What Are Cancer Research Studies?
What is cancer research and why is it important.

Research is the key to progress against cancer and is a complex process involving professionals from many fields. It is also thanks to the participation of people with cancer, cancer survivors, and healthy volunteers that any breakthroughs go on to improve treatment and care for those who need it.
Cancer research studies may lead to discoveries such as new drugs to treat cancer, new therapies to make symptoms less severe, or lifestyle changes to reduce the chances of getting cancer.
Cancer research may also address big picture questions like why cancer is more prevalent in certain populations or how doctors can make existing cancer detection tools more effective in health care settings.
These discoveries can help people with cancer and their caregivers live fuller lives.

Who should join cancer research studies?
When you choose to participate in a research study, you become a partner in scientific discovery. Your generous contribution can make a world of difference for people like you.
As scientists continue to conduct cancer research, anyone can consider joining a research study. The best research includes everyone, and everyone includes you.
Your unique experience with cancer is incredibly valuable and may help current and future generations lead healthier lives.
When more people of all different races, ethnicities, ages, genders, abilities, and backgrounds participate, more people benefit.
It is important for scientists to capture the full genetic diversity of human populations so that the lessons learned are applicable to everyone.
What are the types of cancer research studies?
See below for definitions on the four major types of research and their subtypes:
- basic research
- quality of life/supportive care
- natural history
- longitudinal
- population-based
- epidemiological research
- translational research
Basic Research
Basic cancer research studies explore the very laws of nature. Scientists learn how cancer cells grow and divide, for example, by growing and testing bacteria , viruses , fungi , animal cells, and human cells in a lab. Scientists also study, for example, the genes that make up tumors in mice and rats in the lab. These experiments help build the foundation for further discovery.

Why Participate in a Clinical Trial?
Get information on how to evaluate a clinical trial and what questions to ask.
Clinical Research
Clinical research involves the study of cancer in people. These cancer research studies are further broken down into two types: clinical trials and observational studies .
- Treatment trials test how safe and useful a new treatment or way of using existing treatments is for people with cancer. Test treatments may include drugs, approaches to surgery or radiation therapy , or combinations of treatments.
- Prevention trials are for people who do not have cancer but are at a high risk for developing cancer or for cancer coming back. Prevention clinical trials target lifestyle changes (doing something) or focus on certain nutrients or medicines (adding something).
- Screening trials test how effective screening tests are for healthy people. The goal of these trials is to discover screening tools or methods that reduce deaths from cancer by finding it earlier.
- Quality-of-life/supportive care tests aim to help people with cancer, as well as their family and loved ones, cope with side effects like pain, nutrition problems, nausea and vomiting , sleeping problems, and depression . These trials may involve drugs or activities like therapy and exercising.

Find Observation Studies >
View a studies that are looking for people now.
- Natural history studies look at certain conditions in people with cancer or people who are at a high risk of developing cancer. Researchers often collect information about a person and their family medical history , as well as blood, saliva, and tumor samples. For example, a biomarker test may be used to get a genetic profile of a person’s cancer tissue. This may reveal how certain tumors change over the course of treatment .
- Longitudinal studies gather data on people or groups of people over time, often to see the result of a habit, treatment, or change. For example, two groups of people may be identified as those who smoke and those who do not. These two groups are compared over time to see whether one group is more likely to develop cancer than the other group.
- Population-based studies explore the causes of cancer, cancer trends, and factors that affect cancer care in specific populations. For example, a population-based study may explore the causes of a high cancer rate in a regional Native American population.
Epidemiological Research
Epidemiological research is the study of the patterns, causes, and effects of cancer in a group of people of a certain background. This research encompasses both observational population-based studies but also includes clinical epidemiological studies where the relationship between a population’s risk factors and treatments are tested.
Translational Research
Translational research is when cancer research moves across research disciplines, from basic lab research into clinical settings, and from clinical settings into everyday care. In turn, findings from clinical studies and population-based studies can inform basic cancer research. For example, data from the genetic profile of a tumor during an observational study may help scientists develop a clinical trial to test which drugs to prescribe to cancer patients with specific tumor genes.

Monica Bertagnolli, Director, NIH; former director, NCI; cancer survivor
Participation in Cancer Research Matters
I am so happy to have the opportunity to acknowledge the courage and generosity of an estimated 494,018 women who agreed to participate in randomized clinical trials with results reported between 1971 and 2018.
Their contributions showed that mammography can detect cancer at an early stage, that mastectomies and axillary lymph node dissections are not always necessary, that chemotherapy can benefit some people with early estrogen receptor–positive, progesterone receptor–positive, HER2-negative breast cancer but is not needed for all, and that hormonal therapy can prevent disease recurrence.
For just the key studies that produced these results, it took the strength and commitment of almost 500,000 women. I am the direct beneficiary of their contributions, and I am profoundly grateful.
The true number of brave souls contributing to this reduction in breast cancer mortality over the past 30 years? Many millions. These are our heroes.
— From NCI Director’s Remarks by then-NCI Director Monica M. Bertagnolli, M.D., at the American Society of Clinical Oncology Annual Meeting, June 3, 2023
- News and Features
- Conferences
Clinical Tools
- Special Collections
- Breast Cancer

Gynecologic Cancer

- General Oncology

- General Medicine

Latest News
- Post-Recurrence Treatment Data Lacking in Cancer Trials
- Substituting Lower-Wage Staff for Registered Nurses Tied to Worse Outcomes
- Drug Shortages Still Affecting US Cancer Centers, Survey Shows
- Dabrafenib Plus Trametinib Provides Some Long-Term Benefits in Melanoma
- Acupuncture Reduces Endocrine Symptoms, Hot Flashes in Breast Cancer
- Black Patients More Likely to Experience MACE After ADT for Prostate Cancer
- Krazati Plus Cetuximab Approved for KRAS G12C-Mutated Advanced Colorectal Cancer
- Ready-to-Use Formulation of Akynzeo Now Available
- Taming the Flames of Burnout in Oncology
- Gastroesophageal cancers: Trying to outfox a “tricky entity”
- Surveillance IDs New Tumors in Children With Cancer Predisposition
- Nearly 1 in 4 Do Not Recover From COVID-19 by 90 Days
Latest Features

Conference Coverage

Featured Conference
- Bladder, kidney, and other urologic cancers
- Bone and connective tissue cancer
- Breast cancer
- CNS cancers
- Colorectal and other GI cancers
- Cytoprotective and supportive care agents
- Gynecologic Cancers
- Head and neck cancer
- Kaposi's sarcoma
- Leukemias, lymphomas, and other hematologic cancers
- Melanoma and other skin cancers
- Pancreatic, thyroid, and other endocrine cancers
- Prostate and other male cancers
- Respiratory and thoracic cancers
- Solid tumors
Ritux 3 Regimen Provides Patients With Pemphigus With Sustained Remission
Efficacy of a Co-Located Bridging Recovery Initiative for Opioid Use Disorder
US Oncologists, Hematologists Receive More Industry Payments Than Their Peers
FDA Withdraws Approval of Pepaxto for Multiple Myeloma
PIDS Updates Guidance on COVID-19 Management in Children, Adolescents
- Bladder Cancer
- Central Nervous System Cancers
- Endocrine Cancers
- Gastrointestinal Cancers
- Genitourinary Cancers
- Head and Neck Cancers
- Hematologic Cancers
- Lung Cancer
- Pediatric Cancer
- Prostate Cancer
- Renal Cell Carcinoma
- Skin Cancers
Cases and Controls
A case-control study compares two groups of people: those with the cancer under study (cases) and those who do not have the cancer (controls). Researchers compare the genetic, environmental, lifestyle, and medical histories of the people in the two groups to identify factors associated with cancer.
Selected examples of DCEG case-control studies:
AsiaLymph , an international hospital-based case-control study of lymphoma among Chinese in Eastern Asia to replicate and extend recent and novel observations made in studies of White populations with distinctly different patterns of environmental and occupational risk factors and genetic loci.
African Esophageal Cancer Consortium (AfrECC) facilitates collaborations to coordinate etiologic and molecular studies of esophageal squamous cell carcinoma in East Africa.
Breast, Ovary and Endometrial Cancer Studies in Poland
- Ovarian and Endometrial Cancer Case-Control Study in Poland was conducted among female residents of Warsaw and Lodz (Poland) between 2001 and 2003. Current projects include methylation profiling, tumor gene sequencing, microsatellite instability analysis, and immunohistochemistry to study the etiologic heterogeneity of endometrial and ovarian cancers.
Case-control Studies of Renal Cell Cancer
- Renal Cell Cancer among White and African American Adults in the United States , conducted in the metropolitan areas of Detroit and Chicago in collaboration with Wayne State University and the University of Illinois at Chicago. The aims of this study are to evaluate risk factors for renal cell cancer and examine why rates of this disease are higher among U.S. Black adults than White adults.
- Renal Cell Cancer in Eastern Europe , conducted from 1999 to 2003 in collaboration with the International Agency for Research on Cancer to evaluate kidney cancer risks in relation to occupational and other environmental and lifestyle exposures in six centers across Eastern Europe. Factors being evaluated include occupational exposures, lifestyle factors, medical conditions, markers of genetic susceptibility, and tumor molecular characteristics.
The DETECT Study – Discovery and Evaluation of Testing for Endometrial Cancer in Tampons , a partnership with the University of Alabama Birmingham to evaluate the acceptability and feasibility of self-sampling with vaginal tampons for endometrial cancer detection in a racially-diverse population of women undergoing hysterectomy.
Environment And Genetics in Lung cancer Etiology (EAGLE) is a large population-based case-control study designed and conducted to investigate the genetic and environmental determinants of lung cancer and smoking persistence using an integrative approach that allows combined analysis of genetic, environmental, clinical, and behavioral data.
Epidemiology of Burkitt Lymphoma in East African Children and Minors (EMBLEM) , a large, multidisciplinary epidemiological effort designed to evaluate environmental and host factors associated with childhood Burkitt lymphoma in sub-Saharan Africa.
Nasopharyngeal Case-Control Study , conducted in Taiwan between 1991-1994 and 2010-2014, examines the role of viral, environmental, and genetic factors associated with the development of nasopharyngeal carcinoma (NPC). Factors investigated include Epstein-Barr virus, diet, smoking, occupation, HLA, and other genetic polymorphisms.
NCI-SEER Non-Hodgkin Lymphoma (NHL) Case-Control Study , a multi-center, population-based study of 1,321 NHL cases and 1,057 controls that includes detailed interview data, biospecimens, and environmental samples.
New England Bladder Cancer Study , a population-based, case-control study of bladder cancer in New Hampshire, Vermont, and Maine, is designed to explain the reasons for the persistent excess of rates of bladder cancer in the northern New England area. Investigators collected data on 2,600 participants via personal interviews, biological samples (blood, buccal cells, urine, toenails, and tumor tissue), as well as drinking water samples.
Testicular Cancer among Military Servicemen: the STEED Study , a case-control study of testicular cancer among military servicemen. The project includes obtaining biosamples and questionnaire data from all participants. Pre-diagnostic serum samples are available from the approximately 1,100 cases and 1,100 controls enrolled in the study.
COVID-19 Resources
What people with cancer should know: https://www.cancer.gov/coronavirus
Guidance for cancer researchers: https://www.cancer.gov/coronavirus-researchers
Get the latest public health information from CDC: https://www.cdc.gov/coronavirus
Get the latest research information from NIH: https://www.covid19.nih.gov

Help us improve EBCCP and other cancer control resources on Cancer Control P.L.A.N.E.T.
Help us improve EBCCP
- Teach with EBCCP

EBCCP Case Studies for Teaching
Designed specifically for public health instructors, EBCCP Case Studies for Teaching provide structured learning opportunities that facilitate students' development of public health competencies related to research, evaluation, and implementation science. The first case study focuses on evaluation of research evidence for implementation. Additional case studies are under development.
Available materials for each case include
- Instructor's guide indicating competencies addressed, and complete instructions
- Student's guide with instruction and activity worksheet
- Instructor's presentation "When is a Public Health Intervention Evidence-Based Enough for Broader Implementation?" with speaker notes to set up the activity
- Instructor's answer key to student activity

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Different study designs in the epidemiology of cancer: case-control vs. cohort studies
Affiliation.
- 1 Department of Medicine, University of Manitoba, Winnipeg, MB, Canada.
- PMID: 19109782
- DOI: 10.1007/978-1-59745-416-2_11
It is only since the 1950s that most of the epidemiology studies on cancer have been conducted. The principal study designs for epidemiologic study of cancer etiology are case-control and cohort studies. These study designs have complimentary roles and distinct advantages and disadvantages. This chapter provides historical perspectives, description of the traditional and variant designs of case-control and cohort studies, and the relative advantages and disadvantages of these study designs.
PubMed Disclaimer
Similar articles
- Methods in epidemiology: observational study designs. DiPietro NA. DiPietro NA. Pharmacotherapy. 2010 Oct;30(10):973-84. doi: 10.1592/phco.30.10.973. Pharmacotherapy. 2010. PMID: 20874034 Review.
- Statistical methods in cancer epidemiological studies. Xue X, Hoover DR. Xue X, et al. Methods Mol Biol. 2009;471:239-72. doi: 10.1007/978-1-59745-416-2_13. Methods Mol Biol. 2009. PMID: 19109784
- [Common study designs in epidemiology]. Klug SJ, Bender R, Blettner M, Lange S. Klug SJ, et al. Dtsch Med Wochenschr. 2007;132 Suppl 1:e45-7. doi: 10.1055/s-2007-959041. Dtsch Med Wochenschr. 2007. PMID: 17530597 German. No abstract available.
- Four different study designs to evaluate vaccine safety were equally validated with contrasting limitations. Glanz JM, McClure DL, Xu S, Hambidge SJ, Lee M, Kolczak MS, Kleinman K, Mullooly JP, France EK. Glanz JM, et al. J Clin Epidemiol. 2006 Aug;59(8):808-18. doi: 10.1016/j.jclinepi.2005.11.012. Epub 2006 Mar 15. J Clin Epidemiol. 2006. PMID: 16828674
- Logistics and design issues in the use of biological samples in observational epidemiology. Potter JD. Potter JD. IARC Sci Publ. 1997;(142):31-7. IARC Sci Publ. 1997. PMID: 9354909 Review.
- Diets, Dietary Patterns, Single Foods and Pancreatic Cancer Risk: An Umbrella Review of Meta-Analyses. Gianfredi V, Ferrara P, Dinu M, Nardi M, Nucci D. Gianfredi V, et al. Int J Environ Res Public Health. 2022 Nov 10;19(22):14787. doi: 10.3390/ijerph192214787. Int J Environ Res Public Health. 2022. PMID: 36429506 Free PMC article. Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 June 2024
Why do patients with cancer die?
- Adrienne Boire ORCID: orcid.org/0000-0002-9029-1248 1 na1 ,
- Katy Burke 2 na1 ,
- Thomas R. Cox ORCID: orcid.org/0000-0001-9294-1745 3 , 4 na1 ,
- Theresa Guise 5 na1 ,
- Mariam Jamal-Hanjani 6 , 7 , 8 na1 ,
- Tobias Janowitz ORCID: orcid.org/0000-0002-7820-3727 9 , 10 na1 ,
- Rosandra Kaplan 11 na1 ,
- Rebecca Lee ORCID: orcid.org/0000-0003-2540-2009 12 , 13 na1 ,
- Charles Swanton ORCID: orcid.org/0000-0002-4299-3018 7 , 8 , 14 na1 ,
- Matthew G. Vander Heiden ORCID: orcid.org/0000-0002-6702-4192 15 , 16 na1 &
- Erik Sahai ORCID: orcid.org/0000-0002-3932-5086 12 na1
Nature Reviews Cancer ( 2024 ) Cite this article
6638 Accesses
1 Citations
447 Altmetric
Metrics details
- Cancer models
- Cancer therapy
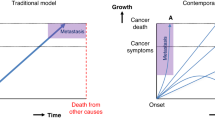
Cancer is a major cause of global mortality, both in affluent countries and increasingly in developing nations. Many patients with cancer experience reduced life expectancy and have metastatic disease at the time of death. However, the more precise causes of mortality and patient deterioration before death remain poorly understood. This scarcity of information, particularly the lack of mechanistic insights, presents a challenge for the development of novel treatment strategies to improve the quality of, and potentially extend, life for patients with late-stage cancer. In addition, earlier deployment of existing strategies to prolong quality of life is highly desirable. In this Roadmap, we review the proximal causes of mortality in patients with cancer and discuss current knowledge about the interconnections between mechanisms that contribute to mortality, before finally proposing new and improved avenues for data collection, research and the development of treatment strategies that may improve quality of life for patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
The changing landscape of cancer in the USA — opportunities for advancing prevention and treatment

Causes of death among people living with metastatic cancer
Deceptive measures of progress in the NHS long-term plan for cancer: case-based vs. population-based measures
Dillekås, H., Rogers, M. S. & Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 8 , 5574–5576 (2019).
Article PubMed PubMed Central Google Scholar
Seyfried, T. N. & Huysentruyt, L. C. On the origin of cancer metastasis. Crit. Rev. Oncog. 18 , 43–73 (2013).
Schnurman, Z. et al. Causes of death in patients with brain metastases. Neurosurgery 93 , 986–993 (2023).
Article PubMed Google Scholar
Gallardo-Valverde, J. M. et al. Obstruction in patients with colorectal cancer increases morbidity and mortality in association with altered nutritional status. Nutr. Cancer 53 , 169–176 (2005).
Swanton, C. et al. Embracing cancer complexity: hallmarks of systemic disease. Cell 187 , 1589–1616 (2024).
Article CAS PubMed Google Scholar
Wheatley-Price, P., Blackhall, F. & Thatcher, N. The influence of sex in non-small cell lung cancer. Onkologie 32 , 547–548 (2009).
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38 , 576 (2020).
Neugut, A. I. et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J. Clin. Oncol. 24 , 2368–2375 (2006).
Sullivan, D. R. et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol. 5 , 1702–1709 (2019).
Sallnow, L. et al. Report of the Lancet Commission on the value of death: bringing death back into life. Lancet 399 , 837–884 (2022).
Abdel-Karim, I. A., Sammel, R. B. & Prange, M. A. Causes of death at autopsy in an inpatient hospice program. J. Palliat. Med. 10 , 894–898 (2007).
Pautex, S. et al. Anatomopathological causes of death in patients with advanced cancer: association with the use of anticoagulation and antibiotics at the end of life. J. Palliat. Med. 16 , 669–674 (2013).
Khorana, A. A., Francis, C. W., Culakova, E., Kuderer, N. M. & Lyman, G. H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 5 , 632–634 (2007).
Levi, M. & Scully, M. How I treat disseminated intravascular coagulation. Blood 131 , 845–854 (2018).
Cines, D. B., Liebman, H. & Stasi, R. Pathobiology of secondary immune thrombocytopenia. Semin. Hematol. 46 , S2 (2009).
Ghanavat, M. et al. Thrombocytopenia in solid tumors: prognostic significance. Oncol. Rev. 13 , 43–48 (2019).
Article CAS Google Scholar
Anker, M. S. et al. Advanced cancer is also a heart failure syndrome: a hypothesis. J. Cachexia Sarcopenia Muscle 12 , 533 (2021).
Asdahl, P. H. et al. Cardiovascular events in cancer patients with bone metastases — a Danish population-based cohort study of 23,113 patients. Cancer Med. 10 , 4885–4895 (2021).
Sinn, D. H. et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS ONE 10 , e0124434 (2015).
Zisman, A. et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J. Urol. 169 , 909–916 (2003).
Suárez, C. et al. Carotid blowout syndrome: modern trends in management. Cancer Manag. Res. 10 , 5617 (2018).
Lin, A. L. & Avila, E. K. Neurologic emergencies in the cancer patient: diagnosis and management. J. Intensive Care Med. 32 , 99 (2017).
Gamburg, E. S. et al. The prognostic significance of midline shift at presentation on survival in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 48 , 1359–1362 (2000).
Mokri, B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56 , 1746–1748 (2001).
Mastall, M. et al. Survival of brain tumour patients with epilepsy. Brain 144 , 3322–3327 (2021).
Steindl, A. et al. Neurological symptom burden impacts survival prognosis in patients with newly diagnosed non-small cell lung cancer brain metastases. Cancer 126 , 4341–4352 (2020).
Girard, N. et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am. J. Surg. Pathol. 33 , 1752–1764 (2009).
Lee, P. et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 69 , 328–333 (2007).
Kookoolis, A. S., Puchalski, J. T., Murphy, T. E., Araujo, K. L. & Pisani, M. A. Mortality of hospitalized patients with pleural effusions. J. Pulm. Respir. Med. 4 , 184 (2014).
PubMed PubMed Central Google Scholar
Cousins, S. E., Tempest, E. & Feuer, D. J. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst. Rev . https://doi.org/10.1002/14651858.CD002764 (2016).
Baker, M. L. et al. Mortality after acute kidney injury and acute interstitial nephritis in patients prescribed immune checkpoint inhibitor therapy. J. Immunother. Cancer 10 , e004421 (2022).
Bhave, P., Buckle, A., Sandhu, S. & Sood, S. Mortality due to immunotherapy related hepatitis. J. Hepatol. 69 , 976–978 (2018).
Lameire, N. H., Flombaum, C. D., Moreau, D. & Ronco, C. Acute renal failure in cancer patients. Ann. Med. 37 , 13–25 (2005).
Ries, F. & Klastersky, J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 8 , 368–379 (1986).
Wong, J. L. & Evans, S. E. Bacterial pneumonia in patients with cancer: novel risk factors and management. Clin. Chest Med. 38 , 263–277 (2017).
Lee, L. Y. W. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395 , 1919–1926 (2020).
Article CAS PubMed PubMed Central Google Scholar
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584 , 430–436 (2020). This study used a platform of 17.4 million pseudo-anonymized health-care records to determine risk factors for COVID-19.
Pelosof, L. C. & Gerber, D. E. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc. 85 , 838–854 (2010).
Donovan, P. J. et al. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J. Clin. Endocrinol. Metab. 100 , 2024–2029 (2015).
Burtis, W. J. et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N. Engl. J. Med. 322 , 1106–1112 (1990). First study to show that patients with cancer-associated hypercalcaemia had elevated concentrations of plasma parathyroid hormone-related protein .
Ellison, D. H. & Berl, T. The syndrome of inappropriate antidiuresis. N. Engl. J. Med. 356 , 2064–2072 (2007).
Okabayashi, T. et al. Diagnosis and management of insulinoma. World J. Gastroenterol. 19 , 829–837 (2013).
Giometto, B. et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch. Neurol. 67 , 330–335 (2010).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4 , 1721 (2018).
Feng, S. et al. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J. Thorac. Oncol. 12 , 1626–1635 (2017).
Coustal, C. et al. Prognosis of immune checkpoint inhibitors-induced myocarditis: a case series. J. Immunother. Cancer 11 , e004792 (2023).
Kuderer, N. M., Dale, D. C., Crawford, J., Cosler, L. E. & Lyman, G. H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106 , 2258–2266 (2006).
Agarwal, M. A. et al. Ventricular arrhythmia in cancer patients: mechanisms, treatment strategies and future avenues. Arrhythm. Electrophysiol. Rev . 12 , e16 (2023).
Zafar, A. et al. The incidence, risk factors, and outcomes with 5-fluorouracil-associated coronary vasospasm. JACC CardioOncol. 3 , 101–109 (2021).
Polk, A. et al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 6 , e012798 (2016).
Safdar, A., Bodey, G. & Armstrong, D. Infections in patients with cancer: overview. Princip. Pract. Cancer Infect. Dis. https://doi.org/10.1007/978-1-60761-644-3_1 (2011).
Foster, D. S., Jones, R. E., Ransom, R. C., Longaker, M. T. & Norton, J. A. The evolving relationship of wound healing and tumor stroma. JCI Insight 3 , e99911 (2018).
Park, S. J. & Bejar, R. Clonal hematopoiesis in cancer. Exp. Hematol. 83 , 105 (2020).
Liebman, H. A. Thrombocytopenia in cancer patients. Thromb. Res. https://doi.org/10.1016/S0049-3848(14)50011-4 (2014).
Chakraborty, R. et al. Characterisation and prognostic impact of immunoparesis in relapsed multiple myeloma. Br. J. Haematol. 189 , 1074–1082 (2020).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26 , 1125–1134 (2020).
Munn, D. H. & Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39 , 1–6 (2016).
Kochar, R. & Banerjee, S. Infections of the biliary tract. Gastrointest. Endosc. Clin. N. Am. 23 , 199–218 (2013).
Valvani, A., Martin, A., Devarajan, A. & Chandy, D. Postobstructive pneumonia in lung cancer. Ann. Transl. Med. 7 , 357–357 (2019).
Rolston, K. V. I. Infections in cancer patients with solid tumors: a review. Infect. Dis. Ther. 6 , 69–83 (2017).
Wu, X. et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLoS One 13 , e0205729 (2018).
The clinicopathological and prognostic role of thrombocytosis in patients with cancer: a meta-analysis. Oncol. Lett . 13 , 5002–5008 (2017).
Kasthuri, R. S., Taubman, M. B. & Mackman, N. Role of tissue factor in cancer. J. Clin. Oncol. 27 , 4834 (2009).
Wade, J. C. Viral infections in patients with hematological malignancies. Hematology 2006 , 368–374 (2006).
Article Google Scholar
Ersvaer, E., Liseth, K., Skavland, J., Gjertsen, B. T. & Bruserud, Ø. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 11 , 1–12 (2010).
Kuter, D. J. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica 107 , 1243 (2022).
Rodgers, G. M. et al. Cancer- and chemotherapy-induced anemia. J. Natl Compr. Canc. Netw. 10 , 628–653 (2012).
Nesher, L. & Rolston, K. V. I. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 42 , 5–13 (2014).
Blijlevens, N. M. A., Logan, R. M. & Netea, M. G. Mucositis: from febrile neutropenia to febrile mucositis. J. Antimicrob. Chemother. 63 , i36–i40 (2009).
Petrelli, F. et al. Association of steroid use with survival in solid tumours. Eur. J. Cancer 141 , 105–114 (2020).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52 , 1219–1226 (2020). This study identified the molecular characteristics of clonal haematopoiesis that increased risk of therapy-related myeloid neoplasms, with different characteristics associated with different treatment exposures.
Bhatia, R. et al. Do cancer and cancer treatments accelerate aging? Curr. Oncol. Rep. 24 , 1401 (2022).
Eisenstein, T. K. The role of opioid receptors in immune system function. Front. Immunol. 10 , 485158 (2019).
Böll, B. et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 100 , 239 (2021).
Ruiz-Giardin, J. M. et al. Blood stream infections associated with central and peripheral venous catheters. BMC Infect. Dis. 19 , 1–9 (2019).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124 , 188–195 (2014).
Brahmer, J. R. et al. Safety profile of pembrolizumab monotherapy based on an aggregate safety evaluation of 8937 patients. Eur. J. Cancer 199 , 113530 (2024). Analysis of the toxicity profile of anti-PD1 therapy in more than 8,000 patients.
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381 , 1535–1546 (2019).
Vozy, A. et al. Increased reporting of fatal hepatitis associated with immune checkpoint inhibitors. Eur. J. Cancer 123 , 112–115 (2019).
Palaskas, N., Lopez-Mattei, J., Durand, J. B., Iliescu, C. & Deswal, A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 9 , e013757 (2020).
Janssen, J. B. E. et al. Immune checkpoint inhibitor-related Guillain–Barré syndrome: a case series and review of the literature. J. Immunother. 44 , 276–282 (2021).
Camelliti, S. et al. Mechanisms of hyperprogressive disease after immune checkpoint inhibitor therapy: what we (don’t) know. J. Exp. Clin. Cancer Res. 39 , 236 (2020).
Kitamura, W. et al. Bone marrow microenvironment disruption and sustained inflammation with prolonged haematologic toxicity after CAR T-cell therapy. Br. J. Haematol. 202 , 294–307 (2023).
Seano, G. et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 3 , 230 (2019).
Madhusoodanan, S., Ting, M. B., Farah, T. & Ugur, U. Psychiatric aspects of brain tumors: a review. World J. Psychiatry 5 , 273 (2015).
Gerstenecker, A. et al. Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J. Neurooncol. 120 , 179 (2014).
Krishna, S. et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 617 , 599–607 (2023). This study demonstrated that high-grade gliomas remodel neural circuits in the human brain, which promotes tumour progression and impairs cognition.
Taylor, K. R. et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature 623 , 366–374 (2023). This study showed that brain-derived neurotrophic factor (BDNF)–tropomyosin-related kinase B (TRKB) signalling promotes malignant synaptic plasticity and augments tumour progression.
Hanahan, D. & Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 41 , 573–580 (2023).
Ahles, T. A. & Root, J. C. Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol . 14 , 425–451 (2018).
Allexandre, D. et al. EEG correlates of central origin of cancer-related fatigue. Neural Plast. 2020 , 8812984 (2020).
Büttner-Teleagă, A., Kim, Y. T., Osel, T. & Richter, K. Sleep disorders in cancer — a systematic review. Int. J. Environ. Res. Public Health 18 , 11696 (2021).
Walsh, D. & Nelson, K. A. Autonomic nervous system dysfunction in advanced cancer. Support. Care Cancer 10 , 523–528 (2002).
Ghandour, F. et al. Presenting psychiatric and neurological symptoms and signs of brain tumors before diagnosis: a systematic review. Brain Sci. 11 , 1–20 (2021).
Akechi, T. et al. Somatic symptoms for diagnosing major depression in cancer patients. Psychosomatics 44 , 244–248 (2003).
Nho, J. H., Kim, S. R. & Kwon, Y. S. Depression and appetite: predictors of malnutrition in gynecologic cancer. Support. Care Cancer 22 , 3081–3088 (2014).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12 , 939–944 (2006). This study linked chronic behavioural stress to higher levels of tissue catecholamines and tumour angiogenesis, resulting in greater tumor burden and invasion in ovarian cancer.
Chang, A. et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med . 15 , eadf1147 (2023).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341 , 1236361 (2013). This study showed that the formation of autonomic nerve fibres in the prostate gland regulates prostate cancer development and dissemination in mouse models.
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4 , 17105 (2018).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12 , 489–495 (2011). International consensus definitions of cancer cachexia.
Bossi, P., Delrio, P., Mascheroni, A. & Zanetti, M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients 13 , 1980 (2021).
Farkas, J. et al. Cachexia as a major public health problem: frequent, costly, and deadly. J. Cachexia Sarcopenia Muscle 4 , 173–178 (2013).
Dennison, E. M., Sayer, A. A. & Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 13 , 340–347 (2017).
Farasat, M. et al. Long-term cardiac arrhythmia and chronotropic evaluation in patients with severe anorexia nervosa (LACE-AN): a pilot study. J. Cardiovasc. Electrophysiol. 31 , 432–439 (2020).
Mehler, P. S., Anderson, K., Bauschka, M., Cost, J. & Farooq, A. Emergency room presentations of people with anorexia nervosa. J. Eat. Disord. 11 , 16 (2023).
Ferrer, M. et al. Cachexia: a systemic consequence of progressive, unresolved disease. Cell 186 , 1824–1845 (2023).
Bourke, C. D., Berkley, J. A. & Prendergast, A. J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 37 , 386–398 (2016).
Tisdale, M. J. Biology of cachexia. J. Natl Cancer Inst. 89 , 1763–1773 (1997).
Babic, A. et al. Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer. Nat. Commun. 14 , 4754 (2023).
Waning, D. L. et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21 , 1262 (2015). This study showed that bone metastases cause TGFβ to be released from the bone marrow, resulting in leakage of calcium from skeletal muscle cells contributing to muscle weakness.
Greco, S. H. et al. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS ONE 10 , e0132786 (2015).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13 , 1333–1340 (2007). This study showed that GDF15 was elevated in patients with cancer-associated weight loss and that this was a central regulator of appetite and therefore a potential therapeutic target.
Al-Sawaf, O. et al. Body composition and lung cancer-associated cachexia in TRACERx. Nat. Med. 29 , 846–858 (2023). This study showed an association among lower skeletal muscle area, subcutaneous adipose tissue and visceral adipose tissue and decreased survival in patients with non-small-cell lung cancer and these were associated with higher levels of circulating GDF15.
Ahmed, D. S., Isnard, S., Lin, J., Routy, B. & Routy, J. P. GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J. Cancer 12 , 1125–1132 (2021).
Rebbapragada, A., Benchabane, H., Wrana, J. L., Celeste, A. J. & Attisano, L. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 23 , 7230 (2003).
Queiroz, A. L. et al. Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat. Commun. 13 , 1–17 (2022).
Loumaye, A. et al. Role of activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 100 , 2030–2038 (2015).
Barton, B. E. & Murphy, T. F. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen. Cytokine 16 , 251–257 (2001).
Webster, J. M., Kempen, L. J. A. P., Hardy, R. S. & Langen, R. C. J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 11 , 597675 (2020).
Strassmann, G., Masui, Y., Chizzonite, R. & Fong, M. Mechanisms of experimental cancer cachexia local involvement of 11-1 in colon-26 tumor. J. Immunol. 150 , 2341–2345 (1993).
Stovroff, M. C., Fraker, D. L., Swedenborg, J. A. & Norton, J. A. Cachectin/tumor necrosis factor: a possible mediator of cancer anorexia in the rat. Cancer Res. 48 , 4567–4572 (1988).
CAS PubMed Google Scholar
Wyke, S. M. & Tisdale, M. J. NF-κB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin–proteasome system in skeletal muscle. Br. J. Cancer 92 , 711 (2005).
Cai, D. et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119 , 285–298 (2004). This study showed that activation of NF-κB, through muscle-specific transgenic expression of activated inhibitor of NF-κB kinase subunit β (IKKβ), causes profound muscle wasting in mice.
Patel, H. J. & Patel, B. M. TNF-α and cancer cachexia: molecular insights and clinical implications. Life Sci. 170 , 56–63 (2017).
Mergenthaler, P., Lindauer, U., Dienel, G. A. & Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36 , 587 (2013).
Sillos, E. M. et al. Lactic acidosis: a metabolic complication of hematologic malignancies case report and review of the literature. Cancer 92 , 2237–46 (2000).
Rampello, E., Fricia, T. & Malaguarnera, M. The management of tumor lysis syndrome. Nat. Clin. Pract. Oncol. 3 , 438–447 (2006).
Delano, M. J. & Moldawer, L. L. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 21 , 68–81 (2006).
Lombardi, A., Villa, S., Castelli, V., Bandera, A. & Gori, A. T-cell exhaustion in Mycobacterium tuberculosis and nontuberculous mycobacteria infection: pathophysiology and therapeutic perspectives. Microorganisms 9 , 2460 (2021).
Moldawer, L. L. & Sattler, F. R. Human immunodeficiency virus-associated wasting and mechanisms of cachexia associated with inflammation. Semin. Oncol. 25 , 73–81 (1998).
von Kobbe, C. Targeting senescent cells: approaches, opportunities, challenges. Aging 11 , 12844 (2019).
Shafqat, S., Chicas, E. A., Shafqat, A. & Hashmi, S. K. The Achilles’ heel of cancer survivors: fundamentals of accelerated cellular senescence. J. Clin. Invest. 132 , e158452 (2022).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22 , 340–355 (2022).
Terry, W., Olson, L. G., Ravenscroft, P., Wilss, L. & Boulton-Lewis, G. Hospice patients’ views on research in palliative care. Intern. Med. J. 36 , 406–413 (2006).
White, C. & Hardy, J. What do palliative care patients and their relatives think about research in palliative care? A systematic review. Support. Care Cancer 18 , 905–911 (2010).
Foster, B., Bagci, U., Mansoor, A., Xu, Z. & Mollura, D. J. A review on segmentation of positron emission tomography images. Comput. Biol. Med. 50 , 76–96 (2014).
Bera, K., Braman, N., Gupta, A., Velcheti, V. & Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19 , 132–146 (2022).
Kaczanowska, S. et al. Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell 42 , 35–51.e8 (2024).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152 , 244–248 (2016).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25 , 487–495 (2019).
Gyawali, B., Hey, S. P. & Kesselheim, A. S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. eClinicalMedicine 21 , 100332 (2020).
Hong, W. et al. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl Acad. Sci. USA 112 , E5351–E5360 (2015).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15 , 234–248 (2018).
Bowden, M. B. et al. Demographic and clinical factors associated with suicide in gastric cancer in the United States. J. Gastrointest. Oncol. 8 , 897–901 (2017).
Zaorsky, N. G. et al. Suicide among cancer patients. Nat. Commun. 10 , 1–7 (2019).
CAS Google Scholar
Hu, X. et al. Suicide risk among individuals diagnosed with cancer in the US, 2000 – 2016 . JAMA Netw. Open 6 , e2251863 (2023).
Google Scholar
Abdel-Rahman, O. Socioeconomic predictors of suicide risk among cancer patients in the United States: a population-based study. Cancer Epidemiol. 63 , 101601 (2019).
Pinquart, M. & Duberstein, P. R. Depression and cancer mortality: a meta-analysis. Psychol. Med. 40 , 1797–1810 (2010).
Fitzgerald, P. et al. The relationship between depression and physical symptom burden in advanced cancer. BMJ Support. Palliat. Care 5 , 381–388 (2015).
Chida, Y., Hamer, M., Wardle, J. & Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 5 , 466–475 (2008).
He, X. Y. et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 42 , 474–486.e12 (2024). This study found that chronic stress shifts the normal circadian rhythm of neutrophils resulting in increased neutrophil extracellular trap (NET) formation via glucocorticoid release, resulting in a metastasis-promoting microenvironment.
Fann, J. R., Ell, K. & Sharpe, M. Integrating psychosocial care into cancer services. J. Clin. Oncol. 30 , 1178–1186 (2012).
Jacobsen, P. B. & Wagner, L. I. A new quality standard: the integration of psychosocial care into routine cancer care. J. Clin. Oncol. 30 , 1154–1159 (2012).
Gorin, S. S. et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J. Clin. Oncol. 30 , 539–547 (2012).
Li, M. et al. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psychooncology 26 , 573–587 (2017).
Bova, G. S. et al. Optimal molecular profiling of tissue and tissue components: defining the best processing and microdissection methods for biomedical applications. Mol. Biotechnol. 29 , 119–152 (2005).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520 , 353–357 (2015). This study found that metastasis-to-metastasis spread was common in prostate cancer evolution and that lesions affecting tumour suppressor genes occurred as single events, whereas mutations in genes involved in androgen receptor signalling commonly involved multiple, convergent events in different metastases.
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173 , 581–594.e12 (2018). This study examined evolutionary trajectories of 100 renal cancers and found that metastasis competence was driven by chromosome complexity, not by driver mutation load, and that loss of 9p and 14q was a common driver.
Spain, L. et al. Late-stage metastatic melanoma emerges through a diversity of evolutionary pathways. Cancer Discov. 13 , 1364–1385 (2023). This study examined evolutionary trajectories of melanoma metastasis and observed frequent whole-genome doubling and widespread loss of heterozygosity, often involving antigen-presentation machinery.
Download references
Acknowledgements
A.B. is funded by National Institutes of Health/National Cancer Institute P30 CA008748 and R01-CA245499. K.B. is employed by the UK National Health Service. T.R.C. acknowledges funding support from the National Health and Medical Research Council (NHMRC) Ideas (2000937), Project (1129766, 1140125), Development (2013881) and Fellowship (1158590) schemes, a Cancer Institute NSW Career Development Fellowship (CDF171105), Cancer Council NSW project support (RG19-09, RG23-11) and Susan G. Komen for the Cure (CCR17483294). T.G. is funded by the Cancer Prevention and Research Institute of Texas Grant 00011633. M.J.-H. has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETs and NIHR. T.J. acknowledges funding from Cancer Grand Challenges (NIH: 1OT2CA278690-01; CRUK: CGCATF-2021/100019), the Mark Foundation for Cancer Research (20-028-EDV), the Osprey Foundation, Fortune Footwear, Cold Spring Harbour Laboratory (CSHL) and developmental funds from CSHL Cancer Center Support Grant 5P30CA045508. R.K. is funded by the Intramural Research Program, the National Cancer Institute, NIH Clinical Center and the National Institutes of Health (NIH NCI ZIABC011332-06 and NIH NCI ZIABC011334-10). R.L. is supported by a Wellcome Early Career Investigator Award (225724/Z/22/Z). E.S. is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (CC2040), the UK Medical Research Council (CC2040) and the Wellcome Trust (CC2040) and the European Research Council (ERC Advanced Grant CAN_ORGANISE, Grant agreement number 101019366). E.S. reports personal grants from Mark Foundation and the European Research Council. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute that receives its core funding from Cancer Research UK (CC2041), the UK Medical Research Council (CC2041) and the Wellcome Trust (CC2041) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (ERC Advanced Grant PROTEUS Grant agreement number 835297). M.G.V.H. reports support from the Lustgarten Foundation, the MIT Center for Precision Cancer Medicine, the Ludwig Center at MIT and NIH grants R35 CA242379 and P30 CA1405141.
Author information
These authors contributed equally: Adrienne Boire, Katy Burke, Thomas R. Cox, Theresa Guise, Mariam Jamal-Hanjani, Tobias Janowitz, Rosandra Kaplan, Rebecca Lee, Charles Swanton, Matthew G. Vander Heiden, Erik Sahai.
Authors and Affiliations
Memorial Sloan Kettering Cancer Center, New York, NY, USA
Adrienne Boire
University College London Hospitals NHS Foundation Trust and Central and North West London NHS Foundation Trust Palliative Care Team, London, UK
Cancer Ecosystems Program, The Garvan Institute of Medical Research and The Kinghorn Cancer Centre, Darlinghurst, New South Wales, Australia
Thomas R. Cox
School of Clinical Medicine, St Vincent’s Healthcare Clinical Campus, UNSW Medicine and Health, UNSW Sydney, Sydney, New South Wales, Australia
Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Theresa Guise
Cancer Metastasis Laboratory, University College London Cancer Institute, London, UK
Mariam Jamal-Hanjani
Department of Oncology, University College London Hospitals, London, UK
Mariam Jamal-Hanjani & Charles Swanton
Cancer Research UK Lung Centre of Excellence, University College London Cancer Institute, London, UK
Cold Spring Harbour Laboratory, Cold Spring Harbour, New York, NY, USA
Tobias Janowitz
Northwell Health Cancer Institute, New York, NY, USA
Paediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Rosandra Kaplan
Tumour Cell Biology Laboratory, The Francis Crick Institute, London, UK
Rebecca Lee & Erik Sahai
Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK
Rebecca Lee
Cancer Evolution and Genome Instability Laboratory, The Francis Crick Institute, London, UK
Charles Swanton
Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA, USA
Matthew G. Vander Heiden
Dana-Farber Cancer Institute, Boston, MA, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors researched data for the article. A.B., K.B., T.R.C., T.G., T.J., C.S., M.G.V.H, R.K., M.J.-H. and E.S. contributed substantially to discussion of the content. T.C., R.L. and E.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Correspondence to Thomas R. Cox or Erik Sahai .
Ethics declarations
Competing interests.
A.B. is an inventor on pending patents 63/449,817, 63/052,139 as well as awarded patents 11,305,014 and 10,413,522; all issued to the Sloan Kettering Institute. She has received personal fees from Apelis Pharmaceuticals and serves as an unpaid member of the Evren Technologies SAB. K.B., T.R.C., T.G., T.J. and R.K. declare no competing interests. M.J.-H. reports support from Achilles Therapeutics Scientific Advisory Board and Steering Committee, Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster and Bristol Myers Squibb outside the submitted work. R.L. reports personal fees from Pierre Fabre and has research funding from BMS, Astra Zeneca and Pierre Fabre outside the submitted work. E.S. reports grants from Novartis, Merck Sharp Dohme, AstraZeneca and personal fees from Phenomic outside the submitted work. C.S. reports grants and personal fees from Bristol Myers Squibb, AstraZeneca, Boehringer-Ingelheim, Roche-Ventana, personal fees from Pfizer, grants from Ono Pharmaceutical, Personalis, grants, personal fees and other support from GRAIL, other support from AstraZeneca and GRAIL, personal fees and other support from Achilles Therapeutics, Bicycle Therapeutics, personal fees from Genentech, Medixci, China Innovation Centre of Roche (CiCoR) formerly Roche Innovation Centre, Metabomed, Relay Therapeutics, Saga Diagnostics, Sarah Canon Research Institute, Amgen, GlaxoSmithKline, Illumina, MSD, Novartis, other support from Apogen Biotechnologies and Epic Bioscience outside the submitted work; in addition, C.S. has a patent for PCT/US2017/028013 licensed to Natera Inc., UCL Business, a patent for PCT/EP2016/059401 licensed to Cancer Research Technology, a patent for PCT/EP2016/071471 issued to Cancer Research Technology, a patent for PCT/GB2018/051912 pending, a patent for PCT/GB2018/052004 issued to Francis Crick Institute, University College London, Cancer Research Technology Ltd, a patent for PCT/GB2020/050221 issued to Francis Crick Institute, University College London, a patent for PCT/EP2022/077987 pending to Cancer Research Technology, a patent for PCT/GB2017/053289 licensed, a patent for PCT/EP2022/077987 pending to Francis Crick Institute, a patent for PCT/EP2023/059039 pending to Francis Crick Institute and a patent for PCT/GB2018/051892 pending to Francis Crick Institute. C.S. is Co-chief Investigator of the NHS Galleri trial funded by GRAIL. He is Chief Investigator for the AstraZeneca MeRmaiD I and II clinical trials and Chair of the Steering Committee. C.S. is cofounder of Achilles Therapeutics and holds stock options. M.G.V.H. is a scientific adviser for Agios Pharmaceuticals, iTeos Therapeutics, Sage Therapeutics, Faeth Therapeutics, Droia Ventures and Auron Therapeutics on topics unrelated to the presented work.
Peer review
Peer review information.
Nature Reviews Cancer thanks Vickie Baracos, Clare M. Isacke, who co-reviewed with Amanda Fitzpatrick and Erica Sloan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An autoimmune encephalitis characterized by complex neuropsychiatric features and the presence of immunoglobulin G (IgG) antibodies against the NR1 subunit of the NMDA receptors in the central nervous system.
Partial collapse or incomplete inflation of the lung.
Pressure-induced movement of brain tissue.
An ageing-associated process in which haematopoiesis becomes dominated by one or a small number of genetically distinct stem or progenitor cells. Clonal haematopoiesis is linked to an increased risk of haematological malignancies.
Inability of the heart to pump blood properly.
Constriction of the arteries that supply blood to the heart.
(CRH). One of the major factors that drives the response of the body to stress.
(DIC). A rare but serious condition in which abnormal blood clotting occurs throughout the blood vessels of the body.
Inflammation of the brain.
An abnormal connection that forms between two body parts, such as an organ or blood vessel and another often unrelated structure in close proximity.
A rare disorder in which the immune system of a body attacks the nerves, which can lead to paralysis.
The stopping of flow of blood, typically associated with the bodies response to prevent and stop bleeding.
A build-up of fluid within the cavities of the brain.
Elevated calcium levels in the blood, often caused by overactive parathyroid glands. Hypercalcaemia is linked to kidney stones, weakened bones, altered digestion and potentially altered cardiac and brain function.
(HPD). Rapid tumour progression sometimes observed during immune checkpoint inhibitor treatment.
The condition that occurs when the level of sodium in the blood is low.
Harm, which is often unavoidable, caused by cancer treatments.
The marked suppression of polyclonal immunoglobulins in the body.
(LEMS). A neuromuscular junction disorder affecting communication between nerves and muscles, which manifests as a result of a paraneoplastic syndrome or a primary autoimmune disorder. Many cases are associated with small-cell lung cancer.
When cancer cells spread to the tissue layers covering the brain and spinal cord (the leptomeninges).
Also known as pulmonary oedema is a condition caused by excess fluid in the lungs. This fluid collects in the alveoli compromising function and making it difficult to breathe.
The observation of displacement of brain tissue across the centre line of the brain, suggestive of uneven intracranial pressure.
Decreased blood flow to the myocardium, commonly called a heart attack.
Inflammation specifically of the middle layer of the heart wall.
A group of rare disorders that occur when the immune system reacts to changes in the body triggered by the presence of a neoplasm.
A dense network of nerves that transmit information from the brain (efferent neurons) to the periphery and conversely transmit information from the periphery to the brain (afferent neurons). A component of the peripheral nervous system is the autonomic nervous system.
A build-up of fluid between the tissues that line the lungs and the chest wall.
A condition characterized by loss of skeletal muscle mass and function.
The lodging of a circulating blood clot within a vessel leading to obstruction. Thromboembolisms may occur in veins (venous thromboembolism) and arteries (arterial thromboembolism).
A key component of the pathway regulating blood clotting, specifically the receptor and cofactor for factor VII/VIIa.
A syndrome occurs when tumour cells release their contents into the bloodstream, either spontaneously or more typically, in response to therapeutic intervention.
Devices worn on the body, typically in the form of accessories or clothing, that incorporate advanced electronics and technology to monitor, track or enhance various aspects of human life. Examples include smartwatches and fitness trackers.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Boire, A., Burke, K., Cox, T.R. et al. Why do patients with cancer die?. Nat Rev Cancer (2024). https://doi.org/10.1038/s41568-024-00708-4
Download citation
Accepted : 15 May 2024
Published : 19 June 2024
DOI : https://doi.org/10.1038/s41568-024-00708-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The road less travelled.
Nature Reviews Cancer (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Open access
- Published: 21 June 2024
End-of-life care needs in cancer patients: a qualitative study of patient and family experiences
- Mario López-Salas 1 ,
- Antonio Yanes-Roldán 1 ,
- Ana Fernández 1 ,
- Ainhoa Marín 1 ,
- Ana I. Martínez 1 ,
- Ana Monroy 1 ,
- José M. Navarro 1 ,
- Marta Pino 1 ,
- Raquel Gómez 1 ,
- Saray Rodríguez 1 ,
- Sergio Garrido 1 ,
- Sonia Cousillas 1 ,
- Tatiana Navas 1 ,
- Víctor Lapeña 1 &
- Belén Fernández 1
BMC Palliative Care volume 23 , Article number: 157 ( 2024 ) Cite this article
191 Accesses
Metrics details
Cancer is a disease that transcends what is purely medical, profoundly affecting the day-to-day life of both patients and family members. Previous research has shown that the consequences of cancer are greatly aggravated in patients at the end of life, at a time when they must also grapple with numerous unmet needs. The main objective of this study was to obtain more in-depth insight into these needs, primarily in patients with end-stage cancer nearing death.
Semi-structured interviews were conducted in Spain with cancer patients at the end of life ( n = 3) and their family members ( n = 12). The findings from the interviews were analyzed using qualitative thematic analysis and a grounded theory approach.
Four major themes emerged from the interviews that explored the needs and concerns of patients with cancer at the end of life: (1) physical well-being (2) emotional well-being (3) social well-being and (4), needs relating to information and autonomous decision-making. The interviews also shed light on the specific needs of family members during this period, namely the difficulties of managing increased caregiver burden and maintaining a healthy work-life balance.
Conclusions
A lack of support, information and transparency during a period of immense vulnerability makes the end-of-life experience even more difficult for patients with cancer. Our findings highlight the importance of developing a more in-depth understanding of the needs of this population, so that informed efforts can be made to improve palliative healthcare and implement more comprehensive care and support at the end of life.
Peer Review reports
The concept of end of life reflects both the irreversible progression of a life-limiting disease, and a life expectancy of six months or less [ 1 ]. Cancer patients in the end-stage of the disease, facing end-of-life issues, undergo significant physical, psychological and social alterations, as do family members, whose lives and environment are drastically changed during this period. It is therefore of vital importance to improve the quality of life of all of those affected by this diagnosis. During this stage, numerous needs emerge regarding the care and well-being of the cancer patient and their caregivers which are not always acknowledged or identified. Recognition and awareness of these needs can ensure early access and comprehensive treatment for cancer patients with palliative care needs, while also meeting the needs of their caregivers [ 2 , 3 ].
In regard to patients, the literature provides some insight into the needs that emerge at this stage of the disease, yet there are still many areas that require further investigation. In a review by Wang et al. [ 4 ], which analyzed patients’ unmet needs in 38 studies, the authors identified seven major categories of needs. These included physical needs and symptom control, the need to maintain functionality and day-to-day activities, the need for information, psychological needs, social and financial needs, and spiritual needs. The relationship between various sociodemographic variables and patients’ unmet needs has also been studied. Several studies found that female patients had a higher frequency of unmet physical and psychological needs than male patients [ 5 , 6 ]. Similarly, patients with a high education level reported more unmet needs in physical domains, functionality, day-to-day activities [ 7 ] and information [ 8 ]. In addition, social needs appeared to a lesser extent in high-income patients [ 9 ]. Severe symptoms of emotional stress, the presence of anxiety and/or depression, a greater lack of problem-focused coping, and poorer quality of life of caregivers were identified as negative predictors of patient satisfaction [ 10 ]. Finally, when regarding primary caregivers, patients reported more unmet needs when their caregivers were male, young, or showed high levels of emotional distress [ 6 ].
For informal (or family) caregivers, the supportive care process is physically and psychologically challenging, especially when caring for patients with advanced cancer at the end of life [ 11 ]. However, assessing the unmet needs of caregivers remains an uncommon practice. Many family members, including those who do not view care giving as a burden, suffer from a wide range of problems, including sleep alterations, anxiety, depression, difficulty balancing caregiving and daily tasks, and financial burdens [ 12 ]. Wang and colleagues [ 4 ] found that caregivers had unmet needs regarding information about the disease and its treatment [ 11 , 13 , 14 , 15 , 16 ], as well as psychological [ 17 , 18 ], economic [ 19 ] and spiritual needs [ 20 ]. Researchers also reported that younger caregivers had a higher number of unmet needs than older caregivers regarding care, information and economic needs [ 13 , 14 ]. Caregivers with physical problems experienced a greater number of unmet needs. Similarly, family caregivers reported more needs when patients suffered from anxiety, depression, or poor physical performance [ 14 ]. Family members also experienced a need for clear and reliable information that would help them prepare for their loved one’s death and the grieving process [ 21 , 22 ].
There is a genuine necessity for studies that integrate patient and caregiver needs, in part to illustrate and conceptualize a unique family unit, given that the different elements that comprise this unit interact and impact on one another. The literature clearly shows that the unmet needs of patients can increase the physical and emotional burden of the caregiver [ 23 ]. In turn, caregiver issues are closely related to patient well-being [ 24 ]. The unresolved issues or unmet needs of caregivers will not only diminish their own quality of life, but also negatively impact on patients’ health outcomes [ 25 ].
Finally, it should be noted that few studies focus on the changing needs of patients and family members at the end of life, or of bereaved caregivers coping with grief. Our research aims to help fill this gap in the literature, using qualitative methodology to identify the unmet needs and potential barriers [ 3 , 26 , 27 , 28 ] encountered by these patients and their families, and thus provide greater insight into what the end of life is, as opposed to what it could and should be, for all those involved.
An exploratory descriptive qualitative study was carried out using semi-structured interviews with patients with end-stage cancer at the end of life and their family members. Fieldwork was conducted in May and June 2023. The Charmaz grounded theory approach to thematic analysis was implemented [ 29 , 30 ]. Categories of analysis have been generated inductively and deductively, following a grounded theory approach, and then grouped into themes. This methodology allows researchers to delve into areas of knowledge and reality from a novel perspective, making it possible to explore common perceptions and experiences as part of the subjective social structure of the participants. The study was designed and developed following the consolidated criteria for reporting qualitative research (COREQ) [ 31 ].
Participants
In this study, purposive sampling was used to identify participants, and obtain the maximum variation of the designed sample. This type of sampling is a strategy used to gather participants in a given context with expertise in the study phenomenon. Participants were recruited by health and social professionals through an association of cancer patients. The health professionals in direct contact with the participants informed them about the study and its objectives. Subsequently, and after signing the written informed consent sheet, the research team contacted the participants directly via e-mail or telephone call to confirm the interview. From an initial contact with 35 participants, the research team finally conducted 11 interviews with 15 participants. The variables chosen for the participants were: phase of the disease/after death (advanced, end of life, recent bereavement, bereavement after 6 + months), place of residence (rural, urban), age (< 65 or > 65) and gender. The sample also had ample experience with palliative care services, both at home and in hospital outpatient visits.
The final sample included 15 participants: 3 patients with end-stage cancer diagnosis and 12 caregivers who cared for terminally ill family members and experienced bereavement. A total of 10 semi-structured in-depth interviews were conducted, two of them in pairs (patient and relative), and another in a group composed of three caregivers. By including relatives of different ages, marital statuses and roles within the family, different narratives can be explored through the diverse perspectives, conflicts and connections collected.
Initially, 16 participants were proposed for the sample. However, theoretical saturation was reached at 15 participants, referring to the point in the research where new interviews added nothing novel or relevant to the study objectives [ 32 ], resulting in the final inclusion of 15 subjects. Table 1 shows the sociodemographic characteristics of these participants.
Data collection
All interviews were conducted online through a videoconferencing platform, except for two that were conducted by telephone at the request of the interviewees. They were conducted by two professionals with experience in qualitative research on healthcare and palliative care who had no previous contact with the participants in order to avoid bias in data collection. The interviews were based on a broad directive script, designed by a team of oncology psychologists and social workers with extensive experience in palliative care. This script was adapted to the context and profile of each person that was interviewed. Family caregivers were asked about the needs of the patients and as well as their own needs. The main topics discussed were: (1) Perceptions about end-of-life and palliative care; (2) Difficulties in the end-of-life process and the needs detected, both met and unmet; (3) The demands and proposals at professional and institutional level for the resolution of these needs (see Supplementary Materials). Interviews lasted an average of 45 min.
The interviews were audio recorded with the consent of the participants, and later verbatim transcribed and anonymized in their entirety. Written informed consent sheet was provided to participants by the professionals who made the recruitment. In addition, the researcher who conducted the interviews asked for oral consent to the participants right before the interviews. They were also provided the opportunity to ask questions and dispel doubts before starting the interview process. No payment was offered in exchange for participation in the study. Personal data and digital rights of participants were protected in accordance with the LOPDGDD 03/2018 [ 33 ].
Data analysis
To get an overview of the information before starting with the initial coding, 5 researchers of the team read the entire transcriptions repeatedly. The transcripts were then segmented into text units relevant to the analysis, which were subsequently read by all researchers. Finally, segments from the interviews were coded based on degree of relevance. A code tree was produced, combining closed categories previously discussed by the research group with categories that emerged inductively from the collected data. This tree was tested in the coding of certain interviews and adapted throughout the field research process to improve its scope and effectiveness. Data collection and analysis occurred concurrently. The information from the codes generated was compared with each other in order to create new categories. The codes were analyzed using both an inductive and a deductive approach that identifies similar, interrelated patterns and allows the theoretic categories described in the study to emerge from the raw data itself. Analytical rigor was assured through a rigorous process of peer review, since other researchers from the team were invited to review the initial analysis, in order to confirm the consistency of the findings and to identify and diminish potential biases. All the researchers agreed with the results of the coding process and the generation of the main categories. Data analysis was performed using ATLAS.ti version 9.
The results were divided into two main sections: the needs of people diagnosed with cancer at the end of life and the needs of family caregivers during this final phase of care.
End-of-life needs for cancer patients
The main results are shown in Table 2 . The needs of these patients are modified in accordance with disease progression and proximity to death.
Physical well-being
One of the main themes that emerged from participant responses was the need to maintain a sense of physical well-being. This set of needs is aimed at improving their quality of life by monitoring the symptoms of both the disease and its treatment. The main codes indicated by participants were the need to control pain and the adverse effects of medication, to find cures and clinical procedures to ensure quality of life, and to organize the administration of medication.
“That I, her daughter, have to act as a nurse, as I watch my mother dying and being told on the phone: ‘inject this, inject that’.” P12 .
The other codes regarding physical well-being were functional problems related to care. These needs include optimal rest and sleep, adapting diet and eating habits to allay weight gain or lack of appetite, and maintaining personal hygiene and physical activity.
“Well, you have to be aware of the circumstances. In terms of strength, it depends on the day…I’ve lost a lot of weight and I don’t sleep well, but I used to sleep very well. And this, of course, limits you.” P1 .
“I have my doubts about what the best diet is. The oncologist doesn’t know. I found a nutritionist, and it’s a little clearer now, but I think I lack information. Nothing is said about the diet…” P1 .
Emotional well-being
Another theme that concerned participants was their emotional well-being. Participants put a high priority on addressing emotional needs related to illness, dependency and death, as well as having access to assistance in effectively expressing and communicating these needs.
“Nobody talks about the psychological burden that this disease can place on the patient. How your life changes, all that it entails. The pain, going to the hospital continuously, and not knowing what will happen in the near future. More help is needed so we can understand how to relate to the disease rather than confront it.” P1 .
Participants also reported the need for the care and protection of their of identity, of their roles and purpose in life, as well as their self-image and self-esteem, all of which are frequent concerns of people facing the end of life.
“So it was very difficult for him … to be aware, and lose those roles and lose the role of doctor, lose the role of father, lose the role of person… in other words, to be sick person.” P6 .
Other relevant codes included the need to maintain affective relationships by spending quality time with your loved ones, and getting support to carry out fulfilling activities, hobbies and everyday pleasures.
“I brought her home, with her granddaughter, who she was mad about, and my kitten. My mother has always loved my cat. When I brought her home on the first day in the wheelchair, the cat climbed onto her lap and gave her a kiss. And now, on her deathbed—the last days of her life—my cat will not leave her side.” P12 .
Social well-being: adapted living space and mobility and economic resources
Most of the participants reported that a well-adapted living space was essential for providing comfort and quality of life at the end of life. This corresponds to the need to adjust the living environment to the patient’s physical circumstances during the end-stage of the disease.
Participants prioritized the following codes: the need for material resources that could improve the patient’s quality of life (medical equipment and supplies, hygiene products, etc.), domestic mobility (wheelchairs, stair lifts, etc.), and comfortable living spaces adapted to the patient’s physical needs.
“Now we must hustle and bustle with a wheelchair, because I cannot walk. We requested a wheelchair and the doctor prescribed it for me, an electric wheelchair. They prescribed it for me, they accepted it and today a letter has come, saying it has been denied.” P2 .
“I have a bathtub and I couldn’t afford it… Also, I pay rent, so I couldn’t replace the bathtub with a shower. I had no time, no disposition, no money. I ultimately had to buy an orthopedic chair so I could shower her in the bathtub” P12 .
Another important aspect of social well-being highlighted by the participants was the need to have access to professional resources that cover daily tasks, mainly through the hiring of professional caregivers.
“And the aid they gave was quite small, that’s also something that could, that should be considered, right? Above all, people who need care, a lot of care. […] In the end, it’s true that you need 24 hours, 7 days a week.” Family Group, P14 .
Information and autonomy in decision-making
Needs related to informed decision-making are associated with a willingness to ensure patient inclusion and autonomy in decisions that are made throughout the end-of-life period. The interviewees emphasized that they often felt as if healthcare professionals were not honest and forthright when sharing information with them. Yet they need this information to cope with doubts and make informed decisions, information on the prognosis of their disease and therapeutic options, legal and bureaucratic procedures, psychological and social resources, and shared experiences of other patients. In addition, they expressed the need for the patient’s will and preferences to prevail.
“Learn more about the process. Like: “The degeneration will progress more and more.” Yes, but how will this degeneration take place? What exactly should I expect? I mean, I know they can’t give me a timeline, I understand that. Learn how the disease really works. You search the Internet, and then have to hope that your search is right.” P9 .
End-of-life needs for family caregivers
The daily tasks of a family caregiver together with the challenges that arise during end-of-life care for a cancer patient can overwhelm the caregiver. In most cases, caregiver overload causes severe stress and physical, emotional and mental exhaustion. Family caregivers must learn to balance caring for their loved ones with caring for themselves. This includes organizing work, home, and leisure activities, improving time management, and building a strong internal support system. Family member interviews also revealed a need for greater awareness of their own specific needs, which are often overlooked. These main results are shown in Table 3 .
Personal health and well-being
Family caregivers reported that they often overlooked their health, neglecting their nutrition and adopting inadequate sleep patterns. Those suffering from an illness also noted greater neglect of their own medical needs and treatment.
“From the time I woke up at 7:00 or 8:00, I didn’t stop, until I went to bed at 12:00. I’ve already lost a lot of weight from the stress. I’ve always said that I have to take care of myself, that I can’t be foolish and neglect myself, because if I fall, we all fall and I have 3 people under my care.” P12 .
Caregivers in the study also highlighted the need for assistance in minimizing uncertainty and emotional distress. They reported feeling overwhelmed with their caregiving responsibilities, which brought on more pressure and feelings of guilt.
Other emotional needs included managing their fear of losing a loved one and coping with grief and life changes after the death of the patient.
“I had prepared a letter for my father, because I hadn’t been capable of saying goodbye yet.” P6 .
Caregiver overload and work-life balance
The main difficulty that family caregivers faced was achieving a healthy balance between work and personal life. The combination of working and family responsibilities (of both the patient and other family members) can virtually do away with a caregiver’s rest and free time.
“My father was on medical leave because of anxiety, until my mother died and then he went back to work. Until it happens to you, you don’t realize how much a person needs to stop working, and that it’s not a whim. In other words, you either stop working or caregiving.” P10 .
The participants emphasized the need for caregivers, in this case, mainly family members. Great importance was given to the possibility of having a formal and informal support network, especially at an emotional level and also to collaborate with daily caregiving tasks.
“ The end was appalling, especially because of the work-life balance. It was very burdensome to have the illness at home. I managed to do it because my father was there, otherwise I wouldn’t have been able to go out […] because I couldn’t disconnect at home .” P10 .
The objective of this study was to explore the needs of people with cancer at the end of life and the needs of their family caregivers. Its novelty lies in its ability to provide relevant information on the unmet needs of these individuals in a qualitative manner during the end-stage of the illness.
There is limited research on this period given the complexities of reaching out to the cancer population and their loved ones at such a difficult time in their lives. Furthermore, there is the complex task of incorporating the experiences of both patients and family members into a single study and viewing them as a unit that interconnects with the social, economic and institutional reality in which they live.
Based on the needs expressed by the participants, our findings suggest that numerous unmet needs may exist among cancer patients and informal caregivers during the end-of-life period. The complexity and magnitude of the challenges they face during this phase of cancer contribute to a significant array of needs. Although there is diversity in the identified needs among patients and family caregivers, there are also a remarkable number of these needs overall. These findings are consistent with results from similar studies conducted on cancer patients in other countries during earlier phases of the end of life.
In terms of the physical well-being of the cancer patient, our findings support other studies in which the need for symptom control, mainly pain and fatigue [ 5 , 13 , 34 , 35 , 36 , 37 ], is considered a priority. Another primary concern is the need for rest and adequate sleep. It should be noted that while caregivers usually attend to the needs of the patient regarding nutrition and hygiene, most of them express a sense of over-delegation when it came to these tasks, as well as a lack of preparation for carrying them out.
The need for emotional well-being is of particular concern to the interviewees, constituting one of the primary sources of vulnerability at the end of life. This phase of the disease is characterized by fear and uncertainty and the need to come to terms with impending death. Priority is given to managing emotions related to illness, dependency, dying and death. Patients also stress the importance of spiritual needs during this period, as they face end-of-life issues and reflect on the purpose and meaning in their lives. Preserving one’s identity, as well as one’s self-image and self-esteem also contributes to emotional well-being for people nearing the end of life. These findings support previous research where patients expressed a fundamental need for managing psychological distress [ 6 , 7 ]. A systematic assessment of the emotional needs of these patients would help identify appropriate coping strategies to reduce emotional distress and lessen its burden on caregivers.
Participants in our study, as in other research, emphasize the unmet need for information that would help them make decisions and express their preferences for end-of-life care [ 7 , 8 , 13 ], thereby ensuring control over their care while easing the burden of decision-making for their caregivers. These findings highlight the need to improve communication between patients and their healthcare providers. By learning and responding to what their patients want to know, these professionals can improve the patient’s understanding of their circumstances and the options available to them at this critical period in their lives.
Regarding social needs, our data show that a well-adapted living space that provides comfort and mobility is vital for dignity at the end of life, as is being able to choose to stay at home rather than move to a hospital. Participants indicated that the main barriers to satisfying these needs were a lack of economic resources to refurbish their home or obtain technical and/or orthotic and prosthetic devices (wheelchair, adjustable beds, etc.) to improve their quality of life. It is necessary to provide assistance and information on adapting the home and devices that facilitate the patient’s mobility. Similarly, more information should be made available regarding economic aid or funds that may be requested to offset these costs and the loss of income resulting from the illness.
Finally, findings on the needs of family caregivers show the challenges that arise during end-of-life care for a patient with cancer cause an overload, which in most cases leads to severe stress and physical and psychological exhaustion. Family caregivers need to learn how to improve their work-life balance. This includes organizing work, home, and leisure activities, improving time management, and building a strong internal support network.
This study contributes to the relatively small number of publications on patients with cancer and their caregivers at the end of life. In addition, this is the first study carried out in Spain that explores the needs of cancer patients in palliative care and their families based on their own personal experiences. The primary strength of our research lies in the inclusion of end-of-life patients nearing death who had diverse experience with the services provided during end-of-life care. Our main findings also corroborate previous research, while providing a more in-depth understanding of these patients’ needs and their connection to the needs of family caregivers. However, while it was possible to saturate the resulting codes theoretically and empirically, our limited sample size prevented the findings from being generalizing. Nevertheless, these results support the growing call for a more in-depth understanding of the needs of this vulnerable population. Only with this first-hand knowledge any real and effective change can be made to improve palliative healthcare and implement more comprehensive care and support at the end of life.
Data availability
The dataset generated and analyzed during the current study is not publicly available because individual privacy could be compromised but is available from the corresponding author on reasonable request.
Hui D, Nooruddin Z, Didwaniya N, Dev R, De La Cruz M, Kim SH, et al. Concepts and definitions for actively dying. End Life Terminally Ill Terminal Care Transition Care: Syst Rev J Pain Symptom Manage. 2014;47(1):77–89.
Google Scholar
Identification of patients with. potential palliative care needs: A systematic review of screening tools in primary care - Yousuf ElMokhallalati, Stephen H Bradley, Emma Chapman, Lucy Ziegler, Fliss EM Murtagh, Miriam J Johnson, Michael I Bennett, 2020. [cited 2024 Jan 5]. https://journals.sagepub.com/doi/full/ https://doi.org/10.1177/0269216320929552 .
Lei L, Lu Y, Gan Q, Hu Z, Luo Y. Awareness and perceptions of Palliative Care among the Elderly: a qualitative study. J Palliat Care. 2022;37(2):204–12.
Article PubMed Google Scholar
Wang T, Molassiotis A, Chung BPM, Tan JY. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018;17(1):96.
Article PubMed PubMed Central Google Scholar
Hasegawa T, Goto N, Matsumoto N, Sasaki Y, Ishiguro T, Kuzuya N, et al. Prevalence of unmet needs and correlated factors in advanced-stage cancer patients receiving rehabilitation. Support Care Cancer. 2016;24(11):4761–7.
Morasso G, Capelli M, Viterbori P, Di Leo S, Alberisio A, Costantini M, et al. Psychological and Symptom Distress in Terminal Cancer patients with Met and Unmet needs. J Pain Symptom Manage. 1999;17(6):402–9.
Article CAS PubMed Google Scholar
Liao YC, Liao WY, Shun SC, Yu CJ, Yang PC, Lai YH. Symptoms, psychological distress, and supportive care needs in lung cancer patients. Support Care Cancer off J Multinatl Assoc Support Care Cancer. 2011;19(11):1743–51.
Voogt E, van Leeuwen AF, Visser AP, van der Heide A, van der Maas PJ. Information needs of patients with incurable cancer. Support Care Cancer off J Multinatl Assoc Support Care Cancer. 2005;13(11):943–8.
Houts PS, Yasko JM, Harvey HA, Kahn SB, Hartz AJ, Hermann JF, et al. Unmet needs of persons with cancer in Pennsylvania during the period of terminal care. Cancer. 1988;62(3):627–34.
Wang T, Molassiotis A, Tan JY, Chung BPM, Huang HQ. Prevalence and correlates of unmet palliative care needs in dyads of Chinese patients with advanced cancer and their informal caregivers: a cross-sectional survey. Support Care Cancer. 2021;29(3):1683–98.
Cui J, Song LJ, Zhou LJ, Meng H, Zhao JJ. Needs of family caregivers of advanced cancer patients: a survey in Shanghai of China: needs of family caregivers of advanced cancer patients. Eur J Cancer Care (Engl). 2014;23(4):562–9.
Lambert SD, Harrison JD, Smith E, Bonevski B, Carey M, Lawsin C, et al. The unmet needs of partners and caregivers of adults diagnosed with cancer: a systematic review. BMJ Support Palliat Care. 2012;2(3):224–30.
Osse BHP, Vernooij-Dassen MJFJ, Schad?? E, Grol RPTM. Problems experienced by the Informal caregivers of Cancer patients and their needs for support. Cancer Nurs. 2006;29(5):378–88.
Chen SC, Chiou SC, Yu CJ, Lee YH, Liao WY, Hsieh PY et al. The unmet supportive care needs—what advanced lung cancer patients’ caregivers need and related factors. Support Care Cancer. 2016 Feb 13 [cited 2023 Dec 4]; http://link.springer.com/ https://doi.org/10.1007/s00520-016-3096-3 .
Fukui S. Information needs and the related variables of Japanese family caregivers of terminally ill cancer patients. Nurs Health Sci. 2004;6(1):29–36.
DuBenske LL, Wen KY, Gustafson DH, Guarnaccia CA, Cleary JF, Dinauer SK, et al. Caregivers’ differing needs across key experiences of the advanced cancer disease trajectory. Palliat Support Care. 2008;6(3):265–72.
Park SM, Kim YJ, Kim S, Choi JS, Lim HY, Choi YS, et al. Impact of caregivers’ unmet needs for supportive care on quality of terminal cancer care delivered and caregiver’s workforce performance. Support Care Cancer off J Multinatl Assoc Support Care Cancer. 2010;18(6):699–706.
Cheng G, Chen C. End-of-life needs of dying patients and their families in Mainland China: a systematic review. OMEGA - J Death Dying. 2023;86(3):1019–45.
Article Google Scholar
Hwang SS, Chang VT, Cogswell J, Alejandro Y, Osenenko P, Morales E, et al. Study of unmet needs in symptomatic veterans with advanced cancer: incidence, independent predictors and unmet needs outcome model. J Pain Symptom Manage. 2004;28(5):421–32.
Bužgová R, Hajnová E, Sikorová L, Jarošová D. Association between unmet needs and quality of life in hospitalised cancer patients no longer receiving anti-cancer treatment. Eur J Cancer Care (Engl). 2014;23(5):685–94.
Hebert RS, Schulz R, Copeland VC, Arnold RM. Preparing family caregivers for death and bereavement. Insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37(1):3–12.
Hebert RS, Prigerson HG, Schulz R, Arnold RM. Preparing caregivers for the death of a loved one: a theoretical Framework and suggestions for Future Research. J Palliat Med. 2006;9(5):1164–71.
Sharpe L, Butow P, Smith C, McConnell D, Clarke S. The relationship between available support, unmet needs and caregiver burden in patients with advanced cancer and their carers. Psychooncology. 2005;14(2):102–14.
Milbury K, Badr H, Fossella F, Pisters KM, Carmack CL. Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Support Care Cancer. 2013;21(9):2371–9.
Hodgkinson K, Butow P, Hunt GE, Wyse R, Hobbs KM, Wain G. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer. 2007;15(4):405–15.
Pyo J, Ock M, Lee M, Kim J, Cheon J, Cho J, et al. Unmet needs related to the quality of life of advanced cancer patients in Korea: a qualitative study. BMC Palliat Care. 2021;20(1):58.
Hirayama K, Kuribara T, Oshikiri M. Experiences of the older spousal caregivers of patients with cancer during palliative chemotherapy: a qualitative descriptive study. BMC Palliat Care. 2023;22(1):188.
Luna-Meza A, Godoy-Casasbuenas N, Calvache JA, Díaz-Amado E, Gempeler Rueda FE, Morales O, et al. Decision making in the end-of-life care of patients who are terminally ill with cancer – a qualitative descriptive study with a phenomenological approach from the experience of healthcare workers. BMC Palliat Care. 2021;20(1):76.
Charmaz K. Grounded theory as an emergent method. Handbook of emergent methods. New York, NY, US: The Guilford; 2008. pp. 155–70.
Charmaz K. Constructing grounded theory. London: SAGE Publications Ltd; 2014.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
De Zarco Colón J, Ramasco Gutiérrez M, Pedraz Marcos A, Palmar Santos, Ana AM. Qualitative research in health. CIS; 2019. p. 349.
Head of State. Organic Law 3/2018, of 5 December, on the Protection of Personal Data and Guarantee of Digital Rights. Section 1, Organic Law 3/2018 Dec 6. 2018 p. 119788–857. https://www.boe.es/eli/es/lo/2018/12/05/3 .
Waller A, Girgis A, Johnson C, Lecathelinais C, Sibbritt D, Forstner D, et al. Improving outcomes for people with Progressive Cancer: interrupted Time Series Trial of a needs Assessment intervention. J Pain Symptom Manage. 2012;43(3):569–81.
Uitdehaag MJ, Verschuur EML, Van Eijck CHJ, Van Der Gaast A, Van Der Rijt CCD, De Man RA, et al. Problems and needs in patients with incurable esophageal and Pancreaticobiliary Cancer: a descriptive study. Gastroenterol Nurs. 2015;38(1):42–54.
Johnsen AT, Petersen MA, Pedersen L, Houmann LJ, Groenvold M. Do advanced cancer patients in Denmark receive the help they need? A nationally representative survey of the need related to 12 frequent symptoms/problems. Psychooncology. 2013;22(8):1724–30.
Rachakonda K, George M, Shafiei M, Oldmeadow C. Unmet supportive Cancer Care needs: an exploratory quantitative study in Rural Australia. World J Oncol. 2015;6(4):387–93.
Download references
Acknowledgements
We are exceptionally grateful to the participants and professionals involved in this study, without whom we could not share the results of this research.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Asociación Española Contra el Cáncer, Madrid, Spain
Mario López-Salas, Antonio Yanes-Roldán, Ana Fernández, Ainhoa Marín, Ana I. Martínez, Ana Monroy, José M. Navarro, Marta Pino, Raquel Gómez, Saray Rodríguez, Sergio Garrido, Sonia Cousillas, Tatiana Navas, Víctor Lapeña & Belén Fernández
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization and design: M.L-S., A.Y-R., B.F.; Acquisition, analysis and interpretation of data: M.L-S., A.Y-R., B.F.; Drafting of the manuscript: M.L-S., A.Y-R., A.F., B.F.; Critical revision of the manuscript for important intellectual content: All authors. All authors have read and approved the final version of this manuscript.
Corresponding author
Correspondence to Mario López-Salas .
Ethics declarations
Ethics approval and consent to participate.
According to the Ethics and Good Governance Committee of the Spanish Association Against Cancer (AECC), this research did not require approval by a medical research ethics committee. Nevertheless, the study complied with the principles of the 2013 Declaration of Helsinki. Each participant was adequately informed of the aims and methods of the study and informed consent was obtained from all individuals to participate in this study. No personal information has been used and the individual’s identity has been protected by removing any personal identifiers from the data. Codes were designated to the respondents to guarantee their anonymity.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
López-Salas, M., Yanes-Roldán, A., Fernández, A. et al. End-of-life care needs in cancer patients: a qualitative study of patient and family experiences. BMC Palliat Care 23 , 157 (2024). https://doi.org/10.1186/s12904-024-01489-1
Download citation
Received : 28 February 2024
Accepted : 14 June 2024
Published : 21 June 2024
DOI : https://doi.org/10.1186/s12904-024-01489-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Palliative care
- Facilitators
- Unmet needs
- Cancer patients
- End-of-life
BMC Palliative Care
ISSN: 1472-684X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Case study 1 - 1st touch communication approach
Full case study.
| This case-study describes the elements of a , introduced by Liverpool University Hospitals NHS Foundation Trust (at Aintree University Hospital), and the Liverpool Heart and Chest Hospital NHS Foundation Trust (at Liverpool Lung Cancer Unit), for newly diagnosed patients with lung cancer. The first touch approach is defined as maintaining a and focuses on the affirmative options for To explore the impact on patients and healthcare professionals we designed a semi-structured workshop with respiratory teams from the respective trusts.
|
Summary 2-pager
A short summary of our full case study report.
Video on key themes

A narrated presentation outlining the key themes which emerged from the workshop
Detailed analysis
Further analysis of the workshop from the Behavioural Science team at CRUK.
- Open access
- Published: 27 June 2024
Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review
- Hyunjoo Kim 1 , 2 ,
- Bomi Hong 3 ,
- Sanghee Kim 4 ,
- Seok-Min Kang 5 &
- Jeongok Park ORCID: orcid.org/0000-0003-4978-817X 4
Systematic Reviews volume 13 , Article number: 167 ( 2024 ) Cite this article
Metrics details
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer.
A scoping review was performed according to an a priori protocol using the Joanna Briggs Institute’s guidelines. The participants were patients with breast cancer. The concept was the literature of specifically reported symptoms directly matched with CRCT and the literature, in English, from 2010, and the context was open. The search strategy included four keywords: “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” All types of research designs were included; however, studies involving patients with other cancer types, animal subjects, and symptoms not directly related to CRCT were excluded. Data were extracted and presented including tables and figures.
A total of 29 articles were included in the study, consisting of 23 case reports, 4 retrospective studies, and 2 prospective studies. There were no restrictions on the participants’ sex; however, all of them were women, except for one case report. The most used chemotherapy regimens were trastuzumab, capecitabine, and doxorubicin or epirubicin. The primary CRCT identified were myocardial dysfunction and heart failure, followed by coronary artery disease, pulmonary hypertension, and other conditions. Major tests used to diagnose CRCT include echocardiography, electrocardiography, serum cardiac enzymes, coronary angiography, computed tomography, and magnetic resonance imaging. In all case reports, CRCT was diagnosed through an incidental checkup according to the patient’s symptom presentation; however, only 10 of these studies showed a baseline checkup before chemotherapy. The five most common CRCT symptoms were dyspnea, chest pain, peripheral edema, fatigue, and palpitations, which were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Dyspnea with trastuzumab treatment and chest pain with capecitabine treatment were particularly characteristic. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days.

Conclusions
This scoping review allowed data mapping according to the study design and chemotherapy regimens. Cardiac assessments for CRCT diagnosis were performed according to the patient’s symptoms. There were approximately five types of typical CRCT symptoms, and the timing of symptom occurrence varied. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Peer Review reports
Breast cancer is currently the most common cancer worldwide. Its incidence and mortality rates in East Asia in 2020 accounted for 24% and 20% of the global rates, respectively, and these rates are expected to continue increasing until 2040 [ 1 ]. In the USA, since the mid-2000s, the incidence rate of breast cancer has been increasing by 0.5% annually, while the mortality rate has been decreasing by 1% per year from 2011 to 2020 [ 2 ]. Despite the improved long-term survival rate in patients with breast cancer due to the development of chemotherapy, the literature has highlighted that cardiotoxicity, a cardiac problem caused by chemotherapy, could be a significant cause of death among these patients [ 3 ]. Chemotherapy-related cardiotoxicity (CRCT) can interfere with cancer treatment and progress to congestive heart failure during or after chemotherapy [ 4 ], potentially lowering the survival rate and quality of life of patients with cancer [ 5 ].
The term cardiotoxicity was first used in the 1970s to describe cardiac complications resulting from chemotherapy regimens, such as anthracyclines and 5-fluorouracil. The early definition of cardiotoxicity centered around heart failure, but the current definition is broad and still imprecise [ 6 ]. The 2022 guidelines on cardio-oncology from the European Society of Cardiology (ESC) define cardiotoxicity as including cardiac dysfunction, myocarditis, vascular toxicity, arterial hypertension, and cardiac arrhythmias. Some of these definitions reflect the symptoms. For example, cardiac dysfunction, which accounts for 48% of cardiotoxicity in patients with cancer, is divided into asymptomatic and symptomatic cardiac dysfunction. Asymptomatic cardiac dysfunction is defined based on left ventricular ejection fraction (LVEF), myocardial global longitudinal strain, and cardiac biomarkers. Symptomatic cardiac dysfunction indicates heart failure and presents with ankle swelling, breathlessness, and fatigue [ 7 ]. The ESC guidelines for heart failure present more than 20 types of symptoms [ 8 ]; however, to the best of our knowledge, few studies have been conducted to determine which heart failure symptoms and their characteristics are associated with CRCT in patients with breast cancer. Similarly, there is a lack of information related to vascular toxicity such as myocardial infarction [ 7 ].
Professional societies in cardiology and oncology have proposed guidelines for the prevention and management of cardiotoxicity in patients with cancer. According to the American Society of Clinical Oncology and the ESC, it is recommended to identify high-risk patients, comprehensively evaluate clinical signs and symptoms associated with CRCT, and conduct cardiac evaluations before, during, and after chemotherapy [ 7 , 9 , 10 ]. In addition, guidelines for patients with cancer, including those for breast cancer survivorship care, emphasize that patients should be aware of the potential risk of CRCT and report symptoms, such as fatigue or shortness of breath to their healthcare providers [ 7 , 11 , 12 ]. Although these guidelines encompass cardiac monitoring as well as symptom observation, many studies have focused solely on objective diagnostic tests, such as echocardiography, cardiac magnetic resonance, and cardiac biomarkers [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ], which means that there is little interest in CRCT symptoms in patients under breast cancer care.
This lack of interest in CRCT symptoms may be related to the absence of a specific symptom assessment tool for CRCT. Symptom monitoring of CRCT in patients with breast cancer was conducted through patient interviews and reported using the appropriate terminology [ 23 ]. In terms of interviews, patients with cancer experienced the burden of expressing symptoms between cardiovascular problems and cancer treatment. Qualitative research on patients with cancer indicates that these patients experience a daily battle to distinguish the symptoms they experience during chemotherapy [ 24 ]. To reduce the burden of identifying CRCT symptoms, it is crucial to educate patients with breast cancer undergoing chemotherapy about these symptoms. To report cardiotoxicity, healthcare providers in oncology can use a dictionary of terms called the Common Terminology Criteria for Adverse Events (CTCAE) for reporting adverse events in patients with cancer [ 25 ]. Patients can also use Patient-Reported Outcome (PRO), which allows unfiltered reporting of symptoms directly to the clinical database [ 26 ]. PRO consists of 78 symptomatic adverse events out of approximately 1,000 types of CTCAE [ 27 ]. Basch et al. suggested that PRO could enable healthcare providers to identify patient symptoms before they worsen, thereby improving the overall survival rate of patients with metastatic cancer [ 28 ]. This finding implies that symptoms can provide valuable clues for enhancing the timeliness and accuracy of clinical assessments of CRCT [ 29 ]. Therefore, it is necessary to explore the scope of research focusing on CRCT symptoms for prevention and early detection of CRCT in patients with breast cancer. The detailed research questions are as follows:
What are the general characteristics of the studies related to CRCT in patients with breast cancer?
What diagnostic tools and monitoring practices are used to detect CRCT?
What are the characteristics and progression of symptoms associated with CRCT?
A scoping review is a research method for synthesizing evidence that involves mapping the scope of evidence on a particular topic [ 30 ]. It aims to clarify key concepts and definitions, identify key characteristics of factors related to a concept, and highlight gaps or areas for further research [ 30 ]. This study used a scoping review methodology based on the Joanna Briggs Institute (JBI) framework. The JBI methodology, refined from the framework initially developed by Arksey and O’Malley [ 31 ], involves developing a research question, establishing detailed inclusion and exclusion criteria, and selecting and analyzing literature accordingly [ 32 ]. In contrast to systematic reviews, scoping reviews can encompass a variety of study designs and are particularly suitable when the topic has not been extensively studied [ 33 ]; hence, the decision was made to conduct a scoping review.
Development of a scoping review protocol
To conduct this review, an a priori scoping review protocol was developed to enhance transparency and increase the usefulness and reliability of the results. The protocol included the title, objective, review questions, introduction, eligibility criteria, participants, concept, context, types of evidence source, methods, search strategy, source of evidence selection, data extraction, data analysis and presentation, and deviation from the protocol [ 34 ] (Supplementary File 1).
Eligibility criteria
A participant-concept-context (PCC) framework was constructed based on the following research criteria. The participants were patients with breast cancer. The concept was that studies that specifically reported symptoms directly matched to CRCT in patients with breast cancer and the literature, published in English since 2010, in line with the year the CRCT guidelines were announced by the Cardio-Oncology Society. The context was open. We included all types of research designs. The exclusion criteria were studies that included patients with other types of cancer, involved animal subjects, and reported symptoms not directly related to CRCT.
Search strategy
The keywords consisted of “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” The keywords for “cardiotoxicity” were constructed according to the clinical cardiotoxicity report and ESC guidelines [ 7 , 35 ]. The keywords for “symptoms” included 40 specific symptoms of arrhythmia, heart failure, and cardiac problems [ 36 , 37 ] (Supplementary Table 1). We used PubMed, Embase, and CINAHL.
Source of evidence selection
Duplicate studies were removed using EndNote 21. The titles and abstracts were then reviewed according to the inclusion criteria, the primary literature was selected, and the final literature was selected through a full-text review. Any disagreements were resolved through discussions between the investigators.
Data extraction
The data from the literature included the general characteristics of the study, as well as information on the patients, chemotherapy, cardiotoxicity, and symptoms. The general characteristics of the study included author, publication year, country of origin, study design; patient information including sample size, sex, age, cancer type, and cancer stage; chemotherapy information including chemotherapy regimen; cardiotoxicity information including type of cardiotoxicity, diagnostic tests, and times of assessment; and symptom information including type of symptom, characteristics of symptom worsening or improvement, onset time, progression time, and time to symptom improvement. Information on whether to receive chemotherapy after the diagnosis of cardiotoxicity was explored.
Data analysis and presentation
The contents of the included studies were divided into three categories: (1) general characteristics, which encompassed study designs, patients, and medications; (2) type of CRCT and cardiac assessment for CRCT; and (3) characteristics and progression of the symptoms associated with CRCT. CRCT symptom-related data are presented in tables and figures.
In total, 487 studies were identified through database searches, and 116 duplicates were subsequently removed. After reviewing the titles and abstracts, we excluded 197 studies in which participants had cancers other than breast cancer, no symptoms, or symptom-related expressions. Of the remaining 174 studies, 146 were excluded after full-text review. Among the excluded studies, 79 were mainly clinical trials that the symptoms were not directly related to CRCT, 62 did not report specific symptoms, four were in the wrong population, and one was unavailable for full-text review. An additional study was included after a review of references, bringing the final count to 29 studies included in the analysis (Fig. 1 ).

Preferred reporting items for systematic reviews flowchart
General characteristics of studies including designs, sex and age, chemotherapy regimen, and CRCT criteria
Table 1 presents the general characteristics of the studies included in this review. The majority of these studies were published in the USA ( n =14), with Japan ( n =3), and Romania ( n =2) following. The study designs primarily consisted of case reports ( n =23), retrospective studies ( n =4), and prospective studies ( n =2).
All case reports involved female patients, except for one involving a male patient. Five quantitative studies did not specify or limit the sex of the participants, and one retrospective study included only female patients. In terms of cancer stage, the majority of studies involved patients with advanced breast cancer ( n =13), while a smaller number involved patients with early-stage breast cancer ( n =4). Twelve studies did not specify the cancer stage. Approximately 20 types of chemotherapy regimens are currently in use. Trastuzumab, which is a human epidermal growth factor receptor 2 (HER2) blocker, was mentioned in the majority of studies ( n =8), followed by capecitabine (an antimetabolite) ( n =7), and doxorubicin or epirubicin (anthracycline-based chemotherapy) ( n =6). Current chemotherapy and previous treatment methods were described together, with the exception of eight studies. Six quantitative studies defined the CRCT criteria, five of which were based on decreased LVEF and one of which was based on significant cardiac symptoms and/or electrocardiogram changes. Twenty-three case reports described the cardiovascular diagnosis as CRCT.
Diagnostic tools and monitoring practice for CRCT
Table 2 displays the types of CRCT, diagnostic tools, and times of cardiac assessment according to chemotherapy regimens. The most prevalent CRCT were myocardial dysfunction and heart failure, identified in 12 case studies, respectively. This was followed by coronary artery disease, represented in 8 case studies, pulmonary hypertension in 2 case studies, and a single case study of periaortitis. The most used test for diagnosing CRCT was echocardiography ( n =22), followed by EKG ( n =20), various types of cardiac enzymes ( n =16), coronary angiography (CAG, n =12), computed tomography ( n =6), and magnetic resonance imaging (MRI, n =4). Regarding the CRCT symptom assessment tools, the CTCAE was used in two studies, the New York Heart Association classification for heart failure in two studies, the dyspnea assessment scale in one study, and symptoms of cardiac origin, which consisted of chest pain, dyspnea, and palpitations in one study.
Regarding the times of cardiac evaluation, two studies performed regular cardiac checkups including before, during, and after chemotherapy. There were 10 case studies and six quantitative studies describing cardiac function testing before chemotherapy, of which seven studies performed regular cardiac screening tests and two studies mentioned cardiac screening even after the completion of chemotherapy. The frequency of regular checkups varied from every 3 months to every two to four cycles. In all case reports ( n =23), CRCT were diagnosed through incidental checkups based on patients’ symptom presentation, and in most cases, several tests were performed subsequentially for CRCT diagnosis. In one case study, cardiac evaluation was conducted 3 days after the patient’s initial symptom presentation, when the symptoms became more severe.
Characteristics and progression of symptoms associated with CRCT
Table 3 shows the descriptive scope of the CRCT-related symptoms according to the chemotherapy regimens used in the included studies. The mapping factors included initial symptoms, symptom onset or severity, symptom progression, medical management, and CRCT results. One of the most frequent symptoms associated with CRCT was dyspnea, which was discussed in 19 studies and described as difficulty in breathing, shortness of breath, or New York Heart Association (NYHA) class II or III. When dyspnea appeared as the initial symptom of CRCT, the symptom progression was worsening in eight case studies and persistent in two cases. Chest pain was described in 12 studies as a symptom characterized by a squeezing, tingling, burning, tightened, or atypical feeling that was relieved by rest and exacerbated by exertion. Other symptoms included peripheral edema ( n =6), fatigue ( n =5), and palpitation ( n =2). The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool.
The symptoms could be categorized based on the type of chemotherapy regimens used. In the case studies involving anthracycline-based regimen and HER2 blockers, dyspnea was the most frequently observed symptom ( n =7), followed by peripheral edema ( n =2), and chest pain or discomfort ( n =2). In case studies where antimetabolites were used, specifically capecitabine, chest pain was a common and prominent symptom. This chest pain typically manifested between 1 and 7 days after drug administration and persisted until treatment. Notably, four out of seven patients reported this symptom on the first day of chemotherapy, according to the case reports. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days. Figure 2 shows the progression of symptoms in case studies, detailing the time of symptom onset, the date of symptom reporting, and the date of treatment completion following the use of chemotherapy. The studies that did not specify any of the dates of symptom onset, reporting, and completion of treatment were excluded from the figure.

Figure 3 shows symptoms according to the main types of chemotherapy regimens reported in case studies. Dyspnea with trastuzumab and chest pain with capecitabine are particularly characteristic. A retrospective study included in this scoping review reported that chest pain was the most common symptom associated with capecitabine, followed by dyspnea and palpitation [ 40 ]. Furthermore, peripheral edema was primarily observed with anthracycline, alkylating, and HER2 blockers, while fatigue was noted with various anticancer drugs, irrespective of the type of chemotherapy regimen.

Ongoing chemotherapy was discontinued after CRCT was detected in 20 case studies. When patients presented symptoms indicative of CRCT, the majority were promptly hospitalized for further evaluation, medication, or interventional treatment. The majority of studies indicated the initiation of cardiac medication ( n =21), with three case studies involving coronary intervention and two involving treatment with wearable devices. Most management procedures were conducted in a general ward or an intensive care unit.
In most case studies, symptoms improved following cardiac treatment, with either complete or partial recovery of LVEF observed in 19 instances. However, a few studies reported a poor prognosis, including two instances of death. LVEF recovered in most patients within 6 months when treated with an anthracycline-based regimen and HER2 blockers (Fig. 2 ). A retrospective study reported that the rates of complete or partial recovery of CRCT following treatment with doxorubicin-based chemotherapy and trastuzumab were 42.9% and 86.1%, respectively [ 39 ]. Another retrospective study noted that the recovery time of CRCT when treated with HER2 blockers increased in correlation with the severity of the NYHA class, ranging from 8 to 80 weeks [ 38 ]. In the case of the antimetabolite capecitabine, all patients recovered within a day to a week, except one patient who did not recover.
This scoping review was conducted to explore the scope of studies focusing on CRCT symptoms, including the general characteristics of the studies, diagnostic tools, monitoring practices related to detecting CRCT, and the characteristics and progression of symptoms associated with CRCT. The primary findings of this review were as follows: (1) common symptoms related to CRCT and differences in symptoms according to the chemotherapy regimens used were identified; (2) the symptoms reported by the patient served as clues to suspect a specific type of CRCT; and (3) regular monitoring practices for CRCT prevention and detection were insufficient.
First, the current study identified common symptoms such as dyspnea, chest pain, peripheral edema, fatigue, and palpitation associated with CRCT, as well as variations in symptoms depending on the chemotherapy regimen used in patients with breast cancer. Among these symptoms, dyspnea, edema, and chest pain were frequently observed in patients receiving anthracycline-based and/or HER2 blocker drugs. These symptoms, which are associated with heart failure, appeared later compared to those observed with capecitabine, as depicted in Fig. 2 . This may be due to the known impact of anthracycline-based and/or HER2 blocker regimens on cardiomyocytes and other cells, leading to myocardial damage [ 42 ]. Therefore, the symptoms are related to heart failure, potentially resulting from the impairment of ventricular filling or ejection in patients undergoing treatment with these regimens [ 43 ].
In a similar vein, Attin et al. (2022) documented the occurrence of symptoms such as lower extremity edema, chest pain, difficulty breathing, and fatigue before the diagnosis of CRCT in women undergoing breast cancer treatment. They conducted a retrospective and longitudinal investigation of the symptoms, signs, and cardiac tests of 15 patients who experienced CRCT, using their electronic medical records. In their study, cardiotoxicity was defined by an echocardiogram or MRI showing a decrease in LVEF of 5 to 10%, with a specialist’s confirmation note. They compared the number of symptom occurrences during the first half of the year with those during the second half of the year prior to the diagnosis of cardiotoxicity. Specifically, the frequency of lower-extremity edema significantly increased from three occurrences in the first half of the year to 17 occurrences in the second half of the year. The frequency of symptoms for dyspnea and chest pain also increased from 10 and 8 times, respectively, to 16 and 14 times in the second half of the year. While there was limited information on the doses or timing of chemotherapy, 87% of the patients received the same chemotherapy regimens, namely anthracyclines and/or HER2 blockers [ 44 ]. This suggests that the increase in symptom occurrence over time may be related to the accumulation of anthracycline and the duration of anti-HER2 therapy [ 45 ].
Salyer et al. (2019) conducted a study on the prevalent symptoms of heart failure and their clustering. They identified three symptom clusters: sickness behavior, gastrointestinal disturbance, and discomfort of illness. Notably, dyspnea, edema, and pain were grouped into the discomfort of illness cluster, which aligns with the symptoms we observed in patients treated with anthracyclines and/or HER2 blockers [ 46 ]. Therefore, it is crucial for patients undergoing treatment with anthracyclines and/or HER2 blockers to be vigilant for symptoms such as dyspnea, edema, or chest pain, as these are indicative of heart failure.
Chest pain caused by vasospasm was a predominant symptom in patients taking antimetabolite regimens such as oral capecitabine, and it manifested as the following types of cardiotoxicities: vasospasm-related arrhythmia, myocardial disease, and ischemia [ 47 ]. Vasospasm can be triggered by endothelial dysfunction, hypersensitive vascular smooth muscle, reactive oxidative stress, or chemotherapy regimens [ 48 , 49 ]. According to previous studies, in patients using antimetabolite drugs such as 5-fluorouracil or capecitabine, chest pain was usually reported to occur from several hours to 72 hours after the first administration [ 47 , 50 , 51 , 52 , 53 ]. To detect chemotherapy-related coronary vasospasm in the early stage, it is recommended to carefully monitor typical or atypical symptoms of chest pain and EKG monitoring during drug infusion [ 54 ]. Muco et al. (2022) reported severe outcomes resulting from delayed management of vasospastic angina symptoms. The patient’s cardiac evaluation was performed 3 days after the onset of symptoms, and unfortunately, she did not recover from brain damage caused by coronary vasospastic sequelae. The authors stressed the importance of medical teams recognizing the symptoms of CRCT through vigilant monitoring and patient education [ 55 ].
As seen in the symptoms of CRCT caused by heart failure and vasospasm, careful observation of symptoms and conducting appropriate tests are crucial to prevent cardiotoxicity and minimize damage. These characteristics of CRCT and the associated symptoms related to chemotherapy regimens can provide crucial educational content for healthcare providers and patients preparing for chemotherapy. In addition, CRCT and symptom progression according to chemotherapy regimens could be used to formulate research questions for future systematic reviews.
Second, the preventive management of CRCT necessitates adherence to recommended guidelines. The 2022 ESC guidelines on cardio-oncology have updated the classification of CRCT and the monitoring protocols based on the chemotherapy regimens used [ 7 ]. The CRCT identified in the current study aligns with the drug toxicity outlined in the 2022 ESC guidelines. These guidelines advocate for regular cardiac monitoring before, during, and after chemotherapy to prevent and manage CRCT induced by anthracycline and HER2 blockers [ 7 , 12 ]. In this scoping review, two of 23 records described cardiac monitoring before, during, and after chemotherapy. An Australian multicenter study revealed that 59% of patients were referred to a cardiologist before CRCT occurred, but only 15% of patients diagnosed with CRCT had consulted a cardiologist before chemotherapy [ 41 ]. Given the declining mortality rates among cancer patients, managing CRCT requires a collaborative approach between oncology and cardiology to minimize mortality and morbidity in patients with breast cancer undergoing chemotherapy [ 7 ]. Therefore, it remains crucial to emphasize adherence to cardiac monitoring guidelines and foster cooperation between oncology and cardiology.
Additionally, symptom assessment is important for the early detection of patients with CRCT. The studies included in the current scoping review assessed whether patients’ symptoms could detect CRCT using interviews with patients, the New York Heart Association classification, a dyspnea assessment scale, and CTCAE tools. The United States National Cancer Institute recommends that healthcare providers use CTCAE and patients with cancer use PRO to report adverse events, including symptoms. CTCAE is a broad and comprehensive terminology that encompasses adverse events related to cancer treatment, has been used since the 1980s [ 25 ], and is not specialized in cardiotoxicity. Additionally, a discrepancy between CTCAE and PRO discovered that healthcare providers often underestimate both the incidence and duration of symptoms compared to the patients [ 56 , 57 , 58 ]. Specifically, healthcare providers tend to report symptom severity as lower than that reported by patients. For instance, there are notable discrepancies between healthcare providers and patients when reporting severe or very severe symptoms of fatigue, dyspnea, and limb edema in patients with early-stage breast cancer undergoing chemotherapy. The reported rates were 8% and 22% for fatigue, 0% and 4% for dyspnea, and 0% and 5% for limb edema, from healthcare providers and patients, respectively. Therefore, it is necessary to develop a user-friendly questionnaire to assess the various symptoms of CRCT.
Finally, we found that once CRCT was confirmed, cardiac treatment was promptly initiated and chemotherapy was frequently halted until CRCT resolution. A Delphi study on the use of anthracycline and trastuzumab proposed altering the treatment schedule or discontinuing treatment until there was an improvement in LVEF [ 59 ]. However, the professional societies did not provide definitive recommendations regarding continuing or ceasing ongoing chemotherapy. Instead, they suggested that the decision to continue or discontinue ongoing chemotherapy should be made based on the patient’s potential risks and benefits [ 60 ]. For example, Polk et al. (2016) reported that out of 22 patients with CRCT resulting from capecitabine, six continued medications with or without cardiac treatment; some of these patients experienced the same symptoms, while others did not exhibit significant symptoms [ 40 ]. Further research is required to explore the continuation or discontinuation of chemotherapy when CRCT is confirmed.
This study has some limitations. First, although we did not restrict the patients’ sex when reviewing the literature, most patients, except for one, were female. This may be related to the lower incidence of breast cancer in men. Second, although this scoping review mapped CRCT symptoms according to chemotherapy regimens, including anthracycline-based drugs, HER2 blockers, and antimetabolites, it did not cover cardiotoxicity related to other types of chemotherapy regimens. Thus, exploring the symptoms by focusing on expanded chemotherapy regimens and cardiovascular toxic diseases will assist in overcoming this limitation. Third, of the 29 studies, 23 were case reports with some grey literature, which may be justified by the nature of scoping reviews that allow for inclusion irrespective of the data source [ 61 ] and the study type. Experimental or observational clinical studies use objective criteria, such as diagnostic tests to generate primary evidence. However, case reports have led to new medical discoveries regarding the prevention and treatment of diseases [ 62 ]. Given the nature of case reports, specific symptoms that could provide clues for evaluating CRCT in patients with breast cancer are most often found in these reports. We incorporated grey literature to gather more comprehensive information on CRCT-related symptoms. However, to mitigate the potential issue of unverified quality in grey literature, we initially organized 16 studies from peer-reviewed literature and subsequently incorporated the grey literature into our findings. This approach helped to clarify the results of the peer-reviewed literature, particularly the types of chemotherapy regimens [ 63 ]. Finally, regarding the literature selection criteria, we examined articles written in English and published since 2010, the year the cardio-oncology guidelines were announced, thereby excluding articles published before 2010.
This scoping review allowed data mapping according to the study design and chemotherapy regimens. The key messages included a type of CRCT, cardiac assessment, and in-depth information regarding the CRCT symptoms. There were approximately five typical CRCT symptoms, including dyspnea, chest pain, peripheral edema, fatigue, and palpitations, and the timing of symptom occurrence varied. The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: global statistics for 2020 and 2040. The Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Agha A, Wang X, Wang M, Lehrer EJ, Horn SR, Rosenberg JC, Trifiletti DM, Diaz R, Louie AV, Zaorsky NG. Long-term risk of death from heart disease among breast cancer patients. Front Cardiovasc Med. 2022;9: 784409.
Oikawa M, Ishida T, Takeishi Y. Cancer therapeutics-related cardiovascular dysfunction: Basic mechanisms and clinical manifestation. J Cardiol. 2023;81(3):253–9.
Piepoli MF, Adamo M, Barison A, Bestetti RB, Biegus J, Böhm M, Butler J, Carapetis J, Ceconi C, Chioncel O, et al. Preventing heart failure: a position paper of the Heart Failure Association in collaboration with the European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(1):143–68.
Chung R, Ghosh AK, Banerjee A: Cardiotoxicity: precision medicine with imprecise definitions. In., vol. 5: Archives of Disease in childhood; 2018: e000774.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European Heart Journal - Cardiovascular Imaging. 2022;23(10):e333–465.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O et al: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022, 24(1):4-131.
Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13(4):270–5.
Lanza O, Ferrera A, Reale S, Solfanelli G, Petrungaro M, Tini Melato G, Volpe M, Battistoni A: New insights on the toxicity on heart and vessels of breast cancer therapies. Med Sci (Basel) 2022, 10(2).
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73.
Lee GA, Aktaa S, Baker E, Gale CP, Yaseen IF, Gulati G, Asteggiano R, Szmit S, Cohen-Solal A, Abdin A, et al. European Society of Cardiology quality indicators for the prevention and management of cancer therapy-related cardiovascular toxicity in cancer treatment. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):1–7.
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, Tsekoura D, Naka K, Mazzocco K, Mauri D et al: New insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers (Basel) 2023, 15(13).
Di Lisi D, Manno G, Madaudo C, Filorizzo C, Intravaia RCM, Galassi AR, Incorvaia L, Russo A, Novo G: Chemotherapy-related cardiac dysfunction: the usefulness of myocardial work indices. Int J Cardiovasc Imaging 2023.
Kar J, Cohen MV, McQuiston SA, Malozzi CM. Can global longitudinal strain (GLS) with magnetic resonance prognosticate early cancer therapy-related cardiac dysfunction (CTRCD) in breast cancer patients, a prospective study? Magn Reson Imaging. 2023;97:68–81.
Article CAS PubMed Google Scholar
Lim A, Jang H, Jeon M, Fadol AP, Kim S. Cancer treatment-related cardiac dysfunction in breast cancer survivors: a retrospective descriptive study using electronic health records from a Korean tertiary hospital. Eur J Oncol Nurs. 2022;59: 102163.
Liu W, Li W, Li H, Li Z, Zhao P, Guo Z, Liu C, Sun L, Wang Z. Two-dimensional speckle tracking echocardiography help identify breast cancer therapeutics-related cardiac dysfunction. BMC Cardiovasc Disord. 2022;22(1):548.
Article CAS PubMed PubMed Central Google Scholar
Mauro C, Capone V, Cocchia R, Cademartiri F, Riccardi F, Arcopinto M, Alshahid M, Anwar K, Carafa M, Carbone A et al: Cardiovascular side effects of anthracyclines and HER2 inhibitors among patients with breast cancer: a multidisciplinary stepwise approach for prevention, early detection, and treatment. J Clin Med 2023, 12(6).
Okushi Y, Saijo Y, Yamada H, Toba H, Zheng R, Seno H, Takahashi T, Ise T, Yamaguchi K, Yagi S et al: Effectiveness of surveillance by echocardiography for cancer therapeutics-related cardiac dysfunction of patients with breast cancer. J Cardiol 2023.
Ositelu K, Trevino A, Tong A, Chen MH, Akhter N: Challenges in cardiovascular imaging in women with breast cancer. Curr Cardiol Rep 2023.
Terui Y, Sugimura K, Ota H, Tada H, Nochioka K, Sato H, Katsuta Y, Fujiwara J, Harada-Shoji N, Sato-Tadano A, et al. Usefulness of cardiac magnetic resonance for early detection of cancer therapeutics-related cardiac dysfunction in breast cancer patients. Int J Cardiol. 2023;371:472–9.
Thavendiranathan P, Shalmon T, Fan CS, Houbois C, Amir E, Thevakumaran Y, Somerset E, Malowany JM, Urzua-Fresno C, Yip P, et al. Comprehensive cardiovascular magnetic resonance tissue characterization and cardiotoxicity in women with breast cancer. JAMA Cardiol. 2023;8(6):524–34.
Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–7.
White J, Byles J, Williams T, Untaru R, Ngo DTM, Sverdlov AL. Early access to a cardio-oncology clinic in an Australian context: a qualitative exploration of patient experiences. Cardiooncology. 2022;8(1):14.
PubMed PubMed Central Google Scholar
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P: CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003, 13(3):176-181.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP et al: Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014, 106(9).
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;36:67–73.
Article Google Scholar
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Liu L, Suo T, Shen Y, Geng C, Song Z, Liu F, Wang J, Xie Y, Zhang Y, Tang T, et al. Clinicians versus patients subjective adverse events assessment: based on patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2020;29(11):3009–15.
Munn Z, Pollock D, Khalil H, Alexander L, Mclnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 2022;20(4):950–2.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H: Chapter 11: scoping reviews (2020 version). In: JBI Manual for Evidence Synthesis. edn. Edited by Aromataris E MZ: JBI; 2020.
Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology. 2018;18:1–7.
Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68.
Bohdan M, Kowalczys A, Mickiewicz A, Gruchala M, Lewicka E: Cancer therapy-related cardiovascular complications in clinical practice: current perspectives. J Clin Med 2021, 10(8).
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63.
Bozkurt B, Coats A, Tsutsui H: Universal definition and classification of heart failure. J Card Fail 2021.
Aldiab A. Cardiotoxicity with adjuvant trastuzumab use in breast cancer: a single institution»s experience. J Saudi Heart Assoc. 2010;22(3):133–6.
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–21.
Polk A, Shahmarvand N, Vistisen K, Vaage-Nilsen M, Larsen FO, Schou M, Nielsen DL: Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016, 6(10).
Clark RA, Marin TS, McCarthy AL, Bradley J, Grover S, Peters R, Karapetis CS, Atherton JJ, Koczwara B. Cardiotoxicity after cancer treatment: a process map of the patient treatment journey. Cardiooncology. 2019;5:14.
Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021;280: 119760.
Malik A, Brito D, Vaqar S, Chhabra L: Congestive heart failure. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023.
Attin M, Reifenstein K, Mehta S, Arcoleo K, Lin CD, Storozynsky E. Reported signs, symptoms, and diagnostic tests before cardiotoxicity among women with breast cancer: a pilot study. J Cardiovasc Nurs. 2022;37(2):104–11.
Huang P, Dai S, Ye Z, Liu Y, Chen Z, Zheng Y, Shao X, Lei L, Wang X. Long-term tolerance and cardiac function in breast cancer patients receiving trastuzumab therapy. Oncotarget. 2017;8(2):2069–75.
Salyer J, Flattery M, Lyon DE. Heart failure symptom clusters and quality of life. Heart Lung. 2019;48(5):366–72.
Padegimas A, Carver JR. How to diagnose and manage patients with fluoropyrimidine-induced chest pain: a single center approach. JACC CardioOncol. 2020;2(4):650–4.
Sheth MA, Widmer RJ, Dandapantula HK. Pathobiology and evolving therapies of coronary artery vasospasm. Proc (Bayl Univ Med Cent). 2021;34(3):352–60.
PubMed Google Scholar
Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, Matsuzawa Y, Mitsutake Y, Mitani Y, Murohara T, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. J Cardiol. 2023;82(4):293–341.
Kanduri J, More LA, Godishala A, Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. 2019;37(4):399–405.
Garbis K, Rafiee MJ, Luu J. 5-fluorouracil-induced coronary vasospasm: a cardiovascular magnetic resonance imaging case report. Glob Cardiol Sci Pract. 2023;2023(3): e202316.
Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020;59(4):475–83.
Becker K, Erckenbrecht JF, Häussinger D, Fueling T. Cardiotoxicity of the antiprolif erative compound fluorouracil. Drugs. 1999;57(4):475–84.
Lestuzzi C, Vaccher E, Talamini R, Lleshi A, Meneguzzo N, Viel E, Scalone S, Tartuferi L, Buonadonna A, Ejiofor L, Schmoll HJ. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059–64.
Muco E, Patail H, Shaik A, McMahon S. Capecitabine-associated coronary vasospasm and cardiac arrest. Cureus. 2022;14(8): e28184.
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S, et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. 2016;2(4):445–52.
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–76.
Galizia D, Milani A, Geuna E, Martinello R, Cagnazzo C, Foresto M, Longo V, Berchialla P, Solinas G, Calori A, et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: a prospective study. Cancer Med. 2018;7(9):4339–44.
Gavila J, Seguí M, Calvo L, López T, Alonso JJ, Farto M. Sánchez-de la Rosa R: Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol. 2017;19(1):91–104.
Leong DP, Lenihan DJ. Clinical practice guidelines in cardio-oncology. Heart Fail Clin. 2022;18(3):489–501.
Munn Z, Pollock D, Khalil H, Alexander L, McLnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evid Synth. 2022;20(4):950–2.
Li YR, Jia Z, Zhu H. Understanding the value of case reports and studies in the context of clinical research, research design and evidence-based practice. J Case Reports and Studies. 2013;1(2):1–4.
Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52(4):256–61.
Download references
Acknowledgements
The authors thank Nawon Kim, a librarian at the Yonsei University Medical Library, for building search terms and guiding the database searches.
This research is supported by the Brain Korea 21 FOUR Project founded by the National Research Foundation (NRF) of Korea, Yonsei University College of Nursing.
Author information
Authors and affiliations.
Graduate School, College of Nursing, Yonsei University, 50 Yonsei-ro, Seoul, South Korea
Hyunjoo Kim
Severance Cardiovascular Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Brain Korea 21 FOUR Project, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Mo-Im Kim Nursing Research Institute, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Sanghee Kim & Jeongok Park
Department of Internal Medicine, Division of Cardiology, Heart Failure Center, Severance Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Seok-Min Kang
You can also search for this author in PubMed Google Scholar
Contributions
HK, BH, SK, and JP contributed to the study conception and design. The literature search and record screening were performed by HK and BH under the supervision of JP. Material preparation, data collection, and analysis were performed by HK, BH, and JP. The first draft of the manuscript was written by HK and JP commented on each updated version of the manuscript. The tables and figures were prepared by BH under the instruction of JP. SK helped to interpret the data and provided critical feedback on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jeongok Park .
Ethics declarations
Ethics approval and consent to participate.
This study was exempted from ethical approval by the Yonsei University Institute Review Board, and consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, H., Hong, B., Kim, S. et al. Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review. Syst Rev 13 , 167 (2024). https://doi.org/10.1186/s13643-024-02588-z
Download citation
Received : 25 December 2023
Accepted : 14 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s13643-024-02588-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Chemotherapy
- Cardiotoxicity
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Equity
- v.3(1); 2019

A Cancer Patient Journey: Complete Review During Acute Treatment Phase
Saima siddiqui.
Department of Family and Community Medicine, University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Purpose: Cancer is a chronic illness with acute episodes lasting for years. Most cancer patients have coexisting comorbidities, which affect cancer treatment outcomes and make a shared care model for chronic diseases essential. There is a considerable gap between the achievable and delivered quality of care for cancer patients.
Methods: We used a case study approach to examine the complexity of cancer management, from the perspective of one person's case as interpreted by the care team. It allowed the complexity of cancer management to retain its holistic and meaningful characteristics. We interviewed the patient, caregiver, primary care physician (PCP), and oncologist. Interviews were audio recorded and analyzed with ATLASti, qualitative statistical software. Participants also completed a basic demographic survey. Common themes were identified, analyzed, and discussed.
Results: Main themes were lack of longitudinal relationship with PCP, communication barriers, and ambiguous health care provider roles. Communication barriers can be associated with the other two main themes.
Conclusion: Our results showed that shared care for cancer management is lacking during the acute cancer treatment phase. Communication barriers between the PCP and oncologist along with lack of continuity of care and unclear role of the PCP are major contributors for fragmented cancer care in U.S. health care system.
Introduction
Cancer is a leading cause of morbidity and mortality worldwide. In 2013, there were ∼1,660,290 new cancer cases and 580,350 cancer deaths in the United States. 1 A diagnosis of cancer is still very stressful and frightening for the majority of patients and families 2–4 although the number of patients living with cancer has increased threefold since 1971. 5 According to the Center for Disease Control and Prevention (CDC), cancer is classified as a chronic disease and described as of long duration and generally slow progression.
Studies have shown that cancer patients receive fragmented care especially during the acute treatment phase 6–9 stemming from system problems such as lack of health insurance, communication problems between health care team members, 7 , 10 and lack of role clarity among team members 11–14 The National Cancer Policy Board has concluded that for many Americans with cancer, there is a wide gulf between what could be construed as the “ideal and the reality of their experience with cancer care.” 15 There is not just a “gap, but a chasm.” 16
The purpose of this study was to gather a qualitative description of collaborative care from viewpoints of cancer patient (Pt), caregiver, primary care physician (PCP), and oncologist to better understand cancer patient's journey in the health care system.
A case study approach was used because it allowed us to examine the complexity of cancer management from the perspective of one person's case as interpreted by multiple people, retaining its holistic and meaningful characteristics while being studied 17 answering how and why questions. 18 Interviews from four participants presented multiple perspectives of the same interested topic, therefore achieving data triangulation.
The study is guided by the chronic care model (CCM), a proactive approach to keep patient healthy through productive collaboration between community and health systems; therefore, the study generalizes to the theoretical propositions of the CCM and not the population. 19 CCM identifies six structural elements: the community, the health system, self-management support, delivery system design, decision support, and clinical information systems. The interview guide was developed based on these areas. Development of the interview guide was an iterative process in which the researchers developed and discussed questions, which contextualized the CCM within cancer management. Once questions were approved, they became part of the official interview guide (12 total questions), which was re-evaluated for consistency and changed if researchers felt that questions were being misinterpreted. Main questions included were as follows: Tell me the story of how you learned you had cancer? Who did you talk to about your cancer diagnosis? How do your PCP and oncologist work together to manage your cancer and general medical care? The researcher conducting the interviews was knowledgeable in social science research of chronic conditions. Her inexperience served as a strength because she was not able to create leading questions or force participants into expected outcomes.
The study utilized a purposeful sampling method. 20 , 21 PCP identified and referred the cancer patient to the study. The patient then identified her caregiver and oncologist.
Data collection consisted of about 1 h long semistructured interview. The approach of starting with the patient and then interviewing the caregiver and the clinicians helped to understand how the same events were viewed from different perspectives. Each interview was recorded and transcribed for analysis. All research activities were reviewed and approved by the University of Texas Health Science Center San Antonio Office of Institutional Review Board.
Our sample included interviews with one cancer patient, her caregiver, PCP, and oncologist. Specifics of sample demographics are in Tables 1 and and2 2 ).
Demographics of Patient and Caregiver in Case Study
| Role | Sex | Age | Ethnicity | Cancer | Insurance | Marital status | Education | Monthly income |
|---|---|---|---|---|---|---|---|---|
| Patient | F | 63 | Hispanic | Leukemia | County system | Divorced | GED | <$1000 |
| Caregiver | F | 31 | Hispanic | n/a | Private | Married | College graduate | $2500–3500 |
Demographics of Providers in Case Study
| Role | Sex | Ethnicity | Specialty | Years of experience |
|---|---|---|---|---|
| PCP | F | White | Family medicine | 25 |
| Oncologist | M | Asian | Hematology | 29 |
M, male; PCP, primary care physician.
The results identified a major breakdown in the delivery system design highlighted in the CCM. The three main themes, which are organized around the patients' experience through the health care system—from cancer diagnosis to treatment, are as follows:
- (a) Lack of longitudinal relationship with PCP
- (b) Communication barriers
- (c) Ambiguous health care provider role.
(a) Lack of longitudinal relationship with PCP: This theme supports a known system problem in which people who are the sickest and need health insurance the most do not have it. This particular patient lost insurance, secondary to unemployment because of uncontrolled hypertension.
The patient ended up in the emergency room (ER) for severe chest pain and was diagnosed as having leukemia.
Pt: “At that time, I did not [have a PCP] … I was one of those persons that go from payday to payday and I could not afford health insurance…Here I am very sick, quitting because I am very sick…So when I went into ER, I did not have a doctor…”
The first PCP visit was 4 months after her leukemia diagnosis and after receiving three cycles of chemotherapy.
Pt: “After my fourth visit to the [cancer treatment center] they told me that I need to call the [healthcare system] and I needed to get a PCP…”
The patient was assigned a new PCP in a teaching facility with residents and faculty members divided into teams and different providers saw the patient every time. The lack of a longitudinal relationship with a PCP appeared normal to the patient; therefore, she began to rely heavily on the oncology team and ER for things that a PCP could manage.
Pt: “Usually I see a different [provider], it is like a set of doctors that all work together. So, I can't say it is one doctor…”
(b) Communication barriers: The communication barriers surrounding cancer treatment began between the patient, caregiver, and health care providers almost immediately. As the patient was diagnosed with cancer in the ER, she felt she was not able to get the answers for her questions,
Pt: “The whole time all I was thinking, I have cancer! What is Leukemia… am I going to die… I heard them talking between themselves that it might be Leukemia…[And] they didn't want to give me the exact diagnosis yet.”
Similarly, the caregiver was not included in any stage of cancer management. After a few months, the oncologist provided a video to share with family members.
Caregiver: “..a lot of questions I had, I just used my own resources… [the providers asked] if you have questions…and then they gave us some packets and pamphlets. I relied mostly on my mom for communication… My mom has a high school education, GED, and she doesn't understand lots of words.”
The teaching practice setting also prevented her from communicating with the health care team. The caregiver's impression was that due to patient's privacy, physicians were not supposed to communicate with her, and residents' learning will be interrupted by her questions.
Caregiver: “I always assume, it's a privacy thing… I just wish there was a means for me to communicate directly with them or staff or nurse. I feel like there's residents that come in as a group with the doctors and learn, so I feel like I didn't want to interrupt their learning with questions.”
Caregiver also identified lack of communication between the PCP and oncologist.
Caregiver: “I feel like there's a lack of communication between them. That's a prescription given to her by her cancer doctor. Then the PCP will say that's not working out for you, let's take them off so that makes me uncomfortable, just in the sense that I feel like you should ask [the oncologist] first… My mom's been bounced back and forth between vitamins and medications that she'll get prescribed by one doctor, and then another doctor will change their mind..”
There was no specific arrangement for cancer patients to contact PCP for early or urgent appointments. When the caregiver called to report a concern, appointment clerk asked her to go to the ER. At times, the ability to provide advice was contingent on the flow of clinic traffic, sometimes the patient and caregiver were able to contact the oncologist but got same advice.
Both the PCP and oncologist identified lack of communication as a barrier for patient management. The health care providers were not able to effectively communicate because of the distance between facilities, a physical difficulty, and relational issue.
Oncologist : “The physical issue of being based in a downtown [building] and having oncology services out at medical center [approximately 12 miles away]. You can't pop over at lunch for a meeting ever, I suppose..”
Time constraint was another factor. PCPs do not have time to serve on cancer boards and oncologists do not communicate with PCP by phone or with follow-up letters.
Oncologist: “No one's going to serve on a board if they're all in clinic full time, of course… Everyone's busy so the communication is lacking…because we in oncology have been very short staffed.”
Use of different electronic health record (EHR) systems break communication further.
PCP: “It is not possible for providers to look into each other notes and management plan. You've got the problem of the two computer systems that don't talk to each other, so they don't see what they are doing in [EHR] and we don't see what they are doing in their EHR, so that makes it very difficult just all around.”
No point of contact within the PCP and oncology clinic was assigned for communication about patients. The PCP was expected to communicate through the oncology on-call resident or the front desk person for any question or concern. It resulted in duplicate laboratory tests and confusion about patient's treatment and patient served as the main communicator between the PCP and oncologist. It also resulted in care delay.
PCP: “These are all unnecessary barriers in communication between the two offices and one of them is the fact that you can't just book the patient before the patient leaves… sometimes you think you are conveying information, sometimes they don't receive it.”
(c) Ambiguous health care provider role: The patient, caregiver, and health care providers agree that the PCP should be an essential part of the management team; however, all ambiguously understands the role. The PCP was viewed as important for the emotional support of the patient and family.
Pt: “She [the PCP] asked me if I ever got depressed. I told her no… She said, it is okay to say it if you are…I told her when I get in the shower I just breakdown crying sometimes for no reason. She says good, let it out. She says it is okay to feel that way. I would feel that way too.”
However, the PCP was not comfortable in managing specific chemotherapy-related side effects. The PCP felt that their strengths were in management of chronic diseases. The oncologist felt that they should be able to rely on the PCP for support of common disease management.
Oncologist: “..honestly my knowledge of ideal hypertension management has declined… even though I am an internist at heart… I quickly need primary care support to manage hypertension, as well as routine health maintenance of immunizations and recommended cancer screenings. We [the oncologist] make the diabetes worse, so we constantly want to work with primary care teams.”
The oncologist identified that the PCP should be seen as (Oncologist): “an educator or tie-breaker in terms of treatment decision making.”
This study reflects a typical journey of an underprivileged uninsured cancer patient through the American health care system. It is unique in that data were collected and interpreted from the patients' perspective but captures several perspectives on the experience. No other studies, focusing on the patient perspective from a case study methodology, were found in the current literature. The patient lost her health insurance due to uncontrolled hypertension resulting in the loss of employment. This resulted in a delay of cancer diagnosis as the patient kept on postponing and neglecting the symptoms as long as she could tolerate. Main barriers identified in our study were the same as identified in earlier studies. Similarities included a lack of longitudinal relationships with the PCP, communication issues between patient, caregiver, PCP, and oncologist, and a lack of role clarification for providers and patient. 14 , 22–25 New finding was the patient and caregiver's inability to communicate with PCP due to the teaching practice setting.
This study identifies the serious gaps and areas of improvement for cancer. Our findings confirm that the PCP is not an active member of patients' management team during chemotherapy. 25–27 The first PCP visit took place after the fourth chemotherapy visit, ∼3½ months into chemotherapy. Studies have shown that one in five Americans reported not getting or delaying medical care, and the percentage of uninsured patients 45–64 years of age increased from 13.1% to 15.6%. 28 , 29 In addition, the patient did not have access to a PCP after obtaining health care insurance due to the PCP's busy schedule and the absence of special arrangements for cancer patients, which resulted in patients using the ER. Previous studies have shown that there is an increased use of health care services by cancer patients when they are undergoing acute cancer treatment by chemotherapy and radiation as well as after treatment. 30 , 31 Ideally, there needs to be special provisions or the identification of a key contact person for cancer patients in PCP offices.
Lack of communication was the most prominent problem identified by the patient, caregiver, and physicians. The main communication failure was between the PCP and the oncology team, confirming similar findings identified in other studies. 32–36 The federal government has offered incentives for meaningful use of information technology as a key tool for improving care coordination, which resulted in an increased use of EHR by physicians and hospitals. 37 , 38 In our study, the use of different EHRs by the oncologist and the PCP office was problematic. The PCP could not access patient information from the oncology visit and there was no formal follow-up letter from oncology. Therefore, the PCP did not have any idea about chemotherapy regime or patient prognosis. Ideally, EHR should account for human factors both tolerating human limitations and augmenting human strengths, 39 and bridging the gap between different segments of patient care rather than collecting numbers and producing reports to fulfill government requirements.
Similarly, the patient and caregiver expressed frustration about the lack of communication because it placed a larger burden on the patient as main communicator between oncologist and PCP, which is not an acceptable practice.
Not knowing the point of contact in the PCP and oncology office was an additional reason for communication breakdown. Good care coordination for safe and appropriate management of chronic conditions such as cancer are essential, but the care coordination remains inadequate and a major cause of health care expenditure and mistakes. 40 , 41 Possible solutions include uniform access to EHRs, clear identification of the patient's PCP and oncologist, a point of contact in each office, and a structured follow-up letter from oncologist to PCP. 42 Further studies are needed to evaluate the efficacy of these measures.
Time constraint was an additional reason identified by the PCP and oncologist for the communication breakdown. There is no formal reimbursement for physician or staff time used for communication and coordination between providers or by insurance companies. 43 In addition, the shortage of PCPs and oncologists, and increased number of cancer patients makes care coordination more difficult. 44 A system-wide change is needed to address these issues and acknowledge that time reimbursement will produce real improvement in patient care and reduce health care cost. The patient and caregiver identified the teaching hospital setting as an inhibitory factor because they felt that asking questions and communicating with health care providers would interfere with learning, which is a new finding by this study. It requires that teaching physicians take extra steps to include the patient and care giver in their discussions and make them feel like part of the team by formally including the patient in discussions.
Lack of PCP role clarification was another barrier identified for effective collaboration. 45–47 The current norm accepts that PCPs will not be a part of the cancer patient health care team. The patient and caregiver expectations were that the PCP would serve as emotional support, manage chronic disease, and perform routine health maintenance such as cancer screening and immunizations. Studies have shown that PCPs can play an important role in the management of cancer patients' coexisting chronic conditions and common side effects of chemotherapy, treating acute conditions such as viral illnesses and helping patient to make informed decisions about management, and end-of-life issues. 48 The oncologist agreed that the PCP was an important part of the health care team, and the PCP was comfortable in fulfilling all these roles. Clear role assignment of health care team members will decrease the role confusion and potentially impact patients' unnecessary ER visits, reducing patient discomfort and health care cost.
Our study revealed many barriers for collaboration during the initial cancer treatment phase between the PCP and oncologist. Even though there is an abundance of resources and expertise available, the lack of collaboration and fragmented effort resulted in a wide gap between possible and actual care delivery for cancer patients.
Limitations
The major weaknesses of this study are that it was conducted in a teaching hospital setting and describes the experience of only one patient. However, the purpose of a case study was to examine the complexity of a phenomenon (cancer management) while it retains its holistic and meaningful characteristics. Major strength of this study is that it describes the complete experience, as it has been understood by an underserved and uninsured patient, caregiver, and patient health care team.
Implications
The barriers identified in this study should be used to devise interventions to be tested in large-scale prospective studies to fill gaps in present system of cancer patient care.
Acknowledgments
This study was funded by American Cancer Society. We thank Robert L. Ferrer, MD, and Sarah Gill, PhD, for helping at every stage of article development and supervising all aspects of this project.
Abbreviations Used
| CCM | chronic care model |
| CDC | Center for Disease Control and Prevention |
| ER | emergency room |
| EHR | electronic health record |
| PCP | primary care physician |
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Siddiqui S, Cruz I (2019) A cancer patient journey: complete review during acute treatment phase, Health Equity 3:1, 403–408, DOI: 10.1089/heq.2019.0046.
The clinical signature of genetic variants and serum levels of macrophage migration inhibitory factor in Egyptian breast cancer patients
- Open access
- Published: 25 June 2024
Cite this article
You have full access to this open access article

- Mahmoud A. Seliem 1 , 2 ,
- Ahmed M. Mohamadin 1 ,
- Mohamed I. Kotb El-Sayed 3 ,
- Yahia Ismail 4 &
- Ahmed A. El-Husseiny ORCID: orcid.org/0000-0003-0153-6555 1 , 5
52 Accesses
Explore all metrics
Macrophage migration inhibitory factor (MIF) is an integral cytokine for the modulation of both innate and adaptive immunity and is involved in the pathogenesis of various cancers. However, conflicting findings on the relationship between MIF polymorphisms and breast cancer (BC) have been reported in earlier research. We investigated the clinical value of serum MIF levels and the association between MIF rs1049829 and rs755622 variants with their serum levels and propensity to develop BC.
A total of 133 treatment-naïve Egyptian BC females and 126 apparently healthy controls were matriculated in this case–control study. The serum MIF protein levels were quantified by ELISA, whereas the genotyping was executed utilizing the TaqMan® allelic discrimination assay.
A significant increase in the serum MIF level in BC cases was observed in comparison to control subjects ( P < 0.0001), with a diagnostic potential to discriminate BC with 92.5% sensitivity and 73.7% specificity at a cut-off value > 9.47 ng/mL. Besides, a significant difference in serum MIF level was observed in BC cases with progesterone receptor (PR) negativity compared to those with PR positivity ( P = 0.046). Moreover, a significant association was depicted between the rs1049829 variant of MIF gene and the protective effect against BC meanwhile the rs755622 variant demonstrated no significant link with BC risk.
Conclusions
This study revealed that serum MIF levels may be regarded as a promising serum tumor marker for BC. Also, the rs1049829 variant of the MIF gene is considered a protective candidate against BC.
Graphical Abstract

Similar content being viewed by others

Association of genetic polymorphisms in MIF with breast cancer risk in Chinese women
Association of tnfrsf1a and ifnlr1 gene polymorphisms with the risk of developing breast cancer and clinical pathologic features, the connection of polymorphisms–238a/g tnf and ile655val her2 influences the risk and molecular features of breast cancer.
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the utmost regularly diagnosed malignancy, with an assessed 2.3 million diagnoses and 685,000 fatalities from cancer worldwide in 2020 [ 1 ]. The BC mortality rates are considerably increasing in developing countries, including Egypt, which represents 10.8% of total cancer cases [ 2 ]. The incidence of BC has noticeably elevated, particularly amongst young females, and its global annual incidence is envisioned to reach 4.4 million in 2070 [ 3 ]. Diagnosing BC in women at earlier stages can noticeably enhance the survival rate and the BC prognosis is intensely complicated by late diagnosis [ 4 ].
Interestingly, several epidemiological studies revealed that patients enduring chronic inflammatory diseases have an amplified risk for cancer incidence. Besides, it is estimated that about 30% of cancer incidence in low- and middle-income nations is related to microbial infections [ 5 ]. With regard to the action of inflammatory mediators in endorsing oncogenesis and tumor progression, evidence is pointing towards a probable correlation amongst macrophage migration inhibitory factor (MIF) expression and tumorigenesis and cancer progression [ 6 ].
Macrophages, which have a fundamental function in immune response, were described as a fundamental contributor throughout the oncogenesis process by endorsing tumor cell proliferation, survival, and migration [ 6 , 7 ]. The MIF gene is located on chromosome 22q11.2 and is considered as a subcomponent of the transforming growth factor-β (TGF-β) superfamily. MIF is released by several cells comprising macrophages, granulocytes, T and B lymphocytes, endothelial cells, as well as cancer cells [ 8 ]. The MIF is regarded as a proinflammatory cytokine and contributes in the progress of oncogenesis by endorsing tumor development, altering immunological responses, enhancing inflammation, and aiding cancer-associated angiogenesis [ 6 , 7 ]. A study by Verjans et al. revealed that MIF protein has a dual role in BC development. Increased MIF protein expression inside the BC cells is beneficial and protective, while increased serum levels of MIF protein are prooncogenic [ 9 ].
Some studies reported that the MIF gene rs755622-G/C polymorphism is associated with the amplified solid tumor risk [ 10 ] and BC risk [ 11 , 12 ]. However, the study by Avalos‐Navarro et al. failed to find a notable association in the Mexican‐mestizo population [ 8 ]. On the other hand, the rs1049829 variant of MIF gene has been studied for a possible correlation with colorectal cancer risk, but no significant association was found [ 13 ]. However, no previous studies were performed to report its association with BC.
There is still no research focusing on the relationship between MIF gene polymorphism and BC susceptibility in Egyptian females. Thus, we investigated the serum MIF levels for the first time in Egyptian BC patients and the association between both MIF gene rs1049829 and rs755622 variants with BC susceptibility.
Patients and methods
Between December 2022 and March 2023, blood samples were drawn from 133 consecutive treatment-naïve primary BC female patients aged ≥ 18 years who presented to the Medical Oncology Department of the National Cancer Institute of Cairo University, and from 126 healthy females as a control group. Ages were closely matched as feasible between the patient and control groups; the median age of the former group was 50 years (range: 18–65). A definite diagnosis of BC was employed by clinical examination, mammography and confirmed by histopathology. Patients’ demographics and clinicopathological data are presented in Table 1 and Table 2 , respectively. Disease stage and tumor grade were based on the American Joint Committee on Cancer (AJCC) [ 14 ] and grading approach [ 15 ], respectively. We included any disease stage at presentation then patients were divided into two groups: curative stage (I, II, and III) and metastatic stage (IV). According to St. Gallen BC expert consensus [ 16 ] and owing to deficient Ki67% information in some BC cases, the remaining patients were classified using immunohistochemistry into classes: luminal A: (ER + and PR + , HER2−, and low-Ki67 index), Luminal B (HER2 negative): (ER + , HER2−, and at least one of high Ki67 or PR-/low), Luminal B (HER2 positive): (ER + , HER2 over-expressed or amplified, and any Ki67 or any PR), triple-negative BC (TNBC): (ER−, PR−, & HER2−), and HER2−enriched (ER−, PR− & HER2+) (Table 2 ). The existing prospective case–control study was conducted based on the ethical principles of the Helsinki Declaration and was approved by the National Cancer Institute’s Institutional Review Board (IRB), located at Cairo University (Cairo, Egypt), IRB approval no. (2212-5051-0443). In advance, the study protocol was explained to every subject in both written and conversational form. Also, control participants and BC patients signed written informed consents. Exclusion criteria included patients with autoimmune diseases, morbid obesity, tobacco users, or those who received immunomodulators. Healthy female controls were recruited from women whose annual physical examinations revealed no cancer.
After the final diagnosis, 6 mL of venous blood specimen was obtained from each subject. 3 mL was collected in a vacutainer tube containing EDTA and stored at −80 °C at Al-Azhar University’s Molecular Biology laboratory until the time of DNA extraction. The other 3 ml was collected in a gel vacutainer tube for serum isolation and used for the determination of serum MIF level.
Measurement of serum MIF levels
Serum MIF levels were quantified by a sandwich enzyme-linked immunosorbent assay (ELISA) utilizing a commercial human MIF ELISA kit supplied by Elabscience®, Texas, USA (Cat. No: E-EL-H1530) adhering to the producer’s instructions. The optical density was determined utilizing a microplate reader (Stat Fax® 2100, Awareness Technology, USA) set to 450 nm.
DNA extraction and genotyping
Utilizing the GeneJET™ DNA purification kit (Thermo scientific, USA), genomic DNA was isolated and purified from subjects’ EDTA whole blood. Aliquots of DNA were then kept at −80 °C until analysis. By measuring DNA’s optical density at 260 nm with a Thermo Scientific NanoDrop 2000 spectrophotometer (Wilmington, USA), the concentration of DNA was calculated. At 260/230 nm and 260/280 nm optical density ratios, the purity of DNA was evaluated. Genotyping of MIF single nucleotide polymorphisms (SNPs) rs755622 and rs1049829 was investigated utilizing a TaqMan® allelic discrimination analysis by design provided by Applied Biosystems, Foster City, USA. The manufacturer’s directions and commonly available probes and primers were applied to conduct this investigation employing an Applied Biosystems International Step One real-time PCR apparatus (Foster City, USA) in a volume for each reaction of 20 μL.
Sample size
Based on Avalos‐Navarro et al. study [ 8 ], a sample size of 108 subjects per group was calculated according to the minor allele frequencies of the MIF rs755622 and 1049829 to detect power of 80% and type 1 error = 0.05. Sample size estimation was performed by the PS statistical package.
Statistical analysis
All statistical assessment was conducted utilizing Graph pad prism (version 9, Inc, USA). The Kolmogorov–Smirnov and Shapiro–Wilk normality tests were applied. The medians and ranges were used to represent quantitative values. On the other hand, numbers ( n ) and percentages (%) were utilized to describe qualitative values. Concerning non-parametric data, the Kruskal–Wallis test was utilized to evaluate the differences across the various studied groups, and if necessary, the Dunn’s adjustment test was performed. Also, the Mann–Whitney U test was applied to evaluate the difference between the two studied groups. Utilizing the receiver operating characteristic (ROC) curve for evaluating the diagnostic accuracy of serum MIF levels, the optimal cut-off point, sensitivity, specificity, as well as area under the curve (AUC) were assessed. Regarding the qualitative data comparison and Hardy–Weinberg equilibrium (HWE) test and genetic association of MIF rs755622 and rs1049829 with risk to BC, the Chi-square test (χ 2 ) was employed. For the risk alleles, odds ratios (ORs) and 95% confidence interval (95% CI) were assessed. SHEsis software ( http://shesisplus.bio-x.cn/ ) was applied for haplotype analysis and investigating the interaction of the two MIF SNPs with the BC susceptibility. The criterion for statistical significance was established at p < 0.05. Our research adheres to Reporting recommendations for tumor marker prognostic studies (REMARK) standards [ 17 ]
The current study comprised 259 females (133 BC patients and 126 healthy controls). The age distributions of the BC patients and controls were matched. In addition, there was no notable difference in menopausal status between the 2 investigated groups ( Table 1 ) . Clinicopathologic features of BC cases are summarized in Table 2 .
The serum level of MIF was significantly increased in the BC cases in comparison to the control subjects ( p < 0.0001), with a diagnostic potential to discriminate BC with a sensitivity of 92.5% and a specificity of 73.7% at a cut-off value > 9.47 ng/mL ( Fig. 1 ) .

Serum MIF level (ng/mL) in BC cases and controls ( A ) and ROC curve for serum MIF as a biomarker of BC ( B ). *Statistically significant at P < 0.05. AUC area under the curve, SN sensitivity, SP specificity
Regarding the comparison of cancer grades and TNM stages in BC patients, there was no notable difference in serum MIF level that was observed. However, a significant increase was demonstrated in BC patients with various TNM stages I, II, III, and IV in contrast to control females with p values 0.0002, < 0.0001, < 0.0001, and < 0.0001, respectively ( Fig. 2 A ) . Also, a significant elevation in serum MIF level was revealed in BC patients with curative and metastatic stages in contrast to control females, with p values < 0.0001 and < 0.0001 respectively, while no notable difference in serum MIF levels was demonstrated between BC cases with curative and metastatic stages ( Fig. 2 B ) .

Serum MIF levels (ng/mL) in control subjects and BC cases with different stages ( A ) and those with curative and metastatic stages ( B ). a Significant different from control group at p < 0.05
Owing to the lack of Ki67 data in 48 BC cases, we were unable to classify those patients according to luminal subclassification; however, no marked difference in serum MIF level was observed between BC molecular subtypes in the rest of the cases ( P = 0.126). Besides, a notable significant difference in serum MIF level in PR-negative compared to PR-positive BC patients ( P = 0.046), while there was no notable difference observed regarding ER and HER2 expression. Moreover, the statistical findings showed that there was no significant change in serum MIF levels amongst BC patients with various pathological tumor stages ( p = 0.69) and nodal status ( p = 0.28) ( Table 2 ) .
Genotype distributions of the MIF gene rs1049829 and rs755622 variants were in accordance with Hardy–Weinberg equilibrium ( p > 0.05) as demonstrated in Tables 3 , 4 Interestingly, a significant association between the T allele of rs1049829 variant of MIF gene and the protective effect against BC (OR = 0.51, 95% CI = 0.34–0.77, p = 0.001). Besides, a notable association with a protective effect against BC was observed under the two codominant models (CT versus CC, OR = 0.46, 95% CI = 0.27–0.76 p = 0. 003, and TT versus CC, OR = 0.24, 95% CI = 0.06–0.85 p = 0. 03), the dominant model (OR = 0.43, 95% CI = 0.26–0.72, p = 0.001). Moreover, a significant association with the risk of BC was revealed only under the over-dominant model (OR = 1.95, 95% CI = 1.16–3.22, p = 0.008), while the recessive model showed no significant association with BC risk ( p = 0.10) (Table 3 ).
On the other hand, the statistical data showed no notable association amongst the rs755622 variant of MIF gene and the susceptibility of BC (p = 0.39 ) . Moreover, no significant association with the risk of BC was observed under the different genetic models used in this study which include the two codominant (GC versus GG, p = 0.45 and CC versus GG, p = 0.56), dominant ( p = 0.4 ) , recessive ( p = 0.61), and over-dominant ( p = 0.48 ) (Table 4 ).
To clarify the association amongst interactions of the two studied MIF SNPs and BC, we have carried out a haplotype analysis performed with in silico analysis by SHEsis software. As a result, the rs755622-G and rs1049829-T haplotypes showed a significant association with a protective impact against BC (OR = 0.51, 95% CI = 0.34–0.79, p = 0.002), while other haplotype frequencies showed no significant association with BC risk (Table 5 ).
In addition, we have investigated the association between serum MIF level and the two investigated MIF SNPs rs755622 and rs1049829 in BC patients, and no statistical association was observed with p values 0.1123 and 0.8455, respectively ( Fig. 3 ) .

Serum MIF levels in BC patients with different genotypes of rs1049829 C/T ( A ) and rs755622G/C ( B ). Statistically significant was established at P < 0.05
Breast cancer is the foremost determinant of cancer mortality in females [ 1 ]. Successful cancer treatment requires the development of early-stage cancer diagnostic techniques and the assessment of a patient’s risk of cancer progression and recurrence [ 18 ]. Several studies have informed that the existence of genetic factors, such as mutations and SNPs, increases the risk for the development and progression of BC [ 19 ], as well as those found in the proinflammatory cytokine MIF gene [ 11 ]. The contribution of the MIF alleles in the progression of cancer was correlated and reported in different types of cancers such as hepatocellular carcinoma [ 20 ], gastric cancer [ 21 ], BC [ 11 ], and prostate cancer [ 22 ]. This is the first study, to our knowledge, to assess the allelic and genotypic frequencies of (rs1049829) and (rs755622) polymorphisms of MIF gene in women with BC in the Egyptian population.
To the best of our knowledge, the association between the MIF gene rs1049829 and BC risk has not been previously studied. This study describes a significant correlation between the rs1049829 variant of the MIF gene and the protective effect against BC in women from the Egyptian population. Although a study by Ramireddy et al. found no notable correlation amongst this polymorphism and the susceptibility to colorectal cancer in the Taiwan population [ 13 ]. This discrepancy may be referred to differences in cancer type, sample size, age, and race.
Consistent with our findings, a study by Avalos‐Navarro et al. evaluated the MIF gene rs755622-G/C polymorphism in women with BC in the Mexican population and showed no significant correlation with BC risk [ 8 ]. Conversely, a study by Lin et al. showed a notable association between the rs755622 variant of the MIF gene and BC susceptibility in women of the Chinese population [ 11 ]. Further studies found a protective effect for this SNP against colorectal cancer [ 23 ] and bladder cancer risks [ 24 , 25 ]. These disagreements may be credited to the criteria for inclusion, sample size, and racial variances amongst nations. Also, some findings from earlier research should be interpreted cautiously because not all genetic models have been evaluated in these investigations.
In accordance with other reports [ 8 , 26 , 27 ], the serum MIF level in BC patients is markedly elevated than that in control subjects, suggesting that MIF has a marked role in endorsing oncogenesis. Furthermore, we demonstrated that serum MIF has the diagnostic potential to distinguish between BC patients and healthy controls at a cut-off value of > 9.47 ng/mL with a 92.5% sensitivity and 73.7% specificity. In harmony with our results, Ciftci et al., observed a significant difference in serum MIF level between BC cases and control group, with a mean level of 10.7 and 5.49 ng/mL, respectively. Also, they revealed a discriminating power of serum MIF level between BC patients and healthy individuals at a cut-off value of 1.1275 ng/ml with sensitivity and specificity of 97% and 71%, respectively [ 27 ]. The difference between our observed cut-off value and that of Ciftci et al. may be attributed to the difference in age of recruited subjects and sample size where Ciftci et al. recruited only 28 control subjects and 96 BC cases. Besides, Fersching et al. revealed a discriminating ability of MIF between BC patients and healthy individuals, and between metastatic BC patients and BC patients with locally confined, with AUC 70.7% and 87.6%, respectively [ 28 ]. Similarly, MIF was found to be a possible diagnostic marker for gastric cancer at a cut-off value of 3.23 ng/mL with 83.5% sensitivity, 92.3% specificity, and 89.7% accuracy [ 29 ]. While the difference observed in cut-off value may be attributed to the difference in cancer type, gender recruitment, and patient demographics. Moreover, Richard et al. revealed a marked increase in the expression level of MIF protein inside the BC tissue describing MIF as a marker to discriminate normal tissue from BC tissue by immunohistochemistry [ 30 ]. These findings might enhance the potentiality of serum MIF level as a diagnostic tumor marker for BC, point out MIF as a possible therapeutic target for pharmacological modulators, and indicate the evidence that cytokines are spawned by immune and tumor cells.
Interestingly, the primary source of MIF in tumors is the epithelial cells themselves, with a little secretory supply from stromal and inflammation-related cells, as well as other components of the tumor microenvironment. Notably, the cytokine MIF drives oncogenesis by supporting tumor growth, regulating immunological responses, boosting inflammation, and promoting tumor-associated angiogenesis [ 31 , 32 ]. Also, MIF enhances the cancerous microenvironment by inducing inflammation and releasing inflammatory mediators like tumor necrosis factor α, interleukin (IL)-1β, and IL-6 [ 33 ].
The present study hasn’t found a statistical significance in serum MIF levels between different grades and stages of BC. These results are endorsed by several studies that reported that neither MIF expression in BC tissue [ 30 , 34 ] nor serum MIF level [ 30 ] showed statistically significant differences between different tumor grades. However, results obtained by Avalos-Navarro et al. revealed a statistically significant elevation in serum MIF levels in BC cases with advanced stages compared to those with stage I [ 35 ]. This contrasting finding may be attributed to the difference in the sample size, patients’ inclusion criteria, and the utilized kit.
Furthermore, we depicted that elevated serum MIF levels were associated with PR negativity, this result comes in harmony with other reports that showed that the expression of the MIF gene is elevated in cells with negative hormonal receptors [ 9 , 35 , 36 ]. Such finding might signify that MIF is a probable marker for aggressive BC conditions characterized by poor prognosis, recurrence, metastasis, and lack of specific therapeutic targets [ 37 ], suggesting a potential use of anti-MIF agents, such as Imalumab which is still processed in clinical trials and showing promising antitumor activity in patients with advanced solid tumors. Imalumab increases apoptosis by suppressing MIF-induced phosphorylation of ERK1/2 and AKT. Imalumab also makes cancer cell lines more susceptible to cytotoxic medications [ 38 ]. Moreover, in clinical practice, serum MIF levels could be utilized to stratify patients based on the aggressiveness of their disease, potentially guiding treatment decisions. This stratification could inform the choice and intensity of therapeutic interventions.
In addition, we have evaluated the probable relationship amongst the functional variants of the MIF gene (rs755622 and rs1049829) with the serum levels of MIF protein in women with BC and showed no significant variation in serum MIF levels neither the BC patients with rs1049829 TT and CT versus CC nor rs755622 CC and GC versus GG. There are limitations in this study, Ki67 data were lacking for some BC patients which decreased the number of cases in the molecular subtyping; moreover, this case–control study did not clarify the underlying mechanisms beyond the effect of MIF polymorphisms on BC risk. Therefore, laboratory validation both in vivo and in vitro is envisioned to illuminate the detailed molecular mechanism, and prospective clinical validation with studies involving larger cohorts of breast cancer patients is required to confirm the prognostic value of serum MIF levels. These studies should aim to correlate serum MIF levels with clinical outcomes, including response to treatment and overall survival.
The findings of this research proposed that serum MIF level may be considered a helpful tumor marker of BC. Also, the rs1049829 variant of MIF gene is considered a protective candidate against BC whereas the rs755622 variant is not regarded as a genetic risk factor for BC amongst Egyptian patients. Moreover, the MIF gene haplotype (rs1049829-T and rs755622-G) showed a significant association with a protective effect against BC.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
American joint committee on cancer
Area under the curve
- Breast cancer
Enzyme-linked immunosorbent assay
Estrogen receptor
Human epidermal growth factor receptor 2
Hardy–Weinberg equilibrium
Institutional Review Board
Invasive ductal carcinoma;
Invasive lobular carcinoma
Macrophage migration inhibitory factor
Pathological node
Progesterone receptor
Pathological tumor
Receiver operating characteristic
Single nucleotide polymorphisms
Transforming growth factor-β
Triple negative breast cancer
Tumor necrosis factor alpha
Tumor-node-metastasis
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. https://doi.org/10.3322/caac.21660
Article PubMed Google Scholar
Cancer Egypt 2020 country profile [ https://www.who.int/publications/m/item/cancer-egy-2020 ]
Soerjomataram I, Bray F (2021) Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol 18(10):663–672. https://doi.org/10.1038/s41571-021-00514-z
Brandt J, Garne JP, Tengrup I, Manjer J (2015) Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol 13:1–11
Article CAS Google Scholar
World Health organization’s cancer newsroom [ https://www.who.int/news-room/fact-sheets/detail/cancer ]
Grieb G, Merk M, Bernhagen J, Bucala R (2010) Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect 23(4):257
Article CAS PubMed PubMed Central Google Scholar
Richard V, Kindt N, Saussez S (2015) Macrophage migration inhibitory factor involvement in breast cancer. Int J Oncol 47(5):1627–1633
Avalos-Navarro G, Del Toro-Arreola A, Daneri-Navarro A, Quintero-Ramos A, Bautista-Herrera LA, Franco Topete RA, Anaya Macias BU, Javalera Castro DI (2020) Morán-Mendoza AdJ, Oceguera-Villanueva A: association of the genetic variants (−794 CATT5-8 and −173 G> C) of macrophage migration inhibitory factor (MIF) with higher soluble levels of MIF and TNFα in women with breast cancer. J Clin Lab Anal 34(5):e23209. https://doi.org/10.1002/jcla.23209
Verjans E, Noetzel E, Bektas N, Schütz AK, Lue H, Lennartz B, Hartmann A, Dahl E, Bernhagen J (2009) Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer 9:1–18. https://doi.org/10.1186/1471-2407-9-230
Vera PL, Meyer-Siegler KL (2011) Association between macrophage migration inhibitory factor promoter region polymorphism (−173 G/C) and cancer: a meta-analysis. BMC Res Notes 4:1–5
Article Google Scholar
Lin S, Wang M, Liu X, Zhu W, Guo Y, Dai Z, Yang P, Tian T, Dai C, Zheng Y (2017) Association of genetic polymorphisms in MIF with breast cancer risk in Chinese women. Clin Exp Med 17:395–401
Article CAS PubMed Google Scholar
Pehlivan S, Işiksaçan N, Pehlivan M, Günaldi M, Oyaci Y, Nursal AF (2020) The MIF rs755622 variant may increase susceptibility of breast cancer but not gastrointestinal cancer in a turkish population. Turk J Oncol. https://doi.org/10.5505/tjo.2020.2186
Ramireddy L, Chen WTL, Peng CT, Hu RM, Ke TW, Chiang HC, Chang SC, Tsai FJ, Lo WY (2015) Association between genetic polymorphism of the MIF gene and colorectal cancer in Taiwan. J Clin Lab Anal 29(4):268–274
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. https://doi.org/10.3322/caac.21388
Article PubMed PubMed Central Google Scholar
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer I the value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathol. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x
Goldhirsch A, Winer EP, Coates A, Gelber R, Piccart-Gebhart M, Thürlimann B, Senn H-J, Albain KS, André F, Bergh J (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24(9):2206–2223
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Statistics subcommittee of the NCIEWGoCD, reporting recommendations for tumor marker prognostic studies. J Clin Oncol. https://doi.org/10.1200/JCO.2004.01.0454
Hartwell L, Mankoff D, Paulovich A, Ramsey S, Swisher E (2006) Cancer biomarkers: a systems approach. Nat Biotechnol 24(8):905–908. https://doi.org/10.1038/nbt0806-905
Dite GS, Mahmoodi M, Bickerstaffe A, Hammet F, Macinnis RJ, Tsimiklis H, Dowty JG, Apicella C, Phillips K-A, Giles GG (2013) Using SNP genotypes to improve the discrimination of a simple breast cancer risk prediction model. Breast Cancer Res Treat 139:887–896
Yuan T, Tang C, Chen M, Deng S, Chen P (2013) Influence of the human MIF promoter polymorphism on hepatocellular carcinoma prognosis. Genet Mol Res 12(4):6629–6635
Ni P, Wang G, Wang Y, Liu K, Chen W, Xiao J, Fan H, Ma X, Li Z, Shen K (2022) Correlation of MIF-AS1 polymorphisms with the risk and prognosis of gastric cancer. Pathol Res Pract 233:153850
Meyer-Siegler K, Vera P, Iczkowski K, Bifulco C, Lee A, Gregersen P, Leng L, Bucala R (2007) Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun 8(8):646–652
Moundir C, Chehab F, Senhaji N, Boufettal R, Idouz K, Erguibi D, Nadifi S (2019) Association of the IL-17A rs2275913 and MIF rs755622 polymorphisms with the risk of gastric and colorectal cancer. Meta Gene 22:100605. https://doi.org/10.1016/j.mgene.2019.100605
AlChalabi R, Mahdi S, Fadhil A, Jawad H (2016) Polymorphism in the promoter region of MIF and risk of bladder cancer in Iraqi patients. Int J Sci Basic Appl Res (IJSBAR) 24:84–93
Google Scholar
Yuan Q, Wang M, Wang M, Zhang Z, Zhang W (2012) Macrophage migration inhibitory factor gene-173G> C polymorphism and risk of bladder cancer in southeast China: a case–control analysis. Mol Biol Rep 39:3109–3115. https://doi.org/10.1007/s11033-011-1075-9
Bando H, Matsumoto G, Bando M, Muta M, Ogawa T, Funata N, Nishihira J, Koike M, Toi M (2002) Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res 93(4):389–396
Ciftci R, Tas F, Aksit E, Vatansever S, Karabulut S, Sen F, Yildiz I, Keskin S, Bozbey HU, Kilic L (2014) Clinical significance of serum macrophage migration inhibitory factor (MIF) level in breast cancer. In Am Soc Clin Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.e11556
Fersching DM, Nagel D, Siegele B, Salat C, Heinemann V, Holdenrieder S, Stoetzer OJ (2012) Apoptosis-related biomarkers sFAS, MIF, ICAM-1 and PAI-1 in serum of breast cancer patients undergoing neoadjuvant chemotherapy. Anticancer Res 32(5):2047–2058
CAS PubMed Google Scholar
Xia HHX, Yang Y, Chu KM, Gu Q, Zhang YY, He H, Wong WM, Leung SY, Yuen ST, Yuen MF (2009) Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer. Cancer 115(23):5441–5449
Richard V, Kindt N, Decaestecker C, Gabius HJ, Laurent G, Noel J-C, Saussez S (2014) Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol Rep 32(2):523–529
Klemke L, De Oliveira T, Witt D, Winkler N, Bohnenberger H, Bucala R, Conradi L-C, Schulz-Heddergott R (2021) Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis 12(2):155
Noe JT, Mitchell RA (2020) MIF-dependent control of tumor immunity. Front Immunol 11:609948. https://doi.org/10.3389/fimmu.2020.609948
Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J (2015) The role of cytokines in breast cancer development and progression. J Interf Cytokine Res 35(1):1–16. https://doi.org/10.1089/jir.2014.0026
Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S (2008) Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett 261(2):147–157
Avalos-Navarro G, Muñoz-Valle JF, Daneri-Navarro A, Quintero-Ramos A, Franco-Topete RA, Morán-Mendoza AdJ, Oceguera-Villanueva A, Bautista-Herrera LA, Topete-Camacho A, Del Toro-Arreola A (2019) Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes: relationship with Th17 cytokine profile. Clin Exp Med 19(385):391
Charan M, Das S, Mishra S, Chatterjee N, Varikuti S, Kaul K, Misri S, Ahirwar DK, Satoskar AR, Ganju RK (2020) Macrophage migration inhibitory factor inhibition as a novel therapeutic approach against triple-negative breast cancer. Cell Death Dis 11(9):774
Cui X, Schiff R, Arpino G, Osborne CK, Lee AV (2005) Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23(30):7721–7735
Mahalingam D, Patel MR, Sachdev JC, Hart LL, Halama N, Ramanathan RK, Sarantopoulos J, Völkel D, Youssef A, de Jong FA (2020) Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br J Clin Pharmacol 86(9):1836–1848
Download references
Acknowledgements
We are deeply grateful to the Al-Azhar University in Cairo, Faculty of Pharmacy’s Biochemistry and Molecular Biology Department for supplying the lab tools and collaborating.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Biochemistry and Molecular Biology Department, Faculty of Pharmacy, Al-Azhar University, Nasr City, Cairo, 11231, Egypt
Mahmoud A. Seliem, Ahmed M. Mohamadin & Ahmed A. El-Husseiny
Department of Biochemistry, Faculty of Pharmacy, Ahram Canadian University, 6th of October City, Giza, Egypt
Mahmoud A. Seliem
Department of Biochemistry and Molecular Biology, Faculty of Pharmacy, Helwan University, Helwan, Cairo, 11790, Egypt
Mohamed I. Kotb El-Sayed
Medical Oncology Department, National Cancer Institute, Cairo University, Cairo, 11796, Egypt
Yahia Ismail
Department of Biochemistry, Faculty of Pharmacy, Egyptian Russian University, Badr City, Cairo, 11829, Egypt
Ahmed A. El-Husseiny
You can also search for this author in PubMed Google Scholar
Contributions
A.M.M., A.A.E., M.I.K.E, and M.A.S. contributed to the study’s conception and design. YI was responsible for patients’ recruitment, diagnosis, and clinicopathologic data collection. A.M.M., A.A.E., M.I.K.E, YI, and M.A.S. contributed to methodology and manuscript writing. A.A.E. and M.A.S. contributed to statistical analysis and investigation. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Ahmed A. El-Husseiny .
Ethics declarations
Conflict of interest.
The authors declare they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the ethical principles of the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of the National Cancer Institute, Cairo University, Cairo, Egypt, with IRB approval number (2212–5051-0443).
Consent to participate
The signed written informed consent of both controls and cases was collected in advance.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Seliem, M.A., Mohamadin, A.M., El-Sayed, M.I.K. et al. The clinical signature of genetic variants and serum levels of macrophage migration inhibitory factor in Egyptian breast cancer patients. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07393-9
Download citation
Received : 23 November 2023
Accepted : 30 May 2024
Published : 25 June 2024
DOI : https://doi.org/10.1007/s10549-024-07393-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Polymorphism
- Tumor marker
- Find a journal
- Publish with us
- Track your research
How IBM helps Wimbledon use generative AI to drive personalised fan engagement
This collaboration with Wimbledon teams extends beyond the fan-facing digital platform, into enterprise-wide transformation.
Re-evaluating data management in the generative AI age
4 min read - A good place to start is refreshing the way organizations govern data, particularly as it pertains to its usage in generative AI solutions.
Top 7 risks to your identity security posture
5 min read - Identity misconfigurations and blind spots stand out as critical concerns that undermine an organization’s identity security posture.
Intesa Sanpaolo and IBM secure digital transactions with fully homomorphic encryption
6 min read - Explore how European bank Intesa Sanpaolo and IBM partnered to deliver secure digital transactions using fully homomorphic encryption.
What is AI risk management?
8 min read - AI risk management is the process of identifying, mitigating and addressing the potential risks associated with AI technologies.
June 27, 2024
IBM announces new AI assistant and feature innovations at Think 2024
June 26, 2024
A major upgrade to Db2® Warehouse on IBM Cloud®
June 25, 2024
Increase efficiency in asset lifecycle management with Maximo Application Suite’s new AI-power...
Achieving operational efficiency through Instana’s Intelligent Remediation
June 24, 2024
Manage the routing of your observability log and event data
Best practices for augmenting human intelligence with AI
2 min read - Enabling participation in the AI-driven economy to be underpinned by fairness, transparency, explainability, robustness and privacy.
Microcontrollers vs. microprocessors: What’s the difference?
6 min read - Microcontroller units (MCUs) and microprocessor units (MPUs) are two kinds of integrated circuits that, while similar in certain ways, are very different in many others.
Mastering budget control in the age of AI: Leveraging on-premises and cloud XaaS for success
2 min read - As organizations harness the power of AI while controlling costs, leveraging anything as a service (XaaS) models emerges as strategic.
Highlights by topic
Use IBM Watsonx’s AI or build your own machine learning models
Automate IT infrastructure management
Cloud-native software to secure resources and simplify compliance
Run code on real quantum systems using a full-stack SDK
Aggregate and analyze large datasets
Store, query and analyze structured data
Manage infrastructure, environments and deployments
Run workloads on hybrid cloud infrastructure
Responsible AI can revolutionize tax agencies to improve citizen services
Generative AI can revolutionize tax administration and drive toward a more personalized and ethical future.
How IBM and AWS are partnering to deliver the promise of responsible AI
4 min read - This partnership between IBM and Amazon SageMaker is poised to play a pivotal role in shaping responsible AI practices across industries
Speed, scale and trustworthy AI on IBM Z with Machine Learning for IBM z/OS v3.2
4 min read - Machine Learning for IBM® z/OS® is an AI platform made for IBM z/OS environments, combining data and transaction gravity with AI infusion.
The recipe for RAG: How cloud services enable generative AI outcomes across industries
4 min read - While the AI is the key component of the RAG framework, other “ingredients” such as PaaS solutions are integral to the mix
Rethink IT spend in the age of generative AI
3 min read - It's critical for organizations to consider frameworks like FinOps and TBM for visibility and accountability of all tech expenditure.
6 hard truths CEOs must confront in the generative AI era
5 min read - A call to action for CEOs to confront the realities of generative AI and to seize its potential for your organization.
Immutable backup strategies with cloud storage
4 min read - IBM Cloud Object Storage is a versatile and scalable solution that is crucial for storing and protecting data backups.
IBM Newsletters

IMAGES
VIDEO
COMMENTS
A Neoadjuvant Chemotherapy Trial for Early Breast Cancer is Impacted by COVID-19: Addressing Vaccination and Cancer Trials Through Education, Equity, and Outcomes, Clinical Cancer Research, 27, 16 ...
Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation, the patient presented to a hospital affiliated with ...
Melanoma is an aggressive disease that accounts for approximately 75% of skin cancer-related deaths. 1 The World Health Organization reports that the incidence of metastatic melanoma has increased over the past 3 decades—estimating 66 000 deaths worldwide every year are due to skin cancer, with approximately 80% due to melanoma. Most cases of malignant melanoma are diagnosed at an early ...
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
Current Problems in Cancer seeks to promote and disseminate innovative, transformative, and impactful data on patient-oriented cancer research and clinical care. Specifically, the journal's scope is focused on reporting the results of well-designed cancer studies that influence/alter practice or … View full aims & scope
Presentation of Case. Dr. Christopher T. Chen: A 34-year-old woman was evaluated in the oncology clinic of this hospital for the management of relapsed, metastatic intrahepatic cholangiocarcinoma ...
Interaction between capecitabine and brivudin in a patient with breast cancer. This Case Study describes a patient with breast cancer who was treated with capecitabine and experienced a severe ...
Introduction. Cervical cancer is one of the major causes of cancer-related death in women worldwide [].It is the fourth most prevalent cause of malignancy in women after breast, colorectal, and lung cancer [].]. The incidence of cervical cancer and mortality rate has declined by 70% in the United States since the 1950s because of age-appropriate screening [].
This review presents five case studies illustrating various successful approaches to addressing such challenges. These efforts are CancerLinQ, the American Association for Cancer Research Project GENIE, Project Data Sphere, the National Cancer Institute Genomic Data Commons, and the Veterans Health Administration Clinical Data Initiative.
Clinical Research. Clinical research involves the study of cancer in people. These cancer research studies are further broken down into two types: clinical trials and observational studies. Clinical trials are research studies that involve an intervention, which is a treatment or change that may affect the results of cancer.These can lead to new treatments, care, and improved results for ...
Background. Worldwide, male breast cancer is extremely rare, accounting for <1% of all breast tumors and <1% of all malignancies in men [1-3].Recently, the incidence of male breast cancer has increased from 1.0 per 100,000 men in the late 1970s to 1.2 per 100,000 men from 2000 to 2004 [4-7].The American Cancer Society reported a similar trend in the incidence of breast cancer in men from ...
Abstract. This paper presents an atypical case of a patient with brain tumor of the glioblastoma multiforme (GBM) type who achieved a 5-year survival. Some general information is provided including epidemiology, diagnostic and treatment procedures (surgery and radio-chemo-therapy), and prognosis of survival related to GBM.
AACR's latest report highlights disparities in cancer incidence and mortality according to race and ethnicity, sexuality and gender identity, and residence. At ASCO 2024, researchers reported ...
Case studies of major research programmes. We fund a number of major research programmes and collaborations led by our world-leading senior cancer researchers. These case studies illustrate the kinds of programmes that we fund to help deliver our ambitious research strategy and vision of 3 in 4 people with cancer surviving their disease by 2034.
A case-control study compares two groups of people: those with the cancer under study (cases) and those who do not have the cancer (controls). Researchers compare the genetic, environmental, lifestyle, and medical histories of the people in the two groups to identify factors associated with cancer. Selected examples of DCEG case-control studies:
The first case study focuses on evaluation of research evidence for implementation. Additional case studies are under development. Available materials for each case include Instructor's guide indicating competencies addressed, and complete instructions; Student's guide with instruction and activity worksheet
Successful applicant case studies. Hear from a selection of our researchers about their research, their career paths, and their experience of applying for funding and of being a part of the CRUK research community. In addition to discovering more about the breadth of research that we fund across cancer research fields, and the depth of support ...
Case 17-2015 — A 44-Year ... For cancer-related pain that is unresponsive to ... JC, Yamamoto, N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung ...
Case study 4 - Dr Rajesh Jena. Personalising radiotherapy - how patient data is helping reduce the side effects of treatment. Radiotherapy is the gold standard of treatment for many types of cancers. In fact, more than 130,000 patients benefit from radiotherapy every year in the UK.
Research Design*. It is only since the 1950s that most of the epidemiology studies on cancer have been conducted. The principal study designs for epidemiologic study of cancer etiology are case-control and cohort studies. These study designs have complimentary roles and distinct advantages and disadvantages. This cha ….
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
This can be the case with brain metastases and glioblastoma or other primary brain cancers, ... a population-based study. Cancer Epidemiol. 63, ... Cancer Research UK Lung Centre of Excellence ...
Case study: Diagnosed with cancer while 29 weeks pregnant. ... I think research focusing the complexities of cancer treatment for females of childbearing age with specific cancers is important to ...
Cancer is a disease that transcends what is purely medical, profoundly affecting the day-to-day life of both patients and family members. Previous research has shown that the consequences of cancer are greatly aggravated in patients at the end of life, at a time when they must also grapple with numerous unmet needs. The main objective of this study was to obtain more in-depth insight into ...
This case-study describes the elements of a first touch communication approach, introduced by Liverpool University Hospitals NHS Foundation Trust ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer. A scoping review was performed according to an a priori protocol ...
Methods. A case study approach was used because it allowed us to examine the complexity of cancer management from the perspective of one person's case as interpreted by multiple people, retaining its holistic and meaningful characteristics while being studied 17 answering how and why questions. 18 Interviews from four participants presented multiple perspectives of the same interested topic ...
Study reveals new opportunities to develop cancer treatments Date: June 24, 2024 Source: Baylor College of Medicine Summary: Researchers uncovered new potential therapeutic targets for cancer and ...
Purpose Macrophage migration inhibitory factor (MIF) is an integral cytokine for the modulation of both innate and adaptive immunity and is involved in the pathogenesis of various cancers. However, conflicting findings on the relationship between MIF polymorphisms and breast cancer (BC) have been reported in earlier research. We investigated the clinical value of serum MIF levels and the ...
News and thought leadership from IBM on business topics including AI, cloud, sustainability and digital transformation.