Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.

As a society-owned publisher with a legacy of serving scientific communities, we are committed to offering a home to all scientifically valid and rigorously reviewed research. In doing so, we aim to accelerate the dissemination of scientific knowledge and the advancement of scholarly communications to benefit all.
Nano Express supports this mission and actively demonstrates our core values of inclusive publishing and trusted science . To find out more about these values and how they can help you publish your next paper with us, visit our journal scope .
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.

Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties
Md Jahidul Haque 1 , Md Masum Bellah 1 , Md Rakibu Hassan 1 and Suhanur Rahman 1
Published 16 March 2020 • © 2020 The Author(s). Published by IOP Publishing Ltd Nano Express , Volume 1 , Number 1 Citation Md Jahidul Haque et al 2020 Nano Ex. 1 010007 DOI 10.1088/2632-959X/ab7a43
Article metrics
29398 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Department of Glass & Ceramic Engineering, Rajshahi University of Engineering & Technology (RUET), Rajshahi-6204, Bangladesh
Md Jahidul Haque https://orcid.org/0000-0001-7945-5937
- Received 23 December 2019
- Revised 3 February 2020
- Accepted 26 February 2020
- Published 16 March 2020
Peer review information
Method : Single-anonymous Revisions: 1 Screened for originality? Yes
Buy this article in print
In this work, two different methods (sol-gel and biosynthesis) were adopted for the synthesis of zinc oxide (ZnO) nanoparticles. The leaf extract of Azadirachta Indica (Neem) was utilized in the biosynthesis scheme. Structural, antibacterial, photocatalytic and optical performances of the two variants were analyzed. Both variants demonstrated a wurtzite hexagonal structure. The biosynthesized variant (25.97 nm) exhibited smaller particles than that of the sol-gel variant (33.20 nm). The morphological analysis revealed that most of the particles of the sol-gel variant remained within the range of 15 nm to 68 nm while for the biosynthesized variant the range was 10–70 nm. The antibacterial assessment was redacted by using the agar well diffusion method in which the bacteria medium was Escherichia coli O157: H7. The zone of inhibition of bacterial growth was higher in the biosynthesized variant (14.5 mm). The photocatalytic performances of the nanoparticles were determined through the degradation of methylene blue dye in which the biosynthesized variant provided better performance. The electron spin resonance (EPR) analysis revealed that the free OH · radicals were the primary active species for this degradation phenomenon. The absorption band of the sol-gel and biosynthesized variants were 363 nm and 356 nm respectively. The optical band gap energy of the biosynthesized variant (3.25 eV) was slightly higher than that of the sol-gel variant (3.23 eV). Nevertheless, the improved antibacterial and photocatalytic responses of the biosynthesized variants were obtained due to the higher rate of stabilization mechanism of the nanoparticles by the organic chemicals (terpenoids) present in the Neem leaf extract.
Export citation and abstract BibTeX RIS
Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
As a rapidly growing sector in materials science, nanotechnology and nanoscience deal with materials that have particles within a size range of 1 to 100 nm and a high surface-to-volume ratio [ 1 ]. In general form, these particles are termed as nanoparticles (NPs) which exhibit highly controllable physical, chemical and biological properties in the atomic and sub-atomic levels. However, these unique features create opportunities to use them in different sectors such as electronics, optoelectronics, agriculture, communications, and biomedicine [ 2 , 3 ].
Although, several NPs are showing their effectiveness in different sectors of technology, but zinc oxide (ZnO) NPs have gained much more importance in the recent years due to their attractive and outstanding properties such as high chemical stability, high photostability, high electrochemical coupling coefficient and a wide range of radiation absorption [ 4 ]. Again, ZnO NPs are also recognized as n-type multi-functional semiconductor materials that have a wide band gap of 3.37 eV and exciton binding energy up to 60 meV even at room temperature [ 1 ]. Nowadays, ZnO NPs are predominantly used as antimicrobial agents, delivering systems vaccines and anti-cancer systems, photocatalyst, biosensors, energy generators and bio-imaging materials [ 5 – 7 ]. Among themselves, the photocatalytic application of ZnO NPs is significant. However, the photocatalytic performance of ZnO NPs can be significantly enhanced by adopting two ways. The first one involves the reduction of particle sizes by using efficient synthesis methods, while the second one involves the change of structural morphology by the incorporation of several elements (such as metal, non-metal, noble metal, transition metal, etc) into the crystal structure of ZnO NPs. However, in this work, we will proceed by adopting the first one.
Several fabrication techniques are used to produce ZnO NPs such as thermal hydrolysis techniques, hydrothermal processing, sol-gel method, vapor condensation method, spray pyrolysis and thermochemical techniques [ 8 ]. Nevertheless, recently a new synthesis method has been introduced and that is called biosynthesis scheme in which the NPs are prepared by using biological materials having significant reducing and stabilizing features. Moreover, NPs with variable size and shape can be achieved through this process.
Researchers proposed several possible plant extracts and fungal biomasses that were used in the green synthesis of ZnO NPs such as Aloe Barbadensis Miller (Aloe Vera) leaf extract [ 9 ], Poncirus trifoliate leaf extract [ 10 ], Parthenium hysterophorus L. (Carrot grass) leaf extract [ 11 ], Aspergillus aeneus [ 12 ], Calotropis procera latex [ 13 ], Sedum alfredii Hance [ 14 ], Physalis alkekengi L. [ 15 ], etc. However, the smaller particle size of ZnO NPs was observed by using Poncirus trifoliate leaf extract (8.48–32.51 nm), while for others, the results were satisfactory. In addition, another potential element for the preparation of ZnO NPs through the biosynthesis method is considered to be a leaf extract of Azadirachta indica (Neem leaf). The leaf extract contains highly active phytochemicals and enzymes that participate in the oxidation or reduction reactions that occur during the fabrication method and manipulate the bulk ZnO to convert into ZnO NPs [ 16 ]. Moreover, Neem leaf provides significant biological restrictions against bacterial growth and fungal growth [ 17 ].
The present study focused on the preparation of ZnO NPs by two different methods. The first one is the sol-gel method, while the second one is the biosynthesis method in which the Neem leaf extract was used as a mandatory element. A comparison of the properties (structural, antibacterial, photocatalytic and optical) between the two variants of ZnO NPs was performed. Here, the sol-gel synthesized and biosynthesized ZnO nanoparticles were nominated as ZnO A NPs and ZnO B NPs respectively.
2. Methodology
2.1. materials.
All the starting raw materials including zinc acetate dihydrate [Zn(CH 3 COO) 2 .2H 2 O, Merck Specialties, India], sodium hydroxide [NaOH, Merck Specialties, India] and absolute ethanol [CH 3 CH 2 OH, Merck Specialties, Germany) were maintained at a high purity level (>99%). However, in the biosynthesis method, another raw material was also used and that was the leaf of Azadirachta indica (Neem leaf).
2.2. Synthesis of ZnO nanoparticles (ZnO A NPs) by sol-gel method
At first, 20 gm Zn(CH 3 COO) 2 .2H 2 O was mixed into 150 ml distilled water and stirred for 20 min at 35 °C to produce a zinc acetate solution. Again, 80 gm NaOH powder was weighed, mixed into 80 ml water and stirred for around 20 min at 35 °C for producing NaOH solution. After mixing both solutions, the titration reaction was performed by the addition of 100 ml ethanol into the drop-wise manner accompanied by vigorous stirring. The stirring was continued for around 90 min to complete the reaction for obtaining a gel-like product. Then the gel was dried at 80 °C overnight and calcined in an oven at 250 °C for 4 h. Finally, ZnO nanoparticles were prepared. However, the overall chemical reaction for the preparation of ZnO nanoparticles by using NaOH can be expressed as:
2.3. Synthesis of ZnO nanoparticles (ZnO B NPs) by biosynthesis method
At first, the neem (A. Indica) leaves were collected from the Azadirachta Indica trees on the campus of Rajshahi University of Engineering and Technology, Bangladesh. After washing with distilled water, the leaves were dried into a dryer for 24 h. Then 20 gm dried leaves were smashed and mixed with 50 ml distilled water. After that, the mixture was stirred by a magnetic stirrer and heated at 60 °C for 1 h. As the mixture displayed a yellow color, it was filtered using the Whatman TM filter paper. However, the extract solution was used for further preparation of ZnO nanoparticles. The overall process for the preparation of Neem leaf extract is stereotyped in figure 1 .

Figure 1. Process flow diagram for the preparation of Neem leaf extract.
Download figure:
The next step included the preparation of the zinc acetate solution. For this, 21.94 gm Zn(CH 3 COO) 2 .2H 2 O was mixed into 50 ml water and stirred for 20 min at 35 °C. Similarly, in order to prepare a NaOH solution, 4 gm NaOH powder was added into 50 ml distilled water and simultaneously stirred for 20 min at 35 °C. Both solutions were then mixed by vigorous stirring. During this stirring process, the neem leaf extract was drop-wise mixed with the solution. As the addition of neem leaf continued, white precipitation of nanoparticles appeared. Then the solution was filtered and the filtered product was dried at 80 °C for 4 h. After that, the dried powder was calcined at 250 °C for 4 h and grounded to obtain the desired ZnO nanoparticles.
2.4. Characterization of ZnO NPs
X-ray diffraction was performed for structural analysis employing 40 kV-40 ma (scanning step of 0.02°) and Cu- K α radiation having wavelengths of K α 1 = 1.54060 Å, K α 2 = 1.54439 Å (Bruker Advance D8, Germany). Morphological characterization was accomplished by scanning electron microscopy (ZEISS EVO 18, UK). The optical properties were determined through UV–vis spectroscopy (SHIMADZU UV/Vis-1650 PC, Japan) into a range of 200–800 nm.
2.5. Antibacterial analysis of ZnO NPs
Escherichia coli bacteria were mainly involved in the determination of the antibacterial performance of ZnO NPs. Initially, the bacteria was stock-cultured in brain heart infusion (BHI) growth medium at −20 °C. Around 3 ml of BHI broth was added to 300 ml of stock-culture and preserved the culture overnight at 36 °C ± 1 °C for 24 h. After 24 h of incubation, dilution of the bacterial suspension (inoculum) was accomplished by using sterile saline. To indicate the bacterial growth during the test, a solution of 2-(4-iodophenyl)−3-(4-nitrophenyl)−5-phenyltetrazolium chloride (INT) in ethanol was added to the bacterial inoculum. Then the inoculum was distributed on a Mueller Hinton Agar Petri Dish in a consistent manner. After that, ZnO A NPs and ZnO B NPs were placed into the wells (prepared by cutting the agar gel) and the systems were preserved at 36 °C ± 1 °C for 24 h to allow successive incubation. After 24 h, the growth of bacteria was monitored and finally, the zone of inhibition for bacterial growth was determined in mm scale.
2.6. Photocatalytic analysis of ZnO NPs
The photocatalytic analysis was performed by monitoring the degradation of Methylene Blue (MB) dye due to ZnO NPs under the influence of UV radiation (having intensity ∼120 μ W cm −2 and wavelength ∼300–400 nm). At first, 5 gm NPs were added into MB solution and mixed properly. The mixture was placed in the dark for 2 h and then irradiated with UV rays with subsequent stirring action and at a variation of time (0, 40, 80, 120, 160, 200 min). The absorbance of the mixture was measured by UV–vis spectroscopy (SHIMADZU UV/Vis-1650 PC, Japan). The efficiency of photodegradation was measured by the following equation:
Where C 0 is the absorption of MB solution before the addition of ZnO NPs and C 1 is the absorption of the mixture solution with respect to time t.
ESR (electron spin resonance) analysis was performed using the EPR spectrometer (Bruker EMX MicroX, Germany) for the identification of the major factor that provides effective photocatalytic performance. During this characterization, DMPO (5,5-dimethyl-1-pyrroline-N-oxide) was used as a spin-trapped reagent in methanol and aqueous state. Moreover, the analysis was performed both in the presence and absence of light irradiation.
3. Results and discussion
3.1. effect analysis of neem leaf extract.
Neem leaf extract contains various phytochemicals such as flavones, quinines, organic acids, aldehyde and ketones which act as reducing agents and significantly reduces the particle sizes. After the successive reduction of particle sizes, the NPs are also affected by the terpenoids. Because of the interaction between the terpenoids and the ZnO NPs become stabilized as terpenoids are effective capping and stabilizing agents. The corresponding mechanism is graphically abstracted into figure 2 . Moreover, the possible seven types of terpenoids that are present in Neem leaf extract are stereotyped in figure 3 .
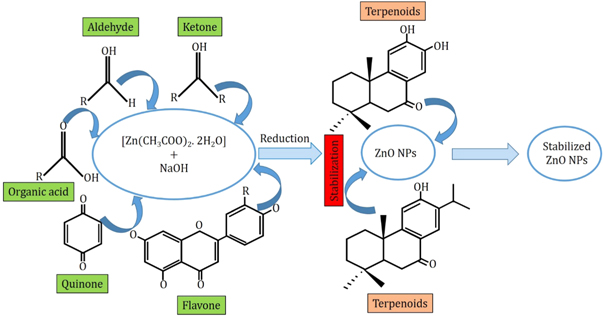
Figure 2. Schematic representation of the mechanism of size reduction and stabilization of ZnO NPs during the biosynthesis fabrication scheme using Neem leaf extract.
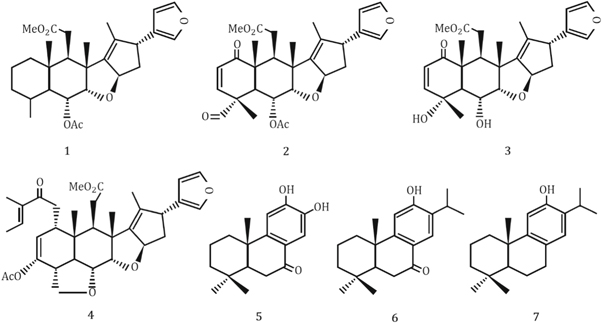
Figure 3. Chemical structures of different types of terpenoids subsisting in the Neem leaf extract.
3.2. X-ray diffraction analysis
Figure 4 represents the corresponding X-ray diffraction patterns of ZnO nanoparticles synthesized by sol-gel and bio-synthesis schemes respectively. The intense peaks at the crystal faces (100), (002), (101), (102), (110) assure the emergence of hexagonal wurtzite structure (as shown in figure 5 ) which belong to the space group of P6 3mc (JCPDS card no. 36-1451) [ 18 ]. The bio-synthesized ZnO nano-particles show more acute diffraction peak value introducing the appearance of the high percentage of crystalline phases. In addition, no impurity phases are present in the samples.
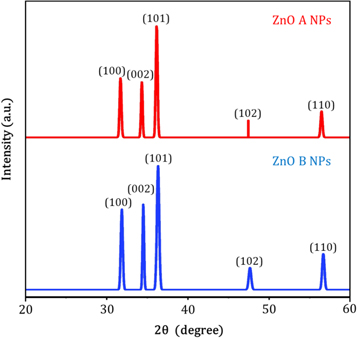
Figure 4. XRD patterns of ZnO A and ZnO B NPs.
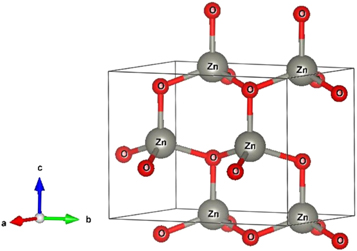
Figure 5. Schematic wurtzite crystal structure of ZnO NPs.
However, considering the most severe diffraction peak (101), the crystallite size (D) can be calculated in accordance with the Debye Scherer formula [ 19 ]:
Hither, β is the Full Width at Half Maxima of the corresponding peak, k is a dimensionless shape factor (∼0.94), while λ is the wavelength of Cu K α radiation (1.54 Å) and ϴ is the Bragg angle. D is mainly the mean size of the ordered domains which is considered to be equal to the particle size (applicable for only particles less than 100 nm). So, the average particle size of ZnO A NPs and ZnO B NPs is 33.20 nm and 25.95 nm respectively [ 19 ]. Again, there remains an inverse relationship between the β and the D which means that narrower peaks are resulted due to larger particles while broader particles are obtained because of smaller particles. The ZnO NPs showed a good agreement with this statement.
Since the crystallite size can be further employed for the determination of defect concentration within the specimen which is designated as the dislocation density ( δ ) and the leading formulae is adopted for this purpose [ 20 ]:
From the exploration of diffraction data, the lattice constant (a & c), inter-planar spacing (d) and unit cell volume (V) of the specimens (table 1 ) can also be enumerated by utilizing the following formulas respectively [ 21 ]:
Where, h, k, l belong to Miller indices.
Table 1. Structural information on ZnO A and ZnO B NPs.
| Structural parameters | ZnO A NPs | ZnO B NPs |
|---|---|---|
| FWHM (°) at (101) | 0.26313 | 0.33652 |
| Lattice constant (Å) | a = 3.50423 | a = 3.49295 |
| c = 4.95573 | c = 4.93979 | |
| Inter-planar spacing, d (Å) | 2.47786 | 2.46988 |
| Cell volume, a c (Å ) | 52.70156 | 52.19439 |
| Average crystallite size (nm) | 33.20 | 25.97 |
| Dislocation density, (nm ) | 0.00907 | 0.01482 |
| Bond length (Zn-O), L (Å) | 2.06488 | 2.05823 |
| Lattice strain, | 0.00107 ± 0.00128 | −0.00038 ± 0.00092 |
Besides, the lengthening of the stricture (L) between Zn and O can be enumerated by the following equation [ 20 ]:
Where u corresponds to parameterized constant belonging to wurtzite structure and can be expressed as:
In accordance with the Williamson-Hall proposition, the lattice strain was calculated by adopting the undermentioned equation [ 20 ]:
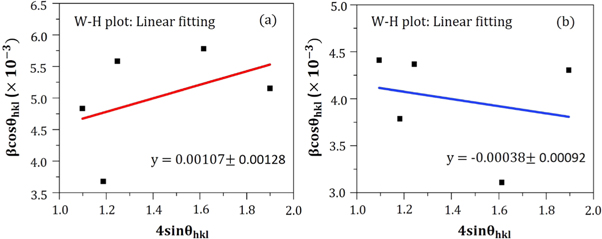
Figure 6. W-H plot of (a) ZnO A NPs and (b) ZnO B NPs for the measurement of lattice strain.
3.3. Morphological analysis
Figures 7 (a) and (b) shows the scanning electron micrographs of ZnO A and ZnO B NPs respectively. From the previous section, we have learned that the average particle size of ZnO B NPs (25.97 nm) is smaller than that of ZnO A NPs (33.20 nm). This can be also caused due to the presence of terpenoids in the Neem leaf extract. The terpenoid act not only as a stabilizing agent but also as a powerful reducing agent that interacts with ZnO NPs and reduces its size significantly [ 8 , 17 ]. Moreover, the maximum particles of ZnO A NPs remain between the range of 15 nm to 68 nm, whereas for ZnO B NPs the range lies from 10 nm to 70 nm.
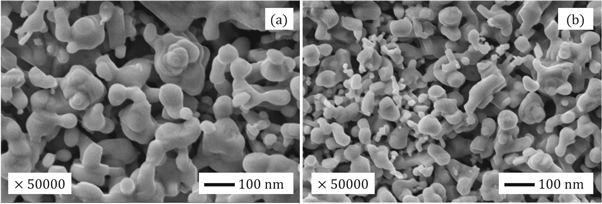
Figure 7. SEM micrographs of (a) ZnO A NPs and (b) ZnO B NPs.
3.4. Antibacterial activity
Antibacterial activity of ZnO A NPs and ZnO B NPs was analyzed by adopting the agar well diffusion method using Escherichia coli O157: H7 as the bacterial medium. Generally, there involve three mechanisms behind the interaction between the bacteria and the NPs. The first one involves the formation of extremely active hydroxyls and the second one involves the deposition of NPs on the bacteria surface. In addition, for the last one, the NPs accumulates in the cytoplasm or in the periplasmic region of bacteria cell which disrupts the cellular operations and simultaneously disorganizes the membrane. However, in consideration of E. coli , ZnO NPs firstly disorganize the membrane of E. coli and enters into the cytoplasmic region. Positioning themselves into the cytoplasm, the NPs neutralizes the respiratory enzymes and increases the emersion of cytoplasmic contents into the outward direction which impairs the membrane and finally kills the E. coli bacteria resulting in a zone of inhibition of bacterial growth around itself [ 3 , 23 ].
From figure 8 , it is observed that the zone of inhibition of bacterial growth due to ZnO A NPs is different from the zone of inhibition that is caused by ZnO B NPs. However, ZnO B NPs introduce a higher zone of inhibition than ZnO A nanoparticles and the measurements of the inhibition zone of bacterial growth are tabulated in table 2 . According to Krishna R Rangupathi, the antibacterial activity of nanoparticles is a size-dependent property and the property enhances with the reduction of particle size [ 23 ]. As the ZnO B NPs have smaller particle size as well as higher surface area, they show more antibacterial potential than that of ZnO A NPs [ 2 ].
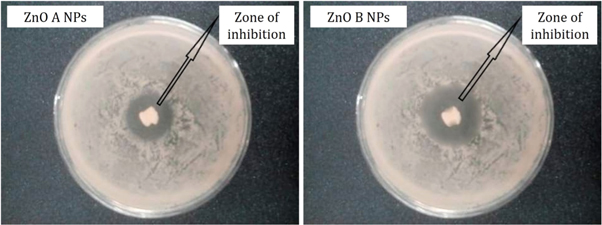
Figure 8. Antibacterial analysis of ZnO NPs showing the zone of inhibition of the growth of Escherichia coli O157: H7.
Table 2. Antibacterial measurements of ZnO A NPs and ZnO B NPs.
| Sample | Weight of the sample (gm) | Bacteria | The scientific name of the bacteria | Bacteria type | Zone of inhibition, D (mm) |
|---|---|---|---|---|---|
| ZnO A NPs | 1.0 | O157:H7 | Gram negative | 9.3 | |
| ZnO B NPs | 1.0 | O157:H7 | Gram negative | 14.5 |
3.5. Photocatalytic activity
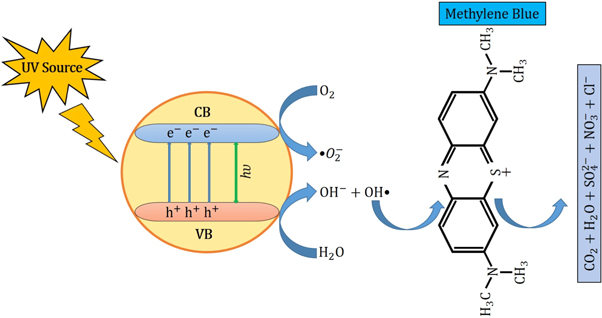
Figure 9. Degradation mechanism of MB dye by ZnO NPs under the influence of UV irradiation.
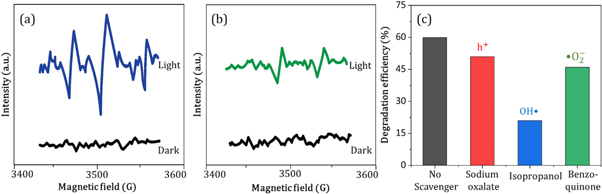
However, the corresponding reactions in the photodegradation scheme can be summarized as below [ 24 , 25 ]:
Figure 11 displays the discoloration of MB dye due to the photocatalytic action of ZnO NPs at different times (0, 40 and 120 min). However, figures 12 (a) and (b) illustrates the absorption spectra of MB dye as a function of wavelength under the influence of UV radiation at a variation of time i.e. 0, 40, 80, 120, 160, 200 min. From the graph, it is observed that the absorption rate of MB containing ZnO B NPs decreases more rapidly than that of ZnO A NPs. Moreover, the degradation efficiency ( η ) of ZnO NPs (biosynthesized and sol-gel synthesized) with respect to time is illustrated in figure 13 . The degradation of MB for sol-gel synthesized ZnO are 35.3%, 45.7%, 56.1% 62.4%, 68.9% at 40, 80, 120, 160 and 200 min respectively. Again, the values for biosynthesized ZnO are 36.9%, 47.5%, 62.7%, 72.1%, and 80.2% at 40, 80, 120, 160 and 200 min respectively. So, MB dye degraded more rapidly in the presence of ZnO B NPs backing the reason for smaller particle sizes than that of ZnO A NPs. As the particles become smaller, the active surface area for the photocatalysis increased which results in enhanced degradation of MB [ 26 ]. Moreover, there remain terpenoids in the neem leaf extract which stabilizes the nanoparticles by capping themselves which also causes in the increment of photocatalytic action [ 27 ].
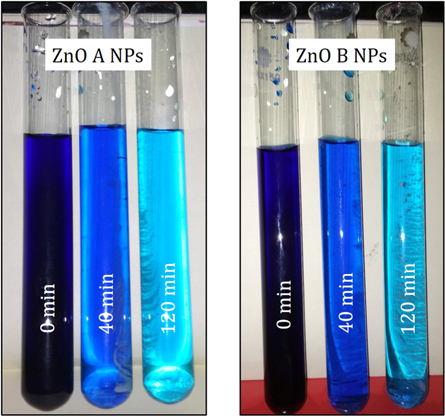
Figure 11. Visual inspection of the degradation phenomenon of MB dye by ZnO NPs.
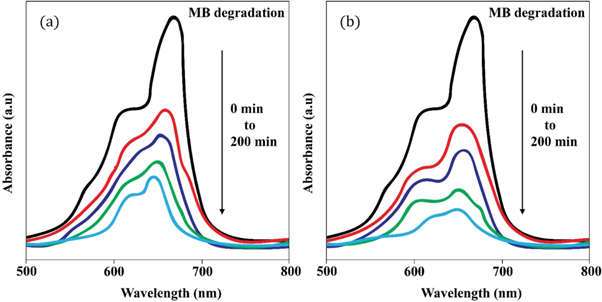
Figure 12. Absorption spectrum of (a) ZnO A NPs and (b) ZnO B NPs as a function of wavelength at 0, 40, 80, 120, 160, 200 min.
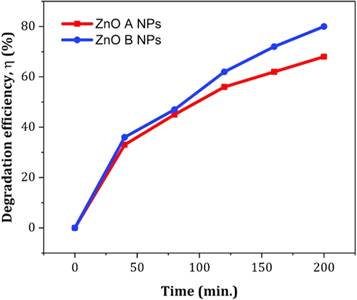
Figure 13. The degradation efficiency of ZnO NPs for methylene blue dye with respect to time.
3.6. Optical analysis
Figures 14 (a) and (b) displays the room temperature absorption spectrum of ZnO nanoparticles fabricated by sol-gel and biosynthesis methods correspondingly. Here, the absorption wavelengths are remaining within the maximum allowable limit of the absorption band of bulk ZnO (∼373 nm). Although the absorption slightly increases up to a wavelength of 363 nm for ZnO A NPs, the maximum incremental value for ZnO B NPs is 356 nm. The slight shift of the absorption peak may be caused due to the variation of particle size and their configuration [ 28 ]. However, this phenomenon results in the presence of a wide range of particle size distribution of ZnO [ 29 ]. Moreover, the redshift of ZnO A NPs compared to ZnO B NPs corresponds to the formation of agglomeration in the specimens significantly. Furthermore, in accordance with Gunanlan Sangeetha et al the shifting of absorption band to the higher wavelength as well as higher energy was associated with the increment of the size of nanoparticles [ 30 ]. Moreover, considering the direct interband transition between the valence band and the conduction band, the absorption band gap energy was measured by adopting the following Tauc's formula [ 31 ]:
Where A is an energy-independent constant, α is the absorption coefficient, h υ is for the photon energy, and E g is the optical band gap energy. The E g of the ZnO NPs was obtained from the ( α h υ ) 2 versus h υ plot (as shown in the inset of figures 14 (a) and (b). Where the extrapolation of the linear segment of the graph to (α h υ ) 2 = 0 provides the value of E g for ZnO NPs. It is observed that the optical band gap energy of ZnO B NPs (3.25 eV) is higher than that of ZnO A NPs (3.23 eV). This incremental phenomenon is mainly attributed to the quantum confinement effect. According to this theory, as the particle size decreases, the electrons in the valence band and the holes in the conduction band confine themselves within a space having a dimension of the de Broglie wavelength. However, this confinement influences the quantization of the energy and the momentum of the corresponding carriers and also enhances the optical transition energy between the valence band and the conduction band resulting in a broad band gap [ 32 ].
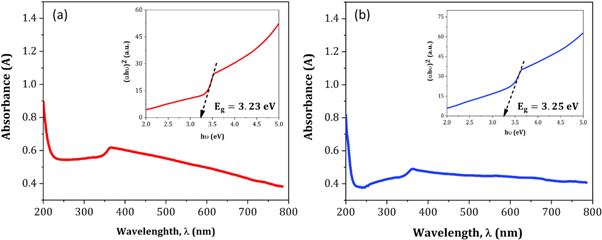
Figure 14. Absorption spectra of (a) ZnO A NPs and (b) ZnO B NPs (inset shows ( α h υ ) 2 versus h υ plot for the determination of band gap energy.
Figure 15 displays the UV visible transmittance spectrum of ZnO A NPs and ZnO B NPs. Here, the transparency of ZnO B NPs is greater than that of ZnO due to the reduced particle size of ZnO B NPs. From the research of Takuya Tsuzuki, it is clear that smaller particles are capable to show higher transparency at the visible range of spectrum [ 33 ]. However, the UV blocking characteristics are almost similar for each of the variants of NPs.
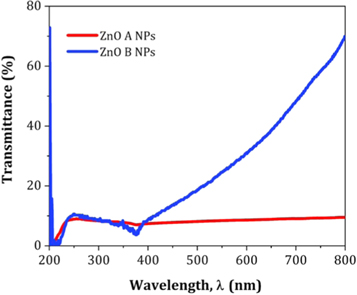
Figure 15. Typical transmittance spectra of ZnO NPs.
4. Conclusion
In summary, ZnO NPs were synthesized by two different methods i.e., sol-gel and biosynthesis method. The green synthesis of ZnO NPs allows avoiding the toxic chemical agents that are used in the sol-gel method for the size reduction. However, the Neem leaf extract possesses some phytochemicals which not only performs in the reduction of the particle sizes but also provide sufficient stabilization. Although, the average particle size of ZnO B NPs (25.97 nm) was smaller than that of ZnO A NPs (33.20 nm), the optical band gap energy of ZnO B NPs was higher than that of ZnO A NPs due to the quantum confinement effect. In addition, the antibacterial and photocatalytic properties of ZnO B NPs were greater than that of ZnO A NPs. Where, the zone of inhibition of bacterial growth for ZnO B NPs was 14.5 mm and for ZnO A NPs, it was 9.3 mm. Moreover, the degradation efficiency of ZnO B NPs at 200 min was 80% while for ZnO A NPs, the corresponding efficiency was 68%. Again, from the ESR analysis, it was proved that the OH · radicals were the main contributing factor for the degradation of MB dye. So, based on the comparison between the properties of the two variants, it is concluded that the biosynthesis method shows more effectiveness than the sol-gel method for the synthesis of ZnO NPs.
Acknowledgments
The authors are grateful to Rajshahi University of Engineering & Technology (RUET) for providing the opportunity to perform various tests. Special thanks go to Tasmia Zaman, Assistant Professor, Department of Glass & Ceramic Engineering, Rajshahi University of Engineering & Technology, Bangladesh for her cordial assistance.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Nanomaterials (Basel)

Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications
Ashok kumar mandal.
1 Natural Product Research Laboratory, Thapathali, Kathmandu 44600, Nepal
Saurav Katuwal
2 Central Department of Chemistry, Tribhuvan University, Kirtipur 44618, Nepal
Felix Tettey
3 Department of Chemical, Biological, and Bioengineering, North Carolina A&T State University, Greensboro, NC 27411, USA
Aakash Gupta
4 Department of Chemistry and Biochemistry, University of Massachusetts Dartmouth, North Dartmouth, MA 02747, USA
Salyan Bhattarai
5 Paraza Pharma, Inc., 2525 Avenue Marie-Curie, Montreal, QC H4S 2E1, Canada
Shankar Jaisi
Devi prasad bhandari, ajay kumar shah.
6 Faculty of Health Sciences, School of Health and Allied Sciences, Pokhara University, Lekhnath 33700, Nepal
Narayan Bhattarai
Niranjan parajuli, associated data.
All data generated or analyzed during this study are available within the article.
Zinc oxide nanoparticles (ZnO-NPs) have piqued the curiosity of researchers all over the world due to their extensive biological activity. They are less toxic and biodegradable with the capacity to greatly boost pharmacophore bioactivity. ZnO-NPs are the most extensively used metal oxide nanoparticles in electronic and optoelectronics because of their distinctive optical and chemical properties which can be readily modified by altering the morphology and the wide bandgap. The biosynthesis of nanoparticles using extracts of therapeutic plants, fungi, bacteria, algae, etc., improves their stability and biocompatibility in many biological settings, and its biofabrication alters its physiochemical behavior, contributing to biological potency. As such, ZnO-NPs can be used as an effective nanocarrier for conventional drugs due to their cost-effectiveness and benefits of being biodegradable and biocompatible. This article covers a comprehensive review of different synthesis approaches of ZnO-NPs including physical, chemical, biochemical, and green synthesis techniques, and also emphasizes their biopotency through antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, and cardioprotective activity. Green synthesis from plants, bacteria, and fungus is given special attention, with a particular emphasis on extraction techniques, precursors used for the synthesis and reaction conditions, characterization techniques, and surface morphology of the particles.
1. Introduction
A diverse application of nanomaterial-based technology has opened a new horizon in material science over the past decades because nanomaterials offer a high surface area and other very distinctive physical, chemical, and biological properties compared to their bulk counterparts [ 1 ]. Nanoparticle (NP) research has gained distinct interest due to the enhanced electrochemical reactivity, thermal conductivity, and nonlinear optical properties of nanoparticles which offer unique applications [ 2 ]. Zinc oxide nanoparticles (ZnO-NPs) are the most commonly used metal oxide nanoparticles because their distinctive optical and chemical properties can be easily modified by altering the morphology and the wide bandgap (3.37 eV) and high excitation binding energy (60 meV) to simulate the ZnO-NPs to be a potent photocatalytic and photo-oxidizing moiety against chemical and biological species [ 3 , 4 ]. They are less toxic to the human body and offer biocompatibility as the Zn ion (Zn 2+ ), a soluble form of ZnO, is a trace element found in the human physiological system. ZnO-based structures have been proven to exhibit biodegradability both in the bulk phase and in the form of nanoparticles [ 5 ]. Zn ions also act as the principal mediators of intracellular bacterial toxicity, disrupting their cell membranes [ 6 ].
Some potential applications where ZnO-NPs have been researched are: therapeutic carriers, biological sensing, gene transfer, nanomedicine discovery, biological labeling, medical implant coatings, electronic sensors, wastewater treatment, and communication [ 4 , 7 , 8 ]. The medical implant coating with zinc oxide and hydroxyapatite exhibited antibacterial and osteoconductive properties, emphasizing the potential of ZnO-NPs in therapeutic diagnostics. ZnO-NPs exhibited cytotoxicity in human cancer cells, resulting in cell death via the apoptotic pathway [ 9 ]. They also promoted antiproliferative activity in triple-negative breast cancer cells [ 10 ], nonautophagic cell death in human lung adenocarcinoma cells with an epidermal growth factor receptor (EGFR) mutation [ 11 ], and anticancer activity via apoptosis in chronic myeloid leukemia cells using a transcriptomic approach [ 12 ]. It has also been shown to induce cytotoxicity in the A549 epithelium and cancer cells [ 13 ]. Recent investigations on the ZnO-Au nanocomposite have developed an electrochemical DNA biosensor [ 14 ], ZnO-NPs for tracing studies in plants [ 15 ], and material in the development of electrochemical sensors in the detection of food additive aspartame [ 16 ]. ZnO-NPs have been shown to influence horizontal gene transfer where it impacts the transformation efficiency of Bacillus subtilis [ 17 ], and the ZnO-Ag NPs have decreased the rate of biofilm formation and gene expression in Staphylococcus aureus at a subminimum inhibitory concentration [ 18 ]. ZnO-NPs have been shown to reduce the parameters responsible for hepatic fibrosis (hydroxyproline) and nephrotoxicity (creatinine, urea, and uric acid) [ 19 ], also attenuating the gonadal toxicity which is induced by cyclophosphamide (an anticancer and immunosuppressant drug) through their antioxidant and antiapoptotic function [ 20 ], and cancer cell death through autophagy induction which supports the release of zinc ions and the generation of reactive oxygen species (ROS) [ 21 ].
In a critical study, zinc ions and ZnO-NPs both showed cytotoxic effects in the earthworm GI tract where it affected the gut epithelium and chlorogenic tissues [ 22 ]. However, ZnO-NPs dissolve slowly in human physiological conditions (pH 6–8), and the United States Food and Drug Administration (USFDA) safety datasheet indicates ZnO as a “Generally Recognized as Safe” (GRAS) substance and nonhemolytic against human red blood cells [ 23 ]. ZnO could be discovered to be a useful nanocarrier to facilitate the drug-delivering and release processes [ 24 , 25 ]. Much research endorses ZnO-NPs as the most beneficial metal nanoparticles, with minimal toxicity and excellent biocompatibility. The structural atom allocation mimics the most bioactive agent, emphasizing its pharmacological effectiveness against various ailments. With all this potential, the objective of this review article is to explore the various synthesis approaches and characterization techniques of ZnO-NPs with a comprehensive mechanistic approach to its biological activity. Although there is an increased number of studies revealing the mutually exclusive and exhaustive area of ZnO-NPs, this review is a comprehensive compilation of recent advances with clear illustrations for a better understanding of the importance of ZnO-NPs in biomedical research.
2. Biological Activities of ZnO-NPs
2.1. antibacterial action of zno-nps.
Bacteria portray a severe threat to human life as the world grapples with escalating antibiotic resistance and bacterial infection. ZnO-NPs have remarkable photo-oxidation and photocatalytic characteristics, and their exceptional antimicrobial properties have led to their recognition as potent agents against MDR [ 26 ]. Although the mechanism of antimicrobial action of ZnO-NPs is not well established, its properties, such as zinc ions and ROS generation, are widely assumed to result in oxidative stress and DNA damage, as well as photocatalytic activity, contributing to antibacterial efficacy ( Figure 1 ). According to Sirelkhatim et al., the oxygen annealing of ZnO increases the number of oxygen atoms on the surface, resulting in increased oxygen atom adsorption and the generation of more ROS, resulting in enhanced oxidation, and hence, a facilitated antimicrobial property [ 27 ]. Moreover, ZnO-NPs cause cytoplasmic shrinkage and the disruption of cell walls leading to cytoplasmic spillage ( Figure 2 ). ZnO-NPs act as an effective bactericidal agent against both Gram-positive as well as Gram-negative bacteria and are found to have direct interaction with the cell wall of bacteria leading to the disruption of its integrity [ 28 ].

Illustration of the antimicrobial property of ZnO-NPs against the bacterial cell wall. They act as potent antibacterial agents through these possible steps: (1) production of reactive oxygen species (ROS) causing oxidative stress, and membrane and DNA damage leading to bacterial death; (2) dissolution of ZnO-NPs into Zn 2+ and interference with bacterial enzymes, proteins, and amino acids; and (3) electrostatic interaction between ZnO-NPs and cell membrane, resulting in membrane plasma damage and intracellular content leakage. (Reprinted from [ 29 ]; open access under CC BY).

Image illustrating antibacterial efficacy against β-lactam-resistant K. pneumoniae obtained using transmission electron microscopy: ( a ) ZnO-NPs in the untreated state and ZnO-NPs in the treated state ( b – e ). Cytoplasmic shrinkage ( b ) disrupted cell wall and membrane ( c ), denatured protein shows as a dark electron-dense patch ( d ), and cytoplasmic spillage ( e , f ). The blue arrow represents an intact cell wall, the yellow arrow represents a disintegrating cell wall and cell membrane, and the violet arrow represents a denatured protein. (Reprinted from [ 30 ]; open access under CC BY).
2.2. Antifungal Action of ZnO-NPs
The antifungal properties of ZnO-NPs have been discovered in various studies in the literature. Their fungicidal activity varies depending on their structure, size, and concentration. The antifungal potency of biofabricated ZnO-NPs against Candida albicans isolates was investigated, and it was revealed that they were more effective against drug-resistant C. albicans isolates, demonstrating ZnO-NPs’ antifungal potency. Furthermore, it was shown that prophylactic treatment with lower concentrations of ZnO-NPs protects G. mellonella from the infection of C. albicans [ 31 , 32 ]. Similarly, the antifungal resistance of a 2% ZnO-NP-based cold cream exceeded the activity compared to a commercial antifungal cream at 2% on clinical isolates of Candida sp. [ 33 ]. ZnO-NPs have antifungal activity against both Aspergillus and Penicillium and have been investigated for their antidermatophytic activity on Trichophyton mentagrophytes and Trichophyton verrucosum [ 34 , 35 ]. Likewise, the bionanocomposite film of the soy protein isolate (SPI), cinnamaldehyde (CIN), and ZnO-NPs exhibited the highest antifungal activity among SPI, SPI-CIN, and SPI-ZnO-NPs films, where it was 1.56-fold stronger compared to the SPI-ZnO film and 1.24-fold stronger compared to the SPI-CIN film [ 36 ]. The antifungal activity studied against two pathogenic fungi— Botrytis cinerea and Penicillium expansum —revealed that activity is also dependent on nanoparticle concentrations, with the efficacy of the ZnO-NP treatment increasing as the concentration of ZnO-NPs rose from 3 to 12 mM. By affecting cellular functions, ZnO-NPs cause deformation in fungal hyphae, inhibiting the growth of B. cinerea . Similarly, P. expansum prevents the formation of conidiophores and conidia, resulting in the death of fungal hyphae, explaining the fact that P. expansum is found to be more sensitive than B. cinerea , i.e., microbe dependent. The activity detected in B. cinerea revealed the stronger the photo-activation, the greater the activity [ 37 , 38 , 39 ].
2.3. Cytotoxic Effect of ZnO-NPs
ZnO-NPs, compared to other metal oxide NPs, have a significant effect on cancer cells. The anticancer potential of ZnO-NPs is strongly influenced by their shape, size, and concentration. It has been discovered that the smaller the size and higher the concentration of NPs, the greater the anticancer activity [ 40 , 41 ]. They showed concentration-dependent anticancer activity against MCF7 human breast cancer cells, where 93% inhibition of proliferation of cells was noted at 100 µg/mL [ 40 ]. Similarly, fabricated ZnO-NPs exhibited concentration-dependent growth inhibition in human pancreatic cancer cell lines, PNAC-1, and AsPC-1, although they were shown to have a relatively smaller effect on the human normal fibroblast cell line (Hu02), which was found by an MTT assay [ 42 ]. The mechanistic approach ( Figure 3 ) underlying its anticancerous activity includes the production of sufficient ROS to cause substantial oxidative stress and DNA damage, disturbances on lipids and proteins in cells, and other cellular components due to their large semiconductor band gap [ 43 ]. Moreover, the establishment of a redox reaction system and the pro-inflammatory response of cells against ZnO-NPs induce cellular apoptosis. Discrimination between cancerous and normal cells has been a major challenge for a drug to be categorized as anticancerous. Failure to achieve selectivity results in systemic toxic effects. Several studies have revealed the selectivity of ZnO-NPs toward cancerous cells. ZnO-NPs have been demonstrated to be selective to Jurkat cancer cells with minimal toxicity toward normal CD4 + T cells [ 44 ]. Similarly, Hanley and the group proposed that ZnO-NPs had 28–35 times the specific cytotoxicity against cancer carcinoma cells compared to normal cells [ 45 ]. Selective localization by enhanced permeability and retention (EPR) time via extravasation toward tumor cells assists in selective activities affecting tumor cells rather than the normal cells. The electrostatic property of ZnO-NPs facilitates the targeting of tumor sites [ 46 ]. Thus, there is ample evidence that ZnO-NPs can exhibit anticancer effects in specific types of tumor cells in the body, which is depicted in Figure 3 .

A schematic representation of cytotoxicity potency of ZnO-NPs leading to the death of cancer cells. ZnO-NPs induce ROS production sequentially, leading to oxidative stress, DNA damage, p53 activation, and apoptosis of cancerous cells.
Despite various biomedical applications such as anticancer therapy, drug delivery, gene therapy, and tumor imaging, ZnO-NPs might have deleterious effects on several key organs including the lungs, kidneys, liver, CNS, reproductive system, and fetal development in animal models. However, the ZnO-NP-induced toxicity is multifactorial, and it is yet unknown just how toxic ZnO-NPs are for these organs [ 47 ].
2.4. Wound Healing Activity of ZnO-NPs
Wound healing is the phenomenon of cell injury responses, involving the activation of fibroblasts, endothelial cells, and macrophages where fibroblasts proliferate; an important step in wound healing for tissue regeneration [ 48 ]. It has been predicted that the delivery of ZnO via poly (lactide-co-glycolic acid) (PLGA)/silk fibroin (SF) nanofibers retains the bioavailability of NPs on the wound area and integrates with the unique structural features of electrospun nanofibers, which stimulate wound closure, re-epithelialization, collagen deposition, cellular migration, and angiogenesis [ 49 ]. Besides this, the ZnO-NPs loaded on bromelain-immobilized silk fibroin (SF-Br) reduced inflammation and promoted wound healing on a second-degree burn dressing [ 50 ]. During the healing process, the low doses of ZnO-NPs favored attachment and proliferation of fibroblasts, but the trend reversed at high doses. Metallic particles in nanocrystalline forms reduce wound infection along with promoting wound healing, as observed in adult male albino Wistar rats [ 51 ] and albino rats [ 52 ]. It was found that the functionalization of ZnO-NPs into triethoxysilane poly(amidoamine) dendrimer to generate a cross-linked collagen scaffold enhances re-epithelization and speedier collagen deposition than other scaffolds, which resulted in instantaneous wound healing [ 53 ]. In addition, the biodegradable thiolated bandage with implanted ZnO-NPs demonstrated an enhanced therapeutic agent for treating surgical site infections, satisfying the criteria for the optimal surgical dressing [ 54 ].
Similarly, the functionalization of bacterial nanocellulose (BNC) grafted with aminoalkyl silane and doped with Pullan-ZnO-NPs electrospun nanofibers (A-g-BNC/Pul-ZnO) exhibited superior performance in blood clotting and antibacterial activity that had a 5 log value higher than BNC, and was found to be safe in terms of cytotoxicity as tested in L929 fibroblast cells. It offers growth and proliferation, which was corroborated by the rat model where the scaffolds revealed rapid wound healing due to re-epithelization, and blood vessel and collagen formation [ 55 ]. An in vitro study reported that the bionanocomposite-based 3D chitosan/pectin/ZnO-NP porous films demonstrated no cytotoxicity (biocompatibility) and cell growth and migration (proliferation) for primary human dermal fibroblast cells (HFCs), suggesting a benign biomaterial for promoting wound healing [ 56 ].
Moreover, 3D-printed alginate-ZnO-NP hydrogels exhibited enhanced pore sizes, stiffness, and no detrimental effect on STO-fibroblasts or cell viability, making them a suitable scaffold for wound healing [ 57 ]. Generally, hydrogels are preferred with ZnO-NPs because they have a slow release of nanoparticles from the preparation, which reduces the cytotoxicity from ROS formation and improves wound healing. The above analyses support the findings of Saddik et al., where it was demonstrated that azithromycin-ZnO-NPs impregnated into an HPMC gel enhanced bacterial clearance and epidermal regeneration, which eventually stimulated tissue formation, leading to the rapid healing of the infected wound [ 58 , 59 ]. Another bioscaffold made from sodium alginate gum acacia ZnO-NP hydrogels showed a similar potential in expediting healing in terms of reducing inflammation and produced no scar at the excision wound on rabbit skin [ 60 ]. Thus, topical zinc application has been shown to improve the process of re-epithelialization, reduce inflammation, and inhibit the growth of bacteria in the case of foot ulcers and other topical wounds [ 61 ].
2.5. Anti-Inflammatory Activity of ZnO-NPs
The inflammatory response in the human body is a complicated process that involves immune system activation and the release of pro-inflammatory cytokines such as interleukin (IL)-1, -6, -12, -18, TNF-α, INFγ, and granulocyte-macrophage colony-stimulating factor (GMS-CF) [ 62 ] ( Figure 4 ). Nuclear factor-kappa b (NF-κβ) is a key transcription factor that regulates the expression of many genes that encode pro-inflammatory mediators, such as COX-2 and iNOS, which increase the synthesis of pro-inflammatory mediators such as PGE2 and nitric oxide [ 63 ]. The ZnO-NPs act as anti-inflammatory agents as they have been shown to inhibit the release of pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) expression, myeloperoxidase, the NF-κβ pathway, and mast cell degranulation [ 64 ]. The mRNA expression of pro-inflammatory cytokines was suppressed by the ZnO-NPs synthesized using Polygala tenuifolia in a dose-dependent manner [ 65 ]. In addition, ZnO-NPs, when doped with aluminum, have been shown to reduce the production of thymic stromal lymphopoietin (TSLP) and caspase-1 activation in mast cells, leading to lowering the expression of pro-inflammatory cytokines, IL-1, IL-6, and TNF-α [ 66 ]. In a comparative study of ZnO-NPs and the ZnO standard form, it was revealed that ZnO-NPs relatively lowered the carrageenan-induced paw edema and amplified the anti-inflammatory activity of the nonsteroidal anti-inflammatory drug, ketoprofen, when administered intraperitoneally [ 67 ]. However, both forms were ineffective when administered per os (po) and guarded the gastric mucosa against the gastric ulcer induced by the administration of ketoprofen. ZnO-NPs have been discovered to have an excellent capping of flavones such as isoorientin, orientin, isovitexin, and vitexin, which have a potent anti-inflammatory response in a variety of ways, including the inhibition of cyclooxygenase, phospholipase A2, and lipoxygenases (enzymes that produce eicosanoids), resulting in a decline in leukotrienes and prostanoids [ 68 ].

Mechanism of anti-inflammatory potency of ZnO-NPs.
2.6. Orthopedic Implants and Bone Healing Activity of ZnO-NPs
Diseases such as osteoporosis, arthritis, and fibrous dysplasia can cause bone abnormalities and lasting disability. The implantation of orthopedic implants and scaffolds has significantly aided in the treatment of these bone diseases and abnormalities since they consist of materials with positive effects on the bone regeneration process [ 69 ]. Orthopedic implants are usually made of metals and alloys such as titanium, nitinol, stainless steel, and Co-Cr alloys [ 70 ]. Over the last several decades, these metals have been excessively utilized for deformity correction, joint replacements, fracture fixation, soft tissue anchorage, and most importantly, for accelerating bone growth [ 71 ]. Unfortunately, orthopedic implants are not free from side effects once placed in the body, leading to infections, limited corrosion resistance, low cell proliferation, excessive inflammation, and poor osseointegration [ 72 , 73 ]. If infection occurs, the implant loosens, bones take longer to heal, and sometimes prolonged suffering leads to death [ 74 ]. If corrosion occurs, toxicity incites, weakening the implant [ 70 ]. Metal oxide nanoparticles such as ZnO, magnesium oxide (MgO), iron oxide, zirconium oxide, titanium oxide, and silver oxide, when used with orthopedic implants, provide a wide range of solutions for the issues mentioned earlier. Figure 5 highlights how the ZnO coating on the implant helps in osteointegration, the prevention of biofilm formation, and the prevention of premature corrosion of the implant.

A diagram showing the effects of metal oxide (e.g., ZnO) coating on the orthopedic implant and bone.
Biodegradable metals (BMs) such as Zn, Mg, Ca, and Fe have additional desirable properties for their applications in orthopedics [ 75 , 76 ]. During biodegradation, these metals release metal ions, metal oxides, and hydroxides. The close interaction between the degraded by-product and the stem-progenitor cells at the interface is what gives bone tissue implants their bioactivity [ 77 ]. Therefore, altering the implant’s chemical composition can have a significant impact on the treatment’s effectiveness [ 77 ]. The integration of growth factors into bone tissue scaffolds and implants is a prominent area of interest in the research. Protein growth factors such as insulin-like growth factors and bone morphogenetic proteins can activate cellular signaling cascades to stimulate active healing [ 78 ], including angiogenesis, a crucial step in bone tissue regeneration [ 79 ].
Zn and ZnO have emerged as a recent alternative among these BMs and are commonly employed in combination with other biomaterials to gain diverse qualities in antibacterial ability, cytocompatibility, and corrosion resistance [ 80 , 81 ] due to their customizable size manipulation from micro to nano [ 82 ]. Bone is the principal repository for Zn since it stores about 30% [ 83 ], and Zn helps in the maintenance of bone mass [ 84 ]. It maintains the shape of cell membranes [ 83 ] and is crucial for bone quality. In osteoblastic cells, Zn can directly activate aminoacyl-tRNA synthetase, a rate-limiting enzyme during protein translation [ 85 ], accelerate cellular protein synthesis [ 86 ] and increase the gene expression of the transcription factor Runx2, which is connected to osteoblast differentiation. Zn also prevents the production of osteoclast-like cells from marrow cells, which minimizes osteoclastic bone resorption [ 87 ]. Bone mineralization is aided by the enzyme alkaline phosphatase, which employs zinc as a co-factor [ 88 , 89 , 90 ]. In an in vitro experiment, Zn doses between 7 and 20 nM enhanced alkaline phosphatase activity, but Zn concentrations over 5 µM decreased alkaline phosphatase activity [ 88 , 91 , 92 ]. These findings imply that a Zn shortage may affect bone growth by impairing osteoid mineralization or calcified cartilage production linked to endochondral ossification. Many distinct types of skeletal defects in prenatal and postnatal development are linked to Zn deficiency, and a study demonstrated that osteoporotic patients had lower skeletal Zn levels than the control [ 93 ]. By promoting collagen production, alkaline phosphatase (ALP) activity, and mineralization of bone nodules, Zn can improve osteogenesis ( Figure 6 ).

The diagram shows the functions of Zn in stimulating osteoblastic bone formation and mineralization. Zinc stimulates gene expression of various proteins including type I collagen, alkaline phosphatase, and osteocalcin in the cells. Zn is also known to increase the production of growth factors such as IGF-I and TGF-β1 in osteoblastic cells.
Yusa et al. showed that eluted Zn ions from Ti surfaces promoted osteoblast activities in human bone marrow-derived mesenchymal stem cells (hBMSCs) and dental pulp stem cells (hDPSCs) [ 94 ]. In both cell types, the eluted Zn ions stimulated the expression of osteoblast marker genes (collagen type I, ALP, and osteocalcin) and calcium deposition. In hDPSCs, Zn ions further stimulated the expression of Runx2, vascular endothelial growth factor A, and transforming growth factor-beta. Additionally, apoptosis rates in MC3T3-E1 cells increased from 7% in normal media to 75% and 90% when the cells were grown in Zn-deficient or Zn-free media, respectively [ 95 ]. Numerous studies have shown that increasing ZnO content improved antibacterial capacity [ 96 , 97 , 98 ], and nanocoating with ZnO may minimize S. epidermidis adherence, thus enhancing the efficacy of orthopedic implants [ 99 ]. Lin, M.-H. et al. detected that the chitosan/ZnO-NP coating showed 1.2-fold stronger antibacterial activity against E. coli than the chitosan coating alone and actively prevented the formation of biofilm [ 100 ].
Similar to Zn and ZnO, another degradable metal such as Mg provides similar benefits for tissue healing [ 101 ]. Adhikari, U. et al. mimicked the nanostructured architecture and chemical makeup of natural bone tissue matrices with a 3D scaffold made from chitosan, carboxymethyl chitosan, calcium phosphate monobasic, and magnesium oxide. This scaffold also served as a source for soluble metal ions that are beneficial to osteoblast cells and offers a favorable background to promote biomineralization [ 102 ]. Pure Mg corrodes too quickly in physiological pH and produces excessive hydrogen gas, which is its biggest drawback; thus, efforts to use the metal oxide coating in orthopedic applications have been limited [ 101 ]. In addition, the inclusion of biodegradable ZnO-NPs in polycaprolactone enables the gradual release of zinc, which has the potential to improve mesenchymal stem cell (MSC) differentiation as an added advantage. Although osteogenic differentiation was improved on scaffolds with an increased concentration of ZnO, MSC chondrogenic differentiation was boosted on scaffolds with a reduced proportion of ZnO [ 103 ].
2.7. Antidiabetic Action of ZnO-NPs
Diabetes is a metabolic disorder characterized by persistent hyperglycemia. Zinc has been discovered to have an important role in the production, storage, and secretion of insulin [ 104 ]. Furthermore, it improves insulin signaling through pathways, such as elevated PI3K activity, insulin receptor tyrosine phosphorylation, and the inhibition of glycogen synthase kinase [ 105 ]. It has been reported that zinc’s insulin-mimicking activity leads to enhanced lipogenesis and decreased nonesterified fatty acid release from adipocytes [ 106 ]. ZnO-NPs are more frequently chosen for antidiabetic effects over other metal nanoparticles because they increase the expression of GLUT-4 and INS genes due to the confluence of factors such as the enhanced cellular permeation of biosynthesized ZnO-NPs, the promotion of glycolysis via hepatic glycogenesis, and the elevation of insulin levels. Moreover, it imposes synergistic effects on the expression and activity of increased glucokinase and the expression levels of IRA and GLUT-2 [ 107 ].
A study revealed that zinc combined with insulin acts as an autocrine molecule, increasing GSIS from rat-isolated pancreatic islets [ 108 ], and interacts with several components of the insulin transduction system, facilitating glucose metabolism and insulin mRNA expression in hepatic tissue of diabetic rats [ 109 ]. In an alloxan-induced diabetic model, rats administered with 96 mg/dL of ZnO-NPs synthesized from the seed extract of Silybum marianum L. had considerably lower fasting blood sugar (FBS) levels than rats fed with 117 mg/dL of insulin, 110 mg/dL of zinc oxide, and 120 mg/dL of crude extract, implying the potent antidiabetic activity of ZnO-NPs. Antidiabetic medicinal plants have also been used to synthesize ZnO-NPs and studied for antidiabetic effects, such as Rheum ribes [ 110 ] and Cosus igneus [ 111 ]. Similarly, the antidiabetic effect of ZnO-NPs synthesized from the flower extract of Senna auriculata [ 112 ] and leaf extract of Andrographis paniculata was studied in terms of α-amylase inhibitory activity, where it showed a lower IC 50 value (121.42 µg/mL) than the leaf extract of A. paniculata (149.65 µg/mL) and ZnNO 3 (178.84 µg/mL) [ 113 ]. Moreover, the antidiabetic activity of ZnO-NPs synthesized from Withania somnifera was monitored in terms of inhibition of α-amylase and α-glucosidase, showing 90% and 95% inhibition, respectively, at 100 µg/mL [ 114 ]. According to the findings of these studies, ZnO-NPs have a substantial antidiabetic effect in terms of glucose and insulin levels, glucose tolerance, and diabetic dyslipidemia.
2.8. Antioxidant Activity of ZnO-NPs
In the modern world, the ingestion of some oxidized meals is associated with numerous serious ailments, such as hepatomegaly or necrosis of epithelial tissues, because they are capable of producing lipid peroxides and other toxic-free radicals [ 115 , 116 , 117 ]. Various natural and synthetic antioxidants are utilized to neutralize these damaging free radicals, but they have drawbacks such as high reactivity and toxicity when compared to the nanoparticles synthesized these days [ 118 , 119 ]. Das et al. investigated the antioxidant potential of ZnO-NPs and revealed that the antioxidant activity of ZnO-NPs is due to the transfer of electron density from oxygen to the odd electron located at the nitrogen atom in DPPH (2,2-diphenyl-1-picrylhydrazyl), resulting in a reduction in the intensity of the n→π* transition at the 517 nm wavelength [ 120 ].
The previous finding showed that the percentage of inhibition of free radicals by ZnO-NPs on DPPH increases along with that of the concentration, explaining the ZnO-NPs’ promising antioxidant potential [ 121 ]. Similarly, the antioxidant activity of ZnO-NPs synthesized using the Aquilegia pubiflora leaf extract was monitored through four different assays (total antioxidant capacity—TAC, total reducing power—TRP, free radical scavenging assay—FRSA (DPPH), and Trolox antioxidant assay—ABTS) for a better evaluation, and the obtained results in terms of ascorbic acid equivalent per milligram (µg AAE/mg) were directly proportional to the concentration of ZnO-NPs in each assay [ 68 ]. In addition to that, similar studies were carried out using ABTS, DPPH, hydrogen peroxide, and super peroxide scavenging assays, where the DPPH assay exhibited direct dose-dependent behavior and the order of antioxidant activity was as follows: ABTS > DPPH > SOR > H 2 O 2 [ 122 ]. Furthermore, several plant sources such as Salvia hispanica [ 123 ], Borassus flabellifer [ 124 ], and Punica granatum [ 125 ] have been utilized for evaluation of the antioxidant activity of ZnO-NPs. Generally, the antioxidant behavior of ZnO-NPs is due to the reducing ability of NPs and the phytochemicals adsorbed/capped on the surface of ZnO-NPs [ 126 ]. This reveals the unparalleled antioxidant capacity of ZnO-NPs.
2.9. Antiviral Action of ZnO-NPs
ZnO-NPs have been reported to exhibit significant antiviral activities against a plethora of viruses, such as herpes simplex virus (HSV), human papillomavirus (HPV), human immunodeficiency virus (HIV), hepatitis C and E virus (HCV, HEV), and severe acute respiratory syndrome coronavirus (SARS-CoV) [ 127 ]. The mechanism of action underlying the antiviral potency of ZnO-NPs is the stimulation of the innate and adaptive immune response via toll-like receptor signaling pathways and proteins down streaming, which results in the production of pro-inflammatory cytokines that inhibit the virus. Zn 2+ ions exhibit antiviral properties by preventing infection, inactivating virus adsorption/entry, blocking coating, impeding replication, assembly, and release during the virus’s life cycle, and producing reactive oxygen species [ 128 , 129 , 130 , 131 , 132 ]. Zinc inhibits the entry of viruses and viral polyprotein translation, as well as inhibiting viral RNA-dependent RNA polymerase activity, and has been shown to modulate the host immune response to limit viral replication. It is a mediator in the LPS (bacterial lipopolysaccharide)-induced TLR4 (toll-like receptor 4)-dependent MyD88 (myeloid differentiation primary response protein 88) signaling cascade, which results in early NF-κβ activation (nuclear factor-kappa b). This triggers the production of pro-inflammatory cytokines such as TNF-α (tumor necrosis factor-α), IL-1 (interleukin-1), and IL-6 to increase (interleukin-6), which plays a crucial role in the control of viral pathogens [ 133 , 134 ]. Moreover, ZnO-NPs can absorb UV–Vis light, dissociate water molecules, and release Zn 2+ ions, generating ROS such as hydrogen peroxide, hydroxyl radicals, and superoxide that disrupt the lipids, proteins, carbohydrates, and DNA of the virus, leading to its death [ 135 ]. According to Jana et al., polysaccharide-encapsulated ZnO-NPs showed exceptional antiviral action against human cytomegalovirus (HCMV), with cell survival rates of 93.6% and 92.4% at 400 µg/mL [ 136 ]. A survey reported that ZnO-NPs and PEGylated ZnO-NPs have inhibitory effects on the H1N1 influenza virus, with PEGylated ZnO-NPs showing higher anti-influenza activity with less cytotoxicity on MDCK-SIAT1 cells than ZnO-NPs, indicating that PEGylation on the surface of ZnO-NPs enhanced antiviral activity while reducing cytotoxicity [ 137 ]. A recent study on ZnO-NPs demonstrated compelling antiviral activity against SARS-CoV-2 at a very low concentration (IC 50 526 ng/mL), and it was found that ZnO-NPs can produce a large number of free radicals which ultimately induce significant damage to the membrane proteins of SARS-CoV-2. However, ZnO-NPs displayed cytotoxic levels (CC 50 292.2 ng/mL) against VERO-E6 cells [ 138 ]. Similarly, they exhibit excellent antiviral activity against the Chikungunya virus [ 139 ], and these findings suggest that ZnO-NPs might be good antiviral agents.
2.10. Cardioprotective Action of ZnO-NPs
As ZnO-NPs possess potent antioxidant activity, this gives us an idea about their use in the scavenging O 2 • — free radicals, which on the other side, possibly have cardioprotective effects. The O 2 • — free radicals are produced from lipid peroxides obtained from today’s fast foods and are made up of several flavoring/bleaching agents such as monosodium glutamate (MSG), which have several adverse effects on the heart, liver, kidney, testis, pancreas, brain, and other various tissues and organs with signs of inflammation [ 140 , 141 , 142 ]. These free radicals must be scavenged using ZnO-NPs to reduce the adverse effects of oxidative stress produced from the heart failure marker, lipid peroxidation (LPO), and lactoperoxidase-like reactive oxygen species free radicals. A study on the alleviation effect of the ZnO-NP/GTE complex on rats, through feeding two dosages of MSG and a dose of ZnO-NP/GTE (10 mg/kg) by oral gavages daily for 30 days, revealed that there was a reduction in LPO markers such as O 2 • — free radicals with a significant improvement in the level of endogenous antioxidants such as SOD, CAT, GSH, and GPx in cardiac tissue, indicating the protection against oxidative stress [ 143 ]. Thus, ZnO-NPs are believed to restore abnormal cardiac myofiber, implying their cardioprotective potential.
2.11. Anthelminthic Action of ZnO-NPs
ZnO-NPs have a strong anthelminthic effect, which is achieved by inducing oxidative stress by producing hydroxyl ions and ROS, which induces helminth membrane damage by electrostatic binding [ 144 , 145 ]. An in vitro study of ZnO-NPs on Gigantocotyle explanatum [ 146 ] revealed that they possess effective anthelminthic properties in higher concentrations. Flukes survive at lower quantities by increasing the activity of their intracellular antioxidant enzymes, SOD and GST, which scavenge reactive oxygen species [ 147 ], whereas with higher concentrations, SOD and GST possibly become saturated due to overproduction of ROS and hydroxyl ions, which leads to detoxification in flukes. These findings demonstrate sufficient evidence for the anthelminthic potential of ZnO-NPs.
3. Approaches for Synthesizing ZnO-NPs
ZnO-NPs are typically synthesized by utilizing physical, chemical, and biological processes that utilize either top-down or bottom-up approaches ( Figure 7 ). The cutting, grinding, or attrition of larger particles, followed by the formation of smaller particles at the nanoscale level, is referred to as a top-down technique. This method is commonly used for nanoparticle synthesis on a small scale [ 148 ]. The bottom-up approach is the process of synthesis of nanoparticles by gathering already miniaturized atoms/molecules through the application of chemical and physical methods. It is a cheaper method and faster than the top-down approach [ 149 ].

Synthesis approaches for ZnO-NPs.
3.1. Physical Methods
Physical methods are used to synthesize ZnO-NPs by attracting smaller molecules and atoms to produce nanoscale-sized particles that employ physical forces. Physical methods comprise ball milling, sputtering, physical vapor deposition, laser ablation, ion implantation, and electric arc deposition. Ball milling is a nonequilibrium phenomenon in which materials of a larger size are crushed with a ball mill due to collision with high-energy balls. The ball milling process has efficient production rates and is easier and more cost-effective. Salah et al. suggested that 15 spherical balls with a circumference of 20 mm concealed in a 500 mL bowl be used to form nanostructures of ZnO in a study on the antibacterial effectiveness of ZnO-NPs [ 149 ]. Laser ablation methods refer to the process of the removal of particles from the solid and liquid interface using a laser beam as an energy source. A study conducted by Mintcheva et al. provides a piece of evidence that the millisecond-pulsed laser ablation technique produced rod-shaped ZnO-NPs with lengths ranging from 40 to 110 nm and an average diameter of 30 nm [ 150 ]. Physical vapor depositions are a frequently used method in which the deposition of metals coating the surface involves two phenomena, such as evaporation and sputtering. Sputtering is the process of expelling particles from the surface by impacting high-energy particles with plasma ions [ 151 ]. Thermal evaporation is another physical approach in which powdered or condensed products are heated to a higher temperature, evaporation occurs, and the resulting vapors condense to form desirable nanoparticles under controlled conditions such as pressure, temperature, humidity, substrate, and so on [ 152 ].
3.2. Chemical Methods
The chemical methods for synthesizing ZnO-NPs are categorized based on their physical state, which includes solid-phase, liquid-phase, and gas-phase synthesis. Liquid-phase synthesis is a widespread method and a viable alternative to gaseous-phase synthesis. For liquid-phase synthesis, the sol-gel process, colloidal methods, precipitation and co-precipitation methods, microemulsion method, hydrothermal synthesis, and solvothermal and sonothermal methods can be utilized, whereas inert gas condensation methods and pyrolysis can be used for vapor-phase synthesis [ 153 ].
3.2.1. Liquid-Phase Synthesis
The sol-gel process is the process of conversion of prepared colloidal solution (sol) into gel through hydrolyzation, condensation, and polymerization reactions. Zinc acetate hydrate in alcohol is the most used precursor for the synthesis of ZnO-NPs [ 154 ]. Khan and companions synthesized pure and uniform thorn-like ZnO-NPs of a size < 50 nm for the first time by the sol-gel method [ 155 ]. Similarly, precipitation and co-precipitation methods involve the formation of a precipitate when inorganic alkalis act as a reducing agent combined with zinc salt. Sodium hydroxide and zinc sulfate heptahydrate are used as precursors, and by adjusting reaction conditions, these precipitates were washed and calcined at the requisite temperature to produce nanoparticles with the desired shape, size, and characteristics [ 156 ].
Solvothermal synthesis is a technique for facilitating a precursor interaction during synthesis by utilizing a solvent at moderate to high pressure (1–10,000 atm) and temperature (100–1000 °C) [ 157 ]. Hydrothermal synthesis, on the other hand, employs water and is normally performed below the supercritical temperature of the water, i.e., 374 °C. The microemulsion is another technique of synthesizing the thermodynamically stable dispersion of two immiscible liquids, namely, water and hydrocarbons. In general, two forms of microemulsions are utilized, such as oil-in-water (O/W) and water-in-oil (W/O), with the latter being predominantly used for the preparation of NPs by dispersing the metal salt (Zinc salt) precursor in the aqueous phase. Surfactant- and co-surfactant-charged hydrophilic groups aid to minimize interfacial tension between two phases and enhancing colloidal stability [ 158 ].
3.2.2. Gas-Phase Synthesis
The aerosol pyrolysis method is the most commonly used gas-phase synthesis method, in which aerosol droplets dispersed in the gas phase generate aerosol droplets of the precursor zinc salts when heated in a flame. The flame heating causes dehydration, which helps to reduce the size of particles in the nanoscale. The required material decomposes and sinters as a result of the heating over the flame [ 159 ]. Inert gas condensation is another major gas-phase synthesis technique. It involves evaporating zinc inside a heat-resistant compartment using a variety of heat sources, such as electron and laser beams or radio frequencies, and then condensing the vapors by migrating them to cooler chambers containing inert gas. Based on the catalyst, this approach is divided into two categories: physical vapor deposition intrigued without catalytic contact and chemical vapor deposition fascinated with catalytic interaction. It may cause agglomeration and coalescence of nanoparticles, which is a typical demerit of this process. Uhm and coworkers synthesized ZnO-NPs of a better shape and size with a 30 nm diameter by the levitational gas condensation method [ 160 ].
3.3. Green Synthesis
The terms “biological synthesis” and “green synthesis” are often used interchangeably. However, for a biological synthesis to be green, it should comply with the basic principles of green chemistry such as being environmentally friendly, no use of toxic chemicals, reduced derivatization, energy consumption, waste, and so on [ 161 ]. Here, green synthesis is the process of synthesizing nanoparticles by incorporating mainly cell extracts (microbial, plant, fungus, algae, etc.) into the substrate involving biofabrication, i.e., the capping of nanoparticles from natural products such as phytochemicals from plants and proteinous extracts from microorganisms and fungus without using any toxic chemicals. Green synthesis is to be nonhazardous, aligning with the principles of green chemistry. These methods provide merits of biocompatibility, cost-effectiveness, large-scale productivity, ecofriendliness, and being devoid of hazardous chemicals and adverse reaction conditions and are, therefore, an attractive alternative to traditional physical and chemical methods [ 162 ]. As such, microbial and plant extracts release phytochemicals that act as reducing agents as well as fabricating or stabilizing agents; this eliminates the dependence on industrial chemicals. On the contrary, if synthetic chemicals/solvents are employed to assist the reduction-stabilization process or to maintain pH in a green synthesis, such synthesis is better described as biochemical synthesis.
3.3.1. Plant-Mediated Synthesis of ZnO-NPs
A multitude of research supports the synthesis of crystalline ZnO-NPs by chelating a zinc complex with plant extracts. The aerial parts of plants, such as leaves and flowers, are commonly used in green synthesis. To optimize ZnO-NP synthesis, usually, reaction parameters such as temperature, pH, concentration, and time are adjusted. The appearance of a yellow coloration generally indicates the formation of ZnO-NPs, which is further confirmed by qualitative investigations such as UV–visible spectroscopy, SEM, and TEM [ 163 ].
The synthesis of ZnO-NPs with regulated shapes and sizes was accomplished by varying the concentration of plant extracts. Madan et al. synthesized NPs of varied sizes ranging from 9–40 nm and different shapes such as bud, cone, closed pine cone, bullet, and hexagonal disk by altering the concentrations of a plant extract from the leaves of Azadirachta indica [ 164 ]. The possible mechanism of the green synthesis has been explained by several researchers and the result is that the secondary metabolites and proteins present in the plant extracts act as capping and reducing agents which promote nanoparticle synthesis, whereas some studies have proposed that the nanoparticles of metal ions are formed due to the electrostatic interaction of plant proteins and metal ions. Proteins would reduce the metal ions, resulting in a change in the protein secondary structure, as well as in the formation of metal oxide nanoparticle seeds [ 163 , 165 ]. Plant components, from leaf to root, are extensively utilized in metal oxide nanoparticle synthesis because phytochemicals such as polyphenolic compounds, vitamins, polysaccharides, amino acids, alkaloids, terpenoids, etc. extracted from plants aid in the efficient bioreduction of metal ions for the synthesis of NPs that are stable and variable in structure and dimension. Bioreduction is the process of reducing metal ions or metal oxides to zero-valence metal NPs, fascinating in maintaining their stability. These techniques yield a large quantity of very pure nanoparticles that are free of contaminants [ 166 , 167 ]. Table 1 summarizes the key findings of extensive research on several plants employed in the synthesis of ZnO NPs.
Summary of the plant-mediated synthesis of zinc oxide nanoparticles.
| Biological Source | Used Plant Parts | Extraction Technique | Zinc Precursors; Condition | Size of Nanoparticles Synthesized (nm) | Morphology of Nanoparticles | References |
|---|---|---|---|---|---|---|
| Stem bark | Decoction at 60 °C | Zinc nitrate hexahydrate and sodium hydroxide, calcined at 350 °C | DLS: 82.31 at 0.05 molar and 110 at 0.01 molar SEM: 66.25, 82.52, 112.87 at 0.1, 0.05, and 0.01 molar concentration | Rod and hexagonal | [ ] | |
| Leaf | Solvent extraction at 90–95 °C | Zinc nitrate hexahydrate | XRD: 16.72 | Spheroid or rodlike | [ ] | |
| Leaf | Soxhlet extraction at 350 °C | Zinc nitrate | XRD: 11–40 | Hexagonal disk | [ ] | |
| Leaf | Boil | Zinc acetate dehydrate, sodium hydroxide | XRD: 5–25 DLS: 90–110 | Needle | [ ] | |
| Solid waste | Decoction | Zinc acetate, pH 12 | XRD: 19.5 | Rod | [ ] | |
| Leaf | Decoction at 70 °C | Zinc acetate dihydrate; 70 °C | XRD: 2.72 DLS: 68.1 | Spherical | [ ] | |
| Leaf | Decoction at 60 °C | Zinc nitrate | TEM: 37.05 ± 18.27 DLS: 50.8 | Spherical | [ ] | |
| Leaf | Boil | Zinc nitrate hexahydrate | TEM: 27 XRD: 17.47 DLS: 27 | Hexagonal wurtzite | [ ] | |
| Leaf | Decoction at 60 °C | Zinc nitrate: pH 10 | HRTEM: 12–53 | Spherical | [ ] | |
| Leaf | Decoction at 70 °C | Zinc acetate dihydrate; 70 °C | XRD: 2.72 DLS: 3.62 | Spherical | [ ] | |
| Leaf | Boil | Zinc nitrate hexahydrate | TEM: 40 FE-SEM: 38–49 XRD: 44.94 | Hexagonal, quasispherical | [ ] | |
| leaf, callus, and stem | Reflux at 100 °C | Zinc nitrate hexahydrate, calcined at 400 °C | XRD L-ZnO-NP: 8 and 15 C-ZnO-NP: 5 and 7 S-ZnO-NP: 9 and 12 | L-ZnO-NP: hexagonal wurtzite C-ZnO-NP and S-ZnO-NP: spherical | [ ] | |
| Fruit | Decoction at 150 °C | Zinc acetate dihydrate; calcined at 500 °C | TEM: 35.5 SEM: 43.3–83.1 XRD: 41.23 | Spherical and hexagonal | [ ] | |
| Oats | Oat biomass | Boil | Zinc nitrate hexahydrate, calcined at 400 °C | DLS, SEM, TEM: 100 XRD: 17.52 | Wurtzite and hexagonal | [ ] |
| Leaf | Decoction at 80 °C | Zinc nitrate hexahydrate at 450 °C | TEM: 20–50 XRD: 36.82 | Hexagonal wurtzite | [ ] |
3.3.2. Green Synthesis Using Bacterial Extracts
The nanoparticle synthesis using bacterial extracts is a complex and time-consuming technique of green synthesis. It is vital to ensure vigilant monitoring of the culture media throughout the process to avoid contamination. Otherwise, synthesized NPs could be less optimized and ineffective [ 2 ]. A study reported that the synthesis of ZnO-NPs can be carried out using Rhodococcus pyridinivorans and zinc sulfate as the substrate. The synthesized NPs were spherically shaped with a 100–130 nm size range confirmed through FE-SEM and XRD analysis [ 181 ]. The synthesis of nanoflowers (40 nm width and 400 nm height) with potent photocatalytic potency was also performed with B. licheniformis using the green synthesis technique [ 182 ]. The excellent antioxidant activity of NPs synthesized using Pseudomonas aeruginosa was also revealed, indicating that enhanced NP stability was attained due to the rhamnolipid of bacteria used. Thus, it is significant to consider that bacteria can be used as a better capping agent with outstanding stability and potency [ 183 ]. Green synthesis using a bacterial strain is well illustrated in Table 2 .
Summary of the bacteria-mediated synthesis of zinc oxide nanoparticles.
| Strain of Bacteria | Family | Size of Nanoparticles Synthesized (nm) | Morphology of Nanoparticles | References |
|---|---|---|---|---|
| | Nocardiaceae | FE-SEM: 100–120 XRD: 120–130 | Hexagonal phase and roughly spherical | [ ] |
| | Pseudomonadaceae | TEM: 35–80 XRD: 27, DLS: 81 | Spherical | [ ] |
| NMJ15 | Pseudomonadaceae | TEM: 6–21 XRD: 21 | Spherical | [ ] |
| | Pseudomonadaceae | AFM: 57.72 XRD: 42–64 | Oval and spherical | [ ] |
| Bacillaceae | TEM: 5–15 XRD: 11 | Hexagonal | [ ] | |
| Bacillaceae | TEM: 200 (nanopetal 40 nm width and 400 nm length) | Nanoflower | [ ] | |
| ( HM475278) | Enterobacteriaceae | SEM: 170–250 (at 30 min), 300–600 (at 60 min), 185–365 (at 90 min) | Spherical and nanoflower | [ ] |
| Microcoleaceae | TEM: 30–55 XRD: ≈45 | Spherical | [ ] | |
| sp. EAZ03 | Desertifilaceae | TEM: 88 XRD: 60–80 | Rod | [ ] |
| sp. 2C8 and sp. VLA (cell-free extract) | Alteromonadaceae Vibrionaceae | 2C8-TEM: 10.23 ± 2.48 VLA-TEM: 20.26 ± 4.44 | Hexagonal wurtzite | [ ] |
3.3.3. Green Synthesis Using Fungal Extracts
Due to the efficient and large-scale productivity, lower cost, and convenient processing, numerous fungal strains are being used for the green synthesis of ZnO-NPs over bacteria [ 2 ]. Fungi are more tolerable and have better metal bioaccumulative properties than bacterial strains, making them a stronger candidate for nanoparticle synthesis [ 191 ]. A study found that fungal strains such as Candida albicans could be employed to synthesize quasispherical-shaped ZnO-NPs [ 192 ]. Similarly, the mycelia of Aspergillus fumigatus were used to make spherical aggregate-shaped NPs, which agglomerate into a larger size after a few days, indicating the stability and potent capping activity of fungus as a substrate [ 193 ]. Some examples of fungal-mediated synthesis are included in Table 3 .
Summary of the fungal-mediated synthesis of zinc oxide nanoparticles.
| Fungal Strain | Family | Size of Nanoparticles Synthesized (nm) | Morphology | References |
|---|---|---|---|---|
| Trichocomaceae | SEM: 61 ± 0.65 XRD: 41 | Spherical Crystalline wurtzite | [ ] | |
| Saccharomycetaceae | XRD: 25, SEM: 15–25, TEM: ~20 | Hexagonal wurtzite, quasispherical | [ ] | |
| Trichocomaceae | DLS: 1.2–6.8 | Oblate spherical and hexagonal | [ ] | |
| Trichocomaceae | SEM: 50–120 | Spherical | [ ] | |
| Xylariaceae | TEM: 30–50, average: 34 SEM: 40–55 DLS: 30–50 XRD: 35–45 | Rod and hexagonal | [ ] |
3.3.4. Green Synthesis Using Microalgae and Macroalgae
Algae are photosynthetic organisms that are made up of single or multiple cells and lack essential components such as roots, stems, and leaves. Algae are classified into two types, macroalgae, and microalgae, as well as three groups, Rhodophyta (red pigmented), Phaeophyta (brown pigmented), and Chlorophyta (green pigmented). Algae have a limited significance in the synthesis of ZnO-NPs and are better suited for the production of other metal nanoparticles such as silver and gold nanoparticles. Microalgae are commonly employed for the green synthesis of NPs because they have a greater potential to minimize metal toxicity through the biodegradation process [ 198 ]. ZnO-NPs are typically synthesized using algae from the Sargassaceae family. Sargassum muticum was employed to make hexagonal wurtzite-shaped ZnO-NPs [ 199 ]. Similarly, nanoparticles of spherical, radial, triangular, hexagonal, and rod shapes were synthesized from S. myriocystum [ 200 ]. Furthermore, Chlamydomonas reinhardtii , a species of the Chlamydomonaceae family, was used to synthesize various-shaped NPs, such as nanorods, nanoflowers, and porous nanosheets [ 201 ]. Table 4 summarizes the ZnO-NPs synthesized by some of the algae.
Summary of the algal-mediated synthesis of zinc oxide nanoparticles.
| Algae Strain | Family | Size of As-Synthesized Nanoparticles (nm) | Morphology of the Nanoparticles | Surface Functional Groups | References |
|---|---|---|---|---|---|
| | Sargassaceae | FE-SEM: 30–57 XRD: 42 | Hexagonal wurtzite | Sulfate group asymmetric with stretching band, asymmetric C–O band coupled with C-O-SO and -OH group, sulfated polysaccharides | [ ] |
| Sargassaceae | SEM: 50 DLS: 25–50 XRD: 15–50 | Spherical | 3432 and 1609 cm presence of O–H stretching, 500 cm below suggests a Zn–O stretching vibration | [ ] | |
| | Chlamydomonaceae | HR-SEM: 55–80 XRD: 21 | Rod | N–H bending band of amide I and amide II, C=O stretching of zinc acetate C=O, and C–O–C stretch of polysaccharide | [ ] |
| Sargassaceae | DLS: 46.6 AFM: 20–36 TEM: 76–186 | Rectangular, triangle, radial hexagonal, rod, and spherical shape | Carboxylic acid, with O–H and C=O stretching bands | [ ] | |
| Ulvaceae | TEM: 10–50, av.: 15 XRD: 5–15 | Triangle, hexagon, rod | 420 cm suggests ZnO, peaks at 1634.00, and 620.93 cm suggests ZnO stretching and deformation vibration | [ ] |
4. Characterization of ZnO-NPs
A plethora of studies suggests that the morphology and surface chemistry of nanoparticles influence their biodistribution, safety, and effectiveness in biological systems ( Figure 8 ). Characterization is the core tool for successful applications and the understanding of nanoparticles. Nanoparticle size characterization is complicated by the polydispersity of materials, yet it is important to determine the morphology since the nanoparticle size’s resemblance to biological moieties is assumed to impart many of their distinct nanomedicine capabilities. Optical microscopy cannot resolve nanostructures; therefore, electron microscopy is used to characterize the nanoparticles. SEM and TEM are used to characterize the shapes and sizes, but TEM is used more often because it uses more powerful electrons and presents high resolution and informative image details regarding the atomic scale-like morphology, aggregation state, and distribution, and observes the functionality of capping agents/phytochemicals in enclosing NPs. Some biological molecules such as liposomes and proteins do not deflect the electron beam sufficiently and are invisible to electromagnetic radiation; therefore, dynamic light scattering (DLS), a nondestructive approach that uses a monochromatic laser and is also known as photon correlation spectroscopy, is used to characterize these compounds in suspensions and solutions. Here, small changes in the intensity of scattered laser light in the nanoparticle solution are regulated with a photon detector to analyze the hydrodynamic diameter and morphology of NPs [ 204 ].

Morphology of ZnO nanostructures: ( A ) needles, rods, and wires; ( B ) helixes and springs; ( C ) nanopellets/nanocapsules; ( D ) flower, snowflake, and dandelion; ( E ) peanut-like; ( F ) interwoven particle hierarchy; ( G ) raspberry, nanosheet/nanoplate; ( H ) circular/round or sphere-shaped. (Reprinted from [ 209 ]; open access under CC BY).
The characterization of nanoparticles in animal tissue is accomplished by energy dispersion X-ray analysis (EDX), which assists in identifying the elemental composition and linkage of metabolites and also facilitates the interpretation of biodistribution of synthesized nanoparticles. Furthermore, atomic force microscopy (AFM) helps in determining the 3D geography (height and volume) of NPs; Fourier transform infrared spectroscopy (FTIR)-attenuated total reflectance (ATR) is an easy and nondestructive technique that contributes metabolites, chemicals, etc. through the synthesis and capping of NPs; UV–visible-diffuse reflectance spectroscopy (UV-DRS) is used to study the optical property of colored samples where the reflectance measurements are utilized to investigate the surface plasmon resonance of metals and hypersensitive biological analysis [ 205 ]; thermal gravimetric-differential thermal analysis (TG-DTA) provides information about the thermal stability, phase transition, and effect of the oxidative as well as reductive environment; photoluminescence (PL) analysis is utilized to determine the band gap, and crystalline purity and impurities; and x-ray photoelectron spectroscopy (XPS) can be used to characterize the morphology, and bioactive surface and material surface chemistry of NPs [ 206 , 207 , 208 ].
ZnO is one of the most significant II-VI compound semiconductor materials in scientific research and technological applications with noncentrosymmetric structures and multiple shape-induced functions. By adjusting the hydrothermal reaction parameters (such as precursor concentration, reaction duration, and pH), several morphologies of ZnO, including microrods, hexagonal pyramid-like rods, and flower-like rod aggregates, have been synthesized, respectively, on glass substrates. The production of ZnO microrods is significantly influenced by the precursor concentration. With longer reaction times, ZnO crystals can change from hexagonal pyramids to rod-like laths. ZnO rod aggregates that resemble flowers are produced at higher pH levels. The findings could provide a strategy for producing ZnO crystals in a certain desirable form [ 210 ]. Similarly, in a recent study, Doustkhah et al. hydrothermally transformed zinc-based metal-organic frameworks into ZnO nanostructures with temperature-dependent tunable structures and catalytic activity, which at an elevated temperature displayed high crystallinity and better dye degradation efficiency than at a lower temperature [ 211 ].
Most of the group II-VI binary compound semiconductors crystallize as hexagonal wurtzite or cubic zinc-blende, with each anion surrounded by four cations at the corners of a tetrahedron. The iconicity of the II-VI compound semiconductor ZnO lies at the interface between covalent and ionic semiconductors. Wurtzite, blende, and rocksalt are potential ZnO crystal formations. Wurtzite is the most thermodynamically stable of these crystal forms at room temperature, but blende is stable when developed on a cubic substrate and rocksalt is stable when synthesized at very high temperatures [ 212 ]. In contrast to the zinc-blende structure, which has two interpenetrating face-centered-cubic (fcc) sublattices that are displaced along the body diagonal by one-quarter of a body diagonal, the wurtzite structure is made up of two interpenetrating hexagonal-closed-packed (hcp) sublattices. Due to the decrease in lattice dimensions, which favors iconicity over a covalent nature, and the structure’s six-fold coordination, wurtzite can undergo the same transformation as other II-VI semiconductors to become rocksalt [ 212 ].
5. Conclusions
This review aimed to explore the synthesis, characterization, and biological activities of ZnO-NPs, illustrating their mechanism of action. Extensive discussion was centered on the green synthesis approach and its biomedical applications. The pathways of different bioactivity were explained, with special emphasis on ZnO-NPs’ biopotency with regard to antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, orthopedic implants, bone healing, and cardioprotective activity, along with the concise interpretation of the green synthesis of nanoparticles using biological sources. The importance and significance of ZnO-NPs in pharmaceutical and biological sectors have attracted scientists to perform an extensive study of their applications in multiple ailments. Green synthesis is an eco-friendly approach that reduces costs, increases production, and improves biocompatibility in humans. Biofabrication with natural compounds helps to stabilize the nanoparticles with reduced toxicity and higher reduction potential. ZnO-NPs possess several compelling pharmacological activities. Special focus should be given to ZnO-NP generation through plant-mediated synthesis, bearing tremendous applications in the fields of pharmaceuticals, food, and cosmetics. The advancement of nanotechnology in the formulation of metal oxide nanoparticles can contribute to the reduction in the dosage used with optimum desired effects and low toxicity.
Acknowledgments
We are thankful to Arpita Roy, Sharda University, India, for her feedback on the manuscript.
Abbreviations
ZnO-NPs: zinc oxide nanoparticles; ROS: reactive oxygen species; SOD: superoxide dismutase; GSTs: glutathione S-transferases; NPs: nanoparticles; SEM: scanning electron microscopy; TEM: transmission electron microscopy; XRD: X-ray diffractometer; DLS: dynamic light scattering; EM: electron microscopy; HRTEM: high-resolution transmission electron microscopy; HRSEM: high-resolution scanning electron microscopy; FE-SEM: filed emission scanning electron microscopy; AFM: atomic force microscopy; GSH: glutathione; GPx: glutathione peroxidase; MSG: monosodium glutamate; DPPH: 2,2-diphenyl-1-picrylhydrazyl; NEFA: nonesterified fatty acid; iNOS: inducible nitric oxide synthase; PGE2: prostaglandin E2; NF-κβ: nuclear factor-kappa b; COX 2: cyclooxygenases 2; IL-1: interleukin-1; TNF: tumor necrosis factor; IL-6: interleukin-6; IL-12: interleukin-12; IL-18: interleukin-18; ATR: attenuated total reflection; EDAX: energy dispersion analysis of X-ray; PL: photoluminescence; XPS: X-ray photoelectron microscopy; TG-DTA: thermal gravimetric-differential thermal analysis; UV-DRS: UV–visible reflectance spectroscopy; BMs: biodegradable metals; ALP: alkaline phosphatase; hBMSCs: human bone marrow-derived mesenchymal stem cells; hDPSCs: human dental pulp stem cells; MDR: multidrug-resistant; HPMC: hydroxypropyl methylcellulose; FBS: fasting blood sugar; CAT: catalase.
Funding Statement
Tettey and Bhattarai acknowledge funding support in part from National Science Foundation (EiR-2100861) for their contribution to this review work.
Author Contributions
Conceptualization, N.P. and N.B.; methodology, A.K.M.; writing—original draft preparation, A.K.M., S.B., A.G., F.T., N.B., A.K.S. and N.P.; writing—review and editing, S.K., S.J. and D.P.B.; editing images, S.B. and F.T.; supervision and project administration, N.P. and N.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Influence of pH on the photocatalytic activity of ZnO nanoparticles
- Published: 08 January 2016
- Volume 27 , pages 4206–4215, ( 2016 )
Cite this article

- Iraj Kazeminezhad 1 &
- Azar Sadollahkhani 1
2157 Accesses
90 Citations
Explore all metrics
In this study, ZnO nanoparticles were fabricated by co-precipitation method. The synthesized nanoparticles possessed monodispersity with the average size 20–30 nm. Since the industrial effluents may not be at neutral pH, the effect of pH on the rate of degradation is important and need to be considered. In order to investigate the effect of pH on ZnO nanoparticles photocatalytic activity, the photocatalytic degradation of Rose Bengal, Methylene blue, and Bromocresol green dyes, was studied with different pH values. It was observed that the adsorption of the dyes onto ZnO nanoparticles surface is strongly dependent on the pH of the solution which plays an important role in photocatalytic degradation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
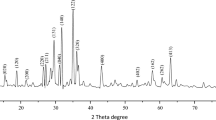
Synthesis and characterization of Zn3V2O8 nanoparticles: mechanism and factors influencing crystal violet photodegradation
Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods.
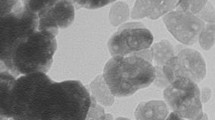
The effect of pH on the photocatalytic degradation of methyl orange using decorated ZnO nanoparticles with SnO2 nanoparticles
Q.I. Rahman, M. Ahmad, S.K. Misra, M. Lohani, Mater. Lett. 91 , 170 (2013)
Article Google Scholar
S.P. Buthelezi, A.O. Olaniran, B. Pillay, Molecules 17 , 14260 (2012)
A.M. Abdulkarem, E.M. Elssfah, N.-N. Yan, G. Demissie, Y. Yu, J. Phys. Chem. Solids 74 , 647 (2013)
S. Suwanboon, P. Amornpitoksuk, A. Sukolrat, N. Muensit, Ceram. Int. 39 , 2811 (2013)
X. Cai, Y. Cai, Y. Liu, H. Li, F. Zhang, Y. Wang, J. Phys. Chem. Solids 74 , 1196 (2013)
T. Madrakian, A. Afkhami, M. Ahmadi, Spectrochim. Acta A 99 , 102 (2012)
I.A. Siddiquey, T. Furusawa, M. Sato, N.M. Bahadur, M.M. Alam, N. Suzuki, Ultrason. Sonochem. 19 , 750 (2012)
G.M. Nair, M. Nirmala, K. Rekha, A. Anukaliani, Mater. Lett. 65 , 1797 (2011)
R. Yousefi, F. Jamali-Sheini, M. Cheraghizade, S. Khosravi-Gandomani, A. Sáaedi, N.M. Huang, W.J. Basirun, M. Azarang, Mater. Sci. Semicond. Process. 32 , 152 (2015)
M. Azarang, A. Shuhaimi, R. Yousefi, A.M. Golsheikh, M. Sookhakian, Ceram. Int. 40 , 10217 (2014)
F. Feng, C. Hao, H. Zhang, W. Xie, X. Wang, Y. Zhao, J. Mater. Sci.: Mater. Electron. 26 , 6704 (2015)
Google Scholar
S.V. Elangovan, N. Sivakumar, V. Chandramohan, J. Mater. Sci.: Mater. Electron. 26 , 8753 (2015)
I. Kazeminezhad, A. Sadollahkhani, M. Farbod, Mater. Lett. 92 , 29 (2013)
K. Vignesh, A. Suganthi, M. Rajarajan, S.A. Sara, Powder Technol. 224 , 331 (2012)
J. Tauc, A. Menth, J. Non-Cryst. Solids 8 , 569 (1972)
S.M. Lam, J.C. Sin, A.Z. Abdullah, A.R. Mohamed, Desalination 41 , 131 (2012)
U.G. Akpan, B.H. Hameed, Hazard. Mater. 170 , 520 (2009)
L.G. Devi, K.M. Reddy, Appl. Surf. Sci. 256 , 3116 (2010)
X. Li, Y. Hou, Q. Zhao, L. Wang, J. Colloid Interface Sci. 358 , 102 (2011)
Download references
Acknowledgments
The authors would like to thank Shahid Chamran University of Ahvaz for financial support.
Author information
Authors and affiliations.
Nanotechnology Lab, Department of Physics, Shahid Chamran University of Ahvaz, Ahvaz, Iran
Iraj Kazeminezhad & Azar Sadollahkhani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Iraj Kazeminezhad .

Rights and permissions
Reprints and permissions
About this article
Kazeminezhad, I., Sadollahkhani, A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J Mater Sci: Mater Electron 27 , 4206–4215 (2016). https://doi.org/10.1007/s10854-016-4284-0
Download citation
Received : 24 October 2015
Accepted : 02 January 2016
Published : 08 January 2016
Issue Date : May 2016
DOI : https://doi.org/10.1007/s10854-016-4284-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Methylene Blue
- Photocatalytic Activity
- Photocatalytic Degradation
- Calcination Temperature
- Rose Bengal
- Find a journal
- Publish with us
- Track your research
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Anna University
- Faculty of Technology
| Title: | Investigations on certain metal oxide based semiconductor nanomaterials for photocatalytic activities |
| Researcher: | Rajeswari, R |
| Guide(s): | |
| Keywords: | Engineering and Technology Engineering Engineering Chemical Semiconductor nanomaterials Metal oxide |
| University: | Anna University |
| Completed Date: | 2020 |
| Abstract: | Photocatalysis is an acceleration of a photoreaction using semiconductor catalyst by the activation of a light energy. It can be referred to the combination of both photochemistry and catalysis. As compared to metal, semiconductors are the best photocatalysts due to their distinctive bandgap structure which makes the photoexcitation. They also act as sensitizers due to their filled valence band and empty conduction band in the light induced redox reaction. Indeed, most of the semiconductor nanomaterials have some intriguing properties such as less toxic, cost efficient, reusable, stable, insoluble and easy to fabrication. Among them, the semiconductor metal oxides (ZnO, MoO3 and Nb2O5), metal sulfide (MoS2) and their heterostructure (ZnO-MoS2) are greatly attracted in the field of heterogeneous photocatalyst for the toxic dye removal applications due to their unique band gap energy and tunable physico-chemical properties. Thus, our focuses on the synthesis of different semiconductor nanomaterials by simple and ecofriendly method for waste water treatment applications. The preparation of zinc oxide (ZnO) nanoparticles (NPs) by green synthesis route using carica papaya leaf extract for photocatalytic application has been demonstrated. In this work, the phase pure ZnO NPs were synthesized via facile green synthesis method, where zinc acetate dihydrate was used as precursor and papaya leaf extract as reducing agent. The structure and phase formation of the synthesized material was confirmed by X-Ray diffraction and FT-IR analysis. The bandgap energy for the prepared ZnO NPs was calculated from the DRS spectra and it is found to be 3.32 eV. The surface morphology and phase purity of ZnO NPs was characterized by FE-SEM and EDX analysis, respectively. Electron microscopic analysis indicates that ZnO NPs are spherical in shape with particle size of ~50 nm. Importantly, the as synthesized ZnO NPs were used as efficient photocatalyst for Methylene Blue (MB) dye degradation. It is persistent to note that the prepared ZnO NPs almost completely degraded the MB dye under UV light condition after 180 min illumination tim newline |
| Pagination: | xxiii,121 p. |
| URI: | |
| Appears in Departments: | |
| File | Description | Size | Format | |
|---|---|---|---|---|
| Attached File | 210.77 kB | Adobe PDF | ||
| 241.25 kB | Adobe PDF | |||
| 700.81 kB | Adobe PDF | |||
| 461.9 kB | Adobe PDF | |||
| 169.17 kB | Adobe PDF | |||
| 633.24 kB | Adobe PDF | |||
| 310.64 kB | Adobe PDF | |||
| 7.61 kB | Adobe PDF | |||
| 194.09 kB | Adobe PDF | |||
| 108.1 kB | Adobe PDF | |||
| 985.87 kB | Adobe PDF | |||
| 307.25 kB | Adobe PDF | |||
| 918.81 kB | Adobe PDF | |||
| 894.66 kB | Adobe PDF | |||
| 1.38 MB | Adobe PDF | |||
| 1.4 MB | Adobe PDF | |||
| 1.11 MB | Adobe PDF | |||
| 230.06 kB | Adobe PDF | |||
| 365.6 kB | Adobe PDF | |||
| 125.35 kB | Adobe PDF | |||
| 372.04 kB | Adobe PDF |
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 November 2023
Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment
- A. El Golli 1 , 3 ,
- S. Contreras 2 &
- C. Dridi 1
Scientific Reports volume 13 , Article number: 20809 ( 2023 ) Cite this article
7984 Accesses
8 Citations
3 Altmetric
Metrics details
- Biosynthesis
- Chemical engineering
- Chemical physics
- Environmental impact
- Green chemistry
- Materials chemistry
- Materials science
- Nanoscale materials
- Nanoscience and technology
- Other nanotechnology
- Physical chemistry
The design of a green photocatalytic system that harnesses renewable and eco-friendly constituents holds the potential to offer valuable insights into alternative strategies for treating toxic multi-components in refinery water effluents. A significant challenge in implementing a practical and viable approach is the utilization of solar energy—an abundant, natural, and cost-effective resource—for photochemical processes within advanced oxidation processes. In this study, we explored the use of zinc oxide nanoparticles (ZnO NPs) as photocatalyst prepared via an environmentally friendly synthesis approach, resulting in the formation of crystalline wurtzite nanoparticles, with an average size of about 14 nm relatively spherical in shape. Notably, the extract derived from Moringa oleifera was employed in this investigation. These nanoparticles were characterized and validated using various characterization techniques, including X-ray diffraction, transmission electron microscopy, field emission scanning electron microscopy, and energy dispersive X-ray spectroscopy. For comparison, conventionally synthesized ZnO NPs were also included in the evaluations. The findings reveal that, under illumination, biosynthesized ZnO nanoparticles (NPs) exhibit photocatalytic performance in effectively breaking down the organic compounds present in synthetic petroleum wastewater. Photochemical analysis further illustrates the degradation efficiency of Green-ZnO, which, within 180 min of irradiation resulted in 51%, 52%, 88%, and 93% of removal for Phenol, O-Cresol. Under optimal loading conditions, NPs produced via the green synthesis approach perform better when compared to chemically synthesized ZnO. This significant improvement in photocatalytic activity underscores the potential of eco-friendly synthesis methods in achieving enhanced water treatment efficiency.
Similar content being viewed by others

Nanoconfinement steers nonradical pathway transition in single atom fenton-like catalysis for improving oxidant utilization

Removal of heavy metal ions from wastewater: a comprehensive and critical review
A photocatalytic redox cycle over a polyimide catalyst drives efficient solar-to-H2O2 conversion
Introduction.
Water is an indispensable valuable resource used in a variety of industrial operations and is essential to all kinds of life. Environmental pollution caused by dangerous chemicals has recently become one of the biggest issues facing industrialized countries. One of the industries that produce a lot of wastewater is the petroleum refining industry. Wastewater produced by the petroleum industries contains a variety of substances, mostly organic molecules (primarily aromatic and aliphatic hydrocarbons) and total solids dissolved (such as salts, barium, and strontium).
Crude oil includes considerable quantities of monoaromatic hydrocarbons including toluene, benzene, ethylbenzene, and xylene (BTEX), which are categorized under volatile organic compounds (VOCs), and trace levels of polycyclic aromatic hydrocarbons (PAHs) 1 . These toxic compounds find their way into the delicate ecological balance via the discharge of wastewater from petroleum industrial facilities, permeating the air, soil, and water, thereby exacerbating environmental pollution 2 . The ecosystem and living species are seriously threatened by a rise in toxins in the water bodies, which can have severe and long-lasting effects Indeed, this can harm aquatic life, disrupt the food chain, and potentially affect human health if contaminated water is used for drinking, irrigation, or other purposes 3 .
Refinery effluents are subject to strict regulations and monitoring to ensure that these potentially harmful compounds are controlled and reduced to safe levels before discharge into the environment. To combat the environmental challenges posed by the release of toxic compounds from petroleum industrial plants, it is essential to promote the adoption of recycling and sustainable practices in the industry. These efforts not only contribute to environmental protection but also offer economic benefits and support long-term water resource preservation 4 .
This urgent concern has prompted a concerted effort to discover renewable technologies for water remediation with the following key tenets: increased efficiency and self-sufficiency. Petroleum refineries commonly employ primary and secondary wastewater treatment techniques. In the primary treatment phase, oil–water separation is achieved through physical methods like sedimentation or dissolved air flotation. To tackle impurities, coagulation with chemicals like aluminum hydroxide or ferric hydroxide is utilized, forming sludge. Nevertheless, these technologies come with inherent drawbacks and constraints. Notably, they generate concentrated sludges necessitating further processing and discharge, which can impose financial constraints due to the substantial initial investments demanded 5 . In lieu of conventional treatment methods, Advanced Oxidation Processes (AOPs) offer a promising alternative for swiftly breaking down contaminants in aquatic environments. These innovative techniques involve the generation of hydroxyl radicals (OH.), among other reactive species, which can effectively interact with organic compounds and facilitate their complete mineralization 6 . Such processes include UV 7 ; O 3 /H 2 O 2 8 ; O 3 /UV 7 , 8 ; photo-Fenton and Fenton processes 4 , 9 and photo-catalysis 4 .
Among these techniques, solar photocatalysis is attracting considerable interest as a sustainable and environmentally friendly technology for petroleum refineries wastewater treatment owing to its ability to oxidize a wide range of organic pollutants.
Presently, a variety of semiconductor-based nanophotocatalysts have been applied in water pollution remediation, with a significant emphasis on metal oxide nanoparticles 10 , 11 , 12 . Notably, zinc oxide (ZnO) has garnered considerable attention in this regard. ZnO stands out due to its outstanding charge transport properties, characterized by a 3.3 eV bandgap and a high excitation binding energy of 60 meV. Moreover, it exhibits excellent chemical stability, non-toxic nature and long-term photo-stability. These distinctive properties together produce an impressive photocatalytic behavior 13 .
Fundamentally, when a semiconductor boasting a suitably wide band gap absorbs light energy surpassing its own, it prompts the migration of valence band electrons (e − ) to the conduction band (CB), creating vacancies, or holes (h + ), in the valence band (VB). These photo-excited electrons and holes subsequently initiate redox reactions with species whose redox potentials align appropriately. This interplay indeed induces reduction and oxidation reactions, giving rise to the production of superoxide (O 2 . ) and hydroxyl (OH . ) radicals, which play a pivotal role in breaking down the organic pollutant 14 , 15 .
Indeed, the generated hydroxyl radicals, known for their strong oxidation properties, will initiate the breakdown of the contaminants adhered to the photocatalyst’s surface, leading to the prompt formation of intermediate substances. These intermediates will ultimately transform into environmentally friendly compounds like carbon dioxide (CO 2 ) and water (H 2 O), as indicated in (Eq. 10 ).
Thus, the process of solar-induced photodegradation of toxic organic substances through redox reactions can be outlined in the following manner 16 , 17 :
Four aromatic and aliphatic hydrocarbons often discovered in refinery effluent were designated as target pollutants to evaluate their removal effectiveness by solar-assisted photocatalysis, specifically, Phenol, O-Cresol, Toluene, and Xylene. Figure 1 illustrates the redox reaction occurring during photocatalysis over ZnO NPs.
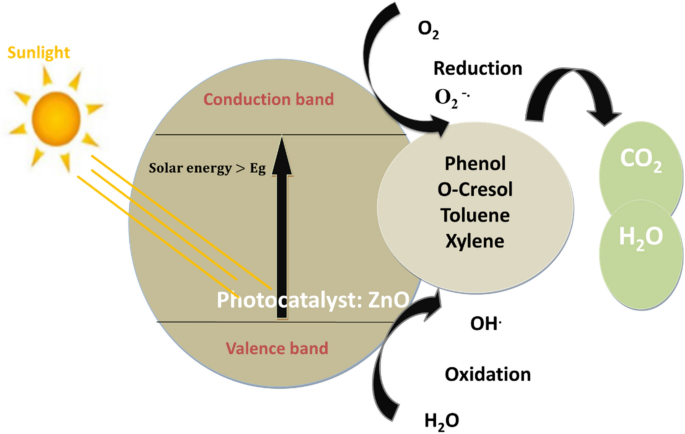
Scheme of PC mechanism occurring over ZnO.
Several methods for NPs synthesis are under consideration. They include both physical and chemical approaches, which, while effective, often entail high costs, time consuming, and environmentally toxic. The environmental compatibility is a significant advantage when it comes to applications involving water treatment, as it ensures that no harmful byproducts or residual toxicity are introduced during the remediation process. Indeed, in conventional processes, common reducing agents, such as sodium citrate, sodium borohydride, and various alcohols are widely known for their hazardous properties, their toxicity, flammability, explosiveness, and resistance to decomposition. Therefore, nowadays different researchers attempted to provide safer, non-toxic, and ecofriendly approaches for NPs fabrication. One such innovative approach is the biological synthesis of NPs. In the process of green synthesis, a natural extract such as microorganisms and/or plant extracts is harnessed as an environmentally sustainable alternative reducing and capping agent. Consequently, the resulting NPs are devoid of any remnants of organic solvents or toxic chemicals, rendering them inherently eco-friendly when introduced into the environment. This approach offers a distinct advantage over conventional methods, due to its simplicity, cost-effectiveness, environmental friendliness, and relative reproducibility 18 , 19 , 20 , 21 , 22 .
Based on several studies, green synthesis presents an alternative and promising approach to produce NPs that are safer, with reduced chemical toxicity, benefiting both human health and the environment 23 , 24 , 25 , 26 . Jayarambabu et al. aimed to synthesize ZnO NPs using Lawsonia inermis leaf extract and explore their potential toxicological impacts. The histopathological assessment revealed the safe use of biosynthesized ZnO NPs, confirming their non-toxicity and compatibility with biological systems, thus indicating their promise in the treatment of various diseases. This study conclusively establishes the harmlessness of the biogenic production of ZnO NPs on all vital organs 27 . Moreover, early literature has shown that, compared to conventionally produced NPs, biosynthesized ZnO NPs significantly suppress both bacterial and fungal diseases 28 . Furthermore, a recent review lends support to the utilization of environmentally-friendly produced ZnO NPs as feed additives, highlighting their potential to enhance immunity against viral infections 24 . ZnO NPs derived from natural resources have received approval from the United States Food and Drug Administration (FDA). They are classified as “GRAS,” which stands for “Generally Recognized As Safe.” 29 . Rashidian et al. 23 conducted a study to assess the toxicological effects of green synthesized versus commercial ZnO NPs on the immune responses within the skin mucus of carp. The results of this investigation revealed that green ZnO NPs exhibited significantly reduced immunosuppressive effects on important components of fish skin mucus. These green NPs hold immense promise for a wide range of applications in the realms of biology, agriculture, and environmental monitoring. In the future, they have the potential to significantly enhance ecological protection and conservation efforts 30 , 31 .
This study presents a green synthesis approach for the production of ZnO photocatalysts, designed to support sustainable and environmentally friendly water remediation processes. This method involves the use of safer precursors, the elimination of hazardous compounds, the reduction of energy consumption and the utilization of renewable natural resources.
The added value of our strategy toward water remediation achievements is articulated in 3 aspects:
Eco-friendly photocatalyst fabrication process, with the addition of few or no chemical compounds during the synthesis, which imposes the respectful aspect to the environment when released into the ecosystem.
Sustainable and cost-effective nanotechnology. On the one hand it employs a green synthesis using readily accessible and cost-effective plant extracts. On the other hand, it utilizes photocatalysis through natural sunlight instead of artificial lighting, which not only has a shorter operational life but also demands a high energy input. This dual-pronged approach simplifies the process, reduces costs, and enhances scalability for widespread application.
Simultaneous degradation of organic compounds, present in refinery effluents (Phenol, O-Cresol, Toluene, Xylene).
Therefore, in the present work, we highlight the utilization of M. Oleifera leaf, a natural substance as a reducing and capping agent to generate crystalline ZnO NPs. This study illustrates the initial efforts to optimize multicomponent synthetic refinery wastewater’s oxidation processes using solar light, coupled with biosynthesized photocatalyst. The prepared materials were investigated by means of X-ray diffraction (XRD), Transmission electron microscope (TEM), field emission scanning electron microscope (FESEM), and energy-dispersive X-Ray spectroscopy analysis (EDXS). Photocatalytic degradation of synthetic refinery wastewater (SRW) with the prepared catalysts is also reported.
Methodology of the study
Biosynthesis of zno nps using moringa oleifera leaves extract (green-zno), declaration statement.
We declare that the collection of plant material is in accordance with relevant institutional, national and international guidelines and legislation.
Preparation of plant extract
Moringa oleifera leaves have served as an eco-friendly alternative natural reducing and capping agent. The “drumstick tree,” also known as Moringa oleifera Lam ., is acknowledged as a plentiful and reasonably priced plant. The phytochemical profile of its leaves showed the presence of essential bioactive compounds; vitamins, phenolic acids, flavonoids, and glucosides 32 , 33 .
Moringa oleifera plant has been cultivated and collected from the Higher Agronomic Institute of Chott Mariem (ISA CM), Tunisia. 10 g of cleaned and dried M. Oleifera leaves were boiled for 30 min in 100 mL of double distilled water at 60 °C under magnetic stirrer. The mixture was brought down to room temperature (for 1h45), then filtered through filter paper and a light-yellow solution. The filtered plant extract was kept in a refrigerator at 4 degrees Celsius for future use 34 .
Biosynthesis of ZnO NPs
A magnetic stirrer was used to heat 60 mL of M. Oleifera leaf aqueous extract to 80 °C before adding 6 g of zinc nitrate hexahydrate (Zn(NO 3 ) 2 .6H 2 O). The mixture was boiled until a yellow-tinted paste formed. After that, it was transferred to a ceramic crucible, then calcined for 2 h in a furnace at 500 °C. Ultimately, a light yellow powder was collected 34 .
Conventional synthesis of ZnO NPs (Chem-ZnO)
With certain adjustments, the chemical synthesis was created in accordance with the earlier work 35 . After vigorously swirling 6 g of zinc nitrate hexahydrate (Zn(NO 3 ) 2 .6H 2 O) into 60 ml of distilled water for 10 min, 2.0 M of ammonium hydroxide was added dropwise until the pH reached 10. The resulting white precipitate was passed through a filter before being calcined for two hours at 500 °C in a furnace.
Characterization methods of ZnO NPs
Several analytical techniques were used to characterize the ZnO synthesized samples that were shown in the preceding sections. The UV–VIS absorbance spectra were acquired in an integrating sphere using a LAMBDA 365 UV/Vis Spectrophotometer in the range 300–600 nm. An X-Ray Diffractometer was used to characterize the structural properties (Siemens D5000). The angular 2 diffraction ranged from 5 to 70. As a sample holder, a low background Si wafer was employed. A copper X-ray tube provided CuKα radiation (0.15414 nm). The particles morphologies were studied by a FESEM (Thermo Scientific Fisher operated by EDS-Software-pathfinder) associated with EDXS (Oxford INCA PentaFET- × 3). TEM-SAED was used to assess shape, size, and crystallinity. TEM figures were collected using a Transmission Electron Microscope (JEOL model 1011). The solid samples were dispersed in ethanol by sonication, and droplets of zinc oxide NPs suspensions were poured onto a carbon coated-copper grid. Further the material was dried at room temperature and transferred to electron microscope for analysis 36 .
The pH drift technique was used to find the point of zero charge (pHpzc) of the biosynthesized ZnO. A series of 0.01 M NaCl solutions (10 mL each) were formed, and their pH values (pH 0 ) were adjusted between 5.0 and 10.0 by adding 0.1 M HCl and 0.1 M NaOH. The suspensions were stirred at 25 °C with 0.02 g of ZnO added to each solution. The solutions’ ultimate pH readings were obtained (pH f ) after 24 h 37 . The variance between the initial (pH 0 ) and final (pH f ) readings was plotted against the starting pH0 (Y-axis) (X axis). The resultant curve’s intersection generated the pHpzc where pH = 0 38 .
Photocatalytic experiments
Four aromatic and aliphatic hydrocarbons often discovered in refinery effluent were designated as target pollutants to evaluate their removal effectiveness by solar-assisted photocatalysis. Table 1 shows the composition of the synthetic water based on earlier studies with Real Refinery Wastewaters (RRWs) 7 , 39 , 40 , 41 , 42 . To prepare the SRW, 900 mL of distilled water was first mixed with 5 mg of Triton-X, the necessary salt quantities, and non-soluble compounds. A homogenizer was then used to emulsify the mixture for 30 min. The mixture was then supplemented with the required amounts of soluble organic materials while being vigorously agitated. Prior to use in the tests, the solution was then adjusted into 1000 mL in distilled and agitated for an additional 30 min to guarantee stable wastewater before use in the studies.
HPLC was used to determine the concentrations of the contaminants (Phenol, O-Cresol, Toluene, and Xylene) in the synthetic modeling water (Shimadzu LC-20AT). The separation was obtained through a C18 TeknoKroma column (4.6 × 250 mm, 5 micron) and detected at wavelength of 254 nm. The concentrations of each component were evaluated based on their respective calibration curves using standards.
The set of experiments was carried out in a borosilicate reactor with 550 mL of synthetic wastewater as a photoreactor. For 30 min, the liquid was mixed in the dark in order to assure the adsorption of compounds on the solid surface. An air-cooled 1500-W Xenon lamp in a solar box that simulates sunlight and emits light in the 300–800 nm range was used to irradiate the reactor (ATLAS, SUNTEST CPS +). The illumination was adjusted at 250 W/m 2 . The pH of solution was adjusted using dilute sodium hydroxide and hydrochloric acid solutions. The samples collected at fixed time intervals were centrifuged for15 minutes at 8000 rpm in advance of the data analysis.
Results and discussion
Characterization of the synthesized nps, uv visible absorption.
The UV–vis absorption spectra of ZnO materials are depicted in Fig. 2 (a). Both samples exhibited UV–vis absorption spectra, with a wide intense absorption from about 350 nm, which may be linked to the intrinsic absorption of the BG of ZnO NPs caused by electron (e−) transfer out from VB towards the CB 34 , 43 , 44 , 45 , 46 .
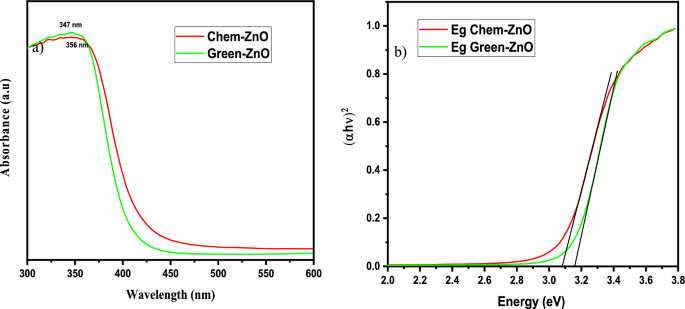
( a ) UV–Visible absorption spectrum ( b ) Inset. Plot of (αhυ) 2 versus photon energy of Green-ZnO and Chem-ZnO.
Since ZnO is a direct band gap Semicond., its ABS coefficient (α) is correlated to the excitation energy by the formula:
where A is a proportionality constant, h is the Planck constant, v is the frequency of vibration, and n is an exponent, 1/2, that characterizes direct allowed optical transitions.
E g is calculated by plotting (αhυ) 1/n vs. (hυ) and extrapolating to (αhυ) 1/n = 0 (Fig. 2 b). The extrapolation of the linear part until its intersection with the photon axis was employed to approximate the optical BG. From Fig. 2 (b), E g values are 3.16, and 3.07 eV for Green-ZnO, and ZnO-Chem respectively 47 , 48 , 49 . It denotes a widening of the optical BG for the Green-ZnO compared to Chem-ZnO. It is thought that a significant contributing element to this blueshift is the quantum size effect. As the grain size decreases, the continuous energy bands split off into discrete levels, causing the effective expand of the BG. Similar earlier reports also noted these results 44 , 50 , 51 .
X-ray diffraction (XRD) analysis
The XRD patterns of green produced ZnO NPs derived from zinc nitrate hexahydrate and Moringa leaf extract, as well as conventionally generated ZnO NPs formed from zinc nitrate hexahydrate and ammonium hydroxide are illustrated in Fig. 3 .
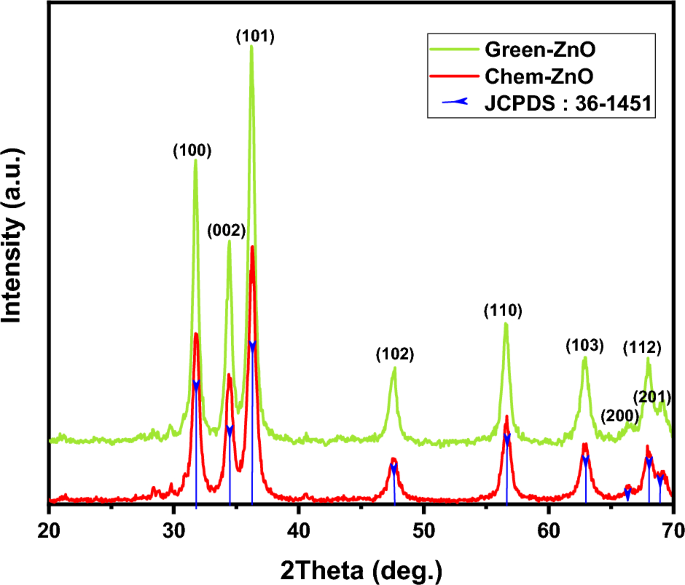
XRD patterns of green and chemically synthesized ZnO NPs.
In both cases, the XRD graph demonstrated that the synthesized product was in crystal and that no further impurities could be found once compared to the structure known (ZnO, 04-016-6648). The reference pattern’s hexagonal structure and each of the prominent peaks in the samples were perfectly correlated.
The ZnO NPs' acquired XRD pattern reveals the positions of 2 degree diffraction peaks at 31.75, 34.43, 36.23, 47.55, 56.58, 62.89, 66.42, 68.17, and 69.11 with matching Miller indexes (hkl) of (100), (002), (101), (102), (110), (103), (200), (112), (201) respectively. This demonstrates ZnO’s hexagonal wurtzite phase (JCPDS: 36-1451).
Scherrer’s formula was applied to the high intensity peak (101) to estimate the crystallite size : D = Kλ/β cos θ Where K is a constant (0.9), λ is the X-ray wavelength, and β is full width at half maximum (FWHM) 52 . ZnO NPs have an average crystallite size of 12.51 nm for Green-ZnO and 11 nm for Chem-ZnO. Based on the Debye–Scherrer equation, Both NPs showed almost the same crystallite size. Indeed, in XRD analysis, it should be noted that the crystallite size is assumed to be the size of a coherently diffracting domain and is not necessarily to be the same as the particle size. Furthermore, according to literature, it has been found that the XRD peak can be widened by defects and internal stress 53 , 54 .
FE-SEM analysis
The surface morphology of chemically produced and green ZnO NPs is examined using a FE-SEM.
The SEM image Fig. 4 (a) of chemically formed ZnO reveals a range of irregularly shaped NPs clustered. We can observe that the chemically obtained ZnO have no defined geometry. On the other hand, the image of biosynthesized ZnO (Fig. 4 b) reveals NPs with well-defined structures at the nanoscale relatively spherical in shape with clear separation 49 . These NPs are surrounded with biomolecules found in the extract, which maintain them apart and avoid agglomeration. This demonstrates that the addition of the plant extract throughout the reaction had a significant influence on the formation mechanism, ending a more defined pattern with less agglomeration 35 , 55 .

FE-SEM: ( a ) chemically synthesized and ( b ) biosynthesized ZnO NPs.
Furthermore, the green method's obtained shape consolidates the physical properties of the NPs, enhancing their qualities and efficiency in many applications. Figure S1 allows us to determine an average size for the biosynthesized ZnO NPs of 13.95 nm. With obvious signals from the atoms of zinc and oxygen and the very low intensity of the carbon atom, the EDX spectrum (Figs. S2 and S3 ) of produced ZnO NPs reveals their chemical content and validates their purity 49 . The spectrum of Fig. S3 revealed additional peaks corresponding to Magnesium (Mg), Sulfur (S), Chlorine (Cl), Potassium (K) and Calcium (Ca) in very small quantities. Generally, these compounds are contained in the leaf extract of Moringa oleifera 55 , 56 , 57 .
TEM analysis
TEM images were used to investigate the in-depth properties of chemically synthesized and biosynthesized ZnO NPs. The TEM micrograph of chemically synthesized ZnO in Fig. S4 (a) depicts the clustering and irregularity of chemically synthesized ZnO structures. On the other hand, Fig. S4 (b) reveals TEM micrograph of the biosynthesized ZnO NPs, giving rise to isolated NPs relatively spherical in shape. This figure (Fig. S4 b) is taken at high resolution and confirms the presence of spheroid-like and hexagonal shapes. The histogram in Fig. 5 shows the particle sizes of Green-ZnO NPs ranging from 9 to 18 nm in diameter, with an average size of about 14 nm and a standard deviation of 2.1. These outcomes validate the SEM analysis.
Particle size distribution histogram of Green-ZnO NPs.
The particles are distributed uniformly which is owing to the existence of organic compounds that encase the particles and act as a capping agent, blocking their aggregation. As a result, it is clear that biosynthesized ZnO has lower particle size and better morphological control than chemically produced ZnO 35 .
The SAED pattern (Fig. S4 c–d) was displaying distinct bright dotty rings, demonstrating the particles' crystalline structure, which is consistent with the XRD pattern shown in Fig. 3 .The corresponding SAED pattern of the chemically synthesized ZnO displays more discrete spots, indicating the single crystalline nature compared to the biosynthesized, which could be because of the residual organic compounds used during the green fabrication process.
Surface area and porosity analyses
Nitrogen adsorption–desorption profiles using BJH and BET techniques were employed to assess the surface properties and the type of porosity of green and chemically produced ZnO NPs. The pore size distribution and porosities were obtained from the desorption isotherm branch by using BJH approach, and the specific surface area was acquired using the BET method.
The specific surface area of the biosynthesized and chemically produced ZnO NPs is 19.789 m 2 /g and 4.923 m 2 /g respectively. The creation of smaller particle sizes may be responsible for the increase in the surface area of green ZnO. Moreover, the pore volumes of Green ZnO and chemically synthesized ZnO are 0.149 cc/g and 0.020 cc/g, respectively. The quantity of ZnO active sites and surface area increase with increasing pore volume 58 , 59 , so increases the adsorption capacity, which therefore boosts the photocatalytic effectiveness. (Fig. S5 a–d).
Heterogeneous photocatalysis for SRW treatment using ZnO NPs
Catalyst loading.
Heterogeneous photocatalysis assays were conducted initially at free pH for 180 min with ZnO catalyst loads of 0.1, 0.25, and 0.5 g/L. All studies proceeded with a previous 30-min adsorption stage in the dark. This period of time was determined based on the results of the catalyst’s 60-min adsorption testing. Before the analytical processes, the samples collected were centrifuged for 10 min at 10,000 rpm.
It can be assumed that 0.1 and 0.25 g/L concentrations show an initial superior adsorption; however, 0.5 g/L concentration exhibits lower adsorption. Considering that the adsorption capacity typically rises with surface area, additional pollutant molecules are adsorbed on the surface given by a high catalyst loading 60 . However, a common tendency of a decline in the removal was seen.
Photocatalysis
As can be observed, 0.25 and 0.5 g/L achieve nearly identical final results at the end of the 180-min experiment. Analyzing the degradation response reveals that raising the concentration has no discernible effect on photocatalytic performance, which makes 0.25 g/L an optimum catalyst concentration.
Effect of initial pH
The surface charge of the semiconductor photocatalyst, the mechanism, and the rate of reactive oxygen species (ROS) formation are all significantly influenced by the solution's pH value 61 . This, in turn, affects the rate of photocatalytic degradation of contaminants 62 , 63 .
The point of Zero charge pH (pHpzc) of ZnO is 8 according to Fig. 6 and in agreement with previous references 64 , 65 .
Point of zero charge (pHpzc) of biosynthesized ZnO nanoparticles.
In this regard, the elimination of each pollutant on ZnO at four distinct pH conditions viz. 5, 7, 8, and 9 have been studied at a constant concentration of catalyst 0.25 g/L. (Fig. 6 ). The C/Ci vs. time graph (where C denotes the concentration at various time intervals and Ci denotes the compound’s starting concentration) using the biosynthesized ZnO NPs (Green-ZnO) under simulated solar irradiation is shown in Fig. 7 (a–d).
Effect of initial pH on the photocatalytic activity of biosynthesized ZnO photocatalyst toward ( a ) Phenol, ( b ) O-Cresol, ( c ) Toluene, ( d ) Xylene after 30 min dark and 180 min of irradiation.
The percentage of phenol destroyed is found to be very low in an acidic media. These observations can be related to the phenomenon having positively charged NPs surfaces, which causes protonation of active sites and hence alters phenol adsorption, thereby affecting its removal 66 . Adsorption on ZnO will be less in the basic pH range, where phenol is predicted to be in the ionized state. As a result, surface-mediated degradation will be reduced 67 . Therefore, the neutral pH was the best suited for the phenol degradation.
Giving the basic nature of o-cresol (pKa = 10.316), under acid media, it tends to be positively charged 14 , 68 . As it is shown, the photodegradation % rose marginally as the pH climbed from 5 to 7. Nevertheless, above the optimum pH 7, there was a decrement in photodegradation%.
The maximum degradation efficiency of Toluene and Xylene (88.30% and 93.13% respectively) was reached with pH 7 following 180 min in the simulated sunlight/ZnO system. Remarkably, the results were comparable at pH values 7 and 9.
The increased removal effectiveness at basic pH can indeed be attributed to the fact that, in alkaline pH, OH . are more readily formed via oxidation of more hydroxyl radicals, that are present on the ZnO surface, thereby boosting the process performance 69 , 70 .
In all the 4 compounds, at pH8, which is the pH pzc the results are the worse ones. This behavior could be related to a possible aggregation of catalyst particles. Indeed, the zero surface charge creates zero electrostatic surface potential for pH levels near to pH zpc , which cannot generate the interaction rejection required to isolate the particles inside the solution. Aggregation occurs as a result, and photocatalyst clusters get bigger 71 .
Green vs chemically synthesized ZnO photocatalyst
Figure 8 (a–d) illustrates a comparison of time-dependent photocatalytic activity of both the Green-ZnO and the Chem-ZnO NPs towards each pollutant degradation. The photo-chemical analysis revealed that the degradation process with Green-ZnO within 180 min of irradiation resulted in 51%, 52%, 88%, and 93% of removal for Phenol, O-Cresol, Toluene, and Xylene respectively. However, in the case of Chem-ZnO, the percentage of degradation was 33%, 34%, 74%, and 89% of removal for Phenol, O-Cresol, Toluene, and Xylene respectively. The biosynthesis of ZnO clearly demonstrated a higher or quite equivalent degradation effectiveness in comparison to ZnO synthesized with conventional chemical route.
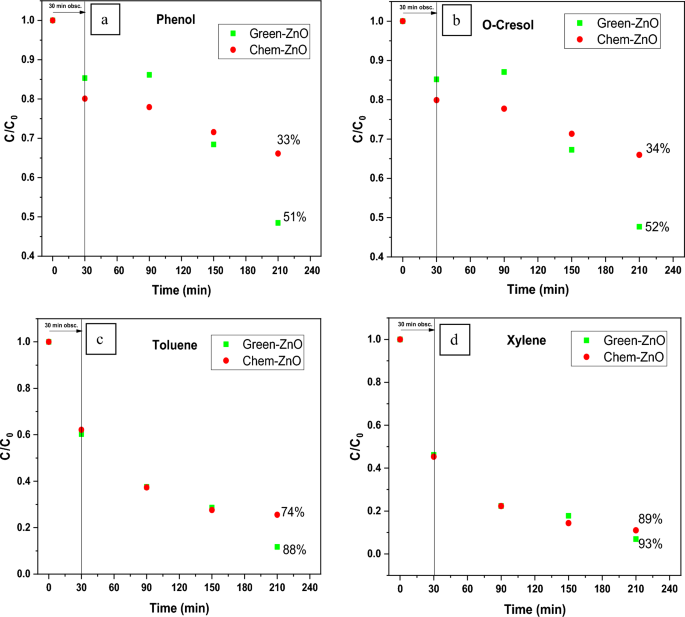
Photocatalytic activity of biosynthesized and chemically synthesized ZnO at optimum conditions toward ( a ) Phenol, ( b ) O-Cresol, ( c ) Toluene, ( d ) Xylene after 30 min dark and 180 min of irradiation.
A similar trend has been documented in prior studies that investigated dye degradation, whether using environmentally friendly or chemical synthesis methods 72 , 73 . It is widely assumed that the morphology, surface, and crystallinity of material are mainly responsible for its photocatalytic activity 74 . Indeed, the plant extract's bioactive substances aid in the development of ZnO nuclei through capping but also stabilizing them. As a result, the green method produces NPs that have greater distribution, structure-tunable, and are size-controlled 75 , 76 , 77 compared to the chemically synthesized sample, which could provide stability, a larger specific surface area, and reduced particle sizes, thus, high photogenerated charge carrier separation capabilities, enhanced light absorption and finally better degradation of pollutant molecules.
Tables S1 , S2 , and S3 in the Supplementary Information section offer a comparative analysis of our research findings with those from previous studies focusing on ZnO synthesized through various methods for the PC degradation of petroleum hydrocarbon contaminants. This comparison underscores the competitiveness of the results we have obtained in this study when compared to existing efficiency standards.
The primary objective of this research is to explore an environmentally sustainable, uncomplicated, and cost-effective solution that can either reduce the volume of waste discharged in effluents or promote the reuse of purified water, thus reducing the consumption of freshwater. The novel approach for photocatalyst synthesis employed in this study represents an initial step towards optimizing a more sustainable process, especially, when paired with sun energy, this allows an economically feasible route in the application of solar photocatalysis. Importantly, According to a previous study, the economical evaluation points out that the highest loads for the cost composition are due to catalyst synthesis, corresponding to 95% in a solar photocatalysis system 34 .
Ultimately, working with photocatalysts in form of powder needs a post-processing removal of the NPs from the liquid solution which could be an inefficient additional process step especially from an industrial perspective. For this reason, the development of photocatalysts immobilized as coatings is thus an improvement. That will require modifying the substrate, using a different thickener, or introducing a linker between ZnO and the substrate. In this case, the stability parameter and a study of reusability would be significant. We consider that an interesting topic for our ongoing and future research.
We reported on the photocatalytic activity of ZnO nanoparticles biosynthesized through a sustainable, cost-effective, easily scalable, and eco-friendly approach. Moringa oleifera leaves extract was used as reducing and stabilizer agent, hence playing a significant role towards structural evolution. The reported results reveal that Green-ZnO can be fruitfully exploited for the removal of toxic compounds present in refinery effluents particularly; Phenol, O-Cresol, Toluene, and Xylene with 51%, 52%, 88%, and 93% in sequence. Indeed, the PC efficiency of green-synthesized ZnO NPs is almost equivalent to that of ZnO via a conventional chemical synthesis. The ability of the proposed approach to use sunlight as the only energy input and photocatalysts with low cost and minimal environmental impact underline its significance in the ongoing efforts towards wastewater remediation.
Data availability
Data are available from the corresponding author Prof. Chérif Dridi, upon reasonable request.
Al-Sabahi, J., Bora, T., Al-Abri, M. & Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 12 , e0189276 (2017).
Article PubMed PubMed Central Google Scholar
Ba-Abbad, M. M. et al. Solar photocatalytic degradation of 2-chlorophenol with ZnO nanoparticles: Optimisation with D-optimal design and study of intermediate mechanisms. Environ. Sci. Pollut. Res. 24 , 2804–2819 (2017).
Article CAS Google Scholar
Elmobarak, W. F., Hameed, B. H., Almomani, F. & Abdullah, A. Z. A review on the treatment of petroleum refinery wastewater using advanced oxidation processes. Catalysts 11 , 782 (2021).
Demir-Duz, H., Ayyildiz, O., Aktürk, A. S., Álvarez, M. G. & Contreras, S. Approaching zero discharge concept in refineries by solar–assisted photo-Fenton and photo-catalysis processes. Appl. Catal. B Environ. 248 , 341–348 (2019).
Tony, M. A., Purcell, P. J. & Zhao, Y. Oil refinery wastewater treatment using physicochemical, Fenton and Photo-Fenton oxidation processes. J. Environ. Sci. Health Part A 47 , 435–440 (2012).
Keramati, M. & Ayati, B. Petroleum wastewater treatment using a combination of electrocoagulation and photocatalytic process with immobilized ZnO nanoparticles on concrete surface. Process Saf. Environ. Prot. 126 , 356–365 (2019).
Coelho, A., Castro, A. V., Dezotti, M. & Sant’Anna, G. L. Treatment of petroleum refinery sourwater by advanced oxidation processes. J. Hazard. Mater. 137 , 178–184 (2006).
Article CAS PubMed Google Scholar
Andreozzi, R. Advanced oxidation processes for the treatment of mineral oil-contaminated wastewaters. Water Res. 34 , 620–628 (2000).
Galvão, S. A. O. et al. Application of the photo-Fenton process to the treatment of wastewaters contaminated with diesel. Sci. Total Environ. 367 , 42–49 (2006).
Article ADS PubMed Google Scholar
Karvekar, O. S. et al. Bos taurus (A-2) urine assisted bioactive cobalt oxide anchored ZnO: A novel nanoscale approach. Sci. Rep. 12 , 15584 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Karvekar, O. S. et al. Biogenic synthesis of silver anchored ZnO nanorods as nano catalyst for organic transformation reactions and dye degradation. Appl. Nanosci. 12 , 2207–2226 (2022).
Sarvalkar, P. D. et al. Bio-mimetic synthesis of catalytically active nano-silver using Bos taurus (A-2) urine. Sci. Rep. 11 , 16934 (2021).
Ani, I. J., Akpan, U. G., Olutoye, M. A. & Hameed, B. H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO 2 - and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 205 , 930–954 (2018).
Abdollahi, Y., Abdullah, A. H., Gaya, U. I., Zainal, Z. & Yusof, N. A. Enhanced photodegradation of o -cresol in aqueous Mn(1%)-doped ZnO suspensions. Environ. Technol. 33 , 1183–1189 (2012).
Peng, X., Urso, M. & Pumera, M. Metal oxide single-component light-powered micromotors for photocatalytic degradation of nitroaromatic pollutants. Npj Clean Water 6 , 21 (2023).
Rajamanickam, D. & Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide—solar process. Arab. J. Chem. 9 , S1858–S1868 (2016).
Rauf, M. A. & Ashraf, S. S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 151 , 10–18 (2009).
Agarwal, H., Venkat Kumar, S. & Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—an eco-friendly approach. Resour.-Eff. Technol. 3 , 406–413 (2017).
Google Scholar
Jebril, S., Jenana, K. B. R. & Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 248 , 122898 (2020).
Jebril, S., Fdhila, A. & Dridi, C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci. Rep. 12 , 22060 (2021).
Article ADS Google Scholar
Shamaila, S. et al. Advancements in nanoparticle fabrication by hazard free eco-friendly green routes. Appl. Mater. Today 5 , 150–199 (2016).
Article Google Scholar
Vishnukumar, P., Vivekanandhan, S., Misra, M. & Mohanty, A. K. Recent advances and emerging opportunities in phytochemical synthesis of ZnO nanostructures. Mater. Sci. Semicond. Process. 80 , 143–161 (2018).
Rashidian, G. et al. Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp ( Cyprinus carpio ) skin mucus. Int. J. Mol. Sci. 22 , 3270 (2021).
Article CAS PubMed PubMed Central Google Scholar
Abdelkhalek, A. & Al-Askar, A. A. Green synthesized ZnO nanoparticles mediated by mentha spicata extract induce plant systemic resistance against tobacco mosaic virus. Appl. Sci. 10 , 5054 (2020).
Salem, S. S. & Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 199 , 344–370 (2021).
Mahdavi, M., Namvar, F., Ahmad, M. & Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe 3 O 4 ) nanoparticles using seaweed ( Sargassum muticum ) aqueous extract. Molecules 18 , 5954–5964 (2013).
Jayarambabu, N., Rao, K. V. & Rajendar, V. Biogenic synthesis, characterization, acute oral toxicity studies of synthesized Ag and ZnO nanoparticles using aqueous extract of Lawsonia inermis . Mater. Lett. 211 , 43–47 (2018).
Chen, X. et al. Preparation of different sized nano-silver loaded on functionalized graphene oxide with highly effective antibacterial properties. J. Mater. Chem. B 3 , 7020–7029 (2015).
Al-Momani, H. et al. The impact of biosynthesized ZnO nanoparticles from Olea europaea (common olive) on Pseudomonas aeruginosa growth and biofilm formation. Sci. Rep. 13 , 5096 (2023).
Gangwar, J. & Sebastian, J. K. Unlocking the potential of biosynthesized zinc oxide nanoparticles for degradation of synthetic organic dyes as wastewater pollutants. Water Sci. Technol. 84 , 3286–3310 (2021).
Weldegebrieal, G. K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 120 , 108140 (2020).
Ngom, I. et al. On the use of Moringa oleifera leaves extract for the biosynthesis of NiO and ZnO nanoparticles. MRS Adv. 5 , 1145–1155 (2020).
Saini, R. K., Sivanesan, I. & Keum, Y.-S. Phytochemicals of Moringa oleifera : A review of their nutritional, therapeutic and industrial significance. 3 Biotech 6 , 203 (2016).
Golli, A. E. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 286 , 112226 (2021).
Abdullah, F. H., Abu Bakar, N. H. H. & Abu Bakar, M. Comparative study of chemically synthesized and low temperature bio-inspired Musa acuminata peel extract mediated zinc oxide nanoparticles for enhanced visible-photocatalytic degradation of organic contaminants in wastewater treatment. J. Hazard. Mater. 406 , 124779 (2021).
Shim, Y. J. et al. Zinc oxide nanoparticles synthesized by Suaeda japonica Makino and their photocatalytic degradation of methylene blue. Optik 182 , 1015–1020 (2019).
Article ADS CAS Google Scholar
Zyoud, A. H. et al. Kaolin-supported ZnO nanoparticle catalysts in self-sensitized tetracycline photodegradation: Zero-point charge and pH effects. Appl. Clay Sci. 182 , 105294 (2019).
Kiwaan, H. A., Atwee, T. M., Azab, E. A. & El-Bindary, A. A. Efficient photocatalytic degradation of Acid Red 57 using synthesized ZnO nanowires. J. Chin. Chem. Soc. 66 , 89–98 (2019).
Alzarooni, M. & Elshorbagy, W. Characterization and assessment of Al Ruwais refinery wastewater. J. Hazard. Mater. 136 , 398–405 (2006).
Dias, I. N., Cerqueira, A. C., Sant’Anna, G. L. & Dezotti, M. Oil refinery wastewater treatment in biofilm reactor followed by sand filtration aiming water reuse. J. Water Reuse Desalin. 2 , 84–91 (2012).
Diya’uddeen, B. H., Daud, W. M. A. W. & Abdul Aziz, A. R. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 89 , 95–105 (2011).
El-Naas, M. H., Alhaija, M. A. & Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2 , 56–62 (2014).
Kahsay, M. H., Tadesse, A., RamaDevi, D., Belachew, N. & Basavaiah, K. Green synthesis of zinc oxide nanostructures and investigation of their photocatalytic and bactericidal applications. RSC Adv. 9 , 36967–36981 (2019).
Osuntokun, J., Onwudiwe, D. C. & Ebenso, E. E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. 12 , 444–457 (2019).
Dumbrava, A., Berger, D., Prodan, G. & Moscalu, F. Functionalized ZnO/CdS composites: Synthesis, characterization and photocatalytic applications. Chalcogenide Lett. 13 , 105–115 (2016).
Zak, A. K. et al. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 37 , 393–398 (2011).
Imran, H. J., Hubeatir, K. A. & Aadim, K. A. A novel method for ZnO@NiO core–shell nanoparticle synthesis using pulse laser ablation in liquid and plasma jet techniques. Sci. Rep. 13 , 5441 (2023).
Moulahi, A. & Sediri, F. Pencil-like zinc oxide micro/nano-scale structures: Hydrothermal synthesis, optical and photocatalytic properties. Mater. Res. Bull. 48 , 3723–3728 (2013).
Siripireddy, B. & Mandal, B. K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 28 , 785–797 (2017).
Karaköse, E. & Çolak, H. Structural, electrical, and antimicrobial characterization of green synthesized ZnO nanorods from aqueous Mentha extract. MRS Commun. 8 , 577–585 (2018).
Wang, J. et al. The Al-doping contents dependence of the crystal growth and energy band structure in Al:ZnO thin films. J. Cryst. Growth 311 , 2305–2308 (2009).
Zhang, X., Chen, Y., Zhang, S. & Qiu, C. High photocatalytic performance of high concentration Al-doped ZnO nanoparticles. Sep. Purif. Technol. 172 , 236–241 (2017).
Othman, A. A., Ali, M. A., Ibrahim, E. M. M. & Osman, M. A. Influence of Cu doping on structural, morphological, photoluminescence, and electrical properties of ZnO nanostructures synthesized by ice-bath assisted sonochemical method. J. Alloys Compd. 683 , 399–411 (2016).
Ashour, A., Kaid, M. A., El-Sayed, N. Z. & Ibrahim, A. A. Physical properties of ZnO thin films deposited by spray pyrolysis technique. Appl. Surf. Sci. 252 , 7844–7848 (2006).
Kumar, M. R. A. et al. Evaluation of bi-functional applications of ZnO nanoparticles prepared by green and chemical methods. J. Environ. Chem. Eng. 7 , 103468 (2019).
Nkechinyere Onyekwere, N. & Felix, I. N. Phytochemical, proximate and mineral composition of leaf extracts of Moringa oleifera Lam. from Nsukka, South-Eastern Nigeria. IOSR J. Pharm. Biol. Sci. 9 , 99–103 (2014).
Mulyaningsih, T. R. & Yusuf, S. Determination of minerals content in leaves of Moringa Olifeira by neutron activation analysis. Ganendra Maj. IPTEK Nukl. 21 , 11 (2018).
Li, D. et al. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO 2 . Nanomaterials 10 , 546 (2020).
Naik, A. P. et al. Super porous TiO 2 photocatalyst: Tailoring the agglomerate porosity into robust structural mesoporosity with enhanced surface area for efficient remediation of azo dye polluted waste water. J. Environ. Manag. 258 , 110029 (2020).
Do, D. D. Adsorption Analysis: Equilibria and Kinetics (Imperial College Press, 1998).
Book Google Scholar
Rubio-Clemente, A., Torres-Palma, R. A. & Peñuela, G. A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total Environ. 478 , 201–225 (2014).
Article ADS CAS PubMed Google Scholar
Ghasemi, Z., Younesi, H. & Zinatizadeh, A. A. Preparation, characterization and photocatalytic application of TiO 2 /Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 159 , 552–564 (2016).
Khodadoust, S., Sheini, A. & Armand, N. Photocatalytic degradation of monoethanolamine in wastewater using nanosized TiO 2 loaded on clinoptilolite. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 92 , 91–95 (2012).
Dhiman, N. & Sharma, N. Removal of pharmaceutical drugs from binary mixtures by use of ZnO nanoparticles. Environ. Technol. Innov. 15 , 100392 (2019).
Ghaffari, S.-B., Sarrafzadeh, M.-H., Fakhroueian, Z., Shahriari, S. & Khorramizadeh, M. R. Functionalization of ZnO nanoparticles by 3-mercaptopropionic acid for aqueous curcumin delivery: Synthesis, characterization, and anticancer assessment. Mater. Sci. Eng. C 79 , 465–472 (2017).
Meshram, S. et al. Continuous flow photocatalytic reactor using ZnO–bentonite nanocomposite for degradation of phenol. Chem. Eng. J. 172 , 1008–1015 (2011).
Anju, G., Jyothi, K. P., Joseph, S., Suguna, Y. & Yesodharan, E. P. Ultrasound assisted semiconductor mediated catalytic degradation of organic pollutants in water: Comparative efficacy of ZnO, TiO 2 and ZnO-TiO 2 . Res. J. Recent Sci. 1 , 191–201 (2012).
CAS Google Scholar
Nguyen, A.-T. & Juang, R.-S. Effect of operating parameters and kinetic study on photocatalytic degradation of o-Cresol in synthetic wastewater with irradiated titanium dioxide. In International Conference on Advances in Engineering and Technology (ICAET’2014) March 29–30, 2014 Singapore (International Institute of Engineers, 2014). https://doi.org/10.15242/IIE.E0314164 .
Alizadeh Fard, M., Aminzadeh, B. & Vahidi, H. Degradation of petroleum aromatic hydrocarbons using TiO 2 nanopowder film. Environ. Technol. 34 , 1183–1190 (2013).
Konstantinou, I. K. & Albanis, T. A. TiO 2 -assisted photocatalytic degradation of AZO dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 49 , 1–14 (2004).
Ferrari-Lima, A. M. et al. Photodegradation of benzene, toluene and xylenes under visible light applying N-doped mixed TiO 2 and ZnO catalysts. Catal. Today 241 , 40–46 (2015).
Nava, O. J. et al. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 1147 , 1–6 (2017).
Stan, M. et al. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 39 , 23–29 (2015).
Tian, G., Fu, H., Jing, L., Xin, B. & Pan, K. Preparation and characterization of stable biphase TiO 2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity. J. Phys. Chem. C 112 , 3083–3089 (2008).
Bala, N. et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5 , 4993–5003 (2015).
Karnan, T. & Selvakumar, S. A. S. Biosynthesis of ZnO nanoparticles using rambutan ( Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 1125 , 358–365 (2016).
Zare, M., Namratha, K., Thakur, M. S. & Byrappa, K. Biocompatibility assessment and photocatalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by Thymus vulgaris leaf extract. Mater. Res. Bull. 109 , 49–59 (2019).
Download references
Acknowledgements
The authors would like to thank the Tunisian MHESR for supporting this work and the University of Sousse for the “Bourse d’alternance” fellowship awarded to Mrs. Asma El Golli. The authors also acknowledge Dr Raoudha Khenfir Ben Jenana from the Higher Agronomic Institute of Chott Mariem (ISA CM), Tunisia, for providing the Moringa oleifera plant.
Author information
Authors and affiliations.
Center of Research on Microelectronics and Nanotechnology of Sousse, NANOMISENE Laboratory LR16CRMN01, Technopole of Sousse, B.P. 334, Sousse, Tunisia
A. El Golli & C. Dridi
Departament d’Enginyeria Química, Universitat Rovira i Virgili, Av. Països Catalans, 26, 43007, Tarragona, Spain
S. Contreras
High School of Sciences and Technology of Hammam Sousse, University of Sousse, Sousse, Tunisia
A. El Golli
You can also search for this author in PubMed Google Scholar
Contributions
A.E.G.: Conceptualization, Investigation, Methodology, Writing– original draft. S.C.: Conceptualization, Methodology, Validation, Writing – review & editing, Funding acquisition. C.D.: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision, Funding acquisition. All authors reviewed and approved the submission of the manuscript.
Corresponding authors
Correspondence to S. Contreras or C. Dridi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
El Golli, A., Contreras, S. & Dridi, C. Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment. Sci Rep 13 , 20809 (2023). https://doi.org/10.1038/s41598-023-47554-2
Download citation
Received : 04 June 2023
Accepted : 15 November 2023
Published : 27 November 2023
DOI : https://doi.org/10.1038/s41598-023-47554-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Green synthesis of zinc oxide nanoparticles using rhus coriaria extract and their anticancer activity against triple-negative breast cancer cells.
- Youssef Mongy
- Thanaa Shalaby
Scientific Reports (2024)
Enhanced Antibiotic Degradation and Antioxidant Activity Using a Novel Biosynthesized PVP-Modified Fe2O3/Fe3O4 Nanocomposite: A Dual Approach to Environmental and Human Health
- Zarah Alqarni
Journal of Cluster Science (2024)
Recent advancements in sustainable synthesis of zinc oxide nanoparticles using various plant extracts for environmental remediation
- Sapana Jadoun
- Jorge Yáñez
- Sampath Chinnam
Environmental Science and Pollution Research (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Cookie Acknowledgement
This website uses cookies to collect information to improve your browsing experience. Please review our Privacy Statement for more information.
- Administration
- Toggle Search
- Find People

Nonlinear Optical and Photovoltaic Studies of Specific Nonconjugated Conductive Polymers and Metallic Nanoparticles
Type of degree.
Mechanical Engineering
In this research, the nonlinear optical properties of specific nonconjugated conductive polymers and gold nanoparticles in transparent dielectric media have been investigated. Photovoltaic devices utilizing nonconjugated conductive polymers have also been studied. Nonconjugated conductive polymers are polymers with at least one double bond in the repeat. 1,4-polyisoprene (cis and trans), styrene butadiene rubber, and poly(β-pinene) are readily available examples of nonconjugated conductive polymers that have been investigated. Nonconjugated conductive polymers exhibit increases of many orders of magnitude in electrical conductivity upon doping with electron acceptors such as iodine. This change in conductivity results from charge-transfer from isolated double bonds in the polymer to the dopant. Exceptionally large optical nonlinearities have been reported for nonconjugated conductive polymers since they form sub-nanometer size metallic domains (quantum dots) upon doping. Nonconjugated conductive polymers have been shown to have many potential applications in nonlinear optics, electro-optics, and photovoltaics. The quadratic electro-optic effect and electroabsorption have been investigated in several nonconjugated conductive polymers including cis-1,4-polyisoprene, trans-polyisoprene, styrene butadiene rubber, and polyethylene terephthalate. The effects of iodine doping on polyethylene terephthalate have also been investigated using spectroscopy. Metallic nanoparticles in a dielectric medium have also been shown to have high magnitude nonlinear optical susceptibilities. Large nonlinear susceptibilities have been reported near the surface plasmon resonance frequencies in the materials. These effects have been attributed to dielectric confinement of charges within the metal nanoparticles and were predicted theoretically to be related to the size of the related charge-system. The nonlinear optical properties of gold nanoparticles in a dielectric medium (glass) and Iodine-doped nonconjugated conductive polymers have been investigated. These studies used the field-induced birefringence method to measure the Kerr effect in these materials. Measurements of Kerr coefficients for the materials investigated have been performed. The magnitudes of the Kerr coefficients for the gold nanoparticle samples have been compared and used to verify theoretical models on the relationship between particle diameter and third-order optical susceptibility. Nonlinear absorption (electroabsorption) has also been measured in these materials. These measurements were made using applied electric fields to change the absorption of the material. The results have been used to gain theoretical understanding of optical nonlinearities of metallic nanoparticles down to the sub-nanometer dimensions. The nonlinearity has been shown to increase as 1/𝑑^3 where d is the diameter of the nanoparticle (quantum dot). Newer nonconjugated conductive polymers such as polyethylene terephthalate have also been studied. Photovoltaic devices utilizing Iodine doped nonconjugated conductive polymers have been constructed and evaluated. These devices were fabricated in a similar way to dye-sensitized solar cells with the polymer used as the absorbing layer. Incident light on the cell excites electrons in the nonconjugated polymer which are then transferred through an electrolyte to a thin layer of titanium dioxide. The created devices were exposed to light and their open-circuit voltages and short-circuit currents were measured. These results have been compared for the various nonconjugated polymers. The stability of the devices has also been investigated. The cells typically show degradation in the photocurrent output over time. Attempts were made to determine the cause of the rapid reduction in output and to find a method of extending the lifetime of the fabricated cells. These studies showed that sealing cells in order to reduce the loss of the liquid electrolyte can extend the lifetime of the cells.
https://etd.auburn.edu//handle/10415/9308

Search form
- Undergraduate
- Message From Chair
- Facts & Figures
- Faculty & Staff
- Academic Employment
- Dept Events
- Discovery News
- Student Testimonials
- Give to BME
- Seminar Series
- E-Newsletter
- Message from Chair
- CEE Affiliates
- Give to CEE
- International Center Form
- MSE Business & Forms
- MSE 298 Seminars
- MSE Diversity & Inclusion
- Support MSE@UCI
- MAE Seminars
- Corporate Affiliates
- Interdisciplinary Graduate Programs
- All faculty & staff
- Dean's Office
- Development and External Relations
- Student Affairs
- Engineering Research Management
- UC Irvine Directory
- Proposal Text and Resources
- Early Career Opportunities
- Purchasing Requests
- Reimbursements
- Purchasing & Reimbursement Mission Statement
- Business Meetings/ Entertainment Guidelines
- Service Agreements
- Travel Guidelines
- Travel Tips
- UC Policies & Procedures
- Dean's Executive Office
- Chief Administrative Officers
- Personnel Unit
- Finance Unit
- Purchasing Unit
- Computing Unit
- Facilities Unit
- Curriculum, Analytical Studies, & Accreditation (CASA)
- Communications Office
- Development and External Relations Office
- Outreach Unit
- Office of Information Technology
- Faculty Websites
- Computer Labs & Laptops
- Engineering Facilities Request Form
- Safety Procedures
- Campus Evacuation Zones
- Environmental Health & Safety
- UCI Police Department
- Helpful Links
- At Your Service
- Zot! Portal
- FAQs for Engineering Instructors
- Spring Awards
- Process Improvement
- Alumni Spotlight
- Hall of Fame
- #ANTEATERENGINEER
- Ways to Give
- UCI Engineering Alumni Society
- UC Irvine Alumni Association
- Dean's Message
- Strategic Plan
- Facts and Figures
- Henry Samueli
- School Leadership
- Engineering Leadership Council
- Accreditation
- Orange County
- Got Questions?
- Enrollment and Degrees Awarded
- How to Apply
- Prospective Students
- Newly Admitted
- Majors and Minors Offered
- Frequently Asked Questions
- Programs and Concentrations
- Accelerated Status Program
- International Fellowships
- Meet Us on the Road
- Anteater Voices
- Ph.D. and Master's Inquiry Form
- Message from the Associate Dean
- UCI Engineering-LANL Graduate Fellowships
- Research Thrusts
- Research by Department
- Research Centers, Institutes and Facilities
- Undergraduate Research
- Interdisciplinary Science and Engineering Building (ISEB)
- Contracts & Grants / ERM
- Research and Proposal Development
- Annual Membership Levels
- Connect with Students
- Prototyping Services
- Sponsored Research
- External Relations Office
- Community College
- International
- IDEA - Inclusion, Diversity, Equity, Access
- Stacey Nicholas Office of Access and Inclusion
- Inclusion in Engineering Education
- Samueli Shoutouts
- Media Watch
- Dean's Report
- Social Media
- Style Guide
CEE@UCI Ph.D. Defense Announcement: Developing a Mechanistic Understanding of Using Engineered Iron Nanoparticles to Sequester Contaminants in Agricultural Systems

Ph.D. Candidate: Ziwei Han University of California, Irvine, 2024 Professor Adeyemi Adeleye
Zoom Link: https://uci.zoom.us/j/92804125616
Abstract: Heavy metal contamination in agricultural systems - soil and irrigation water - is a challenging problem with serious consequences on food safety and human health. Unlike most traditional treatment methods, engineered nanomaterials provide a unique opportunity to prevent and remediate heavy metal contamination in water and farm soils. However, there is a wide knowledge gap on the fundamentals of interactions between nanoparticles and dissolved toxic metallic ions in complex agricultural matrices. This dissertation focuses on understanding the unique chemistry of interactions between nanoscale zerovalent iron (NZVI) and metallic contaminants in water and farm soils and demystifies NZVI-based treatment.
Upcoming Events
- 12 Jul CEE@UCI Ph.D. Defense Announcement:Scheduling is All You Need in Node- and Link-Based Frameworks for Future Traffic Control
News & Events
- Open access
- Published: 28 June 2024
Novel antimicrobial applications of copper oxide nanoparticles after combination with tissue conditioner used in complete prostheses
- Saeed Nikanjam 1 ,
- Aria Yeganegi 1 ,
- Mohammad-Yousef Alikhani 2 ,
- Abbas Farmany 3 ,
- Seyed Amir Ghiasian 4 &
- Roghayeh Hasanzade 5
BMC Oral Health volume 24 , Article number: 752 ( 2024 ) Cite this article
Metrics details
Tissue conditioners are used for treating and improving the tissues supporting complete dentures. On the other hand, recent advances in nanotechnology have revolutionized various fields of science, including dentistry. The present study aimed to investigate novel antimicrobial applications of copper oxide nanoparticle-based tissue conditioner used in complete prostheses.
The present experimental study included 126 tissue conditioner samples with different concentrations of copper oxide nanoparticles (20%, 10%, 5%, 2.5%, 1.25%, 0.625%, and 0% w/w). The samples were incubated with Enterococcus faecalis , Pseudomonas aeruginosa , and Candida albicans in 24-well plates for 24 h. Then, samples from the wells were re-incubated for 24 h, and the microorganisms were counted.
The culture media containing E. faecalis and P. aeruginosa showed significantly different growth between different nanoparticle concentrations following 24 h ( P < 0.001), showing a reduction in bacterial growth with increased nanoparticle concentration. Both bacteria did not show any growth at the 20% concentration. However, C. albicans showed significant differences in growth between different nanoparticle concentrations following 48 h ( P < 0.001), showing a reduction in growth with increased nanoparticle concentration. Also, the least growth was observed at the 20% concentration.
Conclusions
In conclusion, the CuO nanoparticles were prepared using a green synthesis methon in the suitable sizes. Moreover, the tissue conditioners containing CuO nanoparticles showed acceptable antimicrobial properties against E. faecalis , P. aeruginosa , and C. albicans .
Peer Review reports
Replacement of lost teeth is essential for health and high quality of life since edentulism can negatively affect facial aesthetics, speaking, and mastication [ 1 ]. There are different methods for replacing lost teeth, including implant-supported prostheses, implant-supported dental bridges, and removable prostheses [ 2 , 3 ]. However, some of these options, such as dental implants, are less frequently used compared to other options due to limitations of the oral cavity and cost-ineffectiveness [ 4 ]. Considering the increased life expectancy of the middle-aged and the elderly, as well as the high prevalence of edentulism in this population, dental prostheses have become extensively popular in this age group. The prostheses used for tooth restoration should show enough biocompatibility in the oral cavity while improving facial aesthetics [ 5 ]. Moreover, prostheses should be properly designed in order to meet the physiological needs of the oral cavity, support the related soft and hard tissues without causing injuries, and have prolonged durability, thereby making the edentulous patients needless to new prostheses for several years [ 6 ].However, various bacterial and fungal species living in the oral cavity as the natural flora can turn into pathogens under certain conditions, such as prolonged use of dental prostheses. Thus, long-term use of these prostheses may result in stomatitis. Moreover, several factors, such as mucosal trauma, tobacco use, malignancies, endocrinopathic disorders, and the use of antibiotics, which can change the natural flora of the oral cavity, can predispose patients to prosthesis-induced stomatitis [ 7 ].On the other hand, tissue conditioners can be used for treating and improving the tissues supporting complete dentures. Lining the poor-fitting dentures helps in tissue healing and regeneration before molding for a new denture. Moreover, tissue conditioners can be used for temporary reasons, whether accessory or diagnostic, such as restoring the occlusal vertical dimensions and occlusal correction of old prostheses. Also, they can be used for evaluating the need for a permanent soft liner for patients with chronic or denture-induced pain [ 8 ].
Numerous efforts have been made to incorporate antimicrobial additives into the structures of tissue conditioners. These additives include antibiotics, essential oils, herbal oils, and notably, nanoparticles with antimicrobial properties [ 9 ]. Although some of these tissue conditioners show promising results against microorganisms, several deficiencies have been reported for the investigated cases. Among these defects, the lack of stability of the materials added to the tissue conditioner and the harmful effect on the mechanical properties of the tissue conditioner can be mentioned. Despite the positive effect of antimicrobial agents on tissue conditioners, there are no commercial antimicrobial tissue conditioners yet [ 9 , 10 , 11 , 12 , 13 ].
Nanotechnology has made significant advancements in various scientific domains, including dentistry, offering remarkable possibilities. One of the key attributes of nanoparticles is their high surface-to-volume ratio, which contributes to their exceptional properties [ 10 ]. Additionally, nanoparticles possess considerable strength and mechanical characteristics due to the formation of robust cross-links within polymer structures. Fragmenting materials into nanoparticles can be a potent method for creating structures with exceptionally high strength and excellent mechanical properties [ 11 ]. Furthermore, certain nanoparticles, such as silver, gold, copper, or zinc nanoparticles, exhibit antimicrobial properties [ 12 , 13 ].
Despite their considerable optical, catalytic, electrical, and antifungal/antimicrobial properties, copper nanoparticles are less known in the field of nanotechnology compared to other nanoparticles [ 14 ]. However, multiple studies have shown their antimicrobial effects on human pathogens [ 15 , 16 ]. Previous studies have introduced silver, zinc, or chitosan nanoparticles into the tissue conditioners’ structures to investigate their antimicrobial effects. However, despite their beneficial properties, copper nanoparticles are dramatically cost-effective, which justifies their use instead of other metal nanoparticles [ 17 ]. Considering the numerous shortcomings mentioned in relation to various substances added to tissue conditioners, the importance of the present study is to investigate the use of antimicrobial properties of copper nanoparticles in combination with tissue conditioners.
A study by Homsiang et al. used added zinc oxide nanoparticles to tissue conditioners, reporting their antifungal activity [ 18 ]. Moreover, Mousavi et al. have investigated the antimicrobial properties of silver, zinc, and chitosan nanoparticles [ 19 , 20 ].
In dentistry, Pseudomonas aeruginosa infections often develop in patients with apical periodontitis and pulp necrosis [ 21 , 22 ]. Moreover, Enterococcus faecalis , the predominant species of enterococcus genus in humans, is associated with several oral diseases, such as dental caries, root canal infections, periodontitis, and peri-implantitis [ 23 , 24 ]. Also, immunocompromised individuals have increased colonization of Candida albicans in their oral cavity, leading to potential oral candidiasis [ 25 , 26 , 27 ].
To the best of our knowledge, no study has ever investigated the effect of adding copper oxide nanoparticles into the tissue conditioners’ structures on their antimicrobial properties. Thus, the present study aimed to investigate the antibacterial and antifungal properties of tissue conditioners used in complete prostheses following adding different ratios of copper oxide nanoparticles. The antimicrobial effects have been evaluated against P. aeruginosa , E. faecalis , and C. albicans .
Materials and methods
Sample size.
In the present experimental study, the sample size was calculated at a minimum of 6 for each group using a confidence level of 95%, a statistical power of 80%, and the findings of previous studies [ 28 , 29 ]. Thus, we used a total sample size of 126, considering 21 subgroups.
Synthesis and characterization of copper oxide nanoparticle
The hydroalcoholic extract was prepared by grinding 20 g of propolis into a powder, which was then added to 100 mL of a hydroalcoholic solution (3:7 v/v) and kept at room temperature for one week. The hydroalcoholic solution, primarily composed of absolute ethanol, facilitated the extraction of polyphenolic compounds from the propolis, resulting in a higher extraction rate. After one week, the solution was filtered using a Whatman ® filter paper to remove any remaining propolis particles. The filtrate was then subjected to centrifugation at 4000 rpm to separate any solid particles. The resulting supernatant, free from solid particles, was preserved at 4 °C for future experiments. For the preparation of copper oxide nanoparticles, a solution containing 10 ppm of copper chloride was dissolved in deionized water. The copper chloride solution was mixed with the propolis extract solution at a temperature of 80 °C and stirred for 2 h at a uniform speed. The solution was filtered using a Whatman ® filter paper to eliminate impurities, followed by centrifugation at 4000 rpm. The precipitate obtained was isolated and purified. The supernatant from the previous centrifugation step was subjected to further centrifugation at 8000 rpm. The resulting precipitate was rinsed several times and utilized for the identification and characterization of the copper oxide nanoparticles [ 30 , 31 ].
Nanoparticle introduction into the structure of tissue conditioner
The present study used the TDV Soft Provisional tissue conditioner (TDV Dental Ltda, Brasil) to prepare samples with 20%, 10%, 5%, 2.5%, 1.25%, 0.625%, and 0% (w/w) copper oxide nanoparticle. The copper oxide nanoparticles were added to the tissue conditioner powder with a certain powder-to-solution ratio and were mixed for 30 s to become homogenized based on the manufacturer’s instructions. Then, the solution was poured into molds of equal sizes (diameter: 12 mm, depth: 2 mm). The mixed paste was placed between glass slides until it hardened [ 27 ]. Moreover, the samples with undesirable shapes, uneven surfaces, wrong powder-to-liquid ratios, and bubbles were excluded from the study.
Microorganism culture
The present study evaluated the standard human pathogens, including E. faecalis ( ATCC 29,212 ) , P. aeruginosa (ATCC 27,853), and C. albicans ( ATCC 10,261 ) , which were obtained from the Microbial Bank of the Microbiology and Mycology Laboratory, School of Medicine, Hamedan University of Medical Sciences. The bacteria were cultured in blood agar media, while C. albicans was cultured in sabouraud dextrose agar media under laboratory conditions. A suspension with the concentration of 0.5 McFarland (1.5 × 10 8 bacteria/mL) measured using a spectrophotometer (Spectrophotometer Single Beam AE-S60-4 V, A & E Lab co, UK) was prepared from the grown colonies and then was diluted to obtain a suspension in Mueller Hinton broth media with the concentration of 1.5 × 10 5 bacteria/mL. Then, 200 µL of the suspension was added to tissue conditioner samples using a sampler (BRAND, Transferpette S, Germany). Afterward, the samples were incubated at a temperature of 37° C for 24 h, except for the media containing C. albicans that were incubated for 48 h.
Microorganism growth assessment
The broth media containing the microbes on the tissue conditioners was sampled using a sterile swab, and the bacteria or fungi were cultured on blood agar or sabouraud dextrose agar media, respectively, using the lawn culture method. Following incubation for 24 h, the colonies were counted and reported using the CFU/mL.
Nanoparticle characterization
The following methods were used for the characterization of nanoparticles:
X-Ray Diffraction (XRD): This method used the X-ray diffractometer (Xpert Pro MPD, Panalytical, Netherlands) at the wavelength of 1.5405 Å and the power of 40 KV/30 mA to evaluate the crystal structure of nanoparticles.
Fourier-Transform Infrared spectroscopy (FTIR): This method used the FTIR spectrometer (Spectrum400, PerkinElmer, USA) and also was conducted by KBR pellet technique under identical situations in the 500–4000 cm − 1 region.
Transmittance Electron Microscope (TEM): This device was used to examine the surface morphology and size of the nanoparticles. A transmittance electron microscope (TEM, Zeiss; EM10C model, Germany) at an accelerating voltage of 100 kv was used.
Data analysis
Data analysis was performed using the SPSS software version 27 (SPSS Inc., Chicago, Illinois, United States). The mean and Standard Deviation (SD) of growth was calculated in each group. Then, the intergroup comparisons of the 24-hour growth of bacteria between different concentrations of copper oxide nanoparticles were performed using the one-way Analysis Of Variance (ANOVA). In case of significant differences, Turkey’s post hoc test was used to find certain concentrations with significantly different growth of the microorganism. Moreover, the intragroup comparison of C. albicans growth between 24-hour and 48-hour assessments was performed using the Mann-Whitney test. Also, the significance level was set at 0.05 for all tests, except for the post hoc tests, which had a significance level of 0.002.
Copper oxide nanoparticle characterization
Figure 1 presents the X-ray diffraction pattern of the copper oxide nanoparticles, showing monophasic nanoparticles with monoclinic structures. The peaks’ intensity and position in the obtained pattern completely correspond to the previously reported patterns. Figure 2 presents the TEM image taken from the copper oxide nanoparticles, showing crystalline copper oxide nanoparticles with a diameter of 30–70 nm.

X-ray diffraction pattern of copper oxide nanoparticles

TEM image of copper oxide nanoparticles
According to the FTIR of copper oxide nanoparticles in Fig. 3 , the half-broad band at about 3401 cm − 1 shows the stretching frequency of the hydroxyl group, an indicator of the surface morphology of the synthesized nanoparticles. Moreover, a peak in the 1047 cm − 1 corresponds to the bonds between copper and hydroxyl groups.

FTIR of copper oxide nanoparticles
Table 1 presents the intergroup comparison of bacterial growth in different concentrations of copper oxide nanoparticles following 24 h, while Fig. 4 shows the mean bacterial growth in logarithm in different nanoparticle concentrations. According to Table 1 , the culture media containing E. faecalis and P. aeruginosa showed significantly different growth between different nanoparticle concentrations ( P < 0.001), showing a reduction in bacterial growth with increased nanoparticle concentration. Interestingly, both bacteria did not show any growth at the 20% concentration.

The mean bacterial growth in logarithm in different concentrations of copper oxide nanoparticles following 24 h
Table 2 presents the intergroup and intragroup comparison of C. albicans growth in different concentrations of copper oxide nanoparticles following 24 and 48 h, while Fig. 5 shows the mean growth in different nanoparticle concentrations following 48 h. According to Table 2 , C. albicans showed equal growth in all nanoparticle concentrations following 24 h, showing no significant difference ( P > 0.05). However, the growth significantly reduced following 48 h of culture compared to the 24-hour assessment ( P = 0.002), with the mean 24-hour growth being 9.9 × 10 4 folds higher than the 48-hour growth. Moreover, the 48-hour growth was significantly different between different nanoparticle concentrations ( P < 0.001), showing a reduction in growth with increased nanoparticle concentration. Thus, the least growth was observed at the 20% concentration.

The mean growth of C. albicans in different concentrations of copper oxide nanoparticles following 48 h
Considering the significant intergroup differences in all studied pathogens calculated using the one-way ANOVA, pairwise comparisons were performed for each pathogen between different concentrations. Table 3 presents the pairwise intergroup comparisons using Tukey’s post hoc test. According to Table 3 , culture media containing E. faecalis showed significant differences in 0-1.25%, 0-2.5%, 0-5%, 0-10%, 0-20%, 0.625-1.25%, 0.625-2.5%, 0.625-5%, 0.625-10%, and 0.625-20% pairwise comparisons following 24 h of culture ( P < 0.001). Moreover, P. aeruginosa showed significantly different 24-hour growth in 0-0.625%, 0-1.25%, 0-2.5%, 0-5%, 0-10%, and 0-20% pairwise comparisons ( P < 0.001). Also, C. albicans showed significantly different growth between the 0% and 20% concentrations ( P < 0.001), as well as the 0.625% and 20% concentrations ( P = 0.002). Thus, the growth was reduced in all pathogens with increased concentrations of copper oxide nanoparticles.
The present study was the first to investigate the effect of tissue conditioners containing copper oxide nanoparticles on the growth of E. faecalis and P. aeruginosa .
The green biosynthesis of CuO nanoparticles was successfully conducted using a non-toxic, cost-effective, easy, and eco-friendly approach. These copper nanoparticles will probably be used in pharmaceutical formulations, drug delivery systems, and biomedical applications in the future since they can be prepared from natural products using a green biosynthesis method [ 32 ]. According to the findings from XRD, FTIR, and TEM investigations, the CuO nanoparticles made in the present study had monoclinic crystalline structures. Moreover, they had a suitable diameter in the nm range while maintaining their desirable properties and bonds.
Infection with P. aeruginosa is often reported in patients with apical periodontitis and pulp necrosis. Almost all patients with such infections are of lower socio-economic status and have poor oral and dental hygiene, gingivitis, and decayed teeth. The considerable resistance of P. aeruginosa to most antibiotics often makes its treatment extremely difficult, whether systematic or focal. In the present study, P. aeruginosa was used as a representative of gram-negative, antibiotic-resistant bacteria [ 22 ].
On the other hand, Enterococci are the causative agent of various infections, including endocarditis, meningitis, urinary tract, neonatal, and wound infections. Some of these infections are potentially fatal. Moreover, they are globally known as significant nosocomial pathogens, considering the growing emergence of antimicrobial-resistant phenotypes in the last few decades. Enterococci are resistant to vancomycin, tetracyclines, penicillins, cephalosporins, and aminoglycosides. Also, E. faecalis is often the cause of root canal treatment failure due to its high antibiotic resistance. In the present study, E. faecalis is the representative of gram-positive, antibiotic-resistant bacteria [ 23 , 24 ].
Considering the movement limitations of elderly patients using removable prostheses, [ 6 , 7 , 8 ] one of the clinical applications of tissue conditioners containing CuO nanoparticles is to help these patients prevent the growth of pathogenic microorganisms, which is facilitated the observance of hygiene by patients.
Previous research has reported the reactive oxygen species production and resultant oxidative stress as the potential cause of the antibacterial properties of copper nanoparticles. Moreover, these nanoparticles can show direct cytotoxicity by disrupting the membrane function, altering its permeability, and attacking different cellular structures and proteins containing phosphorus and sulfur [ 25 ]. It is worth mentioning that the present study did not use CuO concentrations higher than 20% since they exert cytotoxic effects. Moreover, we used the principles of the Minimal Inhibitory Concentration (MIC) technique to dilute the CuO nanoparticle concentration by half in each step [ 19 , 33 ]. Also, according to Chul Lee et al. the no-observed-adverse-effect levels of Cu nanoparticles and Cu micro particles were determined to be 100 and ≥ 400 mg/kg/day, respectively [ 33 ]. As nanoparticles are solid and in combination with tissue conditioner in our study, they are much harmless compared to the previous study that nanoparticles were used in solution form.
The present study used the colony counting method to assess the number of microorganisms, which is superior to the optical absorption density method since it does not count the non-viable microorganisms [ 34 ]. The reduced growth of E. faecalis and P. aeruginosa in different concentrations of CuO nanoparticles confirmed the benefit of this method. Moreover, the increasing concentration of CuO nanoparticles could directly reduce bacterial growth. Also, the 20% CuO nanoparticle concentration completely stopped the growth in both studied bacteria.
A study by Maqusood et al. evaluated the antimicrobial effect of CuO nanoparticles, reporting compatible results with the present study regarding the effect of these nanoparticles on E. faecalis and P. aeruginosa . Moreover, its effect on E. faecalis was comparable with streptomycin as the standard positive control [ 35 ]. Also, another study by Mardones et al. used CuO nanoparticles inside the root canal to suppress bacterial growth. Furthermore, they conducted an in vitro assessment of E. faecalis growth, reporting a dramatically reduced growth following 24 h compared to the negative control group, which was compatible with our findings. It seems that CuO nanoparticles have higher inhibitory effects on bacterial growth compared to Cu particles with conventional dimensions due to increased bacterial exposure to Cu and facilitated penetration into the cells of the microorganisms [ 36 ].
On the other hand, a study by Mousavi et al. used ZnO-Ag-based tissue conditioners to inhibit the growth of E. faecalis and P. aeruginosa , reporting complete growth arrest in 20% nanoparticle concentration. This study was compatible with the present study regarding the nanoparticle concentration and incubation duration. Thus, it can be concluded that ZnO-Ag and CuO nanoparticles have equal antimicrobial effects [ 27 ]. Moreover, another study investigated the antimicrobial effect of chitosan-based tissue conditioners, reporting complete growth arrest in 5% and 10% chitosan nanoparticle concentrations for P. aeruginosa and E. faecalis , respectively. Thus, it can be concluded that chitosan nanoparticles have a higher inhibitory effect on the growth of these two bacteria compared to CuO nanoparticles [ 20 ]. Also, a study by García Marin et al. compared the antimicrobial effect of Cu nanoparticles on C. albicans compared to common drugs used for treating C. albicans infections, including fluconazole, nystatin, and amphotericin B, reporting a higher antifungal effect for CuO nanoparticles compared to amphotericin B. Furthermore, the observed effect was highly concentration-dependent [ 37 ]. Thus, the mentioned study was compatible with our findings.
Geographically, propolis samples exhibit distinct chemical compositions that directly influence their antioxidant properties. For instance, ethanolic extracts of propolis from Russia and Italy demonstrate similar antioxidant effects due to the presence of shared polyphenols. In contrast, Brazilian propolis has a relatively lower antioxidant effect owing to its diminished polyphenol content [ 38 ]. However, the current understanding of the properties of Iranian propolis is limited and incomplete. Further research is required to comprehensively explore its therapeutic potential. The utilization of propolis is driven by its well-documented therapeutic properties, aiming to augment the economic value of raw propolis and facilitate the development of innovative pharmaceuticals [ 39 ].
According to Amiri et al. study, [ 40 ] copper nanoparticles have a preventive effect on infections caused by different species of Candida. Also, according to the study of Garcia-Marin et al. [ 37 ], copper oxide nanoparticles have a very high effect as a topical antifungal treatment against Candida albicans.
According to the findings of this study on the antimicrobial effects of CuO nanoparticles, it is recommended to do more research regarding the addition of these nanoparticles in heat-cured and 3D-printed denture base resins.
In the end, it is recommended to conduct further studies on the physical and mechanical properties of CuO nanoparticle-based tissue conditioners. Moreover, the antimicrobial effects of such tissue conditioners should be investigated on a more extensive range of microorganisms found in the oral cavity. More research regarding antibiotic resistance tests and biofilm formation is also recommended.
In the present study, the CuO nanoparticles were made properly in suitable sizes. Moreover, the tissue conditioners containing copper oxide nanoparticles showed acceptable antimicrobial effects against E. faecalis , P. aeruginosa , and C. albicans . Also, it is recommended to conduct further studies on this topic to find the optimal concentration of CuO nanoparticles in tissue conditioners, thereby introducing the application of nanoparticles to the field of dental material science to make commercial tissue conditioners containing copper oxide nanoparticles.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.All data generated or analysed during this study are included in this published article [and its supplementary information files].
Wöstmann B, Budtz-Jørgensen E, Jepson N, Mushimoto E, Palmqvist S, Sofou A et al. Indications for removable partial dentures: a literature review. Int J Prosthodont. 2005;18(2).
Tyson K, Yemm R, Scott B. Understanding partial denture design. Oxford University Press; 2006.
Grossmann Y, Nissan J, Levin L. Clinical effectiveness of implant-supported removable partial dentures—a review of the literature and retrospective case evaluation. J Oral Maxillofac Surg. 2009;67(9):1941–6.
Article PubMed Google Scholar
Sharma A, Shashidhara H. A review: flexible removable partial dentures. J Dent Med Sci. 2014;13(12):58–62.
Google Scholar
Geramiuanah F, ASADI G. Post insertion problems of removable prosthesis: Causes, diagnosis and treatment. 2007.
Carr AB, Brown DT. McCracken’s Removable Partial Prosthodontics-E-Book. Elsevier Health Sciences; 2010.
Khasawneh S, al-Wahadni A. Control of denture plaque and mucosal inflammation in denture wearers. J Ir Dent Assoc. 2002;48(4):132–8.
PubMed Google Scholar
Zarb GA. Prosthodontic treatment for edentulous patients: complete dentures and implant-supported prostheses. 13 ed. St. Louis, Mo.: Elsevier Mosby; 2013.
Iqbal Z, Zafar MS. Role of antifungal medicaments added to tissue conditioners: a systematic review. J Prosthodontic Res. 2016;60(4):231–9.
Article Google Scholar
Zhu A, Cai A, Zhou W, Shi Z. Effect of flexibility of grafted polymer on the morphology and property of nanosilica/PVC composites. Appl Surf Sci. 2008;254(13):3745–52.
Article CAS Google Scholar
Sun L, Gibson RF, Gordaninejad F, Suhr J. Energy absorption capability of nanocomposites: a review. Compos Sci Technol. 2009;69(14):2392–409.
Fernando S, Gunasekara T, Holton J. Antimicrobial Nanoparticles: applications and mechanisms of action. 2018.
Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227.
Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA. Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett. 2012;71:114–6.
Meto A, Colombari B, Sala A, Pericolini E, Meto A, Peppoloni S, et al. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent Mater J. 2019;38(4):591–603.
Article CAS PubMed Google Scholar
Xu VW, Nizami MZI, Yin IX, Yu OY, Lung CYK, Chu CH. Application of copper nanoparticles in dentistry. Nanomaterials. 2022;12(5):805.
Article CAS PubMed PubMed Central Google Scholar
Bogdanović U, Lazić V, Vodnik V, Budimir M, Marković Z, Dimitrijević S. Copper nanoparticles with high antimicrobial activity. Mater Lett. 2014;128:75–8.
Homsiang W, Kamonkhantikul K, Arksornnukit M, Takahashi H. Effect of zinc oxide nanoparticles incorporated into tissue conditioner on antifungal, physical, and mechanical properties. Dent Mater J. 2021;40(2):481–6.
Mousavi SA, Khorramdel A, Aghajanzadeh H. Evaluation of antifungal and antibacterial properties of adding Ag, ZnO, Chitosan nanoparticles to tissue conditioners of complete dentures. Res Dent Sci. 2020;17(3):171–81.
Mousavi SA, Ghotaslou R, Kordi S, Khoramdel A, Aeenfar A, Kahjough ST, et al. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int J Biol Macromol. 2018;118:881–5.
Nair VV, Karibasappa GN, Dodamani A, Prashanth VK. Microbial contamination of removable dental prosthesis at different interval of usage: an in vitro study. J Indian Prosthodont Soc. 2016;16(4):346–51.
Article PubMed PubMed Central Google Scholar
Nord CE, Sjöberg L, Wadström T. Pseudomonas Aeruginosa in oral infections. Acta Odontol Scand. 1972;30(3):371–81.
Komiyama EY, Lepesqueur LS, Yassuda CG, Samaranayake LP, Parahitiyawa NB, Balducci I, et al. Enterococcus species in the oral cavity: prevalence, virulence factors and Antimicrobial susceptibility. PLoS ONE. 2016;11(9):e0163001.
Patel M. Oral cavity and Candida albicans: Colonisation to the development of infection. Pathogens. 2022;11(3):335.
Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, et al. Understanding the Antibacterial mechanism of CuO nanoparticles: revealing the Route of Induced oxidative stress. Small. 2012;8(21):3326–37.
Nam KY, Lee CJ. Chapter 14 - characterization of silver nanoparticles incorporated acrylic-based tissue conditioner with antimicrobial effect and cytocompatibility. In: Subramani K, Ahmed W, editors. Nanobiomaterials in Clinical Dentistry. Second Edition): Elsevier; 2019. pp. 335–48.
Mousavi SA, Ghotaslou R, Akbarzadeh A, Azima N, Aeinfar A, Khorramdel A. Evaluation of antibacterial and antifungal properties of a tissue conditioner used in complete dentures after incorporation of ZnO–Ag nanoparticles. J Dent Res Dent Clin Dent Prospects. 2019;13(1):11–8.
Nam K-Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J Adv Prosthodont. 2011;3(1):20–4.
Haghdoost A, Baneshi M, Marzban M. How to Estimate the Sample size in Special conditions? (part two). Iran J Epidemiol. 2011;7(2):67–74.
Habibipour R, Moradi-Haghgou L, Farmany A. Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int J Nanomed. 2019;14:6891–9.
https:// www.thejcdp.com/doi/JCDP/pdf/10.5005/jp-journals-10024-3393 .
Hajizadeh YS, Harzandi N, Babapour E, Yazdanian M, Ranjbar R. Green synthesize and characterization of copper nanoparticles using Iranian Propolis extracts. Adv Mater Sci Eng. 2022;2022:8100440.
Lee I-C, Ko J-W, Park S-H, Shin N-R, Shin I-S, Moon C, et al. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part Fibre Toxicol. 2016;13(1):56.
Pan H, Zhang Y, He GX, Katagori N, Chen H. A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiol. 2014;14:222.
Ahamed M, Alhadlaq HA, Khan MAM, Karuppiah P, Al-Dhabi NA. Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J Nanomaterials. 2014;2014:637858.
Ml JM, C G, Covarrubias DCG. C. In Vitro Antibacterial Properties of Copper Nanoparticles as Endodontic Medicament against Enterococcus faecalis. 2018.
Garcia-Marin LE, Juarez-Moreno K, Vilchis-Nestor AR, Castro-Longoria E. Highly antifungal activity of Biosynthesized Copper Oxide nanoparticles against Candida albicans. Nanomaterials. 2022;12(21):3856.
Freires IA, de Alencar SM, Rosalen PL. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur J Med Chem. 2016;110:267–79.
Zabaiou N, Fouache A, Trousson A, Baron S, Zellagui A, Lahouel M, Lobaccaro J-M A. Biological properties of propolis extracts: something new from an ancient product. Chem Phys Lipids. 2017;207:214–22.
Amiri M, Etemadifar Z, Daneshkazemi A, Nateghi M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. J Dent Biomaterials. 2017;4(1):347–52.
CAS Google Scholar
Download references
This study is part of a PhD thesis at Hamadan University of Medical Sciences. The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (Code number: 140104212693).
Author information
Authors and affiliations.
Department of Prosthodontics, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
Saeed Nikanjam & Aria Yeganegi
Department of Microbiology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Mohammad-Yousef Alikhani
Dental Implant Research Center, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
Abbas Farmany
Department of Medical Parasitology and Mycology Department, School of Medicine, Hamadan University of Medical Sciences and Health Services, Hamadan, Iran
Seyed Amir Ghiasian
Department of Biostatistics, School of Public Health and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
Roghayeh Hasanzade
You can also search for this author in PubMed Google Scholar
Contributions
Saeed Nikanjam: A Aria Yeganegi2: B Mohammad-Yousef Alikhani: C Abbas Farmany: D Seyed Amir Ghiasian: ERoghayeh Hasanzade: Fconception: A, Bdesign of the work : A, Bthe acquisition, analysis: C, D, E, Finterpretation of data: A, B, F the creation of new software used in the work: D.
Corresponding author
Correspondence to Saeed Nikanjam .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by an ethics committee of Hamadan University of Medical Sciences (Ethics No. IR.UMSHA.REC.1400.1004). Informed consent was obtained from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nikanjam, S., Yeganegi, A., Alikhani, MY. et al. Novel antimicrobial applications of copper oxide nanoparticles after combination with tissue conditioner used in complete prostheses. BMC Oral Health 24 , 752 (2024). https://doi.org/10.1186/s12903-024-04534-w
Download citation
Received : 08 January 2024
Accepted : 25 June 2024
Published : 28 June 2024
DOI : https://doi.org/10.1186/s12903-024-04534-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Copper oxidenanoparticle
- C. Albicans
- P. Aeruginosa
- E. Faecalis
BMC Oral Health
ISSN: 1472-6831
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
ZnO nanoparticles were prepared by refluxing precursor zinc acetate dihydrate (0.1 M) in diethylene glycol and triethylene glycol at 180 °C and 220 °C respectively. Reaction time varied for 2 and 3 h with and without sodium acetate (0.01 M). Before refluxing, the solution was kept on a magnetic stirrer at 80 °C for 1.5 h. ...
In the present work, bioaugmented zinc oxide nanoparticles (ZnO-NPs) were prepared from aqueous fruit extracts of Myristica fragrans. The ZnO-NPs were characterized by different techniques such as X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, ultraviolet (UV) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light ...
In this thesis, on one hand, the two-step chemical bath deposition method was used to grown ZnO nanorod arrays (ZNAs) on Si substrates. Firstly, the effects of ZnO nanoparticles, pH value of chemical solution, angel θ between substrate and beaker bottom on the structures of the samples were symmetrically investigated and
When ZnO is photo-induced by solar light with photonic energy (hv) equal to or greater than the excitation energy (E g), e − from the filled valence band (VB) are promoted to an empty conduction band (CB).This photo-induced process produces electron-hole (e − /h +) pairs as shown in (Eq. (1)).The electron-hole pairs can migrate to the ZnO surface and be involved in redox reactions as shown ...
Zinc oxide nanoparticles are categorized. among the materials that have potential. applications in many a reas of nanotechnology. [29, 30]. Z nO possesses one-, two- and three-. dimensional str uc ...
A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Physics UNIVERSITY OF PUERTO RICO MAYAGUEZ CAMPUS 2013 Approved by: Oscar Perales-Perez, PhD Date President, Graduate Committee Sergiy Lysenko, PhD Date Member, Graduate Committee Héctor Jiménez, Ph.D Date ... 3.1 Bare ZnO nanoparticles ...
The zinc oxide (ZnO) nanoparticles were prepared by wet chemical method using zinc nitrate. and sodium hydroxides precursors and soluble starch as stabilizing agent. Different. concentrations of ...
However, ZnO B NPs introduce a higher zone of inhibition than ZnO A nanoparticles and the measurements of the inhibition zone of bacterial growth are tabulated in table 2. According to Krishna R Rangupathi, the antibacterial activity of nanoparticles is a size-dependent property and the property enhances with the reduction of particle size [ 23 ].
ZnO nanoparticles were recommended by Bagabas et al. [106] for environmental applications. They synthesized ZnO nanoparticles from wet chemical route with cyclohexylamine in aqueous and enthanolic medium and detected the photodegradation of cyanide ions. ... (PhD Thesis) (2012) Google Scholar [3] H.N. Bose. Luminescence and allied phenomena ...
Zinc oxide nanoparticles (ZnO-NPs) are the most commonly used metal oxide nanoparticles because their distinctive optical and chemical properties can be easily modified by altering the morphology and the wide bandgap (3.37 eV) and high excitation binding energy (60 meV) to simulate the ZnO-NPs to be a potent photocatalytic and photo-oxidizing ...
are used in the synthesis of nanoparticles.3 Plant-based synthesis is more useful for the synthesis of nanoparticles, Table 1. Typically, plant parts such as the leaf bark, root, stem, seed, flower,fruit, and tubers have been employed to synthesize nanoparticles.4 Many researchers prefer plants for the synthesis Received: November 29, 2022
Biosynthesis and characterization of ZnO nanoparticles. The synthesis of biosynthesized ZnO NPs was carried out by taking 2:2 (v/v) of leaves extract of and zinc nitrate solution to obtain a ...
Figure 5-1. Liquid cell TEM of SnO2/ZnO core/shell nanowires using organic electrolytes. Figure 5-2. Electrochemical cycling of Pt nanoparticles in liquid cell TEM. Figure 5-3. Comparison of the product selectivity of Cu catalyst for CO2RR reaction. Figure 5-4. Time series of bright field TEM images of Cu deposition on Pt electrode under
To harness the unique properties of graphene and ZnO nanoparticles (NPs) for novel applications, the development of graphene-ZnO nanoparticle hybrid materials has attracted great attention and is ...
The potential ecotoxicity of nanosized zinc oxide (ZnO), synthesized by the polyol process, was investigated using common Anabaena flos-aquae cyanobacteria and Euglena gracilis euglenoid microalgae. The photosynthetic activities of these microorganisms, after addition of ZnO nanoparticles, varied with the presence of protective agents such as tri-n-octylphosphine oxide (TOPO) and ...
A thesis submitted in partial fulfilment of the requirements for the degree of Magister Scientiae in the Department of Physics, University of the Western Cape . ... quasi-spherical ZnO nanoparticles with an average crystallite size of 24.6 nm (at 300 °C), 27.2 nm (at 400 °C), 27.6 nm (at 500 °C), and 28.5 nm (at 600 °C) were found. The results
In this study, ZnO nanoparticles were fabricated by co-precipitation method. The synthesized nanoparticles possessed monodispersity with the average size 20-30 nm. Since the industrial effluents may not be at neutral pH, the effect of pH on the rate of degradation is important and need to be considered. In order to investigate the effect of pH on ZnO nanoparticles photocatalytic activity ...
Phd Thesis Zno Nanoparticles - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world's largest social reading and publishing site.
The preparation of zinc oxide (ZnO) nanoparticles (NPs) by green synthesis route using carica papaya leaf extract for photocatalytic application has been demonstrated. In this work, the phase pure ZnO NPs were synthesized via facile green synthesis method, where zinc acetate dihydrate was used as precursor and papaya leaf extract as reducing ...
This is to certify that the thesis entitled, "Synthesis and characterisation of ZnO nanoparticles with various size and their application in biological system" submitted by Mr. Omkar Behera (107BT017) in ful llments for the requirements for the award of Bachelor of Technology degree
PDF | On Aug 31, 2016, Ghata Satish Bhayani published Synthesis Of Zinc Oxide Nanoparticles Through Various Methods, Its Characterization And Probable Applications | Find, read and cite all the ...
Scientific Reports - Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment.
This is to certify that the thesis entitled "Synthesis and Characterization of ZnO nanoparticles" is submitted by Mr. JAYANTA KUMAR BEHERA, (Roll NO- 407PH102) to this Institute in partial fulfillment of the requirement for the award of the degree of Master of Science in Department Physics, is a bonafied record of the work carried out under my
Ph.D.Thesis, Dublin City University, 2013. Google Scholar. ... We found that the ZnO nanoparticles exerted a cytotoxic effect on the human glioma cell lines A172, U87, LNZ308, LN18, and LN229, whereas no cytotoxic effect was obsd. on normal human astrocytes. Similarly, the ZnO nanoparticles induced cell death in breast and prostate cancer cell ...
Investigated a hybrid composite of Hydroxypropyl Methylcellulose (HPMC) and Zinc Oxide (ZnO) nanoparticles for biodegradable TENGs. • The 1% HPMC: ZnO composite achieved the highest performance, with a maximum voltage of 39.8 V, a current of 4.38 μA, and a power density of 0.23 W/m².. The composite films fully degrade in water within 36 hours, demonstrating their potential for sustainable ...
Van Cleave PhD Dissertation Final Version.pdf (2.204Mb) Date 2024-06-27. Author. Van Cleave, Justin. Type of Degree PhD Dissertation. Department. ... The magnitudes of the Kerr coefficients for the gold nanoparticle samples have been compared and used to verify theoretical models on the relationship between particle diameter and third-order ...
However, there is a wide knowledge gap on the fundamentals of interactions between nanoparticles and dissolved toxic metallic ions in complex agricultural matrices. This dissertation focuses on understanding the unique chemistry of interactions between nanoscale zerovalent iron (NZVI) and metallic contaminants in water and farm soils and ...
require small amounts of materials for performing research work [8]. In this thesis, we report a study of structural, optical and electronic properties of. Cobalt incorporated ZnO QDs. The ...
To encapsulate the modified silica nanoparticles in PS-b-PAA vesicles, 3 mg/mL of block copolymer was first dissolved in THF for 24 h and then mixed with the nanoparticle suspension in THF (nanoparticle-to-polymer ratio of 1:10, 1:20, 1:100, and 1:200 or a particle concentration of 0.01, 0.005, 0.001, and 0.0005 wt% at a constant polymer ...
Thus, it can be concluded that ZnO-Ag and CuO nanoparticles have equal antimicrobial effects . Moreover, another study investigated the antimicrobial effect of chitosan-based tissue conditioners, ... This study is part of a PhD thesis at Hamadan University of Medical Sciences. The study was funded by Vice-chancellor for Research and Technology ...