An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Nanomaterials (Basel)


Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications
Vancha harish.
1 School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab 144401, India; moc.liamg@hsirahahcnav (V.H.); moc.liamg@3irawetd (D.T.)
Devesh Tewari
Manish gaur.
2 Centre of Biotechnology, University of Allahabad, Prayagraj, Uttar Pradesh 211002, India; ni.ca.vinudlla@ruaghsinam
Awadh Bihari Yadav
Shiv swaroop.
3 Department of Biochemistry, Central University of Rajasthan, Ajmer 305817, India; ni.ca.jaruc@poorawsvihs
Mikhael Bechelany
4 Institut Européen des Membranes, IEM UMR 5635, University Montpellier, ENSCM, CNRS, 34730 Montpellier, France
Ahmed Barhoum
5 NanoStruc Research Group, Chemistry Department, Faculty of Science, Ain Helwan, Cairo 11795, Egypt
6 National Centre for Sensor Research, School of Chemical Sciences, Dublin City University, D09 Y074 Dublin, Ireland
Associated Data
The data presented in this study are available on request from the corresponding author.
In the last few decades, the vast potential of nanomaterials for biomedical and healthcare applications has been extensively investigated. Several case studies demonstrated that nanomaterials can offer solutions to the current challenges of raw materials in the biomedical and healthcare fields. This review describes the different nanoparticles and nanostructured material synthesis approaches and presents some emerging biomedical, healthcare, and agro-food applications. This review focuses on various nanomaterial types (e.g., spherical, nanorods, nanotubes, nanosheets, nanofibers, core-shell, and mesoporous) that can be synthesized from different raw materials and their emerging applications in bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-foods. Depending on their morphology (e.g., size, aspect ratio, geometry, porosity), nanomaterials can be used as formulation modifiers, moisturizers, nanofillers, additives, membranes, and films. As toxicological assessment depends on sizes and morphologies, stringent regulation is needed from the testing of efficient nanomaterials dosages. The challenges and perspectives for an industrial breakthrough of nanomaterials are related to the optimization of production and processing conditions.
1. Introduction
In the last 50 years, material researchers have been extensively studying how to exploit nanoparticles and nanostructured materials in different biomedical and healthcare sectors [ 1 ]. The term “NP” usually defines minute particles of matter (1 to 100 nm in diameter), but other names can be used to describe larger particles (up to 500 nm in diameter). For example, nanorods, nanowires, and nanofibers are nanoparticles with a diameter in the 1–100 nm range but with one dimension outside the nanoscale dimension [ 2 ]. Nanostructured materials are nanomaterials with one dimension in the nanoscale range (<100 nm) and are made of a single material or multiple materials. Therefore, nanostructured materials are composed of interlinked parts in the nanoscale range [ 3 ]. Nanoparticles and nanostructured materials can be made of simple materials (e.g., metal, carbon, polymer) [ 4 ], of composites (e.g., polymer-metal, silica-metal, graphene-metal), or in the core-shell form [ 5 , 6 , 7 , 8 ].
Nanomaterials are typically synthesized by one of two main approaches, i.e., bottom-up approach and top-down approach. Among all the methods, recently, the synthesis of nanomaterials by physical vapor deposition, chemical vapor deposition, electrospinning, 3D printing, biological synthesis, and supercritical fluid have gained importance, which is mingled with other methods to improve the synthesis efficiency [ 9 , 10 ]. Nanomaterials display many interesting features, such as superior mechanical performance, the possibility of surface functionalization, large surface area, and tunable porosity, compared to their bulk materials [ 11 , 12 , 13 ]. These outstanding features explain why nanomaterials are the perfect candidates in the biomedical sector for the production of tissue-engineered scaffolds (e.g., blood vessels, bone), drug delivery systems (gene therapy, cancer treatments, drugs for chronic respiratory infections), chemical sensors [ 4 , 5 ], biosensors [ 6 , 7 ], and wound dressings [ 14 , 15 ]. Remarkably, several studies suggest that ancient civilizations in India, Egypt, and China used nanotechnology (metallic gold) for therapeutic purposes in 2500 BC [ 16 ]. Nanomaterials’ discrete features can complicate the assessment of the effects and the toxicity risk associated with their use in a biological environment. Indeed, nanomaterials’ chemical composition, size, shape, surface charge, area, and entry route in the body can influence their biological activities and effects [ 17 ].
In bioimaging, tailored fluorescent nanoparticles could outperform traditional molecular probes as fluorescent indicators, particularly in terms of sensitivity [ 18 ]. Tissue-engineered nanofiber scaffolds are considered the best option to manage tissue loss and end-stage organ failure and have already helped millions of patients worldwide [ 15 ]. Three-dimensional nanofibrous scaffolds are polymer-based structures with balanced moisture, absorption, strongly organized porosity (60–90%), and gas permeability, comparable to native extracellular matrices [ 15 ]. One and two-dimensional nanomaterials can be used for signal amplification, are nanosized (≤100 nm), have high electrical conductivity, and are compatible with drugs [ 13 ] and biological molecules [ 12 ]. They have also been used for the early detection of diseases (e.g., virus, bacterial, cancer). Antimicrobial nanomaterials (e.g., Ag, Au, CuO NPs) are frequently employed in dermatology because they contribute to accelerating wound healing and preventing/treating bacterial infections [ 19 , 20 ].
Based on dimensionality, nanomaterials are classified mainly into four groups [ 1 ]: 0D, where length, height, and width are fixed at a single point; 1D, where only one parameter exists (e.g., carbon nanotubes); 2D, where length and width exist (e.g., graphene); and 3D, where length, height, and width exist [ 21 ]. For example, a graphene nanosheet is a typical example of a 2D nanostructure with a thickness in the nanoscale range. Theoretically, single-layer graphene is 0.345 nm thick (one atom thickness) and up to 500 nm in diameter. Based on their chemical composition, nanoparticles and nanostructured materials can be categorized into four types: organic nanomaterials (e.g., micelles, dendrimers, polymersomes, hydrogels, nanoconjugates), inorganic nanomaterials (e.g., metals, metal oxide, and ceramic nanomaterials), carbon-based nanomaterials (fullerenes, carbon nanofibers, diamonds carbon nanotubes, and graphene), and composite nanostructures [ 1 , 22 ]. The synthesis of traditional nanosized products contributes to the present and future economic growth of many countries. Based on porosity, nanomaterials can be classified into porous and non-porous materials [ 3 ]. Porous materials have a less dense molecular structure to allow airflow or the absorption of atoms, ions, and molecules. Non-porous materials are denser, do not absorb well, and allow limited airflow. Mesoporous (or super-nanoporous) nanomaterials are nanoporous materials with pores of 2–50 nm in diameter [ 23 , 24 ]. Recent research has focused on mesoporous nanomaterials for delivering therapeutic agents to tumor cells with little drug leakage into healthy cells. The high porosity, surface functionality, and small pore size of mesoporous nanoparticles allow the controlled release and efficient drug loading at the target site [ 22 ].
Considering the growing use of nanomaterials over the last decades, our group reviewed the key aspects of different types of nanomaterial design and their emerging applications in the biomedical fields [ 1 , 2 , 3 , 15 ]. This review article discusses the unique features of nanomaterials that are exploited for different biomedical applications ( Figure 1 ). It also presents recent trends on nanomaterials use for biomedical engineering, with a particular emphasis on the preparation methods used for designing nanomaterials. The advancement of nanomaterials in the biomedical and health sectors (bioimaging, tissue engineering, wound dressings, drug delivery, biosensors, food industry, and agriculture) is discussed. The review deals with various relevant nanomaterials variables, such as charge, concentration, particle surface modification, and size, which must be considered during the bioimaging of living cells. Various targeted drug delivery systems for treating chronic diseases are described. The use of nanomaterials to improve dental implants is also discussed, as well as the fabrication methods [ 14 ]. As many review articles have already described the biomedical applications of nanomaterials [ 1 , 2 , 3 , 4 ], this review will be extended to discuss the nanomaterial’s toxicity, risks, and regulations in the biomedical sector.

Summary of the recent topic on nanoparticles and nanostructured materials and their applications in bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food sectors. Image created by Biorender.
2. Fluorescent Nanomaterials for Bioimaging
Bioimaging is an advanced non-invasive technology used to visualize internal structures and physiological processes in living cells/organisms in real-time. It is a safe and effective technique for monitoring biological functions without affecting normal life processes (e.g., respiration and movement). It also helps to obtain data on the sample 3D nanostructure [ 25 ] and to investigate tissues at the subcellular and multicellular scale [ 26 ]. Several nanoprobes have been developed for bioimaging and the treatment of many diseases (cancer, heart, and inflammatory diseases) [ 27 , 28 , 29 ]. Nanomaterials are ideal materials for nanoprobes because they can be exactly characterized using nuclear magnetic resonance or gel permeation chromatography and are easily secreted from the body. However, their functions are limited, and researchers are always looking for new materials. In recent days, magnetic nanoparticles have gained great interest because of their progress in image-guided therapy (e.g., fluorescence, magnetic resonance, X-ray CT) and cancer theranosis favorable properties, such as tunable size, generating reactive oxygen species (ROS) or heat, simple fabrication, energy transfer, and X-ray absorption properties ( Figure 2 ). Moreover, their long-term toxicity and dispersion stability must be specifically investigated.

Schematic representation showing the utilization of magnetic nanoparticles in tumor bioimaging and therapy [ 30 ]. Copyright 2016, American Chemical Society.
Fluorescent nanoparticles’ specificity, light-emission (NIR-IR emission), and biocompatibility with the target tissues can be customized by changing their shape, size, and surface properties [ 27 ]. The cell uptake of nanoparticles used for bioimaging is influenced mainly by the following factors [ 31 , 32 ]: (i) size, smaller nanoparticles with identical surface properties are better absorbed than bigger nanoparticles [ 33 ]; (ii) charge, positively charged nanoparticles are preferentially taken up by living cells due to the cell membrane negative charge [ 34 , 35 ]; (iii) cell-specific targeting, this is achieved by conjugating nanoparticles with ligands that can interact with receptors at the cell surface [ 36 , 37 ]; (iv) the conjugation of proteins on the nanoparticles’ surface may promote rapid absorption and endosome bypass [ 38 ]; (v) oligodeoxynucleotide conjugation can help in the presence of complementary cellular DNA at a specific subcellular location [ 39 , 40 ]; (vi) endosome egress of nanoparticles that are positively charged at the surface in the low pH environment of late endosomes. It was reported that small nanoparticles can bypass the degradation pathways more efficiently than larger size particles with the same chemical composition [ 41 ].
The fluorescent nanoparticles used in bioimaging can be classified into two categories: (1) fluorescent nanoparticles that can emit specific optical signals, such as carbon and metallic quantum dots, (2) fluorescent nanoparticles that require labeling with a fluorophore to be visualized, such as fluorescence mesoporous SiO 2 and Fe 2 O 3 NPs, liposomes, protein-based, and polymeric nanoparticles. Many classical fluorophores emit fluorescence at a short wavelength (e.g., the ultraviolet (UV) and visible regions) that can be easily absorbed and scattered by human tissues. This can lead to specific issues, for instance, elevated auto-fluorescence, low signal-to-background ratio, and limited tissue penetration. High-energy light could be used to overcome these drawbacks, but it can cause phototoxicity in human tissues. Optical imaging in the near-infrared (NIR) region is a possible alternative ( Figure 3 ). Fluorophores in the first near-infrared window (NIR-I, 700–900 nm) have been tested and show good sensitivity; however, their application in bioimaging is limited due to their poor tissue penetration (less to 1 cm) and large photon scattering losses in biological matter. Therefore, fluorescent quantum dots (≤10 nm) that allow fluorescent bioimaging in the NIR-II window (1000–1700 nm) have been developed [ 42 ].

Schematic presentation showing fluorescence imaging approaches of traditional methods versus rare-earth-metal doped nanoparticles. ( A ) The spectral range of classical fluorescence imaging methods. NIR, near-infrared region. ( B ) Examples of probes in the NIR-II region: single-walled carbon nanotubes (SWNTs), rare-earth-metal doped nanoparticles (RENPs), organic dyes, conjugated polymers, and quantum dots (QDs). ( C ) Nanoparticles are doped with rare-earth metals (Nd, Tm, Pr, Ho, Er) [ 42 ]. Copyright 2020, Frontiers.
Fluorescent metal quantum dots (e.g., Au, ZnSe, InAs, CdTe, InP, or CdS) with a size in the 1–10 nm range show broad absorbance bands and narrow emission bands and are interesting for bioimaging in the NIR range [ 43 ]. These inorganic nanoparticles are the most frequently used in bioimaging because of their intense color, shape, size, and high-power surface photoluminescence. They allow the non-invasive detection of disease and the monitoring of its progression/response to treatments in humans and animals [ 44 ]. Metal oxide (Fe 3 O 4 , WO 3 , WO 2.9 ) [ 30 , 32 ], lanthanide-doped nanoparticles [ 45 ], and quantum dots (QDs) [ 30 ] have also been tested for bioimaging and therapy. The nanoparticles optical and physical features can be tailored by structure amplification, which is their most distinctive advantage for optical imaging. Ceramic nanomaterials (mesoporous TiO 2 and SiO 2 NPs) also are among the most interesting candidates for bioimaging because of their size-manageable morphology, easy functionalization, and biocompatible hydrophilic surface [ 46 , 47 ]. FDA-approved silica is less toxic and is biocompatible [ 48 ]. Magnetic metal oxide nanoparticles can be exploited as nanocarriers for substances that are optically active or that can emit optical signals upon excitation, depending on their structural composition. However, using magnetic metal oxide nanoparticles in bioimaging is challenging due to their loading capability, synthesis complexity, regulatory hurdles, imaging efficiency, toxicity of the intrinsic ingredients, batch reproducibility, production cost, in vivo stability, and storage [ 49 , 50 ].
Fluorescent carbon nanoparticles are also interesting for bioimaging applications [ 51 ] for several reasons, particularly source abundance, simple synthesis, low cost, and non-toxicity [ 52 ]. Fluorescent carbon nanoparticles, such as fullerenes and carbon quantum dots, are considered to be promising nanomaterials alternatives to fluorescent semiconductor quantum dots, which are composed of toxic heavy metals, such as cadmium [ 53 , 54 ]. Fullerenes and carbon quantum dots are strong candidates for bioimaging applications [ 55 ]. Indeed, they are superior to the currently used inorganic quantum dots and traditional organic fluorophores in terms of photo-bleaching resistance, easy surface functionalization, and chemical inertness [ 56 ]. Their greater aqueous solubility, minimum cytotoxicity, and substantial fluorescence quantum yields explain their suitability for biomedical applications, specifically for in-vitro and in-vivo bioimaging [ 57 , 58 ].
Rare-earth metals are primarily used to develop nanoparticles with persistent luminescence [ 58 ]. However, the major issue linked to the use of rare-earth metal-based nanoparticles in vivo is their potential cytotoxicity and their low tunability for surface modification [ 59 , 60 ]. Therefore, non-toxic conjugated polymer nanoparticles with tremendous optical properties have been developed to act as persistent luminescence materials that can replace the conventional rare-earth metal-based nanoparticles. Conjugated polymer nanoparticles display excellent photoelectronic properties due to the highly localized π electrons on their backbones [ 61 ]. Additionally, due to their light-harvesting and light-amplifying properties, conjugated polymers act as biological photoactive materials. Water-soluble conjugated polymers have been developed and used in drug/gene delivery, biosensing, bioimaging, and antitumor/antimicrobial therapeutics [ 62 , 63 ]. Moreover, it has been demonstrated that conjugated polymers do not have any cytotoxic effect on the host cells and have structure-dependent tunable optical properties [ 64 , 65 ]. Currently, conjugated polymer nanoparticles can be prepared using four different approaches: solvent exchange (nanoprecipitation), mini-emulsion, self-assembly, and emulsion polymerization.
Du et al. [ 66 ] described a fast and multimodal approach for in vivo fluorescence bioimaging through the synthesis of Fe and Zn nanoclusters in HepG2, HeLa, and U87 cancer cells. They showed that fluorescent magnetic Fe 3 O 4 nanoclusters and ZnO nanoclusters are synthesized by cancer cells in which Zn 2+ and Fe 2+ (which are biocompatible) have been introduced. These nanoclusters are promising candidates for multiplexed imaging that integrates fluorescence imaging, computed tomography, and magnetic resonance imaging. Moreover, they did not observe any significant variation in the fluorescence signal before and after the injection of Zn 2+ and Fe 2+ in normal human liver cells (L02) and tissues. This suggests that this multiplexed bioimaging system could be interesting for monitoring the response to cancer treatment and also for cancer diagnosis. Wang et al. manufactured multi-colored conjugated polymer nanoparticles for targeted tumor imaging ( Figure 4 ) [ 67 , 68 ]. Functionalized conjugated polymer nanoparticles with carboxyl groups were fabricated by coprecipitation of poly (styrene co-maleic anhydride) (PSMA) with four conjugated polymers (P1, P2, P3, and P4) with blue, green, yellow, and red fluorescence emission, respectively [ 67 ]. Energy could be transferred from nanoparticles with shorter wavelength emission to nanoparticles with longer wavelength emission, leading to the formation of multi-colored nanoparticles with whole visible light absorption and emission spectrum via only one excitation. For enhancing their specificity towards tumor cells, conjugated polymers (P1-4/PSMA) were labeled with an antibody against EpCAM (a protein overexpressed in many cancer types). Excitation was recorded in antibody-labeled conjugated polymers (P1-4/PSMA/anti-EpCAM) at different wavelengths. Then, due to the antibody cross-reactivity, Wang et al. used P3/PSMA/anti-EpCAM and P3/PSMA/anti-ErbB2 to differentiate between HeLa and MCF-7 cells (EpCAM), and SK-BR-3 cells (ErbB2), although MCF-7 and SK-BR-3 cells were derived from the same patient [ 67 , 69 ].

Multicolor conjugated polymer with carboxyl groups were fabricated from poly (styrene co-maleic anhydride) (PSMA) with four conjugated polymers (P1, P2, P3, and P4), for cancer cell bioimaging and detection. ( a ) UV-vis absorption, and ( b ) fluorescence emission spectra of P1–4/PSMA nanoparticles in water (excitation wavelength: 360 nm). The conjugated polymer nanoparticles were fabricated by precipitation of the tetrahydrofuran solution (2.0 µg/mL of P1, 7.0 µg/mL of P2, 4.0 µg/mL of P3, 12.0 µg/mL of P4, and 20.0 µg/mL of PSMA) into water. ( c ) Multi-channel fluorescence images of MCF-7 cells using P1–4/PSMA/anti-EpCAM polymer nanoparticles. The excitation wavelengths are indicated above the panels [ 67 ]. Copyright 2014, Wiley.
Understanding biological causes and developing therapeutic strategies requires the ability to track cell migration and circulation in vivo. Many attempts have been undertaken to investigate novel and improved cell labeling and imaging techniques, and much has already been learned from these approaches. The capacity to visualize these cells precisely could help in the diagnosis and prognosis of disorders, such as atherosclerosis, neurodegenerative diseases, cancer, and myocardial infarction. Understanding the behavior of circulating cell populations, such as monocytes, macrophages, and circulating tumor cells (CTCs) spread from solid tumors, could provide crucial information on features that could aid in the development of better treatments [ 70 ]. Chen’s team observed CTC behavior in blood arteries surrounding solid tumors using a multiphoton device with a 30 Hz collection rate. The highest number of circulating tumor cells each minute was estimated to be around 100, which could be linked to cancer progression [ 71 ]. Furthermore, by labeling circulating tumor cells with antibody-conjugated quantum dots, the behavior of uncommon subpopulations of circulating tumor cells with higher metastatic potential, such as CD24+ and CD133+ CTCs, has been observed. Jia’s team produced doxorubicin (DOX)-loaded MSNs that are decorated with anti-EpCAM and anti-CD44 aptamers to deliver cancer metastasis chemopreventive medications selectively toward circulating tumor cells. DOX-fluorescence MSNs enable the self-tracking of targeting efficacy and drug administration into colorectal cancer cells. It was possible to track not only how the transplanted CD45-CD541CD1571 lung stem/progenitor cells were precisely located in vivo but also how these cells incorporated and reformed themselves over time at a single-cell resolution using a combination of fluorescent nanodiamonds, fluorescence-activated cell sorting, and fluorescence lifetime imaging microscopy. HNF3 plasmid DNA (pDNA) was efficiently transported by fluorescein isothiocyanate (FITC)-tagged MSNs as a differentiating agent for iPSCs. The indoctrination procedure was simultaneously observed due to their self-monitoring capacity [ 72 ].
Surface functionalization of nanoparticles is a crucial issue for their use in bioimaging applications ( Table 1 ) [ 68 , 69 ]. However, the selection of the most suitable nanoparticles for a specific bioimaging application (e.g., computed tomography) is difficult, and several aspects (biological safety, sensor capacity, size, brightness, and photostability) must be taken into account. After finding the best material for a specific application, all experimental methods must be adapted and adjusted to the selected material. This is not an easy task. A deeper knowledge of the impact of nanoparticles on living cells will help to understand the imaging potential of those nanoparticles and how it is adsorbed by cells. Therefore, more research efforts are needed to promote nanoparticle exploitation in bioimaging applications.
Different nanomaterial types are used for bioimaging applications.
3. Nano-Drug Delivery Systems
Drug delivery systems are quite new, but is a rapidly expanding technology. In these systems, nanoscale materials are used to deliver the therapeutically active drug or the imaging molecule (when used as diagnostic tools) to the targets [ 83 ]. Nanostructures (made of metals, organic/inorganic, and polymeric materials) are often used for the development of drug delivery systems ( Figure 5 ). Nanoparticles and nanostructured materials are crucial components in these carrier systems that play a key role in personalized medicine by improving drug formulation/targeting/controlled release [ 83 , 84 ]. Such systems can deliver a drug to a specific site at a predetermined rate and in a predesigned manner; consequently, the drug bioavailability will be enhanced, while side effects will be reduced. Drugs can be physically or chemically adsorbed into the nanoparticles surface through various adsorption methods, or they can be loaded on nanoparticles during their production [ 83 , 85 ]. The drug and carrier properties, such as drug-carrier solubility, molecular weight, drug-carrier chemical interaction, and carrier size, will determine the drug loading efficacy on/into the carrier [ 83 , 86 ]. The drug release rate from the nanoparticlesis mainly influenced by (1) the release of the adsorbed drug from the surface of the nanoparticles; (2) the drug diffusion from thenanoparticles; and (3) the nanoparticle erosion and drug diffusion from the nanoparticles. Therefore, the drug release rate from the nanoparticles will be governed by polymer biodegradation and drug diffusion. The drug release time and location can be modulated by the nanoparticles composition (e.g., thermosensitive and pH-sensitive materials) and engineering (e.g., monolayer and multilayer nanoparticles, nanocapsules), and also by better understanding the physiological factors involved in this process [ 83 , 87 ] ( Figure 5 ).

Characteristics of nanomaterials that can cross the biological membranes to deliver a drug to a specific site and mechanisms influencing controlled drug release. Image created by Biorender.
Many researchers worldwide are investigating whether and how nanoparticles (e.g., metals, metal oxides, carbon, quantum dots, liposomes, dendrimers) can be used as carriers for different therapeutic agents [ 88 , 89 ]. Graphene oxide nanosheets, graphene quantum dots, single/multiwalled carbon nanotubes, and graphene oxide nanosheets are carbon-based nanomaterials with different drug-loading capacities, targeting specificity, and drug release kinetics. This partly explains the discrepancies in the therapeutic efficiency of these different nanomaterials when used as drug-carrier systems. Biodegradable polymer nanoparticles are used in new drug delivery systems because of their flexibility and many interesting characteristics, such as controlled release, stability in blood, non-immunogenic, and non-toxic nature [ 85 ]. Micelles, liposomes, emulsions, and nanoparticles are colloidal drug carriers that are used to increase the number of drugs that can pass through the blood–brain barrier. Similarly, colloidal systems are used to regulate the drug release rate at target locations (cells or tissues).
Drug delivery via nanocarrier systems presents some strengths compared with conventional drug administration methods, particularly very high accuracy, targeting ability, stability, and sustainability at the target location [ 83 , 90 ]. Delivery systems made of large-size materials display major drawbacks, such as poor absorption, in vivo stability, bioavailability, and solubility, which may decrease their efficacy and target specificity. The major goal of using nanoparticles as drug carriers is to reduce the drug toxicity and increase its bioavailability, target specificity, and delivery without any loss of the therapeutic effects [ 83 , 91 ]. The key challenges when looking for suitable carriers are: (1) drug release kinetics and integration, (2) shelf life and stability of the formulation, (3) biocompatibility of the formulation, (4) biodistribution and targeting, and (5) possible nanocarrier accumulation in the body in the case of prolonged treatment using drug-loaded nanocarriers. Currently, the available data do not allow the determination of the toxicological and environmental impacts of drug-loaded nanocarriers [ 83 , 92 ]. Furthermore, drugs can also be synthesized at the nanoscale, and then they can act as their own “carrier” for delivery. Table 2 lists different nanocarriers used for the delivery of therapeutic molecules/drugs.
Nanomaterials used for drug delivery.
3.1. Nano-Vehicles for Anticancer Drugs
After heart disease, cancer is the second most common cause of death worldwide [ 109 , 110 ]. Functionalized nanocarriers can be used to develop targeted anticancer treatments. Compared with systemic chemotherapy, in these systems, nanoparticles loaded with therapeutics and targeting molecules are specifically delivered to the tumor cells and show good efficacy at lower doses and, consequently, with fewer undesirable effects [ 111 ]. Several studies suggest that nanocarriers are interesting tools to improve cancer diagnosis (as imaging agents) and treatment [ 112 , 113 ]. Over the last two decades, nanoparticles products have been evaluated in several clinical trials [ 114 ].
The efficacy of chemotherapeutic agents at the target site can be increased by encapsulating them in nanoparticles that target the tumor cells actively or passively. Nanoparticles offer various advantages when used for drug delivery: (i) overcoming the stability and solubility issues of chemotherapeutic drugs; (ii) protecting the drug from modifications by enzymes (e.g., proteases and other metabolic enzymes) and, thus, also increasing the drug half-life in blood; (iii) increasing drug targeting and distribution; (iv) modulating the drug release kinetics; and (v) reducing resistance to treatment by delivering multiple drugs [ 84 ].
Often, after reaching the target, the anticancer drug efficacy is significantly reduced for many different reasons (e.g., too low concentration) [ 84 ]. As the therapeutic effect of a drug is possible only if present at the right concentration and in the correct form, nanoparticles used as carriers could increase the local drug concentration inside and around tumor cells. This also decreases the risk of toxicity for healthy cells. The drug-carrying nanoparticles deliver the drug directly into its targeted body area (organ, cellular, and subcellular level of specific tissue) to overcome the specific toxic effect of conventional drug delivery, thereby reducing the amount of drug required for therapeutic efficacy. As a result, the use of nanoparticles in drug delivery opens new possibilities for improving drug distribution and changing cancer management [ 115 ]. The interaction of nanoparticles with ligands (nucleic acid aptamers, peptides, antibodies, carbon dioxide, and tiny molecules) may contribute to the active targeting of cancer cells and organs.
Polymeric nanoparticles can be used for the controlled release of the encapsulated drugs by surface erosion, diffusion, and swelling, followed by diffusion, depending on the time and condition. The most widely used biocompatible polymers for controlling drug release are poly(D, L-lactide), poly(glycolide), and its co-polymer poly(lactic-co-glycolic acid) [ 116 ]. Biodegradable polymer nanoparticles for cancer treatment have been extensively studied [ 117 , 118 ]. Liposomes were the first nanoparticles to be used to administer chemotherapy. Cohen and Bangham were the first to describe liposomes 40 years ago [ 119 ]. Liposomes are structured like vesicles with an aqueous interior and one or more concentrically phospholipid bilayers with diameters ranging from 30 nm to several microns. They are produced using different methods and display different sizes, lipid compositions, and surface chemistry. Liposomes can be used as flexible carriers that can be tailored to various functions and have a specific drug delivery role [ 120 ]. Several materials, such as biodegradable polymers, dendrimers, and nucleic acid-based nanoparticles, have also been used to develop targeted cancer treatments [ 121 , 122 ].
Electrospun nanofibers have also been evaluated for localized anticancer drug delivery [ 113 , 114 ]. They can efficiently deliver the desired drug to the target cancer cells compared with conventional diffusion-based drug delivery vehicles [ 122 , 123 , 124 , 125 ]. Zhang et al. manufactured a nanofiber-based localized anticancer drug delivery system by electrospinning a solution containing self-assembled PEG 2000 -Pt(IV) micelles with dichloroacetate (DCA) that “acts as a prodrug” and with polyvinyl alcohol. This delivery system allowed the quick release of PEG 2000 -Pt(IV)-based micelles and DCA that showed a synergistic apoptotic effect on cancer cells through two apoptotic mechanisms [ 107 ]. Table 3 lists various nanomaterial types exploited in delivery systems for anticancer agents.
Different examples for nanomaterials being used in anticancer drug delivery systems.
3.2. Nanostructured Materials as Drug Delivery Vehicles for Antioxidant Drugs
Antioxidants are reactive molecules that are produced by the body in response to environmental stress and other stimuli and that contribute to limiting cell damage by free radicals. Therefore, they are also called “free-radical scavengers”. Antioxidants are present in many food types and can also be synthesized. However, due to their limited cellular absorption, potency, and lack of precise transportation systems to a specific organ, cell, and tissue, their use for treating diseases in which oxidative stress plays a major role is still limited, particularly for neurodegenerative diseases where the brain targeting is still challenging [ 83 ]. Nanotechnology can address these drawbacks, especially the targeting of dietary antioxidants with neuroprotective properties. Indeed, antioxidant molecules can be protected from degradation using nanotechnology-based delivery mechanisms that improve their bioavailability and physicochemical drug-like properties [ 134 ].
However, most nanoparticle-based formulations tend to cause oxidative stress in the cells, and this hampers their routine clinical use. Importantly, the amount of reactive oxygen species (ROS) produced by the cells is proportional to the concentration of nanoparticles to which the cells were exposed [ 135 ]. Moreover, the cellular redox balance is influenced by nanomaterials, and this can lead to the inhibition or induction of ROS production [ 83 ]. Excess reactive oxygen species production and endogenous antioxidant system overload are common side effects of high nanoparticle concentrations, resulting in cytotoxicity and inflammation [ 83 , 136 ]. As a result, determining the maximum permissible doses is important to minimize negative effects. However, several studies suggest that low nanoparticle exposure levels can unintentionally boost the antioxidant defenses and reduce oxidative stress. Moreover, some nanomaterials have enzyme-like antioxidant properties, and they reduce oxidative damage and scavenge reactive oxygen species and free radicals [ 137 ].
Nanoparticles’ antioxidant properties depend on their surface load, volume surface ratio, chemical composition, particle size, and surface coating [ 138 ]. Nanoparticles offer many advantages compared with traditional methods of antioxidant supplementation, including the environmental safety of bioactive materials, improved bioavailability and selective antioxidant supplementation, and controlled release at the target site [ 139 , 140 ]. The antioxidant function of transition metal oxide nanoparticles (CuO, NiO NPs) has been widely studied and exploited [ 141 , 142 , 143 , 144 ]. Cerium oxide nanoparticles (CeO 2 NPs) are particularly interesting because of their reactive oxygen species (ROS) scavenging and regenerative effects [ 145 ]. These nanoparticles display special features: the coexistence in both oxidation states (Ce 3+ and Ce 4+ ), reversible switching between these states, and reduction potential of 1.52 V [ 146 ]. Gold nanoparticles (Au NPs) have been extensively evaluated by the pharmacology and biomedical sectors due to their inert and non-toxic nature [ 147 ]. Silver nanoparticles (Ag NPs) also have a strong antioxidant capacity (reduction power and free-radical scavenging) [ 148 ]. Arriagada et al. [ 149 ] prepared antioxidant mesoporous SiO 2 nanoparticles on porous nanoplatforms with rosmarinic acid (nano-RA) as an antioxidant that was loaded on the mesoporous SiO 2 nanoparticle surface. Morin flavonoids were incorporated on the antioxidant nanocarrier by the impregnation/solvent evaporation technique with high drug loading (23% wt/wt) compared to bare mesoporous SiO 2 NPs (9% w / w ). In addition, the rosmarinic acid and mesoporous SiO 2 nanoparticle (nano-RA) release profile was evaluated using two biorelevant media. The antioxidant activity of the rosmarinic acid and mesoporous SiO 2 nanoparticles (nano-RA) was maintained, suggesting the correct disposition of the moiety ( Figure 6 ) [ 149 ]. These results suggest a promising antioxidant nanocarrier suitable for future application in drug delivery [ 149 ]. Table 4 lists different nanomaterial types used as carriers for antioxidant delivery.

Schematic presentation showing the preparation of morin-loaded nano-antioxidants of (nano-RA/MH) loaded onto mesoporous silica nanoparticles (MSN). ( a ) The graphic path from the single components to therosmarinic acid nanocarrier (nano-RA) and ( b ) morin loading on the nanocarrier (nano-RA/MH) [ 149 ]. Copyright 2016, MDPI.
Different nanomaterials are used as carriers for antioxidant delivery.
4. Antimicrobial Materials
Antibacterial agents are used in the biomedical sector, textile industry, water treatment, and food industries [ 165 ]. The antimicrobial characteristics of nanoparticles are influenced by several factors [ 166 ], including size, shape, and the type of encapsulated antibiotics drug. For instance, nanoparticles with angular shapes (e.g., triangular, cubic, tetrahedral) cause mechanical harm to the microbial membrane, and this contributes to increased microbial growth inhibition compared to spherical nanoparticles [ 167 ]. Based on their antimicrobial properties, antimicrobial nanoparticles can be classified into four main categories: antibacterial, antifungal, antiviral, and antiparasitic nanoparticles [ 168 ]. Table 5 lists these different types of antimicrobial nanoparticles, their mode of action, and targeted microorganisms. For millennia, metals and metallic salts have been used for their antibacterial properties. For instance, silver pots have been used for drinking water since 4000 BCE [ 169 ].
Classification of antimicrobial nanomaterials based on their antimicrobial properties, their different modes of action, and targeted microorganisms.
Surface functionalization allows the production of antibacterial nanoparticles with two different antibiotics to concomitantly kill, for instance, Gram-positive (encapsulated) and Gram-negative (attached) bacterial strains. Inorganic disinfectants, such as metal oxide nanoparticles, are gaining popularity because of the limitations of organic disinfectants, such as toxicity to humans [ 166 , 170 ]. Currently, nanophysics researchers are investigating the effects of different metallic nanoparticles in bacteria. The antibacterial mechanism of metallic nanoparticles is still debated, but three major mechanisms are proposed: (i) reactive oxygen species (ROS) formation; (ii) metal ion release from metallic nanoparticles; and (iii) metallic nanoparticles interaction with the cell membrane. Metallic nanoparticles display higher antibacterial effects than their salts. The antibacterial function is often influenced by the size of the metallic nanoparticles [ 20 ].
The antimicrobial properties of silver nanoparticles (Ag NPs) are very well known, and when compared to other metallic nanoparticles, they have higher toxicity against microorganisms [ 198 , 199 ]. As they are currently used as an alternative to antibiotic treatment, Ag NPs are often referred to as “next-generation antibiotics”. The antimicrobial potential of Ag NPs has been tested in many different pathogens, including Gram-(+)/(−) bacteria, viruses, and fungi. These particles also show antimicrobial activity in many multidrug-resistant bacteria [ 200 , 201 ]. Despite the many studies on Ag NPs, the precise mechanism of their antimicrobial effect is still unknown [ 202 ]. It is thought that their antibacterial activity relies mainly on the generation of Ag + ions. Some studies showed that Ag + ion generation is influenced by the nanoparticle’s surface area. Specifically, nanoparticles with a larger surface area generate higher Ag + concentrations, which leads to enhanced antimicrobial activity. Conversely, nanoparticles with a smaller surface area generate lower Ag + concentrations and thus display lower antimicrobial activity [ 203 ]. This also indicates that the antibacterial activity is linked to the amount and quality of Ag + ions generated. In addition, Ag NPs’ toxic effects against bacteria are influenced by the nanoparticle’s physicochemical properties. Many studies have shown that Ag NPs disrupt several metabolic pathways and cellular pathways inside the bacterial cell [ 35 , 204 , 205 ]. The antibacterial activity of Ag NPs is influenced by several factors related to the nanoparticles (size, shape, coating) and the medium (light, oxidative species, ligands, ionic strength). The mechanisms whereby these factors can modify the antibacterial activity of Ag NPs are many ( Figure 7 ) and include ligand replacement, oxidative dissolution, Ag + ions reduction, passivation of the Ag surface, passivation layer puncturing, silver speciation, and nanoparticle aggregation [ 206 ]. These phenomena may also be influenced by some chemical species. For instance, chloride can accelerate or slow down corrosion in the function of its concentration. Therefore, the antimicrobial activity of Ag NPs should be assessed in controlled conditions to limit/prevent unexpected changes in the system. Specifically, Ag NPs must be stored in the dark and without oxygen. Antibacterial mechanisms can be classified in two groups: non-oxidative and oxidative mechanisms ( Figure 7 ).

Schematic representation of several factors that influence silver nanoparticles’ (Ag NPs) antibacterial activity.
The antimicrobial and antibiofilm properties of TiO 2 NPs are widely known and are active against pathogens, such as fungi, viruses, parasites, and bacteria. Recently, the use of TiO 2 NPs in the food industry has started and has been approved by the FDA for their use in drugs, food, and cosmetics [ 207 ]. The quantum size effects and photocatalytic effects of these NPs made them ideal in various antimicrobial applications, such as antimicrobial coatings on medical devices, air, and water purification. Anti Gram-positive and Gram-negative effects of TiO 2 NPs are well known, but the bactericidal activity of these NPs has been enhanced by plant extracts, such as Garcinia zeylanica. The combination of inherent antimicrobial activity with plant extract and photocatalytic property of TiO 2 NPs have enhanced their potency as microbicidal agents [ 208 ]. ROS generated by TiO 2 NPs destruct the microorganisms by oxidizing the cell membrane. Apart from antimicrobial activity due to photocatalytic activity TiO 2 NPs, these nanoparticles also show activity in the absence of light by direct contact and adsorption of cells and cause the loss of membrane integrity [ 209 , 210 ]. The antimicrobial properties of Cu NPs are widely known on various species of bacteria (e.g., bacillus subtilis, methicillin-resistant staphylococcus aureus, Pseudomonas aeruginosa, salmonella choleraesuis). The level of agglomeration of Cu NPs decides the microbicidal activity, which is a common issue with them. Sammler-sized Cu NPs result from a reduction in the agglomeration, which increases the surface area and interaction with bacterial membranes that lead to more toxicity. Hydroxyl radicals were produced from the ionic and metallic forms of copper that damage essential DNA and proteins [ 211 , 212 ]. Many other nanoparticles, such as Si NPs, CaO NPs, MgO NPs, and Al 2 O 3 NPs, have good antimicrobial properties with good biocompatibility. Most of them will act by damaging the bacterial cell wall [ 212 ].
5. Gene Therapy
Gene therapy is a method in which genes are modified to prevent and/or treat disease. With this revolutionary technique, clinicians can treat a disease simply by incorporating the modified gene into the patient’s cells, without the need for surgery or drugs. Depending on the disorder, gene therapy may be used to add a functional copy of a gene that is not working properly or to switch off the gene that is causing the problem. These modified genes are delivered into the cells using a vector (i.e., a genetically modified transporter). Due to their nanometric size, large surface-to-volume ratio, and stability, nanoparticles are attractive agents as gene carriers [ 83 ].
Surface modifications may be used to bind an infinite number of ligands and receptors [ 207 ]. Alternatively, nanoparticles may encapsulate and release nucleic acids into target cells, with superior efficacy in gene therapy compared with non-viral vectors and without immunogenicity. Liposomes have been studied as medication and also as a DNA delivery device [ 208 ]. For instance, gene therapy in which liposomes act as vectors has been used to treat corneal diseases [ 209 ], cardiovascular diseases [ 210 ], cystic fibrosis [ 211 ], and cancer [ 212 ]. In lung carcinoma, cancer with low survival rate, DOTAP:cholesterol liposomes were used to deliver the tumor suppressor gene FUS1 in mice harboring lung cancer xenografts [ 213 ]. Liposome–DNA complexes were synthesized using a simple mixing method. The complex efficacy in inhibiting tumor growth and metastasis development was evaluated [ 37 ].
Designing nanoparticles for gene delivery is not easy because many factors must be taken into account. First, nanoparticle functionalization should bring biocompatible layers and contribute to maintaining the structural integrity and activity of the transported genes/drugs in the biological fluids. Second, nanoparticle production, like for any therapeutic product, must take into account the following issues: physicochemical properties, biopharmaceutical properties, and pharmacological properties. Therefore, nanoparticles should have the capacity to treat directly, and this requires new design parameters. Studies on the Administration, Distribution, Metabolism, and Excretion (ADME) of nanoparticles should be designed to take into account their aggregation and surface characteristics ( Figure 8 ) [ 120 ].

Combinatorial approaches based on organic nanoparticles (ONP) for gene therapy are associated with other therapies [ 214 ]. Copyright 2017, Trends in Biotechnology.
In plant science, gene delivery plays a vital part in the growth of new plant varieties and the development of drought-resistant, pest-resistant, high-yield plants [ 215 ]. The main inherent obstacle to gene delivery in plants is the biomolecule movement within cells through the rigid and multilayered cell wall. Many delivery methods have several disadvantages, including low production, contamination, and foreign DNA incorporation into the plant genome [ 83 , 216 ]. The functionalization of biomaterials allows the development of bionanomaterials to deliver a gene into plant cells through a nanoneedle that can overcome the existing limitations in delivering biomolecules to plants [ 217 ]. Intracellular gene delivery has been performed using conventional synthetic vehicles, such as cationic lipids, dendrimers, and polymers. It safeguards DNA from nuclear enzyme degradation, ensures efficient movement inside the cells and tissues, as well as the active gene transfer to the cell nucleus. Mesoporous SiO 2 nanoparticles have also been tested to deliver genetic material to plant cells. Traditional methods of gene transfer have some drawbacks, such as the limited amount of delivered DNA, cell degradation, limited plant diversity, and toxicity; however, new vehicles for activators, nucleic acids, and proteins into vegetable cells are now available showing higher efficiency and additional safety features [ 218 ].
6. Biosensors
Biosensors are devices that combine organic components (e.g., antibodies, enzymes) and an electronic element to yield a detectable signal that can be quantified [ 219 ]. The electronic elements detect the physiological change produced by the interaction of the organic component with environmental chemical or biological elements [ 220 , 221 ]. A typical biosensor consists of five main components ( Figure 9 ): (i) the analyte, a substance the presence and amount of which are detected [ 222 ]; (ii) the receptor, an organic molecule that can detect the analyte [ 221 ]; (iii) the transducer, a device that converts the physiological change occurring following the analyte-receptor interaction to a quantitatively measurable optical or electrical signal [ 220 ]; (iv) the electronic part that receives and quantifies the signals from the transducer; and (v) the display, an interpretation system (a computer and a printer) to display the response output in a manner that can be understood by the user [ 223 ].

Schematic representation of the components of a typical biosensor and of the different types of bioreceptors and transducers. Image created by Biorender.
The efficient signal collection is one of the main challenges in biosensor development (transduction). The interaction of the analyte with the biological element is converted into gravimetric, electrochemical, magnetic, electro-chemiluminescent, or optical signals using a transducer. Engineered nanomaterials have a greater electrical conductivity, are nanosized, may be used to amplify desired signals, and are biocompatible. Due to their potential to trap huge amounts of specific binding units and to operate as a conductive medium, nanomaterials are good candidates to improve the biosensor detection sensitivity for specific molecules. Carbon nanotubes, nanodiamonds, semiconductor quantum dots, polymer nanofibers, and graphene are some of the most studied nanomaterials for biosensing applications. Indeed, carbon compounds can be used to conjugate biomolecules (enzymes, antibodies, DNA, cells). Nanomaterials can improve biosensor performance (better sensitivity and lower limit of detection). Nanomaterials with optimal surface-to-volume ratio, chemical activity, mechanical strength, electrocatalytic capabilities, and diffusivity can profoundly influence biosensor performance. Moreover, nanomaterials biocompatibility is a key feature in building biosensors to monitor bacteria, viruses, DNA, and other biomolecules [ 224 ].
Nanomaterial-based biosensors have been used for diagnostic purposes through the detection of specific biomarkers in biological samples. For example, Tang et al. manufactured a complex biosensor for the detection of prostate cancer biomarkers by using magnetic nanoparticles as a carrier for the labels and as separators. Specifically, the magnetic nanoparticles used for the amperometry detection of four prostate cancer biomarkers (interleukin-6, prostate-specific antigen, platelet factor-4, and prostate-specific membrane antigen) were labeled with horseradish peroxidase (HRP) and secondary antibodies (Ab2) to yield Ab2-MNP-HRP beads. Then, beads were resuspended in a phosphate-based buffer and incubated with a mixture of protein standards to detect the four markers within this mixture. Afterward, the mixture was transferred to the sensing compartment labeled with primary antibodies against the four markers under which ring-shaped magnets were fixed to facilitate the bead migration to the sensing surface. Following the incubation and washing steps, the addition of the HRP substrate enabled the development of a detectable and measurable electrochemical signal [ 225 ]. Table 6 lists the benefits of various nanomaterials.
Examples of different nanomaterials and their key benefits in various applications.
7. Tissue Engineering
Tissue engineering is a multidisciplinary approach to develop structures that are made of biological components (e.g., cells, stimulatory molecules) and biomaterials that can mimic the native organ/tissue. Engineered tissues may be used at the place of conventional organ/tissue transplant procedures to lower the cost burden [ 230 , 231 ]. The rapidly evolving nanotechnological and fabrication techniques have allowed the incorporation of various biocompatible nanomaterials in tissue engineering, including nanoporous scaffolds and nanofiber membranes [ 232 , 233 ]. Nanomaterials have been used in various tissue engineering applications (periodontal, neural, bone, and skin tissue engineering) [ 234 ]. Nanomaterials can contribute to finely tuning the scaffold characteristics, particularly the mechanical strength, and regulating the release of bioactive molecules (growth factors, cytokines, inhibitors, genes, drugs) [ 235 , 236 ].
In dental tissue engineering, novel treatments are required for the effective reconstruction of periodontal tissue damage due to the gradual loss of the self-healing capacities of periodontal tissue with age. Nanomaterials have emerged as promising candidates for the reconstruction of periodontal tissue [ 237 ]. Nanomaterials are interesting materials in dental tissue engineering as (i) nano-coatings for dental implants, (ii) nanofillers to enhance the mechanical properties of the biomaterials used in dental tissue engineering, (iii) antimicrobial agents to prevent oral infections, and (iv) as ingredients for novel personal care products and toothpaste [ 237 ]. Xi et al. prepared multifunctional vesicles by co-assembling poly(ε-caprolactone)-block-poly(lysine-stat-phenylalanine) and poly(ethylene oxide)-block-poly(ε-caprolactone) that were loaded with ciprofloxacin hydrochloride (an antibiotic used to treat periodontitis) ( Figure 10 ). Their in vitro and in vivo experiments showed that these multifunctional vesicles eliminated biofilms made by Escherichia coli and Staphylococcus aureus and greatly contributed to periodontitis treatment [ 238 ].

Vesicles displaying antibacterial activity and good antibiotic delivery capacity for the management of biofilm-induced periodontitis. ( a ) Co-assemblage of multifunctional corona vesicles. ( b ) Encapsulation of ciprofloxacin within the multifunctional corona vesicles. ( c ) Antibacterial activity of the multifunctional corona vesicles to remove dental plaque biofilms produced by bacteria [ 238 ]. Copyright 2019, American Chemical Society.
In humans, the nervous system is an extremely complex system that comprises the peripheral nervous system (PNS, motor, and sensory nerves) and the central nervous system (CNS, spinal cord, and the brain). The nervous system lacks self-healing capacities; therefore, any trauma or disease-related damage to the CNS or PNS is permanent [ 239 ]. The frequency of neurological damage increases with age and is becoming a public health issue due to the aging population [ 240 ]. Currently, protocols of neurological damage treatment are based on autologous or allogeneic cell grafts and neurosurgery. However, these approaches often require the inhibition of the immune response (grafts) and additional surgical procedures and show moderate effects [ 241 ].
Nanofibers were initially defined as fibers with diameters below 100 nm. However, the scope of nanofibers has been broadened in recent years, with all fibers of diameter less than 1 μm included. Several techniques have been reported to prepare nanofibers, including the splitting of bicomponent fibers, melt-blowing, physical drawing, flash-spinning, phase separation, self-assembling, solvent dispersion, centrifugal spinning, hydrothermal, and electrospinning [ 242 , 243 , 244 , 245 ]. Nanofibers present diverse applications, such as molecular filters, bioseparation, bio-sensing, crop protection, bioremediation, anti-counterfeiting, and antibacterial. The high surface area to weight ratio of nanofibers makes an ideal substrate for molecular filtration and shows potential for forming a scaffold for protective clothing applications against biochemical attacks [ 246 , 247 ]. Nanofibrous materials are gaining interest for tissue engineering applications, including skin regeneration. Nanofibrous structures mimic the native extracellular matrix and promote the adhesion of various cells and soluble factors that may promote cell function and tissue regeneration ( Figure 11 ) [ 248 ].

Schematic figure to show how electrospun nanofibers promote the differentiation of various types of pluripotent stem cells into different lineages [ 248 ]. Copyright 2020, Wiely.
Wound healing is a natural process that involves hemostasis, inflammation at the site of injury, the proliferation of keratinocytes, and remodeling [ 249 ]. Based on the healing time, skin wounds are categorized in chronic and acute wounds [ 250 ]. Compared with acute wounds, chronic wounds need much more time to heal, and they are mainly observed in people with comorbidities (e.g., diabetes, obesity) [ 251 ]. The formation of a new blood vessel (angiogenesis) is crucial for the wound healing process because it is required for the flow of nutrients, waste, and oxygen to the wound site. It also accelerates the rate of granulation tissue formation. Any angiogenesis impairment will result in a chronic wound [ 252 ]. Therefore, during the treatment of a skin wound, the successful formation of the new blood supply must be taken into consideration. Currently, various protocols are available for chronic wound management, such as ozone therapy, hyperbaric oxygen therapy, oxygen therapy, and negative pressure wound therapy [ 253 ]. Moreover, for large skin wounds, autologous skin grafts are considered the gold standard. However, their use is limited by the small amount of donor tissue that can be obtained and by the morbidity at the donor site [ 254 ].
Recently, nanomaterials have been proposed as candidates for nanostructured scaffold architectures for the management of large skin wounds. This is explained mainly by their distinct physicochemical features, particularly their nanoscale dimensions and their very high surface-area-to-volume ratio. Nanomaterials can also be used in skin tissue engineering as delivery vehicles of therapeutic molecules [ 19 ]. Randeria et al. prepared Au NPs functionalized with small interfering RNAs against ganglioside-mono sialic acid 3 synthase (GM3S), thiolated ethylene glycol, and dispersed in Aquaphor. GM3S is an enzyme the expression of which is increased in diabetic mice and that causes insulin resistance, thus slowing down wound healing. The authors found that in diabetic mice, these functionalized Au NPs could downregulate GM3S expression and that skin wounds in nanoparticle-treated mice fully healed in 12 days compared with untreated mice [ 255 ].
8. Agriculture and Food Industry
Nanotechnology is used in agriculture to enhance food production and also to improve/preserve the nutritional content, quality, and safety of foods. Fertilizers, insecticides, herbicides, and plant growth factors/regulators are used to enhance agricultural yields. Nanotechnology is also more and more implicated in the development of approaches to stimulate seed germination, plant growth, and plant defenses [ 256 ]. Metal nanoparticles (Ag NPs and Cu NPs) have been particularly studied in plant science. Their organic synthesis is very expensive and involves dangerous chemicals [ 257 ]; however, nanoparticle surface functionalization allows the accommodation of more micronutrients in one nanoparticle for efficient delivery to the plants. These micronutrients may enhance productivity and increase the nutrient content in agriproducts. Carbon nanomaterials are commonly used in agriculture because they can influence the plant’s metabolic functions, and ultimately, its growth. Therefore, these nanomaterials, used at very small concentrations, can go into the plant cells and could be an effective answer to increase crop yield and fruit production [ 258 ].
The plant’s response to nanomaterials is influenced by different factors, including the nanoparticle’s size, shape, application process, and chemical and physical properties. Nanomaterials can be used as nanostructured fertilizers to increase the absorption and efficiency of traditional fertilizers (nutrients and phosphates). It has been shown that soybean growth and seed yield are increased by 33% and 20%, respectively, when treated with hydroxyapatite nanoparticles (phosphorous nano-fertilizers) compared with normal phosphorous fertilizers. Moreover, nano-fertilizers are used at lower concentrations, thus limiting the nutrient spreading to runoff or groundwater and reducing the risks of degradation and toxic effects due to over-application [ 259 ]. Mung bean, cucumber, and rapeseed plants have been treated with ZnO, Fe 2 O 3 , TiO 2 , and CuONPs, as nano-fertilizers, by direct addition to the soil or through irrigation or foliar application [ 260 , 261 ]. In tomato cultures, soil supplements have doubled the number of flowers and fruits, probably by activating plant-based genes/proteins [ 262 ].
In wheat cultures, chitosan nanoparticles have been used to manage the release of nitrogen, phosphorus, and potassium via foliar intake [ 263 ]. In terms of environmental degradation, organic nanoparticles are more appropriate. However, their nutrient supply advantages compared with conventional fertilizers need to be more rigorously demonstrated [ 259 ]. Microgel-based fertilizers biofunctionalized for the delivery of the micronutrients to the plant have been developed for foliar delivery of nutrients [ 264 ]. Nano-fertilizers distributed in a controlled manner can boost crop development, yield, and productivity. Crop enhancement can also be obtained by gene transfer using nano-based target delivery approaches. Nano-pesticides can protect crops effectively. Precision farming is considerably promoted by the development of nanosensors and digital controls. Plant drought tolerance and soil enrichment can both benefit from nanomaterials. Figure 12 shows some potential applications of nanomaterials in the animals and agriculture industry, i.e., nano-fertilizers and nano-pesticides, which stimulate animal and plant growth using nanomaterials; smart monitoring for animals and plants using nanosensors by wireless communication devices and smart packaging.

Potential applications of nanomaterials in the animal and agriculture industry. Increase the productivity of the crop using nano-pesticides and smart packaging; Improve the quality of the soil using nano-fertilizers; Stimulate animal and plant growth using nanomaterials; Provide smart monitoring for animals and plants using nanosensors by wireless communication devices. Image created by Biorender.
Fertilizers significantly increase farm productivity. However, as their over use irreversibly changes the soil chemistry, nanomaterial-based fertilizers are more frequently proposed to increase the fertilizer uptake by plants and to minimize the amount of fertilizer needed, and thus, its concentration in the soil. Sustainable farming implies minimal use of chemicals to protect the environment and various plant/animal species against extinction [ 265 ]. Nanomaterials can boost crop growth/yield by facilitating the fertilizer uptake by the plant, thus ensuring minimal use of inputs and encouraging site-oriented, regulated nutrient supply. Indeed, nanotechnology research (and funding) on plant protection has been growing to guarantee better crop yields. Many plants exposed to toxic concentrations of metal ions and nanoparticles attempt to prevent or reduce uptake into root cells by restricting metal ions (nanoparticles) to the apoplast, binding them to the cell wall or cellular exudates, or by inhibiting long-distance transport [ 266 ].
Through effective farming, irrigation, and utilization of quality seeds, agricultural yields can be improved by 35–40%. The use of nano-formulation fertilizers strongly improves crop productivity. Carbon nanoparticles in fertilizers, for example, increase the yield of rice (by 10.29%), soybean (by 16.74%), winter wheat (by 28.81%), vegetables (by 12.34–19.76%), and spring maize (by 10.93%). Nanomaterials are the best strategy to bring nutrients to plants that are extremely porous at the nanoscale through the activation of different biological plant processes. As a consequence, nano-fertilizers may increase the absorption of nutrients through the plant pores [ 267 ]. Furthermore, extensive research has clearly shown that reducing the nanomaterials size increases the surface mass of particles, thus adsorbing and slowly and steadily desorbing a vast amount of nutrient ions over a long period [ 268 ]. Surface-modified nanomaterials-based fertilizers can accommodate more than one nutrient into the same carrier. Thus, nano-fertilizer formulations enhance the growth of nutritionally healthy crops and increase yields. It should be remembered that improved crop production will push farmers to increase the use of that product ( Figure 12 ) [ 269 ].
To protect the crops from diseases caused by fungi and other plant pathogens and to increase productivity, pesticides and herbicides can be encapsulated into surface-functionalized nanoparticles. The use of this nano-formulation type has increased exponentially. Traditional crop management practices involve the large-scale use of fungicides, herbicides, and insecticides at large concentrations. Nanotechnology has been extensively employed to regulate the application of phytopathogens, and nano-fungicides and nano-pesticides are now commonly used in agriculture [ 270 ]. Encapsulation in nanoparticles allows the gradual and regulated release of the pesticide active ingredients, often leading to the decrease of pesticide utilization and, consequently, of their emissions into the atmosphere. The nanosensor-based detection of pathogens may also reduce the risk of disease in addition to nano-pesticides [ 265 ].
Fast-Moving Consumer Goods (FMCG) are fast-selling consumer items, such as household cleaning materials, toiletries, cosmetics, pharmaceuticals, and also include many non-sustainable items, such as batteries, glassware, and light bulbs. However, the main segment consists of food and beverage items that have a limited shelf life, either because of strong market demand or because the commodity depreciates rapidly or is perishable. Therefore, the packaging of such products is crucial to reduce waste and to avoid product damage during its transport to stores and the consumers’ houses [ 271 ]. Nanotechnology research in the food industry is focused on the development of techniques for food manufacturing, packaging, and distribution. Many food items are now supplemented with nanoparticles that enhance nutrient and bioactive distribution mechanisms, taste and texture, and microbiological safety. In the field of food production and labeling, nanoparticles are also used as antimicrobials or as extremely reactive biosensors for the identification of bacteria, allergens, pollutants, and degradants that may influence food quality and health [ 272 ]. Therefore, today, many food products contain nanoparticles (intentionally introduced or due to contamination). Nanomaterial pollution may also originate in the agricultural environment where many nanomaterials are used (pesticides and fertilizers, cattle protection, poultry development) [ 273 ]. Table 7 summaries the nanomaterial applications in the agri-food sector
Summary of nanotechnology and nanomaterials applications in agri-food sector.
9. Risks of Exposure to Nanomaterials
Despite their advantages, nanomaterials may also be associated with risk factors. Nanoparticles can adversely affect different organs/tissues in the body and can be associated with different disorders ( Figure 13 ). Nanoparticle exposure can promote the development of neurological disorders (e.g., Parkinson’s disease and Alzheimer’s disease), lung (asthma, bronchitis, emphysema, and cancer), and cardiovascular diseases (atherosclerosis, arrhythmia, thrombosis, and hypertension). In addition, exposure to nanoparticles can cause skin irritation, dermatitis, urticaria, and other skin problems.

Disease caused by exposure to nanoparticles and entrances of nanoscale materials into the body through inhalation, dermal exposure, and ingestion, resulting in many potential hazards. Image created by Biorender.
Various factors influence the toxicity of the different nanomaterials, particularly the exposure time and dose. The exposure dose can be determined by multiplying the molar concentration of nanoparticles in the medium by the exposure time [ 63 ]. However, other factors (e.g., aggregation and concentration effects) may also influence the nanoparticle’s toxicity. For instance, some nanoparticles can aggregate. These aggregates (in the micrometer range) might not easily penetrate the body, and thus their toxicity is decreased. Toxicity is also influenced by the nanoparticle’s size because nanoparticles with a size of ~10 nm can go through the cell membrane, and thus, can be more toxic than larger nanoparticles [ 288 ]. Nanoparticles’ shape also influences their toxicity, which may vary in function of the aspect ratio. For instance, 10 µm asbestos fibers cause lung cancer, whereas smaller fibers induce mesothelioma and asbestosis [ 289 ]. Similarly, the nanoparticle effect is inversely proportional to the nanoparticles size and directly proportional to the surface area. The cell uptake, subcellular localization, and oxidative mechanisms can also be influenced by the crystal structure [ 287 ]. For example, a comparison of two polymorphous structures of TiO 2 NPs showed that one causes DNA damage through oxidation but not the other [ 290 ]. The nanoparticle’s surface properties can also contribute to their toxic effects through translocation and oxidation processes [ 291 , 292 ]. Table 8 summarizes the risk of toxicity of different nanoparticles.
Nanoparticles, their toxicity mechanisms and applications.
10. Global Market and Future of Nanomaterials
Nanotechnology has evolved as a solution to many unsolved fundamental biological problems in the field of medicine, healthcare, and human life. This technology helps in the identification of various pathogens, toxins, pesticides, imaging of cancers, and also helps in the transport of active medicaments to the target sites effectively. Currently, many research institutes are involved in nanosystem research. Some of the outcomes of these studies are approved by internal nanotechnology organizations (e.g., the National Center for Nanoscience and Technology and the U.S. Food and Drug Administration) and are commercialized for their better applications in the fields mentioned above, and some of them are in the investigational stage. The biomedical application segment led the market and accounted for the largest revenue share of around 29.98% in 2020. The global nanomaterials market is expected to reach USD 57,608.26 million by 2026, growing at a CAGR of 19.86% during the forecast period (2021–2026). This growth is attributed to the wide range of applications of nanomaterials in the biomedical sector, including imaging, targeted drug delivery, nanorobots for surgery, nanodiagnostics, cell repair, and nanobiosensors [ 1 , 2 , 3 , 4 ].
The importance of nanotechnology and nanosciences open a wider scope for new biomedical-based applications using newly discovered nanomaterials. However, this growth in the use of nanomaterials will be limited by several challenges, including: (1) the ability to deliver cost-effective production of nanomaterials in large volumes; (2) defined production techniques that can be scaled up sufficiently to cover the cost required for targeting volume markets; (3) the rapid identification of priorities in nanoresearch to guarantee nanotechnologies’ and nanomaterial’s safety in the future; (4) address the gaps for current research in risk/toxicity/safety assessment for nanomaterials; and (5) develop an international standard for nanomaterial’s safety; to assist in the determination of appropriate risk management of nanomaterials [ 325 , 326 , 327 ].
The new and future innovation is nanomaterials that have exceptionally extraordinary properties in the food source chain (enhancement of food texture and quality, bioavailability, nutrient values, nano-pesticides, nano-fertilizers, and nano-herbicides) in the world agricultural sector. A large proportion of nanomedicines fail to meet such criteria, and as a result, they are not available in the pharmaceutical sector. Additionally, the scale-up and reproducibility of nanomedicines will be a future issue in formulation development. In the coming decade, nanomaterials will create a new history in the medical field by advancing new nanomaterials for their sustainable development. Synthesis of newer nanomaterials in the near future will revolutionize the treatment strategies for many diseases. There is a lot to achieve in the future in nanomaterial research for their extensive applications in various fields, such as biomedicine (bioimaging, biosensor), agriculture, environmental protection, and food processing.
11. Conclusions
Nanomaterials are interesting materials because of their superior and tunable physical, chemical, and biological features compared with bulk materials. Nanomaterials can be classified in function of their size, shape, composition, and origin. Researchers have exploited nanomaterial features by grafting different groups on them, thus making nanoparticles suitable for biomedical applications. In this review, we presented the applications of nanomaterials in bioimaging, skincare, tissue-engineered scaffolds, drug delivery systems, biosensors, and wound healing, as well as in the food and agricultural industries. The use of nanomaterials for targeted drug delivery has also dramatically progressed with exceptional applications to reduce the limitations of conventional drug delivery systems. Different nanomaterial types (e.g., spherical nanoparticles, core-shell, nanorods, nanowires, hollow, nanofibers, and mesoporous) are studied for the targeted delivery of drugs. Encapsulation techniques have also been tested to deliver various bioactive cytotoxic agents. Nanoparticles also have their place in tissue reengineering for the repair of various tissues. Thanks to their larger surface-area-to-volume ratio, nanostructured scaffolds can act as selective substrates to absorb specific proteins and promote cell adhesion. Carbon and metal-based nanoparticles for biosensor development can lead to many applications in agriculture and in the biomedical sector. The review also presented the fluorescence nanoparticles and the in vivo and in vitro fate of nanoparticles. The applications of fluorescence nanomaterials in bioimaging were explained clearly. The antimicrobial activities of various nanomaterials and their advantages and disadvantages are also discussed individually.
Author Contributions
Conceptualization, A.B.Y., and A.B.; methodology, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B.; software, A.B.; investigation, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B.; resources, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B.; data curation, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B.; writing—original draft preparation, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B., writing—review and editing, V.H., D.T., M.G., A.B.Y., S.S., M.B. and A.B.; supervision, A.B.Y. and A.B.; project administration, A.B.Y. and A.B.; funding acquisition, A.B.Y. and A.B. All authors have read and agreed to the published version of the manuscript.
Ahmed Barhoum (NanoStruc Research Group, Helwan University, Project PIs) Egypt–France Joint Driver (Imhotep, Project No. 43990SF, 2020–2022), Joint Egyptian Japanese Scientific Cooperation (JEJSC, Project No. 42811, 2021–2022), and Irish Research Council (Project ID: GOIPD/2020/340) for financial support. Awadh Bihari Yadav would like to thank the Science and Engineering Research Board (SERB) and Nano mission, Department of Science and Technology (DST), Ministry of Science and Technology, India, for financial support (grant number CRG/2018/002135 and SR/NM/NS-1470/2014).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
Authors declare there is no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
A review on the classification, characterisation, synthesis of nanoparticles and their application
S Anu Mary Ealia 1 and M P Saravanakumar 1
Published under licence by IOP Publishing Ltd IOP Conference Series: Materials Science and Engineering , Volume 263 , Issue 3 Citation S Anu Mary Ealia and M P Saravanakumar 2017 IOP Conf. Ser.: Mater. Sci. Eng. 263 032019 DOI 10.1088/1757-899X/263/3/032019
Article metrics
58949 Total downloads
Share this article
Author e-mails.
Author affiliations
1 School of Civil and Chemical Engineering, VIT University, Vellore, Tamil Nadu 632014, India
Buy this article in print
As per ISO and ASTM standards, nanoparticles are particles of sizes ranging from 1 to 100nm with one or more dimensions. The nanoparticles are generally classified into the organic, inorganic and carbon based particles in nanometric scale that has improved properties compared to larger sizes of respective materials. The nanoparticles show enhanced properties such as high reactivity, strength, surface area, sensitivity, stability, etc. because of their small size. The nanoparticles are synthesised by various methods for research and commercial uses that are classified into three main types namely physical, chemical and mechanical processes that has seen a vast improvement over time. This paper presents a review on nanoparticles, their types, properties, synthesis methods and its applications in the field of environment.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
- Open access
- Published: 07 June 2022
Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists
- Nadeem Joudeh 1 &
- Dirk Linke 1
Journal of Nanobiotechnology volume 20 , Article number: 262 ( 2022 ) Cite this article
82k Accesses
301 Citations
4 Altmetric
Metrics details
Interest in nanomaterials and especially nanoparticles has exploded in the past decades primarily due to their novel or enhanced physical and chemical properties compared to bulk material. These extraordinary properties have created a multitude of innovative applications in the fields of medicine and pharma, electronics, agriculture, chemical catalysis, food industry, and many others. More recently, nanoparticles are also being synthesized ‘biologically’ through the use of plant- or microorganism-mediated processes, as an environmentally friendly alternative to the expensive, energy-intensive, and potentially toxic physical and chemical synthesis methods. This transdisciplinary approach to nanoparticle synthesis requires that biologists and biotechnologists understand and learn to use the complex methodology needed to properly characterize these processes. This review targets a bio-oriented audience and summarizes the physico–chemical properties of nanoparticles, and methods used for their characterization. It highlights why nanomaterials are different compared to micro- or bulk materials. We try to provide a comprehensive overview of the different classes of nanoparticles and their novel or enhanced physicochemical properties including mechanical, thermal, magnetic, electronic, optical, and catalytic properties. A comprehensive list of the common methods and techniques used for the characterization and analysis of these properties is presented together with a large list of examples for biogenic nanoparticles that have been previously synthesized and characterized, including their application in the fields of medicine, electronics, agriculture, and food production. We hope that this makes the many different methods more accessible to the readers, and to help with identifying the proper methodology for any given nanoscience problem.
Nano etymology
The prefix nano is derived from the Greek word nanos, “a dwarf”. In 1947, at the 14th conference of the International Union of Pure and Applied Chemistry (IUPAC), the prefix nano was officially adopted to describe the one-billionth part (10 –9 ) of a unit Footnote 1 . In scientific literature, the prefix nano has been adopted as a popular label in many fields of modern science to describe small entities and processes. These terms include, but are not limited to nanoscience, nanotechnology, nanorobots, nanomagnets, nanoelectronics, nanoencapsulation, etc. [ 1 ]. In all of these cases, the prefix nano is used to describe “very small” entities or processes, most often at actual nanometer scale.
Definitions
Nanoscience is a branch of science that comprises the study of properties of matter at the nanoscale, and particularly focuses on the unique, size-dependent properties of solid-state materials [ 2 ]. Nanotechnology is the branch that comprises the synthesis, engineering, and utilization of materials whose size ranges from 1 to 100 nm, known as nanomaterials [ 3 ]. The birth of nanoscience and nanotechnology concepts is usually linked to the famous lecture of Nobel laureate Richard Feynman at the 1959 meeting of the American Physical Society, ‘‘There’s Plenty of Room at the Bottom’’ [ 4 ]. However, the use of nanotechnology and nanomaterials goes back in history long before that.
History of nanotechnology
Long before the era of nanotechnology, people were unknowingly coming across various nanosized objects and using nano-level processes. In ancient Egypt, dyeing hair in black was common and was for a long time believed to be based on plant products such as henna [ 5 ]. However, recent research on hair samples from ancient Egyptian burial sites showed that hair was dyed with paste from lime, lead oxide, and water [ 6 ]. In this dyeing process, galenite (lead sulfide, PbS) nanoparticles are formed. The ancient Egyptians were able to make the dyeing paste react with sulfur (part of hair keratin) and produce small PbS nanoparticles which provided even and steady dyeing.
Probably the most famous example for the ancient use of nanotechnology is the Lycurgus Cup (fourth century CE). This ancient roman cup possesses unusual optical properties; it changes its color based on the location of the light source. In natural light, the cup is green, but when it is illuminated from within (with a candle), it becomes red. The recent analysis of this cup showed that it contains 50–100 nm Au and Ag nanoparticles [ 7 ], which are responsible for the unusual coloring of the cup through the effects of plasmon excitation of electrons [ 8 ]. The ancient use of nanotechnology does not stop here, in fact, there is evidence for the early use of nanotechnology processes in Mesopotamia, Ancient India, and the Maya [ 9 , 10 ].
Why nanomaterials are different
Today, due to their unique properties, nanomaterials are used in a wide range of applications, such as catalysis, water treatment, energy storage, medicine, agriculture, etc . [ 11 , 12 , 13 ]. Two main factors cause nanomaterials to behave significantly differently than the same materials at larger dimensions: surface effects and quantum effects [ 14 ]. These factors make nanomaterials exhibit enhanced or novel mechanical, thermal, magnetic, electronic, optical, and catalytic properties [ 1 , 15 , 16 ].
Nanomaterials have different surface effects compared to micromaterials or bulk materials, mainly due to three reasons; (a) dispersed nanomaterials have a very large surface area and high particle number per mass unit, (b) the fraction of atoms at the surface in nanomaterials is increased, and (c) the atoms situated at the surface in nanomaterials have fewer direct neighbors [ 1 , 14 ]. As a consequence of each of these differences, the chemical and physical properties of nanomaterials change compared to their larger-dimension counterparts. For instance, having fewer direct neighbor atoms for the atoms situated at the surface results in lowering the binding energy per atom for nanomaterials. This change directly affects the melting temperature of nanomaterials following the Gibbs–Thomson equation, e.g., the melting point of 2.5 nm gold nanoparticles is 407 degrees lower than the melting point of bulk gold [ 14 ]. Larger surface areas and larger surface-to-volume ratios generally increases the reactivity of nanomaterials due to the larger reaction surface [ 1 ], as well as resulting in significant effects of surface properties on their structure [ 17 ]. The dispersity of nanomaterials is a key factor for the surface effects. The strong attractive interactions between particles can result in the agglomeration and aggregation of nanomaterials, which negatively affects their surface area and their nanoscale properties [ 18 ]. Agglomeration can be prevented by increasing the zeta potential of nanomaterials (increasing the repulsive force) [ 19 ], optimizing the degree of hydrophilicity/hydrophobicity of the nanomaterial, or by optimizing the pH and the ionic strength of the suspension medium [ 20 ].
Nanomaterials display distinct size-dependent properties in the 1–100 nm range where quantum phenomena are involved. When the material radius approaches the asymptotic exciton Bohr radius (the separation distance between the electron and hole), the influence of quantum confinement becomes apparent [ 17 ]. In other words, by shrinking the size of the material, quantum effects become more pronounced, and nanomaterials become quantal. Those quantum structures are physical structures where all the charge carriers (electrons and holes) are confined within the physical dimensions [ 21 ]. As a result of quantum confinement effects, for instance, some non-magnetic materials in bulk such as palladium, platinum, and gold become magnetic in the nanoscale [ 14 ]. Quantum confinement can also result in significant changes in electron affinity or the ability to accept or donate electrical charges, which is directly reflected on the catalytic properties of the material. For example, the catalytic activity of cationic platinum clusters in N 2 O decomposition is dictated by the number of atoms in the cluster. 6–9, 11, 12, 15, and 20 atom-containing clusters are very reactive, while clusters with 10, 13, 14, and 19 atoms have low reactivity [ 14 ].
Classification of nanomaterials
The key elements of nanotechnology are the nanomaterials. Nanomaterials are defined as materials where at least one of their dimensions is in the nanoscale, i.e. smaller than 100 nm [ 22 ]. Based on their dimensionalities, nanomaterials are placed into four different classes, summarized in Fig. 1 .
Zero-dimensional nanomaterials (0-D): the nanomaterials in this class have all their three dimensions in the nanoscale range. Examples are quantum dots, fullerenes, and nanoparticles.
One-dimensional nanomaterials (1-D): the nanomaterials in this class have one dimension outside the nanoscale. Examples are nanotubes, nanofibers, nanorods, nanowires, and nanohorns.
Two-dimensional nanomaterials (2-D): the nanomaterials in this class have two dimensions outside the nanoscale. Examples are nanosheets, nanofilms, and nanolayers.
Three-dimensional nanomaterials (3-D) or bulk nanomaterials: in this class the materials are not confined to the nanoscale in any dimension. This class contains bulk powders, dispersions of nanoparticles, arrays of nanowires and nanotubes, etc .
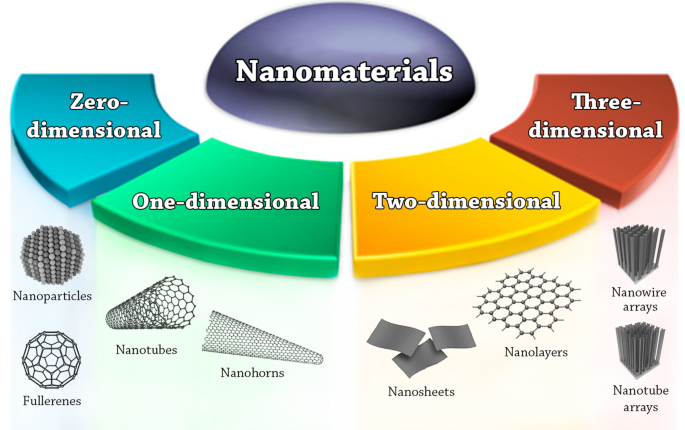
Nanomaterials classification based on dimensionality
Nanoparticles (NPs)
The International Organization for Standardization (ISO) defines nanoparticles as nano-objects with all external dimensions in the nanoscale, where the lengths of the longest and the shortest axes of the nano-object do not differ significantly. If the dimensions differ significantly (typically by more than three times), terms such as nanofibers or nanoplates maybe preferred to the term NPs Footnote 2 .
NPs can be of different shapes, sizes, and structures. They can be spherical, cylindrical, conical, tubular, hollow core, spiral, etc., or irregular [ 23 ]. The size of NPs can be anywhere from 1 to 100 nm. If the size of NPs gets lower than 1 nm, the term atom clusters is usually preferred. NPs can be crystalline with single or multi-crystal solids, or amorphous. NPs can be either loose or agglomerated [ 24 ].
NPs can be uniform, or can be composed of several layers. In the latter case, the layers often are: (a) The surface layer, which usually consists of a variety of small molecules, metal ions, surfactants, or polymers. (b) The shell layer, which is made of a chemically different material from the core layer. (c) The core layer, which is the central portion of the NP [ 25 ].
Classification of NPs
Based on their composition, NPs are generally placed into three classes: organic, carbon-based, and inorganic [ 23 ].
Organic NPs
This class comprises NPs that are made of proteins, carbohydrates, lipids, polymers, or any other organic compounds [ 26 ]. The most prominent examples of this class are dendrimers, liposomes, micelles, and protein complexes such as ferritin (shown in Fig. 2 ). These NPs are typically non-toxic, bio-degradable, and can in some cases, e.g., for liposomes, have a hollow core. Organic NPs are sensitive to thermal and electromagnetic radiation such as heat and light [ 23 ]. In addition, they are often formed by non-covalent intermolecular interactions, which makes them more labile in nature and offers a route for clearance from the body [ 27 ]. There are different parameters that determine the potential field of application of organic NPs, e.g., composition, surface morphology, stability, carrying capacity, etc . Today, organic NPs are mostly used in the biomedical field in targeted drug delivery [ 23 ] and cancer therapy [ 28 ].
Types of organic NPs. A Dendrimers; B liposomes; C micelles; and D ferritin
Carbon-based NPs
This class comprises NPs that are made solely from carbon atoms [ 23 ]. Famous examples of this class are fullerenes, carbon black NPs, and carbon quantum dots (shown in Fig. 3 ). Fullerenes are carbon molecules that are characterized by a symmetrical closed-cage structure. C 60 fullerenes consist of 60 carbon atoms arranged in the shape of a soccer ball [ 29 ], but also other types of fullerenes such as C 70 and C 540 fullerenes have been described [ 30 ]. Carbon black NPs are grape-like aggregates of highly fused spherical particles [ 31 ]. Carbon quantum dots consist of discrete, quasi-spherical carbon NPs with sizes below 10 nm [ 32 ]. Carbon-based NPs unite the distinctive properties of sp 2 -hybridized carbon bonds with the unusual physicochemical properties at the nanoscale. Due to their unique electrical conductivity, high strength, electron affinity, optical, thermal, and sorption properties [ 25 , 33 ], carbon-based NPs are used in a wide range of application such as drug delivery [ 34 ], energy storage [ 35 ], bioimaging [ 36 ], photovoltaic devices, and environmental sensing applications to monitor microbial ecology or to detect microbial pathogens [ 33 ]. Nanodiamonds and carbon nano onions are more complex, carbon-based NPs. Due to their characteristic low toxicity and biocompatibility, they are used in drug delivery and tissue engineering applications [ 37 , 38 ].
Different types of carbon-based NPs. A C 60 fullerene; B carbon black NPs; and C carbon quantum dots
Inorganic NPs
This class comprises NPs that not made of carbon or organic materials. The typical examples of this class are metal, ceramic, and semiconductor NPs. Metal NPs are purely made of metal precursors, they can be monometallic, bimetallic [ 39 ], or polymetallic [ 40 ]. Bimetallic NPs can be made from alloys or formed in different layers (core–shell) [ 39 ]. Due to the localized surface plasmon resonance characteristics, these NPs possess unique optical and electricals properties [ 25 ]. In addition, some metal NPs also possess unique thermal, magnetic, and biological properties [ 23 ]. This makes them increasingly important materials for the development of nanodevices that can be used in numerous physical, chemical, biological, biomedical, and pharmaceutical applications [ 41 , 42 ] (these applications are discussed in detail later in the applications section of the review). In present days, the size-, shape-, and facet-controlled synthesis of metal NPs is important for creating cutting-edge materials [ 43 ].
Semiconductor NPs are made of semiconductor materials, which possess properties between metals and non-metals. These NPs possess unique wide bandgaps and show significant alteration in their properties with bandgap tuning compared to bulk semiconductor materials [ 25 ]. As a result, these NPs are important materials in photocatalysis, optic, and electronic devices [ 44 , 45 ]. Ceramic NPs are inorganic solids made of carbonates, carbides, phosphates, and oxides of metals and metalloids, such as titanium and calcium [ 46 ]. They are usually synthesized via heat and successive cooling and they can be found in amorphous, polycrystalline, dense, porous or hollow forms [ 25 ]. They are mainly used in biomedical applications due to their high stability and high load capacity [ 47 ]. Nevertheless, they are also used in other applications such as catalysis, degradation of dyes, photonics and optoelectronics [ 46 , 48 ].
Physicochemical properties of NPs
As mentioned earlier, NPs can be used in a long list of applications due to their unique physical and chemical properties that do not exist in their larger-dimension counterparts of the same materials. The following sections summarize the most import physicochemical properties that are changing on the nanoscale.
Mechanical properties
Mechanical properties refer to the mechanical characteristics of a material under different conditions, environments, and various external forces. As for traditional materials, the mechanical properties of nanomaterials generally consist of ten parts: strength, brittleness, hardness, toughness, fatigue strength, plasticity, elasticity, ductility, rigidity, and yield stress [ 49 ]. Most inorganic, non-metallic materials are brittle materials and do not have significant toughness, plasticity, elasticity, or ductility properties. Organic materials on the other hand, are flexible materials and do not necessarily have brittleness and rigidity properties.
Due to surface and quantum effects, NPs display different mechanical properties compared to bulk materials [ 49 ]. For example, conventional FeAl powder which is composed of microparticles (larger than 4 µm), is brittle, while ultrafine FeAl alloy powder displays a good combination of strength and ductility as well as enhanced plasticity [ 50 ]. These new properties are believed to arise due to the diverse interaction forces between NPs or between them and a surface. The most important interaction forces involved are van der Waals forces, which consist of three parts, Keesom force, Debye force, and London force [ 51 , 52 , 53 ]. Other relevant interaction forces are electrostatic and electrical double layer forces, normal and lateral capillary forces, solvation, structural, and hydration forces [ 54 ].
There are different theories on how the interaction forces between NPs give them new mechanical properties, such as the DLVO (Derjaguin–Landau–Verwey–Overbeek) theory, JKR (Johnson–Kendall–Roberts) theory, and DMT (Derjaguin–Muller–Toporov) theory. The DLVO theory combines the effects of van der Waals attraction and electrostatic repulsion to describe the stability of colloidal dispersions [ 54 ]. This theory can explain many phenomena in colloidal science, such as the adsorption and the aggregation of NPs in aqueous solutions and the force between charged surfaces interacting through a liquid medium [ 55 , 56 ]. Nevertheless, the DLVO theory is inadequate for the colloidal properties in the aggregated state [ 54 ].
When the size of objects decreases to the nanoscale, the surface forces become a major player in their adhesion, contact, and deformation behaviors. The JRK theory is applicable to easily deformable, large bodies with high surface energies, where it describes the domination of surface interactions by strong, short-range adhesion forces. In contrast to this, the DMT theory is applicable to very small and hard bodies with low surface energies, where it describes the adhesion being caused by the presence of weak, long-range attractive forces. Although the DLVO, JKR and DMT theories have been widely used to describe and study the mechanical properties of NPs [ 57 , 58 ], it is still a matter of debate whether or not continuum mechanics can be used to describe a particle or collection of particles at the nanometer scale [ 54 ].
Thermal properties
Heat transfer in NPs primarily depends on energy conduction due to electrons as well as photons (lattice vibration) and the scattering effects that accompany both [ 59 ]. The major components of thermal properties of a material are thermal conductivity, thermoelectric power, heat capacity, and thermal stability [ 59 , 60 ].
NP size has a direct impact on electrical and thermal conductivity of NPs [ 60 ]. As the NP size decreases, the ratio of particle surface area respective to its volume increases hyperbolically [ 60 ]. Since the conduction of electrons is one of the two main ways in which heat is transferred, the higher surface-to-volume ratio in NPs provides higher number of electrons for heat transfer compared to bulk materials [ 61 ]. Moreover, thermal conductivity in NPs is also promoted by microconvection, which results from the Brownian motion of NPs [ 62 ]. Nevertheless, this phenomenon only happens when solid NPs are dispersed in a liquid (generating a Nanofluid) [ 63 ]. As an example, the addition of Cu NPs to ethylene glycol enhances the thermal conductivity of the fluid up to 40% [ 64 ].
The thermoelectric power of a material depends on its Seebeck coefficient and electrical conductivity ( \(P={S}^{2}\sigma \) , where P is thermoelectric power, S is the Seebeck coefficient, and \(\sigma \) is the electrical conductivity). The scattering of NPs in bulk materials (doping) is known to enhance the thermoelectric power factor [ 65 ]. This enhancement could come from the enhancement of the Seebeck coefficient or the enhancement of electrical conductivity. The embedding of size-controlled NPs in bulk thermoelectric materials helps to reduce the lattice thermal conductivity and enhances the Seebeck coefficient due to electron energy filtering [ 66 , 67 ]. Generally, the enhancement of electrical conductivity is accompanied by the reduction of the Seebeck coefficient and vice versa [ 65 ] However, the doping of InGaAlAs material with 2–3 nm Er NPs resulted in the significant increase of thermoelectric power of the material through the enhancement of the conductivity while keeping the Seebeck coefficient unchanged [ 65 ]. Depending on NP size, volume fraction, and band offset, a NP-doped sample can either enhance or suppress the electrical conductivity in comparison with undoped bulk sample.
Experimental studies have shown that the heat capacity of NPs exceeds the values of analogous bulk materials by up to 10% [ 68 ], e.g. in the case of Al 2 O 3 and SiO 2 NPs [ 69 , 70 ]. The major contribution to heat capacity at ambient temperatures is determined by the vibration degrees of freedom, i.e., the peculiarities of phonon spectra (vibrational energy that arises from oscillating atoms within a crystal) are responsible for the anomalous behavior of heat capacity of NPs [ 68 ]. NPs usually exhibit a significant decrease in melting temperature compared to their analogous bulk materials [ 71 ]. The main reason for this phenomenon is that the liquid/vapor interface energy is generally lower than the average solid/vapor interface energy [ 72 ]. When the particle size decreases, its surface-to-volume ratio increases, and the melting temperature decreases as a result of the improved free energy at the particle surface [ 73 ]. For instance, the melting temperature of 3 nm Au NPs is 300 degrees lower than the melting temperature of bulk gold [ 14 ]. In addition, NP composition plays an important role in thermal stability. For example, the thermal stability of Au in Au 0.8 Fe 0.2 is significantly higher than of pure Au or Au 0.2 Fe 0.8 [ 74 ]. Generally, bimetallic alloy NPs show higher thermal stabilities and melting temperatures than monometallic NPs due to the alloying effect [ 75 , 76 ].
Magnetic properties
All magnetic compounds include a ‘magnetic element’ in their formula, i.e., Fe, Co, or Ni (at ambient temperatures). There are only three known exceptions that are made from mixed diamagnetic elements, Sc 3 In, ZrZn 2 , and TiBe 2-x Cu x [ 77 , 78 , 79 , 80 ]. Otherwise, elements such as Pd, Au, or Ag are diamagnetic. This all changes in the nanoscale. Several materials become magnetic in the form of NPs as a result of uneven electronic distribution [ 25 ]. For instance, FeAl is not magnetic in bulk but in the form of NPs, it is becomes magnetic [ 50 ], other examples include Pd and Au [ 81 ]. In bulk materials, the key parameters for determining magnetic properties are composition, crystallographic structure, magnetic anisotropy, and vacancy defects [ 82 , 83 ]. However, on the nanoscale, two more important parameters are strongly involved, i.e., size and shape [ 84 ].
One of the interesting size-dependent phenomena of NPs is superparamagnetism [ 84 ]. As the size of the NPs decreases, the magnetic anisotropy energy per NP decreases. The magnetic anisotropy energy is the energy keeping the magnetic moment in a particular orientation. At a characteristic size for each type of NPs, the anisotropy energy becomes equal to the thermal energy, which allows the random flipping of the magnetic moment [ 85 ], in this case, the NP is defined as being superparamagnetic [ 86 ]. Superparamagnetic NPs display high magnetization only in the presence of a magnetic field, and once it is removed they do not retain any magnetization [ 87 ]. Superparamagnetism was long believed to form only in small ferromagnetic or ferrimagnetic NPs [ 88 ], but interestingly, other paramagnetic materials show magnetism in the nanoscale too [ 81 ].
NP size effects can also be observed in changes in magnetic coercivity, i.e., the resistance of a magnetic material to changes in magnetization (Fig. 4 ). In contrast to large particles or bulk materials, which possess multiple magnetic domain structures, small NPs possess single magnetic domain structures below a certain critical radius (r c ), where all magnetic spins in the NP align unidirectionally (blue arrows in Fig. 4 ). However, the NP radius has to be lower than the threshold radius for superparamagnetism (r sp ) in order to be superparamagnetic [ 89 ]. In the single-domain regime, between r sp and r c , the magnetic coercivity increases as the size of the NP increases until it reaches the maximum at r c [ 84 ]. In this size regime, due to the high magnetic coercivity, the NPs behave similarly as their larger dimension counterparts despite having a single domain structure, i.e., they become ferromagnetic for ferromagnetic materials or paramagnetic for paramagnetic materials etc . Above r c , the magnetic coercivity starts to decrease when multiple magnetic domains are formed in a single NP. The critical radius represents the size where it is energetically favored for the NP to exist without a domain wall [ 86 ]. The calculated critical radii for some common magnetic materials are 35 nm of Ni, 8 nm for Co, and 1 nm for Fe [ 90 ]. Above that point, multi-domain magnetism begins in which a smaller reversal magnetic field is required to make the net magnetization zero [ 84 ].
The change in magnetic coercivity of NPs as a function of particle radius. Figure adapted from Kalubowilage et al., 2019 [ 89 ]. rc critical radius, rsp threshold radius for superparamagnetism
The second key parameter for determining the magnetic properties of NPs is the shape of NPs. In comparison to the size parameter, there is significant less research on the effect of shape on the magnetic properties of NPs having the same volume [ 86 ]. However, large differences in coercivity were found between a set of cubic and spherical CoFe 2 O 4 NPs [ 91 ]. Unlike the curved topography in spherical CoFe 2 O 4 NPs, cubic CoFe 2 O 4 NPs have fewer missing oxygen atoms, and it was hypothesized that this led to less surface pinning and to lower coercivity for the cubic structures [ 86 ]. Other studies also found differences in magnetism between spherical and cubic Fe 3 O 4 NPs [ 92 , 93 ].
Similar to bulk materials, the composition also affects the magnetism of NPs. The magnetocrystalline phase of the NP is significant in determining its magnetic coercivity [ 94 ]. This effect can be observed in magnetic bimetallic core–shell or alloy NPs with anisotropic crystalline structures. For example, Co@Pt core–shell NPs composed of an isotropically structured face-centered cubic Co core and a non-magnetic Pt shell exhibit superparamagnetic behavior with zero coercivity at room temperature [ 95 ]. In general, the compositional modification of NPs by the adoption of magnetic dopants is known to significantly change the magnetism of NPs [ 96 ].
Electronic and optical properties
Metallic and semiconductor NPs possess interesting linear absorption, photoluminescence emission, and nonlinear optical properties due to the quantum confinement and localized surface plasmon resonance (LSPR) effect [ 97 , 98 ]. LSPR phenomena arise when the incident photon frequency is constant with the collective excitation of the conductive electrons [ 25 ].Due to this phenomenon, noble metal NPs exhibit a strong size-dependent UV–visible extinction band that is not present in the spectra of bulk metals. Generally, the optical properties of NPs depend on the size, shape, and the dielectric environment of the NPs [ 99 ].
The collective excitations of conductive electrons in metals are called plasmons [ 100 ]. Depending on the boundary conditions, bulk plasmons, surface-propagating plasmons, and surface-localized plasmons are distinguished (Fig. 5 A–C). Because of their longitudinal nature, the bulk plasmons cannot be excited by visible light. The surface-propagating plasmons propagate along metal surfaces in a waveguide-like fashion [ 98 ]. In the case of NPs, when they are irradiated by visible light, the oscillating electric field causes the conductive electrons to oscillate coherently. When the electron cloud is displaced relative to the nuclei, a restoring force rises from Coulomb attraction between electrons and nuclei that results in oscillation of the electron cloud relative to the nuclear framework [ 99 ]. This creates uncompensated charges at the NP surface (Fig. 5 D). As the main effect producing the restoring force is the polarization of the NP surface, these oscillations are called surface plasmons and have a well-defined resonance frequency [ 98 ].
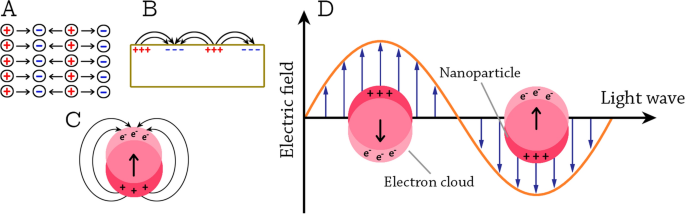
Graphical illustration of the types of plasmons. A bulk; B surface propagating; and C surface localized plasmons (adapted from Khlebtsov et al., 2010 [ 98 ]). D graphical illustration of the localized surface plasmon resonance (LSPR) in NPs (adapted from Kelly et al., 2003 [ 99 ])
Experimental studies on Ag NPs showed significant differences in their optical properties based on the size of NPs. For Ag NPs with 30 nm radius, the main extinction peak was at 369 nm wavelength, while for Ag NPs with 60 nm radius, a totally different behavior was observed [ 99 ]. The same researchers found that the shape of the NPs also is critical for the optical properties, the plasmon resonance wavelength shifts to the red as the NPs become more oblate [ 99 ], demonstrating that plasmon resonance strongly depend on NPs shape. With respect to the dielectric environment of the NPs, both the surrounding solvent and the support (substrate) were found to be critical for the optical properties. For Ag NPs, both experimental and theorical studies on the effect of surrounding solvent show that plasmon wavelength linearly depends on the refractive index of the solvent [ 99 , 101 ]. At the same time, 10 nm Ag NPs supported on mica substrates displayed LSPR wavelength shifts to the red compared to unsupported NPs [ 102 ]. The biogenic synthesis of NPs can also improve the optical properties. Biologically produced CeO 2 NPs using Simarouba glauca leave extract were found to have different absorption bands and higher band gap energies compared to chemically produced CeO 2 NPs. These superior optical properties were attributed to the better crystallinity and small size of biogenic NPs compared to chemical NPs [ 103 ]. Biogenic NPs can also offer higher photocatalytic activities, e.g., ZnO NPs produced by Plectranthus amboinicus leaf extract had higher photocatalytic activity in the photodegradation of methyl red under UV illumination compared to chemical produced ZnO NPs [ 104 ].
Catalytic properties
Nano-catalysis, i.e., the use of NPs as catalysts, is a quickly evolving field within chemical catalysis. Significantly enhanced or novel catalytic properties such as reactivity and selectivity have been reported for NP catalysts compared to their bulk analogues. The catalytic properties of NPs depend on the size, shape, composition, interparticle spacing, the oxidation state, and the support of the NPs [ 76 ].
The dependency of catalytic activity on the size of NPs is well studied. The relation is an inverse one, i.e., the smaller the NPs the more catalytically active they are. This relationship was found e.g., in the electro-catalysis oxidation of CO by size-selected Au NPs (1.5, 4, and 6 nm) deposited on indium tin oxide. The researchers observed that the smallest NPs provided the highest normalized current densities [ 105 ]. The same relationship was also found in several other studies [ 106 , 107 , 108 , 109 , 110 ]. Goodman et al., 1998 [ 111 ] speculated originally that this behavior could be attributed to quantum-size effects generated by the confinement of electrons within a small volume. Later, size-dependent changes in the electronic structure of the clusters [ 112 ] and the resulting larger number of low-coordinated atoms available for interaction by the larger surface-to-volume ratios with smaller NPs were discussed [ 76 ].
The shape is also known to affect the reactivity and selectivity of the NPs. For the oxidation of CO by Au NPs, hemispherical NPs were found to be more active than spherical ones [ 113 ]. For the oxidation of styrene by Ag NPs, nanocubes were found to be fourteen times more efficient than nanoplates and four times more efficient than nanospheres [ 114 ]. The reason for these dramatical changes are attributed to the increase/decrease in the relative area of the catalytically active surface facets [ 76 ] or to the differences in stability for different NP shapes [ 115 ].
As for composition, several studies have shown that the use of alloys in NPs can enhance the catalytic activity as a result of the alloying effect causing changes in the electronic properties of the catalyst, decreasing poisoning effects, and providing distinct selectivities [ 76 ]. For example, the alloying of Pt with other metals such as Ru, Ni, and Co, was reported to enhance the hydrogenation and oxygen reduction activity of the NP catalyst material, as well as enhancing the resistance against CO poisoning [ 116 , 117 , 118 ]. However, the alloying of Pt with Fe, Ru, and Pd, resulted in reduced reactivity for methanol decomposition [ 119 ]. This reduction in reactivity was explained by the possible occupation of the surface with the addition metal atoms, since pure Fe, Ru, and Pd clusters are less reactive for methanol decomposition than similarly-sized pure Pt clusters. In general, the change in the composition of NPs changes the electronic structure of metal surfaces by the formation of bimetallic bonds as well as the modification of metal–metal bond lengths [ 76 ]. In addition, the charge-transfer phenomenon between different metals may favorably change the binding energy of adsorbents, lower the barriers for specific chemical reactions, and enhance resistance against poisoning [ 120 , 121 , 122 ].
The catalytic activity and stability of 2 nm Au NPs dispersed on polycrystalline TiC films displayed a strong dependence on interparticle spacing. In this study, Au NPs having two different interparticle spacing (30 and 80 nm) were analyzed by Thermal Desorption Spectroscopy. It was found that the sample with smaller interparticle spacing was poisoned and subsequently deactivated while the sample with longer interparticle spacing showed longer lifetime [ 123 ]. At the same time, the oxidation state of NPs was shown to affect the catalytic activities. Ru NPs under rich O 2 conditions and moderate temperatures oxidize and form RuO 2 , the reaction of CO oxidation was found to occur on the metal oxide surface not the metal surface [ 124 ]. A similar effect on CO oxidation was also observed with Pt NPs in which the reactivity of PtO 2 was found to be higher than Pt [ 125 ]. The reaction of CO oxidation was compared for several metal NPs (Ru, Pd, Ir, Os, and Pt) and their corresponding oxides, and the oxides were indeed more reactive than the metals [ 126 , 127 ]. The superior catalytic performance of RuO 2 over their metallic counterparts is generally agreed on, nevertheless, the same cannot be said for other catalytically active metals such as Pt [ 76 ]. In general, these differences in catalytic performance are attributed to the electron transfer processes at the metal/metal oxide interfaces. Consequently, the view that NP oxidation is an undesirable process that leads to the reduction of catalytic performance needs to be reconsidered [ 128 ].
An example for the effect of the support material is the role of the MgO support for Au NPs, where MgO was found to be important for CO oxidation and particularly, for controlling the rate of CO oxidation through oxygen vacancies [ 129 ]. Later, the process of electron charge transfer from oxygen vacancies at the metal-substrate interface of supported Au NPs was suggested to be an ideal environment for O 2 activation and oxidation reactions [ 130 ]. A similar behavior was also found in the decomposition of SO 2 and dissociation of water by Au NPs supported on CeO 2 , in which CeO 2 supports played a critical role [ 131 ]. The experiments showed that not only the chemical composition of the support affects the reactivity of the catalyst, but the crystal structure of the support, too [ 132 ]. Enhanced catalytic performance for CO oxidation and SO 2 dissociation have also been reported for Au NPs supported on metal carbides such as TiC [ 108 , 133 ]. In addition to enhanced catalytic reactivities, the support also plays an important role in NP stabilization [ 106 ], i.e., the stabilization of NPs against coarsening, the stabilization of metal oxides at the NP surface, and the stabilization of intermediate reactions species [ 76 ].
Characterization of NPs
The properties of NPs determine their potential applications. Hence, different methods and techniques are used for the analysis and characterization of the various physicochemical properties of NPs. Table 1 summarizes all characterization techniques mentioned in this review and shows what properties and features can be resolved by each technique.
Morphological and topographical characterization
The morphological and topographical features of NPs are of great interest since they influence most of the properties of NPs as described above. These features include the size, shape, dispersity, localization, agglomeration/aggregation, surface morphology, surface area, and porosity of the NPs. The following techniques are regularly used for the characterization of morphological and topographical features of NPs.
Electron microscopy (EM)
Scanning electron microscopy (SEM), scanning tunneling microscopy (STM), and transmission electron microscopy (TEM) are frequently employed for the analysis of NP size, shape, and surface. In SEM, an electron gun is used to produce a beam of electrons that is controlled by a set of lenses to follows a vertical path through the microscope until it hits the samples. Once the sample is hit by the beam, electrons and X-rays are ejected from the sample. Detectors are then used to collect the X-rays and scattered electrons in order to create a 3D image of the sample. SEM provides different information about the NPs such as size, shape, aggregation, and dispersion [ 134 ]. Similarly, TEM provides information about the size, shape, localization, dispersity, and aggregation of NPs in two-dimensional images [ 25 ]. TEM employs an electromagnetic lens that focuses a very fine beam of electrons into an ultrathin section of the sample. This beam passes through the specimen where the electrons either scatter or penetrate the sample and hit a fluorescent screen at the bottom of the microscope. The difference in electron densities is used for the contrast to create an image of the specimen. TEM can be also used for the characterization of NP crystal structure through the use of selected area electron diffraction (SAED), where the electron beam is focused on a selected area in the sample and the scattered electrons are used to obtain a diffraction pattern. STM is based on the phenomenon of quantum tunneling, where a metallic tip is brough very close to the sample surface and used to apply voltage. When voltage is applied, electrons from the sample surface are extracted creating an electrical current that is used to reconstruct an image of the surface with atomic resolution [ 135 ]. STM is mainly used to characterize the topography of NPs. For inorganic NPs, these techniques offer excellent approaches for the determination of morphological features of NPs. For organic NPs (or NPs coated with biological materials), these techniques require sophisticated sample preparations which constitute major restrictions to their use [ 136 ]. The sample preparation for these techniques might cause sample dehydration, which might lead e.g. to sample shrinking and aggregation [ 136 ].
Examples: TEM was used for the characterization of Ag NPs produced by Arbutus unedo leaf extract. In this example, the NPs have a spherical morphology with a uniform size of 30 nm. The NPs were found to agglomerate into small aggregates, each including 5–6 NPs. At the same time, the SAED approach was used to determine the crystal structure of the NPs. The majority of the NPs were found to be single crystalline cubic materials predominately oriented along their (111) direction [ 137 ]. For the characterization of Ag NPs produced by Diospyros kaki leaf extract, SEM helped to show that the NPs were also spherical and the size was 32 nm with some deviations [ 138 ]. STM is less frequently used for the characterization of biogenic NPs. The features of Ag NPs produced by lime, sweet-lime, and orange juices were compared using STM technique [ 139 ].
Dynamic light scattering (DLS)
This technique is a common approach for the analysis of NP size and size distribution. This approach involves the measurement of light interference based on the Brownian motion of NPs in suspension, and on the correlation of NP velocity (diffusion coefficient) with their size using Strokes-Einstein equation [ 140 ]. The size distribution range of NPs is shown as the polydispersity index, which is the output of an autocorrelation function [ 136 ]. The polydispersity index values lie between 0 and 1, where 0 represents a completely homogenous population and 1 represents a highly heterogeneous population. This technique also allows the analysis of non-spherical NPs through the use of multistage DLS [ 136 ]. This technique is also referred to as photon correlation spectroscopy (PCS) [ 141 ].
Examples: DLS was used to measure the size and the size distribution profile of a wide range of biogenic NPs. The average size of Ag NPs produced by Trichoderma koningii fungi was found to be around 25 nm and the size distribution profile was between 14 and 34 nm. The polydispersity index for those NPs was 0.681, which indicates that they are polydispersed [ 142 ]. While the average size of Ag NPs produced by potato ( Solanum tuberosum ) was found to be around 10–12 nm with a wider distribution profile between 3–65 nm [ 143 ]. In a different application, DLS was employed to study the size increase of biogenic MnO 2 NPs overtime, demonstrating that their size is 7.5 nm after 3 min of the initiation of the reaction, then their size grows overtime until it become 54 nm after 31 min [ 144 ].
Nanoparticle tracking analysis (NTA)
This method is used for the analysis of NP size in suspensions based on their Brownian motion. Like in DLS, the rate of NP movement is correlated with their size using Strokes-Einstein equation, allowing the measurement of size distribution profiles for NPs with 10–1000 nm diameter. Its advantage over DLS is that NP motion is analyzed by video. Individual positional changes of NPs are tracked in two dimensions, which are used to determine NP diffusion rates, and by knowing the diffusion coefficient, the hydrodynamic diameter of the particles can be calculated. In DLS, individual NPs are not visualized, but instead, the time-dependent intensity fluctuations caused by Brownian motion are used to calculate the polydispersity index [ 145 ]. NTA was found to be more precise for sizing monodisperse as well as polydisperse organic NPs compared to DLS [ 146 ].
Examples: NTA was used to measure the size and dispersity of Ag NPs produced by Camellia sinensis (green tea) powder, the NPs were found to be well dispersed in an aqueous medium with an average size of 45 ± 12 nm [ 147 ]. For Se NPs produced by lactic acid bacteria, NTA was employed to measure the size and the concentration of NPs. The average size was found to be 187 ± 56 nm with a concentration of (4.67 ± 0.30) × 10 9 Se NPs per ml [ 148 ].
Brunauer–Emmett–Teller (BET) method
This method is based on the adsorption and desorption principle developed by Stephen Brunauer, Paul Emmett, and Edward Teller, and it is considered one of the best methods for the analysis of NP surface area [ 25 ]. In BET analysis, a partial vacuum is created to produce adsorption between the sample and liquid N 2 (because the interaction between solid and gaseous phases is weak, the surface is cooled with liquid N 2 to obtain detectable amounts of adsorption). After the formation of adsorption monolayers, the sample is removed from the N 2 atmosphere and heated to cause the adsorbed N 2 to be released from the material (desorption) and quantified. The data collected is displayed in the form of isotherms (graphs representing the amount of N 2 adsorbed as a function of relative pressure at a constant temperature). The data is displayed in five isotherms where the information is used to determine the surface area of the sample [ 25 , 149 ]. Figure 6 graphically illustrates the principle of this method.

Principles of the BET and BJH methods. The BET method (steps 1–3) is based on the adsorption of nitrogen on the NP surface. After the formation of a monolayer, nitrogen is desorbed, and the surface area is calculated. The BJH method (steps 1, 2, 4, and 5) is based on the complete filling of NP pores with liquid nitrogen. When saturation is reached, nitrogen is desorbed, and pore size is calculated
Examples: The BET method was employed to measure the surface area of CeO 2 NPs produced by Eucalyptus globulus leaf extract. The surface area was found to be 40.96 m 2 /g of biogenic CeO 2 NPs, much higher than the commercial CeO 2 NPs (8.5 m 2 /g) [ 150 ]. BET was also used to measure the surface area of SiO 2 NPs produced by rice husk, CuO NPs produced by Leucaena leucocephala leaf extract, and Ag NPs produced by Acanthospermum hispidum leaf extract. In these examples, the surface area was 7.15 m 2 /g, 47.54 m 2 /g, and 9.91 m 2 /g, respectively [ 151 , 152 , 153 ].
Barrett–Joyner–Halenda (BJH) method
This method is based on the Barrett–Joyner–Halenda principle and is used for the determination of porosity (or pore size) of NPs. Similar to the BET method, this method also involves the use of N 2 gas to adsorb to the sample. In the BJH method, the process is extended so the gas condensates in the sample pores as pressure increases. The pressure is increased until a saturation point is achieved, at which all the pores of the sample are filled with liquid. Afterwards, the condensated gas is allowed to evaporate where the desorption data is calculated and correlated to the pore size using a modified Kelvin equation (Kelvin model of pore filling) [ 154 , 155 ]. Figure 6 graphically illustrates this method.
Examples: The BJH method was employed to study the pore size of a wide range of biogenic NPs, for instance, the pore size of CeO 2 NPs produced by Eucalyptus globulus leaf extract was found to be 7.8 nm [ 150 ], the pore size of CuO NPs produced by Leucaena leucocephala leaf extract was 2.13 nm [ 152 ], the pore size of SiO 2 NPs produced by rice husk and Ag NPs produced by Acanthospermum hispidum leaf extract were much larger, being 29.63 nm and 36.34 nm, respectively [ 151 , 153 ].
Structural and chemical characterization
The structural characterization of NPs and the study of their composition is of high interest due to the strong influence of these parameters on the physicochemical properties. The following techniques are commonly used for the analysis of NP composition, phase, crystallinity, functionalization, chemical state (oxidation), surface charge, polarity, bonding, and electrochemical properties.
X-ray diffraction analysis (XRD)
This technique is based on irradiating a material with incident X-rays and then measuring the intensities and scattering angles of the X-rays that leave the material [ 156 ]. This technique is widely used for the analysis of NP phase and crystallinity. However, the resolution and accuracy of XRD can be affected in cases where the samples have highly amorphous characteristics with varied interatomic distances or when the NPs are smaller than several hundreds of atoms [ 25 ].
Examples: For the characterization of biogenic Ag NPs, the XRD results of Ag NPs produced by Trichoderma koningii [ 142 ], Solanum tuberosum [ 143 ], and Acanthospermum hispidum leaf extract [ 153 ] displayed characteristic peaks occurring at roughly 2θ = 38 o , 44°, and 64 o corresponding to (111), (200), and (220) planes, respectively. These results are in good agreement with the reference to the face-centered cubic structure of crystalline silver. However, the XRD results of Ag NPs produced by Solanum tuberosum were not as clear as the other biogenic Ag NPs and had several impurities. The structural characterization of Pd NPs produced by Garcinia pedunculata Roxb leaf extract by XRD showed the distinct peaks of Pd, however, three other peaks were also observed at 2θ of 34.22˚, 55.72˚, and 86.38˚, indicating the presence of PdO phases along with Pd NPs [ 157 ].
Energy-dispersive X-ray spectroscopy (EDX)
This technique is based on the irradiation of the sample with an electron beam. Electrons of the electron beam when incident on the sample surface eject inner shell electrons, the transition of outer shell electrons to fill up the vacancy in the inner shell produces X-rays. Each element produces a characteristic X-ray emission pattern due to its unique atomic structure, and therefore can be used to perform compositional analysis [ 158 ]. The shortfall of EDX is that the resulting spectra give only qualitative compositional information (it shows the chemical elements present in the sample without quantification). However, the peak intensities to some extent give an estimate of the relative abundance of an element in a sample [ 159 ]. This technique does not require sophisticated additional infrastructures, usually it is a small device that is connected to an existing SEM or TEM. This allows the use of SEM or TEM for the morphological characterization and EDX is used simultaneously for the analysis of chemical composition [ 160 ].
Examples: The EDX technique is usually used for the confirmation of the presence of the element in question in biogenic NPs. For instance, EDX was used to confirm the presence of Au in Au NPs produced by Jasminum auriculatum leaf extract [ 161 ], the presence of Pd in Pd NPs produced by Pulicaria glutinosa extract [ 162 ], the presence of Te in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and the presence of Ag in Ag NPs produced by Trichoderma viride [ 164 ].
High-angle annular dark-field imaging (HAADF)
This method is used for the elemental mapping of a sample using a scanning transmission electron microscope (STEM). The images are formed by the collection of incoherently scattering electrons with an annular dark-field detector [ 165 ]. This method offers high sensitivity to variations in the atomic number of elements of the sample, and it is used for elemental composition analysis usually when the NPs of interest consist of relatively heavy elements. The contrast of the images is strongly correlated with atomic number and specimen thickness [ 166 ].
Examples: The employment of HAADF-STEM in the characterization of biogenic Au–Ag–Cu alloy NPs confirmed the presence of the three elements in the same NP [ 167 ]. Similarly, this approach revealed that Ag NPs produced by Andrographis paniculata stem extract were coated with an organic polymer [ 168 ]. The employment of this approach in the characterization of Cu NPs produced by Shewanella oneidensis revealed that Cu NPs remained stable against oxidization under anaerobic conditions, but when they were exposed to air a thin shell of Cu 2 O develop around the NPs [ 169 ].
X-ray photoelectron spectroscopy (XPS)
This technique is considered the most sensitive approach for the determination of NP exact elemental ratios, chemical state, and exact bonding nature of NP materials [ 25 ]. XPS is based on the photoelectric effect that can identify the elements within a material, or covering a material, as well as their chemical state with high precision [ 170 ]. XPS can also be used to provide in-depth information on electron transfer, e.g., for Pt NPs supported on CeO 2 , it was found that per ten Pt atoms only one electron is transferred to the support [ 171 ].
Examples: The XPS technique can employed for different purposes. For instance, it was used for measuring the purity of Au NPs produced by cumin seed powder [ 172 ]. XPS was used for the determination of the oxidation states of Pt NPs produced by Nigella sativa seeds and Ag NPs produced by Rosa canina . XPS results of Pt NPs showed the presence of three oxidation states for Pt (Pt (0), Pt (II), and Pt (IV)) and two oxidation states for Ag NPs (Ag (0) and Ag (I)). In both cases, the zero-oxidation state was the abundant one, the presence of a small amount of the other oxidation states suggests that some of the NPs were oxidized or had unreduced species [ 173 , 174 ]. XPS was used for the determination of the exact elemental ratios and the bonding nature of FeS NPs produced by Shewanella putrefaciens CN32. For the exact elemental ratios, the researchers compared biogenic and abiotic FeS NPs and found that biogenic FeS NPs had a 2.3:1 Fe:S ratio while the abiotic NPs had a 1.3:1 Fe:S ratio. For the bonding nature, it was determined that the surface of NPs had Fe(II)-S, Fe(III)-S, Fe(II)-O, and Fe(III)-O bonds [ 175 ].
Fourier-transform infrared spectroscopy (FTIR)
This technique is based on irradiating a material with infrared light, where the absorbed or transmitted radiation is recorded. The resulting spectrum represents a unique fingerprint of samples, where information about the nature of the sample can be obtained such as the bonds involved, polarity, and oxidation state of the sample [ 176 , 177 ]. This technique is mainly used for the characterization of organic materials such as the surface chemical composition or functionalization of NPs. It is also used for the identification of contaminants when high purity is sought [ 178 ].
Examples: For biogenic NPs, FTIR is usually used for the identification of probable functional groups present on the surface of NPs that are responsible for the reduction and stabilization of the NPs. For plant-mediated NP synthesis, for instance for Ag NPs produced by Camellia sinensis , the FTIR results indicate the presence of Camellia sinensis phytocompounds, such as caffeine and catechin, on the surface of Ag NPs that could be responsible for the reduction of Ag or act as stabilizing agents [ 147 ]. For Ag NPs produced by Solanum tuberosum , the NPs were found to be capped by amide and amine groups [ 143 ]. For CeO 2 NPs produced by Eucalyptus globulus , the polyphenol groups present in Eucalyptus globulus extract were found on the surface of NPs suggesting their involvement in the reduction/stabilization process [ 150 ]. For microbe-mediated NP synthesis, FTIR results show the presence of protein residues on the surface of NPs confirming the involvement of different proteins in the reduction/stabilization process, such as in Ag NPs produced by Streptomyces sp. NH28 [ 179 ], in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and in Se NPs produced by Azospirillum thiophilum [ 180 ].
Zeta potential analysis
Zeta potential measurements are used for the determination of NP surface charge in colloidal solutions. The surface charge of NPs attracts counter-ions that form a thin layer on the surface of the NPs (called Stern layer). This layer travels with the NPs as they diffuse thought the solution. The electric potential at the boundary of this layer is known as NP zeta potential [ 136 ]. The instruments used to measure this potential are called zeta potential analyzers [ 181 ]. Zeta potential values are indicative for NP stability, where higher absolute value of zeta potential indicate more stable NPs [ 136 ].
Examples: The zeta potential is a good indicator for the stability of NPs, where NPs with zeta potentials of more than + 30 mV or less than − 30 mV are considered stable. Zeta potentials have been measured for a wide range of biogenic NPs. The zeta potential for Ag NPs produced by Ziziphus jujuba leaf extract of − 26.4 mV [ 182 ]. Ag NPs produced by other organisms have different zeta potential values, for example, Ag NPs produced by Punica granatum peel extract have a zeta potential of − 40.6 mV indicating their higher stability [ 183 ], while Ag NPs produced by Aspergillus tubingensis have a zeta potential of + 8.48 indicating their relative instability [ 184 ]. The pH of the sample is another important parameter for zeta potential values, the higher pH the lower the zeta potential value [ 185 ]. Having different zeta potential values for the same type of NPs depending on the organism used for their synthesis is not unique to silver, Se NPs also show different potential values depending on the organism used for their synthesis [ 186 ].
Cyclic voltammetry (CV)
CV is an electrochemical technique for measuring the current response of redox-active solutions to a linearly cycled potential sweep between two or more set values. The CV technique involves the use of three electrodes: a working electrode, reference electrode, and counter electrode. These electrodes are introduced to an electrochemical cell filled with an electrolyte solution and where voltage is in excess, the potential of the working electrode is cycled and the resulting current is measured. This technique is used for determining information about the reduction potential of materials, the kinetics of electron transfer reactions, and the thermodynamics of redox processes [ 187 , 188 , 189 ].
Examples: The CV technique can be employed for two different purposes in the context of biogenic NP characterization. Firstly, it can be used for measuring the stability of NPs in electrocatalysis. For this purpose, the biogenic NPs are assembled on an electrode of the electrolysis cell and are tested for their electrocatalytic behavior against a redox reaction over different cycles. As an example, Ag NPs produced by Citrus sinensis were found to be stable in phenolic compounds redox reactions over multiple cycles [ 190 ]. Secondly, CV can be used for monitoring the progress of reduction of metallic NPs or for the determination of the reducing agent involved in the reduction. For example, for Ag NPs produced by Indian propolis, four cyclic voltammograms were recorded, one for a water extract of Indian propolis, another for an ethanol extract of Indian propolis, and two for the constituent flavonoids of Indian propolis (pinocembrin and galangin). The four cyclic voltammograms showed similar behaviors indicating the involvement of these flavonoids in the reduction of Ag and in forming Ag NPs [ 191 ].
Raman spectroscopy
This technique is based on irradiating a sample with monochromatic light emitted by a laser, in which the interactions between the laser light and molecular vibrations (photons and phonons) are recorded. The technique records the inelastically scattered photons, known as Raman scattering (named after the Indian physician C. V. Raman) [ 192 ]. The output of this technique is a unique fingerprint for each sample, which is used to characterize the chemical and intramolecular bonding of the sample. It can also be used to characterize the crystallographic orientation of the sample [ 193 ]. Surface-enhanced Raman spectroscopy (SERS) enhances Raman scattering of a sample and provides a more sensitive, specific, and selective technique for identifying molecular structures [ 194 ]. Both techniques are also used for the characterization of optical properties, where the recorded photons and phonons are used to understand the plasmonic resonance of NPs [ 25 ].
Examples: Raman spectroscopy was used to characterize Fe 3 O 4 NPs produced by Pisum sativum peel, the researchers found that the NPs were Fe 3 O 4 NPs with face centered cubic phase which was in agreement with their XRD measurements [ 195 ]. Other researchers used Raman spectroscopy for studying the trace deposits of carbohydrates on ferrihydrite NPs produced by Klebsiella oxytoca , the results showed that the pores of NPs had more deposits of carbohydrates that the surface of the NPs [ 196 ]. For Au NPs produced by Raphidocelis subcapitata (green algae), several biomolecules were suggested for their involvement in this process. SERS technique was used to study Au NPs surface-associated biomolecules in order to narrow down the list of biomolecules involved in the bioproduction process. The researchers found that several biomolecules such as, glutathione, β-carotene, chlorophyll a, hydroxyquinoline, and NAD were associated with Au NPs surface, thus, ruling out other molecules such as, glutaraldehyde fixing agent, saccharides, FAD, lipids, and DNA from the list [ 197 ].
Characterization of optical, electronic, and electrical properties
In addition to Raman spectroscopy and SERS, also other techniques can be employed to study and characterize the optical properties of NPs. These techniques give information about the absorption, reflectance, fluorescence, luminescence, electronic state, bandgap, photoactivity, and electrical conductance properties of NPs.
Ultraviolet–visible spectroscopy (UV–vis) and photoluminescence spectroscopy (PL)
In absorption spectroscopy such as UV–vis, the transition of electrons from the ground state to an excited state is measured, while in photoluminescence spectroscopy, the transition of electrons from the excited state to the ground state is measured [ 198 ]. UV–vis spectroscopy uses visible and UV light to measure the absorption or reflectance of a sample. In photoluminescence spectroscopy, usually UV light is used to excite the electron and then measure the luminescence or fluorescence properties of a sample [ 199 ].
Examples: UV–vis spectroscopy is a simple and common technique that is used for the characterization of the optical properties of NPs. For instance, for the characterization of the optical properties of Ag NPs produced by Trichoderma viride , the UV–vis spectrum showed that a Ag surface plasmon band occurs at 405 nm, which is a characteristic band for Ag NPs. The intensity of this band over the reaction time increased as a result of increasing Ag NP concentration in the solution. In the same study, the photoluminescence properties of these NPs were recorded, with an emission in the range between 320–520 nm, which falls in the blue-orange region [ 164 ]. For biogenic Cu NPs, the common absorption peaks are located between 530–590 nm. The difference in NP size and the bio-active molecules used for the reduction process are believed to be the reasons behind the differences in the absorption peaks [ 200 ]. For instance, 15 nm spherical Cu NPs produced by Calotropis procera have an absorption peak at 570 nm [ 201 ], while 76 nm spherical Cu NPs produced by Duranta erecta have an absorption peak at 588 nm [ 202 ]. The same applies to photoluminescence effects, where 27 nm spherical Cu NPs produced by Tilia extract emit light of 563 nm (dark brown) [ 203 ], while 19 nm spherical Cu NPs emit light of 430 nm (green) [ 204 ].
UV–vis diffuse reflectance spectroscopy (DRS)
This technique uses UV and visible light to measure the diffuse reflectance of a material (the reflection of light in many angles, as opposed to specular reflection). The resulting diffuse reflectance spectra are used to determine the electronic state of a sample, which is then used to calculate the bandgap [ 25 ]. Bandgap determination is crucial for determining conductance and photocatalytic properties especially for semiconductor NPs [ 205 ].
Examples: The DRS technique was used to calculate the bandgap for a wide range of biogenic NPs. For instance, TiO 2 NPs produced by Andrographis paniculata exhibit an optical energy bandgap of 3.27 eV [ 206 ]. Interestingly, biogenic ZnO NPs produced by different organism show different bandgaps, for example, ZnO NPs produced by Pseudomonas putida have a bandgap of 4 eV [ 207 ], while ZnO NPs produced by Calotropis procera leaf extract have a bandgap of 3.1 eV [ 208 ].
Spectroscopic ellipsometry
This technique is based on irradiating a sample with polarized light to measures changes in polarization. It is widely used to calculate the optical constants of a material (refractive index and extinction coefficient) [ 209 ]. This technique is also used to characterize the electrical conductivity and dielectric properties of materials [ 210 ].
Examples: Spectroscopic ellipsometry is not a common technique for the characterization of biogenic NPs. For chemically produced NPs, the optical properties for different-sized Au NPs partially embedded in glass substrate were measured by spectroscopic ellipsometry. In this example, a clear transition from LSPR to SPR mode was found as the thickness increases. Moreover, the partially-embedded Au NPs had much higher refractive index sensitivity compared to Au NPs fully immobilized in a glass substrate [ 211 ]. Spectroscopic ellipsometry was also used to measure the changes in the optical constants of a layer of 5 nm ZnO NPs induced by UV illumination. In this case, it was found that the UV illumination of ZnO NPs in inert atmospheres resulted in a clear blue shift in the absorption (Moss-Burstein shift). The UV illumination of ZnO NPs results in the desorption of O 2 from the NPs surface leading to the population of the lowest levels in conduction band with mobile electrons. This phenomenon is reversible, in which the exposure to O 2 from air results in the scavenging of these mobile electrons [ 212 ].
Characterization of magnetic properties
The magnetic properties of NPs are of high importance, as they potentially give NPs great advantages in catalysis, electronics, and medical applications. Several techniques were developed for the detection and quantification of small magnetic moments in NPs.
Magnetic force microscopy (MFM)
This technique is a variety of atomic force microscopy (AFM), in which a magnetic tip is used to scan the sample. The magnetic tip is approached very close to the sample, where the magnetic interactions between the tip and the sample are recorded [ 213 ]. At closer distances to the sample (0–20 nm), other forces such as van der Waals forces also interact with the tip. Therefore, MFM measurements are often operated with two-pass scanning method (also called lift height method) [ 214 ] (Fig. 7 ). In this method, the tip is firstly used to measure the topography of the sample including the molecular forces as van der Waals. Afterwards, the tip is lifted and a second scan is operated following the same topography outline. In the second scan, the short-ranged van der Waals forces disappear and the long-range magnetic forces are almost exclusively recorded. In an experimental study, researchers found that 22 nm was the optimal scanning height for the second scan, at which van der Waals forces are very weak while the distance is still small enough to measure the magnetic interactions for Pd-Fe bimetallic NPs [ 215 ].
Magnetic force microscopy lift height method. The first scan is done very close to the surface to obtain the topography of the sample. Then, the tip is lifted and a second scan is performed following the topography outline obtained in the first scan
Examples: MFM was heavily used for the characterization of magnetite NPs produced by magnetotactic bacteria. For instance, the size and orientation of the magnetic moment of magnetite NPs produced by Magnetospirillum gryphiswaldense strain MSR-1 were studied by MFM [ 216 ], in which the size of the magnetic moment was found to be 1.61 × 10 −17 Am 2 . In a different study, MFM was used to characterize the magnetic properties and to estimate the size of the magnetic kernel of the magnetosomes produced by the same strain, and it was determined that the NPs behaved like single mono-domain nanomagnets [ 217 ]. The magnetic properties of NPs made from materials such as Pd that only exhibit significant magnetism on the nanoscale can also be studied by MFM, however, the magnetic moment of these NPs is much lower than for ferromagnetic NPs. The magnetic decoration of Pd NP samples with Fe 2 O 3 NPs strongly enhances the weak magnetic signal of Pd NPs up to 15 times [ 218 ]. This approach could make the MFM technique useful for the characterization of weak magnetic NPs.
Vibrating-sample magnetometry (VSM)
This technique measures the magnetic properties of materials based on Faraday’s law of induction. In VSM, the sample is placed in a constant magnetic field in a special holder that vibrates vertically. As the holder starts vibrating, the magnetic moment of the sample creates a magnetic field that changes as function of time. The alternating magnetic field created in the sample induces an electric current that is recorded and used to calculate the magnetic properties of the sample [ 219 , 220 ].
Examples: For the characterization of Fe 2 O 3 NPs produced by Tridax leaf extract, VSM studies revealed that the NPs had a saturation magnetization of 7.78 emu/g, a remnant magnetization of 0.054 emu/g, and a coercivity of − 1.6 G [ 221 ]. In other studies, VSM was used to compare the magnetic properties of iron oxide NPs produced Moringa oleifera with the magnetic properties of the same NPs but coated with chitosan. The researchers found that saturation magnetisation, remnant magnetization, and coercivity have lower values when the NPs are coated with chitosan [ 222 ].
Superconducting quantum interference device (SQUID) magnetometry
This technique measures the magnetic properties of materials based on the Josephson effect. Niobium (Nb) or other metal alloys are used in the device which needs to be operated at temperatures very close to the absolute zero to main superconductivity, where liquid helium is used to maintain the cold environment [ 223 ]. However, other kinds of SQUID also exist where high-temperature superconductors are used [ 224 ]. After reaching superconducting environments, the Josephson junctions contained in the device help to create a supercurrent, which is recorded and used to calculate the magnetic properties of the sample [ 225 ].
Examples: For the characterization of iron oxide NPs produced by Cnidium monnieri seed extract, SQUID magnetometry revealed that the NPs had a saturation magnetization of 54.60 emu/g, a remnant magnetization of 1.15 emu/g, a coercivity of 11 Oe, and a magnetic susceptibility of + 1.69 × 10 –3 emu/ cm 3 ⋅ Oe at room temperatures, indicating the superparamagnetic behaviour of these NPs [ 226 ]. SQUID magnetometry was also used for the characterization of the magnetic properties of zinc incorporated magnetite NPs produced by Geobacter sulfurreducens , showing that the loading of only 5% zinc results in the enhancement of saturation magnetization of the NPs by more than 50% [ 227 ].
Electron spin resonance spectroscopy (ESR)
This technique measures the magnetic properties of materials by characterizing and quantifying the unpaired electrons in the sample. Electrons are charged particles that spin around their axis, which can align in two different orientations (+ ½ and − ½) when the sample is placed in strong magnetic field. These two alignments have different energies due to the Zeeman effect. Since unpaired electrons can change their spins by absorbing or emitting photons, in ESR the sample is irradiated with microwave pulses to excite electron spins until a resonance state is reached [ 228 ]. This technique is also referred to as electron paramagnetic resonance spectroscopy (EPR). It can be used to measure the ferromagnetic and antiferromagnetic properties of NPs [ 229 , 230 ].
Examples: ESR was used to characterize the magnetic properties of iron oxide NPs produced by Ficus carica . The trees naturally produce iron oxide NPs as a defence mechanism when are they are subjected to stress. The researchers found that the magnetic properties of iron oxide NPs produced by the same tree but grown in different environmental conditions have different magnetic properties. In addition, a magnetic anisotropy of the signal was visible as the magnetic properties of these NPs varied strongly at different temperatures [ 231 ]. ESR was also used to characterize the magnetic properties of Se nanomaterials produced by anaerobic granular sludge. The ESR results revealed the presence of Fe(III) atoms incorporated in the Se nanomaterial, which enhanced their overall magnetic properties, giving it ferromagnetic behaviour [ 232 ].
Characterization of thermal properties
Several techniques can be used for the characterization of the thermal properties of NPs, such as melting points, crystallization and structural-phase transition points, heat capacity, thermal conductivity, and thermal and oxidative stability.
Differential scanning calorimetry (DSC)
In this technique the analyte and a well-defined reference sample are put at the same temperature, then, the amount of heat required to increase the temperature of the sample and the reference in measured as a function of temperature. This technique is widely used to measure melting points [ 233 ], crystallization points, structural-phase transition points [ 234 ], latent heat capacity [ 235 ], heat of fusion [ 236 ], and oxidative stability [ 237 ].
Examples: For the characterization of Ag NPs produced by Rhodomyrtus tomentosa leaf extract, DSC showed three exothermic peaks at 44, 159, 243, and an endothermic peak at 441 °C. The first peak (at 44 °C) indicates that at this temperature the NPs face a gradual loss of water from their surface. The second peak (at 159 °C) shows that the thermal decomposition of the sample happens at this temperature. The last temperature (441 °C) indicates the melting temperature for those NPs [ 238 ]. For Ag NPs produced by Parthenium hysterophorus leaf extract, DSC showed that their melting temperature was at 750 °C. The researchers also found that these NPs had completely thermally decomposed and crystallized simultaneously [ 239 ].
Differential thermal analysis (DTA)
This technique is based on heating or cooling a sample and an inert reference under identical conditions, where any temperature difference between the sample and the reference is recorded. This technique is primarily used for the study of phase diagrams and transition temperatures [ 240 ]. However, it is also used to measure the melting points, thermal, and oxidative stability [ 241 , 242 ].
Thermogravimetric analysis (TGA)
This technique measures the change in the mass of a sample as a function of temperature and/or time in a controlled atmosphere [ 243 ]. This technique is mainly used to study the thermal stability of materials [ 244 ], in addition, it is also used to measure structural-phase transition points [ 245 ], thermal activation energies [ 246 ], and oxidative stability [ 247 ]. The resulting thermogram is unique for each compound and therefore can also be used for the determination of material composition [ 248 ]. TGA and DTA are usually combined in the same thermal analyzing instrument, called thermogravimetry/differential thermal analysis (TG/DTA) [ 244 ].
Examples: TG/DTA is a common technique for the characterization of thermal properties of biogenic NPs. For instance, the thermal properties of Ag NPs produced by Daphne mucronate leaf extract were studied in the range between 0–1000 °C where the sample was heated at a rate of 10 °C/min. The researchers found that between 400–500 °C the NPs faced a dominant weight loss, while the weight loss below 400 °C and above 500 °C was negligible. The DTA curve showed an intense exothermic peak in the range between 400–500 °C, this indicates that the crystallization of NPs happens in this temperature interval. Some minor weight loss events were seen below 400 °C, this may be caused by the evaporation of water or the degradation of the organic components [ 249 ]. In another study, the thermal properties of Ag NPs produced by two different plants ( Stereospermum binhchauensis and Jasminum subtriplinerve ) were compared. The researchers found that the major weight loss happens between 220–430 °C, which is attributed to the decomposition of biomolecules from the NP surface [ 250 ]. This shows that Ag NPs produced by these plants have much higher content of biomolecules on their surface than Ag NPs produced by Daphne mucronate. TG/DTA showed that Stereospermum binhchauensis Ag NPs crystallize at 315 °C and Jasminum subtriplinerve Ag NPs at 345 °C, around 100 °C less than Daphne mucronate Ag NPs [ 250 ].
Transient hot wire method (THW)
This method is used for the determination of thermal conductivity based on increasing the temperature of a material by a thin hot wire as a function of time, where the heating wire is located directly in the test sample. The advantage of this method over other thermal conductivity measurement methods is the very short measuring time, this gives high accuracy of thermal conductivity due to the negligible values of convection in such short times [ 251 ]. In this method, the NPs are added to a solution (usually water or ethylene glycol) forming a colloidal dispersion called a nanofluid. Then, the thermal conductivity of the nanofluid is measured and compared to the thermal conductivity of the base fluid, giving a thermal conductivity ratio which is used to evaluate the thermal conductivity of different NPs.
Examples: The thermal conductivity ratios of three different concentrations (0.12, 0.18, and 0.24%) of biogenic SnO 2 NPs produced by Punica granatum seed extract were measured in ethylene glycol at 303 K. The researchers found a linear relationship between NPs concentration and the thermal conductivity. The thermal conductivity enhancement of nanofluid to base fluid was between 6 and 24% [ 252 ]. In another study, the thermal conductivity of Fe 2 O 3 NPs produced by Psidium guajava leaf extract was measured in water and in ethylene glycol. The researchers found that the thermal conductivity enhancement in ethylene glycol was better than in water, the thermal conductivity enhancement for 0.025% Fe 2 O 3 NPs in water was 30% while in ethylene glycol was 34%. Moreover, the linear relationship between NPs concentration and thermal conductivity ratio was found for Fe 2 O 3 NPs in both water and ethylene glycol [ 253 ].
Characterization of mechanical properties
Several methods can be used for the characterization of mechanical properties of NPs, such as tensile and compressive strengths, elasticity, viscoelasticity, hardness, and stiffness.
Tensometery
The machine used for this method is called a universal testing machine (UTM) or a tensometer. It is used to measure the elasticity (elastic modulus), tensile and compressive strengths (Young’s modulus) of materials. In this machine, the sample is placed between grips and an extensometer, where changes in gauge length are recorded as a function of load [ 254 ]. However, other mechanical changes in addition to the change in gauge length are also recorded in this machine, such as the elasticity.
Examples: The mechanical properties of different biogenic NP-containing composites can be measured by this machine. For example, the mechanical properties of orthodontic elastic ligatures containing Ag NPs produced by Heterotheca inuloides were studied by comparing the maximum strength, tension, and displacement of the composite with and without the biogenic NPs. The researchers found that maximum strength, tension, and displacement have improved after the addition of Ag NPs [ 255 ]. Interestingly, the addition of biogenic Ag NPs produced by Diospyros lotus fruit extract to starch and polyvinyl alcohol hydrogel membranes resulted in an adverse effect. The tensile strength and modulus of the hydrogel membranes containing 50 and 100 ppm Ag NPs were much lower than of the neat hydrogel membrane. The researchers attributed this adverse effect to the possibility that the addition of Ag NPs could have resulted in blocking the crosslinking between starch and polyvinyl alcohol, or to the possibility of the formation of breakage points in the polymer matrix due to NPs agglomeration [ 256 ].
Instrumented indentation testing
This method is used to characterize the hardness features of materials by using a well-defined hard indenter tip typically made of diamond. The indenter tip is used to make an indentation in the sample by placing incremental loads on the tip, after which the area of indentation in the sample is measured and used to calculate the hardness features [ 257 ]. Light microscopy, SEM, or ATM technique are usually used to visualize the indentation in the sample. The method is also called micro- or nano-indentation testing.
Examples: This method was used to characterize the mechanical properties of calcite NPs produced by Ophiocoma wendtii brittlestar. The arm plates of this brittlestar are covered by hundreds of nanoscale calcite lenses that focus light onto photoreceptor nerve bundles positioned beneath the brittlestar. The researchers used the nanoindentation method to compare Young’s modulus, hardness and fracture toughness of biogenic calcite with geocalcite. The results showed that the biogenic calcite lenses have higher hardness and fracture toughness compared to geocalcite (more than twofold) [ 258 ]. Bamboo is well known for its high silica content in comparison to other wood species. It produces SiO 2 NPs and deposits it in its epidermis in the form of silica cells. The mechanical properties of silica cells compared to other types of cells of Moso bamboo ( Phyllostachys pubescens ) were studied by instrumented indentation testing. The researchers found that the cell wall of silica cells display higher hardness and elastic recovery compared to fibre and epidermal cells, which is attributed to the presence of biogenic SiO 2 NPs in the silica cells [ 259 ].
Dynamic mechanical analysis (DMA)
This method is used to study the mechanical properties of materials by measuring the strain of a material after applying a stress. This method helps to obtain three different values: storage modulus, loss modulus, and loss tangent. These values are important to give an overview about the stiffness and viscoelasticity behavior of materials [ 260 ].
Examples: The DMA method was used to characterize the mechanical properties of polymethyl methacrylate denture base polymer filled with Ag NPs produced by Boesenbergia rotunda . In this study frequency sweep test was used to determine the viscoelastic behavior of this nanocomposite where the temperature was constant at 37 °C and the frequency was increasing from 0.5 to 100 Hz in tension mode. The researchers found a frequency dependence for storage modulus, loss modulus, and loss tangent for the nanocomposite with various Ag NPs loading concentrations. The frequency dependence of storage modulus, loss modulus, and loss tangent indicates the viscoelastic response of this polymer. However, the results showed that the storage modulus for the nanocomposite is much higher than the loss modulus over the range of frequencies, indicating the elastic dominance of the nanocomposite. Moreover, the researchers found that storage and loss moduli increase with increasing Ag NPs loading concentrations, which is due to the interaction between polymethyl methacrylate and Ag NPs [ 261 ].
In a different study, DMA was used to determine the thermomechanical properties of pol(S-co-BuA) polymer filled with cellulose nanocrystals produced by Posidonia oceanica . In this case, the behaviour of storge modulus and loss tangent were studied as a function of temperature for different cellulose nanocrystals loading concentrations. The results showed that the unloaded polymer behaves like an amorphous polymer, the storage modulus remains constant until the temperature reaches 25 °C then it starts to sharply decrease due to glass–rubber transition. A relaxation process was also evident for the unloader polymer, where the loss tangent reaches its maximum at 35 °C then it starts to fall. The addition of cellulose nanocrystals to the polymer positively enhanced both effects. The dramatic drop of storage modulus at 25 °C was less for the nanocomposite, where the drop for the polymer loaded with 15% cellulose nanocrystals was almost cancelled. Similar positive enhancement was found for loss tangent. These enhancements could be attributed to the mechanical coupling effect, in which the NPs connect and form a stiff continuous network linked through hydrogen bonding [ 262 ].
Applications of NPs
NPs, due to their above-mentioned unique or enhanced physicochemical properties, are used in a wide range of applications in different fields. In addition, several potential applications are in research and development. Here we present some examples of these applications.
Applications in medicine and pharma
Metallic and semiconductor NPs have huge potential for cancer diagnosis and therapy based on their enhanced light scattering and absorption properties due to LSPR effect. For instance, Au NPs efficiently absorb light and convert it into localized heat, which can be exploited for selective photothermal therapy of cancer (cancer cell death by heat generated in tumor tissue) [ 263 , 264 ]. In addition, the unique optical properties of Au NPs make them a great candidate for the photodynamic therapy of cancer (the use of a drug that is activated by light to kill cancer cells) [ 265 ]. Gd based NPs have also shown great abilities in tumor growth inhibition [ 266 ], metastasis inhibition [ 267 ], and tumor-specific magnetic resonance contrast enhancement [ 268 ]. Targeted drug delivery is also an important potential application of NPs. ZnO and Fe 3 O 4 NPs were efficiently used for targeted drug delivery and selective destruction of tumor cells [ 269 , 270 , 271 ].
Moreover, NPs have been successfully used in different medical applications such as cellular imaging [ 272 ], or in biosensors for DNA, carbohydrates, proteins, and heavy metal ions [ 273 , 274 ], determination of blood glucose levels [ 275 ], and for medical diagnostics to detect bacteria [ 276 ] and viruses [ 277 ]. For instance, Au NPs were conjugated with SARS-CoV-2 antigens to rapidly detect the presence of SARS-CoV-2 IgM/IgA antibodies in blood samples within 10–15 min [ 278 ], At the same time, due to their antimicrobial and antibacterial activities, NPs such as TiO 2 , ZnO, CuO, and BiVO 4 are being increasing used in various medical products such as catheters [ 279 , 280 ].
Applications in electronics
NPs, due to their novel electronic and optical properties, have a wide range of potential applications in imaging techniques and electronics. For instance, Gd-based NPs can improve the imaging quality and the contrast agent administration dose of magnetic resonance imaging (MRI). The use of Gd 2 O 3 NPs as a contrasting agent was found to be more efficient than the commonly used agent (Gd-DOTA) at the same concentration [ 281 ]. At the same time, GdPO 4 NPs were successfully used for tumor detection using MRI in 1/10 of the dose typically used with Gd-DTPA agent [ 282 ]. Interestingly, NPs also offer the ability to image and track a single molecule, which can reveal important information about cellular processes such as membrane protein organization and interaction with other proteins. For example, Eu 3+ -doped oxide NPs were used to track a single toxin receptor with a localization precision of 30 nm [ 283 ].
Regarding applications in batteries, an important component in lithium-ion batteries is the separators. Their main function is to prevent the physical contact of anode and cathode, and to provide channels for the transport of ions. The commonly used commercial material in battery separators, a polyolefin microporous membrane, suffers from poor electrolyte uptake and poor thermal stability [ 284 ]. Due to the aerogel structure of some NPs (such as ZnO NPs), they are an ideal choice for separator plates in batteries [ 284 ]. This makes the batteries store a significantly higher amount of energy compared to traditional batteries. For lithium-air batteries, using Pt-Au bimetallic NPs strongly enhances oxygen reduction and oxygen evolution reactions [ 285 ]. Moreover, batteries made of nanocrystalline Ni and metal hydrides last longer and require less charging [ 23 ]. In addition to battery applications, several NPs such as CdS and ZnSe are also used in light-emitting diodes (LED) of modern displays to get higher brightness and bigger screens [ 23 , 286 ]. Other NPs such as CdTe NPs are also used in liquid crystal displays (LCDs) [ 287 ]. The addition of a NP layer to LED and LCD enables them to generate more light using the same amount of energy and enhances their lifetime.
Applications in agriculture
NPs have potential to benefit the agriculture field by providing new solutions to current agricultural and environmental problems [ 288 ]. NPs are mainly used in two forms in agriculture, as nanofertilizers and nanopesticides. Chemical fertilizers have poor efficiency due to leaching and volatilization. In these cases, the farmers usually react by using excessive amounts of fertilizers, which increases crops productivity but has an environmental cost [ 288 ]. In contrast, nanofertilizers are compounds that are applied in smaller amounts than regular chemical fertilizers but yet have better efficiencies [ 289 ]. The difference in efficiency comes from the fact that they are able to release the nutrients just when and where they are required by the plants. In that way, they limit the conversion of excess amounts of fertilizer to gaseous forms or from leaking into the ground water [ 290 ]. Several NPs have been employed in the development of fertilizers, including SiO 2 , ZnO, CuO, Fe, and Mg NPs [ 291 , 292 , 293 ]. These nanofertilizers provide the plants with increased nitrogen fixation, improved seed germination, amelioration to drought stress, increased seed weight, and increased photosynthesis ability [ 291 , 292 , 293 ]. The large surface area and small size of these NPs are the main reasons for the better efficiencies of nanofertilizers over conventional fertilizers [ 294 ].
Several NPs have proven antimicrobial, insecticidal, and nematicidal activities, which makes them a promising alternative to chemical pesticides and a potentially cheaper alternative to biopesticides [ 294 ]. For instance, the photocatalytic activity of TiO 2 NPs gives them a potent antimicrobial activity against Xanthomonas perforans , the causing agent of tomato spot disease [ 295 ]. CuO NPs show insecticidal activity against Spodoptera littoralis , known as African cotton leafworm [ 296 ]. Ag NPs show nematicidal activity against Meloidogyne spp. , root-knot nematodes [ 297 ].
Applications in the food industry
NPs, despite toxological concerns, have impactful applications in several food industry-related process such as food production, preservation, and packaging. TiO 2 NPs are a major promising player in this industry. Their photocatalytic antimicrobial activity makes them an interesting material for food packaging [ 298 ]. In addition, they are also used in sensors to detect volatile organic compounds [ 299 ]. Ag NPs are also promising in food packaging due to their antimicrobial activity. They play an important role in reducing the risk of pathogens and extending food shelf-life [ 294 ]. The efficiency of doping Ag and ZnO NPs to degradable and non-degradable packaging materials for meat, bread, fruit, and dairy products was tested against several yeast, molds, aerobic, and anaerobic bacteria [ 300 ]. For instance, polyvinyl chloride doped with Ag NPs was evaluated for packing minced meet at refrigerator temperature (4 °C); the results showed that Ag NPs significantly helped to slow down bacterial growth, increasing the shelf-life of minced meet from 2 to 7 days [ 301 ].
Effects of NPs on biological systems
Although the use of NPs is exponentially growing, their possible toxicological and hazardous impacts to human health and environment cannot be ignored. NPs may get released to the environment during production stages, usage, recycling, or disposal. These NPs may persist in air, soil, water, or biological systems [ 302 ]. NPs can enter the human or animal body though the skin, orally, or via the respiratory tract, and afterwards move to other parts of the body. The exposure to NPs was found to activate proinflammatory cytokines and chemokines with recruitment of inflammatory cells, which impacts the immune system homeostasis and can lead to autoimmune, allergic, or neoplastic diseases [ 302 ]. Moreover, the exposure to ultrafine particles can cause pulmonary, cardiac, and central nervous system diseases [ 303 , 304 , 305 ]. Similarly, NPs can enter plants cells and cause harmful effects [ 306 ]. For instance, the exposure of ZnO and Al NPs was found to cause root growth inhibition in plants [ 307 , 308 ].
Nanoscience and nanotechnology are inherently transdisciplinary fields of science. With new bio-based approaches, there is a need for biologists to understand not only the basic principles of nanoscience, but also the technologies and methods traditionally employed to characterize nanomaterials. We hope that this review can help to inspire new collaborations across different scientific disciplines, by helping biologists to identify the best technologies—and partners—to characterize their nanomaterials. At the same time, we recommend to take potential biological risks of these new materials into careful consideration already during the planning phase of such experiments.
Availability of data and materials
Not applicable.
https://www.etymonline.com/word/nano .
[SOURCE: ISO/TS 80,004‑2:2015, 4.4].
Abbreviations
Atomic force microscopy
Brunauer–Emmett–Teller
Barrett–Joyner–Halenda
Cyclic voltammetry
Dynamic light scattering
Derjaguin–Landau–Verwey–Overbeek
Dynamic mechanical analysis
Derjaguin–Muller–Toporov
UV–vis diffuse reflectance spectroscopy
Differential scanning calorimetry
Differential thermal analysis
Energy-dispersive X-ray spectroscopy
Electron microscopy
Electron paramagnetic resonance spectroscopy
Electron spin resonance spectroscopy
Fourier-transform infrared spectroscopy
High-angle annular dark-field imaging
International Organization for Standardization
Johnson–Kendall–Roberts
Liquid crystal display
Light-emitting diode
Localized surface plasmon resonance
Magnetic force microscopy
Magnetic resonance imaging
Nanoparticles
Nanoparticle tracking analysis
Photoluminescence spectroscopy
Critical radius
Threshold radius for superparamagnetism
Selected area electron diffraction
Scanning electron microscopy
Surface-enhanced Raman spectroscopy
Surface plasmon resonance
Superconducting quantum interference device
Scanning transmission electron microscopy
Scanning tunneling microscopy
Transmission electron microscopy
Thermogravimetry/differential thermal analysis
Thermogravimetric analysis
Transient hot wire
Universal testing machine
Ultraviolet
Ultraviolet–visible spectroscopy
Vibrating-sample magnetometry
X-ray photoelectron spectroscopy
X-ray diffraction analysis
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71.
Article PubMed Google Scholar
Mulvaney P. Nanoscience vs nanotechnology—defining the field. ACS Nano. 2015. https://doi.org/10.1021/acsnano.5b01418 .
Hasan S. A review on nanoparticles: their synthesis and types. Res J Recent Sci. 2015;2277:2502.
Google Scholar
Feynman RP. Plenty of room at the bottom. In: APS annual meeting. 1959.
Tolochko NK. History of nanotechnology (Chapter 1). In: Kharkin V, Bai C, Kapitza S, Awadelkarim OO, editors. Nanoscience and nanotechnologies (vol. 1). ISBN 978-1-78021-531-0. https://www.eolss.net/ebooklib/bookinfo/nanoscience-nanotechnologies.aspx
Walter P, Welcomme E, Hallégot P, Zaluzec NJ, Deeb C, Castaing J, et al. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 2006;6(10):2215–9.
Article CAS PubMed Google Scholar
Barber DJ, Freestone IC. An investigation of the origin of the colour of the Lycurgus Cup by analytical transmission electron microscopy. Archaeometry. 1990;32(1):33–45.
Article Google Scholar
Atwater HA. The promise of plasmonics. Sci Am. 2007;296(4):56–63.
Brill RH, Cahill ND. A red opaque glass from Sardis and some thoughts on red opaques in general. J Glass Stud. 1988;30:16–27. http://www.jstor.org/stable/24190804
Sharon M. History of nanotechnology: from prehistoric to modern times. New Jersey: Wiley; 2019.
Book Google Scholar
Bratovcic A. Different applications of nanomaterials and their impact on the environment. Int J Mater Sci Eng. 2019;5:1–7.
Gajanan K, Tijare SN. Applications of nanomaterials. Mater Today Proc. 2018;5(1):1093–6.
Article CAS Google Scholar
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70.
Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35(7):583–92.
Lines MG. Nanomaterials for practical functional uses. J Alloys Compd. 2008;449(1–2):242–5.
Gade A, Ingle A, Whiteley C, Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. 2010;32(5):593–600.
Ikhmayies SJ. Characterization of nanomaterials. JOM. 2014;66(1):28–9.
Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):1–7.
Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6(1):1–8.
Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4(4):347–63.
Geoffrion LD, Guisbiers G. Quantum confinement: size on the grill! J Phys Chem Solids. 2020;140: 109320.
Kolahalam LA, Viswanath IVK, Diwakar BS, Govindh B, Reddy V, Murthy YLN. Review on nanomaterials: synthesis and applications. Mater Today Proc. 2019;18:2182–90.
Ealia SAM, Saravanakumar MP. A review on the classification, characterisation, synthesis of nanoparticles and their application. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. p. 32019.
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ. 2015;533:76–81.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Pan K, Zhong Q. Organic nanoparticles in foods: fabrication, characterization, and utilization. Annu Rev Food Sci Technol. 2016;7:245–66.
Ng KK, Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev. 2015;115(19):11012–42.
Gujrati M, Malamas A, Shin T, Jin E, Sun Y, Lu Z-R. Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm. 2014;11(8):2734–44.
Article CAS PubMed PubMed Central Google Scholar
Long CM, Nascarella MA, Valberg PA. Carbon black vs black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ Pollut. 2013;181:271–86.
Dresselhaus MS, Dresselhaus G, Eklund PC. Fullerenes. J Mater Res. 1993;8(8):2054–97.
Yuan X, Zhang X, Sun L, Wei Y, Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 2019;16(1):1–27.
Lu K-Q, Quan Q, Zhang N, Xu Y-J. Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J Energy Chem. 2016;25(6):927–35.
Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42(16):5843–59.
Oh W-K, Yoon H, Jang J. Size control of magnetic carbon nanoparticles for drug delivery. Biomaterials. 2010;31(6):1342–8.
Liu M, Zhao F, Zhu D, Duan H, Lv Y, Li L, et al. Ultramicroporous carbon nanoparticles derived from metal–organic framework nanoparticles for high-performance supercapacitors. Mater Chem Phys. 2018;211:234–41.
Chandra S, Das P, Bag S, Laha D, Pramanik P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale. 2011;3(4):1533–40.
Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11–23.
Ahlawat J, Asil SM, Barroso GG, Nurunnabi M, Narayan M. Application of carbon nano onions in the biomedical field: recent advances and challenges. Biomater Sci. 2021. https://doi.org/10.1039/D0BM01476A .
Toshima N, Yonezawa T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem. 1998;22(11):1179–201.
Nascimento MA, Cruz JC, Rodrigues GD, de Oliveira AF, Lopes RP. Synthesis of polymetallic nanoparticles from spent lithium-ion batteries and application in the removal of reactive blue 4 dye. J Clean Prod. 2018;202:264–72.
Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282.
Fedlheim DL, Foss CA. Metal nanoparticles: synthesis, characterization, and applications. Boca Raton: CRC Press; 2001.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–79.
Gupta SM, Tripathi M. An overview of commonly used semiconductor nanoparticles in photocatalysis. High Energy Chem. 2012;46(1):1–9.
Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science (80-). 2000;287(5460):1989–92.
Thomas S, Kumar Mishra P, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21(42):6165–88.
Moreno-Vega A-I, Gomez-Quintero T, Nunez-Anita R-E, Acosta-Torres L-S, Castaño V. Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol. 2012. https://doi.org/10.1155/2012/936041 .
D’Amato R, Falconieri M, Gagliardi S, Popovici E, Serra E, Terranova G, et al. Synthesis of ceramic nanoparticles by laser pyrolysis: from research to applications. J Anal Appl Pyrolysis. 2013;104:461–9.
Wu Q, Miao W, Gao H, Hui D. Mechanical properties of nanomaterials: a review. Nanotechnol Rev. 2020;9(1):259–73.
Pithawalla YB, El-Shall MS, Deevi SC, Ström V, Rao KV. Synthesis of magnetic intermetallic FeAl nanoparticles from a non-magnetic bulk alloy. J Phys Chem B. 2001;105(11):2085–90.
Keesom WH. On the deduction of the equation of state from Boltzmann’s entropy principle’. KNAW Proc. 1912;15:240–56.
Debye P. Molecular forces and their electrical interpretation. Phys Zeitschrift. 1921;22:302–8.
CAS Google Scholar
London F. The general theory of molecular forces. Trans Faraday Soc. 1937;33:8b–26.
Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys. 2013;47(1):13001.
Missana T, Adell A. On the applicability of DLVO theory to the prediction of clay colloids stability. J Colloid Interface Sci. 2000;230(1):150–6.
Brant J, Lecoanet H, Wiesner MR. Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanoparticle Res. 2005;7(4):545–53.
Tan S, Sherman RL, Ford WT. Nanoscale compression of polymer microspheres by atomic force microscopy. Langmuir. 2004;20(17):7015–20.
Armini S, Vakarelski IU, Whelan CM, Maex K, Higashitani K. nanoscale indentation of polymer and composite polymer−silica core−shell submicrometer particles by atomic force microscopy. Langmuir. 2007;23(4):2007–14.
Savage T, Rao AM. Thermal properties of nanomaterials and nanocomposites. In: Thermal conductivity. Springer; 2004. p. 261–84.
Andrievski RA. Review of thermal stability of nanomaterials. J Mater Sci. 2014;49(4):1449–60.
Qiu L, Zhu N, Feng Y, Michaelides EE, Żyła G, Jing D, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys Rep. 2020;843:1–81.
Shima PD, Philip J, Raj B. Role of microconvection induced by Brownian motion of nanoparticles in the enhanced thermal conductivity of stable nanofluids. Appl Phys Lett. 2009;94(22): 223101.
Syam Sundar L, Sharma KV. Thermal conductivity enhancement of nanoparticles in distilled water. Int J Nanoparticles. 2008;1(1):66–77.
Eastman JA, Choi SUS, Li S, Yu W, Thompson LJ. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett. 2001;78(6):718–20.
Zebarjadi M, Esfarjani K, Shakouri A, Bahk J-H, Bian Z, Zeng G, et al. Effect of nanoparticle scattering on thermoelectric power factor. Appl Phys Lett. 2009;94(20): 202105.
Zeng G, Zide JMO, Kim W, Bowers JE, Gossard AC, Bian Z, et al. Cross-plane Seebeck coefficient of Er As: In Ga As/In Ga Al As superlattices. J Appl Phys. 2007;101(3):34502.
Kim W, Singer SL, Majumdar A, Vashaee D, Bian Z, Shakouri A, et al. Cross-plane lattice and electronic thermal conductivities of Er As: In Ga As∕ In Ga Al As superlattices. Appl Phys Lett. 2006;88(24):242107.
Likhachev VN, Vinogradov GA, Alymov MI. Anomalous heat capacity of nanoparticles. Phys Lett A. 2006;357(3):236–9.
Wang L, Tan Z, Meng S, Liang D, Li G. Enhancement of molar heat capacity of nanostructured Al 2 O 3 . J Nanoparticle Res. 2001;3(5):483–7.
Wang L, Tan Z, Meng S, Druzhinina A, Varushchenko RA, Li G. Heat capacity enhancement and thermodynamic properties of nanostructured amorphous SiO 2 . J Non Cryst Solids. 2001;296(1–2):139–42.
Borel J-P. Thermodynamical size effect and the structure of metallic clusters. Surf Sci. 1981;106(1–3):1–9.
Gülseren O, Ercolessi F, Tosatti E. Premelting of thin wires. Phys Rev B. 1995;51(11):7377.
Shim J-H, Lee B-J, Cho YW. Thermal stability of unsupported gold nanoparticle: a molecular dynamics study. Surf Sci. 2002;512(3):262–8.
Naitabdi A, Ono LK, Behafarid F, Cuenya BR. Thermal stability and segregation processes in self-assembled size-selected Au x Fe1-x nanoparticles deposited on TiO 2 (110): composition effects. J Phys Chem C. 2009;113(4):1433–46.
Mottet C, Rossi G, Baletto F, Ferrando R. Single impurity effect on the melting of nanoclusters. Phys Rev Lett. 2005;95(3):35501.
Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films. 2010;518(12):3127–50.
Nealon GL, Donnio B, Greget R, Kappler J-P, Terazzi E, Gallani J-L. Magnetism in gold nanoparticles. Nanoscale. 2012;4(17):5244–58.
Matthias BT, Clogston AM, Williams HJ, Corenzwit E, Sherwood RC. Ferromagnetism in solid solutions of Scandium and Indium. Phys Rev Lett. 1961;7(1):7.
Matthias BT, Bozorth RM. Ferromagnetism of a zirconium–zinc compound. Phys Rev. 1958;109(2):604.
Acker F, Fisk Z, Smith JL, Huang CY. Enhanced paramagnetism of TiBe2 and ferromagnetic transitions in TiBe2-xCux. J Magn Magn Mater. 1981;22(3):250–6.
Hori H, Teranishi T, Nakae Y, Seino Y, Miyake M, Yamada S. Anomalous magnetic polarization effect of Pd and Au nano-particles. Phys Lett A. 1999;263(4–6):406–10.
McCurrie RA. Ferromagnetic materials: structure and properties. Cambridge: Academic Press; 1994.
Edelstein AS, Cammaratra RC. Nanomaterials: synthesis, properties and applications. Boca Raton: CRC Press; 1998.
Jun Y, Seo J, Cheon J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc Chem Res. 2008;41(2):179–89.
Skumryev V, Stoyanov S, Zhang Y, Hadjipanayis G, Givord D, Nogués J. Beating the superparamagnetic limit with exchange bias. Nature. 2003;423(6942):850–3.
Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR. Tuning the magnetic properties of nanoparticles. Int J Mol Sci. 2013;14(8):15977–6009.
Hu M, Butt H-J, Landfester K, Bannwarth MB, Wooh S, Thérien-Aubin H. Shaping the assembly of superparamagnetic nanoparticles. ACS Nano. 2019;13(3):3015–22.
Marghussian V, Marghussian V. Nano-glass ceramics. Amsterdam: Elsevier; 2015.
Kalubowilage M, Janik K, Bossmann SH. Magnetic nanomaterials for magnetically-aided drug delivery and hyperthermia. Appl Sci. 2019;9(14):2927.
Podaru G, Chikan V. Magnetism in nanomaterials: heat and force from colloidal magnetic particles. 2017;
Song Q, Zhang ZJ. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J Am Chem Soc. 2004;126(19):6164–8.
Salazar-Alvarez G, Qin J, Sepelak V, Bergmann I, Vasilakaki M, Trohidou KN, et al. Cubic versus spherical magnetic nanoparticles: the role of surface anisotropy. J Am Chem Soc. 2008;130(40):13234–9.
Zhen G, Muir BW, Moffat BA, Harbour P, Murray KS, Moubaraki B, et al. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2011;115(2):327–34.
Lee W, Kim MG, Choi J, Park J-I, Ko SJ, Oh SJ, et al. Redox-transmetalation process as a generalized synthetic strategy for core-shell magnetic nanoparticles. J Am Chem Soc. 2005;127(46):16090–7.
Park J-I, Cheon J. Synthesis of “solid solution” and “core-shell” type cobalt–platinum magnetic nanoparticles via transmetalation reactions. J Am Chem Soc. 2001;123(24):5743–6.
Lee J-H, Huh Y-M, Jun Y, Seo J, Jang J, Song H-T, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–9.
Kumbhakar P, Ray SS, Stepanov AL. Optical properties of nanoparticles and nanocomposites. Hindawi; 2014.
Khlebtsov NG, Dykman LA. Optical properties and biomedical applications of plasmonic nanoparticles. J Quant Spectrosc Radiat Transf. 2010;111(1):1–35.
Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. Washington: ACS Publications; 2003.
Kreibig U, Vollmer M. Theoretical considerations. In: Optical properties of metal clusters. Springer; 1995. p. 13–201.
Duval Malinsky M, Kelly KL, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of substrate on the localized surface plasmon resonance spectrum of silver nanoparticles. J Phys Chem B. 2001;105(12):2343–50.
Jensen TR, Duval ML, Kelly KL, Lazarides AA, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of the external dielectric medium on the surface plasmon resonance spectrum of a periodic array of silver nanoparticles. J Phys Chem B. 1999;103(45):9846–53.
Rajan AR, Vilas V, Rajan A, John A, Philip D. Synthesis of nanostructured CeO2 by chemical and biogenic methods: optical properties and bioactivity. Ceram Int. 2020;46(9):14048–55.
Fu L, Fu Z. Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int. 2015;41(2):2492–6.
Cuenya BR, Baeck S-H, Jaramillo TF, McFarland EW. Size-and support-dependent electronic and catalytic properties of Au0/Au3+ nanoparticles synthesized from block copolymer micelles. J Am Chem Soc. 2003;125(42):12928–34.
Shaikhutdinov SK, Meyer R, Naschitzki M, Bäumer M, Freund H-J. Size and support effects for CO adsorption on gold model catalysts. Catal Lett. 2003;86(4):211–9.
Lemire C, Meyer R, Shaikhutdinov S, Freund H. Do quantum size effects control CO adsorption on gold nanoparticles? Angew Chem Int Ed. 2004;43(1):118–21.
Ono LK, Sudfeld D, Cuenya BR. In situ gas-phase catalytic properties of TiC-supported size-selected gold nanoparticles synthesized by diblock copolymer encapsulation. Surf Sci. 2006;600(23):5041–50.
Lu Y, Chen W. Size effect of silver nanoclusters on their catalytic activity for oxygen electro-reduction. J Power Sources. 2012;197:107–10.
Shao M, Peles A, Shoemaker K. Electrocatalysis on platinum nanoparticles: particle size effect on oxygen reduction reaction activity. Nano Lett. 2011;11(9):3714–9.
Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science (80-). 1998;281(5383):1647–50.
Zhang P, Sham TK. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys Rev Lett. 2003;90(24): 245502.
Haruta M. Nanoparticulate gold catalysts for low-temperature CO oxidation. ChemInform. 2004. https://doi.org/10.1002/chin.200448226 .
Xu R, Wang D, Zhang J, Li Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem Asian J. 2006;1(6):888–93.
Henry CR. Morphology of supported nanoparticles. Prog Surf Sci. 2005;80(3–4):92–116.
Humbert MP, Murillo LE, Chen JG. Rational design of platinum-based bimetallic catalysts with enhanced hydrogenation activity. ChemPhysChem. 2008;9(9):1262–4.
Toda T, Igarashi H, Uchida H, Watanabe M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J Electrochem Soc. 1999;146(10):3750.
Igarashi H, Fujino T, Zhu Y, Uchida H, Watanabe M. CO tolerance of Pt alloy electrocatalysts for polymer electrolyte fuel cells and the detoxification mechanism. Phys Chem Chem Phys. 2001;3(3):306–14.
Croy JR, Mostafa S, Hickman L, Heinrich H, Cuenya BR. Bimetallic Pt-Metal catalysts for the decomposition of methanol: effect of secondary metal on the oxidation state, activity, and selectivity of Pt. Appl Catal A Gen. 2008;350(2):207–16.
Liu P, Nørskov JK. Ligand and ensemble effects in adsorption on alloy surfaces. Phys Chem Chem Phys. 2001;3(17):3814–8.
Carlsson AF, Naschitzki M, Bäumer M, Freund H-J. The structure and reactivity of Al 2 O 3 -supported cobalt–palladium particles: a CO-TPD, STM, and XPS study. J Phys Chem B. 2003;107(3):778–85.
Besenbacher F, Chorkendorff I, Clausen BS, Hammer B, Molenbroek AM, Nørskov JK, et al. Design of a surface alloy catalyst for steam reforming. Science. 1998;279(5358):1913–5.
Ono LK, Roldan-Cuenya B. Effect of interparticle interaction on the low temperature oxidation of CO over size-selected Au nanocatalysts supported on ultrathin TiC films. Catal Lett. 2007;113(3):86–94.
Knapp M, Crihan D, Seitsonen AP, Over H. Hydrogen transfer reaction on the surface of an oxide catalyst. J Am Chem Soc. 2005;127(10):3236–7.
Hendriksen BLM, Frenken JWM. CO oxidation on Pt (110): scanning tunneling microscopy inside a high-pressure flow reactor. Phys Rev Lett. 2002;89(4):46101.
Gong X-Q, Raval R, Hu P. General insight into CO oxidation: a density functional theory study of the reaction mechanism on platinum oxides. Phys Rev Lett. 2004;93(10): 106104.
Gong X-Q, Liu Z-P, Raval R, Hu P. A systematic study of CO oxidation on metals and metal oxides: density functional theory calculations. J Am Chem Soc. 2004;126(1):8–9.
Over H, Seitsonen AP. Oxidation of metal surfaces. Science (80-). 2002;297(5589):2003–5.
Yoon B, Häkkinen H, Landman U, Wörz AS, Antonietti J-M, Abbet S, et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science (80-). 2005;307(5708):403–7.
Laursen S, Linic S. Oxidation catalysis by oxide-supported Au nanostructures: the role of supports and the effect of external conditions. Phys Rev Lett. 2006;97(2):26101.
Rodriguez JA, Wang X, Liu P, Wen W, Hanson JC, Hrbek J, et al. Gold nanoparticles on ceria: importance of O vacancies in the activation of gold. Top Catal. 2007;44(1–2):73–81.
Yan W, Chen B, Mahurin SM, Dai S, Overbury SH. Brookite-supported highly stable gold catalytic system for CO oxidation. Chem Commun. 2004;17:1918–9.
Rodriguez JA, Liu P, Viñes F, Illas F, Takahashi Y, Nakamura K. Dissociation of SO2 on Au/TiC (001): effects of Au–C interactions and charge polarization. Angew Chemie. 2008;120(35):6787–91.
Vladár AE, Hodoroaba V-D. Characterization of nanoparticles by scanning electron microscopy. In: Characterization of nanoparticles. Elsevier; 2020. p. 7–27.
Kano S, Tada T, Majima Y. Nanoparticle characterization based on STM and STS. Chem Soc Rev. 2015;44(4):970–87.
Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Advances in nanomedicine for the delivery of therapeutic nucleic acids. Elsevier; 2017. p. 43–58.
Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett. 2012;76:18–20.
Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79–84.
Hungund BS, Dhulappanavar GR, Ayachit NH. Comparative evaluation of antibacterial activity of silver nanoparticles biosynthesized using fruit juices. J Nanomed Nanotechnol. 2015;6(2):1.
Li Z, Wang Y, Shen J, Liu W, Sun X. The measurement system of nanoparticle size distribution from dynamic light scattering data. Opt Lasers Eng. 2014;56:94–8.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Basic fundamentals of drug delivery. Elsevier; 2019. p. 369–400.
Tripathi RM, Gupta RK, Shrivastav A, Singh MP, Shrivastav BR, Singh P. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv Nat Sci Nanosci Nanotechnol. 2013;4(3):35005.
Roy K, Sarkar CK, Ghosh CK. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato ( Solanum tuberosum ) infusion. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;146:286–91.
Soldatova AV, Balakrishnan G, Oyerinde OF, Romano CA, Tebo BM, Spiro TG. Biogenic and synthetic MnO 2 nanoparticles: size and growth probed with absorption and Raman spectroscopies and dynamic light scattering. Environ Sci Technol. 2019;53(8):4185–97.
Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27(5):796–810.
Gross J, Sayle S, Karow AR, Bakowsky U, Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: influence of experimental and data evaluation parameters. Eur J Pharm Biopharm. 2016;104:30–41.
Rodrigues MC, Rolim WR, Viana MM, Souza TR, Gonçalves F, Tanaka CJ, et al. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J Dent. 2020;96: 103327.
Moreno-Martin G, Pescuma M, Pérez-Corona T, Mozzi F, Madrid Y. Determination of size and mass-and number-based concentration of biogenic SeNPs synthesized by lactic acid bacteria by using a multimethod approach. Anal Chim Acta. 2017;992:34–41.
Naderi M. Surface area: Brunauer–Emmett–Teller (BET). In: Progress in filtration and separation. Elsevier; 2015. p. 585–608.
Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D. Biogenic ceria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering. 2020;7(1):26.
Article CAS PubMed Central Google Scholar
Sankar S, Sharma SK, Kim DY. Synthesis and characterization of mesoporous SiO 2 nanoparticles synthesized from biogenic rice husk ash for optoelectronic applications. Int J Eng Sci. 2016;17(1):353–8.
Aher YB, Jain GH, Patil GE, Savale AR, Ghotekar SK, Pore DM, et al. Biosynthesis of copper oxide nanoparticles using leaves extract of Leucaena leucocephala L. and their promising upshot against diverse pathogens. Int J Mol Clin Microbiol. 2017;7(1):776–86.
Ghotekar S, Pansambal S, Pawar SP, Pagar T, Oza R, Bangale S. Biological activities of biogenically synthesized fluorescent silver nanoparticles using Acanthospermum hispidum leaves extract. SN Appl Sci. 2019;1(11):1–12.
Bardestani R, Patience GS, Kaliaguine S. Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can J Chem Eng. 2019;97(11):2781–91.
Gelb LD, Gubbins KE. Pore size distributions in porous glasses: a computer simulation study. Langmuir. 1999;15(2):305–8.
Epp J. X-ray diffraction (XRD) techniques for materials characterization. In: Materials characterization using nondestructive evaluation (NDE) methods. Elsevier; 2016. p. 81–124.
Hazarika M, Borah D, Bora P, Silva AR, Das P. Biogenic synthesis of palladium nanoparticles and their applications as catalyst and antimicrobial agent. PLoS ONE. 2017;12(9): e0184936.
Groarke R, Vijayaraghavan RK, Powell D, Rennie A, Brabazon D. Powder characterization—methods, standards, and state of the art. In: Fundamentals of laser powder bed fusion of metals. Elsevier; 2021. p. 491–527.
Nasrollahzadeh M, Atarod M, Sajjadi M, Sajadi SM, Issaabadi Z. Plant-mediated green synthesis of nanostructures: mechanisms, characterization, and applications. In: Interface science and technology. Elsevier; 2019. p. 199–322.
Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC. Scanning electron microscopy and X-ray microanalysis. Cham: Springer; 2017.
Balasubramanian S, Kala SMJ, Pushparaj TL. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J Drug Deliv Sci Technol. 2020;57: 101620.
Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, et al. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalt Trans. 2014;43(24):9026–31.
Barabadi H, Kobarfard F, Vahidi H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: a novel approach in gold biotechnology. Iran J Pharm Res IJPR. 2018;17(Suppl2):87.
CAS PubMed Google Scholar
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R. Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride . Colloids Surf B Biointerfaces. 2010;75(1):175–8.
Otten MT. High-Angle annular dark-field imaging on a tem/stem system. J Electron Microsc Tech. 1991;17(2):221–30.
Utsunomiya S, Ewing RC. Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive X-ray spectrometry, and energy-filtered transmission electron microscopy to the characterization of nanopar. Environ Sci Technol. 2003;37(4):786–91.
Haverkamp RG, Marshall AT, van Agterveld D. Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo. J Nanoparticle Res. 2007;9(4):697–700.
Hossain M, Polash SA, Takikawa M, Shubhra RD, Saha T, Islam Z, et al. Investigation of the antibacterial activity and in vivo cytotoxicity of biogenic silver nanoparticles as potent therapeutics. Front Bioeng Biotechnol. 2019;7:239.
Article PubMed PubMed Central Google Scholar
Kimber RL, Lewis EA, Parmeggiani F, Smith K, Bagshaw H, Starborg T, et al. Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis : application for click chemistry. Small. 2018;14(10):1703145.
Fadley CS. X-ray photoelectron spectroscopy: progress and perspectives. J Electron Spectros Relat Phenomena. 2010;178:2–32.
Lykhach Y, Kozlov SM, Skála T, Tovt A, Stetsovych V, Tsud N, et al. Counting electrons on supported nanoparticles. Nat Mater. 2016;15(3):284–8.
Sneha K, Sathishkumar M, Lee SY, Bae MA, Yun Y-S. Biosynthesis of Au nanoparticles using cumin seed powder extract. J Nanosci Nanotechnol. 2011;11(2):1811–4.
Aygun A, Gülbagca F, Ozer LY, Ustaoglu B, Altunoglu YC, Baloglu MC, et al. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal. 2020;179: 112961.
Gulbagca F, Ozdemir S, Gulcan M, Sen F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon. 2019;5(12): e02980.
Huo Y-C, Li W-W, Chen C-B, Li C-X, Zeng R, Lau T-C, et al. Biogenic FeS accelerates reductive dechlorination of carbon tetrachloride by Shewanella putrefaciens CN32. Enzyme Microb Technol. 2016;95:236–41.
Manor J, Feldblum ES, Zanni MT, Arkin IT. Environment polarity in proteins mapped noninvasively by FTIR spectroscopy. J Phys Chem Lett. 2012;3(7):939–44.
Deepty M, Srinivas C, Kumar ER, Mohan NK, Prajapat CL, Rao TVC, et al. XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn-substituted Zn–ferrite nanoparticles. Ceram Int. 2019;45(6):8037–44.
Chevali V, Kandare E. Rigid biofoam composites as eco-efficient construction materials. In: Biopolymers and biotech admixtures for eco-efficient construction materials. Elsevier; 2016. p. 275–304.
Składanowski M, Golinska P, Rudnicka K, Dahm H, Rai M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol. 2016;205(6):603–13.
Tugarova AV, Mamchenkova PV, Dyatlova YA, Kamnev AA. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum . Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;192:458–63.
Sikora A, Bartczak D, Geißler D, Kestens V, Roebben G, Ramaye Y, et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal methods. 2015;7(23):9835–43.
Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus jujuba leaf extract. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;136:953–60.
Edison TJI, Sethuraman MG. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;104:262–4.
Ballottin D, Fulaz S, Souza ML, Corio P, Rodrigues AG, Souza AO, et al. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res Lett. 2016;11(1):1–9.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol Biol Med. 2010;6(1):103–9.
Menon S, KS SD, Agarwal H, Shanmugam VK. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci Commun. 2019;29:1–8. https://doi.org/10.1016/j.colcom.2018.12.004
Chooto P. Cyclic voltammetry and its applications. In: Voltammetry. IntechOpen; 2019. p. 1.
Saw EN, Grasmik V, Rurainsky C, Epple M, Tschulik K. Electrochemistry at single bimetallic nanoparticles—using nano impacts for sizing and compositional analysis of individual AgAu alloy nanoparticles. Faraday Discuss. 2016;193:327–38.
Testolin A, Cattaneo S, Wang W, Wang D, Pifferi V, Prati L, et al. Cyclic voltammetry characterization of Au, Pd, and AuPd nanoparticles supported on different carbon nanofibers. Surfaces. 2019;2(1):205–15.
Khan AU, Wei Y, Khan ZUH, Tahir K, Khan SU, Ahmad A, et al. Electrochemical and antioxidant properties of biogenic silver nanoparticles. Int J Electrochem Sci. 2015;10(10):7905–16.
Roy N, Mondal S, Laskar RA, Basu S, Mandal D, Begum NA. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf B Biointerfaces. 2010;76(1):317–25.
Long DA. Raman spectroscopy. New York. 1977;1.
Huang M, Yan H, Chen C, Song D, Heinz TF, Hone J. Phonon softening and crystallographic orientation of strained graphene studied by Raman spectroscopy. Proc Natl Acad Sci. 2009;106(18):7304–8.
Lin T, Song Y-L, Liao J, Liu F, Zeng T-T. Applications of surface-enhanced Raman spectroscopy in detection fields. Nanomedicine. 2020;15(30):2971–89.
Prasad C, Yuvaraja G, Venkateswarlu P. Biogenic synthesis of Fe 3 O 4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and methyl orange dye degradation studies. J Magn Magn Mater. 2017;424:376–81.
Anghel L, Balasoiu M, Ishchenko LA, Stolyar S V, Kurkin TS, Rogachev A V, et al. Characterization of bio-synthesized nanoparticles produced by Klebsiella oxytoca. In: Journal of Physics: Conference Series. IOP Publishing; 2012. p. 12005.
Lahr RH, Vikesland PJ. Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sustain Chem Eng. 2014;2(7):1599–608.
Skoog DA, Holler FJ, Crouch SR, editors. Principles of instrumental analysis (7th edn). Boston, USA: Cengage learning; 2017. ISBN 978-1-305-57721-3
Patel S, Patel P, Undre SB, Pandya SR, Singh M, Bakshi S. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. J Mol Liq. 2016;213:304–11.
Al-Hakkani MF. Biogenic copper nanoparticles and their applications: a review. SN Appl Sci. 2020;2(3):1–20.
Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B Biointerfaces. 2012;95:284–8.
Ismail M, Gul S, Khan MI, Khan MA, Asiri AM, Khan SB. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process Synth. 2019;8(1):135–43.
Hassanien R, Husein DZ, Al-Hakkani MF. Biosynthesis of copper nanoparticles using aqueous Tilia extract: antimicrobial and anticancer activities. Heliyon. 2018;4(12): e01077.
Suresh Y, Annapurna S, Bhikshamaiah G, Singh AK. Green luminescent copper nanoparticles. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2016. p. 12187.
Zhang P, Hong RY, Chen Q, Feng WG. On the electrical conductivity and photocatalytic activity of aluminum-doped zinc oxide. Powder Technol. 2014;253:360–7.
Karthik K, Vijayalakshmi S, Phuruangrat A, Revathi V, Verma U. Multifunctional applications of microwave-assisted biogenic TiO 2 nanoparticles. J Clust Sci. 2019;30(4):965–72.
Jayabalan J, Mani G, Krishnan N, Pernabas J, Devadoss JM, Jang HT. Green biogenic synthesis of zinc oxide nanoparticles using Pseudomonas putida culture and its In vitro antibacterial and anti-biofilm activity. Biocatal Agric Biotechnol. 2019;21: 101327.
Gawade VV, Gavade NL, Shinde HM, Babar SB, Kadam AN, Garadkar KM. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J Mater Sci Mater Electron. 2017;28(18):14033–9.
Tompkins H, Irene EA. Handbook of ellipsometry. William Andrew; 2005.
Losurdo M, Bergmair M, Bruno G, Cattelan D, Cobet C, de Martino A, et al. Spectroscopic ellipsometry and polarimetry for materials and systems analysis at the nanometer scale: state-of-the-art, potential, and perspectives. J Nanoparticle Res. 2009;11(7):1521–54.
Moirangthem RS, Yaseen MT, Wei P-K, Cheng J-Y, Chang Y-C. Enhanced localized plasmonic detections using partially-embedded gold nanoparticles and ellipsometric measurements. Biomed Opt Express. 2012;3(5):899–910.
Lakhwani G, Roijmans RFH, Kronemeijer AJ, Gilot J, Janssen RAJ, Meskers SCJ. Probing charge carrier density in a layer of photodoped ZnO nanoparticles by spectroscopic ellipsometry. J Phys Chem C. 2010;114(35):14804–10.
Claxton J, Joudeh N, Røyne A, Linke D, Mikheenko P. Sequential magnetic mapping of bacteria loaded with Pd-Fe nanoparticles. In: 2020 IEEE 10th International conference nanomaterials: applications & properties (NAP). IEEE; 2020. p. 1–5.
Passeri D, Dong C, Reggente M, Angeloni L, Barteri M, Scaramuzzo FA, et al. Magnetic force microscopy: quantitative issues in biomaterials. Biomatter. 2014;4(1): e29507.
Campaña AL, Joudeh N, Høyer H, Røyne A, Linke D, Mikheenko P. Probing van der Waals and magnetic forces in bacteria with magnetic nanoparticles. In: 2020 IEEE 10th International conference nanomaterials: applications & properties (NAP). IEEE; 2020. p. 01NSSA03-1.
Körnig A, Hartmann MA, Teichert C, Fratzl P, Faivre D. Magnetic force imaging of a chain of biogenic magnetite and Monte Carlo analysis of tip–particle interaction. J Phys D Appl Phys. 2014;47(23): 235403.
Albrecht M, Janke V, Sievers S, Siegner U, Schüler D, Heyen U. Scanning force microspy study of biogenic nanoparticles for medical applications. J Magn Magn Mater. 2005;290:269–71.
Campaña AL, Joudeh N, Mikheenko P, Linke D. Magnetic decoration of Escherichia coli loaded with Palladium nanoparticles. In: 2021 IEEE 11th International conference nanomaterials: applications and properties (NAP). IEEE; 2021. p. 1–5.
Foner S. Vibrating sample magnetometer. Rev Sci Instrum. 1956;27(7):548.
Kirupakar BR, Vishwanath BA, Sree MP. Vibrating sample magnetometer and its application in characterisation of magnetic property of the anti cancer drug magnetic microspheres. Int J Pharm Drug Anal. 2016;4(5):227–33.
Yadav VK, Fulekar MH. Biogenic synthesis of maghemite nanoparticles (γ-Fe 2 O 3 ) using Tridax leaf extract and its application for removal of fly ash heavy metals (Pb, Cd). Mater Today Proc. 2018;5(9):20704–10.
Tovar GI, Briceño S, Suarez J, Flores S, González G. Biogenic synthesis of iron oxide nanoparticles using Moringa oleifera and chitosan and its evaluation on corn germination. Environ Nanotechnol Monit Manag. 2020;14: 100350.
Sawicki M, Stefanowicz W, Ney A. Sensitive SQUID magnetometry for studying nanomagnetism. Semicond Sci Technol. 2011;26(6):64006.
Colclough MS, Gough CE, Keene M, Muirhead CM, Thomas N, Abell JS, et al. Radio-frequency SQUID operation using a ceramic high-temperature superconductor. Nature. 1987;328(6125):47–8.
Enpuku K, Minotani T, Gima T, Kuroki Y, Itoh Y, Yamashita M, et al. Detection of magnetic nanoparticles with superconducting quantum interference device (SQUID) magnetometer and application to immunoassays. Jpn J Appl Phys. 1999;38(10A):L1102.
Lingamdinne LP, Chang Y-Y, Yang J-K, Singh J, Choi E-H, Shiratani M, et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem Eng J. 2017;307:74–84.
Byrne JM, Coker VS, Cespedes E, Wincott PL, Vaughan DJ, Pattrick RAD, et al. Biosynthesis of zinc substituted magnetite nanoparticles with enhanced magnetic properties. Adv Funct Mater. 2014;24(17):2518–29.
Atherton NM, Davies MJ, Gilbert BC. Electron spin resonance. Vol. 14. Royal Society of Chemistry; 1994.
Flores-Arias Y, Vázquez-Victorio G, Ortega-Zempoalteca R, Acevedo-Salas U, Ammar S, Valenzuela R. Magnetic phase transitions in ferrite nanoparticles characterized by electron spin resonance. J Appl Phys. 2015;117(17):17A503.
Rubinstein M, Kodama RH, Makhlouf SA. Electron spin resonance study of NiO antiferromagnetic nanoparticles. J Magn Magn Mater. 2001;234(2):289–93.
Nasibova A, Khalilov R, Abiyev H, Trubitsin B, Eftekhari A. Identification of the EPR signals of fig leaves (Ficus carica L.). Eurasian Chem Commun. 2021;3(3):193–9.
Dixit R, Gupta A, Jordan N, Zhou S, Schild D, Weiss S, et al. Magnetic properties of biogenic selenium nanomaterials. Environ Sci Pollut Res. 2021. https://doi.org/10.1007/s11356-020-11683-2 .
Charsley EL, Laye PG, Palakollu V, Rooney JJ, Joseph B. DSC studies on organic melting point temperature standards. Thermochim Acta. 2006;446(1–2):29–32.
Horiuchi K. DSC studies on structural phase transitions and molecular motions in some A2MCl4 compounds. Phys Status Solidi. 2004;201(4):723–6.
Wang J, Xie H, Guo Z, Guan L, Li Y. Improved thermal properties of paraffin wax by the addition of TiO 2 nanoparticles. Appl Therm Eng. 2014;73(2):1541–7.
Illers K-H, Kanig G. Heat of fusion and lamellar structure of polyethylene single crystal mats. Colloid Polym Sci. 1982;260(6):564–9.
Pérez-Alonso C, Cruz-Olivares J, Barrera-Pichardo JF, Rodríguez-Huezo ME, Báez-González JG, Vernon-Carter EJ. DSC thermo-oxidative stability of red chili oleoresin microencapsulated in blended biopolymers matrices. J Food Eng. 2008;85(4):613–24.
Ontong JC, Singh S, Nwabor OF, Chusri S, Voravuthikunchai SP. Potential of antimicrobial topical gel with synthesized biogenic silver nanoparticle using Rhodomyrtus tomentosa leaf extract and silk sericin. Biotechnol Lett. 2020;42(12):2653–64.
Ahsan A, Farooq MA, Ahsan Bajwa A, Parveen A. Green synthesis of silver nanoparticles using Parthenium hysterophorus : optimization, characterization and in vitro therapeutic evaluation. Molecules. 2020;25(15):3324.
Tanzi MC, Farè S, Candiani G. Foundations of biomaterials engineering. Cambridge: Academic Press; 2019.
Thomas S, Thomas R, Zachariah AK, Kumar R. Thermal and rheological measurement techniques for nanomaterials characterization, vol. 3. Amsterdam: Elsevier; 2017.
Song P, Wen D, Guo ZX, Korakianitis T. Oxidation investigation of nickel nanoparticles. Phys Chem Chem Phys. 2008;10(33):5057–65.
Wagner M. Thermal analysis in practice. Munich, Germany: Hanser Publications; 2009. ISBN 978-1-56990-643-9
Ajroudi L, Mliki N, Bessais L, Madigou V, Villain S, Leroux C. Magnetic, electric and thermal properties of cobalt ferrite nanoparticles. Mater Res Bull. 2014;59:49–58.
Loganathan S, Valapa RB, Mishra RK, Pugazhenthi G, Thomas S. Thermogravimetric analysis for characterization of nanomaterials. In: Thermal and rheological measurement techniques for nanomaterials characterization. Elsevier; 2017. p. 67–108.
Rami JM, Patel CD, Patel CM, Patel MV. Thermogravimetric analysis (TGA) of some synthesized metal oxide nanoparticles. Mater Today Proc. 2021;43:655–9.
Pang LSK, Saxby JD, Chatfield SP. Thermogravimetric analysis of carbon nanotubes and nanoparticles. J Phys Chem. 1993;97(27):6941–2.
Saadatkhah N, Carillo Garcia A, Ackermann S, Leclerc P, Latifi M, Samih S, et al. Experimental methods in chemical engineering: thermogravimetric analysis—TGA. Can J Chem Eng. 2020;98(1):34–43.
Shah A, Lutfullah G, Ahmad K, Khalil AT, Maaza M. Daphne mucronata-mediated phytosynthesis of silver nanoparticles and their novel biological applications, compatibility and toxicity studies. Green Chem Lett Rev. 2018;11(3):318–33.
Nguyen TM-T, Huynh TT-T, Dang C-H, Mai D-T, Nguyen TT-N, Nguyen D-T, et al. Novel biogenic silver nanoparticles used for antibacterial effect and catalytic degradation of contaminants. Res Chem Intermed. 2020;46(3):1975–90.
Healy JJ, De Groot JJ, Kestin J. The theory of the transient hot-wire method for measuring thermal conductivity. Physica B + c. 1976;82(2):392–408.
Kumari MM, Philip D. Synthesis of biogenic SnO2 nanoparticles and evaluation of thermal, rheological, antibacterial and antioxidant activities. Powder Technol. 2015;270:312–9.
Rufus A, Sreeju N, Philip D. Synthesis of biogenic hematite (α-Fe 2 O 3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016;6(96):94206–17.
Davis JR. Tensile testing. ASM international; 2004.
Hernández-Gómora AE, Lara-Carrillo E, Robles-Navarro JB, Scougall-Vilchis RJ, Hernández-López S, Medina-Solís CE, et al. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules. 2017;22(9):1407.
Batool S, Hussain Z, Niazi MBK, Liaqat U, Afzal M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J Drug Deliv Sci Technol. 2019;52:403–14.
Schuh CA. Nanoindentation studies of materials. Mater Today. 2006;9(5):32–40.
Polishchuk I, Bracha AA, Bloch L, Levy D, Kozachkevich S, Etinger-Geller Y, et al. Coherently aligned nanoparticles within a biogenic single crystal: a biological prestressing strategy. Science (80-). 2017;358(6368):1294–8.
Xuexia Z. Mechanical properties of silica cells in bamboo measured using in situ imaging nanoindentation. Wood Fiber Sci. 2016;48(4):1–6.
Franck A, Germany TI. Viscoelasticity and dynamic mechanical testing. TA Instruments, New Castle, DE, USA AN004. 1993;
Siripanth J, Wongwitthayakool P. Flexural strength and viscoelastic properties of acrylic resin denture base material containing silver nanoparticle synthesized from fingerroot aqueous extract. In: Key engineering materials. Trans Tech Publ; 2018. p. 178–82.
Bettaieb F, Khiari R, Dufresne A, Mhenni MF, Belgacem MN. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr Polym. 2015;123:99–104.
Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007. https://doi.org/10.2217/17435889.2.5.681 .
El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–35.
Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–56.
Chen C, Xing G, Wang J, Zhao Y, Li B, Tang J, et al. Multihydroxylated [Gd@ C82 (OH) 22] n nanoparticles: antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5(10):2050–7.
Meng H, Xing G, Blanco E, Song Y, Zhao L, Sun B, et al. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomed Nanotechnol Biol Med. 2012;8(2):136–46.
Swanson SD, Kukowska-Latallo JF, Patri AK, Chen C, Ge S, Cao Z, et al. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomed. 2008;3(2):201.
Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063–77.
Chen F-H, Gao Q, Ni JZ. The grafting and release behavior of doxorubincin from Fe 3 O 4 @ SiO 2 core–shell structure nanoparticles via an acid cleaving amide bond: the potential for magnetic targeting drug delivery. Nanotechnology. 2008;19(16): 165103.
Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–96.
Hutter E, Maysinger D. Gold nanoparticles and quantum dots for bioimaging. Microsc Res Tech. 2011;74(7):592–604.
Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–79.
Zeng S, Yong K-T, Roy I, Dinh X-Q, Yu X, Luan F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics. 2011;6(3):491–506.
Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24(8):1415–26.
Phillips RL, Miranda OR, You C, Rotello VM, Bunz UHF. Rapid and efficient identification of bacteria using gold-nanoparticle–poly (para-phenyleneethynylene) constructs. Angew Chemie Int Ed. 2008;47(14):2590–4.
Kairdolf BA, Qian X, Nie S. Bioconjugated nanoparticles for biosensing, in vivo imaging, and medical diagnostics. Anal Chem. 2017;89(2):1015–31.
Ahmadi A, Mirzaeizadeh Z, Omidfar K. Simultaneous detection of SARS-CoV-2 IgG/IgM antibodies, using gold nanoparticles-based lateral flow immunoassay. Monoclon Antib Immunodiagn Immunother. 2021;40(5):210–8.
Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511.
Pant HR, Pant B, Sharma RK, Amarjargal A, Kim HJ, Park CH, et al. Antibacterial and photocatalytic properties of Ag/TiO 2 /ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram Int. 2013;39(2):1503–10.
Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS Nano. 2011;5(11):8488–505.
Hifumi H, Yamaoka S, Tanimoto A, Akatsu T, Shindo Y, Honda A, et al. Dextran coated gadolinium phosphate nanoparticles for magnetic resonance tumor imaging. J Mater Chem. 2009;19(35):6393–9.
Türkcan S, Masson J-B, Casanova D, Mialon G, Gacoin T, Boilot J-P, et al. Observing the confinement potential of bacterial pore-forming toxin receptors inside rafts with nonblinking Eu3+-doped oxide nanoparticles. Biophys J. 2012;102(10):2299–308.
Gu L, Zhang M, He J, Ni P. A porous cross-linked gel polymer electrolyte separator for lithium-ion batteries prepared by using zinc oxide nanoparticle as a foaming agent and filler. Electrochim Acta. 2018;292:769–78.
Lu Y-C, Xu Z, Gasteiger HA, Chen S, Hamad-Schifferli K, Shao-Horn Y. Platinum−gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium−air batteries. J Am Chem Soc. 2010;132(35):12170–1.
Rodríguez-Mas F, Ferrer JC, Alonso JL, Fernández de Ávila S. Expanded electroluminescence in high load CdS nanocrystals PVK-based LEDs. Nanomaterials. 2019;9(9):1212.
Qi H, Hegmann T. Impact of nanoscale particles and carbon nanotubes on current and future generations of liquid crystal displays. J Mater Chem. 2008;18(28):3288–94.
Usman M, Farooq M, Wakeel A, Nawaz A, Cheema SA, Rehman H, et al. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci Total Environ. 2020;721: 137778.
Rameshaiah GN, Pallavi J, Shabnam S. Nano fertilizers and nano sensors—an attempt for developing smart agriculture. Int J Eng Res Gen Sci. 2015;3(1):314–20.
Mastronardi E, Tsae P, Zhang X, Monreal C, DeRosa MC. Strategic role of nanotechnology in fertilizers: potential and limitations. In: Nanotechnologies in food and agriculture. Springer; 2015. p. 25–67.
Changmei L, Chaoying Z, Junqiang W, Guorong W, Mingxuan T. Research of the effect of nanometer materials on germination and growth enhancement of glycine max and its mechanism. Soybean Sci. 2002;21(3):168–71.
Dimkpa CO, Bindraban PS, Fugice J, Agyin-Birikorang S, Singh U, Hellums D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron Sustain Dev. 2017;37(1):5.
Delfani M, Baradarn Firouzabadi M, Farrokhi N, Makarian H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun Soil Sci Plant Anal. 2014;45(4):530–40.
Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, et al. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11(8):902.
Paret ML, Vallad GE, Averett DR, Jones JB, Olson SM. Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology. 2013;103(3):228–36.
Ayoub HA, Khairy M, Elsaid S, Rashwan FA, Abdel-Hafez HF. Pesticidal activity of nanostructured metal oxides for generation of alternative pesticide formulations. J Agric Food Chem. 2018;66(22):5491–8.
Cromwell WA, Yang J, Starr JL, Jo Y-K. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J Nematol. 2014;46(3):261.
CAS PubMed PubMed Central Google Scholar
Othman SH, Abd Salam NR, Zainal N, Kadir Basha R, Talib RA. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int J Photoenergy. 2014;2014:945930. https://doi.org/10.1155/2014/945930
Cui S, Yang L, Wang J, Wang X. Fabrication of a sensitive gas sensor based on PPy/TiO 2 nanocomposites films by layer-by-layer self-assembly and its application in food storage. Sensors Actuators B Chem. 2016;233:337–46.
Carbone M, Donia DT, Sabbatella G, Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. J King Saud Univ. 2016;28(4):273–9.
Mahdi SS, Vadood R, Nourdahr R. Study on the antimicrobial effect of nanosilver tray packaging of minced beef at refrigerator temperature. Glob Vet. 2012;9:284–9.
Roy R, Kumar S, Tripathi A, Das M, Dwivedi PD. Interactive threats of nanoparticles to the biological system. Immunol Lett. 2014;158(1–2):79–87.
Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60(6):455–61.
Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007. https://doi.org/10.1097/01.ede.0000249409.81050.46 .
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–52.
Stark WJ. Nanoparticles in biological systems. Angew Chemie Int Ed. 2011;50(6):1242–58.
Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150(2):243–50.
Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158(2):122–32.
Srivastava SK, Constanti M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt Co, and Li) by Pseudomonas aeruginosa SM1. J Nanoparticle Res. 2012;14(4):1–10.
Arya A, Gupta K, Chundawat TS, Vaya D. Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg Chem Appl. 2018. https://doi.org/10.1155/2018/7879403 .
Mishra A, Ahmad R, Perwez M, Sardar M. Reusable green synthesized biomimetic magnetic nanoparticles for glucose and H 2 O 2 detection. Bionanoscience. 2016;6(2):93–102.
Download references
Acknowledgements
This work was supported by the Research Council of Norway, Grant 294605 (Center for Digital Life) to DL.
Author information
Authors and affiliations.
Department of Biosciences, University of Oslo, Blindern, P.O. Box 1066, 0316, Oslo, Norway
Nadeem Joudeh & Dirk Linke
You can also search for this author in PubMed Google Scholar
Contributions
NJ wrote the manuscript. DL edited the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Correspondence to Dirk Linke .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Joudeh, N., Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol 20 , 262 (2022). https://doi.org/10.1186/s12951-022-01477-8
Download citation
Received : 02 February 2022
Accepted : 23 May 2022
Published : 07 June 2022
DOI : https://doi.org/10.1186/s12951-022-01477-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanomaterials
- Metal nanoparticles
- Biogenic nanoparticles
- Bionanoparticles
- Nanobiotechnology
- Characterization of nanomaterials
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]
Recent progress in carbon-based nanomaterials: critical review
- Review Article
- Published: 17 May 2024
- Volume 26 , article number 106 , ( 2024 )
Cite this article

- Olushola Sunday Ayanda 1 ,
- Augusta Oluchi Mmuoegbulam 2 ,
- Onyemaechi Okezie 2 ,
- Naseer Inuwa Durumin Iya 3 ,
- Sa’adatu Eri Mohammed 3 ,
- Philip Hegarty James 4 ,
- Abba Bashir Muhammad 5 ,
- Augustine Agorye Unimke 2 ,
- Sabur Ajibola Alim 6 ,
- Sharhabil Musa Yahaya 7 ,
- Ayomipo Ojo 1 ,
- Toyin Olanike Adaramoye 8 ,
- Stella Kemilola Ekundayo 9 ,
- Aminu Abdullahi 10 &
- Hamza Badamasi 11
89 Accesses
Explore all metrics
Carbon-based nanomaterials (CBNs) have drawn a lot of attention due to their distinct physical and chemical properties. CBNs, such as fullerenes, carbon nanotubes, carbon nanofibers, carbon quantum dots, graphene, and other derivatives have been thoroughly investigated in environmental remediation, analytical chemistry and sensing, antimicrobial activities, microbial fuel cells, and renewable energy and energy storage. In this study, recent researches on the synthesis, properties, and application of CBNs were detailed, including the toxicity and health implications of CBNs. Conclusively, the various uses of CBNs have shown an exciting result for many applications. However, there are still concerns about some restrictions, including the lack of knowledge on the possible toxicity and health effects of CBNs, difficulties with scalability and cost-effectiveness, and biocompatibility in medical applications; thus, further research is needed to involve the multidisciplinary integration approaches, including Engineering, Medicine, Chemistry, Biology, and Material Science, to open the full potential of CBNs and to further encourage scientists to produce innovative CBNs for usage in a variety of sectors.
Graphical Abstract

This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Source: https://www.energy.gov/eere/solar/perovskite-solar-cells

Similar content being viewed by others

Recent Trends of Recycled Carbon-Based Nanomaterials and Their Applications

Carbon Nanomaterials: A Prominent Emerging Materials Towards Environmental Pollution Study and Control

Biodegradation of Carbon Nanotubes
Lin HY, Nurunnabi M, Chen W, Huang C (2019) Graphene in neuroscience. In: Biomedical applications of graphene and 2D nanomaterials, pp 337–351
Deshmukh MA, Kang BC, Jeon JY, Ha TJ (2020) Stable dispersions of single-wall carbon nanotubes using self-assembled amphiphilic copolymer surfactants for fabricating wafer-scale devices. ACS Appl Nano Mater 3:8829–8839
Article CAS Google Scholar
Park SJ, Deshmukh MA, Kang BC, Jeon JY, Chen C, Ha TJ (2020) A review of advanced electronic applications based on carbon nanomaterials. ECS J Solid State Sci Technol 9:071002
Kothandam G, Singh G, Guan X, Lee JM, Ramadass K, Joseph S, Benzigar M, Karakoti A, Yi A, Kumar J, Vinu PA (2023) Recent advances in carbon-based electrodes for energy storage and conversion. Adv Sci 10:2301045
Cho G (2021) Ink-jet printed carbon nanotubebased transistors as water quality sensors. Thèse de doctorat de l’Institut Polytechnique de Paris préparée à l’ École Polytechnique, École doctorale n°626 École doctorale de l’Institut Polytechnique de Paris (ED IP Paris), Spécialité de doctorat: Physique
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931
Tam NT, Phuong NV, Khoi PH, Minh PN, Afrand M, Trinh PV, Thang BH, Zyła G, Estellé P (2020) Carbon nanomaterial-based nanofluids for direct thermal solar absorption. Nanomaterials 10:1199
Mansuriya BD, Altintas Z (2021) Carbon dots: classification, properties, synthesis, characterization, and applications in health care - an updated review (2018–2021). Nanomater 2021:2525
Article Google Scholar
Hussein SI (2020) Preparation and characterization of polymer blends/ kaolinite nanoclay. Iraqi J Sci:1298–1306
Adeola AO, Duarte MP, Naccache R (2023) Microwave-assisted synthesis of carbon-based nanomaterials from biobased resources for water treatment applications: emerging trends and prospects. Front Carbon 2:220021
Wang L, Chen Y, Lin L, Wang H, Huang X, Xue H (2019) Highly stretchable, anti-corrosive and wearable strain sensors based on the PDMS/CNTs decorated elastomer nanofiber composite. Chem Eng J 362:89–98
Huo Y, Xiu S, Meng LY, Quan B (2023) Solvothermal synthesis and applications of micro/nano carbons: a review. Chem Eng J 451:138572
Ghodrati M, Mousavi-Kamazani M, Zinatloo-Ajabshir S (2020) Zn 3 V 3 O 8 nanostructures: Facile hydrothermal/solvothermal synthesis, characterization, and electrochemical hydrogen storage. Ceram Int 46:28894–28902
Verma A, Goswami P, Diwakar AK (2023) Harnessing the power of 2D nanomaterials for flexible solar cell applications. Res Trends Sci Technol II:1–124
Google Scholar
Salem SS, Eman NH, Asem AM, El-Dougdoug W (2022) A comprehensive review of nanomaterials: types, synthesis, characterization, and applications. Biointerface Res Appl Chem 13:41
Ghaemi F, Ali M, Yunus R, Othman RN (2019) Synthesis of carbon nanomaterials using catalytic chemical vapor deposition technique. Synthesis. Elsevier, Technology and Applications of Carbon Nanomaterials, pp 1–27
Jiang Z, Li L, Huang H, He W, Ming W (2022) Progress in laser ablation and biological synthesis processes: “top-down” and “bottom-up” approaches for the green synthesis of Au/Ag nanoparticles. Int J Mol Sci 23:14658
Article CAS PubMed PubMed Central Google Scholar
Almalkai FA, Khashan KS, Jabir MS, Hadi AA, Sulaiman GM, Abdulameer FA, Albukhaty S, Al-Karagoly H, Albaqami J (2022) Eco-friendly synthesis of carbon nanoparticles by laser ablation in water and evaluation of their antibacterial activity. J Nanomater 2022:7927447
Haider AJ, Alawsi T, Haider MJ, Taha BA, Marhoon HA (2022) A comprehensive review on pulsed laser deposition technique to effective nanostructure production: trends and challenges. Opt Quantum Electron 54:488
Zahid MU, Pervaiz E, Hussain A, Shahzad MI, Niazi MBK (2018) Synthesis of carbon nanomaterials from different pyrolysis techniques: a review. Mater Res Express 5:052002
Yang X, Cui J, Xue K, Fu Y, Li H, Yang H (2021) Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials. Nanotechnol Rev 10:178–186
Yan Y, Li C, Liu C, Mutlu Z, Dong B, Liu J, Ozkan CS, Ozkan M (2019) Bundled and dispersed carbon nanotube assemblies on graphite superstructures as free-standing lithium-ion battery anodes. Carbon 142:38–244
Haj MZ, Fattahi R, Zarei-Behjani Z, Hosseinzadeh S (2022) Carbon nanoparticles for medicine: Current and future. Bull Mater Sci 45:1–19
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2:1821–1871
Liu W, Speranza G (2019) Functionalization of carbon nanomaterials for biomedical applications. C 5:72
Yan Y, Nashath FZ, Chen S, Manickam S, Lim SS, Zhao H, Lester E, Wu T, Pang CH (2020) Synthesis of graphene: potential carbon precursors and approaches. Nanotechnol Rev 9:1284–1314
Pillai SK, Ray SS, Moodley M (2007) Purification of single-walled carbon nanotubes. J Nanosci and Nanotechnol 7:3011–3047
Wu Q, Miao W, Zhang Y, Gao H, Hui D (2020) Mechanical properties of nanomaterials: a review. Nanotechnol Rev 9:259–273
Massetti M, Jiao F, Ferguson AJ, Zhao D, Wijeratne K, Würger A, Blackburn JL, Crispin X, Fabiano S (2021) Unconventional thermoelectric materials for energy harvesting and sensing applications. Chem Rev 121:12465–12547
Article CAS PubMed Google Scholar
Holmannova D, Borsky P, Svadlakova T, Borska L, Fiala Z (2022) Carbon nanoparticles and their biomedical applications. Appl Sci 12:7865
Gupta S, Murthy CN, Ratna Prabha C (2018) Recent advances in carbon nanotube based electrochemical biosensors. Intl J Biol Macromol 108:687–703
Banigo A, Azeez TO, Ejeta KO, Lateef A, Ajuogu E (2020) Nanobiosensors: applications in biomedical technology. Nanotechnology Applications in Africa: opportunities and Constraints, IOP Conf. Series: Mater Sci Eng. 805:012028
CAS Google Scholar
Arduini F, Cinti S, Scognamiglio V, Moscone D (2020) Nanomaterial-based sensors, Chapter 13, Handbook of Nanomaterials in Analytical Chemistry, pp 329–359
Guo K, Xi B, Wei R, Li H, Feng J, Xiong S (2020) Hierarchical microcables constructed by CoP @ C ⊂ carbon framework intertwined with carbon nanotubes for efficient lithium storage. Adv Energy Mater 10:1902913
Lin CH, Dhenadhayalan N, Lin KC (2022) Emergent carbonized polymer dots as versatile featured nanomaterial for latent fingerprints, colorimetric sensor, and photocatalysis applications. Mater Today Nano 20:100246
Upadhyay G, Saxena KK, Sehgal S, Mohammed KA, Prakash C, Dixit S, Buddhi D (2022) Development of carbon nanotube (CNT)-reinforced mg alloys: fabrication routes and mechanical properties. Metals 12:1392
Negri V, Pacheco-Torres J, Calle D, López-Larrubia, P (2022) Carbon nanotubes in biomedicine. Surface-modified Nanobiomaterials for Electrochemical and Biomedicine Applications, pp 177–217
Shewale PS, Yun KS (2020) Synthesis and characterization of Cu-doped ZnO/rGO nanocomposites for room-temperature H 2 S gas sensor. J Alloys Compd 837:155527
Raya I, Kzar HH, Mahmoud ZH, Al Ayub Ahmed A, Ibatova AZ, Kianfar E (2022) A review of gas sensors based on carbon nanomaterial. Carbon Lett 32:339–364
Nayak L, Mohanty S, Nayak SK, Ramadoss A (2019) A review on inkjet printing of nanoparticle inks for flexible electronics. J Mater Chem C 7:8771–8795
Kant T, Shrivas K, Dewangan K, Kumar A, Jaiswal NK, Deb MK, Pervez S (2022) Design and development of conductive nanomaterials for electrochemical sensors: a modern approach. Mater Today Chem 24:100769
Simon J, Flahaut E, Golzio M (2019) Overview of carbon nanotubes for biomedical applications. Materials 12:624
Chen C, Wang J (2020) Optical biosensors: an exhaustive and comprehensive review. Analyst 145:1605–1628
Ramesh M, Janani R, Deepa C, Rajeshkumar L (2023) Nanotechnology-enabled biosensors: a review of fundamentals, design principles, materials and applications. Biosensors 13:40
Hou G, Schulz MJ (2020) Carbon nanotube fibers spun directly from furnace. In Carbon nanotube Fibers and Yarns, pp 37–59
Lee J, Zambrano B, Woo J, Yoon K, Lee T (2020) Recent advances in 1D stretchable electrodes and devices for textile and wearable electronics: materials, fabrications, and applications. Adv Mater 32:1902532
Nag A, Mukhopadhyay SC (2022) Fabrication and implementation of carbon nanotubes for piezoresistive-sensing applications: a review. J Sci - Adv Mater Dev 7:100416
Wang J, Khan M, Hussain A, Khan I, Nawaz A, Ragab AH, Sayqal A, Lei T, Zada A (2023) Carbon foam composites containing carbon nanotubes and graphene oxide as additives for enhanced mechanical, thermal, electrical and catalytic properties. J Mater Res Technol 24:608–622
Zhou Q, Xiab G, Dua M, Lua Y, Xua H (2019) Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl Surf Sci 483:52–59
Farzin MA, Abdoos H (2021) A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta 224:121828
Dua S, Kumar P, Pani B, Kaur A, Khanna M, Bhatt G (2023) Stability of carbon quantum dots: a critical review. RSC Adv 13:13845–13861
Madima N, Mishra SB, Inamuddin I, Mishra AK (2020) Carbon based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ Chem Lett 18:1169–1191
Yaqoob AA, Ibrahim MNM, Akil Ahmad A, Reddy AVB (2021) Toxicology and environmental application of carbon nanocomposite. Environmental remediation through carbon based nano composites, pp 1–18
Ganie AS, Bano S, Khan N, Sultana S, Rehman Z, Rahman MM, Sabir S, Coulon F, Khan MZ (2021) Nanoremediation technologies for sustainable remediation of contaminated environments: recent advances and challenges. Chemosphere 275:130065
Mukhopadhyay R, Sarkar B, Khan E, Alessi D, Biswas J, Manjaiah K, Eguchi M, Wu K, Yamauchi Y, Ok Y (2021) Nanomaterials for sustainable remediation of chemical contaminants in water and soil. Crit Rev Environ Sci Tech 1–50
Ahmed HM, Roy A, Wahab M, Ahmed M, Othman-Qadir G, Elesawy BH, Khandaker MU, Islam MN, Emran TB (2021) Applications of nanomaterials in agrifood and pharmaceutical industry. J Nanomater 2021:1–10
Nasrollahzadeha M, Sajjadia M, Iravanib S, Varmac RS (2021) Carbon-based sustainable nanomaterials for water treatment: state-of-art and future perspectives. Chemosphere 263:128005
Wang Z, Melvin, G.J.H. (2018) Carbon nanomaterials for energy storage devices. Nanotechnology: Applications in Energy, Drug and Food, pp.1–29
Kaur S, Roy A (2021) Bioremediation of heavy metals from wastewater using nanomaterials. Environ Dev Sustain 23:9617–9640
Roy A, Sharma A, Yadav S, Jule LT, Krishnaraj R (2021) Nanomaterials for remediation of environmental pollutants. Bioinorg Chem Appl 2021:1764647
Article PubMed PubMed Central Google Scholar
Del Prado-Audelo ML, Garcia Kerdan I, Escutia-Guadarrama L, Reyna-Gonzalez JM, Magana JJ, Leyva-Gomez G (2021) Nanoremediation: nanomaterials and nanotechnologies for environmental cleanup. Front Environ Sci 9:793765
Wang XD, Vinodgopal K, Dai GP (2019) Synthesis of carbon nanotubes by catalytic chemical vapor deposition. In: H. El-Din Saleh & S. Moawad Mohamed El-Sheikh (Eds.), Perspective of Carbon Nanotubes. IntechOpen, pp 1–19
Liu Z, Ling Q, Cai Y, Xu L, Su J, Yu K, Wu X, Xu J, Hu B, Wang X (2022) Synthesis of carbon-based nanomaterials and their application in pollution management. Nanoscale Adv 4:1246–1262
Madhura L, Singh S, Kanchi S, Sabela M, Bisetty K (2018) Nanotechnology-based water quality management for wastewater treatment. Environ Chem Lett 17:65–121
Ayanda OS, Akintayo CO, Mthembu NL, Emmanuel FCA, Ximba BJ (2016) A comparative study of the kinetics, equilibrium and thermodynamics of the adsorption of Pb (II) ions onto activated carbon and fly ash from aqueous solution. Res J Chem Environ 20:44–54
Crini G, Lichtfouse E, Wilson LD, Morin N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17:195–213
Guerra FD, Attia MF, Whitehead DC, Alexis F (2018) Nanotechnology for environmental remediation: materials and applications. Molecules 23:1760
Amodu OS, Ojumu TV, Ntwampe SK, Ayanda OS (2019) Utilisation of fly ash and magnetite for the synthesis of biosurfactant-modified magnetic zeolites by direct alkali fusion. Nanosistemi Nanomateriali Nanotehnologii 17:439–452
Wang Y, Pan C, Chu W, Vipin AK, Sun L (2019) Environmental remediation applications of carbon nanotubes and graphene oxide: adsorption and catalysis. Nanomater 9:439
Babalola BM, Babalola AO, Adubiaro HO, Ayanda OS, Nelana SM, Naidoo EB (2019) Application of waste Delonix regia pods and leaves for the sorption of Pb (II) ions from aqueous solution: kinetic and equilibrium studies. Water Qual Res J 54:278–289
Afsharnia M, Naghizadeh A, Karimi A, Mansouri B, Ayanda OS (2020) Performance evaluation of multi-walled carbon nanotubes for decolorization of synthetic industrial wastewater: equilibrium, kinetics, and thermodynamics. Desalin Water Treat 188:194–201
Khan WA, Arain MB, Soylak M (2020) Nanomaterials-based solid phase extraction and solid phase microextraction for heavy metals food toxicity. Food Chem Toxicol 145:111704
Aremu OH, Akintayo CO, Nelana SM, Klink MJ, Ayanda OS (2022) Optimization of influential parameters for the degradation of metronidazole contained in aquaculture effluent via sonocatalytic process: kinetics and mechanism. Nat Environ Pollut Technol 21:1875–1885
Sabzehmeidani MM, Mahnaee S, Ghaedi M, Heidari H, Roy VA (2021) Carbon based materials: a review of adsorbents for inorganic and organic compounds. Mater Adv 2:598–627
Gul A, Khaligh NG, Julkapli NM (2021) Surface modification of carbon-based nanoadsorbents for the advanced wastewater treatment. J Mol Struct 1235:130148
Adegoke KA, Maxakato NW (2022) Porous metal oxide electrocatalytic nanomaterials for energy conversion: Oxygen defects and selection techniques. Coord Chem Rev 457:214–389
Mohapatra L, Cheon D, Yoo SH (2023) Carbon-based nanomaterials for catalytic wastewater treatment: a review. Molecules 28:1805
ul Haque S, Nasar A, Duteanu N (2023) Carbon based-nanomaterials used in biofuel cells–a review. Fuel 331:125634
He Y, Chen K, Leung MK, Zhang Y, Li L, Li G, Xuan J, Li J (2022) Photocatalytic fuel cell–a review. Chem Eng J 428:131074
Ahmed SF, Kumar PS, Ahmed B, Mehnaz T, Shafiullah G, Duong XQ, Mofijur M, Badruddin IA, Kamangar S (2024) Carbon-based nanomaterials: Characteristics, dimensions, advances and challenges in enhancing photocatalytic hydrogen production. Int J Hydrog Energy 52:424–442
Lebière PG, Ivan R, del Pino AP, Logofatu C, György E (2023) Boron and nitrogen co-doped carbon-based nanomaterials/nickel oxide/hydroxide hybrids for sunlight induced photocatalytic water cleaning. Colloids Surf 676:132159
Xu D, Huang Y, Ma Q, Qiao J, Guo X, Wu Y (2023) A 3D porous structured cellulose nanofibrils-based hydrogel with carbon dots-enhanced synergetic effects of adsorption and photocatalysis for effective Cr(VI) removal. Chem Eng J 456:141104
Zhang X, Peng J, Qi X, Huang Y, Qiao J, Guo Y, Guo X, Wu Y (2023) Nanocellulose/carbon dots hydrogel as superior intensifier of ZnO/AgBr nanocomposite with adsorption and photocatalysis synergy for Cr(VI) removal. Int J Biol Macromol 233:123566
Yu Y, Wu K, Xu W, Chen D, Fang J, Zhu X, Sun J, Liang Y, Hu X, Li R, Fang Z (2021) Adsorption-photocatalysis synergistic removal of contaminants under antibiotic and Cr(VI) coexistence environment using non-metal g-C 3 N 4 based nanomaterial obtained by supramolecular self-assembly method. J Hazard Mater 404:124171
Huang D, Dai J, Li Z, Wen X, Feng W, Liu H (2023) Effect of carbon-based nanomaterials on the wave absorption properties of hollow ZnFe 2 O 4 . Mater Sci Eng B 293:116462
Gil-Gavilán DG, Cosano D, Castillo-Rodríguez M, de Miguel G, Esquivel D, Jiménez-Sanchidrián C, Ruiz JR, Romero-Salguero FJ (2023) Composites of Co-Al hydrotalcites and carbon nanomaterials for photocatalytic H 2 production. Appl Clay Sci 238:106924
Jian L, Zhao H, Dong Y, Xu J, Mao Q, Ji R, Yan Z, Pan C, Wang G, Zhu Y (2022) Graphite carbon ring modified carbon nitride with a strong built-in electric field for high photocatalysis-self-Fenton performance electronic supplementary information (ESI) available. Catal Sci Technol 12:7379–7388
Cao S, Zhou N, Gao F, Chen H, Jiang F (2017) All-solid-state Z-scheme 3, 4-dihydroxybenzaldehyde-functionalized Ga2O3/graphitic carbon nitride photocatalyst with aromatic rings as electron mediators for visible-light photocatalytic nitrogen fixation. Appl Catal B 218:600–610
Oseghe EO, Akpotu SO, Mombeshora ET, Oladipo AO, Ombaka LM, Maria BB, Idris AO, Mamba G, Ndlwana L, Ayanda OS, Ofomaja AE, Nyamori VO, Feleni U, Nkambule TTI, Msagati TAM, Mamba BB, Bahnemann DW (2021) Multi-dimensional applications of graphitic carbon nitride nanomaterials – a review. J Mol Liq 344:117820
An H, Dat NM, Hai ND, Cong CQ, Nam NTH, Thi DNM, Nghi HB, Huyen NTT, Oanh DTY, Phong MT, Hieu NH (2023) Photocatalytic degradation of organic dyes using zinc oxide-decorated graphitic carbon nitride composite under visible light. Diam Relat Mater 131:109583
Wang J, Suo J, Song Z, Li WJ, Wang Z (2023) Nanomaterial-based flexible sensors for metaverse and virtual reality applications. Int J Extreme Manuf 5:032013
Xie K, Fang J, Li L, Deng J, Chen F (2022) Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J Alloys and Compd 901:163589
Sudhaik A, Hasija V, Selvasembian R, Ahamad T, Singh A, Khan AAP, Raizada P, Singh P (2023) Applications of graphitic carbon nitride-based S-scheme heterojunctions for environmental remediation and energy conversion. Nanofabrication 8:1–36
Wudil Y, Ahmad U, Gondal M, Al-Osta MA, Almohammedi A, Said R, Hrahsheh F, Haruna K, Mohammed J (2023) Tuning of graphitic carbon nitride (g-C 3 N 4 ) for photocatalysis: a critical review. Arab J Chem 16:104542
Nonoguchi Y, Miyao T, Goto C, Kawai T, Funatsu K (2022) Governing factors for carbon nanotube dispersion in organic solvents estimated by machine learning. Adv Mater Interfaces 9:2101723
Dou JB, Tang LX, Mou L, Zhang RF, Jiang XY (2020) Stretchable conductive adhesives for connection of electronics in wearable devices based on metal-polymer conductors and carbon nanotubes. Compos Sci Technol 197:108237
Zheng Q, Cao WQ, Zhai HZ, Cao MS (2023) Tailoring carbon-based nanofibers microstructure for electromagnetic absorbers, shielding and devices. Mater Chem Front 7:1737–1759
Sankhula L, Lavate S, Srivastava R (2023) Advanced carbon-based nanomaterials for photoelectrochemical water splitting. In Solar-driven green hydrogen generation and storage, pp 103–128
Bezzon VDN, Montanheiro TLA, de Menezes BRC (2019) Carbon nanostructure-based sensors: a brief review on recent advances. Adv Mater Sci Eng 2019:4293073
Noah NM (2020) Design and synthesis of nanostructured materials for sensor applications. J Nanomater 2020:1–20
Abdel-Karim R, Reda Y, Abdel-Fattah A (2020) Review-nanostructured materials-based nanosensors. J Electrochem Soc 167:037554
Lone Y, Ansari N, Ali J, Zulfequar M (2021) Nanotechnology, carbon nanotubes (CNTs) and applications. Austin Publishing Group, Importance & Applications of Nanotechnology, pp 71–89
Cardenas JA, Andrews JB, Noyce SG, Franklin AD (2020) Carbon nanotube electronics for IoT sensors. Nano Futures 4:012001
Carbonaro CM, Corpino R, Salis M, Mocci F, Thakkar SV, Olla C, Ricci PC (2019) On the emission properties of carbon dots: reviewing data and discussing models. C 5:60
Speranza G (2021) Carbon nanomaterials: synthesis, functionalization and sensing applications. Nanomater 11:967
Jin X, Feng C, Ponnamma D, Yi Z, Parameswaranpillai J, Sabu T, Salim NV (2020) Review on exploration of graphene in the design and engineering of smart sensors, actuators and soft robotics. Chem Eng J Adv 4:100034
Krishnan SK, Singh E, Singh P, Meyyappan M, Nalwa SH (2019) A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv 9:8778–8881
Speranza G (2019) The role of functionalization in the applications of carbon materials: An overview. C 5:84
Maffeis V, Moni L, Di Stefano D, Giordani S, Riva R (2020) Diversity-oriented synthesis of blue emissive nitrogen heterocycles and their conjugation with carbon nano-onions. Front Chem Sci Eng 14:76–89
Wu X, Zhou B, Zhou J, Chen Y, Chu Y, Huang J (2018) Distinguishable detection of ultraviolet, visible, and infrared spectrum with high-responsivity perovskite-based flexible photosensors. Small 14:1800527
Lu Y, Yu G, Wei X, Zhan C, Jeon J, Wang X, Jeffryes C, Guo Z, Wei S, Wujcik EK (2019) Fabric/multi-walled carbon nanotube sensor for portable on-site copper detection in water. Adv Compos Mater 2:711–719
Khan FU, Ansar MT, Ullah Z, Ali R, Waseem M, Mustafa GM, Atiq S, Naseem S (2021) Acetone sensing and modeling through functionalized Ag-catalysts and CNTs via low cost metal-organic framework-derived ZnO nanostructures. Ceram Int 47:3361–3367
Doshi MS, Thostenson ET (2021) Thin and flexible carbon nanotube-based pressure sensors with ultrawide sensing range. ACS sensors 3:1276–1282
Su X, Jia C, Xiang H, Zhu M (2023) Research progress in preparation, properties, and applications of medical protective fiber materials. Appl Mater Today 32:101792
Yougbaré S, Mutalik C, Okoro G, Lin I, Krisnawati DI, Jazidie A, Nuh M, Chng C, Kuo T (2021) Emerging trends in nanomaterials for antibacterial applications. Int J Nanomed 16:5831–5867
Alehosseini E, Jafari SM (2019) Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci Technol 91:116–128
Salari S, Jafari SM (2020) Application of nanofluids for thermal processing of food products. Trends Food Sci Technol 97:100–113
Kenny RG, Mamion CJ (2019) Toward multi-targeted platinum and ruthenium drugs- a new paradigm in cancer drug treatment regimen? Chem Rev 119:1058–1137
Ganjoo R, Sharma S, Kumar A (2023) Activation of carbon allotropes through covalent and noncovalent functionalization: attempts in modifying properties for enhanced performance. In: Carbon allotropes and composites: Materials for environment protection and remediation, pp 31–50
Bera BA (2017) Review on polymer, graphene and carbon nanotube: properties, synthesis and applications. Imp J Interdiscip Res 3:61–70
Rahman G, Najaf Z, Mehmood A, Bilal S, Shah AU, Mian SA, Ali G (2019) An overview of the recent progress in the synthesis and applications of carbon nanotubes. C (Basel) 5:3
Zhang X, Wen R, Huang Z, Tang C, Huang Y, Liu Y, Fang M, Wu X, Min X, Zu Y (2017) Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build 149:463–470
Kobashi K, Ata S, Yamada T, Futaba DN, Okazaki T, Hata K (2019) Classification of commercialized carbon nanotubes into three general categories as a guide for applications. ACS Appl Nano Mater 2:4043–4047
Liu D, Mao Y, Ding L (2018) Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J Water Health 16:171–180
Article PubMed Google Scholar
Haung CF, Chan YH, Chen LK, Liu CM, Huang WC, Ou SF, Ou KJ (2013) Preparation, characterization, and properties of anticoagulation and antibacterial films of carbon-based nanowires fabricated on surfaces of Ti implants. J Electrochem Soc 160:H392
Xin Q, Shah H, Nawaz A, Xie W, Akram MZ, Batool A, Tian L, Jan SU, Boddula R, Guo B, Liu Q (2019) Antibacterial carbon-based nanomaterials. Adv Mater 31:1804838
Kang S, Pinault M, Pfefferle LD, Elimelech M (2007) Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23:8670–8673
Huang Z, Zheng X, Yan G, Liao X, Kang Y, Yao Y, Huang D, Hao B (2008) Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24:4140–4144
Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10:1066
Nagai H, Toyokuni S (2012) Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: shedding light on fiber entry mechanism. Cancer Sci 103:1378–1390
Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle LD, Van-Tassel PR (2010) Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale 2:1789–1794
Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K (2010) Stone VA (2010) A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicol 4:207–246
Olivi M, Zanni E, De Bellis G, Talora C, Sarto MS, Palleschi C, Flahaut E, Monthiox M, Rapino S, Ucelletti D, Fiorito S (2013) Inhibition of microbial growth by carbon nanotube networks. Nanoscale 5:9023–9029
Chen H, Wang B, Gao D, Guan M, Zheng L, Ouyang H, Chai Z, Zhao Y, Feng W (2013) Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small 9:2735–2746
Kang S, Mauter MS, Elimelech M (2008) Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ Sci Technol 42:7528–7534
Arias LR, Yang L (2009) Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 25:3003–3012
Deryabin DG, Vasilchenko AS, Aleshina ES, Tlyagulova AS, Nikiyan HN (2010) An investigation into the interaction between carbon-based nanomaterials and Escherichia coli cells using atomic force microscopy. Nanotechnol Russ 5:857–863
Raghunath A, Perumal E (2017) Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents 49:137–152
Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE (2012) Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharm 261:121–133
Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura Shinohara N, Uchino K, Honda K (2015) Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal Toxicol 27:207–223
Amiri A, Zare ZH, Shanbedi M, Kazi SN, Taheri-Kafrani A, Chew BT, Zarrabi A (2016) Microbial toxicity of different functional group-treated carbon nanotubes. In: Grumezescu AM, ed. Surface Chemistry of Nanobiomaterials. William Andrew Publishing, pp 33–70
Wang X, Jiao C, Wang T, Yu Z (2016) Study of DNA damage induced by the reactive oxygen species generated in situ based on the multi-walled carbon nanotubes and hemoglobin. J Electroanal Chem 767:183–187
Shu M, He F, Li Z, Zhu X, Ma Y, Zhou Z, Yang Z, Gao F, Zeng M (2020) Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res Lett 15:1–9
Chaudhari AA, Joshi S, Vig K, Sahu R, Dixit S, Baganizi R, Dennis VA, Singh SR, Pillai SA (2019) A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J Biomater Appl 33:924–934
Chaudhari AA, Ashmore D, Nath SD, Kate K, Dennis V, Singh SR, Owen DR, Palazzo C, Arnold RD, Miller ME, Pillai SR (2016) A novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J Nanobiotechnology 14:58
Kumar A, Dalal J, Dahiya S, Punia R, Sharma KD, Ohlan A, Maan AS (2019) In situ decoration of silver nanoparticles on single-walled carbon nanotubes by microwave irradiation for enhanced and durable anti-bacterial finishing on cotton fabric. Ceram Int 45:1011–1019
Levashov PA, Sedov SA, Shipovskov S, Belogurova NG, Levashov AV (2010) Quantitative turbidimetric assay of enzymatic gram-negative bacteria lysis. Anal Chem 82:2161–2163
Mocan T, Matea CT, Pop T, Mosteanu O, Buzoianu AD, Suciu S, Mocan T, Matea CT, Pop T, Mosteanu O, Buzoianu AD, Suciu S, Puia C, Zdrehus C, Iancu C, Mocan L (2017) Carbon nanotubes as anti-bacterial agents. Cell Mol Life Sci 74:3467–3479
Pasquini L, Hashmi S, Sommer T, Elimelech M, Zimmerman J (2012) Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ Sci Technol 46:6297–6305
Neelgund GM, Oki A, Luo Z (2012) Antimicrobial activity of CdS and Ag 2 S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf B100:15–21
Zare-Zardini H, Shanbedi M, Soltaninejad H, Mohammadzadeh M, Amiri A, Hamidieh AA, Ferdowsian F, Alemi A, Hoseinkhani S, Fesahat F, Astani A (2020) The effect of temperature and acidity on antimicrobial activities of pristine MWCNTs and MWCNTs-Arg. Int J Nanosci Nanotechnol 16:127–136
Saleemi MA, Fouladi MH, Yong PVC, Wong EH (2020) Elucidation of antimicrobial activity of non-covalently dispersed carbon nanotubes. Materials 13:1676
Seo Y, Hwang J, Kim J, Jeong Y, Hwang MP, Choi J (2014) Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int J Nanomed 9:4621–4629
Rusen E, Mocanu A, Nistor LC, Dinescu A, Călinescu I, Mustăţea G, Voicu ŞI, Andronescu C, Diacon A (2014) Design of antimicrobial membrane based on polymer colloids/multiwall carbon nanotubes hybrid material with silver nanoparticles. ACS Appl Mater Interfaces 6:17384–17393
Mohan R, Shanmugharaj A, Sung Hun R (2011) An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J Biomed Mater Res Part B Appl Biomater 6:119–126
Sui M, Zhang L, Sheng L, Huang S, She L (2013) Synthesis of ZnO coated multi walled carbon nanotubes and their antibacterial activities. Sci Total Environ 452–453:148–154
Hirschfeld J, Akinoglu EM, Wirtz DC, Hoerauf A, Bekeredjian-Ding I, Jepsen S, Haddouti EM, Limmer A, Giersig M (2017) Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminishes biofilm formation by Staphylococcus epidermidis . Nanomedicine 13:1587–1593
Karthika V, Arumugam A (2017) Synthesis and characterization of MWCNT/TiO 2 /Au nanocomposite for photocatalytic and antimicrobial activity. IET Nanobiotechnol 11:113–118
Grover N, Borkar IV, Dinu CZ, Kane RS, Dordick JS (2012) Laccase-and chloroperoxidase-nanotube paint composites with bactericidal and sporicidal activity. Enzyme Microb Technol 50:271–279
Murugan E, Vimala G (2011) Effective functionalization of multiwalled carbon nanotube amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J Colloid Interface Sci 357:354–365
Neelgund GM, Oki A (2011) Deposition of silver nanoparticles on dendrimer functionalized multiwalled carbon nanotubes: synthesis, characterization and antimicrobial activity. J Nanosci Nanotechnol 11:3621–2629
Slate AJ, Whitehead KA, Brownson DA, Banks CE (2019) Microbial fuel cells: an overview of current technol. Renew Sust Energ Rev 101:60–81
Fan X, Zhou Y, Jin X, Song RB, Li Z, Zhang Q (2021) Carbon material-based anodes in the microbial fuel cells. Carbon Energy 3:449–472
Sarma H, Bhattacharyya PN, Jadhav DA (2021) Fungal-mediated electrochemical system: prospects, applications and challenges. Curr Res Microb 2:179–188
Gaffney AM, Gross J, Borg LE, Hanna KLD, Draper DS, Dygert N, Elkins-Tanton LT, Prissel KB, Prissel TC, Steenstra ES, van Westrenen W (2023) Magmatic evolution I: initial differentiation of the Moon. Rev Mineral Geochem 89:103–145
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027
Leung DHL, Lim YS, Uma K, Engineering S (2021) Oneidensis for performance improvement of microbial fuel cella mini review. Appl Biochem Biotechnol 193:1170–1186
Zohuri B (2020) Energy storage technologies and their role in renewable integration and significance of thermodynamic analysis. Encycl Energy Storage 112–170
Numan M, Subhan F, Khan WZ, Hakak S, Haider S, Reddy GT, Jolfaei A, Alazab M (2020) A systematic review on clone node detection in static wireless sensor networks. IEEE Access 8:65450–65461
Liu Y, Zhang X, Zhang Q, Li C (2020) Microbial fuel cells: nanomaterials based on anode and their application. Energy Technol 8:9–21
Mollaei M, Timmers PHA, SuarezDiez M (2021) Comparative proteomics of Geobacter sulfurreducens PCAT in response to acetate, formate and/or hydrogen as electron donor. Environ Microbiol 23:299–315
Nabil NK, Abd-Elrahman NA, Noor MB, Yas AH, Ahmad U, Sheikh A (2022) Applications of nanomaterials in microbial fuel cells: a review. Molecules 27:74–83
Zengm T, Zhang C, Zhou A, Wu Q, Deng C, Chan SH, Chen J, Foley AM (2021) Enhancing reactant mass transfer inside fuel cells to improve dynamic performance via intelligent hydrogen pressure control. Energy 230:120–146
Tao J, Liao J, Zou Z, Liao G, Li C, Liu S (2021) Polypyrrole-coated manganese dioxide nanowires and multi-walled carbon nanotubes as high-performance electrodes for zinc-ion batteries. J Nanoelectron Optoelectron 16:522–527
Yalikun N, Xu L, Yang XQ, Wang Q (2021) Highly sensitive electrochemical sensor for the detection of hydroquinone and catechol based on biomass derived activated carbon. Sci Adv Mater 13:2178–2184
Niu T, Huang W, Zhang C, Zeng T, Chen J, Li Y, Liu Y (2022) Study of degradation of fuel cell stack based on the collected high-dimensional data and clustering algorithms calculations. Energy AI 10:100184
Jimenez L, Kulko M, Kim R, Jashari T, Choe T (2020) 16S rRNA analysis of electrogenic bacterial communities in microbial fuel cells developed from temperate soils. Bioscience 91:9–20
Muhammad AB, Nasir A, Maina MB, Shuwa M (2022) Scope and potentials of renewable energy hybrid power system in semi-arid region of Northern Nigeria: Maiduguri and its environs. ATBU J Sci Technol Edu 8:250–259
Muchuweni E, Mombeshora ET, Martincigh BS, Nyamori VO (2022) Recent applications of carbon nanotubes in organic solar cells. Front Chem 9:733552
Ashok A, Regmi G, Romero-Núñez A, Solis-López M, Velumani S, Castaneda H (2020) Comparative studies of CdS thin films by chemical bath deposition techniques as a buffer layer for solar cell applications. J Mater Sci Mater Electron 31:7499–7518
Lin Y, Zhang S, Guan L, Tao J (2020) Prospect of Ni-related metal oxides for high-performance supercapacitor electrodes. J Mater Sci 56:1897–1918
Subhan FE, Khan AD, Khan AD, Ullah N, Imran M, Noman M (2020) Optical optimization of double-side-textured monolithic perovskite-silicon tandem solar cells for improved light management. RSC Adv 10:26631–26638
Zhang C, Xie Z, Yang W, Liang Y, Meng D, He X, Liang P, Zhang Z (2020) NiCo 2 O 4 /biomass derived carbon composites as anode for high-performance lithium-ion batteries. J Power Sources 451:227761
Muhammad AB, Ngala GM, Shodiya S, Shuwa M (2016) Modeling and simulation of wind-solar hybrid energy system for power supply to Faculty of Engineering Teaching Workshop in University of Maiduguri. Int J Res Eng Technol 5:229–233
Deshmukh A.N, Chandrakar VK (2021) Power quality issues and their mitigation techniques in grid tied solar photovoltaic systems-a review. In 2021 International Conference on Computer Communication and Informatics (ICCCI), IEEE 2021, pp 1–6
Wang Y, Yang P, Zheng L, Shi X, Zheng H (2020) Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: a review. Energy Storage Mater 26:349–370
Yogeswari B, Khan I, Kumar MS, Vijayanandam N, Devarani PA, Anandaram H, Chaturvedi A, Misganaw W (2022) Role of carbon-based nanomaterials in enhancing the performance of energy storage devices: design small and store big. J Nanomater 2022:4949916
Teprovich JA, Weeks PA, Ward SC, Tinkey C, Huang J, Zhou R, Zidan P (2019) Jena hydrogenated C60 as high-capacity stable anode materials for Li ion batteries. ACS Appl Energy Mater 2:6453
Tuktarov AR, Salikhov RB, Khuzin AA, Popod’ko NR, Safargalin IN, Mullagaliev IN, Dzemilev UM (2019) Photocontrolled organic field effect transistors based on the fullerene C60 and spiropyran hybrid molecule. RSC Adv 9:7505
Umeyama T, Imahori H (2019) Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Acc Chem Res 52:2046
Dutta V, Verma R, Gopalkrishnan C, Yuan MH, Batoo KM, Jayavel R, Chauhan A, Lin KYA, Balasubramani R, Ghotekar S (2022) Bio-inspired synthesis of carbon-based nanomaterials and their potential environmental applications: a state-of-the-art review. Inorganics 10:169
Meng Y, Huang X, Lin H, Zhang P, Gao Q, Li W (2019) Carbon-based nanomaterials as sustainable noble-metal-free electrocatalysts. Front Chem 7:759
Gabris MA, Ping J (2021) Carbon nanomaterial-based nanogenerators for harvesting energy from environment. Nano Energy 90:106494
Díez-Pascual AM (2021) Carbon-based nanomaterials. Int J Mol Sci 22:7726
Onyancha RB, Ukhurebor KE, Aigbe UO, Osibote OA, Kusuma HS, Darmokoesoemo HA (2022) Methodical review on carbon-based nanomaterials in energy-related applications. Adsorp Sci Technol 2022:1–22
Pandey S, Karakoti M, Bhardwaj D, Tatrari G, Sharma RP, Pandey L, Lee MJ, Sahoo NG (2023) Recent advances in carbon-based materials for high-performance perovskite solar cells: gaps, challenges and fulfillment. Nanoscale Adv 5:1492–1526
Lazzarin L, Pasini M, Menna E (2021) Organic functionalized carbon nanostructures for solar energy conversion. Molecules 26:286
Jeong S, Park J, Han T, Zhang F, Zhu K, Kim JS, Park M, Reese MO, Yoo S, Lee T (2020) Characterizing the efficiency of perovskite solar cells and light-emitting diodes. Joule 4:1206–1235
Moore K, Wei W (2021) Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion. Nano Mater Sci 3:276–290
Li X, Zhao Y, Min X, Xiao J, Wu X, Mi R, Liu YG, Huang Z, Fang M (2022) Carbon nanotubes modified graphene hybrid aerogel-based composite phase change materials for efficient thermal storage. Energy and Build 273:112384
Chen TW, Kalimuthu P, Veerakumar P, Lin KC, Chen SM, Ramachandran R, Mariyappan V, Chitra S (2022) Recent developments in carbon-based nanocomposites for fuel cell applications: a review. Molecules 27:761
Andretti Group. Technological advancements in hydrogen fuel: fueling the future https://andretti1.com/technological-advancements-in-hydrogen-fuel/ . Accessed on 18 November 2023
Geiger K (2023) An energy breakthrough: tech researchers create new type of fuel cell. https://www.mtu.edu/news/2023/04/an-energy-breakthrough-tech-researchers-create-new-type-of-fuel-cell.html . Accessed on 05 January 2024
Honorato AMB, Khalid M (2020) Carbon nanomaterials for metal-free electrocatalysis. Appl Chem Eng 3:55–62
Asenbauer J, Eisenmann T, Kuenzel M, Kazzazi A, Chen Z, Bresser D (2020) The success story of graphite as a lithium-ion anode material - fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain Energy Fuels 4:5387
Shang Y, Zhang D, An M, Li Z (2022) Enhanced thermal performance of composite phase change materials based on hybrid graphene aerogels for thermal energy storage. Materials 15:5380
Zhang H, Zhang Z, Luo J, Qi X, Yu J, Cai J, Wei J, Yang ZA (2019) Chemical blowing strategy to fabricate biomass-derived carbon-aerogels with graphene-like nanosheet structures for high-performance supercapacitors. Chemsuschem 12:2462–2470
Lee C, Kort-Kamp W, Yu H, Cullen D, Patterson B, Arman T, Komini Babu S, Mukundan R, Borup R, Spendelow J (2023) Grooved electrodes for high-power-density fuel cells. Nat Energy 25:1–10
Khan I, Vijayanandam N, Devarani P, Anandaram H, Chaturvedi A, Misganaw W (2022) Role of Carbon-based nanomaterials in enhancing the performance of energy storage devices: design small and store big. J Nanomater 2022:1–10
Agudosi ES, Abdullah EC, Mubarak NM, Khalid M (2020) Carbon-based nanomaterials for energy storage and sensing applications. In: Pure and functionalized carbon based nanomaterials, pp 147–174
Notarianni M, Liu J, Vernon K, Motta N (2016) Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J Nanotechnol 7:149–196
Zhao J, Zhang J, Yin H, Zhang X, Xu G, Yuan J, Mo X, Tang J, Wang F (2022) Ultra-fine ruthenium oxide quantum dots/reduced graphene oxide composite as electrodes for high-performance supercapacitors. Nanomater 12:1210
Chen X, Paul R, Gai L (2017) Carbon based supercapacitors for efficient energy storage. Natl Sci Rev 4:453–489
Zheng S, Lim SS, Foo CY, Haw CY, Chiu WS, Chia CH, Khiew PS (2021) Recent progress on the applications of carbonaceous and metal-organic framework nanomaterials for supercapacitors. Front Mater 8:777149
Olabi AG, Abbas Q, Abdelkareem MA, Alami AH, Mirzaeian M, Sayed ET (2022) Carbon-based materials for supercapacitors: recent progress, challenges and barriers. Batteries 9:19
Wang G, Yu M, Feng X (2021) Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem Soc Rev 50:2388–2443
Wang Y, Zhu M, Wei X, Yu J, Li Z, Ding B (2021) A dual-mode electronic skin textile for pressure and temperature sensing. Chem Eng J 425:130599
Bora P, Bhuyan C, Borah A, Hazarika S (2023) Carbon nanomaterials for designing next-generation membranes and their emerging applications. Chem Comm 59:11320–11336
Han T, Xu L, Zhao Y, Zhang S, Hu Z, Li J, Ouyang M (2023) Optimizing catalyst layer structure with carbon nanofiber additive for better performance of proton exchange membrane fuel cell. J Electrochem Soc 170:094508
Jeerapan I, Ma N (2019) Challenges and opportunities of carbon nanomaterials for biofuel cells and supercapacitors: personalized energy for futuristic self-sustainable devices. J Carbon Res 5:62
Ramar V, Balraj A (2022) Critical review on carbon-based nanomaterial for carbon capture: technical challenges, opportunities, and future perspectives. Energy Fuels 36:13479–13505
Madannejad R, Shoaie N, Jahanpeyma F, Darvishi MH, Azimzadeh M, Javadi H (2019) Toxicity of carbon-based nanomaterials: reviewing recent reports in medical and biological systems. Chem Biological Inter 307:206–222
Cheng TM, Chu HY, Huang HM, Li ZL, Chen CY, Shih YJ, Wang K (2022) Toxicologic concerns with current medical nanoparticles. Int J Mol Sci 23:7597
Fu T, Zhang B, Gao X, Cui S, Guan CY, Zhang Y, Peng Y (2023) Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci Total Environ 856:158810
Awashra M, Młynarz P (2023) The toxicity of nanoparticles and their interaction with cells: an in vitro metabolomic perspective. Nanoscale Adv 5:2674–2723
Peng Z, Liu X, Zhang W, Zeng Z, Liu Z, Zhang C, Yuan X (2020) Advances in the application, toxicity and degradation of carbon nanomaterials in environment: a review. Environ Int 134:105298
Yao Y, Zhang T, Tang MA (2022) critical review of advances in reproductive toxicity of common nanomaterials to Caenorhabditis elegans and influencing factors. Environ Pollut 306:119270
Nasirzadeh N, Mohammadian Y, Fakhri Y (2020) Concentration and cancer risk assessment of asbestos in Middle East countries: a systematic review-meta-analysis. Int J Environ Anal Chem 7:1–15
Dalla Colletta A, Pelin M, Sosa S, Fusco L, Prato M, Tubaro A (2022) Carbon-based nanomaterials and SKIN: an overview. Carbon 196:683–698
Garriga R, Herrero-Continente T, Palos M, Cebolla VL, Osada J, Muñoz E, Rodríguez-Yoldi MJ (2020) Toxicity of carbon nanomaterials and their potential application as drug delivery systems: in vitro studies in CaCO-2 and MCF-7 cell lines. Nanomater 10:1617
Debnath SK, Srivastava R (2021) Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Front Nanotechnol 3:644564
Yuan X, Zhang X, Sun L, Wei Y, Wei X (2019) Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicolo 16:1–27
Sajjadi M, Nasrollahzadeh M, Jaleh B, Soufi GJ, Iravani S (2021) Carbon-based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects. J Drug Target 29:716–741
Bessa MJ, Brandão F, Rosário F, Moreira L, Reis AT, Valdiglesias V, Teixeira JP (2023) Assessing the in vitro toxicity of airborne (nano) particles to the human respiratory system: from basic to advanced models. J Toxicol Environ Health B 26:67–96
Ghosh R, Rojanasakul Y (2019) Evaluating carcinogenic potential of carbon nanomaterials. In: Nanotechnology characterization tools for environment, health, and safety, pp 103–144
Sousa SP, Peixoto T, Santos RM, Lopes A, Paiva MDC, Marques AT (2020) Health and safety concerns related to CNT and graphene products, and related composites. J Compos Sci 4:106
Jiang T, Lin Y, Amadei CA, Gou N, Rahman SM, Lan J, Gu AZ (2021) Comparative and mechanistic toxicity assessment of structure-dependent toxicity of carbon-based nanomaterials. J Hazard Mater 418:126282
Romeo D, Clement P, Wick P (2023) Release and toxicity assessment of carbon nanomaterial reinforced polymers during the use and end-of-life phases: a comparative review. NanoImpact 31:100477
Odaudu OR, Akinsiku AA (2022) Toxicity and cytotoxicity effects of selected nanoparticles: a review. IOP Conf Ser: Earth and Environmental Science 1054:012007
Pastrana HF, Cartagena-Rivera AX, Raman A, Ávila A (2019) Evaluation of the elastic Young’s modulus and cytotoxicity variations in fibroblasts exposed to carbon-based nanomaterials. J Nanobiotechnol 17:32
Zhao C, Kang J, Li Y, Wang Y, Tang X, Jiang Z (2023) Carbon-based stimuli-responsive nanomaterials: classification and application. Cyborg Bionic Syst 4:0022
Akçan R, Aydogan HC, Yildirim MŞ, Taştekin B, Sağlam N (2020) Nanotoxicity: a challenge for future medicine. Turk J Med 50:1180–1196
Tabei Y, Fukui H, Nishioka A, Hagiwara Y, Sato K, Yoneda T, Horie M (2019) Effect of iron overload from multi walled carbon nanotubes on neutrophil-like differentiated HL-60 cells. Sci Rep 9:2224
Martínez-Paz P, Negri V, Esteban-Arranz A, Martínez-Guitarte JL, Ballesteros P, Morales M (2019) Effects at molecular level of multi-walled carbon nanotubes (MWCNT) in Chironomus riparius (DIPTERA) aquatic larvae. Aquat Toxicol 209:42–48
Nasirzadeh N, Mohammadian Y, Rasoulzadeh Y, Rezazadeh Azari M, Khodagholi F (2022) Toxicity of carbon-based nanomaterials in the human lung: a comparative in-vitro study. Tanaffos 21:391–400
PubMed PubMed Central Google Scholar
Hassan AA, Mansour MK, El Ahl RMS, El Hamaky AM, Oraby NH (2020) Toxic and beneficial effects of carbon nanomaterials on human and animal health. In: Carbon nanomaterials for agri-food and environmental applications, pp 535–555
Gamoń F, Ziembińska-Buczyńska A, Łukowiec D, Tomaszewski M (2022) Ecotoxicity of selected carbon-based nanomaterials. Int J Environ Sci Technol 20:10153–10162
Sforzini S, Oliveri C, Barranger A, Jha AN, Banni M, Moore MN, Viarengo A (2020) Effects of fullerene C60 in blue mussels: role of mTOR in autophagy related cellular/tissue alterations. Chemosphere 246:125707
Cao Y, Luo Y (2019) Pharmacological and toxicological aspects of carbon nanotubes (CNTs) to vascular system: a review. Toxicol Appl Pharmacol 385:114801
Chen M, Sun Y, Liang J, Zeng G, Li Z, Tang L, Song B (2019) Understanding the influence of carbon nanomaterials on microbial communities. Environ Inter 126:690–698
Malhotra N, Audira G, Castillo AL, Siregar P, Ruallo JMS, Roldan MJ, Hsiao CD (2021) An update report on the biosafety and potential toxicity of fullerene-based nanomaterials toward aquatic animals. Oxid Med Cell Longev 2021
Gangoli VS, Raja PMV, Esquenazi GL, Barron AR (2019) The safe handling of bulk low-density nanomaterials. SN Appl Sci 1:1–6
Issa R, Lozano N, Kostarelos K, Vranic S (2023) Functioning human lung organoids model pulmonary tissue response from carbon nanomaterial exposures. Ann Work Expo Health 67:i73–i74
Seidel C, Sébillaud S, Darne C, Barthel H, Gaté L (2023) 104 Influence of the physicochemical properties of multi-walled carbon nanotubes on their toxicity in lung cells. Ann Work Expo Health 67:i96–i96
Zhang H, Zhang S, Duan Z, Wang L (2022) Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ Inter 162:107177
Russ KA, Thompson JA, Kashon M, Porter DW, Friend SA, McKinney W, Fedan JS (2019) Comparison of multi-wall carbon nanotube and nitrogen-doped multi-wall carbon nanotube effects on lung function and airway reactivity in rats. Toxicol Appl Pharmacol 364:153–163
Halimu G, Zhang Q, Liu L, Zhang Z, Wang X, Gu W, Zhang B, Dai Y, Zhang H, Zhang C, Xu M (2022) Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J Hazard Mater 430:128485
Tsai MH, Chao HR, Jiang JJ, Su YH, Mariene-syne PC, Tayo LL, Hsu WL (2021) Toxicity of low-dose graphene oxide nanoparticles in an in-vivo wild type of Caenorhabditis elegans model. Aerosol Air Qual Res 21:200559
Kalman J, Merino C, Fernández-Cruz ML, Navas JM (2019) Usefulness of fish cell lines for the initial characterization of toxicity and cellular fate of graphene-related materials (carbon nanofibers and graphene oxide). Chemosphere 218:347–358
da Rocha AM, Kist LW, Almeida EA, Silva DGH, Bonan CD, Altenhofen S, Monserrat JM (2019) Neurotoxicity in zebrafish exposed to carbon nanotubes: effects on neurotransmitters levels and antioxidant system. Comp Biochem Physiol C Toxicol Pharma 218:30–35
Kang S, Zhu Y, Chen M, Zeng G, Li Z, Zhang C, Xu P (2019) Can microbes feed on environmental carbon nanomaterials. Nano Today 25:10–12
Xuan L, Ju Z, Skonieczna M, Zhou PK, Huang (2023) Nanoparticles-induced potential toxicity on human health: applications, toxicity mechanisms, and evaluation models. MedComm 4:e327
Thomas DT, Baby A, Raman V, Balakrishnan SP (2022) Carbon-based nanomaterials for cancer treatment and diagnosis: a review. ChemistrySelect 7:e202202455
Goodwin DG Jr, Adeleye AS, Sung L, Ho KT, Burgess RM, Petersen EJ (2018) Detection and quantification of graphene-family nanomaterials in the environment. Environ Sci Technol 52:4491–4513
Adeleye AS, Conway JR, Garner K, Huang Y, Su Y, Keller AA (2016) Engineered nanomaterials for water treatment and remediation: costs, benefits, and applicability. Chem Eng J 86:640–662
Wu W, Deng Y, Chen G (2023) A self-repairing polymer-inorganic composite coating to enable high-performance Zn anodes for zinc-ion batteries. Chin Chem Lett 34:108424
Download references
Author information
Authors and affiliations.
Nanoscience Research Unit, Department of Industrial Chemistry, Federal University Oye Ekiti, P.M.B 373, Oye-Ekiti, Ekiti State, Nigeria
Olushola Sunday Ayanda & Ayomipo Ojo
Department of Microbiology, Faculty of Biological Sciences, University of Calabar, Calabar, Nigeria
Augusta Oluchi Mmuoegbulam, Onyemaechi Okezie & Augustine Agorye Unimke
Department of Chemistry, Federal University Dutse, PMB 7156, Dutse, Jigawa State, Nigeria
Naseer Inuwa Durumin Iya & Sa’adatu Eri Mohammed
Department of Agronomy, Federal University Gashua, Gashua, Yobe State, Nigeria
Philip Hegarty James
Department of Mechanical Engineering, University Of Maiduguri, Maiduguri, Borno State, Nigeria
Abba Bashir Muhammad
Department of Mechanical Engineering, Ahmadu Bello University, Zaria, Nigeria
Sabur Ajibola Alim
Department of Soil Science, Faculty of Agriculture/Institute for Agricultural Research, Ahmadu Bello University, P.M.B. 1044, Zaria, Nigeria
Sharhabil Musa Yahaya
Department of Guidance and Counselling, Faculty of Education, Federal University Oye-Ekiti, Oye-Ekiti, Nigeria
Toyin Olanike Adaramoye
Department of Science Education, Federal University Oye-Ekiti, Oye-Ekiti, Nigeria
Stella Kemilola Ekundayo
Department of Biotechnology, Modibbo Adama University, PMB: 2076, Yola, Adamawa State, Nigeria
Aminu Abdullahi
Department of Chemistry, Faculty of Science, Federal University Dutse, Jigawa, Nigeria
Hamza Badamasi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Olushola Sunday Ayanda .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ayanda, O.S., Mmuoegbulam, A.O., Okezie, O. et al. Recent progress in carbon-based nanomaterials: critical review. J Nanopart Res 26 , 106 (2024). https://doi.org/10.1007/s11051-024-06006-2
Download citation
Received : 17 November 2023
Accepted : 26 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1007/s11051-024-06006-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Carbon-based nanomaterials
- Environmental remediation
- Analytical chemistry and sensing
- Antimicrobial activities
- Microbial fuel cells
- Renewable energy and energy storage
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Nanoparticles articles from across Nature Portfolio
Nanoparticles are particles that exist on a nanometre scale (i.e., below 100 nm in at least one dimension). They can possess physical properties such as uniformity, conductance or special optical properties that make them desirable in materials science and biology.
Liposozyme for wound healing and inflammation resolution
Antibacterial action, along with restoration of redox and immune homeostasis, is achieved using a lipid–nanozyme hybrid for the healing of diabetic foot ulcers.
- Zhichao Deng
- Mingzhen Zhang
Latest Research and Reviews
Engineering nanomaterials for glioblastoma nanovaccination
Developing vaccines for glioblastoma remains challenging owing to the immunosuppressive microenvironment of the tumour and the presence of the blood–brain barrier. In this Perspective, we explore how nanomaterials can be tailored to address the limitations of glioblastoma vaccination, potentially paving the way for important advancements.
- Fatima Hameedat
- Bárbara B. Mendes
- Flávia Sousa
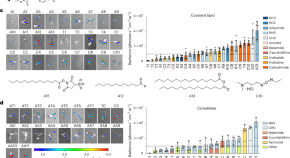
Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells
The ability to genetically modify haematopoietic stem cells would allow the durable treatment of a diverse range of genetic disorders but gene delivery to the bone marrow has not been achieved. Here lipid nanoparticles that target and deliver mRNA to 14 unique cells within the bone marrow are presented.
- Xizhen Lian
- Sumanta Chatterjee
- Daniel J. Siegwart
Assessment of heavy oil recovery mechanisms using in-situ synthesized CeO 2 nanoparticles
- Nafiseh Mehrooz
- Reza Gharibshahi
- Saeid Sadeghnejad
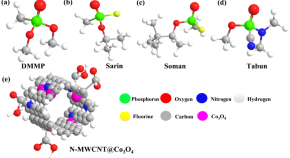
Synthesis and characterization of nitrogen-doped-MWCNT@cobalt oxide for nerve agent simulant detection
- Sanjeeb Lama
- Hyeong-Seon Choi
- Joo Hyung Kim
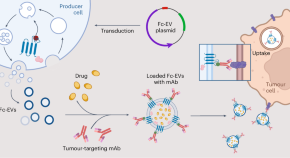
Antibody-displaying extracellular vesicles for targeted cancer therapy
Extracellular vesicles decorated with an antibody-binding moiety specific for the fragment crystallizable domain can be used as a modular delivery system for targeted cancer therapy.
- Oscar P. B. Wiklander
- Doste R. Mamand
- Samir EL Andaloussi
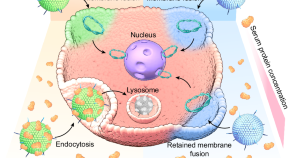
An antifouling membrane-fusogenic liposome for effective intracellular delivery in vivo
Protein corona formation on the surface of liposome nanocarriers can decrease the efficiency of intracellular delivery. Here, the authors develop antifouling membrane-fusogenic liposomes for effective intracellular delivery in vivo.
- Huimin Kong
- Chunxiong Zheng
- Mingqiang Li
News and Comment

Efficient reduction method enables access to rare-earth telluride clusters
Rare-earth telluride clusters enable the construction of highly crystalline rare-earth tellurides, but a general route for preparing such clusters is lacking. Now, a facile reduction approach produces rare-earth clusters supported by (poly)tellurido ligands, including a tri-tellurido ligand with a three-center, four-electron bonding structure.
- Chenyu Wang
Leveraging next-generation materials for cancer neuroscience therapies in the central nervous system
Interdisciplinary strategies bridging oncology, neuroscience, bioelectronics and materials science will facilitate the development of next-generation therapies and devices for cancers of the central nervous system.
- Joshua D. Bernstock
- Benjamin R. Johnston
- Shriya S. Srinivasan
Nanoparticle fix opens up tricky technique to forensic applications
A technique called surface-enhanced Raman spectroscopy can detect tiny quantities of compounds in solution, but has been difficult to use for quantitative analysis. A digital approach involving nanoparticles suggests a way forward.
- Peter J. Vikesland
Reducing greenhouse gas emissions with nanofertilizers
A growing population, climate change and the environmental impacts of conventional agricultural practices are worsening food insecurity. New research shows that use of nanofertilizers can increase food production while simultaneously decreasing the negative environmental effects of agriculture.
- Christian O. Dimkpa
- Christy L. Haynes
- Jason C. White
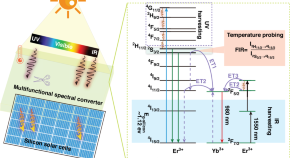
Multifunctional photon conversion materials for enhancing silicon solar cells
- Yiyan Zhang
- Guanying Chen
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Magnetic Nanoparticles for Magnetic Particle Imaging (MPI): Design and Applications
Recent advancements in medical imaging have brought forth various techniques such as magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and ultrasound, each contributing to improved diagnostic capabilities. Most recently, magnetic particle imaging (MPI) has become a rapidly advancing imaging modality with profound implications for medical diagnostics and therapeutics. By directly detecting the magnetization response of these magnetic tracers, MPI surpasses conventional imaging modalities in sensitivity and quantifiability, particularly in stem cell tracking applications. Herein, this comprehensive review explores the fundamental principles, instrumentation, magnetic nanoparticle tracer design, and applications of MPI, offering insights into recent advancements and future directions. Novel tracer designs, such as zinc-doped iron oxide nanoparticles (Zn-IONPs), exhibit enhanced performance, broadening MPI’s utility. Spatial encoding strategies, scanning trajectories, and instrumentation innovations are elucidated, illuminating the technical underpinnings of MPI’s evolution. Moreover, integrating machine learning and deep learning methods enhances MPI’s image processing capabilities, paving the way for more efficient segmentation, quantification, and reconstruction. The potential of superferromagnetic iron oxide nanoparticle chains (SFMIOs) as new MPI tracers further advanced the imaging quality and expanded clinical applications, underscoring the promising future of this emerging imaging modality.
- This article is part of the themed collection: Recent Review Articles
Article information
Download citation, permissions.
B. Rezaei, Z. Tay, S. Mostufa, O. N. Manzari, E. Azizi, S. Ciannella, H. Moni, C. Li, M. Zeng, J. Gomez-Pastora and K. Wu, Nanoscale , 2024, Accepted Manuscript , DOI: 10.1039/D4NR01195C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
REVIEW article
A review on nanoparticles: characteristics, synthesis, applications, and challenges.

- Department of Biology, College of Science, University of Hafr Al Batin, Hafr Al-Batin, Saudi Arabia
The significance of nanoparticles (NPs) in technological advancements is due to their adaptable characteristics and enhanced performance over their parent material. They are frequently synthesized by reducing metal ions into uncharged nanoparticles using hazardous reducing agents. However, there have been several initiatives in recent years to create green technology that uses natural resources instead of dangerous chemicals to produce nanoparticles. In green synthesis, biological methods are used for the synthesis of NPs because biological methods are eco-friendly, clean, safe, cost-effective, uncomplicated, and highly productive. Numerous biological organisms, such as bacteria, actinomycetes, fungi, algae, yeast, and plants, are used for the green synthesis of NPs. Additionally, this paper will discuss nanoparticles, including their types, traits, synthesis methods, applications, and prospects.
1. Introduction
Nanotechnology evolved as the achievement of science in the 21st century. The synthesis, management, and application of those materials with a size smaller than 100 nm fall under the interdisciplinary umbrella of this field. Nanoparticles have significant applications in different sectors such as the environment, agriculture, food, biotechnology, biomedical, medicines, etc. like; for treatment of waste water ( Zahra et al., 2020 ), environment monitoring ( Rassaei et al., 2011 ), as a functional food additives ( Chen et al., 2023 ), and as a antimicrobial agents ( Islam et al., 2022 ). Cutting-edge properties of NPs such as; nature, biocompatibility, anti-inflammatory and antibacterial activity, effective drug delivery, bioactivity, bioavailability, tumor targeting, and bio-absorption have led to a growth in the biotechnological, and applied microbiological applications of NPs.
A particle of matter with a diameter of one to one hundred nanometers (nm) is commonly referred to as a nanoparticle or ultrafine particle. Nanoparticles frequently exhibit distinctive size-dependent features, mostly due to their tiny size and colossal surface area. The periodic boundary conditions of the crystalline particle are destroyed when the size of a particle approaches the nano-scale with the characteristic length scale close to or smaller than the de Broglie wavelength or the wavelength of light ( Guo et al., 2013 ). Because of this, many of the physical characteristics of nanoparticles differ significantly from those of bulk materials, leading to a wide range of their novel uses ( Hasan, 2015 ).
2. Emergence of nanotechnology
Nanotechnology emerged in the 1980s due to the convergence of experimental advances such as the invention of the scanning tunneling microscope in 1981 and the discovery of fullerenes in 1985 ( Bayda et al., 2019 ), with the elucidation. The popularization of a conceptual framework for nanotechnology goals began with the publication of the book Engines of Creation in 1986 ( Bayda et al., 2019 ).
2.1. Early stage of NPs
Carbon nanotubes have been discovered in pottery from Keeladi, India, dating from around 600–300 BC ( Bayda et al., 2019 ; Kokarneswaran et al., 2020 ). Cementite nanowires have been discovered in Damascus steel, a material that dates back to around 900 AD; nevertheless, its origin and creation method are unclear ( Kokarneswaran et al., 2020 ). However, it is unknown how they developed or whether the material containing them was used on purpose.

2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles
Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 ( Chen et al., 2021 ). Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms. They are bent one way and joined to produce a hollow cylindrical cylinder. Carbon nanotubes are carbon allotropes that fall between Fullerene (0 dimensional) and Grapheme (2 dimensional) ( Chen et al., 2021 ).
In addition, M. C. Lea reported that the synthesis of citrate-stabilized silver colloid almost 120 years ago ( Nowack et al., 2011 ). This process produces particles with an average diameter of 7 to 9 nm. Nanoscale size and citrate stabilization are analogous to recent findings on nanosilver production employing silver nitrate and citrate ( Majeed Khan et al., 2011 ). The use of proteins to stabilize nanosilver has also been documented as early as 1902 ( Nowack et al., 2011 ; Beyene et al., 2017 ). Since 1897, a nanosilver known as “Collargol” has been made commercially and used for medicinal purposes ( Nowack et al., 2011 ). Collargol, a type of silver nanoparticle, has a particle size of about 10 nanometers (nm). This was determined as early as 1907, and it was found that the diameter of Collargol falls within the nanoscale range. In 1953, Moudry developed a different type of silver nanoparticle called gelatin-stabilized silver nanoparticles, with a diameter ranging from 2–20 nm. These nanoparticles were produced using another method than Collargol. The necessity of nanoscale silver was recognized by the creators of nanosilver formulations decades ago, as seen by the following remark from a patent: “for optimal efficiency, the silver must be disseminated as particles of colloidal size less than 25 nm in crystallite size”( Nowack et al., 2011 ).
Gold NPs (AuNPs) have a long history in chemistry, going back to the Roman era when they were used to decorate glassware by staining them. With the work of Michael Faraday, who may have been the first to notice that colloidal gold solutions have characteristics different from bulk gold, the contemporary age of AuNP synthesis began more than 170 years ago. Michael Faraday investigated the making and factors of colloidal suspensions of “Ruby” gold in 1857. They are among the magnetic nanoparticles due to their distinctive optical and electrical characteristics. Under specific illumination circumstances, Faraday showed how gold nanoparticles might create solutions of various colors ( Bayda et al., 2019 ; Giljohann et al., 2020 ).
3. Classification of NPs
Nanoparticles (NPs) are categorized into the following classes based on their shape, size, and chemical characteristics
3.1. Carbon-based NPs
Fullerenes and carbon nanotubes (CNTs) are the two essential sub-categories of carbon-based NPs. NPs of globular hollow cages, like allotropic forms of carbon, are found in fullerenes. Due to their electrical conductivity, high strength, structure, electron affinity, and adaptability, they have sparked significant economic interest. These materials have organized pentagonal and hexagonal carbon units, each of which is sp2 hybridized. While CNTs are elongated and form 1–2 nm diameter tubular structures. These fundamentally resemble graphite sheets rolling on top of one another. Accordingly, they are referred to as single-walled (SWNTs), double-walled (DWNTs), or multi-walled carbon nanotubes (MWNTs) depending on how many walls are present in the rolled sheets ( Elliott et al., 2013 ; Astefanei et al., 2015 ).
3.2. Metal NPs
Metal NPs are purely made of metals. These NPs have distinctive electrical properties due to well-known localized surface Plasmon resonance (LSPR) features. Cu, Ag, and Au nanoparticles exhibit a broad absorption band in the visible region of the solar electromagnetic spectrum. Metal NPs are used in several scientific fields because of their enhanced features like facet, size, and shape-controlled synthesis of metal NPs ( Khan et al., 2019 ).
3.3. Ceramics NPs
Ceramic NPs are tiny particles made up of inorganic, non-metallic materials that are heat-treated and cooled in a specific way to give particular properties. They can come in various shapes, including amorphous, polycrystalline, dense, porous, and hollow, and they are known for heat resistance and durable properties. Ceramic NPs are used in various applications, including coating, catalysts, and batteries ( Sigmund et al., 2006 ).
3.4. Lipid-based NPs
These NPs are helpful in several biological applications because they include lipid moieties. Lipid NPs typically have a diameter of 10–1,000 nm and are spherical. Lipid NPs, i.e., polymeric NPs, have a solid lipid core and a matrix consisting of soluble lipophilic molecules ( Khan et al., 2019 ).
3.5. Semiconductor NPs
Semiconductor NPs have qualities similar to metals and non-metals. That is why Semiconductor NPs have unique physical and chemical properties that make them useful for various applications. For example, semiconductor NPs can absorb and emit light and can be used to make more efficient solar cells or brighter light-emitting diodes (LEDs). They can make smaller and faster electronic devices, such as transistors, and can be used in bio imaging and cancer therapy ( Biju et al., 2008 ).
3.6. Polymeric NPs
Polymeric NPs with a size between 1 and 1,000 nm can have active substances surface-adsorbed onto the polymeric core or entrapped inside the polymeric body. These NPs are often organic, and the term polymer nanoparticle (PNP) is commonly used in the literature to refer to them. They resemble Nano spheres or Nano capsules for the most part ( Khan et al., 2019 ; Zielińska et al., 2020 ).
4. Types of different metal-based NPs
Metal NPs are purely made of metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these NPs possess unique optoelectrical properties. NPs of the alkali and noble metals, i.e., Cu, Ag, and Au, have a broad absorption band in the visible zone of the solar electromagnetic spectrum. The facet, size, and shape-controlled synthesis of metal NPs are essential in present-day cutting-edge materials ( Dreaden et al., 2012 ; Khan et al., 2019 ).
4.1. Silver nanoparticles (AgNPs)
AgNPs are particles with a size range of 1–100 nanometers made of silver. They have unique physical and chemical properties due to their small size, high surface area-to-volume ratio, and ability to absorb and scatter light in the visible and near-infrared range. Because of their relatively small size and high surface-to-volume ratios, which cause chemical and physical differences in their properties compared to their bulk counterparts, silver nanoparticles may exhibit additional antimicrobial capabilities not exerted by ionic silver ( Shenashen et al., 2014 ).
Besides, AgNPs can be created in various sizes and forms depending on the manufacturing process, the most common of which is chemical reduction. The AgNPs were created by chemically reducing a 12 mM AgNO3 aqueous solution. The reaction was carried out in an argon environment using 70 mL of this solution containing PVP (keeping the molar ratio of the repeating unit of PVP and Ag equal to 34) and 21 mL of Aloe Vera. The mixture was agitated in ultrasonic for 45 min at ambient temperature, then heated 2°C/min to 80°C and left for 2 h to generate a transparent solution with tiny suspended particles that must be removed by simple filtering ( Shenashen et al., 2014 ; Gloria et al., 2017 ).
4.2. Zinc nanoparticles (ZnONPs)
Zinc nanoparticles (ZnONPs) are particles with a size range of 1–100 nm made of zinc. Zinc oxide (ZnO) NPs are a wide band gap semiconductor with a room temperature energy gap of 3.37 eV. Its catalytic, electrical, optoelectronic, and photochemical capabilities have made it widely worthwhile ( Kumar S.S. et al., 2013 ). ZnO nanostructures are ideal for catalytic reaction processes ( Chen and Tang, 2007 ). Laser ablation, hydrothermal methods, electrochemical depositions, sol-gel method, chemical vapor deposition, thermal decomposition, combustion methods, ultrasound, microwave-assisted combustion method, two-step mechanochemical-thermal synthesis, anodization, co-precipitation, electrophoretic deposition, and precipitation processes are some methods for producing ZnO nanoparticles ( Madathil et al., 2007 ; Moghaddam et al., 2009 ; Ghorbani et al., 2015 ).
4.3. Copper nanoparticles (CuNPs)
Copper nanoparticles (CuNPs) comprise a size range of 1–100 nm of copper-based particles ( Khan et al., 2019 ). Cu and Au metal fluorescence have long been known to exist. For excitation at 488 nm, a fluorescence peak centering on the metals’ interband absorption edge has been noted. Additionally, it was noted that the fluorescence peaked at the same energy at two distinct excitation wavelengths (457.9–514.5 and 300–400 nm), and the high-energy tail somewhat grows with increased photon energy pumping. A unique, physical, top-down EEW approach has been used to create Cu nanoparticles. The EEW method involves sending a current of *1,010 A/m2 (1,010 A/m2) across a thin Cu wire, which explodes on a Cu plate for a duration of 10–6 s ( Siwach and Sen, 2008 ).
4.4. Gold nanoparticles (AuNPs)
Gold nanoparticles(AuNPs) are nanometers made of gold. They have unique physical and chemical properties and can absorb and scatter light in the visible and near-infrared range ( Rad et al., 2011 ; Compostella et al., 2017 ).
Scientists around the turn of the 20th century discovered anisotropic AuNPs. Zsigmond ( Li et al., 2014 ) said that gold particles “are not always spherical when their size is 40 nm or lower” in his book, released in 1909. Additionally, he found anisotropic gold particles of various colors. Zsigmondy won the Nobel Prize in 1925 for “his demonstration of the heterogeneous character of colloidal solutions and the methods he utilized” and for developing the ultramicroscope, which allowed him to see the forms of Au particles. He noticed that gold frequently crystallized into a six-sided leaf shape ( Li et al., 2014 ).
AuNPs are the topic of extensive investigation due to their optical, electrical, and molecular-recognition capabilities, with numerous prospective or promised uses in a wide range of fields, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine ( Rad et al., 2011 ).
4.5. Aluminum nanoparticles (AlNPs)
Aluminum nanoparticles (AlNPs) are nanoparticles made of aluminum. Aluminum nanoparticles’ strong reactivity makes them promising for application in high-energy compositions, hydrogen generation in water processes, and the synthesis of alumina 2D and 3D structures ( Lerner et al., 2016 ).
4.6. Iron nanoparticles (FeNPs)
Iron nanoparticles(FeNPs) are particles with a size range of 1−100 nanometers ( Khan et al., 2019 ) made of iron. FeNPs have several potential applications, including their use as catalysts, drug delivery systems, sensors, and energy storage and conversion. They have also been investigated for use in photovoltaic and solar cells and water purification and environmental remediation. FeNPs can also be used in magnetic resonance imaging (MRI) as contrast agents to improve the visibility of tissues and organs. They can also be used in magnetic recording media, such as hard disk drives ( Zhuang and Gentry, 2011 ; Jamkhande et al., 2019 ).
As with any NPs, there are potential health and safety concerns associated with using FeNPs, e.g., FeNPs are used to deliver drugs to specific locations within the body, such as cancer cells and used in MRI, and used to remove contaminants from water ( Farrell et al., 2003 ; Zhuang and Gentry, 2011 ). Tables 1 , 2 show the characteristics of metal-based nanoparticles and the techniques to study their characteristics, respectively.
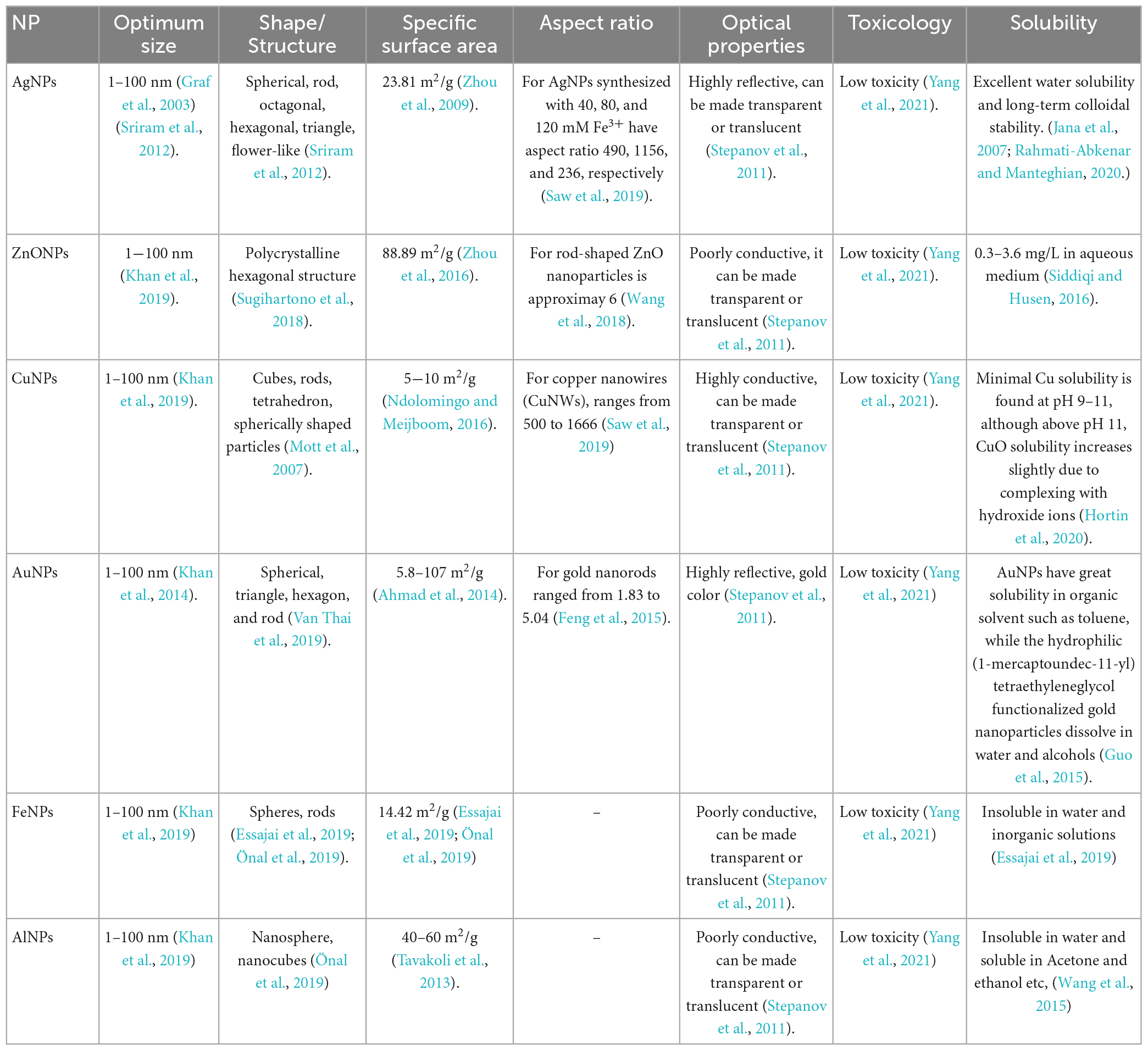
Table 1. Characteristics of metal based nanoparticles.
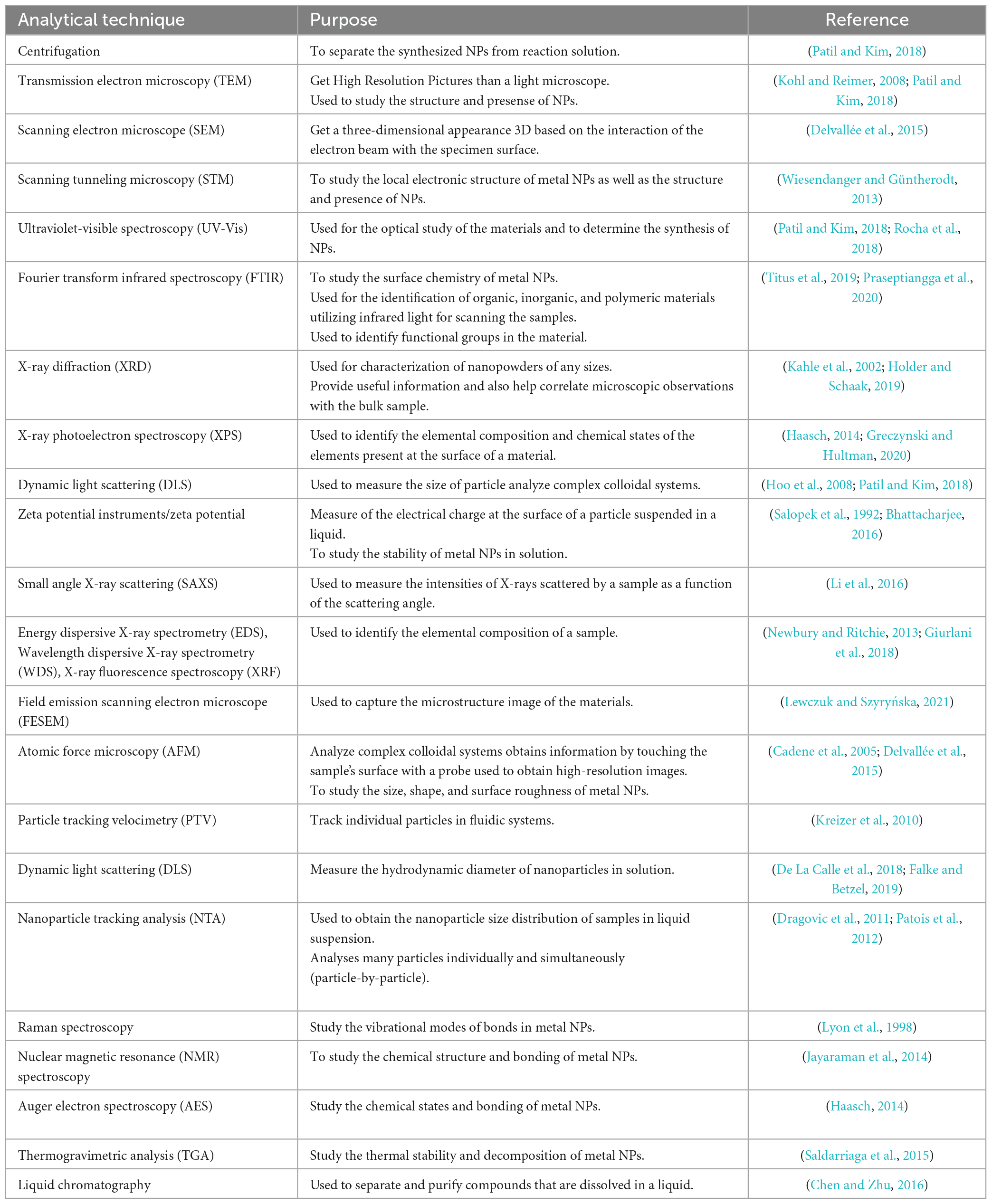
Table 2. Different analytical techniques and their purposes in studying nanoparticles.
5. Approaches for the synthesis of metal NPs
There are mainly three types of approaches for the synthesis of NPs: the physical, chemical, and biological approaches. The physical approach is also called the top-down approach, while chemical and biological approaches are collectively called the bottom-up approach. The biological approach is also named green systems of NPs. All these approaches are further sub-categorized into various types based upon their method adopted. Figure 1 illustrates each approach’s reported methods for synthesizing NPs.
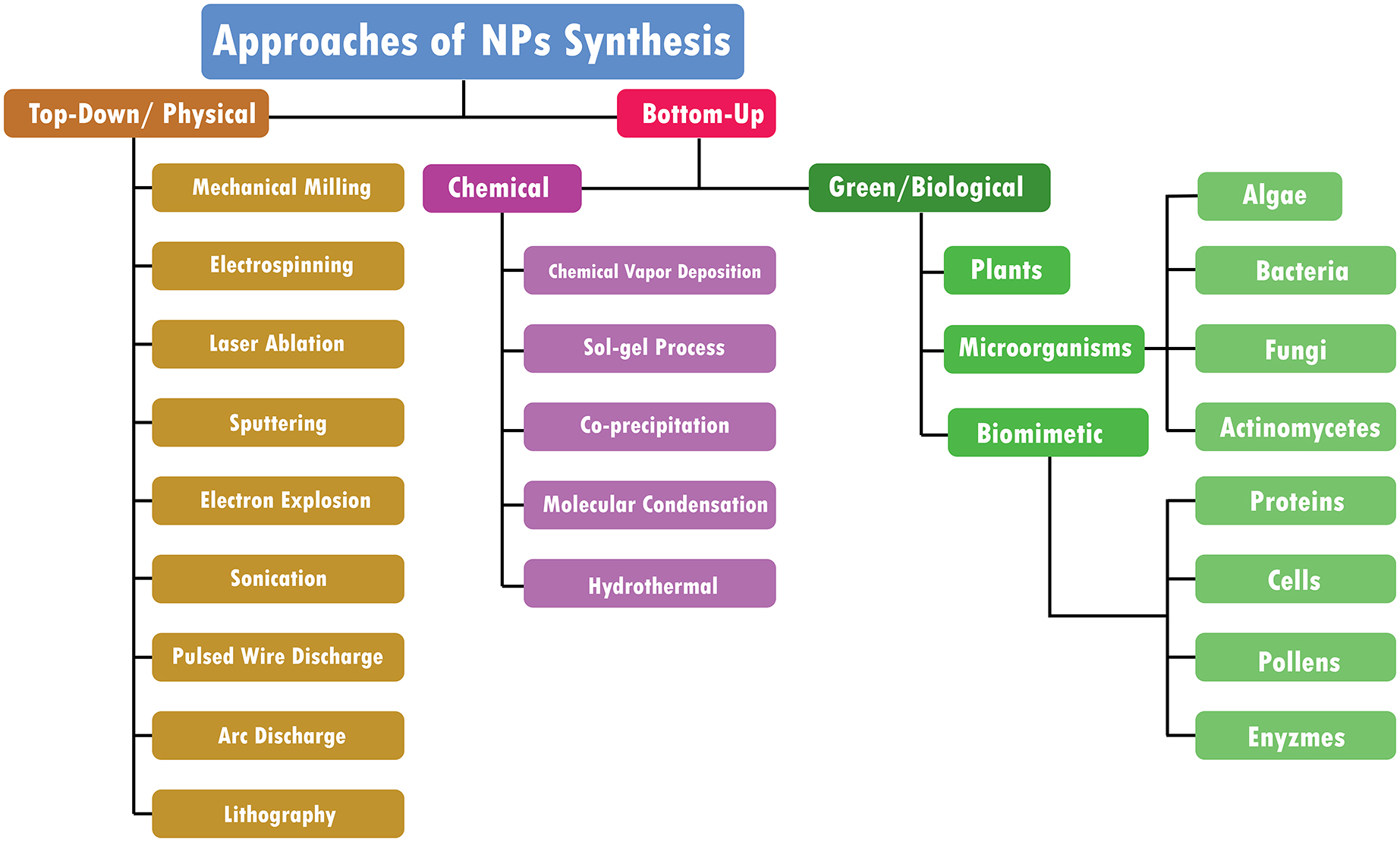
Figure 1. Approaches of NPs synthesis.
5.1. Top down/physical approach
Bulk materials are fragmented in top-down methods to create nano-structured materials ( Figure 2 ). They are additionally known as physical approaches ( Baig et al., 2021 ). The following techniques can achieve a top-down approach
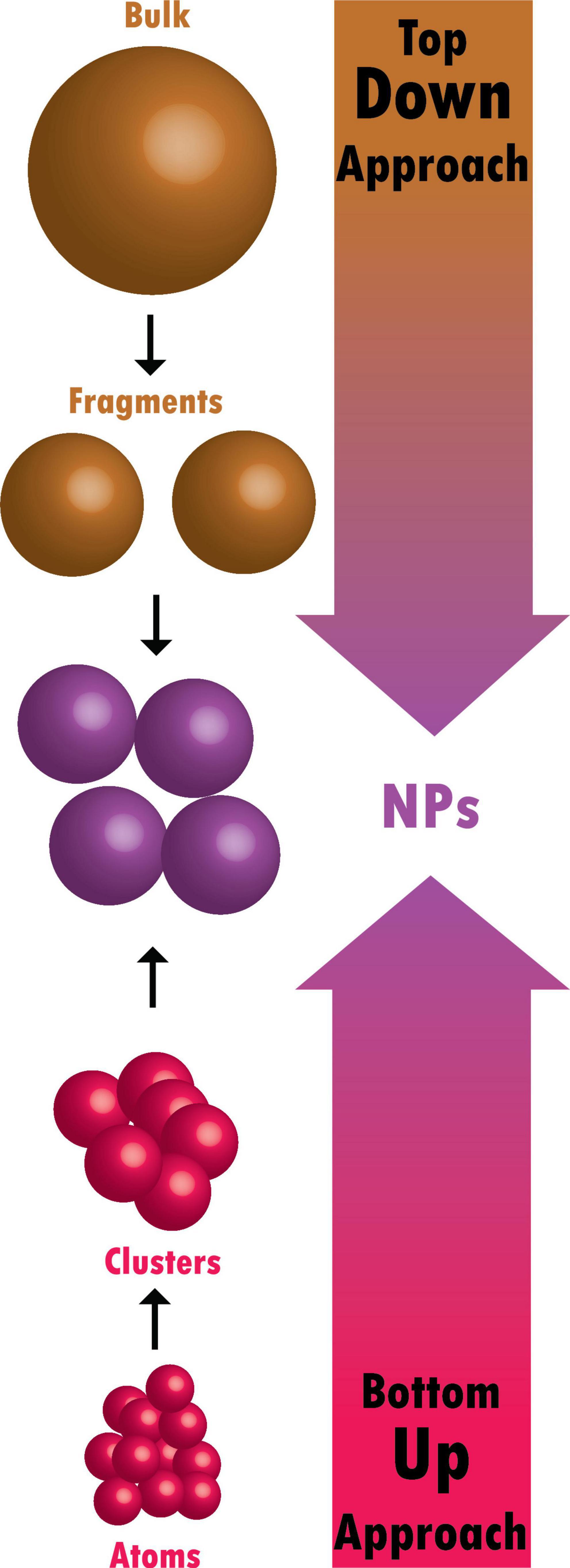
Figure 2. Difference between top-down and bottom-up approaches.
5.1.1. Mechanical milling
The mechanical milling process uses balls inside containers and may be carried out in various mills, typically planetary and shaker mills, which is an impact process with high energy ( Gorrasi and Sorrentino, 2015 ). Mechanical milling is a practical approach for creating materials at the nanoscale from bulk materials. Aluminum alloys that have been strengthened by oxide and carbide, spray coatings that are resistant to wear, nanoalloys based on aluminum, nickel, magnesium, and copper, and a variety of other nanocomposite materials may all be created mechanically. A unique class of nanoparticles known as ball-milled carbon nanomaterials has the potential to meet the needs for energy storage, energy conversion, and environmental remediation ( Yadav et al., 2012 ; Lyu et al., 2017 ).
5.1.2. Electrospinning
Typically, it is used to create nanofibers from various materials, most often polymers ( Ostermann et al., 2011 ). A technique for creating fibers called electrospinning draws charged threads from polymer melts or solutions up to fiber sizes of a few hundred nanometers ( Chronakis, 2010 ). Coaxial electrospinning was a significant advancement in the field of electrospinning. The spinneret in coaxial electrospinning is made up of two coaxial capillaries. Core-shell nanoarchitectures may be created in these capillaries using two viscous liquids, a viscous liquid as the shell and a non-viscous liquid as the core ( Du et al., 2012 ). Core-shell and hollow polymer, inorganic, organic, and hybrid materials have all been developed using this technique ( Kumar R. et al., 2013 ).
5.1.3. Laser ablation
A microfeature can be made by employing a laser beam to vaporize a single material ( Tran and Wen, 2014 ). Laser ablation synthesis produces nanoparticles by striking the target material with an intense laser beam. Due to the high intensity of the laser irradiation used in the laser ablation process, the source material or precursor vaporizes, causing the production of nanoparticles ( Amendola and Meneghetti, 2009 ). Laser ablation is an environmentally friendly for producing noble metal nanoparticles ( Baig et al., 2021 ). This method may be used to create a wide variety of nanomaterials, including metal nanoparticles, carbon nanomaterials, oxide composites, and ceramics ( Su and Chang, 2018 ; Baig et al., 2021 ).
5.1.4. Sputtering
Microparticles of a solid material are expelled off its surface during the phenomenon known as sputtering, which occurs when the solid substance is assaulted by intense plasma or gas particles ( Behrisch, 1981 ). According to the incident gaseous ion energy, energetic gaseous ions used in the sputtering deposition process physically expel tiny atom clusters off the target surface ( Muñoz-García et al., 2009 ). The sputtering method is intriguing because it is more affordable than electron-beam lithography, and the composition of the sputtered nanomaterials is similar to the target material with fewer contaminants ( Baig et al., 2021 ).
5.1.5. Electron explosion
In this technique, a thin metal wire is subjected to a high current pulse that causes an explosion, evaporation, and ionization. The metal becomes vaporized and ionized, expands, and cools by reacting with the nearby gas or liquid medium. The condensed vapor finally forms the nanoparticles ( Joh et al., 2013 ). Electron explosion method because it produces plasma from the electrical explosion of a metallic wire, which may produce nanoparticles from a Pt solution without using a reducing agent ( Joh et al., 2013 ).
5.1.6. Sonication
The most crucial step in the creation of nanofluids is sonication. After the mixture has been magnetically stirred in a magnetic stirrer, sonication is performed in an ultrasonication path, ultrasonic vibrator, and mechanical homogenizer. Sonicators have become the industry standard for Probe sonication and are noticeably more powerful and effective when compared to ultrasonic cleaner baths for nanoparticle applications. Probe sonication is highly effective for processing nanomaterials (carbon nanotubes, graphene, inks, metal oxides, etc.) ( Zheng et al., 2010 ).
5.1.7. Pulsed wire discharge method
This is the most used method for creating metal nanoparticles. A pulsating current causes a metal wire to evaporate, producing a vapor that is subsequently cooled by an ambient gas to form nanoparticles. This plan may quickly produce large amounts of energy ( Patil et al., 2021 ).
5.1.8. Arc discharge method
Two graphite rods are adjusted in a chamber with a constant helium pressure during the Arc Discharge procedure. It is crucial to fill the chamber with helium because oxygen or moisture prevents the synthesis of fullerenes. Arc discharge between the ends of the graphite rods drives the vaporization of carbon rods. Achieving new types of nanoparticles depends significantly on the circumstances in which arc discharge occurs. The creation of several nanostructured materials may be accomplished with this technique ( Berkmans et al., 2014 ). It is well-recognized for creating carbon-based materials such as fullerenes, carbon nanohorns (CNHs), carbon nanotubes ( Shi et al., 2000 ), few-layer graphene, and amorphous spherical carbon nanoparticles ( Kumar R. et al., 2013 ).
5.1.9. Lithography
Lithography typically uses a concentrated beam of light or electrons to create nanoparticles, a helpful technique ( Pimpin and Srituravanich, 2012 ). Masked and maskless lithography are the two primary categories of lithography. Without a mask, arbitrary nano-pattern printing is accomplished in maskless lithography. Additionally, it is affordable and easy to apply ( Brady et al., 2019 ).
5.2. Bottom-up approach
Tiny atoms and molecules are combined in bottom-up methods to create nano-structured particles ( Figure 2 ; Baig et al., 2021 ). These include chemical and biological approaches:
5.2.1. Chemical vapor deposition (CVD)
Through a chemical process involving vapor-phase precursors, a thin coating is created on the substrate surface during CVD ( Dikusar et al., 2009 ). Precursors are deemed appropriate for CVD if they exhibit sufficient volatility, high chemical purity, strong evaporation stability, cheap cost, a non-hazardous nature, and long shelf life. Additionally, its breakdown should not leave behind any contaminants. Vapor phase epitaxy, metal-organic CVD, atomic layer epitaxy, and plasma-enhanced CVD are only a few CVD variations. This method’s benefits include producing very pure nanoparticles that are stiff, homogeneous, and strong ( Ago, 2015 ). CVD is an excellent approach to creating high-quality nanomaterials ( Machac et al., 2020 ). It is also well-known for creating two-dimensional nanoparticles ( Baig et al., 2021 ).
5.2.2. Sol-gel process
A wet-chemical approach, called the sol-gel method, is widely utilized to create nanomaterials ( Das and Srivasatava, 2016 ; Baig et al., 2021 ). Metal alkoxides or metal precursors in solution are condensed, hydrolyzed, and thermally decomposed. The result is a stable solution or sol. The gel gains greater viscosity as a result of hydrolysis or condensation. The particle size may be seen by adjusting the precursor concentration, temperature, and pH levels. It may take a few days for the solvent to be removed, for Ostwald ripening to occur, and for the phase to change during the mature stage, which is necessary to enable the growth of solid mass. To create nanoparticles, the unstable chemical ingredients are separated. The generated material is environmentally friendly and has many additional benefits thanks to the sol-gel technique ( Patil et al., 2021 ). The uniform quality of the material generated, the low processing temperature, and the method’s ease in producing composites and complicated nanostructures are just a few of the sol-gel technique’s many advantages ( Parashar et al., 2020 ).
5.2.3. Co-precipitation
It is a solvent displacement technique and is a wet chemical procedure. Ethanol, acetone, hexane, and non-solvent polymers are examples of solvents. Polymer phases can be either synthetic or natural. By mixing the polymer solution, fast diffusion of the polymer-solvent into the non-solvent phase of the polymer results. Interfacial stress at two phases results in the formation of nanoparticles ( Das and Srivasatava, 2016 ). This method’s natural ability to produce high quantities of water-soluble nanoparticles through a straightforward process is one of its key benefits. This process is used to create many commercial iron oxide NP-based MRI contrast agents, including Feridex, Reservist, and Combidex ( Baig et al., 2021 ; Patil et al., 2021 ).
5.2.4. Inert gas condensation/molecular condensation
Metal NPs are produced using this method in large quantities. Making fine NPs using the inactive gas compression approach has been widespread, which creates NPs by causing a metallic source to disappear in an inert gas. At an attainable temperature, metals evaporate at a tolerable pace. Copper metal nanoparticles are created by vaporizing copper metal inside a container containing argon, helium, or neon. The atom quickly loses its energy by cooling the vaporized atom with an inert gas after it boils out. Liquid nitrogen is used to cool the gases, forming nanoparticles in the range of 2–100 nm ( Pérez-Tijerina et al., 2008 ; Patil et al., 2021 ).
5.2.5. Hydrothermal
In this method, for the production of nanoparticles, hydrothermal synthesis uses a wide temperature range from ambient temperature to extremely high temperatures. Comparing this strategy to physical and biological ones offers several benefits. At higher temperature ranges, the nanomaterials produced by hydrothermal synthesis could become unstable ( Banerjee et al., 2008 ; Patil et al., 2021 ).
5.2.6. Green/biological synthesis
The synthesis of diverse metal nanoparticles utilizing bioactive agents, including plant materials, microbes, and various biowastes like vegetable waste, fruit peel waste, eggshell, agricultural waste, algae, and so on, is known as “green” or “biological” nanoparticle synthesis ( Kumari et al., 2021 ). Developing dependable, sustainable green synthesis technologies is necessary to prevent the formation of undesirable or dangerous byproducts ( Figure 3 ). The green synthesis of nanoparticles also has several advantages, including being straightforward, affordable, producing NPs with high stability, requiring little time, producing non-toxic byproducts, and being readily scaled up for large-scale synthesis ( Malhotra and Alghuthaymi, 2022 ).
Figure 3. Schematic diagram for biosynthesis of NPs.
5.2.6.1. Biological synthesis using microorganisms
Microbes use metal capture, enzymatic reduction, and capping to create nanoparticles. Before being converted to nanoparticles by enzymes, metal ions are initially trapped on the surface or interior of microbial cells ( Ghosh et al., 2021 ). Use of microorganisms (especially marine microbes) for synthesis of metalic NPs is environmental friendly, fast and economical ( Patil and Kim, 2018 ). Several microorganisms are used in the synthesis of metal NPs, including:
Biosynthesis of NPs by bacteria: A possible biofactory for producing gold, silver, and cadmium sulfide nanoparticles is thought to be bacterial cells. It is known that bacteria may produce inorganic compounds either inside or outside of their cells ( Hulkoti and Taranath, 2014 ). Desulforibrio caledoiensis ( Qi et al., 2013 ), Enterococcu s sp. ( Rajeshkumar et al., 2014 ), Escherichia coli VM1 ( Maharani et al., 2016 ), and Ochrobactrum anhtropi ( Thomas et al., 2014 ) based metal NPs are reported previously for their potential photocatalytic properties ( Qi et al., 2013 ), antimicrobial activity ( Rajeshkumar et al., 2014 ), and anticancer activity ( Maharani et al., 2016 ).
Extracellular synthesis of NPs by bacteria: The microorganisms’ extracellular reductase enzymes shrink the silver ions to the nanoscale range. According to protein analysis of microorganisms, the NADH-dependent reductase enzyme carries out the bio-reduction of silver ions to AgNPs. The electrons for the reductase enzyme come from NADH, which is subsequently converted to NAD+. The enzyme is also oxidized simultaneously when silver ions are reduced to nanosilver. It has been noted that bio-reduction can occasionally be caused by nitrate-dependent reductase. The decline occurs within a few minutes in the quick extracellular creation of nanoparticles ( Mathew et al., 2010 ). At pH 7, the bacterium R. capsulata produced gold nanoparticles with sizes ranging from 10−20 nm. Numerous nanoplates and spherical gold nanoparticles were produced when the pH was changed to four ( Sriram et al., 2012 ). By adjusting the pH, the gold nanoparticles’ form may be changed. Gold nanoparticle shape was controlled by regulating the proton content at various pH levels. The bacteria R. capsulata ’s release cofactor NADH and NADH-dependent enzymes may cause the bioreduction of Au (3+) to Au (0) and the generation of gold nanoparticles. By using NADH-dependent reductase as an electron carrier, it is possible to start the reduction of gold ions ( Sriram et al., 2012 ).
Intracellular synthesis of NPs by bacteria: Three processes are involved in the intracellular creation of NPs: trapping, bioreduction, and capping. The cell walls of microorganisms and ions charge contribute significantly to creating NPs in the intracellular route. This entails specific ion transit in the presence of enzymes, coenzymes, and other molecules in the microbial cell. Microbes have a range of polysaccharides and proteins in their cell walls, which function as active sites for the binding of metal ions ( Slavin et al., 2017 ). Not all bacteria can produce metal and metal oxide nanoparticles. The only ions that pose a significant hazard to microorganisms are heavy metal ions, which, in response to a threat, cause the germs to react by grabbing or trapping the ions on the cell wall via electrostatic interactions. This occurs because a metal ion is drawn to the cell wall’s carboxylate groups, including cysteine and polypeptides, and certain enzymes with a negative charge ( Zhang et al., 2011 ).
Additionally, the electron transfers from NADH via NADH-dependent educates, which serves as an electron carrier and is located inside the plasma membrane, causing the trapped ions to be reduced into the elemental atom. The nuclei eventually develop into NPs and build up in the cytoplasm or the pre-plasmic space. On the other hand, the stability of NPs is provided by proteins, peptides, and amino acids found inside cells, including cysteine, tyrosine, and tryptophan ( Mohd Yusof et al., 2019 ).
Biosynthesis of NPs by fungi: Because monodisperse nanoparticles with distinct dimensions, various chemical compositions, and sizes may be produced, the biosynthesis of nanoparticles utilizing fungus is frequently employed. Due to the existence of several enzymes in their cells and the ease of handling, fungi are thought to be great candidates for producing metal and metal sulfide nanoparticles ( Mohanpuria et al., 2008 ).
The nanoparticles were created on the surface of the mycelia. After analyzing the results and noting the solution, it was determined that the Ag + ions are initially trapped on the surface of the fungal cells by an electrostatic interaction between gold ions and negatively charged carboxylate groups, which is facilitated by enzymes that are present in the mycelia’s cell wall. Later, the enzymes in the cell wall reduce the silver ions, causing the development of silver nuclei. These nuclei then increase as more Ag ions are reduced and accumulate on them.
The TEM data demonstrate the presence of some silver nanoparticles both on and inside the cytoplasmic membrane. The findings concluded that the Ag ions that permeate through the cell wall were decreased by enzymes found inside the cytoplasm and on the cytoplasmic membrane. Also possible is the diffusion of some silver nanoparticles over the cell wall and eventual cytoplasmic entrapment ( Mukherjee et al., 2001 ; Hulkoti and Taranath, 2014 ).
It was observed that the culture’s age does not affect the shape of the synthesized gold nanoparticles. However, the number of particles decreased when older cells were used. The different pH levels produce a variety of shapes of gold nanoparticles, indicating that pH plays a vital role in determining the shape. The incubation temperature also played an essential role in the accumulation of the gold nanoparticles. It was observed that the particle growth rate was faster at increased temperature levels ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). The form of the produced gold nanoparticles was shown to be unaffected by the age of the culture. However, when older cells were utilized, the particle count fell. The fact that gold nanoparticles take on various forms at different pH levels suggests that the pH is crucial in determining the shape. The incubation temperature significantly influenced the accumulation of the gold nanoparticles. It was found that higher temperatures caused the particle development rate to accelerate ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). Verticillium luteoalbum is reported to synthesize gold nanoparticles of 20–40 nm in size ( Erasmus et al., 2014 ). Aspergillus terreus and Penicillium brevicompactum KCCM 60390 based metal NPs are reported for their antimicrobial ( Li G. et al., 2011 ) and cytotoxic activities ( Mishra et al., 2011 ), respectively.
Biosynthesis of NPs using actinomycetes: Actinomycetes have been categorized as prokaryotes since they share significant traits with fungi. They are sometimes referred to as ray fungi ( Mathew et al., 2010 ). Making NPs from actinomycetes is the same as that of fungi ( Sowani et al., 2016 ). Thermomonospora sp., a new species of extremophilic actinomycete, was discovered to produce extracellular, monodispersed, spherical gold nanoparticles with an average size of 8 nm ( Narayanan and Sakthivel, 2010 ). Metal NPs synthesized by Rhodococcus sp. ( Ahmad et al., 2003 ) and Streptomyces sp. Al-Dhabi-87 ( Al-Dhabi et al., 2018 ) are reported for their antimicrobial activities.
Biosynthesis of NPs using algae: Algae have a high concentration of polymeric molecules, and by reducing them, they may hyper-accumulate heavy metal ions and transform them into malleable forms. Algal extracts typically contain pigments, carbohydrates, proteins, minerals, polyunsaturated fatty acids, and other bioactive compounds like antioxidants that are used as stabilizing/capping and reducing agents ( Khanna et al., 2019 ). NPs also have a faster rate of photosynthesis than their biosynthetic counterparts. Live or dead algae are used as model organisms for the environmentally friendly manufacturing process of bio-nanomaterials, such as metallic NPs ( Hasan, 2015 ). Ag and Au are the most extensively researched noble metals to synthesized NPs by algae either intracellularly or extracellularly ( Dahoumane et al., 2017 ). Chlorella vulgaris ( Luangpipat et al., 2011 ), Chlorella pyrenoidosa ( Eroglu et al., 2013 ), Nanochloropsis oculata ( Xia et al., 2013 ), Scenedesmus sp. IMMTCC-25 ( Jena et al., 2014 ) based metal NPs are reported for their potential catalytic ( Luangpipat et al., 2011 ; Eroglu et al., 2013 ) and, antimicrobial ( Eroglu et al., 2013 ; Jena et al., 2014 ) activities along with their use in Li-Ion batteries ( Xia et al., 2013 ).
Intracellular synthesis of NPs using algae: In order to create intracellular NPs, algal biomass must first be gathered and thoroughly cleaned with distilled water. After that, the biomass (living algae) is treated with metallic solutions like AgNO3. The combination is then incubated at a specified pH and a specific temperature for a predetermined time. Finally, it is centrifuged and sonicated to produce the extracted stable NPs ( Uzair et al., 2020 ).
Extracellular synthesis of NPs using algae: Algal biomass is first collected and cleaned with distilled water before being used to synthesize NPs extracellularly ( Uzair et al., 2020 ). The following three techniques are frequently utilized for the subsequent procedure:
(i) A particular amount of time is spent drying the algal biomass (dead algae), after which the dried powder is treated with distilled water and filtered.
(ii) The algal biomass is sonicated with distilled water to get a cell-free extract.
(iii) The resultant product is filtered after the algal biomass has been rinsed with distilled water and incubated for a few hours (8–16 h).
5.2.6.2. Biological synthesis using plant extracts
The substance or active ingredient of the desired quality extracted from plant tissue by treatment for a particular purpose is a plant extract ( Jadoun et al., 2021 ). Plant extracts are combined with a metal salt solution at room temperature to create nanoparticles. Within minutes, the response is finished. This method has been used to create nanoparticles of silver, gold, and many other metals ( Li X. et al., 2011 ). Nanoparticles are biosynthesized using a variety of plants. It is known that the kind of plant extract, its concentration, the concentration of the metal salt, the pH, temperature, and the length of contact time all have an impact on how quickly nanoparticles are produced as well as their number and other properties ( Mittal and Chisti, 2013 ). A leaf extract from Polyalthia longifolia was used to create silver nanoparticles, the average particle size was around 58 nm ( Kumar and Yadav, 2009 ; Kumar et al., 2016 ).
Acacia auriculiformis ( Saini et al., 2016 ), Anisomeles indica ( Govindarajan et al., 2016 ), Azadirachta indica ( Velusamy et al., 2015 ), Bergenia ciliate ( Phull et al., 2016 ), Clitoria ternatea , Solanum nigrum ( Krithiga et al., 2013 ), Coffea arabica ( Dhand et al., 2016 ), Coleus forskohlii ( Naraginti et al., 2016 ), Curculigo orchioides ( Kayalvizhi et al., 2016 ), Digitaria radicosa ( Kalaiyarasu et al., 2016 ), Dioscorea alata ( Pugazhendhi et al., 2016 ), Diospyros paniculata ( Rao et al., 2016 ), Elephantopus scaber ( Kharat and Mendhulkar, 2016 ), Emblica officinalis ( Ramesh et al., 2015 ), Euphorbia antiquorum L. ( Rajkuberan et al., 2017 ), Ficus benghalensis ( Nayak et al., 2016 ), Lantana camara ( Ajitha et al., 2015 ), Cinnamomum zeylanicum ( Soni and Sonam, 2014 ), and Parkia roxburghii ( Paul et al., 2016 ) are the few examples of plants which are reported for the green synthesis of metal NPs (i.e., AgNPs). These were evaluated for their antifilaria activity ( Saini et al., 2016 ), mosquitocidal activity ( Govindarajan et al., 2016 ), antibacterial activity ( Velusamy et al., 2015 ), catalytic activity ( Edison et al., 2016 ), antioxidant activity ( Phull et al., 2016 ), and Cytotoxicity ( Patil et al., 2017 ).
5.2.6.3. Biological synthesis using biomimetic
“Biomimetic synthesis” typically refers to chemical processes that resemble biological synthesis carried out by living things ( Dahoumane et al., 2017 ). In the biomimetic approach, proteins, enzymes, cells, viruses, pollen, and waste biomass are used to synthesize NPs. Two categories are used to classify biomimetic synthesis:
Functional biomimetic synthesis uses various materials and approaches to emulate particular characteristics of natural materials, structures, and systems ( Zan and Wu, 2016 ).
Process biomimetic synthesis is a technique that aims to create different desirable nanomaterials/structures by imitating the synthesis pathways, processes, or procedures of natural chemicals and materials/structures. For instance, several distinctive nano-superstructures (such as satellite structures, dendrimer-like structures, pyramids, cubes, 2D nanoparticle arrays, 3D AuNP tubes, etc.) have been put together in vitro by simulating the protein manufacturing process ( Zan and Wu, 2016 ).
6. Applications of NPs
6.1. applications of nps in environment industry.
Due to their tiny size and distinctive physical and chemical characteristics, NPs appeal to various environmental applications. The properties of nanoparticals and their advantages are illustrated in Figure 4 . The following are some possible NP uses in the environment.
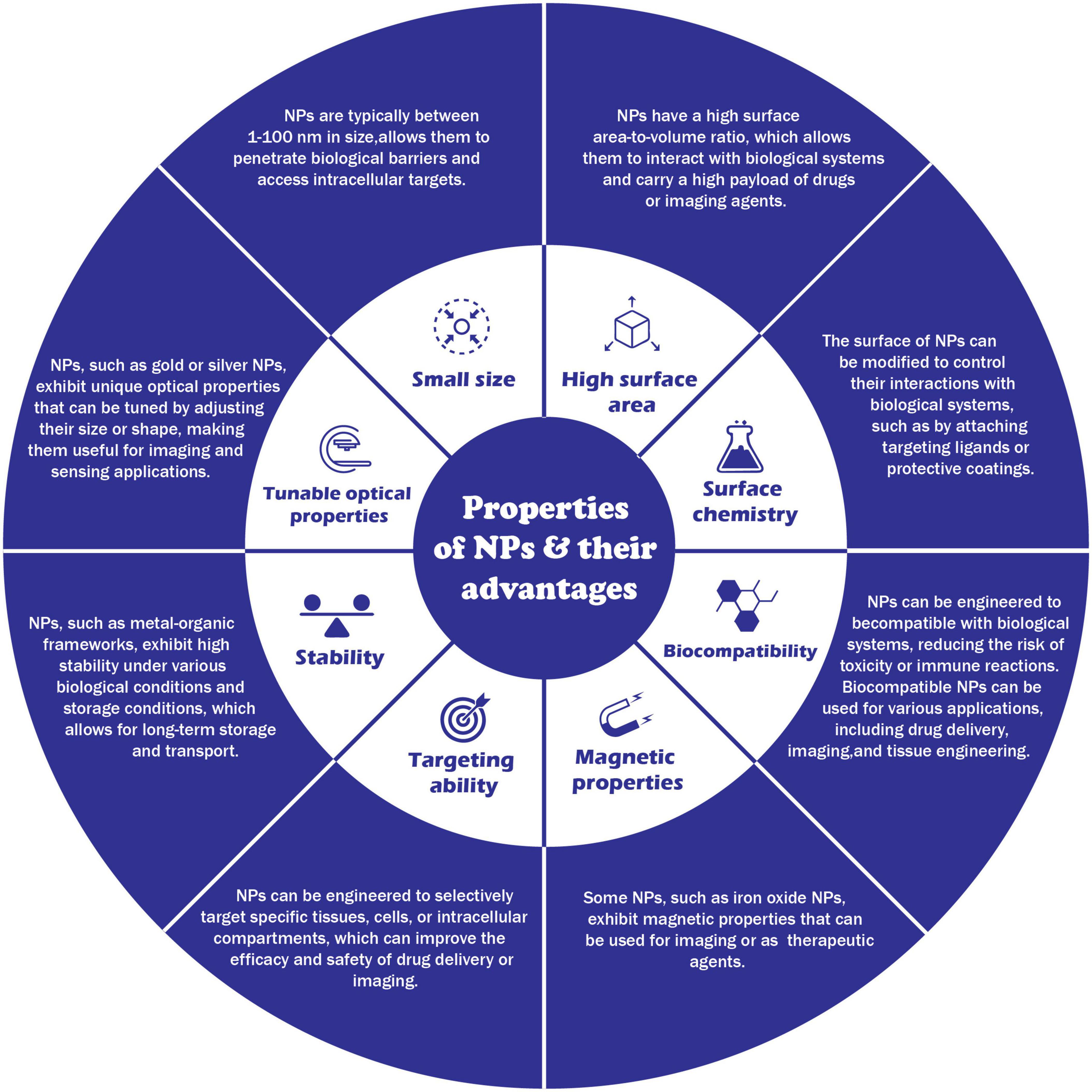
Figure 4. Properties of nanoparticals and their advantages.
6.1.1. Bioremediation
Nanoparticles (NPs) can remove environmental pollutants, such as heavy metals from water or organic contaminants from soil ( Zhuang and Gentry, 2011 ). For example, silver nanoparticles (AgNPs) effectively degrade certain pollutants, such as organic dyes and compounds found in wastewater. Several nanomaterials have been considered for remediation purposes, such as nanoscale zeolites, metal oxides, and carbon nanotubes and fibers ( Zhuang and Gentry, 2011 ). Nanoscale particles used in remediation can access areas that larger particles cannot. They can be coated to facilitate transport and prevent reaction with surrounding soil matrices before reacting with contaminants. One widely used nanomaterial for remediation is Nanoscale zerovalent iron (nZVI). It has been used at several hazardous waste sites to clean up chlorinated solvents that have contaminated groundwater ( Elliott et al., 2013 ). Removing heavy metals such as mercury, lead, thallium, cadmium, and arsenic from natural water has attracted considerable attention because of their adverse effects on environmental and human health. Superparamagnetic iron oxide NPs are an effective sorbent material for this toxic soft material. So, no measurements of engineered NPs in the environment have been available due to the absence of analytical methods able to quantify the trace concentration of NPs ( Elliott et al., 2013 ).
6.1.2. Sensors in environment
Nanotechnology/NPs are already being used to improve water quality and assist in environmental clean-up activities ( Pradeep, 2009 ). Their potential use as environmental sensors to monitor pollutants is also becoming viable NPs can be used as sensors to detect the presence of certain compounds in the environment, such as heavy metals or pollutants. The nano-sensors small size and wide detection range provide great flexibility in practical applications. It has been reported that nanoscale sensors can be used to detect microbial pathogens and biological compounds, such as toxins, in aqueous environments ( Yadav et al., 2010 ). NPS can be designed to selectively bind to specific types of pollutants, allowing them to be detected at low concentrations. For example, gold nanoparticles (AuNPs) have been used as sensors for the detection of mercury in water ( Theron et al., 2010 ).
6.1.3. Catalysts in environment
Nanoparticles (NPs) are used as catalysts in chemical reactions, such as in the production of biofuels or environmental remediation processes, and to catalyze biomass conversion into fuels, such as ethanol or biodiesel. For example, platinum nanoparticles (PtNPs) have been explored for use in the production of biofuels due to their ability to catalyze the conversion of biomass into fuels ( Lam and Luong, 2014 ). PtNPs also showed promising sensing properties; for example, Using Pt NPs, the Hg ions were quantified in the range of 50–500 nM in MilliQ, tap, and groundwater samples, and the limit of quantifications for Hg ions were 16.9, 26, and 47.3 nM. The biogenic PtNPs-based probe proved to be applicable for detecting and quantifying Hg ions ( Kora and Rastogi, 2018 ).
Overall, NPs have significant potential for use in the environment and are being actively researched for a variety of applications.
6.2. Applications of NPs in medicine industry
Nanoparticles (NPs) have unique physical and chemical properties due to their small size, making them attractive for use in various applications, including the medicine industry. Some potential applications of NPs in medicine include:
6.2.1. Drug delivery
Technological interest has been given to AuNPs due to their unique optical properties, ease of synthesis, and chemical stability. The particles can be used in biomedical applications such as cancer treatment ( Sun et al., 2014 ), biological imaging ( Abdulle and Chow, 2019 ), chemical sensing, and drug delivery. Sun et al. (2014) mentioned in detail about two different methods of controlled release of drugs associated with NPs, which were (1) sustained (i.e., diffusion-controlled and erosion-controlled) and (2) stimuli-responsive (i.e., pH-sensitive, enzyme-sensitive, thermoresponsive, and photosensitive). Figure 5 illustrates that how NPs acts as targeted delivery of medicines to treat cancer cells ( Figure 5A ) and therapeutic gene delivery to synthesis proteins of interests in targeted cells ( Figure 5B ). NPs can deliver drugs to specific body areas, allowing for more targeted and effective treatment ( Siddique and Chow, 2020 ). For example AgNPs have been explored for use in drug delivery due to their stability and ability to accumulate in certain types of cancerous tumors ( Siddique and Chow, 2020 ). ZnONPs have also been explored for drug delivery due to their ability to selectively target cancer cells ( Anjum et al., 2021 ). CuNPs have been shown to have antimicrobial properties and are being explored for drug delivery to treat bacterial infections ( Yuan et al., 2018 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for drug delivery due to their ability to accumulate in certain cancerous tumors. Silver NPs (AgNPs) have been incorporated into wound dressings, bone cement, and implants ( Schröfel et al., 2014 ).
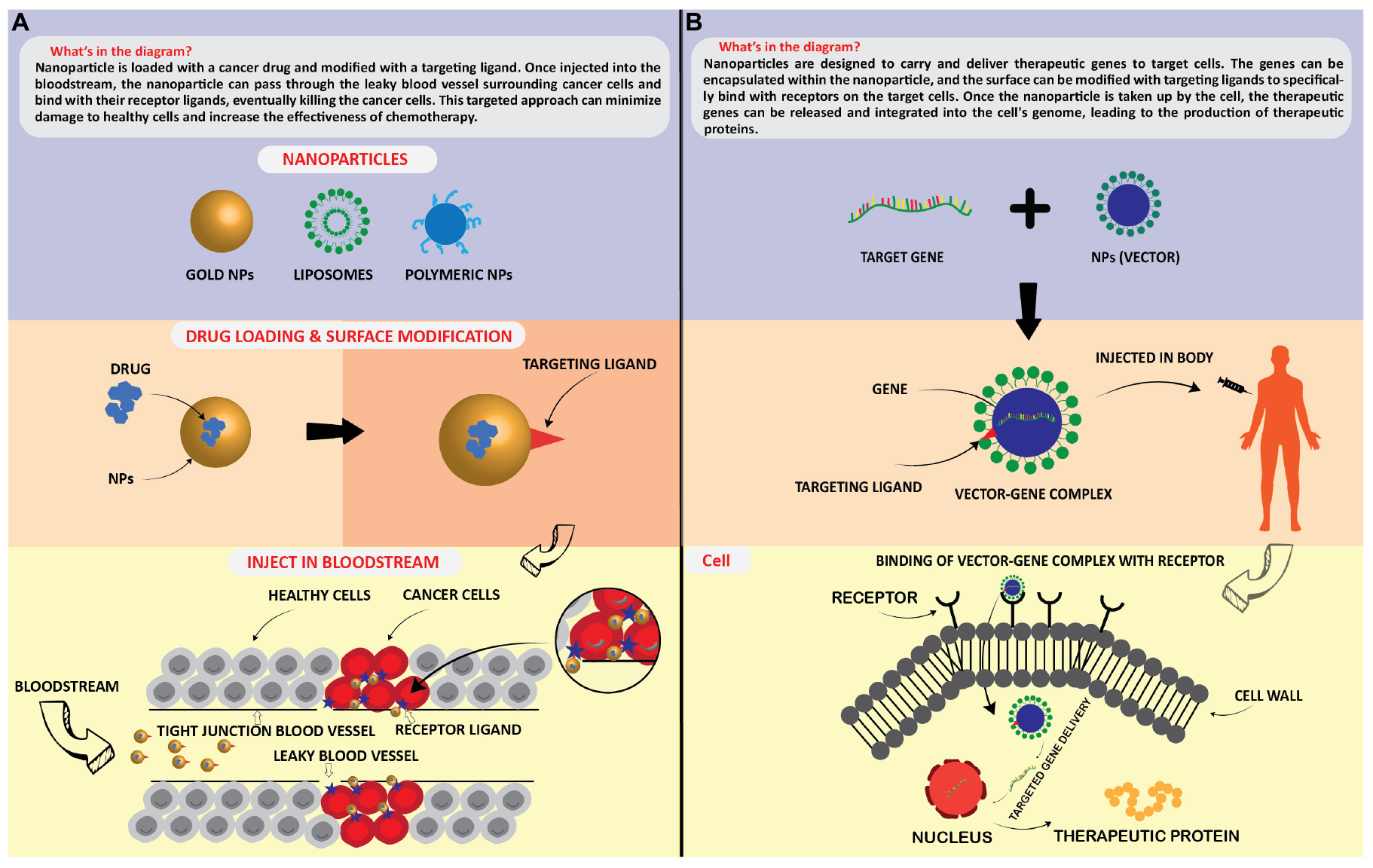
Figure 5. Application of nanoparticles as; targated drug delivery (A) , and therapeutic protein generation in targated cells (B) .
6.2.2. Diagnostics
Nanoparticles (NPs) can be used as imaging agents to help visualize specific body areas. For example, iron oxide nanoparticles (Fe 3 O 4 NPs) have been used as magnetic resonance imaging (MRI) contrast agents to help visualize tissues and organs ( Nguyen et al., 2013 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for diagnostics due to their ability to accumulate in certain cancerous tumors ( Siddique and Chow, 2020 ).
6.2.3. Tissue engineering
Nanoparticles (NPs) can help stimulate the growth and repair of tissues and organs. For example, titanium dioxide nanoparticles (TiO2 NPs) have been explored for tissue engineering due to their ability to stimulate the growth of bone cells ( Kim et al., 2014 ).
6.2.4. Antimicrobials
Some NPs, such as silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs), have strong antimicrobial properties and are being explored for use in a variety of medical products, such as wound dressings and medical devices ( Hoseinzadeh et al., 2017 ).
Overall, NPs have significant potential for use in the medical industry and are being actively researched for various applications. However, it is essential to carefully consider the potential risks and benefits of using NPs in medicine and ensure their safe and responsible use.
6.3. Applications of NPs in agriculture industry
There are several ways in which nanoparticles (NPs) have the potential to alter the agricultural sector. NPs may be used in agriculture for a variety of reasons, including:
6.3.1. Pesticides and herbicides
Nanoparticles (NPs) can be used to deliver pesticides and herbicides in a targeted manner, reducing the number of chemicals needed and minimizing the potential for environmental contamination ( Khan et al., 2019 ). AgNPs and CuNPs have antimicrobial properties, making them potentially useful for controlling pests and diseases in crops. They can also be used as delivery systems for active ingredients, allowing for more targeted application and reducing the potential for environmental contamination ( Hoseinzadeh et al., 2017 ; Dangi and Verma, 2021 ).
It is important to note that using metal NPs in pesticides and herbicides is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment ( Dangi and Verma, 2021 ).
6.3.2. Fertilizers and plant growth
Nano fertilizers offer an opportunity for efficiently improving plant mineral nutrition. Some studies have shown that nanomaterials can be more effective than conventional fertilizers, with a controlled release of nutrients increasing the efficiency of plant uptake and potentially reducing adverse environmental outcomes associated with the loss of nutrients in the broader environment. However, other studies have found that nanomaterial has the same or even less effective effectiveness than conventional fertilizers. NPs used to deliver fertilizers to plants more efficiently, reducing the amount of fertilizer needed, and reducing the risk of nutrient runoff ( Kopittke et al., 2019 ).
Ag ( Jaskulski et al., 2022 ), Zn ( Song and Kim, 2020 ), Cu, Au, Al, and Fe ( Kopittke et al., 2019 ) based NPs have been shown to have fertilizing properties and plant growth-promoting properties, and may help provide essential nutrients to plants and improve plant growth and yield. It is important to note that the use of NPs in fertilizers is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment.
6.3.3. Food safety
Nanoparticles (NPs) can detect and eliminate pathogens in food products, improving food safety, and reducing the risk of foodborne illness ( Zhuang and Gentry, 2011 ).
6.3.4. Water purification
Nanoparticles (NPs) can purify irrigation water, reducing the risk of crop contamination and improving crop yield ( Zhuang and Gentry, 2011 ). Using NPs in agriculture can improve crop yields, reduce agriculture’s environmental impact, and improve food products’ safety and quality.
6.4. Applications of NPs in food industry
Numerous applications for nanoparticles (NPs) in the food sector are possible, including:
6.4.1. Food processing and food preservation/food packaging
Nanoparticles (NPs) can be used to improve the efficiency and performance of food processing operations, such as grinding, mixing, and drying, e.g., AgNPs have been used as a natural antimicrobial agent in food processing operations, helping to prevent the growth of bacteria and other microorganisms ( Dangi and Verma, 2021 ) and also NPs are used to enhance the performance of materials used in food packaging, making them more resistant to pollutants like moisture and gases.
6.4.2. Food fortification
Nanoparticles (NPs) can deliver essential nutrients to food products, such as vitamins and minerals, more efficiently and effectively. e.g., Fe 2 O 3 , and CuNPs have been used to fortify food products with iron, and Cu is an essential nutrient necessary for the metabolism of iron and other nutrients. Iron is an essential nutrient often lacking in many people’s diets, particularly in developing countries ( Kopittke et al., 2019 ).
6.4.3. Sensors
Nanoparticles (NPs) used to improve the sensitivity and specificity of food sensors, allowing them to detect a broader range of substances or signals ( Yadav et al., 2010 ).
Overall, using NPs in the food industry can improve the performance, safety, and nutritional value of a wide range of food products and processes.
6.5. Applications of NPs in electronics industry and automotive industry
In many aspects, nanoparticles (NPs) can transform the electronics sector. NPs may be used in a variety of electrical applications, such as:
6.5.1. Display technologies/storage devices
Nanoparticles (NPs) can be used to improve the performance of displays ( Park and Choi, 2019 ; Bahadur et al., 2021 ; Triana et al., 2022 ), such as LCD and OLED displays, by enhancing the brightness, color, and contrast of the image, such as silver NPs and gold NPs, have been explored for use in LCD and OLED displays as a means of improving the conductivity of the display ( Gwynne, 2020 ). NPs improve the performance and durability of energy storage devices, such as batteries and supercapacitors, by increasing energy density and charging speed. Zinc oxide nanoparticles (ZnO NPs) have the potential to be used in energy storage devices, such as batteries and supercapacitors, due to their ability to store and release energy ( Singh et al., 2011 ).
6.5.2. Data storage
Nanoparticles (NPs) can improve the capacity and speed of data storage devices, such as hard drives and flash drives. Magnetic NPs, such as iron oxide NPs, have been explored for use in data storage devices, such as hard drives, due to their ability to store, and retrieve data using magnetism. These NPs are often composed of a magnetic metal, such as iron, cobalt, or nickel. They can be magnetized and demagnetized, allowing them to store and retrieve data ( Ahmad et al., 2021 ).
Overall, the use of NPs in electronics has the potential to improve the performance and efficiency of a wide range of electronic devices and systems.
Applications of NPs in chemical industry: The chemical industry might be entirely transformed by nanoparticles (NPs) in various ways. The following are potential uses for NPs in the chemical industry ( Salem and Fouda, 2021 ).
6.5.3. Chemical processing/catalysis
Nanoparticles (NPs) can be used as catalysts in chemical reactions, allowing them to be carried out more efficiently and at lower temperatures. Some examples of metal NPs that have been used as catalysts in the chemical industry include: PtNPs have been used as catalysts in a variety of chemical reactions, including fuel cell reactions ( Bhavani et al., 2021 ), hydrogenation reactions, and oxidation reactions ( Lara and Philippot, 2014 ), PdNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions and cross-coupling reactions ( Pérez-Lorenzo, 2012 ), FeNPs have been used as catalysts in a variety of chemical reactions, including hydrolysis reactions ( Jiang and Xu, 2011 ), and oxygen reduction reactions, NiNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions, and hydrolysis reactions ( Salem and Fouda, 2021 ).
6.5.4. Separation and purification
NPs are used to separate and purify chemicals and other substances, such as gases and liquids, by exploiting their size-based properties ( Hollamby et al., 2010 ). Several types of metal nanoparticles (NPs) have been explored for use in separation and purification processes in the chemical industry, including Fe 2 O 3 NPs have been used to separate and purify gases, liquids, and chemicals. They have also been used to remove contaminants from water ( Pradeep, 2009 ; Siddique and Chow, 2020 ). AgNPs have been used to purify water and remove contaminants ( Pradeep, 2009 ), such as bacteria and viruses. They have also been used to remove heavy metals from water and other substances ( Zhuang and Gentry, 2011 ). AuNPs have been used to purify water and remove contaminants, such as bacteria and viruses ( Siddique and Chow, 2020 ). They have also been used to separate and purify gases and liquids ( Zhuang and Gentry, 2011 ). AlNPs have been used to remove contaminants from water and other substances, such as oils and fuels. They have also been used to purify gases ( Zhuang and Gentry, 2011 ).
6.6. Applications of NPs in defense industry
Nanoparticles (NPs) can be used to improve the efficiency and performance of chemical processing operations, such as refining and synthesizing chemicals ( Schröfel et al., 2014 ). Nanoparticles (NPs) have the potential to be used in the defense industry in several ways, including:
6.6.1. Sensors
Nanoparticles (NPs) can improve the sensitivity and specificity of sensors used in defense systems, such as sensors for detecting chemical, biological, or radiological threats ( Zheng et al., 2010 ).
6.6.2. Protective coatings
Nanoparticles (NPs) can improve the performance and durability of protective coatings applied to defense equipment, such as coatings resistant to chemical or biological agents. For example, metal NPs can improve the mechanical properties and durability of the coating, making it more resistant to wear and corrosion. For example, adding Al or Zn based NPs to a polymer coating can improve its corrosion resistance. In contrast, adding Ni or Cr-based NPs can improve their wear resistance ( Rangel-Olivares et al., 2021 ).
6.6.3. Weapons
Nanoparticles (NPs) are used as weapons against viruses, bacteria, etc, ( Ye et al., 2020 ) and as well as in the development of armor and protective materials. There have been some reports of the potential use of NPs in military and defense applications, such as in the development of armor and protective materials. For example, adding nanoparticles, such as ceramic or metal NPs, to polymers or other materials can improve their mechanical properties and make them more resistant to damage. In addition, there have been reports of the use of NPs in developing sensors and detection systems for defense purposes.
6.6.4. Manufacturing
Nanoparticles (NPs) can improve the performance and durability of materials used in defense equipment, such as armor or structural materials. Metal NPs can be used in materials by adding them as a filler or reinforcement in polymers. For example, the addition of metal NPs such as aluminum (Al), copper (Cu), or nickel (Ni) to polymers can improve the mechanical properties, thermal stability, and electrical conductivity of the resulting composite material ( Khan et al., 2019 ).
Metal NPs can also make functional materials, such as catalysts and sensors. For example, metal NPs, such as gold (Au), and platinum (Pt), can be used as catalysts in various chemical reactions due to their high surface area and ability to adsorb reactants ( Zheng et al., 2010 ).
6.6.5. Energy storage
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery’s energy density, rate capability, and cycling stability. For example, lithium cobalt oxide (LiCoO 2 ) nanoparticles have been used as cathode materials in lithium-ion batteries due to their high capacity and good rate performance. In addition, nanoparticles of transition metal oxides, such as iron oxide (Fe 2 O 3 ), and manganese oxide (MnO 2 ), have been used as cathode materials in rechargeable lithium batteries due to their high capacity and good rate performance. In supercapacitors, nanoparticles can be used as the active material in the electrodes to increase the specific surface area, leading to an increase in the device’s capacitance ( Morsi et al., 2022 ). Using NPs in the defense industry can improve defense systems’ performance, efficiency, and safety.
7. Future perspectives
Metal nanoparticles (NPs) have many potential applications in various fields, including electronics, energy storage, catalysis, and medicine. However, there are also several challenges and potential future directions for developing and using metal NPs.
One major challenge is synthesizing and processing metal NPs with precise size and shape control. Many methods for synthesizing metal NPs involve high temperatures and harsh chemical conditions, which can be challenging to scale up for large-scale production. In addition, the size and shape of metal NPs can significantly impact their properties and potential applications, so it is essential to synthesize NPs with precise size and shape control.
Another challenge is the environmental impact of metal NPs. Some metal NPs, such as silver NPs, can be toxic to aquatic life and may have other environmental impacts. There is a need for more research on the environmental effects of metal NPs and the development of more environmentally friendly (Green) synthesis and processing methods.
In terms of future directions, one promising area is the use of metal NPs for energy storage, conversion, and protection of the environment. For example, metal NPs could be used to improve batteries’ performance or develop more efficient solar cells. In addition, metal NPs could be used in catalysis to improve the efficiency of chemical reactions. There is also ongoing research on metal NPs in medicine, including drug delivery and cancer therapy.
Author contributions
KAA: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, and visualization.
Acknowledgments
The author thanks Prof. Dr. Mona M. Sobhy, Department of Reproductive Diseases, Animal Reproduction Research Institute, ARC, Giza, Egypt, and Dr. Omar Hewedy, University of Guelph, Canada, for the critical reading of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdulle, A., and Chow, J. C. (2019). Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 9:920. doi: 10.3390/nano9070920
PubMed Abstract | CrossRef Full Text | Google Scholar
Ago, H. (2015). “CVD growth of high-quality single-layer graphene,” in Frontiers of Graphene and Carbon Nanotubes , Ed. K. Matsumoto (Berlin: Springer), 3–20. doi: 10.1007/978-4-431-55372-4_1
CrossRef Full Text | Google Scholar
Ahmad, A., Alsaad, A., Al-Bataineh, Q. M., Al-Akhras, M.-A. H., Albataineh, Z., Alizzy, K. A., et al. (2021). Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polymer Bull. 78, 1189–1211. doi: 10.1007/s00289-020-03155-x
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., Ramani, R., Srinivas, V., et al. (2003). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete. Rhodococcus species. Nanotechnology 14:824. doi: 10.1088/0957-4484/14/7/323
Ahmad, T., Wani, I. A., Ahmed, J., and Al-Hartomy, O. A. (2014). Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 4, 491–498. doi: 10.1007/s13204-013-0224-y
Ajitha, B., Reddy, Y. A. K., and Reddy, P. S. (2015). Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 49, 373–381. doi: 10.1016/j.msec.2015.01.035
Al-Dhabi, N. A., Mohammed Ghilan, A.-K., and Arasu, M. V. (2018). Characterization of silver nanomaterials derived from marine Streptomyces sp. al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 8:279. doi: 10.3390/nano8050279
Amendola, V., and Meneghetti, M. (2009). Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 11, 3805–3821. doi: 10.1039/b900654k
Anjum, S., Hashim, M., Malik, S. A., Khan, M., Lorenzo, J. M., Abbasi, B. H., et al. (2021). Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 13:4570. doi: 10.3390/cancers13184570
Astefanei, A., Núñez, O., and Galceran, M. T. (2015). Characterisation and determination of fullerenes: a critical review. Anal. Chim. Acta 882, 1–21.
Google Scholar
Bahadur, P. S., Jaiswal, S., Srivastava, R., and Kumar, A. (2021). “Advanced application of nanotechnology in engineering,” in Proceedings of the 2021 International Conference on Technological Advancements and Innovations (ICTAI) , (Piscataway, NJ: IEEE), 92–95.
Baig, N., Kammakakam, I., and Falath, W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2, 1821–1871.
Banerjee, A., Krishna, R., and Das, B. (2008). Size controlled deposition of Cu and Si nano-clusters by an ultra-high vacuum sputtering gas aggregation technique. Appl. Phys. A 90, 299–303.
Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., and Rizzolio, F. (2019). The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25:112. doi: 10.3390/molecules25010112
Behrisch, R. (1981). Sputtering by Particle Bombardment Springer Verlag. Berlin-Heidelberg: Springer.
Berkmans, A. J., Jagannatham, M., Priyanka, S., and Haridoss, P. (2014). Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from PET wastes using the arc discharge method. Waste Manag. 34, 2139–2145. doi: 10.1016/j.wasman.2014.07.004
Beyene, H. D., Werkneh, A. A., Bezabh, H. K., and Ambaye, T. G. (2017). Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 13, 18–23.
Bhattacharjee, S. (2016). DLS and zeta potential–what they are and what they are not? J. Control. Release 235, 337–351.
Bhavani, K. S., Anusha, T., and Brahman, P. K. (2021). Platinum nanoparticles decorated on graphitic carbon nitride-ZIF-67 composite support: An electrocatalyst for the oxidation of butanol in fuel cell applications. Int. J. Hydr. Energy 46, 9199–9214.
Biju, V., Itoh, T., Anas, A., Sujith, A., and Ishikawa, M. (2008). Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 391, 2469–2495.
Brady, B., Wang, P. H., Steenhoff, V., and Brolo, A. G. (2019). “Nanostructuring solar cells using metallic nanoparticles,” in Metal Nanostructures for Photonics , eds L. R. P. Kassab, and C. B. De Araujo (Amsterdam: Elsevier), 197–221.
Cadene, A., Durand-Vidal, S., Turq, P., and Brendle, J. (2005). Study of individual Na-montmorillonite particles size, morphology, and apparent charge. J. Colloid Interf. Sci. 285, 719–730. doi: 10.1016/j.jcis.2004.12.016
Chen, J., and Zhu, X. (2016). Magnetic solid phase extraction using ionic liquid-coated core–shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem. 200, 10–15. doi: 10.1016/j.foodchem.2016.01.002
Chen, J., Guo, Y., Zhang, X., Liu, J., Gong, P., Su, Z., et al. (2023). Emerging nanoparticles in food: sources, application, and safety. J. Agricult. Food Chem. 71, 3564–3582.
Chen, J., Wei, S., and Xie, H. (2021). “A brief introduction of carbon nanotubes: history, synthesis, and properties,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing), 012184. doi: 10.1088/1742-6596/1948/1/012184
Chen, J.-C., and Tang, C.-T. (2007). Preparation and application of granular ZnO/Al2O3 catalyst for the removal of hazardous trichloroethylene. J. Hazardous Mater. 142, 88–96. doi: 10.1016/j.jhazmat.2006.07.061
Chronakis, I. S. (2010). Micro-/nano-fibers by electrospinning technology: processing, properties and applications. Micromanufact. Eng. Technol. 2010, 264–286. doi: 10.1016/B978-0-8155-1545-6.00016-8
Compostella, F., Pitirollo, O., Silvestri, A., and Polito, L. (2017). Glyco-gold nanoparticles: synthesis and applications. Beilstein J. Org. Chem. 13, 1008–1021. doi: 10.3762/bjoc.13.100
Dahoumane, S. A., Mechouet, M., Wijesekera, K., Filipe, C. D., Sicard, C., Bazylinski, D. A., et al. (2017). Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 19, 552–587. doi: 10.1039/C6GC02346K
Dangi, K., and Verma, A. K. (2021). Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45, 3819–3824.
Das, S., and Srivasatava, V. C. (2016). Synthesis and characterization of ZnO–MgO nanocomposite by co-precipitation method. Smart Sci. 4, 190–195.
De La Calle, I., Menta, M., Klein, M., and Séby, F. (2018). Study of the presence of micro-and nanoparticles in drinks and foods by multiple analytical techniques. Food Chem. 266, 133–145. doi: 10.1016/j.foodchem.2018.05.107
Delvallée, A., Feltin, N., Ducourtieux, S., Trabelsi, M., and Hochepied, J. (2015). Direct comparison of AFM and SEM measurements on the same set of nanoparticles. Measur. Sci. Technol. 26:085601.
Dhand, V., Soumya, L., Bharadwaj, S., Chakra, S., Bhatt, D., and Sreedhar, B. (2016). Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 58, 36–43. doi: 10.1016/j.msec.2015.08.018
Dikusar, A., Globa, P., Belevskii, S., and Sidel’nikova, S. (2009). On limiting rate of dimensional electrodeposition at meso-and nanomaterial manufacturing by template synthesis. Surf. Eng. Appl. Electrochem. 45, 171–179.
Dragovic, R. A., Gardiner, C., Brooks, A. S., Tannetta, D. S., Ferguson, D. J., Hole, P., et al. (2011). Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7, 780–788.
Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy, C. J., and El-Sayed, M. A. (2012). The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740–2779. doi: 10.1039/c1cs15237h
Du, P., Song, L., Xiong, J., Li, N., Xi, Z., Wang, L., et al. (2012). Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 78, 392–397.
Edison, T. N. J. I., Lee, Y. R., and Sethuraman, M. G. (2016). Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Pectrochimica Acta Part A 161, 122–129. doi: 10.1016/j.saa.2016.02.044
Elliott, J. A., Shibuta, Y., Amara, H., Bichara, C., and Neyts, E. C. (2013). Atomistic modelling of CVD synthesis of carbon nanotubes and graphene. Nanoscale 5, 6662–6676. doi: 10.1039/c3nr01925j
Erasmus, M., Cason, E. D., Van Marwijk, J., Botes, E., Gericke, M., and Van Heerden, E. (2014). Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull. 47, 245–253.
Eroglu, E., Chen, X., Bradshaw, M., Agarwal, V., Zou, J., Stewart, S. G., et al. (2013). Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 3, 1009–1012.
Essajai, R., Benhouria, Y., Rachadi, A., Qjani, M., Mzerd, A., and Hassanain, N. (2019). Shape-dependent structural and magnetic properties of Fe nanoparticles studied through simulation methods. RSC Adv. 9, 22057–22063. doi: 10.1039/c9ra03047f
Falke, S., and Betzel, C. (2019). “Dynamic light scattering (DLS),” in Radiation in Bioanalysis , eds A. S. Pereira, P. Tavares, P. Limão-Vieira (Berlin: Springer), 173–193.
Farrell, D., Majetich, S. A., and Wilcoxon, J. P. (2003). Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 107, 11022–11030.
Feng, L., Xuan, Z., Ma, J., Chen, J., Cui, D., Su, C., et al. (2015). Preparation of gold nanorods with different aspect ratio and the optical response to solution refractive index. J. Exp. Nanosci. 10, 258–267.
Ghorbani, H. R., Mehr, F. P., Pazoki, H., and Rahmani, B. M. (2015). Synthesis of ZnO nanoparticles by precipitation method. Orient. J. Chem. 31, 1219–1221.
Ghosh, S., Ahmad, R., Zeyaullah, M., and Khare, S. K. (2021). Microbial nano-factories: synthesis and biomedical applications. Front. Chem. 9:194. doi: 10.3389/fchem.2021.626834
Giljohann, D. A., Seferos, D. S., Daniel, W. L., Massich, M. D., Patel, P. C., and Mirkin, C. A. (2020). Gold nanoparticles for biology and medicine. Spherical Nucleic Acids 49, 3280–3294.
Giurlani, W., Innocenti, M., and Lavacchi, A. (2018). X-ray microanalysis of precious metal thin films: thickness and composition determination. Coatings 8:84.
Gloria, E. C., Ederley, V., Gladis, M., César, H., Jaime, O., Oscar, A., et al. (2017). “Synthesis of silver nanoparticles (AgNPs) with antibacterial activity,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing), 012023.
Gorrasi, G., and Sorrentino, A. (2015). Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 17, 2610–2625.
Govindarajan, M., Rajeswary, M., Veerakumar, K., Muthukumaran, U., Hoti, S., and Benelli, G. (2016). Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 161, 40–47. doi: 10.1016/j.exppara.2015.12.011
Graf, C., Vossen, D. L., Imhof, A., and Van Blaaderen, A. (2003). A general method to coat colloidal particles with silica. Langmuir 19, 6693–6700.
Greczynski, G., and Hultman, L. (2020). X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Progr. Mater. Sci. 107:100591.
Guo, D., Xie, G., and Luo, J. (2013). Mechanical properties of nanoparticles: basics and applications. J. Phys. D 47:013001.
Guo, W., Pleixats, R., and Shafir, A. (2015). Water-soluble gold nanoparticles: from catalytic selective Nitroarene reduction in water to refractive index sensing. Chem. An Asian J. 10, 2437–2443. doi: 10.1002/asia.201500290
Gwynne, K. (2020). Enhancement of the Photostability of Blue Phosphorescence Using Plasmonic Surfaces. New Brunswick, NJ: Rutgers University-School of Graduate Studies.
Haasch, R. T. (2014). “X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES),” in Practical Materials Characterization , Ed. M. Sardela (Berlin: Springer), 93–132.
Hasan, S. (2015). A review on nanoparticles: their synthesis and types. Res. J. Recent Sci. 2277:2502.
Holder, C. F., and Schaak, R. E. (2019). Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. Washington, DC: ACS Publications.
Hollamby, M. J., Eastoe, J., Chemelli, A., Glatter, O., Rogers, S., Heenan, R. K., et al. (2010). Separation and purification of nanoparticles in a single step. Langmuir 26, 6989–6994. doi: 10.1021/la904225k
Hoo, C. M., Starostin, N., West, P., and Mecartney, M. L. (2008). A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 10, 89–96.
Hortin, J., Anderson, A., Britt, D., Jacobson, A., and Mclean, J. (2020). Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. 7, 2618–2631.
Hoseinzadeh, E., Makhdoumi, P., Taha, P., Hossini, H., Stelling, J., and Amjad Kamal, M. (2017). A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 18, 120–128.
PubMed Abstract | Google Scholar
Hulkoti, N. I., and Taranath, T. (2014). Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerf. 121, 474–483. doi: 10.1016/j.colsurfb.2014.05.027
Islam, F., Shohag, S., Uddin, M. J., Islam, M. R., Nafady, M. H., Akter, A., et al. (2022). Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 15:2160. doi: 10.3390/ma15062160
Jadoun, S., Arif, R., Jangid, N. K., and Meena, R. K. (2021). Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 19, 355–374.
Jamkhande, P. G., Ghule, N. W., Bamer, A. H., and Kalaskar, M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53, 101174.
Jana, N. R., Earhart, C., and Ying, J. Y. (2007). Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 19, 5074–5082.
Jaskulski, D., Jaskulska, I., Majewska, J., Radziemska, M., Bilgin, A., and Brtnicky, M. (2022). Silver Nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 15:870. doi: 10.3390/ma15030870
Jayaraman, V., Ghosh, S., Sengupta, A., Srivastava, S., Sonawat, H., and Narayan, P. K. (2014). Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reproduct. Genet. 31, 1195–1204. doi: 10.1007/s10815-014-0282-4
Jena, J., Pradhan, N., Nayak, R. R., Dash, B. P., Sukla, L. B., Panda, P. K., et al. (2014). Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. Adv. 24, 522–533. doi: 10.4014/jmb.1306.06014
Jiang, H.-L., and Xu, Q. (2011). Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 170, 56–63.
Joh, D.-W., Jung, T.-K., Lee, H.-S., and Kim, D.-H. (2013). Synthesis of nanoparticles using electrical explosion of Ni wire in Pt solution. J. Nanosci. Nanotechnol. 13, 6092–6094. doi: 10.1166/jnn.2013.7677
Kahle, M., Kleber, M., and Jahn, R. (2002). Review of XRD-based quantitative analyses of clay minerals in soils: the suitability of mineral intensity factors. Geoderma 109, 191–205.
Kalaiyarasu, T., Karthi, N., Sharmila, G. V., and Manju, V. (2016). In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J. Pharm. Clin. Res. 9, 297–302.
Kayalvizhi, T., Ravikumar, S., and Venkatachalam, P. (2016). Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J. Environ. Eng. 142:C4016002.
Khan, A., Rashid, R., Murtaza, G., and Zahra, A. (2014). Gold nanoparticles: synthesis and applications in drug delivery. Trop. J. Pharm. Res. 13, 1169–1177.
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12, 908–931.
Khanna, P., Kaur, A., and Goyal, D. (2019). Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol Methods 163:105656. doi: 10.1016/j.mimet.2019.105656
Kharat, S. N., and Mendhulkar, V. D. (2016). Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 62, 719–724. doi: 10.1016/j.msec.2016.02.024
Kim, J.-H., Sheikh, F. A., Ju, H. W., Park, H. J., Moon, B. M., Lee, O. J., et al. (2014). 3D silk fibroin scaffold incorporating titanium dioxide (TiO2) nanoparticle (NPs) for tissue engineering. Int. J. Biol. Macromol. 68, 158–168. doi: 10.1016/j.ijbiomac.2014.04.045
Kohl, H., and Reimer, L. (2008). Transmission Electron Microscopy. Berlin: Springer Series in Optical Sciences, 36.
Kokarneswaran, M., Selvaraj, P., Ashokan, T., Perumal, S., Sellappan, P., Murugan, K. D., et al. (2020). Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 10, 1–6. doi: 10.1038/s41598-020-76720-z
Kopittke, P. M., Lombi, E., Wang, P., Schjoerring, J. K., and Husted, S. (2019). Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. 6, 3513–3524. doi: 10.3390/biology10111123
Kora, A. J., and Rastogi, L. (2018). Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 254, 690–700.
Kreizer, M., Ratner, D., and Liberzon, A. (2010). Real-time image processing for particle tracking velocimetry. Exp. Fluids 48, 105–110.
Krithiga, N., Jayachitra, A., and Rajalakshmi, A. (2013). Synthesis, characterization and analysis of the effect of copper oxide nanoparticles in biological systems. Ind. J. Ns 1, 6–15.
Kumar, R., Singh, R. K., Dubey, P. K., Kumar, P., Tiwari, R. S., and Oh, I.-K. (2013). Pressure-dependent synthesis of high-quality few-layer graphene by plasma-enhanced arc discharge and their thermal stability. J. Nanopart. Res. 15, 1–10.
Kumar, S. S., Venkateswarlu, P., Rao, V. R., and Rao, G. N. (2013). Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3, 1–6.
Kumar, V., and Yadav, S. K. (2009). Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 84, 151–157.
Kumar, V., Bano, D., Mohan, S., Singh, D. K., and Hasan, S. H. (2016). Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 181, 371–377.
Kumari, S. C., Dhand, V., and Padma, P. N. (2021). Green synthesis of metallic nanoparticles: a review. Nanomaterials 2021, 259–281.
Lam, E., and Luong, J. H. (2014). Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 4, 3393–3410.
Lara, P., and Philippot, K. (2014). The hydrogenation of nitroarenes mediated by platinum nanoparticles: an overview. Catal. Sci. Technol. 4, 2445–2465.
Lerner, M. I., Glazkova, E. A., Lozhkomoev, A. S., Svarovskaya, N. V., Bakina, O. V., Pervikov, A. V., et al. (2016). Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 295, 307–314.
Lewczuk, B., and Szyryńska, N. (2021). Field-emission scanning electron microscope as a tool for large-area and large-volume ultrastructural studies. Animals 11:3390. doi: 10.3390/ani11123390
Li, G., He, D., Qian, Y., Guan, B., Gao, S., Cui, Y., et al. (2011). Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 13, 466–476. doi: 10.3390/ijms13010466
Li, N., Zhao, P., and Astruc, D. (2014). Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angewand. Chem. Int. Edn. 53, 1756–1789.
Li, T., Senesi, A. J., and Lee, B. (2016). Small angle X-ray scattering for nanoparticle research. Chem. Rev. 116, 11128–11180. doi: 10.1021/acs.chemrev.5b00690
Li, X., Xu, H., Chen, Z.-S., and Chen, G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011:270974.
Luangpipat, T., Beattie, I. R., Chisti, Y., and Haverkamp, R. G. (2011). Gold nanoparticles produced in a microalga. J. Nanopart. Res. 13, 6439–6445.
Lyon, L. A., Keating, C. D., Fox, A. P., Baker, B. E., He, L., Nicewarner, S. R., et al. (1998). Raman spectroscopy. Anal. Chem. 70, 341–362.
Lyu, H., Gao, B., He, F., Ding, C., Tang, J., and Crittenden, J. C. (2017). Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sust. Chem. Eng. 5, 9568–9585. doi: 10.1016/j.biortech.2020.123613
Machac, P., Cichon, S., Lapcak, L., and Fekete, L. (2020). Graphene prepared by chemical vapour deposition process. Graph. Technol. 5, 9–17.
Madathil, A. N. P., Vanaja, K., and Jayaraj, M. (2007). “Synthesis of ZnO nanoparticles by hydrothermal method,” in Nanophotonic materials IV , eds Z. Gaburro and S. Cabrini (Bellingham, WA: SPIE), 47–55.
Maharani, V., Sundaramanickam, A., and Balasubramanian, T. J. E. (2016). In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzyme Microb. Technol. 95, 146–154. doi: 10.1016/j.enzmictec.2016.09.008
Majeed Khan, M. A., Kumar, S., Ahamed, M., Alrokayan, S. A., and Alsalhi, M. S. (2011). Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanosc. Res. Lett. 6, 1–8.
Malhotra, S. P. K., and Alghuthaymi, M. A. (2022). Biomolecule-assisted biogenic synthesis of metallic nanoparticles. Agri-Waste Microb. Product. Sust. Nanomater. 2022, 139–163.
Mathew, L., Chandrasekaran, N., and Mukherjee, A. (2010). “Biomimetic synthesis of nanoparticles: science, technology & applicability,” Biomimetics learning from nature , Ed. A. Mukherjee (Norderstedt: Books on Demand).
Mishra, A., Tripathy, S. K., Wahab, R., Jeong, S.-H., Hwang, I., Yang, Y.-B., et al. (2011). Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2 C 12 cells. Appl. Microbiol. Biotechnol. Adv. 92, 617–630. doi: 10.1007/s00253-011-3556-0
Mittal, A., and Chisti, Y. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31, 346–356.
Moghaddam, A. B., Nazari, T., Badraghi, J., and Kazemzad, M. (2009). Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 4, 247–257.
Mohanpuria, P., Rana, N. K., and Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10, 507–517.
Mohd Yusof, H., Mohamad, R., Zaidan, U. H., and Rahman, A. (2019). Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 10, 1–22. doi: 10.1186/s40104-019-0368-z
Morsi, M., Abdelrazek, E., Ramadan, R., Elashmawi, I., and Rajeh, A. (2022). Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polymer Test. 114, 107705.
Mott, D., Galkowski, J., Wang, L., Luo, J., and Zhong, C.-J. (2007). Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23, 5740–5745.
Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S. R., Khan, M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1, 515–519.
Muñoz-García, J., Vázquez, L., Cuerno, R., Sánchez-García, J. A., Castro, M., and Gago, R. (2009). “Self-organized surface nanopatterning by ion beam sputtering,” in Toward Functional Nanomaterials , Ed. Z. M. Wang (Berlin: Springer), 323–398.
Naraginti, S., Kumari, P. L., Das, R. K., Sivakumar, A., Patil, S. H., and Andhalkar, V. V. (2016). Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 62, 293–300. doi: 10.1016/j.msec.2016.01.069
Narayanan, K. B., and Sakthivel, N. (2010). Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interf. Sci. 156, 1–13.
Nayak, D., Ashe, S., Rauta, P. R., Kumari, M., and Nayak, B. (2016). Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 58, 44–52. doi: 10.1016/j.msec.2015.08.022
Ndolomingo, M. J., and Meijboom, R. (2016). Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 390, 224–235.
Newbury, D. E., and Ritchie, N. W. (2013). Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35, 141–168. doi: 10.1002/sca.21041
Nguyen, K. T., Menon, J. U., Jadeja, P. V., Tambe, P. P., Vu, K., and Yuan, B. (2013). Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 3, 152–166.
Nowack, B., Krug, H. F., and Height, M. (2011). 120 Years of Nanosilver History: Implications for Policy Makers. Washington, DC: ACS Publications.
Önal, E. S., Yatkın, T., Aslanov, T., Ergüt, M., and Özer, A. (2019). Biosynthesis and characterization of iron nanoparticles for effective adsorption of Cr (VI). Int. J. Chem. Eng. 2019:2716423.
Ostermann, R., Cravillon, J., Weidmann, C., Wiebcke, M., and Smarsly, B. M. (2011). Metal–organic framework nanofibers via electrospinning. Chem. Commun. 47, 442–444.
Parashar, M., Shukla, V. K., and Singh, R. (2020). Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J. Mater. Sci. 31, 3729–3749.
Park, C. Y., and Choi, B. (2019). Enhanced light extraction from bottom emission oleds by high refractive index nanoparticle scattering layer. Nanomaterials 9:1241. doi: 10.3390/nano9091241
Patil, M. P., and Kim, G.-D. (2018). Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerf. 172, 487–495.
Patil, M. P., Ngabire, D., Thi, H. H. P., Kim, M.-D., and Kim, G.-D. (2017). Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 28, 119–132.
Patil, N., Bhaskar, R., Vyavhare, V., Dhadge, R., Khaire, V., and Patil, Y. (2021). Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharm. Res. 13, 11–16.
Patois, E., Capelle, M., Palais, C., Gurny, R., and Arvinte, T. (2012). Evaluation of nanoparticle tracking analysis (NTA) in the characterization of therapeutic antibodies and seasonal influenza vaccines: pros and cons. J. Drug Deliv. Sci. Technol. 22, 427–433.
Paul, B., Bhuyan, B., Purkayastha, D. D., and Dhar, S. S. (2016). Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf. J. Photochem. Photobiol. B Biol. 154, 1–7. doi: 10.1016/j.jphotobiol.2015.11.004
Pérez-Lorenzo, M. (2012). Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 3, 167–174.
Pérez-Tijerina, E., Pinilla, M. G., Mejía-Rosales, S., Ortiz-Méndez, U., Torres, A., and José-Yacamán, M. (2008). Highly size-controlled synthesis of Au/Pd nanoparticles by inert-gas condensation. Faraday Discuss. 138, 353–362. doi: 10.1039/b705913m
Phull, A.-R., Abbas, Q., Ali, A., Raza, H., Zia, M., and Haq, I.-U. (2016). Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Fut. J. Pharm. Sci. 2, 31–36.
Pimpin, A., and Srituravanich, W. (2012). Review on micro-and nanolithography techniques and their applications. Eng. J. 16, 37–56.
Pradeep, T. (2009). Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517, 6441–6478.
Praseptiangga, D., Zahara, H. L., Widjanarko, P. I., Joni, I. M., and Panatarani, C. (2020). Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. 100005.
Pugazhendhi, S., Sathya, P., Palanisamy, P., and Gopalakrishnan, R. (2016). Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 159, 155–160. doi: 10.1016/j.jphotobiol.2016.03.043
Qi, P., Zhang, D., and Wan, Y. (2013). Sulfate-reducing bacteria detection based on the photocatalytic property of microbial synthesized ZnS nanoparticles. Anal. Chim. Acta 800, 65–70. doi: 10.1016/j.aca.2013.09.015
Rad, A. G., Abbasi, H., and Afzali, M. H. (2011). Gold nanoparticles: synthesising, characterizing and reviewing novel application in recent years. Phys. Proc. 22, 203–208.
Rahmati-Abkenar, M., and Manteghian, M. (2020). Effect of silver nanoparticles on the solubility of methane and ethane in water. J. Nat. Gas Sci. Eng. 82:103505.
Rajeshkumar, S., Ponnanikajamideen, M., Malarkodi, C., Malini, M., and Annadurai, G. (2014). Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 4, 1–7.
Rajkuberan, C., Prabukumar, S., Sathishkumar, G., Wilson, A., Ravindran, K., and Sivaramakrishnan, S. (2017). Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Soc. 21, 911–919.
Ramesh, P., Kokila, T., and Geetha, D. (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A 142, 339–343. doi: 10.1016/j.saa.2015.01.062
Rangel-Olivares, F. R., Arce-Estrada, E. M., and Cabrera-Sierra, R. (2021). Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 11:653.
Rao, N. H., Lakshmidevi, N., Pammi, S., Kollu, P., Ganapaty, S., and Lakshmi, P. (2016). Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 62, 553–557. doi: 10.1016/j.msec.2016.01.072
Rassaei, L., Marken, F., Sillanpää, M., Amiri, M., Cirtiu, C. M., and Sillanpää, M. (2011). Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 30, 1704–1715.
Rocha, F. S., Gomes, A. J., Lunardi, C. N., Kaliaguine, S., and Patience, G. S. (2018). Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 96, 2512–2517.
Saini, P., Saha, S. K., Roy, P., Chowdhury, P., and Babu, S. P. S. (2016). Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 160, 39–48. doi: 10.1016/j.exppara.2015.11.004
Saldarriaga, J. F., Aguado, R., Pablos, A., Amutio, M., Olazar, M., and Bilbao, J. (2015). Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 140, 744–751.
Salem, S. S., and Fouda, A. (2021). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Element Res. 199, 344–370. doi: 10.1007/s12011-020-02138-3
Salopek, B., Krasic, D., and Filipovic, S. (1992). Measurement and application of zeta-potential. Rudarsko-Geolosko-Naftni Zbornik 4:147.
Saw, M. J., Ghosh, B., Nguyen, M. T., Jirasattayaporn, K., Kheawhom, S., Shirahata, N., et al. (2019). High aspect ratio and post-processing free silver nanowires as top electrodes for inverted-structured photodiodes. ACS Omega 4, 13303–13308. doi: 10.1021/acsomega.9b01479
Schröfel, A., Kratošová, G., Šafar̄ík, I., Šafar̄íková, M., Raška, I., and Shor, L. M. (2014). Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater. 10, 4023–4042.
Shenashen, M. A., El-Safty, S. A., and Elshehy, E. A. (2014). Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Char. 31, 293–316.
Shi, Z., Lian, Y., Liao, F. H., Zhou, X., Gu, Z., Zhang, Y., et al. (2000). Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 61, 1031–1036. doi: 10.1166/jnn.2001.012
Siddiqi, K. S., and Husen, A. (2016). Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanosc. Res. Lett. 11, 1–13. doi: 10.1186/s11671-016-1695-z
Siddique, S., and Chow, J. C. (2020). Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 10:3824.
Sigmund, W., Yuh, J., Park, H., Maneeratana, V., Pyrgiotakis, G., and Daga, A. (2006). Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceramic Soc. 89, 395–407.
Singh, R. P., Shukla, V. K., Yadav, R. S., Sharma, P. K., Singh, P. K., and Pandey, A. C. (2011). Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2, 313–317.
Siwach, O. P., and Sen, P. (2008). Synthesis and study of fluorescence properties of Cu nanoparticles. J. Nanopart. Res. 10, 107–114.
Slavin, Y. N., Asnis, J., Häfeli, U. O., and Bach, H. (2017). Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 1–20.
Song, U., and Kim, J. (2020). Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Horticult. Environ. Biotechnol. 61, 625–631.
Soni, N., and Sonam, P. (2014). Green nanoparticles for mosquito control. Sci. World J. 2014, 1–6.
Sowani, H., Mohite, P., Munot, H., Shouche, Y., Bapat, T., Kumar, A. R., et al. (2016). Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Process Biochem. 51, 374–383.
Sriram, M. I., Kalishwaralal, K., Barathmanikanth, S., and Gurunathani, S. (2012). Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci. Methods 1, 56–77.
Stepanov, A. L., Nuzhdin, V. I., Valeev, V. F., and Kreibig, U. (2011). “Optical properties of metal nanoparticles,” in Proceedings of the ICONO 2010: International Conference on Coherent and Nonlinear Optics , (Bellingham, WA: SPIE), 543–552.
Su, S. S., and Chang, I. (2018). “Review of production routes of nanomaterials,” in Commercialization of nanotechnologies–a case study approach , eds D. Brabazon, E. Pellicer, F. Zivic, J. Sort, M. D. Baró, N. Grujovic, K.-L. Choy (Berlin: Springer), 15–29.
Sugihartono, I., Dianisya, D., and Isnaeni, I. (2018). “Crystal structure analyses of ZnO nanoparticles growth by simple wet chemical method,” in Proceedings of the IOP Conference Series: Materials Science and Engineering , (Bristol: IOP Publishing), 012077.
Sun, T., Zhang, Y. S., Pang, B., Hyun, D. C., Yang, M., and Xia, Y. J. A. C. I. E. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 12320–12364.
Tavakoli, A. H., Maram, P. S., Widgeon, S. J., Rufner, J., Van Benthem, K., Ushakov, S., et al. (2013). Amorphous alumina nanoparticles: structure, surface energy, and thermodynamic phase stability. J. Phys. Chem. C 117, 17123–17130. doi: 10.1021/jp405820g
Theron, J., Eugene Cloete, T., and De Kwaadsteniet, M. (2010). Current molecular and emerging nanobiotechnology approaches for the detection of microbial pathogens. Crit. Rev. Microbiol. 36, 318–339. doi: 10.3109/1040841X.2010.489892
Thomas, R., Janardhanan, A., Varghese, R. T., Soniya, E., Mathew, J., and Radhakrishnan, E. (2014). Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 45, 1221–1227. doi: 10.1590/s1517-83822014000400012
Titus, D., Samuel, E. J. J., and Roopan, S. M. (2019). “Nanoparticle characterization techniques,” in Green synthesis, characterization and applications of nanoparticles , eds A. Shukla and S. Iravani (Amsterdam: Elsevier), 303–319. doi: 10.1016/B978-0-08-102579-6.00012-5
Tran, V., and Wen, X. (2014). “Rapid prototyping technologies for tissue regeneration,” in Rapid prototyping of biomaterials , Ed. R. Narayan (Sawston: Woodhead Publishing), 97–155. doi: 10.1533/9780857097217.97
Triana, M. A., Hsiang, E.-L., Zhang, C., Dong, Y., and Wu, S.-T. (2022). Luminescent nanomaterials for energy-efficient display and healthcare. ACS Energy Lett. 7, 1001–1020.
Uzair, B., Liaqat, A., Iqbal, H., Menaa, B., Razzaq, A., Thiripuranathar, G., et al. (2020). Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 7:129. doi: 10.3390/bioengineering7040129
Van Thai, P., Abe, S., Kosugi, K., Saito, N., Takahashi, K., Sasaki, T., et al. (2019). Size/shape control of gold nanoparticles synthesized by alternating current glow discharge over liquid: The role of pH. Mater. Res. Expr. 6:095074.
Velusamy, P., Das, J., Pachaiappan, R., Vaseeharan, B., and Pandian, K. (2015). Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum ( Azadirachta indica L.). Indus. Crops Products 66, 103–109.
Wang, P., Menzies, N. W., Lombi, E., Sekine, R., Blamey, F. P. C., Hernandez-Soriano, M. C., et al. (2015). Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic. Nanotoxicology 9, 1041–1049. doi: 10.3109/17435390.2014.999139
Wang, Z., Li, H., Tang, F., Ma, J., and Zhou, X. (2018). A facile approach for the preparation of nano-size zinc oxide in water/glycerol with extremely concentrated zinc sources. Nanosc. Res. Lett. 13, 1–9. doi: 10.1186/s11671-018-2616-0
Wiesendanger, R., and Güntherodt, H.-J. (2013). Scanning tunneling microscopy III: theory of STM and related scanning probe methods. Berlin: Springer Science & Business Media.
Xia, Y., Xiao, Z., Dou, X., Huang, H., Lu, X., Yan, R., et al. (2013). Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7, 7083–7092. doi: 10.1021/nn4023894
Yadav, R., Dwivedi, S., Kumar, S., and Chaudhury, A. (2010). Trends and perspectives of biosensors for food and environmental virology. Food Environ. Virol. 2, 53–63.
Yadav, T. P., Yadav, R. M., and Singh, D. P. (2012). Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2, 22–48.
Yang, W., Wang, L., Mettenbrink, E. M., Deangelis, P. L., and Wilhelm, S. (2021). Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61, 269–289.
Ye, Q., Chen, W., Huang, H., Tang, Y., Wang, W., Meng, F., et al. (2020). Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 104, 5213–5227. doi: 10.1007/s00253-020-10600-4
Yuan, P., Ding, X., Yang, Y. Y., and Xu, Q. H. (2018). Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv. Healthc. Mater. 7:1701392.
Zahra, Z., Habib, Z., Chung, S., and Badshah, M. A. (2020). Exposure route of TiO2 NPs from industrial applications to wastewater treatment and their impacts on the agro-environment. Nanomaterials 10:1469. doi: 10.3390/nano10081469
Zan, G., and Wu, Q. (2016). Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv. Mater. 28, 2099–2147.
Zhang, X., Yan, S., Tyagi, R., and Surampalli, R. (2011). Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82, 489–494.
Zheng, Z., Zhang, X., Carbo, D., Clark, C., Nathan, C.-A., and Lvov, Y. (2010). Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26, 7679–7681. doi: 10.1021/la101246a
Zhou, C., Wang, Y., Du, L., Yao, H., Wang, J., and Luo, G. (2016). Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Indus. Eng. Chem. Res. 55, 11943–11949.
Zhou, M., Wei, Z., Qiao, H., Zhu, L., Yang, H., and Xia, T. (2009). Particle size and pore structure characterization of silver nanoparticles prepared by confined arc plasma. J. Nanomater. 2009:968058.
Zhuang, J., and Gentry, R. W. (2011). “Environmental application and risks of nanotechnology: a balanced view,” in Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us , eds S. Ripp and T. Henry (Washington, DC: ACS Publications), 41–67. doi: 10.3390/ijerph16234848
Zielińska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Venkatesh, D. N., et al. (2020). Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25:3731. doi: 10.3390/molecules25163731
Keywords : green synthesis, nanoparticles, nanotechnology, biological synthesis, microbial nanotechnology, bionanotechnology
Citation: Altammar KA (2023) A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front. Microbiol. 14:1155622. doi: 10.3389/fmicb.2023.1155622
Received: 31 January 2023; Accepted: 21 March 2023; Published: 17 April 2023.
Reviewed by:
Copyright © 2023 Altammar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadijah A. Altammar, [email protected] ; orcid.org/0000-0002-8691-086X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
share this!
May 20, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study reveals promising development in cancer-fighting nanotechnologies
by Kat Gebauer, University of Oklahoma
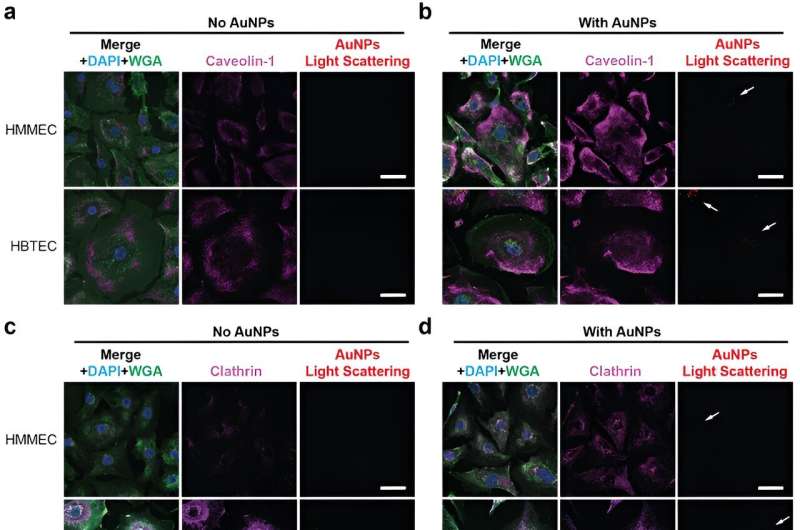
A new study conducted by the Wilhelm Lab at the University of Oklahoma examines a promising development in biomedical nanoengineering. Published in Advanced Materials , the study explores new findings on the transportation of cancer nanomedicines into solid tumors.
A frequent misconception about many malignant solid tumors is that they are comprised only of cancerous cells . However, solid tumors also include healthy cells, such as immune cells and blood vessels. These blood vessels are nutrient transportation highways that tumors need to grow, but they can also be a pathway for medicine delivery, including for cancer nanomedicines.
Blood vessels, and the endothelial cells within them, are the transportation method examined in the new study led by Lin Wang, Ph.D., who was a postdoctoral research associate in the Wilhelm Lab while conducting the study and is the first author of the publication. Endothelial cells line blood vessels and manage the exchange between the bloodstream and surrounding tissues. These cells are the first barrier that the nanotechnologies encounter in the process of being transported into tumors.
The researchers found that endothelial cells in breast cancer tumors are two times more likely to interact with medicine-carrying nanoparticles than endothelial cells in healthy breast tissue. Wang said that the tumor endothelial cells have more transport features than the healthy endothelial cells, making them ideal conduits.
"If you know that the same cell type in tumor tissues is two times more likely to interact with your drug carriers than in healthy tissue, then in theory, you should be able to target those cells to get even more nanoparticles delivered into the tumor," said Stefan Wilhelm, Ph.D., associate professor in the Stephenson School of Biomedical Engineering and corresponding author of the study.
The research was conducted on endothelial cells isolated from breast cancer tissues and isolated from healthy breast tissues. The next steps for the research will involve examining how the nanoparticles react in the context of the whole tissue architecture.
"Cell-culture level experiments are only so good at trying to recapitulate what is happening in the body," said Wilhelm. "Working with colleagues at OU Health Sciences, we hope to get our hands on not just cells but the entire tumor tissue."
The research team is working with the Stephenson Cancer Center to set up an ethics protocol allowing the lab to access stored samples of cancer tissue rather than just isolated cells. The Wilhelm Lab is focused on studying nanomedicine and using nanoparticles for drug delivery and diagnostics. In particular, the team is interested in studying the delivery of drugs into solid tumor tissues.
From an engineering perspective, a unique advantage of using nanoparticles for drug delivery is that they are small and flexible enough to be designed as direct delivery vehicles. In a laboratory setting, the nanoparticles are often designed as tiny spheres and loaded with the necessary drugs. Then, in clinics, they are often administered intravenously to patients. These drugs circulate through the bloodstream, and some of them enter the tumor.
There are challenges associated with this type of medicine transportation. One is that these nanoparticles circulate throughout the body, and consequently, they accumulate in other organs—called off-target organs—such as the liver, spleen and kidneys. Since these organs filter blood, they remove the nanoparticles, which are often considered foreign objects by the body.
The field of nanomedicine has been around for more than 40 years, and there are tens of thousands of publications on using nanoparticles to treat cancers at the preclinical stage. But there is a disconnect between the number of preclinical publications and the number of FDA-approved formulations of nanoparticles that are actually used in clinics.
Of those approved formulations, a fraction are used for solid tumors, and most treat liquid tumors, such as leukemia. Wilhelm speculates that this is partially because there is a lack of full understanding of how the nanoparticle delivery process works.
"And if you don't understand something fully, it's hard to develop solutions to those problems," said Wilhelm.
"Researchers have started to go back to the fundamentals of nanomedicine development to understand the translation from the pre-clinical to the clinical space. Our lab wants to focus on these fundamentals to better understand the field and the delivery mechanisms specifically. If we understand these fundamentals, we can contribute even more to the field," said Wang.
According to Wilhelm, the next big question is this: now that the lab has quantified and shown that endothelial cells are more likely to interact with and transport these nanomedicines, how can that transportation be made more efficient and specific to advance clinical cancer treatments? As these questions are answered, the opportunities for future advances in cancer health care will grow.
"We are just scratching the surface by using breast cancer as our model cancer system, but our findings may be relevant for other types of solid tumors as well," said Wilhelm.
Journal information: Advanced Materials
Provided by University of Oklahoma
Explore further
Feedback to editors
Researchers develop organic photoredox catalysts with enhanced stability and recyclability
9 hours ago

Theory and experiment combine to shine a new light on proton spin

On repeat: Biologists observe recurring evolutionary changes, over time, in stick insects
10 hours ago
New findings on fertility: Sperm can adapt to sexually transmitted microbes
Observing mammalian cells with superfast soft X-rays
11 hours ago

New study challenges conventional wisdom that Americans are 'pocketbook voters'
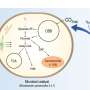
Carbon dioxide, the main culprit of global warming, reborn as an antioxidant substance

New study offers a cleaner path for controlling water, transforming greenhouse gases
12 hours ago

Heavy water: How melting ice sheets and pumped groundwater can lower local sea levels—and boost them elsewhere

Unveiling a novel AAK1 inhibitor: How chemical proteomics unlock therapeutic potential
13 hours ago
Relevant PhysicsForums posts
And now, here comes covid-19 version ba.2, ba.4, ba.5,....
8 hours ago
DNA-maternity test - could you see other relationship than mother?
May 19, 2024
Is it usual for vaccine injection site to hurt again during infection?
May 16, 2024
A Brief Biography of Dr Virgina Apgar, creator of the baby APGAR test
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
The Cass Report (UK)
May 1, 2024
More from Biology and Medical
Related Stories
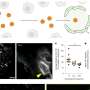
Researchers challenge long-standing theory guiding nanoparticle treatment of tumors
Sep 25, 2023
Biocompatible nanoparticles modified with ATP can enhance systemic delivery of cancer immunotherapy
Mar 27, 2024

Most engineered nanoparticles enter tumours through cells, not between them
Jan 14, 2020
Quantifying intracellular nanoparticle distributions with three-dimensional super-resolution microscopy
May 9, 2023
Targeted delivery: Cancer identity technology makes it easier to find a tumor's 'address'
Nov 15, 2018
New nanocomplex unleashes the immune system on metastases
Sep 7, 2023
Recommended for you

First topological quantum simulator device in strong light-matter interaction regime to operate at room temperatures
19 hours ago
Team develops an intelligent nanodevice based on a component of cinnamon essential oil as an antimicrobial agent
May 23, 2024

Researchers develop a novel strategy for growing two-dimensional transition metal dichalcogenides

New nanostrings can vibrate longer than any previously known solid-state object
May 22, 2024

Scientists develop new battery-free lactic acid sensor

Flexible, biodegradable and wireless magnetoelectric paper for simple in situ personalization of bioelectric implants
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Open access
- Published: 11 May 2024
Natural approach of using nisin and its nanoform as food bio-preservatives against methicillin resistant Staphylococcus aureus and E.coli O157:H7 in yoghurt
- Walaa M. Elsherif 1 , 2 ,
- Alshimaa A. Hassanien 3 ,
- Gamal M. Zayed 2 , 4 &
- Sahar M. Kamal 5
BMC Veterinary Research volume 20 , Article number: 192 ( 2024 ) Cite this article
249 Accesses
Metrics details
Natural antimicrobial agents such as nisin were used to control the growth of foodborne pathogens in dairy products. The current study aimed to examine the inhibitory effect of pure nisin and nisin nanoparticles (nisin NPs) against methicillin resistant Staphylococcus aureus (MRSA) and E.coli O157:H7 during the manufacturing and storage of yoghurt. Nisin NPs were prepared using new, natural, and safe nano-precipitation method by acetic acid. The prepared NPs were characterized using zeta-sizer and transmission electron microscopy (TEM). In addition, the cytotoxicity of nisin NPs on vero cells was assessed using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The minimum inhibitory concentrations (MICs) of nisin and its nanoparticles were determined using agar well-diffusion method. Further, fresh buffalo’s milk was inoculated with MRSA or E.coli O157:H7 (1 × 10 6 CFU/ml) with the addition of either nisin or nisin NPs, and then the inoculated milk was used for yoghurt making. The organoleptic properties, pH and bacterial load of the obtained yoghurt were evaluated during storage in comparison to control group.
The obtained results showed a strong antibacterial activity of nisin NPs (0.125 mg/mL) against MRSA and E.coli O157:H7 in comparison with control and pure nisin groups. Notably, complete eradication of MRSA and E.coli O157:H7 was observed in yoghurt formulated with nisin NPs after 24 h and 5th day of storage, respectively. The shelf life of yoghurt inoculated with nisin nanoparticles was extended than those manufactured without addition of such nanoparticles.
Conclusions
Overall, the present study indicated that the addition of nisin NPs during processing of yoghurt could be a useful tool for food preservation against MRSA and E.coli O157:H7 in dairy industry.
Peer Review reports
Introduction
Using of bacteriocins such as nisin alone or combined with other natural materials such as essential oils, could be represented as a useful candidate for improving the microbiological quality and maintaining the sensory properties of milk and milk products [ 1 , 2 ]. The utility of nisin as a bio preservative in food industry has been approved and this bacteriocins was effective enough to extended shelf life in regions with inadequate preservation facilities such as developing countries [ 3 ]. Nisin is a natural water-soluble antibacterial peptide (AMP) composed of 34 amino acid residues produced by Lactococcus lactis. It has the ability to inhibit the growth of some foodborne pathogens and many of Gram-positive spoilage bacteria [ 4 , 5 ]. This antibacterial peptide is generally regarded as a safe food preservative by the joint Food and Agriculture Organization and World Health Organization (FAO/WHO), also by the US Food and Drug Administration (FDA) [ 6 , 7 ]. Based on aforementioned permissions, it is widely commercialized as a safe and natural food preservative in the food industry in more than 50 countries around the world [ 8 ].
The antibacterial activity of nisin in food is depending on several factors such as its solubility, pH and structural properties of target bacteria. It could exhibit potent antimicrobial activities against many species of Gram-positive pathogens, while it has little effect against Gram-negative bacteria, yeast and fungi due to their outer membrane barriers [ 9 ]. The exact antibacterial mechanism of nisin is attributed to the passage of nisin through the cell wall of bacteria and its interaction with lipid II, which considered as an essential element in the bacterial cell wall [ 9 ].
There are some obstacles that can hinder the antimicrobial efficacy of free nisin as a food bio preservative such as its ability to interact with food components (e.g. proteolytic enzymes, phospholipids, fatty acids and proteins), high pH and many other food additives. These factors could drastically reduce or completely diminish the antimicrobial effect of nisin [ 10 ]. Hence, different strategies were developed to improve the preservative efficacy of nisin such as liposomes [ 11 ] and nanoparticles [ 12 ]. However, these reported techniques are not suitable for applications in food industries due to the utility of inorganic solvents and chemical compounds, in addition to they are expensive and complicated. For these reasons, alternative organic chemicals and solvents or green synthesized nanoparticles were developed to overcome the inactivation of free nisin by many food components through protecting nisin and releasing it in sustained manner [ 13 ]. For instance, acetic acid, a well-known biocompatible organic acid, has no adverse effects, no dietary restrictions and it is generally recognized as a safe food additive. This organic acid is commonly used, as a natural preservative, in the preservation of food especially in cheese and dairy products where it inhibit the development of bacteria, yeast and fungi [ 14 , 15 ]. Besides acetic acid, tween 80 has a great potential to stabilize nanoparticles dispersion through formation of a protective coat around the nanoparticles, so it was used in food without adverse health effect [ 16 , 17 ].
Application of nisin in dairy industry was reported in more than 55 countries due to its prominent antimicrobial, technological characteristics, safety, stability and flavorless. Commercially, nisin was used in several food matrices to ensure safety, extend shelf life, and to improve the microbial quality either through addition of nisin directly in its purified form or through its production in situ by live bacteria [ 18 , 19 , 20 ]. For instance, nisin was added as a bio-preserving ingredient in some kinds of cheese [ 21 , 22 , 23 ], skim milk and whole milk [ 24 , 25 , 26 , 27 ]. Nisin has a potent antibacterial effect against spore-forming bacteria that are the main spoilage concerns in the food industry [ 26 ]. However, several factors such as neutral pH [ 4 ], Fat% [ 25 ], protein% [ 28 ] as well as calcium and magnesium concentrations that can reduce the antimicrobial efficacy of nisin were reported when used directly in dairy foods [ 15 , 29 , 30 ]. Certain previously reported strategies, such as encapsulation and nano-encapsulation of nisin, were applied to increase the antimicrobial efficacy of nisin in dairy industry [ 31 , 32 ]. . Importantly, there is no available data about the use of nisin or nisin NPs as antimicrobial agents during yoghurt preparation.
Accordingly, the current study was designed to prepare nisin NPs by simple nanoprecipitation technique using natural, biocompatible and safe materials. Also the aims of this study were extended to investigate the antibacterial effect of obtained nanoparticles on MRSA and E.coli O157:H7 during manufacturing and storage of yoghurt. Additionally, the effect of the used nisin NPs on the organoleptic properties of yoghurt was addressed.
Materials and methods
Acetic acid (Merck Co., Germany), nisin (Sigma Aldrich from Lactococcus lactis , potency ≥ 900 IU/mg, purity ≥ 95%, CAS Number 1414-45-5), Brain Heart Infusion (BHI) (BBL 11,407, USA), phosphate buffer saline (PBS) (Oxoid, Basingstoke, UK) were purchased and used as received. Polyethylene glycol sorbitan monooleate (Tween 80) was purchased from Sigma Aldrich. Additionally, Mueller Hinton agar (M173) was purchased from HiMedia (Pvt., India), and LAB204 Neogen Company. While, 0.5 McFarland Standard (8.2 log 10 CFU/ml) (Cat. No. TM50) was purchased from Dalynn Biologicals Co. The deionized water was obtained from the Molecular Biology Unit, Assiut University, Egypt.
Preparation of nisin nanoparticles
Nisin (2 mg/mL) was completely dissolved in 100 mL of 0.1 M aqueous acetic acid solution with the aid of sonication using cold probe sonication (UP100H Hielscher Ultrasound). Then, 50 mL of deionized distilled water was gradually added to the nisin solution while maintaining the pH value within the range of 2.5 to 3. Further, 0.01% tween 80 was added as a stabilizer and the mixture was constantly stirred at 25 oC for 7 h to eliminate acetic acid as much as possible. Finally, the nanoparticles suspensions were then sonicated for 5 min before stored at refrigerator temperature for further use. The obtained nanoparticles were examined for size, shape, antibacterial activity and stability after six months.
Characterization of the prepared nisin NPs
Dynamic light scattering (dls).
The prepared nanoparticles was characterized by DLS at a fixed scattered angle of 90° using a Zetasizer, ZS 90 (3000 HS, Malvern Instruments, Malvern, UK) at the Nanotechnology Unit, Al-Azhar University at Assiut, Egypt. Measurements were taken at 25 °C and Zetasizer® software (version 7.03) was used to collect and analyze the data [ 33 ].
Fourier-transform infrared spectroscopy (FTIR)
FTIR was performed at the Chemistry Department at the Faculty of Science, Assiut University. This experiment was used to identify the functional groups and the fingerprint of the molecule. Samples were prepared by compressing potassium bromide with either free nisin or NNPs into small discs. The produced discs were then scanned using FTIR spectrometer (FTIR, NICOLET, iS10, Thermo Scientific) in the wave number ranged from of 4000 to 500 cm − 1 [ 34 ].
High resolution transmission electron microscopy (HRTEM)
The morphology of the prepared nisin NPs was determined using HRTEM (JEM2100, Jeol, Japan) at the Electronic Microscope Unit, National Research Center, Egypt. The sample was diluted with deionized water, and a small drop of nisin NPs was dropped onto 200-mesh copper coated grids at room temperature and negatively stained with uranyl acetate for 3 min. Excess liquid was removed using Whatman filter paper and samples were dried at room temperature [ 35 ].
Bacterial strains and inoculum preparation
The tested pathogens (MRSA and E. coli O157:H7) were previously isolated from dairy products (milk, cheese and yoghurt) samples by culture method and identified using conventional biochemical method and PCR at a certified food lab, Animal Health Research Institute (AHRI), Egypt [ 36 , 37 ]. These isolates were inoculated in trypticase soy broth (Himedia, India) and incubated at 37˚C for 24 h, then co-cultured on selective agars such as MRSA agar base (Acumedia, 7420, USA) and Sorbitol MaCconkey agar (Himedia, India) [ 38 , 39 ] for MRSA and E. coli O157:H7, respectively. The isolates were inoculated in BHI broth and incubated at 37 °C for 24 h until turbidity was comparable to a 0.5 McFarland turbidity standard. Before inoculating bacteria in milk, the inoculum was washed twice in PBS and then re-suspended in skim milk.
Determination of minimum inhibitory concentration (MIC) of free nisin and nisin nanoparticles against MRSA and E. Coli O157:H7
To determine the MIC of nisin NPs against MRSA and E.coli O157:H7, the agar well diffusion method was used according to Suresh et al. [ 40 ] with minor modifications. In brief, 0.1 mL of the previously prepared bacterial suspensions was spread on Mueller Hinton agar plates and left for 10 min to be absorbed. Then, 8 mm wells were punched into the agar plates for testing the antimicrobial activity of nanoparticles. One-hundred µl of different concentrations of free nisin and nisin NPs (from 0.0313 mg/mL to 2 mg/mL) were poured onto the wells. One well in each plate contained 100 µL of sterile deionized water was kept as a negative control. After overnight incubation at 35 ± 2 °C, the diameters of the inhibition zones were observed and measured in mm [ 41 ]. Each concentration was performed in triplicate.
Assessment of nisin nanoparticles cytotoxicity
The biocompatibility and the cytotoxicity of the nisin NPs were evaluated using a MTT assay against a Vero cell line after culture at 37 °C in a humidified incubator with 5% CO 2 in Dulbecco’s Modified Eagle’s Medium supplemented with 10% Fetal Bovine Serum. The cells were seeded into a 96-well plate at a density of 1 × 10 4 cells/well overnight before treatment. Different dilutions (0.5×MIC, MIC, 2×MIC, 4×MIC) of optimized nisin NPs were added to the seeded cells. Cells without nanoparticles served as control group. After 72 h, the consumed media was replaced with phosphate buffered saline, 10 µL from 12 mM MTT stock solution was added to each well and cells were incubated for 4 h at 37 °C. Next, 50 µL DMSO was added to dissolve formazan crystals and then the absorbance was measured at 570 nm using a BMG LABTECH®-FLUO star Omega microplate reader (Ortenberg, Germany). All experiments were performed in triplicate.
Antibacterial efficacy of the free nisin and nisin NPs against MRSA and E. Coli O157:H7 during manufacturing and storage of yoghurt
Fresh milk was heated at 85 °C for 5 min in water bath then suddenly cooled. The prepared inoculums were added to the warmed milk (41 ºC) in a count of 10 6 CFU/mL. The inoculated milk was divided into four parts for further use as following, part 1 is the positive control (contained MRSA or E. coli O157:H7 only, one jar each), part 2 (contained MRSA or E. coli O157:H7 with nisin NPs at MIC and 2×MIC, two jars each), part 3 (contained MRSA or E. coli O157:H7 with free nisin at MIC and 2×MIC, two jars each) and part 4 (negative control; free from pathogens and contained free nisin or nisin NPs only, one jar each). After inoculation of the different treatments, yoghurt was manufactured according to Sarkar [ 42 ] by adding 2% yoghurt starter culture ( Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus ) at 41 °C to milk. The prepared yoghurt was placed in a constant-temperature incubator at 40 °C until pH reached 4.6 to 4.5. Finally, the obtained products were stored at refrigeration temperature (4 ± 1 °C) for 5 days. Samples were collected just after manufacturing of yoghurt and every 2 days during storage, then tested for the count of MRSA using MRSA agar base media [ 43 ], and E. coli O157:H7 using Sorbitol MacConkey (SMAC) agar plates [ 44 ]. In addition, pH values were determined in the examined samples as previously described by Igbabul et al. [ 45 ]. In brief, 10 g o f yoghurt sample was dissolved in 100 mL of distilled water. The mixture was left to equilibrate at room temperature. Then, the pH of the samples was then measured by a pH meter (Microprocessor pH meter, pH 537, WTW, Germany).
Organoleptic assay of manufactured yogurt
Pathogen-free yoghurt jars (negative control) were prepared with two concentrations of either free nisin or nisin NPs (MIC and 2×MIC) as previously mentioned to be used for organoleptic evaluation. Thirty-five panelists were selected in teams of different ages, sex and education. The perception of consumers toward samples with two concentrations of nisin NPs was recorded. Consumers were asked to evaluate the color, flavor, mouth feel, appearance, and overall acceptability (OAA) of the prepared yoghurt samples containing nisin NPs [ 46 ]. The scale points were excellent (5); very good (4); good (3); acceptable (2); and poor (1).
Statistical analysis
One-way analysis of variance (ANOVA) was performed using the SPSS program (SPSS Inc., Chicago, IL, USA, 18) to determine the statistical significance of differences between groups. Results with P < 0.05 were considered statistically significant. The microbiological and cytotoxicity assay data were prepared using Excel software version 2017. While, the FTIR results were performed using Origin Lab 2021 for graphing and analysis. All experiments were carried out in triplicate.
Characterization of the prepared nanoparticles
The freshly prepared nisin NPs had 26.55 nm size and PDI 0.227 as determined by zetasizer. While, the diameter of the same after 6 months at refrigeration temperature was 86.50 nm with a PDI equal to 0.431 (Table 1 ). These results indicated that reasonable small-sized particles of nisin were obtained by precipitation technique using acetic acid. The small size of the prepared particles and the small PDI range (from 0.2 to 0.4) indicated a mono size dispersion and a good stability of the prepared nisin NPs.
The size and morphology of the freshly prepared nisin NPs and after 6 months of storage were measured by HRTEM are presented in Fig. 1 . Both freshly prepared and stored nisin NPs were approximately uniform in size with adequate distribution of particles. The shape of the particles was nearly spherical with slightly a bit of agglomeration just after 6 months of storage. The average size of freshly prepared nisin NPs was 7.35 nm while, after 6 months was 15.4 nm. The size of particles determined by TEM is usually smaller than the dynamic particles determined by zeta-sizer because TEM determine the actual particle diameter while zeta-sizer determine the particles diameter with adjacent moving layers of solvents.
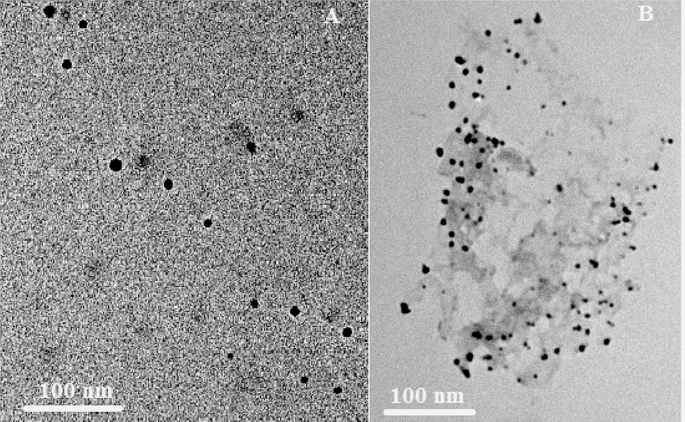
The TEM images of freshly prepared nisin NPs (A) and after 6th months of storage (B)
Figure 2 showed the FTIR of pure and nisin NPs; both spectrum showed the characteristic peaks of nisin at 3425, 1599 and 1493 cm − 1 corresponded to O-H stretching of COOH, C = O stretching of amide I and N-H bending amide II. Bands 1530 cm − 1 in free nisin indicated the stretching of amid II and which, increased to 1549 cm − 1 in nisin NPs that indicated increase the H- bond in nano form than free one. The results of FTIR spectrum confirmed that the formation of nisin NPs did not result in any chemical changes or interaction of nisin with used the materials. These results also demonstrated the suitability of the applied method for the preparation of chemically stable and small-sized nisin NPs.
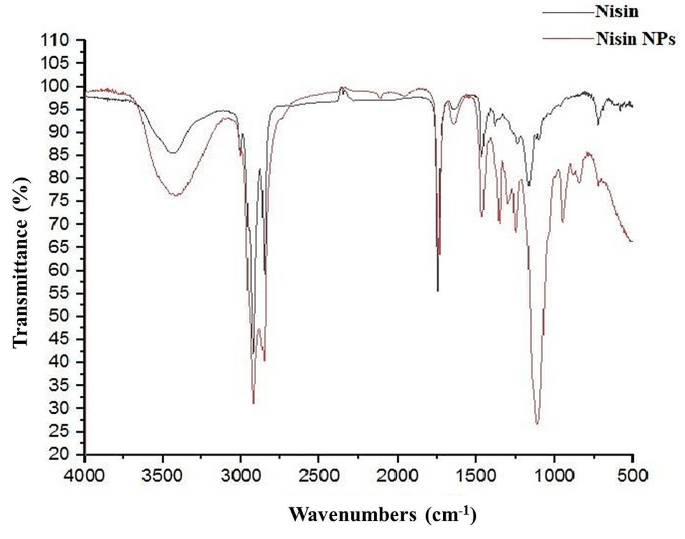
The FTIR of pure nisin and nisin NPs
Assessment of Nisin nanoparticles cytotoxicity
In the present study, Veros cells were exposed to nisin NPs for 48 and 72 h, and the cytotoxicity was measured by MTT assays. Results showed that the MIC did not exhibit an anti-proliferation effect (Fig. 3 ). Interestingly, even at very high concentrations (4xMIC), there were no cytotoxicity effect as the percentage of viable cells reach 92% and 89.98% after 48 and 72 h, respectively. The obtained findings confirmed the safety and good biocompatibility of the prepared nisin NPs at MIC level.
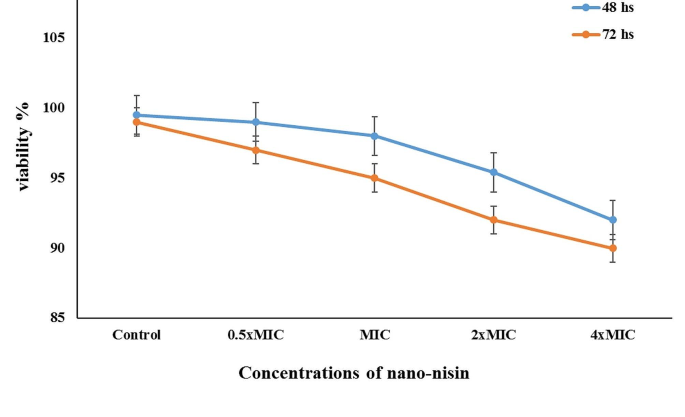
Cytotoxicity and cell viability of different concentrations nisin NPs using Vero cells after 48 and 72 h using MTT assay
MIC of free nisin and nisin NPs against MRSA and E. Coli O157:H7
The efficacy of the free nisin and prepared nisin NPs against MRSA and E. coli O157:H7 was investigated using agar well diffusion assay (Table 2 ). Nisin and its nanoparticles showed potent antibacterial effect against MRSA than E. coli O157:H7. The MICs of nisin and nisin NPs toward MRSA were 0.0625 and 0.0313 mg/mL, respectively. While, 0.125 mg/mL was the MIC of both nisin and nisin NPs against E. coli O157:H7. Of note, growth inhibition zone was not observed against MRSA at 0.0313 mg/mL of nisin, and toward E. coli O157:H7 at both 0.0625 and 0.0313 mg/mL nisin (Table 2 ). On the other hand, the prepared nisin NPs could produce inhibition zones against MRSA with a mean diameter ranged from 25.4 ± 2.1 mm to 7.1 ± 0.89 mm at concentrations of 2 to 0.0313 mg/mL, respectively. Also, the nisin NPs showed anti- E. coli O157:H7 activity at different concentrations of 2, 1, 0.5, 0.25 and 0.125 mg/mL with average size of 20.1, 15.4, 12.7, 9.5 and 7.2 mm of the inhibitory zones, respectively. There were no inhibition zones against E. coli O157:H7 at 0.0625 and 0.0313 mg/mL of nisin NPs. Overall, the obtained findings indicated that the most effective MICs of nisin and nisin NPs for both organisms were 0.125 mg/mL (Table 2 ).
Antibacterial effect of nisin and nisin NPs against MRSA and E. Coli O157:H7 during manufacturing and storage of yoghurt
Figure 4 presented the antibacterial activity of nisin against the examined foodborne pathogens (MRSA and E. coli O157:H7). Here, nisin at 0.125 and 0.25 mg/ml could induce antibacterial effect against MRSA (3.3 and 3 log 10 CFU/g, respectively) after 24 h of yoghurt storage. However the effect was not higher as in case of nisin NPs (2.3 and 1 log 10 CFU/g) at the same concentrations and time of storage. While, the inhibitory impact of the free nisin on E. coli O157:H7 was observed after 24 h (3.7 log 10 CFU/g) and 3 days (3.8 log 10 CFU/g) of storage at the concentrations of 0.25 and 0.125 mg/mL, respectively. The pathogens were still detected till the end of the experiment in nisin treated yoghurt (Fig. 4 ).
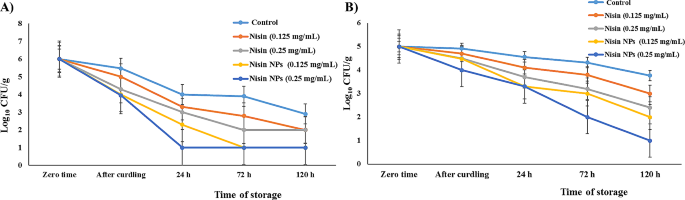
Antibacterial effect of free nisin (A) and nisin NPs (B) on MRSA and E.coli O157:H7 during manufacturing and storage of yoghurt
On the other hand, there was a clear reduction in mean count of MRSA and E.coli O157:H7 in the laboratory-manufactured yoghurt supplemented with different concentrations (0.125 and 0.25 mg/mL) of nisin NPs. A complete inhibition of MRSA was observed after 24 h and at the 3rd day of storage by 0.25 and 0.125 mg/mL of nisin NPs, respectively (Fig. 5 ). While, E. coli O157:H7 was undetectable at the 5th day of storage with 0.25 mg/mL nisin NPs, however it was still detected till the end of the experiment in either yoghurt inoculated with 0.125 mg/mL nisin NPs or in the positive control group (Fig. 4 ). Taken together, the antimicrobial count tests revealed that the free nisin is not effective as the nisin NPs at same time points during processing and storage of yoghurt.
During storage, the pH did not change significantly between different treatments. However, the negative control group showed little decrease in pH in comparison to other groups at the 3rd and 5th day of storage (3.5 and 3, respectively).
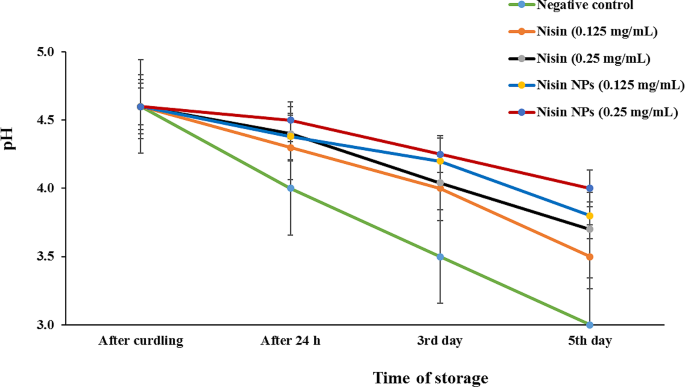
Evaluation of pH levels during processing and storage of yoghurt inoculated with different concentrations of free nisin or nisin NPs
Organoleptic evaluation of the laboratory-manufactured yoghurt
Figure 6 clarified that there was no difference in the sensory properties between the different groups (contained 0.125 or 0.25 mg/mL nisin (Fig. 6A) or nisin NPs (Fig. 6B)) in comparison to the control group. The OAA of yoghurt inoculated with 0.125 mg/mL and 0.25 mg/mL of free nisin was 3 and 2.5, respectively (Fig. 6A). While, the control samples had the highest score in mouth feel (4.5), followed in order with yoghurt loaded with 0.125 mg/mL and 0.25 mg/mL nisin NPs (3.8 and 2.7, respectively). Additionally, the overall acceptability (OOA) of control, 0.125 mg/mL and 0.25 mg/mL nisin NPs groups was 4, 3.7 and 3, respectively (Fig. 6B). Such findings indicated the high acceptability of yoghurt containing different concentrations of nisin NPs than those inoculated with free nisin.
Organoleptic properties of yoghurt inoculated with different concentrations of free nisin and nisin NPs
The current study elucidated for the first time the inhibitory effect of free nisin and nisin NPs on two of the most common foodborne pathogens (MRSA and E. coli O157:H7) during processing and storage of laboratory manufactured yoghurt. Strikingly, adding of nisin NPs to yoghurt could induce much higher antibacterial effect on MRSA and E. coli O157:H7 with high consumer acceptability than free nisin. Accordingly, nisin NPs could be a useful and effective bio-preservative candidate against MRSA and E. coli O157:H7 in dairy industry.
The present study revealed that nisin NPs was prepared by a novel and safe method using natural material such as acetic acid which is commonly applied in food products. Chang et al. [ 47 ]. prepared ultra-small sizes of nisin NPs by nanoprecipitation method using HCL while we obtained much smaller particle size of NNPs using acetic acid which is more safer, less toxic and accepted by consumers. The particle size determined by TEM is smaller than the size measured by DLS this difference could be attributed to the removal of solvent and shrinking of nanoparticles during the drying of nisin NPs samples for TEM investigations. In addition, DLS measures the hydrodynamic diameter of the dispersed moving particles with the surrounding moving layers of solvents [ 48 , 49 ].
The result of FTIR was in consistent with that of Flynn et al. [ 50 ]. Herein, we found that the -OH stretching peak of nisin NPs displayed a greater intensity than that of free nisin, which indicated a stronger hydrogen bonding formation within nisin NPs. In case of free nisin, the peak at 1620 cm − 1 corresponding to COO − was shifted to 1610 cm − 1 in nisin NPs indicating that the hydrogen bonding was increased within nisin NPs. In contrast, the amid II band in free nisin appeared at 1530 cm − 1 became more obvious at 1549 cm − 1 in nisin NPs which was in agreement with Webber et al. [ 51 ]. . Band of amide I at wave number of 1632 cm − 1 could be due to the change in the structure of free nisin when converted into nisin NPs by using natural acetic acid.
In food chain, nisin has been approved for use in over 50 countries due to its safety and its potent antimicrobial activity without inducing microbial resistance [ 52 ]. Of particular note, the FAO/WHO Codex Committee and US FDA allow using nisin as a food additive in dairy products at a concentration up to 250 mg/kg [ 1 , 53 ]. Moreover, European Food Safety Authority [ 54 ] reported that nisin has been shown to be non-toxic to humans and it is safe as a food preservative for dairy and meat products. In the current study, the examined organisms (MRSA and E. coli O157:H7) have been involved in many food outbreaks worldwide as well as their resistance to many antibiotics, considered a challenge to be controlled [ 55 , 56 , 57 ]. Therefore, the present study could be a useful alternative strategy to avoid the possible health hazards of these organisms after consumption of yoghurt using either nisin or nisin NPs as natural food preservatives.
The obtained results revealed that the MICs of nisin and nisin NPs against MRSA were lower than that of E. coli O157:H7. This could be due to the ability of nisin to penetrate the cell wall of Gram-positive bacteria, however, it is difficult for nisin to penetrate the outer membrane barrier of Gram-negative bacteria [ 58 ]. Nisin could destroy bacteria through two mechanisms, either by making pores in the plasma membrane or by inhibiting the cell wall biosynthesis through binding to lipid II [ 59 , 60 , 61 ]. Importantly, the obtained results in the current study showed that that MIC of nisin NPs against MRSA was lower than that of pure nisin. Similarly, Zohri et al. [ 62 ] reported that the MICs of nisin and Nisin-Loaded nanoparticles was 2 and 0.5 mg/mL after 72 h of incubation period with the S. aureus samples, respectively. In addition, Moshtaghi et al. [ 63 ] examined the antibacterial effect of nisin on S. aureus and E. coli at different pH values and they found that the MICs against S. aureus were ranged from 19 to 312 µg/mL of nisin at pH levels from 8 to 5.5, respectively. While for E. coli , the MICs were from 78 to 1250 µg/mL at the same range of pH, respectively [ 63 ].
Interestingly, nisin inhibited the pathogenic foodborne bacteria and many other Gram-positive food spoilage microorganisms [ 13 ]. In the present study, evaluation of the kinetic growth of MRSA and E. coli O157:H7 based on the total counts in the laboratory manufactured yoghurt revealed that nisin NPs was able to inhibit more effectively the growth of such foodborne pathogens than free nisin during manufacturing and storage of yoghurt. These findings were in concurrent with those obtained by Zohri et al. [ 62 ] who demonstrated that nisin-loaded chitosan/alginate nanoparticles showed more antibacterial effect than free nisin on the growth of S. aureus in raw and pasteurized milk samples. Additionally, nisin Z in liposomes can provide a powerful tool to improve nisin stability and inhibitory action against Listeria innocua in the cheddar cheese [ 64 ]. In our study, nisin NPs showed a complete inhibition of MRSA after curdling of yoghurt and reduced the survivability of E. coli O157:H7 when applied at two different concentrations during storage of such product. Nisin NPs with high specific surface area could be easily attached to the target cell surface leading to increased permeability of the cell membrane, and finally cause bacterial cell death. Furthermore, nisin NPs were thermo-tolerant because of the internal non-covalent interactions in the nanoparticles [ 4 , 65 ]. Additionally, the decline in the mean count of the examined pathogens (MRSA and E.coli O157: H7) in the current study may be due to the effect of low pH (high acidity) of yoghurt that leads to shrinkage and death of the bacterial cells [ 66 ]. Similarly, Al-Nabulsi et al. [ 67 ] reported that the combination of a starter culture, low temperature, and pH ( ∼ 5.2) had inhibitory effects on the growth of S. aureus .
The effect of adding different levels of nisin and nisin NPs on OAA scores of yoghurt was recorded and the obtained results were in agreement with Hussain et al. [ 68 ], Radha [ 3 ], and Gharsallaoui et al. [ 4 ] who reported that a Nigerian fermented milk product had acceptable sensory scores till 25th day of storage when loaded with nisin at 400 IU/mL. Additionally, Chang et al. [ 47 ] said that the thermal treatments are known to cause undesirable changes in the sensory, nutritional and/or technological properties of milk. Taking advantage of the antimicrobial action of nisin NPs against several spoilage and pathogenic microorganisms, this innovative non-thermal food preservative offers the inactivation of microorganisms with minimal impact on the quality, safety, nutritional values and acceptability of dairy products.
Overall, as the demand for preservative-free food products increased, natural antimicrobials have gained more and more attention because of their effectiveness and safety. Consequently, the current study investigated that the addition of nisin NPs to milk for manufacturing of yoghurt can be used as an innovative preventive measure to inhibit the contamination with foodborne pathogens. However, further researches are required to determine the effective and safe dose of nisin NPs for application in other dairy products.
The present study prepared nisin NPs using acetic acid by precipitation method and the obtained particles were small in size with good stability and consumer acceptability. The antibacterial effect of nisin and nisin NPs against MRSA and E. coli O157:H7 in yoghurt was impressive. Additionally, the studied nanoparticles did not affect the sensory and textural characteristics of the finished product. Hence, this study could be useful for yoghurt makers and dairy products factories through using this novel preservation technology to inhibit the growth of MRSA and E. coli O157:H7, in yoghurt and dairy products, and subsequently avoid food spoilage and foodborne diseases.
Data availability
All data and materials are available here in the current study.
Ibarra-Sánchez LA, El-Haddad N, Mahmoud D, Miller MJ, Karam L. Invited review: advances in nisin use for preservation of dairy products. J Dairy Sci. 2020;103(3):2041–52.
Article PubMed Google Scholar
Batiha G, Hussein DE, Algammal A, George T, Jeandet P, Al-Snafi A, Tiwari A, Pagnossa J, Gonçalves Lima CM, Thorat N et al. Application of Natural antimicrobials in Food Preservation: recent views. Food Control 2021, 126.
Radha K. Nisin as a biopreservative for pasteurized milk. Indian J Veterinary Anim Sci Res. 2014;10(6):436–44.
Google Scholar
Gharsallaoui A, Oulahal N, Joly C, Degraeve P. Nisin as a Food Preservative: part 1: Physicochemical Properties, Antimicrobial Activity, and Main uses. Crit Rev Food Sci Nutr. 2016;56(8):1262–74.
Article CAS PubMed Google Scholar
Gao S, Zhai X, Cheng Y, Zhang R, Wang W, Hou H. Starch/PBAT blown antimicrobial films based on the synergistic effects of two commercial antimicrobial peptides. Int J Biol Macromol. 2022;204:457–65.
FDA/HHS. Direct food substances affirmed as generally recognized as safe: nisin preparation. Fed Regulations. 1988;53:11247–51.
Thomas LV, Clarkson MR, Delves-Broughton J. In A. S. Naidu, editor, Nisin: Natural food antimicrobial systems (Florida, Boca Raton: CRC) 2000:Press, 463–524.
Pimentel-Filho Nde J, Martins MC, Nogueira GB, Mantovani HC, Vanetti MC. Bovicin HC5 and Nisin reduce Staphylococcus aureus adhesion to polystyrene and change the hydrophobicity profile and Gibbs free energy of adhesion. Int J Food Microbiol. 2014;190:1–8.
Thébault P, Ammoun M, Boudjemaa R, Ouvrard A, Steenkeste K, Bourguignon B, Fontaine-Aupart M-P. Surface functionalization strategy to enhance the antibacterial effect of nisin Z peptide. Surf Interfaces. 2022;30:101822.
Article Google Scholar
Liu G, Nie R, Liu Y, Mehmood A. Combined antimicrobial effect of bacteriocins with other hurdles of physicochemic and microbiome to prolong shelf life of food: a review. Sci Total Environ. 2022;825:154058.
Brum LFW, Dos Santos C, Zimnoch Santos JH, Brandelli A. Structured silica materials as innovative delivery systems for the bacteriocin nisin. Food Chem. 2022;366:130599.
Kazemzadeh S, Abed-Elmdoust A, Mirvaghefi A, Hosseni Seyed V, Abdollahikhameneh H. Physicochemical evaluations of chitosan/nisin nanocapsulation and its synergistic effects in quality preservation in tilapia fish sausage. J Food Process Preserv. 2022;46(3):e16355.
Article CAS Google Scholar
Elsherif W, Zeinab A-E. Effect of nisin as a biopreservative on shelf life of pasteurized milk. Assiut Veterinary Med J. 2019;65:1–24.
Hu Y, Wu T, Wu C, Fu S, Yuan C, Chen S. Formation and optimization of chitosan-nisin microcapsules and its characterization for antibacterial activity. Food Control. 2017;72:43–52.
Khan I, Oh D-H. Integration of nisin into nanoparticles for application in foods. Innovative Food Sci Emerg Technol. 2016;34:376–84.
Zhao Y, Wang Z, Zhang W, Jiang X. Adsorbed Tween 80 is unique in its ability to improve the stability of gold nanoparticles in solutions of biomolecules. Nanoscale. 2010;2(10):2114–9.
Bekhit M, Abu el-naga MN, Sokary R, Fahim RA, El-Sawy NM. Radiation-induced synthesis of tween 80 stabilized silver nanoparticles for antibacterial applications. J Environ Sci Health Part A. 2020;55(10):1210–7.
Cui HY, Wu J, Li CZ, Lin L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J Dairy Sci. 2016;99(11):8598–606.
Kondrotiene K, Kasnauskyte N, Serniene L, Gölz G, Alter T, Kaskoniene V, Maruska AS, Malakauskas M. Characterization and application of newly isolated nisin producing Lactococcus lactis strains for control of Listeria monocytogenes growth in fresh cheese. LWT. 2018;87:507–14.
Santos JCP, Sousa RCS, Otoni CG, Moraes ARF, Souza VGL, Medeiros EAA, Espitia PJP, Pires ACS, Coimbra JSR, Soares NFF. Nisin and other antimicrobial peptides: production, mechanisms of action, and application in active food packaging. Innovative Food Sci Emerg Technol. 2018;48:179–94.
Van Tassell ML, Ibarra-Sánchez LA, Takhar SR, Amaya-Llano SL, Miller MJ. Use of a miniature laboratory fresh cheese model for investigating antimicrobial activities. J Dairy Sci. 2015;98(12):8515–24.
Ibarra-Sánchez LA, Van Tassell ML, Miller MJ. Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018;72:128–34.
Feng Y, Ibarra-Sánchez LA, Luu L, Miller MJ, Lee Y. Co-assembly of nisin and zein in microfluidics for enhanced antilisterial activity in Queso Fresco. LWT. 2019;111:355–62.
Jung D-S, Bodyfelt FW, Daeschel MA. Influence of Fat and emulsifiers on the efficacy of Nisin in inhibiting Listeria monocytogenes in Fluid Milk1. J Dairy Sci. 1992;75(2):387–93.
Bhatti M, Veeramachaneni A, Shelef LA. Factors affecting the antilisterial effects of nisin in milk. Int J Food Microbiol. 2004;97(2):215–9.
Saad MA, Ombarak RA, Abd Rabou HS. Effect of nisin and lysozyme on bacteriological and sensorial quality of pasteurized milk. J Adv Veterinary Anim Res. 2019;6(3):403–8.
Chen H, Zhong Q. Lactobionic acid enhances the synergistic effect of nisin and thymol against Listeria monocytogenes Scott A in tryptic soy broth and milk. Int J Food Microbiol. 2017;260:36–41.
Wirjantoro TI, Lewis MJ, Grandison AS, Williams GC, Delves-Broughton J. The effect of nisin on the keeping quality of reduced heat-treated milks. J Food Prot. 2001;64(2):213–9.
Silva CCG, Silva SPM, Ribeiro SC. Application of Bacteriocins and protective cultures in dairy food preservation. Front Microbiol. 2018;9:594.
Article PubMed PubMed Central Google Scholar
Houlihan AJ, Russell JB. The effect of calcium and magnesium on the activity of bovicin HC5 and nisin. Curr Microbiol. 2006;53(5):365–9.
da Silva Malheiros P, Daroit DJ, da Silveira NP, Brandelli A. Effect of nanovesicle-encapsulated nisin on growth of Listeria monocytogenes in milk. Food Microbiol. 2010;27(1):175–8.
Martinez RCR, Alvarenga VO, Thomazini M, Fávaro-Trindade CS, Sant’Ana AS. Assessment of the inhibitory effect of free and encapsulated commercial nisin (Nisaplin®), tested alone and in combination, on Listeria monocytogenes and Bacillus cereus in refrigerated milk. LWT - Food Sci Technol. 2016;68:67–75.
Lu P-J, Fu W-E, Huang S-C, Lin C-Y, Ho M-L, Chen Y-P, Cheng H-F. Methodology for sample preparation and size measurement of commercial ZnO nanoparticles. J Food Drug Anal. 2018;26(2):628–36.
Bi S, Ahmad N. Green synthesis of palladium nanoparticles and their biomedical applications. Materials Today: Proceedings 2022, 62:3172–3177.
Gruskiene R, Krivorotova T, Staneviciene R, Ratautas D, Serviene E, Sereikaite J. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin. Colloids Surf B. 2018;169:126–34.
Zakaria IM, Elsherif WM. Bactericidal effect of silver nanoparticles on methicillin resistant Staphylococcus aureus (MRSA) isolated from bulk milk tanks. Anim Health Res J. 2018;6(3):42–51.
Elsherif W, Ali DN. Antibacterial effect of silver nanoparticles on antibiotic resistant E. Coli O157:H7 isolated from some dairy products. Bulgarian J Veterinary Med 2020:442.
Bennett RW, Lancette GA. Detection of Staphylococcus aureus in food samples. Bacteriological Analytical Manual (BAM), Ch. 12./ FoodScience Research /Laboratory Methods/ucm071429htm 2016.
Sancak YC, Sancak H, Isleyici O. Presence of Escherichia coli O157 and O157:H7 in raw milk and Van Herby cheese. Bull Veterinary Inst Pulawy 2015, 59.
Suresh S, Karthikeyan S, Saravanan P, Jayamoorthy K. Comparison of antibacterial and antifungal activities of 5-amino-2-mercaptobenzimidazole and functionalized NiO nanoparticles. Karbala Int J Mod Sci. 2016;2(3):188–95.
Hassanien AA, Shaker EM. Investigation of the effect of chitosan and silver nanoparticles on the antibiotic resistance of Escherichia coliO157:H7 isolated from some milk products and diarrheal patients in Sohag City, Egypt. Veterinary World. 2020;13(8):1647–53.
Article CAS PubMed PubMed Central Google Scholar
Sarkar S. Effect of Nisin on Techno- logical and microbiological characteristics of stirred Yoghurt. J Microbiol Microb Technol. 2016;1(1):6.
Sivaraman GK, Gupta S, Sivam V, Muthulakshmi T, Elangovan R, Perumal V, Balasubramanium G, Lodha T, Yadav A. Prevalence of S. Aureus and/or MRSA from seafood products from Indian seafood products. BMC Microbiol 2022, 22.
Anyanwu M, Cugwu I, Okorie-Kanu O, Ngwu M, Kwabugge Y, Chioma A, Chah K. Sorbitol non-fermenting Escherichia coli and E. Coli O157: prevalence and antimicrobial resistance profile of strains in slaughtered food animals in Southeast Nigeria. Access Microbiol 2022, 4.
Igbabul B, Hiikyaa O, Amove J. Effect of fermentation on the Proximate Composition and Functional Properties of Mahogany Bean (Afzelia africana) Flour. Curr Res Nutr Food Sci J. 2014;2:01–7.
Lawless H, Heymann H. Sensory Evaluation of Food Science Principles and Practices. Chapter 1, 2nd Edition, Ithaca, New York 2010: https://doi.org/10.1007/1978-1001-4419-6488-1005 .
Chang R, Lu H, Li M, Zhang S, Xiong L, Sun Q. Preparation of extra-small nisin nanoparticles for enhanced antibacterial activity after autoclave treatment. Food Chem. 2018;245:756–60.
Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol 2008, 7.
Krivorotova T, Cirkovas A, Maciulyte S, Staneviciene R, Budriene S, Serviene E, Sereikaite J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocolloids. 2016;54:49–56.
Flynn J, Durack E, Collins MN, Hudson SP. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J Mater Chem B. 2020;8(18):4029–38.
Webber JL, Namivandi-Zangeneh R, Drozdek S, Wilk KA, Boyer C, Wong EHH, Bradshaw-Hajek BH, Krasowska M, Beattie DA. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci Rep. 2021;11(1):1690.
Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Biomedical applications of nisin. J Appl Microbiol. 2016;120(6):1449–65.
Sobrino-López A, Martín-Belloso O. Use of Nisin and other bacteriocins for preservation of dairy products. Int Dairy J. 2008;18(4):329–43.
European Food Safety Authority. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to the use of nisin (E 234) as a food additive. EFSA J. 2005;3146:1–16.
WHO. Monitoring of Antimicrobial Resistance. Report of an Intercountry Workshop. 2003 Oct 14–17; Tamil Nadu, India 2004.
Titouche Y, Akkou M, Houali K, Auvray F, Hennekinne JA. Role of milk and milk products in the spread of methicillin-resistant Staphylococcus aureus in the dairy production chain. J Food Sci. 2022;87(9):3699–723.
Eltokhy HE, Abdelsamei HM, El barbary H, Nassif, mZ. Prevalence of some pathogenic bacteria in dairy products. Benha Veterinary Med J. 2021;40(2):51–5.
Li Q, Montalban-Lopez M, Kuipers OP. Increasing the antimicrobial activity of Nisin-based lantibiotics against Gram-negative pathogens. Appl Environ Microbiol 2018, 84(12).
Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Sci (New York NY). 2006;313(5793):1636–7.
‘t Hart P, Oppedijk SF, Breukink E, Martin NI. New insights into Nisin’s antibacterial mechanism revealed by binding studies with synthetic lipid II analogues. Biochemistry. 2016;55(1):232–7.
Tol MB, Morales Angeles D, Scheffers DJ. In vivo cluster formation of nisin and lipid II is correlated with membrane depolarization. Antimicrob Agents Chemother. 2015;59(6):3683–6.
Zohri M, Alavidjeh MS, Haririan I, Ardestani MS, Ebrahimi SE, Sani HT, Sadjadi SK. A comparative study between the Antibacterial Effect of Nisin and Nisin-Loaded Chitosan/Alginate Nanoparticles on the growth of Staphylococcus aureus in Raw and pasteurized milk samples. Probiotics Antimicrob Proteins. 2010;2(4):258–66.
Moshtaghi H, Rashidimehr A, Shareghi B. Antimicrobial activity of Nisin and Lysozyme on Foodborne pathogens Listeria Monocytogenes, Staphylococcus Aureus, Salmonella Typhimurium, and Escherichia Coli at different pH. J Nutrtion Food Secur. 2018;3(4):193–201.
Benech RO, Kheadr EE, Laridi R, Lacroix C, Fliss I. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl Environ Microbiol. 2002;68(8):3683–90.
Hegedüs I, Nagy E. Stabilization of activity of hemicellulase enzymes by covering with polyacrylamide layer. Chem Eng Process. 2015;95:143–50.
Normanno G, La Salandra G, Dambrosio A, Quaglia NC, Corrente M, Parisi A, Santagada G, Firinu A, Crisetti E, Celano GV. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int J Food Microbiol. 2007;115(3):290–6.
Al-Nabulsi AA, Osaili TM, AbuNaser RA, Olaimat AN, Ayyash M, Al-Holy MA, Kadora KM, Holley RA. Factors affecting the viability of Staphylococcus aureus and production of enterotoxin during processing and storage of white-brined cheese. J Dairy Sci. 2020;103(8):6869–81.
Hussain SA, Garg FC, Pal D. Effect of different preservative treatments on the shelf-life of sorghum malt based fermented milk beverage. J Food Sci Technol. 2014;51(8):1582–7.
Download references
Acknowledgements
The authors thank the nanotechnology research and synthesis unit at animal health research institute, Assiut, Egypt for their help in preparation of nanomaterials.
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Certified Food Lab, Nanotechnology Research and Synthesis Unit, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Assiut,, Egypt
Walaa M. Elsherif
Faculty of Health Sciences Technology, New Assiut Technological University (NATU), Assiut, Egypt
Walaa M. Elsherif & Gamal M. Zayed
Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
Alshimaa A. Hassanien
Department of Pharmaceutics and Pharmaceutical Technology, Al-Azhar University, Assiut, Egypt
Gamal M. Zayed
Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt
Sahar M. Kamal
You can also search for this author in PubMed Google Scholar
Contributions
W.M.E., A.A.H., G.M.Z., and S.M.K. conceived and designed the experiment. W.M.E., A.A.H., G.M.Z., and S.M.K. collected the experimental data. W.M.E., A.A.H., and S.M.K. performed the microbiological analysis. A.A.H. and G.M.Z. performed the preparation and analysis of nanoparticles. W.M.E. and S.M.K. performed the statistical analysis. All authors interpreted the data. W.M.E. wrote the first draft of the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Sahar M. Kamal .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elsherif, W.M., Hassanien, A.A., Zayed, G.M. et al. Natural approach of using nisin and its nanoform as food bio-preservatives against methicillin resistant Staphylococcus aureus and E.coli O157:H7 in yoghurt. BMC Vet Res 20 , 192 (2024). https://doi.org/10.1186/s12917-024-03985-1
Download citation
Received : 12 October 2023
Accepted : 21 March 2024
Published : 11 May 2024
DOI : https://doi.org/10.1186/s12917-024-03985-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- E.coli O157:H7
- Nanoparticles
- Cytotoxicity
- Food preservative
BMC Veterinary Research
ISSN: 1746-6148
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Abstract. This review is provided a detailed overview of the synthesis, properties and applications of nanoparticles (NPs) exist in different forms. NPs are tiny materials having size ranges from 1 to 100 nm. They can be classified into different classes based on their properties, shapes or sizes.
The unique features of nanomaterials are highlighted throughout the review. This review describes advances in nanomaterials, specifically fullerenes, carbon nanotubes, graphene, carbon quantum dots, nanodiamonds, carbon nanohorns, nanoporous materials, core-shell nanoparticles, silicene, antimonene, MXenes, 2D MOF nanosheets, boron nitride ...
Similarly, a list of autotrophic plants and heterotrophic microbes that help in the formation of Ag NPs along with possible nucleation mechanisms are presented in recent review articles [153,181-184]. This list assists in identifying the crucial factor that induces nanoparticle nucleation.
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery's energy density, rate capability, and cycling stability.
Advances in nanoparticle design could make substantial contributions to personalized and non-personalized medicine. In this Review, Langer, Mitchell, Peppas and colleagues discuss advances in ...
A review. Nanoparticle research is a fascinating branch of science. The strongly size-related properties of nanoparticles offer uncountable opportunities for surprising discoveries. The often unexpected and unprecedented behavior of nanoparticles bears great potential for innovative technol. applications, but also poses great challenges to the ...
This review describes the different nanoparticles and nanostructured material synthesis approaches and presents some emerging biomedical, healthcare, and agro-food applications. ... This review article discusses the unique features of nanomaterials that are exploited for different biomedical applications (Figure 1). It also presents recent ...
The Review discusses the state-of-the-art polymer nanocomposites from three key aspects: dipole activity, breakdown resistance and heat tolerance for capacitive energy storage applications ...
Nanoreactors that isolate chemical precursors within a confined space are promising tools for synthesizing nanoparticles. This Review unifies the many classes of solution-based and substrate ...
In this comprehensive review, we report different synthetic approaches to Cu and Cu-based nanoparticles (metallic copper, copper oxides, and hybrid copper nanostructures) and copper nanoparticles immobilized into or supported on various support materials (SiO 2, magnetic support materials, etc.), along with their applications in catalysis. The ...
The first group of articles is dedicated to gold nanoparticles and clusters. Indeed, this metal was the first for which the small size was shown to be crucial in nanocatalysis. Haruta and Ishida review the Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes.
Nanoparticles developed through the reverse micelle method are amazingly fine and monodispersed in nature. 90 Fig. 11 ... synthesis, 421 modulated synthesis, 422 three-layer synthesis, 423 and surfactant-mediated synthesis. 424 A handful of review articles about 2D-MOFs from a synthetic perspective discuss the challenging issues of the large ...
This paper presents a review on nanoparticles, their types, properties, synthesis methods and its applications in the field of environment. Export citation and abstract BibTeX RIS. Previous article in issue. Next article in issue. Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further ...
Nanotechnology refers to an emerging field of science that. includes synthesis and development of vario us nanomaterials. Nanoparticles can be defined as objects ranging in size from 1-. 100 nm ...
Ningqiang Gong. Neil C. Sheppard. Michael J. Mitchell. Review Article 12 Jan 2021. Nature Nanotechnology ( Nat. Nanotechnol.) ISSN 1748-3395 (online) ISSN 1748-3387 (print) Browse the archive of ...
Interest in nanomaterials and especially nanoparticles has exploded in the past decades primarily due to their novel or enhanced physical and chemical properties compared to bulk material. These extraordinary properties have created a multitude of innovative applications in the fields of medicine and pharma, electronics, agriculture, chemical catalysis, food industry, and many others. More ...
This concise review emphasizes the various preparative methods of polymeric nanoparticles developed in the last few decades and their uses in various branches of science, e.g., therapeutic, optoelectronic, catalytic and magnetic applications along with several challenging issues concerning the commercialization of emerging polymer-based ...
Journal of Nanoparticle Research is a peer-reviewed journal that delves into concepts, properties, phenomena, and processes of structures at the nanoscale. Covered topics include synthesis, assembly, transport, reactivity, and stability of nanoscale structures. Features applications, structures, and devices with novel functions via precursor ...
Carbon-based nanomaterials (CBNs) have drawn a lot of attention due to their distinct physical and chemical properties. CBNs, such as fullerenes, carbon nanotubes, carbon nanofibers, carbon quantum dots, graphene, and other derivatives have been thoroughly investigated in environmental remediation, analytical chemistry and sensing, antimicrobial activities, microbial fuel cells, and renewable ...
Nanoparticles articles from across Nature Portfolio. Nanoparticles are particles that exist on a nanometre scale (i.e., below 100 nm in at least one dimension). They can possess physical ...
The potential of superferromagnetic iron oxide nanoparticle chains (SFMIOs) as new MPI tracers further advanced the imaging quality and expanded clinical applications, underscoring the promising future of this emerging imaging modality. ... Review Article. Submitted 18 Mar 2024. Accepted 23 May 2024. First published 24 May 2024. Download Citation.
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery's energy density, rate capability, and cycling stability.
DOI: 10.1002/adma.202403986. A new study conducted by the Wilhelm Lab at the University of Oklahoma examines a promising development in biomedical nanoengineering. Published in Advanced Materials ...
Background Natural antimicrobial agents such as nisin were used to control the growth of foodborne pathogens in dairy products. The current study aimed to examine the inhibitory effect of pure nisin and nisin nanoparticles (nisin NPs) against methicillin resistant Staphylococcus aureus (MRSA) and E.coli O157:H7 during the manufacturing and storage of yoghurt. Nisin NPs were prepared using new ...