salgado
- Hirotake Komatsu
Type 1 diabetes human enteroid studies reveal major changes in the intestinal epithelial compartment
- Vishwesh Bharadiya
- Varsha Singh
24-h energy expenditure in people with type 1 diabetes: impact on equations for clinical estimation of energy expenditure
- Elvis A. Carnero
- Karen D. Corbin
- Richard E. Pratley
Distinct cellular immune responses in children en route to type 1 diabetes with different first-appearing autoantibodies
Previous studies have reported heterogeneity in the progression to clinical type 1 diabetes in children who develop either insulin- or glutamic acid decarboxylase-specific antibodies as their first autoantibodies. Here, the authors show that children who later develop disease have distinct characteristics in early immune responses, which are dependent on the type of autoantibodies that appear first.
- Inna Starskaia
- Milla Valta
- Riitta Lahesmaa
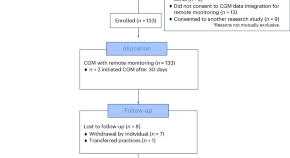
Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes
In a prospective study, a team-based approach combining continuous glucose monitoring with a technology-assisted remote patient monitoring program improved glycemia in a diverse cohort of children, adolescents and young adults with newly diagnosed type 1 diabetes.
- Priya Prahalad
- David Scheinker
- David M. Maahs
Nicotinamide Mononucleotide improves oocyte maturation of mice with type 1 diabetes
- Fucheng Guo
- Xiaoling Zhang
News and Comment

How medical schools can prepare students for new technologies
Patient educators and nurses can demonstrate the real-life use of health technologies.
- Chantal Mathieu
Reply to ‘Slowly progressive insulin dependent diabetes mellitus in type 1 diabetes endotype 2’
- Maria J. Redondo
- Noel G. Morgan
Slowly progressive insulin-dependent diabetes mellitus in type 1 diabetes endotype 2
- Tetsuro Kobayashi
- Takashi Kadowaki
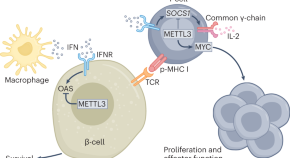
METTL3 restrains autoimmunity in β-cells
Activation of innate immunity has been linked to the progression of type 1 diabetes. A study now shows that overexpression of METTL3, a writer protein of the m 6 A machinery that modifies mRNA, restrains interferon-stimulated genes when expressed in pancreatic β-cells, identifying it as a promising therapeutic target.
- Balasubramanian Krishnamurthy
- Helen E. Thomas
Type 1 diabetes mellitus: a brave new world
One hundred years after the Nobel prize was bestowed on Banting and McLeod for the ‘discovery’ of insulin, we are again seeing major evolutions in the management of type 1 diabetes mellitus, with the prospect of achieving disease control beyond mere management now becoming real. Here, we discuss the latest, most notable developments.
- Pieter-Jan Martens

β-cells protected from T1DM by early senescence programme
- Olivia Tysoe
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes
FDA News Release
Today, the U.S. Food and Drug Administration approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment of type 1 diabetes. Lantidra is approved for the treatment of adults with type 1 diabetes who are unable to approach target glycated hemoglobin (average blood glucose levels) because of current repeated episodes of severe hypoglycemia (low blood sugar) despite intensive diabetes management and education.
“Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels.”
Type 1 diabetes is a chronic autoimmune disease that requires lifelong care including requiring insulin, either through multiple daily injections or continuous infusion using a pump, every day to live. People with type 1 diabetes also perform blood glucose checks several times a day to guide the management of their diabetes.
Some people with type 1 diabetes have trouble managing the amount of insulin needed every day to prevent hyperglycemia (high blood sugar) without causing hypoglycemia. They may also develop hypoglycemia unawareness, where they are unable to detect their blood glucose is dropping and may not have a chance to treat themselves to prevent their blood glucose from further dropping. This makes it difficult to dose insulin. Lantidra provides a potential treatment option for these patients.
The primary mechanism of action of Lantidra is believed to be the secretion of insulin by the infused allogeneic islet beta cells. In some patients with type 1 diabetes, these infused cells can produce enough insulin, so the patient no longer needs to take insulin (by injections or pump) to control their blood sugar levels. Lantidra is administered as a single infusion into the hepatic (liver) portal vein. An additional infusion of Lantidra may be performed depending on the patient’s response to the initial dose.
The safety and effectiveness of Lantidra was evaluated in two non-randomized, single-arm studies in which a total of 30 participants with type 1 diabetes and hypoglycemic unawareness received at least one infusion and a maximum of three infusions. Overall, 21 participants did not need to take insulin for a year or more, with 11 participants not needing insulin for one to five years and 10 participants not needing insulin for more than five years. Five participants did not achieve any days of insulin independence.
Adverse reactions associated with Lantidra varied with each participant depending on the number of infusions they received and the length of time they were followed and may not reflect the rates observed in practice The most common adverse reactions included nausea, fatigue, anemia, diarrhea and abdominal pain. A majority of participants experienced at least one serious adverse reaction related to the procedure for infusing Lantidra into the hepatic portal vein and the use of immunosuppressive medications needed to maintain the islet cell viability. Some serious adverse reactions required discontinuation of immunosuppressive medications, which resulted in the loss of islet cell function and insulin independence. These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra.
The FDA granted approval of Lantidra to CellTrans Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Advertisement
Current and future therapies for type 1 diabetes
- Open access
- Published: 17 February 2021
- Volume 64 , pages 1037–1048, ( 2021 )
Cite this article
You have full access to this open access article

- Bernt Johan von Scholten 1 ,
- Frederik F. Kreiner 1 ,
- Stephen C. L. Gough 1 &
- Matthias von Herrath 1 , 2
26k Accesses
64 Citations
46 Altmetric
Explore all metrics
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts based on immune suppression and modulation have been only partly successful in preventing the key pathophysiological feature in type 1 diabetes: the immune-mediated derangement or destruction of beta cells in the pancreatic islets of Langerhans, leading to low or absent insulin secretion and chronic hyperglycaemia. Evidence now warrants a focus on the beta cell itself and how to avoid its dysfunction, which is putatively caused by cytokine-driven inflammation and other stress factors, leading to low insulin-secretory capacity, autoantigen presentation and immune-mediated destruction. Correspondingly, beta cell rescue strategies are being pursued, which include antigen vaccination using, for example, oral insulin or peptides, as well as agents with suggested benefits on beta cell stress, such as verapamil and glucagon-like peptide-1 receptor agonists. Whilst autoimmune-focused prevention approaches are central in type 1 diabetes and will be a requirement in the advent of stem cell-based replacement therapies, managing the primarily cardiometabolic complications of established type 1 diabetes is equally essential. In this review, we outline selected recent and suggested future attempts to address the evolving profile of the person with type 1 diabetes.
Graphical abstract
Similar content being viewed by others

Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)

Introduction to Diabetes Mellitus

An update on the pathogenesis of Hashimoto’s thyroiditis
Avoid common mistakes on your manuscript.
Introduction
In addition to prolonging the life expectancy of people living with type 1 diabetes, the discovery of insulin a century ago revolutionised the management of this chronic autoimmune disease. Today, type 1 diabetes is the most common type of diabetes in children, and estimates suggest that around 100,000 children develop the disease every year [ 1 ]. Unfortunately, despite the availability of advanced insulins, affected individuals remain at high risk of serious complications, including cardiovascular mortality [ 2 , 3 , 4 ]. New interventions are, therefore, urgently required to improve the prognosis for the increasing number of people who are diagnosed with type 1 diabetes each year.
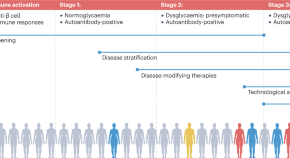
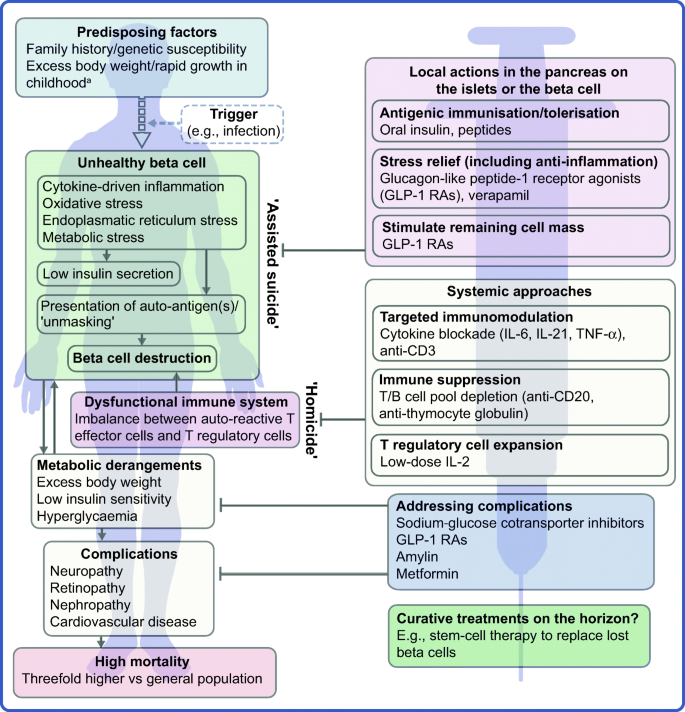
The profile of the person with type 1 diabetes is evolving and, with that, our understanding of the disease. The overall pathophysiological feature is loss of functional beta cell mass in the pancreatic islets of Langerhans (Fig. 1 ) [ 5 ]. Hypotheses suggest that the loss of functional beta cell mass occurs in a chain of events analogous to an ‘assisted suicide’ [ 6 , 7 ], where the demise of the beta cell is likely due to a combination of a dysfunctional beta cell that becomes more visible to the immune system, which, in turn, overreacts and destroys the beta cell.
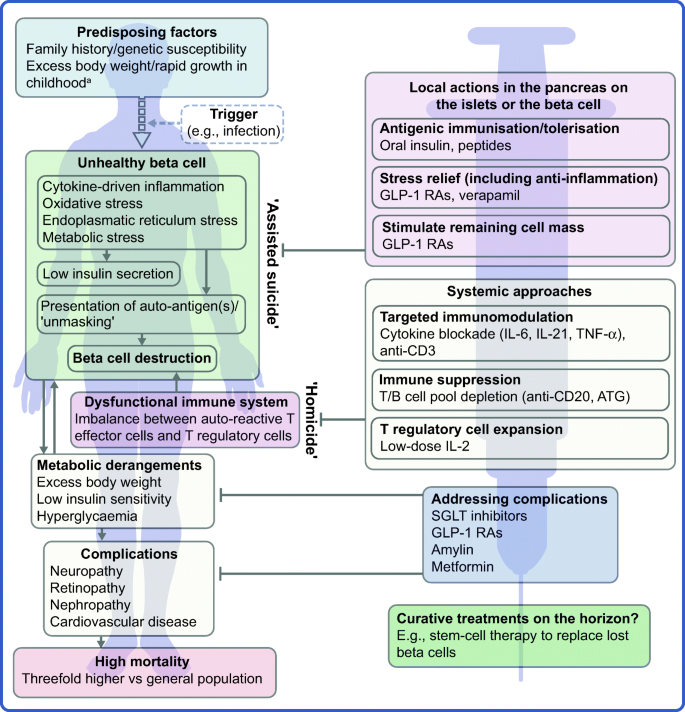
Hallmarks of the evolving profile of the individual with type 1 diabetes, and current and future options for the prevention of this disease and for the management of its associated complications. a According to some recent evidence [ 124 , 125 , 126 , 127 , 128 , 129 , 130 ]. This figure is available as a downloadable slide
In its early stage (Stage 1), type 1 diabetes is usually asymptomatic; however, the development of autoimmunity is often detectable in early life, with circulating autoantibodies targeting insulin or other proteins, such as GAD65, insulinoma-associated protein 2 (IA2) or zinc transporter 8 (ZNT8) [ 5 ]. When a large portion of the beta cell mass has become dysfunctional or lost, asymptomatic dysglycaemia (Stage 2) and, later, symptoms of hyperglycaemia (Stage 3) ensue due to insufficient or absent insulin secretion.
Type 1 diabetes is a polygenic disorder, in which susceptibility loci or genetic variation contributes to disease risk. The HLA region on chromosome 6 is the main susceptibility locus and, in recent years, many other loci across the genome have been associated with an increasing risk of the disease [ 8 ]. However, from studies in monozygotic twins, for whom the onset of type 1 diabetes can vary considerably [ 9 ], it has become evident that non-genetic factors play a major role in triggering or perpetuating overt type 1 diabetes. A multitude of efforts have failed at robustly identifying such factors, strongly indicating that no single pathogen is responsible. Viral infections have been suggested, including enteroviruses and human herpesvirus-6 [ 10 , 11 , 12 , 13 ]. Of note, however, studies (mainly in animals) have also suggested that several viral infections may prevent the development of type 1 diabetes [ 14 , 15 ], in line with the ‘hygiene hypothesis’ [ 16 , 17 ].
People living with type 1 diabetes remain dependent on exogenous insulins as the cornerstone therapeutic option [ 18 ]. Since the isolation of insulin in 1921, novel and versatile formulations, analogues and delivery vehicles have been introduced [ 19 , 20 ]. Together with much improved glucose monitoring, these advances have contributed to the increases in the survival and life expectancy of individuals with type 1 diabetes [ 21 ]. Still, only a minority of people with type 1 diabetes achieve recommended glycaemic and time-in-range targets [ 22 ], and hyperglycaemia continues to be a risk factor for short-term metabolic and long-term macro- and microvascular complications [ 2 , 23 , 24 , 25 ]. Further, the use of exogenous insulins requires unremitting glycaemic monitoring and dose titration to mitigate the risk of hypoglycaemia. The all-cause mortality risk is around threefold higher for the individual with type 1 diabetes than for the general population [ 2 , 3 , 4 , 26 ], and type 1 diabetes has been shown to be linked to cardiovascular outcomes more than any other disease, including type 2 diabetes [ 2 ].
As mentioned earlier, novel interventions are needed for the prevention and management of type 1 diabetes. Whilst progress has been limited, the evolving profile of a person with type 1 diabetes suggests that beyond ensuring accurate titration of exogenous insulin, efficient management of the disease should rely on other additional principles. First, there is an obvious need to act early to prevent or delay the destruction of functional beta cell mass by immunomodulatory intervention or other disease-modifying means. Second, stimulating or reprogramming the remaining beta cell mass to secrete insulin in a balanced way is required to avoid major blood glucose excursions with the lowest possible exogenous insulin dose. Third, reducing the risk of long-term complications, such as cardiovascular and renal outcomes, seems increasingly important (Fig. 1 ). Below we review selected current and in-development interventions meeting these three criteria (Table 1 ).
Immune-focused therapies
The overarching goal of immune-focused therapies in type 1 diabetes is to prevent or delay the loss of functional beta cell mass. The traditional understanding of autoimmunity in type 1 diabetes has focused on systemic immune dysregulation and on autoreactive T cells that have evaded thymic selection and migrated to the periphery, where they destroy islets. This view on the pathogenesis of type 1 diabetes has been referred to as T cell-mediated ‘homicide’ [ 6 ]. Thus, recent efforts have concentrated on cell- or cytokine-directed interventions, which have been successful in other autoimmune diseases. Targeting T cells or proinflammatory cytokines remain valid efforts and many agents are in active development; so far, however, these approaches have been only partly successful. This arguably indicates a need to refocus hypotheses, as discussed later in this review (see ‘ Future perspectives ’ section), where we outline how the beta cell itself contributes to its own demise (the ‘assisted suicide’ hypothesis).
Cell-directed interventions
In line with the traditional immune-centric view on the pathogenesis of type 1 diabetes, many immunomodulatory strategies have focused on antibodies targeting T effector cells. The anti-CD3 antibodies teplizumab and otelixizumab have shown some attenuation of loss of beta cell function [ 27 , 28 , 29 , 30 ]. A Phase II trial with relatives with a high risk of developing type 1 diabetes indicated a more than 50% risk reduction with teplizumab (HR 0.41 vs placebo) and clinical type 1 diabetes diagnosis was delayed by 1.5–2 years [ 31 ]. Accordingly, teplizumab has recently been granted a breakthrough therapy status by the US Food and Drug Administration. An ongoing Phase III trial (PROTECT; ClinicalTrials.gov registration no. NCT03875729) aims to evaluate the benefits and safety of teplizumab in children and adolescents with recently diagnosed type 1 diabetes.
The presence of autoantibodies against beta cell antigens, such as GAD65 and insulin, has spurred attempts targeting B cell-related molecules. These efforts have been somewhat successful in animal models [ 32 , 33 ], as well as clinically, most prominently with the B cell-depleting anti-CD20 antibody rituximab. Although rituximab led to detectable protraction of beta cell function [ 34 ], the effect was transient [ 35 ], exemplifying the fact that B cell-directed therapy alone does not appear to sustainably prevent or ameliorate beta cell autoimmunity. So far, however, B cell-directed agents have not been tested in the early disease stage, precluding conclusions regarding the usefulness of such interventions in delaying or even preventing progression to later stages.
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [ 36 , 37 , 38 ]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the clinical utility of the approach remains to be established. ATG in combination with granulocyte colony stimulating factor (GCSF) was also explored based on the hypothesis of a synergistic benefit of the combination of transient T cell depletion via low-dose ATG with the upregulation of activated T regulatory cells and tolerogenic dendritic cells induced by GCSF. However, the combination did not appear to offer a synergistic effect; in contrast to the use of ATG alone, ATG plus GCSF did not appear to be better than placebo in preserving C-peptide secretion [ 37 ].
Tissue-resident memory T effector cells, which likely play a role in many organ-specific autoimmune diseases, such as type 1 diabetes, are very difficult to eliminate. Alefacept, a T cell-depleting fusion protein that targets CD2 and, therefore, memory T effector cells, was tested in adolescents and young adults with Stage 3 type 1 diabetes in the T1DAL trial [ 39 ]. Although the trial did not complete enrolment as planned, it reported a trend for benefits with regard to beta cell preservation, reduced insulin requirements and low risk of hypoglycaemia that persisted throughout the follow-up of 15 months after treatment.
Importantly, whether considering the targeting of the T or B cell in type 1 diabetes, sufficient long-term benefits via systemic cell pool depletion comes with an inherent risk of introducing equally long-term or even irreversible changes to the immune system. Such changes may predispose the patient to a less favourable prognosis for chronic viral infections. For example, reactivation of Epstein-Barr virus (EBV) has been observed after anti-CD3 therapies [ 40 , 41 ]. Mitigating such risks may be achieved using carefully tailored dosing regimens and monitoring; still, the seriousness of the risks may indicate an unfavourable benefit:risks balance. Therefore, non-depleting immunomodulation has been explored. For example, 24-month blockade of CD80 and CD86 via the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-immunoglobulin fusion molecule abatacept markedly prolonged beta cell function in new-onset type 1 diabetes and was accompanied by increased numbers of naive T cells [ 42 , 43 ].
Cytokine-directed interventions
Anti-inflammatory cytokine-specific compounds, which are successfully used, for example, in rheumatic diseases, have been tested as alternatives to directly targeting the T or B cell in type 1 diabetes, as briefly summarised below. In addition, to stimulate an increase in T regulatory cells, low-dose IL-2 treatment has also been tested and the results have been somewhat promising [ 44 , 45 , 46 , 47 , 48 ], with recent developments mitigating earlier caveats, which included an arguably narrow dose range and lack of full specificity for T regulatory cells.
Blockade or antagonism of the central proinflammatory cytokine TNF-α using infliximab, adalimumab or the receptor fusion protein etanercept have shown some potential in type 1 diabetes, with indications of improved glycaemic control and C-peptide secretion [ 49 , 50 ]. More recently, a C-peptide-sparing effect of TNF-α blockade was reported with golimumab use, after 1 year in children and young adults with type 1 diabetes [ 51 ].
IL-6 is another proinflammatory cytokine that has been targeted with success in multiple other autoimmune diseases [ 52 ]. Although its role in type 1 diabetes is not established, IL-6 has been suggested as a target [ 53 ]. Of note, IL-6 has been shown to protect the beta cell from oxidative stress and is constitutively expressed by pancreatic alpha and beta cells, indicating important physiological roles [ 54 ]. In type 1 diabetes, the EXTEND Phase II trial of tocilizumab, a monoclonal antibody against the IL-6 receptor, was recently completed ( ClinicalTrials.gov registration no. NCT02293837).
IL-21 has been proposed as an attractive target in type 1 diabetes [ 55 , 56 ]. Physiologically, IL-21 is important not only for the function of T helper (Th) cells (Th17 and T follicular helper cells) but also for the generation and migration of CD8 + T cells. CD8 + T cells are now considered the chief T cell type accumulating in and around islets [ 57 , 58 ] with pre-proinsulin emerging as a pivotal autoantigen driving their infiltration in type 1 diabetes [ 59 ]. IL-21 neutralisation has been shown to prevent diabetes in mice [ 60 ], and a C-peptide-sparing benefit of anti-IL-21 alone or in combination with the glucagon-like peptide-1 (GLP-1) receptor agonist (RA) liraglutide has been observed in a clinical proof-of-concept study [ 61 ], as described further below. Reassuringly, non-clinical models, including a viral type 1 diabetes model, showed a minor impact of IL-21 blockade on the immune repertoire [ 55 ].
Antigen vaccination
With the appeal of having no expected effect on acquired immunity, the overall aim of beta cell antigen vaccination is to induce tolerance by balancing the T cell population between auto-aggressive T effector cells and autoantigen-specific T regulatory cells. Induction of T regulatory cells carries the potential benefit of also downregulating the activity of proinflammatory antigen-presenting cells. The topic has been extensively reviewed in the past [ 62 ]. Briefly, inspired by successes with vaccination against, for example, peanut allergy, tolerisation of T effector cells has been attempted using administration of whole antigens, such as oral insulin, or of peptides. Whilst the concepts are promising and under active investigation, their effectiveness in humans is yet to be proven. For example, in at-risk children, oral insulin administration has previously failed to prevent type 1 diabetes [ 63 , 64 ], speculatively due to a suboptimal dose level or unclear effects across risk-specific subgroups [ 65 , 66 ], including those defined by insulin gene polymorphisms. Similar results and considerations have been reported for immunisation with GAD65 [ 67 ] and for peptide-based therapies [ 68 , 69 ]. Further, the lack of full clarity regarding the mechanisms at play with antigen-based therapies outlines a number of shortcomings, including the fact that no biomarker is currently available to assist in establishing the optimal dose regimen.
Non-immunomodulatory adjunctives
We next focus on selected compounds that have gained attention due to their potential benefits as adjuncts to insulin in type 1 diabetes.
Amylin deficiency is a recognised feature of type 1 diabetes [ 70 ]. As a neuroendocrine hormone, amylin inhibits glucagon secretion and contributes to reducing postprandial glucose variability. As an adjunct to meal-time insulin, the injectable amylin analogue pramlintide is approved only in the USA for the treatment of type 1 and type 2 diabetes alike [ 71 ]. In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [ 72 ]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
Metformin is a low-cost agent with glucose-lowering effects that mainly occur via decreased hepatic glucose production. It is not a guideline-recommended option in type 1 diabetes. However, partly because of its ameliorating effect on insulin resistance, metformin has been somewhat promising in managing the disease, especially in children and adolescents, as well as in obese people with type 1 diabetes, with studies indicating reduced insulin requirements and body weight reduction [ 73 , 74 , 75 ]. In the large REducing With MetfOrmin Vascular Adverse Lesions (REMOVAL) trial, however, metformin did not reduce the long-term insulin needs or improve glycaemic control in people with long-standing type 1 diabetes and multiple cardiovascular risk factors [ 76 ].
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter (SGLT) inhibitors lower blood glucose levels by restraining the absorption of glucose in the small intestine and promoting the renal excretion of glucose [ 77 ]. Results with dapagliflozin, empagliflozin and sotagliflozin have indicated benefits of SGLT inhibition in managing type 1 diabetes when added to insulin [ 78 , 79 , 80 , 81 , 82 , 83 ]. Significant benefits included reduced insulin dose requirements, improved glycaemic control and reduced body weight [ 84 ]. So far, sotagliflozin and dapagliflozin are approved in Europe and Japan (but not the USA) as adjuncts to insulin for the management of overweight or obese people with type 1 diabetes when optimally titrated insulin alone does not provide adequate glycaemic control. Importantly, however, data suggest that the use of SGLT inhibitors in type 1 diabetes is associated with markedly increased risk of diabetic ketoacidosis [ 85 , 86 , 87 ]; for sotagliflozin, a 5–17-fold risk increase was noted [ 88 ]. These observations prompted the formation of an international consensus on recommendations for the use of SGLT inhibition in type 1 diabetes [ 89 ] as well as a suggestion that treatment should be overseen by specialists [ 88 ].
GLP-1 is a hormone of the incretin system that is secreted upon food intake. A marked uptake has been seen in the use of GLP-1 RAs in type 2 diabetes due to their pleiotropic glucose-dependent effects that improve glycaemic control and reduce body weight [ 90 ]. In contrast, GLP-1 agonism for the treatment of type 1 diabetes remains unproven, with initial results from smaller investigator-conceived studies being inconclusive. Recently, Phase II findings with the short-acting GLP-1 RA exenatide in adults with type 1 diabetes were negative. In two larger Phase III trials (ADJUNCT ONE and ADJUNCT TWO), the GLP-1 analogue liraglutide used as an adjunct to insulin appeared well-tolerated and improved HbA 1c and reduced body weight [ 91 , 92 ]. Both ADJUNCT trials indicated a minor increase in the risk of hypoglycaemia and hyperglycaemia with ketosis with liraglutide use, whereas the risk of diabetic ketoacidosis was negligible. Subsequently, a plethora of investigations have reached similar conclusions [ 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ]. Nonetheless, the use of GLP-1 RAs in type 1 diabetes remains potentially useful, as discussed below.
Verapamil is a common calcium-channel blocker used for decades as an anti-hypertensive agent. In mouse models of type 1 diabetes, verapamil promoted survival of functional beta cells via a mechanism that involves reduced expression of the cellular redox regulator thioredoxin-interacting protein [ 102 ]. In a smaller Phase II trial, verapamil was better than placebo for preserving meal-stimulated C-peptide secretion in adults with type 1 diabetes and no safety concerns were identified [ 103 ]. Despite these findings, however, the place for verapamil as a disease-modifying agent in type 1 diabetes remains to be fully established.
Future perspectives
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the immunopathophysiology of type 1 diabetes, whilst also exploring the promise of combination therapies that integrate tried or new treatment modalities. In addition, lessons learned from type 2 diabetes with regard to the beneficial effects of certain agents on, for example, body weight and cardiorenal risk may also prove relevant in type 1 diabetes. We review selected future prospects addressing these aspects below.
Of further note, the lack of sufficient efficacy of previously tested therapies may also be related to the fact that type 1 diabetes is a heterogenous disease with diverse disease stages (Stages 1 to 3) and modifiers, such as age of onset or clinical diagnosis. Identifying the optimal timing of each type of intervention relative to the disease stages and the age of the patient is, therefore, important. For example, initiating an immunomodulatory intervention at Stage 1 (i.e. prior to clinical diagnosis) is not a straightforward decision and may be associated with clinical inertia. Moreover, an increased focus on disease endotypes (i.e. different biological processes under the type 1 diabetes umbrella) was recently suggested to ensure a precision-medicine approach to type 1 diabetes research and management [ 104 ].
Immune interventions
It is becoming increasingly clear that autoreactivity to islet antigens is also present in healthy individuals [ 59 ] and autoimmunity recurs after autologous nonmyeloablative haematopoietic stem cell transplantation [ 105 , 106 ]. Thus, in line with the ‘assisted suicide’ theory introduced earlier [ 6 , 7 ], it is also increasingly apparent that the development of type 1 diabetes does not only involve dysfunctional islets, but also beta cells that ‘unmask’ themselves to immune recognition and destruction. This notion supports two central realisations; first, it might explain why, in previous studies, immune therapy alone has failed to protect beta cell function over longer periods of time after onset of diabetes. Second, looking forward, novel type 1 diabetes therapies should pursue the holy grail of type 1 diabetes immune therapy: essentially agents that act locally in the islets, within the pancreas, either targeting the immune cells destroying the beta cell or the beta cell itself. Knowledge gained over the years regarding the beta cell has suggested multiple, yet putative reasons for the ‘unmasking’ of these cells. Potential reasons include the facts that beta cells are especially biosynthetically active and systemically exposed [ 107 ] and, therefore, susceptible to stress-induced production of autoantigenic proteins during, for example, infections [ 108 , 109 , 110 ]. Moreover, the beta cell might be vulnerable to both cytokine-mediated destruction [ 111 ] and various types of endoplasmic reticulum stress [ 112 ]. Relieving the beta cell of these burdens may provide an opportunity to save the beta cell without resorting to aggressive immune suppression.
With this in mind, we see the following two promising avenues as deserving increased focus going forward: (1) therapies aimed at inducing tolerance to beta cell antigens; and (2) the use of GLP-1 RAs that directly target the beta cells to enhance their function whilst also protecting them from immune-mediated inflammatory stress.
As discussed above, achieving antigenic tolerance has, so far, proven elusive but carries the crucial potential of leaving the overall capacity of the immune system intact whilst suppressing only the diabetogenic cell populations. Future studies need to establish whether inducing tolerance in humans can be achieved by clonal anergy or clonal deletion of effector cells, or whether antigen-specific regulatory cells may be able to suppress autoreactivity locally. Moreover, it needs to be clarified to what extent tissue-resident memory effector cells can be eliminated.
Recent evidence from rodent models indicates a role for GLP-1 RAs in protecting beta cells from apoptosis and in promoting beta cell replication and mass [ 113 , 114 , 115 , 116 , 117 ]. As such, although this remains to be confirmed, it is conceivable that GLP-1 RAs may offer a way to prevent the ‘unmasking’ of the beta cell to immune effector cells, for example, by downregulating expression of MHC class I proteins. Intriguingly, unpublished non-clinical evidence shows that liraglutide also limits immune cell infiltration into pseudo-islets (M. von Herrath, unpublished results). In addition, studies in NOD mice have shown that GLP-1 RAs administered in combination with various immunomodulatory agents, including anti-CD3 compounds [ 118 ], were more efficient in inducing diabetes remission than when given as monotherapy [ 119 ]. Furthermore, the anti-inflammatory effects of GLP-1 RAs are well-documented, with liraglutide being associated with reduced systemic levels of C-reactive protein and of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 [ 120 , 121 , 122 , 123 ]. Whilst these findings have mainly been observed in animal models or in type 2 diabetes, their relevance to (clinical) type 1 diabetes is conceivable but, so far, largely unexplored.
Management of cardiometabolic complications
A person diagnosed with type 1 diabetes faces a high risk of serious complications and of premature death, primarily for cardiovascular causes. This warrants a therapeutic focus on the broad pathophysiology of the disease.
Further, whilst the exact connections between excess body weight and type 1 diabetes remain debatable [ 124 ], the increased incidence of type 1 diabetes seems to coincide with the rapid rise in the prevalence of obesity [ 125 , 126 ]. Recent evidence suggests that a high BMI may exacerbate the early-stage immune-mediated beta cell destruction in type 1 diabetes, especially in children and adolescents [ 127 ]. Evidence also points to an impact of rapid growth in early childhood [ 128 ], and a positive correlation between the age of type 1 diabetes onset and BMI has been observed [ 129 ]. The ‘accelerator hypothesis’ views high BMI and low insulin sensitivity as triggers for type 1 diabetes onset [ 130 ] and the term ‘double diabetes’ has been suggested to describe an amalgam of type 1 diabetes with parallel and separate pathophysiological processes typically associated with type 2 diabetes, such as obesity and insulin resistance [ 131 ].
Use of SGLT inhibitors or GLP-1 RAs as adjuncts to insulin admittedly holds promise in ameliorating multiple type 1 diabetes complications. For example, evidence suggests that SGLT inhibitors offer cardiorenal protection [ 132 , 133 ], at least in type 2 diabetes, putatively owing to clinically unproven mechanisms of action beyond improved glucose homeostasis [ 134 ]. Moreover, a few GLP-1 RAs (dulaglutide, liraglutide and semaglutide) are now indicated to reduce cardiovascular risk in people with type 2 diabetes and established cardiovascular disease, and a protective effect of GLP-1 RAs on the kidneys is suggested from a range of cardiovascular outcome trials (CVOTs) in type 2 diabetes [ 135 , 136 , 137 , 138 ]. In addition, both SGLT inhibitors and GLP-1 RAs, especially second-generation GLP-1 RAs (e.g., semaglutide), are associated with a meaningful reducing effect on body weight.
Combination therapies
Combination therapies that work via two mechanistically distinct targets to integrate immune modulation with a beta cell-specific component have been suggested [ 139 , 140 , 141 ] and encouraged [ 142 ]. Truly advantageous combination therapies are arguably those in which the components target different pathogenic pathways (for example, systemic vs beta cell-specific pathways), thereby synergising in terms of the beneficial effects. These combination therapies should also be safe and well-tolerated alone and in combination.
Known ongoing efforts are sparse but include the combination of ATG and GCSF (as discussed above) and the combination of targeted immune modulation via an anti-IL-21 antibody in combination with a GLP-1 analogue (liraglutide). In addition to the potential of preserving functional beta cell mass by leveraging the immunomodulatory and anti-inflammatory properties of both the anti-IL-21 antibody and liraglutide, their combination addresses the need to manage the symptoms and complications of established type 1 diabetes, as discussed earlier. As previously mentioned, results from a clinical proof-of-concept trial recently found that anti-IL-21 plus liraglutide was significantly better than placebo in preserving C-peptide secretion over a period of 54 weeks [ 61 ]. The benefits diminished after treatment cessation; however, the treatment appeared safe and well-tolerated.
Stem cell replacement therapy
On the horizon, we approach the promise of stem cell-based therapies [ 143 ], offering a potential cure by replacing or supplementing beta cells that have been lost or have become dysfunctional. Stem cell-derived beta cells, however, also need to be rescued from immune-mediated destruction, suggesting that some degree of immunomodulation will be needed, even in the advent of viable stem cell therapy in type 1 diabetes, unless a fully effective immune-defying capsule is available [ 144 ]. In this context, better prevention or treatment regimens will also be useful for enabling longer-term beta cell graft acceptance.
Closing thoughts
Whilst many intriguing non-insulin therapies have failed to fully meet their potential in the past few decades, hope remains that the knowledge gained has carved out paths towards better options for the prevention and management of type 1 diabetes. Taken together, in our view, stem cell replacement therapies and a refocused development of safe and well-tolerated combination therapies are the most promising emerging preventive or therapeutic avenues. In parallel, reinforced efforts to predict or diagnose type 1 diabetes as soon as possible are equally important in light of the fact that even the best interventions need to be introduced as early as possible to effectively preserve or rescue beta cells in individuals with this condition.
Abbreviations
Anti-thymocyte globulin
Granulocyte colony stimulating factor
Glucagon-like peptide-1
Receptor agonist
Sodium-glucose cotransporter
Patterson CC, Karuranga S, Salpea P et al (2019) Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107842. https://doi.org/10.1016/j.diabres.2019.107842
Article PubMed Google Scholar
Petrie JR, Sattar N (2019) Excess cardiovascular risk in type 1 diabetes mellitus. Circulation 139(6):744–747. https://doi.org/10.1161/circulationaha.118.038137
Schofield J, Ho J, Soran H (2019) Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther 10(3):773–789. https://doi.org/10.1007/s13300-019-0612-8
Article PubMed PubMed Central Google Scholar
Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM (2006) High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 29(4):798–804. https://doi.org/10.2337/diacare.29.04.06.dc05-1433
Katsarou A, Gudbjörnsdottir S, Rawshani A et al (2017) Type 1 diabetes mellitus. Nat Rev Dis Primers 3:17016. https://doi.org/10.1038/nrdp.2017.16
Atkinson MA, Bluestone JA, Eisenbarth GS et al (2011) How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 60(5):1370–1379. https://doi.org/10.2337/db10-1797
Article CAS PubMed PubMed Central Google Scholar
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A (2020) Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 1–12. https://doi.org/10.1038/s41574-020-00443-4
Pociot F, Lernmark Å (2016) Genetic risk factors for type 1 diabetes. Lancet 387(10035):2331–2339. https://doi.org/10.1016/S0140-6736(16)30582-7
Article CAS PubMed Google Scholar
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T (2008) Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 359(26):2849–2850. https://doi.org/10.1056/NEJMc0805398
Sabouri S, Benkahla MA, Kiosses WB et al (2020) Human herpesvirus-6 is present at higher levels in the pancreatic tissues of donors with type 1 diabetes. J Autoimmun 107:102378. https://doi.org/10.1016/j.jaut.2019.102378
Rodriguez-Calvo T (2018) Enteroviral infections as a trigger for type 1 diabetes. Curr Diab Rep 18(11):106. https://doi.org/10.1007/s11892-018-1077-2
Richardson SJ, Morgan NG (2018) Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention. Curr Opin Pharmacol 43:11–19. https://doi.org/10.1016/j.coph.2018.07.006
Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG (2016) The viral paradigm in type 1 diabetes: who are the main suspects? Autoimmun Rev 15(10):964–969. https://doi.org/10.1016/j.autrev.2016.07.019
Filippi C, von Herrath M (2005) How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol 233(2):125–132. https://doi.org/10.1016/j.cellimm.2005.04.009
Christen U, von Herrath MG (2011) Do viral infections protect from or enhance type 1 diabetes and how can we tell the difference? Cell Mol Immunol 8(3):193–198. https://doi.org/10.1038/cmi.2010.71
Bach JF (2001) Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun 16(3):347–353. https://doi.org/10.1006/jaut.2000.0478
von Mutius E (2007) Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology 212(6):433–439. https://doi.org/10.1016/j.imbio.2007.03.002
Article CAS Google Scholar
American Diabetes Association (2020) 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Supplement 1):S98. https://doi.org/10.2337/dc20-S009
Article Google Scholar
Beck RW, Bergenstal RM, Laffel LM, Pickup JC (2019) Advances in technology for management of type 1 diabetes. Lancet 394(10205):1265–1273. https://doi.org/10.1016/s0140-6736(19)31142-0
Beck RW, Bergenstal RM, Riddlesworth TD et al (2019) Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 42(3):400–405. https://doi.org/10.2337/dc18-1444
Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ (2012) Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 61(11):2987–2992. https://doi.org/10.2337/db11-1625
Weinstock RS, Schütz-Fuhrmann I, Connor CG et al (2016) Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract 122:28–37. https://doi.org/10.1016/j.diabres.2016.09.024
Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM (2017) The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60(10):2084–2091. https://doi.org/10.1007/s00125-017-4374-4
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376(15):1407–1418. https://doi.org/10.1056/NEJMoa1608664
Rawshani A, Sattar N, Franzén S et al (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392(10146):477–486. https://doi.org/10.1016/s0140-6736(18)31506-x
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK (2015) Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 38(2):316–322. https://doi.org/10.2337/dc14-0920
Hagopian W, Ferry RJ, Sherry N et al (2013) Teplizumab preserves C-peptide in recent-onset type 1 diabetes. Diabetes 62(11):3901. https://doi.org/10.2337/db13-0236
Herold KC, Gitelman SE, Ehlers MR et al (2013) Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62(11):3766–3774. https://doi.org/10.2337/db13-0345
Sherry N, Hagopian W, Ludvigsson J et al (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 378(9790):487–497. https://doi.org/10.1016/S0140-6736(11)60931-8
Aronson R, Gottlieb PA, Christiansen JS et al (2014) Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care 37(10):2746–2754. https://doi.org/10.2337/dc13-0327
Herold KC, Bundy BN, Long SA et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381(7):603–613. https://doi.org/10.1056/NEJMoa1902226
Hu CY, Rodriguez-Pinto D, Du W et al (2007) Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 117(12):3857–3867. https://doi.org/10.1172/jci32405
Xiu Y, Wong CP, Bouaziz JD et al (2008) B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol 180(5):2863–2875. https://doi.org/10.4049/jimmunol.180.5.2863
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H et al (2009) Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 361(22):2143–2152. https://doi.org/10.1056/NEJMoa0904452
Pescovitz MD, Greenbaum CJ, Bundy B et al (2014) B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care 37(2):453–459. https://doi.org/10.2337/dc13-0626
Gitelman SE, Gottlieb PA, Rigby MR et al (2013) Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 1(4):306–316. https://doi.org/10.1016/S2213-8587(13)70065-2
Haller MJ, Schatz DA, Skyler JS et al (2018) Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA(1c) in new-onset type 1 diabetes. Diabetes Care 41(9):1917–1925. https://doi.org/10.2337/dc18-0494
Haller MJ, Long SA, Blanchfield JL et al (2019) Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes 68(6):1267–1276. https://doi.org/10.2337/db19-0057
Rigby MR, Harris KM, Pinckney A et al (2015) Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 125(8):3285–3296. https://doi.org/10.1172/JCI81722
Keymeulen B, Candon S, Fafi-Kremer S et al (2010) Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood 115(6):1145–1155. https://doi.org/10.1182/blood-2009-02-204875
Kroll JL, Beam C, Li S et al (2013) Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol 57(2):115–119. https://doi.org/10.1016/j.jcv.2013.01.016
Orban T, Bundy B, Becker DJ et al (2014) Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37(4):1069–1075. https://doi.org/10.2337/dc13-0604
Orban T, Bundy B, Becker DJ et al (2011) Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378(9789):412–419. https://doi.org/10.1016/s0140-6736(11)60886-6
Dwyer CJ, Ward NC, Pugliese A, Malek TR (2016) Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr Diab Rep 16(6):46–46. https://doi.org/10.1007/s11892-016-0739-1
Hartemann A, Bensimon G, Payan CA et al (2013) Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1(4):295–305. https://doi.org/10.1016/s2213-8587(13)70113-x
Rosenzwajg M, Salet R, Lorenzon R et al (2020) Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 63(9):1808–1821. https://doi.org/10.1007/s00125-020-05200-w
Todd JA, Evangelou M, Cutler AJ et al (2016) Regulatory T cell responses in participants with type 1 diabetes after a single dose of interleukin-2: a non-randomised, open label, adaptive dose-finding trial. PLoS Med 13(10):e1002139. https://doi.org/10.1371/journal.pmed.1002139
Khoryati L, Pham MN, Sherve M et al (2020) An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol 5(50):eaba5264. https://doi.org/10.1126/sciimmunol.aba5264
Mastrandrea L, Yu J, Behrens T et al (2009) Etanercept treatment in children with new-onset type 1 diabetes. Diabetes Care 32(7):1244. https://doi.org/10.2337/dc09-0054
Timper K, Hruz P, Beglinger C, Donath MY (2013) Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care 36(7):e90. https://doi.org/10.2337/dc13-0199
Quattrin T, Haller MJ, Steck A et al (2020) 3-LB: golimumab (GLM) preserves ß-cell function and reduces insulin use and hypoglycemia in children and young adults with recently diagnosed type 1 diabetes (T1D): the phase 2 T1GER study. Diabetes 69(Supplement 1):3-LB (Abstract). https://doi.org/10.2337/db20-3-LB
Kang S, Tanaka T, Narazaki M, Kishimoto T (2019) Targeting interleukin-6 signaling in clinic. Immunity 50(4):1007–1023. https://doi.org/10.1016/j.immuni.2019.03.026
Hundhausen C, Roth A, Whalen E et al (2016) Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 8(356):356ra119. https://doi.org/10.1126/scitranslmed.aad9943
Rajendran S, Anquetil F, Quesada-Masachs E et al (2020) IL-6 is present in beta and alpha cells in human pancreatic islets: expression is reduced in subjects with type 1 diabetes. Clin Immunol 211:108320. https://doi.org/10.1016/j.clim.2019.108320
Van Belle TL, Nierkens S, Arens R, von Herrath MG (2012) Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36(6):1060–1072. https://doi.org/10.1016/j.immuni.2012.04.005
Sutherland AP, Van Belle T, Wurster AL et al (2009) Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58(5):1144–1155. https://doi.org/10.2337/db08-0882
Pugliese A (2017) Autoreactive T cells in type 1 diabetes. J Clin Invest 127(8):2881–2891. https://doi.org/10.1172/jci94549
Babon JA, DeNicola ME, Blodgett DM et al (2016) Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 22(12):1482–1487. https://doi.org/10.1038/nm.4203
Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG (2020) The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv 6(42):eabc5586. https://doi.org/10.1126/sciadv.abc5586
McGuire HM, Walters S, Vogelzang A et al (2011) Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60(3):867–875. https://doi.org/10.2337/db10-1157
Mathieu C, von Herrath M, Bain SC et al (2020) OP08-48: efficacy and safety of anti-interleukin (IL)-21 in combination with liraglutide in adults recently diagnosed with type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Roep BO, Wheeler DCS, Peakman M (2019) Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 7(1):65–74. https://doi.org/10.1016/S2213-8587(18)30109-8
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ (2017) Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318(19):1891–1902. https://doi.org/10.1001/jama.2017.17070
Skyler JS, Krischer JP, Wolfsdorf J et al (2005) Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial--Type 1. Diabetes Care 28(5):1068–1076. https://doi.org/10.2337/diacare.28.5.1068
Bonifacio E, Ziegler A-G, Klingensmith G et al (2015) Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT Randomized Clinical Trial. JAMA 313(15):1541–1549. https://doi.org/10.1001/jama.2015.2928
Ziegler AG, Achenbach P, Berner R et al (2019) Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ Open 9(6):e028578. https://doi.org/10.1136/bmjopen-2018-028578
Ludvigsson J, Wahlberg J, Casas R (2017) Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 376(7):697–699. https://doi.org/10.1056/NEJMc1616343
Bovy N, Boitard C, Achenbach P et al (2020) OP09-52: long-term follow-up study of type 1 diabetes patients previously treated with IMCY-0098 or placebo in young adults with recent-onset type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Smith EL, Peakman M (2018) Peptide immunotherapy for type 1 diabetes-clinical advances. Front Immunol 9:392. https://doi.org/10.3389/fimmu.2018.00392
Martin C (2006) The physiology of amylin and insulin: maintaining the balance between glucose secretion and glucose uptake. Diabetes Educ 32(Suppl 3):101s–104s. https://doi.org/10.1177/0145721706288237
Ryan GJ, Jobe LJ, Martin R (2005) Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 27(10):1500–1512. https://doi.org/10.1016/j.clinthera.2005.10.009
Riddle MC, Nahra R, Han J et al (2018) Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: a randomized, two-way crossover study. Diabetes Care 41(11):2346–2352. https://doi.org/10.2337/dc18-1091
Lund SS, Tarnow L, Astrup AS et al (2008) Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS One 3(10):e3363. https://doi.org/10.1371/journal.pone.0003363
Libman IM, Miller KM, DiMeglio LA et al (2015) Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA 314(21):2241–2250. https://doi.org/10.1001/jama.2015.16174
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR (2010) The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 53(5):809–820. https://doi.org/10.1007/s00125-009-1636-9
Petrie JR, Chaturvedi N, Ford I et al (2017) Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 5(8):597–609. https://doi.org/10.1016/s2213-8587(17)30194-8
Ferrannini E (2017) Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 26(1):27–38. https://doi.org/10.1016/j.cmet.2017.04.011
Buse JB, Garg SK, Rosenstock J et al (2018) Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 41(9):1970–1980. https://doi.org/10.2337/dc18-0343
Dandona P, Mathieu C, Phillip M et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 41(12):2552–2559. https://doi.org/10.2337/dc18-1087
Mathieu C, Dandona P, Gillard P et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 41(9):1938–1946. https://doi.org/10.2337/dc18-0623
Rosenstock J, Marquard J, Laffel LM et al (2018) Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41(12):2560–2569. https://doi.org/10.2337/dc18-1749
Garg SK, Henry RR, Banks P et al (2017) Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 377(24):2337–2348. https://doi.org/10.1056/NEJMoa1708337
Henry RR, Thakkar P, Tong C, Polidori D, Alba M (2015) Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 38(12):2258–2265. https://doi.org/10.2337/dc15-1730
Tandon S, Ayis S, Hopkins D, Harding S, Stadler M (2021) The impact of pharmacological and lifestyle interventions on body weight in people with type 1 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 23(2):350–362. https://doi.org/10.1111/dom.14221
Taylor SI, Blau JE, Rother KI (2015) SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100(8):2849–2852. https://doi.org/10.1210/jc.2015-1884
Taylor SI, Blau JE, Rother KI, Beitelshees AL (2019) SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 7(12):949–958. https://doi.org/10.1016/S2213-8587(19)30154-8
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38(9):1687–1693. https://doi.org/10.2337/dc15-0843
Wolfsdorf JI, Ratner RE (2019) SGLT inhibitors for type 1 diabetes: proceed with extreme caution. Diabetes Care 42(6):991. https://doi.org/10.2337/dci19-0008
Danne T, Garg S, Peters AL et al (2019) International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 42(6):1147–1154. https://doi.org/10.2337/dc18-2316
Aroda VR (2018) A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 20(Suppl 1):22–33. https://doi.org/10.1111/dom.13162
Mathieu C, Zinman B, Hemmingsson JU et al (2016) Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 39(10):1702–1710. https://doi.org/10.2337/dc16-0691
Ahrén B, Hirsch IB, Pieber TR et al (2016) Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care 39(10):1693–1701. https://doi.org/10.2337/dc16-0690
Dimitrios P, Michael D, Vasilios K et al (2020) Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: a systematic review and meta-analysis. Curr Diabetes Rev 16(4):313–326. https://doi.org/10.2174/1573399815666190614141918
Ghanim H, Batra M, Green K et al (2020) Liraglutide treatment in overweight and obese patients with type 1 diabetes: a 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab 22(10):1742–1752. https://doi.org/10.1111/dom.14090
Goyal I, Sattar A, Johnson M, Dandona P (2020) Adjunct therapies in treatment of type 1 diabetes. J Diabetes 12(10):742–753. https://doi.org/10.1111/1753-0407.13078
Kuhadiya ND, Prohaska B, Ghanim H, Dandona P (2019) Addition of glucagon-like peptide-1 receptor agonist therapy to insulin in C-peptide-positive patients with type 1 diabetes. Diabetes Obes Metab 21(4):1054–1057. https://doi.org/10.1111/dom.13609
Wang W, Liu H, Xiao S, Liu S, Li X, Yu P (2017) Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther 8(4):727–738. https://doi.org/10.1007/s13300-017-0282-3
Dejgaard TF, Schmidt S, Frandsen CS et al (2020) Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: the Lira Pump trial—a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab 22(4):492–500. https://doi.org/10.1111/dom.13911
Dejgaard TF, Frandsen CS, Hansen TS et al (2016) Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 4(3):221–232. https://doi.org/10.1016/s2213-8587(15)00436-2
Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S (2015) Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care 38(12):2250–2257. https://doi.org/10.2337/dc15-1037
Johansen NJ, Dejgaard TF, Lund A et al (2020) Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 8(4):313–324. https://doi.org/10.1016/s2213-8587(20)30030-9
Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 61(4):848–856. https://doi.org/10.2337/db11-0955
Ovalle F, Grimes T, Xu G et al (2018) Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med 24(8):1108–1112. https://doi.org/10.1038/s41591-018-0089-4
Battaglia M, Ahmed S, Anderson MS et al (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43(1):5–12. https://doi.org/10.2337/dc19-0880
Voltarelli JC, Couri CE, Stracieri AB et al (2007) Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14):1568–1576. https://doi.org/10.1001/jama.297.14.1568
Voltarelli JC, Couri CE, Stracieri AB et al (2008) Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci 1150:220–229. https://doi.org/10.1196/annals.1447.048
Mallone R, Eizirik DL (2020) Presumption of innocence for beta cells: why are they vulnerable autoimmune targets in type 1 diabetes? Diabetologia 63(10):1999–2006. https://doi.org/10.1007/s00125-020-05176-7
James EA, Pietropaolo M, Mamula MJ (2018) Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes 67(6):1035–1042. https://doi.org/10.2337/dbi17-0030
Mauvais FX, Diana J, van Endert P (2016) Beta cell antigens in type 1 diabetes: triggers in pathogenesis and therapeutic targets. F1000Research 5:F1000 Faculty Rev-1728. https://doi.org/10.12688/f1000research.7411.1
Itariu BK, Stulnig TM (2014) Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology 60(3):189–196. https://doi.org/10.1159/000356747
Burrack AL, Martinov T, Fife BT (2017) T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol 8:343. https://doi.org/10.3389/fendo.2017.00343
Marré ML, James EA, Piganelli JD (2015) β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol 3:67. https://doi.org/10.3389/fcell.2015.00067
Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB (2015) Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol 29(7):978–987. https://doi.org/10.1210/me.2015-1030
Lee YS, Jun HS (2014) Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metab Clin Exp 63(1):9–19. https://doi.org/10.1016/j.metabol.2013.09.010
Villalba A, Rodriguez-Fernandez S, Perna-Barrull D et al (2020) Repurposed analog of GLP-1 ameliorates hyperglycemia in type 1 diabetic mice through pancreatic cell reprogramming. Front Endocrinol 11:258
Rowlands J, Heng J, Newsholme P, Carlessi R (2018) Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol 9:672–672. https://doi.org/10.3389/fendo.2018.00672
Buteau J, Foisy S, Joly E, Prentki M (2003) Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 52(1):124–132. https://doi.org/10.2337/diabetes.52.1.124
Sherry NA, Chen W, Kushner JA et al (2007) Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148(11):5136–5144. https://doi.org/10.1210/en.2007-0358
Rother KI, Spain LM, Wesley RA et al (2009) Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 32(12):2251–2257. https://doi.org/10.2337/dc09-0773
Hogan AE, Gaoatswe G, Lynch L et al (2014) Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 57(4):781–784. https://doi.org/10.1007/s00125-013-3145-0
von Scholten BJ, Persson F, Rosenlund S et al (2017) Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: a sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab 19(6):901–905. https://doi.org/10.1111/dom.12884
Pi-Sunyer X, Astrup A, Fujioka K et al (2015) A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373(1):11–22. https://doi.org/10.1056/NEJMoa1411892
Bouchi R, Nakano Y, Fukuda T et al (2017) Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J 64(3):269–281. https://doi.org/10.1507/endocrj.EJ16-0449
Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P (2018) Obesity and diabetes-not only a simple link between two epidemics. Diabetes Metab Res Rev 34(7):e3042. https://doi.org/10.1002/dmrr.3042
Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ (2018) Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 39(5):629–663. https://doi.org/10.1210/er.2017-00191
Liu LL, Lawrence JM, Davis C et al (2010) Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 11(1):4–11. https://doi.org/10.1111/j.1399-5448.2009.00519.x
Ferrara-Cook C, Geyer SM, Evans-Molina C et al (2020) Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care 43(3):580–587. https://doi.org/10.2337/dc19-1167
The EURODIAB Substudy 2 Study Group (2002) Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25(10):1755–1760. https://doi.org/10.2337/diacare.25.10.1755
Giménez M, Aguilera E, Castell C, de Lara N, Nicolau J, Conget I (2007) Relationship between BMI and age at diagnosis of type 1 diabetes in a Mediterranean area in the period of 1990–2004. Diabetes Care 30(6):1593. https://doi.org/10.2337/dc06-2578
Wilkin TJ (2009) The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes 33(7):716–726. https://doi.org/10.1038/ijo.2009.97
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR (2013) Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia 56(7):1462–1470. https://doi.org/10.1007/s00125-013-2904-2
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Neuen BL, Young T, Heerspink HJL et al (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7(11):845–854. https://doi.org/10.1016/s2213-8587(19)30256-6
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43:508–511. https://doi.org/10.2337/dci19-0074
Mann JFE, Ørsted DD, Brown-Frandsen K et al (2017) Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377(9):839–848. https://doi.org/10.1056/NEJMoa1616011
Leiter LA, Bain SC, Bhatt DL et al (2020) The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab 22(9):1690–1695. https://doi.org/10.1111/dom.14079
Gerstein HC, Colhoun HM, Dagenais GR et al (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Tuttle KR, Rayner BL, Lakshmanan M et al (2019) 233-OR: chronic kidney disease (CKD) outcomes with dulaglutide (DU) vs. insulin glargine (IG) in type 2 diabetes (T2D) and moderate-to-severe CKD by albuminuria status: AWARD-7. Diabetes 68(Suppl 1):233-OR (Abstract). https://doi.org/10.2337/db19-233-OR
Kolb H, von Herrath M (2017) Immunotherapy for type 1 diabetes: why do current protocols not halt the underlying disease process? Cell Metab 25(2):233–241. https://doi.org/10.1016/j.cmet.2016.10.009
Bone RN, Evans-Molina C (2017) Combination immunotherapy for type 1 diabetes. Curr Diab Rep 17(7):50. https://doi.org/10.1007/s11892-017-0878-z
von Herrath M, Peakman M, Roep B (2013) Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol 172(2):186–202. https://doi.org/10.1111/cei.12085
Article CAS PubMed Central Google Scholar
Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M (2019) The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 7(1):52–64. https://doi.org/10.1016/s2213-8587(18)30112-8
Chen S, Du K, Zou C (2020) Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther 11(1):275. https://doi.org/10.1186/s13287-020-01793-6
Coppieters K, Winkel L, von Herrath M (2020) Long-term viability through selective permeability. Nat Biomed Eng 4(8):763–764. https://doi.org/10.1038/s41551-020-0602-1
Ratner RE, Dickey R, Fineman M et al (2004) Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 21(11):1204–1212. https://doi.org/10.1111/j.1464-5491.2004.01319.x
Whitehouse F, Kruger DF, Fineman M et al (2002) A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 25(4):724–730. https://doi.org/10.2337/diacare.25.4.724
Download references
Authors’ relationships and activities
All authors are employees of Novo Nordisk A/S, Denmark.
Author information
Authors and affiliations.
Global Chief Medical Office, Novo Nordisk A/S, Søborg, Denmark
Bernt Johan von Scholten, Frederik F. Kreiner, Stephen C. L. Gough & Matthias von Herrath
Type 1 Diabetes Center, The La Jolla Institute for Immunology, La Jolla, CA, USA
Matthias von Herrath
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed substantially to the preparation of the review and approved the version to be published.
Corresponding author
Correspondence to Matthias von Herrath .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure slide.
(PPTX 230 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
von Scholten, B.J., Kreiner, F.F., Gough, S.C.L. et al. Current and future therapies for type 1 diabetes. Diabetologia 64 , 1037–1048 (2021). https://doi.org/10.1007/s00125-021-05398-3
Download citation
Received : 26 October 2020
Accepted : 22 December 2020
Published : 17 February 2021
Issue Date : May 2021
DOI : https://doi.org/10.1007/s00125-021-05398-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adjunctive therapies
- Beta cell preservation
- Immunomodulation
- Type 1 diabetes
- Find a journal
- Publish with us
- Track your research
- Utility Menu
GA4 tracking code

A new therapy for treating Type 1 diabetes
Promising early results show that longstanding harvard stem cell institute (hsci) research may have paved the way for a breakthrough treatment of type 1 diabetes. utilizing research from the melton lab, vertex pharmaceuticals has developed vx-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (t1d). in conjunction with immunosuppressive therapy, vx-880 produced robust restoration of islet cell function on day 90 in the first patient in its phase 1/2 clinical trial..

The patient was treated with a single infusion of VX-880 at half the target dose in conjunction with immunosuppressive therapy. The patient, who was diagnosed with T1D 40 years ago and has been dependent on exogenous (injected) insulin, achieved successful engraftment and demonstrated rapid and robust improvements in multiple measures. These included increases in fasting and stimulated C-peptide, improvements in glycemic control, including HbA1c, and decreases in exogenous insulin requirement, signifying the restoration of insulin-producing islet cells.
VX-880 is not only a potential breakthrough in the treatment of T1D, it is also one of the very first demonstrations of the practical application of embryonic stem cells, using stem cells that have been differentiated into functional islets to treat a patient, explained Doug Melton, Ph.D., co-director of HSCI, is the Xander University Professor at Harvard and an Investigator of the Howard Hughes Medical Institute. Unlike prior treatments, this innovative therapy gives the patient functional hormone producing cells that control glucose metabolism. This potentially obviates the lifelong need for patients with diabetes to self-inject insulin as the replacement cells “provide the patient with the natural factory to make their own insulin,” explained Melton.
These results from the first patient treated with VX-880 are unprecedented. What makes these results truly remarkable is that they were achieved with only half the target dose,” said Bastiano Sanna, Ph.D., Executive Vice President and Chief of Cell and Genetic Therapies at Vertex. “While still early, these results support the continued progression of our VX-880 clinical studies, as well as future studies using our encapsulated islet cells, which hold the potential to be used without the need for immunosuppression.”
“As a surgeon who has worked in the field of islet cell transplantation for decades, this approach, which obviates the need for an organ donor, could be a game changer,” said James Markmann, M.D., Ph.D., Professor of Surgery and Chief of the Division of Transplant Surgery at Massachusetts General Hospital. “We are excited to progress this unique and potentially transformative medicine through clinical trials and to patients.”
“More than a decade ago our lab had a vision for developing an islet cell replacement therapy to provide a functional cure to people suffering from T1D,” said Melton, a founder and one of the first co-chairs of the Harvard Stem Cell and Regenerative Biology Department. “These promising results bring great hope that stem cell-derived, fully differentiated islet cells could deliver a life-changing therapy for people who suffer from the relentless life-long burden of T1D. I'm so grateful that Harvard and the Harvard Stem Cell Institute have supported this work.”
Latest News
How a small fish could lead to better strategies to repair tendon tears, jason buenrostro lands macarthur ‘genius grant’, new model for in vitro production of human brown fat cells lays groundwork for obesity, diabetes cell therapy, share this story, news by topic.
- Alzheimer's Disease (8)
- Bioengineering (31)
- Blood Diseases (52)
- Cancer (55)
- Cardiovascular Disease (25)
- Diabetes (30)
- Endocrine Disease (1)
- Eye Diseases (10)
- Fibrosis (6)
- Gastrointestinal Disease (8)
- Genetics/Epigenetics (24)
- Graft-Versus-Host Disease (1)
- Hearing Loss (5)
- Immunology (15)
- Kidney Disease (12)
- Liver Disease (6)
- Lung Disease (9)
- Multiple Sclerosis (1)
Type 1 Research Highlights
While the Association’s priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes. Read more about the critical research made possible by the American Diabetes Association.
Smart Insulin Patch
American Diabetes Association Pathway to Stop Diabetes Scientist Zhen Gu, PhD, recently published a paper describing the development of an innovative "smart insulin" patch that imitates the body's beta cells by both sensing blood glucose levels and releasing insulin.
A Possible Trigger for Type 1 Diabetes
In order to prevent or reverse the development of type 1 diabetes, it is essential to understand why and how the immune system attacks the body’s own cells. Association-funded Researcher Thomas Delong, PhD, found a possible answer to these questions.
Enhancing Survival of Beta Cells for Successful Transplantation
Islet transplantation has long offered hope as a curative measure for type 1 diabetes. However, more than 80% of transplanted islets die within one week after transplantation. Research efforts are working to improve their survival and the promise of stem cells to reverse diabetes.
Explore: Type 1 Research Highlights
Investments in type 1 diabetes research
The CDC estimates that nearly 1.6 million Americans have it, including about 187,000 children and adolescents. The American Diabetes Association funds a productive research portfolio that offers significant progress and hope for improved outcomes for people with type 1 diabetes.
Identifying type 1 diabetes before beta cell loss
Dr. Hessner is investigating so-called “biomarkers,” which are components in blood or tissue samples that can be measured to predict which individuals are most likely to develop type 1 diabetes.
Beta cell replacement
Both type 1 and type 2 diabetes result from a complete or partial loss of beta cell number and function. Finding a way to successfully replace functional beta cell is key to efforts to one day cure diabetes.
Enhancing survival of beta cells for successful transplantation
Islet transplantation has long offered hope as a curative measure for type 1 diabetes. However, more than 80% of transplanted islets die within one week after transplantation. Research efforts are working to improve their survival and the promise of stem cells to reverse diabetes.
New insight into how diabetes leads to blindness
New research is uncovering how diabetes changes the kinds of proteins that are made in the eye. These changes may lead to diabetic retinopathy, a leading cause of blindness. This information is allowing researchers to identify new targets for therapies that could delay or prevent the development of diabetic retinopathy.

Your support helps drive lifesaving research, advocacy, and programs to help improve the lives of people living with diabetes.
Donate Now and DOUBLE your impact.
Clinical Trials
Type 1 diabetes.
Displaying 71 studies
The purpose of this study is to demonstrate that a morning injection of Toujeo compared to Lantus will provide better glycemic control, as shown by Continuous Glucose Monitoring (CGM), in adult patients with type 1 diabetes mellitus.
The purpose of this study is to identify risk factors for ICI associated diabetes mellitus and to assess the severity and natural course of this immune related adverse effect.
Hypothesis: Increased contact with the diabetes care team throughout pregnancy will lead to improved glucose control during pregnancy.
The purpose of this study is to serve as a comparator group to a group of patients that will be managed with AP for varying periods of time during pregnancy.
The purpose of this study is to evaluate glucose variability in patients with type 1 diabetes (T1D) and insulin antibodies, to evaluate the clinical significance of insulin antibodies, and to establish an in vitro assay that would detect antibodies to insulin and insulin analogs.
This clinical trial will identify exercise-related and emotional stress related effects on glycemic control in patients with type 1 diabetes using sensor-augmented pump (SAP) therapy.
This study will test the efficacy of BKR-017 (colon-targeted 500 mg butyrate tablets) on insulin sensitivity, glucose control and triglycerides in type-1 diabetes subjects.
The purpose of this study is to collect blood samples for biomarker assessment in type 1 diabetes prior to and at specific time points during closed loop control.
Our goal in this pilot study is to test and develop a novel method that will accurately measure, in vivo, glucagon kinetics in healthy humans and generate preliminary data in type 1 diabetes (T1DM) subjects under overnight fasted conditions.
The multi-purpose of this study is to examine the effectiveness of “InsulisiteGuider” in patients with type 1 diabetes (T1D) through a two-group randomized controlled trial, to characterize the RNA biomarkers in skin epithelial cells isolated from the continuous subcutaneous insulin infusion (CSII) cannulas from T1D patients, and to characterize RNA biomarkers in the blood and saliva of TID patients.
The purpose of this research is to test the safety and effectiveness of the interoperable Artificial Pancreas System Smartphone App (iAPS) in managing blood sugars in pregnant patients with type 1 diabetes.
The objective of this study is to evaluate the EWIS in patients with type 1 diabetes on insulin pump therapy.
This study is a multi-center, non-randomized, prospective single arm study with type 1 patients with diabetes on insulin pump therapy with Continuous Glucose Monitoring (CGM).
A total of up to 300 subjects will be enrolled at up to 20 investigational centers in the US in order to have 240 subjects meeting eligibility criteria. Each subject will wear their own MiniMed™ 670G insulin system. Each subject will be given 12 infusion sets to wear (each infusion set for at least 174 hours, or ...
The purpose of this study is to use the USS Virginia Closed-Loop system for overnight insulin delivery in adults with Type 1 Diabetes (T1DM) in an outpatient setting to evaluate the system's ability to significantly improve blood glucose levels. This protocol will test the feasibility of "bedside" closed-loop control - an approach comprised of standard sensor-augmented pump therapy during the day using off-the-shelf devices and overnight closed-loop control using experimental devices in an outpatient setting. The rationale for this study is as follows: we anticipate that closed-loop control may ultimately be adopted by patients with T1DM in a selective manner. ...
The overall objective of this study is to perform baseline and repeat assessments over time of the metabolic and immunologic status of individuals at risk for type 1 diabetes (T1D) to:
- characterize their risk for developing T1D and identify subjects eligible for prevention trials;
- describe the pathogenic evolution of T1D; and
- increase the understanding of the pathogenic factors involved in the development of T1D.
The purpose of this study is to assess a novel informatics approach that incorporates the use of patient’s diabetes self-care data into the design and delivery of individualized education interventions to improve diabetes control.
The purpose of this study is to assess the glycemic variability in patients with complex diabetes admitted in the hospital using a glycemic sensor.
The purpose of this research is to create a single registry for type 1 and type 2 diabetes at Mayo Rochester and affiliated Mayo sites.
The study purpose is to understand patients’ with the diagnosis of Diabetes Mellitus type 1 or 2 perception of the care they receive in the Diabetes clinic or Diabetes technology clinic at Mayo Clinic and to explore and to identify the healthcare system components patients consider important to be part of the comprehensive regenerative care in the clinical setting.
However, before we can implement structural changes or design interventions to promote comprehensive regenerative care in clinical practice, we first need to characterize those regenerative practices occurring today, patients expectations, perceptions and experiences about comprehensive regenerative care and determine the ...
This study is being done to determine the roles that several molecules play in the repair of injured cells that line your blood vessels.
This purpose of this study is to determine if activation of a person's immune system in the small intestine could be a contributing cause of Type 1 Diabetes.
The purpose of this project is to collect data over the first year of clinical use of the FDA approved 670G closed loop insulin delivery system among patients with type 1 diabetes. The goal is to evaluate how this newly approved system impacts both clinical and patient-reported outcomes.
The primary goal of this study protocol is to determine the candidate ratio of pramlintide and insulin co-infusion in individuals with type 1 diabetes (T1DM) to enable stable glucose control during the overnight post-absorptive and in the postprandial periods.
The purpose of this trial is to assess the performance of an Artificial Pancreas (AP) device using the Portable Artificial Pancreas System (pAPS) platform for subjects with type 1 diabetes using an insulin pump and rapid acting insulin. This proposed study is designed to compare closed-loop control with or without optimization of initialization parameters related to basal insulin infusion rates and insulin to carbohydrate (I:C) ratios for meals and snacks. The study consists of an evaluation of the Artificial Pancreas device system during two 24-27.5-hour closed-loop phases in an outpatient/hotel environment. Prior to the closed-loop phases, each subject will undergo ...
The study is being done to find out if low blood sugar (hypoglycemia) can be reduced in people with type 1 diabetes (T1D) 65 years and older with use of automated insulin delivery (AID) system.
The device systems used in this study are approved by the Food and Drug Administration (FDA) for diabetes management. We will be collecting data about how they are used, how well they work, and how safe they are.
This study aims to identify an early stage biomarker for type 1 diabetes. In vitro evidence identified a significant enrichment of the chemokine CXCL10 in β-cell derived EXO upon exposure to diabetogenic pro-inflammatory cytokines. The study also aims to test protocols for efficient isolation of plasma-derived EXO from small volumes of sample, develop an assay for the sensitive detection of CXCL10 in plasma-derived EXO, and characterization of plasma-derived EXO through assessment of concentration, size, and content (proteomics).
The study is designed to understand the confidence and competence level of patients with type 1 diabetes mellitus in their ability to make changes to their insulin pump.
The purpose of this study is to gather preliminary data to better understand acute effects of exercise on glucose metabolism. We will address if subjects with Type 1 Diabetes (T1D) are more insulin sensitive during and following a short bout of exercise compared to healthy controls. We will also determine insulin dependent and insulin independent effects on exercise in people with and without type 1 diabetes.
The purpose of this study is to retrospectively and prospectively compare maternal and fetal/newborn clinical outcomes in age-matched pregnant patients with T1D and healthy controls and to assess the relationship between glycemic variability and pregnancy outcomes in the current era.
The objective for thisstudy is to characterize the impact of glycemic excursions on cognition in Type 1 Diabetes (T1D) and determine mediators and moderators of this relationship. This study will allow us to determine how glycemic excursions impact cognition, as well as to identify mediators and moderators of this relationship that could lead to novel interventions.
The purpose of this study is to compare the effectiveness and safety of an automated insulin delivery (AID) system using a model predictive control (MPC) algorithm versus Sensor-Augmented Pump/Predictive Low Glucose Suspend (SAP/PLGS) therapy with different stress assessments over a 4-week period.
Can QBSAfe be implemented in a clinical practice setting and improve quality of life, reduce treatment burden and hypoglycemia among older, complex patients with type 2 diabetes?
Questionnaire administered to diabetic patients in primary care practice (La Crosse Mayo Family Medicine Residency /Family Health Clinic) to assess patient’s diabetic knowledge. Retrospective chart review will also be done to assess objective diabetic control based on most recent hemoglobin A1c.
The objective of the study is to assess efficacy and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The purpose of this study is to evaluate whether or not a 6 month supply (1 meal//day) of healthy food choices readily available in the patient's home and self management training including understanding of how foods impact diabetes, improved food choices and how to prepare those foods, improve glucose control. In addition, it will evaluate whether or not there will be lasting behavior change modification after the program.
This research study is being done to develop educational materials that will help patients and clinicians talk about diabetes treatment and management options.
The purpose of this study is to measure and characterize specific immune cell abnormalities found in patients who have type 1 diabetes and may or may not be on the waiting list for either a pancreas alone or a pancreas and kidney transplant.
What are the effects of transient insulin deprivation on brain structure, blood flow, mitochondrial function, and cognitive function in T1DM patients? What are the effects of transient insulin deprivation on circulating exosomes and metabolites in T1DM patients?
The primary objective of this study is to determine if continuous glucose monitoring (CGM) can reduce hypoglycemia and improve quality of life in older adults with type 1 diabetes (T1D).
The purpose of this study is to identify novel genetic variants that predispose to Type 1 Diabetes.
The purpose of this study is to demonstrate the safety and effectiveness of the Hybrid Closed Loop system (HCL) in adult and pediatric patients with type 1 diabetes in the home setting. A diverse population of patients with type 1 diabetes will be studied. The study population will have a large range for duration of diabetes and glycemic control, as measured by glycosylated hemoglobin (A1C). They will be enrolled in the study regardless of their prior diabetes regimen, including using Multiple Daily Injections (MDI), Continuous Subcutaneous Insulin Infusion (CSII) or Sensor-Augmented Pump therapy (SAP)
The purpose of this study is to evaluate the safety of utilizing insulin lispro-aabc in the MiniMed™ 780G System to support product and system labeling.
The purpose of this study is to evaluate the effects of improving glycemic control, and/or reducing glycemic variability on gastric emptying, intestinal barrier function, autonomic nerve functions, and epigenetic changes in subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) who are switched to intensive insulin therapy as part of clinical practice.
This study is designed to compare an intensive lifestyle and activity coaching program ("Sessions") to usual care for diabetic patients who are sedentary. The question to be answered is whether the Sessions program improves clinical or patient centric outcomes. Recruitment is through invitiation only.
The purpose of this 3-month extension study (DCLP3 Extension) following a primary trial (DCLP3 or NCT03563313) to assess effectiveness and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The goal of this work is to identify an early stage biomarker for type 1 diabetes. In vitro evidence using rodent models has identified a significant enrichment of the chemokine CXCL10 in β-cell derived sEV upon exposure to diabetogenic pro-inflammatory cytokines. The aims of this project will focus on 1) testing protocols for efficient isolation of plasma-derived sEV from small volumes of sample, 2) development of an assay for the sensitive detection of CXCL10 in plasma-derived sEV, and 3) characterization of plasma-derived sEV through assessment of concentration, size, and content (proteomics). The study plans to include children that ...
This is a study to evaluate a new Point of Care test for blood glucose monitoring.
The objective of the study is to assess the efficacy and safety of home use of a Control-to-Range (CTR) closed-loop (CL) system.
The purpose of this study is assess the feasibility, effectiveness, and acceptability of Diabetes-REM (Rescue, Engagement, and Management), a comprehensive community paramedic (CP) program to improve diabetes self-management among adults in Southeast Minnesota (SEMN) treated for servere hypoglycemia by the Mayo Clinic Ambulance Services (MCAS).
Diabetics are at risk for invasive pneumococcal infections and are more likely to have severe outcomes with infection compared to the general population. The pneumococcal (PPSV23) vaccination is recommended for all people with type 1 diabetes, but whether the vaccine is beneficial for this population has not been established. The purpose of this study is to determine if children with type 1 diabetes have adequate immune response to the PPSV23 vaccination and to assess factors affecting immune response through a pre and post vaccination blood sample.
The purpose of this study is to collect device data to assist in the development of a Personalized Closed Loop (PCL) system.
Although vitreous hemorrhage (VH) from proliferative diabetic retinopathy (PDR) can cause acute and dramatic vision loss for patients with diabetes, there is no current, evidence-based clinical guidance as to what treatment method is most likely to provide the best visual outcomes once intervention is desired. Intravitreous anti-vascular endothelial growth factor (anti-VEGF) therapy alone or vitrectomy combined with intraoperative PRP each provide the opportunity to stabilize or regress retinal neovascularization. However, clinical trials are lacking to elucidate the relative time frame of visual recovery or final visual outcome in prompt vitrectomy compared with initial anti-VEGF treatment. The Diabetic Retinopathy Clinical Research ...
The purpose of this study is to demonstrate feasibility of dynamic 11C-ER176 PET imaging to identify macrophage-driven immune dysregulation in gastric muscle of patients with DG. Non-invasive quantitative assessment with PET can significantly add to our diagnostic armamentarium for patients with diabetic gastroenteropathy.
The purpose of this study is to evaluate the effects of multiple dose regimens of RM-131 on vomiting episodes, stomach emptying and stomach paralysis symptoms in patients with Type 1 and Type 2 diabetes and gastroparesis.
The purpose of this study is to evaluate the effectiveness and safety of brolucizumab vs. aflibercept in the treatment of patients with visual impairment due to diabetic macular edema (DME).
The purpose of this study is to develop a better blood test to diagnose early kidney injury in type 1 diabetes.
The purpose of this study is to use multiple devices to measure blood sugar changes and the reasons for these changes in healthy and diabetic children.
The objectives of this study are to evaluate the safety of IW-9179 in patients with diabetic gastroparesis (DGP) and the effect of treatment on the cardinal symptoms of DGP.
The purpose of this study is to understand why patients with indigestion, with or without diabetes, have gastrointestinal symptoms and, in particular, to understand where the symptoms are related to increased sensitivity to nutrients.Subsequently, look at the effects of Ondansetron on these patients' symptoms.
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and exploratory effectiveness of nimacimab in patients with diabetic gastroparesis.
The purpose of this study is gain the adolescent perspective on living with type 1 diabetes.
The purpose of this study is to demonstrate the performance of the Guardian™ Sensor (3) with an advanced algorithm in subjects age 2 - 80 years, for the span of 170 hours (7 days).
The primary purpose of this study is to prospectively assess symptoms of bloating (severity, prevalence) in patients with diabetic gastroparesis.
The purpose of this study is to track the treatment burden experienced by patients living with Type 2 Diabetes Mellitus (T2DM) experience as they work to manage their illness in the context of social distancing measures.
To promote social distancing during the COVID-19 pandemic, health care institutions around the world have rapidly expanded their use of telemedicine to replace in-office appointments where possible.1 For patients with diabetes, who spend considerable time and energy engaging with various components of the health care system,2,3 this unexpected and abrupt transition to virtual health care may signal significant changes to ...
The purpose of this study is to evaluate the ability of appropriately-trained family physicians to screen for and identify Diabetic Retinopathy using retinal camera and, secondarily, to describe patients’ perception of the convenience and cost-effectiveness of retinal imaging.
Hypothesis: We hypothesize that patients from the Family Medicine Department at Mayo Clinic Florida who participate in RPM will have significantly reduced emergency room visits, hospitalizations, and hospital contacts.
Aims, purpose, or objectives: In this study, we will compare the RPM group to a control group that does not receive RPM. The primary objective is to determine if there are significant group differences in emergency room visits, hospitalizations, outpatient primary care visits, outpatient specialty care visits, and hospital contacts (inbound patient portal messages and phone calls). The secondary objective is to determine if there are ...
The purpose of this research is to determine if CGM (continuous glucose monitors) used in the hospital in patients with COVID-19 and diabetes treated with insulin will be as accurate as POC (point of care) glucose monitors. Also if found to be accurate, CGM reading data will be used together with POC glucometers to dose insulin therapy.
The purpose of this study is to evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening or center-involved diabetic macular edema (CI-DME) with vision loss through 4 years of follow-up in participants with mild to moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
The purpose of this study is to see if there is a connection between bad experiences in the patient's childhood, either by the patient or the parent, and poor blood sugar control, obesity, poor blood lipid levels, and depression in patients with type 1 diabetes.
The purpose of this study is to assess painful diabetic peripheral neuropathy after high-frequency spinal cord stimulation.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
This study (SE2030) will establish a platform of data to build the perfect stress echo test, suitable for all patients, anywhere, anytime, also quantitative and operator independent.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Prevention of Type 1 Diabetes
Book editors, affiliations.
- 1 University of Florida , University of Florida Diabetes Institute , Departments of Pediatrics and Pathology, Immunology, and Laboratory Medicine , Gainesville, FL
- 2 University of Florida, University of Florida Diabetes Institute , Department of Pediatrics , Gainesville, FL
- 3 Yale University , Departments of Immunobiology and Internal Medicine , New Haven, CT
- 4 University of Miami Miller School of Medicine , Diabetes Research Institute , Division of Endocrinology, Diabetes, and Metabolism , Miami, FL
Book Affiliations
- 1 Senior Advisor for Diabetes Epidemiology, Division of Diabetes, Endocrinology and Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
- 2 Social & Scientific Systems, Inc. (A DLH Holdings Company), Silver Spring, MD
- 3 School of Public Health, University of Michigan, Ann Arbor, MI
- 4 Massachusetts General Hospital Diabetes Center, Harvard Medical School, Boston, MA
- 5 Division of Diabetes, Endocrinology and Metabolic Disease, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
- PMID: 38843373
- Bookshelf ID: NBK604182
Type 1 diabetes results from autoimmune destruction of insulin-producing beta cells. There is a genetic predisposition to the disease, with the greatest contribution conferred by alleles present within the major histocompatibility complex (MHC; known as the human leukocyte antigen [HLA] region) on the short arm of chromosome 6. Whether the initiation of this immune response results from environmental triggers remains to be determined. Over years, immune infiltration into pancreatic islets leads to beta cell damage, impairment of cell function, and destruction of beta cells. This notion has led to clinical trials to arrest the progression of disease and potentially prevent or reverse the clinical syndrome.
Clinical trials using various immunologic agents have been conducted at multiple stages of the disease process. Primary prevention trials have been conducted in individuals with genetic predisposition who have not yet developed immunologic markers. Secondary prevention trials have been conducted in individuals with two or more type 1 diabetes-related autoantibodies, either during Stage 1 (normal metabolic function) or Stage 2 (abnormal metabolic function). Intervention trials, also referred to as tertiary prevention trials, have been conducted after diagnosis of hyperglycemia (Stage 3), mostly shortly after clinical onset of disease.
This article provides brief summaries of randomized controlled clinical trials that have been performed and mentions some nonrandomized pilot studies. Success has been limited in primary and secondary prevention trials, with one recent and notable exception (teplizumab). Some tertiary intervention trials have demonstrated improved beta cell function, but these studies have not permanently prevented the decline in beta cell function (eventually declining in parallel to controls). Future interventions with combinations of agents that can target multiple immunologic mechanisms may be needed, including strategies to improve regulatory immunity, as well as to replace and restore beta cell function.
- Introduction
- Primary Prevention Trials
- Secondary Prevention Trials
- Tertiary Prevention Trials
- Conclusions
- List of Abbreviations
- Conversions
- Acknowledgment
- Article History
Publication types


Type 1 Diabetes Research
Through the JDRF – Beyond Type 1 Alliance , Beyond Type 1 has partnered with JDRF—the world’s biggest nonprofit funder of type 1 diabetes research —to educate our community on the important role research plays in the lives of everyone affected by type 1 diabetes (T1D). It was diabetes research that led to the discovery of insulin in 1921. It was research that led to the creation of the first insulin pump in 1963, and research that led to the modern analog insulins used by many living with T1D today. Without research, we wouldn’t have continuous glucose monitors (CGMs), hybrid closed loop systems, or treatment for the complications that arise from living with diabetes. And it is research that will some day lead to the cure for type 1 diabetes.
Cure Research: The most promising cures for Type 1 diabetes will need to address two challenges: the loss of insulin-producing beta cells, and the immune system’s attack on those beta cells.
Vertex VX-880 Clinical Results Lead To Insulin-Independence

Explaining the Research: What Will It Take to Cure Diabetes?

Vertex Acquires ViaCyte, Combining Resources Toward a T1D Cure

Beta Cell Replacement Therapy: A Pathway to a Cure for Type 1 Diabetes?

New Documentary: ‘The Human Trial’ is a Quest to Cure Type 1 Diabetes
Beta Cell Therapies

Immunotherapies
Improving lives: a future cure is not enough for people living with t1d today. research also focuses on improving lives through glucose control and treating complications..

Glucose Control Therapies

Complications Treatment Research
Learn more about t1d research and the importance of trial participation.

Research Trials

Research News

Research Funding
To learn more about all the great T1D research being funded by JDRF, visit their research and impact page here.
WRITTEN BY BT1 Editorial Team, POSTED 02/13/21, UPDATED 01/03/23
The isolated caregiver—a mom’s story -, diabetes + exercise -, diabetes management -, 10 things that can spike your blood sugar -, life with type 1—a photo essay -.

- TERMS OF USE
- 400 CONCAR DR.
- SAN MATEO, CA 94402

- BEYOND TYPE 1 BEYOND TYPE 1 EN ESPAÑOL
- IN ENGLISH EN ESPANOL BEYOND TYPE 1 AUF DEUTSCH BEYOND TYPE 1 IN ITALIANO BEYOND TYPE 1 EN FRANÇAIS BEYOND TYPE 1 IN HET NEDERLANDS BEYOND TYPE 1 PÅ SVENSKA BEYOND TYPE 1 EM PORTUGUÊS BEYOND TYPE 1 بالعربية

- BEYOND TYPE 2 BEYOND TYPE 2 EN ESPAÑOL IN ENGLISH EN ESPANOL BEYOND TYPE 2 AUF DEUTSCH BEYOND TYPE 2 en Français BEYOND TYPE 2 in Italiano BEYOND TYPE 2 Canada (French) BEYOND TYPE 2 Canada (English)
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
Research Gaps Around Type 1 Diabetes
A large body of research on Type 2 diabetes has helped to develop guidance, informing how patients are diagnosed, treated, and manage their lifestyle. In contrast, Type 1 diabetes, often mistakenly associated only with childhood, has received less attention.
In this Q&A, adapted from the April 17 episode of Public Health On Call , Stephanie Desmon speaks to Johns Hopkins epidemiologists Elizabeth Selvin , PhD '04, MPH, and Michael Fang , PhD, professor and assistant professor, respectively, in the Department of Epidemiology, about recent findings that challenge common beliefs about type 1 diabetes. Their conversation touches on the misconception that it’s solely a childhood condition, the rise of adult-onset cases linked to obesity, and the necessity for tailored approaches to diagnosis and care. They also discuss insulin prices and why further research is needed on medications like Ozempic in treating Type 1 diabetes.
I want to hear about some of your research that challenges what we have long understood about Type 1 diabetes, which is no longer called childhood diabetes.
MF: Type 1 diabetes was called juvenile diabetes for the longest time, and it was thought to be a disease that had a childhood onset. When diabetes occurred in adulthood it would be type 2 diabetes. But it turns out that approximately half of the cases of Type 1 diabetes may occur during adulthood right past the age of 20 or past the age of 30.
The limitations of these initial studies are that they've been in small clinics or one health system. So, it's unclear whether it's just that particular clinic or whether it applies to the general population more broadly.
We were fortunate because the CDC has collected new data that explores Type 1 diabetes in the U.S. Some of the questions they included in their national data were, “Do you have diabetes? If you do, do you have Type 1 or Type 2? And, at what age were you diagnosed?”
With these pieces of information, we were able to characterize how the age of diagnosis of Type 1 diabetes differs in the entire U.S. population.
Are Type 1 and Type 2 diabetes different diseases?
ES: They are very different diseases and have a very different burden. My whole career I have been a Type 2 diabetes epidemiologist, and I’ve been very excited to expand work with Type 1 diabetes.
There are about 1.5 million adults with Type 1 diabetes in the U.S., compared to 21 million adults with Type 2 diabetes. In terms of the total cases of diabetes, only 5 to 10 percent have Type 1 diabetes. Even in our largest epidemiologic cohorts, only a small percentage of people have Type 1 diabetes. So, we just don't have the same national data, the same epidemiologic evidence for Type 1 diabetes that we have for Type 2. The focus of our research has been trying to understand and characterize the general epidemiology and the population burden of Type 1 diabetes.
What is it about Type 1 that makes it so hard to diagnose?
MF: The presentation of symptoms varies by age of diagnosis. When it occurs in children, it tends to have a very acute presentation and the diagnosis is easier to make. When it happens in adulthood, the symptoms are often milder and it’s often misconstrued as Type 2 diabetes.
Some studies have suggested that when Type 1 diabetes occurs in adulthood, about 40% of those cases are misdiagnosed initially as Type 2 cases. Understanding how often people get diagnosed later in life is important to correctly diagnose and treat patients.
Can you talk about the different treatments?
MF: Patients with Type 1 diabetes are going to require insulin. Type 2 diabetes patients can require insulin, but that often occurs later in the disease, as oral medications become less and less effective.
ES: Because of the epidemic of overweight and obese in the general population, we’re seeing a lot of people with Type 1 diabetes who are overweight and have obesity. This can contribute to issues around misdiagnosis because people with Type 1 diabetes will have signs and will present similarly to Type 2 diabetes. They'll have insulin resistance potentially as a result of weight gain metabolic syndrome. Some people call it double diabetes—I don't like that term—but it’s this idea that if you have Type 1 diabetes, you can also have characteristics of Type 2 diabetes as well.
I understand that Type 1 used to be considered a thin person's disease, but that’s not the case anymore. MF: In a separate paper, we also explored the issue of overweight and obesity in persons with Type 1 diabetes. We found that approximately 62% of adults with Type 1 diabetes were either overweight or obese, which is comparable to the general U.S. population.
But an important disclaimer is that weight management in this population [with Type 1 diabetes] is very different. They can't just decide to go on a diet, start jogging, or engage in rigorous exercise. It can be a very, very dangerous thing to do.
Everybody's talking about Ozempic and Mounjaro—the GLP-1 drugs—for diabetes or people who are overweight to lose weight and to solve their diabetes. Where does that fit in with this population?
ES: These medications are used to treat Type 2 diabetes in the setting of obesity. Ozempic and Mounjaro are incretin hormones. They mediate satiation, reduce appetite, slow gastric emptying, and lower energy intake. They're really powerful drugs that may be helpful in Type 1 diabetes, but they're not approved for the management of obesity and Type 1 diabetes. At the moment, there aren't data to help guide their use in people with Type 1 diabetes, but I suspect they're going to be increasingly used in people with Type 1 diabetes.
MF: The other piece of managing weight—and it's thought to be foundational for Type 1 or Type 2—is dieting and exercising. However, there isn’t good guidance on how to do this in persons with Type 1 diabetes, whereas there are large and rigorous trials in Type 2 patients. We’re really just starting to figure out how to safely and effectively manage weight with lifestyle changes for Type 1 diabetics, and I think that's an important area of research that should continue moving forward.
ES: Weight management in Type 1 diabetes is complicated by insulin use and the risk of hypoglycemia, or your glucose going too low, which can be an acute complication of exercise. In people with Type 2 diabetes, we have a strong evidence base for what works. We know modest weight loss can help prevent the progression and development of Type 2 diabetes, as well as weight gain. In Type 1, we just don't have that evidence base.
Is there a concern about misdiagnosis and mistreatment? Is it possible to think a patient has Type 2 but they actually have Type 1?
MF: I think so. Insulin is the overriding concern. In the obesity paper, we looked at the percentage of people who said their doctors recommended engaging in more exercise and dieting. We found that people with Type 1 diabetes were less likely to receive the same guidance from their doctor. I think providers may be hesitant to say, “Look, just go engage in an active lifestyle.”
This is why it's important to have those studies and have that guidance so that patients and providers can be comfortable in improving lifestyle management.
Where is this research going next?
ES: What's clear from these studies is that the burden of overweight and obesity is substantial in people with Type 1 diabetes and it's not adequately managed. Going forward, I think we're going to need clinical trials, clear clinical guidelines, and patient education that addresses how best to tackle obesity in the setting of Type 1 diabetes.
It must be confusing for people with Type 1 diabetes who are hearing about people losing all this weight on these drugs, but they go to their doctor who says, “Yeah, but that's not for you.”
ES: I hope it's being handled more sensitively. These drugs are being used by all sorts of people for whom they are not indicated, and I'm sure that people with Type 1 diabetes are accessing these drugs. I think the question is, are there real safety issues? We need thoughtful discussion about this and some real evidence to make sure that we're doing more good than harm.
MF: Dr. Selvin’s group has published a paper, estimating that about 15% of people with Type 1 diabetes are on a GLP-1. But we don't have great data on what potentially can happen to individuals.
The other big part of diabetes that we hear a lot about is insulin and its price. Can you talk about your research on this topic?
MF: There was a survey that asked, “Has there been a point during the year when you were not using insulin because you couldn’t afford it?” About 20% of adults under the age of 65 said that at some point during the year, they couldn't afford their insulin and that they did engage in what sometimes is called “cost-saving rationing” [of insulin].
Medicare is now covering cheaper insulin for those over 65, but there are a lot of people for whom affordability is an issue. Can you talk more about that?
MF: The fight is not over. Just because there are national and state policies, and now manufacturers have been implementing price caps, doesn't necessarily mean that the people who need insulin the most are now able to afford it.
A recent study in the Annals of Internal Medicine looked at states that adopted or implemented out-of-pocket cost caps for insulin versus those that didn't and how that affected insulin use over time. They found that people were paying less for insulin, but the use of insulin didn't change over time. The $35 cap is an improvement, but we need to do more.
ES: There are still a lot of formulations of insulin that are very expensive. $35 a month is not cheap for someone who is on insulin for the rest of their lives.
RELATED:
- Overweight and Obesity in People With Type 1 Diabetes Nearly Same as General Population
- The Impacts of COVID-19 on Diabetes and Insulin
- Why Eli Lilly’s Insulin Price Cap Announcement Matters
Related Content

The Rise of Colorectal Cancers Among Younger People

Nearly One-Third of U.S. Adults Know Someone Who’s Died of Drug Overdose

PFRH’s Patricia Waddy Wins Excellence in Practice Award

Health in All Policies

A Brief History of Traffic Deaths in the U.S.
- Frontiers in Endocrinology
- Clinical Diabetes
- Research Topics
The Recent Advances in Diabetes Mellitus and Glucose Intolerance Associated with Endocrinological or Metabolic Diseases
Total Downloads
Total Views and Downloads
About this Research Topic
Diabetes mellitus is known for its hyperglycemia symptoms resulting from glucose intolerance caused by impaired insulin secretion and/or elevated insulin resistance. Accurate treatment for patients with diabetes mellitus is extremely important for preventing both acute and chronic diabetic complications. While type 2 and type 1 diabetes mellitus are most common, there is increasing recognition that treatment-refractory diabetes cases could have other endocrinological or metabolic disorders. These complications include Cushing’s disease, pheochromocytoma/paraganglioma, primary aldosteronism, acromegaly, adult growth hormone deficiency, hyperthyroidism, and hypothyroidism. The treatment for endocrinological or metabolic diseases may drastically improve glucose intolerance. Meanwhile, the medication for endocrinological or metabolic diseases could be associated with glucose intolerance. Besides, the detailed mechanisms of glucose intolerance in patients with endocrinological or metabolic diseases have been revealed. Hence, updated clinical knowledge is needed to underline the mechanisms. This Research Topic aims to present recent advances and current perspectives on diabetes mellitus and glucose intolerance associated with endocrinological or metabolic diseases. We welcome reviews, mini-reviews, original research, methods, case reports, and perspective articles. We look forward to receiving and compiling a concise source of updated knowledge in this critical area of research that holds great promise for improved patient care.
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, submission deadlines, participating journals.
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
People with Certain Medical Conditions

This information is intended for a general audience. Healthcare professionals should see Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19 for more detailed information.
What You Need to Know
- A person with any of the medical conditions listed below is more likely to get very sick with COVID-19. If you have one of these conditions, talk with your healthcare provider about how best to protect yourself from severe illness from COVID-19.
- The list below does not include all possible conditions that put you at higher risk of severe illness from COVID-19. If you have questions about a condition not included on this list, talk to your healthcare provider about how best to manage your condition and protect yourself from COVID-19.
- If you have symptoms consistent with COVID-19 and you are aged 50 years or older OR are at high risk of getting very sick, you may be eligible for treatment . Contact your healthcare provider and start treatment within the first 5-7 days after you first develop symptoms. You can also visit a Test to Treat location. Treatment can reduce your risk of hospitalization by more than 50% and also reduces the risk of death.
- Staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are important. This is especially important if you are older or have severe health conditions or more than one health condition, including those on the list below.
- Approved and authorized COVID-19 vaccines are safe and effective .
- Some people who are immunocompromised , or people with weakened immune systems, may get additional doses of COVID-19 vaccine.
Medical Conditions
Additional information on children and teens.
Based on the current evidence, a person with any of the conditions listed below is more likely to get very sick from COVID-19. This means that a person with one or more of these conditions who gets very sick from COVID-19 (has severe illness from COVID-19) is more likely to:
- Be hospitalized
- Need intensive care
- Require a ventilator to help them breathe
In addition:
- Older adults are at highest risk of getting very sick from COVID-19. More than 81% of COVID-19 deaths occur in people over age 65. The number of deaths among people over age 65 is 97 times higher than the number of deaths among people ages 18-29 years.
- A person’s risk of severe illness from COVID-19 increases as the number of underlying medical conditions they have increases.
- Studies have shown people from racial and ethnic minority groups are also dying from COVID-19 at younger ages. People in racial and ethnic minority groups are often younger when they develop chronic medical conditions and may be more likely to have more than one medical condition.
- People with disabilities are more likely than those without disabilities to have chronic health conditions, live in shared group (also called “congregate”) settings, and face more barriers in accessing health care . Studies have shown that some people with certain disabilities are more likely to get COVID-19 and have worse outcomes.
Staying up to date with COVID-19 vaccines and taking COVID-19 prevention actions are important. This is especially important if you are older or have severe health conditions or more than one health condition, including those on this list. Learn more about how CDC develops COVID-19 vaccination recommendations. If you have a medical condition, learn more about Actions You Can Take .
- The conditions on this list are in alphabetical order. They are not in order of risk.
- CDC completed a review for each medical condition on this list. This was done to ensure that these conditions met criteria for inclusion on this list. CDC conducts ongoing reviews of additional underlying conditions. If other medical conditions have enough evidence, they might be added to the list.
- Because we are learning more about COVID-19 every day, this list does not include all medical conditions that place a person at higher risk of severe illness from COVID-19. Rare medical conditions, including many conditions that mostly affect children, may not be included on the list below. We will update the list as we learn more.
- A person with a condition that is not listed may still be at greater risk of getting very sick from COVID-19 than other people who do not have the condition. It is important that you talk with your healthcare provider about your risk .
Having cancer can make you more likely to get very sick from COVID-19. Treatments for many types of cancer can weaken your body’s ability to fight off disease. At this time, based on available studies, having a history of cancer may increase your risk.
Get more information:
- COVID-19: What People with Cancer Should Know – National Cancer Institute
Chronic kidney disease
Having chronic kidney disease of any stage can make you more likely to get very sick from COVID-19.
- Chronic Kidney Disease
- National Kidney Foundation: Kidney Disease and COVID-19
Chronic liver disease
Having chronic liver disease can make you more likely to get very sick from COVID-19. Chronic liver disease can include alcohol-related liver disease, non-alcoholic fatty liver disease, autoimmune hepatitis, and cirrhosis (or scarring of the liver).
- Liver Disease
- American Liver Foundation: Your Liver and COVID-19
Chronic lung diseases
Having a chronic lung disease can make you more likely to get very sick from COVID-19. Chronic lung diseases can include:
- Asthma, if it’s moderate to severe
- Bronchiectasis (thickening of the lungs’ airways)
- Bronchopulmonary dysplasia (chronic lung disease affecting newborns)
- Chronic obstructive pulmonary disease (COPD), including emphysema and chronic bronchitis
- Having damaged or scarred lung tissue known as interstitial lung disease (including idiopathic pulmonary fibrosis)
- Pulmonary embolism (blood clot in the lungs)
- Pulmonary hypertension (high blood pressure in the lungs)
- People with Moderate to Severe Asthma
- American Lung Association: Controlling Chronic Lung Diseases Amid COVID-19
- Cystic fibrosis
Having cystic fibrosis, with or without lung or other solid organ transplant (like kidney, liver, intestines, heart, and pancreas) can make you more likely to get very sick from COVID-19.
- Cystic Fibrosis Foundation: CF and Coronavirus (COVID-19)
Dementia or other neurological conditions
Having neurological conditions, such as dementia, can make you more likely to get very sick from COVID-19.
- Alzheimer’s Association: COVID-19, Alzheimer’s and Dementia
Diabetes (type 1 or type 2)
Having either type 1 or type 2 diabetes can make you more likely to get very sick from COVID-19.
- American Diabetes Association: How COVID-19 Impacts People with Diabetes
Disabilities
People with some types of disabilities may be more likely to get very sick from COVID-19 because of underlying medical conditions, living in congregate settings, or systemic health and social inequities, including:
- People with any type of disability that makes it more difficult to do certain activities or interact with the world around them, including people who need help with self-care or daily activities
- People with attention-deficit/hyperactivity disorder (ADHD)
- People with cerebral palsy
- People with birth defects
- People with intellectual and developmental disabilities
- People with learning disabilities
- People with spinal cord injuries
- People with Down syndrome
- People with Disabilities
Heart conditions
Having heart conditions such as heart failure, coronary artery disease, cardiomyopathies, and possibly high blood pressure (hypertension) can make you more likely to get very sick from COVID-19.
- Heart Disease
- American Heart Association: COVID-19
- NHLBI Information and Resources on COVID-19
HIV infection
Having HIV (Human Immunodeficiency Virus) infection can make you more likely to get very sick from COVID-19.
- HIV Infection
- Interim Guidance for COVID-19 and Persons with HIV
Immunocompromised condition or weakened immune system
Some people are immunocompromised or have a weakened immune system because of a medical condition or a treatment for a condition. This includes people who have cancer and are on chemotherapy, or who have had a solid organ transplant, like a kidney transplant or heart transplant, and are taking medication to keep their transplant. Other people have to use certain types of medicines for a long time, like corticosteroids, that weaken their immune system. One example is called primary immunodeficiency. Being immunocompromised can make you more likely to get very sick from COVID-19 or be sick for a longer period of time.
People who are immunocompromised or are taking medicines that weaken their immune system may not be protected even if they are up to date on their vaccines . Talk with your healthcare provider about what additional precautions may be necessary when respiratory viruses are causing a lot of illness in your community . Additionally, people who are moderately or severely immunocompromised may get additional doses of updated COVID-19 vaccine. Because the immune response following COVID-19 vaccination may differ in people who are moderately or severely immunocompromised, specific guidance has been developed.
People who are moderately or severely immunocompromised, are aged 12 and older, and who weigh at least 88 pounds may be eligible to get Pemivibart (Pemgarda™) , a monoclonal antibody authorized to help protect against COVID-19. Pemgarda may provide another layer of protection against COVID-19 in addition to protection provided through vaccination, and can be given at least 2 weeks after receiving a COVID-19 vaccine. Pemgarda is not a treatment for COVID-19. Talk to your healthcare provider to see if Pemgarda is right for you.
- Types of Primary Immune Deficiency Diseases
- Jeffrey Modell Foundation
- Immune Deficiency Foundation
- Primary Immunodeficiency (PI)
Mental health conditions
Having mood disorders, including depression, and schizophrenia spectrum disorders can make you more likely to get very sick from COVID-19.
- National Institute of Mental Health (NIMH) Shareable Resources on Coping with COVID-19
- National Institute of Mental Health (NIMH) Depression
- Mood Disorders
Overweight and obesity
Overweight (defined as a body mass index (BMI) is 25 kg/m 2 or higher, but under 30 kg/m 2 ), obesity (BMI is 30 kg/m 2 or higher, but under 40 kg/m 2 ), or severe obesity (BMI is 40 kg/m 2 or higher), can make you more likely to get very sick from COVID-19. The risk of severe illness from COVID-19 increases sharply with higher BMI.
- Overweight and Obesity
- Obesity, Race/Ethnicity, and COVID-19
- Obesity Action Coalition: COVID-19 and Obesity
Physical inactivity
People who do little or no physical activity are more likely to get very sick from COVID-19 than those who are physically active. Being physically active is important to being healthy. Get more information on physical activity and health, physical activity recommendations, how to become more active, and how to create activity-friendly communities:
- Physical Activity
- Physical Activity Guidelines for Americans , 2nd edition
- Move Your Way ®
- Active People, Healthy Nation SM : Strategies to Increase Physical Activity
- National Center on Health, Physical Activity and Disability – Building Healthy Inclusive Communities
Pregnant and recently pregnant people (for at least 42 days following end of pregnancy) are more likely to get very sick from COVID-19 compared with non-pregnant people.
- Pregnant and Recently Pregnant People
Sickle cell disease or thalassemia
Having hemoglobin blood disorders like sickle cell disease or thalassemia (inherited red blood cell disorders) can make you more likely to get very sick from COVID-19.
- Sickle Cell Disease
- Thalassemia
Smoking, current or former
Being a current or former cigarette smoker can make you more likely to get very sick from COVID-19. If you currently smoke, quit. If you used to smoke, don’t start again. If you’ve never smoked, don’t start.
- Smoking and Tobacco Use
- Tips From Former Smokers
- Health Benefits of Quitting Smoking
Solid organ or blood stem cell transplant
Having had a solid organ or blood stem cell transplant, which includes bone marrow transplants, can make you more likely to get very sick from COVID-19.
- Transplant Safety
- COVID-19 Resources for Transplant Community
Stroke or cerebrovascular disease
Having cerebrovascular disease, such as having a stroke which affects blood flow to the brain, can make you more likely to get very sick from COVID-19.
- COVID19 Stroke Podcast Series for Patients and Caregivers
Substance use disorders
Having a substance use disorder (such as alcohol, opioid, or cocaine use disorder) can make you more likely to get very sick from COVID-19.
- How to Recognize a Substance Use Disorder
- Drug Overdose
Tuberculosis
Having tuberculosis (TB) can make you more likely to get very sick from COVID-19.
- Basic TB Facts
- Public Health Emergencies
People of all ages, including children, can get very sick from COVID-19. Children with underlying medical conditions are at increased risk for getting very sick compared to children without underlying medical conditions.
Current evidence suggests that children with medical complexity, with genetic, neurologic, or metabolic conditions, or with congenital heart disease can be at increased risk for getting very sick from COVID-19. Like adults, children with obesity, diabetes, asthma or chronic lung disease, sickle cell disease, or who are immunocompromised can also be at increased risk for getting very sick from COVID-19. Check out COVID-19 Vaccines for Children and Teens for more information on vaccination information for children.
- COVID-19 Vaccines for Children and Teens
Continue medications and preventive care
- Continue your medicines and do not change your treatment plan without talking to your healthcare provider.
- Have at least a 30-day supply of prescription and non-prescription medicines. Talk to a healthcare provider, insurer, or pharmacist about getting an extra supply (i.e., more than 30 days) of prescription medicines, if possible, to reduce your trips to the pharmacy.
- Follow your current treatment plan (e.g., Asthma Action Plan , dialysis schedule, blood sugar testing, nutrition, and exercise recommendations) to keep your medical condition(s) under control.
- When possible, keep your appointments (e.g., vaccinations and blood pressure checks) with your healthcare provider. Check with your healthcare provider about safety precautions for office visits and ask about telemedicine or virtual healthcare appointment options.
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Type 1 diabetes
Linda a dimeglio.
Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA;
Carmella Evans-Molina
Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;
Richard A Oram
Institute of Biomedical and Clinical Science, University of Exeter Medical School, and The Academic Kidney Unit, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK
Contributors
Type 1 diabetes is a chronic autoimmune disease characterised by insulin deficiency and resultant hyperglycaemia. Knowledge of type 1 diabetes has rapidly increased over the past 25 years, resulting in a broad understanding about many aspects of the disease, including its genetics, epidemiology, immune and β-cell phenotypes, and disease burden. Interventions to preserve β cells have been tested, and several methods to improve clinical disease management have been assessed. However, wide gaps still exist in our understanding of type 1 diabetes and our ability to standardise clinical care and decrease disease-associated complications and burden. This Seminar gives an overview of the current understanding of the disease and potential future directions for research and care.
Introduction
At first consideration, type 1 diabetes pathophysiology and management might seem straightforward; however, the more that is learnt about the disease, the less it seems is truly known. Improved understanding of the disease’s pathogenesis has not led to a single unifying Koch’s postulate for all cases. What once seemed like a single autoimmune disorder, with roots in T-cell mediated attack of insulin-producing β cells, is now recognised to result from a complex interplay between environmental factors and microbiome, genome, metabolism, and immune systems that vary between individual cases.
Despite known genetic underpinnings, most people who are diagnosed with type 1 diabetes do not have a relative with the disease or even the highest risk combination of HLA alleles, making attempts at primary disease prevention difficult. Although survival and patient health have improved considerably, particularly in the past 25 years, a cure for type 1 diabetes remains elusive. 1 , 2 Additionally, despite advances in technology, glycaemic control for most people with type 1 diabetes is not optimised, and many cannot access modern therapies because of the high costs of even basic care.
In 1984, George Eisenbarth developed a conceptual model for the pathogenesis of type 1 diabetes that is still used nowadays ( figure 1 ). 3 The model plots β-cell mass against age, highlighting an event sequence starting with predisposing genetic risk, then a precipitating environmental trigger that causes islet-specific auto-immunity, followed by β-cell loss, dysglycaemia, clinical diabetes, and rapid progression to complete β-cell loss. Although useful, this model does not address the increasingly apparent complexity of type 1 diabetes pathogenesis. Additionally, the disease pathogenesis is shown by a single line of disease course over time; however, at all stages of the disease heterogeneity exists that is not well understood.

Key events of the Eisenbarth model 3 over the course of the disease (measured in years) are shown by dotted lines at different time points. Challenges to this model, taking into account the increasing complexity of type 1 diabetes, include the following: precipitating immune events that might occur prenatally (A); large variation in starting β-cell mass and function, defects in one or both could be developmentally programmed (B); initiation of autoimmunity is measured by autoantibodies, but other immunological abnormalities probably precede the presence of detectable pancreatic antibodies (C); the patient’s environment could affect their entire disease course (D); β-cell loss could relapse or remit (E); dysglycaemia occurs before clinical diagnosis (F); decline in β-cell function might not mirror decline in β-cell mass—methods to measure β-cell mass have not been established (G); and residual C-peptide is detectable in many people who have long duration type 1 diabetes (H). Furthermore, progression through stages A–C is heterogeneous, and will be affected by immune, genetic, environment, and key demographic features (ie, age, body-mass index). Adapted from Atkinson et al. 4
This Seminar provides a review of type 1 diabetes and the status of research in the field. We focus on developments from the past 5 years that highlight the heterogeneity and complexity of the disease.
A diagnosis of diabetes is based on a fasting blood glucose concentration above 7·0 mmol/L (126 mg/dL), a random blood glucose concentration above 11·1 mmol/L (200 mg/dL) with symptoms, or an abnormal result from an oral glucose tolerance test. 5 In the absence of symptoms, abnormal glycaemia must be present on two different occasions. A diagnosis of diabetes can also be made on the basis of a glycated haemoglobin (HbA 1c ) concentration above 48 mmol/mol (6·5%). However, since dysglycaemia progression can be rapid in patients with type 1 diabetes, HbA 1c is less sensitive for diagnosis than fasting or stimulated blood glucose measurements. 5
Children with type 1 diabetes commonly present with symptoms of polyuria, polydipsia, and weight loss; approximately a third present with diabetic ketoacidosis. 6 The onset of type 1 diabetes can be more variable in adults, who might not present with the classic symptoms seen in children. Although traditional definitions classified type 1 diabetes as juvenile onset, the disease can occur at any age, with up to 50% of cases occurring in adulthood. 7 As many as 50% of adults with type 1 diabetes might be initially misclassified as having type 2 diabetes. 8 Similarly, in conjunction with the epidemic of childhood obesity, type 2 diabetes is increasingly common in adolescents (particularly in non-white individuals), and monogenic diabetes (eg, maturity diabetes onset of the young) accounts for 1–6% of childhood diabetes cases. 9 – 11
Although low C-peptide concentration as a marker of severe endogenous insulin deficiency is useful to guide both classification and treatment in cases of diabetes assessed over 3 years after clinical diagnosis, 12 no single clinical feature can perfectly distinguish type 1 from non-type 1 diabetes at diagnosis. Classification depends on an appreciation of other risk factors for type 1 versus other subtypes and the integration of clinical features (eg, age of diagnosis and body-mass index) with biomarkers (eg, pancreatic autoantibodies). 13
Over 90% of people with newly diagnosed type 1 diabetes have measurable antibodies against specific β-cell proteins, including insulin, glutamate decarboxylase, islet antigen 2, zinc transporter 8, and tetraspanin-7. 14 Birth cohort studies 15 , 16 of individuals with a high genetic risk for diabetes have shown a peak incidence of first autoantibody development before age 2 years. Most people with a single autoantibody do not progress to type 1 diabetes, but seroconversion to the presence of two or more serum autoantibodies in children is associated with an 84% risk of clinical type 1 diabetes by the age of 18 years ( figure 2A ). 16 The high risk of progression in the presence of multiple autoantibodies has led to a redefining of type 1 diabetes stages. In this new paradigm, a preclinical stage 1 case of type 1 diabetes is defined as the presence of two or more autoantibodies, while stages 2 and 3 are defined as the progression of metabolic abnormalities from abnormal glycaemia to overt diabetes, diagnosed by standard criteria ( figure 2B ). 18 Since the progression from islet autoantibody positivity to clinical diabetes could take months or years, defining multiple auto-antibody positivity as stage 1 allows targeting of immune interventions to a realistic primary outcome and facilitates early life intervention studies. 19

(A) The probability of developing diabetes in childhood stratified by the number of islet antibodies. In a study by Zeigler and colleagues, 16 13 377 children were identified as at risk in the newborn or infant period on the basis of high-risk HLA genotypes or having a relative with type 1 diabetes, or both, and were followed-up regularly. The numbers at risk are the number of children receiving follow-up at ages 0, 5, 10, 15, and 20 years. Adapted from Ziegler et al 16 with permission of the American Medical Association. (B) Type 1 diabetes progression and stages of type 1 diabetes. Stage 1 is the start of type 1 diabetes, marked by individuals having two or more diabetes-related autoantibodies and normal blood sugar concentrations. In stage 2, individuals have dysglycaemia without symptoms. Stage 3 is the time of clinical diagnosis. Reproduced from Greenbaum et al, 17 with permission from the American Diabetes Association. T1D=type 1 diabetes.
Type 1 diabetes is a heritable polygenic disease with identical twin concordance of 30–70%, 20 sibling risk of 6–7%, and a risk of 1–9% for children who have a parent with diabetes. 21 The overall lifetime risk varies greatly by country and geographical region but overall is around one in 250 people. 22 The disease is slightly more common in men and boys than in women and girls. 23 Two HLA class 2 haplotypes involved in anti gen presentation, HLA DRB1*0301-DQA1*0501-DQ* {"type":"entrez-nucleotide","attrs":{"text":"B10201","term_id":"2091320","term_text":"B10201"}} B10201 ( DR3 ) and HLA DRB1*0401-DQA1*0301-DQB1*0301 (DR4-DQ8) , are linked to approximately 50% of disease heritability and are prevalent in white people. 24 Other haplotypes are known to reduce type 1 diabetes risk, including DRB1*1501-DQA1*0102-DQB1–0602 ( DR15-DQ6 ). 24 The mechanisms by which these HLA haplotypes interact and alter risk are not completely understood. Different HLA associations in other racial groups are recognised but remain poorly characterised. 24 Genome-wide association studies have identified over 60 additional non-HLA loci associated with the risk of type 1 diabetes. These variants have been predominantly associated with the immune system and highlight pathways that are important in disease development—eg, insulin gene expression in the thymus, regulation of T-cell activation, and viral responses. 24 These HLA and non-HLA genetic associations could identify potential targets for future disease-modifying therapies or subgroups of patients who could benefit from specific immune interventions.
Historically, people at high risk of type 1 diabetes have been identified for research by HLA risk or familial risk, or both. 25 By contrast, individual non-HLA loci cannot be used to predict type 1 diabetes or discriminate it from other types of diabetes. Combined measurement of HLA and non-HLA loci into genetic risk scores could offer improved prediction of the risk of developing type 1 diabetes and discrimination of type 1 from type 2 diabetes. 26 , 27 Furthermore, the continuing fall of genotyping costs could facilitate future population-level disease prediction by use of genetic risk scores. 19 , 28
Epidemiology
Globally, type 1 diabetes is increasing both in incidence and prevalence, with overall annual increases in incidence of about 2–3% per year. 29 , 30 US data 31 suggest an overall annualised incidence from 2001 to 2015 of about 22·9 cases per 100 000 people among those younger than 65 years; data from other regions suggest similar incidences. 32 The greatest observed increases in incidence of type 1 diabetes are among children younger than 15 years, particularly in those younger than 5 years. 33 These increases cannot be explained by genetic changes, implicating environmental or behavioural factors, or both. Many environmental exposures are associated with type 1 diabetes, including infant and adult diet, vitamin D sufficiency, early-life exposure to viruses associated with islet inflammation (eg, enteroviruses), and decreased gut-microbiome diversity. 34 Obesity is associated with increasing presentation of type 1 diabetes, with β-cell stress potentially providing a mechanistic underpinning. 34 , 35 The large differences in the incidence of type 1 diabetes in genetically similar populations that are separated by socioeconomic borders 36 and the increasing incidence of type 1 diabetes in genetically low-risk individuals 37 highlight the importance of environmental risk factors regardless of genetic background risk. Further work is being done to understand the role of gene–environment interactions in the pathogenesis of type 1 diabetes, the role of different loci and pathways at different stages of the disease, and whether loci that are independent of disease risk could have a role in disease progression after development of autoimmunity. 38 – 40 Some data 31 , 41 suggest that the observed incidence could be declining in adults or potentially even levelling off across all age ranges; worldwide registry data will eventually reveal if this pattern is indeed true. 42
The incidence of type 1 diabetes varies by country and by region within countries. 31 At northern latitudes, people born in the spring are more likely to develop the disease than those born in the other seasons. 43 The peak incidence of diagnosis is seen in children aged 10–14 years. 31 , 32 Although many people present with type 1 diabetes in adulthood, 44 the higher incidence of type 2 diabetes in adulthood compared with type 1 diabetes and the flawed criteria for distinguishing these forms of disease make assessment of the incidence of type 1 diabetes in adults very difficult. 23 , 45 Most people living with type 1 diabetes are adults. 46
The immune phenotype of type 1 diabetes
The pathogenesis of type 1 diabetes results from a complex interaction between the pancreatic β-cell and innate and adaptive immune systems ( figure 3 ). 47 The question of whether a trigger for the immune response against β cells exists or whether the immune response is a random stochastic event has been a subject of considerable speculation and controversy. Several viral infections are associated with type 1 diabetes, with enterovirus being one of the most commonly associated infections. Enteroviral major capsid protein VP1 and RNA have been detected in islets from people with recent-onset type 1 diabetes, 48 along with hyper-expression of the class 1 major histo compatibility complex 49 and other indices of viral infection. One possibility is that some people with type 1 diabetes have an atypical, chronic viral infection of β cells, leading to chronic inflammation and the development of autoimmunity. The viral hypothesis has been difficult to test, although both antiviral therapy and the development of vaccines targeting enteroviruses are being pursued for this purpose.

The development of type 1 diabetes is thought to be initiated by the presentation of β-cell peptides by antigen-presenting cell (APCs). APCs bearing these autoantigens migrate to the pancreatic lymph nodes where they interact with autoreactive CD4+ T lymphocytes, which in turn mediate the activation of autoreactive CD8+T cells (A). These activated CD8+ T cells return to the islet and lyse β cells expressing immunogenic self-antigens on major histocompatibility complex class I surface molecules (B). β-cell destruction is further exacerbated by the release of proinflammatory cytokines and reactive oxygen species from innate immune cells (macrophages, natural killer cells, and neutrophils; C). This entire process is amplified by defects in regulatory T lymphocytes, which do not effectively suppress autoimmunity (D). Activated T cells within the pancreatic lymph node also stimulate B lymphocytes to produce autoantibodies against β-cell proteins. These autoantibodies can be measured in circulation and are considered a defining biomarker of type 1 diabetes (E).
In the field, much effort has been given to the study of the adaptive immune system in type 1 diabetes by use of assays of peripheral lymphocytes selected for autoreactivity to islet antigens. Increased frequency of islet-specific autoreactive CD8+ T lymphocytes and decreased regulatory immune function have been associated with type 1 diabetes. 50 Experiments, such as the transfer of type 1 diabetes following non-T-cell depleted allogeneic bone-marrow transplantation, 51 development of type 1 diabetes in an individual with B-lymphocyte and antibody deficiency, 52 and inherited genetic defects of T-lymphocyte function causing type 1 diabetes 53 highlight the crucial role of T cells in the pathophysiology of type 1 diabetes. 54 Almost all studies of peripheral autoimmunity in people with type 1 diabetes show overlap of phenotypes seen in the general population, and the proportion of islet autoreactive cells present in the periphery is often tiny (only a few cells among millions of non-autoreactive cells). As a result, connecting the population of autoreactive immune cells that is detectable in blood to the disease process in islets has been difficult. A key development has been the isolation of T lymphocytes that are reactive to β-cell antigen peptides from islets of organ donors with type 1 diabetes. 55 – 57
Histopathologically, these processes are observed as insulitis or immune-infiltrated (insulitic) islets. 58 CD8+ T lymphocytes are the most common immune cells within insulitic lesions, with CD4+ T cells present in lower numbers. Distinct patterns of insulitis that stratify with the aggressiveness of β-cell loss and age of diagnosis have been identified in insulitic islets. 59 Although insulitis is common and intense in animal models of type 1 diabetes, it is much rarer and more variable in human beings ( figure 3 ). 60
The β-cell phenotype of type 1 diabetes
At diagnosis, people with type 1 diabetes have reduced β-cell function compared with healthy controls. 61 With amelioration of hyperglycaemia, these β cells can have a partial recovery of insulin secretory function, leading to a so-called honeymoon period after diagnosis with minimal or no exogenous insulin needed. Over time, many of these residual cells are lost. However, analysis of pancreatic sections from individuals with long-term type 1 diabetes show the presence of residual β cells decades after diagnosis. 62 , 63 When sensitive C-peptide measure ments are performed, 30–80% of people with long-term type 1 diabetes are found to be insulin microsecretors. 64 – 67 So, although endogenous β-cell quantity and function decline with longer disease duration, this decline does not progress to a complete loss of all β cells. 64 – 67 This finding is noteworthy because in the Diabetes Control and Complications Trial 68 , 69 persistent C-peptide secretion was associated with reduced development of retinopathy, nephropathy, and hypoglycaemia. Additionally, the persistence of C-peptide secretion in people with long-term type 1 diabetes could improve glucagon responses to hypoglycaemia. 70 Moreover, the presence of residual C-peptide secretion after the diagnosis of disease could also increase the possibility of an improved effect of interventions targeted at rescuing or augmenting the survival of this residual pool of β cells. Analyses of pancreatic specimens from the Network of Pancreatic Organ Donors repository have not found evidence of either increased neogenesis or proliferation in pancreatic cells from donors with type 1 diabetes. 63 Thus, the mechanisms underlying the persistence of residual β cells in people with long-term type 1 diabetes remain unclear. Identifying pathways that have allowed these cells to escape the autoimmune attack could yield insight into new therapeutic approaches.
β-cell abnormalities might also contribute to type 1 diabetes pathogenesis, leading to the notion of so-called β-cell suicide. β-cell HLA class I overexpression is common in pancreatic sections from cadaveric donors with type 1 diabetes. This overexpression serves as a homing signal for cytotoxic T lymphocytes. 49 However, whether this signal is a primary β-cell defect or a response to a stimulus (eg, a viral infection) is not yet known. Additionally, evidence also exists for increased β-cell endoplasmic reticulum stress linked with accelerated β-cell death. 71 , 72 Endoplasmic reticulum stress in β cells has also been associated with alterations in mRNA splicing and errors in protein translation and folding; the resultant protein products have been proposed as potential immunogenic neoantigens. 73
In addition to these defects in the β-cell compartment, alterations in non-endocrine islet cells and the exocrine pancreas have also been described ( figure 4 ). These defects include abnormalities in the islet extracellular matrix 74 , 75 and in islet innervation and vascularity. 76 – 78 Data have also placed a renewed emphasis on the role of exocrine pancreatic pathology in type 1 diabetes. Compared with healthy individuals, people with type 1 diabetes have a decreased pancreatic weight and volume that continues to decrease with disease duration. 79 , 80 This finding could be explained by developmental defects, or pancreatic atrophy in response to loss of the paracrine and pro-growth effects of insulin or chronic inflammation, or even autoimmune-mediated exocrine destruction. These possibilities are all topics of active investigation.

(A) Type 1 diabetes is characterised by a variety of abnormalities that involve both the islet and the exocrine pancreas. The hallmark of type 1 diabetes is loss of insulin-producing β cells and immune infiltration of islets. However, the presence of insulitis, even within an individual pancreas, can be highly variable. (B) Immunofluorescent image of an insulitic islet from a cadaveric donor with long-term type 1 diabetes. Insulin is shown in blue and CD8+ T cells surrounding the islet are shown in yellow. (C) Haematoxylin and eosin staining of an islet from a cadaveric donor that exhibits a classic pattern of insulitis. The islet is circled with a yellow dotted line. The infiltrating immune cells are circled in red and indicated by arrows. (D) Haematoxylin and eosin staining of an islet, circled in yellow dotted line, from a cadaveric donor with long-term type 1 diabetes without any discernible immune infiltrate. By contrast with the islet in (C), this islet has evidence of peri-islet fibrosis as shown circled in red and indicated by arrows. Images B–D courtesy of M Campbell-Thompson, University of Florida, Gainesville, FL, USA.
Management of clinical disease
Methods of managing type 1 diabetes continue to improve, and although progress is generally slow and incremental, occasionally it is punctuated by rapid change. One such moment of change happened in 1993 with the publication of the Diabetes Control and Complication Trial. 81 This trial and the follow-up observational Epidemiology of Diabetes Interventions and Complications trial convincingly showed that achieving and maintaining glucose concentrations as close to those seen in people without diabetes as possible leads to a reduction in microvascular and cardiovascular type 1 diabetes complications. 82
Although insulin remains the mainstay of therapy, new insulin analogues with varying onsets and durations of action are widely available. Optimal glycaemic control requires multiple-dose insulin regimens that mimic physiological insulin release, with basal insulin for overnight and between-meal control, plus bolus doses of rapid-acting insulin analogues to cover ingested carbohydrate loads and treat hyperglycaemia. Insulin can be taken by injection (with an insulin pen if available) or, preferably for many people, with an insulin pump. 83 Ultra-rapid inhaled insulin is also available, but little enthusiasm for this preparation exists because of its fixed dosing (four or eight unit increments only), issues with consistent delivery, cost, and the need for pulmonary function testing. 84 A faster-acting subcutaneously-administered insulin (via injection or infusion) has also recently become available for clinical use. Appropriate insulin use requires frequent dosing adjustments for ingested carbohydrates, physical activity, and illness or stress.
While pramlintide is the only non-insulin medication approved for improved glycaemic control in patients with type 1 diabetes, metformin, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium-glucose co-transporter-2 (SGLT2) inhibitors have also been used of-label; however, fewer than 5% of patients use these medications. 85 Metformin, an insulin sensitiser, is the most commonly prescribed drug for people with type 1 diabetes who have insulin resistance but it has not been shown to be effective in people younger than 18 years who are overweight or obese and have type 1 diabetes. 86 Use of SGLT2 inhibitors is restricted in part because of early reports of euglycaemic diabetic ketoacidosis in people with type 1 diabetes treated with these compounds. A 2018 meta-analysis of these inhibitors suggests they are safe, 87 but more data are needed.
Glucagon therapy is also poised to undergo a resurgence in management of type 1 diabetes. Although only an emergency kit has been commercially available up until now for cases of severe hypoglycaemia leading to seizure or loss of consciousness, nasal and stable liquid formulations are being developed. The nasal formulation will be available as a rapid rescue therapy only, 88 whereas the stable liquid formulation could also be used in small doses for exercise and in dual hormone (ie, insulin and glucagon) closed-loop systems. 89 , 90
In the past 13 years, continuous glucose monitoring (CGM) and intermittently viewed CGM devices for at-home patient use with minimally invasive devices have become available, which have similar accuracy to capillary blood glucose monitors. 91 Both CGM and intermittently viewed CGM allow examination of glucose concentration patterns over time and, although CGM devices still need periodic calibration, they obviate the need for frequent capillary blood glucose measurements. CGM is more sophisticated than intermittently viewed CGM because it can give the user a warning on the basis of absolute or projected glucose values. When CGM is incorporated into hybrid closed-loop insulin-pump systems that automatically regulate basal infusion rates, but that require manual delivery of meal boluses by trained wearers to cover estimated carbohydrate intakes, substantial improvements in glucose variability and overall glycaemic control are seen ( figure 5 ). 93 Combined use of automated insulin delivery and CGM offers the prospect of an artificial pancreas with little input from the user. The substantial advances that have been made in pump and sensor technology and the increase in the number of trials to test their efficacy show that partially or fully automated systems could become a reality.

Sensor glucose profiles from 124 people with type 1 diabetes, of which 30 were adolescents (14–21 years; A) and 94 were adults (22–75 years; B), before (during run-in phase) and during the study phase using the Medtronic MiniMed 670 g hybrid closed-loop system (Medtronic, Northridge CA, USA) under clinical trial conditions. Median and IQR of sensor glucose values are given as a green line and band for the run-in phase, and a pink line and band for the study phase, respectively. In the run-in phase, the hybrid closed-loop system was in manual mode, with participants making all treatment decisions except for the pump automatically suspending before senor glucose concentrations became too low. In the study phase, the hybrid closed-loop system was in auto mode. Participants had less variability in their blood glucose concentration during auto mode. Reproduced from Garg et al, 92 with permission from Mary Ann Liebert.
Guidelines from the American Diabetes Association, International Society for Pediatric and Adolescent Diabetes, and Candian Diabetes Association suggest a HbA 1c target of less than 53 mmol/mol (7·0%) for adults and less than 58 mmol/mol (7·5%) in paediatric patients with type 1 diabetes; 94 – 96 however, most individuals do not achieve these targets. Although setting more aggressive targets is associated with achieving lower HbA 1c , 97 these targets should be individualised on the basis of many factors including comorbidities, patient capability and attitude, and available care resources 98 —eg, even lower targets are often prescribed for pregnant women and women anticipating pregnancy than those prescribed to other patients. 99 Higher targets might be appropriate for people with hypoglycaemia unawareness, history of severe hypoglycaemia, advanced complications, and short life expectancy. For optimal outcomes, people with diabetes should be cared for by a multidisciplinary care team, including diabetes educators, nurse practitioners, nurses, nutritionists, physician assistants, exercise physiologists, social workers, and psychologists. To optimise glycaemic control, clinical care with skilled and structured patient education and training sessions should be provided—including information on insulin adjustments, carbohydrate counting, and optimal use of available technology. 100
People with type 1 diabetes also risk developing other autoimmune diseases, sometimes as part of a poly-glandular autoimmune syndrome. A study 101 from the Type 1 Diabetes Exchange clinic registry noted the prevalence of autoimmune disease was 27% in a population of over 25 000 people with type 1 diabetes with a mean age of 23 years. The most common autoimmune disease is autoimmune thyroiditis (ie, Hashimoto thyroid itis and Graves’ disease) followed by coeliac disease. Other associated conditions include collagen-vascular diseases (eg, rheumatoid arthritis and lupus), autoimmune gastritis or pernicious anaemia, vitiligo, and Addison’s disease. Guidelines for the care of people with diabetes include periodic screening for these diseases, particularly thyroid and coeliac diseases. 102
Complications of type 1 diabetes
The discovery of insulin in 1922 transformed type 1 diabetes from a terminal to a treatable disease. Despite the advances in care discussed previously, the disease continues to be associated with substantial medical, psychological, and financial burden. Hypoglycaemia and ketoacidosis are persistent potentially life-threatening complications. Severe hypoglycaemic events requiring treatment assistance from another person occur at rates of 16–20 per 100 person-years; hypoglycaemic events leading to loss of consciousness or seizure occur at a rate of 2–8 per 100 person-years. 103 – 105 Recurrent hypoglycaemia results in an increased likelihood of hypoglycaemia unawareness and subsequent severe hypoglycaemic events, since recurrent hypoglycaemia reduces the glucose concentration that triggers the counter-regulatory responses to return to euglycaemia. 106 Hypoglycaemia unawareness can improve with edu cation, support, and glucose targets that are aimed at avoiding biochemical hypoglycaemia, while maintaining overall metabolic control. 107
Hypoglycaemic events are associated with adverse effects on cognitive function, 108 , 109 and are associated with 4–10% of type 1 diabetes-related deaths. 110 – 112 Observational studies suggest poor diabetes control does not reduce the risk of severe hypoglycaemia. 113 Notably, rates of severe hypoglycaemic events have been decreasing over time 104 and with CGM and other advanced diabetes technologies HbA 1c can be lowered into the target range without increasing the risk of severe hypoglycaemia. 114 Treatment in hospital for diabetic ketoacidosis occurs at a rate of 1–10 per 100 patient-years in paediatric populations with established type 1 diabetes, and accounts for 13–19% of type 1 diabetes-related mortality. 105 , 110 , 111 Incidence of diabetic ketoacidosis is higher among women than among men, and among people with higher HbA 1c levels than other people with type 1 diabetes.
Microvascular complications of the disease manifest primarily as retinopathy, neuropathy, and nephropathy, but also can affect cognitive function, the heart, and other organs. Hyperglycaemia is the primary risk factor for microvascular disease, and reducing HbA 1c through intensive diabetes management, particularly early during disease, is associated with striking (about 70%) reductions in incidence and slower progression of microvascular disease. However, differences in HbA 1c do not fully explain the variation in the incidence of complications and the severity of disease between individuals. Variability in glucose concentrations (both during the day and longer term) and glycosylation rates also probably have a role in interindividual differences. 115 , 116 Type 1 diabetes during puberty also appears to accelerate the development of complications. 117
Macrovascular complications of type 1 diabetes include atherosclerosis and thrombosis in the heart, peripheral arteries, and brain. By contrast with microvascular complications, the risk of cardiovascular complications does not appear to be as attenuated by intensive blood sugar control. Diabetic nephropathy, whether manifesting as microalbuminuria, macroalbuminuria, or a reduced glomerular filtration rate progressively augments the overall risk of macrovascular complications. 118 Cardiovascular disease remains the major cause of premature morbidity and mortality, with data 119 , 120 suggesting an 8–13-year shorter life expectancy for people with type 1 diabetes than for healthy individuals.
People with diabetes might also have both chronic and acute neurocognitive changes that include decline in cognitive function with detrimental effects on psychomotor speed, cognitive flexibility, attention, and visual perception. 121 , 122 Although the pathophysiology of neurocognitive changes is poorly understood, their development has been linked with both microvascular and macrovascular changes and changes in brain structure, neuronal loss, and cerebral atrophy. 123 , 124 Risk factors include developing diabetes early in life, chronic hyperglycaemia, and repeated hypoglycaemia.
In the past 25 years, among people with type 1 diabetes the risks of microvascular and macrovascular compli-cations have substantially decreased and outcomes have improved. 125 , 126 These improvements have been largely driven by better glycaemic control and improved management of associated risk factors—eg, hypertension and hyperlipidaemia. Several studies 127 – 130 have identified additional non-glycaemic risk factors for the development of complications. Genetic studies have not yielded strong associations between specific gene variants and complication status. Low levels of education and income have been associated with high risks of both micro-vascular and macrovascular complications. 127 Sex also appears to modify risk, since women with type 1 diabetes have been shown to have higher rates of all-cause premature mortality and vascular events than do men with type 1 diabetes. 128 In the past 5 years, new technologies have been designed to attempt to better predict future risk and complications by combining risk factors into probability models. Two examples are the QDiabetes 129 and QRISK3 130 web calculators that were developed with a prospective general practice dataset of 803 044 people with diabetes (44 440 with type 1 diabetes). These calculators can be used to predict 10-year risk for microvascular and macrovascular complications. However, continued work is needed in this area to combine prediction models with disease-specific bio- markers and disease-modifying therapies that can prevent sequelae.
An additional noteworthy complication of type 1 diabetes is the patient-reported burden of adverse also their family, friends, and caregivers. 131 Fear of hypoglycaemia is a prevalent issue, particularly for the families of very young children with type 1 diabetes. 132 Furthermore, poor quality of life is predictive of subsequent poor glycaemic control. 133
Disease-modifying therapies
For over 30 years, most efforts to cure type 1 diabetes have focused on altering the immune system’s attack on β cells. This approach began with trials of ciclosporin, an immunosuppressant that was given to inhibit T-cell activation. Although ciclosporin was unable to induce a durable disease remission, insulin requirements of patients decreased during active treatment, generating enthusiasm that immune modulation could treat type 1 diabetes. 134 – 136 Subsequently, other strategies have been tested in both primary and secondary prevention paradigms. Most efforts have focused broadly on tolerance induction by use of antigens or modulation of T-lymphocyte, B-lymphocyte, and cytokine responses. Some primary prevention studies have also used dietary approaches. 137 , 138
Antigen-based trials have used various forms of glutamate decarboxylase (GAD) protein, which have shown mixed but mostly negative results. 139 – 141 The Diabetes Prevention Trial—Type 1, tested whether oral or parental insulin prevented the development of type 1 diabetes in people who were autoantibody positive. Neither approach reduced diabetes development, but subgroup analyses suggested a benefit of oral insulin in individuals with the highest titres of insulin auto-antibodies. 142 , 143 Based on this finding, the Type 1 Diabetes TrialNet Network completed a trial 144 of low-dose oral insulin in a second cohort of individuals who were autoantibody positive with similar insulin autoantibody profiles, but this trial was also negative. Negative results were also observed in another trial investigating intranasal insulin. 145
Personalised strategies for tolerance induction are now also being pursued. One study tested repeated intradermal doses of a specific proinsulin peptide fragment in people with the HLA DRB1*0401 genotype, 146 for whom this peptide was identified to be specifically immunogenic. Clinical trials at diagnosis have also tested approaches aimed at modulating T-cell and B-cell responses. Despite many attempts at immune intervention, only four categories of drugs have shown efficacy in preserving C-peptide secretion in recent onset type 1 diabetes in randomised placebocontrolled trials. These drugs include a monoclonal antibody against the B-cell CD20 receptor (rituximab), 147 monoclonal antibodies against the T-cell CD3 receptor (teplizumab 148 , 149 and otelixizumab 150 ), cytotoxic T-lymphocyte protein 4 (CTLA4)-immunoglobulin-mediated co-stimulatory blockade with abatacept, 151 and alefacept, 152 which is a fusion protein that binds CD2 and targets CD4+ and CD8+ effector memory T cells. Although the phase 2 trials of these drugs met their primary or secondary endpoints, defined as an improvement in the C-peptide area-under-the-curve response during a mixed meal tolerance test, no drug has yet been able to induce insulin independence or progressed to a positive phase 3 trial that was translatable into clinical care. This gap in translating results from trials into clinical practice could highlight the need for alternative strategies. Combinatorial approaches that modulate multiple aspects of the immune response could result in better efficacy. For example, low-dose anti-thymocyte globulin in combination with granulocyte colony-stimulating factor has shown early and sustained efficacy in pilot studies 153 , 154 and is being tested in a phase 2 study () in recent-onset type 1 diabetes. Another approach is to intervene earlier in the disease process, at a time when greater β-cell mass remains. To this end, abatacept () and teplizumab () are being tested in stage 1 and stage 2 type 1 diabetes through the TrialNet Network. Even modest preservation of β-cell function could have long-term benefits, and better glycaemic control early in the disease course could mitigate the likelihood of complications. 155 – 157
One potential future therapy for type 1 diabetes is with replacement of β cells from an external source. Pancreas transplants have been performed for over 50 years and have become a standard-of-care treatment in individuals who have developed end-stage renal failure and require kidney transplantation. 158 Simultaneous kidney and pancreas transplantation in experienced centres can offer an up to 80% chance of insulin independence for over 5 years, but there is substantial surgical risk, and the requirement of immunosuppression. 159 Islet transplantation is a low-risk procedure, with donor islets infused into the liver via the portal vein. Shapiro and colleagues’ landmark work, by use of a steroid free Edmonton Protocol, 160 showed that islet transplantation could achieve insulin independence and offered an example of a successful and low-risk cell-based therapy. However, only a minority of islet transplant recipients achieve durable insulin independence. Moreover, morbidity associated with immunosuppression and limitations in the supply of donor islets restricts the number of people who can benefit from islet transplantation. 161 Currently, islet transplantation is used in a small subset of patients who have extremely severe hypoglycaemic unawareness. Even if insulin independence is not achieved, severe life-threatening hypoglycaemia can be prevented with minimal islet transplant function. 162 , 163
Cell therapy as a potential cure for type 1 diabetes remains a field of great interest. 2 Considerable effort has been focused on protocols to generate functional and glucose-responsive β cells from human embryonic stem cells or induced pluripotent stem cells from living donors. This approach offers the possibility of a limitless source of β cells that could be delivered in a semipermeable device that would permit functional insulin secretion but avoid the need for immuno-suppression. 164 Several small molecules, growth factors, hormones, and nutrients have been shown to promote modest β-cell neogenesis and proliferation. However, most positive results come from animal models and have been difficult to replicate in human studies. While stem-cell-based therapies and neogenesis are a source of hope for potential cures, they are not realistic treatments in the immediate future. 2
Other novel approaches include autologous haemopoietic stem-cell transplantation 165 , 166 and autologous T-regulatory cell administration. 167 – 169 In response to growing evidence highlighting an active role for the β cell in disease pathogenesis, several ongoing trials are testing drugs that have successfully targeted β-cell stress responses in mouse models of diabetes. 170
Conclusions
Over the past 50 years, people with type 1 diabetes and their medical-care providers have been tantalised with optimism and subsequently disappointed at the seemingly unobtainable cure on the horizon. However, this long journey has been punctuated by several pivotal successes, including the discovery of insulin in 1922, the first pancreatic transplantation in 1966, 171 the first insulin-pump studies, the first immunomodulatory trial in 1986, 136 and the first definitive evidence linking glycaemic control with complication status in 1993. 81 The past 25 years has brought an upsurge of technological advances, including designer insulin analogues, smart insulin pumps, continuous glucose sensors, and closed-loop insulin delivery systems.
Clinicians, investigators, and patients have gained a better appreciation of the true complexity of type 1 diabetes, and humility in the face of many unsuccessful trials aimed at inducing a durable disease remission. While scientists continue to untangle the complicated pathogenesis of the disease, patients and health-care providers should focus on advocating for improved access to modern advances in diabetes care, especially for affordable insulin analogues and technologies that can reduce the burden of managing this chronic disease. When insulin was discovered, the University of Toronto freely licensed the right to manufacture the drug; yet, people in resource-limited environments continue to die because they have no access to insulin. 172
Additionally, crucial research must continue into strategies to prevent disease onset and preserve or restore β-cell function. These approaches offer the promise of ameliorating or eliminating disease complications, and greatly improving outcomes for those who have the disease. Continued development of new low-cost, low-burden, and highly effective therapies to improve glycaemic control is also needed. These approaches could include investigation into the effects of different dietary composition on glycaemic outcomes, and the safety and efficacy of open-source patient-designed artificial pancreas innovations. Given observed differences in care, health-care providers must be committed to initiatives for continuous quality improvement, with a focus on increasing uptake and implementation of best standards of care. A greater focus on patient-centred outcomes has been present in trials, and further exploration of these important endpoints is also crucial. If stakeholders in the field concentrate on the areas that are most likely to have a long-term effect, management of type 1 diabetes is poised to undergo further radical transformation.
Search strategy and selection criteria
We searched MEDLINE for publications in English published between Jan 1, 2014, and March 1, 2018, using the term “type 1 diabetes” and MEDLINE subheadings and selected papers on the basis of our opinion of their scientific importance. Research published since the 2014 Lancet Seminar on this topic was given particular attention. We provide an overview of type 1 diabetes focusing on updating the reader on recent advances and controversies.
Acknowledgments
This work was partly supported by grants from the National Institutes of Health, JDRF, the Veteran’s Administration, Diabetes UK, the Leona M and Harry B Helmsley Charitable Trust, the BIRAX Regenerative Medicine Initiative, the Ball Brothers Foundation, George and Francis Ball Foundation, Sigma Beta Sorority, Cryptic Masons Medical Research Foundation, and the Luke Weise Research Fund. We thank W Tamborlane, C Matthews, and J Kushner for their review of a draft of this Seminar. We thank M Campbell-Thompson, F Syed, and T Weinzerl for assistance with figures. And we thank T Lewallen and M Wales for administrative support.
Declaration of interests
LAD reports personal fees from Eli Lilly, and grants from Medtronic, Sanofi, Xeris, Caladrius, Dexcom, and Janssen outside the submitted work. RAO holds a UK Medical Research Council institutional Confidence in Concept grant to develop a 10 SNP biochip type 1 diabetes genetic test in collaboration with Randox. CE-M declares no competing interests.
Contributor Information
Linda A DiMeglio, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA;
Carmella Evans-Molina, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;
Richard A Oram, Institute of Biomedical and Clinical Science, University of Exeter Medical School, and The Academic Kidney Unit, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.

IMAGES
VIDEO
COMMENTS
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. ... Research 14 May 2024 European ...
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
The SEARCH for Diabetes in Youth study in the United States found a 1.4% per year increase in T1D incidence from 2002 to 2012, with an unexpected increase in Hispanic youths ( 14 ). This increase in the United States is similar to the gradual worldwide annual increase in T1D incidence over the past 30 years.
In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [ 72 ]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
240-672-8872. Consumer: 888-INFO-FDA. The FDA approved Lantidra, the first cellular therapy for the treatment of adults with type 1 diabetes who are unable to approach average blood glucose levels ...
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts ...
A new therapy for treating Type 1 diabetes. October 20, 2021. Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived ...
Identification of a new player in type 1 diabetes risk. ... Current ways of continuously monitoring glucose are dependent on the activity of an enzyme which can change over time, meaning the potential for inaccurate readings and need for frequent replacement or calibration. ... Determining the genetic risk for gestational diabetes. Research has ...
Management of type 1 diabetes mellitus in children involves close family support and glucose monitoring. ... Diabetes and the American Diabetes Association. 1,2 The current ... Research Group ...
Type 1 Research Highlights. While the Association's priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes. Read more about the critical research made possible by the ...
The purpose of this study is to evaluate glucose variability in patients with type 1 diabetes (T1D) and insulin antibodies, to evaluate the clinical significance of insulin antibodies, and to establish an in vitro assay that would detect antibodies to insulin and insulin analogs. Impact of Non-glucose Signals on Glycemic Control in Patients ...
Beena Akolkar, Ph.D. Clinical research in the prevention and immunopathogenesis of Type 1 Diabetes and the genetics and genomics of Type 1 and Type 2 Diabetes. Guillermo A. Arreaza-Rubín, M.D. Diabetes and endocrine disease bioengineering and glucose sensing. Miranda Broadney, M.D., M.P.H. Pediatrics, Pediatric Endocrinology, Clinical ...
New technologies in type 1 diabetes. Intensive insulin therapy for the management of type 1 diabetes (T1D) was established as the standard of care based on the results of the Diabetes Control and Complication Trial (DCCT), which conclusively demonstrated the benefits of tight glycemic control. 1 However, those who received intensive insulin ...
Type 1 diabetes results from autoimmune destruction of insulin-producing beta cells. There is a genetic predisposition to the disease, with the greatest contribution conferred by alleles present within the major histocompatibility complex (MHC; known as the human leukocyte antigen [HLA] region) on the short arm of chromosome 6.
Through the JDRF - Beyond Type 1 Alliance, Beyond Type 1 has partnered with JDRF—the world's biggest nonprofit funder of type 1 diabetes research —to educate our community on the important role research plays in the lives of everyone affected by type 1 diabetes (T1D).It was diabetes research that led to the discovery of insulin in 1921. It was research that led to the creation of the ...
There are about 1.5 million adults with Type 1 diabetes in the U.S., compared to 21 million adults with Type 2 diabetes. In terms of the total cases of diabetes, only 5 to 10 percent have Type 1 diabetes. Even in our largest epidemiologic cohorts, only a small percentage of people have Type 1 diabetes. So, we just don't have the same national ...
Type 1 Diabetes (T1D) is a chronic health condition (CHC) with a prevalence of 3.9 per 10 000 and incidence of 20 per 100,000 among adults in the United States (Mobasseri et al., 2020). ... Register to receive personalised research and resources by email. Sign me up. Taylor and Francis Group Facebook page. Taylor and Francis Group X Twitter page.
We randomly assigned patients with type 2 diabetes and chronic kidney disease (defined by an estimated glomerular filtration rate [eGFR] of 50 to 75 ml per minute per 1.73 m 2 of body-surface area ...
Diabetes mellitus is known for its hyperglycemia symptoms resulting from glucose intolerance caused by impaired insulin secretion and/or elevated insulin resistance. Accurate treatment for patients with diabetes mellitus is extremely important for preventing both acute and chronic diabetic complications. While type 2 and type 1 diabetes mellitus are most common, there is increasing recognition ...
1. Introduction. Diabetes mellitus (DM) is the most common group of metabolic disorders affecting the population in 2021. More than one in ten people, which is equivalent to 537 million people worldwide, suffers from DM, making it one of the biggest health problems in the world [].It encloses a group of chronic disorders that can be split into four major categories: type 1 diabetes mellitus ...
There are two major types of diabetes, called type 1 and type 2. In type 1 diabetes, formerly called insulin-dependent diabetes mellitus (IDDM), or juvenile-onset diabetes mellitus, the pancreas undergoes an autoimmune attack by the body itself and is rendered incapable of making insulin.Abnormal antibodies have been found in the majority of patients with type 1 diabetes.
Pancreatic islet transplantation is an experimental treatment for people with type 1 diabetes who have trouble controlling their blood glucose levels. Pancreatic islets are clusters of cells in the pancreas that make the hormone insulin. In type 1 diabetes, the body's immune system attacks these cells.
Diabetes (type 1 or type 2) Having either type 1 or type 2 diabetes can make you more likely to get very sick from COVID-19. Get more information: ... Current evidence suggests that children with medical complexity, with genetic, neurologic, or metabolic conditions, or with congenital heart disease can be at increased risk for getting very sick ...
This Seminar provides a review of type 1 diabetes and the status of research in the field. We focus on developments from the past 5 years that highlight the heterogeneity and complexity of the disease. ... Tonoli C, Heyman E, Roelands B, et al. Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J ...