Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 21 March 2024

Diagnosing glaucoma in primary eye care and the role of Artificial Intelligence applications for reducing the prevalence of undetected glaucoma in Australia
- Catherine Jan ORCID: orcid.org/0000-0001-7383-8208 1 , 2 , 3 ,
- Mingguang He 1 , 2 , 4 ,
- Algis Vingrys 1 , 2 , 5 ,
- Zhuoting Zhu ORCID: orcid.org/0000-0002-9897-1192 1 , 2 &
- Randall S. Stafford ORCID: orcid.org/0000-0003-1805-1271 6
Eye volume 38 , pages 2003–2013 ( 2024 ) Cite this article
2347 Accesses
1 Citations
3 Altmetric
Metrics details
- Epidemiology
- Physical examination
Glaucoma is the commonest cause of irreversible blindness worldwide, with over 70% of people affected remaining undiagnosed. Early detection is crucial for halting progressive visual impairment in glaucoma patients, as there is no cure available. This narrative review aims to: identify reasons for the significant under-diagnosis of glaucoma globally, particularly in Australia, elucidate the role of primary healthcare in glaucoma diagnosis using Australian healthcare as an example, and discuss how recent advances in artificial intelligence (AI) can be implemented to improve diagnostic outcomes. Glaucoma is a prevalent disease in ageing populations and can have improved visual outcomes through appropriate treatment, making it essential for general medical practice. In countries such as Australia, New Zealand, Canada, USA, and the UK, optometrists serve as the gatekeepers for primary eye care, and glaucoma detection often falls on their shoulders. However, there is significant variation in the capacity for glaucoma diagnosis among eye professionals. Automation with Artificial Intelligence (AI) analysis of optic nerve photos can help optometrists identify high-risk changes and mitigate the challenges of image interpretation rapidly and consistently. Despite its potential, there are significant barriers and challenges to address before AI can be deployed in primary healthcare settings, including external validation, high quality real-world implementation, protection of privacy and cybersecurity, and medico-legal implications. Overall, the incorporation of AI technology in primary healthcare has the potential to reduce the global prevalence of undiagnosed glaucoma cases by improving diagnostic accuracy and efficiency.
青光眼是全球最常见的不可逆失明性疾病, 超过70%的患者尚未确诊。目前尚无治愈方法。对于青光眼患者来说, 早期诊断对于阻止视力恶化至关重要, 本综述旨在: 找出全球范围内, 特别是澳大利亚普遍存在的青光眼患病率严重被低估的原因;以澳大利亚的医疗保健为例阐明初级医疗保健在青光眼诊断中的作用;讨论如何利用最新的人工智能 (AI) 技术来提高诊断的准确性。青光眼在人口老龄化的国家普遍存在, 通过适当的治疗可以改善视力结局, 因此对于医疗实践至关重要。在澳大利亚、新西兰、加拿大、美国和英国等国家, 验光师是初级眼科保健的守护者, 青光眼检测通常由他们负责。然而, 眼科专业人员在青光眼诊断能力上存在显著差异。利用AI对眼底彩色照片的视神经进行自动化识别技术可帮助验光师快速、高诊断效能地识别高风险变化, 并降低图像解读方面的挑战。尽管具有潜力, 但在将AI应用于初级保健场景之前, 还需要解决一些重要的屏障和挑战, 包括外部验证、高质量的实际应用、保护隐私和网络安全以及医疗法律问题。总之, 将AI纳入初级保健中将通过提高诊断准确性和效率来减少未确诊的青光眼病例的全球发病率。
Similar content being viewed by others
Evaluation of an offline, artificial intelligence system for referable glaucoma screening using a smartphone-based fundus camera: a prospective study
Care pathways for glaucoma detection and monitoring in the UK
Performance of artificial intelligence for the detection of pathological myopia from colour fundus images: a systematic review and meta-analysis
Introduction.
Glaucoma poses a significant public health challenge. Despite its impact, a large percentage of glaucoma cases remain undetected, especially in primary eye care settings [ 1 ]. The primary objective of our review article is to explore the reasons behind the underdiagnosis of glaucoma, particularly in Australia, and discuss how recent advances in Artificial Intelligence (AI) applications can be utilised to improve diagnostic outcomes.
The review article will delve into the global significance of glaucoma and undetected cases, highlighting the prevalence of underdiagnosis in different regions, including Australia. We will examine the challenges faced by primary healthcare providers in accurately diagnosing glaucoma, such as the complexity of the diagnostic process and the lack of specialised training and equipment. Furthermore, we will discuss the potential of AI applications in addressing these challenges and improving glaucoma detection rates.
Our article will provide a comprehensive overview of the clinical detection of glaucoma, focusing on the use of digital imaging technologies, such as monoscopic fundus photos and optical coherence tomography (OCT), as well as the importance of visual field testing. We will also highlight the importance and challenges of glaucoma care at the primary eye care level, emphasising the roles of general practitioners (GPs) and optometrists in glaucoma diagnosis and management.
The section dedicated to AI applications will explore the potential of AI algorithms in glaucoma detection. We will present the performance of AI algorithms using fundus photography, OCT, and visual fields, showcasing their accuracy in diagnosing glaucoma. Additionally, we will discuss the deployment of AI products in clinical practice, addressing potential risks and the need for validation studies and protocols to ensure the reliability and safety of AI-assisted diagnosis.
Global significance of glaucoma and undetected glaucoma
The global significance of glaucoma and its underdiagnosis is a significant public health issue due to its status as the leading cause of irreversible blindness worldwide [ 2 , 3 , 4 ]. Glaucoma is the second leading cause of blindness among people aged 55 and over in Australia [ 5 ]. Despite its impact, over 70% of people with glaucoma remain undiagnosed globally [ 1 ]: 78%−94% for Africa [ 6 ], 72%−84% for Asia [ 7 , 8 ], 57%−68% for Europe [ 9 ], 62%−78% for North America [ 10 ], 75%−88% for Latin America [ 11 ] and, 50%−60% for Australia and Oceania [ 7 , 12 , 13 , 14 ]. The estimated numbers of glaucoma cases worldwide in 2020 are 52.7 million detected versus 43.8 million undetected. These numbers will increase in 2040 to 79.8 million and 67.1 million, respectively [ 1 , 15 ].
The organisation of eye care services, skill levels of involved health care providers, and access to relevant health technologies contribute to glaucoma underdiagnosis. Organisational issues, particularly who provides eye care and who pays for it, varies globally. In Australia, the U.S., and many western European nations, routine eyecare is often fragmented between optometrists and primary healthcare physicians (GP), while ophthalmologists handle specialised patients and issues on referral from each. Inadequate diagnostic technology and provider training further exacerbate underdiagnosis. A new form of technology, Artificial Intelligence (AI), offers a potentially favourable transformation for glaucoma diagnosis. This promise, however, will depend on key financial, logistical, and organisational hurdles. The Australia example of diagnosing and managing glaucoma could be globally useful in addressing the significant public health issue of glaucoma underdiagnosis.
A non-systematic literature review was conducted based on articles published in peer-reviewed journals from the following databases: PubMed, oScopus, Medline-OVID, EMBASE, Cochrane Library, Web of Science and nongovernment organisation reports on 15 April 2023 using search terms to identify articles with no limitations on the publication year. The search combinations included “Glaucoma Care”, “Glaucoma Management”, “Glaucoma Diagnosis”, “Glaucoma Progression”, “Artificial Intelligence”, “Deep Learning”, “Machine Learning”, “Neural Networks”, “Bayesian Networks”, “Clinical Tools”, and “Primary Care”.
Overview of clinical detection of glaucoma
The hallmark of glaucoma is ganglion cell neurodegeneration in the optic nerve that is typically associated with functional loss. Consequently, diagnosis is largely based on optic nerve head (ONH) assessment and visual field (VF) testing, supplemented by several auxiliary tests and patient history (Table 1 ). Since the disorder is irreversible and has no available cure, early detection is vital for halting progressive visual impairment in glaucoma patients [ 16 ].
Detecting structural defects in glaucoma
Digital imaging technologies have become useful not only for documenting ONH and retinal nerve fibre layer (RNFL) changes (glaucomatous optic neuropathy or GON), but also for providing an objective, quantitative and convenient method to assist clinicians in glaucoma diagnosis.
Monoscopic fundus photos
Accurate and consistent detection of the optic disc and RNFL due to progressive retinal ganglion cell death is the key to glaucoma diagnosis [ 17 ]. Traditionally, mydriasis is recommended to obtain a stereoscopic view for optic disc assessment. But a recent study shows that monoscopic optic disc photography provides non-inferior diagnostic accuracy for the clinical evaluation of all optic disc characteristics and glaucoma likelihood [ 18 ]. Monoscopic disc images offer a fast and affordable method to aid in GON detection.
Optical coherence tomography (OCT)
OCT is a non-contact, optical imaging technique that uses low coherence interferometry to measure backscatter from different layers of the optic nerve head and retina [ 19 ]. In a few seconds, it captures structural information such as RNFL thickness, ganglion cell layer thickness, disc size, and minimum rim width [ 20 , 21 ] (the minimum rim width identifies the nerve fibre thickness passing out of the eye back to the brain). Pragmatically, longitudinal data are unlikely to be available to establish the initial glaucoma diagnosis. Therefore, structural information from a single observation (cross-sectional observation) could be considered as standard for diagnosis [ 20 ].
Detecting functional impairment in GON
Visual field testing.
Visual sensitivity progressively declines in glaucoma. Standard automated perimetry (SAP) over the central 24°−30° of the visual field is the current recommended procedure for visual field testing in glaucoma [ 19 , 22 ]. It measures retinal sensitivity to increments of light at many locations across the visual field. The 24−2 grid (central 24 indicates that the central 24 degrees of visual field and the next number indicates how the grid of points is aligned to the visual axis) is the preferred test strategy for this purpose, although the 30−2 is sometimes used to rule out other neurological conditions [ 23 ]. Repetition of visual field testing at baseline using the same threshold strategy is essential to establish a baseline so that the earliest presence of glaucomatous functional defect can be identified, and to accommodate for learning effects in performing the visual field test [ 24 ]. Visual field testing is recommended more than twice a year [ 25 ], with a minimum of three-times per year suggested to overcome test variability and identify progression in newly diagnosed glaucoma patients [ 26 ]. This frequency of testing will obviously challenge health care systems. Recent work indicates that a combination of structural and functional measures (VF and OCT) gives the greatest sensitivity for diagnosis and identifying progression in glaucoma [ 27 ].
Importance and challenges of glaucoma care at primary levels in Australia
Challenges faced by primary healthcare providers in glaucoma diagnosis.
Glaucoma diagnosis is a complex process that requires targeted evaluation by eye care professionals. While primary healthcare providers play an important role in glaucoma screening, they face numerous challenges in accurate diagnosis. The diagnostic process includes history taking, assessment of the ONH and RNFL, measurement of intraocular pressure (IOP), evaluation of the anterior angle and anterior chamber by slit lamp to exclude angle closure or secondary causes of glaucoma, and VF evaluation. Although these assessments are essential for definitive glaucoma diagnosis, not all are required for screening to identify presumptive glaucoma (Box 1 ).
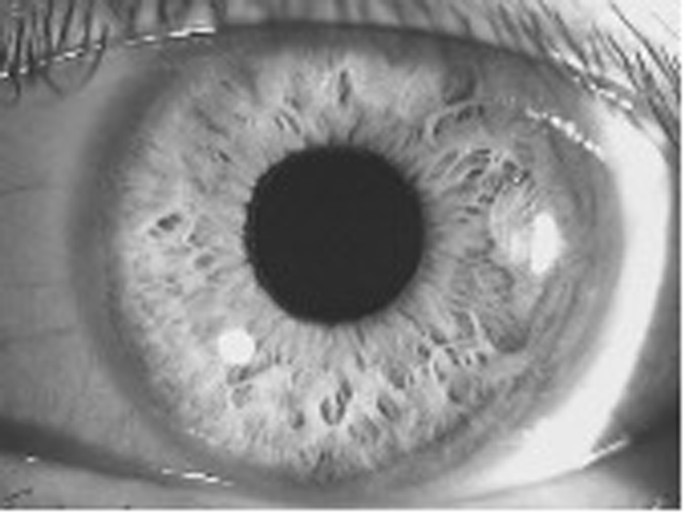
The flashlight is located at the zygomatic arc on the right of the image.
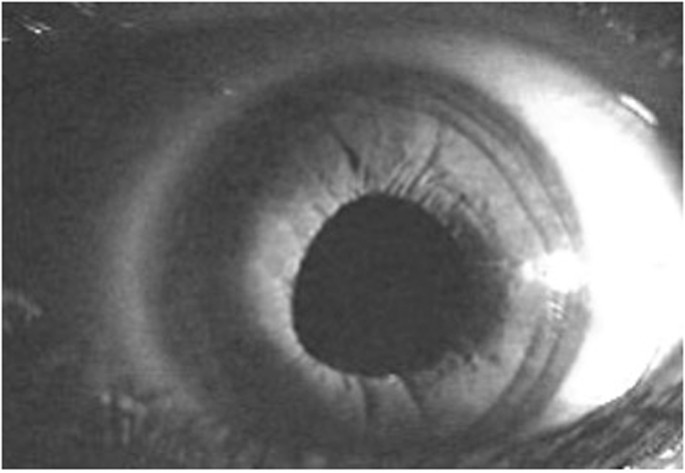
This indicates a prominent and forward placed iris protruding into the anterior chamber, causing angle closure. The flashlight is located at the zygomatic arc on the right of the image.
A study conducted in Victoria, Australia, found that out of 4744 individuals, 72 cases of referable glaucoma were identified, of whom 35 (49%) were undiagnosed [ 1 ]. The primary cause of misclassification was the lack of VF screening, as 97% of their 35 missed cases failed a VF screening test during a subsequent date. The study also found that 66% of the missed cases had an enlarged cup-to-disc ratio, which was not identified during their previous eyecare visits [ 1 ]. Perhaps this finding is not unexpected as cup-disc evaluation using ophthalmoscopy is challenging, and GPs are not versed in performing this procedure, so they may not identify abnormality from normal variations. However, in the case of this study, most participants who had enlarged cup-disc ratios had seen an optometrist or an ophthalmologist in the past year, and their physical state did not provide adequate evidence for further evaluation. This implies that cup-disc evaluation is a complex and challenging undertaking even for ophthalmic practitioners and would likely lead to high false positive or false negative outcomes if undertaken by GPs.
The implementation of AI has the potential to alleviate some of the challenges faced by primary healthcare providers in glaucoma diagnosis Box 2 . AI technology can aid in the interpretation of images of the ONH, RNFL, and VF, providing a more accurate and consistent assessment. However, before AI can be widely adopted in primary healthcare settings, several issues must be resolved first, as we will discuss later in this article.
A case of primary eye care in glaucoma diagnosis in Australia
Australia’s healthcare system is founded on the principles of universal health care and a robust public insurance programme, ensuring that all citizens and permanent residents and New Zealand citizens have access to free and high-quality medical services [ 28 ]. This fundamental aspect has played a pivotal role in the successful implementation of national cancer screening programmes for bowel, cervical and breast cancer [ 29 ]. The achievements of these screening programmes underscore the capability of Australia’s healthcare system in effectively executing universal screening initiatives. Considering the accomplishments in cancer screening, there is a strong foundation for the feasibility of developing and executing a universal glaucoma screening programme. The existing infrastructure, along with the commitment to preventive healthcare, positions Australia favourably to address glaucoma detection and management comprehensively. By leveraging its healthcare system’s strengths and incorporating the latest advancements in medical technology, Australia has the potential to enhance the early diagnosis and treatment of glaucoma, ultimately safeguarding the vision and overall health of its population.
In Australia there are theoretically two models of primary care for glaucoma detection—by GPs and by optometrists. In practice, however, GPs are not trained to diagnose glaucoma and do not have access to testing equipment, therefore the burden of glaucoma diagnosis falls to optometrists. However, optometrists may face challenges in performing all the required procedures for glaucoma diagnosis (Table 1 ), especially when under time pressures, as experienced by all primary health care practitioners.
Starting in 2009, topical glaucoma medications prescribed by optometrists became available at subsidised prices (Pharmaceutical Benefits Scheme, PBS) [ 30 ]. In 2020, there were 6043 registered optometrists in Australia, with 65% having prescribing endorsement [ 31 ]. Despite being given increasing independence in prescribing [ 32 ], optometrists have not widely embraced this role. In 2015, latanoprost was the drug most prescribed by optometrists under PBS. However, while about 40% of these optometry prescriptions are for glaucoma management, they only accounted for 1.3% of all glaucoma prescriptions [ 32 ]. There were around 1000 full-time equivalent (FTE) ophthalmologists and 4800 FTE optometrists employed in Australia in 2019, or 4 FTE ophthalmologists and 19 FTE optometrists per 100,000 population [ 25 ]. Thus, optometrists were not highly active in managing glaucoma with most cases referred to ophthalmology.
Current glaucoma care guidelines for optometry
A patient who presents to an optometrist with glaucoma risk factors during a routine eye examination is recommended to undergo further clinical investigations to determine if glaucoma is present. For this purpose, the Optometry Board of Australia guidelines [ 33 ] recommend that practising optometrists have certain equipment to form a proper evaluation and differential diagnosis (Table 1 ). If this equipment is not available, or if testing cannot be undertaken to satisfy the requirements of Table 1 , then a referral should be made to another optometrist or ophthalmologist for specialised testing and interpretation of the test results.
The glaucoma assessment needs to be made by all optometrists, whether endorsed for the use of scheduled medicines (therapeutically endorsed) or not. If an optometrist is therapeutically endorsed, they are authorised to diagnose and initiate glaucoma treatment independently. Once the diagnosis is established and the treatment started, patients are to be seen by an ophthalmologist within 4 months [ 33 ]. This referral reflects that surgical intervention is sometimes a viable first-choice treatment option and should be considered in all cases [ 34 , 35 ].
Although this surgical related review produces a best-case option, delays in seeing an ophthalmologist can be substantial due to high caseloads [ 31 ]. A patient must be referred by a primary care provider (optometrist or GP) to gain access to specialist eye care in a public hospital in Australia and to be eligible for a rebate under the national health insurance act (Medicare Benefits Schedule) [ 36 ]. A 2020 study found that 72% of glaucoma referrals to the public hospital system came from optometrists [ 37 ]. However, the study also found that the median wait-time for patients to be seen by an ophthalmologist at the hospital can be as long as 400 days [ 37 ]. This delay in treatment can lead to irreversible sight loss, which can be avoided if optometrists/GPs prescribe IOP reducing drugs on diagnosis, given that most optometrists are therapeutically qualified. It will also act to minimise the possibility of vein occlusion associated with lengthy exposure to elevated IOP. Patients can suffer harm and sight loss due to 22 week delay in achieving hospital attendance [ 38 ], which supports an argument for therapeutic intervention on diagnosis to reduce the potential for sight loss prior to hospital attendance.
Box 1 List of clinical procedures recommended for glaucoma screening in primary healthcare settings [ 43 ], where a slit lamp is unavailable
Family history, history on risk factors of glaucoma
Shadow test using flashlight or ophthalmoscope at temple (Figs. 1 , 2 )
Optic Nerve Head assessment in terms of cup-disc ratio
Visual Field screening
Age-specific reviews (40+) doing these tests for all patients for age 40 and above, and repeat every five years up to 60, and biannually after that age.
Box 2 A glossary of key terms
Artificial intelligence (AI) . The study of agents that receive precepts from the environment and perform actions. It is concerned with both understanding and building intelligent entities—machines that can compute how to act effectively and safely in a wide variety of novel situations [ 94 ].
Machine learning (ML) . A subfield of artificial intelligence that is concerned with the study of machines that use algorithms to identify patterns in data [ 95 ].
Supervised ML . A type of machine learning task that aims at predicting the desired output (such as separating “glaucomatous” from “non-glaucomatous”). It involves the use of data labelled by clinical experts to train machines and develop statistical models.
Unsupervised ML . A type of machine learning task that aims at inferring underlying patterns in unlabelled data.
Deep learning (DL) . A subfield of machine learning that employs artificial neural networks with many layers to identify patterns in data [ 96 ].
Why are so many people with glaucoma not diagnosed?
The cause of the high prevalence of undetected glaucoma is multifactorial [ 39 , 40 , 41 ]. Firstly, as above, glaucoma diagnosis requires the consideration of complex diagnostic tests, many of which are not available to GPs and some optometry settings. Additionally, glaucoma (except for acute angle closure glaucoma) is hard to diagnose as patients have few or no symptoms of the disease [ 4 ]. Visual symptoms are rare in early and middle stages of glaucoma, thus glaucoma is coined “the silent thief of vision”[ 1 ].
Secondly, while universal screening for glaucoma is not cost-effective in Western countries [ 42 ], detection of glaucoma relies on primary care providers, namely optometrists and GPs. However, GPs rarely test patients for glaucoma due to a lack of training and specialised equipment [ 43 ]. Fortunately in Australia, we have universal health coverage for routine comprehensive eye examinations in optometry settings, and all optometry students are trained in glaucoma care. However, variation exists in ONH assessment, which could have contributed to the high rate of undetected glaucoma reported in Australia [ 44 ].
A population study [ 1 ] in Victoria found that undiagnosed glaucoma was as high as 63% (Fig. 3 ). Of the undiagnosed cases, 66% had seen an optometrist in the past year, 97% did not have a visual field test performed, and 66% had Cup-to-Disc (CDR) ratios consistent with glaucoma (>0.7) but were not recorded as such. Of note, out of those undiagnosed cases with CDR > 0.7, 65% had seen an optometrist and 48% had seen an ophthalmologist in the past year [ 1 ]. This indicates that glaucomatous disc changes are challenging to detect, which may explain the significant missed diagnosis. The prevalence of undiagnosed disease is even higher in minority populations [ 45 , 46 ].
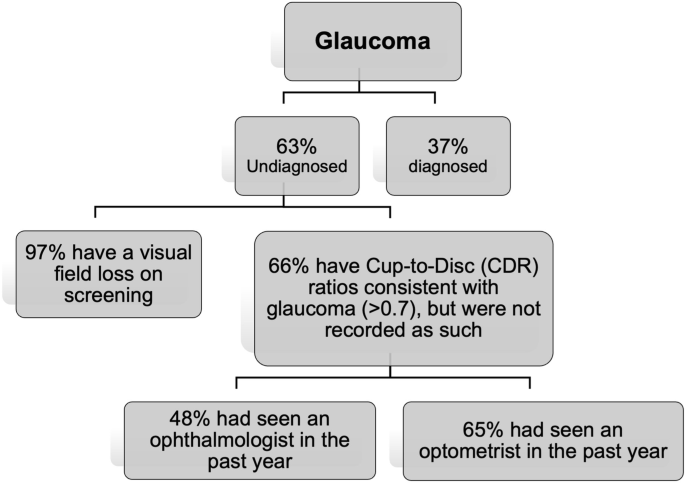
Undiagnosed glaucoma in Victoria, Australia (calculated on data from Wong et al. [ 1 ]).
Standardising the detection of GON based on structural and functional changes can help bridge the gap in glaucoma detection. AI can potentially play a useful role in this regard by providing consistent imaging interpretation and identifying high risk cases for closer consideration by the optometrist.
Potential of AI (artificial intelligence) in glaucoma diagnosis in primary eye care settings
Context and definition.
When Deep Blue (a chess-playing computer developed by IBM [ 47 ]) defeated Garry Kasparov (the youngest world chess champion in history) in 1997, the defenders of human supremacy moved humanity’s battleground to Go (an abstract strategy board game for two players in which the aim is to surround more territory than the opponent. The number of legal board positions in Go has been calculated to be approximately 2.1 × 10 170 [ 48 ], which is far greater than the number of atoms in the observable universe, estimated to be of the order of 10 80 ) [ 49 ]. Piet Hut, an astrophysicist and Go enthusiast, predicted that it would take “a hundred years before a computer beats humans at Go—maybe even longer.” But in just under 20 years since Deep Blue vs. Kasparov, a computer programme developed by neuroscientist and chess prodigy Demis Hassabis and his DeepMind team surpassed all human players at Go [ 50 ].
As in other domains, AI is rapidly changing the healthcare landscape. Recent advancements in AI have introduced promising opportunities for efficient and cost-effective glaucoma detection programmes. Many studies have shown that AI algorithms now equal or exceed expert diagnostic accuracy for many conditions, particularly when the diagnosis is based on image interpretation, such as in dermatology and radiology [ 51 , 52 , 53 , 54 , 55 , 56 , 57 ]. Eye care is the frontrunner of the AI revolution in health care because diagnosing eye conditions heavily depends on imaging. In 2018, IDx-DR (Digital Diagnostics), designed to detect diabetic retinopathy and diabetic macular oedema, became the first FDA-approved autonomous AI device in any field of medicine [ 58 ]. The integration of AI into the glaucoma diagnostic process has the potential to significantly reduce costs and resource burdens and provides a potential to yield more accurate diagnosis.
Overview for performance of AI algorithm in glaucoma detection
Ai using fundus photography.
AI can help address the issue of variation in the assessment of ONH and RNFL changes. Segmentation and structured learning from various studies achieved an accuracy between 94% and 98% [ 59 , 60 ] in reaching a correct diagnosis from fundus photos in glaucoma. Various deep learning algorithms based on fundus features such as the cup-to-disc ratio achieved area under receiver operating curve (AROC) between 0.53 and 0.996 in differentiating healthy from glaucomatous eyes [ 61 , 62 , 63 , 64 ], with a sensitivity ranging from 96% to 100% [ 61 , 65 ], and specificity of 98% [ 64 , 65 ]. Recently, Machine Learning (ML) algorithms developed from the interrogation of 50,000 fundus photos achieved an area under curve (AUC) of 0.986 with 95.6% sensitivity and 92% specificity for identifying referable GON [ 66 ]. AI-based tools can provide standardised and objective assessments, leading to more accurate and consistent diagnoses.
AI using OCT
The RNFL thickness is one common parameter utilised for glaucoma diagnosis [ 67 ] and became a key focus in ML using OCT images. Since 2005, studies have reported the performance of ML algorithms analysing OCT imaging data from peripapillary RNFL thickness maps and the macular ganglion cell complex for detecting GON, with AROC values ranging from 0.69 to 0.99 [ 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 ]. A recent study showed that Deep Learning (DL) network achieved an AROC of 0.94 for detecting GON using unsegmented OCT volumes of the optic nerve head [ 76 ]. Given the cost of OCT this application of AI is likely best retained for specialist or optometry practice where ocular OCT can be applied for many other purposes.
AI using visual fields
AI algorithms to diagnose glaucoma using datasets derived from VF testing have been studied since 1994 [ 77 , 78 , 79 , 80 , 81 ]. Notably, DL algorithms to diagnose glaucoma with data from standard automated perimetry (SAP) with Humphrey VF 24-2 and 30-2 SITA standard VF test outperformed the diagnostic accuracy of glaucoma experts in differentiating normal from glaucomatous VFs, with a sensitivity of 93% and specificity of 83% [ 82 ]. Furthermore, algorithms trained using a combination of OCT images and SAP VF results reached an AROC of 0.98 for identifying patients with glaucoma [ 83 ]. The cost of many dedicated testing devices is prohibitive for general application but the recent advent of a cheap screening option (the Melbourne Rapid Fields app [ 84 ]) makes this suited for such purposes. Figure 4 illustrates examples for such screening options and where the interpretation of results is given in terms of a “probability of abnormality score” (coloured bar) to assist the clinician’s decision making process and identify high risk cases.
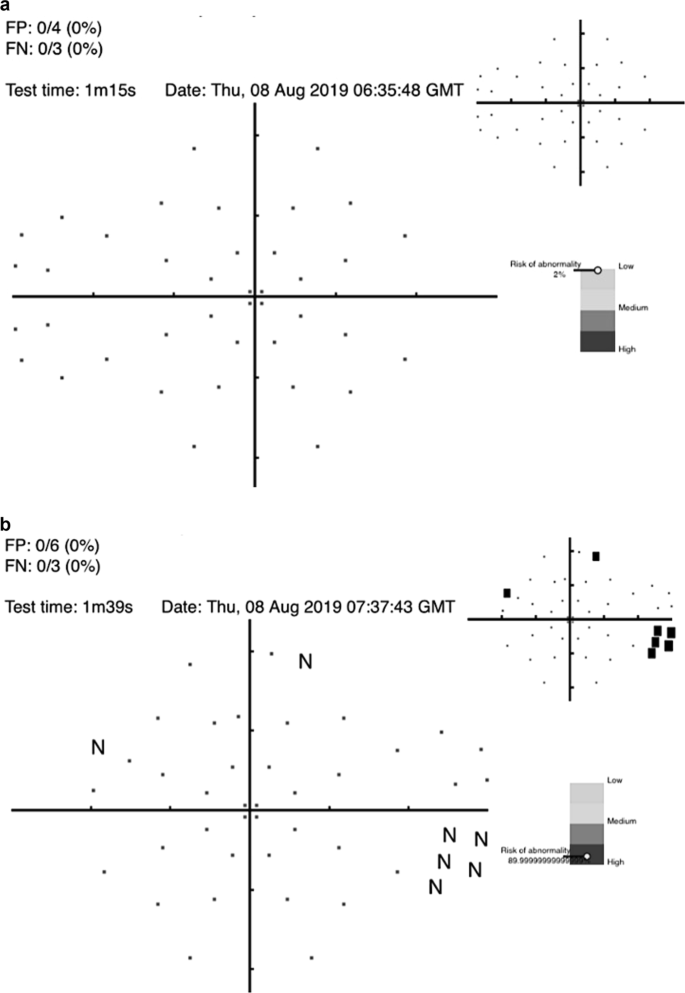
a visual field screening result using the Melbourne Rapid Fields app [ 84 ] for a 57 year old male showing the risk for glaucoma is low. b visual field screening result using the Melbourne Rapid Fields app [ 84 ] for a 68 year old female showing the risk for glaucoma is high. The data were collected using Melbourne Rapid Fields app and required about 1−1.5 min for testing (from Chia et al. [ 93 ]).
Multi-modal AI models
Research has also utilised multimodal structural data to enhance the assessment of glaucomatous structural damage from optic disc photographs for segmentation and detection [ 85 , 86 ]. A recent multimodal model was developed using the Xception model for image feature extraction and various ML algorithms such as random forest (RF), support vector machine (SVM), dense neural network (DNN), and others showed impressive area under the receiver operating characteristic curve (AUROC) values for the different algorithms: RF had an AUROC of 99.56%, SVM had 99.59%, and DNN had 99.10% while analysing the vertical cup-to-disc ratio and mean RNFL thickness in the detection of glaucoma in a population with high incidence of myopia [ 87 ]. Another recent study showed that FusionNet based on bimodal input of VF and OCT paired data demonstrated superior performance to algorithms based on VF or OCT alone [ 88 ].
Incorporation of AI products in Australian primary care
Recent advancements in imaging technologies (such as OCT and retinal photos) allow primary care clinicians and eye specialists to identify the structural damage caused by glaucoma [ 89 ]. However, these advances come with a high cost of equipment and significant time required to interpret the image or results. Moreover, it is becoming increasingly challenging for busy primary eyecare clinics to undertake such imaging without incurring substantial costs.
Furthermore, there is substantial variation in the visual interpretation of ONH features among clinicians [ 90 ]. This variation can be present between different clinicians (inter-observer variation) and between assessments made at different times by the same clinician (intra-observer inter-session variation). A recent study [ 90 ] involving 197 ophthalmic clinicians from 22 countries showed substantial under diagnosis based on optic nerve head photos from patients with known glaucoma. Ophthalmology trainees (22%) and comprehensive ophthalmologists (24%) consistently underestimated the likelihood of glaucoma. This level of underestimation contributes to undetected cases of glaucoma, supporting the need for AI-assisted clinical evaluation.
Automation by AI offers an opportunity to mitigate the time and cost challenges of image analysis and reduce diagnostic variation compared to human clinicians (Fig. 5 ). Figure 5 shows the outcome of an optic nerve image processed with AI that was consistently graded as having glaucoma by AI but returned variable diagnoses between 5 expert clinicians.
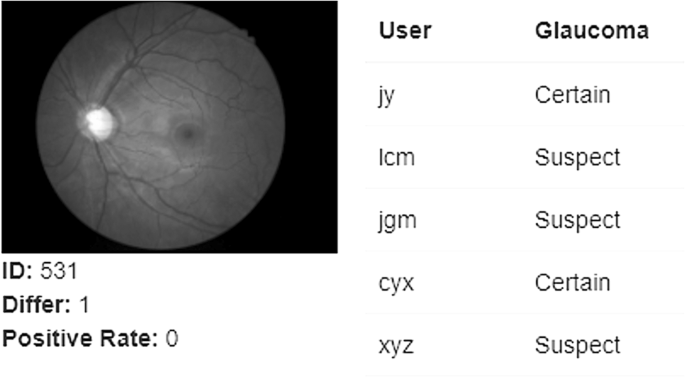
Each user represents one glaucoma specialist (internal data from Prof. Mingguang He, obtained in March, 2022).
Figure 6a, b propose two potential models where AI can be incorporated into primary care settings for glaucoma detection. The assortment of tests was developed based on currently available guidelines for glaucoma detection in primary care settings [ 43 , 91 ] and adopting easy to use and interpret methods for diagnosis. More research is required to investigate the best AI-assisted model for the detection of glaucoma in primary care settings.
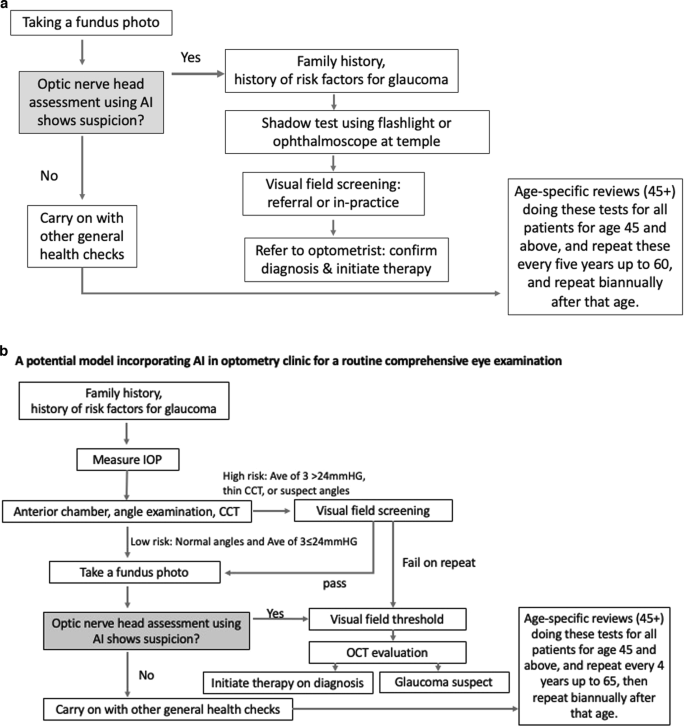
a A potential model incorporating AI in GP clinics for a routine health examination which achieves rapid therapeutic intervention (unpublished protocols). b A potential model incorporating AI in optometry clinics for a routine comprehensive eye examination (unpublished protocols).
Managing potential risks of AI use in glaucoma diagnosis
Significant progress in ML and imaging technologies enable AI to identify glaucoma signs. However, despite rapid progress in AI for glaucoma detection under laboratory settings, its real-world application has not been fully realised. The lack of a unified “gold standard” for glaucoma diagnosis is perhaps one of the biggest challenges that AI faces, as different AI algorithms focus on different aspects of the disease. For instance, the AI that focuses on evaluating the optic nerve head only evaluates structural information alone. A potential area of improvement is to develop algorithms with a more ‘holistic’ evaluation of structural and functional information, as well as information on other glaucoma risk factors such as family history and age.
Furthermore, there is a lack of prospective clinical trials applying AI in real world settings. For example, in the technologies for breast cancer screening with mammography, these issues have been particularly problematic [ 55 ]. Our team is actively trialling several AI models in optometry and GP settings and hope to publish relevant findings on the efficiency (such as accuracy, speed and acceptability of glaucoma screening) and cost-effectiveness of AI incorporation in screening programmes. In addition, real world AI implementation is impeded by challenges such as image quality, legal risks, and regulatory issues, which have barely been systematically summarised. Poor image quality can yield a high amount of ungradable images, which can lead to false positives and high health care cost. Furthermore, there have been concerns that increased reliance on AI may lead to deskilling of ophthalmic clinicians, and around privacy and cyber security as the large amount of personal information contained in the AI systems can increase the risk of data leakage. A systematic review on the patient privacy perspective on health information exchange [ 92 ] has found many patients express concerns about their health data privacy. Evidence-based protocols should be in place to safeguard algorithms and datasets against attacks. The introduction of AI-based technology may increase the cost of care, and if these costs are borne by individual patients, people of lower social economic status may remain undiagnosed. The best way for these tests to become widely available is for them to be included in health screening programmes promoted by national or private insurance companies. Our ongoing research, currently under review, pertains to cost-effectiveness analyses of AI-assisted glaucoma screening models within the context of Australia. The findings from our study indicate that the integration of AI assistance into population-based glaucoma screening programmes is cost-effective compared to traditional screening by optometrists (unpublished data, C. Jan 2023). These results suggest the economic feasibility of policy makers considering the adoption of universal glaucoma screening initiatives throughout Australia. However, it is imperative to note that the precise modalities and logistics for implementing such a programme remain to be defined. Furthermore, the medico-legal ramifications associated with placing reliance on technology for diagnostic purposes are expected to gain increasing significance and should be addressed in the context of healthcare policy and practice.
External validation studies are required to ensure the validity of deep learning algorithms and to better understand the mechanism underlying the technology or “thought process” of AI. Unlike human clinicians, current AI programmes are unable to take a holistic approach to patient care or consider other external contributing factors (such as social and psychological aspects) to management. Clinical trials are required to compare care models from practices with and without AI in real world primary care settings. AI is a tool that assists, not replaces, human clinicians.
AI-development beyond academia
Our review focuses on the prevalence of undiagnosed glaucoma in Australia and the crucial role played by primary healthcare providers in glaucoma care within the Australian healthcare system. While our primary emphasis is not on industrial advancements, it is important to acknowledge the growing prominence of AI-enabled glaucoma screening outside the academic sphere, necessitating an exploration of the latest industry developments. For instance, as of January 2023, Eyenuk (US) has achieved the noteworthy accomplishment of securing the first European Union MDR Certification for autonomous AI detection of glaucomatous optic nerve damage utilising coloured fundus photographs. Furthermore, Eyetelligence (Australia), Digital Diagnostics (US), RetinaLyze (Denmark), and Ophthalmic Sciences (Israel) are actively engaged in the development of AI-based products aimed at facilitating glaucoma screening through the analysis of fundus photographs.
This review highlights the significant challenge of glaucoma underdiagnosis, which can be attributed to variations in optic nerve head assessment, under-performing VF testing, and time constraints, consistent with challenges faced by primary healthcare practitioners in general in Australia. AI has shown the potential to mitigate these problems by carrying out glaucoma assessment (at least partially) in a consistent manner. The emergence of AI technology offers a promising solution to these challenges by enabling a more consistent and objective diagnosis of glaucoma. This is in contrast to the current situation, which is often characterised by inconsistent work-up and interpretation. AI algorithms have demonstrated high accuracy in diagnosing glaucoma and can provide a rapid diagnosis, thereby reducing the risk of misdiagnosis and enabling earlier treatment. Integrating AI into the diagnostic process for glaucoma has the potential to revolutionise the field and improve patient outcomes.
In primary eye care settings, advanced imaging technologies such as fundus photography and OCT, automated visual field testing, electronic health records, and large digital datasets are becoming increasingly available. These technologies can facilitate translational AI research to improve the evidence-based and consistent identification of glaucoma. As optometrists and GPs remain the first point of contact for patients with eye problems in Australia and other countries, the integration of AI into primary eye care settings has the potential to significantly improve glaucoma diagnosis and management.
Data availability
As this is a literature review, no original raw data was used.
Wong EY, Keeffe JE, Rait JL, Vu HT, Le A, McCarty PhD C, et al. Detection of undiagnosed glaucoma by eye health professionals. Ophthalmology. 2004;111:1508–14.
Article PubMed Google Scholar
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull world health Organ. 2004;82:844–51.
PubMed PubMed Central Google Scholar
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8.
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV. et al.Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis.Lancet Glob Health. 2017;5:e1221–34.
The Department of Health. Australia Government. Visual impairment and blindness in Australia, Dec 2008. https://www.aihw.gov.au/getmedia/fc608984-1c92-48d0-b9fc-1ced9acec3ee/bulletin27.pdf.aspx?inline=true#:~:text=The%20leading%20causes%20of%20blindness,%25)%20and%20cataract%20(12%25) . (accessed March 2023).
Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology. 2003;110:376–82.
Soh Z, Yu M, Betzler BK, Majithia S, Thakur S, Tham YC, et al. The global extent of undetected glaucoma in adults: a systematic review and meta-analysis. Ophthalmology. 2021;128:1393–404.
Chua J, Baskaran M, Ong PG, Zheng Y, Wong TY, Aung T, et al. Prevalence, risk factors, and visual features of undiagnosed glaucoma: the Singapore epidemiology of eye diseases study. JAMA Ophthalmol. 2015;133:938–46.
Topouzis F, Coleman AL, Harris A, Koskosas A, Founti P, Gong G, et al. Factors associated with undiagnosed open-angle glaucoma: the Thessaloniki eye study. Am J Ophthalmol. 2008;145:327–35.e1.
Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158:1121–9.e1.
Sakata K, Sakata LM, Sakata VM, Santini C, Hopker LM, Bernardes R, et al. Prevalence of glaucoma in a South brazilian population: projeto glaucoma. Investig Ophthalmol Vis Sci. 2007;48:4974–9.
Article Google Scholar
Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne visual impairment project. Ophthalmology. 1998;105:733–9.
Article CAS PubMed Google Scholar
Foreman J, Xie J, Keel S, Ang GS, Lee PY, Bourne R, et al. Prevalence and causes of unilateral vision impairment and unilateral blindness in Australia: the National Eye Health Survey. JAMA Ophthalmol. 2018;136:240–8.
Article PubMed PubMed Central Google Scholar
Keel S, Xie J, Foreman J, Lee PY, Alwan M, Fahy ET, et al. Prevalence of glaucoma in the Australian national eye health survey. Br J Ophthalmol. 2019;103:191–5.
O'neill EC, Danesh-Meyer HV, Kong GX, Hewitt AW, Coote MA, Mackey DA, et al. Optic disc evaluation in optic neuropathies: the optic disc assessment project. Ophthalmology. 2011;118:964–70.
Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. Bmj. 2005;331:134.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311:1901–11.
Chan HH, Ong DN, Kong YX, O'Neill EC, Pandav SS, Coote MA, et al. Glaucomatous optic neuropathy evaluation (GONE) project: the effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am J Ophthalmol. 2014;157:936–44.
Weinreb RN, Leung CKS, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, et al. Primary open-angle glaucoma. Nat Rev Dis Prim Engl. 2016;2:16067.
Iyer J, Vianna JR, Chauhan BC, Quigley HA. Toward a new definition of glaucomatous optic neuropathy for clinical research. Curr Opin Ophthalmol. 2020;31:85–90.
Chauhan BC, O'leary N, AlMobarak FA, Reis A, Yang H, Sharpe GP, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120:535–43.
Jampel HD, Singh K, Lin SC, Chen TC, Francis BA, Hodapp E, et al. Assessment of visual function in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:986–1002.
Khoury JM, Donahue SP, Lavin PJ, Tsai JC. Comparison of 24-2 and 30-2 perimetry in glaucomatous and nonglaucomatous optic neuropathies. J Neuroophthalmol. 1999;19:100–8.
Yenice O, Temel A. Evaluation of two Humphrey perimetry programs: full threshold and SITA standard testing strategy for learning effect. Eur J Ophthalmol. 2005;15:209–12.
Chauhan BC, Garway-Heath DF, Goñi FJ, Rossetti L, Bengtsson B, Viswanathan AC, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–73.
Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Investig Ophthalmol Vis Sci. 2012;53:2770–6.
Garway-Heath DF, Quartilho A, Prah P, Crabb DP, Cheng Q, Zhu H. Evaluation of visual field and imaging outcomes for glaucoma clinical trials (an American Ophthalomological Society Thesis). Trans Am Ophthalmol Soc. 2017;115:115.
Google Scholar
The Commonwealth Fund. International Profiles of Health Care Systems. Available at https://www.commonwealthfund.org/international-health-policy-center/system-profiles . Accessed 20 Jul 2023.
Australian Institute of Health and Welfare (2023) Cancer screening programs: quarterly data, AIHW, Australian Government, https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation/contents/summary (Accessed 20 Jul 2023).
Optometry Australia. http://archived.optometry.org.au/blog-news/2018/8/24/medicare-a-highlight-in-100-years-of-milestones/ Accessed 27 Jan 2022.
Optometry Board of Australia. https://www.optometry.org.au/workforce/australian-optometrists-top-6000-and-two-thirds-are-therapeutically-endorsed/ . Accessed 4 Feb 2022.
Optometry Australia. A decade on the PBS and nearly 100,000 scripts a year. https://www.optometry.org.au/therapeutics/a-decade-on-the-pbs-and-nearly-100000-scripts-a-year/ . Accessed 25 Jul 2023
Optometry Board of Australia. Optometry Guidelines for Use of Scheduled Medicines. 2018. https://nla.gov.au/nla.obj-2996351198/view (accessed 13 March 2023).
Evidence reviews for selective laser trabeculoplasty in ocular hypertension or chronic open-angle glaucoma adult patients: Glaucoma: diagnosis and management: Evidence review A. London: National Institute for Health and Care Excellence (NICE); 2022.
Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393:1505–16.
Australian Government Department of Health. MBS Online: Medicare Benefits Schedule. 2020. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-202001 . Accessed 26 Apr 2022.
Ford BK, Kim D, Keay L, White AJ. Glaucoma referrals from primary care and subsequent hospital management in an urban Australian hospital. Clin Exp Optom. 2020;103:821–9.
Foot B, MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye. 2017;31:771–5.
Article CAS PubMed PubMed Central Google Scholar
Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12:e11686.
Kirkman JM, Bentley SA, Armitage JA, Woods CA. Could adoption of the rural pipeline concept redress Australian optometry workforce issues? Clin Exp Optom. 2019;102:566–70.
Keeffe JE, Weih LM, McCarty CA, Taylor HR. Utilisation of eye care services by urban and rural Australians. Br J Ophthalmol. 2002;86:24–7.
Burr J, Mowatt G, Hernández R, Siddiqui M, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–190.
US Preventive Services Task F, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, et al. Screening for primary open-angle glaucoma: US preventive services task force recommendation statement. JAMA. 2022;327:1992–7.
Toomey M, Ho KC, Gyawali R, Stapleton F, Wiles L, Hibbert P, et al. The appropriateness of and barriers to glaucoma care delivery by Australian optometrists. Clin Exp Optom. 2022;105:1–9.
Elam AR, Andrews C, Musch DC, Lee PP, Stein JD. Large disparities in receipt of glaucoma care between enrollees in Medicaid and those with commercial health insurance. Ophthalmology. 2017;124:1442–8.
Stein JD, Talwar N, LaVerne AM, Nan B, Lichter PR. Racial disparities in the use of ancillary testing to evaluate individuals with open-angle glaucoma. Arch Ophthalmol. 2012;130:1579–88.
Deep Blue https://www.ibm.com/ibm/history/ibm100/us/en/icons/deepblue/ . Accessed 20 Sept 2023.
Tromp J, Farnebäck G. Combinatorics of go. International Conference on Computers and Games. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 84–99.
Lee KF. AI superpowers: China, Silicon Valley, and the new world order. Houghton Mifflin; 2018.
Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of Go without human knowledge. Nature. 2017;550:354–9.
Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE-an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–4.
Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197:11–7.
Wolf JA, Moreau JF, Akilov O, Patton T, English JC, Ho J, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol. 2013;149:422–6.
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig Ophthalmol Vis Sci. 2016;57:5200–6.
Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. Jama. 2017;318:2199–210.
Manak MS, Varsanik JS, Hogan BJ, Whitfield MJ, Su WR, Joshi N, et al. Live-cell phenotypic-biomarker microfluidic assay for the risk stratification of cancer patients via machine learning. Nat Biomed Eng. 2018;2:761–72.
Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284:574–82.
FDA permits marketing of AI software that autonomously detects diabetic retinopathy. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye (Accessed 10 July 2023).
Fan Z, Rong Y, Cai X, Lu J, Li W, Lin H, et al. Optic disk detection in fundus image based on structured learning. IEEE J Biomed Health Inf. 2018;22:224–34.
Mookiah MR, Acharya UR, Chua CK, Min LC, Ng EY, Mushrif MM, et al. Automated detection of optic disk in retinal fundus images using intuitionistic fuzzy histon segmentation. Proc Inst Mech Eng H J Eng Med. 2013;227:37–49.
Liu H, Li L, Wormstone IM, Qiao C, Zhang C, Liu P, et al. Development and validation of a deep learning system to detect glaucomatous optic neuropathy using fundus photographs. JAMA Ophthalmol. 2019;137:1353–60.
Medeiros FA, Jammal AA, Thompson AC. From machine to machine: an OCT-trained deep learning algorithm for objective quantification of glaucomatous damage in fundus photographs. Ophthalmology. 2019;126:513–21.
Thompson AC, Jammal AA, Medeiros FA. A deep learning algorithm to quantify neuroretinal rim loss from optic disc photographs. Am J Ophthalmol. 2019;201:9–18.
Jammal AA, Thompson AC, Mariottoni EB, Berchuck SI, Urata CN, Estrela T, et al. Human versus machine: comparing a deep learning algorithm to human gradings for detecting glaucoma on fundus photographs. Am J Ophthalmol. 2020;211:123–31.
Raghavendra U, Fujita H, Bhandary SV, Gudigar A, Tan JH, Acharya UR. Deep convolution neural network for accurate diagnosis of glaucoma using digital fundus images. Inf Sci. 2018;441:41–9.
Li Z, He Y, Keel S, Meng W, Chang RT, He M. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology. 2018;125:1199–206.
Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15–9.
Huang ML, Chen HY. Development and comparison of automated classifiers for glaucoma diagnosis using Stratus optical coherence tomography. Investig Ophthalmol Vis Sci. 2005;46:4121–9.
Naithani P, Sihota R, Sony P, Dada T, Gupta V, Kondal D, et al. Evaluation of optical coherence tomography and heidelberg retinal tomography parameters in detecting early and moderate glaucoma. Investig Ophthalmol Vis Sci. 2007;48:3138–45.
Barella KA, Costa VP, Gonçalves Vidotti V, Silva FR, Dias M, Gomi ES. Glaucoma diagnostic accuracy of machine learning classifiers using retinal nerve fiber layer and optic nerve data from SD-OCT. J Ophthalmol. 2013;2013:789129.
Bizios D, Heijl A, Hougaard JL, Bengtsson B. Machine learning classifiers for glaucoma diagnosis based on classification of retinal nerve fibre layer thickness parameters measured by Stratus OCT. Acta Ophthalmol. 2010;88:44–52.
Larrosa JM, Polo V, Ferreras A, García-Martín E, Calvo P, Pablo LE. Neural network analysis of different segmentation strategies of nerve fiber layer assessment for glaucoma diagnosis. J Glaucoma. 2015;24:672–8.
Muhammad H, Fuchs TJ, De Cuir N, De Moraes CG, Blumberg DM, Liebmann JM, et al. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J Glaucoma. 2017;26:1086–94.
Xu J, Ishikawa H, Wollstein G, Bilonick RA, Folio LS, Nadler Z, et al. Three-dimensional spectral-domain optical coherence tomography data analysis for glaucoma detection. PLoS One. 2013;8:e55476.
Burgansky-Eliash Z, Wollstein G, Chu T, Ramsey JD, Glymour C, Noecker RJ, et al. Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest Ophthalmol Vis Sci. 2005;46:4147–52.
Maetschke S, Antony B, Ishikawa H, Wollstein G, Schuman J, Garnavi R. A feature agnostic approach for glaucoma detection in OCT volumes. PLoS One. 2019;14:e0219126.
Andersson S, Heijl A, Bizios D, Bengtsson B. Comparison of clinicians and an artificial neural network regarding accuracy and certainty in performance of visual field assessment for the diagnosis of glaucoma. Acta Ophthalmol. 2013;91:413–7.
Asaoka R, Murata H, Iwase A, Araie M. Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology. 2016;123:1974–80.
Bowd C, Weinreb RN, Balasubramanian M, Lee I, Jang G, Yousefi S, et al. Glaucomatous patterns in Frequency Doubling Technology (FDT) perimetry data identified by unsupervised machine learning classifiers. PLoS One. 2014;9:e85941.
Cai S, Elze T, Bex PJ, Wiggs JL, Pasquale LR, Shen LQ. Clinical correlates of computationally derived visual field defect archetypes in patients from a glaucoma clinic. Curr Eye Res. 2017;42:568–74.
Goldbaum MH, Sample PA, White H, Côlt B, Raphaelian P, Fechtner RD, et al. Interpretation of automated perimetry for glaucoma by neural network. Invest Ophthalmol Vis Sci. 1994;35:3362–73.
CAS PubMed Google Scholar
Li F, Wang Z, Qu G, Song D, Yuan Y, Xu Y, et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med Imaging. 2018;18:35.
Bizios D, Heijl A, Bengtsson B. Integration and fusion of standard automated perimetry and optical coherence tomography data for improved automated glaucoma diagnostics. BMC Ophthalmol. 2011;11:20.
What is MRF Visual Field Test? https://www.appviewmrf.com/what-is-mrf-visual-field-test/ . Accessed 20 Sept 2023.
Wu M, Leng T, de Sisternes L, Rubin DL, Chen Q. Automated segmentation of optic disc in SD-OCT images and cup-to-disc ratios quantification by patch searching-based neural canal opening detection. Opt Express. 2015;23:31216–29.
Babu T, Devi S, Venkatesh R. Optic nerve head segmentation using fundus images and optical coherence tomography images for glaucoma detection. Biomed Pap. 2015;159:607–15.
Lim WS, Ho H-Y, Ho H-C, Chen YW, Lee CK, Chen PJ, et al. Use of multimodal dataset in AI for detecting glaucoma based on fundus photographs assessed with OCT: focus group study on high prevalence of myopia. BMC Med Imaging. 2022;22:1–14.
Xiong J, Li F, Song D, Tang G, He J, Gao K, et al. Multimodal machine learning using visual fields and peripapillary circular OCT scans in detection of glaucomatous optic neuropathy. Ophthalmology. 2022;129:171–80.
Dick HB, Schultz T, Gerste RD. Miniaturization in glaucoma monitoring and treatment: a review of new technologies that require a minimal surgical approach. Ophthalmol Ther. 2019;8:19–30.
O'neill EC, Gurria LU, Pandav SS, Kong YX, Brennan JF, Xie J, et al. Glaucomatous optic neuropathy evaluation project: factors associated with underestimation of glaucoma likelihood. JAMA Ophthalmol. 2014;132:560–6.
Clinical Practice Guide for the Diagnosis and Management of Open Angle Glaucoma 2020. https://www.optometry.org.au/wp-content/uploads/Professional_support/Guidelines/Glaucoma-Clinical-Practice-Guide_Dec-2020_design_v6.pdf (Accessed 13 March 2023).
Shen N, Bernier T, Sequeira L, Strauss J, Silver MP, Carter-Langford A, et al. Understanding the patient privacy perspective on health information exchange: a systematic review. Int J Med Inform. 2019;125:1–12.
Chia MA, Trang E, Agar A, Vingrys AJ, Hepschke J, Kong GY, et al. Screening for glaucomatous visual field defects in rural Australia with an iPad. J Curr Glaucoma Pract. 2021;15:125–31.
Russell, SJ & Norvig, P. A rtificial Intelligence: A Modern Approach (Prentice Hall, New Jersey, 2021).
Murphy, KP & Bach F Machine Learning: A Probabilistic Perspective (MIT Press, Cambridge, 2012).
Goodfellow, I, Bengio, Y, Courville, A & Bengio, Y Deep Learning (MIT Press, Cambridge, 2016).
Download references
Acknowledgements
This project received grant funding from the Australian Government: the National Critical Research Infrastructure Initiative, Medical Research Future Fund (MRFAI00035) and the NHMRC Investigator Grant (APP1175405). The contents of the published material are solely the responsibility of the Administering Institution, a participating institution or individual authors and do not reflect the views of the NHMRC. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. CJ is supported by Research Training Scholarship from the Australian Commonwealth Government. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and affiliations.
Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, VIC, Australia
Catherine Jan, Mingguang He, Algis Vingrys & Zhuoting Zhu
Ophthalmology, Department of Surgery, Faculty of Medicine, Dentistry & Health Sciences, University of Melbourne, Melbourne, VIC, Australia
Lost Child’s Vision Project, Sydney, NSW, Australia
Catherine Jan
Centre for Eye and Vision Research, The Hong Kong Polytechnic University, Kowloon, TU428, Hong Kong SAR
Mingguang He
Department of Optometry and Vision Sciences, The University of Melbourne, Melbourne, VIC, Australia
Algis Vingrys
Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA
Randall S. Stafford
You can also search for this author in PubMed Google Scholar
Contributions
CJ was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, and updating reference lists. MH was responsible for interpreting results and providing critical review of the report. AV was responsible for designing the review protocol, interpreting results, providing additional references, and providing critical review of the report. ZZ was responsible for interpreting results and providing critical review of the report. RSS was responsible for interpreting results and providing critical review of the report.
Corresponding author
Correspondence to Catherine Jan .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jan, C., He, M., Vingrys, A. et al. Diagnosing glaucoma in primary eye care and the role of Artificial Intelligence applications for reducing the prevalence of undetected glaucoma in Australia. Eye 38 , 2003–2013 (2024). https://doi.org/10.1038/s41433-024-03026-z
Download citation
Received : 25 July 2023
Revised : 05 February 2024
Accepted : 08 March 2024
Published : 21 March 2024
Issue Date : August 2024
DOI : https://doi.org/10.1038/s41433-024-03026-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Optimized automated detection of diabetic retinopathy severity: integrating improved multithresholding tunicate swarm algorithm and improved hybrid butterfly optimization.
- Usharani Bhimavarapu
Health Information Science and Systems (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection

Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Updates on the Diagnosis and Management of Glaucoma
Affiliation.
- 1 Department of Ophthalmology, Mayo Clinic School of Medicine, Jacksonville, FL.
- PMID: 36405987
- PMCID: PMC9673042
- DOI: 10.1016/j.mayocpiqo.2022.09.007
Glaucoma is the leading cause of blindness throughout the world (after cataracts); therefore, general physicians should be familiar with the diagnosis and management of affected patients. Glaucomas are usually categorized by the anatomy of the anterior chamber angle (open vs narrow/closed), rapidity of onset (acute vs chronic), and major etiology (primary vs secondary). Most glaucomas are primary (ie, without a contributing comorbidity); however, several coexisting ophthalmic conditions may serve as the underlying etiologies of secondary glaucomas. Chronic glaucoma occurs most commonly; thus, regular eye examinations should be performed in at-risk patients to prevent the insidious loss of vision that can develop before diagnosis. Glaucoma damages the optic nerve and retinal nerve fiber layer, leading to peripheral and central visual field defects. Elevated intraocular pressure (IOP), a crucial determinant of disease progression, remains the only modifiable risk factor; thus, all current treatments (medications, lasers, and operations) aim to reduce the IOP. Pharmacotherapy is the usual first-line therapy, but noncompliance, undesirable adverse effects, and cost limit effectiveness. Laser and surgical treatments may lower IOP significantly over long periods and may be more cost effective than pharmacotherapy, but they are plagued by greater procedural risks and frequent treatment failures. Traditional incisional procedures have recently been replaced by several novel, minimally invasive glaucoma surgeries with improved safety profiles and only minimal decreases in efficacy. Minimally invasive glaucoma surgeries have dramatically transformed the surgical management of glaucoma; nevertheless, large, randomized trials are required to assess their long-term efficacy.
Keywords: ACA, anterior chamber angle; ACG, angle-closure glaucoma; AIT, ab-interno trabeculotomy; CAI, carbonic anhydrase inhibitor; CE, cataract extraction; GDD, glaucoma drainage device; IOP, intraocular pressure; KDB, Kahook Dual Blade; MIGS, minimally invasive glaucoma surgery; MMC, mitomycin C; OAG, open-angle glaucoma; OCT, optical coherence tomography; ONH, optic nerve head; PGA, prostaglandin analog; PGI, PAUL glaucoma implant; POAG, primary open-angle glaucoma; RNFL, retinal nerve fiber layer; SLT, selective laser trabeculoplasty; TM, trabecular meshwork.
© 2022 The Authors.
PubMed Disclaimer
Ultrasound biomicroscopy (UBM) of the…
Ultrasound biomicroscopy (UBM) of the anterior eye segment. A, UBM shows the ciliary…
Comparison of optic nerve head…
Comparison of optic nerve head (ONH), retinal nerve fiber layer (RNFL), and visual…
Similar articles
- Minimally Invasive Glaucoma Surgery. Gurnani B, Tripathy K. Gurnani B, et al. 2023 Aug 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. 2023 Aug 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 35881761 Free Books & Documents.
- Ab interno trabecular bypass surgery with Trabectome for open-angle glaucoma. Hu K, Shah A, Virgili G, Bunce C, Gazzard G. Hu K, et al. Cochrane Database Syst Rev. 2021 Feb 4;2(2):CD011693. doi: 10.1002/14651858.CD011693.pub3. Cochrane Database Syst Rev. 2021. PMID: 33580495 Free PMC article.
- Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, Ambler G, Bunce C, Wormald R, Nathwani N, Barton K, Rubin G, Morris S, Buszewicz M. Gazzard G, et al. Health Technol Assess. 2019 Jun;23(31):1-102. doi: 10.3310/hta23310. Health Technol Assess. 2019. PMID: 31264958 Free PMC article. Clinical Trial.
- Prostaglandin Analogues for Ophthalmic Use: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines [Internet]. Islam S, Spry C. Islam S, et al. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2020 Feb 18. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2020 Feb 18. PMID: 33074623 Free Books & Documents. Review.
- Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Babizhayev MA. Babizhayev MA. Fundam Clin Pharmacol. 2012 Feb;26(1):86-117. doi: 10.1111/j.1472-8206.2011.00969.x. Epub 2011 Aug 24. Fundam Clin Pharmacol. 2012. PMID: 21883446 Review.
- Clinical Outcomes of Excisional Goniotomy with the Kahook Dual Blade: 6-Year Results. Vasu P, Abubaker Y, Boopathiraj N, Wagner IV, Lentz PC, Dorairaj E, Shokair A, Qozat I, Miller DD, Dorairaj S. Vasu P, et al. Ophthalmol Ther. 2024 Aug 16. doi: 10.1007/s40123-024-01016-8. Online ahead of print. Ophthalmol Ther. 2024. PMID: 39150602
- Wound Modulations in Glaucoma Surgery: A Systematic Review. Dave B, Patel M, Suresh S, Ginjupalli M, Surya A, Albdour M, Kooner KS. Dave B, et al. Bioengineering (Basel). 2024 Apr 30;11(5):446. doi: 10.3390/bioengineering11050446. Bioengineering (Basel). 2024. PMID: 38790314 Free PMC article. Review.
- Validation of Diagnostic Codes to Identify Glaucoma in Taiwan's Claims Data: A Multi-Institutional Study. Lu PT, Tsai TH, Lai CC, Chuang LH, Shao SC. Lu PT, et al. Clin Epidemiol. 2024 Apr 3;16:227-234. doi: 10.2147/CLEP.S443872. eCollection 2024. Clin Epidemiol. 2024. PMID: 38586480 Free PMC article.
- Molecular pathways in experimental glaucoma models. Bugara K, Pacwa A, Smedowski A. Bugara K, et al. Front Neurosci. 2024 Mar 18;18:1363170. doi: 10.3389/fnins.2024.1363170. eCollection 2024. Front Neurosci. 2024. PMID: 38562304 Free PMC article. Review.
- Prevalence and Characteristics of Glaucoma Among Patients Presenting to Ophthalmology Clinics in a Tertiary Hospital in the Kingdom of Bahrain. Husain KA, Alaali H, Alarayedh GG. Husain KA, et al. Cureus. 2024 Feb 13;16(2):e54129. doi: 10.7759/cureus.54129. eCollection 2024 Feb. Cureus. 2024. PMID: 38487113 Free PMC article.
- Kang J.M., Tanna A.P. Glaucoma. Med Clin North Am. 2021;105(3):493–510. - PubMed
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. - PubMed
- Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. - PMC - PubMed
- Hollands H., Johnson D., Hollands S., Simel D.L., Jinapriya D., Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 2013;309(19):2035–2042. - PubMed
- Stein J.D., Khawaja A.P., Weizer J.S. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA. 2021;325(2):164–174. - PubMed
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Glaucoma in Adults—Screening, Diagnosis, and Management : A Review
- 1 W.K. Kellogg Eye Center, Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor
- 2 Center for Eye Policy and Innovation, University of Michigan, Ann Arbor
- 3 Department of Health Management and Policy, University of Michigan School of Public Health, Ann Arbor
- 4 NIHR Biomedical Research Centre, Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, United Kingdom
- The Rational Clinical Examination Do Findings on Routine Examination Identify Patients at Risk for Primary Open-Angle Glaucoma? The Rational Clinical Examination Systematic Review Hussein Hollands, MD, MSc (Epid); Davin Johnson, MD; Simon Hollands, BSc; David L. Simel, MD, MHS; Delan Jinapriya, MD, FRCSC; Sanjay Sharma, MD, MBA, MSc JAMA
- From The Medical Letter on Drugs and Therapeutics Glaucoma—Topical Drug Summary JAMA
- JAMA Patient Page Patient Information: Glaucoma Rebecca Voelker, MSJ JAMA
Importance Glaucoma is the most common cause of irreversible blindness worldwide. Many patients with glaucoma are asymptomatic early in the disease course. Primary care clinicians should know which patients to refer to an eye care professional for a complete eye examination to check for signs of glaucoma and to determine what systemic conditions or medications can increase a patient’s risk of glaucoma. Open-angle and narrow-angle forms of glaucoma are reviewed, including a description of the pathophysiology, risk factors, screening, disease monitoring, and treatment options.
Observations Glaucoma is a chronic progressive optic neuropathy, characterized by damage to the optic nerve and retinal nerve fiber layer, that can lead to permanent loss of peripheral or central vision. Intraocular pressure is the only known modifiable risk factor. Other important risk factors include older age, nonwhite race, and a family history of glaucoma. Several systemic medical conditions and medications including corticosteroids, anticholinergics, certain antidepressants, and topiramate may predispose patients to glaucoma. There are 2 broad categories of glaucoma, open-angle and angle-closure glaucoma. Diagnostic testing to assess for glaucoma and to monitor for disease progression includes measurement of intraocular pressure, perimetry, and optical coherence tomography. Treatment of glaucoma involves lowering intraocular pressure. This can be achieved with various classes of glaucoma medications as well as laser and incisional surgical procedures.
Conclusions and Relevance Vision loss from glaucoma can be minimized by recognizing systemic conditions and medications that increase a patient’s risk of glaucoma and referring high-risk patients for a complete ophthalmologic examination. Clinicians should ensure that patients remain adherent with taking glaucoma medications and should monitor for adverse events from medical or surgical interventions used to treat glaucoma.
Read More About
Stein JD , Khawaja AP , Weizer JS. Glaucoma in Adults—Screening, Diagnosis, and Management : A Review . JAMA. 2021;325(2):164–174. doi:10.1001/jama.2020.21899
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Most Popular Articles : Journal of Glaucoma
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Most Popular Articles
Treatment outcomes comparing the paul and baerveldt glaucoma implants after one year of follow-up.
Journal of Glaucoma. 33(8):594-600, August 2024.
- Abstract Abstract
- Permissions
Go to Full Text of this Article
Glaucoma and the Human Microbiome
Journal of Glaucoma. 33(8):529-538, August 2024.
Understanding Patterns of Preserved Retinal Ganglion Cell Layer in Advanced Glaucoma as Seen With Optical Coherence Tomography
Journal of Glaucoma. 33(8):539-548, August 2024.
180- Versus 360-Degree Selective Laser Trabeculoplasty in Open Angle Glaucoma and Ocular Hypertension: A Systematic Review and Meta-Analysis
Journal of Glaucoma. 33(8):566-575, August 2024.
The Effect of Phacoemulsification on the Intraocular Pressure of Patients With Open Angle Glaucoma: A Systematic Review and Meta-Analysis
Journal of Glaucoma. 33(8):576-586, August 2024.
A Prospective Comparison of 180 Versus 360-Degree Gonioscopy-Assisted Transluminal Trabeculotomy Outcomes in Pseudoexfoliation Glaucoma
Journal of Glaucoma. 33(8):559-565, August 2024.
Glaucoma Screening Guidelines Worldwide
Journal of Glaucoma. 33(8S):S9-S12, August 2024.
Anatomy of the Visual Pathways
Journal of Glaucoma. 22:S2-S7, June/July 2013.
Targeted Screening Strategies to Improve Glaucoma Outcomes
Journal of Glaucoma. 33(8S):S1, August 2024.
Oral Ibuprofen is Associated With Reduced Likelihood of Early Bleb Failure After Trabeculectomy in High-Risk Glaucoma Patients
Journal of Glaucoma. 32(4):237-244, April 2023.
Screening of Glaucoma: Consensus and Directions
Journal of Glaucoma. 33(8):S75-S77, August 2024.
Three-Year Outcomes of the Paul Glaucoma Implant for Treatment of Glaucoma
Journal of Glaucoma. 33(7):478-485, July 2024.
Biology of the Extracellular Matrix: An Overview
Journal of Glaucoma. 23:S20-S23, October/November 2014.
Optical Coherence Tomography Versus Optic Disc Photo Assessment in Glaucoma Screening
Journal of Glaucoma. 33(8S):S21-S25, August 2024.
Drug-Induced Liver Injury During a Glaucoma Neuroprotection Clinical Trial
Journal of Glaucoma. 33(8):e58-e59, August 2024.
Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy in Eyes With Prior Failed Glaucoma Surgery
Journal of Glaucoma. 33(8):612-617, August 2024.
Comparison of Different Intraocular Lens Power Calculation Formulas in Eyes With Primary Angle Closure
Journal of Glaucoma. 33(9):665-670, September 2024.
Technology and Methodology in Glaucoma Case Detection
Journal of Glaucoma. 33(8S):S13-S14, August 2024.
Impact of Peripheral Anterior Synechiae on the Outcome of Combined Phacoemulsification, Goniosynechialysis, and Goniotomy for Primary Angle Closure Glaucoma and Cataract: A Multicenter Observational Study
Journal of Glaucoma. 33(8):587-593, August 2024.
The “Pocket” Technique: A Novel Surgical Technique for Repair of Glaucoma Drainage Device Tube Exposure
Journal of Glaucoma. 33(8):e60-e63, August 2024.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Jamie Dietze ; Kyle Blair ; Marco Zeppieri ; Shane J. Havens .
Affiliations
Last Update: March 16, 2024 .
- Continuing Education Activity
Glaucoma is a complex eye condition characterized by elevated intraocular pressure (IOP) that may progress to vision loss over time. This eye condition is categorized into primary or secondary types and further into open-angle or closed-angle variants. Adult glaucoma includes primary open-angle glaucoma (POAG) and angle-closure glaucoma, as well as secondary open and angle-closure glaucoma, with a specific focus on the most prevalent type, POAG. Researchers are investigating genetic and environmental factors contributing to glaucoma development.
Although the available treatments cannot cure existing optic nerve damage or reverse visual field loss, they can help control the disease progression through medication, laser treatment, or incisional glaucoma surgeries to help prevent further vision loss. These treatments aim to lower IOP and mitigate the impact of this vision-threatening condition through an interprofessional healthcare team for optimal treatment outcomes. This activity reviews various forms of glaucoma, including congenital, infantile, developmental, and juvenile variants, emphasizing cases primarily affecting individuals aged 40 and older. This activity also highlights the collaborative approach involving an interprofessional healthcare team for glaucoma management due to its impact on vision. Early detection and regular monitoring are crucial for effectively managing glaucoma—the second leading cause of permanent blindness among older adults in the United States.
- Identify the early signs and risk factors associated with glaucoma, including elevated intraocular pressure, optic nerve changes, and visual field defects.
- Screen patients at risk for glaucoma during routine eye examinations, including those with a family history, advanced age, diabetes, and other relevant medical conditions.
- Apply imaging modalities such as optical coherence tomography and visual field testing in glaucoma diagnosis, monitoring, and management.
- Collaborate with interprofessional healthcare providers to provide comprehensive glaucoma care, ensure timely interventions, optimize outcomes, and prevent irreversible vision loss in patients with glaucoma.
- Introduction
Glaucoma is a complex eye condition characterized by elevated intraocular pressure (IOP) that may progress to vision loss over time. Glaucoma is the second leading cause of permanent blindness in the United States and occurs most often in older adults. [1] Glaucoma can be categorized into either primary or secondary types and further into open-angle or closed-angle variants within each type of glaucoma. Adult glaucoma includes primary open-angle glaucoma (POAG) and angle-closure glaucoma, as well as secondary open and angle-closure glaucoma, [2] [3] with a specific focus on the most prevalent type, POAG. [4] [5]
Glaucoma is an acquired loss of retinal ganglion cells and axons within the optic nerve or optic neuropathy that results in a characteristic optic nerve head appearance and a corresponding progressive loss of vision. [6] This unique pattern of peripheral vision loss serves as a distinguishing feature from other types of vision impairment. [7]
Patients with POAG are often asymptomatic until significant optic nerve damage occurs unless early signs of glaucoma are identified during routine eye examinations. [8] On the other hand, acute angle-closure glaucoma can develop suddenly and lead to a rapid decline in vision, accompanied by symptoms such as corneal edema, eye pain, headache, nausea, and emesis. [9] [10] Secondary glaucoma often arises due to a previous eye injury or underlying medical conditions, resulting in elevated IOP and subsequent optic neuropathy. This category encompasses various subtypes, including congenital, pigmentary, neovascular, exfoliative, traumatic, and uveitic glaucoma. [11] Normal or low-tension type of glaucoma presents as an optic neuropathy with glaucomatous visual loss despite normal or unremarkable IOP readings. [12]
Although congenital, infantile, and developmental glaucoma, along with a juvenile variant of POAG, primarily affect younger individuals, most types of glaucoma are commonly diagnosed in individuals aged 40 and older. While IOP is often associated with glaucoma, a direct causal relationship has not been definitively established. Researchers are investigating genetic and environmental factors contributing to glaucoma development. Evidence from studies involving monozygotic twin pairs, who exhibit a higher concordance rate compared to dizygotic pairs, suggests that environmental factors also have a significant role in the disease's development. [13]
Although the available treatments cannot cure existing optic nerve damage or reverse visual field loss, they can help control the disease progression through medication, laser treatment, or incisional glaucoma surgeries to prevent further vision loss. All therapeutic interventions are focused on lowering IOP and minimizing the impact of this vision-threatening condition. This approach aims to prevent the onset of glaucoma in patients with risk factors and to manage the condition effectively to limit its progression in affected individuals.
The exact etiology of glaucoma is unknown, but a clear correlation with elevated eye pressure exists in most cases. High IOP is the primary risk factor for developing glaucoma and for the progression of the disease, and it is also the sole factor that current treatments can effectively address. [14]
Primary Open-Angle Glaucoma
POAG typically manifests as slow, painless damage to the optic nerve due to an ineffective drainage system in the eye. In glaucoma, the resistance to drainage of aqueous humor most commonly starts at the inner wall of Schlemm canal at the juxtacanalicular trabecular meshwork. This decreased outflow facility or increased resistance to aqueous outflow results in a gradual rise in IOP, leading to characteristic damage patterns in the visual field and the optic nerve ganglion cell nerve fiber layer. [15] Recent studies have highlighted that elevated IOP can also reduce blood flow to the optic nerve fibers, resulting in subtle ischemic damage (see Image. Glaucomatous Optic Nerve Head Showing Inferotemporal Retinal Nerve Fiber Layer Defect). [16]
POAG patients often have elevated IOP readings correlating with characteristic optic nerve damage and visual field defect patterns (see Image. Glaucoma Visual Field Changes in the Left Eye). As the disease progresses, a slow loss of peripheral vision in one or typically both eyes eventually leads to loss of central vision. Because of this loss pattern, affected persons do not notice a change in their vision until their loss is advanced and affects the central vision, in which case damage is permanent and irreversible. [7]
Glaucoma can manifest at different ages, with the age of onset often characterizing its presentation. Although POAG is typically associated with adulthood, it can also affect younger individuals and children, suggesting a significant genetic component. Primary congenital glaucoma is diagnosed in newborns aged up to 1 month, often suspected when there is eye enlargement at birth. [17] Infantile glaucoma affects individuals between the ages of 1 and 36 months, [18] while juvenile glaucoma is used to indicate individuals diagnosed with glaucoma between the ages of 3 and 40. [19] Juvenile open-angle glaucoma shares similarities with POAG in terms of IOP leading to optic nerve damage, but it occurs in a younger age group with higher IOP levels and potentially more severe visual field defects. [20]
Low-Tension or Normal-Tension Glaucoma
This type of glaucoma resembles POAG in terms of characteristic optic disc cupping and peripheral visual-field loss findings. [16] However, what sets it apart is that IOP readings are consistently normal, typically measuring less than 21 mmHg. [12] Theories suggest that patients with this type of glaucoma may have an optic nerve that is abnormally sensitive to pressure or may experience intermittent ischemic changes due to atherosclerosis or vascular insufficiency. These patients often exhibit a higher prevalence of migraines, Raynaud phenomenon, autoimmune diseases, ischemic vascular diseases, and coagulopathies. This observation may suggest the involvement of a vascular autoregulatory defect in the pathogenesis of the disease. [21] [22] [23] [24] In addition, these patients tend to have a greater frequency of nerve fiber layer hemorrhages and a neuroretinal rim that is thinner inferiorly and inferotemporal than those with POAG. Visual field defects in this type of glaucoma are typically more focal, deeper, and closer to fixation rather than following the classic arcuate scotoma pattern seen in open-angle glaucoma. [25]
Angle-Closure Glaucoma
Angle-closure glaucoma is classified based on ocular anatomy and can manifest as a medical emergency in the acute setting or as a chronic condition. [26] In the acute form, this type of glaucoma occurs when the eye's drainage system is abruptly blocked due to the closure of the angle formed between the cornea and the iris. [27] Typically, this blockage arises from age-related lens thickening, leading to a gradual increase in a relative pupillary block that pushes the iris anteriorly. This anteriorly displaced iris, coupled with a natural anatomical variation such as a smaller angle seen in hypermetropia or specific ethnic groups, predisposes easier blockage of the outflow tract. [28]
A pupillary block is considered the underlying cause in the majority of cases. [29] When sudden pupil dilation occurs due to certain stimuli, darkness, or drugs, the iris is thick enough in its contracted state or anteriorly displaced by pupillary block to block fluid drainage via the trabecular meshwork. The pressure rapidly increases within the eye. This rapid change in IOP can cause central and/or peripheral vision loss within a few days of onset without intervention and very high IOP. A significantly elevated IOP and acute angle closure can lead to complications such as retinal vascular occlusion, ischemic optic neuropathy, or glaucomatous optic nerve damage. However, it is important to note that only about 10% of glaucoma cases fall into the acute angle-closure type category. [30]
Angle-closure glaucoma can also occur as a secondary condition due to various causes. Examples include lens subluxation in Marfan syndrome, [31] lens dislocation, [32] and lens-induced glaucoma. [33] The displacement of the lens into the pupil or anterior chamber can lead to an acute pupillary block. [34] Plateau iris configuration can also cause an acute pupillary block and chronic angle closure, attributed to elongated or anteriorly positioned ciliary processes pushing the iris edges forward. [35] [36] In iridocorneal endothelial syndrome, irregular corneal endothelium migration onto the trabecular meshwork and peripheral iris can lead to high peripheral anterior synechiae, closing the angle and hindering outflow. [37] [38]
Neovascularization can cause angle closure in neovascular glaucoma by forming a fibrovascular membrane that flattens and displaces the iris anteriorly. This process, along with new vessel formation in the iris and angle in rubeosis iridis, can lead to total synechial closure of the angle. [39] The most common etiologies of neovascular glaucoma are central retinal or branch retinal vein occlusion, proliferative diabetic retinopathy, and ocular ischemic syndrome. [40] Angle-closure glaucoma can also occur post-ophthalmic surgery due to factors such as ciliary body edema, scleral buckle placement, fibrin deposition, gas, or silicone oil used in retinal surgery. [41] Additionally, sulfa drugs such as topiramate can induce angle closure by causing ciliochoroidal effusion, which compresses the lens-iris diaphragm and anteriorly displaces it, resulting in angle closure. [42]
Secondary Open-Angle Glaucoma
Secondary open-angle glaucoma can result from various factors such as eye injury, eye disease, and occasionally eye surgery. These conditions can elevate IOP, leading to optic nerve damage and functional defects similar to those seen in primary open-angle glaucoma. One notable mechanism of secondary open-angle glaucoma arises from laser surgery, which can trigger pigment release, inflammatory cell accumulation, debris deposition, and mechanical deformation. These factors can collectively obstruct the trabecular meshwork, contributing to elevated IOP levels (see Image. Hypermature Morgagnian Cataract).
Pseudoexfoliation Glaucoma
Pseudoexfoliation syndrome (PEX) is a type of glaucoma characterized by the presence of flaky material within the anterior chamber of the eye that collects in the angle. This material can clog the trabecular meshwork, leading to increased resistance to aqueous outflow and elevated IOP. [43] PEX syndrome is a systemic disorder affecting the extracellular matrix, with ocular manifestations such as white deposits on the pupil's margin and the anterior capsule of the lens, which can occur in one or both eyes. [44] This secondary form of open-angle glaucoma poses additional risks during cataract surgery, including zonular dialysis, capsular bag rupture, and vitreous loss. These risks are attributed to eyes with unstable lens zonules and poor pupillary dilation associated with PEX syndrome. [45]
Individuals with pigment dispersion syndrome (PDS) are at an increased risk of elevated IOP. [46] Ocular manifestations of PDS share similarities with PEX syndrome, except that in PDS, the debris consists of pigment granules from the posterior iris surface. These granules can detach and obstruct the trabecular meshwork, especially in myopic or near-sighted eyes, due to contact with the peripheral lens capsule and zonules. [47] Features of PDS include anterior chamber pigment dispersion, spoke-like iris defects on trans-illumination, central corneal endothelial deposits (known as the Krukenberg spindle), and increased pigmentation in the iridocorneal angle. [48] Some individuals may progress to pigment dispersion glaucoma or pigmentary glaucoma characterized by elevated IOP levels, glaucomatous optic neuropathy, and/or visual field defects. [49]
Steroid-Induced Glaucoma
Steroid-induced glaucoma can occur in susceptible individuals undergoing cortisone therapy due to glucocorticoid's effects. Glucocorticoids can upregulate receptors on cells within the trabecular meshwork, leading to increased outflow resistance. Additionally, the accumulation of glycosaminoglycans in the meshwork pores contributes to this resistance. [50] Steroids can also suppress phagocytic activity, which decreases debris deposition removal from the meshwork and stimulates the expression of extracellular matrix proteins. [51]
Another form of secondary glaucoma is a carotid-cavernous fistula, characterized by an abnormal connection between the cavernous sinus and the carotid artery. [52] This condition causes arterial flow and venous engorgement, leading to elevated episcleral venous pressure. The consequent dilation of retinal veins and swelling of the optic disc can cause concurrent damage to optic nerve fibers. [53]
Secondary glaucoma can also result from posttraumatic or postoperative conditions, elevated episcleral venous pressure, and tumor-related factors. [54] Ellingson syndrome, also known as uveitis-glaucoma-hyphema syndrome, is a complication that can cause elevated IOP following the implantation of an intraocular lens. This elevation in IOP can occur either immediately after surgery or manifest years later as a complication. [55]
Glaucomatocyclitic crisis, also known as Posner-Schlossman syndrome, presents with recurrent acute episodes of elevated IOP that typically resolve spontaneously without treatment. However, repeated episodes have been associated with glaucomatous damage to the optic nerve over time (see Image. Advanced Glaucomatous Damage to the Optic Nerve). [56] [57] [58]
- Epidemiology
Glaucoma is a condition marked by the gradual loss of peripheral vision and irreversible damage to the optic nerve and retinal ganglion cells, making it a significant public health concern. Its multifactorial etiology involves anatomical, genetic, vascular, and immune factors. Glaucoma is a fundamental public health concern, considering that, after cataracts, it is the second cause of irreversible blindness. Currently affecting over 60 million individuals worldwide, this number is expected to surpass 110 million by 2040. [59] POAG is the most prevalent type, affecting approximately 2% to 4% of individuals aged 40 and older and around 10% of those aged 75 and older. [60]
The African population exhibits the highest prevalence of open-angle glaucoma. Individuals of African descent face up to a 15-fold increased risk of blindness from open-angle glaucoma compared to other population groups. [7] On the other hand, the Inuit population has the highest prevalence of angle-closure glaucoma. Women are more commonly affected than men in this group, along with individuals of Asian descent, who generally have a shallower anterior chamber, contributing to the higher rates of angle closure. [61] The normal-tension type of glaucoma is most prevalent in Japanese populations.
Age represents a significant risk factor in the progressive loss of retinal ganglion cells across all types of glaucoma. Additionally, other risk factors for developing glaucoma include a family history of the condition in a primary relative (mother, father, brother, sister, or children) and medical conditions such as diabetes, high blood pressure, and heart disease. Eye trauma, anatomical differences such as thinner corneas, a history of retinal detachment, eye tumors or inflammation, and prolonged use of corticosteroids are also associated with an increased risk of glaucoma. [62]
- Pathophysiology
The optic nerve carries over 1 million nerve fibers that transmit visual signals from the photoreceptors in the outer retina to the visual processing areas of the occipital lobe. Damage to the retinal nerve fiber layer occurs in various types of glaucoma. Aqueous humor, the fluid in the anterior chamber of the eye, is crucial in maintaining intraocular pressure and nourishing ocular tissues. Aqueous humor is produced by the non-pigmented epithelial cells of the ciliary body processes and follows a circadian production pattern. [63] Aqueous humor drains continuously through the pupil, then via the trabecular meshwork anterior to the scleral spur and iris insertion, into Schlemm canal, and finally into the episcleral venous system, larger orbital venous system, and systemic venous circulation. The trabecular meshwork consists of multiple layers of connective tissue and the endothelium of Schlemm canal, forming the primary drainage pathway for aqueous humor. [64]
The conventional outflow pathway regulates fluid drainage from the eye in a pressure-dependent manner, acting as a one-way valve for aqueous humor drainage. In contrast, the uveoscleral outflow pathway allows pressure-independent passage of aqueous humor through the ciliary muscle and iris root into the supraciliary and suprachoroidal space. [65] This pathway is believed to experience reduced outflow with age. Over time, there is a decline in aqueous humor drainage through the trabecular meshwork, while the production of aqueous humor by the ciliary body decreases slightly. This imbalance between outflow and aqueous production leads to elevated average IOP and larger diurnal fluctuations in IOP, which are common features in patients with glaucoma. [66]
Prolonged elevation of IOP leads to the death and atrophy of nerve fibers, resulting in a "cupped" or curved appearance of the optic nerve head observed during fundoscopy. [67] The normal range of IOP is approximately 16±3 mmHg, with values typically falling between 12 and 21 mmHg. [68] However, IOP fluctuates throughout the day due to various factors including heart rate, respiration, exercise, hydration status, systemic medications, time of day, alcohol intake, patient posture, and use of topical medications.
Elevated pressure readings during screening, surpassing 21 mmHg, indicate pressures beyond normal physiological levels and raise concerns about potential future damage to the optic nerve due to glaucoma. However, it is challenging to determine if patients experience transient spikes in pressure during the day, leading to damage that remains undetected during screening. Consequently, elevated screening pressure serves only as a risk factor for developing glaucoma and is not sufficient for a glaucoma diagnosis. Continuous monitoring of IOP throughout the day (diurnal pressure monitoring) may aid in identifying individuals at risk in this patient population. [69]
Patients diagnosed with normal-tension glaucoma often exhibit systemic vascular conditions such as the Raynaud phenomenon, migraines, sleep apnea, carotid artery disease, and significant nocturnal blood pressure fluctuations. [70] [71] In acute angle-closure glaucoma, the trabecular meshwork drainage pathway becomes obstructed due to the iris being pushed forward from pressure, such as an anteriorly displaced lens, or by fibrous tissue pulling the iris forward. The most common cause is a pupillary block, where the iris dilates to a mid-position, bows anteriorly upon contact with the lens, and obstructs the trabecular meshwork, thereby impeding aqueous outflow. Secondary glaucoma, as mentioned earlier, can arise from various causes such as surgery or neovascularization, leading to blockage of the outflow tracts and subsequent elevation of IOP, potentially resulting in glaucomatous optic nerve damage if left untreated.
- History and Physical
Many patients with glaucoma, especially early in the disease, are unaware they have this condition until it is detected during a routine eye examination. Numerous meta-analyses and systematic reviews have indicated that the prevalence of undetected glaucoma in adults exceeds 50% globally, with higher rates observed in Asia and Africa. [72] Individuals typically experience a gradual loss of peripheral vision while retaining central vision until the disease progresses significantly. This can manifest as a classic arcuate pattern on Humphrey visual field testing. During a comprehensive eye examination, optic nerves may exhibit a focally notched neural retinal rim or diffuse cup enlargement, a reduction in peripheral vision detected through visual field testing, and, although not necessary for diagnosis, an elevated IOP reading on tonometry. Usually, changes are observed bilaterally but may progress asymmetrically, leading to an asymmetric optic nerve cup. A cup-to-disc ratio greater than 0.5 is often associated with glaucoma, with initial loss typically observed in the inferotemporal and superotemporal poles of the optic disc (see Image. Optic Nerve Cup-to-Disc Ratio of 0.75). [73]
Patients with normal-tension glaucoma will typically be asymptomatic and have an IOP of less than 21 mmHg, making it challenging to detect and often leading to underdiagnosis due to the normal IOP values. Slit-lamp examination reveals changes in the optic disc, such as an increased cup-to-disc ratio, along with possible disc hemorrhage in the nerve fiber layer. [74] Patients may also have a medical history that includes vasospasm, coagulopathies, nocturnal hypotension, autoimmune diseases, vascular diseases, thyroid dysfunction, or sleep apnea.
In acute angle-closure glaucoma, patients usually experience sudden severe ocular pain, redness, blurry vision or decreased visual acuity, headache, nausea, and vomiting, as well as may report seeing halos of light. On examination, patients have an unresponsive mid-dilated pupil and a firm feeling of eyeball upon palpation. These attacks are often triggered by pupillary dilation from weak mydriatic or dilating drops. IOP is typically significantly elevated and ranges from 30 to 50 mmHg. Predisposing risk factors for acute angle-closure glaucoma include elevated hypermetropia, an angle between the iris and the cornea around 20° or less observed during gonioscopy, and/or an anterior chamber depth of less than 2.5 mm. [75] Patients with these reports should be advised to avoid dilating medications to reduce their risk of experiencing an acute attack. On slit-lamp examination, signs such as a large optic cup with narrowing of the neuroretinal rim and splinter hemorrhages may also be present. [76]
Patients with secondary glaucoma typically have a history of recent ophthalmic procedures, trauma, or underlying health conditions causing neovascularization, such as diabetic retinopathy or previous retinal vascular occlusions. Patients may sometimes not recall a specific precipitating factor, but subtle clinical examination findings can point to the cause of elevated IOP. Examination findings may include exfoliative material on the anterior lens capsule, pigment deposition on the corneal endothelial cells, flare in the anterior chamber indicative of uveitis, abnormal blood vessels on the iris, or signs of trauma, depending on the underlying etiology.
Assessment includes a fundoscopic examination, visual field testing, tonometry, optical coherence tomography (OCT), and gonioscopy. Of these, IOP is the greatest risk factor, thereby making tonometry of utmost importance. [77] Goldmann applanation tonometry is the gold standard for patients with risk factors, elevated IOP, and glaucoma, although several other types of tonometers are available. [78] [79] [80] Alternative tonometers can be considered when Goldmann applanation tonometry is impractical, including bedridden patients, noncollaborative individuals, children, or those allergic to anesthetic drops. [81] [82]
Additional helpful tests in glaucoma evaluation include assessing visual acuity to determine any impact on vision, pachymetry to measure corneal thickness, and retinal scans to monitor progressive changes in the retinal nerve fiber layer. Regular visual field testing using full-threshold strategies is crucial for individuals with risk factors, ocular hypertension, and those undergoing glaucoma treatment. [83] [84] [85] OCT is valuable for monitoring morphological changes in the optic nerve and retinal nerve fiber layer, especially in patients with ocular hypertension and early-to-moderate glaucoma. [86]
Glaucoma diagnosis relies on identifying progressive optic neuropathy and/or visual field defects, often in conjunction with elevated IOP. Ocular hypertension is diagnosed in individuals with IOP levels exceeding 21 mmHg without signs of glaucomatous optic neuropathy or functional visual field defects. Research indicates that around 20% of people with ocular hypertension may progress to glaucoma, highlighting the importance of regular testing, tonometry, and comprehensive eye examinations to initiate appropriate treatment aimed at reducing IOP in the presence of initial glaucomatous damage. [87]
A single gold standard test does not exist for diagnosing glaucoma. Typically, glaucoma is diagnosed during routine eye examinations, as the disease is often asymptomatic without noticeable vision loss. Clinicians rely on recognizing the characteristic appearance of the optic nerve, assessing risk factors, and interpreting ancillary test results to establish an accurate diagnosis and stage of glaucoma. [88] Currently, the American Academy of Ophthalmology recommends routine comprehensive eye examinations for individuals with glaucoma risk factors, with the examination frequency tailored to factors such as age, race, family history, and specific risk factors. [89]
- Treatment / Management
Managing glaucoma involves personalized strategies based on the type and severity of the condition. Available treatments cannot reverse vision loss; they aim to lower IOP, a key risk factor, to prevent further damage and vision loss. Therapeutic options such as eye drops, laser procedures, and surgeries are focused on reducing IOP. Monitoring disease progression involves using tools like tonometry, visual field tests, OCT, and vision loss mapping.
Open-angle glaucoma is typically managed initially with medications aimed at reducing eye pressure. Common medication classes include prostaglandin analogs, β-blockers, carbonic anhydrase inhibitors, α-2 agonists, miotic agents, and more recently, Rho-kinase inhibitors and nitric oxide-donating medications. [90] [91] Laser trabeculoplasty, such as argon laser trabeculoplasty, selective laser trabeculoplasty, and multipulse laser trabeculoplasty, may also be considered in certain cases. However, the benefits of lowering IOP with laser trabeculoplasty often last several months, and retreatments are commonly necessary. [92]
If medical and/or laser management is unsuccessful, procedures such as trabeculectomy, deep sclerectomy, canaloplasty, insertion of a drainage valve/tube shunt, and laser treatment to the ciliary body to reduce aqueous production can help achieve better control of IOP. [93] [94] [95] Minimally invasive glaucoma surgery (MIGS) is an emerging option for individuals with mild-to-moderate glaucoma. [96] Compared to traditional trabeculectomy and tube shunts, MIGS offers a more favorable safety profile, quicker recovery time, and effective reduction of IOP to the mid-high teens. Studies also indicate that MIGS placement can decrease the number of pressure-lowering medications needed to maintain target IOP levels. [97]
Normal-tension glaucoma can be managed with medications to reduce IOP and address any underlying medical conditions. Treatment options include prostaglandin analogs, α-2 agonists, carbonic anhydrase inhibitors, and miotics. The use of β-blockers is debated due to concerns about reduced optic nerve head perfusion, particularly regarding the potential exacerbation of the early morning nadir in blood pressure. If medical therapy proves ineffective, laser trabeculoplasty or filtration surgery may be necessary, especially in cases of progressive vision loss. The collaborative normal-tension glaucoma study demonstrated that patients with this condition can slow or stabilize their field loss after achieving a 30% reduction in IOP. [98]
Angle-closure glaucoma is considered a medical emergency due to the potential for elevated pressures leading to glaucomatous optic nerve damage, ischemic nerve damage, or retinal vascular occlusion. Patients can take medications to reduce eye pressure as quickly as possible but usually require a laser procedure called laser peripheral iridotomy. This procedure involves creating a small hole in the iris to alleviate pupillary blockage. By equalizing the pressure gradient between the posterior and anterior chambers, laser iridotomy resolves the iris bombe and opens up the drainage angle in the anterior chamber, relieving the condition. The peripheral iris can be flattened with laser iridoplasty and, less commonly, with laser pupilloplasty.
A decrease in IOP does not always indicate that the angle has reopened. Ischemic damage to the ciliary body during an attack can reduce aqueous humor production for several weeks. Therefore, it is crucial to perform a follow-up gonioscopy to confirm angle patency. This evaluation also helps assess the percentage of the angle with peripheral anterior synechia from acute or prior subacute attacks. After resolving the acute crisis, patients are at a high risk of experiencing an attack in the contralateral eye. Therefore, they should undergo gonioscopy to assess the angle and consider prophylactic iridotomy in the other eye if the angle is narrow. [99] Treatment for secondary glaucoma should focus on addressing the underlying cause along with the possible inclusion of medications to reduce IOP. [100]
- Differential Diagnosis
When diagnosing POAG, it is crucial to rule out other conditions that can cause optic neuropathy. Differential diagnoses to consider include previous ischemic optic neuropathy, optic atrophy, and compressive non-glaucomatous optic neuropathy, as they can exhibit similar visual field loss patterns and, in some cases, lead to "pseudo-cupping" of the optic nerve. [101] If elevated IOP or characteristic glaucomatous optic nerve changes are observed, performing a gonioscopy is essential to assess the anterior chamber's status (open, narrow, or closed). Moreover, clinicians should be vigilant for subtle signs of various secondary glaucomas, review the patient's medication history for potential idiosyncratic drug reactions or steroid responses, and gather a comprehensive history of prior ocular trauma and surgeries.
With an acute presentation resembling acute angle-closure glaucoma, clinicians should also consider other potential diagnoses such as iritis, traumatic hyphema, conjunctivitis, episcleritis, migraine, cluster headache, subconjunctival hemorrhage, corneal abrasion, endophthalmitis, orbital compartment syndrome, corneal ulcer, periorbital infections, and infectious keratitis. A thorough history-taking and detailed slit-lamp examination are crucial for narrowing down the differentials and arranging appropriate examinations or referrals as needed. [102] [103] [104]
Glaucoma is not a benign disorder, and if left untreated, it can lead to permanent vision loss. The higher the pressure and the duration of elevated IOP, the greater the risk of damaging the optic nerve. Timely diagnosis plays a critical role in mitigating glaucomatous damage, and early intervention is crucial in preventing and slowing down vision loss progression. Effective treatment, especially in maintaining low IOP levels, can often lead to positive outcomes, preserving visual field integrity and halting disease advancement.
- Complications
Complications of glaucoma include visual field loss and can lead to eventual complete blindness, with a progression to no light perception vision in the affected eye. [105]
- Deterrence and Patient Education
Standard screening during a complete eye examination includes checking the IOP of both eyes. Alongside tonometry, periodic eye examinations should encompass a thorough anterior segment evaluation and a fundoscopic exam, with special attention to the optic nerve. These initial screening tests are crucial for identifying glaucoma suspects early in the disease course. High-risk glaucoma suspects require further diagnostic testing such as visual field examination, OCT, pachymetry, and gonioscopy to confirm the diagnosis.
Patients should be educated about the causes, risk factors, and treatment for glaucoma. They should understand that most cases of glaucoma result in slow, progressive vision loss that often goes unnoticed until a significant portion of the visual field is affected. Therefore, regular eye exams are highly recommended to identify high-risk patients promptly and prevent irreversible vision loss.
- Enhancing Healthcare Team Outcomes
Glaucoma represents a chronic and severe condition that may lead to irreversible vision loss if detected late in its progression. Effective management of the condition involves an interprofessional team focused on addressing patients' visual health concerns. A crucial aspect of treatment lies in patient education. Pharmacists should emphasize the importance of medication compliance and the necessity of regular follow-ups with the eye specialist. Ophthalmic technicians must collaborate closely with clinicians to oversee scheduled visual field and OCT assessments. Ophthalmic nurses are responsible for monitoring IOP and communicating these findings promptly to the ophthalmologist for further evaluation and management. This collaborative approach ensures comprehensive care and better outcomes for patients with glaucoma.
Regular eye appointments and compliance with medication are vital to lessening disease progression in glaucoma patients. Given its hereditary nature, educating family members about the increased risk of developing glaucoma is important, prompting them to undergo regular screenings for early detection. Notably, healthcare team members must consult the ophthalmologist before making any changes to medication dosage or frequency. Effective communication among healthcare providers is essential to reduce the morbidity associated with glaucoma. [106]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Glaucomatous Optic Nerve Head Showing Inferotemporal Retinal Nerve Fiber Layer Defect. Increased intraocular pressure can result in reduced blood supply to the optic nerve fibers, resulting in subtle ischemic injury. Contributed by K Tripathy, (more...)
Left Eye Glaucomatous Visual Field Changes. This image displays the results of a visual field test indicating characteristic changes associated with glaucoma in the left eye, as observed on perimetry. Contributed by S Shah, MBBS
Optic Nerve Cup-to-Disc Ratio of 0.75. A cup-to-disc ratio greater than 0.5 is often associated with glaucoma, and initial loss is typically observed in the inferotemporal and superotemporal poles of the optic disc. Contributed by AS Huang, MD (more...)
Hypermature Morgagnian Cataract. This image captures the outcome of treatment for an older male patient who initially presented with severe corneal edema, congestion, and elevated intraocular pressure. Following the administration of (more...)
Advanced Glaucomatous Damage to the Optic Nerve. This image illustrates the severe and advanced glaucomatous damage to the optic nerve observed in a patient with high myopia. Contributed by K Tripathy, MD
Disclosure: Jamie Dietze declares no relevant financial relationships with ineligible companies.
Disclosure: Kyle Blair declares no relevant financial relationships with ineligible companies.
Disclosure: Marco Zeppieri declares no relevant financial relationships with ineligible companies.
Disclosure: Shane Havens declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Dietze J, Blair K, Zeppieri M, et al. Glaucoma. [Updated 2024 Mar 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Glaucoma (Nursing). [StatPearls. 2024] Glaucoma (Nursing). Dietze J, Blair K, Zeppieri M, Havens SJ, Adams M. StatPearls. 2024 Jan
- Routine eye examinations for persons 20-64 years of age: an evidence-based analysis. [Ont Health Technol Assess Ser....] Routine eye examinations for persons 20-64 years of age: an evidence-based analysis. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2006; 6(15):1-81. Epub 2006 Jul 1.
- Review Prostaglandin Analogues for Ophthalmic Use: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines [ 2020] Review Prostaglandin Analogues for Ophthalmic Use: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines Islam S, Spry C. 2020 Feb 18
- Review [Aiming for zero blindness]. [Nippon Ganka Gakkai Zasshi. 2015] Review [Aiming for zero blindness]. Nakazawa T. Nippon Ganka Gakkai Zasshi. 2015 Mar; 119(3):168-93; discussion 194.
- Review Primary open-angle glaucoma. [Nat Rev Dis Primers. 2016] Review Primary open-angle glaucoma. Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR. Nat Rev Dis Primers. 2016 Sep 22; 2:16067. Epub 2016 Sep 22.
Recent Activity
- Glaucoma - StatPearls Glaucoma - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Glaucoma can be defined as a progressive optic neuropathy that induces optic disc cupping and retinal ganglion cell apoptosis. 1 As the world's leading cause of irreversible blindness, the disease currently affects 3.5% of individuals aged between 40 and 80 years. The incidence of glaucoma is increasing, together with life expectancies, in resource-limited countries, and nearly 112 million ...
In western Europe, glaucoma is the second most common cause of irreversible blindness, after age-related macular degeneration . The prevalence of glaucoma in Europe among persons aged 40 to 80 years is 2.93% (figure 3) . Most suffer from open-angle glaucoma, which has a prevalence of 2.51% from age 40 to age 80 .
1. Introduction. Glaucoma is one of the leading causes of blindness in the world, second only to cataracts [ 1, 2 ]. It is a chronic, degenerative disease affecting the optic nerve, but insidious: in fact, when the etiopathogenetic process has started and has already damaged the nerve fibers, the symptomatology is almost silent [ 3, 4 ].
Glaucoma can be divided into several phenotypes. One major distinction of primary glaucomas relates to the anatomy of the anterior chamber angle ().Most have an open angle, but others have angle closure, and in general the disease course in angle closure glaucoma is more severe (see figure 1 for explanations). Globally, about 65 million people have open angle glaucoma and 30 million have angle ...
Xen-DS: a novel technique of ab externo Xen implantation augmented with a modified deep sclerectomy for surgical treatment of glaucoma. A. Elbably. J. Richardson-May. A. Jacob. Research 21 May ...
Importance: Glaucoma is a worldwide leading cause of irreversible vision loss. Because it may be asymptomatic until a relatively late stage, diagnosis is frequently delayed. A general understanding of the disease pathophysiology, diagnosis, and treatment may assist primary care physicians in referring high-risk patients for comprehensive ophthalmologic examination and in more actively ...
Introduction. Glaucoma is a progressive disease characterized by damage to the optic nerve and visual loss which, if left untreated, can lead to blindness [1, 2, 3]. Glaucoma may affect activities ...
Of the reviews of laser and perioperative interventions for glaucoma, we assessed 11 as reliable. Laser trabeculoplasty is as effective as medical treatment as a first-line therapy in controlling IOP in open-angle glaucoma. Of these methods, selective and argon laser trabeculoplasty have similar IOP-lowering efficacy.
Prevention of angle-closure glaucoma: balancing risk and benefit. Eye 36, 2229-2231 (2022) Cite this article. Primary angle-closure glaucoma (PACG) is an important, preventable cause of visual ...
Research has also utilised multimodal structural data to enhance the assessment of glaucomatous structural ... J Glaucoma. 2015;24:672-8. Article PubMed Google Scholar ...
The prevalence rises with age, reaching 10% in persons over 90 years old. The available diagnostic methods include ophthalmoscopy, tonometry, perimetry, and imaging techniques. The treatment of glaucoma is focused on lowering the intraocular pressure with topical drugs, laser therapy, and glaucoma surgery. In patients with manifest glaucoma ...
When Glaucoma Research Foundation first launched Catalyst for a Cure in 2002, our bold idea was to recruit the most promising young researchers from different fields to work collaboratively. Focus on Dr. Xin Duan's Laboratory: Restoring Vision. Xin Duan, PhD is an Associate Professor of Ophthalmology at the UC San Francisco Weill Institute of ...
Glaucoma is a worldwide leading cause of irreversible vision loss. Because it may be asymptomatic until a relatively late stage, diagnosis is frequently delayed. ... (Medeiros), from the National Institutes of Health/National Eye Institute; and an unrestricted grant from Research to Prevent Blindness. Dr Aung is supported by grants from the ...
Observations: Glaucoma is a chronic progressive optic neuropathy, characterized by damage to the optic nerve and retinal nerve fiber layer, that can lead to permanent loss of peripheral or central vision. Intraocular pressure is the only known modifiable risk factor. Other important risk factors include older age, nonwhite race, and a family ...
Glaucoma is a heterogeneous group of diseases characterised by cupping of the optic nerve head and visual-field damage. It is the most frequent cause of irreversible blindness worldwide. Progression usually stops if the intraocular pressure is lowered by 30-50% from baseline. Its worldwide age-standardised prevalence in the population aged 40 years or older is about 3·5%. Chronic forms of ...
The most extensive genome-wide association study of glaucoma to date compared the genes of 34,179 people with the disease to 349,321 control participants. It enabled an international consortium of researchers to identify 44 new gene loci ("genetic street addresses" that denote a specific location on a gene). It confirmed 83 previously ...
In conclusion, the AGS 100 represents an amalgamation of clinically impactful articles generated from a bibliometric analysis, expert panel, and surveys of the AGS membership. The list includes frequently cited as well as newer and "classic" papers spanning 6 decades of progress in glaucoma research and innovation.
Glaucoma Research Foundation is dedicated to scientific research that deepens our understanding of glaucoma, generates new treatments, and will ultimately cure a disease that is the second leading cause of blindness. Research takes resources. That's why, for more than 40 years, we have rallied financial support for science with the potential ...
Abstract. Glaucoma is the leading cause of blindness throughout the world (after cataracts); therefore, general physicians should be familiar with the diagnosis and management of affected patients. Glaucomas are usually categorized by the anatomy of the anterior chamber angle (open vs narrow/closed), rapidity of onset (acute vs chronic), and ...
Importance Glaucoma is the most common cause of irreversible blindness worldwide. Many patients with glaucoma are asymptomatic early in the disease course. Primary care clinicians should know which patients to refer to an eye care professional for a complete eye examination to check for signs of glaucoma and to determine what systemic conditions or medications can increase a patient's risk ...
Glaucoma is a multifactorial optic degenerative neuropathy characterized by the loss of retinal ganglion cells. It is a combination of vascular, genetic, anatomical, and immune factors. ... All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should ...
Journal of Glaucoma. 31 (2):116-122, February 2022. Journal of Glaucoma provides a stimulating forum for discussion of clinical, scientific, and socioeconomic issues of greatest concern to clinicians who care for glaucoma patients. Each issue presents original articles on new approaches to diagnosis, innovations in pharmacological therapy and ...
Our research highlights the need to consider these sociodemographic and psychological factors when developing strategies to improve adherence in glaucoma patients. Newman-Casey et al reported that 61% of patients cited multiple barriers to optimal adherence.
Glaucoma Articles. This is the place for the latest information about our work here at Glaucoma Research Foundation and for current events in the world of eye health. All breakthroughs and insights are made possible through the dedication of researchers, and through the continued financial support and active involvement of the community. CLEAR.
Glaucoma is a complex eye condition characterized by elevated intraocular pressure (IOP) that may progress to vision loss over time. Glaucoma is the second leading cause of permanent blindness in the United States and occurs most often in older adults.[1] Glaucoma can be categorized into either primary or secondary types and further into open-angle or closed-angle variants within each type of ...