Our emergency departments are currently extremely busy, and you may face a long wait to be seen. More information is available on alternative services .


Why is research important?
Research at East Sussex Healthcare NHS Trust
Research helps us increase our knowledge about human health and wellbeing.
This is so we can:
- provide life changing treatments
- diagnose diseases earlier or more accurately
- prevent people from developing conditions
- improve health and care for generations to come
- ensure everyone has a better quality of life.
Overall, the aim is to find out whether what is being tested is better than what is currently available. This can include therapies, medicines and services.
Although health professionals already know a great deal, there are still so many questions that need answers.
Important research discoveries
Every minute in the UK, someone is diagnosed with a disease or a condition. The treatment and support they will receive will, at some point, have been informed by research.
Whether it’s testing a new medicine, a new surgery procedure or scan, or trying healthier lifestyle choices to prevent disease, everyone has an important role to play – if they want to.
Here are some important research discoveries that shape our healthcare today:
Penicillin was discovered in 1928 and developed into a drug in the early 1940s. Today it’s used to treat a broad range of bacterial infections accounting for around 45% of the antibiotics prescribed in the NHS in England.
Research in the 1980s and 1990s showed that low doses of blood-thinning drugs such as aspirin and warfarin significantly reduced the number of heart attacks and strokes in people at risk.
We are conducting multiple clinical research studies, and are committed to improving the quality of care we offer and to making our contribution to wider health improvement.
Cookies on the NHS England website
We’ve put some small files called cookies on your device to make our site work.
We’d also like to use analytics cookies. These send information about how our site is used to a service called Google Analytics. We use this information to improve our site.
Let us know if this is OK. We’ll use a cookie to save your choice. You can read more about our cookies before you choose.
Change my preferences I'm OK with analytics cookies
Maximising the benefits of research: Guidance for integrated care systems
England has a vibrant research and development ecosystem, with well-developed research infrastructure and research expertise within our health and care workforce. The value of research in transforming health and care is significant; additionally, staff satisfaction, recruitment and retention is higher among staff who are involved in research. The inception of integrated care systems (ICSs) provides the opportunity for systems to embed research within health and care for the benefit of our population. Supporting this opportunity, a clear research thread runs through ICS strategies and plans, from joint strategic needs assessments and joint health and wellbeing strategies , integrated care strategies , joint forwards plans , integrated care board (ICB) annual reports and the assessment by NHS England of the discharge of duties by ICBs.
The Health and Care Act 2022 (the 2022 Act) sets new legal duties on ICBs around the facilitation and promotion of research in matters relevant to the health service, and the use in the health service of evidence obtained from research. NHS England will assess ICBs for their discharge of these duties. The ICS design framework sets the expectation that in arranging provision of health services, ICBs will facilitate their partners in the health and care system to work together, combining expertise and resources to foster and deploy research and innovations. This guidance supports ICBs in fulfilling their research duties.
ICSs are encouraged to develop a research strategy that aligns to or could be included in their integrated care strategy. This strategy will enable the unification of research across ICS partners, and be consistently embedded to:
- identify and address local research priorities and needs, and work collaboratively to address national research priorities
- improve the quality of health and care and outcomes for all through the evidence generated by research
- increase the quality, quantity and breadth of research undertaken locally
- extend and expand research in settings such as primary care, community care, mental health services, public health and social care
- drive the use of research evidence for quality improvement and evidence-based practice
- influence the national research agenda to better meet local priorities and needs
- improve co-ordination and standardisation within and between localities for the set up and delivery of research
- harness the patient and economic benefits of commercial contract research
- co-ordinate and develop the research workforce across all settings.
1. Introduction
This guidance sets out what good research practice looks like. It supports integrated care systems (ICSs) to maximise the value of their duties around research for the benefit of their population’s health and care and, through co-ordination across ICSs, for national and international impact. It supports integrated care boards (ICBs), integrated care partnerships (ICPs) and their partners to develop a research strategy that aligns to or can be incorporated into their integrated care strategy, and helps them and their workforce to build on existing research initiatives and activities across health and social care to improve sector-wide performance and best practice
- explains the ICB legal duties and other requirements around research and the use of evidence from research, and that research is included in forward planning and reporting
- encourages system leaders to develop a footprint-wide research strategy that aligns to local and national research priorities, develops and supports their workforce, takes the opportunities offered by commercial research and includes plans to embed research in their system’s governance and leadership
- identifies best practice examples and other resources that ICBs may find useful as they develop their research strategies.
This guidance provides comprehensive information for use by:
- those with senior responsibility, including at board level, for research strategy development and/or operationalising research
- managers responsible for developing joint strategic needs assessments, integrated care strategies, joint health and wellbeing strategies, joint forward plans, other linked strategies, or reporting on ICB activities
- research managers
- research and development/innovation leads
- heads of services
- knowledge and library specialists.
It may also be useful to individuals involved in research, education, and partner organisations such as local authorities, social care services, the voluntary, community and social enterprise sector (VCSE) and other providers of healthcare services.
NHS England provides guidance on embedding research in the NHS and secure data environments, and the Office for Life Sciences (OLS ) champions research, innovation and the use of technology to transform health and care service. Other sources of guidance, support and information are signposted in this guidance to support ICSs in aligning to national visions, strategies and plans around research.
1.1 Definition of research
NHS England uses the UK Policy Framework for Health and Social Care Research definition of research:
“… the attempt to derive generalisable or transferable new knowledge to answer or refine relevant questions with scientifically sound methods. This excludes audits of practice and service evaluation. It includes activities that are carried out in preparation for or as a consequence of the interventional part of the research, such as screening potential participants for eligibility, obtaining participants’ consent and publishing results. It also includes non-interventional health and social care research (that is, projects that do not involve any change in standard treatment, care, or other services), projects that aim to generate hypotheses, methodological research and descriptive research”.
This broad definition encompasses the range of types of research:
- clinical trials and other clinical investigations into the safety and effectiveness of medicines, devices and health technologies
- public health research
- observational studies
- discovery science and experimental medicine
- translational research in which results from basic research are developed into results that directly benefit people
- applied research
- research to support policy-making and commissioning
- social care research and research in social care settings
- research into NHS services and care pathways.
1.2 Why research is important
The UK is a world leader for research and invention in healthcare, with around 25% of the world’s top 100 prescription medicines being discovered and developed in the UK ( The impact of collaboration: The value of UK medical research to EU science and health ). Research in the health and care system is important because it underpins all advances in health and care and is the basis for evidence-based practice. Engaging clinicians and healthcare organisations in research is associated with improvements in delivery of healthcare ( Does the engagement of clinicians and organisations in research improve healthcare performance: a three-stage review) . To benefit service users and the public, the NHS and local government, and achieve return on investment, it is vital that research is disseminated, shared and translated into practice.
The National Institute for Health and Care Research (NIHR) is funded by the Department of Health and Social Care (DHSC) to transform research in the health and social care system, including through support for NHS research. Research led to the first proven treatments for Covid, for example the use of dexamethasone, estimated to have saved over a million lives worldwide . This success was in part due to how research is undertaken in the unique environment of the NHS, innovative trial designs, the support provided by the NIHR, frontline staff enabling research, and the awareness and readiness of the public to support research. We need to learn from these and other successes, and translate this across all health and care settings. ICSs will play a vital role in enabling research to be embedded in evolving patient pathways across their footprints.
Example: PRINCIPLE trial – finding treatments for Covid recovery at home
The Platform Randomised Trial of Treatment in the Community for Epidemic and Pandemic Illnesses (PRINCIPLE) was a UK-wide, clinical study to find Covid treatments for recovery at home without the need to attend hospital. The study was open to all with ongoing Covid symptoms, registration was easy, and the trial was run entirely remotely by delivering ‘participant packs’ to people’s homes. It was one of the first trials in the world to show that azithromycin and doxycycline did not benefit patients with Covid and to identify the effectiveness of a commonly used drug – inhaled budesonide –in reducing time to recovery.
The PRINCIPLE study team demonstrated the integral role that primary, secondary and ambulatory care staff can play in the delivery of studies. Local collaborators were trained in good clinical practice to allow them to assess and confirm the eligibility of potential participants, and were commended specifically for their use of patient data to contact people soon after they received a positive test result. It is this network of local staff contributing to research within their healthcare setting that has enabled over 10,000 people to be recruited onto this study so far – one of the largest at home Covid treatment studies worldwide.
This is an example of a study design that incorporates the vital contributions of healthcare providers across the system.
Policy-makers and commissioners need evidence to support their decision-making around the delivery and system-wide transformation of health and care services, including how health inequalities will be reduced.
There is also evidence that:
- staff involved in research have greater job satisfaction and staff turnover is lower in research active trusts ( Academic factors in medical recruitment: evidence to support improvements in medical recruitment and retention by improving the academic content in medical posts)
- research active hospitals have lower mortality rates, and not just among research participants ( Research activity and the association with mortality )
- 83% of people believe that health research is very important ( Survey of the general public: attitudes towards health research)
- healthcare performance improvements have been seen from the creation of academic research placements ( Experiences of hospital allied health professionals in collaborative student research projects: a qualitative study )
- clinical academic research, and in particular the practice changes resulting from it, is associated with improved patient and carer experiences ( A qualitative systematic review and thematic synthesis exploring the impacts of clinical academic activity by healthcare professionals outside medicine ).
Key to having research embedded in health and care is having staff who can understand, undertake, use and generate new research, and share actionable research finding as part of a pro-research culture. Education and training are therefore critical for research to be sustainably embedded within health and care, and for people to develop careers in research and support it in their clinical or care roles.
DHSC, NHS England, the devolved administrations, NIHR and other partners expect to publish a clinical research workforce strategy in 2023/24 to help the UK realise the national clinical research vision outlined in Saving and Improving Lives: The Future of UK Clinical Research Delivery and deliver the Life Sciences Vision to see research embedded in the NHS as part of health and care pathways.
Research will support ICSs to deliver on their four key aims:
Improving outcomes
The NHS 2023/34 priorities and operational planning guidance emphasises the importance of research in improving patient care, outcomes and experience.
Research evidence will inform commissioning decisions to improve experience and outcomes. Research activities should align with the local health priorities identified through local joint strategic needs assessments, and may be best designed and delivered by collaborating with partners. Research priorities may be best addressed by collaborating with partners nationally to design and deliver research.
Tackling inequalities
Research can give a better understanding of local populations and the wider determinants of health, and with this the steps to maintain health and narrow health inequalities.
Enhancing productivity
The development of ICSs creates the opportunity to consider research delivery within the ICS and across ICS boundaries, increasing flexibility of workforce or recruitment while reducing bureaucracy and improving research productivity and value for money.
Supporting social and economic development
An active research ecosystem working in a co-ordinated way and to national standards brings revenue and jobs to regions. The NIHR Clinical Research Network (CRN) supports service users, the public and health and care organisations across England to participate in high-quality research. The 2019 impact and value report detailed the significant income and cost savings that commercial research generates for NHS trusts. Between 2016/17 and 2018/19 the NHS received on average £9,000 per patient recruited to a commercial clinical trial and saved over £5,800 in drug costs for each of these patients. This equates to income of £355 million and cost savings of £26.8 million in 2018/19.
In 2021 150 members of the Association of Medical Research Charities funded £1.55 billion of medical research, including the salaries of 20,000 researchers. Every £1 million spent by charities on medical research in the UK contributes £1.83 million to the economy.
Example: Research that cut problematic prescribing and generated cost savings in general practice – a local health priority
Analysis of routine patient data identified the need for strategies targeting clinicians and patients to curb rising opioid prescribing. From this, the Campaign to Reduce Opioid Prescription (CROP) was launched in 2016, urging GPs across West Yorkshire to ‘think-twice’ before prescribing opioids. This promoted the NICE guidance on chronic pain , which recommends reducing the use of opioids because there is little or no evidence that they make any difference to people’s quality of life, pain or psychological distress, but they can cause harm, including possible addiction.
Over a year 15,000 fewer people were prescribed opioids (a 5.63% relative reduction), a net saving to the NHS of £700,000. The biggest reduction was in people aged over 75, who are at higher risk of opioid-related falls and death, and there was no compensatory rise in the prescribing of other painkillers or referrals to musculoskeletal services.
The CROP campaign, led by researchers at the University of Leeds, has subsequently been rolled out across all ICBs in Yorkshire and the Humber, and the North East and North Cumbria ICB, and the 1,045 practices to which it has been delivered are reporting results similar to the above.
Foy R, Leaman B, McCrorie C, Petty D, House A, Bennett M, et al (2016) Prescribed opioids in primary care: cross-sectional and longitudinal analyses of influence of patient and practice characteristics | BMJ Open 69(5).
Alderson SL, Faragher TM, Willis TA, Carder P, Johnson S, Foy R (2021) The effects of an evidence- and theory-informed feedback intervention on opioid prescribing for non-cancer pain in primary care: A controlled interrupted time series analysis. PLOS Med .
2. ICS, ICP and ICB responsibilities and requirements
ICBs have legal duties and other requirements that relate to research. These are additional to the duties and responsibilities of individual providers within ICS footprints. This section sets out what these duties mean in practical terms and gives examples of how to meet them.
2.1 Legal duties relating to research in the Health and Care Act 2022
Part 1 of the 2022 Act includes specific legal duties for ICBs and NHS England in respect of research. In the Explanatory Notes to the 2022 Act, government sets out how ICBs could discharge their research duty.
Duty to facilitate or otherwise promote research
The ICB duty builds on the previous clinical commissioning group (CCG) duty to promote research, by requiring each ICB, in the exercise of its functions, to facilitate or otherwise promote research on matters relevant to the health service. This duty is intended to include a range of activities to enable research. Section 3 of this guidance outlines ways in which ICBs can do this.
The NHS Constitution also makes clear that patients should be enabled to take part in research: “the NHS pledges … to inform you of research studies in which you may be eligible to participate”.
The Provider Selection Regime (PSR) will be a new set of rules for arranging healthcare services in England, introduced by regulations made under the 2022 Act. The research component should be referred to once the PSR is published.
Duty to facilitate or otherwise promote the use in the health service of evidence obtained from research
This duty similarly builds on the CCG requirement to promote the use of evidence. ICBs must, in the exercise of their functions, facilitate or otherwise promote the use in the health service of evidence obtained from research. For example, ICBs should facilitate or otherwise promote the use of evidence in care, clinical and commissioning decisions.
Duty for ICSs to include research in their joint forward plans and annual reports
Joint forward plans are five-year plans developed by ICBs and their partner NHS trusts and foundation trusts. Systems are encouraged to use the joint forward plan as a shared delivery plan for the integrated care strategy and joint health and wellbeing strategy, aligned to the NHS’s universal commitments. The plan must explain how the ICB will discharge its duties around research, and the ICB must report on the discharge of its research duties in its annual report. These inclusions will raise the profile of research at board level and help embed research as a business-as-usual activity.
The joint forward plan and NHS Oversight Framework guidance set the minimum requirements for what needs to be included in plans and reports.
NHS England duty to include how each ICB is carrying out its duties relating to research in its annual performance assessment of each ICB
NHS England has a new legal duty to annually assess the performance of each ICB and publish a summary of its findings. For 2022/23 NHS England will complete a narrative assessment, identifying areas of good and/or outstanding performance, areas for improvement and any areas that are particularly challenged, drawing on national expertise as required and having regard to relevant guidance. This assessment will include a section considering how effectively the ICB has discharged its duties to facilitate or otherwise promote research and the use of evidence obtained from research.
This, alongside the implementation of the NHS Long Term Plan commitment to develop research metrics for NHS providers, will increase transparency across the system and enable more targeted support for research. Research metrics from NHS England, the Care Quality Commission (CQC) and NIHR will enable the monitoring of progress over time, and are under development with sector colleagues, including providers.
2.2 Legal requirement to work with people and communities
Working with people and communities is a requirement of ICBs, and statutory guidance is available to support them and their partner providers meet this legal duty. A co-ordinated approach across healthcare delivery and research will make it more likely that research reflects what matters to people and communities.
This will also help ICBs to fulfil their legal duty in the 2022 Act to reduce health inequalities in access to health services and the outcomes achieved. Section 3.9 includes links to resources to help guide engagement with underserved communities around research.
The Public Sector Equality Duty also applies and requires equality of opportunities between persons who share a relevant protected characteristic and persons who do not.
2.3 Research governance
While research can address local priorities, it typically operates across ICS boundaries and at national and international levels. Health and social care research is governed by a range of laws, policies, and international, national and professional standards.
The Health Research Authority (HRA ) is responsible for ensuring such regulation is co-ordinated and standardised across the UK to make it easier to do research that people can trust. The HRA is an executive non-departmental public body created by the Care Act 2014 to protect and promote the interests of patients and the public in health and social care research, including by co-ordinating and standardising the practice of research regulation. Local authorities and the NHS are obliged to have regard to its guidance on the management and conduct of research.
Before a research project can start in the NHS in England it must receive approval from the HRA. This includes research taking place in NHS trusts, NHS foundation trusts, ICBs or primary care providers of NHS commissioned services in England, and all research under an NHS duty of care, including that undertaken by NHS staff working in social care or other non-NHS environments.
The HRA schemes indemnify NHS organisations accepting these assurances against any claim covered by the NHS Litigation Authority arising as a result of incorrect assurances. If an NHS organisation duplicates the HRA assessments, it will be liable for any consequences of the decisions it bases on its own checks.
ICBs and partner organisations should have processes for the set up and delivery of research that comply with national laws and systems, and does not duplicate them. Such national systems include confirmation of capacity, National Contract Value Review (NCVR), management of Excess Treatment Costs (ETCs) and contracting arrangements (see section 2.4).
The UK Policy Framework for Health and Social Care sets out the roles and responsibilities of individuals and organisations involved in research.
2.4 Contractual requirements around research
NHS England mandates commissioner use of the NHS Standard Contract for all contracts for healthcare services other than primary care. The contract is updated annually. References to research in the current NHS Standard Contract and service conditions fall into three main areas.
Recruitment of service users and staff into approved research studies
The NHS Standard Contract obliges every provider of NHS-funded services to assist the recruitment of suitable subjects (whether patients or staff) into approved research studies. This requirement aligns to those in the 2022 Act that require ICBs to facilitate or otherwise promote research (see section 2.1). Section 3 considers how this requirement can best be met. Research involving people or their data requires ethical and potentially other approvals (see section 2.3).
National Directive on Commercial Contract Research Studies
Adherence to the National Directive is mandated as part of the NHS Standard Contract. The directive states that providers must:
- Use the unmodified model agreements for sponsor-to-site contracting; HRA and Health and Care Research Wales (HCRW) approval of studies will be dependent on use of these templates.
- Use the standard costing methodology to set prices for commercial contract research undertaken by NHS providers; this is currently in the NIHR interactive costing tool (NIHR iCT).
- Introduce the National Contract Value Review (NCVR) process in line with national rollout. NCVR is a standardised national approach to costing commercial contract research within the NHS. It currently covers acute, specialist and mental health trusts, but the intention is to roll it out to all NHS providers. The creation of ICSs is the ideal opportunity to explore how commercial study set up can be supported across these footprints, reducing the resource needed and time taken.
Comply with HRA/NIHR research reporting guidance
The provider must comply with HRA/NIHR research reporting guidance, as applicable.
2.5 Excess treatment costs
Patients in a research study may receive healthcare that differs from what is standard in the NHS, requires more clinician time or is delivered in a different location. The associated NHS treatment costs may exceed or be less than those of standard treatment. If greater, the difference is referred to as the NHS Excess Treatment Costs (ETCs).
In the case of commercial contract research, the commercial funder will pay the full cost of the study. In the case of non-commercial research, the commissioner of the service in which the study operates is responsible for funding the ETCs.
ICBs as commissioners of services are responsible for ETCs in services that they commission. Guidance for the management of ETCs is available.
DHSC and NIHR are piloting interim arrangements to support non-NHS ETCs for research in public health and social care (non-NHS intervention costs). Please refer to the further detail on the NIHR website .
2.6 Care Quality Commission
The CQC is currently developing its approach for ICS-level assessments, and its new assessment framework will be introduced towards the end of 2023 .
CQC inspection of NHS providers continue, with research assessed as part of the review of the trust-level Well-led framework. Providers are asked:
- Are divisional staff aware of research undertaken in and through the trust, how it contributes to improvement and the service level needed across departments to support it?
- How do senior leaders support internal investigators initiating and managing clinical studies?
- Does the vision and strategy incorporate plans for supporting clinical research activity as a key contributor to best patient care?
- Does the trust have clear internal reporting systems for its research range, volume, activity, safety and performance?
- How are service users and carers given the opportunity to participate in or become actively involved in clinical research studies in the trust?

3. Developing a research strategy
3.1 why develop a research strategy.
Like the health and care system, the research environment is complex. Developing a research strategy will help bring together the legal and other duties around research in a coherent way, and help the ICS understand its local research capability, workforce, activity and needs, set ambitions around research and maximise the benefits associated with commercial research. It will help demonstrate the benefit of research locally, nationally and internationally, and guide the production of clear plans.
Example: Value of research partnerships and integration with ICSs
Bristol Health Partners (BHP) Academic Health Science Centre (AHSC) has a fully integrated relationship as the new Research and Innovation Steering Group for the Bristol, North Somerset and South Gloucestershire (BNSSG) ICS, and reports directly to ICB chief executives.
The group provides the strategic direction and oversight for all research undertaken and delivered across the system. Membership includes directors of research, clinical strategy, public health, social care, senior innovation and education leaders from its core funding partners. It also includes public contributors and senior representatives from primary care, NIHR Applied Research Collaboration West, NIHR CRN West of England, West of England Academic Health Science Network (WEAHSN), Healthier Together ICS, university research institutes and People in Health West of England.
The group has reviewed ICS programmes, identified current and potential research and innovation connections, and begun to establish new connections. It has also supported work with the ICS Ageing Well programme and secured funding for innovative pilots to improve dementia care and increase physical activity for older adults.
Since 2016 BHP has directly contributed an estimated additional £1.1 million to support ICS priorities through Health Integration Team projects and other activities, and has attracted more than £33 million of external research, service redesign and infrastructure into the region.
3.2 General considerations
In developing its research strategy, the ICS may find it helpful to consider these overarching questions alongside the suggested focused content covered in the sections below:
- What do you hope to achieve within a given timeframe?
- Are all the right organisations involved in developing the research strategy?
- How will the health and care workforce be enabled to deliver the research strategy?
- How can research be embedded in existing health and care delivery and pathways?
- What mechanisms are in place to translate actionable research findings into practice and decision-making?
- What inequalities exist in different areas, communities or groups? How will you ensure planning and delivery of research aligns to CORE20plus5 priorities?
- Are you considering equality, diversity and inclusivity and the Public Sector Equality Duty in facilitating and promoting research opportunities for service users and for health and care staff?
- Is the ICS considering the opportunities of developing their commercial research portfolio?
- Is research informing or being informed by population health management?
- How will you plan and deliver research in a sustainable manner, aligning it to the Greener NHS agenda and the ICB’s duties in relation to climate change ?
Buy-in from NHS staff, patients and the public will be vital if ICBs are to discharge their research duties and deliver on their research plans. An important consideration is how to develop sustainable, routine and accessible information flows to ensure the ICB, partners, staff, patients and public can access up-to-date and appropriate information around local research activity, regional, national and international research opportunities and findings, and contact information.
3.3 Leadership and governance across the ICS
Executive leadership.
The Explanatory Notes to the 2022 Act suggest that ICBs have board-level discussions on research activity, the use of the evidence from research, the research workforce and research culture within the ICS. ICSs should refer to the NHS Leadership Competency Framework for board-level leaders at organisation and ICS level for the competencies relating to the research duties of ICSs, once published.
All ICBs are encouraged to have an executive lead responsible for fulfilling the research duties conferred by the 2022 Act. They should help give the ICB a clear understanding of research across the area, regularly reporting on progress towards agreed aims. An executive lead can take responsibility for ensuring clear research ambitions and a research strategy are developed; oversight of organisational research portfolios, diversity in research, alignment to national priorities; promotion of research skills and the need for research skills training; and succession planning.
Senior leaders could engage, consult and be supported by representatives of each registered health and social care professional group when developing strategic plans, and for oversight of training, succession planning, and equality and inclusivity. They could use the capacity and capability of the research and development leads within provider organisations, although established lead roles across social care settings are rare so extra effort may be needed to garner social care research insight.
Research steering group, board or forum
Some CCGs had research steering groups and some of these have expanded with the widening remit of ICBs. ICSs that do not have a such a group should consider adopting a model similar to one in other ICSs where research is effectively embedded in ICS governance structures.
A dedicated steering research group, board and/or forum can:
- provide dedicated time to plan, oversee and report on research
- bring a range of representatives from research infrastructure organisations, patients and the public together with representation from across the ICS, to develop a common aim and objective
- ensure board-level sight of research
- take a cross-ICS approach to research, increasing participation and diversity in research, and reducing bureaucracy.
Example: A dedicated research and innovation subgroup
East and North Hertfordshire Health Care Partnership established a formal research and innovation subgroup to support its objectives to transform services, reduce health inequalities and improve patient health and wellbeing. This subgroup is dedicated to determining and supporting local research priorities and developing an innovation agenda. With effective patient and public involvement, it is working to ensure the local population has access to more research opportunities.
Bringing together the NIHR, academia, industry and local health and care services, the subgroup develops collaborative work plans that support the design, implementation and evaluation of local transformation needs, sharing resources, staff, expertise and facilities. Its work exemplifies a sustainable approach to partnership working and supports Hertfordshire and West Essex ICS’s developing strategy.
HWE ICS Partnership Board 14 September 2021
3.4 Understanding your research activity and working with local and national research infrastructure
Research in NHS and non-NHS settings across an ICS footprint will be supported by different organisations. In some areas networks or collaboratives already exist to bring these organisations together, but in others the links are not as well formed. ICBs would benefit from having a clear map of the research infrastructure and pre-existing local or national investment into research in their area.
It may be valuable to consider:
- Who are the research leaders in your local health and care system, NIHR, higher education institutions, VCSE sector and businesses?
- Are there any pre-existing local or regional research, researcher or research engagement networks?
- What are the opportunities to inform, participate in, collaborate with or lead national and international research efforts in addition to local opportunities?
A list of organisations involved in research including NIHR-funded infrastructure and programmes is included in Annex 1 .
Much of the research undertaken in NHS and other health and care settings is funded though national calls and grants provided by funders such as NIHR, research charities , UK Research and Innovation (UKRI) , including the Medical Research Council (MRC ) and Economic and Social Research Council (ESRC) , and is aligned to national priorities. Other research may include national or international commercial or non-commercial clinical trials funders.
Partners within ICS systems can use NIHR research portfolio data to monitor and plan research activity; however, not all research is included within the NIHR’s portfolio, so this will not give a full picture of the research within the footprint. Mechanisms to map and monitor research more widely could be incorporated in ICB research strategies.
Some local needs may best be addressed through public health or social care research rather than research in primary, secondary or tertiary healthcare settings. Public health and social care research are described in Annex 2 .
Example: Mapping health and care research activity, expertise, interests and infrastructure
The Nottingham and Nottinghamshire Integrated Care System Research Partners Group meets bi-monthly and is chaired by the ICB Head of Research and Evidence. It brings together senior managers from the NHS providers, ICB, two local authorities, two universities and the NIHR CRN East Midlands, providing a forum for ICS-wide research discussions and the development of a system-wide collaborative approach to health and care research across the ICS. Among its aims, the group seeks to increase participation in research at both the organisational and population level, enable equity of access to research opportunities and generate impact on health and care pathways.
The group have mapped health and care research activity, expertise, interests and infrastructure in the constituent organisations. With this the ICS can see the research capabilities, strengths, expertise, and areas of synergy and opportunities for future collaboration that align to its needs and priorities, and also gaps for future development, recognising that organisations are at different stages of research development.
3.5 Understanding local needs
Universal NHS priorities will be reflected in local research needs, and each ICS footprint is likely to have its own specific local research needs. Joint strategic needs assessments (JSNAs) are undertaken jointly by local authorities and ICBs through health and wellbeing boards (HWBs) to identify current health and social care needs of local communities, where more information is needed to do so or to understand how best to address the need. People and communities should be directly involved in identifying local need, including by working with local charities, specific communities or groups who face inequalities in access to, experience of or outcomes from healthcare, eg to target health research at those areas and populations with greatest need.
ICPs are required to develop an integrated care strategy informed by JSNAs and the joint health and wellbeing strategy (JHWS). The integrated care strategy sets out how the assessed needs can be met through the exercise of the functions of the ICB, partner local authorities or NHS England, and is informed by research and practice-based evidence, as stated in the health and wellbeing guidance. In considering where such evidence is lacking, HWBs should identify in JSNAs those research needs that ICBs, local authorities and NHS England could meet through the exercise of their research functions.
Systems are encouraged to use their joint forward plan to develop a shared delivery plan for the Integrated Care Strategy and the JHWS that is supported by the whole system, including local authorities and VCSE partners. ICBs and trusts must also use their Joint Forward Plan to describe how the ICB will discharge its duty in respect of research.
The Explanatory Notes to the 2022 Act suggest how ICBs can discharge their duties around research. These include the articulating local research needs when assessing local needs and how they will be addressed when preparing strategies and plans, and encouraging partner organisations to play an active and collaborative role in pursuing these.
3.6 Supporting delivery of research
Once an ICS has a clear picture of its local research infrastructure it can consider how best to target and support research and the research workforce across its footprint and how research findings will be used. For this, the ICB should ensure that its approaches reflect national approaches to costing, contracting, approvals and information governance, and that they are also informed by learning from effective practices across equivalent ICBs.
As healthcare shifts into communities, ICSs should support the parallel shift in research by embedding research in health and care. Increasing access to research opportunities will give service users earlier access to new treatments, and faster research set up and delivery may provide the evidence needed to support improvements to local care sooner. Inclusive recruitment practices will be needed to ensure that all groups in society have the opportunity to help shape and take part in research, and benefit from research findings.
In developing its research strategy, an ICS has opportunities to reduce bureaucracy, and make research more efficient and effective across its own and with other ICS footprints, and across NHS and non-NHS boundaries, while meeting national regulatory guidance. ICBs will be expected to work with the HRA to co-develop, build on and implement strategies for further co-ordination and standardisation of study set-up and delivery processes. Any regional systems and processes that ICBs do establish must support consistent national practice in relation to the management and regulation of research, and should not duplicate them. The HRA will work with ICBs to address barriers to efficient and rapid study set-up, including model agreements, information governance and R&D office functions.
Other potential areas for streamlining and cross-organisational working include:
- cross-ICS research proposals to identify research needs
- research delivery – identifying how ICS-wide approaches could accelerate patient recruitment and deployment of research delivery staff
- shared data architecture, including the NHS Secure Data Environment for Research Network and its subnational secure data environments (SDEs). Subnational SDEs cover multiple ICSs to achieve access to multimodal data at a scale of approximately 5 million citizens, and over time will achieve technical and governance interoperability
- a greater focus on translation and implementation of research findings into health and care practice, supporting faster improvements
- sharing access to and funding for knowledge and library services
- shared processes and repositories for research assets.
The Explanatory Notes to the 2022 Act suggest that one way an ICB could discharge its research duty would be to have a dedicated research office or team supporting research.
3.7 Enabling cross-provider research
Health and care priorities can often only be addressed with complex, multiorganisational approaches and as such the research to inform these needs to span organisational boundaries. Organisational policies should promote cross-organisational research and dissemination of research findings, including through participation in collaborative research to address national priorities, joint staff posts, honorary contracts, and administratively easier movement of researchers between health and care organisations and other sector partners, including higher education, industry, charities and local authorities.
The HRA and ICS partners are developing national guidance to support cross-provider research.
The NIHR CRN can offer ICSs opportunities to participate in national and international research studies, including those the NIHR, industry and others commission.
3.8 Commercial research
Commercial contract research is research funded solely by industry, where NHS providers are contracted to carry out the research. Most of these research studies in the NHS are interventional clinical trials, such as the NHS-Galleri trial and Astra Zeneca’s COVID-19 vaccine development . Commercial research can give patients access to a wider range of research opportunities, earlier access to novel therapies and treatments, provide drugs free of charge to patients in trials, accelerate the development of new treatments and devices, generate income for providers, and fund NHS staff. It is vitally important for the benefit of patients, the NHS and the UK economy that we create an environment in the NHS that makes it easy and efficient for the NHS to undertake commercial research. This is particularly important when it comes to international commercial research, where companies can place their studies in a number of different countries and consideration of anticipated set up and recruitment times informs where they place trials.
Data gathered during some commercial research is specific to the study and is the property of the company, as is any Intellectual Property (IP) generated. In other cases, where the NHS contributes to the foreground IP – such as through the use of NHS data for research or where NHS expertise provides important contributions to a commercial product – it is important that the NHS shares in the value of IP generated as a consequence of its contributions.
The establishment of ICSs is an ideal opportunity for their creation of ambitions to enable, grow and benefit from commercial research. ICSs should explore how efficient commercial study set up and delivery could be streamlined across sites within their footprint, and should set ambitions around commercial research.
3.9 Involving patients, service users, carers and the public in research
In developing a research strategy ICSs should set out their approach to diverse public and patient involvement (PPI) in relation to research.
Areas where working with people and communities could add value in the context of research include:
- identification of local research needs, including through JSNAs and JHWSs
- designing research proposals in partnership with local or national experts
- raising awareness of research opportunities and recruitment of participants
- developing research outcome reports and identification of how and when participants will be able to access these
- consideration of how members of the public can access the outputs from publicly-funded research
- how volunteers should be involved and what they should be paid.
The UK Standards for Public Involvement sets out the core components of good public involvement. A guide outlining good practice in engaging underserved communities around research is available from NHS England. Resources about good practice around PPI in designing and delivering research, including around incentivisation , are also available from the HRA and NIHR .
It will be useful to link into established community involvement approaches. NIHR infrastructure organisations may have established networks of expert PPI representatives, and ICSs have extensive VCSE Alliances. A co-ordinated community engagement approach across health and care delivery and research will reduce the risk of overburdening communities with organisations wanting to work with them, and will support the identification of under-served communities.
3.10 Ensuring anyone can participate in research
Making research more visible within communities and increasing the public’s understanding of research can ensure greater diversity in research participation. Research findings will then be more generalisable to a broader range of groups or communities, or can be targeted and specific to relevant communities.
ICSs should seek mechanisms to ensure that opportunities to take part in research are available to all. They should consider encouraging patients and members of the public to register on NIHR Be Part of Research (a national registry where people can express their interest in being contacted about research that is relevant to them), widely disseminate research opportunities and make provision for inclusive access for communities to take part in research. Decentralised or virtual trials are remote access trials recruited to and delivered using electronic tools, making it easier for people to participate in some studies without needing to visit a recruiting hospital or attend appointments in person. ICBs should consider ways in which research delivery can increase access to research opportunities for people within their area. ICBs should also advise the public how they can access research outputs.
NIHR and UK Research and Innovation provide resources that help organisations address issues of equality, diversity and inclusion in research settings.
Example: RELIEVE-IBS decentralised trial
In 2020, Newcastle researchers launched RELIEVE-IBS, one of the first interventional decentralised clinical studies in the UK to trial Enterosgel, a new treatment for irritable bowel syndrome with diarrhoea (IBS-D). Decentralised trials are remote access trials that use electronic tools for trial recruitment and delivery, without the patient needing to visit a recruiting hospital site, which could be miles from their homes – a convenient option for patients with IBS-D. By running the trial remotely, researchers could reach beyond the small proportion of those with this condition who attend specialist clinics, as well as save resource for the sponsor.
Not only did this trial embrace technological developments to deliver research, but it empowered more patients to become involved regardless of where they lived. With in-depth patient input, the research team were able to shape the recruitment approach to be highly accessible to participants and were offered feedback on how to refine the trial design by the sponsors. The resulting patient-centric design ensured a good recruitment response when the trial opened.
NIHR (2020) Virtual trial recruits 67% faster led by NIHR Patient Recruitment Centre in Newcastle in collaboration with Enteromed
NIHR (2021) Pushing virtual boundaries to improve patient engagement and accessibility
NIHR (2022) RELIEVE IBS-D trial case study
3.11 Health data in research
Health data generated through care of service users in the NHS can fuel a revolution in the research and development of new diagnostics and treatments, maximising the potential to improve service user outcomes and experiences, support diversity in research, and minimise health inequalities through research. To do this, researchers need access to high quality and timely data to generate insights. The public expect data to be used legally and efficiently to conduct and support research.
National commitments around data for research can be found in Data saves lives: reshaping health and social care with data . This strategy shows how data will be used to bring benefits to all parts of health and social care. To achieve this vision, the NHS will be making a strategic move away from a system of data dissemination to one of data access when making NHS health and social care data available for research and analysis. This will be facilitated by the implementation of secure data environments (SDEs).
SDEs are data storage and access platforms with features that enable organisations to have greater control and oversight over their data. SDEs allow approved users to view and analyse data without it having to leave the environment. The SDE policy guidelines provide a clear signal to the sector that SDEs will become the default way of accessing NHS data for research.
This change is supported by major investments in digital infrastructure through the Data for Research & Development Programme, which is funding the development of national and subnational SDEs. The subnational SDEs will cover the entirety of England and individual platforms will cover several ICS.
ICBs should seek ways to promote and enable the use of these rich data sources for research and include them in their research strategy.
3.12 Using evidence for planning, commissioning and improving health and care
Evidence-based commissioning has advantages for the commissioner, workforce and service users, as it can:
- lead to innovation in service design and delivery
- enhance the quality of health and care provision
- reduce clinical variation between locations and providers
- improve equity of access to services
- improve patient and population outcomes.
As part of the commissioning process, commissioners are expected to use evidence-based clinical policies, as per the Roadmap for integrating specialised services within integrated care systems . Knowledge and library services can help source and interpret evidence.
The Provider Selection Regime will reflect the research duties of the 2022 Act and should be referred to when commissioning provider services, once it has been published.
NHS knowledge and library services provide access to evidence and support for knowledge management; they train people in searching for, handling and publishing information. The Knowledge for Healthcare strategy encourages and equips NHS knowledge and library services to support NHS organisations with the translation of knowledge for the spread and adoption of research and innovation. To fulfil their obligations under the 2022 Act, ICBs could commit to active knowledge translation.
Evidence for commissioning information is available from a number of sources:
- NHS Library and Knowledge Hub
- Health Libraries and Information Services Directory
- NICE guidance
- NIHR evidence
- NHS evidence works toolkit
- Academy of Medical Royal Colleges: Evidence-based Intervention
- A million decisions
The infographic for the role of research and evidence in commissioning also provides sources for evidence-based commissioning.
Example: Evidence mobilisation, knowledge sharing and improving outcomes
The STEMClub (Sustaining Transformation by Evidence Mobilisation) is a network in the North East and North Cumbria that brings together local policy and decision-makers with NHS knowledge and library specialists to facilitate evidence-based decision-making. The input of knowledge specialists ensures timely access to published research and provides knowledge management expertise to shape how soft intelligence is translated into knowledge assets.
As members within the STEMClub network, knowledge and library specialists are providing ongoing detailed evidence reviews and information management expertise to facilitate system-wide working , eg:
- North East North Cumbria Frailty Framework
- North East and North Cumbria Maternity Clinical Network
- a review of optimal patient transfer times in the North East and North Cumbria
- regular evidence summaries for the ICS Mental Health Evidence and Evaluation subgroup.
3.13 The health and care workforce and research
Staff involved in research have greater job satisfaction and research active trusts have lower staff turnover [3] . Clinical academic roles [7] , having research colleagues within services [8] and taking students on research placements [6] are felt to foster an increase in knowledge and skills across the wider staff workforce. The General Medical Council (GMC) and the Royal College of Physicians (RCP) and NIHR have issued position statements and recommendations around research, with additional signatories including UKRI, UKRD, the Academy of Medical Royal Colleges and the Royal College of Surgeons of England. Learning resources, including programmes for ongoing professional development of the research delivery workforce, are available through NIHR Learn.
In developing a research strategy ICSs could ensure that, as part of their people function and approach to workforce planning :
- Staff roles in leading, delivering or facilitating research and in supervising those developing research skills are recognised, supported and enabled across all staff groups and health and care settings as part of a positive research culture.
- The value of evidence is recognised, and education and training around research are facilitated. Opportunities to develop research careers or in overseeing the development of other researchers are enabled; this may include having protected time, inclusion in job plans and joint appointments across health and care providers and academic institutions.
- Ensuring that there is capacity and systems that support research through services like imaging, pathology and pharmacy, as well as finance and human resources.
- Individual organisations do not always have the necessary skills or services to support effective research and its impact, such as IP management, methodological expertise, regulatory compliance, statistical analysis, knowledge mobilisation expertise, genomics expertise, health informatics and data analytics. Mechanisms are needed to ensure that these can readily and rapidly be accessed across other health and care organisations, including from local authorities and other non-NHS care providers.
A UK Clinical Research Workforce Strategy is under development. ICSs should update their approaches to their research workforce once DHSC publishes this in 2023/24.
Example: Investing in the research workforce – developing capacity for chief investigators
Across the West Midlands NIHR CRN, an investment of approximately £750,000 to develop capacity for chief investigators returned additional research grant income of over £18 million in three years. This was achieved primarily by increasing the programme activity for consultants in areas where chief investigators were underrepresented.
The funding was provided through a competitive process and co-supported by the local NIHR CRN, with several local trusts jointly funded these scholars.
Kirk J, Willcocks J, Boyle P, Brocklehurst P, Morris K, Kearney R, et al (2022) Developing chief investigators within the NHS: the West Midlands clinical trials scholars programme. Clin Med 22(2): 149–52.
Kirk J, Reynolds F, Adey E, Boazman M, Brookes M, Brocklehurst P (2022) Developing paediatric chief investigators within the NHS: the Clinical Trials Scholars programme . Arch Dis Child Educ Pract Published online first: 22 February 2022. doi: 10.1136/archdischild-2021-322186
4. References
- Varnai P, Rentel M, Dave A, De Scalzi M, Timmerman W, Rosemberg-Mantes C, Simmonds P, Technopolis Group (2017) The impact of collaboration: The value of UK medical research to EU science and health .
- Boaz A, Hanney S, Jones T, Soper B (2015) Does the engagement of clinicians and organisations in research improve healthcare performance: a three-stage review. BMJ Open 5: e009415. doi:10.1136/ bmjopen-2015-009415 .
- Rees MR, Bracewell M (2019) Academic factors in medical recruitment: evidence to support improvements in medical recruitment and retention by improving the academic content in medical posts. Postgrad Med J 95(1124): 323-327. doi:10.1136/postgradmedj-2019-136501 .
- Ozdemir BA, Karthikesalingham A, Singha S, Poloniecki JD, Hinchliffe RJ, Thompson MM, et al (2015) Research activity and the association with mortality. PLoS ONE 10(2): doi.org/10.1371/journal.pone.0118253 .
- Hunn A (2017) Survey of the general public: attitudes towards health research . Health Research Authority.
- Angus RL, Hattingh HL, Weir KA (2022) Experiences of hospital allied health professionals in collaborative student research projects: a qualitative study. BMC Health Services Research 22(1). Available at: https://doi.org/10.1186/s12913-022-08119-7 .
- Newington L, Wells M, Adonis A, Bolten L, Bolton Saghdaoui L, Coffey M, et al (2021) A qualitative systematic review and thematic synthesis exploring the impacts of clinical academic activity by healthcare professionals outside medicine. BMC Health Serv Res 21(1). Available at: https://doi.org/10.1186/s12913-021-06354-y .
- Wenke RJ, Hickman I, Hulcombe J, Phillips R, Mickan S (2017) Allied health research positions: A qualitative evaluation of their impact. Health Res Policy Syst 15(6). Available at: https://doi.org/10.1186/s12961-016-0166-4
Annex 1 – Organisations that may be involved in regional or local research
- Clinical Research Networks (CRNs) , which will be retendered and renamed regional research delivery networks (RRDNs) from April 2024
- Applied Research Collaborations (ARCs)
- Biomedical Research Centres (BRCs)
- Experimental Cancer Medicine Centres (ECMCs) , jointly funded with Cancer Research UK
- Research Design Services (RDSs) and Clinical Trials Units (CTUs) which will be replaced by the NIHR Research Support Service from 1 October 2023
- Patient Recruitment Centres (PRCs)
- MedTech and In vitro diagnostic Co-operatives (MICs) , which will be replaced with HealthTech research centres from April 2024
- School of Public Health Research, School of Primary Care Research and School of Social Care Research
- Health Determinants Research Collaborations (HDRCs)
- Clinical Research Facilities (CRFs)
- Patient Safety Research Collaborations (PSRCs)
- Translational Research Collaborations (TRCs)
- Academic Health Science Centres (AHSCs)
- university teaching hospitals and all trusts that deliver research activity
- primary care organisations, including GP practices, that deliver research activity
- higher education institutions (HEIs)
- local authorities
- social care partners
- Local Government Association
- local and national charities that fund, collaborate in or support participation in research
- research and development offices in providers or CSUs, including primary care providers and ambulance, community and mental health trusts, and those in the VCSE sector
- UKRD members
- NHS subnational secure data environments for research
- NHS R&D Forum
- NHS Genomic Medicines Service Research Collaborative
- NHS Knowledge and Library Services
- Academic Health Science Networks (AHSNs) are often well linked with research organisations and infrastructure as part of their roles in development, adoption and spread of innovation.
Annex 2 – Public health and social care research
Public health research investigates issues that impact at a population rather than an individual level. This can be done within the NHS with system-level studies, such as secondary prevention of cardiovascular disease and examining the impact on health inequalities of changes to the NHS resource allocation formula, and outside the NHS for the wider determinants of health such as air quality, transport systems and housing. There is a substantial body of public health evidence for the clinical and cost effectiveness of prevention, health protection, health service redesign and addressing health inequalities.
Social care research aims to improve the lives of children and adults who need to draw on personal or practical care and support, and family members or other unpaid carers. It can include research around the introduction, use and impact of technologies, and changing social care interventions, policies and practice. Social care research also examines issues pertaining to the safeguarding of adults and children and workforce, commissioning of services, and questions about organisational and professional practice, including decision-making, training and the quality of care.
Publication reference: PR1662
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Science, health, and public trust.
September 8, 2021
Explaining How Research Works
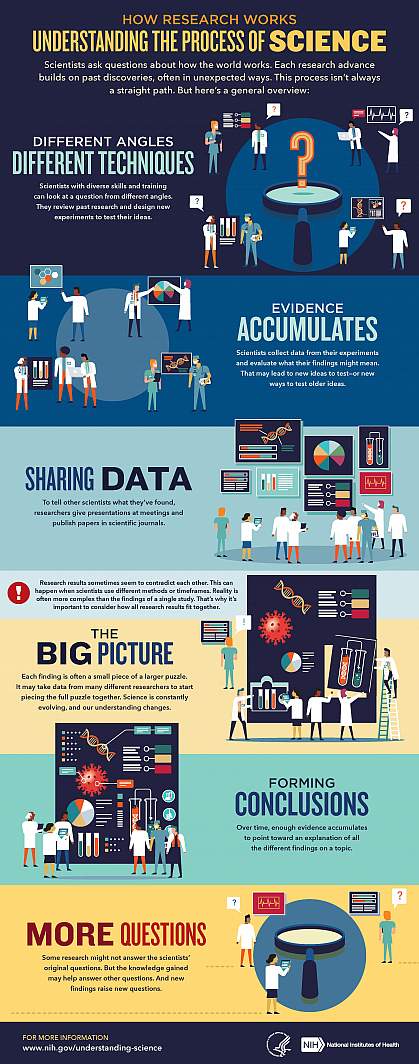
We’ve heard “follow the science” a lot during the pandemic. But it seems science has taken us on a long and winding road filled with twists and turns, even changing directions at times. That’s led some people to feel they can’t trust science. But when what we know changes, it often means science is working.

Explaining the scientific process may be one way that science communicators can help maintain public trust in science. Placing research in the bigger context of its field and where it fits into the scientific process can help people better understand and interpret new findings as they emerge. A single study usually uncovers only a piece of a larger puzzle.
Questions about how the world works are often investigated on many different levels. For example, scientists can look at the different atoms in a molecule, cells in a tissue, or how different tissues or systems affect each other. Researchers often must choose one or a finite number of ways to investigate a question. It can take many different studies using different approaches to start piecing the whole picture together.
Sometimes it might seem like research results contradict each other. But often, studies are just looking at different aspects of the same problem. Researchers can also investigate a question using different techniques or timeframes. That may lead them to arrive at different conclusions from the same data.
Using the data available at the time of their study, scientists develop different explanations, or models. New information may mean that a novel model needs to be developed to account for it. The models that prevail are those that can withstand the test of time and incorporate new information. Science is a constantly evolving and self-correcting process.
Scientists gain more confidence about a model through the scientific process. They replicate each other’s work. They present at conferences. And papers undergo peer review, in which experts in the field review the work before it can be published in scientific journals. This helps ensure that the study is up to current scientific standards and maintains a level of integrity. Peer reviewers may find problems with the experiments or think different experiments are needed to justify the conclusions. They might even offer new ways to interpret the data.
It’s important for science communicators to consider which stage a study is at in the scientific process when deciding whether to cover it. Some studies are posted on preprint servers for other scientists to start weighing in on and haven’t yet been fully vetted. Results that haven't yet been subjected to scientific scrutiny should be reported on with care and context to avoid confusion or frustration from readers.
We’ve developed a one-page guide, "How Research Works: Understanding the Process of Science" to help communicators put the process of science into perspective. We hope it can serve as a useful resource to help explain why science changes—and why it’s important to expect that change. Please take a look and share your thoughts with us by sending an email to [email protected].
Below are some additional resources:
- Discoveries in Basic Science: A Perfectly Imperfect Process
- When Clinical Research Is in the News
- What is Basic Science and Why is it Important?
- What is a Research Organism?
- What Are Clinical Trials and Studies?
- Basic Research – Digital Media Kit
- Decoding Science: How Does Science Know What It Knows? (NAS)
- Can Science Help People Make Decisions ? (NAS)
Connect with Us
- More Social Media from NIH
Evidence-Based Research Series-Paper 1: What Evidence-Based Research is and why is it important?
Affiliations.
- 1 Johns Hopkins Evidence-based Practice Center, Division of General Internal Medicine, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA.
- 2 Digital Content Services, Operations, Elsevier Ltd., 125 London Wall, London, EC2Y 5AS, UK.
- 3 School of Nursing, McMaster University, Health Sciences Centre, Room 2J20, 1280 Main Street West, Hamilton, Ontario, Canada, L8S 4K1; Section for Evidence-Based Practice, Western Norway University of Applied Sciences, Inndalsveien 28, Bergen, P.O.Box 7030 N-5020 Bergen, Norway.
- 4 Department of Sport Science and Clinical Biomechanics, University of Southern Denmark, Campusvej 55, 5230, Odense M, Denmark; Department of Physiotherapy and Occupational Therapy, University Hospital of Copenhagen, Herlev & Gentofte, Kildegaardsvej 28, 2900, Hellerup, Denmark.
- 5 Musculoskeletal Statistics Unit, the Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, Nordre Fasanvej 57, 2000, Copenhagen F, Denmark; Department of Clinical Research, Research Unit of Rheumatology, University of Southern Denmark, Odense University Hospital, Denmark.
- 6 Section for Evidence-Based Practice, Western Norway University of Applied Sciences, Inndalsveien 28, Bergen, P.O.Box 7030 N-5020 Bergen, Norway. Electronic address: [email protected].
- PMID: 32979491
- DOI: 10.1016/j.jclinepi.2020.07.020
Objectives: There is considerable actual and potential waste in research. Evidence-based research ensures worthwhile and valuable research. The aim of this series, which this article introduces, is to describe the evidence-based research approach.
Study design and setting: In this first article of a three-article series, we introduce the evidence-based research approach. Evidence-based research is the use of prior research in a systematic and transparent way to inform a new study so that it is answering questions that matter in a valid, efficient, and accessible manner.
Results: We describe evidence-based research and provide an overview of the approach of systematically and transparently using previous research before starting a new study to justify and design the new study (article #2 in series) and-on study completion-place its results in the context with what is already known (article #3 in series).
Conclusion: This series introduces evidence-based research as an approach to minimize unnecessary and irrelevant clinical health research that is unscientific, wasteful, and unethical.
Keywords: Clinical health research; Clinical trials; Evidence synthesis; Evidence-based research; Medical ethics; Research ethics; Systematic review.
Copyright © 2020 Elsevier Inc. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Biomedical Research* / methods
- Biomedical Research* / organization & administration
- Clinical Trials as Topic / ethics
- Clinical Trials as Topic / methods
- Clinical Trials as Topic / organization & administration
- Ethics, Research
- Evidence-Based Medicine / methods*
- Needs Assessment
- Reproducibility of Results
- Research Design* / standards
- Research Design* / trends
- Systematic Reviews as Topic
- Treatment Outcome

IMAGES
VIDEO
COMMENTS
Health research entails systematic collection or analysis of data with the intent to develop generalizable knowledge to understand health challenges and mount an improved response to them.
1.2 Why research is important. The UK is a world leader for research and invention in healthcare, with around 25% of the world’s top 100 prescription medicines being …
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials. Read and share this infographic (PDF, 317K) to learn why researchers do different kinds …
As a registered nurse (RN), research nurse, or aspiring nurse, it is important to understand how research aids in improving clinical practice, including benefits like evidence-based care and ensuring patient safety.
Increasingly, nursing research is considered essential to the achievement of high-quality patient care and outcomes. In this month's Magnet® Perspectives column, we examine …
We’ve developed a one-page guide, "How Research Works: Understanding the Process of Science" to help communicators put the process of science into perspective. We …
This series introduces evidence-based research as an approach to minimize unnecessary and irrelevant clinical health research that is unscientific, wasteful, and unethical.