- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


Chapter 5: 10 Real Cases on Acute Heart Failure Syndrome: Diagnosis, Management, and Follow-Up
Swathi Roy; Gayathri Kamalakkannan
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Case review, case discussion.
- Full Chapter
- Supplementary Content
Case 1: Diagnosis and Management of New-Onset Heart Failure With Reduced Ejection Fraction
A 54-year-old woman presented to the telemetry floor with shortness of breath (SOB) for 4 months that progressed to an extent that she was unable to perform daily activities. She also used 3 pillows to sleep and often woke up from sleep due to difficulty catching her breath. Her medical history included hypertension, dyslipidemia, diabetes mellitus, and history of triple bypass surgery 4 years ago. Her current home medications included aspirin, atorvastatin, amlodipine, and metformin. No significant social or family history was noted. Her vital signs were stable. Physical examination showed bilateral diffuse crackles in lungs, elevated jugular venous pressure, and 2+ pitting lower extremity edema. ECG showed normal sinus rhythm with left ventricular hypertrophy. Chest x-ray showed vascular congestion. Laboratory results showed a pro-B-type natriuretic peptide (pro-BNP) level of 874 pg/mL and troponin level of 0.22 ng/mL. Thyroid panel was normal. An echocardiogram demonstrated systolic dysfunction, mild mitral regurgitation, a dilated left atrium, and an ejection fraction (EF) of 33%. How would you manage this case?
In this case, a patient with known history of coronary artery disease presented with worsening of shortness of breath with lower extremity edema and jugular venous distension along with crackles in the lung. The sign and symptoms along with labs and imaging findings point to diagnosis of heart failure with reduced EF (HFrEF). She should be treated with diuretics and guideline-directed medical therapy for congestive heart failure (CHF). Telemetry monitoring for arrythmia should be performed, especially with structural heart disease. Electrolyte and urine output monitoring should be continued.
In the initial evaluation of patients who present with signs and symptoms of heart failure, pro-BNP level measurement may be used as both a diagnostic and prognostic tool. Based on left ventricular EF (LVEF), heart failure is classified into heart failure with preserved EF (HFpEF) if LVEF is >50%, HFrEF if LVEF is <40%, and heart failure with mid-range EF (HFmEF) if LVEF is 40% to 50%. All patients with symptomatic heart failure should be started on an angiotensin-converting enzyme (ACE) inhibitor (or angiotensin receptor blocker if ACE inhibitor is not tolerated) and β-blocker, as appropriate. In addition, in patients with New York Heart Association functional classes II through IV, an aldosterone antagonist should be prescribed. In African American patients, hydralazine and nitrates should be added. Recent recommendations also recommend starting an angiotensin receptor-neprilysin inhibitor (ARNI) in patients who are symptomatic on ACE inhibitors.
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 September 2024
Abnormal left atrial strain and left atrial stiffness index are associated with adverse outcomes in children with cardiomyopathies: a pilot study
- Katarzyna Łuczak-Woźniak 1 ,
- Cezary Niszczota 2 ,
- Klaudia Obsznajczyk 2 &
- Bożena Werner 3
Scientific Reports volume 14 , Article number: 21059 ( 2024 ) Cite this article
Metrics details
- Paediatric research
- Risk factors
Conventional diastolic dysfunction parameters seem to be imperfect when applied to the pediatric cardiomyopathy population. The aim of this pilot study was to search for novel echocardiographic parameters associated with adverse outcomes in children with the most common cardiomyopathies. Fifty-six patients with pediatric cardiomyopathies (28 with dilated, 21 with hypertrophic, 7 with left ventricular non-compaction cardiomyopathy) and 28 healthy subjects were included in the study. Left atrial reservoir (LASr), conduit (LAScd) and contraction (LASct) strain, left atrial stiffness index (LASI), as well as conventional diastolic dysfunction parameters were measured using echocardiography. Adverse outcomes were defined as heart failure (including heart transplant) and arrhythmic endpoints. Patients with adverse outcomes presented with significantly lower LASr (16.68% ± 8.64% vs. 33.97% ± 9.99%, p-value < 0.001), lower LAScd (− 10.37% ± 5.83% vs. − 25.50% ± 9.24%, p-value < 0.001) and higher values of LASI (0.69 [IQR 0.34; 1.11] vs. 0.21 [IQR 0.16; 0.31], p-value < 0.001). LASr < 20%, LAScd ≥ − 12%, and LASI ≥ 0.26 were all associated with reduced survival. LASr, LAScd and LASI seem to be promising parameters in predicting adverse outcomes in the most common pediatric cardiomyopathies. Left atrial strain parameters and LASI are helpful in differentiating healthy control subjects from children with hypertrophic and dilated cardiomyopathies.
Similar content being viewed by others
Comparing left atrial indices by CMR in association with left ventricular diastolic dysfunction and adverse clinical outcomes
Prevalence, functional characteristics, and clinical significance of right ventricular involvement in patients with hypertrophic cardiomyopathy
Ventricular longitudinal shortening is an independent predictor of death in heart failure patients with reduced ejection fraction
Introduction.
Conventional echocardiographic ventricular diastolic dysfunction assessment is not perfect in pediatric patients with cardiomyopathies due to variability of the measured parameters with age, difficulty in discriminating cardiomyopathy patients from healthy subjects as well as poor interobserver agreement 1 . Furthermore, some diastolic function parameters may lengthen or shorten depending on the severity of the ventricular stiffness. For instance, mitral valve deceleration time may lengthen in the early diastolic dysfunction stages but shorten with the progression of the disease. Thus, the search for new, easily obtainable, and more objective parameters is necessary in the pediatric population.
Left atrial strain has recently received attention in adult studies on various cardiomyopathy types 2 , 3 , 4 , 5 , 6 , 7 . Left atrial strain measurements reflect both the systolic and diastolic function of the ventricle, while being influenced by the ventricle’s contractility, relaxation, compliance, and intra-ventricular pressures. Atrial filling and emptying abnormalities are dependent on the ventricular conditions. Atrial wall deformation measured by strain may be more accurate in describing ventricular diastolic dysfunction in the pediatric population compared to conventionally measured inflow and annular movement velocities, especially when taking into account the variability of the latter with age.
Because left atrial strain is an easily obtainable and reproducible parameter helpful in outlining adult cardiomyopathy patients at greatest cardiovascular risk, it appears to be worthy of attention also in children 8 , 9 . There are scarce studies concerning left atrial strain in pediatric cardiomyopathies 10 , 11 , 12 . To our knowledge, no studies concerning the association between left atrial strain and survival in pediatric cardiomyopathies have been published so far. Thus, the aim of our prospective pilot study was to assess the value of left atrial strain in predicting adverse events in children with the 3 most common types of cardiomyopathies: dilated, hypertrophic and left ventricular non-compaction. Furthermore, we aimed at outlining whether left atrial strain parameters differ between healthy subjects and patients with early stages of cardiomyopathies.
Patients with dilated, hypertrophic, and left-ventricular non-compaction cardiomyopathies were recruited from the Department of Pediatric Cardiology and General Pediatrics between 2020 and 2023. The diagnosis was based on echocardiography; hypertrophic cardiomyopathy was defined as left ventricular wall thickness z -score > 2 measured in diastole; dilated cardiomyopathy as left ventricular internal end-diastolic dimension (LVIDd) z- score > 2 with concomitant reduced ejection fraction, and left ventricular non-compaction according to the Jenni et al. criteria with non-compaction to compaction (NC:C) ratio in systole > 2:1 13 , 14 , 15 . When borderline cases were present, diagnosis was confirmed using cardiac magnetic resonance (CMR) imaging. Children aged 0–18 years were included in the pilot study.
The exclusion criteria included the following: co-existing co-morbidities including genetic syndromes co-existing with cardiomyopathies (i.e. Noonan syndrome, myopathies, metabolic diseases), ventricular hypertrophy or dilatation due to secondary reasons (i.e. congenital heart defects, hypertension), and lack of consent for participation in the study.
The control group consisted of healthy age- and sex-matched children. They were either recruited from the daily clinic, where they appeared due to a benign heart murmur, or from schools. Two patients from the control group were excluded from the analysis because of newly diagnosed ventricular arrhythmia.
Each patient had an ECG, echocardiography, and ECG Holter monitoring performed at the baseline visit. Moreover, among children with cardiomyopathies serum biomarkers N-Terminal prohormone of Brain Natriuretic Peptide (Nt-proBNP) and high-sensitivity troponin I were additionally analyzed. The medication and family history of each patient was recorded. Previous medical history for malignant arrhythmia (defined as ventricular tachycardia) was evaluated. Heart failure symptoms were assessed using NYHA or Ross scale in younger children 16 . If available, genetic test results were reported; the majority had a TruSight Cardio Sequencing Panel, while in a few patients with familial genotype Sanger sequencing was performed.
Unfavorable outcome were defined as follows: malignant arrhythmia (non-sustained ventricular arrhythmia (nsVT), sustained ventricular arrhythmia (sVT), ventricular fibrillation (VF)), listing for heart transplant, ICD implantation or appropriate ICD shock, and cardiac death. The arrhythmic endpoint included the presence of malignant arrhythmia, qualification for ICD implantation, or sudden cardiac death. The heart failure endpoint was defined as listing for heart transplant, undergoing heart transplant, or death due to heat failure.
This prospective study was approved by the local University Bioethics Committee. The study was performed in accordance with relevant guidelines and regulations (including Declaration of Helsinki and STROBE guidelines). Prior to participating in the study, all participants’ legal guardians as well as children ≥ 16 years signed a written informed consent form.
Echocardiography
Echocardiography was performed using Phillips EPIQ ultrasound system 9.0.1 with X5-1, S5-2 and S8-3 transducers. In each patient left ventricular ejection fraction (LVEF) was recorded using the Simpson biplane method. The left ventricular measurements such as: left ventricular internal diastolic dimension (LVIDd), left ventricular posterior wall thickness in diastole (LVPWd), and interventricular septum thickness in diastole (IVSd) were acquired using M-mode. Z -scores were used to account for the differences between the patients’ height and weight 17 .
Diastolic function was assessed using both pulsed-wave Doppler and tissue Doppler velocities (TDI). Mitral inflow peak E-wave, A-wave velocities and mitral E-wave deceleration time (DT) were measured. In TDI medial (septal) and lateral early (e′) and late (a′) mitral annulus velocities as well as isovolumetric relaxation time (IVRT) were assessed 18 . The left atrial volume was measured using the area-length approximation using the 4-chamber and 2-chamber views and corrected for body surface area (LAVi left atrial volume index) 19 . Mitral valve regurgitation (MR) was graded from 0 to 4 20 .
Myocardial strains were acquired using speckle tracking echocardiography with Philips software. Left ventricular global longitudinal strain (LV GLS) was obtained from the 4-chamber, 3-chamber, and 2-chamber views. Atrial strain was obtained from the 4-chamber view using ventricular end diastole as the zero reference point in accordance with the current recommendations 21 . Left atrial strain during the reservoir (LASr), conduit (LAScd) and contraction phases (LASct) were recorded. Non-invasive left atrial stiffness index (LASI) was defined as the ratio between average E/e′ to LASr 22 . 20% of randomly selected studies of cardiomyopathies patients were reanalyzed by a second observer to look for inter-observer variability in terms of differences in left atrial strain.
Statistical analysis
Statistical analysis was performed using Statistica 13.3 version and R version 4.2.2 GNU General Public License. Continuous data are presented as mean and standard deviation (SD) or median and first and third quartile (IQR), depending on the distribution. Categorical variables were compared using the test for equality of proportions. For continuous variables t -test, Mann–Whitney U test, F test and Kruskal–Wallis test were used depending on the number and distribution of the compared variables. Correlation analysis was performed using Pearson’s or Spearman’s correlations depending on the distribution. Random forest model was used to define the echocardiographic parameters that were most helpful in outlining patients with adverse outcomes in the whole cardiomyopathy group, as well as in dilated and hypertrophic cardiomyopathy subgroups. Kaplan–Meier survival curves were used for survival assessment in the whole cardiomyopathy group. This analysis was not performed on the subgroups due to a limited number of patients. Inter-observer variability was calculated using Lin’s concordance correlation coefficient. A p -value < 0.05 was considered statistically significant.
Conference presentation
Part of the results concerning DCM were presented at the 56th Annual Meeting of the Association for European Paediatric and Congenital Cardiology.
A total of 84 patients was included in the study, 28 with DCM, 21 with HCM, 7 with LVNC, and 28 in the control group. The median age was 8 years (IQR 3; 14). The median observation time of cardiomyopathy patients was 270 days (IQR 158; 525 days). The baseline characteristics of patients with cardiomyopathies and the control group are presented in Table 1 . There were no significant differences between the cardiomyopathy groups and the control group in terms of age, sex, BMI, or heart rate (Table 1 ). Baseline echocardiographic parameters in the 3 cardiomyopathy subgroups are presented in Table S1 ( Supplementary Materials ). DCM group was characterized by reduced LVEF (40.68% [IQR 30.53; 45.95]) and enlarged left ventricle (LVIDd z -score: + 3.74 ± 1.42). In the HCM group hypertrophy of the ventricular septum and left ventricular wall (IVSd z -score + 5.40 [IQR 3.02; 11.30]; LVPWd z -score + 3.08 [IQR 1.74; 5.30]), as well as increased maximal LVOT pressure gradient (9 mmHg [IQR 6; 17]) were present. In the LVNC group the median LVEF was 56.7% (IQR 55.1; 58.3). The 3 cardiomyopathy groups differed only in terms of only some of the diastolic function parameters (Table S1 , Supplementary Materials ). There were no significant differences in LASr, LAScd, LASI, or LAVi between the 3 groups; LASct was borderline significant ( p -value 0.049).
Altogether, 29% of patients (16/56) experienced adverse outcomes, 7 (25%) in the DCM group, 7 (33%) in the HCM group and 2 (29%) in the LVNC group. Among all cardiomyopathy patients 9 (16%) experienced a heart failure endpoint, whereas 12 (21%) experienced an arrhythmic endpoint. During the observation time 9 children were listed for heart transplant.
Eighteen patients (32%) had a positive family history of cardiomyopathies, 6 of whom experienced an adverse outcome. There was no statistically significant difference in terms of positive family history between patients with and without adverse outcomes ( p = 0.59). Genetic testing was performed in 43 patients (78%): in 15 (27%) the panel was positive, in 13 (23%) a variant of uncertain significance was found, in 2 the results were not available yet, and 13 (23%) had a negative panel.
Patients with cardiomyopathies and adverse outcomes had significantly lower LASr (16.68% ± 8.64% vs. 33.97% ± 9.99%), less negative LAScd (− 10.37% ± 5.83% vs. − 25.50% ± 9.24%) and greater LASI (0.69 [IQR 0.34; 1.11] vs. 0.21 [IQR 0.16; 0.31]) when compared to cardiomyopathy patients without adverse outcomes (Table 2 ). In terms of other echocardiographic parameters, patients with adverse outcomes had significantly greater LAVi, lower LVEF, lower E, A, average e′ wave velocities, and greater E/A ratio and lateral IVRT (Table S2 , Supplementary Materials ). There were no significant differences in terms of LASct, MR, and some of the diastolic function parameters (DT, a′, medial and lateral e′/a′, medial IVRT, average E/e′).
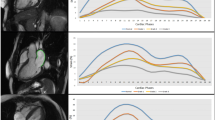
When applying the random forest model to assess echocardiographic parameters most helpful in predicting adverse outcomes LAScd, LASr and LASI were among the top 3 variables, with the model having a 50% sensitivity, 100% specificity, 100% positive predictive value and 90% negative predictive value (Fig. F1 , Supplementary Materials ). On Kaplan–Meier survival curves LASr < 20%, LAScd ≥ − 12%, and LASI ≥ 0.26 were all associated with reduced survival in patients with cardiomyopathies (Figs. 1 , 2 , 3 ).

Kaplan–Meier survival curves in cardiomyopathy patients with different left atrial reservoir strain (LASr).

Kaplan–Meier survival curves in cardiomyopathy patients with different left atrial conduit strain (LAScd).

Kaplan–Meier survival curves in cardiomyopathy patients with different left atrial stiffness index (LASI).
Dilated cardiomyopathy
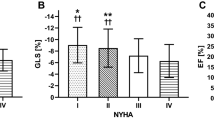
There were 28 children with dilated cardiomyopathy, 7 of whom (33%) experienced an adverse outcome (4 had an arrhythmic event, 7 heart failure adverse outcome). The differences between DCM patients with and without adverse outcomes are presented in Table 3 . Patients with adverse outcomes had significantly greater NT-proBNP serum concentrations than those without, as well as greater NYHA or Ross scale score.
In terms of echocardiographic parameters, patients with adverse outcomes had significantly lower LASr, less negative LAScd and greater LASI (Table 3 ). They presented with significantly lower LV GLS, and LVEF, as well as greater LVIDd z -scores. There were significant differences only in some of the diastolic function parameters between the 2 groups (lower A-wave velocity, greater E/A ratio, lower e′, lateral a′, greater IVRT lateral). However, there were no significant differences in terms of LASct, LAVi, MR and some diastolic dysfunction parameters, such as E-wave velocity, DT, medial IVRT, medial a′, medial and lateral e′/a′ and average E/e′.
Both LASr and LAScd had a strong correlation with LASI, and a moderate correlation with some systolic function parameters (LV GLS, LVEF), as well as some diastolic function parameters (average e′ wave velocity, lateral IVRT). There was a moderate correlation between LASr and LASct and NT-proBNP as well as NYHA/or Ross scale score. The detailed correlation analysis is presented in Table 4 .
LASI showed a strong correlation with NT-proBNP and NYHA, and a moderate correlation with LVEF. Similarly to LASr and LAScd, LASI correlated with only some of the conventional echocardiographic diastolic dysfunction parameters (Table 4 ).
When applying the random forest model, the top 3 parameters associated the strongest with adverse outcomes in patients with dilated cardiomyopathy were LASr, LASI and LAScd. The positive predictive value of the model was 66%, negative predictive value 93%, specificity 93%, sensitivity 66%, and ACC 0.88.
Also among patients with DCM and an arrhythmic endpoint, there were significant differences in terms of LASI (0.90 [IQR 0.57; 2.09] vs. 0.23 [IQR 0.16; 0.52] p -value 0.027), LASr (11.03% ± 6.81 vs. 29.10% ± 13.73, p -value 0.017) and LAScd (− 8.08% ± 5.32 vs. − 23.12% ± 12.13, p -value 0.023).
When compared to the control group, patients with DCM without adverse outcomes differed significantly from the control group in terms of: LASI (0.22 [IQR 0.16; 0.42] vs. 0.12 [IQR 0.10; 0.14] p -value < 0.001), LAVi (31.61 ± 12.29 vs. 17.88 ± 5.12 p -value < 0.001), LASr (30.10% [24.20; 41.40] vs. 50.65% [44.90; 56.10] p -value < 0.001), LAScd (− 25.49% ± 10.99% vs. − 38.77% ± 10.34% p -value < 0.001), and LASct (− 6.21% ± 8.91% vs. − 13.77% ± 4.25% p -value < 0.001). The detailed data concerning differences in the echocardiographic parameters between DCM patients without adverse outcomes and the control group are presented in Table S3 , Supplementary Materials .
Hypertrophic cardiomyopathy
There were 21 patients with hypertrophic cardiomyopathy, 7 of them (33%) experienced an adverse outcome (all of them had an arrhythmic adverse outcome), and one died due to sudden cardiac death. The differences in HCM children with and without adverse outcomes are presented in Table 5 . Patients with adverse outcomes had significantly greater NT-proBNP and troponin levels when compared to patients without adverse outcomes. There were no differences in terms of age or NYHA/Ross scale score.
In terms of echocardiographic parameters there were significant differences in terms of LASI, LAVi, LASr, LAScd, ventricular wall thickness (IVSd and LVPWd z -scores), and some diastolic function parameters (IVRT, e′/a′ medial and lateral, average e′ and E/e′) between patients with and without adverse outcomes (Table 5 ). However, there were no significant differences in terms of LASct, MR, or LVOT gradient. Furthermore, not all diastolic dysfunction parameters were different between the 2 subgroups (Table 5 ).
Correlation analysis showed a strong correlation between LASr and LASI, LVEF, and lateral a′ (Table 4 ). There was a moderate correlation between LASr and LAVI, MR, some diastolic function parameters (medial IVRT, medial E/e′), and NT-proBNP. LAScd showed a strong correlation with NT-proBNP and medial e′, and a moderate correlation with NYHA/Ross scale score, troponin, LASI, LAVi, MR, and some diastolic dysfunction parameters. Detailed correlation analysis is presented in Table 4 .
On the random forest model, the top 3 parameters most helpful in predicting patients with adverse outcomes included the following LAScd, LASr, and left ventricular ejection fraction with a 100% positive predictive value, 89% negative predictive value, 50% sensitivity, 100% specificity, and ACC 0.90.
Patients with HCM, who did not reach the endpoint in comparison to the control group, had significantly different LASI (0.20 [IQR 0.18; 0.27] vs. 0.12 [IQR 0.10; 0.14], p -value < 0.001), LASr (37.40% [32.10; 39.70] vs. 50.65% [44.90; 56.10], p -value < 0.001), LAScd (− 23.75% [− 30.20; − 21.20] vs. − 36.90% [− 44.15; − 31.60], p -value < 0.001) and LASct (− 10.05% [− 12.50; − 7.70] vs. − 14.25% [− 17.50; − 10.65], p -value 0.03). Furthermore, there were significant differences between the 2 subgroups in terms of LV GLS and some diastolic function parameters (e′, e′/a′ medial and lateral, average E/e′, lateral IVRT). The detailed data are presented in Table S4 in the Supplementary Materials .
Interobserver variability was analyzed in terms of left atrial strain parameters using Lin’s concordance coefficient. The agreement between the observers was 0.928 (CI 0.774; 0.979) for LASr, 0.948 (CI 0.843; 0.984) for LAScd, and 0.984 (CI 0.947; 0.995) for LASct.
Conventional echocardiographic diastolic function parameters outlined in the adult guidelines are characterized by low interobserver agreement in assessing differences between healthy children and pediatric patients with cardiomyopathies 1 . Furthermore, classification of diastolic dysfunction into grades is often difficult due to various parameters measured, their variability in time, dependence on the loading conditions, as well as problems with classification of borderline cases. Therefore, in the pediatric population the search for new, easily obtainable and reproducible parameters is crucial. Left atrial strain, which reflects loading abnormalities that occur secondarily to impaired left ventricular relaxation and compliance, seems to be a promising parameter in pediatric patients with primary myocardial diseases such as cardiomyopathies. It was proven to be superior to E/e′ measurements in predicting elevated pulmonary pressure in children qualified for heart transplant 23 . To our knowledge, there are no studies concerning left atrial strain and survival in children with cardiomyopathies. In our pilot study, both LASr and LAScd were associated with adverse outcomes in children with cardiomyopathies, whereas there were no significant differences in terms of LASct. Similarly to what has been reported in adults, pediatric patients with LASr < 20% as well as LAScd ≥ − 12% had worse survival on the Kaplan–Meier curves 2 , 4 . Furthermore, on the random forest model both parameters were among the top 3 parameters most helpful in predicting adverse outcomes, but one must keep in mind the models’ moderate sensitivity and excellent specificity. Thus, both left atrial reservoir and conduit strain appear to be noteworthy parameters in the risk assessment of pediatric cardiomyopathy patients.
Left atrial stiffness index, which is the ratio of left atrial reservoir strain to a conventional echocardiographic diastolic parameter (E/e′), has recently received attention in the adult population 24 , 25 , 26 , 27 . In patients with heart failure with preserved ejection fraction (HFpEF), increased left atrial stiffness index (> 0.26) has been associated with substantial risk of death or heart failure hospitalizations and was superior to conventional echocardiographic measurements of elevated left ventricular filling pressures 24 . It predicted elevated NT-proBNP and was associated with reduced exercise tolerance in adults with HFpEF 26 , 27 . In adults with HCM and DCM it was suggested that LASI might be superior to left atrial strain alone in terms of predicting elevated pulmonary artery pressures and thus be more accurate in detecting ventricular diastolic dysfunction 25 . We did not find any studies on LASI in pediatric cardiomyopathies. In our analysis LASI was significantly greater in patients with adverse outcomes, and, similarly to adults, LASI ≥ 0.26 was associated with significantly reduced survival 24 . Furthermore, LASI was among the top 3 echocardiographic variables helpful in predicting adverse outcomes in pediatric cardiomyopathy patients. LASI was also significantly greater among patients with cardiomyopathies without adverse outcomes compared to control subjects, which shows its potential in assessing early diastolic dysfunction. Therefore, LASI shines as an easily obtainable parameter that might be helpful in assessing risk stratification among cardiomyopathy patients as well as differentiating patients with myocardial diseases from healthy individuals.
In our study patients with dilated cardiomyopathy and adverse outcomes had significantly lower LASr and less negative LAScd. To our knowledge, no previous studies concerning left atrial strain and survival in pediatric DCM have been published. Furthermore, adult studies concerning left atrial function in dilated cardiomyopathy are mostly based on CMR rather than echocardiography 2 , 28 . Raafs et al. showed that LAScd < 12% was a predictor of freedom of adverse events or rehospitalization due to heart failure in adults and was superior in predicting adverse outcomes to LV GLS, LVEF, as well as LAVi 2 . In another adult CMR study on patients with heart failure, left atrial reservoir strain was associated with adverse cardiovascular events independently of late gadolinium enhancement 28 . Our results are in line with previous adult studies, because both LASr and LAScd were associated with adverse outcomes 2 , 29 . Furthermore, they were both, together with LASI, among the top parameters helpful in predicting adverse outcomes among children with DCM, although one has to keep in mind the model’s moderate sensitivity and high specificity. In our study, not all diastolic dysfunction parameters differed between patients with and without adverse outcomes. For instance, there were no differences in terms of DT, E-wave velocity, E/e′, medial IVRT, or medial a′ wave velocity between DCM children with and without adverse outcomes. This seems to agree with previous studies, in which the usefulness of conventional diastolic dysfunction parameters in the pediatric cardiomyopathy population has been questioned 1 . Furthermore, in the random forest model, these parameters were inferior to LASr and LAScd. Thus, conventional echocardiographic diastolic dysfunction parameters seem to be imperfect in outlining patients with and without adverse outcomes.
When compared to the control group, patients with DCM without adverse outcomes differed in terms of all 3 measured left atrial strain parameters, LASI, and only some of the conventional echocardiographic diastolic function parameters. Similarly to Sabatino et al., we observed greater E/e′ ratio and LAVi as well as lower left atrial peak systolic strain 10 . We also did not observe significant differences in terms of DT or E/A ratio, which again points to the imperfection of the conventional diastolic dysfunction parameters in the pediatric population 10 . Because all left atrial strain parameters and LASI were different between the control group and DCM patients without adverse outcomes, it points toward their value not only in assessing pediatric diastolic dysfunction but also in discriminating healthy control subjects from early stages of dilated cardiomyopathy.
In adults, A-wave velocity, E/A wave ratio and LV GLS have been associated with malignant ventricular arrhythmias 30 . Similarly, in our study they were also significantly different between DCM children with and without ventricular arrhythmias. Thus, in our study, we also observed differences in terms of greater LASI, lower LASr, and less negative LAScd, which could be new, additional parameters helpful in predicting arrhythmic events in the pediatric DCM population.
In our study, similarly as reported by Pahl et al., positive family history in children with dilated cardiomyopathy was not associated with adverse outcomes 31 . The role of genetic testing should be stressed because it has been shown that pathogenic or likely pathogenic variants occur independently of family history among pediatric dilated cardiomyopathy patients 32 .
Left atrial strain parameters have been better studied in the HCM adult population; unfavorable outcomes such as heart failure, stroke, or death have been associated with LASr ≤ 23.8% and LAScd ≤ 10.2% on echocardiography 3 . In children, no such values have been defined so far. In our study, LASr and LAScd were significantly different between HCM patients with and without adverse outcomes, which suggests that the abnormalities in the left atrial function and pressures increase with the disease’s progression.
In an adult CMR study, it has been shown that impaired LASct appears with the disease’s progression and is associated with fibrosis, whereas lower LAScd is abnormal in earlier stages of the disease 33 . This observation might not be valid in the pediatric population. In our study, patients with HCM and adverse outcomes did not differ significantly from HCM patients without end-point in terms of LASct, however, they had significantly different LASr and LAScd. Thus, abnormalities associated with the most severe disease progression in the pediatric population might not be identical to the adult population. However, further studies regarding survival in pediatric HCM are necessary to assess this finding.
LASr and LAScd were significantly different between the control group and HCM patients without adverse outcomes, whereas the difference in terms of LASct was borderline significant ( p- value 0.03). In the study by Jhaveri et al. on children and young adults (up to 25 years old) with HCM, both LASr and LASct were reduced in phenotype-positive patients compared to genotype-positive 11 . Phenotype-positive patients and the control group differed in terms of LASr in the 2-chamber view; however, no difference was found in terms of LASct 11 . Thus, both LASr and LAScd seem to be valuable parameters in differentiating healthy individuals from early stages of pediatric hypertrophic cardiomyopathy. The usefulness of LASct in the pediatric population is yet to be determined.
Alis et al. showed that abnormalities in LASr and LAScd on CMR preceded enlargement of the left atrial volumes in children with HCM 12 . This is in agreement with our results, although we did not observe differences in terms of LAVi on echocardiography between control patients and HCM patients without the end-point of the study; thus, we observed significant differences in terms of LASr and LAScd. It appears, that LASr and LAScd both on CMR and echocardiography are more accurate in depicting left atrial abnormalities than LAVi. For this reason, left atrial strain parameters may be more useful in differentiating healthy controls from early stages of pediatric hypertrophic cardiomyopathy.
In children with HCM, it has been suggested that left ventricular outflow tract obstruction leads to abnormalities in left atrial volume, left atrial total strain and conduit strain 34 . However, we believe that abnormalities in the left atrial strain are caused not only by the degree of the left ventricular outflow tract obstruction, but also reflect changes in the diastolic dysfunction and increased stiffness of the ventricle itself. On the contrary, in our study HCM patients with and without adverse outcome did not differ significantly in terms of left ventricular outflow tract obstruction; thus, they differed in terms of LASI, left atrial reservoir and conduit strain.
The relationship between family history and sudden cardiac death in the pediatric hypertrophic cardiomyopathy population is disputable. So far, family history has not been included in the HCM-Risk KIDS scale as a risk factor 35 . Similarly to this finding, in our study positive family history was not associated with increased risk of adverse outcomes.
In adults left atrial volume index (LAVi), left ventricular global longitudinal strain, as well as mechanical dispersion were associated with an adequate ICD therapy, and therefore the occurrence of malignant ventricular arrhythmia 36 . To our knowledge, no studies concerning the association between ventricular arrhythmia and atrial strain echocardiography in children with hypertrophic cardiomyopathy have been published. In our study, in HCM patients with adverse events (who all had arrhythmic adverse events) LAVi was significantly greater than in those without; thus, they did not differ significantly in terms of LV GLS. This suggests that LV GLS abnormalities might progress with age, and pediatric risk factors for adverse outcomes are not identical to the adult ones.
Left ventricular non-compaction
There is scarce literature concerning left atrial strain in left ventricular non-compaction cardiomyopathy. In adults, reduced LASr was a predictor of exacerbation of heart failure 5 . Furthermore, LASr and LASct were different between patients with multiple gene variants with LVNC and genotype-negative patients, which suggests a more severe disease in patients with multiple mutations 6 . In our study, due to a limited number of patients, we could not perform analysis in the LVNC group only. As left ventricular-non compaction is a heterogenous disorder, left atrial strain might be an easily obtainable parameter helpful in outlining those with cardiovascular risk. Thus, the role of genetic testing in left ventricular non-compaction should not be understated, because various cardiomyopathy phenotypes, including hypertrabeculation, may be present among different family members 37 .
Some limitations to the study should be outlined. Because cardiomyopathies in children are rare diseases and this was a single-center study, the sample size was relatively small. For this reason, multicenter studies on a larger number of patients are crucial to more accurately assess left atrial strain values that would be helpful to identify patients with the greatest cardiovascular risk. Furthermore, due to the limited sample size, we did not assess the grade of the diastolic dysfunction abnormalities. Another limitation is that we only used Phillips software for left atrial strain assessment; therefore, variability of left atrial strain in the pediatric population using different software was not assessed. The observation time of the study was relatively short; thus, while this was a pilot study, we believe it sheds light on new and promising echocardiographic parameters helpful in outlining patients with pediatric cardiomyopathies at greatest cardiovascular risk.
Conclusions
Left atrial reservoir and conduit strain as well as left atrial stiffness index appear to be promising new parameters in predicting adverse outcomes in pediatric patients with cardiomyopathies. Moreover, they appear to help differentiate healthy individuals from those with the early stages of dilated and hypertrophic cardiomyopathies.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Dragulescu, A., Mertens, L. & Friedberg, M. K. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: Problems and limitations. Circ. Cardiovasc. Imaging 6 , 254–261. https://doi.org/10.1161/CIRCIMAGING.112.000175 (2013).
Article PubMed Google Scholar
Raafs, A. G. et al. Left atrial strain has superior prognostic value to ventricular function and delayed-enhancement in dilated cardiomyopathy. JACC Cardiovasc. Imaging 15 , 1015–1026. https://doi.org/10.1016/j.jcmg.2022.01.016 (2022).
Vasquez, N. et al. Low left atrial strain is associated with adverse outcomes in hypertrophic cardiomyopathy patients. J. Am. Soc. Echocardiogr. 32 , 593-603 e591. https://doi.org/10.1016/j.echo.2019.01.007 (2019).
Fujimoto, K. et al. Incremental value of left atrial active function measured by speckle tracking echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography 35 , 1138–1148. https://doi.org/10.1111/echo.13886 (2018).
Han, P. L. et al. Prognostic value of left atrial reservoir strain in left ventricular myocardial noncompaction: A 3.0 T cardiac magnetic resonance feature tracking study. J. Magn. Reson. Imaging 57 , 559–575. https://doi.org/10.1002/jmri.28292 (2023).
Zhou, D. et al. CMR characteristics, gene variants and long-term outcome in patients with left ventricular non-compaction cardiomyopathy. Insights Imaging 12 , 184. https://doi.org/10.1186/s13244-021-01130-2 (2021).
Article PubMed PubMed Central Google Scholar
Piras, P. et al. Left atrial trajectory impairment in hypertrophic cardiomyopathy disclosed by geometric morphometrics and parallel transport. Sci. Rep. 6 , 34906. https://doi.org/10.1038/srep34906 (2016).
Article ADS PubMed PubMed Central Google Scholar
Rausch, K. et al. Reproducibility of global left atrial strain and strain rate between novice and expert using multi-vendor analysis software. Int. J. Cardiovasc. Imaging 35 , 419–426. https://doi.org/10.1007/s10554-018-1453-7 (2019).
Paysal, J., Merlin, E., Rochette, E., Terral, D. & Nottin, S. Left atrial remodeling in adolescents with obesity evaluated by speckle-tracking echocardiography. Int. J. Obes. https://doi.org/10.1038/s41366-023-01397-z (2023).
Article Google Scholar
Sabatino, J. et al. Left atrial strain to identify diastolic dysfunction in children with cardiomyopathies. J. Clin. Med. https://doi.org/10.3390/jcm8081243 (2019).
Jhaveri, S. et al. Left atrial strain and function in pediatric hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 34 , 996–1006. https://doi.org/10.1016/j.echo.2021.04.014 (2021).
Alis, D., Asmakutlu, O., Topel, C. & Karaarslan, E. Diagnostic value of left atrial strain in pediatric hypertrophic cardiomyopathy with normal maximum left atrial volume index: Preliminary cardiac magnetic resonance study. Pediatr. Radiol. 51 , 594–604. https://doi.org/10.1007/s00247-020-04884-x (2021).
Lipshultz, S. E. et al. Cardiomyopathy in children: Classification and diagnosis: A scientific statement from the American Heart Association. Circulation 140 , e9–e68. https://doi.org/10.1161/CIR.0000000000000682 (2019).
Authors/Task Force et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35 , 2733–2779. https://doi.org/10.1093/eurheartj/ehu284 (2014).
Jenni, R., Oechslin, E., Schneider, J., Attenhofer Jost, C. & Kaufmann, P. A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 86 , 666–671. https://doi.org/10.1136/heart.86.6.666 (2001).
Ross, R. D. et al. Plasma norepinephrine levels in infants and children with congestive heart failure. Am. J. Cardiol. 59 , 911–914. https://doi.org/10.1016/0002-9149(87)91118-0 (1987).
Lopez, L. et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 23 , 465–495. https://doi.org/10.1016/j.echo.2010.03.019 (2010) ( quiz 576–467 ).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 29 , 277–314. https://doi.org/10.1016/j.echo.2016.01.011 (2016).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16 , 233–270. https://doi.org/10.1093/ehjci/jev014 (2015).
Lancellotti, P. et al. Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 14 (7), 611–644. https://doi.org/10.1093/ehjci/jet105 (2013).
Badano, L. P. et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 19 , 591–600. https://doi.org/10.1093/ehjci/jey042 (2018).
Kurt, M., Wang, J., Torre-Amione, G. & Nagueh, S. F. Left atrial function in diastolic heart failure. Circ. Cardiovasc. Imaging 2 , 10–15. https://doi.org/10.1161/CIRCIMAGING.108.813071 (2009).
Yeh, J., Aiyagari, R., Gajarski, R. J., Zamberlan, M. C. & Lu, J. C. Left atrial deformation predicts pulmonary capillary wedge pressure in pediatric heart transplant recipients. Echocardiography 32 , 535–540. https://doi.org/10.1111/echo.12679 (2015).
Kim, D. et al. Prognostic implications of left atrial stiffness index in heart failure patients with preserved ejection fraction. JACC Cardiovasc. Imaging 16 , 435–445. https://doi.org/10.1016/j.jcmg.2022.11.002 (2023).
Kim, M. et al. Effects of left atrial function on pulmonary arterial pressure in acute myocardial infarction, hypertrophic and dilated cardiomyopathy. BMC Cardiovasc. Disord. 22 , 507. https://doi.org/10.1186/s12872-022-02952-8 (2022).
Liu, L. et al. Reduced left atrial contractile strain with speckle tracking analysis predicts abnormal plasma NTproBNP in an asymptomatic community population. Cardiovasc. Ultrasound 20 , 27. https://doi.org/10.1186/s12947-022-00297-y (2022).
Singleton, M. J. et al. Left atrial stiffness index independently predicts exercise intolerance and quality of life in older, obese patients with heart failure with preserved ejection fraction. J. Cardiac Fail. 28 , 567–575. https://doi.org/10.1016/j.cardfail.2021.10.010 (2022).
Bo, K. et al. Incremental prognostic value of left atrial strain in patients with heart failure. ESC Heart Fail. 9 , 3942–3953. https://doi.org/10.1002/ehf2.14106 (2022).
Abou Kamar, S. et al. Prognostic value of temporal patterns of left atrial reservoir strain in patients with heart failure with reduced ejection fraction. Clin. Res. Cardiol. https://doi.org/10.1007/s00392-023-02244-x (2023).
Negishi, K. et al. Left atrial booster pump function is an independent predictor of subsequent life-threatening ventricular arrhythmias in non-ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 17 , 1153–1160. https://doi.org/10.1093/ehjci/jev333 (2016).
Pahl, E. et al. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: A report from the Pediatric Cardiomyopathy Registry. J. Am. Coll. Cardiol. 59 , 607–615. https://doi.org/10.1016/j.jacc.2011.10.878 (2012).
Khan, R. S. et al. Genotype and cardiac outcomes in pediatric dilated cardiomyopathy. J. Am. Heart Assoc. 11 (1), e022854. https://doi.org/10.1161/JAHA.121.022854 (2022).
Kowallick, J. T. et al. Left atrial performance in the course of hypertrophic cardiomyopathy: Relation to left ventricular hypertrophy and fibrosis. Investig. Radiol. 52 , 177–185. https://doi.org/10.1097/RLI.0000000000000326 (2017).
Mazurkiewicz, L. et al. Biatrial performance in children with hypertrophic cardiomyopathy: CMR study. Eur. Radiol. 28 , 5148–5159. https://doi.org/10.1007/s00330-018-5519-7 (2018).
Norrish, G. et al. Development of a novel risk prediction model for sudden cardiac death in childhood hypertrophic cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 4 (9), 918–927. https://doi.org/10.1001/jamacardio.2019.2861 (2019).
Candan, O. et al. Mechanical dispersion and global longitudinal strain by speckle tracking echocardiography: Predictors of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy. Echocardiography 34 , 835–842. https://doi.org/10.1111/echo.13547 (2017).
Al-Wakeel-Marquard, N. et al. RIKADA study reveals risk factors in pediatric primary cardiomyopathy. J. Am. Heart Assoc. 8 (15), e012531. https://doi.org/10.1161/JAHA.119.012531 (2019).
Download references
Part of the research was funded by the Medical University of Warsaw, grant number 2M6/1/M/MB/N/20.
Author information
Authors and affiliations.
Department of Pediatric Cardiology and General Pediatrics, Doctoral School, Medical University of Warsaw, 02-091, Warsaw, Poland
Katarzyna Łuczak-Woźniak
Department of Pediatric Cardiology and General Pediatrics, Jozef Polikarp Brudzinski Public Pediatric Hospital, 02-091, Warsaw, Poland
Cezary Niszczota & Klaudia Obsznajczyk
Department of Pediatric Cardiology and General Pediatrics, Medical University of Warsaw, 02-091, Warsaw, Poland
Bożena Werner
You can also search for this author in PubMed Google Scholar
Contributions
KLW, BW conceived the experiment; KLW, CN, KO conducted the experiment; KLW analyzed the results and wrote the original manuscript. BW supervised the work on the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Bożena Werner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Łuczak-Woźniak, K., Niszczota, C., Obsznajczyk, K. et al. Abnormal left atrial strain and left atrial stiffness index are associated with adverse outcomes in children with cardiomyopathies: a pilot study. Sci Rep 14 , 21059 (2024). https://doi.org/10.1038/s41598-024-72175-8
Download citation
Received : 04 December 2023
Accepted : 04 September 2024
Published : 10 September 2024
DOI : https://doi.org/10.1038/s41598-024-72175-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
This case study involves a 76 year old female named Mary Lou Poppins, who presented to the ED accompanied by her son. She called her son after having symptoms of shortness of breath and confusion. Her past medical history includes hypertension, hyperlipidemia, coronary artery disease, and she was an everyday smoker for 30 years. She reports her home medications are lisinopril, simvastatin, and baby aspirin. Her current lifestyle includes: being a widow of six years, she lives alone, she walks her dog everyday, she drives to her knitting group three days a week, she makes dinner for her grandchildren once a week, she attempts to eat healthy but admits to consuming salty and high fat foods, and she insists on being very independent.
Mary Lou Poppins initial vitals in the emergency department includes a blood pressure of 138/70, heart rate of 108. respiratory rate of 26, temperature 98.9 degrees fahrenheit, and oxygen saturation of 84%. Her initial assessment included alert and oriented to person and place, dyspnea, inspiratory crackles in bilateral lungs, and a cough with pink frothy sputum. Her labs and diagnostics resulted in a BNP of 740 pg/ml, an echocardiogram showing an ejection fraction of 35%, an ECG that read sinus tachycardia, and a chest x-ray that confirmed pulmonary edema.
The Emergency Department physician diagnosed Mary Lou Poppins with left-sided heart failure. The orders included: supplemental oxygen titrated to keep saturation >93%, furosemide IV, enoxaparin subq, and metoprolol PO. Nursing Interventions included: monitoring oxygen saturation, adjusting oxygen route and dosage according to orders, assessing mentation and confusion, obtaining IV access, reassessing vitals, administering medications, and keeping the head of the bed elevated greater than 45 degrees. She was admitted to the telemetry unit for further stabilization, fluid balance monitoring, and oxygen monitoring.
On day one of hospital admission, Mary Lou Poppins required 4L of oxygen via nasal cannula in order to maintain the goal saturation of >93%. Upon assessment, it was determined that she was oriented to person and place. Auscultation of the lungs revealed bilateral crackles throughout, requiring collaboration with respiratory therapy once in the morning, and once in the afternoon. Physical therapy worked with the patient, but she was only able to ambulate for 100 feet. During ambulation, the patient had a decrease of oxygen saturation and dyspnea, requiring her oxygen to be increased to 6L. At the end of the day, strict intake and output monitoring showed an intake of 1200 mL of fluids, with an urinary output of 2L.
On day two of admission, Mary Lou began demonstrating signs of improvement. She only required 2 L of oxygen via nasal cannula with diminished crackles heard upon auscultation. Morning weight showed a weight loss of 1.3 lbs and the patient was oriented to person, place, and sequence of events. During physical therapy, she was able to ambulate 300 feet without required increased oxygen support. Daily fluid intake was 1400 mL with a urinary output of 1900 mL.
On the third and final day of admission, Mary Lou was AOx4 and did not require any type of oxygen support. When physical therapy arrived, the patient was able to ambulate 500 feet, which was close to her pre-hospital status. When the doctor arrived, the patient informed him that she felt so much better and felt confident going home. The doctor placed orders for discharge.
Upon discharge and throughout the patient’s hospital stay, Mary Lou Poppins was educated regarding the disease process of heart failure; symptoms to monitor for and report to her doctor; the importance of daily monitoring of weight, blood pressure, and heart rate; and the importance of adhering to a diet and exercise regime. Education was also provided regarding her medications and the importance of strictly adhering to them in order to prevent exacerbations of heart failure. Smoking cessation was also included in her plan of care. The patient received an informational packet regarding her treatment plan, symptoms to monitor for, and when to call her physician. Upon discharge, the patient was instructed to schedule a follow up appointment with her cardiologist for continued management of her care.
The patient was put in contact with a home health agency to help manage her care. The home health nurse will help to reinforce the information provided to the patient, assess the patient’s home and modify it to meet her physical limitations, and help to create a plan to meet daily dietary and exercise requirements. Regular follow-up appointments were stressed to Mary Lou Poppins in order to assess the progression of her disease. It will be important to monitor her lab values to also assess her disease progression and for any potential side effects associated with her medications. Repeat echocardiograms will be necessary to monitor her ejection fraction; if it does not improve with the treatment plan, an implanted cardiac defibrillator may be necessary to prevent cardiac death.
Open-Ended Questions
- What were the clinical manifestations that Mary Lou Poppins presented with in the ED that suggested the new onset of CHF?
- What factors most likely contributed to the onset of CHF?
- What patient education should Mary Lou Poppins receive on discharge in regards to managing her CHF?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
- Search Menu
- Sign in through your institution
- Author videos
- ESC Content Collections
- Supplements
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About European Heart Journal Supplements
- About the European Society of Cardiology
- ESC Publications
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case presentation.
- < Previous
Clinical case: heart failure and ischaemic heart disease
- Article contents
- Figures & tables
- Supplementary Data
Giuseppe M C Rosano, Clinical case: heart failure and ischaemic heart disease, European Heart Journal Supplements , Volume 21, Issue Supplement_C, April 2019, Pages C42–C44, https://doi.org/10.1093/eurheartj/suz046
- Permissions Icon Permissions
Patients with ischaemic heart disease that develop heart failure should be treated as per appropriate European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines.
Glucose control in diabetic patients with heart failure should be more lenient that in patients without cardiovascular disease.
Optimization of cardiac metabolism and control of heart rate should be a priority for the treatment of angina in patients with heart failure of ischaemic origin.
This clinical case refers to an 83-year-old man with moderate chronic obstructive pulmonary disease and shows that implementation of appropriate medical therapy according to the European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines improves symptoms and quality of life. 1 The case also illustrates that optimization of glucose metabolism with a more lenient glucose control was most probably important in improving the overall clinical status and functional capacity.
The patient has family history of coronary artery disease as his brother had suffered an acute myocardial infarction (AMI) at the age of 64 and his sister had received coronary artery by-pass. He also has a 14-year diagnosis of arterial hypertension, and he is diabetic on oral glucose-lowering agents since 12 years. He smokes 30 cigarettes per day since childhood.
In February 2009, after 2 weeks of angina for moderate efforts, he suffered an acute anterior myocardial infarction. He presented late (after 14 h since symptom onset) at the hospital where he had been treated conservatively and had been discharged on medical therapy: Atenolol 50 mg o.d., Amlodipine 2.5 mg o.d., Aspirin 100 mg o.d., Atorvastatin 20 mg o.d., Metformin 500 mg tds, Gliclazide 30 mg o.d., Salmeterol 50, and Fluticasone 500 mg oral inhalers.
Four weeks after discharge, he underwent a planned electrocardiogram (ECG) stress test that documented silent effort-induced ST-segment depression (1.5 mm in V4–V6) at 50 W.
He underwent a coronary angiography (June 2009) and left ventriculography that showed a not dilated left ventricle with apical dyskinesia, normal left ventricular ejection fraction (LVEF, 52%); occlusion of proximal LAD, 60% stenosis of circumflex (CX), and 60% stenosis of distal right coronary artery (RCA). An attempt to cross the occluded left anterior descending (LAD) was unsuccessful.
He was therefore discharged on medical therapy with: Atenolol 50 mg o.d., Atorvastatin 20 mg o.d., Amlodipine 2.5 mg o.d., Perindopril 4 mg o.d., oral isosorbide mono-nitrate (ISMN) 60 mg o.d., Aspirin 100 mg o.d., metformin 850 mg tds, Gliclazide 30 mg o.d., Salmeterol 50 mcg, and Fluticasone 500 mcg b.i.d. oral inhalers.
He had been well for a few months but in March 2010 he started to complain of retrosternal constriction associated to dyspnoea for moderate efforts (New York Heart Association (NYHA) II–III, Canadian Class II).
For this reason, he was prescribed a second coronary angiography that showed progression of atherosclerosis with 80% stenosis on the circumflex (after the I obtuse marginal branch) and distal RCA. The LAD was still occluded.
After consultation with the heart team, CABG was avoided because surgical the risk was deemed too high and the patient underwent palliative percutaneous coronary intervention (PCI) of CX and RCA. It was again attempted to cross the occlusion on the LAD. But this attempt was, again, unsuccessful. Collateral circulation from posterior interventricular artery (PDL) to the LAD was found. The pre-PCI echocardiogram documented moderate left ventricular dysfunction (EF 38%), the pre-discharge echocardiogram documented a LVEF of 34%. Because of the reduced LVEF, atenolol was changed for Bisoprolol (5 mg o.d.).
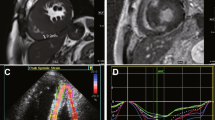
At follow-up visit in December 2012, the clinical status and the haemodynamic conditions had deteriorated. He complained of worsening effort-induced dyspnoea/angina that now occurred for less than a flight of stairs (NYHA III). On clinical examination clear signs of worsening heart failure were detected ( Table 1 ). His medical therapy was modified to: Bisoprolol 5 mg o.d., Atorvastatin 20 mg o.d., Amlodipine 2.5 mg o.d., Perindopil 5 mg o.d., ISMN 60 mg o.d., Aspirin 100 mg o.d., Metformin 500 mg tds, Furosemide 50 mg o.d., Gliclazide 30 mg o.d., Salmeterol 50 mcg oral inhaler, and Fluticasone 500 mcg oral inhaler. A stress perfusion cardiac scintigraphy was requested and revealed dilated ventricles with LVEF 19%, fixed apical perfusion defect and reversible perfusion defect of the antero-septal wall (ischaemic burden <10%, Figure 1 ). He was admitted, and an ICD was implanted.
Clinical parameters during follow-up visits
| . | December 2012 . | March 2013 . | September 2013 . | January 2014 . | January 2015 . |
|---|---|---|---|---|---|
| Weight (kg) | 72 | 71 | 74 | 70 | 68 |
| Height (cm) | 170 | 170 | 170 | 170 | 170 |
| BMI | 24.9 | 24.9 | 25.1 | 24.9 | 24.8 |
| JVP | +2 cm H O | +2 cm H O | +2 cm H O | Normal | Normal |
| Oedema | Bilateral oedema up to mid shins | Bilateral pretibial oedema (2+) | Bilateral pretibial oedema (3+) | No pedal oedema | No pedal oedema |
| Blood pressure (mmHg) | 115/80 | 115/75 | 110/60 | 110/70 | 112/68 |
| Pulse (bpm) | 88 | 86 | 92 | 68 | 56 |
| Auscultation | |||||
| Heart | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex | Systolic murmur 4/6 at apex |
| Lungs | Bilateral fine basilar crackles | Bilateral fine basilar crackles | Bilateral fine basilar and mid lung crackles | Clear | Clear |
| Laboratory findings | |||||
| FPG (mg/dL) | 100 | 98 | 96 | 106 | 112 |
| HbA1c (%) | 6.8 | 6.7 | 6.6 | 7 | 7.3 |
| Plasma creatinine (mg/dL) | 1.1 | 1.2 | 1.5 | 1.1 | 1.2 |
| Triglycerides | 118 mg/dL | NA | NA | 107 mg/dL | 114 mg/dL |
| Total cholesterol | 146 mg/dL | NA | NA | 142 mg/dL | 148 mg/dL |
| LDL-C | 68 mg/dL | NA | NA | 64 mg/dL | 68 mg/dL |
| HDL-C | 51 mg/dL | NA | NA | 48 mg/dL | 54 mg/dL |
| BNP | NA | 862 | 1670 | 276 | 244 |
| LVEF | 19 | 20 | 32 | 32 | |
| . | December 2012 . | March 2013 . | September 2013 . | January 2014 . | January 2015 . |
|---|---|---|---|---|---|
| Weight (kg) | 72 | 71 | 74 | 70 | 68 |
| Height (cm) | 170 | 170 | 170 | 170 | 170 |
| BMI | 24.9 | 24.9 | 25.1 | 24.9 | 24.8 |
| JVP | +2 cm H O | +2 cm H O | +2 cm H O | Normal | Normal |
| Oedema | Bilateral oedema up to mid shins | Bilateral pretibial oedema (2+) | Bilateral pretibial oedema (3+) | No pedal oedema | No pedal oedema |
| Blood pressure (mmHg) | 115/80 | 115/75 | 110/60 | 110/70 | 112/68 |
| Pulse (bpm) | 88 | 86 | 92 | 68 | 56 |
| Auscultation | |||||
| Heart | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex, III sound | Systolic murmur 4/6 at apex | Systolic murmur 4/6 at apex |
| Lungs | Bilateral fine basilar crackles | Bilateral fine basilar crackles | Bilateral fine basilar and mid lung crackles | Clear | Clear |
| Laboratory findings | |||||
| FPG (mg/dL) | 100 | 98 | 96 | 106 | 112 |
| HbA1c (%) | 6.8 | 6.7 | 6.6 | 7 | 7.3 |
| Plasma creatinine (mg/dL) | 1.1 | 1.2 | 1.5 | 1.1 | 1.2 |
| Triglycerides | 118 mg/dL | NA | NA | 107 mg/dL | 114 mg/dL |
| Total cholesterol | 146 mg/dL | NA | NA | 142 mg/dL | 148 mg/dL |
| LDL-C | 68 mg/dL | NA | NA | 64 mg/dL | 68 mg/dL |
| HDL-C | 51 mg/dL | NA | NA | 48 mg/dL | 54 mg/dL |
| BNP | NA | 862 | 1670 | 276 | 244 |
| LVEF | 19 | 20 | 32 | 32 | |

Myocardial perfusion scintigraphy and left ventriculography showing dilated left ventricle with left ventricular ejection fraction 19%. Reversible perfusion defects on the antero-septal wall and fixed apical perfusion defect.
In March 2013, he felt slightly better but still complained of effort-induced dyspnoea/angina (NYHA III, Table 1 ). Medical therapy was updated with bisoprolol changed with Nebivolol 5 mg o.d. and perindopril changed to Enalapril 10 mg b.i.d. The switch from bisoprolol to nebivolol was undertaken because of the better tolerability and outcome data with nebivolol in elderly patients with heart failure. Perindopril was switched to enalapril because the first one has no indication for the treatment of heart failure.
In September 2013, the clinical conditions were unchanged, he still complained of effort-induced dyspnoea/angina (NYHA III) and did not notice any change in his exercise capacity. His BNP was 1670. He was referred for a 3-month cycle of cardiac rehabilitation during which his medical therapy was changed to: Nebivolol 5 mg o.d., Ivabradine 5 mg b.i.d., uptitrated in October to 7.5 b.i.d., Trimetazidine 20 mg tds, Furosemide 50 mg, Metolazone 5 mg o.d., K-canrenoate 50 mg, Enalapril 10 mg b.i.d., Clopidogrel 75 mg o.d., Atorvastatin 40 mg o.d., Metformin 500 mg b.i.d., Salmeterol 50 mcg oral inhaler, and Fluticasone 500 mcg oral inhaler.
At the follow-up visit in January 2014, he felt much better and had symptomatically, he no longer complained of angina, nor dyspnoea (NYHA Class II, Table 1 ). Trimetazidine was added because of its benefits in heart failure patients of ischaemic origin and because of its effect on functional capacity. Ivabradine was added to reduce heart rate since it was felt that increasing nebivolol, that was already titrated to an effective dose, would have had led to hypotension.
He missed his follow-up visits in June and October 2014 because he was feeling well and he had decided to spend some time at his house in the south of Italy. In January and June 2015, he was well, asymptomatic (NYHA I–II) and able to attend his daily activities. He did not complain of angina nor dyspnoea and reported no limitations in his daily activities. Unfortunately, in November 2015 he was hit by a moped while on the zebra crossing in Rome and he later died in hospital as a consequence of the trauma.
This case highlights the need of optimizing both the heart failure and the anti-anginal medications in patients with heart failure of ischaemic origin. This patient has improved dramatically after the up-titration of diuretics, the control of heart rate with nebivolol and ivabradine and the additional use of trimetazidine. 1–3 All these drugs have contributed to improve the clinical status together with a more lenient control of glucose metabolism. 4 This is another crucial point to take into account in diabetic patients, especially if elderly, with heart failure in whom aggressive glucose control is detrimental for their functional capacity and long-term prognosis. 5
IRCCS San Raffaele - Ricerca corrente Ministero della Salute 2018.
Conflict of interest : none declared. The authors didn’t receive any financial support in terms of honorarium by Servier for the supplement articles.
Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JG , Coats AJ , Falk V , González-Juanatey JR , Harjola VP , Jankowska EA , Jessup M , Linde C , Nihoyannopoulos P , Parissis JT , Pieske B , Riley JP , Rosano GM , Ruilope LM , Ruschitzka F , Rutten FH , van der Meer P ; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC . Eur J Heart Fail 2016 ; 18 : 891 – 975 .
Google Scholar
Rosano GM , Vitale C. Metabolic modulation of cardiac metabolism in heart failure . Card Fail Rev 2018 ; 4 : 99 – 103 .
Vitale C , Ilaria S , Rosano GM. Pharmacological interventions effective in improving exercise capacity in heart failure . Card Fail Rev 2018 ; 4 : 1 – 27 .
Seferović PM , Petrie MC , Filippatos GS , Anker SD , Rosano G , Bauersachs J , Paulus WJ , Komajda M , Cosentino F , de Boer RA , Farmakis D , Doehner W , Lambrinou E , Lopatin Y , Piepoli MF , Theodorakis MJ , Wiggers H , Lekakis J , Mebazaa A , Mamas MA , Tschöpe C , Hoes AW , Seferović JP , Logue J , McDonagh T , Riley JP , Milinković I , Polovina M , van Veldhuisen DJ , Lainscak M , Maggioni AP , Ruschitzka F , McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology . Eur J Heart Fail 2018 ; 20 : 853 – 872 .
Vitale C , Spoletini I , Rosano GM. Frailty in heart failure: implications for management . Card Fail Rev 2018 ; 4 : 104 – 106 .
- myocardial ischemia
- cardiac rehabilitation
- heart failure
- older adult
| Month: | Total Views: |
|---|---|
| April 2019 | 719 |
| May 2019 | 146 |
| June 2019 | 86 |
| July 2019 | 98 |
| August 2019 | 112 |
| September 2019 | 144 |
| October 2019 | 281 |
| November 2019 | 263 |
| December 2019 | 229 |
| January 2020 | 254 |
| February 2020 | 303 |
| March 2020 | 271 |
| April 2020 | 380 |
| May 2020 | 372 |
| June 2020 | 427 |
| July 2020 | 320 |
| August 2020 | 307 |
| September 2020 | 376 |
| October 2020 | 553 |
| November 2020 | 459 |
| December 2020 | 473 |
| January 2021 | 305 |
| February 2021 | 424 |
| March 2021 | 479 |
| April 2021 | 445 |
| May 2021 | 251 |
| June 2021 | 307 |
| July 2021 | 228 |
| August 2021 | 248 |
| September 2021 | 383 |
| October 2021 | 414 |
| November 2021 | 442 |
| December 2021 | 367 |
| January 2022 | 276 |
| February 2022 | 354 |
| March 2022 | 537 |
| April 2022 | 373 |
| May 2022 | 451 |
| June 2022 | 253 |
| July 2022 | 161 |
| August 2022 | 208 |
| September 2022 | 281 |
| October 2022 | 424 |
| November 2022 | 568 |
| December 2022 | 442 |
| January 2023 | 305 |
| February 2023 | 357 |
| March 2023 | 533 |
| April 2023 | 474 |
| May 2023 | 403 |
| June 2023 | 235 |
| July 2023 | 242 |
| August 2023 | 230 |
| September 2023 | 339 |
| October 2023 | 456 |
| November 2023 | 477 |
| December 2023 | 262 |
| January 2024 | 226 |
| February 2024 | 283 |
| March 2024 | 282 |
| April 2024 | 400 |
| May 2024 | 298 |
| June 2024 | 103 |
| July 2024 | 119 |
| August 2024 | 125 |
| September 2024 | 71 |
Email alerts
More on this topic, related articles in pubmed, citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1554-2815
- Print ISSN 1520-765X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Managing Heart Failure in Primary Care: A Case Study Approach
- © 2023
- K. Melissa Smith Hayes ORCID: https://orcid.org/0000-0001-8731-4325 0 ,
- Nicole R. Dellise 1
Assistant Professor, Vanderbilt University School of Nursing, Nashville, USA
You can also search for this editor in PubMed Google Scholar
Director, Structural Heart Program, Director, Center for Advanced Heart Failure Therapy, Centennial Heart, Nashville, USA
- Includes a comprehensive review of physical exam findings and common diagnostic testing used to diagnosis heart failure
- Reviews best practice for transitioning the heart failure patient from hospital to home
- Offers many didactical case studies
- Provides a clear and concise overview of the management of heart failure for primary care clinicians
- Offers “Practice Pearls” for the primary care provider treating heart failure
- Discusses goals of care and end of life considerations for patients with heart failure
- Addresses special heart failure considerations in the management and treatment of common diagnoses seen in primary care
- Reflects current heart failure treatment guidelines outlined by AHA/HFSA
12k Accesses
1 Citations
30 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
Similar content being viewed by others.
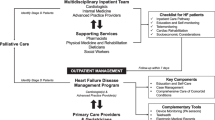
Broadening Heart Failure Care Beyond Cardiology: Challenges and Successes Within the Landscape of Multidisciplinary Heart Failure Care

The heart failure epidemic: a UK perspective

Emergency Department and Observation Unit Discharge Criteria
- Heart failure
- Ejection Fraction
- Co-morbidity
- End of life
- Transitional care
- Diagnostics
- Case studies
Table of contents (19 chapters)
Front matter, pathophysiology of heart failure, heart failure across the population, comprehensive heart failure history.
- Leah A. Carr, Lisa D. Rathman, Roy S. Small
Physical Exam for Presence and Severity of Heart Failure
- Jessica B. Williams, Donna Harmon, JoAnn Lindenfeld
The Cardiology Referral for Heart Failure: Work-up and Expectations
- Kaushik Amancherla, Lisa Mendes
Heart Failure with Reduced Ejection Fraction
- Terri L. Allison, Beth Towery Davidson

Heart Failure with Preserved Ejection Fraction
- Anupam A. Kumar, Deepak K. Gupta
Transitions of Care and Self-Care Strategies for the Heart Failure Patient
- Kelly D. Stamp, Marilyn A. Prasun
Goals of Care for the Heart Failure Patient
- Christine M. Hallman, Krista R. Dobbie
Atrial Fibrillation and Heart Failure
- Tara U. Mudd
Cardiorenal Syndrome, Chronic Kidney Disease, Anemia, and Heart Failure
- Michelle Mason Parker, Mark Wigger
Diabetes and Heart Failure
- Angelina Anthamatten
Chronic Obstructive Pulmonary Disease, Obstructive Sleep Apnea, and Heart Failure
- J. Travis Dunlap, Melissa Glassford, Leslie W. Hopkins
Pulmonary Hypertension in Heart Failure
- Douglas J. Pearce
Liver Disease and Heart Failure
- Mary Lauren Pfieffer, Julie Hannah
Editors and Affiliations
K. Melissa Smith Hayes
Nicole R. Dellise
About the editors
Bibliographic information.
Book Title : Managing Heart Failure in Primary Care: A Case Study Approach
Editors : K. Melissa Smith Hayes, Nicole R. Dellise
DOI : https://doi.org/10.1007/978-3-031-20193-6
Publisher : Springer Cham
eBook Packages : Medicine , Medicine (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
Softcover ISBN : 978-3-031-20192-9 Published: 30 March 2023
eBook ISBN : 978-3-031-20193-6 Published: 29 March 2023
Edition Number : 1
Number of Pages : XIX, 328
Number of Illustrations : 10 b/w illustrations, 10 illustrations in colour
Topics : Nursing , Cardiology , Pharmacology/Toxicology
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Radcliffe Group
- Medical Education
- Content for healthcare professionals only

Editorial Board
For Authors
Current Volume
Special Collections
Previous Volumes
Submit Article
Article Info
Article Text
Figures & Tables
Review Article
Effects of Glucagon-like Peptide-1 Receptor Agonists on Cardiac Function, Exercise Capacity and Quality of Life
Anastasia Shchendrygina
Amina Rakisheva
Ilya Giverts
Yasmin Rustamova
Anzhela Soloveva

Downloads: 4
- Read time: 16m 30s
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are emerging glucose-lowering agents primarily used in managing diabetes and obesity. Recently, GLP-1 RAs have garnered attention for their cardiovascular benefits beyond glycaemic control in patients with type 2 diabetes, exhibiting patterns previously seen in cardiovascular outcomes trials on sodium–glucose cotransporter 2 inhibitors, which now receive a high level of recommendation for the treatment of heart failure (HF). GLP-1 RAs have been increasingly investigated in HF cohorts, but mainly in smallscale studies reporting inconclusive findings regarding clinical outcomes and different safety profiles in HF patients with reduced and preserved ejection fractions. This review discusses the effects of GLP-1 RAs on surrogate HF outcomes, such as cardiac structure and function, exercise capacity and quality of life, in HF patients across the spectrum of left ventricular ejection fraction, to provide insights into the potential of these agents to be investigated in large clinical trials to evaluate clinical outcomes.
Glucagon-like peptide-1 receptor agonists , heart failure , quality of life , cardiac function , exercise capacity ,
Disclosure: AR reports receiving speaker fees from Novartis, Roche Diagnostics and AstraZeneca, and is on the Cardiac Failure Review editorial board; this did not influence peer review. A Soloveva has received grants from the European Society of Cardiology and support from AstraZeneca, Bayer, Novartis and Servier. All other authors have no conflicts of interest to declare.
Received: 15 April 2024
Accepted: 23 June 2024
Published online: 11 September 2024
Citation: Cardiac Failure Review 2024;10:e10.
Select format
DOI: https://doi.org/10.15420/cfr.2024.05
Correspondence Details: Anastasia Shchendrygina, Department of Hospital Therapy No. 2, IM Sechenov First Moscow State Medical University (Sechenov University), 2/4 Bolshaya Pirogovskaya str., Moscow, 119435, Russia. E: [email protected]
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are an emerging class of glucose-lowering drugs that are increasingly used in the treatment of type 2 diabetes (T2D) and obesity. Cardiovascular outcome trials in patients with T2D have demonstrated the efficacy of GLP-1 RAs in reducing major adverse cardiovascular events, including cardiovascular death, MI and stroke, regardless of glycaemic control. 1-8
Recent European Society of Cardiology guidelines for the management of cardiovascular disease (CVD) in patients with diabetes state that GLP-1 RAs (lixisenatide, liraglutide, semaglutide, exenatide extended release [ER], dulaglutide, efpeglenatide) should be considered for glucose-lowering treatment in patients with T2D at risk of or with heart failure (HF; class IIa A recommendation) already taking sodium–glucose cotransporter 2 inhibitors, without differentiating between HF phenotypes. 9 The consensus statement from the American Association of Clinical Endocrinologists supports the use of GLP-1 RAs as first-line therapy in managing hyperglycaemia in T2D patients with established (or at high risk of) atherosclerotic CVD, as well as in those with chronic kidney disease or a history of stroke or transient ischaemic attack, while recommending the administration of sodium–glucose cotransporter 2 inhibitors to HF patients. 10 The American College of Cardiology/American Heart Association primary prevention of cardiovascular disease guideline supports the use of GLP-1 RAs in patients with T2D and high atherosclerotic CVD risk, but does not mention existing HF. 11
Current evidence related to the effects of GLP-1 RAs on HF outcomes remains limited. GLP-1 RA cardiovascular outcome trials in patients with T2D reported a neutral impact of the drugs on HF hospitalisation. Of note, the prevalence of HF varied across these trials from 9% to 24%, and HF events were considered a secondary endpoint. 1–8 Most of these studies do not report HF diagnostic criteria or mention HF therapy, and only one trial reported on left ventricular ejection fraction (LVEF) at baseline. 8 Moreover, one should consider the fact that different GLP-1 RAs were used across the studies, with different chemical structures, durations of action and weight-lowering effects, which may impact their efficacy.
An updated meta-analysis of nine randomised controlled trials (RCTs), including 8,920 patients with HF and T2D, reported a 13% reduction in major adverse cardiovascular events in the GLP-1 RA compared with placebo arm. 12 In contrast, no benefit of GLP-1 RAs was observed in terms of all-cause death, HF hospitalisation or cardiovascular death. 12 Of note, that meta-analysis did not differentiate between HF phenotypes.
In the recent STEP HFpEF DM trial, which studied the efficacy of semaglutide among patients with obesity-related HF with preserved ejection fraction (HFpEF) and T2D, a reduction in the time to first HF events was demonstrated in the intervention arm, but this was a secondary endpoint. 13 No RCTs have primarily tested the effects of GLP-1 RAs on HF hard outcomes and/or mortality in patients with HFpEF or HF with reduced ejection fraction (HFrEF).
Generally, surrogate endpoints are expected to predict clinical benefits. In recent years, data have been accumulated suggesting effects of GLP-1 RAs on surrogate HF outcomes in HF patients across LVEF, including cardiac structure and function, exercise capacity and quality of life. 13
In this review, we summarise the evidence related to the effects of GLP-1 RAs on these outcomes ( Figure 1 ), which may shed light on the potential of these agents to be used in the clinical care of HF patients and investigated in large clinical trials to evaluate clinical outcomes.
Figure 1: Effects of Glucagon-like Peptide-1 Receptor Agonists on Cardiac Function, Exercise Capacity, Quality of Life and Clinical Outcomes in Patients With Heart Failure
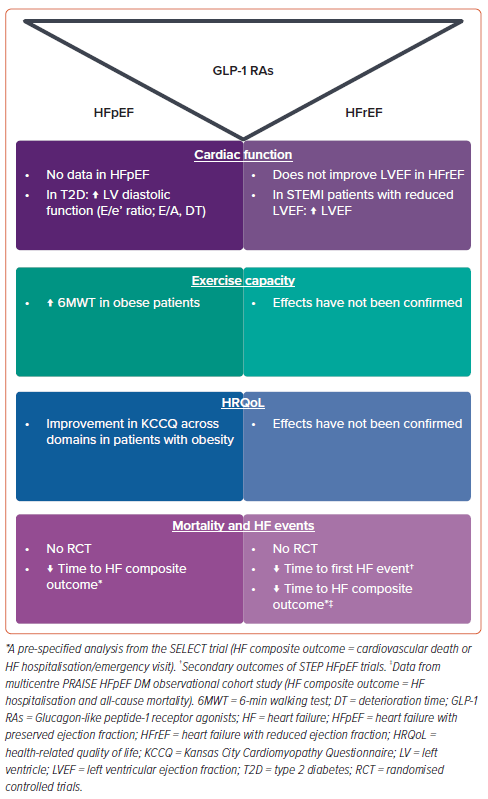
Potential Mechanism Underlying the Effects of GLP-1 RAs in Heart Failure
GLP-1 RAs bind to GLP-1 receptors expressed in various tissues, including pancreatic beta cells, the kidneys, heart, brain and gastric mucosa, among other organs. After binding to GLP-1 receptors in pancreatic beta cells, GLP-1 RAs exert their glucose-lowering effect by stimulating glucose-dependent insulin release. Antihyperglycemic effects aside, GLP-1 RAs reduce gastric emptying and suppress appetite centres in the hypothalamus, resulting in weight loss. Systemic effects of GLP-1 RAs, mediated via GLP-1 receptors in other organs and systems, include enhanced endothelial function and myocardial metabolism, natriuresis and anti-inflammatory and blood pressure-lowering effects. 14–17
All these effects of GLP-1 RAs are crucial for the pathophysiology of HF, with weight loss in obese HF patients being of utmost importance. Obesity is highly prevalent in HF; in HFpEF, the prevalence of obesity reaches up to 80%. 18 Obesity is an important contributor to the pathophysiology of both diabetes and HF, particularly HFpEF. 19 Adipose tissue is a complex endocrine organ that has multiple endocrine and paracrine effects on the heart. 20 Moreover, the high amount of visceral fat and epicardial adipose tissue (EAT), as well as associated plasma volume expansion, in obese individuals results in significant haemodynamic impairment at rest and during exercise, exerting local effects on the heart. 21 Remarkably, even in HF-free patients with morbid obesity, cardiac abnormalities can be seen that are consistent with left ventricular (LV) remodelling and dysfunction, including greater LV mass, LV diastolic dysfunction and elevated LV filling pressure. 22,23
Obese HFpEF patients have greater LV hypertrophy and pulmonary capillary wedge pressure, increased LV filling pressures and severe right ventricular dysfunction compared with non-obese HFpEF patients. 24,25 Increased EAT also results in a greater pericardial restraint, which affects haemodynamics. 26 Overall, the cardiovascular effects of GLP-1 RAs in HF can be realised through multiple pathways, including reducing visceral fat and EAT in obese HF patients; abolishing obesity-related haemodynamic impairment, pathological LV remodelling and myocardial inflammation; and systemic and glucose-lowering effects. 27
Effects of GLP-1 RAs on Cardiac Structure and Function
GLP-1 RAs have garnered significant interest due to their potential benefits beyond glycaemic control, including their effects on cardiac function. 28 A landmark pilot study that investigated the efficacy and safety of 72-h GLP-1 RA infusion in patients with acute MI and impaired LVEF (<40%) revealed a notable improvement in LV function, as assessed by echocardiography, compared with control subjects with similar disease characteristics that was independent of diabetes. 29 Furthermore, in a randomised placebo-controlled trial involving patients with ST-segment elevation MI (STEMI) who underwent balloon angioplasty and stent placement, with daily injections of exenatide or placebo for 3 days, revealed exenatide was associated with a smaller infarct size on cardiac MRI conducted 38 days after reperfusion. 30 Echocardiographic assessment at 6 months demonstrated persistent improvements in diastolic function and global longitudinal strain among patients who received exenatide. 30 The reduction in infarct size was confirmed in a larger study of 172 STEMI patients randomised to receive either intravenous exenatide or placebo. 31,32 However, in that study, no significant difference in changes in LVEF were observed between the groups. 31,32 In contrast, the administration of liraglutide to STEMI patients was associated with a small but significant improvement in LVEF assessed at 3 months in both non-diabetic and diabetic subjects. 33
Although GLP-1 RAs have shown promise in enhancing postischemic LV systolic function in preclinical and clinical settings, studies investigating the effects of GLP-1 RAs on LV diastolic function in patients with T2D reported conflicting findings. 31,34–37 Several small studies reported that liraglutide significantly improved LV diastolic function in T2D patients compared with either placebo or other glucose-lowering agents, which was also associated with improvements in endothelial function and antioxidant and anti-inflammatory activity. 36,37 In contrast, the administration of exenatide was not associated with changes in LV diastolic function. 5 These apparent discrepancies could be explained, in part, by the drug-specific effects of GLP-1 RAs on LV diastolic function, as well as differences in the baseline characteristics of patients, namely less prominent LV diastolic dysfunction in patients in the exenatide cohort compared with patients in the liraglutide studies. 5,36,37
However, a meta-analysis of 10 placebo-controlled RCTs, including 732 individuals with T2D, found that liraglutide therapy did not influence echocardiographic parameters of diastolic function compared with placebo, including the ratio of early diastolic filling velocity (E) to mitral annular early diastolic velocity (e′; weighted mean difference [WMD] −0.763; 95% CI [−2.157, 0.630]; p=0.283), change in e′ (WMD −0.069; 95% CI [−0.481, 0.343]; p=0.742) and change in E/e′ (WMD −0.683; 95% CI [−1.663, 0.298]; p=0.172). 35 LVEF also remained unchanged with liraglutide therapy compared with placebo (WMD −0.651; 95% CI [−1.649, 0.348]; p=0.202). 35
A further meta-analysis of 22 RCTs that included a considerably larger cohort (n=61,412) of T2D patients with or without cardiovascular disease and patients with cardiovascular disease alone revealed that treatment with GLP-1 RAs led to improvements in diastolic function (E-wave; standardised mean difference −0.40; 95% CI [−0.60, −0.20]; p<0.001), early diastolic to late diastolic velocities ratio (WMD −0.10; 95% CI [−0.18, −0.02]; p=0.01), E/e′ ratio (WMD −0.97; 95% CI [−1.54, −0.41; p<0.001) and E-wave deceleration time (WMD −9.96 ms; 95% CI [−18.52, −1.41 ms]; p=0.02), although LVEF was not affected. 38
The effects of GLP-1 RAs on cardiac structure and function were also investigated in HF patients. An early small study of 12 HF patients with New York Heart Association (NYHA) Classes III–IV showed a significant increase in LVEF following 5 weeks of continuous subcutaneous infusion of GLP-1 RAs. 39 However, larger studies have not demonstrated a significant effect of GLP-1 RAs on LV function, including albiglutide, which was administered over a long period (>12 weeks) to non-diabetic, overweight or obese individuals with HF (NYHA Class II–III) and LVEF below 40%. 40 In the FIGHT trial, long-term administration of liraglutide to HF and HFrEF patients with or without diabetes did not significantly improve LVEF after 24 weeks of treatment. 41 However, there was an increase in heart rate and more serious cardiac events, such as arrhythmias and acute coronary syndrome, in patients treated with liraglutide. 41 In a meta-analysis of nine RCTs involving 8,920 patients with HF and coexisting T2D, GLP-1 RAs did not improve LVEF, LV end-diastolic volume or LV end-systolic volume. 12
Although larger-scale studies are yet to explore the effects of GLP-1 RAs on cardiac structure and function, available evidence suggests that GLP-1 RAs may improve systolic and diastolic function in individuals with T2D who are at high risk of CVD and reduce infarct size after acute MI. 33–37 In individuals with HFrEF, GLP-1 RAs do not affect LV systolic function, whereas the effects of GLP-1 RAs in individuals with HFpEF have not yet been investigated.
Effects of GLP-1 RAs on Exercise Capacity
It may be assumed that GLP-1 RAs could potentially increase exercise capacity primarily by promoting weight loss. Other mechanisms underlying the effects of GLP-1 RAs on exercise capacity include improvements in myocardial energetics, enhanced endothelial function, reductions in systemic inflammation and oxidative stress and modulation of skeletal muscle metabolism. 14–17
Overall, clinical evidence regarding the effects of GLP-1 RAs on exercise capacity is limited and inconsistent, and varies across the spectrum of HF. 42 The effects of GLP-1 RAs in HFrEF have been studied in several small trials. In FIGHT, there was no significant effect of liraglutide on 6-min walk test (6MWT) distances compared with placebo. 40 In the LIVE trial, which evaluated the effects of liraglutide on LV function in stable chronic HF patients with and without diabetes, at the end of treatment patients from the liraglutide group were able to walk 28 ± 65 m longer during the 6MWT, compared with 3 ± 89 m for patients in the placebo group, with a mean difference of 24 m. 43 However, more patients in the liraglutide than placebo group experienced serious cardiac adverse events, including significant arrhythmia. 43 A similar trend towards a higher risk of unfavourable outcomes was observed in the post hoc analysis of the FIGHT trial. 40
Another small trial compared the effects of 12 weeks’ treatment with albiglutide (n=27) to placebo (n=30) on cardiac function, cardiac metabolism and exercise capacity in HFrEF. 43 Albiglutide did not improve myocardial glucose use or myocardial oxygen consumption, cardiac efficiency or the 6MWT distance. Surprisingly, a slight improvement in change of peak oxygen consumption (peak VO 2 ) was observed in the albiglutide group compared with placebo (mean 0.9 ± 0.5 ml/kg/min versus −0.6 ± 0.5 ml/kg/min; p=0.02). 40 However, the improvement in peak VO 2 was within the margin of measurement error and was not accompanied by a corresponding improvement in the 6MWT distance or quality of life, so this finding needs to be investigated further. Peak VO 2 improvement was not supported by the pharmacokinetic/pharmacodynamic modelling, indicating no relationship between exposure to albiglutide and peak VO 2 . 40 Therefore, current data suggest that GLP-1 RAs do not improve exercise capacity in HFrEF. 44
More promising evidence is available regarding GLP-1 RAs in obesity-related HFpEF. The benefits of semaglutide in obese HFpEF patients were established in the landmark STEP HFpEF trial. 45 In that trial, patients receiving semaglutide experienced greater reductions in weight (estimated difference −10.7%; p<0.001) and increases in 6MWT distance (estimated difference +20.3 m; p<0.001) compared with those in the control group. 45,46 This result was confirmed by the recent pooled analysis of the STEP HFpEF and STEP HFpEF DM trials, which included 1,145 patients. In that analysis, patients in the semaglutide group showed improvement from baseline to week 52 in both body weight (mean 8.4% reduction; p<0.0001 versus placebo) and 6MWT distance (mean 17.1 m; p<0.001 versus placebo). 47 Because of the high prevalence of frailty and sarcopenic obesity in the HFpEF population, future studies are required to estimate the proportion of lean body mass loss versus fat loss on GLP-1 RA therapy to identify predictors of the disproportionate loss of muscle mass. 45
Conversely, the relative increase in heart rates secondary to GLP-1 RAs may be potentially beneficial in HFpEF due to the high prevalence of chronotropic incompetence in these patients. Another promising strategy for HFpEF patients may be combining exercise training with GLP-1 RAs, mitigating the risk of sarcopenia and frailty and providing a synergistic effect on physical tolerance and quality of life. 46 Exercise training can also potentially attenuate gastrointestinal side effects related to GLP-1 RAs. 45 Similarly, previous studies showed that caloric restriction with or without aerobic training improved peak VO 2 in obese elderly HFpEF patients. 48 Future large studies combining comprehensive cardiac rehabilitation programs and intentional weight loss through GLP-1 RAs, caloric restriction and exercise in obesity-related HFpEF will be highly appreciated. It is hoped that ongoing trials like SUMMIT, which is investigating tirzepatide in participants with HFpEF and obesity, will provide more evidence on incretin-based medications in HFpEF. 49
Effects of GLP-1 RAs on Quality of Life
In addition to reducing the risk of hospital admission and mortality, improving health-related quality of life (HRQoL) is another treatment target in HF. HRQoL is a broad concept that covers individuals’ views on how their disease and treatment affect their overall wellbeing and physical, psychological and social abilities compared to personal expectations. 50 Reduced HRQoL in HF is multifactorial and related to frequent readmissions and symptom burden, exercise intolerance, emotional distress, loss of independence and social limitations. In addition to HF-associated factors, HRQoL is also attributable to comorbidities, the major ones being T2D and obesity. Patients with HFrEF and concomitant T2D show consistently lower Kansas City Cardiomyopathy Questionnaire (KCCQ) scores than those without T2D. 49 Similarly, higher BMI has detrimental effects on perceived health in HFrEF, which is also highly significant in HFpEF. 51–53 Notably, of the different HF phenotypes, HFpEF is associated with the worst HRQoL, whereas among HFpEF patients, the worst HRQoL had been shown in a subgroup with the highest BMI, a higher proportion of at least Grade 2 obesity and T2D. 53 Moreover, there was a linear relationship between HRQoL and BMI. 53
Better T2D control and weight reduction with GLP-1 RA therapy may be beneficial in terms of patient-reported health status among the HF cohort. However, in specifically designed placebo-controlled studies of GLP-1 RAs among patients with HFrEF, there were no improvements in HRQoL. 39,40,43 There may be several possible explanations for the failure of GLP-1 RAs in HFrEF studies. A well-known collider bias, termed the ‘obesity paradox’, should be considered. 54 BMI as a metric of obesity has some limitations, such as not being able to differentiate between lean and fat mass, estimate the magnitude of visceral obesity and predict outcomes. 55 Likewise, data on mean BMI among HFrEF participants may not reflect the presence and severity of obesity as a target for the potential effectiveness of GLP-1 RAs.
The FIGHT study showed that more vulnerable HF patients with severely reduced HRQoL and perhaps more advanced stages of the disease may require complex care planning to improve their health status rather than solely increasing glucose and fatty acid metabolism to enhance cardiac metabolism. 40 Indeed, patients at later stages of HF may respond differently to therapy that improves outcomes in less severe disease states. 56 A short duration of treatment may also have prevented significant changes in HRQoL in HFrEF studies. 39,43
Table 1: Characteristics of Studies Exploring the Effects of Glucagon-like Peptide-1 Receptor Agonists on Quality of Life in Heart Failure
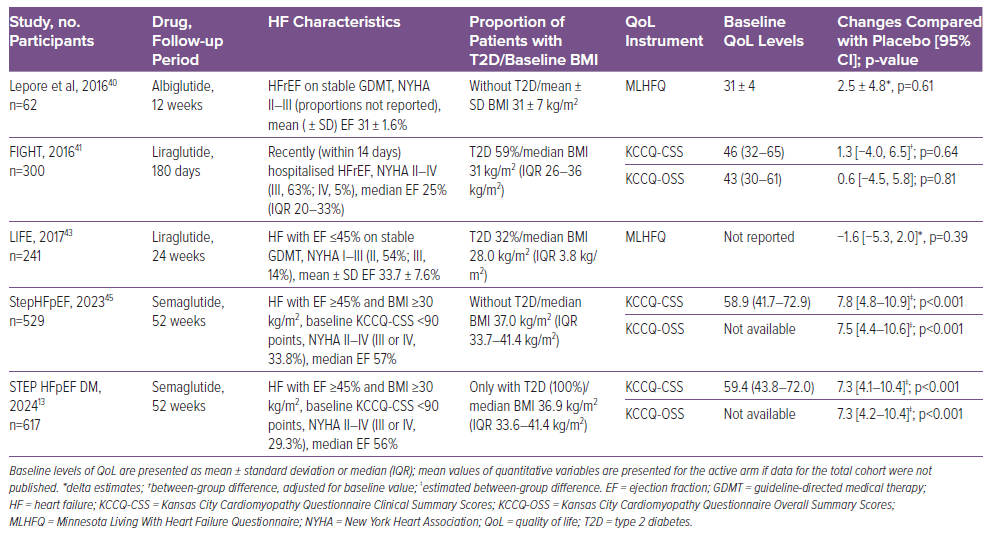
In contrast, among HFpEF cohorts, semaglutide showed clinically meaningful benefits in non-diabetic and diabetic cohorts ( Table 1 ). 13,45 In STEP HFpEF, semaglutide improved in KCCQ clinical summary scores (CSS), one of the study’s primary endpoints, by 7.8 points compared with placebo. 45 In addition, the KCCQ overall summary score improved by 7.5 points compared with placebo. 45 A similar benefit was shown in analyses by baseline KCCQ-CSS tertiles, BMI, and LVEF subgroups. 52,57,58 Moreover, the effect was consistent across all HRQoL domains (e.g. physical limitations score, quality of life score, symptom burden score, symptom frequency score and social limitations score), with estimated treatment differences ranging from 6.7 to 9.6 points. 52 Similarly, the odds of at least 5-, 10-, 15- and 20-point improvements in all KCCQ domains were 1.6- to 2.9-fold higher among semaglutide- than placebo-treated patients. 52 The effect of semaglutide on HRQoL in HFpEF, particularly social and physical function as measured by the KCCQ, is mediated at least in part by the effect on weight reduction. 57
Mental health is an integral part of the HRQoL construct and requires attention in HF. HF patients frequently experience low mood, depression and cognitive impairment, with worse estimates shown for the HFpEF phenotype. 59 Although some effects of GLP-1 RAs, particularly weight loss, are linked to direct effects of GLP-1 RAs in brain areas responsible for appetite, satiety, food behaviour and other central regulatory mechanisms, data regarding the potential impact of therapy on mental health among HF patients are lacking. Some data suggest neuroprotective properties, and there are ongoing randomised trials among patients with neurological and psychiatric disorders (NCT04466345). 60–62 In addition, published reports suggest efficacy of GLP-1 RAs against weight gain related to the use of antipsychotic medication that may affect a patient’s feelings. 63 Once-weekly GLP-1 RAs may also improve HRQoL through greater treatment satisfaction. 64
In contrast with prior anti-obesity medications, concerns about suicide intention have not been confirmed for GLP-1 RA therapy. 65,66 Of all adverse events reported, psychiatric side-effects of GLP-1 RAs are not frequent and account for 1.2–4.4% of events. 67,68 Still, the presence of GLP-1 RA-specific psychiatric adverse events that may also be related to drug intolerance requires the development of a strategy to overcome this barrier for the better implementation of GLP-1RA therapy. 67
Controversy Regarding Effects of GLP-1 RAs in Heart Failure: Direct Drug Effects or Due to Weight Loss?
Previously described cardiovascular effects of GLP-1 RAs are primarily indirect and include enhanced endothelial function and myocardial metabolism, natriuresis and anti-inflammatory and blood pressure-lowering effects. Given that GLP-1 RAs result in weight loss and the pronounced effects of weight loss on haemodynamic disturbance and cardiac reverse remodelling in obese HF patients, the question is, to what extent is the effect of the drugs related to a direct effect on the cardiovascular system in HF?
A study of 5,067 overweight and obese T2D patients failed to demonstrate an association between mild weight reduction and improved cardiac function. 69 In obese HFpEF patients, weight-reduction interventions have significant potential for improving HRQoL. 70 A systematic review of 22 studies investigated the effect of intentional weight loss in overweight and obese patients with HF and demonstrated that all forms of weight loss (lifestyle changes, pharmacotherapy or bariatric surgery) are likely to result in significant improvements of symptoms and HRQoL in HF patients. 70 In the STEP HFpEF trial, higher changes in KCCQ-CSS were linearly associated with weight loss with semaglutide (5.9-point increase in KCCQ-CSS per 10% body weight loss). 45 Remarkably, the recent SELECT trial proved the cardiovascular efficacy of subcutaneous semaglutide in reducing the primary cardiovascular composite endpoint (death from cardiovascular causes, non-fatal MI or non-fatal stroke) in both overweight and obese patients with established atherosclerotic CVD. 71 These data, together with the finding of no effect of prior weight-reduction strategies on hard outcomes in HF, signify very likely beneficial effects of GLP-1RAs that are independent of weight loss. Moreover, a counterintuitive reduction in N-terminal pro B-type natriuretic peptide concentrations on top of weight reduction has been shown in both STEP HFpEF trials, indicating potential direct cardiac effects of the therapies. 72 Still, whether GLP-1RAs in HFpEF exert a disease-modifying effect independent of weight loss remains to be investigated.
The beneficial effects of GLP-1 RAs on HRQoL and other outcomes in HFpEF are likely due to pleiotropic impacts, related or not to weight reduction, such as decongestion (mainly due to the reduction of increased plasma volume associated with obesity and epicardial constrain), reverse cardiac remodelling (mainly due to the decrease in epicardial fat and regression of left atrial and LV myopathy, lowering intracardiac pressure) and anti-inflammatory actions, among others. Additional mechanistic studies are needed to confirm these assumptions.
Future Directions
Recently, Sundaram presented the results of PRAISE HFpEF DM. 73 This was a multicentre observational cohort study from 170 hospitals across the US that investigated the effects of GLP-1 RAs on clinical outcomes, a composite of HF hospitalisation and all-cause mortality, in 1,024 obese HFpEF patients compared with 796 controls receiving dipeptidyl peptidase inhibitor/sulphonylurea therapy. The study showed a 20% risk reduction of the primary outcome in the GLP-1 RA arm (HR 0.80; 95% CI [0.68–0.99]). Furthermore, a prespecified subgroup analysis of HF individuals from the SELECT trial evaluating the effects of semaglutide on cardiovascular outcomes in people with overweight or obesity, which included 2,273 HFpEF and 1,347 HFrEF patients, showed that the therapy was associated with a reduction in the HF composite outcome (cardiovascular death or HF hospitalisation/emergency visit) across LVEF, suggesting that GLP-1 RAs may improve outcomes at least in obese HF patients regardless of diabetes status. 74 However, RCTs are still needed to investigate the effects of GLP-1 RAs on HF outcomes, mortality and clinical outcomes in both HFpEF and HFrEF.
Currently, there is an ongoing randomised double-masked placebo-controlled trial, the SUMMIT trial, studying the efficacy and safety of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and GLP-1 receptors, versus placebo in HFpEF patients with obesity (NCT04847557). The study’s co-primary endpoints are changes in HRQoL and the composite of cardiovascular death and HF events. That study will provide insights into the potential of GLP-1 RAs to modify the disease course in HFpEF patients.
The effects of GLP-1 RAs on HF surrogate endpoints vary in HF patients across the LVEF spectrum. In HFrEF, the administration of GLP-1 RAs did not improve LV systolic function. In HFpEF, the effects of the drugs on cardiac function and structure have not yet been investigated. GLP-1 RA therapy significantly improved both exercise capacity and HRQoL in individuals with obesity-related HFpEF regardless of diabetes status, but had no effect on these parameters in in HFrEF patients. Data from observational studies and subgroup analyses of RCTs show that GLP-1 RAs reduce HF outcomes and mortality in obese HF patients across the LVEF spectrum. However, given the neutral effect of GLP-1 RAs on surrogate HF outcomes in HFrEF and a potentially increased risk of arrhythmias and HF-related hospitalisations, conducting further large-scale trials in the HFrEF cohort seems complicated. Still, it may be relevant to perform pilot studies to address whether the therapy will benefit selected HFrEF cohorts with obesity.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57. Crossref | PubMed
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. Crossref | PubMed
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. Crossref | PubMed
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. Crossref | PubMed
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–39. Crossref | PubMed
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–30; Crossref | PubMed
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–51. Crossref | PubMed
- Fudim M, White J, Pagidipati NJ, et al. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL trial. Circulation 2019;140:1613–22. Crossref | PubMed
- Marx N, Federici M, Schütt K, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 2023;44:4043–140. Crossref | PubMed
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm – 2023 update. Endocr Pract 2023;29:305–40. Crossref | PubMed
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019;140:e563–95. Crossref | PubMed
- Zhao H, Liu Y, Liu M, et al. Clinical outcomes with GLP-1 receptor agonists in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Drugs 2023;83:1293–307. Crossref | PubMed
- Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med 2024;390:1394–1407. Crossref | PubMed
- Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord 2014;15:181–7. Crossref | PubMed
- Muller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130. Crossref | PubMed
- Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol (Lausanne) 2018;9:672. Crossref | PubMed
- Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab 2013;24:85–91. Crossref | PubMed
- Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011;4:324–31. Crossref | PubMed
- Vaduganathan M, Ostrominski JW. Glucagon-like peptide-1 receptor agonists in heart failure: STEPping across the ejection fraction divide. J Am Coll Cardiol 2023;82:2097–100. Crossref | PubMed
- Dzhioeva ON, Timofeev YS, Metelskaya VA, et al. Role of epicardial adipose tissue in the pathogenesis of chronic inflammation in heart failure with preserved ejection fraction. Cardiovasc Ther Prevent 2024;23:3928 [in Russian]. Crossref
- Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev 2017;22:889–902. Crossref | PubMed
- Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013;6:800–7. Crossref | PubMed
- Zarich SW, Kowalchuk GJ, McGuire MP, et al. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol 1991;68:377–81. Crossref | PubMed
- Obokata M, Reddy YNV, Pislaru SV, et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. Crossref | PubMed
- Sorimachi H, Omote K, Omar M, et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail 2022;24:1359–70. Crossref | PubMed
- Koepp KE, Obokata M, Reddy YNV, et al. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:657–66. Crossref | PubMed
- Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol 2023;20:463–74. Crossref | PubMed
- Tate M, Chong A, Robinson E, et al. Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. Br J Pharmacol 2015;172:721–36. Crossref | PubMed
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004;109:962–5. Crossref | PubMed
- Woo JS, Kim W, Ha SJ, et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol 2013;33:2252–60. Crossref | PubMed
- Lønborg J, Vejlstrup N, Kelbæk H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–9. Crossref | PubMed
- Lønborg J, Vejlstrup N, Kelbæk H, et al. Impact of acute hyperglycemia on myocardial infarct size, area at risk, and salvage in patients with STEMI and the association with exenatide treatment: results from a randomized study. Diabetes 2014;63:2474–85. Crossref | PubMed
- Chen WR, Hu SY, Chen YD, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2015;170:845–54. Crossref | PubMed
- Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009;58:975–83. Crossref | PubMed
- Zhong Z, Chen K, Zhao Y, Xia S. Effects of liraglutide on left ventricular function: a meta-analysis of randomized, placebo-controlled trials. Int J Endocrinol 2021;2021:9993229. Crossref | PubMed
- Bizino MB, Jazet IM, Westenberg JJM, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 2019;18:55. Crossref | PubMed
- Hiramatsu T, Asano Y, Mabuchi M, et al. Liraglutide relieves cardiac dilated function than DPP-4 inhibitors. Eur J Clin Investig 2018;48:e13007. Crossref | PubMed
- Huixing L, Di F, Daoquan P. Effect of glucagon-like peptide-1 receptor agonists on prognosis of heart failure and cardiac function: a systematic review and meta-analysis of randomized controlled trials. Clin Ther 2023;45:17–30. Crossref | PubMed
- Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006;12:694–9. Crossref | PubMed
- Lepore JJ, Olson E, Demopoulos L, et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail 2016;4:559–66. Crossref | PubMed
- Margulies KB, Hernandez AF, Redfield MM et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316:500–8. Crossref | PubMed
- Ferreira JP, Saraiva F, Sharma A, et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes Metab 2023;25:1495–502. Crossref | PubMed
- Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE) – a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail 2017;19:69–77. Crossref | PubMed
- Neves JS, Packer M, Ferreira JP. Increased risk of heart failure hospitalization with GLP-1 receptor agonists in patients with reduced ejection fraction: a meta-analysis of the EXSCEL and FIGHT trials. J Card Fail 2023;29:1107–9. Crossref | PubMed
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023;389:1069–84. Crossref | PubMed
- Kambic T, Lavie CJ, Eijsvogels TMH. Seeking synergy for novel weight- and glucose-lowering pharmacotherapy and exercise training in heart failure patients with preserved ejection fraction. Eur Heart J 2024;45:861–3. Crossref | PubMed
- Butler J, Shah SJ, Petrie MC, et al. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomized trials. Lancet 2024;403:1635–48. Crossref | PubMed
- Cimino G, Vaduganathan M, Lombardi CM, et al. Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ESC Heart Fail 2024;11:649–61. Crossref | PubMed
- Vaduganathan M, Fonarow GC, Greene SJ, et al. Health-related quality of life in comorbid heart failure with reduced ejection fraction and diabetes mellitus. J Am Coll Cardiol 2019;74:3176–8. Crossref | PubMed
- Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–9. Crossref | PubMed
- Butler J, Anker SD, Filippatos G, et al. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J 2021;42:1203–12. Crossref | PubMed
- Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020;141:90–9. Crossref | PubMed
- Reddy YNV, Rikhi A, Obokata M, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail 2020;22:1009–18. Crossref | PubMed
- Sato R, von Haehling S. Revisiting the obesity paradox in heart failure: what is the best anthropometric index to gauge obesity? Eur Heart J 2023;44:1154–6. Crossref | PubMed
- Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–9. Crossref | PubMed
- Mann DL, Givertz MM, Vader JM, et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol 2022;7:17–25. Crossref | PubMed
- Borlaug BA, Kitzman DW, Davies MJ, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med 2023;29:2358–65. Crossref | PubMed
- Butler J, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with obesity and heart failure across mildly reduced or preserved ejection fraction. J Am Coll Cardiol 2023;82:2087–96. Crossref | PubMed
- Bekfani T, Nisser J, Derlien S, et al. Psychosocial factors, mental health, and coordination capacity in patients with heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction. ESC Heart Fail 2021;8:3268–78. Crossref | PubMed
- Meissner WG, Remy P, Giordana C, et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med 2024;390:1176–85. Crossref | PubMed
- Kreiner FF, von Scholten BJ, Kurtzhals P, Gough SCL. Glucagon-like peptide-1 receptor agonists to expand the healthy lifespan: current and future potentials. Aging Cell 2023;22:e13818. Crossref | PubMed
- Sass MR, Danielsen AA, Köhler-Forsberg O, et al. Effect of the GLP-1 receptor agonist semaglutide on metabolic disturbances in clozapine-treated or olanzapine-treated patients with a schizophrenia spectrum disorder: study protocol of a placebo-controlled, randomised clinical trial (SemaPsychiatry). BMJ Open 2023;13:e068652. Crossref | PubMed
- Prasad F, De R, Korann V, et al. Semaglutide for the treatment of antipsychotic-associated weight gain in patients not responding to metformin – a case series. Ther Adv Psychopharmacol 2023;13:20451253231165169. Crossref | PubMed
- Billings LK, Handelsman Y, Heile M, et al. Health-related quality of life assessments with once-weekly glucagon-like peptide-1 receptor agonists in type 2 diabetes mellitus. J Manag Care Spec Pharm 2018;24:S30–41. Crossref | PubMed
- Cooper DH, Ramachandra R, Ceban F, et al. Glucagon-like peptide 1 (GLP-1) receptor agonists as a protective factor for incident depression in patients with diabetes mellitus: a systematic review. J Psychiatr Res 2023;164:80–9. Crossref | PubMed
- McIntyre RS, Mansur RB, Rosenblat JD, Kwan ATH. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf 2024;23:47–55. Crossref | PubMed
- Tobaiqy M, Elkout H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int J Clin Pharm 2024;46:488–95. Crossref | PubMed
- Chen W, Cai P, Zou W, Fu Z. Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA Adverse Event Reporting System database. Front Endocrinol (Lausanne) 2024;15:1330936. Crossref | PubMed
- Alonso A, Bahnson JL, Gaussoin SA, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J 2015;170:770–7.e5. Crossref | PubMed
- Lee VYJ, Houston L, Perkovic A, et al. The effect of weight loss through lifestyle interventions in patients with heart failure with preserved ejection fraction – a systematic review and meta-analysis of randomised controlled trials. Heart Lung Circ 2024;33:197–208. Crossref | PubMed
- Peck KH, Dulay MS, Hameed S, et al. Intentional weight loss in overweight and obese patients with heart failure: a systematic review. Eur J Heart Fail 2024. Crossref | PubMed
- Petrie M. Semaglutide 2.4 mg and NTproBNP in obesity-related HFpEF: insights from the STEP-HFpEF programme. Presented at Heart Failure 2024, Lisbon, Portugal, 11–14 May 2024. https://esc365.escardio.org/presentation/283481 (accessed 22 July 2024).
- Sundaram V. Glucagon-like peptide-1 receptor agonist in obese heart failure with preserved ejection fraction and type 2 diabetes mellitus. Presented at Heart Failure 2024, Lisbon, 11–14 May 2024. https://esc365.escardio.org/presentation/283465 (accessed 22 July 2024).
- Deanfield J. Semaglutide and cardiovascular outcomes in patients with overweight or obesity and heart failure: a pre-specified analysis from the SELECT trial. Presented at Heart Failure 2024, Lisbon, Portugal, 11–14 May 2024. https://esc365.escardio.org/presentation/283493 (accessed 22 July 2024).

- Find a Doctor
- Patients & Visitors
- ER Wait Times
- For Medical Professionals
- Piedmont MyChart

- Toggle navigation --> Back to Piedmont.org
- Piedmont Healthcare
Learning Center
- Education and Resources
- Women's Heart Support Network
- Heart Failure Tools and Resources
Case Study: Acute Heart Failure in a 20-year-old Patient
At Piedmont Heart’s Napa Valley Cardiology Conference, Dr. David Dean presents a challenging case of acute heart failure in a 20-year-old patient. Hear Piedmont’s unusual approach to therapy and tips for success from Dr. Dean, surgical director of Piedmont’s Samsky Advanced Heart Failure Center.
Download the Piedmont Now app
- Indoor Hospital Navigation
- Find & Save Physicians
- Online Scheduling
Download the app today!
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Prevention of Heart Failure in Hypertension - the Role of Coronary Heart Disease Events Treated with Versus without Revascularization: the ALLHAT Study
Affiliations.
- 1 Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH.
- 2 University of Texas School of Public Health, Houston, TX.
- 3 Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH.
- 4 Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH; Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH. Electronic address: [email protected].
- PMID: 39243877
- DOI: 10.1016/j.amjcard.2024.08.033
In modern clinical practice, less than half of new-onset heart failure (HF) patients undergo ischemic evaluation, and only a minority undergo revascularization. We aimed to assess the proportion of the effect of hypertension (antihypertensive treatment) on incident HF to be eliminated by prevention of CHD event treated with or without revascularization, considering possible treatment-mediator interaction. Causal mediation analysis of ALLHAT included 42,418 participants (age 66.9±7.7; 35.6% black, 53.2% men). A new CHD event (myocardial infarction or angina) that occurred after randomization but before the incident HF outcome was the mediator. Incident symptomatic congestive HF (CHF) and hospitalized/fatal HF (HHF) were the primary and secondary outcomes. Logistic regression (for mediator) and Cox proportional hazards regression (for outcome) were adjusted for demographics, cardiovascular disease history, and risk factors. During a median 4.5-year follow-up, 2,785 patients developed CHF, including 2,216 HHF events. Participants who developed CHD events had twice the higher incidence rate of CHF than CHD-free (28.5 vs 13.9 events/ 1,000 person-years). The proportion of reference interaction indicating direct harm due to CHD event for lisinopril (234% for CHF; 355% for HHF) and amlodipine (244% for CHF; 468% for HHF) was greater than for chlorthalidone (143% for CHF; 269% for HHF). In patients with revascularized CHD events, chlorthalidone and amlodipine eliminated 21-24%, and lisinopril - 45% of HHF. Antihypertensive treatment was not able to eliminate harm from CHD events treated without revascularization. In conclusion, the antihypertensive drugs (chlorthalidone, lisinopril, amlodipine) prevent HF not principally by preventing CHD events but via other pathways. HF is moderated but not mediated by CHD events. Revascularization of CHD events is paramount for HF prevention.
Keywords: causal mediation analysis; coronary heart disease; heart failure; hypertension; myocardial revascularization.
Copyright © 2024. Published by Elsevier Inc.
PubMed Disclaimer
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Related information
Linkout - more resources, full text sources.
- Elsevier Science
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Br J Clin Pharmacol
- v.47(3); 1999 Mar

Clinical case studies in heart failure management
Robert j macfadyen.
1 Department of Clinical Pharmacology, University of Dundee, Ninewells Hospital and Medical School, Dundee DD1 9SY, United Kingdom
2 Department of Cardiology, University of Dundee, Ninewells Hospital and Medical School, Dundee DD1 9SY, United Kingdom
Paul Shiels
Allan d struthers, therapeutic aspects of heart failure management.
The outlook for patients with cardiac failure has improved substantially in the last 15 years. This is largely due to the application of the results of multicentre clinical trials of new and older drugs and a better understanding of outcomes for individual patients. Interest has focused on systolic dysfunction in both symptomatic and asymptomatic patients. Less is known about the definition and management of diastolic dysfunction.
The following case studies have been chosen to illustrate the basis for therapeutic management of systolic heart failure and outline the remaining gaps in knowledge, of which there are several. The issues apply across the spectrum of patients seen in clinical practice.
Acute stabilization and chronic management of systolic failure
Case history.
A 74-year-old female patient was admitted from home with progressive increase in breathlessness, orthopnoea and ankle oedema over the previous 3 weeks. Her general practitioner had prescribed oral coamoxiclav and coamilofruse (substituting the latter for bendrofluazide). She had suffered from dyspepsia, increasing over recent weeks, and the general practitioner had noted a new murmur.
She was apyrexial yet tachypnoeic (25 beats min −1 ); with a low volume pulse. Sitting blood pressure was 110/70 mm Hg. The apex beat was in the anterior axillary line and a parasternal lift was prominent. A pansystolic murmur was audible and late inspiratory crackles were heard throughout both lung fields. There was sacral oedema.
The ECG confirmed sinus tachycardia (110 beats min −1 ) with antero-lateral Q waves of previous infarction and the chest X-ray confirmed cardiomegaly and interstitial oedema. Routine chemistry showed Na + 128 mmol l −1 ; K + 5.8 mmol l −1 ; urea 9 mmol l −1 ; creatinine 155 mmol l −1 . A creatine kinase series was unremarkable. Echocardiography showed a dilated heart (left ventricular end diastolic distension (LVEDD) 650 mm) with anterior and septal hypokinesis and apical dilatation compatible with previous anterior infarction. The posterior wall was contracting vigorously. A high velocity jet (4.7 m s −1 ) of mitral regurgitation was noted but the left atrial size was normal.
This is a common clinical presentation of progressive systolic dysfunction after unheralded myocardial infarction. A number of therapeutic and general management steps should be considered for all such patients. Whereas in stable patients clinical identification or grading of the severity of heart failure is unreliable [ 1 ], recent studies suggest that in acute heart failure clinical diagnosis is much more secure [ 2 ]. Any abnormality of the ECG in a breathless patient is supportive evidence for a cardiac cause of dyspnoea. A normal ECG usually suggests another diagnosis [ 3 ]. Radiological cardiomegaly is also supportive of a diagnosis of heart failure [ 4 ]. This is not the case for more subtle radiological signs, even in the hands of experienced radiological staff [ 5 ] where the clinical context can significantly affect the interpretation. Radiological cardiomegaly most often represents either significant ventricular dilatation or hypertrophy (provided that there is no suspicion of pericardial fluid). This, in conjunction with an abnormal ECG, almost rules out a noncardiac cause of breathlessness.
Echocardiography provides definitive diagnosis. Although a lack of quantitative images is a frequent practical problem, this may not be of importance to clinical management [ 6 ].
Immediate therapeutic strategies
While there is a lack of controlled clinical trial evidence to support the use of diuretics as initial therapy, effective diuresis and consequent adjustment of the loading conditions of the failing heart is generally regarded as essential [ 7 ]. Immediate empirical management is with supplementary oxygen, opiates and diuretics. Vasodilatation appears to be an important effect of intravenous diuretics although clear evidence for this is scant. However, relief of symptoms appears to precede diuresis and natriuresis.
Intravenous nitrate infusion provides balanced arterio- venous dilatation and is widely employed, supported by beneficial central haemodynamic changes in small studies [ 8 ], although little is known about its effects on either morbidity or mortality [ 9 ]. Intravenous ACE inhibitors also produce beneficial haemodynamic effects [ 10 ]. These drugs are not used routinely intravenously due to difficulties with appropriate dosage regimens, unpredictable and protracted hypotensive effects and concerns over an adverse effect on survival in haemodynamically unstable patients in the early stages after myocardial infarction [ 11 ]. More recent data suggest that there may yet be a role for intravenous administration of ACE inhibitors in unstable patients [ 12 ].
Long-term therapeutic issues
ACE inhibitors It is now clear that patients with either symptomatic or asymptomatic left ventricular dysfunction benefit from ACE inhibitor drugs. Morbidity is reduced with symptomatic improvement, improved exercise duration and improved quality of life indices. Mortality is reduced at all stages of the illness with greater absolute benefit the greater the degree of left ventricular impairment. Treatment is effective regardless of age, gender or aetiology of cardiac impairment. Further coronary events are also reduced in patients with ischaemic cardiomyopathy [ 13 ] although the reasons for this are obscure.
Research now focuses on the reasons why ACE inhibitors remain under used in the management of heart failure [ 14 ]. They are cost-effective in comparison with other cardiovascular treatments [ 15 ] (e.g. lipid lowering in patients with ischaemic heart disease; control of mild hypertension) even when used for treatment of mild (NYHA Grade I/II) heart failure. The reasons why individual patients do not receive an ACE inhibitor need to be clearly defined but may relate to unfounded concerns about side effects. The implications for such patients are a significantly worse prognosis for symptom control, repeated hospitalization and survival.
The degree of neurohormonal suppression by the ACE inhibitor is linked to survival in individual patients [ 16 ]. Some patients taking long-term ACE inhibitor therapy still have normal or elevated levels of the key mediator hormones aldosterone and angiotensin II. The reasons for this are unclear. It may be due to the secondary rise in active renin in response to treatment, failure of compliance, the use of short-acting agents (although these are undoubtedly better than placebo) or to alternative control pathways for generating angiotensin II (the tissue chymase system) and aldosterone (K + or ACTH).
Despite 20 years of study the minimum effective dose of an ACE inhibitor to treat heart failure remains unknown [ 17 ]. It is possible that the symptomatic and mortality benefits occur at different doses and are achieved through a combination of haemodynamic, hormonal or structural effects on the heart and/or the kidneys. One large but short-duration study of enalapril (NETWORK) [ 18 ] has suggested that high dosages may be unnecessary to reduce mortality. The results of a larger study with lisinopril (ATLAS) [ 19 ] suggest high doses may provide additional benefits.
The need for long-term loop diuretic treatment in stable patients taking an ACE inhibitor is unclear and has received little attention. Few studies address the effects of ACE inhibition alone compared with those of an ACE inhibitor plus diuretic, either in the short or long-term. Patients treated with and without a loop diuretic after myocardial infarction in the SAVE study appeared to benefit from captopril [ 20 ]. Those requiring a loop diuretic might have more significant impairment of left ventricular systolic function but this is by no means always the case. Although the left ventricular ejection fraction is an accurate predictor of mortality, it is poorly correlated with exercise capacity. Discontinuation of frusemide in patients with stable heart failure appears to be feasible in a minority of patients, usually those with lesser degrees of impairment of left ventricular function [ 21 ]. In practice this is rarely attempted and chronic diuretic therapy is usual for patients who have had one episode of overt heart failure requiring hospital admission.
Newer agents to suppress the renin angiotensin aldosterone system
Other agents that block the tissue and circulating RAS have been widely studied. Inhibitors of renin have been available for many years but there are few published data from controlled trials [ 22 ]. Low oral bioavailability has seriously compromised the value of this drug class although they are undoubtedly active biochemically and haemodynamically after intravenous administration [ 23 ].
More promising are the selective orally active nonpeptide antagonists of the angiotensin II AT 1 receptor (ARAs) of which there are now several [ 24 ]. Haemodynamic activity is predictable and all produce prolonged hormone suppression. These drugs have a significant advantage for some patients, since they do not produce the dry cough which results from potentiation of kinins during treatment with an ACE inhibitor [ 25 ].
A recent trial has shown a useful improvement in short-term survival with the AT 1 ARA losartan compared with thrice daily captopril in elderly patients with grade II/III heart failure [ 26 ]. This study was designed to examine the safety of losartan with respect to renal function. The reasons for reduced mortality in the group receiving losartan are unclear. The large body of experimental work suggesting that potentiation of the effects of kinins by ACE inhibitors has an important adjuvant role in the therapeutic effects of ACE inhibitors in heart failure [ 27 ] now appears to be in doubt. Before the use of ARAs can be recommended in preference to ACE inhibitors for the management of heart failure, this result must be confirmed in outcome trials. At present, ARAs are a useful alternative for patients intolerant of ACE inhibitors.
Other therapeutic options in systolic heart failure
Combination high dose oral nitrate and hydralazine therapy was shown in the mid 1980s to be effective in relieving symptoms of heart failure [ 28 ] although the impact on survival was less than that achieved by an ACE inhibitor [ 29 ]. The efficacy of nitrates or hydralazine alone has not been tested. The combination should be considered when an ACE inhibitor is contraindicated, e.g. in bilateral reno-vascular stenosis. The main limitation to this therapy is intolerable vasodilator side-effects in a substantial proportion of patients. Nitrate tolerance must be avoided by ensuring an 8 h wash-out phase overnight.
Management and course of the illustrative case
The patient was symptomatically improved by initial intravenous diuretics and supplemental oxygen correction of the fluid imbalance (1.5 l fluid restriction; oral coamilofruse twice daily) resulted in a weight loss of 4.5 kg over 5 days. Although supine blood pressure remained low (≈100 mmHg), an ACE inhibitor (perindopril 2 mg daily) was introduced without difficulty and she experienced no postural symptoms. Renal function improved in response to the changes in therapy. No other therapy was required long-term and diuretic dose was subsequently reduced to coamilofruse one daily). While remaining functionally limited at discharge, she was able to live independently. Having remained well during out-patient review and with no further cardiac admissions, she died suddenly at home some 18 months after presentation.
Optimization of therapy in established cardiac failure
A 53-year-old man who had sustained previous inferior and anterior myocardial infarctions presented with gradually increasing fatigue and oedema despite increasing diuretic therapy. He was being treated with frusemide 80 mg three times daily, captopril 50 mg three times daily, aspirin 150 mg once daily, isosorbide mononitrate SR 60 mg once daily and amlodipine 10 mg once daily.
On examination he had dependent oedema to the mid thigh and basal fine crepitations. He had a resting tachycardia of 105 beats min −1 in sinus rhythm. Mitral and tricuspid regurgitation were evident and the supine blood pressure was low at 95/48 mmHg. Laboratory investigation revealed a reduced serum sodium (125 mmol l −1 ) with impaired renal function (urea 12.8 mmol l −1 ; creatinine 189 μmol l −1 ). The chest radiograph showed minimal interstitial oedema, a right sided pleural effusion and marked cardiomegaly. A 24-h ECG shows repetitive but nonsustained ventricular tachycardia (4 episodes of 10–20 beats with rate >150 beats min −1 ) and multifocal ventricular ectopy (>15 000 aberrant beats/24 h). Repeat echocardiography revealed poor left ventricular contractility with global impairment, marked dilatation (LVEDD 750 mm) and functional mitral regurgitation. Radionuclide scintigraphy indicated a left ventricular ejection fraction of 11%.
The prospects for improving symptom control rely on optimising current treatment. Multiple drug therapy involves the risk of patient confusion over the medicines and a failure of compliance. This is a neglected area in the care of patients with heart failure [ 30 ] yet well known to be associated with poor clinical outcome in cardiac patients [ 31 ]. Most decompensated patients require hospital admission for assessment and observation although community based care is increasingly being studied to reduce costs (see below).
Isolated dependent oedema may be drug-related. Although amlodipine does not appear to cause deterioration in heart failure [ 32 ] unlike some calcium antagonists [ 33 ], marked oedema can occur in some patients. Unless active myocardial ischaemia is present and there are no other management options, withdrawal should be considered.
Diuretic resistance and optimising diuretic therapy
Initiating more effective diuresis and overcoming diuretic resistance is the first goal. Increasing the dosage or frequency of dosing with an oral loop diuretic has limited value for diuretic resistance. Improved efficacy can be achieved by a variety of means [ 34 ]. Oral combination diuretic therapy with the addition of a thiazide drug such as bendrofluazide is one option. Although metolazone is often used, it offers no greater benefit and may cause a greater incidence of adverse effects [ 35 ]. The combination of a loop diuretic with a potassium sparing diuretic is another possibility. If the patient is taking an ACE inhibitor, such an approach is not generally advised but can be used with success given careful control (see below). Combination diuretic treatment requires close monitoring. Once a diuresis is established, it may be possible to stop the additional drug. Long-term combination therapy has not been shown to be beneficial and the risks of electrolyte depletion and induction of dysrhythmia (although this remains controversial) raises concerns. If a combination of diuretics is continued, regular biochemical monitoring is desirable.
Another method to overcome resistance to loop diuretic therapy is intravenous administration either by bolus dosing or by continuous infusion [ 36 ]. The latter is particularly effective as it delivers a constant efficient concentration of frusemide at the tubular lumen. Careful monitoring of renal function and the extent of the diuresis are desirable.
Diuresis can also be enhanced by the addition of low-dose dopamine infusion to facilitate renal vasodilatation [ 37 ]. This may be used in combination with intravenous or oral loop diuretic. Oral dopamine agonist drugs have not been demonstrated to be useful [ 38 ].
Improving neurohormonal blockade of aldosterone and angiotensin II
Failure to suppress plasma angiotensin II and/or aldosterone concentrations may be associated with an adverse outcome in heart failure [ 39 ]. Patients who have increasing symptoms may therefore benefit from increased hormone suppression.
The short-acting agent captopril provides incomplete hormone suppression over 24 h unless used in multiple daily doses. Nevertheless, it is widely used in heart failure. The case for using a longer-acting ACE inhibitor in preference is poorly documented. Comparative studies of long and short-acting ACE inhibitors are few and do not address improvement in symptoms or mortality. Short-acting drugs are sometimes used on the pretext of a lower risk of producing significant biochemical renal dysfunction [ 40 ]. However, adverse renal effects induced by the combination of loop diuretic and ACE inhibitor are generally predictable, minor and are probably unrelated to the duration of action of the ACE inhibitor selected [ 41 ]. Increased dosage or increased dose frequency of the ACE inhibitor might be useful for some patients, although the optimal dose of ACE inhibitor remains uncertain.
Due to the strength of the evidence suggesting that neurohormonal activation in heart failure is integral both to the progression of symptoms and pathology [ 42 ] the combination of ACE inhibitor and ARA is being examined as an option. By blocking the negative feedback loop promoting renin release following treatment with an ACE inhibitor and in addition by providing receptor blockade, levels of bioactive angiotensin II are markedly reduced [ 43 ]. This strategy is currently under investigation using enalapril and valsartan in a multicentre outcome study (ValHeFT).
As aldosterone has control systems independent of the renin-angiotensin axis and since it may be independently detrimental in heart failure, the use of additional aldosterone blockade may have a role. Spironolactone has a variety of beneficial effects on surrogate markers of prognosis in heart failure [ 44 ]. Whether this can be translated to an improved outcome will be demonstrated by the multicentre RALES study [ 45 ]. Preliminary reports of this study suggest that mortality is significantly reduced by this combination. The combination of loop diuretic, ACE inhibitor and spironolactone increases the risk of renal dysfunction and significant electrolyte imbalance, particularly potassium retention. In small trials it is not possible to assess the frequency of such potential adverse events in routine clinical practice, since treatment and biochemical changes are closely monitored.
Additional therapies
Digoxin The role of digoxin in patients with heart failure already treated with an ACE inhibitor and diuretics and who are in sinus rhythm has been a matter of controversy for many years. However, digoxin withdrawal was demonstrated to be detrimental in the PROVED trial [ 46 ]. Recent studies have confirmed that there is no adverse effect on mortality in heart failure [ 47 ] when digoxin is added to patients on diuretics and ACE inhibitors. However, there were useful reductions in recurrent hospitalization after the addition of digoxin. Digoxin should be considered in patients with persistent symptoms despite optimal dosage of a diuretic and an ACE inhibitor. Care should be exercised when there is impairment of renal function and/or suspicion of impaired AV conduction or sinus node disease, in view of the effects of digoxin on cardiac conduction.
β-adrenoceptor blockade
More recently considerable interest has arisen in the use of β-adrenoceptor blockade in heart failure. Importantly these studies have been conducted in patients who were already receiving an ACE inhibitor and diuretic. The combination of data from several studies using carvedilol, a non selective β-adrenoceptor antagonist with additional α 1 -adrenoceptor blocking effects, show its efficacy in unselected patients with heart failure. Carvedilol improves survival in patients already receiving an ACE inhibitor and diuretic [ 48 ]. Worsening heart failure was not more frequent in the β-adrenoceptor blocker treated patients and benefits only emerge over weeks, with a vaguely defined period of increased symptoms during the early phase of treatment. Cardioselective (β 1 )-adrenoceptor blockade has been studied for some years in the management of idiopathic dilated cardiomyopathy. Recently preliminary data from a multicentre trial of bisoprolol in unselected heart failure have shown unequivocal evidence of further reductions in mortality. Although the overall mortality reductions appear small, significant reductions in symptomatic decompensation were evident [ 49 ]. A retrospective (though nonrandomised) analysis of the SAVE study database [ 50 ] suggested that in patients with asymptomatic left ventricular dysfunction after myocardial infarction there is a 30% reduction in cardiovascular death and a 21% reduction in the development of heart failure with the addition of any β-adrenoceptor blocker to captopril.
Small scale studies suggest greater improvements in central haemodynamics and a more significant impact on cardiac adrenergic tone with carvedilol in comparison with metoprolol [ 51 ]. Overall the impact of β-adrenoceptor blockade on exercise capacity is variable, positive in some studies but negative or insubstantial in others dependent on the method of testing [ 52 ].
If β-adrenoceptor blockade is to be widely accepted as a treatment for heart failure, significant practical issues remain to be addressed. There is no clear definition of those patients who stand to benefit most. The treatment appears to be well tolerated in small studies (7–8% intolerance for cardioselective and/or vasodilating β-adrenoceptor blockers; [ 53 ]) but many patients in routine clinical practice will have occult peripheral vascular disease or airways obstruction related to smoking which may complicate the use of these drugs.
The best methods for initiation of treatment is uncertain. Large doses of a β-adrenoceptor bocker can induce hypotension, bradycardia and shock due to the withdrawal of myocardial adrenergic support. As the benefits of β-adrenoceptor blockade appear to emerge gradually during chronic therapy [ 54 ] slow dosage titration is desirable. In most instances weekly medical reviews including daily weight records and regular blood pressure and renal function assessment are recommended for safe treatment [ 53 ]. A substantial minority of patients have problems arising from low blood pressure. As with the ACE inhibitors the optimal dose of β-adrenoceptor blockers is unknown and titration to the ‘maximum tolerated’ dose is currently recommended.
The mechanism of action of β-adrenoceptor blockers in heart failure is unclear. As many patients have overt or covert coronary disease they may simply reduce the impact of ischaemia in otherwise stable cardiac failure. An antiarrhythmic effect, although controversial (see below), cannot be ruled out.
Balanced vasodilator therapy
The use of diuretics can be guided by clinical assessment and body weight. For other treatments, the use of invasive pressure measurements can be valuable. Optimal use of vasodilator/vasoconstrictor agents and inotropic drugs in conjunction with diuretics can sometimes be guided by readings from a central haemodynamic monitoring catheter. This is particularly helpful for patients who have uncontrolled breathlessness and oedema or as a prelude to cardiac transplantation [ 55 ]. This approach can be effective in restoring a compensated state to an otherwise decompensated and deteriorating patient. Most patients who develop major haemodynamic and symptomatic decline despite an ACE inhibitor and effective diuresis are in an almost intractable position. Intensive intravenous therapy, more commonly employed in North America and in younger patients, is not widely used in Europe. Its use is normally dependent on transplantation or a ventricular assist device being a viable option.
Anti-arrhythmic therapy
Control of dysrhythmia in heart failure remains a major concern. Many patients die suddenly, usually attributed to some form of dysrhythmia (both tachydysrhythmias and bradydysrhythmias are common). The numbers who die in this way may be increasing with the increasing use of therapies that reduce uncontrolled pump failure as the mode of death.
The use of antiarrhythmic therapy in left ventricular dysfunction is restricted following the results from the CAST studies which demonstrated increased mortality with some treatments in patients with coexistent left ventricular (LV) dysfunction [ 56 ]. A particular concern reflects the use of potent drugs with the potential for pro-arrhythmic effects in patients who had minimal levels of electrical instability. Amiodarone appears to be an exception. Although results from four major trials have not shown consistent improvement in survival in established LV dysfunction [ 57 , 58 ], amiodarone is the best available drug for overt dysrhythmia in the face of impaired LV function. In general it can be used without preliminary electrophysiological testing [ 59 ].
Nonsustained ventricular tachycardia (VT) is common in symptomatic heart failure and is an independent marker of increased risk of sudden death in severe heart failure [ 60 ]. Whether amiodarone should be prescribed in all patients with heart failure who have non sustained and asymptomatic VT is not yet clear.
The role of drug therapy in comparison with electrical devices allowing sophisticated pacing detection and including the implantable cardioverter/defibrillator remains an important area for study [ 61 ]. At present these devices are generally restricted to patients with documented syncopal dysrhythmia or resuscitated arrest. Patients who survive arrhythmogenic cardiac arrest generally have significant pre-existent heart failure and structurally abnormal hearts. In Europe the application of such technology remains relatively limited and adequate trials comparing this therapy with drug treatments, although underway, are not yet available for guidance. These devices may play a significant role in heart failure in the management of selected patients.
Pulsed inotropic therapy and inotropic-vasodilator drugs
The use of inotropic agents in heart failure has encountered many problems. In the 1980s and early 1990s many agents were developed with effects on the heart and vascular tree combining a central inotropic stimulus with peripheral arterial vasodilatation. These have been uniformly unsuccessful during chronic dosing and often result in increased mortality [ 62 – 64 ] (e.g. flosequinan; ibopamine) As a result, the development of new drugs in this class has been all but halted. The major problems have been selection of appropriate patients and the narrow therapeutic index for such treatments. However, these drugs may have a role in the short term to relieve symptoms without the increase in mortality that occurs during protracted use [ 65 ].
In a different approach to inotropic therapy, Adamopoulos and colleagues employed intermittent dobutamine infusion in 20 patients with severe heart failure in an attempt to up-regulate myocardial β 1 -adrenoceptors [ 66 ]. In the short term, there were no clinical complications of treatment, but also no increase in diuresis. Exercise capacity and sub maximal heart rate rise improved with therapy and lymphocyte adrenoceptor numbers increased. This result is in contrast to the adverse effects of chronic inotropic therapy [ 64 ]. A fine balance may have be struck to improve adrenoreceptor responsiveness but to avoid excessive stimulation or adrenoceptor down-regulation.
Multidisciplinary care and hospitalization
Despite high annual mortality rates a substantial proportion of patients with heart failure face an uncertain yet chronic illness. Many patients are elderly and need repeated hospital admission. These admissions are both psychologically and financially taxing. In common with many chronic diseases, management of these patients can be improved by contact with many varied health professionals concerned not only with cardiac care but social circumstances; promotion of drug compliance and psychological support [ 67 , 68 ]. Non-pharmacological approaches such as increased exercise and training within limits has important and demonstrable physical [ 69 ] as well as psychological effects [ 70 ]. There are few instances where a supervised increase in physical exercise is not of benefit to the patient with heart failure.
Surgical management
Surgical treatment need not be considered as a final line of therapy. Increasingly important is the application of surgical revascularization for occult or overt ischaemia in patients with heart failure. This is often the best way to improve contractility although the operative management may be complicated and associated with increased perioperative mortality. Less well demonstrated are the benefits of scar reduction; cardiomyoplasty or the most recent and most dramatic innovation of ventricular reduction/ventriculotomy. As yet these procedures are limited in their application to a few selected patients and with generally no agreed role.
Cardiac transplantation is a proven therapy but restricted by organ availability and delay prior to intervention. Assessment is usually based on the severity of cardiac impairment on objective testing during treatment with maximal therapies. It is increasingly recognized that many patients with very poor left ventricular function can survive for many years without transplant. Functional exercise capacity is seen as the main delineator of those patients who should be entered onto an urgent transplantation waiting list or those who can safely remain on drug therapy [ 71 , 72 ]. The long-term problems of rejection and accelerated atherosclerosis and their effects on survival after transplantation will not be considered here. The outlook for the transplanted patient is dramatically better than those who await transplantation.
Following emergency admission, initiation of diuresis required bolus doses of intravenous frusemide combined with continuous intravenous infusion of dopamine and fluid and sodium restriction. Although he was considered a good candidate for invasive pulmonary pressure monitoring, this proved unnecessary. Following temporary withdrawal of captopril during stabilisation of renal function and diuresis, an ACE inhibitor was subsequently reintroduced without difficulty (lisinopril 10 mg daily). Renal function improved and blood pressure stabilised. Following discharge he remained symptomatic. Oral digoxin was introduced with only modest benefit. Repeat outpatient ambulatory ECG recording confirmed persistent episodes of non-sustained ventricular tachycardia. He was treated with prophylactic oral amiodarone despite his lack of symptoms. Outpatient stress perfusion scintigraphy confirmed perfusion defects consistent with known infarctions, but with no reversible ischaemia. Maximal cardiopulmonary exercise testing confirmed severe functional incapacity and very poor maximal oxygen consumption at peak exertion (13 ml kg −1 min −1 ). He was therefore referred for consideration of orthotopic cardiac transplantation.
Key therapeutic points
Case 1 acute stabilization and chronic management of systolic failure.
- Establishing aetiology must be part of diagnosis but does not importantly affect therapeutic strategies
- Diuretic therapy is a key element although formally untested in symptomatic patients. Diuretics were given to nearly all the patients in trials assessing the efficacy of ACE inhibitors.
- ACE inhibition must be considered in all patients and failure to provide this treatment requires clear justification. Morbidity and mortality benefits are unequivocal and generally greater the more significant the impairment of LV function. Minor or relative contraindications to use (e.g. asymptomatic hypotension; minor pretreatment renal impairment) should be weighed against the significant consequences of failure to treat.
- Angiotensin receptor antagonists may be an effective substitute for patients unable to tolerate an ACE inhibitor.
Case 2 Optimization of therapy in established heart failure
- In-hospital stabilization is usually necessary if the patient is already taking diuretics and an ACE inhibitor.
- Optimising diuretic therapy may involve:
- intravenous treatment
- combination therapy
- and/or haemodynamic (Swan Ganz catheter) monitoring
- Consider adjuvant digoxin for symptom control even in sinus rhythm
- Consider adjuvant β-adrenoceptor blockade
- dose titration may be protracted and complex
- the effects of selection bias (patient tolerance to treatment) on clinical trial results is unclear and may be important when considering treatment in routine practice
- Pulsed inotropic therapy or balanced vasodilator/inotropic therapy may be useful in selected patients
- Surgical revascularization or myocardial reconstruction may be feasible and useful options but are unproven in formal clinical trials
- Transplantation is effective when available but ‘survivors’ live to receive transplants
- Acute Coronary Syndromes
- Cardiac Surgery
- Heart Failure
- Heart Health
- Heart Rhythm
- Hypertension
- Interventional Cardiology
- Mitral Valve
- Pharmaceutics
- Structural Heart Disease
- Tricuspid Valve
- Vascular & Endovascular
- Chest Pain Guidelines
- Compensation
- Education & Training
- Patient Care
- Policy & Regulations
- Professional Associations
- Cardiac Imaging
- Informatics
- Nuclear Cardiology
- Remote Monitoring
- Experience Stories
- Webinars & Videos
Search form
Nurse-led case management may reduce hf readmissions.
- Case management interventions (intense monitoring of patients following discharge often involving telephone follow-up and home visits);
- Clinic interventions (follow-up in a CHF clinic); and
- Multidisciplinary interventions (holistic approach bridging the gap between hospital admission and discharge home delivered by a team). The components, intensity and duration of the interventions varied, as did the ‘usual care’ comparator provided in different trials.
Related Content
Around the web.
Eleven medical societies have signed on to a consensus statement aimed at standardizing imaging for suspected cardiovascular infections.
Kate Hanneman, MD, explains why many vendors and hospitals want to lower radiology's impact on the environment. "Taking steps to reduce the carbon footprint in healthcare isn’t just an opportunity," she said. "It’s also a responsibility."
Philips introduced a new CT system at ECR aimed at the rapidly growing cardiac CT market, incorporating numerous AI features to optimize workflow and image quality.

IMAGES
VIDEO
COMMENTS
On examination, the temperature was 36.4°C, the heart rate 103 beats per minute, the blood pressure 79/51 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% while ...
Case 4 - A 79-Year-Old Man with Congestive Heart Failure Due to Restrictive Cardiomyopathy. JAP, a 79-year-old male and retired metalworker, born in Várzea Alegre (Ceará, Brazil) and residing in São Paulo was admitted to the hospital in October 2013 due to decompensated heart failure. The patient was referred 1 year before to InCor with a ...
In this case, a patient with known history of coronary artery disease presented with worsening of shortness of breath with lower extremity edema and jugular venous distension along with crackles in the lung. The sign and symptoms along with labs and imaging findings point to diagnosis of heart failure with reduced EF (HFrEF).
Heart Failure Patient Case Quizzes. Unrecognized HFpEF in a Type 2 DM Patient - Reducing CV and HF Risk ; Untreated HFrEF/Ischemic Cardiomyopathy in Type 2 DM - How to Optimize Medical Therapy to Improve Heart Failure Outcomes ; Restrictive Cardiomyopathies Series: Advanced HF Therapies in ATTR Cardiac Amyloidosis (Certified Patient Case Study)
The patient's medical history included a diagnosis of congestive heart failure and coronary artery disease in 2014, after stenting of his left anterior descending artery and right coronary artery.
Dr. Amy A. Sarma: A 54-year-old man was evaluated at this hospital because of new heart failure. One month before this evaluation, a nonproductive cough developed after the patient took a business ...
A 67-year-old woman sought emergency medical care due to prolonged chest pain. In April 2009 the patient had prolonged chest pain and at that time she sought medical care. She was admitted at the hospital and diagnosed with myocardial infarction. The patient had hypertension, diabetes mellitus, dyslipidemia and was a smoker.
Presentation of Case. Dr. Jacqueline B. Henson (Medicine): A 54-year-old man was evaluated at this hospital after cardiac arrest associated with ventricular fibrillation. The patient had been in ...
Common locations reporting heart failure case fatality were the USA (seven studies), Canada (6), India (5) and Israel (4). One-year heart failure case fatality was diagnosed by signs and symptoms (including Framingham, ESC and Boston criteria, and chart review) in 25 studies, and by ICD, ICPC or Read codes in 23 studies (table 3). In these ...
In patients with heart failure with preserved ejection fraction (HFpEF), increased left atrial stiffness index (> 0.26) has been associated with substantial risk of death or heart failure ...
Heart failure (HF) is an important disease entity in aging populations, and despite advances in various treatment options, it remains a significant clinical and public health problem. 1 Left ventricular ejection fraction (EF) is the key factor in HF characterization, treatment decision, and prognosis assessment. 2 Recent guidelines suggest dividing HF into 3 groups on the basis of EF: HF with ...
Heart failure patients presenting to primary care clinics often have multiple, complex comorbidities. Several different disease processes and treatment options may need to be considered simultaneously in the setting of acute on chronic exacerbation of symptoms. This...
Background: Vericiguat has demonstrated efficacy in improving the prognosis of patients with heart failure with reduced ejection fraction (HFrEF) following recent clinical deterioration. However, its real-world impact on reducing N-terminal B-type natriuretic peptide (NT-proBNP) levels and improving ventricular remodeling remains uncertain in stable HFrEF patients receiving guideline-directed ...
Mary Lou Poppins initial vitals in the emergency department includes a blood pressure of 138/70, heart rate of 108. respiratory rate of 26, temperature 98.9 degrees fahrenheit, and oxygen saturation of 84%. Her initial assessment included alert and oriented to person and place, dyspnea, inspiratory crackles in bilateral lungs, and a cough with ...
This case highlights the need of optimizing both the heart failure and the anti-anginal medications in patients with heart failure of ischaemic origin. This patient has improved dramatically after the up-titration of diuretics, the control of heart rate with nebivolol and ivabradine and the additional use of trimetazidine. 1-3 All these drugs ...
The Heart Failure Society of America recently issued a statement, providing guidance on virtual visits, emphasizing the need for patient risk stratification, the importance of having a virtual visit workflow and the role of multidisciplinary HF clinicians such as APPs, physicians, pharmacists and licensed social workers. 1
Early identification of kidney dysfunction in patients with advanced heart failure is crucial for timely interventions. In addition to elevations in serum creatinine, kidney dysfunction encompasses inadequate maintenance of sodium and volume homeostasis, retention of uremic solutes, and disrupted endocrine functions. Hemodynamic derangements and maladaptive neurohormonal upregulations ...
Stress hyperphenylalaninemia predicts elevated mortality rates in patients with acute decompensated heart failure (ADHF). This study investigated the metabolic pathways underlying this association and identified a unique metabolic phenotype underlying the association between stress hyperphenylalaninemia and adverse outcomes in ADHF.
This guide provides a clear and concise overview of heart failure for primary care clinicians. Written by two nurse practitioners for nurses, nurse practitioners, physician assistants, medical students, and pharmacists, it is uniquely designed to bridge the gap between cardiology and primary care. It delivers the most current recommendations ...
Advanced Heart Failure Case Study Patient Case History A 65 y/o man with chronic systolic heart failure comes to the office with progressive heart failure symptoms (dyspnea, fatigue) and a 10# unintentional weight loss. He is having more difficulty carrying on ADLs. He also has nocturnal dyspnea. He complains of chest pain and has
INTRODUCTION AND PURPOSE. Heart failure (HF) has been defined as the inability of the heart to provide the adequate blood supply for the metabolizing tissues of the body. 1 According to the Centers for Disease Control and Prevention, approximately 5 million people in the United States have HF, with an estimated direct cost of $29.6 billion in 2006. 2 Heart failure is the most common reason for ...
Introduction. Heart failure (HF) complicating acute myocardial infarction (AMI) is common. Among patients with AMI, HF is the most powerful predictor of death and it has important implications for treatment. Whereas cardiogenic shock is widely appreciated as the major complication of hemodynamic compromise, less severe HF states on the spectrum are more common and also have major adverse ...
Introduction. This case study maps the journey of a patient and his family following a diagnosis of chronic heart failure secondary to left ventricular systolic dysfunction. It outlines the personal, medical, nursing, and social needs, and highlights the wider implications of heart failure and its progression.
Learn more about the effects of GLP1 Agonists in heart failure patients in this free article from Radcliffe Cardiology. Effects of GLP-1 Receptor Agonists in Heart Failure Patients. ... An early small study of 12 HF patients with New York Heart Association ... et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 ...
At Piedmont Heart's Napa Valley Cardiology Conference, Dr. David Dean presents a challenging case of acute heart failure in a 20-year-old patient. Hear Piedmont's unusual approach to therapy and tips for success from Dr. Dean, surgical director of Piedmont's Samsky Advanced Heart Failure Center.
In modern clinical practice, less than half of new-onset heart failure (HF) patients undergo ischemic evaluation, and only a minority undergo revascularization. We aimed to assess the proportion of the effect of hypertension (antihypertensive treatment) on incident HF to be eliminated by prevention …
Assessing high-density lipoprotein cholesterol's prognostic value in heart failure patients ... 2.97 million new patients. 3 The Chinese Heart Failure Patient Registration Study ... that HDL-C levels may not uniformly predict heart-related health issues in every case. 11-13 The precise mechanisms by which HDL-C is associated with different ...
The following case studies have been chosen to illustrate the basis for therapeutic management of systolic heart failure and outline the remaining gaps in knowledge, of which there are several. ... This is a neglected area in the care of patients with heart failure yet well known to be associated with poor clinical outcome in cardiac patients ...
Among chronic heart failure (CHF) patients who have previously been admitted to the hospital for this condition there is now good evidence that case management type interventions led by a HF specialist nurse reduces CHF related readmissions after one year of follow-up, all-cause readmissions and all-cause mortality, according to a large study published online Sept. 12 in The Cochrane Library.
Heart Failure History of Present Problem: JoAnn Smith is a 72-year-old woman who has a history of myocardial infarction (MI) four years ago and systolic heart failure secondary to ischemic cardiomyopathy with a current ejection fraction (EF) of only 15%. She presents to the emergency department (ED) for shortness of breath (SOB) the past three ...