

Pharmaceutical Business Plan Template
Written by Dave Lavinsky

Pharmaceutical Business Plan
Over the past 20+ years, we have helped over 500 entrepreneurs and business owners create business plans to start and grow their pharmaceutical companies.
If you’re unfamiliar with creating a pharmaceutical business plan, you may think creating one will be a time-consuming and frustrating process. For most entrepreneurs it is, but for you, it won’t be since we’re here to help. We have the experience, resources, and knowledge to help you create a great business plan.
In this article, you will learn some background information on why business planning is important. Then, you will learn how to write a pharmaceutical business plan step-by-step so you can create your plan today.
Download our Ultimate Business Plan Template here >
What is a Pharmaceutical Business Plan?
A business plan provides a snapshot of your pharmaceutical business as it stands today, and lays out your growth plan for the next five years. It explains your business goals and your strategies for reaching them. It also includes market research to support your plans.
Why You Need a Business Plan for a Pharmaceutical Company
If you’re looking to start a pharmaceutical business or grow your existing company, you need a business plan. A business plan will help you raise funding, if needed, and plan out the growth of your pharmaceutical company to improve your chances of success. Your business plan is a living document that should be updated annually as your company grows and changes.
Sources of Funding for Pharmaceutical Businesses
With regards to funding, the main sources of funding for a pharmaceutical business are personal savings, credit cards, bank loans, and angel investors. When it comes to bank loans, banks will want to review your business plan and gain confidence that you will be able to repay your loan and interest. To acquire this confidence, the loan officer will not only want to ensure that your financials are reasonable, but they will also want to see a professional plan. Such a plan will give them the confidence that you can successfully and professionally operate a business. Personal savings and bank loans are the most common funding paths for pharmaceutical businesses.
Finish Your Business Plan Today!
How to write a business plan for a pharmaceutical company.
If you want to start a pharmaceutical company or expand your current one, you need a business plan. The guide below details the necessary information for how to write each essential component of your pharmaceutical business plan.
Executive Summary
Your executive summary provides an introduction to your business plan, but it is normally the last section you write because it provides a summary of each key section of your plan.
The goal of your executive summary is to quickly engage the reader. Explain to them the kind of pharmaceutical business you are running and the status. For example, are you a startup, do you have a company that you would like to grow, or are you operating pharmaceutical companies in multiple markets?
Next, provide an overview of each of the subsequent sections of your plan.
- Give a brief overview of the pharmaceutical industry.
- Discuss the type of pharmaceutical business you are operating.
- Detail your direct competitors. Give an overview of your target customers.
- Provide a snapshot of your marketing strategy. Identify the key members of your team.
- Offer an overview of your financial plan.
Company Overview
In your company overview, you will detail the type of pharmaceutical company you are operating.
For example, you might specialize in one of the following types of pharmaceutical businesses:
- Generic Pharmaceutical Manufacturing : this type of pharmaceutical business develops prescription or over-the-counter drugs products that do not have patent protection.
- Vitamin & Supplement Manufacturing: this type of pharmaceutical company primarily develops products that contain ingredients intended to supplement the diet.
- Brand Name Pharmaceutical Manufacturing: this type of pharmaceutical business engages in significant research and development of patent-protected prescription and over-the-counter medications.
In addition to explaining the type of pharmaceutical business you will operate, the company overview needs to provide background on the business.
Include answers to questions such as:
- When and why did you start the business?
- What milestones have you achieved to date? Milestones could include the number of patents awarded, the extent of your product portfolio, reaching X number of distributors under contract, etc.
- Your legal business Are you incorporated as an S-Corp? An LLC? A sole proprietorship? Explain your legal structure here.
Industry Analysis
In your industry or market analysis, you need to provide an overview of the pharmaceutical industry.
While this may seem unnecessary, it serves multiple purposes.
First, researching the pharmaceutical industry educates you. It helps you understand the market in which you are operating.
Secondly, market research can improve your marketing strategy, particularly if your analysis identifies market trends.
The third reason is to prove to readers that you are an expert in your industry. By conducting the research and presenting it in your plan, you achieve just that.
The following questions should be answered in the industry analysis section:
- How big is the pharmaceutical industry (in dollars)?
- Is the market declining or increasing?
- Who are the key competitors in the market?
- Who are the key suppliers in the market?
- What trends are affecting the industry?
- What is the industry’s growth forecast over the next 5 – 10 years?
- What is the relevant market size? That is, how big is the potential target market for your pharmaceutical company? You can extrapolate such a figure by assessing the size of the market in the entire country and then applying that figure to your local population.
Customer Analysis
The customer analysis section of your business plan must detail the customers you serve and/or expect to serve.
The following are examples of customer segments: healthcare providers, chain pharmacies, independent retailers, and consumers.
As you can imagine, the customer segment(s) you choose will have a great impact on the type of pharmaceutical business you operate. Clearly, individuals would respond to different marketing promotions than hospitals, for example.
Try to break out your target customers in terms of their demographic and psychographic profiles. With regards to demographics, including a discussion of the ages, genders, locations, and income levels of the potential customers you seek to serve.
Psychographic profiles explain the wants and needs of your target customers. The more you can recognize and define these needs, the better you will do in attracting and retaining your customers.
Finish Your Pharmaceutical Business Plan in 1 Day!
Don’t you wish there was a faster, easier way to finish your business plan?
With Growthink’s Ultimate Business Plan Template you can finish your plan in just 8 hours or less!
Competitive Analysis
Your competitive analysis should identify the indirect and direct competitors your business faces and then focus on the latter.
Direct competitors are other pharmaceutical businesses.
Indirect competitors are other options that customers have to purchase from that aren’t directly competing with your product or service. This includes imported alternatives, herbal remedies, or customers’ nutritional self-care. You need to mention such competition as well.
For each such competitor, provide an overview of their business and document their strengths and weaknesses. Unless you once worked at your competitors’ businesses, it will be impossible to know everything about them. But you should be able to find out key things about them such as
- What types of products do they manufacture?
- What are their research and development capabilities?
- What is their pricing (premium, low, etc.)?
- What are they good at?
- What are their weaknesses?
With regards to the last two questions, think about your answers from the customers’ perspective. And don’t be afraid to ask your competitors’ customers what they like most and least about them.
The final part of your competitive analysis section is to document your areas of competitive advantage. For example:
- Will you provide product development?
- Will you offer products or services that your competition doesn’t?
- Will you provide better customer service?
- Will you offer better pricing?
Think about ways you will outperform your competition and document them in this section of your plan.
Marketing Plan
Traditionally, a marketing plan includes the four P’s: Product, Price, Place, and Promotion. For a pharmaceutical company, your marketing strategy should include the following:
Product : In the product section, you should reiterate the type of pharmaceutical business that you documented in your company overview. Then, detail the specific products or services you will be offering. For example, will you manufacture patent-protected prescription medications, or a range of vitamins?
Price : Document the prices you will offer and how they compare to your competitors. Essentially in the product and price sub-sections of your plan, you are presenting the products and/or services you offer and their prices.
Place : Place refers to the site of your pharmaceutical business. Document where your company is situated and mention how the site will impact your success. For example, is your pharmaceutical company located in an industrial district, near a major medical and/or scientific hub, or near input markets? Discuss how your site might be the ideal location for your customers.
Promotions : The final part of your pharmaceutical marketing plan is where you will document how you will drive potential customers to your location(s). The following are some promotional methods you might consider:
- Advertise in local papers, radio stations and/or magazines
- Advertise in trade publications
- Reach out to websites
- Distribute flyers
- Engage in email marketing
- Advertise on social media platforms
- Improve the SEO (search engine optimization) on your website for targeted keywords
Operations Plan
While the earlier sections of your business plan explained your goals, your operations plan describes how you will meet them. Your operations plan should have two distinct sections as follows.
Everyday short-term processes include all of the tasks involved in running your pharmaceutical company, including meeting with potential customers, creating and distributing product information, developing and manufacturing products, etc.
Long-term goals are the milestones you hope to achieve. These could include the dates when you expect to produce your Xth product, or when you hope to reach $X in revenue. It could also be when you expect to expand your pharmaceutical business to a new city.
Management Team
To demonstrate your pharmaceutical company’s potential to succeed, a strong management team is essential. Highlight your key players’ backgrounds, emphasizing those skills and experiences that prove their ability to grow a company.
Ideally, you and/or your team members have direct experience in managing pharmaceutical businesses. If so, highlight this experience and expertise. But also highlight any experience that you think will help your business succeed.
If your team is lacking, consider assembling an advisory board. An advisory board would include 2 to 8 individuals who would act as mentors to your business. They would help answer questions and provide strategic guidance. If needed, look for advisory board members with experience in managing a pharmaceutical business or successfully running a R&D company.
Financial Plan
Your financial plan should include your 5-year financial statement broken out both monthly or quarterly for the first year and then annually. Your financial statements include your income statement, balance sheet, and cash flow statements.
Income Statement
An income statement is more commonly called a Profit and Loss statement or P&L. It shows your revenue and then subtracts your costs to show whether you turned a profit or not.
In developing your income statement, you need to devise assumptions including your sales projections. For example, will you manufacture a line of general sales products, or will you specialize in manufacturing controlled drugs? And will sales grow by 2% or 10% per year? As you can imagine, your choice of assumptions will greatly impact the financial forecasts for your business. As much as possible, conduct research to try to root your assumptions in reality.
Balance Sheets
Balance sheets show your assets and liabilities. While balance sheets can include much information, try to simplify them to the key items you need to know about. For instance, if you spend $50,000 on building out your pharmaceutical company, this will not give you immediate profits. Rather it is an asset that will hopefully help you generate profits for years to come. Likewise, if a lender writes you a check for $50,000, you don’t need to pay it back immediately. Rather, that is a liability you will pay back over time.
Cash Flow Statement
Your cash flow statement will help determine how much money you need to start or grow your business, and ensure you never run out of money. What most entrepreneurs and business owners don’t realize is that you can turn a profit but run out of money and go bankrupt.
When creating your Income Statement and Balance Sheets be sure to include several of the key costs needed in starting or growing a pharmaceutical company:
- Cost of equipment and supplies
- Payroll or salaries paid to staff
- Business insurance
- Other start-up expenses (if you’re a new business) like legal expenses, permits, computer software, and equipment
Attach your full financial projections in the appendix of your plan along with any supporting documents that make your plan more compelling. For example, you might include your facility blueprint or a list of products you manufacture.
Writing a business plan for your pharmaceutical business is a worthwhile endeavor. If you follow the template above, by the time you are done, you will truly be an expert. You will understand the pharmaceutical company industry, your competition, and your customers. You will develop a marketing strategy and will understand what it takes to launch and grow a successful pharmaceutical company.
Don’t you wish there was a faster, easier way to finish your Pharmaceutical business plan?
OR, Let Us Develop Your Plan For You
Since 1999, Growthink has developed business plans for thousands of companies who have gone on to achieve tremendous success. Click here to see how Growthink’s business plan writers can create your business plan for you.
Other Helpful Business Plan Articles & Templates

Business Plan Template for Pharmaceutical Companies
- Great for beginners
- Ready-to-use, fully customizable Subcategory
- Get started in seconds

Securing funding and navigating the highly regulated pharmaceutical industry can be a daunting task for any company. But with ClickUp's Business Plan Template for Pharmaceutical Companies, you can streamline the process and increase your chances of success!
Our template provides you with all the essential elements you need to create a comprehensive and compelling business plan, including:
- Strategic goals to guide your company's growth and development
- Financial projections to showcase the potential profitability of your products and services
- Market analysis to identify key trends, competitors, and target markets
- Operational plans to outline your manufacturing, distribution, and regulatory strategies
Don't miss out on the opportunity to attract investors and secure funding for your pharmaceutical company. Get started with ClickUp's Business Plan Template today and take your business to new heights!
Business Plan Template for Pharmaceutical Companies Benefits
A business plan template designed specifically for pharmaceutical companies offers a range of benefits, including:
- Streamlining the process of creating a comprehensive business plan, saving time and effort
- Providing a clear structure and framework for organizing key information and data
- Ensuring all critical components of a pharmaceutical business plan are included, such as market analysis, competitive analysis, and regulatory compliance
- Presenting a professional and polished document that is attractive to investors and lenders
- Helping pharmaceutical companies articulate their unique value proposition and competitive advantage in the market
- Guiding strategic decision-making and long-term planning for growth and success in the industry.
Main Elements of Pharmaceutical Companies Business Plan Template
ClickUp's Business Plan Template for Pharmaceutical Companies provides a comprehensive solution for creating and managing your pharmaceutical business plan. Here are the main elements of this template:
- Custom Statuses: Keep track of the progress of each section of your business plan with statuses like Complete, In Progress, Needs Revision, and To Do.
- Custom Fields: Add important details to your business plan, such as Reference, Approved, and Section, to ensure accurate documentation and easy reference.
- Custom Views: Access different views, including Topics, Status, Timeline, Business Plan, and Getting Started Guide, to organize and visualize your business plan from different perspectives.
- Collaboration Tools: Utilize ClickUp's collaboration features like comments, mentions, and task assignments to collaborate effectively with your team members and stakeholders.
- Document Management: Store and manage all relevant documents related to your business plan using ClickUp Docs, ensuring easy access and version control.
How To Use Business Plan Template for Pharmaceutical Companies
If you're in the pharmaceutical industry and need to create a business plan, the Business Plan Template for Pharmaceutical Companies in ClickUp can help you get started. Follow these five steps to develop a comprehensive plan for your company's success:
1. Company Overview
Begin by providing a clear and concise overview of your pharmaceutical company. Include details such as your mission statement, vision, values, and a brief history of your organization. Highlight your unique selling proposition and explain how your company stands out in the competitive pharmaceutical market.
Use the Docs feature in ClickUp to write a compelling company overview with all the necessary information.
2. Market Analysis
Conduct a thorough analysis of the pharmaceutical market to identify trends, opportunities, and potential challenges. Research your target audience, competitors, and industry regulations. Provide insights into market size, growth projections, and key market segments that you plan to target.
Utilize the Table view in ClickUp to organize and analyze your market research data effectively.
3. Product Portfolio
Outline your product portfolio and describe the pharmaceutical products or services you offer. Highlight their unique features, benefits, and potential impact on patients' lives. Discuss your research and development efforts, clinical trials, and any patents or intellectual property you have.
Create tasks in ClickUp to track your product development milestones and progress.
4. Marketing and Sales Strategy
Detail your marketing and sales strategies to reach your target audience and generate revenue. Identify your target market segments and outline your marketing channels, such as digital advertising, trade shows, or partnerships with healthcare providers. Define your pricing strategy, distribution channels, and sales forecasting.
Use the Gantt chart feature in ClickUp to visualize and track your marketing and sales activities over time.
5. Financial Projections
Provide financial projections that demonstrate the profitability and sustainability of your pharmaceutical company. Include a comprehensive analysis of your revenue streams, costs, and projected financial statements such as income statements, balance sheets, and cash flow statements. Consider factors such as research and development costs, manufacturing expenses, and pricing strategies.
Utilize the Dashboards feature in ClickUp to create visual representations of your financial data and track your progress towards financial goals.
By using the Business Plan Template for Pharmaceutical Companies in ClickUp and following these steps, you can create a comprehensive business plan that sets your pharmaceutical company up for success in the competitive industry.
Get Started with ClickUp’s Business Plan Template for Pharmaceutical Companies
Pharmaceutical companies can use the Business Plan Template for Pharmaceutical Companies in ClickUp to effectively outline their strategic goals and financial projections, as well as navigate the complex and highly regulated pharmaceutical industry.
First, hit “Add Template” to sign up for ClickUp and add the template to your Workspace. Make sure you designate which Space or location in your Workspace you’d like this template applied.
Next, invite relevant members or guests to your Workspace to start collaborating.
Now you can take advantage of the full potential of this template to create a comprehensive business plan:
- Use the Topics View to organize different sections of your business plan, such as market analysis, product development, and financial projections
- The Status View will help you track the progress of each section, with statuses like Complete, In Progress, Needs Revision, and To Do
- The Timeline View will allow you to set deadlines and milestones for each section of your business plan
- The Business Plan View provides a holistic overview of your entire plan, allowing you to easily navigate and make updates
- The Getting Started Guide View will provide step-by-step instructions on how to use the template and create an effective business plan
- Utilize custom fields like Reference, Approved, and Section to add additional information and categorize different sections of your plan
- Collaborate with team members to gather input, feedback, and revisions to ensure a comprehensive and well-rounded business plan
- Monitor and analyze the progress of each section to ensure your business plan is on track and meets the needs of investors and stakeholders.
- Business Plan Template for Hoteliers
- Business Plan Template for Music Teachers
- Business Plan Template for Vodafone
- Business Plan Template for Advertisers
- Business Plan Template for Mergers And Acquisitions Specialists
Template details
Free forever with 100mb storage.
Free training & 24-hours support
Serious about security & privacy
Highest levels of uptime the last 12 months
- Product Roadmap
- Affiliate & Referrals
- On-Demand Demo
- Integrations
- Consultants
- Gantt Chart
- Native Time Tracking
- Automations
- Kanban Board
- vs Airtable
- vs Basecamp
- vs MS Project
- vs Smartsheet
- Software Team Hub
- PM Software Guide
Try for Free
Step-by-Step Guide to Starting a Pharmaceutical Company
Preview the Next Big Thing with MSB Docs AI

AI Summary Beta
Starting a pharmaceutical company is a challenging but potentially rewarding venture. This summary will provide an overview of key considerations in starting such a business.
Introduction:
Starting a pharmaceutical company is promising but demands substantial time, effort, and resources. Success hinges on market understanding, regulatory compliance, and effective business models. With dedication and knowledge, anyone can embark on this journey.
Thorough research is critical. Dive into the industry, study competitors, and grasp relevant regulations. Analyze market trends, technological advancements, and your competitors’ strengths and weaknesses.
Understanding Regulations:
Pharmaceutical businesses are heavily regulated. Comprehend FDA and local regulations, and consider seeking expert guidance. Stay updated on legal changes, maintain industry standards, and document procedures to avoid legal complications.
Business Model:
Selecting the right business model is crucial. Options include contract manufacturing, retail, and franchising. Each has pros and cons, and your choice should align with your goals, start-up capacity, and customer service strategy.
Launching a pharmaceutical business requires substantial capital for equipment, research, marketing, staffing, and more. Seek funding through government grants, investors, loans, or personal savings, considering tax implications and potential incentives.
Inventory and Supply Chain:
Manage inventory based on business size and product type. Storage conditions and transportation must align with product requirements. Establish efficient supply chains to ensure timely product turnover.
Distribution:
Effective distribution networks are essential for reaching customers. Decide on distribution methods, whether through wholesalers, retailers, or online sales. Choose experienced distributors and continuously evaluate and improve distribution channels.
Technology:
Technology plays a crucial role in pharmaceuticals. Automation enhances efficiency and reduces errors. Invest in secure data storage, cybersecurity, and customer interaction technologies, like websites and social media, to build trust and competitiveness.
Operations:
Operational processes are the backbone of any business. Develop efficient protocols for inventory, production, quality assurance, and regulatory compliance. Quality assurance is paramount in pharmaceuticals.
Prospecting Strategies:
Marketing in the pharmaceutical industry must comply with strict regulations. Consider online advertising, networking, print ads, direct mail, and conferences. Tailor strategies to your target audience and budget.
Insurance and Security:
Protect your business with adequate insurance coverage, considering your company’s size and activities. Implement security measures like surveillance, data encryption, and biometric technology to safeguard assets and sensitive information.
Conclusion:
Starting a pharmaceutical company is a complex but achievable endeavor with careful planning, adherence to regulations, robust business models, and the right technology. Building a reliable distribution network and marketing strategy are vital. Ensure safety and security with insurance and security protocols. Regular monitoring and adaptation are key to success in this dynamic industry. Good luck on your journey!
Unlock the power of our AI Assistant in our cutting-edge digital competition cloud.
Join 10,000+ businesses trusting MSB Docs for contract collaboration.
Table of Contents
Introduction to Starting a Pharmaceutical Company
A pharmaceutical company can be an appealing business venture, as it can offer the potential of substantial revenue growth and a meaningful impact on people’s lives. There is no doubt that starting a pharmaceutical company requires a great deal of time, effort, and resources. However, with the right knowledge and dedication, starting a pharmaceutical company can be a rewarding experience.
It is important to have a thorough understanding of the market, regulations, and business models in order to be successful in this field. With the right combination of research, planning, and dedication, anyone can start a pharmaceutical company and potentially reap the rewards of being at the forefront of medical innovation.
Doing the necessary research is a vital step to starting your own pharmaceutical company. It is important to dive deep into the industry, research potential competitors, and understand the regulations that may affect the business. Research will help establish a strong foundation for a successful business model.
It’s important to understand the current market and how it is evolving. This should include a review of any new trends and technologies that can be used to differentiate the company from its competitors. The research should also include studying the current players in the market, their strengths and weaknesses, and how your company can compete effectively.
Regulations are an important factor to consider when starting a pharmaceutical company. Regulations vary by country, state, and province, so it is important to become familiar with the relevant local regulations. Depending on the scope of the business, some of the regulations may include workplace safety, environmental standards, labeling requirements, etc. It is important to consult legal experts to make sure you remain compliant with the applicable regulations.
Understanding Regulations
Operating a pharmaceutical business can be a daunting task as regulations are placed on the industry. It is important to understand all regulations that can potentially affect the business, such as those put forth by the FDA and other governing institutions. Additionally, having knowledge of the specific regulations in the state where the business is located is essential for success.
Navigating regulations can be a tricky process and may require assistance from an expert. The laws vary from state to state, making it difficult for business owners to be knowledgeable of the specifics. Consulting legal representatives or industry experts can be very beneficial when trying to stay in compliance.
Additionally, keeping up-to-date with any changes in the law is also important. This will help ensure that the business remains compliant and not subject to fines or penalties. Familiarizing oneself with industry standards and proper documentation procedures can go a long way into protecting the business from any potential legal complications.
Business Model for Pharmaceutical Companies
When it comes to running a successful pharmaceutical company, having the right business model in place is essential. There are several different types of business models available for companies in the pharmaceutical industry, and it can be difficult to determine which one is best for you. In this section, we’ll explore the different options and discuss some key considerations you should keep in mind when choosing your business model.
One of the most popular business models for pharmaceutical companies is the contract manufacturing model. This model involves outsourcing the production of your products to an experienced third-party contractor. In this arrangement, the product will be developed and manufactured according to your specifications, and you’ll only have to pay for the manufacturing services. This type of model is great for companies that are just starting out and don’t have the capacity to manage their own production capabilities.
Another option is the retail model, where you manufacturer and sell products directly to customers. This type of model works well for companies with a wide variety of products that require specialized marketing strategies and customer service. However, it requires a significant financial investment upfront and a lot of time commitment from management.
Finally, there’s the franchise model, where you partner with a larger pharmaceutical company to share resources and expertise. This type of model is great for companies that want to benefit from the resources of larger companies without having to build out their own operations.
No matter which business model you choose for your pharmaceutical company, it’s important to do your research and make sure it’s the right fit for your needs. Consider factors such as start-up costs, operational efficiency, and customer service when assessing different models. Additionally, it’s important to weigh the benefits and drawbacks of each model to ensure you’re making an informed decision.
Finance – Funding Requirements and Sources for Starting a Pharmaceutical Company
Starting a pharmaceutical company is no small task. In the modern age, it requires a significant investment of money and resources. Understanding the financial aspects of a pharmaceutical business is essential for success.
In order to launch a successful pharmaceutical business, a tremendous amount of capital will be required. This money will go towards all the necessary steps to get your business up and running, including: production equipment, research and development, marketing, employees, etc. The exact amount of money needed can vary greatly depending on the size and scope of your operations, but it’s safe to say that the cost of starting a business in this industry can be quite high.
When it comes to finding the money for your project, there are several options available. These include government grants, angel investors, venture capital firms, bank loans, and personal savings. Each of these sources carries its own advantages and disadvantages, so it’s important to do the research to find the best option for your specific needs.
Additionally, you should consider the tax implications of each funding source. Not only are specific laws and regulations in place for different types of funding, but there may also be certain deductions or credits available.
Finally, you should keep an eye out for potential incentives and subsidies from the government. Depending on where you are located, there may be programs available to help startup businesses in the pharmaceutical sector.
Funding a pharmaceutical business is a complex process, but it’s definitely achievable. With the right research and preparation, you can be sure to secure the capital you need for success.
Inventory and Supply Chain Considerations for Pharmaceutical Companies
When starting a pharmaceutical business, it’s critical to understand the inventory and supply chain considerations that go into making a successful venture. The necessary inventory components will vary on the size of the business, the scale of operations, and the products. It is important to understand the needs for purchasing, storage, shipping, and distribution.
For smaller businesses, it is important to purchase inventory in small amounts. This will help manage expenses and prevent product expiration. It also helps create flexibility if products or terms are updated frequently. For larger businesses, having sufficient inventory on hand is critical. An efficient supply chain is required to ensure product turnover happens regularly and in a timely manner.
Storage is another key consideration. Depending on the type of products being sold, different environmental conditions may be needed. Temperature control, special packaging, and other considerations must be taken into account. Products must also be protected from theft or damage. Knowing which facilities to use for storage, and the cost of transportation are also key considerations.
Shipping and distribution are two more important components. Clients need to receive the products as quickly as possible. To ensure this, it is essential to select the appropriate methods for transport and to manage the process appropriately. This includes selecting carriers, providing tracking information, and handling returns. Distribution involves getting the product to the end user in a timely, cost-effective manner.
Successfully managing the inventory and supply chain for a pharmaceutical business requires both knowledge and experience with the various processes and components. Having an understanding of these considerations is vital for running a successful venture.
Distribution: The Key to Reaching Customers
Getting products to customers is a critical factor in running a successful pharmaceutical business. Distributors are necessary for a company to reach their target markets effectively. Distribution networks can be complex and challenging to set up, but they are essential for a company’s success.
Once customers have been identified, a company must decide how products will reach them. Companies that distribute internationally require more complex systems than companies that stay local or regional. Different options include using a wholesaler or a third-party distributor, distributing directly to retailers or selling online. Each option has its own advantages and drawbacks, and should be carefully considered when developing a distribution plan.
When selecting distributors, it is important to look for ones with an established reputation, experience in similar products, and a good track record with other customers. Additionally, relevant certifications, such as Good Manufacturing Practices (GMP) certification, need to be taken into consideration. Once selected, distributors must be given the necessary information and resources to effectively market and sell the products.
Finally, setting up distribution channels is not a one-time process – regular evaluations and updates are necessary to ensure maximum customer reach and satisfaction. Distributors must be monitored, and customer feedback should be incorporated into the process. This feedback can help a company improve their product and service offerings to better serve their customers.
In the pharmaceutical industry, technology is vital. As the expectations around quality and delivery continue to increase, organizations need to be equipped with the right technologies and systems. Technology helps ensure that pharmaceutical companies are meeting all regulatory requirements, as well as providing products and services that are reliable and of a high standard.
When it comes to technology for pharmaceutical companies, there are several areas that need to be addressed. The first is automation. Automation can help streamline processes, improve production, and reduce errors. It can also help with inventory management, ensuring that products are quickly and accurately tracked.
Another area of technology is security. Pharmaceutical companies need to ensure that their data is securely stored and kept confidential. They must also have systems in place to detect any unauthorized access attempts. Companies should also investigate cyber insurance policies to provide additional protection.
Finally, pharmaceutical companies need to invest in customer interaction technologies. Having an online presence is essential to developing relationships with customers. This might include a website, social media pages, or even an app. All of these tools can help reach customers and build trust in the company.
By investing in the right technology, pharmaceutical companies can become more efficient and offer better customer service. Technology can also provide a competitive advantage over other companies in the market.
Operations for a Pharmaceutical Company
Operational processes are the backbone of any business, and this is especially true for a pharmaceutical company. Without efficient and effective operational processes in place, a company may struggle to survive. This section looks at what operational processes need to be considered when starting a pharmaceutical company.
The most basic operational processes involve setting up protocols for ordering and receiving inventory, controlling inventory, producing products, dealing with customer service issues, and managing finances. These processes must be able to respond to changing needs and be able to support long-term growth. An effective operational process also allows the company to remain competitive and profitable.
An important part of any operational process is quality assurance. Quality assurance involves procedures that are designed to ensure the safety and effectiveness of products. A company should have qualified personnel to inspect, test, and verify the quality of every product that is produced or sold. Quality assurance is absolutely essential for a successful pharmaceutical company.
Another key operational process for a pharmaceutical company is regulatory compliance. Regulations provide customers and other stakeholders with assurance that a company is adhering to accepted standards of practice and is providing safe products. In order to remain compliant, a company must always keep up with changes in regulations and make sure that their processes adhere to those regulations.
Having an effective and efficient operational process in place is essential for any business, especially a pharmaceutical company. With the right processes in place, a pharmaceutical company can remain competitive and profitable in the long run.
Prospecting Strategies for a Pharmaceutical Company
Marketing and promoting a pharmaceutical company can be quite complex due to the high level of regulations in the industry. Therefore, it is important to find marketing strategies that fit within the legal framework while still providing the visibility required to reach customers. Prospecting strategies for a pharmaceutical company can include techniques such as online advertising, networking, print advertising, direct mail and attending conferences.
For companies just starting out, online advertising is often an ideal option. There are several platforms available, including the increasingly popular social media marketing. This strategy allows companies to gain visibility without spending large amounts of money on advertising and can be tailored to reach a specific audience. It is also a good way to monitor website traffic and gauge customer interest in the products.
Networking is another powerful tool for a pharmaceutical company. Creating partnerships with other companies, medical professionals and research organizations can be beneficial in a number of ways. These partnerships can lead to new contacts, exchanging of knowledge and shared resources. It is also a great way to promote the brand and differentiate it from competitors.
Print advertising and direct mail campaigns can be useful to reach potential customers, although they can be expensive. These methods have the advantage of being able to target a specific demographic and reach people who may not be active online. Attending conferences is also a great way to create visibility and network with relevant individuals or organizations.
Insurance and Security
Starting a pharmaceutical business means taking measures to protect the company and its operations, and this includes insurance and security. It is important to ensure that your business is protected from any unexpected events and that you are able to meet requirements for the industry.
When it comes to insurance, the types and amount of coverage you need depend on a number of factors including the size of the company, the specific products you are manufacturing, and the type of distribution network used. For example, if you are selling products in both domestic and international markets you may need to have additional coverage. Additionally, you may need to acquire product liability insurance, property insurance, and more.
In terms of security, you need to protect your business from any potential theft or vandalism. You may want to consider investing in a surveillance system that monitors the premises in case of break-ins. You should also ensure that any confidential information is stored securely and encrypted to prevent any data breaches. You can also consider using biometric technology to further secure the premises and store confidential information.
By putting the right insurance and security measures in place, you can ensure that your business is protected from any potential harm. A well-thought-out security plan will help you protect your business from unforeseen risks and allow you to focus on running the best possible pharmaceutical company.
Starting a pharmaceutical company can be a daunting task, but with the right research, understanding of regulations, business model, financing, inventory strategy, distribution network, use of technology, operational processes, and marketing tactics, there is no reason why it cannot be successful. The key to success in this endeavor is careful planning and dedication. By following the steps detailed in this guide, you should be well-prepared to begin your journey to starting a successful pharmaceutical company.
Before jumping into anything too quickly, it is important to do your research and be sure that you understand all of the nuances and complexities involved. Companies operating in the pharmaceutical industry are heavily regulated, so it is critical to be aware of and comply with all laws and regulations. Additionally, establishing a strong business model and sound financials is of utmost importance when launching a new venture.
Having the right technology in place is essential to running a successful pharmaceutical business. By incorporating technologies such as artificial intelligence, robotics, and machine learning into operations, companies can become more efficient, reduce costs, and improve customer service. It is also important to remember that building a reliable distribution network and marketing strategy are integral parts of the success of any pharmaceutical business.
To ensure a safe and secure environment for your business, make sure to purchase the necessary insurance and adhere to appropriate security protocols. Finally, don’t forget to regularly monitor the progress of your business and adjust as necessary.
By following the steps outlined in this guide, you should have the knowledge and tools needed to create a successful pharmaceutical company.
Good luck and enjoy the journey!
FAQs about Starting a Pharmaceutical Company
1. what are the benefits of starting a pharmaceutical company.
Starting a pharmaceutical company can provide an opportunity to make a meaningful impact on healthcare and research, by providing innovative treatments and medications for medical conditions. It comes with many advantages such as revenue potential, global reach, and advancing the knowledge and effectiveness of medicines.
2. What research needs to be done when starting a pharmaceutical company?
When launching a pharmaceutical business, research should be conducted to gain a relevant and detailed understanding of the industry. This may include studying the science behind drugs, reviewing the market trends, analyzing competitors, and researching the regulations within the chosen countries or regions.
3. How do regulations affect a pharmaceutical business?
Regulations are an important consideration when setting up a pharmaceutical company – due to the safety and health effects of the products the company manufactures. Depending on the location and type of product, additional tests or licenses may be necessary to meet various regulatory requirements.
4. What types of business models are suitable for a pharmaceutical company?
There are several different business models that a pharmaceutical company may decide to pursue, including wholesalers, generic drug manufacturers, independent virtual companies, and branded drug companies. The choice of which model to pursue depends on the company’s goals, mission, and resources.
5. What does it take to finance a pharmaceutical company?
Starting a pharmaceutical business requires substantial capital investments for activities such as product development, approvals, production, marketing, and hiring employees. Depending on the size of the business, financing may be sourced from personal funds, venture capital investors, loans, or crowdfunding.
6. What inventory strategies should be considered for a pharmaceutical company?
The inventory management strategies for a pharmaceutical business should prioritize safety and efficiency. Companies should ensure they have the right medicines to meet customers’ needs, while avoiding overstocking and expiry. It’s also important to have a reliable and secure supply chain in place to reduce stockouts and waste.
7. What strategies are available to promote a pharmaceutical company?
Promoting a pharmaceutical business requires finding the right channels to reach the target customer base. Strategies may include in-person marketing, digital approaches such as website SEO, social media, email campaigns, and referral programs. Advertising and public relations may also be used to raise brand awareness.
Great, Thank you!
How to Start a Pharma Company in 10 Easy Steps

David Blok | Posted on September 19, 2023 | Updated on March 26, 2024
Introduction
Step 1: market research and pharmaceutical business ideas.
- Step 2: Creating a Business Plan
Step 3: Regulatory Compliance and Legalities
- Step 4: Team Building and Talent Acquisition
- Step 5: Location and Infrastructure
Step 6: Product Development
Step 7: clinical trials and approval process.
- Step 8: Manufacturing and Supply Chain Management
Step 9: Marketing and Sales
- Step 10: Scaling Your Business
- Frequently Asked Questions (FAQs)
Let’s face it: if you are reading this, you are one of two people: the entrepreneurial mind that dreams of building a business in an impactful industry, or you’re passionate about science and research and want to contribute to the better health of humanity.
Whether you envision yourself as a Pharma tycoon or simply long to casually drop ‘I make drugs for a living’ at dinner parties, the time has come for you to admit it – you want to build a Pharma company.
Why is a Pharmaceutical company a good investment?
Starting any company takes time, energy and money, but a Pharma business has even more to consider: it requires a combination of scientific expertise, business acumen, and regulatory knowledge. Whether you’re considering how to start a pharmaceutical business or exploring pharmaceutical business ideas you’re not just selling any products here, you’re impacting the health of people. I know what you are thinking: if it’s so difficult, why do people keep investing in Pharma?
Pharmaceutical manufacturing companies can be extremely profitable:
- These companies show resilience during market downturns, rising in R&D investments to stay competitive & flexible (Grand View Research Report, 2023).
- The industry has experienced significant growth during the past two decades, with Pharma revenues worldwide totaling 1.48 trillion dollars in 2022 (statista, 2024). Still don’t believe it? Check out this blog and discover India’s top Pharma Companies to find out h ow profitable pharma companies are in India.
Being such a lucrative market, it’s a natural good investment, especially if you have a good idea – which I’ll get to in the next chapter.
This brings us to our topic at hand, welcome to your comprehensive guide on how to make your venture a reality. In the upcoming sections, we’ll delve into the why’s and how’s of building a successful Pharma empire. Get ready for insights, anecdotes, and much more.
Let’s dive in!
The first step is always the hardest because there is nothing before it. On the flip side it’s a great opportunity to set the direction of your company. Your business is a blank canvas at this point, and you get to bring your vision to life, so the best way to set the wheel in motion is by understanding everything about the industry gap you are trying to fill, in practical terms, whether your idea is viable or not.
Before you dismiss market research as a formality, ask yourself: “Is there a chance my solution is old news compared to what’s already out there?”.
Unless you have the cure for cancer in your pocket, someone’s advancements in your field are worth taking a look at. At this stage, you should focus on two things:
- Understanding market trends and directions.
- Realizing if your big idea is profitable.
Market research will identify unmet needs within the healthcare system, such as patient preferences. By looking at emerging technologies, you can direct your research and development strategy.
Your company’s direction is in your hands at this point, and isn’t that exciting?
Where to start your research?
Start looking at insightful industry reports, academic journals, and the latest industry news, but remember to use credible and reputable sources.
The most important thing is to stay updated, especially on everything that concerns your big idea; market research will back it up. Staying on top of trends also ensures your product is aligned with market demand, and that you have a competitive edge, also called your unique selling proposition.
Step 2: Business Plan and Funding your Pharmaceutical Company
Once you’ve settled on an idea, it’s time to start shaping it into an actual enterprise. Welcome to a pivotal chapter: creating the blueprint of your operations, aka your business plan. Don’t have the faintest idea of what a business plan consists of? Pharmaoffer has got you covered.
Fundamentals of a bulletproof Business Plan:
- A market analysis – which you already did in Step 1.
- Outline of management and the company organization.
- All your products/services – which you probably already know.
- Customer segmentation.
- Marketing plan.
Pitch Perfect
Why is a detailed business plan crucial, you may ask? This plan isn’t just a formality; it’s your golden ticket. This single document is meant to convince stakeholders and investors that you’re not a risk; you’re a calculated and promising investment, which will increase trust and the chances of getting the funds you need.
So don’t be scared to dive deep into the big questions like who will benefit from your pharmaceutical innovations, and what makes it profitable. Paint the financial portrait of your venture with clarity—how will the funds be utilized, and what’s the return on investment? Venture capitalists, government grant applications, and loans are great routes to explore. Your business plan will prepare you to pitch your idea to the right people.
Okay, this is where it gets tricky, but it’s why pharmaceutical business entrepreneurship isn’t for everybody. You are too far along in this journey to stop now, so it’s better to keep going.
Regulating to triumph
We know that acronyms like FDA, GMP, and CEP may be scary, but like we said from the start of this journey: when it comes to the health of the population, you can’t risk it. Regulations ensure your company is ethical and safe. Dismissing them puts your entire operation at risk of getting a bad reputation, not to mention facing penalties later on. Oh, and besides, no funding without compliance is just not going to happen!
What is your business structure?
Now, let’s talk about how to set up and open a Pharma business. Should you go for an LLC (more partnership-oriented) or a corporation, and why does it matter?
The selection of a business structure is a crucial decision for a company because it impacts various aspects, including:
- Legal responsibilities.
- Liability protection.
- Ability to raise capital – we know this sounds like a broken record on this one, but money it doesn’t grow on trees, right?
Remember, it’s not just about paperwork; your business structure shapes the core of your company. Speaking of structure and shape, it’s time to shape your physical company.
Step 4: Building your A-team
You’re the mastermind behind this venture, but you don’t have to do everything yourself. Like a formula, it takes plenty of ingredients to make one solution. In this section, you’ll select the best people to represent and help grow your business.
The trick to knowing whom to hire is simple: point out the necessary fields for your business to run that you need to hire for, and find people who can excel at them. Think R&D, supply chain management, sales, and, of course, medical professionals. ´
Extra tip: remember that sometimes you don’t want to hire the best technical person, but someone who can complement your team’s attributes, like, for example, someone with a proven track record of adaptability and communication.
Step 5: Location, Location, Location
The perfect location is a no-brainer: it is the one closest to your potential partners. Places like research centers for experiments, hospitals for data, or universities for talent scouting are where you wanna be. Just think of how your idea can improve with these extra resources.
A place to call…office
Ideally, if you picture a creative and productive atmosphere, it won’t be a cramped space, poorly lit with bad chairs, that won’t cut it. Your facilities are the day-to-day of your operation, where some say, the real magic happens, so make sure your spaces are up-to code and optimized for the tasks at hand. Remember, a positive environment boosts morale and increases efficiency.
Admit it, you’ve been thinking of this since the very beginning of this adventure. After all, this is what you came here for. Whether it’s a new drug or medical device, the development phase is the fun, and most important part. Let your R&D work shine to bring your vision to life.
Trials and tribulations
Having a finished product means one thing, and one thing only in the Pharma world: clinical trials. Clinical trials aim to provide a scientific basis for advising and treating patients.
But don’t be discouraged if it doesn’t work out. Even when researchers don’t obtain the outcomes they predicted, the trials results can help point scientists in the correct direction of their research. Trials present their challenges, but as cliché as it is, every challenge is an opportunity in disguise and a testament to your team’s innovation capacities.
Think you heard enough about clinical trials? These are the final stages before your product enters the market, and needless to say, it won’t enter without the green light. How do you make sure it’s approved then?
- Rigorous protocol adherence.
- Collaboration with regulatory bodies.
- Scientifically proven efficacy and safety.
- Real-world testing for validation.
Extra tip: real-world testing is a good option to further validate and solidify product claims that may give you an interesting competitive edge.
Passing this frantic stage is a monumental milestone, and it’s one foot in the door of your Pharmaceutical business success. If you are in this stage, or just looking to dive deeper into the complexities of API’s clinical trials, check out our blog, API Clinical Trials: From Preclinical Trials to Post-Marketing Surveillance.
Step 8: Pharmaceutical Manufacturing and Supply Chain Management
Quality control isn’t just between your lab and office walls most of the time unless you manufacture everything in-house, but is that cost-effective?
We are not going to go into this question but leave it for a promising blog about the pros and cons of API in-house manufacturing or out-sourcing. This time we’re going to talk about the Pharmaceutical company’s supply chain.
As you need to make sure every substance you use is under the same quality control as your business, how can you be positive you are purchasing APIs from a qualified supplier?
We’re not gonna lie, unfortunately this step can be a dead end as many API manufactures aren’t registered in a public contact base.
You really need to know the business and ask around, a total nightmare. This is our mission statement: at Pharmaoffer we want to match the best certified API manufacturers with businesses like yours, so to provide resources for both and enrich Pharmaceutical business supply chain with qualified options.
Your supply ally
When choosing the right suppliers, the biggest worry is compliance standards, so make sure your supplier:
- Meets all compliance standards.
- Is up to speed with industry best practices.
- Has the means to make deliveries on schedule.
You have a finished product, your team is working and the place up and running, so it’s time to find some clients. We do this with a marketing and sales strategy.
What do people think of when they hear your company’s name? Do you have a logo? These are some of the questions you need to clear with the proper professionals.
Branding is understandably the last thing on your mind, but don’t make the mistake to overlook it indefinitely. Nowadays, if your business doesn’t look good it won’t be credible. Branding is how you present your company to the world. It’s your mission statement, your corporate culture, and your values. It will help you find your market and secure a strong position in it.
Getting the word out
Marketing is about business survival, there is no denying it. In this increasingly visual world it’s not enough to have a great product, you need to present it well to elevate it. The way you do so is with marketing tools.
Now don’t fall into cheap marketing tactics: in Pharma you can’t make any false claims, exaggerate benefits, or show dubious testimonials. This will kill your entire operation. Marketing in the Pharma sector demands a careful balance of awareness-raising and ethical considerations. Credibility is central, so keep it real, and let your amazing product and integrity attract customers.
Because we understand how complex marketing strategies can be, at Pharmaoffer we wrote you a startup guide to navigate online marketing in the pharmaceutical sector called Online marketing in pharma; where to start? Feel free to take a look.
Step 10: Scaling Your Pharmaceutical Business
You finally have a Pharma company, congratulations and we take no credit for it. Now that you have a growing business, the question is: How big do you wanna get? We’ve seen how much does it cost to start a pharmaceutical company and remember that scaling and it isn’t just about growth, it’s about smart growth. Scaling is about expanding your operation, and it should be a calculated decision.
To make the right move you need to be on top of market demand, KPIs tracking, and consult your team to know when it’s the right time to do it.Bigger the business, bigger the challenge.
Bigger the business, bigger the challenge
A bigger business is a complex one. From staffing to resource allocation, be prepared to adapt your business strategies as you expand.
Extra tip: keep your staff informed and let them be a part of the change. It will make them more involved in your business success.
You made it to the end yay!
We saw how to start a Pharmaceutical company investment which is no small endeavor, but it’s a rewarding one for sure! You are building something of your own, following your heart and creative dreams and the best of all, you’re saving lives while you do it, how great is that?
Okay, it’s time to get to work, so roll up those sleeves, and get started if you haven’t already. We have no doubts that if you pay attention to these 10 steps, you have what it takes to build your successful Pharmaceutical company.
Needing further assistance to find the right API suppliers for your business? Fill in the form to contact Pharmao ff er and schedule a free meeting to take your business to the next level.
Is it hard to start a pharmaceutical company?
Yes, but it's also rewarding. Be prepared for regulatory hurdles and significant initial investment.
How long does it take to launch pharma company?
On average, it could take 1–3 years, depending on the business model, licensing, and other factors.
Can I start a pharma company without a medical background?
Yes, although you'll need a team of experts in medical and scientific fields. Your role may be more focused on business strategy and growth.
What are the biggest challenges in starting a pharma company?
Regulatory compliance, securing funding, and market competition are some of the biggest hurdles you'll face.
- APIs & Manufacturing (15)
- Education & Career (8)
- Events & Exhibitions (3)
- Industry Insights & Trends (11)
- Interviews & Case Studies (9)
- Market Analysis & Data (14)
- Medicines & Access (17)
- Regulations & Standards (19)
- Sales & Marketing (15)
Latest posts
What is hyaluronic acid, shilpa medicare’s interview, choosing your pharmaceutical path: production, analytical testing, or formulation, from vision to reality: the developers behind pharmaoffer’s platform, chinese drug master file (cdmf), recommended blogs.

APIs & Manufacturing , Medicines & Access
Read time: 5 minutes
Understand how Hyaluronic Acid benefits pharma and cosmetics, its production, market trends, and regulatory requirements across major markets.

Education & Career , Interviews & Case Studies
Read time: 4 minutes
Discover how Shilpa Medicare has grown into a global pharmaceutical leader by focusing on innovation, sustainability, and social responsibility in the healthcare industry.

Education & Career
Thinking about a career in pharma but not sure where to start? Dive into this guide to explore different paths like production, testing, and formulation.

Read time: 3 minutes
Meet the brilliant minds behind Pharmaoffer’s platform. Discover how our developers turn big ideas into reality, driving innovation and efficiency in the pharma world.

Regulations & Standards
Read time: 6 minutes
Learn everything about Chinese Drug Master Files (cDMF) and how to navigate China’s pharmaceutical regulations. Learn key steps, benefits, and expert tips to ensure compliance and market entry success.

Top 10 Biggest CDMO Companies and Market Trends
Market Analysis & Data
Explore the Top 10 Pharmaceutical Companies in India for 2024. Uncover key insights into their financials, innovations, and global impact.
Pharmaoffer is a B2B platform where you can find all qualified API suppliers in one place
- Business Plans Handbook
- Business Plans - Volume 03
- Pharmaceutical Company Business Plan
Pharmaceutical Company

BUSINESS PLAN
PAIN AWAY LTD.
1117 High St. Poughkeepsie, NY 13495
The company described in this plan has moved beyond the initial start-up phase and is now seeking investors to finance its growth. Much of the plan, therefore, is geared toward persuading, explaining, and reassuring potential investors that the company (which produces a therapeutic, topical pain cream), is well-managed and stable. The in-depth analysis of the company's competitors is an outstanding feature of this plan, as is its market research.
EXECUTIVE SUMMARY/OVERVIEW
Competition.
- PROPERTY & FACILITIES
- PATENTS & TRADEMARKS
- RESEARCH & DEVELOPMENT
GOVERNMENT REGULATIONS
Insurance and taxes, corporate structure, risk factors.
- RETURN ON INVESTMENT AND EXIT
- ANALYSIS OF OPERATIONS & PROJECTIONS
FINANCIAL STATEMENTS
Type of business.
Non-prescription drug wholesalers; US SIC Code - 2834 Pharmaceutical Preparations.
Company Summary
Pain Away Ltd. is a going concern, a Delaware corporation formed in January 1995 to manufacture and sell its premier launch product Pain Away, a topical pain remedy using FDA-approved homeopathic ingredients developed for the simple purpose of relieving pain. The company was formed by its parent S-corporation, Peale, Inc. in order to market products nationally and internationally. Peale, Inc. was formed in February 1994 to complete the development of the launch product. The formation of the company was a significant step in a 9-year process of refining and testing a homeopathic formula first used by company founder and CEO Robert Peale to alleviate his pain from carpal tunnel syndrome. The R&D phase of this product began when Mr. Peale purchased the original formula, did a thorough study of homeopathy, and refined the formula to its present marketable state. From the beginning of R&D, Mr. Peale worked within FDA guidelines in order to secure FDA registration. Then, in February 1994, the company was formed to finally manufacture and sell the product. Starting with only a handful of customers, including some professionals, chiropractors, physical therapists, etc., only 19 months of operation have yielded 12,000 individual customers with an 80% reorder rate. The current customer base now includes medical doctors from different specialties, sports trainers, and athletes, both professional and amateur. The company expects to show a profit in 1996 and estimates that it will be very profitable in 3 years.
Mr. Peale is 49 years old and has a 25-year history in sales, sales management, and marketing for a tool distribution company. His deep study of homeopathic medicines started in 1985 and included studies in nutritional supplements. Mr. Peale has been invited to sit on a newly-formed FDA committee addressing the growing national interest in natural medicines.
Curtis Company president, Ms. Alana has 25 years of experience in retail and direct sales. She has been a senior sales director and sales trainer for Beautiful You Cosmetics, has owned and operated a retail sporting goods store, and has managed a 15 person, $1 million department for a major chain retailer. She also has some banking experience.
Vice-president of marketing, Ryan Lemon has 32 years of experience as production manager, buyer, sales manager, and marketing manager. He was director of marketing for Pilgrim Health and was responsible for their first launch into New Jersey which led to their first $18MM in sales (in 3 years). He has a BS degree in textile engineering and has also done independent marketing consulting.
Product and Competition
The R&D mission was to develop a greaseless, odorless, topical cream which was measurably more effective at relieving pain than any other OTC (over the counter) topical product. This mission has been accomplished. The company has collected anecdotal, testimonial, and uncontrolled medical study evidence that Pain Away is more effective than the leading topical analgesics such as Arthritin and others. The product's effectiveness in relieving pain is its most powerful benefit, besides the added benefits of it being greaseless and odorless. What distinguishes Pain Away from any other topical analgesic in this still-growing $402.1MM market is its advanced homeopathic formula - a refined blend of 11 FDA approved pure and natural ingredients. The typical OTC topical analgesic works to either block the sensation of pain or distract perception of deep pain by "counterirritating" another localized area near the pain. Pain Away's formula is different. Pain Away treats pain at its source. It stimulates improved circulation in the micro-capillary system in the ligaments and tendons, where most pain is felt. Pain-relief from Pain Away is the result of the body's own self-healing. It also can be applied several times a day because it is odorless and greaseless. The US pain management market ($15.2 billion by 1997) is a mature market with intense, established competition ("The Market for Pain Management Products in the US - Introduction, Drugs, Devices, Trends, and Market Structure," in FIND-SVP). With future pharmaceutical market growth dependent upon new and innovative product additions, Pain Away is entering the field at the right time. The company will distinguish itself and its market position by dedication to the development of only natural-ingredient products. Since its unique formula of ingredients already has FDA approval, the company aims to penetrate the OTC pharmaceutical market, where new products traditionally find success. Here Pain Away will compete with topical as well as internal analgesics, including aspirin, acetaminophin and ibuprofen. An estimated 4,000 people a year die from aspirin overdose. A condition known as "analgesic neuropathy" can result from extended or inappropriate use of analgesics. Medical studies linking heavy usage to health problems have affected aspirin, acetaminophin, and ibuprofen. Pain Away can be marketed as a substitute for (reducing overdose risk with internal analgesics), or as a supplement to (using Pain Away can reduce needed dosage of internal analgesics) internal analgesics when used for certain pain relief. Furthermore, Pain Away is not contraindicated for use with any other medication. This broad-based appeal is built upon the reliability of Pain Away's effectiveness in relieving pain, inflammation, and spasm associated with arthritis, bursitis, sciatic spasm, neck/back pain, tendonitis, tennis elbow, tension headache, achilles tendonitis, and carpal tunnel syndrome.
A second product, a natural anti-inflammatory nutritional support system formula known as "Pain Away Plus," will soon be marketed as a companion product to Pain Away. This multistaged formula is a combination of trace minerals, herbs, and a natural cartilage-derived substance. The company has long-term plans to develop more health-related products.
Funds Requested
Company principals have invested all available personal assets into the product development and operations to date. The need for capital is in the context of the readiness of the product for mass marketing. Management is seeking a $1,500,000 equity investment in exchange for a suggested 30% ownership of the company. All terms of financing are negotiable in order to meet the financial requirements of the investor.
Use of Proceeds
Advertising & promotion campaign - $1,200,000 (see below); Market research - $300,000. The company anticipates the need for follow-on financing after 24 months of business.

| Magazines | $330,000 |
| Radio | $200,000 |
| Shows & Conventions | $140,000 |
| TV | $400,000 |
| Retail Shops | $70,000 |
| Sample - POP Display | $60,000 |

Financial History

| Sales | 543,633 |
| Net Income | (226,600) |
| Assets | 56,987 |
| Liabilities | 224,253 |
Sales were first made in 5/94 under Peale Inc. ($143,881). As sales expanded nationally, Pain Away Ltd was formed in January 1995. All sales since then have been under Pain Away Ltd.
Financial Projections
| Sales | 3,000,000 | 8,000,000 | 18,000,000 | 32,000,000 | 50,000,000 |
| Net Inc. | 360,000 | 2,160,000 | 4,860,000 | 8,640,000 | 13,500,000 |
With capital request accomodated, the company believes that Pain Away will jump in sales starting in 1996.
The company will attempt a public offering based on year 2000 earnings. If there is no public market and no prospect for a public market in the near future, then the company will offer to buy back the stock owned by the venture capitalist. A predetermined price could be set ahead of time, if desired by the venture capitalist.
The product effectiveness, evidenced largely through anecdotal evidence, personal testimonials, and repeat sales, has formed the basis for the future growth of the company. Together with a second, complementary product (nearly ready for market), the launch product will be aggressively mass marketed as a pain management system for the next five to ten years. Past and current sales have been to end-users, health professionals, and to some retail chains. The company and product are now poised for first stage expansion. Over 30 target wholesale markets have been identified. While the company uses its marketing strategy to enter these wholesale markets, simultaneous efforts will be made to develop research protocols. Management is confident that the anecdotal evidence and personal testimonials will be strengthened by controlled studies, designed to test the effectiveness of the product and demonstrate the physiological healing activity stimulated by the formula. With scientific credibility, the product will not only build its position in the $150 million homeopathic product category but will also strengthen its transition into the formidable mainstream topical analgesic category.
Future research is planned, based upon inquiry, in order to adapt the formula for animal use (Pain Away currently being tested on thoroughbred horses).
At the end of five years, the company intends to have at least one additional health product and should be able to go public off its revenues. The long-term goal for the company is to become an entrepreneurial leader in the development of natural products for various segments of the health care market. The company plans to capture enough share of the topical analgesic market to become either a viable joint venture partner or an acquisition candidate.
The product formula and delivery system are proprietary. The formula is uniquely advanced and is nearly immediately effective in relieving pain. Homeopathy and immunization have much in common, namely the principle of similars, which states that whatever a substance causes in a large dose, it can stimulate an immune response to defend against it in a small dose. It works by the principles of stimulation to the body's own self-healing mechanism and by the scientific balancing of its natural active ingredients through a dilution process called micro-dosing. Micro-dosing has given homeopathy its 200-year history of safety with no known side effects or toxicity. This self-healing process is in contrast to the majority of commercially successful topical analgesics, which contain counter-irritants, including the newer capsaicin-based products. These ingredients cause a superficial inflammation on the skin which masks pain by deadening the sensation of pain in the epidermal nerve endings only, or by distracting from the perception of pain by irritating an area near the pain source. The Pain Away formula has been developed with precision and balance and is a product that is effective and safe for use on all skin types. Pain Away's eleven active ingredients stimulate improved circulation in the micro-capillary system to ligaments and tendons, where most pain is felt. Pain relief is the result of the body's self-healing.
The manufacturing is sub-contracted out to a highly respected FDA-licensed manufacturer of homeopathic products.
An important unique feature of Pain Away which distinguishes it from other homeopathic remedies is that Pain Away is a topical treatment and is not a systemic treatment. As such, it requires little knowledge to use and is conducive to cross-merchandising in the mainstream analgesic category. Furthermore, since Pain Away is a formula of ingredients, it provides a broad spectrum of effects as compared to single remedies.
The personal commitment of the founder to relieve his own pain also adds a unique value to the story of this product - a story which can enhance marketability - to anyone who is in pain or anyone who knows someone in pain.
Although Pain Away is an homeopathic product, the company will position itself as a natural ingredients company - not necessarily homeopathic. All the company principals plan to engage both septics and advocates of complementary medicine by applying rigorous scientific standards equally across the board, for both conventional and unconventional treatments. Contacts have already been made with the National Institute of Health regarding future research.
Product Description
The product is a specialty consumer good carrying a suggested retail price of $19.95 for a 3.7 oz. jar (1.9 oz. jar also available at $12.95). The jar is designed with a medical appearance. The jar is easy to ship in multiples, is easy to stack on a shelf, is aesthetically pleasing, and has an easy-to-handle screw cap. The actual cream is greaseless, easy and pleasant to apply, and is odorless. Pain Away has, to date, largely been sold directly to end-users, and wholesale to retailers, distributors, and catalogues. The markets have supported the suggested retail price, which was arrived at by surveying market research supporting the $19.95 price along with the perceived value of the product compared to similar products at about the same price. This price also yielded a gross profit of $3.75 per jar and allowed for 100% markup from wholesale.
The eleven ingredients are readily available through top-quality labs which control for purity and authenticity. The cream is compatible with any medication being taken. The product carries a money-back 30-day guarantee.
Purchasers of the Product
Preliminary studies done by independent treatment professionals (no control group used) have shown that Pain Away has been effective for relieving the pain, inflammation, and spasm associated with arthritis, bursitis, sciatic spasm, neck and back pain, tendonitis, tennis elbow, tension headache, achilles tendonitis, and carpal tunnel syndrome. Anyone suffering these ailments, treating these ailments, or caring about anyone suffering these ailments is a potential purchaser of the product. A New Jersey hockey team uses Pain Away prior to workouts, competition, and for pain relief. The head trainer for the team says, "There's no product better for contusion of the quadriceps." He has reported shorter recovery times as a result of using Pain Away. Reports from athletes are that using Pain Away before and after workouts yields less cramping, fatigue, and soreness.
Top purchasers of TPR to date:
| Mall Booth Marketing | $11,000/month | 800/month |
| Direct Selling-Retail | $16,000/month | 1200/month |
The total market for OTC internal and topical analgesics is estimated at $3.6 billion for 1995 and is projected to be $4 billion by 1997. With over 400 brands saturating this mature market, growth is still occurring through new products and product innovations. Driving this growth are:
- increasing use of pain management products for the over-50 population segment, whose numbers are increasing
- increasing awareness that pain does not have to be tolerated and can be treated
- price increases
Body/Muscle Pain Market
The market is dominated by internal analgesics:
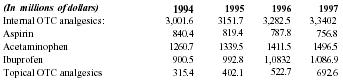
| Internal OTC analgesics: | 3,001.6 | 3151.7 | 3,282.5 | 3,340.2 |
| Aspirin | 840.4 | 819.4 | 787.8 | 756.8 |
| Acetaminophen | 1260.7 | 1339.5 | 1411.5 | 1496.5 |
| Ibuprofen | 900.5 | 992.8 | 1,083.2 | 1/086.9 |
| Topical OTC analgesics | 315.4 | 402.1 | 522.7 | 692.6 |
Pain Away is a new product to this sizable OTC pain-relief market. It will enter this large arena riding on its effectiveness and coming from the new and growing alternative health care market segment. As a new OTC product, Pain Away has such a broad-based appeal that it will be sold to a large portion of the total OTC pain-relief market (both internal & topical), estimated to be 84% of all US adults and growing as the baby boom population ages and concerns regarding age-related ailments, such as arthritis, increase. Of this 84%, about 25% alone use pain-relief products for body/muscle pain for which Pain Away is especially suited. Just this one type of ailment offers a substantial market potential:
| 161.3 Million X | 40.3 Million X | 156x/year | = | 6.3 Billion uses |
If only 40.3 million Americans (25% of 84% of adults) use an OTC pain-relief product three times a week for body/muscle pain alone, then the market potential is 6.3 billion uses of a pain-relieving product per year. Past use of Pain Away has indicated that a minimum of 3 applications per week would use about one 3.7 oz. jar per month. A conservative yearly estimate would be 10 jars per year, with consistent use. In order to reach a five-year sales goal of $50 million (6.7 MM jars), 667 MM consistent purchasers (10 jars/yr.) are needed. Product history has indicated a consistent 80% reorder rate, so at this rate, 833,000 original purchasers are required. This figure is 2.07% of just this one market segment. The company is very confident that it can capture 2.07% of this market segment within five years, especially considering that the roughly 40 million Americans who exercise on a regular basis, and who are aging, are included in this segment. Anecdotal reports from athletes who use Pain Away are that it can prevent injuries by "warming up" vulnerable muscles and joints prior to a workout. The product has wide applicability within this segment. The table below shows the percentage of the body/muscle pain market segment required to meet the next 5 years of sales projections.

| (in millions) | (with 80% reorder rate) | ||
| 1996 | $3 | 50,000 | .12% |
| 1997 | $8 | 133,000 | .33% |
| 1998 | $18 | 300,000 | .74% |
| 1999 | $32 | 533,000 | 1.32% |
| 2000 | $50 | 833,000 | 2.07% |
These numbers are based upon a wholesale price of $7.50 per jar and a usage rate of 10 jars/year with a segment population of 40.3 million potential purchasers.
The prescription pain relief market is a distinct market which Pain Away will not attempt to penetrate. Pain Away can, however, compete directly with nearly all pain-relief products because of its unique identity of being both a substitute and a supplement to ail competing products. This uniqueness fits a projected market shift from internal to topical analgesic use as the population ages, and derives from 2 factors: 1) Use of Pain Away can reduce the needed dosage of any pain-relieving medication and 2) Pain Away is already part of a rapidly growing segment (25%-30%/year) of consumers who use alternative health care because of a disenchantment with OTC drugs and a concern about side effects with adverse reactions. Use of Pain Away can reduce needed dosage of other pain-relieving medications. As stated earlier, Pain Away's effectiveness is based upon the homeopathic principle of microdosing. While it promotes self-healing by stimulating blood flow to micro-capillaries, it remains safe for all skin types and with use of any other medication. Anecdotal evidence (from hospitals, some doctors, and occupational rehab center) has indicated that use of Pain Away alone has yielded positive results and use of Pain Away, along with other treatments, has seemed to accelerate recovery. As always, this kind of evidence will be scientifically studied. The salient point is that Pain Away can be a substitute and/or a supplement in pain management, and thereby reduce needed dosages of other medications.
Alternative Health Care Segment
Homeopathy, being an established (officially recognized by UK National Health Service) and significant alternative mode of treatment, is gaining increasing acceptance in mainstream American health care. The National Institute of Health has even awarded grant money for research in alternative treatments, including homeopathy. Drug retailers report that homeopathy may be the fastest-growing category in the trade class of drug chains. Since homeopathy is gaining acceptance as an alternative treatment, the market segments which are already embracing these alternatives will continue to be targeted in the company's initial expansion. These segments include people ages 25-elderly, who seek improved quality in life, and whose lifestyle values involve "newness." This segment includes most of the "baby-boomer" population, estimated at over 75 million. The market of alternative health care seekers is characterized by patients who can and will pay for their own care. As much as 70% of alternative medical treatments are still paid for by patients themselves rather than insurers. This kind of purchasing indicates a willingness to try an alternative product and continue purchasing based upon perceived value of the product's effectiveness. Company management has been encouraged by the consistent 80% reorder rate and knows sales will be sustained once initial purchases are made. The alternative health care market is of respectable proportion. According to the New England Journal of Medicine (1/28/93), 34% of Americans spend $13 billion/year on alternative treatments such as chiropractic, acupuncture, massage, and homeopathy. Pain Away is already marketed to all of these treatment specialties so it will reach the spectrum of alternative treatment. This 34% of Americans are familiar with the term "homeopathic," so there's a consumer predisposition to being further educated about homeopathy as a value-added natural ingredient alternative.
The company will build an early market position on the alternative health-care market and will join the growth of the homeopathic segment as it moves from the fringes to the mainstream of the OTC pharmaceutical market.
Alternative Market Potential:
| 262 Million × | 89.1 M (34%) × | 24x/Yr. | = | 2,000 Billion |
If only about one third of Americans use an an alternative pain-reliever just twice per month, then the market potential is 2 trillion uses of an alternative pain-relieving product per year. Market indicators are that both the number of users and the frequency of use will increase as the population ages. The use rate of 2 times per month converts to 2 jars of Pain Away per year with consistent use. Again, in order to reach the 6.7 million jar sales goal ($50 MM), at the re-order rate of 80%, Pain Away would have to make 4.2 million initial sales in order to sustain 3.3 million consistent purchasers. This size customer base comprises 4.71% of the growing alternative health care market. The company believes that this sales goal is attainable within the next five years. The table below shows the percentage of the alternative health care market segment required to meet projected sales.
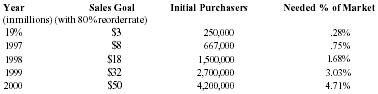
| (in millions) | (with 80% reorder rate) | ||
| 1996 | $3 | 250,000 | .28% |
| 1997 | $8 | 667,000 | .75% |
| 1998 | $18 | 1,500,000 | 1.68% |
| 1999 | $32 | 2,700,000 | 3.03% |
| 2000 | $50 | 4,200,000 | 4.71% |
These numbers are based upon a wholesale price of $7.50 per jar and a usage rate of 2 jars/year with a segment population of 89.1 million potential purchasers.
Narrowing the Market Focus 2X
The market potential for pain relief products is huge. By narrowing the focus to product category sales, the potential becomes more exact. Pain Away's product category is within the topical analgesic market, estimated at $402.1 MM annually with a projected $522.7 MM market in 1996 (30% growth) and $692.6 in 1997 (32.5% growth). Starting with $522.7 as the base market volume, and with 30% growth per year for the next 5 years, Pain Away would have to capture 3.33% of the year 2000 market volume to make its sales goal of $50MM. Management believes that these goals are attainable.
The table below shows what percentage of the topical analgesic market will meet Pain Away's sales projections.
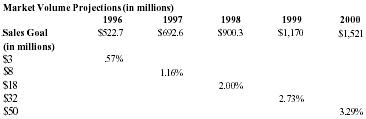
| $522.7 | $692.6 | $900.3 | $1,170 | $1,521 | |
| $3 | .57% | ||||
| $8 | 1.16% | ||||
| $18 | 2.00% | ||||
| $32 | 2.73% | ||||
| $50 | 3.29% | ||||
The focus can be narrowed further to the homeopathic product category, which is growing at a rapid rate at this time. The dollar volume of this segment is estimated at present to be between $150 million and $215 million and expected to grow at a rate of 25% to 30% a year. Some market-trackers say that retail sales haven't grown enough to support the existing number of homeopathic manufacturers and that a shakeout will consolidate sales in the hands of fewer manufacturers. The forseeable trend, however, is progressive growth from the fringes to mainstream markets, and at a rapid rate. The table below again shows percentages of this dollar volume required to meet sales projections.
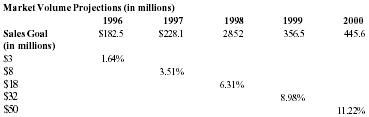
| $182.5 | $228.1 | 285.2 | 356.5 | 445.6 | |
| $3 | 1.64% | ||||
| $8 | 3.51% | ||||
| $18 | 6.31% | ||||
| $32 | 8.98% | ||||
| $50 | 11.22% | ||||
These numbers are based upon a 1996 volume mid-point between the projected volume range of $150 MM and $215 MM. Growth rate is 25% a year. At first glance these percentages may seem daunting. However, the manufacturers supplying this niche are relatively few in number and therefore hold significant market shares A new player can get a reasonable market share with the right product and marketing plan. The mainstream merchandising of homeopathic products started in the early '90's and has been tested as a lucrative direction. Company management is very confident that Pain Away will gain enough share points to capitalize on the rapid growth of this product category. Pain Away will not remain in the homeopathic niche. Its effectiveness will make it competitive with mainstream topical angalgesics.
International Markets
The company will also develop an international market. A 10,000-unit order has already been received from a distribution company in Hungary and is awaiting final approval from the Hungarian State Department of Pharmacy. A small order was also sent to well-known sports figure in Spain. Discussions are underway for this individual to start large-scale distribution. The homeopathy market in the UK is estimated at 18M pounds and in Germany at 120M pounds, so European marketing could be strengthened by the homeopathic identity alone. In Germany, an independent division of the German Federal Health Agency publishes monographs on the safety and efficacy of herbal medicines. The company believes that Pain Away would fare excellently under such review and will carefully research and plan when and how to reach such markets.
There are many companies competing for shares of the 3.6 billion dollar OTC analgesic market. The major players are the internal analgesic manufacturers:

| Reynolds | Aspernol | 1.2B | 34% |
| Pharmacorp | Aspiril | 612 MM | 17% |
| American Pharmacy | Anaprin | 180 MM | 5% |
| Oxford Co. | Maraprin | 180 MM | 5% |
| Jones-Smythe Benton | Aspirin | 144 MM | 4% |
The balance of the OTC internal analgesic market is held by private label companies and "others." The major strengths of this level of competition are obvious in comparison to Pain Away's present market position. The major players have:
- a manufacturing cost advantage,
- sophisticated market knowledge and access,
- established sales capability,
- strong R&D capacity,
- and of course, brand name loyalty.
An important competitive strength of Pain Away is that it is topical - pain relief is accomplished without risk of overdose and consequent risk of serious side effects. This competitive strength derives from a previously noted shift in the market from internal to topical analgesic use. This shift in consumer preference, along with Pain Away's effectiveness, can position the product as a substitute/supplement among these large competitors. Management is ever mindful that mainstream pharmaceutical companies are watchful of the homeopathic market and will act accordingly should market share be lost to homeopathic remedies. Becoming a viable acquisition candidate to any one of its major competitors is a realistic goal. Pain Away management is committed to quality product development and is also open to strategic alliances which would enhance its market capability.
The competition in the topical analgesic market is head-to-head. The top competitors are:

| Pepper Co. | Pepperub | Menthol | 60.3 MM | 15% |
| Athens | Vapol | Menthol | 47.9 MM | 11.9% |
| Lucia | Menthol Plus | Menthol | 35.8MM | 8.9% |
| Skin Care Corp. | Zanprin | Capsaicin | 44.2 MM | 8.7% |
| Bioderm | Aspratin | Salycin | 18.8 MM | 5% |
| Capcreme | Capsaicin | NA | ||
| Men-Thol Co. | Menthoflex | Menthol | NA | |
| Capthol | Capsaicin | Menthol | NA | |
| Bianco-Picard | Salicreme | Methylsalicilate | 86 MM | |
| Synergy | Lyptum | Eucalyptus | NA |
The basis for the competitive analysis is Pain Away's most competitive feature:
- It doesn't have any of the aforementioned advantages held by the major, well-known players in this market - yet.
- It doesn't have widespread brand name recognition - yet.
- It doesn't have appreciable market share in topical analgesics, alternative health, or homeopathy - yet.
- It does have a unique formula of safe and effective ingredients which none of the above products have.
All topical analgesics contain counter-irritants, including camphor, menthol, methyl salicylate, eucalyptus, wintergreen, and even the popular capsaicin. These ingredients, even when blended, act primarily to cause a superficial inflammation on the skin. This inflammation serves to hide the pain by deadening pain receptors in the skin.
What distinguishes Pain Away from all of the above products is that the eleven active homeopathic ingredients stimulate the blood flow in the body's micro-capillaries and act synergistically with the body tissue. This stimulates the body's own self-healing. Pain is treated at its source. Company management believes that the unique effectiveness of Pain Away will give it competitive clout. The issue then becomes how to compete.
Although Pepperub (Pepper) and Vapol (Athens) enjoy the largest market share, they are vulnerable to new product introductions. Menthol Plus (Lucia) held the top position in this category last year until Pepperub was re-packaged and relaunched with line extensions. That relaunch along with a relaunch of Zanprin boosted sales of both brands and put Pepperub back on top. Pepperub, Vapol, and Mentholplus are all menthol-based products. Zanprin is a capsaicin-based product and has boosted usage of its relatively new ingredient. Other relatively new capsaicin products are Capcreme (Bioderm) and Capthol (Men-Thol Co.).
Company management believes that Pain Away is generally more effective than Pepperub and Vapol. However, these venerated brand names, large advertising budgets, and consumer loyalty are formidable competitive advantages. Pain Away will focus on other competitors in order to gain a market position.
The key competitors are Menthol Plus, made by Lucia and Zanprin, made by Skin Care Corp.. Menthol Plus is a menthol-based product which Pain Away has encountered head to head in the sports market. Menthol Plus has a retail price advantage in the mass market, selling for $4 for a 2 oz. tube. This price difference is of little concern because Pain Away will promote itself as a high value product. The topical analgesic, alternative health, and homeopathic markets all support pricing based on perceived product value. Menthol Plus' manufacturer has reduced the advertising budget for this product (about $2 million) recognizing from a 21% decrease in 1994 sales that the product has matured. The company plans to acquire other brands (no topical analgesics) and extend its other lines in order to generate sales growth. The company sells another topical analgesic which is doing well in sales but has not reached the same position as Menthol Plus. Pain Away will monitor the life cycle of Menthol Plus and move to gain any market share it might lose.
Zanprin, made by Skin Care Corp., is gaining market share because Zanprin (.025%) and Zanprin- X (.075%) are capsaicin-based products. Capsaicin, derived from cayenne peppers, has created a new segment in the market and is very popular. Other companies are making capsaic in products but Skin Care Corp. attracted market attention by relaunching Zanprin as an OTC consumer product. It had been marketed for seven years to physicians and kept behind the counter, carrying the credibility of a prescription product. In early 1995, the product was re-packaged for shelf space and supported by TV ads. Despite commanding premium prices ($19.95/2oz of Zanprin-X), the product has done dramatically well.
Skin Care Corp. claims that Zanprin is the "only brand with physician endorsement and specific clinical support." This is a credible claim, cultivated for seven years, and obviously contributing to sales of the product.
Skin Care claims to be the first in the industry to develop their highly purified version of capsaicin for a pharmaceutical base. Zanprin distinguishes itself by promoting controlled clinical studies which have supported its effectiveness. Skin Care claims that such clinical trials don't apply to other, less pure, capsaicin formulas. This scientific feature enhances product credibility among physicians and pharmacists.
The management of Pain Away Ltd. recognizes the effective marketing strategy used by Skin Care because it is similar to their own strategy. Advertising and promotion expense is critical. With proper capitalization, Pain Away can compete because the Pain Away homeopathic formula is unique and effective. Many capsaicin users, including Zanprin users, have complained about the burning sensation caused by capsaicin. Pain Away will stand up to any topical analgesic on the market and do very well with comfort, safety, and effectiveness. The company needs to get this message out. The seven-year product life of Zanprin, supported by unique and heavy TV advertising, gives Zanprin quite an edge. Zanprin is now a "new" growth product and Pain Away can grow behind it, by comparing ingredients and effectiveness at every turn. Pain Away is also in the same price range as Zanprin, doing slightly better with $19.95 for a 3.7 oz. jar or $12.95 for a 1.9 oz. jar.
Zanprin is not the "only brand with physician endorsement and specific clinical support." Pain Away has been cultivating health professional support since the R&D phase. The product is heavily endorsed, and more medical support is developing. Many of Pain Away's sales to date have been to health professionals. Regarding clinical support, Skin Care's success with this strategy underscores the strategic importance of Pain Away's plans for controlled clinical studies.
Speaking of "highly purified" formulas, Pain Away can compete strongly with any formula on the market, especially capsaicin-based. The company wants to discuss purity of ingredients and formula and will do so in all promotional efforts.
The remainder of the products listed in the top competitor list have of course the same advantages that any established company with significant market share has. Beyond these immediate competitive advantages, Pain Away can compete, again, on the ingredient effectiveness basis.
Aspratin, an odorless rub which contains Salycin, sold well when it was introduced in 1992. It held third place among topical analgesics at the end of 1993. It has since been surpassed by capsaicin-based Zanprin. Bioderm developed Capcreme and lowered its price when Zanprin was relaunched.
Capthol was recently developed by the long-established Men-Thol Co. and is a capsaicin-menthol blend designed to compensate for the sometimes delayed pain relief when using capsaicin alone.
Salicreme is a methylsalicylate product which has shown flat growth and has lost market share.
Lyptum was a rapid-growth product in 1990-1991 but has since lost market share. Besides the well-established brands like Pepperub, the products which are gaining in this market are the capsaicin-based. This product category is known to be affected by product innovation and development. With proper support, Pain Away will take a respectable market share.
Homeopathic Competition
The competition takes place in the drug chain arena. Homeopathy may well be the fastest-growing category in the trade class of drug chains (20% of all homeopathic product sales). Among the growing number of drug chains which are giving shelf space to homeopathic products are: Walgreens, Medicine Shoppes International, Thrifty Payless, Eckerd Corp., Edgehill Drugs, Genovese and FEDCO, a California supermarket chain. Research published in the Journal of Clinical Pharmacy and Therapeutics states that 27% of US pharmacists consider homeopathic medicines helpful while only 18% consider them useless. The crossover of homeopathy from health food stores, where sales are still strong, to mass markets is gaining momentum.
As mentioned earlier, there are relatively few companies supplying homeopathic products to the mass market. There are five major producers/distributors of homeopathic products.

| Health System Products | Full line of products |
| Homeopathic Co. | Full line under brand name Organa |
| Life-Right Corp. | Full line |
| Del Sol Inc. | Full line |
| Scandinavian Co. | Full line |
| Bio Health | Full line |
Health System, Homeopathic Co., and Life-Right pioneered the distribution of homeopathic products to chain drug stores in the early 1990's and are now market leaders, although more companies are entering this lucrative market. Health System Products now has about 40% market share. Homeopathic Co. and Del Sol are aggressively developing the crossover into mass marketing with line development and heavy TV advertising.
All the topical analgesics listed above are arnica-based, with few other ingredients. Arnica Montana is the premier homeopathic medicine for the treatment of shock and trauma to the muscle. These formulas come the closest to Pain Away's because they contain some of the essential homeopathic pain-reducing ingredients. Pain Away's formula, however, blends more ingredients than any other homeopathic topical analgesic on the market. This more inclusive formula gives the product wider applicability. Price-wise, Pain Away is more expensive than most of the competing homeopathic products, where prices are in the $5-$10 range for 2oz.-4oz. sizes. But, this is a value-priced market, so price is not a critical variable. Since Pain Away is very competitive on an ingredient/effectiveness basis, the critical factor is having the resources to promote the product.
Future Competition
As has been noted, the topical analgesic category, including natural ingredient, is rapidly influenced by new clinical studies and product innovations. There are three main sources of new competition:
- New ingredients and/or new innovations of existing ingredients. Examples are new products which employ the medicinal benefits of ammonium compounds. These products are designed to provide pain relief without the objectionable training room smells, burning sensations and stinging of abraded skin that are often caused by the majority of topical analgesics that contain menthol, methyl salicylate or capsaicin as active ingredients. Pain Away's formula has solved this sensation problem and is a less "high-tech" product, for which consumers are showing a preference.
- Companies currently in this market who could increase market share and become major players. Pain Away Ltd. is in this category.
- Chain drug companies may produce their own private label homeopathic products and corral a significant share of this growing market - much as they did in the non-homeopathic analgesic market. This scenario is more likely to happen as homeopathic companies expand the sales volume in this market and there are share points to be taken away by private labeling.
Pain Away Ltd. can be very competitive with the right promotional support.
Marketing Strategies
Increase market share by reducing market share of competitors. This strategy will capitalize on the market development to date and capture a share of markets held by existing pain-relieving topical applications. The key benefit is that conventional pain-relievers mask pain while Pain Away stimulates the body's own healing ability to directly battle an ailment. Another benefit is that homeopathic remedies have no known side effects while many pain-relievers, especially those ingested, have side effects. Neither will Pain Away interfere with any medication. This strategy requires extensive advertising in mainstream media, including infomercial, QVC (Pain Away already under review), 60 second commercial, cable TV, interactive TV, direct mail, independent sales reps, POP displays, and educational inserts/newsletters. One objective of planned controlled studies on the effectiveness of Pain Away is to use scientific evidence to help bridge the narrowing gap between natural and conventional medicine. Product studies will support this marketing strategy. In this context, the company will pursue preliminary inquiries from a favored vendor to use Pain Away in the workplace to study any reduction of lost work time and/or medical costs precipitated by repetitive stress injuries.
Expand a growing new market for alternative health care by positioning to lead this growing market. This strategy involves specialty catalogues (placed in 5 currently), placement on retail shelves of health food stores, educational product inserts/newsletters, media appearances discussing product, and independent sales reps. This strategy addresses the 89.1 million users of alternative health care.
The company has already been approached by two large Multi-Level Marketing companies. This strategy would involve creating private labels for a large customer. Of utmost consideration with this strategy is product identity and how this channel of distribution would affect it. This channel of distribution usually requires more price mark-up than the product would tolerate.
The company will create its own "competition" by developing private labels and/or separate companies to market to different niches.
Keep capital outlay to a minimum by licensing/franchising Pain Away to a brand-name company. This strategy would add value to the product in the form of brand name loyalty, manufacturing strength, and a strong sales/service force already in place. The company envisions its role in this type of strategic alliance as conducting scientific studies to increase the credibility of TPR and in developing new products. This strategy remains an option which could preclude other strategies under mutually acceptable terms.
Building on an initial order from a health product distribution company in Hungary, Pain Away Ltd. will penetrate the European market by targeting England and Germany, where homeopathy is an accepted form of treatment. This strategy would be developed only after a US market position was established.
Marketing Plan
The company is moving from start-up stage into its first growth stage. Market strategy to date can be succinctly described as selling "one jar at a time." Direct personal selling has been the mainstay in sales growth. This strategy has targeted any end-user willing to try the product. These early customers were reached through health care professionals and direct selling through state/county fairs, shopping mall space, health food store chains, and most recently lifestyle catalogues. As the company moves away from direct selling, a strategy which proved to be an excellent market test, into mass-marketing, identified market segments are being matched with appropriate distribution channels. The plan now is to expand and concentrate more on helping the consumer develop product preference by heavy advertising of the brand name, the benefits of the product, the ease of use, and the guarantee. Company expectations are that all advertising will be enhanced by results of controlled studies of product effectiveness.
The company intends to expand regionally, based on existing markets and consumer profiles (e.g., households from the South are likely heavy users of analgesics). The national market will only be tested by placement in catalogues with a distribution of 200 million. As regional sales grow and as the product gains recognition, then a national marketing strategy will take shape. Company management have begun discussions with a major marketing communications agency (Fortune 500 client list) who themselves approached Pain Away. The marketing and sales outline is as follows.
Marketing Function
- A complete review and analysis of the topical analgesic market.
- Utilization of Triad Groups conducted with the professional community and general consumers. Purpose is to identify professional and consumer preferences.
- Based on research, create a product identity.
- From product identity, establish professional and consumer strategic directions, which would affect product design, packaging, advertising, consumer promotion, and product publicity.
- Test both professional and consumer strategic direction via two more Triad Groups.
- Develop launch marketing plan with all elements and budget for both professional and consumer.
- Actual implementation of the plan to include product design changes, packaging, advertising, consumer promotion, display, and product publicity.
Sales Function
Utilize a sales organization enabling direct-call coverage on the top 25 customers, which generally account for 80% of retail sales, and broker-managed coverage for the remainder. Launch plan would include a national sales meeting and all necessary materials.
Professional
Concentrate on the pharmacist community via co-op direct mail. Pharmacist recommendation at the purchase counter does affect sales.
The production process takes place in a standard homeopathic laboratory where raw materials are blended. There are no significant health or safety risks involved. Production orders are processed by purchase order for finished product. Some raw materials are usually on hand but more are ordered against purchase order requirements. Jars are ordered from a separate manufacturer and sent to the homeopathic laboratory to be filled, packaged, and shipped to Pain Away Ltd., where fulfillment is done.
The homeopathic laboratory has the capacity to fill all projected orders. As orders increase, Pain Away management will consider using a fulfillment service and more drop-shipping to wholesale customers. Cost of goods is estimated at 18% of gross sales. This figure has been consistent throughout production to date and is based on the complete production cycle.
There is no backlog.
Production Characteristics
The production process does not require any specialized or proprietary machinery. The critical factors in the production process are the highest quality of raw materials and the incubation process, which assures a stable finished product. Water is added to a base of vegetable/plant emollients. The eleven active ingredients are then mixed into the emulsion, which incubates for about 48 hours in large vats, while monitored for any fungal invasion. The finished product is then lab-tested for potency, which is done by lot number (the company gets lot samples). Filling is currently done by gravity-feed. The manufacturer might advance to computerized filling. One batch is 500 gallons. Lead time from order to packaged product is 4 weeks. Only a skilled and experienced manufacturer can produce the formula. Even other homeopathic manufacturers not familiar with a cream-based product would have difficulty with the production process. General topical analgesic manufacturers would need to become familiar with the raw materials and the production process in order to blend Pain Away's eleven active and ten inert ingredients. The company currently has one back-up manufacturer, which has never been used.
Labor Force and Employees
The company administrative staff consists of 5 people (recently reduced by 3) including the 3 officers. The two employees are paid an hourly wage. The staff are not unionized and there is no expectation of such. The labor supply in the region is more than sufficient to meet all future staffing needs. The sales force is comprised of independent agents who are paid on commission.

| Herbal Laboratories | 35,000 jars | all raw materials |
| Portland, Oregon | jars & caps | |
| labels | ||
| packaging | ||
| shipping boxes |
Currently, the laboratory procures all production materials. There are no shortages of key components, and multiple sources are available.
Subcontractors
All production is sub contracted out. Only fulfillment and shipping are done in-house. The company has formed a strong working relationship with Herbal Laboratories, which is the key subcontractor. Although management has selected a back-up manufacturer, the existing relationship with Herbal Labs has been more than satisfactory, so no change is foreseen. Other subcontractors supplying jars, labels, and boxes are used based upon price and service and can be replaced.
Standard office equipment is used for administrative functions. All production equipment at Herbal Laboratories is new and there is nothing that would cause production to be stopped for any appreciable time.
PROPERTY AND FACILITIES
The company facility is a single-story 1,950 square foot, cement block structure on about a two-acre cleared lot that is leased in one-year increments. The facility is located in northern Dutchess County, NY. All necessary commercial and industrial infrastructure is in place. The facility is easily accessible from major thoroughfares. The general area has been and is recovering from the closing of 2 large industrial facilities, so there's been anoticeable decline in property values. There is, however, a regional effort to re-direct the area to rely more upon small and entrepreneurial business. Management plans to purchase the building in order to add an appreciable fixed asset and to reduce expenses. The structure is easily expandable, so the company will not have to move during its critical growth stage.
PATENTS AND TRADEMARKS
Active homeopathics are not patentable. Management is exploring establishing a trademark and a formula patent.
RESEARCH AND DEVELOPMENT
The three principals have invested collectively $100,000, which has been capitalized. Plans for the immediate future include forming a research alliance with a university, hospital, or research group in order to develop a protocol for applying the "rigorous scientific standards" against which the effectiveness of Pain Away can be proven. Management has projected R&D expenses at $ 30,000 for the next 12-month period. These expenditures are intended for controlled studies proving effectiveness, and for continuation of developing applications for animals. Management is sales-marketing oriented and does not want to develop only a research lab. Any R&D will be designed to enhance sales and profits. Company management is currently investigating an SBIR grant.
There are no particular federal, state or local laws/regulations that affect the conduct of business. The manufacturer meets OSHA requirements, as does the Pain Away administrative facility. The FDA regulates homeopathy as an OTC non-prescription medicine. Pain Away's ingredients are in total compliance with FDA standards. Mr. Peale cultivated a working relationship with FDA representatives during the initial research and wisely intends to sustain such.
Product liability insurance is underwritten. A buy-sell agreement among officers exists but is not yet backed by insurance. Key employee insurance is also yet to be written.
All taxes are current. The company pays standard payroll, Social Security, and corporate taxes. The product is sales tax exempt in many states.
Company principals first formed an S-corporation under the name Peale Inc. The realization of the likelihood of international sales prompted management to form Pain Away Ltd. as the operational company. Peale Inc. serves a limited partnership which was formed to attract investors. Both companies are run by the same management team. All R&D is done through Peale Inc. There is comingling of funds. This proposal seeks financing for Pain Away Ltd. Return on the investment will derive from the sale of the product Pain Away itself and any other products which the company sells.
Pain Away Ltd. is a member of the Homeopathic Manufacturers Association. The officers were invited to participate in an annual meeting of the newly formed FDA committee on natural medicines. This committee works on the bases for regulations, compliance, and claims for the natural ingredient industry, covering vitamins, herbs, and homeopathy.
Management subscribes to the following publications:
- Homeopathy Today
- Natural Foods Merchandiser
- American Health
- Prevention Magazine
- Let's Live
- New England Journal of Medicine letter
Directors and Officers
A board of directors will be developed in the near future. There is interest from the medical, nutritional, and professional sports communities, as well as from a local bank. Officers are:
Robert Peale - CEO Alana Curtis - President Ryan Lemon - Vice-President, Marketing
Profit and loss responsibilities are shared by the officers.
The officers are primary key employees (backgrounds in executive summary). Other key employees include:
Key Employees
Leslie Ottaviani - bookkeeper and office manager - known by management for 5 years and described as "a dedicated innovator with a true grasp for details." She has experience supervising 20 employees in the accounting department of Worldwide Airlines and has worked as an independent bookkeeper for several companies in Hudson Valley, NY.
Julia Allen - administrative assistant - known by management for 6 years and described as "having people and problem-solving skills and works incredibly well under pressure." Her background includes sales in a successful business which included business consulting.
Remuneration
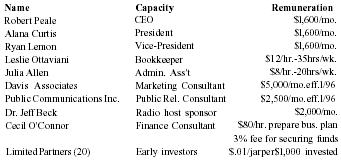
| Robert Peale | CEO | $1,600/mo. |
| Alana Curtis | President | $1,600/mo. |
| Ryan Lemon | Vice-President | $1,600/mo. |
| Leslie Ottaviani | Bookkeeper | $12/hr.-35hrs/wk. |
| Julia Allen | Admin. Ass't | $8/hr.-20hrs/wk. |
| Davis Associates | Marketing Consultant | $5,000/mo.eff. 1/96 |
| Public Communications Inc. | Public Rel. Consultant | $2,500/mo.eff. 1/96 |
| Dr. Jeff Beck | Radio host sponsor | $2,000/mo. |
| Cecil O'Connor | Finance Consultant | $80/hr. prepare bus. plan |
| 3% fee for securing funds | ||
| Limited Partners (20) | Early investors | $.01/jarper $1,000 invested |
Accountant and Banker
| Jonathan Wainwright | Accountant | no retained\fee for service only |
| Arnold Lee | Banker | no remuneration |
All other fees paid on an ad hoc basis. Different attorneys have been used on an ad hoc basis (finance closing fees will be paid by the company).
Principal Shareholders
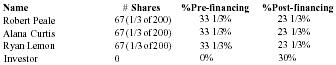
| Robert Peale | 67 (1/3 of 200) | 33 1/3% | 23 1/3% |
| Alana Curtis | 67 (1/3 of 200) | 33 1/3% | 23 1/3% |
| Ryan Lemon | 67 (1/3 of 200) | 33 1/3% | 23 1/3% |
| Investor | 0 | 0% | 30% |
Proposed Financing
Management is willing to negotiate any structure which suits the investor. The company is seeking an equity investor. Management will provide a seat on the company's board of directors. Ongoing reports of key ratios, profit-loss statements, balance sheets, and annual audits would be provided to the investor. It is management's intent that the investor will enjoy returns on investment in excess of that of alternative investments, as a privately held company, while providing investor liquidity of his investment by taking the company public at its earliest opportunity.
Capital Structure
The existing capital structure includes a $50,000 unsecured line of credit with Poughkeepsie National Savings Bank. This line of credit was just brought to maturity in 1/96 for a 30-day period, at which time the line was renewed. If the current financing proposal is accomodated, then the line of credit can be increased.
Additional financing to date has derived from the sale of limited partnerships offering $.01 per 3.7 oz. jar royalty for every $1,000 invested. Each limited partner has been given the right to convert his/her capital investment into common stock when the company goes public, or, to receive back his/her original capital investment when the company goes public. Total amount of financing raised through the limited partnership is $100,000.
As mentioned earlier, officers have collectively invested about $100,000 in the company, mostly through the R&D phase. Officers' "sweat equity" is immeasurable.
As stated in the executive summary: Advertising & promotion campaign - $1,200,000 (see below); Market research - $300,000. The company anticipates the need for follow-on financing after 24 months of business.

| Magazines | $330,000 |
| Radio | $200,000 |
| Shows & Conventions | $140,000 |
| TV | $400,000 |
| Retail Shops | $70,000 |
| Sample-POP Display | $60,000 |
Management intends to preserve cash flow by factoring much of the receivables. With the current lead time of 4 weeks, however, some capital may be used to increase merchandising inventory in order to fulfill initial large orders. It is hoped that any follow-on financing can and will be debt financing, serviced by cash flow.
The following table sets forth the capitalization of Pain Away Ltd. as of 12/31/95 and as adjusted to reflect the proposed sale of common stock.

| Common Stock, no par value, | (167,268) | 1,332,732 |
| 200 shares authorized; | ||
| 0 outstanding | ||
| Additional Paid-in Capital | 1,500,000 | |
| Accumulated Earnings (deficit) | (167,268) | (167,268) |
| Total Stockholders' Equity | (334,536) | 1,165,464 |
Dilution: The net tangible book value of the company as of 12/31/95 was minus $1,673 per share. Without taking into account any other changes in such net tangible book value after 12/31/96, other than to give effect to the sale of 60 shares (proposed 30% equity share) hereby, the pro forma net tangible book value of the company on 12/31/95 will be $5,827 per share, representing an immediate dilution of $13,597 per share to new investors.

| Price per share to Investor | 19,424 |
| Net tangible book value before the sale | (1,673) |
| Increase attributable to new investor | 7,500 |
| Pro forma net tangible book value after the purchase | 5,827 |
Management recognizes that this proposed financing implies a large premium value on the existing equity and so will negotiate any other conditions which would induce the investor to make the investment.
At the time of the company's IPO, limited partners who opt for common stock will receive their shares from the officers' share of owned stock. The negotiated ownership held by the investor will not be further diluted.
Investor Involvement
Management seeks a close working relationship with the investor. The investor will be given one seat on the board of directors. Management would solicit consultations (for a fee) on financial matters, or any other area of investor expertise (e.g., planning, management development), but voting power is not an option. Fees will also be paid for any future financing and/or profitable business connections arranged by the investor.
Limited Operating History
Even though management feels that the company is at first-stage expansion, it is definitely still an early-stage company. Two obvious risks inherent in early-stage companies are undercapitalization and poor liquidity. Management has capitalized the business operations to date well enough to have developed the product and identified penetrable market segments. The current proposed financing will provide enough capital to handle the anticipated growth.
Limited Resources
Management believes that it has the resources to continue at the present pace of business. An anticipated increase in sales through advertising media such as QVC , regional/national catalogues, retail outlets, and some European distribution can be financed by factoring. These "bootstrapping" approaches have sustained the company to date and will accommodate slow growth. Management believes, however, that more rapid expansion is desirable in order to penetrate its identified market segments. More rapid expansion requires more resources.
Limited Management Experience
All officers have successful backgrounds in marketing. Additional experience in manufacturing/distribution has been gained in the past nine years of product development. Management has consistently shown a willingness to leverage themselves with accomplished professional consulting relationships. The company culture is one which reinforces sharing of expertise with mutual benefit to all concerned.
Market Uncertainties
Any consumer product business is subject to the changing preferences of the marketplace. As presented in the marketing section of this proposal, the target markets are showing substantial growth, which limits uncertainty. There is currently a growing consumer preference for homeopathic topical remedies. More uncertainty is evident when considering competition, but can be made tolerable by on-going research and analysis.
Production Uncertainties
The only uncertainty at present is whether or not the lead time (4 weeks) from purchase order to finished product can consistently be reduced. This uncertainty is of material concern as sales increase. Herbal Laboratories is a sound company with a promising long-term future and has always been customer-friendly, so no more serious uncertainties exist at present. Management believes that vertical integration of manufacturing is feasible in the long-term but is not practical in the near-term.
Liquidation
In the event that liquidation becomes necessary, management believes that the most value could be realized from the sale of the product formula itself. The formula is not patented, so valuation remains uncertain. However, the sales history, along with the testimonials attesting to the effectiveness of this "ready-made" product, should determine value. Office equipment would yield limited value, and unless the company building was purchased prior to liquidation, no value would be realized. Management believes that the company can and will generate increasing value in the near future, evidenced by increasing sales.
Dependence on Key Management
At present, CEO Robert Peale is considered the primary key manager/officer. His knowledge of the product ingredients, his history of public appearances promoting the product, his increasing recognition by the health community as an expert in natural medicine, and his charisma as a business professional highlight his key role. Managerially, the other officers are thoroughly competent and could manage the company and market its products without Mr. Peale. At this critical early stage, however, the product needs an identity and a market position before the loss of any key managers could be overcome. Once the premier product is securely launched and the product line is expanded, the loss of any officer could be absorbed by continued proper management of the company. Management believes that such a development is not far off, once the company is properly capitalized. Until such time, key person life insurance will be purchased.
What Could Go Wrong?
Upgraded advertising campaigns could not lead to any substantial increase in sales. This problem can be avoided by using experienced advertising/marketing consultants who have familiarity with the targeted markets. Furthermore, properly designed test runs on any advertising campaign would provide objective indicators of expected returns. Capital investment in advertising should be gradual and progressively based upon certain expected levels of return.
Stronger competition could capitalize on and stall Pain Away's early success by replicating the product and its marketing strategy. This problem can be solved in two ways: First, with proper capitalization, Pain Away can make an entry into targeted markets rapidly and with enough strength to grab market share. Keeping market share can be easier than getting it. This market requires extensive advertising. Increasing market share could mean an increasing advertising budget. An increasing advertising budget can easily reduce profit margin, so strategic planning is required. The second way to solve the competition problem is in the formula itself. Management will seek to patent the formula. The nature of the homeopathic ingredients is likely to inhibit any mainstream non-homeopathic company from replicating the product. Acquisition of a homeopathic company would make more sense. Narrowing the competition, then, to other homeopathic companies gives Pain Away more of a fighting chance, since its formula is more sophisticated and user-friendly than any homeopathic topical analgesic on the market.
Governmental controls could conceivably impede sales. This problem is unlikely because the ingredients are already FDA-approved. Furthermore, management's participation in the FDA committee to develop regulatory standards for the natural medicine field would provide early warnings of any such prohibitory controls.
The company could be controlled by non-investor stockholders. This problem is not likely to develop because the management team would hold a majority. Management is dedicated to the principles of increasing value and profits and is confident that its efforts will be in concert with those of the investor.
RETURN ON INVESTMENT ANDEXIT
Public offering.
Management plans for an IPO in 5-7 years. The investor's shares would be sold to provide the targeted return on investment. Should there be no public market, then a buy back would occur.
Management will negotiate a buy back formula with the investor and will target milestones in planning for this possibility. Management aims for returning 6 times the original investment in five years.
ANALYSIS OF OPERATIONS AND PROJECTIONS
The business has not shown a profit since sales activity began in May 1994. This lack of profit is not unusual for an early-stage company. Losses were incurred in the start-up phase, where the objective was to get consumers to try the product. Gross profit margins have remained stable, however. Management focus was targeted on getting professionals and consumers to try the product in order to collect anecdotal evidence and testimonials of its effectiveness. Not enough focus was on asset management, as evidenced by a low return on assets ratio (p.32). Now that the product has gotten some recognition, especially in professional circles, the focus will shift toward mass marketing. Management intends to improve inventory management by using factoring of receivables in conjunction with JIT inventory control. As sales volume increases, drop-shipping from plant to wholesale customer, will also be arranged.
Balance Sheet
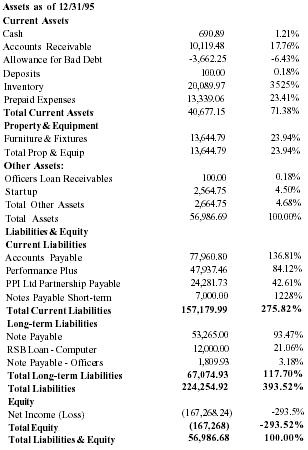
| Cash | 690.89 | 1.21% |
| Accounts Receivable | 10,119.48 | 17.76% |
| Allowance for Bad Debt | −3,662.25 | −6.43% |
| Deposits | 100.00 | 0.18% |
| Inventory | 20,089.97 | 35.25% |
| Prepaid Expenses | 13,339.06 | 23.41% |
| 40,677.15 | 71.38% | |
| Furniture & Fixtures | 13,644.79 | 23.94% |
| Total Prop & Equip | 13,644.79 | 23.94% |
| Officers Loan Receivables | 100.00 | 0.18% |
| Startup | 2,564.75 | 4.50% |
| Total Other Assets | 2,664.75 | 4.68% |
| Total Assets | 56,986.69 | 100.00% |
| Accounts Payable | 77,960.80 | 136.81% |
| Performance Plus | 47,937.46 | 84.12% |
| PPI Ltd Partnership Payable | 24,281.73 | 42.61% |
| Notes Payable Short-term | 7,000.00 | 12.28% |
| Note Payable | 53,265.00 | 93.47% |
| RSB Loan - Computer | 12,000.00 | 21.06% |
| Note Payable - Officers | 1,809.93 | 3.18% |
| Net Income (Loss) | (167,268.24) | −293.5% |
Monthly Income Statements 1995
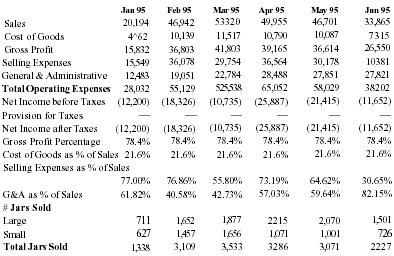
| Sales | 20,194 | 46,942 | 53,320 | 49,955 | 46,701 | 33,865 |
| Cost of Goods | 4,362 | 10,139 | 11,517 | 10,790 | 10,087 | 7,315 |
| Gross Profit | 15,832 | 36,803 | 41,803 | 39,165 | 36,614 | 26,550 |
| Selling Expenses | 15,549 | 36,078 | 29,754 | 36,564 | 30,178 | 10,381 |
| General & Administrative | 12,483 | 19,051 | 22,784 | 28,488 | 27,851 | 27,821 |
| 28,032 | 55,129 | 525,538 | 65,052 | 58,029 | 38,202 | |
| Net Income before Taxes | (12,200) | (18,326) | (10,735) | (25,887) | (21,415) | (11,652) |
| Provision for Taxes | — | — | — | — | — | — |
| Net Income after Taxes | (12,200) | (18,326) | (10,735) | (25,887) | (21,415) | (11,652) |
| Gross Profit Percentage | 78.4% | 78.4% | 78.4% | 78.4% | 78.4% | 78.4% |
| Cost of Goods as % of Sales | 21.6% | 21.6% | 21.6% | 21.6% | 21.6% | 21.6% |
| Selling Expenses as % of Sales | 77.00% | 76.86% | 55.80% | 73.19% | 64.62% | 30.65% |
| G&A as % of Sales | 61.82% | 40.58% | 42.73% | 57.03% | 59.64% | 82.15% |
| Large | 711 | 1,652 | 1,877 | 2,215 | 2,070 | 1,501 |
| Small | 627 | 1,457 | 1,656 | 1,071 | 1,001 | 726 |
| 1,338 | 3,109 | 3,533 | 3,286 | 3,071 | 2,227 | |
Income Statement - 12/31/95
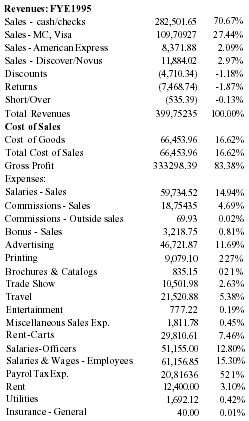
| Sales - cash/checks | 282,501.65 | 70.67% |
| Sales - MC, Visa | 109,709.27 | 27.44% |
| Sales - American Express | 8,371.88 | 2.09% |
| Sales - Discover/Novus | 11,884.02 | 2.97% |
| Discounts | (4,710.34) | −1.18% |
| Returns | (7,468.74) | −1.87% |
| Short/Over | (535.39) | −0.13% |
| Total Revenues | 399,752.35 | 100.00% |
| Cost of Goods | 66,453.96 | 16.62% |
| Total Cost of Sales | 66,453.96 | 16.62% |
| Gross Profit | 333,298.39 | 83.38% |
| Expenses: | ||
| Salaries - Sales | 59,734.52 | 14.94% |
| Commissions - Sales | 18,754.35 | 4.69% |
| Commissions - Outside sales | 69.93 | 0.02% |
| Bonus - Sales | 3,218.75 | 0.81% |
| Advertising | 46,721.87 | 11.69% |
| Printing | 9,079.10 | 2.27% |
| Brochures & Catalogs | 835.15 | 0.21% |
| Trade Show | 10,501.98 | 2.63% |
| Travel | 21,520.88 | 5.38% |
| Entertainment | 777.22 | 0.19% |
| Miscellaneous Sales Exp. | 1,811.78 | 0.45% |
| Rent-Carts | 29,810.61 | 7.46% |
| Salaries-Officers | 51,155.00 | 12.80% |
| Salaries & Wages - Employees | 61,156.85 | 15.30% |
| Payrol Tax Exp. | 20,816.36 | 5.21% |
| Rent | 12,400.00 | 3.10% |
| Utilities | 1,692.12 | 0.42% |
| Insurance - General | 40.00 | 0.01% |
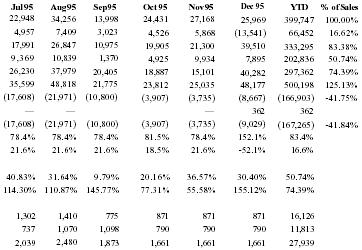
| 22,948 | 34,256 | 13,998 | 24,431 | 27,168 | 25,969 | 399,747 | 100.00% |
| 4,957 | 7,409 | 3,023 | 4,526 | 5,868 | (13,541) | 66,452 | 16.62% |
| 17,991 | 26,847 | 10,975 | 19,905 | 21,300 | 39,510 | 333,295 | 83.38% |
| 9,369 | 10,839 | 1,370 | 4,925 | 9,934 | 7,895 | 202,836 | 50.74% |
| 26,230 | 37,979 | 20,405 | 18,887 | 15,101 | 40,282 | 297,362 | 74.39% |
| 35,599 | 48,818 | 21,775 | 23,812 | 25,035 | 48,177 | 500,198 | 125.13% |
| (17,608) | (21,971) | (10,800) | (3,907) | (3,735) | (8,667) | (166,903) | −41.75% |
| — | — | — | 362 | 362 | |||
| (17,608) | (21,971) | (10,800) | (3,907) | (3,735) | (9,029) | (167,265) | −41.84% |
| 78.4% | 78.4% | 78.4% | 81.5% | 78.4% | 152.1% | 83.4% | |
| 21.6% | 21.6% | 21.6% | 18.5% | 21.6% | −52.1% | 16.6% | |
| 40.83% | 31.64% | 9.79% | 20.16% | 36.57% | 30.40% | 50.74% | |
| 114.30% | 110.87% | 145.77% | 77.31% | 55.58% | 155.12% | 74.39% | |
| 1,302 | 1,410 | 775 | 871 | 871 | 871 | 16,126 | |
| 737 | 1,070 | 1,098 | 790 | 790 | 790 | 11,813 | |
| 2,039 | 2,480 | 1,873 | 1,661 | 1,661 | 1,661 | 27,939 |
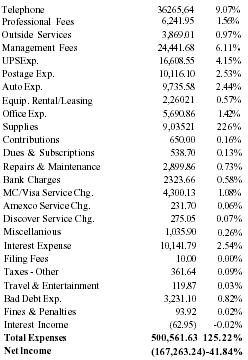
| Telephone | 36,265,64 | 9.07% |
| Professional Fees | 6,241.95 | 1.56% |
| Outside Services | 3,869.01 | 0.97% |
| Management Fees | 24,441.68 | 6.11% |
| UPS Exp. | 16,608.55 | 4.15% |
| Postage Exp. | 10,116.10 | 2.53% |
| Auto Exp. | 9,735.58 | 2.44% |
| Equip. Rental/Leasing | 2,260.21 | 0.57% |
| Office Exp. | 5,690.86 | 1.42% |
| Supplies | 9,035.21 | 2.26% |
| Contributions | 650.00 | 0.16% |
| Dues & Subscriptions | 538.70 | 0.13% |
| Repairs & Maintenance | 2,899.86 | 0.73% |
| Bank Charges | 2,323.66 | 0.58% |
| MC/Visa Service Chg. | 4,300.13 | 1.08% |
| Amexco Service Chg. | 231.70 | 0.06% |
| Discover Service Chg. | 275.05 | 0.07% |
| Miscellanious | 1,035.90 | 0.26% |
| Interest Expense | 10,141.79 | 2.54% |
| Filing Fees | 10.00 | 0.00% |
| Taxes - Other | 361.64 | 0.09% |
| Travel & Entertainment | 119.87 | 0.03% |
| Bad Debt Exp. | 3,231.10 | 0.82% |
| Fines & Penalties | 93.92 | 0.02% |
| Interest Income | (62.95) | −0.02% |
Key Ratio Analysis
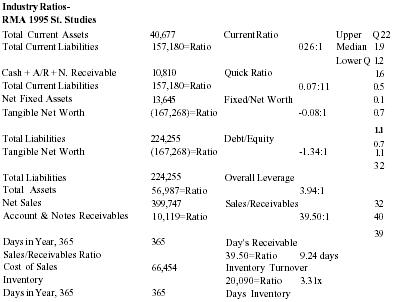
| Total Current Assets | 40,677 | Current Ratio | Upper | Q2.2 | |
| Total Current Liabilities | 157,180=Ratio | 026:1 | Median | 1.9 | |
| Lower Q | 1.2 | ||||
| Cash + A/R + N. Receivable | 10,810 | Quick Ratio | 1.6 | ||
| Total Current Liabilities | 157,180=Ratio | 0.07:11 | 0.5 | ||
| Net Fixed Assets | 13,645 | Fixed/Net Worth | 0.1 | ||
| Tangible Net Worth | (167,268)=Ratio | −0.08:1 | 0.7 | ||
| 1.1 | |||||
| Total Liabilities | 224,255 | Debt/Equity | 0.7 | ||
| Tangible Net Worth | (167,268)=Ratio | −1.34:1 | 1.1 | ||
| 3.2 | |||||
| Total Liabilities | 224,255 | Overall Leverage | |||
| Total Assets | 56,987=Ratio | 3.94:1 | |||
| Net Sales | 399,747 | Sales/Receivables | 32 | ||
| Account & Notes Receivables | 10,119=Ratio | 39.50:1 | 40 | ||
| 39 | |||||
| Days in Year, 365 | 365 | Day's Receivable | |||
| Sales/Receivables Ratio | 39.50=Ratio | 9.24 days | |||
| Cost of Sales | 66,454 | Inventory Turnover | |||
| Inventory | 20,090=Ratio | 3.31x | |||
| Days in Year, 365 | 365 | Days Inventory | |||

| Cost of Goods/Inventory Ratio | 3.31 = Ratio | 110.34 days | |||
| Net Sales | 399,747 | Sales to Working Capital | |||
| Curr. Assets - Curr. Liabilities | (116,503) = Ratio | −3.43x | RMA Ratios | NA | |
| Net Profit + Depr. + Amort. | (167,268) | Cash Flow/Long Term Debt | |||
| Current Portion L T Debt | 7,000 = Ratio | −23.90x | |||
| Profit, before Tax | (167,268) | Return on Equity | |||
| Tangible Net Worth (Equity) | (167,268) = Ratio | 1.00% | |||
| Profit, before Tax | (167,268) | Return on Assets | |||
THIS PORTION OF PAGE INTENTIONALLY LEFT BLANK SEE NEXT PAGE FOR PROJECTED CASH FLOW TABLE
Projected Cash Flow
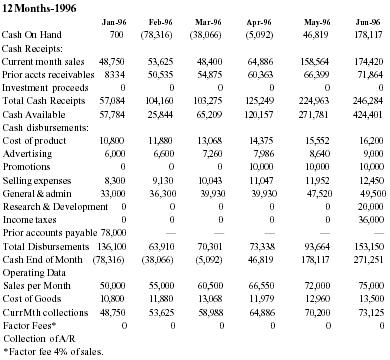
| *Factor fee 4% of sales. | ||||||
| Cash On Hand | 700 | (78,316) | (38,066) | (5,092) | 46,819 | 178,117 |
| Cash Receipts: | ||||||
| Current month sales | 48,750 | 53,625 | 48,400 | 64,886 | 158,564 | 174,420 |
| Prior accts receivables | 8,334 | 50,535 | 54,875 | 60,363 | 66,399 | 71,864 |
| Investment proceeds | 0 | 0 | 0 | 0 | 0 | 0 |
| Total Cash Receipts | 57,084 | 104,160 | 103,275 | 125,249 | 224,963 | 246,284 |
| Cash Available | 57,784 | 25,844 | 65,209 | 120,157 | 271,781 | 424,401 |
| Cash disbursements: | ||||||
| Cost of product | 10,800 | 11,880 | 13,068 | 14,375 | 15,552 | 16,200 |
| Advertising | 6,000 | 6,600 | 7,260 | 7,986 | 8,640 | 9,000 |
| Promotions | 0 | 0 | 0 | 10,000 | 10,000 | 10,000 |
| Selling expenses | 8,300 | 9,130 | 10,043 | 11,047 | 11,952 | 12,450 |
| General & admin | 33,000 | 36,300 | 39,930 | 39,930 | 47,520 | 49,500 |
| Research & Development | 0 | 0 | 0 | 0 | 0 | 20,000 |
| Income taxes | 0 | 0 | 0 | 0 | 0 | 36,000 |
| Prior accounts payable | 78,000 | — | — | — | — | — |
| Total Disbursements | 136,100 | 63,910 | 70,301 | 73,338 | 93,664 | 153,150 |
| Cash End of Month | (78,316) | (38,066) | (5,092) | 46,819 | 178,117 | 271,251 |
| Operating Data | ||||||
| Sales per Month | 50,000 | 55,000 | 60,500 | 66,550 | 72,000 | 75,000 |
| Cost of Goods | 10,800 | 11,880 | 13,068 | 11,979 | 12,960 | 13,500 |
| Curr Mth collections | 48,750 | 53,625 | 58,988 | 64,886 | 70,200 | 73,125 |
| Factor Fees* | 0 | 0 | 0 | 0 | 0 | 0 |
| Collection of A/R | ||||||
Projected Annual Financial Statements
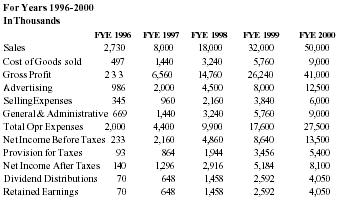
| Sales | 2,730 | 8,000 | 18,000 | 32,000 | 50,000 |
| Cost of Goods sold | 497 | 1,440 | 3,240 | 5,760 | 9,000 |
| Gross Profit | 2,233 | 6,560 | 14,760 | 26,240 | 41,000 |
| Advertising | 986 | 2,000 | 4,500 | 8,000 | 12,500 |
| Selling Expenses | 345 | 960 | 2,160 | 3,840 | 6,000 |
| General & Administrative | 669 | 1,440 | 3,240 | 5,760 | 9,000 |
| Total Opr Expenses | 2,000 | 4,400 | 9,900 | 17,600 | 27,500 |
| Net Income Before Taxes | 233 | 2,160 | 4,860 | 8,640 | 13,500 |
| Provision for Taxes | 93 | 864 | 1,944 | 3,456 | 5,400 |
| Net Income After Taxes | 140 | 1,296 | 2,916 | 5,184 | 8,100 |
| Dividend Distributions | 70 | 648 | 1,458 | 2,592 | 4,050 |
| Retained Earnings | 70 | 648 | 1,458 | 2,592 | 4,050 |
Assumptions

| % Cost of Goods Sold | 18% |
| % Selling Expenses | 12% |
| % General & Administrative | 18% |
| % Tax Provision | 40% |
| Dividends - of NATP | 50% |
| Advertising -1996 | 40% |
| Advertising - all other years | 25% |
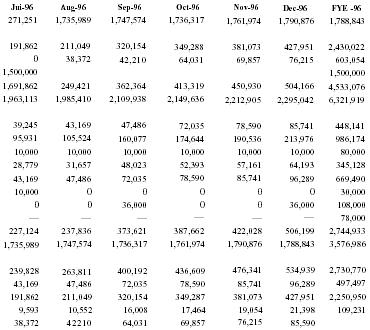
| 271,251 | 1,735,989 | 1,747,574 | 1,736,317 | 1,761,974 | 1,790,876 | 1,788,843 |
| 191,862 | 211,049 | 320,154 | 349,288 | 381,073 | 427,951 | 2,430,022 |
| 0 | 38,372 | 42,210 | 64,031 | 69,857 | 76,215 | 603,054 |
| 1,500,000 | 1,500,000 | |||||
| 1,691,862 | 249,421 | 362,364 | 413,319 | 450,930 | 504,166 | 4,533,076 |
| 1,963,113 | 1,985,410 | 2,109,938 | 2,149,636 | 2,212,905 | 2,295,042 | 6,321,919 |
| 39,245 | 43,169 | 47,486 | 72,035 | 78,590 | 85,741 | 448,141 |
| 95,931 | 105,524 | 160,077 | 174,644 | 190,536 | 213,976 | 986,174 |
| 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 80,000 |
| 28,779 | 31,657 | 48,023 | 52,393 | 57,161 | 64,193 | 345,128 |
| 43,169 | 47,486 | 72,035 | 78,590 | 85,741 | 96,289 | 669,490 |
| 10,000 | 0 | 0 | 0 | 0 | 0 | 30,000 |
| 0 | 0 | 36,000 | 0 | 0 | 36,000 | 108,000 |
| — | — | — | — | — | — | 78,000 |
| 227,124 | 237,836 | 373,621 | 387,662 | 422,028 | 506,199 | 2,744,933 |
| 1,735,989 | 1,747,574 | 1,736,317 | 1,761,974 | 1,790,876 | 1,788,843 | 3,576,986 |
| 239,828 | 263,811 | 400,192 | 436,609 | 476,341 | 534,939 | 2,730,770 |
| 43,169 | 47,486 | 72,035 | 78,590 | 85,741 | 96,289 | 497,497 |
| 191,862 | 211,049 | 320,154 | 349,287 | 381,073 | 427,951 | 2,250,950 |
| 9,593 | 10,552 | 16,008 | 17,464 | 19,054 | 21,398 | 109,231 |
| 38,372 | 42,210 | 64,031 | 69,857 | 76,215 | 85,590 |
User Contributions:
Comment about this article, ask questions, or add new information about this topic:.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Pharmaceutical Business Plan

Related papers
Health spending accounted for 9.3% of GDP on average across OECD countries in 2011. The Pharmaceutical expenditure, as a percentage of total expenditure on health, accounted for 15%. The pharmaceutical sector is highly regulated. On describe the major characteristics of the world pharmaceutical industry as one increased globalization, changing structure of competition and increased competitiveness. This are growing pressures on discovery and development. Drug liabilities become more frequent and more costly. The pharmaceutical industry is under immense pressure by external and internal stakeholders. Government and National Health Services are monopsonic practices. Pharmaceutical companies are criticised for high prices, over-intensive sales and marketing activities, presents to medical doctors, clinical trials and industry – government alliances. Lawyers, medical journals, physicians, politicians, and the media use product liabilities and marketing activities to denounce pharmaceutical companies as culprits. The pharmaceutical sector needs to demonstrate responsibility and take steps to increase awareness. Transparency would increase the credibility of the pharmaceutical industry. Corporate governance will prevent corruption by being in compliance with the legislation and establishing their own internal policies designed to prevent corruption. All firms will act more responsibly. In order to rebuild the trust the industry needs to work together and quickly.
Continuous innovation is one of the pharmaceutical industry's most defi ning characteristics. New medications can be crucial for maintaining the quality of human life, and may even affect its duration. The sales potential is staggering: the global pharmaceutical market is expected to reach $1.1 trillion by 2015. The pressure to succeed is tremendous. Yet, pharmaceutical innovation is hardly an orderly, predictable process. It follows a technology-push model dependent on a meandering path of scientifi c breakthroughs with uneven timing and hard to foresee outcomes. Technological competency, decades of rigorous research, and profound understanding of unmet customer needs, while necessary, may prove insuffi cient for market success as the critical decision for commercialization remains outside the fi rm. Drug innovation as a business process requires savvy strategic, organizational, and managerial decisions. It is already enjoying intensive research coverage, giving rise to abundant but relatively dispersed knowledge of the mechanisms driving drug discovery and development. In this chapter, we present a comprehensive overview of the process of drug innovation from a business and academic perspective. We discuss the evolving organizational forms and models for collaboration, summarize signifi cant empirical regularities, and highlight differences in market positions related to fi rms' strategic orientation, innovation emphasis, attitudes to risk, and specialized resources. As a guide to future research, critical drivers and modes for drug innovation are systematized in a unifying framework of characteristics and process decisions, and multiple areas in need of further scrutiny, analysis, and optimization are suggested. Because of its rich potential and high signifi cance, research on drug innovation seems poised to gain increasing momentum in the years to come.
Marketing Intelligence & Planning, 2009
Journal of Personal Selling and Sales Management, 2008
A critical exploration of the history and present dynamics of drug advertising in the United States reveals how advertising practices exemplify the extent to which late capitalist market rationality impacts on popular under- standings of medicine, well being, and disease. An historical overview of drug advertising and regulation thereof contextualizes a closer analysis of the ways in which the pharmaceutical industry acts to maximize profits through marketing efforts and the creation of diseases as platforms for the expansion of drug product markets. In this way U.S. drug manufacturers operate on a variety of communicative levels to ensure that their promotional messages are among the most widely disseminated. As medical and pharmaceutical technology develops and plays an increasingly pronounced role in everyday life from casual to more profound levels via the mass media, a critical and historical understanding of how and for whom it acts may become a vital focus of future inquiry on social and communicative phenomena and processes.
Entrepreneurship Theory and Practice, 2010
Opt-e-scrip, Inc., has developed a patented test for determining the efficacy of drugs in individual patients so that doctors can prescribe the drug that really works for each person. In addition, test results show that older, less expensive drugs are often as or more efficacious than the newer, expensive drugs pushed by manufacturers. It, thus, benefits physicians, patients, and drug benefit payers. However, the company has had difficulty entering the market, given its complexity and their lack of resources. The test remains a product in search of an application and a customer willing to pay for its usage.
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
Innovation and internationalization, 2004
Clinical Therapeutics, 2003
Marketing Letters, 2005
Journal of Pharmaceutical Marketing & Management, 2004
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024





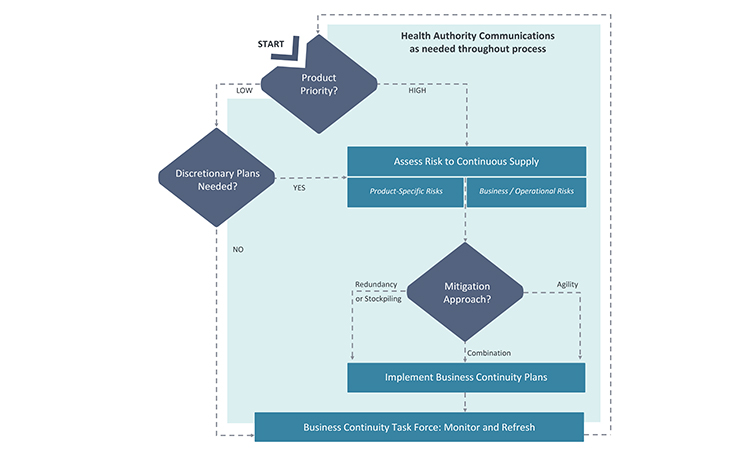
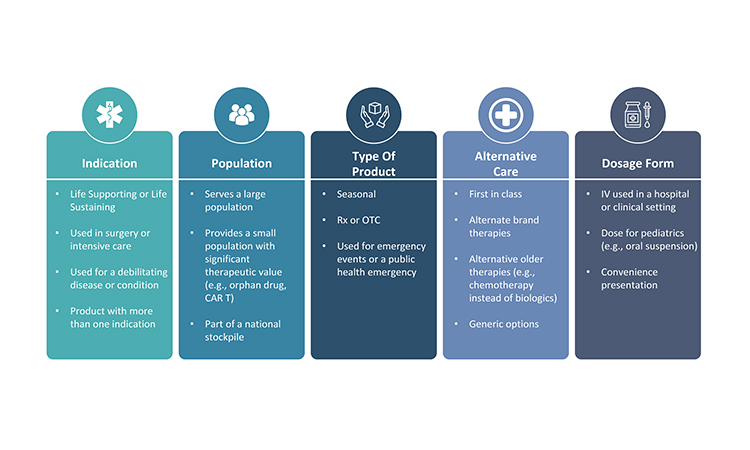
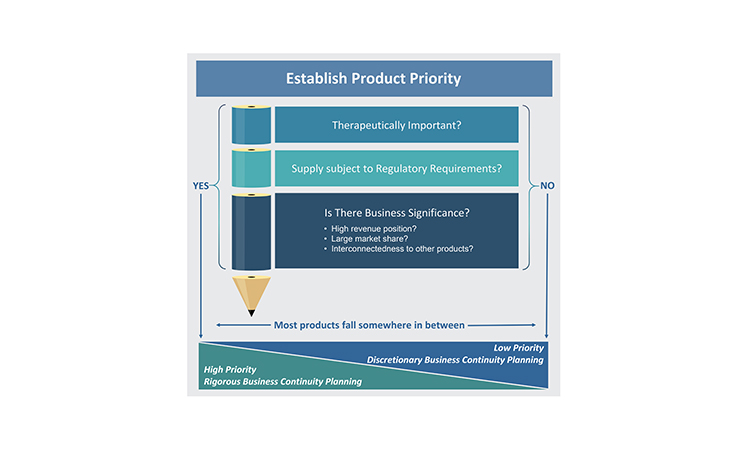
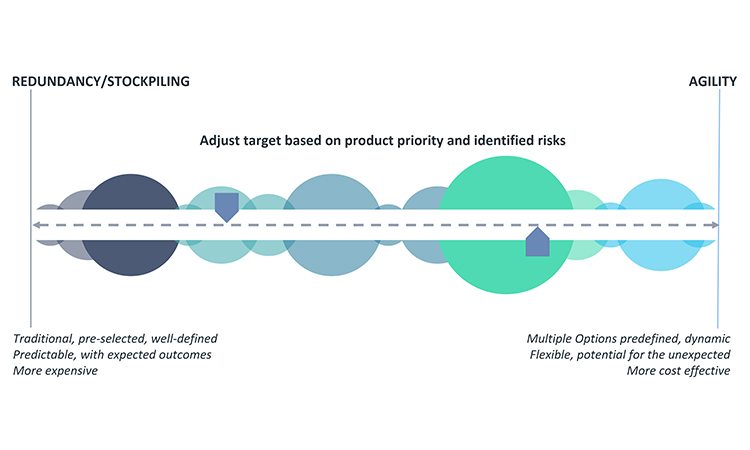
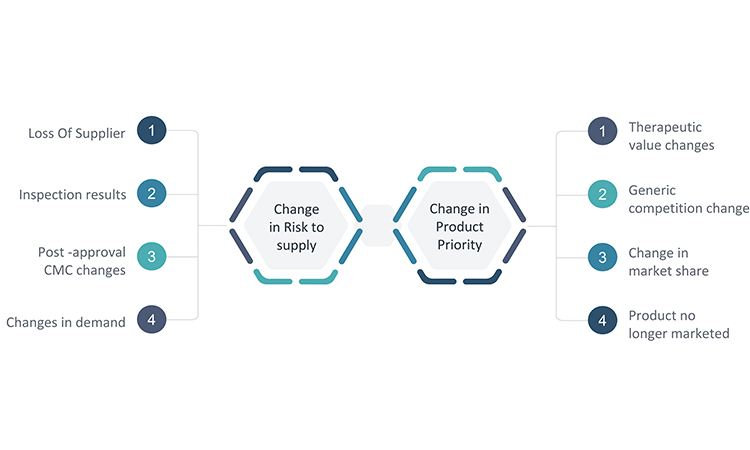




)
)
)
)
)

IMAGES
COMMENTS
Traditionally, a marketing plan includes the four P's: Product, Price, Place, and Promotion. For a pharmaceutical company, your marketing strategy should include the following: Product: In the product section, you should reiterate the type of pharmaceutical business that you documented in your company overview.
Below is the sales projection for Harry Tancredo® Pharmaceuticals, LLC, it is based on the location of our business and other factors as it relates to small scale and medium scale generic pharmaceutical manufacturing company start - ups in the United States; First Fiscal Year-: $250,000. Second Fiscal Year-: $550,000.
A business plan has 2 main parts: a financial forecast outlining the funding requirements of your pharmaceutical manufacturer and the expected growth, profits and cash flows for the next 3 to 5 years; and a written part which gives the reader the information needed to decide if they believe the forecast is achievable.
ClickUp's Business Plan Template for Pharmaceutical Companies provides a comprehensive solution for creating and managing your pharmaceutical business plan. Here are the main elements of this template: Custom Statuses: Keep track of the progress of each section of your business plan with statuses like Complete, In Progress, Needs Revision, and ...
Research. Doing the necessary research is a vital step to starting your own pharmaceutical company. It is important to dive deep into the industry, research potential competitors, and understand the regulations that may affect the business. Research will help establish a strong foundation for a successful business model.
The next step to start a pharmaceutical manufacturing business is to choose the company's market positioning. Market positioning refers to the place your product and service offering occupies in customers' minds and how it differs from how competitors are perceived. Being perceived as a high-end solution, for example.
How to Start a Pharma Company. David Blok | Posted on September 19, 2023 | Updated on March 26, 2024. Introduction. Step 1: Market Research and Pharmaceutical Business Ideas. Step 2: Creating a Business Plan. Step 3: Regulatory Compliance and Legalities. Step 4: Team Building and Talent Acquisition. Step 5: Location and Infrastructure.
pharmaceutical products including African Traditional Medicines that are relevant to local health problems. The pharmaceutical programme is one of the priorities under the RISDP. 1.3.3 It is against this background that a Business Plan has been developed in order to operationalize the Programme. The Plan has six main sections which include:
Biopharma M&A deal value more than doubled between 2017 and 2019, from $138 billion to $336 billion, and valuations reached all-time highs. Most of those deals involved midsized biotech companies, for which the average premium paid was close to 70%, with an average EV/sales multiple of nearly 8x. All in all, close to 60% of new therapeutic ...
Building a new facility can cost up to $2 billion and take 5 to 10 years. Expanding existing facilities, transferring a single product to a new manufacturing site or retrofitting an existing facility for a new product can take several years. As large-scale manufacturing processes are developed, companies develop plans for sourcing all the ...
Encyclopedia of Business, 2nd ed. Pharmaceutical Company Business Plan: Business Plans - Volume 03. Toggle navigation. ... Pharmaceutical Company Business Plan; Pharmaceutical Company Photo by: mangostock. BUSINESS PLAN ... The manufacturing is sub-contracted out to a highly respected FDA-licensed manufacturer of homeopathic products.
Research and development: Since it takes an average of 10 years for a drug to reach the market, and typically six to seven of those years are in clinical trials, plan for approximately three years of research and development. Remember, 10 years is just the average—it could take longer. Make sure to plan around that.
Health spending accounted for 9.3% of GDP on average across OECD countries in 2011. The Pharmaceutical expenditure, as a percentage of total expenditure on health, accounted for 15%. The pharmaceutical sector is highly regulated. On describe the major characteristics of the world pharmaceutical industry as one increased globalization, changing ...
Flexibility: A pharmaceutical facility should be designed to be flexible in order to accommodate future changes and expansion. This means that the layout should be able to be easily modified as needed, and that there should be ample space for future expansion. 3. Safety and Security: Safety is of the utmost importance in a pharmaceutical ...
outcomes. For a pharmaceutical company, quality is closely connected to current Good Manufacturing Practice (cGMP), which helps your business produce pharmaceuticals which meet customer requirements for quality, strength, and reliability. Without a comprehensive QA plan, your pharmaceutical organization cannot
This Business Plan was developed by a partnership of the African Union Commission (AUC) and the United Nations Industrial Development Organization (UNIDO) to accel-erate the implementation of the Pharmaceutical Manufacturing Plan for Africa (PMPA), with funding from the German government. The Commission therefore extends special
Cost of launching a website - $1,000. Cost of throwing an opening party - $5,000. Miscellaneous - $20,000. From the above breakdown, we would need an estimate of $1,180,000 in order to successfully start up and operate our pharmaceutical distribution company here in Louisville - Kentucky.
Pharma 2.0 is a service company consisting of a web portal and additional services that offer. a peer-to-peer networking environment for corporations, universities, and research facilities. to locate, coordinate and collaborate on various projects without the necessity of extensive. resources.
Strategic alliance with Otsuka Pharmaceutical Co., Ltd. (Sold European Business, strengthened the business structure in China & Asia ) Launched new businesses (Regenerative Medicine/Cell Therapy Business and Frontier Business) Shifted regional strategies napabucasin, alvocidib, DSP-7888, dasotraline Extended LATUDA®'s exclusive
As noted in the ISPE Drug Shortages Prevention Plan,1 business continuity planning is a powerful, methodology, essential for enabling an uninterrupted supply of critical medicines for patients in challenging manufacturing circumstances. This "30,000-foot view" article shares an executive summary of principles and best practices of business continuity planning to ensure continued ...
MiKO Pharma Gh. Ltd is set to break ground on a cutting-edge manufacturing plant in the Akosombo-Akwamu Traditional Area on October 1, 2024. President Nana Addo Dankwa Akufo-Addo will attend the ...
SeQuent Scientific, a player in the global animal health industry, and Viyash Lifesciences, an integrated pharmaceutical company, have announced a strategic merger to form a global leader in animal healthcare. The merger aims to create a unique and differentiated platform, combining their respective ...
Tabuk Pharmaceutical Manufacturing Company is a fully-owned subsidiary of Astra Industrial Group, a leading pharmaceutical company in the Middle East and North Africa (MENA) region. Click here to connect with us on WhatsApp. Glucagon-like peptide-1 (GLP-1) are medications that help lower blood sugar levels and promote weight loss.
The plan would reduce chip manufacturing costs for companies like Taiwan Semiconductor Manufacturing Company, the world's largest chip producer. Image The plan would allow Nvidia to churn out ...