- Search by keyword
- Search by citation
Page 1 of 33

Extracellular vesicle therapy in neurological disorders
Extracellular vesicles (EVs) are vital for cell-to-cell communication, transferring proteins, lipids, and nucleic acids in various physiological and pathological processes. They play crucial roles in immune mo...
- View Full Text

Machine learning enabled classification of lung cancer cell lines co-cultured with fibroblasts with lightweight convolutional neural network for initial diagnosis
Identification of lung cancer subtypes is critical for successful treatment in patients, especially those in advanced stages. Many advanced and personal treatments require knowledge of specific mutations, as w...
Beyond glycan barriers: non-cognate ligands and protein mimicry approaches to elicit broadly neutralizing antibodies for HIV-1
Human immunodeficiency virus type 1 (HIV-1) vaccine immunogens capable of inducing broadly neutralizing antibodies (bNAbs) remain obscure. HIV-1 evades immune responses through enormous diversity and hides its...

Plexin C1 influences immune response to intracellular LPS and survival in murine sepsis
Intracellular sensing of lipopolysaccharide (LPS) is essential for the immune response against gram-negative bacteria and results in activation of caspase-11 and pyroptotic cell death with fatal consequences i...
EpCAM-targeted betulinic acid analogue nanotherapy improves therapeutic efficacy and induces anti-tumorigenic immune response in colorectal cancer tumor microenvironment
Betulinic acid (BA) has been well investigated for its antiproliferative and mitochondrial pathway-mediated apoptosis-inducing effects on various cancers. However, its poor solubility and off-target activity h...

Low-level HIV-1 viremia affects T-cell activation and senescence in long-term treated adults in the INSTI era
Around 10% of people with HIV (PWH) exhibit a low-level viremia (LLV) under antiretroviral therapy (ART). However, its origin and clinical significance are largely unknown, particularly at viremias between 50 ...
Gene therapy for ultrarare diseases: a geneticist’s perspective
Gene therapy has made considerable strides in recent years. More than 4000 protein-coding genes have been implicated in more than 6000 genetic diseases; next-generation sequencing has dramatically revolutioniz...
Targeted nanotherapeutics for the treatment of Helicobacter pylori infection
Helicobacter pylori infection is involved in gastric diseases such as peptic ulcer and adenocarcinoma. Approved antibiotherapies still fail in 10 to 40% of the infected patients and, in this scenario, targeted na...
Variants of human DECTIN-1 rs16910526 are linked to differential reactive oxygen species production and susceptibility to tuberculosis
Dectin-1 is a transmembrane receptor that plays a pivotal role in recognising fungi and Mycobacterium tuberculosis (Mtb) . A specific variant, DECTIN-1 rs16910526, results in a truncated receptor that disrupts mem...

Retraction Note: Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of Serenoa repens , selenium and lycopene
Development of novel antimicrobials with engineered endolysin lysecd7-smap to combat gram-negative bacterial infections.
Among the non-traditional antibacterial agents in development, only a few targets critical Gram-negative bacteria such as carbapenem-resistant Pseudomonas aeruginosa , Acinetobacter baumannii or cephalosporin-resi...
BUB1B monoallelic germline variants contribute to prostate cancer predisposition by triggering chromosomal instability
Prostate cancer (PrCa) is the most frequently diagnosed cancer in men. Variants in known moderate- to high-penetrance genes explain less than 5% of the cases arising at early-onset (< 56 years) and/or with fam...
Enteroviruses: epidemic potential, challenges and opportunities with vaccines
Enteroviruses (EVs) are the most prevalent viruses in humans. EVs can cause a range of acute symptoms, from mild common colds to severe systemic infections such as meningitis, myocarditis, and flaccid paralysi...
Intracellular domain of epithelial cell adhesion molecule induces Wnt receptor transcription to promote colorectal cancer progression
Epithelial cell adhesion molecule (EpCAM) has been widely studied as a tumor antigen due to its expression in varieties of solid tumors. Moreover, the glycoprotein contributes to critical cancer-associated cel...
Unraveling the differential mechanisms of revascularization promoted by MSCs & ECFCs from adipose tissue or umbilical cord in a murine model of critical limb-threatening ischemia
Critical limb-threatening ischemia (CLTI) constitutes the most severe manifestation of peripheral artery disease, usually induced by atherosclerosis. CLTI patients suffer from high risk of amputation of the lo...
A glimpse into viral warfare: decoding the intriguing role of highly pathogenic coronavirus proteins in apoptosis regulation
Coronaviruses employ various strategies for survival, among which the activation of endogenous or exogenous apoptosis stands out, with viral proteins playing a pivotal role. Notably, highly pathogenic coronavi...
CPEB2-activated axonal translation of VGLUT2 mRNA promotes glutamatergic transmission and presynaptic plasticity
Local translation at synapses is important for rapidly remodeling the synaptic proteome to sustain long-term plasticity and memory. While the regulatory mechanisms underlying memory-associated local translatio...
Dual inhibition of SUMOylation and MEK conquers MYC-expressing KRAS -mutant cancers by accumulating DNA damage
KRAS mutations frequently occur in cancers, particularly pancreatic ductal adenocarcinoma, colorectal cancer, and non-small cell lung cancer. Although KRAS G12C inhibitors have recently been approved, effective pr...
Exosomes: a review of biologic function, diagnostic and targeted therapy applications, and clinical trials
Exosomes are extracellular vesicles generated by all cells and they carry nucleic acids, proteins, lipids, and metabolites. They mediate the exchange of substances between cells,thereby affecting biological pr...
Cholestasis-induced phenotypic transformation of neutrophils contributes to immune escape of colorectal cancer liver metastasis
Cholestasis is a common yet severe complication that occurs during the advancement of liver metastasis. However, how cholestasis impacts the development, treatment, and tumor microenvironment (TME) of liver me...

Enterovirus-A71 exploits RAB11 to recruit chaperones for virus morphogenesis
Enterovirus 71 (EV-A71) causes Hand, Foot and Mouth Disease (HFMD) in children and has been associated with neurological complications. The molecular mechanisms involved in EV-A71 pathogenesis have remained el...
The double whammy of ER-retention and dominant-negative effects in numerous autosomal dominant diseases: significance in disease mechanisms and therapy
The endoplasmic reticulum (ER) employs stringent quality control mechanisms to ensure the integrity of protein folding, allowing only properly folded, processed and assembled proteins to exit the ER and reach ...
Beyond traditional translation: ncRNA derived peptides as modulators of tumor behaviors
Within the intricate tapestry of molecular research, noncoding RNAs (ncRNAs) were historically overshadowed by a pervasive presumption of their inability to encode proteins or peptides. However, groundbreaking...
Adipocyte pyroptosis occurs in omental tumor microenvironment and is associated with chemoresistance of ovarian cancer
Ovarian carcinoma (OC) is a fatal malignancy, with most patients experiencing recurrence and resistance to chemotherapy. In contrast to hematogenous metastasizing tumors, ovarian cancer cells disseminate withi...
Correction: Excess glucose alone depress young mesenchymal stromal/stem cell osteogenesis and mitochondria activity within hours/days via NAD + / SIRT1 axis
The original article was published in Journal of Biomedical Science 2024 31 :49
The glycosylation deficiency of flavivirus NS1 attenuates virus replication through interfering with the formation of viral replication compartments
Flavivirus is a challenge all over the world. The replication of flavivirus takes place within membranous replication compartments (RCs) derived from endoplasmic reticulum (ER). Flavivirus NS1 proteins have be...
Osteosarcoma in a ceRNET perspective
Osteosarcoma (OS) is the most prevalent and fatal type of bone tumor. It is characterized by great heterogeneity of genomic aberrations, mutated genes, and cell types contribution, making therapy and patients ...
Immunoglobulin and T cell receptor repertoire changes induced by a prototype vaccine against Chagas disease in naïve rhesus macaques
A vaccine against Trypanosoma cruzi , the agent of Chagas disease, would be an excellent additional tool for disease control. A recombinant vaccine based on Tc24 and TSA1 parasite antigens was found to be safe and...
Revolution in sepsis: a symptoms-based to a systems-based approach?
Severe infection and sepsis are medical emergencies. High morbidity and mortality are linked to CNS dysfunction, excessive inflammation, immune compromise, coagulopathy and multiple organ dysfunction. Males ap...
Development of a highly effective combination monoclonal antibody therapy against Herpes simplex virus
Infections with Herpes simplex virus (HSV)-1 or -2 usually present as mild chronic recurrent disease, however in rare cases can result in life-threatening conditions with a large spectrum of pathology. Monoclo...
USP9X-mediated REV1 deubiquitination promotes lung cancer radioresistance via the action of REV1 as a Rad18 molecular scaffold for cystathionine γ-lyase
Radioresistance is a key clinical constraint on the efficacy of radiotherapy in lung cancer patients. REV1 DNA directed polymerase (REV1) plays an important role in repairing DNA damage and maintaining genomic...
Tumor necrosis factor-inducible gene 6 protein and its derived peptide ameliorate liver fibrosis by repressing CD44 activation in mice with alcohol-related liver disease
Alcohol-related liver disease (ALD) is a major health concern worldwide, but effective therapeutics for ALD are still lacking. Tumor necrosis factor-inducible gene 6 protein (TSG-6), a cytokine released from m...
Characterization of the genetic variation and evolutionary divergence of the CLEC18 family
The C-type lectin family 18 (CLEC18) with lipid and glycan binding capabilities is important to metabolic regulation and innate immune responses against viral infection. However, human CLEC18 comprises three p...
Pivotal functions and impact of long con-coding RNAs on cellular processes and genome integrity
Recent advances in uncovering the mysteries of the human genome suggest that long non-coding RNAs (lncRNAs) are important regulatory components. Although lncRNAs are known to affect gene transcription, their m...
Somatic PDGFRB activating variants promote smooth muscle cell phenotype modulation in intracranial fusiform aneurysm
The fusiform aneurysm is a nonsaccular dilatation affecting the entire vessel wall over a short distance. Although PDGFRB somatic variants have been identified in fusiform intracranial aneurysms, the molecular...
A G-quadruplex-binding platinum complex induces cancer mitochondrial dysfunction through dual-targeting mitochondrial and nuclear G4 enriched genome
G-quadruplex DNA (G4) is a non-canonical structure forming in guanine-rich regions, which play a vital role in cancer biology and are now being acknowledged in both nuclear and mitochondrial (mt) genome. Howev...

Excess glucose alone depress young mesenchymal stromal/stem cell osteogenesis and mitochondria activity within hours/days via NAD + /SIRT1 axis
The impact of global overconsumption of simple sugars on bone health, which peaks in adolescence/early adulthood and correlates with osteoporosis (OP) and fracture risk decades, is unclear. Mesenchymal stromal...
The Correction to this article has been published in Journal of Biomedical Science 2024 31 :61
Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction
Retinal degenerative diseases, including diabetic retinopathy (DR) and age-related macular degeneration (AMD), loom as threats to vision, causing detrimental effects on the structure and function of the retina...
Exploiting urine-derived induced pluripotent stem cells for advancing precision medicine in cell therapy, disease modeling, and drug testing
The field of regenerative medicine has witnessed remarkable advancements with the emergence of induced pluripotent stem cells (iPSCs) derived from a variety of sources. Among these, urine-derived induced pluri...
Targeting cathepsin S promotes activation of OLF1-BDNF/TrkB axis to enhance cognitive function
Cathepsin S (CTSS) is a cysteine protease that played diverse roles in immunity, tumor metastasis, aging and other pathological alterations. At the cellular level, increased CTSS levels have been associated wi...
Campylobacter jejuni virulence factors: update on emerging issues and trends
Campylobacter jejuni is a very common cause of gastroenteritis, and is frequently transmitted to humans through contaminated food products or water. Importantly, C. jejuni infections have a range of short- and l...
Membrane lipid remodeling eradicates Helicobacter pylori by manipulating the cholesteryl 6'-acylglucoside biosynthesis
Helicobacter pylori , the main cause of various gastric diseases, infects approximately half of the human population. This pathogen is auxotrophic for cholesterol which it converts to various cholesteryl α-glucosi...

Dengue virus pathogenesis and host molecular machineries
Dengue viruses (DENV) are positive-stranded RNA viruses belonging to the Flaviviridae family. DENV is the causative agent of dengue, the most rapidly spreading viral disease transmitted by mosquitoes. Each yea...
Targeting NLRP3 signaling reduces myocarditis-induced arrhythmogenesis and cardiac remodeling
Myocarditis substantially increases the risk of ventricular arrhythmia. Approximately 30% of all ventricular arrhythmia cases in patients with myocarditis originate from the right ventricular outflow tract (RV...
T cell expressions of aberrant gene signatures and Co-inhibitory receptors (Co-IRs) as predictors of renal damage and lupus disease activity
Systemic lupus erythematosus (SLE) is distinguished by an extensive range of clinical heterogeneity with unpredictable disease flares and organ damage. This research investigates the potential of aberrant sign...
Applications of peptides in nanosystems for diagnosing and managing bacterial sepsis
Sepsis represents a critical medical condition stemming from an imbalanced host immune response to infections, which is linked to a significant burden of disease. Despite substantial efforts in laboratory and ...

Enhancement of NETosis by ACE2-cross-reactive anti-SARS-CoV-2 RBD antibodies in patients with COVID-19
High levels of neutrophil extracellular trap (NET) formation or NETosis and autoantibodies are related to poor prognosis and disease severity of COVID-19 patients. Human angiotensin-converting enzyme 2 (ACE2) ...
Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice
Mitochondria are essential organelles involved in cellular energy production. Changes in mitochondrial function can lead to dysfunction and cell death in aging and age-related disorders. Recent research sugges...

Longitudinal alterations in brain perfusion and vascular reactivity in the zQ175DN mouse model of Huntington’s disease
Huntington’s disease (HD) is marked by a CAG-repeat expansion in the huntingtin gene that causes neuronal dysfunction and loss, affecting mainly the striatum and the cortex. Alterations in the neurovascular co...
Antimicrobial peptide thanatin fused endolysin PA90 (Tha-PA90) for the control of Acinetobacter baumannii infection in mouse model
This study addresses the urgent need for infection control agents driven by the rise of drug-resistant pathogens such as Acinetobacter baumannii . Our primary aim was to develop and assess a novel endolysin, Tha-P...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal

Journal of Biomedical Science is supported by the National Science and Technology Council (NSTC) , Taiwan.
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 9.0 5-year Journal Impact Factor: 10.7 Source Normalized Impact per Paper (SNIP): 2.014 SCImago Journal Rank (SJR): 2.606
Speed 2023 Submission to first editorial decision (median days): 14 Submission to acceptance (median days): 107
Usage 2023 Downloads: 1,698,723 Altmetric mentions: 3,813
- More about our metrics
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 September 2023
A systematic review of retractions in biomedical research publications: reasons for retractions and their citations in Indian affiliations
- Pratibha Sharma 1 nAff4 ,
- Bhavya Sharma 2 ,
- Asad Reza 1 ,
- Krishna Kishore Inampudi 1 &
- Rajinder K Dhamija 3
Humanities and Social Sciences Communications volume 10 , Article number: 597 ( 2023 ) Cite this article
3647 Accesses
8 Citations
1 Altmetric
Metrics details
- Health humanities
- Medical humanities
- Science, technology and society
Retractions of peer-reviewed biomedical journal articles with Indian authorship have been on the rise for many years. Our study aimed to investigate the reason behind these retractions, namely plagiarism, falsification, fabrication, duplicate publication, author conflicts, ethical issues, fake peer-reviews, and data-related issues, besides providing year-wise trends regarding retraction, authorship, impact factor, and citations. We retrieved retracted publications with Indian affiliations indexed in MEDLINE between 1 January 1990 to 31 December 2021. During this period a total of 619 papers from 372 different journals with median values (interquartile range) pertaining to impact factor [3.2 (1.5, 5.2)], retraction time [24 (10, 51)] months, pre-retraction citations [4 (1, 12)], and post-retraction citations [4 (2, 12)] were retracted. While retractions still account for a small fraction of all publications (0.1%), the overall rate of retractions, that is, the number of retractions relative to the number of newly published journal articles in a given year, has been increasing. The reasons for retractions included plagiarism (27%), falsification and fabrication (26%), duplicate publication (21%), erroneous data (12%), authorship issues (4%), fake-peer reviews (3%), and ethical and funding issues (2%). We have analysed these reasons separately and compared them with each other. Besides a spurt in retraction due to plagiarism, instances of falsification have been escalating over the past decade. Half of the papers retracted on grounds of falsification were published by repeat offender authors in high-impact journals. Furthermore, 82% of retracted papers continued to accumulate citations even after the release of the journal retraction notices. The increase in retractions raises concerns over research quality as well as the wastage of scientific resources, which is especially pressing considering the present environment of scarce funding. The problem of retractions due to reasons such as plagiarism, duplicate publication, authorship issues, and, ethical issues as well as post-retraction citations can be mitigated by educating and raising awareness on publication ethics and responsible research conduct of researchers and journal publishers. Retractions due to fabrication, falsification, and fake peer reviews are more challenging to difficult to address and require further research for the identification of effective solutions.
Similar content being viewed by others
Systematic review and meta-analyses of studies analysing instructions to authors from 1987 to 2017
Analysis of retracted articles in the ophthalmic literature

Evidence and consequences of academic drift in the field of dental research: A bibliometric analysis 2000–2015
Introduction.
Retractions in the field of biomedical research have become a growing concern, eroding the trust placed in the scientific integrity of past and future research. The frequency of these retractions can indicate shifts in scientific conduct, and efficient removal of flawed publications from the literature can help maintain the scientific integrity of the research in this field (Fanelli, 2013 ; Ana et al., 2013 ; Fang et al., 2012 ). Retractions in the form of journal retraction notices can be considered an indication of critical reading by the scientific community and should deter future researchers from engaging in research misconduct or publishing flawed data. These notices can also offer valuable insights for conducting reason analyses.
There are ambiguities concerning the classification of retraction reasons. Previous studies have reported differently on the increase in retraction due to errors or misconduct (Fanelli, 2013 ; Fang et al., 2012 ; Steen, 2011a ). Some studies have characterised plagiarism as an inadvertent mistake (Fang et al., 2012 ; Steen, 2011a ), while others have categorised it as intentional fraud or misconduct (Nath et al., 2006 ; Smith, 2006 ; Gupta, 2013 ). Further clarity is required on the classification of plagiarism as either misconduct or error, along with solutions for addressing plagiarism cases (US Office of Research Integrity (ORI), 2022 ; Harriman and Patel, 2014 ). A study conducted in India (Abinandanan, 2011 ) highlighted 69 retracted papers from the period 2001 to 2010, with subsequent research projects furnishing similar findings (Damineni et al., 2015 ; Sabir et al., 2015 ; Elango et al., 2019 ; Elango, 2021 ). These studies focused on limited aspects of retraction reasons and involved a small time frame. Thus, conclusions cannot be drawn on specific retraction reasons and the citations of retracted studies in the field of Indian biomedical research. The retractions in biomedical research in India exerted a notable impact on public health (Kalita et al., 2015 ) and have led to substantial expenditure of funds (Dandona et al., 2017 ). Flawed biomedical research is often associated with the duped health of the patients (Steen, 2011b ; Nath et al., 2006 ; Rapani et al., 2020 ).
The editorial policies and practices of journals that influence the issuance of “retraction notices” are crucial for determining the rationale behind retractions, and reforms are required to enhance the transparency of these influencing factors (Vuong, 2020a ). Continued citations of these retracted papers, even following the release of retraction notices, have introduced additional concerns (Atlas, 2004 ; Fang et al., 2012 ). Citations of retracted papers may affect the findings of subsequent research. The practice of citing retracted papers post-retraction has been ongoing since 1990 (Pfeifer and Snodgrass, 1990 ). It remains unclear whether the presence of these citations is merely due to subsequent researchers’ unawareness or ignorance, or if they indicate a deliberate act by journals of not representing retraction notices properly along with the publication (Bolboacă et al., 2019 ; Resnik et al., 2009 ).
No national research council in India has issued any retraction guidelines to date. Therefore, our study had two primary research objectives, first, to identify the main reasons behind the retraction of publications in the field of clinical biomedical and basic biomedical research in Indian affiliations. Second, to identify the year-wise trends pertaining to each retraction reason, the time taken for retraction, the impact factor of retracted research (IF), and the post-retraction citations of retracted biomedical research. We collected data on retraction reasons from journal retraction notices, considering all categories, including plagiarism, falsification, fabrication, duplicate publication, error in data, authorship concerns, ethical issues, funding problems, data issues, and fake peer reviews. We also collected information on citation(s) of retracted publications. The data obtained were analysed to identify trends in retraction by year, citations, number of authors, journal IF, and time taken for retraction.
Retracted papers from India from the field of biomedical research were obtained from the PubMed database, using search options “retracted publication”, “retraction of publication”, and “duplicate publication” with the search term “India”. The search exclusively targeted studies in the English language. Every retracted paper from 1 January 1990 (the first retracted paper reported from India (Abinandanan, 2011 )) to 31 December 2021 was evaluated. PubMed was searched on 5 August 2022. Following the PRISMA guidelines we identified 619 retracted papers from the biomedical research field (Fig. 1 ; Moher D et al., 2009 ). Complete articles and PDFs of retraction notices were downloaded from journal websites and dichotomised. The retraction notices for 16 papers were inaccessible on journal websites. Therefore, we searched for the print versions at AIIMS and IHBAS library facilities (primarily old subscribed print-only journals that had published retraction notices prior to 2010). The PRISMA 2020 checklist is available in Supplementary file.
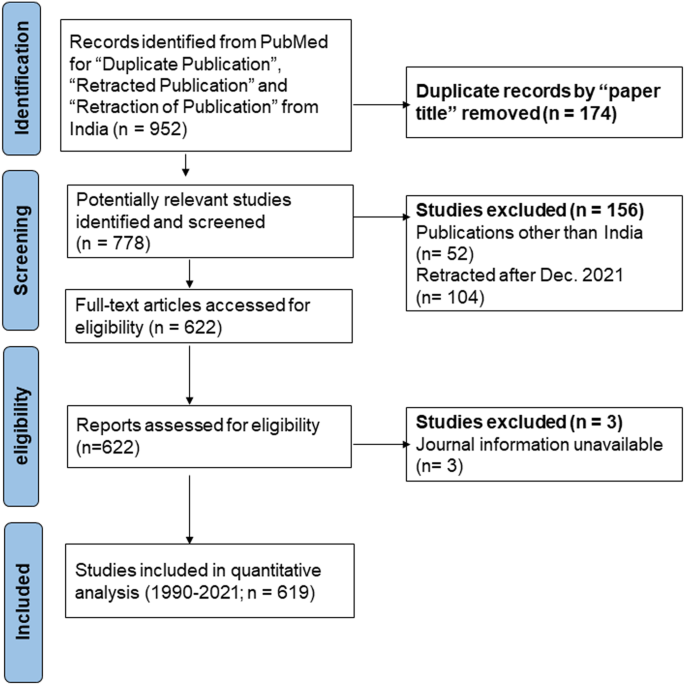
The flowchart showing the study identification and selection of retracted articles from an initial number of records in PubMed to final sample retrieval according to PRISMA guidelines. Data accessed Aug 2022.
Relevant MEDLINE data encompassing paper titles, publication dates, retraction dates, types (noticed/retracted/withdrawn/statement/requested/erratum/removed), retraction initiators (author(s)/editor/journal), journals’ names, authors’ names, and authors’ counts were succinctly added to an Excel sheet. Only retracted papers that were published by Indian affiliations were considered. The retracted papers with international collaborations were selected on the basis of either first authorship or corresponding authorship of Indian origin. The sentences mentioned in the retraction notices were reviewed word-by-word thrice to classify the reason for retraction. For papers retracted for more than one reason, the primary reason was identified by determining the most predominantly repeated word in the retraction notice. For duplicate publications, the duplications within the publications were verified by cross-referencing the paper title, authors’ names, and cited references. Furthermore, journal notices were consulted to ascertain the reason for retraction. Two independent reviewers conducted the selection process for determining the retracted studies to be included in the current research. Discrepancies, if any, were reviewed by the senior authors.
The IFs of the journals were assessed utilising Journal Citation Reports (JCR, Clarivate Analytics 2021). Scopus Cite Score 2021 was used in cases where IFs of journals were unavailable in JCR 2021. UGC-CARE list 2021 was also referred for information on Indian journals. Papers with missing IF were excluded from the statistical analysis. Web of Science was employed for checking citations of retracted papers. Pre-retraction citations refer to citations of a retracted publication that appear in papers of other authors prior to the issuance of the retraction notice. Post-retraction citations constitute the continued citations of a retracted publication in papers of other authors even after the release of the journal retraction notice. Citation pertaining to “retraction notice” itself was considered under pre-retraction citations, for our data analysis. The time taken for retraction was calculated in months. Journals issued alerts for retracted papers in various forms, such as by citing the main article, appending the retraction notice to the main articles’ PDF, applying a cross mark on both the retraction notice and the main paper, and using a watermark with the terms “retracted/retraction/withdrawn” on the retracted paper. These journal-initiated alerts that aimed to inform readers about paper retractions were identified and noted in our study.
Statistical analysis was performed using GraphPad Prism version 8 software (San Diego, CA). Descriptive statistics was employed to compare retraction time (in months), IF and citations. The Kolmogorov–Smirnov test was used to test for normality of variable distributions. Variables that did not reject the hypothesis of normal distribution were expressed as mean ± standard deviation (SD), whereas those that rejected the hypothesis were presented as median and described using the measure of the interquartile range (IQR). Correlations were estimated using the Pearson correlation coefficient and Spearmans’ rank correlation coefficient. The non-parametric Mann–Whitney test was employed to test for differences between the two study groups (clinical and basic biomedical retractions) and citations. Publications retracted for unknown reasons were excluded from the statistical analysis. Categorical variables were expressed as frequencies with percentages and were compared using the Chi-square test or Fisher exact test, as appropriate. For all tests, statistical significance was considered as p -value < 0.05.
Number of retractions
A total of 619 biomedical research publications were retracted from PubMed from the years 1990–2021. These retracted papers had overall median values (IQR), pertaining to IF [3.2 (1.5, 5.2)], retraction time [months; 24 (10, 51)], pre-retraction citations [4 (1, 12)], and post-retraction citations [4 (2, 12)]. Year-wise trends of publication retraction in Indian biomedical research have been illustrated in Fig. 2 and provided in Supplementary Table S1 . The retracted papers account for 0.1% of the total published papers in India during the selected period. Papers with Indian affiliations numbered 130,813 and 516,513 during the periods 1990–2010 and 2011–2021, respectively. The number of retracted biomedical research publications was 76 and 543 during the periods 1990–2010 and 2011–2021, respectively (Fig. 2 ). An increase in publication by four times and an increase in retraction by seven times were observed for the years 2011–2021 in comparison with the years 1990–2010. The first retraction of a paper from India occurred in 1992 from the Japanese Journal of Medical Science & Biology (published 1990) for duplicate publication by authors as they published identical data in the Journal of Medical Microbiology . The second retraction took place in 1993 from the Indian Journal of Gastroenterology due to duplicate publication. At the end of the 20th century, three papers from the field of biomedical research were retracted.
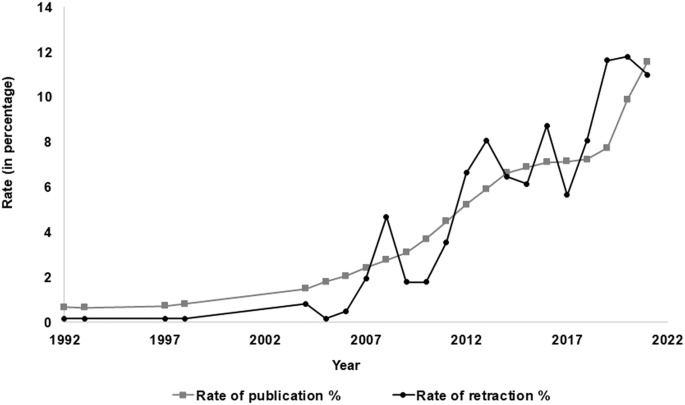
Comparison of year-wise rate of publication and rate of retractions in India.
The 619 retracted papers (including 30 papers featuring international collaborations where either the first or the corresponding author belonged to India) were published in 372 unique journals. Among these, 290 journals retracted one paper, while 82 journals retracted two or more papers. PloS One recorded the highest ( n = 27) number of retractions within a 54-month time frame (median value), followed by the Journal of Biological Chemistry ( n = 26) in 56.5 months and the Journal of Hazardous Materials ( n = 11) in 11 months. An increase in retractions of papers by repeat offenders was observed in 2008 from the Journal of Hazardous Material , which has an IF of 10.59 (Fig. 2 , Table 1 ). Life Science and FEMS Immunology and Medical Microbiology each retracted four papers, which took a long time of approximately 105 months. The average time taken for retraction was 24 months for all retracted papers. We would like to highlight that we could not obtain the International Standard Serial Number for two journals and found featured on the ceased list by the University Grants Commission (UGC) of India.
Classification of reasons for retraction
After a thorough reading of journal retraction notices, the following terms were noted: fabrication and falsification (of image and table data); plagiarism (encompassing text, images, tables, dissertations, books, multiple papers, study design, copyright concerns, improper citations, data overlap, content and scope); duplicate publication (pertaining to text, images, data, complete articles, scope, and content); authorship issues (involving conflict among authors, gifting of authorship, disputes, discrepancies, uncredited contributions, and absence of acknowledgement); ethical issues (pertaining to absence of patient consent for use of image, institutional ethics, unethical practices, and infringement of code of conduct); fake peer reviews (involving falsified emails, and compromised identity); data issues (encompassing unavailability of raw or original data, doubts concerning data integrity and authenticity, misinterpretation of results, inadequate information, irreproducibility of results, and invalid article findings); technical errors (such as premature publishing, accidental repetition, and unintentional actions); and unknown. The retraction notices issued for unknown reasons were also made available by the journals, in which the reasons were explained in ambiguous terms. The terms plagiarism, duplicate publication, and fabrication and falsification are most predominantly used in the retraction notices. These terms were used as the basis for the classification of retraction reasons. The following are the operational definitions of the terminology used:
The author copied text, images, data, or ideas from other author(s) in the field and published the same without citation(s) as their own original work.
Duplicate publication
The author published their own texts, images, or data repetitively without providing proper citation(s) to their previous work and presented the same as new or recent content.
Falsification and fabrication
Falsification—refers to the act of an author manipulating data (image, material, and equipment used) or selectively omitting data to fit their results to justify their hypothesis. Fabrication—occurs when an author generates false data by either creating it or cooking raw data, which is then recorded, and reported in the results and subsequent publications. Due to challenges in clearly differentiating between these two acts in the retraction notices. We considered these two actions as a combined reason for retraction to avoid bias.
This category encompasses genuine mistakes or technical errors encountered by the journal or publisher during the republishing process, genuine mistakes made by authors, unavailability of old or original raw data, irreproducibility of results, and errors in data, later acknowledged by authors in notices with apologies. This category includes voluntary withdrawals initiated by authors in coordination with the journals.
Misconduct (or other reasons)
This terminology was used distinctly to address papers retracted due to authorship issues, ethical issues, funding issues, or fake peer reviews.
Clinical biomedical research
Studies in this domain primarily focus on human patient material or sampling (in the form of case reports and patient data).
Basic biomedical research
Studies in this domain do not directly involve human patient material or sampling and are mainly focused on biological processes and disease pathways.
Unknown reason
The exact reason for retraction could not be determined for ~5% ( n = 34) of the retracted papers (including eight papers from the Journal of Biological Chemistry alone). Although the journals had released retraction notices, the reasons remained undisclosed. Additionally, announcements were unavailable for seven papers leading to the classification of the retraction reason of these papers under “unknown”. These articles shared some characteristics, including an IF of 2.4, a retraction time of 18.5 months, 2.5 pre-retraction citations, and 5 post-retraction citations.
Explanation of the categories retraction reasons
Plagiarism, duplicate publication, falsification and fabrication, authorship issues, ethical issues, and fake peer reviews are forms of intentional misconduct according to the US Office of Research Integrity and Medical Research Council (MRC) (US Office of Research Integrity (ORI), 2022 ; MRC, 1997 ). Our study classified each of these reasons separately. Some publications were also retracted due to unintended errors in data or technical issues. The year-wise data obtained for each retraction for 619 retracted papers have been presented in Fig. 3a . Plagiarism emerged as the predominant reason for retraction accounting for the retraction of 187 (27%) papers. More frequent plagiarism of text by authors was evidenced in 68% of these papers. The remaining papers exhibited plagiarism in data, similarity in scope and content, copyright concerns, and unattributed quotations. Duplicate publication and falsification and fabrication were noted in 143 (21%) papers and 177 (26%) retractions, respectively (Fig. 3a ). A total of 47 retracted papers were published by authors in different journals using the same intellectual materials and text without citation to previous research. A few of these papers were published in another journal while still being under peer review by the first journal, resulting in subsequent publication. Five papers were retracted due to plagiarism, as the journals identified titles, content, and data similar to those published by different authors with separate university affiliations. This constitutes an infringement of professional ethics and the Code of Conduct stipulated by the Committee on Publication Ethics (COPE), which states that “submission of a paper implies that it reports unpublished work and that it is not under consideration for publication elsewhere”, in forming the guidelines of good publication ethics (COPE, 2000 ).
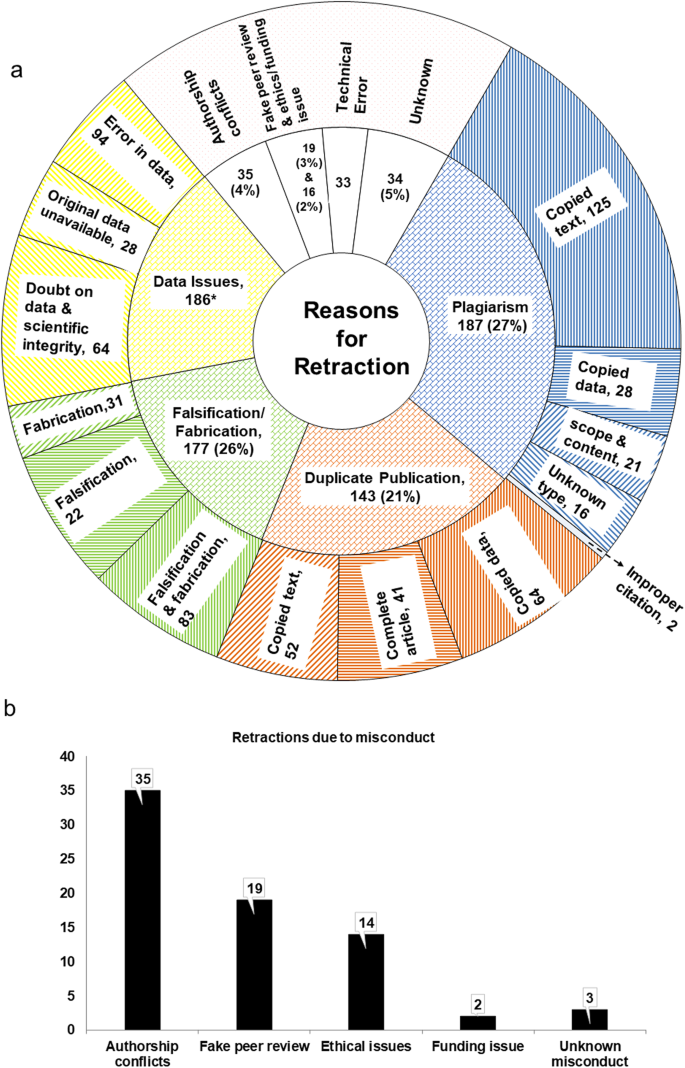
Classification based on the reason for retraction ( a and b ).
Data-related issues were cited as an associated reason for the retraction of 186 papers in retraction notices along with other reasons. A total of 58 papers were retracted for two predominant reasons (31 papers for duplicate publication and falsification, 6 papers for duplicate publication and plagiarism, 14 papers for falsification and plagiarism, 2 papers for duplicate publication and misconduct, 4 papers for falsification and misconduct and 1 paper for plagiarism and misconduct). Two papers were retracted for plagiarism, duplicate publication, and falsification. To avoid bias, we considered these reasons separately under distinct categories for statistical analysis. The misconduct category encompassed authorship issues (uncredited, ghost, or gift authorship); compromised peer-review process due to the use of fake emails; ethical issues on grounds of failure to obtain patients’ consent (breach of privacy); and funding issues. A total of 59 papers under the misconduct category were retracted for 70 reasons (Fig. 3b ; including papers retracted for more than one reason). For three retracted papers misconduct was discovered later upon investigation by the journal; however, details regarding the nature of the misconduct were not mentioned. These three papers were included in the category of “unknown misconduct”. Falsification and fabrication accounted for 26% of the retracted papers. Furthermore, image manipulation was responsible for the retraction of 78% of the papers, while the remaining papers were retracted due to manipulation in table data for experiments and results. A total of 82 papers were retracted due to errors (or mistakes) in data, with the retraction taking 11 months. Overall, these papers were retracted for 682 distinct reasons (21 papers with 3 reasons, 77 papers for two reasons, and the remainder for one reason) (see Supplementary Table S2 ).
Study type: Clinical biomedical research and basic biomedical research
The retracted papers’ abstracts and complete texts were evaluated to classify the papers into two study types: clinical biomedical research papers ( n = 172; involved human materials, including 57 case reports) and basic biomedical research papers ( n = 447; did not utilise human sampling or materials). The retracted basic biomedical papers had a higher average IF of 3.8 compared with the clinical biomedical papers, which had an average IF of 1.4. Although plagiarism was the most common reason for retractions in both clinical and basic biomedical research categories, falsification was responsible for the retraction of a large number of papers within the basic biomedical research category. Retraction reasons such as authorship issues and ethical issues were predominantly observed in clinical biomedical research retractions ( n = 23). The retracted clinical biomedical papers raise concerns regarding the quality and reliability of these articles, more so because of their focus on human health. Total citations were higher for basic biomedical papers than for clinical biomedical papers. Interestingly, post-citations and pre-citations were 436 and 382, respectively, for clinical biomedical publications. The overall number of citations for all retracted publications has been presented in Supplementary Fig. S1 .
Time taken for retraction, year-wise trends in retraction reasons, and journal impact factor
The time taken for retraction was 27 months for basic biomedical papers and 15.5 months for clinical publications. Journals took less time to identify and retract publications that primarily had authorship issues, ethical issues, and fake peer reviews. Journals took longer time to identify and retract publications on grounds of due to fabrication and falsification than they did for cases with text overlaps in duplicate publications and plagiarism (Supplementary Table S2 ). One paper which was retracted due to plagiarism, stood out due to its lengthiest retraction time of 266 months . Retractions due to plagiarism were prevalent in India by the year 2004 and those due to fabrication or falsification by the year 2008 (Fig. 4a ). Plagiarism saw an increase after 2010 (reaching a peak of 21 plagiarised papers in 2012). Misconduct was most prevalent in the year 2016, with a majority of analysed papers published in the Scientific World Journal facing retraction for reason fake-peer review processes undertaken by authors using fake email accounts. Retraction notices for repeat offender authors (same set of authors and affiliations) were published in the Breast Cancer Journal . In India, retractions for duplicate publications have been recorded from before 2000 (retraction time = 105.7 months). This trend has shown a decline with the minimum average retraction time reaching 2.8 months in 2018 ( R 2 = 0.69, p < 0.001). Notably, 32% of the publications ( n = 200) were retracted within 12 months of publication, with thirteen papers being retracted on the same day of publication due to technical errors and author conflicts. One paper was retracted from the New England Journal of Medicine due to image fabrication within 18 days of publication.
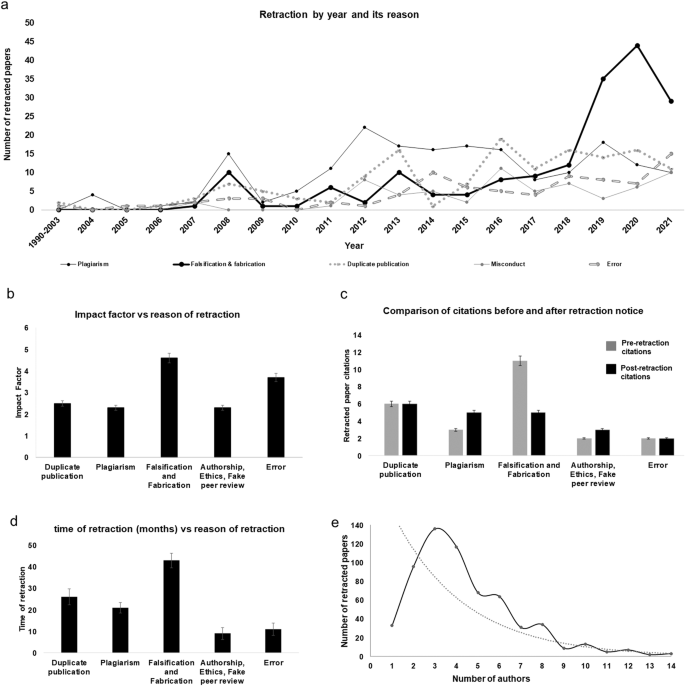
The number of retracted papers and their reasons per year ( a ), the median impact factor of journals ( b ), comparison of the pre-retraction citations and post-retraction citations ( c ), the median time taken (in months) for retraction ( d ), and the number of author distribution for each retracted paper ( e ).
Overall, each retracted paper had a median IF of 3, out of which 69% had an IF < 5 and 41 retracted papers had an IF > 10. The journals with very high IF (>10) completed retraction in 25 months, while journals with low IF (<5) accomplished the same in 21 months. The papers retracted for falsification and fabrication had a median IF of 4.6. The IF of these papers ranged from 0.2 to 12.54, with the exception of two that had the highest IF of 91.25 and were retracted in 2013 and 2019 from the New England Journal of Medicine . An outlier in this case, the 2013 paper was retracted due to falsification and fabrication of data and images, in 22 months, with 79 pre-citations and 116 post-citations. The paper retracted in the year 2019 had been published just 18 days prior to publication (Fig. 4 b and d ). Falsification was the most cited reason accounting for the retraction of 49 papers by five journals, namely Acta Biomaterialia, Biosensors and Bioelectronics, Life Sciences, Journal of Hazardous Materials and PLoS ONE (Table 1 ). Journals that retracted papers for plagiarism, duplicate publication, and misconduct had IFs ranging from 2 to 2.5. The IFs for 21 retracted papers were unavailable. Therefore, these were excluded from the statistical analysis.
Pre-retraction and post-retraction citations
Overall, the retracted papers had 5809 citations before the issuance of the retraction notice and garnered 4027 citations after the notice’s release in 372 unique journals. Furthermore, 25% of the retracted papers had a greater number of post-retraction citations compared with their pre-retraction citations. Papers retracted on grounds of fabrication and falsification had a total of 4752 citations (average of 27 citations per paper), whereas those retracted due to plagiarism had a total of 3286 citations (average of 18 citations per paper). Basic biomedical research papers accumulated a significant number of citations primarily due to their publication in the form of review papers (1172 citations post-issuance of retraction notices with a median of 14 citations for each retracted review paper).
Number of authors
The 619 retracted papers were published by 2753 authors, including 199 authors among which 199 authors were “repeat offenders” with at least two retracted publications and another 119 were repeat offenders with 3–14 retracted publications. The mean ± SD for the number of authors per retracted paper was 4.4 ± 2.5, and the mode was 136 for three authors. The graph in Fig. 4e for the number of retracted papers and the number of authors can be seen in two phases: The first phase reveals a rapid expansion starting with 5.3% ( n = 33) of retracted publications authored by single author, followed by, 15.5% ( n = 96) authored by two authors, and 22% ( n = 136) involving three authors, and the median retraction time of these papers was 17, 25.5, and 20 months, respectively (Fig. 4e ). The second phase demonstrates a decline with 117 articles authored by four authors, and 68 by five authors, decreasing to as low as two papers by 13 authors, and one paper each by 15, 16, and 23 authors. The second phase reveals an inverse relationship with the increase in the number of co-authors, a decrease in the number of retractions was observed. However, the presence of multiple co-authors does not guarantee immunity from retractions, as certain cases with ≥10 co-authors were still retracted due to falsification and plagiarism (Steen, 2011c ). Retractions involving a single author indicate a lower likelihood of employment of unfair practices compared with cases involving two, or three authors, in which the burden and risk are seemingly shared.
Whistle-blowers
In the case of 33 papers, readers acted as whistle-blowers, identifying incidences of misconduct and alerting the journal editor. Journal editors then investigated these papers, resulting in erratum, correction, and retraction. A majority of retractions were issued due to requests by editors-in-chief and/or the journals (498); followed by agreements between editors and authors (68); authors initiated appeals (36); and collaboration between editors-in-chief, journal publishers and institutional investigation committees (24). When authors requested retractions, it was primarily due to errors in data and other data-related issues, as described in previous research (Vuong, 2020b ). Papers retracted primarily due to falsification reasons involved institutional panels or committees, in addition to editors-in-chief, other editors, and journals to in the retraction decision. Final decisions were taken by the editors-in-chief of journals.
Phrases used for retraction and issuance of alerts by journals
The different types of retraction notices employed various phrases, which are listed in Table 2 . “ Retracted (requested)” should not be confused with the retractions requested by authors. The latter was evaluated by assessing the words or sentences mentioned in the retraction notices . Journals bore the responsibility of alerting their readers about retraction through various ways including labelling on their websites, appending the PDF of the retraction notice to the main article, utilising watermarks, and placing a cross mark on the paper. The alerts employed by the journals to inform readers about the paper retraction are listed in Table 3 . Although 603 retracted papers were accompanied by PDFs of retraction notices on journal websites (except 16 retracted papers that had only print versions of retraction notices available and were retracted before the year 2010), only 304 papers were watermarked. These watermarks, which were red or grey conveyed the phrase “retracted”, “retraction of publication” or “withdrawn”. The Chinese Journal of Lung Cancer used the colour blue in its retraction watermarks. Cross marks in red were found in only 113 retracted articles. Furthermore, 25.4% of the downloaded complete papers were accompanied by PDFs of retraction notices. It was surprising to note that only 139 articles contained both watermarks and cross marks, while only 31 articles had all three. The journals did not employ prominent markers for alerting readers for 31% of the papers, which might have led to them receiving post-retraction citations.
In this study we report evidence confirming that research papers with Indian affiliation are retracted more due to misconduct including plagiarism, duplicate publication, falsification, authorship issues, ethical issues, and fake peer reviews than errors (Fig. 3 ). Although we found a progressive increase in biomedical research publications from India, it does not take away from the fact that retractions are increasing at a higher rate than before, as observed in recent years (Fig. 2 ; Supplementary Fig. S2 ). This is not a healthy trend. Retractions due to duplicate publication were first reported in India in the year 1990, and such cases have been persisting at a steady rate since then. Plagiarism first appeared in 2004 (the highest number of retracted papers [22] observed in 2012), followed by falsification in 2008 (the highest number of retracted papers [44] observed in 2020). It is evident from the data that 48% of the papers were retracted due to plagiarism and duplicate publication. This can be attributed to the “publish and perish culture” (De Rond and Miller, 2005 ). Furthermore, the readily available electronic access to publications and technological advancements in word processing have greatly promoted plagiarism over the past years. Retraction due to plagiarism has been decreasing in recent years due to the implementation of facilitation of plagiarism detection software and tools and strict guidelines (Horrom, 2012 ; UGC, 2021 ; Fig. 4a ). However, we still lack software and guidelines for addressing the dealing falsification of images and data. This could possibly be the primary reason behind the trend of increasing retractions due to falsification and fabrication. The number of research publications is indicative of a scholar’s talent and their standing among peers. It leads to invitations to talks, committee membership at institutions, and opportunities to serve as reviewers or editors for journals, bestowing recognition, and credibility on the scholar (Sabir et al., 2015 ). Publication makes scholars more appealing to funding agencies, which improves their chances of obtaining grants and enhances their candidacy for recruitment or promotion (De Rond and Miller, 2005 ). In India, many scientists face disparities in research funding, which forces them to resort to publishing subpar work (Kalita et al., 2015 ; Dandona et al., 2017 ). These authors prioritise having their names on articles over upholding basic publication ethics, often resorting to plagiarism or duplication. Most of the time, faculty members and research scholars are bound by their institutional degrees/course work/project guidelines to publish, which puts them under pressure to perform this duty (Smith, 2006 ; Elango et al., 2019 ). Publications by new and untrained researchers often face retractions due to proofreading oversight by senior colleagues or faculty members. Furthermore, the absence of proper guidelines to follow exacerbates these issues.
COPE classifies plagiarism and duplicate publications as distinct categories of retraction reasons (COPE, 2000 guidelines). The classification of plagiarism involving data but not text under the category of misconduct is contradictory (Steen, 2011a ; Fang, 2012 ). Fang ( 2012 ) considered plagiarism separately in a subsequent paper, highlighting that India and China collectively accounted for a greater number of retractions due to plagiarism and duplicate publication than the United States (Fang et al., 2012 ). The MRC code classifies plagiarism, falsification, and fabrication as forms of misconduct, but it does not include honest errors (MRC, 1997 ). We believe that plagiarism of text, ideas, data, and images all of which are grounds for paper retraction should be taken seriously. Therefore, to bring the retraction reasons of plagiarism and duplicate publication to the attention of scholars and journals, we classified them separately. Authors have republished their data and images to earn credit without due reference and have largely duplicated texts within their Introduction, Method, Discussion, and Conclusion sections. In case there is a chance of the same article being published by two journals, it is the duty of the author to request the withdrawal of the article from the first journal prior to submitting it to another journal.
However, with time plagiarism and duplicate publications are becoming easy to detect through plagiarism detection software. These programs have been made available (free or on a payment basis) at large to all Indian institutions and their faculties aiding the detection of text overlap in research articles. Furthermore, the Indian Government’ (UGC), has issued a strict guideline mandating the use of “plagiarism-detection programs” for submitted manuscripts and theses, which is largely followed by numerous journals. However, these programs cannot detect “idea plagiarism” in manuscripts. The UGC has also introduced a two-credit course on publication ethics and publication misconduct, which is mandatory for all PhD students (UGC, 2019 ). Academic Integrity Panels have been established to investigate complaints concerning plagiarism and impose appropriate penalties on guilty researchers according to the graded level of plagiarism, ranging from minor actions to termination of service (UGC, 2021 ). This could be the reason behind the decrease in the number of retractions due to plagiarism and duplicate publication, as evident from our analysis (Fig. 4a ).
India has strict defamation laws, and Article 19(1)(a) of the Constitution of India safeguards the right to free speech. However, this right does not extend to defaming individuals and harming their reputations through libel or slander (Constitution of India). These laws could apply to the research field as well. But, to the best of our knowledge, no Indian authority has published any guidelines regarding research integrity and publication, to best of our knowledge. It is challenging to determine whether defamation laws have any influence on the 5% of cases from India that have been retracted for reasons that have not been disclosed by journals in their retraction notices (Fig. 3a , Supplementary Table S2 ). Our data lacks details regarding this aspect.
In addition to being the authors’ responsibility, it is also the duty of journals to review manuscripts carefully for plagiarism before sending them to external reviewers. In one of the retractions notices the editor-in-chief of the journal Pharmacy Practice discussed the concepts of plagiarism and misconduct, stating that “plagiarism is a concept without a clear definition…all types of scientific misconduct constitute a frequent and serious problem that we all should be aware of and address together” (Fernandez-Llimos, 2012 ). In cases of duplicate publication, journals may ask authors to submit the previously published papers along with their manuscripts.
Fabrication and falsification have been increasing rapidly and continuously at twice the rate of plagiarism (Figs. 3 a and 4a ). These papers are primarily published in high IF journals and boast a high number of citations (Fig. 4 b and c ). Fraud takes a longer time to identify compared with other retraction reasons (Fig. 4d ). Deliberate fraud (fabrication or falsification of data) by multiple collaborating authors has been observed. These fraudulent authors typically target high IF journals and have other fraudulent publications with shared authorship (Steen, 2011c ). Even in our data analysis, we discovered that retracted papers involving falsification and fabrication of data and images were published by “repeat offenders” who are co-authors in multiple papers. These repeat offenders were found to be published extensively in journals with IFs ranging from 3 to 12 and mostly target high IF journals. More than 50% of such retracted papers have ≥4 authors who are repeat offenders. In addition, these papers were published repeatedly in primarily the same type of journals (Table 1 ). Identifying and investigating repeat offenders might have taken journals a significant amount of time (Fig. 4d ). The repeat offenders were authors found guilty of repetitively using the same data and figures to validate the results of different scientific experiments in various publications. Restrictions were imposed on future activities at their universities. The veracity of their published research project across all journals has become doubtful, and they are regarded as potentially fraudulent. Publications in high IF journals are associated with the prestige of a scientist, which places significant pressure on them, leading them towards increased errors and falsification for publication. These issues need to be subjected to critical examination and strong observation during the review process. These papers may have come to attention late because of the limited and restricted availability of software for detecting falsification and fabrication of image data, an issue that remains unknown at many research institutions. However, due to clear policies established by a majority of high IF journals, post-retraction citations of these fraudulent journals are comparatively less (Fig. 4c ). An analysis of retracted biomedical research papers revealed that 53 papers in the field of dental research were retracted primarily due to falsification. Similar trends have been observed for retractions in dental research from India, with 49 out of 180 retracted papers originating in other countries (Rapani et al., 2020 ). We wish to report a specific retraction notice involving a dental research paper. The author of this paper had previously served as a reviewer for the International Journal of Paediatric Dentistry , which was when he rejected a manuscript submitted by another researcher. The same manuscript was later published in the Journal of Conservative Dentistry under this authorship (Retraction, 2016 ). The journal’s editorial office later retracted this paper.
Fake peer review is a type of manipulated peer review, wherein an author uses fake email addresses to provide review suggestions. The author fabricates favourable reviews, facilitating the acceptance of their own paper (Misra et al., 2018 ; Fig. 4b ). Researchers must obtain ethical permissions from Institutional Ethical Committees prior to publication for human or animal sampling and seek patients’ consent before using their images in case reports or clinical research studies to avoid retractions in future.
Authorship disputes among researchers are common; however, the contribution of an author can be assessed through careful discussions in lab research groups or among research collaborators before submission. The ranking of authors constitutes a critical aspect of scientific research and should be decided on prior to publication to avoid retractions. Arguments are common in the research field; however, they should be approached carefully to avoid misconduct accusations.
Committing honest errors in scientific research cannot be deemed as misconduct. Errata are published seeking corrections in papers when the errors are not severe enough to warrant full retraction (Nath et al., 2006 ). Errata for correction was published for 16 papers; however, the papers were later retracted by journals on grounds of failure to produce results, unavailability of raw data by authors and technical issues. Papers retracted due to errors may be a result of honest mistakes by journals or publishers due to technical issues leading to the publication of the same article twice. Such retracted papers can spark constructive debates among researchers. Authors should shoulder the responsibility of promptly any error in the data to report journals. Such actions constitute a component of good research practice and foster a culture of robust scientific explorations.
The retraction announcements issued by journals were discovered to have similar patterns in disclosing the reason for retraction. However, the Journal of Biological Chemistry does not disclose the retraction reason in its notices. There must be a common guideline and policy concerning retractions that can be followed by all journals without bias (Wager et al., 2009 ). This will reduce the long time taken by journals for retraction. The retraction of the most highly cited papers can be largely attributed to plagiarism in the form of review articles. The most cited retracted articles from India and the reasons for their retraction have been listed (Supplementary Table S3 ). Overall, the number of post-retraction citations is found to be higher than that of pre-retraction citations for the retracted papers (Fig. 4c ). Falsification led to the retraction of 75% of the papers published in high IF journals. These papers were either watermarked or cross-marked by the journals (Table 3 ). A significant difference can be expected once retraction notices are made readily available, enabling the removal of retracted articles to prevent their further use. Journals may take a long time to retract papers, and ignoring alerts by capitalising on ambiguities and discrepancies in retraction notices helps journals maintain their IF, which upholds their reputation and helps them reap benefits. Citations of papers tend to increase over time, and we found a positive correlation between citations and retraction time. As more time passes, more researchers will read and cite an article. Post-retraction citations can lead to another form of misconduct often overlooked by journals and cited by researchers due to unawareness or oversight, possibly indicating that the alerts are not being read or are being purposely ignored. However, studies published by authors who have cited retracted articles become questionable. The onus of pre-retraction citations rests on the authors and the publishing journal. Simultaneously, the onus of post-retraction citations rests mainly on the researcher who has included these citations. Therefore, such studies should also be scrutinised to avoid such citations. Journals play a critical role here in alerting readers about retractions (Table 3 ). To avoid misconduct in research publications and maintain scientific research integrity, we would like to suggest the following recommendations based on our findings:
Training of researchers : First and foremost, the training of undergraduate, postgraduate, and post-doctoral research students is necessary. The UGC has mandated a two-credit course on publication ethics and publication misconduct for all PhD students (UGC, 2019). These courses should be conducted at regular intervals for all researchers at any stage, ranging from undergraduate to faculty levels.
Careful use of citations : Scholars must maintain research integrity by citing previous literature carefully. Self-citations are also crucial for avoiding retractions due to duplications.
Contribution of authors : Authorship ranking and contributions must be determined before writing the manuscript. The international guidelines on authorship issued by COPE ( 2000 ), CSE ( 2021 ) and ICMJE ( 2021 ) must be followed.
For biomedical research papers, ethical clearances must be obtained before seeking grants or at least before drafting the manuscript. Patients’ consent must be sought for using patient data or images well in advance.
National and international guidelines : Indian researchers should follow national and international guidelines while publishing to ensure responsible research conduct (RCR) (DBT, 2016 ; ICMR RIPE, 2019 ; UGC, 2018 ; UGC, 2019 ) and international guidelines (COPE, 2000 ; CSE, 2021 ; ICMJE, 2021 ). These guidelines concerning publication ethics and responsible research conduct must be revised and updated regularly.
Webinars, seminars, or workshops must be regularly organised at all institutions for raising awareness and researchers who abide by these guidelines must be awarded.
Appropriate addressal of retraction reasons : Different forms of misconduct (namely plagiarism, duplicate publication, falsification, fabrication, authorship issues, ethical issues, and fake peer reviews) must be addressed with distinct solutions. The degree of misconduct should be decided and subsequent penalties should be imposed accordingly.
Journals and editors must adopt transparency and clarity when releasing retraction notices and cite reasons without bias. All journals must follow a universal guideline for issuing retraction notices. Further reforms to enhance the transparency of retractions have been suggested by Vuong ( 2020a ).
Issues such as plagiarism, duplicate publication and falsification can be avoided by early use of plagiarism and falsification detection tools at the time of peer review itself to avoid retractions in the future.
The issue of duplicate publication can be avoided through a thorough investigation of all previously published studies by authors at the time of the peer review process. Journals can ask authors to provide all their published studies along with a plagiarism report at the time of manuscript submission.
The journals must meticulously provide alerts to the readers informing them about paper retractions of paper by attaching PDFs of retraction notices, applying watermarks, or placing cross-marks on the entire paper. This will help reduce future citations of retracted papers.
All citations in a manuscript should be checked carefully for retracted papers by journals before sending it for external peer review and publication as well as by authors at the time of submission. It is unethical to cite an article solely based on its previous citations. The full text of the articles must be thoroughly read by authors before citing the same (Bolboacă et al., 2019 ).
To avoid paper retractions due to genuine mistakes or errors in data, authors should be given the opportunity to make scientific corrections in their papers through published errata/ or demand for corrections. This move should be adopted instead of resorting to full retractions.
In India, the UGC has released guidelines to avoid predatory publishing and has provided a list of cloned and predatory journals in the UGC-CARE list, which is revised regularly (Patwardhan and Nagarkar, 2021 ).
The strength of this research lies in its qualitative analysis of the distinct reasons that lead to retraction and its uncovering of the ambiguities pertaining to research integrity. The primary aim of this paper was to highlight the exact reasons for retraction in Indian biomedical research as well as the inclusion and exclusion criteria for the retracted papers. Therefore, other retraction sources, subjects or countries have not been covered here. Retraction notices issued during 1990–2021 have been covered, and they provide a lower estimate of the number of papers potentially retracted during this period. The analysis of the retraction reasons is based on the information obtained from the words and sentences employed by journals in their retraction notices. The data provided by journals concerning retraction reasons may have informed the results and could have also undermined its credibility if the reasons were not disclosed transparently. Additionally, the study findings might present an underestimation of the total number of retractions due to the study’s exclusive reliance on the PubMed database (which started recording errata from 1987 onwards). However, the significant strength of this study lies in its complete coverage of biomedical research papers from India. Scopus Cite Score 2021 was utilised for cases where the IFs of papers were unavailable in JCR 2021. This paper does not delve into an empirical analysis of the impact of retractions on the number of subsequent citations. To perform such analysis, information on additional “matching papers” that were not retracted would be required. Conducting such an analysis would be useful for future research. This paper did not assess the research quality of papers and retraction-based relationships. The quality and validity of retracted papers need to be reviewed further, as these retractions do not entirely invalidate the retracted papers. However, retractions in medical research, especially those of case reports, are of utmost importance as they influence patient health and treatment decisions.
Science requires a comprehensive system to safeguard its underlying values, and retraction constitutes an important tool for upholding these values. Today’s problem with eroded trust in the sciences, there is a need for a renewal of integrity, transparency, and intellectual honesty. Ignoring the issues highlighted and explored in this research article may lead to inflated costs of doing science and could pose obstacles to securing additional or future funding (Vuong, 2018 ). The problem of retractions due to plagiarism, duplicate publication, authorship issues, ethical issues, and the like as well as the issues of post-retraction citations can be avoided by educating and increasing the awareness of researchers and journal publishers. Retractions due to fabrication, falsification, and fake peer reviews are issues that stand as pressing concerns that require further investigations for the identification and implementation of potentially ideal solutions. The results presented concerning the various reasons for retraction in India-affiliated biomedical research, year-wise trends, impact of retractions on clinical and basic biomedical research, and post-retraction citations will help raise awareness among researchers. This paper has the potential to improve scientific research by authors by encouraging them to exercise caution and avoid misconduct as well as post-retraction citations while publishing. The findings from our study can serve as a reference for future investigations and the development of guidelines.
Data availability
The datasets supporting this article will be provided on reasonable request email to the corresponding author.
Abinandanan TA (2011) Scientific misconduct in India: an analysis of retracted papers in PubMed. In Chennai. Workshop on Academic Ethics, Institute of Mathematical Sciences. https://www.imsc.res.in/~ethicsmeet/abstracts/abinandanan. html
Ana J, Koehlmoos T, Smith R, Yan LL (2013) Research misconduct in low-and middle-income countries. PLoS Med 10(3):e1001315. https://doi.org/10.1371/journal.pmed.1001315
Article PubMed PubMed Central Google Scholar
Atlas MC (2004) Retraction policies of high-impact biomedical journals. J Med Libr Assoc 92(2):242
PubMed PubMed Central Google Scholar
Bolboacă SD, Buhai DV, Aluaș M, Bulboacă AE (2019) Post retraction citations among manuscripts reporting a radiology-imaging diagnostic method. PLoS ONE 14(6):e0217918. https://doi.org/10.1371/journal.pone.0217918
Article CAS PubMed PubMed Central Google Scholar
Committee on Publication Ethics (COPE) (2000) Guidelines on good publication practice. J Urol 163(1):249–252
Council of Science Editors (CSE) (2021) White paper on publication ethics. https://www.councilscienceeditors.org/resource-library/editorial-policies/white-paper-on-publication-ethics/
Damineni RS, Sardiwal KK, Waghle SR, Dakshyani MB (2015) A comprehensive comparative analysis of articles retracted in 2012 and 2013 from the scholarly literature. J Int Soc Prev Community Dent 5(1):19. https://doi.org/10.4103/2231-0762.151968
Dandona L, Dandona R, Kumar GA, Cowling K, Titus P, Katoch VM, Swaminathan S (2017) Mapping of health research funding in India. Natl Med J India 30(6):309. https://doi.org/10.4103/0970-258X.239069
Article PubMed Google Scholar
De Rond M, Miller AN (2005) Publish or perish: bane or boon of academic life. J Manag Inq 14(4):321–329. https://doi.org/10.1177/1056492605276850
Article Google Scholar
Department of Biotechnology (DBT) (2016) Statement on the handling of allegations of research misconduct 2016. http://dbtindia.gov.in/sites/default/files/DBTresearch-misconduct13042016.pdf
Elango B, Kozak M, Rajendran P (2019) Analysis of retractions in Indian science. Scientometrics 119(2):1081–1094. https://doi.org/10.1007/s11192-019-03079-y
Elango B (2021) Retracted articles in the biomedical literature from Indian authors. Scientometrics 126(5):3965–3981. https://doi.org/10.1007/s11192-021-03895-1
Fanelli D (2013) Why growing retractions are (mostly) a good sign. PLoS Med 10(12):e1001563. https://doi.org/10.1371/journal.pmed.1001563
Fang FC, Steen RG, Casadevall A (2012) Misconduct accounts for the majority of retracted scientific publications. Proc Natl Acad Sci USA 109(42):17028–17033. https://doi.org/10.1073/pnas.1212247109
Article PubMed PubMed Central ADS Google Scholar
Fernandez-Llimos F (2012) Pharmacy practice suffered a plagiarism case. Pharm Pract 10(1):1–2. https://doi.org/10.4321/s1886-36552012000100001
Gupta A (2013) Fraud and misconduct in clinical research: a concern. Perspect Clin Res 4(2):144–147. https://doi.org/10.4103/2229-3485.111800
Harriman S, Patel J (2014) Text recycling: acceptable or misconduct? BMC Med 12:148. https://doi.org/10.1186/s12916-014-0148-8
Horrom TA (2012) The perils of copy and paste: plagiarism in scientific publishing. J Rehabil Res Dev 49(8):VII. https://doi.org/10.1682/jrrd.2012.09.0165
ICMR RIPE (2019) ICMR Policy on research integrity and publication ethics 2019. https://ethics.ncdirindia.org//asset/pdf/ICMR_PRIPE2019.pdf
International Committee of Medical Journal Editors (ICMJE) (2021) Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. https://www.icmje.org/icmje-recommendations.pdf
Kalita A, Shinde S, Patel V (2015) Public health research in India in the new millennium: a bibliometric analysis. Global Health Action 8(1):27576. https://doi.org/10.3402/gha.v8.27576
Medical Research Council (Great Britain) (1997) MRC policy and procedure for inquiring into allegations of scientific misconduct. Medical Research Council, London
Misra DP, Ravindran V, Agarwal V (2018) Integrity of authorship and peer review practices: challenges and opportunities for improvement. J Korean Med Sci 33(46). https://doi.org/10.3346/jkms.2018.33.e287
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group* T (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–9. https://doi.org/10.1136/bmj.b2535
Nath SB, Marcus SC, Druss BG (2006) Retractions in the research literature: misconduct or mistakes? Med J Aust 185(3):152–154. https://doi.org/10.5694/j.1326-5377.2006.tb00504.x
Patwardhan B, Nagarkar S (2021) The UGC-CARE initiative: Indian academia’s quest for research and publishing integrity. First Monday https://doi.org/10.5210/fm.v26i10.10349
Pfeifer MP, Snodgrass GL (1990) The continued use of retracted, invalid scientific literature. JAMA 263(10):1420–1423
Article CAS PubMed Google Scholar
Rapani A, Lombardi T, Berton F, Del Lupo V, Di Lenarda R, Stacchi C (2020) Retracted publications and their citation in dental literature: a systematic review. Clin Exp Dent Res 6(4):383–390. https://doi.org/10.1002/cre2.292
Resnik DB, Peddada S, Brunson Jr W (2009) Research misconduct policies of scientific journals. Account Res 16(5):254–67. https://doi.org/10.1080/08989620903190299
Retraction (2016) Role of casein phosphopeptide amorphous calcium phosphate in remineralization of white spot lesions and inhibition of Streptococcus mutans ? J Conserv Dent 19(2):198. https://doi.org/10.4103/0972-0707.178710
Sabir H, Kumbhare S, Parate A, Kumar R, Das S (2015) Scientific misconduct: a perspective from India. Med Health Care Philos 18(2):177–184. https://doi.org/10.1007/s11019-014-9603-8
Smith R (2006) Research misconduct: the poisoning of the well. J R Soc Med 99(5):232–237. https://doi.org/10.1177/014107680609900514
Steen RG (2011a) Retractions in the scientific literature: is the incidence of research fraud increasing. J Med Eth 37(4):249–253. https://doi.org/10.1136/jme.2010.040923
Steen RG (2011b) Retractions in the medical literature: how many patients are put at risk by flawed research? J Med Eth 37(11):688–692. https://doi.org/10.1136/jme.2011.043133
Steen RG (2011c) Retractions in the scientific literature: do authors deliberately commit research fraud? J Med Eth 37(2):113–117. https://doi.org/10.1136/jme.2010.038125
University Grant Commission (2018) UGC-Promotion of academic integrity and prevention of plagiarism in higher educational institutions. Regulations 2018. https://www.ugc.ac.in/pdfnews/7771545_academic-integrity-Regulation2018.pdf
University Grants Commission in its 543” meeting held on 9th August 2019 approved two. https://www.ugc.ac.in/pdfnews/9836633_Research-and-Publication-Ethics.pdf
University Grants Commission (2019) Consortium for Academic and Research Ethics Approved list of journals. Updated 14th June 2019. https://ugccare.unipune.ac.in/apps1/home/index . Accessed April 2022
University Grants Commission (2021) Academic Integrity and Research Quality
US Office of Research Integrity (ORI) (2022) https://ori.hhs.gov/definition-research-misconduct . Accessed in April 2022
Vuong QH (2020a) Reform retractions to make them more transparent. Nature 582:149. https://doi.org/10.1038/d41586-020-01694-x
Article CAS ADS Google Scholar
Vuong QH (2020b) The limitations of retraction notices and the heroic acts of authors who correct the scholarly record: an analysis of retractions of papers published from 1975 to 2019. Learn Publ 33(2):119–130. https://doi.org/10.1002/leap.1282
Vuong QH (2018) The (ir) rational consideration of the cost of science in transition economies. Nat Hum Behav 2(1):5–5. https://doi.org/10.1038/s41562-017-0281-4
Wager E, Barbour V, Yentis S, Kleinert S (2009) Retractions: guidance from the Committee on Publication Ethics (COPE). Croat Med J 50(6):532–535. https://doi.org/10.3325/cmj.2009.50.532
Download references
Acknowledgements
We are thankful to Miss Meenakshi Chawla, library staff, B.B. Dixit Library, AIIMS for helping me in finding print versions of old retraction notices unavailable on the journal website. We are thankful to the Library facility, IHBAS for providing support in terms of access and a peaceful environment of work. The corresponding author was supported by a fellowship grant from the Department of Health Research, Ministry of Health & Family Welfare (R.12013/07/2019-HR) at the time of writing this paper.
Author information
Pratibha Sharma
Present address: Human Behaviour Department, Institute of Human Behaviour and Allied Sciences, Delhi, India
Authors and Affiliations
Department of Biophysics, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India
Pratibha Sharma, Asad Reza & Krishna Kishore Inampudi
Department of Statistics, Ramlal Anand College, University of Delhi, Delhi, India
Bhavya Sharma
Institute of Human Behaviour and Allied Sciences, Delhi, India
Rajinder K Dhamija
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Pratibha Sharma .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Additional information.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary file, additional data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Cite this article.
Sharma, P., Sharma, B., Reza, A. et al. A systematic review of retractions in biomedical research publications: reasons for retractions and their citations in Indian affiliations. Humanit Soc Sci Commun 10 , 597 (2023). https://doi.org/10.1057/s41599-023-02095-x
Download citation
Received : 03 November 2022
Accepted : 07 September 2023
Published : 21 September 2023
DOI : https://doi.org/10.1057/s41599-023-02095-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Writing a Biomedical Research Paper
A Guide to Structure and Style
- © 2009
- Brian Stephen Budgell 0
Universitä du Quäbec à Trois-Riviäres, Trois-Riviäres, Canada
You can also search for this author in PubMed Google Scholar
- Focuses very precisely on the biomedical research article. Biomedical language is quite different in many respects from general English and other scientific dialects, and so biomedical language deserves to be treated separately
- Deals with research articles, the publications which biomedical scientists have to produce in order to keep their jobs
- Derived largely from analyses of biomedical corpora. Hence, the advice offered is evidence-based rather than opinion-based, and is derived from examination of successful biomedical writing, i.e. that which has been published in peer-reviewed journals
- Web-based resources are available for exercises
- Includes supplementary material: sn.pub/extras
33k Accesses
1 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
Similar content being viewed by others.

A brief guide to the science and art of writing manuscripts in biomedicine

The Introduction Section
Beyond substance: grammar, syntax and style.
- biomedical research
- wissenschaftliche Publikation
Table of contents (13 chapters)
Front matter, beginning a manuscript, the title: your last chance to make a first impression, writing an eff ective introduction, ensuring the flow of discourse: conjunctions and conjuncts, hedging your bets and minding your modals, writing an eff ective methods section, the passive voice and i, writing an eff ective results section, the special case of case studies, writing an eff effctive discussion, is it a discussion or a systematic review, writing an eff ective abstract, the process of manuscript submission and review, back matter, authors and affiliations.
Brian Stephen Budgell
About the author
Bibliographic information.
Book Title : Writing a Biomedical Research Paper
Book Subtitle : A Guide to Structure and Style
Authors : Brian Stephen Budgell
DOI : https://doi.org/10.1007/978-4-431-88037-0
Publisher : Springer Tokyo
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer-Verlag Tokyo 2009
Softcover ISBN : 978-4-431-88036-3 Published: 08 January 2009
eBook ISBN : 978-4-431-88037-0 Published: 05 December 2008
Edition Number : 1
Number of Pages : VIII, 66
Topics : Medicine/Public Health, general , Biomedicine general
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Biomedical Research Paper Topics

This page offers students an extensive list of biomedical research paper topics , expert advice on how to choose these topics, and guidance on how to write a compelling biomedical research paper. The guide also introduces the services of iResearchNet, an academic assistance company that caters to the unique needs of each student. Offering expert writers, custom-written works, and a host of other features, iResearchNet provides the tools and support necessary for students to excel in their biomedical research papers.
100 Biomedical Research Paper Topics
Biomedical research is a vibrant field, with an extensive range of topics drawn from various sub-disciplines. It encompasses the study of biological processes, clinical medicine, and even technology and engineering applied to the domain of healthcare. Given the sheer breadth of this field, choosing a specific topic can sometimes be overwhelming. To help you navigate this rich landscape, here is a list of biomedical research paper topics, divided into ten categories, each with ten specific topics.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code.
1. Genetics and Genomics
- Role of genetics in rare diseases
- Advances in gene editing: CRISPR technology
- Human genome project: findings and implications
- Genetic basis of cancer
- Personalized medicine through genomics
- Epigenetic modifications and disease progression
- Genomic data privacy and ethical implications
- Role of genetics in mental health disorders
- Prenatal genetic screening and ethical considerations
- Gene therapy in rare genetic disorders
2. Bioengineering and Biotechnology
- Tissue engineering in regenerative medicine
- Bioprinting of organs: possibilities and challenges
- Role of nanotechnology in targeted drug delivery
- Biosensors in disease diagnosis
- Bioinformatics in drug discovery
- Development and application of biomaterials
- Bioremediation and environmental cleanup
- Biotechnology in agriculture and food production
- Therapeutic applications of stem cells
- Role of biotechnology in pandemic preparedness
3. Neuroscience and Neurology
- Pathophysiology of Alzheimer’s disease
- Advances in Parkinson’s disease research
- Role of neuroimaging in mental health diagnosis
- Understanding the brain-gut axis
- Neurobiology of addiction
- Role of neuroplasticity in recovery from brain injury
- Sleep disorders and cognitive function
- Brain-computer interfaces: possibilities and ethical issues
- Neural correlates of consciousness
- Epigenetic influence on neurodevelopmental disorders
4. Immunology
- Immune response to COVID-19
- Role of immunotherapy in cancer treatment
- Autoimmune diseases: causes and treatments
- Vaccination and herd immunity
- The hygiene hypothesis and rising allergy prevalence
- Role of gut microbiota in immune function
- Immunosenescence and age-related diseases
- Role of inflammation in chronic diseases
- Advances in HIV/AIDS research
- Immunology of transplantation
5. Cardiovascular Research
- Advances in understanding and treating heart failure
- Role of lifestyle factors in cardiovascular disease
- Cardiovascular disease in women
- Hypertension: causes and treatments
- Pathophysiology of atherosclerosis
- Role of inflammation in heart disease
- Novel biomarkers for cardiovascular disease
- Personalized medicine in cardiology
- Advances in cardiac surgery
- Pediatric cardiovascular diseases
6. Infectious Diseases
- Emerging and re-emerging infectious diseases
- Role of antiviral drugs in managing viral diseases
- Antibiotic resistance: causes and solutions
- Zoonotic diseases and public health
- Role of vaccination in preventing infectious diseases
- Infectious diseases in immunocompromised individuals
- Role of genomic sequencing in tracking disease outbreaks
- HIV/AIDS: prevention and treatment
- Advances in malaria research
- Tuberculosis: challenges in prevention and treatment
7. Aging Research
- Biological mechanisms of aging
- Impact of lifestyle on healthy aging
- Age-related macular degeneration
- Role of genetics in longevity
- Aging and cognitive decline
- Social aspects of aging
- Advances in geriatric medicine
- Aging and the immune system
- Role of physical activity in aging
- Aging and mental health
8. Endocrinology
- Advances in diabetes research
- Obesity: causes and health implications
- Thyroid disorders: causes and treatments
- Role of hormones in mental health
- Endocrine disruptors and human health
- Role of insulin in metabolic syndrome
- Advances in treatment of endocrine disorders
- Hormones and cardiovascular health
- Reproductive endocrinology
- Role of endocrinology in aging
9. Mental Health Research
- Advances in understanding and treating depression
- Impact of stress on mental health
- Advances in understanding and treating schizophrenia
- Child and adolescent mental health
- Mental health in the elderly
- Impact of social media on mental health
- Suicide prevention and mental health services
- Role of psychotherapy in mental health
- Mental health disparities
10. Oncology
- Advances in cancer immunotherapy
- Role of genomics in cancer diagnosis and treatment
- Lifestyle factors and cancer risk
- Early detection and prevention of cancer
- Advances in targeted cancer therapies
- Role of radiation therapy in cancer treatment
- Cancer disparities and social determinants of health
- Pediatric oncology: challenges and advances
- Role of stem cells in cancer
- Cancer survivorship and quality of life
These biomedical research paper topics represent a wide array of studies within the field of biomedical research, providing a robust platform to delve into the intricacies of human health and disease. Each topic offers a unique opportunity to explore the remarkable advancements in biomedical research, contributing to the ongoing quest to enhance human health and wellbeing.
Choosing Biomedical Research Paper Topics
The selection of a suitable topic for your biomedical research paper is a critical initial step that will largely influence the course of your study. The right topic will not only engage your interest but will also be robust enough to contribute to the existing body of knowledge. Here are ten tips to guide you in choosing the best topic for your biomedical research paper.
- Relevance to Your Coursework and Interests: Your topic should align with the courses you have taken or are currently enrolled in. Moreover, a topic that piques your interest will motivate you to delve deeper into research, resulting in a richer, more nuanced paper.
- Feasibility: Consider the practicality of your proposed research. Do you have access to the necessary resources, including the literature, laboratories, or databases needed for your study? Ensure that your topic is one that you can manage given your resources and time constraints.
- Novelty and Originality: While it is essential to ensure your topic aligns with your coursework and is feasible, strive to select a topic that brings a new perspective or fresh insight to your field. Originality enhances the contribution of your research to the broader academic community.
- Scope: A well-defined topic helps maintain a clear focus during your research. Avoid choosing a topic too broad that it becomes unmanageable, or so narrow that it lacks depth. Balancing the scope of your research is key to a successful paper.
- Future Career Goals: Consider how your chosen topic could align with or benefit your future career goals. A topic related to your future interests can provide an early start to your career, showcasing your knowledge in that particular field.
- Available Supervision and Mentoring: If you’re in a setting where you have a mentor or supervisor, choose a topic that fits within their area of expertise. This choice will ensure you have the best possible guidance during your research process.
- Ethical Considerations: Some topics may involve ethical considerations, particularly those involving human subjects, animals, or sensitive data. Make sure your topic is ethically sound and you’re prepared to address any related ethical considerations.
- Potential Impact: Consider the potential impact of your research on the field of biomedical science. The best research often addresses a gap in the current knowledge or has the potential to bring about change in healthcare practices or policies.
- Literature Gap: Literature review can help identify gaps in the existing body of knowledge. Choosing a topic that fills in these gaps can make your research more valuable and unique.
- Flexibility: While it’s essential to start with a clear topic, remain open to slight shifts or changes as your research unfolds. Your research might reveal a different angle or a more exciting question within your chosen field, so stay flexible.
Remember, choosing a topic should be an iterative process, and your initial ideas will likely evolve as you conduct a preliminary literature review and discuss your thoughts with your mentors or peers. The ultimate goal is to choose a topic that you are passionate about, as this passion will drive your work and make the research process more enjoyable and fulfilling.
How to Write a Biomedical Research Paper
Writing a biomedical research paper can be a daunting task. However, with careful planning and strategic execution, the process can be more manageable and rewarding. Below are ten tips to help guide you through the process of writing a biomedical research paper.
- Understand Your Assignment: Before you begin your research or writing, make sure you understand the requirements of your assignment. Know the expected length, due date, formatting style, and any specific sections or components you need to include.
- Thorough Literature Review: A comprehensive literature review allows you to understand the current knowledge in your research area and identify gaps where your research can contribute. It will help you shape your research question and place your work in context.
- Clearly Define Your Research Question: A well-defined research question guides your research and keeps your writing focused. It should be clear, specific, and concise, serving as the backbone of your study.
- Prepare a Detailed Outline: An outline helps organize your thoughts and create a roadmap for your paper. It should include all the sections of your research paper, such as the introduction, methods, results, discussion, and conclusion.
- Follow the IMRaD Structure: Most biomedical research papers follow the IMRaD format—Introduction, Methods, Results, and Discussion. This structure facilitates the orderly and logical presentation of your research.
- Use Clear and Concise Language: Biomedical research papers should be written in a clear and concise manner to ensure the reader understands the research’s purpose, methods, and findings. Avoid unnecessary jargon and ensure that complex ideas are explained clearly.
- Proper Citation and Reference: Always properly cite the sources of information you use in your paper. This not only provides credit where it’s due but also allows your readers to follow your line of research. Be sure to follow the citation style specified in your assignment.
- Discuss the Implications: In your discussion, go beyond simply restating your findings. Discuss the implications of your results, how they relate to previous research, and how they contribute to the existing knowledge in the field.
- Proofread and Edit: Never underestimate the importance of proofreading and editing. Checking for grammatical errors, punctuation mistakes, and clarity of language can enhance the readability of your paper.
- Seek Feedback Before Final Submission: Before submitting your paper, seek feedback from peers, mentors, or supervisors. Fresh eyes can often spot unclear sections or errors that you may have missed.
Writing a biomedical research paper is a significant academic endeavor, but remember that every researcher started where you are right now. It’s a process that requires time, effort, and patience. Remember, the ultimate goal is not just to get a good grade but also to contribute to the vast body of biomedical knowledge.
iResearchNet’s Custom Writing Services
Navigating the process of writing a biomedical research paper can be complex and demanding. At iResearchNet, we understand these challenges and strive to offer a stress-free, seamless solution to support your academic journey. With our roster of highly skilled, degree-holding writers, we are committed to delivering top-quality, custom-written papers tailored specifically to your individual requirements and desired outcomes.
- Expert Degree-Holding Writers: iResearchNet takes pride in our team of knowledgeable and experienced writers who hold advanced degrees in diverse fields. These writers are not only academic experts but are also keenly in tune with the complex landscape of biomedical research. This breadth and depth of expertise ensure that your paper benefits from a thorough understanding of the topic, resulting in a well-informed, academically credible document.
- Custom Written Works: We appreciate the unique academic goals and distinct requirements of each student. That’s why iResearchNet specializes in providing custom-written papers. Our aim is to capture your individual academic voice and perspective, blending it with our professional acumen to create a paper that reflects your specific academic needs and aspirations.
- In-Depth Research: Every paper that we produce is founded on the bedrock of extensive and in-depth research. Our writers are committed to exploring a wide range of credible and reputable sources to enrich your paper with diverse viewpoints and comprehensive information. This dedication to rigorous research ensures that your paper is not only thoroughly informed but also accurately referenced, adding to its academic integrity.
- Custom Formatting: Academic institutions often require different formatting styles. Be it APA, MLA, Chicago/Turabian, or Harvard, our writers are adept at all these academic formatting styles. We strive to adhere strictly to your specified formatting style, contributing to the polished and professional presentation of your paper.
- Top Quality: Quality is the cornerstone of our services at iResearchNet. We believe that each paper we craft should demonstrate a high standard of scholarship. Our writers dedicate their skills and effort to ensure every aspect of your paper, from clarity of language to depth of analysis and precision of information, reflects top-quality work.
- Customized Solutions: Recognizing that each research paper brings a distinct set of challenges and requirements, we offer customized solutions. Our approach is to thoroughly understand your specific needs and shape our writing services accordingly. We ensure that every aspect of your paper, from its overarching structure to the smallest details, aligns with your expectations.
- Flexible Pricing: We believe that high-quality academic writing services should be accessible. That’s why we offer our top-quality services at competitive prices, striking a careful balance between affordability and excellence. We provide a range of pricing options designed to cater to various budget needs without compromising on the quality of our services.
- Short Deadlines: Facing a fast-approaching deadline can be nerve-wracking. But with iResearchNet, you need not worry. Our expert writers are skilled at delivering high-quality papers under tight deadlines, even as short as three hours. Despite the time pressure, we ensure that the quality of your paper remains top-notch.
- Timely Delivery: At iResearchNet, we understand the critical importance of adhering to deadlines in the academic world. We commit to the timely delivery of all orders, ensuring that you are always able to submit your work on time. With our service, you can put aside worries about late submissions.
- 24/7 Support: Academic queries or concerns can arise at any time, and we are here to assist you around the clock. We have a dedicated support team ready to answer your questions, address your concerns, or simply provide guidance about your project, at any time of the day or night.
- Absolute Privacy: Your privacy is of utmost importance to us. All personal and financial information you share with us is handled with the highest level of confidentiality and security. Our strict privacy policies ensure that your details remain safe and private.
- Easy Order Tracking: We believe in providing a seamless experience for our clients. With our user-friendly platform, you can track your order’s progress easily and stay updated on your paper’s status. This feature provides real-time status reports, giving you peace of mind and assurance about the progress of your work.
- Money Back Guarantee: Your satisfaction is our ultimate goal. We strive to meet your expectations, but if for any reason the final work falls short, we offer a money-back guarantee. This policy is a testament to our confidence in the quality of our services and our commitment to your academic success.
At iResearchNet, we strive to be more than just a writing service provider. We aspire to be a trusted academic partner, providing support and expertise to help you navigate the complexities of writing a biomedical research paper. With our combination of expert knowledge, high commitment to quality, and excellent customer service, we are the ideal choice for all your academic writing needs.
Start Your Journey to Academic Success with iResearchNet Today!
Are you ready to elevate your academic journey and achieve your full potential? At iResearchNet, we are prepared to be your trusted partner every step of the way. Our team of expert writers, experienced in biomedical research, are ready and waiting to transform your academic vision into a top-quality, custom-written biomedical research paper that meets all your requirements.
Navigating the complexities of biomedical research can be overwhelming, but with iResearchNet, you don’t have to do it alone. Our dedicated team of professionals is committed to taking the stress out of the writing process, allowing you to focus on your learning. Imagine the relief of knowing your assignment is in the hands of experienced, degree-holding experts who are passionate about your success. With our meticulous research and thorough understanding of biomedical topics, we guarantee a paper that not only meets but surpasses your expectations.
From in-depth research and custom formatting to a final product that reflects the highest academic standards, iResearchNet provides a comprehensive solution for your academic needs. And it’s not just about delivering excellent papers. Our commitment extends to providing an exceptional experience marked by 24/7 support, absolute privacy, and a transparent order tracking system.
The clock is ticking, and your academic success is just a click away. Don’t let the opportunity to excel in your biomedical research paper slip through your fingers. Reach out to us today to start your journey with iResearchNet. You bring your academic aspirations, and we’ll bring our expertise and commitment. Together, let’s make your academic dreams come true.
ORDER HIGH QUALITY CUSTOM PAPER

- British Journal of Biomedical Science
- Special Issues
Current Reviews in the Biomedical Sciences
Total Downloads
Total Views and Downloads
About this Special Issue
This special issue will publish high-quality scholarly review papers on key topics in the biomedical sciences. It aims to highlight recent advances in the field, whilst emphasizing important directions and new possibilities for future inquiries. We anticipate this will promote discussion in the biomedical ...
Keywords : microbiome, Diabetes, Neurodegeneration, Inflammatory, toxicology
Issue Editors
Recent articles, participating journals.
Manuscripts can be submitted to this Special Issue via the following journals:
total views
- Demographics
No records found
total views article views downloads issue views
Top countries
Top referring sites.
- Open access
- Published: 10 November 2020
A brief guide to the science and art of writing manuscripts in biomedicine
- Diego A. Forero 1 , 2 ,
- Sandra Lopez-Leon ORCID: orcid.org/0000-0001-7504-3441 3 &
- George Perry 4
Journal of Translational Medicine volume 18 , Article number: 425 ( 2020 ) Cite this article
19k Accesses
22 Citations
211 Altmetric
Metrics details
Publishing articles in international scientific journals is the primary method for the communication of validated research findings and ideas. Journal articles are commonly used as a major input for evaluations of researchers and institutions. Few articles have been published previously about the different aspects needed for writing high-quality articles. In this manuscript, we provide an updated and brief guide for the multiple dimensions needed for writing manuscripts in the health and biological sciences, from current, international and interdisciplinary perspectives and from our expertise as authors, peer reviewers and editors. We provide key suggestions for writing major sections of the manuscript (e.g. title, abstract, introduction, methods, results and discussion), for submitting the manuscript and bring an overview of the peer review process and of the post-publication impact of the articles.
Introduction
Publishing articles in international scientific journals is the current primary approach for the communication of validated research findings and ideas. Scientific papers are commonly used as a major input for evaluations of researchers and institutions [ 1 , 2 ]. However, taking into account the evolving and multidimensional landscape of the publishing process, there is a need for additional updated training in the science and art of writing manuscripts for scientific journals.
Few articles have been published previously about the different aspects needed for writing high-quality articles [ 3 , 4 , 5 , 6 ]. In this article, we provide an updated and brief guide for the multiple dimensions needed for writing manuscripts in the health and biological sciences, from current, international and interdisciplinary perspectives and from our expertise as authors, peer reviewers and editors, extending and complementing previous publications about this topic. The writing of manuscripts in biomedicine has its own standards, including the availability of multiple guidelines for reporting different types of studies, which are discussed in this article.
General recommendations
One of the first steps before starting to write an article should be to read the main papers that have been previously published on the subject. The first search might be focused on the available literature reviews and meta-analyses, and key for a scientist, the technique of performing a proper literature review [ 7 ]. Science advances by building on what it is known and there is no point in re-inventing the wheel [ 8 ].
It has been suggested, when writing scientific papers, to keep it short, compact and simple, avoiding the excessive use of adjectives and adverbs [ 9 ]. If you read a word or sentence and it does not add anything, delete it.
The success of an article depends on the quality of primary data and their analyses, on the way it is written and on the clearness of the tables and figures. It is fundamental to follow the current standards of research integrity (such as avoiding plagiarism and data manipulation) [ 10 ]. Both negative and positive results should be published, to avoid publication bias [ 11 ].
Authors should keep in mind that scientific writing is a process that involves multiple steps, takes time, dedication and inspiration, and involves patience, motivation, analytical thinking and adherence to high-quality standards [ 86 ]. Table 1 provides an important number of online resources that facilitate the writing of scientific manuscripts.
Following international recommendations for the authorship of articles in the biomedical sciences, such as the ones from the International Committee of Medical Journal Editors (ICMJE), is a fundamental topic in scientific publications, in order to avoid ghost and gift authorship practices [ 12 , 13 ]. In general, authors should have a significant involvement in these 4 points: (1) study concept/design, data collection or data analysis/interpretation (2) drafting/revising the manuscript, (3) approving the final version and (4) holding responsibility for accuracy and integrity of all aspects of the reported research [ 14 ].
There is a trend for the increase of the number of authors over the years [ 15 ], which is a reflection of globalization and the increasing complexity of medical research [ 16 ]. In the last two decades, there has been an increased use of consortia authorship with very long lists of authors, usually derived from international mega-collaborations. Authors from non-English speaking countries might have to take into account the current standards for names (two first names and one last name), to avoid confusion in the indexing processes in databases. Authors with two last names can hyphen their two last names to avoid confusing their first last name with a middle name, although the use of ORCID identifiers facilitates the disambiguation of author profiles.
The meaning of the order of the listed authors varies between fields. In many disciplines, the author order indicates the magnitude of the contribution, with the last author usually representing the principal investigator [ 17 ]. It is possible to have an equal co-authorship, either for the first or corresponding authors [ 18 ].
Title and abstract
The Title [ 19 ] and the Abstract [ 20 ] are the two most visible items of the article [ 21 ], as they are the main sections indexed in bibliographic databases. These two elements compete for the reader’s attention; therefore both should be informative, accurate, attractive, concise, clear and specific [ 19 , 20 ]. It is advisable that the title of the manuscript reflects the actual findings of the work and be concise.
The Abstract section should provide a brief description of the main sections of the manuscript, describing key methods, findings and conclusions. It is recommended that the abstract be specific, clear, unbiased, honest, concise, precise, stand-alone, complete, and scholarly [ 22 ]. An important number of medical journals ask for structured abstracts. Usually, keywords are provided at the end of the Abstract section and the use of Medical Subject Headings (MeSH) as keywords is quite helpful.
Introduction section
Although the standards of the length of the Introduction vary between scientific fields (for example, they are longer in psychology journals), it is recommended that the introduction section should be concise, avoiding long reviews about the topics of the article. It has been proposed that the introduction section be designed as a cone or funnel, starting with the main points of the general topic, followed by a highlight of the existing knowledge gap, the hypothesis or main question of the article and ending with a brief overview of the approach of the current work [ 23 ].
Another recommendation is to keep it simple, including three main paragraphs: the first paragraph explaining what is known, the second what is not known and the third what the objective of the study is and explain what it will add to the scientific knowledge. When stating what is known, it should not be a full review of the literature, but it should be the essential information needed to understand the background. Information from the introduction should not overlap with the discussion. The paragraph explaining what is unknown should be focused on helping the reader understand why the research is being performed. The last paragraph should state the research question or hypothesis [ 24 ]. It is important to cite key articles (both recent reviews and related primary works) and to highlight the novelty of the current work.
Methods section
This section is essential and should be written to facilitate other researchers enabling them to replicate the study. This section has been compared to a recipe, which includes all the ingredients and how they need to be combined [ 25 ].
Key details of methods employed, such as overall design of the study, inclusion and exclusion criteria, sample sizes and statistical power, should be described [ 26 ]. Another way to subdivide it is with subheadings that might include: study design, setting, subjects, data collection and data analysis [ 25 ]. The incorporation of data about the origins of samples and validated criteria for diagnoses is indispensable, including key references to validated instruments and methodologies. Description of approval by institutional ethics committees and use of informed consent, when needed, is fundamental. In the case of the use of equipment and reagents, details of the respective manufacturers are needed. Statistical and bioinformatic analyses should be described clearly, including the details of statistical tests and the software used [ 27 , 28 , 29 , 30 ]. It is fundamental that all the results described in the Results section correlate with the procedures described in the Methods section.
Results section
The Results section should provide an adequate and complete description of the main findings of the work carried out. It is suggested to avoid the repetition of the same exact content of the Tables or Figures and to leave the interpretation of the results of the findings to the Discussion section [ 31 ]. The main messages and details of the Results section should be provided in the Figures and Tables. No interpretation should be provided in this section.
The results section should be seen as a mirror of the methods: for every method provided, there should be a corresponding result. Subheadings can be included and some suggestions might be: recruitment/response, characteristics of the sample, findings from primary analyses, secondary analyses and additional findings [ 32 ]. Exact p values should be presented and must always be shown together with the estimates and confidence intervals. There should be a consistency with the number of decimal places presented in the results section and in the tables. It is common to present one or two decimals places. Always present the absolute number of cases, in addition to relative measures (e.g. percentage was 22% -33/150-) [ 32 ].
Tables and figures
Tables facilitate the detailed presentation of the results and they should be constructed adequately. Abbreviations are useful for avoiding repetitions of phrases and should be explained in the footnotes [ 33 ]. Each table or figure should be self-explanatory, and there should be no need to read the text to be able to understand it. They have to be presented in the same chronological order, following how they are presented in the text [ 34 ].
For tables where a lot of information is presented, the p values that are statistically significant can be presented in bold. In case of long or complex tables, it is helpful to provide them as supplementary files, leaving the key data in the tables of the main text. It is important to provide details of statistical significance in the table, in order to avoid going back and forth between the tables and the text to read key data.
The creation of figures for scientific articles involves data visualization. A major element in the creation of figures is their focus on the representation of key findings without biases, avoiding the generation of overly complex figures. In addition, it is important to remove the repetition of the same data that is also presented as tables in the main manuscript. Description of key conventions should be provided in detail in the figure legends and it is important to avoid the misrepresentation of data [ 35 ], particularly digital enhancement. As the large majority of journals are published and distributed in digital formats, there are no actual restrictions for the adequate use of colors in scientific images. In case of photographs, it is important to follow the guidelines of the journals regarding image size and resolution. In addition, other recommendations are related to the use of adequate tools and parameters for the generation of figures [ 36 ].
Discussion section
It has been proposed that the general outline of the discussion can be seen as an inverted funnel. Thus, it has been suggested that the configurations of the introduction and discussion sections can have, together, the form of an hourglass [ 37 ] (Fig. 1 ). The first paragraph is usually a summary of the important results, focused on answering the research question. The next paragraphs should focus on integrating the findings with what is known in the literature. If there are different findings, each should have a separate paragraph. The discussion of each result should follow the same order of the methods and results. A balanced contextualization of findings of the current study should be provided by citing the key previous original articles and related reviews that put the results in perspective [ 38 ]. If there are differences between the findings and previously published studies, the differences and similarities of the results and studies should be stated.
A graphical overview of the general structure of research articles
It is important to list the strengths and the limitations of the study. An explanation of the implications of those limitations should be included. An essential point is to include the needs and the perspectives for future studies. It can be stated that the results need replication or to highlight new questions that appeared after the analyses. This point can be of great guidance for future studies and can help the advance of science. It is highly advisable to avoid very long discussion sections and overstatements about the actual findings. The discussion section should not have results that were not described in the Results section. The last paragraph should include a conclusion that clearly states what the study adds to the knowledge.
References section
Although each journal usually has its own citation style, the Vancouver style is quite common in medical journals. There are several freely and commercially available programs (such as EndNote, Zotero or Mendeley) that facilitate the citations process and the generation of the bibliography, including the details for multiple citation styles. They can help to organize, store, download -and most importantly- format the references to the style requirements of the journal you want to submit to. By having the references in these programs, it is easy to reformat the style for any other journal in a matter of seconds.
Always try to cite the original source behind a key statement, making sure that the reference you mention is not only mentioning another source. If you need to choose among several references, take into consideration the level of evidence, the year of publication and the quality of the work [ 39 ].
It is important to verify that the bibliography includes all the publications cited and to check issues with names of authors or journals. Several journals have limitations in the number of citations for certain types of publications.
Acknowledgments and other sections
Usually, the authors thank their funding agencies for their economical support for the studies carried out. In addition, it is possible to include acknowledgements to people who helped with the development of the work (technical support, for example) or in the writing of the manuscript (such as corrections of use of the English language) [ 40 ]. In several cases, the journals ask for declarations about ethical considerations and declarations of the roles of individual authors (such as the design of the study and/or the collection or analysis of the data) [ 41 ]. Declarations of potential conflicts of interest is fundamental for the transparency of scientific activities [ 12 , 42 ].
Supplementary data
With modern high-throughput methods, the size of the analyzed datasets is becoming larger and larger. This means that there is a growing need to provide access to the large datasets as supplementary files (such as spreadsheets or pdf files) or to include them in publicly available repositories (such as OSF or figshare) [ 43 ]. In addition, certain fields have specific guidelines asking authors to submit their data to specific online repositories (such as the NCBI GEO database for whole genome expression data) [ 44 ].
Review articles and other types of publications
There are two main types of review articles: systematic reviews and narrative reviews. In the case of systematic reviews and meta-analyses there are important standards to follow, including the need for well-defined search strategies [ 45 ]. For the writing of narrative reviews [ 46 , 47 ], it is essential to define its scope and current needs and it is highly advisable to construct tables and figures to consolidate and visualize the key information. Articles for case reports follow a different structure and there are recommendations about their development [ 48 ].
Reporting guidelines
It is important to follow published guidelines for the reporting of studies in clinical research, such as STROBE for observational studies [ 49 ], STROBE-ME for molecular epidemiology studies [ 50 ], STREGA for genetic association studies [ 51 ], PRISMA for systematic reviews and meta-analyses [ 52 ], TRIPOD for prediction models of diagnosis or prognosis [ 53 ], CONSORT for clinical trials [ 54 ], CARE for case reports [ 55 ] and AGREE II for practice guidelines [ 56 ], in addition to ARRIVE 2.0 for animal research [ 57 ]. For molecular and cellular analyses, there are several important guidelines, such as MIQE for qPCR [ 58 , 59 ], flow cytometry [ 60 ], cell death [ 61 ], mutational analyses [ 62 ], simulation experiments [ 63 ] and gene nomenclatures [ 64 , 65 ].
Find the best candidate journals
There are several aspects that the authors should take into account in the selection of a journal, such as local standards of publications, the visibility or impact of the journals and their affinities with the topics of the manuscripts. It is highly advisable to verify the indexing of the journals in key databases, such as PubMed, Scopus/Scimago (quartiles) and Journal Citation Reports (impact factor) [ 66 , 67 ]. Finally, authors should be careful with the growing number of predatory journals [ 68 ], which commonly mention spurious impact factors [ 69 ]. Another way to determine which journal is suitable is to see the list of the references in your study. Before selecting the journal, read all the instructions and make sure the scope of the journal and editor preference fits your manuscript. Make a list of 3 to 5 journals, and rank them [ 70 ]. In several cases, sending a pre-submission enquiry to the editor of the journal is helpful [ 71 ]. There is a growing trend for the initial divulgation of manuscripts as preprints, in repositories such as bioRxiv and medRxiv [ 72 ].
Submission and peer review
It is fundamental to follow the guidelines for authors of the selected journal. In addition to manuscript files, tables, figures and supplementary data, it is common that the authors provide a cover letter (highlighting the main contributions of the work) in their submissions. In the cover letter it is recommended to include: (1) Your request to submit your work (mentioning the title). (2) 2–3 sentences summarizing the significance of the work (importance, main finding, message) (3) A statement of the relevance to the journal audience (eg. A related work published in the journal) (4) Any statement required from the journal, such as that the material has not been submitted/published elsewhere [ 73 ].
There are differences in peer review practices between journals. In many cases, there are two or more peer reviewers in a single-blind approach (the authors do not know the identities of the reviewers). In other cases, there is an approach based in double-blind, in which the reviewers also do not know the identities of the authors. In recent years, there has been an increase in the implementation of open peer review, in which the identities and concepts of the reviewers are publicly available.
Answer to peer reviewers
When addressing the comments and questions of the peer reviewers do it in a new document. Copy/paste all comments and number them. For each comment briefly respond and indicate where the change was made in the manuscript. The response should be in present tense or past present (e.g. We now present; we have added to the first paragraph).
Make the changes in the paper with “track changes” or highlighting the change in another color. Be thankful and respectful to each reviewer and editor and take each comment very seriously. If you disagree with the comment, add solid evidence, adding references or key data [ 74 ].
The process of providing adequate answers to peer reviewers and editors and of the incorporation of their suggestions into the revised manuscript is an important challenge [ 75 ] in the publishing of an article.
Open science
Interest in Open Science practices has been growing in recent years, considering their advantages to facilitate the access to information and their potential to increase the reproducibility and the quality of research findings [ 76 , 77 , 78 , 79 , 80 ]. It has been shown that open access articles [ 81 ] have advantages in terms of the amount of citations [ 82 ] and that articles that provide links to repositories with primary data have also have a higher citation count [ 43 ]. Open Science, in addition to open peer review, also involves open protocols, materials [ 8 , 83 ] and data (Fig. 2 ).
An overview of the different dimensions and components of Open Science
Post-publication impact
Citation counts are one of the main ways to measure the scientific impact of publications, allowing the development of multiple metrics, such as the H index [ 84 ], to measure the influence and visibility of scientists and research groups [ 1 ]. Recently, there is a growing use of alternative metrics [ 85 ], which measure other types of article mentions (such as social networks, blogs and news, recorded by Altmetric) or downloads. There are platforms (such as PubPeer and Retraction Watch) that allow comments on published articles, facilitating divulgation of possible issues on reported findings (among others) and to visualize information about retracted articles.
Conclusions
The quality of scientific publications is directly related to the careful revision by peer reviewers of the manuscript, in order to improve the submitted manuscript. This process means that receiving feedback is a constant process and that authors should have the resilience to receive rejections and recommendations for major changes [ 2 ]. In addition, authors can have feedback from collaborators before submitting the manuscript (including revision of the use of the English language) and they can benefit themselves from the experience of being peer reviewers [ 86 ]. In the current scientific environment, publishing an article is not the end of a process; it is the beginning: the article is beginning its journey of being read and analyzed by people around the world.
The writing of a scientific article is a work of art that is honed with experience. The more publications you have, the easier it is to write a manuscript. The collaboration between authors can be very enriching and give rise to new projects and new learnings. The contribution to science and to following generations comes with every single article one publishes. Therefore, one should always strive for the best.
Availability of data and materials
Not applicable.
Ioannidis JPA, Baas J, Klavans R, Boyack KW. A standardized citation metrics author database annotated for scientific field. PLoS Biol. 2019;17:e3000384.
Article CAS PubMed PubMed Central Google Scholar
Forero DA, Moore JH. Considerations for higher efficiency and productivity in research activities. BioData Min. 2016;9:35.
Article PubMed PubMed Central Google Scholar
Zhang W. Ten simple rules for writing research papers. PLoS Comput Biol. 2014;10:e1003453.
Article PubMed PubMed Central CAS Google Scholar
Vitse CL, Poland GA. Writing a scientific paper-A brief guide for new investigators. Vaccine. 2017;35:722–8.
Article PubMed Google Scholar
Meo SA. Anatomy and physiology of a scientific paper. Saudi J Biol Sci. 2018;25:1278–83.
Cook DA. Twelve tips for getting your manuscript published. Med Teach. 2016;38:41–50.
Cronin P, Ryan F, Coughlan M. Undertaking a literature review: a step-by-step approach. Br J Nurs. 2008;17:38–43.
Masum H, Rao A, Good BM, Todd MH, Edwards AM, Chan L, Bunin BA, Su AI, Thomas Z, Bourne PE. Ten simple rules for cultivating open science and collaborative R&D. PLoS Comput Biol. 2013;9:e1003244.
Weinberger CJ, Evans JA, Allesina S. Ten simple (empirical) rules for writing science. PLoS Comput Biol. 2015;11:e1004205.
Shaw DM, Erren TC. Ten simple rules for protecting research integrity. PLoSComputBiol. 2015;11:e1004388.
Google Scholar
van Aert RCM, Wicherts JM, van Assen M. Publication bias examined in meta-analyses from psychology and medicine: a meta-meta-analysis. PLoS ONE. 2019;14:e0215052.
Rohwer A, Young T, Wager E, Garner P. Authorship, plagiarism and conflict of interest: views and practices from low/middle-income country health researchers. BMJ Open. 2017;7:e018467.
Kornhaber RA, McLean LM, Baber RJ. Ongoing ethical issues concerning authorship in biomedical journals: an integrative review. Int J Nanomedicine. 2015;10:4837–46.
Gotzsche PC, Kassirer JP, Woolley KL, Wager E, Jacobs A, Gertel A, Hamilton C. What should be done to tackle ghostwriting in the medical literature? PLoS Med. 2009;6:e23.
Gulen S, Fonnes S, Andresen K, Rosenberg J. Increasing number of authors in Cochrane reviews. J Evid Based Med. 2020;13:34–41.
Weeks WB, Wallace AE, Kimberly BC. Changes in authorship patterns in prestigious US medical journals. Soc Sci Med. 2004;59:1949–54.
Tscharntke T, Hochberg ME, Rand TA, Resh VH, Krauss J. Author sequence and credit for contributions in multiauthored publications. PLoS Biol. 2007;5:e18.
Hosseini M. Equal co-authorship practices: review and recommendations. Sci Eng Ethics. 2020;26:1133–48.
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. The principles of biomedical scientific writing: title. Int J EndocrinolMetab. 2019;17:e98326.
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. The principles of biomedical scientific writing: abstract and keywords. Int J EndocrinolMetab. 2020;18:e100159.
Cook DA, Bordage G. Twelve tips on writing abstracts and titles: how to get people to use and cite your work. Med Teach. 2016;38:1100–4.
Tullu MS. Writing the title and abstract for a research paper: being concise, precise, and meticulous is the key. Saudi J Anaesth. 2019;13:S12–7.
Annesley TM. “It was a cold and rainy night”: set the scene with a good introduction. Clin Chem. 2010a;56:708–13.
Article CAS PubMed Google Scholar
Cals JW, Kotz D. Effective writing and publishing scientific papers, part III: introduction. J Clin Epidemiol. 2013a;66:702.
Kotz D, Cals JW. Effective writing and publishing scientific papers, part IV: methods. J Clin Epidemiol. 2013a;66:817.
Annesley TM. Who, what, when, where, how, and why: the ingredients in the recipe for a successful Methods section. Clin Chem. 2010b;56:897–901.
Lang T. Twenty statistical errors even you can find in biomedical research articles. Croat Med J. 2004;45:361–70.
PubMed Google Scholar
Worthy G. Statistical analysis and reporting: common errors found during peer review and how to avoid them. Swiss Med Wkly. 2015;145:w14076.
Patnala R, Clements J, Batra J. Candidate gene association studies: a comprehensive guide to useful in silico tools. BMC Genet. 2013;14:39.
Makin TR, de OrbanXivry JJ. Ten common statistical mistakes to watch out for when writing or reviewing a manuscript. Elife. 2019;8:e48175.
Annesley TM. Show your cards: the results section and the poker game. Clin Chem. 2010c;56:1066–70.
Kotz D, Cals JW. Effective writing and publishing scientific papers, part V: results. J Clin Epidemiol. 2013b;66:945.
Annesley TM. Bring your best to the table. Clin Chem. 2010d;56:1528–34.
Kotz D, Cals JW. Effective writing and publishing scientific papers, part VII: tables and figures. J Clin Epidemiol. 2013c;66:1197.
Annesley TM. Put your best figure forward: line graphs and scattergrams. Clin Chem. 2010e;56:1229–33.
Rougier NP, Droettboom M, Bourne PE. Ten simple rules for better figures. PLoS Comput Biol. 2014;10:e1003833.
Cals JW, Kotz D. Effective writing and publishing scientific papers, part VI: discussion. J Clin Epidemiol. 2013b;66:1064.
Annesley TM. The discussion section: your closing argument. Clin Chem. 2010f;56:1671–4.
Cals JW, Kotz D. Effective writing and publishing scientific papers, part VIII: references. J Clin Epidemiol. 2013c;66:1198.
Paul-Hus A, Desrochers N. Acknowledgements are not just thank you notes: A qualitative analysis of acknowledgements content in scientific articles and reviews published in 2015. PLoS ONE. 2019;14:e0226727.
Brand A, Allen L, Altman M. Hlava M. Scott JJLP: Beyond authorship: attribution, contribution, collaboration, and credit. 2015;28:151–5.
Ancker JS, Flanagin A. A comparison of conflict of interest policies at peer-reviewed journals in different scientific disciplines. Sci Eng Ethics. 2007;13:147–57.
Colavizza G, Hrynaszkiewicz I, Staden I, Whitaker K, McGillivray B. The citation advantage of linking publications to research data. PLoS ONE. 2020;15:e0230416.
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–71.
Forero DA, Lopez-Leon S, Gonzalez-Giraldo Y, Bagos PG. Ten simple rules for carrying out and writing meta-analyses. PLoS Comput Biol. 2019;15:e1006922.
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–17.
Gregory AT, Denniss AR. An introduction to writing narrative and systematic reviews—tasks, tips and traps for aspiring authors. Heart Lung Circ. 2018;27:893–8.
Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296.
Article Google Scholar
Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch-Volders M, Matullo G, Phillips DH, Schoket B, Stromberg U, et al. STrengthening the Reporting of OBservational studies in Epidemiology-Molecular Epidemiology (STROBE-ME): an extension of the STROBE Statement. PLoS Med. 2011;8:e1001117.
Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, et al. STrengthening the REporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009;6:e22.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med. 2015;13:1.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, Group C. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013;7:223.
Hoffmann-Esser W, Siering U, Neugebauer EA, Brockhaus AC, Lampert U, Eikermann M. Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. PLoS ONE. 2017;12:e0174831.
du Percie Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoSBiol. 2020;18:e3000410.
Article CAS Google Scholar
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22.
dMIQE Group, Huggett JF. The digital MIQE guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. ClinChem. 2020;66:1012–29.
Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, Aghaeepour N, Akdis M, Allez M, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019;49:1457–973.
Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–107.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Waltemath D, Adams R, Beard DA, Bergmann FT, Bhalla US, Britten R, Chelliah V, Cooling MT, Cooper J, Crampin EJ, et al. Minimum Information About a Simulation Experiment (MIASE). PLoS Comput Biol. 2011;7:e1001122.
Bruford EA, Braschi B, Denny P, Jones TEM, Seal RL, Tweedie S. Guidelines for human gene nomenclature. Nat Genet. 2020;52:754–8.
Seal RL, Chen LL, Griffiths-Jones S, Lowe TM, Mathews MB, O’Reilly D, Pierce AJ, Stadler PF, Ulitsky I, Wolin SL, Bruford EA. A guide to naming human non-coding RNA genes. EMBO J. 2020;39:e103777.
Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22:338–42.
Martín-Martín A, Orduna-Malea E, Thelwall M, López-Cózar ED. Google Scholar, Web of Science, and Scopus: a systematic comparison of citations in 252 subject categories. JOI. 2018;12:1160–77.
Forero DA, Oermann MH, Manca A, Deriu F, Mendieta-Zeron H, Dadkhah M, Bhad R, Deshpande SN, Wang W, Cifuentes MP. Negative effects of “Predatory” journals on global health research. Ann Glob Health. 2018;84:584–9.
Gutierrez FR, Beall J, Forero DA. Spurious alternative impact factors: the scale of the problem from an academic perspective. BioEssays. 2015;37:474–6.
Cals JW, Kotz D. Effective writing and publishing scientific papers, part X: choice of journal. J Clin Epidemiol. 2014;67:3.
Lengauer T, Nussinov R. How to write a presubmission inquiry. PLoS Comput Biol. 2015;11:e1004098.
Abdill RJ, Blekhman R. Tracking the popularity and outcomes of all bioRxiv preprints. Elife. 2019. https://doi.org/10.7554/eLife.45133 .
Kotz D, Cals JW. Effective writing and publishing scientific papers, part XI: submitting a paper. J Clin Epidemiol. 2014a;67:123.
Kotz D, Cals JW. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014b;67:243.
Annesley TM. Top 10 tips for responding to reviewer and editor comments. Clin Chem. 2011;57:551–4.
Allen C, Mehler DMA. Open science challenges, benefits and tips in early career and beyond. PLoS Biol. 2019;17:e3000246.
Munafò MR, Nosek BA, Bishop DV, Button KS, Chambers CD, Du Sert NP, Simonsohn U, Wagenmakers E-J, Ware JJ. A manifesto for reproducible science. Nat Hum Behav. 2017;1:1–9.
Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, Buck S, Chambers CD, Chin G, Christensen G, et al. Promoting an open research culture. Science. 2015;348:1422–5.
Carroll MW. Creative commons and the openness of open access. N Engl J Med. 2013;368:789–91.
Carroll MW. Sharing research data and intellectual property law: a primer. PLoSBiol. 2015;13:e1002235.
Piwowar H, Priem J, Lariviere V, Alperin JP, Matthias L, Norlander B, Farley A, West J, Haustein S. The state of OA: a large-scale analysis of the prevalence and impact of Open Access articles. PeerJ. 2018;6:e4375.
Eysenbach G. Citation advantage of open access articles. PLoS Biol. 2006;4:e157.
McKiernan EC. Imagining the “open” university: sharing scholarship to improve research and education. PLoS Biol. 2017;15:e1002614.
Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569–72.
Thelwall M, Haustein S, Lariviere V, Sugimoto CR. Do altmetrics work? Twitter and ten other social web services. PLoS ONE. 2013;8:e64841.
Bourne PE. Ten simple rules for getting published. PLoS Comput Biol. 2005;1:e57.
Download references
Acknowledgements
DAF has been previously supported by research grants from MinCiencias. GP is supported by the NIH and the Alzheimer´s Association. The authors thank Leon Ruiter Lopez for his help in the creation of Fig. 1 .
No specific funding was received for this work.
Author information
Authors and affiliations.
Health and Sport Sciences Research Group, School of Health and Sport Sciences, Fundación Universitaria del Área Andina, Bogotá, Colombia
Diego A. Forero
MSc Program in Epidemiology, School of Health and Sport Sciences, Fundación Universitaria del Área Andina, Bogotá, Colombia
Global Drug Development, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
Sandra Lopez-Leon
Department of Biology and Neurosciences Institute, The University of Texas at San Antonio, San Antonio, TX, USA
George Perry
You can also search for this author in PubMed Google Scholar
Contributions
DAF wrote an initial draft of the manuscript; DAF, SLL and GP contributed to different sections of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Diego A. Forero or Sandra Lopez-Leon .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
DAF does not report conflicts of interest. SLL is employee of Novartis Pharmaceutical Company; the statements presented in the paper do not necessarily represent the position of the company. GP is on the boards of Neurotrope and Neurotez. DAF, SL-L and GP are members of the editorial boards of several scientific journals.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Forero, D.A., Lopez-Leon, S. & Perry, G. A brief guide to the science and art of writing manuscripts in biomedicine. J Transl Med 18 , 425 (2020). https://doi.org/10.1186/s12967-020-02596-2
Download citation
Received : 15 September 2020
Accepted : 29 October 2020
Published : 10 November 2020
DOI : https://doi.org/10.1186/s12967-020-02596-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Peer review
- Abstracting and indexing
- Publications
- Biological science disciplines
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

The landscape of biomedical research
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Dmitry Kobak
- For correspondence: [email protected]
- Info/History
- Preview PDF
The number of publications in biomedicine and life sciences has rapidly grown over the last decades, with over 1.5 million papers now published every year. This makes it difficult to keep track of new scientific works and to have an overview of the evolution of the field as a whole. Here we present a 2D atlas of the entire corpus of biomedical literature, and argue that it provides a unique and useful overview of the life sciences research. We base our atlas on the abstract texts of 21 million English articles from the PubMed database. To embed the abstracts into 2D, we use a large language model PubMedBERT, combined with t -SNE tailored to handle samples of our size. We use our atlas to study the emergence of the Covid-19 literature, the evolution of the neuroscience discipline, the uptake of machine learning, and the distribution of gender imbalance in academic authorship. Furthermore, we present an interactive web version of our atlas that allows easy exploration and will enable further insights and facilitate future research.
Competing Interest Statement
Benjamin M. Schmidt is Vice President of Information at Nomic AI. The other authors declare no conflicts of interest.
https://static.nomic.ai/pubmed.html
https://github.com/berenslab/pubmed-landscape
https://zenodo.org/record/7695390
View the discussion thread.
Thank you for your interest in spreading the word about bioRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Scientific Communication and Education
- Animal Behavior and Cognition (5573)
- Biochemistry (12660)
- Bioengineering (9538)
- Bioinformatics (31014)
- Biophysics (15948)
- Cancer Biology (13030)
- Cell Biology (18640)
- Clinical Trials (138)
- Developmental Biology (10081)
- Ecology (15067)
- Epidemiology (2067)
- Evolutionary Biology (19258)
- Genetics (12806)
- Genomics (17639)
- Immunology (12770)
- Microbiology (29896)
- Molecular Biology (12465)
- Neuroscience (65149)
- Paleontology (483)
- Pathology (2015)
- Pharmacology and Toxicology (3486)
- Physiology (5382)
- Plant Biology (11167)
- Scientific Communication and Education (1731)
- Synthetic Biology (3079)
- Systems Biology (7718)
- Zoology (1739)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Biomed Eng

BMC Biomedical Engineering: a home for all biomedical engineering research
Alexandros houssein.
1 Springer Nature, 4 Crinan Street, London, N1 9XW UK
Alan Kawarai Lefor
2 Department of Surgery, Jichi Medical University, Shimotsuke, Tochigi Japan
Antonio Veloso
3 Laboratory of Biomechanics and Functional Morphology, Faculty of Human Kinetics, Lisbon, Portugal
4 Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN USA
Jong Chul Ye
5 Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea
Dimitrios I. Zeugolis
6 Regenerative, Modular and Developmental Engineering Laboratory (REMODEL), Biomedical Sciences Building, National University of Ireland Galway (NUI Galway), Galway, Ireland
Sang Yup Lee
7 Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea
Associated Data
Not applicable.
This editorial accompanies the launch of BMC Biomedical Engineering , a new open access, peer-reviewed journal within the BMC series, which seeks to publish articles on all aspects of biomedical engineering. As one of the first engineering journals within the BMC series portfolio, it will support and complement existing biomedical communities, but at the same time, it will provide an open access home for engineering research. By publishing original research, methodology, database, software and review articles, BMC Biomedical Engineering will disseminate quality research, with a focus on studies that further the understanding of human disease and that contribute towards the improvement of human health.
Introduction
Biomedical engineering is a multidisciplinary field that integrates principles from engineering, physical sciences, mathematics and informatics for the study of biology and medicine, with the ultimate goal of improving human health and quality of life.
Biomedical engineering is not a new concept; however, it was not until the 1900s when rapid technological advancements in the chemical, physical and life sciences influenced breakthroughs in the prevention, diagnosis and treatment of disease. The invention of the electrocardiograph, the concept of x-ray imaging, the electron microscope, the mechanical heart valve and human genome sequencing, are just a few examples of technological innovations that revolutionised science and medicine and changed the approach to human healthcare. Current biomedical engineering technologies are a growing part of clinical decision making, which can now be influenced from multiscale observations, ranging from the nano to the macro-scale.
Today, the need for innovation in health technologies is ever more prominent. The annual global healthcare spending has seen continued growth and is projected to reach a staggering $8.7 trillion by 2020 [ 1 ]. Global health challenges are becoming more complex, wide spread and difficult to control. Resources are scarce and with a growing population, our society has a need for affordable, portable and sustainable solutions. The World Health Organisation has pledged to make a billion lives healthier by 2023 [ 2 ], a goal that will require widespread commitment by governments, funding agencies, researchers and clinicians. Biomedical engineers will be at the heart of this movement and face a responsibility for continuous innovation. Biomedical engineering research is expected to create health technologies that will drastically improve the prevention, diagnosis and treatment of disease, as well as patient rehabilitation. As an example, the NIH 2016–2020 strategic plan focuses on point of care and precision medicine technologies including genetic engineering, microfluidics, nanomedicine, imaging, digital/mobile-Health and big data [ 3 ].
BMC Biomedical Engineering will strive to complement these efforts and provide an open access venue for the dissemination of all biomedical engineering research. As part of the BMC series, a portfolio of journals serving communities across all sciences, the Journal will act as a resource for a wide range of disciplines. It aims to support scientists, engineers and clinicians by making their research openly and permanently available, irrespective of their location or affiliation.
Aims and scope
BMC Biomedical Engineering considers articles on all aspects of biomedical engineering, including fundamental, translational and clinical research. It combines tools and methods from biology and medicine with mathematics, physical sciences and engineering towards the understanding of human biology and disease and the improvement of human health. The Journal will publish a range of article types, including research, methodology, software, database and review articles.
As part of the BMC series, a collection of open access, peer-reviewed and community focused journals covering all areas of science, editorial decisions will not be made on the basis of the interest of a study or its likely impact. Studies must be scientifically valid. For research articles this includes a scientifically sound research question, the use of suitable methods and analysis, and following community-agreed standards relevant to the research field.
BMC Biomedical Engineering aims to publish work that undergoes a thorough peer review process by appropriate peer-reviewers and is deemed to be a coherent and valid addition to the scientific knowledge. It aims to provide an open access venue which allows for immediate and effective dissemination of research and enables our readers to explore and understand the latest developments, trends and practices in biomedical engineering. We believe that open access and the Creative Commons Attribution License [ 4 ] are essential to this, allowing universal and free access to all articles published in the Journal and allowing them to be read and the data re-used without restrictions. BMC Biomedical Engineering will work closely with the rest of the journals in the BMC series portfolio [ 5 ] to help authors find the right home for their research. We will highlight selected journal content through various promotional channels to ensure the research reaches its target audience and receives the attention it deserves.
Editorial sections
Many new technologies that have revolutionised biomedical engineering require the coalition of previously independent communities. 3D bioprinting of tissues and organs brings together methods from cell biology, biomaterials, nanotechnology and engineering and is being used for the transplantation of tissues, including skin, bone, muscle, soft tissue, cartilage and others [ 6 , 7 ]. The concept of tissue and disease modelling is being driven towards drug discovery and toxicology studies, aiming to increase the yield of drug testing by tackling limitations of current cell and animal models [ 8 ].
New approaches in natural and synthetic biomaterials have redefined bioelectronics. Silk fibroins and other unconventional interfaces can form flexible electronics and challenge the use of silicon-based technologies. For biomedical applications, these new approaches present advantages not only due to their biocompatibility and low cost, but also for their electromechanical and optical virtues [ 9 ]. Implantable probes are being redesigned so that they facilitate long term stability and high resolution, without perturbing the biological system or creating an immune response. Such technologies are now able to facilitate recordings of single neurons in vivo, in a chronically stable manner, with applications to the restoration of vision and retinal prosthetics [ 10 ].
For many years biomedical imaging has been connecting microscopic discoveries with macroscopic observations. Photoacoustic tomography (PAT) is now able to image large spatial scales, from organelles to small animals, at very high speeds [ 11 ]. In fact, single-shot real-time imaging can operate at 10 trillion frames per second and is finding applications in breast cancer diagnosis [ 12 , 13 ].
In the field of medical robotics, new approaches combine machine learning and artificial intelligence to strengthen the clinician’s decision making. Others are leveraging augmented reality (AR) to facilitate better immersion and more natural surgical workflows for computer assisted orthopaedic surgery [ 14 ].
BMC Biomedical Engineering celebrates the interdisciplinary nature of the field. In order to navigate the wide range of biomedical engineering research, the Journal is structured in six editorial sections.
- Biomaterials, nanomedicine and tissue engineering
- Medical technologies, robotics and rehabilitation engineering
- Biosensors and bioelectronics
- Computational and systems biology
- Biomechanics
- Biomedical Imaging
We are delighted to welcome our founding Section Editors along with a growing international group of Editorial board Members [ 15 , 16 ]. The Journal is supported by an expert Editorial Advisory group of senior engineers and scientists, which is chaired by Distinguished Professor Sang Yup Lee. Together with the in-house Editor, this group will provide academic leadership and expertise and will work together to transverse into multiple clinical and engineering disciplines. The Editorial Board will keep growing and developing to reflect and adapt to the nature of this diverse community.
Biomaterials, nanomedicine and tissue engineering section
This section primarily focuses on the development of biofunctional tissue substitutes, which possess the highest level of biomimicry, through recapitulation of nature’s innate sophistication and thorough processes. It considers research, methods, clinical trials, leading opinion and review articles on the development, characterisation and application of nano- and micro- biofunctional biomaterials, cell-assembled tissue substitutes, diagnostic tools, microfluidic devices and drug/gene discovery and delivery methods. Manuscripts focusing on permanently differentiated, engineered and stem cell biology and application are welcome. This section will place a substantial focus on clinical translation and technologies that advance the current status-quo. As such, articles that enhance the scalability and robustness of tissue engineering methodologies, or that enable new and improved industrial or clinical applications of biomedical engineering discoveries, tools and technologies are strongly encouraged.
Medical technologies, robotics and rehabilitation engineering section
This section seeks to represent research in engineering that encompasses a wide range of interests across medical specialties, including orthopaedic, cardiovascular, musculoskeletal, craniofacial, neurological, urologic and other medical technologies. It will consider research on medical robotics, computer assisted technologies, medical devices, e/m-health and other medical instrumentation. It aims to improve the prevention, diagnosis, intervention and treatment of injury or disease and it welcomes articles that represent new approaches to engineering that may be useful in the care of patients. Technical and practical aspects of rehabilitation engineering, from concept to clinic and papers on improving the quality of life of patients with a disability are encouraged. The section also seeks to represent clinically important research that is based on new and emerging technologies. This could include clinical studies of new approaches to robotic-assisted surgery, clinical studies of new devices, or other studies that are close to patient care or rehabilitation.
Biosensors and bioelectronics section
This section considers articles on the theory, design, development and application on all aspects of biosensing and bioelectronics technologies. The section will consider approaches that combine biology and medicine with sensing and circuits and systems technologies on a wide variety of subjects, including lab-on-chips, microfluidic devices, biosensor interfaces, DNA chips and bioinstrumentation. It also considers articles on the development of computational algorithms (such as deep learning, reinforcement learning, etc.) that interpret the acquired signals, hardware acceleration and implementation of the algorithms, brain-inspired or brain-like computational schemes, and bioelectronics technologies that can have a wide impact in the research and clinical community. Articles on implantable and wearable electronics, low-power, wireless and miniaturised imaging systems, organic semiconductors, smart sensors and neuromorphic circuits and systems are strongly encouraged.
Computational and systems biology section
Computational, integrative and systemic approaches are at the heart of biomedical engineering. This section considers papers on all aspects of mathematical, computational, systems and synthetic biology that result in the improvement of patient health. Integrative and multi-scale approaches, in the network and mechanism-based definition of injury and disease, or its prevention, diagnosis and treatment are welcome. Papers on high precision, interactive and personalised medicine, on digital/mobile health, on complex/big data analytics and machine learning, or on systemic and informatics approaches in a healthcare or clinical setting are encouraged.
Biomechanics section
This section represents the interdisciplinary field of biomechanics and investigates the relationship of structure with function in biological systems from the micro- to the macro- world. It considers papers on all aspects of analytical and applied biomechanics at all scales of observation, that improve the diagnosis, therapy and rehabilitation of patients or that advance their kinetic performance. The topics of interest range from mechanobiology and cell biomechanics to clinical biomechanics, orthopaedic biomechanics and human kinetics. Articles on the mechanics and wear of bones and joints, artificial prostheses, body-device interaction, musculoskeletal modelling biomechanics and solid/fluid computational approaches are strongly encouraged.
Biomedical imaging section
Biomedical imaging has been connecting microscopic discoveries with macroscopic observations for the diagnosis and treatment of disease and has seen considerable advances in recent years. This section will consider articles on all biomedical imaging modalities including medical imaging (MRI, CT, PET, ultrasound, x-ray, EEG/MEG), bio-imaging (microscopy, optical imaging) and neuroimaging across all scales of observation. Its primary focus will be to foster integrative approaches that combine techniques in biology, medicine, mathematics, computation, hardware development and image processing. Articles on new methodologies or on technical perspectives involving novel imaging concepts and reconstruction methods, machine learning, sparse sampling and statistical analysis tool development are encouraged.
The motivation for the launch of BMC Biomedical Engineering is to create an authoritative, unbiased and community-focused open access journal. We are committed to working together with our authors, editors and reviewers to provide an inclusive platform for the publication of high-quality manuscripts that span all aspects of biomedical engineering research. We welcome articles from all over the world and we will devote our efforts to ensure a robust and fair peer-review process for all. We believe in continuous improvement and we encourage the community to get in touch with us to provide ideas and feedback on how to improve the Journal and serve the community better.
We hope you will find the first group of articles an interesting and valuable read, and we look forward to working with you all to disseminate research into the exciting field of biomedical engineering.
Acknowledgements
Availability of data and materials, abbreviations.
| AR | Augmented Reality |
| CT | Computed Tomography |
| EEG | Electroencephalogram |
| MEG | Magnetoencephalography |
| PAT | Photoacoustic Tomography |
| PET | Positron Emission Tomography |
Authors’ contributions
AH wrote the introduction, aims and scope and conclusion. AH, AKL, AV, ZY, JCY, DIZ and SYL wrote the editorial sections. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Consent for publication, competing interests.
AH is the Editor of BMC Biomedical Engineering and an employee of Springer Nature. AL, AV, ZY, JY, DZ and SL are members of the Editorial Board of BMC Biomedical Engineering .
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
In 1987, the New York Times Magazine characterized the Human Genome Project as the "biggest, costliest, most provocative biomedical research project in history." 2 But in the years between the ...
B. Linju Yen, Li-Tzu Wang, Hsiu-Huang Wang, Chin-Pao Hung, Pei-Ju Hsu, Chia-Chi Chang, Chien-Yu Liao, Huey-Kang Sytwu and Men-Luh Yen. Journal of Biomedical Science 2024 31 :49. Research Published on: 13 May 2024. The Correction to this article has been published in Journal of Biomedical Science 2024 31 :61.
1. Context. The "results section" is the heart of the paper, around which the other sections are organized ().Research is about results and the reader comes to the paper to discover the results ().In this section, authors contribute to the development of scientific literature by providing novel, hitherto unknown knowledge ().In addition to the results, this section contains data and ...
Abstract. The materials and methods (M&M) section is the heart of a scientific paper and is subject to initial screening of the editor to decide whether the manuscript should be sent for external review. If the M&M section of a scientific paper be considered as a recipe, its ingredients would be who, what, when, where, how, and why.
The past 200 years have seen rapid advances in western biomedicine. A model arising from western Europe and North America, current biomedical science is largely driven by efforts to prevent or cure diseases. It uses hierarchies of evidence generated from observational and experimental research,1 and is arguably driven by the interests of scientists who hold this underlying philosophy, with ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on BIOMEDICAL RESEARCH. Find methods information, sources, references or conduct a literature review on ...
A free repository of open-access full-text biomedical and life sciences research articles. Includes citation information and text-mining tools that link to external molecular and medical databases. ... aim to cite the original research paper rather than a review article that subsequently discussed the work. This accurately assigns credit and ...
Our review highlights the significance and content of the "result section", structuring and presenting findings, utilizing effective presentation techniques, reporting statistics, and maintaining language and logical flow. We suggested guidelines for authors to consider when writing "results sections" in biomedical scientific papers.
Multimodal machine learning (also referred to as multimodal learning) is a subfield of machine learning that aims to develop and train models that can leverage multiple different types of data and ...
Basic biomedical research papers accumulated a significant number of citations primarily due to their publication in the form of review papers (1172 citations post-issuance of retraction notices ...
An abstract is a self-contained, short, powerful statement that describes a larger body of work. It may be incorporated as part of a published paper, book, grant proposal, thesis, research report, or a conference paper. An abstract of a scientific paper will be published online independently, so it should make sense when it is read alone.
Exchange of knowledge in every field of Biomedical Science | Explore the latest full-text research PDFs, articles, conference papers, preprints and more on BIOMEDICAL SCIENCE. Find methods ...
BioMed Research International is an open access journal publishing original research articles, review articles, and clinical studies covering a wide range of subjects within the biomedical sciences. As part of Wiley's Forward Series, this journal offers a streamlined, faster publication experience with a strong emphasis on integrity.
Here, we highlight the most recent progress of these biomedical engineering (BME) technologies applied to COVID-19 diagnosis in this review, as well as provide insights into how the research direction in this field has shifted in response to practical demands for disease surveillance and personal healthcare.
In this paper, we reviewed the latest developments in the application of AI in biomedicine, including disease diagnostics and prediction, living assistance, biomedical information processing, and biomedical research. AI has interesting applications in many other biomedical areas as well.
Overview. Focuses very precisely on the biomedical research article. Biomedical language is quite different in many respects from general English and other scientific dialects, and so biomedical language deserves to be treated separately. Derived largely from analyses of biomedical corpora. Hence, the advice offered is evidence-based rather ...
The review also suggests experiences on how to effectively present data in numbers such as percentages, statistical measures such as p-values, and other advanced forms of statistics, Finally, the review recommends relevant points to keep language brevity and logical flow in writing the "results section" of a scientific paper.
Biomedical research is a vibrant field, with an extensive range of topics drawn from various sub-disciplines. It encompasses the study of biological processes, clinical medicine, and even technology and engineering applied to the domain of healthcare. Given the sheer breadth of this field, choosing a specific topic can sometimes be overwhelming.
This special issue will publish high-quality scholarly review papers on key topics in the biomedical sciences. It aims to highlight recent advances in the field, whilst emphasizing important directions and new possibilities for future inquiries. We anticipate this will promote discussion in the biomedical science community that will translate to best practice applications in clinical, and ...
Reviewing Manuscripts for Biomedical Journals. Writing for publication is a complex task. For many professionals, producing a well-executed manuscript conveying one's research, ideas, or educational wisdom is challenging. Authors have varying emotions related to the process of writing for scientific publication.
Following international recommendations for the authorship of articles in the biomedical sciences, such as the ones from the International Committee of Medical Journal Editors (ICMJE), is a fundamental topic in scientific publications, in order to avoid ghost and gift authorship practices [12, 13].In general, authors should have a significant involvement in these 4 points: (1) study concept ...
The number of publications in biomedicine and life sciences has rapidly grown over the last decades, with over 1.5 million papers now published every year. This makes it difficult to keep track of new scientific works and to have an overview of the evolution of the field as a whole. Here we present a 2D atlas of the entire corpus of biomedical literature, and argue that it provides a unique ...
For research articles this includes a scientifically sound research question, the use of suitable methods and analysis, and following community-agreed standards relevant to the research field. BMC Biomedical Engineering aims to publish work that undergoes a thorough peer review process by appropriate peer-reviewers and is deemed to be a ...