Benefits of Using Plants in Indoor Environments: Exploring Common Research Gaps
- October 2021
- ACE Arquitectura Ciudad y Entorno 1(2):83-98

- Technische Universität München

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- SCI TOTAL ENVIRON

- BUILD ENVIRON

- Lana Van Galen
- Wong Lai Kee

- Joan Villanueva
- Antoni Rosell
- Gaia Stringari

- Int J Environ Res Publ Health

- Li-Shih Liao

- Philip Gibbons

- Ming fang Tang
- SENSORS-BASEL

- Yuko Yokota
- Marni Barnes

- J EXPO SCI ENV EPID
- Bryan E. Cummings
- Michael S. Waring
- Chen Qibing
- Liu Yinggao
- Liu Shiliang
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The basic roles of indoor plants in human health and comfort
Affiliations.
- 1 School of Energy Science and Engineering, Central South University, Changsha, 410083, China.
- 2 School of Energy Science and Engineering, Central South University, Changsha, 410083, China. [email protected].
- 3 XiangYa School of Public Health, Central South University, Changsha, 410078, Hunan, China. [email protected].
- PMID: 30387059
- DOI: 10.1007/s11356-018-3554-1
Humans have a close relationship with nature, and so integrating the nature world into indoor space could effectively increase people's engagement with nature, and this in turn may benefit their health and comfort. Since people spend 80-90% of their time indoors, the indoor environment is very important for their health. Indoor plants are part of natural indoor environment, but their effect on the indoor environment and on humans has not been quantified. This review provides a comprehensive summary of the role and importance of indoor plants in human health and comfort according to the following four criteria: photosynthesis; transpiration; psychological effects; and purification. Photosynthesis and transpiration are important mechanisms for plants, and the basic functions maintaining the carbon and oxygen cycles in nature. Above all have potential inspiration to human's activities that people often ignored, for example, the application of solar panel, artificial photosynthesis, and green roof/facades were motivated by those functions. Indoor plants have also been shown to have indirect unconscious psychological effect on task performance, health, and levels of stress. Indoor plants can act as indoor air purifiers, they are an effective way to reduce pollutants indoor to reduce human exposure, and have been widely studied in this regard. Indoor plants have potential applications in other fields, including sensing, solar energy, acoustic, and people's health and comfort. Making full use of various effects in plants benefit human health and comfort.
Keywords: Health; Indoor plants; Photosynthesis; Psychological effect; Purification; Transpiration.
PubMed Disclaimer
Similar articles
- Plants for Sustainable Improvement of Indoor Air Quality. Brilli F, Fares S, Ghirardo A, de Visser P, Calatayud V, Muñoz A, Annesi-Maesano I, Sebastiani F, Alivernini A, Varriale V, Menghini F. Brilli F, et al. Trends Plant Sci. 2018 Jun;23(6):507-512. doi: 10.1016/j.tplants.2018.03.004. Epub 2018 Apr 19. Trends Plant Sci. 2018. PMID: 29681504
- Variation of Al concentrations depending on the growing environment in some indoor plants that used in architectural designs. Cetin M, Abo Aisha AES. Cetin M, et al. Environ Sci Pollut Res Int. 2023 Feb;30(7):18748-18754. doi: 10.1007/s11356-022-23434-6. Epub 2022 Oct 11. Environ Sci Pollut Res Int. 2023. PMID: 36219289
- An Experiment with Air Purifiers in Delhi during Winter 2015-2016. Vyas S, Srivastav N, Spears D. Vyas S, et al. PLoS One. 2016 Dec 15;11(12):e0167999. doi: 10.1371/journal.pone.0167999. eCollection 2016. PLoS One. 2016. PMID: 27978542 Free PMC article.
- Towards practical indoor air phytoremediation: A review. Pettit T, Irga PJ, Torpy FR. Pettit T, et al. Chemosphere. 2018 Oct;208:960-974. doi: 10.1016/j.chemosphere.2018.06.048. Epub 2018 Jun 6. Chemosphere. 2018. PMID: 30068040 Review.
- The use of indoor plant as an alternative strategy to improve indoor air quality in Indonesia. Susanto AD, Winardi W, Hidayat M, Wirawan A. Susanto AD, et al. Rev Environ Health. 2020 Sep 14;36(1):95-99. doi: 10.1515/reveh-2020-0062. Print 2021 Mar 26. Rev Environ Health. 2020. PMID: 32920542 Review.
- Critical factors influencing visitor emotions: analysis of "restorativeness" in urban park visits in Fuzhou, China. Wu Y, Liu J, Quevedo JMD, Cheng H, Yu K, Kohsaka R. Wu Y, et al. Front Public Health. 2023 Nov 21;11:1286518. doi: 10.3389/fpubh.2023.1286518. eCollection 2023. Front Public Health. 2023. PMID: 38074738 Free PMC article.
- Effects of nature on restorative and cognitive benefits in indoor environment. Rhee JH, Schermer B, Han G, Park SY, Lee KH. Rhee JH, et al. Sci Rep. 2023 Aug 14;13(1):13199. doi: 10.1038/s41598-023-40408-x. Sci Rep. 2023. PMID: 37580348 Free PMC article.
- Phytoremediation for the indoor environment: a state-of-the-art review. Matheson S, Fleck R, Irga PJ, Torpy FR. Matheson S, et al. Rev Environ Sci Biotechnol. 2023;22(1):249-280. doi: 10.1007/s11157-023-09644-5. Epub 2023 Feb 27. Rev Environ Sci Biotechnol. 2023. PMID: 36873270 Free PMC article. Review.
- Impact of COVID-19 containment zone built-environments on students' mental health and their coping mechanisms. Asim F, Chani PS, Shree V. Asim F, et al. Build Environ. 2021 Oct;203:108107. doi: 10.1016/j.buildenv.2021.108107. Epub 2021 Jul 1. Build Environ. 2021. PMID: 36567701 Free PMC article.
- Effects of Indoor Plants on Human Functions: A Systematic Review with Meta-Analyses. Han KT, Ruan LW, Liao LS. Han KT, et al. Int J Environ Res Public Health. 2022 Jun 17;19(12):7454. doi: 10.3390/ijerph19127454. Int J Environ Res Public Health. 2022. PMID: 35742700 Free PMC article. Review.
- Plant Physiol. 1995 Dec;109(4):1427-1433 - PubMed
- Bull Environ Contam Toxicol. 2000 Feb;64(2):302-8 - PubMed
- J Allergy Clin Immunol. 2008 Mar;121(3):585-91 - PubMed
- J Hazard Mater. 2011 Aug 15;192(1):314-8 - PubMed
- J Altern Complement Med. 2009 Sep;15(9):975-80 - PubMed
Publication types
- Search in MeSH
Grants and funding
- 51576214/National Natural Science Foundation of China
- 21777193/National Natural Science Foundation of China
- 2017SK2091/the Key Research and Development Program of Hunan Province
LinkOut - more resources
Full text sources.
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 06 November 2019
Potted plants do not improve indoor air quality: a review and analysis of reported VOC removal efficiencies
- Bryan E. Cummings 1 &
- Michael S. Waring 1
Journal of Exposure Science & Environmental Epidemiology volume 30 , pages 253–261 ( 2020 ) Cite this article
12k Accesses
40 Citations
1214 Altmetric
Metrics details
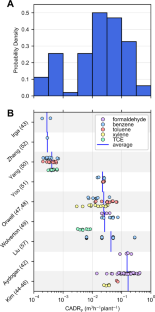
Potted plants have demonstrated abilities to remove airborne volatile organic compounds (VOC) in small, sealed chambers over timescales of many hours or days. Claims have subsequently been made suggesting that potted plants may reduce indoor VOC concentrations. These potted plant chamber studies reported outcomes using various metrics, often not directly applicable to contextualizing plants’ impacts on indoor VOC loads. To assess potential impacts, 12 published studies of chamber experiments were reviewed, and 196 experimental results were translated into clean air delivery rates (CADR, m 3 /h), which is an air cleaner metric that can be normalized by volume to parameterize first-order loss indoors. The distribution of single-plant CADR spanned orders of magnitude, with a median of 0.023 m 3 /h, necessitating the placement of 10–1000 plants/m 2 of a building’s floor space for the combined VOC-removing ability by potted plants to achieve the same removal rate that outdoor-to-indoor air exchange already provides in typical buildings (~1 h −1 ). Future experiments should shift the focus from potted plants’ (in)abilities to passively clean indoor air, and instead investigate VOC uptake mechanisms, alternative biofiltration technologies, biophilic productivity and well-being benefits, or negative impacts of other plant-sourced emissions, which must be assessed by rigorous field work accounting for important indoor processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
251,40 € per year
only 41,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Carbon dioxide guidelines for indoor air quality: a review
Exploring toilet plume bioaerosol exposure dynamics in public toilets using a Design of Experiments approach

Recent progress in indoor CO 2 capture for urban decarbonization
Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Exposure Sci Environ Epidemiol. 2001;11:231–52.
Article CAS Google Scholar
Weschler CJ. Ozone’s impact on public health: contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ Health Perspect. 2006;114:1489–96.
Wallace L. Indoor particles: a review. J Air Waste Manag Assoc. 1996;46:98–126.
Wallace L. Indoor sources of ultrafine and accumulation mode particles: size distributions, size-resolved concentrations, and source strengths. Aerosol Sci Technol. 2006;40:348–60.
Weschler CJ, Shields HC. Production of the hydroxyl radical in indoor air. Environ Sci Technol. 1996;30:3250–8.
Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos Environ. 2008;42:9018–40.
Brown SK, Sim MR, Abramson MJ, Gray CN. Concentrations of volatile organic compounds in indoor air—a review. Indoor Air. 1994;4:123–34.
Morawska L, Afshari A, Bae GN, Buonanno G, Chao CYH, Hänninen O, et al. Indoor aerosols: from personal exposure to risk assessment. Indoor Air. 2013;23:462–87.
Johnson AM, Waring MS, DeCarlo PF. Real-time transformation of outdoor aerosol components upon transport indoors measured with aerosol mass spectrometry. Indoor Air. 2017;27:230–40.
Avery AM, Waring MS, DeCarlo PF. Seasonal variation in aerosol composition and concentration upon transport from the outdoor to indoor environment. Environ Sci: Process Impacts. 2019;21:528–47.
CAS Google Scholar
Uhde E, Salthammer T. Impact of reaction products from building materials and furnishings on indoor air quality—a review of recent advances in indoor chemistry. Atmos Environ. 2007;41:3111–28.
Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos Environ. 2004;38:2841–65.
Huang Y, Ho SSH, Ho KF, Lee SC, Yu JZ, Louie PKK. Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J Hazard Mater. 2011;186:344–51.
Brinke JT, Selvin S, Hodgson AT, Fisk WJ, Mendell MJ, Koshland CP, et al. Development of new volatile organic compound (VOC) exposure metrics and their relationship to “sick building syndrome” symptoms. Indoor Air 1998;8:140–52.
Article Google Scholar
Jones AP. Indoor air quality and health. Atmos Environ. 1999;33:4535–64.
Wallace LA. Human exposure to volatile organic pollutants: implications for indoor air studies. Annu Rev Energy Environ. 2001;26:269–301.
Wieslander G, Norbäck D, Edling C. Airway symptoms among house painters in relation to exposure to volatile organic compounds (VOCs)—a longitudinal study. Ann Occup Hyg. 1997;41:155–66.
Yu C, Crump D. A review of the emission of VOCs from polymeric materials used in buildings. Build Environ. 1998;33:357–74.
Waring MS. Secondary organic aerosol in residences: predicting its fraction of fine particle mass and determinants of formation strength. Indoor Air. 2014;24:376–89.
Youssefi S, Waring MS. Predicting secondary organic aerosol formation from terpenoid ozonolysis with varying yields in indoor environments. Indoor Air. 2012;22:415–26.
Waring MS, Wells JR. Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: Magnitudes and impacts of oxidant sources. Atmos Environ (1994). 2015;106:382–91.
Cummings BE, Waring MS. Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 2019;29:616–29.
Youssefi S, Waring MS. Indoor transient SOA formation from ozone+α-pinene reactions: Impacts of air exchange and initial product concentrations, and comparison to limonene ozonolysis. Atmos Environ. 2015;112:106–15.
Youssefi S, Waring MS. Transient secondary organic aerosol formation from limonene ozonolysis in indoor environments: impacts of air exchange rates and initial concentration ratios. Environ Sci Technol. 2014;48:7899–908.
Yang Y, Waring MS. Secondary organic aerosol formation initiated by α-terpineol ozonolysis in indoor air. Indoor Air. 2016;26:939–52.
Rohr AC. The health significance of gas- and particle-phase terpene oxidation products: a review. Environ Int. 2013;60:145–62.
Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys. 2009;9:5155–236.
Lin Y-H, Arashiro M, Clapp PW, Cui T, Sexton KG, Vizuete W, et al. Gene expression profiling in human lung cells exposed to isoprene-derived secondary organic aerosol. Environ Sci Technol. 2017;51:8166–75.
Wargocki P, Sundell J, Bischof W, Brundrett G, Fanger PO, Gyntelberg F, et al. Ventilation and health in non-industrial indoor environments: report from a European multidisciplinary scientific consensus meeting (EUROVEN). Indoor Air. 2002;12:113–28.
Mendell MJ, Eliseeva EA, Davies MM, Spears M, Lobscheid A, Fisk WJ, et al. Association of classroom ventilation with reduced illness absence: a prospective study in California elementary schools. Indoor Air. 2013;23:515–28.
Wargocki P, Wyon DP, Fanger PO. The performance and subjective responses of call-center operators with new and used supply air filters at two outdoor air supply rates. Indoor Air. 2004;14 Suppl 8:7–16.
Haverinen‐Shaughnessy U, Moschandreas DJ, Shaughnessy RJ. Association between substandard classroom ventilation rates and students’ academic achievement. Indoor Air 2011;21:121–31.
Carrer P, Wargocki P, Fanetti A, Bischof W, De Oliveira Fernandes E, Hartmann T, et al. What does the scientific literature tell us about the ventilation–health relationship in public and residential buildings? Build Environ. 2015;94:273–86.
Fisk WJ, Mirer AG, Mendell MJ. Quantification of the association of ventilation rates with sick building syndrome symptoms. Berkeley, CA, USA: Lawrence Berkeley National Lab. (LBNL); 2009. Report No.: LBNL-2035E. https://www.osti.gov/biblio/962711
Rackes A, Ben‐David T, Waring MS. Outcome-based ventilation: a framework for assessing performance, health, and energy impacts to inform office building ventilation decisions. Indoor Air. 2018;28:585–603.
Quang TN, He C, Morawska L, Knibbs LD. Influence of ventilation and filtration on indoor particle concentrations in urban office buildings. Atmos Environ. 2013;79:41–52.
Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269–88.
Ben-David T, Wang S, Rackes A, Waring MS. Measuring the efficacy of HVAC particle filtration over a range of ventilation rates in an office building. Build Environ. 2018;144:648–56.
Benne K, Griffith B, Long N, Torcellini P, Crawley D, Logee T. Assessment of the energy impacts of outside air in the commercial sector. 2009. Report No.: NREL/TP-550-41955, 951796. http://www.osti.gov/servlets/purl/951796-l2ErYY/
Rackes A, Waring MS. Alternative ventilation strategies in U.S. offices: Comprehensive assessment and sensitivity analysis of energy saving potential. Build Environ. 2017;116:30–44.
Ben-David T, Rackes A, Waring MS. Alternative ventilation strategies in U.S. offices: Saving energy while enhancing work performance, reducing absenteeism, and considering outdoor pollutant exposure tradeoffs. Build Environ. 2017;116:140–57.
Aydogan A, Montoya LD. Formaldehyde removal by common indoor plant species and various growing media. Atmos Environ. 2011;45:2675–82.
Irga PJ, Torpy FR, Burchett MD. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos Environ. 2013;77:267–71.
Kim KJ, Kil MJ, Song JS, Yoo EH, Son K-C, Kays SJ. Efficiency of volatile formaldehyde removal by indoor plants: contribution of aerial plant parts versus the root zone. J Am Soc Hort Sci. 2008;133:521–6.
Kim KJ, Jeong MI, Lee DW, Song JS, Kim HD, Yoo EH, et al. Variation in formaldehyde removal efficiency among indoor plant species. HortScience 2010;45:1489–95.
Kim KJ, Kim HJ, Khalekuzzaman M, Yoo EH, Jung HH, Jang HS. Removal ratio of gaseous toluene and xylene transported from air to root zone via the stem by indoor plants. Environ Sci Pollut Res. 2016;23:6149–58.
Orwell RL, Wood RL, Tarran J, Torpy F, Burchett MD. Removal of benzene by the indoor plant/substrate microcosm and implications for air quality. Water Air Soil Pollut. 2004;157:193–207.
Orwell RL, Wood RA, Burchett MD, Tarran J, Torpy F. The potted-plant microcosm substantially reduces indoor air VOC pollution: II. Laboratory study. Water Air Soil Pollut. 2006;177:59–80.
Wolverton BC, Johnson A, Bounds K. Interior landscape plants for indoor air pollution abatement. 1989. Report No.: NASA-TM-101766. https://ntrs.nasa.gov/search.jsp?R=19930073077
Yang DS, Pennisi SV, Son K-C, Kays SJ. Screening indoor plants for volatile organic pollutant removal efficiency. HortScience 2009;44:1377–81.
Yoo MH, Kwon YJ, Son K-C, Kays SJ. Efficacy of indoor plants for the removal of single and mixed volatile organic pollutants and physiological effects of the volatiles on the plants. J Am Soc Horticultural Sci 2006;131:452–8.
Zhang L, Routsong R, Strand SE. Greatly enhanced removal of volatile organic carcinogens by a genetically modified houseplant, pothos Ivy ( Epipremnum aureum ) expressing the mammalian cytochrome P450 2e1 gene. Environ Sci Technol. 2019;53:325–31.
Rackes A, Waring MS. Do time-averaged, whole-building, effective volatile organic compound (VOC) emissions depend on the air exchange rate? A statistical analysis of trends for 46 VOCs in U.S. offices. Indoor Air 2016;26:642–59.
Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, et al. Relationship of indoor, outdoor and personal air (RIOPA) study: study design, methods and quality assurance/control results. J Expo Sci Environ Epidemiol. 2005;15:123–37.
Turpin BJ, Weisel CP, Morandi M, Colome S, Stock T, Eisenreich S, et al. Relationships of indoor, outdoor, and personal air (RIOPA): part II. Analyses of concentrations of particulate matter species. Res Rep Health Eff Inst. 2007;130 Pt 2:1–77. discussion 79–92.
Google Scholar
Xu Z, Wang L, Hou H. Formaldehyde removal by potted plant–soil systems. J Hazard Mater. 2011;192:314–8.
Liu Y-J, Mu Y-J, Zhu Y-G, Ding H, Crystal Arens N. Which ornamental plant species effectively remove benzene from indoor air? Atmos Environ. 2007;41:650–4.
Cruz MD, Müller R, Svensmark B, Pedersen JS, Christensen JH. Assessment of volatile organic compound removal by indoor plants—a novel experimental setup. Environ Sci Pollut Res. 2014;21:7838–46.
Hörmann V, Brenske K-R, Ulrichs C. Suitability of test chambers for analyzing air pollutant removal by plants and assessing potential indoor air purification. Water Air Soil Pollut. 2017;228:402.
Girman J, Phillips T, Levin H. Critical review: how well do house plants perform as indoor air cleaners? 2009;5.
Dingle P, Tapsell P, Hu S. Reducing formaldehyde exposure in office environments using plants. Bull Environ Contam Toxicol. 2000;64:302–8.
Wood RA, Burchett MD, Alquezar R, Orwell RL, Tarran J, Torpy F. The potted-plant microcosm substantially reduces indoor air VOC pollution: I. Office field-study. Water Air Soil Pollut. 2006;175:163–80.
Levin H. Can house plants solve IAQ problems?. Indoor Air Bull. 1992;2:1–7.
Wood RA, Orwell RL, Tarran J, Torpy F, Burchett M. Potted-plant/growth media interactions and capacities for removal of volatiles from indoor air. J Horticult Sci Biotechnol. 2002;77:120–9.
Waring MS, Siegel JA, Corsi RL. Ultrafine particle removal and generation by portable air cleaners. Atmos Environ. 2008;42:5003–14.
Kim H-J, Han B, Kim Y-J, Yoon Y-H, Oda T. Efficient test method for evaluating gas removal performance of room air cleaners using FTIR measurement and CADR calculation. Build Environ. 2012;47:385–93.
Chen W, Zhang J, Zhang ZB. Performance of air cleaners for removing multi-volatile organic compounds in indoor air. ASHRAE Trans. 2005;111:1101–14.
Russell JA, Hu Y, Chau L, Pauliushchyk M, Anastopoulos I, Anandan S, et al. Indoor-biofilter growth and exposure to airborne chemicals drive similar changes in plant root bacterial communities. Appl Environ Microbiol. 2014;80:4805–13.
Darlington A, Chan M, Malloch D, Pilger C, Dixon MA. The biofiltration of indoor air: implications for air quality. Indoor Air 2000;10:39–46.
Darlington AB, Dat JF, Dixon MA. The biofiltration of indoor air: air flux and temperature influences the removal of toluene, ethylbenzene, and xylene. Environ Sci Technol. 2001;35:240–6.
Alraddadi O, Leuner H, Boor B, Rajkhowa B, Hutzel W, Dana M. Air cleaning performance of a biowall for residential applications. International High Performance Buildings Conference. 2016. https://docs.lib.purdue.edu/ihpbc/185
Wang Z, Zhang JS. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build Environ. 2011;46:758–68.
Soreanu G, Dixon M, Darlington A. Botanical biofiltration of indoor gaseous pollutants—a mini-review. Chem Eng J. 2013;229:585–94.
Mikkonen A, Li T, Vesala M, Saarenheimo J, Ahonen V, Kärenlampi S, et al. Biofiltration of airborne VOCs with green wall systems—microbial and chemical dynamics. Indoor Air. https://onlinelibrary.wiley.com/doi/abs/10.1111/ina.12473 . 2018.
Bringslimark T, Hartig T, Patil GG. The psychological benefits of indoor plants: A critical review of the experimental literature. J Environ Psychol. 2009;29:422–33.
Peñuelas J, Llusià J. Plant VOC emissions: making use of the unavoidable. Trends Ecol Evolution. 2004;19:402–4.
Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010;15:176–84.
Download references
Author information
Authors and affiliations.
Department of Civil, Architectural and Environmental Engineering, Drexel University, 3141 Chestnut, St. Philadelphia, PA, 19104, USA
Bryan E. Cummings & Michael S. Waring
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Michael S. Waring .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental information, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Cummings, B.E., Waring, M.S. Potted plants do not improve indoor air quality: a review and analysis of reported VOC removal efficiencies. J Expo Sci Environ Epidemiol 30 , 253–261 (2020). https://doi.org/10.1038/s41370-019-0175-9
Download citation
Received : 28 February 2019
Revised : 18 June 2019
Accepted : 12 July 2019
Published : 06 November 2019
Issue Date : 01 March 2020
DOI : https://doi.org/10.1038/s41370-019-0175-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Empirical/statistical models
- Volatile organic compounds
- Exposure modeling
This article is cited by
Effective mitigation strategies for reducing workers’ exposure to formaldehyde: a systematic review.
- Federica Castellani
- Matteo Vitali
- Carmela Protano
Air Quality, Atmosphere & Health (2024)
Phytoremediation for the indoor environment: a state-of-the-art review
- S. Matheson
- F. R. Torpy
Reviews in Environmental Science and Bio/Technology (2023)
Potted plants can remove the pollutant nitrogen dioxide indoors
- Curtis Gubb
- Tijana Blanusa
- Christian Pfrang
Air Quality, Atmosphere & Health (2022)
Effects of airflow rate and plant species on formaldehyde removal by active green walls
Environmental Science and Pollution Research (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Users agree that automated translations may not effectively convert the intended design, meaning, and/or context of the website, may not translate images or PDF content, and may not take into account regional language differences. Any person that uses the translated site does so at that person’s own risk. UGA Extension is not responsible for any damages, costs, liability, or risk associated with any use, functionality, and/or content of the website translations.
For more information, visit the Language Translation page.
Growing Indoor Plants with Success
Bodie V. Pennisi, Extension Floriculture Specialist
Introduction
Factors affecting plant growth, temperature, relative humidity, soil/growing medium, what to look for when shopping for indoor plants, selecting containers.
- Pruning, Grooming, Cleaning and Repotting

Pest Management
What to do for plant problems, summary of cultural care, bibliography.
Much of the scenic beauty of nature has been replaced by densely populated areas that sprawl for miles from urban centers. This visual pollution affects us all and leaves us with a longing for a closer connection with nature. We spend about 90 percent of our time indoors. Interior plants are an ideal way to create attractive and restful settings while enhancing our sense of well being. In addition, houseplants can be a satisfying hobby and can help purify the air in our homes. Indoor plants not only convert carbon dioxide to oxygen, but they also trap and absorb many pollutants. Many of these chemical compounds, which are released into our air through a process called “off-gassing,” come from everyday items present in our homes and offices.
To be a successful indoor gardener, you need to understand how the interior environment affects plant growth and how cultivation differs from growing plants outdoors.
Plant growth is affected by light, temperature, humidity, water, nutrition, and soil.
Of all of the factors affecting plant growth in interiors, adequate light is by far the most important. Light is needed for plants to produce food and survive — generally, the more light available, the more food produced for growth. Light is measured in units called foot candles. One footcandle (ft-c) is the amount of light cast by a candle on a white surface 1 foot away in a completely dark room. Outdoors, the light levels on a bright day range from 10,000 ft-c in an open sunny area to 250 ft-c or less in the shade of a large tree.
It is very helpful to have a general idea of how much light is present in a given location in your house. You can get a fairly good estimate with a handheld light meter, or you can use a 35 mm camera and do the following:
- Set the film speed indicator to ASA 25 and the shutter speed to 1/60th second.
- Place a piece of white paper where you want to measure the light levels, aim the camera toward the paper close enough to fill the view, and adjust the f/stop so that the meter indicates a correct exposure.
- Read the approximate light level from Table 1.
| Indoor light levels and appropriate f/stop settings | |
| f/2 | 40 ft-c |
| f/2 | 75 ft-c |
| f/4 | 150 ft-c |
| f/5.6 | 300 ft-c |
| f/8 | 600 ft-c |
| f/11 | 1,200 ft-c |
| f/16 | 2,400 ft-c |
With the help of this table, you can obtain the light intensity reading from anywhere in your home. For example, if the f/stop setting is f/16, the approximate light level is 2,400 ft-c.
Using the light readings, your home can be divided into four areas, which have the following light levels for 8 hours per day:
- Low-light areas: 25 ft-c – 75 ft-c
- Medium-light areas: 75 ft-c – 200 ft-c
- High-light areas: over 200 ft-c but not direct sunlight
- Sunny light areas: at least 4 hours of direct sunlight

In your home, the amount of light in a given location is variable — it is affected by the presence of trees outdoors (may shade at certain times), roof overhangs (may shade at certain times), wall color (reflectance), window curtains, day length, time of day, and time of year.
When shopping for indoor plants, select plants for a given location based on the approximate light levels in the spot. The plant’s label will usually contain information on the light requirements of the plant. If the plant label lists “high light” but the selected area in the home does not provide adequate light, artificial light sources such as fluorescent and/or special incandescent lights may be used to supplement the natural light.
Increasing the number of hours of light exposure can also help—for example, 16 hours of light and 8 hours of dark. This extends the number of hours during which plants receive light.
While adequate light is crucial for plant growth, too much light can be damaging (Figure 1).
Indoor plants are classified according to the amount of light needed for growth. (A list of plants and their light requirements is provided in Table 3.) Look for this information in general terms on the plant’s label:
- Low: minimum 25 ft-c – 75 ft-c, 75ft-c – 200 ft-c for good growth
- Medium: minimum 75 ft-c – 150 ft-c, 200 ft-c – 500 ft-c preferred
- High: minimum 150 ft-c – 1,000 ft-c, 500 ft-c – 1,000 ft-c preferred
- Very high: minimum 1,000 ft-c, 1,000+ ft-c preferred
Windows with eastern exposure within the home generally provide the best light and temperature conditions for most indoor plant growth because plants receive direct morning light from sunrise until nearly midday. Footcandle readings at these windows can reach 5,000-8,000. As the morning progresses, the direct sun recedes from the room.
An eastern room is cooler than southern or western rooms because the house absorbs less radiant heat. Light from the east is cooler than that from the south or the west, and thus it causes less water loss from the plants.
Windows with southern exposure give the largest variation of light and temperature conditions. The low winter sun shines across the room for most of the daylight hours.
In the summer, when the sun is farther north than it is in the winter, the sun rises at a sharp angle in the morning and is high in the sky by noon. Direct light comes into a south window only at midday. If there is a wide overhang covering the windows outside, the sun may not enter the room at all. The sun at noon on a summer day may measure 10,000 ft-c. Indoors, however, a southern window with wide eaves on the outside will receive about the same amount of light as a window with northern exposure. Southern and western exposures are interchangeable for most plants. In the winter, most plants, except those with definite preference for northern exposure, can be placed in a room with southern exposure.
Windows with northern exposure provide the least light and the lowest temperature. Because the United States is in the northern hemisphere, it receives most of its sunlight from the south. Out of the four exposures, the northern exposure receives the least light and heat year round.
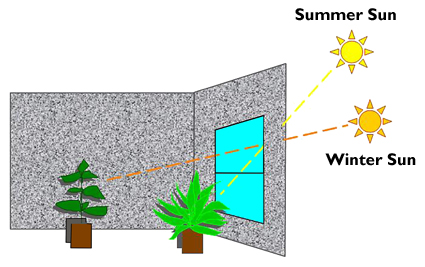
Because of the low-light levels, maintaining healthy plants can be a challenge. A northern windowsill can measure light levels as low as 200 ft-c on a clear winter day, which is optimal for some plants, such as the African violet. This exposure is best for plants with green foliage because the coloration on variegated foliage tends to disappear under low-light conditions. Although most plants grown indoors will not grow in a northern room, they may tolerate it for short periods of time.
Seasons change the amount of natural light entering through windows. For example, the summer sun reaches a higher zenith compared to the winter sun (Figure 2). Therefore, sunlight penetrates farther into a room during winter.
How can you tell if your plant is not receiving adequate light?
- The plant does not grow.
- The internodes (spaces between the leaves) on the new growth are much longer than the internodes on the older part of the plant.
- The new leaves are smaller than the older leaves.
- The leaf color is a lighter green on the newer foliage than on the older foliage.
- The older leaves are dead.
Temperature is the second most important factor influencing plant growth in interior environments. People feel comfortable in the range of 72 degrees F-82 degrees F, and interior plants can tolerate and grow well in the 58 degrees F-86 degrees F range because most indoor plants originate from tropical and subtropical areas of the world.
Temperature and light are linked through the processes of photosynthesis and respiration. These processes can be thought of as the “yin and yang” of plant life — two parts of a circle. Photosynthesis builds sugars and starch, which are then broken down by respiration to provide energy for the development of new tissues (growth) and the maintenance of existing ones. High temperature speeds up respiration. If the plant is not producing sufficient sugars (as under low light), then high temperatures may break down what little sugars are made, leaving little to none for growth. Maintenance takes precedence over growth; therefore, under insufficient light, plants do not grow. If light is so low that sugars produced are insufficient for maintenance, the plant eventually dies.
When sugar levels are low, the plant takes nutrients and sugars from older leaves to maintain new leaves. To help plants in an indoor environment, two options are available: (1) raise light levels to increase photosynthesis and sugar production or (2) reduce night temperature to lower respiration rates and allow more sugars for growth.
What temperatures are likely to occur in homes? During the summer, air conditioning that may have been turned off at night or weekend thermostat settings that may have been raised result in higher than desirable night temperatures. During the winter, heating that may have been turned off at night or weekend thermostat settings that may have been lowered may result in lower night temperatures. Be especially careful not to allow temperatures to drop below 50 degrees F, or chill damage will result on some sensitive foliage plants (e.g., Chinese Evergreen, Aglaonema). Chill damage is manifested with the yellowing of lower leaves and/or defoliation.
Plants vary in their minimum and maximum temperature requirements. Examples of cool-loving plants suitable for locations where temperatures drop to the low 50s at night and 60s during the day are Cyclamen, Wonder Plant, Fatshedera, Japanese Aralia, and Fatsia. A list of plants and their temperature requirements is provided in Table 3 .
Not all interior plants have the same temperature requirements for optimal growth. For example, Cast Iron Plant, Aspidistra, and ferns actually grow better with cooler temperatures (72°F), while other tropical plants grow best if the temperatures are 90 degrees F – 95 degrees F. Such temperatures are rarely allowed indoors.
The best temperature range for indoor plants is 70 degrees F – 80 degrees F day and 65 degrees F – 70 degrees F night.
Relative humidity is the amount of moisture contained in the air. For interior plants, relative humidity below 20 percent is considered low, 40 percent – 50 percent is medium, and above 50 percent is high. Relative humidity is a very important factor, but it is easily overlooked. In a greenhouse, relative humidity is 50 percent or higher. Rapid transpiration and water loss may result when newly purchased plants are placed in the 10 percent – 20 percent relative humidity typical of most homes (Figure 3). Most indoor plants come from the tropics where high relative humidity is common. Therefore, take the following steps to help your plants adjust to the low relative humidity in your home.
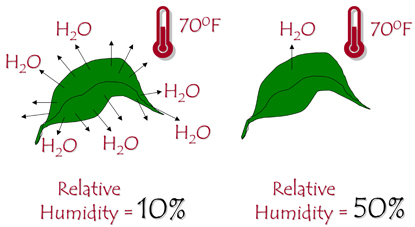
- Place plants close together to create a microenvironment with a higher relative humidity.
- Use a shallow container filled with water and lava rocks or gravel, which will provide evaporation from a large surface area and increase relative humidity.
- Use a humidifier.
- Use mist bottles to spray water around the plant; however, in reality, you would need to mist every few minutes for an indefinite amount of time to make a difference in relative humidity around the plant.
- The foliage and flowers of plants with hairy leaves should not be sprayed with water. Water on such leaves may stay longer, providing opportunities for disease spores to germinate.
Water Quantity
Learning to water is one of the most important skills in plant care. Applying too much water can suffocate plant roots and too little water causes growth to become erratic and stunted. Watering frequency will depend on the conditions under which the plants are growing. When dealing with how much water to apply, consider the following:
- Plant type: A list of plants and their moisture requirements is provided in Table 3 . Not all plants are similar in their water requirements. This information, along with the light preference, is usually included on the plant label. For example, a croton, which prefers high light, will likely need more frequent watering compared with a succulent plant such as Opuntia cactus. Both have similar light needs but dissimilar water requirements.
- Plant size: Larger plants need more water compared to smaller plants.
- Container volume: If the growing container is too small, watering may be required more frequently.
- Soil moisture: The amount of water already present in the growing medium will also affect your watering frequency.
- Light intensity: Plants under high light transpire more water compared with plants under low light.
Improper watering causes many problems. Containers with saucers may cause an excessive build-up of soluble salts (from the applied fertilizer). High levels of soluble salts can cause damage to plant roots and a decline in growth. Discard any water that had drained in the saucer after irrigation, and apply large quantities of water to the soil to leach the accumulated soluble salts. In deciding when you should water, feel the soil by pushing a finger an inch or so below the surface. If the soil is still moist, no further water is needed. Water devices or water meters are also available to simplify watering.
Water Quality
The quality of the irrigation water is an issue with plants that are susceptible to fluorine and chlorine, such as Corn Plant ( Dracaena ), Ti Plant ( Cordyline ), Peacock Plant ( Maranta ), and Rattlesnake Plant ( Calathea ) (Figure 4). Alleviate this problem by letting the water stand for several days — so that some chlorine and fluorine will be released from it — before applying the water to the plants. Move susceptible plants away from the edge of the pool to prevent water splashes from reaching the foliage. Do not use susceptible plants around enclosed pools. In general, plants with long linear leaves (such as the Spider Plant) are more susceptible to fluorine.

Many indoor gardeners have the same problem with fertilizer that they have with water — they want to give their plants too much. Danger from over-fertilization occurs because any fertilizer used, whether in liquid, powder, or tablet form, will dissolve in soil water and will form salts in the water. When you over-fertilize, the water in the soil becomes so salty that it “burns” the plant’s roots by removing water from them (Figure 5). Excess soluble salts accumulate as a whitish crust on the surface of the growing medium and/or near the rim of the container.

Before feeding plants, consider the following:
Plant type: Some plants are heavy feeders (e.g., Ficus species), while others need little or no additional fertilizer for months (e.g., succulents). Volume of soil: The growing medium that is present — smaller pots require less fertilizer compared with larger pots because they contain less soil. Light intensity: The higher the light levels, the more nutrients needed for plant growth.
A newly purchased, healthy plant rarely needs an immediate application of fertilizer. In most cases, the amount of fertilizer applied by the commercial producer will supply enough nutrients for two to three months in the home. This rule is flexible — if deficiency symptoms are evident, fertilizer application is desirable.
The secret to fertilizing plants indoors is to apply small amounts of fertilizer as the plant grows. Without new growth, the plant has a limited need for more fertilizer. During the winter when light levels are low, a plant’s need for fertilizer reduces. During the summer when light levels increase and the plant is actively growing, its need for fertilizer increases. As a starting point, use about one-fourth the label rate for monthly applications. If the overall plant color becomes lighter green, fertilize every two weeks. If the new growth is dark green but the leaves are small and internodes seem longer than on the older growth, decrease the fertilizer rate.
Varying fertilizer formulations are available to the indoor gardener. Many fertilizers come in specially designed formulas for indoor plants. Generally, they contain a lower percentage of the required mineral elements to prevent over-fertilization problems.
The growing medium provides anchorage, water, and minerals. When repotting plants, make sure that the new mix is well drained and aerated, holds water and nutrients well, and is within the right pH range (5.0-6.5). A good potting mix provides ample amounts of oxygen to the root system. Most professional mixes are good to use. Some plants require special mixes, e.g., bromeliads, orchids, and African violets. Either purchase these mixes or prepare your own. Below are some formulas that can be used to prepare a homemade potting mix.
Growing Mix for Flowering House Plants
The following potting mix will grow acceptable flowering plants in most homes for most gardeners:
- 1 part garden loam or potting soil
- 1 part sand or perlite or vermiculite
- 1 part peat moss
Add 2 to 3 ounces of 20 percent superphosphate and ¾ ounce of either bonemeal or dolomitic limestone (by weight) to 4 gallons of potting mix. After sterilizing the soil (see “How to Sterilize Soil”), add 3 tablespoons of a 6-6-6 or similarly balanced fertilizer to every 4 gallons (½ bushel) of mix. Add a minor element formulation according to the manufacturer’s recommendations.
Growing Mixes for Foliage Plants
Although most foliage plants will grow satisfactorily in the growing mix recommended for flowering house plants, they will grow better if the mix contains a higher percentage of organic matter.
- 1 part sand or 2 parts peat moss
- 1 part pine bark
- 2 parts peatmoss
- 1 part sand
Add 2 to 3 ounces (dry weight) of dolomitic limestone to 4 gallons (½ bushel) of mix. For fluoride-sensitive plants, adjust the pH so it is no lower than pH 6.5. Superphosphate contains enough fluoride to cause foliar burn on sensitive plants. After sterilizing the soil, add 3 tablespoons of a 6-6-6 or another fertilizer such as 5-10-5 to each ½ bushel. Plastic-coated fertilizers also can be used; most of them require about 2 ounces per ½ bushel. Add a minor element formulation to the potting mix per the manufacturer’s recommendation.
Growing Mixes for Bromeliads
Bromeliads are plants from Central and South America, which are either epiphytic (they grow on tree branches or in the crotches of trees) or terrestrial (they grow in the ground). Although most of the bromeliads can be grown successfully in foliage plant mixes, most grow better in specially designed soil mixes. Any mix for bromeliads must be well aerated and drained.
- 2 parts peat moss
- 1 part perlite
- 1 part fir bark
- 1 part peat
- 1 part cypress shavings
Add 2 ounces of dolomitic limestone to 4 gallons (½ bushel) of soil mix and a minor element mix. Dissolve 1 ounce of 10-10-10 water-soluble fertilizer in 3 gallons of water. Use this solution after repotting and again monthly when watering. Also, add enough water to fill the vase formed by the overlapping leaf bases.
Growing Mixes for Orchids
Orchids have a great deal in common with bromeliads because they also grow on trees as epiphytes and on the ground as terrestrials. A mix for orchids should have excellent drainage and aeration, too. Some soil mixes that can be used are:
- 3 parts osmunda tree fern fiber (moisten before use by soaking in water for 12 hours)
- 1 part redwood bark
- 5 parts fir bark
Tree fern slabs may also be used to grow epiphytic orchids.Add 1 ounce (dry weight) of dolomitic limestone per 4 gallons (½ bushel) of soil mix. Do not add fertilizer to the mix. After the plants are potted, add ¼ ounce of liquid 10-10-10 with minor elements per gallon of water and fertilize once every 6 weeks (if the plants are growing in osmunda fern fibers). If plants are growing in fir bark, use a liquid 30-10-10 with minor elements every 6 weeks instead of a 10-10-10 fertilizer.
Growing Mix for Succulents and Cacti
Cacti and other succulents grow best in a well-drained and aerated soil.
- 2 parts garden loam or potting soil
- 2 parts sand
- 2 parts peat
- 1 part perlite (crushed charcoal can be substituted)
Add 2 ounces (dry weight) of dolomitic limestone to 4 gallons (½ bushel) of soil mix, 2 ounces (by weight) of bonemeal, and ½ ounce of superphosphate. After sterilizing the soil, add a minor element supplement according to the manufacturer’s recommendation.
Growing Mix for Ferns
Ferns grow well in most recommended mixes that have a high proportion of organic matter with good soil aeration and drainage characteristics. Use any of the suggested foliage plant mixes. However, most ferns kept indoors grow better in the following mix:
- 1 part coarse sand
Add 2 ounces (dry weight) of dolomitic limestone to each ½ bushel (4 gallons) of soil mix and ½ ounce of either bonemeal or 20 percent superphosphate. After pasteurizing the soil mix, add minor elements to the mix. Add 1 tablespoon of a 6-6-6 or similarly balanced fertilizer to each ½ bushel of soil mix.
Growing Mix for African Violets
Any number of soil mixes for African violets exist, and most of them will grow high quality plants. A good mix should be well drained and aerated.
- 1 part vermiculite
Add 2½ tablespoons of dolomite and 1½ tablespoons of 20 percent superphosphate to each ½ bushel of soil mix. Add 3 tablespoons of a high phosphorous fertilizer such as 5-10-5 or a similar ratio of fertilizer.
How to Sterilize Soil
Sterilization reduces the number of diseased organisms and weeds present in the soil. First, mix the soil with an equal portion of vermiculite or peat moss (otherwise, the soil will become very hard). Next, moisten the mixture and place it in the oven. Allow it to “bake” at 180 degrees F–200 degrees F for 1 hour. Once the soil cools, it is ready to use. To treat soil in the microwave, first mix the portion with an equal amount of vermiculite or peat moss and moisten. Place the mixture in a plastic bag. Next, consult the manufacturer’s manual to determine the amount of time and power level needed to heat the quantity of soil to about 180 degrees F (most portions of soil will generally require about 10 to 15 minutes). Insert a probe into the soil and make sure it has heated to 180 degrees F – 200 degrees F. Allow the soil to cool before using it or storing it for future use.
Make sure that the soil or potting mix you want to sterilize does not contain perlite. At high temperatures, toxic levels of fluoride may be released and subsequently damage your plants.
Acclimatization
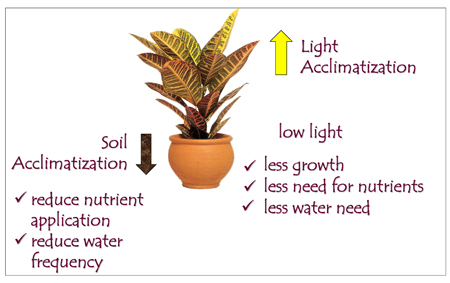
Acclimatization is the adaptation of a plant to a new environment, and it is very important for the health and growth of indoor plants. In greenhouses, plants are accustomed to high light, nutrition, water supply, temperatures, and relative humidity — conditions ideal for fast growth (Figure 6). Residential homes, with low-light interiors and low relative humidity, will most likely produce a stressful experience for plants — the greater the difference between the previous environment and the environment of the house, the greater the stress the plant endures.
Acclimatization is generally done in the greenhouse or the nursery. Plants are grown for a period of time under low-light levels and with fewer nutrients. Because this slows down plant growth, acclimatized plants are not ready for the market as early as nonacclimatized plants. Acclimatized plants cost more compared to nonacclimatized plants, but this is money well spent. Figure 7 and Table 2 describe the symptoms and appearance of acclimatized plants.

To acclimatize plants at home, place newly purchased plants in bright areas for at least 3 to 4 weeks and then move them to their final location. Porches and patios are ideal bright places for your plants in the warm months, as long as the plants are not in direct sunlight. The most common symptom occurring in plants placed indoors is defoliation. As long as it is not extensive and it slows down after a few weeks, the plants will adjust to the particular location. Keep in mind, however, that each time the plant is moved around, it will experience an acclimatization period, and such changes may become evident.
Learn as much as possible about the extent of acclimatization of the chosen plants. The retailer should be able to provide this information. When shopping for plants at a garden center, ask if the plants have been acclimatized.
Remember that the most important factors of indoor plant growth are adequate light, fertilizer, and water at reduced rates.
| Symptoms of acclimatized plants vs. nonacclimatized plants | |
| Medium to dark green leaves Large leaves Flat leaves Thin leaves Widely spaced leaves | Yellowish to light green leaves Small leaves Partially folder leaves Thick leaves Closely spaced leaves |
| Long internodes Thin to medium stems Horizontal or slightly flexed leaf position | Short internodes Thick stems Upright leaf position |
| Few new leaves Wide branch angles | Many new leaves Acute angles |

Purchase only healthy looking plants with medium to dark green foliage (unless foliage is supposed to be a different color).
Avoid plants with unnaturally spotted, yellow, or brown leaves. If the plant is unhealthy at the nursery, chances are that it will die soon after consumer purchase. Look for pests on the undersides of leaves. Remove the plant from the pot and examine the root system. Healthy roots generally are and should be visible along the outside of the soil ball and should have an earthy smell (Figure 8).
Any discolorations, generally brown or blackened roots, are signs of problems. Some plants, such as Dracaenas, have roots with colors other than white. Unhealthy roots also may smell foul. If shopping for ferns, do not be alarmed if you see brown-colored spots or long rows of structures on the lower leaf surface; these “spots” are reproductive structures called spores.
Planters can enhance the decorative value of the plants. Consider the following when selecting a planter:
- Suitability for the plant’s needs
- Suitability for the needs of the individual and the environment
- Cost and availability
- Strength and durability
The style, shape, and size of the container should complement the plants grown. Small containers are best for small slow-growing plants, while fast-growing plants are better suited for large containers.
Containers can be made from a wide range of materials — terra cotta, clay, plastic, or ceramic. Terra cotta pots, made of fired clay, are some of the most popular choices, with designs ranging from plain to ornate. Plants perform very well in terra cotta pots, as the porous surface allows good air exchange between the plant roots and the environment. Other clay containers (not considered terra cotta) range from gray to brown in color, depending on the clay used. Clay pots can be glazed or unglazed. The glazed pots restrict air exchange but offer more design choices. Unglazed pots evaporate water faster and plants in them may need more frequent watering. Disadvantages of clay containers include their weight (especially large pots) and the chance they will chip or break.
Constructed of materials such as polyethylene, polyurethane, recycled plastic, and fiberglass, plastic pots have evolved from very simple to quite elaborate. They have the advantage of being lightweight as well as chip- and break-resistant. Air exchange and water evaporation rates are generally lower in plastic containers compared with clay containers. Plants in plastic pots will not dry out as quickly as plants in clay pots, increasing the danger of over-watering.
In general, there are two types of containers — ones with drainage holes and ones without. Do not allow plants in containers with drainage holes to sit in saucers filled with water, unless the plant is suspended above the water level by a layer of rocks. To avoid salt buildup, leach the soil once a month by applying a gallon of water to every cubic foot of potting medium; after a few hours, follow with ½ gallon of water. If the potting medium contains garden soil, apply 5 gallons of water per every cubic foot of growing medium.
Containers without drainage holes work well for plants such as the Peace Lily (Spathiphyllum), which needs plenty of water, but they should not be used for cacti and succulents.
Pruning, Grooming, Cleaning, and Repotting
When is the best time to prune? “When the knife is sharp” goes the old saying, and it means using the natural life cycles as a guide. For example, when the plant is growing rapidly and you want to maintain a certain size, prune lightly and frequently, removing shoots or shoot tips when they are small. When removing the very immature tips, the practice is known as pinching. Pinching and light pruning also increase branching of the stem and result in a stockier, fuller plant.
When the plant has outgrown its container, root pruning is advisable. Pull roots away from the root mass then cut them back to within 1 inch of the soil mass. An alternative method is to make three or four vertical cuts 1 inch deep in the soil ball on the opposite sides of the root ball.
If you are re-using containers, make sure that they are clean by washing out any old compost, chemical, or paint residues. Sterilize the container by placing it in a 10 percent bleach solution and rinse well.
A clean plant is a healthy plant. Water flow causes salt accumulation along the leaf margins and/or tips, creating necrotic areas. Dust dulls normal leaf coloration, lessening plant value, but it also shades plant surfaces, reflecting light that can be used in photosynthesis. Dust on lower leaf surfaces may clog stomata (specialized cells involved in water transpiration), inhibiting gas exchange within the leaf. Leaves with thick, shiny cuticles (Croton, Ficus, Peace Lily, Bromeliads) should be cleaned with a damp sponge.
If the plant is small, dip the foliage in tepid water and swirl it around. Water should not be used when cleaning cacti, African violet leaves, and other plants with hairy leaves. Instead, use a clean, small paintbrush brush to remove dust. Remove dead flowers and leaves regularly. Leaves with tip and/or marginal necrosis, such as fluoride damage, should be trimmed to the healthy part.
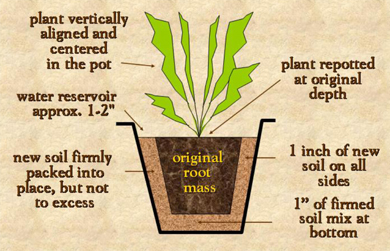
If the plant has been growing well, it will likely need repotting. The decision to repot should be based on plant appearance—if it is top-heavy, if it fills the container with new shoots, or if it has extensive root growth out of the pot’s drainage holes. Ideally, plants should be repotted in 1 inch increments. Planting into too large a container will give the roots more soil than they initially need. The excess soil will hold extra moisture, creating overly wet conditions. Increase pot size through smaller increments rather than doubling the pot size in one step (Figure 9).

Very few plants stay pest-free forever. Pest insects are more likely to be encountered on indoor plants than diseases because the interior environment rarely offers favorable conditions for foliar diseases to develop. However, when plants are grown under stressful conditions (such as low light and excess water), soil-borne pathogens often develop.
Scales are ⅛ inch to ⅓ inch long with various colors, depending upon the species. The three main families of scales are armored (the body covering can be separated from the body), soft (the body covering cannot be separated from the body), and mealybugs. Scales suck plant juices from leaves and stems, causing stunting, leaf discoloration, and death of the tissue. As a result of their feeding, sticky “honeydew” (digested plant sap) is excreted (the exception is armored scales). Honeydew offers a growing medium for a fungus called sooty mold, which, when present, can detract from the plant appearance and block light from reaching the leaf surface. Scales are usually inconspicuous; by the time infestation is noticed, the population is usually very large (Figure 10 and Figure 11).
Mealybugs are soft bodied, 1/5 inch to 1/3 inch long, and covered by white, waxy filaments, giving them a white, cottony appearance. Insects are frequently found on the new growth at the stem apex, where they suck plant juices, causing leaf wilting and abscission (Figure 12). Some species of mealybugs appear first on the undersides of leaves. Mealybugs excrete sticky honeydew, which attracts sooty mold.

Aphids are soft bodied, pear shaped, 1/25 inch to 1/8 inch long, and are usually green in color (but may be pink, blue, brown, yellow, or black). Aphids reside on new growth or on the underside of young leaves, where they suck plant juices, causing deformed, curled growth of new leaves, buds, and flowers. Aphids also excrete honeydew. Aphids are usually wingless but develop winged forms when colonies become too large (Figure 13).
Spider mites are the second most common pest problem on houseplants (Figure 14). The adult females are about 1/50 inch long, hardly visible with the unaided eye. Mites feed on the undersides of young leaves. Infected areas are grayish or yellow speckled.
Webs form as a means of dispersal. Spider mites thrive in hot and dry conditions.

Thrips, while uncommon on houseplants, predominantly feed on plants in patios and other outdoor areas (Figure 15). Thrips are small, slender, 1/25 inch to 1/12 inch long, and tan, black, or brown in color, with lighter markings. Adults and larvae feed on shoot tips, flowers, and leaves by sucking sap and cell contents. Injured tissue has a whitish or silver-flecked appearance due to the light reflecting from the empty cell walls of the dead cells.
- The best method is prevention — purchase pest-free plants.
- Remove a light infestation of mealybugs or aphids with a cotton swab dipped in rubbing alcohol.
- If outdoor conditions permit, take the affected houseplant outside in a protected area, where natural predators will eventually come and rid the plant of the pest.
- Treat with insecticidal soap. The best results occur on plants that have been hardened off in the interior environment. New plants, if they have not been acclimatized (accustomed to lower light, fertilizer, and water levels), are going to be tender and should be treated after the first couple of weeks. Add 2 teaspoons of insecticidal soap per gallon of water and wipe foliage and stems with the soapy water and soft cloth.
- Heavy infestations may be too extensive to treat. Discard these plants and do not place them in your compost pile.
- Do not introduce beneficial insects indoors! They may work great in the greenhouse with a large number of plants and pests, but there is just not enough food in your home to sustain their population. Most pests can be controlled culturally on indoor plants without the use of chemicals.
Another potential problem in the indoor garden is the occurrence of various diseases. For a disease to happen, three factors must be present: (1) a susceptible plant, (2) a viable pathogen, and (3) a favorable environment. Because the home has very low relative humidity and water is often applied directly to the growing medium (thus keeping the foliage dry), chances of a foliar disease occurring are minimal.
Leaf spots are the most common problem, but they are usually not caused by a disease. For example, leaf scalds occur when water droplets on the leaves act as lenses and focus excessive light in one spot, bleaching the chlorophyll and killing the underlying tissue. Spots with patterns are signs of a disease, including a tan center, dark borders, and/or light-colored borders called “halos.” Dark structures may be present on the underside; these contain a means of dispersal called spores.
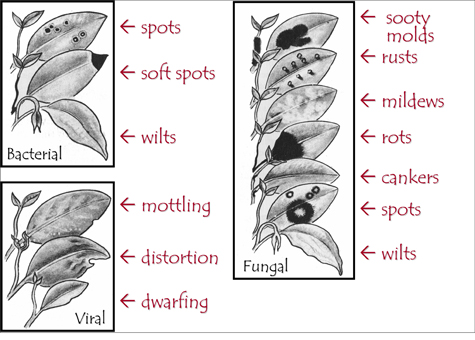
Most importantly, avoid causing stress to plants. A healthy plant is much more likely to fight off a disease than a stressed one. Use a simplified key for identifying the causal agent for a disease (Figure 16).
Soil-borne pathogens are commonly found on stressed plants. Soil-borne pathogens affect plants at or below the soil line; disease development is usually well underway before symptoms are noted on plant parts aboveground. Soil-borne diseases commonly occur when the growing medium is kept excessively moist and fertility levels are high. Low light and over-watering create favorable environments for soil-borne diseases indoors.
The most common causes of stress in interiors are low light and over-watering.
Table 3 provides a listing of more than 200 plants and their cultural requirements. To summarize cultural care guidelines, the following abbreviations and coding numbers are used. These guidelines apply to actively growing indoor plants.
- Sunny light areas: At least 4 hours of direct sun
- High-light areas: Over 200 ft-c, but not direct sun
- Medium-light areas: 75 ft-c to 200 ft-c
- Low-light areas: 25 ft-c to 75 ft-c
T = Temperature
- Cool: 50°F night, 65°F day temperatures
- Average: 65°F night, 75°F day temperatures
- Warm: 70°F night, 85°F day temperatures
H = Relative Humidity
- High: 50% or higher
- Average: 25% to 49%
- Low: 5% to 24%
W = Watering
- Keep soil mix moist
- Surface of soil mix should dry before re-watering
- Soil mix can become moderately dry before re-watering
S = Suggested Soil Mix
For specific ingredients, refer to the various growing mixes in “Soil/Growing Medium.” The soil mixes are keyed as follows:
- Flowering house plants
- Foliage plants
- Succulents and cacti
- African violets and other Gesneriads
| Indoor plants and their cultural requirements (adopted from McConnell, D. B. 1978) | ||||||
| Flowering Maple | 1 | 1 | 2 | 2 | 1 | |
| Chenile Plant | 1 | 2 | 2 | 2 | 1 | |
| Magic Flower | 2 | 2 | 2 | 1 | 7 | |
| Sweet Flag | 2-3 | 2 | 2 | 1 | 2 | |
| Miniature Sweet Flag | 2-3 | 2 | 2 | 1 | 2 | |
| Maidenhair Fern | 2-3 | 2 | 1 | 1 | 6 | |
| Crinkle-Leaf Plant | 2-3 | 2 | 2 | 2 | 5 | |
| Plover Eggs | 2-3 | 2 | 2 | 2 | 5 | |
| Silver Vase | 2-3 | 2 | 2 | 2 | 3 | |
| ‘Discolor’ | Purplish Coral Berry | 2-3 | 2 | 2 | 2 | 3 |
| ‘Royal Wine’ | Royal Wine Bromeliad | 2-3 | 2 | 2 | 1 | 3 |
| Zebra Basket Vine | 2 | 2 | 2 | 1 | 7 | |
| Lipstick Vine | 2 | 2 | 2 | 1 | 7 | |
| ‘Marginata’ | Variegated Century Plant | 1 | 2 | 3 | 3 | 5 |
| Queen Agave | 1 | 2 | 2 | 2 | 5 | |
| Chinese Evergreen | 3-4 | 2 | 2 | 2 | 2 | |
| ‘Silver King’ | Silver King | 3-4 | 2 | 2 | 2 | 2 |
| ‘Silver Queen’ | Silver Queen | 3-4 | 2 | 2 | 2 | 2 |
| Allamanda | 1 | 2 | 1-2 | 2 | 1 | |
| Miniature Pouch Flower | 2-3 | 2 | 1-2 | 1 | 7 | |
| Candelabra Plant | 1 | 3 | 3 | 3 | 5 | |
| Medicine Plant | 1 | 3 | 3 | 3 | 5 | |
| Brevifolia Aloe | 1 | 3 | 3 | 3 | 5 | |
| Pineapple | 1-2 | 2 | 2 | 1 | 3 | |
| Dwarf Crystal Anthurium | 2-3 | 2 | 1-2 | 1 | 2 | |
| Bird’s Nest Anthurium | 2-3 | 2 | 1-2 | 1 | 2 | |
| Flamingo Flower | 2-3 | 2 | 1-2 | 1 | 6 | |
| Zebra Plant | 2 | 2 | 2 | 1 | 2 | |
| Norfolk Island Pine | 2-3 | 2 | 2 | 1 | 2 | |
| Ardisia | 2-3 | 2 | 2 | 1 | 2 | |
| ‘Myers’ | Plume Asparagus | 2-3 | 2 | 2 | 2 | 2 |
| ‘Sprengeri’ | Foxtail Fern | 2-3 | 2 | 2 | 2 | 2 |
| Sickle Thorn | 2-3 | 2 | 2 | 2 | 2 | |
| Cast Iron Plant | 3-4 | 2 | 3 | 2 | 2 | |
| Mother Fern | 3 | 2 | 2 | 1 | 6 | |
| Bird’s Nest Fern | 3 | 2 | 2 | 1 | 6 | |
| Bishop’s Cap | 2 | 2 | 3 | 3 | 5 | |
| Ponytail | 1 | 2 | 3 | 3 | 5 | |
| Cuban Holly | 2-3 | 2 | 2 | 2 | 2 | |
| Metallic Leaf Begonia | 2-3 | 2 | 2 | 2 | 2 | |
| Rex Begonia | 2-3 | 2 | 2 | 2 | 2 | |
| Wax Begonia | 1-2 | 1 | 2 | 2 | 1 | |
| Queen’s Tears | 2-3 | 2 | 2 | 2 | 3 | |
| Urn Plant | 2-3 | 2 | 2 | 2 | 3 | |
| Zebra Plant | 2-3 | 2 | 2 | 2 | 3 | |
| Bougainvillea | 1 | 2 | 3 | 3 | 1 | |
| Schefflera | 2-3 | 2 | 2 | 2 | 2 | |
| Dwarf Schefflera | 2-3 | 2 | 2 | 2 | 2 | |
| Caladium | 2 | 2 | 1 | 1 | 2 | |
| Rattlesnake Plant | 2-3 | 2 | 2 | 1 | 2 | |
| Peacock Plant | 2-3 | 2 | 2 | 1 | 2 | |
| Miniature Maranta | 2-3 | 2 | 2 | 1 | 2 | |
| Rose Calathea | 2-3 | 2 | 2 | 1 | 2 | |
| Slipperwort | 2 | 1 | 1 | 1 | 1 | |
| Striped Inch Plant | 2-3 | 2 | 2 | 2 | 2 | |
| ‘Bonsai’ | Bonsai Natal Plum | 1-2 | 2-3 | 2 | 2 | 1 |
| ‘Boxwood Beauty’ | Boxwood Beauty | 1-2 | 2-3 | 2 | 2 | 1 |
| Fishtail Palm | 2-3 | 2 | 2 | 2 | 2 | |
| Madagascar Periwinkle | 1-2 | 2 | 1-2 | 2 | 1 | |
| Peruvian Apple Cactus | 1 | 2-3 | 3 | 3 | 5 | |
| Rosary Vine | 2-3 | 2 | 2 | 2 | 5 | |
| Parlor Palm | 3-4 | 2 | 2 | 2 | 2 | |
| Bamboo Palm | 3-4 | 2 | 2 | 2 | 2 | |
| European Fan Palm | 2-3 | 2 | 2 | 2 | 2 | |
| Hindustan Gentian | 2-3 | 2 | 1-2 | 1 | 7 | |
| Variegatum’ | Variegated Spider Plant | 2-3 | 2 | 2 | 1 | 2 |
| ‘Vittatum’ | Spider Plant | 2-3 | 2 | 2 | 1 | 2 |
| Areca Palm | 2-3 | 2 | 2 | 1 | 2 | |
| Chrysanthemum | 1 | 2 | 2 | 1 | 1 | |
| Kangaroo Vine | 2-3 | 2 | 2 | 2 | 2 | |
| Grape Leaf Ivy | 2-3 | 2 | 2 | 2 | 2 | |
| Wax Cissus | 2 | 2 | 3 | 3 | 2 | |
| Miniature Grape Ivy | 2-3 | 2 | 2 | 2 | 2 | |
| Calamondin Orange | 1-2 | 1 | 2 | 2 | 1 | |
| ‘Grandiflora’ | Kafir Lily | 2 | 2 | 2 | 2 | 1 |
| Croton | 1 | 2 | 1 | 1 | 2 | |
| Coffee | 2 | 2 | 2 | 2 | 1 | |
| Coleus | 2-3 | 2 | 2 | 2 | 1 | |
| Goldfish Plant | 2-3 | 2 | 1-2 | 1 | 7 | |
| Ti Plant | 2 | 1-2 | 2 | 2 | 2 | |
| Jade Plant | 2-3 | 2 | 2 | 2 | 2 | |
| Propeller Plant | 1-2 | 2 | 2 | 3 | 5 | |
| Arab’s Turban | 1-2 | 2 | 2 | 3 | 5 | |
| Toy Cypress | 1-2 | 2 | 2 | 2 | 5 | |
| Red Flowering Crassula | 2-3 | 2 | 2 | 2 | 5 | |
| Rattlesnake Tail | 2-3 | 2 | 3 | 3 | 5 | |
| Crossandra | 2 | 2 | 2 | 1 | 1 | |
| ‘Minor’ | Dwarf Rose Stripe Star | 2 | 2 | 2 | 2 | 3 |
| Stiff Pheasant Leaf | 2 | 2 | 2 | 2 | 3 | |
| Zebra Plant | 2 | 2 | 2 | 2 | 3 | |
| ‘Rochfordianum’ | House Holly Fern | 2-3 | 2 | 2 | 2 | 6 |
| Rabbit’s Foot Fern | 2-3 | 2 | 1 | 1 | 3 | |
| ‘Exotica Perfection’ | Exotica Perfection | 2-3 | 2 | 2 | 2 | 2 |
| Spotted Dumb Cane | 3 | 2 | 2 | 2 | 2 | |
| False Aralia | 2-3 | 2 | 2 | 2 | 2 | |
| ‘Janet Craig’ | Janet Craig | 2-4 | 2 | 2 | 2 | 2 |
| ‘Warneckii’ | Warneckii | 2-4 | 2 | 2 | 2 | 2 |
| ‘Massangeana’ | Corn Plant | 2-3 | 2 | 2 | 2 | 2 |
| Marginata | 2-4 | 2 | 2 | 2 | 2 | |
| Gold Dust Dracaena | 2-4 | 2 | 2 | 2 | 2 | |
| Miniature Agave | 1-2 | 2 | 3 | 2-3 | 2 | |
| Silver and Gold Dyckia | 1-2 | 2 | 3 | 2-3 | 3 | |
| Molded Wax | 1-2 | 2 | 3 | 3 | 5 | |
| Mexican Snowball | 1-2 | 2 | 3 | 3 | 5 | |
| Lace Cactus | 1-2 | 2 | 3 | 3 | 5 | |
| Spice Orchid | 2 | 2 | 1-2 | 1 | 4 | |
| Orchid Cacti | 2 | 2 | 2 | 2 | 1 | |
| Golden Pothos | 2-4 | 2 | 2 | 2 | 2 | |
| ‘Marble Queen’ | Marble Queen | 2-4 | 2 | 2 | 2 | 2 |
| Flame Violet | 2 | 2-3 | 1 | 1 | 7 | |
| Lace-Flower Vine | 2 | 2-3 | 1 | 1 | 7 | |
| Scarlet Violet | 2 | 2-3 | 1 | 1 | 7 | |
| Blue Euphorbia | 2-3 | 2 | 2-3 | 2-3 | 5 | |
| Corncob Cactus | 1 | 2 | 2-3 | 3 | 5 | |
| Crown-of-Thorns | 1 | 2 | 2-3 | 3 | 5 | |
| Poinsettia | 1-2 | 2 | 2 | 2 | 1 | |
| Milkbush | 1-2 | 2 | 2 | 2 | 1 | |
| Botanical Wonder Plant | 2-3 | 1-2 | 2 | 2 | 2 | |
| Japanese Aralia | 3-4 | 1-2 | 2 | 2 | 2 | |
| Weeping Fig | 1-3 | 2 | 2 | 2 | 2 | |
| Mistletoe Ficus | 2-3 | 2 | 2 | 2 | 2 | |
| ‘Decora’ | Rubber Plant | 1-3 | 2-3 | 2 | 2 | 2 |
| Fiddle-Leaf Fig | 1-3 | 2 | 2 | 2 | 2 | |
| ‘Minima’ | Dwarf Creeping Fig | 2-3 | 2 | 2 | 2 | 2 |
| Cuban Laurel | 2-3 | 2 | 2 | 2 | 2 | |
| Rooting Fig | 2-3 | 2 | 2 | 2 | 2 | |
| Dwarf Fiddle-Leaf Fig | 2-3 | 2 | 2 | 2 | 2 | |
| Red-Nerved Fittonia | 2-3 | 2 | 1 | 1 | 2 | |
| Silver-Nerved Fittonia | 2-3 | 2 | 1 | 1 | 2 | |
| Fuchsias | 2 | 1-2 | 1 | 1 | 1 | |
| Ox Tongue | 2 | 2 | 2 | 3 | 5 | |
| Jewel Leaf Plant | 2-3 | 2 | 2-3 | 3 | 5 | |
| ‘Major’ | Scarlet Star | 2 | 2 | 1 | 2 | 3 |
| Striped Torch | 2 | 2 | 1 | 2 | 3 | |
| ‘Purple Passion’ | Purple Passion | 2-3 | 2 | 2 | 2 | 2 |
| Star Window Plant | 1-2 | 2 | 3 | 2-3 | 5 | |
| Zebra Haworthia | 1-2 | 2 | 3 | 2-3 | 5 | |
| Little Zebra Plant | 2 | 2 | 3 | 2-3 | 5 | |
| Clipped Window Plant | 1-2 | 2 | 3 | 3 | 5 | |
| Algerian Ivy | 2-3 | 1-2 | 2 | 2 | 1 | |
| English Ivy | 2-3 | 1-2 | 2 | 2 | 1 | |
| Waffle Plant | 2-3 | 2 | 2 | 2 | 2 | |
| Chinese Hibiscus | 1 | 2 | 2 | 2 | 1 | |
| Amaryllis | 2 | 2 | 2 | 2 | 1 | |
| Belmore Sentry Palm | 3-2 | 2 | 2 | 2 | 2 | |
| Kentia Palm | 2-4 | 2 | 2 | 2 | 2 | |
| ‘Variegata’ | Wax Plant | 2-3 | 2 | 2-3 | 2 | 2 |
| Sweetheart Hoya | 2 | 2 | 2 | 2 | 2 | |
| Hyacinth | 2 | 1-2 | 2 | 1 | 1 | |
| ‘Variegata’ | Busy Lizzie Impatiens | 2-3 | 2 | 2 | 2 | 1 |
| Ixora | 1 | 2 | 2 | 2 | 1 | |
| Peregrian | 1 | 2 | 2 | 2 | 1 | |
| Shrimp Plant | 1-2 | 2 | 2 | 2 | 1 | |
| Christmas Kalanchoe | 1-2 | 2 | 2 | 2 | 1 | |
| Dwarf Purple Kalanchoe | 1-2 | 2 | 2-3 | 3 | 5 | |
| Panda Plant | 1-2 | 2 | 2-3 | 3 | 5 | |
| Turk’s Cap | 1 | 2 | 2 | 1 | 1 | |
| Powder Puff | 1-2 | 2 | 3 | 3 | 5 | |
| Firecracker Plant | 2 | 2 | 1-2 | 2 | 1 | |
| Red Nerve Plant | 2-3 | 2 | 2 | 2 | 2 | |
| Prayer Plant | 2-3 | 2 | 2 | 2 | 2 | |
| Plush Vine | 2-3 | 2 | 2 | 2 | 2 | |
| Philodendron Pertusum | 2-4 | 2 | 2 | 2 | 2 | |
| Window Leaf | 3 | 2 | 2 | 2 | 2 | |
| Black Alloplectus | 2-3 | 2 | 2 | 1 | 7 | |
| ‘Tricolor’ | Tricolor Bromeliad | 2-3 | 2 | 2 | 2 | 3 |
| Fingernail Plant | 2-3 | 2 | 2 | 2 | 3 | |
| Zonata | 2-3 | 2 | 2 | 2 | 3 | |
| ‘Bostoniensis’ | Boston Fern | 2-3 | 2 | 1-2 | 2 | 6 |
| ‘Fluffy Ruffles’ | Fluffy Ruffles | 2-3 | 2 | 1 | 2 | 6 |
| Miniature Bird’s Nest | 2-3 | 2 | 2 | 1 | 3 | |
| Little Tree Cactus | 1-2 | 2 | 3 | 3 | 5 | |
| Irish Mittens | 1-2 | 2 | 3 | 3 | 5 | |
| Finger Oxalis | 1-2 | 2 | 2 | 2 | 1 | |
| Red Oxalis | 1-2 | 2 | 2 | 2 | 1 | |
| Pearly Moonstones | 1-2 | 2 | 2-3 | 2-3 | 5 | |
| Yellow Shrimp Plant | 2-3 | 2 | 2 | 2 | 1 | |
| Ladyslipper Orchids | 2-3 | 2 | 2 | 1-2 | 4 | |
| ‘Variegatus’ | Devil’s Backbone | 2-3 | 2 | 2 | 2 | 5 |
| House Geranium | 1-2 | 1-2 | 2-3 | 2 | 1 | |
| Ivy Geranium | 1-2 | 1-2 | 2 | 2 | 1 | |
| Button Fern | 2-3 | 2 | 2 | 1-2 | 6 | |
| Satin Pellionia | 2-3 | 2 | 2 | 1-2 | 2 | |
| Egyptian Star Cluster | 1 | 2 | 2 | 2 | 1 | |
| Emerald Ripple | 2-3 | 2 | 2 | 2 | 2 | |
| Leather Peperomia | 2-3 | 2 | 2 | 2 | 2 | |
| Baby Rubber Tree | 2-3 | 2 | 2 | 2 | 2 | |
| Fiddle-Leaf Philodendron | 3-4 | 2 | 2 | 2 | 2 | |
| ‘Emerald Queen’ | Emerald Queen | 2-4 | 2 | 2 | 2 | 2 |
| ‘Florida’ | Florida | 2-4 | 2 | 2 | 2 | 2 |
| Heart-Leaf Philodendron | 2-4 | 2 | 2 | 2 | 2 | |
| Selloum | 2-4 | 2 | 2 | 2 | 2 | |
| Pigmy Date Palm | 2-3 | 2 | 2 | 2 | 2 | |
| Aluminum Plant | 2-3 | 2 | 1-2 | 1 | 2 | |
| Artillery Plant | 2-3 | 2 | 1 | 1 | 2 | |
| Staghorn Fern | 2-3 | 2 | 2 | 2 | 6 | |
| Swedish Ivy | 2-3 | 2 | 2 | 2 | 2 | |
| Candle Plant | 2-3 | 2 | 2 | 2 | 2 | |
| Podocarpus | 2-3 | 2 | 2 | 2 | 2 | |
| ‘Marginata’ | Variegated Balfour Aralia | 2-3 | 2 | 2 | 2 | 2 |
| Ming Aralia | 2-3 | 2 | 2 | 2 | 2 | |
| Lady Palm | 2-3 | 2 | 2 | 2 | 2 | |
| Azaleas | 2 | 1-2 | 1 | 1 | 2 | |
| Red-Spray Ruellia | 1-2 | 2 | 2 | 2 | 1 | |
| African Violets | 2-3 | 2 | 2 | 1 | 7 | |
| Parva Sansevieria | 2-3 | 2 | 3 | 2-3 | 5 | |
| ‘Hahnii’ | Birdsnest Sansevieria | 2-4 | 2 | 3 | 2-3 | 5 |
| ‘Laurentii’ | Gold-Banded Sansevieria | 2-4 | 2 | 3 | 2-3 | 5 |
| Strawberry Geranium | 2-3 | 1-2 | 2 | 2 | 2 | |
| Christmas Cactus | 2-3 | 2 | 2 | 2 | 2 | |
| Christmas Cactus | 2-3 | 2 | 2 | 2 | 2 | |
| Silver Pothos | 3 | 2 | 2 | 2 | 2 | |
| Showy Sedum | 1-2 | 1-2 | 2-3 | 2-3 | 5 | |
| Cow Web Houseleek | 1-2 | 1-2 | 2-3 | 2-3 | 5 | |
| ‘Purple Heart’ | Purple Heart | 1-2 | 2 | 2 | 2 | 2 |
| Gloxinia | 2-3 | 2 | 1-2 | 2 | 7 | |
| Baby Tears | 2-3 | 2 | 1-2 | 1 | 2 | |
| ‘Clevelandii’ | Peace Lily | 2-3 | 2 | 2 | 1 | 2 |
| ‘Mauna Loa’ | Mauna Loa | 2-3 | 2 | 2 | 1 | 2 |
| Carrion Flower | 1-2 | 2 | 2-3 | 2-3 | 5 | |
| Cape Primrose | 2-3 | 2 | 2 | 2 | 7 | |
| Strobilanthes dyeranum | Persian Shield | 2-3 | 2 | 2 | 2 | 2 |
| Nephthytis | 2-4 | 2 | 2 | 2 | 2 | |
| Dancing Bulb | 2 | 2 | 2 | 2 | 3 | |
| Blue-Flowered Torch | 2 | 2 | 2 | 2 | 3 | |
| Piggyback Plant | 2 | 1-2 | 2 | 2 | 2 | |
| Flowering Inch Plant | 2-3 | 2 | 2 | 2 | 2 | |
| White Velvet | 2-3 | 2 | 2 | 2 | 2 | |
| Flaming Sword | 2 | 2 | 2 | 2 | 3 | |
| Spineless Yucca | 2 | 2 | 3 | 2 | 2 | |
| Wandering Jew | 2-3 | 2 | 2 | 2 | 2 | |
Manaker, G. H. (1997). Interior plantscapes: Installation, maintenance, and management (3rd ed.). Prentice Hall.
McConnell, D. B. (1978). The indoor gardener’s companion: A definitive, color-illustrated guide to the selection and care of houseplants. Van Nostrand Reinhold Company.
Status and Revision History Published on Dec 15, 2006 Published on Oct 13, 2009 Published with Full Review on Dec 01, 2012 Published with Full Review on Jan 05, 2017 Published with Full Review on May 27, 2020 Published with Full Review on Jul 21, 2022
Have a question?
Related publications.
- Native Plants for Georgia Part II: Ferns (B 987-2)
- Home & Garden Georgia Pest Management Handbook Series: Ornamentals (SB 48-10)
- Growing Ferns (B 737)
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Effects of indoor plants on human functions: a systematic review with meta-analyses.

1. Introduction
2.1. eligibility criteria, 2.2. information sources, 2.3. search, 2.4. study selection, 2.5. data collection process, 2.6. data items, 2.7. risk of bias in individual studies, 2.8. summary measures, 2.9. planned methods of analysis, 2.10. risk of bias across studies, 2.11. additional analyses, 3.1. study selection, 3.2. study characteristics.
| Source | Participant | Interventions | Comparator | Exposure Duration | Distance to Plants | Room Size | Room Climate | Study Design | Functions | Function Category | Funding | Publication Language |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ ] | 96 US adults (48 males and 48 females, 80 of whom were college students), age: 18 to 46 | Presence or absence of 17 potted plants in a computer lab | Control | 13.5 × 7.3 × 2.6 m | 27 °C, 38% RH, 420 lux | Field experiment (RCT) | SBP, reaction time | Physiology, cognition | English | |||
| [ ] | 81 US adults | 10 potted plants (accounting 7.16% of the space), 22 potted plants (accounting 17.88% of the space), or no plants in an office | Control | 15–20 min | 12.08 m , 31.3 m | Field experiment (RCT) | A sorting task, a productivity task | Cognition | English | |||
| [ ] | 814 Chinese participants (347 males and 467 females), ethnicity: Asian | A building with or without indoor greening | Survey (non-RCT) | Neurobehavioral Functioning Evaluation System Testing | Cognition | Sciences and Technology Commission of Shanghai | Chinese | |||||
| [ ] | 198 US adults (71 males and 127 females), 176 of whom were college students | 5 potted plants, nonplant objects, no plants in a room | Nonplant objects, control | about 17 min | 3.5 × 6 × 2.4 m | 23 °C, 34% RH, 703 lux | Experiment (RCT) | Skin temperature, blood pressure, pain tolerance | Physiology, behavior | English | ||
| [ ] | 150 US college students (75 males and 75 females), mean age: 19.6 | 9 potted red-flowering geraniums, 9 potted non-flowering geraniums, no plants in a lab | Non-flowering plants, control | 5 min | 1.8 m | 22.4 °C | Experiment (RCT) | EEG, EDA, finger skin temperature | Physiology | American Horticultural Therapy Association | English | |
| [ ] | 146 Japanese college students (83 males and 63 females), ethnicity: Asian | 1 potted 1-m-tall plant placed in front of the participant, the same plant placed on the right-hand side of the participant, no plants in a room | Control | 15 min | 2.345 m in front of and 1.75 m at the side of the participants | 5.81 × 2.78 × 2.35 m | Experiment (RCT) | An association task, a sorting task | Cognition | English | ||
| [ ] | 66 US college students (32 males and 34 females), age: 91% from 18 to 24 | 1 potted flower arrangement (45 × 45 × 45 cm), lavender fragrance, flower and fragrance, or no plants and no fragrance in a lab | Control | 30 min | 3.5 × 2.7 × 2.4 m | 21 °C, 10.6 μmol·m ·s | Experiment (RCT) | EEG, EDA, skin temperature | Physiology | English | ||
| [ ] | 90 US college female students, mean age: 18.9 | Foliage and flowing plants, flowing plants, or no plants in a lab | Control | 5 min maximum | 1.4 m | 3.9 × 2.3 × 2.7 m | 21.7 °C, 904 lux | Experiment (RCT) | Pain tolerance, EEG, EDA, finger skin temperature | Behavior, physiology | English | |
| [ ] | 90 Japanese college students (35 males and 55 females), ethnicity: Asian | 1 potted 1.5-m-tall plant, a magazine rack put at the same location, or no plants and no magazine racks in a room | A magazine rack, control | 15 min | About 2.9 m in front of the participant | 2.78 × 5.81 × 2.35 m | Experiment (RCT) | An association task | Cognition | English | ||
| [ ] | 38 Taiwanese college students (10 males and 28 females), ethnicity: Asian | Presentation of 6 slides (office without a window view nor indoor plants, office without a window view but with indoor plants, office with a city window view but without indoor plants, office with a city window view and with indoor plants, office with a nature window view but without indoor plants, and office with a nature window view and with indoor plants) in a lab | Control | 15 s for each slide | 3 m | 7 × 5 m | 25 °C | Experiment (non-RCT) | EEG, EMG, BVP | Physiology | English | |
| [ ] | 364 Norwegian office workers, mean age: 43.1 | Presence or absence of potted plants on desks or shelves in an office | Survey (non-RCT) | Sick leave | Health | English | ||||||
| [ ] | 50 healthy Swedish people (23 males and 27 females), mean age: 39.2 | 1 potted flowering begonias (Begonia Elatior) approximately 22 cm high (control plant irrigated with ordinary local tap water; experiment plant irrigated with vortex-rotated local tap water) in an office | Plant irrigated with ordinary local tap water | 10 min for each plant | 5.6 × 3.0 × 2.4 m | 23–24 °C, 36–38% RH, 570–650 lux | Experiment (RCT) | Heart rate, heart rate variability, power spectral density | Physiology | The Swedish Flower Corporations | English | |
| [ ] | 90 South Korean patients who had received appendectomy (52 males and 38 females), mean age: 37.6, ethnicity: Asian | Presence or absence of 12 potted flowering plants in a ward | Control | Field experiment (RCT) | Pain killer consumption, blood pressure, body temperature, heart rate, respiratory rate | Health, physiology | English | |||||
| [ ] | 140 South Korean female high school students, ethnicity: Asian | Presence or absence of plants in 2 classrooms (accounting for 5% of the space) | Control | 14 weeks of school time | Field quasi-experiment (non-RCT, pre-post design) | Cortisol level, health | Physiology, health | English | ||||
| [ ] | 89 US sophomores | Presence or absence of plants in a classroom | Control | 1 semester of class time | Field quasi-experiment (non-RCT) | Course grade | Cognition | English | ||||
| [ ] | 76 Taiwanese junior high school students (58 males and 18 females), mean age: 13.55, ethnicity: Asian | Presence or absence of 6 potted plants (about 135 × 80 cm, having a green coverage ratio of 6%) in a classroom | Control | 12 weeks of school time | Field quasi-experiment (non-RCT) | Sick leave, misconduct | Health, behavior | English | ||||
| [ ] | 80 South Korean female patients who had received thyroidectomy, mean age: 36.2, ethnicity: Asian | Presence or absence of 12 potted flowering plants in a ward | Control | Field experiment (RCT) | Pain killer consumption, hospitalization days | Health | English | |||||
| [ ] | 34 Norwegian college students (12 males and 22 females), mean age: 24.15 | Presence or absence of 4 potted plants (2 flowering pink Phalaenopsis, 1 30-cm-tall Aglaonema commutatum, and 1 120-cm-tall Schefflera arboricola) in an office | Control | 60 min | 3.9 × 2.1 × 3.6 m | Experiment (RCT) | The Reading Span Task | Cognition | English | |||
| [ ] | 36 Taiwanese junior high school students (18 males and 18 females), mean age: 12.41, ethnicity: Asian | Taking care of 34 potted plants inside and outside a classroom (with a green coverage ratio of 6.3% indoors) | Control | 18 weeks of school time | Field experiment (RCT) | Examination score | Cognition | Chinese | ||||
| [ ] | 30 Chinese college students (15 males and 15 females), ethnicity: Asian | Presentation of 5 photos of vegetation landscapes and a blank in a room | Control | 2 min | 0.5 m | 7 × 4 × 3 m | 25 °C, 40% RH | Experiment (RCT) | ECG, blood pressure, heart rate, GSR, fingertip pulse | Physiology | English | |
| [ ] | 30 Chinese college students (15 males and 15 females), age: 18 to 24, ethnicity: Asian | Presentation of 12 photos of flowers and a blank in a room | Control | 2 min | 0.5 m | 7 × 4 × 3 m | 25 °C, 40% RH | Experiment (RCT) | Blood pressure, heart rate, GSR, fingertip plus | Physiology | National Key Technology Research and Development Program in China | English |
| [ ] | 29 Japanese college students (14 males and 15 females), age: 19 to 24, ethnicity: Asian | Potted Hedera helix L. (60 × 40 cm) of 5 different colors on a table in a room | Different colors of the plant | 1 min for each plant color | 0.5 m | Experiment (RCT) | Brain activity, eye movement | Cognition | Egyptian Ministry of Higher Education | English | ||
| [ ] | 28 Japanese undergraduate and graduate students (14 males and 14 females), mean age: 21.42, ethnicity: Asian | Placement of 1 potted plant of 3 different colors on a table in a room | Different colors of plants | 1 min for each plant color | 1.5 m | 59.4 m | 23 °C, 55% RH, 700 lux | Experiment (RCT) | Eye movement, brain activity | Cognition | Egyptian Ministry of Higher Education | English |
| [ ] | 30 South Korean college students (15 males and 15 females), mean age: 23.5, ethnicity: Asian | Placement of potted plants (60 × 40 cm) of 5 different colors on a box in a classroom | Different colors of plants | 3 min for each plant color | 1 m | 7 × 4.5 × 2.8 m | 25 °C, 70% RH, 700 lux | Experiment (RCT) | EEG | Physiology | English | |
| [ ] | Study 3: 33 British adult office workers (16 males and 17 females), mean age: 28 | Study 3: presence or absence of 8 potted plants (average height 90 cm) in an office | Control | Study 3: Field experiment (RCT) | An information management and processing task, a vigilance task | Cognition | English | |||||
| [ ] | 16 Chinese college students (8 males and 8 females), mean age: 23.5, ethnicity: Asian | Presence of potted plants of the combinations of 3 colors, 3 scents, and 3 sizes on a table in an office | Combinations of plant colors, scents, and sizes | 10–15 min | 22 °C, 41.65% RH, 0.2 ms wind velocity | Experiment (RCT) | EEG, ECG, oxyhaemoglobin saturation, fingertip blood flow, skin resistance, respiration rate | Physiology | Sciences and Technology Commission of Shanghai | English | ||
| [ ] | 24 South Korean male adults, mean age: 24.9, ethnicity: Asian | A plant transplanting task, a computer operation task on a table in a greenhouse room | A computer task | 15 min | 20.8 °C, 57.7% RH, 1365.5 lux | Experiment (RCT) | Heart rate variability, blood pressure, pulse rate | Physiology | English | |||
| [ ] | 565 Norwegian office workers | Outdoor nature contact, indoor nature contact, and outdoor view through windows | Survey (non-RCT) | Sick leave | Health | English | ||||||
| [ ] | 270 Pakistani surgical patients, ethnicity: Asian | Presence or absence of foliage plants and flower arrangements in a ward | Control | Field experiment (RCT) | Blood pressure, heart rate, respirationrate, body temperature, hospitalization days, analgesics consumption | Physiology, health | The University of Agriculture Peshawar in Pakistan | English | ||||
| [ ] | 30 Egyptian male college students, age: 22 to 37, ethnicity: African | Potted Hedera helix L. (60 × 40 cm) of 5 different colors on a table in a room | Different colors of the plant | 1 min for each plant color | 0.5 m | 59.4 m | 21 °C, 55% RH | Experiment (RCT) | Eye movements, brain activity | Cognition, physiology | Egyptian Ministry of Higher Education | English |
| [ ] | 5 Indonesians, ethnicity: Asian | A room with 5 potted plants and a room without plants | Control | 30 min | Experiment (non-RCT) | Heart rate, blood pressure | Physiology | Ministry of National Education in Indonesia | English | |||
| [ ] | 66 Hong Kongese college students (40 males and 26 females), mean age: 25.6, ethnicity: Asian | A basement room with plants, with a fake window, with plants and a fake window, and without plants nor a window | Control | At least 8 min | 3.3 × 2.2 × 2 m | 24 °C | Experiment (non-RCT) | EDA, a response time task | Physiology, cognition | Hong Kong Polytechnic University | English | |
| [ ] | 28 US adults (12 males and 16 females), age: 23 to 42 | Presence or absence of plants in an actual environment and a virtual one | Control | 5 min | Experiment (RCT) | Heart rate, EDA, blood pressure, a visual reaction time task, The Stroop task, a visual backward digit span task | Physiology, cognition | Campus Sustainability Innovation Fund, Harvard University Office for Sustainability | English | |||
| [ ] | 36–41 Japanese office workers, mean age: 33.95, ethnicity: Asian | Presence (3–10% green coverage ratio) or absence of plants in 2 offices | Control | 16 weeks of working hours | 132 m (321 m ), 270 m (675 m ) | Field quasi-experiment (non-RCT) | Heart rate, salivary amylase activity, critical flicker fusion frequency, fingertip pulse wave | Physiology | Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science | English | ||
| [ ] | 50 Chinese female elders with hypertension, mean age: 79.2, ethnicity: Asian | Presence or absence of 1 potted plant on a table in a room | Control | 5 min | 0.38 m | 23 °C, 40% RH, 500 lux, | Experiment (RCT) | Blood pressure, EEG | Physiology | English | ||
| [ ] | 100 Taiwanese elders, age: >65, ethnicity: Asian | Presence or absence of plants in houses | 1 year | Survey (non-RCT) | Blood pressure, heart rate | Physiology | Ministry of Science and Technology in Taiwan | English | ||||
| [ ] | 63 adult Japanese office workers (33 males and 30 females), mean age: 40.15, ethnicity: Asian | Presence or absence of 1 potted plant (15–20 cm tall, 7–10 cm wide) on the desk in an office | Control | 3 min | 1260 m | 20–24 °C, 40–50% RH, 500–700 lux | Field experiment (non-RCT, pre-post design) | Pulse rate | Physiology | English | ||
| [ ] | 30 Chinese female office workers, mean age: 29.42, ethnicity: Asian | Presence or absence of 1 potted plant with blue or purple flowers on a desk in an office | Control | 3 min | 0.4 m | 21 °C, 50% RH, 300 lux | Field quasi-experiment (non-RCT, pre-post design) | EEG, heart rate variability, skin conductance | Physiology | National Nature Science Foundation of China | English | |
| [ ] | 33 Chinese elders, age: 65 to 99, ethnicity: Asian | Combination of potted succulents (3–10 cm tall, 3 cm wide) or flower arrangement (50–60 cm tall, 5–18 cm wide) performed indoors | Flower arrangement | 25 min | Experiment (RCT) | Salivary cortisol | Physiology | National Nature Science Foundation of China | Chinese | |||
| [ ] | 34 Chinese elders with dementia (13 males and 21 females), ethnicity: Asian | With or without a treatment course of indoor horticultural activities (sowing, transplanting seedlings, succulents potting, and herbal flower potting) | Control | 30 min | Experiment (non-RCT) | Blood pressure, heart rate, ECG | Physiology | National Nature Science Foundation of China, Beijing Science and Technology Project Foundation | Chinese | |||
| [ ] | 44 Chinese elders living alone, ethnicity: Asian | Four kinds of indoor horticultural activities (sowing, transplanting seedlings, succulents potting, and herbal flower potting) | Within- participants, between-participants | 30 min | Experiment (non-RCT) | Blood pressure, heart rate, ECG | Physiology | Beijing Science and Technology Commission Green Communication Foundation | Chinese | |||
| [ ] | Study 1: 120 South Africans, mean age: 33.72, ethnicity: African | Presence of 3 potted plants, 6 plant pictures on 3 walls (80 × 80 cm), and no potted plants and plant pictures in an office | Control | 35 min | 3 × 3 m | 21 °C, 510 lux | Experiment (RCT) | A card-sorting task, a reading task | Cognition | English |
3.3. Risk of Bias within Studies
3.4. results of individual studies, 3.5. synthesis of results, 3.7. eeg α waves, 3.8. eeg β waves, 3.9. attention, 3.10. academic achievement, 3.11. response time, 3.12. risk of bias across studies, 3.13. additional analysis, 4. discussion, 4.1. summary of evidence, 4.2. limitations, 4.3. suggestions, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Hartig, T.; Mang, M.; Evans, G.W. Restorative effects of natural environment experiences. Environ. Behav. 1991 , 23 , 3–26. [ Google Scholar ] [ CrossRef ]
- Ulrich, R.S.; Parsons, R. Influences of passive experiences with plants on individual well-being and health. In The Role of Horticulture in Human Well-Being and Social Development ; Relt, P., Ed.; Timber Press: Portland, OR, USA, 1992; pp. 93–105. [ Google Scholar ]
- Ulrich, R.S. Aesthetic and affective response to natural environment. In Behavior and the Natural Environment ; Altman, I., Wohwill, J.F., Eds.; Plenum: New York, NY, USA, 1983; pp. 85–125. [ Google Scholar ]
- Wilson, E.O. Biophilia ; Harvard University Press: Cambridge, MA, USA, 1984. [ Google Scholar ]
- Kellert, S.R.; Wilson, E.O. The Biophilia Hypothesis ; Island Press: Washington, DC, USA, 1993. [ Google Scholar ]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991 , 11 , 201–230. [ Google Scholar ] [ CrossRef ]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective ; Cambridge University Press: New York, NY, USA, 1989. [ Google Scholar ]
- Han, K.-T. Restorative Nature: An Overview of the Positive Influences of Natural Landscapes on Humans ; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [ Google Scholar ]
- Hartig, T.; Mitchell, R.; de Vries, S.; Frumkin, H. Nature and health. Annu. Rev. Public Health 2014 , 35 , 207–228. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- van den Bosch, M.; Sang, Å.O. Urban natural environments as nature-based solutions for improved public health-A systematic review of reviews. Environ. Res. 2017 , 158 , 373–384. [ Google Scholar ] [ CrossRef ]
- Bowler, D.E.; Buyung-Ali, L.M.; Knight, T.M.; Pullin, A.S. A systematic review of evidence for added benefits to health of exposure to natural environments. BMC Public Health 2010 , 10 , 456–465. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Britton, E.; Kindermann, G.; Domegan, C.; Carlin, C. Blue care: A systematic review of blue space interventions for health and wellbeing. Health Promot. Int. 2020 , 35 , 50–69. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Rojas-Rueda, D.; Plaséncia, A.; Nieuwenhuijsen, M.J. Residential green spaces and mortality: A systematic review. Environ. Int. 2016 , 86 , 60–67. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lakhani, A.; Norwood, M.; Watling, D.; Zeeman, H.; Kendall, E. Using the natural environment to address the psychosocial impact of neurological disability: A systematic review. Health Place 2019 , 55 , 188–201. [ Google Scholar ] [ CrossRef ]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Forns, J.; Plaséncia, A.; Nieuwenhuijsen, M.J. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. Int. J. Environ. Res. Public Health 2015 , 12 , 4354–4379. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Coventry, P.A.; Brown, J.V.E.; Pervin, J.; Brabyn, S.; Pateman, R.; Breedvelt, J.; Gilbody, S.; Stancliffe, R.; McEachan, R.; White, P.C.L. Nature-based outdoor activities for mental and physical health: Systematic review and meta-analysis. SSM Popul. Health 2021 , 16 , 100934. [ Google Scholar ] [ CrossRef ]
- McMahan, E.A.; Estes, D. The effect of contact with natural environments on positive and negative affect: A meta-analysis. J. Posit. Psychol. 2015 , 10 , 507–519. [ Google Scholar ] [ CrossRef ]
- Menardo, E.; Brondino, M.; Hall, R.; Pasini, M. Restorativeness in natural and urban environments: A meta-analysis. Psychol. Rep. 2021 , 124 , 417–437. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ohly, H.; White, M.P.; Wheeler, B.W.; Bethel, A.; Ukoumunne, O.C.; Nikolaou, V.; Garside, R. Attention Restoration Theory: A systematic review of attention potential of exposure to natural environments. J. Toxicol. Environ. Health Part B 2016 , 19 , 305–343. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Stevenson, M.P.; Schilhab, T.; Bentsen, P. Attention Restoration Theory II: A systematic review to clarify attention processes affected by exposure to natural environments. J. Toxicol. Environ. Health Part B 2018 , 21 , 227–268. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018 , 166 , 626–637. [ Google Scholar ] [ CrossRef ]
- Yang, B.-Y.; Zhao, T.; Hu, L.-X.; Browning, M.H.E.M.; Heinrich, J.; Dharmage, S.C.; Jalaludin, B.; Knibbs, L.D.; Liu, X.-X.; Luo, Y.-N.; et al. Greenspace and human health: An umbrella review. Innovation 2021 , 2 , 100164. [ Google Scholar ] [ CrossRef ]
- United Nations, Department of Economic and Social Affairs, Population Division. 2018 Revision of World Urbanization Prospects ; United Nations: New York, NY, USA, 2018. [ Google Scholar ]
- World Health Organization. Urban Green Spaces and Health ; WHO Regional Office for Europe: Copenhagen, Denmark, 2016. [ Google Scholar ]
- World Health Organization. European Healthy Cities Network. 2016. Available online: http://www.euro.who.int/en/health-topics/environment-and-health/urban-health/activities/healthy-cities/who-european-healthy-cities-network/what-is-a-healthy-city (accessed on 10 July 2020).
- Schweizer, C.; Edwards, R.D.; Bayer-Oglesby, L.; Gauderman, W.J.; Ilacqua, V.; Jantunen, M.J.; Lai, H.K.; Nieuwenhuijsen, M.; Künzli, N. Indoor time-microenvironment–activity patterns in seven regions of Europe. J. Expo. Sci. Environ. Epidemiol. 2007 , 17 , 170–181. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Fjeld, T.; Veiersted, B.; Sandvik, L.; Riise, G.; Levy, F. The effect of indoor foliage plants on health and discomfort symptoms among office workers. Indoor Built Environ. 1998 , 7 , 204–209. [ Google Scholar ] [ CrossRef ]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001 , 11 , 231–252. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Marć, M.; Śmiełowska, M.; Namiesnik, J.; Zabiegała, B. Indoor air quality of everyday use spaces dedicated to specific purposes—A review. Environ. Sci. Pollut. Res. 2018 , 25 , 2065–2082. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Beyer, K.; Szabo, A.; Hoormann, K.; Stolley, M. Time spent outdoors, activity levels, and chronic disease among American adults. J. Behav. Med. 2018 , 41 , 494–503. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bringslimark, T.; Hartig, T.; Patil, G.G. The psychological benefits of indoor plants: A critical review of the experimental literature. J. Environ. Psychol. 2009 , 29 , 422–433. [ Google Scholar ] [ CrossRef ]
- Smardon, R.C. Perception and aesthetics of the urban environments: Review of the role of vegetation. Landsc. Urban Plan. 1988 , 15 , 85–106. [ Google Scholar ] [ CrossRef ]
- Centers for Disease Control. Public Health Terms for Planners & Planning Terms for Public Health Professionals. 2013. Available online: https://www.cdc.gov/healthyplaces/terminology.htm (accessed on 10 July 2020).
- Taylor, L.; Hochuli, D. Defining greenspace: Multiple uses across multiple disciplines. Landsc. Urban Plan. 2017 , 158 , 25–38. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- van den Bogerd, N.; Dijkstra, S.C.; Koole, S.L.; Seidell, J.C.; de Vries, R.; Maas, J. Nature in the indoor and outdoor study environment and secondary and tertiary education students’ well-being, academic outcomes, and possible mediating pathways: A systematic review with recommendations for science and practice. Health Place 2020 , 66 , 102403–102418. [ Google Scholar ] [ CrossRef ]
- Yeo, N.L.; Elliott, L.R.; Bethel, A.; White, M.P.; Dean, S.G.; Garside, R. Indoor nature interventions for health and wellbeing of older adults in residential settings: A systematic review. Gerontologist 2020 , 60 , e184–e199. [ Google Scholar ] [ CrossRef ]
- Han, K.-T. Effects of indoor plants on the physical environment with respect to distance and green coverage ratio. Sustainability 2019 , 11 , 3679. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Han, K.-T. Effects of visible greenness, quantity and distance of indoor plants on human perceptions and physical parameters. Indoor Build Environ. 2020 , 30 , 1353–1372. [ Google Scholar ] [ CrossRef ]
- Kim, H.-H.; Lee, J.-Y.; Kim, H.-J.; Lee, Y.-W.; Kim, K.-J.; Park, J.-H.; Shin, D.-C.; Lim, Y.-W. Impact of foliage plant interventions in classrooms on actual air quality and subjective health complaints. J. Am. Soc. Hortic. Sci. 2013 , 82 , 255–262. [ Google Scholar ] [ CrossRef ]
- Deng, L.; Deng, Q. The basic roles of indoor plants in human health and comfort. Environ. Sci. Pollut. Res. 2018 , 25 , 36087–36101. [ Google Scholar ] [ CrossRef ]
- Moya, T.A.; van den Dobbelsteen, A.; Ottelé, M.; Bluyssen, P.M. A review of green systems within the indoor environment. Indoor Built Environ. 2019 , 28 , 298–309. [ Google Scholar ] [ CrossRef ]
- Moher, D.; Liberati, A.; Tetzla, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systemic review and meta-analysis: The PRISMA statement. PLoS Med. 2009 , 6 , e1000097. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis ; John Wiley & Sons: Hoboken, NJ, USA, 2009. [ Google Scholar ]
- Centre for Reviews and Dissemination. Systematic Reviews , 3rd ed.; CRD: York, UK, 2009. [ Google Scholar ]
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on self-reported perceptions: A systemic review. Sustainability 2019 , 11 , 4506. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Stone, A.A.; Bachrach, C.A.; Jobe, J.B.; Kurtzman, H.S.; Cain, V.S. The Science of Self-Report: Implications for Research and Practice ; Psychology Press: East Sussex, UK, 1999. [ Google Scholar ]
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020 , 27 , 16019–16051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chiou, H.-J. Quantitative Research and Statistical Analysis in Social and Behavior Sciences ; Wunan: Taipei, Taiwan, 2004. [ Google Scholar ]
- Frankfort-Nachmias, C.; Nachmias, D. Research Methods in the Social Sciences ; Arnold: London, UK, 1996. [ Google Scholar ]
- Cook, T.C.; Campbell, D.T. Quasi-Experimentation: Design & Analysis Issues for Field Settings ; Houghton Mifflin: Boston, MA, USA, 1979. [ Google Scholar ]
- Critical Appraisal Skills Programme. CASP UK Critical Appraisal Checklists ; Critical Appraisal Skills Programme: Oxford, UK, 2013. [ Google Scholar ]
- Effective Public Health Practice Project. Quality Assessment Tool for Quantitative Studies. 2013. Available online: http://www.ephpp.ca/tools.html (accessed on 25 March 2019).
- Larsen, L.; Adams, J.; Deal, B.; Kweon, B.-S.; Tyler, E. Plants in the workplace: The effects of plant density on productivity, attitudes, and perceptions. Environ. Behav. 1998 , 30 , 261–281. [ Google Scholar ] [ CrossRef ]
- Bjørnstad, S.; Patil, G.G.; Raanaas, R.K. Nature contact and organizational support during office working hours: Benefits relating to stress reduction, subjective health complaints, and sick leave. Work 2016 , 53 , 9–20. [ Google Scholar ] [ CrossRef ]
- Bringslimark, T.; Hartig, T.; Patil, G.G. Psychological benefits of indoor plants in workplaces: Putting experimental results into context. HortScience 2007 , 42 , 581–587. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Elasdek, M.; Liu, B. Effects of viewing flowering plants on employees’ wellbeing in an office-like environment. Indoor Built Environ. 2020 , 30 , 1429–1440. [ Google Scholar ] [ CrossRef ]
- Genjo, K.; Matsumoto, H.; Ogata, N.; Nakano, T. Feasibility study on mental health-care effects of plant installations in office spaces. Jpn. Archit. Rev. 2019 , 2 , 376–388. [ Google Scholar ] [ CrossRef ]
- Nieuwenhuis, M.; Knight, C.; Postmes, T.; Haslam, S.A. The relative benefits of green versus lean office space: Three field experiments. J. Exp. Psychol. Appl. 2014 , 20 , 199–214. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Toyoda, M.; Yokota, Y.; Barnes, M.; Kaneko, M. Potential of a small indoor plant on the desk for reducing office workers’ stress. HortTechnology 2020 , 30 , 55–63. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hassan, A.; Qibing, C.; Yinggao, L.; Tao, J.; Li, G.; Jiang, M.; Nian, L.; Bing-Yang, L. Psychological and physiological effects of viewing a money plant by older adults. Brain Behav. 2019 , 9 , e01359. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Khan, M.A.; Amin, N.; Khan, A.; Imtiaz, M.; Khan, F.; Ahmad, I.; Ali, A.; Islam, B. Plant therapy: A nonpharmacological and noninvasive treatment approach medically beneficial to the wellbeing of hospital patients. Gesunde Pflanz. 2016 , 68 , 191–200. [ Google Scholar ] [ CrossRef ]
- Li, X.; Huang, Q.; Li, S.; Chen, C. Research on the physical and psychological health effect of the horticultural plant cultivation activity on the elderly with dementia. J. Northwest Univ. (Nat. Sci. Ed.) 2020 , 50 , 867–880. [ Google Scholar ] [ CrossRef ]
- Park, S.-H.; Mattson, R.H. Effects of flowering and foliage plants in hospital rooms on patients recovering from abdominal surgery. HortTechnology 2008 , 18 , 563–568. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Park, S.-H.; Mattson, R.H. Therapeutic influences of plants in hospital rooms on surgical recovery. HortScience 2009 , 44 , 1–4. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Han, K.-T. Influence of limitedly visible leafy indoor plants on the psychology, behavior, and health of students at a junior high school in Taiwan. Environ. Behav. 2009 , 41 , 658–692. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Han, K.-T.; Hung, C.-Y. Influences of physical interactions and visual contacts with plants on students’ psycho-physiology, behaviors, academic performance and health. Sci. Agric. 2012 , 59 , 195–210. [ Google Scholar ]
- Park, S.-Y.; Song, J.-S.; Kim, H.-D.; Yamane, K.; Son, K.C. Effects of interior plantscapes on indoor environments and stress level of high school students. J. Jpn. Soc. Hortic. Sci. 2008 , 77 , 447–454. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Johansson, B. Heart rate and heart rate variability response to the Transpiration of Vortex-Water by Begonia Eliator Plants to the air in an office during visual display terminal work. J. Altern. Complementary Med. 2008 , 14 , 993–1003. [ Google Scholar ] [ CrossRef ]
- Liu, M.; Kim, E.; Mattson, R.H. Physiological and emotional influences of cut flower arrangements and lavender fragrance on university students. J. Ther. Hortic. 2003 , 14 , 18–27. [ Google Scholar ]
- Shibata, S.; Suzuki, N. Effects of the foliage plant on task performance and mood. J. Environ. Psychol. 2002 , 22 , 265–272. [ Google Scholar ] [ CrossRef ]
- Shibata, S.; Suzuki, N. Effects of an indoor plant on creative task performance and mood. Scand. J. Psychol. 2004 , 45 , 373–381. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chang, C.-Y.; Chen, P.-K. Human response to window views and indoor plants in the workplace. HortScience 2005 , 40 , 1354–1359. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Li, X.; Zhang, Z.; Gu, M.; Jiang, D.-Y.; Wang, J.; Lv, Y.-M.; Zhang, Q.-X.; Pan, H.-T. Effects of plantscape colors on psycho-physiological responses of university students. J. Food Agric. Environ. 2012 , 10 , 702–708. [ Google Scholar ]
- Li, X.; Lu, Y.-M.; Zhang, Z.; Wang, J.; Pan, H.-T.; Zhang, Q.-X. The visual effects of flower colors on university students psycho-physiological responses. J. Food Agric. Environ. 2012 , 10 , 1294–1300. [ Google Scholar ]
- Yin, J.; Zhu, S.; MacNaughton, P.; Allen, J.G.; Spengler, J.D. Physiological and cognitive performance of exposure to biophilic indoor environment. Build. Environ. 2018 , 132 , 255–262. [ Google Scholar ] [ CrossRef ]
- Chen, R.-U.; Ho, K.-F.; Hong, G.-B.; Chuang, K.-J. Houseplant, indoor air pollution, and cardiovascular effects among elderly subjects in Taipei, Taiwan. Sci. Total Environ. 2020 , 705 , 135770. [ Google Scholar ] [ CrossRef ]
- Kim, J.; Chaa, S.-H.; Koo, C.; Tang, S.-K. The effects of indoor plants and artificial windows in an underground environment. Build. Environ. 2018 , 138 , 53–62. [ Google Scholar ] [ CrossRef ]
- Lohr, V.I.; Pearson-Mims, C.H.; Goodwin, G.K. Interior plants may improve worker productivity and reduce stress in a windowless environment. J. Environ. Hortic. 1996 , 14 , 97–100. [ Google Scholar ] [ CrossRef ]
- Jang, H.S.; Kim, J.; Kim, K.S.; Pak, C.H. Human brain activity and emotional responses to plant color stimuli. Color Res. Appl. 2014 , 39 , 307–316. [ Google Scholar ] [ CrossRef ]
- Lohr, V.I.; Pearson-Mims, C.H. Physical discomfort may be reduced in the presence of interior plants. HortTechnology 2000 , 10 , 53–58. [ Google Scholar ] [ CrossRef ]
- Qin, J.; Sun, C.; Zhou, X.; Leng, H.; Lian, Z. The effect of indoor plants on human comfort. Indoor Built Environ. 2014 , 23 , 709–723. [ Google Scholar ] [ CrossRef ]
- Lee, M.-S.; Lee, J.; Park, B.-J.; Miyazaki, Y. Interaction with indoor plants may reduce psychological and physiological stress by suppressing autonomic nervous system activity in young adults: A randomized crossover study. J. Physiol. Anthropol. 2015 , 34 , 21. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Kim, E.; Mattson, R.H. Stress recovery effects of viewing red-flowering geraniums. J. Ther. Hortic. 2002 , 13 , 4–12. [ Google Scholar ]
- Chen, J.-G.; Xiang, C.-Q.; Ruan, S.-U.; Zheng, Y.-Y.; Zhang, S.N. Application of Neurobehavioral Evaluation System Testing in the sealed building study on population Health. J. Labour Med. 2000 , 17 , 134–137. [ Google Scholar ]
- Park, S.-H.; Mattson, R.H.; Kim, E. Pain tolerance effects of ornamental plants in a simulated hospital patient room. Acta Hortic. 2004 , 639 , 241–247. [ Google Scholar ] [ CrossRef ]
- Doxey, J.S.; Waliczek, T.M. The impact of interior plants in university classrooms on student course performance and on student perceptions of the course and instructor. Hortscience 2009 , 44 , 384–391. [ Google Scholar ] [ CrossRef ]
- Raanaas, R.K.; Evensen, K.H.; Rich, D.; Sjøstrøm, G.; Patil, G. Benefits of indoor plants on attention capacity in an office setting. J. Environ. Psychol. 2011 , 31 , 99–105. [ Google Scholar ] [ CrossRef ]
- Elsadek, M.; Sayaka, S.; Fujii, E.; Koriesh, E.; Moghazy, E.; Elfatah, Y.A. Human emotional and psycho-physiological responses to plant color stimuli. J. Food Agric. Environ. 2013 , 11 , 1584–1591. [ Google Scholar ]
- Elsadek, M.; Fujii, E. People’s psycho-physiological responses to plantscape colors stimuli: A pilot study. Int. J. Psychol. Behav. Sci. 2014 , 4 , 70–78. [ Google Scholar ]
- Elsadek, M.; Sun, M.; Fujii, E. Psycho-physiological responses to plant variegation as measured through eye movement, self-reported emotion and cerebral activity. Indoor Built Environ. 2017 , 26 , 758–770. [ Google Scholar ] [ CrossRef ]
- Sugiono Swara, S.E.; Wijanarko, W.; Sulistyarini, D.H. Investigating the impact of ornamental plants correlated with indoor thermal comfort and eco-energy. Int. Rev. Civ. Eng. 2017 , 8 , 221–226. [ Google Scholar ] [ CrossRef ]
- Huang, Q.; Kang, N.; Li, X.; Li, S. Effects of different horticultural activities on the negative emotions of the elderly. J. Northwest Univ. (Nat. Sci. Ed.) 2020 , 50 , 887–896. [ Google Scholar ] [ CrossRef ]
- Wei, Y.; Dong, Z.; Yu, W.; Huang, Q.; Li, S. Study on the physical and psychological effect of the four different horticultural activities on the elderly without family members. J. Northwest Univ. (Nat. Sci. Ed.) 2020 , 50 , 923–933. [ Google Scholar ] [ CrossRef ]
- Thatcher, A.; Adamson, K.; Bloch, L.; Kalantzis, A. Do indoor plants improve performance and well-being in offices? Divergent results from laboratory and field studies. J. Environ. Psychol. 2020 , 71 , 101487. [ Google Scholar ] [ CrossRef ]
- Sutton, A.J.; Abrams, K.R.; Jones, D.R.; Trevor, A.; Sheldon, T.A.; Song, F. Methods for Meta-Analysis in Medical Research ; John Wiley & Sons: Hoboken, NJ, USA, 2000. [ Google Scholar ]
- Hsu, C.-H.; Chang, Y.-C.; Tang, S.-M.; Lee, W.-C.; Hsiao, J.-L.; Chen, Y.-Y. Physiology ; New Wun Ching: New Taipei, Taiwan, 2014. [ Google Scholar ]
- Fazio, R.H.; Cooper, J. Arousal in the dissonance process. In Social Psychophysiology: A Sourcebook ; Cacioppo, J.T., Petty, R.E., Eds.; Guilford: New York, NY, USA, 1983; pp. 122–152. [ Google Scholar ]
- Gola, M.; Magnuski, M.; Szumska, I.; Wróbel, A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int. J. Psychophysiol. 2013 , 89 , 334–341. [ Google Scholar ] [ CrossRef ]
- Jena, S.K. Examination stress and its effect on EEG. Int. J. Med. Sci. Public Health 2015 , 4 , 1493–1497. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Gupta, P.C. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011 , 2 , 109–112. [ Google Scholar ] [ CrossRef ]
- Noseworthy, J.H.; Ebers, G.C.; Vandervoort, M.K.; Farquhar, R.E.; Yetisir, E.; Roberts, R. The impact of blinding on the results of a randomized, placebo-controlled multiple sclerosis clinical trial. Neurology 1994 , 44 , 16–20. [ Google Scholar ] [ CrossRef ]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences ; Erbaum: Hillsdale, NJ, USA, 1988. [ Google Scholar ]
- Suresh, K.P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011 , 4 , 8–11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patino, C.M.; Ferreira, J.C. Inclusion and exclusion criteria in research studies: Definitions and why they matter. J. Brasialeiro De Pneumol. 2018 , 44 , 84. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Blinding: Who, what, when, why, how? Can. J. Surg. 2010 , 53 , 345–348. [ Google Scholar ] [ PubMed ]
- Fanelli, D. Negative results are disappearing from most disciplines and countries. Scientometrics 2012 , 90 , 891–904. [ Google Scholar ] [ CrossRef ]
- Every-Palmer, S.; Howick, J. How evidence-based medicine is failing due to biased trials and selective publication. J. Eval. Clin. Pract. 2014 , 20 , 908–914. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lamé., G. Systematic Literature Reviews: An Introduction. In Proceedings of the International Conference on Engineering Design 2019, Delft, The Netherlands, 5–8 August 2019; pp. 1633–1642. [ Google Scholar ]
- Chen, S.-L.; Chen, Y.-M. Health Care Statistics and Meta-Analysis: RevMan 5 Software Operation ; Wunan: Taipei, Taiwan, 2016. [ Google Scholar ]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Br. Med. J. 2010 , 340 , 698–702. [ Google Scholar ] [ CrossRef ]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004 , 94 , 361–366. [ Google Scholar ] [ CrossRef ]
- American Psychological Association. Publication Manual of the American Psychological Association , 7th ed.; American Psychological Association: Washington, DC, USA, 2020. [ Google Scholar ]
- Jiang, B.; Larsen, L.; Deal, B.; Sullivan, W.C. A dose-response curve describing the relationship between tree cover density and landscape preference. Landsc. Urban Plan. 2015 , 139 , 16–25. [ Google Scholar ] [ CrossRef ]
- Jiang, B.; Li, D.; Larsen, L.; Sullivan, W.C. A dose-response curve describing the relationship between urban tree cover density and self-reported stress recovery. Environ. Behav. 2016 , 48 , 607–629. [ Google Scholar ] [ CrossRef ]
- Shanahan, D.F.; Fuller, R.A.; Bush, R.; Lin, B.B.; Gaston, K.J. The health benefits of urban nature: How much do we need? BioScience 2015 , 65 , 476–485. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hong, X.-M. A study on Restoration Effects of Greening Levels of Plant at Indoor Working Environment. Master’s Thesis, University of Feng Chia, Taichung, Taiwan, 2009. [ Google Scholar ]
- Fjeld, T.; Bonnevie, C. The Effect of Plants and Artificial Day-Light on the Well-Being and Health of Office Workers ; School Children and Health Care Personnel, Medicine, Lippincott: Philadelphia, PA, USA, 2002. [ Google Scholar ]
- Godish, T.; Guindon, C. An assessment of botanical air purification as a formaldehyde mitigation measure under dynamic laboratory chamber conditions. Environ. Pollut. 1989 , 62 , 13–20. [ Google Scholar ] [ CrossRef ]
- Han, K.-T. Urban Forestry: Theories and Applications ; Lamper: Taipei, Taiwan, 1998. [ Google Scholar ]
- Vimalanathan, K.; Ramesh Babu, T. The effect of indoor office environment on the work performance, health and well-being of office workers. J. Environ. Health Sci. Eng. 2014 , 12 , 113. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Persiani, S. Benefits of using plants in indoor environments: Exploring common research gaps. Architecture 2021 , 1 , 83–98. [ Google Scholar ] [ CrossRef ]
- Robins, R.; Fraley, C.; Krueger, R. Handbook of Research Methods in Personality Psychology ; The Guilford Press: New York, NY, USA, 2007. [ Google Scholar ]
- Wood, L.; Egger, M.; Gluud, L.L.; Schulz, K.F.; Jüni, P.; Altman, D.G.; Gluud, C.; Martin, R.M.; Wood AJ, G.; Sterne, J.A.C. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. Br. Med. J. 2008 , 336 , 601–605. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Obling, K.H.; Hansen, A.L.; Overgaard, K.; Normann, K.; Sandbaek, A.; Maindal, H.T. Association between self-reported and objectively measured physical fitness level in a middle-aged population in primary care. Prev. Med. Rep. 2015 , 2 , 462–466. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Orta, O.R.; Barbosa, C.; Velez, J.C.; Gelaye, B.; Chen, X.; Stoner, L.; Williams, M.A. Associations of self-reported and objectively measured sleep disturbances with depression among primary caregivers of children with disabilities. Nat. Sci. Sleep 2016 , 8 , 181–188. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Neupane, S.; Karstad, K.; Hallman, D.M.; Rugulies, R.; Holtermann, A. Objectively measured versus self-reported occupational physical activity and multisite musculoskeletal pain: A prospective follow-up study at 20 nursing homes in Denmark. Int. Arch. Occup. Environ. Health 2020 , 93 , 381–389. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- World Health Organization. Housing: Sick Building Syndrome (Pamphlet No. 2), Copenhagen, World Health Organization Regional Office for Europe. 2005. Available online: http://www.emro.who.int/ceha/Publication_Details.asp?ID=46 (accessed on 10 July 2020).
- Fisk, W. How IEQ affects health, productivity. ASHRAE J. 2002 , 44 , 56–60. [ Google Scholar ]
- Cincinelli, A.; Martellini, T.; Amore, A.; Dei, L.; Marrazza, G.; Carretti, E.; Belosi, F.; Ravegnani, F.; Leva, P. Measurement of volatile organic compounds (VOCs) in libraries and archives in Florence (Italy). Sci. Total Environ. 2016 , 572 , 333–339. [ Google Scholar ] [ CrossRef ]
- Fleck, R.; Pettit, T.J.; Douglas, A.N.J.; Irga, P.J.; Torpy, F.R. Bio-Based Materials and Biotechnologies for Eco-Efficient Construction ; Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2020; pp. 305–327. [ Google Scholar ]
- Torpy, F.; Clements, N.; Pollinger, M.; Dengel, A.; Mulvihill, I.; He, C.; Irga, P. Testing the single-pass VOC removal efficiency of an active green wall using methyl ethyl ketone (MEK). Air Qual. Atmos. Health 2018 , 11 , 163–170. [ Google Scholar ] [ CrossRef ] [ Green Version ]
Click here to enlarge figure
| Publication Year | Publication Language | Total | ||||
|---|---|---|---|---|---|---|
| Chinese | English | |||||
| Number of Papers | Percentage (%) | Number of Papers | Percentage (%) | Number of Papers | Percentage (%) | |
| 1996–2000 | 1 | 20 | 3 | 8.1 | 4 | 9.5 |
| 2001–2005 | 0 | 0 | 6 | 16.2 | 6 | 14.3 |
| 2006–2010 | 0 | 0 | 7 | 18.9 | 7 | 16.7 |
| 2011–2015 | 1 | 20 | 9 | 24.3 | 10 | 23.8 |
| 2016–2020 | 3 | 60 | 12 | 32.4 | 15 | 35.7 |
| Total | 5 | 100.0 | 37 | 100.0 | 42 | 100.0 |
| Participant Location | Number of Records | Percentage (%) |
|---|---|---|
| China (Asia, Global North) | 10 | 23.8 |
| United States (America, Global North) | 8 | 19.0 |
| Japan (Asia, Global North) | 6 | 14.3 |
| South Korea (Asia, Global North) | 5 | 11.9 |
| Taiwan (Asia, Global North) | 4 | 9.5 |
| Norway (Europe, Global North) | 3 | 7.1 |
| United Kingdom (Europe, Global North) | 1 | 2.4 |
| Sweden (Europe, Global North) | 1 | 2.4 |
| Pakistan (Asia, Global North) | 1 | 2.4 |
| Egypt (Africa, Global North) | 1 | 2.4 |
| South Africans (Africa, Global South) | 1 | 2.4 |
| Indonesia (Asia, Equatorial) | 1 | 2.4 |
| Total | 42 | 100.0 |
| Experimental Condition | Maximum | Minimum | Number of Records | |
|---|---|---|---|---|
| 1 year | 15 s. | 34 | ||
| [ ] | [ ] | |||
| Floor area | 1260 m | 7.26 m | 19 | |
| [ ] | [ ] | |||
| Volume | 675 m | 14.52 m | 14 | |
| [ ] | [ ] | |||
| 3 m | 0.38 m | 13 | ||
| [ ] | [ ] | |||
| 27 °C | 20 °C | 19 | ||
| [ ] | [ ] | |||
| 70% | 34% | 13 | ||
| [ ] | [ ] | |||
| 0.2 m·s | 1 | |||
| [ ] | ||||
| Illuminance | 1365.5 lux | 300 lux | 11 | |
| [ ] | [ ] | |||
| Quantum | 10.6 μmol·m ·s | 1 | ||
| [ ] | ||||
| Quality Indicators | [ ] | [ ] | [ ] | [ ] | [ ] | [ ] | [ ] | |
|---|---|---|---|---|---|---|---|---|
| Power calculation reported | No | No | No | No | No | No | No | |
| Inclusion/exclusion criteria reported | No | No | No | No | No | No | No | |
| Individual level allocation | No | Yes | NA | Yes | Yes | Yes | Yes | |
| Random allocation to groups/condition/order | Yes | NA | Yes | Yes | Yes | Yes | ||
| Randomization procedure appropriate | Unclear | NA | Unclear | Unclear | Unclear | Unclear | ||
| Groups similar (sociodemographic) | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Yes | |
| Group balanced at baseline | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | |
| Participants blind to research question | Yes | Unclear | Unclear | Unclear | Unclear | |||
| Clear description of intervention and control | Yes | Yes | NA | Yes | Yes | Yes | Yes | |
| Consistency of intervention (within and between groups) | Yes | No | NA | Yes | Yes | Yes | No | |
| Outcome assessors blind to group allocation | Unclear | Unclear | Unclear | Unclear | ||||
| Baseline measures taken before the intervention | Yes | Unclear | NA | Yes | Yes | No | Yes | |
| Consistency of data collection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| All outcomes reported (means and SD/SE) | No | Yes | No | No | No | Yes | No | |
| All participants accounted for (i.e., losses/exclusions) | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| ITT analysis conducted (all data included after allocation) | Unclear | Unclear | NA | Unclear | Unclear | No | Unclear | |
| Individual level analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Sample representative of target population | No | No | No | No | No | No | No | |
| Total number of points (out of possible 38) | 20 | 18 | 8 | 20 | 20 | 16 | 22 | |
| Quality rating as percent | 52.6 (M) | 47.4 (M) | 21.1 (L) | 52.6 (M) | 52.6 (M) | 42.1 (M) | 57.9 (M) | |
| Responded to query about “uncertain” ratings | Yes | Yes | NA | No | NA | Yes | ||
| ] | ] | ] | ] | ] | ] | ] | ||
| Power calculation reported | No | No | No | No | No | No | No | |
| Inclusion/exclusion criteria reported | No | No | Yes | No | No | Yes | No | |
| Individual level allocation | Yes | Yes | Yes | NA | Yes | Yes | No | |
| Random allocation to groups/condition/order | Yes | Yes | Unclear | NA | Yes | Yes | No | |
| Randomization procedure appropriate | Unclear | Unclear | Unclear | NA | Unclear | Unclear | NA | |
| Groups similar (sociodemographic) | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes | |
| Group balanced at baseline | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Yes | |
| Participants blind to research question | Unclear | Unclear | Yes | Unclear | Yes | Unclear | ||
| Clear description of intervention and control | Yes | Yes | Yes | NA | Yes | Yes | Partial | |
| Consistency of intervention (within and between groups) | No | No | No | NA | Yes | Yes | No | |
| Outcome assessors blind to group allocation | Unclear | Unclear | NA | Unclear | Unclear | Unclear | ||
| Baseline measures taken before the intervention | Yes | Yes | No | NA | Yes | No | Yes | |
| Consistency of data collection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| All outcomes reported (means and SD/SE) | No | Yes | Yes | No | Yes | No | No | |
| All participants accounted for (i.e., losses/exclusions) | Yes | Yes | Yes | No | Yes | Yes | No | |
| ITT analysis conducted (all data included after allocation) | Unclear | Unclear | Unclear | NA | Unclear | Unclear | No | |
| Individual level analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | Yes | Yes | No | Yes | Yes | Yes | No | |
| Sample representative of target population | No | No | No | No | No | No | No | |
| Total number of points (out of possible 38) | 18 | 20 | 18 | 8 | 20 | 20 | 11 | |
| Quality rating as percent | 47.4 (M) | 52.6 (M) | 47.4 (M) | 21.1 (L) | 52.6 (M) | 52.6 (M) | 28.9 (L) | |
| Responded to query about “uncertain” ratings | NA | Yes | No | No | ||||
| ] | ] | ] | ] | ] | ] | ] | ||
| Power calculation reported | No | No | No | No | No | No | No | |
| Inclusion/exclusion criteria reported | No | Yes | Yes | No | No | Yes | Yes | |
| Individual level allocation | No | No | Yes | Yes | Yes | Yes | Yes | |
| Random allocation to groups/condition/order | No | No | Yes | Yes | Yes | Yes | Yes | |
| Randomization procedure appropriate | NA | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Groups similar (sociodemographic) | Partial | Partial | Unclear | Unclear | Yes | Yes | Yes | |
| Group balanced at baseline | Unclear | Unclear | Unclear | Partial | Unclear | Yes | Yes | |
| Participants blind to research question | Unclear | Yes | Yes | Yes | Yes | Unclear | Unclear | |
| Clear description of intervention and control | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Consistency of intervention (within and between groups) | Yes | Yes | Yes | Yes | No | No | No | |
| Outcome assessors blind to group allocation | Unclear | No | Unclear | Unclear | No | Unclear | Unclear | |
| Baseline measures taken before the intervention | No | Yes | No | Yes | Yes | Yes | Yes | |
| Consistency of data collection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| All outcomes reported (means and SD/SE) | Yes | No | No | No | No | Yes | Yes | |
| All participants accounted for (i.e., losses/exclusions) | No | Yes | Yes | Yes | Yes | Yes | Yes | |
| ITT analysis conducted (all data included after allocation) | No | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Individual level analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Sample representative of target population | No | No | No | No | No | No | No | |
| Total number of points (out of possible 38) | 13 | 19 | 20 | 21 | 20 | 24 | 24 | |
| Quality rating as percent | 34.2 (M) | 50.0 (M) | 52.6 (M) | 55.3 (M) | 52.6 (M) | 63.2 (M) | 63.2 (M) | |
| Responded to query about “uncertain” ratings | No | No | No | |||||
| ] | ] | ] | ] | ] | ] | ] | ||
| Power calculation reported | No | No | No | Study 3: No | Yes | No | No | |
| Inclusion/exclusion criteria reported | Yes | Yes | Yes | Study 3: No | Yes | Yes | Yes | |
| Individual level allocation | Yes | Yes | Yes | Study 3: No | No | Yes | NA | |
| Random allocation to groups/condition/order | Yes | Yes | Yes | Study 3: Yes | Unclear | Yes | NA | |
| Randomization procedure appropriate | Unclear | Unclear | Unclear | Study 3: Unclear | Unclear | Unclear | NA | |
| Groups similar (sociodemographic) | Yes | Yes | Yes | Study 3: Unclear | Yes | Yes | Unclear | |
| Group balanced at baseline | Yes | Yes | Yes | Study 3: Unclear | Yes | Yes | Unclear | |
| Participants blind to research question | Unclear | Unclear | Study 3: | Unclear | No | Unclear | ||
| Clear description of intervention and control | Yes | Yes | Yes | Study 3: Yes | Yes | Yes | NA | |
| Consistency of intervention (within and between groups) | No | No | No | Study 3: No | No | Yes | NA | |
| Outcome assessors blind to group allocation | Unclear | Unclear | Study 3: | Unclear | Unclear | Unclear | ||
| Baseline measures taken before the intervention | No | No | No | Study 3: No | No | Yes | NA | |
| Consistency of data collection | Yes | Yes | Yes | Study 3: Yes | Yes | Yes | Yes | |
| All outcomes reported (means and SD/SE) | No | No | Yes | Study 3: No | No | No | No | |
| All participants accounted for (i.e., losses/exclusions) | Yes | Yes | Yes | Study 3: Yes | Yes | Yes | No | |
| ITT analysis conducted (all data included after allocation) | Unclear | Unclear | Unclear | Study 3: Unclear | Unclear | Unclear | NA | |
| Individual level analysis | Yes | Yes | Yes | Study 3: Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | Yes | Yes | No | Study 3: Yes | No | Yes | Yes | |
| Sample representative of target population | No | No | No | Study 3: No | No | No | No | |
| Total number of points (out of possible 38) | 20 | 20 | 20 | Study 3: 14 | 16 | 24 | 8 | |
| Quality rating as percent | 52.6 (M) | 52.6 (M) | 52.6 (M) | Study 3: 36.8 (M) | 42.1 (M) | 63.2 (M) | 21.1 (L) | |
| Responded to query about “uncertain” ratings | Yes | No | No | Yes | ||||
| ] | ] | ] | ] | ] | ] | ] | ||
| Power calculation reported | No | No | No | No | No | No | No | |
| Inclusion/exclusion criteria reported | Yes | Yes | No | No | Yes | No | Yes | |
| Individual level allocation | Yes | Yes | Unclear | Yes | Yes | No | Yes | |
| Random allocation to groups/condition/order | Yes | Yes | Unclear | Unclear | Yes | No | Yes | |
| Randomization procedure appropriate | Unclear | Yes | Unclear | Unclear | Unclear | NA | Unclear | |
| Groups similar (sociodemographic) | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear | |
| Group balanced at baseline | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear | |
| Participants blind to research question | Unclear | No | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Clear description of intervention and control | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Consistency of intervention (within and between groups) | Yes | No | No | No | Yes | No | Yes | |
| Outcome assessors blind to group allocation | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Baseline measures taken before the intervention | No | No | No | Yes | Yes | Yes | Partial | |
| Consistency of data collection | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| All outcomes reported (means and SD/SE) | No | No | No | No | No | No | No | |
| All participants accounted for (i.e., losses/exclusions) | Yes | Yes | Yes | Yes | No | No | Yes | |
| ITT analysis conducted (all data included after allocation) | Unclear | Unclear | Unclear | Unclear | No | Unclear | Unclear | |
| Individual level analysis | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | No | No | Unclear | Yes | Yes | No | Yes | |
| Sample representative of target population | No | No | No | No | No | No | No | |
| Total number of points (out of possible 38) | 16 | 16 | 10 | 14 | 22 | 6 | 19 | |
| Quality rating as percent | 42.1 (M) | 42.1 (M) | 26.3 (L) | 36.8 (M) | 58.9 (M) | 15.8 (L) | 50.0 (M) | |
| Responded to query about “uncertain” ratings | ||||||||
| ] | ] | ] | ] | ] | ] | ] | ||
| Power calculation reported | No | No | No | No | No | No | Yes | |
| Inclusion/exclusion criteria reported | Yes | No | Yes | Yes | No | Yes | No | |
| Individual level allocation | NA | No | Yes | No | Unclear | Unclear | Yes | |
| Random allocation to groups/condition/order | NA | No | No | Yes | Unclear | Unclear | Yes | |
| Randomization procedure appropriate | NA | NA | NA | Unclear | Unclear | Unclear | Unclear | |
| Groups similar (sociodemographic) | Unclear | Yes | Yes | Unclear | Yes | Unclear | Unclear | |
| Group balanced at baseline | Unclear | Yes | Yes | Unclear | Yes | Unclear | Unclear | |
| Participants blind to research question | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | |
| Clear description of intervention and control | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Consistency of intervention (within and between groups) | Yes | No | No | Yes | No | No | No | |
| Outcome assessors blind to group allocation | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Baseline measures taken before the intervention | No | Yes | Yes | Yes | Yes | Yes | No | |
| Consistency of data collection | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| All outcomes reported (means and SD/SE) | Yes | No | No | Yes | Yes | Yes | Yes | |
| All participants accounted for (i.e., losses/exclusions) | Yes | Yes | Yes | No | Yes | No | Yes | |
| ITT analysis conducted (all data included after allocation) | NA | Unclear | Unclear | No | Unclear | No | Unclear | |
| Individual level analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Statistical analysis methods appropriate for study design | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Sample representative of target population | No | No | No | No | No | No | No | |
| Total number of points (out of possible 38) | 16 | 12 | 20 | 18 | 18 | 14 | 20 | |
| Quality rating as percent | 42.1 (M) | 31.6 (L) | 52.6 (M) | 47.4 (M) | 47.4 (M) | 36.8 (M) | 52.6 (M) | |
| Responded to query about “uncertain” ratings | ||||||||
| Yes | Partial | No | Unclear | NA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | (%) | Frequency | (%) | Frequency | (%) | Frequency | (%) | Frequency | (%) | |
| Power Calculation Reported | 2 | 5 | 0 | 0 | 39 | 95 | 0 | 0 | 0 | 0 |
| Inclusion/exclusion Criteria Reported | 20 | 49 | 0 | 0 | 21 | 51 | 0 | 0 | 0 | 0 |
| Individual Level Allocation | 26 | 63 | 0 | 0 | 8 | 20 | 3 | 7 | 4 | 10 |
| Random Allocation to Groups/Condition/Order | 25 | 61 | 0 | 0 | 6 | 15 | 6 | 15 | 4 | 10 |
| Randomization Procedure Appropriate | 2 | 5 | 0 | 0 | 0 | 0 | 30 | 73 | 9 | 22 |
| Groups Similar (Sociodemographic) | 19 | 46 | 2 | 5 | 0 | 0 | 20 | 49 | 0 | 0 |
| Group Balanced at Baseline | 15 | 37 | 1 | 2 | 0 | 0 | 25 | 61 | 0 | 0 |
| Participants Blind to Research Question | 11 | 27 | 0 | 0 | 3 | 7 | 27 | 66 | 0 | 0 |
| Clear Description of Intervention and Control | 37 | 90 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 7 |
| Consistency of Intervention (within and between groups) | 16 | 39 | 0 | 0 | 22 | 54 | 0 | 0 | 3 | 7 |
| Outcome Assessors Blind to Group Allocation | 1 | 2 | 0 | 0 | 6 | 15 | 33 | 80 | 1 | 2 |
| Baseline Measures Taken before the Intervention | 22 | 54 | 1 | 2 | 14 | 34 | 1 | 2 | 3 | 7 |
| Consistency of Data Collection | 39 | 95 | 0 | 0 | 2 | 5 | 0 | 0 | 0 | 0 |
| All Outcomes Reported (Means and SD/SE) | 14 | 34 | 0 | 0 | 27 | 66 | 0 | 0 | 0 | 0 |
| All Participants Accounted for (i.e., losses/exclusions) | 32 | 78 | 0 | 0 | 9 | 22 | 0 | 0 | 0 | 0 |
| ITT Analysis Conducted (all data included after allocation) | 0 | 0 | 0 | 0 | 6 | 15 | 31 | 76 | 4 | 10 |
| Individual Level Analysis | 40 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Statistical Analysis Methods Appropriate for Study Design | 32 | 78 | 0 | 0 | 8 | 20 | 1 | 2 | 0 | 0 |
| Sample Representative of Target Population | 0 | 0 | 0 | 0 | 41 | 100 | 0 | 0 | 0 | 0 |
| Source | Outcomes |
|---|---|
| [ ] | When conducting a computer task, participants had a smaller SBP increase with the presence of plants than without plants. After accomplishing the task, the participants also exhibited a faster SBP decrease when plants were present than when plants were absent. Participants’ reaction time was 12% faster when plants were present than when they were absent. |
| [ ] | Participants had the lowest productivity when the office was furnished with 22 potted plants, whereas the highest productivity was observed when no plants were present. |
| [ ] | Participants had a significantly lower search error rate with indoor greening than without indoor greening. |
| [ ] | The percentage of participants putting their hands in ice water for more than 5 min was higher with the presence of plants than without plants. |
| [ ] | Female participants’ decreases in EEG β waves and EDA were significantly faster when red-flowering geraniums were present than when flowerless geraniums were present and when plants were absent. |
| [ ] | Male participants had a lower score in the association task than their female counterparts when plants were absent, whereas female participants had higher scores on the sorting task regardless of the presence or absence of plants. |
| [ ] | Female participants’ EEG β waves and EDA were significantly lower when flower arrangements were present than when flower arrangements were absent. |
| [ ] | Participants’ time of hand immersion in ice water was significantly longer when green-leaf and flowering plants were simultaneously present than when only green-leaf plants or flowering plants were in the room and when plants were not in the room. Participants’ EDA was significantly lower when the plants were in the room than when the plants were not in the room. |
| [ ] | Female participants showed significantly higher scores of the association task than male participants in the three interventions. Female participants had significantly higher scores of the association task when plants were present than when the magazine-rack was present. |
| [ ] | Participants had the greatest effect of EEG β waves when viewing the slide of the office with a nature window view and indoor plants than other slides. |
| [ ] | A weak but significant correlation was observed between the number of potted plants and sick leave days in the workplace. |
| [ ] | The increased humidity of the indoor potted plants improved the vagus-induced sympathovagal balance of the heart of the participant. |
| [ ] | Participants’ frequency of pain killer consumption, SBP, and heart rate were significantly lower when plants were in the room than when plants were not in the room. |
| [ ] | Participants’ frequency of visiting the school infirmary was significantly lower when plants were in the room than when plants were not in the room. |
| [ ] | Participants’ grade point averages wer significantly higher when plants were present than when plants were absent. |
| [ ] | Participants’ sick leave hours and misconduct were significantly less when plants were present than when plants were absent. |
| [ ] | Participants’ frequency of pain killer use and hospitalization days were significantly lower when plants were in the room than when plants were not in the room. |
| [ ] | Participants’ attention improved significantly from the baseline to after the proofreading task was completed when plants were present, whereas no improvement was noted when plants were absent. |
| [ ] | Participants who took care of plants had greater academic achievement than those who did not. |
| [ ] | Red, yellow, and green plants significantly reduced participants’ DBP and fingertip pulse. Red, purple, and yellow plants significantly reduced participants’ fingertip pulse. Changes in fingertip pulse were more significant in male participants than in female participants. |
| [ ] | Except for yellow African daisies, the other flowers significantly reduced participants’ SBP. Pink and white African daisies, pink and white carnations, and pink and white roses significantly reduced participants’ DBP. |
| [ ] | Male participants spent significantly more time looking at white Hedera helix L. than at the dark green variety. Female participants had a greater frequency of looking at yellow-green plants than looking at dark green and green-white plants. |
| [ ] | Male participants spent significantly more time looking at green plants than at red-green ones. The number of fixings at red–green plants was greater than at green and white–green plants. Female participants spent significantly more time looking at green and red–green plants and with greater frequency than green–white plants. |
| [ ] | Relative to green plants with white, yellow, pink, and red flowers, green-leaf plants resulted in a greater increase in participants’ relative slow α power, relative fast α power, relative low β power, and relative moderate β power spectra. By contrast, green-leaf plants with yellow flowers increased participants’ relative θ power spectrum. |
| [ ] | Participants spent less time completing the vigilance and information processing tasks when plants were present than when plants were absent. |
| [ ] | Participants had a significantly higher δ waves and significantly lower α and β waves when plants were present than when plants were absent. |
| [ ] | After transplanting plants, participants had a significantly lower DBP than their counterparts did after a computer operation task. |
| [ ] | The indoor nature contact during work was significantly negatively correlated with sick leave days. |
| [ ] | The percentage of patients with stable blood pressure, heart rate, respiration rate, and body temperature was significantly higher in the ward with plants than in the one without plants. These patients also received a significantly lower dose of pain killers and had significantly shorter hospitalization. |
| [ ] | Yellow–green Hedera helix L. received more attention than did the plants of other colors. |
| [ ] | Participants had lower heart rate in the room when the plants were present than when the plants were not present. |
| [ ] | Participants had a significantly faster reaction rate when plants were present than when plants were absent. |
| [ ] | In both the actual and virtual environments with plants, participants exhibited greater changes in SBP, DBP, and EDA than in the plantless environment. They also had greater performance in the visual backward digit span task in the plant setting. |
| [ ] | Participants had the least flicker fusion frequency (eye fatigue) when flowering plants were provided than with other plants and controls. |
| [ ] | Participants had significantly lower SBP and a significant increase in the amplitude of high β waves when plants were present than when plants were absent. |
| [ ] | Participants without houseplants had significantly higher SBP and heart rate than those with houseplants. |
| [ ] | Participants had a significantly greater proportion of significantly decreased pulse rate when the plant was present than when the plant was absent. |
| [ ] | Participants had a significant increase in α relative waves in the prefrontal and occipital lobes and in parasympathetic nervous activity when the plant was present than when the plant was absent. |
| [ ] | There were significant differences between the two horticultural activities and between the pretest and the posttest. |
| [ ] | There were significant differences between the experimental and the control groups in heart rate variability (standard deviation of the NN intervals, root mean square of the successive differences, low frequency, high frequency, and low frequency/high frequency). Within the treatment, male participants’ standard deviation of the NN intervals was significantly different between sowing and transplanting seedlings. |
| [ ] | Participants had a significantly lower heart rate after sowing, transplanting seedlings, and potting succulents. Among the four kinds of horticultural activities, sowing yielded the greatest heart rate reduction while herbal flower potting was the worst. |
| [ ] | Participants had significantly fewer errors and faster time of task completion when the plants and pictures were present than when they were absent. |
| Study | Study Design | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ] | Experiment (RCT) | Moderate | 24 | 71.75 | 0.78 | 24 | 65.26 | 0.69 |
| [ ] | Experiment (RCT) | Moderate | 50 | 68.2 | 5.77 | 50 | 67.3 | 9.05 |
| [ ] | Survey (non-RCT) | Moderate | 300 | 74.20 | 6.20 | 300 | 70.10 | 6.00 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 3 | −0.644 | 0.077 | <0.001 | 81.782 | 2 | <0.001 | 97.554 |
| Random | 3 | −2.526 | 0.825 | 0.002 | ||||
| Study | Study Design | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ] | Experiment (non-RCT) | Moderate | 38 | 0.130 | 0.210 | 38 | 0.090 | 0.170 |
| [ ] | Experiment (RCT) | Moderate | 17 | 0.043 | 0.020 | 17 | 0.112 | 0.027 |
| [ ] | Field quasi- experiment (non-RCT) | Moderate | 30 | 0.160 | 0.054 | 60 | 0.210 | 0.054 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 3 | 0.605 | 0.156 | <0.001 | 36.285 | 2 | <0.001 | 94.488 |
| Random | 3 | 1.140 | 0.714 | 0.110 | ||||
| Study | Study Design | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ] | Experiment (non-RCT) | Moderate | 38 | 0.160 | 0.240 | 38 | 0.120 | 0.220 |
| [ ] | Experiment (RCT) | Moderate | 17 | 0.051 | 0.046 | 17 | 0.214 | 0.057 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 2 | 0.381 | 0.210 | 0.069 | 34.885 | 1 | <0.001 | 97.133 |
| Random | 2 | 1.455 | 1.660 | 0.381 | ||||
| Study | StudyDesign | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ]_1 | Experiment (RCT) | Moderate | 28 | 43.55 | 6.76 | 27 | 40.28 | 6.94 |
| [ ]_2 | Experiment (RCT) | Moderate | 28 | 43.55 | 6.76 | 26 | 38.24 | 8.64 |
| [ ] | Experiment (RCT) | Moderate | 18 | 64.67 | 20.08 | 18 | 78.77 | 21.89 |
| [ ] | Experiment (RCT) | Moderate | 30 | 4.69 | 1.18 | 30 | 5.29 | 1.13 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 4 | −0.038 | 0.143 | 0.789 | 16.749 | 3 | 0.001 | 82.088 |
| Random | 4 | −0.005 | 0.340 | 0.988 | ||||
| Study | Study Design | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ] | Field quasi-experiment (non-RCT) | Low | 39 | 2.62 | 0.847 | 44 | 3.14 | 0.795 |
| [ ] | Field experiment (RCT) | Moderate | 19 | 0.133 | 0.009 | 17 | 0.154 | 0.098 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 2 | 0.534 | 0.187 | 0.004 | 0.639 | 1 | 0.424 | 0.000 |
| Random | 2 | 0.534 | 0.187 | 0.004 | ||||
| Study | Study Design | Appraisal Quality | Without Plant | With Plant | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| [ ] | Field experiment (RCT) | Moderate | 17 | 20.390 | 5.870 | 16 | 17.390 | 3.850 |
| [ ] | Experiment (non-RCT) | Moderate | 317 | 289.900 | 51.115 | 319 | 286.100 | 40.377 |
| [ ] | Experiment (RCT) | Moderate | 40 | 1228.000 | 258.720 | 40 | 738.650 | 186.180 |
| Model | Number of Studies | Pooled Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size | Standard Error | p-Value | Q-Value | df (Q) | p-Value | I-Squared | ||
| Fixed | 3 | −0.252 | 0.075 | 0.001 | 51.872 | 2 | <0.001 | 96.144 |
| Random | 3 | −0.939 | 0.684 | 0.170 | ||||
| Egger’s Regression Test | ||
|---|---|---|
| Effect | Intercept | p-Value |
| DBP | −5.892 | 0.527 |
| EEG α waves | 10.005 | 0.374 |
| attention | 7.251 | 0.656 |
| response time | −5.679 | 0.424 |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Han, K.-T.; Ruan, L.-W.; Liao, L.-S. Effects of Indoor Plants on Human Functions: A Systematic Review with Meta-Analyses. Int. J. Environ. Res. Public Health 2022 , 19 , 7454. https://doi.org/10.3390/ijerph19127454
Han K-T, Ruan L-W, Liao L-S. Effects of Indoor Plants on Human Functions: A Systematic Review with Meta-Analyses. International Journal of Environmental Research and Public Health . 2022; 19(12):7454. https://doi.org/10.3390/ijerph19127454
Han, Ke-Tsung, Li-Wen Ruan, and Li-Shih Liao. 2022. "Effects of Indoor Plants on Human Functions: A Systematic Review with Meta-Analyses" International Journal of Environmental Research and Public Health 19, no. 12: 7454. https://doi.org/10.3390/ijerph19127454
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 134 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Environ Health Perspect
- v.119(10); 2011 Oct

Planting Healthier Indoor Air
Poor indoor air quality has been linked to health problems, especially in children. Asthma has reached epidemic proportions among multiple age groups and is considered the most common chronic disease in urban-dwelling children. 1 The American Academy of Allergy, Asthma and Immunology Indoor Allergen Committee suggested in a 2010 report that allergists consider indoor air filtration to be part of a comprehensive strategy to improve respiratory health. 2 Air cleaners with HEPA filters have been shown to improve symptoms of asthma. 2 However, filtration systems and air purifiers do not reduce levels of all indoor air pollutants, and some types can actually aggravate the problem. For example, one study showed that some air purifiers raise indoor concentrations of ozone above safety levels established by the U.S. Environmental Protection Agency. 3
A more benign addition to air filtration could be the use of houseplants. In addition to basic photosynthesis that removes carbon dioxide and returns oxygen to the air, plants can remove toxicants from air, soil, and water in at least two ways. First, they can metabolize some toxic chemicals, releasing harmless by-products, and second, they can incorporate toxicants such as heavy metals into plant tissues, thus sequestering them.
Data on plant-mediated indoor air quality come from experiments conducted by the U.S. National Aeronautics and Space Administration (NASA). As NASA researchers explored the possibilities of long-term space habitation, it became evident that the air in a tightly sealed space capsule would quickly become contaminated with volatile organic compounds (VOCs) and other chemicals released by the materials used to manufacture the capsule interior. 4
This is similar to the situation in newly constructed energy-efficient dwellings. If energy-efficient construction is not carefully designed to maintain indoor–outdoor air exchange, one unintended consequence can be increased concentrations of pollutants indoors. For example, in a study recently published in the American Journal of Public Health , Gary Adamkiewicz and colleagues used a simulation model to demonstrate that in homes with low air exchange rates and multiple sources of air pollution, up to 90% of exposure to fine particulate matter came from indoor sources. 5 Besides particles and VOCs, indoor air and dust can also contain brominated flame retardants, pesticides, toxic metals, and other pollutants. 6
For more than 30 years, B.C. “Bill” Wolverton, a retired civilian scientist for NASA, investigated the use of plants as air- and water-purifying systems for enclosed environments in space missions. Through his research, Wolverton found the air-cleaning capacity of houseplants can be improved exponentially by increasing air circulation to the roots of the plants, where symbiotic microorganisms help make the substances culled from air bioavailable to the plant.

For maximum benefit, multiple species of houseplants would likely be needed on a site to remove the relevant toxicants in a particular space, given that houseplants vary in the types of chemicals they are able to remove from the environment and the efficiency with which they do their work. “For effective phytoremediation, the number and type of plants selected would need to be tailored to each individual building,” says Stanley J. Kays of the University of Georgia.
Shutterstock.com
In those studies, Wolverton and colleagues tested several types of low-light houseplants. 7 For example, golden pothos ( Epipremnum aureum , also known as devil’s ivy) grown on an activated carbon filter system reduced air levels of benzene and trichloroethylene inside a Plexiglas chamber measuring 0.58 cubic yard from approximately 36 ppm to barely detectable levels within 2 hours. 4 Experiments conducted elsewhere by Stanley J. Kays and colleagues at the University of Georgia also documented the ability of different plant species to remove VOCs such as benzene, toluene, octane, and trichloroethylene. 8
One indoor contaminant of particular concern is formaldehyde, which is released by many household products, among them pressed woods, some types of foam insulation, paper products, some paints and varnishes, and permanent-press fabrics. The National Toxicology Program lists formaldehyde as reasonably anticipated to be a human carcinogen. 9
In an unpublished 2006 study, Wolverton tested a small fan-assisted planter/air filter inside a travel trailer that had been used as temporary housing for displaced Hurricane Katrina victims. This trailer, like similar units, had been found to be highly contaminated with formaldehyde. The plant/air filter contained a plant growing in a mixture of activated carbon and expanded clay pebbles. Wolverton’s tests showed that the levels of formaldehyde were reduced from potentially toxic levels of 0.18 ppm to 0.03 ppm, within the safety limits defined by the World Health Organization. 10
Those studies fit well with evidence on the biochemical mechanisms involved in plant detoxification of formaldehyde. In studies published this year Zhongjun Xu and colleagues tested three kinds of potted plants for their capacity to remove formaldehyde from indoor air in test chambers. They found that the formaldehyde-removal capacity of the plants depended on the dehydrogenase activity in the leaves and root system—that is, how efficiently the plant could metabolize formaldehyde. 11 As Wolverton found earlier, these investigators also found that formaldehyde removal by plants was diffusion-limited. That means increasing the circulation of contaminated air through the root system and leaves improved the formaldehyde-removal effect.

Top 10 Houseplant Air Cleaners
Based on an assessment 17 of 50 houseplants by four criteria: 1) removal of chemical vapors, 2) ease of growth and maintenance, 3) resistance to insect infestation, and 4) transpiration rates. Wolverton says studies suggest houseplants are most effective in removing VOCs in energyefficient, nonventilated buildings; in highly ventilated buildings, the rapid exchange of inside and outside air makes the benefits of houseplants mostly limited to their psychologic and aesthetic values.
In another recent study, Kays and colleagues tested 86 species of houseplants from five general classes for their ability to remove formaldehyde. In their experiments, ferns had the highest formaldehyde-removal efficiency of all the plants tested, especially Osmunda japonica , commonly known as Japanese royal fern, or zenmai. 12
Another important air contaminant that is amenable to plants’ cleanup abilities is mercury vapor. Mercury can make its way into homes through accidental spills (for instance, breakage of thermometers and fluorescent bulbs) as well as through its use in certain cultural and religious practices. 13 Mercury vapor is neurotoxic and lingers in the air even after new sources have been eliminated from the environment. 14
Joao Paulo Machado Torres, a senior scientist at the Radioisotopes Laboratory of the Federal University of Rio de Janeiro, Brazil, and his group have published many studies on the use of plants in indoor and outdoor mercury-contaminated settings. 15 “We have used plants of the bromeliad family and Spanish moss ( Tillandsia usneoides ) as sentinel species to detect and absorb mercury from the air in shops contaminated by the gold trade in the Amazon,” he says. The use of plants can be uniquely useful in these environments where other kinds of remediation technology may be impractical or difficult to deploy.
But as has been shown with many natural remedies, “natural” does not necessarily equate to “absolutely harmless.” A study by Kays and colleagues published in 2009 pointed out that some houseplants—as well as the media and plastic pots they are grown in, the microorganisms that inhabit them, and the pesticides used to treat them—can potentially contaminate indoor air with VOCs. 16 “It is not yet possible to project the true potential of plants for purifying indoor air,” Kays says. “At this time the role of plants, though appearing [generally] positive, is not totally clear. The absence of funding for phytoremediation research has greatly impeded solving the problem.”
Kays also notes the lack of an accurate means for the public to determine if the VOCs in their home or office represent a significant health problem. “The absence of a relatively inexpensive method available to the public results in situations where it takes two and a half years to determine that the Katrina trailers had toxic levels of formaldehyde even though there had been health complaints by the occupants almost as soon as the trailers were in place,” he says. “If an accurate, reasonably priced method was available from a credible source such as a university extension analytical laboratory, the public would be able to ascertain their potential health risk before buying or renting a house, apartment, or office.”
NTRS - NASA Technical Reports Server
Available downloads, related records.

COMMENTS
2.1. Eligibility Criteria. The eligibility criteria for inclusion of a study in this research were as follows: (1) participants of any type were recruited; (2) no criteria were set for the type of indoor plant to be used in interventions; (3) the comparator was participants in an indoor environment without any plants or with other elements; (4) the outcome included any type of objectively ...
These physiological benefits may result from multiple natural stimuli acting on the senses of vision, hearing, touch, and smell; this effect is also seen in forest therapy research [26-29]. Although many studies reported positive effects of indoor plants, most of them have been focused on the benefits of passive interaction [ 25 ] with indoor ...
In addition, the Stage of Change Model, which was employed in this study to theoretically identify participants' current behaviour of house planting, contributed to unbalanced numbers of participants in each group, further relevant research can group participants into (a) actively growing houseplants; and (b) not currently growing houseplants.
In this paper, we consider whether indoor plants offer them some of the same benefits provided by experiences of nature outdoors. Specifically, we overview and critique the experimental evidence on psychological benefits of indoor plants. We also comment on some overarching theoretical issues in the research area.
This paper examines some of the studies that have documented the beneficial effects of plants, focusing on those used indoors, and then examines some of ... 2002). Further research has shown that plants remove many indoor air pollutants, including ozone, toluene, and benzene (Darlington et al., 2001; Wood et al., 2002; Papinchak et al., 2009).
An increasing number of plant studies for the interiorscape is highly desirable concerning the growing "work from home" trend. This research aims to empirically 1) identify the research trends of indoor plants, 2) identify the most popular plants for the indoor landscape, and 3) investigate the practical aspects currently appearing in the ...
By focusing on people's experiences at home with plants, the research provides a situated examination of people's motivations for keeping plants, their embodied care practices, and wider connections formed through keeping houseplants. The paper proceeds by outlining relevant research that enables thinking about the contributions and the ...
This paper reviews past literature from 1990 to 2010s, to examine the relationship of plants with indoor environment and identifies how they influence people, psychologically and physiologically ...
Indoor greenery measures included the number of house plants, gardening activities, and digital nature exposure as well as semantic image segmentation applied to photographs from the most viewed ...
Abstract: The introduction of green plants in indoor spaces has raised a great amount of interest. motivated by plants' supposed capacity to improve the quality of indoor built environments. Sub ...
The introduction of green plants in indoor spaces has raised a great amount of interest motivated by plants' supposed capacity to improve the quality of indoor built environments. Subsequent studies have covered a broad range of topics, testing plants in indoor environments for their climate-mitigating effects, acoustic benefits, potential energy savings and the enhancement of the indoor ...
This paper reviews the state of art of vegetation systems and their effect on the indoor environmental quality (IEQ), based on scientific studies from the past 30 years. ... Research experiences from peer-reviewed journal articles were considered as base material for this review. ... the common tropical house plants Janet Craig and Peace Lily ...
Indoor plants can act as indoor air purifiers, they are an effective way to reduce pollutants indoor to reduce human exposure, and have been widely studied in this regard. Indoor plants have potential applications in other fields, including sensing, solar energy, acoustic, and people's health and comfort. Making full use of various effects in ...
1. Introduction. Built environments affect our health, behaviour and mental well-being [1, 2].The adverse impacts of indoor air pollution and poor thermal comfort on the health, well-being and productivity of building occupants are well documented, and as people spend more time indoors in tightly sealed buildings these concerns are rising [[3], [4], [5]].
Many studies which carried out these experiments subsequently draw conclusions that potted plants may improve indoor air quality, spurring a presence of nonacademic resources (predominantly online ...
After the plants are potted, add ¼ ounce of liquid 10-10-10 with minor elements per gallon of water and fertilize once every 6 weeks (if the plants are growing in osmunda fern fibers). If plants are growing in fir bark, use a liquid 30-10-10 with minor elements every 6 weeks instead of a 10-10-10 fertilizer.
The influences of indoor plants on people have been examined by only three systematic reviews and no meta-analyses. The objective of this study was therefore to investigate the effects of indoor plants on individuals' physiological, cognitive, health-related, and behavioral functions by conducting a systematic review with meta-analyses to fill the research gap. The eligibility criteria of ...
This protocol can minimize data errors and provide useful research guidelines that will eventually lead to a healthier human environment. In brief, implementing indoor plants to reduce SARS-CoV-2 transmission is an idea worth pursuing in the near future with a well-design experiment and controlled parameters to aid in our fight against the ...
The main research questions can be summarised as follows: 1. ... no gender differences were detected with regard to the positive influence of house plants on the respondents' emotional state during the health crisis. Regarding the age groups, although older people felt negative emotions less frequently, they acknowledged a higher positive ...
Based on an assessment 17 of 50 houseplants by four criteria: 1) removal of chemical vapors, 2) ease of growth and maintenance, 3) resistance to insect infestation, and 4) transpiration rates. Wolverton says studies suggest houseplants are most effective in removing VOCs in energyefficient, nonventilated buildings; in highly ventilated ...
Interior Landscape Plants for Indoor Air Pollution Abatement In this study, the leaves, roots, soil, and associated microorganisms of plants have been evaluated as a possible means of reducing indoor air pollutants. Additionally, a novel approach of using plant systems for removing high concentrations of indoor air pollutants such as cigarette smoke, organic solvents, and possibly radon has ...
In three studies, this paper first looked to replicate the findings of previous laboratory studies for the South African context and then to assess whether these findings were robust in two call centre field studies. In the laboratory study, the condition with indoor plants performed statistically better on three measures of work performance ...